
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 47
First draft prepared by S. Czerczak, The Nofer Institute of Occupational Medicine, Lodz, Poland; second draft prepared by L. Fishbein, Fairfax, Virginia, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2002
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Arsine: Human health aspects.
(Concise international chemical assessment document ; 47)
1. Arsenic - toxicity 2. Arsenic - poisoning 3. Arsenic - physiology 4. Occupational exposure
5. Environmental exposure 6. Hazardous substances - adverse effects
I. International Programme on Chemical Safety II.Series
ISBN 92 4 153047 2 (NLM Classification: QV 294)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. Applications and enquiries should be addressed to the Office of Publications, World Health Organization, Geneva, Switzerland, which will be glad to provide the latest information on any changes made to the text, plans for new editions, and reprints and translations already available.
©World Health Organization 2002
Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the World Health Organization concerning the legal status of any country, territory, city, or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
|
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION |
|
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
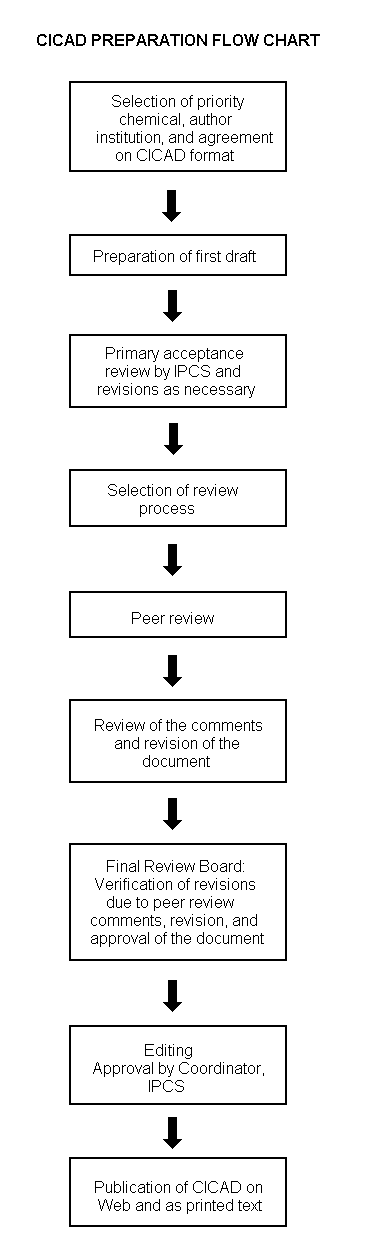
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, a priority chemical typically
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
The first draft of this CICAD on human health aspects of arsine was prepared by Dr S. Czerczak from the Nofer Institute of Occupational Medicine, Lodz, Poland. The literature searches performed cover data identified up to June 2000. The US Environmental Protection Agency’s Integrated Risk Information System document (US EPA, 1994b) and the German MAK document (Greim, 2001) were used as additional source documents. The source documents and their peer review are described in Appendix 1. The peer review of this CICAD is described in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Monks Wood, England, on 16–19 September 2002. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card for arsine (ICSC 0222), produced by the International Programme on Chemical Safety in a separate, peer-reviewed process (IPCS, 2001b), has also been reproduced in this document.
The information presented in this CICAD focuses on effects associated with short-term exposure to arsine. Within the body, arsine is oxidized to other arsenic species. Effects (notably cancer and genotoxic effects) associated with exposure to arsenic and arsenic compounds have been recently reviewed by IPCS (2001a). Arsenic and arsenic compounds are carcinogenic to humans and induce genotoxic effects in experimental systems and in humans.
Arsine (CAS No.
For the determination of arsine in air at the workplace, the air sample is collected in a charcoal tube equipped with a cellulose filter to eliminate aerosols of arsenic compounds. The measurement is done by graphite furnace atomic absorption spectrometry after nitric acid desorption and using nickel as a matrix modifier. The working range of the method is 1–200 µg/m3 in a 10-litre air sample. Commercial continuously recording instruments with specified sensitivity of 1 µg/m3 are available.
Arsine may be formed by microorganisms by biotransformation of non-volatile arsenic compounds, such as arsenites and arsenates.
The main anthropogenic sources of arsine include formation from reaction of acids with reducing metals containing arsenic impurities, which occurs primarily as a by-product of the refining of non-ferrous metals, such as zinc, copper, and cadmium. It is predicted that arsine can be formed in the environment in such places as hazardous waste deposits.
Arsine is extensively used in the semiconductor industry for epitaxial growth of gallium arsenide, as a doping agent for silicon-based solid-state electronic devices, and in the manufacture of light-emitting diodes.
In the environment, arsine is transformed into other arsenic compounds.
There are no reports on concentrations of arsine in the environment, and reports on the levels of occupational exposure to arsine are scarce.
In humans and animals, arsine is absorbed via the lungs and mucous surface of the respiratory tract. After exposure, the concentration of arsine increases rapidly in blood, whereas the distribution to liver, kidneys, and other organs is much slower.
In humans and animals, arsine is metabolized to trivalent arsenic (As(III)) as well as pentavalent arsenic (As(V)). As(III) is methylated to monomethylarsonate and dimethylarsinate. Arsine metabolites are mainly excreted via urine.
The acute toxicity of arsine in different species, including humans, is high. The target organ of arsine poisoning is the haematopoietic system, in particular the erythrocytes. Arsine induces haemolysis, causing haemoglobinuria and subsequent kidney damage; a large number of fatal intoxications in humans have been described, and they continue to occur. The LC50 for mice is 250 mg/m3 for a 10-min exposure. Inhalation of arsine by mice caused an increase in the relative spleen weight after a 6-h exposure to 16 mg/m3 and a decrease in haematocrit value after a 1-h exposure to 30 mg/m3. Histopathological changes observed included haemosiderosis and extramedullary haematopoietic activity in the spleen.
No studies or relevant data are available on the irritant effects of arsine on skin or eyes or on the sensitizing activity of arsine.
Repeated exposure to arsine caused persistent splenomegaly and slight suppression of bone marrow erythroid precursors in rats and mice at concentrations of >1.6 mg/m3 and in Syrian Golden hamsters at concentrations of >8.1 mg/m3. Methaemoglobinaemia was observed in mice exposed to arsine at a concentration of 8.1 mg/m3. In rats, mice, and Syrian Golden hamsters, a lowest-observed-adverse-effect level (LOAEL) for haematological effects of 1.6 mg/m3 was observed. A no-observed-adverse-effect level (NOAEL) was established at 0.08 mg/m3, recognizing that a reversible, compensatory change in mean corpuscular volume (MCV), not considered to be adverse, was observed in mice even at the lowest exposure level studied.
In the only study available, arsine did not induce developmental toxicity in mice or rats at exposure levels that induced splenomegaly.
In humans, arsine induces haemolysis with an increase in plasma haemoglobin, iron, and potassium concentrations and subsequent anaemia and kidney damage. No reliable information is available on exposure levels at which these effects occur. Myocardial and pulmonary failures are other causes of death. Severe liver lesion is rare.
Anaemia is observed to varying degrees, accompanied by Heinz-Ehrlich corpuscles and increased leukocytosis. Haemoglobin, haemosiderin, erythrocytes, proteins, and casts are found in the urine.
Effects of long-term exposure to low levels of arsine are not well characterized. There are no data on the carcinogenicity or mutagenicity of arsine to humans or experimental animals. Exposure to other arsenic compounds to which arsine is metabolized can induce lung, bladder, kidney, and skin cancer in humans.
From the NOAEL of 0.08 mg/m3, using an overall uncertainty factor of 300 and adjusting for the exposure pattern, a guidance value of 0.05 µg/m3 can be derived.
Arsine (CAS No.
The conversion factors2 for arsine in air (20 °C, 101.3 kPa) are as follows:
1 mg/m3 = 0.309 ppm
1 ppm = 3.24 mg/m3
Numerous methods have been developed to determine arsine in atmospheric and workplace air (i.e., in workers’ breathing zones). The most widely applied include colorimetric methods, spectrophotometry (Mazur et al., 1983), graphite furnace atomic absorption spectrometry (Denyszyn et al., 1978; NIOSH, 1985, 1994), and X-ray fluorescence spectrometry (Keech & West, 1980).
The method of Mazur et al. (1983) is based on the reaction of arsine with molybdenum reagent and determination of the blue-coloured reaction product by spectrophotometry at a wavelength of 800 nm. The air sample is collected into a bubbler with potassium permanganate and sulfuric acid. The detection limit is 0.05 mg/m3. However, this method does not allow specific analysis of arsine.
In the graphite furnace atomic absorption spectrometric methods, Matsumura (1988) used synthetic resin active carbon for the sample collection, whereas Denyszyn et al. (1978) and NIOSH (1985, 1994) used activated charcoal. All three desorbed arsine in nitric acid and analysed it with graphite furnace atomic absorption spectrometry, using nickel as a matrix modifier. NIOSH (1985, 1994) used a cellulose filter to prevent aerosols of arsenic compounds from entering the adsorbent. The limits of detection were 1 µg/m3 for the method of Matsumura (1988) and 2 µg/m3 in a 15-litre air sample for the method of Denyszyn et al. (1978); the working range of the NIOSH (1985, 1994) method was 1–200 µg/m3 in a 10-litre air sample.
The content of arsine in workplace air can be determined by X-ray fluorescence spectrometry. Arsine gas was collected on a filter paper impregnated with silver nitrate and then subjected to X-ray fluorescence analysis. The limit of detection of the method for a 60-litre air sample was 4 µg/m3 (Keech & West, 1980).
Commercial continuously recording instruments with specified sensitivity of 1 µg/m3 are available (G. Franz, personal communication, 2002).
Arsenic concentrations in urine, blood, hair, and nails have been used for the biological monitoring of arsenic exposure. However, these measures are not specific to arsine exposure, and exposure to other arsenic species may confound the findings. Extensive description of these methods has been presented in the Environmental Health Criteria document on arsenic (IPCS, 2001a).
Arsine may be generated from other arsenic compounds by some fungi and bacteria (Cheng & Focht, 1979; Tamaki & Frankenberger, 1992). Cheng & Focht (1979) studied the production of arsine from some arsenical substrates (arsenate, arsenite, monomethylarsonate, and dimethylarsinate) added to three different soils. Both Pseudomonas and Alcaligenes reduced arsenate and arsenite to arsine under anaerobic conditions. Many microorganisms isolated from anaerobic sewage sludge have also been reported to produce arsine (Michalke et al., 2000). However, the liberation of arsine from dimethylarsinate and monomethylarsonate from silty clay was a very minor degradation pathway (<0.4% in 70 days) compared with mineralization, which was the major degradation pathway for these arsenic species (3–87%) (Gao & Burau, 1997).
The main anthropogenic sources of arsine include its accidental formation, particularly in the chemical and non-ferrous (e.g., zinc, copper, and cadmium) metallurgical industries, and production or use of the gas itself during manufacture of semiconductors as a doping agent (Wald & Becker, 1986; Badman & Jaffe, 1996; Aposhian, 1997; Winski et al., 1997) and in battery production as an alloy with lead (Wald & Becker, 1986). Arsine intoxications are often unexpected, since even very small amounts of arsenic present as an impurity can produce dangerous quantities of arsine (Johnson, 1953). Arsine may also be produced in the use of lead and batteries (Marr & Smaga, 1987). It is predicted that arsine can be formed in the environment in such places as hazardous waste deposits.
In investigating a possible relationship between exposure to arsine and sudden infant death syndrome, Richardson (1990) detected the generation of arsine from polyvinyl chloride-covered cot mattresses. The fungus Scopulariopsis brevicaulis could be cultivated from all mattresses studied, and it was suggested that 10,10'-oxybisphenoxyarsine used in plasticized polyvinyl chloride as the preservative was converted to arsine by these arsine-tolerant organisms (Richardson, 1990).
Arsine is produced commercially by the reaction of aluminium arsenide with water or hydrochloric acid or as a result of electrochemical reduction of arsenic compounds in acid solutions. Arsine is manufactured in the USA, Belgium, Italy, and Germany (IARC, 1980).
Arsine is extensively used in the semiconductor industry for epitaxial growth of gallium arsenide and as a dopant for silicon-based electronic devices (Zukauskas & Gavriusinas, 2002). Arsine is also used in organic synthesis (Lewis, 1993), as an agent in the manufacture of light-emitting diodes, and for manufacturing certain glass dyes (HSDB, 1999). Arsine has been investigated as a chemical warfare agent, but no such use has been documented (Lewis, 1993; Suchard & Wu, 2001).
No data are available on the environmental transport or distribution of arsine between media. Arsine decomposes on exposure to light or when it comes into contact with moisture in the air, depositing shiny black arsenic (Windholz, 1983); in water, it rapidly hydrolyses to other arsenic compounds (HSDB, 1999).
