
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 2
3,3'-Dichlorobenzidine
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Ms R. Gomes and Ms M.E. Meek,
Environmental Health Directorate,
Health Canada
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization Geneva, 1998
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
3,3'-Dichlorobenzidine.
(Concise international chemical assessment document ; 2)
1.3,3'-Dichlorobenzidine - toxicity 2.Environmental exposure
3.Occupational exposure I.International Programme on
Chemical Safety II.Series
ISBN 92 4 153002 2 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1998
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND
HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1. Aquatic environment
10.2. Terrestrial environment
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response
assessment
11.1.2. Criteria for setting guidance values for
3,3'-dichlorobenzidine
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
13.1. Human health hazards
13.2. Advice to physicians
13.3. Health surveillance advice
13.4. Fire hazards
13.5. Spillage and disposal
13.5.1. Spillage
13.5.2. Disposal
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
INTERNATIONAL CHEMICAL SAFETY CARD
REFERENCES
APPENDIX 1 - SOURCE DOCUMENTS
APPENDIX 2 - CICAD PEER REVIEW
APPENDIX 3 - CICAD FINAL REVIEW BOARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACION
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) - a cooperative programme of the
World Health Organization (WHO), the International Labour Organisation
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents have undergone extensive peer review by internationally
selected experts to ensure their completeness, accuracy in the way in
which the original data are represented, and the validity of the
conclusions drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary
of all available data on a particular chemical; rather, they include
only that information considered critical for characterization of the
risk posed by the chemical. The critical studies are, however,
presented in sufficient detail to support the conclusions drawn. For
additional information, the reader should consult the identified
source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based guidance values.
1 International Programme on Chemical Safety (1994) Assessing
human health risks of chemicals: derivation of guidance values for
health-based exposure limits. Geneva, World Health Organization
(Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact the IPCS to inform it of the new information.
Procedures
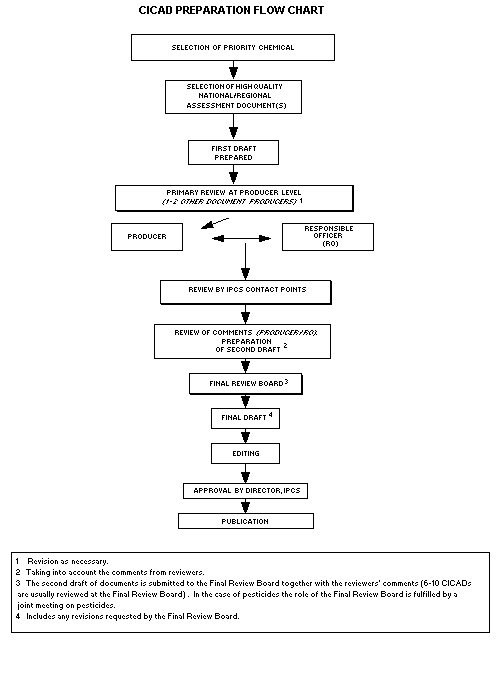
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world - expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS and one or
more experienced authors of criteria documents to ensure that it meets
the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments
into account and revise their draft, if necessary. The resulting
second draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards
are chosen according to the range of expertise required for a meeting
and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on 3,3'-dichlorobenzidine was prepared by the
Environmental Health Directorate of Health Canada, based principally
on reviews of the Government of Canada (1993) and the Agency for Toxic
Substances and Disease Registry (ATSDR, 1989), to assess the potential
effects on human health of indirect exposure to 3,3'-dichlorobenzidine
in the general environment as well as the chemical's environmental
effects. Data relevant to the assessment of the effects of
3,3'-dichlorobenzidine on the environment and human health identified
as of April 1992 (environmental effects) and October 1992 (human
health effects) were considered in the Government of Canada (1993)
review; data were subsequently updated to September 1995 from the
International Register of Potentially Toxic Chemicals and on-line
databases. Information concerning the nature of peer review and the
availability of the Government of Canada (1993) and ATSDR (1989)
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0481) for 3,3'-dichlorobenzidine, produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
3,3'-Dichlorobenzidine (CAS no. 91-94-1) is a synthetic
chlorinated primary aromatic amine and is commercially available as
the dihydrochloride salt, 3,3'-dichlorobenzidine dihydrochloride
(C12H10N2Cl2.2HCl). 3,3'-Dichlorobenzidine (and some of its
derivatives) is used primarily as an intermediate in the manufacture
of pigments for printing inks, textiles, paints, and plastics. It is
also used in the analytical determination of gold and as a curing
agent in the synthesis of polyurethane elastomers.
Occupational exposure to 3,3'-dichlorobenzidine may occur in the
pigment manufacturing industry and in industrial applications where
3,3'-dichlorobenzidine-based pigments are heated for prolonged
periods. Levels of 3,3'-dichlorobenzidine ranging from <0.6 to 25
µg/m3 have been measured in the workroom air of production and
pigment manufacturing plants in Germany and Japan.
Owing to its relatively low volatility, very short persistence,
and low concentrations in the atmosphere, 3,3'-dichlorobenzidine is
not expected to contribute to the greenhouse effect, depletion of the
ozone layer, or the formation of ground-level ozone. Based on
available data, it is likely that 3,3'-dichlorobenzidine poses
negligible risk to aquatic organisms.
Available data are inadequate to serve as a basis for the
development of tolerable intakes of 3,3'-dichlorobenzidine based on
non-neoplastic effects. Based upon sufficient evidence of
carcinogenicity of 3,3'-dichlorobenzidine in multiple animal species
in limited studies and substantial evidence of genotoxicity both
in vitro and in vivo, 3,3'-dichlorobenzidine is considered to be a
potential human carcinogen. In view of its potential carcinogenicity
in humans and in the absence of adequate epidemiological data, a
sample health-based guidance value for 3,3'-dichlorobenzidine has been
derived based upon the carcinogenic potency (i.e. TD0.05) of this
chemical, which has been calculated from the best reported, although
limited, chronic study in which ChR-CD rats were fed 1000 ppm
3,3'-dichlorobenzidine in the diet for up to 488 days. The TD0.05 was
calculated based on linear interpolation, incorporating a body weight
to surface area correction (power of 2/3, owing to the lack of
identification of the active metabolite[s]) and correcting for less
than 2 years of exposure, and ranges from 0.74 to 1.4 mg/kg body
weight per day. A value obtained by dividing the low end of this
TD0.05 range (i.e. 0.74 mg/kg body weight per day) by, for example,
5000-50 000 (i.e. 1.48 × 10-4 to 1.48 × 10-5 mg/kg body weight per
day) might be considered appropriate as a guidance value in ingested
media. This margin affords protection similar to that associated with
the range for low-dose risk estimates generally considered by various
agencies to be "essentially negligible" (i.e. 10-5 to 10-6). Based
upon a sample estimate of exposure that involved predicted
concentrations or limited monitoring data from the USA and Canada,
where the chemical has generally not been detected, the estimated
total daily intake of 3,3'-dichlorobenzidine by the general population
(from indirect exposure in the general environment) is several orders
of magnitude less than the sample guidance value derived above.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3,3'-Dichlorobenzidine (CAS no. 91-94-1;
3,3'-dichloro-4,4'-biphenyldiamine,
3,3'-dichloro-4,4'-diaminobiphenyl,
4,4'-diamino-3,3'-dichlorobiphenyl), a synthetic chlorinated primary
aromatic amine, is a grey to purple crystalline solid at room
temperature. It has a relatively low vapour pressure (1 mPa at 22°C),
a low water solubility (0.4 mg/100 ml at 22°C), and a log
n-octanol/water partition coefficient of 3.02.
3,3'-Dichlorobenzidine is usually commercially available as the
dihydrochloride salt, 3,3'-dichlorobenzidine dihydrochloride
(C12H10N2Cl2.2HCl) (Government of Canada, 1993). Additional
physical/chemical properties of 3,3'-dichlorobenzidine are presented
in the International Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Analysis of 3,3'-dichlorobenzidine in environmental samples is
most commonly achieved by gas chromatography/mass spectrometry,
capillary column gas chromatography/Fourier transform infrared
spectrometry, and high-performance liquid chromatography. A detection
limit of 3 µg/m3 has been reported for 3,3'-dichlorobenzidine in air,
whereas detection limits for 3,3'-dichlorobenzidine in water range
from 0.05 to 50 µg/litre (IARC, 1982; ATSDR, 1989). A detection limit
of <20 ppb (µg/kg) has been reported for 3,3'-dichlorobenzidine in
food (i.e. fish) using gas chromatographic techniques with either
nitrogen-phosphorus detection or electron capture detection. No
analytical methods for the determination of 3,3'-dichlorobenzidine in
soil or sediment were identified (ATSDR, 1989). A detection limit of
60 ppt (ng/litre) has been reported for 3,3'-dichlorobenzidine in
biological samples (i.e. urine) using gas chromatographic techniques
with either electron capture detection or rubidium-sensitized
thermionic detection (HSDB, 1995).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 3,3'-dichlorobenzidine.
3,3'-Dichlorobenzidine (and some of its derivatives) is used primarily
as an intermediate in the manufacture of diarylide yellow or azo red
pigments for printing inks, textiles, paints, and plastics. It is
also used in the analytical determination of gold and as a curing
agent in the synthesis of polyurethane elastomers (Gerarde & Gerarde,
1974; Government of Canada, 1993).
The world production of 3,3'-dichlorobenzidine was estimated to
range from 7000 to 10 000 t in 1983, with 3900-4200 t being
synthesized in Europe. In Japan, approximately 3170 t and 3400 t of
3,3'-dichlorobenzidine were produced in 1992 and 1993, respectively.
In Germany, approximately 2500 t of 3,3'-dichlorobenzidine were
produced in 1989 (none of which was exported). In 1983, 1.1 million
pounds (500 t) of 3,3'-dichlorobenzidine base and salts were imported
into the USA. In 1987, 9.9 million pounds (4490 t) of
3,3'-dichlorobenzidine were consumed in the USA.
3,3'-Dichlorobenzidine is not produced in Canada but has been imported
on a regular basis. The reported volumes of importation into Canada
have ranged from 21 to 109 t over the period 1986-1989 (ATSDR, 1989;
Chemicals Daily, 1993, 1994; Government of Canada, 1993; Law, 1995).
3,3'-Dichlorobenzidine can enter the environment from any stage
in the production, storage, transport, use, or disposal of
3,3'-dichlorobenzidine-containing materials. Although there are no
quantitative data, the largest releases would likely be through direct
emissions from plants that manufacture 3,3'-dichlorobenzidine-
containing materials and from the degradation of
3,3'-dichlorobenzidine-containing pigments (Government of Canada,
1993).
Total industrial emissions of 3,3'-dichlorobenzidine to the
environment in the USA in 1988 were estimated to be 6 t. In Germany,
emissions of 3,3'-dichlorobenzidine into wastewater from various
industrial facilities were estimated to be less than 0.095 t in 1989.
Total industrial emissions of 3,3'-dichlorobenzidine to the
environment in Canada were estimated to be 0.1 t in 1989 (Government
of Canada, 1993; Law, 1995).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The half-life for volatilization of 3,3'-dichlorobenzidine from
surface water to the atmosphere has been estimated to be 72 days. In
water, 3,3'-dichlorobenzidine may be degraded by photooxidation,
photolysis, and biodegradation; the estimated half-life of
3,3'-dichlorobenzidine in water is less than 10 minutes. The
half-life of 3,3'-dichlorobenzidine in aqueous media is proportional
to the light intensity, with some of the major photodegradation
products including monochlorobenzidine, benzidine, as well as a number
of brightly coloured water-insoluble materials; under intense light or
sunlight, very little of the 3,3'-dichlorobenzidine,
monochlorobenzidine, and benzidine remain in the water after 15
minutes (Banerjee et al., 1978; Government of Canada, 1993).
3,3'-Dichlorobenzidine is fairly resistant to degradation by
naturally occurring aquatic microorganisms. Half-lives of 4-26 weeks
and 16-101 weeks have been estimated for the biodegradation of
3,3'-dichlorobenzidine in surface water and anaerobic groundwater,
respectively (Government of Canada, 1993).
3,3'-Dichlorobenzidine is strongly bound to soil, sediment, and
sludge and therefore is highly immobile. In soil,
3,3'-dichlorobenzidine is mineralized very slowly under aerobic and
anaerobic conditions. The half-life for the aerobic degradation of
3,3'-dichlorobenzidine in soil has been estimated to range from 4 to
26 weeks. 3,3'-Dichlorobenzidine may also be oxidized by metal ions
(e.g. ferric iron) present in clay (BUA, 1993; Government of Canada,
1993; HSDB, 1995).
Half-lives of 1.5-5 minutes and 0.9-9 hours have been estimated
for the photolysis and photooxidation of 3,3'-dichlorobenzidine in
air, respectively (Government of Canada, 1993).
3,3'-Dichlorobenzidine can accumulate in aquatic biota. A
bioconcentration factor of approximately 500 has been reported for
bluegill sunfish (Lepomis macrochirus), based on a study in which
the fish were exposed to 5 or 100 µg [14C]3,3'-dichlorobenzidine per
litre; equilibria were achieved within 96-168 hours (Appleton & Sikka,
1980). In other studies, a 3-day bioaccumulation factor of 610 in
fish (golden orfe, Leuciscus idus melanotus), a 5-day
bioaccumulation factor of 3100 in activated sludge, and a 1-day
bioaccumulation factor of 940 in algae (Chlorella fusca) have been
reported (Freitag et al., 1985).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Available data on the levels of 3,3'-dichlorobenzidine in air
were limited to reports from the USA in which 3,3'-dichlorobenzidine
was not detected (detection limits 0.1 and 5 ng/m3) in ambient air in
the vicinity of two dyestuff production facilities (Narang et al.,
1982; Riggin et al., 1983).
Levels of 3,3'-dichlorobenzidine in surface water have ranged
from not detected (the only reported detection limit was 0.25
µg/litre) to 0.3 µg/litre, based on studies conducted between 1987 and
1992 in France, England, the Netherlands, Switzerland, Germany, Spain,
and the USA (Staples et al., 1985; Valls et al., 1990; Slobodnik et
al., 1993).
In the only relevant study identified, 3,3'-dichlorobenzidine was
not detected (detection limit 0.02 ng/litre) in samples of
drinking-water collected from two municipalities in Ontario, Canada
(Malaiyandi et al., 1987).
Data were not identified concerning the levels of
3,3'-dichlorobenzidine in uncontaminated soil; however, this compound
was not detected (detection limits not reported) in sediment in the
USA (location of sample acquisition not reported) and Spain (samples
collected from the Besos River, Barcelona) (Staples et al., 1985;
Valls et al., 1990). Information concerning the levels (or
occurrence) of 3,3'-dichlorobenzidine in foodstuffs was not
identified. Although 3,3'-dichlorobenzidine has the potential to
bioaccumulate in aquatic organisms, this compound was not detected
(detection limit not reported) in samples of biota collected in the
USA (Staples et al., 1985).
Concentrations of 3,3'-dichlorobenzidine at contaminated
(industrial and municipal) sites within the USA have ranged from not
detected (detection limit 20 µg/litre) to 120 µg/litre in landfill
leachate and groundwater and from not detected (detection limit 0.66
mg/kg) to 20 mg/kg dry weight in landfill soil and sewage sludge
(Fricke et al., 1985; Smith & Weber, 1990; US EPA, 1990a,b; BUA,
1993).
Estimates of the fate and concentrations of
3,3'-dichlorobenzidine in the Canadian environment have been generated
by the Level III fugacity computer model of Mackay and Paterson
(1991), incorporating data on the chemical's physical and chemical
properties and transformation half-lives (Howard et al., 1991) and
assuming that 1% of the total amount produced and imported into Canada
is released into various media in proportions similar to those
reported in the US Toxic Release Inventory. The results indicate
that, at steady state, the proportion of released
3,3'-dichlorobenzidine found in air, water, and soil would be
<0.001%, 99.75%, and <0.001%, respectively. Average concentrations
of 3,3'-dichlorobenzidine in air, water, and soil, predicted on the
basis of the model, were 7.6 × 10-16 µg/m3, 3.4 × 10-7 ng/litre, and
1.1 × 10-16 µg/g dry weight, respectively (Government of Canada,
1993). As 3,3'-dichlorobenzidine binds strongly to soil and aquatic
sediment, it is likely to have reduced bioavailability.
6.2 Human exposure
Exposure of the general population to 3,3'-dichlorobenzidine in
environmental media may be estimated based on measured and predicted
concentrations in various media and reference values for body weight
and consumption patterns. Owing to the availability of relevant data,
exposure has been estimated here based primarily on data from Canada
and the USA. In view of the extremely limited nature of the available
data, these estimates are presented primarily in an attempt to
identify typical relative proportions of exposure from various media.
Countries are encouraged to estimate total exposure on the basis of
national data, possibly in a manner similar to that outlined here.
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the most
sensitive detection limit for 3,3'-dichlorobenzidine (0.1 ng/m3) in
reports in which this compound was not detected in the air surrounding
dye production facilities in the USA, mean intake from air for the
general population is estimated to be less than 3.4 × 10-5 µg/kg body
weight per day. Based on a predicted concentration (by fugacity
modelling) of 3,3'-dichlorobenzidine in ambient air in Canada of 7.6 ×
10-16 µg/m3, the mean intake of 3,3'-dichlorobenzidine from air for
the general population is estimated to be much lower (i.e. 2.6 × 10-16
µg/kg body weight per day).
Based on a daily volume of water consumption for adults of 1.4
litres, a mean body weight of 64 kg (IPCS, 1994), and the limit of
detection for 3,3'-dichlorobenzidine (0.02 ng/litre) in a survey in
which this compound was not detected in drinking-water from two
municipalities in Canada, the mean intake of 3,3'-dichlorobenzidine
from drinking-water for the general population is estimated to be less
than 4.4 × 10-7 µg/kg body weight per day. However, based on a
predicted concentration of 3,3'-dichlorobenzidine in surface water in
Canada of 3.4 × 10-7 ng/litre, the estimated mean intake of
3,3'-dichlorobenzidine from drinking-water for the general population
would be 7.4 × 10-12 µg/kg body weight per day.
Available data were inadequate to estimate the intake of
3,3'-dichlorobenzidine from food. Studies were not identified
concerning measured levels of 3,3'-dichlorobenzidine in soil; however,
based on a predicted concentration of 3,3'-dichlorobenzidine in soil
in Canada of 1.1 × 10-16 µg/g, a daily ingestion of 20 mg of soil by
adults, and a mean body weight of 64 kg (IPCS, 1994), the estimated
intake of 3,3'-dichlorobenzidine from soil would be 3.4 × 10-20 µg/kg
body weight per day.
The general population may be exposed to 3,3'-dichlorobenzidine
during the use of commercial products, including pressurized spray
containers of paints, lacquers, and enamels containing traces of
benzidine yellow, an azo dye derived from 3,3'-dichlorobenzidine
(ATSDR, 1989). However, no quantitative data were identified
concerning the levels of 3,3'-dichlorobenzidine in products containing
pigments or dyes derived from this substance.
Occupational exposure (principally by inhalation or dermal
contact) to 3,3'-dichlorobenzidine may occur in the pigment
manufacturing industry and in industrial applications where
3,3'-dichlorobenzidine-based pigments are heated for prolonged periods
(e.g. the production of coloured plastics and films; the spin dying of
polypropylene fibres) (US EPA, 1990c; US DHHS, 1994). Levels of
3,3'-dichlorobenzidine ranging from <0.6 to 25 µg/m3 have been
measured in the workroom air of production and pigment manufacturing
plants in Germany (ATSDR, 1989) and Japan (US EPA, 1980).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
It is generally believed that the hepatic metabolism of
3,3'-dichlorobenzidine in rodents (i.e. rats) involves its oxidation
to highly reactive N-oxygenated intermediates by the microsomal
cytochrome P-450 and flavin monooxygenase enzyme complexes. Although
the 3,3'-dichlorobenzidine-derived N-oxygenated intermediates have
not been unequivocally identified, they are believed to be responsible
for the mutagenic and genotoxic effects (e.g. related to DNA binding)
of 3,3'-dichlorobenzidine in bacterial and mammalian systems
(Government of Canada, 1993).
Few quantitative data on the metabolism of 3,3'-dichlorobenzidine
in humans were identified. Small amounts (approximately 1-2%) of free
and glucuronide-conjugated N-hydroxyacetyl derivatives of
3,3'-dichlorobenzidine were excreted in the urine of volunteers orally
administered 3,3'-dichlorobenzidine. Based upon studies in which
3,3'-dichlorobenzidine has been detected in the urine (at
concentrations up to 296 µg/litre) of workers occupationally exposed
to this substance, 3,3'-dichlorobenzidine appears to be readily
absorbed from the skin following dermal exposure (BUA, 1993).
3,3'-Dichloro- N-acetylbenzidine,
3,3'-dichloro- N,N'-diacetylbenzidine, and conjugated metabolites
(the identities of which were not confirmed) have been detected in
the urine of rats administered 3,3'-dichlorobenzidine orally. The
covalent binding of 3,3'-dichlorobenzidine metabolites to haemoglobin,
hepatic lipids, and DNA in the intestinal and bladder epithelium or
liver has been observed in experimental animals (e.g. rodents) exposed
in vivo (Government of Canada, 1993).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of 3,3'-dichlorobenzidine (either as the free
base or as dihydrochloride salt) in experimental animals is low, with
oral LD50s in rats and mice being higher than 3820 mg/kg body weight
and 488 mg/kg body weight, respectively (Government of Canada, 1993).
