
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 46
First draft prepared by R. Newhook and M.E. Meek, Existing Substances Division, Health Canada, Ottawa, Ontario, Canada; and D. Caldbick, Commercial Chemicals Evaluation Branch, Environment Canada, Hull, Quebec, Canada
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2002
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Carbon disulfide.
(Concise international chemical assessment document ; 46)
1. Carbon disulfide - adverse effects 2. Risk assessment 3. Environmental exposure
4. Occupational exposure I. International Programme on Chemical Safety II.Series
ISBN 92 4 153046 4 (NLM Classification: QV 633)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available.
©World Health Organization 2002
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
|
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION |
|
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS |
|
11.1.3 Sample risk characterization for the general population |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) – a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose-response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
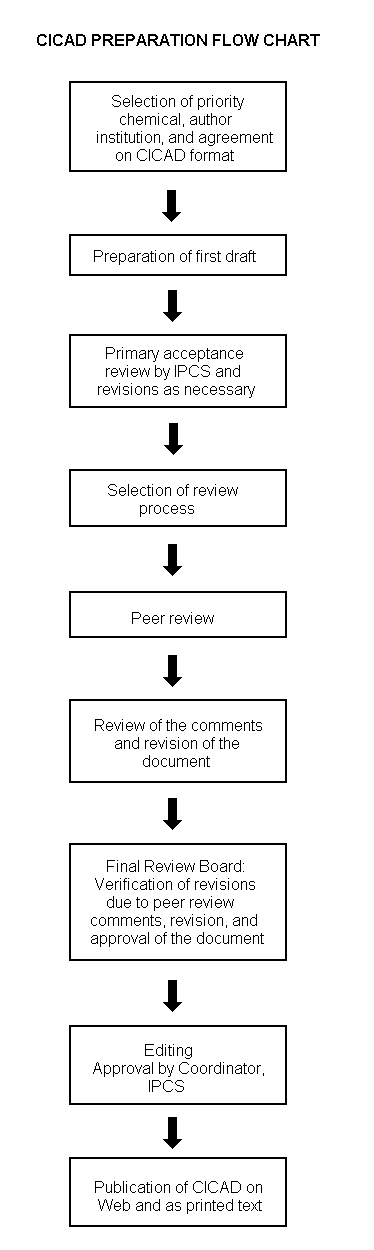
The flow chart shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world – expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, a priority chemical typically
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers' comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers' comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on carbon disulfide was prepared by the Environmental Health Directorate of Health Canada and the Commercial Chemicals Evaluation Branch of Environment Canada based on documentation prepared concurrently as part of the Priority Substances Program under the Canadian Environmental Protection Act (CEPA). The objective of assessments on priority substances under CEPA is to assess potential effects of indirect exposure in the general environment on human health as well as environmental effects. Data identified as of the end of May 1999 were considered in these reviews.2 Information on the nature of the peer review and availability of the source document is presented in Appendix 1. Other source documents for the health and environmental effects include reports prepared by IPCS (1979), the Nofer Institute (Rolecki & Tarkowski, 2000), and the United Kingdom Department of Environment (Crookes et al., 1993). Other reviews that were also consulted include BUA (1993) and ATSDR (1996). Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, on 29 October - 1 November 2001. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card (ICSC 0022) for carbon disulfide, produced by the International Programme on Chemical Safety (IPCS, 2000), has also been reproduced in this document.
The worldwide production capacity of carbon disulfide (CAS No.
Carbon disulfide is ubiquitous throughout the environment. It has been detected in air, water, sediment, and soil; however, it is present primarily in air. The highest concentrations of carbon disulfide in air in the source country for this CICAD (i.e., Canada) have been measured near industrial sources, in particular near natural gas processing plants and sites with sulfur-containing natural gas flares. Carbon disulfide is removed from the air primarily by reaction with hydroxyl radicals, resulting in a half-life of 1-2 weeks. This half-life in air makes it a candidate for long-range transport; however, it is rapidly diluted to natural background levels. Carbon disulfide is rapidly metabolized by organisms and does not bioconcentrate or biomagnify.
Available data upon which to base estimates of human exposure to carbon disulfide are extremely limited; however, air appears to be the major route of exposure for members of the general population. Airborne exposures are estimated to be elevated for populations in the vicinity of industrial point sources.
Carbon disulfide is extensively absorbed by inhalation, but also via the skin. It is metabolized to several metabolites, one of which (2-thiothiazolidine-4-carboxylic acid) is the basis for biomonitoring of exposure at the workplace.
Available data to serve as a basis for assessment of the potential of carbon disulfide to induce irritancy or sensitization are limited. While it has been reported in secondary accounts of seemingly limited early studies that carbon disulfide is severely irritating to the eyes and skin, it has not been possible to verify these data. While inhalation in viscose rayon plants is irritating to the mucous membranes, including the respiratory system, the role of concomitant exposure to hydrogen sulfide and sulfuric acid in induction of these effects is unknown.
Based on the results of studies of workers exposed to carbon disulfide and supporting data from experiments conducted on animals, the nervous system appears to be the critical target for carbon disulfide-induced toxicity, manifested most often as reduced conduction velocity in the peripheral nerves and impaired performance in psychomotor testing. Other effects for which there is considerable weight of evidence in humans exposed to carbon disulfide include alterations in serum lipids and blood pressure that are associated with increased risk of cardiovascular disease, systemic ophthalmological effects, including those on colour vision and damage to the blood vessels of the retina, and (with higher exposures) increased mortality from heart disease.
No evidence of carcinogenicity has been observed in limited epidemiological studies; long-term studies on carcinogenicity in experimental animals have not been reported. There is no clear evidence of genotoxicity, although there is some evidence of weak and/or equivocal clastogenicity in vitro or in vivo.
There are several reports of decreased libido and/or impotence among males occupationally exposed to high concentrations of carbon disulfide, but there is no consistent evidence based on limited study of other adverse reproductive effects in humans. In experimental animals, carbon disulfide is embryotoxic and fetotoxic at high concentrations and causes terata at exposure levels toxic to the dam.
In the sample risk characterization, the estimated mean airborne exposure to carbon disulfide for the general population and for populations in the vicinity of point sources is considerably less than a tolerable concentration of 100 µg/m3. This tolerable concentration was derived based on the benchmark concentration estimated for a 5% excess risk of an abnormal response (defined based on the 5th percentile of the unexposed workers in the critical study) for the most sensitive response variable – i.e., peroneal motor nerve conduction velocity (MCV)3 in viscose rayon workers – adjusting to continuous exposure (24 h/day, 7 days/week) and applying an overall uncertainty factor of 50.
Since most carbon disulfide is released to air, for environmental effects, it is terrestrial organisms in the vicinity of industrial sources that are at greatest potential risk. Aquatic organisms close to discharge points might also be potentially affected. However, based on the sample risk characterization, conservative comparisons of estimated exposure with no-effect values indicate that it is unlikely that carbon disulfide causes adverse effects on populations of terrestrial or aquatic organisms.
Carbon disulfide (also known as carbon disulphide, carbon bisulfide, carbon sulfide, and dithiocarbonic anhydride) is a clear, colourless or faintly yellow, mobile liquid at room temperature. Its relative molecular mass is 76.14, its Chemical Abstracts Service (CAS) registry number is
S = C = S
Analysis of carbon disulfide in air usually involves preconcentration on a sorbent tube followed by thermal or solvent desorption followed by gas chromatography (mass spectrometry, electron capture, photoionization, or flame photometric detector). Detection limits range from 0.6 ng/m3 to 10 µg/m3 (ATSDR, 1996). In samples of water and soil, carbon disulfide is often analysed by purge and trap methods followed by gas chromatography and mass spectrometry. Detection limits are in the low microgram per litre (or kilogram) or milligram per litre (or kilogram) range (ATSDR, 1996). In food, carbon disulfide is usually extracted with acetone and analysed by gas chromatography (electron capture or Sievers chemiluminescence detection). Detection limits are in the nanogram per sample range (ATSDR, 1996). For biological fluids, various purge and trap or solvent extraction methods are applied before analysis by high-performance liquid chromatography or gas chromatography and mass spectrometry. Detection limits are in the nanogram per litre range (ATSDR, 1996).
For the biological monitoring of carbon disulfide, mainly at the workplace, analysis of the metabolite 2-thiothiazolidine-4-carboxylic acid (TTCA) in urine has been extensively applied. The methods most often used are based on high-performance liquid chromatography; the detection limit is <0.1 mg/litre, adequate for assessing 8-h time-weighted average (TWA) exposures below 0.3 mg/m3 (Lowry, 1996).
Wherever possible, data on sources and emissions in a global context are presented. Where such information was not identified, data from the source country of the national assessment on which the CICAD is based (i.e., Canada) are presented as an example.
Carbon disulfide is released into the environment from a wide variety of natural sources. Soils, marshes, and coastal regions tend to be the largest biogenic sources. Production of carbon disulfide from soil and plants occurs naturally from the metabolic action of soil bacteria and plants during the growing season. Increases in soil moisture, temperature, organic content, and light resulted in a direct increase in the rate of production from soil (Staubes et al., 1987). Up to 35 000 tonnes of carbon disulfide may be added to the Canadian environment annually from this natural source alone (Environment Canada, 1980). It is estimated that up to 2280 tonnes of carbon disulfide per year could be released globally as a result of the weathering of sulfide minerals (Stedman et al., 1984). Carbon disulfide is also produced by forest and grass fires and by volcanoes.
Although there is a great deal of uncertainty in the estimates, worldwide, at least 40% and possibly as much as 80% of releases are a result of natural or biogenic activity (Environment Canada & Health Canada, 2000).
Carbon disulfide is used primarily as a solvent in the viscose rayon (about 65%) and cellophane (about 10-12%) industries (BUA, 1993; ATSDR, 1996). In the production of viscose fibres and cellophane film, carbon disulfide is not bound to the end product. Thus, unless appropriate measures are taken, most of the carbon disulfide used in these processes is likely to end up in the atmosphere. If approximately 75% of the world production of about 1 million tonnes is used for these two processes (see section 4.3), some 700 kilotonnes would be released annually.
The 1999 Toxic Release Inventory indicates that approximately 16 kilotonnes of carbon disulfide were released to the environment in the USA from manufacturing and processing facilities in 1999. Virtually all of this was released to the air (TRI, 1999). The estimated annual release of carbon disulfide in the United Kingdom from viscose fibre and cellophane film manufacturing was 15-21 kilotonnes/year (Crookes et al., 1993).
Between 2120 and 2465 tonnes of carbon disulfide were released from Canadian industrial sources in 1996. Nearly all of this was emitted into the atmosphere in the gas sector. Total reported releases from all other industrial sources – including commercial manufacture, distribution, and use of carbon disulfide – were less than 100 tonnes (Environment Canada, 1997b).
Carbon disulfide has been used as a fumigant on stored grain, although its registration for this purpose has been withdrawn in most countries (C. Warfield, personal communication, 1996). It is also released to the environment in cigarette smoke.
Worldwide production of carbon disulfide was estimated to be 1.025 million tonnes in 1984 and 0.9 million tonnes in 1990 (Rolecki & Tarkowski, 2000). In 1985, production in the USA alone was around 143 kilotonnes (ATSDR, 1996). Annual production in the United Kingdom prior to 1992 was estimated to be between 25 and 35 kilotonnes (Crookes et al., 1993).
The major use of carbon disulfide worldwide is the production of viscose (rayon) fibres (65%) and cellophane film (10-15%) (Rolecki & Tarkowski, 2000). Other uses include the manufacture of carbon tetrachloride (apparently only very small amounts at present), sodium sulfite, mineral flotation agents, xanthates, mercaptans, and thioureas. Carbon disulfide is also used as a solvent for fats, lipids, resins, rubbers, sulfur monochloride, and white phosphorus (Crookes et al., 1993).
In 1996, 3.1 kilotonnes of carbon disulfide were manufactured for commercial purposes in Canada (Environment Canada, 1997b). Camford Information Services (1995) reported Canadian domestic production of 10.9 kilotonnes in 1993, down from 25 kilotonnes in 1976. The much lower production figure in recent years reflects the closure in Canada of the rayon and cellulose fibre industry, which had been the major user of carbon disulfide.
In Canada, nearly 1.7 kilotonnes of carbon disulfide were used in 1996 as a precursor in the manufacture of xanthates, which are used as flotation agents in mineral refining processes (Environment Canada, 1997b). Carbon disulfide is also used to produce drilling mud additives to dissolve waxes that interfere with the efficiency and yields of oil and gas wells and in the manufacture of rubber curing accelerators, which are used in the production of rubber tires for vehicles (Camford Information Services, 1995).
In air, carbon disulfide is primarily degraded through photo-oxidation by reactions with hydroxyl radicals and by a secondary route involving triplet oxygen (O(3P)). Based on an assumed concentration of hydroxyl radicals of 5 ´ 105 radicals/cm3, a half-life of about 5.5-15 days is calculated from rate constants between 1.1 ´ 10-12 and 2.9 ´ 10-12 cm3/molecule per second (BUA, 1993). Wine et al. (1981) likewise estimated that photo-oxidation in the troposphere results in a half-life in air of 7-14 days. Reaction products include carbonyl sulfide and sulfur dioxide. Carbonyl sulfide has a much longer lifetime (2 years) than carbon disulfide in the atmosphere.
Photolysis of carbon disulfide by radiation at wavelengths above 290 nm occurs in the troposphere. An atmospheric lifetime of 11 days (half-life of 7.7 days) was calculated assuming 12 h of sunlight (Peyton et al., 1976). Wood & Heicklen (1971) demonstrated that direct photolysis of carbon disulfide at 313 nm produces reaction products similar to those of the photo-oxidation reaction – that is, carbon monoxide, carbonyl sulfide, sulfur dioxide, plus an unidentified polymeric material. Wet deposition from the atmosphere is probably a minor removal process, because carbon disulfide interacts only weakly with water (Lovejoy, 1989).
The overall reactivity-based half-life of carbon disulfide in air, as estimated for ChemCAN4 steady-state fugacity modelling (section 5.5), is about 1 week (DMER & AEL, 1996).
Carbon disulfide has little potential to exert effects on the atmospheric environment. Since carbon disulfide is non-halogenated, its ozone-depleting potential is zero, and it will therefore not contribute to the depletion of stratospheric ozone. It is estimated that carbon disulfide has less than 1% of the global warming potential of the reference compound CFC-11, and it is therefore not considered to be involved in climate change. (Carbon disulfide may have an indirect impact on climate change through its main atmospheric transformation product, carbonyl sulfide, but the magnitude of this impact is considered to be small.) Except in proximity to strong point sources, average annual concentrations of carbon disulfide in ambient air are low relative to those for the volatile organic compounds with similar photochemical ozone creation potential that contribute most to the formation of ground-level ozone. Therefore, the contribution of carbon disulfide to ground-level ozone formation is not expected to be significant (Environment Canada & Health Canada, 2000).
Based on a Henry's law constant of 1748 Pa× m3/mol at 25 °C and a vapour pressure of 48.2 kPa at 25 ° C, carbon disulfide released into water is expected to volatilize, with a half-life ranging between 11 min in water (saturated solution) and 2.6 h in a model river (Peyton et al., 1976; Howard, 1989). Carbon disulfide is resistant to hydrolysis in water within the biological pH range (4-10), with a hydrolysis half-life extrapolated to pH 9 of 1.1 years (Peyton et al., 1976). Its predicted rate of biodegradation in water is negligible compared with its rate of volatilization from surface water (ATSDR, 1996). The mean degradation half-life assumed for fugacity modelling by DMER & AEL (1996) (section 5.5) of 5500 h (7.4 months) was based on the estimate of biodegradation half-life by Abrams et al. (1975).
Owing to its low affinity for sorption to organic substances (organic carbon/water partition coefficient [log Koc] = 1.79), very little carbon disulfide is likely to partition to or remain in sediment. In one study, the soil/sediment microorganism Thiobacillus thioparus (grown aerobically, incubated anaerobically) was able to metabolize carbon disulfide to produce carbonyl sulfide and hydrogen sulfide (Smith & Kelly, 1988). Thus, some biodegradation is expected to occur. The estimated mean reactivity half-life assumed for fugacity modelling (section 5.5) was 5500 h (7.4 months), based on the estimate of biodegradation half-life by Abrams et al. (1975).
No estimates of a half-life for carbon disulfide in soil were identified in the literature. Carbon disulfide has been aerobically degraded by a strain of Thiobacillus thioparus. This particular strain was able to hydrolytically oxidize carbon disulfide sequentially to carbonyl sulfide and hydrogen sulfide; all the carbon was released as carbon dioxide, followed by oxidation of the sulfide to sulfate (Smith & Kelly, 1988). For soil, DMER & AEL (1996) assumed a mean degradation half-life of 5500 h for their fugacity modelling (section 5.5), based on the estimate of biodegradation half-life by Abrams et al. (1975). In the natural environment, carbon disulfide is highly mobile in soil (log Koc = 1.79) and is subject to rapid volatilization, so it is unlikely to remain in soil long enough to undergo significant biodegradation.
Carbon disulfide is expected to have little or no tendency to bioaccumulate or biomagnify in biota, owing to its relatively low log Kow value (2.14) and rapid metabolism in most animals (Beauchamp et al., 1983).
Fugacity modelling provides an overview of key reaction, intercompartment, and advection (movement out of a system) pathways for carbon disulfide and its overall distribution in the environment (DMER & AEL, 1996). A steady-state, non-equilibrium EQC model (Level III fugacity model) was run using the methods developed by Mackay (1991) and Mackay & Paterson (1991). Values for physical/chemical properties utilized in the modelling are presented in section 2; those for half-lives in various media are presented in sections 5.1-5.3, above. Modelling was based on an assumed default emission rate of 1000 kg/h into a region of 100 000 km2, which includes a 10 000-km2 area of surface water (20 m deep). The height of the atmosphere is 1000 m. Sediments and soils have an organic carbon content of 4% and 2% and a depth of 1 cm and 10 cm, respectively. The estimated percent distribution predicted by this model is not affected by the assumed emission rate.
Modelling indicates that carbon disulfide partitions differently depending on the medium to which it is released. For example, if emitted into air, 99.8% of the carbon disulfide is present in air; if emitted into soil, the fraction in air is reduced to 73%, with most of the rest in soil. When carbon disulfide is released to water, it is present primarily in water (85%) and, to a lesser extent, in air (15%) (DMER & AEL, 1996). Thus, while the predicted distributions indicate little intermedia transport when carbon disulfide is discharged to air, release to each of soil and (to a lesser extent) water has the potential for substantial transport of carbon disulfide to air (see also sections 5.1-5.3).
Data on concentrations in the environment from the source country of the national assessment on which the CICAD is based (i.e., Canada) are presented here as a basis for the sample risk characterization. Where identified, data from other countries are also presented.
Based on continuous monitoring in ambient air over 2 years at a remote site and at two sites in the vicinity of a sour gas processing plant (carbon disulfide is a minor component of the waste gases emitted from the processing of sour gas), carbon disulfide was not detected in the majority of samples – e.g., in 85-90% of samples at the remote site – and was detected somewhat more frequently at the sour gas sites. Based on extensive data from conventional gas chromatography, combined with some limited data collected over an 8-min sampling period by a sensitive cryofocusing technique, the mean and maximum levels were higher at the sites near the sour gas plant (0.61 and 88 µg/m3, respectively, at an upwind site, and 1.40 and 156 µg/m3, respectively, at a downwind site) than at the remote site (0.51 and 12.5 µg/m3, respectively) (Legge et al., 1990a, 1990b).
The concentration of carbon disulfide in the air downwind from another gas processing site was predicted by the ISC 3 view plume dispersion model (reported release of 1287 tonnes to the atmosphere in 1995, the largest release reported for Canada that year; NPRI, 1996). The highest calculated concentration in air 1 km downwind (a 1-h average) was about 114 µg/m3. The 24-h average maximum ground-level concentration 10 km downwind was 14.3 µg/m3 (The, 1998).
The results of other modelling studies indicate that levels near smaller sour gas wells are somewhat less than those near larger wells. Based on concentrations of carbon disulfide measured in flare gases from a sour gas facility in central Alberta, in combination with plume dispersion modelling, Strosher (1996) predicted the maximum ground-level concentration at 2.02 µg/m3 for a daily average and 0.16 µg/m3 for an annual average.
Carbon disulfide levels were also elevated on the site of Prospec Chemicals, Fort Saskatchewan, Alberta, which uses the compound on-site as a feedstock for xanthates. In monitoring of ambient air outside of the property line (at the point of impingement predicted by dispersion modelling) during the summer of 1997, monthly average concentrations of carbon disulfide ranged from 3 to 6 µg/m3, and hourly maximum concentrations were between 56 and 100 µg/m3 (L. Fu, personal communication, 1997; E. Weiss, personal communication, 1998).
