
INTERNATIONAL PREGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 60
PRINCIPLES AND METHODS FOR THE
ASSESSMENT OF NEUROTOXICITY
ASSOCIATED WITH EXPOSURE TO CHEMICALS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
ISBN 92 4 154260 8
(c) World Health Organization 1986
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
ISSN 0250-863X
CONTENTS
PRINCIPLES AND METHODS FOR THE ASSESSMENT OF NEUROTOXICITY ASSOCIATED
WITH EXPOSURE TO CHEMICALS
PREFACE
1. INTRODUCTION
1.1. The importance of studying the nervous system
1.1.1. Methylmercury
1.1.2. Carbon disulfide (CS2)
1.2. Need to establish a comprehensive strategy for neurotoxicity
testing
1.3. Scope of the book
1.4. Purpose of the publication
2. GENERAL PRINCIPLES FOR THE ASSESSMENT OF TOXIC EFFECTS OF
CHEMICALS ON THE NERVOUS SYSTEM
2.1. Factors to be considered in the design of neurotoxicity
studies
2.1.1. General considerations
2.1.2. Objectives
2.1.3. Choice of animals
2.1.4. Dosing regimen
2.1.5. Functional reserve and adaptation
2.1.6. Other factors
2.2. Statistical analysis
2.2.1. Type I and Type II errors
2.2.2. Selection of the appropriate statistical test(s)
3. TEST METHODS IN BEHAVIOURAL TOXICOLOGY
3.1. Introduction
3.2. Classes of behaviour
3.2.1. Respondent behaviour
3.2.2. Operant behaviour
3.3. Test methods
3.3.1. General attributes of behavioural methods
3.3.1.1 Sensitivity and specificity
3.3.1.2 Validity
3.3.1.3 Replicability
3.3.1.4 Costs
3.3.2. Primary tests
3.3.2.1 Functional observation battery
3.3.2.2 Motor activity
3.3.3. Secondary tests
3.3.3.1 Intermittent schedules of reinforcement
3.3.3.2 Motor function
3.3.3.3 Sensory function
3.3.3.4 Cognitive function
3.3.3.5 Eating and drinking behaviour
3.3.3.6 Social behaviour
3.3.4. Strengths and weaknesses of various methods
3.4. Research needs
3.4.1. Compensatory mechanisms
3.4.2. Method development and refinement
4. NEUROPHYSIOLOGICAL METHODS IN NEUROTOXICOLOGY
4.1. Introduction
4.2. Methods for evaluation of the peripheral nervous system
4.2.1. Conduction velocity
4.2.2. Peripheral nerve terminal function
4.2.3. Electromyography (EMG)
4.2.4. Spinal reflex excitability
4.3. Methods for evaluation of the autonomic nervous system
4.3.1. Electrocardiography (EKG)
4.3.2. Blood pressure
4.4. Methods for the evaluation of the central nervous system
4.4.1. Spontaneous activity - electroencephalography (EEG)
4.4.2. Sensory systems
4.4.3. General excitability
4.4.3.1 Convulsive phenomena
4.4.3.2 Stimulation of the cerebral motor cortex
4.4.3.3 Recovery functions
4.4.4. Cognitive function
4.4.5. Synaptic and membrane activity
4.5. Interpretation issues
4.6. Summary and conclusions
5. MORPHOLOGICAL METHODS
5.1. Introduction
5.1.1. Role of morphology
5.1.2. Basis for morphological assessment
5.2. The nervous system and toxic injuries
5.2.1. The nervous system
5.2.2. Cellular structure of the nervous system
5.2.3. Neurocellular reaction to injury
5.2.3.1 Biological principles
5.2.3.2 Neurons
5.2.3.3 Myelinating cells
5.3. Experimental design and execution
5.3.1. General principles and procedure
5.3.2. Gross morphology
5.3.3. The role of histology
5.3.3.1 Biological principles dictating tissue
response
5.3.4. Use of controls
5.3.5. Pattern of response
5.3.6. Data acquired
5.4. Principles, limitations, and pitfalls of the morphological
approach
5.4.1. Tissue state
5.4.2. Principles of fixation
5.4.3. Principles of tissue sampling
5.4.4. Preparation of tissue for examination
5.4.5. Recognition of artefact
5.4.6. Recognition of normal structural variations
5.4.7. Qualitative versus quantitative approaches
5.5. Specific procedures
5.5.1. Introduction
5.5.2. Primary methods
5.5.2.1 Formaldehyde/paraffin method
5.5.2.2 Glutaraldehyde/epoxy method
5.5.3. Special methods
5.5.3.1 Peripheral nerve microdissection
5.5.3.2 Frozen sections
5.5.3.3 Histochemical methods
5.5.3.4 Golgi method
5.5.3.5 Transmission electron microscopy
5.5.3.6 Other anatomical methods
5.6. Conclusions
6. BIOCHEMICAL AND NEUROENDOCRINOLOGICAL METHODS
6.1. Introduction
6.2. Fractionation methods
6.2.1. Brain dissection
6.2.2. Isolation of specific cell types
6.2.3. Subcellular fractionation
6.3. DNA, RNA, and protein synthesis
6.4. Lipids, glycolipids, and glycoproteins
6.5. Neurotransmitters
6.5.1. Synthesis/degradation
6.5.2. Transport/release
6.5.3. Binding
6.5.4. Ion channels
6.5.5. Cyclic nucleotides
6.5.6. Summary of nerve terminal function
6.6. Energy metabolism
6.7. Biochemical correlates of axonal degeneration
6.8. Neuroendocrine assessments
6.8.1. Anterior pituitary hormones
6.8.2. Disruption of neuroendocrine function
6.8.2.1 Direct pituitary effects
6.8.2.2 Peripheral target effects
6.8.2.3 Disruption of hypothalamic control of
pituitary secretions
6.8.2.4 Other sites of action
6.8.3. Sex differences
6.9. Recommendations for future research
7. CONCLUSIONS AND RECOMMENDATIONS
REFERENCES
WHO TASK GROUP ON PRINCIPLES AND METHODS FOR THE ASSESSMENT OF
NEUROTOXICITY ASSOCIATED WITH EXPOSURE TO CHEMICALS
Members
b Dr M.B. Abou-Donia, Department of Pharmacology, Duke
University Medical Center, Durham, North Carolina,
USA
b,c Dr W.K. Anger, Neurobehavioral Research Section,
Division of Biomedical and Behavioral Science,
National Institute for Occupational Safety and
Health, Cincinatti, Ohio, USA
b,c Dr G. Bignami, Section of Neurobehavioural
Pathophysiology, Laboratory of Organ and System
Pathophysiology, High Institute of Health, Rome,
Italy
b Dr T.J. Bonashevskaya, Sysin Institute of General and
Community Hygiene, Moscow, USSR
b Dr E. Bonilla, Institute of Clinical Research, Faculty
of Medicine, University of Zulia, Maracaibo,
Venezuela
b,c Professor J. Cavanagh, West Norwood, London, England
(Rapporteurb,c)
b Dr V.A. Colotla, National Autonomous University, City
University, Coyoacan, Mexico
b Dr I. Desi, Division of Toxicology, National Institute
of Hygiene, Budapest, Hungary
a Dr L. Di Giamberardino, Département de Biologie,
Commissariat à l'Energie Atomique, Centre d'Etudes
Nucléaùres de Saclay, Gif-sur-Yvette, France
b Dr S. Frankova, Institute of Psychology, Czechoslovak
Academy of Sciences, Prague, Czechoslovakia
b Dr E. Frantik, Institute of Hygiene and Epidemiology,
Prague, Czechoslovakia
c Dr. I. Goto, Department of Neurology, Neurological
Institute, Faculty of Medicine, Kyushu University,
Higashiku, Fukuoka, Japan
b,c Professor Dr M. Hasan, Brain Research Centre, J.N.
Medical College, Aligharh Muslim University,
Aligharh, India
b Dr L. Hinkova, Institute of Hygiene and Occupational
Health, Sofia, Bulgaria
a,b,c Associate Professor Dr M. Horvath, Institute of Hygiene
and Epidemiology, Prague, Czechoslovakia
(Vice-Chairmanc)
c Professor A. Korczyn, Neurology Department, Tel Aviv
Medical Centre, Tel Aviv University, Tel Aviv,
Israel
b Dr N.N. Litvinov, Sysin Institute of General and
Community Hygiene, Moscow, USSR
a,b Professor R.V. Merkureva, Department of Medical
Biological Research, Sysin Institute of General and
Community Hygiene, Moscow, USSR
(Vice-Chairmana)
a,b,c Dr C. Mitchell, Laboratory of Behavioural and
Neurological Toxicology, National Institute of
Environmental Health Sciences, Research Triangle
Park, North Carolina, USA (Chairmana,b,c)
b,c Professor J.E. Murad, Medical School and Chairman, Al.
Ezequiel Dins, 275, Drugs Orientation Centre, Belo
Horizonte, Brazil
b,c Professor O.B. Osuntokun, Department of Medicine,
University of Ibadan, WHO Collaborating Centre for
Research and Training in Neurosciences, University
of Ibadan, Nigeria
a,b,c Dr L. Reiter, Division of Neurotoxicology (MD-74B), US
Environmental Protection Agency, Health Effects
Research Laboratory, Research Triangle Park, North
Carolina, USA
b Dr D.C. Rice, Toxicology Research Division, Food
Directorate, Health Protection Branch, Department
of National Health and Welfare, Tunney's Pasture,
Ottawa, Ontario, Canada
b,c Dr M. Rudnev, Kiev Research Institute of General and
Communal Hygiene, Kiev, USSR
c Dr M. Ruscak, Centre of Physiological Sciences, Slovak
Academy of Sciences, Bratislava, Czechoslovakia
a Dr H. Savolainen, Institute of Occupational Health,
Helsinki, Finland (Rapporteur)
b,c Professor V. Schreiber, Laboratory for Endodrinology,
Faculty of Medicine, Charles University, Prague,
Czechoslovakia
b,c Professor P. Spencer, Institute of Neurotoxicology,
Albert Einstein College of Medicine, New York, USA
b Dr T. Tanimura, Department of Anatomy, Kinki University
School of Medicine, Osaka, Japan (invited, but
could not attend)
b,c Dr H. Tilson, Laboratory of Behavioural and
Neurological Toxicology, National Institute of
Environmental Health Sciences, Research Triangle
Park, North Carolina, USA
b,c Dr L. Uphouse, Department of Biology, Texas Woman's
University, Denton, Texas, USA
b,c Dr T. Vergieva, Department of Toxicology, Institute of
Hygiene and Occupational Health, Sofia, Bulgaria
c Professor D. Warburton, Psychopharmacology Group, Early,
Reading, United Kingdom
a,b Dr G. Winneke, Institute of Environmental Hygiene,
University of Düsseldorf, Düsseldorf, Federal
Republic of Germany
b Dr V.G. Zilov, I.M. Sechnov Medical Institute,
Department of Normal Physiology, Moscow, USSR
(Vice-Chairmanb)
Secretariat
c Dr G. Becking, International Programme on Chemical
Safety, World Health Organization, Research
Triangle Park, North Carolina, USA
a Dr C.L. Bolis, Neurosciences Programme, World Health
Organization, Geneva, Switzerland
a Dr A. David, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
a,b,c Dr M. Draper, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland
b Dr M. Gounar, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland
a Mr E. Hellen, Occupational Safety and Health Branch,
International Labour Organisation, Geneva,
Switzerland
a Dr A.I. Koutcherenko, International Register for
Potentially Toxic Chemicals, United Nations
Environment Programme, Geneva, Switzerland
a Dr M. Mercier, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland
b Dr L.A. Moustafa, International Programme on Chemical
Safety, World Health Organization, Research
Triangle Park, North Carolina, USA
a Dr J. Parizek, International Programme on Chemical
Safety, World Health Organization, Geneva,
Switzerland (Secretary)
a Dr J. Purswell, Section on Hygiene, International Labour
Organisation, Geneva, Switzerland
a Dr C. Satkunananthan, International Programme on
Chemical Safety, World Health Organization, Geneva,
Switzerland (Temporary Adviser)
a Dr C. Xintaras, Office of Occupational Health, World
Health Organization, Geneva, Switzerland
a Preparatory consultation, Geneva, 14-16 September 1981.
b First Task Group meeting, Moscow, 1-7 June 1983.
c Second Task Group meeting, Prague, 17-21 September 1984.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
PREFACE
The need for generally accepted scientific principles and
requirements in all areas of toxicology particularly applies to the
newly developed field of neurotoxicology. Methods continue to be
developed in isolation, and the comparability of results is often in
doubt. Furthermore, until scientific principles have been agreed on,
internationally accepted strategies to test the effects of chemicals
on the many functions of the mammalian nervous system will not be
developed. At the suggestion of the two participating institutions
(Sysin Institute of General and Community Hygiene, USSR, and the
National Institute of Environmental Health Sciences, USA), the
International Programme on Chemical Safety (IPCS) undertook a study of
the principles and methods used to study the effects of chemicals on
the nervous system (neurotoxicology) in order to lay the foundations
for further developments in this important area of toxicology. The
publication of the results of the study in a monograph seemed the most
effective means of achieving this goal.
Members of an International Task Group of experts from 18
countries generously devoted much time to the preparation of the
monograph, and the IPCS wishes to express its deep gratitude to all
the members of the Task Group for their efforts.
The scope and plans for the development of the monograph were
discussed at a consultation, chaired by Dr C.L. Mitchell with Dr R.V.
Merkureva as Vice-Chairman. Representatives from the IPCS institutions
that had expressed a particular interest in the assessment of
neurotoxic effects of chemicals attended the meeting at the invitation
of the Manager, IPCS, Dr M. Mercier. The following institutions were
represented: National Institute of Environmental Health Sciences
(NIEHS), Research Triangle Park, North Carolina, USA; Sysin Institute,
Academy of Medical Sciences, Moscow, USSR; Institute of Hygiene and
Epidemiology, Czechoslovak Academy of Sciences, Prague,
Czechoslovakia; US Environmental Protection Agency (US EPA), Health
Effects Research Laboratories, Research Triangle Park, North Carolina,
USA; Atomic Energy Commission, Department of Biology, Gif-sur-Yvette,
France; Institute of Occupational Health, Helsinki, Finland; and
Institute of Environmental Hygiene, University of Düsseldorf, Federal
Republic of Germany. It was agreed that nine background papers would
be used as the basis for the preparation of sections in a monograph
reviewing the principles and methods for the evaluation of effects on
the nervous system associated with exposure to chemicals.
On 1-7 June 1983, the first meeting of the Task Group was convened
in Moscow, hosted by the USSR Ministry of Health, the USSR Commission
for UNEP, and the Centre for International Projects, GKNT. The Sysin
Institute of General and Community Hygiene collaborated in the
preparations for this meeting. Dr S.N. Bajbakov, Director, Centre of
International Projects, GKNT, formally opened the meeting and Dr A.M.
Pisarev, USSR Ministry of Health and Dr N.N. Litvinov of the Sysin
Institute added greetings to the Task Group. It was agreed that
Dr C.L. Mitchell would be Chairman and Dr V.G. Zilov, Vice-Chairman.
Dr J.B. Cavanagh was asked to be Rapporteur of the meeting.
The scope and content of ten background papers made available by
the Secretariat were discussed thoroughly by the experts, and
agreement was reached on the appropriate sections needed. After a
detailed discussion on the issues to be addressed in each section, the
meeting divided into five working groups to draft the texts for the
following sections: General Principles, Morphology/Pathology,
Biochemistry, Neuroendocrinology, Neurophysiology, and Behaviour. At
the end of the meeting, most of the text was completed, and Dr
Mitchell was asked to act as an overall editor to assist the Working
Group leaders in completing their sections.
A revised text was submitted to the Task Group before its second
meeting, held in Prague on 17-21 September 1984. Dr C.L. Mitchell was
again asked to be Chairman and Dr M. Horvath, Vice-chairman.
Dr J. Cavanagh was appointed Rapporteur. The meeting was hosted by the
Ministry of Health of the Czech Socialist Republic, Prague, and
Professor Dr D. Zuskova welcomed the experts on behalf of the Minister
of Health. The final text for the document was agreed upon by the end
of the meeting. Dr Mitchell was asked to continue as overall editor to
ensure the incorporation of all material discussed at the meeting,
including the appropriate references.
The International Programme on Chemical Safety wishes to
acknowledge the valuable help provided by many scientists who were not
members of the Task Group, and, in particular, that of Dr R. Dyer
(Neurophysiology), Dr R. McPhail and the late Dr P. Ruppert
(Behaviour) of the US EPA, and Dr T. Damstra (Neurochemistry) of the
National Institute of Environmental Health Sciences.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of Health
and Human Services, through a contract from the National Institute of
Environmental Health Sciences, Research Triangle Park, North Carolina,
USA -- a WHO Collaborating Centre for Environmental Health Effects.
The United Kingdom Department of Health and Social Security generously
covered the costs of printing.
1. INTRODUCTION
Interest in nervous system toxicology has been growing in recent
years, not only because of increased public concern over the impact of
toxic agents on human health and the quality of life, but also because
the nervous system has been shown to be particularly vulnerable to
chemical insult. Thus, there has been an increased demand for improved
methods for the detection of neurotoxic effects and the assessment of
health risks within the field of occupational and environmental health
and safety research related to the science known as "Neurotoxicology"
(Spencer & Schaumburg, 1980; O'Donoghue, 1985).
Neurotoxicology includes studies on the actions of chemical,
biological, and certain physical agents that produce adverse effects
on the nervous system and/or behaviour during development and at
maturity. Toxic disorders of the nervous system of human beings and
animals may occur following abuse of such substances as ethanol,
inhalants, narcotics, therapeutic drugs, products or components of
living organisms (e.g., bacteria, fungi, plants, animals), chemicals
designed to affect certain organisms (e.g., pest-control products),
industrial chemicals, chemical warfare agents, additives and natural
components of food, raw materials for perfumes, and certain other
types of chemicals encountered in the environment.
This document is intended to aid in the design and assessment of
studies concerned with exploring the association between exposure to
chemicals and the development of adverse neurobehavioural changes. The
emphasis is on animals as systems to model and predict adverse
reactions of the human nervous system to exogenous chemicals.
1.1 The Importance of Studying the Nervous System
The brain is an extremely complex organ, the function of which is
to receive and integrate signals and then to respond appropriately, to
maintain bodily functions. It supports a diversity of complex
processes including cognition, awareness, memory, and language. Sexual
behaviour, locomotion, and the use of a vast array of tools ranging
from the slingshot to the microcomputer, suggest the range of
responses available to the human organism. Moreover, the nervous
system is influenced by the functioning of other organ systems (e.g.,
hepatic, cardiovascular, and endocrine systems). Thus, toxicant-
induced alterations in any of these organ systems can be reflected in
changes in neurobehavioural output. This fact alone suggests that
nervous system function should be among the first to be thoroughly
assessed in cases of exposure to known or potentially hazardous
agents.
Major outbreaks of neurotoxicity in human populations of various
sizes have emphasized the importance of neurotoxicology as an
independent discipline. Poisoning episodes have resulted from
exposures in the environment (e.g., methylmercury, lead), at the work-
place (e.g., hexanes, carbon disulfide, leptophos, chlordecone), as
well as from food toxins (cassava) and food contaminants
(triorthocresyl phosphate (TOCP)). There are numerous sources of
reference dealing with chemicals reported to produce behavioural and
neurological effects in human beings and laboratory animals including
Horvath (1973), Xintaras et al. (1974), Weiss & Laties (1975), Spencer
& Schaumburg (1980), and O'Donoghue (1985). These references should be
consulted by the reader unfamiliar with this area of toxicology.
At present, it is not possible to give a precise estimate of the
number of chemicals that exert behavioural or neurotoxic effects.
Anger & Johnson (1985) list more than 850 chemicals that have been
reported to produce such effects. Anger (1984) states that, of the 588
chemicals listed in the 1982 edition of the American Conference of
Governmental Industrial Hygienists publication "Threshold Limit Values
for Chemical Substances and Physical Agents in the Workroom
Environment", 167 (29%) "have threshold limit values (recommended
exposure maxima) based, at least in part, on direct neurological or
behavioural effects, or on factors associated with the nervous system
(viz., cholinesterase inhibition)." The neurotoxic properties of
chemicals have been identified because of the conspicuous nature of
severe signs and symptoms. It is not known how often insidious
problems of neurotoxicity may lie undetected because the effects are
incorrectly attributed to other conditions (e.g., advancing age, mood
disorders) or misdiagnosed. The early and incipient stages of
intoxications produced by environmental agents are frequently marked
by vagueness and ambiguity (Mello, 1975; US NAS, 1975), and many
complaints are subjective (e.g., fatigue, anxiety, irritability,
lethargy, headache, weakness, depression). Thus, the potential is
large for the occurrence of subtle, undetected effects, which
nonetheless have an important bearing on the quality of life.
The following examples illustrate the types of neurotoxic
outbreaks that have occurred and exemplify the usefulness of animal
models to further characterize this neurotoxicity.
1.1.1 Methylmercury
The neurotoxicity of mercury compounds has received world-wide
attention for centuries (Hunter et al., 1940). However, it was not
until the outbreak of methylmercury poisoning in Minamata, Japan in
the 1950s that the extreme toxicological consequences of human
exposure were fully recognized (Takeuchi, 1968). During this outbreak
of poisoning, thousands of inhabitants were exposed to methylmercury.
The source of exposure was found to be industrial effluent that
contained large amounts of mercury. The mercury made its way into
Minamata Bay where it was converted to methylmercury by marine biota.
This accumulated in fish and shellfish, which were eventually consumed
by the local residents. This consumption of contaminated seafood
continued for several years and resulted in hundreds of reported cases
of methylmercury poisoning. Since that time, methylmercury poisoning
has been referred to as "Minamata Disease".
A second major outbreak of methylmercury poisoning occurred in
Iraq during 1971-72 (Bakir et al., 1973). In this case, the source of
exposure was the ingestion of seed grain treated with a methylmercury
fungicide; the grain had been ground into flour to make bread.
Approximately 6500 people were hospitalized due to poisoning, and at
least 400 died.
The clinical manifestations of methylmercury poisoning are quite
extensive and include disturbances in sensory, motor, and cognitive
functions. The earliest complaints are associated with sensory loss
in the extremities, perioral numbness and concentric constriction of
the visual fields, followed by the development of ataxic gait,
dyarthria, incoordination, intention tremor, hearing problems, and
muscle weakness (Takeuchi et al., 1979). Mental disturbances, as well
as alterations in taste, smell, and salivation have also been reported
(Takeuchi, 1968; Chang, 1977, 1980; Reuhl & Chang, 1979, Takeuchi et
al., 1979).
The pattern of sensory neural damage in the monkey resembles that
of man. Visual system deficits in primates include constriction of
visual fields, deficit in scotopic (low light) vision (Evans et al.,
1975), and deficit in ability to detect flicker (Merigan, 1980).
Spatial visual function was impaired in monkeys dosed from birth (Rice
& Gilbert, 1982). Hypoesthesia (impaired sense of touch) has been
reported in the monkey (Evans et al., 1975) as well as man (Hunter et
al., 1940). Moreover, the body burden at which signs are observed in
primates is similar to that reported for human beings.
In rodents, neurobehavioural research on methylmercury has
concentrated primarily on an evaluation of the later motor effects,
but some of the sensory deficits have not been found (Shaw et al.,
1975, 1980; Evans et al., 1977). Several investigators have observed a
progressive weakness of the hindlimbs followed by decrease in forelimb
function (Diamond & Sleight, 1972; Klein et al., 1972; Herman et al.,
1973; Snyder & Braun, 1977; Ohi et al., 1978). Grip strength was
reported to be reduced in rats following long-term dosing with
methylmercury (Pryor et al., 1983). Decreased motor activity in mice
exposed to methylmercury in the drinking-water was reported by
MacDonald & Harbison (1977), but horizontally-directed motor activity
did not appear to be markedly affected by repeated exposure to
methylmercury (Salvaterra et al., 1973; Morganti et al., 1976).
Responsiveness to noxious stimuli was reportedly intact in
methylmercury-exposed rats, even in the presence of gross neuromotor
deficits (Herman et al., 1973; Salvaterra et al., 1973). Similarly,
Pryor et al. (1983) did not observe any significant alteration in
startle responsiveness to an acoustic stimulus in methylmercury-
exposed rats. Finally, in an attempt to investigate the effects of
mercury on development, prepubescent rats were exposed to a single
dose of methylmercury. Ability to learn an active avoidance response
at 70 days of age was impaired (Reuhl & Chang, 1979).
1.1.2 Carbon disulfide (CS2)
Carbon disulfide (CS2) is a volatile solvent that has been used
for a variety of industrial purposes. Since its discovery in 1776,
there have been numerous examples of CS2-induced neurotoxicity.
Many cases of human CS2 poisoning occurred in the viscose industry
during and after the second world war and consisted of various
neurological and behavioural effects. According to Braceland (1942),
the psychological effects consisted of personality changes,
irritability, memory deficits, insomnia, bad dreams, decreased libido,
and constant fatigue.
Paraesthesia and dysesthesia from CS2 exposure tend to occur in
a "stocking and glove" distribution characteristic of peripheral nerve
injury and indeed Seppalainen et al. (1972) reported a slowing of
motor conduction in peripheral nerves. Various sensory alterations
have been reported following CS2 exposure, particularly in vision.
Central field loss, disturbances in colour vision, enlargement of the
blind spot, and reduction in peripheral vision have been reported.
Alterations in auditory, vestibular, and olfactory senses have also
been reported (Wood, 1981).
While there have been few studies that have attempted to quantify
the sensory and psychological effects of CS2 in laboratory animals
(Wood, 1981), the neuropathological changes and effects on motor
behaviour have been extensively studied. CS2 produces filamentous
inclusions in axons in both the central and peripheral nervous
systems. Experimental CS2 neuropathy consisting of weakness or loss
of power in the limbs, depressed reflexes, and general behavioural
suppression has been reported in dogs (Lewey, 1941), rabbits
(Seppalainen & Linnoila, 1975), and rats (Frantik, 1970; Lukas, 1970).
Tilson et al. (1979b) reported decreases in grip strength and motor
activity in rats following CS2 exposure; Horvath (1973) found
decreases in motor activity in rats following exposure to high
concentrations of CS2, but motor activity increased following
exposure to low concentrations.
The cases illustrated above are unfortunately typical in that the
neurotoxicity of the chemicals was first discovered when human beings
exposed to them became ill. Later, research using animal models in a
controlled setting provided experimental evidence that the chemical
believed to have caused the illness in the human population produced
similar effects in animals. One purpose of this book is to provide the
background through which this process can be reversed; i.e., the
chemical can be tested in an animal model before human beings become
overexposed, seriously ill, or irreversibly harmed by it.
There are a few examples where this principle has been put into
practice. Some compounds have been discovered to be neurotoxic in
animals and, as a result, further human exposure has been
discontinued. Other chemicals, shown to be neurotoxic for animals,
have only later been discovered to produce comparable disorders in
human beings.
The former is illustrated by Musk Tetralin, a synthetic musk
introduced in 1955 as a raw material and as an artificial flavouring
substance. Apparently, the minimal acute toxicity testing in use at
that time suggested that the compound was acceptable for human
consumption. After 20 years of widespread use in the domestic
environment, a new set of long-term animal toxicity studies showed
that the substance was a potent neurotoxin inducing behavioural
changes and irreversible structural damage throughout the nervous
system of dermally- or orally-exposed animals (Spencer et al., 1980).
Two examples illustrate that experimental animal studies may
reveal the neurotoxic properties of substances before they are
discovered in human beings. One concerns aluminium, a substance that
was shown to induce central nervous system toxicity in animals as
early as 1886. Subsequent studies confirmed this, before the first
case of suspected human aluminum encephalopathy was reported in 1962.
With the introduction of aluminium-containing phosphate-binding gels
for the haemodialysis of individuals with kidney dysfunction, large
numbers of treated patients developed a progressive dementing illness
that often proved fatal (Crapper & DeBoni, 1980). Another recent
example of this phenomenon concerns the peripheral neurotoxicity of
megavitamin doses of pyridoxine phosphate (vitamin B6), recently
reported in human beings taking the drug in prescribed amounts for
therapeutic purposes (Schaumburg, 1982). The clinical neurological
syndrome had been reproduced in animals some 40 years previously and
would not have been recognized in human beings but for more recent
experimental animal work that had highlighted the surprising
neurotoxic potential of this essential nutrient (Krinke et al., 1981).
Both examples demonstrate the power of animal studies in
predicting human neurotoxicity and both illustrate the need for the
medical world to pay close attention to discoveries in experimental
neurotoxicology. With the further refinement and validation of methods
used in this discipline, it should be possible to discover many other
potentially harmful agents, presently in use, and to prevent new
compounds with neurotoxic properties from reaching the human
environment. Compounds that have been identified, through various
types of research, are listed in Table 1 according to important
nervous system targets.
Table 1. Illustrative manifestations of human neurotoxicity
Function affected Manifestation Chemical example
Sensorium change irritability carbon disulfide
apathy/lethargy carbon monoxide
attention difficulty anticholinesterases
illusions, delusions, ergot
hallucinations
dementia aluminium
depression, euphoria ozocerite
stupor, coma dicyclopentadiene
Sensory special Abnormalities of:
smell cadmium
vision methanol
taste selenium
hearing toluene
balance methyl nitrite
Somatosensory skin senses (e.g., trichloroethylene
numbness, pain) thallium
proprioception acrylamide
Motor muscle weakness:
paralysis, organophosphates
spasticity, Lathyrus sativus
rigidity methyl phenyl
tetrahydropyridine
tremor chlordecone
dystonia manganese
incoordination organomercury
hyperactivity lead
myoclonus toluene
fasciculatton anticholinesterases
cramps styrene
seizures, convulsions acetonitrile
Autonomic Abnormalities of:
sweating acrylamide
temperature control chlordane
gastrointestinal lead
function
appetite, body-weight, dinitrobenzene
cardiovascular control, vocor
urination dimethylamino-
propionitrile
sexual function ß-chloroprene
1.2 Need to Establish A Comprehensive Strategy for Neurotoxicity
Testing
The need to establish a comprehensive strategy for neurotoxicity
testing is made clear by estimates recently provided by the US EPA
Office of Toxic Substances. In the USA alone, between 40 000 and
60 000 chemicals are currently in commercial use; furthermore,
approximately 1000 new chemicals are introduced into commerce each
year (Reiter, 1980). It is not surprising that few or no toxicological
data exist for many of these chemicals. Therefore, any toxicity
testing strategy intended for general use must be flexible enough to
evaluate a wide variety of chemicals and chemical classes and must be
able to take into account the potential for chemical interaction, as
most people are exposed to combinations of chemicals. The
toxicological data that are available for a given chemical will
influence testing requirements for that chemical. For example, the
desired approach will depend on whether the investigation has been
initiated to evaluate the toxicity of the chemical prior to its
commercial use or to confirm reports of chemically-induced disease in
man.
Risk from exposure to a toxic substance is a function of both the
intrinsic toxicity of the chemical and the human exposure pattern.
These factors will influence where it enters the testing scheme; its
potency and the human exposure pattern will influence the extent to
which testing must be pursued. In both cases, steps must be
incorporated that facilitate decision-making about acceptance,
rejection, or continuation of testing. However, the eventual goal is
to understand the mechanism(s) by which chemicals adversely alter
nervous system function.
1.3 Scope of the Book
Emphasis in this book is placed on animal test data. An important
role of the toxicologist, of course, is to provide data that can be
used for quantitative estimation of the risks associated with exposure
of human populations to toxic chemicals. Even in extensively explored
areas such as carcinogenesis, risk assessment is an extremely
difficult undertaking for which there is no concise research strategy.
A major problem in dealing with most chemicals is that the available
toxicological data base concerning precise information on human health
effects is relatively sparse. The neurotoxicological data base is no
exception in this respect; in particular, adequate human behavioural
data are available for only a few chemicals (Anger & Johnson, 1985).
Furthermore, a number of experimental problems inherent in most
published human studies cloud interpretation of data from these
studies. Perhaps the most serious problem is that of adequately
defining the exposure level (dose) in human populations, a critical
factor in risk assessment. Other factors that influence behavioural
measures include sex, age, cultural variables, disease states, and
possible exposure to additional toxic substances (Johnson & Anger,
1983). As these critical variables cannot be adequately controlled in
human field studies, in all or even most cases, the neurotoxicologist
must rely to a great extent on animal test data to establish accurate
dose-effect relationships.
A word should be said about related and important areas excluded
from this publication. Developmental and human neurotoxicology are not
discussed in detail, since they have already been addressed in other
publications of the World Health Organization (WHO, 1984; WHO, in
preparation). The usefulness of tissue culture techniques will be
considered in a future publication. The closely related area of
psychopharmacology, which assesses the effects of drugs on the nervous
system, is not within the scope of this document and has been
addressed in other publications (CIOMS, 1983). However, many of the
methods identified in this manuscript have been used in this area.
The very important role played by the autonomic nervous system in
the regulation of many physiological processes is well recognized
(Dyck et al., 1984). However, it has not been possible to discuss in
any detail the principles of the methods available to study the
effects of chemicals on this system. A detailed discussion of such
methods will be presented in a WHO publication that is in preparation
and deals with methods for the assessment of toxicity from exposure to
chemicals.
1.4 Purpose of the Publication
Given the magnitude of the problem and the potential for subtle
damage to the nervous system, there is a pressing need to assess the
behavioural and neurological effects of the mass of chemicals found in
the work-place and the ambient environment.
The purpose of this book is to provide an overview of the
principles of neurobehavioural assessment and to identify methods that
have been successfully applied to the study of neurotoxicity in the
past. These methods, which may eventually be modified or supplemented
by other better methods, have been generally established and can be
relied on to provide an assessment of chemicals for their
neurotoxicity. The references for each method can be consulted for
further details.
The book is divided into five sections. The first deals with
principles of assessment, and the remaining sections deal with methods
in the four major research disciplines that contribute substantially
to the assessment of the neurotoxicity of chemicals found in the
occupational or community environment. Each discipline is not in a
comparable stage of development as it relates to neurotoxic assessment
of environmental chemicals. Behavioural and neurophysiological methods
have been extensively applied, but there is limited agreement on which
of the many methods described are the most appropriate for the initial
screening of an unknown chemical and, thus, the approach of tiered
testing is suggested or general guidelines are given for selecting
methods. On the other hand, the neuromorphological section presents a
relatively more methodological approach to the neuropathological
assessment of nervous tissue that has been validated in other areas of
research, recognizing that it is the experience of the
neuropathologist that is critical to an adequate assessment rather
than the test methods, as in the sections mentioned above. Finally,
the section on endocrinological and biochemical methods reflects the
fact that these methods have been applied far less to the neurotoxic
assessment of environmental contaminants. HoweVer, their inclusion is
important because of the critical role they play in exposure
monitoring and the elucidation of mechanisms.
2. GENERAL PRINCIPLES FOR THE ASSESSMENT OF TOXIC EFFECTS OF CHEMICALS
ON THE NERVOUS SYSTEM
2.1 Factors to be Considered in the Design of Neurotoxicity Studies
2.1.1 General considerations
Many factors must be taken into consideration with regard to any
toxicology study. These include the choice and number of animals,
dosage, route and duration of administration, metabolism and
pharmacokinetics, and testing procedures. These have been discussed in
detail elsewhere (US NAS/NRC, 1970; US NAS, 1975; WHO, 1978) and the
various reference sources should be consulted, as only aspects of
special relevance to neurotoxicology will be emphasized here.
The nervous system is protected from undesirable external
influences by both physical and chemical barriers. This protection,
however, is not complete. The blood-brain barrier has an important
function in preserving the chemical constitution of the nervous
system, but some noxious substances, particularly those that are lipid
soluble, may still cross it. Another mode of entry is by uptake into
the peripheral terminals of nerves, which may then transfer the
substances into their cell bodies in the central nervous system
through retrograde axonal flow. Such a mechanism operates for
substances as remote as tetanus toxin and some viruses. The peripheral
nervous system is, of course, more likely to be exposed to
neurotoxicity. The neurons of the autonomic nervous system and the
sensory ganglia are outside the blood-brain barrier, as are small
regions of the CNS, circumventricular organs (e.g., area postrema)
and, to a limited extent, the retina.
As might be expected, the nervous system may be particularly
vulnerable either during development or in senescence. Some aspects of
this have been alluded to elsewhere (WHO, 1984). Physical changes or
the presence of toxins may also disrupt the blood-brain barrier and,
thus, allow substances normally excluded from the brain to reach and
affect it adversely.
2.1.2 Objectives
The objectives of neurotoxicity testing are to:
(a) identify whether the nervous system is altered by the toxicant
(detection);
(b) characterize the nervous system alterations associated with
exposure;
(c) ascertain whether the nervous system is the primary target for
the chemical; and
(d) determine dose- and time-effect relationships aimed at
establishing a no-observed-adverse-effect level.
In a sense, these objectives translate into a series of questions
about the toxicity of a chemical, and achieving them requires
behavioural, neurophysiological, biochemical, and neuropathological
information.
When faced with a chemical for which no toxicological data are
available, the first question is whether the nervous system is or is
not affected by the chemical. This represents the most fundamental
level of investigation and entails procedures that "screen" for
neurotoxicity. Such tests must not only forecast the potential of a
substance to produce adverse effects, but must also be simple, rapid,
and economical to administer. Once a chemical is known to produce
neurotoxic effects, further studies must be performed in order to
characterize the nature and mechanism of the alterations. These
studies explore the consequences of toxicant exposure and give an
indication of whether or not the nervous system is the primary target
organ.
Many functions are mediated by unique neural substrates, and
chemicals may produce selective effects. Thus, it is important to use
a variety of tests that measure different functions, in order to
maximize the probability of detecting a toxic effect. It is clear that
the methods used may differ depending on the following factors:
(a) the objective of the study;
(b) the age of the animal; and
(c) the species examined.
If the objective of the study is to provide an initial evaluation of
the effect of a new substance on the nervous system, the methods used
may differ considerably from those used when a great deal is known
about a substance, and its mechanism of action is being investigated,
or environmental or occupational standards are being set for
acceptable levels in the biosphere. If the objective is to contribute
substantively to the overall toxic risk assessment for the chemical,
the methods used should attempt to model human disease states. Thus,
it is important that the purpose of the study is clear to both the
investigator and the evaluator. In many situations, the evaluation of
reference substances in the same protocols will help determine the
specificity and validity of the observed changes. It also makes it
possible to evaluate the relative potency of different chemicals
(Horvath & Frantik, 1973), and this is always essential for the novice
investigator or for the investigator using a new technique.
Although certain chemicals produce selective damage in the nervous
system, a more common finding is one of widespread damage and
disruption of a variety of functions. Ideally, characterization of
such generalized neurotoxicity by a variety of methods will establish
a profile of the disrupted functions.
Once a chemical has been identified as neurotoxic, the next
objective is to determine dose-effect and time-effect relationships.
One aim of these studies is to establish no-observed-adverse-effect
levels, but to prove that a certain dose produces no effect may
require a very large number of experimental animals (Dews, 1982). To
be useful in risk assessment, threshold determinations must be
obtained by the most sensitive tests available. For example, as the
toxic effects of methylmercury became known, studies using subhuman
primates began to focus on its effects on the visual system.
Alterations in visual function in monkeys following methylmercury
exposure has now been well documented using sophisticated visual
psychophysical techniques (Evans et al., 1975).
The question of how to define toxicity is of critical importance
for the ultimate goal of risk assessment and the establishment of
hygienic standards. Considerable controversy exists concerning what
constitutes an adverse effect in toxicology. According to one view,
any evidence of a behavioural or biological change is considered to be
an adverse effect. According to others, evidence is required of both
an irreversible decrement in the ability of the organism to maintain
homeostasis and/or an enhanced susceptibility to the deleterious
effects of other environmental influences. In this latter view,
differentiation between "nonadversive" and "adverse" effects requires
considerable knowledge of the importance of reversible changes and
subtle departures from "normal" behaviour, physiology, biochemistry,
and morphology in terms of the organism's overall economy of life,
ability to adapt to other stresses, and their possible effects on life
span (WHO, 1978). Real or potential risks to the nervous system are
difficult to assess because of its complexity. Some of the problems in
assessment are associated with the wide variations that can occur but
are still considered to be within the "normal" range. Some are
associated with the plasticity of the nervous system. Other problems
in assessment are related to incomplete understanding of what is being
measured by certain tests. It is clear, therefore, that no single test
will suffice to examine the functional capacity of the nervous system.
The above comments suggest tiered testing approaches, such as
those recommended in the section on behavioural methods, where a
variety of testing schemes, ranging from simple to complex, have been
proposed (Pavlenko, 1975; US NAS, 1975; Tilson & Cabe, 1978). Such
schemes typically begin with simple, rapid, inexpensive tests for
detecting the presence of neurobehavioural effects. The tests in
successive stages are designed to answer increasingly specific
questions about the toxicity of the chemical. Each stage should also
incorporate decision points as to whether the available information is
sufficient for determining the toxicity of the chemical. When combined
with estimates of potential exposure of human populations, this
information can provide a basis for evaluating the justification for
proceeding to the next level of testing. The advantage of the tiered
approach is that decisions are made at each level of testing and,
therefore, scarce resources are directed towards chemicals for which
the greatest hazard or risk potential exists.
Obviously, the amount of available information about the substance
will determine the level at which the chemical will enter this testing
scheme. Another inherent assumption is that a chemical's pattern of
use, in combination with its toxic potency, will be considered in any
decision about further testing. Testing requirements for a chemical
that is indigenous the environment and to which large segments of the
population are exposed will require extensive investigation leading,
ideally, to determination of the no-observed-adverse-effect level.
This information is extremely useful to governmental agencies
responsible for setting exposure or hygienic standards. On the other
hand, compounds that are being introduced into commerce and/or for
which the projected exposure is limited may require less testing.
2.1.3 Choice of animals
For obvious reasons of safety and ethics, it is necessary to use
animals in toxicity assessments. However, the extrapolation of animal
toxicological data to human beings is always tenuous and should be
carried out with caution. In preliminary mass screening of known or
suspected environmental toxicants, there are economic factors that
must be taken into account. It is also important that there be
adequate anatomical, physiological, pharmacological, and toxicological
data bases on the species chosen for study, so that meaningful
interpretations of effects can be made and appropriate hypotheses
about mechanisms and loci of action can be framed. For these reasons,
the mouse or rat is usually preferred in a preliminary screen, though
the rodent differs from man in many significant ways. For more
detailed studies, other species may provide a more appropriate model.
For example, the adult chicken is the animal of choice to test
organophosphate-induced delayed neurotoxicity (Abou-Donia, 1981).
Other variables, besides species, must also be considered. One of
these is the strain of animal used. For example, it has been
demonstrated that rats inbred from the Fischer strain are
behaviourally different from Zivic-Miller rats, which are derived from
the Sprague Dawley strain (Barrett et al., 1973; Ray & Barrett, 1975).
Rats of the Fischer strain rapidly learn both where and when to run in
a discriminated Y-maze avoidance task, whereas Sprague Dawley rats
eventually learn where to escape but not when to avoid shock. The
administration of amphetamine produces a dramatic improvement in the
avoidance response of Sprague Dawley rats, whereas little or no
behavioural facilitation is observed in the Fischer strain of rat
(Barrett et al., 1974). Festing (1979) has reviewed the properties of
isogenic and nonisogenic stocks and their relation to toxicity
testing.
Since an environmental agent may have a selective effect on either
the male or female, gender cannot be ignored when assessing
neurotoxicity. For example, sex differences have been seen for the
toxic effects of polychlorinated hydrocarbons (Lamartiniere et al.,
1979); gonadal hormones influence the biotransformation and toxicity
of DDT (Durham et al., 1956) and parathion (Agrawal et al., 1982).
Another important factor is the age of the animal. It is well
known that the effects of a toxic agent may vary dramatically
depending on the stage of maturation of the animal (Damstra & Bondy,
1982; Hunt et al., 1982). For example, it has been established that
young animals of otherwise sensitive species are not susceptible to
organophosphate-induced delayed neurotoxicity (Abou-Donia, 1981). It
has been suggested that, under conditions where exposure may occur
pre- or perinatally, animals of both sexes should be tested at all
stages of maturation (Spyker, 1975).
Each of these considerations relates to extrapolation, a subject
discussed in detail in WHO (1978). Quantitative and qualitative
differences in sensitivity to, and body distribution of, chemicals
affect extrapolation significantly. A better understanding of
structure-activity relationships, pharmacokinetics, and mechanisms of
toxicity will facilitate cross-species extrapolation (Dixon, 1976).
2.1.4 Dosing regimen
Some compounds produce toxic effects following a single exposure
(e.g., trimethyltin, organophosphates); for others, cumulative effects
follow prolonged or repeated exposure (e.g., acrylamide). In
environmental toxicology, the detection of cumulative toxicity
following continued (or intermittent) exposure is a major goal. Thus,
a multiple-dosing regimen is most frequently used. It is important to
assess the toxicity at various intervals, since both quantitative and
qualitative changes in the response to environmental factors can occur
on repeated exposure, or even with time following a single exposure
(Evans & Weiss, 1978). Assessments should be made for some time
following cessation of the dosing regimen, since it is of interest to
determine the reversibility of any effects noted during the dosing
phase and to note any post-dosing effects.
2.1.5 Functional reserve and adaptation
Functional reserve is the excess capacity possessed by the nervous
system. Thus, a portion of the nervous system can be damaged, and this
damage can go undetected by the usual functional tests. The situation
in which a change in function was observed at one time, but can no
longer be detected by the usual functional tests, is referred to as
adaptation and presumably reflects compensatory processes.
If a part of a redundant system is damaged, it is reasonable to
assume that the reserve potential has been reduced. If compensatory
changes have occurred, the ability of a system to make further
compensatory changes may also have been reduced. One way to assess
such changes is to incorporate in the test procedures one or more
conditions in which the system(s) or organism(s) are placed under
stress. The combination of the test substance plus stress may result
in a greater deficit in performance than can be seen in animals
receiving either the stress or the toxicant only. Examples of
stressors that have been used are pharmacological changes such as
ethanol, muscular or work stress, exposure to cold, or auditory and
electrical stimuli (Lehrer, 1974; Pavlenko, 1975); it is expected that
other powerful stressors include those referred to as psychological or
psychic.
2.1.6 Other factors
Several additional factors should be carefully considered in
designing neurotoxicological tests. One condition that may affect
toxicity is the nutritional state of the animal. Changes attributed to
exposure to toxicants might be due to relatively nonspecific effects
related to inhibition of growth or decreases in food or water
consumption. This is particularly true in studies involving developing
organisms.
Another variable is the housing conditions of the experimental
animal. In some cases, animals are housed individually in home cages
during pharmacological or toxicological studies. This arrangement can
alter the responsiveness of the subjects to drugs. Pirch & Rech (1968)
found that alpha-methyltyrosine, a depletor of brain norepinephrine
and dopamine, produced less depression of motor activity and rotorod
performance in rats isolated for 34 days than in grouped rats. The
potentiating effects of crowding on the toxicity of amphetamine in
mice are also well known (Chance, 1946).
It has been well established that numerous biological systems,
ranging from metabolic pathways to behaviour of the whole organism,
exhibit rhythmic changes in amplitude (Scheving et al., 1974). Classes
of behaviour that show circadian rhythms include feeding, drinking,
sleeping, motor activity, and mating (Rusak & Zucker, 1975). There is
growing literature on how biological rhythms influence the
pharmacological and toxicological response to chemicals (Reiter &
MacPhail, 1982). These biological rhythms cannot be ignored and must
be either controlled for in the study or studied explicitly.
2.2 Statistical Analysis
2.2.1 Type I and Type II errors
All fields of biological research have at least one feature in
common: inherent variability in their data. When there is considerable
variation in the experimental material and when it is not feasible to
examine the entire population, the research worker is forced to give a
probability statement concerning any treatment differences observed.
In order to do this, it is necessary for the study to be designed in
such a way that statistical analysis of the data will yield a valid
answer to the question, "What is the likelihood that the differences
observed could have occurred by chance?" Thus, a null hypothesis is
set up, i.e., a statement that there is no difference between the
parameters or the distributions being estimated by the samples. Taking
the simplest case (e.g., Student's t-test), the null hypothesis is
that there is no difference between the mean values for 2 populations
(e.g., control versus treated). When the null hypothesis is accepted,
this may be either right or wrong. A Type I error (false positive) is
made when the null hypothesis is rejected and is, in fact, true. A
Type II error (false negative) is made when the null hypothesis is
accepted and is, in fact, false.
The probability of making a Type I error is called alpha and is
fixed before the study is carried out. If something is statistically
significant at P = 0.05, this means that the probability that these
results could have occurred by chance is 0.05. The inference is that
there is a "real" difference, but this can be wrong, and, in fact,
there is a one in twenty chance that it is.
There are two characteristics about neurotoxicology that make it
highly likely that one or more Type I errors may occur in any given
study. These are (a) the use of multiple tests (measuring multiple
parameters), and (b) repeated measurements using the same animal in
the same test. Multiple tests are used because of the complexity of
the nervous system and the need to assess sensory and motor function
as well as more complex behaviour such as discrimination and learning
processes. Repeated testing is done because changes in the response to
agents can occur on repeated exposure, or even with time following a
single exposure (Evans & Weiss, 1978). Thus, in any given study, there
are a number of statistical tests of hypotheses. The greater the
number of statistical tests of hypotheses, the higher the probability
of obtaining Type I errors (false positives). Although problems
created by multiple comparisons can be dealt with statistically, to a
certain extent, it is imperative to look at the internal consistency
of the data and not simply at the presence or absence of statistical
significance. Nothing, of course, can take the place of a well-
designed study with a clear statement of its purpose. In any case,
when in doubt, repetition of the study is in order, if it is
sufficiently important.
Variable data can increase the probability of a Type II error
(false negative). A Type II error is made when the null hypothesis is
accepted and is, in fact, false. The probability of making such an
error is called Beta. The value of Beta is seldom, if ever, known. Its
relative magnitude can be approximated and depends on:
(a) the distance between the population parameters being estimated
by the samples (population means in the case of Student's t);
(b) the value selected for alpha (the probability of making a
Type I error or rejecting the null hypothesis when it is, in
fact, true); and
(c) the sample size.
The smaller the distance between the population parameters, the larger
will be beta. Beta varies inversely with alpha, and both decrease as
sample size increases. Thus, if the data are highly variable, a large
sample size is needed to detect a small effect. In selecting sample
size, these factors must be taken into consideration. When the sample
size is determined, the size of the difference that is detectable has
also been determined. The smaller the sample size, the larger the
change has to be in order to be statistically significant. Techniques
are available that identify the sample size needed to detect either a
given incidence of occurrence (power function) or a given change in
magnitude on the basis of an estimate of the variability in the
population(s). Their use is strongly urged. Too many studies have been
conducted with sample sizes that were not adequate to detect any real
but subtle effect. This topic has been discussed in detail by Dews
(1982).
Unfortunately, it is not possible to increase the sample size at
will. Therefore, other means must be used in an attempt to reveal
individual sensitivity to a given toxicant. In addition to statistical
analysis, the raw data should be examined (this is always true,
regardless of the results). If any trends exist, it may be necessary
to repeat the study. Alternatively, the data may be examined for a
change in variability, since a common observation of near-threshold
doses of environmental toxicants seems to be an increase in the
variability of the data (Evans & Weiss, 1978). This increase in
variability is a clue that certain individuals in the population are
being affected differentially. Moreover, statistical procedures exist
for comparing the degree of variability between control and treated
groups (e.g., Steel & Torrie, 1960).
2.2.2 Selection of the appropriate statistical test(s)
In the selection of statistical test(s), it is essential for the
investigator to:
(a) be able to choose a technique that is appropriate for the data
and hypothesis;
(b) understand the assumptions being made when carrying out the
statistical test;
(c) be able to execute the procedure correctly;
(d) be able to interpret the results correctly; and
(e) recognize the existence of controversies or differences of
opinion in any of the above areas (Bennett & Bowers, 1976).
Clearly, it is beyond the scope of this section to discuss these at
length. Similarly, it should be clear that to abide by these
principles, frequent and close consultation with a biostatistican may
be necessary. Moreover, such consultation should occur before the
study is conducted, not afterwards. Consulted beforehand, the
statistician can give the guidance necessary for the proper design of
the study. Once the study is completed, statistical manipulation
cannot compensate for an ill-conceived experimental design.
Different statistical techniques are based on different
assumptions, either with respect to the nature of the data, their
distribution, or both. Also, the power of different techniques
differs. In statistics, power refers to the ability of a test to
detect the alternative hypothesis (i.e., that there is a difference)
when it is true. Given two tests and a particular level of alpha
(e.g., P = 0.05), the test with the greater power will detect a
significant difference with a smaller sample size compared with the
test having lesser power, providing that the assumptions underlying
the tests are true. Non-parametric alternatives are available in most
cases where the assumptions of parametric tests are not valid. Gad &
Wiel (1982) present a "statistical testing decision tree" for
selecting the most appropriate test based on whether or not the data
are quantitative (continuous) or qualitative (discontinuous) in nature
and/or normally distributed. Gad (1982) has also examined the
statistical tests most commonly used in behavioural toxicology for
different types of observations (data) and suggests procedures that
are more appropriate.
Although much more could be said about statistics, it is hoped
that the above comments will serve as a warning that the prudent
investigator should become facile with experimental design and
statistics or work together with a biostatistician, or both.
3. TEST METHODS IN BEHAVIOURAL TOXICOLOGY
3.1 Introduction
Behaviour has been used to study the adverse effects of chemical
and/or physical agents on intact organisms. Behavioural toxicology
draws on the fields of experimental psychology, behavioural
pharmacology, and behavioural brain research.
Behavioural toxicology plays an important role within the broader
field of neurotoxicology for two reasons. The first is that the
behaviour of an organism is important in itself. As mentioned in the
introductory chapter, the nervous system (and consequently behaviour)
is influenced by the functioning of other organ systems. Thus,
regardless of whether the site of action is the nervous system or some
other organ system, toxicant-induced changes in performance,
sensorimotor function, or cognitive function adversely affect the
organism and its ability to interact with its environment. The second
reason is that behaviour is the final product of nervous system
activity and, therefore, toxicant-induced changes in the nervous
system may be reflected by behavioural changes. Thus, behavioural
analysis serves as a useful tool for measuring neurotoxicity (i.e.,
the direct action of a chemical on neural tissue). This approach can
be compared to measuring blood flow or cardiac output as an index of
cardiovascular toxicity (i.e., the use of functional measures to
evaluate the status of a target organ).
This section deals with the use of animal behavioural testing to
estimate neurotoxicity in human beings. It should be noted that
Citovic (1930), a student of Pavlov, reported the use of conditioned
reflexes to study the neurotoxic effects of gasoline and acetone.
Soviet and Eastern European toxicologists have commonly incorporated
behavioural testing in their studies. However, scientists in Canada,
western Europe, and the USA placed heavy emphasis in chemical toxicity
studies on defining pathological changes following exposure, and it
was not until 1969 that the Annual Review of Pharmacology in the USA
included a section on behavioural toxicology (Weiss & Laties, 1969).
Since then, behavioural toxicology has been the subject of numerous
books, symposia, and reviews (Anger & Johnson, 1985). Thus, it is a
relatively new discipline, and its specific methodology continues to
evolve rapidly. In this section, the major emphasis is placed on the
basic principles of behavioural toxicity testing. General approaches
are discussed in relation to their use in a comprehensive test
strategy for evaluating behavioural toxicity. As discussed later, any
such strategy should include tests that adequately evaluate each of
the 5 main functional categories listed in Table 1 (p. 17). Strengths
and weaknesses of existing methods are also considered, together with
future methodological directions and needs.
3.2 Classes of Behaviour
In the experimental analysis of behaviour, the primary focus is on
defining the functional relationships between an organism's behaviour
and its environment, i.e., in this context, everything that has an
effect on the organism. Behaviour is a dynamic process, since it
reflects changes in the interaction of an organism with its
environment. Thus, an important feature of behavioural toxicology is
that the effects of a toxic agent may depend largely on environmental
circumstances. In other words, with a given toxic effect on the
nervous system, the observed behavioural effects may (and probably
will) depend on environmental factors.
The basic units of behaviour are termed responses. Aspects of the
external or internal environment that affect behaviour are termed
stimuli. Behavioural responses have been typically divided into two
classes based on the functional relations that control their
occurrence. One class of behaviour is controlled mainly or exclusively
by the prior occurrence of an event (stimulus) in the environment.
Such responses are referred to as elicited or respondent behaviour.
The events are called eliciting stimuli, and the responses are called
respondents. The other class of behaviour is controlled mainly or
exclusively by its consequences and is referred to as operant or
emitted behaviour. Behaviour may be either unconditioned (unlearned)
or conditioned (learned). Conditioning refers to the modification of a
response that results from an organism's interaction with its
environment. In general, however, when a response is said to have been
conditioned, this usually implies that the conditioning was done
explicitly as part of an experimental procedure rather than as a
result of some other experience the organism encountered.
Descriptions of conditioning as either "operant" or "respondent"
can be confusing, in that behaviour is also characterized by these
terms. The issue becomes clearer if it is realized that operant and
respondent conditioning are operationally defined procedures of
behavioural modification, whereas operant and respondent responses are
descriptions of two classes of behaviours, both of which are
modifiable through conditioning.
3.2.1 Respondent behaviour
Respondent behaviours are those that are reliably elicited by a
specific observable stimulus. Two major features of a respondent
behaviour are: (a) its occurrence depends on the frequency of
occurrence of the eliciting stimulus; and (b) its consequences do not
affect its frequency, or affects it only to a minor extent.
Respondents frequently take the form of simple or complex reflexes
and typically involve smooth muscles, glandular secretion, autonomic
responses, or environmentally-elicited effector responses. Examples
are the auditory startle response (Hoffman & Fleshler, 1963),
olfactory responses, such as homing behaviour (Gregory & Pfaff, 1971),
visually guided responses, such as optokinetic nystagmus (King &
Vestal, 1974), and responses elicited by somaesthetic cues, such as
negative geotaxis (Alder & Zbinden, 1977).
Respondent behaviour can be quantified in a number of ways,
including: (a) latency of the response from onset of the stimulus;
(b) stimulus intensity required to elicit the response (threshold);
and (c) magnitude of the response. Response magnitude can be measured
either directly (e.g., force, duration) or by the use of rating scales
(Irwin, 1968). A respondent generally occurs in close temporal
contiguity with its eliciting stimulus, and its occurrence is
independent of stimulus parameters such as duration, intensity, and
frequency.
Various types of respondent behaviour, both unconditioned and
conditioned, have been used in behavioural toxicology (Irwin, 1968;
Pavlenko, 1975). Several features of unconditioned respondent
behaviour argue for its use. Perhaps the most important features are
that: (a) stimulus-response relations are well defined and easily
controlled; and (b) such methods require no prior training of the
animal and, therefore, are easily and rapidly administered.
The use of unconditioned respondent behaviour has generally been
focused on 2 response classes:
(a) reflexes, in which the response is limited to a specific
effector system, such as skeletal muscle (motor response) or
smooth muscle (autonomic response); and
(b) taxis, in which the whole animal orients itself towards or
away from a particular stimulus.
Reflexes have been more extensively studied. An example is the
acoustic startle response, which occurs following an intense auditory
stimulus. A principle motor component of this response-forelimb
extension can be readily measured. The acoustic startle response has
been used widely in the study of drugs (Davis, 1980) and more recently
in the study of other neurotoxic substances (Reiter et al., 1980;
Squibb & Tilson, 1982).
Unconditioned respondent behaviour has also found specific
application in the study of effects of toxic substances on the
developing nervous system. Developmental profiles for many types of
unconditioned respondent behaviour have been well described for the
rat (Altman & Sudarshan, 1975) and mouse (Fox, 1965). Because normal
development of these responses is very closely timed, measurements of
their developmental time-course have been widely used as an index of
nervous system development. A number of investigators have used
measurement of this behaviour as an index of toxicity (Rodier, 1978;
Butcher & Vorhees, 1979).
Respondent behaviour can also be conditioned using the classical
techniques initially described by Pavlov (1927). This involves the
pairing of a previously neutral stimulus (one that does not normally
elicit a response) with an eliciting stimulus. Through repeated
pairings, the neutral (conditioned) stimulus comes to elicit a
conditioned response. Respondent conditioning is illustrated in the
following example: when food (unconditioned stimulus) is placed in a
dog's mouth, it elicits salivation (unconditioned response). If a tone
(conditioned stimulus) is sounded just before food is placed in the
dog's mouth, the tone itself eventually comes to elicit salivation
(conditioned response) in the absence of food presentation. Although a
variety of different controlling stimuli can be used in classical
conditioning, responses are limited to those for which there is an
initial unconditioned eliciting stimulus. The conditioned response can
be quantified in terms of its latency, magnitude, and frequency of
occurrence. If the conditioned stimulus occurs repeatedly in the
absence of the unconditioned stimulus, the conditioned response
becomes progressively weaker and eventually disappears; this process
is called extinction.
Since movement is the basic measurement of behaviour, the majority
of conditioned reflex procedures evaluate motor responses. The
acquisition of a conditioned response can be studied by gradually
producing it. This requires careful control over external conditions,
particularly the intensity and timing of both the conditioned and
unconditioned stimuli. Since the rate of acquisition of a conditioned
response depends on the functional status of the nervous system, such
procedures are useful in the study of behavioural toxicity.
Pavlenko (1975) reviewed the extensive use of conditioning by
behavioural toxicologists in the USSR. Classical conditioning methods
were divided into 2 groups. The first group, "defence-motor reflexes",
generally involved a motor response to an electrical stimulation
applied to the skin. The second group, "alimentary-motor reflexes",
generally involved the response of a hungry animal to the presentation
of food. Soviet investigators have frequently studied the rate of
acquisition of such conditioned responses in the presence of toxic
substances. According to Pavlenko (1975), small dosages of toxic
agents more readily affect the acquisition of conditioned responses
than the performance of firmly established conditioned responses
(Shandala et al., 1980).
3.2.2 Operant behaviour
Behaviour that appears to occur in the absence of an eliciting
stimulus is referred to as an emitted or operant response. Operant
responses are movements of the organism that operate on (or change)
the environment. Although these responses may occur in the presence of
many environmental stimuli, they are not readily associated with an
identifiable eliciting stimulus, and their occurrence is controlled
mainly by their consequences. However, some responses are known to
include both respondent and operant components. The best-known example
is provided by bird pecks, which are controlled partly by eliciting
stimuli and partly by response consequences, apparently in relation to
their consummatory and non-consummatory functions, respectively.
Emitted behaviour generally occurs with a close temporal
relationship to the deprivation and presentation of particular
environmental conditions, regardless of whether the deprivation
produces an obvious physiological change. For example, an animal given
access to a novel environment will show a characteristic temporal
pattern of "exploratory" activity, with initial high levels of
activity diminishing to low levels. Availability of the novel
environment is associated with motor activity, but the novel
environment is not an eliciting stimulus. Under these conditions,
operant behaviour can be studied by observing all or part of the
animal's behaviour during a specified period of time.
Various rating scales have been used to quantify the frequency of
occurrence and/or magnitude of selected operant responses (Irwin,
1968). A more detailed approach has been to quantify the frequency,
duration, and temporal patterning of selected emitted responses using
time-lapse photographic analysis (Norton, 1973).
Operant or instrumental conditioning refers to the modification of
an operant behaviour by the control of its consequences. The following
example illustrates operant conditioning: a food-deprived rat is
placed in a chamber equipped with a food dispenser and with a lever
projecting from a wall. If depression of the lever is followed by
presentation of food, there is an increased likelihood that this
response (lever press) will occur again. That is, the consequences of
behaviour (e.g., receipt of food) come to control the occurrence of
the response (pressing the lever).
In contrast to classical conditioning, in operant conditioning,
mere temporal contiguity between stimulus events is not sufficient for
learning to take place and it is the consequences of behaviour that
control the learning process. This concept was first introduced by
Thorndike (1932) in his "Law of Effect," which dealt with the nature
of events that can control operant behaviour. When the occurrence of
an event following a response increases the probability that the
response will occur again, the event is termed a reinforcing stimulus
or reinforcer. The presentation of the reinforcing stimulus is termed
reinforcement. Events that serve as reinforcers when presented (e.g.,
food) are termed positive reinforcers; events that reinforce when
terminated (e.g., electric shock) are termed negative reinforcers. If,
on the other hand, the probability of occurrence of a response is
decreased by its consequences, the consequences are termed punishment.
An operant is defined as the properties of behaviour upon which
reinforcement (or punishment) is contingent. In the previous example,
for instance, pressing the lever is the operant. There are, of course,
many ways that the rat can press the lever during operant
conditioning. Nevertheless, each press is considered to belong to a
single response class: the operant.
Some response outputs, such as the bird pecks mentioned above, can
be modified by experience as a function both of stimulus-response
contingencies (so-called autoshaping) and response-reinforcement
contingencies. In other words, both classical and operant conditioning
can contribute to the changes observed in a particular response
output, and this may have to be taken into account when assessing
treatment effects.
One of the strong features of operant conditioning is the broad
range of behaviour that can be controlled and the new responses that
can be generated. In practice, the responses most commonly selected
for study meet four basic criteria: (a) they are easily identified and
readily counted; (b) they are easily recorded with automated
equipment; (c) their emission requires little time; and (d) they are
readily repeatable (Kelleher & Morse, 1968).
Reinforcement need not accompany every response in order to
maintain that response. More commonly, reinforcement occurs
intermittently and according to a schedule defining a sequential
and/or temporal relationship between the response and its
reinforcement. Schedules of reinforcement are of critical importance
in determining both an organism's rate and pattern of responding, and
the effects of a chemical (Kelleher & Morse, 1968). Different
schedules of reinforcement have been shown to generate characteristic
patterns of behaviour between species, even when using a wide variety
of response topographies and reinforcing stimuli. Through the
systematic development of schedules of reinforcement (Ferster &
Skinner, 1957), operant conditioning has become an important tool in
behavioural pharmacology and, more recently, in behavioural toxicology
(Laties, 1982).
3.3 Test Methods
3.3.1 General attributes of behavioural methods
Behavioural toxicity test methods vary in sensitivity,
specificity, validity, replicability, and cost. These factors, in
combination with the "question(s) to be asked" at a particular stage
of testing, will strongly influence the choice of method. It is also
important to take into account the extent to which the results of such
tests may be applicable to human beings.
3.3.1.1 Sensitivity and specificity
Sensitivity refers to the ability of a test method to detect the
occurrence of a toxicant-induced behavioural change. If the objective
of a study is to detect whether a chemical produces behavioural
toxicity, a test with moderate sensitivity may be sufficient,
particularly when there is no limitation on dose range. However, if
the intention is to define threshold levels of exposure associated
with behavioural effects, sensitivity becomes extremely important.
Specificity has been used in several contexts. In one, it refers
to the ability to give a negative finding when no behavioural effect
has occurred. In another, it refers to the number of nervous system
functions it reflects. Some tests are relatively specific. Many are of
limited specificity in that they reflect changes in a number of
nervous system functions (which, in turn, may reflect changes in other
organ systems). In this context, specificity is interrelated with
validity (section 3.3.1.2). Since behavioural tests rely on motor
performance, all lack specificity to a certain extent.
3.3.1.2 Validity
Validity is concerned with whether a chemically-induced change in
a behavioural response reflects only the change(s) in the behaviour to
be measured. If, for example, a test is used to assess learning, the
possibility must be considered that factors other than learning (e.g.,
motivational levels, motor function) may influence the test results.
Some understanding of test validity is essential for the proper
interpretation of behavioural toxicity test results. The use of a
variety of behavioural tests, thereby determining a behavioural
profile, can be useful in establishing the validity of a particular
test.
3.3.1.3 Replicability
Replicability refers to the ability of a test method to give
consistent results in repeated studies (both within and across
laboratories); it is somewhat analogous to precision. Precise
protocols and strict attention to experimental detail will lessen
variability and, therefore, increase replicability.
3.3.1.4 Costs
The costs of performing different types of behavioural analyses
will have some influence on test selection. During the early stages of
testing, when the focus is on detecting the presence of behavioural
toxicity, cost will considerably influence the choice of method. It is
unreasonable to use a highly sophisticated procedure requiring
expensive equipment to detect whether a compound has potential
behavioural toxicity whenever a cheaper alternative is available. In
contrast, studies to determine the no-observed-adverse-effect levels
of a chemical may justify the use of expensive, time-consuming
methods.
3.3.2 Primary tests
Tests can be divided into primary and secondary categories.
Primary tests are used for screening neurotoxicity, but such tests
must forecast the potential of such chemicals to produce effects.
Secondary tests are used to further characterize the nature of these
effects. Since many functions are mediated by unique neural substrates
and many chemicals produce rather selective effects, it is important
to employ a variety of tests that measure different behavioural
functions.
3.3.2.1 Functional observation battery
Functional observation batteries are designed to detect major
overt neurotoxic effects. A number of investigators have proposed
series of tests that are generally intended to evaluate various
aspects of behavioural, neurological, and autonomic status (Irwin,
1968; Pavlenko, 1975; Pryor et al., 1983). These batteries consist of
series of semiquantitative measurements appropriate for the initial
level of behavioural assessment (e.g., tremor, convulsions, ataxia,
autonomic signs, paralysis). The tests are, in effect, rating scales
concerning the presence or absence (and, in some cases, the relative
degree of presence) of certain reflexes. In addition, eating and
drinking behaviour and body weight should also be considered in the
context of primary behavioural assessment.
The major advantages of these tests are that they can be easily
administered and can provide some indication of the possible
functional alterations produced by exposure. Potential problems
include insufficient interobserver reliability, difficulty in defining
certain measures (e.g., stupor), and the tendency towards subjective
bias. As a consequence, it is essential that observations be carried
out by individuals who are blind to the groups.
Many types of screening tests are currently used to assess the
effects of neurotoxic agents on motor and reflex function. The
simplest of these include observational assessments of body posture,
muscle tone, equilibrium and gait, and righting reflexes (Irwin, 1968;
Snyder & Braun, 1977). These tests are quantal or categorical at best,
and are generally subjective. Larger animals permit a conventional
neurological examination similar to that used with human beings
(Abou-Donia et at., 1983).
3.3.2.2 Motor activity
Spontaneous motor activity in rodents has been extensively used in
behavioural toxicology (Reiter, 1978; Reiter & MacPhail, 1979).
Movement within the living space or environment is a high-probability
response in animals and can be easily manipulated by environmental
changes, including exposure to neurotoxic agents. Although seemingly
simple, locomotor activity is very complex behaviour comprising a
variety of motor acts, such as horizontally- and vertically-directed
movement, sniffing, and grooming. Rating scales have been developed to
fractionate locomotor activity into its relative components (Draper,
1967). The measures used most often in behavioural toxicology are
horizontally- and vertically-directed activity (Reiter & MacPhail,
1979). A large variety of devices, automated and nonautomated, have
been invented to measure motor activity. Following exposure to a
neurotoxic agent, various qualitative and quantitative changes can be
observed, depending on the apparatus that is used. For example, the
figure-eight maze has been used extensively and successfully to detect
effects produced by a number of chemicals (Reiter, 1983).
Although the figure-eight maze has been used as a residential maze
to measure toxic effects on diurnal activity patterns, recent research
has almost exclusively employed shorter time intervals (Reiter, 1983).
Elsner et al. (1979) have reported a method for the continuous
monitoring of spontaneous locomotor patterns in rats. Using computer-
assisted techniques, these investigators found that methylmercury
treatment lowered activity during the night portion of the diurnal
cycle.
The complexity of motor activity is emphasized by the finding that
low-level exposure to volatile organic solvents increases activity,
whereas high level exposure decreases it (Horvath & Frantik, 1973).
Positive results in a motor activity test usually require further
testing to identify the precise function affected. Activity is not a
unitary measure and a change in the frequency of this behaviour can
reflect toxicant-induced changes in one or more sensory or motor
functions, alterations in reactivity (excitability) or motivational
states, or perturbations of a variety of regulatory states (e.g.,
diurnal cycles, energy balance of the animal). For example, a decrease
in activity might mean that the animal is paralysed or, perhaps, that
it suffers from "general malaise". Thus, if a change in motor activity
is observed, additional tests are needed to determine the cause.
3.3.3 Secondary tests
3.3.3.1 Intermittent schedules of reinforcement
Performance generated by intermittent schedules of reinforcement
(Ferster & Skinner, 1957; Reynolds, 1958; Kelleher & Morse, 1968;
Schoenfeld, 1970) has played an important role in behavioural
pharmacology and is proving a useful tool in behavioural toxicology
(Laties, 1982).
Most intermittent schedules involve reinforcement as a function of
the number of responses emitted, some temporal requirements for
emission of responses, or both. Ratio schedules require the animal to
emit a fixed number of responses (fixed ratio or FR) or a number
distributed around some average (variable ratio or VR) in order to be
reinforced. As the ratio requirement is increased, the latency of the
first response increases; however, once responding begins, it
typically proceeds at a high and constant rate. Interval schedules
require that a certain length of time should elapse before the
response is reinforced. This may be a fixed time (fixed interval or
FI) or time distributed around an average (variable interval, VI).
Although only one response need be emitted at the end of the interval
to cause reinforcement, the organism typically emits many responses
during the interval. Interval schedules usually generate lower rates
of responding than ratio schedules. The FI schedule generates a
characteristic pattern of responding for which a variety of parameters
potentially sensitive to disruption by neurotoxic agents can be
analysed (Kelleher & Morse, 1968). Other commonly-used intermittent
schedules specify the temporal spacing of responses. In the
differential reinforcement of low rate (DRL) schedule, the organism is
required to wait a specific time between responses in order to be
reinforced. In the differential reinforcement of high rate (DRH)
schedule, the organism is required to emit a specified number of
responses within a specified (short) time, and thus responds at a high
rate.
Intermittent schedules of reinforcement can be combined to form
more complicated "multiple" schedules of reinforcement. A classic
example is the combination of FR and FI schedules presented in
succession during a single test session; the resulting multiple
schedule is termed a multiple FR-FI schedule. Each component of the
multiple schedule is independent and occurs in the presence of a
different external discriminative stimulus, which signals it. The
schedule components are typically presented in alternating fashion,
allowing the investigator to collect data on both types of behaviour
almost simultaneously. Individual schedule components can be combined
in other ways with different levels of complexity (Tilson & Harry,
1982). Such schedules are not yet in general use in behavioural
toxicology.
The most common measure of performance with intermittent schedules
is response rate (responses per unit time). This measure may be
sufficient to establish whether there is an effect in a particular
schedule or schedule component before grossly toxic levels are
reached. A measure that may be more sensitive to the effects of toxic
agents is the inter-response time (IRT) distribution (Schoenfeld,
1970). For example, IRT distribution was a sensitive indicator of lead
toxicity in rats performing on a multiple FI-FR schedule (Angell &
Weiss, 1982).
Another measure that may be a sensitive indicator of behavioural
toxicity is variability in performance, both within and across
sessions (Schoenfeld, 1970). Near the no-observed-adverse-effect
level, variability between animals may be increased in the exposed
group(s); variability between groups may be a sensitive indicator of
toxicity. For example, Laties (1982) studied the effects of
methylmercury exposure in the pigeon using a fixed consecutive number
(FCN) procedure, in which the animal was required to respond on one
key a specified number of times consecutively before responding on
another key to be reinforced. Methylmercury exposure decreased the
mean number of times pigeons responded on the first key before
switching (run length) and increased the standard deviation of the run
length within a session. Rice (in press) found increases in both
within- and between-session variability in FI response rate in monkeys
exposed developmentally to lead at doses insufficient to produce
changes in rate.
Marked differences in individual susceptibility to the effects of
lead exposure on development have been observed using FI performance
in the rat (Cory-Slechta & Thompson, 1979) and monkey (Rice et al.,
1979). In general, lower doses increased, and higher doses decreased,
the response rate. In the monkey, the IRT distribution remained
different between treated and control groups, even though changes in
rate disappeared after about 40 min. Remarkable differences in
individual susceptibility were also observed on a response duration
schedule (Cory-Slechta et al., 1981). In this schedule, the animal was
required to depress a lever for a specified time (several seconds)
before releasing it. Some of the exposed animals gave performances
indistinguishable from those of the controls, while others performed
much more poorly than controls. Differences in individual
susceptibility may be a common phenomenon, particularly in behavioural
toxicology, and should be considered in the determination of
no-observed-adverse-effect levels (Dews & Wenger, 1979). An increase in
group variability may signal the presence of toxicity in some portion
of the population of responders (Good, 1979).
Simple schedules, such as FR, FI, VI, DRL, and continuous
avoidance, have been used to detect effects produced by a number of
industrial and environmental toxic agents (Padich & Zenick, 1977;
Dietz et al., 1978; Geller et al., 1979; Zenick et al., 1979; Leander
& MacPhail, 1980; Alfano & Petit, 1981; McMillan, 1982). Perinatal
lead exposure resulting in a relatively low body burden of lead
produced effects in a DRH schedule in the absence of changes in motor
activity (Gross-Selbeck & Gross-Selbeck, 1981).
Multiple schedules offer the opportunity to study behaviour
controlled by different variables, which may be differentially
sensitive to the effects of toxic agents (this is known to be true for
pharmacological agents). For example, toluene decreased the response
rate in the FR component and increased the rate in the DRL component
of a multiple schedule (Colotla et al., 1979); furthermore, the
relative sensitivities of the two components were different. The
multiple FI-FR schedule has proved particularly useful in detecting
behavioural toxicity (Levine, 1976; Dews & Wenger, 1979; Leander &
MacPhail, 1980; Angell & Weiss, 1982; McMillan, 1982).
Intermittent schedules of reinforcement can be used to monitor
effects other than, or in addition to, direct effects on the central
nervous system. These may include damage to the peripheral nervous
system or to some other organ system that results in general malaise
(Laties, 1982). For example, acrylamide, an organic solvent that
produces a "dying back" axonopathy, produced decreases in FR response
rate (Tilson et al., 1980). The schedules typically produce high
response rates and thus may be sensitive to impairment in motor
function. Exposure to ozone resulted in decreased responding on an FI
schedule; this was interpreted as resulting from general discomfort
produced by ozone (Weiss et al., 1981).
In addition to schedules that maintain behaviour by positive
reinforcement, responding can be maintained by negative reinforcement.
Avoidance schedules are either continuous (i.e., each response
postpones shock by a fixed amount of time) or discrete-trial (each
shock is preceded by a warning signal during which a response will
prevent punishment). Avoidance schedules have been used extensively in
the study of anticholinesterase compounds, including pesticides, in
order to assess dose-response relationships, the time course of
behavioural depression during acute intoxication, and the effects of
repeated exposure (Bignami et al., 1975). Their usefulness in the
assessment of treatment effects is shown in the extensive drug
literature. In fact, the confounding of treatment effects with
"motivational" changes is postulated to be less of a problem with
avoidance tasks than with appetitive tasks (positive reinforcement).
Furthermore, once acquired, avoidance responses can be maintained at
fairly stable levels without the precautions necessary in the study of
appetitive responses (e.g., daily control of food or fluid intake
and/or body weight), though induction of stress responses and changes
in pain sensitivity must be considered.
3.3.3.2 Motor function
A variety of techniques developed to evaluate motor function have
been used; these include performance on a rotating rod or treadmill
(Frantik, 1970), swimming to exhaustion (Bhagat & Wheeler, 1973), or
suspension from a horizontal rod (Molinengo & Orsetti, 1976). One
increasingly used technique is the quantification of hindlimb splay.
Edwards & Parker (1977) inked the feet of rats, dropped the animals
from a specified height, and measured the distance between the digit
marks. Schallert et al. (1978) used a similar technique of inking
paws to evaluate abnormal gait in rats treated centrally with
6-hydroxydopamine.
A negative geotaxis procedure was used by Pryor et al. (1983) to
evaluate neurotoxic agent-induced alteration in motor coordination.
Reduction in grip strength is a frequently reported neurological sign
in human beings; fore and hindlimb grip strength in rats and mice has
been quantified using commercially available strain gauges (Meyer et
al., 1979).
Tremor is a common neurotoxic effect. A number of rating scales
and semiquantitative procedures to measure tremor are available
(Gerhart et al., 1982). A simple but expensive spectral analysis
technique that permits rapid evaluation of tremor in freely moving
animals has been reported by Gerhart et al. (1982).
Other more complicated techniques have been devised to measure
motor deficits in laboratory animals. For example, Falk (1970) trained
animals to press a lever within a designated range of force for a
given period. Falk and others (Fowler & Price, 1978) used this
procedure to study the effects of toxic agents on fine motor control.
3.3.3.3 Sensory function
Exposure to toxic chemicals can cause a wide range of sensory
effects. Alterations in sensory processes, such as paraesthesia or
visual or auditory impairment, are frequently among the first signs of
toxicity in human beings exposed to toxic agents (Damstra, 1978). In
animals, "psychophysical" methods are used to arrive at some
estimation of differential response in the presence of a stimulus
varied across some physical dimension (Stebbins, 1970). The great
majority of psychophysical studies have been carried out on non-human
primates and birds; ideally, such studies should be conducted on
species in which sensory function closely resembles that of human
beings. Psychophysical methods range from those that assess a gross
loss of sensation to those that provide a sensitive and precise
analysis of changes in threshold levels and other ancillary or complex
sensory phenomena.
One of the least complex approaches to the study of sensory
deficits is based on the localization or orientation response.
Marshall and colleagues (Marshall & Teitelbaum, 1974; Marshall et al.,
1974; Marshall, 1975) have described a battery of observational tests
in which a visual, auditory, olfactory, or dermal stimulus is
delivered to the organism. The presence or absence of a localization
or orientation response to the source of this stimulus is then
recorded. Such techniques have been used to demonstrate sensory
inattention as well as hyperexcitability in rats having lesions in
various regions of the brain. Pavlenko (1975) has described a variety
of stimulus-elicited orientation reflexes used in the USSR. Despite
the fact that observational tests are simple to perform, they are
labour intensive, especially if the necessary inter-rater reliability
scales are used. However, the scoring of the tests is frequently
subjective and necessitates testing under "blind conditions." Finally,
the data are usually quantal (i.e., the response is scored as either
present or absent) or categorical (scored on a rating scale). Thus,
interpretation of the results is difficult, particularly in repeated-
measure designs.
Several attempts have been made to develop simple yet objective
tests for sensory dysfunction in rodents. Some investigators have used
measurement of the acoustic startle reflex (i.e., measurement of the
presence (and magnitude) or absence of response to a novel sound or
tone) as a screen for auditory dysfunction. Pain sensitivity can be
assessed using standard psychopharmacological techniques measuring
reaction times to a noxious stimulus (Pryor et al., 1983). Electrical
stimulation of the tooth pulp is a sensitive method to detect change
in pain sensitivity (Costa & Murad, 1969). The flinch-jump technique
also is used extensively to determine changes in pain threshold
(Evans, 1962). Taste reactivity has been assessed using taste aversion
procedures (Kodama et al., 1978). Depth perception has been assessed
using a visual cliff procedure, which measures whether or not an
animal chooses to step onto a nearby platform or floor ("shallow"
floor) in preference to one that may be perceived as more distant
("deep" floor) (Sloane et al., 1978). Another simple test of visual
function is the optokinetic drum, which relies on the optokinetic
nystagmus or optomotor response (i.e., tracking a moving object with
the eyes and head for a certain distance until the head is
repositioned back into the frontal plane). On the basis of this
measure, a procedure was developed that was believed to assess visual
acuity as well as colour vision (Wallman, 1975).
Changes in reflex response have been monitored in a variety of
ways. The ability of a stimulus to inhibit a reflex response has been
used to detect changes in auditory threshold in the rat (Young &
Fechter, 1983). Reflex responses (e.g., front-paw withdrawal or ear
flexion) following electric shock have been used extensively in the
USSR to determine changes in pain threshold or detection of
electromagnetic fields (Speranskij, 1965).
More complicated paradigms have been used to assess sensory
dysfunction in more precise ways. Mazes and maze-like apparatus appear
to have some use for evaluating certain sensory deficits (e.g., visual
or somesthetic) in rats (Overmann, 1977; Post et al., 1973). However,
as Evans (1978) pointed out, the relative contributions of motor and
higher-level functions should be carefully distinguished when
interpreting results of such studies.
Some of the more precise methods for evaluating subtle sensory
deficits involve operant techniques. In these studies, a response
(e.g., pressing a lever) is maintained by food or electric shock. Once
the animal has learned to make the response only under certain
stimulus conditions, the intensity of the stimulus can be varied and
the response determined as a function of the intensity. Such
techniques have been used to show auditory loss following exposure to
kanamycin (Harpur & d'Arcy, 1975; Chiba & Ando, 1976). Merigan (1979)
used reinforcement of the identification of spots of light by a monkey
with a fixed gaze to demonstrate the presence of scotomas following
methylmercury exposure.
Olfactory (Wood, 1978), shock or pain (Weiss & Laties, 1961), and
vibration (Maurissen, 1979) thresholds have been determined using
operant techniques. Operant methods also have been used to study the
effects of toxic exposure on more complex sensory phenomena, including
light flicker discrimination (Schechter & Winter, 1971), critical
flicker frequency (Merigan, 1979), and discrimination of the duration
of a visual or auditory stimulus (Johnson et al., 1975).
Chemical agents can act as reinforcing and internal discriminative
stimuli and gain control of a variety of behavioural responses. In
fact, Wood (1979) demonstrated the abuse potential of toluene in an
operant paradigm analogous to drug self-administration.
Pryor et al. (1983) reported on the use of a relatively simple
psychophysical technique to assess 3 sensory modalities concurrently
in the same animal. In this procedure, rats learned to climb or pull a
rope to avoid a noxious electric footshock. Eventually, the response
was brought under the control of three conditioned stimuli; a tone, a
low-intensity, nonaversive current on the floor, and a change in
intensity of the chamber house light. Various intensities of each
stimulus were presented, permitting generation of a quasipsycho-
physical response function. Once the animals were trained, they were
exposed to toxic agents and the changes in responding were measured.
Another procedure that shows promise in the assessment of sensory
function is the use of prepulse inhibition of the acoustic startle
reflex. By varying the intensity of the prepulse stimulus, treatment
effects on sensory acuity can be assessed. Such a procedure has been
used to study the effects of triethyltin on auditory functions in
experimental animals (Young & Fechter, 1983).
3.3.3.4 Cognitive function
Within general psychology, "cognition" refers to the processes by
which knowledge of surroundings is gained; perception, thinking,
learning, and memory refer to different aspects of cognitive
processes. The attribute "cognitive" has been extended by analogy to
classify animal behaviour, as well. Tolman (1948) used the concept of
"cognitive maps" to account for the fact that rats are able to acquire
and retain information about spatial relationships in experimental
mazes, and to use this "latent" information for subsequent learning.
Another example of the study of cognitive processes in animals are the
"insight" studies on chimpanzees (Kohler, 1976), in which behaviour
resembling human problem-solving has been demonstrated. Thus,
cognitive functions cover a much broader field than just approach-
avoidance learning (the prevailing paradigm in today's behavioural
toxicology). In practice, however, learning and memory are the
cognitive functions that have received particular attention in animal
studies, because they are amenable to quantification and because the
abilities to learn and to remember have obvious adaptive value for an
organism. The capacity to learn permits an organism to escape or avoid
situations, approach desirable objects, and store these contingencies
for future use.
Behavioural toxicologists have used a variety of experimental
paradigms to assess learning and memory in laboratory animals. A few
procedures have been developed to measure the ability to adjust to a
new contingency once an initial task has been learned. Studies have
involved conditioned respondent as well as conditioned operant
behaviours.
(a) Procedures using negative reinforcement
In passive avoidance techniques, the animal is trained to withhold
a response, to avoid punishment. A standard procedure is to put the
animal on the lighted side of a shuttle box and to shock it as it
enters the dark compartment (Wolf, 1976). After a given interval of
time, the animal is returned to the apparatus and is given no shock.
Dependent variables include the initial response latency, subsequent
response latencies, number of positive responses, number of
compartment crossings, and total time spent in either compartment on
retesting for a fixed interval of time. Walsh et al. (1982b) used
one-way passive avoidance to demonstrate trimethyl tin-induced memory
deficits in adult rats in the absence of changes in sensitivity to
shock. This technique was used to demonstrate learning/memory deficit
in rats exposed neonatally to chlordecone (Mactutus et al., 1982).
Passive avoidance techniques have the advantages of speed, ease of
performance, and low cost. They have the disadvantage of producing
highly variable results if performed under inadequate test conditions
or if the appropriate retention intervals are not used. Finally, it is
imperative that the nonassociative variables mentioned above be
measured and that alterations in motivational factors (e.g., changes
in "pain thresholds" to footshock) be evaluated.
One-way active avoidance tasks require the animal to respond in
order to escape or avoid negative reinforcement. Typically, an animal
is placed in one compartment of a shuttle box, where it can be
shocked. Once the active avoidance or escape response to the
neighbouring compartment has been registered, the animal is returned
to the original compartment and the process is repeated. Using this
type of test, Tilson et al. (1979a) reported that rats with long-term
exposure to chlordecone learned to avoid as rapidly as controls but
displayed a marked retention deficit when retested several days later.
Another variant of the shock-motivated learning task is the
two-way shuttle box paradigm. In this procedure, rats learn to shuttle
from one compartment to another in the presence of a warning signal in
order to escape or avoid electric footshock. Unlike one-way avoidance,
the animals must learn to return to a compartment where they have just
been shocked. Interesting differences in effects can be observed
between one- and two-way procedures. For example, Sobotka et al.
(1975) reported that rats exposed neonatally to lead performed as well
as controls in a one-way shock avoidance test but displayed
significant deficits in a two-way paradigm.
In general, one- and two-way avoidance tasks entail one training
trial or several discrete massed training trials. These are followed
by one or more trials in which retention is assessed. Dependent
variables for both types of avoidance tasks are avoidance latencies,
number of correct responses, and number of trials to a predetermined
criterion of learning. A useful measure of activity is number of
inter-trial crossings; this information can aid in interpretation of
treatment effects. Tilson et al. (1982a) reported a triethyl lead-
induced facilitation of two-way shuttle-box performance that was not
associated with altered motor activity (inter-trial crossings) or
flinch-jump threshold.
The symmetrical Y maze is a somewhat more complete learning task
than either one- or two-way avoidance. In this procedure, a light or
tone is activated in one of two arms of a maze not occupied by the
animal. The animal is given a predetermined amount of time to run to
the proper arm to avoid electric shock. Dependent variables include
all those previously mentioned for avoidance procedures as well as
number of correct choices. The Y maze has been used very successfully
by Vorhees (1974) to study learning ability in rats exposed in utero
to vitamin A. The paradigm involves two types of learning: when to run
and where to run. These may be affected differently by treatment (Ray
& Barrett, 1975). As pointed out in section 2, strain differences must
also be considered: rats of the Fischer strain easily learn when and
where to avoid, but Sprague Dawley rats do not readily learn when to
avoid (Tilson & Harry, 1982).
Experimental paradigms that involve other tasks and types of
negative reinforcement have been used to assess learning. In the water
maze, animals are placed in a maze filled with water and are required
to learn a series of correct turns in order to gain access to an exit
ramp. Learning trials are preceded by straight-channel swimming trials
as an adaptive procedure and to determine if there are any measurable
neuromotor deficits. Frequently, initial learning is tested in 6
trials on day 1, retention is tested after 7 days, and then reversal-
learning is tested by changing the position of the escape ramp.
Performance measures are swimming time, number of errors, and number
of correct choices. Water maze studies have revealed learning deficits
in animals exposed developmentally to vitamin A (Vorhees et al., 1978)
and to lead or mercury (Brady et al., 1976; Zenick et al., 1979).
Vergieva & Zaikov (1981) studied the performance in a water maze of
adult rats after short- and long-term inhalation of styrene.
(b) Procedures using positive reinforcement
Learning and memory can also be assessed in paradigms that use
positive reinforcement. A number of techniques involve positive
reinforcement of discrimination tasks. The type of discrimination can
be spatial or sensory (visual, auditory). Spatial discrimination tasks
usually involve simple T mazes or more complicated versions of the
T maze, such as the Hebb-Williams maze, which actually is a sequence
of successive T mazes. Dependent measures are the number of correct
trials or the time needed to reach the goalbox. Snowdon (1973) found
that rats exposed to lead neonatally showed impaired performance in a
Hebb-Williams maze, whereas rats exposed post-weaning or as adults did
not.
Visual discrimination tasks frequently take the form of
simultaneous two-choice pattern discrimination. Dependent variables
usually are the number of correct discriminations or number of trials
needed to reach a predetermined criterion. Winneke et al. (1977)
trained food-deprived rats to make a discrimination based on either
the size (circle size) or orientation (horizontal versus vertical
stripes) of a cue placed on a door in a maze leading to food
reinforcement. Lead-exposed animals took longer to acquire the more
difficult size discrimination but resembled controls in learning the
orientation-cued response. Since visual deficit, as assessed by visual
evoked potentials, did not occur until blood-lead levels exceeded
400 µg/litre (Winneke, 1979), visual dysfunction could be ruled out as
an alternative explanation for the lead-induced learning deficit,
which occurred at blood-lead levels below 200 µg/litre (Winneke et
al., 1983).
In a similar two-choice visual discrimination task (Tilson et al.,
1982b), animals were trained to make a nose-poke response to the side
of a cue panel that contained a visual cue. After an animal had
learned the correct discrimination (which occurred over a period of
days), the contingency was reversed, i.e., a response to the side in
which the cue lamp remained unlit was reinforced. Rats exposed to
chlordecone during development showed a trend towards altered
acquisition performance and were markedly different in the way that
they responded during the reversal phase of this test.
Visual discrimination learning has also been used to study the
effects on monkeys of lead exposure during the developmental period
(Bushnell & Bowman, 1979; Rice & Willes, 1979). In these studies,
lead-induced deficit was observed only in the more demanding
discrimination reversal paradigm. This constitutes evidence for
"silent" toxicities that become apparent only with tasks of increased
complexity. Similar conclusions can be drawn from studies on rats in
which lead-induced learning deficit was demonstrated for difficult but
not simple discrimination tasks (Winneke et al., 1977, 1983).
The previous measures of learning have involved between-subject
designs that can require large numbers of animals, if there is large
inter-animal variability. A within-subject design enables the effects
of chemical agents to be evaluated with reference to the animal's
stable pre-exposure baseline and so controls for inter-animal
variability. One such paradigm is repeated chain acquisition: an
animal is given a series of 4 buttons that must be pressed in a
specific order. The order is changed each day, so that a new but
generally similar repeatable task is presented to the animal each day.
In this paradigm, carbaryl affected the rate of performance of
monkeys, but errors were not as clearly affected (Anger & Setyes,
1979). A similar method was used to detect alteration of response
patterning by lead (Dietz et al., 1978) and mercuric chloride (Leander
et al., 1977). In addition to studies of the effects of chemical
agents on the acquisition of new behaviour, there have been studies of
memory, and the persistence or lack of persistence of acquired
information with the passage of time. Memory has been studied using
between-subject designs employing a radial maze.
The radial-arm maze (RAM) is a complex spatial learning task in
which animals must "remember" a list of previously entered and
unentered feeders during a free-choice test session (Olton et al.,
1979, 1980). The RAM is believed to be useful in studying working
(information relevant to a single trial), as well as reference memory
(information relevant to all trials). The most commonly used RAM
consists of a circular arena from which eight equidistant arms radiate
like spokes from a wheel. A trial begins with all arms baited and ends
when all pellets have been consumed or when a fixed period of time has
elapsed. The most effective strategy for solving the maze is to enter
and eat in each of the arms only once. Results can be expressed in
several ways such as: (a) number of correct choices in the first eight
selections (control rats will often obtain all eight pellets without
an error); (b) total number of errors made in obtaining the eight
pellets. Walsh et al. (1982b) reported that trimethyltin-exposed rats
displayed impaired performance in this task and that the behavioural
deficit might be due to an alteration in the integrity of limbic
forebrain structures such as the hippocampus. The RAM has also been
used to study loss of spatial memory as a function of isolation in
darkness and of time elapsed since initial learning (Buresova & Bures,
1982).
Memory has been studied using operant discrete trial techniques,
which enable greater control over the stimuli applied in this study
and thus more sensitivity, but require more training. Animals are
trained to respond in a series of trials that are separated by time
intervals. Performance in a trial depends on information presented in
the previous trial. These operant techniques always use within-subject
designs and have proved useful in the study of drugs because they make
it possible to dissociate the effects on memory from the effects on
motivation or motor control. For example, it has been shown that
scopolamine impairs memory but does not interfere with responding in
delayed, go-no go, alternation (Heise & Milar, 1984). This technique
was applied in behavioural toxicology using delayed, spatial
alternation, which combined two toxicologically sensitive tasks,
discrimination and reversal spatial memory (Heise, 1983). It was found
that carbaryl decreased both memory and responding.
Modifications of Harlow's "learning set formation paradigm" for
use with primates also deserves mention in this context. In this task,
the animal is given a great number of discrimination problems to be
solved successively. As training progresses, new problems are solved
faster and faster (i.e., the number of trials necessary to solve each
problem decreases with increasing number of problems). Lilienthal et
al. (1983) used this task to demonstrate lead-induced cognitive
deficit in rhesus monkeys; simple discrimination learning was not
affected, but transfer of learning was. Thus, acquisition of
information was not impaired but memory for previously acquired
information was disrupted.
3.3.3.5 Eating and drinking behaviour
Many of the behavioural tests described above use food as the
primary reinforcer to get the animal to perform an instrumental
response. Eating is thus involved in the results of the various tests
employed. However, eating can also be used in the assessment of the
potential behavioural toxicity of chemical compounds. Eating and
drinking are naturally occurring behaviour that can be measured in the
animals' laboratory environment; once a stable eating and drinking
pattern is established, toxic agents can be introduced and the
resulting alteration in behaviour measured (Tilson & Cabe, 1978). For
example, since it has been shown that carbon monoxide and hypoxia
depress food intake, Annau (1975) compared the effects of both
conditions on food and water intake in naive rats. Two control groups
were compared with groups exposed to 250, 500, and 1000 ppm carbon
monoxide and 16%, 14%, and 10% oxygen. Although both experimental
conditions produced a decrease in body weight and in food and water
intake, the shapes of the resulting curves were very different,
suggesting that carbon monoxide may not act on these biological
systems in an identical manner to hypoxia; in fact, it appears that
hypoxia has a more severe effect on behaviour than the equivalent
concentration of carbon monoxide. A more recent investigation (Bloom
et al., 1983) showed that subconvulsive doses of pyrethroid
insecticides reduced variable interval performance as well as food
intake, indicating that further attention should be devoted to the
relationship between eating and drinking and other behavioural
response changes.
Eating responses in rodents also altered when a food having a
specific taste was paired with an illness produced either by
irradiation or by the administration of a toxic substance, thus making
it possible to assess the aversive properties of the agent tested.
This conditioned taste aversion has been employed as an experimental
preparation in the evaluation of the unconditioned stimulus functions
of several toxic substances, such as chlordimeform (MacPhail &
Leander, 1982), trialkyltin (MacPhail, 1982), and industrial solvents
(Vila & Colotla, 1981).
Schedule-induced, or adjunctive, drinking is another type of
consummatory behaviour (Colotla, 1981) of interest to the
neurobehavioural toxicologist for two reasons; first, it has been
demonstrated that the procedure can generate "voluntary" consumption
of alcohol and several other drugs, and second, it produces a
consistent and regular post-reinforcement behaviour pattern that is
sensitive to the effects of several drugs (Colotla, 1981). Although,
to date, no reports have appeared in the neurotoxicological literature
on the effects of toxic substances on adjunctive behaviour, this type
of eating response may be useful as part of a test battery for
neurobehavioural toxicity.
3.3.3.6 Social behaviour
Social behaviour implies behaviour involving two or more
individuals (Hinde, 1974), which means that almost all activities of
an animal are, or can be, social. Since social behaviour represents a
complex set of interactions, its investigation in the laboratory is
not simple: each behavioural response of an animal living in a group
(including a pair) may be influenced by previous interactions with
members of the group and by the behavioural characteristics of
individuals forming the group.
Although considerable literature exists on the social behaviour of
laboratory animals, additional methodological development is necessary
before it can be included in routine toxicological evaluations.
Methodological and theoretical problems include the definition of
individual classes of social behaviour, and the generalization of
results obtained with different species. There are problems dealing
with the objectivity of measurement, standardization of testing
procedure, and statistical evaluation. Use of laboratory animals
(mice, rats) raises the question of adequacy of the social environment
created by the research worker because the laboratory environment is
very different from the social environment of the species in the wild.
Although social behaviour has not been used extensively in
toxicology, it has found some use as a diagnostic tool in
psychopharmacology, where various kinds of social interaction have
differentiated between the effects of drugs of different
pharmacological classes (Miczek & Barry, 1976). This body of
psychopharmacological work demonstrates that the behavioural effects
of different chemicals can be modified by the social environment and
thus can differ from the effects in isolated animals.
Two basic approaches have been used to study social behaviour in
animals. The first evaluates the impact of social setting on the
various types of behaviour in animals living for long periods in semi-
natural or artificial conditions. Under this test situation, housing
conditions and population density can be shown to affect various
behavioural and physiological responses. Litter size, for example, was
shown to influence an animal's response to various stimuli; the effect
depended not only on nutritional status but also on social behaviour
(Frankova, 1970). Overcrowding has been shown to affect behaviour, as
well as endocrine and other organ systems functions (Thiessen, 1964).
Chemicals such as amphetamine have greater toxicity when administered
to grouped mice (Chance, 1946). There are differences between
individually-and colony-housed rats in the oral ingestion of morphine
(Alexander et al., 1981), aversiveness to Naloxone (Pilcher & Jones,
1981), and ethanol consumption (Kulkosky et al., 1980).
A second more common approach to the study of social behaviour is
the short-term observation of an animal's behavioural response to
another individual (e.g., cage mate, sexual partner) or group. Here
the pair or group is observed out of the home cage, usually in a
specially equipped box. Various chemically-induced disruptions of
different social interactions have been reported for rodents and
primates. Silverman (1965) developed criteria for paired interactions
in rats (e.g., mating aggression, submission, escape) and demonstrated
the effects of drugs on these categories of social behaviour. Various
other studies (Frankova, 1977; Krsiak, 1979; File, 1980) have
evaluated effects of drugs on isolated and group-bound mice and rats.
Cutler (1977) demonstrated that lead exposure disrupts social
behaviour in mice.
Other studies have investigated the effects of chemicals on the
social behaviour of non-human primates. Apfelbach & Delgado (1974)
administered chlordiazepoxide to gibbon colonies and observed a
decrease in mobility and aggressive acts and increased play, grooming,
and water intake. Bushnell & Bowman (1979) showed that long-term lead
ingestion affected play and other social behaviour in infant rhesus
monkeys.
The types of social behaviour most frequently examined for effects
of chemicals (especially drugs) are dominance and submission
(Baenninger, 1966), isolation-induced aggression (Krsiak, 1979;
Eichelman et al., 1981), sexual behaviour, and maternal behaviour
(Grota & Ader, 1969; Frankova, 1971, 1977).
3.3.4 Strengths and weaknesses of various methods
Many of the test methods described in this section have been used
successfully to model human neurotoxicity, while the relationship of
findings of other tests to human disease is not known. Also, of
course, each method or group of methods has its proponents; often the
individuals or groups who have developed and used it extensively.
However, over the past decade, there has been a broadening in the
exchange of test methods between research laboratories and countries.
Some obvious examples are the use in the USSR of T-maze and shuttle-
box avoidance testing (Kholodov & Solov'eva 1971; Asabayev et al.,
1972) and of unique test devices for teaching simple discrimination
using positive reinforcement (Kotliarevski, 1957; Masterov, 1974;
Medvedev, 1975); the use in Eastern Europe and South America of
operant techniques (Colotla et al., 1979; Vergieva & Zaikov, 1981);
and the use in the USA and South America of reflex conditioning to
elucidate pharmacological and toxicological effects (Costa & Murad,
1969; Young & Fechter, 1983).
It is probably true that investigators who have perfected the
technical aspects of their own paradigms "get the most out of them."
Behavioural science, as any other science, involves a set of
techniques of such sophistication that many are best learned in the
laboratories in which they were developed. This presents some
difficulties in inter-country transfer of methods and the unrestrained
endorsement of methods developed in another country with other
behavioural/psychological traditions. Those who adopt a method for
which they have no ready contacts with experienced investigators may
abandon it as "insensitive", when the only problem may be technical
errors in application.
Respondent behaviours have the advantage of being rapidly formed.
In most cases, only 40-60 trials or tests in a single day are required
for their formation, and the behaviours may be elicited periodically
during long-term exposure studies with no diminution in their
usefulness. Pure muscle responses or integrated motor activity (as in
a shuttle box or startle response chamber) can be measured. These
methods can reflect both increases (stimulation) and decreases
(depression) in responding, and can be completely automated, thus
eliminating subjective factors.
Operant methods can entail the kinds of complex behaviour needed
to evaluate such complex factors as learning. Their proponents also
point out that they can be used to measure fundamental behavioural
properties related to the control of behaviour. However, operant
paradigms require several weeks for the animals to reach stability on
a schedule of reinforcement and a great deal of time of a highly
skilled research worker to train animals to perform complex tasks. As
with respondent methods, operant methods can detect both increases and
decreases in responding and can be completely automated.
In addition, it is axiomatic that behaviour is essentially an
integration of sensory, cognitive, and motor processes. The
specificity of a toxic effect on any behavioural parameters must
always be evaluated in the context of the experimental design and in
conjunction with controls for effects on the other systems.
3.4 Research Needs
3.4.1 Compensatory mechanisms
Because the functional redundancy of the nervous system can mask
some perturbations, procedures need to be devised and used that will
reveal toxic effects that are not apparent under normal test
conditions. Challenges by environmental and pharmacological agents
have proved to be useful in the search for subclinical toxic effects,
and their use is recommended. By creating additional demands on
behavioural integration, challenges can reveal "hidden" functional
deficits (Hughes & Sparber, 1978; Tilson et al., 1979b; Tilson &
Squibb, 1982). The pharmacological challenges most commonly used are
psychoactive drugs and substances that mimic or block the actions of
putative neurotransmitters or alter their synthesis, storage, or
release. Some common stresses that have been used as environmental
challenges are alterations in circadian rhythms, density of housing,
noise level, and ambient temperature (MacPhail et al., 1983). Studies
to increase the understanding of subclinical toxic effects and provide
information concerning appropriate rationale for the selection of
specific challenges represent an important research need (Tilson &
Mitchell, 1984).
Adaptive changes in the nervous system can also occur after
repeated exposures to high levels of a toxic agent. There is an
initial decrement or increment in behaviour after the first exposure,
but then there is a recovery of function to the pre-exposure levels as
exposure continues. This apparent return to normal function results
from adaptive changes in the systems that control behaviour. Sometimes
this change is due to tolerance within the affected systems, but in
other cases, there could be a redundant system that enables the return
to the pre-exposure state. Research on these forms of compensatory
mechanisms is to be encouraged.
3.4.2 Method development and refinement
Development of methods, evaluation, and refinement remain basic
needs in behavioural toxicology. Many behavioural test strategies and
test batteries have been proposed for the longitudinal assessment of
behavioural function in animals exposed to toxic agents either during
development or as adults (Butcher, 1976; Grant, 1976; Rodier, 1978;
Buelke-Sam & Kimmel, 1979; Butcher & Vorhees, 1979; Tilson et al.,
1979c; Zbinden, 1981; Mitchell et al., 1982; Vorhees & Butcher, 1982).