No data are available on the occurrence of arsine per se in the environment. Environmental levels of arsenic can be found in IPCS (2001a).
No quantitative data are available on arsine exposures except at work; even information on occupational exposures is very limited. Table 1 illustrates a number of workplace conditions and localities where exposures to arsine have occurred, usually at levels sufficient to cause acute arsine intoxication. Studies where information is available on exposure levels are briefly described below.
Table 1: Workplace conditions that may lead to arsine exposure.a
|
Workplace |
Particular conditions |
References |
|
Slag-washing plant |
Process of washing arsenic-contaminated slag during copper–aluminium alloy production. From this slag, arsine can be generated by: - hydrolysis of aluminium arsenide; - the production of nascent hydrogen by finely separated aluminium in an alkaline medium; or - an electrolytic action between the revolving iron drum and the aluminium. |
Kipling & Fothergill, 1964 |
|
Chemical manufacturing company |
An antifreeze concentrate containing water, caustic, and sodium arsenate was loaded into an aluminium tank. A worker periodically placed his head into the dome opening to check the fill level. Arsine was evolved in a reaction between antifreeze concentrate and the aluminium tank. |
Konzen & Dodson, 1966 |
|
Trucking company |
An aluminium tank trailer was cleaned with a phosphoric acid solution. The tank had been used previously for storage of sodium arsenite solution. Most probably, arsenic was deposited on the aluminium and was converted to arsine when acid was added. |
Elkins & Fahy, 1967 |
|
Handling and transporting cylinders containing arsenic compounds |
A solution containing sodium hydroxide and arsenic trioxide was removed from an aluminium tank by workers who entered the tank and flushed out the residue with water for about 30 min. Most probably, arsine was evolved in the reaction of the aluminium wall of the tank with sodium hydroxide and arsenic trioxide. |
Muehrcke & Pirani, 1968 |
|
Chemical plant, in which a herbicide was synthesized by reacting methyl chloride with sodium arsenite |
An aluminium ladder was placed into a tank with a moist mixture containing sodium arsenite. The three ingredients necessary for the evolution of arsine gas were present in the tank: arsenic, water and/or acid, and aluminium. The workers saw "bubbling" at the foot of the ladder. |
De Palma, 1969 |
|
Copper smelting and refinery |
A galvanized bucket was accidentally used instead of a plastic one to transfer sulfuric acid containing both arsenic and antimony impurities. It was estimated that the interior of the galvanized pail could produce 2300 mg of arsenic (in the form of arsine) as a result of reaction with sulfuric acid solution. |
Pinto, 1976 |
|
"Bronzing" process |
During the technological process of bronze plating, the alloy containing zinc was by mistake placed in the bronzing solution, instead of brass. The solution was composed of arsenic and ferric chlorides in concentrated hydrochloric acid. This reaction led to arsine formation. |
Clay et al., 1977 |
|
Chemical company — cleaning a clogged drain |
Arsine was formed by the action of a drain cleaner containing sodium hydroxide, sodium nitrate, and aluminium chips on an arsenic residue. It is not clear whether the arsenic came mainly from the liquid in the storage tank (1% solution arsenical herbicide) or from arsenic residue in the drain, which had collected arsenic trioxide. |
Parish et al., 1979 |
|
Transistor industry |
The valve on one of the cylinders containing arsine was half opened and leaking during its delivery to a semiconductor division and the process of unloading the cylinders. |
Kleinfeld, 1980 |
|
Zinc metallurgical plants |
Arsine was generated in a zinc refining furnace and during furnace repair. |
Braszczyńska et al., 1983 |
|
Blackening operations on zinc/aluminium alloy parts |
Arsine was formed when zinc/aluminium alloy parts ware treated with acid solutions. |
Marchiori et al., 1989 |
|
Transmission repair shop |
Aluminium transmission casings of trucks used for arsenical herbicide applications were cleaned in a hot acidic detergent bath. Arsenic-containing pesticides were deposited on the casings after spraying. The acidic detergent caused rapid evolution of arsine gas. |
Risk & Fuortes, 1991 |
|
Ferrous metal foundry |
An immediate increase of arsine concentration was recorded when hot dross, which contained arsenical impurity, was coated with water. |
Mora et al., 1992 |
|
Burnishing of metals |
Small zinc–tin alloy components were treated with solutions containing hydrochloric acid and arsenous anhydride. Arsenic compounds were reduced to arsine in the presence of nascent hydrogen in an acid environment. |
Romeo et al., 1997 |
|
a |
Quantitative data are not available for the incidents described; rather, they are illustrative of situations where arsine exposures have been so high that acute intoxications have been produced. |
Unintentional formation of arsine can occur principally in the metallurgical industry as a result of arsenic contamination of many ores, such as zinc, lead, copper, cadmium, antimony, gold, silver, and tin. When acid comes into contact with these arsenic-bearing ores or metals, arsine is formed (Stokinger, 1981; Suess et al., 1985). Many processes, including electrolytic refining, galvanizing, soldering, etching, lead plating, metal smelting, and extraction, may expose workers to toxic concentrations of arsine.
Workers in the electronics industry using gallium arsenide to manufacture gallium arsenide optoelectronic, microwave, and integrated circuit products are potentially exposed to arsine (Sheehy & Jones, 1993). Arsine has also been demonstrated to be formed from gallium arsenide in the presence of hydrochloric acid (Scott et al., 1989). Evaluation of arsenic and arsine levels was conducted in three electronics industry facilities according to NIOSH Method 6001 (NIOSH, 1985, 1994). Personal samples collected for arsenic (or arsine) analysis indicated that there was exposure to both particulate and gaseous arsenic species; quantification of arsine and other arsenic species was not reported (Sheehy & Jones, 1993).
In a factory involved with bronzing of brass products, the concentration of arsine in the bronzing tank was 2.6 mg/m3. The concentration was similar at the lip of the bronzing tank when the lid was opened and the work was started, but dropped to 0.28 mg/m3 during the work. The concentration in the breathing zone of the bronzing workers was 0.08 mg/m3 (Clay, 1977).
Measurements of arsine in the workroom air breathing zone of a lead battery manufacturing plant found concentrations ranging from non-detectable to 49 µg/m3 (8-h time-weighted average). The greatest arsine exposure was recorded during electrical formation as a result of contact between lead–arsenic alloy and battery acid (Landrigan et al., 1982).
Jones & Gamble (1984) reported arsine concen-trations in five plants involved in the manufacturing of lead acid storage batteries. Arsine was detected in the charging and forming areas, where its concentrations in area samples ranged from non-detectable to 0.18 mg/m3 (average 43 µg/m3).
Mora et al. (1992) reported arsine concentrations in a ferrous metal foundry. To determine arsine concentrations in the work environment, continuous measurements were performed using Dräger pipes. The arsine concentration in the dry atmosphere was 0.2–0.8 mg/m3; after water was poured onto the slag, the concentration rose momentarily to 200 mg/m3. The highest concentration in the breathing zone of the workers, as a 1- to 1.5-h average, was 0.081 mg/m3.
Arsine poisoning was suspected in some workers employed at a leaching operation for the recovery of cadmium as a secondary output of zinc smelter operations. Several tests for arsine in the ambient air were positive. Urinary arsenic concentrations in workers who were suspected to be arsine poisoned were 0.6, 0.7, 1.0, 1.0, 1.0, and 2.0 mg/litre. The urinary arsenic levels of the employees on other shifts were usually between 0.05 and 0.20 mg/litre (for health effects observed in this study, see section 9.2) (Johnson, 1953).
Uldall et al. (1970) reported a case of acute poisoning of three workers employed in the metallurgical industry that involved the cleaning of tanks with zinc sulfide solution. Arsine was probably released when the tank was opened and zinc added. Arsenic levels were 1.6–8.2 mg/kg in nails and 2–8 mg/kg in hair of the poisoned workers. Hair arsenic concentration was measured in one non-exposed person from the same factory and was 1 mg/kg. Although the concentrations were measured after an acute poisoning, previous incidents of arsine poisoning had been observed in the same facility, and it is likely that exposure to arsine was not limited to a single short-term incident.
Only limited quantitative information is available on the kinetics and metabolism of arsine in experimental animals and humans. It should be pointed out that tissues and urine were mostly analysed for total arsenic in older human studies.
In an old but carefully controlled study, an average of 64% of inhaled arsine was absorbed in mice exposed by inhalation at concentrations of 25–2500 mg/m3 for periods ranging from 0.40 min to 24 h (Levvy, 1947).
Blair et al. (1990b) measured the arsenic content in liver after exposure of rats (both males and females) to arsine at concentrations of 0.08, 1.6, and 8.1 mg/m3 for 6 h/day for 90 days. The arsenic concentration in liver increased with airborne arsine concentration and was higher in females than in males. The arsenic level in liver 3–4 days after a 90-day exposure at the concentration of 8.1 mg/m3 was 6–8 µg/g (compared with approximately 1.5 µg/g in the controls).
After arsine exposure of rabbits, high arsenic concentrations were found in liver, lungs, and kidneys. Following a 5-min exposure at 940 mg arsine/m3, the following arsenic levels were measured: 4.6 mg/kg (liver, wet weight), 3.6 mg/kg (lungs), and 1.2 mg/kg (kidneys). The corresponding values for arsenic concentrations following a 20-min exposure to arsine at 960 mg/m3 were 22 mg/kg (liver), 9.9 mg/kg (lungs), and 8.9 mg/kg (kidneys) (Levvy, 1947).
The main route of arsine excretion is via urine after metabolism. The elimination of arsenic in arsine-exposed mice was compared with that resulting from exposure to sodium arsenite. It was found that arsenic from arsenite administered intravenously was excreted monoexponentially; after 24 h, less than 10% of the dose remained. On the other hand, arsenic arising from arsine (inhalation exposure to 180 mg/m3 for 20 min) was excreted more slowly; after 24 h, about 45% remained in the exposed mice (Levvy, 1947).
When rats were exposed to 4–80 mg arsine/m3 for 1 h, the major metabolites were As(III), As(V), monomethylarsonate, and dimethylarsenate. Importantly (see section 7.2), no arsenobetaine was observed. As the exposure exceeded 60 mg/m3, the proportional urinary excretion increased, indicating saturation of arsine/arsenic binding (Buchet et al., 1998).
Arsine is rapidly absorbed into blood through the respiratory tract; quantitative information in humans is not available (Thienes & Haley, 1972; Venugopal & Luckey, 1978).
In the first days after arsine poisoning, arsenic can be detected in blood. In persons fatally poisoned with arsine, the highest quantities of arsenic were found in liver, kidney, and spleen, and smaller amounts of arsenic were also found in hair of workers occupationally exposed to arsine (Łazariew, 1956).
The presence of arsenic was detected in the tissues, blood, and urine of workers in the petroleum industry who were poisoned with arsine. In cases of fatal poisoning, the following arsenic levels were found: in lungs, 400 µg/litre (probably means µg/kg); in urine, 260 µg/litre; and in blood, 434 µg/litre. No arsenic was detected in the gastric contents. Neither doses nor exposure times were specified in this study (Teitelbaum & Kier, 1969).
In a fatal case of arsine poisoning involving a worker at a zinc plant, arsenic was found in the liver at a concentration of 11.8 mg/g, in the spleen at 7.9 mg/g, in the kidneys at 3.2 mg/g, in the brain at 0.6 mg/g, and in the urine at 0.6 mg/g; trace amounts were also found in blood (Fowler & Weissberg, 1974).
The analysis of a case of lethal arsine poisoning showed the presence of arsenic in the liver at concentrations as high as 15 mg/kg; concentrations above 1 mg arsenic/kg were also found in kidneys and spleen (Macaulay & Stanley, 1956).
Inhaled arsine was rapidly dissolved in body fluids and oxidized to As(III) (Pershagen et al., 1982). Part of As(III) is further oxidized to As(V), as indicated by the appearance of As(V) in urine of humans exposed to arsenic 1–2 days following exposure (Romeo et al., 1997). Trivalent arsenic is methylated to monomethylarsonate and dimethylarsinate.
Various forms of arsenic are excreted mainly via urine (about 60% in 24 days) (Apostoli et al., 1997). Information on possible sequestration of arsenic in the human body after exposure to arsine is not available. After an acute occupational intoxication with arsine, the highest urinary excretion occurred in the first 5 days after exposure. The urinary clearance equalled 7.8 ml/h per kg body weight; through other routes (non-renal excretion), it was 5.27 ml/h per kg body weight. As in experimental animals, after exposure to inorganic arsenic species, the following arsenic species are usually found in urine: dimethylarsinate, monomethylarsonate, As(III), and, to a lesser extent, As(V) (Apostoli et al., 1997; Romeo et al., 1997). Apostoli and co-workers (1997) also reported the presence of arsenobetaine in the urine of a person after an acute arsenic poisoning; it is not excluded, however, that this was from dietary origin.
