
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 49
First draft prepared by Dr K. Ziegler-Skylakakis, German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (BUA), currently with the European Commission, DG Employment and Social Affairs; and Drs J. Kielhorn, G. Könnecker, J. Koppenhöfer, and I. Mangelsdorf, Fraunhofer Institute of Toxicology and Aerosol Research, Drug Research and Clinical Inhalation, Hanover, Germany
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Thiourea.
(Concise international chemical assessment document ; 49)
1.Thiourea - adverse effects 2.Risk assessment 3.Environmental exposure
4.Occupational exposure I.International Programme on Chemical Safety II.Series
ISBN 92 4 153049 9 (NLM Classification: WK 202)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: ). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on thiourea was prepared jointly by the German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (BUA) and the Fraunhofer Institute of Toxicology and Aerosol Research, Germany. It is based on the BUA (1995) report on thiourea and the German MAK Commission (MAK, 1988, 1997) documentation. A comprehensive literature search of relevant databases was conducted in November 2001 to identify any relevant references published subsequent to those incorporated in these reports. Information on the preparation and peer review of the source documents is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Monks Wood, United Kingdom, on 16–19 September 2002. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card for thiourea (ICSC 0680), produced by the International Programme on Chemical Safety (IPCS, 2000), has also been reproduced in this document.
Thiourea (CAS No.
In 1993, the global annual production of thiourea was about 10 000 tonnes. A more recent global production figure is not available. Thiourea has a wide range of uses; for example, it is used in the production and modification of textile and dyeing auxiliaries, in the leaching of ores, in the production of pharmaceuticals and pesticides, as a vulcanization accelerator, and as an auxiliary agent in diazo paper.
Based on thiourea’s use pattern, the hydrosphere is expected to be its main environmental target compartment. Measured concentrations of the chemical in surface waters are not available. Thiourea is not expected to evaporate from water. It is resistant to hydrolysis in water and direct photolysis in water and air, and it undergoes photochemical oxidation by hydroxyl radicals in the atmosphere (calculated half-life 2.4 h). Thiourea will be biodegraded by an adapted microflora only after extended acclimation periods. Thus, under conditions not favouring biotic or abiotic removal, thiourea may be present in surface waters and sediments over longer periods. Adsorption to sediment particles, however, is not to be expected, as indicated by low soil sorption coefficients. Leaching of thiourea from soil to groundwater seems possible, particularly under conditions unfavourable for biotic degradation. The available experimental data on bioaccumulation indicate no bioaccumulation potential for thiourea in aquatic organisms.
There are only few data on exposure levels at the workplace. One study from a thiourea production factory gives a concentration of 0.6–12 mg thiourea/m3 in air. Another occupational exposure study giving measured data from the production and packing of thiourea reported an average air concentration (thiourea in total dust) of 0.085 mg/m3 (maximum 0.32 mg/m3).
There is possible consumer exposure due to dermal contact with cloth finished with thiourea. There is also a possibility of contact with blueprint paper at the workplace (architects, engineers, engineering draughtsmen). When diazo copy paper is used, thiourea is readily released from the surface coating. Further exposure could occur from the use of thiourea-containing metal polish and from the metabolism of thiourea-based pharmaceuticals.
Thiourea is an antioxidant. After oral administration to humans and animals, it is almost completely absorbed and is excreted largely unchanged via the kidneys. However, some metabolic transformation catalysed by microsomal flavin-containing monooxygenase to formamidine sulfinic acid can take place.
Based upon studies conducted primarily in laboratory animals, the major adverse health effect associated with exposure to thiourea is the inhibition of thyroid gland function, although effects on lungs, liver, haematopoietic system, and kidneys have also been described. Thiourea produces pulmonary oedema secondary to permeability changes in the lung.
Thiourea has mitogenic properties. The chemical did not induce gene mutations in bacteria. Inconsistent results, with the majority being negative, were obtained in assays in mammalian cells. Thiourea induced chromosomal recombination in yeast and Drosophila. It is not considered to be a genotoxic carcinogen.
At high doses, thiourea can cause thyroid hyperplasia in mice and thyroid adenomas and carcinomas, hepatocellular adenomas, and tumours of the Zymbal or Meibomian gland in rats. However, none of the studies of carcinogenicity would meet present-day standards. Although no definite conclusion regarding the mechanism of carcinogenicity can be made, it is probable that thiourea acts via the known mechanism for non-genotoxic thyroid carcinogens.
Although thiourea has been shown to be a carcinogen in rats, the weight of evidence suggests that rodents are more sensitive than humans to thyroid tumour induction due to hormonal imbalances that cause elevated thyroid-stimulating hormone (TSH) levels.
Hypothyroidism caused by the administration of 50 mg thiourea/kg body weight to sheep for 2, 4, or 6 months adversely influences somatic development, reproductive/gestational performance of animals, and growth of developing fetuses in utero. A similar study with male lambs showed adverse effects on male reproductive development.
Exposure to thiourea can induce contact and photocontact allergies in humans. Thiourea yielded negative results in a sensitization test in animals.
In a Russian study, thyroid hyperplasia was observed in 17 of 45 workers exposed to air concentrations of 0.6–12 mg/m3, equivalent to a dose of 0.07–1.4 mg thiourea/kg body weight per day. Tolerable intakes should be much below 0.07 mg thiourea/kg body weight per day.
From data on its use as a thyroid depressant, <15 mg thiourea/day (<0.2 mg/kg body weight per day) had no effect, whereas 70 mg/day (about 1.0 mg/kg body weight per day) showed an effect.
The sample risk characterization compares the data reported in the Russian study above with the average air concentration (thiourea in total dust) of 0.085 mg/m3 and the maximum concentration of 0.32 mg/m3 measured in a German factory. It is likely that a health risk may exist in the German factory, at least at the maximum level, if no hygienic precautions are taken.
Exposure of the general population to thiourea has not been quantified, so no risk characterization was possible.
From valid test results available on the toxicity of thiourea to various aquatic organisms, thiourea can be classified as moderately to highly toxic in the aquatic compartment. The lowest no-observed-effect concentrations (NOECs) were found in two long-term studies on reproduction of the water flea (Daphnia magna, 21-day NOEC <0.25 mg/litre and 0.25 mg/litre).
According to the reliable experimental data available for toxicity to aquatic and terrestrial species, the low bioaccumulation potential, and the expected environmental fate when released to water or soil, thiourea is not expected to pose a significant risk for organisms in both environmental compartments (except in the case of accidental spill).
Thiourea (CAS No.
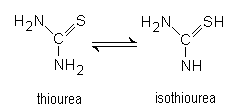
and thus has three functional groups: amino, imino, and thiol (BUA, 1995).
The substance has no sharp melting point, as rearrangement to ammonium thiocyanate (NH4SCN) occurs at temperatures above about 135 °C (Mertschenk et al., 1995). Data on melting between 167 and 182 °C are reported in the literature (BUA, 1995). Information on the boiling point is not available, as decomposition occurs. The temperature of decomposition is not known.
Thiourea is soluble in water (137 g/litre at 20 °C), soluble in polar protic and aprotic organic solvents, and insoluble in non-polar solvents (BUA, 1995). A UV absorption maximum at 238 nm was measured in water at pH 7.4 (Weast & Astle, 1979). A significant pH dependence of the n-octanol/water partition coefficient (log Kow) was not detected (Govers et al., 1986).
Additional physicochemical properties for thiourea are presented in Table 1 and in the International Chemical Safety Card (ICSC 0680) reproduced in this document.
Table 1: Physicochemical properties of thiourea.
|
Property |
Value |
Reference |
|
Relative molecular mass |
76.1 |
|
|
Density (g/cm3) |
1.405 |
Mertschenk et al. (1995) |
|
Vapour pressure (kPa) at 20 °C |
9.98 x 10–9 |
Mertschenk et al. (1995) |
|
n-Octanol/water partition coefficient |
–1.61 to –0.92 |
BUA (1995) |
|
Water solubility (g/litre) |
95 at 10 °C |
Mertschenk et al. (1995) |
|
Henry’s law constant (Pa·m3/mol) |
5.6 x 10–9 |
BUA (1995) |
The determination of thiourea in workplace air can be carried out by adsorption on a glass fibre filter, filter elution with water in an ultrasonic bath, C18 reversed-phase HPLC with water as the mobile phase, and UV detection at 245 nm. The detection limit is 0.4 µg thiourea/litre sample solution; a recovery rate of 106 ± 6% is given (BUA, 1995).
This method can also be applied to the detection of thiourea in water. The detection limit is 0.1 mg/litre water. Thiourea concentrations above 10 mg/litre have to be diluted before analysis; solutions with very low concentrations of the chemical can be concentrated in a rotary evaporator (sample solution 2.1 µg/litre; BUA, 1995).
In soil, thiourea can be determined by HPLC, but with a cationic exchange resin as the separating phase and under salting-out conditions (with an aqueous solution of ammonium sulfate as the mobile phase). Detection is carried out by UV absorption at 240 nm. The method works especially well at a column temperature of 60 °C. At a substance concentration of 160 µg/litre, a recovery rate of 99.3 ± 2.7% is given. The detection limit is 2.7 ng absolute (Hashimoto, 1979).
For the detection of thiourea in biological material, reversed-phase HPLC with methanol/water as the mobile phase and UV detection (240 nm) is applied. For rat plasma, extraction with ethanol, enrichment by evaporation, and purification on silica gel with methanolic trichloromethane are described (Kobayashi et al., 1981).
Thiourea has been detected but not quantified in laburnum shrubs (Laburnum anagyroides) and is a natural metabolite of the fungi Verticillium alboatrum and Bortrylius cinerea (IARC, 1974).
Thiourea is industrially produced by the reaction between technical-grade calcium cyanamide (CaCN2) and hydrogen sulfide (H2S) or one of its precursors in aqueous solution — e.g., ammonium sulfide ((NH4)2S) or calcium hydrogen sulfide (Ca(HS)2). Calcium cyanamide must not contain calcium carbide, as explosive acetylene can be liberated with water or hydrogen disulfide. In Germany, thiourea is produced by a continuous process in a closed reaction vessel (BUA, 1995; Mertschenk et al., 1995).
In 1993, the global annual production of thiourea was about 10 000 tonnes (BUA, 1995). Of this, about 40% (4000 tonnes) was produced by the German manufacturer, which is the sole manufacturer in Western Europe; 20% (2000 tonnes) was contributed by a Japanese manufacturer; and another 40% (4000 tonnes) was contributed by at least seven Chinese companies. A more recent global production figure is not available.
Table 2 gives use patterns derived from 1993 global data (BUA, 1995), but the use pattern may vary widely between countries.
Table 2: Estimated global use pattern of thiourea.a
|
Use |
Share of market (%) |
|
Direct use |
|
|
Ore leaching (e.g., gold and silver extraction from minerals) |
25 |
|
Auxiliary agent (diazo paper) |
16 |
|
Isomerization catalyst (conversion of maleic to fumaric acid) |
12 |
|
Additive (slurry explosives) |
4 |
|
Metal refinement (copper) |
1.5 |
|
Metal cleaning (including silver polish) |
1 |
|
Other (e.g., drilling auxiliary in petroleum industry, fertilizer) |
1 |
|
Processing |
|
|
Production of thiourea dioxide |
27.5 |
|
Modification of resins |
4 |
|
Production and modification of textile and dyeing auxiliaries |
4 |
|
Various chemical intermediates |
4 |
a From BUA (1995).
In the USA, thiourea is used in animal hide glue, which contains thiourea at a concentration of 10–20% as a liquefying agent. Reports indicate its use in the production of flame retardant resins and as a vulcanization accelerator (NTP, 2000). In Germany, thiourea is not used in the leaching of ore mines and not processed to thiourea dioxide. Instead, the following use pattern is reported (BUA, 1995): auxiliary agent in diazo paper (light-sensitive photocopy paper) and almost all other types of copy paper (19%); metal cleaning, including silver polish (4%); precipitation of heavy metals (3%); additive in slurry explosives (3%); electroplating/electroforming (1%); corrosion inhibitor (1%); processing to organic intermediates (41%); mercaptosilanes (6.5%); vulcanization accelerators (0.5%); resin modification (4.5%); and chemicals industry and miscellaneous (16.5%) (BUA, 1995). In Japan, thiourea is added to fertilizers to inhibit the nitrification process (Hashimoto, 1979; Kubota & Asami, 1985). Data on the quantities used are not available.
Thiourea is emitted by manufacturers of electronic components and accessories and manufacturers of aircraft and aircraft parts (CARB, 1997).
Organic thiourea derivatives are used as vulcanization accelerators, pharmaceuticals (antiseptic, thyrotherapeutic, narcotic, and tuberculostatic agents), and plant protection agents and pesticides (e.g., chloromethiuron, diafenthiuron, thiophanate, and thiophanate-methyl) (Mertschenk et al., 1995).
The global release of thiourea during production, use, and processing cannot be estimated with the available data. As the use pattern varies widely, it is to be concluded that emissions also differ between countries. The US Toxics Release Inventory (US EPA, 1999) states that 4.85 tonnes were released in 1995 and 1.13 tonnes in 1999. The following data are for Germany, the country of the primary source document (BUA, 1995).
Releases into air from production at the German manufacturer, which was the sole Western European manufacturer in 1993, were approximately 14 g/tonne produced; releases into surface water were not relevant (waste mother liquor from the production process is used to remove nitrogen dioxide in high-temperature incineration processes or is incinerated). The annual wastes are given as about 15 kg/tonne produced ("white sludge"), containing 20% w/w thiourea at a maximum (i.e., 3 kg thiourea/tonne produced). These wastes are disposed of by incineration. In addition, 2.8 tonnes of lime (calcium carbonate) per tonne thiourea produced emerge during production. The thiourea content of this waste is <0.1% w/w. More than 96% of the lime (residual thiourea: <10.8 tonnes/year) is used by brick and cement industries or similar industries. The remainder (residual thiourea: maximum 400 kg/year) is disposed of in an authorized dump. The leachate of this dump is collected and completely reintroduced into the production process as so-called make-up water. Therefore, emissions into soil or groundwater from this site are not to be expected.
No significant emissions into the air are expected from the industrial use of thiourea as a catalyst in the synthesis of fumaric acid, diazo paper, or metal polish, whereas releases to surface water are unclear.
The releases from the processing of thiourea at German manufacturers (synthesis of organic intermediates) in 1993 were <1 kg/tonne processed for each reported site into air (from registry limit of emission declaration of 25 kg/year) and <5 kg/tonne processed for each reported site into surface water. Wastes from processing are incinerated. Waste air is also in general incinerated. At some processing sites, liquor from the process or active carbon used for purification is incinerated; therefore, emissions into surface water are not expected.
A major use of thiourea in Germany is as an auxiliary agent in blueprint (diazo) paper. Thiourea emissions may occur, especially from the disposal of waste paper. It is assumed, however, that only 10% of this paper is recycled, since blueprint paper often contains confidential information (e.g., construction plans). The remaining 90% is assumed to be shredded and disposed of with domestic waste. Assuming further that blueprint paper contains 0.5 g thiourea/m2 at a maximum, that 100% of production-related paper cuttings are recycled, that the de-inking removes 67%, and that the chemical adsorption onto de-inking sludge is about 80%, an annual emission into wastewater treatment plants of 3.1 tonnes thiourea can be calculated. Landfill disposal of diazo paper may also release thiourea into soil and groundwater. However, a quantification is not possible with the available data.
The use of thiourea in metal polish occurs in industrial and consumer products as well. From this type of application (aqueous solutions), it can be assumed, as a worst case, that the total amount used is released into wastewater. In Germany, this is about 13.2 tonnes/year.
In all of the vulcanization accelerators, pharmaceuticals, and pesticides being synthesized from thiourea, the basic structure of the substance is maintained. It is therefore possible that thiourea can be released from these agents by metabolic or hydrolytic degradation. However, a quantification of the thiourea releases into the environment is not possible with the available data.
From its very low vapour pressure (see section 2), a significant adsorption of thiourea onto airborne particles is not expected. Due to its solubility in water (137 g/litre at 20 °C), the washout from the atmosphere by wet deposition (fog, rain, snow) is assumed to be significant. Measured data on this are not available.
From water solubility and vapour pressure data, a Henry’s law constant in the range of 5.58 × 10–9 – 8.44 × 10–9 Pa·m3/mol can be calculated, indicating that thiourea is not expected to volatilize from aqueous solutions, according to the classification of Thomas (1990). Based on the physicochemical properties of thiourea and its use pattern, the hydrosphere is expected to be the main target compartment for this compound.
Soil sorption coefficients (Koc) in the range of 26–315 were determined in studies conducted according to Organisation for Economic Co-operation and Development (OECD) Guideline 106 (adsorption/desorption). According to the classification scheme of Blume & Ahlsdorf (1993), the sorption of thiourea onto organic matter of three different soils may be characterized as low (spodosol) to moderate (entisol/alfisol). Fesch et al. (1998) stated that neutral thiourea did not undergo any significant ion exchange or other sorption processes in investigations with sorbents such as pure quartz sand, quartz sand coated with polyvinyl alcohol, and quartz sand coated with a mixture of the clay mineral montmorillonite and polyvinyl alcohol.
Based on its physicochemical properties, a significant evaporation of thiourea from soil is not to be expected.
Thiourea is hydrolytically stable, as measured according to OECD Guideline A-79.74 D (Korte & Greim, 1981).