Most authors agree that it is essential that a core of functions
should be assessed, since it is unlikely that any single one will be
sensitive to all toxic agents. The guidelines for reproductive testing
in France, Japan, and the United Kingdom require assessment of several
functions but do not specify exact tests. Many fear premature
standardization of specific tests while behavioural toxicology is
still in its initial period of growth (Weiss & Laties, 1979).
Development, validation, and standardization of primary (screening)
tests need to continue and could be facilitated by the use of
reference compounds (Horvath & Frantik, 1973).
An easily obtained source of information that deserves more
attention, especially in long-term studies, is the uninterrupted
behaviour of animals in their home cages. Food and water intake can
provide information on homeostatic functioning, while a measure of
activity can assess changes in movement patterns or in the circadian
rhythm of activity.
There does not yet appear to be an animal model of fatigue.
Further elaboration of animal models to evaluate fatigue dissociated
from motivational factors needs consideration.
It is generally accepted that complex tasks are more sensitive to
chemical disruption than simple tasks, and that a behaviour that is
not fully learned or practiced is more sensitive to chemical
disruption than one that is well learned and established. These and
many related assumptions require testing. Publication of skilfully
selected comparative data from a variety of laboratories will assist
all investigators in the future selection of test methods. In
particular, more and better methods involving complex tasks that may
be relatable to human beings are required.
Increased and improved automation of test methods is another need.
Automation can eliminate observer (and even trainer) bias, and should
be pursued where feasible. The increasing availability of inexpensive
microprocessors continues to put more and more laboratories in a
position to acquire the central tool for automation of more complex
methods.
4. NEUROPHYSIOLOGICAL METHODS IN NEUROTOXICOLOGY
4.1 Introduction
The term neurophysiology may refer to all studies of the function
of the nervous system. As such, it could include studies of
conditioning and behaviour as well as electrical recordings obtained
from the nervous system. However, for the purposes of this section, a
more restricted definition of neurophysiology will be used, with few
exceptions, to mean the study, by measurement of electrical activity,
of nervous system activity.
Even with this restricted definition, an enormous array of
neurophysiological methods is available to the neurotoxicologist.
Depending on the methods selected, neurophysiological studies can be
used to achieve goals as diverse as detecting neurotoxicity,
characterizing neurotoxicity (i.e., which neural systems are involved)
and unravelling mechanisms of neurotoxicity. Each of the methods
selected for presentation below can be used to achieve at least one of
these goals. In addition, some of the methods can be used in human
studies as well as those on laboratory animals, thereby providing
ready opportunity for cross-species extrapolation.
While the use of physiological methods to assess the impact of
toxic chemicals on the nervous system has a long history (Citovich,
1930; Zakusov, 1936), the development of more sophisticated recording
devices has led to an expansion of their use. As the technology to use
these techniques develops, it becomes progressively more affordable,
and should therefore become even more popular in the near future.
In this section, the neurophysiological evaluation of
neurotoxicity will be discussed in terms of assessment of the
peripheral nervous system, the autonomic nervous system, and the
central nervous system. With careful reading of the different
sections, it should become evident that some methods described in one
place may be useful under other circumstances. In the final section, a
few issues pertaining to the interpretation of neurophysiological data
will be addressed.
4.2 Methods for Evaluation of the Peripheral Nervous System
4.2.1 Conduction velocity
Many substances are known to produce alterations in the peripheral
nervous system (Spencer & Schaumburg, 1980), and evaluation of the
functional integrity of peripheral nerves is the subject of the
clinical science known as electrodiagnosis (Johnson, B.L., 1980).
Conduction velocity, the speed at which action potentials are
conducted along axons and nerves, is the most widely used measure of
peripheral nerve function. Conduction velocity is usually measured in
such a way that the activity of the fastest conducting axons is
assessed. Changes in conduction velocity that occur following exposure
to toxic substances producing axonopathy are reliable, but usually not
large, often ranging from 10 to 30% of control values (Gilliatt,
1973). On the other hand, demyelination produces large decrements
(50%) in conduction velocity (McDonald, 1963).
Principles involved in performing conduction velocity studies have
been presented by Daube (1980), and techniques using rodents have been
described, in detail, by others; some of these toxicological studies
have been reviewed by Johnson, B.L. (1980) and Fox et al. (1982).
Many other techniques besides conduction velocity have been
applied in the assessment of peripheral nerve function. They include
assessment of the refractory period (Hopf & Eysholdt, 1978; Lowitzsch
et al., 1981), assessment of the extent to which axons and nerves can
follow trains of stimuli occurring at high rates (Lehmann & Tachmann,
1974), accommodation indices (Quevedo et al., 1980), and the use of
collision techniques for selectively blocking activity of some nerve
axons to study others (Kimura, 1976). Some of these techniques will
certainly provide even greater sensitivity than the simple velocity
measurements in common use.
4.2.2 Peripheral nerve terminal function
Methods for evaluating function in peripheral sensory receptors
are particularly valuable in neurotoxicology. Toxic agents that
exhibit a preference for the distal ends of long peripheral nerves, a
dying-back neuropathy, or distal axonopathy might be expected to alter
or impair the sensory function of these receptors (Fox et al., 1982).
Indeed, in the case of proprioceptors, such as muscle spindles,
neurotoxic insult can contribute to ataxia, areflexia, and
incoordination (Lowndes et al., 1978a,b). Detailed discussion of the
methods can be found in Fox et al. (1982). Although measuring
peripheral nerve terminal function is more difficult than measuring
peripheral nerve conduction velocity, such measurements are important
since alteration of function in the terminal portions of axons
frequently precedes alterations in conduction velocity. For example,
function of muscle spindles (Lowndes et al, 1978a,b), motor nerve
terminals (Lowndes & Baker 1976), and primary afferent terminals
(Goldstein et al., 1981) has been reported to be compromised by toxic
agents, long before any alterations are detectable in conduction
parameters. Another advantage of these techniques is that, not only
can the presence of neurotoxicity be detected, but the site(s) of
neurotoxic action can also be investigated.
4.2.3 Electromyography (EMG)
The recording of biopotentials from muscle (electromyography) has
been extensively used in human clinical studies in the diagnosis of
certain diseases of the muscle (Johnson, E.W., 1980). EMG is an
objective and sensitive method for the detection of changes in
neuromuscular function. Altered neuromuscular function using EMG was
detected in organophosphorus insecticide workers who did not exhibit
other detectable signs and symptoms of poisoning including depressed
blood cholinesterase activity (Drenth et al., 1972; Roberts, 1977).
EMG methods have been little used for the study of neurotoxic
substances in experimental animals. According to Johnson, B.L. (1980),
there are probably two reasons for this. First, few toxicologists are
trained in EMG procedures. Second, one important component of an EMG
examination involves the evaluation of the voluntary graded
contraction of the muscles. This is sometimes difficult to control in
experimental animals. Nevertheless, methods using experimental animals
are available (Johnson, B.L., 1980), and have been used successfully
to study the neurotoxic effects of methyl n-butyl ketone (Mendell et
al., 1974) and manganese (Ulrich et al., 1979).
Electromyography is used to study direct toxic effects on muscles.
Evoked muscle responses to nerve stimulation are invaluable in
examining the neuromuscular junction, which can be affected by various
neurotoxic agents (e.g., botulinum and tetanus toxins and
organophosphate insecticides).
4.2.4 Spinal reflex excitability
Segmental spinal monosynaptic and polysynaptic reflexes are
relatively simple functions of the central nervous system that can be
easily evaluated by quantitative techniques (Mikiskova & Mikiska,
1968; Fox et al., 1982). Many of the methods used in animals are
direct laboratory counterparts of some of the clinically used
neurological tests in human beings. There are two basic approaches.
One does not requires any invasive procedures and thus is most akin to
the tests used in human beings. The other involves electrophysio-
logical techniques for examining the effects of neurotoxic agents on
mono- and polysynaptic reflexes.
In the non-invasive techniques, the functional state of the reflex
arch is inferred either from the latency and size of the reflex
response evoked by stimuli of a predetermined intensity (Zakusov,
1953) or from the stimulus intensity (threshold) just sufficient to
elicit a detectable response (Mikiskova & Mikiska, 1968). The
threshold approach has been used by Mikiskova & Mikiska (1966, 1968)
to study a variety of volatile solvents. In their view, the procedure
is useful both as a screening test and in estimating the relative
toxicities of substances. It is critical that a stimulator with a
constant current output is used so that the stimulating current does
not depend on the resistance of the skin and electrodes.
The time required for a stimulus to a peripheral nerve to reach
the spinal cord and return to the site of stimulation directly
(F response), particularly after crossing a single synapse
(H response), can indicate the excitability of the motoneuron pool.
Fox et al. (1982) present electrophysiological techniques for
examining the effects of neurotoxic agents on mono- and polysynaptic
reflexes. This approach can provide better clues than the non-invasive
approach concerning possible site(s) of action for the neurotoxic
agent, but is considerably more time consuming. Moreover, the manner
in which it is generally carried out (decerebrate animals) precludes
repeated testing on the same animal. Thus, for most types of
investigations (screening, determining threshold concentrations for
effect) the non-invasive approach is preferable.
4.3 Methods for Evaluation of the Autonomic Nervous System
Compared to other approaches, relatively little effort has been
expended in assessing the impact of suspected toxic agents on the
activity of the autonomic nervous system. Most neurophysiological
methods are designed to measure relatively rapid events, and therefore
they are not particularly well suited to the evaluation of the
autonomic nervous system. However, a few exceptions are noteworthy.
4.3.1 Electrocardiography (EKG)
Electrocardiography supplements other neurophysiological methods
by providing data on the central and peripheral nervous control of
autonomic functions. However, the interpretation of EKG changes is
complex and must take into account direct effects on the myocardium
(Mikiskova & Mikiska, 1968).
4.3.2 Blood pressure
Concomitant recording of EKG and blood pressure using
environmental and pharmacological challenges is an approach worthy of
consideration for evaluating animals exposed to suspected toxic
agents. Altered circulatory responses to these types of stimuli may
yield important information concerning the functional status of the
autonomic nervous system. Indeed, exaggerated responses to vasopressor
and cardiac acceleratory stimuli would suggest that such exposure
might be accompanied by a higher risk of cardiovascular disease.
4.4 Methods for Evaluation of the Central Nervous System
4.4.1 Spontaneous activity - electroencephalography (EEG)
EEG analysis was one of the first forms of electro-diagnosis of
nervous system dysfunction. Since its discovery by Berger (1929),
there have been progressively more sophisticated attempts at analysis,
and progressively greater promises for its value. At present, the EEG
is used widely in clinical settings, and consequently a variety of
sources are available describing the technical details of recording,
analysis, and interpretation (Basar, 1980; Niedermeyer & Lopes da
Silva, 1982). In clinical neurology, the EEG has been used for the
diagnosis and description of epilepsy, localization of tumours,
description of sleep stage, as well as many other neurological
disorders (Niedermeyer & Lopes da Silva, 1982). It has been used less
often for the detection of subtle toxic agent-induced dysfunction,
though it is an integral part of Soviet and Eastern European
neurotoxicological studies (Horvath & Frantik, 1973, 1976).
The normal EEG, whether recorded from the scalp or with indwelling
electrodes in specific brain regions, has an amplitude of up to about
100 uv. The useful frequency spectrum of the EEG is below 50 Hz,
though higher frequencies are encountered in certain brain regions
(Johnson, B.L., 1980). It is common to analyse the scalp-recorded EEG
according to the amount of electrical activity contained within
specific frequency bands, specifically: delta (2-4 Hz), theta
(4-8 Hz), alpha (8-13 Hz), ß1 (13-20), and ß2 (20-30 Hz) (Lindsley
& Wicke, 1974). A variety of electronic frequency analysers, as well
as computer procedures, are available for use in analysing the power
spectrum of the EEG throughout the entire frequency range or within
selected frequency bands (Lindsley & Wicke, 1974).
When electrodes are implanted in specific brain areas, the
resultant EEG can be used to assess the effects of toxicants on these
different brain areas or structures. Also, specific brain regions
(e.g., the hippocampus) have particular patterns of after-discharge
following chemical or electrical stimulation, which can be
quantitatively examined and used as a tool in neurotoxicology (Dyer et
al., 1979).
It should be pointed out, however, that disassociation between the
EEG pattern and behaviour can occur. For example, high voltage-slow
activity (generally associated with sleep) has been seen following
administration of atropine in animals that seemed to be excited
(Bradley, 1958). Also, low voltage-fast activity (generally associated
with arousal or with REM sleep) has been reported following
physostigmine in animals that seemed to be asleep (Bradley, 1958).
Thus, caution must be used in the interpretation of EEG changes alone.
Extensive discussions and references, concerning the use of the
EEG in neurotoxicology, are presented in Horvath & Michalova (1956),
Mikiskova & Mikiska (1968), Johnson, B.L. (1980), and Fox et al.
(1982). The full potential of this technique in neurotoxicology has
not yet been fully exploited. However, it is clear that modern
electronic and computer facilities are required to maximize its use.
Moreover, changes in the pattern of the EEG elicited by stimuli
producing arousal (light, sound, electrical stimulation) (Desi & Sos,
1962; 1963) or produced by sleep (Fodor, et al., 1973) will most
probably enhance its usefulness in neurotoxicology (Zilov et al.,
1983). It should be noted that changes in the EEG have been reported
after treatment with organophosphate compounds before depression of
acetylcholinesterase activity was noted in the blood or brain tissue
(Desi et al., 1974). In a more recent study, Desi (1983) compared
changes in EEG with a behavioural test (maze), cholinesterase enzyme
activity, and several general toxicological tests following exposure
to 13 different pesticides. He concluded that the EEG was the most
sensitive test for the detection of early and mild changes caused by
these pesticides.
4.4.2 Sensory systems
In studies of sensory systems, the response of the nervous system
to a well defined, yet physiological, input stimulus can be evaluated.
Precise specification and control of the input stimulus reduces
variability in the measured end-points, and increases the clarity with
which toxicant-induced alterations are detected. Certain neurotoxic
agents seem to have a particular affinity for sensory systems (e.g.,
methanol). Furthermore, there are few cases when toxicant-induced
damage to a non-sensory system is not parallelled by damage in sensory
systems.
The overall functional integrity of a sensory system is most
directly assessed using evoked potential techniques. These techniques
require the application of a discrete sensory stimulus, and averaging
of the electrical activity from an appropriate neural pathway or brain
area during a brief (e.g., 0.3 second) post stimulus epoch over
repeated (e.g., 100) trials. Such averaging reveals a characteristic
waveform that is specific to a particular modality, stimulus,
electrode location, or species. Alterations in the latency from the
stimulus to the specific peaks in the waveform, or in the amplitude of
specific peaks in the waveform, are diagnostic of dysfunction.
Sensory-evoked potential techniques are widely used in
neurological clinics (Beck et al., 1975), and are becoming
increasingly used in neurotoxicity evaluations. Since they may be
readily recorded from unanaesthetized unrestrained animals, sensory
evoked potentials may be obtained during, and correlated with,
behavioural studies. They may detect, with various levels of
sensitivity, alterations resulting from exposure to such chemicals as
organometals (Dyer et al., 1978) and pesticides (Boyes et al., 1985).
More detailed description of the rationale, methods, interpretation,
strengths and limitations of sensory evoked potential techniques can
be found in Dyer (1985a,b).
4.4.3 General excitability
A common readily observed consequence of exposure to high
concentrations of neurotoxic agents is an alteration in behavioural
arousal. While some compounds produce sleep and coma, others produce
seizures (Holmstedt, 1959; Lipp, 1968; Joy et al., 1980; Joy, 1982).
These alterations may be presumed to reflect disordered excitability
of the brain, and have led to the assumption that quantitative
measures of excitability would be useful for the detection of
dysfunction in this dimension. Four main approaches have been taken in
the assessment of general excitability; (a) convulsive phenomena;
(b) stimulation of motor cortex; (c) recovery functions; and (d) EEG
recordings. EEG recordings have already been discussed in section
4.4.1.
4.4.3.1 Convulsive phenomena
While there are many experimental models of epilepsy (Purpura et
al., 1972), not all of these are suitable for the detection and
characterization of neurotoxicity. In neurotoxicology, seizure
susceptibility has been most often assessed using either electrical
stimulation or systemically administered drugs to produce seizures.
Pharmacological stimulation, using agents such as pentylenete-
trazol (Metrazol), is simple, quick, and cheap. While interpretation
may be complicated if the toxic agent under study alters the
metabolism of the convulsant agent, practice has failed to reveal many
such instances. Depending on the convulsant selected, the integrity of
selected neurochemical systems may be assessed. For example,
picrotoxin is presumed to act by blocking activity in GABA-ergic
systems.
Electrically induced seizures have been widely used in
neurotoxicology. Horvath & Frantik (1979) and Frantik & Benes (1984)
evaluated the effects and relative potentials of a wide variety of
organic solvents on the extensor phase of electrically induced
seizures in rats. They compared the severity of these seizures with
other behavioural effects obtained in both laboratory animals and man,
thus providing a comparative measure of the relative toxicity of these
chemicals. The seizures were suppressed by relatively low air
concentrations of most of the solvents tested and this occurred in a
concentration-dependent manner.
Electrically-induced after discharges offer the opportunity to
study seizure activity in specific brain regions with implanted
electrodes in the absence of behavioural correlates. These tests have
been used particularly in areas of the limbic system known to be
sensitive to neurotoxic agents and to have low seizure thresholds
(Dyer et al., 1979).
Repeated elicitation of after-discharges from certain brain
regions leads to the progressive recruitment of behavioural correlates
by a process known as kindling. The development of kindling-induced
seizures, while more time consuming than other methods, may provide
more detailed information regarding the nature of alterations produced
by the toxic agent. A discussion of kindling as a model for the study
of neurotoxic agents can be found in Joy (1985).
4.4.3.2 Stimulation of the cerebral motor cortex
The motor area of the cerebral cortex is preferred because
stimulation results in constant, clearly defined motor responses. The
procedure is discussed in detail by Benesova et al. (1956), Mikiska
(1960), and Mikiskova & Mikiska (1968). Basically, "excitability" is
determined by measuring the current required to evoke minimal movement
of the contralateral fore limb. Studies can be conducted acutely or
with the long-term implantation in animals of electrodes (generally
fine screws) touching the motor cortex. Depressant agents uniformly
raise the threshold to electrical stimulation (by as much as several
fold) whereas stimulant type agents lower it (but to a lesser extent).
Effects of drugs and toxic agents in this procedure are cited by
Mikiska (1960) and Mikiskova & Mikiska (1968). It should be noted that
direct electrical stimulation of central nervous tissues has far more
complex consequences than stimulation of peripheral nerve. The
stimulus excites thousands of neurons mutually connected by excitatory
and inhibitory synapses, which often form reverberating circuits
(Mikiskova & Mikiska, 1968). Thus, interpretation concerning the
possible mechanism of effect is difficult.
4.4.3.3 Recovery functions
Repeated stimulations may alter the threshold for evoking a
response. Neuronal recovery processes are among several neurophysio-
logical challenges reviewed for their relevance for the detection and
characterization of neurotoxicity by Dyer & Boyes (1983). While these
methods have only recently been used in neurotoxicology, there are a
number of instances in which they detect neurotoxic effects earlier
than other methods (Dyer & Boyes, 1983).
4.4.4 Cognitive function
Not all evoked potentials are directly related to eliciting
sensory stimuli. Parts of some waveforms (most often the "late" or
"slow" components) are presumed to be elicited by events that are
internal, and may reflect cognitive activity or initiation of motor
activity (Otto, 1978). In human beings, changes in one such potential
have been associated with extremely low body burdens of lead (Otto et
al., 1981). Unambiguous interpretation of such findings is not yet
possible, and the methods have not been applied extensively to animal
studies. However, increased research activity in this area should make
assessing the value of these "slow potentials" more straightforward.
4.4.5 Synaptic and membrane activity
Using appropriate microelectrode neurophysiological techniques, it
is possible to determine in a direct way whether exposure to a toxic
agent impairs the response properties, synaptic function, or membrane
properties of neurons (Fadeev & Andrianov, 1971; Andrianov et al.,
1977; Fadeev, 1980; Barker & McKelvy, 1983; Dingledine, 1984). While
exquisite in the precision of the information they provide, these
techniques are technically difficult and expensive to use, and are
most profitably used to assess mechanism of toxicity. Data and
discussion of the value of these techniques can be found in Joy
(1982), Narahashi (1982), and Narahashi & Haas (1967, 1968); all of
these reports consider the effects of organochlorine insecticides and
pyrethroid insecticides on properties of neuronal membranes.
4.5 Interpretation Issues
Most neurophysiological methods provide information at several
different levels. For purposes of organization, it is convenient to
divide the brain into discrete functional systems such as the
somatosensory system, the visual system, the extrapyramidal system,
etc. While these systems are clearly interrelated, they are almost
always treated separately. Most such systems are constructed of
neurons with similar membrane and axonal properties, but they differ
with respect to their connections, neurotransmitters, and metabolic
activity. Thus, evaluation of any one system provides a combination of
information, some unique to the system, and some common to most or all
neural systems.
The choice of the appropriate neurophysiological methods for a
given study depends on the questions posed. If emphasis is on
identification of systems involved in the toxicity produced by a
specific compound, then gross measures of the functional activity of
whole systems are desirable (e.g., evoked potentials in the visual
system). On the other hand, if a compound is known to be toxic for a
particular system, for example the hippocampus, and the emphasis is on
the mechanism of toxicity, then microelectrode techniques may be
appropriate. Investigators about to perform neurophysiological studies
of neurotoxicity should become familiar with the strengths and
limitations of the methods mentioned in this review before selecting
one. Furthermore, attention should be paid to the possibility that the
toxicant-induced alterations observed reflect secondary dysfunction,
which in turn is produced by primary dysfunction in another system.
For example, a compound that produces hypothalamic dysfunction and
compromises the body's ability to thermo-regulate will appear to
produce changes in other systems that are really secondary to
hypothermia.
A major advantage of many of the neurophysiological techniques
mentioned above (EEG and evoked potential) is that they are readily
recorded in human beings as well as laboratory animals. This feature
provides a ready framework for cross species extrapolation of data,
though considerable work must still be performed before the health
implications of alterations in some of these end-points is fully
understood. A second important feature is that the relationships
between behavioural and electrophysiological alterations produced by
toxic agents can be observed in awake subjects, as can any
dissociation between these two types of end-points.
Finally, it cannot be emphasized too strongly that the CNS is
constantly receiving afferent input and sending efferent output to all
the organs of the body. This principle of self-regulation of
physiological functions, incorporating the cybernetic principle of
feedback gave rise to the functional system theory of Anokhin (1935).
This theory has been described in detail by Anokhin (1968, 1974) and
Sudakov (1982). According to this theory, functional systems are
dynamic self-regulated organizations, the components of which
contribute to the attainment of a useful adaptive result for an
organism. The components of any given functional system are united or
integrated by the afferent and efferent CNS influences impinging on
it. The importance of this theory to toxicologists lies in its focus
on: (a) adaptive mechanisms; and (b) the inseparable nature between
the CNS and the other organs via the afferent input and efferent
outflow. First, it is the interference with adaptive mechanisms that
gives rise to the signs and symptoms produced by a toxic agent.
Second, the inseparable nature of the CNS and other organs complicates
localization of the primary target of a toxic agent. Thus, the
electrophysiological changes seen in the CNS or the alteration in
behaviour may not be due to a primary effect on the CNS, but may
rather be the result of alterations in afferent input due to
disturbances in peripheral systems such as the gastrointestinal tract,
liver, or kidney. Or, the chemical could interfere with a metabolic
process common to a number of "functional systems" subserving
different biological needs. The lesson, as stated previously in both
sections 2 and 3, is that neurotoxicologists must not be too hasty in
concluding that any effect observed is due to a direct effect on the
CNS. They should, in fact, demonstrate that it is not due to an effect
on another organ.
4.6 Summary and Conclusions
Neurophysiological methods are important in animal neuro-
toxicology. They provide direct laboratory counterparts for many of
the tests used in human beings. They can give insight into possible
site(s) and mechanism(s) of actions. They can be particularly useful
when used concomitantly with behavioural methods. The use of evocative
techniques (arousal, stimuli, work loads, interactions with
psychopharmacological agents) can increase the sensitivity of the
tests for detecting toxicant effects. As with behavioural methods, it
is incumbent on the investigator to determine whether an effect on the
CNS is due to a primary action of the toxic agent or a secondary one
as a result of damage to some other organ, thus altering the afferent
input into the CNS or its processing within the CNS.
The most useful techniques for screening are those that are
minimally invasive, relatively cheap and rapid to perform, and test
neural function in a broad sense. Depending on the particular
application, these may include evoked potential, EEG, EMG,
excitability, or conduction velocity studies. More restricted use of
these techniques, or use of microelectrode techniques, may be useful
for further characterizing the systems affected and the mechanisms of
action of neurotoxic agents. In some instances, neurophysiological
techniques have been reported to be more sensitive than behavioural,
biochemical, or neuropathological measurements. Further research using
direct comparison with other methods is needed to determine the
conditions under which this is so and the significance of this in
human risk assessment.
5. MORPHOLOGICAL METHODS
5.1 Introduction
5.1.1 Role of morphology
Just as neurobehavioural methods find their special use in the
description and analysis of neurotoxic diseases for which there are no
pathological correlates, the morphologist's special contribution is to
describe neurotoxic conditions associated with structural alterations
of nervous tissue (Table 2). Commonly, as in most chronic neurotoxic
diseases, pathological alterations in the nervous system lead to
changes in behaviour and function. These changes may provide important
clues as to the site and even the nature of the underlying structural
damage. Conversely, analysis of the location and type of pathological
change often allows the morphologist to predict the type of
accompanying dysfunction, the likely duration of the abnormality, and
the degree of reversibility. This type of structural-functional
correlation, so widely used in the evaluation of human neurological
disorders, is especially helpful for assessing the severity of
neurotoxic disease in experimental animals and, thereby, the
implications for human exposure.
A properly conducted morphological examination of experimental
animals establishes or rules out the existence of structural damage,
identifies the most vulnerable sites within the nervous system and
traces the temporal evolution of pathological changes. Armed with this
information, the morphologist often is able to advise the electro-
physiologist and biochemist where to focus their attention for the
early detection and detailed analysis of the underlying neurocellular
dysfunction.
5.1.2 Basis for morphological assessment
An understanding of the organization, structure, and function of
the normal nervous system is the point of departure for any assessment
of pathological changes induced by exposure to chemical substances.
This requires not only a working knowledge of neuroanatomy (Williams &
Warwick, 1975; Pansky & Allen, 1980), but also an understanding of
structural variations that may occur in relation to factors such as
the species under study and the age of the animal. In addition, since
the morphologist usually studies dead tissue, an understanding of
possible post-mortem changes is required.
5.2 The Nervous System and Toxic Injuries
5.2.1 The nervous system
The nervous system may be separated anatomically into central and
peripheral divisions. The peripheral nervous system (PNS) is composed
of nerve cells (neurons) and their processes (axons) which conduct
Table 2. Morphological assessment in neurotoxic injuries
Type of neurotoxic Chemical Pathological change
injury
Neurons
excitable (neuronal) pyrethroid none expected
membrane
neurotransmitter anticholinesterase none or terminal and
systems muscle swelling
anabolic disturbance doxorubicin chromatolysis, or somal
degeneration and neuronophagia,
Wallerian degeneration,
muscle atrophy (PNS),
or transynaptic
neuronal degeneration (CNS)
catabolic disturbance swainsonine increase of axonal and/or
somal lysosomes
axonal transport acrylamide accumulation of cytoskeletal
elements and/or organelles,
Wallerian degeneration
dedrite lathyrus toxin swelling, variable involvement
of soma
Special sense organs
retina methanol oedema
inner ear arsenical degeneration of stria
vascularis
Glial and myelinating cells
astrocyte 6-aminonlcotinamide swelling and degeneration
oligodendrocyte isoniazid degeneration and myelin
vacuolation
central myelin triethyltin myelin vacuolation and loss
Table 2 (Cont'd)
Type of neurotoxic Chemical Pathological change
injury
Schwann cell diptheria toxin degeneration and local
demyelination
peripheral myelin hexachlorophene myelin vacuolation and loss
Blood vessels
cadmium haemorrhage and associated
neurocellular degeneration
information between muscles, glands, sense organs, and the spinal cord
or brain. The PNS includes afferent (sensory) and efferent (motor)
fibres, and both types are represented in the somatic and visceral
(autonomic) components of the nervous system. Somatic afferent fibres
carry information from special organs and sensory receptors in skin
and muscles, while visceral afferents convey impulses from the gut,
glands, and various organs. On the motor side, somatic efferents
innervate striated muscle, while visceral efferents supply smooth
muscles of blood vessels, glands, and gut. In toxic states involving
the PNS, degeneration of somatic sensory-motor fibres leads to
peripheral neuropathies associated with sensory loss (e.g., decreased
sensitivity to vibration, touch, and position sense) and motor
weakness in distal extremities, while dysfunction or breakdown of
autonomic fibres may lead to abnormal sweating, cardiovascular
changes, or gastrointestinal, urinary-tract, genital, and other types
of dysfunction (Schaumburg et al., 1983; Dyck et al., 1984).
Manifestations of central nervous system (CNS) disorders depend
largely on the site and nature of the induced functional or structural
change (Collins, 1982). The CNS consists of the parts of the nervous
system contained within the skull and vertebral column. The spinal
cord receives information from PNS afferents supplying skin, muscles,
and glands, transmits signals for motor function by way of efferent
fibres, and communicates via specific pathways with coordination
centres within the brain. The brain is immensely complex and
responsible for initiating, receiving, and integrating signals needed
to maintain internal homeostasis, cognition, awareness, memory,
language, personality, sexual behaviour, sleep and wakefulness,
locomotion, sensation, vision, audition, balance, and many other body
functions. Most of the available information on the structural-
functional correlates of brain come from the study of higher mammals,
including man (Truex & Carpenter, 1983). The brainstem, consisting of
the midbrain, ports, and medulla oblongata, receives and processes
information from skin, muscles, and special sense organs (e.g., inner
ear) and, in turn, controls these muscles, as well as certain
autonomic functions. The cerebellum and basal ganglia are required for
the modulation and coordination of muscle movement. The diencephalon,
including the thalamus and hypothalamus, is a relay zone for
transmitting information about sensation and movement, and also
contains important control mechanisms to maintain the internal
homeostasis of the body. The hypothalamus functions as the primary
control centre for the visceral system and serves to integrate the
activity of the endocrine and other systems. The cerebral hemispheres,
capped by the cerebral cortex, are concerned with perceptual,
cognitive, motor, sensory, visual, and other functions. The optic
nerves and their radiations conduct visual information from the
retina, through the thalamus to the occipital cortex.
5.2.2 Cellular structure of the nervous system
Neurons and glial cells are the fundamental cellular elements of
the nervous system, and these are associated with blood vessels and
other specialized epithelial and connective tissue cells (Williams &
Warwick, 1975). Neurons are equipped with multiple short processes
(dendrites) that receive information from other nerve cells, and a
single long axon that conducts electrical signals to other neurons and
muscles, and to or from the skin, muscles, and glands (Fig. 1). The
axon terminates at a synapse where chemically-encoded information is
conveyed to neurons or muscle.
Glial cells in the CNS include astrocytes, oligodendrocytes, and
microglia (Fig. 2). Astrocytes are divisible into protoplasmic and
fibrous forms, are closely associated with neurons and blood vessels,
and may play a nutritive role in maintaining neurons and other cells.
Oligodendrocytes are responsible for elaborating short lengths of
myelin around multiple axons, and microgila have a phagocytic
function. In the PNS, Schwann cells envelop multiple small axons
(unmyelinated fibres) or associate with and elaborate lengths of
myelin (internodes) around single axons. Myelinated and unmyelinated
fibres are separated from each other by endoneurial connective tissue
composed of fibroblasts and collagen, and these elements are bound
together in fasciles surrounded by a fibrocellular sleeve, the
perineurium. Bundles of fascicles held together by epineurial
connective tissue form a peripheral nerve.
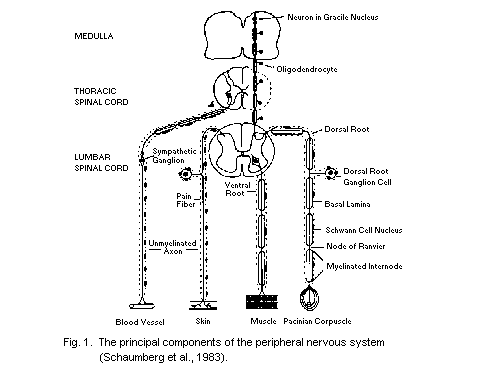
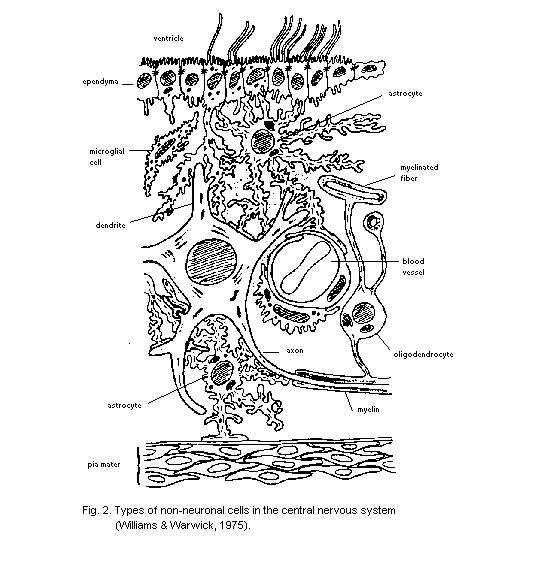 5.2.3 Neurocellular reaction to injury
5.2.3.1 Biological principles
Neurons are highly atypical cells because their cytoplasmic
processes often occupy a much greater volume than their cell somata.
This unusual cellular architecture provides an enormous surface area
for chemical attack and places a great demand on the soma, since it
alone has the metabolic machinery required to maintain the cellular
processes. Specialized transport systems have evolved to convey
information to the cell body, the axon, and its terminal regions
(Ochs, 1982). The functional problem can be illustrated by considering
a peripheral motor neuron, located in the lumbar spinal cord and
innervating muscle in the foot, which must maintain the structure and
function of an axon that is about a metre in length. Failure to
maintain the entire length of this enormous column of cytoplasm, by
interruption of axonal transport or by some other mechanism, may
account for the vulnerability of distal axons in many types of
neurotoxic disease. Other types of neurotoxins (e.g., doxorubicin) may
interfere with the metabolic machinery of the soma, thereby resulting
in degeneration of the entire neuron. Comparable explanations can be
offered to explain the vulnerability to chemical attack of myelinating
cells, their cytoplasmic processes, and myelin sheath (Spencer &
Schaumburg, 1980).
There are two considerations when contemplating toxic damage to an
organ or tissue. First, there are the changes that can be related to
the action of the chemical agent; second, there is the reaction of the
tissue to these changes. When toxic damage to neurons and myelinating
cells is considered, it is often difficult to separate the two phases
of the pathological process being studied. The situation is further
complicated as the type of change observed is often dependent on dose,
and may also vary with other factors, such as the species. In general,
however, chemical attack leads to two types of primary change in
neural cells:
(a) the accumulation, proliferation, or rearrangement of
structural elements (e.g., intermediate filaments,
microtubules) or organelles (e.g., mitochondria, lysosomes);
and/or
(b) the breakdown (degeneration) of cells, in whole or in part.
The latter is usually followed by regenerative processes, and these
may occur during the period of intoxication. Changes in the cellular
elements of intraneural blood vessels may occur, and secondary changes
may develop in other organ systems, notably voluntary muscle (Walton,
1974).
Neural cells appear to have a limited repertoire of pathological
responses, and the consequences of many types of toxic damage can be
predicted from an understanding of the biology and function of the
cells that are involved. As in any organ system, changes in one cell
type -- the primary target -- usually lead to secondary and tertiary
responses in related cells, so that the net effect is a predictable
cascade of pathological responses. For the nervous system, however,
the neuropathological changes often have to be considered with regard
to the fact that, while cellular responses to toxic injury may occur
locally, they may also occur at distant sites. Changes may also
develop in different locations as a function of time, dose, and/or
duration of intoxication.
5.2.3.2 Neurons
Loss of the cell body of neurons (neuronopathy) is an irreversible
event seen in many types of intoxication. Similar tissue reactions
occur in the CNS and PNS. Fig. 3 illustrates the changes occurring
with loss of primary sensory neurons in dorsal root ganglia. Glial
cells and macrophages proliferate around the dying cell (neurono-
phagia) and axon processes under Wallerian degeneration. This involves
breakdown of the entire length of the axon, dissolution and removal of
the myelin sheath, and the proliferation of phagocytic and glial
cells. Degenerated myelin is removed more slowly in the CNS than in
the PNS and astrocytes increase the number and size of their fiberous
processes to occupy the space left by the degenerating nerve fibre.
Axon degeneration also produces significant secondary changes in the
denervated cells, such as muscle (neurogenic atrophy) or other neurons
(transynaptic and retrograde transynaptic degeneration) (Blackwood et
al., 1971).
Neurons survive and recover from certain types of toxic assault,
notably those that cause structural damage to their processes.
Abnormalities of axons may be expressed in the form of generalized
atrophy or localized swellings containing excessive numbers of
structural elements or organelles, with associated secondary changes
in the myelin sheath in the form of corrugation and displacement
(secondary demyelination), respectively. Disruption of axonal
integrity leads to axon atrophy and/or degeneration (axonopathy) below
the site of injury (Fig. 4), with secondary changes in target cells,
such as muscle (neurogenic atrophy) or neuron (transynaptic
degeneration). Oligodendrocytes and Schwann cells lose their ability
to maintain myelin and then undergo cell division, while phagocytic
cells remove the myelin debris. The cell bodies of affected neurons
may also undergo responses secondary to axonal lesions, including cell
necrosis or, more commonly, rearrangement of cellular components
(chromatolysis) as a prelude to axon regeneration. Regrowth of the
axon commences promptly at the position of axon interruption and, in
the PNS, the elongating neuronal process reassociates with Schwann
cells, which elaborate a new myelin sheath and conduct the
5.2.3 Neurocellular reaction to injury
5.2.3.1 Biological principles
Neurons are highly atypical cells because their cytoplasmic
processes often occupy a much greater volume than their cell somata.
This unusual cellular architecture provides an enormous surface area
for chemical attack and places a great demand on the soma, since it
alone has the metabolic machinery required to maintain the cellular
processes. Specialized transport systems have evolved to convey
information to the cell body, the axon, and its terminal regions
(Ochs, 1982). The functional problem can be illustrated by considering
a peripheral motor neuron, located in the lumbar spinal cord and
innervating muscle in the foot, which must maintain the structure and
function of an axon that is about a metre in length. Failure to
maintain the entire length of this enormous column of cytoplasm, by
interruption of axonal transport or by some other mechanism, may
account for the vulnerability of distal axons in many types of
neurotoxic disease. Other types of neurotoxins (e.g., doxorubicin) may
interfere with the metabolic machinery of the soma, thereby resulting
in degeneration of the entire neuron. Comparable explanations can be
offered to explain the vulnerability to chemical attack of myelinating
cells, their cytoplasmic processes, and myelin sheath (Spencer &
Schaumburg, 1980).
There are two considerations when contemplating toxic damage to an
organ or tissue. First, there are the changes that can be related to
the action of the chemical agent; second, there is the reaction of the
tissue to these changes. When toxic damage to neurons and myelinating
cells is considered, it is often difficult to separate the two phases
of the pathological process being studied. The situation is further
complicated as the type of change observed is often dependent on dose,
and may also vary with other factors, such as the species. In general,
however, chemical attack leads to two types of primary change in
neural cells:
(a) the accumulation, proliferation, or rearrangement of
structural elements (e.g., intermediate filaments,
microtubules) or organelles (e.g., mitochondria, lysosomes);
and/or
(b) the breakdown (degeneration) of cells, in whole or in part.
The latter is usually followed by regenerative processes, and these
may occur during the period of intoxication. Changes in the cellular
elements of intraneural blood vessels may occur, and secondary changes
may develop in other organ systems, notably voluntary muscle (Walton,
1974).
Neural cells appear to have a limited repertoire of pathological
responses, and the consequences of many types of toxic damage can be
predicted from an understanding of the biology and function of the
cells that are involved. As in any organ system, changes in one cell
type -- the primary target -- usually lead to secondary and tertiary
responses in related cells, so that the net effect is a predictable
cascade of pathological responses. For the nervous system, however,
the neuropathological changes often have to be considered with regard
to the fact that, while cellular responses to toxic injury may occur
locally, they may also occur at distant sites. Changes may also
develop in different locations as a function of time, dose, and/or
duration of intoxication.
5.2.3.2 Neurons
Loss of the cell body of neurons (neuronopathy) is an irreversible
event seen in many types of intoxication. Similar tissue reactions
occur in the CNS and PNS. Fig. 3 illustrates the changes occurring
with loss of primary sensory neurons in dorsal root ganglia. Glial
cells and macrophages proliferate around the dying cell (neurono-
phagia) and axon processes under Wallerian degeneration. This involves
breakdown of the entire length of the axon, dissolution and removal of
the myelin sheath, and the proliferation of phagocytic and glial
cells. Degenerated myelin is removed more slowly in the CNS than in
the PNS and astrocytes increase the number and size of their fiberous
processes to occupy the space left by the degenerating nerve fibre.
Axon degeneration also produces significant secondary changes in the
denervated cells, such as muscle (neurogenic atrophy) or other neurons
(transynaptic and retrograde transynaptic degeneration) (Blackwood et
al., 1971).
Neurons survive and recover from certain types of toxic assault,
notably those that cause structural damage to their processes.
Abnormalities of axons may be expressed in the form of generalized
atrophy or localized swellings containing excessive numbers of
structural elements or organelles, with associated secondary changes
in the myelin sheath in the form of corrugation and displacement
(secondary demyelination), respectively. Disruption of axonal
integrity leads to axon atrophy and/or degeneration (axonopathy) below
the site of injury (Fig. 4), with secondary changes in target cells,
such as muscle (neurogenic atrophy) or neuron (transynaptic
degeneration). Oligodendrocytes and Schwann cells lose their ability
to maintain myelin and then undergo cell division, while phagocytic
cells remove the myelin debris. The cell bodies of affected neurons
may also undergo responses secondary to axonal lesions, including cell
necrosis or, more commonly, rearrangement of cellular components
(chromatolysis) as a prelude to axon regeneration. Regrowth of the
axon commences promptly at the position of axon interruption and, in
the PNS, the elongating neuronal process reassociates with Schwann
cells, which elaborate a new myelin sheath and conduct the
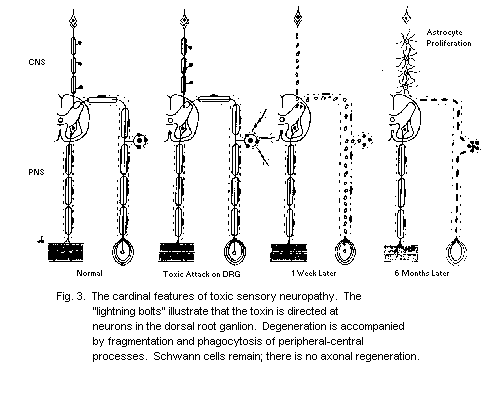
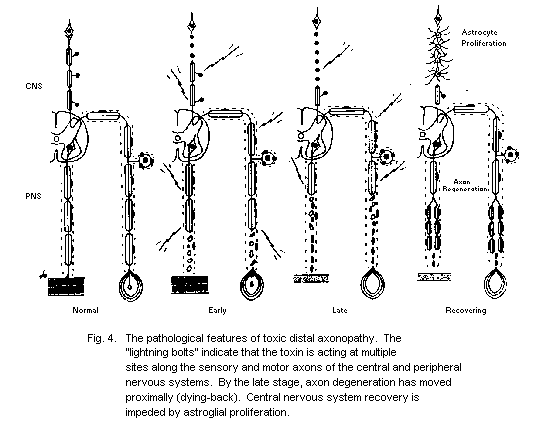 regenerating axon to its target organ (muscle or sense organ)
(Schaumburg et al., 1983). In the CNS, astrocytes respond to injury by
increasing the number and size of their fibrous processes, and these
appear to inhibit regeneration of all but the smallest (e.g.,
monoaminergic) axons. Prominent axon swellings develop at the viable
end of larger fibres, a process known as axonal dystrophy.
5.2.3.3 Myelinating cells
Some chemical compounds induce primary morphological changes in
myelinating cells or their myelin sheaths. It is usual to separate
agents that induce changes in the cell body of Schwann cells or
oligodendrocytes from those that cause abnormalities selectively in
the myelin sheath, though this distinction may be misleading as many
compounds can produce both types of change, depending on dose and
length of exposure. The net result of either type of CNS or PNS insult
is primary demyelination (myelinopathy) (Fig. 5). Loss of
oligodendrocyte somata is associated with the arrival of phagocytic
cells which remove myelin and become filled with droplets of neutral
fat. Astrocytes are commonly affected by chemical agents that perturb
oligodendrocytes but, if they survive, astrocytes may also take up
small amounts of myelin debris, divide, hypertrophy and, if
remyelination does not occur, surround the demyelinated axons. More
usually, however, new oligodendrocytes are recruited, their processes
contact the demyelinated axon and these elaborate new but
foreshortened internodal lengths of myelin (remyelination). Loss of
Schwann cells also results in primary demyelination, cell
multiplication, and remyelination. Another common type of myelinopathy
involves the processes of CNS and PNS myelinating cells: these
disorders are usually visualized by the accumulation of oedema fluid
within the myelin sheath or its associated cellular processes (Spencer
& Schaumburg, 1980). Vacuolation of the myelin sheath is usually a
reversible process, though severe intramyelinic swelling may constrict
the axon and induce Wallerian degeneration.
5.3 Experimental Design and Execution
5.3.1 General principles and procedure
Morphological examination is primarily concerned with studying
structural changes in the nervous system that might explain
behavioural disturbances. These changes are most commonly found when
there is repeated dosing, but permanent neurological damage may also
occur after single doses of some compounds. On occasion, such changes
appear to be minor but, if restricted to a few neurons of a nucleus of
considerable importance, may result in profound functional effects.
This is particularly true of the developing nervous system where,
because of stepwise, interdependent development, damage induced by a
chemical agent at one stage may have a "domino effect" on later stages
of development. Damage to CNS structures, especially when loss of
regenerating axon to its target organ (muscle or sense organ)
(Schaumburg et al., 1983). In the CNS, astrocytes respond to injury by
increasing the number and size of their fibrous processes, and these
appear to inhibit regeneration of all but the smallest (e.g.,
monoaminergic) axons. Prominent axon swellings develop at the viable
end of larger fibres, a process known as axonal dystrophy.
5.2.3.3 Myelinating cells
Some chemical compounds induce primary morphological changes in
myelinating cells or their myelin sheaths. It is usual to separate
agents that induce changes in the cell body of Schwann cells or
oligodendrocytes from those that cause abnormalities selectively in
the myelin sheath, though this distinction may be misleading as many
compounds can produce both types of change, depending on dose and
length of exposure. The net result of either type of CNS or PNS insult
is primary demyelination (myelinopathy) (Fig. 5). Loss of
oligodendrocyte somata is associated with the arrival of phagocytic
cells which remove myelin and become filled with droplets of neutral
fat. Astrocytes are commonly affected by chemical agents that perturb
oligodendrocytes but, if they survive, astrocytes may also take up
small amounts of myelin debris, divide, hypertrophy and, if
remyelination does not occur, surround the demyelinated axons. More
usually, however, new oligodendrocytes are recruited, their processes
contact the demyelinated axon and these elaborate new but
foreshortened internodal lengths of myelin (remyelination). Loss of
Schwann cells also results in primary demyelination, cell
multiplication, and remyelination. Another common type of myelinopathy
involves the processes of CNS and PNS myelinating cells: these
disorders are usually visualized by the accumulation of oedema fluid
within the myelin sheath or its associated cellular processes (Spencer
& Schaumburg, 1980). Vacuolation of the myelin sheath is usually a
reversible process, though severe intramyelinic swelling may constrict
the axon and induce Wallerian degeneration.
5.3 Experimental Design and Execution
5.3.1 General principles and procedure
Morphological examination is primarily concerned with studying
structural changes in the nervous system that might explain
behavioural disturbances. These changes are most commonly found when
there is repeated dosing, but permanent neurological damage may also
occur after single doses of some compounds. On occasion, such changes
appear to be minor but, if restricted to a few neurons of a nucleus of
considerable importance, may result in profound functional effects.
This is particularly true of the developing nervous system where,
because of stepwise, interdependent development, damage induced by a
chemical agent at one stage may have a "domino effect" on later stages
of development. Damage to CNS structures, especially when loss of
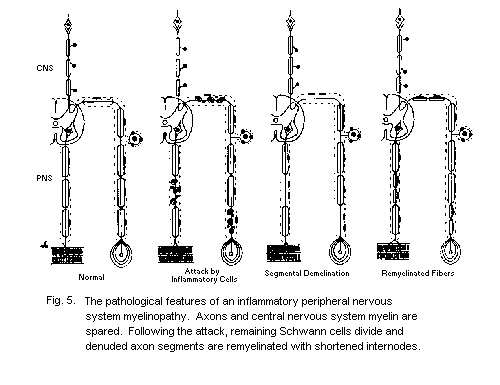 nerve cells is involved, is more likely to be associated with
permanent behavioural changes than disorders predominantly affecting
the peripheral nervous system, where satisfactory regeneration is more
often the rule. Rapidly reversible effects on behaviour are
occasionally, but not often, associated with detectable structural
damage to the nervous system.
5.3.2 Gross morphology
While most neurotoxic damage is most evident at the microscopic
level, it is important to pay attention to the weight of the brain as
well as to macroscopic manifestations such as discolouration (for
example, due to bilirubin), discrete or massive haemorrhage, or other
localized lesions (for example, transverse myelitis).
The new methods of nuclear magnetic resonance (NMR) can supply
data concerning structural changes, for example, demyelination, both
in vivo and post-mortem.
5.3.3 The role of histology
There is no substitute for a thorough light-microscope examination
of the nervous system and other organs for the initial assessment of
animals suspected of having neurotoxic injury. The same careful
attention to anatomical detail should be given in the examination of
experimental animal tissues as would be given by a pathologist
studying autopsy tissue from a patient who has suffered from a new
type of neurological disease. The results of this type of analysis
will provide direction for additional studies that focus on more
specific questions such as the type and extent of structural damage,
the location where changes first occurred, the time-to-onset of
structural damage, the reversibility of these changes, the lowest dose
that caused pathological changes, and the highest no-observed-adverse-
effect dose.
5.3.3.1 Biological principles dictating tissue response
The study design must take into account several important
principles of underlying cellular responses to injury. These
principles will influence when and where tissue should be sampled for
morphological study.
Often, there is a delay of days or weeks between dosing and the
first appearance of structural changes in animals treated with
neurotoxic agents. The extent of the delay depends largely on the
chemical used, the dose, and the species studied. Once the
pathological process has been initiated, a chain of degenerative and
regenerative events may be set in motion, and these may or may not
cease to evolve after dosing has stopped. Most neurotoxic substances
that damage neurocellular elements produce a largely symmetrical
pattern of structural change, whether in the CNS or PNS. Some neurons
or nerve fibres may be more vulnerable than others to chemical attack,
so that at any point in the pathological process, the observer may be
presented with many or all of the stages in the reaction of individual
cellular elements. The active pathological process may also move in
space with time, as in dying-back axonpathies in which degeneration
proceeds retrogradely along affected nerve and fibre tracts (Cavanagh,
1964). Careless sampling of animals with these or other types of
neurotoxic diseases may reveal little or no pathological changes. By
contrast, the careful and thorough investigator will be able to
distinguish between early and later changes, and thereby develop a
hypothesis of the spatial-temporal sequence of the pathological
process. This can be tested in subsequent studies in which animals are
examined at different stages in the development of the disease.
Investigations of this type will also provide information on the
relationship between the dose and duration of treatment required to
induce neurotoxic injury. No-observed-adverse-effect levels can be
established by determining doses that do not produce any
characteristic changes after a specific period of chemical treatment.
Quantitative estimation of structural abnormalities in treated versus
control animals becomes increasingly important as the no-observed-
adverse-effect dose is approached.
5.3.4 Use of controls
Parallel study of age-matched control animals is mandatory,
whenever treated animals are subjected to neuropathological
examination. Control groups should include untreated animals, vehicle-
treated animals, and positive controls. The may receive a neurotoxic
dose of the agent under study or of another compound with well-
characterized neurotoxic properties that elicits a comparable type of
neurotoxic damage. Demonstrating the development of an appropriate
neurotoxic response in the positive control strengthens the validity
of data obtained from animals treated with the agent under study,
especially if these prove to lack pathological changes. The use of
positive controls is imperative for the inexperienced investigator who
needs to gain confidence from the self-demonstration that a known
neurotoxic agent produces the expected changes. Inclusion of these
results in a published report will also reassure the audience that the
investigator has reproduced the expected pattern of structural damage
associated with a known neurotoxic chemical.
There are important advantages in studying the structure of
nervous tissue without knowledge of the treatment group. The
investigator is forced to be more critical in seeking and describing
abnormal findings, and the final assessment is a more objective
statement of the similarities and differences between treatment and
control groups.
5.3.5 Pattern of response
It is not the purpose of this publication to provide a detailed
analysis of the various problems of selective neuronal damage
encountered under neurotoxic conditions. However, it is emphasised
that occasionally the localization is very precise, limited to one or
two nuclei in the brain stem, or to distal regions of long fibre
tracts and peripheral nerves. Selective pathological changes are given
in Table 3 for reference in interpreting new situations.
5.3.6 Data acquired
At the end of the survey, clear answers must be found to the
following questions about the CNS:
1. Are there changes in nerve cells in the area of the cerebral
cortex, basal ganglia, hypothalamus, limbic system, diencephalon,
brain stem, cerebellum, or spinal cord?
2. Are there any changes in shape or number of astroglia and/or
microglial cells?
3. Is there any loss or other change in oligodendroglia?
4. Are there any changes in the myelinated areas of the brain or
spinal cord, such as myelin fragmentation or myelin vacuolation?
Is this associated with axonal swellings and/or degeneration?
5. If 4 is positive, are there glial responses?
6. Are there focal, vascular, and necrotic changes? If so, where?
7. Are tumours present?
8. Are there any changes in special sense organs to account for
functional changes?
For the peripheral nerves, the following questions must be
answered:
1. Are there any changes in peripheral nerves?
2. If so, is it axon degeneration or primary demyelination, or both?
3. What is the distribution of degeneration? Is it distal, affecting
only long and large-diameter fibres, or are shorter fibres such as
cranial nerves also affected?
4. Are sensory, motor, or both types of fibres involved?
Table 3. Examples of toxic effects illustrating the specific
patterns of damage that may be found
Pattern Cause Reference
Neuronal changes
laminar cortical necrosis anoxia Briefly (1976)
hippocampal pyramidal cell trimethylin Brown et al.
damage (1979)
selective granule cell loss methylmercury Hunter & Russell
from the cerebellum (1954)
selective loss of inferior 3-acetylpyridine Desclin (1974)
olivary cells
selective degeneration of doxorubicin Cho et al. (1980)
perikarya of sensory
ganglion cells
selective degeneration of methylmercury Cavanagh & Chen
axons of sensory ganglion (1971)
cells
retrograde degeneration of organophosphorus, Cavanagh (1964);
long sensory and motor acrylamide, Spencer &
axons in CNS and PNS hexanedione Schaumburg (1977)
retrograde degenerations of clioquinol Krinke et al. (1979)
axons of long spinal cord
tracts
Myelin changes
CNS myelin vacuolation triethyltin Aleu et al. (1963)
CNS and PNS myelin hexachlorphene Towfighi et al.
vacuolatin (1975)
focal degeneration of PNS diphtheria Cavanagh & Jacobs
myelin without axon loss (1954)
focal degeneration of PNS lead Fullerton (1966)
myelin with some axon loss
Table 3 (Cont'd)
Pattern Cause Reference
Vascular and neurotic changes
symmetrical lesions in misonidazole Griffin et al.
brain-stem nuclei (1980)
non-symmetrical brain-stem lead Wells et al. (1976)
and cortical lesions
5. What is the state of nerve cell bodies (i.e., sensory ganglion
cells, anterior horn cells)?
6. If the cells in 5 show changes, are those in the medulla and
trigeminal ganglia (i.e., cranial nerves) similarly affected?
7. Are neurons normal, chromatolytic, or degenerated?
8. Are automatic ganglia or nerves similarly affected or not?
9. Are muscles affected by denervation and/or myopathic changes?
10. At dose levels below those that produce clinical signs, is there
histochemical and/or morphometric evidence of metabolic neuronal
changes that is brought out by using these more sensitive
techniques?
5.4. Principles, Limitations, and Pitfalls of the Morphological
Approach
5.4.1 Tissue state
Although under special circumstances the morphologist is able to
study the reactions of living tissue to chemical exposure, either
in vivo or in tissue culture (Yonesawa et al., 1980), the vast
majority of pathological studies involve the assessment of dead
tissue. The structural changes that occur in the nervous system as a
function of time post-mortem can obscure the recognition of toxic
injury and may confuse the inexperienced neuropathologist. Chemical,
or rarely, physical (freezing) fixation methods are used to prevent
the development of post-mortem changes.
5.4.2 Principles of fixation
The use of a reliable fixation method is mandatory for the
morphological examination of nervous tissue (Thompson, 1963; Pease,
1964; Hayat, 1973; Gabe, 1976; Bancroft & Stevens, 1977; Glausert,
1980). The purpose of fixation is to preserve cellular architecture as
closely as possible to that in the living state, to inhibit the loss
of chemical components required for the maintenance of morphology, and
to prepare the tissue to accept stains that enhance tissue density for
clear resolution of cytological detail. The ideal method of fixation
is one that instantaneously terminates life processes in the tissue of
interest without distorting cytological detail. Rapid freezing of
living tissue approaches this ideal. Excision and freezing of tissue
is routinely employed for the assessment of human biopsy tissue and is
particularly suitable for light-microscope histochemistry and
immunocytochemistry. A few specialized laboratories use rapid freezing
for the examination of tissue, or metal replicas thereof, by
transmission electron microscopy.
Chemical methods are far more commonly used in neurotoxicology for
the preparation of nervous tissue. Although the ideal chemical
fixative should instantaneously kill living tissue without inducing
structural changes, all known methods fall short of this ideal.
Instead, the morphologist must strive to induce small, consistent and
readily recognizable changes that will not compromise tissue
examination and assessment. The most satisfactory and widely used
chemical fixatives are dilute, aqueous solutions of aldehydes
(formaldehyde and/or glutaraldehyde), which rapidly cross-link
proteins and stabilize associated lipids, prevent post-mortem changes,
and introduce rigidity into the tissue to facilitate handling. A
secondary phase of fixation using a dilute aqueous solution of osmium
tetroxide to fix lipids is routinely added after glutaraldehyde
fixation for improved preservation of tissue. Occasionally, osmium
tetroxide is used as the primary fixative. Structural artefacts
induced by fixation are minimized if the fixative solutions are
buffered to match tissue pH and osmolality. Temperature should also be
controlled; low temperatures may affect the preservation of fine
structural elements (e.g., microtubles).
Chemical fixatives must be delivered to the site of interest as
rapidly as possible if post-mortem artefacts are to be minimized. Most
effective is systemic perfusion (Pease, 1964; Hayat, 1973; Spencer et
al., 1980; Spencer & Bischoff, 1982), in which the fixative is
introduced via the ascending aorta of the deeply anaesthetized animal,
and distributed throughout the vascular network under controlled
pressure. Spaces normally occupied by blood and tissue fluid are
rapidly replaced by the chosen fixative solution, and the animal
expires painlessly within seconds. Perfusion of individual organs is
an alternative, but more difficult and less satisfactory technique. An
alternative method, suitable only for readily accessible parts of the
nervous system (e.g., peripheral nerves), is to bathe the tissue
in situ with a repeatedly replenished solution of fixative.
In practical terms, the requirements for tissue sampling, organ
weighing, etc., have to be balanced against the need to get good
fixation, and this frequently presents conflicts of interest between
those involved in analysing the animal tissues. Short perfusion
fixation (section 5.5.2), with paraformaldehyde and subsequent
secondary fixation in formalin or formal-acetic acid in the case of
paraffin sections and light microscopy, and glutaraldehyde and osmium
tetroxide in the case of epoxy resin, semi-thin sections, may help to
provide a compromise. When the protocol for killing requires only
immersion fixation, then a few animals in each group must be kept for
perfusion fixation, if adequate tissue examination is going to be
achieved. A study using only immersion fixation is scarcely worth the
labour and cost involved.
The selection of the chemical fixative and its method of delivery
to the tissue site are chosen according to the type of information
that is required. Systemic perfusion with the chosen fixative is
always to be preferred. In general, formaldehyde is suitable for
studies that require widespread tissue sampling, low resolution of
cytological detail, or the use of special histochemical stains to
assess the chemical content of abnormal cells and tissues. Conversely,
in studies focusing on more selected areas of the nervous system or
requiring resolution of fine structural detail, the use of
glutaraldehyde and osmium tetroxide is mandatory. While the latter
method was originally introduced for use in transmission electron
microscopy, an increasing number of laboratories use the technique
routinely to exploit the remarkable resolution afforded by the light
microscope.
5.4.3 Principles of tissue sampling
Extensive sampling of tissue is essential for the initial
assessment of a suspected neurotoxic injury. Organs, other than the
nervous system, which should be examined, are listed in WHO (1978).
Neural tissue should include: brain, including cerebellum, brain stem,
pituitary gland, eye with occulomotor muscles and optic nerve
attached, spinal cord, several sensory ganglia, sciatic nerve and its
branches from its exit from the vertebral column to the level of the
ankle, and selected muscles innervated by the sciatic nerve and its
branches. Other regions less commonly sampled include: olfactory
epithelium and tubercles, inner ear and labyrinths, plantar nerves and
skin receptors, autonomic ganglia, and nerves and organs of
innervation, such as the gut. The brain contains many sites known to
be vulnerable to chemical agents and should be examined as thoroughly
as possible. The use of an atlas such as that for the rat brain (Zeman
& Innes, 1963) will be valuable. The brain stem should be sectioned
just rostral to the cerebellum, the slice passing through the inferior
colliculi. The cerebral hemispheres can then be sliced coronally
(transversely). Olfactory lobes may be taken if questions of
inhalation and olfaction are being studied. The cerebellum is removed
by cutting through the peduncles that attach it to the brain stem.
Sagittal sections are obtained by cutting through the midline, and
parallel sections are also taken. Emphasis is usually placed on the
retina and optic nerves, since these are often heavily involved in
toxic diseases affecting neurons, axons, and myelin. Ototoxicity is
also a common event, though the technical difficulties associated with
the examination of the inner ear and labyrinths have prevented
widespread study of this phenomenon. Olfactory lobes and associated
epithelium are rarely examined, but should be taken if questions of
inhalation and olfaction are being studied.
The spinal cord contains ascending and descending nerve fibre
tracts that commonly undergo changes in myelinopathies and
axonopathies (Table 3). Since the latter are tractable diseases with
distal accentuation, it is critical to sample various levels from the
medulla oblongata (where the gracile tract terminates) to the sacral
region. By taking transverse sections of the spinal cord, it is
possible to make a simultaneous evaluation of white matter (myelinated
tracts and glial cells), gray matter (neurons, dendrites, proximal
axons, glial cells), blood vessels, and associated tissues.
Dorsal root and cranial nerve ganglia contain primary sensory
neurons that may display pathological changes in certain
neuronopathies and axonopathies (Figs. 3, 4). Structural alterations
in blood vessels and myelin may also occur under certain conditions.
The sensory ganglia of the Vth cranial nerve, at least 4 sensory
ganglia from the cervical bulb region (C5-T1 spinal levels), and 4
from the lumbosacral regions (L3-S2 spinal levels), should be sampled
for examination in longitudinal section. Corresponding dorsal and
ventral spinal roots, vulnerable in toxic demyelinating diseases,
should also be studied.
Peripheral nerves commonly display changes in neurotoxic diseases
affecting myelin, neurons, and axons (Table 3). As the last display
distal, retrograde changes in dying-back axonopathies, a common
response to chemical attack, it is critical to examine several levels
of vulnerable nerves. Samples of the sciatic nerve and its branches
are usually taken commencing adjacent to the vertebral column and
terminating in the distal regions of the sural nerve, peroneal nerve,
and/or the tibial nerve. The fine branches of the tibial nerve that
leave the main trunk below the knee are especially vulnerable in toxic
neuropathies, because they contain very large myelinated nerve fibres.
Terminal regions of sensory and motor nerve fibres can be examined by
searching for twigs supplying intrafusal (spindles) and extrafusal
muscle fibres, respectively (Schaumburg et al., 1974). Routinely,
anterior tibial muscles, and gastronemius with suralis muscles are
examined. Muscle tissue is also valuable for assessing whether there
is denervation atrophy, evidence of toxic myopathy, nerve sprouting,
or other regenerative activity (Pearson & Mostofi, 1973).
Tissue sampling should not only be tailored to the goal of the
study but also to the method of fixation. In general, blocks of tissue
several millimeters thick may be taken if the tissue has been fixed
with formaldehyde and is destined for paraffin embedding. For tissue
fixed with glutaraldehyde, the relatively slow rate of penetration of
osmium tetroxide necessitates the use of thin (1 µm) slices of tissue.
It is often helpful to mark a small area of tissue (with Indian ink or
a small cut) to maintain the orientation. Elongate structures, such as
peripheral nerves and spinal cord, are most readily studied in
transverse sections, though important information is acquired by
complementary examination of longitudinal sections. Particularly
informative for the identification of pathological changes in
peripheral nerves is the technique of microdissection (teasing apart
intrafascicular tissue), which yields long lengths of individual
myelinated nerve fibres suitable for light microscope examination.
Inspection of these preparations can demonstrate rapidly the presence
of primary demyelination and remyelination and axonal degeneration and
regeneration. The minor changes characteristic of early toxic
neuropathies, readily missed in the examination of sections, can be
easily detected by this method. Other parts of the nervous system are
technically difficult to examine by microdissection because of the
absence of a collagen network to support the individual nerve fibres.
While it is prudent to embed tissues in paraffin wax or epoxy
resin promptly, regions that will not be examined immediately can be
stored in a solution and at a temperature appropriate to the method of
fixation. Loss of tissue quality will occur in proportion to the
length of storage.
5.4.4 Preparation of tissue for examination
While gross tissue changes can be visualized with the naked eye or
by examination with a stereoscopic binocular microscope, and surface
detail can be resolved with the scanning electron microscopy, routine
examination of cellular structure requires the use of tissue sections.
This inevitably places severe limitations on the amount of tissue that
is practical to study. Sections must be such that the energy beam
(light, electrons, X-rays) of the chosen microscope can be transmitted
through the tissue. Relatively thick sections containing several
layers of cells can be visualized with the light microscope, but the
ability to resolve cytological detail decreases as section thickness
increases. The use of semi-thin sections allows the investigator to
exploit the maximum available resolution of the light microscope. Much
thinner sections are required for the assessment of tissue by
transmission electron microscopy (Pease, 1964; Hayat, 1973).
Chemically-fixed tissue must be supported by a pliable material to
facilitate the sectioning process and to prevent disintegration of the
sectioned tissue. Paraffin wax or epoxy resin are routinely used: the
former for routine work, the latter for more specialized studies.
Since both types of embedding media are water insoluble and therefore
immiscible with the fixative solutions, water is first removed by
stepwise dehydration in ethanol or methanol immersing the tissue in an
aromatic hydrocarbon (clearing solution) that is miscible both with
ethanol and the embedding medium. Dehydration, clearing, and
infiltration with an embedding medium are procedures requiring
considerable care and time to avoid tissue drying and distortion.
Automatic systems are available and, for paraffin embedding, these are
routinely used in histological laboratories. Once the tissue is
infiltrated and embedded, the medium must be allowed to harden by
cooling (paraffin wax) or heating (epoxy resin).
Sections of tissue are prepared with the aid of knives mounted in
microtomes. Steel knives are suitable to cut thick (4-15 µm) sections
of paraffin embedded tissues, whereas knives prepared by scoring and
breaking strips of special glass are needed to prepare sections (1 µm)
of tissue embedded in the harder epoxy resin. The sections of tissue
prepared with steel knives permits study of larger tissue areas than
is possible with sections prepared from epoxy blocks, though the
latter afford a higher resolution of cytological detail. With new
developments in the preparation of glass knives and the design of
microtomes, ever larger sections can be routinely prepared by the
latter technique. Diamond knives mounted in ultramicrotomes are used
to obtain the thin sections (50 nm) required for transmission electron
microscopy.
Contrast of cytological detail in sectioned material can be
enhanced by the use of special light-microscope methods (phase,
Nomarski, fluorescence) or, more commonly, by the introduction of
stains that render structures light-dense. A wealth of cytological
stains is available for use with paraffin sections, whereas only a few
general purpose stains are usually used to enhance structural detail
for the light-microscope assessment of epoxy sections. Heavy metal
stains that are electron dense are needed to visualize fine structure
by transmission electron microscopy.
5.4.5 Recognition of artefact
Some degree of artefact is unavoidable, irrespective of the care
taken in preparing tissue for morphological examination, and
recognition and identification of the sources of artefact are of the
utmost importance in the morphological assessment. Possible artefacts
are legion and stem from various sources: e.g., poor tissue
preservation from failure of fixative to penetrate tissue; shrinkage
induced by inappropriate dehydration or drying; traumatic changes
associated with excision of the tissue (bending, stretching) or rough
handling during processing; wrinkling of sections and precipitation of
stain (Spencer & Bischoff, 1982).
5.4.6 Recognition of normal structural variations
While the gross and fine structure of the nervous system of any
species and age is remarkably constant from animal to animal, it is
critical to recognize the irregular occurrence of normal variations in
cellular structure that may lead the inexperienced observer to an
inappropriate conclusion. These variations include ectopic structures
(e.g., sensory neurons misplaced in the sciatic nerve), local
oedematous or proliferative changes (e.g., at a site of unrecognized
trauma or infection), and those that accompany advancing age (Johnson,
1981). The latter are especially important in long-term neurotoxicity
studies, and there is no substitute for rigorous comparative study of
control animals of the same age. Examples of normal age-related
changes include neuronal pigmentation by lipofuscin, localized
demyelination and remyelination, scattered neuronal loss, and axonal
dystrophy and degeneration.
5.4.7 Qualitative versus quantitative approaches
While there can be no substitute for a thorough qualitative
assessment of the morphological state of the nervous system,
morphometric methods are required for a more precise description of
these changes (Weibel, 1979; Bonashevskaya et al., 1983). This is
especially important for the description of minor changes, or when the
tissue reactions of different groups of animals are being compared.
Randomization of samples is essential to minimize subjective
influences, and a statistical approach is required both for the design
of the morphometric study and the analysis of the data. Tissue
sampling, group size, age, sex, body weight, and length are important
considerations in this regard. This type of morphometric approach has
been helpful in measuring tissue reactions to toxic chemicals in the
form of: the numbers of fibres affected in specific pathways subject
to degeneration, changes in cell populations in vulnerable parts of
the brain, and the relationship between fibre diameter and intermodal
length of peripheral myelinated nerve fibres prepared by
microdissection (Dyck et al., 1984).
5.5 Specific Procedures
5.5.1 Introduction
Strategic considerations in planning how to proceed must depend on
the state of knowledge of the possible effects. When it is unknown
whether damage occurs in nervous tissue, a wide-ranging examimation of
the nervous system with the light microscope is appropriate. Most
structural changes induced by neurotoxic chemicals can be detected
using the primary methods given below. More specialized, secondary
methods can be applied subsequently for more detailed studies.
5.5.2 Primary methods
There are strong convictions among experienced experimental
neuropathologists as to which fixation method should be chosen for the
primary screening. Some believe the conventional approach using
formaldehyde-based fixatives and paraffin embedding is most
appropriate; others prefer the more contemporary method of examining
epoxy sections of tissue fixed in glutaraldehyde and osmium tetroxide.
Each method has significant advantages and disadvantages and both
complement each other. Since it is impractical to use both methods for
the same animal, a prudent investigator will include a sufficient
number of animals to permit the parallel use of both procedures.
5.5.2.1 Formaldehyde/paraffin method
(a) Tissue fixation and excision
This technique is most suited for the study of large areas of
tissue at low resolution and with special histochemical stains
(section 5.5.3.3). Tissue can be removed from the carcass and fixed by
immersion in formalin (10%) containing 2% acetic acid (added just
before use) to improve penetration and hardening. Making up the
solution in 80% alcohol rather than water to hasten penetration
further has sometimes been recommended, but this is unnecessary and
may make dissection more difficult.
Rapid and careful transfer of tissues from the carcass to the
fixative solution will retard artefactual cell distortion. In a
general post-mortem dissection, the brain, including the cerebellum
and brainstem, are removed and transferred to fixative, prior to the
removal of other tissues. Retrieval of the spinal cord from its spinal
column is difficult and time consuming; it is recommended that the
whole spinal column with cord be excised quickly, trimmed of as much
muscle as possible, and immersed in the fixative intact. Once this
portion is fixed, the spinal cord and ganglia can be separated from
the column with greater care. Alternatively, after approximately 24 h
of fixation, the spinal column can be immersed in 5% formic acid
decalcifying solution. The column can then be sliced with little
difficulty.
To assist with the examination of peripheral nerves, tissue is
placed on a stiff card before immersion to retain orientation.
Optimally, the nerve to be excised should be exposed and, prior to
removal from the carcass, bathed with fixative for approximately
5 min. This will begin to introduce rigidity into the tissue and
retard decay artefact. A length of nerve can then be excised, quickly
placed on a card and pinned at the tip of both cut ends to retain
position after immersion. Care should be taken to avoid overstretching
the nerve as it is pinned to the card. Samples of these tissues can
then be sectioned in a transverse or longitudinal direction. Muscle
can be treated in the same manner. Another technique is to take off
the limb at the hip or shoulder joint, remove the skin and fix en bloc
in formalacetic acid solution. The limb can then be decalcified in 5%
formic acid solution for approximately two weeks as noted earlier for
the spinal column. It can then either be embedded in paraffin wax as
one large block or further dissected.
Tissue preservation is greatly improved by perfusing animals
systemically for 10 min with phosphate-buffered paraformaldehyde
(section 5.5.2.2), a method that simultaneously preserves all body
organs. Tissue can then be sampled in a more leisurely manner without
fear of introducing post-mortem changes (section 5.5.2.2).
(b) Dehydration and embedding
Relatively large tissue samples can be embedded in paraffin wax,
and this is particularly suitable for localizing and identifying
lesions in the brain. Automated processing for paraffin embedding has
greatly diminished the time requirements for preparing tissue samples.
Most automated systems will conform to a variety of preparation
schedules and process great numbers of tissue samples at one time.
There are systems that operate under combined heat and vacuum, the
great advantage being rapid processing. This combination is
deleterious and may cause unnecessary shrinkage and brittleness, which
will distort cell structure and hinder interpretation. Paraffin
sections routinely cut 4-6 µm thick, are suitable for certain
histochemical techniques and can easily be treated with special stain
to enhance particular cellular components.
(c) Section staining
Four staining methods are routinely used to enhance cellular
detail in paraffin sections.
Hematoxylin and eosin
Routinely used on paraffin sections, this method stains nuclei
blue to purple, cytoplasm and Nissl substance (and mast cell granules)
blue, and myelin sheaths pink. Nuclear density can be readily
assessed, as well as an increase or decrease in the size of nuclei and
the number of cells. Neuronal damage is readily detectable. Nuclei and
cytoplasm of other cell types, such as microgila and vascular
endothelial cells, are also well defined, though cresyl violet may be
preferred.
Cresyl fast violet
This method enhances identification of cell-population changes and
is more aesthetically pleasing than other basic aniline dyes, such as
thionine or gallocyanine-chrome alum. DNA stains pale blue and RNA
purple-blue. Hypertrophy of astroglial cytoplasm and changes in
neuronal nuclei and cytoplasm are readily observed. Chromatolysis,
poorly demonstrated in hematoxylin- and eosin-stained sections, is
more apparent when stained with cresyl violet, as are most cells in
peripheral nerves.
Glees and Marsland's stain
This method is easy to carry-out and selectively stains
neurofibrillary components in axons (Marsland et al., 1954).
Longitudinal sections demonstrate the state of terminal innervation in
motor and sensory fibres. Empty endplates are often recognized by the
observation of remaining clumps of nuclei on the muscle surface.
Sudan Black B
This method is useful to reveal nerve terminals in muscles
(Cavanagh et al., 1964).
5.5.2.2 Glutaraldehyde/epoxy method
(a) Tissue fixation and excision
This technique is best suited for the resolution of early
pathological changes with the light microscope and is required for
transmission electron microscopy (section 5.5.3.5). Whole body
perfusion with buffered fixators (paraformaldehyde) followed by
glutaraldehyde) delivered under pressure (100-150 mm Hg) is the only
satisfactory method to prepare brain and spinal cord sections, and
this method has the advantage of optimally fixing all other organs
simultaneously. For this purpose, the animal is deeply anaesthetized
with a solution (e.g., sodium pentobarbital) containing a small
percentage of heparin to facilitate circulation. Subsequent procedures
should be performed rapidly and smoothly to prevent the development of
anoxic damage and to ensure global fixation. The animal is secured on
its back and the rib cage cut bilaterally and reflected backwards to
expose the heart. After slitting the pericardium, the right atrium is
opened to drain circulating blood. The ventricular apex is then
excised to provide access to the aorta, and a cannula spurting a
column of fixative is inserted through the ventricular opening to the
apex of the aorta and clamped in place. The circulatory system is
cleared by a brief, initial perfusion with paraformaldehyde or saline,
and followed by more prolonged (e.g., 10-15 min) perfusion with
glutaraldehyde.
Rigid tissues from a fixed carcass are less susceptible to
handling artefact. The nervous system can be sampled in any order. In
some laboratories, the entire nervous system, including brain with
optic tract and nerves, spinal cord, spinal roots, ganglia, and
peripheral nerves are exposed in the carcass, then carefully detached
intact and removed. When this is accomplished, there is no need
initially to mark tissues for orientation. It is imperative to avoid
tissue drying during dissection and excision. Exposed tissues should
be bathed with fixative and tissues should be manipulated as little as
possible with forceps.
(b) Dehydration and embedding
After glutaraldehyde fixation, tissue samples are post-fixed for
2-3 h by immersion in buffered 1-2% osmium tetroxide in the cold. The
samples are then dehydrated stepwise in ascending concentrations of
ethanol, immersed in acetone or toluene, infiltrated with a solution
of epoxy resin and finally, embedded and polymerized in epoxy resin.
For light-microscope examination, 1 µm sections are cut with specially
prepared glass knives mounted in an ultramicrotome (an instrument
designed specifically for the purpose of obtaining the thin sections
suitable for electron microscope examination). New techniques and
equipment have recently become available which allow larger tissue
samples to be prepared for sectioning in epoxy resin. Sections one
micrometer thick can be examined by phase-contrast optics or by bright
field after a brief staining with 1% toluidine blue.
(c) Section staining
Thick (1 µm) sections of tissue fixed in glutaraldehyde and osmium
tetroxide and stained with borate-buffered 1% toluidine blue allow
resolution of structures as small as a single mitochondrion. Brain and
spinal cord neurons display clearly defined nuclei, nucleoli, issl
substance, and lipofuscin. Dendrites stand out against a more darkly-
stained neuropil. Small, densely staining oligodendrocytes are readily
distinguished from large, pale astrocytes. Myelinated nerve fibres
contain a pale axon and darkly-stained myelin. In cross-sections of
peripheral nerves, large and small diameter myelinated fibres, as well
as the smallest unmyelinated axons, can be seen. Motor and sensory
nerve terminals are detectable in muscle.
5.5.3 Special methods
5.5.3.1 Peripheral nerve microdissection
Isolated fibre preparations provide information that may be missed
in cross-sections, and it is recommended that this procedure be
included in any study concerned with toxic neuropathy. Several
fixation methods are available to prepare peripheral nerves for
microdissection. Perfused tissue is ideal, though satisfactory
preparations can be obtained by bathing the tissue with fixative prior
to excision. Post-excision immersion in fixative can also be used,
though the act of transecting the nerve introduces major fibre
artefacts that are not restricted to the cut ends and are readily
mistaken for pathological changes.
Optimal preparations suitable for high-resolution light microscopy
require the use of glutaraldehyde, post-fixation with osmium
tetroxide, stepwise dehydration, and infiltration with a low-viscosity
epoxy resin. This is an ideal medium to tease apart nerve fibres and
is suitable for the storage of tissue at low temperature. Mounted
fibres can be polymerized by heat to affix them to a glass slide and
prevent squashing by a coverslip (Spencer & Thomas, 1970). Bright-
field examination will reveal changes in overall structure, and
Nomarski differential interference microscopy can be used to study the
axon.
Older techniques yield poorer preservation, low resolution of
detail, and isolated fibres are susceptible to squashing, an important
consideration if morphometric study is planned. One method involves
formalin fixation, post-fixation in osmium tetroxide, and infiltration
with a dilute solution of glycerol, which is used to support the
fibres during microdissection. Single fibres are mounted on the
microscope slide, cleared with cresol, blotted dry, and mounted with
pure glycerin (Thomas, 1970). Sudan Black B can also be used to stain
tissue prior to microdissection. Nerves are microdissected with the
aid of a pair of mounted needles and stereoscopic dissecting
microscope. Epineurial connective tissue is removed and a small
fascicle selected. After splitting the perineurium surrounding the
fascicle, the sleeve is removed to expose the intrafascicular tissue
containing the nerve fibres. The tissue is then repeatedly split into
progressively smaller longitudinal bundles until individual fibres are
obtained. These are picked up on the end of sharpened wooden
applicator sticks and transferred to a clean glass slide and provided
with a coverslip. Alternatively, a rapid "squash" preparation can be
obtained by teasing a small bundle of fibres loosely apart to create a
mesh formation on a microscope slide; a coverslip is then added for
microscope examination.
5.5.3.2 Frozen sections
Frozen sections lend themselves well to enzyme histochemical,
immunocytochemical, and silver-impregnation procedures, but for the
purposes of a large-scale study, this method is impractical. Tissues
fixed instantaneously by immersion in liquid nitrogen are particularly
vulnerable to cell distortion and/or destruction caused by excessive
temperature changes. Encasing tissue samples in gelatin or albumin is
advisable, especially for brain tissues (Crane & Goldman, 1979).
Ice-crystal formation is a disruptive artefact that produces a
vacuolated appearance in frozen sections. To minimize crystal
formation, it is important to freeze tissues as rapidly as possible
and maintain critical temperatures throughout the cutting procedure to
avoid thawing and refreezing. Typically, frozen sections are between
10 and 15 µm thick.
5.5.3.3 Histochemical methods
Using these procedures, it is possible to assess the activity of
cell energy systems, the rate of use of substrates for processes of
oxidative phosphorylation, and the functional state of intracellular
organelles. The activities of succinate dehydrogenase and malate
dehydrogenase, enzymes of the Krebs cycle, are well known, the former
being closely linked with mitochondrial membranes. Enzymes of the
hexose monophosphate shunt (glucose-6-phosphate dehyrogenase and
gluconate-6-phosphate dehydrogenase) and those of the electron
transport chain (NAD and NADP disphorase) should also be studied. The
rate of exchange of nitrogenous bases can be examined by the
determination of activity of dihydroorotate dehydrogenase for fatty
acids; monoamine oxidase for biogenic amines; and acetylcholinesterase
for acetylcholine.
Histochemical enzyme assays are performed on freshly frozen
sections cut on a cryostat (Dubowitz & Brooks, 1973). Tissues are
quick-frozen in liquid nitrogen. Control and experimental tissues
should be mounted on one slide and reactions carried out using the
methods outlined in Pearse (1968).
The following methods can be used to demonstrate the functional
state of nerve cells (Pearse, 1968):
(a) RNA (Brachet's method with ribonuclease control);
(b) DNA (Feulgen's method with deoxyribonuclease control);
(c) Total nucleic acids (Einarsen's method);
(d) Total protein (sublimate with bromophenol blue);
(e) Sulfhydryl groups (Barnet and Zeligman's method);
(f) Glycogen and glycosaminoglycans (amylase control-PAS
reaction); and
(g) Lipids (Sudan III, Sudan Black, Nile Blue methods).
To express histochemical reactions quantitatively, the following
formula for Mean Histochemical Index (MHI) can be used:
MHI = 3a + 2b + 1c + 0d
N
where 3, 2, 1, and 0 are the degrees of intensity of colour (from 3
to 0), and a, b, c, and d are the numbers of the cells with the given
intensity of colour; the denominator N is the number of cells counted.
The results allow statistical calculation of the assays and estimation
of the reliability of the results.
5.5.3.4 Golgi method
This unique silver-impregnation method demonstrates the shape and
surface characteristics of entire neurons, including cell bodies and
their processes. Golgi preparations are particularly useful in the
study of dendritic arborizations and synaptic associations (Santini,
1975). However, the technique is capricious in that only a few neurons
are unpredictably demonstrated: many preparations must therefore be
examined to gather a representative sample.
In the Rapid Golgi method, tissue is fixed with formaldehyde or
glutaraldehyde and post-fixed with osmium tetroxide. The tissue is
then impregnated with silver nitrate, dehydrated, and infiltrated with
celloidin. Thick (120 µm) sections are prepared, mounted, and examined
by bright-field microscopy.
5.5.3.5 Transmission electron microscopy
This technique is laborious and requires extensive training.
However, it has resulted in extensive advances in the understanding of
cellular processes in neurotoxicity. There is little need for such
exacting methods to determine the presence of changes in cell
structure, few experimental protocols require electron microscopy. The
transmission electron microscope is properly used to confirm and study
further the nature of lesions already shown and mapped by light
microscopic methods. It is all too easy to seek, and find, changes to
which no significance will ultimately be attached. Until the pattern
of change induced by the chemical under study has been well identified
by light microscopy, electron microscopy should not be considered. On
rare occasions, such as in toxic disorders of unmyelinated axons,
examination by transmission electron microscopy is necessary to
localize cellular changes not revealed by the methods discussed
previously. Thin (50 nm) plastic sections are prepared with the aid of
a diamond knife mounted in an ultramicrotome and subsequently
impregnated with heavy metals for electron-microscope examination
(Pease, 1964; Hayat, 1973).
5.5.3.6 Other anatomical methods
There are numerous additional specialized anatomical methods that
are waiting to be applied in the study of neurotoxicological issues.
These include histological techniques to trace anatomical pathways
(with horseradish peroxidase) and cellular activity. Examples of the
latter are the use of autoradiography to localize the distribution of
radiolabelled precursors such as thymidine and 2-dexoyglucose
(Sokoloff et al., 1977). Other methods susceptible to light and
electron microscope analysis include immunocytochemical studies of
receptors, proteins, and other cell structures (Emson, 1983).
Fluorescence microscopy, particularly for the localization and
detection of regional concentrations of catecholamines using the
formaldehyde vapour or glyoxylic acid technique of Falck et al.
(1962), supplements quantitative biochemical methods in evaluating
alterations in catecholamine levels. Fluorescence microscopy is also
of value in antigen and antibody localization techniques. Certain
light and electron microscope techniques may be combined, such as the
examination of fine structure of single teased nerve fibres (Spencer &
Lieberman, 1971; Ochoa, 1972) or of Golgi-stained preparations.
Advanced methods in electron microscopy include scanning (for surface
features), energy-dispersive X-ray analysis (for detection of elements
of high relative atomic mass) and energy-loss (for detection of
elements of low atomic mass). Freeze-fracturing tissue can reveal
details of the internal surfaces of cellular membranes when replicas
are examined by transmission electron microscopy (Hayat, 1973).
5.6 Conclusions
The information derived from morphological studies is highly
relevant to the interpretation of biochemical, neurophysiological, and
behavioural data. Structural changes have always been the firm
foundation on which analysis of clinical neurological disease has been
based. Both diagnosis and prognosis depend heavily on previous
neuropathological experience for their accuracy. Moreover, treatment
of neurological disease can only be satisfactorily planned by using
the knowledge gained from experimental and morphological studies that
have provided an understanding of the pattern by which neural cells
are affected. Indeed, in the majority of human intoxications of the
nervous system, knowledge of the structural changes is based almost
exclusively on animal studies. There is no reason to believe that it
will be less so in the future.
6. BIOCHEMICAL AND NEUROENDOCRINOLOGICAL METHODS
6.1 Introduction
Biochemical tools are valuable for the study of neurotoxicity.
They have the potential for both identifying toxic compounds and
delineating mechanisms of action of known toxic substances. However,
the range of biochemical techniques is vast and careful decisions must
be made in devising an effective research strategy. The most important
decision is identification of the objective of the research, which may
be conveniently categorized into three groups: determining mechanisms
of action of neurotoxic substances, identifying exposed individuals,
and screening for toxic conditions.
Devising an effective biochemical screen is the most challenging
of the three objectives. It requires the selection of biochemical
parameters that are general enough to indicate toxicity resulting from
multiple sites of damage, but specific enough to prevent the
accumulation of false positives. No single biochemical parameter is
likely to suffice. An effective screen must include indices of each of
the major functions of nervous tissue (e.g., cellular metabolism,
neuronal propagation, and neurotransmission). It might include
estimates of RNA and/or protein synthesis, lysosomal enzymes, membrane
transport, channel and neurotransmitter receptors, or their turnover.
The precise choice of biochemical indices must be limited by the
expertise and equipment of individual laboratories, but every attempt
must be made to consider a range of functional events rather than a
single parameter.
Considerable discussion has centred on the use of in vitro
procedures for screening potential neurotoxic compounds. The
advantages of an in vitro approach are obvious. It is less expensive
and less time-consuming than whole animal study and biochemical
techniques can be employed under precise, standard conditions.
In vitro procedures must include both parent compounds and potential
toxic metabolites. However, the exclusive use of in vitro methods
would fail to detect compounds in which the neurotoxicity results from
alterations in non-neuronal systems.
Theoretically, the choice of biochemical tools for the
identification of mechanisms of toxicity is the simplest of the three
objectives. Biochemical tools are virtually limitless, but they can be
chosen on the basis of known information about the toxicity of the
compound. Scientists can then proceed systematically from one level of
analysis to another. By synthesizing data from behavioural, neuro-
physiological, neuroendocrinological, neuropharmacological, and
neuropathological studies, the biochemist can employ successively more
selective tools to determine the initial biochemical lesion induced by
the toxic compound.
The purpose of this section is to review the biochemical
approaches that may be useful for identifying and further
understanding the effects of toxic compounds on the nervous system.
6.2 Fractionation Methods
Neural tissue has several features not shared by other tissues. In
particular, neural tissue exhibits considerable cellular,
morphological, and chemical heterogeneity. It is often desirable to
precede biochemical procedures with some attempt to decrease this
complexity. One approach is to separate the tissue into discrete parts
of brain prior to analyses. A second approach is to separate specific
cell types. A third involves subcellular fractionation procedures. In
many cases, it may be desirable to use a combination of these
fractionation procedures.
6.2.1 Brain dissection
Brain tissue can be fractionated into any number of potential
sections. However, the most often used scheme is based on that
described by Glowinski & Iverson (1966). In this method the brain is
divided into relatively discrete units of cerebellum, thalamus,
hypothalamus, striatum, hippocampus, etc., by using visible anatomical
landmarks. Such dissection procedures have been very useful in
describing the neurochemistry of different brain areas and are
essential in evaluating the effects of toxic compounds on molecules
(such as neurotransmitters) that are not uniformally distributed in
neural tissue. Because of the regional variability of brain chemistry,
whole brain analyses are seldom useful for evaluating brain function.
More importantly, the use of the whole brain for studies of toxic
compounds may fail to detect functionally-relevant alterations in
specific brain areas. For example, effects of lead on GABA levels have
been reported for cerebellar tissue (Silbergeld et al., 1980) and
acrylamide effects have been noted in striatal dopamine receptors
(Bondy et al., 1981).
Differences in the response to toxic compounds would be
anticipated from differences in neurochemistry in various regions of
the brain. In addition, many toxic substances are not distributed
uniformly across brain areas (e.g., chlordecone (Fujimori et al.,
1982) and manganese (Bonilla et al., 1982). Although not predictive,
examination of such regions can provide a biochemical clue regarding
possible molecular sites of interaction of the toxic compounds.
In more precise dissection procedures, a small needle, of defined
diameter, is used to obtain samples within anatomically defined areas
(Palkovits, 1973; O'Callaghan et al., 1983). Such finite samples have
been used in identifying several neurochemical events, e.g.,
neurotransmitter concentrations (Palkovits, 1973) and neuro-
transmitter-induced phosphorylation of specific phosphoproteins
(Dolphin & Greengard, 1981), and they may be especially valuable in
locating the site of neural responses to toxic compounds.
6.2.2 Isolation of specific cell types
Although neural tissue is recognized by a marked heterogeneity of
cellular elements, based on function and embyronic origin, these cell
types are conveniently categorized as neurons or glia. Each cell type
makes a unique contribution to neural function and their sensitivity
to toxic compounds may differ. Several methods have been described for
the bulk separation of neuronal and glial cell populations from whole
brain or specific brain regions (Rose & Sinha, 1970; Appel & Day,
1976; Magata & Tsukada, 1978). A dissociated cell suspension that
avoids disruption of the cells is prepared by forcing the tissues
through fine sieves. Proteolytic enzymes are used to facilitate the
dissociation. The fraction enriched in neuronal perikarya is separated
from the smaller glial cells (also containing some synaptosomes) by
centrifugation. The cell separations are never complete and the
procedures generally provide low yields. However, enrichment of the
neurons and glia is usually sufficient fox determining whether a toxic
agent interferes primarily with neuronal or glial metabolism. Isolated
cell populations have been used to study the lipid and protein
composition of membranes and the several metabolic differences between
neuron and glia. Such information may provide baseline data for future
studies of neurotoxic chemicals. Disadvantages of this method include
loss of cell processes from the perikarya, reduction of cell surface
area, and damage to the cell membrane. These factors must be
considered in any metabolic study.
To date, there have been few attempts to use such separation
procedures in neurotoxicity and they are not recommended as an initial
study. The method is laborious and time consuming and must be
performed on fresh tissue. Only a limited number of samples can be
simultaneously processed and it is never clear if the low yields
result from random or specific loss of cell types.
6.2.3 Subcellular fractionation
The neuron can be divided into relatively discrete functional
units. The cell body contains the metabolic machinery for the
synthesis and packaging of macromolecules and for the general
maintenance of cellular homeostatis. The long axonal process acts as a
communication link, propagating electrical impulses from the cell body
to the terminal and transporting vital nutrients and cellular
components to more distal regions including the nerve terminal.
Finally, the synapse functions to transfer chemically encoded
information from one cell to another. Toxic substances may disrupt
neuronal biochemistry at any one, or all, of these cellular sites.
The isolation of subcellular organelles and specific membrane
fractions can be a first approximation in determining the subcellular
sites of action of toxic agents. Differential centrifugation
procedures provide a rapid means of obtaining fractions consisting
predominantly of a single cellular component (Gray & Whittaker, 1962;
Cotman & Barker, 1974; Rose and Sinha, 1970). Subcellular
fractionation is never totally effective, but it can produce an
enrichment of organelles and cellular subfractions. Some of the more
commonly used biochemical markers that can help identify the degree of
enrichment are listed in Table 4. These markers can be used as
potential indices of toxicity. For example, the effects of triethyltin
on myelination in the developing brain has been studied by examining
the activity of the myelin marker, 2',3'cyclic nucleotide 3'
phosphohydrolase (Konat & Clausen, 1977). Merkuryva & Tsapkova (1982)
have used several marker enzymes in their study on the toxic effects
of ethanol on the nervous system.
When subcellular fractionation is combined with regional analysis,
the number of potential samples requiring examination can be
overwhelming. Therefore, careful selection must be used in deciding
which brain area and which subcellular fraction to investigate. This
selection can be very difficult, especially when knowledge concerning
a particular compound is limited. The recent introduction of
immunochemical methods promises to facilitate this task, not by
reducing the difficulty of the selection, but by increasing the number
of chemicals that can be subjected for analysis.
Immunochemical methods have been used to identify molecules
associated with various cell types, for localizing enzymes responsible
for the synthesis of neurotransmitters and for identifying proteins
specific to the nervous system. While the procedures initially require
the preparation of antisera to highly purified proteins, once the
antisera are available, they can be sensitive tools for identifying
specific changes and for characterizing different cell populations. In
addition, monoclonal antibodies can be used to determine the mechanism
of action of toxins.
These procedures are especially valuable in studying neuronal
peptides. These peptides (e.g., substance P, encephalins, hypothalamic
releasing factors, somatostatin, etc.) play a significant role as
neuromodulators (Snyder & Inms, 1979). Many are potent at low
concentrations. Prior to the use of immunological procedures, peptide
molecules could only be examined by using bioassay techniques and
these were not always specific for particular molecular species.
Radioimmunoassay techniques are more specific. Assay kits for various
peptides are now available commercially, and their number is rapidly
increasing.
Table 4. Examples of biochemical markers of brain subcellular fractions
and cell types
Fraction Marker Reference
nuclei DNA Steele & Busch (1963)
nuclear membrane RNA polymerase Roeder & Rutter (1969)
cytosol lactate dehydrogenase Kornberg (1955)
microsomes NADPH; cytochrome c Miller & Dawson (1972)
oxidoreductase (rote
none insensitive)
Mitochondria
inner membrane D-3-hydroxybutyrate Fitzgerald et al.
NAD+ oxidoreductase (1974)
outer membrane monoamine oxidase Schnaitman et al.
(1967)
lysosomes ß-glucuronidase Fishman & Bernfeld
(1955)
ß-glucosidase Robins et al. (1968)
ß-galactosidase Robins et al. (1968)
N-arylamidase Boer (1974)
myelin 2',3'-cyclic nucleotide- Konat & Calusen (1977)
3'-phosphohydrolase
synaptosomes Na+K+-activated Verity (1972)
oubain-sensitive
ATPase
neuronal cells guanyl cyclase Goridis et al. (1974)
tyrosine hydroxylase Kuczenski & Mandell
(1972)
oligodendroglial cells glyceride galactosyl Radin et al. (1972)
transferase
Because the method is very recent, radioimmunoassay procedures are
only beginning to be use in neurotoxicology. However, using such
methods, Hong and his colleagues (Ali et al., 1982; Hong & All, 1982)
have successfully identified several peptide responses following
chlordecone treatment. The major limitation of the method for
identifying toxic compounds is the small number of purified compounds,
the small number of available RIA kits, and the expense of obtaining
radioiodinated compounds. With increasing availability of RIA kits,
the procedure could become a powerful screening tool.
6.3 DNA, RNA, and Protein Synthesis
Changes in the amount of DNA can be used to detect whether toxic
agents affect cellular proliferation and cell death. Polyploidy is
uncommon in the nervous system, thus the DNA content of brain regions
can be taken as an index of cell number. This can be related to tissue
weight and RNA or protein content to estimate cell size. For example,
Krigman & Hogan (1974) reported that lead exposure during early
development reduced brain weight and decreased total brain proteins,
but the number of brain cells was unchanged. DNA measurements have
been particularly useful in studying the toxic effects of drugs on
retinal photoreceptors (Dewar et al., 1977). It is also well-known
that the DNA content changes after nutritional restriction during
development, or after exposure to various toxic chemicals that
influence nutritional status.
Although the measurement of DNA content is relatively simple and
in the nervous system can be an index of cell number, it is probably
not a very sensitive or early indicator of neurotoxicity. For example,
neuronal loss might be accompanied by glial proliferation and produce
no change in total DNA. The earliest effects of a toxic substance on
DNA will probably involve: (a) the disturbance of DNA-repair enzymes;
(b) the intercalation of the substance into the DNA molecule; or
(c) the direct binding of the substance to the nucleic acid moiety or
its associated chromosomal proteins. Two to three percent of added
mercuric chloride was reported to bind to the chromatin in cultured
glial cells (Ramanujam et al., 1970). Since this mode of action will
ultimately interfere with the functional integrity of the CNS, it
offers great promise for the identification of toxic compounds.
The total amount of RNA or protein could also be examined after
toxic insult. However, neither total RNA nor total protein is likely
to provide a sensitive index of toxic damage. Only in the extreme
stages of neurotoxicity will total or even regional levels of these
macromolecules be likely to change. More sensitive indices of their
metabolism rely on estimates of synthesis or degradation.
The rate of RNA or protein synthesis can be evaluated by using
radiolabelled precursors and measuring their rate of incorporation
into the macromolecule in vitro or in vivo. Although this
technique is simple and sensitive, it assumes that the rate of
incorporation of the precursor directly reflects the rate of
macromolecular synthesis and this may not always be true (Dunn, 1977).
Changes in labelling could also occur as a result of changes in
cerebral blood flow, precursor transport, precursor pool size, energy
charge, etc. Inhibition of amino acid transport across the blood-brain
barrier has been reported for mercury (Partridge, 1976) and lead
(Lorenzo & Gerwitz, 1977). Thus, it is also important to consider
whether a toxic agent can cause changes in the amino acid or nucleic-
acid-precursor pool sizes by using one of several methods available
for compensating for precursor pool fluctations (Munro et al., 1964;
Dunlop et al., 1975; Dunn, 1975). Changes in brain-protein synthesis
have been reported after exposure to methylmercury (Verity et al.,
1977), and carbon disulfide (Savolaiene & Jarvisalo, 1977). However,
in many cases, only the absolute levels of incorporation of
radioactive amino acids into protein fractions were studied and it is
difficult to understand the primary mechanisms of action.
Although these relatively crude methods are subject to several
criticisms, all the variations that influence the incorporation of the
radioactive precursor can ultimately influence cellular metabolism.
These methods can, therefore, be useful for identifying metabolic
disturbances. The relative amount of protein synthesis can also be
estimated simply by measuring the polyribosome to free ribosome pool.
This method is based on the assumption that during high rates of
protein synthesis, the greatest proportion of the total ribosomes will
be attached to an mRNA molecule. The relative polyribosome to monosome
ratio has the advantage of allowing for normalization across animals
and therefore reducing the interanimal variability that often plagues
neurochemical assessments. Furthermore, the use of this method would
detect toxic effects not only on protein synthesis but on the
stability or synthesis of the ribosome itself.
The rate of RNA and protein synthesis in the brain is very high
and nerve cell functioning is dependent on protein metabolism. The
turnover rates of brain macromolecules may vary considerably, and it
is always going to be difficult to interpret any data dealing with the
turnover of total RNA or protein. However, with the availability of
newer separation methods for proteins (detergent gel electrophoresis
combined with chromatofocusing cellulose ion exchange chromatography,
etc.), soluble and bound polypeptides can be separated to study the
synthesis of individual proteins at the subcellular level, in specific
cell types and in localized brain regions. Large classes of RNA can be
analysed by hybridization procedures or specific mRNA molecules could
be targeted for investigation by using specific cDNA probes.
6.4 Lipids, Glycolipids, and Glycoproteins
Complex lipids, glycolipids, and glycoproteins have several
important functions in neural tissue (Zuber, 1978). They constitute
structural elements of the plasma membrane, act as components of ion
channels, comprise portions of neurotransmitter receptors, and are
major constituents of myelin. Complex lipids (e.g., phospholipids,
cerebrosides, sulfatides, and gangliosides) make up neuronal
membranes. Phosphatides (phosphatidylethanolamine, phosphatidyl-
choline, phosphatidylinositol, phosphatidylserine) are the most
important group of phospholipids. They consist of some of the most
metabolically active of the phospholipids (Prokhorova, 1974) and
participate in the movement of Na and K ions through the neural
membrane. A closely related group of phospholipids, the plasmalogens,
are concentrated in myelin and constitute 18-30% of total brain
phospholipids.
Ganliosides are localized primarily in the plasma membrane
(Karpova et al., 1978), but small amounts of monosialoganglioside are
detectable in myelin. Receptors of neurotransmitters, such as
serotonin, and other biologically-active compounds may contain
ganglioside (Avrona, 1971), and evidence suggests that gangliosides
may bind biologically-active compounds, as well as various
neurotoxins, through their terminal N-acetylneuraminic acid moiety
(Kryzhavosky, 1973). Glycosaminoglycans are also functional and
structural elements of the neuronal membrane and have been reported to
exhibit disturbed metabolism in various hereditary diseases of the
mucopolysaccharidosis type (Constantopoulos et al., 1976; Vasan &
Chase, 1976).
The most interesting of the functionally-active, minor components
of neuronal membranes are sialic acids, and particularly
N-acetylneuraminic acid. As sialo-glycoproteins and gangliosides,
N-acetylneuraminic acid participates in carrying out specific
neuronal functions such as the establishment of synaptic contact
neurotransmission, and axonal propagation (Partingron & Daly, 1979).
Gangliosides and sialo-glycoproteins bind Ca++ at their hydrophilic
ends and thus, by influencing the concentration of this ion, affect
depolarization and neurotransmitter release.
Myelin metabolism may be affected by a variety of toxic agents
(Cammer, 1980). After administration of these compounds, there is
often a delay before the appearance of neurotoxic signs and symptoms
such as the ataxia indicative of peripheral demyelination. Central
demyelination often occurs to a lesser extent. Compounds that cause
disturbances in myelin metabolism may exert their effect directly on
myelin-forming cells, or demyelination may be a secondary response to
a disturbance of neuronal metabolism.
Several compounds have been reported to alter complex lipids or
their metabolism. Neonatal exposure to lead has been reported to
decrease the total brain content of phospholipids, galactolipids,
plasmalogens, and cholesterol (Van Gelder, 1978). Hydrogen sulfide and
sulfur dioxide decrease total brain lipids and/or phospholipids
(Haider et al., 1980). Meta-SystoxR (phosphorothioic acid
O-[2-(ethylthio)ethyl] O,O-dimethyl ester mixture with
S-[2-(ethylthio)ethyl] O,O-dimethyl phosphorothioate)
(O,O-dimethyl-S-2 (ethylsulfinyl) ethylthiophosphate), an
organophosphorus pesticide, has been reported to decrease brain levels
of total lipids, phospholipids, cholesterol, esterfied fatty acids,
and gangliosides (Islam et al., 1983). Merkuryva et al. (1978) found
one of the early affects of carbon disulfide intoxication to be
changes in the enzyme substrate system of N-acetylneuraminic acid,
N-acetylneuraminate lyase. In the olfactory bulb of the rabbit,
carbon disulfide led to an accumulation of N-acetylneuraminic acid
because of a reduced degradation by ß-acetylneuraminate lyase. In some
cases, the carbon disulfide-induced disturbance of the metabolism of
cerebral glycoconjugates could be correlated with the changing of
physiological parameters (e.g., lowering of the amplitude of the
cortical evoked potential (Bokina et al., 1976)). Long-term exposure
to lead acetate in aging rats was reported to decrease
N-acetylneuraminic acid content in cerebral tissue and also to
decrease the activity of the degradative enzyme (Merkuryva &
Bushinskaya 1982). Long-term exposure to ethanol led to an
accumulation of N-acetylneuraminic acid in the subfornical region of
the hypothalamus and in the midbrain reticular formation (Merkuryva et
al., 1980).
A recently applied approach to the study of neurotoxicology
involves the investigation of lipid peroxidation. Peroxidation
involves the direct reaction of oxygen and lipids to form free radical
intermediates and semistable peroxides. Lipid peroxidation is damaging
because of the subsequent reactivity of these free radicals (Tappel,
1970). Biomembranes and subcellular organelles are the major cellular
components damaged by lipid peroxidation. Increased lipid peroxidation
has been reported after exposure to thallium, nickel, or cobalt (Hasan
& Ali, 1981). Since the estimate of lipid perioxidation is a
relatively simple technique, this method may prove to be an effective
tool for screening for neurotoxic compounds.