In a small study, the mean level of carbon disulfide in six outdoor air samples in New York City, USA, was 0.30 µg/m3 (Phillips, 1992). Concentrations up to 1.1 and 1.2 µg/m3 in ambient air were measured in the USA and Europe, respectively (Sandalls & Penkett, 1977; Maroulis & Bandy, 1980; Crookes et al., 1993; ATSDR, 1996).
In a small study, carbon disulfide was detected in all nine indoor air samples from a hospital in New York City, USA; the mean concentration was 0.63 µg/m3, which was not significantly higher than the mean level in six outdoor air samples (0.30 µg/m3) (Phillips, 1992).
Concentrations of carbon disulfide were below the limit of detection in samples of indoor air from several office buildings in the USA (Fuortes, 1990; Oldaker et al., 1995).
Data on levels of carbon disulfide in surface waters are limited to southern Ontario, Canada. Background levels at remote sites in Ontario, largely due to biogenic production, ranged between about 0.005 and 0.4 µg/litre (Caron & Kramer, 1994). In Lake Ontario, in 1981, a median concentration of 0.4 µg/litre and a maximum of 3.9 µg/litre were measured (Kaiser et al., 1983). The authors considered that the lower levels in the open lake were likely due to biogenic activity, while the elevated levels were due mainly to the influence of nearby urban/industrial areas (B. Scott, personal communication, 1998). The highest measured concentration in surface water, 25.0 µg/litre, was associated with a chemical plant on Thompson Creek in the Niagara region that has since closed (Kaiser & Comba, 1983).
In seawater, Lovelock (1974) reported concentrations in the open Atlantic of 0.52 and 0.78 ng/litre off the coast of Ireland and 5.4 ng/litre in stagnant bay water near Ireland. Leck & Rodhe (1991) measured levels of carbon disulfide between 0.83 and 1.18 ng/litre in the open Baltic and North seas. Kim & Andreae (1987) reported concentrations of carbon disulfide in surface waters in the North Atlantic ranging between 0.01 and 4.6 ng/litre.
Data on levels in groundwater were not identified.
Very few data on the levels of carbon disulfide in drinking-water supplies were identified. In a 1982-1983 survey of raw and treated water samples from 10 Ontario municipalities, carbon disulfide was frequently detected at low levels in each of spring, summer, and winter samplings. Concentrations over the three seasons ranged from non-detectable (<0.1 µg/litre) to trace levels in most cities, from non-detectable to 0.2 µg/litre in Cornwall, and from non-detectable to 0.3 µg/litre in Hamilton (Otson, 1987; R. Otson, personal communication, 1996).
Only limited data on concentrations of carbon disulfide in soils were identified. In a 1985-1986 study of background sites in the general vicinity of petrochemical refinery facilities west of Toronto, Ontario, carbon disulfide was detected at one of five sites in Port Credit at 0.000 11 µg/g, but not at any of six sites from Oakville/Burlington (Golder Associates, 1987). In a 1987 survey of organic compounds in surface soils in background areas in the same municipalities, carbon disulfide was detected at 3 of 30 urban residential and parkland sites in Port Credit, Oakville, and Burlington, at concentrations of 0.10, 0.10, and 0.14 µg/g, respectively (Golder Associates, 1987). However, reported levels were near the method detection limit (0.10 µg/g), and the values were not corrected for the observed contamination of the method blank.
In 1988, carbon disulfide was measured in sedi-ment suspensions taken from Lake Ontario, near Burlington, Ontario, and in Harp Lake, near Huntsville, Ontario. Caron & Kramer (1994), using a sulfur-specific gas chromatographic method, were able to detect 5.9 ng carbon disulfide/litre in Lake Ontario sediments and 9.7 ng carbon disulfide/litre in Harp Lake sediments.
Reliable data to serve as a basis for estimation of exposure to carbon disulfide in food were not identified. Carbon disulfide has been used as a fumigant on stored grain, although its registration for this purpose has been withdrawn in most countries (C. Warfield, personal communication, 1996). Carbon disulfide is produced during the metabolism of certain pesticides, such as dithiocarbamates, in plants and soil. Carbon disulfide is also a metabolite produced by plants from naturally occurring sulfur compounds (section 4.1).
The results of a number of food surveys from the USA in which the levels of carbon disulfide were determined have been published (Heikes & Hopper, 1986; Daft, 1987, 1988, 1989; Heikes, 1987). However, the results of these studies are considered to be of limited relevance because they appear to have been conducted before the use of carbon disulfide as a grain fumigant was cancelled and/or the analytical methodology was relatively insensitive.
Based on analysis of mainstream smoke from seven samples of commercial and experimental cigarettes and a single cigar and marijuana cigarette, each of these products delivered approximately 2 µg of carbon disulfide per cigarette/cigar smoked (Horton & Guerin, 1974).
Carbon disulfide (at the micrograms per litre or micrograms per cubic metre level) and its metabolite TTCA were detected in virtually all samples of breath, blood, urine, and breast milk of subjects with no known occupational exposure in a number of studies (Pellizzari et al., 1982; Phillips, 1992; Brugnone et al., 1994). At least some of the carbon disulfide and/or TTCA may have arisen from exposure to other chemicals of which they are known to be metabolites, such as disulfiram, captan, or dithiocarbamate fungicides, and TTCA is present naturally in brassica vegetables and may be found in the urine at levels above 10 µmol/litre after consumption of such items (Simon et al., 1994, and references therein; Kivisto, 2000).
Data on levels of carbon disulfide in environmental media to serve as the basis for development of sample estimates of population exposure are limited to a small number of surveys of ambient air conducted at few locations in Canada or the USA and limited Canadian surveys in drinking-water and soil in which carbon disulfide was seldom detected. Meaningful probabilistic assessment is precluded, therefore. In this section, mean deterministic estimates of environmental intake from air, water, and soil by members of the general population of Canada have been derived. These are followed by consideration of mean estimates of potential airborne exposures by populations in the vicinity of point sources in Canada, based on the very limited available data.
Point estimates of total daily intake of carbon disulfide by six age groups of the general population of Canada were developed (Table 1), primarily to determine the relative contributions from various media. These estimates indicate that intake from environmental exposure to carbon disulfide is virtually all from inhalation. That air is the principal route of exposure is supported by the results of the EQC fugacity modelling, which indicate that virtually all of the carbon disulfide released to air (industrial releases in Canada are almost entirely to air) will tend to remain in that compartment. Exposure from ingestion of drinking-water and soil appears to be negligible in comparison with that from air. Based on the absence of registered uses for carbon disulfide on food and the results of the fugacity modelling for southern Alberta, which predicted that very low levels of the compound (<1 × 10-6 µg/g) will accumulate in biota (Environment Canada & Health Canada, 2000), it was assumed that exposure via food will be negligible. For smokers, it is estimated that cigarette smoking can increase the intake of carbon disulfide severalfold.
Table 1: Estimated mean intakes of carbon disulfide for the general population.
|
|
Mean intake of carbon disulfide (µg/kg body weight per day) |
|||||
|
0-0.5 yearsa |
0.5-4 yearsb |
5-11 yearsc |
12-19 yearsd |
20-59 yearse |
60+ yearsf |
|
|
Outdoor airg |
0.01 |
0.02 |
0.02 |
0.01 |
0.01 |
0.01 |
|
Indoor airh |
0.15 |
0.33 |
0.26 |
0.15 |
0.13 |
0.11 |
|
Drinking-wateri |
0.007 |
0.003 |
0.002 |
0.001 |
0.001 |
0.001 |
|
Soilj |
1 × 10-7 |
2 × 10-7 |
7 × 10-8 |
2 × 10-8 |
1 × 10-8 |
1 × 10-8 |
|
Total intake (not food or cigarettes) |
0.17 |
0.36 |
0.28 |
0.16 |
0.14 |
0.12 |
|
Intake by cigarette smokersk |
- |
- |
- |
0.67 |
0.57 |
0.57 |
|
a |
Assumed to weigh 7.5 kg, breathe 2.1 m3 of air per day, drink 0.8 litres of water used in the preparation of powdered infant formula per day, and ingest 30 mg of soil per day (EHD, 1998). |
|
b |
Assumed to weigh 15.5 kg, breathe 9.3 m3 of air per day, drink 0.7 litres of water per day, and ingest 100 mg of soil per day (EHD, 1998). |
|
c |
Assumed to weigh 31.0 kg, breathe 14.5 m3 of air per day, drink 1.1 litres of water per day, and ingest 65 mg of soil per day (EHD, 1998). |
|
d |
Assumed to weigh 59.4 kg, breathe 15.8 m3 of air per day, drink 1.2 litres of water per day, and ingest 30 mg of soil per day (EHD, 1998). |
|
e |
Assumed to weigh 70.9 kg, breathe 16.2 m3 of air per day, drink 1.5 litres of water per day, and ingest 30 mg of soil per day (EHD, 1998). |
|
f |
Assumed to weigh 72.0 kg, breathe 14.3 m3 of air per day, drink 1.6 litres of water per day, and ingest 30 mg of soil per day (EHD, 1998). |
|
g |
Based on the mean concentration of carbon disulfide in ambient (outdoor) air (0.30 µg/m3) at six randomly selected sites in New York, USA (Phillips, 1992; section 6.1.2), assuming 3 of 24 h are spent outdoors daily (EHD, 1998). |
|
h |
Based on the mean concentration of carbon disulfide in nine samples of indoor air (0.63 µg/m3) from a hospital office room in New York, USA (Phillips, 1992; section 6.1.2), assuming that 21 of 24 h are spent indoors daily (EHD, 1998). |
|
i |
Based on the average concentration of carbon disulfide over three seasons (0.065 µg/litre) reported in treated drinking-water samples from a 1982-1983 survey of 10 Ontario municipalities (Otson, 1987; R. Otson, personal communication, 1996). In calculating the mean, a value of one-half the detection limit (i.e., half of 0.1 µg/litre, or 0.05 µg/litre) was assigned to samples that did not contain detectable levels of carbon disulfide. |
|
j |
Based on analysis of a limited number of samples of urban soils removed from point sources in a 1985-1986 survey conducted in Port Credit and in Oakville/Burlington, Canada, in which carbon disulfide was detected at one of five sites in Port Credit at 0.000 11 µg/g (Golder Associates, 1987). In calculating the mean, a value of one-half the detection limit (0.000 015 µg/g) was assigned to samples that did not contain detectable levels of carbon disulfide. |
|
k |
Based on the approximate content of carbon disulfide in mainstream smoke from cigarettes reported by Horton & Guerin (1974) (2 µg/cigarette) and consumption of 20 cigarettes per day, the approximate number smoked by regular Canadian smokers aged 15 years or older as of 1995 (M. Kaiserman, personal communication, 1997). |
It is also known that concentrations of carbon disulfide in ambient air are elevated in the vicinity of some point sources in Canada (section 5.1). Based on the range of mean concentrations measured in the vicinity of natural gas processing (1.40 µg/m3; Legge et al., 1990b) and xanthate production facilities (3-6 µg/m3; L. Fu, personal communication, 1997; E. Weiss, personal communication, 1998) in Canada, average exposures by inhalation near such facilities may be increased between 2- and 10-fold over those for the general population.
The database on occupational exposure to carbon disulfide in rayon fibre production is extensive. Concentrations of carbon disulfide in this industry are known to have declined substantially over the several decades encompassed by the available epidemiological studies (Price et al., 1997). The figures below have been selected from relatively recent studies (confined to the 1990s) in different countries.
In a Finnish viscose rayon fibre factory, the mean concentration of carbon disulfide in the air (8-h TWA) was 9.4 mg/m3 (range 4.7-25 mg/m3); in a viscose sheeting production factory, it was 13 mg/m3 (range 0.6-28 mg/m3) (Riihimaki et al., 1992). In a viscose fibre plant in Yugoslavia, exposure to carbon disulfide (TWA) concentrations measured by personal samplers was 63 mg/m3 in the spinning rooms and 19 mg/m3 in the viscose manufacturing departments (Krstev et al., 1993). In a viscose rayon factory in Taiwan, fixed-point airborne concentrations of carbon disulfide were 470-940 mg/m3 in the cutting areas and 47-310 mg/m3 in the spinning areas. The estimated 8-h TWA concentrations in the fibre cutting areas were 125-210 mg/m3 (Chu et al., 1995). In a synthetic fibres factory in Poland, the concentration of carbon disulfide ranged from 9.4 to 23 mg/m3 (Kuligowski, 1996). Geometric mean concentrations of carbon disulfide in a rayon factory in Singapore ranged from 8.4 to 63 mg/m3 (Yang et al., 1996a). Among workers in Taiwan in viscose manufacturing, cellophane processing and ripening, and filament spinning, concentrations of carbon disulfide were highest in the ripening area (170 mg/m3) and the filament spinning area (61 mg/m3) (Kuo et al., 1997). Levels of carbon disulfide fluctuated between <0.6 and 210 mg/m3 in a German viscose rayon factory (Reinhardt et al., 1997b). In a Belgian rayon factory, exposures ranged from 3.1 mg/m3 (centrifuge operator) to 150 mg/m3 (spinning) (Vanhoorne et al., 1991). In a Bulgarian viscose rayon production facility, levels ranged between 9.4 and 63 mg/m3 (Kotseva & De Bacquer, 2000). In a chemical company in Canada, peak exposures to carbon disulfide were in the order of 310-630 mg/m3 (Guidotti & Hoffman, 1999). The average concentrations of carbon disulfide in the workplace in China have decreased to around 10 mg/m3 in recent decades (Yang et al., 1996b; Sun et al., 1998; Lu & Wang, 1999; Q. Wang et al., 1999; Wang & Shiu, 2000).
Dermal absorption can also contribute substantially to occupational exposure for some tasks, although quantitative data were not identified. In a study of German viscose rayon workers, Drexler et al. (1995) observed that the slope of the regression between levels of carbon disulfide in personal air and of TTCA in urine was significantly greater for spinners than for other workers, particularly if they had skin diseases or skin irritation, which they attributed to the increased physical requirements and potential for dermal exposure associated with this task.
There is also exposure of laboratory workers to carbon disulfide, as a consequence of its common use as an analytical solvent, although quantitative data were not identified.
Carbon disulfide is absorbed primarily via the lungs but also through the skin. At the outset of exposure, pulmonary retention is about 80% and declines steadily, reaching a plateau of about 40% within 2 h. Extensive dermal absorption takes place from liquid and even gaseous carbon disulfide, although the available data are limited. Dutkiewicz & Baranowska (1967) reported that rates of absorption of carbon disulfide, determined from analysis of aqueous solutions into which subjects had immersed their hands for 1 h, ranged from 0.232 to 0.789 mg/m2 per hour. The experimental procedure was reported only briefly, and hence it is not clear whether factors (other than absorption through the skin) that would have affected these results, such as evaporation, were taken into account. Gastrointestinal absorption has been observed in experimental animals and in case reports in humans (Environment Canada & Health Canada, 2000).
Carbon disulfide is extensively metabolized, with the main metabolites being 2-mercapto-2-thiazolinone-5, thiocarbamide, and TTCA. TTCA represents 2-6% of the total carbon disulfide absorbed in humans and has been utilized for biomonitoring. The information on metabolism of carbon disulfide is somewhat limited. Few data are available on the biotransformation of carbon disulfide in humans, and the metabolic products of carbon disulfide are not completely known. Although the available data indicate that the metabolism of carbon disulfide is generally similar between humans and animals, oxidation to inorganic sulfate may not occur significantly in humans, in contrast to the results obtained in some animal studies (ATSDR, 1996). Different studies show a constant, close correlation between 8-h TWA airborne exposure to carbon disulfide and urinary TTCA concentrations. The concentration of TTCA in an after-shift urine specimen collected towards the end of the work week following exposure to an 8-h TWA of 31 mg carbon disulfide/m3 was approximately 4 mmol/mol creatinine (Lowry, 1996; Rolecki & Tarkowski, 2000).
Carbon disulfide can be metabolized in the liver by the cytochrome P-450 mono-oxygenase system to an unstable oxygen intermediate that either spontaneously generates atomic sulfur, carbonyl sulfide, and carbon dioxide or hydrolyses to form atomic sulfur and mono-thiocarbonate, yielding carbonyl sulfide and carbon dioxide in breath and inorganic sulfates and organosulfur compounds in urine. Alternatively, dithiocarbamates are formed in humans and animals by reaction with amino acids; conjugation of carbon disulfide or carbonyl sulfide with endogenous glutathione forms TTCA and 2-oxythiazolidine-4-carboxylic acid, respectively, which are excreted in urine (ATSDR, 1996).
Owing to the relatively extensive database in humans for critical neurological and cardiovascular effects, emphasis for the review of toxicological data in this context is on their contribution to assessment of biological plausibility and mode of action and characterization of other end-points for which studies in humans are limited or unavailable.
The LC50 for male mice exposed for 60 min to carbon disulfide by inhalation was approximately 690 mg/m3 (Gibson & Roberts, 1972), whereas no mortality occurred in rats exposed to as much as 2470 mg/m3 for 15 h, although neurological effects were observed (HSE, 1981). The oral LD50 for mice over a 24-h period was 3020 mg carbon disulfide/kg body weight. Single oral doses of up to 1260 mg/kg body weight did not cause any deaths or overt toxicity in rats, and only minimal lesions were noted at autopsy (HSE, 1981; ATSDR, 1996).
Although it has been reported that carbon disulfide is irritating to the eyes and skin in secondary accounts of seemingly limited early studies (CEC, 1988), it has not been possible to verify these data.
The repeated-dose toxicity of carbon disulfide has predominantly been investigated in specialized studies of effects on the nervous system. In numerous studies, medium- or long-term exposure of rats to carbon disulfide levels of between 800 and 2500 mg/m3 has been associated with reductions in the nerve conduction velocity in the peripheral nerves or spinal cord (Environment Canada & Health Canada, 2000). In a number of these studies, this effect was accompanied in later stages by neurological impairment and atrophy of the hind limbs and was only partially reversible upon cessation of exposure. In rats exposed to carbon disulfide, hydrogen sulfide, or both, reductions in peripheral nerve conduction velocity were observed only in those exposed to carbon disulfide, and there was no interaction between the compounds (Gagnaire et al., 1986). These reductions in nerve conduction velocity have also been observed in the central nervous system and in the optic pathway, as indicated by increased latencies and decreased amplitudes of somatosensory-, visual-, or brainstem auditory-evoked potentials in rats exposed to 2500 mg carbon disulfide/m3 for periods of 11-15 weeks (Rebert & Becker, 1986; Hirata et al., 1992). In the latter study, there was also a transient increase in the latency of some components of the brainstem auditory-evoked potential at 630 mg/m3 (Hirata et al., 1992). Bokina et al. (1976, 1979) observed deviations in visual-evoked potentials in rabbits exposed for 6 weeks to 0.2 or 2 mg/m3, but these results cannot be critically evaluated, owing to limitations in their reporting. However, it is noted that this end-point was affected only at much higher levels (i.e., 2500 mg/m3) in the (well reported) study in rats by Rebert & Becker (1986).
The reductions in nerve conduction velocity observed in animal studies are accompanied by charac-teristic histopathological lesions in the axon. In a number of studies, rats exposed to between 800 and 2500 mg carbon disulfide/m3 for between 3 and 15 months developed an axonopathy in the peripheral nerves and/or spinal cord (Environment Canada & Health Canada, 2000). The distal portions of the largest and longest myelinated axons (which are the most rapidly conducting axons) are affected first. Structural changes proceed through the development of large axonal swellings composed of disorganized masses of neurofilaments proximal to the nodes of Ranvier, followed by axonal atrophy and Wallerian-like degeneration proximal and distal to the swellings, respectively. These features are characteristic of giant neurofilament axonopathies induced by other compounds, such as 2,5-hexanedione, the neurotoxic metabolite of hexane (Graham et al., 1995).
Neurobehavioural effects have been observed in a number of studies in rats. Neuromuscular effects, most notably reductions in grip strength and gait alterations, were observed following 2-4 weeks of exposure to 1600 and 2500 mg/m3. Gait was also significantly affected after 13 weeks of exposure to 160 mg/m3, although the test values were usually within the normal range (Moser et al., 1998). Exposure to between 610 mg/m3 and roughly 800 mg/m3 or greater inhibited avoidance behaviour in short-term studies (Goldberg et al., 1964a, 1964b) and affected some measures of locomotor activity in chronic studies (Frantik, 1970; Opacka et al., 1984). In those studies that included a recovery period, these neurobehavioural effects were reversible.