Experimental data on direct photolysis are not available. From the UV spectrum of the substance (see section 2), direct photolysis in air and water is not to be expected. The extinction coefficients epsilonmax at lambdamax (235 and 238 nm) are in the range of 11 000–12 590/mol per second (Weast & Astle, 1979; Fesch et al., 1998). However, in the atmosphere, the main degradation pathway is probably the reaction of thiourea with hydroxyl radicals. An estimation of the photo-oxidation of thiourea by hydroxyl radicals according to Atkinson and the Atmospheric Oxidation Program (Version 1.90, 12 h sunlight, hydroxyl radical concentration 1.5 × 106/cm3) revealed a half-life of 2.4 h. For the hydrosphere, specific rate constants for the reaction of thiourea with hydrated electrons and hydroxyl radicals are given as 3.0 × 109/mol per second (pH 6.4) and 4.7 × 109/mol per second (pH 7), respectively (Anbar & Neta, 1967). Based on a hydroxyl radical concentration of 1 × 10–16 mol/litre in water, a half-life of 17 days can be calculated.
Numerous tests have been performed on the biodegradability of thiourea. Tests performed according to internationally accepted standard procedures under aerobic conditions are summarized in Table 3. In two studies on ready biodegradability, no mineralization of thiourea was observed (TNO, 1990; MITI, 1992). On the other hand, removal of up to 97% was reported from laboratory tests on inherent biodegradation (Semi-Continuous Activated Sludge, or SCAS, Test), in which the inoculum was very slowly adapted to increasing thiourea concentrations prior to incubation.
Cultures of different fungi isolated from soil and grown on glucose and thiourea were shown to degrade thiourea more or less effectively. Whereas Aspergillus glaucus, Penicillium citrinum, and Trichoderma viride took up only 30–50% of an initial thiourea concentration of 0.01% even after long incubation periods of 46 and 106 days and converted not more than 15–17% of thiourea sulfur to sulfate (Jensen, 1957), concentrations in the range of 0.1–0.5 g thiourea/litre were completely removed within 7 days of incubation by Penicillium rugulosum (Lashen & Starkey, 1970).
Rheinheimer et al. (1990) investigated the aerobic biodegradability of environmentally relevant concentrations of organic chemicals (including, among others, thiourea) in water and sediment samples of the river Elbe (including its estuary) and the western reaches of the Baltic Sea. In all water samples from the Elbe estuary, very slow but continuous degradation of thiourea was observed over the incubation period of 85 days (maximum 9% within the first 8 days, maximum 68% at the end of observation; based on carbon dioxide production). In sediment samples, 40–70% degradation was observed. In samples taken from the Baltic Sea, biodegradation varied widely between 50 and 87% in water and between 28 and 72% in sediment.
Degradation of thiourea by soil microorganisms was observed by Lashen & Starkey (1970). Twenty-two per cent of an initial concentration of 1.5 g/litre was degraded within 1 week and 96% within 15 weeks of incubation. Thiourea concentrations exceeding 7.6 g/litre inhibited microbial transformation. In aerobic batch laboratory microcosm experiments, half-lives of 12.8 days (basic soil) and 18.7 days (acid soil) were determined. Although no abiotic controls were performed, removal of thiourea was attributed mainly to biotic processes, assuming abiotic mechanisms (e.g., oxidation, evaporation) to be of minor importance (Loehr & Matthews, 1992). After applying thiourea concentrations of 5 and 200 mg/litre to soil in the frame of a plant growth test, Günther & Pestemer (1990) observed a marked increase in mineral nitrogen within 4 weeks of incubation, which was explained by primary degradation of thiourea.
From the available degradation tests and taking into account the expected environmental distribution of thiourea, leaching of this compound from soil to ground-water seems possible, particularly under conditions unfavourable for biotic degradation.
Table 3: Elimination of thiourea in standard biodegradation tests under aerobic conditions.
|
Test |
Thiourea concentration (mg/litre) |
Adaptation (days) |
Test duration (days) |
Removal (%) |
Reference |
|
Tests on ready biodegradation |
|||||
|
OECD 301C (modified MITI Test)a |
10/30 |
No |
34 |
No ready biodegradation |
TNO (1990) |
|
OECD 301C |
30 |
No |
14 |
2.6 |
MITI (1992) |
|
Tests on inherent biodegradation |
|||||
|
GSF Test |
0.05 |
No |
5 |
17 |
Rott et al. (1982) |
|
OECD 302A (SCAS Test) |
20 mg carbon/litre |
25 + 39 |
No data |
0 |
Fischer (1985) |
|
OECD 302A (SCAS Test) |
20 mg carbon/litre |
11 |
26–28b |
45 |
Fischer (1985) |
|
OECD 302A (SCAS Test) |
20 mg carbon/litre |
<13 |
13–29b |
80 |
Fischer (1985) |
|
OECD 302A (SCAS Test) |
20 mg carbon/litre |
43 |
43/69/84b |
93 |
Friesel et al. (1984) |
|
OECD 302A (SCAS Test) |
20 |
5 |
24 |
97 |
Broecker et al. (1984); Fischer (1985) |
|
a |
Additional nitrogen and carbon source. |
|
b |
Date of measurement. |
Based on the available data on soil sorption, biodegradation in soil, and the calculated Koc value, accumulation of thiourea in the geosphere is unlikely.
Due to the low n-octanol/water partition coefficient (see section 2), bioaccumulation of thiourea is expected to be insignificant. This assumption is confirmed by the available experimental data. In a study conducted according to OECD Guideline 305C, bioconcentration factors determined for carp (Cyprinus carpio) were in the range of <0.2 to <2 (related to whole fish) (MITI, 1992). Freitag et al. (1984, 1985) and Geyer et al. (1984) obtained accumulation factors in the range of <10–90 for golden orfe (Leuciscus idus), algae (Chlorella fusca), and activated sludge.
Data on thiourea concentrations in ambient air are not available.
From the physicochemical data on thiourea, it is concluded that the hydrosphere is its main target compartment. In 1977, thiourea was not detected in any of six seawater or six sediment samples from bay areas (Yokaichi, Dokaiwan) and a strait (Kanmonkaikyo) in Japan (detection limits: 0.0011–0.4 µg/litre for the water phase; 0.055 and 1 µg/kg for the sediment) (Environment Agency Japan, 1985). In 1992, a thiourea concentration of 130 mg/litre was detected in groundwater in Germany in the vicinity of an old landfill in which thiourea-containing lime had been deposited at the site where the leachate flows into the aquifer. Ten metres downstream, the thiourea level was below the detection limit of 1 mg/litre (BUA, 1995).
Further data on the occurrence of thiourea in the hydrosphere and data on the occurrence of thiourea in soil or in the biosphere are not available.
In a Russian thiourea manufacturing factory, reported air concentrations of thiourea were in the range 0.6–12 mg/m3. In the middle of the production hall, the air concentration was 3.9 ± 1.0 mg/m3, and concentrations around loading and cleaning were higher (9.0 ± 0.9 mg/m3) (Talakin et al., 1985).
In 1988–1991, workplace measurements (12 personal and stationary samples) from the production and packing of thiourea at the German manufacturer gave an average air concentration (thiourea in total dust) of 0.085 mg/m3 (maximum 0.32 mg/m3) (BUA, 1995).
Thiourea is used in dyeing and finishing processes in the textile industry. Finishing involves the application of thiourea as a fire retardant to the cloth, which will typically contain <0.02% thiourea after this stage. An investigation at a textile factory on the prevalence of hypothyroidism gave typical concentrations of 5 µg thiourea/m3 at an inlet of the local exhaust ventilation of the finishing machines. No thiourea was found in the atmosphere of the process area (Roberts et al., 1990).
There is the possibility of contact with blueprint paper at the workplace (architects, engineers, engineering draughtsmen). When diazo copy paper is used, thiourea is readily released from the surface coating (MAK, 1997). Measured data are not available.
Consumer exposure to thiourea can occur from the metabolism of thiourea-based pharmaceuticals.
There is possible dermal contact with blueprint paper.
Metal polish can contain up to 10% thiourea. If silver cutlery is not washed thoroughly after dipping in the cleaner, thiourea could be ingested. Dermal contact from the cleaning process could be relevant for those occupationally exposed.
In humans and animals, thiourea is rapidly absorbed from the gastrointestinal tract. A single oral dose of 28.57 mg thiourea/kg body weight in humans was completely eliminated within 48 h in urine, while a peak concentration in blood was measured within 30 min. In rats administered 5 mg intravenously, 30% of the thiourea was recovered from the carcasses after 3 h, and only traces after 25 h (Williams & Kay, 1947).
There is no information available on kinetics following inhalation of thiourea.
Thiourea has been identified as one of the metabolites in workers exposed to carbon disulfide (Pergal et al., 1972).
Thiourea is also absorbed to a lesser degree through the skin. Following dermal application of 2000 mg/kg body weight to rabbits in the form of an aqueous solution (26 ml of a 25% w/v solution), approximately 4% of the applied dose was found in the animals’ urine; when applied in solid form, only 0.1% was found in the urine (TNO, 1979a, 1980).
In rats, there is a direct and linear correlation between the quantities present in the horny layer 30 min after topical application of thiourea and the subsequent percutaneous absorption and excretion measured over 4 days. Thiourea at 200 nmol/cm2 was applied to the dorsal skin for 30 min, and the total body distribution was measured after 96 h (Schaefer & Jamoulle, 1988). The quantity of thiourea present in the stratum corneum of the application area was measured by liquid scintillation counting after tape-stripping the treated area (Rougier et al., 1983).
Pregnant mice were injected intravenously with 14C-labelled thiourea. Autoradiography revealed that radioactivity began to accumulate in the thyroid gland of mothers and fetuses after only 5 min and remained higher in this tissue than in any other organ during the entire 4-day observation period. Increased levels of radioactivity were also found in the walls of the large blood vessels, the cortex of the adrenal glands, the mammary glands, liver, lungs, and kidneys (Slanina et al., 1973). In rats, [14C]thiourea administered intravenously was found to be uniformly distributed in lung, liver, and kidney proteins 24 h after application (Hollinger et al., 1974, 1976).
In a study in which rats were given thiourea (100 mg/kg body weight) intraperitoneally, the half-time in plasma was calculated to be 3.3 h (Giri & Combs, 1972).
Thiourea is oxidized by thyroid gland peroxidase in the presence of iodine or iodide and hydrogen peroxide to form formamidine disulfide (NH2(NH)CSSC(NH)NH2). Formamidine disulfide is unstable and decomposes at pH values above 3.0, forming cyanamide, elementary sulfur, and thiourea. It was shown in vitro and in vivo that both cyanamide and thiourea are inhibitors of thyroid peroxidase (Davidson et al., 1979).
In liver microsomes, it has been shown that flavin-containing monooxygenase (FMO) catalyses the S-oxygenation of thiourea to the reactive electrophilic formamidine sulfenic acid and formamidine sulfinic acid (Fig. 1) (Ziegler, 1978). Oxidation of thiourea also occurs in the intact rat liver (Krieter et al., 1984). In the presence of glutathione, formamidine sulfenic acid is rapidly reduced to thiourea with concomitant formation of glutathione disulfide both in vitro and in vivo (Ziegler, 1978; Krieter et al., 1984). Whether significant S-oxygenation of thiourea occurs in organs other than liver is not known.
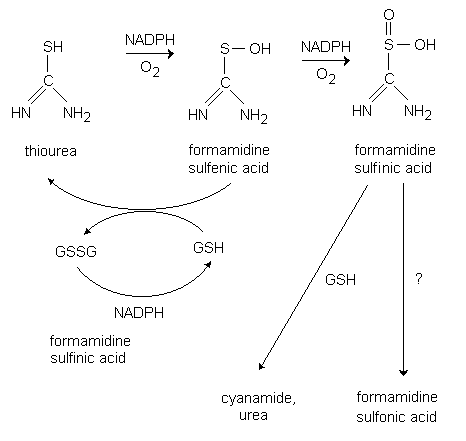
Fig. 1: Metabolism of thiourea by the microsomal FAD-dependent monooxygenase (Ziegler, 1978).
[GSSG = oxidized glutathione, GSH = reduced glutathione, NADPH = reduced nicotinamide adenine dinucleotide phosphate]
For more details on the studies in this section, the reader is referred to MAK (1988).
The acute toxicity of thiourea varies with the species, strain, and age of the animals exposed to the chemical and with the iodine content of their diet. Oral LD50s are about 1000 mg/kg body weight for mice, 125–1930 mg/kg body weight for rats, depending on the strain, and 10 000 mg/kg body weight for rabbits. The intraperitoneal LD50 for the rat ranges between 4 and 1340 mg/kg body weight, according to the strain. Death at these doses is due to lung oedema, and the survivors exhibit pleural effusion. Accordingly, thiourea at doses between 10 and 500 mg/kg body weight has been employed in experimental animal studies as a model agent for the elicitation of lung oedema and pleural effusion. The pathological effects are prevented by pretreatment of the animals with cysteine or glutathione, which reduces the irreversible binding of radioactivity to lung proteins after administration of [14C]thiourea. Toxic doses of thiourea also resulted in hyperglycaemia, glucosuria, polyuria, and a reduction in the liver glycogen level in rats (MAK, 1988).
The LC50 of a 10% aqueous solution for rats (4 h of inhalation) is above 195 mg/m3 (TNO, 1979b). The dermal LD50 for New Zealand White rabbits is above 2800 mg/kg body weight. Thiourea was applied on the shaved skin as solutions in water in amounts of 9 ml/kg body weight for each dose level (TNO, 1978).
An intraperitoneal dose of thiourea in male Sprague-Dawley rats (10 mg/kg body weight) resulted in significant elevations in plasma histamine as well as in lung vascular permeability and 100% mortality within 24 h. A non-lethal dose (0.5 mg/kg body weight) given as pretreatment followed by the lethal dose at 1, 4, 8, 16, and 32 days provided complete protection against death for 8 days and partial protection until 24 days. This decrease in mortality correlated quite closely with reduced plasma histamine levels and diminished pulmonary vascular permeability. The authors concluded that the degree of tolerance to thiourea developed is related to plasma histamine concentration and pulmonary vascular permeability (Giri et al., 1991b).
Experimental pulmonary oedema was induced in adult male Sprague-Dawley rats injected intraperitoneally with thiourea at doses of 3, 6, or 10 mg/kg body weight. Induction of pulmonary oedema was observed by a significant increase in the ratio of lung weight to body weight in all three groups of experimental rats. An increase in plasma calcium and a decrease in plasma copper and ceruloplasmin were observed in the rats in the two highest dose groups (Sarkar et al., 1988).
A 24-h exposure to undiluted thiourea applied to the intact and abraded skin of rabbits resulted in mild to marked erythema with a slight degree of oedema (TNO, 1983a). When rabbit skin was exposed to 0.5 g of thiourea for a period of 4 h, the substance was tolerated without reaction (Korte & Greim, 1981).
A single application of a 10% (w/w) aqueous solution of thiourea to the eye was tolerated without reaction (TNO, 1983b). In another study, the application of 100 mg thiourea to the conjunctiva of the rabbit eye resulted in reddening (1–2 using Draize scoring) and swelling (1–2 using Draize scoring) (Korte & Greim, 1981).
Thiourea yielded negative results in a sensitization test carried out with guinea-pigs according to the method of Magnusson & Kligman (1970) (Korte & Greim, 1981).
When 28-day-old male rats (strain not given) were treated daily for 2 weeks with thiourea administered at 600 ± 60 mg/kg body weight via gastric intubation, about a 50% reduction of body weight gain was observed (Smith, 1950). Daily ingestion of 131 mg thiourea/kg body weight in drinking-water by 21- to 30-day-old female rats (strain not given) for 10 consecutive days led to hyperplasia of the thyroid, which could be demonstrated both macroscopically and microscopically. No such effect resulted from treatment with 12 mg thiourea/kg body weight (Astwood, 1943). Another study demonstrated a reduction of the basal metabolic rate, which could be prevented by simultaneous administration of thyroxine (tetraiodothyronine, or T4) (MacKenzie & MacKenzie, 1943). Rats received, over a 2-week period, 0.05% thiourea (25 mg/kg body weight per day) up to 2% thiourea (1000 mg/kg body weight per day) in food. The weight of the thyroid glands was increased maximally in rats that received 0.5% thiourea (250 mg/kg body weight per day); the basal metabolic rate showed a definite depression in rats receiving 1% thiourea (500 mg/kg body weight per day). The basal metabolic rate was determined in rats that were starved for 20 h (no further details are given).
The iodine level of the thyroid gland was reduced from 73 to 13 mg/100 g tissue upon the oral administration of thiourea at 70 mg/kg body weight for 10 days (Astwood et al., 1945). Thiourea also resulted in a reduction of thyroid iodine uptake when administered in rats at 1% (500 mg/kg body weight per day) in the diet for 2 months (Keston et al., 1944). Concomitant with reduced thyroid activity, the weight of the pituitary gland increased and signs of pituitary overactivity were evident both histologically and biochemically; the weights of the ovary, uterus, and prostate gland all declined. Haemosiderosis in the spleen, lymph nodes, and intestinal villi of rats was observed subsequent to the administration of 16–50 daily doses of 1 ml of a 1% aqueous solution of thiourea by gavage. The repeated administration of high doses (no quantitative data given) of thiourea in the diet, in the drinking-water, or by intraperitoneal injection resulted in manifold effects: reduced osmotic resistance of the erythrocytes, congestion, haemosiderosis and atrophy of the spleen, anaemia, leukocytopenia, granulocytopenia, increased erythropoiesis in the bone marrow, reduced clotting times, and increased phospholipid levels of the blood (MAK, 1988).
Mice appear to be less sensitive to thiourea than rats, in that daily subcutaneous administration at 500 mg/kg body weight for 10 days resulted in only a slight reduction in the colloid content of the thyroid (Jones, 1946).
When 0.25% thiourea (350 mg/kg body weight per day) was administered to rats in the drinking-water for 65–122 days, an enlargement of the pituitary gland was observed, in addition to structural changes in the pars intermedia, hyperplasia of the parathyroid gland, and fibrotic inflammation of the bones (Malcolm et al., 1949).
