
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 55
POLYCHLORINATED BIPHENYLS:
HUMAN HEALTH ASPECTS
First draft prepared by Dr Obaid M. Faroon, Mr L. Samuel Keith, Ms Cassandra Smith-Simon, and Dr Christopher T. De Rosa, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia, USA
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2003
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Polychlorinated biphenyls : Human health aspects.
(Concise international chemical assessment document ; 55)
1.Polychlorinated biphenyls - toxicity 2.Polychlorinated biphenyls - adverse effects
3.Risk assessment 4.Environmental exposure I.International Programme on Chemical
Safety II.Series
ISBN 92 4 153055 3 (LC/NLM Classification: QV 633)
ISSN 1020-6167
©World Health Organization 2003
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
The Federal Ministry for the Environment, Nature Conservation and Nuclear Safety, Germany, provided financial support for the printing of this publication.
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., EHC or CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is based on an existing national, regional, or international review. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
The Agency for Toxic Substances and Disease Registry, Division of Toxicology, prepared this CICAD on polychlorinated biphenyls (PCBs) based on the updated Toxicological profile for polychlorinated biphenyls (PCBs) (ATSDR, 2000). In addition, several articles based on the source document can be consulted for details on each of several health end-points considered important in this CICAD (Faroon et al., 2000, 2001a,b). Information on the nature of the peer review and the availability of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Ottawa, Canada, from 29 October to 1 November 2001. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Card (ICSC 0939) for polychlorinated biphenyl (Aroclor 1254), produced by the International Programme on Chemical Safety (IPCS, 2000), has also been reproduced in this document.
PCBs are synthetic chlorinated hydrocarbon compounds that consist of two benzene rings linked by a single carbon–carbon bond, with from 1 to all 10 of the hydrogen atoms replaced with chlorines. PCBs have been produced commercially since 1929. They have been used in plasticizers, surface coatings, inks, adhesives, flame retardants, pesticide extenders, paints, and microencapsulation of dyes for carbonless duplicating paper. Because PCBs resist both acids and alkalis and are relatively heat-stable, they have been used in dielectric fluids in transformers and capacitors. Further environmental contamination may occur from the disposal of old electrical equipment containing PCBs. The pyrolysis of PCB mixtures produces hydrogen chloride and polychlorinated dibenzofurans (PCDFs), and pyrolysis of mixtures containing chlorobenzenes also produces polychlorinated dibenzodioxins (PCDDs). Many countries have severely restricted or banned the production of PCBs.
The more highly chlorinated PCB congeners adsorb strongly to soil and sediment and are generally persistent in the environment. The various congeners in soil and sediment have half-lives that extend from months to years. Adsorption of PCBs generally increases with the extent of chlorination of the congener and with the organic carbon and clay contents of the soil or sediment. Volatilization and biodegradation — two very slow processes — are the major pathways of PCB removal from water and soil.
PCBs accumulate in the food-chain. They are rapidly absorbed from the gastrointestinal tract and distribute to and accumulate in the liver and adipose tissue. They also cross the placenta, are excreted in milk, and accumulate in the fetus/infant. PCBs are metabolized by hydroxylation and subsequent conjugation. The rates of metabolism and subsequent excretion vary markedly between different congeners.
For the purpose of this CICAD, the health end-points and risk characterization associated with PCB exposures have been based on the approach for mixtures. This is justified on the basis that populations in the general and occupational environments are commonly exposed to mixtures of PCBs, the components of which have different modes of action. It is recognized that in some cases, the mixtures to which various populations are exposed differ considerably from those on which this assessment is based. In such cases, it may be more appropriate to adopt a toxic equivalence (TEQ) approach for individual congeners for which modes of action are known to be similar. Another alternative approach is the use of total body burden of PCB mixtures, since it is done on humans rather than laboratory animals or in vitro, so as to eliminate the need for species extrapolation. Additional information on the various approaches can be found in the source document.2
Humans may be exposed to PCBs by inhaling contaminated air and ingesting contaminated water and food. In 1978, the estimated dietary intake of PCBs by adults in the USA was 0.027 µg/kg body weight per day, but it declined to 0.0005 µg/kg body weight per day in 1982–1984 and <0.001 µg/kg body weight per day for the period 1986–1991.
Some studies on the health effects of PCBs are confounded by exposure to other halogenated environmental contaminants and by impurities in the PCBs, notably chlorinated dibenzofurans. This CICAD deals only minimally with the toxicity of contaminants that result from either the manufacturing process or the heating of PCBs (e.g., PCDDs, PCDFs, or even other persistent organic pollutants); however, studies dealing with the Yusho and Yu-Cheng contaminated cooking oil accidents are briefly summarized in this document.
In studies on humans exposed to PCBs, effects on sperm motility, fetal growth rate (lower birth weight, smaller head circumference) and development (shorter gestational age, neuromuscular immaturity), and neurological functions of the offspring (impaired autonomic function, increased number of abnormally weak reflexes, reduced memory capacity, lower IQ scores, and attention deficit) have been observed. Some of the neurological deficiencies at early ages may disappear later during childhood.
Epidemiological studies suggest exposure-related increases in cancers of the digestive system, especially liver cancer, and malignant melanoma. However, the limitations of exposure information, the inconsistency of the results, and, in some cases, the presence of confounding exposures preclude a clear identification of an exposure–response relationship.
No increase in the incidence of respiratory tract infections during the first 18 months of life was observed, but changes in the relative amounts of different circulating lymphocyte types were observed among children born to PCB-exposed mothers. Decreased numbers of natural killer cells have been observed in consumers of PCB-contaminated fish. The prevalence of recurrent middle-ear infections and chicken pox was related to plasma PCB concentrations in 3.5-year-old children.
Adverse health effects were observed in rats, mice, monkeys, and other mammalian species. Effects were seen in most animal health end-points, such as immunological, developmental, reproductive, hepatic, and body weight. Several studies consistently report an increase in liver cancer incidence among rodents exposed to different PCBs. The severity of the health effects depended on dose, species, PCB mixture, duration and timing of the exposure, and other factors.
Limited studies indicate that PCBs are not genotoxic by direct mechanisms.
Secondary challenge with sheep red blood cells after exposing monkeys to PCBs for 55 months showed decreasing trends in the IgM and IgG anamnestic responses, with IgM significantly lower than in controls for all doses. Based on a lowest-observed-adverse-effect level (LOAEL) of 5 μg/kg body weight per day for several end-points, a tolerable intake of 0.02 μg/kg body weight per day for an Aroclor 1254 mixture was derived, using an overall uncertainty factor of 300 (10 for use of a LOAEL rather than a no-observed-adverse-effect level [NOAEL], 3 for interspecies variation, and 10 for intraspecies variation).
PCBs are a class of chemical compounds in which chlorine atoms replace some or all of the hydrogen atoms on a biphenyl molecule. The general chemical structure of chlorinated biphenyls is shown below in Figure 1.
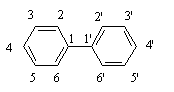
Fig. 1: Biphenyl molecule with the numbering system. In PCBs, some or all of the 10 hydrogens
(attached to carbon atoms numbered 2–6 and 2'–6') are substituted with chlorines.
PCBs were manufactured and sold as mixtures with a variety of trade names, including Aroclor, Pyranol, Pyroclor (USA), Phenochlor, Pyralene (France), Clopehn, Elaol (Germany), Kanechlor, Santotherm (Japan), Fenchlor, Apirolio (Italy), and Sovol (USSR).
Two different but correlated nomenclature systems are currently used. The IUPAC name (according to IUPAC rules A-52.3 and A-52.4) identifies the numbered carbons to which chlorines are attached and lists the numbers sequentially (e.g., the PCB congener with chlorines on carbons 2, 3, 4, and 3' is identified as 233'4); a variant of that system lists the chlorinated ring positions separately, sometimes eliminating the prime symbols for clarity and ease of typing (e.g., 234-3' or 234-3). A second widely used system was developed by Ballschmiter & Zell (1980) as a way to simplify referring to specific congeners. It correlates the structural arrangement of the PCB congeners in an ascending order of number of chlorine substitutions within each sequential homologue. An unprimed number is considered lower (higher priority) than the same number when primed. This results in the congeners being numbered from PCB 1 through PCB 209. Original typographical errors in the Ballschmiter & Zell (1980) numbering system have subsequently been resolved (i.e., old PCB numbers 107, 108, 109, 199, 200, and 201 are now numbered 109, 107, 108, 200, 201, and 199, respectively). Table 1 shows the relationship between the IUPAC and revised PCB numbering systems. Some of the congeners that are either prevalent in commercial PCB products or considered by some researchers to be more toxic than average PCBs are shown in the tables cited later in this section. The primary focus of this CICAD is on commercial mixtures of PCBs and mixtures generated from them in the environment and food-chain.
Table 1: PCB nomenclature conversion table.a
|
Chlorine positions on each ring |
None |
2 |
3 |
4 |
23 |
24 |
25 |
26 |
34 |
35 |
234 |
235 |
236 |
245 |
246 |
345 |
2345 |
2346 |
2356 |
23456 |
|
23456 |
209 |
|||||||||||||||||||
|
2356 |
202 |
208 |
||||||||||||||||||
|
2346 |
197 |
201 |
207 |
|||||||||||||||||
|
2345 |
194 |
196 |
199 |
206 |
||||||||||||||||
|
345 |
169 |
189 |
191 |
193 |
205 |
|||||||||||||||
|
246 |
155 |
168 |
182 |
184 |
188 |
204 |
||||||||||||||
|
245 |
153 |
154 |
167 |
180 |
183 |
187 |
203 |
|||||||||||||
|
236 |
136 |
149 |
150 |
164 |
174 |
176 |
179 |
200 |
||||||||||||
|
235 |
133 |
135 |
146 |
148 |
162 |
172 |
175 |
178 |
198 |
|||||||||||
|
234 |
128 |
130 |
132 |
138 |
140 |
157 |
170 |
171 |
177 |
195 |
||||||||||
|
35 |
80 |
107 |
111 |
113 |
120 |
121 |
127 |
159 |
161 |
165 |
192 |
|||||||||
|
34 |
77 |
79 |
105 |
109 |
110 |
118 |
119 |
126 |
156 |
158 |
163 |
190 |
||||||||
|
26 |
54 |
71 |
73 |
89 |
94 |
96 |
102 |
104 |
125 |
143 |
145 |
152 |
186 |
|||||||
|
25 |
52 |
53 |
70 |
72 |
87 |
92 |
95 |
101 |
103 |
124 |
141 |
144 |
151 |
185 |
||||||
|
24 |
47 |
49 |
51 |
66 |
68 |
85 |
90 |
91 |
99 |
100 |
123 |
137 |
139 |
147 |
181 |
|||||
|
23 |
40 |
42 |
44 |
46 |
56 |
58 |
82 |
83 |
84 |
97 |
98 |
122 |
129 |
131 |
134 |
173 |
||||
|
4 |
15 |
22 |
28 |
31 |
32 |
37 |
39 |
60 |
63 |
64 |
74 |
75 |
81 |
114 |
115 |
117 |
166 |
|||
|
3 |
11 |
13 |
20 |
25 |
26 |
27 |
35 |
36 |
55 |
57 |
59 |
67 |
69 |
78 |
106 |
108 |
112 |
160 |
||
|
2 |
4 |
6 |
8 |
16 |
17 |
18 |
19 |
33 |
34 |
41 |
43 |
45 |
48 |
50 |
76 |
86 |
88 |
93 |
142 |
|
|
None |
0 |
1 |
2 |
3 |
5 |
7 |
9 |
10 |
12 |
14 |
21 |
23 |
24 |
29 |
30 |
38 |
61 |
62 |
65 |
116 |
|
a |
Example (illustrated by shaded area in table): To determine IUPAC and alternative names for PCB 156: |
|
[1] Locate PCB 156 within table. |
|
|
[2] Identify the associated column heading (2345) and row heading (34) values. |
|
|
[3] The IUPAC name for PCB 156 is 2,3,3',4,4',5-hexachlorobiphenyl. |
|
|
Various additional names for this congener include 2,3,4,5,3',4'-hexachlorobiphenyl, 2345-3'4'-hexachlorobiphenyl (group starting with lower number appears first), |
|
|
Adapted from Frame et al. (1996). |
As evidenced in Table 1, 209 chlorinated compounds, called congeners, are possible. PCBs can also be categorized by degree and location of chlorination. The term "homologue" is used for all compounds with the same number of chlorines (e.g., congeners with three chlorines attached are termed trichlorobiphenyls). PCBs of a given homologue with different substitution patterns are called isomers. There are, for example, 12 isomers in the dichlorobiphenyl homologue.
The benzene rings can rotate around the bond connecting them, but the rings are forced towards either the same plane (called planar or coplanar) or perpendicular planes (termed non-planar) by the electrostatic repulsion of the highly electronegative chlorine atoms. The degree to which the rings can twist beyond these two extremes is a function of steric hindrance produced by chlorine atoms in different positions on the two rings. A non-planar orientation is produced by multiple substitutions in the ortho positions (2, 2', 6, and 6'), and an increase from two to four substitutions results in a progressively stronger rotational hindrance. Conversely, some mono-ortho-substituted and all non-ortho-substituted PCBs are considered to be planar, otherwise called coplanar or mono-ortho coplanar, implying that the rings of some congeners can twist but not turn completely. Additionally, solely ortho-substituted congeners, on one or both rings, may be polar molecules with an ability to form hydrogen bonds and thus may be more water soluble. Meta- and para-saturated congeners would be more non-polar and so more lipid-soluble. The congeners considered to be most toxic, based on combined health effects considerations, are coplanar.
An important property of PCBs is their general inertness. PCBs resist both acids and alkalis and have thermal stability, making them useful in a wide variety of applications, including dielectric fluids in transformers and capacitors, heat transfer fluids, and lubricants (Afghan & Chau, 1989).
At high temperatures, PCBs are combustible, and the products of combustion may be more hazardous than the original material. Combustion by-products include hydrogen chloride and PCDFs. Combustion of technical-grade materials containing PCBs and chlorobenzenes (such as some dielectric fluids) may also produce PCDDs (IPCS, 1993; ATSDR, 2000). PCDFs are also produced during commercial production and handling of PCBs. The amount of PCDFs formed depends upon manufacturing conditions. The impurities 2,3,7,8-tetrachlorodibenzofuran and 2,3,4,7,8-pentachlorodibenzofuran were found at concentrations of 0.33 and 0.83 mg/kg, respectively, in Aroclor 1248; and at 0.11 and 0.12 mg/kg, respectively, in Aroclor 1254. Concentrations of PCDFs in commercial PCB mixtures, including Clophen A-60, Phenoclor DP-6, and Kanechlor 400, have been reported.
Physical properties such as solubility, vapour pressure, and Henry’s law constant have been reported for individual congeners. Experimentally determined octanol/water partition coefficients (Kow values) for 19 congeners and an estimation method for the determination of log Kow values of other PCB congeners are also available. The congeners reported are important due to their toxicity or because they occur in high concentrations in the environment. A comprehensive database of chemical and physical data exists (Syracuse, 2000). Table 2 contains such values for the most toxic and the most environmentally prevalent congeners. Tables 3 and 4 summarize the compositions of common Aroclor mixtures by congener prevalence and congener toxicity, while Table 5 categorizes the Aroclors by homologue. Generally, PCBs are relatively insoluble in water, with the highest solubilities among the ortho-chlorinated congeners (5 mg/litre for PCB 1), which may be due to hydrogen bonding associated with the more polar character of these molecules. Solubility decreases rapidly in ortho-vacant congeners, especially as the para positions are filled, which may result in greater and more uniform perimeter electronegativity and interference with hydrogen bonding. PCBs are freely soluble in non-polar organic solvents and biological lipids (US EPA, 1980), and the shift from water to lipid solubility is shown in Table 2 as an increasing Kow with increased chlorination. PCBs, especially the more chlorinated congeners, are also relatively non-volatile, with partial pressures and Henry’s law constants that tend to decrease with increased chlorination, especially for meta- and para-saturated congeners.
Table 2: Physical and chemical properties of some of the most toxic and/or environmentally prevalent PCB congeners.a,b
|
PCB 1 |
PCB 77 |
PCB 81 |
PCB 105 |
PCB 118 |
PCB 126 |
PCB 138 |
PCB 153 |
PCB 156 |
PCB 163 |
PCB 169 |
PCB 180 |
|
|
Chlorine substitution (IUPAC No.) |
2 |
34-3'4' |
345-4' |
234-3'4' |
245-3'4' |
345-3'4' |
234-2'4'5' |
245-2'4'5' |
2345-3'4' |
2356-3'4' |
345-3'4'5' |
2345-2'4'5' |
|
CAS No. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Relative molecular mass |
188.7 |
292.0 |
292.0 |
326.4 |
326.4 |
326.4 |
360.9 |
360.9 |
390.6 |
390.6 |
360.9 |
395.3 |
|
Molecular formula |
C12H9Cl |
C12H6Cl4 |
C12H6Cl4 |
C12H5Cl5 |
C12H5Cl5 |
C12H5Cl5 |
C12H4Cl6 |
C12H4Cl6 |
C12H4Cl6 |
C12H4Cl6 |
C12H4Cl6 |
C12H3Cl7 |
|
Boiling point (°C) |
274 |
360 (calc.) |
400 (calc.) |
240–280 |
||||||||
|
Water solubility (mg/litre at 25 °C) |
4.83 |
0.175 |
0.0034 |
0.0134 (20 °C) |
0.0159 (calc.) |
0.000 91 |
0.005 33 |
0.001 195 |
0.000 036– |
0.000 31– |
||
|
Log Kow |
4.53 |
6.04–6.63 |
6.98 |
7.12 |
6.50–7.44 (calc.) |
8.35 |
7.60 |
7.20 |
7.408 |
6.70–7.21 (calc.) |
||
|
Vapour pressure |
1.38 × 10–3 |
4.4 × 10–7 |
6.531 × 10–6 |
8.974 × 10–6 |
4 × 10–6 |
3.80 × 10–7 |
1.61 × 10–6 |
5.81 × 10–7 |
4.02 × 10–7 |
9.77 × 10–7 |
||
|
Henry’s law constant |
7.36 × 10–4 |
0.43 × 10–4 |
8.25 × 10–4 |
2.88 × 10–4 |
1.07 × 10–4 |
2.78 × 10–4 |
1.43 × 10–4 |
0.15 × 10–4 |
0.15 × 10–4 |
1.07 × 10–4 |
||
|
Atmospheric hydroxyl radical rate constant (cm3/mol·s at 25 °C) |
2.82 × 10–12 |
7.301 × 10–13 |
3.348 × 10–13 |
3.348 × 10–13 |
1.64 × 10–13 |
1.64 × 10–13 |
2.11 × 10–13 |
2.11 × 10–13 |
3.04 × 10–13 |
1.046 × 10–13 |
|
a |
Adapted from ATSDR (2000) and Syracuse (2000). |
|
b |
Included PCB 77, PCB 126, and PCB 169 per Patterson et al. (1994) and PCB 81 based on configuration. Included PCB 1 based on its significantly different solubility. |
|
c |
1 mmHg = 0.1333 kPa. |
|
d |
1 atm·m3/mol = 101.325 kPa·m3/mol. |
Table 3: Most prevalent congeners (mol%) of common commercial PCB products.a
|
Congener No. |
Chlorine substitution |
Aroclor |
Aroclor |
Aroclor |
Aroclor |
Aroclor |
|
4 |
2,2' |
4.36 |
3.99 |
|||
|
8 |
2,4' |
10.30 |
8.97 |
|||
|
18 |
2,5,2' |
10.87 |
9.36 |
9.95 |
||
|
28 |
2,4,4' |
14.48 |
13.30 |
|||
|
31 |
2,5,4' |
4.72 |
4.53 |
9.31 |
||
|
42 |
2,3,2',4' |
7.05 |
||||
|
52 |
2,5,2',5' |
4.35 |
4.08 |
8.36 |
||
|
53 |
2,5,2',6' |
6.30 |
||||
|
70 |
2,5,3',4' |
6.38 |
4.75 |
|||
|
91 |
2,3,6,2',4' |
5.00 |
||||
|
99 |
2,5,2',3',4' |
6.10 |
||||
|
101 |
2,4,5,2',5' |
6.98 |
5.04 |
|||
|
110 |
2,3,6,3',4' |
8.51 |
||||
|
118 |
2,4,5,3',4' |
8.09 |
||||
|
138 |
2,3,4,2',4',5' |
5.01 |
||||
|
149 |
2,3,6,2',4',5' |
9.52 |
||||
|
153 |
2,4,5,2',4',5' |
8.22 |
||||
|
180 |
2,3,4,5,2',4',5' |
7.20 |
||||
|
185 |
2,3,4,5,6,2',5' |
5.65 |
a Values less than approximately 4% are not included.
Table 4: Percent composition for some of the most toxic congeners in commercial Aroclors (mol%).a
|
PCB 105 |
PCB 118 |
PCB 138 |
PCB 153 |
PCB 156 |
PCB 163 |
PCB 180 |
PCB 183 |
|
|
(234-3'4') |
(245-3'4') |
(234-2'4'5') |
(245-2'4'5') |
(2345-3'4') |
(2356-3'4') |
(2345-2'4'5') |
(2346-2'4'5') |
|
|
Aroclor 1016 |
0.00 |
– |
– |
– |
– |
– |
– |
– |
|
Aroclor 1221 |
0.04 |
0.07 |
– |
0.00 |
– |
– |
– |
– |
|
Aroclor 1232 |
0.21 |
0.27 |
0.06 |
0.05 |
– |
0.01 |
0.02 |
– |
|
Aroclor 1242 |
0.47 |
0.66 |
0.10 |
0.06 |
0.01 |
0.01 |
0.00 |
– |
|
Aroclor 1248b |
1.60/1.45 |
2.29/2.35 |
0.38/0.41 |
0.23/0.43 |
0.06/0.04 |
0.06/0.08 |
0.02/0.21 |
–/0.08 |
|
Aroclor 1254c |
7.37/2.99 |
13.59/7.35 |
5.95/5.80 |
3.29/3.77 |
1.13/0.82 |
0.70/1.03 |
0.42/0.67 |
0.09/0.18 |
|
Aroclor 1260 |
0.22 |
0.48 |
6.54 |
9.39 |
0.52 |
2.42 |
11.38 |
2.41 |
|
Aroclor 1262 |
0.09 |
0.15 |
2.74 |
7.10 |
0.16 |
1.52 |
14.13 |
2.88 |
|
a |
Adapted from ATSDR (2000) and Frame et al. (1996). |
|
b |
The two values represent batches A3.5 and G3.5, respectively. |
|
c |
The two values represent batches A4 and A6, respectively. |
Table 5: Estimated homologue composition (%) of different Aroclors.
|
No. of chlorine substitutions |
Aroclor |
Aroclor |
Aroclor |
Aroclor |
Aroclor |
Aroclor |
|
1 |
31.3 |
<1 |
<1 |
<0.2 |
– |
– |
|
2 |
23.7 |
21.2 |
14.7 |
<1 |
<0.1 |
– |
|
3 |
23.4 |
51.5 |
46 |
20.9 |
1.8 |
<0.3 |
|
4 |
15.7 |
27.3 |
30.6 |
60.3 |
17.1 |
<0.3 |
|
5 |
5.8 |
<0.6 |
8.7 |
18.1 |
49.3 |
9.2 |
|
6 |
– |
– |
<0.3 |
0.8 |
27.8 |
46.9 |
|
7 |
– |
– |
– |
<0.3 |
3.9 |
36.9 |
|
8 |
<0.05 |
6.3 |
||||
|
9 |
<0.05 |
0.7 |
The quantification of PCBs in biological samples usually consists of three distinct steps: extraction of PCBs from the sample matrix by a solvent or a combination of solvents, cleanup of PCBs (i.e., removal of impurities) on single or multiple columns, and quantification by gas chromatography (GC) with a suitable detector. Authors may report PCB concentrations as Aroclors, as sum of homologues, or as individual congeners.
PCBs are extracted from blood or serum using hexane, benzene, or mixed solvents, such as hexane/ethyl ether. A variety of adsorbents may be used for cleanup and/or fractionation of extracts: deactivated silica gel (Burse et al., 1989), Florisil, alumina (Koopman-Esseboom et al., 1994), or multiple columns. Supercritical fluid extraction (SFE) has also been used to extract PCBs from adipose tissue samples (Djordjevic et al., 1994). GC coupled with an electron capture detector (GC/ECD) is used most often to determine PCBs (Burse et al., 1989; ATSDR, 2000), but confirmation by mass spectrometry (MS) is recommended when multiple individual congener measurements are required. Detection limits for individual Aroclors are in the low- to sub-microgram per litre range, and recoveries, where reported, range from 80% to 96% (Koopman-Esseboom et al., 1994; ATSDR, 2000). The accuracy and precision of the results of PCB analysis in serum, using a packed column GC/ECD method, were examined in a collaborative study (Burse et al., 1989). Capillary or high-resolution gas chromatography (HRGC) has made it possible to achieve lower detection limits and better separation of PCB congeners for quantification (Mullin et al., 1984). Although complete separation with a single column has not yet been achieved, advances have been made in analysing for specific coplanar PCBs (77, 126, 169), which some consider to be the most toxic congeners. Equipment can be calibrated to yield results by congener; however, because of the time required to analyse all congeners, results may be reported only for selected ones or for homologues, using the areas or heights of selected peaks or ranges to estimate the concentration in a sample. This tends to reduce analytical costs but can also complicate study intercomparisons. Detection limits have been reported as 144 ng/g for adipose tissue, 2 ng/g for blood, 0.01 ng/g for plasma, and 1–2.5 ng/ml for serum.
Traditional PCB analytical methods quantify PCBs as Aroclor mixtures, and one assumption has been that the original congener formulation is retained in the environment. The validity of this assumption suffers as individual congeners undergo different physical, chemical, and biological interactions with the environment that alter the congener mixture relative to the original formulation. Analysis by Aroclor also has the disadvantage of being insensitive to the 4-to-6-chlorine coplanar congeners that are of highest potential biological significance for some health effects end-points. An adjusted approach is to analyse the isomer classes (C1–C10), nine of which are addressed in Table 5.
The most appropriate approach is to analyse for individual congeners. Results may then be adjusted to compare with older studies, where mixtures were assessed from multiple peaks. Summing of selected congeners is also often done; sums can be weighted for biological importance, and summing systems form the basis for long-term monitoring programmes such as the National Oceanic and Atmospheric Administration Mussel Watch Program (NOAA, 1989). McFarland & Clarke (1989) recommended including priority congeners 49, 77, 87, 101, 105, 118, 126, 128, 138, 153, 156, 158, 169, 170, 180, 183, and 184. These include congeners with greatest environmental importance based on potential toxicity, frequency of occurrence in environmental samples, and relative abundance in animal tissue. Congener analysis can be performed using high-resolution capillary gas chromatograph columns and electron capture detectors (HRGC/ECD) or mass spectrometric (HRGC/MS) techniques; the latter has individual congener detection levels approaching 0.01 ng/litre in human serum. Ballschmiter & Zell (1980) and Safe et al. (1985b) reported a congener-specific analysis of a commercial PCB preparation and the PCB composition of a human milk sample.
Studies have described the analysis of non-ortho coplanar and mono-ortho coplanar PCBs in breast milk and coplanar PCBs in serum and adipose tissue. Determination of these congeners, which are the most toxic, is useful in assessing the toxic potential of breast milk for infants and of dietary intake for adults.
Sampling method variables may also greatly influence results. Random error, interlaboratory variations in procedure, and methods used for reporting data may have considerable impact on the reported PCB levels in human tissues. Since there is no standard method or approach for analysing PCBs in biological samples, caution is appropriate when comparing exposure estimates or health effects studies reported by different investigators.
Methods are available for measuring concentrations of PCBs in fish and animal tissues. Tissues are homogenized, then dried by blending with sodium sulfate prior to Soxhlet or column extraction. Few methods are available to determine PCBs in foods. A minimum of performance data has been reported as well. A method is available to determine Aroclors in poultry fat, fish, and dairy products. Congener-specific analytical methods for coplanar PCBs 77, 126, and 169 are amenable to these media.
Air samples are usually collected by pumping air through a sampler containing a glass fibre filter and adsorbent trap to separate the particle-bound and vapour-phase fractions. Adsorbents used most often include Florisil and polyurethane foam. Florisil traps are solvent desorbed, and XAD-2 traps are Soxhlet extracted. PCBs are determined by GC/ECD or HRGC/MS. Detection limits of low nanograms per cubic metre for individual Aroclors to low picograms per cubic metre for individual congeners have been reported. Recovery, where reported, is good, greater than 80%.
Drinking-water samples are typically extracted with solvent prior to analysis by GC/ECD or HRGC/ECD. Composite PCB detection limits are in the sub-microgram per litre range, and recovery is greater than 80%. Method 508A of the US Environmental Protection Agency (EPA), which converts all the PCBs to decachlorobiphenyl, is a screening method for quantifying total PCBs. The method is likely to show interference because of perchlorination of biphenyl or related compounds and because the method cannot quantify either the individual commercial Aroclors in a PCB mixture or the individual congeners present.
Soil, sediment, and solid waste samples are usually Soxhlet extracted. Ultrasonic extraction with various solvent combinations and SFE are also used. Analyses for screening PCB contamination in soils, using the enzyme-linked immunosorbent assay, are commercially available. These methods are inexpensive and provide fast turnaround time.
A number of Standard Reference Materials (SRMs) with certified PCB congener concentrations are available from the US National Institute of Standards and Technology. These include SRM 1588 for cod liver oil, SRM 1939 for river sediment, SRM 1941 for marine sediment, and SRM 1974 for mussel tissue.
PCB production started in the late 1920s. Since 1929, about 2 × 109 kg of PCBs have been produced commercially, of which about 2 × 108 kg remain in mobile environmental reservoirs. PCB pollution may occur during the incineration of municipal waste. PCB concentrations of 0.01–1.5 mg/kg were detected in fly ash from five municipal incinerators operating under different technological and working conditions. Stack effluents from several municipal refuse and sewage incinerators in the midwestern USA contained PCB concentrations of 0.3–3.0 μg/m3. The total PCB concentration measured in the flue gas effluent from a municipal waste incinerator in Ohio, USA, was 0.26 μg/m3. PCB levels of 2–10 ng/m3 were detected in effluents from coal and refuse combustion in Ames, Iowa, USA (US EPA, 1988a). An additional source of PCB pollution is volatilization from landfills containing transformers, capacitors, and other PCB waste and from contaminated bodies of water, such as the Great Lakes in North America. Because of possible health implications and environmental impacts, the use and production of PCBs are severely restricted or banned in many countries. Sweden restricted their use and production in 1972, the USA in 1977, Norway in 1980, Finland in 1985, and Denmark in 1986.