Gastrointestinal congestion and haemorrhage were the principal effects
in these studies (ACGIH, 1991). In their review of acute toxicity
data, the American Conference of Governmental and Industrial
Hygienists (ACGIH, 1991) reported mortality in four of five rabbits
following dermal application of 3,3'-dichlorobenzidine at 1 g/kg body
weight for 24 hours; however, additional data were not presented.
Following the acute administration (route not specified) of up to 50
mg of 3,3'-dichlorobenzidine per kg body weight in cats, there was
only a slight increase (up to 1.3%) in methaemoglobin content in the
blood, whereas the number of Heinz bodies was increased fourfold (BUA,
1993).
8.2 Irritation and sensitization
Data on the sensitization potential of 3,3'-dichlorobenzidine in
experimental animals were not identified, and only limited data were
available concerning the irritation potential of this chemical. BUA
(1993) reported no irritation or "symptoms of intolerance" in rabbits
exposed by conjunctival application of 100 mg of
3,3'-dichlorobenzidine; however, additional data were not presented in
this published account.
8.3 Short-term exposure
Studies on the toxicological effects produced following the
short-term repeated exposure of experimental animals to
3,3'-dichlorobenzidine have not been identified.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Subchronic studies in which non-neoplastic effects of
3,3'-dichlorobenzidine have been assessed have not been identified.
8.4.2 Chronic exposure and carcinogenicity
Chronic studies in which non-neoplastic effects of
3,3'-dichlorobenzidine have been adequately assessed have not been
identified. In several early limited carcinogenesis bioassays
(summarized in Table 1), 3,3'-dichlorobenzidine increased the
incidence of tumours in experimental animals, sometimes following
relatively short periods of administration. The incidence of mammary
gland carcinomas in ChR-CD rats administered diets containing 0 or
1000 ppm 3,3'-dichlorobenzidine for up to 488 days was 3/44 and 26/44
in females and 0/44 and 7/44 in males, respectively (Stula et al.,
1975). In male rats, the incidence of Zymbal gland carcinomas was
0/44 and 8/44 and the incidence of granulocytic leukaemias was 2/44
and 9/44 in the control and 3,3'-dichlorobenzidine-exposed animals,
respectively. In a limited study in which a small group of beagle
dogs received approximately 10.4 mg 3,3'-dichlorobenzidine per kg body
weight in gelatin capsules 3 or 5 times/week for up to 7.1 years, the
incidence of hepatocellular carcinoma and bladder carcinoma was
increased ( p < 0.025) compared with controls (Stula et al., 1978).
Hepatic tumours occurred early (after 6 and 12 months) in male ICR/JCL
mice administered a diet containing 0 or 1000 ppm
3,3'-dichlorobenzidine over a 12-month period; after 12 months, the
incidence of hepatomas was 2/21 and 18/18 ( p < 0.001) in the
control and exposed groups, respectively (Osanai, 1976).
Compared with controls, the incidence of lymphoid leukaemias in
offspring born to female mice administered five subcutaneous
injections of 3,3'-dichlorobenzidine during the last week of pregnancy
was increased (0/30 versus 7/24; statistical significance unspecified)
(Golub et al., 1975). Results in studies with hamsters have been
equivocal (Saffiotti et al., 1967; Sellakumar et al., 1969). Other
identified studies (Pliss, 1959, 1963; Tsuda et al., 1977) contribute
little to the assessment of the weight of evidence of carcinogenicity
for 3,3'-dichlorobenzidine, owing to their limitations of protocol and
reporting (Table 1).
8.5 Genotoxicity and related end-points
There is convincing evidence that 3,3'-dichlorobenzidine is
genotoxic both in vivo and in vitro (Table 2). In in vitro
assays, 3,3'-dichlorobenzidine was mutagenic in Salmonella
typhimurium, increased the frequency of sister chromatid exchange
and unscheduled DNA synthesis in human cells, and "morphologically
transformed" rat embryo cells. In in vivo studies,
3,3'-dichlorobenzidine increased the frequency of micronuclei in the
bone marrow cells of mice, increased unscheduled DNA synthesis in the
liver of rats, and increased the frequency of chromosomal aberrations
in the bone marrow cells of mice.
8.6 Reproductive and developmental toxicity
The incidence of "hyperplastic changes" in kidney explants
obtained from mouse embryos exposed to 3,3'-dichlorobenzidine
in utero (following subcutaneous injection of dams) was increased,
compared with controls (Shabad et al., 1972).
8.7 Immunological and neurological effects
Studies concerning the immunological effects of
3,3'-dichlorobenzidine have not been identified. Data on neurotoxic
effects were limited to the observation (Stula et al., 1978) of
convulsions and neuronal degeneration in one female beagle dog orally
administered 3,3'-dichlorobenzidine (100 mg 3 times weekly for 6 weeks
and then 5 times weekly for the duration of the experiment) for 42
months.
Table 1: Carcinogenicity of 3,3'-dichlorobenzidine.
Test protocol Results Comments Reference
Female beagle dogs (n = 6) were The incidence of bladder Small number of anumals and Stula et al., 1978
administered 3,3'-dichlorobenzidine carcinomas and hepatocellular single sex exposed for a limited
(100 mg in gelatin capsules) 3 carcinomas in 3,3'-dichloro- proportion of life span
times/week for 6 weeks, and then 5 benzidine-exposed animals that
times/week for up to 7.1 years (mean survived longer than 6.6 years was
dose approximately 10.4 mg/kg body 5/5 (p < 0.025) and 4/5 (p < 0.025),
weight per exposure); six unexposed respectively. No liver or bladder
controls were sacrificed after 8.3-9.0 tumours were found in the
years. unexposed controls, although 4/6
controls were reported to have
developed mammary carcinomas
(attributed to the age of these
animals).
Groups of ChR-CD rats (n = 50 per sex) The incidence of mammary Exposure for a limited proportion Stula et al., 1975
were administered diets containing 0 or carcinomas in rats administered 0 or of life span of one sex
1000 ppm (0.1% w/w) 3,3'-dichloro- 1000 ppm 3,3'-dichlorobenzidine in
benzidine (0 or 50 mg/kg body weight the diet was 3/44 and 26/44 (p <
per day) for up to 488 days. 0.05) in females and 0/44 and 7/44
(p < 0.05) in males. In male rats,
the incidence of Zymbal gland
carcinomas was 0/44 and 8/44 (p <
0.05) and the incidence of
granulocytic leukaemias was 2/44
and 9/44 (p < 0.05) in the control
and 3,3'-dichlorobenzidine-exposed
animals, respectively.
Table 1 (continued)
Test protocol Results Comments Reference
Male (n = 35) and female (n = 15) Tumours (in the Zymbal and Small group sizes, single dose Pliss, 1959
Rappolovo rats were administered a mammary glands, bladder, and level, lack of appropriate
diet containing 3,3'-dichlorobenzidine haematopoietic system) were controls, and incomplete
(0.5-1.0 ml of a 4.4% solution) observed in 79% (23/29) of animals reporting of results (i.e. the sex
dissolved in sunflower oil for 6 surviving at the time (not specified) of the animals with tumours, and
days/week for 12 months. "Control" the first tumours were detected the nature and incidence of
animals (n = 130) were administered following exposure to 3,3'-dichloro- tumours)
(by injection) octadecylamine and benzidine; no tumours were
stearylamine for 10 months and observed in the controls.
observed until 23 months.
Rappolovo rats (sex and number not Tumours (in bone, mammary Lack of appropriate controls and Pliss, 1963
reported) were administered a diet glands, bladder, "etc.") were incomplete reporting of the
containing an amount (not clearly observed in 86% of rats exposed to protocol and results (i.e. nature
specified) of 3,3'-dichlorobenzidine for 3,3'-dichlorobenzidine in the diet, and incidence of the tumours)
10-13 months. A group of 50 rats (25 whereas tumours (liver sarcoma)
of which were injected with sunflower were observed in only one of the
oil) served as controls. controls.
Groups of male Wistar rats (n = 18) No evidence of histopathological Limited period of exposure, Tsuda et al., 1977
were administered diets containing 0 or effects in either the bladder or liver small number of animals, single
0.03% (w/w) (0 or 300 ppm) 3,3'- was observed following the sex, and limited histopatho-
dichlorobenzidine (0 or 15 mg/kg body administration of diets containing 0 logical examination
weight per day) for 40 weeks. or 300 ppm 3,3'-dichlorobenzidine
for 40 weeks.
Table 1 (continued)
Test protocol Results Comments Reference
Groups of male ICR/JCL mice (n = 26) After 12 months, the incidence of Small numbers of animals, Osanai, 1976
were administered a diet containing 0 hepatomas was 2/21 and 18/18 single sex, and incomplete
or 0.1% (w/w) (0 or 1000 ppm) 3,3'- (p < 0.001) in the control and 3,3'- description of the nature of
dichlorobenzidine (0 or 130 mg/kg body dichlorobenzidine-exposed groups, hepatic tumours
weight per day) for up to 12 months. respectively.
Male (n = 51) and female (n = 22) After 18.5 months, the incidence of Small group sizes, single dose Pliss, 1959
strain D mice were administered a diet hepatomas and haemangiomas in level, lack of appropriate
containing 3,3'-dichlorobenzidine (0.1 mice exposed to 3,3'-dichloro- controls, and incomplete
ml of a 1.1% solution) for a period of benzidine was 2/18 and 2/18, reporting of results (i.e. the sex
10 months and were maintained until respectively. of animals with tumours, and the
18.5 months. No information was nature and incidence of the
presented concerning the use of tumours)
controls.
Groups of Syrian golden hamsters (n = Exposure to 1000 ppm 3,3'-dichloro- Small group sizes and Saffiotti et al.,
30 per sex) were administered diets benzidine in the diet failed to inadequate reporting of data in 1967
containing 0 or 0.1% (w/w) (0 or 1000 produce "any significant carcinogenic the published account (abstract
ppm) 3,3'-dichlorobenzidine (0 or 90 effect or bladder pathology" in only)
mg/kg body weight per day) for life. hamsters; however, no quantitative
experimental results were
presented.
Groups of male and female hamsters Exposure to 3,3'-dichlorobenzidine Small number of animals and Sellakumar et al.,
(n = 30 per sex) were administered "induced 4 transitional cell bladder incomplete reporting of data (i.e. 1969
diets containing 0 or 0.3% (w/w) (0 or carcinomas, some liver-cell and the sex of the animals with
3000 ppm) 3,3'-dichlorobenzidine (0 or cholangiomatous tumours and diffuse tumours, the incidence of the
270 mg/kg body weight per day). chronic intrahepatic obstructing various tumour types, and the
cholangitis (63%)." results for unexposed controls)
Table 2: Genotoxicity of 3,3'-dichlorobenzidine.
Result
With Without
Test species/cell line End-point activation activation Reference
IN VITRO STUDIES
Salmonella typhimurium Gene mutation Garner et al., 1975; Anderson &
TA98 + + Styles, 1978; Lazear et al.,
TA100 + - 1979; Reid et al., 1984; Savard
TA1535 +/- ND & Josephy, 1986; Iba, 1987;
TA1538 + + Messerly et al., 1987; Ghosal &
Iba, 1992; You et al., 1993
Human B-lymphoblastoid cells Sister chromatid exchange + + Shiraishi, 1986
HeLa S3 cells Unscheduled DNA synthesis + ND Martin et al., 1978
Rat embryo cells Transformation ND + Freeman et al., 1973
IN VIVO STUDIES
ICR-SPF mice (oral administration) Micronuclei + NA Cihak & Vontorkova, 1987
Alpk:AP rats (oral administration) Unscheduled DNA synthesis + NA Ashby & Mohammed, 1988
Micea (intraperitoneal injection) Chromosomal aberrations + NA You et al., 1993
NA = not applicable; ND = no data were identified
a Strain not specified.
9. EFFECTS ON HUMANS
Case reports concerning the effects of exposure of humans to
3,3'-dichlorobenzidine were not identified. Although clinical studies
concerning the potential for 3,3'-dichlorobenzidine to induce dermal
and ocular irritation in humans have not been identified, dermatitis
was reported among workers occupationally exposed to
3,3'-dichlorobenzidine in one (limited) study (Gerarde & Gerarde,
1974).
Identified epidemiological data are restricted to three limited
studies of cancer in production workers. No increase in the incidence
of or death due to bladder cancer was reported in historical cohort
studies of groups of 109 (MacIntyre, 1975), 35 (Gadian, 1975), or 207
(Gerarde & Gerarde, 1974) workers occupationally exposed to
3,3'-dichlorobenzidine. Owing to the limitations of all three of
these studies, including the small number of individuals examined, the
relatively short periods of follow-up (i.e. less than 20 years), and
methodological limitations, such as lack of appropriate control groups
(Gerarde & Gerarde, 1974; Gadian, 1975), these studies have only
limited power to detect an exposure-related effect. The workers in
these studies were also exposed to other substances.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
There are very few data on the acute toxicity of
3,3'-dichlorobenzidine to aquatic organisms. An IC50 of 0.06
mg/litre was reported for bacteria in the Microtox assay (Dutka &
Kwan, 1981). Sikka et al. (1978) reported a 48-hour LC100 value for
bluegill sunfish (Lepomis macrochirus) of 2 mg/litre; 50% mortality
was observed following exposure to 3,3'-dichlorobenzidine at 0.5
mg/litre for 96-120 hours. Based on quantitative structure-activity
relationships, 96-hour LC50 values for fathead minnow (Pimephales
promelas), rainbow trout (Oncorhynchus mykiss), and golden orfe
(Leuciscus idus melanotus) have been estimated to be >3 mg/litre,
3 mg/litre, and 1.5 mg/litre, respectively (Government of Canada,
1993).
10.2 Terrestrial environment
No data were identified concerning the toxicity of
3,3'-dichlorobenzidine to wild mammals, terrestrial organisms, birds,
or sediment or soil biota.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Epidemiological investigations concerning the carcinogenicity of
3,3'-dichlorobenzidine are restricted to three limited studies in
which the health of occupationally exposed individuals was examined
(Gerarde & Gerarde, 1974; Gadian, 1975; MacIntyre, 1975). Although no
evidence of a relationship between occupational exposure to
3,3'-dichlorobenzidine and an increased incidence of tumours or death
due to cancer was reported in these investigations, the available
information, owing to the limitations of these studies, is considered
inadequate to assess the carcinogenicity of 3,3'-dichlorobenzidine in
humans.
Available data were inadequate to assess the non-neoplastic
effects of 3,3'-dichlorobenzidine. 3,3'-Dichlorobenzidine has been
found to be carcinogenic to rats, mice, dogs, and possibly hamsters
(Pliss 1959, 1963; Sellakumar et al., 1969; Stula et al., 1975, 1978;
Osanai, 1976). However, it should be noted that all of these studies
were limited owing to small group sizes, single dose levels,
relatively short periods of exposure, or inadequate histopathological
analysis and reporting of data.
In view of the sufficient evidence of the carcinogenicity of
3,3'-dichlorobenzidine in multiple animal species and the substantial
evidence of its genotoxicity in bacterial and mammalian cells, both
in vitro and in vivo, 3,3'-dichlorobenzidine is considered to be a
genotoxic carcinogen. Carcinogenicity is the critical end-point.
The carcinogenic potency (TD0.05), the dose of
3,3'-dichlorobenzidine associated with a 5% increase in tumour
incidence, has been characterized based on the increased incidence of
mammary tumours (fibroadenomas and adenocarcinomas [combined] in males
and females), granulocytic leukaemias (males), and Zymbal gland
carcinomas (males) in ChR-CD rats administered 1000 ppm
3,3'-dichlorobenzidine in the diet in the study conducted by Stula et
al. (1975). This study was considered most appropriate for
quantitative assessment, owing to the size ( n = 50) of the study
groups, which were relatively large compared with those in other
available investigations, and to the relative adequacy of
documentation of the protocol and results. It should be noted,
however, that only one dose level was administered in this study and
that the period of administration was less than 2 years (i.e. up to
488 days). A TD0.05 was not developed based on tumour incidence in
the Osanai (1976) study owing to its limitations, including small
group size, use of one sex only, single dose level, relatively short
period of follow-up, and inadequate histopathological analysis and
reporting of data; however, it would likely be less than that derived
from Stula et al. (1975).
Based on the dietary study (50 mg/kg body weight per day)
conducted in rats by Stula et al. (1975), the TD0.05 calculated based
on linear interpolation, incorporating a body weight to surface area
correction (power of 2/3, owing to the lack of identification of the
active metabolite[s]) and correcting for less than 2 years of
exposure, ranges from 0.74 mg/kg body weight per day (based on mammary
tumours in females) to 1.4 mg/kg body weight per day (based on
granulocytic leukaemias in males).
Available data were inadequate to serve as a basis for developing
a TC0.05 for inhalation of 3,3'-dichlorobenzidine in air.
11.1.2 Criteria for setting guidance values for 3,3'-dichlorobenzidine
As available data indicate that 3,3'-dichlorobenzidine is a
genotoxic carcinogen, exposure should be reduced to the extent
possible. The following quantitative guidance is provided as a
possible basis for derivation of limits of exposure and judgement of
the quality of environmental media by relevant authorities, based on
the carcinogenic potency of 3,3'-dichlorobenzidine. Based on very
limited available data, the principal medium of exposure to
3,3'-dichlorobenzidine for the general population is unclear, although
it is likely to be inhalation and dermal exposure in the occupational
environment. Available data are considered inadequate to serve as a
basis for the development of tolerable intakes of
3,3'-dichlorobenzidine based on non-neoplastic effects.
Although it is desirable to reduce exposure to genotoxic
carcinogens to the extent possible, a value derived by dividing the
low end of the TD0.05 range (i.e. 0.74 mg/kg body weight per day) by,
for example, 5000-50 000 (i.e. 1.48 × 10-4 to 1.48 × 10-5 mg/kg body
weight per day) might be considered appropriate as a guidance value
for ingested media. This margin affords protection similar to that
associated with the range for low-dose risk estimates generally
considered by various agencies to be "essentially negligible" (i.e.
10-5 to 10-6). The limitations of the critical study (Stula et al.,
1975) upon which this guidance value is based should be borne in mind,
however, in its interpretation.
Available data are inadequate to serve as a basis for developing
a guidance value for 3,3'-dichlorobenzidine in air.
11.1.3 Sample risk characterization
The extremely limited nature of the available data that serve as
a basis for estimation of exposure should be borne in mind in
interpreting the comparison presented here for indirect population
exposure to 3,3'-dichlorobenzidine in the general environment.
Based upon the sample estimates of exposure presented in section
6.2 involving primarily detection limits in monitoring studies in
which the compound was not detected or predicted concentrations in
Canada, the total daily intake of 3,3'-dichlorobenzidine by the
general population via indirect exposure in the general environment is
several orders of magnitude less than the sample guidance values
derived above. Identified data are inadequate to serve as a basis for
estimating exposure to 3,3'-dichlorobenzidine in the occupational
environment.
11.2 Evaluation of environmental effects
Owing to its relatively low volatility, very short residence
time, and low concentrations in the atmosphere, 3,3'-dichlorobenzidine
is not expected to contribute to the greenhouse effect, depletion of
the ozone layer, or the formation of ground-level ozone.
The most sensitive aquatic species of those examined, or for
which predictions have been made, was bacteria in the Microtox assay,
with an IC50 value of 0.06 mg/litre. This value is 200 to >240
times greater than measured levels in surface water (<0.25-0.3
µg/litre) and about 1.8 × 1011 times greater than the concentration
predicted in water (3.4 × 10-7 ng/litre) using worst-case assumptions
for release in Canada. Similarly, the 96-hour LC50 for
3,3'-dichlorobenzidine in the most sensitive fish species (i.e. 0.5
mg/litre in bluegill sunfish) is approximately 1670 to >2000 times
greater than measured levels in surface water and about 1.4 × 1012
times greater than the predicted concentration in water.
3,3'-Dichlorobenzidine binds strongly to soil and aquatic sediment and
is therefore likely to have reduced bioavailability. Therefore, on
the basis of the limited available data, it is likely that
3,3'-dichlorobenzidine poses negligible risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
3,3'-dichlorobenzidine in group 2B ("possibly carcinogenic to
humans"), based on inadequate evidence for carcinogenicity in humans
and sufficient evidence for carcinogenicity in animals (IARC, 1987).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0481) reproduced in this
document.
13.1 Human health hazards
3,3'-Dichlorobenzidine is considered to be a potential human
carcinogen. It also has the potential to adversely affect the
developing embryo, based upon studies conducted in animals.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Special attention
should be given to pregnant women exposed to 3,3'-dichlorobenzidine.
13.3 Health surveillance advice
Workers potentially exposed to 3,3'-dichlorobenzidine should
undergo regular urine cytology surveillance. This should be followed
by more specific procedures in the case of positive results and should
be included in the health surveillance programme in parallel with the
monitoring of liver function.
13.4 Fire hazards
3,3'-Dichlorobenzidine is combustible; toxic gases (hydrogen
chloride, nitrogen oxides) may be produced in a fire.
13.5 Spillage and disposal
13.5.1 Spillage
In the event of spillage, measures should be taken to prevent
3,3'-dichlorobenzidine from reaching drains or watercourses, owing to
potential bioaccumulation and toxicity in aquatic species.