Thiourea was administered to Sprague-Dawley rats (10 per sex per dose group) at concentrations of 0, 0.02, 0.1, 0.5, or 2.5 mg/litre (0, 0.0028, 0.014, 0.070, or 0.350 mg/kg body weight per day) in the drinking-water for 13 weeks (Hazleton, 1987). Animals were observed for mortality and moribundity and for overt signs of toxicity. Detailed physical examinations and individual body weight and food consumption measurements were performed. Clinical pathology parameters (haematology, clinical chemistry, urinalysis, triiodothyronine [T3], T4, and TSH levels in blood) were evaluated. There was no evidence of substance-related clinical or histopathological effects.
In mice, no effect on body weight was observed upon inclusion of 2.5 g thiourea/kg in the diet (125 mg/kg body weight per day) for 13 weeks (Morris et al., 1946).
Twenty-seven female lambs (2–3 months old) were orally administered 0 or 50 mg thiourea/kg body weight daily for 2, 4, or 6 months (six treated and three controls per group) (Nasseri & Prasad, 1987a; see section 8.7.2). Slight to moderate facial oedema, significant reduction in weight gain, stunted growth, weakness, profound depression, and loss of appetite were observed. Alopecia became evident from the second month on. The thyroid gland was moderately to severely enlarged, although there was no direct correlation with length of dosing. Muscular weakness and difficulty standing and walking were noted with increased dosing. Hypoglycaemia, hyperlipidaemia/hypercholesterolaemia, and a significant fall in serum T4 were related to length of treatment.
Eight male lambs aged 3–3.5 months were orally administered 50 mg thiourea/kg body weight daily for 3.5 months together with four control lambs (Sokkar et al., 2000; see section 8.7.2). The dosed animals became weak, emaciated, anaemic, and significantly reduced in body weight, with facial oedema and alopecia at thigh, legs, and abdomen. Clinical analysis showed significant reduction in erythrocyte and leukocyte numbers and in levels of T3 and testosterone at the end of the experiment. Histopathology of the thyroid gland revealed hyperplasia of the follicle-lining epithelial cells that project into the lumen. The lumina were devoid of colloid. The testes showed ill developed, small, empty seminiferous tubules. Hepatocytes in the liver showed degeneration and vacuolation with proliferation of Kupffer cells. The kidney showed glomerular lipidosis with accumulation of haemosiderin pigment in the cytoplasm of the renal tubules. Hyperkeratosis of the epidermis was associated with excessive keratin formation within the hair follicles.
In a chronic toxicity study, thiourea was administered daily in drinking-water at concentrations of 1.72, 6.88, or 27.5 mg/kg body weight to mice for 2 years and to rats for the duration of their lifetimes or a maximum of 3 years. A reduction in body weight gain and an enlargement of the thyroid gland were observed only in the rats in the highest dose group, and no other changes were detected, either macroscopically or microscopically (Hartzell, 1942, 1945). A lowest-observed-adverse-effect level (LOAEL) of 27.5 mg/kg body weight per day (reduction of body weight and enlargement of thyroid gland) and a no-observed-adverse-effect level (NOAEL) of 6.88 mg/kg body weight per day for rats can be given.
Thiourea has not been tested in a standard bioassay of carcinogenicity in rodents. Several older carcinogenicity studies were carried out prior to the mid-1960s (Table 4). They described the occurrence of tumours at numerous locations other than the thyroid gland, but the distribution of these varied from one study to another. Unfortunately, most of these reports are highly unsatisfactory. They lack important details regarding dosages or the frequencies of spontaneous tumour formation, and the doses administered were often sufficiently toxic to result in 100% mortality (IARC, 1974, 2001). In several studies involving different strains of mice, thyroid hyperplasia, but not thyroid tumours, was reported after oral administration. In rats given thiourea orally, a high incidence of thyroid follicular cell adenomas and carcinomas and increased incidences of hepatocellular adenomas and tumours of the Zymbal or Meibomian gland were reported (IARC, 1974, 2001).
In an experiment in which thiourea (3 × 200 mg/kg body weight) given in water by gavage was followed by 2 × 10 mg of a technical mixture of polychlorinated biphenyls (PCBs) ("promoter") weekly for 11 weeks in Sprague-Dawley rats, thiourea demonstrated no initiation capacity, as expressed by the number or size of ATP-free islets in the liver. Similarly, when thiourea (0.2% in drinking-water for 12 weeks) was administered after a dose of 8 mg diethylnitrosamine/kg ("initiator"), it expressed no "promotion" activity in the liver (Oesterle & Deml, 1988).
Male F344 rats initiated with N-bis(2-hydroxypropyl)nitrosamine (DHPN) at 2000 mg/kg body weight in a single subcutaneous injection were given a diet containing 0 or 0.1% thiourea from weeks 2 to 20 for 19 weeks. Histopathological examination revealed altered hepatocellular foci and/or hepatocellular adenomas in the rats in incidences of 40% and 93% in control and treated rats, respectively. In addition, proliferative lesions in the thyroid consisting of adenomatous nodules and neoplasias and proliferative lesions in the lung were seen in the rats that received thiourea (Shimo et al., 1994b).
In a study with male 4-week-old Fischer 344 rats, 0.1% thiourea was given to them in the drinking-water starting 1 week after they had received a single subcutaneous dose of 2000 mg DHPN/kg body weight. Animals were sacrificed at weeks 1, 2, 4, 8, 12, or 16. Serum T4 levels were decreased by approximately 60% at week 1 and remained significantly lower than in rats treated with DHPN only throughout the experiment, while serum TSH levels were elevated and peaked at 4 weeks (20-fold increase), returning to normal at 12 weeks. Thyroid weights were significantly increased. Hyperplasia was observed at 2 weeks, and adenomas were observed at 4 weeks. Proliferation was greatest when TSH levels were elevated. In 5 of 20 rats treated with DHPN and thiourea, thyroid follicular cell adenomas occurred. In contrast, no tumours were induced in rats treated with DHPN alone (Shimo et al., 1994a).
Table 4: Studies on the carcinogenicity of thiourea.
|
Species (strain) |
Number, sex,a age |
Dose, treatment period |
Observations |
Reference |
|
Mouse (five strains) |
4–65 m, f per group controls: 4–51 m, f |
2% in diet (1000 mg/kg body weight per day), up to 21 months |
Thyroid hyperplasia, no carcinomas |
Gorbman (1947) |
|
Mouse (C3H) |
21 f |
0.25% in diet (125 mg/kg body weight per day), 13 weeks; then 0.375% (187.5 mg/kg body weight per day), 3–45 weeks; killed on appearance of tumours |
Thyroid hyperplasia, no tumours |
Dalton et al. (1948) |
|
Mouse (C3H) |
25 m + 25 f |
0.3% in diet (150 mg/kg body weight per day), 7 months |
Thyroid hyperplasia |
Casas & Koppisch (1952) |
|
Mouse (C3H) |
49 f |
0.1–0.2% in drinking-water (140–280 mg/kg body weight per day), 4–6 months |
No thyroid hyperplasia (1/20 hypertrophy), mammary tumours in 54%, less in controls: 28% |
Vasquez-Lopez (1949) |
|
Mouse (R3) with high incidence of mammary tumours |
11 f |
0.2–0.5% in drinking-water (280–700 mg/kg body weight per day), average 10 months |
Thyroid hyperplasia |
Vasquez-Lopez (1949) |
|
Mouse (ICR Swiss) |
42 (not specified) |
1 x 2500 mg/kg body weight subcutaneously; killed after 6 months |
Incidence of lung adenomas: 5% |
Gargus et al. (1969) |
|
Rat (Norway) |
9 f |
0.25% in drinking-water (350 mg/kg body weight per day), 12–23 months |
Thyroid: 4 carcinomas from month 20, 7 adenomas |
Purves & Griesbach (1947) |
|
Rat (Norway) |
8 f |
0.25% in drinking-water (350 mg/kg body weight per day), 12–24 months |
Thyroid: 3 carcinomas from month 20, 8 adenomas |
Purves & Griesbach (1947) |
|
Rat (Wistar) |
8 f |
0.25% in drinking-water (350 mg/kg body weight per day), 12–22 months |
Thyroid: 6 adenomas |
Purves & Griesbach (1947) |
|
Rats from the above three groups |
8 f with adenomas |
0.25% in drinking-water (350 mg/kg body weight per day), 17–18 months, plus thyroid extract, thyroxine injected from month 16 |
No thyroid gland tumours |
Purves & Griesbach (1947) |
|
Rat (albino) |
19 m |
0.2% in drinking-water (280 mg/kg body weight per day), 13–26 months |
1 nasal tumour, 6 tumours in the ear, 6 orbital tumours; 5 animals with tumours in both of the latter localities |
Rosin & Rachmilewitz (1954); Rosin & Ungar (1957) |
|
Rat (Wistar) |
9 m |
0.2% in drinking-water (280 mg/kg body weight per day), 12–23 weeks |
Squamous cell carcinoma of the Zymbal gland and/or Meibomian glands in 8/9 animals |
Ungar & Rosin (1960) |
|
Rat (Osborne-Mendel) |
30 m + 30 f |
80 mg/kg in the diet (4 mg/kg body weight per day), 24 months |
No increased tumour frequencies |
Radomski et al. (1965) |
|
Rat (Osborne-Mendel) |
30 m + 30 f |
50 mg/kg in the diet (2.5 mg/kg body weight per day), 26 months |
21 tumours, 4 of them malignant |
Deichmann et al. (1967) |
|
Rat (albino) |
18 m/f per group |
0.01–1% in the diet (5–500 mg/kg body weight per day), 24 months |
From 0.25%: thyroid hyperplasia |
Fitzhugh & Nelson (1948) |
|
Rat (albino) |
12 m/f |
3–4 ml 10% solution intraperitoneally (857–1142 mg/kg body weight), 3 times per week for 6 months, then 0.2% in drinking-water (280 mg/kg body weight per day) to 15 months |
6 animals died or were killed after 6 weeks to 8 months: no effects |
Rosin & Ungar (1957) |
a m = male; f = female.
In a study in which male Fischer 344 rats were given 0.2% thiourea in the drinking-water for 10 weeks, starting 1 week after initial subcutaneous application of DHPN at 2800 mg/kg body weight, the treated animals showed decreased body weights, 5-fold increased thyroid weights, 25% decreased T4 levels, and 5-fold increased TSH levels. Administration of thiourea induced an increased incidence (P < 0.01) of thyroid follicular cell tumours: 10/10 in the DHPN and thiourea group compared with 1/10 in the DHPN-only group (Takegawa et al., 1997).
A further study (Mitsumori et al., 1996) confirmed that thiourea, given after DHPN, increased the frequency of thyroid follicular cell tumours in Fischer rats and showed that this increase was observed for tumours with both adenomatous and solid growth patterns. A single subcutaneous injection of 2.8 g DHPN/kg body weight followed by thiourea at a concentration of 0.2% (280 mg/kg body weight per day) in the drinking-water for 19 weeks increased the incidence of thyroid follicular cell neoplasms in rats after 20 weeks, when the study was terminated.
In summary, it has been shown that thiourea can promote thyroid follicular cell tumours initiated by DHPN.
Thiourea has been tested in numerous assays. It did not induce gene mutations in bacteria. Inconsistent results, the majority of which were negative, were obtained in mammalian cells. Thiourea induced chromosomal recombination in yeast and insects. Thiourea is not considered to be a genotoxic carcinogen.
Several research groups have investigated the effect of thiourea on Salmonella typhimurium strains TA 97, TA 98, TA 100, and TA 1535 in both the absence and presence of a metabolic activation system. Yamaguchi (1980) reported the doubling of a number of revertants in strain TA 100 at 100 µg thiourea/plate. However, all other authors found no positive effects due to this chemical.
Thiourea tested in the SOS chromotest at concentrations ranging between 7.6 ng/ml and 7.6 mg/ml with a 2-h incubation period both with and without metabolic activation did not induce an increase in the revertants (Brams et al., 1987).
In the umu-test with S. typhimurium strain TA 1535/pSK1002, thiourea was not found to be genotoxic in either the absence or presence of metabolic activation, even at the highest applied concentration of 1670 µg/ml (Nakamura et al., 1987).
Thiourea was tested for its genotoxic potential with Saccharomyces cerevisiae at concentrations of 0, 5, 10, 20, and 40 mg/ml (Schiestl, 1989; Galli & Schiestl, 1996). Deletion and intrachromosomal recombinations were observed to be induced at the two highest concentrations. These concentrations (20 and 40 mg/ml) of thiourea also proved to be highly cytotoxic to the yeast cells, with only 11 and and 1% surviving, respectively. In another study, the application of 0.12–0.4 mol thiourea/litre (about 9.1–30.4 mg/ml) to S. cerevisiae D7 resulted in a 1.5- to 7.5-fold increase in gene conversion at the trp locus over that of the control organisms (Jiang et al., 1989). The effect of thiourea on the permeable yeast mutant S. cerevisiae C658-k42 at concentrations of 0, 0.5, 1.0, and 2.0 mg/ml was tested in both the absence and presence of metabolic activation. Whereas only negative results were obtained without metabolic activation, with it, the concentrations of 0.5 and 1.0 mg/ml led to 6.7- and 4.5-fold increases in trp+ revertants, respectively, in comparison with the control. The concentration of 2.0 mg/ml proved to be ineffective in this regard. The cytotoxicity was less than 15% (Morita et al., 1989).
The genotoxicity of thiourea was investigated with Aspergillus nidulans using concentrations of 65.7–197.1 mmol/litre of the chemical at 99% purity in tests in both the absence and presence of metabolic activation (Crebelli et al., 1986). Neither forward mutations nor chromosomal malsegregations were observed to result from thiourea treatment, although the higher doses of the chemical were generally toxic.
A concentration of 60 mmol thiourea/litre inhibited DNA synthesis in human fibroblasts in the so-called "DNA synthesis inhibition test" (Painter, 1977). Yanagisawa et al. (1987) considered this to be evidence for a genotoxic effect of the chemical.
Thiourea at concentrations of 10–40 mmol/litre induced a 5-fold increase in the frequency of azaguanine-resistant V79 Chinese hamster cells (while the cytotoxicity was less than 15%) in the absence of a metabolic activation system (Ziegler-Skylakakis et al., 1985).
Two studies on the effect of thiourea on L5178Y mouse lymphoma cells in tests in both the presence and the absence of a metabolic activation system (S9-mix from Aroclor 1254-induced rat liver) have been carried out. In one (Caspary et al., 1988), the tests were carried out by two independent contract institutes (A and B), which used similar protocols, in which thiourea concentrations of 0–5000 µg/ml and 0–6000 µg/ml were tested without and with metabolic activation, respectively. The chemical was shown to be non-genotoxic and non-toxic by both institutes in the test without metabolic activation and by institute A in the presence of the metabolic activation system. However, institute B found thiourea to have a positive effect in the test with metabolic activation, although no data on toxicity were provided. Overall, the effect of the chemical was described as being negative in one case (institute A) and positive in the other (institute B). In the second study (Wangenheim & Bolcsfoldi, 1988), thiourea was tested at concentrations of 0, 0.068, 1.37, 2.05, and 2.74 mg/ml in the absence of metabolic activation and at concentrations of 0, 0.63, 0.95, 1.26, 1.89, and 2.52 mg/ml in the presence of the S9-mix. The mutation frequency in the tests without metabolic activation increased 1.3-fold in comparison with the control at the concentrations of 1.37 and 2.05 mg/ml and increased 1.8-fold at 2.74 mg/ml (P < 0.001). The cytotoxicity at these concentrations was estimated to be between 30 and 60%. The corresponding increase at the highest tested concentration with metabolic activation (2.52 mg/ml) was 1.6-fold (P < 0.001). The investigators considered that a positive effect was detectable only by means of statistical evaluation and deemed that a 2-fold or higher mutation frequency would represent a suitable criterion for an unequivocally positive effect. Thiourea was thus concluded to be only weakly mutagenic in this study.
When rats were treated with two successive oral doses of 350 mg thiourea/kg body weight (corresponding to 20% of the LD50; the second oral dose was administered 24 h after the first), no positive results were obtained in a micronucleus test. No symptoms of toxicity or any cytotoxic effects resulted from the treatment (TNO, 1979c).
Seiler (1977) found no inhibition of the incorporation of [3H]thymidine into testicular DNA due to thiourea in vivo using the Friedman-Staub test (Friedman & Staub, 1976).
Thiourea in concentrations of 0.5 and 1.0 mmol/litre nutrient solution had a positive effect in the zeste-white test system of Drosophila melanogaster, whereas equivocal results were obtained with the same concentrations in the white-ivory test system (Batiste-Alentorn et al., 1991, 1994). In the eye mosaic assay with D. melanogaster, the application of 0.5 mmol thiourea/litre yielded positive results with respect to end-point interchromosomal mitotic recombination, whereas the concentration of 1.0 mmol/litre proved to be lethal (Vogel & Nivard, 1993).
A single intraperitoneal dose of 125 mg thiourea/kg body weight administered to mice led to a weak increase in mutation rate (up to a factor of 3.6) in Salmonella strains TA 1530 and TA 1538 in a host-mediated assay, but negative results were obtained in Saccharomyces cerevisiae following a single intraperitoneal dose of 1000 mg/kg body weight. The examined tissue was the peritoneum (Simmon et al., 1979).
The effect of thiourea was investigated by means of the unscheduled DNA synthesis (UDS) test with primary rat hepatocyte cultures at concentrations ranging from 0.064 to 10 000 µg/litre as part of a collaborative study involving seven laboratories. None of the laboratories identified any induction of UDS. A further laboratory investigated the possible induction of DNA strand breaks by thiourea in primary rat hepatocytes using an alkaline elution technique. Thiourea also proved to have no positive effect in this study (Fautz et al., 1991).
A DNA repair test was carried out with Escherichia coli K-12343/113 at thiourea concentrations up to 329 mmol/litre (equivalent to 25 mg/ml; no further details on the concentration range were provided) in both the absence and presence of metabolic activation provided by the S9-mix from Aroclor 1254-induced rat liver. Thiourea had no effect with metabolic activation, but had a positive effect in its absence (Hellmér & Bolcsfoldi, 1992).