Values for estimated Henry’s law constants for Aroclors, ranging from 29 to 47 Pa·m3/mol, indicate that vaporization can be an important environmental transport process for PCBs dissolved by surface waters (Thomas, 1982) when the concentrations in silt force the water levels to remain elevated and evaporation represents a significant portion of the overall water loss. The volatility and solubility differences among the various congeners can be expected to cause redistribution in both surface water and bottom sediment; this emphasizes the need for congener-specific analysis of environmental and biological samples. A study of Lake Michigan in North America indicates that vaporization may be the major process for the removal of PCBs from lakes (Swackhamer & Armstrong, 1986). PCBs may vaporize even more significantly from dam spillways and outfalls, waterfalls, and other waterways with markedly higher aeration rates (McLachlan et al., 1990). Nonetheless, adsorption to sediment significantly decreases the rate of vaporization of highly chlorinated Aroclors from the aquatic phase (Lee et al., 1979; US EPA, 1985a,b).
In water, adsorption to sediments and suspended particulates is also a major process that partitions PCBs from the water to the solid phase. A study of the Saginaw River in Michigan, USA (Verbrugge et al., 1995), reports that the ratio of total PCBs bound to suspended particulates relative to dissolved PCBs was 2:1 and that this ratio remained fairly constant for river discharges of less than 400 m3/s. The adsorption usually increases as organic matter, clay, and micro-particle contents in the water increase (US EPA, 1980).
Adsorption and subsequent sedimentation may immobilize PCBs for a long time in aquatic systems. Redissolution of PCBs into the water column and vaporization from the water surface into the air occur in the environment. Therefore, the substantial quantities of PCBs contained in aquatic sediments can act as an environmental reservoir from which PCBs may be released slowly over a long period. The rate of redissolution of PCBs from sediment to water and of evaporation from water to air is always greater in summer than in winter, because these parameters increase with temperature (Larsson & Soedergren, 1987).
PCBs in the atmosphere are physically removed by wet and dry deposition (Eisenreich et al., 1981; Leister & Baker, 1994). Dry deposition of PCBs occurs from gravitational settling of particulates and from impaction of vapour-phase PCBs on land or aquatic surfaces. Wet deposition of PCBs occurs through rain, snow, and fog (Hart et al., 1993). The atmospheric wet and dry deposition fluxes of PCBs over Chesapeake Bay in Maryland, USA, in 1990–1991 were 1.9 μg/m3 per year and 1.4 μg/m3 per year, respectively (Leister & Baker, 1994). Thus, wet depositional flux in the bay represented 58% of the overall flux. This is a function of the local precipitation pattern; overall, more wet deposition results from particle washout (99%) than from vapour washout (1%) (Atlas & Giam, 1987).
The measured atmospheric concentration of PCBs near a Superfund6 hazardous waste site was higher than atmospheric concentrations monitored 15 km from the site (Hermanson & Hites, 1989), suggesting that PCBs may be transported from soil to air through vaporization and subsequently diluted downwind. The vaporization rate was greater from soil with low organic carbon content, because of the weaker adsorption of PCBs (Shen & Tofflemire, 1980). Vaporization rates are also greater in moist soils because of the co-evaporation of PCBs with water. Storm water runoff can also transport PCBs through soil erosion.
Because of their large and mobile biomass, position in the food-chain, and biotransformation potential, insects may significantly contribute to the global transport and transformation of PCBs (Saghir et al., 1994).
The bioconcentration factor (BCF, or ratio of the concentration of PCBs in the organism to the concentration of PCBs in water) values of PCBs are expected to increase with an increase in chlorine substitution and a decrease in water solubility (Zhang et al., 1983). However, the maximum bioaccumulation was observed for hexachlorobiphenyls and not hepta- or octachlorophenyls (Porte & Albaiges, 1993; Bremle et al., 1995). The lower BCF values for the latter two classes of higher chlorinated compounds may be due to lower uptake rates.
The elimination of PCBs from aquatic organisms is both species- and congener-specific. Generally, congeners containing two vicinal hydrogen atoms at the meta and para positions in at least one aromatic ring are easily metabolized (Pruell et al., 1993). The biotransformation of PCBs in vertebrates is mediated by the cytochrome P450-dependent mixed-function oxygenase (MFO) (Safe et al., 1985a). There is evidence that different cytochrome P450 enzymes metabolize specific PCB congeners. In rats, the di-ortho PCBs are preferably metabolized by the CYP2B family, while the CYP1A enzymes preferably metabolize the non-ortho PCBs (Kaminsky et al., 1981). Cytochrome P450-dependent MFO activities are species-dependent, and MFO activities are generally much lower in mussels than in crabs and fish (Porte & Albaiges, 1993). The PCB congeners 110, 138, 149, 153, and 187 are most recalcitrant in mussels. The most stable PCB congeners are 138, 153, 170, and 180 in crabs; 138, 153, 170, 180, and 187 in mullet; and 84, 110, 118, and 138 in tuna (Porte & Albaiges, 1993).
Bioaccumulation in tuna of PCB congeners 84 and 110, which are rapidly metabolized by birds and mammals (Hansen, 1987), is unusual and may be related to the seasonal shallow surface feeding habit of tuna. The 2,3,6 congeners (including 149) are also more volatile (Mullin et al., 1984) and are likely found at higher concentrations near the air and water interface. Bioaccumulation of PCBs in aquatic animals may also depend on the water zone in which the animals predominantly reside and feed. When airborne PCBs are deposited onto the surface of water, they become enriched in the surface strata. The PCB levels are 500 times higher in the surface strata than in deeper water. Consequently, bioaccumulation by fish is several times higher in this zone (Soedergren et al., 1990). Because the concentration of PCBs in sediments is several times higher than in water, the bioaccumulation of PCBs in bottom-feeding species is also high. Loss of accumulated PCB residues from the tissues is slow, because PCBs tend to remain stored in lipids. Therefore, greater bioaccumulation occurs in the fatty tissues than in the muscles or whole body of aquatic organisms (US EPA, 1980). However, studies of fish indicate that stored PCBs may become more mobilized from lipids of organs that contain higher polar lipid components, such as phospholipids (Boon et al., 1984).
PCBs generally biomagnify within aquatic food-chains, as indicated by the PCB concentrations detected in higher trophic levels of aquatic organisms (LeBlanc, 1995; Wilson et al., 1995). This biomagnification is evident in shellfish that accumulate PCBs from consumption of phytoplankton and zooplankton (Secor et al., 1993) and in marine mammals (seals, dolphins, and whales) that accumulate PCBs from consumption of plankton and fish (Andersson et al., 1988; Kuehl et al., 1994; Kuehl & Haebler, 1995; Lake et al., 1995; Salata et al., 1995). Food-chain biomagnification also occurs in several species of fish-consuming birds (Mackay, 1989). Concentrations of PCBs in common (Sterna hirundo) and Forster’s terns (S. forsteri) (which are primarily piscivores) are higher than concentrations in tree swallows (Tachycineta bicolor) and red-winged blackbirds (Agelaius phoeniceus) (which are insectivores) (Ankley et al., 1993), which shows evidence of biomagnification of PCBs in the aquatic food-chain. The biomagnification of PCBs in the aquatic food-chain is also congener-specific (Koslowski et al., 1994). For example, although the concentrations of congener 138 increased from plankton (1 μg/kg) to piscivores (1388 μg/kg in silver bass [Morone chrysops] muscle tissue) to herring gulls (Larus argentatus) (30 063 μg/kg), the three toxic congeners 77, 126, and 169 showed no obvious biomagnification in these species. The lack of biomagnification in congener 77 was attributed to its rapid elimination by aquatic species (Koslowski et al., 1994). These samples were collected during the summer of 1991.
Biotransfer factors for organic chemicals in beef and milk are directly proportional to their Kow values. The biotransfer factors of Aroclor 1254 (concentration in food [mg/kg]/daily intake of Aroclor [mg/day]) for beef and milk are estimated at 0.052 and 0.011 kg/day, respectively, using the estimation procedures of Travis & Arms (1988). Based on summary data from Canada, the estimated mean BCF value (the ratio of the concentration of PCBs in tissues to the concentration of PCBs in the diet) for PCBs in human fat is 128 on a wet weight basis and 192 on a lipid weight basis (Geyer et al., 1986). The biomagnification of PCBs in the terrestrial food-chain occurs through accumulation of PCBs from soil/plant to earthworm to bird (Hebert et al., 1994) and from oak leaves to caterpillars to birds (Winter & Streit, 1992). While total PCBs were not detected in soil or plants, the ranges in concentrations (wet weight) observed were 14.8–18.6 μg/kg in earthworms; not detected to 208.8 μg/kg in mammals; 39.2–68.3 μg/kg in starlings; 71.5–157.2 μg/kg in robins; and 56.0–219.9 μg/kg in kestrels (Hebert et al., 1994). In addition, the authors reported that the sum of PCBs in pooled egg samples ranged from 0.066 to 0.477 mg/kg for kestrels and from 0.032 to 0.061 mg/kg for bluebirds. A concentration of 5.298 mg/kg was detected in a single pooled egg sample from herring gulls, a piscivorous species. Juvenile great tits (Parus major) received PCBs from the mother bird through egg transfer and from caterpillars, their primary food source (Winter & Streit, 1992).
The ability of PCBs to be degraded or transformed in the environment depends on the degree of chlorination of the biphenyl molecule and on the chlorination pattern (Callahan et al., 1979; Leifer et al., 1983; US EPA, 1988a). Generally, the persistence of PCB congeners increases as the degree of chlorination and structural uniformity increase. Adjacent unchlorinated carbons allow the formation of arene oxide intermediates and thus facilitate metabolism. Kubatova et al. (1998) examined the biodegradation of PCB congener 11 (3,3'-dichlorobiphenyl) in the soil. PCB 11, labelled with 14C, was chosen as a low chlorinated coplanar congener and assumed to be readily degraded by Pseudomonas species and the oyster mushroom (Pleurotus ostreatus). After a 2-month incubation, results showed that the mineralization of PCB 11 was <0.4%, volatilization was <3.1%, and 30% of the radioactivity was irreversibly bound to the soil matrix. The major biodegradation product was 3-chlorobenzoic acid. The concentrations of the coplanar congeners were significantly lowered by reductive dechlorination and by anaerobic bacteria (Quensen et al., 1998).
In the atmosphere, the vapour-phase reaction of PCBs with hydroxyl radicals (which are photochemically formed by sunlight) may be the dominant transformation process. The estimated tropospheric half-times for this reaction increase as the number of chlorine substitutions increases. The half-times are 3.5–7.6 days for monochlorobiphenyl, 5.5–11.8 days for dichlorobiphenyl, 9.7–20.8 days for trichlorobiphenyl, 17.3–41.6 days for tetrachlorobiphenyl, and 41.6–83.2 days for pentachlorobiphenyl (Atkinson, 1987). Photochemical studies conducted under simulated and natural sunlight with a number of chlorobiphenyl congeners and commercial PCB mixtures in aqueous suspension, thin film, or vapour state resulted in several degradative reactions that produced dechlorination, polymerization, and polar (hydroxy- and carboxy-) products (Hutzinger et al., 1972).
In water, transformation processes such as hydrolysis and oxidation do not significantly degrade PCBs (Callahan et al., 1979). Photolysis appears to be the only viable chemical degradation process in water. PCBs containing up to six chlorine substitutions do not significantly absorb sunlight, and the estimated photolysis half-times of mono- through tetrachlorobiphenyls with summer sunlight at a shallow water depth (<0.5 m) range from 17 to 210 days. Photolysis rates with sunlight are even slower during winter. Nonetheless, as the number of chlorine substitutions increases, the light absorption band shifts towards longer wavelengths, and the photolysis rate for hepta- through decachlorobiphenyls increases.
Biodegradation of PCBs in water, although slow, can occur under both aerobic and anaerobic conditions. However, biodegradation is probably more significant in soil and sediment than in water, given the higher numbers of microorganisms. The use of adapted (pre-exposed) microbial populations and the addition of amenable substrates for co-metabolic and co-oxidative biotransformation can enhance the biotransformation and biodegradation of PCBs.
Biodegradation occurs under both aerobic and anaerobic conditions and is the major degradation process for PCBs in soil and sediment. No abiotic process is known to significantly degrade PCBs in sediment and soil; however, photolysis of PCBs on surface soil may occur. Higson (1992) and Robinson & Lenn (1994) provide a review of biodegradation of PCBs in soil and sediment. Experiments with pure and mixed cultures of microorganisms show that some congeners of PCBs, usually containing six or fewer chlorine substituents, biodegrade under aerobic conditions (Leifer et al., 1983; US EPA, 1988a; Sugiura, 1992; Thomas et al., 1992; Dowling et al., 1993; Fava et al., 1993; Gibson et al., 1993). Biodegradation rates are highly variable because they depend on several factors, including the amount and location of chlorination, PCB concentration, type of microbial population, available nutrients, and temperature. The most common process for the aerobic degradation of PCBs by bacterial cultures proceeds by two distinct steps, one involving bioconversion of PCBs to chlorinated benzoic acids and the other involving mineralization of chlorobenzoates to carbon dioxide and inorganic chlorides (Thomas et al., 1992; Robinson & Lenn, 1994). Complete mineralization of biodegradable PCBs requires the presence of two clusters of genes for the two-step bioconversion process (Sondossi et al., 1992); therefore, complete degradation requires mixed microbial cultures (Afghan & Chau, 1989).
Reliable evaluation of the potential for human exposure to PCBs depends partly on the reliability of supporting analytical data from environmental samples and biological specimens. With respect to PCB analysis, comparisons among various studies are complicated by the fact that authors may report PCB concentrations as Aroclors, homologues, or congeners.
Table 6 lists PCB levels relevant to human exposure in air and water in various countries. The mean atmospheric concentration of PCBs in urban areas in the USA was 5 ng/m3 (range 1–10 ng/m3) (Eisenreich et al., 1992). The mean atmospheric concentrations of PCBs in two rural areas (rural Ontario, Canada, and Adirondack, New York, USA) were 0.2 and 0.95 ng/m3, respectively (Knap & Binkley, 1991; Hoff et al., 1992). These values are within the range for continental areas (0.2–1.5 ng/m3) given by Eisenreich et al. (1992). For two remote areas (the Arctic and the Antarctic), the mean PCB value was 0.2 ng/m3 (range 0.02–0.5 ng/m3) (Tanabe et al., 1983; Baker & Eisenreich, 1990). The air concentration in the eastern Arctic in 1996 was 0.074 ng/m3 (Harner et al., 1998).
Table 6: PCB levels in air and water in various countries.a
|
Medium |
Country |
Location |
Total PCB concentration |
|
Air |
Canada |
Northwest Territories |
0.002–0.07 ng/m3 |
|
Germany |
Industrial area |
3.3 ng/m3 |
|
|
Non-contaminated area |
0.003 ng/m3 |
||
|
Japan |
North and South Pacific, Indian, Antarctic, and South Atlantic oceans |
0.1–0.3 ng/m3 |
|
|
Sweden |
Several locations |
0.8–3.9 ng/m3 |
|
|
Water |
Germany |
Several rivers |
5–103 ng/litre |
|
The Netherlands |
Rhine River |
100–500 ng/litre |
|
|
Sweden |
Water entering a treatment plant |
0.5 ng/litre |
a Adapted from IPCS (1993).
The mean atmospheric PCB concentration in marine and coastal areas was 0.1 ng/m3 (range 0.01–0.7 ng/m3). Over the Great Lakes in North America, the mean concentration was 1.0 ng/m3 (range 0.2–4.0 ng/m3). These samples were collected in the late 1980s to early 1990s (Eisenreich et al., 1992).
Since the early 1980s, PCB concentrations in the air have shown a slightly decreasing trend for urban, rural, and marine/coastal areas (Eisenreich et al., 1981). Over the same approximate period (late 1970s to early 1980s compared with late 1980s to early 1990s), rainwater PCB concentrations from continental areas significantly declined by 4- to 5-fold, with values decreasing from 20 to 5 ng/litre in rural areas and from 50 to 10 ng/litre in urban areas.
When indoor air in a number of laboratories, offices, and homes was monitored for various Aroclors, the "normal" indoor air concentrations of PCBs were at least 1 order of magnitude higher than those in the surrounding ambient outdoor atmosphere (MacLeod, 1981). For example, average PCB levels were 100 ng/m3 inside an industrial research building and 210 ng/m3 inside the laboratories, compared with <20 ng/m3 in air outside the facility. The mean PCB indoor air concentration in one home was 310 ng/m3, while the average air concentration outside the home on the same day was 4 ng/m3. Certain electrical appliances and devices (e.g., fluorescent lighting ballasts) and building materials (elastic sealant), which have PCB-containing components, may emit PCBs into the indoor air, thereby elevating indoor PCB levels significantly above outdoor background levels (Balfanz et al., 1993).
The average PCB concentration (Aroclors 1242 and 1260) in emissions from gas vents at a hazardous waste landfill in North Carolina, USA, was 126 000 ng/m3 (Lewis et al., 1985). The maximum total PCB concentration detected in air samples collected at Raquette Point within the Mohawk Nation Reservation at Akwesasne, New York, USA (adjacent to a Superfund site), was 50 ng/m3 (ATSDR, 1995).
PCB concentrations reported for seawater from various oceans include 0.04–0.59 ng/litre in the North Pacific, 0.035–0.069 ng/litre in the Antarctic, and 0.02–0.20 ng/litre in the North Atlantic (Giam et al., 1978; Tanabe et al., 1983, 1984). PCB levels were several orders of magnitude higher in sea surface microlayer samples taken from industrial areas than in those taken from sites farther offshore (Cross et al., 1987). This indicates that the PCB contribution from anthropogenic sources is higher in nearshore samples than in offshore samples. PCB concentrations of 0.3–3 ng/litre, which are higher than the seawater concentrations reported above, have been detected in surface seawater from the North Sea (Boon & Duinker, 1986). Analysis of water from eight sites in Galveston Bay, a highly industrialized area in Texas, USA, showed an average PCB concentration of 3.1 ng/litre between 1978 and 1979 (Murray et al., 1981). In 1993, the total dissolved plus particulate PCB concentration in Great Lakes water ranged, in increasing order, from 0.07 to 0.10 mg/litre, from 0.12 to 0.16 mg/litre, from 0.17 to 0.27 mg/litre, from 0.19 to 0.25 mg/litre, and from 0.2 to 1.6 mg/litre for Lakes Superior, Huron, Michigan, Ontario, and Erie, respectively (Anderson et al., 1999).
The historic profiles of PCB concentrations in sediments of the lower Passaic River, New Jersey, USA, were studied by determining the concentrations at different depths. The authors concluded that total PCB sediment concentrations peaked at 4.7 mg/kg dry weight in the 1970s and then decreased to 1.1 mg/kg dry weight in the 1990s (Wenning et al., 1994). A similar study of dated sediments from the Newark Bay Estuary, New Jersey (including the Passaic River), also reported that the highest concentration of PCBs occurred in buried sediments from the Passaic River and Newark Bay, corresponding to historic deposition during the 1960s and 1970s, the peak manufacturing period for Aroclors (Iannuzzi et al., 1995).
Yao et al. (2000) analysed sediment core samples attributable to the period from 1935 to 1993 in Tokyo Bay, Japan. Coplanar PCB concentrations peaked from 1967 to 1972 (reflecting the historical production and use patterns), decreased rapidly from 1972 to 1977, following the ban on production and use of PCBs, and then slowly levelled off to about one-third of the peak level. Kang et al. (2000) reported a decline in PCB levels in fish from 1953 to 1975 that was similar to the decline in PCB levels in sediment.
An 80% decrease (from 9.08 μg/g wet weight in 1976 to 1.72 μg/g in 1994) in PCB concentration in trouts from Lake Ontario was reported by Huestis et al. (1996); a similar decrease was noted in the two species of trout tested (lake trout [Salvelinus namaycush] and rainbow trout [Oncorhynchus mykiss]) from both Lake Ontario and Lake Huron. In several fish species from the Great Lakes, the PCB concentrations in samples collected in the 1990s were generally below 1 µg/g wet weight (ATSDR, 2000).
The general population is exposed to PCBs via air, drinking-water, and food.
Typically, outdoor air in urban areas contains an average PCB concentration of 5 ng/m3 (Eisenreich et al., 1992). As the average adult male inhales 23 m3/day (IPCS, 1994), the average daily exposure through inhalation is approximately 100 ng. However, the concentration of PCBs can be at least an order of magnitude higher in indoor air than in outdoor air. PCB concentrations in the workplace air of unspecified PCB disposal facilities in the USA ranged from 850 to 40 000 ng/m3. In 95 of the 96 air samples collected for analysis from such facilities, PCB concentrations exceeded 1000 ng/m3 (Bryant et al., 1989).
Based on a US EPA (1988a) study, PCB levels in drinking-water were below 100 ng/litre, and not all water samples had detectable PCB levels. Using a conservative adult consumption rate of 2 litres/day, the general US population is exposed to <200 ng PCBs/day from drinking-water.
In a Canadian drinking-water survey conducted in 1985–1988 (O’Neill et al., 1992), PCBs were detected in 1 out of the 280 municipal drinking-water samples, at a level of 6 ng/litre.
The primary exposure pathway appears to be through consumption of contaminated foods, particularly meat, fish, and poultry (ATSDR, 2000). In the USA, the dietary intake of PCBs for adults continually decreased from 1978 until 1986–1991 (Table 7). The mean daily intake was <0.001 µg/kg body weight per day for individuals of ages 6 months and 25–30 years and 0.002 µg/kg body weight per day for 2-year-olds between 1986 and 1991 (based on average total diet composition) (Gunderson, 1995). In a study on dietary intake during 1991–1997, the decreasing trend did not continue, and the dietary exposure was 3–5 ng/kg body weight per day for adults and 2–12 ng/kg body weight per day for children of different ages (based on calculated intakes from 265 different food items) (P.M. Bolger, personal communication, 1999, as cited in ATSDR, 2000).
Table 7: Estimated daily dietary intake of PCBs in the USA.a
|
Year |
Dietary intake (µg/kg body weight per day) |
||
|
Adult |
Toddler |
Infants |
|
|
1986–1991 |
<0.001 |
0.002 |
<0.001 |
|
1982–1984 |
0.0005 |
0.0008 |
0.0012 |
|
1981–1982 |
0.003 |
NDb |
ND |
|
1980 |
0.008 |
ND |
ND |
|
1979 |
0.014 |
ND |
ND |
|
1978 |
0.027 |
0.099 |
0.011 |
|
1977 |
0.016 |
0.030 |
0.025 |
|
1976 |
Trace |
ND |
Trace |
|
a |
ATSDR (2000). Estimated intakes are based on an average "total diet" composition (which varies slightly) and not on individual food items. Average body weights are assumed to be 9 kg for infants, 13 kg for toddlers, and 70 kg for adults. Accordingly, the average dietary intake for 1982–1984 would be 0.0108 μg, 0.0104 μg, and 0.035 μg for the infant, toddler, and adult, respectively. |
|
b |
ND = not detected. |
A total diet study using market basket methodology based on an analysis of 120 food items purchased in 10 locations in Japan was performed in 1997–1998 to estimate the intake of dioxin-like chemicals from those food items (Toyoda et al., 1999a,b). The concentrations of the coplanar PCBs 3,3',4,4'-tetrachlorobiphenyl, 3,3',4,4',5-pentachlorobiphenyl, and 3,3',4,4',5,5'-hexachlorobiphenyl were highest in the fish and shellfish group (8.39–25.7 pg/g wet weight basis), among the 14 food groups that were categorized following the method of the National Nutrition Survey in Japan. The total intake of these three coplanar PCBs from all food items was 1.45 pg/kg body weight per day (assuming 50-kg body weight), which contributes to 60% of the total TEQ intake for Japanese persons from foods. This intake represents a relatively high level of PCB contamination in Japan.
The average PCB concentration in human milk fat ranges from 0.5 to 4 mg/kg (Jensen, 1987). The mean concentration of PCBs in whole breast milk in Canadian women steadily increased from 6 μg/kg in 1970 to 12 μg/kg in 1975 to 26 μg/kg in 1982, before declining to 6 μg/kg in 1986 (Mes, 1994). Norιn & Meironyté (2000) reported a steady decrease (from 910 to 324 ng/g lipid) of total PCB levels in the breast milk of Swedish women for the period 1967–1997. In Japan, breast milk samples were analysed for PCB content to determine their yearly trend; the average PCB level increased from 1972 (1.302 µg/g fat basis) to its highest in 1974 (1.514 µg/g fat basis), and then decreased to about 13% of that level (0.200 µg/g fat basis) in 1998. Daily intake of PCBs from breast milk was estimated to decrease from 22.3 µg/g per day to 0.31 µg/g per day during this period. This trend was thought to reflect a change in levels of PCBs in food (both a decrease in contamination and more dependence on imported foods, which were less contaminated than domestic ones) (Konishi et al., 2001) and is consistent with a decline in the levels of PCBs in both the environment and human tissues. Concentrations of different PCB congeners in breast milk from different countries are given in Table 8.
Table 8: Concentration of different PCB isomers in pooled human milk samples (pool size 5–20) from different countries in 1993.a
|
PCB number |
Chlorine substitution |
Concentration in milk (ng/g fat) |
||||||||
|
Albania (Tirana) |
Albania (Librazhd) |
Austria (Vienna) |
Belgium (Brussels) |
Canada (Quebec) |
Canada (Hudson Bay) |
Netherlands |
Pakistan (Lahore) |
Ukraine (Kiev) |
||
|
Non-ortho-substituted PCBs |
||||||||||
|
77 |
34-3'4' |
0.0009 |
0.0006 |
0.014 |
0.0044b |
<0.02 |
<0.02 |
0.0055 |
0.0198 |
0.0076 |
|
126 |
34-3'4'5' |
0.0124 |
0.0097 |
0.077 |
0.0372 |
0.049 |
0.112 |
0.0822 |
0.0183 |
0.0908 |
|
169 |
345-3'4'5' |
0.0048 |
0.0039 |
0.063 |
0.0241 |
0.023 |
0.213 |
0.0557 |
0.0042 |
0.026 |
|
Mono-ortho-substituted PCBs |
||||||||||
|
105 |
234-3'4' |
2.4 |
1.3 |
4.0 |
11.0 |
<2.05 |
<3.19 |
4.7 |
1.2 |
12.2 |
|
118 |
245-3'4 |
8.1 |
5.6 |
30.0 |
27.8 |
16.6 |
80.2 |
20.7 |
2.9 |
44.0 |
|
Marker PCBs |
||||||||||
|
28 |
24-4' |
1.6 |
<1.5 |
3.9 |
3.8 |
3.9 |
3.3 |
3.9 |
3.3 |
4.2 |
|
52 |
25-2'5' |
<1.0 |
<1.0 |
1.7 |
<1.0 |
1.6 |
2.3 |
0.4 |
<1.0 |
c |
|
101 |
25-2'4'5' |
<1.0 |
<1.0 |
2.0 |
1.5 |
<1.03 |
6.4 |
0.8 |
1.2 |
2.7 |
|
138 |
234-2'4'5' |
18.0 |
12.8 |
149.0 |
78.6 |
48.0 |
342.1 |
77.2 |
5.2 |
93.8 |
|
153 |
245-2'4'5 |
32.2 |
22.2 |
146.0 |
122.1 |
59.5 |
716.7 |
113.3 |
5.7 |
122.7 |
|
180 |
2345-2'4'5' |
11.6 |
7.5 |
78.0 |
54.5 |
24.3 |
290.6 |
57.6 |
3.6 |
40.1 |
|
Other PCBs |
||||||||||
|
60 |
234-4' |
0.6 |
<0.5 |
1.6 |
1.2 |
1.0 |
||||
|
66 |
24-3'4' |
1.5 |
5.6 |
|||||||
|
74 |
245-4' |
3.4 |
2.0 |
14.9 |
15.1 |
32.2 |
13.0 |
4.0 |
18.0 |
|
|
110 |
236-3'4' |
<1.37 |
<2.13 |
|||||||
|
156 |
2345-3'4' |
3.5 |
2.3 |
14.2 |
9.1 |
13.2 |
13.8 |
0.8 |
14.9 |
|
|
157 |
234-3'4'5' |
1.0 |
<1.0 |
3.4 |
<3.42 |
<5.32 |
2.8 |
<1.0 |
3.4 |
|
|
167 |
245-3'4'5' |
1.1 |
1.0 |
5.0 |
3.7 |
0.5 |
6.3 |
|||
|
170 |
234-2'3'4'5' |
15.6 |
115.5 |
|||||||
|
183 |
2346-2'4'5'6' |
2.7 |
20.2 |
|||||||
|
187 |
2356-2'4'5' |
7.4 |
89.6 |
|||||||
|
189 |
2345-3'4'5' |
0.5 |
<0.5 |
1.6 |
0.9 |
<0.5 |
<2.5 |
|||
|
a |
From WHO (1996). |
|
b |
Uncertain because of elevated analytical background. |
|
c |
Empty cell denotes that the isomer was not analysed. |
A large number of studies have reported serum PCB concentrations (ATSDR, 2000). In the USA, mean serum PCB levels were generally between 4 and 8 μg/litre, with 95% of the individuals having levels below 20 μg/litre (Kreiss, 1985). Hanrahan et al. (1999) reported that the geometric means for consumers of sport-caught fish from the Great Lakes were 4.8 μg/litre for males and 2.1 μg/litre for females, and the geometric means for non-frequent fish consumers were 1.5 μg/litre for males and 0.9 μg/litre for females. A direct relationship was found between serum PCB levels and quantities of fish consumed or numbers of fish meals consumed (Humphrey & Budd, 1996). The range of geometric means of PCB serum levels for non-occupationally exposed individuals who do not consume fish from PCB-contaminated waters is 0.9–15 ng/ml in the US population (ATSDR, 2000).
Kutz et al. (1991) reported that in samples collected between 1970 and 1983, 66.4% of Americans studied had PCB concentrations below 1 mg/kg, 28.9% had concentrations above 1 mg/kg, and 5.1% had concentrations above 3 mg/kg in their adipose tissues.
Data regarding the toxicokinetics of PCBs in humans are limited to information derived from cases of accidental ingestion of food contaminated with PCBs and to cases of occupational exposure by inhalation and dermal routes.
Humans can absorb PCBs administered by the inhalation, oral, and dermal routes. PCBs are well absorbed by experimental animals when administered orally, but less so when administered by the dermal route. Inhalation absorption data are insufficient for estimating absorption rates. In the gastrointestinal tract, PCBs diffuse passively into the lipophilic cell membranes and into the blood vessels and, via lipids, are absorbed by the lymphatic system that empties into the subclavian vein.
The predominant PCB carriers in human plasma are in the lipoprotein fraction. The distribution pattern of PCBs does not differ significantly between humans and animals and among animal species. Because of their lipophilic nature, PCBs, especially the highly chlorinated congeners, tend to accumulate in lipid-rich tissues. The highest concentrations of PCBs are usually found in the liver, adipose tissue, brain, and skin. Mean PCB concentrations of 5.1, 3.2, and 0.76 mg/kg of extractable fat were determined in samples of abdominal fat, liver, and brain, respectively, obtained from cadavers autopsied in Denmark in 1972 and 1973 (Kraul & Karlog, 1976). Concentrations of PCBs are much higher in cord blood in humans than in breast milk. The concentrations in placenta (average 5027 ng/g fat) were 2.8 times higher than those in breast milk (1770 ng/g fat) (DeKoning & Karmaus, 2000).