The sequence of neurotoxic effects of carbon disulfide was elucidated in a recent collaborative study at the US National Institute for Environmental Health Sciences. In this study, in which rats were exposed to 160, 1600, or 2500 mg/m3, 6 h/day, 5 days/week, for up to 13 weeks, neurofilament protein cross-linking in the spinal cord was observed as early as 2-4 weeks at all exposure levels (Valentine et al., 1997, 1998). Other early indicators were increased expression of nerve growth factor receptor mRNA in the sciatic nerve (an indicator of alterations in the axon-Schwann cell relationship) (Toews et al., 1998) and gait abnormalities (Moser et al., 1998). By 4 weeks, the neuromotor alterations progressed to reductions in grip strength of the hind limbs and forelimbs (Moser et al., 1998). Axonal swelling and degeneration (Sills et al., 1998) and electrophysiological alterations (Herr et al., 1998) in the peripheral nerves and/or spinal cord occurred only in the later stages of the study and at the two highest dose levels.
The effect of carbon disulfide on lipid metabolism has been extensively studied. In several studies, exposure of rats to between 230 and 1700 mg/m3 for periods of between 6 and 15 months resulted in significant increases in serum levels of cholesterol (and often phospholipids and triglycerides). The content of total cholesterol and cholesterol esters in the aorta was sig-nificantly increased in rats and rabbits as a result of medium- or long-term exposure to 1000 mg carbon disulfide/m3. Exposure to 1000 mg carbon disulfide/m3 exacerbated the effect of an atherogenic diet on the levels of lipids in the serum, heart, or walls of the coronary blood vessels (Environment Canada & Health Canada, 2000).
There is only limited evidence of other effects induced by inhalation of carbon disulfide. In the collaborative National Institute for Environmental Health Sciences study, medium-term exposure to 160-2500 mg/m3 did not cause histopathological lesions in a range of organs (brain, heart, aorta, lung, and female reproductive tract), with the exception of the peripheral nervous system and spinal cord (Sills et al., 1998). However, there are a number of reports in which elevated exposure to levels of several hundred milligrams per cubic metre or greater affected visual function and optic nerve/retinal cellular structure in monkeys and rats, renal histopathology in mice and rabbits, and hepatic metabolism in rats and mice (BUA, 1993; ATSDR, 1996).
While it has often been assumed that the cardiovascular effects of carbon disulfide are secondary to its arteriosclerotic effects, the results of several studies in rats suggest that these may be the result of a direct effect on the heart. Short-term exposure of restrained and anaesthetized rats to between 126 and 253 mg/kg body weight per day had a cardiodepressive effect on electrophysiological and mechanical parameters and decreased left ventricular contractility, increased blood pressure, and caused electrocardiograph alterations indicative of myocardial ischaemia following administration of epinephrine or norepinephrine (Hoffmann & Klapperstück, 1990; Hoffmann & Müller, 1990; Klapperstück et al., 1991). However, in conscious unrestrained normotensive rats, the highest dose did not alter mean arterial blood pressure or heart rate, although it significantly reduced body weight (Hoffmann & Klapperstück, 1990).
Short-term administration of 300 mg carbon disulfide/kg body weight per day to mice was not hepatotoxic but reduced the hepatic microsomal cytochrome P-450 content and the activities of several associated mono-oxygenases (Masuda et al., 1986).
While short-term exposure of mice to between 138 and 1102 mg/kg body weight per day altered thymus weight, it was not immunotoxic, as indicated by white blood cell differentials, spleen weight, and natural killer cell activity (Keil et al., 1996).
Available data are inadequate as a basis for assessment of the carcinogenicity of carbon disulfide. Identified data are confined to a single screening study of lung tumour induction in strain A mice (Adkins et al., 1986).
There is no clear evidence from in vitro studies that carbon disulfide is genotoxic (Environment Canada & Health Canada, 2000). In several studies in bacteria, carbon disulfide did not induce point mutations in Salmonella typhimurium or in Escherichia coli, both with and without metabolic activation. In studies of mammalian cells exposed to carbon disulfide in the presence of metabolic activation, there were small and/or equivocal increases in chromatid gaps in human lymphocytes, in unscheduled DNA synthesis in diploid WI-38 cells derived from human embryonic lung tissue, and in sister chromatid exchanges in human lymphocytes. In one study (Le & Fu, 1996), in human sperm exposed to carbon disulfide in vitro, there was a significant increase in the frequency of chromosomal aberrations and in the frequency of chromosomal breaks.
Available in vivo data on the genotoxicity of carbon disulfide are limited. In male and female rats inhaling 63 or 125 mg carbon disulfide/m3, 7 h/day for 1 or 5 days, there was no significant increase in the frequency of chromosomal aberrations in bone marrow cells (Belisles et al., 1980). In contrast, Vasil'eva (1982) reported that oral exposure to carbon disulfide induced chromosomal aberrations and polyploid cells in the bone marrow of female rats and in rat embryos exposed on days 10-13 of gestation. It is difficult to assess the validity of these findings, as the reporting was brief (e.g., the statistical significance was often not indicated) and the effective dose was not reported, except to indicate that it was one-tenth of the LD50.
When male rats were exposed to 63-125 mg carbon disulfide/m3, 7 h/day for 5 days, there was no significant increase in dominant lethal mutations, nor was there a dose-related increase in sperm abnormalities in rats or mice exposed according to the same protocol (Belisles et al., 1980), although lack of an effect on sperm abnormalities in positive control rats undermines somewhat the significance of these observations.
In a small number of studies, exposure of male rats to 1875 mg carbon disulfide/m3 (but not to 1090 mg/m3), 5 h/day, 5 days/week, for several weeks, affected copulatory behaviour, reducing times to mount and ejaculate. There were no clear effects on sperm counts, circulating levels of reproductive hormones, or testicular histology (Tepe & Zenick, 1984; Zenick et al., 1984).
In an early study, prolonged estrous cycle in rats was reported after exposure to 10 mg/m3 or more for 4 months (Acadzhanova, 1978). However, in a more recent study (WIL Research Laboratories, Inc., 1992), there were no effects on estrous cycling, mating index, or fertility index in rats exposed to up to 1560 mg carbon disulfide/m3, 6 h/day, before and during mating and throughout gestation. This dose adversely affected maternal weight and weight gain and increased pup mortality, decreased pup viability, and decreased live litter size, but development of the pups was otherwise unaffected. There were no effects at 780 mg/m3, except for a small increase in the length of gestation (also at 1560 mg/m3), which was within the range of historical controls.
There was a significant reduction in the number of viable fetuses in rats and mice after exposure to 2000 mg carbon disulfide/m3 for 2 h/day during pregnancy; no significant gross toxicological effects in the dams were reported at this high level of exposure (Yaroslavskii, 1969). In another early series of investigations in rats, inhalation of 100 or 200 mg/m3 for several hours daily during gestation was reported to be fetotoxic and cause malformations, most often club foot and hydrocephalus (Tabacova et al., 1978, 1983), while 10 mg/m3 reduced postnatal survival, delayed the development of postnatal milestones, and impaired motor coordination (Tabacova et al., 1981).
Behavioural changes in the offspring, most often reduced exploratory activity in open field tests, were also reported at levels between 0.03 and 200 mg/m3 (Hinkova & Tabacova, 1978; Tabacova et al., 1978, 1981, 1983). Exposure over two generations appeared to result in greatly increased sensitivity to the teratogenic effects of carbon disulfide, causing malformations at as little as 0.03 mg/m3 in the second generation, compared with 100 mg/m3 in the first (Tabacova et al., 1983). In general, no significant maternal toxicity was reported at exposure levels of 100 mg/m3 and below. However, it is difficult to evaluate the validity of these findings. The studies are generally only briefly reported, and important information (e.g., concerning maternal toxicity in each case) is often not provided. There is also some inconsistency in the findings; for example, Tabacova et al. (1981) reported that in utero exposure to as little as 0.03 and 10 mg/m3 increased motor activity in open field tests, whereas motor activity was impaired at the same doses in their other studies. Moreover, the results of subsequent studies, most of which are better reported, have generally failed to confirm the teratogenic findings reported by Tabacova and colleagues, although it should be noted that some of the studies conducted by these investigators differed somewhat in their design (e.g., exposure over two generations). In utero exposure of rats to levels of 1250 or 2500 mg/m3 did not induce a significant increase in the incidence of club foot, although it did cause maternal and fetal toxicity and minor skeletal anomalies (Saillenfait et al., 1989). (There was no effect at 625 mg/m3.) In another study (Belisles et al., 1980; Hardin et al., 1981), there was no evidence of embryo/fetotoxicity or of teratogenicity in rats exposed to levels of 63 or 125 mg/m3. However, there is some weak support for the behavioural effects reported in the early studies from a small study by Lehotzky et al. (1985), in which the latency of a conditioned avoidance response was significantly lengthened by in utero exposure to concentrations of 10-2000 mg/m3, although there was no clear dose-response.
In rabbits, inhalation of 1875 or 3750 mg carbon disulfide/m3 during organogenesis decreased fetal body weight and increased post-implantation losses. Significant increases in visceral and skeletal malformations were also observed at the higher, maternally toxic, dose level (PAI, 1991). In another study, there was no evidence of embryo/fetotoxicity or teratogenicity in rabbits exposed to much lower concentrations (63 or 125 mg/m3) prior to and during gestation (Belisles et al., 1980; Hardin et al., 1981). However, it is difficult to assess the validity of these results, owing to mortality among the dams from causes that were apparently unrelated to the chemical exposure.
There was no compound-related increase in malformations, and no clear evidence of embryo- or fetotoxicity, in rats exposed orally to maternally toxic doses of carbon disulfide (between 100 and 600 mg/kg body weight per day) during the period of organogenesis. Fetal body weights were decreased in rats exposed to 200 mg/kg body weight per day and more (Jones-Price et al., 1984a). In contrast, in rabbits gavaged with 25, 75, or 150 mg/kg body weight per day in a similar study, carbon disulfide was embryo- and fetotoxic at all dose levels (increases in percent resorptions, non-live fetuses, and affected [non-live and malformed] fetuses), although this was accompanied by maternal toxicity at the two highest doses. The highest dose also induced significant increases in the frequency of malformed fetuses (Jones-Price et al., 1984b).
As reviewed by Graham et al. (1995), it has been postulated that the axonal degeneration that underlies the central-peripheral neuropathy caused by carbon disulfide is the result of the reaction of carbon disulfide and carbonyl sulfide with protein amino groups to yield initial adducts (dithiocarbamate derivatives). The adducts decompose to an electrophile (isothiocyanate for carbon disulfide and isocyanate for carbonyl sulfide), which in turn reacts with protein nucleophiles on the neurofilaments to cause protein cross-linking. (Such cross-links have been demonstrated in vitro and in erythrocyte spectrin and neurofilaments in rats exposed to carbon disulfide [Valentine et al., 1993, 1995, 1997].) Progressive cross-linking of the neurofilament occurs during its transport along the axon, and covalently cross-linked masses of neurofilaments may occlude axonal transport at the nodes of Ranvier, ultimately resulting in axonal swelling and degeneration.
In a number of early reports of poisoning following pulmonary exposure to 1560-3125 mg carbon disulfide/m3, a range of psychiatric disturbances was reported, while concentrations of approximately 15 625 mg/m3 resulted in central nervous system depression, coma, respiratory paralysis, and death. In several case reports, ingestion of approximately 18 g caused neurological signs, cyanosis, peripheral vascular collapse, and hypothermia, followed by death due to central nervous system depression and respiratory paralysis within a few hours (HSE, 1981).
The majority of the available epidemiological studies are of workers in the viscose rayon production industry, in which there is exposure to airborne carbon disulfide, along with lesser quantities of hydrogen sulfide,5 at several stages during the process. Concentrations of carbon disulfide in this industry are known to have declined substantially over the several decades encompassed by the available epidemiological studies (Price et al., 1997), and the results of epidemiological studies suggest that some of the effects induced by carbon disulfide (e.g., reductions in peripheral nerve conduction velocity) are not completely reversible. In addition, it is clear that exposures are markedly higher for certain tasks (e.g., operators of spinning machinery) than for the workplace as a whole. Consequently, when possible, those studies in which exposures and/or processes were reported to have remained the same for many years, and those in which personal monitoring data were collected, have been highlighted in the following sections.
There are numerous early clinical reports of pronounced psychological and central nervous system damage following single or long-term exposure to high, but poorly characterized, levels of carbon disulfide in the rubber and viscose rayon industries. Extended exposure under such conditions led to the recognition of chronic carbon disulfide intoxication, characterized by psychoses, polyneuropathy of the lower extremities, gastrointestinal disturbances, myopathy of the calf muscles, nerasthenic syndrome, optic neuritis, and atherosclerotic vasculencephalopathy (IPCS, 1979; O'Donoghue, 1985). Exposures in the workplace were reduced in order to prevent such overt toxicity; more recent studies in which effects of these lower levels of exposure were examined are the focus of the discussion that follows.
Neurophysiological effects on both the peripheral and central nervous systems, as well as behavioural and neuropathological effects, have been reported in a number of cross-sectional studies of workers exposed to carbon disulfide in the viscose rayon industry. The most common observations are of effects on the peripheral nervous system, most often characterized by reduced conduction velocity in the motor and, in some instances, sensory nerves, and generally most pronounced in the more distal portions of the nervous system (e.g., in the lower limbs).
In an early neurophysiological study, male Finnish viscose rayon workers with long-term exposure to carbon disulfide and hydrogen sulfide at 31-94 mg/m3 (with higher peak and historical levels) were compared with unexposed paper mill workers of similar age distribution (Seppäläinen & Tolonen, 1974). In exposed workers as a whole, there were significant reductions in MCV of the deep peroneal, posterior tibial, and ulnar nerves and in slow motor fibre conduction velocities in the deep peroneal and ulnar nerves. Results were comparable in workers who were currently exposed and in those removed from exposure for a number of years.
Effects on peripheral nervous system conduction were also associated with lower exposures to carbon disulfide in a cross-sectional study of 156 white male workers employed for at least 1 year (actual mean of 12.2 years) in a US viscose rayon plant (Johnson et al., 1983). After excluding data from workers with other possible neurotoxic exposures/conditions (diabetes, excessive alcohol consumption, or elevated blood lead) and adjusting for age, exposed workers had significantly reduced MCV and amplitude ratio of muscle action potentials following peroneal nerve stimulation and reduced MCV and increased discrete amplitude of the nerve action potential in the sural nerve, compared with 233 unexposed workers at two synthetic textile plants at the same site. These differences were observed primarily in the workers who were most highly exposed at the time of the study, with median 8-h personal airborne levels of 24 mg/m3, although conduction velocities in both nerves were slightly lower in workers with moderate (median 13 mg/m3) and low (median 3 mg/m3) exposures (the mean peroneal MCV in the high, medium, and low exposed workers were 43.7, 43.4, and 41.8 m/s, respectively, compared with 45.3 m/s in the unexposed workers). Personal air sampling was conducted at the same time as the investigation of a wide range of health effects; 81.8% of exposed employees had worked in their current job assignment for the duration of their employment with the company, and, based on area samples, exposures were stable over more than 20 years prior to the study. In contrast to the findings for nerves in the legs, none of the neurophysiological variables in the ulnar nerve was associated with carbon disulfide exposure. In behavioural testing of this population, there were no remarkable findings in psychological, psychomotor, cognitive-perceptual, or vision testing, although exposed workers reported symptoms of neurobehavioural ailments signifiantly more frequently (Putz-Anderson et al., 1983).
In another study in which exposures were well characterized, there were significant reductions in MCV of the peroneal nerve, after adjustment for potential confounders (age, weight, height, glucose tolerance, and cigarette and alcohol consumption), in workers exposed to carbon disulfide (median 13 mg/m3 in personal air). There were also reductions in sensory nerve conduction velocity (SCV) of the sural nerve in workers from those departments with high exposure compared with workers from departments with low exposure (Reinhardt et al., 1997a). The authors questioned the significance of these results, based primarily on the lack of effects on other neurophysiological parameters and the lack of significant dose-response among exposed workers. However, it is considered that the changes observed by Reinhardt et al. (1997a) represent a compound-related effect.
The results of several other studies confirm that exposure to carbon disulfide at mean concentrations of 15-<30 mg/m3 is associated with reductions in MCV and SCV in the peripheral nerves, most often in the lower limbs, although exposures were not well characterized in most of these studies (Vasilescu & Florescu, 1980; Sandrini et al., 1983; Hirata et al., 1996; Takebayashi et al., 1998).
In contrast, there was little indication of effects on the peripheral nervous system in a small study of Italian viscose rayon workers who had been exposed to slightly lower carbon disulfide levels – i.e., mostly less than 10 mg/m3 (Cirla & Graziano, 1981). In this study, MCV of the peroneal nerve was non-significantly slower in exposed workers than in well matched controls. Based on needle electromyography and neurological examinations, 5 out of 50 exposed subjects had peripheral nerve impairment, compared with only 2 out of 50 controls. There were no significant differences in the results of neuropsychological testing of intelligence, performance, and memory conducted on half of the subjects.
In several studies in which exposures were substantially higher, effects on the peripheral nervous system were more pronounced, as indicated by reductions in the MCV and SCV of a wider range of nerves (including those in the upper limbs) and/or alterations in other peripheral neurophysiological variables (Gilioli et al., 1978; Ruijten et al., 1993; Chu et al., 1995; Vanhoorne et al., 1995). In the subset of these studies in which subgroup analyses were conducted, there was an exposure-response relationship, with reductions in peroneal MCV among exposed workers being related to the exposure concentrations (Gilioli et al., 1978; Vanhoorne et al., 1995) or most pronounced in workers engaged in tasks that would most likely have entailed the heaviest exposure to carbon disulfide (Chu et al., 1995).
Chu et al. (1996) reported histopathological findings in a male viscose rayon worker exposed to a TWA concentration of 125-209 mg/m3, with clinical and neurophysiological signs of peripheral neuropathy. The results of sural nerve biopsy revealed ultrastructural changes similar to those in the peripheral nervous system of animals exposed to carbon disulfide (axonal degeneration with disorganized neurofilaments) (section 8.2.1).
In four studies, workers with long-term exposure to approximately 30-90 mg carbon disulfide/m3 (often with higher historical exposures) performed significantly more poorly than unexposed workers on a variety of neurobehavioural tests, most often on psychomotor tests of motor speed or dexterity (Hänninen, 1971; Cassitto et al., 1978; Hänninen et al., 1978; De Fruyt et al., 1998). The evidence that such effects are associated with lower exposures is conflicting, although there were no remark-able differences in the results of extensive neurobehavioural testing in workers exposed to similar or slightly higher levels of carbon disulfide in several well described studies (Cirla & Graziano, 1981; Putz-Anderson et al., 1983; Reinhardt et al., 1997b; Takebayashi et al., 1998); however, there were significant increases in the frequency of reported central nervous system symptoms in some of these studies (Cirla & Graziano, 1981; Putz-Anderson et al., 1983; Takebayashi et al., 1998).
There was no clear evidence of effects on the results of electroencephalography conducted on workers exposed to carbon disulfide (Environment Canada & Health Canada, 2000), although this end-point has not been extensively investigated.
In epidemiological studies of more specific effects on the nervous system, exposure to mean levels of carbon disulfide in the range of 15-30 mg/m3 was associated with vestibular alterations, changes in the wave pattern of brainstem auditory-evoked potentials, and effects on the dopaminergic system. However, in all of these studies, the group sizes were fairly small, and there was often historical exposure to higher levels (Environment Canada & Health Canada, 2000).
In an early study, Hernberg et al. (1970, 1971, 1973; Tolonen et al., 1975) reported a significant excess of deaths from coronary heart disease over the first 5 years in 343 workers exposed to carbon disulfide in a Finnish viscose rayon plant, compared with a well matched group of workers from a paper mill (14 exposed, 3 control deaths, relative risk [RR] 4.8, P < 0.007). There were also significant increases in indicators of cardiovascular morbidity (non-fatal myocardial infarction, chest pain) and of risk factors for coronary heart disease (increased blood pressure). The workers had been exposed to airborne concentrations of carbon disulfide of 31-94 mg/m3 during the period when the study was initiated, although short-term and historical exposures were much higher. After these results were reported, exposures were reduced to less than 31 mg/m3, and the majority of the cohort was removed from exposure. In a subsequent (13-year) follow-up (Tolonen et al., 1979; Hernberg & Tolonen, 1981; Nurminen et al., 1982), there was still a significant excess of deaths from coronary heart disease, but this was entirely due to the almost 5-fold excess in the initial 5 years.