When primary cultures of isolated rat hepatocytes were treated with 5–25 mmol thiourea/litre, the induction of a relatively small linear increase in UDS was observed in the cells (Ziegler-Skylakakis et al., 1985). Very similar results had already been reported previously (Lonati-Galligani et al., 1983), although they were (presumably erroneously: see Rossberger & Andrae, 1987) interpreted as constituting a negative response.
Thiourea at concentrations of 30–300 mmol/litre induced single strand breaks in the DNA of primary cultures of isolated rat hepatocytes (Sina et al., 1983). The inhibitory effect of thiourea on the induction of DNA strand breakage due to various intercalating substances in mouse leukaemia cells might be the result of a change in chromatin structure. This could alter the activity of a topoisomerase responsible for the occurrence of strand breaks in cooperation with the intercalating substances (Pommier et al., 1983). The methods of detection of UDS were either autoradiography or liquid scintillation counting, and the DNA single strand breaks were detected with the alkaline elution assay.
Thiourea has mitogenic properties. Older studies with high doses of thiourea (0.4 g, 1–14 times, intraperitoneal; unclear whether per animal or per kg body weight) produced a high mitosis rate in the liver without hepatocellular necrosis. Studies on partially hepatectomized rats showed similar results (MAK, 1988).
Thiourea can affect fertility as a result of hypothyroidism.
Thiourea was included in the diet of rats at concentrations of between 0.01 and 1% for 24 months, which were equivalent to doses ranging from 5 to 500 mg/kg body weight per day (see Table 4). A reduction or cessation of spermatogenesis and effects on the thyroid gland or other organs were observed at doses higher than 35 mg/kg body weight per day (Fitzhugh & Nelson, 1948).
Thiourea had neither a maternally toxic nor a teratogenic effect when administered to rats on the 12th or 13th day of gestation as a single oral dose of 480 mg/kg body weight (Ruddick et al., 1976).
In a study in which 66 female sheep (18 growing lambs, 18 maiden ewes, 9 pregnant ewes; controls: 9 growing lambs, 9 maiden ewes, 3 pregnant ewes) were orally administered 0 or 50 mg thiourea/kg body weight daily for 2, 4, or 6 months (six treated and three controls per group), external genitalia were infantile and stunted in growing lambs, while they were pale anaemic and dry in maiden ewes. None of the growing lambs showed signs of estrus. Mammary development was retarded (Nasseri & Prasad, 1987b).
Thiourea (50 mg/kg body weight per day) was administered orally to four female lambs 6–8 months of age for 80 days (Alavi Shoushtari & Safaii, 1993). Size and weight of the reproductive tract (ovaries, uterine horn, and vagina) revealed a slight, although not statistically significant, decrease. Histological examination showed that follicles in the ovaries were atretic and that the endometrial cells were shorter than the controls, indicating that hypothyroidism probably suppresses the ovarian and other reproductive functions of female lambs.
[35S]Thiourea was shown to cross the placenta in mice and rats and to be preferentially stored in the thyroid gland, depending on the stage of development of this organ, where it affects iodine metabolism (Shepard, 1963). In a study in which groups of CF4 rats were treated with 0.2% thiourea in the drinking-water on days 1–14 of gestation, growth retardation and malformations of the nervous system and skeleton were present in treated offspring, although specific incidences of fetal effects were not given (Kern et al., 1980). Maternally toxic oral doses of 1000 mg thiourea/kg body weight administered to mice on the 10th day and to rats on the 12th or 14th days of gestation were likewise found to be embryotoxic. The rate of absorption of thiourea increased in live fetuses on the 18th and 20th days of gestation in mice and rats, respectively, without any evidence of malformations (Teramoto et al., 1981). Maturation defects were apparent on the 20th day of gestation in the fetuses of dams that had been treated with 0.25% thiourea in the drinking-water during the first 14 days (Kern et al., 1980). These effects can be attributed to the depressing action of thiourea on thyroid activity. It is thus not to be expected that such effects would occur at levels of thiourea that do not result in an inhibition of thyroid function.
In studies with pregnant ewes administered 50 mg thiourea/kg body weight daily for 2, 4, or 6 months, abortion, stillbirth, birth of weak/low-weight lambs, dystokia, and retention of placenta were common features. The severity of changes was dependent upon the stage of gestation when hypothyroidism was induced (Nasseri & Prasad, 1987b).
Eight male lambs aged 3–3.5 months were orally administered 50 mg thiourea/kg body weight daily for 3.5 months (Sokkar et al., 2000). There were four control lambs. The secondary iodine deficiency resulting from the administered thiourea caused hypothyroidism, which led to retardation of growth and interfered with the sexual maturity of the growing male lambs. The treated males did not show any sexual desire when introduced to ewes in estrus compared with control animals. Palpation of the testes of treated lambs revealed hydrocoele with small testes. The average weight of the testes of the hypothyroid lambs was significantly reduced (3.2 ± 0.255 g) compared with that of control lambs (8.9 ± 1.00 g). The testes showed ill developed, small, empty seminiferous tubules with thick basement membranes. The Sertoli cells were primitive and non-functional. The level of testosterone in the plasma of these hypothyroid lambs was not detectable.
Acute intoxication with thiourea has been linked with an increase in the level of histamine in the lungs and plasma (4.38 µg histamine/100 ml plasma was determined for rats administered thiourea intraperitoneally at 10 mg/kg body weight compared with 2.08 µg/100 ml in the controls) and with an increase in lung vessel permeability (Giri et al., 1991a). Rats developed tolerance to an otherwise lethal dose of thiourea (10 mg/kg body weight) when pretreated with a non-lethal dose (0.5 mg/kg body weight) over a period of 8 days. This tolerance was accompanied by a reduction in both lung vessel permeability and plasma histamine levels (Giri et al., 1991b).
Adult and sexually immature Sprague-Dawley rats were given Evans-Blue dye intravenously at 60 mg/kg body weight, followed by 10 or 100 mg thiourea/kg body weight, and sacrificed after 2 h. No difference was seen in lung permeability between control and 26-day-old treated animals. Increased permeability was seen in 50- and 65-day-old rats after treatment. The histamine content of the lung increased with age and after treatment with thiourea. The increased vascular permeability in response to thiourea in mature rats is associated with corresponding increases in lung and plasma histamine levels (Giri et al., 1991a).
[14C]Thiourea (0.6 mg/kg body weight) admin-istered intravenously to adult rats results in binding to lung protein (Hollinger & Giri, 1990).
The oedema-inducing effect of thiourea is probably due to the action of its oxidation product cyanamide and can be alleviated by treatment with hydroxyl radical scavengers such as dimethyl sulfoxide, ethanol, or mannitol (Fox et al., 1983). The adverse action of thiourea on the lungs of rats injected intraperitoneally with 0.3 mg/kg body weight could also be diminished by intraperitoneal treatment with the antiarrhythmic agents procainamide (at 4 mg/kg body weight), quinidine gluconate (20 mg/kg body weight), and lidocaine (30 mg/kg body weight) (Stelzner et al., 1987).
Treatment in vitro with 75 mmol thiourea/litre results in an inhibition of interleukin-8 production in human whole blood, the toxic effect of which can be suppressed by the administration of glutathione or cysteine (DeForge et al., 1992).
Administration of thiourea to healthy animals or humans leads to depression of thyroid function. It acts by inhibiting the peroxidase in the thyroid gland, resulting in decreased thyroid hormone production and increased proliferation due to an increase in the secretion of TSH (MAK, 1988; IARC, 2001). This could lead to tumour formation. This is a well recognized mechanism of action for non-genotoxic thyroid carcinogens (Capen et al., 1999). However, no definite conclusion regarding the mechanism of carcinogenicity can be made for thiourea, since it cannot totally be excluded that the possible genotoxicity of thiourea also plays a role.
It was shown in liver microsomes, in mammalian cells in culture (Ziegler, 1978; Poulsen et al., 1979; Ziegler-Skylakakis, 1998), and in intact rat liver (Krieter et al., 1984) that thiourea can form S-oxygenated products such as the reactive electrophiles formamidine sulfenic acid and formamidine sulfinic acid. The latter has been shown to be genotoxic in cultured mammalian cells (Ziegler-Skylakakis, 1998). The importance of the oxidative thiourea metabolites for the genotoxicity of thiourea needs further elucidation.
On the other hand, under the assumption that there is no direct interaction of thiourea with DNA, it was concluded that thyroid follicular neoplasia involves a non-linear dose–response process and would not develop unless there is prolonged interference with the thyroid–pituitary feedback mechanism (Hard, 1998).
There are several important species differences in thyroid gland physiology, which are important for the development of thyroid tumours. The half-life of T4 is much shorter in rats (12–24 h) than in humans (5–9 days), and the serum levels of TSH are 25 times higher in rodents than in humans. Further, rats require about a 10-fold higher production of T4 than do humans. In addition, the human plasma high-affinity T4-binding globulin is absent in rodents, cats, and rabbits. As a result, more free T4 is transported in the blood in these species, and therefore there are higher levels of metabolism and excretion of T4 in rodents, cats, and rabbits than in humans (Dohler et al., 1979; McClain, 1995; Dybing & Sanner, 1999). The weight of evidence suggests that rodents are more sensitive than humans to thyroid tumour induction due to hormonal imbalances that cause elevated TSH levels. Nevertheless, there are gaps in the available information (Hard, 1998; Capen et al., 1999).
There are reports on disorders of workers coming into contact with thiourea during the course of, for example, maintenance of machinery or packing, without providing any details as to exposure levels. The symptoms observed were typical of hypothyroidism, as evidenced by facial oedema, hypotonia, bradycardia, electrocardiograph alterations associated with reduced basal metabolism, constipation, flatulence, polyuria, and granulocytopenia, accompanied by lymphocytosis and monocytosis. The first perturbations of the blood count were observed after 5–6 months of exposure, and the highest incidence of the symptoms was evident in those workers who had been in contact with the chemical for 5–15 years (Zaslawska, 1964; Speranski et al., 1969).
Indications of reduced thyroid function were observed in a Russian study of workers employed in thiourea manufacture. The study covered 45 exposed workers and 20 unexposed controls. Reported air concentrations of thiourea were in the range 0.6–12 mg/m3 (see section 6.1). The workers had been exposed for 9.5 ± 1.1 years; 73% had been exposed for at least 5 years; and 54.5% of them were over 40 years of age. The concentrations of thyroid hormones T4 and T3 were significantly lower in the exposed workers than in the controls (T4: 78.0 ± 5.2 versus 109.4 ± 2.0 nmol/litre, P < 0.05; T3: 1.2 ± 0.1 versus 3.8 ± 0.1 nmol/litre, P < 0.001). Thyroid hyperplasia was observed in 17 of the 45 exposed workers. Concentrations of T4 and T3 in this subgroup were 80.6 ± 1.8 and 0.9 ± 0.1 nmol/litre, respectively (Talakin et al., 1985).
Slightly elevated levels of immunoglobulin A (1.2 mg/ml compared with 1.03 mg/ml in controls) and immunoglobulin M (1.4 mg/ml compared with a control value of 0.91 mg/ml) were determined for workers in a thiourea processing plant in a Russian study in which details as to exposure were not provided. A decrease in T3 levels (<60 ng/100 ml) at normal levels of T4 and a decrease in the leukocyte count were interpreted by the authors to be indicative of thiourea intoxication (Talakin et al., 1990).
Cases of contact dermatitis have been described in thiourea production workers; the contact dermatitis disappeared rapidly after the workers had been transferred to another workplace (Speranski et al., 1969).
Reports of individual cases of contact dermatitis related to the use or processing of thiourea and thiourea compounds have been reviewed (Dooms-Goossens et al., 1987; Kanerva et al., 1994; McCleskey & Swerlick, 2001). Most cases have been reported from the use of thiourea as an antioxidant in diazo copy paper (light-sensitive photocopy paper) and almost all other types of copy paper (Van der Leun et al., 1977; Nurse, 1980; Kellett et al., 1984; Liden, 1984; Dooms-Goossens et al., 1987; Niinimäki, 1989; Pasche-Koo & Grosshans, 1991; Torres et al., 1992; Geier & Fuchs, 1993; Bartels & Schauder, 1994; van Gerwen et al., 1996; Kanerva et al., 2000). Some cases showed increased sensitivity to UV light (photocontact dermatitis). Contact dermatitis from thiourea in silver polish has also been reported (Dooms-Goossens et al., 1988). Thiourea derivatives such as dimethyl, diethyl, dibutyl, diphenyl, ethylbutyl, and ethylene thiourea are used as accelerators in the vulcanization process in the rubber industry. Products such as wet suits, swimming goggles, orthopaedic devices, protective gloves, and shoes containing these compounds have been shown to produce allergic contact dermatitis (Kanerva et al., 1994; McCleskey & Swerlick, 2001).
It was reported that thiourea compound allergy is relatively rare. An allergic patch test reaction was provoked in only 5 patients out of 423 (1.2%) (Kanerva et al., 1994). Relative to the number of persons exposed to thiourea, the number of reported contact and photocontact allergies to thiourea is small (MAK, 1997).
Thiourea had a former use in the treatment of excessive thyroid gland activity. The doses of thiourea recommended vary considerably. Originally, a dose of 2–3 g daily was used, especially as an initial dose. This was later reduced because of the associated side-effects. The side-effects of thiourea have been described from observations of the former therapeutic use of thiourea in the 1940s as a thyroid depressant (MAK, 1988). Forty-nine (i.e., 9.3%) of 525 patients who were treated with thiourea suffered from one or more of the following side-effects as specified by the respective number of individual cases quoted in parentheses: agranulocytosis (1), leukopenia (4), elevated temperature (24), erythema (9), swollen lymph nodes (1), pains in muscles and joints (4), gastrointestinal disorders (17), and various other symptoms (Vanderlaan & Storrie, 1955). Elevated temperature was observed almost immediately after commencement of the therapy and regressed upon its termination. Both attacks of feverishness, which occur within 7–14 days after the onset of the therapy, and skin reactions have been attributed to sensitization (Peters et al., 1949).
In an early study with hyperthyroid patients (n = 12), it was shown that a dose of 15 mg (about 0.2 mg/kg body weight per day for a 70-kg person) daily for 10–12 weeks was insufficient to depress thyroid activity, as judged by the concentrations of serum precipitable iodine, while a dose of 70 mg daily (1.0 mg/kg body weight per day) in conjunction with iodine solution produced a remission in hyperthyroidism (Winkler et al., 1947).
Four cases of hypothyroidism occurred over a period of 6 years among 539 employees at a textile factory where thiourea and resorcinol were used in the dyeing and finishing processes. A typical level of thiourea at the inlet of the local exhaust ventilation of the stenters was 5 µg/m3, and resorcinol levels were less than 20 µg/m3. The prevalence of hypothyroidism among men appeared to be higher than the rate of <1/1000 found for men in a large epidemiological survey of the adult population in the mixed urban and rural area of Wickham, near Newcastle-upon-Tyne, United Kingdom. The prevalence for women was less remarkable when compared with the rate of 19/1000 found for women in the same survey. The authors concluded that since the employees were exposed to thiourea and resorcinol, both compounds with antithyroid properties, the occurrence of hypothyroidism in this working population could have been work-related (Roberts et al., 1990).
Numerous tests have been performed on the toxicity of thiourea to all trophic levels of aquatic organisms. Experimental test results for the most sensitive species are summarized in Table 5. Additional data on the toxicity of thiourea to aquatic organisms are cited in the BUA (1995) report. Among the tested organisms, green algae (Scenedesmus subspicatus) and water flea (Daphnia magna) proved to be the most sensitive freshwater species. The lowest EC50 value determined in a 96-h cell multiplication inhibition test was reported to be 3.8 mg/litre for S. subspicatus. For immobilization of D. magna, a 96-h EC50 value of 1.8 mg/litre was determined. In two long-term tests with D. magna, 21-day NOEC values of <0.25 mg/litre and 0.25 mg/litre were established for reproduction. It has to be taken into account that concentration–response curves in many acute tests on daphnia are very flat and difficult to reproduce, leading to very variable effect values (BUA, 1995). With respect to freshwater fish, all tests available for short-term exposure revealed LC50 values (48- and 96-h) at or above 100 mg/litre. Experimental results from long-term fish studies conducted to standard test procedures are not available. However, many authors have studied the effects of long-term exposure of teleosts and other kinds of fish to thiourea. Effects of thiourea (exposure concentrations: 20–330 mg/litre) on thyroid gland metabolism and the endocrine system have been described (Mackay, 1973; McBride & Van Overbeeke, 1975; Sathyanesan et al., 1978; Saxena & Mani, 1979).