6.5 Neurotransmitters
Chemical synaptic transmission involves a complex series of events
(synthesis and storage of neurotransmitters, release of neurotrans-
mitters, re-uptake or degradation of transmitters, interaction of
transmitters with postsynaptic membrane), any or all of which could be
disturbed by neurotoxins. In addition to the classical neurotrans-
mitters (Table 5), a number of peptides have been discovered, which
may act as chemical messengers (Burgen et al., 1980). Most
toxicological studies have focused on the neurotransmitters listed in
Table 5, because considerable background information on these
neurotransmitters is already available. Nearly every class of neuron
contains some marker enzyme (Table 5), and these have been useful in
studies in which attempts have been made to identify neurotransmitter
specific cells and in the determination of the regional distribution
of neurotransmitter systems.
Table 5. Enzyme markers for neurotransmitter specific cells
Neurotransmitter Marker enzyme Receptor types
acetylcholine choline acetyltransferase mucarinic, nicotinic
dopamine tyrosine hydroxylase DA1 and DA2
noradrenaline dopamine-ß-hydroxylase alpha1, alpha2, ß1,
ß2 adrenoreceptors
GABA(gamma- glutamate decarboxylase GABAA and GABAB
aminobutyric acid) (strychnine-insensitive;
picrotoxin-sensitive)
glycine unknown strychnine-sensitive
serotonin 1-amino acid decarboxylase 5 HT1, 5 HT2
6.5.1 Synthesis/degradation
Many neurotoxic compounds have been reported to alter steady-state
levels of neurotransmitters, but: it is difficult to draw functional
inferences from such data since the steady-state levels of
neurotransmitters can be influenced via multiple mechanisms (e.g.,
rate of synthesis, rate of release, rate of degradation). More
informative data are obtained by examination of the turnover rates,
reflecting the metabolic half-life of the neurotransmitter (Costa,
1970). Turnover of 5-HT, after disulfiram treatment, was examined by
observing the accumulation of 5-HT following administration of
pargyline (Minegishi et al., 1979). It has been speculated that carbon
disulfide acts by altering brain catecholamine concentrations. Carbon
disulfide increased DA and decreased NE by inhibiting dopamine
ß-hydroxylase, the enzyme responsible for converting dopamine (DA)
into norepinephrine (NE) (McKenna & DiStefano, 1975). Manganese is
known to inhibit DA formation by blocking tyrosine hydroxylase in the
striatum (Bonilla, 1980) and many acute effects of organophosphorus
compounds are related to inhibition of AChE activity (O'Brien, 1976).
Soon after their introduction, the inhibition of AChE was accepted as
a mechanism for the acute toxic effects of organophosphorus
insecticides (DuBois et al., 1949). However, this mechanism does not
account for the delayed neurotoxicity seen after organophosphate
poisoning (Abou-Donia & Preissig, 1976; Abou-Donia, 1981).
Interference with axonal transport may also disrupt
neurotransmitter function by altering the availability of
neurotransmitter enzymes or by decreasing the transport of peptide
precursors. The transport of materials along axons is bidirectional:
anterograde and retrograde. Anterograde transport conveys materials
from cell body to axon and terminals at slow (1-3 mm per day) and fast
(410 mm per day) rates (Ochs, 1972; Hoffman & Lasek, 1975). The former
contains the bulk of axoplasmic proteins (notably of neurofilaments
and neurotubules) and the latter, membrane and other components.
Retrograde transport, a system that conveys materials at rapid rates
(300 mm per day) may also be affected early in toxic axonopathies.
Retrograde transport may be a critical factor in the toxicity
resulting from exposure to various compounds.
6.5.2 Transport/release
Neurotransmitters or their precursors can rapidly concentrate in
nerve endings through specific high-affinity uptake systems associated
with each transmitter-specific class of neurons (Iverson, 1971). The
high-affinity, Na+-dependent transport mechanisms are specific to
nerve cells and can be distinguished from the ubiquitous low-affinity
transport systems that concentrate a large variety of amino acids,
sugars, and nucleosides, or their precursors. High-affinity uptake
phenomena may be studied in brain homogenates, brain minces, or
synaptosomal preparations. Lead (Silbergeld & Goldberg, 1975), the
insecticide chlordecone (Chang-tsui & Ho, 1979), and erythrosin B
(Lafferman & Silbergeld, 1979) have been reported to interfere with
neurotransmitter uptake processes. However, a variety of nonspecific
events may influence apparent uptake, so caution must be exercised in
interpreting results of these studies. For example, the effects of
erythrosin B on uptake mechanisms were reported to be non-specific
(Mailman et al., 1980).
The release of neurotransmitters from synaptic endings occurs
through an exocytotic process that is triggered by an influx of
calcium ions on depolarization of the nerve endings (Cotman et al.,
1976). Neurotransmitter release can be measured by preloading nerve
endings with labelled neurotransmitters, exposing tissue slices or
synaptosomal fractions maintained on filter beds to calcium ions, and
measuring the appearance of labelled or unlabelled compounds in the
supernatant medium (Bondy & Harrington, 1979). Heavy metals such as
lead have been reported to interfere with calcium-dependent
neurotransmitter release (Bondy et al., 1979; Ramsay et al., 1980),
and manganese has been reported to block transmitter release (Kirpekar
et al., 1970; Balnave & Gage, 1973; Kostial et al., 1974), possibly by
blocking inward Ca2+ current (Baker et al., 1971). Again, caution
must be used in comparing the results from different assay systems.
6.5.3 Binding
Binding of neurotransmitters to specific membrane receptors is the
first step in a complex series of events initiated in the
post-synaptic cell. Such binding interactions are reversible,
stereospecific, nonenzymatic, have equilibria with low dissociation
contants, and involve configurational recognition (Yamamura et al,
1981). Studies on receptor binding have been made possible by the
availability of specific radioactive analogues of neurotransmitters.
In general, the most potent binding ligands are the pharmacological
antagonists or agonists of a given neurotransmitter, rather than the
transmitter itself. Kinetic characteristics of binding and receptor
density are estimated by incubating receptor-enriched membranes with
radioactive ligands. Excess ligands, not bound to membranes, can be
removed by filtration or centrifugation. Non-specific binding of the
labelled compound is estimated by repeating the incubation in the
presence of a high concentration (10-4 to 10-6 mol) of an
unlabelled analogue. The solubility of ligands either in lipids or in
water has to be considered when interpreting results of ligand-binding
studies. This method is relatively simple and has been suggested as a
useful screening procedure for the detection of neurotoxic compounds
(Damstra & Bondy, 1980, 1982). If properly employed, such analyses can
provide valuable information. However, interpretation of the data can
be complicated by the often low degree of specificity of the ligand
and the location of the receptor, which may be pre- or post-synaptic
or on non-neuronal glial elements. Furthermore, for many
neurotransmitters, several subtypes of receptors exist and may produce
different effects on the post-synaptic membrane. Functional
extrapolations from neurotransmitter binding studies, therefore,
should be made with caution.
Receptor binding techniques have only recently been applied to
neurotoxicological studies. However, in a short time, many compounds
have been reported to increase or decrease estimates of receptor
density. Acrylamide (Bondy et al., 1981), chlordecone (Seth et al.,
1981), lead and mercury (Bondy & Agrawal, 1980), and cadmium (Hedlund
et al., 1979) have all been reported to alter putative
neurotransmitter receptors.
6.5.4 Ion channels
Many neuronal functions are regulated by ion channels. The Na+
channel is responsible for depolarizing the membrane, and K+
channels are responsible for repolarizing the membrane. Maintenance of
appropriate Na+ and K+ concentrations requires the classical
Na+/K+ ATPase. Disturbed functioning of these ion channels or
disturbance of ATP availability can severely disturb neuronal
functioning. Several naturally-occurring toxins affect Na+ channels
(Hille, 1976; Ritchie, 1979; Catterall, 1977). Tetrodotoxin and
saxitoxin inactivate the channel at nanomolar concentrations and can
be used as probes for measuring channel density or isolation of
channel polypeptides (Agnew et al., 1978). Batrachotoxin and
veratridine bind the channels and produce persistent activation by
preventing channel inactivation (Albuquerque & Daly, 1976). Peptide
toxins, such as scorpion toxin (Couraud et al., 1978), exhibit either
voltage sensitive or insensitive binding to the extracellular surface
of the Na+ channel (Catterall, 1977). Several potassium channels
probably exist in neurons (Reichardt & Kelly, 1983), but purification
and characterization of these channels has lagged behind that of the
Na+ channel. However, a scorpion toxin that exibited K+ channel
affinity has been described (Carbone et al., 1982).
Na+/K+-ATPase consists of 2 polypeptide chains. The smaller
polypeptide is a glycoprotein (Carilli et al., 1982). The larger
polypeptide binds ATP internally and ouabain externally. Hormones and
neurotransmitters such as catecholamines regulate the ATPase (Clausen
& Flatman, 1977), and neurotoxicants could alter neuronal functioning
by disturbing this control of ionic gradients. Desaiah et al. (1980)
have reported inhibition of Na+/K+-ATPase and decreased ouabain
binding to synaptosomes after treatment of mice with the insecticide
chlordecone. The evaluation of ion balance and their control will
probably be used increasingly in neurotoxicology.
For the most part, transmitter release is regulated by Ca2+
entry through a Ca2+-selective channels. Depolarization,
neurotransmitters, and hormones regulate Ca2+-selective channel
(Reichardt & Kelly, 1983). Nerve terminals contain several Ca2+
calmodulin-dependent protein kinases and a Ca2+-phospholipid
activated kinase, each of which has a distinct set of protein
substrates. Cytoplasmic Ca2+ binds calmodulin and other Ca2+
binding proteins, which directly or indirectly activate other enzymes
via their Ca2+-dependent kinases. How such activation facilitates
exocytosis is not known, but presumably the phosphorylation state of
synapsin I, a phosphoprotein present primarily in nerve terminals and
associated with synaptic vesicles, is believed to regulate transmitter
vesicle release (Dolphin & Greengard, 1981).
Changes in Ca2+ concentration induced by neurotoxicants could
have significant consequences for neuronal function. Cytoplasmic
Ca2+ stimulates not only exocytosis, but also glucogenolysis and
mitochondrial respiration (Landowne & Ritchie, 1976), endocytosis
(Ceccarelli & Hurlbut, 1980), and neurotransmitter synthesis (Collier
& Ilson, 1977). Even though many of these events are homeostatic
responses to transmitter utilization, neurotoxic disruption of calcium
influx or sequestering could produce disturbances not restricted to
neurotransmitter release.
Few neurotoxic compounds have been investigated for their
influence on calcium channels. Manganese is reported to block
neurotransmitter release by blocking inward Ca2+ current (Kirpekar
et al., 1970), and other heavy metals have been reported to interfere
with calcium-mediated neurotransmitter release (Bondy et al., 1979;
Ramsay et al., 1980). Similar effects of heavy metals were also
observed for the Na+-Ca2+ exchange system. Ion channels and
Na+-Ca2+ probes may be a valuable screening approach for some
types of neurotoxic compounds.
6.5.5 Cyclic nucleotides
Nerve terminal function and the effects of neurotransmitters are
often regulated by cyclic nucleotides. Binding of agonists to
receptors on the nerve membrane can result in activation of second
messenger systems. Activation of different receptors results in
specific changes in cyclic AMP and cyclic GMP levels and consequent
alterations in protein kinases. It is thought that on phosphorylation,
conformational changes occur in membrane proteins that may change ion
permeabilities. Thus, the responsiveness of the nervous system to some
toxic agents can also be measured by determining changes in the
activities of adenylate cyclase, cyclic nucleotides, and protein
phosphorylation. For example, lead has been shown to inhibit
cerebellar adenylate cyclase (Nathanson & Bloom, 1975) and
dopamine-sensitive adenylate cyclase in the striatum (Wilson, 1982).
Various pesticides cause increases in the levels of cyclic nucleotides
in brain tissue (Aldridge et al., 1978). For example, tri-o-cresyl
phosphate enhanced Ca2+-calmodulin-dependent in vivo
phosphorylation of proteins in chicken brain (Patton et al., 1983).
6.5.6 Summary of nerve terminal function
The analysis of synaptic function and neurotransmitter metabolism
is reasonably complex, and it should be borne in mind that disturbance
at many levels of neuronal organization will ultimately alter synaptic
function. However, examination of the nerve terminal is an excellent
approach for the initial study of toxic chemicals. However, it is
important to recognize the dynamic nature of the nerve terminal and to
remain alert to the possibility of false positives.
6.6 Energy Metabolism
Nervous tissue, and brain tissue in particular, require
disproportionately large amounts of energy to sustain the
translocation of ions important for electrical activity and to
maintain the highly-active biosynthetic machinery of the tissue.
Since neural tissue has only limited stores of energy (e.g., glycogen
and creatine phosphate), glucose availability and enzymes critical for
energy production are vital to neuronal functioning. A number of
neurotoxic chemicals have been shown to interfere with glucose and
energy metabolism in both the central and peripheral nervous system.
These include methylmercury (Bull & Lutkenhoff, 1975), hexachlorophene
(Cammer & Moore, 1972), organotin compounds (Lock, 1976), and alcohol
(Merkuryva et al., 1980). Glycolytic enzymes have been shown to be
inhibited by alcohol (Merkuryva et al., 1980) and by chemicals known
to cause distal axonopathy, e.g., acrylamide (Howland et al., 1980)
carbon disulfide, and methyl-n-butyl ketone (Sabri et al., 1979).
Active brain areas have a higher rate of glucose consumption than
less active regions. The brain regional metabolic activity is
determined by the 14C-2-deoxyglucose method (2-DG) of Sokoloff et
al. (1977). This method is based on the use of 2-deoxy-D-14C-glucose
as a tracer for the exchange of glucose between the plasma and the
blood and its phosphorylation by hexokinase. The product,
2-deoxyglucose-6-phosphate (2-DG-P), is trapped in the tissue and its
accumulation in the various regions of the brain might be a measure of
the glucose use and neuronal activity. The method provides an index of
the in vivo glucose metabolic activity of brain regions and has been
used in experimental animals to determine which brain regions are
depressed or activated during acute Soman intoxication (McDonough et
al., 1983).
Metabolic compartmentalization in nerve tissue could provide an
additional tool in toxicological studies. It is well established that
neurons mainly use glucose in their energy metabolism, while glia use
substrates other than glucose (Hertz, 1981). Thus, it may be possible
to differentiate between the toxicological effects of chemical
substances on neuronal and glial populations in vitro or in vivo.
6.7 Biochemical Correlates of Axonal Degeneration
A relatively large class of neurotoxic agents is known to produce
axonal degeneration (Griffin & Price, 1980; Sabri & Spencer, 1980;
Thomas, 1980). Some, such as acrylamide and organophosphorus
compounds, produce the distal to proximal "dying back" pattern of
degeneration, while others, such as ß,ß-iminodipropionitrile, lead to
proximal to distal degeneration. Since many of these compounds produce
Wallerian degeneration, biochemical correlates of Wallerian
degeneration have been suggested as toxicological indices (Dewar &
Moffett, 1979). These authors tabulated the chemical changes in
peripheral nerves that occurred during degeneration and suggested that
ß-gluocorinadase and ß-galactosidase might be good indices of
toxicity. Acrylamide, methylmercury, and dimethyl sulfoxide were shown
to increase these lysosomal enzymes (Dewar & Moffett, 1979). The use
of lysosomal enzymes involves relatively simple procedures and might,
therefore, be appropriate for the initiation of a biochemical screen.
Disturbance of axoplasmic transport has been suggested as an
alternative approach to detecting compounds producing axonal
degeneration. Several substances, e.g., organophosphates (Reichart &
About-Donia, 1980) and acrylamide (Pleasure et al., 1969) disrupt
axonal transport. However, these methods are more difficult, more
expensive, and more time-consuming than the lysosomal enzyme assays.
6.8 Neuroendocrine Assessments
The number of toxic compounds being recognized for their
neuroendocrine actions is increasing. Hormonal balance results from
the integrated action of the hypothalamus, pituitary, and endocrine
target organ. Each site is susceptible to disruption by environmental
toxic agents. This disruption may result from the direct interaction
of the toxic agent with the endocrine organ, pituitary, or
hypothalamus. Alternatively, neuroendocrine dysfunction may occur
because of a disturbance in the regulation and/or modulatory elements
of the complex neuroendocrine feedback systems. Any such disturbances
would ultimately modify anterior pituitary secretions. Thus, the
analysis of blood levels is the most appropriate starting point
(section 6.8.2.1).
6.8.1 Anterior pituitary hormones
The main effector organ in the neuroendocrine system is the
pituitary. This "master gland" consists of an anterior
adenohypophysis, and a posterior neurohypophysis. Based on
histological, immunocytochemical, and electron microscopic examination,
the following cell types are differentiated in the anterior pituitary
(Junqueira et al., 1977); follicular cells, which do not contain any
secretory granules but form the support stroma of the glandular cells;
chromophobe cells, which are undifferentiated, nonsecretory and
secretory cells without discernible granules in the light microscope;
somatotropic cells, which are acidophilic with immunocytochemically-
detectable growth hormone and contain granules of about 350 nm
diameter; prolactin cells, which are acidophilic and contain prolactin
granules of 600-900 nm diameter; gonadotropic cells, which consist of
2 basophilic subgroups: follicle-stimulating hormone secreting and
luteinizing-hormone secreting; follicle-stimulating hormone secreting
cells, which are large round cells with dense 200 nm diameter
granules; luteinizing hormone-containing cells, which are small and
contain 200-250 nm diameter granules; thyrotropic cells, which are
basophilic with thyroid-stimulating hormone granules of 120-200 nm
diameter; and finally, cortico cells, which are the least abundant of
the cell types and contain basophilic granules 100-200 nm in diameter.
The glandular cells of the anterior pituitary secrete seven
endocrine hormones, of which four act directly on other endocrine
glands. Follicle-stimulating hormone (FSH) promotes spermatogenesis in
the male testes and facilitates follicular maturation and estrogen
secretion in the female ovary. Lutenizing hormone (LH) acts on the
male testes to facilitate testosterone secretion from the interstitial
cells of Leydig and is a key hormone that promotes follicular rupture
(ovulation) in the female ovary. Thyroid-stimulating hormone (TSH)
triggers the secretion of thyroxine from the thyroid gland, and
adrenocorticotropic hormone (ACTH) causes the adrenal cortex to
secrete its products, especially glucocorticoids. The secretion of
these tropic, anterior pituitary hormones is in turn regulated by the
hormones of the endocrine gland. In most cases, this is a negative
feedback regulation. Estrogen decreases the output of pituitary FSH
and LH; thyroxine decreases the secretion of TSH; and glucocorticoids
reduce the output of ACTH.
The three other anterior pituitary hormones are prolactin (PRL),
melanocyte-stimulating hormone (MSH), and growth hormone (GH). A major
target for prolactin is the mammary gland, where prolactin promotes
milk production. PRL also plays a role in maintaining the ovarian
corpus luteum after ovulation. The entire body is a target for GH,
which promotes growth and maintenance of cellular integrity. GH
facilitates growth, in part, because it increases the transport of
amino acids into the cell, where they can be used for protein
synthesis. MSH increases the production of melanin by the melanocytes
of the skin.
Hypothalamic control of anterior pituitary secretions occurs
through the release of hypothalamic-hypophysiotropic hormones (HHH).
The hypothalmus secretes these HHH into vessels of the hypothalamic-
hypophyseal portal system, by which they are transported to the
anterior pituitary. One of the major advances of the last two decades
has been the isolation and characterization of these HHH.
6.8.2 Disruption of neuroendocrine function
Disruption of neuroendocrine function can occur at any one or all
of the levels of hypothalamic-pituitary-target organ integration.
Alternatively, neuroendocrine effects may result from modification of
"higher" levels of neuronal processing or from alterations in
peripheral metabolism. In the following discussion, approaches will be
indicated for the study of each of these possible sites of neurotoxic
action.
6.8.2.1 Direct pituitary effects
At the most dramatic level, massive changes in the weight or size
of the pituitary, such as occur in the adenohypophysis after large
doses of estrogen (Schreiber & Pribyl, 1980) might be seen. However,
by far the most accessible method for studying pituitary function
relies on the examination of blood levels of pituitary hormones by
radioimmunoassay. Furthermore, the analysis of blood-hormone levels is
one of the few techniques applicable for screening human populations.
A large number of toxic substances have been reported to modify
adenohypophyseal secretions. Methallibure and allied substances
inhibit thyrotropin (Tulloch et al., 1963), and prolactin (Benson &
Zagni, 1965) secretion. Modifications of pituitary hormone secretions
have been reported for acrylamide (Uphouse et al., 1982), alcohol
(Mendelson et al., 1977), 1,4-DDT (Gellert et al., 1972), and
chlordecone (Uphouse et al., 1984). Dimethylsulfoxide may influence
adrenal glucocorticoids by elevating pituitary secretions (Allen &
Allen, 1975). In spite of the fact that changes in the blood levels of
pituitary hormones may be the most sensitive index of neuroendocrine
modification by toxic compounds, they have been examined for
relatively few compounds. The high sensitivity of this approach
results from the fact that the blood levels represent the final
consequence of the complex neuroendocrine integration. Regardless of
the actual site of modification, neuroendocrine disturbances will
usually be revealed in modified levels of circulating hormones.
Therefore, such changes, alone, cannot be regarded as evidence of
direct neuroendocrine disruption. The major disadvantage of serum-
hormone measurements is the responsiveness of the hypothalamic-
pituitary axis to a variety of environmental and chemical stimuli. It
can be difficult to identify the toxic agent as the causal agent.
Because hormones are secreted in a pulsatile manner, single point
analyses of serum hormones must be cautiously interpreted. However,
even with these limitations, analysis of blood levels of pituitary
hormones remains the most appropriate starting point for evaluating
potential neuroendocrine toxicity.
Similar analytical methods may be used to investigate the
pituitary content of respective pituitary secretions. The pituitary is
removed, homogenized, and extracted for use with the appropriate RIA.
For most pituitary secretions, neurotoxicologists have not yet studied
pituitary tissue directly. However, pituitary endorphins has been
reported to be modified by chlordecone (Hong & All, 1982).
6.8.2.2 Peripheral target effects
Most anterior pituitary hormones are subject to negative feedback
control by peripheral endocrine glands. If the neurotoxic agent
modifies peripheral secretions, neuroendocrine changes can result from
this altered feedback. Modifications in the functioning of these
endocrine secretions could occur after toxic exposure. Such approaches
have been widely applied in the experimental and clinical literature
and have shown that a number of compounds alter blood levels of
glucocorticoids, thyroxine, estrogen, and testosterones (Chapman,
1983).
Target-tissue effects can also be evaluated by a variety of
additional techniques. One of the simplest assessments is the change
in the weight, morphology, or biochemistry of the target organ.
Various environmental contaminants have been reported to produce such
changes. Lesions of the thyroid have been reported after exposure to
carbon disulfide (Cavalleri, 1975) and strong magnetic fields
(Persinger et al., 1978). Marked changes occur in the adrenals during
antimony and lead poisoning (Minkins et al., 1973) and in response to
very long chain saturated fatty acids (Powers et al., 1980).
Chlordecone causes adrenal, cortical, and medullary hypertrophy
(Eroschenko & Wilson, 1975) and alters the epinephrine and
norephinephrine content of the adrenal medulla (Baggett et al., 1980).
DDT (McBlain et al., 1976) and other chlorinated hydrocarbons
(Dicksith & Datta, 1972; Fellegiova et al., 1977), carbon disulfide
(Rosewickyi et al., 1973), and cadmium salts (Parizek, 1956;
Zylber-Haran et al., 1982) have all been described as having toxic
effects on the gonads. Although such changes are not necessarily due
to direct neuroendocrine effects, target organ changes can often be a
first indication of neuroendocrine changes. In some cases, even
relatively gross target organ events have been a first clue towards
the mode of action of a neurotoxic agent. For example, the chlorinated
pesticides, DDT and chlordecone, modify gonads in a manner reminiscent
of the natural steroid, estrogen (Gellert et al., 1972; Eroschenko &
Palmiter, 1980; Kupfer & Bulger, 1980). Uterine and/or oviduct weight
changes in the immature animal were the first evidence that the
pesticides might exert estrogenic action.
6.8.2.3 Disruption of hypothalamic control of pituitary secretions
Hypothalamic control of anterior pituitary secretions includes
direct regulation through hypothalamic hypophysiotropic hormones and
modulation through neurotransmitters and neurally-active peptides.
Evaluation of the effect of a neurotoxic agent on HHH is directly
measureable only for the HHH for which antisera are available. For
these compounds, it is possible (though difficult) to measure release
into the portal blood supply, to evaluate the effects of toxic agents
on either amounts or release, and to identify pituitary responsiveness
to the hypothalamic factors. Where identified hormones are not
available, hypothalamic extracts may still be used with in vitro
pituitary responsiveness as a bioassay. Details of such methods can be
found in several sources (Burgus & Guillemin, 1970; Oliver et al.,
1974). However, the value of such approaches for neurotoxicology has
yet to, be tested. Such methods will be of greatest value in testing
hypotheses regarding the mechanism of action of known neuroendocrine
toxic agents. They are not recommended for initial screens.
Biochemical changes in the hypothalamus can also be used as
indices of potential neuroendocrine disruption. Such hypothalamic
effects of neurotoxic compounds have received much less attention than
brain areas such as the striatum, cortex, or cerebellum. Methods for
studying the hypothalamus are the same as those used for other brain
areas. However, the hypothalamic neurons that regulate pituitary
function receive numerous synaptic inputs, both conventional and from
putative neurotransmitters. Consequently, the neuroendocrine
significance of changes in hypothalamic neurotransmitters and
neuropeptides is usually only inferential. However, any disruption of
hypothalamic biochemistry has the potential to alter neuroendocrine
function. When combined with more direct measurements of
neuroendocrine function, such studies are very important. There have
been a few reports of biochemical changes in the hypothalamus and/or
pituitary, correlated with neuroendocrine toxicity. One approach has
been to examine the effects of toxic compounds on hormone-mediated
changes. Administration of estrogens is followed by hypothalamic
ascorbic acid depletion (Schreiber et al., 1982) and by increases in
polyphenol oxidase (ceruloplasmin) activity in the hypothalamus and
the blood (Schreiber & Pribyl, 1980). This can be inhibited by the
simultaneous administration of silver nitrate. Disulfiram
(tetraethylthiuram disulfide) inhibits the reaction of the
adenohypophysis (Schreiber et al., 1979). Disulfiram also inhibits
dopamine-ß-hydroxylase (Szmigielski, 1975) and lowers the
blood-prolactin level (Cavalleri et al., 1978). The effect of
disulfirams on prolactin may be due to a "sparing of dopamine" through
inhibition of dopamine-ß-hydroxylase.
Hypothalamic peptides have been studied most extensively after
chlordecone treatment. Long-term exposure to the pesticide reduced
hypothalamic ß-endorphin levels under conditions where substance P,
neurotensin, and met-enkephalin were unchanged (Ali et al., 1982).
6.8.2.4 Other sites of action
For many neurotoxic agents, there may be no, identifiable effects
on neuroendocrine function, but it may be altered through the indirect
effects of the toxic compound. Such interruption could occur via
modification of neural integration leading to an altered response of
the organism to environmental challenge. Detection of such integrative
action and its relevance to neuroendocrine function might include
treatment with the toxic agent followed by environmental,
pharmacological, or hormonal challenges known to produce a
neuroendocrine response. Several toxic agents have been reported to
produce behavioural changes, such as stress-induced analgesia, and
these undoubtedly involve some aspect of neuroendocrine function. CNS
opiate systems are important components of the CNS response to stress
and studies of peripheral and brain endorphins and encephalins are
potentially relevant to such disturbances. For example, neonatal
treatment with chlordecone dissolved in dimethyl sulfoxide produces an
elevated corticosterone response to footshock (Rosecrans et al.,
1982), and long-term exposure of female rats to chlordecone has been
reported to decrease hypothalamic ß-endorphin levels (Ali et al.,
1982).
A toxic agent could also indirectly affect neuroendocrine function
by altering the peripheral metabolism of endocrine secretions. Such
metabolic differences could indirectly influence CNS function.
However, hormone transformation and the formation of active
metabolites occurs in the CNS so that metabolic disturbances may even
have a direct effect on CNS functioning. Many metabolic variables have
been reported to change after treatment with a toxic agent.
Aromatization of testosterone to estrogen, an important step in the
metabolism of estrogen, took place in the brain of animals (Gallard et
al., 1978). Its inhibition by aminoglutethimide or other blockers of
aromatization (Morali et al., 1977) markedly altered the sexual
behaviour of experimental animals. Aminoglutethimide also inhibited
total adrenal steroidogenesis as well as gonadal steroidogenesis and
thyroid function (Schreiber et al., 1969). The transformation of
thyroxine to the more active metabolite triiodothyronine was inhibited
by a series of toxic substances such as ethanol (Shimizu et al.,
1978), propylthiouracil (Leonard & Rosenberg, 1980), iopanoic acid
(Kaplan, 1980), and sodium salicylate (Chopra et al., 1980). Since the
monodeiodination of thryoxine to triiodothyronine also takes place in
the adenohypophysis, these factors may be an important component of
neuroendocrine reactions to neurotoxic substances. There is evidence
that demonstrates that the direct dopaminergic effect of estrogen on
the pituitary may require conversion of estrogen to catechol estrogen
(Paul et al., 1980). Such conversion occurs not only in the pituitary,
but also in the hypothalamus and cerebral cortex and may mediate some
of the effects of estrogen on the CNS. Study of this enzyme promises
to be important for future research, especially for the investigation
of estrogen-like toxic agents.
A final way in which the toxic agent might disrupt neuroendocrine
function is by altering the peripheral metabolism of the endocrine
secretions within liver tissue. Although beyond the scope of this
publication, several neurotoxic agents (chlordecone, dieldrin,
heptachlor, lindane, p,p'-DDE, and toxaphene) stimulate the
metabolism of estrone by liver microsomal enzymes (Welch et al.,
1971). Biochemical approaches for identifying these metabolic
disturbances are the same as those discussed in other sections.
However, the precise metabolic end-point should be carefully chosen,
when inferences are to be made about neuroendocrine function.
6.8.3 Sex differences
Because neurotoxic agents may produce changes in neuroendocrine
function and, since males and females differ with regard to a variety
of metabolic variables, a valuable approach to any neurotoxicological
investigation is the comparison of the toxic response in the sexes.
Whenever neuroendocrine effects are suspected, sex differences should
be a routine aspect of the overall investigation. Sex-related
differences have been observed for many environmental compounds.
Often, these result from the influence of gonadal hormones on the
metabolism of the compound. Hormonal influences on the
biotransformation and toxicity of DDT (Durham et al., 1956) and several
organophosphates (Murphy & DuBois, 1958; DuBois & Puchala, 1961) have
been demonstrated. Parathion is an organophosphate insecticide that
exerts its toxicity through its active metabolite paraoxon phosphate.
Agrawal et al. (1982) have recently shown that the sex difference in
AChE inhibition after parathion treatment was not evident with
paraoxon treatment. This suggests that the sex difference was in the
rate of metabolism to the toxic product. The female's increased
sensitivity to amobarbital (Castro & Gillette, 1967) may also be due
to sex differences in hepatic metabolism. Sex differences have also
been reported for the rate of disposition of chlordiazepoxide
(Greenblatt et al., 1977; Roberts et al., 1979) and for the toxic
effects of ethylmorphine, aniline, p-nitroanisole (Nicholas &
Barron, 1932; Holck et al., 1937; Quinn et al., 1958) and
polychlorinated hydrocarbons (Lamartiniere et al., 1979).
6.9 Recommendations for Future Research
Several biochemical and neuroendocrine approaches are available
that have not yet been applied to the study of neurotoxic compounds.
These have the advantage of increasing the sensitivity of the
biochemical approach and furthering understanding of the ways in which
compounds disrupt nervous system function. For example, some enzymes
involved in neurotransmitter synthesis have been purified and used for
the production of specific antibodies (John et al., 1973). It is now
possible to use immunological titration to determine whether changes
in enzyme activity result from activity alterations or variations in
the amount of enzyme protein.
Identification of direct pituitary modification can be
accomplished by preparation of anterior pituitary cell cultures and
the measurement of hormone release in response to hypothalamic
releasing factors, neurotransmitters, or suspect toxic compounds. The
pituitary cells are removed, dispersed, and agitated with collagenase.
After resuspension in medium, the cells are plated and used for
examination. Under such culture conditions, pituitary cells respond to
releasing factors and neurotransmitter regulation (Enjalbert et al.,
1978; Drouin & Labrie, 1981). Thus, it is possible to determine
whether the toxic agent modifies the responsiveness of the pituitary
to hypothalamic control. By comparing the effects of the toxic agent
in vivo with those in vitro, direct versus indirect effects of the
compound can be identified.
Furthermore, for suspect steroid-like compounds, direct evaluation
of receptor interactions is possible. Steroid receptors modify their
target tissue by binding to intracellular receptors. Measurement of
intracellular steroid receptors involves the preparation of a high
speed cytosol (180 000 g) fraction from the appropriate target tissue.
Identification of binding is accomplished by incubating the cytosol
in vitro in the presence of radioactively-labelled hormone. Bound
label is removed from unbound by a variety of techniques distinct for
the particular receptor of interest. Specific binding is assessed by
incubating the labelled hormone in the presence of excess unlabelled
hormone as competitor. Specific binding refers to bound molecules that
are removed in the presence of this unlabelled competitor. When a
neurotoxic agent is suspected of interacting with hormone receptors,
the toxic agent may be substituted for the unlabelled competitor and
its ability to compete for binding determined. This procedure has
demonstrated competition by both chlordecone (Palmiter & Mulvihill,
1978) and o,p'-DDT (Kupfer & Bulger, 1976) for the estradiol
cytosol receptor in the uterus. Using an estradiol exchange method,
investigators have shown that these pesticides produce translocation
of the estradiol receptor to the nucleus (Hammond et al., 1979).
Employment of the exchange method necessitates the in vivo treatment
of the organism with the toxic agent. Nuclei and cytosol are prepared
from the target tissue and incubated in vitro with the
radioactively-labelled hormone. Since a major movement of cytosol
receptors into the nucleus occurs only after binding to the hormone,
the presence of the receptor in the nucleus after exposure to the
toxic agent suggests direct binding of the toxic agent to the steroid
cytosol receptor.
Modifications of the procedures described for neurotransmitter
receptor binding are used in the measurement of membrane receptors.
However, no neurotoxic agents have yet been tested for their ability
to modify these hormone receptors. For both intracellular and
extracellular hormone receptors, most studies have been on peripheral
tissues. However, the brain and the pituitary contain receptors for a
variety of steroid and peptide hormones, and these offer a number of
targets for disruption by environmental compounds. Such analyses have
great potential for future studies of neurotoxic compounds. However,
they will usually be applied for the investigation of mechanisms of
action rather than as screens for neurotoxic compounds.
Finally, hypothalamic regulation of pituitary function should
receive further emphasis. Neurotransmitters also regulate pituitary
secretions via neurotransmitter receptors of the pituitary gland. The
potential for a direct modification of these receptor interactions by
neurotoxic compounds is only just being realized. The most thoroughly
described pituitary neurotransmitter receptor is that of dopamine,
which regulates the inhibition of prolactin release by dopamine.
Steroid hormones, such as estrogen, by conversion to catechol
estrogens, increase prolactin secretion by direct interaction with the
pituitary dopamine receptors (Gudelsky et al., 1981; Fishman, 1982).
Neuroleptic drugs elevate prolactin secretion by antagonistic action
on pituitary dopamine receptors (Horowski & Graf, 1979; Besser et al.,
1980).
7. CONCLUSIONS AND RECOMMENDATIONS
There is ample evidence of real and potential hazards of
environmental chemicals for nervous system function. Changes or
disturbances in central nervous function, many times manifest by vague
complaints and alterations in behaviour, reflect on the quality of
life; however, they have not yet received attention.
Neurotoxicological assessment is therefore an important area for
toxicological research.
It has become evident, particularly in the last decade, that
low-level exposure to certain toxic agents can produce deleterious
neural effects that may be discovered only when appropriate procedures
are used. While there are still episodes of large-scale poisoning,
concern has shifted to the more subtle deficits that reduce
functioning of the nervous system in less obvious, but still important
ways, so that intelligence, memory, emotion, and other complex neural
functions are affected.
Information on neurobehaviour, neurochemistry, neurophysiology,
neuroendocrinology, and neuropathology is vital for understanding the
mechanisms of neurotoxicity. One of the major objectives of a
multifaceted approach to toxicological studies is to understand
effects across all levels of neural organization. Such a multifaceted
approach is necessary for confirmation that the nervous system is the
target organ for the effect. Interdisciplinary studies are also
necessary to understand the significance of any behavioural changes
observed and thus, to aid in extrapolation to human beings by
providing specific neurotoxic profiles. Concomitant measurements at
different levels of neural organization can improve the validity of
results.
The following recommendations are made on the basis of the
information contained in this book:
1. Health personnel should take into account the possible role of
exposure to chemicals whenever a patient presents with any
neurobehavioural complaint.
2. Neurotoxicological testing should be an essential consideration in
any profile developed by the agencies responsible for the control
of toxic chemicals.
3. In the determination of the potential of a chemical to produce
neurotoxic effects, a multidisciplinary strategy should be used.
4. Priority should be given to obtaining clinical and epidemiological
data when exposure occurs to chemicals suspected of being
neurotoxic.
5. Test development in preclinical neurotoxicology has evolved to the
point where interlaboratory validation of procedures using
prototypic neurotoxic agents could be attempted.
REFERENCES
ABOU-DONIA, M.B. (1981) Organophosphorus ester-induced delayed
neurotoxicity. Ann. Rev. Pharmacol. Toxicol., 21 511-548.
ABOU-DONIA, M.B. & PREISSIG, S.H. (1976) Delayed neurotoxicity of
leptophos: toxic effects on the nervous system of hens. Toxicol.
appl. Pharmacol., 35 269-282.
ABOU-DONIA, M.G., JENSEN, D.N., & LAPADULA, D.M. (1983) Neurologic
manifestations of tri-o-cresyl phosphate delayed neurotoxicity in
cats. Neurobehav. Toxicol. Teratol., 5: 431-442.
AGNEW, W.S., LEVINSON, S.R., BRABSON, J.S., & RAFTERY, M.A. (1978)
Purification of the tetrodotoxin-binding component associated with the
voltage-sensitive sodium channel from Electrophorus electricus
electroplax membranes. Proc. Natl Acad. Sci. (USA), 75: 2606-2610.
AGRAWAL, A.K., SQUIBB, R.E., & BONDY, S,C. (1981) The effects of
acrylamide treatment upon the dopamine receptor. Toxicol. appl.
Pharmacol., 58 89-99.
AGRAWAL, D.K., MISRA, D., AGARWAL, S,, SETH, P.K., & KOHLI, J.D.
(1982) Influence of sex hormones on parathion toxicity in rats;
antiacetylcholinesterase activity of parathion and paraoxon in plasma,
erythrocytes, and brain. J. Toxicol. environ. Health, 9: 451-459.
ALBURQUERQUE, E.X. & DALY, J.W. (1976) Steroidal alkaloid toxins and
ion transport in electrogenic membranes. In: Cuatracasas, P., ed. The
specificity of animal, bacterial, and plant toxins, London, Chapman
and Hall, pp. 297-338.
ALDER, S. & ZBINDEN, G. (1977) Methods for the evaluation of physical,
neuromuscular, and behavioral development in rats in early postnatal
life. In: Newbert, D., Merber, H.J., & Kwasigroch, T.E., ed. Methods
in prenatal toxicology, Stuttgart, George Thiene, pp. 175-185.
ALDRIDGE, W.M., CLOTHIER, B., FORSHAW, P., JOHNSON, M.K., PARKER,
V.H., PRICE, R.J., SKILLETER, D.N., VERSCHOYLE, P.D., & STEVENS, C.
(1978) The effect of DDT and pyrethroids cismethrin and decamethrin on
the acetylcholine and cyclic nucliotide content of rat brain.
Biochem. Pharmacol., 27: 1703-1706.
ALEU, F.P., KATZMAN, R., & TERRY, R.D. (1963) Fine structures and
electrolyte analyses of cerebral oedema induced by alkyl tin
intoxication. J. Neuropathol. exp. Neurol., 22: 403-413.
ALEXANDER, B.K. BEYERSTEIN, B.L., HADAWAY, P.F., & COAMBS, R.B.
(1981) Effect of early and later colony housing on oral ingestion of
morphine in rats. Pharmacol. Biochem. Behav., 15: 571-576.
ALFANO, D.P. & PETIT, T.L. (1981) Behavioral effects of postnatal lead
exposure. Possible relationship to hippocampal dysfunction. Behav.
neural Biol., 32: 319-333.
ALI, S.F., HONG, J.S., WILSON, W.E., LAMB, J.C., MOORE, J.A., MASON,
G.A., & BONDY, S.C. (1982) Subchronic dietary exposure of rats to
chlordecone (kepone) modifies levels of hypothalamic beta-endorphin.
Neurotoxicology, 3: 119-124.
ALLEN, J.P. & ALLEN, C.F. (1975) The effect of dimethyl sulfoxide on
hypothalamic-pituitary-adrenal functions in the rat. Ann. NY Acad.
Sci., 243: 325-336.
ALTMAN, J. & SUDARSHAN, K. (1975) Postnatal development of locomotion
in the laboratory rat. Anim. Behav., 23: 896-920.
AMIN-ZAKI, L., MAJEED, M.A., ELHASSANI, S.B., CLARKSON, T.W.,
GREENWOOD, M.R. & DOHERTY, R.A. (1979) Prenatal methylmercury
poisoning: clinical observations over five years. J. Dis. Child,
133: 172-177.
ANDRIANOV, V.V., ZAKHAROV, N.C., & FADEEV, JU.A. (1977) Microiono-
phoretic analysis of chemical properties of cortical units in freely
moving animals. Physiol. J. (USSR), 63(4): 602-605.
ANGELL, F. & WEISS, B. (1982) Operant behavior of rats exposed to lead
before or after weaning. Toxicol. appl. Pharmacol., 63: 62-71.
ANGER, W.K. (1984) Neurobehavioral testing of chemicals: impact on
recommended standards. Neurobehav. Toxicol. Teratol., 6(2): 147-153.
ANGER, W.K. & JOHNSON, B.L. (1985) Chemicals affecting behavior. In:
O'Donoghue, J.L., ed. Neurotoxicity of industrial and commercial
chemicals, Boca Raton, Florida, CRC Press.
ANGER, W.K. & SETYES, J.V. (1979) Effects of oral and intramuscular
carbaryl administration on repeated chain acquisition in monkeys.
J. Toxicol. environ. Health, 5: 793-808.
ANNAU, W. (1975) The comparative effects of carbon monoxide and
hypoxia on behaviour. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Press, pp. 105-127.
ANOKHIN, P.K. (1935) [Problem of center and periphery in contemporary
physiology of nervous system.] In: Anokhin, P.K., ed. [Problem of
centre and periphery in physiology of nervous activity], Gorkiy,
Gorkiy Publishing House, pp. 9-71 (in Russian).
ANOKHIN, P.K. (1964) [Comparative electrophysical analysis of
alterations of laility of the cortical potential components in
postnatal ontogenesis.] Fiziol. Zh. SSSR, 50: 773-778 (in Russian).
ANOKHIN, P.K. (1968) [Biology and neurophysiology of the conditioned
reflex,] Moscow, Meditsina, pp. 547 (in Russian).
ANOKHIN, P.K. (1974) The functional system as a basis of the
physiological architecture of the behavioural act. In: Anokhin, P.K.,
ed. Biology and neurophysiology of the conditioned reflex and its
role in adaptive behaviour, New York, Pergamon Press, pp. 190-254.
APFELBACH, R. & DELGADO, J.M.R. (1974) Social hierarchy in monkeys
(Macaca mulatta) modified by chlordiazepoxide hydrochloride.
Neuropharmacology, 13: 11-20.
APPEL, S.H. & DAY, E.D. (1976) Cellular and subcellular fractionation.
In: Seigel, G.L., Albers, R.W., Katzman, R., & Agranoff, B.W., ed.
Basic neurochemistry, Boston, Toronto, Little, Brown and Company,
pp. 34-57.
ARBUKHANAOVA, M.S. (1981) Metabolism of glutamic acid in the cerebral
mitochondria during cooling disease. In: Mitochondria: the
mechanisms of conjugation and regulation, Puschino, p. 76.
ASABAYEV, CH., BONCHKOVSKAYA, T.YU., & ZHEGALLO, I.G. (1972) [A study
of the reaction of the animal nervous system to the effect of
low-strength electromagnetic fields.] In: [Labour hygiene and the
biological effect of radio frequency electromagnetic fields,]
Moscow, pp. 48-49 (in Russian).
AVROVA, N.F. (1971) Brain ganglioside patterns of vertebrates.
J. Neurochem., 18: 667-674.
BAENNINGER, L.P. (1966) The reliability of dominance orders in rats.
Anim. Behav., 14: 367-371.
BAGGETT, J.MCC., THURESON-KLEIN, A., & KLEIN, R.L. (1980) Effects of
chlordecone on the adrenal medulla of the rat. Toxicol. appl.
Pharmacol., 52: 313-322.
BAKER, P.F., HODGKIN, A.L., & RIDGWAY, E.B. (1971) Depolarization and
calcium entry in giant squid axons. J. Physiol., 218: 709-775.
BAKIR, F., DAMLUJI, S.F., AMIN-ZAKI, L., MURTADHA, M., KHALIDI, A.
AL-RAWL, N.Y. TIKRITI, S. DHARIR, H.I., CLARKSON, T.W., SMITH, J.C.,
& DOHERTY, R.A. (1973) Methylmercury poisoning in Iraq: an
inter-university report. Science, 181: 230-241.
BALNAVE, R.J. & GAGE, P.W. (1973) The inhibitory effect of manganese
on transmitter release at the neuromuscular junctions of the toad.
Br. J. Pharmacol., 47: 339-352.
BANCROFT, J.D. & STEVENS, A. (1977) Theory and practice of
histological techniques, New York, Churchill Livingston.
BARKER, J.L. & MCKELVY, J.F., ed. (1983) Current methods in cellular
neurobiology: electrophysiological and optical recording techniques,
New York, John Wiley and Sons, Vol. 3.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1973) A behavioral and
pharmacological analysis of variables mediating active avoidance
behaviour in rats. J. comp. Physiol. Psychol., 82: 489-500.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1974) An analysis of the
facilitation of avoidance acquisition produced by delta-amphetamine
and scopolamine. Behav. Biol., 11: 189-203.
BASAR, E. (1980) EEG-brain dynamics. In: Relation between EEG and
brain-evoked potentials, Amsterdam, Oxford, New York, Elsevier
Science Publishers.
BAUMGARTNER, G. GAWEL, M.J. KAESER, H.E., PALLIS, C.A., CLIFFORD,
F.R., SCHAUMBURG, H.H., THOMAS, P.K., & WADIA, N.H. (1979)
Neurotoxicity of halogenated hydroxyquinolines: clinical analysis of
cases reported outside Japan. J. Neurol. Neurosurg. Psychiatr.,
42: 1073-1083.
BEARD, R.R. & GRANDSTAFF, N. (1975) Carbon monoxide and human
function. In: Weiss, B. & Laties, V., ed. Behavioural toxicology,
New York, Plenum Press, pp. 1-26.
BECK, E.C., DUSTMAN, R.E., & LEWIS, E.G. (1975) The use of the
averaged evoked potential in the evaluation of central nervous system
disorders. Int. J. Neurol., 9: 211-232.
BENESOVA, O., HORVATH, M., & MIKISKA, A. (1956) [Assessment of the
depth and duration of narcosis according to the electrophysiological
characteristics of the cerebral cortex.] Physiol. bohemoslov.,
5: 188-194 (in German).
BENNETT, S. & BOWERS, D. (1976) An introduction to multivariate
techniques for social and behavioral sciences, New York, Halsted
Press.
BENSON, C.K. & ZAGNI, P.A. (1965) The effect of 1-alpha-
methylallylthiocarbamyl-2-methylthiocarbamylhydrazine (ICI 33828) on
lactation in the rat. J. Endocr., 32: 275-285.
BERGER, H. (1929) [On the electroencephalogram in man.] Arch.
Psychiat. Nervenkr., 87: 527-550 (in German).
BERNARD, C. (1927) An introduction to the study of experimental
medicine, New York, MacMillan Inc., p. 127.
BESSER, G.M., DELITALA, G., GROSSMAN, A., STUBBS, W.A., & YEO, T.
(1980) Chlorpromazine, haloperidol, metoclopramide, and domperidone
release prolactin through dopamine antagonism at low concentrations
but paradoxically inhibit prolactin release at high concentrations.
Br. J. Pharmacol., 71: 569-573.
BHAGAT, B. & WHEELER, M. (1973) Effect of amphetamine on the swimming
endurance of rats. Neuropharmacology, 12: 1161-1165.
BIGNAMI, G., ROSIC, N., MICHALEK, H., MILOSEVIC, M., & GATTI, G.L.
(1975) Behavioral toxicity of anticholinesterase agents:
methodological, neurochemical and neuropsychological aspects. In:
Weiss, B. & Laties, V.G., ed. Behavioral toxicology, New York,
Plenum Press, pp. 155-215.
BLACKWOOD, W., MCMENEMEY, W.H., MEYER, A., NORMAN, R.M., & RUSSELL,
D.S. (1971) Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd.
BLOOM, A.S., STOOTZ, C.G., & DIERINGER, T. (1983) Pyrethroid effects
on operant responding and feeding. Neurobehav. Toxicol. Teratol.,
5: 321-324.
BOER, G.J. (1974) A microassay for arylamidase activity. Anal.
Biochem., 59: 410-411.
BOKINA, A.I., EKSLER, N.D., SEMENENKO, A.D., & MERKURYVA, R.V. (1976)
Investigation of the mechanism of action of atmospheric pollutants on
the central nervous system and comparative evaluation of methods of
study. Environ. Health Perspect., 13: 37-42.
BOKINS, A.I., KAN, T.M., BEREZINA, O.V., & PINIGINA, I.A. (1979)
[Study of complex forms of animal behavior in a hygenic evaluation of
toxic substances.] Gig. i Sanit., 7: 49-51 (in Russian).
BONASHEVSKAYA, T.I., SHAMARIN, A.A., KESAREV, V.S., & YURASOVA, O.I.
(1983) [Morphological criteria and a system of indexes for the
evaluation of neurotoxic effects produced by environmental chemical
factors (on the basis of a carbon monoxide model).] Gig. i Sanit.,
10: 31-33 (in Russian, with English summary).
BONDY, S.C. & AGRAWAL, A.K. (1980) The inhibition of cerebral high
affinity receptor sites by lead and mercury compounds. Arch.
Toxicol., 46: 249-256.
BONDY, S.C. & HARRINGTON, M.L. (1979) Calcium-dependent release of
putative neurotransmitters in the chick visual system. Neuroscience,
4: 1521-1527.
BONDY, S.C., HARRINGTON, M.L., ANDERSON, C.L., & PRASAD, K.N. (1979)
Low concentration of an organic lead compound alters transport and
release of putative neurotransmitters. Toxicol. Lett., 3: 35-41.
BONDY, S.C., TILSON, H.A., & AGRAWAL, A.K. (1981) Neurotransmitter
receptors in brains of acrylamide-treated rats. II. Effects of
extended exposure to acrylamide. Pharmacol. Biochem. Behav.,
14: 533-537.
BONILLA, E. (1980) L-tyrosine hydroxylase activity in the rat brain
after chronic oral administration of manganese chloride. Neurobehav.
Toxicol., 2: 37-41.
BONILLA, E. SALAZAR, E. VILLASMIL, J.J., & VILLALOBOS, R. (1982) The
regional distribution of manganese in the normal human brain.
Neurochem. Res., 7: 221-227.
BOYES, W.K., JENKINS, D.E., & DYER, R.S. (1985) Chlordimeform produces
contrast-dependent changes in visual-evoked potentials in hooded rats.
Exp. Neurol., 89: 391-407.
BRACELAND, F.J. (1942) Mental symptoms following carbon disulfide
absorption and intoxication. Ann. intern. Med., 16: 246-261.
BRADLEY, P.B. (1958) The central action of certain drugs in relation
to the reticular formation of the brain. In: Jasper, H.H., Proctor,
L.D., Knighton, R.S., Noshay, W.C., & Costello, R.T., ed. Reticular
formation of the brain, Boston, Toronto, Little, Brown and Company,
pp. 123-149.
BRADY, K., HERRERA, Y., & ZENICK, H. (1976) Influence of parental lead
exposure on subsequent learning ability of offspring. Pharmacol.
Biochem. Behav., 3: 561-565.
BRIERLEY, J.B. (1976) Cerebral hypoxia. In: Blackwood, W. & Corsellis,
J., ed. Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd., pp. 43-85.
BROWN, A.W., ALDRIDGE, W.N., STREET, B.W., & VERSCHOYLE, R.D. (1979)
The behavioural and neuropathological sequelae of intoxication by
trimethyltin compounds in the rat. Am. J. Pathol., 97: 59-82.
BUELKE-SAM, J. & KIMMEL, C.A. (1979) Development and standardization
of screening methods for behavioral teratology. Teratology,
20: 17-29.
BULL, R.J. & LUTKENHOFF, S.D. (1975) Changes in the metabolic
responses of brain tissue to stimulation, in vitro, produced by in
vivo administration of methylmercury. Neuropharmacology,
14: 351-359.
BURES, J. & BURESOVA, O. (1979) New methods in memory research. In:
Proceedings of the 21st Annual Meeting of the Czechoslovak
Psychopharmacological Society, Jesenik Spa, 9-14 January, 1979,
pp. 7-12.
BURESOVA, O. & BURES, J. (1982) Capacity of working memory in rats as
determined by performance on a radial maze. Behav. Processes,
7: 63-72.
BURGEN, A.S.V., KOSTERLITZ, H.W., & IVERSEN, L.L., ed. (1980)
Neuroactive peptides, London, Royal Society.
BURGUS, R. & GUILLEMIN, R. (1970) Chemistry of thyrotropin releasing
factor (TRF). In: Meites, J., ed. Hypophysiotropic hormones of the
hypothalamus: assays and chemistry, Baltimore, Maryland, Williams
and Wilkins Company, pp. 227-241.
BUSHNELL, P.J. & BOWMAN, R.E. (1979) Effects of chronic lead ingestion
on social development in infant rhesus monkeys. Neurobehav.
Toxicol., 1: 207-219.
BUTCHER, R.E. (1976) Behavioral testing as a method for assessing
risk. Environ. Health Perspect., 18: 75-81.
BUTCHER, R.E. & VORHEES, C.V. (1979) A preliminary test battery for
the investigation of the behavioral teratology of selected
psychotropic drugs. Neurobehav. Toxicol., 1(Suppl. 1): 207-212.
CABE, P.A. & TILSON, H.A. (1978) Hind limb extensor response: a method
for assessing motor dysfunction in rats. Pharmacol. Biochem. Behav.,
9: 133-136.
CAMMER, W. (1980) Toxic demyelination: biochemical studies and
hypothetical mechanisms. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 239-256.
CAMMER, W. & MOORE, C.L. (1972) The effect of hexachlorophene on the
respiration of brain and liver mitochondria. Biochem. biophys. Res.
Comm., 46: 1887-1894.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W., HAYES, C.,
STRAUB, W.E., LANDRIGAM, P.J., & LIDDLE, J.A. (1978) Epidemic kepone
poisoning in chemical workers. Am. J. Epidemiol., 17: 529.
CARBONE, E., WANKE, E., PRESTIPINO, G., POSSANI, L.D. & MAELICKE, A.
(1982) Selective blockage of voltage-dependent K+ channels by a
novel scorpion toxin. Nature (Lond.), 296: 90-91.
CARILLI, C.T., FARLEY, R.A., PERLMAN, D.M., & GANTHEY, L.C. (1982)
The active site structure of Na+- and K+- stimulated ATPase. J.
biol. Chem., 257: 5601-5606.
CASARETT, L.J. (1975) Toxicologic evaluation. In: Casarett, L.J. &
Doull, J., ed. Toxicology; the basic science of poisons, New York,
MacMillan Inc., pp. 11-25.
CASTRO, J.A. & GILLETTE, J.R. (1967) Species and sex differences in
the kinetic constants for the N-demethylation of ethyl-morphine by
liver microsomes. Biochem. biophys. Res. Comm., 28: 426-430.
CATTERALL, W.A. (1977) Activation of the action potential Na+
ionophore by neurotoxins. J. biol. Chem., 252: 8669-8676.
CAVALLERI, A. (1975) Serum thyroxine in the early diagnosis of carbon
disulfide poisoning. Arch. environ. Health, 30: 85-87.
CAVALLERI, A., POLATTI, F., & BOLIS, P.F. (1978) Acute effects of
tetramethylthiuram disulfide on serum levels of hypophyseal hormones
in humans. Scand. Work environ. Health, 4: 66-72.
CAVANAGH, J.B. (1964) The significance of the "dying-back" process in
experimental and human neurological disease. Int. Rev. exp. Pathol.,
3: 219-267.
CAVANAGH, J.B. & CHEN, F.C.-K. (1971) The effects of methyl-mercury-
dicyandiamide on the peripheral nerves and spinal cord of rats. Acta
neuropathol., 19: 208-215.
CAVANAGH, J.B. & JACOBS, J.M. (1954) Some quantitative aspects of
diphtheritic neuropathy. Br. J. exp. Pathol., 45: 309-327.
CAVANAGH, J.B., PASSINGHAM, R.J., & VOGT, J.A. (1964) Staining of
sensory and motor nerves in muscles with Sudan Black B. J. Pathol.
Bacteriol., 88: 89-92.
CECCARELLI, B. & HURLBUT, W.P. (1980) Ca-dependent recycling of
synaptic vesicles at the frog neuromuscular junction. J. cell Biol.,
87: 297-303
CHANCE, M.R.A. (1946) Aggregation as a factor influencing the toxicity
of sympathomimetic amines in mice. J. Pharmacol. exp. Ther.,
87: 214-219.
CHANG, L. (1977) Neurotoxic effects of mercury -- a review. Environ.
Res., 14: 329-373.
CHANG, L. (1980) Mercury. In: Spencer, P.S. and Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 508-526.
CHANG-TSUI, Y.H. & HO, I.K. (1979) Effects of kepone (chlordecone) on
synaptosomal gamma-aminobutryic acid uptake in the mouse.
Neurotoxicology, 1: 357-361.
CHAPMAN, R.M. (1983) Gonadal injury resulting from chemotherapy. Am.
J. ind. Med., 4: 149-161.
CHIBA, S. & ANDO, K. (1976) Effects of chronic administration of
kanamycin on conditioned suppression to auditory stimulus in rats.
Jpn. J. Pharmacol., 26: 419-426.
CHO, E.S., SPENCER, P.S., JORTNER, B.S., & SCHAUMBURG, H.H. (1980) A
single intravenous injection of doxorubicin (AdriamycinR) induces
sensory neuropathy in rats. Neurotoxicology, 1: 583-591.
CHOPRA, I.J., SOLOMON, D.J., TECO, G.N.C., & NGUYEN, A.H. (1980)
Inhibition of hepatic outer ring monodeiodination of thyroxine and
3,3',5'-triiodothyronine by sodium salicylate. Endocrinology,
106: 1728-1734.
CIOMS (1983) Safety requirements for the first use of new drugs and
diagnostic agents in man, Geneva, Council for International
Organizations of Medical Sciences.
CITOVIC, I.S. (1930) [The procedure of studying the effects of oil
products on the organism of animals.] Inst. Work Saf., 1: 92
(in Russian).
CLAUSEN, T. & FLATMAN, J.A. (1977) The effects of catecholamines on
Na-K transport and membrane potential in rat soleus muscle.
J. Physiol., 270: 383-414.
COLLIER, B. & ILSON, D. (1977) The effect of preganglionic nerve
stimulation on the accumulation of certain analogues of choline by a
sympathetic ganglion. J. Physiol., 264: 489-509.
COLLINS, R.D. (1982) Illustrated manual of neurologic diagnosis, 2nd
ed., Philadelphia, Pennsylvania, J.B. Lippincott Company.
COLOTLA, V.A. (1981) Adjunctive polydipsia as a model of alcoholism.
Neurosci. Biobehav. Rev., 5: 335-342.
COLOTLA, A., BAUTISTA, M., LORENZANA-JIMENEZ, M., & RODRIGUEZ, R.
(1979) Effects of solvents on schedule-controlled behavior.
Neurobehav. Toxicol., 1(Suppl. 1): 113-118.
CONSTANTOPOULOS, G., MCCOMB, R.D., & DECABAN, A. (1976) Neurochemistry
of the mucopolysaccharidoses: brain glycosaminoglycans in normal and
four types of mucopolysaccharidoses. J. Neurochem., 26: 901-908.
CORY-SLECHTA, D.A. & THOMPSON, T. (1979) Behavioral toxicity of
chronic postweaning lead exposure in the rat. Toxicol. appl.
Pharmacol., 47: 151-159.
CORY-SLECHTA, D.A., BISSEN, T., YOUNG, M., & THOMPSON, T. (1981)
Chronic postweaning lead exposure and response duration performance.
Toxicol. appl. Pharmacol., 60: 78-84.
COSTA, A. & MURAD, J.E. (1969) Study of the association between
chlorpromazine and analgesics through the method of electrical
stimulation of the rabbit dental pulp. Rev. Bras de Pesgnisa Med.
Biol., 2: 235-240.
COSTA, E. (1970) Simple neuronal models to estimate turnover rate of
noradrenergic transmitters in vivo. In: Costa, E. & Giabocini, E.,
ed. Advances in biochemical psychopharmacology. II. Biochemistry of
simple neuronal models, New York, Raven Press.
COTMAN, C.W. & BARKER, G.A. (1974) The making of a synapse. Rev.
Neurosci., 1: 1-62.
COTMAN, C.W., HAYCOCK, J.W., & WHITE, W.F. (1976) Stimulus secretion
coupling processes in brain: analysis of noradrenaline and gamma-
aminobutyric acid release. J. Physiol., 254: 475-505.
COURAUD, F.C., ROCHAT, H., & LISSITSKY, S. (1978) Binding of scorpion
and sea anemone neurotoxins to a common site related to the action
potential Na+ ionophore in neuroblastoma cells. Biochem. biophys.
Res. Comm., 83: 1525-1530.
CRANE, A.M. & GOLDMAN, P.S. (1979) An improved method for embedding
brain tissue in albumin-gelatin. Stain Technol., 54: 71-75.
CRAPPER, D.R. & DE BONI, U. (1980) Aluminum. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 326-335.
CUTLER, M.G. (1977) Effects of exposure to lead on social behaviour in
the laboratory mouse. Psychopharmacology, 52: 279-282.
DAMSTRA, T. (1978) Environmental chemicals and nervous system
dysfunction. Yale J. Biol. Med., 51: 457-468.
DAMSTRA, T. & BONDY, S.C. (1980) The current status and future of
biochemical assays for neurotoxicity. In: Spencer, P.S. & Schaumburg,
H.H., ed. Experimental and clinical neurotoxicology, Baltimore,
Maryland, Williams and Wilkins Company, pp. 820-833.
DAMSTRA, T. & BONDY, S.C. (1982) Neurochemical approaches to the
detection of neurotoxicity. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 349-373.
DAUBE, J. (1980) Nerve conduction studies. In: Aminoff, J., ed.
Electrodiagnosis in clinical neurology, New York, Churchill
Livingstone, pp. 229-264.
DAVIS, M. (1980) Neurochemical modulation of sensory-motor reactivity:
acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev.,
4: 241-263.
DE JESUS, C.P.V., TOCOFIGHI, J., & SNYDER, D.R. (1978) Sural nerve
conduction study in the rat: a new technique for studying experimental
neuropathies. Muscle Nerve, 1: 162-167.
DESAIAH, D., GILLILAND, T., HO, I.K., & MEHENDALE, H.M. (1980)
Inhibition of mouse brain synaptosomal ATPases and ouabain binding by
chlordecone. Toxicol. Lett., 6: 275-285.
DESCLIN, J.C. (1974) Histological evidence supporting the inferior
olive as the major source of cerebellar climbing fibres in the rat.
Brain Res., 77: 365-384.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I. & SOS, J. (1962) Central nervous injury by a chemical
herbicide. Acta Med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1963) Experimental lesions of the central nervous
system induced by triorthocresyl phosphate. Acta Physiol. Acad. Sci.
Hung., 23: 63-68.
DESI, I., GONCZI, L., SIMON, G., FARKAS, I., & KNEFFEL, Z. (1974)
Neurotoxicologic studies of two carbamate pesticides in subacute
animal experiments. Toxicol. appl. Pharmacol., 27: 465-476.
DEWAR, A.J. & MOFFETT, B.J. (1979) Biochemical methods for detecting
neurotoxicity. A short review. Pharmacol. Ther., 5: 545-562.
DEWAR, A.J., MOFFETT, B.J., & SITTON, M.F. (1977) The effect of
anti-inflammatory drugs on retinal dystrophy in the rat. Toxicol.
appl. Pharmacol., 42: 65-74.
DEWS, P.B. (1982) Epistemology of screening for behavioral toxicity.
In: Mitchell, C.L., ed. Nervous system toxicology, New York, Raven
Press, pp. 229-236.
DEWS, P.B. & WENGER, G.R. (1979) Testing for behavioral effects of
agents. Neurobehav. Toxicol., 1(Suppl. 1): 119-127.
DIAMOND, S.S. & SLEIGHT, S.D. (1972) Acute and subchronic
methylmercury toxicosis in the rat. Toxicol. appl. Pharmacol.,
23: 197-207.
DICKSITH, T.S.S. & DATTA, K.K. (1972) Effect of intratesticular
injection of lindane and endrine on the testes of rats. Acta
pharmacol., 31: 1-10.
DIETZ, D.D., MCMILLAN, D.E., GRANT, L.D., & KIMMEL, C.A. (1978)
Effects of lead on temporally-spaced responding in rats. Drug chem.
Toxicol., 1(4): 401-419.
DINGLEDINE, R., ed. (1984) Brain slices, New York, Plenum Press,
442 pp.
DIXON, R.L. (1976) Problems in extrapolating toxicity data for
laboratory animals to man. Environ. Health Perspect., 13: 43-50.
DOLPHIN, A.C. & GREENGARD, P. (1981) Neurotransmitter- and
neuromodulator-independent alterations in phosphorylation of protein I
in slices of rat facial nucleus. J. Neurosci., 1: 192-203.
DRAPER, W.A. (1967) A behavioural study of the home-cage activity of
the white rat. Behaviour, 28: 280-306.
DRENTH, H.J., ENSBERG, I.F.G., ROBERTS, D.V., & WILSON, A. (1972)
Neuromuscular function in agriculture workers using pesticides. Arch.
environ. Health, 25: 395-398.
DROUIN, J. & LABRIE, F. (1981) Interactions between 17 beta-estradiol
and progesterone in the control of luteinizing hormone and follicle
stimulating hormone release in rat pituitary cells in culture.
Endocrinology, 108: 52-57.
DUBOIS, K.P. & PUCHALA, E. (1961) Studies on the sex differences in
the toxicity of cholinergic phosphorothioate (26791). Proc. Soc. Exp.
Biol. Med., 107: 908-911.
DUBOIS, K.P., DOULL, J., SALERNO, P.R., & COON, J.M. (1949) Studies on
the toxicity and mechanisms of action of p-nitro-phenyl diethyl-
thionosphosphate (parathion). J. Pharmacol. exp. Ther., 95: 79-91.
DUBOWITZ, V. & BROOKE, M.H. (1973) Muscle biopsy: a modern approach,
London, W.B. Saunders.
DUNLOP, D.S., VAN ELDEN, W., & LAJTHA, A. (1975) Optimal conditions
for protein synthesis in incubated slices of rat brain. Brain Res.,
99: 303-318.
DUNN, A.J. (1975) Intercerebral injections inhibit amino acid
incorporation into brain protein. Brain Res., 99: 405-409.
DUNN, A.J. (1977) Measurements of the rate of brain protein synthesis.
In: Roberts, S., Lajtha, A., & Gispen, W.H., ed. Mechanisms,
regulation, and special function of protein synthesis in the brain,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 97-105.
DURHAM, W.F., CUETO, C., Jr, & HAYES, W.J., Jr (1956) Hormonal
influence on DDT metabolism in the white rat. Am. J. Physiol.,
187: 373-377.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
DYER, R.S. (1980) Effects of prenatal and postnatal exposure to carbon
monoxide on visually-evoked responses in rats. In: Weiss, B. &
Merigan, W., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 17-33.
DYER, R.S. (1985a) The use of sensory-evoked potentials in toxicology.
Fundam. appl. Toxicol., 5: 24-40.
DYER, R.S. (1985b) Neurophysiological measures following exposure to
toxic substances. In: Annau, Z., ed. Behavioral toxicology,
Baltimore, Maryland, Johns Hopkins.
DYER, R.S. & BOYES, W.K. (1983) Use of neurophysiological challenges
for the detection of neurotoxicity. Fed. Proc., 42: 3201-3206.
DYER, R.S., ECCLES, C.U., & ANNAU, Z. (1978) Evoked potential
alterations following prenatal methylmercury exposure. Pharmacol.
Biochem. Behav., 8: 137-141.
DYER, R.S., SWARTZWELDER, H.S., ECCLES, C.W., & ANNAU, Z. (1979)
Hippocampal after discharges and their post-ictal sequelae in rats: a
potential tool for assessment of CNS neurotoxicity. Neurobehav.
Toxicol., 1: 5-19.
ECKERMAN, D.A., GORDON, W.A., EDWARDS, J.D., MACPHAIL, R.C., & GAGE,
M.I. (1980) Effects of scopolamine, pentabarbital, and amphetamine on
radial arm maze performance in the rat. Pharmacol. Biochem. Behav.,
12: 595-602.
EDWARDS, P.M. & PARKER, V.H. (1977) A simple, sensitive, and objective
method for early assessment of acrylamide neuropathy in rats.
Toxicol. appl. Pharmacol., 40: 589-591.
EGAN, G.F., LEWIS, S.C., & SCALA, R.A. (1980) Experimental design for
animal toxicity studies. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams & Wilkins Company, pp. 708-725.
EICHELMAN, B., HEGSTRAND, L.R., MCMURRAY, T., & KANTAX, K.M. (1981)
Cyclic nucleotides and aggressive behaviour. Psychopharmacology of
aggression and social behaviour. Pharmacol. Biochem. Behav.,
14(Suppl. 1): 7-12.
ELSNER, J., LOOSER, R., & ZBINDEN, G. (1979) Quantitative analysis of
rat behaviour patterns in a residential maze. Neurobehav. Toxicol.,
1(Suppl. 1): 163-174.
EMSON, P.C. (1983) Chemical neuroanatomy, New York, Raven Press.
ENJALBERT, A., RUBERG, M., & KORDON, C. (1978) Neuroendocrine control
of prolactin secretion. In: Robyn, C. & Harter, M., ed. Progress in
prolactin physiology and pathology, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 83-94.
EROSCHENKO, V.P. & PALMITER, R.D. (1980) Estrogenicity of kepone in
birds and mammals. In: McLachlan, J., ed. Estrogens in the
environment, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 305-325.
EROSCHENKO, V.O. & WILSON, W.O. (1975) Cellular changes in the gonads,
liver, and adrenal glands of Japanese quail as affected by the
insecticide kepone. Toxicol. appl. Pharmacol., 31: 491-504.
EVANS, H.L. (1978) Behavioral assessment of visual toxicity. Environ.
Health Perspect., 26: 53-58.
EVANS, H.L. & WEISS, B. (1978) Behavioral toxicology. In: Blackman,
D.E. & Sanger, D.J., ed. Contemporary research in behavioral
pharmacology, New York, Plenum Press, pp. 449-487.
EVANS, H.L., LATIES, V.G., & WEISS, B. (1975) Behavioral effects of
mercury and methylmercury. Fed. Proc., 34: 1858-1867.
EVANS, H.L., GARMAN, R.H., & WEISS, B. (1977) Methylmercury: exposure
duration and regional distribution as determinants of neurotoxicity in
non-human primates. Toxicol. appl. Pharmacol., 41: 15-33.
EVANS, W.O. (1962) A comparison of the analgetic potency of some
analgesics as measured by the "flinch-jump" procedure.
Psychopharmacologia, 3: 51-54.
FADEEV, JU.A. (1980) [Unit activity of the brain cortex in realization
of goal-directed behaviour.] Usp. fiziol. nauk., 11(3): 12-46 (in
Russian).
FADEEV, JU.A. & ANDRIANOV, V.V. (1971) Methods of registration of
neuronal activity in freely-moving animals. Physiol. J. (USSR),
57(8): 1217-1219.
FALCK, B., HILLARP, N.A., THIEME, G., & TORP, A. (1962) Fluorescence
of catecholamines and related compounds condensed with formaldehyde.
J. Histochem. Cytochem., 10: 348-354.
FALK, J.L. (1970) Readings in behavioral pharmacology, New York,
Appleton-Century-Crofts.
FELLEGIOVA, M., ADAMEC, O., & DAHKOVA, A. (1977) [The effect of
lindane on the metabolism of testosterone in the rat.] Cs. Hyg.,
22: 115-120 (in Slovak).
FERSTER, C.B. & SKINNER, B.F. (1957) Schedules of reinforcement, New
York, Appleton-Century-Crofts.
FESTING, M.F. (1979) Properties of inbred strains and outbred stocks,
with special reference to toxicity testing. J. environ. Health
Toxicol., 5: 53-68.
FILE, S. (1980) The use of social interaction as a method for
detecting anxiolytic activity of chlordiarepoxide-like drugs.
J. Neurosci. Methods, 2: 219-238.
FINK, G. (1979) Feedback actions of target hormones on hypothalamus
and pituitary with special reference to gonadal steroids. Ann. Rev.
Physiol., 41: 571-585.
FISHMAN, J. (1982) Catechol estrogens and pituitary function. In:
Muldoon, T.G., Mahesh, V.B., & Perez-Ballester, B., ed. Recent
advances in fertility research. Part A. Developments in reproductive
endocrinology, New York, Alan R. Lyss, pp. 183-189.
FISHMAN, W.H. & BERNFELD, P. (1955) Glucuronidases. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 262.
FITZGERALD, G.J., KAUFMAN, E.E., SOKOLOFF, L., & SHEIN, H.M. (1974)
D(-)-beta-hydroxybutyrote dehydrogenase activity in closed cell lines
of glial and neuronal origin. J. Neurochem., 22: 1163-1166.
FODOR, G.G., SCHLIPKOTER, H.W., & ZIMMERMAN, M. (1973) The objective
study of sleeping behaviour in animals as a test of behaviour
toxicity. In: Horvath, M. & Frantik, M., ed. Adverse effects of
environmental chemicals and psychotropic drugs, Amsterdam, London,
New York, Elsevier Science Publishers, Vol. 1, pp. 115-122.
FOLCH, J., ASCOLI, I., LEES, M., MEATH, J.A., & LEBARON, F.N. (1951)
Preparation of lipid extracts from brain tissue. J. biol. Chem.,
191: 833-841.
FOWLER, S.C. & PRICE, A.W. (1978) Some effects of chlordiaz-epoxide
and delta-amphetamine on response force during punished responding in
rats. Psychopharmacology, 56: 211-215.
FOX, D.A., LOWNDES, H.E., & BIERKAMPER, G.G. (1982) Electro-
physiological techniques in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 299-335.
FOX, W.M. (1965) Reflex-ontogeny and behavioral development of the
mouse. Anim. Behav., 13: 234-241.
FRANKOVA, S. (1970) Behavioral responses of rats to early
overnutrition. Nutr. Metab., 12: 228-230.
FRANKOVA, S. (1971) Relationship between nutrition during lactation
and maternal behavior of rats. Act. Nerv. Super. (Praha), 13: 1-8.
FRANKOVA, S. (1977) Drug-induced changes in the maternal behavior of
rats. Psychopharmacology, 3: 83-87.
FRANTIK, E. (1970) The development of motor disturbances in
experimental chronic carbon disulfide intoxication. Med. Lav.,
61: 309-313.
FRANTIK, E. & BENES, V. (1984) Central nervous effect and blood level
regressions on exposure time paralleled in solvents (toluene, carbon
tetrachloride, and chloroform). Act. Nerv. super. (Praha), 26: 131.
FUJIMORI, K., BENET, H., MEHENDALE, H.M., & HO, I.K. (1982) Comparison
of brain discrete area distribution of chlordecone and mirex in the
mouse. Neurotoxicology, 3: 125-130.
FULLERTON, P.M. (1966) Chronic peripheral neuropathy produced by lead
poisoning in guinea-pigs. J. Neuropathol. exp. Neurol., 25: 214-236.
GABE, M. (1976) Histological techniques, New York, Paris,
Springer-Verlag, Masson.
GAD, S.C. (1982) Statistical analysis of behavioral toxicology data
and studies. Arch. Toxicol., Suppl. 5: 256-266.
GAD, S.C. & WEIL, C.S. (1982) Statistics for toxicologists. In: Hayes,
A.W., ed. Principles and methods of toxicology, New York, Raven
Press, pp. 270-320.
GALLARD, G.V., PETRO, Z., & RYAN, K.J. (1978) Phylogenetic
distribution of aromatase and other androgen-converting enzymes in the
central nervous system. Endocrinology, 10: 2283-2290.
GELLER, I., MENDEZ, V., HAMILTON, M., HARTMANN, R.J., & GAUSE, E.
(1979) Effects of carbon monoxide on operant behavior of laboratory
rats and baboons. Neurobehav. Toxicol., 1(Suppl. 1): 179-184.
GELLERT, R.J., HEINRICHS, W.L., & SWERDLOFF, R.S. (1972) DDT
homologues: estrogen-like effects on the vagina, uterus, and pituitary
of the rat. Endocrinology, 91: 1095-1100.
GERHART, J.M., HONG. J.S., UPHOUSE, L.L., & TILSON, H.A. (1982)
Chlordecone-induced tremor: quantification and pharmacological
analysis. Toxicol. appl. Pharmacol., 66(2): 234-243.
GILLIATT, R.W. (1973) Recent advances in the pathophysiology of nerve
conduction. In: Desmedt, J.E., ed. New developments in
electromyography and clinical neurophysiology, Basel, Karger,
Vol. 2, pp. 2-18.
GLATT, A.F., TALAAT, H.N., & KOELLA, W.P. (1979) Testing of peripheral
nerve function in chronic experiments with rats. Pharmacol. Ther.,
5: 539-543.
GLAUSERT, A.M. (1980) Fixation, dehydration, and embedding of
biological specimens, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
GLOWINSKI, J. & IVERSEN, L.L. (1966) Regional studies of
catecholamines in the rat brain. I. The disposition of 3H-norephine-
phrine, 3H-dopamine, and 3H-DOPA in various regions of the brain. J.
Neurochem., 13: 655-699.
GOLDSTEIN, B.D., LOWNDES, H.E., & CHO, E.S. (1981) Neurotoxicology of
vincristine in the cat: electrophysiological studies. Arch.
Toxicol., 48: 253-264.
GONCHARUK, V.D. (1981) Alteration of activity of glutamate
dehydrogenase in ganglionated neurons of rabbits under acute
experimental emotional stress. Bull. exp. Biol. Med., 10: 494-496.
GOOD, P. (1979) Detection of a treatment effect when not all
experimental subjects will respond to treatment. Biometrics,
35: 483-489
GORIDIS, C., MASSARELLI, R., SERSENBRENNER, M., & MANDEL, P. (1974)
Guanyl cyclase in chick embryo brain cell cultures: evidence of
neuronal localization. J. Neurochem., 23: 135-139.
GOYER, R.A. & RHYNE, B.C. (1973) Pathological effects of lead. Int.
Rev. exp. Pathol., 12: 1-7.
GRANT, L.D. (1976) Research strategies for behavioral toxicology
studies. Environ. Health Perspect., 18: 85-94.
GRAY, E.G. & WHITTAKER, V.P. (1962) The isolation of nerve endings
from brain: an electron microscopic study of the cell fragments of
homogenation and centrifugation. J. Anat., 96: 79-87.
GREENBLATT, D.J., SHADER, R.I., FRANKE, K.K., MACLAUGHLIN, E.D.,
RONSIL, G.J., & KOCH-WESER, J. (1977) Kinetics of intravenous
chlordiazepoxide: sex differences in drug metabolism. Clin.
Pharmacol. Ther., 22: 893-903.
GREENGARD, P. (1979) Cyclic nucleotides, phosphorylated proteins and
the neuronal system. Fed. Proc., 38: 2208-2217.
GREGORY, E.H. & PFAFF, D.W. (1971) Development of olfactory-guided
behavior in infant rats. Physiol. Behav., 6: 573-576.
GRIFFIN, J.W. & PRICE, D.L. (1980) Proximal axonopathies induced by
toxic chemicals. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 161-178.
GRIFFIN, J.W., PRICE, D.L., KLUTHE, D.O., & GOLDBERG, M.A. (1980)
Neurotoxicity of misonidazole in rats. Neuropathol. appl.
Neurobiol., 6: 656-666.
GROSS-SELBECK, E. & GROSS-SELBECK, M. (1981) Changes in operant
behavior of rats exposed to lead at the accepted no-effect level.
Clin. Toxicol., 18: 1247-1256.
GROTA, L.S. & ADER, R. (1969) Continuous recording of maternal
behavior in the rat. Anim. Behav., 17: 72-729.
GUDELSKY, G.A., NANSEL, D.D., & PORTER, J.C. (1981) Role of estrogen
in the dopaminergic control of prolactin secretion. Endocrinology,
108: 440-444.
HAIDER, S.S., HANSAN, M., & ISLAM, F. (1980) Effect of air pollutant
hydrogen sulfide on the levels of total lipids, phospholipids, and
cholesterol in different regions of the guinea-pig brain. Ind. J.
exp. Biol., 18: 418-420.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., & MCCONNEL, J.
(1979) Estrogenic activity of the insecticide chlordecone (kepone) and
interaction with uterine estrogen receptors. Proc. Natl Acad. Sci.
(USA), 76: 6641-6645.
HARPUR, E.S. & D'ARCY, P.F. (1975) The quantification of kanamycin
ototoxicity in the rat using conditioned tone discrimination.
J. Pharm. Pharmacol., 27: 907-913.
HARRY, G.J. & TILSON, H.A. (1982) Postpartum exposure to triethyltin
produces long-term alterations in responsiveness to apomorphine.
Neurotoxicology, 3: 64-71.
HASAN, M. & ALI, S.F. (1981) Effects of thallium, nickel, and cobalt
administration on the lipid peroxidation in different regions of the
rat brain. Toxicol. appl. Pharmacol., 57: 8-13.
HAY, A. (1978) Neurotoxins may go unrecognized. Nature (Lond.),
274: 206.
HAYAT, M.A. (1973) Principles and techniques of electron microscopy,
New York, Van Rostrand Reinhold Company, Vols. 1 and 2.
HEDLUND, B., GAMARA, M., & BARTFAI, I. (1979) Inhibition of striatal
muscarinic receptors in vivo by cadium. Brain Res., 168: 216-218.
HEISE, G.A. (1983) Toward a behavioural toxicology of learning and
memory. In: Zbinden, G., Cuomo, V., Racagni, G., & Weiss, B., ed.
Application of behaviour pharmacology and toxicology, New York,
Raven Press, pp. 27-38.
HEISE, G.A. & MILAR, C. (1984) Drugs and stimulus control. In:
Iverson, S.D. & Snyder, S.H., ed. Handbook of psychopharmacology,
New York, Plenum Press, pp. 129-190.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930.
HERMAN, S.P., KLEIN, R., TALLEY, F.A., & KRIGMAN, M. (1973) An
ultrastructural study of methylmercury-induced primary sensory
neuropathy in the rat. Lab. Inv., 28: 104-118.
HERTZ, L. (1981) Gamma amino butyric-acid glutamate pathway in glia.
5th European Neuroscience Congress, Liege, Belgium, 14-18 September,
1981. Neurosci. Lett, Suppl. 7: 172.
HETZLER, B.E., HEILBRONNER, R.L., GRIFFIN, J., & GRIFFIN, G. (1981)
Acute effects of alcohol on evoked potentials in visual cortex and
superior colliculus of the rat. Electroencephalogr. clin.
Neurophysiol., 51 69-79.
HILLE, B. (1976) Gating in sodium channels of nerve. Ann. Rev.
Physiol., 38 139-152.
HINDE, R. (1974) Biological basis of human social behaviour, New
York, McGraw Hill, Inc.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930
HOFFMAN, P.N. & LASEK, R.J. (1975) The slow component of axonal
transport. Identification of major structural polypeptides of the axon
and their generality among mammalian neurons. J. cell Biol.,
66: 351.
HOLCK, H.G.O., MUNIR, A.K., MILLS, L.M., & SMITH, E.L. (1937) Studies
upon the sex differences in rats in tolerance to certain barbiturates
and to nicotine. J. Pharmacol. exp. Ther., 60: 323-346.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HONG, J.S. & ALI, S.F. (1982) Chlordecone (kepone) exposure in the
neonate selectively alters brain and pituitary endorphin levels in
prepuberal and adult rats. Neurotoxicology, 3: 111-118.
HOPF, H.C. & EYSHOLDT, M. (1978) Impaired refractory periods of
peripheral sensory nerves in multiple sclerosis. Ann. Neurol.,
4: 499-501.
HOROWSKI, D. & GRAF, K.J. (1979) Neuroendocrine effects of
neuropsychotropic drugs and their possible influence on toxic
reactions in animals and man: the role of dopamine-prolactin system.
Arch. Toxicol. Suppl. 2: 93-104.
HORVATH, M., ed. (1973) Adverse effects of environmental chemicals
and psychotropic drugs. Quantitative interpretation of functional
tests, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 1, 292 pp.
HORVATH, M. & FRANTIK, E. (1973) Quantitative interpretation of
experimental toxicological data: the use of reference substances. In:
Horvath, M., ed. Adverse effects of environmental chemicals and
psychotropic drugs, Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Co., Vol. 1, pp. 11-21.
HORVATH, M. & FRANTIK, M., ed. (1976) Adverse effects of
environmental chemicals and psychotropic drugs: neurophysiological
and behavioural tests, Amsterdam, New York, Oxford, Elsevier,
Vol. 2, 334 pp.
HORVATH, M. & FRANTIK, E. (1979) Industrial chemicals and drugs
lowering central nervous systems activation level: quantitative
assessment of hazard in man and animals. Act. Nerv. super. (Praha),
21: 269-272.
HORVATH, M. & MICHALOVA, C. (1956) Changes of EEG in experimental
exposure to carbon disulfide in the light of Vvedenskys theory of
parabiosis. Arch. Gewerbepathol., 15: 131-136.
HORVATH, M., FRANTIK, E., & KREKULE, P. (1981) Diazepam impairs
alertness and potentiates a similar effect of toluene. Act. Nerv.
super. (Praha), 22: 17-19.
HOWLAND, R.D., VYAS, I.L., LOWNDES, H.E., & ARGENTIERI, T.M. (1980)
The etiology of toxic peripheral neuropathies; in vitro effects of
acrylamide and 2,5-hexanedione on brain enolase and other glycolytic
enzymes. Brain Res., 202: 131-142.
HUGHES, J.A. & SPARBER, S.B. (1978) Delta-amphetamine unmasks
postnatal consequences of exposure to methylmercury in utero:
methods for studying behavioral teratogenesis. Pharmacol. Biochem.
Behav., 8: 365-375.
HUNT, V.R., SMITH, M.K., & WORK, D., ed. (1982) Banbury Report II -
Environmental factors in human growth and development, New York,
Cold Spring Harbor Laboratory.
HUNTER, D. & RUSSELL, D.S. (1954) Focal cerebral and cerebellar
atrophy in human subjects due to organic mercury compounds.
J. Neurol. Neurosurg. Psychiatr., 17: 235-241.
HUNTER, D., BORNFORD, R., & RUSSELL, D. (1940) Poisoning by
methylmercury compounds. Q. S. Med., 9: 193-213.
HUTCHINGS, D.E. (1978) Behavioral teratology: embryopathic and
behavioural effects of drugs during pregnancy. In: Gottlieb, G., ed.
Studies on the development of behaviour and nervous system: early
influences, New York, Academic Press, pp. 7-34.
IRWIN, S. (1968) Comprehensive observational assessment: a systematic
quantitative procedure for assessing the behavioral and psychologic
state of the mouse. Psychopharmacologia, 13: 222-257.
ISLAM, F., TAYYABA, K., & HASAN, M. (1983) Organophosphate metasystox-
induced increment of lipase activity and lipid peroxidation in
cerebral hemisphere: diminution of lipids in discrete areas of the
brain. Acta pharmacol. toxicol., 53: 121-124.
IVERSEN, L.L. (1971) Role of transmitter uptake mechanisms in synaptic
neurotransmission. Br. J. Pharmacol., 44: 571-591.
JACOBWITZ, D.M. (1974) Removal of discrete fresh regions of the rat
brain. Brain Res., 80: 111-115.
JOHN, T.H., GEGHMAN, C., & REIS, D.J. (1973) Immunochemical
demonstration of increased accumulation of tyrosine hydroxylase
protein in sympathetic ganglia and adrenal medulla elicited by
reserpine. Proc. Natl Acad. Sci. (USA), 70: 2767-2771.
JOHNSON, B.L. (1980) Electrophysiological methods in neurotoxicity
testing. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental and
clinical neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 726-742.
JOHNSON, B.L. & ANGER, W.K. (1983) Behavioral toxicology. In: Rom,
W.N., ed. Environmental and occupational medicine, Boston, Little
Brown and Co., pp. 329-350.
JOHNSON, B.L., ANGER, W.K., SETZIER, J.V., & XINTARAS, C. (1975) The
application of a computer-controlled time discrimination performance
to problems in behavioral toxicology. In: Weiss, B. & Laties, V.G.,
ed. Behavioral toxicology, New York, London, Plenum Press,
pp. 129-153.
JOHNSON, E.W. (1980) Practical electromyography, Baltimore,
Maryland, Williams and Wilkins Company.
JOHNSON, J.E., Jr (1981) Aging and cell structure, New York, Plenum
Press, Vol. 1.
JOY, R.M. (1982) Chlorinated hydrocarbon insecticides. In: Ecobichon,
D.J. & Joy, R.M., ed. Pesticides and neurological diseases, Boca
Raton, Florida, CRC Press, pp. 91-150.
JOY, R.M. (1985) Kindling as a tool in neurotoxicology. Fund. appl.
Pharmacol., 5: 41-65.
JOY, R.M., STARK, L.G., PETERSON, S.L., BOWYER, J.T., & ALBERTSON,
T.E. (1980) The kindled seizure; production of and modification by
dieldrin in rats. Neurobehav. Toxicol., 2: 117-124.
JUNQUEIRA, L.C., CARNEIRO, J., & CONTOPOULOS, A. (1977) Basic
Histology, 2nd ed., Los Altos, Lange.
KAPLAN, K.L. (1980) Thyroxine 5'-monodeiodination in rat pituitary
homogenates. Endocrinology, 106: 567-576.
KARPOVA, O.B., OBEHOVA, E.L., AVIOVA, N.F., & SCHVARTS, E.G. (1978)
[Content and composition of cerebral gangliosides during Down's
Syndrome.] Vopros. med. Xhim., 4: 524-527 (in Russian).
KELLEHER, R.T. & MORSE, W.H. (1968) Determinations of the specificity
of behavioral effects of drugs. Ergeb. Physiol., 60: 1-56.
KHOLODOV, Ju.A. & SOLOV'EVA, G.R. (1971) [Occasional publication:
news on medical equipment manufacture,] fasc. 3, pp. 69-72
(in Russian).
KIMURA, J. (1976) Collision techniques: physiological block of nerve
impulses in studies of motor nerve conduction velocity. Neurology,
26: 680-682.
KING, J.A. & VESTAL, B.M. (1974) Visual acuity of peromyscus.
J. Mammal., 55: 238-243.
KIRPEKAR, S.M., DIXON, W., & PRATT, J.C. (1970) Inhibitory effect of
manganese on norephinephrine release from the splenic nerves of cat.
J. Pharmacol. exp. Ther., 174: 72-76.
KLEIN, R., HERMAN, S., BRUBAKER, P.E., & LUCIER, G.W. (1972) A model
of acute methylmercury intoxication in rats. Arch. Path.,
93: 404-418.
KODAMA, J., FUKUSHIMA, M., & SAKATA, T. (1978) Impaired taste
discrimination against quinine following chronic administration of
theophylline in rats. Physiol. Behav., 20: 151-155.
KOHLER, W. (1976) The mentality of ages, New York, Liveright,
336 pp.
KONAT, G. & CLAUSEN, J. (1977) Triethyl lead-induced intoxication as
an experimental model of hypomyelination. Proc. Int. Soc.
Neurochem., 6: 215.
KORNBERG, A. (1955) Lactic dehydrogenase in muscle. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 441.
KOSTIAL, K., LANDEKA, M., & SLAT, B. (1974) Manganese ions and
synaptic transmission in the superior cervical ganglion of the cat.
Br. J. Pharmacol., 51: 231-235.
KOTLIAREVSKI, L.I. (1951) [Methodology for studying motor conditioned
reflexes in some small animals (white rats and guinea-pigs.]
Zh. Vyssh. nerv. deyat. imeni, 1(5): 753-761 (in Russian).
KOTLIAREVSKI, L.I. (1957) [Procedure for the investigation of motor-
food conditioned reflexes in small animals.] Tr. Inst. Vyssh. nerv.
deyat. AN SSR, 3: 23-28 (in Russian).
KRIGMAN, M.R. & HOGAN, E.L. (1974) Effect of lead intoxication on
the postnatal growth of the rat nervous system. Environ. Health
Perspect., 7: 187-199.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., SUTER, Y., THOMANN, G., &
HESS, R. (1981) Pyridoxine megavitaminosis produces degeneration of
peripheral sensory neurons (sensory neuronopathy) in the dog.
Neurotoxicology, 2: 13-24.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., THOMANA, P., & HESS, R.
(1979) Clinoquinol and 2,5-hexanedione induce different types of
distal axonopathy in the dog. Acta neuropathol., 47: 213.
KRSIAK, M. (1979) Effects of drugs on behavior of aggressive mice.
Br. J. Pharmacol., 65: 525-533.
KRUSIUS, T. & FINNE, J. (1977) Structural features of tissue
glycoproteins. Fractionation and methylation analysis of gylcopeptides
derived from rat brain, kidney, and liver. Eur. J. Biochem.,
78: 369-379.
KRYZHANOVSKY, G.N. (1973) Mechanism of action of tetanus toxin. Effect
on synaptic processes and some particular features of toxin binding by
the nervous tissue. Jaunyn-Schmiedeberg's Arch., 276: 247-270.
KUCZENSKI, R.T. & MANDELL, A.J. (1972) Regulatory properties of
soluble and particulate rat brain tyrosine hydroxylase. J. biol.
Chem., 247: 3114-3116.
KULKOSKY, P.J., ZELLNER, D.A., HYSON, R.L., & RILEY, A.L. (1980)
Ethanol consumption of rats in individual, group, and colonial housing
conditions. Physiol. Psychol., 8: 56-60.
KUPFER, D. & BULGER, W.H. (1976) Studies on the mechanism of
estrogenic actions of o-p'-DDT; interactions with the estrogen
receptor. Pestic. Biochem. Physiol., 6: 561-570.
KUPFER, D. & BULGER, W.H. (1980) Estrogenic properties of DDT and its
analogues. In: McLachlan, J., ed. Estrogens in the environment,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 239-263.
LAFFERMAN, J.A. & SILBERGELD, E.K. (1979) Erythrosin B inhibition of
neurotransmitter accumulation by rat brain homogenate. Science,
206: 363-365.
LAJTHA, A. & MARKS, N. (1971) Protein turnover. In: Lajtha, A., ed.
Handbook Neurochemistry, New York, Plenum Press, Vol. 5, pp. 551-629
LAMARTINIERE, C.A., DIERINGER, C.S., & LUCIER, G.W. (1979) Altered
ontogeny of glutathione S-transferases by 2,4,5-2',4',5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 51: 233-238.
LANDOWNE, D. & RITCHIE, J.M. (1976) On the control of glycogenolysis
in mammalian nervous tissue by calcium. J. Physiol., 212: 503-517.
LATIES, V.G. (1982) Contributions of operant conditioning to
behavioral toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 67-80.
LAZAROVA, M., BENDOTTI, C., & SAMANIN, R. (1984) Evidence that the
dorsal raphe area is involved in the effect of clonidine against
pentylenetetrazol-induced seizures in rats. Naunyn-Schmiedebergs
Arch. Pharmacol., 325: 12-16.
LEANDER, J.D. & MACPHAIL, R.C. (1980) Effect of chlordimeform
(a formamidine pesticide) on schedule-controlled responding of
pigeons. Neurobehav. Toxicol., 2: 315-321.
LEANDER, J.D., MCMILLAN, D.E., & BARLOW, T.S. (1977) Chronic mercuric
chloride: behavioural effects in pigeons. Environ. Res.,
14: 424-435.
LEHMANN, H.J. & TACKMANN, E. (1974) Neurographic analysis of trains of
frequent electrical stimuli in the diagnosis of peripheral nerve
disease. Eur. Neurol., 12: 293-308.
LEHRER, G.M. (1974) Measurement of minimal brain dysfunction. In:
Xintaras, C., Johnson, B.L., & DeGroot, I., ed. Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, pp. 450-454 (US DREW (NIOSH) Publication No. 74-126).
LEONARD, J.L. & ROSENBERG, L.H. (1980) Characterization of essential
enzyme sulfhydryl groups of thryoxine 5'deiodinase from rat kidney.
Endocrinology, 108: 444-451.
LEVINE, T.E. (1976) Effects of carbon disulfide and FLA-63 on operant
behavior in pigeons. J. Pharmacol. exp. Ther., 199: 669-678.
LEWEY, F.H. (1941) Experimental chronic carbon disulfide poisoning in
dogs. J. ind. Hyg. Toxicol., 23: 415-535.
LILIENTHAL, H., WINNEKE, G., BROCKHAUS, A., MOLIK, B., & SCHLIPKOTER,
H.-W. (1983) Learning set-formation in rhesus monkeys pre- and
postnatally exposed to lead. In: Proceedings of the International
Conference on Heavy metals in the Environment, Heidelberg, September,
1983, Edinburgh, CEP Consultants, p. 901.
LINDSLEY, D.B. & WICKE, J.D. (1974) The electroencephalogram:
autonomous electrical activity in man and animals. In: Thompson, R. &
Patterson, M., ed. Bioelectric recording techniques, Part B, New
York, Academic Press.
LIPP, J.A. (1968) Cerebral electrical activity following soman
administration. Arch. int. Pharmacodyn., 175: 161-169.
LOCK, E.A. (1976) Increase in cerebral fluids in rats after treatment
with hexachlorophene or triethyltin. Biochem. Pharmacol.,
25: 1455-1458.
LORENZO, A.V. & GERWITZ, M. (1977) Inhibition of 14C-tryptophane
transport into brain of lead-exposed neonatal rabbits. Brain Res.,
132: 386-392.
LOWITZSCH, K., GOHRING, U., HECKING, E., & KOHLER, H. (1981)
Refractory period, sensory conduction velocity, and visual-evoked
potentials before and after haemodialysis. J. Neurol. Neurosurg.
Psychiatr., 44: 121-128.
LOWNDES, H.E. & BAKER, T. (1976) Studies on drug-induced neuropathies.
III. Motor nerve deficit in cats with acrylamide neuropathy. Eur. J.
Pharmacol., 35: 177-184.
LOWNDES, H.E., BAKER, T., CHO, E.S., & JORTNER, B.S. (1978a) Position
sensitivity of deafferented muscle spindles in experimental acrylamide
neuropathy. J. Pharmacol. exp. Ther., 205: 40-48.
LOWNDES, H.E., BAKER, T., MICHAELSON, L.P., & VINCENT-ABLAZEY, M.
(1978b) Attenuated dynamic responses of primary endings of muscle
spindles; a basis for depressed tendon responses in acrylamide
neuropathy. Ann. Neurol., 3: 435-437.
LUKAS, E. (1970) Stimulation electrohypography in experimental
toxicology (carbon disulfide neuropathy in rats). Med. Lav.,
61: 302-308.
MCBLAIN, W.A., LEWIN, V., & WOLFE, F.H. (1976) Differing estrogenic
activities for the enatiomers of o,p'-DDT in immature female rat.
Can. J. Physiol., 54: 629-632.
MACDONALD, J.S. & HARBISON, R.D. (1977) Methylmercury induced
encephalopathy in mice. Toxicol. appl. Pharmacol., 39: 195-205.
MCDONALD, W.I. (1963) The effects of experimental demyelination on
conduction in peripheral nerve. II. Electrophysical observations.
Brain, 86: 501-524.
MCDONOUGH, J.H., Jr, HACKLEY, B.E., Jr, CROSS, R., SAMSON, F., &
NELSON, S. (1983) Brain regional glucose use during soman-induced
seizures. Neurotoxicology, 4: 203-210.
MCKENNA, M.J. & DESTEFANO, V. (1975) A proposed mechanism of action of
carbon disulfide on carbon disulfide on dopamine-beta-hydroxylase.
Toxicol. appl. Pharmacol., 33: 137.
MCMILLAM, D.E. (1982) Effects of chronic administration of pesticides
on schedule-controlled responding by rats and pigeons. In: Chambers,
J.E. & Yarborough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 211-226.
MACPHAIL, R.C. (1982) Studies on the flavour aversions induced by
trialkyltin compounds. Neurobehav. Toxicol. Teratol., 4: 225-230.
MACPHAIL, R.C. & LEANDER, J.D. (1980) Flavour aversions induced by
chlordimeform. Neurobehav. Toxicol., 2: 363-365.
MACPHAIL, R.C., CROFTON, K.M., & REITER, L.W. (1983) Use of
environmental challenges in behavioral toxicology. Fed. Proc.,
42: 3196-3200.
MACTUTUS, C.F., UNGER, K.L., & TILSON, H.A. (1982) Neonatal
chlordecone exposure impairs early learning and memory in the rat on a
multiple measure passive avoidance task. Neurotoxicology, 3: 24-44.
MAGATA, Y. & TSUKADA, W. (1978) Bulk separation of neuronal cell
bodies and glial cells from mammalian brain and some of their
biochemical properties. Rev. Neurosci., 3: 18-27.
MAILMAN, R.B., FERRIS, R.M., VOGEL, R.A., KILTS, C.D., LIPTOM, M.A.,
TANG, F.L., SMITH, D.A., MUELLER, R.A., & BREESE, G.R. (1980)
Non-specificity of the biochemical actions of erythrosine (Red No. 3):
what relation to behavioural changes. Science, 207: 535-537.
MARES, P. & VELISEK, L. (1983) Influence of ethosuximide on metrazol-
induced seizures during ontogenesis in rats. Act. Nerv. super
(Praha), 25: 292-298.
MARSHALL, J.F. (1975) Increased orientation to sensory stimuli
following lateral hypothalamic damage in rats. Brain Res.,
86: 373-387.
MARSHALL, J.F. & TEITELBAUM, P. (1974) Further analysis of sensory
inattention following lateral hypothalamic damage in rats. J. comp.
physiol. Psychol., 86: 375-395.
MARSHALL, J.F., TURNER, D.H., & TEITELBAUM, P. (1971) Sensory neglect
produced by lateral hypothaLamic damage. Science, 174: 523-525.
MARSHALL, J.F., RICHARDSON, J.S., & TEITELBAUM, P. (1974)
Nigrostriatal bundle damage and the lateral hypothalamic syndrome.
J. comp. Physiol. Psychol., 87: 808-830.
MARSLAND, T.A., GLEES, P., & ERIKSON, L.B. (1954) Modification of
Glee's silver impregnation for paraffin sections. J. Neuropathol.
exp. Neurol., 13: 587-591.
MASTEROV, A.V. (1974) [Features of the interaction of rats in an
electromagnetic field following extinction of conditioned food
reactions.] In: [Problems of clinical and experimental medicine,]
Moscow, pp. 61-63 (in Russian).
MAURISSEN, J.P.J. (1979) Effects of toxicants on the somatosensory
system. Neurobehav. Toxicol., 1(Suppl. 1): 23-32.
MEDVEDEV, B.M. (1975) [The effect of a static electric field on
conditioned reflex activity.] Fiziol. Zh., 3: 320-325 (in Russian).
MELLO, N.K. (1975) Behavioral toxicology; a developing discipline.
Fed. Proc., 34: 1832-1834.
MENDELL, J.R., SAIDA, K., GANANSIA, M.F., JACKSON, D.B., WEISS, H.,
GARDIER, R.S., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J., MARKS,
B., & HETLAND, L. (1974) Toxic polyneuropathy produced by methyl
n-butyl ketone. Science, 185: 787-789.
MENDELSON, J.H., MELLO, N.K., & ELLINGBOE, J. (1977) Effects of acute
alcohol intake on pituitary-gonadal hormones in normal human males.
J. Pharmacol. exp. Ther., 202: 675-682.
MERIGAN, W.H. (1979) Effects of toxicants on visual systems.
Neurobehav. Toxicol., 1: 15-22.
MERIGAN, W.H. (1980) Visual fields and flicker thresholds in
methylmercury poisoned monkeys. In; Merigan, W.H. & Weiss, B., ed.
Neurotoxicity of the visual system, New York, Raven Press,
pp. 149-163.
MERKURYVA, R.V. & BUSHINSKAYA, L.I. (1982) [Metabolism of
sialo-containing glycoproteins and lysosomal enzyme activity under
neurotropic effect of chemical environmental factors.] Ukranian
Biochem. J., 53: 31-35 (in Russian).
MERKURYVA, R.V. & TSAPKOVA, N.N. (1982) Hygienic significance of a
system of biochemical criteria for the evaluation of hepatotoxic and
neurotoxic effects of some chemical factors in the environment.
J. Hyg. Epidemiol. Microbiol. Immunol., 26: 336-341.
MERKURYVA, R.V., BUSHINSKAIA, L.J., AULIKA, B.V., SHATERNIKOVA, J.S.,
PROTSENKO, S.J., KULYGINA, A.A., & EKSLER, N.D. (1978) The activity of
lyosomal and cytoplasmic enzymes under the experimental influence of
bisulfide of carbon. Vop. Med. Xhim., 2: 151-156.
MERKURYVA, R.V., KOTOV, A.V., SILOV, V.G., BUSHINSKAIA, L.J.,
MUCHAMBETOVA, L.KH., & TSAPKOVA, N.N. (1980) Experimental study of
biochemical and physiological mechanisms of cerebral activity
influenced by alcohol, Nauka, Minsk Publishing House, pp. 93-94.
MEYER, O.A., TILSON, H.A., BYRD, W.C., & RILEY, H.T. (1979) A method
for the routine assessment of fore- and hindlimb grip strength of rats
and mice. Neurobehav. Toxicol., 1: 233-236.
MICZEK, K.A. & BARRY, H. (1976) Pharmacology of sex and aggression.
In: Glick, S.D. & Goldforb, J., ed. Behavioral pharmacology,
St. Louis, Missouri, C.V. Mosby Co., pp. 176-257.
MIKISKA, A. (1960) [Determination of the susceptibility to electrical
activity of the cerebral motor cortex, and its pharmacological and
toxicological applications. I. Methodology, controlled trials and
physiological basis.] Arch. Gewerbepathol. Gewerbehyg., 18: 286-299
(in German).