Cardiovascular mortality was significantly greater in the most highly exposed workers in a cohort of 2939 male workers at a viscose rayon factory in the United Kingdom (Sweetnam et al., 1987). Among spinners with at least 10 years of employment in the industry, who were considered to have the highest continuous exposures, mortality was significantly in excess for all causes, ischaemic heart disease (73 deaths, standardized mortality ratio [SMR] 172, P < 0.001), and other circulatory diseases combined (33 deaths, SMR 165, P < 0.01), compared with the general population. There was also a significant excess of mortality from ischaemic heart disease in non-process fitters (nine deaths, SMR 290, P < 0.01). A significant trend between mortality from ischaemic heart disease among long-term older workers and cumulative exposure score or exposure score over the last 2 years was observed. These patterns were not evident in workers who had left employment or those with less than 10 years of exposure. Based on a report of an earlier follow-up, levels in the spinning department frequently exceeded 63 mg/m3 (Tiller et al., 1968). While there was concomitant exposure to hydrogen sulfide, the excess of mortality from ischaemic heart disease was similar among workers with high-level exposure to carbon disulfide alone or to both compounds.
Findings were similar in a larger cohort of 10 418 male workers employed for at least 1 year at one of four US viscose rayon plants (MacMahon & Monson, 1988). In workers with the greatest exposure (based on their job titles – principally spinners and cutters), there was a significant excess of mortality from arteriosclerotic heart disease compared with the general population (242 deaths, SMR 124, P < 0.01); this occurred principally in workers with 15 or more years of exposure. No data were presented on exposures to carbon disulfide or other chemicals, nor on other known risk factors for heart disease.
In a historical cohort study of 3322 Dutch male viscose rayon workers, mortality from circulatory diseases was significantly increased among the 1434 workers exposed to carbon disulfide compared with the general population (Swaen et al., 1994). Among workers from the bleaching and spinning departments, who had continuous exposure to carbon disulfide, there was a significant excess of mortality from cardiovascular diseases (103 deaths, SMR 126, 95% confidence interval [CI] 103-154) and a non-significant excess from ischaemic heart disease (65 deaths, SMR 125, CI 96-162). Among these workers, mortality from cardiovascular diseases and ischaemic heart disease was inversely related to cumulative exposure, although this was estimated from personal air samples collected late in the study period, and historical exposures were most likely higher. The risk for cardiovascular disease was reported to be most pronounced 20-30 years after the first exposure. In contrast to the results of other studies (Hernberg & Tolonen, 1981; Sweetnam et al., 1987), the risk for cardiovascular mortality did not decrease after termination of exposure. No information was available on other risk factors for heart disease, but there was no excess of cardiovascular diseases in unexposed workers, who were considered to be similar to the exposed workers in terms of lifestyle.
Mancuso (1981) conducted a historical cohort study of more than 9000 males and females employed at a US viscose rayon plant. In the 26-year follow-up, there was significant excess mortality from coronary heart disease among males (453 deaths, SMR 111, CI 101-122). There were no quantitative exposure data, but the SMRs for coronary heart disease increased with increasing duration of exposure and were significantly increased in male workers employed for more than 10 years in those tasks for which exposure was considered high (spinning and twisting, maintenance, and mechanics). In females, findings were similar but less pronounced and generally not statistically significant.
Among a historical cohort of 2291 Polish viscose rayon workers who had been diagnosed with chronic carbon disulfide poisoning, there were significant excesses of deaths from diseases of the circulatory system (SMR 139, CI 125-154), including ischaemic heart disease (SMR 137, CI 114-164) and cerebrovascular disease (SMR 188, CI 143-242), and a non-significant excess of mortality from arteriosclerosis among males (SMR 120, CI 94-151) (Peplonska et al., 1996). Results were similar among women but were based on few cases and were often not statistically significant. Exposures to carbon disulfide, although apparently heavy, were poorly characterized.
There is also evidence from cross-sectional studies of overt toxicity to the cardiovascular system, most often reported as an increased frequency of angina or non-fatal myocardial infarction or of abnormal electrocardiograph. However, the increases were often non-significant and were based on small numbers of cases, and there is no clear dose-response across studies (although exposures were poorly characterized in most of these investigations) (Environment Canada & Health Canada, 2000).
As discussed in Drexler et al. (1995), shift work, as is common in viscose production, tends to be associated with a series of risk factors for cardiovascular disease and hence is a potential confounding factor in epidemiological studies. However, in those studies in which shift work was taken into account (Vanhoorne et al., 1992; Drexler et al., 1995), the results were similar to those of other studies (Environment Canada & Health Canada, 2000).
Egeland et al. (1992) observed a significant association between increases in serum levels of low-density lipoprotein cholesterol (LDL-C) and diastolic blood pressure and increasing exposure to carbon disulfide among male workers exposed to median levels of 3-24 mg/m3 at a US viscose rayon plant, compared with unexposed workers at three synthetic textile plants, and after adjustment for potential confounders. There was no association between exposure and high-density lipoprotein cholesterol (HDL-C), triglyceride, blood glucose, or systolic blood pressure. The levels of LDL-C, total cholesterol, and diastolic blood pressure were significantly greater in the high-exposure group than in the low-exposure workers. The results of area sampling indicated that exposures were stable for more than 20 years prior to the study.
In a study of 237 Polish women exposed to levels of carbon disulfide in the same range (i.e., 16-22 mg/m3) in viscose fibre production (Stanosz et al., 1994), exposed women had significantly increased levels of total cholesterol and LDL-C and significantly reduced levels of HDL-C compared with a control group of female textile workers of similar age. Effects on these blood lipids were confined to women aged 40-55 and to those with greater than 10 years of exposure. No subgroup analyses by exposure level were conducted.
These findings are supported by two studies of viscose rayon workers (Wronksa-Nofer & Laurman, 1987; Vanhoorne et al., 1992) in which exposure to carbon disulfide at levels generally in excess of 31 mg/m3 was associated with significant increases in serum cholesterol and LDL-C and decreases in HDL-C and, in the latter study (which adjusted for several potential confounders), with increases in blood pressure.
In contrast to the above studies, findings were negative in two investigations in which exposure levels were slightly less than those for the US workers studied by Egeland et al. (1992). In a study of German male viscose rayon workers exposed to a median personal airborne concentration of 13 mg/m3 (Drexler et al., 1995), there was no association between various measures of exposure (exposure category, levels in personal air, or TTCA levels in urine) and blood pressure or blood levels of cholesterol, LDL-C, HDL-C, triglycerides, apolipoproteins, electrolytes, or glucose. HDL-C and apolipoprotein levels were associated with duration of employment in jobs with exposure, but this was also observed in the controls, and the authors suggested that this was the result of long-term shift work. Similarly, Cirla & Graziano (1981) reported no significant differences in blood pressure or serum levels of blood lipid and lipoproteins between workers exposed to mean carbon disulfide levels of 5.0-20 mg/m3 and controls who were well matched for age and a series of lifestyle factors.
There are a number of early clinical reports of decreased glucose tolerance and increased prevalence of diabetes among patients with severe carbon disulfide poisoning (reviewed in Candura et al., 1979; HSE, 1981). In some cross-sectional studies, viscose rayon workers exposed to unknown concentrations of carbon disulfide had a significantly increased prevalence of diabetes mellitus (Goto & Hotta, 1967) or decreased glucose tolerance (Goto et al., 1971; Candura et al., 1979). However, in a number of similar studies in which exposure levels were characterized, there was no association between exposure to carbon disulfide or glucose tolerance in workers exposed to mean or median concentrations of approximately 3-90 mg/m3, often with higher historical exposures (Hernberg et al., 1971; Cirla & Graziano, 1981; Franco et al., 1981, 1982; Egeland et al., 1992; Chrostek Maj & Czeczotko, 1995; Drexler et al., 1995; Takebayashi et al., 1998).
Exposure to carbon disulfide at levels greater than 31 mg/m3 was associated with damage to the retinal capillaries, in the form of microaneurysms or haemorrhages, in a number of cross-sectional studies. However, there appears to be considerable variation in the susceptibility to this effect among populations, and there is no clear evidence that exposure to lower levels of carbon disulfide is associated with retinopathy. In addition, such effects are of uncertain clinical significance (Environment Canada & Health Canada, 2000).
The association of exposure to carbon disulfide with other effects on the eye has not been extensively investigated. There are two reports of effects on colour vision in viscose rayon workers with current or historical exposures to carbon disulfide at levels greater than 31 mg/m3 (Raitta et al., 1981; Vanhoorne et al., 1996), while colour vision was not affected in workers exposed to lower levels (Albright et al., 1984; Ruijten et al., 1990). In these populations, there were no other effects on measures of vision, including visual acuity, visual field, eye motility, depth perception, and pupillary reaction.
In those epidemiological studies in which mortality from non-cardiovascular causes was presented, there was no consistent excess of mortality from all cancers combined or from cancers at any specific site. However, the number of cancer deaths at any given site was small or modest in all of these studies (Environment Canada & Health Canada, 2000).
With the exception of several reports of reduced libido and/or impotence in male workers exposed to (mostly) high concentrations of carbon disulfide in the viscose rayon industry (Cirla et al., 1978; Cirla & Graziano, 1981; Wägar et al., 1981; Vanhoorne et al., 1994), there is no clear evidence of effects on human reproduction and development.
Semen quality, fertility, and pregnancy outcomes were not associated with exposure of male viscose rayon workers to carbon disulfide in the better documented of the available studies (Meyer, 1981; Selevan et al., 1983; Vanhoorne et al., 1994). The potential effects of carbon disulfide on female reproduction have not been adequately investigated, and there are conflicting reports of the frequency of abnormal menstrual duration or pain/bleeding in populations of female Chinese viscose rayon workers (Cai & Bao, 1981; Zhou et al., 1988; He et al., 1996; Q. Wang et al., 1999; Zhang et al., 1999). The findings of two early overlapping, small exploratory Finnish studies (Hemminki et al., 1980; Hemminki & Niemi, 1982) of an increased frequency of spontaneous abortions associated with parental employment in the viscose rayon industry were not confirmed in several subsequent studies, some of which were of inherently stronger design, although in all cases the number of abortions was small (Cai & Bao, 1981; Selevan et al., 1983; Zhou et al., 1988; Bao et al., 1991; Lindbohm et al., 1991; He et al., 1996; Q. Wang et al., 1999; Zhang et al., 1999).6
In a study reported as an abstract, the incidence of all birth defects was increased (RR 2.0, CI 1.1-3.6) among Chinese women who had worked at least 6 months in a viscose factory; the difference remained after adjusting for several possible confounding factors, but was not related to estimated exposure (>10 versus <10 mg/m3) (Bao et al., 1991). No effect on terata was observed in three other small Chinese studies, where the reporting was similarly limited (He et al., 1996; Q. Wang et al., 1999; Zhang et al., 1999).
There are a number of epidemiological investigations of the association between exposure to carbon disulfide and a variety of other effects, most often alterations in circulating levels of thyroid hormones, gonadotropins, or adrenal and/or testicular hormones and increases in the prevalence of diabetes or decreased glucose tolerance (Environment Canada & Health Canada, 2000). However, the findings in the available studies of these effects were inconsistent and, in some instances, contradictory and have often not been confirmed in those studies in which the study design was stronger and/or the reporting was more detailed.
The mode of action for the toxicity of carbon disulfide varies from species to species. In microorganisms, carbon disulfide may interfere with the general metabolism of a nitrifier species or with the primary oxidative reactions. In higher life forms, carbon disulfide can form dithiocarbamates, which are metal chelating, or it can form elemental sulfur during oxidative desulfurization in the liver (Beauchamp et al., 1983). Acute toxicity is confined mainly to neurotoxic effects.
Mammals appear to have relatively high tolerance to single or short-term exposure to carbon disulfide (Crookes et al., 1993). While investigations of effects on wild mammals have not been reported, effects on laboratory mammals have been extensively studied. In a flow-through inhalation study using mice, an approximate 1-h LC50 for vapour exposure of 690 mg/m3 was estimated by Gibson & Roberts (1972). This was the lowest acute toxicity value identified from the literature (see section 8.1).
Taylor & Selvidge (1984) investigated the effects of gaseous carbon disulfide on bush beans (Phaseolus vulgaris) in a closed system, with three replicate exposures, and reported no effect on transpiration or photosynthesis at any of the measured concentrations (0.42 ´ 106 to 5.6 ´ 106 µg/m3 for 6 h) or visible injury at the single measured concentration at which it was assessed (1.0 ´ 107 µg/m3). In another study, of all the reduced sulfur gases examined, carbon disulfide had the lowest internal flux rate from leaf surface to leaf interior in all three plant species (bush bean Phaseolus vulgaris, soybean Glycine max, and tomato Lycopersicon esculentum) tested (Taylor et al., 1983). This may account in part for its relatively low toxicity compared with that of other sulfur gases, since flux to the interior of a leaf is the major determinant of the ability of a compound to cause leaf injury.
Few other studies on plants were identified in the literature; however, the effects on seeds from the use of carbon disulfide as a fumigant were examined by two separate investigators (Kamel et al., 1975; Verma, 1991). The most sensitive species was seed of the wheat plant, Giza 135 variety. The reduction in germination was 55% when grains with a 15% moisture content were exposed to a concentration of 5.05 × 108 µg carbon disulfide/m3 (Kamel et al., 1975). In general, seeds with higher moisture content were more sensitive. Overall, a concentration of 2.53 × 108 µg carbon disulfide/m3 for a 24-h period can be considered to be without adverse effect for wheat seed when the moisture content does not exceed 15%.
Carbon disulfide fumigation affects all life stages of invertebrates with varying degrees of toxicity (Crookes et al., 1993). The 7-day LC50 value for the most sensitive identified species, the mite Lepidoglyphus destructor, was 1.1 × 106 µg/m3 (Barker, 1982).
In a 5-day study of the effects of carbon disulfide on nitrification in soils using sealed containers, Bremner & Bundy (1974) reported nearly complete inhibition at nominal concentrations as low as 0.5 µg/g. The ecological significance of this result is uncertain, however, because concentrations in test soils were not measured, and the effect nearly disappeared when the test duration was increased to 14 days.
Van Leeuwen et al. (1985) investigated the effects of carbon disulfide on several aquatic species, from algae to the guppy (Poecilia reticulata). Under controlled conditions in a sealed container to prevent evaporative loss, the most sensitive species was Daphnia magna, with a 48-h LC50 of 2.1 mg/litre. At higher concentrations, 3 mg/litre and above, reduced hatching and developmental effects, particularly notochord deformities, were observed in the frog Microhyla ornata (Ghate, 1985). The most sensitive fish species studied was the guppy, with a 96-h LC50 of 4 mg/litre (van Leeuwen et al., 1985). The 96-h EC50 for the green alga Chlorella pyrenoidosa was 21 mg/litre, based on inhibition of growth (van Leeuwen et al., 1985).
The most relevant data for hazard characterization are those from epidemiological studies of populations exposed to carbon disulfide in the workplace. In this section, the available data for those effects that are potentially critical (i.e., effects on the nervous and cardiovascular systems) are evaluated in the context of the traditional criteria for causality for epidemiological studies. (While the epidemiological data regarding the association between exposure to carbon disulfide and damage to the retinal capillaries [section 9.2.4] would satisfy some of the criteria for causality, such effects are considered to be of uncertain clinical significance. The weight of evidence for the remaining categories of effects discussed in sections 8 and 9, including carcinogenicity, genotoxicity, reproductive, developmental and other systemic or organ system effects, is considered inadequate.)
Effects on the nervous system, including neurophysiological, behavioural, and pathological effects, were reported in a large number of cross-sectional studies of viscose rayon workers (section 9.2.1). The most common, consistent findings were reduced conduction velocity in the motor and sensory nerves, generally most pronounced in the more distal portions of the nervous system (e.g., in the lower limbs). There are also a small number of reports of impaired performance on neuropsychological testing, most often on psychomotor tests of motor speed or dexterity, in workers exposed to relatively high levels of carbon disulfide.
In most instances when subgroups of the study populations were analysed separately, the reductions in nerve conduction velocities were most pronounced in those workers with exposure to the highest concentrations, those employed in tasks that were considered to entail the highest exposures, or those with the greatest cumulative exposures. Considering the most reliable studies as a whole, there was an apparent gradient in response across studies, with reductions in the nerve conduction velocities of a wider range of nerves, including those in the upper limbs, observed in the most highly exposed populations, compared with effects only on the lower limbs in populations with exposure to moderate or low concentrations, and no significant effects on conduction velocities in the least exposed populations. In addition, effects on peripheral nerve conduction velocity were generally observed at lower levels than were other effects on the nervous system, particularly the psychomotor effects, both within and between studies. Reductions in nerve conduction velocity in workers who were removed from exposure for a number of years were less pronounced than in workers who were currently exposed in some studies, but not in others.
The effects reported on the peripheral nervous system are supported by the results of studies of animals exposed over the medium or long term by inhalation, in which nerve conduction velocity in the peripheral nerves or spinal cord was consistently reduced, accompanied by histopathological lesions and biochemical changes similar to those induced by certain other compounds that cause axonopathy (e.g., 2,5-hexanedione, the neurotoxic metabolite of hexane) (section 8.2.1). In several studies in rats, exposure to carbon disulfide also affected performance in neurobehavioural testing or altered levels of catecholamines in the brain or adrenals (section 8.2.1).
While the populations of viscose rayon workers in which effects on the nervous system were observed had concomitant exposure to carbon disulfide and hydrogen sulfide, the available evidence indicates that the effects on peripheral nerve conduction velocity were due to carbon disulfide alone. In these studies, concentrations of hydrogen sulfide were typically much less than concentrations of carbon disulfide. In one study, motor tail nerve conduction velocity in rats was reduced by exposure to carbon disulfide but was not affected by hydrogen sulfide alone, nor did hydrogen sulfide modify the effect of carbon disulfide in combined exposures. Medium-term exposure of Sprague-Dawley rats to up to 114 mg hydrogen sulfide/m3 (a concentration that reduced body weights) did not cause neuropathology (CIIT, 1983).
Excess mortality from coronary heart disease has been observed in a number of cohorts of viscose rayon workers exposed to carbon disulfide. While there was inadequate account taken of factors known to affect heart disease (e.g., smoking) in most of these studies, there were consistent excesses in all of the more powerful investigations. The strength of the association was moderate to high, with relative risks ranging from 1.1 to 4.8. There was evidence of a dose-response relationship in most of the studies in which it was examined. The excesses were generally much less pronounced following elimination or reduction of exposure.
In a number of cross-sectional studies in which potential confounding factors were accounted for, occupational exposure to carbon disulfide was associated with clinical changes that increase the risk of heart disease, including increases in blood pressure and in serum levels of total cholesterol and LDL-C and decreases in serum levels of HDL-C. In those studies where internal comparisons were made, these effects were related to the extent of exposure, although there are a number of inconsistencies in this context across studies. There have been some reports of increases in overt manifestations of coronary heart disease, such as angina and coronary electrocardiograph, in workers exposed to carbon disulfide; in the available studies, however, there was often no precise information on the extent of exposure, and the increases were often non-significant and/or based on small numbers of cases.
The biological plausibility of the associations between exposure to carbon disulfide and clinical changes or adverse outcomes for heart disease is supported by the results of studies in animals, in which long-term exposure of rats to high levels of airborne carbon disulfide consistently altered lipid metabolism, resulting in increased serum levels of cholesterol and other blood lipids and exacerbating the atherogenic effects of a lipid-rich diet. Hence, the traditional criteria for causality for associations observed in epidemiological studies are fulfilled, at least in part, for cardiovascular effects associated with exposure to carbon disulfide.
There are several reports of decreased libido and/or impotence among males occupationally exposed to high concentrations of carbon disulfide, but there is no consistent evidence based on limited study of other adverse reproductive effects in humans. In experimental animals, carbon disulfide is embryo- and fetotoxic at high concentrations and causes terata at exposure levels toxic to the dam.
It is not possible at present to identify a quantitative relationship between a given decrease in nerve conduction velocity and an expected degree of loss of function. However, it is noted that nerve conduction velocity is a relatively crude indicator of effects of carbon disulfide on the nerves, because function is not impaired until axonal degeneration has actually occurred, in contrast to agents that produce demyelination or have a direct effect on conduction. An additional concern is that, although the effect is measured on the peripheral nervous system, because carbon disulfide produces a central-peripheral distal axonopathy, it is likely that the long axons of the central nervous system are also affected. Further, there is only limited capability for regeneration of the peripheral nervous system, and even less in the central nervous system. In short, while reduced nerve conduction velocity (subclinical in the key available studies) by itself may not produce an adverse health outcome, it is indicative of, and a precursor of, other changes that clearly are adverse; given the limited reversibility of this effect, a precautionary approach is warranted. Consequently, the critical effect for the characterization of exposure-response is defined as a statistically significant, compound-related decrease in peripheral nerve conduction velocity.