In a driver who was poisoned when unloading cylinders containing arsine (approximate exposure time about 1–2 min), the level of total arsenic in his urine amounted to 0.72 mg/litre on the day of exposure, decreasing to 0.01 mg/litre by the fourth day (Kleinfeld, 1980).
Parish et al. (1979) described acute arsine poisoning in two workers employed at cleaning clogged drains who used a cleaner containing sodium hydroxide and aluminium chips. Both workers were hospitalized because of rapid acute haemolytic anaemia and kidney failure. Arsenic concentrations in the urine of the patients, analysed within 3 days after the incident (but after exchange transfusion and dialysis), were 0.85 and 0.97 mg/kg, and in blood, 0.18 mg/kg and 0.20 mg/kg; in two persons working near the gutter, arsenic was found at concentrations of 0.30 and 0.12 mg/kg in urine and <0.082 and <0.96 mg/kg in blood.
Arsenic concentrations in urine, blood, hair, and nails have been used for the biological monitoring of arsenic exposure. However, these measures are not specific to arsine exposure, and exposure to other arsenic species may confound the findings (Vahter, 1988; Aitio et al., 1996).
The arsenic concentration in urine has been conventionally used as an indicator of occupational exposure to arsine. Positive correlations between urinary elimination of arsenic in individuals exposed to arsine and the level of their occupational exposure have been reported (Elkins & Fahy, 1967; Landrigan et al., 1982; Apostoli et al., 1997). Speciation of arsenic in the urine is a prerequisite for the meaningful interpretation of the results in biological monitoring (Aitio et al., 1996).
A close relationship was observed between urinary arsenic concentrations and arsine exposure level (N = 47, r = 0.84, P = 0.0001) among employees at a lead acid battery manufacturing factory (Landrigan et al., 1982). From this correlation, it was estimated that concentrations of arsine in air above 15.6 µg/m3 are associated with total arsenic concentrations in urine in excess of 0.67 µmol/litre.
Blood arsenic concentration is a poor indicator of exposure to arsine. Since most arsenic compounds contained in human blood disappear quickly, the arsenic level in blood reflects exposure to arsine for a short period only (Romeo et al., 1997).
Since the trivalent form of arsenic accumulates in hair, as a result of its binding to the thiol group of the scleroprotein keratin, the arsenic content in hair may be used for monitoring long-term exposures to arsine (compared with the arsenic content in urine, which reflects relatively short-term exposures only). The determination of arsenic levels in hair as an indicator of exposure to arsenic in the atmosphere is limited, however, since there is no reliable method to distinguish hair arsenic adsorbed as a result of external exposure from that absorbed and metabolized in the individual.
Increased arsenic concentrations in pubic hair and toenails were observed in individuals exposed to arsenic and arsine in workplace atmospheres. Two cases of arsine poisoning were reported in workers employed at bronzing of brass products, where the arsine level in the breathing zone of the bronzing workers was 0.08 mg/m3 for sampling times of 35–40 min (Clay et al., 1977). The concentrations of arsenic were 10.8–76.1 mg/kg in hair, 8.6–63.1 mg/kg in pubic hair, 15–188 mg/kg in fingernails, and 37.1–60.9 mg/kg in toenails. In workers employed at bronzing of brass products in other departments, where no cases of arsine poisoning had been reported, arsenic concentrations were lower: 3.33–16.0 mg/kg in hair, 5.61–38.5 in pubic hair, 9.03–53.3 mg/kg in fingernails, and 1.06–5.79 in toenails. In adults not exposed to arsenic at work, concentrations of arsenic in hair are usually <1 mg/kg, whereas those in nails are <3 mg/kg (Vahter, 1988).
The acute toxicity of inhaled arsine is summarized in Table 2, and the relationship between the LC50 value and duration of exposure is shown in Table 3.
Table 2: Acute toxicity of inhaled arsine in different species.
|
Species |
Parameter |
Exposure concentration |
Duration of exposure (min) |
|
Rat |
LC50 |
390 |
10 |
|
Mouse |
LC50 |
250 |
10 |
|
Dog |
LC50 |
350 |
30 |
|
Rabbit |
LC50 |
650 |
10 |
Table 3: Dependence of acute inhalation LC50 of arsine on the duration of exposure in mice. a
|
LC50 (mg/m3) |
Duration of exposure (min) |
|
2500 |
0.40 |
|
1000 |
1.18 |
|
500 |
2.4 |
|
250 |
12 |
|
100 |
50 |
|
25 |
1440 |
a From Levvy (1947).
The acute toxicity of arsine in animals is attributable chiefly to its haemolytic effects. Arsine also induces haemolysis in vitro (see section 8.6); approximately 20% haemolysis was observed after a 2-h incubation of rat and dog erythrocytes in the presence of 0.56 mmol arsine/litre (Hatlelid et al., 1995).
Morgan (1992) studied the acute toxicity of arsine in Fischer-344 rats, B6C3F1 and C57BL/6 mice, and Syrian Golden hamsters. One hundred per cent mortality was observed in all these species when they were exposed for 6 h to 81 mg arsine/m3.
Peterson & Bhattecharyya (1985) studied the haematological responses of mice to exposure for 1 h at 16–84 mg arsine/m3. The decrease in haematocrit at 24 h was linear with increasing exposure concentration and statistically significant for exposures >30 mg/m3; the changes in numbers of erythrocytes paralleled changes in haematocrit.
In B6C3F1 mice, no changes in body weight gain were observed in either sex exposed to arsine by inhalation for 6 h at concentrations of 1.6, 8.1, or 16 mg/m3. Significant exposure-related increases in relative spleen weights occurred in both sexes in all exposure groups. Female mice exposed to 16 mg arsine/m3 for 6 h had significantly enlarged spleens after 2 days (Blair et al., 1990b).
In repeated-exposure studies in animals, similarly to acute arsine poisoning, blood is mainly affected, as shown by haemolysis, decrease in erythrocyte count, and changes in haemoglobin level (Stokinger, 1981).
A series of studies at the National Institute of Environmental Health Sciences in the USA (Hong et al., 1989; Rosenthal et al., 1989; Blair et al., 1990a,b,), in which mice, rats, and Syrian Golden hamsters were exposed to arsine by inhalation for 1–90 days, forms the basis of the hazard characterization of arsine (see section 10.1.1).
In a study in mice, in which only peripheral blood was studied as the end-point, mice were exposed for 6 h/day, 5 days/week, for 5–90 days to arsine at concentrations of 0.08, 1.6, or 8.1 mg/m3 (Blair et al., 1990a). Moderate anaemia was observed at the highest exposure level, and the reticulocyte count, MCV, and mean corpuscular haemoglobin (MCH) were increased. With continued exposure, the anaemia was less severe, and the compensatory increase in the reticulocyte count was greater. An increased concentration of methaemoglobin was observed in animals exposed to 8.1 mg arsine/m3. In this study, the NOAEL was 1.6 mg/m3, and the LOAEL was 8.1 mg/m3 (Blair et al., 1990a).
Blair et al. (1990b) exposed B6C3F1 mice (14 or 90 days), Fischer-344 rats (14, 28, or 90 days), and Syrian Golden hamsters (28 days) of both sexes to arsine with a regimen of 6 h/day, 5 days/week. The short-term experiments were performed with arsine at concentrations of 1.6, 8.1, and 16 mg/m3. In the 90-day studies, arsine at 0.08, 1.6, and 8.1 mg/m3 was used.
Histopathological studies in all species on 29 (males) or 31 (females) organs revealed effects on the bone marrow, liver, and spleen only. A decrease of body weight gain was observed only in male rats exposed to 16 mg arsine/m3 for 28 days. Inconsistent increases in liver weight were observed at exposures of >8.1 mg/m3.
In rats, significant dose-related increases in relative spleen weights were recorded in both sexes (35% in females and 20% in males at 1.6 mg/m3 after 90 days) in groups exposed to arsine at >1.6 mg/m3. Increased haemosiderosis and splenic haematopoiesis and medullary hyperplasia were observed in rats exposed to 16 mg/m3.
Anaemia (reduced haemoglobin, haematocrit, red blood cell count) was observed in all exposed groups in females (4–18% drop in haemoglobin at exposure levels of 0.08–8.1 mg/m3) (but was not observed in two other groups with the low exposure after a recovery period of 3 or 4 days) and in males at exposure levels of >1.6 mg/m3 (7–16% drop in haemoglobin). MCV, MCH, and erythrocyte aminolevulinate dehydratase activity were elevated in rats exposed to >1.6 mg/m3 (indicating a compensatory increase in the proportion of reticulocytes in blood) (Blair et al., 1990b).
Blair et al. (1990b) concluded that a significant anaemia was observed at the lowest exposure level (0.08 mg/m3). This conclusion is based on a decrease of haemoglobin, red blood cell count, and haematocrit of 4–5% (P < 0.05) in female (but not in male) rats exposed for 80–81 days to arsine. However, when a group of female rats was exposed for 90 days and studied 3 days later, no effect on haematocrit was observed. Similarly no effect was observed in a further group exposed for 90 days and analysed after 4 days. Thus, 0.08 mg/m3 is considered to be a NOAEL rather than a LOAEL in rats; the LOAEL is 1.6 mg/m3.
In mice, significant dose-related increases in relative spleen weights were recorded in males exposed to arsine at >1.6 mg/m3 (20–30% at 1.6 mg/m3). In females, the same was observed after 14 days of exposure; after 90 days of exposure, the increase in spleen weight was significant only for the highest exposure studied, 16 mg/m3 (for the groups studied after 3 days of recovery and after 4 days of recovery). Consistent decreases in packed cell volume were observed in animals exposed to >8.1 mg/m3. Increases in aminolevulinate dehydratase activity were first seen in animals exposed to 1.6 mg/m3 and consistently seen in animals exposed to >8.1 mg/m3. Intracanalicular bile stasis in the liver was observed in mice exposed to 8.1 mg/m3 for 90 days (highest dose studied for 90 days).
In a mechanistic study on female mice (for results of the mechanistic studies, see below) of the same strain, using a similar exposure pattern (14-day inhalation of 1.6, 8.1, or 16 mg/m3 or 12-week inhalation of 0.08, 1.6, or 8.1 mg/m3, 6 h/day, 5 days/week) (Hong et al., 1989), anaemia was similarly observed in animals exposed to 8.1 mg/m3 (highest exposure studied) for 2 or 12 weeks; this effect subsided in 2 weeks. After an exposure of 12 weeks to 0.08 mg/m3, a 2% (P < 0.05) increase in MCV was also observed. In contrast to the study of Blair et al. (1990b), an increase in the absolute (24%) and relative (25%) spleen weights (P < 0.05) was observed after a 12-week exposure (and 3-day recovery) to the lowest concentration, 0.08 mg/m3; the spleen weight returned to normal in this group after a further 17-day recovery period. It should be noted that the spleen weight varied markedly in different control groups in the experiment and was low in the referent group for the group with a 12-week exposure and 4-day recovery (but returned to the common level during the 17-day additional recovery period).
In the mechanistic parts of this study, bone marrow cellularity, granulocyte-macrophage progenitors (CFU-GM), colony-forming unit erythroids (CFU-E) (Hong et al., 1989), and relative abundance of different lymphocyte populations (Rosenthal et al., 1989) were studied. No changes were observed in bone marrow cellularity. CFU-GM showed a >8% dose-dependent decrease after a 14-day exposure to >1.6 mg/m3 (i.e., lowest exposure studied), which was no longer observed after a 12-week exposure. CFU-E showed a similar >11% dose-dependent decrease after exposure to >1.6 mg/m3, which was reduced in magnitude but did not completely disappear when exposure was continued for 12 weeks.
There was no effect on the total number of splenic lymphocytes, but the percentage of lymphocytes of all cells fell in all arsine-exposed groups (from 83.4% in controls to 45.6% in the 16 mg/m3 group). Splenic T-cell percentage was similarly decreased in all arsine-treated groups, but B cells were decreased in the high-dose group only in the 14-day study (Rosenthal et al., 1989).
As the compensatory, reversible 2% increase in MCV (Hong et al., 1989) is not considered adverse and the observed effect on spleen weight at 0.08 mg/m3 in the Hong et al. (1989) study is in striking contrast to the findings of Blair et al. (1990b) (no similar effect at a 20 times higher dose), it is concluded that 0.08 mg/m3 is a NOAEL, rather than a LOAEL, and the LOAEL is 1.6 mg/m3 in mice.
In Syrian Golden hamsters, significant dose-related increases in relative spleen weights were recorded in both sexes exposed to >8.1 mg/m3, and packed cell volume and aminolevulinate dehydratase activity in the erythrocytes showed dose-dependent decreases from the lowest exposure level studied (1.6 mg/m3). The spleen was enlarged and dark in animals exposed to >8.1 mg arsine/m3. A LOAEL of 1.6 mg/m3 is deduced for Syrian Golden hamsters from these studies; as this was the lowest exposure investigated, a NOAEL cannot be identified (Blair et al., 1990b).