In biota, PCBs can easily be transformed to hydroxy and methyl sulfone metabolites that are not readily excreted, but instead can be retained and accumulated in specific tissues and body fluids. These persistent metabolites have been identified in humans as well as wildlife species. Certain P450 enzymes, especially those of the CYP2B family, are known to be involved with the formation of these metabolites. The methyl sulfone PCB patterns that have been identified in different biological matrices, including those from humans, indicate that the biological half-time depends on the structure of the parent PCB (Letcher et al., 2000).
PCBs are metabolized by the microsomal monooxygenase system catalysed by cytochrome P450 into polar metabolites that can undergo conjugation with glutathione and glucuronic acid. A flow-limited pharmacokinetic model was constructed to describe the disposition of some PCB congeners in adults of various animal species (Lutz & Dedrick, 1987). The concept of blood flow-limited uptake was used because experimental data indicate that PCBs leave the blood and enter tissue very rapidly. In this model, metabolism of PCBs was assumed to occur in the liver compartment as a single step leading to the formation of one metabolite, which is excreted in the urine and bile as the glucuronide conjugate. Model simulations of tissue disposition of parent compounds in monkeys were in agreement with experimental data. However, in dogs, the simulations underpredicted the experimental data at longer time points, except for 2,2',4,4',5,5'-hexachlorobiphenyl. While many similarities exist from species to species, there were some important differences. The most important parameter in the model appeared to be the metabolic rate. Knowledge of this parameter in a species of interest is crucial if reliable predictions of PCB disposition are to be made.
The rate of metabolism is determined by the following factors: 1) hydroxylation is favoured at the para position in the least chlorinated phenyl ring unless this site is sterically hindered (such as 3,5-dichloro substitution); 2) in the lower chlorinated biphenyls, the para position of both phenyl rings and carbon atoms that are para to the chloro substituent are all readily hydroxylated; 3) the availability of two vicinal unsubstituted carbon atoms (particularly C5 and C4) also facilitates oxidative metabolism of the PCB substrate, but is not a necessary requirement for metabolism; 4) as the degree of chlorination increases on both phenyl rings, the rate of metabolism decreases; and 5) the metabolism of specific PCB isomers by different species can result in considerable variations in metabolite distribution (Safe, 1980). Trichlorobiphenyls metabolize faster and produce a greater number of metabolites than tetra- or pentachlorobiphenyls. Thus, for example, sheep liver microsomes converted 2,2',5-trichlorobiphenyl to at least 5 more polar metabolites within 1 min and at least 10 metabolites within 15 min. However, within the homologous series, 2,2',5,5'-tetrachlorobiphenyl and 2,2',4,5,5'-pentachlorobiphenyl were oxidized to only three metabolites at rates 7- and 14-fold slower, respectively, than was 2,2',5-trichlorobiphenyl (Hansen, 1987). Rats excreted four symmetrical hexachlorobiphenyls at different rates, depending on chlorine positions (Kato & Yoshida, 1980).
Apparent blood half-times of a PCB mixture were reported to be shorter for children than for their mothers after dietary exposure to Kanechlor 300; this may be partly explained by the growth of the children (i.e., disposition of PCBs in the increasing tissue mass results in accelerated decrease of the blood PCB concentrations due to dilution rather than elimination) (Yakushiji et al., 1984). Enzyme induction over long-term occupational exposure can render some PCBs less persistent in occupationally exposed humans than in the general population (Taylor & Lawrence, 1992).
The main PCB elimination routes are through the faeces, urine, and breast milk.
Single-dose oral LD50 values of 4250, 1010–1295, and 1315 mg/kg body weight have been reported for Aroclors 1242, 1254, and 1260, respectively, in rats. In minks, single-dose oral LD50 values were 750–1000, >3000, and 4000 mg/kg body weight for Aroclors 1221, 1242, and 1254, respectively (Kimbrough et al., 1972; Bruckner et al., 1973; Fishbein, 1974; Grant & Phillips, 1974; Linder et al., 1974; Aulerich & Ringer, 1977; Garthoff et al., 1981). The variation in LD50 values may be related to factors such as animal strain, age, sex, or formulation purity. For example, there is evidence that immature rats (3–4 weeks old) are more susceptible to PCB mixtures than adults (Grant & Phillips, 1974; Linder et al., 1974). Clinical signs in rats include diarrhoea, respiratory depression, dehydration, decreased response to pain stimuli, unusual gait and stance, oliguria, and coma. Vacuolar degeneration and fatty infiltration in renal and hepatic cells (Bruckner et al., 1973) and decreased dopamine concentration in caudate nucleus (Seegal et al., 1986) have been observed after a single high dose of a PCB mixture. Pathological findings in the dead rats were haemorrhagic lung, stomach, and pancreas. Foci of ulceration surrounded by severe inflammatory reaction, primarily in the duodenum and occasionally in the glandular part of the stomach, were observed at necropsy (Linder et al., 1974). Single dermal exposure to Aroclor 1254 at 2273 mg/kg body weight induced death in mice (Puhvel et al., 1982).
Pubertal Sprague-Dawley rats (six females) were treated orally with 0 or 25 mg Aroclor 1221/kg body weight per day in sesame oil on postpartum days 25, 27, 29, and 31. Controls received vehicle only, and rats were killed 18 h after the last treatment. Treatment with Aroclor 1221 had slight, statistically not significant effects on cell proliferation, mammary gland development, body weight, uterine weight, and mammary gland size.
Wistar rats (n = 4) were administered Aroclor 1254 in their diet at doses of 0, 2.5, or 7.5 mg/kg body weight per day for 7 days. Significant increases in relative liver weight and decreases in liver glucose-6-phosphatase activity and serum thyroxine (T4) were observed in both dose groups. A dose of 2.5 mg/kg body weight per day was identified as the LOAEL for the liver weight and T4 level end-points in Wistar rats (Price et al., 1988).
Most of the long-term toxicity studies on PCBs have been performed using commercial mixtures of PCBs, the exact composition of which is not known; the impurities in the PCB mixtures may thus have contributed to the findings. Where the impurities have been analysed, they are noted in the study descriptions below.
Mayes and co-workers (Brunner et al., 1996; Mayes et al., 1998) conducted a comprehensive chronic toxicity and carcinogenicity study on male and female Sprague-Dawley rats (650 per sex), which is summarized in Table 9. The rats were exposed to Aroclor 1016, 1242, 1254, or 1260 at dietary concentrations ranging from 25 to 200 mg/kg. The total PCDD concentrations analysed in the Aroclors 1016, 1242, 1254, and 1260 used in the study were 0.6, 0, 20, and 0 µg/kg, respectively, and the total PCDF concentrations were 0.05, 2.2, 0.13, and 5.5 mg/kg, respectively. Liver enzyme levels (aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase), body weights, mortality, and histopathological examinations were conducted for 2 years. All Aroclors tested induced a dose-dependent increase in the incidence of hepatic tumours in female rats; the carcinogenic potency was in the following decreasing order: Aroclor 1254 > 1260 ≈ 1242 >> 1016. A significant response in males was observed after the administration of Aroclor 1260 at the highest dose level only (Mayes et al., 1998). There were no changes in appearance, behaviour, and mortality rates to indicate systemic toxicity. Total thyroid tumours among males (follicular cell adenoma plus follicular cell carcinoma) were significantly (P < 0.05) higher than in the control group in animals treated with Aroclor 1242 and Aroclor 1254 or the two lowest doses of Aroclor 1260; no statistically significant increase in thyroid tumours was observed after the administration of Aroclor 1016 (Mayes et al., 1998). A statistically significant decreasing trend in the incidence of neoplastic mammary gland lesions was observed in females administered Aroclor 1242, 1254, or 1260.
Aroclor 1254 (purity not reported) was administered at doses of 0, 25, 50, or 100 mg/kg in the diet to groups of Fischer-344 rats (24 per sex per dose level) for 104 weeks. A low and statistically not significant incidence of hepatocellular adenomas and carcinomas (<3 per dose group) was observed in the dosed groups; these tumours were not observed in the controls. A dose-related increase in "non-neoplastic hyperplastic nodules" of the liver was observed in both male and female animals (see Table 9). The combined incidence of lymphomas and leukaemias showed a significant dose-related trend in males, although none of the differences between the individual dosed groups and the matched controls was significant (NCI, 1978).
Table 9: Liver tumour induction in rats after dietary exposure to various PCB mixtures.a
|
PCB mixture |
Sex of rat |
Doseb (mg/kg body weight per day) |
Duration |
Animals with hepatic tumours/ |
Reference |
|
Aroclor 1260 |
F |
Control |
23 months |
1/173 |
Kimbrough et al., 1975d |
|
5.0 |
170/184e |
||||
|
M |
Control |
105 weeks |
7/98 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
|
1.0 |
3/50 |
||||
|
2.0 |
6/49 |
||||
|
4.1 |
10/49e |
||||
|
F |
Control |
105 weeks |
1/85 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998 f,g |
|
|
1.4 |
10/49e |
||||
|
2.8 |
11/45e |
||||
|
5.8 |
24/50e |
||||
|
Aroclor 1254 |
M |
Control |
104 weeks |
0/24 |
NCI, 1978h |
|
1.5 |
5/24 |
||||
|
2.5 |
9/24e |
||||
|
5.0 |
15/24e |
||||
|
F |
Control |
104 weeks |
0/23 |
NCI, 1978h |
|
|
1.5 |
6/24e |
||||
|
2.5 |
10/24e |
||||
|
5.0 |
19/24e |
|
|||
|
M |
Control |
105 weeks |
7/98 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
|
1.0 |
4/48 |
||||
|
2.0 |
4/49 |
||||
|
4.3 |
6/47 |
||||
|
F |
Control |
105 weeks |
1/85 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
|
1.4 |
19/45e |
||||
|
2.9 |
28/49e |
||||
|
6.6 |
28/49e |
||||
|
Aroclor 1242 |
M |
Control |
105 weeks |
7/98 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
2.0 |
1/50 |
||||
|
4.0 |
4/46 |
||||
|
F |
Control |
105 weeks |
1/85 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
|
2.8 |
11/49e |
||||
|
5.7 |
15/45e |
||||
|
Aroclor 1016 |
M |
Control |
105 weeks |
7/98 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
2.0 |
2/48 |
||||
|
4.0 |
2/50 |
||||
|
8.0 |
4/49 |
||||
|
F |
Control |
105 weeks |
1/85 |
Brunner et al., 1996; Cogliano, 1998; Mayes et al., 1998f,g |
|
|
2.7 |
1/48 |
||||
|
5.4 |
6/45e |
||||
|
11.2 |
5/50e |
||||
|
Clophen A-30 |
M |
Control |
832 days |
1/92 |
Schaeffer, 1984i,j |
|
5.0 |
4/107 |
||||
|
Clophen A-60 |
5.0 |
832 days |
61/115e |
|
a |
Studies selected based on number of animals, duration of exposure, and dose–response observed. |
|
b |
Conversion formula for mg/kg in food to mg/kg body weight per day dose: mg/kg in food x 0.05 kg food/kg body weight per day (e.g., 100 mg/kg in diet x 0.05 kg food/kg body weight per day= 5 mg/kg body weight per day) (US EPA, 1988b). |
|
c |
Number of animals bearing specified hepatic tumours (with only the highest stage of carcinogenicity for an animal considered) / effective number of animals. |
|
d |
Tumours included neoplastic nodules and hepatocellular carcinoma. |
|
e |
Incidence significantly (P < 0.05 or P < 0.01) different from respective controls. |
|
f |
For all experiments by Mayes et al. (1998) and Brunner et al. (1996), there was one common control group for all PCB mixtures. |
|
g |
In the studies of Mayes et al. (1998) and Brunner et al. (1996), the tumours include hepatocellular adenoma and carcinoma, as well as cholangioma and cholangiocarcinoma. |
|
h |
In the National Cancer Institute study, tumours include "non-neoplastic hyperplastic nodules" and hepatocellular carcinoma. |
|
i |
A common control group for both Clophen A-30 and Clophen A-60. |
|
j |
Tumours include hepatocellular carcinoma in animals that had survived 800 days and were killed after 832 days at the end of the experiment. |
Male and female Sprague-Dawley rats (63 controls and 70 exposed per sex) were administered Aroclor 1260 (purity not reported) in the diet at doses of 0 or 100 mg/kg for 16 months followed by 50 mg/kg for 8 months (total treatment period 24 months), then kept on the control diet for 3 months. Hepatocellular neoplasms developed in 52/93 treated and 1/81 control rats that survived at least 18 months. High incidences of hepatocellular neoplasm were observed in female groups (95%) compared with male groups (15%), indicating a sex-related effect (Norback & Weltman, 1985). The hepatocellular lesions progressed as follows: centrilobular cell hypertrophy at 1 month, foci of cell alteration at 3 months, areas of cell alteration at 6 months, and adenocarcinoma at 24 months. These lesions were relatively unaggressive malignancies, as they did not metastasize to lungs or invade blood vessels.
Groups of male Wistar rats (n = 139–152 per group at the start of the experiment) were treated with Clophen A-60 or Clophen A-30 (reported to be free of furans) at 100 mg/kg in their diet for 832 days. The control group received a diet without added PCBs. The mortality rate was investigated at 100-day intervals. First hepatocellular carcinomas were detected after 700 days and developed in 61/115 (53%), 4/107 (4%), and 1/92 (1%) rats fed Clophen A-60, Clophen A-30, and control diets, respectively. Neoplastic nodules started to appear after 500 days and were observed in 62/125 (50%), 38/130 (29%), and 5/122 (4%) animals (Schaeffer et al., 1984).
Three groups of male Wistar rats were fed Aroclor 1260 (purity not reported) in the diet at 0, 50, or 100 mg/kg for 120 days. Treatment with Aroclor 1260 significantly increased the incidences of neoplastic nodules in the liver of the 50 mg/kg (24/32) and 100 mg/kg (16/32) groups. Neoplastic nodules apparently occurred with adenofibrosis. No macroscopic changes were observed in tissues other than the liver (Rao & Banerji, 1988). Dose–response cannot be established in this study.
In more limited studies (IARC, 1978; IPCS, 1993), PCB mixtures have also induced hepatic tumours in different strains of mice.
Oral multi-stage carcinogenicity studies have established that Aroclor 1254 and other PCBs of similar chlorine content can promote liver tumorigenesis in rats and mice (Beebe et al., 1993) and lung tumorigenesis in mice (Anderson et al., 1994) following initiation with various genotoxic carcinogens. Promotion by less highly chlorinated PCBs (<50% chlorine by weight) has not been evaluated.
Several studies in which low doses (0.1 mg/mouse) of Aroclor 1254 were applied to the skin of mice showed little or no initiation or promotion activity (DiGiovanni et al., 1977; Berry et al., 1978, 1979; Poland et al., 1983).
In summary, PCBs, particularly the highly chlorinated mixtures (>42%), are hepatocarcinogenic in animals.
PCBs did not exhibit mutagenic activity when tested in the prokaryotic organism Salmonella typhimurium with or without activation systems.
In vitro testing in eukaryotic organisms resulted in negative mutagenic responses in Chinese hamster V79 cells (Hattula, 1985). Results with Aroclor 1254 in human lymphocytes are inconclusive, because Hoopingarner et al. (1972) found no evidence of chromosomal damage at a concentration of 100 μg/ml, whereas Sargent et al. (1989) observed chromosomal damage at a concentration of 1.1 μg/ml using Aroclor 1254. Aroclor 1254 induced DNA damage in rat liver cells in culture as judged by an increase in unscheduled DNA synthesis (Althaus et al., 1982). However, whether the genotoxic doses also caused cytotoxicity was not stated.
Assays on the genotoxicity of PCBs, conducted in vivo on rats and mice, gave generally negative results. Single doses of <500 mg Aroclor 1242/kg body weight administered by gavage to rats did not induce chromosomal damage in bone marrow cells or spermatogonial cells (Green et al., 1975). In the same study, Aroclor 1254 doses of <750 mg/kg body weight per day administered for a 5-day period did not increase the incidence of chromosomal abnormalities in rat bone marrow cells. Similar results were reported for Aroclor 1254 after intraperitoneal administration to mice (Bruce & Heddle, 1979). Rats treated with a single dose of 1295 mg Aroclor 1254/kg body weight exhibited evidence of DNA damage in hepatocytes 4–12 h after treatment; however, the DNA damage appeared to be transient, as it was completely repaired 48 h after treatment (Robbiano & Pino, 1981).
Whysner et al. (1998) administered Aroclor 1260 to mice as either a single 50 mg/kg body weight dose by gavage or an estimated daily dose of 37.5 mg/kg body weight for 14 days in the diet. Liver weights were increased in mice fed for 14 days but not in those administered a single dose. No other liver toxicity end-points were included in either study. Adduct formation was not detected in the liver of mice.
Human lymphocytes in whole blood or isolated cultures were exposed to PCB 77 at concentrations ranging from 0.1 to 100 μg/ml in an in vitro cytochalasin micronucleus test (Belpaeme et al., 1996). PCB 77 did not induce chromosome breakage or produce micronuclei in this study. Dubois et al. (1995) used 32P post-labelling to detect DNA adducts in the human cell line Hep G2 exposed to Aroclor 1254 or 3,3',4,4'-tetrachlorobiphenyl. DNA adducts were found after exposure to 3,3',4,4'-tetrachlorobiphenyl, but not Aroclor 1254.
In summary, the results of in vitro and in vivo genotoxicity studies on PCB mixtures are generally negative and suggest that PCB mixtures do not pose a direct genotoxic threat to humans. Although the mechanistic basis of the hepatocarcinogenicity of PCB mixtures in rodents is not clearly understood, it apparently is not due to genotoxicity.
Toxic effects on fertility and reproductive organs in animals exposed orally to PCBs have been established, but studies of reproductive toxicity following inhalation and dermal exposure were not located.
After oral exposure to relatively high doses of PCBs, effects have been observed in several species, including rats (12.5 mg/kg body weight per day, decreased conception rate; 35.4 mg/kg body weight per day, decreased litter size; and 25 mg/kg body weight per day, decreased seminal vesicle and caudal epididymal weight and reduced sperm count), mice (12.5 mg/kg body weight per day, decreased conception; and 3.5 mg/kg body weight per day, increased testes weight), and monkeys (0.1 mg/kg body weight per day, prolonged menstruation; and 0.2 mg/kg body weight per day, decreased conception). Above-mentioned dose levels represent the lowest at which effects were induced. Adverse reproductive effects have been observed in both male and female offspring of dams treated with Aroclor 1254 during lactation (Sager, 1983; Sager et al., 1987; Sager & Girard, 1994), suggesting that PCBs transferred during breast-feeding can have a long-lasting effect. In those studies, Holtzman rats were exposed once daily to 0, 8, 32, or 64 mg Aroclor 1254/kg body weight per day by gavage on lactation days 1, 3, 5, 7, and 9. Offspring were examined at 2–4.5 months (young), 5–8 months (mature), and 8.5–13 months (older adults). Young rats were mated to untreated males at age 112 days, mature males at 200 days, and older males at 350 days. Autopsies were performed on day 11 or 12 of gestation. In the offspring, the day of vaginal opening and the day of first estrus were significantly delayed at the 32 and 64 mg/kg body weight per day doses. A dose-related reduction in preweaning weight gain was observed and was statistically significant in the same 32 and 64 mg/kg body weight per day groups. Mating rate (sperm-positive) was not significantly altered in young or older offspring, but was significantly reduced in the mature offspring at all doses.
Arnold et al. (1995) treated five groups of female monkeys (16 monkeys per group) with 0, 5, 20, 40, or 80 µg/kg body weight per day of Aroclor 1254 containing 5.2 mg PCDFs/kg and undetectable levels of PCDDs. The doses were given daily by capsules to ensure the exact dosage of the chemicals. Pharmacokinetic steady state was attained at the 25th month of treatment. On the 27th month of treatment, each female was coupled with a compatible untreated male until impregnation came about or the 29th month of the breeding phase of the study was completed. There was a significant decrease in the conception rate at the three highest dose levels. After adjusting for the total number of matings, effects observed at the lowest dose (5 µg/kg body weight per day) were not statistically significant (P = 0.085) and was therefore identified as the NOAEL.
Neonatal male rats were treated subcutaneously for 25 days with Aroclor 1242 (0.4, 1.6, or 3.3 mg/day) or Aroclor 1254 (0.4 or 1.6 mg/day) in 0.04 ml of corn oil. The control group received corn oil only. The pups were then weaned and housed 3–4 animals per cage until they were 135 days old. Paired testes weight, body weight, serum T4, and daily sperm production were measured. The 0.4 mg/day dose of Aroclor 1242 did not increase testicular weight significantly, and therefore this dose was considered as a NOAEL. The doses of Aroclor 1254 (0.4 or 1.6 mg/day) both increased the testicular weight significantly over the control group. Daily sperm production also increased significantly (P < 0.05) for the dose of 1.6 mg Aroclor 1242/day and doses of 0.4 and 1.6 mg Aroclor 1254/day compared with the control (Cooke et al., 1996). Testicular weight and testosterone levels were also assessed in offspring of female rats kept on a diet containing a reconstituted PCB mixture similar to that found in human breast milk from 50 days prior to mating until birth of the offspring. The adult male offspring (170 days old) showed a 30% reduction in testicular weight and a 60% decline in serum testosterone levels. There was also a decrease in aromatase activity in the brains of the newborn male pups, and feminine behaviour was observed among the adult male offspring (Hany et al., 1999).
Several studies have investigated the estrogen-linked effects of PCBs. Intraperitoneal injection of 1.4 mg Aroclor 1242/kg body weight into immature rats significantly increased uterine weight, similar to the effect of 17β-estradiol (Jansen et al., 1993). This effect was also induced by the di-ortho-substituted 2,2',5,5'-tetrachlorobiphenyl, but not by the dioxin-like congener 3,3',4,4'-tetrachlorobiphenyl, which also diminished the increase in uterine weight due to either Aroclor 1242 or estradiol treatment. The conclusion is that PCBs can exert estrogenic or antiestrogenic effects, depending on the congener composition of the mixture. In contrast to the increase in uterine weight produced by Aroclor 1242 after intraperitoneal injection (Jansen et al., 1993), a much higher dose (25 mg/kg body weight) of either Aroclor 1254 or Aroclor 1221 given by gavage to pubertal rats did not induce an increase in uterine weight (Brown & Lamartiniere, 1995). However, a dose of 8 mg Aroclor 1254/kg body weight on lactation days 1, 3, 5, 7, and 9 reduced preweaning uterine weight (Sager & Girard, 1994). The difference in the results may reflect the different dosing procedures and/or qualitative and quantitative differences in the congener composition of the Aroclor as well as differences in the concentrations of impurities (PCDFs) in commercial PCBs. PCB congeners that showed appreciable receptor binding activity were also active in vivo in inducing uterine weight increase. Jansen et al. (1993) reported that Aroclor 1242 increased basal gonadotropin release from pituitary cells in vitro and potentiated pituitary responsiveness to gonadotropin-releasing hormone. Nesaretnam et al. (1996) reported estrogenic effects of 3,3',4,4'-tetrachlorobiphenyl in vitro and in vivo. The congener exhibited ligand binding activity at the estrogen receptor, ligand ability to induce estrogen receptor binding to DNA, ligand regulation of estrogen-regulated genes, estrogen-like effects on cell growth in human breast cancer cells (MCF-7 and ZR-75-1), and uterotrophic effects in vivo in premature mice. To a lesser extent, these effects were also found with 3,3',5,5'-tetrachlorobiphenyl. In these assays, no estrogenic activity was detected for 2,2',5,5'-tetrachlorobiphenyl. Krishnan & Safe (1993) studied the effects of various Aroclor mixtures and PCB congeners in the aryl hydrocarbon (Ah)-responsive MCF-7 human breast cancer cell line. The response measured was the inhibition of the 17β-estradiol-induced secretion of the 52-kilodalton protein procathepsin D. The order of antiestrogenic potency of PCBs was 3,3',4,4',5-pentachlorobiphenyl > 3,3',4,4',5,5'-hexachlorobiphenyl ~ 3,3',4,4'-tetrachlorobiphenyl > 2,3,3',4,4',5'-hexa-, 2,3,3',4,4'-penta-, and 2,3,4,4',5-pentachlorobiphenyl. Aroclors 1221, 1232, 1248, 1254, and 1260 were inactive as antiestrogens at the highest dose used in this experiment. Results from in vitro studies with oocytes from superovulated B6D2F1 mice showed that Aroclors 1221, 1254, and 1268 and 3,3',4,4'-tetrachlorobiphenyl significantly reduced fertilization rate while increasing the incidence of degenerative ova and abnormal two-cell embryos (Kholkute et al., 1994a). Of the four chemicals tested, Aroclor 1254 was the most effective. In the range of concentrations tested (1–10 μg/ml), Aroclor 1254 did not show an effect on sperm function (Kholkute et al., 1994b). Korach et al. (1988) showed that hydroxylated metabolites of lower chlorinated non-planar PCBs bind to estrogen receptors in vitro and are uterotrophic in vivo. It should be noted that those hydroxylated PCB metabolites that exhibit a significant estrogen receptor binding (Connor et al., 1997; Fielden et al., 1997; Ramamoorthy et al. 1997) were derived from parent compounds with limited environmental relevance. Both in vivo and in vitro studies indicate potential estrogenic and antiestrogenic effects associated with exposure to PCBs, and the apparent dichotomy may have been caused by differences in dosing regimen or congener composition of the mixtures.
Developmental effects of PCBs administered orally have been tested in several animal species. The most sensitive developmental end-points appear to be those involving neurobehavioural functions.
The study of Arnold and co-workers (1995) in which five groups of female monkeys (16 monkeys per group) were given 0, 0.005, 0.020, 0.040, or 0.080 mg Aroclor 1254/kg body weight per day, described above in section 8.5.1, showed a significant increase and dose-related trend in fetal mortality (14 deaths from 25 impregnations in the treated groups combined vs. 2/11 in the control group), which became even more pronounced if postpartum/infant deaths were included in the analysis (18/25 vs. 2/11). At the high dose, some of the adult animals showed wasting.
Female rhesus monkeys (n = 8) were administered 2.5 mg Aroclor 1248/kg in their diet (0.03 mg/kg body weight per day) for 18.2 months during the gestation and lactation periods (Levin et al., 1988; Schantz et al., 1991). Animals were allowed to conceive at 12 months (group I) (four offspring tested at 6 years of age, four controls) and again at 32 months after the end of Aroclor 1248 treatment (group II) (three offspring tested at 4 years of age, six controls). No overt signs of maternal toxicity were reported. In group I, seven of the eight dams gave birth to live infants. One infant died of Shigella infection shortly after weaning and at necropsy showed clear signs of PCB immunotoxicity and keratinization of hair follicles of the face and eyelashes. Another infant died at 16 months of age of a condition unrelated to PCB treatment. In group II, three of seven mothers gave birth to live infants. The three available offspring were tested at 4 years of age, with six controls. In both groups of infants, significant differences were detected in delayed spatial alternation between the control and PCB-treated monkeys. This effect was associated with impairment in attentional or associational processes, rather than to an impairment in memory processes. Furthermore, at the time of testing (6 years for group I and 4 years for group II), the offspring of the exposed mothers had mild dermatological lesions and hyperpigmentation about the hairlines. The LOAEL for Aroclor 1248 was thus 0.03 mg/kg body weight per day.
In a similar study on Aroclor 1016, groups of eight female rhesus monkeys received 0.25 or 1.0 mg/kg of Aroclor 1016 (containing no measurable dibenzofurans) in their diet (0.007 or 0.03 mg/kg body weight per day) for 7 months before conception and continued until the offspring were weaned at 4 months of age (average dosing period = 21.8 ± 2.2 months for mothers). Offspring were tested at 4 years of age (seven offspring for the low-dose group [0.007 mg/kg body weight per day] and six offspring for the high-dose group [0.03 mg/kg body weight per day], with six offspring controls for both treatments). No overt signs of maternal toxicity were reported (Schantz et al., 1989, 1991). Mean birth weights in the infants in the control, 0.007, and 0.03 mg/kg body weight per day groups were 521, 491, and 442 g, respectively, the difference being significant for the high-dose group. The deficit was still present at weaning (Levin et al., 1988). A significant decrease was observed in the performance in spatial discrimination reversal in the high-dose group compared with the low-dose group (but not compared with the control group) (Schantz et al., 1989). Therefore, 0.03 mg/kg body weight per day was identified as a LOAEL and 0.007 mg/kg body weight per day as a NOAEL for Aroclor 1016.
Neurodevelopmental deficits were also observed in female rats after exposure to higher levels (>4 mg/kg body weight per day) of ortho-substituted individual PCB isomers (2,4,4'-trichlorobiphenyl, 2,3',4,4',5-pentachlorobiphenyl, 2,2',4,4',5,5'-hexachlorobiphenyl) (Schantz et al., 1995), but not after exposure to coplanar PCB congeners (3, 3',4,4'-tetrachlorobiphenyl, 3,3'4,4'5-pentachlorobiphenyl). Maternal health was assessed and found to be unaffected by the PCB exposure (Schantz et al., 1996). Neurodevelopmental deficits have also been reported in offspring of rats treated during gestation and lactation with relatively low doses of Fenclor 42. Maternal weight was unaffected, and general health was assessed but not reported (Pantaleoni et al., 1988). Female Fischer rats (eight per group) were exposed to Fenclor 42 during the following periods: 2 weeks before mating (for 5 days); during gestation (days 6–15); and after delivery (from day 1 to day 21). These rats received 5–10 mg/kg body weight per day intraperitoneal injection in corn oil before conception, 2–4 mg/kg body weight per day by gavage in corn oil during pregnancy, and 1–2 mg/kg body weight per day by gavage during the lactation period. Behavioural assessments of the pups were started on days 3–6 for surface righting, days 4–10 for cliff avoidance, days 10 and 15 for negative geotaxis, and days 14 and 21 for open field activity. The control group received corn oil only. Females and males were housed for mating in a 2:1 ratio. The day of birth was considered as day 0 in this experiment. Dose-dependent differences in behavioural outcomes between the offspring of control and treated groups were observed. The development of cliff avoidance response, swimming ability, and open field activity were significantly different in treated groups compared with the control group. The doses of 2 mg/kg body weight per day and 4 mg/kg body weight per day were identified as the NOAEL and "serious LOAEL" for behavioural alteration, respectively (Pantaleoni et al., 1988). Studies in which rats received PCBs only through lactation showed that such transfer of PCBs through milk can have adverse reproductive effects (such as infertility) later in life (Sager, 1983; Sager et al., 1987; Sager & Girard, 1994).