The lowest levels associated with the reductions in peripheral nerve conduction velocity in exposed humans are very similar among the key studies (i.e., those in which exposure and/or processes were reported to be the same for many years and for which personal monitoring data were collected), ranging from 13 to <31 mg/m3. Those levels without significant effect in the key studies are also very similar, ranging from <10 to 13 mg/m3; in both studies, however, there were reductions in nerve conduction velocity, albeit not statistically significant, in the peroneal and/or sural nerves even at these concentrations.
However, in only one of the available epidemiological studies in which there was an association between exposure to carbon disulfide and reductions in peripheral nerve conduction velocity was the exposure of the study population adequately characterized to permit quantitative exposure-response analyses – i.e., in the study reported by Johnson et al. (1983). In addition, the design of the study by Johnson et al. (1983) was among the strongest of the available studies – it was conducted on a sizable population, for which a range of exposure levels were well characterized using personal sampling and for which exposures had been stable for more than 20 years; the exclusionary criteria and analyses would have taken account of a number of potential confounders; and the study also included examination of other manifestations of effects on the nervous system, including peripheral nervous system symptoms and neurobehavioural testing.
Based on the results of the study by Johnson et al. (1983), a benchmark concentration (BMC) for the association between exposure to carbon disulfide and effects on peripheral nerve conduction has been calculated as a measure of exposure-response (Appendix 4).
To convert continuous end-points to quantal ones for which meaningful benchmark doses can be developed, it is necessary to define an "abnormal" response. An abnormal response in this case was defined as that for the 5th percentile of the unexposed population (i.e., measures more extreme than this were considered to be abnormal). The benchmark is then defined as the concentration at which the excess risk of an abnormal response is 5%. On this basis, the BMCL05s (the lower 95% confidence limits for the BMC05s) were 20 mg/m3 (6.3 ppm) for peroneal MCV and 31 mg/m3 (9.9 ppm) for sural SCV (Table A-1; see Appendix 4 for details of the calculations).7 While serum LDL-C was also significantly associated with exposure to carbon disulfide, it is noted that the weight of evidence for cardiovascular effects is not as great as for effects on the nervous system, and the BMC calculated for this end-point was greater than those for the peroneal MCV. A tolerable concentration of 100 µg/m3 was derived based on the BMCL05 estimated for a 5th percentile-based definition of abnormal for the most sensitive response variable – i.e., peroneal MCV8 – at 20 mg/m3 (6.3 ppm) by adjusting to continuous exposure (24 h/day, 7 days/week), and applying an overall uncertainty factor of 50 (×10 for intraspecies [interindividual] variation;9 ×5 to account for potential for effects on neurobehavioural development, since limited available data, although inadequate to serve as a basis for developing a tolerable concentration, indicate that the developing offspring may be more sensitive to the neurological effects of carbon disulfide). An additional uncertainty factor to account for less than lifetime exposure was not considered necessary, in light of the long duration of exposure for the population on which the tolerable concentration is based (mean of 12.2 years), the lesser association of peroneal MCV with cumulative exposure in the regression analysis, and the limited life span of neurofilaments as they traverse the axon (approximately 3-8 months). An uncertainty factor was also not incorporated for inadequacies in the available data for some other effects (e.g., reproductive), because available data indicate that the critical effect is likely to be limiting.
A tolerable intake for oral exposure to carbon disulfide has not been derived, owing to the limitations of the available data. However, it is noted that a tolerable intake derived on the basis of the lowest-observed-adverse-effect level (LOAEL) for developmental toxicity of 25 mg/kg body weight per day in the study in rabbits conducted by Jones-Price et al. (1984b) would be almost identical to one that might be derived by taking into account the volume of air inhaled and the body weights for various age classes of the Canadian population (EHD, 1998), from the tolerable concentration presented above.
Available data upon which to base estimates of human exposure to carbon disulfide are limited; however, air is likely the principal source of exposure (Table 1). Exposure from ingestion of drinking-water and soil appears to be negligible in comparison with that from air. Based on the absence of registered uses for carbon disulfide on food in the sample country (i.e., Canada) and the results of fugacity modelling for southern Alberta, which predicted that very low levels of the compound (<1 × 10-6 µg/g) will accumulate in biota (Environment Canada & Health Canada, 2000), it is assumed that exposure via food will be negligible. For smokers, it is estimated that moderate tobacco use (i.e., 20 cigarettes per day) can increase the intake of carbon disulfide severalfold.
Exposure of the general population has been estimated based on the mean airborne concentrations of carbon disulfide of 0.63 µg/m3 in indoor air and 0.30 µg/m3 in outdoor air (Phillips, 1992), which are not dissimilar to long-term values in the vicinity of point sources. Assuming that people spend on average 21 h indoors and 3 h outdoors each day (EHD, 1998), they would be exposed to a TWA concentration of 0.58 µg/m3. This concentration is 172-fold less than the tolerable concentration derived above. The mean concentrations of carbon disulfide measured in air in limited available studies in the vicinity of point sources in Canada ranged from 1.4 to 6 µg/m3, while the maximum 24-h average concentration predicted in dispersion modelling of the largest anthropogenic source in Canada was 14 µg/m3. These concentrations are 7- to 71-fold less than the tolerable concentration.
While some limitations of the data on exposure have been indicated above, this text addresses uncertainties in data on effects, since it is this information that is likely most relevant in an international context. Exposure estimates and resulting risk characterizations presented in CICADs are examples only.
There is a fair degree of confidence in the results of the critical study by Johnson et al. (1983), which was part of a large investigation in which a wide range of end-points (including those that have historically been associated with exposure to carbon disulfide) was examined in a sizable population, and for which a range of exposures and potential confounders was fairly well characterized. Further, there is consistent support for the critical effect (i.e., reduced peripheral nerve conduction velocity) identified in this study from the results of other rigorously conducted epidemiological studies in which exposure levels were relatively low (as for the study by Johnson et al., 1983) and from the results of studies in animals for both the nature and plausible mechanism of action of the critical effect. However, while the exposure characterization in the critical study was based on personal monitoring of a population for whom a large majority had had the same work assignment for the duration of their employment, it is noted that the personal monitoring was conducted only over a few days at the time of the study and on a minority of the study population. Further, in the exposure-response analyses in the original paper by Johnson et al. (1983), as well as in this assessment, each worker was assigned the mean concentration in personal air for his job task, while it is known that the range of measured concentrations for some job categories was quite wide, more than 2 orders of magnitude (Egeland et al., 1992).
Additional uncertainty results from the critical effect (i.e., reduced conduction velocity in the peripheral nerves) being a somewhat crude indicator of effects on the nervous system, being secondary to axonal damage. Moreover, it is likely that similar effects are occurring in the long axons of the central nervous system (indeed, Hirata et al. [1992] observed effects on latency of some components of the brainstem auditory-evoked potential in rats at exposure levels that were slightly lower than those that affected peripheral nerve conduction velocity), although this has not been as well studied as the peripheral nervous system.
Finally, there is considerable uncertainty introduced by the limitations of the available database, particularly with respect to the effects of carbon disulfide on neurobehavioural development. While neurobehavioural end-points in animals were consistently affected by lower concentrations of carbon disulfide in developing offspring (Hinkova & Tabacova, 1978; Tabacova et al., 1981, 1983; Lehotzky et al., 1985) than in adults (Goldberg et al., 1964a, 1964b; Frantik, 1970; Opacka et al., 1984; Moser et al., 1998), limitations of the available data preclude adequate characterization of exposure-response in this context, as a consequence of such factors as the variety of end-points examined at different time points and inadequate dose spacing and reporting of some of the key studies. While the available data concerning reproductive effects of exposure to carbon disulfide are also inadequate, it is noted that these are unlikely to be limiting, since it appears that they are associated with exposures that are greater than those for the critical neurological effects. While there is no clear evidence of genotoxicity of carbon disulfide, it is noted that this area has not been extensively investigated, and there have been positive results in isolated studies. Adequate studies of carcinogenicity are not available.
Nearly all carbon disulfide is released to air. Therefore, terrestrial organisms living near industrial sources are the populations potentially most impacted, owing to their greatest likelihood of exposure. Aquatic organisms close to discharge points could also be potentially affected.
Relevant results for terrestrial plants, invertebrates, and vertebrates have been identified. The most sensitive organism identified in these studies was the mouse.
The critical toxicity value (CTV) for terrestrial organisms is 6.9 × 105 µg/m3, the 1-h LC50 for mice exposed to carbon disulfide via inhalation. Dividing this CTV by an application factor of 100 (to account for the conversion of an LC50 to a long-term no-effects value, extrapolation from laboratory to field conditions, and interspecies and intraspecies variations in sensitivity) results in an estimated no-effects value (ENEV) of 6.9 × 103 µg/m3.
The estimated exposure value (EEV) for terrestrial organisms is 156 µg/m3, the maximum concentration measured in air over an 8-min period, downwind from a gas plant. The quotient EEV/ENEV, comparing a short-term (acute) exposure value with an estimate of long-term (chronic) effects, is 156/(6.9 × 103) = 0.023. Since this quotient is less than 1, it is unlikely that carbon disulfide causes adverse effects on populations of terrestrial organisms in Canada.
Relevant results for algae, Daphnia, an amphibian, and several fish species have been identified. The most sensitive organism identified in these tests was the invertebrate, Daphnia magna. Aquatic invertebrates are key consumers in the aquatic food web and are themselves consumed by other species of invertebrates and by vertebrates.
The CTV is 2.1 × 103 µg/litre, the 48-h LC50 for the most sensitive aquatic invertebrate, Daphnia magna. Dividing this CTV by a factor of 100 (to account for the conversion of an LC50 to a long-term no-effects value, extrapolation from laboratory to field conditions, and a somewhat limited toxicity data set) (Environment Canada, 1997a) results in an ENEV of 21 µg/litre.
The EEV for aquatic biota is 3.9 µg/litre (the maximum concentration of carbon disulfide measured in Lake Ontario in 1981). This value is believed to be conservative, because emissions of carbon disulfide to the environment have decreased significantly since the early 1980s. The quotient EEV/ENEV is 3.9/21 = 0.19. Since this quotient is less than 1, it is unlikely that carbon disulfide causes adverse effects on populations of aquatic organisms in Canada.
Regarding effects of carbon disulfide on terrestrial and aquatic organisms, there is uncertainty in the extrapolation from available data on acute toxicity to prediction of long-term effects on the ecosystem. For wildlife, and especially small mammals, inhalation exposure in laboratory animals was used as a surrogate for actual exposure in the field. While the aquatic toxicity data set included studies on organisms from a variety of ecological niches and taxa, there are no chronic studies available for invertebrates or fish.
The World Health Organization air quality guideline for carbon disulfide is 100 µg/m3 averaged over 24 h (WHO, 2001). This was based on the lowest concentration of carbon disulfide at which an adverse effect was observed in the occupational environment being considered to be about 10 mg/m3 and an uncertainty factor of 100, to take into account the expected variability in the susceptibility of the general population. Based on the sensory effects of carbon disulfide, a guideline value of 20 µg/m3 (averaging time, 30 min) was recommended.
Abrams EF, Derkics D, Fong CV, Guinan DK, Slimak KM (1975) Identification of organic compounds in effluents from industrial sources. Washington, DC, US Environmental Protection Agency (EPA-560/3-75-002; PB 241 641 OBA).
Acadzhanova AA (1978) Occupational hygiene of women in viscose fibre production. Gigiena Truda i Professional'naya Zabolevaniya, 4:10-13.
Adkins B Jr, Van Stee EW, Simmons JE, Eustis SL (1986) Oncogenic response of strain A/J mice to inhaled chemicals. Journal of Toxicology and Environmental Health, 17:311-322.
Albright BE, Burg JR, Fagen J, Johnson BL, Lee ST, Leffingwell S, Meyer CR, Putz-Anderson VR, Smith AB (1984) Health effects of occupational exposures to carbon disulfide. Cincinnati, OH, National Institute for Occupational Safety and Health (NTIS PB85-110229).
ATSDR (1996) Toxicological profile for carbon disulfide (update). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 219 pp.
ATSDR (1999) Toxicological profile for hydrogen sulfide. Atlanta, GA, US Department of Health and Human Services, Public Health Service, 179 pp.
Bao YS, Cai S, Zhao SF, Xhang XC, Huang MY, Zheng OM, Jiang H (1991) Birth defects in the offspring of female workers occupationally exposed to carbon disulfide in China. Teratology, 43(5):451-452.
Barker PS (1982) Control of a mite, Lepidoglyphus destructor, including hypopi, in wheat with carbon disulfide. Journal of Economic Entomology, 75:436-439.
Beauchamp RO, Bus JS, Popp JA, Boreiko CJ, Goldberg L (1983) A critical review of the literature on carbon disulfide toxicity. CRC Critical Reviews in Toxicology, 11:169-278.
Belisles RP, Brusick DJ, Melcher FJ (1980) Teratogenic-mutagenic risk of workplace contaminants: trichloroethylene, perchloroethylene, and carbon disulphide. Report prepared by Litton Bionetics Inc. for the National Institute for Occupational Safety and Health, Cincinnati, OH, May (NTIS PB82-185075).
Bokina AI, Eksler ND, Semenenko AD, Merkur'yeva RV (1976) Investigation of the mechanism of action of atmospheric pollutants on the central nervous system and comparative evaluation of methods of study. Environmental Health Perspectives, 13:37-42.
Bokina AI, Merkur'yeva RV, Eksler ND, Oleynik AA, Pinigina II (1979) Experimental study of the mechanism and indices of harmful effects of certain chemical substances on the central nervous system. Environmental Health Perspectives, 30:31-38.
Bremner JM, Bundy LG (1974) Inhibition of nitrification in soils by volatile sulfur compounds. Soil Biology and Biochemistry, 6:161-165.
Brugnone F, Perbellini L, Giuliari C, Cerpelloni M, Soave M (1994) Blood and urine concentrations of chemical pollutants in the general population. Medicina del Lavoro, 85:370-389.
BUA (1993) Carbon disulfide. German Chemical Society, GDCh Advisory Committee on Existing Chemicals of Environmental Relevance (Beratergremium für Umweltrelevante Alstoffe). Stuttgart, S. Hirzel Verlag (Report No. 83).
Cai SX, Bao YS (1981) Placental transfer, secretion into mother [sic] milk of carbon disulphide and the effects on maternal function of female viscose rayon workers. Industrial Health, 19:15-29.
Camford Information Services (1995) CPI product profiles: Carbon disulfide. Don Mills, Ontario, Camford Information Services Inc.
Candura F, Franco T, Malamani T, Piazza A (1979) Altered glucose tolerance in carbon disulfide exposed workers. Acta Diabetologica Latina, 16:259-263.
Caron F, Kramer JR (1994) Formation of volatile sulfides in freshwater environments. The Science of the Total Environment, 153:177-194.
Cassitto MG, Bertazzi PA, Camerino D, Bulgheroni C, Cirla AM, Gilioli R, Graziano C, Tomasini M (1978) Subjective and objective behavioural alterations in carbon disulphide workers. Medicina del Lavoro, 69(2):144-150.
CEC (1988) Draft summary record of Meeting 23 of the Working Group "Classification and Labelling of Dangerous Substances," Brussels, 14-15 January. Brussels, Commission of the European Communities.
Chrostek Maj J, Czeczotko B (1995) The evaluation of the health state of the workers occupationally exposed to low concentration of carbon disulphide (CS2). Part one: General medical examination and laboratory tests. Przeglad Lekarski, 52(5):249-251.
Chu C-C, Huang C-C, Chen R-S, Shih T-S (1995) Polyneuropathy induced by carbon disulphide in viscose rayon workers. Occupational and Environmental Medicine, 52(6):404-407.
Chu C-C, Huang C-C, Chu N-S, Wu T-N (1996) Carbon disulfide induced polyneuropathy: sural nerve pathology, electrophysiology, and clinical correlation. Acta Neurologica Scandinavica, 94:258-263.
CIIT (1983) 90-day vapor inhalation toxicity study of hydrogen sulfide in Sprague-Dawley rats. Report prepared by Toxigenics, Inc., for the Chemical Industry Institute of Toxicology, Research Triangle Park, NC (CIIT Docket No. 32063) [cited in ATSDR, 1999].
Cirla AM, Graziano C (1981) Health impairment in viscose-rayon workers with carbon disulfide risk below 30 mg/m3. An exposed-controls study. Giornale Italiano di Medicina del Lavoro, 3:69-73.
Cirla AM, Bertazzi PA, Tomasini M, Villa A, Graziano C, Invernizzi R, Gilioli R (1978) Study of endocrinological functions and sexual behaviour in carbon disulphide workers. Medicina del Lavoro, 69(2):118-129.
Crookes MJ, Diment J, Dobson S (1993) Environmental hazard assessment: carbon disulfide. Garston, Watford, United Kingdom Department of the Environment, Building Research Establishment (TSD/14).
Crump K (1995) Calculation of benchmark doses from continuous data. Risk Analysis, 15(1):79-89.
Daft J (1987) Determining multifumigants in whole grains and legumes, milled and low-fat grain products, spices, citrus fruit, and beverages. Journal of the Association of Official Analytical Chemists, 70:734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical residues in food. Journal of the Association of Official Analytical Chemists, 71:748-760.
Daft JL (1989) Determination of fumigants and related chemicals in fatty and nonfatty foods. Journal of Agricultural and Food Chemistry, 37:560-564.
De Fruyt F, Thiery E, De Bacquer D, Vanhoorne M (1998) Neuropsychological effects of occupational exposures to carbon disulfide and hydrogen sulfide. International Journal of Occupational and Environmental Health, 4:139-146.
DMER, AEL (1996) Pathways analysis using fugacity modeling of carbon disulfide for the second Priority Substances List. Peterborough, Ontario, Don Mackay Environmental Research; and Don Mills, Ontario, Angus Environmental Limited.
Drexler H, Ulm K, Hubmann M, Hardt R, Goen T, Mondorf W, Lang E, Angerer J, Lehnert G (1995) Carbon disulphide. III. Risk factors for coronary heart diseases in workers in the viscose industry. International Archives of Occupational and Environmental Health, 67:243-252.
Dutkiewicz T, Baranowska B (1967) The significance of absorption of carbon disulphide through the skin in the evaluation of exposure. In: Brieger H, Teisinger J, eds. Toxicology of carbon disulphide. Proceedings of a symposium, Prague, 15-17 September 1966. Amsterdam, Excerpta Medica Foundation, pp. 50-51.
Egeland GM, Burkhart GA, Schnorr TM, Hornung RW, Fajen JM, Lee ST (1992) Effects of exposure to carbon disulphide on low density lipoprotein cholesterol concentration and diastolic blood pressure. British Journal of Industrial Medicine, 49:287-293.
EHD (1998) Exposure factors for assessing total daily intake of priority substances by the general population of Canada. Ottawa, Ontario, Health Canada, Environmental Health Directorate, Bureau of Chemical Hazards, Priority Substances Section, March.
Environment Canada (1980) National inventory of natural sources and emissions of sulphur compounds. Ottawa, Ontario, Environment Canada, Environmental Protection Service, Air Pollution Control Directorate (Report EPS 3-AP-79-2).
Environment Canada (1997a) Environmental assessments of priority substances under the Canadian Environmental Protection Act. Guidance manual Version 1.0 (March 1997). Ottawa, Ontario, Environment Canada.
Environment Canada (1997b) Results of the CEPA Section 16 Notice respecting the second Priority Substances List and di(2-ethylhexyl) phthalate. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch, Use Patterns Section.
Environment Canada (1999) Canadian Environmental Protection Act – Priority Substances List – Supporting document for the environmental assessment of carbon disulfide. Hull, Quebec, Environment Canada, Commercial Chemicals Evaluation Branch.
Franco G, Malamani T, Adami V, Germani L, Suraci A, Tempini G, Tornaghi G, Aliotta A (1981) Glucose tolerance, glycosylated haemoglobin and blood lipids in viscose workers exposed to 30 mg/m3. Giornale Italiano di Medicina del Lavoro, 3:113-116.
Franco G, Malamani T, Germani L, Candura F (1982) Assessment of coronary heart disease risk among viscose rayon workers exposed to carbon disulfide at concentrations of about 30 mg/m3. Scandinavian Journal of Work, Environment and Health, 8:113-120.
Frantik E (1970) The development of motor disturbances in experimental chronic carbon disulphide intoxication. Medicina del Lavoro, 61(5):309-313.