13.5.2 Disposal
Disposal of contaminated waste is achieved by high-temperature
incineration with hydrochloric acid scrubber or microwave plasma
detoxification.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
3,3'-DICHLOROBENZIDINE ICSC:0481
3,3'-DICHLOROBENZIDINE
3,3'Dichlorobiphenyl-4,4'ylenediamine
4,4'-Diamino-3,3'-dichlorobiphenyl
C6H3ClNH2C6ClNH2/C12H10Cl2N2
Molecular mass: 253.1
CAS # 91-94-1
RTECS # KI0525000
ICSC # 0250
UN # 1184
EC # 602-012-00-7
1. EXECUTIVE SUMMARY
This CICAD on 3,3'-dichlorobenzidine was prepared by the
Environmental Health Directorate of Health Canada, based principally
on reviews of the Government of Canada (1993) and the Agency for Toxic
Substances and Disease Registry (ATSDR, 1989), to assess the potential
effects on human health of indirect exposure to 3,3'-dichlorobenzidine
in the general environment as well as the chemical's environmental
effects. Data relevant to the assessment of the effects of
3,3'-dichlorobenzidine on the environment and human health identified
as of April 1992 (environmental effects) and October 1992 (human
health effects) were considered in the Government of Canada (1993)
review; data were subsequently updated to September 1995 from the
International Register of Potentially Toxic Chemicals and on-line
databases. Information concerning the nature of peer review and the
availability of the Government of Canada (1993) and ATSDR (1989)
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0481) for 3,3'-dichlorobenzidine, produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
3,3'-Dichlorobenzidine (CAS no. 91-94-1) is a synthetic
chlorinated primary aromatic amine and is commercially available as
the dihydrochloride salt, 3,3'-dichlorobenzidine dihydrochloride
(C12H10N2Cl2.2HCl). 3,3'-Dichlorobenzidine (and some of its
derivatives) is used primarily as an intermediate in the manufacture
of pigments for printing inks, textiles, paints, and plastics. It is
also used in the analytical determination of gold and as a curing
agent in the synthesis of polyurethane elastomers.
Occupational exposure to 3,3'-dichlorobenzidine may occur in the
pigment manufacturing industry and in industrial applications where
3,3'-dichlorobenzidine-based pigments are heated for prolonged
periods. Levels of 3,3'-dichlorobenzidine ranging from <0.6 to 25
µg/m3 have been measured in the workroom air of production and
pigment manufacturing plants in Germany and Japan.
Owing to its relatively low volatility, very short persistence,
and low concentrations in the atmosphere, 3,3'-dichlorobenzidine is
not expected to contribute to the greenhouse effect, depletion of the
ozone layer, or the formation of ground-level ozone. Based on
available data, it is likely that 3,3'-dichlorobenzidine poses
negligible risk to aquatic organisms.
Available data are inadequate to serve as a basis for the
development of tolerable intakes of 3,3'-dichlorobenzidine based on
non-neoplastic effects. Based upon sufficient evidence of
carcinogenicity of 3,3'-dichlorobenzidine in multiple animal species
in limited studies and substantial evidence of genotoxicity both
in vitro and in vivo, 3,3'-dichlorobenzidine is considered to be a
potential human carcinogen. In view of its potential carcinogenicity
in humans and in the absence of adequate epidemiological data, a
sample health-based guidance value for 3,3'-dichlorobenzidine has been
derived based upon the carcinogenic potency (i.e. TD0.05) of this
chemical, which has been calculated from the best reported, although
limited, chronic study in which ChR-CD rats were fed 1000 ppm
3,3'-dichlorobenzidine in the diet for up to 488 days. The TD0.05 was
calculated based on linear interpolation, incorporating a body weight
to surface area correction (power of 2/3, owing to the lack of
identification of the active metabolite[s]) and correcting for less
than 2 years of exposure, and ranges from 0.74 to 1.4 mg/kg body
weight per day. A value obtained by dividing the low end of this
TD0.05 range (i.e. 0.74 mg/kg body weight per day) by, for example,
5000-50 000 (i.e. 1.48 × 10-4 to 1.48 × 10-5 mg/kg body weight per
day) might be considered appropriate as a guidance value in ingested
media. This margin affords protection similar to that associated with
the range for low-dose risk estimates generally considered by various
agencies to be "essentially negligible" (i.e. 10-5 to 10-6). Based
upon a sample estimate of exposure that involved predicted
concentrations or limited monitoring data from the USA and Canada,
where the chemical has generally not been detected, the estimated
total daily intake of 3,3'-dichlorobenzidine by the general population
(from indirect exposure in the general environment) is several orders
of magnitude less than the sample guidance value derived above.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3,3'-Dichlorobenzidine (CAS no. 91-94-1;
3,3'-dichloro-4,4'-biphenyldiamine,
3,3'-dichloro-4,4'-diaminobiphenyl,
4,4'-diamino-3,3'-dichlorobiphenyl), a synthetic chlorinated primary
aromatic amine, is a grey to purple crystalline solid at room
temperature. It has a relatively low vapour pressure (1 mPa at 22°C),
a low water solubility (0.4 mg/100 ml at 22°C), and a log
n-octanol/water partition coefficient of 3.02.
3,3'-Dichlorobenzidine is usually commercially available as the
dihydrochloride salt, 3,3'-dichlorobenzidine dihydrochloride
(C12H10N2Cl2.2HCl) (Government of Canada, 1993). Additional
physical/chemical properties of 3,3'-dichlorobenzidine are presented
in the International Chemical Safety Card reproduced in this document.
3. ANALYTICAL METHODS
Analysis of 3,3'-dichlorobenzidine in environmental samples is
most commonly achieved by gas chromatography/mass spectrometry,
capillary column gas chromatography/Fourier transform infrared
spectrometry, and high-performance liquid chromatography. A detection
limit of 3 µg/m3 has been reported for 3,3'-dichlorobenzidine in air,
whereas detection limits for 3,3'-dichlorobenzidine in water range
from 0.05 to 50 µg/litre (IARC, 1982; ATSDR, 1989). A detection limit
of <20 ppb (µg/kg) has been reported for 3,3'-dichlorobenzidine in
food (i.e. fish) using gas chromatographic techniques with either
nitrogen-phosphorus detection or electron capture detection. No
analytical methods for the determination of 3,3'-dichlorobenzidine in
soil or sediment were identified (ATSDR, 1989). A detection limit of
60 ppt (ng/litre) has been reported for 3,3'-dichlorobenzidine in
biological samples (i.e. urine) using gas chromatographic techniques
with either electron capture detection or rubidium-sensitized
thermionic detection (HSDB, 1995).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
There are no known natural sources of 3,3'-dichlorobenzidine.
3,3'-Dichlorobenzidine (and some of its derivatives) is used primarily
as an intermediate in the manufacture of diarylide yellow or azo red
pigments for printing inks, textiles, paints, and plastics. It is
also used in the analytical determination of gold and as a curing
agent in the synthesis of polyurethane elastomers (Gerarde & Gerarde,
1974; Government of Canada, 1993).
The world production of 3,3'-dichlorobenzidine was estimated to
range from 7000 to 10 000 t in 1983, with 3900-4200 t being
synthesized in Europe. In Japan, approximately 3170 t and 3400 t of
3,3'-dichlorobenzidine were produced in 1992 and 1993, respectively.
In Germany, approximately 2500 t of 3,3'-dichlorobenzidine were
produced in 1989 (none of which was exported). In 1983, 1.1 million
pounds (500 t) of 3,3'-dichlorobenzidine base and salts were imported
into the USA. In 1987, 9.9 million pounds (4490 t) of
3,3'-dichlorobenzidine were consumed in the USA.
3,3'-Dichlorobenzidine is not produced in Canada but has been imported
on a regular basis. The reported volumes of importation into Canada
have ranged from 21 to 109 t over the period 1986-1989 (ATSDR, 1989;
Chemicals Daily, 1993, 1994; Government of Canada, 1993; Law, 1995).
3,3'-Dichlorobenzidine can enter the environment from any stage
in the production, storage, transport, use, or disposal of
3,3'-dichlorobenzidine-containing materials. Although there are no
quantitative data, the largest releases would likely be through direct
emissions from plants that manufacture 3,3'-dichlorobenzidine-
containing materials and from the degradation of
3,3'-dichlorobenzidine-containing pigments (Government of Canada,
1993).
Total industrial emissions of 3,3'-dichlorobenzidine to the
environment in the USA in 1988 were estimated to be 6 t. In Germany,
emissions of 3,3'-dichlorobenzidine into wastewater from various
industrial facilities were estimated to be less than 0.095 t in 1989.
Total industrial emissions of 3,3'-dichlorobenzidine to the
environment in Canada were estimated to be 0.1 t in 1989 (Government
of Canada, 1993; Law, 1995).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The half-life for volatilization of 3,3'-dichlorobenzidine from
surface water to the atmosphere has been estimated to be 72 days. In
water, 3,3'-dichlorobenzidine may be degraded by photooxidation,
photolysis, and biodegradation; the estimated half-life of
3,3'-dichlorobenzidine in water is less than 10 minutes. The
half-life of 3,3'-dichlorobenzidine in aqueous media is proportional
to the light intensity, with some of the major photodegradation
products including monochlorobenzidine, benzidine, as well as a number
of brightly coloured water-insoluble materials; under intense light or
sunlight, very little of the 3,3'-dichlorobenzidine,
monochlorobenzidine, and benzidine remain in the water after 15
minutes (Banerjee et al., 1978; Government of Canada, 1993).
3,3'-Dichlorobenzidine is fairly resistant to degradation by
naturally occurring aquatic microorganisms. Half-lives of 4-26 weeks
and 16-101 weeks have been estimated for the biodegradation of
3,3'-dichlorobenzidine in surface water and anaerobic groundwater,
respectively (Government of Canada, 1993).
3,3'-Dichlorobenzidine is strongly bound to soil, sediment, and
sludge and therefore is highly immobile. In soil,
3,3'-dichlorobenzidine is mineralized very slowly under aerobic and
anaerobic conditions. The half-life for the aerobic degradation of
3,3'-dichlorobenzidine in soil has been estimated to range from 4 to
26 weeks. 3,3'-Dichlorobenzidine may also be oxidized by metal ions
(e.g. ferric iron) present in clay (BUA, 1993; Government of Canada,
1993; HSDB, 1995).
Half-lives of 1.5-5 minutes and 0.9-9 hours have been estimated
for the photolysis and photooxidation of 3,3'-dichlorobenzidine in
air, respectively (Government of Canada, 1993).
3,3'-Dichlorobenzidine can accumulate in aquatic biota. A
bioconcentration factor of approximately 500 has been reported for
bluegill sunfish (Lepomis macrochirus), based on a study in which
the fish were exposed to 5 or 100 µg [14C]3,3'-dichlorobenzidine per
litre; equilibria were achieved within 96-168 hours (Appleton & Sikka,
1980). In other studies, a 3-day bioaccumulation factor of 610 in
fish (golden orfe, Leuciscus idus melanotus), a 5-day
bioaccumulation factor of 3100 in activated sludge, and a 1-day
bioaccumulation factor of 940 in algae (Chlorella fusca) have been
reported (Freitag et al., 1985).
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
Available data on the levels of 3,3'-dichlorobenzidine in air
were limited to reports from the USA in which 3,3'-dichlorobenzidine
was not detected (detection limits 0.1 and 5 ng/m3) in ambient air in
the vicinity of two dyestuff production facilities (Narang et al.,
1982; Riggin et al., 1983).
Levels of 3,3'-dichlorobenzidine in surface water have ranged
from not detected (the only reported detection limit was 0.25
µg/litre) to 0.3 µg/litre, based on studies conducted between 1987 and
1992 in France, England, the Netherlands, Switzerland, Germany, Spain,
and the USA (Staples et al., 1985; Valls et al., 1990; Slobodnik et
al., 1993).
In the only relevant study identified, 3,3'-dichlorobenzidine was
not detected (detection limit 0.02 ng/litre) in samples of
drinking-water collected from two municipalities in Ontario, Canada
(Malaiyandi et al., 1987).
Data were not identified concerning the levels of
3,3'-dichlorobenzidine in uncontaminated soil; however, this compound
was not detected (detection limits not reported) in sediment in the
USA (location of sample acquisition not reported) and Spain (samples
collected from the Besos River, Barcelona) (Staples et al., 1985;
Valls et al., 1990). Information concerning the levels (or
occurrence) of 3,3'-dichlorobenzidine in foodstuffs was not
identified. Although 3,3'-dichlorobenzidine has the potential to
bioaccumulate in aquatic organisms, this compound was not detected
(detection limit not reported) in samples of biota collected in the
USA (Staples et al., 1985).
Concentrations of 3,3'-dichlorobenzidine at contaminated
(industrial and municipal) sites within the USA have ranged from not
detected (detection limit 20 µg/litre) to 120 µg/litre in landfill
leachate and groundwater and from not detected (detection limit 0.66
mg/kg) to 20 mg/kg dry weight in landfill soil and sewage sludge
(Fricke et al., 1985; Smith & Weber, 1990; US EPA, 1990a,b; BUA,
1993).
Estimates of the fate and concentrations of
3,3'-dichlorobenzidine in the Canadian environment have been generated
by the Level III fugacity computer model of Mackay and Paterson
(1991), incorporating data on the chemical's physical and chemical
properties and transformation half-lives (Howard et al., 1991) and
assuming that 1% of the total amount produced and imported into Canada
is released into various media in proportions similar to those
reported in the US Toxic Release Inventory. The results indicate
that, at steady state, the proportion of released
3,3'-dichlorobenzidine found in air, water, and soil would be
<0.001%, 99.75%, and <0.001%, respectively. Average concentrations
of 3,3'-dichlorobenzidine in air, water, and soil, predicted on the
basis of the model, were 7.6 × 10-16 µg/m3, 3.4 × 10-7 ng/litre, and
1.1 × 10-16 µg/g dry weight, respectively (Government of Canada,
1993). As 3,3'-dichlorobenzidine binds strongly to soil and aquatic
sediment, it is likely to have reduced bioavailability.
6.2 Human exposure
Exposure of the general population to 3,3'-dichlorobenzidine in
environmental media may be estimated based on measured and predicted
concentrations in various media and reference values for body weight
and consumption patterns. Owing to the availability of relevant data,
exposure has been estimated here based primarily on data from Canada
and the USA. In view of the extremely limited nature of the available
data, these estimates are presented primarily in an attempt to
identify typical relative proportions of exposure from various media.
Countries are encouraged to estimate total exposure on the basis of
national data, possibly in a manner similar to that outlined here.
Based on a daily inhalation volume for adults of 22 m3, a mean
body weight for males and females of 64 kg (IPCS, 1994), and the most
sensitive detection limit for 3,3'-dichlorobenzidine (0.1 ng/m3) in
reports in which this compound was not detected in the air surrounding
dye production facilities in the USA, mean intake from air for the
general population is estimated to be less than 3.4 × 10-5 µg/kg body
weight per day. Based on a predicted concentration (by fugacity
modelling) of 3,3'-dichlorobenzidine in ambient air in Canada of 7.6 ×
10-16 µg/m3, the mean intake of 3,3'-dichlorobenzidine from air for
the general population is estimated to be much lower (i.e. 2.6 × 10-16
µg/kg body weight per day).
Based on a daily volume of water consumption for adults of 1.4
litres, a mean body weight of 64 kg (IPCS, 1994), and the limit of
detection for 3,3'-dichlorobenzidine (0.02 ng/litre) in a survey in
which this compound was not detected in drinking-water from two
municipalities in Canada, the mean intake of 3,3'-dichlorobenzidine
from drinking-water for the general population is estimated to be less
than 4.4 × 10-7 µg/kg body weight per day. However, based on a
predicted concentration of 3,3'-dichlorobenzidine in surface water in
Canada of 3.4 × 10-7 ng/litre, the estimated mean intake of
3,3'-dichlorobenzidine from drinking-water for the general population
would be 7.4 × 10-12 µg/kg body weight per day.
Available data were inadequate to estimate the intake of
3,3'-dichlorobenzidine from food. Studies were not identified
concerning measured levels of 3,3'-dichlorobenzidine in soil; however,
based on a predicted concentration of 3,3'-dichlorobenzidine in soil
in Canada of 1.1 × 10-16 µg/g, a daily ingestion of 20 mg of soil by
adults, and a mean body weight of 64 kg (IPCS, 1994), the estimated
intake of 3,3'-dichlorobenzidine from soil would be 3.4 × 10-20 µg/kg
body weight per day.
The general population may be exposed to 3,3'-dichlorobenzidine
during the use of commercial products, including pressurized spray
containers of paints, lacquers, and enamels containing traces of
benzidine yellow, an azo dye derived from 3,3'-dichlorobenzidine
(ATSDR, 1989). However, no quantitative data were identified
concerning the levels of 3,3'-dichlorobenzidine in products containing
pigments or dyes derived from this substance.
Occupational exposure (principally by inhalation or dermal
contact) to 3,3'-dichlorobenzidine may occur in the pigment
manufacturing industry and in industrial applications where
3,3'-dichlorobenzidine-based pigments are heated for prolonged periods
(e.g. the production of coloured plastics and films; the spin dying of
polypropylene fibres) (US EPA, 1990c; US DHHS, 1994). Levels of
3,3'-dichlorobenzidine ranging from <0.6 to 25 µg/m3 have been
measured in the workroom air of production and pigment manufacturing
plants in Germany (ATSDR, 1989) and Japan (US EPA, 1980).
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
It is generally believed that the hepatic metabolism of
3,3'-dichlorobenzidine in rodents (i.e. rats) involves its oxidation
to highly reactive N-oxygenated intermediates by the microsomal
cytochrome P-450 and flavin monooxygenase enzyme complexes. Although
the 3,3'-dichlorobenzidine-derived N-oxygenated intermediates have
not been unequivocally identified, they are believed to be responsible
for the mutagenic and genotoxic effects (e.g. related to DNA binding)
of 3,3'-dichlorobenzidine in bacterial and mammalian systems
(Government of Canada, 1993).
Few quantitative data on the metabolism of 3,3'-dichlorobenzidine
in humans were identified. Small amounts (approximately 1-2%) of free
and glucuronide-conjugated N-hydroxyacetyl derivatives of
3,3'-dichlorobenzidine were excreted in the urine of volunteers orally
administered 3,3'-dichlorobenzidine. Based upon studies in which
3,3'-dichlorobenzidine has been detected in the urine (at
concentrations up to 296 µg/litre) of workers occupationally exposed
to this substance, 3,3'-dichlorobenzidine appears to be readily
absorbed from the skin following dermal exposure (BUA, 1993).
3,3'-Dichloro- N-acetylbenzidine,
3,3'-dichloro- N,N'-diacetylbenzidine, and conjugated metabolites
(the identities of which were not confirmed) have been detected in
the urine of rats administered 3,3'-dichlorobenzidine orally. The
covalent binding of 3,3'-dichlorobenzidine metabolites to haemoglobin,
hepatic lipids, and DNA in the intestinal and bladder epithelium or
liver has been observed in experimental animals (e.g. rodents) exposed
in vivo (Government of Canada, 1993).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
The acute toxicity of 3,3'-dichlorobenzidine (either as the free
base or as dihydrochloride salt) in experimental animals is low, with
oral LD50s in rats and mice being higher than 3820 mg/kg body weight
and 488 mg/kg body weight, respectively (Government of Canada, 1993).
Gastrointestinal congestion and haemorrhage were the principal effects
in these studies (ACGIH, 1991). In their review of acute toxicity
data, the American Conference of Governmental and Industrial
Hygienists (ACGIH, 1991) reported mortality in four of five rabbits
following dermal application of 3,3'-dichlorobenzidine at 1 g/kg body
weight for 24 hours; however, additional data were not presented.
Following the acute administration (route not specified) of up to 50
mg of 3,3'-dichlorobenzidine per kg body weight in cats, there was
only a slight increase (up to 1.3%) in methaemoglobin content in the
blood, whereas the number of Heinz bodies was increased fourfold (BUA,
1993).
8.2 Irritation and sensitization
Data on the sensitization potential of 3,3'-dichlorobenzidine in
experimental animals were not identified, and only limited data were
available concerning the irritation potential of this chemical. BUA
(1993) reported no irritation or "symptoms of intolerance" in rabbits
exposed by conjunctival application of 100 mg of
3,3'-dichlorobenzidine; however, additional data were not presented in
this published account.
8.3 Short-term exposure
Studies on the toxicological effects produced following the
short-term repeated exposure of experimental animals to
3,3'-dichlorobenzidine have not been identified.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Subchronic studies in which non-neoplastic effects of
3,3'-dichlorobenzidine have been assessed have not been identified.
8.4.2 Chronic exposure and carcinogenicity
Chronic studies in which non-neoplastic effects of
3,3'-dichlorobenzidine have been adequately assessed have not been
identified. In several early limited carcinogenesis bioassays
(summarized in Table 1), 3,3'-dichlorobenzidine increased the
incidence of tumours in experimental animals, sometimes following
relatively short periods of administration. The incidence of mammary
gland carcinomas in ChR-CD rats administered diets containing 0 or
1000 ppm 3,3'-dichlorobenzidine for up to 488 days was 3/44 and 26/44
in females and 0/44 and 7/44 in males, respectively (Stula et al.,
1975). In male rats, the incidence of Zymbal gland carcinomas was
0/44 and 8/44 and the incidence of granulocytic leukaemias was 2/44
and 9/44 in the control and 3,3'-dichlorobenzidine-exposed animals,
respectively. In a limited study in which a small group of beagle
dogs received approximately 10.4 mg 3,3'-dichlorobenzidine per kg body
weight in gelatin capsules 3 or 5 times/week for up to 7.1 years, the
incidence of hepatocellular carcinoma and bladder carcinoma was
increased ( p < 0.025) compared with controls (Stula et al., 1978).
Hepatic tumours occurred early (after 6 and 12 months) in male ICR/JCL
mice administered a diet containing 0 or 1000 ppm
3,3'-dichlorobenzidine over a 12-month period; after 12 months, the
incidence of hepatomas was 2/21 and 18/18 ( p < 0.001) in the
control and exposed groups, respectively (Osanai, 1976).