After feeding female rats a low chlorinated PCB mixture (Clophen A-30; 0, 5, or 30 mg/kg diet, relating to a daily oral intake of about 0, 0.5, or 3 mg/kg body weight starting 60 days prior to mating and continued PCB feeding in the offspring after weaning), no overt signs of maternal toxicity were reported, but alterations in three activity-dependent behavioural tests were found in adult male offspring of the 30 mg/kg diet group. No effects were found in the 5 mg/kg diet group. The exposure resulted in PCB concentrations of 0.05 and 0.18 mg/kg tissue in brains and about 4 and 15 mg/kg tissue in the adipose tissue of adult male offspring in the 5 mg/kg and 30 mg/kg diet groups, respectively (sum of six congeners). These values were about the same at the age of 120 and 420 days (Lilienthal et al., 1990). Using the same exposure scheme and a 0 or 30 mg/kg diet, but using a cross-fostering design for examination of sensitive periods for PCB effects, a subsequent study revealed the importance of the prenatal exposure period for these neurobehavioural alterations. Only groups with a prenatal exposure period (prenatal and permanent groups) exhibited deficits on two behavioural tasks, while the postnatal group was not different from controls. At the time of testing, the PCB tissue concentration was 0.03 mg/kg or lower in the brain of prenatally exposed rats, while it was 2–3 times higher in the postnatal group (Lilienthal & Winneke, 1991).
Rice & Hayward (1997) studied the effects of postnatal exposure to a mixture of 15 PCB congeners constructed to resemble the PCB composition in human milk (with an estimated correspondence of 80%) on learning in cynomolgus monkeys. Eight male monkeys were dosed from birth to 20 weeks of age with 0.0075 mg PCBs/kg body weight per day. Five monkeys were used as a control group (they received vehicle only). The daily dose was delivered from a syringe inserted directly into each infant’s mouth 3 times/day, 7 days/week. Infants were weighed daily and doses adjusted accordingly. Samples were taken from the nuchal fat pad at 20 weeks, then analysed for adipose tissue PCB levels. Blood samples were collected at 20 weeks of age and analysed for PCB levels. At 20 weeks of age, the PCB levels were 1.7–3.6 mg/kg and 2–3 μg/litre in fat and blood, respectively. Monkeys were tested on a series of non-spatial discrimination reversal problems followed by a spatial delayed alternation task. At the beginning of their 3rd year, exposed monkeys had decreased median response latencies and variable increases in mean response latencies across three tasks of non-spatial discrimination reversal. Overall accuracy tests showed no difference. Treated monkeys also displayed retarded acquisition of a delayed alternation task and increased errors at short delayed task responses. In a separate portion of this study (Rice, 1997), treated monkeys displayed shorter mean inter-response times compared with controls. The increase in pause time for fixed-interval performance emerged more slowly across the 48 sessions in treated monkeys. For fixed-ratio performance tasks, the control monkeys decreased their mean pause time across 10 sessions, whereas the treated monkeys did not. The dose of 0.0075 mg/kg body weight per day was considered as a LOAEL for behavioural impairment in monkeys. From day 7 through day 18 of gestation, Hany et al. (1999) injected Long-Evans rats daily with coplanar PCB 77 (0.5 or 1.5 mg/kg body weight), PCB 47 (1.5 mg/kg body weight), or a congener mixture (0.5 mg PCB 77/kg body weight + 1.0 mg PCB 47/kg body weight). In the PCB-treated group, an elevation of distance travelled and rearing behaviour on postnatal day 340 was observed. A step-down passive avoidance task revealed decreased latencies in the PCB 77 and combined exposure on postnatal day 80. On the haloperidol-induced catalepsy test, only PCB 77-treated rats showed increased latencies on postnatal day 100.
Exposure in utero and through suckling may lead to neurobehavioural deficits in offspring in mice also. Eriksson & Fredriksson (1998) studied postnatal effects of exposure to doses of 0.23, 0.46, or 4.6 mg/kg body weight of PCB 105 or PCB 126 in MRI mice. In this experiment, behavioural effects (learning and memory) were seen in male mice exposed to 0.46 mg PCB 126/kg body weight or higher. The neurochemical changes in rats (see section 8.7), albeit at relatively high doses, as well as neurobehavioural deficits in mice and non-spatial discrimination reversal problems in monkeys receiving PCBs similar to those found in human breast milk (in terms of dose and composition), give support to similar findings in human studies (see section 9.3.2).
Other developmental effects of PCBs include impaired immune function in monkeys (Truelove et al., 1982), focal liver necrosis in rabbits (Thomas & Hinsdill, 1980), cellular alterations of the thyroid in rats (Collins & Capen, 1980), vacuolation of hepatocytes in rats (Linder et al., 1974), and hydronephrosis in mice (Haake et al., 1987); the latter effect was observed after a single administration of a high dose of Aroclor 1254 during gestation.
Smialowicz et al. (1989) exposed young rats to PCBs to assess their effect on the developing immune system. Weanling male Fischer-344 rats (five per group) were administered Aroclor 1254 at doses of 0, 0.1, 1, 10, or 25 mg/kg body weight per day by oil gavage for 5, 10, or 15 weeks. The rats were then killed, and their immune function was evaluated in vitro. The following immune parameters were examined: lymphoid organ weights, mitogen-stimulated lymphoproliferative response, natural killer cell activity, mixed lymphocyte reaction, cytotoxic T-lymphocyte response, and other parameters. Spleen weight was not affected by treatment. Thymus weight was reduced (P < 0.05) with the 10 and 25 mg/kg body weight per day doses at 5, 10, and 15 weeks. Natural killer cell activity, tested against rat and mouse lymphoma cells, was significantly reduced at week 15 by the two highest dose levels. The decrease in natural killer cells at 15 weeks and the decrease of thymus weight suggested that the dose level of 10 mg/kg body weight per day should be considered as a serious LOAEL. Additionally, a NOAEL for all parameters was identified at 1 mg/kg body weight per day for immune response.
Highly chlorinated Aroclors were more immunosuppressive to mice than those with low chlorine content when administered intraperitoneally (Davis & Safe, 1989). Experimental evidence in animals indicates that exposure in utero and through suckling may lead to immunological abnormalities, such as underdeveloped thymus, cellular alterations in lymph nodes, and hypocellularity of the bone marrow in the offspring at a dose level that was overtly toxic to the offspring (Allen & Barsotti, 1976; Truelove et al., 1982). Maternal toxicity was not addressed by Allen & Barsotti (1976); however, Truelove et al. (1982) reported loss of maternal fingernails at all doses studied.
One infant born from female rhesus monkeys administered 0.03 mg Aroclor 1248/kg body weight per day in the diet for 18.2 months during the gestation and lactation periods (Levin et al., 1988; Schantz et al., 1989, 1991) died of Shigella infection shortly after weaning and at necropsy showed clear signs of PCB intoxication (hypocellularity of thymus, spleen, and lymph nodes). For details, refer to section 8.5.3.
In summary, in a range of experimental animals, including primates, PCBs induced fetal or neonatal mortality, neurological defects, and immunological changes at doses not causing maternal toxicity except for loss of fingernails in the monkey.
Immunological effects of PCBs have been evaluated in laboratory animals by determining the infection rates of PCB-exposed animals, monitoring lymphocyte proliferation in response to mitogen stimulation, and assessing morphological changes in the thymus, spleen, and lymph nodes. Evidence suggests that certain immunological parameters, such as antibody production against sheep red blood cells, are sensitive end-points for assessing PCB effects.
Female rhesus monkeys (16 per group) were exposed for 55 months to Aroclor 1254 containing 5.2 mg PCDFs/kg and undetectable levels of PCDDs at doses of 0, 0.005, 0.02, 0.04, or 0.08 mg/kg body weight per day in their diet. PCBs were administered in gelatin capsules containing glycerol/corn oil. The ratio between the vehicle oils and PCBs was 1:1. The monkeys were challenged with injected sheep red blood cells at 23 months of exposure and received a second challenge at 55 months. The animals had achieved an apparent pharmacokinetic steady state at 23 months based on concentrations in blood and fat. Treatment with Aroclor 1254 induced a significant (all doses) and dose-related decrease in antibody levels (IgG and IgM) in response to immunization with sheep red blood cells. The lowest dose (0.005 mg/kg body weight per day) was identified as the LOAEL (Tryphonas et al., 1989). A follow-up study (Tryphonas et al., 1991) showed that the same monkeys had a reduced anamnestic response (only IgM significant) following secondary immunization with sheep red blood cells after 55 months of treatment with Aroclor 1254. These results suggest that Aroclor 1254 is probably affecting the T-lymphocyte system only, because other immunological parameters monitored were not significantly affected (lymphocyte proliferation in response to three different mitogens, mixed lymphocyte culture assay, monocyte activation, interleukin 1 production after stimulation with Escherichia coli, and lymphocyte subpopulation analysis). No signs of microbial infection were noticed throughout the treatment period; however, the monkeys were kept in a clean environment throughout the study. Treatment with up to 0.08 mg Aroclor 1254/kg body weight per day did not alter the response to pneumococcus antigen, although the monkeys were not challenged with live bacteria.
No cross-species generalizations can be made regarding effects on the thymus, spleen, and lymph nodes. Rats experienced decreases in thymus weight at 10 mg/kg body weight per day (Smialowicz et al., 1989), but guinea-pigs and mice at 22 mg/kg body weight per day did not (Vos & de Roij, 1972; Loose et al., 1978a; Thomas & Hinsdill, 1980). Spleen weight was not affected in rats administered 50 mg/kg body weight per day (Allen & Abrahamson, 1973), guinea-pigs administered 0.8 or 4.0 mg/kg body weight per day, or mice administered 22 mg/kg body weight per day (Vos & de Roij, 1972; Loose et al., 1978b). Furthermore, no PCB-attributed histological alterations were reported in the reticuloendothelial organs and tissues of guinea-pigs at 0.8 or 4.0 mg/kg body weight per day or mice at 22 mg/kg body weight per day (Vos & de Roij, 1972; Loose et al., 1978b). In contrast, marked thymic atrophy was reported in rabbits at a relatively low dose (0.18 mg/kg body weight per day) of Aroclor 1254 (Street & Sharma, 1975). Effects on the thymus and spleen are largely due to the Ah receptor actions of the dioxin-like PCBs. They are dose-dependent and occur in all species examined that were administered a high enough dose. The differences in response across species are probably due to a combination of dose differences, type of PCB mixture, and species-specific sensitivity.
In summary, immunological effects on adult animals are dose- and congener-dependent and occur in all species examined at high doses and in some species, such as the monkey, at low dose levels. PCBs can cause a reduction in antibody production against sheep erythrocytes (seen as decreased IgG and IgM levels) and and other immunological effects, such as increased susceptibility to disease, reduced anamnestic response, and decreased thymus weight and thymic atrophy (especially in rabbits). Adult animals appear to be less sensitive than the fetus to the effects of PCBs.
PCB isomers 153 and 128 and Aroclors 1254 and 1260 induced changes in neurotransmitters and their metabolite levels in different areas in the central nervous system in the rat. Levels of dopamine were also lowered in several anatomical locations of the brain in monkeys fed Aroclor 1016 (ATSDR, 2000).
PCBs exhibit a wide range of mechanisms of action that depend on the chlorine substitution pattern in the molecule. The most noticeable difference in mode of action is found due to the presence or absence of chlorine molecules on the ortho (2, 2', 6, 6') positions. Those PCBs that do not contain ortho chlorines and have two pairs of adjacent chlorines on the meta and para positions can have high-affinity binding to the Ah receptor (e.g., PCBs 77, 126, and 169). Both the biochemical and the toxicological modes of action have a striking resemblance to those of the 2,3,7,8-substituted chlorinated dioxins (PCDDs) and dibenzofurans (PCDFs) (Safe, 1990). With an increasing number of ortho chlorines, the possible planar configuration of the PCB molecule becomes increasingly more difficult. As a result, the binding affinity of these (multiple) ortho PCBs to the Ah receptor decreases drastically, and so do the associated mechanisms of action. In this group of ortho-substituted congeners, only some mono-ortho-substituted PCBs (e.g., PCBs 105 and 118) exhibit some binding to the Ah receptor, which results in dioxin-like toxicity and biochemical effects (Safe, 1994). PCB congeners that possess two or more ortho chlorines (e.g., PCB 153) do not exhibit any significant dioxin-like toxicity due to the lack of relevant binding to the Ah receptor. Nevertheless, multiple-ortho-substituted PCBs do have other pronounced mechanisms of action, including effects on neurological development, dopamine levels, and tumour promotion, which will be described in the following paragraphs. However, it should be noted that, in general, the more specific effects of multiple-ortho PCBs are seen at considerably higher dose levels than the pronounced dioxin-like effects that are associated with some non- and mono-ortho PCBs that are potent Ah receptor agonists.
Perhaps the most distinct difference between various groups of PCB congeners is found in the way they induce cytochrome P450 enzymes. Those congeners that show high-affinity binding to the Ah receptor, such as non-ortho PCBs, can be potent inducers of the CYP1 family, including CYP1A1, CYP1A2, and CYP1B1. In contrast, those PCBs that have an ortho substitution pattern induce enzymes from the CYP2 and CYP3 families, which resembles the induction by phenobarbital. In this respect, the mono-ortho PCBs take an intermediate position, as they can induce enzymes from both the CYP1 family as well as the CYP2 and CYP3 families, a property that is generally lost for the di-ortho-substituted PCBs (Safe, 1990, 1994).
Furthermore, activation of the Ah receptor leads to changes in gene expression and signal transduction, inducing changes in cell proliferation and differentiation, inhibition of body weight gain, and thymic atrophy. However, an alternative to the Ah receptor mechanism of toxicity (Loose et al., 1978a,b) involves an increased endotoxin sensitivity and enhanced susceptibility to a malarial parasite in mice treated with Aroclor 1242, suggesting that the immunosuppressive effect may have been caused by a PCB blockade of the hepatic, splenic, and thymic components of the reticuloendothelial system.
Estrogen-like PCB congeners and endocrine disruptors bind to estrogenic receptors. These PCBs may cause subtle endocrine disturbances and adversely affect reproductive performance. Jansen et al. (1993) discussed various possibilities for the mechanism of estrogen-like action of some PCBs. Some PCB congeners may increase gonadotropin-releasing hormone or produce effects beyond the receptor for gonadotropin-releasing hormone. Furthermore, PCBs may affect production and release of luteinizing hormone from the pituitary by mechanisms unrelated to estrogenic action. Studies in breast cancer cell lines (Krishnan & Safe, 1993) indicate that the Ah receptor may be important in mediating the antiestrogenic response. It is important to realize that any hormonal effect is likely to be species-, tissue-, and developmental stage-specific.
Besides the biological and toxicological effects of the parent compounds, the possible endocrine disrupting role of certain hydroxylated and methyl sulfone PCB metabolites is of special toxicological interest (Letcher et al., 2000). Due to the strong binding of some hydroxylated metabolites (e.g., that of PCB 77) to the T4 binding protein (transthyretin), a negative effect on circulating thyroid hormone levels can result. As transthyretin also forms a complex with the retinol binding protein, effects of PCBs on vitamin A can also (partly) be explained (Brouwer, 1991; Brouwer et al., 1998).
In addition, the methyl sulfone PCBs have been shown to inhibit the in vitro CYP11B enzyme activity in the adrenals of mice and grey seals (Halichoerus grypus). This type of mechanism could explain the observed in vivo modulation of glucocorticoid synthesis (Johansson et al., 1998a,b). Furthermore, reproductive toxicity, interaction with the estrogen receptor, and pulmonary metabolism have been observed after exposure to methyl sulfone PCBs (Letcher et al., 2000).
Some studies (Seegal et al., 1991) indicate that mono- and di-ortho-substituted PCBs have a more pronounced adverse effect on neurological development; this toxicity is thus probably mediated by a mechanism independent of the Ah receptor. Schantz et al. (1997) suggested that neurological effects are at least partially mediated via binding to the ryanodine receptor.
The mechanistic basis of PCB-induced hepatocarcinogenesis in rodents is not known, but apparently it is not due to genotoxicity, and assessments of structure–activity relationships of PCB congeners are unclear (Hayes, 1987; Safe, 1989; Buchmann et al., 1991; Laib et al., 1991; Luebeck et al., 1991; Sargent et al., 1992). However, inhibition of intercellular communication was suggested as a probable mechanism for PCB-induced tumour promotion (De Haan et al., 1994; Krutovskikh et al., 1995). Another mechanism involving oxidative stress was proposed by Brunner et al. (1996) and Mayes et al. (1998).
The relationship between exposure to PCBs and human health effects is reflective of the large variation in human exposure to the many different congeners and contaminants present in PCB formulations and to combustion by-products of PCBs. The evidence suggests that exposure to PCBs is associated with increases in risk of cancers of the digestive system, notably of the liver, and of malignant melanoma. However, limitations of exposure information and the presence, in some cases, of further confounding exposures preclude a clear identification of an exposure–response relationship. PCB exposure is also associated with reproductive deficiencies, such as reduced growth rates, retarded development, and neurological effects (although some neurological deficiencies at early ages may disappear later during childhood); immunological changes, manifested as increased infection rates and changes in circulating lymphocyte populations; and dermatological changes, including chloracne and pigmentation disturbances of skin, nails, and gingivaes, as well as nail deformation after exposure to highly chlorinated congeners. The studies in this section were selected mainly based on soundness of study design, quality of exposure assessment, assessment of confounding and bias, and statistical power.
The review in this section examines some 50 studies published since 1976 describing cancer incidence or mortality among populations considered to be exposed to PCBs. Details of those studies can be found in ATSDR (2000). These studies were conducted mainly among people exposed to PCBs at work, and they show increases in mortality from cancer of the gastrointestinal tract, liver, and haematopoietic system and malignant melanoma; however, no consistent picture emerges for any cancer site. Exposures for most study populations were mixed, and many studies were limited by the small numbers of observed deaths and incomplete exposure assessments.
Bertazzi et al. (1987) conducted a retrospective cancer mortality study of 544 male and 1556 female workers employed in the manufacture of PCB-impregnated capacitors at a plant in Italy for 1 or more weeks from 1946 to 1978 and followed through 1982. PCB mixtures containing 54% chlorine were used until 1964, when they were replaced with PCB mixtures containing 42% chlorine until 1970; Pyralenes 3010 and 3011 were used until 1978. Three air PCB concentration measurements (Aroclor 1254) were available from 1954: 5.2, 6.4, and 6.8 mg/m3. In 1977, concentrations of Pyralene 3010 in the air ranged from 0.048 to 0.275 mg/m3. Concentrations of PCBs in 1977 ranged from 0.2 to 159 µg/cm2 and from 0.3 to 9.2 µg/cm2 on workplace surfaces and workers’ hands, respectively. In 1977 and 1982, mean concentrations of 54% chlorine PCBs in blood were 282.8 and 202.8 μg/litre, respectively. In males, the mortality from all cancers (14 observed vs. 7.6 expected; standardized mortality ratio [SMR] = 183; 95% confidence interval [CI] = 104–300) and from cancer of the digestive tract and peritoneum (ICD-8 codes 150–159) (6 observed vs. 2.2 expected; SMR = 274; CI = 112–572) was significantly elevated. In females, the overall mortality from malignant tumours (12 observed vs. 5.3 expected; SMR = 226; CI = 123–385) was significantly increased, and the mortality from haematological cancer (4 observed vs. 1.1 expected) was significantly higher than expected. In a follow-up study, adding 9 years of latency, none of the excess mortalities remained statistically significant, but that from cancer of the digestive system remained elevated in males (10 observed vs. 5.1 expected; SMR = 195; CI = 94–359) (Tironi et al., 1996).
Brown (1987) conducted a retrospective cohort mortality study of 1270 male and 1318 female workers employed for at least 3 months to more than 25 years between 1938 and 1977 and followed through 1987 in areas of two US capacitor manufacturing plants with heavy potential for exposure to PCBs. Over time, the production of PCBs at both plants was changed from Aroclor 1254 to Aroclor 1242 to Aroclor 1016, but the years were not specified. Personal air sampler concentrations ranged between 0.024 and 0.393 mg/m3 at the New York plant and between 0.170 and 1.260 mg/m3 at the Massachusetts plant. Compared with mortality rates for the US population, a statistically significant excess risk was observed for combined cancers of the liver, gall bladder, and biliary tract (5 observed vs. 1.9 expected; SMR = 263; P < 0.05). The excess mostly occurred in women (4 observed vs. 0.9 expected; SMR = 444; P < 0.05) employed at the Massachusetts plant.
Sinks et al. (1992) conducted a retrospective cohort mortality analysis of 3588 workers employed for at least 1 day at an electric capacitor manufacturing plant in Indiana, USA, where Aroclor 1242 was used from 1957 through 1970 and Aroclor 1016 from 1971 through 1977. Mortality from all causes (192 observed vs. 283.3 expected; SMR = 70; CI = 60–80) and from cancer (54 observed vs. 63.7 expected; SMR = 80; CI = 60–110) was lower than expected. There was a statistically significant excess mortality from malignant melanoma of the skin (7 observed vs. 2 expected; SMR = 350; CI = 140–730). No relationship was found between this excess and latency or average estimated cumulative PCB exposure; however, the strength of the association increased with duration of employment. A non-significant excess in mortality from cancers of the brain and nervous system (SMR = 180; CI = 60–420) was also observed. There was co-exposure to solvents, such as 1,1,1-trichloroethane, trichloroethylene, toluene, methyl ethyl ketone, and xylene, but exposure to metals from brazing and soldering operations was considered minimal based on environmental sample results.
Greenland and co-workers (1994) performed an exploratory multi-site case–control study on cancer deaths among employees of a transformer assembly facility using lists of insurance claims after deaths in 1969–1984 among employees who had been employed before 1984. Exposure to pyranol (nominally composed of 50% chlorinated PCBs, trichlorobenzene, and trace amounts of dibenzofurans), benzene, trichloroethylene, other solvents, machining fluids, asbestos, and resin systems was assessed from a job exposure matrix of over 1000 job titles from 50 separate departments in 100 buildings. For exposure to pyranol, the most elevated odds ratios (OR) were observed for liver and biliary cancer (OR = 2.40; CI = 0.59–9.71) and lymphomas combined (OR = 3.26; CI = 1.14–9.32). The authors noted that the study power was limited for most cancer sites, and several biases may have affected the results.
Svensson et al. (1995a) conducted a cancer incidence study in commercial fishermen from the east (n = 2896) and west (n = 8477) coasts of Sweden. Plasma total PCB concentrations in each of four groups of fishermen were 1336 ng/g for the west coast and 2200 ng/g for the Sea of Bothnia, 1696 ng/g for Baltic Proper, and 3076 ng/g for Baltic South, all on the east coast. For non-fishermen in these areas, the corresponding PCB concentrations were 56, 908, 976, and 1337 ng/g (Svensson et al., 1995b). While the overall cancer incidence was not elevated among fishermen, the incidences of lip cancer (standardized incidence ratio [SIR] = 260; CI = 105–536), stomach cancer (SIR = 159; CI = 103–239), and non-melanoma cancer of the skin (SIR = 230; CI = 145–350) were elevated in east coast fishermen. West coast fishermen showed a higher incidence of cancer of the lip (SIR = 192; CI = 129–280) and non-melanoma cancer of the skin (SIR = 112; CI = 88–143). Both fishermen populations showed a deficit in the incidence of cutanous melanoma, which reached statistical significance among the east coast fishermen. The authors considered that differential exposure to sunlight did not explain the patterns of skin and lip cancer. Cancer of the liver showed a small, statistically non-significant excess (SIR = 131; CI = 38–285) among east coast, but not among west coast, fishermen (SIR = 97). Fishermen also had elevated PCDD and PCDF concentrations in their blood.
Gustavsson & Hogstedt (1997) performed a retrospective cohort study of 242 males employed for at least 6 months between 1 January 1965 and 31 December 1978 at a Swedish capacitor manufacturing facility and followed from 1965 through 1991. PCB exposure was classified as low or high by job task based on company records and took into account exposure by inhalation and dermal contact. The overall incidence of cancer was 18 cases versus 20.8 expected (SIR = 86; CI = 51–137). A statistically non-significant excess of incidence of (and mortality from) all cancers was observed among the high exposure group (8 observed vs. 6.15 expected; SIR = 130; CI = 56–256), but not in the entire cohort (18 observed vs. 20.81 expected; SIR = 86; CI = 51–137). Two cases of cancer of the liver and bile ducts were reported (0.78 expected; SIR = 256; CI = 31–926). The study was limited by small cohort size, short follow-up, and limited information on exposure levels.
Loomis et al. (1997) conducted a retrospective cohort mortality study among 138 905 male electrical utility workers employed at power plants for at least 6 months between 1 January 1950 and 31 December 1986. Panels of industrial hygienists, safety personnel, and managers from each of the five participating companies estimated, for each occupational category, the frequency and duration of exposure to insulating fluids containing PCBs during the average workweek. Overall cancer mortality and mortality from cancer of the liver or brain were not related to cumulative exposure to dielectric fluids containing PCBs. Mortality from malignant melanoma increased with increasing cumulative exposure, with rate ratios (RR) of 1.23 (CI = 0.59–2.52), 1.71 (CI = 0.68–7.14), and 1.93 (CI = 0.52–7.14) for cumulative exposure of <2000, 2000–10 000, and >10 000 h, respectively. For the same cumulative exposures, lagging exposure by 20 years yielded a statistically significant increase in mortality from malignant melanoma for the higher two exposure groups, for which the RRs were 2.56 (CI = 1.09–5.97) and 4.81 (CI = 1.49–15.1), respectively. Mortality from all skin cancer (melanoma included) was not elevated. This study was limited by small numbers of subjects in the higher exposure and longer latency groups.
Kimbrough et al. (1999) conducted a mortality study of 7075 workers with at least 90 days of exposure to PCBs between 1946 and 1977 and followed to 1993. The workers were employed at one of two capacitor manufacturing/repairing plants in New York, USA. One plant was included in the Brown studies (Brown & Jones, 1981; Brown, 1987) and involved 2567 workers. PCB exposures were predominantly to Aroclor 1254 from 1946 to 1954, Aroclor 1242 from 1954 to 1971, and Aroclor 1016 from 1971 to 1977. Exposures were classified as high (227–1500 μg/m3), low (3–50 μg/m3), or undefined, based on job types and locations, potential for direct dermal or inhalation PCB contact, and some area measurements. The study found lower than expected mortality rates from all cancers and a statistically significant elevation in mortality from intestinal cancer among women with >20 years’ latency (SMR = 189; P < 0.05). This study is limited by possible exposure misclassification and the small number of long-term high-exposure workers (median number of years in high-exposure groups was 1.6–3.2 in different employee categories).
In 1968, approximately 2000 people of Japan who consumed rice oil contaminated with Kanechlor 400 (a PCB mixture containing 48% chlorine by weight and small amounts of polychlorinated quaterphenyls [PCQs] and PCDFs as contaminants) were afflicted with a condition termed "Yusho." The range of PCB levels in one batch of rice oil was 2000–3000 mg/kg, and a concentration of 3 mg PCDFs/kg was reported in a few other samples that contained about 1000 mg PCBs/kg (Nagayama et al., 1981). The mean estimated intakes were 157 μg/kg body weight per day for PCBs, 0.9 μg/kg body weight per day for PCDFs, and 148 μg/kg body weight per day for PCQs. Serum levels were measured at approximately 40–60 µg PCBs/litre and 13.5 ng PCDFs/litre (Chen et al., 1992). Several months after the episode, the PCB concentrations in tissues were 13.1 mg/kg, 75.5 mg/kg, and 59 mg/kg in the abdomen, subcutaneous fat, and nails, respectively. Approximately 5 years after the accident, the body tissue concentrations were 1.9 ± 1.4 mg/kg fat, 8.08 ± 0.06 mg/kg liver, and 6.7 ± 0.3 μg/litre blood. These levels are about twice as high as in the general control population.
Kuratsune et al. (1987) conducted a retrospective study of 887 males and 874 females 11 years following registration as Yusho victims. Compared with local death rates, statistically significantly increased mortality from liver cancer was observed in males (9 observed vs. 2.34 expected; SMR = 385; P < 0.01); for cases occurring less than 9 years after consumption of the contaminated rice oil, a lower mortality was observed (4 observed vs. 1.04 expected; SMR = 385; P < 0.05). Increased mortality, although not statistically significant, from liver cancer was also observed in females (2 observed vs. 0.79 expected; SMR = 253; P > 0.05) (Kuratsune et al., 1987). No statistically significant (P < 0.05) increased mortality from cancer of the stomach or oesophagus or for leukaemia was observed in either males or females.
In 1979 in Taiwan, more than 2000 people consumed rice-bran oil contaminated with PCBs. The resulting food poisoning condition was termed Yu-Cheng. At an unspecified time, but less than 6 months after the consumption occurred, blood PCB levels averaged 89.1 μg/litre (range 3–1156 μg/litre) in 278 poisoned individuals. Six months after the consumption of the contaminated rice oil, blood PCB, PCDF, and PCQ levels ranged from 12 to 50 μg/litre, from 0.062 to 0.24 μg/litre, and from 1.7 to 11 μg/litre, respectively. One year later, the mean blood PCB level in a subset of the exposed population was 99 ± 163 μg/litre, while the background level for the general population was 1.2 ± 0.7 μg/litre (Hsieh et al., 1996).
Hsieh et al. (1996) conducted a retrospective cohort mortality study following 1940 Yu-Cheng cases (929 males, 1011 females, >95% of all registered cases) for 12 years post-exposure (between 1980 and 1991). The average age of the subjects was 27 years at the beginning of the study. The study reported a significant excess in mortality from non-malignant liver diseases (SMR = 3.22; CI = 1.8–5.1); for all cancers, a non-significant deficit was observed (SMR = 0.58; CI = 0.29–1.04). For individual cancer sites, the study was not very informative because of the small number of expected and observed cases (2 observed cases of lung cancer, 1 or 0 for the others); however, the mortality from cancer of the liver and intrahepatic bile ducts was elevated in females (SMR = 1.08, CI = 0.03–6.02, for national referents; SMR = 1.23, CI = 0.03–6.87, for local referents), but not in males (SMR = 0.29, CI = 0.01–1.62, for national referents; SMR = 0.32, CI = 0.01–1.80, for local referents).
An additional limitation of the Yusho andYu-Cheng studies was the co-exposure to dibenzofurans and chloroquaterphenyls.
As part of a prospective study of determinants of health (Campaign against Cancer and Stroke) among 25 802 adults in Maryland, USA, a nested case–control study on non-Hodgkin lymphoma (NHL) (74 cases, 147 matched controls) was performed (Rothman et al., 1997). The relationship between concentrations of PCBs, DDT/DDT metabolites, and metabolites of five other organochlorine compounds, determined in specimens collected in 1974, and NHL diagnosed in 1975–1989 was studied. Average concentrations of PCBs in plasma were higher among patients than among referents (medians 951 and 864 ng/g lipid), and a strong dose–response relationship between the risk and serum PCB levels was observed. The means of the PCB concentration quartiles were 526, 727, 924, and 1430 ng/g lipid, and the ORs (CI) of developing NHL for the PCB concentration quartiles were 1.0, 1.3 (0.5–3.3), 2.8 (1.1–7.6), and 4.5 (1.7–12). The trend was dose-related, with a P-value of 0.0008. Adjustment of the results for education or smoking or exclusion of the one case diagnosed within 2 years of enrolment did not affect the results. There was no relationship between NHL and the other organochlorine compounds measured.