MIKISKOVA, H. & MIKISKA, A. (1962) [A contribution to the application
of defensive skin stimulation in the toxicity of substances acting on
the CNS. II. Measuring stimulus threshold intensity in guinea-pigs.]
Int. Arch. Gewerbepathol. Gewerbehyg., 19: 68-75 (in German).
MIKISKOVA, H. & MIKISKA, A. (1966) Trichloroethanol in
trichloroethylene poisoning. Br. J. ind. Med., 23: 116-125.
MIKISKOVA, H. & MIKISKA, A. (1968) Some electrophysiological methods
for studying the action of narcotic agents in animals, with special
reference to industrial solvents: a review. Br. J. ind. Med.,
25: 81-105.
MILLER, D.B. (1983) Neurotoxicity of the pesticidal carbamates.
Neurobehav. Toxicol. Teratol., 4: 779-788.
MILLER, E.K. & DAWSON, R.M.C. (1972) Can mitochondria and synaptosomes
of guinea-pig brain synthesize phospholipids? Biochem. J.,
126: 805-807.
MINEGISHI, A., FUJUMORI, R., SATOH, T., KILAGAWA, H., & YANAURA, S.
(1979) Changes in serotonin turnover and the brain sensitivity to
barbituates by disulfiram treatment in rats. Res. Comm. Chem. Pathol.
Pharmacol., 24: 273-285.
MINKINS, C., CHEKUNOVA, M.P., & LEVINA, E.N. (1973) [Effect of
antimony and lead on the adrenal glands and biogenic amines.]
Gig. Tr. Prof. Zabol., 17: 21-24 (in Russian).
MITCHELL, C.L. & TILSON, H.A. (1982) Behavioral toxicology in risk
assessment: problems and research needs, Boca Raton, Florida, CRC
Press, pp. 265-274 (CRC Critical Reviews in Toxicology 10).
MITCHELL, C.L., TILSON, H.A., & CABE, P.A. (1982) Screening for
neurobehavioral toxicity: factors to consider. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 237-245.
MIYOSHI, T. & GOTO, I. (1973) Serial in vivo determinations of nerve
conduction velocity in rat tails: physiological and pathological
changes. Electroencephal. clin. Neurophysiol., 35: 125-131.
MOLINENGO, L. & ORSETTI, M. (1976) Drug action on the "grasping"
reflex and on swimming endurance: an attempt to characterize
experimentally anti-depressant drugs. Neuropharmacology,
15: 257-260.
MORALI, G., LARSSON, K., & BEYER, C. (1977) Inhibition of
testosterone-induced sexual behaviour in the castrated male rat by
aromatization blockers. Horm. Behav., 9: 203-213.
MORGANTI, J.B., LOWNE, B.A., SALVATERRA, P., & MASSARO, E.J. (1976)
Effects of open-field behaviour of mice exposed to multiple doses of
methylmercury. Gen. Pharmacol., 7(1): 41-44.
MUNRO, A.J., JACKSON, R.J., & KORNER, A. (1964) Disaggregation of
brain polysomes. Biochem. J., 92: 289-294.
MURPHY, S.D. & DUBOIS, K.P. (1958) The influence of various factors on
the enzymatic conversion of organic thiophosphates to
anticholinesterase agents. J. Pharmacol. exp. Ther., 124: 194-202.
NARAHASHI, T. (1982) Cellular and molecular mechanisms of action of
insecticides: neurophysiological approach. Neurobehav. Toxicol.
Teratol., 4: 753-758.
NARAHASHI, T. & HAAS, H.G. (1967) DDT: interaction with nerve membrane
conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & HAAS, H.G. (1968) Interaction with DDT with the
components of lobster nerve membrane conductance. J. gen. Physiol.,
51: 177-198.
NATHANSON, J.A. & BLOOM, F.E. (1975) Lead-induced inhibition of brain
adenylcyclase. Nature (Lond.), 255: 419-420.
NICHOLAS, G.S. & BARRON, D.H. (1932) The use of sodium amytal in the
production of anaesthesia in the rat. J. Pharmacol. exp. Ther., 46;
125-129.
NIEDERMEYER, E. & LOPES DA SILVA, F. (1982) Electroencephalography:
basic principles, clinical applications, and related fields,
Baltimore, Maryland, Munich, Urban Schwartzenberg.
NIOKESARYISKY, A.A. & TUROVSKY, V.S. (1980) N-acetylneuraminic acid
content in water-soluble and membranous tissue fractions of various
analysers of cerebral cortex. Biochimia, 45: 1829-1932.
NORTON, S. (1973) Amphetamine as a model for hyperactivity in the rat.
Physiol. Behav., 11: 181-186.
O'BRIEN, R.D. (1976) Acetylcholinesterase and its inhibition. In:
Wilkerson, C.F., ed. Insecticide biochemistry and physiology, New
York, Plenum Press, pp. 271-296.
O'CALLAGHAN, J.P., LAVIN, K.L., CHESS, Q., & CLOUET, D.H. (1983) A
method for dissection of discrete regions of rat brain following
microwave irradiation. Brain Res., 11: 31-42.
O'DONOGHUE, J.L., ed. (1985) Neurotoxicity of industrial and
commercial chemicals, Boca Raton, Florida, CRC Press.
OCHOA, J. (1972) Ultrathin longitudinal sections of single myelinated
fibres for electron microscopy. J. neurol. Sci., 17: 103.
OCHS, S. (1972) Fast transport of materials in mammalian nerve fibres.
Science, 176: 252.
OCHS, S. (1982) Axoplasmic transport and its relation to other nerve
functions, New York, John Wiley and Sons.
OHI, G., NISHIGAKI, S., SEKI, H., TAMURA, Y., MIZOGUCHI, I., YAGYA,
H., & NAGASHIMA, K. (1978) Tail rotation, an early neurological sign
of methylmercury poisoning in the rat. Environ. Res., 16: 353-359.
OLIVER, C., ESKAY, R.L., BEN-JONATHAN, N., & PORTER, J.C. (1974)
Distribution and concentration of TRH in rat brain. Endocrinology,
96: 540-546.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1979) Hippocampus
space, and memory. Behav. Brain Sci., 2: 313-365.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1980) Hippocampal
function: working memory or cognitive mapping? Physiol. Psychol.,
8: 239-246.
OTTO, D.A. (1977) Neurobehavioral toxicology: problems and methods in
human research. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 10/1-10/31
(EPA 600/9-77-042).
OTTO, D.A. (1978) Multidisciplinary perspectives in event-related
brain potential research. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Washington
DC, US Environmental Protection Agency (EPA 600/9-77-043).
OTTO, D.A., BENIGNUS, V.A., MULLER, K.E., & BARTON, C.N. (1981)
Effects of age and body lead burden on CNS function in young children.
I. Slow cortical potentials. Electroencephalogr. clin.
Neurophysiol., 52: 229-239.
OVERMANN, S.R. (1977) Behavioral effects of asymptomatic lead exposure
during neonatal development in rats. Toxicol. appl. Pharmacol.,
41: 459-471.
PADICH, R. & ZENICK, M. (1977) The effects of developmental and/or
direct lead exposure on FR behavior in the rat. Pharmacol. Biochem.
Behav., 6: 371-375.
PALKOVITS, M. (1973) Isolated removal of hypothalamic or other brain
nuclei of the rat. Brain Res., 59: 449-450.
PALMITER, R.D. & MULVIHILL, E.D. (1978) Estrogenic activity of the
insecticide kepone on the chick oviduct. Science, 201: 356-358.
PANCHENKO, L.F., DUDCHENKO, A.M., & SHPAKOV, A.A. (1975) The influence
of acute hypoxic hypoxia on the activity of rats cerebral cortex:
mitochondria enzymes possessing different sensibility to oxygen
deficiency. In: Gorky, ed. Biochemistry of hypoxia, pp. 59-64.
PANSKY, B. & ALLEN, D.J. (1980) Review of neuroscience, New York,
McMillan Inc.
PARIZEK, J. (1956) Effects of cadmium salts on testicular tissue.
Nature (Lond.), 177: 1036-1037.
PARTINGRON, C.R. & DALY, J.W. (1979) Effect of gangliosides on
adenylate cyclase activity in rat cerebral cortical membranes. Mol.
Pharmacol., 15: 484-491.
PARTRIDGE, W.M. (1976) Inorganic mercury: selective effects on
blood-brain barrier transport systems. J. Neurochem., 27: 333-335.
PATTON, S.E., O'CALLAGHAN, J.P., MILLER, D.B., & ABOU-DONIA, M.B.
(1983) Effect of oral adminstration of tri-o-cresyl phosphate on in
vitro phosphorylation of membrane and cytosolic proteins from
chicken brain. J. Neurochem., 41: 897-901.
PAUL, S.M., HOFFMAN, A.R., & AXELROD, A. (1980) Catechol estrogens:
synthesis and metabolism in brain and other endocrine tissues. In:
Martini, L. & Ganong, W.F., ed. Frontiers in neuroendocrinology, New
York, Raven Press, pp. 203-217.
PAVLENKO, S.M. (1975) Methods for the study of the central nervous
system in toxicological tests. In: Methods used in the USSR for
establishing biologically safe levels of toxic substances, Geneva,
World Health Organization, pp. 86-108.
PAVLOV, I.P (1927) Conditioned reflexes, Oxford, Oxford University
Press.
PEARSE, A.G.E. (1968) Histochemistry: theoretical and applied, 3rd
ed., Baltimore, Maryland, Williams and Wilkins Company.
PEARSON, C.M. & MOSTOFI, F.K. (1973) The striated muscle, Baltimore,
Maryland, Williams and Wilkins Company.
PEASE, D.E. (1964) Histological techniques for electron microscopy,
2nd ed., New York, Academic Press.
PERSINGER, M.A., LAFREINIERE, G.P., & CARREY, N.V. (1978) Thyroid
morphology and wet organ weight changes in rats exposed to different
low intensity 0.5-Hz magnetic fields and pre-experimental caging
conditions. Biometeorology, 22: 67-73.
PILCHER, C.W.T. & JONES, S.M. (1981) Social crowding enhances
aversiveness of naloxone in rats. Pharmacol. Biochem. Behav.,
14: 299-303.
PIRCH, J.H. & RECH, R.H. (1968) Effect of isolation on alpha-
methyltyrosine-induced behavioral depression. Life Sci., 7: 173-182.
PLEASURE, D.E., MISHLER, K.C., & ENGEL, W.K. (1969) Axonal transport
of proteins in experimental neuropathies. Science, 166: 524-525.
POST, E.M., YANG, M.G., KING, J.A., & SANGER, V.L. (1973) Behavioral
changes of young rats force-fed methylmercury chloride. Proc. Soc.
Exp. Biol. Med., 143: 1113-1116.
POWERS, J.M., SCHAUMBURG, H.H., JOHNSON, A.E., & RAINE, C.S. (1980) A
correlative study of the adrenal cortex in adrenoleukodystrophy:
evidence for a fatal intoxication with very long-chain saturated fatty
acids. Invest. cell Pathol., 3: 353-376.
PRABHU, V.G. (1972) Amphetamine aggregation effect in mice under
conditions of altered microsomal enzymes. Arch. int. Pharmacodyn.
Ther., 195: 81-86.
PROKHOROVA, M.J. (1974) Structure and function of phosphornositides
and gangliosides of the brain. In: Progress of neurochemistry,
Leningrad, Nauka, pp. 61-73.
PRYOR, G.T., UYENO, E.T., TILSON, H.A., & MITCHELL, C.L. (1983)
Assessment of chemicals using a battery of neurobehavioral tests: a
comparative study. Neurobehav. Toxicol. Teratol., 5: 91-117.
PURPURA, D.P., PENRY, J.K, TOWER, P., WOODBURY, D.M., & WALTER, R.
(1972) Experimental models of epilepsy, New York, Raven Press.
QUEVEDO, L., CONCHA, J., MARCHANT, Z., ESKUCHE, W., & MADARIAGA, J.
(1980) Nerve accommodation in chronic alcoholic subjects.
Pharmacology, 21: 229-232.
QUINN, G.P., AXELROD, J., & BRODIE, B.B. (1958) Species, strain, and
sex differences in metabolism of hexobarbitone, amidopyrine,
antipyrine, and aniline. Biochem. Pharmacol., 1: 152-159.
RADIN, N.S., BRENHERT, A., ARORA, R.C., SELLINGER, O.Z., & FLANGAS,
A.L. (1972) Glial and neuronal localization of cerebroside-
metabolizing enzymes. Brain Res., 39: 163-166.
RAFALES, L.S., BORNSCHEIN, R.L., MICHAELSON, A., LOCH, R.K., & BARKER,
G.F. (1979) Drug-induced activity in lead-exposed mice. Pharmacol.
Biochem. Behav., 10: 95-104.
RAMSAY, R.B., KRIGMAN, M.R., & MORELL, P. (1980) Developmental studies
of the uptake of choline, GABA, and dopamine by crude synaptosomal
preparations after in vivo or in vitro lead treatment. Brain
Res., 187: 383-387.
RAY, O.S. & BARRETT, R.J. (1975) Behavioral, pharmacological, and
biochemical analysis of genetic differences in rats. Behav. Biol.,
15: 391-417.
REICHARDT, L.F. & KELLY, R.B. (1983) A molecular description of nerve
terminal function. Ann. Rev. Biochem., 52: 871-926.
REICHERT, B.L. & ABOU-DONIA, M.B. (1980) Inhibition of fast axoplasmic
transport by delayed neurotoxic organophosphorus esters: a possible
mode of action. Mol. Pharmacol., 17: 56-60.
REITER, L.W. (1978) Use of activity measures in behavioral toxicology.
Environ. Health Perspect., 26: 9-20.
REITER, L.W. (1980) Neurotoxicology -- meet the real world.
Neurobehav. Toxicol., 2: 73-74.
REITER, L.W. (1983) Chemical exposure and animal activity: utility of
the Figure-Eight Maze. In: Hayes, A.H., Schnell, R.C., & Miya, T.S.,
ed. Developments in the science and practice of toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 78-84.
REITER, L.W. & MACPHAIL, R.C. (1979) Motor activity: a survey of
methods with potential use in toxicity testing. Neurobehav.
Toxicol., 1(Suppl. 1): 53-66.
REITER, L.W. & MACPHAIL, R.C. (1982) Factors influencing motor
activity measurements in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 45-65.
REITER, L.W., KIDD, K., HEAVNER, G., & RUPPERT, P. (1980) Behavioral
toxicity of acute and subacute exposure to triethyltin in the rat.
Neurotoxicology, 2: 97-112.
REUHL, K. & CHANG, L. (1979) Effects of methylmercury on the
development of the nervous system: a review. Neurotoxicology,
1: 21-55.
REYNOLDS, G.S. (1958) A primer of operant conditioning, Glenview,
Illinois, Scott, Foresman and Co.
RICE, D.C. & GILBERT, S.G. (1982) Early chronic low-level
methylmercury poisoning in monkeys impairs spatial vision. Science,
216: 759-761.
RICE, D.C. & WILLES, R.F. (1979) Neonatal low-level lead exposure in
monkeys (Macaca fascicularis): effects on two choice non-spatial
form discrimination. J. environ. Pathol. Toxicol., 2: 1195-1203.
RICE, D.C., GILBERT, S.G., & WILLES, R.F. (1979) Neonatal low-level
lead exposure in monkeys: locomotor activity, schedule-control
behavior, and the affects of amphetamine. Toxicol. appl. Pharmacol.,
51: 503-513.
RICE, D.F. (1985) Central nervous system effects of perinatal exposure
to lead or methylmercury in the monkey. In: Clarkson, T.W. & Nordberg,
G., ed. Reproductive developmental toxicity of metals, New York,
Plenum Press.
RITCHIE, J.M. (1979) A pharmacological approach to the structure of
sodium channels in myelinated axons. Ann. Rev. Neurosci.,
2: 341-362.
ROBERTS, D.V. (1977) A longitudinal electromyographic study of six men
occupationally exposed to organophosphorus compounds. Int. Arch.
occup. environ. Health, 38: 221-229.
ROBERTS, R.K., DESMOND, P.V., WILKINSON, G.R., & SCHENKER, S. (1979)
Disposition of chlordiazepoxide; sex differences and effects of oral
contraceptives. Clin. Pharmacol. Ther., 25: 826-831.
ROBINS, E., HIRSCH, H.E., & EMMONS, S.S. (1968) Glycosidases in the
nervous system. I. Assay, some properties, and distribution of
beta-galactosidase, beta-glucoronidase, and beta-glucosidase.
J. biol. Chem., 243: 4246-4249.
RODIER, P. (1978) Behavioral teratology. In: Wilson, J.G. & Fraser,
F.C., ed. Handbook of teratology, New York, Plenum Press, Vol. 4,
pp. 397-428.
ROEDER, R.J. & RUTTER, W.J. (1969) Multiple forms of DNA-dependent RNA
polymerase in eukaryotic organisms. Nature (Lond.), 224: 234-236.
ROSE, S.P.R. & SINHA, A.K. (1970) Separation of neuronal and neuropil
cell fractions: a modified procedure. Life Sci., 9: 907-912.
ROSECRANS, J.A., HONG, J.S., SQUIBB, R.E., JOHNSON, J.H., WILSON,
W.E., & TILSON, H.A. (1982) Effects of perinatal exposure to
chlordecone (kepone) on neuroendocrine and neurochemical
responsiveness of rats to environmental challenges. Neurotoxicology,
3: 131-142.
ROSEWICKYI, S., STANOSZ, S., & SAMOCHOWIEC, L. (1973) [The effect of
protracted exposure to carbon disulfide on the hormonal activity of
the ovaries in rats.] Med. Pracy., 24: 133-136 (in Polish).
ROZIER, L., SHIRAKI, H., & GRCEVIC, N., ed. (1977) Neurotoxicology,
New York, Raven Press, Vol. 1, 658 pp.
RUDNEV, M., BOKINA, A., ESKLER, N., & NAVAKATIKYAN, M. (1978) The use
of evoked potential and behavioural measures in the assessment of
environmental insult. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency, pp. 444-447 (EPA 600/9-77-0443).
RUSSELL, R.W., WARBURTON, D.M., & SEGAL, D.S. (1969) Behavioral
tolerance during chronic changes in the cholinergic system. Comm.
Behav. Biol., 4: 121-128.
RUSAK, B. & ZUCKER, I. (1975) Biological rhythms and animal behavior.
Ann. Rev. Psychol., 27: 137-171.
SABRI, M.I. & SPENCER, P.S. (1980) Toxic distal axonopathy:
biochemical studies and hypothetical mechanisms. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 206-219.
SABRI, M.I., MOORE, C.L., & SPENCER, P.S. (1979) Studies on the
biochemical basis of distal axonopathies. Inhibition of glycolysis
produced by neurotoxic hexacarbon compounds. J. Neurochem.,
32: 683-690.
SAKMANN, B. & NEHER, E. (1983) Single channel recording, New York,
Plenum Press, 503 pp.
SALVATERRA, P., LOWN, B., MORGANTI, J., & MARSARO, E.J. (1973)
Alterations in neurochemical and behavioural parameters in the mouse
induced by low doses of methylmercury. Acta pharmacol. toxicol.,
33(3): 177-190.
SANTINI, M. (1975) Golgi Centennial Symposium: Perspectives in
Neurobiology, New York, Raven Press.
SAVOLAINEN, H. (1983) Trends and prospects in experimental
neurotoxicology. Scand. J. Work environ. Health, 9: 214-218.
SAVOLAINEN, H. & JARVISALO, J. (1977) Effects of acute CS2
intoxication on protein metabolism in rat brain. Chem.-biol.
Interact., 17: 51-59.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINO, H. (1977)
Biochemical and behavioural effects of inhalation exposure to
tetrachloroethylene and dichloromethane. J. Neuropathol. exp.
Neurol., 36: 941-949.
SCHALLERT, T., WHISHAW, I.Q., RAMIREZ, V.D., & TEITELBAUM, P. (1978)
Compulsive, abnormal walking caused by anticholinergies in akinetic, 6
hydroxydopamine-treated rats. Science, 199: 1461-1463.
SCHAUMBURG, H.H. (1982) Pyridoxine megavitaminosis produces sensory
neuropathy in humans. Ann. Neurol., 12: 107-108.
SCHAUMBURG, H.H., SPENCER, P.S., & THOMAS, P.K. (1983) Disorders of
peripheral nerves, Philadelphia, Pennsylvania, F.A. Davis.
SCHAUMBURG, H.H., WISNIEWSKI, H., & SPENCER, P.S. (1974)
Ultrastructural studies of the dying-back process. I. Peripheral nerve
terminal and axon degeneration in systemic acrylamide intoxication.
J. Neuropathol. exp. Neurol., 33: 260-284.
SCHECHTER, M.D. & WINTER, J.C. (1971) Effect of mescaline and lysergic
acid diethylamide on flicker discrimination in the rat. J. Pharmacol.
exp. Ther., 177: 461-467
SCHEUHAMMER, A.M. & CHERIAN, M.G. (1982) The regional distribution of
lead in normal rat brain. Neurotoxicology, 3: 85-92.
SCHEVING, L.E., HALBERG, F., & PAULY, J.E., ed. (1974)
Chronobiology, Tokyo, Igaku Shoin Ltd.
SCHNAITMAN, C., ERWIN, V.G., & GREENSWALT, J.W. (1967) The
submitochondrial localization of monoamine oxidase. An enzymatic
marker for the outer membrane of rat liver mitochondria. J. cell
Biol., 32: 719-720.
SCHOENFELD, W.N., ed., (1970) The theory of reinforcement schedules,
New York, Appleton-Century-Crofts.
SCHOTMAN, P., GIPON, L., JENNEKENS, F.G.I., & GISPEN, W.H. (1978)
Polyneuropathies and CNS protein metabolism. III. Changes in protein
synthesis induced by acrylamide intoxication. J. Neuropathol. exp.
Neurol., 37: 820-837.
SCHREIBER, V. & PRIBYL, T. (1980) Possible role of ceruloplasmin in
endocrine regulation. In: Ishü, S., Hirano, T., & Wade, M., ed.
Hormones, adaptation, and evolution, Tokyo, Japan Science Society
Press/Berlin, Springer-Verlag.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1982) Inhibition by an
ergoline derivative (dopaminergic agonist) of estradiol-induced
adenohypophyseal growth and of the decrease in the hypothalamic
ascorbic acid (HA) concentration in rats. Physiol. Bohemoslov.,
31: 97-100.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1979) Effect of a dopamine
p-hydroxylase inhibitor (disulfiram) on the response of
adenohypophysis, serum ceruloplasmin, and hypothalamic ascorbic acid
to estradiol treatment. Endoc. Exper. (Bratislava), 13: 131-138.
SCHREIBER, V., ZBUZEK, V., & ZBUZKOVA-KMENTOVA, V. (1969) Reactions of
the rat and hamster endocrine system to aminoglutethimide. Physiol.
Bohemoslov., 18: 249-261.
SEPPALAINEN, A.M. & LINNOILA, I. (1975) Electrophysiological studies
on rabbits in long-term exposure to carbon disulphide. Scand. J. Work
environ. Health, 1: 178-183.
SEPPALAINEN, A.M., TOLONEN, M., KARLI, P., HANNINEN, H., & HEINBERG,
S. (1972) Neurophysiological findings in chronic carbon disulfide
poisoning: a descriptive study. Work Environ. Health, 9: 71-75.
SETH, P.K., AGRAWAL, A.K., & BONDY, S.C. (1981) Biochemical changes in
the brain consequent to dietary exposure of developing and mature rats
to chlordecone (kepone). Toxicol. appl. Pharmacol., 26: 262-267.
SHANDALA, M.G., RUDNEV, M.I., & NAVAKATIKYAN, M.A. (1980) [Behavioural
reactions in experimental hygienic research.] Gig. i Sanit.,
6: 43-48 (in Russian).
SHAW, C.M., MOTTET, N.K., & CHEN, W.J. (1980) Effects of methylmercury
on the visual system of rhesus macaque (Macaca mulatta). II.
Neuropathological findings (with emphasis on vascular lesions in the
brain). In: Merigan, W.H. & Weiss, B., ed. Neurotoxicity of the
visual system, New York, Raven Press, pp. 123-134.
SHAW, C.M., MOTTET, N.K., BODY, R.L., & LUSCHEI, E.S. (1975)
Variability of neuropathological lesions in experimental methylmercury
encephalopathy in primates. Am. J. Pathol., 80: 451-470.
SHIBUSAWA, K., YAMAMOTO, T., NISHI, K., ABE, C., & TOMIE, S. (1959)
Urinary secretion of TRF in various functional states of the thyroid.
Endocrinol. Jpn., 6: 131-136.
SHILJAGINA, N.N. (1971) [Evoked potentials in the projection of sign
stimulus during conditional reflex formation in ontogenesis.]
Zh. Vyssh. nerv. deyat. imeni, 21: 492-500 (in Russian).
SHIMIZU, T., TAKAISHI, M., & SHISHIBE, Y. (1978) Effect of ethanol on
the peripheral metabolism of thyroxine. Endocrinol. Jpn,
25: 201-204.
SHUGALOV, N.P. (1969) Primary and associative evoked responses to
optic irritants during various stages of conditioned reflex formation
in cats. In: Summary of Reports of the 22nd Meeting on the Problems
of Higher Nervous Activity, Ryazan, USSR, p. 268.
SHUMILINA, A.I. (1971) Characteristics of summary electric activity
and evoked potentials under motivating stimulation of various
biological significance. In: Proceedings of the Sixth All-Union
Conference on Electrophysiology of the Central Nervous System,
pp. 301-302.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974a) Hyperactivity: a
lead-induced behavior disorder. Environ. Health Perspect.,
7: 227-232.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974b) Lead-induced behavioral
dysfunction: an animal model of hyperactivity. Exp. Neurol.,
42: 146-157.
SILBERGELD, E.K. & GOLDBERG, A.M. (1975) Pharmacological and
neurochemical investigations of lead-induced hyperactivity.
Neuropharmacology, 14: 431-444.
SILBERGELD, E.K., HRUSKA, R.E., MILLER, L.P., & ENG, N. (1980) Effects
of lead in vivo and in vitro on GABA energic neurochemistry.
J. Neurochem., 34: 1711-1718.
SILVERMAN, A.P. (1965) Ethological and statistical analysis of drug
effects on the social behaviour of laboratory rats. Br. J.
Pharmacol., 24: 579-590.
SLOANE, S.A., SHEA, S.L., PROCTER, M.M., & DEWSBURY, D. (1978) Visual
cliff performance in 10 species of muroid rodents. Anim. Learn.
Behav., 6: 244-248.
SNOWDON, C.T. (1973) Learning deficits in lead injected rats.
Pharmacol. Biochem. Behav., 1: 599-603.
SNYDER, D.R. & BRAUN, J.J. (1977) Dissociation between behavioral and
physiological indices of organomercurial ingestion. Toxicol. appl.
Pharmacol., 41: 277-284.
SNYDER, S.H. & INMS, R.B. (1979) Peptide neurotransmitters. Ann. Rev.
Biochem., 48: 755-782.
SOBOTKA, T.J., BRODIE, R.E., & COOK, M.P. (1975) Psychophysiologic
effects of early lead exposure. Toxicology, 5: 175-191.
SOKOLOFF, L., REIVICH, M., KENNEDY, C., DES ROSIERS, M.H., PATLAK,
C.S., PETTIGREW, K.D., SAKURADA, O., & SHINOHARA, M. (1977) The
14C-deoxyglucose method for the measurement of local cerebral glucose
utilization: theory, procedure, and normal values in the conscious and
anaesthetized albino rat. J. Neurochem., 28: 897-916.
SPENCER, P.S. & BISCHOFF, M.C. (1982) Contemporary neuropathological
methods in toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 259-275.
SPENCER, P.S. & LIEBERMAN, A.R. (1971) Scanning electron microscopy of
isolated peripheral nerve fibres. Normal surface structure and
alterations proximal to neuromas. Z. Zellforsch. Mikrosk. Anat.,
119: 534-551.
SPENCER, P.S. & SCHAUMBURG, H.H. (1980) Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
929 pp.
SPENCER, P.S. & THOMAS, P.K. (1970) The examination of isolated nerve
fibres by light and electron microscopy, with observations on
demyelination proximal to neuromas. Acta neuropathol., 16: 177-186.
SPENCER, P.S., BISCHOFF, M., & SCHAUMBURG, H.H. (1980)
Neuropathological methods for the detection of neurotoxic disease. In:
Spencer, P.S. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 743-757.
SPERANSKIJ, S.V. (1965) [Advantages incident to the use of mounting
current in analysing the ability of Albino mice to summate subliminal
impulses.] Farmakol. i Toksikol., 1: 123-124 (in Russian).
SPYKER, J. (1975) Behavioral teratology and toxicology. In: Weiss, B.
& Laties, V.G., ed. Behavioral toxicology, New York, Plenum Press,
pp. 311-349.
SQUIBB, R.E. & TILSON, H.A. (1982) Neurobehavioural changes in adult
Fisher 344 rats exposed to dietary levels of chlordecone (KeponeR):
a 90-day chronic dosing study. Neurotoxicology, 3: 59-66.
STEBBINS, W.C. (1970) Animal psychophysics: the design and conduct of
animal experiments, New York, Appleton-Century-Crofts.
STEEL, R.G.D. & TORRIE, J.H. (1960) Principles and procedures of
statistics, New York, McGraw-Hill, pp. 83-347.
STEELE, W.J. & BUSCH, H. (1963) Studies on acidic nuclear proteins of
the Walker tumour and liver. Cancer Res., 23: 1153-1157.
STOCKINGER, H.E. (1974) Behavioral toxicology in the development of
threshold limit values. In: Xintaras, C., Johnson, B.L., & DeGroot,
I., ed. Behavioral toxicology, Cincinnati, Ohio, US Department of
Health, Education and Welfare, pp. 18-19 (US DHEW (NIOSH) Publication
No. 74-126).
SUDAKOV, K.V. (1982) Functional system theory in physiology.
Agressologie, 23: 167-176.
SZMIGIELSKI, A. (1975) Effect of disulfiram and sodium
diethyldithiocarbamate on thyroxine and dopamine hydroxylases in rat
brain and breast. Pol. J. Pharmacol. Pharm., 27: 153-159.
TAKEUCHI, T. (1968) Pathology of Minamata disease. In: Katsuma, M.,
ed. Minamata disease. Study Group of Minamata Disease, Japan,
Kumamoto University, pp. 141-228.
TAKEUCHI, T., ETO, N., & ETO, K. (1979) Neuropathology of childhood
cases of methylmercury poisoning (Minamata disease) with prolonged
symptoms with particular reference to the decortication syndrome.
Neurotoxicology, 1: 1-20.
TALKE, T., HIRABAGASHI, J., KONDO, R., MATSUMOTO, M., & KOJIMA, K.
(1979) Effect of butyrate on glycolipid metabolism of two cell types
of rat ascites hepatomes with different ganglioside biosynthesis.
J. Biochem., 96: 1139-1402.
TAPPEL, A.L. (1970) Lipid peroxidation damage to cell components.
Fed. Proc., 29: 239.
TAYLOR, J.R., SELHORST, J.B., & CALABRESE, V.P. (1980) Chlordecone.
In: Spencer, P. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 407-421.
THIESSEN, D.D. (1964) Population density and behavior: a review of
theoretical and physiological contributions. Texas Rep. Biol. Med.,
22: 266-314.
THOMAS, P.K. (1970) The quantitation of nerve biopsy findings.
J. neurol. Sci., 11: 285-295.
THOMAS, P.K. (1980) The peripheral nervous system as a target for
toxic substances. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 35-47.
THOMPSON, S.W. (1963) Selected histochemical and histopathological
methods, Springfield, Illinois, Charles C. Thomas.
THORNDIKE, E.L. (1932) Reward and punishment in animal learning.
Comp. Psychol. Monogr., 39: 8.
TILSON, H.A. & CABE, P.A. (1978) Strategy for the assessment of
neurobehavioral consequences of environmental factors. Environ.
Health Perspect., 26: 287-299.
TILSON, H.A. & HARRY, G.J. (1982) Behavioral principles for use in
behavioral toxicology and pharmacology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 1-27.
TILSON, H.A. & MITCHELL, C.L. (1984) Neurobehavioral techniques to
assess the effects of chemicals on the nervous system. Ann. Rev.
Pharmacol. Toxicol., 24: 425-450.
TILSON, H.A. & SQUIBB, R.D. (1982) The effects of acrylamide on the
behavioral suppression produced by psychoactive agents.
Neurotoxicology, 3: 113-120.
TILSON, H.A., BYRD, N., & RILEY, M. (1979a) Neurobehavioral effects of
exposing rats to kepone via the diet. Environ. Health Perspect.,
33: 321.
TILSON, H.A., CABE, P.A., ELLINWOOD, E.H., Jr, & GONZALEZ, L.P.
(1979b) Effects of carbon disulfide on motor function and
responsiveness to delta-amphetamine in rats. Neurobehav. Toxicol.,
1: 57-63.
TILSON, H.A., MITCHELL, C.L., & CABE, P.A. (1979c) Screening for
neurobehavioral toxicity: the need for and examples of validation of
testing procedures. Neurobehav. Toxicol., 1(Suppl. 1): 137-148.
TILSON, H.A., CABE, P.A., & BURNE, T.A. (1980) Behavioral procedures
used in the assessment of neurotoxicity. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 758-766.
TILSON, H.A., MACTUTUS, C.F., MCLAMB, R.L., & BURNE, T.A. (1982a)
Characterization of triethyl lead chloride neurotoxicity in adult
rats. Neurobehav. Toxicol. Teratol., 4: 671-681.
TILSON, H.A., SQUIBB, R.E., & BURNE, T.A. (1982b) Neurobehavioral
effects following a single dose of chlordecone (kepone) administered
neonatally to rats. Neurotoxicology, 3: 45-52.
TOLMAN, E.C. (1948) Cognitive maps in rats and men. Psychol. Rev.,
55: 189-208.
TOWFIGHI, J., GONATAS, N.K., & MCCREE, L. (1975) Hexachlorophene
retinopathy in rats. Lab. Invest., 32: 330-338.
TROPKINA, J.K., SELZNEVA, S.N., & POGODAEV, K.V. (1981) Prenatal
influence of hypoxia on metabolic structure of cerebral mitochondria
in the oncogenesis with epileptic superactivity. In: Mitochondria:
mechanisms of conjugation and regulation, Puscino, p. 39.
TRUEX, R.C. & CARPENTER, M.B. (1983) Human neuroanatomy, 8th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
TSUJI, S. & FUJIHSIMA H. (1975) Paraplegias: clinical statistics,
Fukuoka, Kyushu Rosai Hospital, Department of Orthopedics and
Neurology.
TULLOCH, M.L., CROOKS, J., & BROWN, P.S. (1963]) Inhibition of thyroid
function by a dithiocarbamoylhydrazine. Nature (Lond.),
199: 288-289.
ULRICH, C.E., RINEHART, W., & BRANDT, M. (1979) Evaluation of the
chronic inhalation toxicity of a manganese oxide aerosol. III.
Pulmonary function, electromyograms, limb tremor, and tissue manganese
data. Am. Ind. Hyg. Assoc. J., 40: 349-353.
UPHOUSE, L., NEMEROFF, C.B., MASON, G., PRANGE, A.J., & BONDY, S.C.
(1982) Interactions between "handling" and acrylamide on endocrine
responses in rats. Neurotoxicology, 3: 121-125.
UPHOUSE, L., MASON, G., & HUNTER, V. (1984) Persistent vaginal estrus
and serum hormones after chlordecone (kepone) treatment of adult rats.
Toxicol. appl. Pharmacol., 72: 177-186.
US FDA (1978) Nonclinical laboratory studies. Good laboratory
practice regulation, Washington DC, US Food and Drug Administration,
Department of Health, Education and Welfare (Federal Register 43:
59986-60025).
US NAS (1975) Principles for evaluating chemicals in the
environment, Washington DC, National Academy of Science (A Report of
the Committee for the Working Conference on Principles of Protocols
for Evaluating Chemicals in the Environment).
US NAS/NRC (1970) Evaluating the safety of food chemicals,
Washington DC, National Academy of Sciences, National Research
Council, Food Protection Committee, Food Nutrition Branch, Division of
Biology and Agriculture.
VAN GELDER, G.A. (1978) Lead and the nervous system. In: Oehme, F.W.,
ed. Toxicity of heavy metals in the environment, Part 1, Basel, New
York, Marcel Dekker, Inc., pp. 101-121.
VANDERWOLF, C.H. & LEUNG, L.-W.S. (1983) Hippocampal rhythmic slow
activity: a brief history and the effects of entorhinal lesions and
phencyclidine. In: Seifert, W., ed. Neurobiology of the hippocampus,
New York, Academic Press, pp. 275-302.
VASAN, N.S. & CHASE, H.P. (1976) Brain glucosaminoglycans
(mucopolysaccharides) following intrauterine growth retardation.
Biol. Neonate, 28: 196-206.
VEKSLER, M.S. & ARBUKHANOVA, M.S. (1981) Cerebral and spinal cord
mitochondria under strenuous physical work. In: Mitochondria:
mechanism of conjugation and regulation, Puscino, p. 81.
VERGIEVA, T. & ZAIKOV, H.R. (1981) Behavioral changes in rats with
inhalation of styrene. Hig. Zdraveopazvane, 24: 242-247.
VERITY, M.A. (1972) Cation modulation of synaptosomal respiration.
J. Neurochem., 19: 1305-1309.
VERITY, M.A., BROWN, W.J., CHEUNG, M., & DZER, G. (1977) Methylmercury
inhibition of synaptosomes and brain slices protein synthesis: in
vivo and in vitro studies. J. Neurochem., 29: 673-679.
VILA, J. & COLOTLA, V.A. (1981) Some stimulus properties of inhalants:
preliminary findings. Neurobehav. Toxicol., 3: 447-480.
VINAR, O., FRANTIK, E., & HORVATH, M. (1981) Subjectively-perceived
effects of diazepam, toluene, and their combination. Act. Nerv.
super. (Praha), 23: 179-182.
VORHEES, C.V. (1974) Some behavioral effects of maternal
hypervitaminosis A in rats. Teratology, 17: 269-273.
VORHEES, C.V. & BUTCHER, R.E. (1982) Behavioral teratogenicity. In:
Snell, L., ed. Developmental toxicology, London, Croom Helm Press,
pp. 247-298.
VORHEES, C.V., BRUNNER, R.L., MCDANIEL, C.R., & BUTCHER, R.E. (1978)
The relationship of gestational age to vitamin A induced postnatal
dysfunction. Teratology, 17: 271-276.
WALLMAN, J. (1975) A simple technique using an optomotor response for
visual psychophysical measurements in animals. Vision Res., 15: 3-8.
WALSH, J.M., CURLEY, M.D., BURCH, L.S., & KURLANSIK, L. (1982) The
behavioral toxicity of a tributyltin ester in the rat. Neurobehav.
Toxicol. Teratol., 4: 241-246.
WALSH, R.N. (1980) On the necessity for a shift in emphasis from
means-oriented to problems-oriented research in developmental
psychobiology. Dev. Psychobiol., 13: 229-231.
WALSH, T.J., MILLER, D.B., & DYER, R.S. (1982b) Trimethyltin, a
selective limbic system neurotoxicant, impairs radial-arm maze
performance. Neurobehav. Toxicol. Teratol., 4: 177-184.
WALTON, J.N. (1974) Disorders of voluntary muscle, 3rd ed.,
Edinburg, London, Churchill Livingston.
WANG, G.H. (1932) Relation between spontaneous activity and oestrous
cycle in the white rat. Comp. Psychol. Monogr., 6: 1-32.
WEIBEL, E.R. (1979) Stereological methods. In: Practical methods for
biological morphometry, New York, Academic Press, Vol. 1.
WEISS, B., FERRIN, J., MERIGAN, M., & COX, C. (1981) Modification of
rat operant behaviour by ozone exposure. Toxicol. appl. Pharmacol.,
58: 244-251.
WEISS, B. & LATIES, V. (1961) Changes in pain tolerance and other
behavior produced by salicylates. J. Pharmacol. exp. Ther.,
131: 120-129.
WEISS, B. & LATIES, V. (1969) Behavioral pharmacology and toxicology.
Ann. Rev. Pharmacol., 9: 297-326.
WEISS, B. & LATIES, V.G., ed. (1975) Behavioral toxicology, New
York, Plenum Press, 469 pp.
WEISS, B. & LATIES, V.G. (1979) Assays fox behavioural toxicity: a
strategy for the Environmental Protection Agency. Neurobehav.
Toxicol., 1(Suppl. 1): 213-215.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, J., CONNEY, A.H.
(1971) Effect of halogenated hydrocarbon insecticides on the
metabolism and uterotropic action of estrogens in rats and mice.
Toxicol. appl. Pharmacol., 19: 234-246.
WELLS, G.A.H., HOWELL, J.M., & GOPINATH, C. (1976) Experimental lead
encephalopathy of calves: histological observations on the nature and
distribution of the lesions. Neuropathol. appl. Neurobiol.,
2: 175-190.
WHEATLEY, B., BARBEAU, A., CLARKSON, T.W., & LAPHAM, L.W. (1979)
Methylmercury poisoning in Canadian indians -- the elusive diagnosis.
Can. J. neurol. Sci., 6: 417-422.
WHO (1975) Methods used in the USSR for establishing biologically
safe levels of toxic substances, Geneva, World Health Organization,
171 pp.
WHO (1978) Principles and methods of evaluating the toxicity of
chemicals. Part 1, Geneva, World Health Organization (Environmental
Health Criteria Series No. 6, Part 1).
WHO (1984) Principles for evaluating health risks to progeny
associated with exposure to chemicals during pregnancy, Geneva,
World Health Organization (Environmental Health Criteria Series
No. 30).
WHO (in preparation) Principles and methods of evaluating the
toxicity of chemicals. Part 2, Geneva, World Health Organization
(Environmental Health Criteria Series No. 6, Part 2).
WILLIAMS, P.L. & WARWICK, R. (1975) Functional neuroanatomy of man,
Philadelphia, Pennsylvania, W.B. Saunders Company.
WILSON, W.E. (1982) Dopamine-sensitive adenylate cyclase inactivation
by organolead compounds. Neurotoxicology, 3: 100-107.
WINNEKE, G. (1979) Modification of visual evoked potentials in rats
after long-term blood lead elevation. Act. Nerv. super. (Praha),
21: 282-284.
WINNEKE, G., BROCKHAUS, A., & BALTISSEN, R. (1977) Neurobehavioral and
systematic effects of longterm blood lead-elevation in rats. I.
Discrimination learning and open field behavior. Arch. Toxicol.,
37: 247-263.
WINNEKE, G., FODOR, G., & SCHLIPKOTER, H. (1978) Carbon monoxide,
trichloroethylene, and alcohol: reliability and validity of
neurobehavioural effects. Multidisciplinary perspectives in event-
related brain potentials. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency (EPA 600/9-77-0443).
WINNEKE, G., LILIENTHAL, H., & ZIMMERMANN, U. (1984) Neurobehavioural
effects of lead and cadmium. In: Proceedings of the Third
International Congress on Toxicology, San Diego, 28 August-2
September 1983, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
WOLF, D.L. (1976) Rotating rod, spontaneous activity, and passive
avoidance conditioning: their suitability as functional tests in
industrial toxicology. In: Horvath, M. & Frantik, E., ed. Adverse
effects of environmental chemicals and psycho- tropic drugs,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 293-303.
WOOD, R.W. (1978) Stimulus properties of inhaled substances. Environ.
Health Perspect., 26: 69-76.
WOOD, R.W. (1979) Reinforcing properties of inhaled substances.
Neurobehav. Toxicol., 1(Suppl. 1): 67-72.
WOOD, R.W. (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav. Toxicol. Teratol., 3: 397-405.
WOOLLEY, D.E. (1977) Electrophysiological techniques in
neurotoxicology. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 9-1 -- 9-25 (EPA
600/9-77-042).
XINTARAS, C. & BURG, J.R. (1981) Neurotoxicity studies of workers
exposed to carbon disulfide. G. Ital. Med. Lav., 3: 83-86.
XINTARAS, C., JOHNSON, B.L., & DEGROOT, I., ed. (1974) Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, 507 pp (US DHEW (NIOSH) Publication No. 74-126).
YAMAMURA, H.I., ENNA, S.J., & KUHAR, M.J. (1981) Neurotransmitter
receptor binding, New York, Raven Press.
YONESAWA, T., BORNSTEIN, M.B., & PETERSON, E.R. (1980) Organotypic
cultures of nerve tissue as a model for neurotoxicity investigation
and screening. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental
and clinical neurotoxicology, Baltimore, Maryland, Williams and
Wilkins Company, pp. 788-802.
YOUNG, J.S. & FECHTER, L.D. (1983) Reflex inhibition procedures for
animal audiometry: a technique for assessing ototoxicity. J. Acoust.
Soc. Am., 73: 1686-1693.
ZAKUSOV, V.V. (1936) [Estimation of the reflex time under the
influence of some narcotic substances.] Fiziol. Zh. USSR,
23: 276-278 (in Russian).
ZAKUSOV, V.V. (1953) [Pharmacology of the nervous system,]
Leningrad, Medgiz, 258 pp (in Russian).
ZBINDEN, G. (1981) Experimental methods in behavioral teratology.
Arch. Toxicol., 48: 69-88.
ZEMAN, W. & INNES, J.R.M. (1963) Craigie's neuroanatomy of the rat,
New York, Academic Press.
ZENICK, H., RODRIQUEZ, W., WARD, J., & ELKINGTON, B. (1979) Deficits
in fixed-interval performance following prenatal and postnatal lead
exposure. Dev. Psychobiol., 12(5): 509-514.
ZILOV, V.G., ROGACHEVA, S.K., & IVANOVA, L.I. (1983)
[Electroencephalographic analysis of limbic-reticular interrelations
in various motivational reactions of rabbits under ethanol.] Bull.
exp. Biol. Med., 11: 74-77 (in Russian).
ZUBER, V.L. (1978) Intensity of metabolism of various fractions of
cerebral phospholipids under hyperphenyl-alaninaemia. Vop. med.
Xhim., 2: 163-166.
ZYLBER-HARAN, E.A., GERSHMAN, H., ROSENMANN, E., & SPITZ, I.M. (1982)
Gonadotrophin, testosterone, and prolactin interrelationships in
cadmium-treated rats. J. Endocrinol., 92: 123-130.
nerve cells is involved, is more likely to be associated with
permanent behavioural changes than disorders predominantly affecting
the peripheral nervous system, where satisfactory regeneration is more
often the rule. Rapidly reversible effects on behaviour are
occasionally, but not often, associated with detectable structural
damage to the nervous system.
5.3.2 Gross morphology
While most neurotoxic damage is most evident at the microscopic
level, it is important to pay attention to the weight of the brain as
well as to macroscopic manifestations such as discolouration (for
example, due to bilirubin), discrete or massive haemorrhage, or other
localized lesions (for example, transverse myelitis).
The new methods of nuclear magnetic resonance (NMR) can supply
data concerning structural changes, for example, demyelination, both
in vivo and post-mortem.
5.3.3 The role of histology
There is no substitute for a thorough light-microscope examination
of the nervous system and other organs for the initial assessment of
animals suspected of having neurotoxic injury. The same careful
attention to anatomical detail should be given in the examination of
experimental animal tissues as would be given by a pathologist
studying autopsy tissue from a patient who has suffered from a new
type of neurological disease. The results of this type of analysis
will provide direction for additional studies that focus on more
specific questions such as the type and extent of structural damage,
the location where changes first occurred, the time-to-onset of
structural damage, the reversibility of these changes, the lowest dose
that caused pathological changes, and the highest no-observed-adverse-
effect dose.
5.3.3.1 Biological principles dictating tissue response
The study design must take into account several important
principles of underlying cellular responses to injury. These
principles will influence when and where tissue should be sampled for
morphological study.
Often, there is a delay of days or weeks between dosing and the
first appearance of structural changes in animals treated with
neurotoxic agents. The extent of the delay depends largely on the
chemical used, the dose, and the species studied. Once the
pathological process has been initiated, a chain of degenerative and
regenerative events may be set in motion, and these may or may not
cease to evolve after dosing has stopped. Most neurotoxic substances
that damage neurocellular elements produce a largely symmetrical
pattern of structural change, whether in the CNS or PNS. Some neurons
or nerve fibres may be more vulnerable than others to chemical attack,
so that at any point in the pathological process, the observer may be
presented with many or all of the stages in the reaction of individual
cellular elements. The active pathological process may also move in
space with time, as in dying-back axonpathies in which degeneration
proceeds retrogradely along affected nerve and fibre tracts (Cavanagh,
1964). Careless sampling of animals with these or other types of
neurotoxic diseases may reveal little or no pathological changes. By
contrast, the careful and thorough investigator will be able to
distinguish between early and later changes, and thereby develop a
hypothesis of the spatial-temporal sequence of the pathological
process. This can be tested in subsequent studies in which animals are
examined at different stages in the development of the disease.
Investigations of this type will also provide information on the
relationship between the dose and duration of treatment required to
induce neurotoxic injury. No-observed-adverse-effect levels can be
established by determining doses that do not produce any
characteristic changes after a specific period of chemical treatment.
Quantitative estimation of structural abnormalities in treated versus
control animals becomes increasingly important as the no-observed-
adverse-effect dose is approached.
5.3.4 Use of controls
Parallel study of age-matched control animals is mandatory,
whenever treated animals are subjected to neuropathological
examination. Control groups should include untreated animals, vehicle-
treated animals, and positive controls. The may receive a neurotoxic
dose of the agent under study or of another compound with well-
characterized neurotoxic properties that elicits a comparable type of
neurotoxic damage. Demonstrating the development of an appropriate
neurotoxic response in the positive control strengthens the validity
of data obtained from animals treated with the agent under study,
especially if these prove to lack pathological changes. The use of
positive controls is imperative for the inexperienced investigator who
needs to gain confidence from the self-demonstration that a known
neurotoxic agent produces the expected changes. Inclusion of these
results in a published report will also reassure the audience that the
investigator has reproduced the expected pattern of structural damage
associated with a known neurotoxic chemical.
There are important advantages in studying the structure of
nervous tissue without knowledge of the treatment group. The
investigator is forced to be more critical in seeking and describing
abnormal findings, and the final assessment is a more objective
statement of the similarities and differences between treatment and
control groups.
5.3.5 Pattern of response
It is not the purpose of this publication to provide a detailed
analysis of the various problems of selective neuronal damage
encountered under neurotoxic conditions. However, it is emphasised
that occasionally the localization is very precise, limited to one or
two nuclei in the brain stem, or to distal regions of long fibre
tracts and peripheral nerves. Selective pathological changes are given
in Table 3 for reference in interpreting new situations.
5.3.6 Data acquired
At the end of the survey, clear answers must be found to the
following questions about the CNS:
1. Are there changes in nerve cells in the area of the cerebral
cortex, basal ganglia, hypothalamus, limbic system, diencephalon,
brain stem, cerebellum, or spinal cord?
2. Are there any changes in shape or number of astroglia and/or
microglial cells?
3. Is there any loss or other change in oligodendroglia?
4. Are there any changes in the myelinated areas of the brain or
spinal cord, such as myelin fragmentation or myelin vacuolation?
Is this associated with axonal swellings and/or degeneration?
5. If 4 is positive, are there glial responses?
6. Are there focal, vascular, and necrotic changes? If so, where?
7. Are tumours present?
8. Are there any changes in special sense organs to account for
functional changes?
For the peripheral nerves, the following questions must be
answered:
1. Are there any changes in peripheral nerves?
2. If so, is it axon degeneration or primary demyelination, or both?
3. What is the distribution of degeneration? Is it distal, affecting
only long and large-diameter fibres, or are shorter fibres such as
cranial nerves also affected?
4. Are sensory, motor, or both types of fibres involved?
Table 3. Examples of toxic effects illustrating the specific
patterns of damage that may be found
Pattern Cause Reference
Neuronal changes
laminar cortical necrosis anoxia Briefly (1976)
hippocampal pyramidal cell trimethylin Brown et al.
damage (1979)
selective granule cell loss methylmercury Hunter & Russell
from the cerebellum (1954)
selective loss of inferior 3-acetylpyridine Desclin (1974)
olivary cells
selective degeneration of doxorubicin Cho et al. (1980)
perikarya of sensory
ganglion cells
selective degeneration of methylmercury Cavanagh & Chen
axons of sensory ganglion (1971)
cells
retrograde degeneration of organophosphorus, Cavanagh (1964);
long sensory and motor acrylamide, Spencer &
axons in CNS and PNS hexanedione Schaumburg (1977)
retrograde degenerations of clioquinol Krinke et al. (1979)
axons of long spinal cord
tracts
Myelin changes
CNS myelin vacuolation triethyltin Aleu et al. (1963)
CNS and PNS myelin hexachlorphene Towfighi et al.
vacuolatin (1975)
focal degeneration of PNS diphtheria Cavanagh & Jacobs
myelin without axon loss (1954)
focal degeneration of PNS lead Fullerton (1966)
myelin with some axon loss
Table 3 (Cont'd)
Pattern Cause Reference
Vascular and neurotic changes
symmetrical lesions in misonidazole Griffin et al.
brain-stem nuclei (1980)
non-symmetrical brain-stem lead Wells et al. (1976)
and cortical lesions
5. What is the state of nerve cell bodies (i.e., sensory ganglion
cells, anterior horn cells)?
6. If the cells in 5 show changes, are those in the medulla and
trigeminal ganglia (i.e., cranial nerves) similarly affected?
7. Are neurons normal, chromatolytic, or degenerated?
8. Are automatic ganglia or nerves similarly affected or not?
9. Are muscles affected by denervation and/or myopathic changes?
10. At dose levels below those that produce clinical signs, is there
histochemical and/or morphometric evidence of metabolic neuronal
changes that is brought out by using these more sensitive
techniques?
5.4. Principles, Limitations, and Pitfalls of the Morphological
Approach
5.4.1 Tissue state
Although under special circumstances the morphologist is able to
study the reactions of living tissue to chemical exposure, either
in vivo or in tissue culture (Yonesawa et al., 1980), the vast
majority of pathological studies involve the assessment of dead
tissue. The structural changes that occur in the nervous system as a
function of time post-mortem can obscure the recognition of toxic
injury and may confuse the inexperienced neuropathologist. Chemical,
or rarely, physical (freezing) fixation methods are used to prevent
the development of post-mortem changes.
5.4.2 Principles of fixation
The use of a reliable fixation method is mandatory for the
morphological examination of nervous tissue (Thompson, 1963; Pease,
1964; Hayat, 1973; Gabe, 1976; Bancroft & Stevens, 1977; Glausert,
1980). The purpose of fixation is to preserve cellular architecture as
closely as possible to that in the living state, to inhibit the loss
of chemical components required for the maintenance of morphology, and
to prepare the tissue to accept stains that enhance tissue density for
clear resolution of cytological detail. The ideal method of fixation
is one that instantaneously terminates life processes in the tissue of
interest without distorting cytological detail. Rapid freezing of
living tissue approaches this ideal. Excision and freezing of tissue
is routinely employed for the assessment of human biopsy tissue and is
particularly suitable for light-microscope histochemistry and
immunocytochemistry. A few specialized laboratories use rapid freezing
for the examination of tissue, or metal replicas thereof, by
transmission electron microscopy.
Chemical methods are far more commonly used in neurotoxicology for
the preparation of nervous tissue. Although the ideal chemical
fixative should instantaneously kill living tissue without inducing
structural changes, all known methods fall short of this ideal.
Instead, the morphologist must strive to induce small, consistent and
readily recognizable changes that will not compromise tissue
examination and assessment. The most satisfactory and widely used
chemical fixatives are dilute, aqueous solutions of aldehydes
(formaldehyde and/or glutaraldehyde), which rapidly cross-link
proteins and stabilize associated lipids, prevent post-mortem changes,
and introduce rigidity into the tissue to facilitate handling. A
secondary phase of fixation using a dilute aqueous solution of osmium
tetroxide to fix lipids is routinely added after glutaraldehyde
fixation for improved preservation of tissue. Occasionally, osmium
tetroxide is used as the primary fixative. Structural artefacts
induced by fixation are minimized if the fixative solutions are
buffered to match tissue pH and osmolality. Temperature should also be
controlled; low temperatures may affect the preservation of fine
structural elements (e.g., microtubles).
Chemical fixatives must be delivered to the site of interest as
rapidly as possible if post-mortem artefacts are to be minimized. Most
effective is systemic perfusion (Pease, 1964; Hayat, 1973; Spencer et
al., 1980; Spencer & Bischoff, 1982), in which the fixative is
introduced via the ascending aorta of the deeply anaesthetized animal,
and distributed throughout the vascular network under controlled
pressure. Spaces normally occupied by blood and tissue fluid are
rapidly replaced by the chosen fixative solution, and the animal
expires painlessly within seconds. Perfusion of individual organs is
an alternative, but more difficult and less satisfactory technique. An
alternative method, suitable only for readily accessible parts of the
nervous system (e.g., peripheral nerves), is to bathe the tissue
in situ with a repeatedly replenished solution of fixative.
In practical terms, the requirements for tissue sampling, organ
weighing, etc., have to be balanced against the need to get good
fixation, and this frequently presents conflicts of interest between
those involved in analysing the animal tissues. Short perfusion
fixation (section 5.5.2), with paraformaldehyde and subsequent
secondary fixation in formalin or formal-acetic acid in the case of
paraffin sections and light microscopy, and glutaraldehyde and osmium
tetroxide in the case of epoxy resin, semi-thin sections, may help to
provide a compromise. When the protocol for killing requires only
immersion fixation, then a few animals in each group must be kept for
perfusion fixation, if adequate tissue examination is going to be
achieved. A study using only immersion fixation is scarcely worth the
labour and cost involved.
The selection of the chemical fixative and its method of delivery
to the tissue site are chosen according to the type of information
that is required. Systemic perfusion with the chosen fixative is
always to be preferred. In general, formaldehyde is suitable for
studies that require widespread tissue sampling, low resolution of
cytological detail, or the use of special histochemical stains to
assess the chemical content of abnormal cells and tissues. Conversely,
in studies focusing on more selected areas of the nervous system or
requiring resolution of fine structural detail, the use of
glutaraldehyde and osmium tetroxide is mandatory. While the latter
method was originally introduced for use in transmission electron
microscopy, an increasing number of laboratories use the technique
routinely to exploit the remarkable resolution afforded by the light
microscope.
5.4.3 Principles of tissue sampling
Extensive sampling of tissue is essential for the initial
assessment of a suspected neurotoxic injury. Organs, other than the
nervous system, which should be examined, are listed in WHO (1978).
Neural tissue should include: brain, including cerebellum, brain stem,
pituitary gland, eye with occulomotor muscles and optic nerve
attached, spinal cord, several sensory ganglia, sciatic nerve and its
branches from its exit from the vertebral column to the level of the
ankle, and selected muscles innervated by the sciatic nerve and its
branches. Other regions less commonly sampled include: olfactory
epithelium and tubercles, inner ear and labyrinths, plantar nerves and
skin receptors, autonomic ganglia, and nerves and organs of
innervation, such as the gut. The brain contains many sites known to
be vulnerable to chemical agents and should be examined as thoroughly
as possible. The use of an atlas such as that for the rat brain (Zeman
& Innes, 1963) will be valuable. The brain stem should be sectioned
just rostral to the cerebellum, the slice passing through the inferior
colliculi. The cerebral hemispheres can then be sliced coronally
(transversely). Olfactory lobes may be taken if questions of
inhalation and olfaction are being studied. The cerebellum is removed
by cutting through the peduncles that attach it to the brain stem.
Sagittal sections are obtained by cutting through the midline, and
parallel sections are also taken. Emphasis is usually placed on the
retina and optic nerves, since these are often heavily involved in
toxic diseases affecting neurons, axons, and myelin. Ototoxicity is
also a common event, though the technical difficulties associated with
the examination of the inner ear and labyrinths have prevented
widespread study of this phenomenon. Olfactory lobes and associated
epithelium are rarely examined, but should be taken if questions of
inhalation and olfaction are being studied.
The spinal cord contains ascending and descending nerve fibre
tracts that commonly undergo changes in myelinopathies and
axonopathies (Table 3). Since the latter are tractable diseases with
distal accentuation, it is critical to sample various levels from the
medulla oblongata (where the gracile tract terminates) to the sacral
region. By taking transverse sections of the spinal cord, it is
possible to make a simultaneous evaluation of white matter (myelinated
tracts and glial cells), gray matter (neurons, dendrites, proximal
axons, glial cells), blood vessels, and associated tissues.
Dorsal root and cranial nerve ganglia contain primary sensory
neurons that may display pathological changes in certain
neuronopathies and axonopathies (Figs. 3, 4). Structural alterations
in blood vessels and myelin may also occur under certain conditions.
The sensory ganglia of the Vth cranial nerve, at least 4 sensory
ganglia from the cervical bulb region (C5-T1 spinal levels), and 4
from the lumbosacral regions (L3-S2 spinal levels), should be sampled
for examination in longitudinal section. Corresponding dorsal and
ventral spinal roots, vulnerable in toxic demyelinating diseases,
should also be studied.
Peripheral nerves commonly display changes in neurotoxic diseases
affecting myelin, neurons, and axons (Table 3). As the last display
distal, retrograde changes in dying-back axonopathies, a common
response to chemical attack, it is critical to examine several levels
of vulnerable nerves. Samples of the sciatic nerve and its branches
are usually taken commencing adjacent to the vertebral column and
terminating in the distal regions of the sural nerve, peroneal nerve,
and/or the tibial nerve. The fine branches of the tibial nerve that
leave the main trunk below the knee are especially vulnerable in toxic
neuropathies, because they contain very large myelinated nerve fibres.
Terminal regions of sensory and motor nerve fibres can be examined by
searching for twigs supplying intrafusal (spindles) and extrafusal
muscle fibres, respectively (Schaumburg et al., 1974). Routinely,
anterior tibial muscles, and gastronemius with suralis muscles are
examined. Muscle tissue is also valuable for assessing whether there
is denervation atrophy, evidence of toxic myopathy, nerve sprouting,
or other regenerative activity (Pearson & Mostofi, 1973).
Tissue sampling should not only be tailored to the goal of the
study but also to the method of fixation. In general, blocks of tissue
several millimeters thick may be taken if the tissue has been fixed
with formaldehyde and is destined for paraffin embedding. For tissue
fixed with glutaraldehyde, the relatively slow rate of penetration of
osmium tetroxide necessitates the use of thin (1 µm) slices of tissue.
It is often helpful to mark a small area of tissue (with Indian ink or
a small cut) to maintain the orientation. Elongate structures, such as
peripheral nerves and spinal cord, are most readily studied in
transverse sections, though important information is acquired by
complementary examination of longitudinal sections. Particularly
informative for the identification of pathological changes in
peripheral nerves is the technique of microdissection (teasing apart
intrafascicular tissue), which yields long lengths of individual
myelinated nerve fibres suitable for light microscope examination.
Inspection of these preparations can demonstrate rapidly the presence
of primary demyelination and remyelination and axonal degeneration and
regeneration. The minor changes characteristic of early toxic
neuropathies, readily missed in the examination of sections, can be
easily detected by this method. Other parts of the nervous system are
technically difficult to examine by microdissection because of the
absence of a collagen network to support the individual nerve fibres.
While it is prudent to embed tissues in paraffin wax or epoxy
resin promptly, regions that will not be examined immediately can be
stored in a solution and at a temperature appropriate to the method of
fixation. Loss of tissue quality will occur in proportion to the
length of storage.
5.4.4 Preparation of tissue for examination
While gross tissue changes can be visualized with the naked eye or
by examination with a stereoscopic binocular microscope, and surface
detail can be resolved with the scanning electron microscopy, routine
examination of cellular structure requires the use of tissue sections.
This inevitably places severe limitations on the amount of tissue that
is practical to study. Sections must be such that the energy beam
(light, electrons, X-rays) of the chosen microscope can be transmitted
through the tissue. Relatively thick sections containing several
layers of cells can be visualized with the light microscope, but the
ability to resolve cytological detail decreases as section thickness
increases. The use of semi-thin sections allows the investigator to
exploit the maximum available resolution of the light microscope. Much
thinner sections are required for the assessment of tissue by
transmission electron microscopy (Pease, 1964; Hayat, 1973).
Chemically-fixed tissue must be supported by a pliable material to
facilitate the sectioning process and to prevent disintegration of the
sectioned tissue. Paraffin wax or epoxy resin are routinely used: the
former for routine work, the latter for more specialized studies.
Since both types of embedding media are water insoluble and therefore
immiscible with the fixative solutions, water is first removed by
stepwise dehydration in ethanol or methanol immersing the tissue in an
aromatic hydrocarbon (clearing solution) that is miscible both with
ethanol and the embedding medium. Dehydration, clearing, and
infiltration with an embedding medium are procedures requiring
considerable care and time to avoid tissue drying and distortion.
Automatic systems are available and, for paraffin embedding, these are
routinely used in histological laboratories. Once the tissue is
infiltrated and embedded, the medium must be allowed to harden by
cooling (paraffin wax) or heating (epoxy resin).
Sections of tissue are prepared with the aid of knives mounted in
microtomes. Steel knives are suitable to cut thick (4-15 µm) sections
of paraffin embedded tissues, whereas knives prepared by scoring and
breaking strips of special glass are needed to prepare sections (1 µm)
of tissue embedded in the harder epoxy resin. The sections of tissue
prepared with steel knives permits study of larger tissue areas than
is possible with sections prepared from epoxy blocks, though the
latter afford a higher resolution of cytological detail. With new
developments in the preparation of glass knives and the design of
microtomes, ever larger sections can be routinely prepared by the
latter technique. Diamond knives mounted in ultramicrotomes are used
to obtain the thin sections (50 nm) required for transmission electron
microscopy.
Contrast of cytological detail in sectioned material can be
enhanced by the use of special light-microscope methods (phase,
Nomarski, fluorescence) or, more commonly, by the introduction of
stains that render structures light-dense. A wealth of cytological
stains is available for use with paraffin sections, whereas only a few
general purpose stains are usually used to enhance structural detail
for the light-microscope assessment of epoxy sections. Heavy metal
stains that are electron dense are needed to visualize fine structure
by transmission electron microscopy.
5.4.5 Recognition of artefact
Some degree of artefact is unavoidable, irrespective of the care
taken in preparing tissue for morphological examination, and
recognition and identification of the sources of artefact are of the
utmost importance in the morphological assessment. Possible artefacts
are legion and stem from various sources: e.g., poor tissue
preservation from failure of fixative to penetrate tissue; shrinkage
induced by inappropriate dehydration or drying; traumatic changes
associated with excision of the tissue (bending, stretching) or rough
handling during processing; wrinkling of sections and precipitation of
stain (Spencer & Bischoff, 1982).
5.4.6 Recognition of normal structural variations
While the gross and fine structure of the nervous system of any
species and age is remarkably constant from animal to animal, it is
critical to recognize the irregular occurrence of normal variations in
cellular structure that may lead the inexperienced observer to an
inappropriate conclusion. These variations include ectopic structures
(e.g., sensory neurons misplaced in the sciatic nerve), local
oedematous or proliferative changes (e.g., at a site of unrecognized
trauma or infection), and those that accompany advancing age (Johnson,
1981). The latter are especially important in long-term neurotoxicity
studies, and there is no substitute for rigorous comparative study of
control animals of the same age. Examples of normal age-related
changes include neuronal pigmentation by lipofuscin, localized
demyelination and remyelination, scattered neuronal loss, and axonal
dystrophy and degeneration.
5.4.7 Qualitative versus quantitative approaches
While there can be no substitute for a thorough qualitative
assessment of the morphological state of the nervous system,
morphometric methods are required for a more precise description of
these changes (Weibel, 1979; Bonashevskaya et al., 1983). This is
especially important for the description of minor changes, or when the
tissue reactions of different groups of animals are being compared.
Randomization of samples is essential to minimize subjective
influences, and a statistical approach is required both for the design
of the morphometric study and the analysis of the data. Tissue
sampling, group size, age, sex, body weight, and length are important
considerations in this regard. This type of morphometric approach has
been helpful in measuring tissue reactions to toxic chemicals in the
form of: the numbers of fibres affected in specific pathways subject
to degeneration, changes in cell populations in vulnerable parts of
the brain, and the relationship between fibre diameter and intermodal
length of peripheral myelinated nerve fibres prepared by
microdissection (Dyck et al., 1984).
5.5 Specific Procedures
5.5.1 Introduction
Strategic considerations in planning how to proceed must depend on
the state of knowledge of the possible effects. When it is unknown
whether damage occurs in nervous tissue, a wide-ranging examimation of
the nervous system with the light microscope is appropriate. Most
structural changes induced by neurotoxic chemicals can be detected
using the primary methods given below. More specialized, secondary
methods can be applied subsequently for more detailed studies.
5.5.2 Primary methods
There are strong convictions among experienced experimental
neuropathologists as to which fixation method should be chosen for the
primary screening. Some believe the conventional approach using
formaldehyde-based fixatives and paraffin embedding is most
appropriate; others prefer the more contemporary method of examining
epoxy sections of tissue fixed in glutaraldehyde and osmium tetroxide.
Each method has significant advantages and disadvantages and both
complement each other. Since it is impractical to use both methods for
the same animal, a prudent investigator will include a sufficient
number of animals to permit the parallel use of both procedures.
5.5.2.1 Formaldehyde/paraffin method
(a) Tissue fixation and excision
This technique is most suited for the study of large areas of
tissue at low resolution and with special histochemical stains
(section 5.5.3.3). Tissue can be removed from the carcass and fixed by
immersion in formalin (10%) containing 2% acetic acid (added just
before use) to improve penetration and hardening. Making up the
solution in 80% alcohol rather than water to hasten penetration
further has sometimes been recommended, but this is unnecessary and
may make dissection more difficult.
Rapid and careful transfer of tissues from the carcass to the
fixative solution will retard artefactual cell distortion. In a
general post-mortem dissection, the brain, including the cerebellum
and brainstem, are removed and transferred to fixative, prior to the
removal of other tissues. Retrieval of the spinal cord from its spinal
column is difficult and time consuming; it is recommended that the
whole spinal column with cord be excised quickly, trimmed of as much
muscle as possible, and immersed in the fixative intact. Once this
portion is fixed, the spinal cord and ganglia can be separated from
the column with greater care. Alternatively, after approximately 24 h
of fixation, the spinal column can be immersed in 5% formic acid
decalcifying solution. The column can then be sliced with little
difficulty.
To assist with the examination of peripheral nerves, tissue is
placed on a stiff card before immersion to retain orientation.
Optimally, the nerve to be excised should be exposed and, prior to
removal from the carcass, bathed with fixative for approximately
5 min. This will begin to introduce rigidity into the tissue and
retard decay artefact. A length of nerve can then be excised, quickly
placed on a card and pinned at the tip of both cut ends to retain
position after immersion. Care should be taken to avoid overstretching
the nerve as it is pinned to the card. Samples of these tissues can
then be sectioned in a transverse or longitudinal direction. Muscle
can be treated in the same manner. Another technique is to take off
the limb at the hip or shoulder joint, remove the skin and fix en bloc
in formalacetic acid solution. The limb can then be decalcified in 5%
formic acid solution for approximately two weeks as noted earlier for
the spinal column. It can then either be embedded in paraffin wax as
one large block or further dissected.
Tissue preservation is greatly improved by perfusing animals
systemically for 10 min with phosphate-buffered paraformaldehyde
(section 5.5.2.2), a method that simultaneously preserves all body
organs. Tissue can then be sampled in a more leisurely manner without
fear of introducing post-mortem changes (section 5.5.2.2).
(b) Dehydration and embedding
Relatively large tissue samples can be embedded in paraffin wax,
and this is particularly suitable for localizing and identifying
lesions in the brain. Automated processing for paraffin embedding has
greatly diminished the time requirements for preparing tissue samples.
Most automated systems will conform to a variety of preparation
schedules and process great numbers of tissue samples at one time.
There are systems that operate under combined heat and vacuum, the
great advantage being rapid processing. This combination is
deleterious and may cause unnecessary shrinkage and brittleness, which
will distort cell structure and hinder interpretation. Paraffin
sections routinely cut 4-6 µm thick, are suitable for certain
histochemical techniques and can easily be treated with special stain
to enhance particular cellular components.
(c) Section staining
Four staining methods are routinely used to enhance cellular
detail in paraffin sections.
Hematoxylin and eosin
Routinely used on paraffin sections, this method stains nuclei
blue to purple, cytoplasm and Nissl substance (and mast cell granules)
blue, and myelin sheaths pink. Nuclear density can be readily
assessed, as well as an increase or decrease in the size of nuclei and
the number of cells. Neuronal damage is readily detectable. Nuclei and
cytoplasm of other cell types, such as microgila and vascular
endothelial cells, are also well defined, though cresyl violet may be
preferred.
Cresyl fast violet
This method enhances identification of cell-population changes and
is more aesthetically pleasing than other basic aniline dyes, such as
thionine or gallocyanine-chrome alum. DNA stains pale blue and RNA
purple-blue. Hypertrophy of astroglial cytoplasm and changes in
neuronal nuclei and cytoplasm are readily observed. Chromatolysis,
poorly demonstrated in hematoxylin- and eosin-stained sections, is
more apparent when stained with cresyl violet, as are most cells in
peripheral nerves.
Glees and Marsland's stain
This method is easy to carry-out and selectively stains
neurofibrillary components in axons (Marsland et al., 1954).
Longitudinal sections demonstrate the state of terminal innervation in
motor and sensory fibres. Empty endplates are often recognized by the
observation of remaining clumps of nuclei on the muscle surface.
Sudan Black B
This method is useful to reveal nerve terminals in muscles
(Cavanagh et al., 1964).
5.5.2.2 Glutaraldehyde/epoxy method
(a) Tissue fixation and excision
This technique is best suited for the resolution of early
pathological changes with the light microscope and is required for
transmission electron microscopy (section 5.5.3.5). Whole body
perfusion with buffered fixators (paraformaldehyde) followed by
glutaraldehyde) delivered under pressure (100-150 mm Hg) is the only
satisfactory method to prepare brain and spinal cord sections, and
this method has the advantage of optimally fixing all other organs
simultaneously. For this purpose, the animal is deeply anaesthetized
with a solution (e.g., sodium pentobarbital) containing a small
percentage of heparin to facilitate circulation. Subsequent procedures
should be performed rapidly and smoothly to prevent the development of
anoxic damage and to ensure global fixation. The animal is secured on
its back and the rib cage cut bilaterally and reflected backwards to
expose the heart. After slitting the pericardium, the right atrium is
opened to drain circulating blood. The ventricular apex is then
excised to provide access to the aorta, and a cannula spurting a
column of fixative is inserted through the ventricular opening to the
apex of the aorta and clamped in place. The circulatory system is
cleared by a brief, initial perfusion with paraformaldehyde or saline,
and followed by more prolonged (e.g., 10-15 min) perfusion with
glutaraldehyde.
Rigid tissues from a fixed carcass are less susceptible to
handling artefact. The nervous system can be sampled in any order. In
some laboratories, the entire nervous system, including brain with
optic tract and nerves, spinal cord, spinal roots, ganglia, and
peripheral nerves are exposed in the carcass, then carefully detached
intact and removed. When this is accomplished, there is no need
initially to mark tissues for orientation. It is imperative to avoid
tissue drying during dissection and excision. Exposed tissues should
be bathed with fixative and tissues should be manipulated as little as
possible with forceps.
(b) Dehydration and embedding
After glutaraldehyde fixation, tissue samples are post-fixed for
2-3 h by immersion in buffered 1-2% osmium tetroxide in the cold. The
samples are then dehydrated stepwise in ascending concentrations of
ethanol, immersed in acetone or toluene, infiltrated with a solution
of epoxy resin and finally, embedded and polymerized in epoxy resin.
For light-microscope examination, 1 µm sections are cut with specially
prepared glass knives mounted in an ultramicrotome (an instrument
designed specifically for the purpose of obtaining the thin sections
suitable for electron microscope examination). New techniques and
equipment have recently become available which allow larger tissue
samples to be prepared for sectioning in epoxy resin. Sections one
micrometer thick can be examined by phase-contrast optics or by bright
field after a brief staining with 1% toluidine blue.
(c) Section staining
Thick (1 µm) sections of tissue fixed in glutaraldehyde and osmium
tetroxide and stained with borate-buffered 1% toluidine blue allow
resolution of structures as small as a single mitochondrion. Brain and
spinal cord neurons display clearly defined nuclei, nucleoli, issl
substance, and lipofuscin. Dendrites stand out against a more darkly-
stained neuropil. Small, densely staining oligodendrocytes are readily
distinguished from large, pale astrocytes. Myelinated nerve fibres
contain a pale axon and darkly-stained myelin. In cross-sections of
peripheral nerves, large and small diameter myelinated fibres, as well
as the smallest unmyelinated axons, can be seen. Motor and sensory
nerve terminals are detectable in muscle.
5.5.3 Special methods
5.5.3.1 Peripheral nerve microdissection
Isolated fibre preparations provide information that may be missed
in cross-sections, and it is recommended that this procedure be
included in any study concerned with toxic neuropathy. Several
fixation methods are available to prepare peripheral nerves for
microdissection. Perfused tissue is ideal, though satisfactory
preparations can be obtained by bathing the tissue with fixative prior
to excision. Post-excision immersion in fixative can also be used,
though the act of transecting the nerve introduces major fibre
artefacts that are not restricted to the cut ends and are readily
mistaken for pathological changes.
Optimal preparations suitable for high-resolution light microscopy
require the use of glutaraldehyde, post-fixation with osmium
tetroxide, stepwise dehydration, and infiltration with a low-viscosity
epoxy resin. This is an ideal medium to tease apart nerve fibres and
is suitable for the storage of tissue at low temperature. Mounted
fibres can be polymerized by heat to affix them to a glass slide and
prevent squashing by a coverslip (Spencer & Thomas, 1970). Bright-
field examination will reveal changes in overall structure, and
Nomarski differential interference microscopy can be used to study the
axon.
Older techniques yield poorer preservation, low resolution of
detail, and isolated fibres are susceptible to squashing, an important
consideration if morphometric study is planned. One method involves
formalin fixation, post-fixation in osmium tetroxide, and infiltration
with a dilute solution of glycerol, which is used to support the
fibres during microdissection. Single fibres are mounted on the
microscope slide, cleared with cresol, blotted dry, and mounted with
pure glycerin (Thomas, 1970). Sudan Black B can also be used to stain
tissue prior to microdissection. Nerves are microdissected with the
aid of a pair of mounted needles and stereoscopic dissecting
microscope. Epineurial connective tissue is removed and a small
fascicle selected. After splitting the perineurium surrounding the
fascicle, the sleeve is removed to expose the intrafascicular tissue
containing the nerve fibres. The tissue is then repeatedly split into
progressively smaller longitudinal bundles until individual fibres are
obtained. These are picked up on the end of sharpened wooden
applicator sticks and transferred to a clean glass slide and provided
with a coverslip. Alternatively, a rapid "squash" preparation can be
obtained by teasing a small bundle of fibres loosely apart to create a
mesh formation on a microscope slide; a coverslip is then added for
microscope examination.
5.5.3.2 Frozen sections
Frozen sections lend themselves well to enzyme histochemical,
immunocytochemical, and silver-impregnation procedures, but for the
purposes of a large-scale study, this method is impractical. Tissues
fixed instantaneously by immersion in liquid nitrogen are particularly
vulnerable to cell distortion and/or destruction caused by excessive
temperature changes. Encasing tissue samples in gelatin or albumin is
advisable, especially for brain tissues (Crane & Goldman, 1979).
Ice-crystal formation is a disruptive artefact that produces a
vacuolated appearance in frozen sections. To minimize crystal
formation, it is important to freeze tissues as rapidly as possible
and maintain critical temperatures throughout the cutting procedure to
avoid thawing and refreezing. Typically, frozen sections are between
10 and 15 µm thick.
5.5.3.3 Histochemical methods
Using these procedures, it is possible to assess the activity of
cell energy systems, the rate of use of substrates for processes of
oxidative phosphorylation, and the functional state of intracellular
organelles. The activities of succinate dehydrogenase and malate
dehydrogenase, enzymes of the Krebs cycle, are well known, the former
being closely linked with mitochondrial membranes. Enzymes of the
hexose monophosphate shunt (glucose-6-phosphate dehyrogenase and
gluconate-6-phosphate dehydrogenase) and those of the electron
transport chain (NAD and NADP disphorase) should also be studied. The
rate of exchange of nitrogenous bases can be examined by the
determination of activity of dihydroorotate dehydrogenase for fatty
acids; monoamine oxidase for biogenic amines; and acetylcholinesterase
for acetylcholine.
Histochemical enzyme assays are performed on freshly frozen
sections cut on a cryostat (Dubowitz & Brooks, 1973). Tissues are
quick-frozen in liquid nitrogen. Control and experimental tissues
should be mounted on one slide and reactions carried out using the
methods outlined in Pearse (1968).
The following methods can be used to demonstrate the functional
state of nerve cells (Pearse, 1968):
(a) RNA (Brachet's method with ribonuclease control);
(b) DNA (Feulgen's method with deoxyribonuclease control);
(c) Total nucleic acids (Einarsen's method);
(d) Total protein (sublimate with bromophenol blue);
(e) Sulfhydryl groups (Barnet and Zeligman's method);
(f) Glycogen and glycosaminoglycans (amylase control-PAS
reaction); and
(g) Lipids (Sudan III, Sudan Black, Nile Blue methods).
To express histochemical reactions quantitatively, the following
formula for Mean Histochemical Index (MHI) can be used:
MHI = 3a + 2b + 1c + 0d
N
where 3, 2, 1, and 0 are the degrees of intensity of colour (from 3
to 0), and a, b, c, and d are the numbers of the cells with the given
intensity of colour; the denominator N is the number of cells counted.
The results allow statistical calculation of the assays and estimation
of the reliability of the results.
5.5.3.4 Golgi method
This unique silver-impregnation method demonstrates the shape and
surface characteristics of entire neurons, including cell bodies and
their processes. Golgi preparations are particularly useful in the
study of dendritic arborizations and synaptic associations (Santini,
1975). However, the technique is capricious in that only a few neurons
are unpredictably demonstrated: many preparations must therefore be
examined to gather a representative sample.
In the Rapid Golgi method, tissue is fixed with formaldehyde or
glutaraldehyde and post-fixed with osmium tetroxide. The tissue is
then impregnated with silver nitrate, dehydrated, and infiltrated with
celloidin. Thick (120 µm) sections are prepared, mounted, and examined
by bright-field microscopy.
5.5.3.5 Transmission electron microscopy
This technique is laborious and requires extensive training.
However, it has resulted in extensive advances in the understanding of
cellular processes in neurotoxicity. There is little need for such
exacting methods to determine the presence of changes in cell
structure, few experimental protocols require electron microscopy. The
transmission electron microscope is properly used to confirm and study
further the nature of lesions already shown and mapped by light
microscopic methods. It is all too easy to seek, and find, changes to
which no significance will ultimately be attached. Until the pattern
of change induced by the chemical under study has been well identified
by light microscopy, electron microscopy should not be considered. On
rare occasions, such as in toxic disorders of unmyelinated axons,
examination by transmission electron microscopy is necessary to
localize cellular changes not revealed by the methods discussed
previously. Thin (50 nm) plastic sections are prepared with the aid of
a diamond knife mounted in an ultramicrotome and subsequently
impregnated with heavy metals for electron-microscope examination
(Pease, 1964; Hayat, 1973).
5.5.3.6 Other anatomical methods
There are numerous additional specialized anatomical methods that
are waiting to be applied in the study of neurotoxicological issues.
These include histological techniques to trace anatomical pathways
(with horseradish peroxidase) and cellular activity. Examples of the
latter are the use of autoradiography to localize the distribution of
radiolabelled precursors such as thymidine and 2-dexoyglucose
(Sokoloff et al., 1977). Other methods susceptible to light and
electron microscope analysis include immunocytochemical studies of
receptors, proteins, and other cell structures (Emson, 1983).
Fluorescence microscopy, particularly for the localization and
detection of regional concentrations of catecholamines using the
formaldehyde vapour or glyoxylic acid technique of Falck et al.
(1962), supplements quantitative biochemical methods in evaluating
alterations in catecholamine levels. Fluorescence microscopy is also
of value in antigen and antibody localization techniques. Certain
light and electron microscope techniques may be combined, such as the
examination of fine structure of single teased nerve fibres (Spencer &
Lieberman, 1971; Ochoa, 1972) or of Golgi-stained preparations.
Advanced methods in electron microscopy include scanning (for surface
features), energy-dispersive X-ray analysis (for detection of elements
of high relative atomic mass) and energy-loss (for detection of
elements of low atomic mass). Freeze-fracturing tissue can reveal
details of the internal surfaces of cellular membranes when replicas
are examined by transmission electron microscopy (Hayat, 1973).
5.6 Conclusions
The information derived from morphological studies is highly
relevant to the interpretation of biochemical, neurophysiological, and
behavioural data. Structural changes have always been the firm
foundation on which analysis of clinical neurological disease has been
based. Both diagnosis and prognosis depend heavily on previous
neuropathological experience for their accuracy. Moreover, treatment
of neurological disease can only be satisfactorily planned by using
the knowledge gained from experimental and morphological studies that
have provided an understanding of the pattern by which neural cells
are affected. Indeed, in the majority of human intoxications of the
nervous system, knowledge of the structural changes is based almost
exclusively on animal studies. There is no reason to believe that it
will be less so in the future.
6. BIOCHEMICAL AND NEUROENDOCRINOLOGICAL METHODS
6.1 Introduction
Biochemical tools are valuable for the study of neurotoxicity.
They have the potential for both identifying toxic compounds and
delineating mechanisms of action of known toxic substances. However,
the range of biochemical techniques is vast and careful decisions must
be made in devising an effective research strategy. The most important
decision is identification of the objective of the research, which may
be conveniently categorized into three groups: determining mechanisms
of action of neurotoxic substances, identifying exposed individuals,
and screening for toxic conditions.
Devising an effective biochemical screen is the most challenging
of the three objectives. It requires the selection of biochemical
parameters that are general enough to indicate toxicity resulting from
multiple sites of damage, but specific enough to prevent the
accumulation of false positives. No single biochemical parameter is
likely to suffice. An effective screen must include indices of each of
the major functions of nervous tissue (e.g., cellular metabolism,
neuronal propagation, and neurotransmission). It might include
estimates of RNA and/or protein synthesis, lysosomal enzymes, membrane
transport, channel and neurotransmitter receptors, or their turnover.
The precise choice of biochemical indices must be limited by the
expertise and equipment of individual laboratories, but every attempt
must be made to consider a range of functional events rather than a
single parameter.
Considerable discussion has centred on the use of in vitro
procedures for screening potential neurotoxic compounds. The
advantages of an in vitro approach are obvious. It is less expensive
and less time-consuming than whole animal study and biochemical
techniques can be employed under precise, standard conditions.
In vitro procedures must include both parent compounds and potential
toxic metabolites. However, the exclusive use of in vitro methods
would fail to detect compounds in which the neurotoxicity results from
alterations in non-neuronal systems.
Theoretically, the choice of biochemical tools for the
identification of mechanisms of toxicity is the simplest of the three
objectives. Biochemical tools are virtually limitless, but they can be
chosen on the basis of known information about the toxicity of the
compound. Scientists can then proceed systematically from one level of
analysis to another. By synthesizing data from behavioural, neuro-
physiological, neuroendocrinological, neuropharmacological, and
neuropathological studies, the biochemist can employ successively more
selective tools to determine the initial biochemical lesion induced by
the toxic compound.
The purpose of this section is to review the biochemical
approaches that may be useful for identifying and further
understanding the effects of toxic compounds on the nervous system.
6.2 Fractionation Methods
Neural tissue has several features not shared by other tissues. In
particular, neural tissue exhibits considerable cellular,
morphological, and chemical heterogeneity. It is often desirable to
precede biochemical procedures with some attempt to decrease this
complexity. One approach is to separate the tissue into discrete parts
of brain prior to analyses. A second approach is to separate specific
cell types. A third involves subcellular fractionation procedures. In
many cases, it may be desirable to use a combination of these
fractionation procedures.
6.2.1 Brain dissection
Brain tissue can be fractionated into any number of potential
sections. However, the most often used scheme is based on that
described by Glowinski & Iverson (1966). In this method the brain is
divided into relatively discrete units of cerebellum, thalamus,
hypothalamus, striatum, hippocampus, etc., by using visible anatomical
landmarks. Such dissection procedures have been very useful in
describing the neurochemistry of different brain areas and are
essential in evaluating the effects of toxic compounds on molecules
(such as neurotransmitters) that are not uniformally distributed in
neural tissue. Because of the regional variability of brain chemistry,
whole brain analyses are seldom useful for evaluating brain function.
More importantly, the use of the whole brain for studies of toxic
compounds may fail to detect functionally-relevant alterations in
specific brain areas. For example, effects of lead on GABA levels have
been reported for cerebellar tissue (Silbergeld et al., 1980) and
acrylamide effects have been noted in striatal dopamine receptors
(Bondy et al., 1981).
Differences in the response to toxic compounds would be
anticipated from differences in neurochemistry in various regions of
the brain. In addition, many toxic substances are not distributed
uniformly across brain areas (e.g., chlordecone (Fujimori et al.,
1982) and manganese (Bonilla et al., 1982). Although not predictive,
examination of such regions can provide a biochemical clue regarding
possible molecular sites of interaction of the toxic compounds.
In more precise dissection procedures, a small needle, of defined
diameter, is used to obtain samples within anatomically defined areas
(Palkovits, 1973; O'Callaghan et al., 1983). Such finite samples have
been used in identifying several neurochemical events, e.g.,
neurotransmitter concentrations (Palkovits, 1973) and neuro-
transmitter-induced phosphorylation of specific phosphoproteins
(Dolphin & Greengard, 1981), and they may be especially valuable in
locating the site of neural responses to toxic compounds.
6.2.2 Isolation of specific cell types
Although neural tissue is recognized by a marked heterogeneity of
cellular elements, based on function and embyronic origin, these cell
types are conveniently categorized as neurons or glia. Each cell type
makes a unique contribution to neural function and their sensitivity
to toxic compounds may differ. Several methods have been described for
the bulk separation of neuronal and glial cell populations from whole
brain or specific brain regions (Rose & Sinha, 1970; Appel & Day,
1976; Magata & Tsukada, 1978). A dissociated cell suspension that
avoids disruption of the cells is prepared by forcing the tissues
through fine sieves. Proteolytic enzymes are used to facilitate the
dissociation. The fraction enriched in neuronal perikarya is separated
from the smaller glial cells (also containing some synaptosomes) by
centrifugation. The cell separations are never complete and the
procedures generally provide low yields. However, enrichment of the
neurons and glia is usually sufficient fox determining whether a toxic
agent interferes primarily with neuronal or glial metabolism. Isolated
cell populations have been used to study the lipid and protein
composition of membranes and the several metabolic differences between
neuron and glia. Such information may provide baseline data for future
studies of neurotoxic chemicals. Disadvantages of this method include
loss of cell processes from the perikarya, reduction of cell surface
area, and damage to the cell membrane. These factors must be
considered in any metabolic study.
To date, there have been few attempts to use such separation
procedures in neurotoxicity and they are not recommended as an initial
study. The method is laborious and time consuming and must be
performed on fresh tissue. Only a limited number of samples can be
simultaneously processed and it is never clear if the low yields
result from random or specific loss of cell types.
6.2.3 Subcellular fractionation
The neuron can be divided into relatively discrete functional
units. The cell body contains the metabolic machinery for the
synthesis and packaging of macromolecules and for the general
maintenance of cellular homeostatis. The long axonal process acts as a
communication link, propagating electrical impulses from the cell body
to the terminal and transporting vital nutrients and cellular
components to more distal regions including the nerve terminal.
Finally, the synapse functions to transfer chemically encoded
information from one cell to another. Toxic substances may disrupt
neuronal biochemistry at any one, or all, of these cellular sites.
The isolation of subcellular organelles and specific membrane
fractions can be a first approximation in determining the subcellular
sites of action of toxic agents. Differential centrifugation
procedures provide a rapid means of obtaining fractions consisting
predominantly of a single cellular component (Gray & Whittaker, 1962;
Cotman & Barker, 1974; Rose and Sinha, 1970). Subcellular
fractionation is never totally effective, but it can produce an
enrichment of organelles and cellular subfractions. Some of the more
commonly used biochemical markers that can help identify the degree of
enrichment are listed in Table 4. These markers can be used as
potential indices of toxicity. For example, the effects of triethyltin
on myelination in the developing brain has been studied by examining
the activity of the myelin marker, 2',3'cyclic nucleotide 3'
phosphohydrolase (Konat & Clausen, 1977). Merkuryva & Tsapkova (1982)
have used several marker enzymes in their study on the toxic effects
of ethanol on the nervous system.
When subcellular fractionation is combined with regional analysis,
the number of potential samples requiring examination can be
overwhelming. Therefore, careful selection must be used in deciding
which brain area and which subcellular fraction to investigate. This
selection can be very difficult, especially when knowledge concerning
a particular compound is limited. The recent introduction of
immunochemical methods promises to facilitate this task, not by
reducing the difficulty of the selection, but by increasing the number
of chemicals that can be subjected for analysis.
Immunochemical methods have been used to identify molecules
associated with various cell types, for localizing enzymes responsible
for the synthesis of neurotransmitters and for identifying proteins
specific to the nervous system. While the procedures initially require
the preparation of antisera to highly purified proteins, once the
antisera are available, they can be sensitive tools for identifying
specific changes and for characterizing different cell populations. In
addition, monoclonal antibodies can be used to determine the mechanism
of action of toxins.
These procedures are especially valuable in studying neuronal
peptides. These peptides (e.g., substance P, encephalins, hypothalamic
releasing factors, somatostatin, etc.) play a significant role as
neuromodulators (Snyder & Inms, 1979). Many are potent at low
concentrations. Prior to the use of immunological procedures, peptide
molecules could only be examined by using bioassay techniques and
these were not always specific for particular molecular species.
Radioimmunoassay techniques are more specific. Assay kits for various
peptides are now available commercially, and their number is rapidly
increasing.
Table 4. Examples of biochemical markers of brain subcellular fractions
and cell types
Fraction Marker Reference
nuclei DNA Steele & Busch (1963)
nuclear membrane RNA polymerase Roeder & Rutter (1969)
cytosol lactate dehydrogenase Kornberg (1955)
microsomes NADPH; cytochrome c Miller & Dawson (1972)
oxidoreductase (rote
none insensitive)
Mitochondria
inner membrane D-3-hydroxybutyrate Fitzgerald et al.
NAD+ oxidoreductase (1974)
outer membrane monoamine oxidase Schnaitman et al.
(1967)
lysosomes ß-glucuronidase Fishman & Bernfeld
(1955)
ß-glucosidase Robins et al. (1968)
ß-galactosidase Robins et al. (1968)
N-arylamidase Boer (1974)
myelin 2',3'-cyclic nucleotide- Konat & Calusen (1977)
3'-phosphohydrolase
synaptosomes Na+K+-activated Verity (1972)
oubain-sensitive
ATPase
neuronal cells guanyl cyclase Goridis et al. (1974)
tyrosine hydroxylase Kuczenski & Mandell
(1972)
oligodendroglial cells glyceride galactosyl Radin et al. (1972)
transferase
Because the method is very recent, radioimmunoassay procedures are
only beginning to be use in neurotoxicology. However, using such
methods, Hong and his colleagues (Ali et al., 1982; Hong & All, 1982)
have successfully identified several peptide responses following
chlordecone treatment. The major limitation of the method for
identifying toxic compounds is the small number of purified compounds,
the small number of available RIA kits, and the expense of obtaining
radioiodinated compounds. With increasing availability of RIA kits,
the procedure could become a powerful screening tool.
6.3 DNA, RNA, and Protein Synthesis
Changes in the amount of DNA can be used to detect whether toxic
agents affect cellular proliferation and cell death. Polyploidy is
uncommon in the nervous system, thus the DNA content of brain regions
can be taken as an index of cell number. This can be related to tissue
weight and RNA or protein content to estimate cell size. For example,
Krigman & Hogan (1974) reported that lead exposure during early
development reduced brain weight and decreased total brain proteins,
but the number of brain cells was unchanged. DNA measurements have
been particularly useful in studying the toxic effects of drugs on
retinal photoreceptors (Dewar et al., 1977). It is also well-known
that the DNA content changes after nutritional restriction during
development, or after exposure to various toxic chemicals that
influence nutritional status.
Although the measurement of DNA content is relatively simple and
in the nervous system can be an index of cell number, it is probably
not a very sensitive or early indicator of neurotoxicity. For example,
neuronal loss might be accompanied by glial proliferation and produce
no change in total DNA. The earliest effects of a toxic substance on
DNA will probably involve: (a) the disturbance of DNA-repair enzymes;
(b) the intercalation of the substance into the DNA molecule; or
(c) the direct binding of the substance to the nucleic acid moiety or
its associated chromosomal proteins. Two to three percent of added
mercuric chloride was reported to bind to the chromatin in cultured
glial cells (Ramanujam et al., 1970). Since this mode of action will
ultimately interfere with the functional integrity of the CNS, it
offers great promise for the identification of toxic compounds.
The total amount of RNA or protein could also be examined after
toxic insult. However, neither total RNA nor total protein is likely
to provide a sensitive index of toxic damage. Only in the extreme
stages of neurotoxicity will total or even regional levels of these
macromolecules be likely to change. More sensitive indices of their
metabolism rely on estimates of synthesis or degradation.
The rate of RNA or protein synthesis can be evaluated by using
radiolabelled precursors and measuring their rate of incorporation
into the macromolecule in vitro or in vivo. Although this
technique is simple and sensitive, it assumes that the rate of
incorporation of the precursor directly reflects the rate of
macromolecular synthesis and this may not always be true (Dunn, 1977).
Changes in labelling could also occur as a result of changes in
cerebral blood flow, precursor transport, precursor pool size, energy
charge, etc. Inhibition of amino acid transport across the blood-brain
barrier has been reported for mercury (Partridge, 1976) and lead
(Lorenzo & Gerwitz, 1977). Thus, it is also important to consider
whether a toxic agent can cause changes in the amino acid or nucleic-
acid-precursor pool sizes by using one of several methods available
for compensating for precursor pool fluctations (Munro et al., 1964;
Dunlop et al., 1975; Dunn, 1975). Changes in brain-protein synthesis
have been reported after exposure to methylmercury (Verity et al.,
1977), and carbon disulfide (Savolaiene & Jarvisalo, 1977). However,
in many cases, only the absolute levels of incorporation of
radioactive amino acids into protein fractions were studied and it is
difficult to understand the primary mechanisms of action.
Although these relatively crude methods are subject to several
criticisms, all the variations that influence the incorporation of the
radioactive precursor can ultimately influence cellular metabolism.
These methods can, therefore, be useful for identifying metabolic
disturbances. The relative amount of protein synthesis can also be
estimated simply by measuring the polyribosome to free ribosome pool.
This method is based on the assumption that during high rates of
protein synthesis, the greatest proportion of the total ribosomes will
be attached to an mRNA molecule. The relative polyribosome to monosome
ratio has the advantage of allowing for normalization across animals
and therefore reducing the interanimal variability that often plagues
neurochemical assessments. Furthermore, the use of this method would
detect toxic effects not only on protein synthesis but on the
stability or synthesis of the ribosome itself.
The rate of RNA and protein synthesis in the brain is very high
and nerve cell functioning is dependent on protein metabolism. The
turnover rates of brain macromolecules may vary considerably, and it
is always going to be difficult to interpret any data dealing with the
turnover of total RNA or protein. However, with the availability of
newer separation methods for proteins (detergent gel electrophoresis
combined with chromatofocusing cellulose ion exchange chromatography,
etc.), soluble and bound polypeptides can be separated to study the
synthesis of individual proteins at the subcellular level, in specific
cell types and in localized brain regions. Large classes of RNA can be
analysed by hybridization procedures or specific mRNA molecules could
be targeted for investigation by using specific cDNA probes.
6.4 Lipids, Glycolipids, and Glycoproteins
Complex lipids, glycolipids, and glycoproteins have several
important functions in neural tissue (Zuber, 1978). They constitute
structural elements of the plasma membrane, act as components of ion
channels, comprise portions of neurotransmitter receptors, and are
major constituents of myelin. Complex lipids (e.g., phospholipids,
cerebrosides, sulfatides, and gangliosides) make up neuronal
membranes. Phosphatides (phosphatidylethanolamine, phosphatidyl-
choline, phosphatidylinositol, phosphatidylserine) are the most
important group of phospholipids. They consist of some of the most
metabolically active of the phospholipids (Prokhorova, 1974) and
participate in the movement of Na and K ions through the neural
membrane. A closely related group of phospholipids, the plasmalogens,
are concentrated in myelin and constitute 18-30% of total brain
phospholipids.
Ganliosides are localized primarily in the plasma membrane
(Karpova et al., 1978), but small amounts of monosialoganglioside are
detectable in myelin. Receptors of neurotransmitters, such as
serotonin, and other biologically-active compounds may contain
ganglioside (Avrona, 1971), and evidence suggests that gangliosides
may bind biologically-active compounds, as well as various
neurotoxins, through their terminal N-acetylneuraminic acid moiety
(Kryzhavosky, 1973). Glycosaminoglycans are also functional and
structural elements of the neuronal membrane and have been reported to
exhibit disturbed metabolism in various hereditary diseases of the
mucopolysaccharidosis type (Constantopoulos et al., 1976; Vasan &
Chase, 1976).
The most interesting of the functionally-active, minor components
of neuronal membranes are sialic acids, and particularly
N-acetylneuraminic acid. As sialo-glycoproteins and gangliosides,
N-acetylneuraminic acid participates in carrying out specific
neuronal functions such as the establishment of synaptic contact
neurotransmission, and axonal propagation (Partingron & Daly, 1979).
Gangliosides and sialo-glycoproteins bind Ca++ at their hydrophilic
ends and thus, by influencing the concentration of this ion, affect
depolarization and neurotransmitter release.
Myelin metabolism may be affected by a variety of toxic agents
(Cammer, 1980). After administration of these compounds, there is
often a delay before the appearance of neurotoxic signs and symptoms
such as the ataxia indicative of peripheral demyelination. Central
demyelination often occurs to a lesser extent. Compounds that cause
disturbances in myelin metabolism may exert their effect directly on
myelin-forming cells, or demyelination may be a secondary response to
a disturbance of neuronal metabolism.
Several compounds have been reported to alter complex lipids or
their metabolism. Neonatal exposure to lead has been reported to
decrease the total brain content of phospholipids, galactolipids,
plasmalogens, and cholesterol (Van Gelder, 1978). Hydrogen sulfide and
sulfur dioxide decrease total brain lipids and/or phospholipids
(Haider et al., 1980). Meta-SystoxR (phosphorothioic acid
O-[2-(ethylthio)ethyl] O,O-dimethyl ester mixture with
S-[2-(ethylthio)ethyl] O,O-dimethyl phosphorothioate)
(O,O-dimethyl-S-2 (ethylsulfinyl) ethylthiophosphate), an
organophosphorus pesticide, has been reported to decrease brain levels
of total lipids, phospholipids, cholesterol, esterfied fatty acids,
and gangliosides (Islam et al., 1983). Merkuryva et al. (1978) found
one of the early affects of carbon disulfide intoxication to be
changes in the enzyme substrate system of N-acetylneuraminic acid,
N-acetylneuraminate lyase. In the olfactory bulb of the rabbit,
carbon disulfide led to an accumulation of N-acetylneuraminic acid
because of a reduced degradation by ß-acetylneuraminate lyase. In some
cases, the carbon disulfide-induced disturbance of the metabolism of
cerebral glycoconjugates could be correlated with the changing of
physiological parameters (e.g., lowering of the amplitude of the
cortical evoked potential (Bokina et al., 1976)). Long-term exposure
to lead acetate in aging rats was reported to decrease
N-acetylneuraminic acid content in cerebral tissue and also to
decrease the activity of the degradative enzyme (Merkuryva &
Bushinskaya 1982). Long-term exposure to ethanol led to an
accumulation of N-acetylneuraminic acid in the subfornical region of
the hypothalamus and in the midbrain reticular formation (Merkuryva et
al., 1980).
A recently applied approach to the study of neurotoxicology
involves the investigation of lipid peroxidation. Peroxidation
involves the direct reaction of oxygen and lipids to form free radical
intermediates and semistable peroxides. Lipid peroxidation is damaging
because of the subsequent reactivity of these free radicals (Tappel,
1970). Biomembranes and subcellular organelles are the major cellular
components damaged by lipid peroxidation. Increased lipid peroxidation
has been reported after exposure to thallium, nickel, or cobalt (Hasan
& Ali, 1981). Since the estimate of lipid perioxidation is a
relatively simple technique, this method may prove to be an effective
tool for screening for neurotoxic compounds.
6.5 Neurotransmitters
Chemical synaptic transmission involves a complex series of events
(synthesis and storage of neurotransmitters, release of neurotrans-
mitters, re-uptake or degradation of transmitters, interaction of
transmitters with postsynaptic membrane), any or all of which could be
disturbed by neurotoxins. In addition to the classical neurotrans-
mitters (Table 5), a number of peptides have been discovered, which
may act as chemical messengers (Burgen et al., 1980). Most
toxicological studies have focused on the neurotransmitters listed in
Table 5, because considerable background information on these
neurotransmitters is already available. Nearly every class of neuron
contains some marker enzyme (Table 5), and these have been useful in
studies in which attempts have been made to identify neurotransmitter
specific cells and in the determination of the regional distribution
of neurotransmitter systems.
Table 5. Enzyme markers for neurotransmitter specific cells
Neurotransmitter Marker enzyme Receptor types
acetylcholine choline acetyltransferase mucarinic, nicotinic
dopamine tyrosine hydroxylase DA1 and DA2
noradrenaline dopamine-ß-hydroxylase alpha1, alpha2, ß1,
ß2 adrenoreceptors
GABA(gamma- glutamate decarboxylase GABAA and GABAB
aminobutyric acid) (strychnine-insensitive;
picrotoxin-sensitive)
glycine unknown strychnine-sensitive
serotonin 1-amino acid decarboxylase 5 HT1, 5 HT2
6.5.1 Synthesis/degradation
Many neurotoxic compounds have been reported to alter steady-state
levels of neurotransmitters, but: it is difficult to draw functional
inferences from such data since the steady-state levels of
neurotransmitters can be influenced via multiple mechanisms (e.g.,
rate of synthesis, rate of release, rate of degradation). More
informative data are obtained by examination of the turnover rates,
reflecting the metabolic half-life of the neurotransmitter (Costa,
1970). Turnover of 5-HT, after disulfiram treatment, was examined by
observing the accumulation of 5-HT following administration of
pargyline (Minegishi et al., 1979). It has been speculated that carbon
disulfide acts by altering brain catecholamine concentrations. Carbon
disulfide increased DA and decreased NE by inhibiting dopamine
ß-hydroxylase, the enzyme responsible for converting dopamine (DA)
into norepinephrine (NE) (McKenna & DiStefano, 1975). Manganese is
known to inhibit DA formation by blocking tyrosine hydroxylase in the
striatum (Bonilla, 1980) and many acute effects of organophosphorus
compounds are related to inhibition of AChE activity (O'Brien, 1976).
Soon after their introduction, the inhibition of AChE was accepted as
a mechanism for the acute toxic effects of organophosphorus
insecticides (DuBois et al., 1949). However, this mechanism does not
account for the delayed neurotoxicity seen after organophosphate
poisoning (Abou-Donia & Preissig, 1976; Abou-Donia, 1981).