Compared with controls, the incidence of lymphoid leukaemias in
offspring born to female mice administered five subcutaneous
injections of 3,3'-dichlorobenzidine during the last week of pregnancy
was increased (0/30 versus 7/24; statistical significance unspecified)
(Golub et al., 1975). Results in studies with hamsters have been
equivocal (Saffiotti et al., 1967; Sellakumar et al., 1969). Other
identified studies (Pliss, 1959, 1963; Tsuda et al., 1977) contribute
little to the assessment of the weight of evidence of carcinogenicity
for 3,3'-dichlorobenzidine, owing to their limitations of protocol and
reporting (Table 1).
8.5 Genotoxicity and related end-points
There is convincing evidence that 3,3'-dichlorobenzidine is
genotoxic both in vivo and in vitro (Table 2). In in vitro
assays, 3,3'-dichlorobenzidine was mutagenic in Salmonella
typhimurium, increased the frequency of sister chromatid exchange
and unscheduled DNA synthesis in human cells, and "morphologically
transformed" rat embryo cells. In in vivo studies,
3,3'-dichlorobenzidine increased the frequency of micronuclei in the
bone marrow cells of mice, increased unscheduled DNA synthesis in the
liver of rats, and increased the frequency of chromosomal aberrations
in the bone marrow cells of mice.
8.6 Reproductive and developmental toxicity
The incidence of "hyperplastic changes" in kidney explants
obtained from mouse embryos exposed to 3,3'-dichlorobenzidine
in utero (following subcutaneous injection of dams) was increased,
compared with controls (Shabad et al., 1972).
8.7 Immunological and neurological effects
Studies concerning the immunological effects of
3,3'-dichlorobenzidine have not been identified. Data on neurotoxic
effects were limited to the observation (Stula et al., 1978) of
convulsions and neuronal degeneration in one female beagle dog orally
administered 3,3'-dichlorobenzidine (100 mg 3 times weekly for 6 weeks
and then 5 times weekly for the duration of the experiment) for 42
months.
Table 1: Carcinogenicity of 3,3'-dichlorobenzidine.
Test protocol Results Comments Reference
Female beagle dogs (n = 6) were The incidence of bladder Small number of anumals and Stula et al., 1978
administered 3,3'-dichlorobenzidine carcinomas and hepatocellular single sex exposed for a limited
(100 mg in gelatin capsules) 3 carcinomas in 3,3'-dichloro- proportion of life span
times/week for 6 weeks, and then 5 benzidine-exposed animals that
times/week for up to 7.1 years (mean survived longer than 6.6 years was
dose approximately 10.4 mg/kg body 5/5 (p < 0.025) and 4/5 (p < 0.025),
weight per exposure); six unexposed respectively. No liver or bladder
controls were sacrificed after 8.3-9.0 tumours were found in the
years. unexposed controls, although 4/6
controls were reported to have
developed mammary carcinomas
(attributed to the age of these
animals).
Groups of ChR-CD rats (n = 50 per sex) The incidence of mammary Exposure for a limited proportion Stula et al., 1975
were administered diets containing 0 or carcinomas in rats administered 0 or of life span of one sex
1000 ppm (0.1% w/w) 3,3'-dichloro- 1000 ppm 3,3'-dichlorobenzidine in
benzidine (0 or 50 mg/kg body weight the diet was 3/44 and 26/44 (p <
per day) for up to 488 days. 0.05) in females and 0/44 and 7/44
(p < 0.05) in males. In male rats,
the incidence of Zymbal gland
carcinomas was 0/44 and 8/44 (p <
0.05) and the incidence of
granulocytic leukaemias was 2/44
and 9/44 (p < 0.05) in the control
and 3,3'-dichlorobenzidine-exposed
animals, respectively.
Table 1 (continued)
Test protocol Results Comments Reference
Male (n = 35) and female (n = 15) Tumours (in the Zymbal and Small group sizes, single dose Pliss, 1959
Rappolovo rats were administered a mammary glands, bladder, and level, lack of appropriate
diet containing 3,3'-dichlorobenzidine haematopoietic system) were controls, and incomplete
(0.5-1.0 ml of a 4.4% solution) observed in 79% (23/29) of animals reporting of results (i.e. the sex
dissolved in sunflower oil for 6 surviving at the time (not specified) of the animals with tumours, and
days/week for 12 months. "Control" the first tumours were detected the nature and incidence of
animals (n = 130) were administered following exposure to 3,3'-dichloro- tumours)
(by injection) octadecylamine and benzidine; no tumours were
stearylamine for 10 months and observed in the controls.
observed until 23 months.
Rappolovo rats (sex and number not Tumours (in bone, mammary Lack of appropriate controls and Pliss, 1963
reported) were administered a diet glands, bladder, "etc.") were incomplete reporting of the
containing an amount (not clearly observed in 86% of rats exposed to protocol and results (i.e. nature
specified) of 3,3'-dichlorobenzidine for 3,3'-dichlorobenzidine in the diet, and incidence of the tumours)
10-13 months. A group of 50 rats (25 whereas tumours (liver sarcoma)
of which were injected with sunflower were observed in only one of the
oil) served as controls. controls.
Groups of male Wistar rats (n = 18) No evidence of histopathological Limited period of exposure, Tsuda et al., 1977
were administered diets containing 0 or effects in either the bladder or liver small number of animals, single
0.03% (w/w) (0 or 300 ppm) 3,3'- was observed following the sex, and limited histopatho-
dichlorobenzidine (0 or 15 mg/kg body administration of diets containing 0 logical examination
weight per day) for 40 weeks. or 300 ppm 3,3'-dichlorobenzidine
for 40 weeks.
Table 1 (continued)
Test protocol Results Comments Reference
Groups of male ICR/JCL mice (n = 26) After 12 months, the incidence of Small numbers of animals, Osanai, 1976
were administered a diet containing 0 hepatomas was 2/21 and 18/18 single sex, and incomplete
or 0.1% (w/w) (0 or 1000 ppm) 3,3'- (p < 0.001) in the control and 3,3'- description of the nature of
dichlorobenzidine (0 or 130 mg/kg body dichlorobenzidine-exposed groups, hepatic tumours
weight per day) for up to 12 months. respectively.
Male (n = 51) and female (n = 22) After 18.5 months, the incidence of Small group sizes, single dose Pliss, 1959
strain D mice were administered a diet hepatomas and haemangiomas in level, lack of appropriate
containing 3,3'-dichlorobenzidine (0.1 mice exposed to 3,3'-dichloro- controls, and incomplete
ml of a 1.1% solution) for a period of benzidine was 2/18 and 2/18, reporting of results (i.e. the sex
10 months and were maintained until respectively. of animals with tumours, and the
18.5 months. No information was nature and incidence of the
presented concerning the use of tumours)
controls.
Groups of Syrian golden hamsters (n = Exposure to 1000 ppm 3,3'-dichloro- Small group sizes and Saffiotti et al.,
30 per sex) were administered diets benzidine in the diet failed to inadequate reporting of data in 1967
containing 0 or 0.1% (w/w) (0 or 1000 produce "any significant carcinogenic the published account (abstract
ppm) 3,3'-dichlorobenzidine (0 or 90 effect or bladder pathology" in only)
mg/kg body weight per day) for life. hamsters; however, no quantitative
experimental results were
presented.
Groups of male and female hamsters Exposure to 3,3'-dichlorobenzidine Small number of animals and Sellakumar et al.,
(n = 30 per sex) were administered "induced 4 transitional cell bladder incomplete reporting of data (i.e. 1969
diets containing 0 or 0.3% (w/w) (0 or carcinomas, some liver-cell and the sex of the animals with
3000 ppm) 3,3'-dichlorobenzidine (0 or cholangiomatous tumours and diffuse tumours, the incidence of the
270 mg/kg body weight per day). chronic intrahepatic obstructing various tumour types, and the
cholangitis (63%)." results for unexposed controls)
Table 2: Genotoxicity of 3,3'-dichlorobenzidine.
Result
With Without
Test species/cell line End-point activation activation Reference
IN VITRO STUDIES
Salmonella typhimurium Gene mutation Garner et al., 1975; Anderson &
TA98 + + Styles, 1978; Lazear et al.,
TA100 + - 1979; Reid et al., 1984; Savard
TA1535 +/- ND & Josephy, 1986; Iba, 1987;
TA1538 + + Messerly et al., 1987; Ghosal &
Iba, 1992; You et al., 1993
Human B-lymphoblastoid cells Sister chromatid exchange + + Shiraishi, 1986
HeLa S3 cells Unscheduled DNA synthesis + ND Martin et al., 1978
Rat embryo cells Transformation ND + Freeman et al., 1973
IN VIVO STUDIES
ICR-SPF mice (oral administration) Micronuclei + NA Cihak & Vontorkova, 1987
Alpk:AP rats (oral administration) Unscheduled DNA synthesis + NA Ashby & Mohammed, 1988
Micea (intraperitoneal injection) Chromosomal aberrations + NA You et al., 1993
NA = not applicable; ND = no data were identified
a Strain not specified.
9. EFFECTS ON HUMANS
Case reports concerning the effects of exposure of humans to
3,3'-dichlorobenzidine were not identified. Although clinical studies
concerning the potential for 3,3'-dichlorobenzidine to induce dermal
and ocular irritation in humans have not been identified, dermatitis
was reported among workers occupationally exposed to
3,3'-dichlorobenzidine in one (limited) study (Gerarde & Gerarde,
1974).
Identified epidemiological data are restricted to three limited
studies of cancer in production workers. No increase in the incidence
of or death due to bladder cancer was reported in historical cohort
studies of groups of 109 (MacIntyre, 1975), 35 (Gadian, 1975), or 207
(Gerarde & Gerarde, 1974) workers occupationally exposed to
3,3'-dichlorobenzidine. Owing to the limitations of all three of
these studies, including the small number of individuals examined, the
relatively short periods of follow-up (i.e. less than 20 years), and
methodological limitations, such as lack of appropriate control groups
(Gerarde & Gerarde, 1974; Gadian, 1975), these studies have only
limited power to detect an exposure-related effect. The workers in
these studies were also exposed to other substances.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
10.1 Aquatic environment
There are very few data on the acute toxicity of
3,3'-dichlorobenzidine to aquatic organisms. An IC50 of 0.06
mg/litre was reported for bacteria in the Microtox assay (Dutka &
Kwan, 1981). Sikka et al. (1978) reported a 48-hour LC100 value for
bluegill sunfish (Lepomis macrochirus) of 2 mg/litre; 50% mortality
was observed following exposure to 3,3'-dichlorobenzidine at 0.5
mg/litre for 96-120 hours. Based on quantitative structure-activity
relationships, 96-hour LC50 values for fathead minnow (Pimephales
promelas), rainbow trout (Oncorhynchus mykiss), and golden orfe
(Leuciscus idus melanotus) have been estimated to be >3 mg/litre,
3 mg/litre, and 1.5 mg/litre, respectively (Government of Canada,
1993).
10.2 Terrestrial environment
No data were identified concerning the toxicity of
3,3'-dichlorobenzidine to wild mammals, terrestrial organisms, birds,
or sediment or soil biota.
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Epidemiological investigations concerning the carcinogenicity of
3,3'-dichlorobenzidine are restricted to three limited studies in
which the health of occupationally exposed individuals was examined
(Gerarde & Gerarde, 1974; Gadian, 1975; MacIntyre, 1975). Although no
evidence of a relationship between occupational exposure to
3,3'-dichlorobenzidine and an increased incidence of tumours or death
due to cancer was reported in these investigations, the available
information, owing to the limitations of these studies, is considered
inadequate to assess the carcinogenicity of 3,3'-dichlorobenzidine in
humans.
Available data were inadequate to assess the non-neoplastic
effects of 3,3'-dichlorobenzidine. 3,3'-Dichlorobenzidine has been
found to be carcinogenic to rats, mice, dogs, and possibly hamsters
(Pliss 1959, 1963; Sellakumar et al., 1969; Stula et al., 1975, 1978;
Osanai, 1976). However, it should be noted that all of these studies
were limited owing to small group sizes, single dose levels,
relatively short periods of exposure, or inadequate histopathological
analysis and reporting of data.
In view of the sufficient evidence of the carcinogenicity of
3,3'-dichlorobenzidine in multiple animal species and the substantial
evidence of its genotoxicity in bacterial and mammalian cells, both
in vitro and in vivo, 3,3'-dichlorobenzidine is considered to be a
genotoxic carcinogen. Carcinogenicity is the critical end-point.
The carcinogenic potency (TD0.05), the dose of
3,3'-dichlorobenzidine associated with a 5% increase in tumour
incidence, has been characterized based on the increased incidence of
mammary tumours (fibroadenomas and adenocarcinomas [combined] in males
and females), granulocytic leukaemias (males), and Zymbal gland
carcinomas (males) in ChR-CD rats administered 1000 ppm
3,3'-dichlorobenzidine in the diet in the study conducted by Stula et
al. (1975). This study was considered most appropriate for
quantitative assessment, owing to the size ( n = 50) of the study
groups, which were relatively large compared with those in other
available investigations, and to the relative adequacy of
documentation of the protocol and results. It should be noted,
however, that only one dose level was administered in this study and
that the period of administration was less than 2 years (i.e. up to
488 days). A TD0.05 was not developed based on tumour incidence in
the Osanai (1976) study owing to its limitations, including small
group size, use of one sex only, single dose level, relatively short
period of follow-up, and inadequate histopathological analysis and
reporting of data; however, it would likely be less than that derived
from Stula et al. (1975).
Based on the dietary study (50 mg/kg body weight per day)
conducted in rats by Stula et al. (1975), the TD0.05 calculated based
on linear interpolation, incorporating a body weight to surface area
correction (power of 2/3, owing to the lack of identification of the
active metabolite[s]) and correcting for less than 2 years of
exposure, ranges from 0.74 mg/kg body weight per day (based on mammary
tumours in females) to 1.4 mg/kg body weight per day (based on
granulocytic leukaemias in males).
Available data were inadequate to serve as a basis for developing
a TC0.05 for inhalation of 3,3'-dichlorobenzidine in air.
11.1.2 Criteria for setting guidance values for 3,3'-dichlorobenzidine
As available data indicate that 3,3'-dichlorobenzidine is a
genotoxic carcinogen, exposure should be reduced to the extent
possible. The following quantitative guidance is provided as a
possible basis for derivation of limits of exposure and judgement of
the quality of environmental media by relevant authorities, based on
the carcinogenic potency of 3,3'-dichlorobenzidine. Based on very
limited available data, the principal medium of exposure to
3,3'-dichlorobenzidine for the general population is unclear, although
it is likely to be inhalation and dermal exposure in the occupational
environment. Available data are considered inadequate to serve as a
basis for the development of tolerable intakes of
3,3'-dichlorobenzidine based on non-neoplastic effects.
Although it is desirable to reduce exposure to genotoxic
carcinogens to the extent possible, a value derived by dividing the
low end of the TD0.05 range (i.e. 0.74 mg/kg body weight per day) by,
for example, 5000-50 000 (i.e. 1.48 × 10-4 to 1.48 × 10-5 mg/kg body
weight per day) might be considered appropriate as a guidance value
for ingested media. This margin affords protection similar to that
associated with the range for low-dose risk estimates generally
considered by various agencies to be "essentially negligible" (i.e.
10-5 to 10-6). The limitations of the critical study (Stula et al.,
1975) upon which this guidance value is based should be borne in mind,
however, in its interpretation.
Available data are inadequate to serve as a basis for developing
a guidance value for 3,3'-dichlorobenzidine in air.
11.1.3 Sample risk characterization
The extremely limited nature of the available data that serve as
a basis for estimation of exposure should be borne in mind in
interpreting the comparison presented here for indirect population
exposure to 3,3'-dichlorobenzidine in the general environment.
Based upon the sample estimates of exposure presented in section
6.2 involving primarily detection limits in monitoring studies in
which the compound was not detected or predicted concentrations in
Canada, the total daily intake of 3,3'-dichlorobenzidine by the
general population via indirect exposure in the general environment is
several orders of magnitude less than the sample guidance values
derived above. Identified data are inadequate to serve as a basis for
estimating exposure to 3,3'-dichlorobenzidine in the occupational
environment.
11.2 Evaluation of environmental effects
Owing to its relatively low volatility, very short residence
time, and low concentrations in the atmosphere, 3,3'-dichlorobenzidine
is not expected to contribute to the greenhouse effect, depletion of
the ozone layer, or the formation of ground-level ozone.
The most sensitive aquatic species of those examined, or for
which predictions have been made, was bacteria in the Microtox assay,
with an IC50 value of 0.06 mg/litre. This value is 200 to >240
times greater than measured levels in surface water (<0.25-0.3
µg/litre) and about 1.8 × 1011 times greater than the concentration
predicted in water (3.4 × 10-7 ng/litre) using worst-case assumptions
for release in Canada. Similarly, the 96-hour LC50 for
3,3'-dichlorobenzidine in the most sensitive fish species (i.e. 0.5
mg/litre in bluegill sunfish) is approximately 1670 to >2000 times
greater than measured levels in surface water and about 1.4 × 1012
times greater than the predicted concentration in water.
3,3'-Dichlorobenzidine binds strongly to soil and aquatic sediment and
is therefore likely to have reduced bioavailability. Therefore, on
the basis of the limited available data, it is likely that
3,3'-dichlorobenzidine poses negligible risk to aquatic organisms.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer has classified
3,3'-dichlorobenzidine in group 2B ("possibly carcinogenic to
humans"), based on inadequate evidence for carcinogenicity in humans
and sufficient evidence for carcinogenicity in animals (IARC, 1987).
Information on international hazard classification and labelling
is included in the International Chemical Safety Card reproduced in
this document.
13. HUMAN HEALTH PROTECTION AND EMERGENCY ACTION
Human health hazards, together with preventative and protective
measures and first aid recommendations, are presented in the
International Chemical Safety Card (ICSC 0481) reproduced in this
document.
13.1 Human health hazards
3,3'-Dichlorobenzidine is considered to be a potential human
carcinogen. It also has the potential to adversely affect the
developing embryo, based upon studies conducted in animals.
13.2 Advice to physicians
In case of poisoning, treatment is supportive. Special attention
should be given to pregnant women exposed to 3,3'-dichlorobenzidine.
13.3 Health surveillance advice
Workers potentially exposed to 3,3'-dichlorobenzidine should
undergo regular urine cytology surveillance. This should be followed
by more specific procedures in the case of positive results and should
be included in the health surveillance programme in parallel with the
monitoring of liver function.
13.4 Fire hazards
3,3'-Dichlorobenzidine is combustible; toxic gases (hydrogen
chloride, nitrogen oxides) may be produced in a fire.
13.5 Spillage and disposal
13.5.1 Spillage
In the event of spillage, measures should be taken to prevent
3,3'-dichlorobenzidine from reaching drains or watercourses, owing to
potential bioaccumulation and toxicity in aquatic species.
13.5.2 Disposal
Disposal of contaminated waste is achieved by high-temperature
incineration with hydrochloric acid scrubber or microwave plasma
detoxification.
14. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
Information on national regulations, guidelines, and standards is
available from the International Register of Potentially Toxic
Chemicals (IRPTC) legal file.
The reader should be aware that regulatory decisions about
chemicals taken in a certain country can be fully understood only in
the framework of the legislation of that country. The regulations and
guidelines of all countries are subject to change and should always be
verified with appropriate regulatory authorities before application.
INTERNATIONAL CHEMICAL SAFETY CARD
3,3'-DICHLOROBENZIDINE ICSC:0481
3,3'-DICHLOROBENZIDINE
3,3'Dichlorobiphenyl-4,4'ylenediamine
4,4'-Diamino-3,3'-dichlorobiphenyl
C6H3ClNH2C6ClNH2/C12H10Cl2N2
Molecular mass: 253.1
CAS # 91-94-1
RTECS # KI0525000
ICSC # 0250
UN # 1184
EC # 602-012-00-7
 TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF DUST! IN ALL CASES CONSULT A
PREVENT GENERATION OF MISTS! DOCTOR!
AVOID ALL CONTACT!
* INHALATION Cough. Sore throat. Avoid inhalation of fine dust Fresh air, rest. Refer for
and mist. Local exhaust or medical attention.
breathing protection.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection if powder. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth. Refer for
during work. Wash hands medical attention.
before eating.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Sweep spilled substance into sealable Separated from food and feedstuffs. Do not transport with food and
containers; if appropriate, moisten Well closed. feedstuffs.
first to prevent dusting. Carefully
collect remainder, then remove to safe T symbol
place. Do NOT let this chemical enter
the environment (extra personal R: 45-21-43
protection: complete protective S: 53-45
clothing including self-contained
breathing apparatus). Note: E
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
GREY TO PURPLE CRYSTALS. The substance irritates the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance decomposes on heating producing Repeated or prolonged contact with skin may
toxic and corrosive fumes including nitrogen cause dermatitis. The substance may have
oxides and hydrogen chloride. effects on the liver. This substance is
probably carcinogenic to humans.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: A2 (skin) (ACGIH 1993-1994).
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however be reached quickly when dispersed,
especially if powdered.
PHYSICAL Boiling point: 368°C
PROPERTIES Melting point: 132-133°C
Solubility in water: none
Auto-ignition temperature: 350°C
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA
NOTES
The substance is combustible but no flash point is available in literature.
Curithane C126 is a trade name.
ICSC: 0481 1.1 Transport Emergency Card: TEC (R)-61G12b
3,3'-DICHLOROBENZIDINE
REFERENCES
ACGIH (1991) 3,3'-Dichlorobenzidine. In: Documentation of the
threshold limit values and biological exposure indices, 6th ed.
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 417-419.
Anderson D, Styles J (1978) The bacterial mutation test. British
journal of cancer, 37:924-930.
Appleton H, Sikka H (1980) Accumulation, elimination and metabolism of
dichlorobenzidine in the bluegill sunfish. Environmental science
and technology, 14:50-54.