In old studies at higher arsine concentrations (Nau, 1948; Łazariew, 1956), haemolytic anaemia with
a dose–response relationship was reported in dogs and guinea-pigs.No data were identified on the carcinogenicity of arsine in experimental animals. In a study only published as an abstract, administration of sodium arsenate (500 µg/litre) in drinking-water to C57Bl/6J mice for 26 months led to an increased incidence of tumours in the intestinal tract, lungs, liver, and, to a smaller extent, other organs. Administration of dimethylarsinate in drinking-water induced a dose-dependent increase in the incidence of urinary bladder tumours in rats (IPCS, 2001a).
No relevant data were available on skin or eye irritation or on sensitizing effects of arsine on skin or respiratory tract in animals.
Swiss (CD-1) mice and Fischer-344 rats were exposed to arsine by inhalation at concentrations of 0.08, 1.6, or 8.1 mg/m3 from gestation days 6 through 15. In rats, splenomegaly (in the 8.1 mg/m3 group) and decreased packed red cell volume were detected in dams. No developmental toxicity was observed. In mice, dams dosed with 8.1 mg arsine/m3 developed significant splenomegaly, whereas the number of living fetuses, mean fetal body weight, and the percentages of resorptions and malformations per litter did not differ from the findings in controls (Morrissey et al., 1990).
No studies are available regarding the genotoxicity of arsine per se. Inorganic arsenic does not induce point mutations, but it does induce chromosomal abnormalities in vitro, including changes in structure and number of chromosomes, endoreduplication, and sister chromatid exchange, and it affects methylation and repair of DNA. Limited studies indicate that arsenite is also clastogenic in vivo (IPCS, 2001a).
The mechanisms of toxicity and modes of action of arsine in humans and animals have not been fully elaborated. While some authors have postulated an oxidative stress (Pernis & Magistretti, 1960; Blair et al., 1990a; Hatlelid et al., 1995, 1996; Hatlelid & Carter, 1997), others have suggested a mechanism dependent on reaction with sulfhydryl groups (Levinsky et al., 1970; Winski et al., 1997).
Hatlelid & Carter (1997) postulated that the haemolytic activity of arsine is connected with oxidative stress through the formation of hydrogen peroxide and arsine adducts with haemoglobin, according to the following sequences:
H2As–H + HbO2 --> H2As* + Hb–O2–H
These products may then react to form methaemoglobin and arsine peroxide, or, alternatively, the reaction may produce hydrogen peroxide and an arsenic adduct such as H2As–Hb or H2As–haem:
H2As* + Hb–O2–H --> adduct + H2O2
Such an adduct may probably damage haemoglobin molecules, leading to the rapid denaturation and precipitation of the proteins (Hatlelid & Carter, 1997).
The denaturation of proteins and the aggregation of precipitated molecules and their binding to the inner surface of red blood cells (Heinz bodies) lead to a redistribution of membrane proteins and increased membrane rigidity. Oxidation of the haem iron induces the formation of haemin, which results in the oxidation of membrane protein sulfhydryl groups, dissociation of membrane skeletal protein, and perturbation of membrane ion gradients (Hatlelid et al., 1996). Ultimately, the Heinz bodies and haemins increase the fragility of red blood cell membranes and predispose the cells to fragmentation (Weed & Reed, 1966). In this manner, the degradation of haemoglobin may lead to haemolysis.
Some studies have suggested that the sulfhydryl groups of glutathione prevented haemoglobin oxidation and in this manner are essential for the maintenance of the intact erythrocyte structure (Blair et al., 1990a). In in vitro studies, a decrease in reduced glutathione concentration in human red blood cells was found to correlate with the haemolytic action of arsine (Pernis & Magistretti, 1960). Blair et al. (1990a) recorded a 60% decrease in reduced glutathione level in erythrocytes exposed to arsine in vitro. However, later studies of Hatlelid et al. (1995) showed that the depletion of reduced glutathione in red blood cells in dogs neither preceded nor coincided with haemolysis.
According to the hypothesis of the sulfhydryl-dependent mechanism of arsine toxicity (Levinsky et al., 1970), arsine reacts with the sulfhydryl group of Na+K+-ATPase, causing an impairment in the sodium–potassium pump mechanism, with subsequent red cell swelling and haemolysis. The affinity of trivalent arsenic for the sulfhydryl group is well known. However, dog erythrocytes, which do not have Na+K+-ATPase, were haemolysed when they were exposed to arsine (Hatlelid et al., 1995), which may suggest that Na+K+-ATPase is only partially responsible for red cell damage (Hatlelid et al., 1995; Winski et al., 1997).
Winski et al. (1997) observed immediate profound abnormalities in membrane ultrastructure and in red blood cell volume, which were manifested by potassium leakage, sodium influx, and increases in haematocrit in arsine-exposed red cells. Additionally, ATP levels did not significantly decrease, and ATPase was not inhibited by arsine. Winski et al. (1997) then suggested that haemolysis in arsine-exposed red cells depended on membrane disruption caused by arsine–haemoglobin metabolites, which are the ultimate toxic species.
Although it is generally held that the kidney failure in arsine intoxication is due to the effects of free haemoglobin and degradation products, arsine has also been demonstrated to have a direct toxic effect on kidney glomeruli and tubules (Ayala-Fierro et al., 2000).
The toxicity to humans of arsine was demonstrated dramatically in 1815 when a German chemist, in the course of an experiment, inhaled arsine vapour, became ill within an hour, and soon died (Vallee et al., 1960). Following this demonstration of the toxicity of arsine, 454 cases of poisoning were reported by 1974 (Elkins & Fahy, 1967; Guajardo et al., 1970; Levinsky et al., 1970; Fowler & Weissberg, 1974; Wilkinson et al., 1975); of 207 cases of arsine toxicity reported between 1928 and 1974 (4.5 cases per year), 25% were fatal (Fowler & Weissberg, 1974). Prior to 1974, anuria was a common cause of death following short-term exposure to high concentrations of arsine. Between 1974 and 1986, there were an additional approximately 30 cases reported (2.5 cases per year), with no fatalities (Guajardo et al., 1970; Hocken & Bradshaw, 1970; Levinsky et al., 1970; Wilkinson et al., 1975; Conrad et al., 1976; Frank, 1976; Pinto, 1976; Levy et al., 1979; Parish et al., 1979; Rathus et al., 1979; Kleinfeld, 1980; Williams et al., 1981; Dlhopolcek et al., 1982; Gosselin et al., 1982; Rogge et al., 1983; Phoon et al., 1984; Togaybayev et al., 1984; Hesdorffer et al., 1986; Wald & Becker, 1986; Marchiori et al., 1989; Hotz & Boillat, 1991; Mora et al., 1992; Pairon et al., 1992; US EPA, 1994a,b; Romeo et al., 1997).
The data on arsine concentrations in the workplace atmosphere are relatively scant (see section 6). A case report indicated that exposure to arsine by inhalation for a few hours at a concentration of 10–32 mg/m3 might induce symptoms of poisoning, whereas exposure to 810 mg/m3 for 30 min might be fatal (Steel & Feltham, 1950). Morse & Setterlind (1950) reported that concentrations of 230–970 mg arsine/m3 were associated with fatalities.
A number of reports indicated that clinical manifestations of arsine intoxication appeared within 1–24 h of exposure (usually within a few hours). The period of latency was dependent on concentration and time of exposure. The initial symptoms included headache, malaise, weakness, dyspnoea, dizziness, abdominal pain, nausea, and vomiting. The urine was dark red, usually 4–6 h following exposure, and jaundice of the skin and mucous membranes was seen usually 24–48 h after exposure (Kipling & Fothergill, 1964; Jenkins et al., 1965; Anthonisen et al., 1968; De Palma, 1969; Guajardo et al., 1970; Levinsky et al., 1970; Fowler & Weissberg, 1974; Rogge et al., 1983). In some cases, hepatomegaly and splenomegaly with tenderness of costovertebral angle, fever, tachycardia, and tachypnoea occur (Klimecki & Carter, 1995). Information on the concentration of arsine in the air or on the duration of the exposure in relation to the effects observed is mostly not available.
Haemolytic anaemia is the most consistent clinical finding in humans (Levinsky et al., 1970; Fowler & Weissberg, 1974; Wald & Becher, 1986). Massive haemoglobinuria may lead to anuria, which, if untreated, is often the cause of death (Uldall et al., 1970; Fowler & Weissberg, 1974; Klimecki & Carter, 1995). Both central and peripheral nervous systems may also be affected (Anthonisen et al., 1968; De Palma, 1969; Risk & Fuortes, 1991). Toxic pulmonary oedema and acute circulatory failure have also been reported as the cause of death in arsine poisoning (Vallee et al., 1960; Stokinger, 1981; Matthews, 1989).
Late consequences of acute arsine poisoning include chronic renal damage (Muehrcke & Pirani, 1968), haematological changes (Muehrcke & Pirani, 1968), polyneuritis (De Palma, 1969; Frank, 1976; Gosselin et al., 1982), and neuropsychological symptoms (e.g., irritation, confusion, memory losses, agitation, and disorientation) (De Palma, 1969; Levinsky et al., 1970).
Muehrcke & Pirani (1968) reported morphological changes in the kidneys of a truck driver with arsine-induced anuria. Six months after arsine poisoning, the patient had anaemia and azotaemia. Twenty-three months after recovery from acute renal failure, interstitial fibrosis was focal, and severe nephrosclerosis with renal insufficiency was present.
Gosselin and co-workers (1982) described reversible polyneuritis of the upper and lower extremities that was observed 3 months after exposure. Peripheral neuropathy was still present 6 months after exposure (De Palma, 1969; Levinsky et al., 1970).
Extreme restlessness, loss of memory, agitation, and disorientation occurred several days after exposure and lasted about 10 days in two patients heavily exposed to arsine (Levinsky et al., 1970).
An increase in total cell count and macrophages in bronchoalveolar lavage was observed in an arsine-exposed worker. Progressive improvement in diffusing capacity of lungs was observed only after 2 months of treatment (Romeo et al., 1997).
Pinto and co-workers (1950) reported that electrocardiographic (ECG) changes in one case lasted for 10 months.
Vertical white lines on the nails (Mee’s lines) were observed in many cases 10 days to 3 weeks following arsine exposure (Vallee et al., 1960; De Palma, 1969; Levinsky et al., 1970).
Mora and co-workers (1992) noted hepatitis in an arsine-poisoned patient on the 20th day after the acute haemolysis.
Long-term exposure may cause symptoms similar to those observed in acutely poisoned individuals (Klimecki & Carter, 1995). The main differences from acute poisoning were in a delay in onset and development of peripheral neuritis (Stokinger, 1981), development of gastrointestinal tract involvement (Mueller & Benowitz, 1989), and development of haemolysis and renal impairment (Kensler et al., 1946; Risk & Fuortes, 1991).
The degree of anaemia in chronic arsine-poisoned workers employed at facilities involving the cyanide extraction of gold was found to be proportional to the duration of exposure to arsine (Bulmer et al., 1940).
Lowered haemoglobin levels were also found in zinc ore smelting workers who were exposed to arsine for long periods and who had urinary arsenic concentrations (analytical method not specified) below 0.2 mg/litre. These urinary concentrations were estimated to correspond to air arsine concentrations below 0.16 mg/m3; the basis for the relationship was not given. Once a special ventilation system had been installed, the haemoglobin levels in the workers gradually returned to their normal values (Johnson, 1953).
Rapidly progressing intravascular haemolysis within a few hours is characteristic of arsine poisoning (Fowler & Weissberg, 1974; Gosselin et al., 1982). The haematological changes in humans were consistent with those observed in experimental animals and included anaemia, damage to red blood cells (such as basophilic stippling, Heinz-Ehrlich bodies, anisocytosis, poikilocytosis, red cell fragments and ghost cells, and reticulocytosis), and leukocytosis, accompanied by increased plasma free haemoglobin, iron, and potassium concentrations (Jenkins et al., 1965; Teitelbaum & Kier, 1969; Levinsky et al., 1970; Fowler & Weissberg, 1974; Wilkinson et al., 1975; Parish et al., 1979; Kleinfeld, 1980; Bogdanicka, 1988; Klimecki & Carter, 1995). In cases of severe poisoning due to arsine exposure, almost complete haemolysis was found, with a haematocrit value of 0 and serum haemoglobin concentration of 70 g/litre (Wilkinson et al., 1975).
Wilkinson et al. (1975) reported a decrease in the number of blood platelets and a decrease in reticulocyte count to a level of less than 1% for a period of almost 2 weeks, followed by an increase to 4.5% after 20 days. After a period of 12 weeks, the haemoglobin level started to increase.