Fuortes L (1990) A sick house syndrome, possibly resulting from a landfill geologic effluvia. Veterinary and Human Toxicology, 32(6):528-530.
Gagnaire F, Simon P, Bonnet P, De Ceaurriz J (1986) The influence of simultaneous exposure to carbon disulfide and hydrogen sulfide on the peripheral nerve toxicity and metabolism of carbon disulfide in rats. Toxicology Letters, 34:175-183.
Ghate HV (1985) Toxicity and teratogenic effects in the frog embryo. Rivista di Biologia, 78:129-131.
Gibson JD, Roberts RJ (1972) Effect of carbon disulfide on liver function in vivo and in the isolated perfused liver. Journal of Pharmacology and Experimental Therapeutics, 181(1):176-182 [cited in ASTDR, 1996].
Gilioli R, Bulgheroni G, Bertazzi PA, Cirla AM, Tomasini M, Cassitto MG, Jacovone MT (1978) Study of neurological and neurophysiological impairment in carbon disulphide workers. Medicina del Lavoro, 69(2):130-143.
Goldberg ME, Johnson HE, Pozzani UC, Smyth HF Jr (1964a) Effect of repeated inhalation of vapors of industrial solvents on animal behaviour. I. Evaluation of nine solvent vapors on pole-climb performance in rats. American Industrial Hygiene Association Journal, 25:369-375.
Goldberg ME, Johnson HE, Pozzani UC, Smyth HF Jr (1964b) Behavioural response of rats during inhalation of trichloroethylene and carbon disulphide vapours. Acta Pharmacologica et Toxicologica, 21:36-44.
Golder Associates (1987) Testing of specific organic compounds in soils in background urban areas – Port Credit and Oakville/Burlington, Ontario. Working paper prepared for Shell Canada Limited and Texaco Canada Limited.
Goto S, Hotta R (1967) The medical and hygienic prevention of carbon disulphide poisoning in Japan. In: Brieger H, Teisinger J, eds. Toxicology of carbon disulphide. Proceedings of a symposium, Prague, 15-17 September 1966. Amsterdam, Excerpta Medica Foundation, pp. 219-230.
Goto S, Hotta R, Sugimoto K (1971) Studies on chronic carbon disulfide poisoning. Pathogenesis of retinal microaneurysms due to carbon disulfide, with special reference to a subclinical defect of carbohydrate metabolism. Internationales Archiv für Arbeitsmedizin, 28:115-126.
Environment Canada, Health Canada (2000) Canadian Environmental Protection Act – Priority Substances List assessment report – Carbon disulfide. Ottawa, Ontario, Health Canada; and Hull, Quebec, Environment Canada.
Graham DG, Amarnath V, Valentine WM, Pyle SJ, Anthony DC (1995) Pathogenetic studies of hexane and carbon disulfide neurotoxicity. Crititcal Reviews in Toxicology, 25(2):91-112.
Guidotti TL, Hoffman H (1999) Indicators of cardiovascular risk among workers exposed to high intermittent levels of carbon disulphide. Occupational Medicine (London), 49(8):507-515.
Hänninen H (1971) Psychological picture of manifest and latent carbon disulphide poisoning. British Journal of Industrial Medicine, 28:374-381.
Hänninen H, Nurminen M, Tolonen M, Martelin T (1978) Psychological tests as indicators of excessive exposure to carbon disulfide. Scandinavian Journal of Psychology, 19:163-174.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Belisles RP, Niemeier RW (1981) Testing of selected workplace chemicals for teratogenic potential. Scandinavian Journal of Work, Environment and Health, 7(Suppl. 4):66-75.
He F, Shi M, Xiu R (1996) [Investigation on the impact of carbon disulfide exposure on menstruation and reproduction in female workers.] Industrial Medicine, 13:128 (in Chinese).
Heikes DL (1987) Purge and trap method for determination of volatile hydrocarbons and carbon disulfide in table-ready foods. Journal of the Association of Official Analytical Chemists, 70:215-226.
Heikes DL, Hopper ML (1986) Purge and trap method for determination of fumigants in whole grains, milled grain products, and intermediate grain-based foods. Journal of the Association of Official Analytical Chemists, 69:990-998.
Hemminki K, Niemi ML (1982) Community study of spontaneous abortions: relation to occupation and air pollution by sulfur dioxide, hydrogen sulfide, and carbon disulfide. International Archives of Occupational and Environmental Health, 51:55-63.
Hemminki K, Franssila E, Vainio H (1980) Spontaneous abortions among female chemical workers in Finland. International Archives of Occupational and Environmental Health, 45:123-126.
Hernberg S, Tolonen M (1981) Epidemiology of coronary heart disease among viscose rayon workers. Giornale Italiano di Medicina del Lavoro, 3:49-52.
Hernberg S, Partanen T, Nordman C-H, Sumari P (1970) Coronary heart disease among workers exposed to carbon disulphide. British Journal of Industrial Medicine, 27:313-325.
Hernberg S, Nordman C-H, Partanen T, Christiansen V, Virkola P (1971) Blood lipids, glucose tolerance and plasma creatinine in workers exposed to carbon disulphide. Work, Environment, Health, 8:11-16.
Hernberg S, Nurminen M, Tolonen M (1973) Excess mortality from coronary heart disease in viscose rayon workers exposed to carbon disulphide. Work, Environment, Health, 10:93-99.
Herr DW, Vo KT, Morgan DL, Sills RC (1998) Carbon disulfide neurotoxicity in rats: VI. Electrophysiological examination of caudal tail nerve compound action potentials and nerve conduction velocity. Neurotoxicology, 19(1):129-146.
Hinkova L, Tabacova S (1978) Open field exploration in two successive generations of rats treated with carbon disulphide throughout gestation. Activitas Nervosa Superior (Praha), 20(1):12-14.
Hirata M, Ogawa Y, Okayama A, Goto S (1992) Changes in auditory brainstem response in rats chronically exposed to carbon disulfide. Archives of Toxicology, 66(5):334-338.
Hirata M, Ogawa Y, Goto S (1996) A cross-sectional study on nerve conduction velocities among workers exposed to carbon disulphide. Medicina del Lavoro, 87(1):29-34.
Hoffmann P, Klapperstück M (1990) Effects of carbon disulfide on cardiovascular function after acute and subacute exposure of rats. Biomedica Biochimica Acta, 49(1):121-128.
Hoffmann P, Müller S (1990) Subacute carbon disulfide exposure modifies adrenergic cardiovascular actions in rats. Biomedica Biochimica Acta, 49(1):115-120.
Horton AD, Guerin MR (1974) Quantitative determination of sulfur compounds in the gas phase of cigarette smoke. Journal of Chromatography, 90:63-70.
Howard P (1989) Handbook of environmental fate and exposure data for organic chemicals. Volume 2. Solvents. Boca Raton, FL, Lewis Publishers.
HSE (1981) Toxicity review 3. Carbon disulphide. London, Health and Safety Executive, 42 pp.
IPCS (1979) Carbon disulfide. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 10).
IPCS (1994) Assessing human health risks of chemicals: Derivation of guidance values for health-based exposure limits. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 170).
IPCS (2000) International Chemical Safety Card – Carbon disulfide. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0022).
Johnson BL, Boyd J, Burg JR, Lee ST, Xintaras C, Albright BE (1983) Effects on the peripheral nervous system of workers' exposure to carbon disulfide. Neurotoxicology, 4(1):53-66.
Jones-Price C, Wolkowski-Tyl R, Marr MC, Kimmel CA (1984a) Teratologic evaluation of carbon disulfide (CAS No.
Jones-Price C, Wolkowski-Tyl R, Marr MC, Kimmel CA (1984b) Teratologic evaluation of carbon disulfide (CAS No.
Kaiser KLE, Comba ME (1983) Volatile contaminants in the Welland River watershed. Journal of Great Lakes Research, 9:274-280.
Kaiser KLE, Comba ME, Huneault H (1983) Volatile halocarbon contaminants in the Niagara River and in Lake Ontario. Journal of Great Lakes Research, 9:212-223.
Kamel AH, Fam EZ, Mahdi MT, Sheltawi EM (1975) The phytotoxic effect of carbon bisulfide, methyl bromide, and hydrogen phosphide on the germination of seeds of certain field crops. Bulletin of the Entomological Society of Egypt, Economic Series, 8:75-80 [cited in BUA, 1993].
Keil DE, Padgett EL, Barnes DB, Pruett SB (1996) Role of decomposition products in sodium methyldithiocarbamate-induced immunotoxicity. Journal of Toxicology and Environmental Health, 47:479-492.
Kim KH, Andreae MO (1987) Carbon disulfide in sea water and the marine atmosphere over the North Atlantic. Journal of Geophysical Research, 92(D12):14 733-14 738.
Kivisto H (2000) TTCA measurements in biomonitoring of low-level exposure to carbon disulphide. International Archives of Occupational and Environmental Health, 73(4):263-269.
Klapperstück M, Müller S, Hoffman P (1991) Carbon disulfide exposure attenuates adrenergic inotropic response in rats. Journal of Hygiene, Epidemiology, Microbiology, and Immunology, 35(2):113-120.
Krstev S, Perunicic B, Farkic B, Varagic M (1993) Environmental and biological monitoring in carbon disulfide exposure assessment. Medicina del Lavoro, 84(6):473-481.
Kuligowski D (1996) [The influence of chronic exposure to carbon disulfide on metabolism of catecholamines and serotonin in women.] Annales Academiae Medicae Stetinensis, 42(6):139-156 (in Polish).
Kuo H-W, Lai J-S, Lin M, Su E-S (1997) Effects of exposure to carbon disulfide (CS2) on electrocardiographic features of ischemic heart disease among viscose rayon factory workers. International Archives of Occupational and Environmental Health, 70:61-66.
Leck C, Rodhe H (1991) Emissions of marine biogenic sulfur to the atmosphere. Journal of Atmospheric Chemistry, 12:63-86.
Le JY, Fu XM (1996) Human sperm chromosome analysis – study on human sperm chromosome mutagens induced by carbon disulfide. Biomedical and Environmental Sciences, 9:37-40.
Legge AH, Nosal M, Peake E, Strosher M, Hansen M, Lefohn AS (1990a) Air quality of an area proximal to anthropogenic emissions. In: Legge AH, Krupka SV, eds. Acidic deposition: Sulphur and nitrogen oxides – the Alberta government/industry Acid Deposition Research Program (ADRP). Chelsea, MI, Lewis Publishers, pp. 249-345.
Legge AH, Peake E, Strosher M, Nosal M, McVehil GE, Hansen M (1990b) Characteristics of the background air quality. In: Legge AH, Krupka SV, eds. Acidic deposition: Sulphur and nitrogen oxides – the Alberta government/industry Acid Deposition Research Program (ADRP). Chelsea, MI, Lewis Publishers, pp. 129-240.
Lehotzky K, Szeberényi JM, Ungváry G, Kiss A (1985) Behavioural effects of prenatal exposure to carbon disulphide and to Aromatol in rats. Archives of Toxicology, Supplement 8:442-446.
Li P, Wang Z, Han L, Qiu Y, Zhang G, Ho G, Wu Z (1999) [Life-table analysing the effects of carbon disulphide on pregnancy probability.] Journal of Weifang Medical College, 27:244-246 (in Chinese).
Lindbohm M-L, Hemminki K, Bonhomme MG, Anttila A, Rantala K, Heikkilä P, Rosenberg MJ (1991) Effects of paternal occupational exposure on spontaneous abortions. American Journal of Public Health, 81:1029-1033.
Lovejoy ER (1989) The kinetics and products of the OH initiated oxidation of CS2 and O2. PhD thesis, University of Colorado, Boulder, CO.
Lovelock JE (1974) CS2 and the natural sulfur cycle. Nature, 248:625-626.
Lowry LK (1996) Carbon disulfide. In: Biological monitoring of chemical exposure in the workplace. Volume 1. Geneva, World Health Organization, pp. 156-167.
Lu, KF, Wang YB (1999) [Observation on the neurological effects on male workers associated with CS2 exposure.] Journal of Labour and Medicine, 16(3):167-168 (in Chinese).
Mackay D (1991) Multimedia environmental models: the fugacity approach. Chelsea, MI, Lewis Publishers.
Mackay D, Paterson S (1991) Evaluating the multimedia fate of organic chemicals: a Level III fugacity model. Environmental Science and Technology, 25:427-436.
MacMahon B, Monson RR (1988) Mortality in the US rayon industry. Journal of Occupational Medicine, 30(9):699-705.
Mancuso TF (1981) Epidemiological study of workers employed in the viscose rayon industry. Pittsburgh, PA, University of Pittsburgh (NTIS PB82-151275).
Maroulis PJ, Bandy AR (1980) Measurements of atmospheric concentrations of CS2 in the eastern United States. Geophysical Research Letters, 7(9):681-684.
Masuda Y, Yasoshima M, Nakayama N (1986) Early, selective and reversible suppression of cytochrome P-450 dependent monooxygenase of liver microsomes following the administration of low doses of carbon disulfide in mice. Biochemical Pharmacology, 35(22):3941-3947.
Meyer CR (1981) Semen quality in workers exposed to carbon disulfide compared to a control group from the same plant. Journal of Occupational Medicine, 23(6):435-439.
Moser VC, Phillips PM, Morgan DL, Sills RC (1998) Carbon disulfide neurotoxicity in rats: VII. Behavioural evaluations using a functional observational battery. Neurotoxicology, 19(1):147-158.
Neter J, Wasserman W, Kutner M (1989) Applied linear regression models, 2nd ed. Homewood, IL, Irwin.
NPRI (1996) Summary report 1995, National Pollutant Release Inventory, Canadian Environmental Protection Act. Hull, Quebec, Environment Canada.
Nurminen M, Mutanen P, Tolonen M, Hernberg S (1982) Quantitated effects of carbon disulfide exposure, elevated blood pressure and aging on coronary mortality. American Journal of Epidemiology, 115(1):107-118.
O'Donoghue JL (1985) Carbon disulfide and organic sulfur-containing compounds. In: O'Donoghue JL, ed. Neurotoxicity of industrial and commercial chemicals. Volume II. Boca Raton, FL, CRC Press, pp. 39-60.
Oldaker GB, Taylor WD, Parrish KB (1995) Investigations of ventilation rate, smoking activity and indoor air quality at four large office buildings. Environmental Technology, 16:173-180.
Opacka J, Baranski B, Wronska-Nofer T (1984) Effect of alcohol intake on some disturbances induced by chronic exposure to carbon disulphide in rats. I. Behavioural alterations. Toxicology Letters, 23:91-97.
Otson R (1987) Purgeable organics in Great Lakes raw and treated water. Journal of Environmental and Analytical Chemistry, 31:41-53.
PAI (1991) Developmental inhalation toxicity study of carbon disulfide in the New Zealand white rabbit. Contract report prepared by Pathology Associates, Inc., Frederick, MD, for Akzo Chemicals Inc., Chicago, IL, 31 January.
Pellizzari E, Hartwell TD, Harris BSH III, Waddell RD, Whitaker DA, Erickson MD (1982) Purgeable organic compounds in mothers' milk. Bulletin of Environmental Contamination and Toxicology, 28:322-328.
Peplonska B, Szeszenia-Dabrowska N, Sobala W, Wilczynska U (1996) A mortality study of workers with reported chronic occupational carbon disulfide poisoning. International Journal of Occupational Medicine and Environmental Health, 9(3):291-299.
Peyton TO, Steele RV, Mabey WR (1976) Carbon disulfide, carbonyl sulfide: literature review and environmental assessment. Washington, DC, US Environmental Protection Agency (EPA-600/9-78-009).
Phillips M (1992) Detection of carbon disulfide in breath and air: a possible new risk factor for coronary artery disease. International Archives of Occupational and Environmental Health, 64:119-123.
Price B, Berner T, Henrich R, Stewart J, Moran E (1996) A benchmark concentration for carbon disulfide: analysis of the NIOSH carbon disulfide exposure database. Regulatory Toxicology and Pharmacology, 24:171-176.
Price B, Bergman TS, Rodriguez M, Henrich RT, Moran EJ (1997) A review of carbon disulfide exposure data and the association between carbon disulfide exposure and ischemic heart disease mortality. Regulatory Toxicology and Pharmacology, 26, Part 1:119-128.
Putz-Anderson V, Albright BE, Lee ST, Johnson BL, Chrislip DW, Taylor BJ, Brightwell WS, Dickerson N, Culver M, Zentmeyer D, Smith P (1983) A behavioral examination of workers exposed to carbon disulfide. Neurotoxicology, 4(1):67-78.
Raitta C, Teir H, Tolonen M, Nurminen M, Helpiö E, Malström S (1981) Impaired color discrimination among viscose rayon workers exposed to carbon disulfide. Journal of Occupational Medicine, 23(3):189-192.
Rebert CS, Becker E (1986) Effects of inhaled carbon disulfide on sensory-evoked potentials of Long-Evans rats. Neurobehavioural Toxicology and Teratology, 8:533-541.
Reinhardt F, Drexler H, Bickel A, Claus D, Angerer J, Ulm K, Lehnert G, Neundörfer B (1997a) Neurotoxicity of long-term low-level exposure to carbon disulphide: results of questionnaire, clinical neurological examination and neuropsychological testing. International Archives of Occupational and Environmental Health, 69:332-338.
Reinhardt F, Drexler H, Bickel A, Claus D, Ulm K, Angerer J, Lehnert G, Neundörfer B (1997b) Electrophysiological investigation of central, peripheral and autonomic nerve function in workers with long-term low-level exposure to chronic carbon disulphide in the viscose industry. International Archives of Occupational and Environmental Health, 70:249-256.
Riihimaki V, Kivisto H, Peltonen K, Helpio E, Aitio A (1992) Assessment of exposure to carbon disulfide in viscose production workers from urinary 2-thiothiazolidine-4-carboxylic acid determinations. American Journal of Industrial Medicine, 22:85-87.
Rolecki R, Tarkowski S (2000) Draft document for carbon disulfide. Lodz, The Nofer Institute of Occupational Medicine.
Ruijten MWMM, Sallé HJA, Verberk MM, Muijser H (1990) Special nerve functions and colour discrimination in workers with long term low level exposure to carbon disulphide. British Journal of Industrial Medicine, 47:589-595.
Ruijten MWMM, Sallé HJA, Verberk MM (1993) Verification of effects on the nervous system of low level occupational exposure to CS2. British Journal of Industrial Medicine, 50:301-307.
Saillenfait AM, Bonnet P, de Ceaurriz J (1989) Effects of inhalation exposure to carbon disulfide and its combination with hydrogen sulfide on embryonal and fetal development in rats. Toxicology Letters, 48:57-66.
Sandalls FJ, Penkett SA (1977) Measurements of carbonyl sulphide and carbon disulphide in the atmosphere. Atmospheric Environment, 11:197-199.
Sandrini G, Bosso A, Biscaldi G, Malamani T, Franco G, Grampella D, Alfonsi E, Moglia A, Arrigo A (1983) Electromyographic investigation in early diagnosis of carbon disulphide neuropathy: a study on 216 workers with different degrees of exposure. Giornale Italiano di Medicina del Lavoro, 5:199-202.
Selevan SG, Hornung R, Fajen J, Cottrill C (1983) Paternal exposure to carbon disulfide and spouse's pregnancy experience. Cincinnati, OH, US Department of Health and Human Services, Centers for Disease Control, National Institute for Occupational Safety and Health (NTIS PB85-220754).
Seppäläinen AM, Tolonen M (1974) Neurotoxicity of long-term exposure to carbon disulfide in the viscose rayon industry. A neurophysiological study. Work, Environment, Health, 11:145-153.
Sills RC, Harry GJ, Morgan DL, Valentine WM, Graham DG (1998) Carbon disulfide neurotoxicity in rats: V. Morphology of axonal swelling in the muscular branch of the posterior tibial nerve and spinal cord. Neurotoxicology, 19(1):117-128.
Simon P, Nicot T, Dieudonné M (1994) Dietary habits, a non-negligible source of 2-thiothiazolidine-4-carboxylic acid and possible overestimation of carbon disulfide exposure. International Archives of Occupational and Environmental Health, 66:85-90.
Smith NA, Kelly DP (1988) Oxidation of carbon disulfide as the sole source of energy for the autotrophic growth of Thiobacillus thioparus, strain TK-m. Journal of General Microbiology, 134:3041-3048.
Stanosz S, Kuligowski D, Pieleszek A, Zuk E, Rzechula D, Chlubek D (1994) Concentration of dopamine in plasma, activity of dopamine beta-hydroxylase in serum and urinary excretion of free catecholamines and vanillylmandelic acid in women chronically exposed to carbon disulphide. International Journal of Occupational Medicine and Environmental Health, 7(3):257-261.