Ashby J, Mohammed R (1988) UDS activity in the rat liver of the human
carcinogens benzidine and 4-aminobiphenyl, and the rodent carcinogens
3,3'-dichlorobenzidine and Direct Black 38. Mutagenesis, 3:69-71.
ATSDR (1989) Toxicological profile for 3,3'-dichlorobenzidine.
Atlanta, GA, US Department of Health and Human Services, Public Health
Service, Agency for Toxic Substances and Disease Registry
(PB90-168691).
Banerjee S, Sikka H, Gary R, Kelly C (1978) Photodegradation of
3,3'-dichlorobenzidine. Environmental science and technology,
12:1425-1427.
BUA (1993) 3,3'-Dichlorobenzidine. GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, March 1989. New York,
NY, VCH Publishers, Inc. (BUA Report No. 30).
Chemicals Daily (1993) 12493 chemical products. Chemicals Daily Co.,
Ltd., p. 751.
Chemicals Daily (1994) 12394 chemical products. Chemicals Daily Co.,
Ltd., p. 767.
Cihak R, Vontorkova M (1987) Benzidine and 3,3'-dichlorobenzidine
(DCB) induce micronuclei in the bone marrow and the fetal liver of
mice after gavage. Mutagenesis, 2:267-269.
Dutka B, Kwan K (1981) Comparison of three microbial toxicity
screening tests with the Microtox test. Bulletin of environmental
contamination and toxicology, 27:753-757.
Freeman A, Weisburger E, Weisburger J, Wolford R, Maryak J, Huebner R
(1973) Transformation of cell cultures as an indication of the
carcinogenic potential of chemicals. Journal of the National Cancer
Institute, 51:799-808.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile for organic chemicals. An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C-labelled chemicals.
Chemosphere, 14:1589-1616.
Fricke C, Clarkson C, Lomnitz E, O'Farrel T (1985) Comparing priority
pollutants in municipal sludges. Biocycle, 26:35-37.
Gadian T (1975) Carcinogens in industry, with special reference to
dichlorobenzidine. Chemistry & industry (London), 4:821-831.
Garner R, Walpole A, Rose F (1975) Testing of some benzidine analogues
for microsomal activation to bacterial mutagens. Cancer letters,
1:39-42.
Gerarde H, Gerarde D (1974) Industrial experience with
3,3'-dichlorobenzidine: an epidemiology study of a chemical
manufacturing plant. Journal of occupational medicine, 16:322-344.
Ghosal A, Iba M (1992) Enhancement of butylated hydroxytoluene of the
in vitro activation of 3,3'-dichlorobenzidine. Mutation research,
278:31-41.
Golub N, Kolesnichenko T, Shabad L (1975) [Oncogenic action of some
nitrogen compounds on the progeny of experimental mice.] Bulletin
of experimental biology and medicine, 78:1402-1404 (in Russian)
[cited in US EPA (1988) Health and environmental effects document
for 3,3'-dichlorobenzidine. Washington, DC, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-G034)].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for
3,3'-dichlorobenzidine. Prepared by Health Canada and Environment
Canada. Ottawa, Ontario, Canada Communication Group Publishing (ISBN
0-662-21070-9).
Howard P, Boethling R, Jarvis W, Meylan W, Michalenk E (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers, Inc.
HSDB (1995) Data profile for 3,3'-dichlorobenzidine. Hamilton,
Ontario, Canadian Centre for Occupational Health and Safety, Hazardous
Substances Databank.
IARC (1982) 3,3'-Dichlorobenzidine and its dihydrochloride. Lyon,
International Agency for Research on Cancer, pp. 239-256 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Vol. 29).
IARC (1987) 3,3'-Dichlorobenzidine (Group 2B). Lyon, International
Agency for Research on Cancer, pp. 193-194 (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Suppl. 7).
Iba M (1987) Comparative activation of 3,3'-dichlorobenzidine and
related benzidines to mutagens in Salmonella typhimurium assays by
hepatic S9 and microsomes from rats pretreated with different inducers
of cytochrome P-450. Mutation research, 182:231-241.
IPCS (1993) International Chemical Safety Card -
3,3'-Dichlorobenzidine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0481).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Law R (1995) 3,3'-Dichlorobenzidine: a candidate for inclusion in
marine monitoring programmes? Chemosphere, 30:1791-1797.
Lazear E, Shaddock J, Barren P, Louie S (1979) The mutagenicity of
some of the proposed metabolites of Direct Black 38 and Pigment Yellow
12 in the Salmonella typhimurium assay system. Toxicology
letters, 4:519-525.
MacIntyre I (1975) Experience of tumours in a British plant handling
3,3'-dichlorobenzidine. Journal of occupational medicine, 17:23-26.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic
chemicals: a level III fugacity model. Environmental science and
technology, 25:427-436.
Malaiyandi M, Wightman R, LaFerriere C (1987) Concentration of
selected organic pollutants: comparison of adsorption and reverse
osmosis techniques. In: Malaiyandi M, ed. Organic pollutants in
water. Sampling, analysis and toxicity testing. Washington, DC,
American Chemical Society, pp. 163-179 (American Chemical Society
Symposium Series No. 214).
Martin C, McDermid A, Garner R (1978) Testing of known carcinogens and
noncarcinogens for their ability to induce unscheduled DNA synthesis.
Cancer research, 38:2621-2627.
Messerly E, Fekete J, Wade D, Sinsheimer J (1987) Structure-
mutagenicity relationships of benzidine analogues. Environmental
and molecular mutagenesis, 10:263-274.
Narang A, Choudhury D, Richards A (1982) Separation of aromatic amines
by thin-layer and high performance liquid chromatography. Journal
of chromatographic science, 20:235-237.
Osanai H (1976) [An experimental study on hepatoma caused by aromatic
amines.] Journal of science of labour, 52:179-201 (in Japanese).
Pliss G (1959) [Dichlorobenzidine as a blastomogenic agent.]
Voprosy Onkologii, 5:524-533 (in Russian).
Pliss G (1963) On some regular relationships between carcinogenicity
of aminodiphenyl derivatives and the structure of substances. Acta
Unio Internationalis Contra Cancrum, 19:499-501.
Reid T, Wang C, King C, Morton K (1984) Mutagenicity of some benzidine
congeners and their N-acetylated and N,N'-diacetylated derivatives
in different strains of Salmonella typhimurium. Environmental
mutagenesis, 6:145-151.
Riggin R, Howard C, Scott D, Hedgecoke R (1983) Determination of
benzidine related congeners and pigments in atmospheric particulate
matter. Journal of chromatography, 27:321-325.
Saffiotti U, Cefis F, Montessano R, Sellakumar A (1967) Induction of
bladder cancer in hamsters fed aromatic amines. In: Deichman W, Lampe
K, eds. Bladder cancer. Birmingham, AL, Aesculapis Publishing Co.,
pp. 129-135.
Savard S, Josephy P (1986) Synthesis and mutagenicity of
3,3'-dihalogenated benzidines. Carcinogenesis, 7:1239-1241.
Sellakumar A, Montesano R, Saffiotti U (1969) Aromatic amines:
carcinogenicity in hamsters. Proceedings of the American
Association for Cancer Research, 10:78 (abstract).
Shabad L, Sorokina J, Golub N, Bogovski S (1972) Transplacental effect
of some chemical compounds on organ cultures of embryonic tissue.
Cancer research, 32:617-627.
Shiraishi Y (1986) Hypersensitive character of Bloom syndrome
B-lymphoblastoid cell lines usable for sensitive carcinogen detection.
Mutation research, 175:179-187.
Sikka H, Appleton H, Banerjee S (1978) Fate of
3,3'-dichlorobenzidine in aquatic environments. Athens, GA, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-78-068).
Slobodnik J, Groenewegen M, Brouwer E, Lingeman H, Brinkman U (1993)
Fully automated multi-residue method for trace level monitoring of
polar pesticides by liquid chromatography. Journal of
chromatography, 642:359-370.
Smith E, Weber W (1990) Comparative assessment of the chemical and
adsorptive characteristics of leachates from a municipal and an
industrial landfill. Water, air, and soil pollution, 53:279-295.
Staples C, Werner A, Hoogheem T (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Stula E, Sherman H, Zapp J, Clayton J (1975) Experimental neoplasia in
rats from oral administration of 3,3'-dichlorobenzidine,
4,4'-methylene-bis(2-chloroaniline), and
4,4'-methylene-bis(2-methylaniline). Toxicology and applied
pharmacology, 31:159-176.
Stula E, Barnes J, Sherman H, Reinhardt C, Zapp J (1978) Liver and
urinary bladder tumours in dogs from 3,3'-dichlorobenzidine.
Journal of environmental pathology and toxicology, 1:475-490.
Tsuda H, Miyata Y, Murasaki G, Kinoshita H, Fukushima S, Ito N (1977)
Synergistic effect of urinary bladder carcinogenesis in rats treated
with N-butyl- N-(4-hydroxybutyl)nitrosamine,
N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide,
N-2-fluorenylacetamide and 3,3'-dichlorobenzidine. Gann,
68:183-192.
US DHHS (1994) Seventh annual report on carcinogens. Summary 1994.
Research Triangle Park, NC, US Department of Health and Human
Services, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences.
US EPA (1980) Ambient water quality criteria for dichlorobenzidine.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards (PB81-117517).
US EPA (1990a) Remedial investigation report, landfill area,
Greenville, South Carolina. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-900000412).
US EPA (1990b) Subsurface investigation, Glenholden Laboratory,
Glenholden, Pennsylvania. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-910000001).
US EPA (1990c) Letter from PMS Consolidated to USEPA containing
information on study of potential workplace exposure to
3,3'-dichlorobenzidine, with attachment. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Document
No. 89-900000363).
Valls M, Bayona J, Albaigés J (1990) Broad spectrum analysis of ionic
and non-ionic organic contaminants in urban wastewaters and coastal
receiving aquatic systems. International journal of environmental
analytical chemistry, 39:329-348.
You Z, Brezzell M, Das S, Espadas-Torre M, Hooberman B, Sinsheimer J
(1993) Ortho-substituent effects on the in vitro and in vivo
genotoxicity of benzidine derivatives. Mutation research, 319:19-30.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List assessment report for
3,3'-dichlorobenzidine (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A OH3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above. Copies of the unpublished
Supporting Documentation related to effects on the environment that
formed the basis for preparation of the above-mentioned report may be
obtained from the Commercial Chemicals Branch at the address noted
above.
Initial drafts of the Supporting Documentation and Assessment
Report for 3,3'-dichlorobenzidine were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed by Drs C.M. Auer and W.H. Farland of the US Environmental
Protection Agency. Sections related to the assessment of human health
effects were approved by an interdirectorate Standards and Guidelines
Rulings Committee of the Bureau of Chemical Hazards of Health Canada.
The final Assessment Report was reviewed and approved by the
Environment Canada/Health Canada CEPA Management Committee.
Agency for Toxic Substances and Disease Registry (1989)
Copies of the ATSDR Toxicological profile for
3,3'-dichlorobenzidine (ATSDR, 1989) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicological profile for
3,3'-dichlorobenzidine were reviewed by scientists from the Agency
for Toxic Substances and Disease Registry, the US Environmental
Protection Agency, the US Centre for Disease Control and Prevention,
and the US National Toxicology Program. The document was also
reviewed by an expert panel of nongovernmental reviewers, consisting
of the following members: Dr Paul Mushak, Private Consultant, Durham,
North Carolina; Dr David Jollow, Professor, Medical University of
South Carolina; and Dr T. Kneip, Professor, New York University
Medical Center.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 3,3'-dichlorobenzidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Hoechst Aktiengesellschaft, Frankfurt, Germany
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (Document international succinct sur l'évaluation des risques
chimiques) sur la 3,3'-dichlorobenzidine en s'inspirant principalement
des évaluations du Gouvernement du Canada (1993) et de l'Agency for
Toxic Substances and Disease Registry (ATSDR, 1989) visant à
déterminer les effets potentiels sur la santé humaine d'une exposition
indirecte à la 3,3'-dichlorobenzidine dans l'environnement général,
ainsi que les effets de cette substance sur l'environnement.
L'évaluation du Gouvernement du Canada (1993) a pris en compte les
données relatives à la détermination des effets de la
3,3'-dichlorobenzidine sur l'environnement et la santé humaine qui
étaient disponibles en avril 1992 (effets sur l'environnement) et en
octobre 1992 (effets sur la santé humaine); ces données ont été mises
à jour en septembre 1995 à partir des données du Registre
international des substances chimiques potentiellement toxiques et de
bases de données en ligne. Des informations concernant la nature de
l'évaluation par les pairs et la disponibilité des documents du
Gouvernement du Canada (1993) et de l'ATSDR (1989) figurent à
l'appendice 1. Des informations sur l'évaluation par les pairs du
présent CICAD figurent à l'appendice 2. Ce CICAD a été approuvé pour
publication à une réunion du Comité d'évaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à la réunion du Comité d'évaluation finale figure à
l'appendice 3. La fiche d'information sur la sécurité chimique (ICSC
0481) pour la 3,3'-dichlorobenzidine, préparée par le Programme
international sur la sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La 3,3'-dichlorobenzidine (CAS N° 91-94-1) est une amine
aromatique primaire chlorée synthétique disponible dans le commerce
sous la forme de dichlorhydrate de 3,3'-dichlorobenzidine
(C12H10N2Cl2.2HCl). La 3,3'-dichlorobenzidine et certains de ses
dérivés sont utilisés principalement comme intermédiaires dans la
fabrication de pigments pour les encres d'imprimerie, les textiles,
les peintures et les matières plastiques. Elle est également utilisée
pour le titrage de l'or et comme agent de polymérisation dans la
synthèse d'élastomères de la famille des polyuréthanes.
L'exposition professionnelle à la 3,3'-dichlorobenzidine est
possible lors de la fabrication de pigments et dans les installations
industrielles où des pigments qui en contiennent sont chauffés pendant
des périodes prolongées. Des concentrations de 3,3'-dichlorobenzidine
comprises entre <0,6 à 25 µg/m3 ont été mesurées dans l'air des
ateliers de production et des usines de fabrication de pigments en
Allemagne et au Japon.
Étant donné sa volatilité relativement faible, sa très courte
persistance et sa faible concentration dans l'atmosphère, la
3,3'-dichlorobenzidine ne devrait pas contribuer à l'effet de serre, à
la destruction de la couche d'ozone ou à la formation d'ozone au
niveau du sol. D'après les données disponibles, il est probable que
la 3,3'-dichlorobenzidine présente un risque négligeable pour les
organismes aquatiques.
Les données disponibles sont insuffisantes pour établir des doses
tolérables de 3,3'-dichlorobenzidine fondées sur ses effets non
néoplasiques. Des études limitées ont apporté des preuves suffisantes
de sa cancérogénicité chez de nombreuses espèces animales et sa
génotoxicité a été démontrée in vitro +et in vivo; en conséquence,
la 3,3'-dichlorobenzidine est considérée comme une substance
potentiellement cancérogène pour l'homme. Compte tenu de cette
cancérogénicité potentielle et de l'absence de données
épidémiologiques adéquates, une valeur guide fondée sur des critères
de santé peut être établie sur la base du potentiel cancérogène
(c'est-à-dire sur la DT0,05) déterminé à partir des données de la
mieux présentée des études de toxicité chronique. Dans cette étude,
certes limitée, des rats ChR-CD ont consommé pendant 488 jours une
nourriture contenant 1000 ppm de 3,3'-dichlorobenzidine. La DT0,05 a
été calculée par interpolation linéaire en incorporant un facteur de
correction du poids corporel en fonction de la surface corporelle
(puissance 2/3 en raison de l'absence d'identification du ou des
métabolites actifs) et un autre facteur tenant compte de la durée
d'exposition inférieure à deux ans. La fourchette obtenue a été de
0,74 à 1,4 mg/kg de poids corporel par jour. En divisant la valeur
inférieure de cette fourchette (soit 0,74 mg/kg de poids corporel par
jour) par 5000 - 50 000, on obtient 1,48 × 10-4 à 1,48 × 10-5 mg/kg
de poids corporel par jour, ce qui peut être considéré comme une
valeur guide appropriée pour l'ingestion. Cette marge offre une
protection analogue à celle des fourchettes d'exposition aux faibles
doses qui sont généralement considérées par les organismes compétents
comme présentant un risque "pratiquement négligeable" (soit 10-5 à
10-6). Si l'on se base sur une estimation de l'exposition effectuée
à partir des concentrations prédites ou des quelques données
recueillies aux États-Unis d'Amérique et au Canada, où la substance
n'a généralement pas été détectée, la dose quotidienne totale
estimative absorbée par la population générale (des suites d'une
exposition indirecte à l'environnement général) est inférieure de
plusieurs ordres de grandeur à la valeur guide indiquée ci-dessus.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional de la
3,3'-diclorobencidina fue preparada por la Dirección de Higiene del
Medio de Health Canada, principalmente sobre la base de revisiones del
Gobierno del Canadá (1993) y de la Agencia para el Registro de
Sustancias Tóxicas y Enfermedades (ATSDR, 1989), a fin de evaluar los
efectos potenciales en la salud humana de la exposición indirecta a la
3,3'-diclorobencidina en el medio ambiente general, así como sus
efectos ambientales. En su estudio (1993), el Gobierno del Canadá
examinó los datos pertinentes para la evaluación de los efectos de la
3,3'-diclorobencidina en el medio ambiente y la salud humana
identificados hasta abril de 1992 (efectos ambientales) y octubre de
1992 (efectos en la salud humana). Posteriormente los datos se
actualizaron hasta septiembre de 1995 utilizando el Registro
Internacional de Sustancias Químicas Potencialmente Tóxicas y bases de
datos en línea. En el apéndice 1 se presenta información sobre el
carácter de la revisión científica y la disponibilidad de los
documentos del Gobierno del Canadá (1993) y del ATSDR (1989). En el
apéndice 2 se presenta información sobre la revisión científica de
esta reseña. Esta reseña fue aprobada para publicación en una reunión
de la Junta de Revisión Final celebrada en Bruselas, Bélgica, del 18
al 20 de noviembre de 1996. En el apéndice 3 figuran los
participantes en dicha reunión. En el presente documento también se
reproduce la Ficha Internacional de Seguridad Química (ICSC 0481) de
la 3,3'-diclorobencidina, producida por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993).
La 3,3'-diclorobencidina (CAS N° 91-94-1) es una amina aromática
primaria clorada sintética que se puede obtener comercialmente en
forma de sal del dihidrocloruro, dihidrocloruro de
3,3'-diclorobencidina (C12H10N2Cl2.2HCl). La 3,3'-diclorobencidina
(y algunos de sus derivados) se utiliza principalmente como
intermediario en la fabricación de pigmentos para tintas de imprenta,
textiles, pinturas y plásticos. También se utiliza en la
determinación analítica del oro y como agente de curado en la síntesis
de elastómeros de poliuretano.
La exposición ocupacional a la 3,3'-diclorobencidina puede
presentarse en la industria productora de pigmentos y en aplicaciones
industriales en las que los pigmentos a base de 3,3'-diclorobencidina
se calientan durante periodos prolongados. Se han detectado niveles
de 3,3'-diclorobencidina que oscilaban entre <0,6 a 25 µg/m3 en el
aire ambiente de las plantas de producción y plantas de fabricación de
pigmentos en Alemania y el Japón.
Debido a su baja volatilidad relativa, persistencia muy breve y
baja concentración en la atmósfera, no se cree que la
3,3'-diclorobencidina contribuya al efecto de invernadero, al
agotamiento de la capa de ozono ni a la formación de ozono
troposférico. Los datos disponibles indican que la
3,3'-diclorobencidina probablemente constituya un riesgo
insignificante para los organismos acuáticos.
Los datos disponibles son insuficientes para establecer ingestas
tolerables de 3,3'-diclorobencidina en función de los efectos no
neoplásticos. Sobre la base de indicios suficientes de
carcinogenicidad de la 3,3'-diclorobencidina en múltiples especies
animales en estudios limitados e indicios sustanciales de
genotoxicidad tanto in vitro como in vivo, se considera que la
3,3'-diclorobencidina es un posible carcinógeno humano. En vista de
su carcinogenicidad potencial para el ser humano y a falta de
suficientes datos epidemiológicos, sobre la base del potencial
carcinogénico de la 3,3'-diclorobencidina se ha derivado un valor de
orientación para la salud (DT0,05), calculado a partir de los mejores
estudios crónicos notificados, si bien limitados, en los que se
alimentó a ratas ChR-CD con 1000 ppm de 3,3'-diclorobencidina en la
dieta durante un máximo de 488 días. La DT0,05 se calculó sobre la
base de una interpolación linear que incorporaba una corrección del
peso respecto de la superficie corporal (potencia de 2/3, debido a la
falta de identificación de metabolitos activos) y una corrección por
menos de dos años de exposición, con márgenes de variación de 0,74 a
1,4 mg/kg de peso corporal por día. Para obtener un valor de
orientación respecto de los medios ingeridos se podría dividir el
extremo menor de este margen de DT0,05 (es decir, 0,74 mg/kg de peso
corporal por día), por ejemplo por 5000-50 000 (es decir, 1,48 × 10-4
a 1,48 × 10-5 mg/kg de peso corporal por día). Este margen permite
una protección semejante a la asociada con el margen correspondiente a
las estimaciones de riesgo de dosis bajas generalmente considerados
por diversos organismos como «esencialmente insignificantes» (es
decir, 10-5 a 10-6) . Sobre la base de una estimación de muestra de
la exposición que comportaba concentraciones previstas o datos de
vigilancia limitados procedentes de los Estados Unidos de América y
del Canadá, donde en general no se ha detectado esta sustancia
química, la ingesta diaria total estimada de 3,3'-diclorobencidina por
la población general (por exposición indirecta a través del medio
ambiente) es varios órdenes de magnitud inferior al valor de
orientación de muestra derivado más arriba.