Interference with axonal transport may also disrupt
neurotransmitter function by altering the availability of
neurotransmitter enzymes or by decreasing the transport of peptide
precursors. The transport of materials along axons is bidirectional:
anterograde and retrograde. Anterograde transport conveys materials
from cell body to axon and terminals at slow (1-3 mm per day) and fast
(410 mm per day) rates (Ochs, 1972; Hoffman & Lasek, 1975). The former
contains the bulk of axoplasmic proteins (notably of neurofilaments
and neurotubules) and the latter, membrane and other components.
Retrograde transport, a system that conveys materials at rapid rates
(300 mm per day) may also be affected early in toxic axonopathies.
Retrograde transport may be a critical factor in the toxicity
resulting from exposure to various compounds.
6.5.2 Transport/release
Neurotransmitters or their precursors can rapidly concentrate in
nerve endings through specific high-affinity uptake systems associated
with each transmitter-specific class of neurons (Iverson, 1971). The
high-affinity, Na+-dependent transport mechanisms are specific to
nerve cells and can be distinguished from the ubiquitous low-affinity
transport systems that concentrate a large variety of amino acids,
sugars, and nucleosides, or their precursors. High-affinity uptake
phenomena may be studied in brain homogenates, brain minces, or
synaptosomal preparations. Lead (Silbergeld & Goldberg, 1975), the
insecticide chlordecone (Chang-tsui & Ho, 1979), and erythrosin B
(Lafferman & Silbergeld, 1979) have been reported to interfere with
neurotransmitter uptake processes. However, a variety of nonspecific
events may influence apparent uptake, so caution must be exercised in
interpreting results of these studies. For example, the effects of
erythrosin B on uptake mechanisms were reported to be non-specific
(Mailman et al., 1980).
The release of neurotransmitters from synaptic endings occurs
through an exocytotic process that is triggered by an influx of
calcium ions on depolarization of the nerve endings (Cotman et al.,
1976). Neurotransmitter release can be measured by preloading nerve
endings with labelled neurotransmitters, exposing tissue slices or
synaptosomal fractions maintained on filter beds to calcium ions, and
measuring the appearance of labelled or unlabelled compounds in the
supernatant medium (Bondy & Harrington, 1979). Heavy metals such as
lead have been reported to interfere with calcium-dependent
neurotransmitter release (Bondy et al., 1979; Ramsay et al., 1980),
and manganese has been reported to block transmitter release (Kirpekar
et al., 1970; Balnave & Gage, 1973; Kostial et al., 1974), possibly by
blocking inward Ca2+ current (Baker et al., 1971). Again, caution
must be used in comparing the results from different assay systems.
6.5.3 Binding
Binding of neurotransmitters to specific membrane receptors is the
first step in a complex series of events initiated in the
post-synaptic cell. Such binding interactions are reversible,
stereospecific, nonenzymatic, have equilibria with low dissociation
contants, and involve configurational recognition (Yamamura et al,
1981). Studies on receptor binding have been made possible by the
availability of specific radioactive analogues of neurotransmitters.
In general, the most potent binding ligands are the pharmacological
antagonists or agonists of a given neurotransmitter, rather than the
transmitter itself. Kinetic characteristics of binding and receptor
density are estimated by incubating receptor-enriched membranes with
radioactive ligands. Excess ligands, not bound to membranes, can be
removed by filtration or centrifugation. Non-specific binding of the
labelled compound is estimated by repeating the incubation in the
presence of a high concentration (10-4 to 10-6 mol) of an
unlabelled analogue. The solubility of ligands either in lipids or in
water has to be considered when interpreting results of ligand-binding
studies. This method is relatively simple and has been suggested as a
useful screening procedure for the detection of neurotoxic compounds
(Damstra & Bondy, 1980, 1982). If properly employed, such analyses can
provide valuable information. However, interpretation of the data can
be complicated by the often low degree of specificity of the ligand
and the location of the receptor, which may be pre- or post-synaptic
or on non-neuronal glial elements. Furthermore, for many
neurotransmitters, several subtypes of receptors exist and may produce
different effects on the post-synaptic membrane. Functional
extrapolations from neurotransmitter binding studies, therefore,
should be made with caution.
Receptor binding techniques have only recently been applied to
neurotoxicological studies. However, in a short time, many compounds
have been reported to increase or decrease estimates of receptor
density. Acrylamide (Bondy et al., 1981), chlordecone (Seth et al.,
1981), lead and mercury (Bondy & Agrawal, 1980), and cadmium (Hedlund
et al., 1979) have all been reported to alter putative
neurotransmitter receptors.
6.5.4 Ion channels
Many neuronal functions are regulated by ion channels. The Na+
channel is responsible for depolarizing the membrane, and K+
channels are responsible for repolarizing the membrane. Maintenance of
appropriate Na+ and K+ concentrations requires the classical
Na+/K+ ATPase. Disturbed functioning of these ion channels or
disturbance of ATP availability can severely disturb neuronal
functioning. Several naturally-occurring toxins affect Na+ channels
(Hille, 1976; Ritchie, 1979; Catterall, 1977). Tetrodotoxin and
saxitoxin inactivate the channel at nanomolar concentrations and can
be used as probes for measuring channel density or isolation of
channel polypeptides (Agnew et al., 1978). Batrachotoxin and
veratridine bind the channels and produce persistent activation by
preventing channel inactivation (Albuquerque & Daly, 1976). Peptide
toxins, such as scorpion toxin (Couraud et al., 1978), exhibit either
voltage sensitive or insensitive binding to the extracellular surface
of the Na+ channel (Catterall, 1977). Several potassium channels
probably exist in neurons (Reichardt & Kelly, 1983), but purification
and characterization of these channels has lagged behind that of the
Na+ channel. However, a scorpion toxin that exibited K+ channel
affinity has been described (Carbone et al., 1982).
Na+/K+-ATPase consists of 2 polypeptide chains. The smaller
polypeptide is a glycoprotein (Carilli et al., 1982). The larger
polypeptide binds ATP internally and ouabain externally. Hormones and
neurotransmitters such as catecholamines regulate the ATPase (Clausen
& Flatman, 1977), and neurotoxicants could alter neuronal functioning
by disturbing this control of ionic gradients. Desaiah et al. (1980)
have reported inhibition of Na+/K+-ATPase and decreased ouabain
binding to synaptosomes after treatment of mice with the insecticide
chlordecone. The evaluation of ion balance and their control will
probably be used increasingly in neurotoxicology.
For the most part, transmitter release is regulated by Ca2+
entry through a Ca2+-selective channels. Depolarization,
neurotransmitters, and hormones regulate Ca2+-selective channel
(Reichardt & Kelly, 1983). Nerve terminals contain several Ca2+
calmodulin-dependent protein kinases and a Ca2+-phospholipid
activated kinase, each of which has a distinct set of protein
substrates. Cytoplasmic Ca2+ binds calmodulin and other Ca2+
binding proteins, which directly or indirectly activate other enzymes
via their Ca2+-dependent kinases. How such activation facilitates
exocytosis is not known, but presumably the phosphorylation state of
synapsin I, a phosphoprotein present primarily in nerve terminals and
associated with synaptic vesicles, is believed to regulate transmitter
vesicle release (Dolphin & Greengard, 1981).
Changes in Ca2+ concentration induced by neurotoxicants could
have significant consequences for neuronal function. Cytoplasmic
Ca2+ stimulates not only exocytosis, but also glucogenolysis and
mitochondrial respiration (Landowne & Ritchie, 1976), endocytosis
(Ceccarelli & Hurlbut, 1980), and neurotransmitter synthesis (Collier
& Ilson, 1977). Even though many of these events are homeostatic
responses to transmitter utilization, neurotoxic disruption of calcium
influx or sequestering could produce disturbances not restricted to
neurotransmitter release.
Few neurotoxic compounds have been investigated for their
influence on calcium channels. Manganese is reported to block
neurotransmitter release by blocking inward Ca2+ current (Kirpekar
et al., 1970), and other heavy metals have been reported to interfere
with calcium-mediated neurotransmitter release (Bondy et al., 1979;
Ramsay et al., 1980). Similar effects of heavy metals were also
observed for the Na+-Ca2+ exchange system. Ion channels and
Na+-Ca2+ probes may be a valuable screening approach for some
types of neurotoxic compounds.
6.5.5 Cyclic nucleotides
Nerve terminal function and the effects of neurotransmitters are
often regulated by cyclic nucleotides. Binding of agonists to
receptors on the nerve membrane can result in activation of second
messenger systems. Activation of different receptors results in
specific changes in cyclic AMP and cyclic GMP levels and consequent
alterations in protein kinases. It is thought that on phosphorylation,
conformational changes occur in membrane proteins that may change ion
permeabilities. Thus, the responsiveness of the nervous system to some
toxic agents can also be measured by determining changes in the
activities of adenylate cyclase, cyclic nucleotides, and protein
phosphorylation. For example, lead has been shown to inhibit
cerebellar adenylate cyclase (Nathanson & Bloom, 1975) and
dopamine-sensitive adenylate cyclase in the striatum (Wilson, 1982).
Various pesticides cause increases in the levels of cyclic nucleotides
in brain tissue (Aldridge et al., 1978). For example, tri-o-cresyl
phosphate enhanced Ca2+-calmodulin-dependent in vivo
phosphorylation of proteins in chicken brain (Patton et al., 1983).
6.5.6 Summary of nerve terminal function
The analysis of synaptic function and neurotransmitter metabolism
is reasonably complex, and it should be borne in mind that disturbance
at many levels of neuronal organization will ultimately alter synaptic
function. However, examination of the nerve terminal is an excellent
approach for the initial study of toxic chemicals. However, it is
important to recognize the dynamic nature of the nerve terminal and to
remain alert to the possibility of false positives.
6.6 Energy Metabolism
Nervous tissue, and brain tissue in particular, require
disproportionately large amounts of energy to sustain the
translocation of ions important for electrical activity and to
maintain the highly-active biosynthetic machinery of the tissue.
Since neural tissue has only limited stores of energy (e.g., glycogen
and creatine phosphate), glucose availability and enzymes critical for
energy production are vital to neuronal functioning. A number of
neurotoxic chemicals have been shown to interfere with glucose and
energy metabolism in both the central and peripheral nervous system.
These include methylmercury (Bull & Lutkenhoff, 1975), hexachlorophene
(Cammer & Moore, 1972), organotin compounds (Lock, 1976), and alcohol
(Merkuryva et al., 1980). Glycolytic enzymes have been shown to be
inhibited by alcohol (Merkuryva et al., 1980) and by chemicals known
to cause distal axonopathy, e.g., acrylamide (Howland et al., 1980)
carbon disulfide, and methyl-n-butyl ketone (Sabri et al., 1979).
Active brain areas have a higher rate of glucose consumption than
less active regions. The brain regional metabolic activity is
determined by the 14C-2-deoxyglucose method (2-DG) of Sokoloff et
al. (1977). This method is based on the use of 2-deoxy-D-14C-glucose
as a tracer for the exchange of glucose between the plasma and the
blood and its phosphorylation by hexokinase. The product,
2-deoxyglucose-6-phosphate (2-DG-P), is trapped in the tissue and its
accumulation in the various regions of the brain might be a measure of
the glucose use and neuronal activity. The method provides an index of
the in vivo glucose metabolic activity of brain regions and has been
used in experimental animals to determine which brain regions are
depressed or activated during acute Soman intoxication (McDonough et
al., 1983).
Metabolic compartmentalization in nerve tissue could provide an
additional tool in toxicological studies. It is well established that
neurons mainly use glucose in their energy metabolism, while glia use
substrates other than glucose (Hertz, 1981). Thus, it may be possible
to differentiate between the toxicological effects of chemical
substances on neuronal and glial populations in vitro or in vivo.
6.7 Biochemical Correlates of Axonal Degeneration
A relatively large class of neurotoxic agents is known to produce
axonal degeneration (Griffin & Price, 1980; Sabri & Spencer, 1980;
Thomas, 1980). Some, such as acrylamide and organophosphorus
compounds, produce the distal to proximal "dying back" pattern of
degeneration, while others, such as ß,ß-iminodipropionitrile, lead to
proximal to distal degeneration. Since many of these compounds produce
Wallerian degeneration, biochemical correlates of Wallerian
degeneration have been suggested as toxicological indices (Dewar &
Moffett, 1979). These authors tabulated the chemical changes in
peripheral nerves that occurred during degeneration and suggested that
ß-gluocorinadase and ß-galactosidase might be good indices of
toxicity. Acrylamide, methylmercury, and dimethyl sulfoxide were shown
to increase these lysosomal enzymes (Dewar & Moffett, 1979). The use
of lysosomal enzymes involves relatively simple procedures and might,
therefore, be appropriate for the initiation of a biochemical screen.
Disturbance of axoplasmic transport has been suggested as an
alternative approach to detecting compounds producing axonal
degeneration. Several substances, e.g., organophosphates (Reichart &
About-Donia, 1980) and acrylamide (Pleasure et al., 1969) disrupt
axonal transport. However, these methods are more difficult, more
expensive, and more time-consuming than the lysosomal enzyme assays.
6.8 Neuroendocrine Assessments
The number of toxic compounds being recognized for their
neuroendocrine actions is increasing. Hormonal balance results from
the integrated action of the hypothalamus, pituitary, and endocrine
target organ. Each site is susceptible to disruption by environmental
toxic agents. This disruption may result from the direct interaction
of the toxic agent with the endocrine organ, pituitary, or
hypothalamus. Alternatively, neuroendocrine dysfunction may occur
because of a disturbance in the regulation and/or modulatory elements
of the complex neuroendocrine feedback systems. Any such disturbances
would ultimately modify anterior pituitary secretions. Thus, the
analysis of blood levels is the most appropriate starting point
(section 6.8.2.1).
6.8.1 Anterior pituitary hormones
The main effector organ in the neuroendocrine system is the
pituitary. This "master gland" consists of an anterior
adenohypophysis, and a posterior neurohypophysis. Based on
histological, immunocytochemical, and electron microscopic examination,
the following cell types are differentiated in the anterior pituitary
(Junqueira et al., 1977); follicular cells, which do not contain any
secretory granules but form the support stroma of the glandular cells;
chromophobe cells, which are undifferentiated, nonsecretory and
secretory cells without discernible granules in the light microscope;
somatotropic cells, which are acidophilic with immunocytochemically-
detectable growth hormone and contain granules of about 350 nm
diameter; prolactin cells, which are acidophilic and contain prolactin
granules of 600-900 nm diameter; gonadotropic cells, which consist of
2 basophilic subgroups: follicle-stimulating hormone secreting and
luteinizing-hormone secreting; follicle-stimulating hormone secreting
cells, which are large round cells with dense 200 nm diameter
granules; luteinizing hormone-containing cells, which are small and
contain 200-250 nm diameter granules; thyrotropic cells, which are
basophilic with thyroid-stimulating hormone granules of 120-200 nm
diameter; and finally, cortico cells, which are the least abundant of
the cell types and contain basophilic granules 100-200 nm in diameter.
The glandular cells of the anterior pituitary secrete seven
endocrine hormones, of which four act directly on other endocrine
glands. Follicle-stimulating hormone (FSH) promotes spermatogenesis in
the male testes and facilitates follicular maturation and estrogen
secretion in the female ovary. Lutenizing hormone (LH) acts on the
male testes to facilitate testosterone secretion from the interstitial
cells of Leydig and is a key hormone that promotes follicular rupture
(ovulation) in the female ovary. Thyroid-stimulating hormone (TSH)
triggers the secretion of thyroxine from the thyroid gland, and
adrenocorticotropic hormone (ACTH) causes the adrenal cortex to
secrete its products, especially glucocorticoids. The secretion of
these tropic, anterior pituitary hormones is in turn regulated by the
hormones of the endocrine gland. In most cases, this is a negative
feedback regulation. Estrogen decreases the output of pituitary FSH
and LH; thyroxine decreases the secretion of TSH; and glucocorticoids
reduce the output of ACTH.
The three other anterior pituitary hormones are prolactin (PRL),
melanocyte-stimulating hormone (MSH), and growth hormone (GH). A major
target for prolactin is the mammary gland, where prolactin promotes
milk production. PRL also plays a role in maintaining the ovarian
corpus luteum after ovulation. The entire body is a target for GH,
which promotes growth and maintenance of cellular integrity. GH
facilitates growth, in part, because it increases the transport of
amino acids into the cell, where they can be used for protein
synthesis. MSH increases the production of melanin by the melanocytes
of the skin.
Hypothalamic control of anterior pituitary secretions occurs
through the release of hypothalamic-hypophysiotropic hormones (HHH).
The hypothalmus secretes these HHH into vessels of the hypothalamic-
hypophyseal portal system, by which they are transported to the
anterior pituitary. One of the major advances of the last two decades
has been the isolation and characterization of these HHH.
6.8.2 Disruption of neuroendocrine function
Disruption of neuroendocrine function can occur at any one or all
of the levels of hypothalamic-pituitary-target organ integration.
Alternatively, neuroendocrine effects may result from modification of
"higher" levels of neuronal processing or from alterations in
peripheral metabolism. In the following discussion, approaches will be
indicated for the study of each of these possible sites of neurotoxic
action.
6.8.2.1 Direct pituitary effects
At the most dramatic level, massive changes in the weight or size
of the pituitary, such as occur in the adenohypophysis after large
doses of estrogen (Schreiber & Pribyl, 1980) might be seen. However,
by far the most accessible method for studying pituitary function
relies on the examination of blood levels of pituitary hormones by
radioimmunoassay. Furthermore, the analysis of blood-hormone levels is
one of the few techniques applicable for screening human populations.
A large number of toxic substances have been reported to modify
adenohypophyseal secretions. Methallibure and allied substances
inhibit thyrotropin (Tulloch et al., 1963), and prolactin (Benson &
Zagni, 1965) secretion. Modifications of pituitary hormone secretions
have been reported for acrylamide (Uphouse et al., 1982), alcohol
(Mendelson et al., 1977), 1,4-DDT (Gellert et al., 1972), and
chlordecone (Uphouse et al., 1984). Dimethylsulfoxide may influence
adrenal glucocorticoids by elevating pituitary secretions (Allen &
Allen, 1975). In spite of the fact that changes in the blood levels of
pituitary hormones may be the most sensitive index of neuroendocrine
modification by toxic compounds, they have been examined for
relatively few compounds. The high sensitivity of this approach
results from the fact that the blood levels represent the final
consequence of the complex neuroendocrine integration. Regardless of
the actual site of modification, neuroendocrine disturbances will
usually be revealed in modified levels of circulating hormones.
Therefore, such changes, alone, cannot be regarded as evidence of
direct neuroendocrine disruption. The major disadvantage of serum-
hormone measurements is the responsiveness of the hypothalamic-
pituitary axis to a variety of environmental and chemical stimuli. It
can be difficult to identify the toxic agent as the causal agent.
Because hormones are secreted in a pulsatile manner, single point
analyses of serum hormones must be cautiously interpreted. However,
even with these limitations, analysis of blood levels of pituitary
hormones remains the most appropriate starting point for evaluating
potential neuroendocrine toxicity.
Similar analytical methods may be used to investigate the
pituitary content of respective pituitary secretions. The pituitary is
removed, homogenized, and extracted for use with the appropriate RIA.
For most pituitary secretions, neurotoxicologists have not yet studied
pituitary tissue directly. However, pituitary endorphins has been
reported to be modified by chlordecone (Hong & All, 1982).
6.8.2.2 Peripheral target effects
Most anterior pituitary hormones are subject to negative feedback
control by peripheral endocrine glands. If the neurotoxic agent
modifies peripheral secretions, neuroendocrine changes can result from
this altered feedback. Modifications in the functioning of these
endocrine secretions could occur after toxic exposure. Such approaches
have been widely applied in the experimental and clinical literature
and have shown that a number of compounds alter blood levels of
glucocorticoids, thyroxine, estrogen, and testosterones (Chapman,
1983).
Target-tissue effects can also be evaluated by a variety of
additional techniques. One of the simplest assessments is the change
in the weight, morphology, or biochemistry of the target organ.
Various environmental contaminants have been reported to produce such
changes. Lesions of the thyroid have been reported after exposure to
carbon disulfide (Cavalleri, 1975) and strong magnetic fields
(Persinger et al., 1978). Marked changes occur in the adrenals during
antimony and lead poisoning (Minkins et al., 1973) and in response to
very long chain saturated fatty acids (Powers et al., 1980).
Chlordecone causes adrenal, cortical, and medullary hypertrophy
(Eroschenko & Wilson, 1975) and alters the epinephrine and
norephinephrine content of the adrenal medulla (Baggett et al., 1980).
DDT (McBlain et al., 1976) and other chlorinated hydrocarbons
(Dicksith & Datta, 1972; Fellegiova et al., 1977), carbon disulfide
(Rosewickyi et al., 1973), and cadmium salts (Parizek, 1956;
Zylber-Haran et al., 1982) have all been described as having toxic
effects on the gonads. Although such changes are not necessarily due
to direct neuroendocrine effects, target organ changes can often be a
first indication of neuroendocrine changes. In some cases, even
relatively gross target organ events have been a first clue towards
the mode of action of a neurotoxic agent. For example, the chlorinated
pesticides, DDT and chlordecone, modify gonads in a manner reminiscent
of the natural steroid, estrogen (Gellert et al., 1972; Eroschenko &
Palmiter, 1980; Kupfer & Bulger, 1980). Uterine and/or oviduct weight
changes in the immature animal were the first evidence that the
pesticides might exert estrogenic action.
6.8.2.3 Disruption of hypothalamic control of pituitary secretions
Hypothalamic control of anterior pituitary secretions includes
direct regulation through hypothalamic hypophysiotropic hormones and
modulation through neurotransmitters and neurally-active peptides.
Evaluation of the effect of a neurotoxic agent on HHH is directly
measureable only for the HHH for which antisera are available. For
these compounds, it is possible (though difficult) to measure release
into the portal blood supply, to evaluate the effects of toxic agents
on either amounts or release, and to identify pituitary responsiveness
to the hypothalamic factors. Where identified hormones are not
available, hypothalamic extracts may still be used with in vitro
pituitary responsiveness as a bioassay. Details of such methods can be
found in several sources (Burgus & Guillemin, 1970; Oliver et al.,
1974). However, the value of such approaches for neurotoxicology has
yet to, be tested. Such methods will be of greatest value in testing
hypotheses regarding the mechanism of action of known neuroendocrine
toxic agents. They are not recommended for initial screens.
Biochemical changes in the hypothalamus can also be used as
indices of potential neuroendocrine disruption. Such hypothalamic
effects of neurotoxic compounds have received much less attention than
brain areas such as the striatum, cortex, or cerebellum. Methods for
studying the hypothalamus are the same as those used for other brain
areas. However, the hypothalamic neurons that regulate pituitary
function receive numerous synaptic inputs, both conventional and from
putative neurotransmitters. Consequently, the neuroendocrine
significance of changes in hypothalamic neurotransmitters and
neuropeptides is usually only inferential. However, any disruption of
hypothalamic biochemistry has the potential to alter neuroendocrine
function. When combined with more direct measurements of
neuroendocrine function, such studies are very important. There have
been a few reports of biochemical changes in the hypothalamus and/or
pituitary, correlated with neuroendocrine toxicity. One approach has
been to examine the effects of toxic compounds on hormone-mediated
changes. Administration of estrogens is followed by hypothalamic
ascorbic acid depletion (Schreiber et al., 1982) and by increases in
polyphenol oxidase (ceruloplasmin) activity in the hypothalamus and
the blood (Schreiber & Pribyl, 1980). This can be inhibited by the
simultaneous administration of silver nitrate. Disulfiram
(tetraethylthiuram disulfide) inhibits the reaction of the
adenohypophysis (Schreiber et al., 1979). Disulfiram also inhibits
dopamine-ß-hydroxylase (Szmigielski, 1975) and lowers the
blood-prolactin level (Cavalleri et al., 1978). The effect of
disulfirams on prolactin may be due to a "sparing of dopamine" through
inhibition of dopamine-ß-hydroxylase.
Hypothalamic peptides have been studied most extensively after
chlordecone treatment. Long-term exposure to the pesticide reduced
hypothalamic ß-endorphin levels under conditions where substance P,
neurotensin, and met-enkephalin were unchanged (Ali et al., 1982).
6.8.2.4 Other sites of action
For many neurotoxic agents, there may be no, identifiable effects
on neuroendocrine function, but it may be altered through the indirect
effects of the toxic compound. Such interruption could occur via
modification of neural integration leading to an altered response of
the organism to environmental challenge. Detection of such integrative
action and its relevance to neuroendocrine function might include
treatment with the toxic agent followed by environmental,
pharmacological, or hormonal challenges known to produce a
neuroendocrine response. Several toxic agents have been reported to
produce behavioural changes, such as stress-induced analgesia, and
these undoubtedly involve some aspect of neuroendocrine function. CNS
opiate systems are important components of the CNS response to stress
and studies of peripheral and brain endorphins and encephalins are
potentially relevant to such disturbances. For example, neonatal
treatment with chlordecone dissolved in dimethyl sulfoxide produces an
elevated corticosterone response to footshock (Rosecrans et al.,
1982), and long-term exposure of female rats to chlordecone has been
reported to decrease hypothalamic ß-endorphin levels (Ali et al.,
1982).
A toxic agent could also indirectly affect neuroendocrine function
by altering the peripheral metabolism of endocrine secretions. Such
metabolic differences could indirectly influence CNS function.
However, hormone transformation and the formation of active
metabolites occurs in the CNS so that metabolic disturbances may even
have a direct effect on CNS functioning. Many metabolic variables have
been reported to change after treatment with a toxic agent.
Aromatization of testosterone to estrogen, an important step in the
metabolism of estrogen, took place in the brain of animals (Gallard et
al., 1978). Its inhibition by aminoglutethimide or other blockers of
aromatization (Morali et al., 1977) markedly altered the sexual
behaviour of experimental animals. Aminoglutethimide also inhibited
total adrenal steroidogenesis as well as gonadal steroidogenesis and
thyroid function (Schreiber et al., 1969). The transformation of
thyroxine to the more active metabolite triiodothyronine was inhibited
by a series of toxic substances such as ethanol (Shimizu et al.,
1978), propylthiouracil (Leonard & Rosenberg, 1980), iopanoic acid
(Kaplan, 1980), and sodium salicylate (Chopra et al., 1980). Since the
monodeiodination of thryoxine to triiodothyronine also takes place in
the adenohypophysis, these factors may be an important component of
neuroendocrine reactions to neurotoxic substances. There is evidence
that demonstrates that the direct dopaminergic effect of estrogen on
the pituitary may require conversion of estrogen to catechol estrogen
(Paul et al., 1980). Such conversion occurs not only in the pituitary,
but also in the hypothalamus and cerebral cortex and may mediate some
of the effects of estrogen on the CNS. Study of this enzyme promises
to be important for future research, especially for the investigation
of estrogen-like toxic agents.
A final way in which the toxic agent might disrupt neuroendocrine
function is by altering the peripheral metabolism of the endocrine
secretions within liver tissue. Although beyond the scope of this
publication, several neurotoxic agents (chlordecone, dieldrin,
heptachlor, lindane, p,p'-DDE, and toxaphene) stimulate the
metabolism of estrone by liver microsomal enzymes (Welch et al.,
1971). Biochemical approaches for identifying these metabolic
disturbances are the same as those discussed in other sections.
However, the precise metabolic end-point should be carefully chosen,
when inferences are to be made about neuroendocrine function.
6.8.3 Sex differences
Because neurotoxic agents may produce changes in neuroendocrine
function and, since males and females differ with regard to a variety
of metabolic variables, a valuable approach to any neurotoxicological
investigation is the comparison of the toxic response in the sexes.
Whenever neuroendocrine effects are suspected, sex differences should
be a routine aspect of the overall investigation. Sex-related
differences have been observed for many environmental compounds.
Often, these result from the influence of gonadal hormones on the
metabolism of the compound. Hormonal influences on the
biotransformation and toxicity of DDT (Durham et al., 1956) and several
organophosphates (Murphy & DuBois, 1958; DuBois & Puchala, 1961) have
been demonstrated. Parathion is an organophosphate insecticide that
exerts its toxicity through its active metabolite paraoxon phosphate.
Agrawal et al. (1982) have recently shown that the sex difference in
AChE inhibition after parathion treatment was not evident with
paraoxon treatment. This suggests that the sex difference was in the
rate of metabolism to the toxic product. The female's increased
sensitivity to amobarbital (Castro & Gillette, 1967) may also be due
to sex differences in hepatic metabolism. Sex differences have also
been reported for the rate of disposition of chlordiazepoxide
(Greenblatt et al., 1977; Roberts et al., 1979) and for the toxic
effects of ethylmorphine, aniline, p-nitroanisole (Nicholas &
Barron, 1932; Holck et al., 1937; Quinn et al., 1958) and
polychlorinated hydrocarbons (Lamartiniere et al., 1979).
6.9 Recommendations for Future Research
Several biochemical and neuroendocrine approaches are available
that have not yet been applied to the study of neurotoxic compounds.
These have the advantage of increasing the sensitivity of the
biochemical approach and furthering understanding of the ways in which
compounds disrupt nervous system function. For example, some enzymes
involved in neurotransmitter synthesis have been purified and used for
the production of specific antibodies (John et al., 1973). It is now
possible to use immunological titration to determine whether changes
in enzyme activity result from activity alterations or variations in
the amount of enzyme protein.
Identification of direct pituitary modification can be
accomplished by preparation of anterior pituitary cell cultures and
the measurement of hormone release in response to hypothalamic
releasing factors, neurotransmitters, or suspect toxic compounds. The
pituitary cells are removed, dispersed, and agitated with collagenase.
After resuspension in medium, the cells are plated and used for
examination. Under such culture conditions, pituitary cells respond to
releasing factors and neurotransmitter regulation (Enjalbert et al.,
1978; Drouin & Labrie, 1981). Thus, it is possible to determine
whether the toxic agent modifies the responsiveness of the pituitary
to hypothalamic control. By comparing the effects of the toxic agent
in vivo with those in vitro, direct versus indirect effects of the
compound can be identified.
Furthermore, for suspect steroid-like compounds, direct evaluation
of receptor interactions is possible. Steroid receptors modify their
target tissue by binding to intracellular receptors. Measurement of
intracellular steroid receptors involves the preparation of a high
speed cytosol (180 000 g) fraction from the appropriate target tissue.
Identification of binding is accomplished by incubating the cytosol
in vitro in the presence of radioactively-labelled hormone. Bound
label is removed from unbound by a variety of techniques distinct for
the particular receptor of interest. Specific binding is assessed by
incubating the labelled hormone in the presence of excess unlabelled
hormone as competitor. Specific binding refers to bound molecules that
are removed in the presence of this unlabelled competitor. When a
neurotoxic agent is suspected of interacting with hormone receptors,
the toxic agent may be substituted for the unlabelled competitor and
its ability to compete for binding determined. This procedure has
demonstrated competition by both chlordecone (Palmiter & Mulvihill,
1978) and o,p'-DDT (Kupfer & Bulger, 1976) for the estradiol
cytosol receptor in the uterus. Using an estradiol exchange method,
investigators have shown that these pesticides produce translocation
of the estradiol receptor to the nucleus (Hammond et al., 1979).
Employment of the exchange method necessitates the in vivo treatment
of the organism with the toxic agent. Nuclei and cytosol are prepared
from the target tissue and incubated in vitro with the
radioactively-labelled hormone. Since a major movement of cytosol
receptors into the nucleus occurs only after binding to the hormone,
the presence of the receptor in the nucleus after exposure to the
toxic agent suggests direct binding of the toxic agent to the steroid
cytosol receptor.
Modifications of the procedures described for neurotransmitter
receptor binding are used in the measurement of membrane receptors.
However, no neurotoxic agents have yet been tested for their ability
to modify these hormone receptors. For both intracellular and
extracellular hormone receptors, most studies have been on peripheral
tissues. However, the brain and the pituitary contain receptors for a
variety of steroid and peptide hormones, and these offer a number of
targets for disruption by environmental compounds. Such analyses have
great potential for future studies of neurotoxic compounds. However,
they will usually be applied for the investigation of mechanisms of
action rather than as screens for neurotoxic compounds.
Finally, hypothalamic regulation of pituitary function should
receive further emphasis. Neurotransmitters also regulate pituitary
secretions via neurotransmitter receptors of the pituitary gland. The
potential for a direct modification of these receptor interactions by
neurotoxic compounds is only just being realized. The most thoroughly
described pituitary neurotransmitter receptor is that of dopamine,
which regulates the inhibition of prolactin release by dopamine.
Steroid hormones, such as estrogen, by conversion to catechol
estrogens, increase prolactin secretion by direct interaction with the
pituitary dopamine receptors (Gudelsky et al., 1981; Fishman, 1982).
Neuroleptic drugs elevate prolactin secretion by antagonistic action
on pituitary dopamine receptors (Horowski & Graf, 1979; Besser et al.,
1980).
7. CONCLUSIONS AND RECOMMENDATIONS
There is ample evidence of real and potential hazards of
environmental chemicals for nervous system function. Changes or
disturbances in central nervous function, many times manifest by vague
complaints and alterations in behaviour, reflect on the quality of
life; however, they have not yet received attention.
Neurotoxicological assessment is therefore an important area for
toxicological research.
It has become evident, particularly in the last decade, that
low-level exposure to certain toxic agents can produce deleterious
neural effects that may be discovered only when appropriate procedures
are used. While there are still episodes of large-scale poisoning,
concern has shifted to the more subtle deficits that reduce
functioning of the nervous system in less obvious, but still important
ways, so that intelligence, memory, emotion, and other complex neural
functions are affected.
Information on neurobehaviour, neurochemistry, neurophysiology,
neuroendocrinology, and neuropathology is vital for understanding the
mechanisms of neurotoxicity. One of the major objectives of a
multifaceted approach to toxicological studies is to understand
effects across all levels of neural organization. Such a multifaceted
approach is necessary for confirmation that the nervous system is the
target organ for the effect. Interdisciplinary studies are also
necessary to understand the significance of any behavioural changes
observed and thus, to aid in extrapolation to human beings by
providing specific neurotoxic profiles. Concomitant measurements at
different levels of neural organization can improve the validity of
results.
The following recommendations are made on the basis of the
information contained in this book:
1. Health personnel should take into account the possible role of
exposure to chemicals whenever a patient presents with any
neurobehavioural complaint.
2. Neurotoxicological testing should be an essential consideration in
any profile developed by the agencies responsible for the control
of toxic chemicals.
3. In the determination of the potential of a chemical to produce
neurotoxic effects, a multidisciplinary strategy should be used.
4. Priority should be given to obtaining clinical and epidemiological
data when exposure occurs to chemicals suspected of being
neurotoxic.
5. Test development in preclinical neurotoxicology has evolved to the
point where interlaboratory validation of procedures using
prototypic neurotoxic agents could be attempted.
REFERENCES
ABOU-DONIA, M.B. (1981) Organophosphorus ester-induced delayed
neurotoxicity. Ann. Rev. Pharmacol. Toxicol., 21 511-548.
ABOU-DONIA, M.B. & PREISSIG, S.H. (1976) Delayed neurotoxicity of
leptophos: toxic effects on the nervous system of hens. Toxicol.
appl. Pharmacol., 35 269-282.
ABOU-DONIA, M.G., JENSEN, D.N., & LAPADULA, D.M. (1983) Neurologic
manifestations of tri-o-cresyl phosphate delayed neurotoxicity in
cats. Neurobehav. Toxicol. Teratol., 5: 431-442.
AGNEW, W.S., LEVINSON, S.R., BRABSON, J.S., & RAFTERY, M.A. (1978)
Purification of the tetrodotoxin-binding component associated with the
voltage-sensitive sodium channel from Electrophorus electricus
electroplax membranes. Proc. Natl Acad. Sci. (USA), 75: 2606-2610.
AGRAWAL, A.K., SQUIBB, R.E., & BONDY, S,C. (1981) The effects of
acrylamide treatment upon the dopamine receptor. Toxicol. appl.
Pharmacol., 58 89-99.
AGRAWAL, D.K., MISRA, D., AGARWAL, S,, SETH, P.K., & KOHLI, J.D.
(1982) Influence of sex hormones on parathion toxicity in rats;
antiacetylcholinesterase activity of parathion and paraoxon in plasma,
erythrocytes, and brain. J. Toxicol. environ. Health, 9: 451-459.
ALBURQUERQUE, E.X. & DALY, J.W. (1976) Steroidal alkaloid toxins and
ion transport in electrogenic membranes. In: Cuatracasas, P., ed. The
specificity of animal, bacterial, and plant toxins, London, Chapman
and Hall, pp. 297-338.
ALDER, S. & ZBINDEN, G. (1977) Methods for the evaluation of physical,
neuromuscular, and behavioral development in rats in early postnatal
life. In: Newbert, D., Merber, H.J., & Kwasigroch, T.E., ed. Methods
in prenatal toxicology, Stuttgart, George Thiene, pp. 175-185.
ALDRIDGE, W.M., CLOTHIER, B., FORSHAW, P., JOHNSON, M.K., PARKER,
V.H., PRICE, R.J., SKILLETER, D.N., VERSCHOYLE, P.D., & STEVENS, C.
(1978) The effect of DDT and pyrethroids cismethrin and decamethrin on
the acetylcholine and cyclic nucliotide content of rat brain.
Biochem. Pharmacol., 27: 1703-1706.
ALEU, F.P., KATZMAN, R., & TERRY, R.D. (1963) Fine structures and
electrolyte analyses of cerebral oedema induced by alkyl tin
intoxication. J. Neuropathol. exp. Neurol., 22: 403-413.
ALEXANDER, B.K. BEYERSTEIN, B.L., HADAWAY, P.F., & COAMBS, R.B.
(1981) Effect of early and later colony housing on oral ingestion of
morphine in rats. Pharmacol. Biochem. Behav., 15: 571-576.
ALFANO, D.P. & PETIT, T.L. (1981) Behavioral effects of postnatal lead
exposure. Possible relationship to hippocampal dysfunction. Behav.
neural Biol., 32: 319-333.
ALI, S.F., HONG, J.S., WILSON, W.E., LAMB, J.C., MOORE, J.A., MASON,
G.A., & BONDY, S.C. (1982) Subchronic dietary exposure of rats to
chlordecone (kepone) modifies levels of hypothalamic beta-endorphin.
Neurotoxicology, 3: 119-124.
ALLEN, J.P. & ALLEN, C.F. (1975) The effect of dimethyl sulfoxide on
hypothalamic-pituitary-adrenal functions in the rat. Ann. NY Acad.
Sci., 243: 325-336.
ALTMAN, J. & SUDARSHAN, K. (1975) Postnatal development of locomotion
in the laboratory rat. Anim. Behav., 23: 896-920.
AMIN-ZAKI, L., MAJEED, M.A., ELHASSANI, S.B., CLARKSON, T.W.,
GREENWOOD, M.R. & DOHERTY, R.A. (1979) Prenatal methylmercury
poisoning: clinical observations over five years. J. Dis. Child,
133: 172-177.
ANDRIANOV, V.V., ZAKHAROV, N.C., & FADEEV, JU.A. (1977) Microiono-
phoretic analysis of chemical properties of cortical units in freely
moving animals. Physiol. J. (USSR), 63(4): 602-605.
ANGELL, F. & WEISS, B. (1982) Operant behavior of rats exposed to lead
before or after weaning. Toxicol. appl. Pharmacol., 63: 62-71.
ANGER, W.K. (1984) Neurobehavioral testing of chemicals: impact on
recommended standards. Neurobehav. Toxicol. Teratol., 6(2): 147-153.
ANGER, W.K. & JOHNSON, B.L. (1985) Chemicals affecting behavior. In:
O'Donoghue, J.L., ed. Neurotoxicity of industrial and commercial
chemicals, Boca Raton, Florida, CRC Press.
ANGER, W.K. & SETYES, J.V. (1979) Effects of oral and intramuscular
carbaryl administration on repeated chain acquisition in monkeys.
J. Toxicol. environ. Health, 5: 793-808.
ANNAU, W. (1975) The comparative effects of carbon monoxide and
hypoxia on behaviour. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Press, pp. 105-127.
ANOKHIN, P.K. (1935) [Problem of center and periphery in contemporary
physiology of nervous system.] In: Anokhin, P.K., ed. [Problem of
centre and periphery in physiology of nervous activity], Gorkiy,
Gorkiy Publishing House, pp. 9-71 (in Russian).
ANOKHIN, P.K. (1964) [Comparative electrophysical analysis of
alterations of laility of the cortical potential components in
postnatal ontogenesis.] Fiziol. Zh. SSSR, 50: 773-778 (in Russian).
ANOKHIN, P.K. (1968) [Biology and neurophysiology of the conditioned
reflex,] Moscow, Meditsina, pp. 547 (in Russian).
ANOKHIN, P.K. (1974) The functional system as a basis of the
physiological architecture of the behavioural act. In: Anokhin, P.K.,
ed. Biology and neurophysiology of the conditioned reflex and its
role in adaptive behaviour, New York, Pergamon Press, pp. 190-254.
APFELBACH, R. & DELGADO, J.M.R. (1974) Social hierarchy in monkeys
(Macaca mulatta) modified by chlordiazepoxide hydrochloride.
Neuropharmacology, 13: 11-20.
APPEL, S.H. & DAY, E.D. (1976) Cellular and subcellular fractionation.
In: Seigel, G.L., Albers, R.W., Katzman, R., & Agranoff, B.W., ed.
Basic neurochemistry, Boston, Toronto, Little, Brown and Company,
pp. 34-57.
ARBUKHANAOVA, M.S. (1981) Metabolism of glutamic acid in the cerebral
mitochondria during cooling disease. In: Mitochondria: the
mechanisms of conjugation and regulation, Puschino, p. 76.
ASABAYEV, CH., BONCHKOVSKAYA, T.YU., & ZHEGALLO, I.G. (1972) [A study
of the reaction of the animal nervous system to the effect of
low-strength electromagnetic fields.] In: [Labour hygiene and the
biological effect of radio frequency electromagnetic fields,]
Moscow, pp. 48-49 (in Russian).
AVROVA, N.F. (1971) Brain ganglioside patterns of vertebrates.
J. Neurochem., 18: 667-674.
BAENNINGER, L.P. (1966) The reliability of dominance orders in rats.
Anim. Behav., 14: 367-371.
BAGGETT, J.MCC., THURESON-KLEIN, A., & KLEIN, R.L. (1980) Effects of
chlordecone on the adrenal medulla of the rat. Toxicol. appl.
Pharmacol., 52: 313-322.
BAKER, P.F., HODGKIN, A.L., & RIDGWAY, E.B. (1971) Depolarization and
calcium entry in giant squid axons. J. Physiol., 218: 709-775.
BAKIR, F., DAMLUJI, S.F., AMIN-ZAKI, L., MURTADHA, M., KHALIDI, A.
AL-RAWL, N.Y. TIKRITI, S. DHARIR, H.I., CLARKSON, T.W., SMITH, J.C.,
& DOHERTY, R.A. (1973) Methylmercury poisoning in Iraq: an
inter-university report. Science, 181: 230-241.
BALNAVE, R.J. & GAGE, P.W. (1973) The inhibitory effect of manganese
on transmitter release at the neuromuscular junctions of the toad.
Br. J. Pharmacol., 47: 339-352.
BANCROFT, J.D. & STEVENS, A. (1977) Theory and practice of
histological techniques, New York, Churchill Livingston.
BARKER, J.L. & MCKELVY, J.F., ed. (1983) Current methods in cellular
neurobiology: electrophysiological and optical recording techniques,
New York, John Wiley and Sons, Vol. 3.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1973) A behavioral and
pharmacological analysis of variables mediating active avoidance
behaviour in rats. J. comp. Physiol. Psychol., 82: 489-500.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1974) An analysis of the
facilitation of avoidance acquisition produced by delta-amphetamine
and scopolamine. Behav. Biol., 11: 189-203.
BASAR, E. (1980) EEG-brain dynamics. In: Relation between EEG and
brain-evoked potentials, Amsterdam, Oxford, New York, Elsevier
Science Publishers.
BAUMGARTNER, G. GAWEL, M.J. KAESER, H.E., PALLIS, C.A., CLIFFORD,
F.R., SCHAUMBURG, H.H., THOMAS, P.K., & WADIA, N.H. (1979)
Neurotoxicity of halogenated hydroxyquinolines: clinical analysis of
cases reported outside Japan. J. Neurol. Neurosurg. Psychiatr.,
42: 1073-1083.
BEARD, R.R. & GRANDSTAFF, N. (1975) Carbon monoxide and human
function. In: Weiss, B. & Laties, V., ed. Behavioural toxicology,
New York, Plenum Press, pp. 1-26.
BECK, E.C., DUSTMAN, R.E., & LEWIS, E.G. (1975) The use of the
averaged evoked potential in the evaluation of central nervous system
disorders. Int. J. Neurol., 9: 211-232.
BENESOVA, O., HORVATH, M., & MIKISKA, A. (1956) [Assessment of the
depth and duration of narcosis according to the electrophysiological
characteristics of the cerebral cortex.] Physiol. bohemoslov.,
5: 188-194 (in German).
BENNETT, S. & BOWERS, D. (1976) An introduction to multivariate
techniques for social and behavioral sciences, New York, Halsted
Press.
BENSON, C.K. & ZAGNI, P.A. (1965) The effect of 1-alpha-
methylallylthiocarbamyl-2-methylthiocarbamylhydrazine (ICI 33828) on
lactation in the rat. J. Endocr., 32: 275-285.
BERGER, H. (1929) [On the electroencephalogram in man.] Arch.
Psychiat. Nervenkr., 87: 527-550 (in German).
BERNARD, C. (1927) An introduction to the study of experimental
medicine, New York, MacMillan Inc., p. 127.
BESSER, G.M., DELITALA, G., GROSSMAN, A., STUBBS, W.A., & YEO, T.
(1980) Chlorpromazine, haloperidol, metoclopramide, and domperidone
release prolactin through dopamine antagonism at low concentrations
but paradoxically inhibit prolactin release at high concentrations.
Br. J. Pharmacol., 71: 569-573.
BHAGAT, B. & WHEELER, M. (1973) Effect of amphetamine on the swimming
endurance of rats. Neuropharmacology, 12: 1161-1165.
BIGNAMI, G., ROSIC, N., MICHALEK, H., MILOSEVIC, M., & GATTI, G.L.
(1975) Behavioral toxicity of anticholinesterase agents:
methodological, neurochemical and neuropsychological aspects. In:
Weiss, B. & Laties, V.G., ed. Behavioral toxicology, New York,
Plenum Press, pp. 155-215.
BLACKWOOD, W., MCMENEMEY, W.H., MEYER, A., NORMAN, R.M., & RUSSELL,
D.S. (1971) Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd.
BLOOM, A.S., STOOTZ, C.G., & DIERINGER, T. (1983) Pyrethroid effects
on operant responding and feeding. Neurobehav. Toxicol. Teratol.,
5: 321-324.
BOER, G.J. (1974) A microassay for arylamidase activity. Anal.
Biochem., 59: 410-411.
BOKINA, A.I., EKSLER, N.D., SEMENENKO, A.D., & MERKURYVA, R.V. (1976)
Investigation of the mechanism of action of atmospheric pollutants on
the central nervous system and comparative evaluation of methods of
study. Environ. Health Perspect., 13: 37-42.
BOKINS, A.I., KAN, T.M., BEREZINA, O.V., & PINIGINA, I.A. (1979)
[Study of complex forms of animal behavior in a hygenic evaluation of
toxic substances.] Gig. i Sanit., 7: 49-51 (in Russian).
BONASHEVSKAYA, T.I., SHAMARIN, A.A., KESAREV, V.S., & YURASOVA, O.I.
(1983) [Morphological criteria and a system of indexes for the
evaluation of neurotoxic effects produced by environmental chemical
factors (on the basis of a carbon monoxide model).] Gig. i Sanit.,
10: 31-33 (in Russian, with English summary).
BONDY, S.C. & AGRAWAL, A.K. (1980) The inhibition of cerebral high
affinity receptor sites by lead and mercury compounds. Arch.
Toxicol., 46: 249-256.
BONDY, S.C. & HARRINGTON, M.L. (1979) Calcium-dependent release of
putative neurotransmitters in the chick visual system. Neuroscience,
4: 1521-1527.
BONDY, S.C., HARRINGTON, M.L., ANDERSON, C.L., & PRASAD, K.N. (1979)
Low concentration of an organic lead compound alters transport and
release of putative neurotransmitters. Toxicol. Lett., 3: 35-41.
BONDY, S.C., TILSON, H.A., & AGRAWAL, A.K. (1981) Neurotransmitter
receptors in brains of acrylamide-treated rats. II. Effects of
extended exposure to acrylamide. Pharmacol. Biochem. Behav.,
14: 533-537.
BONILLA, E. (1980) L-tyrosine hydroxylase activity in the rat brain
after chronic oral administration of manganese chloride. Neurobehav.
Toxicol., 2: 37-41.
BONILLA, E. SALAZAR, E. VILLASMIL, J.J., & VILLALOBOS, R. (1982) The
regional distribution of manganese in the normal human brain.
Neurochem. Res., 7: 221-227.
BOYES, W.K., JENKINS, D.E., & DYER, R.S. (1985) Chlordimeform produces
contrast-dependent changes in visual-evoked potentials in hooded rats.
Exp. Neurol., 89: 391-407.
BRACELAND, F.J. (1942) Mental symptoms following carbon disulfide
absorption and intoxication. Ann. intern. Med., 16: 246-261.
BRADLEY, P.B. (1958) The central action of certain drugs in relation
to the reticular formation of the brain. In: Jasper, H.H., Proctor,
L.D., Knighton, R.S., Noshay, W.C., & Costello, R.T., ed. Reticular
formation of the brain, Boston, Toronto, Little, Brown and Company,
pp. 123-149.
BRADY, K., HERRERA, Y., & ZENICK, H. (1976) Influence of parental lead
exposure on subsequent learning ability of offspring. Pharmacol.
Biochem. Behav., 3: 561-565.
BRIERLEY, J.B. (1976) Cerebral hypoxia. In: Blackwood, W. & Corsellis,
J., ed. Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd., pp. 43-85.
BROWN, A.W., ALDRIDGE, W.N., STREET, B.W., & VERSCHOYLE, R.D. (1979)
The behavioural and neuropathological sequelae of intoxication by
trimethyltin compounds in the rat. Am. J. Pathol., 97: 59-82.
BUELKE-SAM, J. & KIMMEL, C.A. (1979) Development and standardization
of screening methods for behavioral teratology. Teratology,
20: 17-29.
BULL, R.J. & LUTKENHOFF, S.D. (1975) Changes in the metabolic
responses of brain tissue to stimulation, in vitro, produced by in
vivo administration of methylmercury. Neuropharmacology,
14: 351-359.
BURES, J. & BURESOVA, O. (1979) New methods in memory research. In:
Proceedings of the 21st Annual Meeting of the Czechoslovak
Psychopharmacological Society, Jesenik Spa, 9-14 January, 1979,
pp. 7-12.
BURESOVA, O. & BURES, J. (1982) Capacity of working memory in rats as
determined by performance on a radial maze. Behav. Processes,
7: 63-72.
BURGEN, A.S.V., KOSTERLITZ, H.W., & IVERSEN, L.L., ed. (1980)
Neuroactive peptides, London, Royal Society.
BURGUS, R. & GUILLEMIN, R. (1970) Chemistry of thyrotropin releasing
factor (TRF). In: Meites, J., ed. Hypophysiotropic hormones of the
hypothalamus: assays and chemistry, Baltimore, Maryland, Williams
and Wilkins Company, pp. 227-241.
BUSHNELL, P.J. & BOWMAN, R.E. (1979) Effects of chronic lead ingestion
on social development in infant rhesus monkeys. Neurobehav.
Toxicol., 1: 207-219.
BUTCHER, R.E. (1976) Behavioral testing as a method for assessing
risk. Environ. Health Perspect., 18: 75-81.
BUTCHER, R.E. & VORHEES, C.V. (1979) A preliminary test battery for
the investigation of the behavioral teratology of selected
psychotropic drugs. Neurobehav. Toxicol., 1(Suppl. 1): 207-212.
CABE, P.A. & TILSON, H.A. (1978) Hind limb extensor response: a method
for assessing motor dysfunction in rats. Pharmacol. Biochem. Behav.,
9: 133-136.
CAMMER, W. (1980) Toxic demyelination: biochemical studies and
hypothetical mechanisms. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 239-256.
CAMMER, W. & MOORE, C.L. (1972) The effect of hexachlorophene on the
respiration of brain and liver mitochondria. Biochem. biophys. Res.
Comm., 46: 1887-1894.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W., HAYES, C.,
STRAUB, W.E., LANDRIGAM, P.J., & LIDDLE, J.A. (1978) Epidemic kepone
poisoning in chemical workers. Am. J. Epidemiol., 17: 529.
CARBONE, E., WANKE, E., PRESTIPINO, G., POSSANI, L.D. & MAELICKE, A.
(1982) Selective blockage of voltage-dependent K+ channels by a
novel scorpion toxin. Nature (Lond.), 296: 90-91.
CARILLI, C.T., FARLEY, R.A., PERLMAN, D.M., & GANTHEY, L.C. (1982)
The active site structure of Na+- and K+- stimulated ATPase. J.
biol. Chem., 257: 5601-5606.
CASARETT, L.J. (1975) Toxicologic evaluation. In: Casarett, L.J. &
Doull, J., ed. Toxicology; the basic science of poisons, New York,
MacMillan Inc., pp. 11-25.
CASTRO, J.A. & GILLETTE, J.R. (1967) Species and sex differences in
the kinetic constants for the N-demethylation of ethyl-morphine by
liver microsomes. Biochem. biophys. Res. Comm., 28: 426-430.
CATTERALL, W.A. (1977) Activation of the action potential Na+
ionophore by neurotoxins. J. biol. Chem., 252: 8669-8676.
CAVALLERI, A. (1975) Serum thyroxine in the early diagnosis of carbon
disulfide poisoning. Arch. environ. Health, 30: 85-87.
CAVALLERI, A., POLATTI, F., & BOLIS, P.F. (1978) Acute effects of
tetramethylthiuram disulfide on serum levels of hypophyseal hormones
in humans. Scand. Work environ. Health, 4: 66-72.
CAVANAGH, J.B. (1964) The significance of the "dying-back" process in
experimental and human neurological disease. Int. Rev. exp. Pathol.,
3: 219-267.
CAVANAGH, J.B. & CHEN, F.C.-K. (1971) The effects of methyl-mercury-
dicyandiamide on the peripheral nerves and spinal cord of rats. Acta
neuropathol., 19: 208-215.
CAVANAGH, J.B. & JACOBS, J.M. (1954) Some quantitative aspects of
diphtheritic neuropathy. Br. J. exp. Pathol., 45: 309-327.
CAVANAGH, J.B., PASSINGHAM, R.J., & VOGT, J.A. (1964) Staining of
sensory and motor nerves in muscles with Sudan Black B. J. Pathol.
Bacteriol., 88: 89-92.
CECCARELLI, B. & HURLBUT, W.P. (1980) Ca-dependent recycling of
synaptic vesicles at the frog neuromuscular junction. J. cell Biol.,
87: 297-303
CHANCE, M.R.A. (1946) Aggregation as a factor influencing the toxicity
of sympathomimetic amines in mice. J. Pharmacol. exp. Ther.,
87: 214-219.
CHANG, L. (1977) Neurotoxic effects of mercury -- a review. Environ.
Res., 14: 329-373.
CHANG, L. (1980) Mercury. In: Spencer, P.S. and Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 508-526.
CHANG-TSUI, Y.H. & HO, I.K. (1979) Effects of kepone (chlordecone) on
synaptosomal gamma-aminobutryic acid uptake in the mouse.
Neurotoxicology, 1: 357-361.
CHAPMAN, R.M. (1983) Gonadal injury resulting from chemotherapy. Am.
J. ind. Med., 4: 149-161.
CHIBA, S. & ANDO, K. (1976) Effects of chronic administration of
kanamycin on conditioned suppression to auditory stimulus in rats.
Jpn. J. Pharmacol., 26: 419-426.
CHO, E.S., SPENCER, P.S., JORTNER, B.S., & SCHAUMBURG, H.H. (1980) A
single intravenous injection of doxorubicin (AdriamycinR) induces
sensory neuropathy in rats. Neurotoxicology, 1: 583-591.
CHOPRA, I.J., SOLOMON, D.J., TECO, G.N.C., & NGUYEN, A.H. (1980)
Inhibition of hepatic outer ring monodeiodination of thyroxine and
3,3',5'-triiodothyronine by sodium salicylate. Endocrinology,
106: 1728-1734.
CIOMS (1983) Safety requirements for the first use of new drugs and
diagnostic agents in man, Geneva, Council for International
Organizations of Medical Sciences.
CITOVIC, I.S. (1930) [The procedure of studying the effects of oil
products on the organism of animals.] Inst. Work Saf., 1: 92
(in Russian).
CLAUSEN, T. & FLATMAN, J.A. (1977) The effects of catecholamines on
Na-K transport and membrane potential in rat soleus muscle.
J. Physiol., 270: 383-414.
COLLIER, B. & ILSON, D. (1977) The effect of preganglionic nerve
stimulation on the accumulation of certain analogues of choline by a
sympathetic ganglion. J. Physiol., 264: 489-509.
COLLINS, R.D. (1982) Illustrated manual of neurologic diagnosis, 2nd
ed., Philadelphia, Pennsylvania, J.B. Lippincott Company.
COLOTLA, V.A. (1981) Adjunctive polydipsia as a model of alcoholism.
Neurosci. Biobehav. Rev., 5: 335-342.
COLOTLA, A., BAUTISTA, M., LORENZANA-JIMENEZ, M., & RODRIGUEZ, R.
(1979) Effects of solvents on schedule-controlled behavior.
Neurobehav. Toxicol., 1(Suppl. 1): 113-118.
CONSTANTOPOULOS, G., MCCOMB, R.D., & DECABAN, A. (1976) Neurochemistry
of the mucopolysaccharidoses: brain glycosaminoglycans in normal and
four types of mucopolysaccharidoses. J. Neurochem., 26: 901-908.
CORY-SLECHTA, D.A. & THOMPSON, T. (1979) Behavioral toxicity of
chronic postweaning lead exposure in the rat. Toxicol. appl.
Pharmacol., 47: 151-159.
CORY-SLECHTA, D.A., BISSEN, T., YOUNG, M., & THOMPSON, T. (1981)
Chronic postweaning lead exposure and response duration performance.
Toxicol. appl. Pharmacol., 60: 78-84.
COSTA, A. & MURAD, J.E. (1969) Study of the association between
chlorpromazine and analgesics through the method of electrical
stimulation of the rabbit dental pulp. Rev. Bras de Pesgnisa Med.
Biol., 2: 235-240.
COSTA, E. (1970) Simple neuronal models to estimate turnover rate of
noradrenergic transmitters in vivo. In: Costa, E. & Giabocini, E.,
ed. Advances in biochemical psychopharmacology. II. Biochemistry of
simple neuronal models, New York, Raven Press.
COTMAN, C.W. & BARKER, G.A. (1974) The making of a synapse. Rev.
Neurosci., 1: 1-62.
COTMAN, C.W., HAYCOCK, J.W., & WHITE, W.F. (1976) Stimulus secretion
coupling processes in brain: analysis of noradrenaline and gamma-
aminobutyric acid release. J. Physiol., 254: 475-505.
COURAUD, F.C., ROCHAT, H., & LISSITSKY, S. (1978) Binding of scorpion
and sea anemone neurotoxins to a common site related to the action
potential Na+ ionophore in neuroblastoma cells. Biochem. biophys.
Res. Comm., 83: 1525-1530.
CRANE, A.M. & GOLDMAN, P.S. (1979) An improved method for embedding
brain tissue in albumin-gelatin. Stain Technol., 54: 71-75.
CRAPPER, D.R. & DE BONI, U. (1980) Aluminum. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 326-335.
CUTLER, M.G. (1977) Effects of exposure to lead on social behaviour in
the laboratory mouse. Psychopharmacology, 52: 279-282.
DAMSTRA, T. (1978) Environmental chemicals and nervous system
dysfunction. Yale J. Biol. Med., 51: 457-468.
DAMSTRA, T. & BONDY, S.C. (1980) The current status and future of
biochemical assays for neurotoxicity. In: Spencer, P.S. & Schaumburg,
H.H., ed. Experimental and clinical neurotoxicology, Baltimore,
Maryland, Williams and Wilkins Company, pp. 820-833.
DAMSTRA, T. & BONDY, S.C. (1982) Neurochemical approaches to the
detection of neurotoxicity. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 349-373.
DAUBE, J. (1980) Nerve conduction studies. In: Aminoff, J., ed.
Electrodiagnosis in clinical neurology, New York, Churchill
Livingstone, pp. 229-264.
DAVIS, M. (1980) Neurochemical modulation of sensory-motor reactivity:
acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev.,
4: 241-263.
DE JESUS, C.P.V., TOCOFIGHI, J., & SNYDER, D.R. (1978) Sural nerve
conduction study in the rat: a new technique for studying experimental
neuropathies. Muscle Nerve, 1: 162-167.
DESAIAH, D., GILLILAND, T., HO, I.K., & MEHENDALE, H.M. (1980)
Inhibition of mouse brain synaptosomal ATPases and ouabain binding by
chlordecone. Toxicol. Lett., 6: 275-285.
DESCLIN, J.C. (1974) Histological evidence supporting the inferior
olive as the major source of cerebellar climbing fibres in the rat.
Brain Res., 77: 365-384.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I. & SOS, J. (1962) Central nervous injury by a chemical
herbicide. Acta Med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1963) Experimental lesions of the central nervous
system induced by triorthocresyl phosphate. Acta Physiol. Acad. Sci.
Hung., 23: 63-68.
DESI, I., GONCZI, L., SIMON, G., FARKAS, I., & KNEFFEL, Z. (1974)
Neurotoxicologic studies of two carbamate pesticides in subacute
animal experiments. Toxicol. appl. Pharmacol., 27: 465-476.
DEWAR, A.J. & MOFFETT, B.J. (1979) Biochemical methods for detecting
neurotoxicity. A short review. Pharmacol. Ther., 5: 545-562.
DEWAR, A.J., MOFFETT, B.J., & SITTON, M.F. (1977) The effect of
anti-inflammatory drugs on retinal dystrophy in the rat. Toxicol.
appl. Pharmacol., 42: 65-74.
DEWS, P.B. (1982) Epistemology of screening for behavioral toxicity.
In: Mitchell, C.L., ed. Nervous system toxicology, New York, Raven
Press, pp. 229-236.
DEWS, P.B. & WENGER, G.R. (1979) Testing for behavioral effects of
agents. Neurobehav. Toxicol., 1(Suppl. 1): 119-127.
DIAMOND, S.S. & SLEIGHT, S.D. (1972) Acute and subchronic
methylmercury toxicosis in the rat. Toxicol. appl. Pharmacol.,
23: 197-207.
DICKSITH, T.S.S. & DATTA, K.K. (1972) Effect of intratesticular
injection of lindane and endrine on the testes of rats. Acta
pharmacol., 31: 1-10.
DIETZ, D.D., MCMILLAN, D.E., GRANT, L.D., & KIMMEL, C.A. (1978)
Effects of lead on temporally-spaced responding in rats. Drug chem.
Toxicol., 1(4): 401-419.
DINGLEDINE, R., ed. (1984) Brain slices, New York, Plenum Press,
442 pp.
DIXON, R.L. (1976) Problems in extrapolating toxicity data for
laboratory animals to man. Environ. Health Perspect., 13: 43-50.
DOLPHIN, A.C. & GREENGARD, P. (1981) Neurotransmitter- and
neuromodulator-independent alterations in phosphorylation of protein I
in slices of rat facial nucleus. J. Neurosci., 1: 192-203.
DRAPER, W.A. (1967) A behavioural study of the home-cage activity of
the white rat. Behaviour, 28: 280-306.
DRENTH, H.J., ENSBERG, I.F.G., ROBERTS, D.V., & WILSON, A. (1972)
Neuromuscular function in agriculture workers using pesticides. Arch.
environ. Health, 25: 395-398.
DROUIN, J. & LABRIE, F. (1981) Interactions between 17 beta-estradiol
and progesterone in the control of luteinizing hormone and follicle
stimulating hormone release in rat pituitary cells in culture.
Endocrinology, 108: 52-57.
DUBOIS, K.P. & PUCHALA, E. (1961) Studies on the sex differences in
the toxicity of cholinergic phosphorothioate (26791). Proc. Soc. Exp.
Biol. Med., 107: 908-911.
DUBOIS, K.P., DOULL, J., SALERNO, P.R., & COON, J.M. (1949) Studies on
the toxicity and mechanisms of action of p-nitro-phenyl diethyl-
thionosphosphate (parathion). J. Pharmacol. exp. Ther., 95: 79-91.
DUBOWITZ, V. & BROOKE, M.H. (1973) Muscle biopsy: a modern approach,
London, W.B. Saunders.
DUNLOP, D.S., VAN ELDEN, W., & LAJTHA, A. (1975) Optimal conditions
for protein synthesis in incubated slices of rat brain. Brain Res.,
99: 303-318.
DUNN, A.J. (1975) Intercerebral injections inhibit amino acid
incorporation into brain protein. Brain Res., 99: 405-409.
DUNN, A.J. (1977) Measurements of the rate of brain protein synthesis.
In: Roberts, S., Lajtha, A., & Gispen, W.H., ed. Mechanisms,
regulation, and special function of protein synthesis in the brain,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 97-105.
DURHAM, W.F., CUETO, C., Jr, & HAYES, W.J., Jr (1956) Hormonal
influence on DDT metabolism in the white rat. Am. J. Physiol.,
187: 373-377.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
DYER, R.S. (1980) Effects of prenatal and postnatal exposure to carbon
monoxide on visually-evoked responses in rats. In: Weiss, B. &
Merigan, W., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 17-33.
DYER, R.S. (1985a) The use of sensory-evoked potentials in toxicology.
Fundam. appl. Toxicol., 5: 24-40.
DYER, R.S. (1985b) Neurophysiological measures following exposure to
toxic substances. In: Annau, Z., ed. Behavioral toxicology,
Baltimore, Maryland, Johns Hopkins.
DYER, R.S. & BOYES, W.K. (1983) Use of neurophysiological challenges
for the detection of neurotoxicity. Fed. Proc., 42: 3201-3206.
DYER, R.S., ECCLES, C.U., & ANNAU, Z. (1978) Evoked potential
alterations following prenatal methylmercury exposure. Pharmacol.
Biochem. Behav., 8: 137-141.
DYER, R.S., SWARTZWELDER, H.S., ECCLES, C.W., & ANNAU, Z. (1979)
Hippocampal after discharges and their post-ictal sequelae in rats: a
potential tool for assessment of CNS neurotoxicity. Neurobehav.
Toxicol., 1: 5-19.
ECKERMAN, D.A., GORDON, W.A., EDWARDS, J.D., MACPHAIL, R.C., & GAGE,
M.I. (1980) Effects of scopolamine, pentabarbital, and amphetamine on
radial arm maze performance in the rat. Pharmacol. Biochem. Behav.,
12: 595-602.
EDWARDS, P.M. & PARKER, V.H. (1977) A simple, sensitive, and objective
method for early assessment of acrylamide neuropathy in rats.
Toxicol. appl. Pharmacol., 40: 589-591.
EGAN, G.F., LEWIS, S.C., & SCALA, R.A. (1980) Experimental design for
animal toxicity studies. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams & Wilkins Company, pp. 708-725.
EICHELMAN, B., HEGSTRAND, L.R., MCMURRAY, T., & KANTAX, K.M. (1981)
Cyclic nucleotides and aggressive behaviour. Psychopharmacology of
aggression and social behaviour. Pharmacol. Biochem. Behav.,
14(Suppl. 1): 7-12.
ELSNER, J., LOOSER, R., & ZBINDEN, G. (1979) Quantitative analysis of
rat behaviour patterns in a residential maze. Neurobehav. Toxicol.,
1(Suppl. 1): 163-174.
EMSON, P.C. (1983) Chemical neuroanatomy, New York, Raven Press.
ENJALBERT, A., RUBERG, M., & KORDON, C. (1978) Neuroendocrine control
of prolactin secretion. In: Robyn, C. & Harter, M., ed. Progress in
prolactin physiology and pathology, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 83-94.
EROSCHENKO, V.P. & PALMITER, R.D. (1980) Estrogenicity of kepone in
birds and mammals. In: McLachlan, J., ed. Estrogens in the
environment, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 305-325.
EROSCHENKO, V.O. & WILSON, W.O. (1975) Cellular changes in the gonads,
liver, and adrenal glands of Japanese quail as affected by the
insecticide kepone. Toxicol. appl. Pharmacol., 31: 491-504.
EVANS, H.L. (1978) Behavioral assessment of visual toxicity. Environ.
Health Perspect., 26: 53-58.
EVANS, H.L. & WEISS, B. (1978) Behavioral toxicology. In: Blackman,
D.E. & Sanger, D.J., ed. Contemporary research in behavioral
pharmacology, New York, Plenum Press, pp. 449-487.
EVANS, H.L., LATIES, V.G., & WEISS, B. (1975) Behavioral effects of
mercury and methylmercury. Fed. Proc., 34: 1858-1867.
EVANS, H.L., GARMAN, R.H., & WEISS, B. (1977) Methylmercury: exposure
duration and regional distribution as determinants of neurotoxicity in
non-human primates. Toxicol. appl. Pharmacol., 41: 15-33.
EVANS, W.O. (1962) A comparison of the analgetic potency of some
analgesics as measured by the "flinch-jump" procedure.
Psychopharmacologia, 3: 51-54.
FADEEV, JU.A. (1980) [Unit activity of the brain cortex in realization
of goal-directed behaviour.] Usp. fiziol. nauk., 11(3): 12-46 (in
Russian).
FADEEV, JU.A. & ANDRIANOV, V.V. (1971) Methods of registration of
neuronal activity in freely-moving animals. Physiol. J. (USSR),
57(8): 1217-1219.
FALCK, B., HILLARP, N.A., THIEME, G., & TORP, A. (1962) Fluorescence
of catecholamines and related compounds condensed with formaldehyde.
J. Histochem. Cytochem., 10: 348-354.
FALK, J.L. (1970) Readings in behavioral pharmacology, New York,
Appleton-Century-Crofts.
FELLEGIOVA, M., ADAMEC, O., & DAHKOVA, A. (1977) [The effect of
lindane on the metabolism of testosterone in the rat.] Cs. Hyg.,
22: 115-120 (in Slovak).
FERSTER, C.B. & SKINNER, B.F. (1957) Schedules of reinforcement, New
York, Appleton-Century-Crofts.
FESTING, M.F. (1979) Properties of inbred strains and outbred stocks,
with special reference to toxicity testing. J. environ. Health
Toxicol., 5: 53-68.
FILE, S. (1980) The use of social interaction as a method for
detecting anxiolytic activity of chlordiarepoxide-like drugs.
J. Neurosci. Methods, 2: 219-238.
FINK, G. (1979) Feedback actions of target hormones on hypothalamus
and pituitary with special reference to gonadal steroids. Ann. Rev.
Physiol., 41: 571-585.
FISHMAN, J. (1982) Catechol estrogens and pituitary function. In:
Muldoon, T.G., Mahesh, V.B., & Perez-Ballester, B., ed. Recent
advances in fertility research. Part A. Developments in reproductive
endocrinology, New York, Alan R. Lyss, pp. 183-189.
FISHMAN, W.H. & BERNFELD, P. (1955) Glucuronidases. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 262.
FITZGERALD, G.J., KAUFMAN, E.E., SOKOLOFF, L., & SHEIN, H.M. (1974)
D(-)-beta-hydroxybutyrote dehydrogenase activity in closed cell lines
of glial and neuronal origin. J. Neurochem., 22: 1163-1166.
FODOR, G.G., SCHLIPKOTER, H.W., & ZIMMERMAN, M. (1973) The objective
study of sleeping behaviour in animals as a test of behaviour
toxicity. In: Horvath, M. & Frantik, M., ed. Adverse effects of
environmental chemicals and psychotropic drugs, Amsterdam, London,
New York, Elsevier Science Publishers, Vol. 1, pp. 115-122.
FOLCH, J., ASCOLI, I., LEES, M., MEATH, J.A., & LEBARON, F.N. (1951)
Preparation of lipid extracts from brain tissue. J. biol. Chem.,
191: 833-841.
FOWLER, S.C. & PRICE, A.W. (1978) Some effects of chlordiaz-epoxide
and delta-amphetamine on response force during punished responding in
rats. Psychopharmacology, 56: 211-215.
FOX, D.A., LOWNDES, H.E., & BIERKAMPER, G.G. (1982) Electro-
physiological techniques in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 299-335.
FOX, W.M. (1965) Reflex-ontogeny and behavioral development of the
mouse. Anim. Behav., 13: 234-241.
FRANKOVA, S. (1970) Behavioral responses of rats to early
overnutrition. Nutr. Metab., 12: 228-230.
FRANKOVA, S. (1971) Relationship between nutrition during lactation
and maternal behavior of rats. Act. Nerv. Super. (Praha), 13: 1-8.
FRANKOVA, S. (1977) Drug-induced changes in the maternal behavior of
rats. Psychopharmacology, 3: 83-87.
FRANTIK, E. (1970) The development of motor disturbances in
experimental chronic carbon disulfide intoxication. Med. Lav.,
61: 309-313.
FRANTIK, E. & BENES, V. (1984) Central nervous effect and blood level
regressions on exposure time paralleled in solvents (toluene, carbon
tetrachloride, and chloroform). Act. Nerv. super. (Praha), 26: 131.
FUJIMORI, K., BENET, H., MEHENDALE, H.M., & HO, I.K. (1982) Comparison
of brain discrete area distribution of chlordecone and mirex in the
mouse. Neurotoxicology, 3: 125-130.
FULLERTON, P.M. (1966) Chronic peripheral neuropathy produced by lead
poisoning in guinea-pigs. J. Neuropathol. exp. Neurol., 25: 214-236.
GABE, M. (1976) Histological techniques, New York, Paris,
Springer-Verlag, Masson.
GAD, S.C. (1982) Statistical analysis of behavioral toxicology data
and studies. Arch. Toxicol., Suppl. 5: 256-266.
GAD, S.C. & WEIL, C.S. (1982) Statistics for toxicologists. In: Hayes,
A.W., ed. Principles and methods of toxicology, New York, Raven
Press, pp. 270-320.
GALLARD, G.V., PETRO, Z., & RYAN, K.J. (1978) Phylogenetic
distribution of aromatase and other androgen-converting enzymes in the
central nervous system. Endocrinology, 10: 2283-2290.
GELLER, I., MENDEZ, V., HAMILTON, M., HARTMANN, R.J., & GAUSE, E.
(1979) Effects of carbon monoxide on operant behavior of laboratory
rats and baboons. Neurobehav. Toxicol., 1(Suppl. 1): 179-184.
GELLERT, R.J., HEINRICHS, W.L., & SWERDLOFF, R.S. (1972) DDT
homologues: estrogen-like effects on the vagina, uterus, and pituitary
of the rat. Endocrinology, 91: 1095-1100.
GERHART, J.M., HONG. J.S., UPHOUSE, L.L., & TILSON, H.A. (1982)
Chlordecone-induced tremor: quantification and pharmacological
analysis. Toxicol. appl. Pharmacol., 66(2): 234-243.
GILLIATT, R.W. (1973) Recent advances in the pathophysiology of nerve
conduction. In: Desmedt, J.E., ed. New developments in
electromyography and clinical neurophysiology, Basel, Karger,
Vol. 2, pp. 2-18.
GLATT, A.F., TALAAT, H.N., & KOELLA, W.P. (1979) Testing of peripheral
nerve function in chronic experiments with rats. Pharmacol. Ther.,
5: 539-543.
GLAUSERT, A.M. (1980) Fixation, dehydration, and embedding of
biological specimens, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
GLOWINSKI, J. & IVERSEN, L.L. (1966) Regional studies of
catecholamines in the rat brain. I. The disposition of 3H-norephine-
phrine, 3H-dopamine, and 3H-DOPA in various regions of the brain. J.
Neurochem., 13: 655-699.
GOLDSTEIN, B.D., LOWNDES, H.E., & CHO, E.S. (1981) Neurotoxicology of
vincristine in the cat: electrophysiological studies. Arch.
Toxicol., 48: 253-264.
GONCHARUK, V.D. (1981) Alteration of activity of glutamate
dehydrogenase in ganglionated neurons of rabbits under acute
experimental emotional stress. Bull. exp. Biol. Med., 10: 494-496.
GOOD, P. (1979) Detection of a treatment effect when not all
experimental subjects will respond to treatment. Biometrics,
35: 483-489
GORIDIS, C., MASSARELLI, R., SERSENBRENNER, M., & MANDEL, P. (1974)
Guanyl cyclase in chick embryo brain cell cultures: evidence of
neuronal localization. J. Neurochem., 23: 135-139.
GOYER, R.A. & RHYNE, B.C. (1973) Pathological effects of lead. Int.
Rev. exp. Pathol., 12: 1-7.
GRANT, L.D. (1976) Research strategies for behavioral toxicology
studies. Environ. Health Perspect., 18: 85-94.
GRAY, E.G. & WHITTAKER, V.P. (1962) The isolation of nerve endings
from brain: an electron microscopic study of the cell fragments of
homogenation and centrifugation. J. Anat., 96: 79-87.
GREENBLATT, D.J., SHADER, R.I., FRANKE, K.K., MACLAUGHLIN, E.D.,
RONSIL, G.J., & KOCH-WESER, J. (1977) Kinetics of intravenous
chlordiazepoxide: sex differences in drug metabolism. Clin.
Pharmacol. Ther., 22: 893-903.
GREENGARD, P. (1979) Cyclic nucleotides, phosphorylated proteins and
the neuronal system. Fed. Proc., 38: 2208-2217.
GREGORY, E.H. & PFAFF, D.W. (1971) Development of olfactory-guided
behavior in infant rats. Physiol. Behav., 6: 573-576.
GRIFFIN, J.W. & PRICE, D.L. (1980) Proximal axonopathies induced by
toxic chemicals. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 161-178.
GRIFFIN, J.W., PRICE, D.L., KLUTHE, D.O., & GOLDBERG, M.A. (1980)
Neurotoxicity of misonidazole in rats. Neuropathol. appl.
Neurobiol., 6: 656-666.
GROSS-SELBECK, E. & GROSS-SELBECK, M. (1981) Changes in operant
behavior of rats exposed to lead at the accepted no-effect level.
Clin. Toxicol., 18: 1247-1256.
GROTA, L.S. & ADER, R. (1969) Continuous recording of maternal
behavior in the rat. Anim. Behav., 17: 72-729.
GUDELSKY, G.A., NANSEL, D.D., & PORTER, J.C. (1981) Role of estrogen
in the dopaminergic control of prolactin secretion. Endocrinology,
108: 440-444.
HAIDER, S.S., HANSAN, M., & ISLAM, F. (1980) Effect of air pollutant
hydrogen sulfide on the levels of total lipids, phospholipids, and
cholesterol in different regions of the guinea-pig brain. Ind. J.
exp. Biol., 18: 418-420.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., & MCCONNEL, J.
(1979) Estrogenic activity of the insecticide chlordecone (kepone) and
interaction with uterine estrogen receptors. Proc. Natl Acad. Sci.
(USA), 76: 6641-6645.
HARPUR, E.S. & D'ARCY, P.F. (1975) The quantification of kanamycin
ototoxicity in the rat using conditioned tone discrimination.
J. Pharm. Pharmacol., 27: 907-913.
HARRY, G.J. & TILSON, H.A. (1982) Postpartum exposure to triethyltin
produces long-term alterations in responsiveness to apomorphine.
Neurotoxicology, 3: 64-71.
HASAN, M. & ALI, S.F. (1981) Effects of thallium, nickel, and cobalt
administration on the lipid peroxidation in different regions of the
rat brain. Toxicol. appl. Pharmacol., 57: 8-13.
HAY, A. (1978) Neurotoxins may go unrecognized. Nature (Lond.),
274: 206.
HAYAT, M.A. (1973) Principles and techniques of electron microscopy,
New York, Van Rostrand Reinhold Company, Vols. 1 and 2.
HEDLUND, B., GAMARA, M., & BARTFAI, I. (1979) Inhibition of striatal
muscarinic receptors in vivo by cadium. Brain Res., 168: 216-218.
HEISE, G.A. (1983) Toward a behavioural toxicology of learning and
memory. In: Zbinden, G., Cuomo, V., Racagni, G., & Weiss, B., ed.
Application of behaviour pharmacology and toxicology, New York,
Raven Press, pp. 27-38.
HEISE, G.A. & MILAR, C. (1984) Drugs and stimulus control. In:
Iverson, S.D. & Snyder, S.H., ed. Handbook of psychopharmacology,
New York, Plenum Press, pp. 129-190.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930.
HERMAN, S.P., KLEIN, R., TALLEY, F.A., & KRIGMAN, M. (1973) An
ultrastructural study of methylmercury-induced primary sensory
neuropathy in the rat. Lab. Inv., 28: 104-118.
HERTZ, L. (1981) Gamma amino butyric-acid glutamate pathway in glia.
5th European Neuroscience Congress, Liege, Belgium, 14-18 September,
1981. Neurosci. Lett, Suppl. 7: 172.
HETZLER, B.E., HEILBRONNER, R.L., GRIFFIN, J., & GRIFFIN, G. (1981)
Acute effects of alcohol on evoked potentials in visual cortex and
superior colliculus of the rat. Electroencephalogr. clin.
Neurophysiol., 51 69-79.
HILLE, B. (1976) Gating in sodium channels of nerve. Ann. Rev.
Physiol., 38 139-152.
HINDE, R. (1974) Biological basis of human social behaviour, New
York, McGraw Hill, Inc.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930
HOFFMAN, P.N. & LASEK, R.J. (1975) The slow component of axonal
transport. Identification of major structural polypeptides of the axon
and their generality among mammalian neurons. J. cell Biol.,
66: 351.
HOLCK, H.G.O., MUNIR, A.K., MILLS, L.M., & SMITH, E.L. (1937) Studies
upon the sex differences in rats in tolerance to certain barbiturates
and to nicotine. J. Pharmacol. exp. Ther., 60: 323-346.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HONG, J.S. & ALI, S.F. (1982) Chlordecone (kepone) exposure in the
neonate selectively alters brain and pituitary endorphin levels in
prepuberal and adult rats. Neurotoxicology, 3: 111-118.
HOPF, H.C. & EYSHOLDT, M. (1978) Impaired refractory periods of
peripheral sensory nerves in multiple sclerosis. Ann. Neurol.,
4: 499-501.
HOROWSKI, D. & GRAF, K.J. (1979) Neuroendocrine effects of
neuropsychotropic drugs and their possible influence on toxic
reactions in animals and man: the role of dopamine-prolactin system.
Arch. Toxicol. Suppl. 2: 93-104.
HORVATH, M., ed. (1973) Adverse effects of environmental chemicals
and psychotropic drugs. Quantitative interpretation of functional
tests, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 1, 292 pp.
HORVATH, M. & FRANTIK, E. (1973) Quantitative interpretation of
experimental toxicological data: the use of reference substances. In:
Horvath, M., ed. Adverse effects of environmental chemicals and
psychotropic drugs, Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Co., Vol. 1, pp. 11-21.
HORVATH, M. & FRANTIK, M., ed. (1976) Adverse effects of
environmental chemicals and psychotropic drugs: neurophysiological
and behavioural tests, Amsterdam, New York, Oxford, Elsevier,
Vol. 2, 334 pp.
HORVATH, M. & FRANTIK, E. (1979) Industrial chemicals and drugs
lowering central nervous systems activation level: quantitative
assessment of hazard in man and animals. Act. Nerv. super. (Praha),
21: 269-272.
HORVATH, M. & MICHALOVA, C. (1956) Changes of EEG in experimental
exposure to carbon disulfide in the light of Vvedenskys theory of
parabiosis. Arch. Gewerbepathol., 15: 131-136.
HORVATH, M., FRANTIK, E., & KREKULE, P. (1981) Diazepam impairs
alertness and potentiates a similar effect of toluene. Act. Nerv.
super. (Praha), 22: 17-19.
HOWLAND, R.D., VYAS, I.L., LOWNDES, H.E., & ARGENTIERI, T.M. (1980)
The etiology of toxic peripheral neuropathies; in vitro effects of
acrylamide and 2,5-hexanedione on brain enolase and other glycolytic
enzymes. Brain Res., 202: 131-142.
HUGHES, J.A. & SPARBER, S.B. (1978) Delta-amphetamine unmasks
postnatal consequences of exposure to methylmercury in utero:
methods for studying behavioral teratogenesis. Pharmacol. Biochem.
Behav., 8: 365-375.
HUNT, V.R., SMITH, M.K., & WORK, D., ed. (1982) Banbury Report II -
Environmental factors in human growth and development, New York,
Cold Spring Harbor Laboratory.
HUNTER, D. & RUSSELL, D.S. (1954) Focal cerebral and cerebellar
atrophy in human subjects due to organic mercury compounds.
J. Neurol. Neurosurg. Psychiatr., 17: 235-241.
HUNTER, D., BORNFORD, R., & RUSSELL, D. (1940) Poisoning by
methylmercury compounds. Q. S. Med., 9: 193-213.
HUTCHINGS, D.E. (1978) Behavioral teratology: embryopathic and
behavioural effects of drugs during pregnancy. In: Gottlieb, G., ed.
Studies on the development of behaviour and nervous system: early
influences, New York, Academic Press, pp. 7-34.
IRWIN, S. (1968) Comprehensive observational assessment: a systematic
quantitative procedure for assessing the behavioral and psychologic
state of the mouse. Psychopharmacologia, 13: 222-257.
ISLAM, F., TAYYABA, K., & HASAN, M. (1983) Organophosphate metasystox-
induced increment of lipase activity and lipid peroxidation in
cerebral hemisphere: diminution of lipids in discrete areas of the
brain. Acta pharmacol. toxicol., 53: 121-124.
IVERSEN, L.L. (1971) Role of transmitter uptake mechanisms in synaptic
neurotransmission. Br. J. Pharmacol., 44: 571-591.
JACOBWITZ, D.M. (1974) Removal of discrete fresh regions of the rat
brain. Brain Res., 80: 111-115.
JOHN, T.H., GEGHMAN, C., & REIS, D.J. (1973) Immunochemical
demonstration of increased accumulation of tyrosine hydroxylase
protein in sympathetic ganglia and adrenal medulla elicited by
reserpine. Proc. Natl Acad. Sci. (USA), 70: 2767-2771.
JOHNSON, B.L. (1980) Electrophysiological methods in neurotoxicity
testing. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental and
clinical neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 726-742.
JOHNSON, B.L. & ANGER, W.K. (1983) Behavioral toxicology. In: Rom,
W.N., ed. Environmental and occupational medicine, Boston, Little
Brown and Co., pp. 329-350.
JOHNSON, B.L., ANGER, W.K., SETZIER, J.V., & XINTARAS, C. (1975) The
application of a computer-controlled time discrimination performance
to problems in behavioral toxicology. In: Weiss, B. & Laties, V.G.,
ed. Behavioral toxicology, New York, London, Plenum Press,
pp. 129-153.
JOHNSON, E.W. (1980) Practical electromyography, Baltimore,
Maryland, Williams and Wilkins Company.
JOHNSON, J.E., Jr (1981) Aging and cell structure, New York, Plenum
Press, Vol. 1.
JOY, R.M. (1982) Chlorinated hydrocarbon insecticides. In: Ecobichon,
D.J. & Joy, R.M., ed. Pesticides and neurological diseases, Boca
Raton, Florida, CRC Press, pp. 91-150.
JOY, R.M. (1985) Kindling as a tool in neurotoxicology. Fund. appl.
Pharmacol., 5: 41-65.
JOY, R.M., STARK, L.G., PETERSON, S.L., BOWYER, J.T., & ALBERTSON,
T.E. (1980) The kindled seizure; production of and modification by
dieldrin in rats. Neurobehav. Toxicol., 2: 117-124.
JUNQUEIRA, L.C., CARNEIRO, J., & CONTOPOULOS, A. (1977) Basic
Histology, 2nd ed., Los Altos, Lange.
KAPLAN, K.L. (1980) Thyroxine 5'-monodeiodination in rat pituitary
homogenates. Endocrinology, 106: 567-576.
KARPOVA, O.B., OBEHOVA, E.L., AVIOVA, N.F., & SCHVARTS, E.G. (1978)
[Content and composition of cerebral gangliosides during Down's
Syndrome.] Vopros. med. Xhim., 4: 524-527 (in Russian).
KELLEHER, R.T. & MORSE, W.H. (1968) Determinations of the specificity
of behavioral effects of drugs. Ergeb. Physiol., 60: 1-56.
KHOLODOV, Ju.A. & SOLOV'EVA, G.R. (1971) [Occasional publication:
news on medical equipment manufacture,] fasc. 3, pp. 69-72
(in Russian).
KIMURA, J. (1976) Collision techniques: physiological block of nerve
impulses in studies of motor nerve conduction velocity. Neurology,
26: 680-682.
KING, J.A. & VESTAL, B.M. (1974) Visual acuity of peromyscus.
J. Mammal., 55: 238-243.
KIRPEKAR, S.M., DIXON, W., & PRATT, J.C. (1970) Inhibitory effect of
manganese on norephinephrine release from the splenic nerves of cat.
J. Pharmacol. exp. Ther., 174: 72-76.
KLEIN, R., HERMAN, S., BRUBAKER, P.E., & LUCIER, G.W. (1972) A model
of acute methylmercury intoxication in rats. Arch. Path.,
93: 404-418.
KODAMA, J., FUKUSHIMA, M., & SAKATA, T. (1978) Impaired taste
discrimination against quinine following chronic administration of
theophylline in rats. Physiol. Behav., 20: 151-155.
KOHLER, W. (1976) The mentality of ages, New York, Liveright,
336 pp.
KONAT, G. & CLAUSEN, J. (1977) Triethyl lead-induced intoxication as
an experimental model of hypomyelination. Proc. Int. Soc.
Neurochem., 6: 215.
KORNBERG, A. (1955) Lactic dehydrogenase in muscle. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 441.
KOSTIAL, K., LANDEKA, M., & SLAT, B. (1974) Manganese ions and
synaptic transmission in the superior cervical ganglion of the cat.
Br. J. Pharmacol., 51: 231-235.
KOTLIAREVSKI, L.I. (1951) [Methodology for studying motor conditioned
reflexes in some small animals (white rats and guinea-pigs.]
Zh. Vyssh. nerv. deyat. imeni, 1(5): 753-761 (in Russian).
KOTLIAREVSKI, L.I. (1957) [Procedure for the investigation of motor-
food conditioned reflexes in small animals.] Tr. Inst. Vyssh. nerv.
deyat. AN SSR, 3: 23-28 (in Russian).
KRIGMAN, M.R. & HOGAN, E.L. (1974) Effect of lead intoxication on
the postnatal growth of the rat nervous system. Environ. Health
Perspect., 7: 187-199.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., SUTER, Y., THOMANN, G., &
HESS, R. (1981) Pyridoxine megavitaminosis produces degeneration of
peripheral sensory neurons (sensory neuronopathy) in the dog.
Neurotoxicology, 2: 13-24.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., THOMANA, P., & HESS, R.
(1979) Clinoquinol and 2,5-hexanedione induce different types of
distal axonopathy in the dog. Acta neuropathol., 47: 213.
KRSIAK, M. (1979) Effects of drugs on behavior of aggressive mice.
Br. J. Pharmacol., 65: 525-533.
KRUSIUS, T. & FINNE, J. (1977) Structural features of tissue
glycoproteins. Fractionation and methylation analysis of gylcopeptides
derived from rat brain, kidney, and liver. Eur. J. Biochem.,
78: 369-379.
KRYZHANOVSKY, G.N. (1973) Mechanism of action of tetanus toxin. Effect
on synaptic processes and some particular features of toxin binding by
the nervous tissue. Jaunyn-Schmiedeberg's Arch., 276: 247-270.
KUCZENSKI, R.T. & MANDELL, A.J. (1972) Regulatory properties of
soluble and particulate rat brain tyrosine hydroxylase. J. biol.
Chem., 247: 3114-3116.
KULKOSKY, P.J., ZELLNER, D.A., HYSON, R.L., & RILEY, A.L. (1980)
Ethanol consumption of rats in individual, group, and colonial housing
conditions. Physiol. Psychol., 8: 56-60.
KUPFER, D. & BULGER, W.H. (1976) Studies on the mechanism of
estrogenic actions of o-p'-DDT; interactions with the estrogen
receptor. Pestic. Biochem. Physiol., 6: 561-570.
KUPFER, D. & BULGER, W.H. (1980) Estrogenic properties of DDT and its
analogues. In: McLachlan, J., ed. Estrogens in the environment,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 239-263.
LAFFERMAN, J.A. & SILBERGELD, E.K. (1979) Erythrosin B inhibition of
neurotransmitter accumulation by rat brain homogenate. Science,
206: 363-365.
LAJTHA, A. & MARKS, N. (1971) Protein turnover. In: Lajtha, A., ed.
Handbook Neurochemistry, New York, Plenum Press, Vol. 5, pp. 551-629
LAMARTINIERE, C.A., DIERINGER, C.S., & LUCIER, G.W. (1979) Altered
ontogeny of glutathione S-transferases by 2,4,5-2',4',5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 51: 233-238.
LANDOWNE, D. & RITCHIE, J.M. (1976) On the control of glycogenolysis
in mammalian nervous tissue by calcium. J. Physiol., 212: 503-517.
LATIES, V.G. (1982) Contributions of operant conditioning to
behavioral toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 67-80.
LAZAROVA, M., BENDOTTI, C., & SAMANIN, R. (1984) Evidence that the
dorsal raphe area is involved in the effect of clonidine against
pentylenetetrazol-induced seizures in rats. Naunyn-Schmiedebergs
Arch. Pharmacol., 325: 12-16.
LEANDER, J.D. & MACPHAIL, R.C. (1980) Effect of chlordimeform
(a formamidine pesticide) on schedule-controlled responding of
pigeons. Neurobehav. Toxicol., 2: 315-321.
LEANDER, J.D., MCMILLAN, D.E., & BARLOW, T.S. (1977) Chronic mercuric
chloride: behavioural effects in pigeons. Environ. Res.,
14: 424-435.
LEHMANN, H.J. & TACKMANN, E. (1974) Neurographic analysis of trains of
frequent electrical stimuli in the diagnosis of peripheral nerve
disease. Eur. Neurol., 12: 293-308.
LEHRER, G.M. (1974) Measurement of minimal brain dysfunction. In:
Xintaras, C., Johnson, B.L., & DeGroot, I., ed. Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, pp. 450-454 (US DREW (NIOSH) Publication No. 74-126).
LEONARD, J.L. & ROSENBERG, L.H. (1980) Characterization of essential
enzyme sulfhydryl groups of thryoxine 5'deiodinase from rat kidney.
Endocrinology, 108: 444-451.
LEVINE, T.E. (1976) Effects of carbon disulfide and FLA-63 on operant
behavior in pigeons. J. Pharmacol. exp. Ther., 199: 669-678.
LEWEY, F.H. (1941) Experimental chronic carbon disulfide poisoning in
dogs. J. ind. Hyg. Toxicol., 23: 415-535.
LILIENTHAL, H., WINNEKE, G., BROCKHAUS, A., MOLIK, B., & SCHLIPKOTER,
H.-W. (1983) Learning set-formation in rhesus monkeys pre- and
postnatally exposed to lead. In: Proceedings of the International
Conference on Heavy metals in the Environment, Heidelberg, September,
1983, Edinburgh, CEP Consultants, p. 901.
LINDSLEY, D.B. & WICKE, J.D. (1974) The electroencephalogram:
autonomous electrical activity in man and animals. In: Thompson, R. &
Patterson, M., ed. Bioelectric recording techniques, Part B, New
York, Academic Press.
LIPP, J.A. (1968) Cerebral electrical activity following soman
administration. Arch. int. Pharmacodyn., 175: 161-169.
LOCK, E.A. (1976) Increase in cerebral fluids in rats after treatment
with hexachlorophene or triethyltin. Biochem. Pharmacol.,
25: 1455-1458.
LORENZO, A.V. & GERWITZ, M. (1977) Inhibition of 14C-tryptophane
transport into brain of lead-exposed neonatal rabbits. Brain Res.,
132: 386-392.
LOWITZSCH, K., GOHRING, U., HECKING, E., & KOHLER, H. (1981)
Refractory period, sensory conduction velocity, and visual-evoked
potentials before and after haemodialysis. J. Neurol. Neurosurg.
Psychiatr., 44: 121-128.
LOWNDES, H.E. & BAKER, T. (1976) Studies on drug-induced neuropathies.
III. Motor nerve deficit in cats with acrylamide neuropathy. Eur. J.
Pharmacol., 35: 177-184.
LOWNDES, H.E., BAKER, T., CHO, E.S., & JORTNER, B.S. (1978a) Position
sensitivity of deafferented muscle spindles in experimental acrylamide
neuropathy. J. Pharmacol. exp. Ther., 205: 40-48.
LOWNDES, H.E., BAKER, T., MICHAELSON, L.P., & VINCENT-ABLAZEY, M.
(1978b) Attenuated dynamic responses of primary endings of muscle
spindles; a basis for depressed tendon responses in acrylamide
neuropathy. Ann. Neurol., 3: 435-437.
LUKAS, E. (1970) Stimulation electrohypography in experimental
toxicology (carbon disulfide neuropathy in rats). Med. Lav.,
61: 302-308.
MCBLAIN, W.A., LEWIN, V., & WOLFE, F.H. (1976) Differing estrogenic
activities for the enatiomers of o,p'-DDT in immature female rat.
Can. J. Physiol., 54: 629-632.
MACDONALD, J.S. & HARBISON, R.D. (1977) Methylmercury induced
encephalopathy in mice. Toxicol. appl. Pharmacol., 39: 195-205.
MCDONALD, W.I. (1963) The effects of experimental demyelination on
conduction in peripheral nerve. II. Electrophysical observations.
Brain, 86: 501-524.
MCDONOUGH, J.H., Jr, HACKLEY, B.E., Jr, CROSS, R., SAMSON, F., &
NELSON, S. (1983) Brain regional glucose use during soman-induced
seizures. Neurotoxicology, 4: 203-210.
MCKENNA, M.J. & DESTEFANO, V. (1975) A proposed mechanism of action of
carbon disulfide on carbon disulfide on dopamine-beta-hydroxylase.
Toxicol. appl. Pharmacol., 33: 137.
MCMILLAM, D.E. (1982) Effects of chronic administration of pesticides
on schedule-controlled responding by rats and pigeons. In: Chambers,
J.E. & Yarborough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 211-226.
MACPHAIL, R.C. (1982) Studies on the flavour aversions induced by
trialkyltin compounds. Neurobehav. Toxicol. Teratol., 4: 225-230.
MACPHAIL, R.C. & LEANDER, J.D. (1980) Flavour aversions induced by
chlordimeform. Neurobehav. Toxicol., 2: 363-365.
MACPHAIL, R.C., CROFTON, K.M., & REITER, L.W. (1983) Use of
environmental challenges in behavioral toxicology. Fed. Proc.,
42: 3196-3200.
MACTUTUS, C.F., UNGER, K.L., & TILSON, H.A. (1982) Neonatal
chlordecone exposure impairs early learning and memory in the rat on a
multiple measure passive avoidance task. Neurotoxicology, 3: 24-44.
MAGATA, Y. & TSUKADA, W. (1978) Bulk separation of neuronal cell
bodies and glial cells from mammalian brain and some of their
biochemical properties. Rev. Neurosci., 3: 18-27.
MAILMAN, R.B., FERRIS, R.M., VOGEL, R.A., KILTS, C.D., LIPTOM, M.A.,
TANG, F.L., SMITH, D.A., MUELLER, R.A., & BREESE, G.R. (1980)
Non-specificity of the biochemical actions of erythrosine (Red No. 3):
what relation to behavioural changes. Science, 207: 535-537.
MARES, P. & VELISEK, L. (1983) Influence of ethosuximide on metrazol-
induced seizures during ontogenesis in rats. Act. Nerv. super
(Praha), 25: 292-298.
MARSHALL, J.F. (1975) Increased orientation to sensory stimuli
following lateral hypothalamic damage in rats. Brain Res.,
86: 373-387.
MARSHALL, J.F. & TEITELBAUM, P. (1974) Further analysis of sensory
inattention following lateral hypothalamic damage in rats. J. comp.
physiol. Psychol., 86: 375-395.
MARSHALL, J.F., TURNER, D.H., & TEITELBAUM, P. (1971) Sensory neglect
produced by lateral hypothaLamic damage. Science, 174: 523-525.
MARSHALL, J.F., RICHARDSON, J.S., & TEITELBAUM, P. (1974)
Nigrostriatal bundle damage and the lateral hypothalamic syndrome.
J. comp. Physiol. Psychol., 87: 808-830.
MARSLAND, T.A., GLEES, P., & ERIKSON, L.B. (1954) Modification of
Glee's silver impregnation for paraffin sections. J. Neuropathol.
exp. Neurol., 13: 587-591.
MASTEROV, A.V. (1974) [Features of the interaction of rats in an
electromagnetic field following extinction of conditioned food
reactions.] In: [Problems of clinical and experimental medicine,]
Moscow, pp. 61-63 (in Russian).
MAURISSEN, J.P.J. (1979) Effects of toxicants on the somatosensory
system. Neurobehav. Toxicol., 1(Suppl. 1): 23-32.
MEDVEDEV, B.M. (1975) [The effect of a static electric field on
conditioned reflex activity.] Fiziol. Zh., 3: 320-325 (in Russian).
MELLO, N.K. (1975) Behavioral toxicology; a developing discipline.
Fed. Proc., 34: 1832-1834.
MENDELL, J.R., SAIDA, K., GANANSIA, M.F., JACKSON, D.B., WEISS, H.,
GARDIER, R.S., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J., MARKS,
B., & HETLAND, L. (1974) Toxic polyneuropathy produced by methyl
n-butyl ketone. Science, 185: 787-789.
MENDELSON, J.H., MELLO, N.K., & ELLINGBOE, J. (1977) Effects of acute
alcohol intake on pituitary-gonadal hormones in normal human males.
J. Pharmacol. exp. Ther., 202: 675-682.
MERIGAN, W.H. (1979) Effects of toxicants on visual systems.
Neurobehav. Toxicol., 1: 15-22.
MERIGAN, W.H. (1980) Visual fields and flicker thresholds in
methylmercury poisoned monkeys. In; Merigan, W.H. & Weiss, B., ed.
Neurotoxicity of the visual system, New York, Raven Press,
pp. 149-163.
MERKURYVA, R.V. & BUSHINSKAYA, L.I. (1982) [Metabolism of
sialo-containing glycoproteins and lysosomal enzyme activity under
neurotropic effect of chemical environmental factors.] Ukranian
Biochem. J., 53: 31-35 (in Russian).
MERKURYVA, R.V. & TSAPKOVA, N.N. (1982) Hygienic significance of a
system of biochemical criteria for the evaluation of hepatotoxic and
neurotoxic effects of some chemical factors in the environment.
J. Hyg. Epidemiol. Microbiol. Immunol., 26: 336-341.
MERKURYVA, R.V., BUSHINSKAIA, L.J., AULIKA, B.V., SHATERNIKOVA, J.S.,
PROTSENKO, S.J., KULYGINA, A.A., & EKSLER, N.D. (1978) The activity of
lyosomal and cytoplasmic enzymes under the experimental influence of
bisulfide of carbon. Vop. Med. Xhim., 2: 151-156.
MERKURYVA, R.V., KOTOV, A.V., SILOV, V.G., BUSHINSKAIA, L.J.,
MUCHAMBETOVA, L.KH., & TSAPKOVA, N.N. (1980) Experimental study of
biochemical and physiological mechanisms of cerebral activity
influenced by alcohol, Nauka, Minsk Publishing House, pp. 93-94.
MEYER, O.A., TILSON, H.A., BYRD, W.C., & RILEY, H.T. (1979) A method
for the routine assessment of fore- and hindlimb grip strength of rats
and mice. Neurobehav. Toxicol., 1: 233-236.
MICZEK, K.A. & BARRY, H. (1976) Pharmacology of sex and aggression.
In: Glick, S.D. & Goldforb, J., ed. Behavioral pharmacology,
St. Louis, Missouri, C.V. Mosby Co., pp. 176-257.
MIKISKA, A. (1960) [Determination of the susceptibility to electrical
activity of the cerebral motor cortex, and its pharmacological and
toxicological applications. I. Methodology, controlled trials and
physiological basis.] Arch. Gewerbepathol. Gewerbehyg., 18: 286-299
(in German).
MIKISKOVA, H. & MIKISKA, A. (1962) [A contribution to the application
of defensive skin stimulation in the toxicity of substances acting on
the CNS. II. Measuring stimulus threshold intensity in guinea-pigs.]
Int. Arch. Gewerbepathol. Gewerbehyg., 19: 68-75 (in German).
MIKISKOVA, H. & MIKISKA, A. (1966) Trichloroethanol in
trichloroethylene poisoning. Br. J. ind. Med., 23: 116-125.
MIKISKOVA, H. & MIKISKA, A. (1968) Some electrophysiological methods
for studying the action of narcotic agents in animals, with special
reference to industrial solvents: a review. Br. J. ind. Med.,
25: 81-105.
MILLER, D.B. (1983) Neurotoxicity of the pesticidal carbamates.
Neurobehav. Toxicol. Teratol., 4: 779-788.
MILLER, E.K. & DAWSON, R.M.C. (1972) Can mitochondria and synaptosomes
of guinea-pig brain synthesize phospholipids? Biochem. J.,
126: 805-807.
MINEGISHI, A., FUJUMORI, R., SATOH, T., KILAGAWA, H., & YANAURA, S.
(1979) Changes in serotonin turnover and the brain sensitivity to
barbituates by disulfiram treatment in rats. Res. Comm. Chem. Pathol.
Pharmacol., 24: 273-285.
MINKINS, C., CHEKUNOVA, M.P., & LEVINA, E.N. (1973) [Effect of
antimony and lead on the adrenal glands and biogenic amines.]
Gig. Tr. Prof. Zabol., 17: 21-24 (in Russian).
MITCHELL, C.L. & TILSON, H.A. (1982) Behavioral toxicology in risk
assessment: problems and research needs, Boca Raton, Florida, CRC
Press, pp. 265-274 (CRC Critical Reviews in Toxicology 10).
MITCHELL, C.L., TILSON, H.A., & CABE, P.A. (1982) Screening for
neurobehavioral toxicity: factors to consider. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 237-245.
MIYOSHI, T. & GOTO, I. (1973) Serial in vivo determinations of nerve
conduction velocity in rat tails: physiological and pathological
changes. Electroencephal. clin. Neurophysiol., 35: 125-131.
MOLINENGO, L. & ORSETTI, M. (1976) Drug action on the "grasping"
reflex and on swimming endurance: an attempt to characterize
experimentally anti-depressant drugs. Neuropharmacology,
15: 257-260.
MORALI, G., LARSSON, K., & BEYER, C. (1977) Inhibition of
testosterone-induced sexual behaviour in the castrated male rat by
aromatization blockers. Horm. Behav., 9: 203-213.
MORGANTI, J.B., LOWNE, B.A., SALVATERRA, P., & MASSARO, E.J. (1976)
Effects of open-field behaviour of mice exposed to multiple doses of
methylmercury. Gen. Pharmacol., 7(1): 41-44.