Information on dose–response relationships of arsine-induced haemolysis is limited. In an acute poisoning case with extensive haemolysis (haematocrit drop to 0.25), a urinary level of arsenic as high as 3940 µg/litre and a blood arsenic level of 1150 µg/litre (approximately 24 h after the exposure) were recorded. The urinary arsenic concentration (24 h after the cessation of the exposure) was estimated to correspond to an airborne arsine concentration of approximately 1.6 mg/m3 (Romeo et al., 1997).
In some cases of acute poisoning, free haemoglobin, haemosiderin, erythrocytes, proteins, and casts (Kipling & Fothergill, 1964), as well as methaemoglobin, have been found in the urine (Uldall et al., 1970; Fowler & Weissberg, 1974). Massive haemoglobinuria has led to renal tubule obturation, oliguria, or even anuria (Klimecki & Carter, 1995). Anuria may develop within 2 days after exposure. Untreated renal failure resulting from severe arsine intoxication usually leads to death (Fowler & Weissberg, 1974). Persistent morphological and functional changes in the renal tissue of individuals have been reported (Muehrcke & Pirani, 1968; Teitelbaum & Kier, 1969).
Examination of two arsine-poisoned patients revealed acute oliguric renal failure in one and a moderate reduction of renal function in the other (Pedersen et al., 1968). Renal blood flow was reduced to approximately one-sixth and one-third in the oliguric and the non-oliguric patient, respectively. The changes in the renal blood flow were observed 24 h after the onset of the disease, and the mean circulation time for blood was prolonged and the vascular volume reduced. The reduction in kidney function was related to the degree of severity of the haemolysis (Pedersen et al., 1968).
Muehrcke & Pirani (1968) reported a clinicopathological study and observations over 23 months of kidney damage by arsine in a 32-year-old truck driver who was exposed to arsine formed as a result of the reaction of arsenic in the aluminium wall of the tank he was cleaning with sodium hydroxide. Anuria was observed 24 h after the exposure, but no changes in renal glomeruli were observed in the first week following arsine exposure. During the phase of diuresis restoration, progressive thickenings of the tubular basement membranes in the glomeruli were noted. The most serious damage, however, occurred in the proximal and distal portions of the tubules in the renal cortex, and these changes in the tubules were degenerative, reparative, and regenerative. Cells of the proximal portion of convoluted tubules still did not appear normal 66 days after the exposure. Six months after arsine exposure, the patient had anaemia and azotaemia. Twenty-three months later, focal endothelial thickening and common nephrosclerosis were observed, and the patient suffered from chronic kidney insufficiency.
When anuria develops in arsine poisoning, mortality without dialysis may be as high as 100%. Patients’ survival depends on a correct treatment modality: early blood exchange transfusion and dialysis may be life-saving (Muehrcke & Pirani, 1968; Teitelbaum & Kier, 1969; Hesdorffer et al., 1986).
Severe liver damage has occasionally been found as a consequence of arsine poisoning (Vallee et al., 1960; Anthonisen et al., 1968; Fowler & Weissberg, 1974; Stokinger, 1981; Togaybayev et al., 1984).
Acute jaundice and tenderness of the liver have been noted after 24 h in cases of acute arsine poisoning (Vallee et al., 1960). One month after the exposure in patients who survived arsine poisoning (Parish et al., 1979), alanine transferase levels in the serum were normal. Six months after an exposure, the patient’s liver was palpable and tender, and 12 months after the exposure, the liver was clinically normal (Hocken & Bradshaw, 1970).
In a study of an arsine-poisoned patient, Teitelbaum & Kier (1969) noted increased levels of aspartate aminotransferase, alanine transferase, and lactate dehydrogenase. After the transfusion, haemodialysis, and treatment with dimercaprol, the enzyme levels quickly returned to normal, and it appeared that the elevated level of lactate dehydrogenase was due to release of that enzyme from erythrocyte debris.
Symptoms of effects on the central and peripheral nervous systems include disorientation, chills, convulsion, and paraesthesia, which may appear shortly after exposure at high concentrations (Anthonisen et al., 1968; De Palma, 1969; Risk & Fuortes, 1991).
Following arsine poisoning, symptoms of peripheral neuropathy may occur after latency periods of 10 days (Wilkinson et al., 1975) to 6 months (Frank, 1976). Toxic polyneuropathy and a mild psycho-organic syndrome have been reported in six workers after acute arsine poisoning. The severity of those symptoms was directly related to the time of exposure. Nerve biopsy revealed myelin fragmentation and atrophy of the axon (Frank, 1976).
Wilkinson et al. (1975) reported confusion and disorientation in an arsine-poisoned patient. These symptoms disappeared after exchange transfusion and administration of dexamethasone. On day 10 after the exposure, the patient complained of paraesthesia, burning sensation in the hands and feet, and, later on, muscular weakness in arms and legs. On day 30, the weakness was so severe that the patient was unable to walk. Muscular atrophy involved proximal and distal muscles, but the sensory changes were limited to the periphery of the extremities. Sensory and motor nerve conduction velocity and amplitude were decreased in the upper and lower extremities. The neuropathy started to recede after 7 weeks; however, even as long as after 6 months, the patient had difficulty walking.
Gosselin et al. (1982) additionally reported regressive polyneuritis in the lower and upper extremities in arsine-poisoned individuals 3 months after exposure.
Toxic pulmonary oedema or acute circulatory failure has been reported as the cause of death in arsine poisoning (Vallee et al., 1960; Stokinger, 1981; Matthews, 1989).
Arsine appeared to exert a direct effect on cardiac function (Rosenman, 1979). Pinto et al. (1950) reported that in 13 arsine-poisoned males, laboratory results and autopsy findings suggested that the cause of death among the 4 of 13 exposed workers who died was not anaemia but an acute myocardial failure. Tachycardia and ECG abnormalities (changes in repolarization, such as S-T segment and T-wave changes, reflecting mainly hyperkalaemia) were reported in acute arsine poisoning (Pinto et al., 1950; Konzen & Dodson, 1966; Pinto, 1976; Parish et al., 1979; Togaybayev et al., 1984; Klimecki & Carter, 1995). Among the nine survivors in the case series of Pinto and co-workers (1950), all reached normal state in about 2 weeks, but significant ECG changes persisted for several weeks longer. In one case, definite changes were observed for over 10 months.
No interpretable information is available on the reproductive toxicity of arsine.
There is no reported evidence for the carcinogenic effect of arsine per se in humans.
Exposure to airborne arsenic compounds at work induces cancer of the lungs in a dose-dependent manner; a statistically significant increase in lung cancer risk has been observed after a cumulative exposure of 75 mg/m3 per year, corresponding, for example, to a 15-year exposure to an average airborne arsenic concentration of 50 µg/m3 (IPCS, 2001a).
Exposure to inorganic arsenic in drinking-water causes cancer of the lung, kidney, bladder, and skin in humans; elevated risk of lung and bladder cancer has been observed in cohorts whose drinking-water arsenic concentration was, respectively, 30–50 and 10–50 µg/litre (IPCS, 2001a).
The information presented in this CICAD on arsine focuses on effects associated with short-term exposure. Within the body, arsine is oxidized to other arsenic species. Effects (notably cancer and genotoxic effects) associated with exposure to arsenic and arsenic compounds have been recently reviewed by IPCS (2001a). Arsenic and arsenic compounds are carcinogenic to humans and induce genotoxic effects in experimental systems and in humans.
The target organ of arsine poisoning is the haematopoietic system, in particular the erythrocytes. Arsine induces haemolysis, causing haemoglobinuria and sub-sequent renal damage; a large number of fatal intoxications have been described, and they continue to occur.
There are no data on the carcinogenicity of arsine in humans or in experimental animals. However, arsine is oxidized to the same trivalent and pentavalent forms of arsenic as those seen after drinking-water or inhalation exposure to arsenic compounds known to present a cancer hazard.
There are no data on the genotoxicity of arsine. Arsine oxidation products do not induce point mutations in bacteria or mammalian cells but are clastogenic in vitro and in experimental animals and humans in vivo.
The results of a 13-week rat and mouse and a 28-day hamster inhalation study (Blair et al., 1990a,b) and a 12-week mouse inhalation study (Hong et al., 1989) indicated that there were no qualitative differences in the effects produced by arsine in mice, rats, and Syrian Golden hamsters. These studies indicated that the most sensitive end-points of arsine exposure were increased haemolysis, abnormal red blood cell morphology, increased spleen weight, and impaired compensatory erythropoiesis; these effects resulted in splenic changes due to increased removal of damaged red blood cells and increased splenic haematopoiesis.
In rats, significant dose-related increases in relative spleen weights were recorded in both sexes exposed to arsine at >1.6 mg/m3. Increased haemosiderosis and splenic haematopoiesis and medullary hyperplasia were observed at exposure to 16 mg/m3. In males rats, anaemia was observed at exposure levels of >1.6 mg/m3. In female rats, a 4–5% reduction in the haemoglobin, haematocrit, and red blood cell count was observed after 80–81 days of exposure in the low exposure group (0.08 mg/m3). However, no decrease in haematocrit was observed in the two low-exposure female groups studied at the end of the experiment, i.e., after 90 days of exposure and 3 or 4 days of recovery. Because of the lack of reproducibility of the findings, uncertainty of the clinical significance of the findings at the exposure level of 0.08 mg/m3, and flat dose–response curve (less than 10% effect on haematocrit, even at a 20 times higher exposure level), 0.08 mg/m3 is considered to be a NOAEL rather than a LOAEL in rats; the LOAEL is 1.6 mg/m3.
In mice, significant dose-related increases in relative spleen weights were recorded in males exposed to arsine at >1.6 mg/m3. In females, one study reported a significant increase in spleen weight at the lowest exposure level (0.08 mg/m3); however, in another study using the same mouse strain and similar duration of exposure, the increase in spleen weight was significant only for the highest exposure studied, 16 mg/m3. Consistent decreases in packed cell volume were observed in animals exposed to >8.1 mg/m3. Increases in aminolevulinate dehydratase activity were first seen in animals exposed to 1.6 mg/m3. Intracanalicular bile stasis in the liver was observed in mice exposed to 8.1 mg/m3. Anaemia was observed in animals exposed to 8.1 mg/m3. After a 12-week exposure to 0.08 mg/m3, a 2% (P < 0.05) increase in MCV was observed in female mice, in the absence of an effect on other haematological parameters. No changes were observed in bone marrow cellularity. CFU-GM and CFU-E showed a decrease after a 14-day exposure to >1.6 mg/m3, the lowest exposure studied.
As the compensatory, reversible 2% increase in MCV is not considered adverse and the observed effect on spleen weight at 0.08 mg/m3 in the Hong et al. (1989) study is in striking contrast to the findings of the Blair et al. (1990b) study (no similar effect at a 20 times higher dose), it is concluded that 0.08 mg/m3 is a NOAEL rather than a LOAEL in mice, and the LOAEL is 1.6 mg/m3.
In Syrian Golden hamsters, significant dose-related increases in relative spleen weights were recorded in both sexes exposed to >8.1 mg/m3, and packed cell volume and aminolevulinate dehydratase activity in the erythrocytes showed dose-dependent decreases from the lowest exposure level studied (1.6 mg/m3). The spleen was enlarged and dark in animals exposed to >8.1 mg arsine/m3. A LOAEL of 1.6 mg/m3 is deduced for Syrian Golden hamsters from these studies; as this was the lowest exposure investigated, a NOAEL cannot be identified.
An analysis of these studies is presented in Table 4. These studies support a NOAEL of 0.08 mg/m3; the LOAEL is 1.6 mg/m3.
Table 4: Basis for the derivation of a guidance value for arsine.a
|
Species, sex |
Study duration |
NOAEL |
LOAEL |
Reference |
|
Mouse, female |
14–90 days |
0.08 |
1.6 |
Hong et al., 1989; Rosenthal et al., 1989; Blair et al., 1990a,b |
|
Mouse, male |
14–90 days |
0.08 |
1.6 |
Blair et al., 1990b |
|
Rat, female |
14–90 days |
0.08 |
1.6 |
|
|
Rat, male |
14–90 days |
0.08 |
1.6 |
|
|
Syrian Golden hamster, female |
28 days |
– |
1.6b |
|
|
Syrian Golden hamster, male |
28 days |
– |
1.6b |
|
a |
In all studies, the critical end-point was haemolysis and its sequelae (anaemia, increased splenic weight, etc.). |
|
b |
Lowest concentration studied. |
From a NOAEL of 0.08 mg/m3, a guidance value for non-cancer end-points may be derived:
|
Guidance value |
= NOAEL • Time adjustment / Uncertainty factor |
|
|
= 0.08 mg/m3 • 5/7 • 6/24 / 300 |
|
|
= 0.05 µg/m3 |
where the overall uncertainty factor is 300 (10 for interindividual differences in humans, 3 for interspecies extrapolation [as differences between several species in direct haemolytic effects have been demonstrated to be small], and a composite factor of 10 to account for both less than long-term duration of exposure and database deficiencies [specifically the lack of a two-generation reproductive study]). The factors 5/7 and 6/24 adjust the experimental exposure parameters to a constant exposure.