Table 5: Aquatic toxicity of thiourea.
|
Most sensitive species (end-point/test method) |
End-point (effect) |
Concentration |
Reference |
|
Bacteria |
|||
|
Nitrifying enrichment culture from domestic sewage |
IC50 |
0.33 |
Wagner & Kayser (1990) |
|
Municipal activated sludge (nitrification inhibition/ |
EC20 |
0.19 |
FV (1991) |
|
Microbial culture enriched from nitrifying sewage plant (nitrification inhibition test) |
IC50 |
0.8 |
König & Riedel (1998) |
|
Unadapted nitrifying activated sludge (nitrification inhibition test) |
2- to 4-h IC75 |
0.075 |
Downing et al. (1964) |
|
Algae |
|||
|
Scenedesmus subspicatus (biomass reduction) |
96-h EC10 |
0.3–0.6 |
Geyer et al. (1985) |
|
Scenedesmus subspicatus (growth rate) |
96-h EC10 |
0.5–0.7 |
Friesel et al. (1984) |
|
Invertebrates |
|||
|
Daphnia magna (immobilization/static) |
24-h EC0 |
2 |
Friesel et al. (1984) |
|
Daphnia magna (immobilization/static) |
96-h EC50 |
1.8 |
NAPM (1974a,b) |
|
Daphnia magna (reproduction rate/semistatic) |
21-day NOEC |
0.25–1.0 |
Friesel et al. (1984) |
|
Daphnia magna (reproduction rate/semistatic, EEC Directive 79/831) |
21-day NOEC |
<0.25 |
Broecker et al. (1984) |
|
Fish |
|||
|
Fathead minnow (Pimephales promelas) (static test conducted according to US Standard Method) |
96-h LC0 |
100 |
NAPM (1974a,b) |
|
Fathead minnow (Pimephales promelas) (US EPA-600/3-75-009, modified) |
96-h LC0 |
600 |
Curtis et al. (1981) |
|
Zebrafish (Brachydanio rerio) (semistatic) |
21-day NOEC |
>5000 |
Friesel et al. (1984) |
Numerous investigations have been performed on the inhibition of microbial nitrification by thiourea (see Table 5), leading to very heterogeneous results. In short-term toxicity tests conducted with non-adapted activated sludge, inhibition of nitrification was observed at thiourea concentrations as low as 0.075 mg/litre (2- to 4-h IC75), whereas NAPM (1974a,b) determined an IC0 of 100 mg/litre for this end-point. Sensitivity is obviously highly dependent on the origin and adaptation of the specific microbial consortium. Tests on respiration inhibition revealed IC0 values of >100 mg/litre for activated sludge (NAPM, 1974a,b; Grünwald, 1984) and IC50 values of up to 4500 mg/litre. From the available studies, it can be concluded that microorganisms are able to adapt to thiourea.
Laboratory tests on the toxicity of thiourea to terrestrial species have been performed with microorganisms, higher plants, and invertebrates (earthworms, nematodes, insects). Experimental test results for the most sensitive species are summarized below. Additional data on the toxicity of thiourea to terrestrial species are cited in the BUA (1995) report. Among the tested organisms, different stages of the red cotton bug (Dysdercus similis) proved to be most sensitive, exhibiting EC50 values of 0.03 and 0.025 mg/litre for egg survival and hatching, respectively.
Different fungi were found to be relatively insensitive to thiourea exposure. Complete growth inhibition was observed for Penicillium rugulosum after a 7-day exposure to 2000 mg thiourea/litre (Lashen & Starkey, 1970) and for Helminthosporium sativum and Fusarium oxysporum after a 15-day exposure to 750 mg/litre and 1000 mg/litre, respectively (Pandey et al., 1976).
Terrestrial plants proved to be generally more sensitive. Whereas thiourea concentrations below 12 mg/litre increased the growth of excised tomato roots (Lycopersicum esculentum) within 4 weeks of exposure in a defined basal medium, 18, 23, and 46 mg/litre reduced growth by about 45%, 60%, and 30%, respectively (Glazer & Orion, 1984). Friesel et al. (1984) obtained 14-day EC50 values of 15 mg/kg soil dry weight (turnip, Brassica rapa) and 190 mg/kg soil dry weight (common oat, Avena sativa) in a study conducted according to the draft of the OECD guideline "Growth Test with Higher Plants" (1981; adopted in 1984 as OECD Guideline 208). Rudolph & Boje (1985) reported 14-day EC50 values in the range of 205–618 mg/kg soil dry weight and 190–618 mg/kg soil dry weight for B. rapa and A. sativa, respectively. In greenhouse experiments, Günther & Pestemer (1990) determined a 10-day EC50 of 52.1 mg/kg for the end-point growth/germination of B. rapa. In experiments with 8 weeks of exposure of A. sativa to thiourea in soil solution, Günther & Pestemer (1990) observed that during the first 4 weeks of exposure, the EC50 value for growth reduction dropped from 170 mg/litre after 2 weeks over 80 mg/litre after 3 weeks to 30 mg/litre after 4 weeks. This value remained constant during the course of the following 4 weeks.
Friesel et al. (1984) investigated the toxicity of thiourea towards the earthworm Eisenia fetida according to the OECD draft "Guideline on Testing the Toxicity of Chemicals and Plant Protection Agents towards the Earth Worm" (adopted as OECD Guideline 207 in 1984). They determined a 28-day LC50 of 3550 mg/kg soil dry weight. Rudolph & Boje (1985) reported a 28-day LC50 of >1000 mg/kg soil dry weight for E. fetida.
Glazer & Orion (1984) investigated the effects of thiourea on the development of nematodes. Excised tomato roots, growing on basal medium and inoculated with eggs of Meloidogyne javanica, were exposed to thiourea concentrations in the range of 6–46 mg/litre. After 96 h of exposure, thiourea concentrations of 12 mg/litre inhibited nematode development. Only 36% matured to adults (in the untreated control: 90%) after an observation period of 4 weeks. For M. javanica (second larval stage), Tylenchulus semipenetrans (second larval stage), and Pratylenchus thornei (adult and juvenile organisms), no increased mortality was found after incubation in aqueous solutions of thiourea at concentrations up to 100 mg/litre for 96 h. The authors furthermore demonstrated that thiourea is taken up via the plant roots and that the nematicidal effect is systemic.
Bhide (1991) investigated the effect of different thiourea concentrations on eggs and nymphs of the red cotton bug (Dysdercus similis), a cotton plant pest. Solutions were applied topically to larval stages 1–5 and, for imagos, additionally in the diet. EC50 values of 0.03 mg/litre and 0.025 mg/litre were determined for egg survival and hatching, respectively. Thiourea concentrations in the range of 0.01–0.025 mg/litre reduced adult emergence by 50%. When nymphal instars were exposed topically, a thiourea concentration of 100 mg/litre proved to be lethal, with all the nymphs at all the various stages of development dying within 6 h.
The database is old and insufficient to derive quantitative estimates of tolerable intakes or tolerable concentrations for exposure to thiourea. Species differences in toxicity are large, and there is evidence of tolerance after relatively low exposure, which makes the extrapolation of animal data to humans difficult. In addition, the mechanism of toxic action, which is based on disturbance of hormonal balance and possibly involvement of immune response, may be different in humans and animals.
The critical effect of thiourea is inhibition of thyroid function, which has been shown in humans and in animal studies.
There are only a few reports of adverse effects on health after occupational exposure. Inhibition of thyroid function, as shown by reduction in the concentrations of thyroid hormones T4 and T3, has been reported at a thiourea manufacturing factory in Russia. Thyroid hyperplasia was reported in 17 out of 45 workers exposed to a reported 0.6–12 mg/m3 (Talakin et al., 1985). In other studies, stomach and intestinal disorders as well as blood count changes have also been described.
Thiourea was used in former times as a thyroid depressant in patients with hyperthyroidism. A daily dose of <15 mg (<0.2 mg/kg body weight per day for a 70-kg adult) in adults did not lead to measurable depression of the thyroid gland function, while a dose of 70 mg/day (about 1.0 mg/kg body weight per day) produced a remission of hyperthyroidism (Winkler et al., 1947).
Contact dermatitis and photocontact dermatitis upon dermal exposure have been described during thiourea production and also after handling products containing thiourea, such as diazo copy paper and silver polish. However, thiourea gave a negative result in a guinea-pig sensitization assay.
Administration of thiourea to laboratory animals has caused a reduction in weight gain and enlargement of the thyroid gland and resulting symptoms of hypothyroidism.
Most of the studies in experimental animals were not performed according to current standards and were in some cases not suitable for the overall assessment. There was only one study in which a LOAEL/NOAEL could be derived.
A LOAEL of 27.5 mg/kg body weight per day (reduction of body weight and enlargement of thyroid gland) and a NOAEL of 6.88 mg/kg body weight per day for rats were given for a 2-year drinking-water study (Hartzell, 1942, 1945).
Studies of genotoxicity in vitro and in vivo gave inconsistent results, with the majority being negative. Therefore, thiourea is not considered to be a genotoxic carcinogen.
There are no reports of carcinogenicity due to thiourea exposure in humans.
In several strains of mice, thyroid hyperplasia, but not thyroid tumours, was induced after oral administration of high doses of thiourea. In rats, a high incidence of thyroid follicular cell adenomas and carcinomas or increased incidences of hepatocellular adenomas or tumours of Zymbal or Meibomian glands were observed after oral administration of thiourea. However, there were deficiencies in each of these studies.
Thiourea promoted thyroid tumours in rats initiated by DHPN, but did not show any promoting activity in a rat liver foci bioassay after initiation with diethylnitrosamine or DHPN.
Thiourea passes the placental barrier. In rats, thiourea at maternally toxic doses (0.25% in drinking-water; 350 mg/kg body weight per day) was toxic to the fetuses of the dams.
Hypothyroidism caused by administration of 50 mg thiourea/kg body weight per day to sheep for 2, 4, or 6 months adversely influences somatic development, reproductive/gestational behaviour of animals, and growth of developing fetuses in utero. A similar study with male lambs showed adverse effects on male reproductive development. In limited studies in rodents, no teratogenic effects have been observed.
Thyroid hyperplasia was observed in 17 of the 45 workers exposed to air concentrations of 0.6–12 mg/m3. If it is assumed that the workers weighed 70 kg and inhaled 1 m3/h for 8 h/day and that the uptake was complete, this air concentration is equivalent to a dose of 0.07–1.4 mg thiourea/kg body weight per day. At these levels, there was a clear effect. Therefore, tolerable intakes should be much below 0.07 mg thiourea/kg body weight per day.
From data on its use as a thyroid depressant, <15 mg thiourea/day (<0.2 mg/kg body weight per day for a 70-kg adult) had no effect, whereas 70 mg/day (about 1.0 mg/kg body weight per day) showed an effect (Winkler et al., 1947).
Due to a lack of suitable studies and due to the species differences in thyroid gland biochemistry and physiology, it is difficult to set a tolerable intake or tolerable concentration based on animal studies.
Although thiourea has been shown to be a carcinogen in rats, the weight of evidence suggests that rodents are more sensitive than humans to thyroid tumour induction due to hormonal imbalances that cause elevated TSH levels. Up to now, radiation is the only well defined risk factor for thyroid cancer, although an excess risk of thyroid cancer has, in some studies, been associated with goitre (hypothyroidism) (Hill et al., 1998; Franceshi & Dal Maso, 1999).
In occupational settings, dermal contact with thiourea (and resulting sensitization) is a relevant exposure scenario.
An occupational exposure study giving measured data from the production and packing of thiourea in a German factory reported an average air concentration (thiourea in total dust) of 0.085 mg/m3 (maximum 0.32 mg/m3) (BUA, 1995). From the data reported in the Russian study, it is likely that at least at these maximum levels, a health risk may exist if no hygienic precautions are taken.
The accuracy of the occupational exposure data (Talakin et al., 1985) is uncertain.
Although the clinical experience from the use of thiourea as an antithyroid drug is rather extensive, the estimate of the no-effect level is based on very limited information from rather old studies, where the assessment of thyroid function was not performed with the sensitive methods of today. Furthermore, these were patients with hyperthyroidism and not healthy workers.
High doses of thiourea have induced hypothyroidism and thyroid tumours and promote nitrosamine-induced carcinogenesis in the thyroid in rats and hypothyroidism without thyroid tumours in mice. Although these tumours are likely to be induced by the hypothyroidism, thiourea has also, in some studies, shown weak genotoxic potential, and the mechanism of carcinogenesis is not fully settled. There are no studies on the possible carcinogenic effect of thiourea in exposed humans.
There are possible species differences in thyroid gland biochemistry and physiology, which indicate that the rodent thyroid gland is more active and operates at a higher level with respect to thyroid hormone turnover compared with the human gland.
The estimation of workplace exposure is based on very limited data.
The main environmental target compartment of thiourea from its physicochemical properties and its uses is expected to be the hydrosphere.
The calculated Henry’s law constant indicates that thiourea is not expected to volatilize from aqueous solution. In water, thiourea is resistant to hydrolysis. Whereas direct photolysis is not to be expected, thiourea undergoes photochemical oxidation via reaction with hydroxyl radicals. Half-lives of 17 days and 2.4 h can be calculated for the hydrosphere and the atmosphere, respectively. According to the available data, thiourea will be biodegraded by an adapted microflora only after extended acclimation periods. Thus, under conditions not favouring biotic or abiotic removal, thiourea may be present in surface waters and sediments over longer time periods. Adsorption to sediment particles, however, is not to be expected.
The available experimental data on bioaccumulation as well as the measured n-octanol/water partition coefficients indicate no bioaccumulation potential for thiourea in aquatic organisms.
A sample risk characterization with respect to the aquatic environment may be performed by calculating the ratio between a (local or regional) predicted environmental concentration (PEC; based on measured or model concentrations) and a predicted no-effect concentration (PNEC) (EC, 1996).
A quantification of the thiourea releases from all the different industrial sources is not possible with the available data. Furthermore, there are no present-day monitoring data available. Thus, a PEC for thiourea in the hydrosphere could not be defined.
A PNEC for surface waters may be calculated by dividing the lowest valid NOEC obtained in chronic studies by an appropriate uncertainty or characterization factor:
PNEC = (0.25 mg/litre)/50 = 0.005 mg/litre
where:
In European Union jurisdiction, for substances exhibiting PEC/PNEC ratios of less than or equal to one, further information and/or testing as well as risk reduction measures beyond those that are already being applied are not required. Therefore, measured or calculated thiourea concentrations in surface waters below 0.005 mg/litre will not lead to any regulatory actions.
The lack of current monitoring data for relevant aquatic compartments does not allow a quantitative risk assessment to be performed; however, from the reliable data available on environmental fate, bioaccumulation, and ecotoxicity, a significant risk of thiourea to aquatic organisms is not to be expected (except in the case of an accidental spill).
Due to the measured soil sorption coefficients and the observed (slow) biodegradation in soil, accumulation of thiourea in the geosphere is not to be expected. A leaching of residual thiourea from soil into groundwater cannot be excluded.
For the terrestrial compartment, toxicity tests on microorganisms, higher plants, earthworms, nematodes, and insects are available. The lowest effect value is reported for turnip (Brassica rapa; 14-day EC50 = 15 mg/kg soil dry weight). No studies on the toxicity of thiourea to terrestrial vertebrates or effects on ecosystems are available.
As no measured soil concentrations of thiourea are available, a quantitative risk characterization could not be performed. However, according to the experimental data available for toxicity to terrestrial species, the low bioaccumulation potential, and the expected environmental fate when released to soil, thiourea is not expected to pose a significant risk for terrestrial species (except in the case of an accidental spill).
Due to the lack of measured thiourea concentrations in surface waters and soil, a quantitative risk assessment for these environmental compartments could not be performed.
IARC (2001) has concluded that there is inadequate evidence in humans and limited evidence in experimental animals for the carcinogenicity of thiourea. Overall, therefore, thiourea is not classifiable as to its carcinogenicity to humans (Group 3).
Alavi Shoushtari SM, Safaii M (1993) Effects of hypothyroidism on the reproductive system of female lambs. Journal of the Veterinary Faculty of the University of Tehran, 48:40–35.
Anbar M, Neta P (1967) A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. International Journal of Applied Radiation and Isotopes, 18:493–523.
Astwood EB (1943) The chemical nature of compounds which inhibit the function of the thyroid gland. Journal of Pharmacology and Experimental Therapeutics, 78:79–89.
Astwood EB, Bissell A, Hughes AM (1945) Further studies on the chemical nature of compounds which inhibit the function of the thyroid gland. Endocrinology, 37:456–481.
Bartels S, Schauder S (1994) [Photoallergic contact dermatitis and persistent light reaction from thiourea in blueprint paper.] Aktuelle Dermatologie, 20:182–184 (in German).
Batiste-Alentorn M, Xamena N, Creus A, Marcos R (1991) Genotoxicity studies with the unstable zeste-white (UZ) system of Drosophila melanogaster: results with ten carcinogenic compounds. Environmental and Molecular Mutagenesis, 18:120–125.
Batiste-Alentorn M, Xamena N, Creus A, Marcos R (1994) Further studies with the somatic white-ivory system of Drosophila melanogaster: genotoxicity testing of ten carcinogens. Environmental and Molecular Mutagenesis, 24:143–147.
Bhide M (1991) Thiourea as a xenobiotic, showing its adverse effects on mortality, behaviour and metamorphosis and on histopathological and cytological changes in the developing ovaries of Dysdercus similis. Functional and Developmental Morphology, 1(1):27–34.
Blume H, Ahlsdorf B (1993) Prediction of pesticide behavior in soil by means of simple field tests. Ecotoxicology and Environmental Safety, 26:313–332.
Brams A, Buchet JP, Crutzen-Fayt MC, De Meester C, Lauwerys R, Leonaed A (1987) A comparative study, with 40 chemicals, of the efficiency of the Salmonella assay and the SOS chromotest (kit procedure). Toxicology Letters, 38:123–133.
Broecker B, Fischer R, Gerber HG, Görlitz G, Markert M, Wellens H (1984) Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufe 1 und 2 des Chemikaliengesetzes. FE-Vorhaben 10604011/07. Berlin, Umweltbundesamt.
BUA (1995) Thiourea. German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). Stuttgart, S. Hirzel, Wissenschaftliche Verlagsgesellschaft (BUA Report 179).
Capen CC, Dybing E, Rice JM, Wilbourn JD, eds. (1999) Species differences in thyroid, kidney and urinary bladder carcinogenesis. IARC Scientific Publications, 147:1–225.
CARB (1997) Thiourea. Sacramento, CA, California Air Resources Board, 4 pp. (Toxic Air Contaminant Fact Sheets; available at http://).
Casas CB, Koppisch E (1952) The thyroid and adrenal glands of castrated C3H mice treated with thiourea. Endocrinology, 51:322.
Caspary WJ, Daston DS, Myhr BC, Mitchell AD, Rudd CJ, Lee PS (1988) Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay: interlaboratory reproducibility and assessment. Environmental and Molecular Mutagenesis, 12(Suppl. 13):195–229.