MUNRO, A.J., JACKSON, R.J., & KORNER, A. (1964) Disaggregation of
brain polysomes. Biochem. J., 92: 289-294.
MURPHY, S.D. & DUBOIS, K.P. (1958) The influence of various factors on
the enzymatic conversion of organic thiophosphates to
anticholinesterase agents. J. Pharmacol. exp. Ther., 124: 194-202.
NARAHASHI, T. (1982) Cellular and molecular mechanisms of action of
insecticides: neurophysiological approach. Neurobehav. Toxicol.
Teratol., 4: 753-758.
NARAHASHI, T. & HAAS, H.G. (1967) DDT: interaction with nerve membrane
conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & HAAS, H.G. (1968) Interaction with DDT with the
components of lobster nerve membrane conductance. J. gen. Physiol.,
51: 177-198.
NATHANSON, J.A. & BLOOM, F.E. (1975) Lead-induced inhibition of brain
adenylcyclase. Nature (Lond.), 255: 419-420.
NICHOLAS, G.S. & BARRON, D.H. (1932) The use of sodium amytal in the
production of anaesthesia in the rat. J. Pharmacol. exp. Ther., 46;
125-129.
NIEDERMEYER, E. & LOPES DA SILVA, F. (1982) Electroencephalography:
basic principles, clinical applications, and related fields,
Baltimore, Maryland, Munich, Urban Schwartzenberg.
NIOKESARYISKY, A.A. & TUROVSKY, V.S. (1980) N-acetylneuraminic acid
content in water-soluble and membranous tissue fractions of various
analysers of cerebral cortex. Biochimia, 45: 1829-1932.
NORTON, S. (1973) Amphetamine as a model for hyperactivity in the rat.
Physiol. Behav., 11: 181-186.
O'BRIEN, R.D. (1976) Acetylcholinesterase and its inhibition. In:
Wilkerson, C.F., ed. Insecticide biochemistry and physiology, New
York, Plenum Press, pp. 271-296.
O'CALLAGHAN, J.P., LAVIN, K.L., CHESS, Q., & CLOUET, D.H. (1983) A
method for dissection of discrete regions of rat brain following
microwave irradiation. Brain Res., 11: 31-42.
O'DONOGHUE, J.L., ed. (1985) Neurotoxicity of industrial and
commercial chemicals, Boca Raton, Florida, CRC Press.
OCHOA, J. (1972) Ultrathin longitudinal sections of single myelinated
fibres for electron microscopy. J. neurol. Sci., 17: 103.
OCHS, S. (1972) Fast transport of materials in mammalian nerve fibres.
Science, 176: 252.
OCHS, S. (1982) Axoplasmic transport and its relation to other nerve
functions, New York, John Wiley and Sons.
OHI, G., NISHIGAKI, S., SEKI, H., TAMURA, Y., MIZOGUCHI, I., YAGYA,
H., & NAGASHIMA, K. (1978) Tail rotation, an early neurological sign
of methylmercury poisoning in the rat. Environ. Res., 16: 353-359.
OLIVER, C., ESKAY, R.L., BEN-JONATHAN, N., & PORTER, J.C. (1974)
Distribution and concentration of TRH in rat brain. Endocrinology,
96: 540-546.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1979) Hippocampus
space, and memory. Behav. Brain Sci., 2: 313-365.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1980) Hippocampal
function: working memory or cognitive mapping? Physiol. Psychol.,
8: 239-246.
OTTO, D.A. (1977) Neurobehavioral toxicology: problems and methods in
human research. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 10/1-10/31
(EPA 600/9-77-042).
OTTO, D.A. (1978) Multidisciplinary perspectives in event-related
brain potential research. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Washington
DC, US Environmental Protection Agency (EPA 600/9-77-043).
OTTO, D.A., BENIGNUS, V.A., MULLER, K.E., & BARTON, C.N. (1981)
Effects of age and body lead burden on CNS function in young children.
I. Slow cortical potentials. Electroencephalogr. clin.
Neurophysiol., 52: 229-239.
OVERMANN, S.R. (1977) Behavioral effects of asymptomatic lead exposure
during neonatal development in rats. Toxicol. appl. Pharmacol.,
41: 459-471.
PADICH, R. & ZENICK, M. (1977) The effects of developmental and/or
direct lead exposure on FR behavior in the rat. Pharmacol. Biochem.
Behav., 6: 371-375.
PALKOVITS, M. (1973) Isolated removal of hypothalamic or other brain
nuclei of the rat. Brain Res., 59: 449-450.
PALMITER, R.D. & MULVIHILL, E.D. (1978) Estrogenic activity of the
insecticide kepone on the chick oviduct. Science, 201: 356-358.
PANCHENKO, L.F., DUDCHENKO, A.M., & SHPAKOV, A.A. (1975) The influence
of acute hypoxic hypoxia on the activity of rats cerebral cortex:
mitochondria enzymes possessing different sensibility to oxygen
deficiency. In: Gorky, ed. Biochemistry of hypoxia, pp. 59-64.
PANSKY, B. & ALLEN, D.J. (1980) Review of neuroscience, New York,
McMillan Inc.
PARIZEK, J. (1956) Effects of cadmium salts on testicular tissue.
Nature (Lond.), 177: 1036-1037.
PARTINGRON, C.R. & DALY, J.W. (1979) Effect of gangliosides on
adenylate cyclase activity in rat cerebral cortical membranes. Mol.
Pharmacol., 15: 484-491.
PARTRIDGE, W.M. (1976) Inorganic mercury: selective effects on
blood-brain barrier transport systems. J. Neurochem., 27: 333-335.
PATTON, S.E., O'CALLAGHAN, J.P., MILLER, D.B., & ABOU-DONIA, M.B.
(1983) Effect of oral adminstration of tri-o-cresyl phosphate on in
vitro phosphorylation of membrane and cytosolic proteins from
chicken brain. J. Neurochem., 41: 897-901.
PAUL, S.M., HOFFMAN, A.R., & AXELROD, A. (1980) Catechol estrogens:
synthesis and metabolism in brain and other endocrine tissues. In:
Martini, L. & Ganong, W.F., ed. Frontiers in neuroendocrinology, New
York, Raven Press, pp. 203-217.
PAVLENKO, S.M. (1975) Methods for the study of the central nervous
system in toxicological tests. In: Methods used in the USSR for
establishing biologically safe levels of toxic substances, Geneva,
World Health Organization, pp. 86-108.
PAVLOV, I.P (1927) Conditioned reflexes, Oxford, Oxford University
Press.
PEARSE, A.G.E. (1968) Histochemistry: theoretical and applied, 3rd
ed., Baltimore, Maryland, Williams and Wilkins Company.
PEARSON, C.M. & MOSTOFI, F.K. (1973) The striated muscle, Baltimore,
Maryland, Williams and Wilkins Company.
PEASE, D.E. (1964) Histological techniques for electron microscopy,
2nd ed., New York, Academic Press.
PERSINGER, M.A., LAFREINIERE, G.P., & CARREY, N.V. (1978) Thyroid
morphology and wet organ weight changes in rats exposed to different
low intensity 0.5-Hz magnetic fields and pre-experimental caging
conditions. Biometeorology, 22: 67-73.
PILCHER, C.W.T. & JONES, S.M. (1981) Social crowding enhances
aversiveness of naloxone in rats. Pharmacol. Biochem. Behav.,
14: 299-303.
PIRCH, J.H. & RECH, R.H. (1968) Effect of isolation on alpha-
methyltyrosine-induced behavioral depression. Life Sci., 7: 173-182.
PLEASURE, D.E., MISHLER, K.C., & ENGEL, W.K. (1969) Axonal transport
of proteins in experimental neuropathies. Science, 166: 524-525.
POST, E.M., YANG, M.G., KING, J.A., & SANGER, V.L. (1973) Behavioral
changes of young rats force-fed methylmercury chloride. Proc. Soc.
Exp. Biol. Med., 143: 1113-1116.
POWERS, J.M., SCHAUMBURG, H.H., JOHNSON, A.E., & RAINE, C.S. (1980) A
correlative study of the adrenal cortex in adrenoleukodystrophy:
evidence for a fatal intoxication with very long-chain saturated fatty
acids. Invest. cell Pathol., 3: 353-376.
PRABHU, V.G. (1972) Amphetamine aggregation effect in mice under
conditions of altered microsomal enzymes. Arch. int. Pharmacodyn.
Ther., 195: 81-86.
PROKHOROVA, M.J. (1974) Structure and function of phosphornositides
and gangliosides of the brain. In: Progress of neurochemistry,
Leningrad, Nauka, pp. 61-73.
PRYOR, G.T., UYENO, E.T., TILSON, H.A., & MITCHELL, C.L. (1983)
Assessment of chemicals using a battery of neurobehavioral tests: a
comparative study. Neurobehav. Toxicol. Teratol., 5: 91-117.
PURPURA, D.P., PENRY, J.K, TOWER, P., WOODBURY, D.M., & WALTER, R.
(1972) Experimental models of epilepsy, New York, Raven Press.
QUEVEDO, L., CONCHA, J., MARCHANT, Z., ESKUCHE, W., & MADARIAGA, J.
(1980) Nerve accommodation in chronic alcoholic subjects.
Pharmacology, 21: 229-232.
QUINN, G.P., AXELROD, J., & BRODIE, B.B. (1958) Species, strain, and
sex differences in metabolism of hexobarbitone, amidopyrine,
antipyrine, and aniline. Biochem. Pharmacol., 1: 152-159.
RADIN, N.S., BRENHERT, A., ARORA, R.C., SELLINGER, O.Z., & FLANGAS,
A.L. (1972) Glial and neuronal localization of cerebroside-
metabolizing enzymes. Brain Res., 39: 163-166.
RAFALES, L.S., BORNSCHEIN, R.L., MICHAELSON, A., LOCH, R.K., & BARKER,
G.F. (1979) Drug-induced activity in lead-exposed mice. Pharmacol.
Biochem. Behav., 10: 95-104.
RAMSAY, R.B., KRIGMAN, M.R., & MORELL, P. (1980) Developmental studies
of the uptake of choline, GABA, and dopamine by crude synaptosomal
preparations after in vivo or in vitro lead treatment. Brain
Res., 187: 383-387.
RAY, O.S. & BARRETT, R.J. (1975) Behavioral, pharmacological, and
biochemical analysis of genetic differences in rats. Behav. Biol.,
15: 391-417.
REICHARDT, L.F. & KELLY, R.B. (1983) A molecular description of nerve
terminal function. Ann. Rev. Biochem., 52: 871-926.
REICHERT, B.L. & ABOU-DONIA, M.B. (1980) Inhibition of fast axoplasmic
transport by delayed neurotoxic organophosphorus esters: a possible
mode of action. Mol. Pharmacol., 17: 56-60.
REITER, L.W. (1978) Use of activity measures in behavioral toxicology.
Environ. Health Perspect., 26: 9-20.
REITER, L.W. (1980) Neurotoxicology -- meet the real world.
Neurobehav. Toxicol., 2: 73-74.
REITER, L.W. (1983) Chemical exposure and animal activity: utility of
the Figure-Eight Maze. In: Hayes, A.H., Schnell, R.C., & Miya, T.S.,
ed. Developments in the science and practice of toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 78-84.
REITER, L.W. & MACPHAIL, R.C. (1979) Motor activity: a survey of
methods with potential use in toxicity testing. Neurobehav.
Toxicol., 1(Suppl. 1): 53-66.
REITER, L.W. & MACPHAIL, R.C. (1982) Factors influencing motor
activity measurements in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 45-65.
REITER, L.W., KIDD, K., HEAVNER, G., & RUPPERT, P. (1980) Behavioral
toxicity of acute and subacute exposure to triethyltin in the rat.
Neurotoxicology, 2: 97-112.
REUHL, K. & CHANG, L. (1979) Effects of methylmercury on the
development of the nervous system: a review. Neurotoxicology,
1: 21-55.
REYNOLDS, G.S. (1958) A primer of operant conditioning, Glenview,
Illinois, Scott, Foresman and Co.
RICE, D.C. & GILBERT, S.G. (1982) Early chronic low-level
methylmercury poisoning in monkeys impairs spatial vision. Science,
216: 759-761.
RICE, D.C. & WILLES, R.F. (1979) Neonatal low-level lead exposure in
monkeys (Macaca fascicularis): effects on two choice non-spatial
form discrimination. J. environ. Pathol. Toxicol., 2: 1195-1203.
RICE, D.C., GILBERT, S.G., & WILLES, R.F. (1979) Neonatal low-level
lead exposure in monkeys: locomotor activity, schedule-control
behavior, and the affects of amphetamine. Toxicol. appl. Pharmacol.,
51: 503-513.
RICE, D.F. (1985) Central nervous system effects of perinatal exposure
to lead or methylmercury in the monkey. In: Clarkson, T.W. & Nordberg,
G., ed. Reproductive developmental toxicity of metals, New York,
Plenum Press.
RITCHIE, J.M. (1979) A pharmacological approach to the structure of
sodium channels in myelinated axons. Ann. Rev. Neurosci.,
2: 341-362.
ROBERTS, D.V. (1977) A longitudinal electromyographic study of six men
occupationally exposed to organophosphorus compounds. Int. Arch.
occup. environ. Health, 38: 221-229.
ROBERTS, R.K., DESMOND, P.V., WILKINSON, G.R., & SCHENKER, S. (1979)
Disposition of chlordiazepoxide; sex differences and effects of oral
contraceptives. Clin. Pharmacol. Ther., 25: 826-831.
ROBINS, E., HIRSCH, H.E., & EMMONS, S.S. (1968) Glycosidases in the
nervous system. I. Assay, some properties, and distribution of
beta-galactosidase, beta-glucoronidase, and beta-glucosidase.
J. biol. Chem., 243: 4246-4249.
RODIER, P. (1978) Behavioral teratology. In: Wilson, J.G. & Fraser,
F.C., ed. Handbook of teratology, New York, Plenum Press, Vol. 4,
pp. 397-428.
ROEDER, R.J. & RUTTER, W.J. (1969) Multiple forms of DNA-dependent RNA
polymerase in eukaryotic organisms. Nature (Lond.), 224: 234-236.
ROSE, S.P.R. & SINHA, A.K. (1970) Separation of neuronal and neuropil
cell fractions: a modified procedure. Life Sci., 9: 907-912.
ROSECRANS, J.A., HONG, J.S., SQUIBB, R.E., JOHNSON, J.H., WILSON,
W.E., & TILSON, H.A. (1982) Effects of perinatal exposure to
chlordecone (kepone) on neuroendocrine and neurochemical
responsiveness of rats to environmental challenges. Neurotoxicology,
3: 131-142.
ROSEWICKYI, S., STANOSZ, S., & SAMOCHOWIEC, L. (1973) [The effect of
protracted exposure to carbon disulfide on the hormonal activity of
the ovaries in rats.] Med. Pracy., 24: 133-136 (in Polish).
ROZIER, L., SHIRAKI, H., & GRCEVIC, N., ed. (1977) Neurotoxicology,
New York, Raven Press, Vol. 1, 658 pp.
RUDNEV, M., BOKINA, A., ESKLER, N., & NAVAKATIKYAN, M. (1978) The use
of evoked potential and behavioural measures in the assessment of
environmental insult. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency, pp. 444-447 (EPA 600/9-77-0443).
RUSSELL, R.W., WARBURTON, D.M., & SEGAL, D.S. (1969) Behavioral
tolerance during chronic changes in the cholinergic system. Comm.
Behav. Biol., 4: 121-128.
RUSAK, B. & ZUCKER, I. (1975) Biological rhythms and animal behavior.
Ann. Rev. Psychol., 27: 137-171.
SABRI, M.I. & SPENCER, P.S. (1980) Toxic distal axonopathy:
biochemical studies and hypothetical mechanisms. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 206-219.
SABRI, M.I., MOORE, C.L., & SPENCER, P.S. (1979) Studies on the
biochemical basis of distal axonopathies. Inhibition of glycolysis
produced by neurotoxic hexacarbon compounds. J. Neurochem.,
32: 683-690.
SAKMANN, B. & NEHER, E. (1983) Single channel recording, New York,
Plenum Press, 503 pp.
SALVATERRA, P., LOWN, B., MORGANTI, J., & MARSARO, E.J. (1973)
Alterations in neurochemical and behavioural parameters in the mouse
induced by low doses of methylmercury. Acta pharmacol. toxicol.,
33(3): 177-190.
SANTINI, M. (1975) Golgi Centennial Symposium: Perspectives in
Neurobiology, New York, Raven Press.
SAVOLAINEN, H. (1983) Trends and prospects in experimental
neurotoxicology. Scand. J. Work environ. Health, 9: 214-218.
SAVOLAINEN, H. & JARVISALO, J. (1977) Effects of acute CS2
intoxication on protein metabolism in rat brain. Chem.-biol.
Interact., 17: 51-59.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINO, H. (1977)
Biochemical and behavioural effects of inhalation exposure to
tetrachloroethylene and dichloromethane. J. Neuropathol. exp.
Neurol., 36: 941-949.
SCHALLERT, T., WHISHAW, I.Q., RAMIREZ, V.D., & TEITELBAUM, P. (1978)
Compulsive, abnormal walking caused by anticholinergies in akinetic, 6
hydroxydopamine-treated rats. Science, 199: 1461-1463.
SCHAUMBURG, H.H. (1982) Pyridoxine megavitaminosis produces sensory
neuropathy in humans. Ann. Neurol., 12: 107-108.
SCHAUMBURG, H.H., SPENCER, P.S., & THOMAS, P.K. (1983) Disorders of
peripheral nerves, Philadelphia, Pennsylvania, F.A. Davis.
SCHAUMBURG, H.H., WISNIEWSKI, H., & SPENCER, P.S. (1974)
Ultrastructural studies of the dying-back process. I. Peripheral nerve
terminal and axon degeneration in systemic acrylamide intoxication.
J. Neuropathol. exp. Neurol., 33: 260-284.
SCHECHTER, M.D. & WINTER, J.C. (1971) Effect of mescaline and lysergic
acid diethylamide on flicker discrimination in the rat. J. Pharmacol.
exp. Ther., 177: 461-467
SCHEUHAMMER, A.M. & CHERIAN, M.G. (1982) The regional distribution of
lead in normal rat brain. Neurotoxicology, 3: 85-92.
SCHEVING, L.E., HALBERG, F., & PAULY, J.E., ed. (1974)
Chronobiology, Tokyo, Igaku Shoin Ltd.
SCHNAITMAN, C., ERWIN, V.G., & GREENSWALT, J.W. (1967) The
submitochondrial localization of monoamine oxidase. An enzymatic
marker for the outer membrane of rat liver mitochondria. J. cell
Biol., 32: 719-720.
SCHOENFELD, W.N., ed., (1970) The theory of reinforcement schedules,
New York, Appleton-Century-Crofts.
SCHOTMAN, P., GIPON, L., JENNEKENS, F.G.I., & GISPEN, W.H. (1978)
Polyneuropathies and CNS protein metabolism. III. Changes in protein
synthesis induced by acrylamide intoxication. J. Neuropathol. exp.
Neurol., 37: 820-837.
SCHREIBER, V. & PRIBYL, T. (1980) Possible role of ceruloplasmin in
endocrine regulation. In: Ishü, S., Hirano, T., & Wade, M., ed.
Hormones, adaptation, and evolution, Tokyo, Japan Science Society
Press/Berlin, Springer-Verlag.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1982) Inhibition by an
ergoline derivative (dopaminergic agonist) of estradiol-induced
adenohypophyseal growth and of the decrease in the hypothalamic
ascorbic acid (HA) concentration in rats. Physiol. Bohemoslov.,
31: 97-100.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1979) Effect of a dopamine
p-hydroxylase inhibitor (disulfiram) on the response of
adenohypophysis, serum ceruloplasmin, and hypothalamic ascorbic acid
to estradiol treatment. Endoc. Exper. (Bratislava), 13: 131-138.
SCHREIBER, V., ZBUZEK, V., & ZBUZKOVA-KMENTOVA, V. (1969) Reactions of
the rat and hamster endocrine system to aminoglutethimide. Physiol.
Bohemoslov., 18: 249-261.
SEPPALAINEN, A.M. & LINNOILA, I. (1975) Electrophysiological studies
on rabbits in long-term exposure to carbon disulphide. Scand. J. Work
environ. Health, 1: 178-183.
SEPPALAINEN, A.M., TOLONEN, M., KARLI, P., HANNINEN, H., & HEINBERG,
S. (1972) Neurophysiological findings in chronic carbon disulfide
poisoning: a descriptive study. Work Environ. Health, 9: 71-75.
SETH, P.K., AGRAWAL, A.K., & BONDY, S.C. (1981) Biochemical changes in
the brain consequent to dietary exposure of developing and mature rats
to chlordecone (kepone). Toxicol. appl. Pharmacol., 26: 262-267.
SHANDALA, M.G., RUDNEV, M.I., & NAVAKATIKYAN, M.A. (1980) [Behavioural
reactions in experimental hygienic research.] Gig. i Sanit.,
6: 43-48 (in Russian).
SHAW, C.M., MOTTET, N.K., & CHEN, W.J. (1980) Effects of methylmercury
on the visual system of rhesus macaque (Macaca mulatta). II.
Neuropathological findings (with emphasis on vascular lesions in the
brain). In: Merigan, W.H. & Weiss, B., ed. Neurotoxicity of the
visual system, New York, Raven Press, pp. 123-134.
SHAW, C.M., MOTTET, N.K., BODY, R.L., & LUSCHEI, E.S. (1975)
Variability of neuropathological lesions in experimental methylmercury
encephalopathy in primates. Am. J. Pathol., 80: 451-470.
SHIBUSAWA, K., YAMAMOTO, T., NISHI, K., ABE, C., & TOMIE, S. (1959)
Urinary secretion of TRF in various functional states of the thyroid.
Endocrinol. Jpn., 6: 131-136.
SHILJAGINA, N.N. (1971) [Evoked potentials in the projection of sign
stimulus during conditional reflex formation in ontogenesis.]
Zh. Vyssh. nerv. deyat. imeni, 21: 492-500 (in Russian).
SHIMIZU, T., TAKAISHI, M., & SHISHIBE, Y. (1978) Effect of ethanol on
the peripheral metabolism of thyroxine. Endocrinol. Jpn,
25: 201-204.
SHUGALOV, N.P. (1969) Primary and associative evoked responses to
optic irritants during various stages of conditioned reflex formation
in cats. In: Summary of Reports of the 22nd Meeting on the Problems
of Higher Nervous Activity, Ryazan, USSR, p. 268.
SHUMILINA, A.I. (1971) Characteristics of summary electric activity
and evoked potentials under motivating stimulation of various
biological significance. In: Proceedings of the Sixth All-Union
Conference on Electrophysiology of the Central Nervous System,
pp. 301-302.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974a) Hyperactivity: a
lead-induced behavior disorder. Environ. Health Perspect.,
7: 227-232.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974b) Lead-induced behavioral
dysfunction: an animal model of hyperactivity. Exp. Neurol.,
42: 146-157.
SILBERGELD, E.K. & GOLDBERG, A.M. (1975) Pharmacological and
neurochemical investigations of lead-induced hyperactivity.
Neuropharmacology, 14: 431-444.
SILBERGELD, E.K., HRUSKA, R.E., MILLER, L.P., & ENG, N. (1980) Effects
of lead in vivo and in vitro on GABA energic neurochemistry.
J. Neurochem., 34: 1711-1718.
SILVERMAN, A.P. (1965) Ethological and statistical analysis of drug
effects on the social behaviour of laboratory rats. Br. J.
Pharmacol., 24: 579-590.
SLOANE, S.A., SHEA, S.L., PROCTER, M.M., & DEWSBURY, D. (1978) Visual
cliff performance in 10 species of muroid rodents. Anim. Learn.
Behav., 6: 244-248.
SNOWDON, C.T. (1973) Learning deficits in lead injected rats.
Pharmacol. Biochem. Behav., 1: 599-603.
SNYDER, D.R. & BRAUN, J.J. (1977) Dissociation between behavioral and
physiological indices of organomercurial ingestion. Toxicol. appl.
Pharmacol., 41: 277-284.
SNYDER, S.H. & INMS, R.B. (1979) Peptide neurotransmitters. Ann. Rev.
Biochem., 48: 755-782.
SOBOTKA, T.J., BRODIE, R.E., & COOK, M.P. (1975) Psychophysiologic
effects of early lead exposure. Toxicology, 5: 175-191.
SOKOLOFF, L., REIVICH, M., KENNEDY, C., DES ROSIERS, M.H., PATLAK,
C.S., PETTIGREW, K.D., SAKURADA, O., & SHINOHARA, M. (1977) The
14C-deoxyglucose method for the measurement of local cerebral glucose
utilization: theory, procedure, and normal values in the conscious and
anaesthetized albino rat. J. Neurochem., 28: 897-916.
SPENCER, P.S. & BISCHOFF, M.C. (1982) Contemporary neuropathological
methods in toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 259-275.
SPENCER, P.S. & LIEBERMAN, A.R. (1971) Scanning electron microscopy of
isolated peripheral nerve fibres. Normal surface structure and
alterations proximal to neuromas. Z. Zellforsch. Mikrosk. Anat.,
119: 534-551.
SPENCER, P.S. & SCHAUMBURG, H.H. (1980) Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
929 pp.
SPENCER, P.S. & THOMAS, P.K. (1970) The examination of isolated nerve
fibres by light and electron microscopy, with observations on
demyelination proximal to neuromas. Acta neuropathol., 16: 177-186.
SPENCER, P.S., BISCHOFF, M., & SCHAUMBURG, H.H. (1980)
Neuropathological methods for the detection of neurotoxic disease. In:
Spencer, P.S. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 743-757.
SPERANSKIJ, S.V. (1965) [Advantages incident to the use of mounting
current in analysing the ability of Albino mice to summate subliminal
impulses.] Farmakol. i Toksikol., 1: 123-124 (in Russian).
SPYKER, J. (1975) Behavioral teratology and toxicology. In: Weiss, B.
& Laties, V.G., ed. Behavioral toxicology, New York, Plenum Press,
pp. 311-349.
SQUIBB, R.E. & TILSON, H.A. (1982) Neurobehavioural changes in adult
Fisher 344 rats exposed to dietary levels of chlordecone (KeponeR):
a 90-day chronic dosing study. Neurotoxicology, 3: 59-66.
STEBBINS, W.C. (1970) Animal psychophysics: the design and conduct of
animal experiments, New York, Appleton-Century-Crofts.
STEEL, R.G.D. & TORRIE, J.H. (1960) Principles and procedures of
statistics, New York, McGraw-Hill, pp. 83-347.
STEELE, W.J. & BUSCH, H. (1963) Studies on acidic nuclear proteins of
the Walker tumour and liver. Cancer Res., 23: 1153-1157.
STOCKINGER, H.E. (1974) Behavioral toxicology in the development of
threshold limit values. In: Xintaras, C., Johnson, B.L., & DeGroot,
I., ed. Behavioral toxicology, Cincinnati, Ohio, US Department of
Health, Education and Welfare, pp. 18-19 (US DHEW (NIOSH) Publication
No. 74-126).
SUDAKOV, K.V. (1982) Functional system theory in physiology.
Agressologie, 23: 167-176.
SZMIGIELSKI, A. (1975) Effect of disulfiram and sodium
diethyldithiocarbamate on thyroxine and dopamine hydroxylases in rat
brain and breast. Pol. J. Pharmacol. Pharm., 27: 153-159.
TAKEUCHI, T. (1968) Pathology of Minamata disease. In: Katsuma, M.,
ed. Minamata disease. Study Group of Minamata Disease, Japan,
Kumamoto University, pp. 141-228.
TAKEUCHI, T., ETO, N., & ETO, K. (1979) Neuropathology of childhood
cases of methylmercury poisoning (Minamata disease) with prolonged
symptoms with particular reference to the decortication syndrome.
Neurotoxicology, 1: 1-20.
TALKE, T., HIRABAGASHI, J., KONDO, R., MATSUMOTO, M., & KOJIMA, K.
(1979) Effect of butyrate on glycolipid metabolism of two cell types
of rat ascites hepatomes with different ganglioside biosynthesis.
J. Biochem., 96: 1139-1402.
TAPPEL, A.L. (1970) Lipid peroxidation damage to cell components.
Fed. Proc., 29: 239.
TAYLOR, J.R., SELHORST, J.B., & CALABRESE, V.P. (1980) Chlordecone.
In: Spencer, P. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 407-421.
THIESSEN, D.D. (1964) Population density and behavior: a review of
theoretical and physiological contributions. Texas Rep. Biol. Med.,
22: 266-314.
THOMAS, P.K. (1970) The quantitation of nerve biopsy findings.
J. neurol. Sci., 11: 285-295.
THOMAS, P.K. (1980) The peripheral nervous system as a target for
toxic substances. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 35-47.
THOMPSON, S.W. (1963) Selected histochemical and histopathological
methods, Springfield, Illinois, Charles C. Thomas.
THORNDIKE, E.L. (1932) Reward and punishment in animal learning.
Comp. Psychol. Monogr., 39: 8.
TILSON, H.A. & CABE, P.A. (1978) Strategy for the assessment of
neurobehavioral consequences of environmental factors. Environ.
Health Perspect., 26: 287-299.
TILSON, H.A. & HARRY, G.J. (1982) Behavioral principles for use in
behavioral toxicology and pharmacology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 1-27.
TILSON, H.A. & MITCHELL, C.L. (1984) Neurobehavioral techniques to
assess the effects of chemicals on the nervous system. Ann. Rev.
Pharmacol. Toxicol., 24: 425-450.
TILSON, H.A. & SQUIBB, R.D. (1982) The effects of acrylamide on the
behavioral suppression produced by psychoactive agents.
Neurotoxicology, 3: 113-120.
TILSON, H.A., BYRD, N., & RILEY, M. (1979a) Neurobehavioral effects of
exposing rats to kepone via the diet. Environ. Health Perspect.,
33: 321.
TILSON, H.A., CABE, P.A., ELLINWOOD, E.H., Jr, & GONZALEZ, L.P.
(1979b) Effects of carbon disulfide on motor function and
responsiveness to delta-amphetamine in rats. Neurobehav. Toxicol.,
1: 57-63.
TILSON, H.A., MITCHELL, C.L., & CABE, P.A. (1979c) Screening for
neurobehavioral toxicity: the need for and examples of validation of
testing procedures. Neurobehav. Toxicol., 1(Suppl. 1): 137-148.
TILSON, H.A., CABE, P.A., & BURNE, T.A. (1980) Behavioral procedures
used in the assessment of neurotoxicity. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 758-766.
TILSON, H.A., MACTUTUS, C.F., MCLAMB, R.L., & BURNE, T.A. (1982a)
Characterization of triethyl lead chloride neurotoxicity in adult
rats. Neurobehav. Toxicol. Teratol., 4: 671-681.
TILSON, H.A., SQUIBB, R.E., & BURNE, T.A. (1982b) Neurobehavioral
effects following a single dose of chlordecone (kepone) administered
neonatally to rats. Neurotoxicology, 3: 45-52.
TOLMAN, E.C. (1948) Cognitive maps in rats and men. Psychol. Rev.,
55: 189-208.
TOWFIGHI, J., GONATAS, N.K., & MCCREE, L. (1975) Hexachlorophene
retinopathy in rats. Lab. Invest., 32: 330-338.
TROPKINA, J.K., SELZNEVA, S.N., & POGODAEV, K.V. (1981) Prenatal
influence of hypoxia on metabolic structure of cerebral mitochondria
in the oncogenesis with epileptic superactivity. In: Mitochondria:
mechanisms of conjugation and regulation, Puscino, p. 39.
TRUEX, R.C. & CARPENTER, M.B. (1983) Human neuroanatomy, 8th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
TSUJI, S. & FUJIHSIMA H. (1975) Paraplegias: clinical statistics,
Fukuoka, Kyushu Rosai Hospital, Department of Orthopedics and
Neurology.
TULLOCH, M.L., CROOKS, J., & BROWN, P.S. (1963]) Inhibition of thyroid
function by a dithiocarbamoylhydrazine. Nature (Lond.),
199: 288-289.
ULRICH, C.E., RINEHART, W., & BRANDT, M. (1979) Evaluation of the
chronic inhalation toxicity of a manganese oxide aerosol. III.
Pulmonary function, electromyograms, limb tremor, and tissue manganese
data. Am. Ind. Hyg. Assoc. J., 40: 349-353.
UPHOUSE, L., NEMEROFF, C.B., MASON, G., PRANGE, A.J., & BONDY, S.C.
(1982) Interactions between "handling" and acrylamide on endocrine
responses in rats. Neurotoxicology, 3: 121-125.
UPHOUSE, L., MASON, G., & HUNTER, V. (1984) Persistent vaginal estrus
and serum hormones after chlordecone (kepone) treatment of adult rats.
Toxicol. appl. Pharmacol., 72: 177-186.
US FDA (1978) Nonclinical laboratory studies. Good laboratory
practice regulation, Washington DC, US Food and Drug Administration,
Department of Health, Education and Welfare (Federal Register 43:
59986-60025).
US NAS (1975) Principles for evaluating chemicals in the
environment, Washington DC, National Academy of Science (A Report of
the Committee for the Working Conference on Principles of Protocols
for Evaluating Chemicals in the Environment).
US NAS/NRC (1970) Evaluating the safety of food chemicals,
Washington DC, National Academy of Sciences, National Research
Council, Food Protection Committee, Food Nutrition Branch, Division of
Biology and Agriculture.
VAN GELDER, G.A. (1978) Lead and the nervous system. In: Oehme, F.W.,
ed. Toxicity of heavy metals in the environment, Part 1, Basel, New
York, Marcel Dekker, Inc., pp. 101-121.
VANDERWOLF, C.H. & LEUNG, L.-W.S. (1983) Hippocampal rhythmic slow
activity: a brief history and the effects of entorhinal lesions and
phencyclidine. In: Seifert, W., ed. Neurobiology of the hippocampus,
New York, Academic Press, pp. 275-302.
VASAN, N.S. & CHASE, H.P. (1976) Brain glucosaminoglycans
(mucopolysaccharides) following intrauterine growth retardation.
Biol. Neonate, 28: 196-206.
VEKSLER, M.S. & ARBUKHANOVA, M.S. (1981) Cerebral and spinal cord
mitochondria under strenuous physical work. In: Mitochondria:
mechanism of conjugation and regulation, Puscino, p. 81.
VERGIEVA, T. & ZAIKOV, H.R. (1981) Behavioral changes in rats with
inhalation of styrene. Hig. Zdraveopazvane, 24: 242-247.
VERITY, M.A. (1972) Cation modulation of synaptosomal respiration.
J. Neurochem., 19: 1305-1309.
VERITY, M.A., BROWN, W.J., CHEUNG, M., & DZER, G. (1977) Methylmercury
inhibition of synaptosomes and brain slices protein synthesis: in
vivo and in vitro studies. J. Neurochem., 29: 673-679.
VILA, J. & COLOTLA, V.A. (1981) Some stimulus properties of inhalants:
preliminary findings. Neurobehav. Toxicol., 3: 447-480.
VINAR, O., FRANTIK, E., & HORVATH, M. (1981) Subjectively-perceived
effects of diazepam, toluene, and their combination. Act. Nerv.
super. (Praha), 23: 179-182.
VORHEES, C.V. (1974) Some behavioral effects of maternal
hypervitaminosis A in rats. Teratology, 17: 269-273.
VORHEES, C.V. & BUTCHER, R.E. (1982) Behavioral teratogenicity. In:
Snell, L., ed. Developmental toxicology, London, Croom Helm Press,
pp. 247-298.
VORHEES, C.V., BRUNNER, R.L., MCDANIEL, C.R., & BUTCHER, R.E. (1978)
The relationship of gestational age to vitamin A induced postnatal
dysfunction. Teratology, 17: 271-276.
WALLMAN, J. (1975) A simple technique using an optomotor response for
visual psychophysical measurements in animals. Vision Res., 15: 3-8.
WALSH, J.M., CURLEY, M.D., BURCH, L.S., & KURLANSIK, L. (1982) The
behavioral toxicity of a tributyltin ester in the rat. Neurobehav.
Toxicol. Teratol., 4: 241-246.
WALSH, R.N. (1980) On the necessity for a shift in emphasis from
means-oriented to problems-oriented research in developmental
psychobiology. Dev. Psychobiol., 13: 229-231.
WALSH, T.J., MILLER, D.B., & DYER, R.S. (1982b) Trimethyltin, a
selective limbic system neurotoxicant, impairs radial-arm maze
performance. Neurobehav. Toxicol. Teratol., 4: 177-184.
WALTON, J.N. (1974) Disorders of voluntary muscle, 3rd ed.,
Edinburg, London, Churchill Livingston.
WANG, G.H. (1932) Relation between spontaneous activity and oestrous
cycle in the white rat. Comp. Psychol. Monogr., 6: 1-32.
WEIBEL, E.R. (1979) Stereological methods. In: Practical methods for
biological morphometry, New York, Academic Press, Vol. 1.
WEISS, B., FERRIN, J., MERIGAN, M., & COX, C. (1981) Modification of
rat operant behaviour by ozone exposure. Toxicol. appl. Pharmacol.,
58: 244-251.
WEISS, B. & LATIES, V. (1961) Changes in pain tolerance and other
behavior produced by salicylates. J. Pharmacol. exp. Ther.,
131: 120-129.
WEISS, B. & LATIES, V. (1969) Behavioral pharmacology and toxicology.
Ann. Rev. Pharmacol., 9: 297-326.
WEISS, B. & LATIES, V.G., ed. (1975) Behavioral toxicology, New
York, Plenum Press, 469 pp.
WEISS, B. & LATIES, V.G. (1979) Assays fox behavioural toxicity: a
strategy for the Environmental Protection Agency. Neurobehav.
Toxicol., 1(Suppl. 1): 213-215.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, J., CONNEY, A.H.
(1971) Effect of halogenated hydrocarbon insecticides on the
metabolism and uterotropic action of estrogens in rats and mice.
Toxicol. appl. Pharmacol., 19: 234-246.
WELLS, G.A.H., HOWELL, J.M., & GOPINATH, C. (1976) Experimental lead
encephalopathy of calves: histological observations on the nature and
distribution of the lesions. Neuropathol. appl. Neurobiol.,
2: 175-190.
WHEATLEY, B., BARBEAU, A., CLARKSON, T.W., & LAPHAM, L.W. (1979)
Methylmercury poisoning in Canadian indians -- the elusive diagnosis.
Can. J. neurol. Sci., 6: 417-422.
WHO (1975) Methods used in the USSR for establishing biologically
safe levels of toxic substances, Geneva, World Health Organization,
171 pp.
WHO (1978) Principles and methods of evaluating the toxicity of
chemicals. Part 1, Geneva, World Health Organization (Environmental
Health Criteria Series No. 6, Part 1).
WHO (1984) Principles for evaluating health risks to progeny
associated with exposure to chemicals during pregnancy, Geneva,
World Health Organization (Environmental Health Criteria Series
No. 30).
WHO (in preparation) Principles and methods of evaluating the
toxicity of chemicals. Part 2, Geneva, World Health Organization
(Environmental Health Criteria Series No. 6, Part 2).
WILLIAMS, P.L. & WARWICK, R. (1975) Functional neuroanatomy of man,
Philadelphia, Pennsylvania, W.B. Saunders Company.
WILSON, W.E. (1982) Dopamine-sensitive adenylate cyclase inactivation
by organolead compounds. Neurotoxicology, 3: 100-107.
WINNEKE, G. (1979) Modification of visual evoked potentials in rats
after long-term blood lead elevation. Act. Nerv. super. (Praha),
21: 282-284.
WINNEKE, G., BROCKHAUS, A., & BALTISSEN, R. (1977) Neurobehavioral and
systematic effects of longterm blood lead-elevation in rats. I.
Discrimination learning and open field behavior. Arch. Toxicol.,
37: 247-263.
WINNEKE, G., FODOR, G., & SCHLIPKOTER, H. (1978) Carbon monoxide,
trichloroethylene, and alcohol: reliability and validity of
neurobehavioural effects. Multidisciplinary perspectives in event-
related brain potentials. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency (EPA 600/9-77-0443).
WINNEKE, G., LILIENTHAL, H., & ZIMMERMANN, U. (1984) Neurobehavioural
effects of lead and cadmium. In: Proceedings of the Third
International Congress on Toxicology, San Diego, 28 August-2
September 1983, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
WOLF, D.L. (1976) Rotating rod, spontaneous activity, and passive
avoidance conditioning: their suitability as functional tests in
industrial toxicology. In: Horvath, M. & Frantik, E., ed. Adverse
effects of environmental chemicals and psycho- tropic drugs,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 293-303.
WOOD, R.W. (1978) Stimulus properties of inhaled substances. Environ.
Health Perspect., 26: 69-76.
WOOD, R.W. (1979) Reinforcing properties of inhaled substances.
Neurobehav. Toxicol., 1(Suppl. 1): 67-72.
WOOD, R.W. (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav. Toxicol. Teratol., 3: 397-405.
WOOLLEY, D.E. (1977) Electrophysiological techniques in
neurotoxicology. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 9-1 -- 9-25 (EPA
600/9-77-042).
XINTARAS, C. & BURG, J.R. (1981) Neurotoxicity studies of workers
exposed to carbon disulfide. G. Ital. Med. Lav., 3: 83-86.
XINTARAS, C., JOHNSON, B.L., & DEGROOT, I., ed. (1974) Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, 507 pp (US DHEW (NIOSH) Publication No. 74-126).
YAMAMURA, H.I., ENNA, S.J., & KUHAR, M.J. (1981) Neurotransmitter
receptor binding, New York, Raven Press.
YONESAWA, T., BORNSTEIN, M.B., & PETERSON, E.R. (1980) Organotypic
cultures of nerve tissue as a model for neurotoxicity investigation
and screening. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental
and clinical neurotoxicology, Baltimore, Maryland, Williams and
Wilkins Company, pp. 788-802.
YOUNG, J.S. & FECHTER, L.D. (1983) Reflex inhibition procedures for
animal audiometry: a technique for assessing ototoxicity. J. Acoust.
Soc. Am., 73: 1686-1693.
ZAKUSOV, V.V. (1936) [Estimation of the reflex time under the
influence of some narcotic substances.] Fiziol. Zh. USSR,
23: 276-278 (in Russian).
ZAKUSOV, V.V. (1953) [Pharmacology of the nervous system,]
Leningrad, Medgiz, 258 pp (in Russian).
ZBINDEN, G. (1981) Experimental methods in behavioral teratology.
Arch. Toxicol., 48: 69-88.
ZEMAN, W. & INNES, J.R.M. (1963) Craigie's neuroanatomy of the rat,
New York, Academic Press.
ZENICK, H., RODRIQUEZ, W., WARD, J., & ELKINGTON, B. (1979) Deficits
in fixed-interval performance following prenatal and postnatal lead
exposure. Dev. Psychobiol., 12(5): 509-514.
ZILOV, V.G., ROGACHEVA, S.K., & IVANOVA, L.I. (1983)
[Electroencephalographic analysis of limbic-reticular interrelations
in various motivational reactions of rabbits under ethanol.] Bull.
exp. Biol. Med., 11: 74-77 (in Russian).
ZUBER, V.L. (1978) Intensity of metabolism of various fractions of
cerebral phospholipids under hyperphenyl-alaninaemia. Vop. med.
Xhim., 2: 163-166.
ZYLBER-HARAN, E.A., GERSHMAN, H., ROSENMANN, E., & SPITZ, I.M. (1982)
Gonadotrophin, testosterone, and prolactin interrelationships in
cadmium-treated rats. J. Endocrinol., 92: 123-130.

 5.2.3 Neurocellular reaction to injury
5.2.3.1 Biological principles
Neurons are highly atypical cells because their cytoplasmic
processes often occupy a much greater volume than their cell somata.
This unusual cellular architecture provides an enormous surface area
for chemical attack and places a great demand on the soma, since it
alone has the metabolic machinery required to maintain the cellular
processes. Specialized transport systems have evolved to convey
information to the cell body, the axon, and its terminal regions
(Ochs, 1982). The functional problem can be illustrated by considering
a peripheral motor neuron, located in the lumbar spinal cord and
innervating muscle in the foot, which must maintain the structure and
function of an axon that is about a metre in length. Failure to
maintain the entire length of this enormous column of cytoplasm, by
interruption of axonal transport or by some other mechanism, may
account for the vulnerability of distal axons in many types of
neurotoxic disease. Other types of neurotoxins (e.g., doxorubicin) may
interfere with the metabolic machinery of the soma, thereby resulting
in degeneration of the entire neuron. Comparable explanations can be
offered to explain the vulnerability to chemical attack of myelinating
cells, their cytoplasmic processes, and myelin sheath (Spencer &
Schaumburg, 1980).
There are two considerations when contemplating toxic damage to an
organ or tissue. First, there are the changes that can be related to
the action of the chemical agent; second, there is the reaction of the
tissue to these changes. When toxic damage to neurons and myelinating
cells is considered, it is often difficult to separate the two phases
of the pathological process being studied. The situation is further
complicated as the type of change observed is often dependent on dose,
and may also vary with other factors, such as the species. In general,
however, chemical attack leads to two types of primary change in
neural cells:
(a) the accumulation, proliferation, or rearrangement of
structural elements (e.g., intermediate filaments,
microtubules) or organelles (e.g., mitochondria, lysosomes);
and/or
(b) the breakdown (degeneration) of cells, in whole or in part.
The latter is usually followed by regenerative processes, and these
may occur during the period of intoxication. Changes in the cellular
elements of intraneural blood vessels may occur, and secondary changes
may develop in other organ systems, notably voluntary muscle (Walton,
1974).
Neural cells appear to have a limited repertoire of pathological
responses, and the consequences of many types of toxic damage can be
predicted from an understanding of the biology and function of the
cells that are involved. As in any organ system, changes in one cell
type -- the primary target -- usually lead to secondary and tertiary
responses in related cells, so that the net effect is a predictable
cascade of pathological responses. For the nervous system, however,
the neuropathological changes often have to be considered with regard
to the fact that, while cellular responses to toxic injury may occur
locally, they may also occur at distant sites. Changes may also
develop in different locations as a function of time, dose, and/or
duration of intoxication.
5.2.3.2 Neurons
Loss of the cell body of neurons (neuronopathy) is an irreversible
event seen in many types of intoxication. Similar tissue reactions
occur in the CNS and PNS. Fig. 3 illustrates the changes occurring
with loss of primary sensory neurons in dorsal root ganglia. Glial
cells and macrophages proliferate around the dying cell (neurono-
phagia) and axon processes under Wallerian degeneration. This involves
breakdown of the entire length of the axon, dissolution and removal of
the myelin sheath, and the proliferation of phagocytic and glial
cells. Degenerated myelin is removed more slowly in the CNS than in
the PNS and astrocytes increase the number and size of their fiberous
processes to occupy the space left by the degenerating nerve fibre.
Axon degeneration also produces significant secondary changes in the
denervated cells, such as muscle (neurogenic atrophy) or other neurons
(transynaptic and retrograde transynaptic degeneration) (Blackwood et
al., 1971).
Neurons survive and recover from certain types of toxic assault,
notably those that cause structural damage to their processes.
Abnormalities of axons may be expressed in the form of generalized
atrophy or localized swellings containing excessive numbers of
structural elements or organelles, with associated secondary changes
in the myelin sheath in the form of corrugation and displacement
(secondary demyelination), respectively. Disruption of axonal
integrity leads to axon atrophy and/or degeneration (axonopathy) below
the site of injury (Fig. 4), with secondary changes in target cells,
such as muscle (neurogenic atrophy) or neuron (transynaptic
degeneration). Oligodendrocytes and Schwann cells lose their ability
to maintain myelin and then undergo cell division, while phagocytic
cells remove the myelin debris. The cell bodies of affected neurons
may also undergo responses secondary to axonal lesions, including cell
necrosis or, more commonly, rearrangement of cellular components
(chromatolysis) as a prelude to axon regeneration. Regrowth of the
axon commences promptly at the position of axon interruption and, in
the PNS, the elongating neuronal process reassociates with Schwann
cells, which elaborate a new myelin sheath and conduct the
5.2.3 Neurocellular reaction to injury
5.2.3.1 Biological principles
Neurons are highly atypical cells because their cytoplasmic
processes often occupy a much greater volume than their cell somata.
This unusual cellular architecture provides an enormous surface area
for chemical attack and places a great demand on the soma, since it
alone has the metabolic machinery required to maintain the cellular
processes. Specialized transport systems have evolved to convey
information to the cell body, the axon, and its terminal regions
(Ochs, 1982). The functional problem can be illustrated by considering
a peripheral motor neuron, located in the lumbar spinal cord and
innervating muscle in the foot, which must maintain the structure and
function of an axon that is about a metre in length. Failure to
maintain the entire length of this enormous column of cytoplasm, by
interruption of axonal transport or by some other mechanism, may
account for the vulnerability of distal axons in many types of
neurotoxic disease. Other types of neurotoxins (e.g., doxorubicin) may
interfere with the metabolic machinery of the soma, thereby resulting
in degeneration of the entire neuron. Comparable explanations can be
offered to explain the vulnerability to chemical attack of myelinating
cells, their cytoplasmic processes, and myelin sheath (Spencer &
Schaumburg, 1980).
There are two considerations when contemplating toxic damage to an
organ or tissue. First, there are the changes that can be related to
the action of the chemical agent; second, there is the reaction of the
tissue to these changes. When toxic damage to neurons and myelinating
cells is considered, it is often difficult to separate the two phases
of the pathological process being studied. The situation is further
complicated as the type of change observed is often dependent on dose,
and may also vary with other factors, such as the species. In general,
however, chemical attack leads to two types of primary change in
neural cells:
(a) the accumulation, proliferation, or rearrangement of
structural elements (e.g., intermediate filaments,
microtubules) or organelles (e.g., mitochondria, lysosomes);
and/or
(b) the breakdown (degeneration) of cells, in whole or in part.
The latter is usually followed by regenerative processes, and these
may occur during the period of intoxication. Changes in the cellular
elements of intraneural blood vessels may occur, and secondary changes
may develop in other organ systems, notably voluntary muscle (Walton,
1974).
Neural cells appear to have a limited repertoire of pathological
responses, and the consequences of many types of toxic damage can be
predicted from an understanding of the biology and function of the
cells that are involved. As in any organ system, changes in one cell
type -- the primary target -- usually lead to secondary and tertiary
responses in related cells, so that the net effect is a predictable
cascade of pathological responses. For the nervous system, however,
the neuropathological changes often have to be considered with regard
to the fact that, while cellular responses to toxic injury may occur
locally, they may also occur at distant sites. Changes may also
develop in different locations as a function of time, dose, and/or
duration of intoxication.
5.2.3.2 Neurons
Loss of the cell body of neurons (neuronopathy) is an irreversible
event seen in many types of intoxication. Similar tissue reactions
occur in the CNS and PNS. Fig. 3 illustrates the changes occurring
with loss of primary sensory neurons in dorsal root ganglia. Glial
cells and macrophages proliferate around the dying cell (neurono-
phagia) and axon processes under Wallerian degeneration. This involves
breakdown of the entire length of the axon, dissolution and removal of
the myelin sheath, and the proliferation of phagocytic and glial
cells. Degenerated myelin is removed more slowly in the CNS than in
the PNS and astrocytes increase the number and size of their fiberous
processes to occupy the space left by the degenerating nerve fibre.
Axon degeneration also produces significant secondary changes in the
denervated cells, such as muscle (neurogenic atrophy) or other neurons
(transynaptic and retrograde transynaptic degeneration) (Blackwood et
al., 1971).
Neurons survive and recover from certain types of toxic assault,
notably those that cause structural damage to their processes.
Abnormalities of axons may be expressed in the form of generalized
atrophy or localized swellings containing excessive numbers of
structural elements or organelles, with associated secondary changes
in the myelin sheath in the form of corrugation and displacement
(secondary demyelination), respectively. Disruption of axonal
integrity leads to axon atrophy and/or degeneration (axonopathy) below
the site of injury (Fig. 4), with secondary changes in target cells,
such as muscle (neurogenic atrophy) or neuron (transynaptic
degeneration). Oligodendrocytes and Schwann cells lose their ability
to maintain myelin and then undergo cell division, while phagocytic
cells remove the myelin debris. The cell bodies of affected neurons
may also undergo responses secondary to axonal lesions, including cell
necrosis or, more commonly, rearrangement of cellular components
(chromatolysis) as a prelude to axon regeneration. Regrowth of the
axon commences promptly at the position of axon interruption and, in
the PNS, the elongating neuronal process reassociates with Schwann
cells, which elaborate a new myelin sheath and conduct the

 regenerating axon to its target organ (muscle or sense organ)
(Schaumburg et al., 1983). In the CNS, astrocytes respond to injury by
increasing the number and size of their fibrous processes, and these
appear to inhibit regeneration of all but the smallest (e.g.,
monoaminergic) axons. Prominent axon swellings develop at the viable
end of larger fibres, a process known as axonal dystrophy.
5.2.3.3 Myelinating cells
Some chemical compounds induce primary morphological changes in
myelinating cells or their myelin sheaths. It is usual to separate
agents that induce changes in the cell body of Schwann cells or
oligodendrocytes from those that cause abnormalities selectively in
the myelin sheath, though this distinction may be misleading as many
compounds can produce both types of change, depending on dose and
length of exposure. The net result of either type of CNS or PNS insult
is primary demyelination (myelinopathy) (Fig. 5). Loss of
oligodendrocyte somata is associated with the arrival of phagocytic
cells which remove myelin and become filled with droplets of neutral
fat. Astrocytes are commonly affected by chemical agents that perturb
oligodendrocytes but, if they survive, astrocytes may also take up
small amounts of myelin debris, divide, hypertrophy and, if
remyelination does not occur, surround the demyelinated axons. More
usually, however, new oligodendrocytes are recruited, their processes
contact the demyelinated axon and these elaborate new but
foreshortened internodal lengths of myelin (remyelination). Loss of
Schwann cells also results in primary demyelination, cell
multiplication, and remyelination. Another common type of myelinopathy
involves the processes of CNS and PNS myelinating cells: these
disorders are usually visualized by the accumulation of oedema fluid
within the myelin sheath or its associated cellular processes (Spencer
& Schaumburg, 1980). Vacuolation of the myelin sheath is usually a
reversible process, though severe intramyelinic swelling may constrict
the axon and induce Wallerian degeneration.
5.3 Experimental Design and Execution
5.3.1 General principles and procedure
Morphological examination is primarily concerned with studying
structural changes in the nervous system that might explain
behavioural disturbances. These changes are most commonly found when
there is repeated dosing, but permanent neurological damage may also
occur after single doses of some compounds. On occasion, such changes
appear to be minor but, if restricted to a few neurons of a nucleus of
considerable importance, may result in profound functional effects.
This is particularly true of the developing nervous system where,
because of stepwise, interdependent development, damage induced by a
chemical agent at one stage may have a "domino effect" on later stages
of development. Damage to CNS structures, especially when loss of
regenerating axon to its target organ (muscle or sense organ)
(Schaumburg et al., 1983). In the CNS, astrocytes respond to injury by
increasing the number and size of their fibrous processes, and these
appear to inhibit regeneration of all but the smallest (e.g.,
monoaminergic) axons. Prominent axon swellings develop at the viable
end of larger fibres, a process known as axonal dystrophy.
5.2.3.3 Myelinating cells
Some chemical compounds induce primary morphological changes in
myelinating cells or their myelin sheaths. It is usual to separate
agents that induce changes in the cell body of Schwann cells or
oligodendrocytes from those that cause abnormalities selectively in
the myelin sheath, though this distinction may be misleading as many
compounds can produce both types of change, depending on dose and
length of exposure. The net result of either type of CNS or PNS insult
is primary demyelination (myelinopathy) (Fig. 5). Loss of
oligodendrocyte somata is associated with the arrival of phagocytic
cells which remove myelin and become filled with droplets of neutral
fat. Astrocytes are commonly affected by chemical agents that perturb
oligodendrocytes but, if they survive, astrocytes may also take up
small amounts of myelin debris, divide, hypertrophy and, if
remyelination does not occur, surround the demyelinated axons. More
usually, however, new oligodendrocytes are recruited, their processes
contact the demyelinated axon and these elaborate new but
foreshortened internodal lengths of myelin (remyelination). Loss of
Schwann cells also results in primary demyelination, cell
multiplication, and remyelination. Another common type of myelinopathy
involves the processes of CNS and PNS myelinating cells: these
disorders are usually visualized by the accumulation of oedema fluid
within the myelin sheath or its associated cellular processes (Spencer
& Schaumburg, 1980). Vacuolation of the myelin sheath is usually a
reversible process, though severe intramyelinic swelling may constrict
the axon and induce Wallerian degeneration.
5.3 Experimental Design and Execution
5.3.1 General principles and procedure
Morphological examination is primarily concerned with studying
structural changes in the nervous system that might explain
behavioural disturbances. These changes are most commonly found when
there is repeated dosing, but permanent neurological damage may also
occur after single doses of some compounds. On occasion, such changes
appear to be minor but, if restricted to a few neurons of a nucleus of
considerable importance, may result in profound functional effects.
This is particularly true of the developing nervous system where,
because of stepwise, interdependent development, damage induced by a
chemical agent at one stage may have a "domino effect" on later stages
of development. Damage to CNS structures, especially when loss of
 nerve cells is involved, is more likely to be associated with
permanent behavioural changes than disorders predominantly affecting
the peripheral nervous system, where satisfactory regeneration is more
often the rule. Rapidly reversible effects on behaviour are
occasionally, but not often, associated with detectable structural
damage to the nervous system.
5.3.2 Gross morphology
While most neurotoxic damage is most evident at the microscopic
level, it is important to pay attention to the weight of the brain as
well as to macroscopic manifestations such as discolouration (for
example, due to bilirubin), discrete or massive haemorrhage, or other
localized lesions (for example, transverse myelitis).
The new methods of nuclear magnetic resonance (NMR) can supply
data concerning structural changes, for example, demyelination, both
in vivo and post-mortem.
5.3.3 The role of histology
There is no substitute for a thorough light-microscope examination
of the nervous system and other organs for the initial assessment of
animals suspected of having neurotoxic injury. The same careful
attention to anatomical detail should be given in the examination of
experimental animal tissues as would be given by a pathologist
studying autopsy tissue from a patient who has suffered from a new
type of neurological disease. The results of this type of analysis
will provide direction for additional studies that focus on more
specific questions such as the type and extent of structural damage,
the location where changes first occurred, the time-to-onset of
structural damage, the reversibility of these changes, the lowest dose
that caused pathological changes, and the highest no-observed-adverse-
effect dose.
5.3.3.1 Biological principles dictating tissue response
The study design must take into account several important
principles of underlying cellular responses to injury. These
principles will influence when and where tissue should be sampled for
morphological study.
Often, there is a delay of days or weeks between dosing and the
first appearance of structural changes in animals treated with
neurotoxic agents. The extent of the delay depends largely on the
chemical used, the dose, and the species studied. Once the
pathological process has been initiated, a chain of degenerative and
regenerative events may be set in motion, and these may or may not
cease to evolve after dosing has stopped. Most neurotoxic substances
that damage neurocellular elements produce a largely symmetrical
pattern of structural change, whether in the CNS or PNS. Some neurons
or nerve fibres may be more vulnerable than others to chemical attack,
so that at any point in the pathological process, the observer may be
presented with many or all of the stages in the reaction of individual
cellular elements. The active pathological process may also move in
space with time, as in dying-back axonpathies in which degeneration
proceeds retrogradely along affected nerve and fibre tracts (Cavanagh,
1964). Careless sampling of animals with these or other types of
neurotoxic diseases may reveal little or no pathological changes. By
contrast, the careful and thorough investigator will be able to
distinguish between early and later changes, and thereby develop a
hypothesis of the spatial-temporal sequence of the pathological
process. This can be tested in subsequent studies in which animals are
examined at different stages in the development of the disease.
Investigations of this type will also provide information on the
relationship between the dose and duration of treatment required to
induce neurotoxic injury. No-observed-adverse-effect levels can be
established by determining doses that do not produce any
characteristic changes after a specific period of chemical treatment.
Quantitative estimation of structural abnormalities in treated versus
control animals becomes increasingly important as the no-observed-
adverse-effect dose is approached.
5.3.4 Use of controls
Parallel study of age-matched control animals is mandatory,
whenever treated animals are subjected to neuropathological
examination. Control groups should include untreated animals, vehicle-
treated animals, and positive controls. The may receive a neurotoxic
dose of the agent under study or of another compound with well-
characterized neurotoxic properties that elicits a comparable type of
neurotoxic damage. Demonstrating the development of an appropriate
neurotoxic response in the positive control strengthens the validity
of data obtained from animals treated with the agent under study,
especially if these prove to lack pathological changes. The use of
positive controls is imperative for the inexperienced investigator who
needs to gain confidence from the self-demonstration that a known
neurotoxic agent produces the expected changes. Inclusion of these
results in a published report will also reassure the audience that the
investigator has reproduced the expected pattern of structural damage
associated with a known neurotoxic chemical.
There are important advantages in studying the structure of
nervous tissue without knowledge of the treatment group. The
investigator is forced to be more critical in seeking and describing
abnormal findings, and the final assessment is a more objective
statement of the similarities and differences between treatment and
control groups.
5.3.5 Pattern of response
It is not the purpose of this publication to provide a detailed
analysis of the various problems of selective neuronal damage
encountered under neurotoxic conditions. However, it is emphasised
that occasionally the localization is very precise, limited to one or
two nuclei in the brain stem, or to distal regions of long fibre
tracts and peripheral nerves. Selective pathological changes are given
in Table 3 for reference in interpreting new situations.
5.3.6 Data acquired
At the end of the survey, clear answers must be found to the
following questions about the CNS:
1. Are there changes in nerve cells in the area of the cerebral
cortex, basal ganglia, hypothalamus, limbic system, diencephalon,
brain stem, cerebellum, or spinal cord?
2. Are there any changes in shape or number of astroglia and/or
microglial cells?
3. Is there any loss or other change in oligodendroglia?
4. Are there any changes in the myelinated areas of the brain or
spinal cord, such as myelin fragmentation or myelin vacuolation?
Is this associated with axonal swellings and/or degeneration?
5. If 4 is positive, are there glial responses?
6. Are there focal, vascular, and necrotic changes? If so, where?
7. Are tumours present?
8. Are there any changes in special sense organs to account for
functional changes?
For the peripheral nerves, the following questions must be
answered:
1. Are there any changes in peripheral nerves?
2. If so, is it axon degeneration or primary demyelination, or both?
3. What is the distribution of degeneration? Is it distal, affecting
only long and large-diameter fibres, or are shorter fibres such as
cranial nerves also affected?
4. Are sensory, motor, or both types of fibres involved?
Table 3. Examples of toxic effects illustrating the specific
patterns of damage that may be found
Pattern Cause Reference
Neuronal changes
laminar cortical necrosis anoxia Briefly (1976)
hippocampal pyramidal cell trimethylin Brown et al.
damage (1979)
selective granule cell loss methylmercury Hunter & Russell
from the cerebellum (1954)
selective loss of inferior 3-acetylpyridine Desclin (1974)
olivary cells
selective degeneration of doxorubicin Cho et al. (1980)
perikarya of sensory
ganglion cells
selective degeneration of methylmercury Cavanagh & Chen
axons of sensory ganglion (1971)
cells
retrograde degeneration of organophosphorus, Cavanagh (1964);
long sensory and motor acrylamide, Spencer &
axons in CNS and PNS hexanedione Schaumburg (1977)
retrograde degenerations of clioquinol Krinke et al. (1979)
axons of long spinal cord
tracts
Myelin changes
CNS myelin vacuolation triethyltin Aleu et al. (1963)
CNS and PNS myelin hexachlorphene Towfighi et al.
vacuolatin (1975)
focal degeneration of PNS diphtheria Cavanagh & Jacobs
myelin without axon loss (1954)
focal degeneration of PNS lead Fullerton (1966)
myelin with some axon loss
Table 3 (Cont'd)
Pattern Cause Reference
Vascular and neurotic changes
symmetrical lesions in misonidazole Griffin et al.
brain-stem nuclei (1980)
non-symmetrical brain-stem lead Wells et al. (1976)
and cortical lesions
5. What is the state of nerve cell bodies (i.e., sensory ganglion
cells, anterior horn cells)?
6. If the cells in 5 show changes, are those in the medulla and
trigeminal ganglia (i.e., cranial nerves) similarly affected?
7. Are neurons normal, chromatolytic, or degenerated?
8. Are automatic ganglia or nerves similarly affected or not?
9. Are muscles affected by denervation and/or myopathic changes?
10. At dose levels below those that produce clinical signs, is there
histochemical and/or morphometric evidence of metabolic neuronal
changes that is brought out by using these more sensitive
techniques?
5.4. Principles, Limitations, and Pitfalls of the Morphological
Approach
5.4.1 Tissue state
Although under special circumstances the morphologist is able to
study the reactions of living tissue to chemical exposure, either
in vivo or in tissue culture (Yonesawa et al., 1980), the vast
majority of pathological studies involve the assessment of dead
tissue. The structural changes that occur in the nervous system as a
function of time post-mortem can obscure the recognition of toxic
injury and may confuse the inexperienced neuropathologist. Chemical,
or rarely, physical (freezing) fixation methods are used to prevent
the development of post-mortem changes.
5.4.2 Principles of fixation
The use of a reliable fixation method is mandatory for the
morphological examination of nervous tissue (Thompson, 1963; Pease,
1964; Hayat, 1973; Gabe, 1976; Bancroft & Stevens, 1977; Glausert,
1980). The purpose of fixation is to preserve cellular architecture as
closely as possible to that in the living state, to inhibit the loss
of chemical components required for the maintenance of morphology, and
to prepare the tissue to accept stains that enhance tissue density for
clear resolution of cytological detail. The ideal method of fixation
is one that instantaneously terminates life processes in the tissue of
interest without distorting cytological detail. Rapid freezing of
living tissue approaches this ideal. Excision and freezing of tissue
is routinely employed for the assessment of human biopsy tissue and is
particularly suitable for light-microscope histochemistry and
immunocytochemistry. A few specialized laboratories use rapid freezing
for the examination of tissue, or metal replicas thereof, by
transmission electron microscopy.
Chemical methods are far more commonly used in neurotoxicology for
the preparation of nervous tissue. Although the ideal chemical
fixative should instantaneously kill living tissue without inducing
structural changes, all known methods fall short of this ideal.
Instead, the morphologist must strive to induce small, consistent and
readily recognizable changes that will not compromise tissue
examination and assessment. The most satisfactory and widely used
chemical fixatives are dilute, aqueous solutions of aldehydes
(formaldehyde and/or glutaraldehyde), which rapidly cross-link
proteins and stabilize associated lipids, prevent post-mortem changes,
and introduce rigidity into the tissue to facilitate handling. A
secondary phase of fixation using a dilute aqueous solution of osmium
tetroxide to fix lipids is routinely added after glutaraldehyde
fixation for improved preservation of tissue. Occasionally, osmium
tetroxide is used as the primary fixative. Structural artefacts
induced by fixation are minimized if the fixative solutions are
buffered to match tissue pH and osmolality. Temperature should also be
controlled; low temperatures may affect the preservation of fine
structural elements (e.g., microtubles).
Chemical fixatives must be delivered to the site of interest as
rapidly as possible if post-mortem artefacts are to be minimized. Most
effective is systemic perfusion (Pease, 1964; Hayat, 1973; Spencer et
al., 1980; Spencer & Bischoff, 1982), in which the fixative is
introduced via the ascending aorta of the deeply anaesthetized animal,
and distributed throughout the vascular network under controlled
pressure. Spaces normally occupied by blood and tissue fluid are
rapidly replaced by the chosen fixative solution, and the animal
expires painlessly within seconds. Perfusion of individual organs is
an alternative, but more difficult and less satisfactory technique. An
alternative method, suitable only for readily accessible parts of the
nervous system (e.g., peripheral nerves), is to bathe the tissue
in situ with a repeatedly replenished solution of fixative.
In practical terms, the requirements for tissue sampling, organ
weighing, etc., have to be balanced against the need to get good
fixation, and this frequently presents conflicts of interest between
those involved in analysing the animal tissues. Short perfusion
fixation (section 5.5.2), with paraformaldehyde and subsequent
secondary fixation in formalin or formal-acetic acid in the case of
paraffin sections and light microscopy, and glutaraldehyde and osmium
tetroxide in the case of epoxy resin, semi-thin sections, may help to
provide a compromise. When the protocol for killing requires only
immersion fixation, then a few animals in each group must be kept for
perfusion fixation, if adequate tissue examination is going to be
achieved. A study using only immersion fixation is scarcely worth the
labour and cost involved.
The selection of the chemical fixative and its method of delivery
to the tissue site are chosen according to the type of information
that is required. Systemic perfusion with the chosen fixative is
always to be preferred. In general, formaldehyde is suitable for
studies that require widespread tissue sampling, low resolution of
cytological detail, or the use of special histochemical stains to
assess the chemical content of abnormal cells and tissues. Conversely,
in studies focusing on more selected areas of the nervous system or
requiring resolution of fine structural detail, the use of
glutaraldehyde and osmium tetroxide is mandatory. While the latter
method was originally introduced for use in transmission electron
microscopy, an increasing number of laboratories use the technique
routinely to exploit the remarkable resolution afforded by the light
microscope.
5.4.3 Principles of tissue sampling
Extensive sampling of tissue is essential for the initial
assessment of a suspected neurotoxic injury. Organs, other than the
nervous system, which should be examined, are listed in WHO (1978).
Neural tissue should include: brain, including cerebellum, brain stem,
pituitary gland, eye with occulomotor muscles and optic nerve
attached, spinal cord, several sensory ganglia, sciatic nerve and its
branches from its exit from the vertebral column to the level of the
ankle, and selected muscles innervated by the sciatic nerve and its
branches. Other regions less commonly sampled include: olfactory
epithelium and tubercles, inner ear and labyrinths, plantar nerves and
skin receptors, autonomic ganglia, and nerves and organs of
innervation, such as the gut. The brain contains many sites known to
be vulnerable to chemical agents and should be examined as thoroughly
as possible. The use of an atlas such as that for the rat brain (Zeman
& Innes, 1963) will be valuable. The brain stem should be sectioned
just rostral to the cerebellum, the slice passing through the inferior
colliculi. The cerebral hemispheres can then be sliced coronally
(transversely). Olfactory lobes may be taken if questions of
inhalation and olfaction are being studied. The cerebellum is removed
by cutting through the peduncles that attach it to the brain stem.
Sagittal sections are obtained by cutting through the midline, and
parallel sections are also taken. Emphasis is usually placed on the
retina and optic nerves, since these are often heavily involved in
toxic diseases affecting neurons, axons, and myelin. Ototoxicity is
also a common event, though the technical difficulties associated with
the examination of the inner ear and labyrinths have prevented
widespread study of this phenomenon. Olfactory lobes and associated
epithelium are rarely examined, but should be taken if questions of
inhalation and olfaction are being studied.
The spinal cord contains ascending and descending nerve fibre
tracts that commonly undergo changes in myelinopathies and
axonopathies (Table 3). Since the latter are tractable diseases with
distal accentuation, it is critical to sample various levels from the
medulla oblongata (where the gracile tract terminates) to the sacral
region. By taking transverse sections of the spinal cord, it is
possible to make a simultaneous evaluation of white matter (myelinated
tracts and glial cells), gray matter (neurons, dendrites, proximal
axons, glial cells), blood vessels, and associated tissues.
Dorsal root and cranial nerve ganglia contain primary sensory
neurons that may display pathological changes in certain
neuronopathies and axonopathies (Figs. 3, 4). Structural alterations
in blood vessels and myelin may also occur under certain conditions.
The sensory ganglia of the Vth cranial nerve, at least 4 sensory
ganglia from the cervical bulb region (C5-T1 spinal levels), and 4
from the lumbosacral regions (L3-S2 spinal levels), should be sampled
for examination in longitudinal section. Corresponding dorsal and
ventral spinal roots, vulnerable in toxic demyelinating diseases,
should also be studied.
Peripheral nerves commonly display changes in neurotoxic diseases
affecting myelin, neurons, and axons (Table 3). As the last display
distal, retrograde changes in dying-back axonopathies, a common
response to chemical attack, it is critical to examine several levels
of vulnerable nerves. Samples of the sciatic nerve and its branches
are usually taken commencing adjacent to the vertebral column and
terminating in the distal regions of the sural nerve, peroneal nerve,
and/or the tibial nerve. The fine branches of the tibial nerve that
leave the main trunk below the knee are especially vulnerable in toxic
neuropathies, because they contain very large myelinated nerve fibres.
Terminal regions of sensory and motor nerve fibres can be examined by
searching for twigs supplying intrafusal (spindles) and extrafusal
muscle fibres, respectively (Schaumburg et al., 1974). Routinely,
anterior tibial muscles, and gastronemius with suralis muscles are
examined. Muscle tissue is also valuable for assessing whether there
is denervation atrophy, evidence of toxic myopathy, nerve sprouting,
or other regenerative activity (Pearson & Mostofi, 1973).
Tissue sampling should not only be tailored to the goal of the
study but also to the method of fixation. In general, blocks of tissue
several millimeters thick may be taken if the tissue has been fixed
with formaldehyde and is destined for paraffin embedding. For tissue
fixed with glutaraldehyde, the relatively slow rate of penetration of
osmium tetroxide necessitates the use of thin (1 µm) slices of tissue.
It is often helpful to mark a small area of tissue (with Indian ink or
a small cut) to maintain the orientation. Elongate structures, such as
peripheral nerves and spinal cord, are most readily studied in
transverse sections, though important information is acquired by
complementary examination of longitudinal sections. Particularly
informative for the identification of pathological changes in
peripheral nerves is the technique of microdissection (teasing apart
intrafascicular tissue), which yields long lengths of individual
myelinated nerve fibres suitable for light microscope examination.
Inspection of these preparations can demonstrate rapidly the presence
of primary demyelination and remyelination and axonal degeneration and
regeneration. The minor changes characteristic of early toxic
neuropathies, readily missed in the examination of sections, can be
easily detected by this method. Other parts of the nervous system are
technically difficult to examine by microdissection because of the
absence of a collagen network to support the individual nerve fibres.
While it is prudent to embed tissues in paraffin wax or epoxy
resin promptly, regions that will not be examined immediately can be
stored in a solution and at a temperature appropriate to the method of
fixation. Loss of tissue quality will occur in proportion to the
length of storage.
5.4.4 Preparation of tissue for examination
While gross tissue changes can be visualized with the naked eye or
by examination with a stereoscopic binocular microscope, and surface
detail can be resolved with the scanning electron microscopy, routine
examination of cellular structure requires the use of tissue sections.
This inevitably places severe limitations on the amount of tissue that
is practical to study. Sections must be such that the energy beam
(light, electrons, X-rays) of the chosen microscope can be transmitted
through the tissue. Relatively thick sections containing several
layers of cells can be visualized with the light microscope, but the
ability to resolve cytological detail decreases as section thickness
increases. The use of semi-thin sections allows the investigator to
exploit the maximum available resolution of the light microscope. Much
thinner sections are required for the assessment of tissue by
transmission electron microscopy (Pease, 1964; Hayat, 1973).
Chemically-fixed tissue must be supported by a pliable material to
facilitate the sectioning process and to prevent disintegration of the
sectioned tissue. Paraffin wax or epoxy resin are routinely used: the
former for routine work, the latter for more specialized studies.
Since both types of embedding media are water insoluble and therefore
immiscible with the fixative solutions, water is first removed by
stepwise dehydration in ethanol or methanol immersing the tissue in an
aromatic hydrocarbon (clearing solution) that is miscible both with
ethanol and the embedding medium. Dehydration, clearing, and
infiltration with an embedding medium are procedures requiring
considerable care and time to avoid tissue drying and distortion.
Automatic systems are available and, for paraffin embedding, these are
routinely used in histological laboratories. Once the tissue is
infiltrated and embedded, the medium must be allowed to harden by
cooling (paraffin wax) or heating (epoxy resin).
Sections of tissue are prepared with the aid of knives mounted in
microtomes. Steel knives are suitable to cut thick (4-15 µm) sections
of paraffin embedded tissues, whereas knives prepared by scoring and
breaking strips of special glass are needed to prepare sections (1 µm)
of tissue embedded in the harder epoxy resin. The sections of tissue
prepared with steel knives permits study of larger tissue areas than
is possible with sections prepared from epoxy blocks, though the
latter afford a higher resolution of cytological detail. With new
developments in the preparation of glass knives and the design of
microtomes, ever larger sections can be routinely prepared by the
latter technique. Diamond knives mounted in ultramicrotomes are used
to obtain the thin sections (50 nm) required for transmission electron
microscopy.
Contrast of cytological detail in sectioned material can be
enhanced by the use of special light-microscope methods (phase,
Nomarski, fluorescence) or, more commonly, by the introduction of
stains that render structures light-dense. A wealth of cytological
stains is available for use with paraffin sections, whereas only a few
general purpose stains are usually used to enhance structural detail
for the light-microscope assessment of epoxy sections. Heavy metal
stains that are electron dense are needed to visualize fine structure
by transmission electron microscopy.
5.4.5 Recognition of artefact
Some degree of artefact is unavoidable, irrespective of the care
taken in preparing tissue for morphological examination, and
recognition and identification of the sources of artefact are of the
utmost importance in the morphological assessment. Possible artefacts
are legion and stem from various sources: e.g., poor tissue
preservation from failure of fixative to penetrate tissue; shrinkage
induced by inappropriate dehydration or drying; traumatic changes
associated with excision of the tissue (bending, stretching) or rough
handling during processing; wrinkling of sections and precipitation of
stain (Spencer & Bischoff, 1982).
5.4.6 Recognition of normal structural variations
While the gross and fine structure of the nervous system of any
species and age is remarkably constant from animal to animal, it is
critical to recognize the irregular occurrence of normal variations in
cellular structure that may lead the inexperienced observer to an
inappropriate conclusion. These variations include ectopic structures
(e.g., sensory neurons misplaced in the sciatic nerve), local
oedematous or proliferative changes (e.g., at a site of unrecognized
trauma or infection), and those that accompany advancing age (Johnson,
1981). The latter are especially important in long-term neurotoxicity
studies, and there is no substitute for rigorous comparative study of
control animals of the same age. Examples of normal age-related
changes include neuronal pigmentation by lipofuscin, localized
demyelination and remyelination, scattered neuronal loss, and axonal
dystrophy and degeneration.
5.4.7 Qualitative versus quantitative approaches
While there can be no substitute for a thorough qualitative
assessment of the morphological state of the nervous system,
morphometric methods are required for a more precise description of
these changes (Weibel, 1979; Bonashevskaya et al., 1983). This is
especially important for the description of minor changes, or when the
tissue reactions of different groups of animals are being compared.
Randomization of samples is essential to minimize subjective
influences, and a statistical approach is required both for the design
of the morphometric study and the analysis of the data. Tissue
sampling, group size, age, sex, body weight, and length are important
considerations in this regard. This type of morphometric approach has
been helpful in measuring tissue reactions to toxic chemicals in the
form of: the numbers of fibres affected in specific pathways subject
to degeneration, changes in cell populations in vulnerable parts of
the brain, and the relationship between fibre diameter and intermodal
length of peripheral myelinated nerve fibres prepared by
microdissection (Dyck et al., 1984).
5.5 Specific Procedures
5.5.1 Introduction
Strategic considerations in planning how to proceed must depend on
the state of knowledge of the possible effects. When it is unknown
whether damage occurs in nervous tissue, a wide-ranging examimation of
the nervous system with the light microscope is appropriate. Most
structural changes induced by neurotoxic chemicals can be detected
using the primary methods given below. More specialized, secondary
methods can be applied subsequently for more detailed studies.
5.5.2 Primary methods
There are strong convictions among experienced experimental
neuropathologists as to which fixation method should be chosen for the
primary screening. Some believe the conventional approach using
formaldehyde-based fixatives and paraffin embedding is most
appropriate; others prefer the more contemporary method of examining
epoxy sections of tissue fixed in glutaraldehyde and osmium tetroxide.
Each method has significant advantages and disadvantages and both
complement each other. Since it is impractical to use both methods for
the same animal, a prudent investigator will include a sufficient
number of animals to permit the parallel use of both procedures.
5.5.2.1 Formaldehyde/paraffin method
(a) Tissue fixation and excision
This technique is most suited for the study of large areas of
tissue at low resolution and with special histochemical stains
(section 5.5.3.3). Tissue can be removed from the carcass and fixed by
immersion in formalin (10%) containing 2% acetic acid (added just
before use) to improve penetration and hardening. Making up the
solution in 80% alcohol rather than water to hasten penetration
further has sometimes been recommended, but this is unnecessary and
may make dissection more difficult.
Rapid and careful transfer of tissues from the carcass to the
fixative solution will retard artefactual cell distortion. In a
general post-mortem dissection, the brain, including the cerebellum
and brainstem, are removed and transferred to fixative, prior to the
removal of other tissues. Retrieval of the spinal cord from its spinal
column is difficult and time consuming; it is recommended that the
whole spinal column with cord be excised quickly, trimmed of as much
muscle as possible, and immersed in the fixative intact. Once this
portion is fixed, the spinal cord and ganglia can be separated from
the column with greater care. Alternatively, after approximately 24 h
of fixation, the spinal column can be immersed in 5% formic acid
decalcifying solution. The column can then be sliced with little
difficulty.
To assist with the examination of peripheral nerves, tissue is
placed on a stiff card before immersion to retain orientation.
Optimally, the nerve to be excised should be exposed and, prior to
removal from the carcass, bathed with fixative for approximately
5 min. This will begin to introduce rigidity into the tissue and
retard decay artefact. A length of nerve can then be excised, quickly
placed on a card and pinned at the tip of both cut ends to retain
position after immersion. Care should be taken to avoid overstretching
the nerve as it is pinned to the card. Samples of these tissues can
then be sectioned in a transverse or longitudinal direction. Muscle
can be treated in the same manner. Another technique is to take off
the limb at the hip or shoulder joint, remove the skin and fix en bloc
in formalacetic acid solution. The limb can then be decalcified in 5%
formic acid solution for approximately two weeks as noted earlier for
the spinal column. It can then either be embedded in paraffin wax as
one large block or further dissected.
Tissue preservation is greatly improved by perfusing animals
systemically for 10 min with phosphate-buffered paraformaldehyde
(section 5.5.2.2), a method that simultaneously preserves all body
organs. Tissue can then be sampled in a more leisurely manner without
fear of introducing post-mortem changes (section 5.5.2.2).
(b) Dehydration and embedding
Relatively large tissue samples can be embedded in paraffin wax,
and this is particularly suitable for localizing and identifying
lesions in the brain. Automated processing for paraffin embedding has
greatly diminished the time requirements for preparing tissue samples.
Most automated systems will conform to a variety of preparation
schedules and process great numbers of tissue samples at one time.
There are systems that operate under combined heat and vacuum, the
great advantage being rapid processing. This combination is
deleterious and may cause unnecessary shrinkage and brittleness, which
will distort cell structure and hinder interpretation. Paraffin
sections routinely cut 4-6 µm thick, are suitable for certain
histochemical techniques and can easily be treated with special stain
to enhance particular cellular components.
(c) Section staining
Four staining methods are routinely used to enhance cellular
detail in paraffin sections.
Hematoxylin and eosin
Routinely used on paraffin sections, this method stains nuclei
blue to purple, cytoplasm and Nissl substance (and mast cell granules)
blue, and myelin sheaths pink. Nuclear density can be readily
assessed, as well as an increase or decrease in the size of nuclei and
the number of cells. Neuronal damage is readily detectable. Nuclei and
cytoplasm of other cell types, such as microgila and vascular
endothelial cells, are also well defined, though cresyl violet may be
preferred.
Cresyl fast violet
This method enhances identification of cell-population changes and
is more aesthetically pleasing than other basic aniline dyes, such as
thionine or gallocyanine-chrome alum. DNA stains pale blue and RNA
purple-blue. Hypertrophy of astroglial cytoplasm and changes in
neuronal nuclei and cytoplasm are readily observed. Chromatolysis,
poorly demonstrated in hematoxylin- and eosin-stained sections, is
more apparent when stained with cresyl violet, as are most cells in
peripheral nerves.
Glees and Marsland's stain
This method is easy to carry-out and selectively stains
neurofibrillary components in axons (Marsland et al., 1954).
Longitudinal sections demonstrate the state of terminal innervation in
motor and sensory fibres. Empty endplates are often recognized by the
observation of remaining clumps of nuclei on the muscle surface.
Sudan Black B
This method is useful to reveal nerve terminals in muscles
(Cavanagh et al., 1964).
5.5.2.2 Glutaraldehyde/epoxy method
(a) Tissue fixation and excision
This technique is best suited for the resolution of early
pathological changes with the light microscope and is required for
transmission electron microscopy (section 5.5.3.5). Whole body
perfusion with buffered fixators (paraformaldehyde) followed by
glutaraldehyde) delivered under pressure (100-150 mm Hg) is the only
satisfactory method to prepare brain and spinal cord sections, and
this method has the advantage of optimally fixing all other organs
simultaneously. For this purpose, the animal is deeply anaesthetized
with a solution (e.g., sodium pentobarbital) containing a small
percentage of heparin to facilitate circulation. Subsequent procedures
should be performed rapidly and smoothly to prevent the development of
anoxic damage and to ensure global fixation. The animal is secured on
its back and the rib cage cut bilaterally and reflected backwards to
expose the heart. After slitting the pericardium, the right atrium is
opened to drain circulating blood. The ventricular apex is then
excised to provide access to the aorta, and a cannula spurting a
column of fixative is inserted through the ventricular opening to the
apex of the aorta and clamped in place. The circulatory system is
cleared by a brief, initial perfusion with paraformaldehyde or saline,
and followed by more prolonged (e.g., 10-15 min) perfusion with
glutaraldehyde.
Rigid tissues from a fixed carcass are less susceptible to
handling artefact. The nervous system can be sampled in any order. In
some laboratories, the entire nervous system, including brain with
optic tract and nerves, spinal cord, spinal roots, ganglia, and
peripheral nerves are exposed in the carcass, then carefully detached
intact and removed. When this is accomplished, there is no need
initially to mark tissues for orientation. It is imperative to avoid
tissue drying during dissection and excision. Exposed tissues should
be bathed with fixative and tissues should be manipulated as little as
possible with forceps.
(b) Dehydration and embedding
After glutaraldehyde fixation, tissue samples are post-fixed for
2-3 h by immersion in buffered 1-2% osmium tetroxide in the cold. The
samples are then dehydrated stepwise in ascending concentrations of
ethanol, immersed in acetone or toluene, infiltrated with a solution
of epoxy resin and finally, embedded and polymerized in epoxy resin.
For light-microscope examination, 1 µm sections are cut with specially
prepared glass knives mounted in an ultramicrotome (an instrument
designed specifically for the purpose of obtaining the thin sections
suitable for electron microscope examination). New techniques and
equipment have recently become available which allow larger tissue
samples to be prepared for sectioning in epoxy resin. Sections one
micrometer thick can be examined by phase-contrast optics or by bright
field after a brief staining with 1% toluidine blue.
(c) Section staining
Thick (1 µm) sections of tissue fixed in glutaraldehyde and osmium
tetroxide and stained with borate-buffered 1% toluidine blue allow
resolution of structures as small as a single mitochondrion. Brain and
spinal cord neurons display clearly defined nuclei, nucleoli, issl
substance, and lipofuscin. Dendrites stand out against a more darkly-
stained neuropil. Small, densely staining oligodendrocytes are readily
distinguished from large, pale astrocytes. Myelinated nerve fibres
contain a pale axon and darkly-stained myelin. In cross-sections of
peripheral nerves, large and small diameter myelinated fibres, as well
as the smallest unmyelinated axons, can be seen. Motor and sensory
nerve terminals are detectable in muscle.
5.5.3 Special methods
5.5.3.1 Peripheral nerve microdissection
Isolated fibre preparations provide information that may be missed
in cross-sections, and it is recommended that this procedure be
included in any study concerned with toxic neuropathy. Several
fixation methods are available to prepare peripheral nerves for
microdissection. Perfused tissue is ideal, though satisfactory
preparations can be obtained by bathing the tissue with fixative prior
to excision. Post-excision immersion in fixative can also be used,
though the act of transecting the nerve introduces major fibre
artefacts that are not restricted to the cut ends and are readily
mistaken for pathological changes.
Optimal preparations suitable for high-resolution light microscopy
require the use of glutaraldehyde, post-fixation with osmium
tetroxide, stepwise dehydration, and infiltration with a low-viscosity
epoxy resin. This is an ideal medium to tease apart nerve fibres and
is suitable for the storage of tissue at low temperature. Mounted
fibres can be polymerized by heat to affix them to a glass slide and
prevent squashing by a coverslip (Spencer & Thomas, 1970). Bright-
field examination will reveal changes in overall structure, and
Nomarski differential interference microscopy can be used to study the
axon.
Older techniques yield poorer preservation, low resolution of
detail, and isolated fibres are susceptible to squashing, an important
consideration if morphometric study is planned. One method involves
formalin fixation, post-fixation in osmium tetroxide, and infiltration
with a dilute solution of glycerol, which is used to support the
fibres during microdissection. Single fibres are mounted on the
microscope slide, cleared with cresol, blotted dry, and mounted with
pure glycerin (Thomas, 1970). Sudan Black B can also be used to stain
tissue prior to microdissection. Nerves are microdissected with the
aid of a pair of mounted needles and stereoscopic dissecting
microscope. Epineurial connective tissue is removed and a small
fascicle selected. After splitting the perineurium surrounding the
fascicle, the sleeve is removed to expose the intrafascicular tissue
containing the nerve fibres. The tissue is then repeatedly split into
progressively smaller longitudinal bundles until individual fibres are
obtained. These are picked up on the end of sharpened wooden
applicator sticks and transferred to a clean glass slide and provided
with a coverslip. Alternatively, a rapid "squash" preparation can be
obtained by teasing a small bundle of fibres loosely apart to create a
mesh formation on a microscope slide; a coverslip is then added for
microscope examination.
5.5.3.2 Frozen sections
Frozen sections lend themselves well to enzyme histochemical,
immunocytochemical, and silver-impregnation procedures, but for the
purposes of a large-scale study, this method is impractical. Tissues
fixed instantaneously by immersion in liquid nitrogen are particularly
vulnerable to cell distortion and/or destruction caused by excessive
temperature changes. Encasing tissue samples in gelatin or albumin is
advisable, especially for brain tissues (Crane & Goldman, 1979).
Ice-crystal formation is a disruptive artefact that produces a
vacuolated appearance in frozen sections. To minimize crystal
formation, it is important to freeze tissues as rapidly as possible
and maintain critical temperatures throughout the cutting procedure to
avoid thawing and refreezing. Typically, frozen sections are between
10 and 15 µm thick.
5.5.3.3 Histochemical methods
Using these procedures, it is possible to assess the activity of
cell energy systems, the rate of use of substrates for processes of
oxidative phosphorylation, and the functional state of intracellular
organelles. The activities of succinate dehydrogenase and malate
dehydrogenase, enzymes of the Krebs cycle, are well known, the former
being closely linked with mitochondrial membranes. Enzymes of the
hexose monophosphate shunt (glucose-6-phosphate dehyrogenase and
gluconate-6-phosphate dehydrogenase) and those of the electron
transport chain (NAD and NADP disphorase) should also be studied. The
rate of exchange of nitrogenous bases can be examined by the
determination of activity of dihydroorotate dehydrogenase for fatty
acids; monoamine oxidase for biogenic amines; and acetylcholinesterase
for acetylcholine.
Histochemical enzyme assays are performed on freshly frozen
sections cut on a cryostat (Dubowitz & Brooks, 1973). Tissues are
quick-frozen in liquid nitrogen. Control and experimental tissues
should be mounted on one slide and reactions carried out using the
methods outlined in Pearse (1968).
The following methods can be used to demonstrate the functional
state of nerve cells (Pearse, 1968):
(a) RNA (Brachet's method with ribonuclease control);
(b) DNA (Feulgen's method with deoxyribonuclease control);
(c) Total nucleic acids (Einarsen's method);
(d) Total protein (sublimate with bromophenol blue);
(e) Sulfhydryl groups (Barnet and Zeligman's method);
(f) Glycogen and glycosaminoglycans (amylase control-PAS
reaction); and
(g) Lipids (Sudan III, Sudan Black, Nile Blue methods).
To express histochemical reactions quantitatively, the following
formula for Mean Histochemical Index (MHI) can be used:
MHI = 3a + 2b + 1c + 0d
N
where 3, 2, 1, and 0 are the degrees of intensity of colour (from 3
to 0), and a, b, c, and d are the numbers of the cells with the given
intensity of colour; the denominator N is the number of cells counted.
The results allow statistical calculation of the assays and estimation
of the reliability of the results.
5.5.3.4 Golgi method
This unique silver-impregnation method demonstrates the shape and
surface characteristics of entire neurons, including cell bodies and
their processes. Golgi preparations are particularly useful in the
study of dendritic arborizations and synaptic associations (Santini,
1975). However, the technique is capricious in that only a few neurons
are unpredictably demonstrated: many preparations must therefore be
examined to gather a representative sample.
In the Rapid Golgi method, tissue is fixed with formaldehyde or
glutaraldehyde and post-fixed with osmium tetroxide. The tissue is
then impregnated with silver nitrate, dehydrated, and infiltrated with
celloidin. Thick (120 µm) sections are prepared, mounted, and examined
by bright-field microscopy.
5.5.3.5 Transmission electron microscopy
This technique is laborious and requires extensive training.
However, it has resulted in extensive advances in the understanding of
cellular processes in neurotoxicity. There is little need for such
exacting methods to determine the presence of changes in cell
structure, few experimental protocols require electron microscopy. The
transmission electron microscope is properly used to confirm and study
further the nature of lesions already shown and mapped by light
microscopic methods. It is all too easy to seek, and find, changes to
which no significance will ultimately be attached. Until the pattern
of change induced by the chemical under study has been well identified
by light microscopy, electron microscopy should not be considered. On
rare occasions, such as in toxic disorders of unmyelinated axons,
examination by transmission electron microscopy is necessary to
localize cellular changes not revealed by the methods discussed
previously. Thin (50 nm) plastic sections are prepared with the aid of
a diamond knife mounted in an ultramicrotome and subsequently
impregnated with heavy metals for electron-microscope examination
(Pease, 1964; Hayat, 1973).
5.5.3.6 Other anatomical methods
There are numerous additional specialized anatomical methods that
are waiting to be applied in the study of neurotoxicological issues.
These include histological techniques to trace anatomical pathways
(with horseradish peroxidase) and cellular activity. Examples of the
latter are the use of autoradiography to localize the distribution of
radiolabelled precursors such as thymidine and 2-dexoyglucose
(Sokoloff et al., 1977). Other methods susceptible to light and
electron microscope analysis include immunocytochemical studies of
receptors, proteins, and other cell structures (Emson, 1983).
Fluorescence microscopy, particularly for the localization and
detection of regional concentrations of catecholamines using the
formaldehyde vapour or glyoxylic acid technique of Falck et al.
(1962), supplements quantitative biochemical methods in evaluating
alterations in catecholamine levels. Fluorescence microscopy is also
of value in antigen and antibody localization techniques. Certain
light and electron microscope techniques may be combined, such as the
examination of fine structure of single teased nerve fibres (Spencer &
Lieberman, 1971; Ochoa, 1972) or of Golgi-stained preparations.
Advanced methods in electron microscopy include scanning (for surface
features), energy-dispersive X-ray analysis (for detection of elements
of high relative atomic mass) and energy-loss (for detection of
elements of low atomic mass). Freeze-fracturing tissue can reveal
details of the internal surfaces of cellular membranes when replicas
are examined by transmission electron microscopy (Hayat, 1973).
5.6 Conclusions
The information derived from morphological studies is highly
relevant to the interpretation of biochemical, neurophysiological, and
behavioural data. Structural changes have always been the firm
foundation on which analysis of clinical neurological disease has been
based. Both diagnosis and prognosis depend heavily on previous
neuropathological experience for their accuracy. Moreover, treatment
of neurological disease can only be satisfactorily planned by using
the knowledge gained from experimental and morphological studies that
have provided an understanding of the pattern by which neural cells
are affected. Indeed, in the majority of human intoxications of the
nervous system, knowledge of the structural changes is based almost
exclusively on animal studies. There is no reason to believe that it
will be less so in the future.
6. BIOCHEMICAL AND NEUROENDOCRINOLOGICAL METHODS
6.1 Introduction
Biochemical tools are valuable for the study of neurotoxicity.
They have the potential for both identifying toxic compounds and
delineating mechanisms of action of known toxic substances. However,
the range of biochemical techniques is vast and careful decisions must
be made in devising an effective research strategy. The most important
decision is identification of the objective of the research, which may
be conveniently categorized into three groups: determining mechanisms
of action of neurotoxic substances, identifying exposed individuals,
and screening for toxic conditions.
Devising an effective biochemical screen is the most challenging
of the three objectives. It requires the selection of biochemical
parameters that are general enough to indicate toxicity resulting from
multiple sites of damage, but specific enough to prevent the
accumulation of false positives. No single biochemical parameter is
likely to suffice. An effective screen must include indices of each of
the major functions of nervous tissue (e.g., cellular metabolism,
neuronal propagation, and neurotransmission). It might include
estimates of RNA and/or protein synthesis, lysosomal enzymes, membrane
transport, channel and neurotransmitter receptors, or their turnover.
The precise choice of biochemical indices must be limited by the
expertise and equipment of individual laboratories, but every attempt
must be made to consider a range of functional events rather than a
single parameter.
Considerable discussion has centred on the use of in vitro
procedures for screening potential neurotoxic compounds. The
advantages of an in vitro approach are obvious. It is less expensive
and less time-consuming than whole animal study and biochemical
techniques can be employed under precise, standard conditions.
In vitro procedures must include both parent compounds and potential
toxic metabolites. However, the exclusive use of in vitro methods
would fail to detect compounds in which the neurotoxicity results from
alterations in non-neuronal systems.
Theoretically, the choice of biochemical tools for the
identification of mechanisms of toxicity is the simplest of the three
objectives. Biochemical tools are virtually limitless, but they can be
chosen on the basis of known information about the toxicity of the
compound. Scientists can then proceed systematically from one level of
analysis to another. By synthesizing data from behavioural, neuro-
physiological, neuroendocrinological, neuropharmacological, and
neuropathological studies, the biochemist can employ successively more
selective tools to determine the initial biochemical lesion induced by
the toxic compound.
The purpose of this section is to review the biochemical
approaches that may be useful for identifying and further
understanding the effects of toxic compounds on the nervous system.
6.2 Fractionation Methods
Neural tissue has several features not shared by other tissues. In
particular, neural tissue exhibits considerable cellular,
morphological, and chemical heterogeneity. It is often desirable to
precede biochemical procedures with some attempt to decrease this
complexity. One approach is to separate the tissue into discrete parts
of brain prior to analyses. A second approach is to separate specific
cell types. A third involves subcellular fractionation procedures. In
many cases, it may be desirable to use a combination of these
fractionation procedures.
6.2.1 Brain dissection
Brain tissue can be fractionated into any number of potential
sections. However, the most often used scheme is based on that
described by Glowinski & Iverson (1966). In this method the brain is
divided into relatively discrete units of cerebellum, thalamus,
hypothalamus, striatum, hippocampus, etc., by using visible anatomical
landmarks. Such dissection procedures have been very useful in
describing the neurochemistry of different brain areas and are
essential in evaluating the effects of toxic compounds on molecules
(such as neurotransmitters) that are not uniformally distributed in
neural tissue. Because of the regional variability of brain chemistry,
whole brain analyses are seldom useful for evaluating brain function.
More importantly, the use of the whole brain for studies of toxic
compounds may fail to detect functionally-relevant alterations in
specific brain areas. For example, effects of lead on GABA levels have
been reported for cerebellar tissue (Silbergeld et al., 1980) and
acrylamide effects have been noted in striatal dopamine receptors
(Bondy et al., 1981).
Differences in the response to toxic compounds would be
anticipated from differences in neurochemistry in various regions of
the brain. In addition, many toxic substances are not distributed
uniformly across brain areas (e.g., chlordecone (Fujimori et al.,
1982) and manganese (Bonilla et al., 1982). Although not predictive,
examination of such regions can provide a biochemical clue regarding
possible molecular sites of interaction of the toxic compounds.
In more precise dissection procedures, a small needle, of defined
diameter, is used to obtain samples within anatomically defined areas
(Palkovits, 1973; O'Callaghan et al., 1983). Such finite samples have
been used in identifying several neurochemical events, e.g.,
neurotransmitter concentrations (Palkovits, 1973) and neuro-
transmitter-induced phosphorylation of specific phosphoproteins
(Dolphin & Greengard, 1981), and they may be especially valuable in
locating the site of neural responses to toxic compounds.
6.2.2 Isolation of specific cell types
Although neural tissue is recognized by a marked heterogeneity of
cellular elements, based on function and embyronic origin, these cell
types are conveniently categorized as neurons or glia. Each cell type
makes a unique contribution to neural function and their sensitivity
to toxic compounds may differ. Several methods have been described for
the bulk separation of neuronal and glial cell populations from whole
brain or specific brain regions (Rose & Sinha, 1970; Appel & Day,
1976; Magata & Tsukada, 1978). A dissociated cell suspension that
avoids disruption of the cells is prepared by forcing the tissues
through fine sieves. Proteolytic enzymes are used to facilitate the
dissociation. The fraction enriched in neuronal perikarya is separated
from the smaller glial cells (also containing some synaptosomes) by
centrifugation. The cell separations are never complete and the
procedures generally provide low yields. However, enrichment of the
neurons and glia is usually sufficient fox determining whether a toxic
agent interferes primarily with neuronal or glial metabolism. Isolated
cell populations have been used to study the lipid and protein
composition of membranes and the several metabolic differences between
neuron and glia. Such information may provide baseline data for future
studies of neurotoxic chemicals. Disadvantages of this method include
loss of cell processes from the perikarya, reduction of cell surface
area, and damage to the cell membrane. These factors must be
considered in any metabolic study.
To date, there have been few attempts to use such separation
procedures in neurotoxicity and they are not recommended as an initial
study. The method is laborious and time consuming and must be
performed on fresh tissue. Only a limited number of samples can be
simultaneously processed and it is never clear if the low yields
result from random or specific loss of cell types.
6.2.3 Subcellular fractionation
The neuron can be divided into relatively discrete functional
units. The cell body contains the metabolic machinery for the
synthesis and packaging of macromolecules and for the general
maintenance of cellular homeostatis. The long axonal process acts as a
communication link, propagating electrical impulses from the cell body
to the terminal and transporting vital nutrients and cellular
components to more distal regions including the nerve terminal.
Finally, the synapse functions to transfer chemically encoded
information from one cell to another. Toxic substances may disrupt
neuronal biochemistry at any one, or all, of these cellular sites.
The isolation of subcellular organelles and specific membrane
fractions can be a first approximation in determining the subcellular
sites of action of toxic agents. Differential centrifugation
procedures provide a rapid means of obtaining fractions consisting
predominantly of a single cellular component (Gray & Whittaker, 1962;
Cotman & Barker, 1974; Rose and Sinha, 1970). Subcellular
fractionation is never totally effective, but it can produce an
enrichment of organelles and cellular subfractions. Some of the more
commonly used biochemical markers that can help identify the degree of
enrichment are listed in Table 4. These markers can be used as
potential indices of toxicity. For example, the effects of triethyltin
on myelination in the developing brain has been studied by examining
the activity of the myelin marker, 2',3'cyclic nucleotide 3'
phosphohydrolase (Konat & Clausen, 1977). Merkuryva & Tsapkova (1982)
have used several marker enzymes in their study on the toxic effects
of ethanol on the nervous system.
When subcellular fractionation is combined with regional analysis,
the number of potential samples requiring examination can be
overwhelming. Therefore, careful selection must be used in deciding
which brain area and which subcellular fraction to investigate. This
selection can be very difficult, especially when knowledge concerning
a particular compound is limited. The recent introduction of
immunochemical methods promises to facilitate this task, not by
reducing the difficulty of the selection, but by increasing the number
of chemicals that can be subjected for analysis.
Immunochemical methods have been used to identify molecules
associated with various cell types, for localizing enzymes responsible
for the synthesis of neurotransmitters and for identifying proteins
specific to the nervous system. While the procedures initially require
the preparation of antisera to highly purified proteins, once the
antisera are available, they can be sensitive tools for identifying
specific changes and for characterizing different cell populations. In
addition, monoclonal antibodies can be used to determine the mechanism
of action of toxins.
These procedures are especially valuable in studying neuronal
peptides. These peptides (e.g., substance P, encephalins, hypothalamic
releasing factors, somatostatin, etc.) play a significant role as
neuromodulators (Snyder & Inms, 1979). Many are potent at low
concentrations. Prior to the use of immunological procedures, peptide
molecules could only be examined by using bioassay techniques and
these were not always specific for particular molecular species.
Radioimmunoassay techniques are more specific. Assay kits for various
peptides are now available commercially, and their number is rapidly
increasing.
Table 4. Examples of biochemical markers of brain subcellular fractions
and cell types
Fraction Marker Reference
nuclei DNA Steele & Busch (1963)
nuclear membrane RNA polymerase Roeder & Rutter (1969)
cytosol lactate dehydrogenase Kornberg (1955)
microsomes NADPH; cytochrome c Miller & Dawson (1972)
oxidoreductase (rote
none insensitive)
Mitochondria
inner membrane D-3-hydroxybutyrate Fitzgerald et al.
NAD+ oxidoreductase (1974)
outer membrane monoamine oxidase Schnaitman et al.
(1967)
lysosomes ß-glucuronidase Fishman & Bernfeld
(1955)
ß-glucosidase Robins et al. (1968)
ß-galactosidase Robins et al. (1968)
N-arylamidase Boer (1974)
myelin 2',3'-cyclic nucleotide- Konat & Calusen (1977)
3'-phosphohydrolase
synaptosomes Na+K+-activated Verity (1972)
oubain-sensitive
ATPase
neuronal cells guanyl cyclase Goridis et al. (1974)
tyrosine hydroxylase Kuczenski & Mandell
(1972)
oligodendroglial cells glyceride galactosyl Radin et al. (1972)
transferase
Because the method is very recent, radioimmunoassay procedures are
only beginning to be use in neurotoxicology. However, using such
methods, Hong and his colleagues (Ali et al., 1982; Hong & All, 1982)
have successfully identified several peptide responses following
chlordecone treatment. The major limitation of the method for
identifying toxic compounds is the small number of purified compounds,
the small number of available RIA kits, and the expense of obtaining
radioiodinated compounds. With increasing availability of RIA kits,
the procedure could become a powerful screening tool.
6.3 DNA, RNA, and Protein Synthesis
Changes in the amount of DNA can be used to detect whether toxic
agents affect cellular proliferation and cell death. Polyploidy is
uncommon in the nervous system, thus the DNA content of brain regions
can be taken as an index of cell number. This can be related to tissue
weight and RNA or protein content to estimate cell size. For example,
Krigman & Hogan (1974) reported that lead exposure during early
development reduced brain weight and decreased total brain proteins,
but the number of brain cells was unchanged. DNA measurements have
been particularly useful in studying the toxic effects of drugs on
retinal photoreceptors (Dewar et al., 1977). It is also well-known
that the DNA content changes after nutritional restriction during
development, or after exposure to various toxic chemicals that
influence nutritional status.