In the absence of exposure data, a sample risk characterization cannot be performed. Arsine continues to cause serious, even fatal, intoxications, especially in colour metal industries.
There is disagreement between approaches taken in different national assessments of arsine — specifically, whether the 0.08 mg/m3 exposure level in the key study on haemolysis represents a NOAEL or a LOAEL. This reflects the limitations of the NOAEL/LOAEL approach to developing guidance values, rather than any real scientific disagreement on the significance of the minor effects seen at this dose. An alternative approach to developing a guidance value based on the dose–response might be more appropriate for arsine.
The second major source of uncertainty is that the guidance value is based on non-cancer end-points only. There is no information in either humans or experimental systems on the mutagenicity or carcinogenicity of arsine, but arsine is oxidized to other arsenic species that are carcinogenic to humans and have induced genotoxic effects in experimental systems and in humans.
Quantitative information on human exposure is practically non-existent.
IARC (1980, 1987) evaluated inorganic arsenic compounds and concluded that there is sufficient evidence for the carcinogenicity of "arsenic and arsenic compounds" in humans and limited evidence of carcinogenicity in experimental animals; the overall conclusion was that arsenic and arsenic compounds are carcinogenic to humans (Group 1). In making this evaluation, IARC noted that this evaluation applies to the group of chemicals as a whole and not necessarily to all individual chemicals within the group (IARC, 1987).
Aitio A, Hakala E, Pyy L (1996) Arsenic. In: Biological monitoring of chemical exposure in the workplace. Vol. 2. Geneva, World Health Organization, pp. 18–34.
Anthonisen P, Nielsen B, Pedersen K, Raaschou F (1968) Clinical picture and treatment in arsine poisoning. Acta Medica Scandinavica, Supplementum, 496:14–22.
Aposhian HV (1997) Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annual Reviews of Pharmacological Toxicology, 37:397–419.
Apostoli P, Alessio L, Romeo L, Buchet JP, Leone R (1997) Metabolism of arsenic after acute occupational arsine intoxication. Journal of Toxicology and Environmental Health, 52(4):331–342.
Ayala-Fierro F, Baldwin AL, Wilson LM, Valeski JE, Carter DE (2000) Structural alterations in the rat kidney after acute arsine exposure. Laboratory Investigation, 80(1):87–97.
Badman DG, Jaffe ER (1996) Blood and air pollution: State of knowledge and research needs. Otolaryngology — Head and Neck Surgery, 114(2):205–208.
Blair PC, Thompson MB, Bechtold M, Wilson RE, Moorman MP, Fowler BA (1990a) Evidence for oxidative damage to red blood cells in mice induced by arsine gas. Toxicology, 63(1):25–34.
Blair PC, Thompson MB, Morrissey RE, Moorman MP, Sloane RA, Fowler BA (1990b) Comparative toxicity of arsine gas in B6C3F1 mice, Fischer 344 rats, and Syrian Golden hamsters: system organ studies and comparison of clinical indices of exposure. Fundamental and Applied Toxicology, 14(4):776–787.
Bogdanicka T (1988) [Clinical toxicology.] Warsaw, Panstwowy Zakład Wydawnictw Lekarskich, pp. 393–394 (in Polish).
Braszczyńska Z, Linscheid D, Osińska R, Kłopotowski J (1983) Evaluation of the exposure to arsenic compounds in zinc smelters. Medycyna Pracy, 34(5–6): 413–417.
Buchet JP, Apostoli P, Lison D (1998) Arsenobetaine is not a major metabolite of arsine gas in the rat. Archives of Toxicology, 72:706–710.
Bulmer FMR, Rothwell HE, Polack SS, Stewart DW (1940) Chronic arsine poisoning among workers employed in the cyanide extraction of gold: A report of fourteen cases. Journal of Industrial Hygiene and Toxicology, 22:111–124.
Cheng CN, Focht DD (1979) Production of arsine and methylarsines in soil and in culture. Applied Environmental Microbiology, 38(3):494–498.
Clay JE, Dale I, Cross JD (1977) Arsenic absorption in steel bronze workers. Journal of the Society of Occupational Medicine, 27(3):102–104.
Conrad ME, Mazey RM, Reed JE (1976) Industrial arsine poisoning: report of three cases. Alabama Journal of Medical Sciences, 13:65–66.
Denyszyn RB, Grohse PM, Wagoner DE (1978) Sampling and atomic absorption spectrometric determination of arsine at the 2 µg/m3 level. Analytical Chemistry, 50(8):1094–1096.
De Palma AE (1969) Arsine intoxication in a chemical plant. Report of three cases. Journal of Occupational Medicine, 11(11):582–587.
Dlhopolcek P, Hrnciar J, Dalik R, Kolacny J, Find’o P, Szentivanyi M (1982) [Acute industrial poisoning with arsine.] Vnitrni Lekarstvi, 28:393–398 (in Slovak).
Elkins HB, Fahy JP (1967) Arsine poisoning from aluminum tank cleaning. Industrial Medicine and Surgery, 36:747–749.
Fowler BA, Weissberg JB (1974) Arsine poisoning. New England Journal of Medicine, 291(22):1171–1174.
Frank G (1976) Neurologische und psychiatrische Folgesymptome bei akuter Arsen-Wasserstoff-Vergiftung. Journal of Neurology, 213:59–70.
Gao S, Burau RG (1997) Environmental factors affecting rates of arsine formation from and mineralization of arsenicals in soil. Journal of Environmental Quality, 26: 753–763.
Gosselin B, Mathieu D, Desprez-Nolf M, Cosson A, Goudemand J, Haguenoer JM, Wattel F (1982) [Acute arsenious hydride intoxication. Four cases.] Nouvelle Presse Medicale, 11(6):439–442 (in French).
Greim H, ed. (2001) Critical data evaluation for MAK values and classification of carcinogens. Weinheim, German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (Occupational Toxicants, Vol. 15).
Guajardo AS, García AM, Heredia EM, Sicilia LS (1970) Hemolisis y fracaso renal agudo en intoxicacion colectiva por arsina. Revista Clinica Espanola, 119(6):525–532.
Hatlelid KM, Carter DE (1997) Reactive oxygen species do not cause arsine-induced hemoglobin damage. Journal of Toxicology and Environmental Health, 50:463–474.
Hatlelid KM, Brailsford C, Carter DE (1995) An in vitro model for arsine toxicity using isolated red blood cells. Fundamental and Applied Toxicology, 25:302–306.
Hatlelid KM, Brailsford C, Carter DE (1996) Reactions of arsine with hemoglobin. Journal of Toxicology and Environmental Health, 47:145–157.
Hesdorffer CS, Milne FJ, Terblanche J, Meyers AM (1986) Arsine gas poisoning: the importance of exchange transfusions in severe cases. British Journal of Industrial Medicine, 43:353–355.
Hocken AG, Bradshaw G (1970) Arsine poisoning. British Journal of Industrial Medicine, 27(1):56–60.
Hong HL, Fowler BA, Boorman GA (1989) Hematopoietic effects in mice exposed to arsine gas. Toxicology and Applied Pharmacology, 97(1):173–182.
Hotz P, Boillat MA (1991) Acute poisoning with arsine and with phosphine. Revue Medicale de la Suisse Romande, 111:169–173.
HSDB (1999) Hazardous Substances Data Bank. Bethesda, MD, US National Library of Medicine (Chem-Bank CD-ROM).
IARC (1980) Arsenic and arsenic compounds. In: Some metal and metallo compounds. Lyon, International Agency for Research on Cancer, pp. 39–42 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 23).
IARC (1987) Arsenic and arsenic compounds. In: Overall evaluation of carcinogenicity: an updating of IARC monographs volumes 1 to 42. Lyon, International Agency for Research on Cancer, pp. 100–106 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Supplement 7).
IPCS (2001a) Arsenic and arsenic compounds, 2nd ed. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 224).
IPCS (2001b) International Chemical Safety Card — Arsine. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0222).
Jenkins GC, Ind JE, Kazantzis G, Owen R (1965) Arsine poisoning: massive haemolysis with minimal impairment of renal function. British Medical Journal, 2(5433):78–80.
Johnson GA (1953) An arsine problem — engineering notes. Industrial Hygiene Quarterly, 14(3):188–190.
Jones W, Gamble J (1984) Epidemiological-environmental study of lead acid battery workers. Environmental Research, 35(1):1–10.
Keech DJ, West NG (1980) The determination of arsine in workplace air by X-ray fluorescence spectrometry. Annals of Occupational Hygiene, 23(3):273–282.
Kensler CJ, Abels JC, Rhoads CP (1946) Arsine poisoning, mode of action and treatment. Journal of Pharmacology and Experimental Therapeutics, 88:99–108.
Kipling MD, Fothergill R (1964) Arsine poisoning in a slag-washing plant. British Journal of Industrial Medicine, 21:74–77.
Kleinfeld MJ (1980) Arsine poisoning. Journal of Occupational Medicine, 22(12):820–821.
Klimecki WT, Carter DE (1995) Arsine toxicity: Chemical and mechanistic implications [invited review]. Journal of Toxicology and Environmental Health, 46:399–409.
Konzen JL, Dodson VN (1966) Arsine — a cause of hemolytic anaemia. Journal of Occupational Medicine, 8(10):540–541.
Landrigan PJ, Costello RJ, Stringer WT (1982) Occupational exposure to arsine; an epidemiologic reappraisal of current standards. Scandinavian Journal of Work, Environment and Health, 8(3):169–177.
Łazariew NW (1956) [Toxic substances in industry. Vol. 2.] Warsaw, Panstwowe Wydawnictwa Techniczne, p. 163 (in Polish).
Levinsky WJ, Smalley RV, Hillyer PN, Shindler RL (1970) Arsine hemolysis. Archives of Environmental Health, 20:436–440.
Levvy GA (1947) A study of arsine poisoning. Quarterly Journal of Experimental Physiology, 34:47–67.
Levy H, Lewin JR, Ninin DT, Schneider HR, Milne FJ (1979) Asymptomatic arsine nephrotoxicity. South African Medical Journal, 56(5):192–194.
Lewis RJ Sr, ed. (1993) Hawley’s condensed chemical dictionary, 12th ed. New York, NY, Van Nostrand Reinhold Co., p. 98.
Macaulay DB, Stanley A (1956) Arsine poisoning. British Journal of Industrial Medicine, 13:217–221.
Marchiori L, Rozio L, Bressan A, Biasoli S, Cesaro A, Peretti A, Tommasi I, Perbellini L (1989) [Occupational arsine poisoning: a case report.] Medicina del Lavoro, 80(4):330–334 (in Italian).
Marr JJ, Smaga JA (1987) Measurement of stibine and arsine generation from the Exide 3100-Ah lead-acid module. Washington, DC, US Department of Energy (Government Reports Announcements & Index, Issue 20; NTIS/DE7009461).
Matsumura Y (1988) Determination of airborne arsine by adsorption sampling with synthetic resin active carbon and graphite furnace AAS. Industrial Health, 26:135–146.
Matthews G (1989) Toxic gases. Postgraduate Medical Journal, 65(762):224–232.
Mazur IA, Pazynich VM, Mandrichenko BE, Chinchevich VI (1983) [Spectrophotometric determination of arsine in atmospheric air.] Gigiena i Sanitariya, 5:52–53 (in Russian).
Michalke K, Wickenheiser EB, Mehring M, Hirner AV, Hensel R (2000) Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Applied Environmental Microbiology, 66:2791–2796.
Mora V, Pairon JC, Garnier R, Laureillard J, Lionnet F, Hoguet L, Schaeffer A, Efthymiou ML, Brochard P (1992) Acute arsine poisoning in a ferrous metal foundry. A report of two cases. Archives des Maladies Professionelles, de Médecine du Travail et de Sécurité Social (Paris), 53(3):167–173 (in French).
Morgan DL (1992) Mechanisms of arsine gas and gallium arsenide toxicity. CRISP (Computer Retrieval of Information on Scientific Projects) database, National Institute of Environmental Health Sciences, US National Institutes of Health, at web site http://commons.cit.nih.gov/crisp3/CRISP_LIB.getdoc?textkey=3840995&p_grant_num=1Z01ES021090-07&p_query=&ticket=1865153& p_audit_session_id=9292954&p_keywords=.
Morrissey RE, Fowler BA, Harris MW, Moorman MP, Jameson CW, Schwetz BA (1990) Arsine: absence of developmental toxicity in rats and mice. Fundamental and Applied Toxicology, 15:350–356.
Morse KM, Setterlind AN (1950) Arsine poisoning in the smelting and refining industry. Archives of Industrial Hygiene and Occupational Medicine, 2:148–169.