Staubes R, Georgii H-W, Ockelmann G (1987) Emissions of biogenic sulfur compounds from various soils. In: Angeletti G, Restelli G, eds. Proceedings of the 4th European symposium on physico-chemical behaviour of atmospheric pollutants. Dordrecht, D. Reidel Publishing Co., pp. 427-433 (EUR 10832).
Stedman DH, Creech MZ, Cloke PL, Kesler SE, Gardner M (1984) Formation of CS2 and OCS from decomposition of metal sulfides. Geophysical Research Letters, 11:858-860.
Strosher M (1996) Investigations of flare gas emissions in Alberta. Final report to Environment Canada (Conservation and Protection), the Alberta Energy and Utilities Board, and the Canadian Association of Petroleum Producers. Calgary, Alberta, Alberta Research Council, Environmental Technologies, November.
Sun H, Zhang AG, Liu CF, Tao TF, Zhu J, Gao YH (1998) [The effects on cardiovascular system of workers associated with long-term low-level concentration exposure to CS2.] Journal of Labour and Medicine, 15(1):28 (in Chinese).
Swaen GMH, Braun C, Slangen JJM (1994) Mortality of Dutch workers exposed to carbon disulfide. International Archives of Occupational and Environmental Health, 66:103-110.
Sweetnam PM, Taylor SWC, Elwood PC (1987) Exposure to carbon disulphide and ischaemic heart disease in a viscose rayon factory. British Journal of Industrial Medicine, 44:220-227.
Tabacova S, Hinkova L, Balabaeva L (1978) Carbon disulphide teratogenicity and postnatal effects in rat. Toxicology Letters, 2:129-133.
Tabacova S, Hinkova L, Nikiforov B, Balabaeva L (1981) Hazards for the progeny after maternal exposure to low carbon disulfide concentrations. Giornale Italiano di Medicina del Lavoro, 3:121-125.
Tabacova S, Nikiforov B, Balabaeva L (1983) Carbon disulphide intrauterine sensitization. Journal of Applied Toxicology, 3(5):223-229.
Takebayashi T, Omae K, Ishizuka C, Nomiyama T, Sakurai H (1998) Cross sectional observation of the effects of carbon disulphide on the nervous system, endocrine system, and subjective symptoms in rayon manufacturing workers. Occupational and Environmental Medicine, 55:473-479.
Taylor GE, Selvidge WJ (1984) Phytotoxicity in bush beans of five sulfur-containing gases released from advanced fossil energy technologies. Journal of Environmental Quality, 13:224-230.
Taylor GE Jr, McLaughlin SB Jr, Shriner DS, Selvidge WD (1983) The flux of sulfur-containing gases to vegetation. Atmospheric Environment, 17(4):789-796.
Tepe SJ, Zenick H (1984) The effects of carbon disulfide on the reproductive system of the male rat. Toxicology, 32:47-56.
The JL (1998) Carbon disulfide study. Waterloo, Ontario, Lakes Environmental Consultants Inc.
Tiller JR, Schilling RSF, Morris JN (1968) Occupational toxic factor in mortality from coronary heart disease. British Medical Journal, 4:407-411.
Toews AD, Harry GJ, Lowrey KB, Morgan DL, Sills RC (1998) Carbon disulfide neurotoxicity in rats: IV. Increased mRNA expression of low-affinity nerve growth factor receptor – a sensitive and early indicator of PNS damage. Neurotoxicology, 19(1):109-116.
Tolonen M, Hernberg S, Nurminen M, Tiitola K (1975) A follow-up study of coronary heart disease in viscose rayon workers exposed to carbon disulphide. British Journal of Industrial Medicine, 32:1-10.
Tolonen M, Nurminen M, Hernberg S (1979) Ten-year coronary mortality of workers exposed to carbon disulfide. Scandinavian Journal of Work, Environment and Health, 5:109-114.
TRI (1999) Toxic Release Inventory. US Environmental Protection Agency (http://www.epa.gov/tri/tridata/tri99/).
Valentine WM, Graham DG, Anthony DC (1993) Covalent cross-linking of erythrocyte spectrin by carbon disulfide in vivo. Toxicology and Applied Pharmacology, 121:71-77.
Valentine WM, Amarnath V, Amarnath K, Rimmele F, Graham DG (1995) Carbon disulfide mediated protein cross-linking by N,N-diethyldithiocarbamate. Chemical Research in Toxicology, 8:96-102.
Valentine WM, Amarnath V, Graham DG, Morgan DL, Sills RC (1997) CS2-mediated cross-linking of erythrocyte spectrin and neurofilament protein: dose-response and temporal relationship to the formation of axonal swellings. Toxicology and Applied Pharma-cology, 142:95-105.
Valentine WM, Amarnath V, Amarnath K, Erve JCL, Graham DG, Morgan DL, Sills RC (1998) Covalent modifications of hemoglobin by carbon disulfide: III. A potential biomarker of effect. Neurotoxicology, 19(1):99-108.
Vanhoorne M, Van Den Berge LP, Devreese A, Tijtgat E, Van Poucke L, Van Peteghem C (1991) Survey of chemical exposures in a viscose rayon plant. Annals of Occupational Hygiene, 36:619-631.
Vanhoorne M, De Bacquer D, De Backer G (1992) Epidemiological study of the cardiovascular effects of carbon disulphide. International Journal of Epidemiology, 21(4):745-752.
Vanhoorne M, Comhaire F, De Bacquer D (1994) Epidemiological study of the effects of carbon disulfide on male sexuality and reproduction. Archives of Environmental Health, 49(4):273-278.
Vanhoorne MH, Ceulemans L, De Bacquer DA, De Smet FP (1995) An epidemiologic study of the effects of carbon disulfide on the peripheral nerves. Occupational and Environmental Health, 1(4):295-302.
Vanhoorne M, De Rouck A, Bacquer D (1996) Epidemiological study of the systemic ophthalmological effects of carbon disulfide. Archives of Environmental Health, 51(3):181-188.
van Leeuwen CJ, Maas-Diepeveen JL, Niebeek G, Vergouw WHA, Griffioen PS, Luijken MW (1985) Aquatic toxicological aspects of dithiocarbamates and related compounds. I. Short-term toxicity tests. Aquatic Toxicology, 7:145-164.
Vasilescu C, Florescu A (1980) Clinical and electrophysiological studies of carbon disulphide polyneuropathy. Journal of Neurology, 224:59-70.
Vasil'eva IA (1982) Investigation of the action of carbon disulfide on the chromosome apparatus of adult and embryonic rat cells. Tsitologiya i Genetica, 16(2):57-59.
Verma BR (1991) Vacuum fumigation schedule for seed inhabiting chalcidoids. Journal of Entomological Research, 15:229-232.
Wägar G, Tolonen M, Stenman U-H, Helpiö E (1981) Endocrinologic studies in men occupationally exposed to carbon disulfide. Journal of Toxicology and Environmental Health, 7:363-371.
Wang Q, Fu K, Wu Q (1999) [Effects on fertility and menstrual cycle of female workers exposed to carbon disulfide.] Chinese Public Health, 15(3):215-217 (In Chinese).
Wang YF, Shiu YF (2000) [Investigation on eye injury of workers exposed to CS2.] Journal of Labour and Medicine, 17(2):89 (in Chinese).
Wang Z, Hou G, Li P, Zhang C, Han L, Du J, Wang Z, Ren Y, Hong X (1997) [Time-to-pregnancy among women occupationally exposed to carbon disulphide.] Chinese Journal of Industrial Hygiene and Occupational Disease, 15:288-290 (in Chinese).
Wang Z, Ho G, Li P, Zhang C, Han L, Du J, Zhang G, Cui Q, Qui Y, Ren Y, Hong X, Wang C (1999) [Very early pregnancy loss of women occupationally exposed to carbon disulphide.] Chinese Journal of Industrial Hygiene and Occupational Disease, 17:204-207 (in Chinese).
WHO (2001) 1999 WHO air quality guidelines for Europe, 2nd ed. Copenhagen, World Health Organization, Regional Office for Europe (WHO Regional Publications, European Series; Internet address: http://www.who.int/peh/air/Airqualitygd.htm).
WIL Research Laboratories, Inc. (1992) An assessment of reproduction in female rats exposed to CS2 via inhalation. Contract report prepared by WIL Research Laboratories, Inc., Ashland, OH, for Chemical Manufacturers Association, Washington, DC, 2 September (Sponsor Project No. CDS-2.0-REPRO/WIL).
Wine PH, Chameides WL, Ravishankara AR (1981) Potential role of CS photooxidation in tropospheric sulfur chemistry. Geophysical Research Letters, 8(5):543-546.
Wood WP, Heicklen J (1971) The photooxidation of carbon disulfide. Journal of Physical Chemistry, 75:854-860.
Wronska-Nofer T, Laurman W (1987) The lipid risk factor for coronary heart disease in human exposure to carbon disulphide. In: Foa V, ed. Occupational and environmental hazards: Cellular and biochemical indices for monitoring toxicity. Proceedings of a symposium. Chichester, E. Horwood; New York, NY, Halsted Press, pp. 187-191.
Yang XF, Lee BL, New AL, Ong HY, Ma L, Zhang Q, Ong CN (1996a) Urinary homovanillic acid and vanillylmandelic acid in workers exposed to carbon disulfide. American Journal of Industrial Medicine, 29:269-274.
Yang XF, Gao YH, Liang YX (1996b) [Neurobehavioural effects associated with occupational exposure to CS2.] Journal of Labour and Medicine, 23(6):1-3 (in Chinese).
Yaroslavskii VK (1969) Toxic action of carbon disulphide on reproductive function and potentiation of the effect by tryptophan. Byulleten Eksperimental'noi Biologii i Meditsiny, 68:88-91.
Zenick H, Blackburn K, Hope E, Baldwin D (1984) An evaluation of the copulatory, endocrinologic, and spermatotoxic effects of carbon disulfide in the rat. Toxicology and Applied Pharmacology, 73:275-283.
Zhang W, Lian X, Wang B, Liu B, Pan Z (1999) [Toxic effects of carbon disulfide on gonadal and reproductive health in occupationally exposed women.] Chinese Public Health, 15:217-218 (in Chinese).
Zhou SY, Liang YX, Chen ZQ, Wang YL (1988) Effects of occupational exposure to low-level carbon disulfide (CS2) on menstruation and pregnancy. Industrial Health, 26:203-214.
Environment Canada & Health Canada (2000)
Copies of the Canadian Environmental Protection Act Priority Substances List Assessment Report (Environment Canada & Health Canada, 2000) are available on the Internet at:
www.hc-sc.gc.ca/ehp/ehd/bch/env_contaminants/psap/psap.htm
Unpublished supporting documentation, which presents additional information, is available upon request from:
Commercial Chemicals Evaluation Branch
Environment Canada
14th Floor, Place Vincent Massey
351 St. Joseph Blvd.
Hull, Quebec
Canada K1A 0H3
or
Room 104, Environmental Health Centre
Health Canada
Tunney's Pasture
Ottawa, Ontario
Canada K1A 0L2
Initial drafts of the supporting documentation and Assessment Report for carbon disulfide were prepared by staff of Health Canada and Environment Canada. Sections of the supporting documentation and Assessment Report on genotoxicity were reviewed by D. Blakey (Environmental and Occupational Toxicology Division, Health Canada). L. Turner and H. Hirtle contributed additional information in the preparation of the draft CICAD.
Environmental sections of the Assessment Report and supporting documentation (Environment Canada, 1999) were reviewed externally by E. Moran, Chemical Manufacturers Association, USA; and C. Williams, CRW Consulting Inc.
In order to address primarily adequacy of coverage, sections of the supporting documentation pertaining to human health were reviewed externally by:
H. Drexler, Technical University at Aachen
S. Gabos, Alberta Health
D. Graham, Vanderbilt University Medical Center
R. Henrich, Akzo Nobel Chemicals Inc.
W. Valentine, Vanderbilt University Medical Center
M. Vanhoorne, State University of Ghent
Accuracy of reporting, adequacy of coverage, and defensibility of conclusions with respect to hazard characterization and dose-response analyses were considered in written review by staff of the Information Department of BIBRA International and by H. Kappus, Humbolt University, as well as at a panel meeting of the following members, convened by Toxicology Excellence in Risk Assessment (TERA), on 17 May 1999 in Ottawa, Ontario:
R. Bornschein, University of Cincinnati
J. Christopher, California Environmental Protection Agency
H. Clewell III, ICF Kaiser International
M. Dourson, TERA
M. Prince, National Institute of Occupational Safety and Health
W. Valentine, Vanderbilt University Medical Center
A draft of the Assessment Report was also made available for a 60-day public comment period (23 October to 22 December 1999) A summary of the comments and responses is available on the Internet at:
www.ec.gc.ca/cceb1/eng/public/index_e.html
The draft CICAD on carbon disulfide was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
American Chemistry Council, USA
M. Baril, International Programme on Chemical Safety/
Institut de Recherche en Santé et en Sécurité du Travail, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, USA
C.D. Carrington, Food and Drug Administration, USA
R. Cary, Health and Safety Executive, United Kingdom
M Cikrt, National Institute of Public Health, Czech Republic
S. Dobson, Centre for Ecology and Hydrology, United Kingdom
H. Drexler, University of Erlangen, Germany
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, USA
R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Germany
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Germany
M. Vanhoorne, Ghent University, Belgium
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Ottawa, Canada,
29 October - 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Since all variables for critical end-points are of a continuous nature, an abnormal response was considered to be that outside of normal physiological range. This effectively reduces the continuous end-point to a quantal end-point. The BMC is then chosen as the concentration at which the risk of an abnormal response is increased by a specified quantity (Crump, 1995). The mean observed response may then be modelled as a function of other confounding factors (such as age, weight, and height). This method of computing BMCs was applied to the data from the study of workers exposed to carbon disulfide by Johnson et al. (1983).
The original study data10 from the population studied by Johnson et al. (1983) were used to calculate the BMC. The data file contained measurements on 165 exposed and 245 unexposed workers. The measurements consisted of indicators (i.e., response variables) relating to ischaemic heart disease and the peripheral nervous system as well as potential confounding information.11 Exposures were represented as either current job exposures to carbon disulfide in parts per million (ppm), cumulative exposure in ppm-months, or average exposure (ppm), defined as a worker's cumulative exposure divided by the duration of exposure.
Following Johnson et al. (1983) and Price et al. (1996), workers were eliminated from the nervous system analysis if they were diabetic, had excessive alcohol consumption (>35 units), or had high blood lead levels (>40 µg/dl). These conditions can cause peripheral neuropathy and therefore potentially mask an exposure-effect relationship. Following Egeland et al. (1992), workers were eliminated from the blood pressure analysis if they used antihypertensive drugs, from the fasting glucose analysis if they used hypoglycaemic drugs, and from the lipoprotein analysis if they used corticosteroids or lipid-lowering or thyroid medications.
Stepwise regression was performed to determine which confounding variables (including the three exposure measures – current, cumulative, and average) could be used to explain the response variables. For those responses showing a significant relationship with exposure, BMCs were calculated using the following procedure.
First, the regression was obtained of exposure and all other significant confounders on the response:

where y is the response, d is exposure, x is a vector of confounding variables, and b and γ are parameters estimated in the regression. For the purpose at hand, the response y is thought of as the mean response as a function of exposure. That is, y = µ(d).
Next, the responses were discretized following the method of Crump (1995), modified to use excess risk rather than additional risk. In this method, it is assumed that a proportion, P0, of the control group will be abnormal. This proportion is chosen to be small (e.g., 5% or 1%) so that most unexposed individuals will not be abnormal. This is equivalent to choosing a cut-off level x0, above which a response in the control group would be considered abnormal. The probability of a response in the unexposed population being abnormal is described by
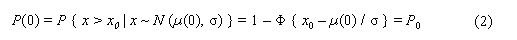
where Φ is the normal cumulative density function (i.e., Φ(
z) is the probability that a standard normal variable is less than z), µ is the mean response as a function of exposure, and σ is the standard deviation, assumed to be constant for all exposures. As a consequence, equation 2 indicates that, knowing x0, P0 can be calculated from normal tables, and vice versa. For this analysis, P0 is specified as either 1% or 5%. Given P0 (and hence x0), the probability of a response being abnormal at dose d is given by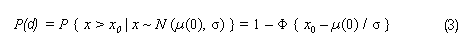
The BMC is computed by setting the excess risk equal to BMR, the specific benchmark risk level; that is,
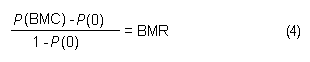
By solving equation 2 for x0, substituting into equation 3, and then substituting equations 2 and 3 into 4, it can be shown that solving equation 4 for BMC is equivalent to solving
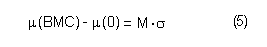
for BMC, with
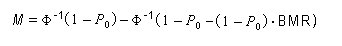
and µ defined by equation 1. This effectively reduces the continuous end-point to a quantal end-point; the BMC05 is chosen as the concentration at which the excess risk of an abnormal response is 5%.
Note that this argument assumes that larger responses are adverse. Blood pressure is an example of a case where a larger response is adverse, since higher blood pressure levels are associated with an increased risk of heart disease. If smaller responses are more severe, such as with nerve conduction velocities, where slower velocities are detrimental, a similar argument would hold and equation 5 would be identical, except that M would be replaced by - M.
The BMC was calculated by substituting equation 1 into 5, with y = µ(d) and solving for BMC. The b ' x terms cancel, and the BMC is given by
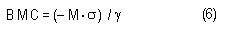
Finally, BMCL, the lower bound on the BMC, was obtained using a standard formula in linear regression for the lower bound on an inverse prediction (i.e., when the response is known and the exposure is estimated by equation 6). This formula is presented, for example, in Neter et al. (1989). BMCs computed on the basis of cumulative exposures were converted to a daily exposure in ppm by dividing by 12.2 years, which is the average exposure duration of exposed workers in the cohort.
The stepwise regression indicated that, of the nervous system outcomes, maximum MCV for the peroneal nerve and SCV for the sural nerve were significantly related to all three exposure measures. If given the choice, average exposure for peroneal MCV and cumulative exposure for sural SCV would be selected by the stepwise model. Average exposure was chosen to model both outcomes, however, since the model including cumulative exposure fit the sural SCV data nearly as well (r2 of 0.166 versus r2 of 0.158 for average exposure), and since average exposure gives a more accurate estimate of ambient levels for each worker (i.e., the cumulative exposure was divided by employment duration for each worker, as opposed to dividing the final BMC by the average employment duration for the entire exposed cohort). Sural distal latency was significantly related to current exposure; when one large outlier was removed (a value of 39.1, whereas the median sural distal latency for the cohort was 4.2), however, the relationship with exposure was no longer significant. As a result, sural distal latency was not utilized for BMC calculation. Among the risk factors for heart disease, LDL-C was significantly related to current exposure.
The variables selected for inclusion in the linear regression models by the stepwise procedure were age, height, race, and average exposure for the maximum MCV of the peroneal nerve; age, height, weight, and average exposure for the SCV of the sural nerve; and age, current exposure, weight, and height for LDL-C. For each of peroneal MCV, sural SCV, and LDL-C, the corresponding contributing variables were input into the linear regression in equation 1, and the resulting parameter estimates were obtained.
BMC05s were calculated by applying equation 6 with M equal to either 0.77 for a 1% adverse response rate or 0.35 for a 5% adverse response rate, σ equal to the standard error, and γ equal to the regression coefficient for exposure. For an abnormal response based on the 5th percentile of the control population (i.e., a 5% adverse response), the BMCL05s (the lower 95% confidence limits for the BMC05s) were 20 mg/m3 (6.3 ppm) for peroneal MCV and 31 mg/m3 (9.9 ppm) for sural SCV. (While serum LDL-C was also significantly associated with exposure to carbon disulfide, it is noted that the weight of evidence for cardiovascular effects is not as great as for effects on the nervous system, and the BMC calculated for this end-point was greater than those for the peroneal MCV, in any case.) The BMC05 point estimates are quite similar to the lower bounds. If nerve conduction velocities below the 1st percentile of the unexposed population are considered abnormal, the estimated BMC05s and BMCL05s are approximately 2-fold higher than those for a 5% adverse response (Table A-1).