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF DUST! IN ALL CASES CONSULT A
PREVENT GENERATION OF MISTS! DOCTOR!
AVOID ALL CONTACT!
* INHALATION Cough. Sore throat. Avoid inhalation of fine dust Fresh air, rest. Refer for
and mist. Local exhaust or medical attention.
breathing protection.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection if powder. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth. Refer for
during work. Wash hands medical attention.
before eating.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Sweep spilled substance into sealable Separated from food and feedstuffs. Do not transport with food and
containers; if appropriate, moisten Well closed. feedstuffs.
first to prevent dusting. Carefully
collect remainder, then remove to safe T symbol
place. Do NOT let this chemical enter
the environment (extra personal R: 45-21-43
protection: complete protective S: 53-45
clothing including self-contained
breathing apparatus). Note: E
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
GREY TO PURPLE CRYSTALS. The substance irritates the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance decomposes on heating producing Repeated or prolonged contact with skin may
toxic and corrosive fumes including nitrogen cause dermatitis. The substance may have
oxides and hydrogen chloride. effects on the liver. This substance is
probably carcinogenic to humans.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: A2 (skin) (ACGIH 1993-1994).
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however be reached quickly when dispersed,
especially if powdered.
PHYSICAL Boiling point: 368°C
PROPERTIES Melting point: 132-133°C
Solubility in water: none
Auto-ignition temperature: 350°C
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA
NOTES
The substance is combustible but no flash point is available in literature.
Curithane C126 is a trade name.
ICSC: 0481 1.1 Transport Emergency Card: TEC (R)-61G12b
3,3'-DICHLOROBENZIDINE
REFERENCES
ACGIH (1991) 3,3'-Dichlorobenzidine. In: Documentation of the
threshold limit values and biological exposure indices, 6th ed.
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 417-419.
Anderson D, Styles J (1978) The bacterial mutation test. British
journal of cancer, 37:924-930.
Appleton H, Sikka H (1980) Accumulation, elimination and metabolism of
dichlorobenzidine in the bluegill sunfish. Environmental science
and technology, 14:50-54.
Ashby J, Mohammed R (1988) UDS activity in the rat liver of the human
carcinogens benzidine and 4-aminobiphenyl, and the rodent carcinogens
3,3'-dichlorobenzidine and Direct Black 38. Mutagenesis, 3:69-71.
ATSDR (1989) Toxicological profile for 3,3'-dichlorobenzidine.
Atlanta, GA, US Department of Health and Human Services, Public Health
Service, Agency for Toxic Substances and Disease Registry
(PB90-168691).
Banerjee S, Sikka H, Gary R, Kelly C (1978) Photodegradation of
3,3'-dichlorobenzidine. Environmental science and technology,
12:1425-1427.
BUA (1993) 3,3'-Dichlorobenzidine. GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, March 1989. New York,
NY, VCH Publishers, Inc. (BUA Report No. 30).
Chemicals Daily (1993) 12493 chemical products. Chemicals Daily Co.,
Ltd., p. 751.
Chemicals Daily (1994) 12394 chemical products. Chemicals Daily Co.,
Ltd., p. 767.
Cihak R, Vontorkova M (1987) Benzidine and 3,3'-dichlorobenzidine
(DCB) induce micronuclei in the bone marrow and the fetal liver of
mice after gavage. Mutagenesis, 2:267-269.
Dutka B, Kwan K (1981) Comparison of three microbial toxicity
screening tests with the Microtox test. Bulletin of environmental
contamination and toxicology, 27:753-757.
Freeman A, Weisburger E, Weisburger J, Wolford R, Maryak J, Huebner R
(1973) Transformation of cell cultures as an indication of the
carcinogenic potential of chemicals. Journal of the National Cancer
Institute, 51:799-808.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile for organic chemicals. An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C-labelled chemicals.
Chemosphere, 14:1589-1616.
Fricke C, Clarkson C, Lomnitz E, O'Farrel T (1985) Comparing priority
pollutants in municipal sludges. Biocycle, 26:35-37.
Gadian T (1975) Carcinogens in industry, with special reference to
dichlorobenzidine. Chemistry & industry (London), 4:821-831.
Garner R, Walpole A, Rose F (1975) Testing of some benzidine analogues
for microsomal activation to bacterial mutagens. Cancer letters,
1:39-42.
Gerarde H, Gerarde D (1974) Industrial experience with
3,3'-dichlorobenzidine: an epidemiology study of a chemical
manufacturing plant. Journal of occupational medicine, 16:322-344.
Ghosal A, Iba M (1992) Enhancement of butylated hydroxytoluene of the
in vitro activation of 3,3'-dichlorobenzidine. Mutation research,
278:31-41.
Golub N, Kolesnichenko T, Shabad L (1975) [Oncogenic action of some
nitrogen compounds on the progeny of experimental mice.] Bulletin
of experimental biology and medicine, 78:1402-1404 (in Russian)
[cited in US EPA (1988) Health and environmental effects document
for 3,3'-dichlorobenzidine. Washington, DC, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-G034)].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for
3,3'-dichlorobenzidine. Prepared by Health Canada and Environment
Canada. Ottawa, Ontario, Canada Communication Group Publishing (ISBN
0-662-21070-9).
Howard P, Boethling R, Jarvis W, Meylan W, Michalenk E (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers, Inc.
HSDB (1995) Data profile for 3,3'-dichlorobenzidine. Hamilton,
Ontario, Canadian Centre for Occupational Health and Safety, Hazardous
Substances Databank.
IARC (1982) 3,3'-Dichlorobenzidine and its dihydrochloride. Lyon,
International Agency for Research on Cancer, pp. 239-256 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Vol. 29).
IARC (1987) 3,3'-Dichlorobenzidine (Group 2B). Lyon, International
Agency for Research on Cancer, pp. 193-194 (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Suppl. 7).
Iba M (1987) Comparative activation of 3,3'-dichlorobenzidine and
related benzidines to mutagens in Salmonella typhimurium assays by
hepatic S9 and microsomes from rats pretreated with different inducers
of cytochrome P-450. Mutation research, 182:231-241.
IPCS (1993) International Chemical Safety Card -
3,3'-Dichlorobenzidine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0481).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Law R (1995) 3,3'-Dichlorobenzidine: a candidate for inclusion in
marine monitoring programmes? Chemosphere, 30:1791-1797.
Lazear E, Shaddock J, Barren P, Louie S (1979) The mutagenicity of
some of the proposed metabolites of Direct Black 38 and Pigment Yellow
12 in the Salmonella typhimurium assay system. Toxicology
letters, 4:519-525.
MacIntyre I (1975) Experience of tumours in a British plant handling
3,3'-dichlorobenzidine. Journal of occupational medicine, 17:23-26.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic
chemicals: a level III fugacity model. Environmental science and
technology, 25:427-436.
Malaiyandi M, Wightman R, LaFerriere C (1987) Concentration of
selected organic pollutants: comparison of adsorption and reverse
osmosis techniques. In: Malaiyandi M, ed. Organic pollutants in
water. Sampling, analysis and toxicity testing. Washington, DC,
American Chemical Society, pp. 163-179 (American Chemical Society
Symposium Series No. 214).
Martin C, McDermid A, Garner R (1978) Testing of known carcinogens and
noncarcinogens for their ability to induce unscheduled DNA synthesis.
Cancer research, 38:2621-2627.
Messerly E, Fekete J, Wade D, Sinsheimer J (1987) Structure-
mutagenicity relationships of benzidine analogues. Environmental
and molecular mutagenesis, 10:263-274.
Narang A, Choudhury D, Richards A (1982) Separation of aromatic amines
by thin-layer and high performance liquid chromatography. Journal
of chromatographic science, 20:235-237.
Osanai H (1976) [An experimental study on hepatoma caused by aromatic
amines.] Journal of science of labour, 52:179-201 (in Japanese).
Pliss G (1959) [Dichlorobenzidine as a blastomogenic agent.]
Voprosy Onkologii, 5:524-533 (in Russian).
Pliss G (1963) On some regular relationships between carcinogenicity
of aminodiphenyl derivatives and the structure of substances. Acta
Unio Internationalis Contra Cancrum, 19:499-501.
Reid T, Wang C, King C, Morton K (1984) Mutagenicity of some benzidine
congeners and their N-acetylated and N,N'-diacetylated derivatives
in different strains of Salmonella typhimurium. Environmental
mutagenesis, 6:145-151.
Riggin R, Howard C, Scott D, Hedgecoke R (1983) Determination of
benzidine related congeners and pigments in atmospheric particulate
matter. Journal of chromatography, 27:321-325.
Saffiotti U, Cefis F, Montessano R, Sellakumar A (1967) Induction of
bladder cancer in hamsters fed aromatic amines. In: Deichman W, Lampe
K, eds. Bladder cancer. Birmingham, AL, Aesculapis Publishing Co.,
pp. 129-135.
Savard S, Josephy P (1986) Synthesis and mutagenicity of
3,3'-dihalogenated benzidines. Carcinogenesis, 7:1239-1241.
Sellakumar A, Montesano R, Saffiotti U (1969) Aromatic amines:
carcinogenicity in hamsters. Proceedings of the American
Association for Cancer Research, 10:78 (abstract).
Shabad L, Sorokina J, Golub N, Bogovski S (1972) Transplacental effect
of some chemical compounds on organ cultures of embryonic tissue.
Cancer research, 32:617-627.
Shiraishi Y (1986) Hypersensitive character of Bloom syndrome
B-lymphoblastoid cell lines usable for sensitive carcinogen detection.
Mutation research, 175:179-187.
Sikka H, Appleton H, Banerjee S (1978) Fate of
3,3'-dichlorobenzidine in aquatic environments. Athens, GA, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-78-068).
Slobodnik J, Groenewegen M, Brouwer E, Lingeman H, Brinkman U (1993)
Fully automated multi-residue method for trace level monitoring of
polar pesticides by liquid chromatography. Journal of
chromatography, 642:359-370.
Smith E, Weber W (1990) Comparative assessment of the chemical and
adsorptive characteristics of leachates from a municipal and an
industrial landfill. Water, air, and soil pollution, 53:279-295.
Staples C, Werner A, Hoogheem T (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Stula E, Sherman H, Zapp J, Clayton J (1975) Experimental neoplasia in
rats from oral administration of 3,3'-dichlorobenzidine,
4,4'-methylene-bis(2-chloroaniline), and
4,4'-methylene-bis(2-methylaniline). Toxicology and applied
pharmacology, 31:159-176.
Stula E, Barnes J, Sherman H, Reinhardt C, Zapp J (1978) Liver and
urinary bladder tumours in dogs from 3,3'-dichlorobenzidine.
Journal of environmental pathology and toxicology, 1:475-490.
Tsuda H, Miyata Y, Murasaki G, Kinoshita H, Fukushima S, Ito N (1977)
Synergistic effect of urinary bladder carcinogenesis in rats treated
with N-butyl- N-(4-hydroxybutyl)nitrosamine,
N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide,
N-2-fluorenylacetamide and 3,3'-dichlorobenzidine. Gann,
68:183-192.
US DHHS (1994) Seventh annual report on carcinogens. Summary 1994.
Research Triangle Park, NC, US Department of Health and Human
Services, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences.
US EPA (1980) Ambient water quality criteria for dichlorobenzidine.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards (PB81-117517).
US EPA (1990a) Remedial investigation report, landfill area,
Greenville, South Carolina. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-900000412).
US EPA (1990b) Subsurface investigation, Glenholden Laboratory,
Glenholden, Pennsylvania. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-910000001).
US EPA (1990c) Letter from PMS Consolidated to USEPA containing
information on study of potential workplace exposure to
3,3'-dichlorobenzidine, with attachment. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Document
No. 89-900000363).
Valls M, Bayona J, Albaigés J (1990) Broad spectrum analysis of ionic
and non-ionic organic contaminants in urban wastewaters and coastal
receiving aquatic systems. International journal of environmental
analytical chemistry, 39:329-348.
You Z, Brezzell M, Das S, Espadas-Torre M, Hooberman B, Sinsheimer J
(1993) Ortho-substituent effects on the in vitro and in vivo
genotoxicity of benzidine derivatives. Mutation research, 319:19-30.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List assessment report for
3,3'-dichlorobenzidine (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A OH3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above. Copies of the unpublished
Supporting Documentation related to effects on the environment that
formed the basis for preparation of the above-mentioned report may be
obtained from the Commercial Chemicals Branch at the address noted
above.
Initial drafts of the Supporting Documentation and Assessment
Report for 3,3'-dichlorobenzidine were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed by Drs C.M. Auer and W.H. Farland of the US Environmental
Protection Agency. Sections related to the assessment of human health
effects were approved by an interdirectorate Standards and Guidelines
Rulings Committee of the Bureau of Chemical Hazards of Health Canada.
The final Assessment Report was reviewed and approved by the
Environment Canada/Health Canada CEPA Management Committee.
Agency for Toxic Substances and Disease Registry (1989)
Copies of the ATSDR Toxicological profile for
3,3'-dichlorobenzidine (ATSDR, 1989) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicological profile for
3,3'-dichlorobenzidine were reviewed by scientists from the Agency
for Toxic Substances and Disease Registry, the US Environmental
Protection Agency, the US Centre for Disease Control and Prevention,
and the US National Toxicology Program. The document was also
reviewed by an expert panel of nongovernmental reviewers, consisting
of the following members: Dr Paul Mushak, Private Consultant, Durham,
North Carolina; Dr David Jollow, Professor, Medical University of
South Carolina; and Dr T. Kneip, Professor, New York University
Medical Center.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 3,3'-dichlorobenzidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Hoechst Aktiengesellschaft, Frankfurt, Germany
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (Document international succinct sur l'évaluation des risques
chimiques) sur la 3,3'-dichlorobenzidine en s'inspirant principalement
des évaluations du Gouvernement du Canada (1993) et de l'Agency for
Toxic Substances and Disease Registry (ATSDR, 1989) visant à
déterminer les effets potentiels sur la santé humaine d'une exposition
indirecte à la 3,3'-dichlorobenzidine dans l'environnement général,
ainsi que les effets de cette substance sur l'environnement.
L'évaluation du Gouvernement du Canada (1993) a pris en compte les
données relatives à la détermination des effets de la
3,3'-dichlorobenzidine sur l'environnement et la santé humaine qui
étaient disponibles en avril 1992 (effets sur l'environnement) et en
octobre 1992 (effets sur la santé humaine); ces données ont été mises
à jour en septembre 1995 à partir des données du Registre
international des substances chimiques potentiellement toxiques et de
bases de données en ligne. Des informations concernant la nature de
l'évaluation par les pairs et la disponibilité des documents du
Gouvernement du Canada (1993) et de l'ATSDR (1989) figurent à
l'appendice 1. Des informations sur l'évaluation par les pairs du
présent CICAD figurent à l'appendice 2. Ce CICAD a été approuvé pour
publication à une réunion du Comité d'évaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à la réunion du Comité d'évaluation finale figure à
l'appendice 3. La fiche d'information sur la sécurité chimique (ICSC
0481) pour la 3,3'-dichlorobenzidine, préparée par le Programme
international sur la sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La 3,3'-dichlorobenzidine (CAS N° 91-94-1) est une amine
aromatique primaire chlorée synthétique disponible dans le commerce
sous la forme de dichlorhydrate de 3,3'-dichlorobenzidine
(C12H10N2Cl2.2HCl). La 3,3'-dichlorobenzidine et certains de ses
dérivés sont utilisés principalement comme intermédiaires dans la
fabrication de pigments pour les encres d'imprimerie, les textiles,
les peintures et les matières plastiques. Elle est également utilisée
pour le titrage de l'or et comme agent de polymérisation dans la
synthèse d'élastomères de la famille des polyuréthanes.
L'exposition professionnelle à la 3,3'-dichlorobenzidine est
possible lors de la fabrication de pigments et dans les installations
industrielles où des pigments qui en contiennent sont chauffés pendant
des périodes prolongées. Des concentrations de 3,3'-dichlorobenzidine
comprises entre <0,6 à 25 µg/m3 ont été mesurées dans l'air des
ateliers de production et des usines de fabrication de pigments en
Allemagne et au Japon.
Étant donné sa volatilité relativement faible, sa très courte
persistance et sa faible concentration dans l'atmosphère, la
3,3'-dichlorobenzidine ne devrait pas contribuer à l'effet de serre, à
la destruction de la couche d'ozone ou à la formation d'ozone au
niveau du sol. D'après les données disponibles, il est probable que
la 3,3'-dichlorobenzidine présente un risque négligeable pour les
organismes aquatiques.
Les données disponibles sont insuffisantes pour établir des doses
tolérables de 3,3'-dichlorobenzidine fondées sur ses effets non
néoplasiques. Des études limitées ont apporté des preuves suffisantes
de sa cancérogénicité chez de nombreuses espèces animales et sa
génotoxicité a été démontrée in vitro +et in vivo; en conséquence,
la 3,3'-dichlorobenzidine est considérée comme une substance
potentiellement cancérogène pour l'homme. Compte tenu de cette
cancérogénicité potentielle et de l'absence de données
épidémiologiques adéquates, une valeur guide fondée sur des critères
de santé peut être établie sur la base du potentiel cancérogène
(c'est-à-dire sur la DT0,05) déterminé à partir des données de la
mieux présentée des études de toxicité chronique. Dans cette étude,
certes limitée, des rats ChR-CD ont consommé pendant 488 jours une
nourriture contenant 1000 ppm de 3,3'-dichlorobenzidine. La DT0,05 a
été calculée par interpolation linéaire en incorporant un facteur de
correction du poids corporel en fonction de la surface corporelle
(puissance 2/3 en raison de l'absence d'identification du ou des
métabolites actifs) et un autre facteur tenant compte de la durée
d'exposition inférieure à deux ans. La fourchette obtenue a été de
0,74 à 1,4 mg/kg de poids corporel par jour. En divisant la valeur
inférieure de cette fourchette (soit 0,74 mg/kg de poids corporel par
jour) par 5000 - 50 000, on obtient 1,48 × 10-4 à 1,48 × 10-5 mg/kg
de poids corporel par jour, ce qui peut être considéré comme une
valeur guide appropriée pour l'ingestion. Cette marge offre une
protection analogue à celle des fourchettes d'exposition aux faibles
doses qui sont généralement considérées par les organismes compétents
comme présentant un risque "pratiquement négligeable" (soit 10-5 à
10-6). Si l'on se base sur une estimation de l'exposition effectuée
à partir des concentrations prédites ou des quelques données
recueillies aux États-Unis d'Amérique et au Canada, où la substance
n'a généralement pas été détectée, la dose quotidienne totale
estimative absorbée par la population générale (des suites d'une
exposition indirecte à l'environnement général) est inférieure de
plusieurs ordres de grandeur à la valeur guide indiquée ci-dessus.
RESUMEN DE ORIENTACION
Esta reseña de la evaluación química internacional de la
3,3'-diclorobencidina fue preparada por la Dirección de Higiene del
Medio de Health Canada, principalmente sobre la base de revisiones del
Gobierno del Canadá (1993) y de la Agencia para el Registro de
Sustancias Tóxicas y Enfermedades (ATSDR, 1989), a fin de evaluar los
efectos potenciales en la salud humana de la exposición indirecta a la
3,3'-diclorobencidina en el medio ambiente general, así como sus
efectos ambientales. En su estudio (1993), el Gobierno del Canadá
examinó los datos pertinentes para la evaluación de los efectos de la
3,3'-diclorobencidina en el medio ambiente y la salud humana
identificados hasta abril de 1992 (efectos ambientales) y octubre de
1992 (efectos en la salud humana). Posteriormente los datos se
actualizaron hasta septiembre de 1995 utilizando el Registro
Internacional de Sustancias Químicas Potencialmente Tóxicas y bases de
datos en línea. En el apéndice 1 se presenta información sobre el
carácter de la revisión científica y la disponibilidad de los
documentos del Gobierno del Canadá (1993) y del ATSDR (1989). En el
apéndice 2 se presenta información sobre la revisión científica de
esta reseña. Esta reseña fue aprobada para publicación en una reunión
de la Junta de Revisión Final celebrada en Bruselas, Bélgica, del 18
al 20 de noviembre de 1996. En el apéndice 3 figuran los
participantes en dicha reunión. En el presente documento también se
reproduce la Ficha Internacional de Seguridad Química (ICSC 0481) de
la 3,3'-diclorobencidina, producida por el Programa Internacional de
Seguridad de las Sustancias Químicas (IPCS, 1993).
La 3,3'-diclorobencidina (CAS N° 91-94-1) es una amina aromática
primaria clorada sintética que se puede obtener comercialmente en
forma de sal del dihidrocloruro, dihidrocloruro de
3,3'-diclorobencidina (C12H10N2Cl2.2HCl). La 3,3'-diclorobencidina
(y algunos de sus derivados) se utiliza principalmente como
intermediario en la fabricación de pigmentos para tintas de imprenta,
textiles, pinturas y plásticos. También se utiliza en la
determinación analítica del oro y como agente de curado en la síntesis
de elastómeros de poliuretano.
La exposición ocupacional a la 3,3'-diclorobencidina puede
presentarse en la industria productora de pigmentos y en aplicaciones
industriales en las que los pigmentos a base de 3,3'-diclorobencidina
se calientan durante periodos prolongados. Se han detectado niveles
de 3,3'-diclorobencidina que oscilaban entre <0,6 a 25 µg/m3 en el
aire ambiente de las plantas de producción y plantas de fabricación de
pigmentos en Alemania y el Japón.