Muehrcke RC, Pirani CL (1968) Arsine-induced anuria: A correlative clinicopathological study with electron microscopic observations. Annals of Internal Medicine, 68(4):853–866.
Mueller PD, Benowitz NL (1989) Toxicologic causes of acute abdominal disorders. Emergency Medicine Clinics of North America, 7:667–683.
Nau CA (1948) The accidental generation of arsine gas in industry. Southern Medical Journal, 41:1341–1344.
NIOSH (1985) Arsine. Method No. 6001. In: Manual of analytical methods, 3rd ed. Cincinnati, OH, National Institute for Occupational Safety and Health.
NIOSH (1994) Arsine. Method No. 6001. In: Manual of analytical methods, 4th ed. Cincinnati, OH, National Institute for Occupational Safety and Health.
Pairon JC, Mora V, Garnier R, Laureillard J, Hoguet L, Schaeffer A, Efthymiou ML, Brochard P (1992) Acute poisoning by arsine in a ferrous metal foundry. Journal de Toxicologie Clinique et Experimentale, 12:289–291.
Parish GG, Glass R, Kimbrough R (1979) Acute arsine poisoning in two workers cleaning a clogged drain. Archives of Environmental Health, 34(4):224–227.
Pedersen F, Ladefoged J, Winkler K, Baunøe B, Munck O (1968) The renal circulation in acute arsine poisoning. Acta Medica Scandinavica, Supplementum, 496:27–31.
Pernis B, Magistretti M (1960) A study of the mechanism of acute hemolytic anaemia from arsine. Medicina del Lavoro, 51(1):37–41.
Pershagen G, Lind B, Bjorklund NE (1982) Lung retention and toxicity of some inorganic arsenic compounds. In: Pershagen G, ed. Arsenic and lung cancer with special reference to interacting factors. Thesis, Department of Environmental Hygiene, Karolinska Institute and the National Institute of Environmental Medicine, Stockholm.
Peterson DP, Bhattecharyya MH (1985) Hematological responses to arsine exposure: quantitation of exposure response in mice. Fundamental and Applied Toxicology, 5:499–505.
Phoon WH, Chan MOY, Goh CH, Edmondson RPS, Kwek YK, Gan SL, Ngui SJ, Kwok SF (1984) Five cases of arsine poisoning. Annals of the Academy of Medicine, 13(2) (Supplement):394–398.
Pinto SS (1976) Arsine poisoning: evaluation of the acute phase. Case report. Journal of Occupational Medicine, 18(9):633–635.
Pinto SS, Petronella SJ, Johns DR, Arnold MF (1950) Arsine poisoning. Archives of Industrial Hygiene, 1:437–451.
Rathus E, Stinton RG, Putman JL (1979) Arsine poisoning, country style. Medical Journal of Australia, 1:163–166.
Richardson BA (1990) Cot mattress biodeterioration and SIDS. The Lancet, 335:670.
Risk M, Fuortes L (1991) Chronic arsenicalism suspected from arsine exposure: a case report and literature review. Veterinary and Human Toxicology, 33(6):590–595.
Rogge H, Fassbinder W, Martin H (1983) Arsin (AsH3) - Intoxikation: Hämolyse und Nierenversagen. Deutsche Medizinische Wochenschrift, 108:1720–1725.
Romeo L, Apostoli P, Kovacic M, Martini S, Brugnone F (1997) Acute arsine intoxication as a consequence of metal burnishing operations. American Journal of Industrial Medicine, 32:211–216.
Rosenman KD (1979) Cardiovascular disease and environmental exposure. British Journal of Industrial Medicine, 36(2):85–97.
Rosenthal GJ, Fort MM, Germolec DR, Ackermann MF, Lamm KR, Blair PC, Fowler BA, Luster MI, Thomas PT (1989) Effect of subchronic arsine inhalation on immune function and host resistance. Inhalation Toxicology, 1:113–127.
Scott N, Carter DE, Fernando Q (1989) Reaction of gallium arsenide with concentrated acids: formation of arsine. American Industrial Hygiene Association Journal, 50(7):379–381.
Sheehy JW, Jones JH (1993) Assessment of arsenic exposures and controls in gallium arsenide production. American Industrial Hygiene Association Journal, 54(2):61–69.
Steel M, Feltham DVG (1950) Arsine poisoning in industry: Report of a case. The Lancet, 1:108–110.
Stokinger HE (1981) Arsine, AsH3. In: Patty’s industrial hygiene and toxicology. New York, NY, Wiley-Interscience, pp. 1528–1530.
Suchard JR, Wu IY (2001) Arsenicals, arsine. eMedicine Journal 2, Internet communication of 10 December 2001 at web site http://www.emedicine.com/emerg/topic920.htm.
Suess MJ, Grefen K, Reinisch DW, eds. (1985) Ambient air pollutants from industrial sources. Amsterdam, Elsevier, pp. 419, 420, 457, 458.
Tamaki S, Frankenberger WT (1992) Environmental biochemistry of arsenic. Reviews of Environmental Contamination and Toxicology, 124:79–110.
Teitelbaum DT, Kier LC (1969) Arsine poisoning: Report of five cases in the petroleum industry and a discussion of the indications for exchange transfusion and hemodialysis. Archives of Environmental Health, 19(1):133–143.
Thienes C, Haley TJ (1972) Clinical toxicology, 5th ed. Philadelphia, PA, Lea and Febiger, pp. 171–175.
Togaybayev AA, Lapin VI, Sheynin GD, Sultanbayev BK, Shel FG (1984) [Intensive therapy of hydrogen arsenous poisoning.] Anesthesiology and Reanimatology, 4:46–47 (in Russian).
Uldall PR, Khan HA, Ennis JE, McCallum RI, Grimson TA (1970) Renal damage from industrial arsine poisoning. British Journal of Industrial Medicine, 27(4):372–377.
US EPA (1994a) Arsine (CASRN
US EPA (1994b) Toxicological review on arsine. Washington, DC, US Environmental Protection Agency.
Vahter ME (1988) Arsenic. In: Clarkson TW, Friberg L, Nordberg GF, Sager PR, eds. Biological monitoring of toxic metals. New York, NY, Plenum Press, pp. 303–321.
Vallee BL, Ulmer DD, Wacker WEC (1960) Arsenic toxicology and biochemistry. Archives of Industrial Health, 21(2):56–75.
Venugopal B, Luckey TD (1978) Toxicity of group V metals and metalloids. In: Metal toxicity in mammals. Vol. 2. New York, NY, Plenum Press, pp. 207–213.
Wald PH, Becker CE (1986) Toxic gases used in the microelectronics industry. Occupational Medicine, 1(1):105–117.
Weed RI, Reed CF (1966) Membrane alterations leading to red cell destruction. American Journal of Medicine, 41:681–698.
Wilkinson SP, McHugh P, Horsley S, Tubbs H, Lewis M, Thould A, Winterton M, Parsons V, Williams R (1975) Arsine toxicity aboard the Asia freighter. British Medical Journal, 3:559–563.
Williams PL, Spain WH, Rubenstein M (1981) Suspected arsine poisoning during the restoration of a large cyclorama painting. American Industrial Hygiene Association Journal, 42:911–913.
Windholz M, ed. (1983) The Merck index, 10th ed. Rahway, NJ, Merck & Co., pp. 117–118.
Winski SL, Barber DS, Rael LT, Carter DE (1997) Sequence of toxic events in arsine-induced hemolysis in vitro: Implications for the mechanism of toxicity in human erythrocytes. Fundamental and Applied Toxicology, 38:123–128.
Zukauskas A, Gavriusinas V (2002) Semiconductor device technology. In: Functional combinations in solid states at web site http://www.mtmi.vu.lt/pfk/funkc_dariniai/technology.htm.
US EPA (1994b): Toxicological review on arsine
Copies of the document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (telephone)
513-569-7159 (fax)
rih.iris@epa.gov (e-mail address)
www.epa.gov/iris (IRIS web site)
This document received internal peer review by US Environmental Protection Agency (EPA) scientists, an external review by well qualified scientists, and consensus review by EPA Program Offices and the 10 Regional Offices. Summaries of significant comments from external peer review are included in an appendix to the document.
Greim (2001): Critical data evaluation for MAK values and classification of carcinogens
The scientific documents of the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK) are based on critical evaluations of the available toxicological and occupational medical data from extensive literature searches and from well documented industrial data. The evaluation documents involve a critical examination of the quality of the database indicating inadequacy or doubtful validity of data and identification of data gaps. This critical evaluation and the classification of substances are the result of an extensive discussion process by the members of the Commission proceeding from draft documentation prepared by members of the Commission, by ad hoc experts, or by the Scientific Secretariat of the Commission. Scientific expertise is guaranteed by the members of the Commission, consisting of experts from the scientific community, industry, and employer associations.
The draft CICAD on arsine was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
M. Baril, International Programme on Chemical Safety/Institut de Recherches en Santé et en Sécurité du Travail du Québec, Montreal, Quebec, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
J.P. Buchet, Industrial Toxicology and Occupational Medicine Unit, Catholic University of Louvain, Brussels, Belgium
R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
P.C. Chan, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
C. Cooke, Health and Safety Executive, Bootle, Merseyside, United Kingdom
J. Descotes, Poison Centre, Hôpital Edouard Herriot, Lyon, France
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Research Triangle Park, NC, USA
J. Gift, National Center for Environmental Assessment, US Environmental Protection Agency, Research Triangle Park, NC, USA
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
C. Hiremath, National Center for Environmental Assessment, US Environmental Protection Agency, Research Triangle Park, NC, USA
J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
D. Lison, Industrial Toxicology and Occupational Medicine Unit, Catholic University of Louvain, Brussels, Belgium
R. Liteplo, Existing Substances Division, Environmental Contaminants Bureau, Health Canada, Ottawa, Ontario, Canada
R. Lomax, Health and Safety Executive, Bootle, Merseyside, United Kingdom
M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
M. Vojtísek, Centre of Industrial Hygiene and Occupational Diseases, Prague, Czech Republic
K. Ziegler-Skylakakis, European Commission, DG Employment & Social Affairs, Luxembourg
Monks Wood, United Kingdom,
16–19 September 2002
Members
Dr R. Benson, US Environmental Protection Agency, Region VIII, Denver, CO, USA
Mr R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA, USA
Dr S. Czerczak, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health, Jozsef Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr Y. Hayashi, Division of Chem-Bio Informatics, National Institute of Health Sciences, Ministry of Health, Labour and Welfare, Tokyo, Japan
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, Division of Risk Assessment, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Prof. J. Jeyaratnam, Colombo, Sri Lanka
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
Prof. Y.-X. Liang, School of Public Health, Fudan University, Shanghai Medical College, Shanghai, People’s Republic of China
Dr R. Liteplo, Existing Substances Division, Environmental Contaminants Bureau, Health Canada, Ottawa, Ontario, Canada
Ms M.E. Meek, Existing Substances Division, Safe Environments Programme, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr F.P. Simeonova, Sofia, Bulgaria
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, Australia
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, European Commission, DG Employment & Social Affairs, Luxembourg
Resource Persons
Dr C. Cowles, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr C. Elliott-Minty, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr K. Fuller, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Observers
Mr A.G. Berends, Solvay S.A., Brussels, Belgium; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr C. Newsome, Dow Chemical Company Limited, West Drayton, Middlesex, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr M.A. Pemberton, Wilmslow, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Stott, Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr J.M. Waechter, Jr, The Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr H. Malcolm, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Ms C. Vickers, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
La première version du présent CICAD relatif aux effets de l’arsine sur la santé humaine a été préparé par le Dr S. Czerczak, de l’Institut Nofer de médecine du travail de Lodz (Pologne). Les recherches bibliographiques ont permis de rassembler des données qui vont jusqu’à juin 2000. D’autres sources documentaires ont été utilisées, à savoir un document émanant du Système intégré d’information sur les risques qui relève de l’Agence des Etats-Unis pour la protection de l’environnement (US Environmental Protection Agency) (US EPA, 1994b) et un document rédigé par le MAK de la République fédérale d’Allemagne (Greim, 2001). L’appendice 1 donne des informations sur les sources documentaires et sur leur examen par des pairs. Des renseignements sur l’examen par des pairs du présent CICAD sont donnés à l’appendice 2. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Monks Wood (Angleterre) du 16 au 19 septembre 2002. La liste des participants à cette réunion figure à l’appendice 3. La fiche internationale sur la sécurité chimique de l’arsine (ICSC 0222) établie par le Programme international sur la sécurité chimique après un autre examen par des pairs (IPCS, 2001b), est également reproduite dans le présent document.