Table A-1: Final BMC05s and BMCL05s for selected outcome variables.a
|
Outcome |
1st/99th percentile abnormalb |
|
5th/95th percentile abnormal |
||
|
BMC05 |
BMCL05 |
|
BMC05 |
BMCL05 |
|
|
Peroneal motor nerve conduction velocity |
16.3 |
14.9 |
|
7.6 |
6.3 |
|
Sural sensory nerve conduction velocity |
25.9 |
23.7 |
|
12.1 |
9.9 |
|
Low-density lipoprotein cholesterol |
20.9 |
19.2 |
|
9.8 |
8.1 |
|
a |
Note that 1 ppm = 3.125 mg/m3. |
|
b |
An abnormal response was defined on the basis of a low (or, in the case of low-density lipoprotein cholesterol, high) percentile of the end-point in question in unexposed workers. For nerve conduction velocities, the 1st and 5th percentiles were used, while the 99th and 95th percentiles were used for low-density lipoprotein cholesterol. The benchmark concentration was chosen as the concentration at which the risk of an abnormal response is estimated to be increased by a specified quantity, in this case 5%. |
For illustration, peroneal MCV (adjusted for age, height, and race) is plotted against average exposure to carbon disulfide in Figure A-1. The regression line is also plotted. There is considerable scatter among the data points, and, while the regression with exposure to carbon disulfide is significant, it explains a relatively small proportion of the variability in the data. Average exposure accounts for 5.0% of the total variation in the data, which is similar to the association with age (8.5%) and height (6.7%) and greater than that with race (1.1%).
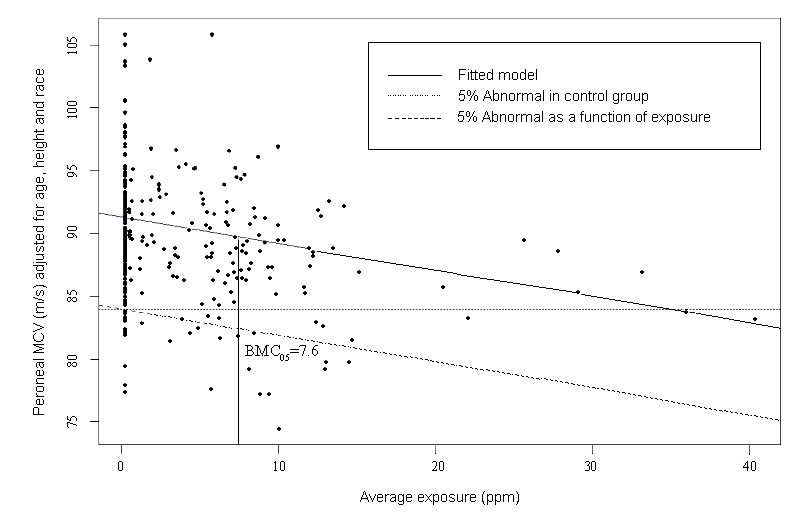
Figure A-1. Exposure-response relationship for peroneal MCV
Ce CICAD relatif au sulfure de carbone a été préparé conjointement par la Direction de l'Hygiène du Milieu de Santé Canada et la Direction de l'Evaluation des produits chimiques commerciaux d'Environnement Canada à partir d'une documentation rédigée simultanément dans le cadre du programme sur les substances prioritaires prévu par la Loi canadienne sur la protection de l'environnement (LCPE). Les études sur les substances prioritaires prescrites par la LCPE ont pour objectif d'évaluer les effets potentiels sur la santé humaine d'une exposition indirecte à celles de ces substances qui sont présentes dans l'environnement ainsi que leurs effets sur l'environnement lui-même. La présente mise au point prend en compte les données recensées jusqu'à fin mai 1999.12 Des renseignements sur la nature de l'examen par des pairs et la disponibilité du document de base sont donnés à l'appendice 1. L'IPCS (1979) et l'institut Nofer (Rolecki & Tarkowski, 2000) ont également préparé des documents de base sur les effets environnementaux et sanitaires, de même que le Département de l'environnement du Royaume-Uni (Crookes et al., 1993). D'autres mises au point ont été également consultées, à savoir celles de la BUA (1993) et de l'ATSDR (1996). Les informations concernant l'examen par des pairs du présent CICAD figurent à l'appendice 2. Ce CICAD a été approuvé en tant qu'évaluation internationale lors d'une réunion du Comité d'évaluation finale qui s'est tenue à Ottawa (Canada) du 29 octobre au 1er novembre 2001. La liste des participants à cette réunion se trouve à l'appendice 3. La fiche internationale sur la sécurité chimique du sulfure de carbone (ICSC 0022), établie par le Programme international sur la sécurité chimique (IPCS, 2000), est également reproduite dans le présent document.
La capacité mondiale de production de sulfure de carbone (No CAS
Le sulfure de carbone est présent dans tout l'environnement. On en a décelé dans l'air, l'eau, les sédiments et le sol, mais c'est dans l'air qu'il prédomine. Dans le pays à l'origine du présent CICAD (le Canada), c'est à proximité de sources industrielles et en particulier près d'installations de traitement du gaz naturel et de torchères de gaz naturel soufré que l'on mesure les concentrations atmosphériques de CS2 les plus élevées. L'élimination du sulfure de carbone atmosphérique est principalement due à la réaction de la molécule avec les radicaux hydroxyles, la demi-vie étant dans ce cas de 1 à 2 semaines. Avec une telle demi-vie, le CS2 peut être transporté sur de longues distances, mais il se dilue rapidement dans l'air pour redescendre à sa concentration de fond. Il est rapidement métabolisé par les êtres vivants sans subir de bioconcentration ni de bioamplification.
On ne dispose que de données extrêmement limitées sur lesquelle se baser pour évaluer l'exposition humaine au sulfure de carbone. Cela étant, c'est l'air qui constitue la principale voie d'exposition pour la population dans son ensemble. On estime que les populations qui vivent à proximité de sources ponctuelles de CS2 sont fortement exposés au sulfure de carbone présent dans l'air.
Le sulfure de carbone est fortement absorbé par inhalation, mais également par voie percutanée. Il donne naissance à plusieurs métabolites dont un, l'acide 2-thiothiazolidine-4-carboxylique, est utilisé pour le contrôle biologique de l'exposition sur le lieu de travail.
Les données susceptibles d'être utilisées pour évaluer le pouvoir irritant ou sensibilisateur du sulfure de carbone n'existent qu'en nombre limité. D'après des informations citant des études anciennes qui paraissent de toute manière limitées, le sulfure de carbone serait très fortement irritant pour les yeux et la peau, mais on n'a pas été en mesure de vérifier ces résultats. Dans les usines produisant de la rayonne, on constate des cas d'irritation, notamment de la muqueuse respiratoire après inhalation; toutefois, on ignore si l'exposition concomitante à l'hydrogène sulfuré et aux vapeurs d'acide sulfurique joue un rôle dans le déclenchement de cet effet.
Selon des études effectuées sur des travailleurs exposés au sulfure de carbone et sur la base d'autres données tirées de l'expérimentation animale, il semble que le système nerveux soit le point d'aboutissement de l'action toxique de ce composé. Celle-ci se manifeste la plupart du temps par une réduction de la vitesse de conduction au niveau des nerfs périphériques et par une moindre performance lors des tests psychomoteurs. Il existe des données très probantes concernant d'autres effets chez les sujets humains exposés au sulfure de carbone, notamment des taux anormaux de lipides sériques et une HTA pouvant être à l'origine d'un risque accru de cardiopathies. Par ailleurs, on observe aussi des effets ophtalmologiques généraux tels qu'une dyschromatopsie ou des lésions vasculaires rétiniennes et, à dose plus élevée, un accroissement de la mortalité par affections cardiaques.
Des études épidémiologiques de portée limitée n'ont révélé aucun signe de cancérogénicité et il n'existe pas d'études de cancérogénicité à long terme chez l'animal qui aient été publiées. On ne possède pas non plus preuves indiscutables d'une activité génotoxique, encore que quelques signes peu marqués ou ambigus d'effets clastogènes aient été observés in vivo et in vitro.
Un certain nombre de rapports médicaux font état d'une diminution de la libido accompagnée ou non d'impuissance chez des travailleurs de sexe masculin professionnellement exposés à de fortes concentrations de sulfure de carbone, mais les études limitées concernant d'éventuels effets sur l'appareil reproducteur humain ne fournissent pas de preuves cohérentes d'une action toxique à ce niveau. Chez l'animal de laboratoire, le CS2 se révèle embryotoxique et foetotoxique à forte concentration et il est également tératogène à des doses toxiques pour la mère.
S'agissant de la caractérisation du risque type, l'exposition estimative moyenne de la population générale au sulfure de carbone présent dans l'air et celle des populations vivant à proximité de sources ponctuelles de CS2, sont très inférieures à la concentration tolérable, fixée à 100 m g/m3. Cette valeur de la concentration tolérable a été obtenue à partir de la concentration de référence pour un excès de 5 % du risque de réaction anormale (basé sur le 5ème centile des travailleurs non exposés dans l'étude retenue) concernant la variable la plus sensible, à savoir la vitesse de conduction du nerf moteur péronier13 chez des travailleurs employés à la fabrication de rayonne, en tenant compte d'une exposition continue (24 h par jour et 7 jours par semaine) et en appliquant un coefficient d'incertitude global de 50.
Etant donné que la majeure partie du sulfure de carbone est rejetée dans l'air, ce sont les organismes terrestres vivant à proximité des sources industrielles qui courent le plus grand risque. Les organismes aquatiques qui se trouvent dans le voisinage de points de décharge pourraient également subir des effets indésirables. Toutefois, en se fondant sur la caractérisation du risque type et en comparant avec prudence l'exposition estimative à la concentration sans effet observable, on peut conclure que le sulfure de carbone ne devrait pas causer d'effets nocifs sur les populations d'organismes terrestres ou aquatiques.
Este CICAD sobre el disulfuro de carbono, preparado conjuntamente por la Dirección de Higiene del Medio del Ministerio de Sanidad del Canadá y la División de Evaluación de Productos Químicos Comerciales del Ministerio de Medio Ambiente del Canadá, se basó en la documentación preparada al mismo tiempo como parte del Programa de Sustancias Prioritarias en el marco de la Ley Canadiense de Protección del Medio Ambiente (CEPA). Las evaluaciones de sustancias prioritarias previstas en la CEPA tienen por objeto valorar los efectos potenciales para la salud humana de la exposición indirecta en el medio ambiente general, así como los efectos ecológicos. En estos exámenes se analizaron los datos identificados hasta el final de mayo de 1999.14 La información relativa al carácter del examen colegiado y a la disponibilidad del documento original figura en el apéndice 1. Otros documentos originales relativos a la salud y el medio ambiente son los informes preparados por el IPCS (1979), el Instituto Nofer (Rolecki y Tarkowski, 2000) y el Departamento de Medio Ambiente del Reino Unido (Crookes et al., 1993). También se consultaron otros exámenes, entre ellos los del BUA (1993) y la ATSDR (1996). La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Ottawa (Canadá) del 29 de octubre al 1º de noviembre de 2001. La lista de participantes en esta reunión figura en el apéndice 3. La Ficha internacional de seguridad química (ICSC 0022) para el disulfuro de carbono, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000), también se reproduce en este documento.
La capacidad de producción mundial de disulfuro de carbono (CAS Nº
El disulfuro de carbono es ubicuo en todo el medio ambiente. Se ha detectado en el aire, el agua, los sedimentos y el suelo; sin embargo, está presente fundamentalmente en el aire. Las concentraciones más altas de disulfuro de carbono en el aire en el país de origen para este CICAD (es decir, el Canadá) se han medido cerca de fuentes industriales, sobre todo en las proximidades de instalaciones de tratamiento de gas natural y de lugares donde se quema gas natural que contiene azufre. El disulfuro de carbono se elimina del aire principalmente por reacción con radicales hidroxilo, con una semivida de 1-2 semanas. Esta semivida en el aire favorece el transporte a larga distancia; sin embargo, se diluye con rapidez hasta alcanzar los niveles de fondo naturales. El disulfuro de carbono se metaboliza en el organismo con rapidez y no experimenta procesos de bioconcentración o biomagnificación.
Son muy limitados los datos disponibles en los cuales basar las estimaciones de la exposición humana al disulfuro de carbono; sin embargo, el aire parece ser la principal vía de exposición para los miembros de la población general. Se considera que las poblaciones próximas a fuentes puntuales industriales tienen una exposición elevada a las partículas suspendidas en el aire.
El disulfuro de carbono se absorbe ampliamente por inhalación, pero también por vía cutánea. Se degrada formando varios metabolitos, uno de los cuales (ácido 2-tiotiazolidín-4-carboxílico) se utiliza para la biovigilancia de la exposición en el lugar de trabajo.
Se dispone de datos limitados como base de la evaluación del potencial del disulfuro de carbono para inducir irritación o sensibilización. Si bien se han notificado en informes secundarios de estudios iniciales al parecer limitados que el disulfuro de carbono irrita gravemente los ojos y la piel, no ha sido posible verificar estos datos. Aunque la inhalación en las fábricas de rayón viscosa es irritante para las membranas mucosas, incluido el sistema respiratorio, no se conoce la función de la exposición concomitante al ácido sulfídrico y al ácido sulfúrico en la inducción de estos efectos.
Sobre la base de los resultados obtenidos de estudios con trabajadores expuestos al disulfuro de carbono y los datos justificativos de experimentos realizados con animales, parece que el sistema nervioso es el más afectado por su toxicidad inducida, manifestada la mayoría de las veces por una disminución de la velocidad de conducción en los nervios periféricos y un rendimiento deficiente en las pruebas psicomotoras. Otros efectos en personas expuestas al disulfuro de carbono para los cuales hay pruebas considerables son las alteraciones de los lípidos del suero y la presión sanguínea, que están asociados con un mayor riesgo de enfermedades cardiovasculares, efectos oftalmológicos sistémicos, entre ellos los relativos a la visión de los colores y el daño de los vasos sanguíneos de la retina y (con exposiciones más altas) aumento de la mortalidad por enfermedades cardíacas.
No se han observado pruebas de carcinogenicidad en estudios epidemiológicos limitados; no se han notificado estudios prolongados sobre carcinogenicidad en animales de experimentación. No hay pruebas claras de genotoxicidad, aunque existen algunos indicios de clastogenicidad débil y/o ambigua in vitro o in vivo.
Hay varios informes de reducción de la libido y/o impotencia de varones profesionalmente expuestos a concentraciones elevadas de disulfuro de carbono, pero no hay pruebas coherentes basadas en un estudio limitado de otros efectos reproductivos adversos en las personas. En animales de experimentación, el disulfuro de carbono es embriotóxico y fetotóxico a concentraciones elevadas y provoca deformaciones con niveles de exposición tóxicas para las madres.
En la caracterización del riesgo de muestra, la exposición media estimada al disulfuro de carbono suspendido en el aire para la población general y para las poblaciones de las proximidades de fuentes puntuales es considerablemente inferior a la concentración tolerable de 100 µg/m3. Esta concentración tolerable se obtuvo a partir de la concentración de referencia estimada para un exceso de riesgo de una respuesta anormal del 5% (establecida a partir del percentil 5 de los trabajadores no expuestos en el estudio crítico) para la variable más sensible de la respuesta - es decir, la velocidad de conducción del nervio motor peroneal15 en los trabajadores del rayón viscosa - ajustada a una exposición continua (24 horas/día, siete días/semana) y aplicando un factor de incertidumbre global de 50.
Puesto que la mayor parte del disulfuro de carbono se libera en el aire, para los efectos ecológicos son los organismos terrestres de las cercanías de fuentes industriales los que corren el mayor riesgo potencial. Los organismos acuáticos próximos a puntos de descarga también podrían verse potencialmente afectados. Sin embargo, basándose en la caracterización del riesgo de muestra, las comparaciones prudentes de la exposición estimada con valores sin efecto indican que es poco probable que el disulfuro de carbono provoque efectos adversos en poblaciones de organismos terrestres o acuáticos.
ENDNOTES:
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
Critical new information has been scoped to indicate its likely impact on the essential conclusions of this assessment, primarily to establish priority for their consideration in an update. This ensures appropriate consideration in the context of the complete identified database through the several stages of internal and external national review and subsequent international review. More recent information not critical to the hazard characterization or exposure-response analysis, considered by reviewers to add to informational content, has been added. |
|
|
A tolerable concentration calculated on the basis of the no-observed-effect level (NOEL) of 13 mg/m3 from the same study would be quite similar. |
|
|
In keeping with WHO policy, which is to provide measurements in SI units, all concentrations of gaseous chemicals in air will be given in SI units in the CICAD series. Where the original study or source document has provided concentrations in SI units, these will be cited here. Where the original study or source document has provided concentrations in volumetric units, conversions will be done using the conversion factors given here, assuming a temperature of 20 °C and a pressure of 101.3 kPa. Conversions are usually to no more than two significant digits. |
|
|
It is noted that hydrogen sulfide is typically present in these workplaces at much lower concentrations than carbon disulfide, and that the profile of effects from exposure to hydrogen sulfide is quite distinct from that for carbon disulfide (ATSDR, 1999). Further, as discussed in section 11.1.1, the available information indicates that the critical effect is caused by carbon disulfide, rather than hydrogen sulfide. |
|
|
In a series of studies in which few details are given on the study design and performance, notably exposure and exposure assessment, delayed pregnancy or very early pregnancy loss (detected from serial human chorionic gonadotropin measurements only) has been reported among Chinese women exposed to carbon disulfide at average area levels of 21 mg/m3 (Li et al., 1999), 9 mg/m3 (Wang et al., 1997), and 24 mg/m3 (Z. Wang et al., 1999). |
|
|
BMC estimates based on the 1st percentile of the unexposed workers as the cut-off for abnormal have also been included in Table A-1 of Appendix 4 for illustrative purposes. |
|
|
A tolerable concentration on the basis of the NOEL of 13 mg/m3 from the same study would be quite similar. |
|
|
Available quantitative data are insufficient to replace default values for the components of this uncertainty factor with data-derived values (see IPCS, 1994). For example, knowledge regarding the respective contributions of the parent compound and oxidative metabolites to the critical effect is inadequate (section 7). In addition, the metabolism of carbon disulfide is not fully known, particularly in humans, and there are potential sensitive subpopulations that would not have been included in the occupational epidemiological studies (e.g., the elderly [because of the age-related decrease in nerve conduction velocity, this group would have less reserve] and diabetics [who are prone to polyneuropathy]). |
|
|
The cooperation of the Chemical Manufacturers Association in the provision of these data is gratefully acknowledged. |
|
|
For ischaemic heart disease: total serum cholesterol, LDL-C, HDL-C, triglyceride, fasting glucose, systolic and diastolic blood pressure. For peripheral nerve conduction: maximal MCV, distal latency, and amplitude ratio of the ulnar and peroneal nerves, and SCV, distal latency, and discrete amplitude ratio of the sural nerve. For confounders: age, height, weight, race, body mass index, education, current smoking status, current alcohol consumption, blood lead level, haemoglobin concentration, pulse rate, and diabetes. |
|
|
Les nouvelles données importantes ont été examinées compte tenu de leur influence probable sur les conclusions essentielles de la présente évaluation, le but étant avant tout d'établir si leur prise en compte serait prioritaire lors d'une prochaine mise à jour. On pourra ainsi leur accorder toute l'attention voulue dans le contexte de la base de donnée complète qui aura été établie lors des phases successives des examens interne et externe au niveau national, puis de l'examen au niveau international. Les auteurs ayant estimé qu'elles apportaient des éléments d'information supplémentaires, on a ajouté des données plus récentes encore que non essentielles pour la caractérisation des dangers ou l'analyse des relations dose-réponse. |
|
|
La valeur de la concentration tolérable calculée à partir de la concentration sans effet observable, qui est égale à 13 mg/m3 et qui est tirée de la même étude serait sensiblement identique. |
|
|
Se ha incluido nueva información crítica para señalar sus probables repercusiones en las conclusiones esenciales de esta evaluación, principalmente con objeto de establecer la prioridad para su examen en una actualización. De esta manera se garantiza un examen adecuado en el marco de la base de datos completa establecida mediante las distintas fases del examen nacional interno y externo y el posterior examen internacional. Se ha añadido información más reciente no decisiva para la caracterización del peligro o el análisis de la exposición-respuesta que los examinadores consideraban que aumentaba el contenido informativo. |
|
|
Sería bastante similar una concentración tolerable sobre la base del nivel sin efectos observados (NOEL) de 13 mg/m3 obtenida del mismo estudio. |
See Also:
Toxicological Abbreviations
Carbon disulfide (EHC 10, 1979)
Carbon disulfide (ICSC)
Carbon disulfide (PIM 102)
Carbon disulfide (FAO Meeting Report PL/1965/10/2)
Carbon disulfide (FAO/PL:1967/M/11/1)
Carbon disulfide (FAO/PL:1968/M/9/1)
Carbon disulfide (WHO Pesticide Residues Series 1)