Debido a su baja volatilidad relativa, persistencia muy breve y
baja concentración en la atmósfera, no se cree que la
3,3'-diclorobencidina contribuya al efecto de invernadero, al
agotamiento de la capa de ozono ni a la formación de ozono
troposférico. Los datos disponibles indican que la
3,3'-diclorobencidina probablemente constituya un riesgo
insignificante para los organismos acuáticos.
Los datos disponibles son insuficientes para establecer ingestas
tolerables de 3,3'-diclorobencidina en función de los efectos no
neoplásticos. Sobre la base de indicios suficientes de
carcinogenicidad de la 3,3'-diclorobencidina en múltiples especies
animales en estudios limitados e indicios sustanciales de
genotoxicidad tanto in vitro como in vivo, se considera que la
3,3'-diclorobencidina es un posible carcinógeno humano. En vista de
su carcinogenicidad potencial para el ser humano y a falta de
suficientes datos epidemiológicos, sobre la base del potencial
carcinogénico de la 3,3'-diclorobencidina se ha derivado un valor de
orientación para la salud (DT0,05), calculado a partir de los mejores
estudios crónicos notificados, si bien limitados, en los que se
alimentó a ratas ChR-CD con 1000 ppm de 3,3'-diclorobencidina en la
dieta durante un máximo de 488 días. La DT0,05 se calculó sobre la
base de una interpolación linear que incorporaba una corrección del
peso respecto de la superficie corporal (potencia de 2/3, debido a la
falta de identificación de metabolitos activos) y una corrección por
menos de dos años de exposición, con márgenes de variación de 0,74 a
1,4 mg/kg de peso corporal por día. Para obtener un valor de
orientación respecto de los medios ingeridos se podría dividir el
extremo menor de este margen de DT0,05 (es decir, 0,74 mg/kg de peso
corporal por día), por ejemplo por 5000-50 000 (es decir, 1,48 × 10-4
a 1,48 × 10-5 mg/kg de peso corporal por día). Este margen permite
una protección semejante a la asociada con el margen correspondiente a
las estimaciones de riesgo de dosis bajas generalmente considerados
por diversos organismos como «esencialmente insignificantes» (es
decir, 10-5 a 10-6) . Sobre la base de una estimación de muestra de
la exposición que comportaba concentraciones previstas o datos de
vigilancia limitados procedentes de los Estados Unidos de América y
del Canadá, donde en general no se ha detectado esta sustancia
química, la ingesta diaria total estimada de 3,3'-diclorobencidina por
la población general (por exposición indirecta a través del medio
ambiente) es varios órdenes de magnitud inferior al valor de
orientación de muestra derivado más arriba.
 1. EXECUTIVE SUMMARY
This CICAD on 3,3'-dichlorobenzidine was prepared by the
Environmental Health Directorate of Health Canada, based principally
on reviews of the Government of Canada (1993) and the Agency for Toxic
Substances and Disease Registry (ATSDR, 1989), to assess the potential
effects on human health of indirect exposure to 3,3'-dichlorobenzidine
in the general environment as well as the chemical's environmental
effects. Data relevant to the assessment of the effects of
3,3'-dichlorobenzidine on the environment and human health identified
as of April 1992 (environmental effects) and October 1992 (human
health effects) were considered in the Government of Canada (1993)
review; data were subsequently updated to September 1995 from the
International Register of Potentially Toxic Chemicals and on-line
databases. Information concerning the nature of peer review and the
availability of the Government of Canada (1993) and ATSDR (1989)
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0481) for 3,3'-dichlorobenzidine, produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
3,3'-Dichlorobenzidine (CAS no.
1. EXECUTIVE SUMMARY
This CICAD on 3,3'-dichlorobenzidine was prepared by the
Environmental Health Directorate of Health Canada, based principally
on reviews of the Government of Canada (1993) and the Agency for Toxic
Substances and Disease Registry (ATSDR, 1989), to assess the potential
effects on human health of indirect exposure to 3,3'-dichlorobenzidine
in the general environment as well as the chemical's environmental
effects. Data relevant to the assessment of the effects of
3,3'-dichlorobenzidine on the environment and human health identified
as of April 1992 (environmental effects) and October 1992 (human
health effects) were considered in the Government of Canada (1993)
review; data were subsequently updated to September 1995 from the
International Register of Potentially Toxic Chemicals and on-line
databases. Information concerning the nature of peer review and the
availability of the Government of Canada (1993) and ATSDR (1989)
documents is presented in Appendix 1. Information on the peer review
of this CICAD is presented in Appendix 2. This CICAD was approved for
publication at a meeting of the Final Review Board, held in Brussels,
Belgium, on 18-20 November 1996. Participants at the Final Review
Board meeting are presented in Appendix 3. The International Chemical
Safety Card (ICSC 0481) for 3,3'-dichlorobenzidine, produced by the
International Programme on Chemical Safety (IPCS, 1993), has also been
reproduced in this document.
3,3'-Dichlorobenzidine (CAS no.  TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF DUST! IN ALL CASES CONSULT A
PREVENT GENERATION OF MISTS! DOCTOR!
AVOID ALL CONTACT!
* INHALATION Cough. Sore throat. Avoid inhalation of fine dust Fresh air, rest. Refer for
and mist. Local exhaust or medical attention.
breathing protection.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection if powder. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth. Refer for
during work. Wash hands medical attention.
before eating.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Sweep spilled substance into sealable Separated from food and feedstuffs. Do not transport with food and
containers; if appropriate, moisten Well closed. feedstuffs.
first to prevent dusting. Carefully
collect remainder, then remove to safe T symbol
place. Do NOT let this chemical enter
the environment (extra personal R: 45-21-43
protection: complete protective S: 53-45
clothing including self-contained
breathing apparatus). Note: E
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
GREY TO PURPLE CRYSTALS. The substance irritates the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance decomposes on heating producing Repeated or prolonged contact with skin may
toxic and corrosive fumes including nitrogen cause dermatitis. The substance may have
oxides and hydrogen chloride. effects on the liver. This substance is
probably carcinogenic to humans.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: A2 (skin) (ACGIH 1993-1994).
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however be reached quickly when dispersed,
especially if powdered.
PHYSICAL Boiling point: 368°C
PROPERTIES Melting point: 132-133°C
Solubility in water: none
Auto-ignition temperature: 350°C
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA
NOTES
The substance is combustible but no flash point is available in literature.
Curithane C126 is a trade name.
ICSC: 0481 1.1 Transport Emergency Card: TEC (R)-61G12b
3,3'-DICHLOROBENZIDINE
REFERENCES
ACGIH (1991) 3,3'-Dichlorobenzidine. In: Documentation of the
threshold limit values and biological exposure indices, 6th ed.
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 417-419.
Anderson D, Styles J (1978) The bacterial mutation test. British
journal of cancer, 37:924-930.
Appleton H, Sikka H (1980) Accumulation, elimination and metabolism of
dichlorobenzidine in the bluegill sunfish. Environmental science
and technology, 14:50-54.
Ashby J, Mohammed R (1988) UDS activity in the rat liver of the human
carcinogens benzidine and 4-aminobiphenyl, and the rodent carcinogens
3,3'-dichlorobenzidine and Direct Black 38. Mutagenesis, 3:69-71.
ATSDR (1989) Toxicological profile for 3,3'-dichlorobenzidine.
Atlanta, GA, US Department of Health and Human Services, Public Health
Service, Agency for Toxic Substances and Disease Registry
(PB90-168691).
Banerjee S, Sikka H, Gary R, Kelly C (1978) Photodegradation of
3,3'-dichlorobenzidine. Environmental science and technology,
12:1425-1427.
BUA (1993) 3,3'-Dichlorobenzidine. GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, March 1989. New York,
NY, VCH Publishers, Inc. (BUA Report No. 30).
Chemicals Daily (1993) 12493 chemical products. Chemicals Daily Co.,
Ltd., p. 751.
Chemicals Daily (1994) 12394 chemical products. Chemicals Daily Co.,
Ltd., p. 767.
Cihak R, Vontorkova M (1987) Benzidine and 3,3'-dichlorobenzidine
(DCB) induce micronuclei in the bone marrow and the fetal liver of
mice after gavage. Mutagenesis, 2:267-269.
Dutka B, Kwan K (1981) Comparison of three microbial toxicity
screening tests with the Microtox test. Bulletin of environmental
contamination and toxicology, 27:753-757.
Freeman A, Weisburger E, Weisburger J, Wolford R, Maryak J, Huebner R
(1973) Transformation of cell cultures as an indication of the
carcinogenic potential of chemicals. Journal of the National Cancer
Institute, 51:799-808.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile for organic chemicals. An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C-labelled chemicals.
Chemosphere, 14:1589-1616.
Fricke C, Clarkson C, Lomnitz E, O'Farrel T (1985) Comparing priority
pollutants in municipal sludges. Biocycle, 26:35-37.
Gadian T (1975) Carcinogens in industry, with special reference to
dichlorobenzidine. Chemistry & industry (London), 4:821-831.
Garner R, Walpole A, Rose F (1975) Testing of some benzidine analogues
for microsomal activation to bacterial mutagens. Cancer letters,
1:39-42.
Gerarde H, Gerarde D (1974) Industrial experience with
3,3'-dichlorobenzidine: an epidemiology study of a chemical
manufacturing plant. Journal of occupational medicine, 16:322-344.
Ghosal A, Iba M (1992) Enhancement of butylated hydroxytoluene of the
in vitro activation of 3,3'-dichlorobenzidine. Mutation research,
278:31-41.
Golub N, Kolesnichenko T, Shabad L (1975) [Oncogenic action of some
nitrogen compounds on the progeny of experimental mice.] Bulletin
of experimental biology and medicine, 78:1402-1404 (in Russian)
[cited in US EPA (1988) Health and environmental effects document
for 3,3'-dichlorobenzidine. Washington, DC, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-G034)].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for
3,3'-dichlorobenzidine. Prepared by Health Canada and Environment
Canada. Ottawa, Ontario, Canada Communication Group Publishing (ISBN
0-662-21070-9).
Howard P, Boethling R, Jarvis W, Meylan W, Michalenk E (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers, Inc.
HSDB (1995) Data profile for 3,3'-dichlorobenzidine. Hamilton,
Ontario, Canadian Centre for Occupational Health and Safety, Hazardous
Substances Databank.
IARC (1982) 3,3'-Dichlorobenzidine and its dihydrochloride. Lyon,
International Agency for Research on Cancer, pp. 239-256 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Vol. 29).
IARC (1987) 3,3'-Dichlorobenzidine (Group 2B). Lyon, International
Agency for Research on Cancer, pp. 193-194 (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Suppl. 7).
Iba M (1987) Comparative activation of 3,3'-dichlorobenzidine and
related benzidines to mutagens in Salmonella typhimurium assays by
hepatic S9 and microsomes from rats pretreated with different inducers
of cytochrome P-450. Mutation research, 182:231-241.
IPCS (1993) International Chemical Safety Card -
3,3'-Dichlorobenzidine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0481).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Law R (1995) 3,3'-Dichlorobenzidine: a candidate for inclusion in
marine monitoring programmes? Chemosphere, 30:1791-1797.
Lazear E, Shaddock J, Barren P, Louie S (1979) The mutagenicity of
some of the proposed metabolites of Direct Black 38 and Pigment Yellow
12 in the Salmonella typhimurium assay system. Toxicology
letters, 4:519-525.
MacIntyre I (1975) Experience of tumours in a British plant handling
3,3'-dichlorobenzidine. Journal of occupational medicine, 17:23-26.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic
chemicals: a level III fugacity model. Environmental science and
technology, 25:427-436.
Malaiyandi M, Wightman R, LaFerriere C (1987) Concentration of
selected organic pollutants: comparison of adsorption and reverse
osmosis techniques. In: Malaiyandi M, ed. Organic pollutants in
water. Sampling, analysis and toxicity testing. Washington, DC,
American Chemical Society, pp. 163-179 (American Chemical Society
Symposium Series No. 214).
Martin C, McDermid A, Garner R (1978) Testing of known carcinogens and
noncarcinogens for their ability to induce unscheduled DNA synthesis.
Cancer research, 38:2621-2627.
Messerly E, Fekete J, Wade D, Sinsheimer J (1987) Structure-
mutagenicity relationships of benzidine analogues. Environmental
and molecular mutagenesis, 10:263-274.
Narang A, Choudhury D, Richards A (1982) Separation of aromatic amines
by thin-layer and high performance liquid chromatography. Journal
of chromatographic science, 20:235-237.
Osanai H (1976) [An experimental study on hepatoma caused by aromatic
amines.] Journal of science of labour, 52:179-201 (in Japanese).
Pliss G (1959) [Dichlorobenzidine as a blastomogenic agent.]
Voprosy Onkologii, 5:524-533 (in Russian).
Pliss G (1963) On some regular relationships between carcinogenicity
of aminodiphenyl derivatives and the structure of substances. Acta
Unio Internationalis Contra Cancrum, 19:499-501.
Reid T, Wang C, King C, Morton K (1984) Mutagenicity of some benzidine
congeners and their N-acetylated and N,N'-diacetylated derivatives
in different strains of Salmonella typhimurium. Environmental
mutagenesis, 6:145-151.
Riggin R, Howard C, Scott D, Hedgecoke R (1983) Determination of
benzidine related congeners and pigments in atmospheric particulate
matter. Journal of chromatography, 27:321-325.
Saffiotti U, Cefis F, Montessano R, Sellakumar A (1967) Induction of
bladder cancer in hamsters fed aromatic amines. In: Deichman W, Lampe
K, eds. Bladder cancer. Birmingham, AL, Aesculapis Publishing Co.,
pp. 129-135.
Savard S, Josephy P (1986) Synthesis and mutagenicity of
3,3'-dihalogenated benzidines. Carcinogenesis, 7:1239-1241.
Sellakumar A, Montesano R, Saffiotti U (1969) Aromatic amines:
carcinogenicity in hamsters. Proceedings of the American
Association for Cancer Research, 10:78 (abstract).
Shabad L, Sorokina J, Golub N, Bogovski S (1972) Transplacental effect
of some chemical compounds on organ cultures of embryonic tissue.
Cancer research, 32:617-627.
Shiraishi Y (1986) Hypersensitive character of Bloom syndrome
B-lymphoblastoid cell lines usable for sensitive carcinogen detection.
Mutation research, 175:179-187.
Sikka H, Appleton H, Banerjee S (1978) Fate of
3,3'-dichlorobenzidine in aquatic environments. Athens, GA, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-78-068).
Slobodnik J, Groenewegen M, Brouwer E, Lingeman H, Brinkman U (1993)
Fully automated multi-residue method for trace level monitoring of
polar pesticides by liquid chromatography. Journal of
chromatography, 642:359-370.
Smith E, Weber W (1990) Comparative assessment of the chemical and
adsorptive characteristics of leachates from a municipal and an
industrial landfill. Water, air, and soil pollution, 53:279-295.
Staples C, Werner A, Hoogheem T (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Stula E, Sherman H, Zapp J, Clayton J (1975) Experimental neoplasia in
rats from oral administration of 3,3'-dichlorobenzidine,
4,4'-methylene-bis(2-chloroaniline), and
4,4'-methylene-bis(2-methylaniline). Toxicology and applied
pharmacology, 31:159-176.
Stula E, Barnes J, Sherman H, Reinhardt C, Zapp J (1978) Liver and
urinary bladder tumours in dogs from 3,3'-dichlorobenzidine.
Journal of environmental pathology and toxicology, 1:475-490.
Tsuda H, Miyata Y, Murasaki G, Kinoshita H, Fukushima S, Ito N (1977)
Synergistic effect of urinary bladder carcinogenesis in rats treated
with N-butyl- N-(4-hydroxybutyl)nitrosamine,
N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide,
N-2-fluorenylacetamide and 3,3'-dichlorobenzidine. Gann,
68:183-192.
US DHHS (1994) Seventh annual report on carcinogens. Summary 1994.
Research Triangle Park, NC, US Department of Health and Human
Services, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences.
US EPA (1980) Ambient water quality criteria for dichlorobenzidine.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards (PB81-117517).
US EPA (1990a) Remedial investigation report, landfill area,
Greenville, South Carolina. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-900000412).
US EPA (1990b) Subsurface investigation, Glenholden Laboratory,
Glenholden, Pennsylvania. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-910000001).
US EPA (1990c) Letter from PMS Consolidated to USEPA containing
information on study of potential workplace exposure to
3,3'-dichlorobenzidine, with attachment. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Document
No. 89-900000363).
Valls M, Bayona J, Albaigés J (1990) Broad spectrum analysis of ionic
and non-ionic organic contaminants in urban wastewaters and coastal
receiving aquatic systems. International journal of environmental
analytical chemistry, 39:329-348.
You Z, Brezzell M, Das S, Espadas-Torre M, Hooberman B, Sinsheimer J
(1993) Ortho-substituent effects on the in vitro and in vivo
genotoxicity of benzidine derivatives. Mutation research, 319:19-30.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List assessment report for
3,3'-dichlorobenzidine (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A OH3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above. Copies of the unpublished
Supporting Documentation related to effects on the environment that
formed the basis for preparation of the above-mentioned report may be
obtained from the Commercial Chemicals Branch at the address noted
above.
Initial drafts of the Supporting Documentation and Assessment
Report for 3,3'-dichlorobenzidine were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed by Drs C.M. Auer and W.H. Farland of the US Environmental
Protection Agency. Sections related to the assessment of human health
effects were approved by an interdirectorate Standards and Guidelines
Rulings Committee of the Bureau of Chemical Hazards of Health Canada.
The final Assessment Report was reviewed and approved by the
Environment Canada/Health Canada CEPA Management Committee.
Agency for Toxic Substances and Disease Registry (1989)
Copies of the ATSDR Toxicological profile for
3,3'-dichlorobenzidine (ATSDR, 1989) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicological profile for
3,3'-dichlorobenzidine were reviewed by scientists from the Agency
for Toxic Substances and Disease Registry, the US Environmental
Protection Agency, the US Centre for Disease Control and Prevention,
and the US National Toxicology Program. The document was also
reviewed by an expert panel of nongovernmental reviewers, consisting
of the following members: Dr Paul Mushak, Private Consultant, Durham,
North Carolina; Dr David Jollow, Professor, Medical University of
South Carolina; and Dr T. Kneip, Professor, New York University
Medical Center.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 3,3'-dichlorobenzidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Hoechst Aktiengesellschaft, Frankfurt, Germany
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (Document international succinct sur l'évaluation des risques
chimiques) sur la 3,3'-dichlorobenzidine en s'inspirant principalement
des évaluations du Gouvernement du Canada (1993) et de l'Agency for
Toxic Substances and Disease Registry (ATSDR, 1989) visant à
déterminer les effets potentiels sur la santé humaine d'une exposition
indirecte à la 3,3'-dichlorobenzidine dans l'environnement général,
ainsi que les effets de cette substance sur l'environnement.
L'évaluation du Gouvernement du Canada (1993) a pris en compte les
données relatives à la détermination des effets de la
3,3'-dichlorobenzidine sur l'environnement et la santé humaine qui
étaient disponibles en avril 1992 (effets sur l'environnement) et en
octobre 1992 (effets sur la santé humaine); ces données ont été mises
à jour en septembre 1995 à partir des données du Registre
international des substances chimiques potentiellement toxiques et de
bases de données en ligne. Des informations concernant la nature de
l'évaluation par les pairs et la disponibilité des documents du
Gouvernement du Canada (1993) et de l'ATSDR (1989) figurent à
l'appendice 1. Des informations sur l'évaluation par les pairs du
présent CICAD figurent à l'appendice 2. Ce CICAD a été approuvé pour
publication à une réunion du Comité d'évaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à la réunion du Comité d'évaluation finale figure à
l'appendice 3. La fiche d'information sur la sécurité chimique (ICSC
0481) pour la 3,3'-dichlorobenzidine, préparée par le Programme
international sur la sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La 3,3'-dichlorobenzidine (CAS N°
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
FIRE Combustible. Gives off NO open flames. Powder, water spray, foam,
irritating or toxic fumes carbon dioxide.
(or gases) in a fire.
EXPLOSION
EXPOSURE PREVENT DISPERSION OF DUST! IN ALL CASES CONSULT A
PREVENT GENERATION OF MISTS! DOCTOR!
AVOID ALL CONTACT!
* INHALATION Cough. Sore throat. Avoid inhalation of fine dust Fresh air, rest. Refer for
and mist. Local exhaust or medical attention.
breathing protection.
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
TYPES OF ACUTE HAZARDS/ PREVENTION FIRST AID/
HAZARD/ SYMPTOMS FIRE FIGHTING
EXPOSURE
* SKIN MAY BE ABSORBED! Protective gloves. Protective Remove contaminated clothes.
clothing. Rinse and then wash skin with
water and soap. Refer for
medical attention.
* EYES Face shield or eye protection First rinse with plenty of
in combination with breathing water for several minutes
protection if powder. (remove contact lenses if
easily possible), then take
to a doctor.
* INGESTION Do not eat, drink, or smoke Rinse mouth. Refer for
during work. Wash hands medical attention.
before eating.
SPILLAGE DISPOSAL STORAGE PACKAGING & LABELLING
Sweep spilled substance into sealable Separated from food and feedstuffs. Do not transport with food and
containers; if appropriate, moisten Well closed. feedstuffs.
first to prevent dusting. Carefully
collect remainder, then remove to safe T symbol
place. Do NOT let this chemical enter
the environment (extra personal R: 45-21-43
protection: complete protective S: 53-45
clothing including self-contained
breathing apparatus). Note: E
INTERNATIONAL CHEMICAL SAFETY CARD (continued)
IMPORTANT DATA PHYSICAL STATE; APPEARANCE: EFFECTS OF SHORT-TERM EXPOSURE:
GREY TO PURPLE CRYSTALS. The substance irritates the respiratory tract.