Several small case–control studies (see ATSDR, 2000) have reported on the relationship between the risk of breast cancer and serum PCB concentrations at the time of diagnosis. No consistent picture emerges from these studies; the same is true for the smaller number of studies on PCB concentrations in breast tissue and breast cancer risk (Moysich et al., 1998; Welp et al., 1998; Aronson et al., 2000). Several relatively large prospective studies on the relationship between PCBs in blood and breast cancer risk are consistent in not suggesting any association between PCB serum or plasma concentrations and breast cancer (Krieger et al., 1994; Hunter et al., 1997; Hoyer et al., 1998; Dorgan et al., 1999; Helzlsouer et al., 1999).
Limited information was found concerning genotoxic effects in humans after exposure to PCBs. One study described a moderate increase in sister chromatid exchanges and chromosome breaks in lymphocytes after exposure to PCBs and other compounds after an electrical facility fire in Italy (Melino et al., 1992). Another study reported an increase in chromosomal aberrations and sister chromatid exchanges in peripheral lymphocytes from workers involved in manufacturing PCBs in Czechoslovakia. The exposed workers were also exposed to benzene and formaldehyde (Kalina et al., 1991).
In a questionnaire survey within the New York State angler cohort, 575 female anglers or anglers’ wives who had discontinued birth control in order to become pregnant were questioned concerning their frequency and duration of use of locally caught fish and the time to pregnancy. Based on concentrations of PCBs in different fish species in different locations in Lake Ontario, a PCB index was also generated. Fecundability of women decreased with years of local fish use, frequency of fish meals, and PCB index and reached statistical significance for the frequency of fish meals category >1 fish meal per week. Paternal fish consumption was not related to fecundability (Buck et al., 2000).
Time to pregnancy was also assessed among the wives of the Swedish east coast and west coast fishermen (for cohort description, see Svensson et al., 1995a in section 9.1) using a questionnaire (Axmon et al., 2000a). Lower fecundability was observed among the heavy smokers in the east coast cohort (higher PCB exposure) than among the west coast heavy smokers; no such difference was observed among non-smokers or light smokers. Among the more exposed east coast cohort, no relationship was observed between growing up in a fishing village or high current fish consumption and low fecundability. Presumed exposure to PCBs was not associated with miscarriages or stillbirths (Axmon et al., 2000b).
Emmett et al. (1988a,b) conducted a cross-sectional study of 55 transformer repairmen (exposed primarily to Aroclor 1260 and less to Aroclor 1242 for an average 3.75 years at a rate of 8 h/day, 5 days/week) and 56 non-exposed controls to assess reproductive and neurological effects. Eight-hour personal breathing zone time-weighted average air PCB concentrations were assessed at four areas: 0.0167–0.0240, 0.0032–0.0070, 0.000 01–0.0004, and 0.0007–0.0124 mg/m3. The study found no association between exposure to PCB and sperm counts.
Bush et al. (1986) collected 170 seminal samples from fertile men, men with idiopathic oligospermia or azoospermia, and vasectomized men who were apparently environmentally exposed to PCBs. The mean total PCB concentration in serum was 5.8 ng/g wet weight. The concentrations of three coplanar PCB congeners (2,2',4,4',5,5'-hexachlorobiphenyl, 2,2',3',4,4',5-hexachlorobiphenyl, and 2,3',4,4',5-pentachlorobiphenyl) were inversely correlated with sperm motility index in samples with a sperm count of less than 20 million sperm per millilitre. Pines et al. (1987) reported higher tetra- and pentachlorobiphenyl levels in infertile men compared with the control population. DDE was determined to be a minor component in these samples. Because of limitations in these studies, including a limited number of patients, as well as exposure to DDT and other organochlorine pesticides, possible effects on sperm counts and motility cannot be solely attributed to PCB exposure.
Neurodevelopmental effects of prenatal exposure to PCBs have been investigated extensively in a prospective longitudinal study of 242 infant–mother pairs where the women consumed PCB-contaminated fish from Lake Michigan and 71 infant–mother controls (Jacobson et al., 1985; Jacobson & Jacobson, 1996). Each woman included in the study had consumed more than 11.8 kg of contaminated fish (containing unspecified concentrations of PCBs, PCDDs, and PCDFs) over a 6-year period. Cord and maternal serum samples collected after delivery averaged 2.5 (SD 1.9) and 5.5 (SD 3.7) ng PCBs/ml. Milk samples were collected between 1 and 16 weeks postpartum. Overall fish consumption and levels of PCBs in cord serum were positively correlated with lower birth weight, smaller head circumference, and shorter gestational age. Fish consumption during pregnancy was related to neuromuscular immaturity. Contaminated fish consumption was also positively correlated with impaired autonomic function, increased number of abnormally weak reflexes, and decreased range of state (Jacobson et al., 1984). Cord serum PCB levels were a stronger predictor of poorer mean visual recognition memory than overall consumption of fish (Jacobson et al., 1985). Postnatal exposure from nursing was not related to recognition memory performance. Preference for novelty was inversely related to PCB levels in cord serum.
Seventy-five per cent of the children in the original study were re-examined at age 4 (Jacobson et al., 1990a,b). Prenatal exposure to PCBs, assessed by cord serum PCB levels, was associated with poorer performance on two specific tests involving short-term memory. There was no indication of perceptive-motor deficits or alteration of long-term memory. Exposure from nursing was unrelated to cognitive performance. At the follow-up at the age of 11 years, it was observed that prenatal exposure to PCBs was associated with lower full-scale and verbal IQ scores after control for potential confounding variables such as socioeconomic status (P = 0.02). The strongest effects were related to memory and attention. The most highly exposed children were 3 times as likely to have low average IQ scores (P < 0.001) and twice as likely to be at least 2 years behind in reading comprehension (P = 0.03) (Jacobson & Jacobson, 1996).
A large study was carried out on 418 mother–infant pairs that were recruited from 1990 to 1992 in the Netherlands. The Groningen and Rotterdam subset of this study group consisted of 207 healthy mother–infant pairs, of which 105 infants were breast-fed for at least 6 weeks and 102 infants were bottle-fed. Prenatal PCB exposure was estimated by the PCB sum (PCB congeners 118, 138, 153, and 180) in maternal blood and the total TEQ level in human milk (17 individual dioxins and 8 dioxin-like PCB congeners). The mean PCB plasma sum in the total group was 2.25 µg/litre, and the mean total TEQ level was 66.59 pg/g fat. The infants were born at term (37–42 weeks) and without congenital anomalies or complications during delivery. Weight was measured at birth, and weight, length, and head circumference were measured at 10 days and 3, 7, 18, and 42 months of age. The neurological examinations were conducted between the 10th and the 21st day, as well as at 18 and 42 months, after birth. Blood samples were collected from mothers between the 36th and 40th weeks of pregnancy and from the umbilical cord to measure the PCB concentrations as an indication of prenatal exposure. During the 2nd week after delivery, breast milk samples were collected before each feeding for a full day. This study indicated that in utero exposure to PCBs was associated with lower psychomotor scores at 3 months of age. However, no effects due to PCB exposure were detected at 18 months of age (Huisman et al., 1995; Koopman-Esseboom et al., 1996). Higher PCB/dioxin levels, expressed as TEQs, correlated significantly with lower plasma levels of maternal triiodothyronine (T3) and total T4 and with higher plasma levels of thyroid stimulating hormone in infants in the 2nd week and 3rd month after birth (Koopman-Esseboom et al., 1994). Infants exposed to high cord plasma PCB levels (0.80 µg/litre) weighed 165 g less than infants exposed to low cord plasma PCB levels (0.20 µg/litre). Both cord and maternal plasma PCB levels were significantly associated with lower growth rates until 3 months of age (Patandin et al., 1998). Patandin et al. (1999) reported a four-point IQ difference between high (SigmaPCB > 3 µg/litre maternal plasma) and low (SigmaPCB < 1.5 µg/litre) exposed infants using the Kaufman assessment battery test. A potential limitation of these studies was a co-exposure to dioxin.
Winneke et al. (1998) investigated the neurodevelopmental toxicity of SigmaPCBs (sum of congeners 138, 153, and 180 in cord plasma and maternal milk) of 170 healthy mother–infant pairs in Germany. The PCB concentrations were found to be 0.52 (range 0.17–1.36) ng/ml and 389.6 (range 97–1011.0) ng/g in cord plasma and breast milk fat, respectively. A visual recognition memory test was administered to the infants at 7 months of age, along with the Bayley-Scales of Infant Development Test, for which mental scale (MDI) and psychomotor (PDI) scores were determined. The MDI score assesses memory, learning, problem solving, language development, and personal/social development, while the PDI score assesses fine and gross motor development of children. Multilinear regression analysis was used to carry out the statistical analysis. After adjusting for confounders (education of mother, quality of the home environment, verbal IQ of mother, mother’s age, duration and type of feeding), the only significant (P < 0.05) negative association was found between SigmaPCBs in milk and MDI score.
Stewart et al. (2000) examined a subset of data collected for a prospective longitudinal study initiated in Oswego, New York, USA, in 1991. The study consisted of 141 newborns whose mothers consumed at least 18 PCB-equivalent kilograms of Lake Ontario fish over their lifetime and of 152 newborns whose mothers had not consumed fish. Cord blood PCBs, DDE, hexachlorobenzene, mirex, and lead and hair mercury were measured for all 293 women. Cord blood total PCB concentrations were 0.174, 0.525, and 1.11 ng/g wet weight for the 25th, 50th, and 75th quartiles, respectively. Cord blood levels of heavily chlorinated PCBs (Cl7–Cl9) correlated significantly with breast milk concentrations of both heavily chlorinated (P < 0.005) and total (P < 0.05) PCBs. Impaired performance (such as habituation, autonomic, and reflex clusters of Neonatal Behavioral Assessment Scale [NBAS]) was associated with consumption of PCB-contaminated fish. A significant linear dose–response trend relationship was observed between exposure to only the most heavily chlorinated PCBs and performance impairments on the habituation scores (f [1,221] = 3.95; P < 0.05) and autonomic clusters scores (f[1,261] = 4.40; P < 0.05) of the NBAS at 25–48 h after birth.
Rogan et al. (1986, 1987), Gladen et al. (1988), and Gladen & Rogan (1991) conducted studies and follow-ups on 912 children born to women with background PCB body burdens. Breast milk concentrations ranged from 0 to >4 mg/kg in milk fat at birth. Higher levels of PCBs in milk were associated with decreased muscle tone and hyper-reflexia for infants tested at day 3 or earlier. Further evaluation of the children showed that transplacental exposure was associated with poorer performance on the PDI at 6 and 12 months of age, whereas exposure through breast-feeding had no apparent relation with performance (Gladen et al., 1988). PDI patterns at 18 and 24 months were similar to those at 6 and 12 months, the deficit achieving statistical significance in the 24-month follow-up (Rogan & Gladen, 1991). A subsequent report (Gladen & Rogan, 1991) found that the deficits observed in children through 2 years of age were no longer apparent at age 3, 4, and 5 years. Fein et al. (1984) reported similar findings.
As part of the analysis of the effects of methylmercury exposure on child development, possible confounding of these findings by exposure to PCBs was investigated in the Faroe Islands (Steuerwald et al., 2000). PCB concentrations were determined in maternal blood and milk (geometric means 1.12 μg/g lipid and 1.52 μg/g lipid, respectively), and a neurological optimality score (NOS) was determined at the age of 2 weeks. A weak positive association was found between NOS and maternal serum PCB levels, and a weak negative association was found with maternal milk PCB levels. Resin T3 uptake in cord serum appeared to change inversely with PCB concentration (r = –0.21, P = 0.01), and thyroid stimulating hormone concentrations showed a weak negative correlation with both cord blood and maternal serum (r = –0.04, P = 0.66; and r = –0.13, P = 0.09).
Japanese and Taiwanese residents who ingested PCB-contaminated rice oil (these episodes also resulted in exposure to relatively high concentrations of PCDF and PCQ impurities) were examined for developmental outcomes. Reported effects included an approximately 15% decrease in birth weight (Taki et al., 1969; Funatsu et al., 1971; Yamaguchi et al., 1971; Lan et al., 1987; Rogan et al., 1988; Rogan, 1989) that continued to the second but not the third birth (Lan et al., 1987) and a decrease in rate of growth (height and weight gains) during the first 6 years of age that tended to be reversed (Yoshimura & Ikeda, 1978). More details are available in the source document (ATSDR, 2000).
Svensson et al. (1994) conducted a retrospective immunological competence cohort study of 23 Swedish males (mean age 39.4 years, range 23–62 years) who consumed a large amount of fish from the Baltic Sea (dose not specified) and a control group of 20 males (mean age 45.9 years, range 23–69 years) who did not consume any fish. The range of mean blood plasma measurements for various PCB congeners and groupings was 0.2–3.6 pg/g for high consumers and 0.08–1.7 pg/g for non-consumers. The high fish consumers had lower proportions and numbers of natural killer cells (identified by the CD 56 marker) in the peripheral blood than the control, indicating a negative correlation between weekly intake of fatty fish and natural killer cells (Rs = –0.32, P = 0.04). A subgroup of 11 individuals showed a significant negative correlation between numbers of natural killer cells and non-ortho PCB congener (PCB 126: Rs = –0.68, P = 0.02) and a mono-ortho congener (PCB 118: Rs = –0.76, P = 0.01). DDT, but not mercury, was determined to be a significant confounder.
The Dutch study (for study description, see section 9.3.2) also investigated the immunological effects of pre- and postnatal PCB/dioxin exposure from birth to 18 months of age (Weisglas-Kuperus et al., 1995). The number of periods with rhinitis, bronchitis, and tonsillitis during the first 18 months of life was used as an estimate of the health status of the infants. Humoral immunity was measured at 18 months of age by detecting antibody levels to mumps, measles, and rubella. White blood cell counts (monocytes, granulocytes, and lymphocytes) and immunological markers (CD4+CD45RA+ and CD4+CD45R0+ T-lymphocytes, CD8+ T-lymphocytes, activated T-lymphocytes [HLA-DR+CD3], and other immunological markers) in cord blood and venous blood at 3 and 18 months of age were assessed in a subgroup of 55 infants. This study found no relationship between the numbers of upper or lower respiratory tract symptoms or humoral antibody production and pre- or postnatal PCB/dioxin exposure. No significant correlations existed between the number of periods with rhinitis, bronchitis, tonsillitis, and otitis during the first 18 months of life and pre- and postnatal exposure to PCBs/dioxins. However, higher prenatal PCB/dioxin exposure was associated with an increase in the number of TcR gamma delta+ T cells at birth and with an increase in the total number of T cells and the number of CD8+ (cytotoxic), TcR alpha beta+, and TcR gamma delta+ T cells at 18 months of age. In a follow-up assessment, the sum of four PCB congeners in plasma of these children at age 42 months was found to be associated with a higher prevalence of recurrent middle-ear infections and chicken pox (Weisglas-Kuperus, 2000).
Limited information exists regarding neurological effects in adult humans following exposure to PCBs. Occupationally exposed workers reported symptoms such as headache, dizziness, depression, fatigue, and a tingling sensation in the hands (Fischbein et al., 1979; Smith et al., 1982; Emmett et al., 1988a). Persons regularly consuming fish from waters contaminated with PCBs performed poorly on tests that required cognitive ability, word naming, auditory recall, and complex motor tasks (Mergler et al., 1998). A limitation of this investigation is that fish contaminated with PCBs are also usually contaminated with heavy metals, pesticides, and other chemicals. Significantly higher concentrations of structurally similar PCB 153 and PCB 180, as well as total PCBs, were found in the caudate nuclei of Parkinson disease patients than in control groups (Corrigan et al., 1998). PCB/DDE exposure through consumption of contaminated fish from the Great Lakes did not significantly reduce visual coordination (as measured by the grooved pegboard test) or affect hand steadiness (as measured by the Static Motor Steadiness test) in 50- to 90-year-old individuals.
Japanese and Taiwanese residents who ingested PCB-contaminated rice oil (these episodes also resulted in exposure to relatively high concentrations of PCDF and PCQ impurities) were examined for neuropsychological outcomes. Children exposed in utero scored lower on the Stanford-Binet test (ages 4–7 years), Wechsler Intelligence Scale (ages 4–7 years, other than the WISC-R at age 6 years), Rutter’s Child Behavior Scale A (emotional or behavioural disorders), modified Werry-Weiss-Peters Activity Scale (activity levels), MDI and PDI tests (between ages 6 months and 2 years), and Raven’s Colored Progressive Matrices (at age 9 years). After accounting for confounders (neighbourhood, age, sex, mother’s age, and parental education and occupation), Yu-Cheng children showed lower intelligence test scores, more behavioural disorders, higher activity levels, and lower psychomotor scores than the controls (Chen et al., 1992, 1994; Lai et al., 1994; Guo et al., 1995). More details are available in the source document (ATSDR, 2000).
Adult victims of the Yusho and Yu-Cheng incidents were examined for neurological and neurophysiological symptoms and signs. Exposure resulted in various neurological symptoms (numbness, weakness, and neuralgia of limbs, hypoaesthesia, and headaches) (Chia & Chu, 1984, 1985; Kuratsune, 1989; Rogan, 1989). Exposure also caused a reduction in sensory nerve (radial and/or sural, median, and ulnar) and motor nerve (ulnar, tibial, and peroneal) conduction velocities (Kuroiwa et al., 1969; Chia & Chu, 1984, 1985; Chen et al., 1985). The findings from the studies of these groups cannot be attributed solely to exposure to PCBs, since the victims were also exposed to PCDFs and other chlorinated chemicals (ATSDR, 1994, 2000).
Dermal effects, particularly chloracne and skin rashes, have been associated with occupational exposure to Aroclors (Meigs et al., 1954; Ouw et al., 1976; Fischbein et al., 1979, 1982, 1985; Baker et al., 1980; Maroni et al., 1981; Smith et al., 1982; Bertazzi et al., 1987; Emmett et al., 1988a). Chloracne is a type of acneiform eruption caused by some chlorinated organic chemicals. It is characterized by keratinous plugs (comedones) and skin-coloured cysts with a central opening in the pilosebaceous orifices (Kimbrough, 1987).
Mild to moderate chloracne was observed in 7 out of 14 workers exposed to Aroclors (formulation not specified) at 0.1 mg/m3 for an average duration of 14.3 months (Meigs et al., 1954). Because PCBs were used as a heat exchange material, it is possible that the workers were exposed to such pyrolysis products as PCDFs. In these workers, the chloracne was found primarily on the face, especially the cheeks, forehead, and ears. Three cases of chloracne occurred among an unspecified number of autoclave operators exposed to 5.2–6.8 mg Aroclor 1254/m3 for 4–7 months in 1954 (Bertazzi et al., 1987). In 1977, four more cases of chloracne were diagnosed among 67 workers from the same plant who were engaged in impregnating capacitors with Pyralene 3010 (0.048–0.275 mg/m3) and had skin contact confirmed as a major exposure route. An increased incidence of non-adolescent acneiform was reported in workers exposed to mean concentrations of various Aroclors of 0.007–11 mg/m3 for >5 years; 40% of the workers were exposed for >20 years (Fischbein et al., 1979, 1982). This chloracne often appears first as facial comedones and cysts, but sometimes manifests itself as severe skin lesions, probably resulting from a systemic response.
Effects on the skin were widely reported among Japanese and Taiwanese residents who ingested PCB-contaminated rice oil (these episodes also resulted in exposure to relatively high concentrations of PCDF and PCQ impurities) (Lu & Wu, 1985; Kuratsune, 1989; Rogan, 1989; Guo et al., 1999). Characteristic skin changes included marked enlargement, elevation, and keratotic plugging of follicular orifices, comedo formation, acneiform eruptions, hyperpigmentation, hyperkeratosis, and deformed nails. The acne most commonly developed on the face and other parts of the head, axillae, trunk, and external genitalia, with follicular plugging occurring in the axillae, groin, glenoid regions such as elbow and knee flexures, trunk, thigh, and outer aspect of the forearm. Dark-coloured pigmentation frequently occurred in the gingival and buccal mucosa, lips, and nails and improved only gradually in most patients (Fu, 1984; Lu & Wu, 1985; Kuratsune, 1989; Rogan, 1989). Improvement of the dermal changes was gradual. Evaluation of Yu-Cheng subjects 14 years after the poisoning incident showed that men and women exposed to PCBs/PCDFs had a higher lifetime prevalence of chloracne, abnormal nails, hyperkeratosis, and gum pigmentation (Guo et al., 1999). Skin lesions were commonly observed in children born to mothers with Yusho or Yu-Cheng exposure. The dermal changes are consistent with those observed in exposed adults and included hyperpigmentation of the skin, nails, and gingivae, deformed nails, and acne (Taki et al., 1969; Funatsu et al., 1971; Yamaguchi et al., 1971; Yoshimura, 1974; Hsu et al., 1985; Rogan et al., 1988; Gladen et al., 1990). These effects generally diminished as the babies grew older.
Among the methods that have been considered for assessing the dose–response relationship for exposure to PCBs, the mixtures approach was the one selected to set the tolerable intake for PCBs. The bases for this decision are that analytical data for performing this analysis are generally available and that workers have been occupationally exposed to identical Aroclor mixtures; workers and groups in the general population have been and are potentially still exposed accidentally to these PCB mixtures. In addition, populations have been exposed environmentally to broad mixtures of PCB congeners via food, air, and water due to global environmental transport and distribution. Although the composition of these exposures is not identical to the commercial mixtures, studies of environmentally relevant mixtures (such as that in breast milk) have been found to have comparable toxicity. An alternative approach is the TEQ method, which is based on the analysis of the congeners with known toxicity equivalence factors (TEFs) relative to health effects mediated by the Ah receptor. This method assesses the toxicity of an individual congener for a specific end-point and estimates the combined effect for those congeners. It is being developed to simplify risk assessment and regulatory control (Ahlborg et al., 1994). A further approach is the total body burden method for PCB mixtures. It is derived from human data rather than from laboratory animal or in vitro studies, so as to eliminate the need for species extrapolation (Tilson et al., 1990).
Human studies have identified associations between exposure to PCB mixtures and adverse immunological, reproductive, and dermatological effects and cancer. However, the human studies are limited by limited exposure data, inconsistency among some results, and the presence of confounding factors; these limitations make it impossible to use them as a basis of quantitative risk estimations. Therefore, this document has chosen to use animal rather than human study results for the purpose of risk characterization.
Several studies demonstrate that PCBs, notably the more chlorinated congeners and mixtures, induce benign and malignant hepatic tumours in rodents. The cancer incidences were dose-, time-, and congeneric-mixture-type dependent, and cancer occurred only at dose levels far in excess of those inducing other effects. The weight of evidence indicates that these lesions were not induced by a genotoxic mechanism.
The evidence from epidemiological studies suggests that PCB exposure is associated with increases in risk of cancers of the digestive system, notably of the liver, and of malignant melanoma. However, limitations of exposure information and the presence, in some cases, of confounding exposures preclude a clear identification of an exposure–response relationship.
Rhesus monkeys receiving daily doses of Aroclor 1254 for several months showed a dose-related increase in liver weight and decreases in the IgG and IgM immunoglobulin response to a sheep red blood cell challenge. The lowest dose studied, 0.005 mg/kg body weight per day, was identified as the LOAEL.
One study reported a positive association between some PCB congeners and recurrent middle-ear infections and chicken pox during the first few years of life. Another study described a relationship between prenatal PCB exposure and changes in circulating lymphocyte subpopulations. A further study demonstrated a decreased number of natural killer cells among fishermen exposed to PCBs.
Neurodevelopmental effects after exposure to PCBs have been observed in several animal species. Monkeys exposed to 0.03 mg Aroclor 1016/kg body weight per day or 0.07 mg Aroclor 1248/kg body weight per day for approximately 20 months showed a reduced ability to learn a simple spatial discrimination task.
Cynomolgus monkeys administered a mixture of 15 PCB congeners similar to the congeners found in human milk for 20 weeks starting at birth showed decreased median response latencies and variable increases in mean response latency across three tasks of non-spatial discrimination reversal. The dose of 0.0075 mg/kg body weight per day was considered as a LOAEL for behavioural impairment in monkeys.
In a series of studies on infants born to mothers consuming PCB-contaminated fish from Lake Michigan, prenatal exposure to PCBs was associated with lower birth weight and smaller head circumference; cord PCB levels were associated with poor performance in tests for short-term memory at 4 years of age and with lower verbal IQ and achievement tests at 11 years of age, after controlling for potential confounding variables.
In a Dutch study, exposure to PCBs, as assessed from PCB concentrations in cord blood, was associated with lower psychomotor scores at the age of 3 months, while no such effects were observed at the age of 18 months. There was a co-exposure to chlorinated dioxins. In a German study, the sum of three PCB isomers in cord plasma showed a negative association with MDI of the infants. Impaired performance in an NBAS was associated with cord blood concentrations of heavily chlorinated PCB congeners among infants born to heavy consumers of fish from Lake Ontario.
Studies performed by Tryphonas et al. (1989, 1991) formed the basis for setting a tolerable intake for PCBs. Those studies found that rhesus monkeys exposed to Aroclor 1254 suffered adverse immune health effects. Those studies were selected on the following basis:
Rhesus monkeys receiving daily doses of Aroclor 1254 for several months showed a dose-related increase in liver weight and decreases in the IgG and IgM immunoglobulin response to a sheep red blood cell challenge. The lowest dose studied, 0.005 mg/kg body weight per day, was identified as the LOAEL. Using an uncertainty factor of 300 (based on individual factors of 3 for interspecies variation, 10 for intraspecies variation, and 10 for extrapolation from a LOAEL to a NOAEL), a tolerable intake of 0.02 μg/kg body weight per day can be derived for mixtures of PCBs. The lower-than-default interspecies uncertainty value of 3 was justified based on oral Aroclor study observations that confirm non-human primates as among the most sensitive species. The available data are insufficient to derive tolerable con-centrations for airborne PCBs or tolerable intakes for short-term oral exposures.
In a developmental neurotoxicity study in monkeys, slight changes in neurobehavioural tests were observed at the only exposure level studied, 0.0075 mg/kg body weight per day. In this study, the PCB mixture given to the monkeys was engineered to mimic the congener pattern in mother’s milk. As the LOAELs from the two studies converge, they strengthen each other and also increase confidence in the applicability of the mixtures approach to dietary exposures, at least for infants.
In the USA, the current estimated PCB intake from drinking-water is <0.2 ng/kg body weight per day, based on a 70-kg individual drinking 2 litres/day with a level of 6 ng/litre, the maximum concentration measured in a Canadian finished drinking-water study conducted from 1985 to 1988. The current estimate of PCB intake from air is 0.3–3 ng/kg body weight per day, based on the typical range of urban levels (1–10 ng/m3) and a breathing volume of 23 m3/day. The mean intake from food is estimated to range from 0.5 to 5 ng/kg body weight per day, based on total diet studies in adults in 1982–1984 and 1991–1997. Fish, poultry, and meat are the primary contributors. The total from all sources is in the range of 1–8 ng/kg body weight per day (0.2 ng/kg body weight per day in water + 0.3–3 ng/kg body weight per day in air + 0.5–5 ng/kg body weight per day in food), which is approximately 1000 times lower than the minimum level that caused adverse effects in animals (5000 ng/kg body weight per day) and 4 times lower than the tolerable intake (20 ng/kg body weight per day).
Immunotoxicological effects were limited to T-cell-derived parameters in rhesus monkeys, and their health significance to humans is not clear-cut. Thus, the LOAEL based on immunotoxicological findings may represent an overly conservative estimate. In addition, rhesus monkeys have exhibited other frank toxicities from PCBs at body burdens equivalent to or significantly below those found in the general or working populations with no clinical effects. Thus, rhesus monkeys may be a species unusually sensitive to PCBs. However, very similar LOAELs were observed in studies on neurodevelopmental effects and on reproductive toxicity in other monkeys and in rats.
The health risk assessment is based on studies using a limited set of PCB mixtures, mostly Aroclors 1242 and 1254. The health risks from exposure to PCB mixtures that have lower chlorine contents are likely to be smaller. However, PCB mixtures with a higher content of more toxic congeners, notably non- and mono-ortho congeners, may pose a larger risk.
For the purpose of this CICAD, the health effects end-points and risk assessments associated with PCB exposures have been based upon the approach for intakes of mixtures rather than either of the alternative approaches, i.e., the individual congeners that might have a common mechanism of action, or body burdens in humans. This approach is practical for occupational, accidental, and environmental exposures, because people are exposed to mixtures of PCB congeners having different modes of action, and the approach does not require very sophisticated analytical methodology. When the pattern of PCB isomers is different from the commercial mixtures, another approach may be preferable, if feasible.
Certain scenarios, such as eating large amounts of PCB-contaminated fish, breathing indoor air with high PCB concentrations, or breathing outdoor air containing elevated PCB levels downwind of contaminated sites, may result in a total PCB exposure that is significantly higher than and qualitatively different from that of the general population for which the sample risk characterization is made. A site-specific risk assessment should take such potential exposures into consideration.
Based on limited evidence of carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals, the International Agency for Research on Cancer (IARC, 1987) has concluded that PCBs are probably carcinogenic to humans (Category 2A).
IPCS has previously assessed the toxicity and health effects of PCBs (IPCS, 1976, 1993).
Afghan BK, Chau ASY (1989) Analysis of trace organics in the aquatic environment. Boca Raton, FL, CRC Press, pp. 33–68.
Ahlborg UG, Becking GC, Birnbaum LS, Brouwer A, Derks HJGM, Feeley M, Golor G, Hanberg A, Larsen JC, Liem AKD, Safe SH, Schlatter C, Waern F, Younes M, Yrjänheikki E (1994) Toxic equivalency factors for dioxin-like PCBs. Chemosphere, 28:1049–1067.
Allen JR, Abrahamson LJ (1973) Morphological and biochemical changes in the liver of rats fed polychlorinated biphenyls. Archives of Environmental Contamination and Toxicology, 1:265–280.
Allen JR, Barsotti DA (1976) The effects of transplacental and mammary movement of PCBs on infant rhesus monkeys. Toxicology, 6:331–340.
Althaus FR, Lawrence SD, Sattler GL, Longfellow DG, Pitot HC (1982) Chemical quantification of unscheduled DNA synthesis in cultured hepatocytes as an assay for the rapid screening of potential chemical carcinogens. Cancer Research, 42:3010–3015.
Anderson DJ, Bloem TB, Blankenbaker PK, Stanko TA (1999) Concentrations of polychlorinated biphenyls in the water column of the Laurentian Great Lakes: Spring 1993. Journal of Great Lakes Research, 25(1):160–170.
Anderson LM, Logsdon D, Ruskie S, Fox SD, Issaq HJ, Kovatch RM, Riggs CM (1994) Promotion by polychlorinated biphenyls of lung and liver tumors in mice. Carcinogenesis, 15:2245–2248.
Andersson O, Linder CE, Olsson M, Reutergardh L, Uvemo UB, Wideqvist U (1988) Spatial differences and temporal trends of organochlorine compounds in biota from the northwestern hemisphere. Archives of Environmental Contamination and Toxicology, 17:755–765.