Les données qui figurent dans ce CICAD portent essentiellement sur les effets résultant d’une exposition de courte durée à l’arsine. Dans l’organisme, ce composé subit une oxydation qui conduit à d’autres dérivés arsenicaux. Les effets de l’exposition à l’arsenic et à ses dérivés (notamment les effets cancérogènes et génotoxiques) ont récemment été examinés par l’IPCS (2001a). Il ressort de cette étude que l’arsenic et ses dérivés sont cancérogènes pour l’Homme et qu’ils ont des effets génotoxiques sur les systèmes expérimentaux ainsi que chez les sujets humains.
L’arsine (No CAS
Pour doser l’arsine dans l’atmosphère des lieux de travail, on recueille un échantillon d’air dans un tube à billes de charbon muni d’un filtre cellulosique afin d’éliminer les aérosols de composés arsenicaux. Le dosage s’effectue par spectrométrie d’absorption atomique avec four graphite après désorption par l’acide nitrique et utilisation de nickel comme modificateur de matrice. Cette méthode est utilisable dans les limites de concentration de 1 à 200 μg/m3 pour un échantillon de 10 litres d’air. Il existe dans le commerce des appareils qui permettent un enregistrement continu de la teneur en arsine avec une sensibilité nominale de 1 μg/m3.
Certains microorganismes sont capables de transformer en arsine par voie biologique des composés arsenicaux non volatils comme les arsénites et les arséniates.
La principale source d’arsine due à l’activité humaine consiste dans le traitement par divers acides de métaux réducteurs contenant de l’arsenic comme impureté et ce gaz est ainsi un sous-produit du raffinage de métaux non ferreux tels que le cuivre, le zinc ou le cadmium. On peut s’attendre à ce que de l’arsine se forme dans l’environnement en des lieux tels que les décharges de produits dangereux.
L’arsine est largement utilisée dans l’industrie des semi-conducteurs pour la croissance épitaxique de l’arséniure de gallium, comme agent dopant dans les circuits intégrés à base de silicium et dans la fabrication de diodes photoémettrices.
Dans l’environnement, l’arsine subit des transformations conduisant à d’autres dérivés arsenicaux.
On ne possède pas de documentation relative à la concentration de l’arsine dans l’environnement et les données concernant le niveau d’exposition professionnelle à ce gaz sont rares.
Chez l’Homme et les animaux, l’arsine est absorbé au niveau des poumons et de la muqueuse respiratoire. Après exposition, la concentration sanguine d’arsine augmente rapidement, le composé étant par contre beaucoup plus lent à se répartir dans le foie, le rein et les autres viscères. La métabolisation conduit à l’arsenic trivalent (As(III)) et à l’arsenic pentavalent (As(V)). L’arsenic (III) est méthylé en monométhylarsonate et en diméthylarsinate. Les métabolites de l’arsine sont principalement excrétés par la voie urinaire.
Chez les différentes espèces, et notamment chez l’Homme, l’arsine présente une forte toxicité aiguë. C’est le système hématopoïétique qui est la cible de l’intoxication par l’arsine et en particulier les érythrocytes. L’arsine provoque une hémolyse qui entraîne une hémoglobinurie puis une atteinte rénale. De nombreuses intoxications mortelles ont été décrites chez l’Homme et il continue de s’en produire. Chez la souris, la CL50 est égale à 250 mg/m3 pour une exposition de 10 minutes. Chez ce même animal, l’inhalation d’arsine a entraîné une augmentation du poids relatif de la rate après 6 heures d’exposition à une concentration de 16 mg/m3 et une diminution de l’hématocrite après une exposition de 1 heure à une concentration de 30 mg/m3. Les anomalies histopathologiques observées consistaient notamment en une hémosidérose et une activité hématopoïétique extramédullaire au niveau de la rate.
On n’a pas connaissance d’études ou de données intéressantes concernant les effets irritants de l’arsine sur la peau et les yeux ou qui portent sur son activité sensibilisatrice.
Une exposition répétée à l’arsine a provoqué une splénomégalie persistante et une légère dépression des précurseurs érythroïdes de la moelle osseuse chez des rats et des souris à des concentrations > 1,6 mg/m3 et chez des hamsters dorés à les concentrations > 8,1 mg/m3. On a observé une méthémoglobinémie chez des souris exposées à de l’arsine à la concentration de 8,1 mg/m3. Chez le rat, la souris et le hamster doré, la dose la plus faible produisant un effet hématologique indésirable observable (LOAEL) a été estimée à 1,6 mg/m3. La dose sans effet indésirable observable (NOAEL) a été estimée à 0,08 mg/m3, tout en reconnaissant que même pour l’exposition la plus faible étudiée, on pouvait observer chez la souris une modification compensatoire réversible du volume globulaire moyen, modification considérée comme non pathologique.
Selon la seule étude dont on ait connaissance, l’arsine n’a pas d’effet indésirable sur le développement de la souris ou du rat à des niveaux d’exposition entraînant une splénomégalie.
Chez l’Homme, l’arsine provoque une hémolyse avec augmentation de la concentration plasmatique d’hémoglobine, de fer et de potassium suivie d’une anémie et de lésions rénales. On ne possède aucune donnée fiable sur les niveaux d’exposition auxquels ces effets se produisent. La mort peut également survenir par suite d’une insuffisance cardiaque ou respiratoire. Les lésions hépatiques graves sont rares.
On observe une anémie de gravité variable qui s’accompagne de la formation de corps d’Ehrlich-Heinz et d’une hyperleucocytose. On note la présence d’hémoglobine, d’hémosidérine, d’érythrocytes, de protéines et de cylindres dans les urines.
Les effets à long terme d’une exposition à de faibles concentrations d’arsine ne sont pas très bien caractérisés. On ne possède pas de données sur la cancérogénicité ou la mutagénicité de l’arsine pour l’Homme et les animaux de laboratoire. Chez l’Homme, l’exposition aux autres composés arsenicaux résultant de la métabolisation de l’arsine peut provoquer des cancers du poumon, de la vessie, du rein et de la peau.
Sur la base d’une NOAEL de 0,08 mg/m3, en appliquant un facteur d’incertitude de 300 et en tenant compte du mode d’exposition, on peut énoncer une valeur-guide de 0,05 μg/m3.
El primer borrador de este CICAD sobre los aspectos relativos a la salud humana de la arsina fue preparado por el Dr. S. Czerczak, del Instituto Nofer de Medicina del Trabajo de Lodz (Polonia). La búsqueda bibliográfica realizada comprende los datos identificados hasta junio de 2000. Se utilizaron también como documentos originales el del Sistema de Información Integrada del Riesgo, del Organismo para la Protección del Medio Ambiente de los Estados Unidos (US EPA, 1994b), y el documento alemán MAK (Greim, 2001). Los documentos originales y su examen colegiado se describen en el apéndice 1. El examen colegiado de este CICAD figura en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Monks Wood (Inglaterra) del 16 al 19 de septiembre de 2002. La lista de participantes en esta reunión figura en el apéndice 3. La Ficha internacional de seguridad química para la arsina (ICSC 0222), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas en un proceso de examen colegiado independiente (IPCS, 2001b), también se reproduce en el presente documento.
La información que se presenta en este CICAD se concentra en los efectos asociados con la exposición breve a la arsina. En el organismo, la arsina se oxida para formar otras especies de arsénico. En el IPCS (2001a) se han examinado recientemente los efectos (en particular los carcinogénicos y genotóxicos) asociados con la exposición al arsénico y sus compuestos. Éstos son carcinógenicos para el ser humano e inducen efectos genotóxicos en sistemas experimentales y en las personas.
La arsina (CAS Nş
Para la determinación de la arsina en el aire del lugar de trabajo se recoge una muestra de aire en un tubo de carbón vegetal dotado de un filtro de celulosa a fin de eliminar los aerosoles de compuestos de arsénico. La medición se realiza mediante espectrometría de absorción atómica en horno de grafito, tras la desorción con ácido nítrico y utilizando níquel como modificador de la matriz. La escala de funcionamiento del método es de 1-200 µg/m3 en una muestra de aire de 10 litros. Existen instrumentos comerciales de registro continuo con una sensibilidad especificada de 1 µg/m3.
Algunos microorganismos pueden formar arsina mediante la biotransformación de compuestos de arsénico no volátiles, como los arsenitos y arsenatos.
Las principales fuentes antropogénicas de arsina son su formación a partir de la reacción de ácidos con metales reductores que contienen impurezas de arsénico, principalmente como subproducto del refinado de metales no ferrosos, como el zinc, el cobre y el cadmio. Se prevé que se puede formar arsina en el medio ambiente de lugares como los depósitos de desechos peligrosos.
La arsina se utiliza ampliamente en la industria de los semiconductores para el crecimiento epitaxial del arseniuro de galio, como agente de impurificación para dispositivos electrónicos en estado sólido a base de silicio y en la fabricación de diodos emisores de luz.
En el medio ambiente, la arsina se transforma en otros compuestos de arsénico.
No hay información sobre las concentraciones de arsina en el medio ambiente y son escasos los datos relativos a los niveles de exposición en el lugar de trabajo.
En las personas y los animales, la arsina se absorbe a través de los pulmones y la superficie mucosa del tracto respiratorio. Tras la exposición, la concentración de arsina aumenta con rapidez en la sangre, mientras que la distribución al hígado, los riñones y otros órganos es mucho más lenta.
En las personas y los animales la arsina se metaboliza a arsénico trivalente (As (III)), así como pentavalente (As (V)). El As (III) se metila para formar monometilarsonato y dimetilarsinato. Los metabolitos de la arsina se excretan fundamentalmente en la orina.
La toxicidad aguda de la arsina en diferentes especies, incluidas las personas, es alta. El órgano destinatario de su toxicidad es el sistema hematopoyético, en particular los eritrocitos. La arsina induce hemólisis, provocando hemoglobinuria y posteriormente lesiones renales; se han descrito un gran número de intoxicaciones mortales en las personas y se siguen produciendo. La CL50 en ratones es de 250 mg/m3 para una exposición de 10 minutos. La inhalación de arsina por los ratones provocó un aumento del peso relativo del bazo tras una exposición de seis horas a 16 mg/m3 y una disminución del valor hematócrito tras una exposición de una hora a 30 mg/m3. Los cambios histopatológicos observados fueron hemosiderosis y actividad hematopoyética extramedular en el bazo.
No se dispone de estudios o datos pertinentes sobre sus efectos de irritación cutánea u ocular o sobre su actividad sensibilizadora.
La exposición repetida a la arsina provocó esplenomegalia persistente y una ligera supresión de los precursores eritroides de la médula ósea en ratas y ratones con concentraciones >1,6 mg/m3 y en hámsteres dorados sirios con concentraciones >8,1 mg/m3. Se observó metahemoglobinemia en ratones expuestos a concentraciones de arsina de 8,1 mg/m3. En ratas, ratones y hámsteres dorados sirios, la concentración más baja con efectos adversos observados (LOAEL) para los efectos hematológicos fue de 1,6 mg/m3. Se estableció una concentración sin efectos adversos observados (NOAEL) de 0,08 mg/m3, reconociendo que se detectó en ratones un cambio compensatorio reversible del volumen corpuscular medio, no considerado como adverso, incluso con los niveles de exposición más bajos estudiados.
En el único estudio disponible, la arsina no indujo toxicidad en el desarrollo de ratones o ratas con niveles de exposición que inducían esplenomegalia.
En las personas, la arsina induce hemólisis con un aumento de la concentración de hemoglobina, hierro y potasio en el plasma y los efectos posteriores de anemia y lesiones renales. No se dispone de información fidedigna sobre los niveles de exposición a los cuales se producen estos efectos. Otras causas de muerte son la miocardiopatía y la insuficiencia pulmonar. Son raras las lesiones hepáticas graves.
Se observa anemia en grado variable, acompañada de corpúsculos de Heinz-Ehrlich y un aumento de la leucocitosis. En la orina se encuentran hemoglobina, hemosiderina, eritrocitos, proteínas y cilindros.
No están bien caracterizados los efectos de la exposición prolongada a niveles bajos de arsina. No hay datos sobre la carcinogenicidad o mutagenicidad de la arsina para las personas o los animales de experimentación. La exposición a otros compuestos de arsénico a los cuales se metaboliza la arsina puede inducir cáncer de pulmón, vejiga, riñón y piel.
A partir de una NOAEL de 0,08 mg/m3, utilizando un factor de incertidumbre general de 300 y ajustándolo a la pauta de exposición, se puede obtener un valor de orientación de 0,05 µg/m3.
ENDNOTES
|
1. |
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
2. |
In keeping with WHO policy, which is to provide measurements in SI units, all concentrations of gaseous chemicals in air will be given in SI units in the CICAD series. Where the original study or source document has provided concentrations in SI units, these will be cited here. Where the original study or source document has provided concentrations in volumetric units, conversions will be done using the conversion factors given here, assuming a temperature of 20 °C and a pressure of 101.3 kPa. Conversions are to no more than two significant digits. |
See Also:
Toxicological Abbreviations