Although the measurement of DNA content is relatively simple and
in the nervous system can be an index of cell number, it is probably
not a very sensitive or early indicator of neurotoxicity. For example,
neuronal loss might be accompanied by glial proliferation and produce
no change in total DNA. The earliest effects of a toxic substance on
DNA will probably involve: (a) the disturbance of DNA-repair enzymes;
(b) the intercalation of the substance into the DNA molecule; or
(c) the direct binding of the substance to the nucleic acid moiety or
its associated chromosomal proteins. Two to three percent of added
mercuric chloride was reported to bind to the chromatin in cultured
glial cells (Ramanujam et al., 1970). Since this mode of action will
ultimately interfere with the functional integrity of the CNS, it
offers great promise for the identification of toxic compounds.
The total amount of RNA or protein could also be examined after
toxic insult. However, neither total RNA nor total protein is likely
to provide a sensitive index of toxic damage. Only in the extreme
stages of neurotoxicity will total or even regional levels of these
macromolecules be likely to change. More sensitive indices of their
metabolism rely on estimates of synthesis or degradation.
The rate of RNA or protein synthesis can be evaluated by using
radiolabelled precursors and measuring their rate of incorporation
into the macromolecule in vitro or in vivo. Although this
technique is simple and sensitive, it assumes that the rate of
incorporation of the precursor directly reflects the rate of
macromolecular synthesis and this may not always be true (Dunn, 1977).
Changes in labelling could also occur as a result of changes in
cerebral blood flow, precursor transport, precursor pool size, energy
charge, etc. Inhibition of amino acid transport across the blood-brain
barrier has been reported for mercury (Partridge, 1976) and lead
(Lorenzo & Gerwitz, 1977). Thus, it is also important to consider
whether a toxic agent can cause changes in the amino acid or nucleic-
acid-precursor pool sizes by using one of several methods available
for compensating for precursor pool fluctations (Munro et al., 1964;
Dunlop et al., 1975; Dunn, 1975). Changes in brain-protein synthesis
have been reported after exposure to methylmercury (Verity et al.,
1977), and carbon disulfide (Savolaiene & Jarvisalo, 1977). However,
in many cases, only the absolute levels of incorporation of
radioactive amino acids into protein fractions were studied and it is
difficult to understand the primary mechanisms of action.
Although these relatively crude methods are subject to several
criticisms, all the variations that influence the incorporation of the
radioactive precursor can ultimately influence cellular metabolism.
These methods can, therefore, be useful for identifying metabolic
disturbances. The relative amount of protein synthesis can also be
estimated simply by measuring the polyribosome to free ribosome pool.
This method is based on the assumption that during high rates of
protein synthesis, the greatest proportion of the total ribosomes will
be attached to an mRNA molecule. The relative polyribosome to monosome
ratio has the advantage of allowing for normalization across animals
and therefore reducing the interanimal variability that often plagues
neurochemical assessments. Furthermore, the use of this method would
detect toxic effects not only on protein synthesis but on the
stability or synthesis of the ribosome itself.
The rate of RNA and protein synthesis in the brain is very high
and nerve cell functioning is dependent on protein metabolism. The
turnover rates of brain macromolecules may vary considerably, and it
is always going to be difficult to interpret any data dealing with the
turnover of total RNA or protein. However, with the availability of
newer separation methods for proteins (detergent gel electrophoresis
combined with chromatofocusing cellulose ion exchange chromatography,
etc.), soluble and bound polypeptides can be separated to study the
synthesis of individual proteins at the subcellular level, in specific
cell types and in localized brain regions. Large classes of RNA can be
analysed by hybridization procedures or specific mRNA molecules could
be targeted for investigation by using specific cDNA probes.
6.4 Lipids, Glycolipids, and Glycoproteins
Complex lipids, glycolipids, and glycoproteins have several
important functions in neural tissue (Zuber, 1978). They constitute
structural elements of the plasma membrane, act as components of ion
channels, comprise portions of neurotransmitter receptors, and are
major constituents of myelin. Complex lipids (e.g., phospholipids,
cerebrosides, sulfatides, and gangliosides) make up neuronal
membranes. Phosphatides (phosphatidylethanolamine, phosphatidyl-
choline, phosphatidylinositol, phosphatidylserine) are the most
important group of phospholipids. They consist of some of the most
metabolically active of the phospholipids (Prokhorova, 1974) and
participate in the movement of Na and K ions through the neural
membrane. A closely related group of phospholipids, the plasmalogens,
are concentrated in myelin and constitute 18-30% of total brain
phospholipids.
Ganliosides are localized primarily in the plasma membrane
(Karpova et al., 1978), but small amounts of monosialoganglioside are
detectable in myelin. Receptors of neurotransmitters, such as
serotonin, and other biologically-active compounds may contain
ganglioside (Avrona, 1971), and evidence suggests that gangliosides
may bind biologically-active compounds, as well as various
neurotoxins, through their terminal N-acetylneuraminic acid moiety
(Kryzhavosky, 1973). Glycosaminoglycans are also functional and
structural elements of the neuronal membrane and have been reported to
exhibit disturbed metabolism in various hereditary diseases of the
mucopolysaccharidosis type (Constantopoulos et al., 1976; Vasan &
Chase, 1976).
The most interesting of the functionally-active, minor components
of neuronal membranes are sialic acids, and particularly
N-acetylneuraminic acid. As sialo-glycoproteins and gangliosides,
N-acetylneuraminic acid participates in carrying out specific
neuronal functions such as the establishment of synaptic contact
neurotransmission, and axonal propagation (Partingron & Daly, 1979).
Gangliosides and sialo-glycoproteins bind Ca++ at their hydrophilic
ends and thus, by influencing the concentration of this ion, affect
depolarization and neurotransmitter release.
Myelin metabolism may be affected by a variety of toxic agents
(Cammer, 1980). After administration of these compounds, there is
often a delay before the appearance of neurotoxic signs and symptoms
such as the ataxia indicative of peripheral demyelination. Central
demyelination often occurs to a lesser extent. Compounds that cause
disturbances in myelin metabolism may exert their effect directly on
myelin-forming cells, or demyelination may be a secondary response to
a disturbance of neuronal metabolism.
Several compounds have been reported to alter complex lipids or
their metabolism. Neonatal exposure to lead has been reported to
decrease the total brain content of phospholipids, galactolipids,
plasmalogens, and cholesterol (Van Gelder, 1978). Hydrogen sulfide and
sulfur dioxide decrease total brain lipids and/or phospholipids
(Haider et al., 1980). Meta-SystoxR (phosphorothioic acid
O-[2-(ethylthio)ethyl] O,O-dimethyl ester mixture with
S-[2-(ethylthio)ethyl] O,O-dimethyl phosphorothioate)
(O,O-dimethyl-S-2 (ethylsulfinyl) ethylthiophosphate), an
organophosphorus pesticide, has been reported to decrease brain levels
of total lipids, phospholipids, cholesterol, esterfied fatty acids,
and gangliosides (Islam et al., 1983). Merkuryva et al. (1978) found
one of the early affects of carbon disulfide intoxication to be
changes in the enzyme substrate system of N-acetylneuraminic acid,
N-acetylneuraminate lyase. In the olfactory bulb of the rabbit,
carbon disulfide led to an accumulation of N-acetylneuraminic acid
because of a reduced degradation by ß-acetylneuraminate lyase. In some
cases, the carbon disulfide-induced disturbance of the metabolism of
cerebral glycoconjugates could be correlated with the changing of
physiological parameters (e.g., lowering of the amplitude of the
cortical evoked potential (Bokina et al., 1976)). Long-term exposure
to lead acetate in aging rats was reported to decrease
N-acetylneuraminic acid content in cerebral tissue and also to
decrease the activity of the degradative enzyme (Merkuryva &
Bushinskaya 1982). Long-term exposure to ethanol led to an
accumulation of N-acetylneuraminic acid in the subfornical region of
the hypothalamus and in the midbrain reticular formation (Merkuryva et
al., 1980).
A recently applied approach to the study of neurotoxicology
involves the investigation of lipid peroxidation. Peroxidation
involves the direct reaction of oxygen and lipids to form free radical
intermediates and semistable peroxides. Lipid peroxidation is damaging
because of the subsequent reactivity of these free radicals (Tappel,
1970). Biomembranes and subcellular organelles are the major cellular
components damaged by lipid peroxidation. Increased lipid peroxidation
has been reported after exposure to thallium, nickel, or cobalt (Hasan
& Ali, 1981). Since the estimate of lipid perioxidation is a
relatively simple technique, this method may prove to be an effective
tool for screening for neurotoxic compounds.
6.5 Neurotransmitters
Chemical synaptic transmission involves a complex series of events
(synthesis and storage of neurotransmitters, release of neurotrans-
mitters, re-uptake or degradation of transmitters, interaction of
transmitters with postsynaptic membrane), any or all of which could be
disturbed by neurotoxins. In addition to the classical neurotrans-
mitters (Table 5), a number of peptides have been discovered, which
may act as chemical messengers (Burgen et al., 1980). Most
toxicological studies have focused on the neurotransmitters listed in
Table 5, because considerable background information on these
neurotransmitters is already available. Nearly every class of neuron
contains some marker enzyme (Table 5), and these have been useful in
studies in which attempts have been made to identify neurotransmitter
specific cells and in the determination of the regional distribution
of neurotransmitter systems.
Table 5. Enzyme markers for neurotransmitter specific cells
Neurotransmitter Marker enzyme Receptor types
acetylcholine choline acetyltransferase mucarinic, nicotinic
dopamine tyrosine hydroxylase DA1 and DA2
noradrenaline dopamine-ß-hydroxylase alpha1, alpha2, ß1,
ß2 adrenoreceptors
GABA(gamma- glutamate decarboxylase GABAA and GABAB
aminobutyric acid) (strychnine-insensitive;
picrotoxin-sensitive)
glycine unknown strychnine-sensitive
serotonin 1-amino acid decarboxylase 5 HT1, 5 HT2
6.5.1 Synthesis/degradation
Many neurotoxic compounds have been reported to alter steady-state
levels of neurotransmitters, but: it is difficult to draw functional
inferences from such data since the steady-state levels of
neurotransmitters can be influenced via multiple mechanisms (e.g.,
rate of synthesis, rate of release, rate of degradation). More
informative data are obtained by examination of the turnover rates,
reflecting the metabolic half-life of the neurotransmitter (Costa,
1970). Turnover of 5-HT, after disulfiram treatment, was examined by
observing the accumulation of 5-HT following administration of
pargyline (Minegishi et al., 1979). It has been speculated that carbon
disulfide acts by altering brain catecholamine concentrations. Carbon
disulfide increased DA and decreased NE by inhibiting dopamine
ß-hydroxylase, the enzyme responsible for converting dopamine (DA)
into norepinephrine (NE) (McKenna & DiStefano, 1975). Manganese is
known to inhibit DA formation by blocking tyrosine hydroxylase in the
striatum (Bonilla, 1980) and many acute effects of organophosphorus
compounds are related to inhibition of AChE activity (O'Brien, 1976).
Soon after their introduction, the inhibition of AChE was accepted as
a mechanism for the acute toxic effects of organophosphorus
insecticides (DuBois et al., 1949). However, this mechanism does not
account for the delayed neurotoxicity seen after organophosphate
poisoning (Abou-Donia & Preissig, 1976; Abou-Donia, 1981).
Interference with axonal transport may also disrupt
neurotransmitter function by altering the availability of
neurotransmitter enzymes or by decreasing the transport of peptide
precursors. The transport of materials along axons is bidirectional:
anterograde and retrograde. Anterograde transport conveys materials
from cell body to axon and terminals at slow (1-3 mm per day) and fast
(410 mm per day) rates (Ochs, 1972; Hoffman & Lasek, 1975). The former
contains the bulk of axoplasmic proteins (notably of neurofilaments
and neurotubules) and the latter, membrane and other components.
Retrograde transport, a system that conveys materials at rapid rates
(300 mm per day) may also be affected early in toxic axonopathies.
Retrograde transport may be a critical factor in the toxicity
resulting from exposure to various compounds.
6.5.2 Transport/release
Neurotransmitters or their precursors can rapidly concentrate in
nerve endings through specific high-affinity uptake systems associated
with each transmitter-specific class of neurons (Iverson, 1971). The
high-affinity, Na+-dependent transport mechanisms are specific to
nerve cells and can be distinguished from the ubiquitous low-affinity
transport systems that concentrate a large variety of amino acids,
sugars, and nucleosides, or their precursors. High-affinity uptake
phenomena may be studied in brain homogenates, brain minces, or
synaptosomal preparations. Lead (Silbergeld & Goldberg, 1975), the
insecticide chlordecone (Chang-tsui & Ho, 1979), and erythrosin B
(Lafferman & Silbergeld, 1979) have been reported to interfere with
neurotransmitter uptake processes. However, a variety of nonspecific
events may influence apparent uptake, so caution must be exercised in
interpreting results of these studies. For example, the effects of
erythrosin B on uptake mechanisms were reported to be non-specific
(Mailman et al., 1980).
The release of neurotransmitters from synaptic endings occurs
through an exocytotic process that is triggered by an influx of
calcium ions on depolarization of the nerve endings (Cotman et al.,
1976). Neurotransmitter release can be measured by preloading nerve
endings with labelled neurotransmitters, exposing tissue slices or
synaptosomal fractions maintained on filter beds to calcium ions, and
measuring the appearance of labelled or unlabelled compounds in the
supernatant medium (Bondy & Harrington, 1979). Heavy metals such as
lead have been reported to interfere with calcium-dependent
neurotransmitter release (Bondy et al., 1979; Ramsay et al., 1980),
and manganese has been reported to block transmitter release (Kirpekar
et al., 1970; Balnave & Gage, 1973; Kostial et al., 1974), possibly by
blocking inward Ca2+ current (Baker et al., 1971). Again, caution
must be used in comparing the results from different assay systems.
6.5.3 Binding
Binding of neurotransmitters to specific membrane receptors is the
first step in a complex series of events initiated in the
post-synaptic cell. Such binding interactions are reversible,
stereospecific, nonenzymatic, have equilibria with low dissociation
contants, and involve configurational recognition (Yamamura et al,
1981). Studies on receptor binding have been made possible by the
availability of specific radioactive analogues of neurotransmitters.
In general, the most potent binding ligands are the pharmacological
antagonists or agonists of a given neurotransmitter, rather than the
transmitter itself. Kinetic characteristics of binding and receptor
density are estimated by incubating receptor-enriched membranes with
radioactive ligands. Excess ligands, not bound to membranes, can be
removed by filtration or centrifugation. Non-specific binding of the
labelled compound is estimated by repeating the incubation in the
presence of a high concentration (10-4 to 10-6 mol) of an
unlabelled analogue. The solubility of ligands either in lipids or in
water has to be considered when interpreting results of ligand-binding
studies. This method is relatively simple and has been suggested as a
useful screening procedure for the detection of neurotoxic compounds
(Damstra & Bondy, 1980, 1982). If properly employed, such analyses can
provide valuable information. However, interpretation of the data can
be complicated by the often low degree of specificity of the ligand
and the location of the receptor, which may be pre- or post-synaptic
or on non-neuronal glial elements. Furthermore, for many
neurotransmitters, several subtypes of receptors exist and may produce
different effects on the post-synaptic membrane. Functional
extrapolations from neurotransmitter binding studies, therefore,
should be made with caution.
Receptor binding techniques have only recently been applied to
neurotoxicological studies. However, in a short time, many compounds
have been reported to increase or decrease estimates of receptor
density. Acrylamide (Bondy et al., 1981), chlordecone (Seth et al.,
1981), lead and mercury (Bondy & Agrawal, 1980), and cadmium (Hedlund
et al., 1979) have all been reported to alter putative
neurotransmitter receptors.
6.5.4 Ion channels
Many neuronal functions are regulated by ion channels. The Na+
channel is responsible for depolarizing the membrane, and K+
channels are responsible for repolarizing the membrane. Maintenance of
appropriate Na+ and K+ concentrations requires the classical
Na+/K+ ATPase. Disturbed functioning of these ion channels or
disturbance of ATP availability can severely disturb neuronal
functioning. Several naturally-occurring toxins affect Na+ channels
(Hille, 1976; Ritchie, 1979; Catterall, 1977). Tetrodotoxin and
saxitoxin inactivate the channel at nanomolar concentrations and can
be used as probes for measuring channel density or isolation of
channel polypeptides (Agnew et al., 1978). Batrachotoxin and
veratridine bind the channels and produce persistent activation by
preventing channel inactivation (Albuquerque & Daly, 1976). Peptide
toxins, such as scorpion toxin (Couraud et al., 1978), exhibit either
voltage sensitive or insensitive binding to the extracellular surface
of the Na+ channel (Catterall, 1977). Several potassium channels
probably exist in neurons (Reichardt & Kelly, 1983), but purification
and characterization of these channels has lagged behind that of the
Na+ channel. However, a scorpion toxin that exibited K+ channel
affinity has been described (Carbone et al., 1982).
Na+/K+-ATPase consists of 2 polypeptide chains. The smaller
polypeptide is a glycoprotein (Carilli et al., 1982). The larger
polypeptide binds ATP internally and ouabain externally. Hormones and
neurotransmitters such as catecholamines regulate the ATPase (Clausen
& Flatman, 1977), and neurotoxicants could alter neuronal functioning
by disturbing this control of ionic gradients. Desaiah et al. (1980)
have reported inhibition of Na+/K+-ATPase and decreased ouabain
binding to synaptosomes after treatment of mice with the insecticide
chlordecone. The evaluation of ion balance and their control will
probably be used increasingly in neurotoxicology.
For the most part, transmitter release is regulated by Ca2+
entry through a Ca2+-selective channels. Depolarization,
neurotransmitters, and hormones regulate Ca2+-selective channel
(Reichardt & Kelly, 1983). Nerve terminals contain several Ca2+
calmodulin-dependent protein kinases and a Ca2+-phospholipid
activated kinase, each of which has a distinct set of protein
substrates. Cytoplasmic Ca2+ binds calmodulin and other Ca2+
binding proteins, which directly or indirectly activate other enzymes
via their Ca2+-dependent kinases. How such activation facilitates
exocytosis is not known, but presumably the phosphorylation state of
synapsin I, a phosphoprotein present primarily in nerve terminals and
associated with synaptic vesicles, is believed to regulate transmitter
vesicle release (Dolphin & Greengard, 1981).
Changes in Ca2+ concentration induced by neurotoxicants could
have significant consequences for neuronal function. Cytoplasmic
Ca2+ stimulates not only exocytosis, but also glucogenolysis and
mitochondrial respiration (Landowne & Ritchie, 1976), endocytosis
(Ceccarelli & Hurlbut, 1980), and neurotransmitter synthesis (Collier
& Ilson, 1977). Even though many of these events are homeostatic
responses to transmitter utilization, neurotoxic disruption of calcium
influx or sequestering could produce disturbances not restricted to
neurotransmitter release.
Few neurotoxic compounds have been investigated for their
influence on calcium channels. Manganese is reported to block
neurotransmitter release by blocking inward Ca2+ current (Kirpekar
et al., 1970), and other heavy metals have been reported to interfere
with calcium-mediated neurotransmitter release (Bondy et al., 1979;
Ramsay et al., 1980). Similar effects of heavy metals were also
observed for the Na+-Ca2+ exchange system. Ion channels and
Na+-Ca2+ probes may be a valuable screening approach for some
types of neurotoxic compounds.
6.5.5 Cyclic nucleotides
Nerve terminal function and the effects of neurotransmitters are
often regulated by cyclic nucleotides. Binding of agonists to
receptors on the nerve membrane can result in activation of second
messenger systems. Activation of different receptors results in
specific changes in cyclic AMP and cyclic GMP levels and consequent
alterations in protein kinases. It is thought that on phosphorylation,
conformational changes occur in membrane proteins that may change ion
permeabilities. Thus, the responsiveness of the nervous system to some
toxic agents can also be measured by determining changes in the
activities of adenylate cyclase, cyclic nucleotides, and protein
phosphorylation. For example, lead has been shown to inhibit
cerebellar adenylate cyclase (Nathanson & Bloom, 1975) and
dopamine-sensitive adenylate cyclase in the striatum (Wilson, 1982).
Various pesticides cause increases in the levels of cyclic nucleotides
in brain tissue (Aldridge et al., 1978). For example, tri-o-cresyl
phosphate enhanced Ca2+-calmodulin-dependent in vivo
phosphorylation of proteins in chicken brain (Patton et al., 1983).
6.5.6 Summary of nerve terminal function
The analysis of synaptic function and neurotransmitter metabolism
is reasonably complex, and it should be borne in mind that disturbance
at many levels of neuronal organization will ultimately alter synaptic
function. However, examination of the nerve terminal is an excellent
approach for the initial study of toxic chemicals. However, it is
important to recognize the dynamic nature of the nerve terminal and to
remain alert to the possibility of false positives.
6.6 Energy Metabolism
Nervous tissue, and brain tissue in particular, require
disproportionately large amounts of energy to sustain the
translocation of ions important for electrical activity and to
maintain the highly-active biosynthetic machinery of the tissue.
Since neural tissue has only limited stores of energy (e.g., glycogen
and creatine phosphate), glucose availability and enzymes critical for
energy production are vital to neuronal functioning. A number of
neurotoxic chemicals have been shown to interfere with glucose and
energy metabolism in both the central and peripheral nervous system.
These include methylmercury (Bull & Lutkenhoff, 1975), hexachlorophene
(Cammer & Moore, 1972), organotin compounds (Lock, 1976), and alcohol
(Merkuryva et al., 1980). Glycolytic enzymes have been shown to be
inhibited by alcohol (Merkuryva et al., 1980) and by chemicals known
to cause distal axonopathy, e.g., acrylamide (Howland et al., 1980)
carbon disulfide, and methyl-n-butyl ketone (Sabri et al., 1979).
Active brain areas have a higher rate of glucose consumption than
less active regions. The brain regional metabolic activity is
determined by the 14C-2-deoxyglucose method (2-DG) of Sokoloff et
al. (1977). This method is based on the use of 2-deoxy-D-14C-glucose
as a tracer for the exchange of glucose between the plasma and the
blood and its phosphorylation by hexokinase. The product,
2-deoxyglucose-6-phosphate (2-DG-P), is trapped in the tissue and its
accumulation in the various regions of the brain might be a measure of
the glucose use and neuronal activity. The method provides an index of
the in vivo glucose metabolic activity of brain regions and has been
used in experimental animals to determine which brain regions are
depressed or activated during acute Soman intoxication (McDonough et
al., 1983).
Metabolic compartmentalization in nerve tissue could provide an
additional tool in toxicological studies. It is well established that
neurons mainly use glucose in their energy metabolism, while glia use
substrates other than glucose (Hertz, 1981). Thus, it may be possible
to differentiate between the toxicological effects of chemical
substances on neuronal and glial populations in vitro or in vivo.
6.7 Biochemical Correlates of Axonal Degeneration
A relatively large class of neurotoxic agents is known to produce
axonal degeneration (Griffin & Price, 1980; Sabri & Spencer, 1980;
Thomas, 1980). Some, such as acrylamide and organophosphorus
compounds, produce the distal to proximal "dying back" pattern of
degeneration, while others, such as ß,ß-iminodipropionitrile, lead to
proximal to distal degeneration. Since many of these compounds produce
Wallerian degeneration, biochemical correlates of Wallerian
degeneration have been suggested as toxicological indices (Dewar &
Moffett, 1979). These authors tabulated the chemical changes in
peripheral nerves that occurred during degeneration and suggested that
ß-gluocorinadase and ß-galactosidase might be good indices of
toxicity. Acrylamide, methylmercury, and dimethyl sulfoxide were shown
to increase these lysosomal enzymes (Dewar & Moffett, 1979). The use
of lysosomal enzymes involves relatively simple procedures and might,
therefore, be appropriate for the initiation of a biochemical screen.
Disturbance of axoplasmic transport has been suggested as an
alternative approach to detecting compounds producing axonal
degeneration. Several substances, e.g., organophosphates (Reichart &
About-Donia, 1980) and acrylamide (Pleasure et al., 1969) disrupt
axonal transport. However, these methods are more difficult, more
expensive, and more time-consuming than the lysosomal enzyme assays.
6.8 Neuroendocrine Assessments
The number of toxic compounds being recognized for their
neuroendocrine actions is increasing. Hormonal balance results from
the integrated action of the hypothalamus, pituitary, and endocrine
target organ. Each site is susceptible to disruption by environmental
toxic agents. This disruption may result from the direct interaction
of the toxic agent with the endocrine organ, pituitary, or
hypothalamus. Alternatively, neuroendocrine dysfunction may occur
because of a disturbance in the regulation and/or modulatory elements
of the complex neuroendocrine feedback systems. Any such disturbances
would ultimately modify anterior pituitary secretions. Thus, the
analysis of blood levels is the most appropriate starting point
(section 6.8.2.1).
6.8.1 Anterior pituitary hormones
The main effector organ in the neuroendocrine system is the
pituitary. This "master gland" consists of an anterior
adenohypophysis, and a posterior neurohypophysis. Based on
histological, immunocytochemical, and electron microscopic examination,
the following cell types are differentiated in the anterior pituitary
(Junqueira et al., 1977); follicular cells, which do not contain any
secretory granules but form the support stroma of the glandular cells;
chromophobe cells, which are undifferentiated, nonsecretory and
secretory cells without discernible granules in the light microscope;
somatotropic cells, which are acidophilic with immunocytochemically-
detectable growth hormone and contain granules of about 350 nm
diameter; prolactin cells, which are acidophilic and contain prolactin
granules of 600-900 nm diameter; gonadotropic cells, which consist of
2 basophilic subgroups: follicle-stimulating hormone secreting and
luteinizing-hormone secreting; follicle-stimulating hormone secreting
cells, which are large round cells with dense 200 nm diameter
granules; luteinizing hormone-containing cells, which are small and
contain 200-250 nm diameter granules; thyrotropic cells, which are
basophilic with thyroid-stimulating hormone granules of 120-200 nm
diameter; and finally, cortico cells, which are the least abundant of
the cell types and contain basophilic granules 100-200 nm in diameter.
The glandular cells of the anterior pituitary secrete seven
endocrine hormones, of which four act directly on other endocrine
glands. Follicle-stimulating hormone (FSH) promotes spermatogenesis in
the male testes and facilitates follicular maturation and estrogen
secretion in the female ovary. Lutenizing hormone (LH) acts on the
male testes to facilitate testosterone secretion from the interstitial
cells of Leydig and is a key hormone that promotes follicular rupture
(ovulation) in the female ovary. Thyroid-stimulating hormone (TSH)
triggers the secretion of thyroxine from the thyroid gland, and
adrenocorticotropic hormone (ACTH) causes the adrenal cortex to
secrete its products, especially glucocorticoids. The secretion of
these tropic, anterior pituitary hormones is in turn regulated by the
hormones of the endocrine gland. In most cases, this is a negative
feedback regulation. Estrogen decreases the output of pituitary FSH
and LH; thyroxine decreases the secretion of TSH; and glucocorticoids
reduce the output of ACTH.
The three other anterior pituitary hormones are prolactin (PRL),
melanocyte-stimulating hormone (MSH), and growth hormone (GH). A major
target for prolactin is the mammary gland, where prolactin promotes
milk production. PRL also plays a role in maintaining the ovarian
corpus luteum after ovulation. The entire body is a target for GH,
which promotes growth and maintenance of cellular integrity. GH
facilitates growth, in part, because it increases the transport of
amino acids into the cell, where they can be used for protein
synthesis. MSH increases the production of melanin by the melanocytes
of the skin.
Hypothalamic control of anterior pituitary secretions occurs
through the release of hypothalamic-hypophysiotropic hormones (HHH).
The hypothalmus secretes these HHH into vessels of the hypothalamic-
hypophyseal portal system, by which they are transported to the
anterior pituitary. One of the major advances of the last two decades
has been the isolation and characterization of these HHH.
6.8.2 Disruption of neuroendocrine function
Disruption of neuroendocrine function can occur at any one or all
of the levels of hypothalamic-pituitary-target organ integration.
Alternatively, neuroendocrine effects may result from modification of
"higher" levels of neuronal processing or from alterations in
peripheral metabolism. In the following discussion, approaches will be
indicated for the study of each of these possible sites of neurotoxic
action.
6.8.2.1 Direct pituitary effects
At the most dramatic level, massive changes in the weight or size
of the pituitary, such as occur in the adenohypophysis after large
doses of estrogen (Schreiber & Pribyl, 1980) might be seen. However,
by far the most accessible method for studying pituitary function
relies on the examination of blood levels of pituitary hormones by
radioimmunoassay. Furthermore, the analysis of blood-hormone levels is
one of the few techniques applicable for screening human populations.
A large number of toxic substances have been reported to modify
adenohypophyseal secretions. Methallibure and allied substances
inhibit thyrotropin (Tulloch et al., 1963), and prolactin (Benson &
Zagni, 1965) secretion. Modifications of pituitary hormone secretions
have been reported for acrylamide (Uphouse et al., 1982), alcohol
(Mendelson et al., 1977), 1,4-DDT (Gellert et al., 1972), and
chlordecone (Uphouse et al., 1984). Dimethylsulfoxide may influence
adrenal glucocorticoids by elevating pituitary secretions (Allen &
Allen, 1975). In spite of the fact that changes in the blood levels of
pituitary hormones may be the most sensitive index of neuroendocrine
modification by toxic compounds, they have been examined for
relatively few compounds. The high sensitivity of this approach
results from the fact that the blood levels represent the final
consequence of the complex neuroendocrine integration. Regardless of
the actual site of modification, neuroendocrine disturbances will
usually be revealed in modified levels of circulating hormones.
Therefore, such changes, alone, cannot be regarded as evidence of
direct neuroendocrine disruption. The major disadvantage of serum-
hormone measurements is the responsiveness of the hypothalamic-
pituitary axis to a variety of environmental and chemical stimuli. It
can be difficult to identify the toxic agent as the causal agent.
Because hormones are secreted in a pulsatile manner, single point
analyses of serum hormones must be cautiously interpreted. However,
even with these limitations, analysis of blood levels of pituitary
hormones remains the most appropriate starting point for evaluating
potential neuroendocrine toxicity.
Similar analytical methods may be used to investigate the
pituitary content of respective pituitary secretions. The pituitary is
removed, homogenized, and extracted for use with the appropriate RIA.
For most pituitary secretions, neurotoxicologists have not yet studied
pituitary tissue directly. However, pituitary endorphins has been
reported to be modified by chlordecone (Hong & All, 1982).
6.8.2.2 Peripheral target effects
Most anterior pituitary hormones are subject to negative feedback
control by peripheral endocrine glands. If the neurotoxic agent
modifies peripheral secretions, neuroendocrine changes can result from
this altered feedback. Modifications in the functioning of these
endocrine secretions could occur after toxic exposure. Such approaches
have been widely applied in the experimental and clinical literature
and have shown that a number of compounds alter blood levels of
glucocorticoids, thyroxine, estrogen, and testosterones (Chapman,
1983).
Target-tissue effects can also be evaluated by a variety of
additional techniques. One of the simplest assessments is the change
in the weight, morphology, or biochemistry of the target organ.
Various environmental contaminants have been reported to produce such
changes. Lesions of the thyroid have been reported after exposure to
carbon disulfide (Cavalleri, 1975) and strong magnetic fields
(Persinger et al., 1978). Marked changes occur in the adrenals during
antimony and lead poisoning (Minkins et al., 1973) and in response to
very long chain saturated fatty acids (Powers et al., 1980).
Chlordecone causes adrenal, cortical, and medullary hypertrophy
(Eroschenko & Wilson, 1975) and alters the epinephrine and
norephinephrine content of the adrenal medulla (Baggett et al., 1980).
DDT (McBlain et al., 1976) and other chlorinated hydrocarbons
(Dicksith & Datta, 1972; Fellegiova et al., 1977), carbon disulfide
(Rosewickyi et al., 1973), and cadmium salts (Parizek, 1956;
Zylber-Haran et al., 1982) have all been described as having toxic
effects on the gonads. Although such changes are not necessarily due
to direct neuroendocrine effects, target organ changes can often be a
first indication of neuroendocrine changes. In some cases, even
relatively gross target organ events have been a first clue towards
the mode of action of a neurotoxic agent. For example, the chlorinated
pesticides, DDT and chlordecone, modify gonads in a manner reminiscent
of the natural steroid, estrogen (Gellert et al., 1972; Eroschenko &
Palmiter, 1980; Kupfer & Bulger, 1980). Uterine and/or oviduct weight
changes in the immature animal were the first evidence that the
pesticides might exert estrogenic action.
6.8.2.3 Disruption of hypothalamic control of pituitary secretions
Hypothalamic control of anterior pituitary secretions includes
direct regulation through hypothalamic hypophysiotropic hormones and
modulation through neurotransmitters and neurally-active peptides.
Evaluation of the effect of a neurotoxic agent on HHH is directly
measureable only for the HHH for which antisera are available. For
these compounds, it is possible (though difficult) to measure release
into the portal blood supply, to evaluate the effects of toxic agents
on either amounts or release, and to identify pituitary responsiveness
to the hypothalamic factors. Where identified hormones are not
available, hypothalamic extracts may still be used with in vitro
pituitary responsiveness as a bioassay. Details of such methods can be
found in several sources (Burgus & Guillemin, 1970; Oliver et al.,
1974). However, the value of such approaches for neurotoxicology has
yet to, be tested. Such methods will be of greatest value in testing
hypotheses regarding the mechanism of action of known neuroendocrine
toxic agents. They are not recommended for initial screens.
Biochemical changes in the hypothalamus can also be used as
indices of potential neuroendocrine disruption. Such hypothalamic
effects of neurotoxic compounds have received much less attention than
brain areas such as the striatum, cortex, or cerebellum. Methods for
studying the hypothalamus are the same as those used for other brain
areas. However, the hypothalamic neurons that regulate pituitary
function receive numerous synaptic inputs, both conventional and from
putative neurotransmitters. Consequently, the neuroendocrine
significance of changes in hypothalamic neurotransmitters and
neuropeptides is usually only inferential. However, any disruption of
hypothalamic biochemistry has the potential to alter neuroendocrine
function. When combined with more direct measurements of
neuroendocrine function, such studies are very important. There have
been a few reports of biochemical changes in the hypothalamus and/or
pituitary, correlated with neuroendocrine toxicity. One approach has
been to examine the effects of toxic compounds on hormone-mediated
changes. Administration of estrogens is followed by hypothalamic
ascorbic acid depletion (Schreiber et al., 1982) and by increases in
polyphenol oxidase (ceruloplasmin) activity in the hypothalamus and
the blood (Schreiber & Pribyl, 1980). This can be inhibited by the
simultaneous administration of silver nitrate. Disulfiram
(tetraethylthiuram disulfide) inhibits the reaction of the
adenohypophysis (Schreiber et al., 1979). Disulfiram also inhibits
dopamine-ß-hydroxylase (Szmigielski, 1975) and lowers the
blood-prolactin level (Cavalleri et al., 1978). The effect of
disulfirams on prolactin may be due to a "sparing of dopamine" through
inhibition of dopamine-ß-hydroxylase.
Hypothalamic peptides have been studied most extensively after
chlordecone treatment. Long-term exposure to the pesticide reduced
hypothalamic ß-endorphin levels under conditions where substance P,
neurotensin, and met-enkephalin were unchanged (Ali et al., 1982).
6.8.2.4 Other sites of action
For many neurotoxic agents, there may be no, identifiable effects
on neuroendocrine function, but it may be altered through the indirect
effects of the toxic compound. Such interruption could occur via
modification of neural integration leading to an altered response of
the organism to environmental challenge. Detection of such integrative
action and its relevance to neuroendocrine function might include
treatment with the toxic agent followed by environmental,
pharmacological, or hormonal challenges known to produce a
neuroendocrine response. Several toxic agents have been reported to
produce behavioural changes, such as stress-induced analgesia, and
these undoubtedly involve some aspect of neuroendocrine function. CNS
opiate systems are important components of the CNS response to stress
and studies of peripheral and brain endorphins and encephalins are
potentially relevant to such disturbances. For example, neonatal
treatment with chlordecone dissolved in dimethyl sulfoxide produces an
elevated corticosterone response to footshock (Rosecrans et al.,
1982), and long-term exposure of female rats to chlordecone has been
reported to decrease hypothalamic ß-endorphin levels (Ali et al.,
1982).
A toxic agent could also indirectly affect neuroendocrine function
by altering the peripheral metabolism of endocrine secretions. Such
metabolic differences could indirectly influence CNS function.
However, hormone transformation and the formation of active
metabolites occurs in the CNS so that metabolic disturbances may even
have a direct effect on CNS functioning. Many metabolic variables have
been reported to change after treatment with a toxic agent.
Aromatization of testosterone to estrogen, an important step in the
metabolism of estrogen, took place in the brain of animals (Gallard et
al., 1978). Its inhibition by aminoglutethimide or other blockers of
aromatization (Morali et al., 1977) markedly altered the sexual
behaviour of experimental animals. Aminoglutethimide also inhibited
total adrenal steroidogenesis as well as gonadal steroidogenesis and
thyroid function (Schreiber et al., 1969). The transformation of
thyroxine to the more active metabolite triiodothyronine was inhibited
by a series of toxic substances such as ethanol (Shimizu et al.,
1978), propylthiouracil (Leonard & Rosenberg, 1980), iopanoic acid
(Kaplan, 1980), and sodium salicylate (Chopra et al., 1980). Since the
monodeiodination of thryoxine to triiodothyronine also takes place in
the adenohypophysis, these factors may be an important component of
neuroendocrine reactions to neurotoxic substances. There is evidence
that demonstrates that the direct dopaminergic effect of estrogen on
the pituitary may require conversion of estrogen to catechol estrogen
(Paul et al., 1980). Such conversion occurs not only in the pituitary,
but also in the hypothalamus and cerebral cortex and may mediate some
of the effects of estrogen on the CNS. Study of this enzyme promises
to be important for future research, especially for the investigation
of estrogen-like toxic agents.
A final way in which the toxic agent might disrupt neuroendocrine
function is by altering the peripheral metabolism of the endocrine
secretions within liver tissue. Although beyond the scope of this
publication, several neurotoxic agents (chlordecone, dieldrin,
heptachlor, lindane, p,p'-DDE, and toxaphene) stimulate the
metabolism of estrone by liver microsomal enzymes (Welch et al.,
1971). Biochemical approaches for identifying these metabolic
disturbances are the same as those discussed in other sections.
However, the precise metabolic end-point should be carefully chosen,
when inferences are to be made about neuroendocrine function.
6.8.3 Sex differences
Because neurotoxic agents may produce changes in neuroendocrine
function and, since males and females differ with regard to a variety
of metabolic variables, a valuable approach to any neurotoxicological
investigation is the comparison of the toxic response in the sexes.
Whenever neuroendocrine effects are suspected, sex differences should
be a routine aspect of the overall investigation. Sex-related
differences have been observed for many environmental compounds.
Often, these result from the influence of gonadal hormones on the
metabolism of the compound. Hormonal influences on the
biotransformation and toxicity of DDT (Durham et al., 1956) and several
organophosphates (Murphy & DuBois, 1958; DuBois & Puchala, 1961) have
been demonstrated. Parathion is an organophosphate insecticide that
exerts its toxicity through its active metabolite paraoxon phosphate.
Agrawal et al. (1982) have recently shown that the sex difference in
AChE inhibition after parathion treatment was not evident with
paraoxon treatment. This suggests that the sex difference was in the
rate of metabolism to the toxic product. The female's increased
sensitivity to amobarbital (Castro & Gillette, 1967) may also be due
to sex differences in hepatic metabolism. Sex differences have also
been reported for the rate of disposition of chlordiazepoxide
(Greenblatt et al., 1977; Roberts et al., 1979) and for the toxic
effects of ethylmorphine, aniline, p-nitroanisole (Nicholas &
Barron, 1932; Holck et al., 1937; Quinn et al., 1958) and
polychlorinated hydrocarbons (Lamartiniere et al., 1979).
6.9 Recommendations for Future Research
Several biochemical and neuroendocrine approaches are available
that have not yet been applied to the study of neurotoxic compounds.
These have the advantage of increasing the sensitivity of the
biochemical approach and furthering understanding of the ways in which
compounds disrupt nervous system function. For example, some enzymes
involved in neurotransmitter synthesis have been purified and used for
the production of specific antibodies (John et al., 1973). It is now
possible to use immunological titration to determine whether changes
in enzyme activity result from activity alterations or variations in
the amount of enzyme protein.
Identification of direct pituitary modification can be
accomplished by preparation of anterior pituitary cell cultures and
the measurement of hormone release in response to hypothalamic
releasing factors, neurotransmitters, or suspect toxic compounds. The
pituitary cells are removed, dispersed, and agitated with collagenase.
After resuspension in medium, the cells are plated and used for
examination. Under such culture conditions, pituitary cells respond to
releasing factors and neurotransmitter regulation (Enjalbert et al.,
1978; Drouin & Labrie, 1981). Thus, it is possible to determine
whether the toxic agent modifies the responsiveness of the pituitary
to hypothalamic control. By comparing the effects of the toxic agent
in vivo with those in vitro, direct versus indirect effects of the
compound can be identified.
Furthermore, for suspect steroid-like compounds, direct evaluation
of receptor interactions is possible. Steroid receptors modify their
target tissue by binding to intracellular receptors. Measurement of
intracellular steroid receptors involves the preparation of a high
speed cytosol (180 000 g) fraction from the appropriate target tissue.
Identification of binding is accomplished by incubating the cytosol
in vitro in the presence of radioactively-labelled hormone. Bound
label is removed from unbound by a variety of techniques distinct for
the particular receptor of interest. Specific binding is assessed by
incubating the labelled hormone in the presence of excess unlabelled
hormone as competitor. Specific binding refers to bound molecules that
are removed in the presence of this unlabelled competitor. When a
neurotoxic agent is suspected of interacting with hormone receptors,
the toxic agent may be substituted for the unlabelled competitor and
its ability to compete for binding determined. This procedure has
demonstrated competition by both chlordecone (Palmiter & Mulvihill,
1978) and o,p'-DDT (Kupfer & Bulger, 1976) for the estradiol
cytosol receptor in the uterus. Using an estradiol exchange method,
investigators have shown that these pesticides produce translocation
of the estradiol receptor to the nucleus (Hammond et al., 1979).
Employment of the exchange method necessitates the in vivo treatment
of the organism with the toxic agent. Nuclei and cytosol are prepared
from the target tissue and incubated in vitro with the
radioactively-labelled hormone. Since a major movement of cytosol
receptors into the nucleus occurs only after binding to the hormone,
the presence of the receptor in the nucleus after exposure to the
toxic agent suggests direct binding of the toxic agent to the steroid
cytosol receptor.
Modifications of the procedures described for neurotransmitter
receptor binding are used in the measurement of membrane receptors.
However, no neurotoxic agents have yet been tested for their ability
to modify these hormone receptors. For both intracellular and
extracellular hormone receptors, most studies have been on peripheral
tissues. However, the brain and the pituitary contain receptors for a
variety of steroid and peptide hormones, and these offer a number of
targets for disruption by environmental compounds. Such analyses have
great potential for future studies of neurotoxic compounds. However,
they will usually be applied for the investigation of mechanisms of
action rather than as screens for neurotoxic compounds.
Finally, hypothalamic regulation of pituitary function should
receive further emphasis. Neurotransmitters also regulate pituitary
secretions via neurotransmitter receptors of the pituitary gland. The
potential for a direct modification of these receptor interactions by
neurotoxic compounds is only just being realized. The most thoroughly
described pituitary neurotransmitter receptor is that of dopamine,
which regulates the inhibition of prolactin release by dopamine.
Steroid hormones, such as estrogen, by conversion to catechol
estrogens, increase prolactin secretion by direct interaction with the
pituitary dopamine receptors (Gudelsky et al., 1981; Fishman, 1982).
Neuroleptic drugs elevate prolactin secretion by antagonistic action
on pituitary dopamine receptors (Horowski & Graf, 1979; Besser et al.,
1980).
7. CONCLUSIONS AND RECOMMENDATIONS
There is ample evidence of real and potential hazards of
environmental chemicals for nervous system function. Changes or
disturbances in central nervous function, many times manifest by vague
complaints and alterations in behaviour, reflect on the quality of
life; however, they have not yet received attention.
Neurotoxicological assessment is therefore an important area for
toxicological research.
It has become evident, particularly in the last decade, that
low-level exposure to certain toxic agents can produce deleterious
neural effects that may be discovered only when appropriate procedures
are used. While there are still episodes of large-scale poisoning,
concern has shifted to the more subtle deficits that reduce
functioning of the nervous system in less obvious, but still important
ways, so that intelligence, memory, emotion, and other complex neural
functions are affected.
Information on neurobehaviour, neurochemistry, neurophysiology,
neuroendocrinology, and neuropathology is vital for understanding the
mechanisms of neurotoxicity. One of the major objectives of a
multifaceted approach to toxicological studies is to understand
effects across all levels of neural organization. Such a multifaceted
approach is necessary for confirmation that the nervous system is the
target organ for the effect. Interdisciplinary studies are also
necessary to understand the significance of any behavioural changes
observed and thus, to aid in extrapolation to human beings by
providing specific neurotoxic profiles. Concomitant measurements at
different levels of neural organization can improve the validity of
results.
The following recommendations are made on the basis of the
information contained in this book:
1. Health personnel should take into account the possible role of
exposure to chemicals whenever a patient presents with any
neurobehavioural complaint.
2. Neurotoxicological testing should be an essential consideration in
any profile developed by the agencies responsible for the control
of toxic chemicals.
3. In the determination of the potential of a chemical to produce
neurotoxic effects, a multidisciplinary strategy should be used.
4. Priority should be given to obtaining clinical and epidemiological
data when exposure occurs to chemicals suspected of being
neurotoxic.
5. Test development in preclinical neurotoxicology has evolved to the
point where interlaboratory validation of procedures using
prototypic neurotoxic agents could be attempted.
REFERENCES
ABOU-DONIA, M.B. (1981) Organophosphorus ester-induced delayed
neurotoxicity. Ann. Rev. Pharmacol. Toxicol., 21 511-548.
ABOU-DONIA, M.B. & PREISSIG, S.H. (1976) Delayed neurotoxicity of
leptophos: toxic effects on the nervous system of hens. Toxicol.
appl. Pharmacol., 35 269-282.
ABOU-DONIA, M.G., JENSEN, D.N., & LAPADULA, D.M. (1983) Neurologic
manifestations of tri-o-cresyl phosphate delayed neurotoxicity in
cats. Neurobehav. Toxicol. Teratol., 5: 431-442.
AGNEW, W.S., LEVINSON, S.R., BRABSON, J.S., & RAFTERY, M.A. (1978)
Purification of the tetrodotoxin-binding component associated with the
voltage-sensitive sodium channel from Electrophorus electricus
electroplax membranes. Proc. Natl Acad. Sci. (USA), 75: 2606-2610.
AGRAWAL, A.K., SQUIBB, R.E., & BONDY, S,C. (1981) The effects of
acrylamide treatment upon the dopamine receptor. Toxicol. appl.
Pharmacol., 58 89-99.
AGRAWAL, D.K., MISRA, D., AGARWAL, S,, SETH, P.K., & KOHLI, J.D.
(1982) Influence of sex hormones on parathion toxicity in rats;
antiacetylcholinesterase activity of parathion and paraoxon in plasma,
erythrocytes, and brain. J. Toxicol. environ. Health, 9: 451-459.
ALBURQUERQUE, E.X. & DALY, J.W. (1976) Steroidal alkaloid toxins and
ion transport in electrogenic membranes. In: Cuatracasas, P., ed. The
specificity of animal, bacterial, and plant toxins, London, Chapman
and Hall, pp. 297-338.
ALDER, S. & ZBINDEN, G. (1977) Methods for the evaluation of physical,
neuromuscular, and behavioral development in rats in early postnatal
life. In: Newbert, D., Merber, H.J., & Kwasigroch, T.E., ed. Methods
in prenatal toxicology, Stuttgart, George Thiene, pp. 175-185.
ALDRIDGE, W.M., CLOTHIER, B., FORSHAW, P., JOHNSON, M.K., PARKER,
V.H., PRICE, R.J., SKILLETER, D.N., VERSCHOYLE, P.D., & STEVENS, C.
(1978) The effect of DDT and pyrethroids cismethrin and decamethrin on
the acetylcholine and cyclic nucliotide content of rat brain.
Biochem. Pharmacol., 27: 1703-1706.
ALEU, F.P., KATZMAN, R., & TERRY, R.D. (1963) Fine structures and
electrolyte analyses of cerebral oedema induced by alkyl tin
intoxication. J. Neuropathol. exp. Neurol., 22: 403-413.
ALEXANDER, B.K. BEYERSTEIN, B.L., HADAWAY, P.F., & COAMBS, R.B.
(1981) Effect of early and later colony housing on oral ingestion of
morphine in rats. Pharmacol. Biochem. Behav., 15: 571-576.
ALFANO, D.P. & PETIT, T.L. (1981) Behavioral effects of postnatal lead
exposure. Possible relationship to hippocampal dysfunction. Behav.
neural Biol., 32: 319-333.
ALI, S.F., HONG, J.S., WILSON, W.E., LAMB, J.C., MOORE, J.A., MASON,
G.A., & BONDY, S.C. (1982) Subchronic dietary exposure of rats to
chlordecone (kepone) modifies levels of hypothalamic beta-endorphin.
Neurotoxicology, 3: 119-124.
ALLEN, J.P. & ALLEN, C.F. (1975) The effect of dimethyl sulfoxide on
hypothalamic-pituitary-adrenal functions in the rat. Ann. NY Acad.
Sci., 243: 325-336.
ALTMAN, J. & SUDARSHAN, K. (1975) Postnatal development of locomotion
in the laboratory rat. Anim. Behav., 23: 896-920.
AMIN-ZAKI, L., MAJEED, M.A., ELHASSANI, S.B., CLARKSON, T.W.,
GREENWOOD, M.R. & DOHERTY, R.A. (1979) Prenatal methylmercury
poisoning: clinical observations over five years. J. Dis. Child,
133: 172-177.
ANDRIANOV, V.V., ZAKHAROV, N.C., & FADEEV, JU.A. (1977) Microiono-
phoretic analysis of chemical properties of cortical units in freely
moving animals. Physiol. J. (USSR), 63(4): 602-605.
ANGELL, F. & WEISS, B. (1982) Operant behavior of rats exposed to lead
before or after weaning. Toxicol. appl. Pharmacol., 63: 62-71.
ANGER, W.K. (1984) Neurobehavioral testing of chemicals: impact on
recommended standards. Neurobehav. Toxicol. Teratol., 6(2): 147-153.
ANGER, W.K. & JOHNSON, B.L. (1985) Chemicals affecting behavior. In:
O'Donoghue, J.L., ed. Neurotoxicity of industrial and commercial
chemicals, Boca Raton, Florida, CRC Press.
ANGER, W.K. & SETYES, J.V. (1979) Effects of oral and intramuscular
carbaryl administration on repeated chain acquisition in monkeys.
J. Toxicol. environ. Health, 5: 793-808.
ANNAU, W. (1975) The comparative effects of carbon monoxide and
hypoxia on behaviour. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Press, pp. 105-127.
ANOKHIN, P.K. (1935) [Problem of center and periphery in contemporary
physiology of nervous system.] In: Anokhin, P.K., ed. [Problem of
centre and periphery in physiology of nervous activity], Gorkiy,
Gorkiy Publishing House, pp. 9-71 (in Russian).
ANOKHIN, P.K. (1964) [Comparative electrophysical analysis of
alterations of laility of the cortical potential components in
postnatal ontogenesis.] Fiziol. Zh. SSSR, 50: 773-778 (in Russian).
ANOKHIN, P.K. (1968) [Biology and neurophysiology of the conditioned
reflex,] Moscow, Meditsina, pp. 547 (in Russian).
ANOKHIN, P.K. (1974) The functional system as a basis of the
physiological architecture of the behavioural act. In: Anokhin, P.K.,
ed. Biology and neurophysiology of the conditioned reflex and its
role in adaptive behaviour, New York, Pergamon Press, pp. 190-254.
APFELBACH, R. & DELGADO, J.M.R. (1974) Social hierarchy in monkeys
(Macaca mulatta) modified by chlordiazepoxide hydrochloride.
Neuropharmacology, 13: 11-20.
APPEL, S.H. & DAY, E.D. (1976) Cellular and subcellular fractionation.
In: Seigel, G.L., Albers, R.W., Katzman, R., & Agranoff, B.W., ed.
Basic neurochemistry, Boston, Toronto, Little, Brown and Company,
pp. 34-57.
ARBUKHANAOVA, M.S. (1981) Metabolism of glutamic acid in the cerebral
mitochondria during cooling disease. In: Mitochondria: the
mechanisms of conjugation and regulation, Puschino, p. 76.
ASABAYEV, CH., BONCHKOVSKAYA, T.YU., & ZHEGALLO, I.G. (1972) [A study
of the reaction of the animal nervous system to the effect of
low-strength electromagnetic fields.] In: [Labour hygiene and the
biological effect of radio frequency electromagnetic fields,]
Moscow, pp. 48-49 (in Russian).
AVROVA, N.F. (1971) Brain ganglioside patterns of vertebrates.
J. Neurochem., 18: 667-674.
BAENNINGER, L.P. (1966) The reliability of dominance orders in rats.
Anim. Behav., 14: 367-371.
BAGGETT, J.MCC., THURESON-KLEIN, A., & KLEIN, R.L. (1980) Effects of
chlordecone on the adrenal medulla of the rat. Toxicol. appl.
Pharmacol., 52: 313-322.
BAKER, P.F., HODGKIN, A.L., & RIDGWAY, E.B. (1971) Depolarization and
calcium entry in giant squid axons. J. Physiol., 218: 709-775.
BAKIR, F., DAMLUJI, S.F., AMIN-ZAKI, L., MURTADHA, M., KHALIDI, A.
AL-RAWL, N.Y. TIKRITI, S. DHARIR, H.I., CLARKSON, T.W., SMITH, J.C.,
& DOHERTY, R.A. (1973) Methylmercury poisoning in Iraq: an
inter-university report. Science, 181: 230-241.
BALNAVE, R.J. & GAGE, P.W. (1973) The inhibitory effect of manganese
on transmitter release at the neuromuscular junctions of the toad.
Br. J. Pharmacol., 47: 339-352.
BANCROFT, J.D. & STEVENS, A. (1977) Theory and practice of
histological techniques, New York, Churchill Livingston.
BARKER, J.L. & MCKELVY, J.F., ed. (1983) Current methods in cellular
neurobiology: electrophysiological and optical recording techniques,
New York, John Wiley and Sons, Vol. 3.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1973) A behavioral and
pharmacological analysis of variables mediating active avoidance
behaviour in rats. J. comp. Physiol. Psychol., 82: 489-500.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1974) An analysis of the
facilitation of avoidance acquisition produced by delta-amphetamine
and scopolamine. Behav. Biol., 11: 189-203.
BASAR, E. (1980) EEG-brain dynamics. In: Relation between EEG and
brain-evoked potentials, Amsterdam, Oxford, New York, Elsevier
Science Publishers.
BAUMGARTNER, G. GAWEL, M.J. KAESER, H.E., PALLIS, C.A., CLIFFORD,
F.R., SCHAUMBURG, H.H., THOMAS, P.K., & WADIA, N.H. (1979)
Neurotoxicity of halogenated hydroxyquinolines: clinical analysis of
cases reported outside Japan. J. Neurol. Neurosurg. Psychiatr.,
42: 1073-1083.
BEARD, R.R. & GRANDSTAFF, N. (1975) Carbon monoxide and human
function. In: Weiss, B. & Laties, V., ed. Behavioural toxicology,
New York, Plenum Press, pp. 1-26.
BECK, E.C., DUSTMAN, R.E., & LEWIS, E.G. (1975) The use of the
averaged evoked potential in the evaluation of central nervous system
disorders. Int. J. Neurol., 9: 211-232.
BENESOVA, O., HORVATH, M., & MIKISKA, A. (1956) [Assessment of the
depth and duration of narcosis according to the electrophysiological
characteristics of the cerebral cortex.] Physiol. bohemoslov.,
5: 188-194 (in German).
BENNETT, S. & BOWERS, D. (1976) An introduction to multivariate
techniques for social and behavioral sciences, New York, Halsted
Press.
BENSON, C.K. & ZAGNI, P.A. (1965) The effect of 1-alpha-
methylallylthiocarbamyl-2-methylthiocarbamylhydrazine (ICI 33828) on
lactation in the rat. J. Endocr., 32: 275-285.
BERGER, H. (1929) [On the electroencephalogram in man.] Arch.
Psychiat. Nervenkr., 87: 527-550 (in German).
BERNARD, C. (1927) An introduction to the study of experimental
medicine, New York, MacMillan Inc., p. 127.
BESSER, G.M., DELITALA, G., GROSSMAN, A., STUBBS, W.A., & YEO, T.
(1980) Chlorpromazine, haloperidol, metoclopramide, and domperidone
release prolactin through dopamine antagonism at low concentrations
but paradoxically inhibit prolactin release at high concentrations.
Br. J. Pharmacol., 71: 569-573.
BHAGAT, B. & WHEELER, M. (1973) Effect of amphetamine on the swimming
endurance of rats. Neuropharmacology, 12: 1161-1165.
BIGNAMI, G., ROSIC, N., MICHALEK, H., MILOSEVIC, M., & GATTI, G.L.
(1975) Behavioral toxicity of anticholinesterase agents:
methodological, neurochemical and neuropsychological aspects. In:
Weiss, B. & Laties, V.G., ed. Behavioral toxicology, New York,
Plenum Press, pp. 155-215.
BLACKWOOD, W., MCMENEMEY, W.H., MEYER, A., NORMAN, R.M., & RUSSELL,
D.S. (1971) Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd.
BLOOM, A.S., STOOTZ, C.G., & DIERINGER, T. (1983) Pyrethroid effects
on operant responding and feeding. Neurobehav. Toxicol. Teratol.,
5: 321-324.
BOER, G.J. (1974) A microassay for arylamidase activity. Anal.
Biochem., 59: 410-411.
BOKINA, A.I., EKSLER, N.D., SEMENENKO, A.D., & MERKURYVA, R.V. (1976)
Investigation of the mechanism of action of atmospheric pollutants on
the central nervous system and comparative evaluation of methods of
study. Environ. Health Perspect., 13: 37-42.
BOKINS, A.I., KAN, T.M., BEREZINA, O.V., & PINIGINA, I.A. (1979)
[Study of complex forms of animal behavior in a hygenic evaluation of
toxic substances.] Gig. i Sanit., 7: 49-51 (in Russian).
BONASHEVSKAYA, T.I., SHAMARIN, A.A., KESAREV, V.S., & YURASOVA, O.I.
(1983) [Morphological criteria and a system of indexes for the
evaluation of neurotoxic effects produced by environmental chemical
factors (on the basis of a carbon monoxide model).] Gig. i Sanit.,
10: 31-33 (in Russian, with English summary).
BONDY, S.C. & AGRAWAL, A.K. (1980) The inhibition of cerebral high
affinity receptor sites by lead and mercury compounds. Arch.
Toxicol., 46: 249-256.
BONDY, S.C. & HARRINGTON, M.L. (1979) Calcium-dependent release of
putative neurotransmitters in the chick visual system. Neuroscience,
4: 1521-1527.
BONDY, S.C., HARRINGTON, M.L., ANDERSON, C.L., & PRASAD, K.N. (1979)
Low concentration of an organic lead compound alters transport and
release of putative neurotransmitters. Toxicol. Lett., 3: 35-41.
BONDY, S.C., TILSON, H.A., & AGRAWAL, A.K. (1981) Neurotransmitter
receptors in brains of acrylamide-treated rats. II. Effects of
extended exposure to acrylamide. Pharmacol. Biochem. Behav.,
14: 533-537.
BONILLA, E. (1980) L-tyrosine hydroxylase activity in the rat brain
after chronic oral administration of manganese chloride. Neurobehav.
Toxicol., 2: 37-41.
BONILLA, E. SALAZAR, E. VILLASMIL, J.J., & VILLALOBOS, R. (1982) The
regional distribution of manganese in the normal human brain.
Neurochem. Res., 7: 221-227.
BOYES, W.K., JENKINS, D.E., & DYER, R.S. (1985) Chlordimeform produces
contrast-dependent changes in visual-evoked potentials in hooded rats.
Exp. Neurol., 89: 391-407.
BRACELAND, F.J. (1942) Mental symptoms following carbon disulfide
absorption and intoxication. Ann. intern. Med., 16: 246-261.
BRADLEY, P.B. (1958) The central action of certain drugs in relation
to the reticular formation of the brain. In: Jasper, H.H., Proctor,
L.D., Knighton, R.S., Noshay, W.C., & Costello, R.T., ed. Reticular
formation of the brain, Boston, Toronto, Little, Brown and Company,
pp. 123-149.
BRADY, K., HERRERA, Y., & ZENICK, H. (1976) Influence of parental lead
exposure on subsequent learning ability of offspring. Pharmacol.
Biochem. Behav., 3: 561-565.
BRIERLEY, J.B. (1976) Cerebral hypoxia. In: Blackwood, W. & Corsellis,
J., ed. Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd., pp. 43-85.
BROWN, A.W., ALDRIDGE, W.N., STREET, B.W., & VERSCHOYLE, R.D. (1979)
The behavioural and neuropathological sequelae of intoxication by
trimethyltin compounds in the rat. Am. J. Pathol., 97: 59-82.
BUELKE-SAM, J. & KIMMEL, C.A. (1979) Development and standardization
of screening methods for behavioral teratology. Teratology,
20: 17-29.
BULL, R.J. & LUTKENHOFF, S.D. (1975) Changes in the metabolic
responses of brain tissue to stimulation, in vitro, produced by in
vivo administration of methylmercury. Neuropharmacology,
14: 351-359.
BURES, J. & BURESOVA, O. (1979) New methods in memory research. In:
Proceedings of the 21st Annual Meeting of the Czechoslovak
Psychopharmacological Society, Jesenik Spa, 9-14 January, 1979,
pp. 7-12.
BURESOVA, O. & BURES, J. (1982) Capacity of working memory in rats as
determined by performance on a radial maze. Behav. Processes,
7: 63-72.
BURGEN, A.S.V., KOSTERLITZ, H.W., & IVERSEN, L.L., ed. (1980)
Neuroactive peptides, London, Royal Society.
BURGUS, R. & GUILLEMIN, R. (1970) Chemistry of thyrotropin releasing
factor (TRF). In: Meites, J., ed. Hypophysiotropic hormones of the
hypothalamus: assays and chemistry, Baltimore, Maryland, Williams
and Wilkins Company, pp. 227-241.
BUSHNELL, P.J. & BOWMAN, R.E. (1979) Effects of chronic lead ingestion
on social development in infant rhesus monkeys. Neurobehav.
Toxicol., 1: 207-219.
BUTCHER, R.E. (1976) Behavioral testing as a method for assessing
risk. Environ. Health Perspect., 18: 75-81.
BUTCHER, R.E. & VORHEES, C.V. (1979) A preliminary test battery for
the investigation of the behavioral teratology of selected
psychotropic drugs. Neurobehav. Toxicol., 1(Suppl. 1): 207-212.
CABE, P.A. & TILSON, H.A. (1978) Hind limb extensor response: a method
for assessing motor dysfunction in rats. Pharmacol. Biochem. Behav.,
9: 133-136.
CAMMER, W. (1980) Toxic demyelination: biochemical studies and
hypothetical mechanisms. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 239-256.
CAMMER, W. & MOORE, C.L. (1972) The effect of hexachlorophene on the
respiration of brain and liver mitochondria. Biochem. biophys. Res.
Comm., 46: 1887-1894.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W., HAYES, C.,
STRAUB, W.E., LANDRIGAM, P.J., & LIDDLE, J.A. (1978) Epidemic kepone
poisoning in chemical workers. Am. J. Epidemiol., 17: 529.
CARBONE, E., WANKE, E., PRESTIPINO, G., POSSANI, L.D. & MAELICKE, A.
(1982) Selective blockage of voltage-dependent K+ channels by a
novel scorpion toxin. Nature (Lond.), 296: 90-91.
CARILLI, C.T., FARLEY, R.A., PERLMAN, D.M., & GANTHEY, L.C. (1982)
The active site structure of Na+- and K+- stimulated ATPase. J.
biol. Chem., 257: 5601-5606.
CASARETT, L.J. (1975) Toxicologic evaluation. In: Casarett, L.J. &
Doull, J., ed. Toxicology; the basic science of poisons, New York,
MacMillan Inc., pp. 11-25.
CASTRO, J.A. & GILLETTE, J.R. (1967) Species and sex differences in
the kinetic constants for the N-demethylation of ethyl-morphine by
liver microsomes. Biochem. biophys. Res. Comm., 28: 426-430.
CATTERALL, W.A. (1977) Activation of the action potential Na+
ionophore by neurotoxins. J. biol. Chem., 252: 8669-8676.
CAVALLERI, A. (1975) Serum thyroxine in the early diagnosis of carbon
disulfide poisoning. Arch. environ. Health, 30: 85-87.
CAVALLERI, A., POLATTI, F., & BOLIS, P.F. (1978) Acute effects of
tetramethylthiuram disulfide on serum levels of hypophyseal hormones
in humans. Scand. Work environ. Health, 4: 66-72.
CAVANAGH, J.B. (1964) The significance of the "dying-back" process in
experimental and human neurological disease. Int. Rev. exp. Pathol.,
3: 219-267.
CAVANAGH, J.B. & CHEN, F.C.-K. (1971) The effects of methyl-mercury-
dicyandiamide on the peripheral nerves and spinal cord of rats. Acta
neuropathol., 19: 208-215.
CAVANAGH, J.B. & JACOBS, J.M. (1954) Some quantitative aspects of
diphtheritic neuropathy. Br. J. exp. Pathol., 45: 309-327.
CAVANAGH, J.B., PASSINGHAM, R.J., & VOGT, J.A. (1964) Staining of
sensory and motor nerves in muscles with Sudan Black B. J. Pathol.
Bacteriol., 88: 89-92.
CECCARELLI, B. & HURLBUT, W.P. (1980) Ca-dependent recycling of
synaptic vesicles at the frog neuromuscular junction. J. cell Biol.,
87: 297-303
CHANCE, M.R.A. (1946) Aggregation as a factor influencing the toxicity
of sympathomimetic amines in mice. J. Pharmacol. exp. Ther.,
87: 214-219.
CHANG, L. (1977) Neurotoxic effects of mercury -- a review. Environ.
Res., 14: 329-373.
CHANG, L. (1980) Mercury. In: Spencer, P.S. and Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 508-526.
CHANG-TSUI, Y.H. & HO, I.K. (1979) Effects of kepone (chlordecone) on
synaptosomal gamma-aminobutryic acid uptake in the mouse.
Neurotoxicology, 1: 357-361.
CHAPMAN, R.M. (1983) Gonadal injury resulting from chemotherapy. Am.
J. ind. Med., 4: 149-161.
CHIBA, S. & ANDO, K. (1976) Effects of chronic administration of
kanamycin on conditioned suppression to auditory stimulus in rats.
Jpn. J. Pharmacol., 26: 419-426.
CHO, E.S., SPENCER, P.S., JORTNER, B.S., & SCHAUMBURG, H.H. (1980) A
single intravenous injection of doxorubicin (AdriamycinR) induces
sensory neuropathy in rats. Neurotoxicology, 1: 583-591.
CHOPRA, I.J., SOLOMON, D.J., TECO, G.N.C., & NGUYEN, A.H. (1980)
Inhibition of hepatic outer ring monodeiodination of thyroxine and
3,3',5'-triiodothyronine by sodium salicylate. Endocrinology,
106: 1728-1734.
CIOMS (1983) Safety requirements for the first use of new drugs and
diagnostic agents in man, Geneva, Council for International
Organizations of Medical Sciences.
CITOVIC, I.S. (1930) [The procedure of studying the effects of oil
products on the organism of animals.] Inst. Work Saf., 1: 92
(in Russian).
CLAUSEN, T. & FLATMAN, J.A. (1977) The effects of catecholamines on
Na-K transport and membrane potential in rat soleus muscle.
J. Physiol., 270: 383-414.
COLLIER, B. & ILSON, D. (1977) The effect of preganglionic nerve
stimulation on the accumulation of certain analogues of choline by a
sympathetic ganglion. J. Physiol., 264: 489-509.
COLLINS, R.D. (1982) Illustrated manual of neurologic diagnosis, 2nd
ed., Philadelphia, Pennsylvania, J.B. Lippincott Company.
COLOTLA, V.A. (1981) Adjunctive polydipsia as a model of alcoholism.
Neurosci. Biobehav. Rev., 5: 335-342.
COLOTLA, A., BAUTISTA, M., LORENZANA-JIMENEZ, M., & RODRIGUEZ, R.
(1979) Effects of solvents on schedule-controlled behavior.
Neurobehav. Toxicol., 1(Suppl. 1): 113-118.
CONSTANTOPOULOS, G., MCCOMB, R.D., & DECABAN, A. (1976) Neurochemistry
of the mucopolysaccharidoses: brain glycosaminoglycans in normal and
four types of mucopolysaccharidoses. J. Neurochem., 26: 901-908.
CORY-SLECHTA, D.A. & THOMPSON, T. (1979) Behavioral toxicity of
chronic postweaning lead exposure in the rat. Toxicol. appl.
Pharmacol., 47: 151-159.
CORY-SLECHTA, D.A., BISSEN, T., YOUNG, M., & THOMPSON, T. (1981)
Chronic postweaning lead exposure and response duration performance.
Toxicol. appl. Pharmacol., 60: 78-84.
COSTA, A. & MURAD, J.E. (1969) Study of the association between
chlorpromazine and analgesics through the method of electrical
stimulation of the rabbit dental pulp. Rev. Bras de Pesgnisa Med.
Biol., 2: 235-240.
COSTA, E. (1970) Simple neuronal models to estimate turnover rate of
noradrenergic transmitters in vivo. In: Costa, E. & Giabocini, E.,
ed. Advances in biochemical psychopharmacology. II. Biochemistry of
simple neuronal models, New York, Raven Press.
COTMAN, C.W. & BARKER, G.A. (1974) The making of a synapse. Rev.
Neurosci., 1: 1-62.
COTMAN, C.W., HAYCOCK, J.W., & WHITE, W.F. (1976) Stimulus secretion
coupling processes in brain: analysis of noradrenaline and gamma-
aminobutyric acid release. J. Physiol., 254: 475-505.
COURAUD, F.C., ROCHAT, H., & LISSITSKY, S. (1978) Binding of scorpion
and sea anemone neurotoxins to a common site related to the action
potential Na+ ionophore in neuroblastoma cells. Biochem. biophys.
Res. Comm., 83: 1525-1530.
CRANE, A.M. & GOLDMAN, P.S. (1979) An improved method for embedding
brain tissue in albumin-gelatin. Stain Technol., 54: 71-75.
CRAPPER, D.R. & DE BONI, U. (1980) Aluminum. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 326-335.
CUTLER, M.G. (1977) Effects of exposure to lead on social behaviour in
the laboratory mouse. Psychopharmacology, 52: 279-282.
DAMSTRA, T. (1978) Environmental chemicals and nervous system
dysfunction. Yale J. Biol. Med., 51: 457-468.
DAMSTRA, T. & BONDY, S.C. (1980) The current status and future of
biochemical assays for neurotoxicity. In: Spencer, P.S. & Schaumburg,
H.H., ed. Experimental and clinical neurotoxicology, Baltimore,
Maryland, Williams and Wilkins Company, pp. 820-833.
DAMSTRA, T. & BONDY, S.C. (1982) Neurochemical approaches to the
detection of neurotoxicity. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 349-373.
DAUBE, J. (1980) Nerve conduction studies. In: Aminoff, J., ed.
Electrodiagnosis in clinical neurology, New York, Churchill
Livingstone, pp. 229-264.
DAVIS, M. (1980) Neurochemical modulation of sensory-motor reactivity:
acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev.,
4: 241-263.
DE JESUS, C.P.V., TOCOFIGHI, J., & SNYDER, D.R. (1978) Sural nerve
conduction study in the rat: a new technique for studying experimental
neuropathies. Muscle Nerve, 1: 162-167.
DESAIAH, D., GILLILAND, T., HO, I.K., & MEHENDALE, H.M. (1980)
Inhibition of mouse brain synaptosomal ATPases and ouabain binding by
chlordecone. Toxicol. Lett., 6: 275-285.
DESCLIN, J.C. (1974) Histological evidence supporting the inferior
olive as the major source of cerebellar climbing fibres in the rat.
Brain Res., 77: 365-384.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I. & SOS, J. (1962) Central nervous injury by a chemical
herbicide. Acta Med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1963) Experimental lesions of the central nervous
system induced by triorthocresyl phosphate. Acta Physiol. Acad. Sci.
Hung., 23: 63-68.
DESI, I., GONCZI, L., SIMON, G., FARKAS, I., & KNEFFEL, Z. (1974)
Neurotoxicologic studies of two carbamate pesticides in subacute
animal experiments. Toxicol. appl. Pharmacol., 27: 465-476.
DEWAR, A.J. & MOFFETT, B.J. (1979) Biochemical methods for detecting
neurotoxicity. A short review. Pharmacol. Ther., 5: 545-562.
DEWAR, A.J., MOFFETT, B.J., & SITTON, M.F. (1977) The effect of
anti-inflammatory drugs on retinal dystrophy in the rat. Toxicol.
appl. Pharmacol., 42: 65-74.
DEWS, P.B. (1982) Epistemology of screening for behavioral toxicity.
In: Mitchell, C.L., ed. Nervous system toxicology, New York, Raven
Press, pp. 229-236.
DEWS, P.B. & WENGER, G.R. (1979) Testing for behavioral effects of
agents. Neurobehav. Toxicol., 1(Suppl. 1): 119-127.
DIAMOND, S.S. & SLEIGHT, S.D. (1972) Acute and subchronic
methylmercury toxicosis in the rat. Toxicol. appl. Pharmacol.,
23: 197-207.
DICKSITH, T.S.S. & DATTA, K.K. (1972) Effect of intratesticular
injection of lindane and endrine on the testes of rats. Acta
pharmacol., 31: 1-10.
DIETZ, D.D., MCMILLAN, D.E., GRANT, L.D., & KIMMEL, C.A. (1978)
Effects of lead on temporally-spaced responding in rats. Drug chem.
Toxicol., 1(4): 401-419.
DINGLEDINE, R., ed. (1984) Brain slices, New York, Plenum Press,
442 pp.
DIXON, R.L. (1976) Problems in extrapolating toxicity data for
laboratory animals to man. Environ. Health Perspect., 13: 43-50.
DOLPHIN, A.C. & GREENGARD, P. (1981) Neurotransmitter- and
neuromodulator-independent alterations in phosphorylation of protein I
in slices of rat facial nucleus. J. Neurosci., 1: 192-203.
DRAPER, W.A. (1967) A behavioural study of the home-cage activity of
the white rat. Behaviour, 28: 280-306.
DRENTH, H.J., ENSBERG, I.F.G., ROBERTS, D.V., & WILSON, A. (1972)
Neuromuscular function in agriculture workers using pesticides. Arch.
environ. Health, 25: 395-398.
DROUIN, J. & LABRIE, F. (1981) Interactions between 17 beta-estradiol
and progesterone in the control of luteinizing hormone and follicle
stimulating hormone release in rat pituitary cells in culture.
Endocrinology, 108: 52-57.
DUBOIS, K.P. & PUCHALA, E. (1961) Studies on the sex differences in
the toxicity of cholinergic phosphorothioate (26791). Proc. Soc. Exp.
Biol. Med., 107: 908-911.
DUBOIS, K.P., DOULL, J., SALERNO, P.R., & COON, J.M. (1949) Studies on
the toxicity and mechanisms of action of p-nitro-phenyl diethyl-
thionosphosphate (parathion). J. Pharmacol. exp. Ther., 95: 79-91.
DUBOWITZ, V. & BROOKE, M.H. (1973) Muscle biopsy: a modern approach,
London, W.B. Saunders.
DUNLOP, D.S., VAN ELDEN, W., & LAJTHA, A. (1975) Optimal conditions
for protein synthesis in incubated slices of rat brain. Brain Res.,
99: 303-318.
DUNN, A.J. (1975) Intercerebral injections inhibit amino acid
incorporation into brain protein. Brain Res., 99: 405-409.
DUNN, A.J. (1977) Measurements of the rate of brain protein synthesis.
In: Roberts, S., Lajtha, A., & Gispen, W.H., ed. Mechanisms,
regulation, and special function of protein synthesis in the brain,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 97-105.
DURHAM, W.F., CUETO, C., Jr, & HAYES, W.J., Jr (1956) Hormonal
influence on DDT metabolism in the white rat. Am. J. Physiol.,
187: 373-377.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
DYER, R.S. (1980) Effects of prenatal and postnatal exposure to carbon
monoxide on visually-evoked responses in rats. In: Weiss, B. &
Merigan, W., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 17-33.
DYER, R.S. (1985a) The use of sensory-evoked potentials in toxicology.
Fundam. appl. Toxicol., 5: 24-40.
DYER, R.S. (1985b) Neurophysiological measures following exposure to
toxic substances. In: Annau, Z., ed. Behavioral toxicology,
Baltimore, Maryland, Johns Hopkins.
DYER, R.S. & BOYES, W.K. (1983) Use of neurophysiological challenges
for the detection of neurotoxicity. Fed. Proc., 42: 3201-3206.
DYER, R.S., ECCLES, C.U., & ANNAU, Z. (1978) Evoked potential
alterations following prenatal methylmercury exposure. Pharmacol.
Biochem. Behav., 8: 137-141.
DYER, R.S., SWARTZWELDER, H.S., ECCLES, C.W., & ANNAU, Z. (1979)
Hippocampal after discharges and their post-ictal sequelae in rats: a
potential tool for assessment of CNS neurotoxicity. Neurobehav.
Toxicol., 1: 5-19.
ECKERMAN, D.A., GORDON, W.A., EDWARDS, J.D., MACPHAIL, R.C., & GAGE,
M.I. (1980) Effects of scopolamine, pentabarbital, and amphetamine on
radial arm maze performance in the rat. Pharmacol. Biochem. Behav.,
12: 595-602.
EDWARDS, P.M. & PARKER, V.H. (1977) A simple, sensitive, and objective
method for early assessment of acrylamide neuropathy in rats.
Toxicol. appl. Pharmacol., 40: 589-591.
EGAN, G.F., LEWIS, S.C., & SCALA, R.A. (1980) Experimental design for
animal toxicity studies. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams & Wilkins Company, pp. 708-725.
EICHELMAN, B., HEGSTRAND, L.R., MCMURRAY, T., & KANTAX, K.M. (1981)
Cyclic nucleotides and aggressive behaviour. Psychopharmacology of
aggression and social behaviour. Pharmacol. Biochem. Behav.,
14(Suppl. 1): 7-12.
ELSNER, J., LOOSER, R., & ZBINDEN, G. (1979) Quantitative analysis of
rat behaviour patterns in a residential maze. Neurobehav. Toxicol.,
1(Suppl. 1): 163-174.
EMSON, P.C. (1983) Chemical neuroanatomy, New York, Raven Press.
ENJALBERT, A., RUBERG, M., & KORDON, C. (1978) Neuroendocrine control
of prolactin secretion. In: Robyn, C. & Harter, M., ed. Progress in
prolactin physiology and pathology, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 83-94.
EROSCHENKO, V.P. & PALMITER, R.D. (1980) Estrogenicity of kepone in
birds and mammals. In: McLachlan, J., ed. Estrogens in the
environment, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 305-325.
EROSCHENKO, V.O. & WILSON, W.O. (1975) Cellular changes in the gonads,
liver, and adrenal glands of Japanese quail as affected by the
insecticide kepone. Toxicol. appl. Pharmacol., 31: 491-504.
EVANS, H.L. (1978) Behavioral assessment of visual toxicity. Environ.
Health Perspect., 26: 53-58.
EVANS, H.L. & WEISS, B. (1978) Behavioral toxicology. In: Blackman,
D.E. & Sanger, D.J., ed. Contemporary research in behavioral
pharmacology, New York, Plenum Press, pp. 449-487.
EVANS, H.L., LATIES, V.G., & WEISS, B. (1975) Behavioral effects of
mercury and methylmercury. Fed. Proc., 34: 1858-1867.
EVANS, H.L., GARMAN, R.H., & WEISS, B. (1977) Methylmercury: exposure
duration and regional distribution as determinants of neurotoxicity in
non-human primates. Toxicol. appl. Pharmacol., 41: 15-33.
EVANS, W.O. (1962) A comparison of the analgetic potency of some
analgesics as measured by the "flinch-jump" procedure.
Psychopharmacologia, 3: 51-54.
FADEEV, JU.A. (1980) [Unit activity of the brain cortex in realization
of goal-directed behaviour.] Usp. fiziol. nauk., 11(3): 12-46 (in
Russian).
FADEEV, JU.A. & ANDRIANOV, V.V. (1971) Methods of registration of
neuronal activity in freely-moving animals. Physiol. J. (USSR),
57(8): 1217-1219.
FALCK, B., HILLARP, N.A., THIEME, G., & TORP, A. (1962) Fluorescence
of catecholamines and related compounds condensed with formaldehyde.
J. Histochem. Cytochem., 10: 348-354.
FALK, J.L. (1970) Readings in behavioral pharmacology, New York,
Appleton-Century-Crofts.
FELLEGIOVA, M., ADAMEC, O., & DAHKOVA, A. (1977) [The effect of
lindane on the metabolism of testosterone in the rat.] Cs. Hyg.,
22: 115-120 (in Slovak).
FERSTER, C.B. & SKINNER, B.F. (1957) Schedules of reinforcement, New
York, Appleton-Century-Crofts.
FESTING, M.F. (1979) Properties of inbred strains and outbred stocks,
with special reference to toxicity testing. J. environ. Health
Toxicol., 5: 53-68.
FILE, S. (1980) The use of social interaction as a method for
detecting anxiolytic activity of chlordiarepoxide-like drugs.
J. Neurosci. Methods, 2: 219-238.
FINK, G. (1979) Feedback actions of target hormones on hypothalamus
and pituitary with special reference to gonadal steroids. Ann. Rev.
Physiol., 41: 571-585.
FISHMAN, J. (1982) Catechol estrogens and pituitary function. In:
Muldoon, T.G., Mahesh, V.B., & Perez-Ballester, B., ed. Recent
advances in fertility research. Part A. Developments in reproductive
endocrinology, New York, Alan R. Lyss, pp. 183-189.
FISHMAN, W.H. & BERNFELD, P. (1955) Glucuronidases. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 262.
FITZGERALD, G.J., KAUFMAN, E.E., SOKOLOFF, L., & SHEIN, H.M. (1974)
D(-)-beta-hydroxybutyrote dehydrogenase activity in closed cell lines
of glial and neuronal origin. J. Neurochem., 22: 1163-1166.
FODOR, G.G., SCHLIPKOTER, H.W., & ZIMMERMAN, M. (1973) The objective
study of sleeping behaviour in animals as a test of behaviour
toxicity. In: Horvath, M. & Frantik, M., ed. Adverse effects of
environmental chemicals and psychotropic drugs, Amsterdam, London,
New York, Elsevier Science Publishers, Vol. 1, pp. 115-122.
FOLCH, J., ASCOLI, I., LEES, M., MEATH, J.A., & LEBARON, F.N. (1951)
Preparation of lipid extracts from brain tissue. J. biol. Chem.,
191: 833-841.
FOWLER, S.C. & PRICE, A.W. (1978) Some effects of chlordiaz-epoxide
and delta-amphetamine on response force during punished responding in
rats. Psychopharmacology, 56: 211-215.
FOX, D.A., LOWNDES, H.E., & BIERKAMPER, G.G. (1982) Electro-
physiological techniques in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 299-335.
FOX, W.M. (1965) Reflex-ontogeny and behavioral development of the
mouse. Anim. Behav., 13: 234-241.
FRANKOVA, S. (1970) Behavioral responses of rats to early
overnutrition. Nutr. Metab., 12: 228-230.
FRANKOVA, S. (1971) Relationship between nutrition during lactation
and maternal behavior of rats. Act. Nerv. Super. (Praha), 13: 1-8.
FRANKOVA, S. (1977) Drug-induced changes in the maternal behavior of
rats. Psychopharmacology, 3: 83-87.
FRANTIK, E. (1970) The development of motor disturbances in
experimental chronic carbon disulfide intoxication. Med. Lav.,
61: 309-313.
FRANTIK, E. & BENES, V. (1984) Central nervous effect and blood level
regressions on exposure time paralleled in solvents (toluene, carbon
tetrachloride, and chloroform). Act. Nerv. super. (Praha), 26: 131.
FUJIMORI, K., BENET, H., MEHENDALE, H.M., & HO, I.K. (1982) Comparison
of brain discrete area distribution of chlordecone and mirex in the
mouse. Neurotoxicology, 3: 125-130.
FULLERTON, P.M. (1966) Chronic peripheral neuropathy produced by lead
poisoning in guinea-pigs. J. Neuropathol. exp. Neurol., 25: 214-236.
GABE, M. (1976) Histological techniques, New York, Paris,
Springer-Verlag, Masson.
GAD, S.C. (1982) Statistical analysis of behavioral toxicology data
and studies. Arch. Toxicol., Suppl. 5: 256-266.
GAD, S.C. & WEIL, C.S. (1982) Statistics for toxicologists. In: Hayes,
A.W., ed. Principles and methods of toxicology, New York, Raven
Press, pp. 270-320.
GALLARD, G.V., PETRO, Z., & RYAN, K.J. (1978) Phylogenetic
distribution of aromatase and other androgen-converting enzymes in the
central nervous system. Endocrinology, 10: 2283-2290.
GELLER, I., MENDEZ, V., HAMILTON, M., HARTMANN, R.J., & GAUSE, E.
(1979) Effects of carbon monoxide on operant behavior of laboratory
rats and baboons. Neurobehav. Toxicol., 1(Suppl. 1): 179-184.
GELLERT, R.J., HEINRICHS, W.L., & SWERDLOFF, R.S. (1972) DDT
homologues: estrogen-like effects on the vagina, uterus, and pituitary
of the rat. Endocrinology, 91: 1095-1100.
GERHART, J.M., HONG. J.S., UPHOUSE, L.L., & TILSON, H.A. (1982)
Chlordecone-induced tremor: quantification and pharmacological
analysis. Toxicol. appl. Pharmacol., 66(2): 234-243.
GILLIATT, R.W. (1973) Recent advances in the pathophysiology of nerve
conduction. In: Desmedt, J.E., ed. New developments in
electromyography and clinical neurophysiology, Basel, Karger,
Vol. 2, pp. 2-18.
GLATT, A.F., TALAAT, H.N., & KOELLA, W.P. (1979) Testing of peripheral
nerve function in chronic experiments with rats. Pharmacol. Ther.,
5: 539-543.
GLAUSERT, A.M. (1980) Fixation, dehydration, and embedding of
biological specimens, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
GLOWINSKI, J. & IVERSEN, L.L. (1966) Regional studies of
catecholamines in the rat brain. I. The disposition of 3H-norephine-
phrine, 3H-dopamine, and 3H-DOPA in various regions of the brain. J.
Neurochem., 13: 655-699.
GOLDSTEIN, B.D., LOWNDES, H.E., & CHO, E.S. (1981) Neurotoxicology of
vincristine in the cat: electrophysiological studies. Arch.
Toxicol., 48: 253-264.
GONCHARUK, V.D. (1981) Alteration of activity of glutamate
dehydrogenase in ganglionated neurons of rabbits under acute
experimental emotional stress. Bull. exp. Biol. Med., 10: 494-496.
GOOD, P. (1979) Detection of a treatment effect when not all
experimental subjects will respond to treatment. Biometrics,
35: 483-489
GORIDIS, C., MASSARELLI, R., SERSENBRENNER, M., & MANDEL, P. (1974)
Guanyl cyclase in chick embryo brain cell cultures: evidence of
neuronal localization. J. Neurochem., 23: 135-139.
GOYER, R.A. & RHYNE, B.C. (1973) Pathological effects of lead. Int.
Rev. exp. Pathol., 12: 1-7.
GRANT, L.D. (1976) Research strategies for behavioral toxicology
studies. Environ. Health Perspect., 18: 85-94.
GRAY, E.G. & WHITTAKER, V.P. (1962) The isolation of nerve endings
from brain: an electron microscopic study of the cell fragments of
homogenation and centrifugation. J. Anat., 96: 79-87.
GREENBLATT, D.J., SHADER, R.I., FRANKE, K.K., MACLAUGHLIN, E.D.,
RONSIL, G.J., & KOCH-WESER, J. (1977) Kinetics of intravenous
chlordiazepoxide: sex differences in drug metabolism. Clin.
Pharmacol. Ther., 22: 893-903.
GREENGARD, P. (1979) Cyclic nucleotides, phosphorylated proteins and
the neuronal system. Fed. Proc., 38: 2208-2217.
GREGORY, E.H. & PFAFF, D.W. (1971) Development of olfactory-guided
behavior in infant rats. Physiol. Behav., 6: 573-576.
GRIFFIN, J.W. & PRICE, D.L. (1980) Proximal axonopathies induced by
toxic chemicals. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 161-178.
GRIFFIN, J.W., PRICE, D.L., KLUTHE, D.O., & GOLDBERG, M.A. (1980)
Neurotoxicity of misonidazole in rats. Neuropathol. appl.
Neurobiol., 6: 656-666.
GROSS-SELBECK, E. & GROSS-SELBECK, M. (1981) Changes in operant
behavior of rats exposed to lead at the accepted no-effect level.
Clin. Toxicol., 18: 1247-1256.
GROTA, L.S. & ADER, R. (1969) Continuous recording of maternal
behavior in the rat. Anim. Behav., 17: 72-729.
GUDELSKY, G.A., NANSEL, D.D., & PORTER, J.C. (1981) Role of estrogen
in the dopaminergic control of prolactin secretion. Endocrinology,
108: 440-444.
HAIDER, S.S., HANSAN, M., & ISLAM, F. (1980) Effect of air pollutant
hydrogen sulfide on the levels of total lipids, phospholipids, and
cholesterol in different regions of the guinea-pig brain. Ind. J.
exp. Biol., 18: 418-420.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., & MCCONNEL, J.
(1979) Estrogenic activity of the insecticide chlordecone (kepone) and
interaction with uterine estrogen receptors. Proc. Natl Acad. Sci.
(USA), 76: 6641-6645.
HARPUR, E.S. & D'ARCY, P.F. (1975) The quantification of kanamycin
ototoxicity in the rat using conditioned tone discrimination.
J. Pharm. Pharmacol., 27: 907-913.
HARRY, G.J. & TILSON, H.A. (1982) Postpartum exposure to triethyltin
produces long-term alterations in responsiveness to apomorphine.
Neurotoxicology, 3: 64-71.
HASAN, M. & ALI, S.F. (1981) Effects of thallium, nickel, and cobalt
administration on the lipid peroxidation in different regions of the
rat brain. Toxicol. appl. Pharmacol., 57: 8-13.
HAY, A. (1978) Neurotoxins may go unrecognized. Nature (Lond.),
274: 206.
HAYAT, M.A. (1973) Principles and techniques of electron microscopy,
New York, Van Rostrand Reinhold Company, Vols. 1 and 2.
HEDLUND, B., GAMARA, M., & BARTFAI, I. (1979) Inhibition of striatal
muscarinic receptors in vivo by cadium. Brain Res., 168: 216-218.
HEISE, G.A. (1983) Toward a behavioural toxicology of learning and
memory. In: Zbinden, G., Cuomo, V., Racagni, G., & Weiss, B., ed.
Application of behaviour pharmacology and toxicology, New York,
Raven Press, pp. 27-38.
HEISE, G.A. & MILAR, C. (1984) Drugs and stimulus control. In:
Iverson, S.D. & Snyder, S.H., ed. Handbook of psychopharmacology,
New York, Plenum Press, pp. 129-190.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930.
HERMAN, S.P., KLEIN, R., TALLEY, F.A., & KRIGMAN, M. (1973) An
ultrastructural study of methylmercury-induced primary sensory
neuropathy in the rat. Lab. Inv., 28: 104-118.
HERTZ, L. (1981) Gamma amino butyric-acid glutamate pathway in glia.
5th European Neuroscience Congress, Liege, Belgium, 14-18 September,
1981. Neurosci. Lett, Suppl. 7: 172.
HETZLER, B.E., HEILBRONNER, R.L., GRIFFIN, J., & GRIFFIN, G. (1981)
Acute effects of alcohol on evoked potentials in visual cortex and
superior colliculus of the rat. Electroencephalogr. clin.
Neurophysiol., 51 69-79.
HILLE, B. (1976) Gating in sodium channels of nerve. Ann. Rev.
Physiol., 38 139-152.
HINDE, R. (1974) Biological basis of human social behaviour, New
York, McGraw Hill, Inc.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930
HOFFMAN, P.N. & LASEK, R.J. (1975) The slow component of axonal
transport. Identification of major structural polypeptides of the axon
and their generality among mammalian neurons. J. cell Biol.,
66: 351.
HOLCK, H.G.O., MUNIR, A.K., MILLS, L.M., & SMITH, E.L. (1937) Studies
upon the sex differences in rats in tolerance to certain barbiturates
and to nicotine. J. Pharmacol. exp. Ther., 60: 323-346.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HONG, J.S. & ALI, S.F. (1982) Chlordecone (kepone) exposure in the
neonate selectively alters brain and pituitary endorphin levels in
prepuberal and adult rats. Neurotoxicology, 3: 111-118.
HOPF, H.C. & EYSHOLDT, M. (1978) Impaired refractory periods of
peripheral sensory nerves in multiple sclerosis. Ann. Neurol.,
4: 499-501.
HOROWSKI, D. & GRAF, K.J. (1979) Neuroendocrine effects of
neuropsychotropic drugs and their possible influence on toxic
reactions in animals and man: the role of dopamine-prolactin system.
Arch. Toxicol. Suppl. 2: 93-104.
HORVATH, M., ed. (1973) Adverse effects of environmental chemicals
and psychotropic drugs. Quantitative interpretation of functional
tests, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 1, 292 pp.
HORVATH, M. & FRANTIK, E. (1973) Quantitative interpretation of
experimental toxicological data: the use of reference substances. In:
Horvath, M., ed. Adverse effects of environmental chemicals and
psychotropic drugs, Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Co., Vol. 1, pp. 11-21.
HORVATH, M. & FRANTIK, M., ed. (1976) Adverse effects of
environmental chemicals and psychotropic drugs: neurophysiological
and behavioural tests, Amsterdam, New York, Oxford, Elsevier,
Vol. 2, 334 pp.
HORVATH, M. & FRANTIK, E. (1979) Industrial chemicals and drugs
lowering central nervous systems activation level: quantitative
assessment of hazard in man and animals. Act. Nerv. super. (Praha),
21: 269-272.
HORVATH, M. & MICHALOVA, C. (1956) Changes of EEG in experimental
exposure to carbon disulfide in the light of Vvedenskys theory of
parabiosis. Arch. Gewerbepathol., 15: 131-136.
HORVATH, M., FRANTIK, E., & KREKULE, P. (1981) Diazepam impairs
alertness and potentiates a similar effect of toluene. Act. Nerv.
super. (Praha), 22: 17-19.
HOWLAND, R.D., VYAS, I.L., LOWNDES, H.E., & ARGENTIERI, T.M. (1980)
The etiology of toxic peripheral neuropathies; in vitro effects of
acrylamide and 2,5-hexanedione on brain enolase and other glycolytic
enzymes. Brain Res., 202: 131-142.
HUGHES, J.A. & SPARBER, S.B. (1978) Delta-amphetamine unmasks
postnatal consequences of exposure to methylmercury in utero:
methods for studying behavioral teratogenesis. Pharmacol. Biochem.
Behav., 8: 365-375.
HUNT, V.R., SMITH, M.K., & WORK, D., ed. (1982) Banbury Report II -
Environmental factors in human growth and development, New York,
Cold Spring Harbor Laboratory.
HUNTER, D. & RUSSELL, D.S. (1954) Focal cerebral and cerebellar
atrophy in human subjects due to organic mercury compounds.
J. Neurol. Neurosurg. Psychiatr., 17: 235-241.
HUNTER, D., BORNFORD, R., & RUSSELL, D. (1940) Poisoning by
methylmercury compounds. Q. S. Med., 9: 193-213.
HUTCHINGS, D.E. (1978) Behavioral teratology: embryopathic and
behavioural effects of drugs during pregnancy. In: Gottlieb, G., ed.
Studies on the development of behaviour and nervous system: early
influences, New York, Academic Press, pp. 7-34.
IRWIN, S. (1968) Comprehensive observational assessment: a systematic
quantitative procedure for assessing the behavioral and psychologic
state of the mouse. Psychopharmacologia, 13: 222-257.
ISLAM, F., TAYYABA, K., & HASAN, M. (1983) Organophosphate metasystox-
induced increment of lipase activity and lipid peroxidation in
cerebral hemisphere: diminution of lipids in discrete areas of the
brain. Acta pharmacol. toxicol., 53: 121-124.
IVERSEN, L.L. (1971) Role of transmitter uptake mechanisms in synaptic
neurotransmission. Br. J. Pharmacol., 44: 571-591.
JACOBWITZ, D.M. (1974) Removal of discrete fresh regions of the rat
brain. Brain Res., 80: 111-115.
JOHN, T.H., GEGHMAN, C., & REIS, D.J. (1973) Immunochemical
demonstration of increased accumulation of tyrosine hydroxylase
protein in sympathetic ganglia and adrenal medulla elicited by
reserpine. Proc. Natl Acad. Sci. (USA), 70: 2767-2771.
JOHNSON, B.L. (1980) Electrophysiological methods in neurotoxicity
testing. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental and
clinical neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 726-742.
JOHNSON, B.L. & ANGER, W.K. (1983) Behavioral toxicology. In: Rom,
W.N., ed. Environmental and occupational medicine, Boston, Little
Brown and Co., pp. 329-350.
JOHNSON, B.L., ANGER, W.K., SETZIER, J.V., & XINTARAS, C. (1975) The
application of a computer-controlled time discrimination performance
to problems in behavioral toxicology. In: Weiss, B. & Laties, V.G.,
ed. Behavioral toxicology, New York, London, Plenum Press,
pp. 129-153.
JOHNSON, E.W. (1980) Practical electromyography, Baltimore,
Maryland, Williams and Wilkins Company.
JOHNSON, J.E., Jr (1981) Aging and cell structure, New York, Plenum
Press, Vol. 1.
JOY, R.M. (1982) Chlorinated hydrocarbon insecticides. In: Ecobichon,
D.J. & Joy, R.M., ed. Pesticides and neurological diseases, Boca
Raton, Florida, CRC Press, pp. 91-150.
JOY, R.M. (1985) Kindling as a tool in neurotoxicology. Fund. appl.
Pharmacol., 5: 41-65.
JOY, R.M., STARK, L.G., PETERSON, S.L., BOWYER, J.T., & ALBERTSON,
T.E. (1980) The kindled seizure; production of and modification by
dieldrin in rats. Neurobehav. Toxicol., 2: 117-124.
JUNQUEIRA, L.C., CARNEIRO, J., & CONTOPOULOS, A. (1977) Basic
Histology, 2nd ed., Los Altos, Lange.
KAPLAN, K.L. (1980) Thyroxine 5'-monodeiodination in rat pituitary
homogenates. Endocrinology, 106: 567-576.
KARPOVA, O.B., OBEHOVA, E.L., AVIOVA, N.F., & SCHVARTS, E.G. (1978)
[Content and composition of cerebral gangliosides during Down's
Syndrome.] Vopros. med. Xhim., 4: 524-527 (in Russian).
KELLEHER, R.T. & MORSE, W.H. (1968) Determinations of the specificity
of behavioral effects of drugs. Ergeb. Physiol., 60: 1-56.
KHOLODOV, Ju.A. & SOLOV'EVA, G.R. (1971) [Occasional publication:
news on medical equipment manufacture,] fasc. 3, pp. 69-72
(in Russian).
KIMURA, J. (1976) Collision techniques: physiological block of nerve
impulses in studies of motor nerve conduction velocity. Neurology,
26: 680-682.
KING, J.A. & VESTAL, B.M. (1974) Visual acuity of peromyscus.
J. Mammal., 55: 238-243.
KIRPEKAR, S.M., DIXON, W., & PRATT, J.C. (1970) Inhibitory effect of
manganese on norephinephrine release from the splenic nerves of cat.
J. Pharmacol. exp. Ther., 174: 72-76.
KLEIN, R., HERMAN, S., BRUBAKER, P.E., & LUCIER, G.W. (1972) A model
of acute methylmercury intoxication in rats. Arch. Path.,
93: 404-418.
KODAMA, J., FUKUSHIMA, M., & SAKATA, T. (1978) Impaired taste
discrimination against quinine following chronic administration of
theophylline in rats. Physiol. Behav., 20: 151-155.
KOHLER, W. (1976) The mentality of ages, New York, Liveright,
336 pp.
KONAT, G. & CLAUSEN, J. (1977) Triethyl lead-induced intoxication as
an experimental model of hypomyelination. Proc. Int. Soc.
Neurochem., 6: 215.
KORNBERG, A. (1955) Lactic dehydrogenase in muscle. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 441.
KOSTIAL, K., LANDEKA, M., & SLAT, B. (1974) Manganese ions and
synaptic transmission in the superior cervical ganglion of the cat.
Br. J. Pharmacol., 51: 231-235.
KOTLIAREVSKI, L.I. (1951) [Methodology for studying motor conditioned
reflexes in some small animals (white rats and guinea-pigs.]
Zh. Vyssh. nerv. deyat. imeni, 1(5): 753-761 (in Russian).
KOTLIAREVSKI, L.I. (1957) [Procedure for the investigation of motor-
food conditioned reflexes in small animals.] Tr. Inst. Vyssh. nerv.
deyat. AN SSR, 3: 23-28 (in Russian).
KRIGMAN, M.R. & HOGAN, E.L. (1974) Effect of lead intoxication on
the postnatal growth of the rat nervous system. Environ. Health
Perspect., 7: 187-199.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., SUTER, Y., THOMANN, G., &
HESS, R. (1981) Pyridoxine megavitaminosis produces degeneration of
peripheral sensory neurons (sensory neuronopathy) in the dog.
Neurotoxicology, 2: 13-24.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., THOMANA, P., & HESS, R.
(1979) Clinoquinol and 2,5-hexanedione induce different types of
distal axonopathy in the dog. Acta neuropathol., 47: 213.
KRSIAK, M. (1979) Effects of drugs on behavior of aggressive mice.
Br. J. Pharmacol., 65: 525-533.
KRUSIUS, T. & FINNE, J. (1977) Structural features of tissue
glycoproteins. Fractionation and methylation analysis of gylcopeptides
derived from rat brain, kidney, and liver. Eur. J. Biochem.,
78: 369-379.
KRYZHANOVSKY, G.N. (1973) Mechanism of action of tetanus toxin. Effect
on synaptic processes and some particular features of toxin binding by
the nervous tissue. Jaunyn-Schmiedeberg's Arch., 276: 247-270.
KUCZENSKI, R.T. & MANDELL, A.J. (1972) Regulatory properties of
soluble and particulate rat brain tyrosine hydroxylase. J. biol.
Chem., 247: 3114-3116.
KULKOSKY, P.J., ZELLNER, D.A., HYSON, R.L., & RILEY, A.L. (1980)
Ethanol consumption of rats in individual, group, and colonial housing
conditions. Physiol. Psychol., 8: 56-60.
KUPFER, D. & BULGER, W.H. (1976) Studies on the mechanism of
estrogenic actions of o-p'-DDT; interactions with the estrogen
receptor. Pestic. Biochem. Physiol., 6: 561-570.
KUPFER, D. & BULGER, W.H. (1980) Estrogenic properties of DDT and its
analogues. In: McLachlan, J., ed. Estrogens in the environment,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 239-263.
LAFFERMAN, J.A. & SILBERGELD, E.K. (1979) Erythrosin B inhibition of
neurotransmitter accumulation by rat brain homogenate. Science,
206: 363-365.
LAJTHA, A. & MARKS, N. (1971) Protein turnover. In: Lajtha, A., ed.
Handbook Neurochemistry, New York, Plenum Press, Vol. 5, pp. 551-629
LAMARTINIERE, C.A., DIERINGER, C.S., & LUCIER, G.W. (1979) Altered
ontogeny of glutathione S-transferases by 2,4,5-2',4',5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 51: 233-238.
LANDOWNE, D. & RITCHIE, J.M. (1976) On the control of glycogenolysis
in mammalian nervous tissue by calcium. J. Physiol., 212: 503-517.
LATIES, V.G. (1982) Contributions of operant conditioning to
behavioral toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 67-80.
LAZAROVA, M., BENDOTTI, C., & SAMANIN, R. (1984) Evidence that the
dorsal raphe area is involved in the effect of clonidine against
pentylenetetrazol-induced seizures in rats. Naunyn-Schmiedebergs
Arch. Pharmacol., 325: 12-16.
LEANDER, J.D. & MACPHAIL, R.C. (1980) Effect of chlordimeform
(a formamidine pesticide) on schedule-controlled responding of
pigeons. Neurobehav. Toxicol., 2: 315-321.
LEANDER, J.D., MCMILLAN, D.E., & BARLOW, T.S. (1977) Chronic mercuric
chloride: behavioural effects in pigeons. Environ. Res.,
14: 424-435.
LEHMANN, H.J. & TACKMANN, E. (1974) Neurographic analysis of trains of
frequent electrical stimuli in the diagnosis of peripheral nerve
disease. Eur. Neurol., 12: 293-308.
LEHRER, G.M. (1974) Measurement of minimal brain dysfunction. In:
Xintaras, C., Johnson, B.L., & DeGroot, I., ed. Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, pp. 450-454 (US DREW (NIOSH) Publication No. 74-126).
LEONARD, J.L. & ROSENBERG, L.H. (1980) Characterization of essential
enzyme sulfhydryl groups of thryoxine 5'deiodinase from rat kidney.
Endocrinology, 108: 444-451.
LEVINE, T.E. (1976) Effects of carbon disulfide and FLA-63 on operant
behavior in pigeons. J. Pharmacol. exp. Ther., 199: 669-678.
LEWEY, F.H. (1941) Experimental chronic carbon disulfide poisoning in
dogs. J. ind. Hyg. Toxicol., 23: 415-535.
LILIENTHAL, H., WINNEKE, G., BROCKHAUS, A., MOLIK, B., & SCHLIPKOTER,
H.-W. (1983) Learning set-formation in rhesus monkeys pre- and
postnatally exposed to lead. In: Proceedings of the International
Conference on Heavy metals in the Environment, Heidelberg, September,
1983, Edinburgh, CEP Consultants, p. 901.
LINDSLEY, D.B. & WICKE, J.D. (1974) The electroencephalogram:
autonomous electrical activity in man and animals. In: Thompson, R. &
Patterson, M., ed. Bioelectric recording techniques, Part B, New
York, Academic Press.
LIPP, J.A. (1968) Cerebral electrical activity following soman
administration. Arch. int. Pharmacodyn., 175: 161-169.
LOCK, E.A. (1976) Increase in cerebral fluids in rats after treatment
with hexachlorophene or triethyltin. Biochem. Pharmacol.,
25: 1455-1458.
LORENZO, A.V. & GERWITZ, M. (1977) Inhibition of 14C-tryptophane
transport into brain of lead-exposed neonatal rabbits. Brain Res.,
132: 386-392.
LOWITZSCH, K., GOHRING, U., HECKING, E., & KOHLER, H. (1981)
Refractory period, sensory conduction velocity, and visual-evoked
potentials before and after haemodialysis. J. Neurol. Neurosurg.
Psychiatr., 44: 121-128.