Ankley GT, Niemi GJ, Lodge KB, Harris HJ, Beaver DL, Tillitt DE, Schwartz TR, Giesy JP, Jones PD, Hagley C (1993) Uptake of planar polychlorinated biphenyls and 2,3,7,8-substituted polychlorinated dibenzofurans and dibenzo-p-dioxins by birds nesting in the lower Fox River and Green Bay, Wisconsin. Archives of Environmental Contamination and Toxicology, 24:332–344.
Arnold DL, Bryce F, McGuire PF (1995) Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys. Part 2. Reproduction and infant findings. Food and Chemical Toxicology, 33:457–474.
Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, Fish EB, Hiraki GY, Holloway C, Ross T, Hanna WM, SenGupta SK, Weber J-P (2000) Breast adipose tissue concentrations of polychlorinated biphenyls on other organochlorines and breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 9:65–71.
Atkinson R (1987) Estimation of OH radical reaction rate constants and atmospheric lifetimes for polychlorobiphenyls, dibenzo-p-dioxins, and dibenzofurans. Environmental Science & Technology, 21:305–307.
Atlas E, Giam CS (1987) Ambient concentration and precipitation scavenging of atmospheric organic pollutants. Water, Air and Soil Pollution, 38:19–36.
ATSDR (1994) Toxicological profile for chlorodibenzofurans. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
ATSDR (1995) Exposure to PCBs from hazardous waste among Mohawk women and infants at Akwesasne. Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry.
ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs). Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, 765 pp. + app.
Aulerich RJ, Ringer PK (1977) Current status of PCB toxicity to mink and effects on their reproduction. Archives of Environmental Contamination and Toxicology, 6:279–292.
Axmon A, Rylander L, Strömberg U, Hagmar L (2000a) Miscarriages and stillbirths in women with a high intake of fish contaminated with persistent organochlorine compounds. International Archives of Occupational and Environmental Health, 73:204–208.
Axmon A, Rylander L, Strömberg U, Hagmar L (2000b) Time to pregnancy and infertility among women with a high intake of fish contaminated with persistent organochlorine compounds. Scandinavian Journal of Work, Environment & Health, 26(3):199–206.
Baker EL Jr, Landrigan PJ, Glueck CJ, Zack MM Jr, Liddle JA, Burse VW, Housworth WJ, Needham LL (1980) Metabolic consequences of exposure to polychlorinated biphenyls (PCB) in sewage sludge. American Journal of Epidemiology, 112:553–563.
Baker JE, Eisenreich SJ (1990) Concentrations and fluxes of polycyclic aromatic hydrocarbons and polychlorinated biphenyls across the air–water interface. Environmental Science & Technology, 24:342–352.
Balfanz E, Fuchs J, Kieper H (1993) Sampling and analysis of polychlorinated biphenyls (PCBs) in indoor air due to permanently elastic sealants. Chemosphere, 26(5):871–880.
Ballschmiter K, Zell M (1980) Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography: Composition of technical Aroclor and Clophen–PCB mixtures. Fresenius’ Zeitschrift für Analytische Chemie, 302:20–31.
Beebe LE, Kim YE, Amin S, Riggs CW, Kovatch RM, Anderson LM (1993) Comparison of transplacental and neonatal initiation of mouse lung and liver tumors by N-nitrosodimethylamine (NDMA) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and promotability by a polychlorinated biphenyls mixture (Aroclor 1254). Carcinogenesis, 14:1545–1548.
Belpaeme K, Delbeke K, Zhu L, Kirsch-Volders M (1996) PCBs do not induce DNA breakage in vitro in human lymphocytes. Mutagenesis, 11:383–389.
Berry DL, DiGiovanni J, Juchau MR, Bracken WM, Gleason GL, Slaga TJ (1978) Lack of tumor-promoting ability of certain environmental chemicals in a two-stage mouse skin tumorigenesis assay. Research Communications in Chemical Pathology and Pharmacology, 20:101–108.
Berry DL, Slaga TJ, DiGiovanni J, Juchau RM (1979) Studies with chlorinated dibenzo-p-dioxins, polybrominated biphenyls, and polychlorinated biphenyls in a two-stage system of mouse skin tumorigenesis: Potent anticarcinogenic effects. Annals of the New York Academy of Sciences, 320:405–414.
Bertazzi PA, Ribaldi L, Pesatori A, Radice L, Zocchetti C (1987) Cancer mortality of capacitor manufacturing workers. American Journal of Industrial Medicine, 11:165–176.
Boon JP, Duinker JC (1986) Monitoring of cyclic organochlorines in the marine environment. Environmental Monitoring and Assessment, 7:189–208.
Boon JP, Oudejans RCHM, Duinkeys JC (1984) Kinetics of individual polychlorinated biphenyl (PCB) components in juvenile sole (Solea solea) in relation to their concentrations in food and to lipid metabolism. Comparative Biochemistry and Physiology C: Comparative Pharmacology and Toxicology, 79:131–142.
Bremle G, Okla L, Larsson P (1995) Uptake of PCBs in fish in contaminated river system: Bioconcentration factors measured in the field. Environmental Science & Technology, 29:2010–2015.
Brouwer A (1991) Role of biotransformation in PCB-induced alterations in vitamin A and thyroid hormone metabolism in laboratory and wildlife species. Biochemical Society Transactions, 19:731–737.
Brouwer A, Morse DC, Schuur AG, Murk AJ, Klasson-Wehler E, Bergman A, Visser T (1998) Interaction of persistent environmental organohalogens with the thyroid hormone system: Mechanism and possible consequences for animal and human health. Toxicology and Industrial Health, 14:59–84.
Brown DP (1987) Mortality of workers exposed to polychlorinated biphenyls — an update. Archives of Environmental Health, 42:333–339.
Brown DP, Jones M (1981) Mortality and industrial hygiene study of workers exposed to polychlorinated biphenyls. Archives of Environmental Health, 36:120–129.
Brown NM, Lamartiniere CA (1995) Xenoestrogens alter mammary gland differentiation and cell proliferation in the rat. Environmental Health Perspectives, 103:708–713.
Bruce RW, Heddle JA (1979) The mutagenic activity of 61 agents as determined by the micronucleus, Salmonella, and sperm abnormality assays. Canadian Journal of Genetics and Cytology, 21:319–334.
Bruckner JV, Khanna KL, Cornish HH (1973) Biological responses of the rat to polychlorinated biphenyls. Toxicology and Applied Pharmacology, 24:434–448.
Brunner MJ, Sullivan TM, Singer AW, Ryan MJ, Toft JD II, Menton RS, Graves SW, Peters AC (1996) Assessment of the chronic toxicity and oncogenicity of Aroclor-1016, Aroclor-1242, Aroclor-1254, and Aroclor-1260 administered in diet to rats. Columbus, OH, Battelle (Battelle Study No. Sc920192).
Bryant CJ, Hartle RW, Crandall MS (1989) Polychlorinated biphenyl, polychlorinated dibenzo-p-dioxin, and polychlorinated dibenzofuran contamination in PCB disposal facilities. Chemosphere, 18:569–576.
Buchmann A, Ziegler S, Wolf A, Robertson LW, Durham SK, Schwartz M (1991) Effects of polychlorinated biphenyls in rat liver: Correlation between primary subcellular effects and promoting activity. Toxicology and Applied Pharmacology, 111:454–468.
Buck GM, Vena JE, Schisterman EF, Dmochowski J, Mendola P, Sever LE, Fitzgerald E, Kostyniak P, Greizerstein H, Olson J (2000) Parental consumption of contaminated sport fish from Lake Ontario and predicted fecundability. Epidemiology, 11:388–393.
Burse VW, Korver MP, Needham LL, Lapeza CR, Boozer EL, Head SL, Liddle JA, Bayse DD (1989) Gas chromatographic determination of polychlorinated biphenyls (as Aroclor 1254) in serum: Collaborative study. Journal of the Association of Official Analytical Chemists, 72:649–659.
Bush B, Bennett AH, Snow JT (1986) Polychlorobiphenyl congeners, p,p'-DDE, and sperm function in humans. Archives of Environmental Contamination and Toxicology, 15:333–341.
Callahan MA, Slimak MW, Gabel NW, May IP, Fowler CF, Freed JR, Jennings P, Durfee RL, Whitmore FC, Maestri B, Mabey WR, Holt BR, Gould C (1979) Water-related environmental fate of 129 priority pollutants. Vol. I. Washington, DC, US Environmental Protection Agency (EPA 440/4-79-029a).
Chen RC, Tang SY, Miyata H, Kashimoto T, Chang YC, Chang KJ, Tung TC (1985) Polychlorinated biphenyl poisoning: correlation of sensory and motor nerve conduction, neurologic symptoms, and blood levels of polychlorinated biphenyls, quaterphenyls, and dibenzofurans. Environmental Research, 37:340–348.
Chen YC, Guo YL, Hsu CC, Rogan WJ (1992) Cognitive development of Yu-Cheng ("oil disease") children prenatally exposed to heat-degraded PCBs. Journal of the American Medical Association, 268:3213–3218.
Chen YC, Yu ML, Rogan WJ, Gladen BC, Hsu CC (1994) A 6-year follow-up of behavior and activity disorders in the Taiwan Yu-Cheng children. American Journal of Public Health, 84:415–421.
Chia LG, Chu FL (1984) Neurological studies on polychlorinated biphenyl (PCB)-poisoned patients. American Journal of Industrial Medicine, 5:117–126.
Chia LG, Chu FL (1985) A clinical and electrophysiological study of patients with polychlorinated biphenyl poisoning. Journal of Neurology, Neurosurgery, and Psychiatry, 48:894–901.
Cogliano VJ (1998) Assessing the cancer risk from environmental PCBs. Environmental Health Perspectives, 106:317–323.
Collins WT, Capen CC (1980) Fine structural lesions and hormonal alterations in thyroid glands of perinatal rats exposed in utero and by milk to polychlorinated biphenyls. American Journal of Pathology, 99:125–142.
Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P (1997) Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure–activity relationships. Toxicology and Applied Pharmacology, 145(1):111–123.
Cooke PS, Zhao Y-D, Hansen LG (1996) Neonatal polychlorinated treatment increases adult testis size and sperm production in the rat. Toxicology and Applied Pharmacology, 136:112–117.
Corrigan FM, Murray L, Wyatt CL, Shore RF (1998) Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson’s disease. Experimental Neurology, 150(2):339–342.
Cross JN, Hardy JT, Hose JE, Hershelman GP, Antrim LD, Gossett RW, Creelius EA (1987) Contaminant concentrations and toxicity of sea-surface microlayer near Los Angeles, California. Marine Environmental Research, 23:307–324.
Davis D, Safe S (1989) Dose–response immunotoxicities of commercial polychlorinated biphenyls (PCBs) and their interaction with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology Letters, 48:35–43.
De Haan LH, Simons JW, Bos AT, Aarts JM, Denison MS, Brouwer A (1994) Inhibition of intercellular communication by 2,3,7,8-tetrachlorodibenzo-p-dioxin and dioxin-like PCBs in mouse hepatoma cells (Hepa1c1c7): involvement of the Ah receptor. Toxicology and Applied Pharmacology, 129:283–293.
DeKoning EP, Karmaus W (2000) PCB exposure in utero and via breast milk: A review. Journal of Exposure Analysis and Environmental Epidemiology, 10:285–293.
DiGiovanni J, Viaje A, Berry DL, Slaga TJ, Juchau MR (1977) Tumor-initiating ability of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and Aroclor 1254 in the two-stage system of mouse skin carcinogenesis. Bulletin of Environmental Contamination and Toxicology, 18:552–557.
Djordjevic MV, Hoffmann D, Fan J, Prokopczyk B, Citron ML, Stellman SD (1994) Assessment of chlorinated pesticides and polychlorinated biphenyls in adipose breast tissue using a supercritical fluid extraction method. Carcinogenesis, 15:2581–2585.
Dorgan JF, Brock J, Rothman N, Needham LL, Miller R, Stephenson HEJ, Schussler N, Taylor PR (1999) Serum organochlorine pesticides and PCBs and breast cancer risk: results from a prospective analysis (USA). Cancer Causes & Control, 10:1.
Dowling DN, Pipke R, Dwyer DF (1993) A DNA module encoding bph genes for the degradation of polychlorinated biphenyls (PCBs). FEMS Microbiology Letters, 113(2):149–154.
Dubois M, Pfohl-Leszkowicz A, Grosse Y, Kremers P (1995) DNA adducts and P450 induction in human, rat and avian liver cells after exposure to polychlorobiphenyls. Mutation Research, 345:181–190.
Eisenreich SJ, Looney BB, Thornton JD (1981) Airborne organic contaminants in the Great Lakes ecosystem. Environmental Science & Technology, 15:30–38.
Eisenreich SJ, Baker JE, Franz T, Swanson M, Rapaport RA, Strachan WMJ, Hites RA (1992) Atmospheric deposition of hydrophobic organic contaminants to the Laurentian Great Lakes. In: Schnoor JL, ed. Fate of pesticides and chemicals in the environment. New York, NY, John Wiley & Sons, pp. 51–78.
Emmett EA, Maroni M, Schmith JM, Levin BK, Jefferys J (1988a) Studies of transformer repair workers exposed to PCBs: I. Study design, PCB concentrations, questionnaire, and clinical examination results. American Journal of Industrial Medicine, 13:415–427.
Emmett EA, Maroni M, Schmith JM, Levin BK, Jefferys J (1988b) Studies of transformer repair workers exposed to PCBs: II. Results of clinical laboratory investigations. American Journal of Industrial Medicine, 14:47–62.
Eriksson P, Fredriksson A (1998) Neurotoxic effects in adult mice neonatally exposed to 3,3',4,4',5'-pentachlorobiphenyl or 2,3,3',4,4'-pentachlorobiphenyl. Changes in brain nicotinic receptors and behavior. Environmental Toxicology and Pharmacology, 5(1):17–27.
Faroon O, Jones D, De Rosa C (2000) Effects of polychlorinated biphenyls on the nervous system. Toxicology and Industrial Health, 16:305–333.
Faroon O, Keith S, Jones D, De Rosa C (2001a) Carcinogenic effects of polychlorinated biphenyls. Toxicology and Industrial Health, 17(2):41–62.
Faroon O, Keith S, Jones D, De Rosa C (2001b) Effects of polychlorinated biphenyls on reproduction and development. Toxicology and Industrial Health, 17(3):63–93.
Fava F, Di Gioia D, Marchetti L, Quattroni G, Marraffa V (1993) Aerobic mineralization of chlorobenzoates by a natural polychlorinated biphenyl-degrading mixed bacterial culture. Applied Microbiology and Biotechnology, 40(4):541–548.
Fein GG, Jacobson JL, Jacobson SW, Schwartz PM, Dowler JK (1984) Prenatal exposure to polychlorinated biphenyls: Effects on birth size and gestation age. Journal of Pediatrics, 105:315–320.
Fielden MR, Chen I, Chittim B, Safe SH, Zacharewski TR (1997) Examination of the estrogenicity of 2,4,6,2',6'-pentachlorobiphenyl (PCB 104), its hydroxylated metabolite 2,4,6,2',6'-pentachloro-4-biphenylol (HO-PCB 104), and a further chlorinated derivative, 2,4,6,2',4',6'-hexachlorobiphenyl (PCB 155). Environmental Health Perspectives, 105(11):1238–1248.
Fischbein A, Wolff MS, Lilis R, Thornton J, Selikoff IJ (1979) Clinical findings among PCB-exposed capacitor manufacturing workers. Annals of the New York Academy of Sciences, 320:703–715.
Fischbein A, Thornton J, Wolff MS, Bernstein J, Selifoff IJ (1982) Dermatological findings in capacitor manufacturing workers exposed to dielectric fluids containing polychlorinated biphenyls (PCBs). Archives of Environmental Health, 37:69–74.
Fischbein A, Rizzo JN, Solomon SJ, Wolff MS (1985) Oculodermatological findings in workers with occupational exposure to polychlorinated biphenyls (PCBs). British Journal of Industrial Medicine, 42:426–430.
Fishbein L (1974) Toxicity of chlorinated biphenyls. Annual Review of Pharmacology, 14:139–156.
Frame GM, Cochran JW, Bowadt SS (1996) Complete PCB congener distributions for 17 Aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis. Journal of High Resolution Chromatography, 19(12):657–668.
Fu YA (1984) Ocular manifestation of polychlorinated biphenyls intoxication. American Journal of Industrial Medicine, 5:127–132.
Funatsu I, Yamashita F, Yoshikane T, Funatsu T, Ito Y, Tsugawa S, Hayashi M, Kato T, Yakushiji M, Okamoto G, Arima A, Adachi N, Takahashi K, Miyahara M, Tashiro Y, Shimomura M, Yamasaki S, Arima T, Kuno T, Ide H, Ide I (1971) A chlorobiphenyl induced fetopathy. Fukuoka Igaku Zasshi, 62(1):139–149.
Garthoff LH, Cerra FE, Marks EM (1981) Blood chemistry alteration in rats after single and multiple gavage administration of polychlorinated biphenyls. Toxicology and Applied Pharmacology, 60:33–44.
Geyer H, Scheunert I, Korte F (1986) Bioconcentration potential of organic environmental chemicals in humans. Regulatory Toxicology and Pharmacology, 6:313–347.
Giam CS, Chan HS, Neff GS, Atlas EL (1978) Phthalate ester plasticizers: A new class of marine pollutant. Science, 199:419–421.
Gibson DT, Cruden DL, Haddock JD, Zylstra GJ, Brand JM (1993) Oxidation of polychlorinated biphenyls by Pseudomonas sp. strain LB400 and Pseudomonas pseudoalcaligenes KF707. Journal of Bacteriology, 175:4561–4564.
Gladen BC, Rogan WJ (1991) Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethane on later development. Journal of Pediatrics, 119:58–63.
Gladen BC, Rogan WJ, Hardy P, Thullen J, Tingelstad J, Tully M (1988) Development after exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene transplacentally and through human milk. Journal of Pediatrics, 113(6):991–995.
Gladen BC, Taylor JS, Wu YC, Ragan NB, Rogan WJ, Hsu CC (1990) Dermatological findings in children exposed transplacentally to heat-degraded polychlorinated biphenyls in Taiwan. British Journal of Dermatology, 122:799–808.
Grant DL, Phillips WEJ (1974) The effect of age and sex on the toxicity of Aroclor 1254, a polychlorinated biphenyl, in the rat. Bulletin of Environmental Contamination and Toxicology, 12:145–152.
Green S, Carr JV, Palmer KA, Oswald EJ (1975) Lack of cytogenetic effects in bone marrow and spermatogonial cells in rats treated with polychlorinated biphenyls (Aroclors 1242 and 1254). Bulletin of Environmental Contamination and Toxicology, 13:14–22.
Greenland S, Salvan A, Wegman DH, Hallock MF, Smith TJ (1994) A case–control study of cancer mortality at a transformer-assembly facility. International Archives of Occupational and Environmental Health, 66:49–54.
Gunderson EL (1995) FDA total diet study, July 1986 – April 1991: Dietary intakes of pesticides, selected elements, and other chemicals. Journal of the Association of Official Analytical Chemists, 78:1353–1363.
Guo YL, Lai TJ, Chen SJ, Hsu CC (1995) Gender-related decrease in Raven’s progressive matrices scores in children prenatally exposed to polychlorinated biphenyls and related contaminants. Bulletin of Environmental Contamination and Toxicology, 55:8–13.
Guo YL, Yu ML, Hsu CC, Rogan WJ (1999) Chloracne, goiter, arthritis, and anemia after polychlorinated biphenyl poisoning: 14-year follow-up of the Taiwan Yucheng cohort. Environmental Health Perspectives, 107:715–719.
Gustavsson P, Hogstedt C (1997) A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). American Journal of Industrial Medicine, 32:234–239.
Haake JM, Safe S, Mayura K, Phillips TD (1987) Aroclor 1254 as an antagonist of the teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology Letters, 38:299–306.
Hanrahan LP, Falk C, Anderson HA, Draheim L, Kanarek MS, Olson J (1999) Serum PCB and DDE levels of frequent Great Lakes sport fish consumers — first look. The Great Lakes Consortium. Environmental Research, 80(2 Pt 2):S26–S37.
Hansen LG (1987) Environmental toxicology of polychlorinated biphenyls. In: Safe S, ed. Polychlorinated biphenyls (PCBs): Mammalian and environmental toxicology. Berlin, Springer-Verlag, pp. 15–48.
Hany J, Lilienthal H, Roth-Harer A, Ostendorp G, Heinzow B, Winneke G (1999) Behavioral effects following single and combined maternal exposure to PCB 77 (3,4,3',4'-tetrachlorobiphenyl) and PCB 47 (2,4,2',4'-tetrachlorobiphenyl) in rats. Neurotoxicology and Teratology, 21(2):147–156.
Harner T, Kylin H, Bidleman TF, Halsall C, Strachan WMJ (1998) Polychlorinated naphthalenes and coplanar polychlorinated biphenyls in Arctic air. Environmental Science & Technology, 32:3257–3265.
Hart KM, Tremp J, Molinar E, Eawag WG (1993) The occurrence and the fate of organic pollutants in the atmosphere. Water, Air and Soil Pollution, 68(1–2):91–112.
Hattula ML (1985) Mutagenicity of PCBs and their pyrosynthetic derivatives in cell-mediated assay. Environmental Health Perspectives, 60:255–257.
Hayes MA (1987) Carcinogenic and mutagenic effects of PCBs. In: Safe S, ed. Polychlorinated biphenyls (PCBs): Mammalian and environmental toxicology. Berlin, Springer-Verlag, pp. 77–95.
Hebert CE, Weseloh DV, Kot L, Glooschenko V (1994) Organochlorine contaminants in a terrestrial foodweb on the Niagara Peninsula, Ontario, Canada 1987–89. Archives of Environmental Contamination and Toxicology, 26:356–366.
Helzlsouer KJ, Alberg AJ, Brock JW, Burse VW, Needham LL, Bell DA, Lavigne JA, Yager JD, Comstock GW (1999) Serum concentrations of organochlorine compounds and the subsequent development of breast cancer. Cancer Epidemiology, Biomarkers & Prevention, 8:525–532.
Hermanson MH, Hites RA (1989) Long-term measurements of atmospheric polychlorinated biphenyls in the vicinity of Superfund dumps. Environmental Science & Technology, 23:1253–1258.
Higson FK (1992) Microbial degradation of biphenyl and its derivatives. Advances in Applied Microbiology, 37:135–164.
Hoff RM, Muir DCG, Grist NP (1992) Annual cycle of polychlorinated biphenyls and organohalogen pesticides in air in Southern Ontario. 1: Air contamination data. Environmental Science & Technology, 26:266–275.
Hoopingarner R, Samuel A, Krause D (1972) Polychlorinated biphenyl interactions with tissue culture cells. Environmental Health Perspectives, 1:155–158.
Hoyer AP, Grandjean P, Jorgensen T, Brock JW, Hartvig HB (1998) Organochlorine exposure and risk of breast cancer. The Lancet, 352:1816–1820.
Hsieh SF, Yen YY, Lan SJ, Hsieh CC, Lee CH, Ko YC (1996) A cohort study on mortality and exposure to polychlorinated biphenyls. Archives of Environmental Health, 51:417–424.
Hsu ST, Ma CI, Hsu SK, Wu SS, Hsu NH, Yeh CC, Wu SB (1985) Discovery and epidemiology of PCB poisoning in Taiwan: a four-year followup. Environmental Health Perspectives, 59:5–10.
Huestis SY, Servos MR, Whittle DM, Dixon DG (1996) Temporal and age-related trends in levels of polychlorinated biphenyl congeners and organochlorine contaminants in Lake Ontario lake trout (Salvelinus namaycush). Journal of Great Lakes Research, 22(2):310–330.
Huisman M, Koopman-Esseboom C, Lanting CI, van der Paauw CG, Tuinstra LG, Fidler V, Weisglas-Kuperus N, Sauer PJ, Boersma ER, Touwen BC (1995) Neurological condition in 18-month-old children perinatally exposed to polychlorinated biphenyls and dioxins. Early Human Development, 43:165–176.
Humphrey HEB, Budd ML (1996) Michigan’s fisheater cohorts: A prospective history of exposure. Toxicology and Industrial Health, 12(3–4):499–505.
Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE, Willett WC, Speizer FE, Wolff MS (1997) Plasma organochlorine levels and the risk of breast cancer. New England Journal of Medicine, 337:1253–1258.
Hutzinger O, Safe S, Zitko V (1972) Photochemical degradation of chlorobiphenyls (PCBs). Environmental Health Perspectives, 1:15–20.
Iannuzzi TJ, Huntley SL, Bonnevie NL, Finely BL, Wenning RJ (1995) Distribution and possible sources of polychlorinated biphenyls in dated sediments from the Newark Bay Estuary, New Jersey. Archives of Environmental Contamination and Toxicology, 28:108–117.
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls. Lyon, World Health Organization, International Agency for Research on Cancer (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume 18).
IARC (1987) Update of IARC monographs volumes 1–42. Lyon, World Health Organization, International Agency for Research on Cancer (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Supplement 7).
IPCS (1976) Polychlorinated biphenyls and terphenyls. Geneva, World Health Organization, International Programme on Chemical Safety, 85 pp. (Environmental Health Criteria 2).
IPCS (1993) Polychlorinated biphenyls and terphenyls, 2nd ed. Geneva, World Health Organization, International Programme on Chemical Safety, 682 pp. (Environmental Health Criteria 140).
IPCS (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 170).
IPCS (2000) International Chemical Safety Card — Polychlorinated biphenyl (Aroclor 1254). Geneva, World Health Organization, International Programme on Chemical Safety (ICSC 0939).
Jacobson JL, Jacobson SW (1996) Intellectual impairment in children exposed to polychlorinated biphenyls in utero. New England Journal of Medicine, 335:783–789.
Jacobson JL, Jacobson SW, Schwartz PM, Fein GG, Dowler JK (1984) Prenatal exposure to an environmental toxin: A test of the multiple effects model. Developmental Psychobiology, 20:523–532.
Jacobson JL, Jacobson SW, Humphrey HEB (1990a) Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicology and Teratology, 12:319–326.
Jacobson JL, Jacobson SW, Humphrey HEB (1990b) Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. Journal of Pediatrics, 116:38–45.
Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK (1985) The effect of intrauterine PCB exposure on visual recognition memory. Child Development, 56:853–860.
Jansen HT, Cooke PS, Porcelli J, Liu TC, Hansen LG (1993) Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies. Reproductive Toxicology, 7(3):237–248.
Jensen AA (1987) Polychlorobiphenyls (PCBs), polychlorodibenzo-p-dioxins (PCDDs) and polychlorodibenzofurans (PCDFs) in human milk, blood and adipose tissue. The Science of the Total Environment, 64:259–293.
Johansson M, Larsson C, Bergman A, Lund BO (1998a) Structure–activity relationship for inhibition of CYP11B1-dependent glucocorticoid synthesis in Y1 cells by aryl methyl sulfones. Pharmacology and Toxicology, 83(5):225–230.
Johansson M, Nilsson S, Lund BO (1998b) Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environmental Health Perspectives, 106(12):769–772.
Kalina I, Sram RJ, Konecna H, Ondrussekova A (1991) Cytogenetic analysis of peripheral blood lymphocytes in workers occupationally exposed to polychlorinated biphenyls. Teratogenesis, Carcinogenesis, and Mutagenesis, 11:77–82.
Kaminsky LS, Kennedy MW, Adams SM, Guengerich FP (1981) Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P450. Biochemistry, 20:7379–7384.
Kang Y, Taniuchi T, Masunaga S, Nakanishi J (2000) Concentration profiles of PCDD/DFs and dioxin-like PCBs in fish from Tokyo Bay. In: Proceedings of the 3rd international workshop on risk evaluation and management of chemicals. Yokohama, Yokohama National University, pp. 70–77.
Kato N, Yoshida A (1980) Effect of dietary PCB on hepatic cholesterogenesis in rats. Nutrition Reports International, 21:107–112.
Kholkute SD, Rodriguez J, Dukelow WR (1994a) Effects of polychlorinated biphenyls (PCBs) on in vitro fertilization in the mouse. Reproductive Toxicology, 8:69–73.
Kholkute SD, Rodriguez J, Dukelow WR (1994b) Reproductive toxicity of Aroclor 1254: effects on oocyte, spermatozoa, in vitro fertilization, and embryo development in the mouse. Reproductive Toxicology, 8:487–493.
Kimbrough RD (1987) Human health effect of polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs). Annual Review of Pharmacology and Toxicology, 27:87–111.
Kimbrough RD, Linder RE, Gaines TB (1972) Morphological changes in livers of rats fed polychlorinated biphenyls. Archives of Environmental Health, 25:354–364.
Kimbrough RD, Squire RA, Linder RE, Strandberg JD, Montali RJ, Burse VW (1975) Induction of liver tumors in Sherman strain female rats by polychlorinated biphenyl Aroclor 1260. Journal of the National Cancer Institute, 55:1453–1459.
Kimbrough RD, Doemland ML, LeVois ME (1999) Mortality in male and female capacitor workers exposed to polychlorinated biphenyls. Journal of Occupational and Environmental Medicine, 41:161–171.
Knap AH, Binkley KS (1991) Chlorinated organic compounds in the troposphere over the western North Atlantic Ocean measured by aircraft. Atmospheric Environment, 25A:1507–1516.
Konishi Y, Kuwabara K, Hori S (2001) Continuous surveillance of organochlorine compounds in human breast milk samples from 1972 to 1998 in Osaka, Japan. Archives of Environmental Contamination and Toxicology, 40:571–578.
Koopman-Esseboom C, Huisman M, Weisglas-Kuperus N, Van der Paauw CG, Tuinstra LG, Boersma ER, Sauer PJJ (1994) PCB and dioxin levels in plasma and human milk of 418 Dutch women and their infants. Predictive value of PCB congener levels in maternal plasma for fetal and infant’s exposure to PCBs and dioxins. Chemosphere, 28(9):1721–1732.
Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, Van der Paauw CG, Tuinstra LG, Sauer PJ (1996) Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants’ mental and psychomotor development. Pediatrics, 97:700–706.
Korach KS, Sarver P, Chae K, McLachlan JA, McKinney JD (1988) Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls: conformationally restricted structural probes. Molecular Pharmacology, 33:120–126.
Koslowski SE, Metcalfe CD, Lazar R, Haffner GD (1994) The distribution of 42 PCBs, including three coplanar congeners, in the food web of the western basin of Lake Erie. Journal of Great Lakes Research, 20:260–270.
Kraul I, Karlog O (1976) Persistent organochlorinated compounds in human organs collected in Denmark: 1972–73. Acta Pharmacologica Toxicologica, 38:38–48.
Kreiss K (1985) Studies on populations exposed to polychlorinated biphenyls. Environmental Health Perspectives, 60:193–199.
Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N (1994) Breast cancer and serum organochlorines: a prospective study among white, black, and Asian women. Journal of the National Cancer Institute, 86(8):589–599.
Krishnan V, Safe S (1993) Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), and dibenzofurans (PCDFs) as antiestrogens in MCF-7 human breast cancer cells: quantitative structure–activity relationships. Toxicology and Applied Pharmacology, 120(1):55–61.