Crebelli R, Bellincampi D, Conti G, Conti L, Morpurgo G, Carere A (1986) A comparative study on selected chemical carcinogens for chromosome malsegregation, mitotic crossing-over and forward mutation induction in Aspergillus nidulans. Mutation Research, 172:138–149.
Curtis M, Curran C, Ward C (1981) Aquatic toxicity testing as fundament for a spill prevention program. In: Proceedings of the 1980 National Conference on Control of Hazardous Material Spills. New York, NY, American Institute of Chemical Engineers, pp. 284–288.
Dalton AJ, Morris HP, Dubnik CS (1948) Morphologic changes in the organs of female C3H mice after long-term ingestion of thiourea and thiouracil. Journal of the National Cancer Institute, 9:201.
Davidson B, Soodak M, Strout HV, Neary JT, Nakamura C, Maloof F (1979) Thiourea and cyanamide as inhibitors of thyroid peroxidase: the role of iodide. Endocrinology, 104:919.
DeForge LE, Fantone JC, Kenney JS, Remick DG (1992) Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. Journal of Clinical Investigation, 90:2123–2129.
Deichmann WB, Keplinger M, Sala F, Glass E (1967) Synergism among oral carcinogens. IV. The simultaneous feeding of four tumorigens to rats. Toxicology and Applied Pharmacology, 11:88–103.
Dohler KD, Wong CC, von zur Muhlen A (1979) The rat as a model for the study of drug effects on thyroid function: Consideration of methodological problems. Pharmacology and Therapeutics, 5:305–318.
Dooms-Goossens A, Chrispeels MT, de Veylder H, Roelandts R, Willems L, Degreef H (1987) Contact and photocontact sensitivity problems associated with thiourea and its derivatives: a review of the literature and case reports. British Journal of Dermatology, 116:573–579.
Dooms-Goossens A, Debusschere K, Morren M, Roelandts R, Coopman S (1988) Silver polish: another source of contact dermatitis reactions to thiourea. Contact Dermatitis, 19:133–135.
Downing A, Tomlinson T, Truesdale G (1964) Effect of inhibitors on nitrification in the activated-sludge process. Journal of the Institute of Sewage Purification, 6:537–554.
Dybing E, Sanner T (1999) Species differences in chemical carcinogenesis of the thyroid gland, kidney and urinary bladder. IARC Scientific Publications, 147:15–32.
EC (1996) Technical guidance document in support of the Commission Directive 93/EEC on risk assessment for new notified substances and the Commission Regulation (EC)1488/94 on risk assessment for existing substances. Brussels, European Commission.
Environment Agency Japan (1985) Report on environmental survey and wildlife monitoring in F.Y. 1982 and 1983. In: Chemicals in the environment. Tokyo, Department of Environmental Health, Office of Health Studies, pp. 1–76.
Fautz R, Forster R, Hechenberger CMA, von der Hude W, Kaufmann G, Madle H, Madle S, Miltenberger HG, Mueller L, Pool-Zobel BL, Puri EC, Schmezer P, Seeberg AH, Strobel R, Suter W, Baumeister M (1991) Report of a comparative study of DNA damage and repair assays in primary rat hepatocytes with five coded chemicals. Mutation Research, 260:281–294.
Fesch C, Simon W, Haderlein S, Reichert P, Schwarzenbach R (1998) Nonlinear sorption and nonequilibrium solute transport in aggregated porous media: Experiments, process identification and modeling. Journal of Contaminant Hydrology, 31:373–407.
Fischer H (1985) Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufen 1 und 2 des Chemikaliengesetzes – 25-Stoffe-Programm – FE-Vorhaben 10604 011. Abschlußseminar am 17./18.01.1985: Auswertung und Übersicht ueber die durchgeführten Prüfungen: Akkumulation; Bioabbau (SCAS-Test). Berlin, Umweltbundesamt.
Fitzhugh OG, Nelson AA (1948) Liver tumors in rats fed thiourea or thioacetamide. Science, 108:626–628.
Fox RB, Harada RN, Tate RM, Repine JE (1983) Prevention of thiourea-induced pulmonary edema by hydroxyl-radical scavengers. Journal of Applied Physiology, Respiratory Environmental and Exercise Physiology, 55:1456–1459.
Franceschi S, Dal Maso L (1999) Hormonal imbalances and thyroid cancers in humans. IARC Scientific Publications, 147:33–43.
Freitag D, Lay J, Korte F (1984) Environmental hazard profile — test results as related to structures and translation into the environment. In: Kaiser KE, ed. QSAR in environmental toxicology: proceedings of the workshop on quantitative structure–activity relationships (QSAR) in environmental toxicology. Dordrecht, D. Reidel Publishing Company, pp. 111–136.
Freitag D, Ballhorn L, Geyer H, Korte F (1985) Environmental hazard profile of organic chemicals — an experimental method for the assessment of the behavior of organic chemicals in the ecosphere by simple laboratory tests with carbon-14-labeled chemicals. Chemosphere, 14:1589–1616.
Friedman MA, Staub J (1976) Inhibition of mouse testicular DNA synthesis by mutagens and carcinogens as a potential simple mammalian assay for mutagenesis. Mutation Research, 37:67.
Friesel P, Hansen P-D, Kühn R, Trénel J (1984) Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Stufen 1 und 2 des Chemikaliengesetzes – Teil VI – FE-Vorhaben 10604 011/08. Berlin, Umweltbundesamt.
FV (1991) Forschungsvorhaben "Bewertung wassergefährdender Stoffe - Teil III" Nitrifikationshemmtest - Leuchtbakterienhemmtest. Ergebnisprotokoll vom 23.5.1991.
Galli A, Schiestl RH (1996) Effects of Salmonella assay negative and positive carcinogens on intrachromosomal recombination in G1-arrested yeast cells. Mutation Research, 370:209–221.
Gargus JL, Paynter OE, Reese WH (1969) Utilization of newborn mice in the bioassay of chemical carcinogens. Toxicology and Applied Pharmacology, 15:552.
Geier J, Fuchs T (1993) Contact allergy due to 4-N,N-dimethylaminobenzene diazonium chloride and thiourea in diazo copy paper. Contact Dermatitis, 28:304–305.
Geyer H, Politzki G, Freitag D (1984) Prediction of ecotoxicological behaviour of chemicals: relationship between n-octanol/water partition coefficient and bioaccumulation of organic chemicals by alga Chlorella. Chemosphere, 13: 269–284.
Geyer H, Scheunert I, Korte F (1985) The effects of organic environmental chemicals on the growth of the alga Scenedesmus subspicatus: a contribution to environmental biology. Chemosphere, 14:1355–1369.
Giri S, Combs A (1972) Thiourea binding by rat erythrocyte, resistant to trichloroacetic acid denaturation of protein. Chemico-Biological Interactions, 5:97–105.
Giri SN, Hollinger MA, Rice SA (1991a) Effects of thiourea on pulmonary vascular permeability and on lung and plasma histamine levels in rats. Toxicology Letters, 57:283–290.
Giri SN, Hollinger MA, Rice SA (1991b) Effects of thiourea tolerance on plasma histamine, and lung vascular permeability. Archives of Toxicology, 65:603–605.
Glazer I, Orion D (1984) Influence of urea, hydroxurea and thiourea on Meloidogyne javanica and infected excised tomato roots in culture. Journal of Nematology, 16(2):125–130.
Gorbman A (1947) Thyroidal and vascular changes in mice following chronic treatment with goitrogens and carcinogens. Cancer Research, 7:746.
Govers H, Ruepert C, Stevens T, Van Leeuwen C (1986) Experimental determination and prediction of partition coefficients of thioureas and their toxicity to Photobacterium phosphoreum. Chemo-sphere, 15:383–393.
Grünwald R (1984) Einfluß von Thioharnstoff auf aerobe biologische Prozesse insbesondere auf die Nitrifikation. Diplomarbeit im Fachbereich Chemie der Fachhochschule Aalen, pp. 1–70.
Günther P, Pestemer W (1990) Risk assessment for selected xenobiotics by bioassay methods with higher plants. Environmental Management, 14:381–388.
Hard GC (1998) Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environmental Health Perspectives, 106:427–457.
Hartzell A (1942) Adult life span animal feeding experiments with thiourea (thiocarbamide). Contributions from the Boyce Thompson Institute, 12:471–480.
Hartzell A (1945) Thiourea (thiocarbamide): adult life span feeding experiments with rats. Contributions from the Boyce Thompson Institute, 13:501–513.
Hashimoto A (1979) Salting-out chromatography applied to separation and analysis of mixtures of thioureas and thioacetamide by high performance liquid chromatography. Analytical Chemistry, 51(3):385–387.
Hazleton (1987) 13-week drinking water study in rats with thiourea. Hazleton Laboratories America Inc. for SKW Trostberg AG, Trostberg, pp. 1–360 (HLA Study No. 2319-119).
Hellmér L, Bolcsfoldi G (1992) An evaluation of the E. coli K-12 uvrB/recA DNA repair host-mediated assay. I. In vitro sensitivity of the bacteria to 61 compounds. Mutation Research, 272:145–160.
Hill RN, Crisp TM, Hurley PM, Rosenthal SL, Singh DV (1998) Risk assessment of thyroid follicular cell tumors. Environmental Health Perspectives, 106(8):447–457.
Hollinger MA, Giri SN (1990) Interaction of thiourea with rat lung protein. Toxicology, 60:245–251.
Hollinger MA, Giri SN, Budd E, Hwang F (1974) Tissue distribution and binding of radioactivity from 14C-thiourea in the rat. Drug Metabolism and Disposition, 2:521–525.
Hollinger MA, Giri SN, Budd E (1976) A pharmacodynamic study of [14C]-thiourea toxicity in mature, immature, tolerant and nontolerant rats. Toxicology and Applied Pharmacology, 37:545–556.
IARC (1974) Some anti-thyroid and related substances, nitrofurans and industrial chemicals. Lyon, International Agency for Research on Cancer, pp. 95–109 (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man, Volume 7).
IARC (2001) Thiourea. In: Some thyrotropic agents. Lyon, International Agency for Research on Cancer, pp. 703–725 (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 79).
IPCS (2000) International Chemical Safety Card — Thiourea. Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0680).
Jensen H (1957) Biological transformation of thiourea. Archives of Microbiology, 23:145–152.
Jiang Z et al. (1989) Genotoxicity of 23 chemicals to S. cerevisiae. Environmental and Molecular Mutagenesis, 14(Suppl. 15):95 (Environmental Mutagen Society Abstract 272).
Jones RP (1946) Studies on the effect of thiourea and allied substances on the thyroid gland and other organs in rats and mice. Journal of Pathology and Bacteriology, 58:483.
Kanerva L, Estlander T, Jolanki R (1994) Occupational allergic contact dermatitis caused by thiourea compounds. Contact Dermatitis, 31(4):242–248.
Kanerva L, Estlander T, Jolanki R (2000) Occupational allergic contact dermatitis from trichlorozincates of 4-(dimethylamino)benzendiazonium (Diazo A) and 3-methyl-4-(pyrrolidin-1-yl)benzenediazonium (Diazo Y) and thiourea in diazo copy paper. Contact Dermatitis, 43(3):170–171.
Kellett JK, Beck MH, Auckland G (1984) Contact sensitivity to thiourea in photocopy paper. Contact Dermatitis, 11:124.
Kern M, Tatár-Kiss Z, Kertai P, Foeldes I (1980) Teratogenic effect of 2'-thiourea in the rat. Acta Morphologica Academiae Scientiarum Hungaricae, 28:259.
Keston AS, Goldsmith ED, Gordon AS, Charipper HA (1944) The effect of thiourea upon the metabolism of iodine by rat thyroid. Journal of Biological Chemistry, 152:241–244.
Kobayashi H, Matano O, Goto S (1981) Simultaneous quantitation of thioureas in rat plasma by high-performance liquid chromatography. Journal of Chromatography, 207:281–285.
König A, Riedel K (1998) A microbial sensor for detecting inhibitors of nitrification in wastewater. Biosensors and Bioelectronics, 13:869–874.
Korte F, Greim H (1981) Überprüfung der Durchführbarkeit von Prüfungsvorschriften und der Aussagekraft der Grundprüfung des E. Chem. G. Forschungsbericht 10704 006/01. Neuherberg, Gesellschaft für Strahlen- und Umweltforschung München mbH (GSF).
Krieter PA, Ziegler DM, Hill KE, Burk RF (1984) Increased biliary GSSG efflux from rat livers perfused wth thiocarbamide substrates for the flavin-containing monooxygenase. Molecular Pharmacology, 26:122–127.
Kubota M, Asami T (1985) Source of nitrous acid volatilized from upland soils. Soil Science and Plant Nutrition (Tokyo), 31:35–42.
Lashen E, Starkey R (1970) Decomposition of thioureas by a penicillium species and soil and sewage-sludge microflora. Journal of General Microbiology, 64:139–150.
Liden C (1984) Contact allergy to the photographic chemical PBA-1. Contact Dermatitis, 11:156.
Loehr R, Matthews J (1992) Loss of organic chemicals in soil: Pure compound treatability studies. Journal of Soil Contamination, 1(4):339–360.
Lonati-Galligani M, Lohman PHM, Berends F (1983) The validity of the autoradiographic method for detecting DNA repair synthesis in rat hepatocytes in primary culture. Mutation Research, 113:145–160.
Mackay N (1973) The effects of methallibure (I.C.I.33,828) and thiourea on gametogenesis in the firetail gudgeon, Hypseleotris galii. General Comparative Endocrinology, 20:221–235.
MacKenzie CG, MacKenzie JB (1943) Effect of sulfonamides and thioureas on the thyroid gland and basal metabolism. Endocrinology, 32:185–209.
Magnusson B, Kligman A (1970) Allergic contact dermatitis in the guinea pig. Identification of contact allergens. Springfield, IL, Charles C. Thomas, pp. 1–139.
MAK (1988) Thiourea. In: Henschler D, ed. Occupational toxicants: Critical data evaluation for MAK values and classification of carcinogens. Volume 1. Deutsche Forschungsgemeinschaft (DFG); Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, VCH Verlagsgesellschaft mbH, pp. 301–312.
MAK (1997) Thiourea. In: Greim H, ed. Occupational toxicants: Critical data evaluation for MAK values and classification of carcinogens. Volume 14. Deutsche Forschungsgemeinschaft (DFG); Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, Wiley-VCH, pp. 143–148.
Malcolm J, Griesbach W, Bielschowsky F (1949) Hyperplasia of the parathyroids associated with osteitis fibrosa in rats treated with thiouracil and related compounds. British Journal of Experimental Pathology, 30:17.
McBride J, Van Overbeeke A (1975) Effects of thiourea treatment on sexually maturing and gonadectomized male sockeye salmon (Oncorhynchus nerka). Journal of the Fisheries Research Board of Canada, 32:11–19.
McClain RM (1995) Mechanistic consideration for the relevance of animal data on thyroid neoplasia to human risk assessment. Mutation Research, 333:131–142.
McCleskey PE, Swerlick RA (2001) Clinical review: thioureas and allergic contact dermatitis. Cutis, 68(6):387–396.
Mertschenk B, Beck F, Bauer W (1995) Thiourea and thiourea derivates. In: Elvers B, ed. Ullmann’s encyclopedia of industrial chemistry, 5th ed. Volume A26. Weinheim, VCH, pp. 803–815.
MITI (1992) Biodegradation and bioaccumulation. Data of existing chemicals based on the CSCL Japan. Tokyo, Ministry of International Trade and Industry, Chemicals Inspection & Testing Institute Japan, pp. 3–99.
Mitsumori T, Onodera H, Takahashi M, Shimo T, Yasuhara K, Takegawa K, Takahashi M, Hayashi Y (1996) Promoting effect of large amounts of vitamin A on cell proliferation of thyroid proliferative lesions induced by simultaneous treatment with thiourea. Cancer Letters, 103:19–39.
Morita T, Iwamoto Y, Shimizu T, Masuzawa T, Yanagihara Y (1989) Mutagenicity tests with a permeable mutant of yeast on carcinogens showing false-negative in the Salmonella assay. Chemical and Pharmaceutical Bulletin, 37:407–409.
Morris HP, Dubnik A, Dalton A (1946) Effect of prolonged ingestion of thiourea on mammary glands and the appearance of mammary tumors in adult C3H mice. Journal of the National Cancer Institute, 7:159.
Nakamura S, Oda Y, Shimada T, Oki I, Sugimoto K (1987) SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: Examination with 151 chemicals. Mutation Research, 192:239–246.
NAPM (1974a) Environmental effect of photoprocessing chemicals. Volume 1. Harrison, NY, National Association of Photographic Manufacturers, Inc.
NAPM (1974b) Environmental effect of photoprocessing chemicals. Volume 2. Harrison, NY, National Association of Photographic Manufacturers, Inc.
Nasseri AA, Prasad MC (1987a) Experimental hydrothyroidism in lambs: clinicobiochemical studies. Indian Journal of Animal Science, 57:383–387.
Nasseri AA, Prasad MC (1987b) Effects of hypothyroidism on reproductive behaviour in female sheep: clinical studies. Indian Veterinary Medicine Journal, 11:191–199.
Niinimäki A (1989) Photocontact allergy from photocopy paper: a report of two cases. In: Frosch PJ, Dooms-Goossens A, Lachapelle J-M, Rycroft RJG, Scheper RJ, eds. Current topics in contact dermatitis. Berlin, Springer, pp. 507–509.
NTP (2000) Eighth annual report on carcinogens. Research Triangle Park, NC, US Department of Health and Human Services, National Institute of Environmental Health Sciences, National Toxicology Program.
Nurse D (1980) Sensitivity to thiourea in plain printing paper. Contact Dermatitis, 6:153–154.
Oesterle D, Deml E (1988) Lack of initiating and promoting activity of thiourea in rat liver foci bioassay. Cancer Letters, 41:245–249.
Painter RB (1977) Rapid test to detect agents that damage human DNA. Nature (London), 265:650.