CHEMICAL DANGERS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
The substance decomposes on heating producing Repeated or prolonged contact with skin may
toxic and corrosive fumes including nitrogen cause dermatitis. The substance may have
oxides and hydrogen chloride. effects on the liver. This substance is
probably carcinogenic to humans.
OCCUPATIONAL EXPOSURE LIMITS (OELs):
TLV: A2 (skin) (ACGIH 1993-1994).
INHALATION RISK:
Evaporation at 20°C is negligible; a harmful
concentration of airborne particles can,
however be reached quickly when dispersed,
especially if powdered.
PHYSICAL Boiling point: 368°C
PROPERTIES Melting point: 132-133°C
Solubility in water: none
Auto-ignition temperature: 350°C
ENVIRONMENTAL The substance is toxic to aquatic organisms.
DATA
NOTES
The substance is combustible but no flash point is available in literature.
Curithane C126 is a trade name.
ICSC: 0481 1.1 Transport Emergency Card: TEC (R)-61G12b
3,3'-DICHLOROBENZIDINE
REFERENCES
ACGIH (1991) 3,3'-Dichlorobenzidine. In: Documentation of the
threshold limit values and biological exposure indices, 6th ed.
Cincinnati, OH, American Conference of Governmental Industrial
Hygienists, pp. 417-419.
Anderson D, Styles J (1978) The bacterial mutation test. British
journal of cancer, 37:924-930.
Appleton H, Sikka H (1980) Accumulation, elimination and metabolism of
dichlorobenzidine in the bluegill sunfish. Environmental science
and technology, 14:50-54.
Ashby J, Mohammed R (1988) UDS activity in the rat liver of the human
carcinogens benzidine and 4-aminobiphenyl, and the rodent carcinogens
3,3'-dichlorobenzidine and Direct Black 38. Mutagenesis, 3:69-71.
ATSDR (1989) Toxicological profile for 3,3'-dichlorobenzidine.
Atlanta, GA, US Department of Health and Human Services, Public Health
Service, Agency for Toxic Substances and Disease Registry
(PB90-168691).
Banerjee S, Sikka H, Gary R, Kelly C (1978) Photodegradation of
3,3'-dichlorobenzidine. Environmental science and technology,
12:1425-1427.
BUA (1993) 3,3'-Dichlorobenzidine. GDCh-Advisory Committee on
Existing Chemicals of Environmental Relevance, March 1989. New York,
NY, VCH Publishers, Inc. (BUA Report No. 30).
Chemicals Daily (1993) 12493 chemical products. Chemicals Daily Co.,
Ltd., p. 751.
Chemicals Daily (1994) 12394 chemical products. Chemicals Daily Co.,
Ltd., p. 767.
Cihak R, Vontorkova M (1987) Benzidine and 3,3'-dichlorobenzidine
(DCB) induce micronuclei in the bone marrow and the fetal liver of
mice after gavage. Mutagenesis, 2:267-269.
Dutka B, Kwan K (1981) Comparison of three microbial toxicity
screening tests with the Microtox test. Bulletin of environmental
contamination and toxicology, 27:753-757.
Freeman A, Weisburger E, Weisburger J, Wolford R, Maryak J, Huebner R
(1973) Transformation of cell cultures as an indication of the
carcinogenic potential of chemicals. Journal of the National Cancer
Institute, 51:799-808.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard
profile for organic chemicals. An experimental method for the
assessment of the behaviour of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C-labelled chemicals.
Chemosphere, 14:1589-1616.
Fricke C, Clarkson C, Lomnitz E, O'Farrel T (1985) Comparing priority
pollutants in municipal sludges. Biocycle, 26:35-37.
Gadian T (1975) Carcinogens in industry, with special reference to
dichlorobenzidine. Chemistry & industry (London), 4:821-831.
Garner R, Walpole A, Rose F (1975) Testing of some benzidine analogues
for microsomal activation to bacterial mutagens. Cancer letters,
1:39-42.
Gerarde H, Gerarde D (1974) Industrial experience with
3,3'-dichlorobenzidine: an epidemiology study of a chemical
manufacturing plant. Journal of occupational medicine, 16:322-344.
Ghosal A, Iba M (1992) Enhancement of butylated hydroxytoluene of the
in vitro activation of 3,3'-dichlorobenzidine. Mutation research,
278:31-41.
Golub N, Kolesnichenko T, Shabad L (1975) [Oncogenic action of some
nitrogen compounds on the progeny of experimental mice.] Bulletin
of experimental biology and medicine, 78:1402-1404 (in Russian)
[cited in US EPA (1988) Health and environmental effects document
for 3,3'-dichlorobenzidine. Washington, DC, US Environmental
Protection Agency, Environmental Criteria and Assessment Office
(ECAO-CIN-G034)].
Government of Canada (1993) Canadian Environmental Protection Act.
Priority Substances List assessment report for
3,3'-dichlorobenzidine. Prepared by Health Canada and Environment
Canada. Ottawa, Ontario, Canada Communication Group Publishing (ISBN
0-662-21070-9).
Howard P, Boethling R, Jarvis W, Meylan W, Michalenk E (1991)
Handbook of environmental degradation rates. Chelsea, MI, Lewis
Publishers, Inc.
HSDB (1995) Data profile for 3,3'-dichlorobenzidine. Hamilton,
Ontario, Canadian Centre for Occupational Health and Safety, Hazardous
Substances Databank.
IARC (1982) 3,3'-Dichlorobenzidine and its dihydrochloride. Lyon,
International Agency for Research on Cancer, pp. 239-256 (IARC
Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man,
Vol. 29).
IARC (1987) 3,3'-Dichlorobenzidine (Group 2B). Lyon, International
Agency for Research on Cancer, pp. 193-194 (IARC Monographs on the
Evaluation of Carcinogenic Risk of Chemicals to Man, Suppl. 7).
Iba M (1987) Comparative activation of 3,3'-dichlorobenzidine and
related benzidines to mutagens in Salmonella typhimurium assays by
hepatic S9 and microsomes from rats pretreated with different inducers
of cytochrome P-450. Mutation research, 182:231-241.
IPCS (1993) International Chemical Safety Card -
3,3'-Dichlorobenzidine. Geneva, World Health Organization,
International Programme on Chemical Safety (No. 0481).
IPCS (1994) Assessing human health risks of chemicals: derivation
of guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
Law R (1995) 3,3'-Dichlorobenzidine: a candidate for inclusion in
marine monitoring programmes? Chemosphere, 30:1791-1797.
Lazear E, Shaddock J, Barren P, Louie S (1979) The mutagenicity of
some of the proposed metabolites of Direct Black 38 and Pigment Yellow
12 in the Salmonella typhimurium assay system. Toxicology
letters, 4:519-525.
MacIntyre I (1975) Experience of tumours in a British plant handling
3,3'-dichlorobenzidine. Journal of occupational medicine, 17:23-26.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic
chemicals: a level III fugacity model. Environmental science and
technology, 25:427-436.
Malaiyandi M, Wightman R, LaFerriere C (1987) Concentration of
selected organic pollutants: comparison of adsorption and reverse
osmosis techniques. In: Malaiyandi M, ed. Organic pollutants in
water. Sampling, analysis and toxicity testing. Washington, DC,
American Chemical Society, pp. 163-179 (American Chemical Society
Symposium Series No. 214).
Martin C, McDermid A, Garner R (1978) Testing of known carcinogens and
noncarcinogens for their ability to induce unscheduled DNA synthesis.
Cancer research, 38:2621-2627.
Messerly E, Fekete J, Wade D, Sinsheimer J (1987) Structure-
mutagenicity relationships of benzidine analogues. Environmental
and molecular mutagenesis, 10:263-274.
Narang A, Choudhury D, Richards A (1982) Separation of aromatic amines
by thin-layer and high performance liquid chromatography. Journal
of chromatographic science, 20:235-237.
Osanai H (1976) [An experimental study on hepatoma caused by aromatic
amines.] Journal of science of labour, 52:179-201 (in Japanese).
Pliss G (1959) [Dichlorobenzidine as a blastomogenic agent.]
Voprosy Onkologii, 5:524-533 (in Russian).
Pliss G (1963) On some regular relationships between carcinogenicity
of aminodiphenyl derivatives and the structure of substances. Acta
Unio Internationalis Contra Cancrum, 19:499-501.
Reid T, Wang C, King C, Morton K (1984) Mutagenicity of some benzidine
congeners and their N-acetylated and N,N'-diacetylated derivatives
in different strains of Salmonella typhimurium. Environmental
mutagenesis, 6:145-151.
Riggin R, Howard C, Scott D, Hedgecoke R (1983) Determination of
benzidine related congeners and pigments in atmospheric particulate
matter. Journal of chromatography, 27:321-325.
Saffiotti U, Cefis F, Montessano R, Sellakumar A (1967) Induction of
bladder cancer in hamsters fed aromatic amines. In: Deichman W, Lampe
K, eds. Bladder cancer. Birmingham, AL, Aesculapis Publishing Co.,
pp. 129-135.
Savard S, Josephy P (1986) Synthesis and mutagenicity of
3,3'-dihalogenated benzidines. Carcinogenesis, 7:1239-1241.
Sellakumar A, Montesano R, Saffiotti U (1969) Aromatic amines:
carcinogenicity in hamsters. Proceedings of the American
Association for Cancer Research, 10:78 (abstract).
Shabad L, Sorokina J, Golub N, Bogovski S (1972) Transplacental effect
of some chemical compounds on organ cultures of embryonic tissue.
Cancer research, 32:617-627.
Shiraishi Y (1986) Hypersensitive character of Bloom syndrome
B-lymphoblastoid cell lines usable for sensitive carcinogen detection.
Mutation research, 175:179-187.
Sikka H, Appleton H, Banerjee S (1978) Fate of
3,3'-dichlorobenzidine in aquatic environments. Athens, GA, US
Environmental Protection Agency, Environmental Research Laboratory
(EPA 600/3-78-068).
Slobodnik J, Groenewegen M, Brouwer E, Lingeman H, Brinkman U (1993)
Fully automated multi-residue method for trace level monitoring of
polar pesticides by liquid chromatography. Journal of
chromatography, 642:359-370.
Smith E, Weber W (1990) Comparative assessment of the chemical and
adsorptive characteristics of leachates from a municipal and an
industrial landfill. Water, air, and soil pollution, 53:279-295.
Staples C, Werner A, Hoogheem T (1985) Assessment of priority
pollutant concentrations in the United States using STORET database.
Environmental toxicology and chemistry, 4:131-142.
Stula E, Sherman H, Zapp J, Clayton J (1975) Experimental neoplasia in
rats from oral administration of 3,3'-dichlorobenzidine,
4,4'-methylene-bis(2-chloroaniline), and
4,4'-methylene-bis(2-methylaniline). Toxicology and applied
pharmacology, 31:159-176.
Stula E, Barnes J, Sherman H, Reinhardt C, Zapp J (1978) Liver and
urinary bladder tumours in dogs from 3,3'-dichlorobenzidine.
Journal of environmental pathology and toxicology, 1:475-490.
Tsuda H, Miyata Y, Murasaki G, Kinoshita H, Fukushima S, Ito N (1977)
Synergistic effect of urinary bladder carcinogenesis in rats treated
with N-butyl- N-(4-hydroxybutyl)nitrosamine,
N-[4-(5-nitro-2-furyl)-2-thiazolyl]formamide,
N-2-fluorenylacetamide and 3,3'-dichlorobenzidine. Gann,
68:183-192.
US DHHS (1994) Seventh annual report on carcinogens. Summary 1994.
Research Triangle Park, NC, US Department of Health and Human
Services, Public Health Service, National Institutes of Health,
National Institute of Environmental Health Sciences.
US EPA (1980) Ambient water quality criteria for dichlorobenzidine.
Washington, DC, US Environmental Protection Agency, Office of Water
Regulations and Standards (PB81-117517).
US EPA (1990a) Remedial investigation report, landfill area,
Greenville, South Carolina. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-900000412).
US EPA (1990b) Subsurface investigation, Glenholden Laboratory,
Glenholden, Pennsylvania. Washington, DC, US Environmental
Protection Agency, Office of Toxic Substances (Document No.
86-910000001).
US EPA (1990c) Letter from PMS Consolidated to USEPA containing
information on study of potential workplace exposure to
3,3'-dichlorobenzidine, with attachment. Washington, DC, US
Environmental Protection Agency, Office of Toxic Substances (Document
No. 89-900000363).
Valls M, Bayona J, Albaigés J (1990) Broad spectrum analysis of ionic
and non-ionic organic contaminants in urban wastewaters and coastal
receiving aquatic systems. International journal of environmental
analytical chemistry, 39:329-348.
You Z, Brezzell M, Das S, Espadas-Torre M, Hooberman B, Sinsheimer J
(1993) Ortho-substituent effects on the in vitro and in vivo
genotoxicity of benzidine derivatives. Mutation research, 319:19-30.
APPENDIX 1 - SOURCE DOCUMENTS
Government of Canada (1993)
Copies of the Canadian Environmental Protection Act (CEPA)
Priority Substances List assessment report for
3,3'-dichlorobenzidine (Government of Canada, 1993) may be obtained
from the:
Commercial Chemicals Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A OH3
Environmental Health Centre
Health Canada
Address Locator: 0801A
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Copies of the unpublished Supporting Documentation related to
human health effects that formed the basis for preparation of the
above-mentioned report may be obtained from the Environmental Health
Centre at the address noted above. Copies of the unpublished
Supporting Documentation related to effects on the environment that
formed the basis for preparation of the above-mentioned report may be
obtained from the Commercial Chemicals Branch at the address noted
above.
Initial drafts of the Supporting Documentation and Assessment
Report for 3,3'-dichlorobenzidine were prepared by staff of Health
Canada and Environment Canada. The environmental sections were
reviewed by Drs C.M. Auer and W.H. Farland of the US Environmental
Protection Agency. Sections related to the assessment of human health
effects were approved by an interdirectorate Standards and Guidelines
Rulings Committee of the Bureau of Chemical Hazards of Health Canada.
The final Assessment Report was reviewed and approved by the
Environment Canada/Health Canada CEPA Management Committee.
Agency for Toxic Substances and Disease Registry (1989)
Copies of the ATSDR Toxicological profile for
3,3'-dichlorobenzidine (ATSDR, 1989) may be obtained from the:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road, E-29
Atlanta, Georgia 30333
USA
Initial drafts of the Toxicological profile for
3,3'-dichlorobenzidine were reviewed by scientists from the Agency
for Toxic Substances and Disease Registry, the US Environmental
Protection Agency, the US Centre for Disease Control and Prevention,
and the US National Toxicology Program. The document was also
reviewed by an expert panel of nongovernmental reviewers, consisting
of the following members: Dr Paul Mushak, Private Consultant, Durham,
North Carolina; Dr David Jollow, Professor, Medical University of
South Carolina; and Dr T. Kneip, Professor, New York University
Medical Center.
APPENDIX 2 - CICAD PEER REVIEW
The draft CICAD on 3,3'-dichlorobenzidine was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS national Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Department of Health, London, United Kingdom
Department of Public Health, Albert Szent-Gyorgyi University
Medical School, Szeged, Hungary
Direccion General de Salud Ambiental, Subsecretario de Regulacion
y Fomento Sanitario, San Luis Potosi, Mexico
Fraunhofer Institute of Toxicology and Aerosol Research, Hanover,
Germany
Guy's & St. Thomas' Hospital Trust, Medical Toxicology Unit,
London, United Kingdom
Health and Safety Executive, Bootle, United Kingdom
Hoechst Aktiengesellschaft, Frankfurt, Germany
International Agency for Research on Cancer, Lyon, France
Ministry of Health, National Centre of Hygiene, Medical Ecology
and Nutrition, Sofia, Bulgaria
Ministry of Health and Welfare, International Affairs Division,
Government of Japan, Tokyo, Japan
National Institute for Working Life, Solna, Sweden
United States Department of Health and Human Services (Agency for
Toxic Substances and Disease Registry; National Institute of
Environmental Health Sciences)
United States Environmental Protection Agency (Office of
Pollution Prevention and Toxics; National Center for
Environmental Assessment, Office of Research and Development;
Office of Drinking Water)
APPENDIX 3 - CICAD FINAL REVIEW BOARD
Brussels, Belgium, 18-20 November 1996
Members
Dr A. Aitio, Institute of Occupational Health, Helsinki, Finland
Dr K. Bentley, Director, Environment Policy Section, Commonwealth
Department of Human Services and Health, Canberra, Australia
Mr R. Cary, Toxicology and Existing Substances Regulation Unit, Health
and Safety Executive, Merseyside, United Kingdom
Dr J. de Fouw, National Institute of Public Health and Environmental
Protection, Bilthoven, The Netherlands
Dr C. DeRosa, Director, Division of Toxicology, Agency for Toxic
Substances and Disease Registry, Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr W. Farland, Director, National Center for Environmental Assessment,
Office of Research and Development, US Environmental Protection
Agency, Washington, DC, USA (Chairperson)
Dr T.I. Fortoul, Depto. Biologia Celular y Tisular, National
University of Mexico and Environmental Health Directorate of the
Health Ministry, Mexico D.F., Mexico
Dr H. Gibb, National Center for Environmental Assessment, US
Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers &
Veterinary Medicine, Berlin, Germany
Mr J.R. Hickman, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Dr T. Lakhanisky, Head, Division of Toxicology, Institute of Hygiene
and Epidemiology, Brussels, Belgium (Vice-Chairperson)
Dr I. Mangelsdorf, Documentation and Assessment of Chemicals,
Fraunhofer Institute for Toxicology and Aerosol Sciences, Hanover,
Germany
Ms E. Meek, Head, Priority Substances Section, Environmental Health
Directorate, Health Canada, Ottawa, Ontario, Canada
Dr K. Paksy, National Institute of Occupational Health, Budapest,
Hungary
Mr D. Renshaw, Department of Health, London, United Kingdom
Dr J. Sekizawa, Division of Chemo-Bio Informatics, National Institute
of Hygienic Sciences, Tokyo, Japan
Dr H. Sterzl-Eckert, GSF-Forschungszentrum für Umwelt und Gesundheit
GmbH, Institut für Toxikologie, Oberschleissheim, Germany
Professor S. Tarkowski, Department of Environmental Health Hazards,
The Nofer Institute of Occupational Medicine, Lodz, Poland
Dr M. Wallen, National Chemicals Inspectorate (KEMI), Solna, Sweden
Observers
Professor F.M.C. Carpanini,1 Director, Centre for Ecotoxicology and
Toxicology of Chemicals (ECETOC), Brussels, Belgium
Mr R. Haigh,1 Head of Unit, Health and Safety Directorate, European
Commission, Luxembourg
Mr B.U. Hildebrandt, Federal Ministry for the Environment, Nature
Conservation and Nuclear Safety, Bonn, Germany
Mr P. Hurst,1 Chemical and Consumer Policy Officer, Conservation
Policy Division, World Wide Fund for Nature, Gland, Switzerland
Dr A. Lombard (Representative of CEFIC), ELF-ATOCHEM, Paris, France
Dr P. McCutcheon,1 Environment, Consumer Protection and Nuclear
Safety, European Commission, Brussels, Belgium
Dr R. Montaigne, Counsellor, Technical Affairs Department, European
Chemical Industry Council (CEFIC), Brussels, Belgium
Dr M. Pemberton, ICI Acrylics, Lancashire, United Kingdom
Dr A. Smith, Organisation for Economic Co-operation and Development,
Environment Division, Paris, France
Secretariat
Dr M. Baril, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
1 Invited but unable to attend.
Dr L. Harrison, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M. Mercier, Director, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland
Dr P. Toft, Associate Director, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland
RÉSUMÉ D'ORIENTATION
La Direction de l'Hygiène du Milieu de Santé Canada a rédigé ce
CICAD (Document international succinct sur l'évaluation des risques
chimiques) sur la 3,3'-dichlorobenzidine en s'inspirant principalement
des évaluations du Gouvernement du Canada (1993) et de l'Agency for
Toxic Substances and Disease Registry (ATSDR, 1989) visant à
déterminer les effets potentiels sur la santé humaine d'une exposition
indirecte à la 3,3'-dichlorobenzidine dans l'environnement général,
ainsi que les effets de cette substance sur l'environnement.
L'évaluation du Gouvernement du Canada (1993) a pris en compte les
données relatives à la détermination des effets de la
3,3'-dichlorobenzidine sur l'environnement et la santé humaine qui
étaient disponibles en avril 1992 (effets sur l'environnement) et en
octobre 1992 (effets sur la santé humaine); ces données ont été mises
à jour en septembre 1995 à partir des données du Registre
international des substances chimiques potentiellement toxiques et de
bases de données en ligne. Des informations concernant la nature de
l'évaluation par les pairs et la disponibilité des documents du
Gouvernement du Canada (1993) et de l'ATSDR (1989) figurent à
l'appendice 1. Des informations sur l'évaluation par les pairs du
présent CICAD figurent à l'appendice 2. Ce CICAD a été approuvé pour
publication à une réunion du Comité d'évaluation finale qui s'est
tenue à Bruxelles (Belgique) du 18 au 20 novembre 1996. La liste des
participants à la réunion du Comité d'évaluation finale figure à
l'appendice 3. La fiche d'information sur la sécurité chimique (ICSC
0481) pour la 3,3'-dichlorobenzidine, préparée par le Programme
international sur la sécurité chimique (IPCS, 1993), est également
reproduite dans le présent document.
La 3,3'-dichlorobenzidine (CAS N° 