Krutovskikh VA, Mesnil M, Mazzoleni G, Yamasaki H (1995) Inhibition of rat liver gap junction intercellular communication by tumor-promoting agents in vivo. Association with aberrant localization of connexin proteins. Laboratory Investigation, 72:571–577.
Kubatova A, Matucha M, Erbanova P, Novotny C, Vlasakova V, Sasek V (1998) Investigation into PCB biodegradation using uniformly 14C-labelled dichlorobiphenyl. Isotopes in Environmental and Health Studies, 34(4):325–334.
Kuehl DW, Haebler R (1995) Organochlorine, organobromine, metal and selenium residues in bottlenose dolphins (Tursiops truncatus) collected during an unusual mortality event in the Gulf of Mexico, 1990. Archives of Environmental Contamination and Toxicology, 28:494–499.
Kuehl DW, Haebler R, Potter C (1994) Coplanar PCB and metal residues in dolphins from the US Atlantic Coast including Atlantic bottlenose obtained during the 1987/88 mass mortality. Chemosphere, 28(6):1245–1253.
Kuratsune M (1989) Yusho, with reference to Yu-Cheng. In: Kimbrough RD, Jensen AA, eds. Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products, 2nd ed. Amsterdam, Elsevier Science Publishers, pp. 381–400.
Kuratsune M, Nakamura Y, Ikeda M, Hirohata T (1987) Analysis of deaths seen among patients with Yusho — A preliminary report. Chemosphere, 16:2085–2088.
Kuroiwa Y, Murai Y, Santa T (1969) Neurological and nerve conduction velocity studies on 23 patients with chlorobiphenyl poisoning. Fukuoka Igaku Zasshi, 60:462–463.
Kutz FW, Wood PH, Bottimore DP (1991) Residues in human adipose tissue. Reviews of Environmental Contamination and Toxicology, 120:61–81.
Lai TJ, Guo YL, Yu ML, Ko HC, Hsu CC (1994) Cognitive development in Yucheng children. Chemosphere, 299(11):2405–2411.
Laib RJ, Rose J, Brunn H (1991) Hepatocarcinogenicity of PCB congeners. I. Initiation and promotion of enzyme-altered rat liver foci by 2,2',4,5'-tetra- and 2,2',4,4',5,5'-hexachlorobiphenyl. Toxicological and Environmental Chemistry, 34:19–22.
Lake CA, Lake JL, Haebler R, McKinney R, Boothman WS, Sadove SS (1995) Contaminant levels in harbor seals from the northeastern United States. Archives of Environmental Contamination and Toxicology, 29:128–134.
Lan SJ, Yen YY, Yang CH, Yang CY, Chen ER (1987) [A study on the birth weight of transplacental Yu-Cheng babies.] Gaoxiong Yi Xue Ke Xue Za Zhi, 3(4):273–282.
Larsson P, Soedergren A (1987) Transport of polychlorinated biphenyls (PCBs) in freshwater mesocosms from sediment to water and air. Water, Air and Soil Pollution, 36:33–46.
LeBlanc GA (1995) Trophic-level differences in the bioconcentration of chemicals: Implications in assessing environmental biomagnification. Environmental Science & Technology, 29:154–160.
Lee MC, Griffin RA, Miller ML, Chian ESK (1979) Adsorption of water-soluble polychlorinated biphenyl Aroclor 1242 and used capacitor fluid by soil materials and coal chars. Journal of Environmental Science and Health Part A — Environmental Science and Engineering & Toxic and Hazardous Substance Control, 14:415–442.
Leifer A, Brink RH, Thom GC, Partymiller KG (1983) Environmental transport and transformation of polychlorinated biphenyls. Washington, DC, US Environmental Protection Agency, Office of Pesticides and Toxic Substances (NTIS PB84-142579; EPA-560/5-83-025).
Leister DL, Baker JE (1994) Atmospheric deposition of organic contaminants to the Chesapeake Bay. Atmospheric Environment, 28(8):1499–1520.
Letcher RJ, Klasson-Wehler E, Bergman A (2000) Methyl sulfone and hydroxylated metabolites of polychlorinated biphenyls. In: Paasivirta J, ed. The handbook of environmental chemistry. Vol. 3. Part K. New types of persistent halogenated compounds. Berlin, Springer-Verlag.
Levin ED, Schantz SL, Bowman RE (1988) Delayed spatial alternation deficits resulting from perinatal PCB exposure in monkeys. Archives of Toxicology, 62:267–273.
Lewis RG, Martin BE, Sgontz DL, Sgontz DL, Howe JE (1985) Measurements of fugitive atmospheric emissions of polychlorinated biphenyls from hazardous waste landfills. Environmental Science & Technology, 19:986–991.
Lilienthal H, Winneke G (1991) Sensitive periods for behavioral toxicity of polychlorinated biphenyls: Determination by cross-fostering in rats. Fundamental and Applied Toxicology, 17:368–375.
Lilienthal H, Manfred N, Carmen M, Winneke G (1990) Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundamental and Applied Toxicology, 15:457–467.
Linder RE, Gaines TB, Kimbrough RD (1974) The effect of polychlorinated biphenyls on rat reproduction. Food and Cosmetics Toxicology, 12:63–77.
Loomis D, Browning SR, Schenck AP, Gregory E, Savitz DA (1997) Cancer mortality among electric utility workers exposed to polychlorinated biphenyls. Occupational and Environmental Medicine, 54:720–728.
Loose LD, Pittman KA, Benitz KF, Silkworth JB, Mueller W, Coulston F (1978a) Environmental chemical induced immune dysfunction. Ecotoxicology and Environmental Safety, 2:173–198.
Loose LD, Silkworth JB, Pittman KA, Benitz KF, Mueller W (1978b) Impaired host resistance to endotoxin and malaria in polychlorinated biphenyl- and hexachlorobenzene-treated mice. Infection and Immunity, 20:30–35.
Lu Y-C, Wu Y-C (1985) Clinical findings and immunological abnormalities in Yu-Cheng patients. Environmental Health Perspectives, 59:17–29.
Luebeck EG, Moolgavkar SH, Buchmann A, Schwarz M (1991) Effects of polychlorinated biphenyls in rat liver: Quantitative analysis of enzyme-altered foci. Toxicology and Applied Pharmacology, 111:469–484.
Lutz RJ, Dedrick RL (1987) Physiologic pharmacokinetic modeling of polychlorinated biphenyls. In: Safe S, ed. Polychlorinated biphenyls (PCBs): Mammalian and environmental toxicology. Berlin, Springer-Verlag, pp. 111–131.
Mackay D (1989) Modeling the long-term behavior of an organic contaminant in a large lake: application to PCBs in Lake Ontario. Journal of Great Lakes Research, 15:283–297.
MacLeod KE (1981) Polychlorinated biphenyls in indoor air. Environmental Science & Technology, 15:926–928.
Maroni M, Colombi A, Cantoni S, Ferioli E, Foa V (1981) Occupational exposure to polychlorinated biphenyls in electrical workers. I. Environmental and blood polychlorinated biphenyls concentrations. British Journal of Industrial Medicine, 38:49–54.
Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, Peters AC, Ryan MJ, Toft JD, Singer AW, Brown JF Jr, Menton RG, Moore JA (1998) Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicological Sciences, 41:62–76.
McFarland VA, Clarke JU (1989) Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environmental Health Perspectives, 81:225–239.
McLachlan M, Mackay D, Jones PH (1990) A conceptual model of organic chemical volatilization at waterfalls. Environmental Science & Technology, 24:252–257.
Meigs JW, Albom JJ, Kartin BL (1954) Chloracne from an unusual exposure to Aroclor. Journal of the American Medical Association, 154:1417–1418.
Melino G, Vernole P, Antinori M, Nicoletti B, Concato C, Finazzi-Agro A (1992) Immunological and cytogenetic damage in workers accidentally exposed to polychlorinated biphenyls (PCB). Clinical Chemistry and Enzymology Communications, 4(5):341–353.
Mergler D, Belanger S, Larribe F, Panisset M, Bowler R, Baldwin M, Lebel J, Hudnell K (1998) Preliminary evidence of neurotoxicity associated with eating fish from the Upper St. Lawrence River lakes. Neurotoxicology, 19:691–702.
Mes J (1994) Temporal changes in some chlorinated hydrocarbon residue levels of Canadian breast milk and infant exposure. Environmental Pollution, 84(3):261–268.
Moysich KB, Ambrosone CB, Vena JE, Shields PG, Mendola P, Kostyniak P, Greizerstein H, Graham S, Marshall JR, Schisterman EF, Freudenheim JL (1998) Environmental organochlorine exposure and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers & Prevention, 7:181–188.
Mullin MD, Pochini CM, McCrindle S, Romkes M, Safe SH, Safe LM (1984) High resolution PCB analysis: Synthesis and chromatographic properties of all 209 PCB congeners. Environmental Science & Technology, 18:468–476.
Murray HE, Ray LE, Giam CS (1981) Phthalic acid esters, total DDT and polychlorinated biphenyls in marine samples from Galveston Bay, Texas. Bulletin of Environmental Contamination and Toxicology, 26:769–774.
Nagayama J, Kuratsune M, Masuda Y (1981) Formation of polychlorinated dibenzofurans by heating polychlorinated biphenyls. Fukuoka Acta Medica, 72:136–141.
NCI (1978) Bioassay of Aroclor 1254 for possible carcinogenicity. Bethesda, MD, National Cancer Institute (NCI-GC-TR-38; NTIS PB279624).
Nesaretnam K, Corcoran D, Dils RR, Darbre P (1996) 3,4,3',4'-Tetrachlorobiphenyl acts as an estrogen in vitro and in vivo. Molecular Endocrinology, 10:923–936.
NOAA (1989) Standard analytical procedures of the NOAA National Analytical Facility, 2nd ed. Rockville, MD, National Oceanic and Atmospheric Administration (NOAA Technical Memorandum NMFS F/NWC-92, 1985-86; to obtain a copy, contact National Status and Trends Program, NOAA N/OMA32, 11400 Rockville Pike, Rockville, MD 20852, USA).
Norback DH, Weltman RH (1985) Polychlorinated biphenyl induction of hepatocellular carcinoma in the Sprague-Dawley rat. Environmental Health Perspectives, 60:97–105.
Norén K, Meironyté D (2000) Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere, 40:1111–1123.
O’Neill HJ, Pollock TL, Brun GL, Doull JA, Léger DA, Bailey HS (1992) Toxic chemical survey of municipal drinking water sources in Atlantic Canada 1985–1988. Water Pollution Research Journal of Canada, 27:715–732.
Ouw HK, Simpson GR, SiIyali DS (1976) Use and health effects of Aroclor 1242, a polychlorinated biphenyl in an electrical industry. Archives of Environmental Health, 31:189–194.
Pantaleoni G, Fanini D, Sponta AM, Palumbo G, Giorgi R, Adams PM (1988) Effects of maternal exposure to polychlorobiphenyls (PCBs) on F1 generation behavior in the rat. Fundamental and Applied Toxicology, 11:440–449.
Patandin S, Koopman-Esseboom C, De Ridder MA, Weisglas-Kuperus N, Sauer PJ (1998) Effects of environmental exposure to polychlorinated biphenyls and dioxin on birth size and growth in Dutch children. Pediatric Research, 44(4):538–545.
Patandin S, Lanting C, Mulder P, Boersma E, Sauer P, Weisglas-Kuperus N (1999) Effects of environmental exposure to polychlorinated biphenyls and dioxins on cognitive abilities in Dutch children at 42 months of age. Journal of Pediatrics, 134:33–41.
Patterson DG Jr, Todd GD, Turner WE, Maggio V, Alexander LR, Needham LL (1994) Levels of non-ortho-substituted (coplanar), mono- and di-ortho-substituted polychlorinated biphenyls, dibenzo-p-dioxins, and dibenzofurans in human serum and adipose tissue. Environmental Health Perspectives, 102(Suppl. 1):195–204 [cited in ATSDR, 2000].
Pines A, Cucos S, Ever-Hadani P, Ron M (1987) Some organochlorine insecticide and polychlorinated biphenyl blood residues in infertile males in the general Israeli population of the middle 1980’s. Archives of Environmental Contamination and Toxicology, 16:587–597.
Poland A, Knutson J, Glover E, Kende A (1983) Tumor promotion in the skin of hairless mice by halogenated aromatic hydrocarbons. In: Weinstein IB, Vogel HJ, eds. Genes and proteins in oncogenesis. New York, NY, Academic Press, pp. 143–161.
Porte C, Albaiges J (1993) Bioaccumulation patterns of hydrocarbons and polychlorinated biphenyls in bivalves, crustaceans, and fishes. Archives of Environmental Contamination and Toxicology, 263:273–281.
Price SC, Ozalp S, Weaver R (1988) Thyroid hyperactivity caused by hypolipodaemic compounds and polychlorinated biphenyls. Archives of Toxicology, Supplement 12:85–92.
Pruell RJ, Rubinstein NI, Taplin BK, LiVolsi JA, Bowen RD (1993) Accumulation of polychlorinated organic contaminants from sediment by three benthic marine species. Archives of Environmental Contamination and Toxicology, 24(3):290–297.
Puhvel SM, Sakamoto M, Ertl DC, Reisner RM (1982) Hairless mice as models for chloracne: A study of cutaneous changes induced by topical application of established chloracnegens. Toxicology and Applied Pharmacology, 64:492–503.
Quensen JF III, Mousa MA, Boyd SA, Sanderson JT, Froese KL, Giesy JP (1998) Reduction of aryl hydrocarbon receptor-mediated activity of polychlorinated biphenyl mixtures due to anaerobic microbial dechlorination. Environmental Toxicology and Chemistry, 17(5):806–813.
Ramamoorthy K, Vyhlidal C, Wang F, Chen I, Safe S, McDonnell DP, Leonard LS, Gaido KW (1997) Additive estrogenic activities of a binary mixture of 2',4',6'-trichloro- and 2',3',4',5'-tetrachloro-4-biphenylol. Toxicology and Applied Pharmacology, 147(1):93–100.
Rao CV, Banerji SA (1988) Induction of liver tumors in male Wistar rats by feeding polychlorinated biphenyls (Aroclor 1260). Cancer Letters, 39:59–67.
Rice DC (1997) Effect of postnatal exposure to a PCB mixture in monkeys on multiple fixed interval–fixed ratio performance. Neurotoxicology and Teratology, 19:429–434.
Rice DC, Hayward S (1997) Effects of postnatal exposure to a PCB mixture in monkeys on nonspatial discrimination reversal and delayed alternation performance. Neurotoxicology, 18(2):479–494.
Robbiano L, Pino A (1981) Induction in rats of liver DNA single-strand breaks by the polychlorinated biphenyl Aroclor 1254. Bolletino della Societa Italiana di Biologia Sperimentale, 57:407–413.
Robinson GK, Lenn MJ (1994) The bioremediation of polychlorinated biphenyls (PCBs): Problems and perspectives. Biotechnology and Genetic Engineering Reviews, 12:139–188.
Rogan WJ (1989) Yu-Cheng. In: Kimbrough RD, Jensen AA, eds. Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products, 2nd ed. Amsterdam, Elsevier Science Publishers, pp. 401–415.
Rogan WJ, Gladen BC (1991) PCBs, DDE, and child development at 18 and 24 months. Annals of Epidemiology, 1:407–413.
Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tinglestad J, Tully M (1986) Neonatal effects of transplacental exposure to PCBs and DDE. Journal of Pediatrics, 109:335–341.
Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tinglestad J, Tully M (1987) Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: Effects on growth, morbidity and duration of lactation. American Journal of Public Health, 77:1294–1297.
Rogan WJ, Gladen BC, Hung KL, Koong SL, Shih LY, Taylor JS, Wu YC, Yang D, Ragan NB, Hsu CC (1988) Congenital poisoning by polychlorinated biphenyls and their contaminants in Taiwan. Science, 241:334–336.
Rothman N, Cantor KP, Blair A, Bush D, Brock JW, Helzlsouer K, Zahm SH, Needham LL, Pearson GR, Hoover RN, Comstock GW, Strickland PT (1997) A nested case–control study of non-Hodgkin lymphoma and serum organochlorine residues. The Lancet, 350(9073):240–244.
Safe S (1980) Metabolism, uptake, storage and bioaccumulation of halogenated aromatic pollutants. In: Kimbrough RD, Jensen AA, eds. Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products, 2nd ed. Amsterdam, Elsevier Science Publishers, pp. 81–107.
Safe S (1989) Polyhalogenated aromatics: Uptake, disposition and metabolism. In: Kimbrough R, Jensen S, eds. Halogenated biphenyls, terphenyls, naphthalenes, dibenzodioxins and related products, 2nd ed. Amsterdam, Elsevier Science Publishers, pp. 131–159.
Safe S (1990) Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Critical Reviews in Toxicology, 21:51–88.
Safe S (1994) Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Critical Reviews in Toxicology, 24:87–149.
Safe S, Bandiera S, Sawyer T, Robertson L, Safe L, Parkinson A, Thomas PE, Ryan DE, Reik LM, Levin W, Denomme MA, Fujita T (1985a) PCBs: structure–function relationships and mechanism of action. Environmental Health Perspectives, 60:47–56.
Safe S, Safe L, Mullin M (1985b) Polychlorinated biphenyls: Congener-specific analysis of a commercial mixture and a human milk extract. Journal of Agricultural and Food Chemistry, 33:24–29.
Sager DB (1983) Effect of postnatal exposure to polychlorinated biphenyls on adult male reproductive function. Environmental Research, 31:76–94.
Sager DB, Girard DM (1994) Long-term effects on reproductive parameters in female rats after translactational exposure to PCBs. Environmental Research, 66(1):52–76.
Sager DB, Shih-Schroeder W, Girard D (1987) Effect of early postnatal exposure to polychlorinated biphenyls (PCBs) on fertility in male rats. Bulletin of Environmental Contamination and Toxicology, 38:946–953.
Saghir SA, Koritz GD, Hansen LG (1994) Toxicokinetics of 2,2',4,4'- and 3,3',4,4'-tetrachlorobiphenyl in house flies following topical administration. Pesticide Biochemistry and Physiology, 49:94–113.
Salata GG, Wade TL, Sericano JL, Davis JW, Brooks JM (1995) Analysis of Gulf of Mexico bottlenose dolphins for organochlorine pesticides and PCBs. Environmental Pollution, 88:167–175.
Sargent L, Roloff B, Meisner L (1989) In vitro chromosome damage due to PCB interactions. Mutation Research, 224:79–88.
Sargent LM, Sattler GL, Roloff B, Xu YH, Sattler CA, Meisner L, Pitot HC (1992) Ploidy and specific karyotypic changes during promotion with phenobarbital, 2,5,2',5'-tetrachlorobiphenyl, and/or 3,4,3',4-tetrachlorobiphenyl in rat liver. Cancer Research, 52:955–962.
Schaeffer E, Grein H, Goessner W (1984) Pathology of chronic polychlorinated biphenyl (PCB) feeding in rats. Toxicology and Applied Pharmacology, 75:278–288.
Schantz SL, Levin ED, Bowman RE, Heironimus MP, Laughlin NK (1989) Effects of perinatal PCB exposure on discrimination-reversal learning in monkeys. Neurotoxicology and Teratology, 11:243–250.
Schantz SL, Levin ED, Bowman RE (1991) Long-term neurobehavioral effects of perinatal polychlorinated biphenyl (PCB) exposure in monkeys. Environmental Toxicology and Chemistry, 10:747–756.
Schantz SL, Moshtaghian J, Ness DK (1995) Spatial learning deficits in adult rats exposed to ortho-substituted PCB congeners during gestation and lactation. Fundamental and Applied Toxicology, 26:117–126.
Schantz SL, Seo BW, Moshtaghian J, Peterson RE, Moore RW (1996) Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicology and Teratology, 18:305–313.
Schantz SL, Seo BW, Wong PW, Pessah IN (1997) Long-term effects of developmental exposure to 2,2',3,5',6-pentachlorobiphenyl (PCB95) on locomotor activity, spatial learning and memory, and brain ryanodine binding. Neurotoxicology, 18:457–467.
Secor CL, Mills EL, Harshbarger J, Kuntz HT, Gutenmann WH, Lisk DJ (1993) Bioaccumulation of toxicants, element and nutrient composition, and soft tissue histology of zebra mussels (Dreissena polymorpha) from New York State waters. Chemosphere, 26(8):1559–1575.
Seegal RF, Brosch KO, Bush B (1986) Regional alterations in serotonin metabolism induced by oral exposure of rats to polychlorinated biphenyls. Neurotoxicology, 7:155–166.
Seegal RF, Bush B, Brosch KO (1991) Comparison of effects of Aroclors 1016 and 1260 on non-human primate catecholamine function. Toxicology, 66:145–163.
Shen TT, Tofflemire TJ (1980) Air pollution aspects of land disposal of toxic wastes. Journal of the Environmental Engineering Division (American Society of Civil Engineers), 106:211–226.
Sinks T, Steele G, Smith AB, Watkins K, Shults RA (1992) Mortality among workers exposed to polychlorinated biphenyls. American Journal of Epidemiology, 136:389–398.
Smialowicz RJ, Andrews JE, Riddle MM (1989) Evaluation of the immunotoxicity of low level PCB exposure in the rat. Toxicology, 56:197–211.
Smith AB, Schloemer J, Lowry LK, Smallwood AW, Ligo RN, Tanaka S, Stringer W, Jones M, Hervin R, Glueck CJ (1982) Metabolic and health consequences of occupational exposure to polychlorinated biphenyls. British Journal of Industrial Medicine, 39:361–369.
Soedergren A, Larsson P, Knulst J, Bergovist C (1990) Transport of incinerated organochlorine compounds to air, water, microlayer, and organisms. Marine Pollution Bulletin, 21:18–24.
Sondossi M, Sylvestre M, Ahmad D (1992) Effects of chlorobenzoate transformation on the Pseudomonas testosteroni biphenyl and chlorobiphenyl degradation pathway. Applied and Environmental Microbiology, 58(2):485–495.
Steuerwald U, Weihe P, Jorgensen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jorgensen E, Grandjean P (2000) Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. Journal of Pediatrics, 136(5):599–605.
Stewart P, Reihman J, Lonky E, Darvill T, Pagano J (2000) Prenatal PCB exposure and Neonatal Behavioral Assessment Scale (NBAS) performance. Neurotoxicology and Teratology, 22:21–29.
Street JC, Sharma RP (1975) Alteration of induced cellular and humoral immune responses by pesticides and chemicals of environmental concern: Quantitative studies of immunosuppression by DDT, Aroclor 1254, carbaryl, carbofuran, and methylparathion. Toxicology and Applied Pharmacology, 32:587–602.
Sugiura K (1992) Microbial degradation of polychlorinated biphenyls in aquatic environments. Chemosphere, 24(7):881–890.
Svensson BG, Hallberg T, Nilsson A, Schutz A, Hagmar L (1994) Parameters of immunological competence in subjects with high consumption of fish contaminated with persistent organochlorine compounds. International Archives of Occupational and Environmental Health, 65(6):351–358.
Svensson B-G, Mikoczy Z, Strömberg U, Hagmar L (1995a) Mortality and cancer incidence among fishermen with a high dietary intake of persistent organochlorine compounds. Scandinavian Journal of Work, Environment & Health, 21:106–115.
Svensson B-G, Nilsson A, Jonsson E, Schütz A, Åkesson B, Hagmar L (1995b) Fish consumption and exposure to persistent organochlorine compounds, mercury, selenium and methylamines among Swedish fishermen. Scandinavian Journal of Work, Environment & Health, 21:96–105.
Swackhamer DL, Armstrong DE (1986) Estimation of the atmospheric and nonatmospheric contributions and losses of polychlorinated biphenyls for Lake Michigan on the basis of sediment records of remote lakes. Environmental Science & Technology, 20:879–883.
Syracuse (2000) SRC PhysProp database. Available at http://esc.syrres.com/interkow/webprop.exe?CAS(enter congener or Aroclor CAS#). North Syracuse, NY, Syracuse Research Corporation, 30 November 2000.
Taki I, Hisanaga S, Amagase Y (1969) Report on Yusho (chlorobiphenyls poisoning) pregnant women and their fetuses. Fukuoka Acta Medica, 60:471–474.
Tanabe S, Hidaka H, Tatsukawa R (1983) PCBs and chlorinated hydrocarbon pesticides in Antarctic atmosphere and hydrosphere. Chemosphere, 12(2):277–288.
Tanabe S, Tanaka H, Tatsukawa R (1984) Polychlorobiphenyls, σ-DDT and hexachlorocyclohexane isomers in the western North Pacific ecosystem. Archives of Environmental Contamination and Toxicology, 13:731–738.
Taylor PR, Lawrence CE (1992) Polychlorinated biphenyls: estimated serum half lives. British Journal of Industrial Medicine, 49:527–528.
Thomas DR, Carswell KS, Georgiou G (1992) Mineralization of biphenyl and PCBs by the white rot fungus Phanerochaete chrysosporium. Biotechnology & Bioengineering, 40(11):1395–1402.
Thomas PT, Hinsdill RD (1980) Perinatal PCB exposure and its effect on the immune system of young rabbits. Drug and Chemical Toxicology, 3:173–184.
Thomas RG (1982) Volatilization from water. In: Lyman WJ, Reehl WF, Rosenblatt DH, eds. Handbook of chemical property estimation methods. New York, NY, McGraw-Hill Book Co., pp. 16–16 [cited in ATSDR, 2000].
Tilson HA, Jacobson JL, Rogan WJ (1990) Polychlorinated biphenyls and the developing nervous system cross-species comparisons. Neurotoxicology and Teratology, 12:239–248.
Tironi A, Pesatori A, Consonni D, Zocchetti C, Bertazzi PA (1996) Mortality among women workers exposed to PCB. Epidemiologia e Prevenzione, 20:200–202.
Toyoda M, Iida T, Hori T, Yanagi T, Kono Y, Uchibe H (1999a) Concentrations of PCDDs, PCDFs, and coplanar PCBs in Japanese retail foods. Journal of the Food Hygienic Society of Japan, 40:111–121.
Toyoda M, Uchibe H, Yanagi T, Kono Y, Hori T, Iida T (1999b) Dietary daily intake of PCDDs, PCDFs, and coplanar PCBs by total diet study in Japan. Journal of the Food Hygienic Society of Japan, 40:98–110.
Travis CC, Arms AD (1988) Bioconcentration of organics in beef, milk and vegetation. Environmental Science & Technology, 22:271–274.
Truelove J, Grant D, Mes J, Tryphonas H, Tryphonas L, Zawidzka Z (1982) Polychlorinated biphenyl toxicity in the pregnant cynomolgus monkey: A pilot study. Archives of Environmental Contamination and Toxicology, 11:583–588.
Tryphonas H, Hayward S, O’Grady L, Loo JCK, Arnold DL, Bryce F, Zawidzka ZZ (1989) Immunotoxicity studies of PCB (Aroclor 1254) in the adult rhesus (Macaca mulatta) monkey — preliminary report. International Journal of Immunopharmacology, 11:199–206.
Tryphonas H, Luster MI, Schiffman G, Dawson LL, Hodgen M, Germolec D, Hayward S, Bryce F, Loo JC, Mandy F, Arnold D (1991) Effect of chronic exposure of PCB (Aroclor 1254) on specific and nonspecific immune parameters in the rhesus (Macaca mulatta) monkey. Fundamental and Applied Toxicology, 16:773–786.
US EPA (1980) Code of Federal Regulations. 40 CFR 707.60. Washington, DC, US Environmental Protection Agency.
US EPA (1985a) Code of Federal Regulations. 40 CFR 302.4. Washington, DC, US Environmental Protection Agency [cited in ATSDR, 2000].
US EPA (1985b) Code of Federal Regulations. 40 CFR 797. Washington, DC, US Environmental Protection Agency [cited in ATSDR, 2000].
US EPA (1988a) Drinking water criteria document for polychlorinated biphenyls (PCBs). Final. Cincinnati, OH, US Environmental Protection Agency, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office (ECAO-CIN-414).
US EPA (1988b) Polychlorinated biphenyls (PCBs): Manufacturing, processing, distribution in commerce, and use prohibitions. Code of Federal Regulations. 40 CFR 761. Washington, DC, US Environmental Protection Agency.
Verbrugge DA, Giesy JP, Mora MA, Williams LL, Rossmann R, Moll RA, Tuchman M (1995) Concentrations of dissolved and particulate polychlorinated biphenyls in water from the Saginaw River, Michigan. Journal of Great Lakes Research, 21(2):219–233.
Vos JG, de Roij T (1972) Immunosuppressive activity of a polychlorinated biphenyl preparation on the humoral immune response in guinea pigs. Toxicology and Applied Pharmacology, 21:549–555.
Weisglas-Kuperus N, Sas TC, Koopman-Esseboom C, van der Zwan CW, De Ridder MA, Beishuizen A, Hooijkaas H, Sauer PJ (1995) Immunologic effects of background prenatal and postnatal exposure to dioxins and polychlorinated biphenyls in Dutch infants. Pediatric Research, 38:404–410.
Weisglas-Kuperus N, Patendin S, Berbers GAM, Sas TCJ, Mulder PGH, Sauer PJJ, Hooijkaas H (2000) Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environmental Health Perspectives, 108(12):1203–1207.
Welp EA, Weiderpass E, Boffetta P, Vaino H, Vasama-Neuvonen K, Petralia S, Partanen TJ (1998) Environmental risk factors of breast cancer. Scandinavian Journal of Work, Environment & Health, 24(1):3–7.
Wenning RJ, Bonnevie NL, Huntley SL (1994) Accumulation of metals, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons in sediments from the lower Passaic River, New Jersey. Archives of Environmental Contamination and Toxicology, 27(1):64–81.
WHO (1996) Levels of PCBs, PCDDs and PCDFs in human milk. Second round of WHO-coordinated exposure study. Bilthoven, World Health Organization, European Centre for Environment and Health.
WHO (2002) WHO consultation on risk assessment of non-dioxin-like PCBs, 12 March 2002, available at website http://www.who.int/pcs/docs/consultation_%20pcb.htm.
Whysner J, Montandon F, McClain RM, Downing J, Verna LK, Steward RE III, Williams GM (1998) Absence of DNA adduct formation by phenobarbital, polychlorinated biphenyls, and chlordane in mouse liver using the 32P-postlabeling assay. Toxicology and Applied Pharmacology, 148:14–23.
Wilson R, Allen-Gil S, Griffin D, Landers D (1995) Organochlorine contaminants in fish from an Arctic lake in Alaska, USA. The Science of the Total Environment, 160–161:511–519.
Winneke G, Bucholski A, Heinzow B, Krämer U, Schmidt E, Walkowiak J, Wiener JA, Steingrüber HJ (1998) Developmental neurotoxicity of polychlorinated biphenyls (PCBs): cognitive and psychomotor functions in 7-month-old children. Toxicology Letters, 102–103:423–428.
Winter S, Streit B (1992) Organochlorine compounds in a three-step terrestrial food chain. Chemosphere, 24(12):1765–1774.
Yakushiji T, Watanabe I, Kuwabara K, Tanaka R, Kashimoto T, Kunita N, Hara I (1984) Postnatal transfer of PCBs from exposed mothers to their babies: influence of breast-feeding. Archives of Environmental Health, 39:368–375.