Pandey K, Mishra G, Grover S (1976) Some studies on chemosterilants. I. Thiourea as fungus growth inhibitor. Science and Culture, 42:476–477.
Pasche-Koo F, Grosshans E (1991) [Contact dermatitis from thiourea.] Nouvelles Dermatologiques, 10:694–696 (in French).
Pergal M, Vukojevic N, Djuric D (1972) Carbon disulfide metabolites excreted in the urine of exposed workers. II. Isolation and identification of thiocarbamide. Archives of Environmental Health, 25:42–44.
Peters JP, Man EB, Kydd DM, Engstrom WW, Waters LL (1949) Toxic effects of antithyroid drugs. Yale Journal of Biological Medicine, 22:139–197.
Pommier Y, Zwelling LA, Mattern MR, Erickson LC, Kerrigan D, Schwartz R, Kohn KW (1983) Effects of dimethyl sulfoxide and thiourea upon intercalator-induced DNA single-strand breaks in mouse leukemia (L1210) cells. Cancer Research, 43:5718–5724.
Poulsen LL, Hyslop RM, Ziegler DM (1979) S-Oxygenation of N-substituted thioureas catalyzed by the pig liver microsomal FAD-containing monooxygenase. Archives of Biochemistry and Biophysics, 198(1):78–88.
Purves HD, Griesbach WE (1947) Studies on experimental goitre. VIII: Thyroid tumours in rats treated with thiourea. Journal of Experimental Pathology, 28:46.
Radomski JL, Deichmann WB, MacDonald WE, Glass EM (1965) Synergism among oral carcinogens. I. Results of the simultaneous feeding of four tumorigens to rats. Toxicology and Applied Pharmacology, 7(5):652–656.
Rheinheimer G, Gericke H, Wesnigk J (1990) Prüfung der biologischen Abbaubarkeit von organischen Chemikalien im umweltrelevanten Konzentrationsbereich. Forschungsbericht 106 02 051. Kiel, Institut für Meereskunde der Christian-Albrechts-Universistät.
Roberts FP, Wright AL, O’Hagan SA (1990) Hypothyroidism in textile workers. Journal of the Society of Occupational Medicine, 40(4):153–156.
Rosin A, Rachmilewitz M (1954) The development of malignant tumors of the face in rats after prolonged treatment with thiourea. Cancer Research, 174:494–496.
Rosin A, Ungar H (1957) Malignant tumors in the eyelids and the auricular region of thiourea-treated rats. Cancer Research, 17:302–305.
Rossberger S, Andrae U (1987) Background DNA repair synthesis in rat hepatocyte cultures used for genotoxicity testing. Toxicology in Vitro, 1:215–223.
Rott B, Viswanathan R, Freitag D, Korte F (1982) Vergleichende Untersuchung der Anwendbarkeit verschiedener Tests zur Überprüfung der Abbaubarkeit von Umweltchemikalien. Chemosphere, 11:531–538.
Rougier A, Dupius D, Lotte C, Roguet R, Schaefer H (1983) In vivo correlation between stratum corneum reservoir function and percutaneous absorption. Journal of Investigative Dermatology, 81:275–278.
Ruddick JA, Newsome WH, Nash L (1976) Correlation of teratogenicity and molecular structure: ethylenethiourea and related compounds. Teratology, 13:263–266.
Rudolph P, Boje R (1986) Ökotoxikologie. Grundlagen für die ökotoxikologische Bewertung von Umweltchemikaliennach dem Chemikaliengesetz. In: Vogl J, Heigl A, Schäfer K, eds. Handbuch des Umweltschutzes. Landsberg, Ecomed Verlag.
Sarkar SR, Singh L, Uniyal BP (1988) Changes in plasma calcium, magnesium, iron and copper in experimental pulmonary edema in rats. Journal of Health Science, XIV:65–68.
Sathyanesan A, Joy K, Kulkarni R (1978) Endocrine changes in fishes in response to pollutants. Quarterly Journal of Surgical Science, 14:64–77.
Saxena P, Mani K (1979) Ovarian recrudescence in freshwater teleost Channa punctatus (Bl.), during thiourea treatment. Indian Journal of Experimental Biology, 17:1301–1304.
Schaefer H, Jamoulle JC (1988) Skin pharmacokinetics. International Journal of Dermatology, 27:351–359.
Schiestl RH (1989) Nonmutagenic carcinogens induce intrachromosomal recombination in yeast. Nature (London), 337:285–288.
Seiler JP (1977) Inhibition of testicular DNA synthesis by chemical mutagens and carcinogens. Preliminary results in the validation of a novel short term test. Mutation Research, 46:305–310.
Shepard TH (1963) Metabolism of thiourea 35S by the fetal thyroid of the rat. Endocrinology, 72:223–230.
Shimo T, Mitsumori K, Onodera H, Yasuhara K, Takahashi M, Ueno Y, Hayashi Y (1994a) Time course observation of thyroid proliferative lesions and serum TSH levels in rats treated with thiourea after DHPN initiation. Cancer Letters, 85:141–149.
Shimo T, Mitsumori K, Onodera H, Yasuhara K, Kitaura K, Takahashi M, Kanno J, Hayashi Y (1994b) Synergistic effects of phenobarbital and thiourea on proliferative lesions in the rat liver. Cancer Letters, 81:45–52.
Simmon VF, Rosenkranz HS, Zeiger E, Poirier LA (1979) Mutagenic activity of chemical carcinogens and related compounds in the intraperitoneal host-mediated assay. Journal of the National Cancer Institute, 62:911–918
Sina JF, Bean CL, Dysart GL, Taylor VI, Bradley MO (1983) Evaluation of the alkaline elution/rat hepatocyte assay as a predictor of carcinogenic/mutagenic potential. Mutation Research, 113:357–391.
Slanina P, Ullberg S, Hammarstroem L (1973) Distribution and placenta transfer of 14C-thiourea and 14C-thiouracil in mice studied by whole-body autoradiography. Acta Pharmacologica et Toxicologica, 32:358–368.
Smith CC (1950) A short term chronic toxicity test. Journal of Pharmacology and Experimental Therapeutics, 100:408–420.
Sokkar S, Soror A, Ahmed Y, Ezzo O, Hamouda M (2000) Pathological and biochemical studies on experimental hypothyroidism in growing lambs. Journal of Veterinary Medicine, Series B, 47(9):641–652.
Speranski NJ, Zacharow IR, Taranucha NM (1969) [Occupational skin diseases in workers at a thiourea-processing factory.] Gigiena Truda i Professional’nye Zabolevaniya, 13:50–51 (in Russian) [cited in MAK, 1988].
Stelzner TJ, Welsh CH, Berger E, McCullough RG, Morris K, Repine JE, Wel JV (1987) Antiarrhythmic agents diminish thiourea-induced pulmonary vascular protein leak in rats. Journal of Applied Physiology, 63:1877–1883.
Swedish Criteria Group for Occupational Standards (1999) Scientific basis for Swedish occupational standards. XX. Consensus report for thiourea. Arbete och hälsa, 26:97–109.
Takegawa K, Mitsumori K, Onodera H, Mutai M, Kitaura K, Takahashi M, Uneyama C, Yasuhara K, Yanai M, Masegi T, Hayashi T (1997) UDP-GT involvement in the enhancement of cell proliferation in thyroid follicular cell proliferative lesions in rats treated with thio-urea and vitamin A. Archives of Toxicology, 71:661–667.
Talakin YUN, Kolornoiskaya M, Meleknin UD, Grishina KA, Chernykh LY, Kondratenko LA (1985) Functional status of the thyroid gland of workers employed in thiourea manufacture. Gigiena Truda i Professional’nye Zabolevaniya, 9:50–51 [cited in Swedish Criteria Group for Occupational Standards, 1999].
Talakin Y, Kurilova V, Savchenko M, Ivanova L, Kostezkaia N (1990) Immune reactivity state in the workers engaged in the processing of thiourea, ammonium thiocyanate, and cobalt and manganese salts. Gigiena Truda i Professional’nye Zabolevaniya, 11:18–20 [cited in BUA, 1995].
Teramoto S, Kaneda M, Aoyama H, Shiramu Y (1981) Correlation between the molecular structure of N-alkylureas and N-alkylthioureas and their teratogenic properties. Teratology, 23:335.
Thomas RG (1990) Volatilization from water. In: Lyman WJ, Reehl WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods. Washington, DC, American Chemical Society.
TNO (1978) Acute dermal toxicity study with the product "Thioharnstoff" in albino rabbits. Central Institute for Nutrition and Food Research, Netherlands, for SKW Trostberg AG, Trostberg, 8 pp. (Report No. R 5693).
TNO (1979a) Study on the percutaneous absorption of thiourea by the rabbit. Central Institute for Nutrition and Food Research, Netherlands, for SKW Trostberg AG, Trostberg, 7 pp. (Report No. R 6259).
TNO (1979b) Acute inhalation toxicity study of a 10% aqueous solution of "Thioharnstoff" with rats. Central Institute for Nutrition and Food Research, Netherlands, for SKW Trostberg AG, Trostberg, 5 pp. (Report No. R 6264).
TNO (1979c) Evaluation of "Kalkstickstoff" and "Thioharnstoff" in the micronucleus test. Central Institute for Nutrition and Food Research, Netherlands, for SKW Trostberg AG, Trostberg (Report No. R 6012).
TNO (1980) Study on the percutaneous absorption of thiourea by the rabbit. Part II. Central Institute for Nutrition and Food Research, Netherlands, for SKW Trostberg AG, Trostberg, 6 pp. (Report No. R 6369).
TNO (1983a) Primary skin irritation test with thiourea in albino rabbits. Institute CIVO–Toxicology and Nutrition, TNO, Netherlands, for SKW Trostberg AG, Trostberg (Report B 83-61/36).
TNO (1983b) Eye irritation test with thiourea, 10% aqueous solution, in albino rabbits. Institute CIVO–Toxicology and Nutrition, TNO, Netherlands, for SKW Trostberg AG, Trostberg (Report No. R 83.312/230061-36).
TNO (1990) Biodegradability of thiourea according to a modified MITI test (OECD 301C). TNO Division of Technology for Society, Netherlands, for SKW Trostberg AG, Trostberg, pp. 1–25 (Report No. R 89/218).
Torres V, Campos Lopes J, Lobo L, Pinto Soares A (1992) Occupational contact dermatitis to thiourea and dimethylthiourea from diazo copy paper. American Journal of Contact Dermatitis, 3:37–39.
Ungar H, Rosin A (1960) The histogenesis of thiourea-induced carcinoma of the auditory duct sebaceous (Zymbal’s) glands in rats. Archivio "De Vecchi" per l’Anatomia Patologica e la Medicina Clinica, 31:419.
US EPA (1999) Toxics Release Inventory. US Environmental Protection Agency, at website http://www.epa.gov/tri/.
Vanderlaan WP, Storrie VM (1955) A survey of the factors controlling thyroid function, with especial reference to newer views on antithyroid substances. Pharmacological Reviews, 7:301.
Van der Leun J, De Kreek E, Deenstra-van Leeuwen H, van Weelden H (1977) Photosensitivity owing to thiourea. Archives of Dermatology, 113:1611.
van Gerwen HJL, Alkemade JAC, van der Valk PGM (1996) [Photocontact allergy to thiourea as a component of photocopy paper: photocontact eczema of "airborne allergic contact dermatitis."] Nederlands Tijdschrift voor Dermatologie en Venereologie, 6:194–195 (in Dutch).
Vasquez-Lopez E (1949) The effects of thiourea on the development of spontaneous tumours on mice. British Journal of Cancer Research, 3:401.
Vogel EW, Nivard MJM (1993) Performance of 181 chemicals in a Drosophila assay predominantly monitoring interchromosomal mitotic recombination. Mutagenesis, 8:57–81.
Wagner R, Kayser G (1990) Laboruntersuchungen zur Hemmung der Nitrifikation durch spezielle Inhaltsstoffe industrieller und gewerblicher Abwässer. GWF-Wasser/Abwasser, 131(4):165–177.
Wangenheim J, Bolcsfoldi G (1988) Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis, 3:193–205.
Weast R, Astle M, eds. (1979) Physical constants of organic compounds. In: CRC handbook of chemistry and physics. Boca Raton, FL, CRC Press, p. C-540.
Williams RH, Kay GA (1947) Thiouracils and thioureas: comparisons of the absorption, distribution, destruction and excretion. Archives of Internal Medicine, 80:37–52.
Winkler AW, Man EB, Danowski TS (1947) Minimum dosage of thiourea, given together with iodine medication, necessary for the production amd maintenance of a remission in hyperthyroidism. Journal of Clinical Investigation, 26:446–452.
Yamaguchi T (1980) Mutagenicity of isothiocyanates, isocyanates and thioureas on Salmonella typhimurium. Agricultural and Biological Chemistry, 44:3017–3018.
Yanagisawa K, Nishio K, Gotoh S (1987) Screening for carcinogens by the DNA synthesis inhibition test using human fibroblasts. Mutation Research, 183:89–94.
Zaslawska AG (1964) [Changes in blood and organs through continuous intoxication with thiourea.] Klinicheskaya Meditsina (Moscow), 42:129–132 (in Russian) [cited in MAK, 1988].
Ziegler DM (1978) Intermediate metabolites of thiocarbamides, thioureylenes and thioamides; mechanism of formation and reactivity. Biochemical Society Transactions, 6:94–96.
Ziegler-Skylakakis K, Rossberger S, Andrae U (1985) Thiourea induces DNA repair synthesis in primary rat hepatocyte cultures and gene mutations in V79 Chinese hamster cells. Archives of Toxicology, 58:5–9.
Ziegler-Skylakakis K, Nill S, Pan JF, Andrae U (1998) S-Oxygenation of thiourea results in the formation of genotoxic products. Environmental and Molecular Mutagenesis, 31:362–373.
BUA (1995) Thiourea. German Chemical Society (GDCh) Advisory Committee on Existing Chemicals of Environmental Relevance (BUA). Stuttgart, S. Hirzel, Wissenschaftliche Verlagsgesellschaft (BUA Report 179)
The objective of BUA assessments is to serve as a basis for the instigation of administrative measures when there are indications of risks of a chemical to health or to the environment.
For the BUA review process, the company that is in charge of writing the report (usually the largest manufacturer in Germany) prepares a draft report using literature from an extensive literature search as well as internal company studies. This draft is subject to a peer review in several readings of a working group consisting of representatives from government agencies, the scientific community, and industry.
The English translation of this BUA report was published in 1998.
MAK (1988) Thiourea. In: Henschler D, ed. Occupational toxicants: Critical data evaluation for MAK values and classification of carcinogens. Volume 1. Deutsche Forschungsgemeinschaft (DFG); Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, VCH Verlagsgesellschaft mbH, pp. 301–312
MAK (1997) Thiourea. In: Greim H, ed. Occupational toxicants: Critical data evaluation for MAK values and classification of carcinogens. Volume 14. Deutsche Forschungsgemeinschaft (DFG); Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission). Weinheim, Wiley-VCH, pp. 143–148
The scientific documentations of the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK Commission) are based on critical evaluations of the available toxicological and occupational medical data from extensive literature searches and of well documented industrial data. The evaluation documents involve a critical examination of the quality of the database, indicating inadequacy or doubtful validity of data and identification of data gaps. This critical evaluation and the classification of substances are the result of an extensive discussion process by the members of the Commission, proceeding from a draft documentation prepared by members of the Commission, by ad hoc experts, or by the Scientific Secretariat of the Commission. Scientific expertise is guaranteed by the members of the Commission, which consists of experts from the scientific community, industry, and employer associations.
The draft CICAD on thiourea was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
R. Benson, US Environmental Protection Agency, Denver, CO, USA
R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
M. Cikrt, National Institute of Public Health, Prague, Czech Republic
G. Dura, Fodor Jozsef National Public Health Centre, Budapest, Hungary
E. Dybing, Norwegian Public Health Institute, Oslo, Norway
C. Elliott-Minty, Health and Safety Executive, Bootle, Merseyside, United Kingdom
L. Fishbein, Fairfax, VA, USA
E. Frantik, National Institute of Public Health, Prague, Czech Republic
R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
A. Hirose, National Institute of Health Sciences, Tokyo, Japan
H. Kivisto, Finnish Institute of Occupational Health, Helsinki, Finland
H. Malcolm, Centre for Ecology and Hydrology, Monks Wood, United Kingdom
M. Mercier, Scientific Institute of Public Health, Brussels, Belgium
H. Nagy, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
E. Savigny, Health and Safety Executive, Bootle, Merseyside, United Kingdom
J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
F. Simeonova, Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
E. Soderlund, Norwegian Institute of Public Health, Oslo, Norway
J. Stauber, Centre for Advanced Analytical Chemistry, Bangor, Australia
M. Sweeney, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
J.H.M. Temmink, Wageningen Agricultural University, Wageningen, The Netherlands
M. Warholm, Institute of Environmental Medicine, Stockholm, Sweden
Monks Wood, United Kingdom,
16–19 September 2002
Members
Dr R. Benson, US Environmental Protection Agency, Region VIII, Denver, CO, USA
Mr R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry (ATSDR), Atlanta, GA, USA
Dr S. Czerczak, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environmental Health, Jozsef Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr Y. Hayashi, Division of Chem-Bio Informatics, National Institute of Health Sciences, Ministry of Health, Labour and Welfare, Tokyo, Japan
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, Division of Risk Assessment, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Prof. J. Jeyaratnam, Colombo, Sri Lanka
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany
Prof. Y.-X. Liang, School of Public Health, Fudan University, Shanghai Medical College, Shanghai, People’s Republic of China
Dr R. Liteplo, Existing Substances Division, Environmental Contaminants Bureau, Health Canada, Ottawa, Ontario, Canada
Ms M.E. Meek, Existing Substances Division, Safe Environments Programme, Health Canada, Ottawa, Ontario, Canada
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr O. Sabzevari, Department of Toxicology & Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr F.P. Simeonova, Sofia, Bulgaria
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, Australia
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr K. Ziegler-Skylakakis, European Commission, DG Employment & Social Affairs, Luxembourg
Resource Persons
Dr C. Cowles, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr C. Elliott-Minty, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Dr K. Fuller, Health and Safety Executive, Industrial Chemicals Unit HD, Bootle, Merseyside, United Kingdom
Observers
Mr A.G. Berends, Solvay S.A., Brussels, Belgium; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr C. Newsome, Dow Chemical Company Limited, West Drayton, Middlesex, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr M.A. Pemberton, Wilmslow, United Kingdom; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr W. Stott, Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Mr J.M. Waechter, Jr, The Dow Chemical Company, Midland, MI, USA; European Chemical Industry Council / European Centre for Ecotoxicology and Toxicology of Chemicals (CEFIC/ECETOC)
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr H. Malcolm, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Ms C. Vickers, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Le présent CICAD consacré à la thiourée a été préparé conjointement par le Comité consultatif sur les substances chimiques d’importance écologique (BUA) de la Société allemande de Chimie (GDCh) et l’Institut Fraunhofer de recherche sur la toxicologie et les aérosols (Allemagne). Il reprend le rapport que le BUA avait déjà rédigé en 1995 au sujet de ce composé et s’inspire d’une documentation établie par la MAK-Komission allemande (1988, 1997). Il a été procédé en novembre 2001 à un dépouillement bibliographique exhaustif des bases de données existantes afin de rechercher des références à des publications postérieures à celles qui sont citées dans les rapports en question. Des informations sur la préparation et l’examen par des pairs des sources documentaires utilisées sont données à l’appendice 1. L’appendice 2 fournit des renseignements sur l’examen par des pairs du présent CICAD. Ce CICAD a été approuvé en tant qu’évaluation internationale lors d’une réunion du Comité d’évaluation finale qui s’est tenue à Monks Wood (Royaume-Uni) du 16 au 19 septembre 2002. La liste des participants à cette réunion figure à l’appendice 3. La Fiche internationale sur la sécurité chimique de la thiourée (ICSC 0680), établie par le Programme international sur la sécurité chimique (IPCS, 2000) est également reproduite dans le présent document.