Yamaguchi A, Yoshimura T, Kuratsune M (1971) Investigation concerning babies born from women who consumed oil contaminated with chlorobiphenyl. Fukuoka Igaku Zasshi, 62:117–122.
Yao Y, Takada H, Masunaga S, Nakanishi J (2000) Study on the historical trends of PCDD/Fs and coplanar PCBs in Tokyo Bay, Japan. In: Proceedings of the 3rd international workshop on risk evaluation and management of chemicals. Yokohama, Yokohama National University, pp. 61–68.
Yoshimura T (1974) Epidemiological study on Yusho babies born to mothers who had consumed oil contaminated by PCB. Fukuoka Igaku Zasshi, 65:74–80.
Yoshimura T, Ikeda M (1978) Growth of school children with polychlorinated biphenyl poisoning or Yusho. Environmental Research, 17:416–425.
Zhang Y, Rott B, Freitag D (1983) Accumulation and elimination of 14C-PCBs by Daphnia magna strains 1820. Chemosphere, 12:1645–1651.
ATSDR (2000) Toxicological profile for polychlorinated biphenyls (PCBs). Atlanta, GA, US Department of Health and Human Services, Agency for Toxic Substances and Disease Registry
The Agency for Toxic Substances and Disease Registry (ATSDR), Division of Toxicology, prepared this CICAD on PCBs based on the Toxicological profile for polychlorinated biphenyls (2000 update). During the development process, the document received several important reviews by substance-specific experts and the public to enhance its credibility and ensure its transparency. The first version of the PCB profile was issued in 1988, and it was subsequently revised and updated in 1993, 1997, and 2000. Each revision involved an initial peer review, a public comment period, and at least one post-public peer review. In addition to the regular pre- and post-public peer reviews, the 2000 update was also reviewed by an extensive panel of experts on PCBs that consisted of 37 scientists from academia, research institutes, and government, with 3 observers representing environmental advocacy and industry. Altogether, the 2000 profile update (CICAD source document) received peer reviews in 1998 and 2000, public comment in 1999, and the expert panel review in 2000. July 1998 was the cutoff of literature search for the pre-public draft of the source document, which extended back to 1995, when the literature search for the 1997 version of the profile ended. A second literature search for the post-public update of the source document was conducted in April 2000, and additional articles were subsequently included based on peer reviewers’ and expert panel recommendations.
The profile has undergone the following ATSDR internal reviews:
Health Effects Review (HER). The HER Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying end-points.
Minimal Risk Level (MRL) Review. The MRL Workgroup considers issues relevant to substance-specific MRLs, reviews the health effects database of each profile, and makes recommendations for derivation of MRLs.
Review panels were assembled to review the profile before the public comment period began, after compiling the public comments to discuss the public comments and conduct a further comprehensive review of the profile, and after the public comments were addressed.
The pre-public review panel consisted of the following members:
Loren Koller, College of Veterinary Medicine, Oregon State University, Corvallis, OR
Ernest McConnel, Consultant, Raleigh, NC
Shane Que Hee, Department of Environmental Health Sciences, School of Public Health, University of California at Los Angeles, Los Angeles, CA
The special expert review panel consisted of the following members:
Annette E. Ashizawa, Division of Toxicology/Research Implementation Branch, ATSDR, Atlanta, GA
Mike Bolger, Contaminants Branch, ATSDR, Washington, DC
Stephen Bosch, Syracuse Research Corporation, North Syracuse, NY
Frank J. Bove, Division of Health Studies, ATSDR, Atlanta, GA
Mr. Virlyn Burse, Environmental Health Laboratory, National Center for Environmental Health, Chamblee, GA
Rick Canady, Diplomate American Board of Toxicology, Division of Health Assessment and Consultation, ATSDR, Atlanta, GA
David O. Carpenter, School of Public Health, University at Albany, State University of New York, Rensselaer, NY
John Cicmanec, US Environmental Protection Agency, Cincinnati, OH
Jim Cogliano, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC
Thomas Darvill, Department of Psychology, Oswego State University, Oswego, NY
Christopher De Rosa, Division of Toxicology, ATSDR, Atlanta, GA
Henry Falk, ATSDR, Atlanta, GA
Obaid Faroon, ATSDR, Atlanta, GA
Ginger Gist, Division of Health Studies, ATSDR, Atlanta, GA
Anthony Gray, Syracuse Research Corporation, North Syracuse, NY
Larry Hansen, College of Veterinary Medicine, University of Illinois, Urbana, IL
Heraline E. Hicks, Division of Toxicology, ATSDR, Atlanta, GA
Joseph L. Jacobson, Department of Psychology, Wayne State University, Detroit, MI
Edwin M. Kilbourne, Centers for Disease Control and Prevention/National Center for Environmental Health, Chamblee, GA
Fernando Llados, Syracuse Research Corporation, North Syracuse, NY
Mark McClanahan, National Center for Environmental Health, Chamblee, GA
Stephanie Miles-Richardson, Division of Toxicology/Research Implementation Branch, ATSDR, Atlanta, GA
Ralph O’Connor, Division of Health Education and Promotion, ATSDR, Atlanta, GA
Victoria Persky, University of Illinois, Chicago, IL
Hana Pohl, ATSDR, Atlanta, GA
Walter Rogan, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC
Susan L. Schantz, Department of Veterinary Biosciences, University of Illinois, Urbana, IL
Arnold Schecter, School of Public Health, University of Texas – Houston, Dallas, TX
Tom Sinks, Centers for Disease Control and Prevention, Chamblee, GA
Barbara Slade, ATSDR, Atlanta, GA
Allan S. Susten, Diplomate American Board of Toxicology, Division of Health Assessment and Consultation, ATSDR, Atlanta, GA
Helen Tryphonas, Toxicology Research Division, Bureau of Chemical Safety, Health Protection Branch, Health Canada, Ottawa, Ontario
John Vena, Social and Preventive Medicine, University of Buffalo, Buffalo, NY
John Wheeler, Diplomate American Board of Toxicology, Division of Toxicology, ATSDR, Atlanta, GA
Elizabeth Whelan, National Institute for Occupational Safety and Health, Cincinnati, OH
Mary C. White, Health Investigation Branch, Division of Health Studies, ATSDR, Atlanta, GA
Malcolm Williams, ATSDR, Atlanta, GA
Observers
Stephen B. Hamilton, Jr., General Electric Company, Fairfield, CN
Brett D. Hulsey (invited to the meeting, but did not attend), Sierra Club, Madison, WI
Robert Kaley, Solutia, Inc., St. Louis, MO
The post-public peer review panel consisted of the following members:
Larry Hensen, College of Veterinary Medicine, University of Illinois, Urbana, IL
Joseph Jacobsen, Wayne State University, Detroit, MI
Helen Tryphonas, Toxicology Research Division, Bureau of Chemical Safety, Health Protection Branch, Health Canada, Ottawa, Ontario
John Vena, Social and Preventive Medicine, University of Buffalo, Buffalo, NY
These experts collectively have knowledge of PCBs’ physical and chemical properties, toxicokinetics, key health end-points, mechanisms of action, human and animal exposure, and quantification of risk to humans. All reviewers were selected in conformity with the conditions for peer review specified in Section 104(i)(13) of the Comprehensive Environmental Response, Compensation, and Liability Act, as amended.
Scientists from ATSDR have reviewed the peer reviewers’ comments and determined how they should be addressed in the profile. A listing of the peer reviewers’ comments not incorporated in the profile, with a brief explanation of the rationale for their exclusion, exists as part of the administrative record for this compound. A list of databases reviewed and a list of unpublished documents cited are also included in the administrative record.
The citation of the peer review panel should not be understood to imply its approval of the profile’s final content. The responsibility for the content of this profile lies with ATSDR.
The draft CICAD on PCBs was sent for review to IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
L. Birnbaum, National Center for Environmental Assessment, US Environmental Protection Agency, Research Triangle Park, NC, USA
R. Cary, Health and Safety Executive, Bootle, Merseyside, United Kingdom
R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
J. Curless, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
M.E. Davis, American Chemistry Council, Arlington, VA, USA
T. Feldman, American Chemistry Council, Arlington, VA, USA
H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
B. Gladden, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
A. Hanberg, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden
P. Harvey, Health and Safety Executive, Bootle, Merseyside, United Kingdom
R. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
C. Hiremath, National Center for Environmental Assessment, US Environmental Protection Agency, Research Triangle Park, NC, USA
C.T. Howlett, Jr., American Chemistry Council, Arlington, VA, USA
H. Lilienthal, Medizinisches Institut für Umwelthygiene an der Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany
D. Mukerjee, National Center for Environmental Assessment, US Environmental Protection Agency, Cincinnati, OH
H. Nagy, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
C.M. Price, American Chemistry Council, Arlington, VA, USA
D.C. Rice, US Environmental Protection Agency Headquarters, Washington, DC, USA
W.J. Rogan, National Institute for Environmental Health Sciences, Research Triangle Park, NC, USA
J. Sekizawa, National Institute for Health Sciences, Tokyo, Japan
M.H. Sweeney, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
M. van den Berg, Research Institute of Toxicology, Utrecht University, Utrecht, The Netherlands
G. Winnneke, Medizinisches Institut für Umwelthygiene an der Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany
K. Ziegler-Skylakakis, Commission of the European Communities, European Union, Luxembourg
Ottawa, Canada,
29 October – 1 November 2001
Members
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr T. Chakrabarti, National Environmental Engineering Research Institute, Nehru Marg, India
Dr B.-H. Chen, School of Public Health, Fudan University (formerly Shanghai Medical University), Shanghai, China
Dr R. Chhabra, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA (teleconference participant)
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA (Chairman)
Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Vice-Chairman)
Dr O. Faroon, Agency for Toxic Substances and Disease Registry, Department of Health and Human Services, Atlanta, GA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Ms R. Gomes, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr M. Gulumian, National Centre for Occupational Health, Johannesburg, South Africa
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers and Veterinary Medicine, Berlin, Germany
Dr A. Hirose, National Institute of Health Sciences, Tokyo, Japan
Mr P. Howe, Centre for Ecology and Hydrology, Huntingdon, Cambridgeshire, United Kingdom (Co-Rapporteur)
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol Research, Hanover, Germany (Co-Rapporteur)
Dr S.-H. Lee, College of Medicine, The Catholic University of Korea, Seoul, Korea
Ms B. Meek, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario, Canada
Dr J.A. Menezes Filho, Faculty of Pharmacy, Federal University of Bahia, Salvador, Bahia, Brazil
Dr R. Rolecki, Nofer Institute of Occupational Medicine, Lodz, Poland
Dr J. Sekizawa, Division of Chem-Bio Informatics, National Institute of Health Sciences, Tokyo, Japan
Dr S.A. Soliman, Faculty of Agriculture, Alexandria University, Alexandria, Egypt
Dr M.H. Sweeney, Document Development Branch, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Temmink, Department of Agrotechnology & Food Sciences, Wageningen University, Wageningen, The Netherlands
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme (NICNAS), Sydney, Australia
Representative of the European Union
Dr K. Ziegler-Skylakakis, European Commission, DG Employment and Social Affairs, Luxembourg
Observers
Dr R.M. David, Eastman Kodak Company, Rochester, NY, USA
Dr R.J. Golden, ToxLogic LC, Potomac, MD, USA
Mr J.W. Gorsuch, Eastman Kodak Company, Rochester, NY, USA
Mr W. Gulledge, American Chemistry Council, Arlington, VA, USA
Mr S.B. Hamilton, General Electric Company, Fairfield, CN, USA
Dr J.B. Silkworth, GE Corporate Research and Development, Schenectady, NY, USA
Dr W.M. Snellings, Union Carbide Corporation, Danbury, CN, USA
Dr E. Watson, American Chemistry Council, Arlington, VA, USA
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
|
Ah |
aryl hydrocarbon |
|
ATSDR |
Agency for Toxic Substances and Disease Registry |
|
BCF |
bioconcentration factor |
|
CI |
95% confidence interval |
|
CICAD |
Concise International Chemical Assessment Document |
|
CYP |
cytochrome P450 |
|
DDE |
1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene |
|
DDT |
1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane |
|
DNA |
deoxyribonucleic acid |
|
ECD |
electron capture detector |
|
EPA |
Environmental Protection Agency |
|
GC |
gas chromatography |
|
HRGC |
high-resolution gas chromatography |
|
ICD-8 |
International Classification of Diseases, 8th revision |
|
IgG |
immunoglobulin G |
|
IgM |
immunoglobulin M |
|
IPCS |
International Programme on Chemical Safety |
|
IQ |
Intelligence Quotient |
|
Kow |
octanol/water partition coefficient |
|
LD50 |
lethal dose to 50% of the exposed population |
|
LOAEL |
lowest-observed-adverse-effect level |
|
MDI |
Mental Development Index |
|
MFO |
mixed-function oxygenase |
|
MS |
mass spectrometry |
|
NBAS |
Neonatal Behavioral Assessment Scale |
|
NHL |
non-Hodgkin lymphoma |
|
NOAEL |
no-observed-adverse-effect level |
|
NOS |
neurological optimality score |
|
OR |
odds ratio |
|
PCB |
polychlorinated biphenyl |
|
PCDD |
polychlorinated dibenzodioxin |
|
PCDF |
polychlorinated dibenzofuran |
|
PCQ |
polychlorinated quaterphenyl |
|
PDI |
Psychomotor Development Index |
|
RR |
rate ratio |
|
SD |
standard deviation |
|
SFE |
supercritical fluid extraction |
|
SIR |
standardized incidence ratio |
|
SMR |
standardized mortality ratio |
|
SRM |
Standard Reference Material |
|
T3 |
triiodothyronine |
|
T4 |
thyroxine |
|
TEF |
toxicity equivalence factor |
|
TEQ |
toxicity equivalence |
|
WHO |
World Health Organization |
|
WISC-R |
Wechsler Intelligence Scale R |
POLYCHLORINATED BIPHENYL (AROCLOR 1254) ICSC:0939
Le présent CICAD sur les biphényles polychlorés (PCB) a été préparé par l’Agence des Etats-Unis pour les produits toxiques et le Registre des maladies (Division de toxicologie) à partir d’une mise à jour du Profil toxicologique des biphényles polychlorés (PCB) (ATSDR, 2000). On pourra en outre, pour plus de détails sur plusieurs points d’aboutissement de l’action toxique jugés importants dans ce CICAD, se reporter à un certain nombre d’articles inspirés du document de base (Faroon et al., 2000, 2001a,b). Des renseignements sur la nature de l’examen par des pairs et sur la disponibilité du document de base sont donnés à l’appendice 1. L’appendice 2 donne des indications sur l’examen par des pairs du présent CICAD. Ce CICAD a été approuvé en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui a eu lieu à Ottawa (Canada) du 29 octobre au 1er novembre 2001. La liste des participants à cette réunion figure à l’appendice 3. La fiche internationale sur la sécurité d’un biphényle polychloré, l’Aroclor 1254 (ICSC 0939) établie par le Programme international sur la sécurité chimique (IPCS, 2000), est également reproduite dans le présent document.
Les biphényles polychlorés ou polychlorobiphényles (PCB) sont des hydrocarbures chlorés de synthèse formés de deux cycles benzéniques reliés par une liaison simple carbone-carbone et dont les atomes d’hydrogène (de 1 jusqu’à tous les 10) peuvent être remplacés par du chlore. On les produit industriellement depuis 1929. Ils sont utilisés dans les plastifiants, les revêtements de surface, les encres, les adhésifs, les retardateurs de flamme, les diluants pour pesticides, les peintures ou encore dans la microencapsulation de colorants destinés aux papiers pour duplication sans carbone. Comme les PCB résistent à la fois aux acides et aux alcalis et qu’ils sont relativement stables à la chaleur, on les utilise comme fluides diélectriques dans les transformateurs et les condensateurs. Il peut donc y avoir une contamination supplémentaire de l’environnement lors de la mise au rebut de vieux matériel électrique contenant des PCB. La pyrolyse des mélanges de PCB produit du gaz chlorhydrique et des polychlorodibenzofuranes (PCDF); par ailleurs la pyrolyse de mélanges contenant des chlorobenzènes conduit également à la formation de polychlorodibenzodioxines (PCDD). Dans de nombreux pays, les PCB sont interdits ou soumis à de sévères restrictions.
Les PCB les plus chlorés s’adsorbent fortement aux particules du sol et aux sédiments et en général, ils persistent dans l’environnement. Les divers PCB présents dans le sol et les sédiments ont une demi-vie allant de quelques mois à plusieurs années. L’adsorption augmente en général avec le degré de chloration ainsi qu’avec la teneur en carbone organique et en argile du sol ou des sédiments. Les PCB sont éliminés de l’eau et du sol par deux voies principales : la volatilisation et la biodégradation, deux processus très lents.
Les PCB s’accumulent le long de la chaîne alimentaire. Ils sont rapidement absorbés au niveau des voies digestives, après quoi ils se répartissent dans le foie et les tissus adipeux où ils ont tendance à s’accumuler. Ils traversent la barrière placentaire, ils sont excrétés dans le lait et ils s’accumulent dans l’organisme du foetus ou du nourrisson. Les PCB sont métabolisés par hydroxylation puis conjugaison. La vitesse de métabolisation et d’excrétion varie sensiblement d’un PCB à l’autre.
Pour les points d’aboutissement des effets toxiques pris en considération dans le présent CICAD ainsi que la caractérisation du risque liée à l’exposition aux PCB, on a utilisé la méthode applicable aux mélanges. Cette manière de procéder est justifiée par le fait que les populations exposées aux PCB dans l’environnement général ou sur le lieu de travail sont effectivement exposées à des mélanges de ces composés, qui, individuellement, peuvent avoir différents modes d’action. Il est vrai que dans certains cas, les mélanges auxquels telle ou telle population est exposée diffèrent largement de ceux sur lesquels repose la présente évaluation. En pareille circonstance, il serait plus indiqué de recourir à la notion d’équivalence toxique (TEQ) pour les PCB dont on sait qu’ils ont un mode d’action similaire. Une autre méthode consiste à utiliser la charge corporelle totale en mélanges de PCB, car on la détermine chez l’Homme plutôt que chez l’animal ou in vitro, ce qui évite d’avoir ensuite à extrapoler d’une espèce à une autre. Pour plus de détails sur les différentes méthodes, on peut se reporter au document de base.7
L’Homme peut être exposé aux PCB en inhalant de l’air ou en ingérant des aliments et de l’eau contaminés par ces composés. On estime qu’en 1978, l’apport journalier de PCB d’origine alimentaire était de 0,027 μg/kg de poids corporel chez l’adulte aux Etats-Unis, mais il est tombé à 0,0005 μg/kg p.c. en 1982-1984 et à moins de 0,001 μg/kg p.c. pendant la période 1986-1991.
Certaines études consacrées aux effets sanitaires des PCB souffrent de la confusion créée par la présence d’autres polluants halogénés dans l’environnement et d’impuretés des PCB eux-mêmes, comme les chlorodibenzofuranes. Le présent CICAD n’aborde que superficiellement la toxicité de contaminants qui résultent soit du processus de fabrication, soit du chauffage des PCB (comme les PCDD, les PCDF ou même d’autres polluants organiques persistants); toutefois, les études relatives aux accidents provoqués à Yusho et Yu-Cheng par l’utilisation d’huile de cuisine contaminée y sont briévement résumées.
Les études sur des sujets humains exposés à des PCB ont permis d’observer des effets sur la mobilité des spermatozoïdes, le taux de croissance foetale (moindre poids de naissance, réduction de la circonférence crânienne), le développement (réduction de l’âge gestationnel, immaturité neuromusculaire) et les fonctions neurologiques de la descendance (perturbations des fonctions autonomes, augmentation du nombre de réflexes anormalement faibles, réduction de la capacité de mémorisation, baisse du QI et de l’attention). Certains déficits neurologiques observés aux premiers âges de la vie sont susceptibles de disparaître ultérieurement au cours de l’enfance.
Les études épidémiologiques donnent à penser qu’il y aurait une augmentation des cancers digestifs liée à l’exposition, en particulier des cancers du foie, ainsi d’ailleurs que des mélanomes malins. Toutefois vu les insuffisances des données concernant l’exposition, l’irrégularité des résultats et, dans certains cas, la présence de facteurs de confusion dus à d’autres types d’exposition, il est exclu de pouvoir établir une relation exposition-réponse claire.
Au cours des 18 premiers mois de la vie, on n’observe aucune augmentation de l’incidence des infections respiratoires, mais on a pu constater un changement dans la proportion des différents types de lymphocytes circulants chez les enfants nés de mères exposées à des PCB. On a également observé une diminution du nombre de cellules tueuses naturelles (cellules NK) chez des consommateurs de poisson contaminé par des PCB. En outre, on a établi que chez des enfants de 3,5 ans, la prévalence des rechutes d’otite moyenne et de varicelle était liée à la concentration plasmatique des PCB.
Des effets indésirables ont été observés chez des rats, des souris, des singes et d’autres espèces de mammifères. Ces effets ont été notés à la plupart des points d’aboutissement toxicologiques habituels chez l’animal : système immunitaire, développement, appareil reproducteur, foie et poids du corps. Un certain nombre d’études mettent systématiquement en évidence une augmentation de l’incidence des cancers du foie chez des rongeurs exposés à divers PCB. La gravité des effets sanitaires dépend de la dose, de l’espèce, du mélange de PCB en cause, de la durée et du moment de l’exposition, entre autres facteurs.
Selon des études limitées, les PCB n’auraient pas d’action génotoxique directe.
En soumettant le sérum de singes exposés à des PCB pendant 55 mois à une épreuve secondaire avec des érythrocytes de mouton, on a constaté que les réponses anamnestiques en IgM et IgG avaient tendance à diminuer, le titre des IgM étant sensiblement plus faible que chez les témoins à toutes les doses. En se basant sur la valeur de 5 μg/kg de poids corporel par jour pour la dose la plus faible produisant un effet observable (LOAEL) concernant un certain nombre de points d’aboutissement, on obtient une valeur de 0,02 μg/kg p.c. par jour pour la dose tolιrable par ingestion dans le cas de l’Aroclor 1254 après application d’un facteur global d’incertitude de 300 (10 pour l’utilisation de la LOAEL plutôt que la dose sans effet observable [NOAEL], 3 pour tenir compte des variations interspécifiques et 10 pour les variations intraspécifiques).
La Agencia para el Registro de Sustancias Tóxicas y Enfermedades preparó este CICAD sobre los bifenilos policlorados (PCB) basándose en el Perfil toxicológico de los bifenilos policlorados (ATSDR, 2000). Además, se pueden consultar diversos artículos basados en el documento original para obtener más detalles sobre cada uno de los distintos efectos finales en la salud considerados importantes en este CICAD (Faroon et al., 2000, 2001a,b). La información relativa al carácter del examen colegiado y a la disponibilidad de los documentos originales se presenta en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final, celebrada en Ottawa (Canadá) del 29 de octubre al 1ş de noviembre de 2001. La lista de participantes en esta reunión figura en el apéndice 3. También se reproduce en este documento la Ficha internacional de seguridad química (ICSC 0939) para el bifenilo policlorado (Aroclor 1254), preparada por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000).
Los PCB son hidrocarburos clorados sintéticos formados por dos anillos de benceno unidos por un enlace carbono-carbono sencillo, que tienen entre 1 y los 10 átomos de hidrógeno sustituidos por átomos de cloro. Los PCB se producen comercialmente desde 1929. Se han utilizado en plastificantes, revestimientos de superficies, tintas, adhesivos, pirorretardantes, excipientes de plaguicidas, pinturas y microencapsulación de colorantes para papel de copia sin carbón. Debido a que los PCB resisten tanto los ácidos como los álcalis y son relativamente estables al calor, se han utilizado en los fluidos dieléctricos de transformadores y capacitores. También se puede contaminar el medio ambiente por la eliminación de equipo eléctrico viejo que contiene PCB. La pirólisis de mezclas de PCB produce ácido sulfhídrico y dibenzofuranos policlorados y la pirólisis de mezclas con clorobencenos también producen dibenzodioxinas policloradas. Muchos países han restringido rigurosamente o prohibido la producción de PCB.
Los PCB más clorados se adsorben con fuerza al suelo y los sedimentos y suelen ser persistentes en el medio ambiente. Los distintos compuestos tienen en el suelo y los sedimentos semividas que oscilan entre meses y años. La adsorción de los PCB generalmente aumenta con el grado de cloración del compuesto y con el contenido de carbono orgánico y arcilla del suelo o los sedimentos. La volatilización y la biodegradación - dos procesos muy lentos - son las vías principales de eliminación de PCB del agua y el suelo.
Los PCB se acumulan en la cadena trófica. Se absorben con rapidez del tracto gastrointestinal y se distribuyen y acumulan en el hígado y el tejido adiposo. También atraviesan la placenta, se excretan en la leche y se acumulan en el feto/lactante. Los PCB se metabolizan por hidroxilación y posterior conjugación. La velocidad del metabolismo y de la posterior excreción varía considerablemente entre los distintos compuestos.
A efectos de este CICAD, los efectos finales en la salud y la caracterización del riesgo asociado con la exposición a los PCB se han basado en el método para mezclas. Esto se justifica por el hecho de que las poblaciones en el medio ambiente general y el entorno profesional suelen estar expuestas a mezclas de PCB, cuyos componentes tienen mecanismos de acción diferentes. Se reconoce que en algunos casos las mezclas a las cuales están expuestas diversas poblaciones son muy diferentes de las utilizadas para realizar la presente evaluación. En tales casos, tal vez sea más adecuado adoptar un método de equivalencia tóxica para cada uno de los compuestos cuyo mecanismo de acción se sabe que es semejante. Otro sistema alternativo es la utilización de la carga corporal total de las mezclas de PCB, puesto que se hace en personas más que en animales de laboratorio o in vitro, de manera que se elimina la necesidad de extrapolación de especies. En el documento original se puede encontrar información adicional sobre los diversos procedimientos.8
Las personas pueden estar expuestas a los PCB por inhalación de aire contaminado y la ingestión de agua y alimentos contaminados. En 1978, la ingesta alimenticia estimada de PCB por adultos en los Estados Unidos fue de 0,027 µg/kg de peso corporal al día, pero disminuyó a 0,0005 µg/kg de peso corporal al día en 1982-1984 y a <0,001 µg/kg de peso corporal al día en el período de 1986-1991.
En algunos estudios sobre los efectos de los PCB en la salud han creado confusión la exposición a otros contaminantes halogenados del medio ambiente y las impurezas contenidas en los PCB, en particular los dibenzofuranos clorados. Este CICAD se ocupa sólo mínimamente de la toxicidad de los contaminantes derivados del proceso de fabricación o bien del calentamiento de los PCB (por ejemplo dibenzodioxinas policloradas, dibenzofuranos policlorados o incluso otros contaminantes orgánicos persistentes); sin embargo, en este documento se resumen brevemente los estudios de Yusho y Yu-Cheng que se ocupan de los accidentes con aceite de cocinar contaminado.
En estudios con personas expuestas a los PCB, se han observado efectos en la movilidad de los espermatozoides, la tasa de crecimiento fetal (peso más bajo al nacer, circunferencia de la cabeza más pequeña) y el desarrollo (período de gestación más corto, inmadurez neuromuscular) y las funciones neurológicas de la progenie (función autonómica dañada, mayor número de reflejos anormalmente débiles, capacidad de memoria reducida, coeficiente de inteligencia más bajo y déficit de atención). Algunas de las deficiencias neurológicas iniciales pueden desaparecer más tarde durante la infancia.
Los estudios epidemiológicos parecen indicar un aumento relacionado con la exposición de la aparición de cáncer del sistema digestivo, en particular cáncer de hígado y melanoma maligno. Sin embargo, la limitada información relativa a la exposición, la discrepancia de los resultados y, en algunos casos, la presencia de exposiciones que crean confusión impiden determinar con claridad la relación exposición-respuesta.
No se observó aumento en la incidencia de infecciones del tracto respiratorio durante los 18 primeros meses de vida, pero se detectaron cambios en la cantidad relativa de distintos tipos de linfocitos circulantes en niños nacidos de madres expuestas a PCB. Se ha observado en consumidores de pescado contaminado por PCB una disminución del número de células citolíticas. Se ha relacionado la prevalencia de infecciones recurrentes del oído medio y de varicela en niños de 3.5 años con las concentraciones de PCB en plasma.
Se observaron efectos adversos para la salud en ratas, ratones, monos y otras especies de mamíferos. Se registraron cambios en la mayor parte de los efectos finales relativos a la salud de los animales, por ejemplo de tipo inmunitario, del desarrollo, reproductivos y de peso corporal. Varios estudios han informado de manera uniforme de un aumento en la incidencia de cáncer de hígado en roedores expuestos a diferentes PCB. La gravedad de los efectos en la salud depende de la dosis, la especie, la mezcla de PCB, la duración y la oportunidad de la exposición y otros factores.
Estudios limitados indican que los PCB no son genotóxicos por mecanismos directos.
Una prueba de inmunidad secundaria con eritrocitos de oveja tras la exposición de monos a PCB durante 55 meses puso de manifiesto una tendencia decreciente en las respuestas anamnésticas de la IgM y la IgG, siendo la IgM notablemente más baja que en los testigos para todas las dosis. Basándose en la concentración más baja con efectos adversos observados (LOAEL) de 5 µg/kg de peso corporal al día para varios efectos finales, se obtuvo una ingesta tolerable de 0,02 µg/kg de peso corporal al día para una mezcla de Aroclor 1254, utilizando un factor de incertidumbre global de 300 (10 para el uso de una LOAEL en lugar de una concentración sin efectos adversos observados [NOAEL], 3 para la variación interespecífica y 10 para la variación intraespecífica).
ENDNOTES
|
International Programme on Chemical Safety (1994) Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. Geneva, World Health Organization (Environmental Health Criteria 170) (also available at http://www.who.int/pcs/). |
|
|
WHO (2002) has recently started an evaluation of the risks from non-dioxin-like PCBs (http://www.who.int/pcs/docs/consultation_%20pcb.htm). |
|
|
Unless otherwise noted, the references for this section may be found in the source document (ATSDR, 2000). |
|
|
Unless otherwise noted, the references for this section may be found in the source document (ATSDR, 2000). |
|
|
Unless otherwise noted, the references for this section may be found in the source document (ATSDR, 2000). |
|
|
A Superfund site is any land in the USA that has been contaminated by hazardous waste and identified by the US EPA as a candidate for cleanup because it poses a risk to human health and/or the environment (http://cfpub.epa.gov/superapps/index.cfm/fuseaction/faqs.viewAnswer/question_id/111/category_id/7/faqanswr.cfm). |
|
|
L’OMS (2002) a récemment commencé l’évaluation du risque imputable aux PCB non dioxiniques (http://www.who.int/pcs/docs/consultation_%20pcb.htm). |
|
|
La OMS (2002) ha comenzado recientemente una evaluación del riesgo de los PCB no semejantes a la dioxina (http://www.who.int/pcs/docs/consultation_%20pcb.htm). |
See Also:
Toxicological Abbreviations