La thiourée (No CAS
En 1993, la production annuelle mondiale de thiourée avoisinait les 10 000 tonnes.On ne possède pas de données plus récentes sur la production au niveau mondial. La thiourée a de nombreuses applications; on l’utilise par exemple dans la production et la modification des produits auxiliaires pour les textiles et les teintures, pour la lixiviation des minerais, dans la production de produits pharmaceutiques et de pesticides, comme accélérateur de vulcanisation et comme produit auxiliaire dans la préparation du papier diazo.
Des différents usages de la thiourée, on peut déduire que ce composé va principalement aboutir dans l’hydrosphère. On ne possède pas de mesures de la concentration de la thiourée dans les eaux de surface. Le composé ne devrait pas s’évaporer des eaux dans lesquelles il se trouve. Il résiste à l’hydrolyse dans l’eau ainsi qu’à la photolyse directe dans l’air et dans l’eau, mais il subit une oxydation photochimique sous l’effet des radicaux hydroxyle présents dans l’atmosphère (demi-vie calculée : 2,4 heures). Une microflore adaptée est capable de dégrader la thiourée, mais seulement après une longue période d’acclimatation. Par conséquent, lorsque les conditions ne favorisent pas son élimination biotique ou abiotique, la thiourée peut persister longtemps dans les eaux de surface et les sédiments. Toutefois, étant donné la faiblesse de son coefficient de sorption par les particules du sol, il ne semble pas que le composé puisse s’accumuler sur les particules sédimentaires. Il n’est pas exclu que la thiourée puisse passer du sol aux eaux souterraines par lessivage, en particulier lorsque les conditions ne sont pas favorables à une biodégradation. Les données expérimentales que l’on possède au sujet de la bioaccumulation de ce composé indiquent qu’il n’existe pas de possibilité de bioaccumulation de la thiourée dans les organismes aquatiques.
Les données relatives à l’exposition sur le lieu de travail sont peu nombreuses. Selon une étude effectuée dans une usine de production de thiourée, la concentration serait de 0,6 à 12 mg/m3 dans l’aire. Une autre étude sur l’exposition professionnelle, qui rend compte de mesures effectuées dans la zone de production et d’emballage d’une unité de production de thiourée, donne un chiffre de 0,085 mg/m3 (maximum 0,32 mg/m3) pour la concentration moyenne de la thiourée dans les poussières totales.
Il existe un risque d’exposition des consommateurs du fait de la possibilité de contacts cutanés avec des tissus ayant subi un finissage à la thiourée. Des contacts cutanés sont également possibles lors de manipulation d’ozalids sur le lieu de travail (architectes, ingénieurs et dessinateurs industriels). Lorsqu’on utilise du papier diazo pour la reproduction, l’enduit superficiel libère facilement de la thiourée. Il peut également y avoir exposition lors de l’utilisation de produits à faire briller les métaux à base de thiourée ou lors de la métabolisation de produits pharmaceutiques contenant cette molécule.
La thiourée est un antioxydant. Après administration par voie orale à des sujets humains ou à des animaux, elle est presque totalement absorbée puis excrétée en grande partie telle quelle par la voie rénale. Elle peut toutefois subir une certaine métabolisation en acide formamidine-sulfinique, catalysée par la monooxygénase microsomique contenant de la flavine.
Selon des études portant essentiellement sur les animaux de laboratoire, les principaux effets indésirables d’une exposition à la thiourée consistent en une inhibition de la fonction thyroïdienne, mais des effets sur le poumon, le foie, le système hématopoïétique et le rein ont également été décrits. La thiourée provoque également un oedème pulmonaire consécutif à une modification de la perméabilité pulmonaire.
La thiourée est dotée de propriétés mitogènes. Elle ne provoque pas de mutations géniques chez les bactéries. Les épreuves effectuées sur des cellules mammaliennes ont donné des résultats incohérents, mais la plupart du temps négatifs. La thiourée provoque des recombinaisons chromosomiques chez les levures ainsi que chez la drosophile. On ne la considère pas comme un composé cancérogène génotoxique.
A forte dose, la thiourée peut provoquer une hyperplasie de la thyroïde chez la souris ainsi que des adénomes ou des carcinomes thyroïdiens ainsi que des adénomes hépatocellulaires et des tumeurs des glandes de Zymbal et de Meibom chez le rat. Toutefois, aucune des études de cancérogénicité effectuées par le passé ne satisferait aux exigences actuelles en la matière. On ne peut tirer de conclusion définitive quant au mécanisme de l’action cancérogène de la thiourée, mais il est probable qu’il s’agit du mécanisme habituel par lequel agissent les cancérogènes thyroïdiens non génotoxiques.
La cancérogénicité de la thiourée a certes été mise en évidence chez le rat, mais on a la preuve que les rongeurs sont plus sensibles que l’Homme à la formation de tumeurs thyroïdienne sous l’effet de déséquilibres hormonaux qui se traduisent par une élévation du taux de thyréostimuline (TSH).
L’hypothyroïdie provoquée par l’administration de 50 mg de thiourée par kg de poids corporel à des moutons pendant des durées de 2, 4 ou 6 mois a une influence défavorable sur le développement somatique et les performances reproductives et gestationnelles des animaux; elle affecte également la croissance des foetus en cours de développement dans l’utérus. Une étude du même genre effectuée sur des agneaux mâles a montré que la thiourée avait un effet nocif sur le développement de l’appareil reproducteur.
Chez l’Homme, l’exposition à la thiourée peut provoquer des allergies et des photo-allergies de contact. Un test de sensibilisation sur l’animal a donné des résultats négatifs.
Une étude russe relate l’observation d’une hyperplasie thyroïdienne sur 17 membres d’un groupe de 45 travailleurs exposés à des concentrations atmosphériques de thiourée de 0,6 à 12 mg/m3, soit l’équivalent d’une dose journalière de 0,07 à 1,4 mg de composé par kg de poids corporel. Les prises supportables devraient être très inférieures à 0,07 mg de thiourée par kg de poids corporel et par jour.
Les données tirées de l’utilisation de la thiourée comme thyréostatique, montrent que ce composé n’a aucun effet aux doses journalières inférieures à 15 mg (< 0,2 mg /kg de poids corporel par jour), mais que l’effet est visible à la dose de 70 mg par jour (soit l’équivalent d’environ 1,0 mg/kg de poids corporel par jour).
Pour caractériser le risque, on a comparé les données tirées de l’étude russe mentionnée ci-dessus à la concentration atmosphérique moyenne (de thiourée dans les poussières totales) de 0,085 mg/m3 et à la concentration maximale de 0,32 mg/m3 mesurées dans une usine allemande. Il est vraisemblable qu’il existerait un risque sanitaire dans cette usine allemande, tout au moins en cas d’exposition à la concentration maximale, si aucune mesure d’hygiène n’était prise.
On n’a pas chiffré l’exposition de la population générale à la thiourée, ne sorte qu’il n’ est pas possible de caractériser le risque à ce niveau.
En se basant sur des tests toxicologiques valables portant sur divers organismes aquatiques, on peut considérer la thiourée comme modérément à fortement toxique pour la faune aquatique. Deux études à long terme portant sur la reproduction de la daphnie (Daphnia magna) ont permis d’obtenir pour la concentration sans effet observable (NOEC) à 21 jours les valeurs de < 0,25 mg/litre et de 0,25 mg/litre.
D’après les données expérimentales fiables dont on dispose au sujet de la toxicité de ce composé pour les espèces terrestres et aquatiques, compte tenu en outre de son faible potentiel de bioaccumulation et de son devenir environnemental probable après rejet dans l’eau ou le sol, il ne semble pas que la thiourée présente un risque important pour les organismes terrestres ou aquatiques, sauf en cas de déversement accidentel.
El presente CICAD sobre la tiourea es el resultado de la labor conjunta realizada por el Comité Consultivo Alemán sobre las Sustancias Químicas Importantes para el Medio Ambiente (BUA) y el Instituto Fraunhofer de Toxicología y de Investigación sobre los Aerosoles (Alemania). Se basa en un informe del BUA (1995) sobre la tiourea y en la documentación de la Comisión MAK Alemana (1988, 1997). En noviembre de 2001 se realizó una búsqueda bibliográfica amplia de bases de datos pertinentes para localizar cualquier referencia oportuna publicada después de las incorporadas a estos informes. La información relativa a la preparación y el examen colegiado de los documentos originales figura en el Apéndice 1. La información sobre el examen colegiado de este CICAD se presenta en el Apéndice 2. Este CICAD se aprobó en una reunión de la Junta de Evaluación Final celebrada en Monks Wood (Reino Unido) del 16 al 19 de septiembre de 2002. La lista de participantes en la Junta de Evaluación Final figura en el Apéndice 3. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 0680) para la tiourea, preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000).
La tiourea (CAS Nş
La producción anual mundial de tiourea en 1993 fue de unas 10 000 toneladas. No se dispone de una cifra más reciente de la producción mundial. La tiourea tiene una gran variedad de usos; por ejemplo, se utiliza en la producción y modificación de coadyuvantes de los textiles y el teñido, en la lixiviación de menas, en la fabricación de productos farmacéuticos y plaguicidas, como acelerador de la vulcanización y como agente coadyuvante en el papel diazo.
Basándose en las pautas de uso de la tiourea, cabe prever que será la hidrosfera su principal compartimento destinatario en el medio ambiente. No se dispone de mediciones de la concentración del producto químico en las aguas superficiales. No es previsible la evaporación de tiourea a partir del agua. Es resistente a la hidrólisis en agua y la fotolisis directa en el agua y el aire y sufre oxidación fotoquímica por radicales hidroxilo en la atmósfera (semivida calculada de 2,4 h). La tiourea sólo se degrada mediante una microflora adaptada tras largos periodos de aclimatación. Así pues, en condiciones no favorables a la eliminación biótica o abiótica la tiourea puede estar presente en las aguas superficiales y los sedimentos durante períodos más prolongados. Sin embargo, no cabe esperar su acumulación en las partículas de los sedimentos, como indican sus bajos coeficientes de sorción en el suelo. Parece posible la lixiviación de tiourea del suelo hacia el agua freática, particularmente en condiciones desfavorables para la degradación biótica. Los datos experimentales disponibles sobre la bioacumulación indican que la tiourea no tiene potencial para ello en los organismos acuáticos.
Son pocos los datos que hay sobre los niveles de exposición en el lugar de trabajo. En un estudio de una fábrica de producción de tiourea se da una concentración de 0,6 a 12 mg de tiourea/m3 en el aire. En otro estudio de exposición profesional con datos medidos a partir de la producción y el envasado de tiourea se notifica una concentración media en el aire (tiourea en el polvo total) de 0,085 mg/m3 (máximo 0,32 mg/m3).
Es posible la exposición del consumidor debido al contacto cutáneo con tejidos acabados con tiourea. También hay posibilidad de contacto con el papel para copias heliográficas en el lugar de trabajo (arquitectos, ingenieros, delineantes). Cuando se utiliza papel diazo de copia, la tiourea se desprende fácilmente del revestimiento de la superficie. También se puede producir exposición por el uso de limpiadores de metales con tiourea y a partir del metabolismo de productos farmacéuticos a base de tiourea.
La tiourea es antioxidante. Tras la administración oral a personas y animales, se absorbe casi por completo y se excreta en su mayor parte inalterada a través de los riñones. Sin embargo, se puede producir alguna transformación metabólica catalizada por la monooxigenasa microsomal con flavina para formar ácido formamidínsulfínico.
Basándose en los estudios realizados fundamentalmente en animales de laboratorio, el principal efecto adverso para la salud asociado con la exposición a la tiourea es la inhibición de la función glandular del tiroides, aunque se han descrito también efectos en los pulmones, el hígado, el sistema hematopoyético y los riñones. La tiourea produce edema pulmonar derivado de los cambios en la permeabilidad pulmonar.
La tiourea tiene propiedades mitogénicas. El producto químico no induce mutaciones genéticas en bacterias. En valoraciones realizadas con células de mamíferos se obtuvieron resultados discrepantes, siendo la mayoría negativos. La tiourea indujo recombinación cromosómica en levaduras y en Drosophila. No se considera un carcinógeno genotóxico.
En dosis elevadas, la tiourea puede provocar hiperplasia tiroidea en ratones y adenomas y carcinomas tiroideos, adenormas hepatocelulares y tumores de la glándula de Zymbal o de Meibomio en ratas. Sin embargo, ninguno de los estudios de carcinogenicidad se ajusta a las normas actuales. Aunque no se ha llegado a conclusiones definitivas con respecto al mecanismo de la carcinogenicidad, es probable que la tiourea actúe mediante el mecanismo conocido para los carcinógenos tiroideos no genotóxicos.
Aunque se ha demostrado que la tiourea es carcinogénica en ratas, el valor probatorio parece indicar que los roedores son más sensibles que las personas a la inducción de tumores tiroideos debido a desequilibrios hormonales que producen concentraciones elevadas de la hormona estimulante del tiroides.
El hipotiroidismo provocado por la administración a ovejas de 50 mg de tiourea/kg de peso corporal durante dos, cuatro o seis meses afecta negativamente al desarrollo somático, el rendimiento reproductivo/gestacional de los animales y el crecimiento de los fetos en el útero. En un estudio semejante con corderos macho se observaron efectos adversos en su desarrollo reproductivo.
La exposición a la tiourea puede inducir en las personas alergia por contacto y por fotocontacto. La tiourea dio resultados negativos en una prueba de sensibilización en animales.
En un estudio realizado en Rusia se observó hiperplasia del tiroides en 17 de 45 trabajadores expuestos a concentraciones de 0,6 a 12 mg/m3 en el aire, equivalentes a una dosis de 0,07 a 1,4 mg de tiourea/kg de peso corporal al día. Las tomas tolerables deberían ser muy inferiores a 0,07 mg de tiourea/kg de peso corporal al día.
Según los datos sobre su uso como depresor del tiroides, <15 mg de tiourea/día (<0,2 mg/kg de peso corporal al día) no tuvieron ningún efecto, mientras que 70 mg/día (alrededor de 1,0 mg/kg de peso corporal al día) sí lo tuvieron.
En la caracterización del riesgo de muestra se comparan los datos notificados en el estudio de Rusia antes mencionado con la concentración media en el aire (tiourea en el polvo total) de 0,085 mg/m3 y la concentración máxima de 0,32 mg/m3 medida en una fábrica de Alemania. Es probable que si no se adoptan precauciones higiénicas en la fábrica de Alemania pueda haber riesgo para la salud, por lo menos al nivel máximo.
No se ha cuantificado la exposición de la población general a la tiourea, de manera que no es posible realizar una caracterización del riesgo.
Basándose en los resultados válidos de pruebas disponibles sobre la toxicidad de la tiourea para diversos organismos acuáticos, se puede clasificar como producto de toxicidad entre moderada y alta para el compartimento acuático. Las concentraciones más bajas sin efectos observados (NOEC) se encontraron en dos estudios prolongados sobre la reproducción de la pulga de agua (Daphnia magna, NOEC de 21 días <0,25 mg/l y 0,25 mg/l).
De acuerdo con los datos experimentales fidedignos disponibles sobre la toxicidad para las especies acuáticas y terrestres, el bajo potencial de bioacumulación y el destino previsto en el medio ambiente cuando se libera en el agua o el suelo hacen suponer que la tiourea no representa un riesgo significativo para los organismos de ambos compartimentos del medio ambiente (excepto en el caso de derrame accidental).
ENDNOTES
1. International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/).
See Also:
Toxicological Abbreviations
Thiourea (ICSC)
Thiourea (IARC Summary & Evaluation, Volume 7, 1974)
Thiourea (IARC Summary & Evaluation, Volume 79, 2001)