LOWNDES, H.E. & BAKER, T. (1976) Studies on drug-induced neuropathies.
III. Motor nerve deficit in cats with acrylamide neuropathy. Eur. J.
Pharmacol., 35: 177-184.
LOWNDES, H.E., BAKER, T., CHO, E.S., & JORTNER, B.S. (1978a) Position
sensitivity of deafferented muscle spindles in experimental acrylamide
neuropathy. J. Pharmacol. exp. Ther., 205: 40-48.
LOWNDES, H.E., BAKER, T., MICHAELSON, L.P., & VINCENT-ABLAZEY, M.
(1978b) Attenuated dynamic responses of primary endings of muscle
spindles; a basis for depressed tendon responses in acrylamide
neuropathy. Ann. Neurol., 3: 435-437.
LUKAS, E. (1970) Stimulation electrohypography in experimental
toxicology (carbon disulfide neuropathy in rats). Med. Lav.,
61: 302-308.
MCBLAIN, W.A., LEWIN, V., & WOLFE, F.H. (1976) Differing estrogenic
activities for the enatiomers of o,p'-DDT in immature female rat.
Can. J. Physiol., 54: 629-632.
MACDONALD, J.S. & HARBISON, R.D. (1977) Methylmercury induced
encephalopathy in mice. Toxicol. appl. Pharmacol., 39: 195-205.
MCDONALD, W.I. (1963) The effects of experimental demyelination on
conduction in peripheral nerve. II. Electrophysical observations.
Brain, 86: 501-524.
MCDONOUGH, J.H., Jr, HACKLEY, B.E., Jr, CROSS, R., SAMSON, F., &
NELSON, S. (1983) Brain regional glucose use during soman-induced
seizures. Neurotoxicology, 4: 203-210.
MCKENNA, M.J. & DESTEFANO, V. (1975) A proposed mechanism of action of
carbon disulfide on carbon disulfide on dopamine-beta-hydroxylase.
Toxicol. appl. Pharmacol., 33: 137.
MCMILLAM, D.E. (1982) Effects of chronic administration of pesticides
on schedule-controlled responding by rats and pigeons. In: Chambers,
J.E. & Yarborough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 211-226.
MACPHAIL, R.C. (1982) Studies on the flavour aversions induced by
trialkyltin compounds. Neurobehav. Toxicol. Teratol., 4: 225-230.
MACPHAIL, R.C. & LEANDER, J.D. (1980) Flavour aversions induced by
chlordimeform. Neurobehav. Toxicol., 2: 363-365.
MACPHAIL, R.C., CROFTON, K.M., & REITER, L.W. (1983) Use of
environmental challenges in behavioral toxicology. Fed. Proc.,
42: 3196-3200.
MACTUTUS, C.F., UNGER, K.L., & TILSON, H.A. (1982) Neonatal
chlordecone exposure impairs early learning and memory in the rat on a
multiple measure passive avoidance task. Neurotoxicology, 3: 24-44.
MAGATA, Y. & TSUKADA, W. (1978) Bulk separation of neuronal cell
bodies and glial cells from mammalian brain and some of their
biochemical properties. Rev. Neurosci., 3: 18-27.
MAILMAN, R.B., FERRIS, R.M., VOGEL, R.A., KILTS, C.D., LIPTOM, M.A.,
TANG, F.L., SMITH, D.A., MUELLER, R.A., & BREESE, G.R. (1980)
Non-specificity of the biochemical actions of erythrosine (Red No. 3):
what relation to behavioural changes. Science, 207: 535-537.
MARES, P. & VELISEK, L. (1983) Influence of ethosuximide on metrazol-
induced seizures during ontogenesis in rats. Act. Nerv. super
(Praha), 25: 292-298.
MARSHALL, J.F. (1975) Increased orientation to sensory stimuli
following lateral hypothalamic damage in rats. Brain Res.,
86: 373-387.
MARSHALL, J.F. & TEITELBAUM, P. (1974) Further analysis of sensory
inattention following lateral hypothalamic damage in rats. J. comp.
physiol. Psychol., 86: 375-395.
MARSHALL, J.F., TURNER, D.H., & TEITELBAUM, P. (1971) Sensory neglect
produced by lateral hypothaLamic damage. Science, 174: 523-525.
MARSHALL, J.F., RICHARDSON, J.S., & TEITELBAUM, P. (1974)
Nigrostriatal bundle damage and the lateral hypothalamic syndrome.
J. comp. Physiol. Psychol., 87: 808-830.
MARSLAND, T.A., GLEES, P., & ERIKSON, L.B. (1954) Modification of
Glee's silver impregnation for paraffin sections. J. Neuropathol.
exp. Neurol., 13: 587-591.
MASTEROV, A.V. (1974) [Features of the interaction of rats in an
electromagnetic field following extinction of conditioned food
reactions.] In: [Problems of clinical and experimental medicine,]
Moscow, pp. 61-63 (in Russian).
MAURISSEN, J.P.J. (1979) Effects of toxicants on the somatosensory
system. Neurobehav. Toxicol., 1(Suppl. 1): 23-32.
MEDVEDEV, B.M. (1975) [The effect of a static electric field on
conditioned reflex activity.] Fiziol. Zh., 3: 320-325 (in Russian).
MELLO, N.K. (1975) Behavioral toxicology; a developing discipline.
Fed. Proc., 34: 1832-1834.
MENDELL, J.R., SAIDA, K., GANANSIA, M.F., JACKSON, D.B., WEISS, H.,
GARDIER, R.S., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J., MARKS,
B., & HETLAND, L. (1974) Toxic polyneuropathy produced by methyl
n-butyl ketone. Science, 185: 787-789.
MENDELSON, J.H., MELLO, N.K., & ELLINGBOE, J. (1977) Effects of acute
alcohol intake on pituitary-gonadal hormones in normal human males.
J. Pharmacol. exp. Ther., 202: 675-682.
MERIGAN, W.H. (1979) Effects of toxicants on visual systems.
Neurobehav. Toxicol., 1: 15-22.
MERIGAN, W.H. (1980) Visual fields and flicker thresholds in
methylmercury poisoned monkeys. In; Merigan, W.H. & Weiss, B., ed.
Neurotoxicity of the visual system, New York, Raven Press,
pp. 149-163.
MERKURYVA, R.V. & BUSHINSKAYA, L.I. (1982) [Metabolism of
sialo-containing glycoproteins and lysosomal enzyme activity under
neurotropic effect of chemical environmental factors.] Ukranian
Biochem. J., 53: 31-35 (in Russian).
MERKURYVA, R.V. & TSAPKOVA, N.N. (1982) Hygienic significance of a
system of biochemical criteria for the evaluation of hepatotoxic and
neurotoxic effects of some chemical factors in the environment.
J. Hyg. Epidemiol. Microbiol. Immunol., 26: 336-341.
MERKURYVA, R.V., BUSHINSKAIA, L.J., AULIKA, B.V., SHATERNIKOVA, J.S.,
PROTSENKO, S.J., KULYGINA, A.A., & EKSLER, N.D. (1978) The activity of
lyosomal and cytoplasmic enzymes under the experimental influence of
bisulfide of carbon. Vop. Med. Xhim., 2: 151-156.
MERKURYVA, R.V., KOTOV, A.V., SILOV, V.G., BUSHINSKAIA, L.J.,
MUCHAMBETOVA, L.KH., & TSAPKOVA, N.N. (1980) Experimental study of
biochemical and physiological mechanisms of cerebral activity
influenced by alcohol, Nauka, Minsk Publishing House, pp. 93-94.
MEYER, O.A., TILSON, H.A., BYRD, W.C., & RILEY, H.T. (1979) A method
for the routine assessment of fore- and hindlimb grip strength of rats
and mice. Neurobehav. Toxicol., 1: 233-236.
MICZEK, K.A. & BARRY, H. (1976) Pharmacology of sex and aggression.
In: Glick, S.D. & Goldforb, J., ed. Behavioral pharmacology,
St. Louis, Missouri, C.V. Mosby Co., pp. 176-257.
MIKISKA, A. (1960) [Determination of the susceptibility to electrical
activity of the cerebral motor cortex, and its pharmacological and
toxicological applications. I. Methodology, controlled trials and
physiological basis.] Arch. Gewerbepathol. Gewerbehyg., 18: 286-299
(in German).
MIKISKOVA, H. & MIKISKA, A. (1962) [A contribution to the application
of defensive skin stimulation in the toxicity of substances acting on
the CNS. II. Measuring stimulus threshold intensity in guinea-pigs.]
Int. Arch. Gewerbepathol. Gewerbehyg., 19: 68-75 (in German).
MIKISKOVA, H. & MIKISKA, A. (1966) Trichloroethanol in
trichloroethylene poisoning. Br. J. ind. Med., 23: 116-125.
MIKISKOVA, H. & MIKISKA, A. (1968) Some electrophysiological methods
for studying the action of narcotic agents in animals, with special
reference to industrial solvents: a review. Br. J. ind. Med.,
25: 81-105.
MILLER, D.B. (1983) Neurotoxicity of the pesticidal carbamates.
Neurobehav. Toxicol. Teratol., 4: 779-788.
MILLER, E.K. & DAWSON, R.M.C. (1972) Can mitochondria and synaptosomes
of guinea-pig brain synthesize phospholipids? Biochem. J.,
126: 805-807.
MINEGISHI, A., FUJUMORI, R., SATOH, T., KILAGAWA, H., & YANAURA, S.
(1979) Changes in serotonin turnover and the brain sensitivity to
barbituates by disulfiram treatment in rats. Res. Comm. Chem. Pathol.
Pharmacol., 24: 273-285.
MINKINS, C., CHEKUNOVA, M.P., & LEVINA, E.N. (1973) [Effect of
antimony and lead on the adrenal glands and biogenic amines.]
Gig. Tr. Prof. Zabol., 17: 21-24 (in Russian).
MITCHELL, C.L. & TILSON, H.A. (1982) Behavioral toxicology in risk
assessment: problems and research needs, Boca Raton, Florida, CRC
Press, pp. 265-274 (CRC Critical Reviews in Toxicology 10).
MITCHELL, C.L., TILSON, H.A., & CABE, P.A. (1982) Screening for
neurobehavioral toxicity: factors to consider. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 237-245.
MIYOSHI, T. & GOTO, I. (1973) Serial in vivo determinations of nerve
conduction velocity in rat tails: physiological and pathological
changes. Electroencephal. clin. Neurophysiol., 35: 125-131.
MOLINENGO, L. & ORSETTI, M. (1976) Drug action on the "grasping"
reflex and on swimming endurance: an attempt to characterize
experimentally anti-depressant drugs. Neuropharmacology,
15: 257-260.
MORALI, G., LARSSON, K., & BEYER, C. (1977) Inhibition of
testosterone-induced sexual behaviour in the castrated male rat by
aromatization blockers. Horm. Behav., 9: 203-213.
MORGANTI, J.B., LOWNE, B.A., SALVATERRA, P., & MASSARO, E.J. (1976)
Effects of open-field behaviour of mice exposed to multiple doses of
methylmercury. Gen. Pharmacol., 7(1): 41-44.
MUNRO, A.J., JACKSON, R.J., & KORNER, A. (1964) Disaggregation of
brain polysomes. Biochem. J., 92: 289-294.
MURPHY, S.D. & DUBOIS, K.P. (1958) The influence of various factors on
the enzymatic conversion of organic thiophosphates to
anticholinesterase agents. J. Pharmacol. exp. Ther., 124: 194-202.
NARAHASHI, T. (1982) Cellular and molecular mechanisms of action of
insecticides: neurophysiological approach. Neurobehav. Toxicol.
Teratol., 4: 753-758.
NARAHASHI, T. & HAAS, H.G. (1967) DDT: interaction with nerve membrane
conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & HAAS, H.G. (1968) Interaction with DDT with the
components of lobster nerve membrane conductance. J. gen. Physiol.,
51: 177-198.
NATHANSON, J.A. & BLOOM, F.E. (1975) Lead-induced inhibition of brain
adenylcyclase. Nature (Lond.), 255: 419-420.
NICHOLAS, G.S. & BARRON, D.H. (1932) The use of sodium amytal in the
production of anaesthesia in the rat. J. Pharmacol. exp. Ther., 46;
125-129.
NIEDERMEYER, E. & LOPES DA SILVA, F. (1982) Electroencephalography:
basic principles, clinical applications, and related fields,
Baltimore, Maryland, Munich, Urban Schwartzenberg.
NIOKESARYISKY, A.A. & TUROVSKY, V.S. (1980) N-acetylneuraminic acid
content in water-soluble and membranous tissue fractions of various
analysers of cerebral cortex. Biochimia, 45: 1829-1932.
NORTON, S. (1973) Amphetamine as a model for hyperactivity in the rat.
Physiol. Behav., 11: 181-186.
O'BRIEN, R.D. (1976) Acetylcholinesterase and its inhibition. In:
Wilkerson, C.F., ed. Insecticide biochemistry and physiology, New
York, Plenum Press, pp. 271-296.
O'CALLAGHAN, J.P., LAVIN, K.L., CHESS, Q., & CLOUET, D.H. (1983) A
method for dissection of discrete regions of rat brain following
microwave irradiation. Brain Res., 11: 31-42.
O'DONOGHUE, J.L., ed. (1985) Neurotoxicity of industrial and
commercial chemicals, Boca Raton, Florida, CRC Press.
OCHOA, J. (1972) Ultrathin longitudinal sections of single myelinated
fibres for electron microscopy. J. neurol. Sci., 17: 103.
OCHS, S. (1972) Fast transport of materials in mammalian nerve fibres.
Science, 176: 252.
OCHS, S. (1982) Axoplasmic transport and its relation to other nerve
functions, New York, John Wiley and Sons.
OHI, G., NISHIGAKI, S., SEKI, H., TAMURA, Y., MIZOGUCHI, I., YAGYA,
H., & NAGASHIMA, K. (1978) Tail rotation, an early neurological sign
of methylmercury poisoning in the rat. Environ. Res., 16: 353-359.
OLIVER, C., ESKAY, R.L., BEN-JONATHAN, N., & PORTER, J.C. (1974)
Distribution and concentration of TRH in rat brain. Endocrinology,
96: 540-546.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1979) Hippocampus
space, and memory. Behav. Brain Sci., 2: 313-365.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1980) Hippocampal
function: working memory or cognitive mapping? Physiol. Psychol.,
8: 239-246.
OTTO, D.A. (1977) Neurobehavioral toxicology: problems and methods in
human research. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 10/1-10/31
(EPA 600/9-77-042).
OTTO, D.A. (1978) Multidisciplinary perspectives in event-related
brain potential research. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Washington
DC, US Environmental Protection Agency (EPA 600/9-77-043).
OTTO, D.A., BENIGNUS, V.A., MULLER, K.E., & BARTON, C.N. (1981)
Effects of age and body lead burden on CNS function in young children.
I. Slow cortical potentials. Electroencephalogr. clin.
Neurophysiol., 52: 229-239.
OVERMANN, S.R. (1977) Behavioral effects of asymptomatic lead exposure
during neonatal development in rats. Toxicol. appl. Pharmacol.,
41: 459-471.
PADICH, R. & ZENICK, M. (1977) The effects of developmental and/or
direct lead exposure on FR behavior in the rat. Pharmacol. Biochem.
Behav., 6: 371-375.
PALKOVITS, M. (1973) Isolated removal of hypothalamic or other brain
nuclei of the rat. Brain Res., 59: 449-450.
PALMITER, R.D. & MULVIHILL, E.D. (1978) Estrogenic activity of the
insecticide kepone on the chick oviduct. Science, 201: 356-358.
PANCHENKO, L.F., DUDCHENKO, A.M., & SHPAKOV, A.A. (1975) The influence
of acute hypoxic hypoxia on the activity of rats cerebral cortex:
mitochondria enzymes possessing different sensibility to oxygen
deficiency. In: Gorky, ed. Biochemistry of hypoxia, pp. 59-64.
PANSKY, B. & ALLEN, D.J. (1980) Review of neuroscience, New York,
McMillan Inc.
PARIZEK, J. (1956) Effects of cadmium salts on testicular tissue.
Nature (Lond.), 177: 1036-1037.
PARTINGRON, C.R. & DALY, J.W. (1979) Effect of gangliosides on
adenylate cyclase activity in rat cerebral cortical membranes. Mol.
Pharmacol., 15: 484-491.
PARTRIDGE, W.M. (1976) Inorganic mercury: selective effects on
blood-brain barrier transport systems. J. Neurochem., 27: 333-335.
PATTON, S.E., O'CALLAGHAN, J.P., MILLER, D.B., & ABOU-DONIA, M.B.
(1983) Effect of oral adminstration of tri-o-cresyl phosphate on in
vitro phosphorylation of membrane and cytosolic proteins from
chicken brain. J. Neurochem., 41: 897-901.
PAUL, S.M., HOFFMAN, A.R., & AXELROD, A. (1980) Catechol estrogens:
synthesis and metabolism in brain and other endocrine tissues. In:
Martini, L. & Ganong, W.F., ed. Frontiers in neuroendocrinology, New
York, Raven Press, pp. 203-217.
PAVLENKO, S.M. (1975) Methods for the study of the central nervous
system in toxicological tests. In: Methods used in the USSR for
establishing biologically safe levels of toxic substances, Geneva,
World Health Organization, pp. 86-108.
PAVLOV, I.P (1927) Conditioned reflexes, Oxford, Oxford University
Press.
PEARSE, A.G.E. (1968) Histochemistry: theoretical and applied, 3rd
ed., Baltimore, Maryland, Williams and Wilkins Company.
PEARSON, C.M. & MOSTOFI, F.K. (1973) The striated muscle, Baltimore,
Maryland, Williams and Wilkins Company.
PEASE, D.E. (1964) Histological techniques for electron microscopy,
2nd ed., New York, Academic Press.
PERSINGER, M.A., LAFREINIERE, G.P., & CARREY, N.V. (1978) Thyroid
morphology and wet organ weight changes in rats exposed to different
low intensity 0.5-Hz magnetic fields and pre-experimental caging
conditions. Biometeorology, 22: 67-73.
PILCHER, C.W.T. & JONES, S.M. (1981) Social crowding enhances
aversiveness of naloxone in rats. Pharmacol. Biochem. Behav.,
14: 299-303.
PIRCH, J.H. & RECH, R.H. (1968) Effect of isolation on alpha-
methyltyrosine-induced behavioral depression. Life Sci., 7: 173-182.
PLEASURE, D.E., MISHLER, K.C., & ENGEL, W.K. (1969) Axonal transport
of proteins in experimental neuropathies. Science, 166: 524-525.
POST, E.M., YANG, M.G., KING, J.A., & SANGER, V.L. (1973) Behavioral
changes of young rats force-fed methylmercury chloride. Proc. Soc.
Exp. Biol. Med., 143: 1113-1116.
POWERS, J.M., SCHAUMBURG, H.H., JOHNSON, A.E., & RAINE, C.S. (1980) A
correlative study of the adrenal cortex in adrenoleukodystrophy:
evidence for a fatal intoxication with very long-chain saturated fatty
acids. Invest. cell Pathol., 3: 353-376.
PRABHU, V.G. (1972) Amphetamine aggregation effect in mice under
conditions of altered microsomal enzymes. Arch. int. Pharmacodyn.
Ther., 195: 81-86.
PROKHOROVA, M.J. (1974) Structure and function of phosphornositides
and gangliosides of the brain. In: Progress of neurochemistry,
Leningrad, Nauka, pp. 61-73.
PRYOR, G.T., UYENO, E.T., TILSON, H.A., & MITCHELL, C.L. (1983)
Assessment of chemicals using a battery of neurobehavioral tests: a
comparative study. Neurobehav. Toxicol. Teratol., 5: 91-117.
PURPURA, D.P., PENRY, J.K, TOWER, P., WOODBURY, D.M., & WALTER, R.
(1972) Experimental models of epilepsy, New York, Raven Press.
QUEVEDO, L., CONCHA, J., MARCHANT, Z., ESKUCHE, W., & MADARIAGA, J.
(1980) Nerve accommodation in chronic alcoholic subjects.
Pharmacology, 21: 229-232.
QUINN, G.P., AXELROD, J., & BRODIE, B.B. (1958) Species, strain, and
sex differences in metabolism of hexobarbitone, amidopyrine,
antipyrine, and aniline. Biochem. Pharmacol., 1: 152-159.
RADIN, N.S., BRENHERT, A., ARORA, R.C., SELLINGER, O.Z., & FLANGAS,
A.L. (1972) Glial and neuronal localization of cerebroside-
metabolizing enzymes. Brain Res., 39: 163-166.
RAFALES, L.S., BORNSCHEIN, R.L., MICHAELSON, A., LOCH, R.K., & BARKER,
G.F. (1979) Drug-induced activity in lead-exposed mice. Pharmacol.
Biochem. Behav., 10: 95-104.
RAMSAY, R.B., KRIGMAN, M.R., & MORELL, P. (1980) Developmental studies
of the uptake of choline, GABA, and dopamine by crude synaptosomal
preparations after in vivo or in vitro lead treatment. Brain
Res., 187: 383-387.
RAY, O.S. & BARRETT, R.J. (1975) Behavioral, pharmacological, and
biochemical analysis of genetic differences in rats. Behav. Biol.,
15: 391-417.
REICHARDT, L.F. & KELLY, R.B. (1983) A molecular description of nerve
terminal function. Ann. Rev. Biochem., 52: 871-926.
REICHERT, B.L. & ABOU-DONIA, M.B. (1980) Inhibition of fast axoplasmic
transport by delayed neurotoxic organophosphorus esters: a possible
mode of action. Mol. Pharmacol., 17: 56-60.
REITER, L.W. (1978) Use of activity measures in behavioral toxicology.
Environ. Health Perspect., 26: 9-20.
REITER, L.W. (1980) Neurotoxicology -- meet the real world.
Neurobehav. Toxicol., 2: 73-74.
REITER, L.W. (1983) Chemical exposure and animal activity: utility of
the Figure-Eight Maze. In: Hayes, A.H., Schnell, R.C., & Miya, T.S.,
ed. Developments in the science and practice of toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 78-84.
REITER, L.W. & MACPHAIL, R.C. (1979) Motor activity: a survey of
methods with potential use in toxicity testing. Neurobehav.
Toxicol., 1(Suppl. 1): 53-66.
REITER, L.W. & MACPHAIL, R.C. (1982) Factors influencing motor
activity measurements in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 45-65.
REITER, L.W., KIDD, K., HEAVNER, G., & RUPPERT, P. (1980) Behavioral
toxicity of acute and subacute exposure to triethyltin in the rat.
Neurotoxicology, 2: 97-112.
REUHL, K. & CHANG, L. (1979) Effects of methylmercury on the
development of the nervous system: a review. Neurotoxicology,
1: 21-55.
REYNOLDS, G.S. (1958) A primer of operant conditioning, Glenview,
Illinois, Scott, Foresman and Co.
RICE, D.C. & GILBERT, S.G. (1982) Early chronic low-level
methylmercury poisoning in monkeys impairs spatial vision. Science,
216: 759-761.
RICE, D.C. & WILLES, R.F. (1979) Neonatal low-level lead exposure in
monkeys (Macaca fascicularis): effects on two choice non-spatial
form discrimination. J. environ. Pathol. Toxicol., 2: 1195-1203.
RICE, D.C., GILBERT, S.G., & WILLES, R.F. (1979) Neonatal low-level
lead exposure in monkeys: locomotor activity, schedule-control
behavior, and the affects of amphetamine. Toxicol. appl. Pharmacol.,
51: 503-513.
RICE, D.F. (1985) Central nervous system effects of perinatal exposure
to lead or methylmercury in the monkey. In: Clarkson, T.W. & Nordberg,
G., ed. Reproductive developmental toxicity of metals, New York,
Plenum Press.
RITCHIE, J.M. (1979) A pharmacological approach to the structure of
sodium channels in myelinated axons. Ann. Rev. Neurosci.,
2: 341-362.
ROBERTS, D.V. (1977) A longitudinal electromyographic study of six men
occupationally exposed to organophosphorus compounds. Int. Arch.
occup. environ. Health, 38: 221-229.
ROBERTS, R.K., DESMOND, P.V., WILKINSON, G.R., & SCHENKER, S. (1979)
Disposition of chlordiazepoxide; sex differences and effects of oral
contraceptives. Clin. Pharmacol. Ther., 25: 826-831.
ROBINS, E., HIRSCH, H.E., & EMMONS, S.S. (1968) Glycosidases in the
nervous system. I. Assay, some properties, and distribution of
beta-galactosidase, beta-glucoronidase, and beta-glucosidase.
J. biol. Chem., 243: 4246-4249.
RODIER, P. (1978) Behavioral teratology. In: Wilson, J.G. & Fraser,
F.C., ed. Handbook of teratology, New York, Plenum Press, Vol. 4,
pp. 397-428.
ROEDER, R.J. & RUTTER, W.J. (1969) Multiple forms of DNA-dependent RNA
polymerase in eukaryotic organisms. Nature (Lond.), 224: 234-236.
ROSE, S.P.R. & SINHA, A.K. (1970) Separation of neuronal and neuropil
cell fractions: a modified procedure. Life Sci., 9: 907-912.
ROSECRANS, J.A., HONG, J.S., SQUIBB, R.E., JOHNSON, J.H., WILSON,
W.E., & TILSON, H.A. (1982) Effects of perinatal exposure to
chlordecone (kepone) on neuroendocrine and neurochemical
responsiveness of rats to environmental challenges. Neurotoxicology,
3: 131-142.
ROSEWICKYI, S., STANOSZ, S., & SAMOCHOWIEC, L. (1973) [The effect of
protracted exposure to carbon disulfide on the hormonal activity of
the ovaries in rats.] Med. Pracy., 24: 133-136 (in Polish).
ROZIER, L., SHIRAKI, H., & GRCEVIC, N., ed. (1977) Neurotoxicology,
New York, Raven Press, Vol. 1, 658 pp.
RUDNEV, M., BOKINA, A., ESKLER, N., & NAVAKATIKYAN, M. (1978) The use
of evoked potential and behavioural measures in the assessment of
environmental insult. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency, pp. 444-447 (EPA 600/9-77-0443).
RUSSELL, R.W., WARBURTON, D.M., & SEGAL, D.S. (1969) Behavioral
tolerance during chronic changes in the cholinergic system. Comm.
Behav. Biol., 4: 121-128.
RUSAK, B. & ZUCKER, I. (1975) Biological rhythms and animal behavior.
Ann. Rev. Psychol., 27: 137-171.
SABRI, M.I. & SPENCER, P.S. (1980) Toxic distal axonopathy:
biochemical studies and hypothetical mechanisms. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 206-219.
SABRI, M.I., MOORE, C.L., & SPENCER, P.S. (1979) Studies on the
biochemical basis of distal axonopathies. Inhibition of glycolysis
produced by neurotoxic hexacarbon compounds. J. Neurochem.,
32: 683-690.
SAKMANN, B. & NEHER, E. (1983) Single channel recording, New York,
Plenum Press, 503 pp.
SALVATERRA, P., LOWN, B., MORGANTI, J., & MARSARO, E.J. (1973)
Alterations in neurochemical and behavioural parameters in the mouse
induced by low doses of methylmercury. Acta pharmacol. toxicol.,
33(3): 177-190.
SANTINI, M. (1975) Golgi Centennial Symposium: Perspectives in
Neurobiology, New York, Raven Press.
SAVOLAINEN, H. (1983) Trends and prospects in experimental
neurotoxicology. Scand. J. Work environ. Health, 9: 214-218.
SAVOLAINEN, H. & JARVISALO, J. (1977) Effects of acute CS2
intoxication on protein metabolism in rat brain. Chem.-biol.
Interact., 17: 51-59.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINO, H. (1977)
Biochemical and behavioural effects of inhalation exposure to
tetrachloroethylene and dichloromethane. J. Neuropathol. exp.
Neurol., 36: 941-949.
SCHALLERT, T., WHISHAW, I.Q., RAMIREZ, V.D., & TEITELBAUM, P. (1978)
Compulsive, abnormal walking caused by anticholinergies in akinetic, 6
hydroxydopamine-treated rats. Science, 199: 1461-1463.
SCHAUMBURG, H.H. (1982) Pyridoxine megavitaminosis produces sensory
neuropathy in humans. Ann. Neurol., 12: 107-108.
SCHAUMBURG, H.H., SPENCER, P.S., & THOMAS, P.K. (1983) Disorders of
peripheral nerves, Philadelphia, Pennsylvania, F.A. Davis.
SCHAUMBURG, H.H., WISNIEWSKI, H., & SPENCER, P.S. (1974)
Ultrastructural studies of the dying-back process. I. Peripheral nerve
terminal and axon degeneration in systemic acrylamide intoxication.
J. Neuropathol. exp. Neurol., 33: 260-284.
SCHECHTER, M.D. & WINTER, J.C. (1971) Effect of mescaline and lysergic
acid diethylamide on flicker discrimination in the rat. J. Pharmacol.
exp. Ther., 177: 461-467
SCHEUHAMMER, A.M. & CHERIAN, M.G. (1982) The regional distribution of
lead in normal rat brain. Neurotoxicology, 3: 85-92.
SCHEVING, L.E., HALBERG, F., & PAULY, J.E., ed. (1974)
Chronobiology, Tokyo, Igaku Shoin Ltd.
SCHNAITMAN, C., ERWIN, V.G., & GREENSWALT, J.W. (1967) The
submitochondrial localization of monoamine oxidase. An enzymatic
marker for the outer membrane of rat liver mitochondria. J. cell
Biol., 32: 719-720.
SCHOENFELD, W.N., ed., (1970) The theory of reinforcement schedules,
New York, Appleton-Century-Crofts.
SCHOTMAN, P., GIPON, L., JENNEKENS, F.G.I., & GISPEN, W.H. (1978)
Polyneuropathies and CNS protein metabolism. III. Changes in protein
synthesis induced by acrylamide intoxication. J. Neuropathol. exp.
Neurol., 37: 820-837.
SCHREIBER, V. & PRIBYL, T. (1980) Possible role of ceruloplasmin in
endocrine regulation. In: Ishü, S., Hirano, T., & Wade, M., ed.
Hormones, adaptation, and evolution, Tokyo, Japan Science Society
Press/Berlin, Springer-Verlag.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1982) Inhibition by an
ergoline derivative (dopaminergic agonist) of estradiol-induced
adenohypophyseal growth and of the decrease in the hypothalamic
ascorbic acid (HA) concentration in rats. Physiol. Bohemoslov.,
31: 97-100.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1979) Effect of a dopamine
p-hydroxylase inhibitor (disulfiram) on the response of
adenohypophysis, serum ceruloplasmin, and hypothalamic ascorbic acid
to estradiol treatment. Endoc. Exper. (Bratislava), 13: 131-138.
SCHREIBER, V., ZBUZEK, V., & ZBUZKOVA-KMENTOVA, V. (1969) Reactions of
the rat and hamster endocrine system to aminoglutethimide. Physiol.
Bohemoslov., 18: 249-261.
SEPPALAINEN, A.M. & LINNOILA, I. (1975) Electrophysiological studies
on rabbits in long-term exposure to carbon disulphide. Scand. J. Work
environ. Health, 1: 178-183.
SEPPALAINEN, A.M., TOLONEN, M., KARLI, P., HANNINEN, H., & HEINBERG,
S. (1972) Neurophysiological findings in chronic carbon disulfide
poisoning: a descriptive study. Work Environ. Health, 9: 71-75.
SETH, P.K., AGRAWAL, A.K., & BONDY, S.C. (1981) Biochemical changes in
the brain consequent to dietary exposure of developing and mature rats
to chlordecone (kepone). Toxicol. appl. Pharmacol., 26: 262-267.
SHANDALA, M.G., RUDNEV, M.I., & NAVAKATIKYAN, M.A. (1980) [Behavioural
reactions in experimental hygienic research.] Gig. i Sanit.,
6: 43-48 (in Russian).
SHAW, C.M., MOTTET, N.K., & CHEN, W.J. (1980) Effects of methylmercury
on the visual system of rhesus macaque (Macaca mulatta). II.
Neuropathological findings (with emphasis on vascular lesions in the
brain). In: Merigan, W.H. & Weiss, B., ed. Neurotoxicity of the
visual system, New York, Raven Press, pp. 123-134.
SHAW, C.M., MOTTET, N.K., BODY, R.L., & LUSCHEI, E.S. (1975)
Variability of neuropathological lesions in experimental methylmercury
encephalopathy in primates. Am. J. Pathol., 80: 451-470.
SHIBUSAWA, K., YAMAMOTO, T., NISHI, K., ABE, C., & TOMIE, S. (1959)
Urinary secretion of TRF in various functional states of the thyroid.
Endocrinol. Jpn., 6: 131-136.
SHILJAGINA, N.N. (1971) [Evoked potentials in the projection of sign
stimulus during conditional reflex formation in ontogenesis.]
Zh. Vyssh. nerv. deyat. imeni, 21: 492-500 (in Russian).
SHIMIZU, T., TAKAISHI, M., & SHISHIBE, Y. (1978) Effect of ethanol on
the peripheral metabolism of thyroxine. Endocrinol. Jpn,
25: 201-204.
SHUGALOV, N.P. (1969) Primary and associative evoked responses to
optic irritants during various stages of conditioned reflex formation
in cats. In: Summary of Reports of the 22nd Meeting on the Problems
of Higher Nervous Activity, Ryazan, USSR, p. 268.
SHUMILINA, A.I. (1971) Characteristics of summary electric activity
and evoked potentials under motivating stimulation of various
biological significance. In: Proceedings of the Sixth All-Union
Conference on Electrophysiology of the Central Nervous System,
pp. 301-302.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974a) Hyperactivity: a
lead-induced behavior disorder. Environ. Health Perspect.,
7: 227-232.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974b) Lead-induced behavioral
dysfunction: an animal model of hyperactivity. Exp. Neurol.,
42: 146-157.
SILBERGELD, E.K. & GOLDBERG, A.M. (1975) Pharmacological and
neurochemical investigations of lead-induced hyperactivity.
Neuropharmacology, 14: 431-444.
SILBERGELD, E.K., HRUSKA, R.E., MILLER, L.P., & ENG, N. (1980) Effects
of lead in vivo and in vitro on GABA energic neurochemistry.
J. Neurochem., 34: 1711-1718.
SILVERMAN, A.P. (1965) Ethological and statistical analysis of drug
effects on the social behaviour of laboratory rats. Br. J.
Pharmacol., 24: 579-590.
SLOANE, S.A., SHEA, S.L., PROCTER, M.M., & DEWSBURY, D. (1978) Visual
cliff performance in 10 species of muroid rodents. Anim. Learn.
Behav., 6: 244-248.
SNOWDON, C.T. (1973) Learning deficits in lead injected rats.
Pharmacol. Biochem. Behav., 1: 599-603.
SNYDER, D.R. & BRAUN, J.J. (1977) Dissociation between behavioral and
physiological indices of organomercurial ingestion. Toxicol. appl.
Pharmacol., 41: 277-284.
SNYDER, S.H. & INMS, R.B. (1979) Peptide neurotransmitters. Ann. Rev.
Biochem., 48: 755-782.
SOBOTKA, T.J., BRODIE, R.E., & COOK, M.P. (1975) Psychophysiologic
effects of early lead exposure. Toxicology, 5: 175-191.
SOKOLOFF, L., REIVICH, M., KENNEDY, C., DES ROSIERS, M.H., PATLAK,
C.S., PETTIGREW, K.D., SAKURADA, O., & SHINOHARA, M. (1977) The
14C-deoxyglucose method for the measurement of local cerebral glucose
utilization: theory, procedure, and normal values in the conscious and
anaesthetized albino rat. J. Neurochem., 28: 897-916.
SPENCER, P.S. & BISCHOFF, M.C. (1982) Contemporary neuropathological
methods in toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 259-275.
SPENCER, P.S. & LIEBERMAN, A.R. (1971) Scanning electron microscopy of
isolated peripheral nerve fibres. Normal surface structure and
alterations proximal to neuromas. Z. Zellforsch. Mikrosk. Anat.,
119: 534-551.
SPENCER, P.S. & SCHAUMBURG, H.H. (1980) Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
929 pp.
SPENCER, P.S. & THOMAS, P.K. (1970) The examination of isolated nerve
fibres by light and electron microscopy, with observations on
demyelination proximal to neuromas. Acta neuropathol., 16: 177-186.
SPENCER, P.S., BISCHOFF, M., & SCHAUMBURG, H.H. (1980)
Neuropathological methods for the detection of neurotoxic disease. In:
Spencer, P.S. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 743-757.
SPERANSKIJ, S.V. (1965) [Advantages incident to the use of mounting
current in analysing the ability of Albino mice to summate subliminal
impulses.] Farmakol. i Toksikol., 1: 123-124 (in Russian).
SPYKER, J. (1975) Behavioral teratology and toxicology. In: Weiss, B.
& Laties, V.G., ed. Behavioral toxicology, New York, Plenum Press,
pp. 311-349.
SQUIBB, R.E. & TILSON, H.A. (1982) Neurobehavioural changes in adult
Fisher 344 rats exposed to dietary levels of chlordecone (KeponeR):
a 90-day chronic dosing study. Neurotoxicology, 3: 59-66.
STEBBINS, W.C. (1970) Animal psychophysics: the design and conduct of
animal experiments, New York, Appleton-Century-Crofts.
STEEL, R.G.D. & TORRIE, J.H. (1960) Principles and procedures of
statistics, New York, McGraw-Hill, pp. 83-347.
STEELE, W.J. & BUSCH, H. (1963) Studies on acidic nuclear proteins of
the Walker tumour and liver. Cancer Res., 23: 1153-1157.
STOCKINGER, H.E. (1974) Behavioral toxicology in the development of
threshold limit values. In: Xintaras, C., Johnson, B.L., & DeGroot,
I., ed. Behavioral toxicology, Cincinnati, Ohio, US Department of
Health, Education and Welfare, pp. 18-19 (US DHEW (NIOSH) Publication
No. 74-126).
SUDAKOV, K.V. (1982) Functional system theory in physiology.
Agressologie, 23: 167-176.
SZMIGIELSKI, A. (1975) Effect of disulfiram and sodium
diethyldithiocarbamate on thyroxine and dopamine hydroxylases in rat
brain and breast. Pol. J. Pharmacol. Pharm., 27: 153-159.
TAKEUCHI, T. (1968) Pathology of Minamata disease. In: Katsuma, M.,
ed. Minamata disease. Study Group of Minamata Disease, Japan,
Kumamoto University, pp. 141-228.
TAKEUCHI, T., ETO, N., & ETO, K. (1979) Neuropathology of childhood
cases of methylmercury poisoning (Minamata disease) with prolonged
symptoms with particular reference to the decortication syndrome.
Neurotoxicology, 1: 1-20.
TALKE, T., HIRABAGASHI, J., KONDO, R., MATSUMOTO, M., & KOJIMA, K.
(1979) Effect of butyrate on glycolipid metabolism of two cell types
of rat ascites hepatomes with different ganglioside biosynthesis.
J. Biochem., 96: 1139-1402.
TAPPEL, A.L. (1970) Lipid peroxidation damage to cell components.
Fed. Proc., 29: 239.
TAYLOR, J.R., SELHORST, J.B., & CALABRESE, V.P. (1980) Chlordecone.
In: Spencer, P. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 407-421.
THIESSEN, D.D. (1964) Population density and behavior: a review of
theoretical and physiological contributions. Texas Rep. Biol. Med.,
22: 266-314.
THOMAS, P.K. (1970) The quantitation of nerve biopsy findings.
J. neurol. Sci., 11: 285-295.
THOMAS, P.K. (1980) The peripheral nervous system as a target for
toxic substances. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 35-47.
THOMPSON, S.W. (1963) Selected histochemical and histopathological
methods, Springfield, Illinois, Charles C. Thomas.
THORNDIKE, E.L. (1932) Reward and punishment in animal learning.
Comp. Psychol. Monogr., 39: 8.
TILSON, H.A. & CABE, P.A. (1978) Strategy for the assessment of
neurobehavioral consequences of environmental factors. Environ.
Health Perspect., 26: 287-299.
TILSON, H.A. & HARRY, G.J. (1982) Behavioral principles for use in
behavioral toxicology and pharmacology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 1-27.
TILSON, H.A. & MITCHELL, C.L. (1984) Neurobehavioral techniques to
assess the effects of chemicals on the nervous system. Ann. Rev.
Pharmacol. Toxicol., 24: 425-450.
TILSON, H.A. & SQUIBB, R.D. (1982) The effects of acrylamide on the
behavioral suppression produced by psychoactive agents.
Neurotoxicology, 3: 113-120.
TILSON, H.A., BYRD, N., & RILEY, M. (1979a) Neurobehavioral effects of
exposing rats to kepone via the diet. Environ. Health Perspect.,
33: 321.
TILSON, H.A., CABE, P.A., ELLINWOOD, E.H., Jr, & GONZALEZ, L.P.
(1979b) Effects of carbon disulfide on motor function and
responsiveness to delta-amphetamine in rats. Neurobehav. Toxicol.,
1: 57-63.
TILSON, H.A., MITCHELL, C.L., & CABE, P.A. (1979c) Screening for
neurobehavioral toxicity: the need for and examples of validation of
testing procedures. Neurobehav. Toxicol., 1(Suppl. 1): 137-148.
TILSON, H.A., CABE, P.A., & BURNE, T.A. (1980) Behavioral procedures
used in the assessment of neurotoxicity. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 758-766.
TILSON, H.A., MACTUTUS, C.F., MCLAMB, R.L., & BURNE, T.A. (1982a)
Characterization of triethyl lead chloride neurotoxicity in adult
rats. Neurobehav. Toxicol. Teratol., 4: 671-681.
TILSON, H.A., SQUIBB, R.E., & BURNE, T.A. (1982b) Neurobehavioral
effects following a single dose of chlordecone (kepone) administered
neonatally to rats. Neurotoxicology, 3: 45-52.
TOLMAN, E.C. (1948) Cognitive maps in rats and men. Psychol. Rev.,
55: 189-208.
TOWFIGHI, J., GONATAS, N.K., & MCCREE, L. (1975) Hexachlorophene
retinopathy in rats. Lab. Invest., 32: 330-338.
TROPKINA, J.K., SELZNEVA, S.N., & POGODAEV, K.V. (1981) Prenatal
influence of hypoxia on metabolic structure of cerebral mitochondria
in the oncogenesis with epileptic superactivity. In: Mitochondria:
mechanisms of conjugation and regulation, Puscino, p. 39.
TRUEX, R.C. & CARPENTER, M.B. (1983) Human neuroanatomy, 8th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
TSUJI, S. & FUJIHSIMA H. (1975) Paraplegias: clinical statistics,
Fukuoka, Kyushu Rosai Hospital, Department of Orthopedics and
Neurology.
TULLOCH, M.L., CROOKS, J., & BROWN, P.S. (1963]) Inhibition of thyroid
function by a dithiocarbamoylhydrazine. Nature (Lond.),
199: 288-289.
ULRICH, C.E., RINEHART, W., & BRANDT, M. (1979) Evaluation of the
chronic inhalation toxicity of a manganese oxide aerosol. III.
Pulmonary function, electromyograms, limb tremor, and tissue manganese
data. Am. Ind. Hyg. Assoc. J., 40: 349-353.
UPHOUSE, L., NEMEROFF, C.B., MASON, G., PRANGE, A.J., & BONDY, S.C.
(1982) Interactions between "handling" and acrylamide on endocrine
responses in rats. Neurotoxicology, 3: 121-125.
UPHOUSE, L., MASON, G., & HUNTER, V. (1984) Persistent vaginal estrus
and serum hormones after chlordecone (kepone) treatment of adult rats.
Toxicol. appl. Pharmacol., 72: 177-186.
US FDA (1978) Nonclinical laboratory studies. Good laboratory
practice regulation, Washington DC, US Food and Drug Administration,
Department of Health, Education and Welfare (Federal Register 43:
59986-60025).
US NAS (1975) Principles for evaluating chemicals in the
environment, Washington DC, National Academy of Science (A Report of
the Committee for the Working Conference on Principles of Protocols
for Evaluating Chemicals in the Environment).
US NAS/NRC (1970) Evaluating the safety of food chemicals,
Washington DC, National Academy of Sciences, National Research
Council, Food Protection Committee, Food Nutrition Branch, Division of
Biology and Agriculture.
VAN GELDER, G.A. (1978) Lead and the nervous system. In: Oehme, F.W.,
ed. Toxicity of heavy metals in the environment, Part 1, Basel, New
York, Marcel Dekker, Inc., pp. 101-121.
VANDERWOLF, C.H. & LEUNG, L.-W.S. (1983) Hippocampal rhythmic slow
activity: a brief history and the effects of entorhinal lesions and
phencyclidine. In: Seifert, W., ed. Neurobiology of the hippocampus,
New York, Academic Press, pp. 275-302.
VASAN, N.S. & CHASE, H.P. (1976) Brain glucosaminoglycans
(mucopolysaccharides) following intrauterine growth retardation.
Biol. Neonate, 28: 196-206.
VEKSLER, M.S. & ARBUKHANOVA, M.S. (1981) Cerebral and spinal cord
mitochondria under strenuous physical work. In: Mitochondria:
mechanism of conjugation and regulation, Puscino, p. 81.
VERGIEVA, T. & ZAIKOV, H.R. (1981) Behavioral changes in rats with
inhalation of styrene. Hig. Zdraveopazvane, 24: 242-247.
VERITY, M.A. (1972) Cation modulation of synaptosomal respiration.
J. Neurochem., 19: 1305-1309.
VERITY, M.A., BROWN, W.J., CHEUNG, M., & DZER, G. (1977) Methylmercury
inhibition of synaptosomes and brain slices protein synthesis: in
vivo and in vitro studies. J. Neurochem., 29: 673-679.
VILA, J. & COLOTLA, V.A. (1981) Some stimulus properties of inhalants:
preliminary findings. Neurobehav. Toxicol., 3: 447-480.
VINAR, O., FRANTIK, E., & HORVATH, M. (1981) Subjectively-perceived
effects of diazepam, toluene, and their combination. Act. Nerv.
super. (Praha), 23: 179-182.
VORHEES, C.V. (1974) Some behavioral effects of maternal
hypervitaminosis A in rats. Teratology, 17: 269-273.
VORHEES, C.V. & BUTCHER, R.E. (1982) Behavioral teratogenicity. In:
Snell, L., ed. Developmental toxicology, London, Croom Helm Press,
pp. 247-298.
VORHEES, C.V., BRUNNER, R.L., MCDANIEL, C.R., & BUTCHER, R.E. (1978)
The relationship of gestational age to vitamin A induced postnatal
dysfunction. Teratology, 17: 271-276.
WALLMAN, J. (1975) A simple technique using an optomotor response for
visual psychophysical measurements in animals. Vision Res., 15: 3-8.
WALSH, J.M., CURLEY, M.D., BURCH, L.S., & KURLANSIK, L. (1982) The
behavioral toxicity of a tributyltin ester in the rat. Neurobehav.
Toxicol. Teratol., 4: 241-246.
WALSH, R.N. (1980) On the necessity for a shift in emphasis from
means-oriented to problems-oriented research in developmental
psychobiology. Dev. Psychobiol., 13: 229-231.
WALSH, T.J., MILLER, D.B., & DYER, R.S. (1982b) Trimethyltin, a
selective limbic system neurotoxicant, impairs radial-arm maze
performance. Neurobehav. Toxicol. Teratol., 4: 177-184.
WALTON, J.N. (1974) Disorders of voluntary muscle, 3rd ed.,
Edinburg, London, Churchill Livingston.
WANG, G.H. (1932) Relation between spontaneous activity and oestrous
cycle in the white rat. Comp. Psychol. Monogr., 6: 1-32.
WEIBEL, E.R. (1979) Stereological methods. In: Practical methods for
biological morphometry, New York, Academic Press, Vol. 1.
WEISS, B., FERRIN, J., MERIGAN, M., & COX, C. (1981) Modification of
rat operant behaviour by ozone exposure. Toxicol. appl. Pharmacol.,
58: 244-251.
WEISS, B. & LATIES, V. (1961) Changes in pain tolerance and other
behavior produced by salicylates. J. Pharmacol. exp. Ther.,
131: 120-129.
WEISS, B. & LATIES, V. (1969) Behavioral pharmacology and toxicology.
Ann. Rev. Pharmacol., 9: 297-326.
WEISS, B. & LATIES, V.G., ed. (1975) Behavioral toxicology, New
York, Plenum Press, 469 pp.
WEISS, B. & LATIES, V.G. (1979) Assays fox behavioural toxicity: a
strategy for the Environmental Protection Agency. Neurobehav.
Toxicol., 1(Suppl. 1): 213-215.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, J., CONNEY, A.H.
(1971) Effect of halogenated hydrocarbon insecticides on the
metabolism and uterotropic action of estrogens in rats and mice.
Toxicol. appl. Pharmacol., 19: 234-246.
WELLS, G.A.H., HOWELL, J.M., & GOPINATH, C. (1976) Experimental lead
encephalopathy of calves: histological observations on the nature and
distribution of the lesions. Neuropathol. appl. Neurobiol.,
2: 175-190.
WHEATLEY, B., BARBEAU, A., CLARKSON, T.W., & LAPHAM, L.W. (1979)
Methylmercury poisoning in Canadian indians -- the elusive diagnosis.
Can. J. neurol. Sci., 6: 417-422.
WHO (1975) Methods used in the USSR for establishing biologically
safe levels of toxic substances, Geneva, World Health Organization,
171 pp.
WHO (1978) Principles and methods of evaluating the toxicity of
chemicals. Part 1, Geneva, World Health Organization (Environmental
Health Criteria Series No. 6, Part 1).
WHO (1984) Principles for evaluating health risks to progeny
associated with exposure to chemicals during pregnancy, Geneva,
World Health Organization (Environmental Health Criteria Series
No. 30).
WHO (in preparation) Principles and methods of evaluating the
toxicity of chemicals. Part 2, Geneva, World Health Organization
(Environmental Health Criteria Series No. 6, Part 2).
WILLIAMS, P.L. & WARWICK, R. (1975) Functional neuroanatomy of man,
Philadelphia, Pennsylvania, W.B. Saunders Company.
WILSON, W.E. (1982) Dopamine-sensitive adenylate cyclase inactivation
by organolead compounds. Neurotoxicology, 3: 100-107.
WINNEKE, G. (1979) Modification of visual evoked potentials in rats
after long-term blood lead elevation. Act. Nerv. super. (Praha),
21: 282-284.
WINNEKE, G., BROCKHAUS, A., & BALTISSEN, R. (1977) Neurobehavioral and
systematic effects of longterm blood lead-elevation in rats. I.
Discrimination learning and open field behavior. Arch. Toxicol.,
37: 247-263.
WINNEKE, G., FODOR, G., & SCHLIPKOTER, H. (1978) Carbon monoxide,
trichloroethylene, and alcohol: reliability and validity of
neurobehavioural effects. Multidisciplinary perspectives in event-
related brain potentials. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency (EPA 600/9-77-0443).
WINNEKE, G., LILIENTHAL, H., & ZIMMERMANN, U. (1984) Neurobehavioural
effects of lead and cadmium. In: Proceedings of the Third
International Congress on Toxicology, San Diego, 28 August-2
September 1983, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
WOLF, D.L. (1976) Rotating rod, spontaneous activity, and passive
avoidance conditioning: their suitability as functional tests in
industrial toxicology. In: Horvath, M. & Frantik, E., ed. Adverse
effects of environmental chemicals and psycho- tropic drugs,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 293-303.
WOOD, R.W. (1978) Stimulus properties of inhaled substances. Environ.
Health Perspect., 26: 69-76.
WOOD, R.W. (1979) Reinforcing properties of inhaled substances.
Neurobehav. Toxicol., 1(Suppl. 1): 67-72.
WOOD, R.W. (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav. Toxicol. Teratol., 3: 397-405.
WOOLLEY, D.E. (1977) Electrophysiological techniques in
neurotoxicology. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 9-1 -- 9-25 (EPA
600/9-77-042).
XINTARAS, C. & BURG, J.R. (1981) Neurotoxicity studies of workers
exposed to carbon disulfide. G. Ital. Med. Lav., 3: 83-86.
XINTARAS, C., JOHNSON, B.L., & DEGROOT, I., ed. (1974) Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, 507 pp (US DHEW (NIOSH) Publication No. 74-126).
YAMAMURA, H.I., ENNA, S.J., & KUHAR, M.J. (1981) Neurotransmitter
receptor binding, New York, Raven Press.
YONESAWA, T., BORNSTEIN, M.B., & PETERSON, E.R. (1980) Organotypic
cultures of nerve tissue as a model for neurotoxicity investigation
and screening. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental
and clinical neurotoxicology, Baltimore, Maryland, Williams and
Wilkins Company, pp. 788-802.
YOUNG, J.S. & FECHTER, L.D. (1983) Reflex inhibition procedures for
animal audiometry: a technique for assessing ototoxicity. J. Acoust.
Soc. Am., 73: 1686-1693.
ZAKUSOV, V.V. (1936) [Estimation of the reflex time under the
influence of some narcotic substances.] Fiziol. Zh. USSR,
23: 276-278 (in Russian).
ZAKUSOV, V.V. (1953) [Pharmacology of the nervous system,]
Leningrad, Medgiz, 258 pp (in Russian).
ZBINDEN, G. (1981) Experimental methods in behavioral teratology.
Arch. Toxicol., 48: 69-88.
ZEMAN, W. & INNES, J.R.M. (1963) Craigie's neuroanatomy of the rat,
New York, Academic Press.
ZENICK, H., RODRIQUEZ, W., WARD, J., & ELKINGTON, B. (1979) Deficits
in fixed-interval performance following prenatal and postnatal lead
exposure. Dev. Psychobiol., 12(5): 509-514.
ZILOV, V.G., ROGACHEVA, S.K., & IVANOVA, L.I. (1983)
[Electroencephalographic analysis of limbic-reticular interrelations
in various motivational reactions of rabbits under ethanol.] Bull.
exp. Biol. Med., 11: 74-77 (in Russian).
ZUBER, V.L. (1978) Intensity of metabolism of various fractions of
cerebral phospholipids under hyperphenyl-alaninaemia. Vop. med.
Xhim., 2: 163-166.
ZYLBER-HARAN, E.A., GERSHMAN, H., ROSENMANN, E., & SPITZ, I.M. (1982)
Gonadotrophin, testosterone, and prolactin interrelationships in
cadmium-treated rats. J. Endocrinol., 92: 123-130.
nerve cells is involved, is more likely to be associated with
permanent behavioural changes than disorders predominantly affecting
the peripheral nervous system, where satisfactory regeneration is more
often the rule. Rapidly reversible effects on behaviour are
occasionally, but not often, associated with detectable structural
damage to the nervous system.
5.3.2 Gross morphology
While most neurotoxic damage is most evident at the microscopic
level, it is important to pay attention to the weight of the brain as
well as to macroscopic manifestations such as discolouration (for
example, due to bilirubin), discrete or massive haemorrhage, or other
localized lesions (for example, transverse myelitis).
The new methods of nuclear magnetic resonance (NMR) can supply
data concerning structural changes, for example, demyelination, both
in vivo and post-mortem.
5.3.3 The role of histology
There is no substitute for a thorough light-microscope examination
of the nervous system and other organs for the initial assessment of
animals suspected of having neurotoxic injury. The same careful
attention to anatomical detail should be given in the examination of
experimental animal tissues as would be given by a pathologist
studying autopsy tissue from a patient who has suffered from a new
type of neurological disease. The results of this type of analysis
will provide direction for additional studies that focus on more
specific questions such as the type and extent of structural damage,
the location where changes first occurred, the time-to-onset of
structural damage, the reversibility of these changes, the lowest dose
that caused pathological changes, and the highest no-observed-adverse-
effect dose.
5.3.3.1 Biological principles dictating tissue response
The study design must take into account several important
principles of underlying cellular responses to injury. These
principles will influence when and where tissue should be sampled for
morphological study.
Often, there is a delay of days or weeks between dosing and the
first appearance of structural changes in animals treated with
neurotoxic agents. The extent of the delay depends largely on the
chemical used, the dose, and the species studied. Once the
pathological process has been initiated, a chain of degenerative and
regenerative events may be set in motion, and these may or may not
cease to evolve after dosing has stopped. Most neurotoxic substances
that damage neurocellular elements produce a largely symmetrical
pattern of structural change, whether in the CNS or PNS. Some neurons
or nerve fibres may be more vulnerable than others to chemical attack,
so that at any point in the pathological process, the observer may be
presented with many or all of the stages in the reaction of individual
cellular elements. The active pathological process may also move in
space with time, as in dying-back axonpathies in which degeneration
proceeds retrogradely along affected nerve and fibre tracts (Cavanagh,
1964). Careless sampling of animals with these or other types of
neurotoxic diseases may reveal little or no pathological changes. By
contrast, the careful and thorough investigator will be able to
distinguish between early and later changes, and thereby develop a
hypothesis of the spatial-temporal sequence of the pathological
process. This can be tested in subsequent studies in which animals are
examined at different stages in the development of the disease.
Investigations of this type will also provide information on the
relationship between the dose and duration of treatment required to
induce neurotoxic injury. No-observed-adverse-effect levels can be
established by determining doses that do not produce any
characteristic changes after a specific period of chemical treatment.
Quantitative estimation of structural abnormalities in treated versus
control animals becomes increasingly important as the no-observed-
adverse-effect dose is approached.
5.3.4 Use of controls
Parallel study of age-matched control animals is mandatory,
whenever treated animals are subjected to neuropathological
examination. Control groups should include untreated animals, vehicle-
treated animals, and positive controls. The may receive a neurotoxic
dose of the agent under study or of another compound with well-
characterized neurotoxic properties that elicits a comparable type of
neurotoxic damage. Demonstrating the development of an appropriate
neurotoxic response in the positive control strengthens the validity
of data obtained from animals treated with the agent under study,
especially if these prove to lack pathological changes. The use of
positive controls is imperative for the inexperienced investigator who
needs to gain confidence from the self-demonstration that a known
neurotoxic agent produces the expected changes. Inclusion of these
results in a published report will also reassure the audience that the
investigator has reproduced the expected pattern of structural damage
associated with a known neurotoxic chemical.
There are important advantages in studying the structure of
nervous tissue without knowledge of the treatment group. The
investigator is forced to be more critical in seeking and describing
abnormal findings, and the final assessment is a more objective
statement of the similarities and differences between treatment and
control groups.
5.3.5 Pattern of response
It is not the purpose of this publication to provide a detailed
analysis of the various problems of selective neuronal damage
encountered under neurotoxic conditions. However, it is emphasised
that occasionally the localization is very precise, limited to one or
two nuclei in the brain stem, or to distal regions of long fibre
tracts and peripheral nerves. Selective pathological changes are given
in Table 3 for reference in interpreting new situations.
5.3.6 Data acquired
At the end of the survey, clear answers must be found to the
following questions about the CNS:
1. Are there changes in nerve cells in the area of the cerebral
cortex, basal ganglia, hypothalamus, limbic system, diencephalon,
brain stem, cerebellum, or spinal cord?
2. Are there any changes in shape or number of astroglia and/or
microglial cells?
3. Is there any loss or other change in oligodendroglia?
4. Are there any changes in the myelinated areas of the brain or
spinal cord, such as myelin fragmentation or myelin vacuolation?
Is this associated with axonal swellings and/or degeneration?
5. If 4 is positive, are there glial responses?
6. Are there focal, vascular, and necrotic changes? If so, where?
7. Are tumours present?
8. Are there any changes in special sense organs to account for
functional changes?
For the peripheral nerves, the following questions must be
answered:
1. Are there any changes in peripheral nerves?
2. If so, is it axon degeneration or primary demyelination, or both?
3. What is the distribution of degeneration? Is it distal, affecting
only long and large-diameter fibres, or are shorter fibres such as
cranial nerves also affected?
4. Are sensory, motor, or both types of fibres involved?
Table 3. Examples of toxic effects illustrating the specific
patterns of damage that may be found
Pattern Cause Reference
Neuronal changes
laminar cortical necrosis anoxia Briefly (1976)
hippocampal pyramidal cell trimethylin Brown et al.
damage (1979)
selective granule cell loss methylmercury Hunter & Russell
from the cerebellum (1954)
selective loss of inferior 3-acetylpyridine Desclin (1974)
olivary cells
selective degeneration of doxorubicin Cho et al. (1980)
perikarya of sensory
ganglion cells
selective degeneration of methylmercury Cavanagh & Chen
axons of sensory ganglion (1971)
cells
retrograde degeneration of organophosphorus, Cavanagh (1964);
long sensory and motor acrylamide, Spencer &
axons in CNS and PNS hexanedione Schaumburg (1977)
retrograde degenerations of clioquinol Krinke et al. (1979)
axons of long spinal cord
tracts
Myelin changes
CNS myelin vacuolation triethyltin Aleu et al. (1963)
CNS and PNS myelin hexachlorphene Towfighi et al.
vacuolatin (1975)
focal degeneration of PNS diphtheria Cavanagh & Jacobs
myelin without axon loss (1954)
focal degeneration of PNS lead Fullerton (1966)
myelin with some axon loss
Table 3 (Cont'd)
Pattern Cause Reference
Vascular and neurotic changes
symmetrical lesions in misonidazole Griffin et al.
brain-stem nuclei (1980)
non-symmetrical brain-stem lead Wells et al. (1976)
and cortical lesions
5. What is the state of nerve cell bodies (i.e., sensory ganglion
cells, anterior horn cells)?
6. If the cells in 5 show changes, are those in the medulla and
trigeminal ganglia (i.e., cranial nerves) similarly affected?
7. Are neurons normal, chromatolytic, or degenerated?
8. Are automatic ganglia or nerves similarly affected or not?
9. Are muscles affected by denervation and/or myopathic changes?
10. At dose levels below those that produce clinical signs, is there
histochemical and/or morphometric evidence of metabolic neuronal
changes that is brought out by using these more sensitive
techniques?
5.4. Principles, Limitations, and Pitfalls of the Morphological
Approach
5.4.1 Tissue state
Although under special circumstances the morphologist is able to
study the reactions of living tissue to chemical exposure, either
in vivo or in tissue culture (Yonesawa et al., 1980), the vast
majority of pathological studies involve the assessment of dead
tissue. The structural changes that occur in the nervous system as a
function of time post-mortem can obscure the recognition of toxic
injury and may confuse the inexperienced neuropathologist. Chemical,
or rarely, physical (freezing) fixation methods are used to prevent
the development of post-mortem changes.
5.4.2 Principles of fixation
The use of a reliable fixation method is mandatory for the
morphological examination of nervous tissue (Thompson, 1963; Pease,
1964; Hayat, 1973; Gabe, 1976; Bancroft & Stevens, 1977; Glausert,
1980). The purpose of fixation is to preserve cellular architecture as
closely as possible to that in the living state, to inhibit the loss
of chemical components required for the maintenance of morphology, and
to prepare the tissue to accept stains that enhance tissue density for
clear resolution of cytological detail. The ideal method of fixation
is one that instantaneously terminates life processes in the tissue of
interest without distorting cytological detail. Rapid freezing of
living tissue approaches this ideal. Excision and freezing of tissue
is routinely employed for the assessment of human biopsy tissue and is
particularly suitable for light-microscope histochemistry and
immunocytochemistry. A few specialized laboratories use rapid freezing
for the examination of tissue, or metal replicas thereof, by
transmission electron microscopy.
Chemical methods are far more commonly used in neurotoxicology for
the preparation of nervous tissue. Although the ideal chemical
fixative should instantaneously kill living tissue without inducing
structural changes, all known methods fall short of this ideal.
Instead, the morphologist must strive to induce small, consistent and
readily recognizable changes that will not compromise tissue
examination and assessment. The most satisfactory and widely used
chemical fixatives are dilute, aqueous solutions of aldehydes
(formaldehyde and/or glutaraldehyde), which rapidly cross-link
proteins and stabilize associated lipids, prevent post-mortem changes,
and introduce rigidity into the tissue to facilitate handling. A
secondary phase of fixation using a dilute aqueous solution of osmium
tetroxide to fix lipids is routinely added after glutaraldehyde
fixation for improved preservation of tissue. Occasionally, osmium
tetroxide is used as the primary fixative. Structural artefacts
induced by fixation are minimized if the fixative solutions are
buffered to match tissue pH and osmolality. Temperature should also be
controlled; low temperatures may affect the preservation of fine
structural elements (e.g., microtubles).
Chemical fixatives must be delivered to the site of interest as
rapidly as possible if post-mortem artefacts are to be minimized. Most
effective is systemic perfusion (Pease, 1964; Hayat, 1973; Spencer et
al., 1980; Spencer & Bischoff, 1982), in which the fixative is
introduced via the ascending aorta of the deeply anaesthetized animal,
and distributed throughout the vascular network under controlled
pressure. Spaces normally occupied by blood and tissue fluid are
rapidly replaced by the chosen fixative solution, and the animal
expires painlessly within seconds. Perfusion of individual organs is
an alternative, but more difficult and less satisfactory technique. An
alternative method, suitable only for readily accessible parts of the
nervous system (e.g., peripheral nerves), is to bathe the tissue
in situ with a repeatedly replenished solution of fixative.
In practical terms, the requirements for tissue sampling, organ
weighing, etc., have to be balanced against the need to get good
fixation, and this frequently presents conflicts of interest between
those involved in analysing the animal tissues. Short perfusion
fixation (section 5.5.2), with paraformaldehyde and subsequent
secondary fixation in formalin or formal-acetic acid in the case of
paraffin sections and light microscopy, and glutaraldehyde and osmium
tetroxide in the case of epoxy resin, semi-thin sections, may help to
provide a compromise. When the protocol for killing requires only
immersion fixation, then a few animals in each group must be kept for
perfusion fixation, if adequate tissue examination is going to be
achieved. A study using only immersion fixation is scarcely worth the
labour and cost involved.
The selection of the chemical fixative and its method of delivery
to the tissue site are chosen according to the type of information
that is required. Systemic perfusion with the chosen fixative is
always to be preferred. In general, formaldehyde is suitable for
studies that require widespread tissue sampling, low resolution of
cytological detail, or the use of special histochemical stains to
assess the chemical content of abnormal cells and tissues. Conversely,
in studies focusing on more selected areas of the nervous system or
requiring resolution of fine structural detail, the use of
glutaraldehyde and osmium tetroxide is mandatory. While the latter
method was originally introduced for use in transmission electron
microscopy, an increasing number of laboratories use the technique
routinely to exploit the remarkable resolution afforded by the light
microscope.
5.4.3 Principles of tissue sampling
Extensive sampling of tissue is essential for the initial
assessment of a suspected neurotoxic injury. Organs, other than the
nervous system, which should be examined, are listed in WHO (1978).
Neural tissue should include: brain, including cerebellum, brain stem,
pituitary gland, eye with occulomotor muscles and optic nerve
attached, spinal cord, several sensory ganglia, sciatic nerve and its
branches from its exit from the vertebral column to the level of the
ankle, and selected muscles innervated by the sciatic nerve and its
branches. Other regions less commonly sampled include: olfactory
epithelium and tubercles, inner ear and labyrinths, plantar nerves and
skin receptors, autonomic ganglia, and nerves and organs of
innervation, such as the gut. The brain contains many sites known to
be vulnerable to chemical agents and should be examined as thoroughly
as possible. The use of an atlas such as that for the rat brain (Zeman
& Innes, 1963) will be valuable. The brain stem should be sectioned
just rostral to the cerebellum, the slice passing through the inferior
colliculi. The cerebral hemispheres can then be sliced coronally
(transversely). Olfactory lobes may be taken if questions of
inhalation and olfaction are being studied. The cerebellum is removed
by cutting through the peduncles that attach it to the brain stem.
Sagittal sections are obtained by cutting through the midline, and
parallel sections are also taken. Emphasis is usually placed on the
retina and optic nerves, since these are often heavily involved in
toxic diseases affecting neurons, axons, and myelin. Ototoxicity is
also a common event, though the technical difficulties associated with
the examination of the inner ear and labyrinths have prevented
widespread study of this phenomenon. Olfactory lobes and associated
epithelium are rarely examined, but should be taken if questions of
inhalation and olfaction are being studied.
The spinal cord contains ascending and descending nerve fibre
tracts that commonly undergo changes in myelinopathies and
axonopathies (Table 3). Since the latter are tractable diseases with
distal accentuation, it is critical to sample various levels from the
medulla oblongata (where the gracile tract terminates) to the sacral
region. By taking transverse sections of the spinal cord, it is
possible to make a simultaneous evaluation of white matter (myelinated
tracts and glial cells), gray matter (neurons, dendrites, proximal
axons, glial cells), blood vessels, and associated tissues.
Dorsal root and cranial nerve ganglia contain primary sensory
neurons that may display pathological changes in certain
neuronopathies and axonopathies (Figs. 3, 4). Structural alterations
in blood vessels and myelin may also occur under certain conditions.
The sensory ganglia of the Vth cranial nerve, at least 4 sensory
ganglia from the cervical bulb region (C5-T1 spinal levels), and 4
from the lumbosacral regions (L3-S2 spinal levels), should be sampled
for examination in longitudinal section. Corresponding dorsal and
ventral spinal roots, vulnerable in toxic demyelinating diseases,
should also be studied.
Peripheral nerves commonly display changes in neurotoxic diseases
affecting myelin, neurons, and axons (Table 3). As the last display
distal, retrograde changes in dying-back axonopathies, a common
response to chemical attack, it is critical to examine several levels
of vulnerable nerves. Samples of the sciatic nerve and its branches
are usually taken commencing adjacent to the vertebral column and
terminating in the distal regions of the sural nerve, peroneal nerve,
and/or the tibial nerve. The fine branches of the tibial nerve that
leave the main trunk below the knee are especially vulnerable in toxic
neuropathies, because they contain very large myelinated nerve fibres.
Terminal regions of sensory and motor nerve fibres can be examined by
searching for twigs supplying intrafusal (spindles) and extrafusal
muscle fibres, respectively (Schaumburg et al., 1974). Routinely,
anterior tibial muscles, and gastronemius with suralis muscles are
examined. Muscle tissue is also valuable for assessing whether there
is denervation atrophy, evidence of toxic myopathy, nerve sprouting,
or other regenerative activity (Pearson & Mostofi, 1973).
Tissue sampling should not only be tailored to the goal of the
study but also to the method of fixation. In general, blocks of tissue
several millimeters thick may be taken if the tissue has been fixed
with formaldehyde and is destined for paraffin embedding. For tissue
fixed with glutaraldehyde, the relatively slow rate of penetration of
osmium tetroxide necessitates the use of thin (1 µm) slices of tissue.
It is often helpful to mark a small area of tissue (with Indian ink or
a small cut) to maintain the orientation. Elongate structures, such as
peripheral nerves and spinal cord, are most readily studied in
transverse sections, though important information is acquired by
complementary examination of longitudinal sections. Particularly
informative for the identification of pathological changes in
peripheral nerves is the technique of microdissection (teasing apart
intrafascicular tissue), which yields long lengths of individual
myelinated nerve fibres suitable for light microscope examination.
Inspection of these preparations can demonstrate rapidly the presence
of primary demyelination and remyelination and axonal degeneration and
regeneration. The minor changes characteristic of early toxic
neuropathies, readily missed in the examination of sections, can be
easily detected by this method. Other parts of the nervous system are
technically difficult to examine by microdissection because of the
absence of a collagen network to support the individual nerve fibres.
While it is prudent to embed tissues in paraffin wax or epoxy
resin promptly, regions that will not be examined immediately can be
stored in a solution and at a temperature appropriate to the method of
fixation. Loss of tissue quality will occur in proportion to the
length of storage.
5.4.4 Preparation of tissue for examination
While gross tissue changes can be visualized with the naked eye or
by examination with a stereoscopic binocular microscope, and surface
detail can be resolved with the scanning electron microscopy, routine
examination of cellular structure requires the use of tissue sections.
This inevitably places severe limitations on the amount of tissue that
is practical to study. Sections must be such that the energy beam
(light, electrons, X-rays) of the chosen microscope can be transmitted
through the tissue. Relatively thick sections containing several
layers of cells can be visualized with the light microscope, but the
ability to resolve cytological detail decreases as section thickness
increases. The use of semi-thin sections allows the investigator to
exploit the maximum available resolution of the light microscope. Much
thinner sections are required for the assessment of tissue by
transmission electron microscopy (Pease, 1964; Hayat, 1973).
Chemically-fixed tissue must be supported by a pliable material to
facilitate the sectioning process and to prevent disintegration of the
sectioned tissue. Paraffin wax or epoxy resin are routinely used: the
former for routine work, the latter for more specialized studies.
Since both types of embedding media are water insoluble and therefore
immiscible with the fixative solutions, water is first removed by
stepwise dehydration in ethanol or methanol immersing the tissue in an
aromatic hydrocarbon (clearing solution) that is miscible both with
ethanol and the embedding medium. Dehydration, clearing, and
infiltration with an embedding medium are procedures requiring
considerable care and time to avoid tissue drying and distortion.
Automatic systems are available and, for paraffin embedding, these are
routinely used in histological laboratories. Once the tissue is
infiltrated and embedded, the medium must be allowed to harden by
cooling (paraffin wax) or heating (epoxy resin).
Sections of tissue are prepared with the aid of knives mounted in
microtomes. Steel knives are suitable to cut thick (4-15 µm) sections
of paraffin embedded tissues, whereas knives prepared by scoring and
breaking strips of special glass are needed to prepare sections (1 µm)
of tissue embedded in the harder epoxy resin. The sections of tissue
prepared with steel knives permits study of larger tissue areas than
is possible with sections prepared from epoxy blocks, though the
latter afford a higher resolution of cytological detail. With new
developments in the preparation of glass knives and the design of
microtomes, ever larger sections can be routinely prepared by the
latter technique. Diamond knives mounted in ultramicrotomes are used
to obtain the thin sections (50 nm) required for transmission electron
microscopy.
Contrast of cytological detail in sectioned material can be
enhanced by the use of special light-microscope methods (phase,
Nomarski, fluorescence) or, more commonly, by the introduction of
stains that render structures light-dense. A wealth of cytological
stains is available for use with paraffin sections, whereas only a few
general purpose stains are usually used to enhance structural detail
for the light-microscope assessment of epoxy sections. Heavy metal
stains that are electron dense are needed to visualize fine structure
by transmission electron microscopy.
5.4.5 Recognition of artefact
Some degree of artefact is unavoidable, irrespective of the care
taken in preparing tissue for morphological examination, and
recognition and identification of the sources of artefact are of the
utmost importance in the morphological assessment. Possible artefacts
are legion and stem from various sources: e.g., poor tissue
preservation from failure of fixative to penetrate tissue; shrinkage
induced by inappropriate dehydration or drying; traumatic changes
associated with excision of the tissue (bending, stretching) or rough
handling during processing; wrinkling of sections and precipitation of
stain (Spencer & Bischoff, 1982).
5.4.6 Recognition of normal structural variations
While the gross and fine structure of the nervous system of any
species and age is remarkably constant from animal to animal, it is
critical to recognize the irregular occurrence of normal variations in
cellular structure that may lead the inexperienced observer to an
inappropriate conclusion. These variations include ectopic structures
(e.g., sensory neurons misplaced in the sciatic nerve), local
oedematous or proliferative changes (e.g., at a site of unrecognized
trauma or infection), and those that accompany advancing age (Johnson,
1981). The latter are especially important in long-term neurotoxicity
studies, and there is no substitute for rigorous comparative study of
control animals of the same age. Examples of normal age-related
changes include neuronal pigmentation by lipofuscin, localized
demyelination and remyelination, scattered neuronal loss, and axonal
dystrophy and degeneration.
5.4.7 Qualitative versus quantitative approaches
While there can be no substitute for a thorough qualitative
assessment of the morphological state of the nervous system,
morphometric methods are required for a more precise description of
these changes (Weibel, 1979; Bonashevskaya et al., 1983). This is
especially important for the description of minor changes, or when the
tissue reactions of different groups of animals are being compared.
Randomization of samples is essential to minimize subjective
influences, and a statistical approach is required both for the design
of the morphometric study and the analysis of the data. Tissue
sampling, group size, age, sex, body weight, and length are important
considerations in this regard. This type of morphometric approach has
been helpful in measuring tissue reactions to toxic chemicals in the
form of: the numbers of fibres affected in specific pathways subject
to degeneration, changes in cell populations in vulnerable parts of
the brain, and the relationship between fibre diameter and intermodal
length of peripheral myelinated nerve fibres prepared by
microdissection (Dyck et al., 1984).
5.5 Specific Procedures
5.5.1 Introduction
Strategic considerations in planning how to proceed must depend on
the state of knowledge of the possible effects. When it is unknown
whether damage occurs in nervous tissue, a wide-ranging examimation of
the nervous system with the light microscope is appropriate. Most
structural changes induced by neurotoxic chemicals can be detected
using the primary methods given below. More specialized, secondary
methods can be applied subsequently for more detailed studies.
5.5.2 Primary methods
There are strong convictions among experienced experimental
neuropathologists as to which fixation method should be chosen for the
primary screening. Some believe the conventional approach using
formaldehyde-based fixatives and paraffin embedding is most
appropriate; others prefer the more contemporary method of examining
epoxy sections of tissue fixed in glutaraldehyde and osmium tetroxide.
Each method has significant advantages and disadvantages and both
complement each other. Since it is impractical to use both methods for
the same animal, a prudent investigator will include a sufficient
number of animals to permit the parallel use of both procedures.
5.5.2.1 Formaldehyde/paraffin method
(a) Tissue fixation and excision
This technique is most suited for the study of large areas of
tissue at low resolution and with special histochemical stains
(section 5.5.3.3). Tissue can be removed from the carcass and fixed by
immersion in formalin (10%) containing 2% acetic acid (added just
before use) to improve penetration and hardening. Making up the
solution in 80% alcohol rather than water to hasten penetration
further has sometimes been recommended, but this is unnecessary and
may make dissection more difficult.
Rapid and careful transfer of tissues from the carcass to the
fixative solution will retard artefactual cell distortion. In a
general post-mortem dissection, the brain, including the cerebellum
and brainstem, are removed and transferred to fixative, prior to the
removal of other tissues. Retrieval of the spinal cord from its spinal
column is difficult and time consuming; it is recommended that the
whole spinal column with cord be excised quickly, trimmed of as much
muscle as possible, and immersed in the fixative intact. Once this
portion is fixed, the spinal cord and ganglia can be separated from
the column with greater care. Alternatively, after approximately 24 h
of fixation, the spinal column can be immersed in 5% formic acid
decalcifying solution. The column can then be sliced with little
difficulty.
To assist with the examination of peripheral nerves, tissue is
placed on a stiff card before immersion to retain orientation.
Optimally, the nerve to be excised should be exposed and, prior to
removal from the carcass, bathed with fixative for approximately
5 min. This will begin to introduce rigidity into the tissue and
retard decay artefact. A length of nerve can then be excised, quickly
placed on a card and pinned at the tip of both cut ends to retain
position after immersion. Care should be taken to avoid overstretching
the nerve as it is pinned to the card. Samples of these tissues can
then be sectioned in a transverse or longitudinal direction. Muscle
can be treated in the same manner. Another technique is to take off
the limb at the hip or shoulder joint, remove the skin and fix en bloc
in formalacetic acid solution. The limb can then be decalcified in 5%
formic acid solution for approximately two weeks as noted earlier for
the spinal column. It can then either be embedded in paraffin wax as
one large block or further dissected.
Tissue preservation is greatly improved by perfusing animals
systemically for 10 min with phosphate-buffered paraformaldehyde
(section 5.5.2.2), a method that simultaneously preserves all body
organs. Tissue can then be sampled in a more leisurely manner without
fear of introducing post-mortem changes (section 5.5.2.2).
(b) Dehydration and embedding
Relatively large tissue samples can be embedded in paraffin wax,
and this is particularly suitable for localizing and identifying
lesions in the brain. Automated processing for paraffin embedding has
greatly diminished the time requirements for preparing tissue samples.
Most automated systems will conform to a variety of preparation
schedules and process great numbers of tissue samples at one time.
There are systems that operate under combined heat and vacuum, the
great advantage being rapid processing. This combination is
deleterious and may cause unnecessary shrinkage and brittleness, which
will distort cell structure and hinder interpretation. Paraffin
sections routinely cut 4-6 µm thick, are suitable for certain
histochemical techniques and can easily be treated with special stain
to enhance particular cellular components.
(c) Section staining
Four staining methods are routinely used to enhance cellular
detail in paraffin sections.
Hematoxylin and eosin
Routinely used on paraffin sections, this method stains nuclei
blue to purple, cytoplasm and Nissl substance (and mast cell granules)
blue, and myelin sheaths pink. Nuclear density can be readily
assessed, as well as an increase or decrease in the size of nuclei and
the number of cells. Neuronal damage is readily detectable. Nuclei and
cytoplasm of other cell types, such as microgila and vascular
endothelial cells, are also well defined, though cresyl violet may be
preferred.
Cresyl fast violet
This method enhances identification of cell-population changes and
is more aesthetically pleasing than other basic aniline dyes, such as
thionine or gallocyanine-chrome alum. DNA stains pale blue and RNA
purple-blue. Hypertrophy of astroglial cytoplasm and changes in
neuronal nuclei and cytoplasm are readily observed. Chromatolysis,
poorly demonstrated in hematoxylin- and eosin-stained sections, is
more apparent when stained with cresyl violet, as are most cells in
peripheral nerves.
Glees and Marsland's stain
This method is easy to carry-out and selectively stains
neurofibrillary components in axons (Marsland et al., 1954).
Longitudinal sections demonstrate the state of terminal innervation in
motor and sensory fibres. Empty endplates are often recognized by the
observation of remaining clumps of nuclei on the muscle surface.
Sudan Black B
This method is useful to reveal nerve terminals in muscles
(Cavanagh et al., 1964).
5.5.2.2 Glutaraldehyde/epoxy method
(a) Tissue fixation and excision
This technique is best suited for the resolution of early
pathological changes with the light microscope and is required for
transmission electron microscopy (section 5.5.3.5). Whole body
perfusion with buffered fixators (paraformaldehyde) followed by
glutaraldehyde) delivered under pressure (100-150 mm Hg) is the only
satisfactory method to prepare brain and spinal cord sections, and
this method has the advantage of optimally fixing all other organs
simultaneously. For this purpose, the animal is deeply anaesthetized
with a solution (e.g., sodium pentobarbital) containing a small
percentage of heparin to facilitate circulation. Subsequent procedures
should be performed rapidly and smoothly to prevent the development of
anoxic damage and to ensure global fixation. The animal is secured on
its back and the rib cage cut bilaterally and reflected backwards to
expose the heart. After slitting the pericardium, the right atrium is
opened to drain circulating blood. The ventricular apex is then
excised to provide access to the aorta, and a cannula spurting a
column of fixative is inserted through the ventricular opening to the
apex of the aorta and clamped in place. The circulatory system is
cleared by a brief, initial perfusion with paraformaldehyde or saline,
and followed by more prolonged (e.g., 10-15 min) perfusion with
glutaraldehyde.
Rigid tissues from a fixed carcass are less susceptible to
handling artefact. The nervous system can be sampled in any order. In
some laboratories, the entire nervous system, including brain with
optic tract and nerves, spinal cord, spinal roots, ganglia, and
peripheral nerves are exposed in the carcass, then carefully detached
intact and removed. When this is accomplished, there is no need
initially to mark tissues for orientation. It is imperative to avoid
tissue drying during dissection and excision. Exposed tissues should
be bathed with fixative and tissues should be manipulated as little as
possible with forceps.
(b) Dehydration and embedding
After glutaraldehyde fixation, tissue samples are post-fixed for
2-3 h by immersion in buffered 1-2% osmium tetroxide in the cold. The
samples are then dehydrated stepwise in ascending concentrations of
ethanol, immersed in acetone or toluene, infiltrated with a solution
of epoxy resin and finally, embedded and polymerized in epoxy resin.
For light-microscope examination, 1 µm sections are cut with specially
prepared glass knives mounted in an ultramicrotome (an instrument
designed specifically for the purpose of obtaining the thin sections
suitable for electron microscope examination). New techniques and
equipment have recently become available which allow larger tissue
samples to be prepared for sectioning in epoxy resin. Sections one
micrometer thick can be examined by phase-contrast optics or by bright
field after a brief staining with 1% toluidine blue.
(c) Section staining
Thick (1 µm) sections of tissue fixed in glutaraldehyde and osmium
tetroxide and stained with borate-buffered 1% toluidine blue allow
resolution of structures as small as a single mitochondrion. Brain and
spinal cord neurons display clearly defined nuclei, nucleoli, issl
substance, and lipofuscin. Dendrites stand out against a more darkly-
stained neuropil. Small, densely staining oligodendrocytes are readily
distinguished from large, pale astrocytes. Myelinated nerve fibres
contain a pale axon and darkly-stained myelin. In cross-sections of
peripheral nerves, large and small diameter myelinated fibres, as well
as the smallest unmyelinated axons, can be seen. Motor and sensory
nerve terminals are detectable in muscle.
5.5.3 Special methods
5.5.3.1 Peripheral nerve microdissection
Isolated fibre preparations provide information that may be missed
in cross-sections, and it is recommended that this procedure be
included in any study concerned with toxic neuropathy. Several
fixation methods are available to prepare peripheral nerves for
microdissection. Perfused tissue is ideal, though satisfactory
preparations can be obtained by bathing the tissue with fixative prior
to excision. Post-excision immersion in fixative can also be used,
though the act of transecting the nerve introduces major fibre
artefacts that are not restricted to the cut ends and are readily
mistaken for pathological changes.
Optimal preparations suitable for high-resolution light microscopy
require the use of glutaraldehyde, post-fixation with osmium
tetroxide, stepwise dehydration, and infiltration with a low-viscosity
epoxy resin. This is an ideal medium to tease apart nerve fibres and
is suitable for the storage of tissue at low temperature. Mounted
fibres can be polymerized by heat to affix them to a glass slide and
prevent squashing by a coverslip (Spencer & Thomas, 1970). Bright-
field examination will reveal changes in overall structure, and
Nomarski differential interference microscopy can be used to study the
axon.
Older techniques yield poorer preservation, low resolution of
detail, and isolated fibres are susceptible to squashing, an important
consideration if morphometric study is planned. One method involves
formalin fixation, post-fixation in osmium tetroxide, and infiltration
with a dilute solution of glycerol, which is used to support the
fibres during microdissection. Single fibres are mounted on the
microscope slide, cleared with cresol, blotted dry, and mounted with
pure glycerin (Thomas, 1970). Sudan Black B can also be used to stain
tissue prior to microdissection. Nerves are microdissected with the
aid of a pair of mounted needles and stereoscopic dissecting
microscope. Epineurial connective tissue is removed and a small
fascicle selected. After splitting the perineurium surrounding the
fascicle, the sleeve is removed to expose the intrafascicular tissue
containing the nerve fibres. The tissue is then repeatedly split into
progressively smaller longitudinal bundles until individual fibres are
obtained. These are picked up on the end of sharpened wooden
applicator sticks and transferred to a clean glass slide and provided
with a coverslip. Alternatively, a rapid "squash" preparation can be
obtained by teasing a small bundle of fibres loosely apart to create a
mesh formation on a microscope slide; a coverslip is then added for
microscope examination.
5.5.3.2 Frozen sections
Frozen sections lend themselves well to enzyme histochemical,
immunocytochemical, and silver-impregnation procedures, but for the
purposes of a large-scale study, this method is impractical. Tissues
fixed instantaneously by immersion in liquid nitrogen are particularly
vulnerable to cell distortion and/or destruction caused by excessive
temperature changes. Encasing tissue samples in gelatin or albumin is
advisable, especially for brain tissues (Crane & Goldman, 1979).
Ice-crystal formation is a disruptive artefact that produces a
vacuolated appearance in frozen sections. To minimize crystal
formation, it is important to freeze tissues as rapidly as possible
and maintain critical temperatures throughout the cutting procedure to
avoid thawing and refreezing. Typically, frozen sections are between
10 and 15 µm thick.
5.5.3.3 Histochemical methods
Using these procedures, it is possible to assess the activity of
cell energy systems, the rate of use of substrates for processes of
oxidative phosphorylation, and the functional state of intracellular
organelles. The activities of succinate dehydrogenase and malate
dehydrogenase, enzymes of the Krebs cycle, are well known, the former
being closely linked with mitochondrial membranes. Enzymes of the
hexose monophosphate shunt (glucose-6-phosphate dehyrogenase and
gluconate-6-phosphate dehydrogenase) and those of the electron
transport chain (NAD and NADP disphorase) should also be studied. The
rate of exchange of nitrogenous bases can be examined by the
determination of activity of dihydroorotate dehydrogenase for fatty
acids; monoamine oxidase for biogenic amines; and acetylcholinesterase
for acetylcholine.
Histochemical enzyme assays are performed on freshly frozen
sections cut on a cryostat (Dubowitz & Brooks, 1973). Tissues are
quick-frozen in liquid nitrogen. Control and experimental tissues
should be mounted on one slide and reactions carried out using the
methods outlined in Pearse (1968).
The following methods can be used to demonstrate the functional
state of nerve cells (Pearse, 1968):
(a) RNA (Brachet's method with ribonuclease control);
(b) DNA (Feulgen's method with deoxyribonuclease control);
(c) Total nucleic acids (Einarsen's method);
(d) Total protein (sublimate with bromophenol blue);
(e) Sulfhydryl groups (Barnet and Zeligman's method);
(f) Glycogen and glycosaminoglycans (amylase control-PAS
reaction); and
(g) Lipids (Sudan III, Sudan Black, Nile Blue methods).
To express histochemical reactions quantitatively, the following
formula for Mean Histochemical Index (MHI) can be used:
MHI = 3a + 2b + 1c + 0d
N
where 3, 2, 1, and 0 are the degrees of intensity of colour (from 3
to 0), and a, b, c, and d are the numbers of the cells with the given
intensity of colour; the denominator N is the number of cells counted.
The results allow statistical calculation of the assays and estimation
of the reliability of the results.
5.5.3.4 Golgi method
This unique silver-impregnation method demonstrates the shape and
surface characteristics of entire neurons, including cell bodies and
their processes. Golgi preparations are particularly useful in the
study of dendritic arborizations and synaptic associations (Santini,
1975). However, the technique is capricious in that only a few neurons
are unpredictably demonstrated: many preparations must therefore be
examined to gather a representative sample.
In the Rapid Golgi method, tissue is fixed with formaldehyde or
glutaraldehyde and post-fixed with osmium tetroxide. The tissue is
then impregnated with silver nitrate, dehydrated, and infiltrated with
celloidin. Thick (120 µm) sections are prepared, mounted, and examined
by bright-field microscopy.
5.5.3.5 Transmission electron microscopy
This technique is laborious and requires extensive training.
However, it has resulted in extensive advances in the understanding of
cellular processes in neurotoxicity. There is little need for such
exacting methods to determine the presence of changes in cell
structure, few experimental protocols require electron microscopy. The
transmission electron microscope is properly used to confirm and study
further the nature of lesions already shown and mapped by light
microscopic methods. It is all too easy to seek, and find, changes to
which no significance will ultimately be attached. Until the pattern
of change induced by the chemical under study has been well identified
by light microscopy, electron microscopy should not be considered. On
rare occasions, such as in toxic disorders of unmyelinated axons,
examination by transmission electron microscopy is necessary to
localize cellular changes not revealed by the methods discussed
previously. Thin (50 nm) plastic sections are prepared with the aid of
a diamond knife mounted in an ultramicrotome and subsequently
impregnated with heavy metals for electron-microscope examination
(Pease, 1964; Hayat, 1973).
5.5.3.6 Other anatomical methods
There are numerous additional specialized anatomical methods that
are waiting to be applied in the study of neurotoxicological issues.
These include histological techniques to trace anatomical pathways
(with horseradish peroxidase) and cellular activity. Examples of the
latter are the use of autoradiography to localize the distribution of
radiolabelled precursors such as thymidine and 2-dexoyglucose
(Sokoloff et al., 1977). Other methods susceptible to light and
electron microscope analysis include immunocytochemical studies of
receptors, proteins, and other cell structures (Emson, 1983).
Fluorescence microscopy, particularly for the localization and
detection of regional concentrations of catecholamines using the
formaldehyde vapour or glyoxylic acid technique of Falck et al.
(1962), supplements quantitative biochemical methods in evaluating
alterations in catecholamine levels. Fluorescence microscopy is also
of value in antigen and antibody localization techniques. Certain
light and electron microscope techniques may be combined, such as the
examination of fine structure of single teased nerve fibres (Spencer &
Lieberman, 1971; Ochoa, 1972) or of Golgi-stained preparations.
Advanced methods in electron microscopy include scanning (for surface
features), energy-dispersive X-ray analysis (for detection of elements
of high relative atomic mass) and energy-loss (for detection of
elements of low atomic mass). Freeze-fracturing tissue can reveal
details of the internal surfaces of cellular membranes when replicas
are examined by transmission electron microscopy (Hayat, 1973).
5.6 Conclusions
The information derived from morphological studies is highly
relevant to the interpretation of biochemical, neurophysiological, and
behavioural data. Structural changes have always been the firm
foundation on which analysis of clinical neurological disease has been
based. Both diagnosis and prognosis depend heavily on previous
neuropathological experience for their accuracy. Moreover, treatment
of neurological disease can only be satisfactorily planned by using
the knowledge gained from experimental and morphological studies that
have provided an understanding of the pattern by which neural cells
are affected. Indeed, in the majority of human intoxications of the
nervous system, knowledge of the structural changes is based almost
exclusively on animal studies. There is no reason to believe that it
will be less so in the future.
6. BIOCHEMICAL AND NEUROENDOCRINOLOGICAL METHODS
6.1 Introduction
Biochemical tools are valuable for the study of neurotoxicity.
They have the potential for both identifying toxic compounds and
delineating mechanisms of action of known toxic substances. However,
the range of biochemical techniques is vast and careful decisions must
be made in devising an effective research strategy. The most important
decision is identification of the objective of the research, which may
be conveniently categorized into three groups: determining mechanisms
of action of neurotoxic substances, identifying exposed individuals,
and screening for toxic conditions.
Devising an effective biochemical screen is the most challenging
of the three objectives. It requires the selection of biochemical
parameters that are general enough to indicate toxicity resulting from
multiple sites of damage, but specific enough to prevent the
accumulation of false positives. No single biochemical parameter is
likely to suffice. An effective screen must include indices of each of
the major functions of nervous tissue (e.g., cellular metabolism,
neuronal propagation, and neurotransmission). It might include
estimates of RNA and/or protein synthesis, lysosomal enzymes, membrane
transport, channel and neurotransmitter receptors, or their turnover.
The precise choice of biochemical indices must be limited by the
expertise and equipment of individual laboratories, but every attempt
must be made to consider a range of functional events rather than a
single parameter.
Considerable discussion has centred on the use of in vitro
procedures for screening potential neurotoxic compounds. The
advantages of an in vitro approach are obvious. It is less expensive
and less time-consuming than whole animal study and biochemical
techniques can be employed under precise, standard conditions.
In vitro procedures must include both parent compounds and potential
toxic metabolites. However, the exclusive use of in vitro methods
would fail to detect compounds in which the neurotoxicity results from
alterations in non-neuronal systems.
Theoretically, the choice of biochemical tools for the
identification of mechanisms of toxicity is the simplest of the three
objectives. Biochemical tools are virtually limitless, but they can be
chosen on the basis of known information about the toxicity of the
compound. Scientists can then proceed systematically from one level of
analysis to another. By synthesizing data from behavioural, neuro-
physiological, neuroendocrinological, neuropharmacological, and
neuropathological studies, the biochemist can employ successively more
selective tools to determine the initial biochemical lesion induced by
the toxic compound.
The purpose of this section is to review the biochemical
approaches that may be useful for identifying and further
understanding the effects of toxic compounds on the nervous system.
6.2 Fractionation Methods
Neural tissue has several features not shared by other tissues. In
particular, neural tissue exhibits considerable cellular,
morphological, and chemical heterogeneity. It is often desirable to
precede biochemical procedures with some attempt to decrease this
complexity. One approach is to separate the tissue into discrete parts
of brain prior to analyses. A second approach is to separate specific
cell types. A third involves subcellular fractionation procedures. In
many cases, it may be desirable to use a combination of these
fractionation procedures.
6.2.1 Brain dissection
Brain tissue can be fractionated into any number of potential
sections. However, the most often used scheme is based on that
described by Glowinski & Iverson (1966). In this method the brain is
divided into relatively discrete units of cerebellum, thalamus,
hypothalamus, striatum, hippocampus, etc., by using visible anatomical
landmarks. Such dissection procedures have been very useful in
describing the neurochemistry of different brain areas and are
essential in evaluating the effects of toxic compounds on molecules
(such as neurotransmitters) that are not uniformally distributed in
neural tissue. Because of the regional variability of brain chemistry,
whole brain analyses are seldom useful for evaluating brain function.
More importantly, the use of the whole brain for studies of toxic
compounds may fail to detect functionally-relevant alterations in
specific brain areas. For example, effects of lead on GABA levels have
been reported for cerebellar tissue (Silbergeld et al., 1980) and
acrylamide effects have been noted in striatal dopamine receptors
(Bondy et al., 1981).
Differences in the response to toxic compounds would be
anticipated from differences in neurochemistry in various regions of
the brain. In addition, many toxic substances are not distributed
uniformly across brain areas (e.g., chlordecone (Fujimori et al.,
1982) and manganese (Bonilla et al., 1982). Although not predictive,
examination of such regions can provide a biochemical clue regarding
possible molecular sites of interaction of the toxic compounds.
In more precise dissection procedures, a small needle, of defined
diameter, is used to obtain samples within anatomically defined areas
(Palkovits, 1973; O'Callaghan et al., 1983). Such finite samples have
been used in identifying several neurochemical events, e.g.,
neurotransmitter concentrations (Palkovits, 1973) and neuro-
transmitter-induced phosphorylation of specific phosphoproteins
(Dolphin & Greengard, 1981), and they may be especially valuable in
locating the site of neural responses to toxic compounds.
6.2.2 Isolation of specific cell types
Although neural tissue is recognized by a marked heterogeneity of
cellular elements, based on function and embyronic origin, these cell
types are conveniently categorized as neurons or glia. Each cell type
makes a unique contribution to neural function and their sensitivity
to toxic compounds may differ. Several methods have been described for
the bulk separation of neuronal and glial cell populations from whole
brain or specific brain regions (Rose & Sinha, 1970; Appel & Day,
1976; Magata & Tsukada, 1978). A dissociated cell suspension that
avoids disruption of the cells is prepared by forcing the tissues
through fine sieves. Proteolytic enzymes are used to facilitate the
dissociation. The fraction enriched in neuronal perikarya is separated
from the smaller glial cells (also containing some synaptosomes) by
centrifugation. The cell separations are never complete and the
procedures generally provide low yields. However, enrichment of the
neurons and glia is usually sufficient fox determining whether a toxic
agent interferes primarily with neuronal or glial metabolism. Isolated
cell populations have been used to study the lipid and protein
composition of membranes and the several metabolic differences between
neuron and glia. Such information may provide baseline data for future
studies of neurotoxic chemicals. Disadvantages of this method include
loss of cell processes from the perikarya, reduction of cell surface
area, and damage to the cell membrane. These factors must be
considered in any metabolic study.
To date, there have been few attempts to use such separation
procedures in neurotoxicity and they are not recommended as an initial
study. The method is laborious and time consuming and must be
performed on fresh tissue. Only a limited number of samples can be
simultaneously processed and it is never clear if the low yields
result from random or specific loss of cell types.
6.2.3 Subcellular fractionation
The neuron can be divided into relatively discrete functional
units. The cell body contains the metabolic machinery for the
synthesis and packaging of macromolecules and for the general
maintenance of cellular homeostatis. The long axonal process acts as a
communication link, propagating electrical impulses from the cell body
to the terminal and transporting vital nutrients and cellular
components to more distal regions including the nerve terminal.
Finally, the synapse functions to transfer chemically encoded
information from one cell to another. Toxic substances may disrupt
neuronal biochemistry at any one, or all, of these cellular sites.
The isolation of subcellular organelles and specific membrane
fractions can be a first approximation in determining the subcellular
sites of action of toxic agents. Differential centrifugation
procedures provide a rapid means of obtaining fractions consisting
predominantly of a single cellular component (Gray & Whittaker, 1962;
Cotman & Barker, 1974; Rose and Sinha, 1970). Subcellular
fractionation is never totally effective, but it can produce an
enrichment of organelles and cellular subfractions. Some of the more
commonly used biochemical markers that can help identify the degree of
enrichment are listed in Table 4. These markers can be used as
potential indices of toxicity. For example, the effects of triethyltin
on myelination in the developing brain has been studied by examining
the activity of the myelin marker, 2',3'cyclic nucleotide 3'
phosphohydrolase (Konat & Clausen, 1977). Merkuryva & Tsapkova (1982)
have used several marker enzymes in their study on the toxic effects
of ethanol on the nervous system.
When subcellular fractionation is combined with regional analysis,
the number of potential samples requiring examination can be
overwhelming. Therefore, careful selection must be used in deciding
which brain area and which subcellular fraction to investigate. This
selection can be very difficult, especially when knowledge concerning
a particular compound is limited. The recent introduction of
immunochemical methods promises to facilitate this task, not by
reducing the difficulty of the selection, but by increasing the number
of chemicals that can be subjected for analysis.
Immunochemical methods have been used to identify molecules
associated with various cell types, for localizing enzymes responsible
for the synthesis of neurotransmitters and for identifying proteins
specific to the nervous system. While the procedures initially require
the preparation of antisera to highly purified proteins, once the
antisera are available, they can be sensitive tools for identifying
specific changes and for characterizing different cell populations. In
addition, monoclonal antibodies can be used to determine the mechanism
of action of toxins.
These procedures are especially valuable in studying neuronal
peptides. These peptides (e.g., substance P, encephalins, hypothalamic
releasing factors, somatostatin, etc.) play a significant role as
neuromodulators (Snyder & Inms, 1979). Many are potent at low
concentrations. Prior to the use of immunological procedures, peptide
molecules could only be examined by using bioassay techniques and
these were not always specific for particular molecular species.
Radioimmunoassay techniques are more specific. Assay kits for various
peptides are now available commercially, and their number is rapidly
increasing.
Table 4. Examples of biochemical markers of brain subcellular fractions
and cell types
Fraction Marker Reference
nuclei DNA Steele & Busch (1963)
nuclear membrane RNA polymerase Roeder & Rutter (1969)
cytosol lactate dehydrogenase Kornberg (1955)
microsomes NADPH; cytochrome c Miller & Dawson (1972)
oxidoreductase (rote
none insensitive)
Mitochondria
inner membrane D-3-hydroxybutyrate Fitzgerald et al.
NAD+ oxidoreductase (1974)
outer membrane monoamine oxidase Schnaitman et al.
(1967)
lysosomes ß-glucuronidase Fishman & Bernfeld
(1955)
ß-glucosidase Robins et al. (1968)
ß-galactosidase Robins et al. (1968)
N-arylamidase Boer (1974)
myelin 2',3'-cyclic nucleotide- Konat & Calusen (1977)
3'-phosphohydrolase
synaptosomes Na+K+-activated Verity (1972)
oubain-sensitive
ATPase
neuronal cells guanyl cyclase Goridis et al. (1974)
tyrosine hydroxylase Kuczenski & Mandell
(1972)
oligodendroglial cells glyceride galactosyl Radin et al. (1972)
transferase
Because the method is very recent, radioimmunoassay procedures are
only beginning to be use in neurotoxicology. However, using such
methods, Hong and his colleagues (Ali et al., 1982; Hong & All, 1982)
have successfully identified several peptide responses following
chlordecone treatment. The major limitation of the method for
identifying toxic compounds is the small number of purified compounds,
the small number of available RIA kits, and the expense of obtaining
radioiodinated compounds. With increasing availability of RIA kits,
the procedure could become a powerful screening tool.
6.3 DNA, RNA, and Protein Synthesis
Changes in the amount of DNA can be used to detect whether toxic
agents affect cellular proliferation and cell death. Polyploidy is
uncommon in the nervous system, thus the DNA content of brain regions
can be taken as an index of cell number. This can be related to tissue
weight and RNA or protein content to estimate cell size. For example,
Krigman & Hogan (1974) reported that lead exposure during early
development reduced brain weight and decreased total brain proteins,
but the number of brain cells was unchanged. DNA measurements have
been particularly useful in studying the toxic effects of drugs on
retinal photoreceptors (Dewar et al., 1977). It is also well-known
that the DNA content changes after nutritional restriction during
development, or after exposure to various toxic chemicals that
influence nutritional status.
Although the measurement of DNA content is relatively simple and
in the nervous system can be an index of cell number, it is probably
not a very sensitive or early indicator of neurotoxicity. For example,
neuronal loss might be accompanied by glial proliferation and produce
no change in total DNA. The earliest effects of a toxic substance on
DNA will probably involve: (a) the disturbance of DNA-repair enzymes;
(b) the intercalation of the substance into the DNA molecule; or
(c) the direct binding of the substance to the nucleic acid moiety or
its associated chromosomal proteins. Two to three percent of added
mercuric chloride was reported to bind to the chromatin in cultured
glial cells (Ramanujam et al., 1970). Since this mode of action will
ultimately interfere with the functional integrity of the CNS, it
offers great promise for the identification of toxic compounds.
The total amount of RNA or protein could also be examined after
toxic insult. However, neither total RNA nor total protein is likely
to provide a sensitive index of toxic damage. Only in the extreme
stages of neurotoxicity will total or even regional levels of these
macromolecules be likely to change. More sensitive indices of their
metabolism rely on estimates of synthesis or degradation.
The rate of RNA or protein synthesis can be evaluated by using
radiolabelled precursors and measuring their rate of incorporation
into the macromolecule in vitro or in vivo. Although this
technique is simple and sensitive, it assumes that the rate of
incorporation of the precursor directly reflects the rate of
macromolecular synthesis and this may not always be true (Dunn, 1977).
Changes in labelling could also occur as a result of changes in
cerebral blood flow, precursor transport, precursor pool size, energy
charge, etc. Inhibition of amino acid transport across the blood-brain
barrier has been reported for mercury (Partridge, 1976) and lead
(Lorenzo & Gerwitz, 1977). Thus, it is also important to consider
whether a toxic agent can cause changes in the amino acid or nucleic-
acid-precursor pool sizes by using one of several methods available
for compensating for precursor pool fluctations (Munro et al., 1964;
Dunlop et al., 1975; Dunn, 1975). Changes in brain-protein synthesis
have been reported after exposure to methylmercury (Verity et al.,
1977), and carbon disulfide (Savolaiene & Jarvisalo, 1977). However,
in many cases, only the absolute levels of incorporation of
radioactive amino acids into protein fractions were studied and it is
difficult to understand the primary mechanisms of action.
Although these relatively crude methods are subject to several
criticisms, all the variations that influence the incorporation of the
radioactive precursor can ultimately influence cellular metabolism.
These methods can, therefore, be useful for identifying metabolic
disturbances. The relative amount of protein synthesis can also be
estimated simply by measuring the polyribosome to free ribosome pool.
This method is based on the assumption that during high rates of
protein synthesis, the greatest proportion of the total ribosomes will
be attached to an mRNA molecule. The relative polyribosome to monosome
ratio has the advantage of allowing for normalization across animals
and therefore reducing the interanimal variability that often plagues
neurochemical assessments. Furthermore, the use of this method would
detect toxic effects not only on protein synthesis but on the
stability or synthesis of the ribosome itself.
The rate of RNA and protein synthesis in the brain is very high
and nerve cell functioning is dependent on protein metabolism. The
turnover rates of brain macromolecules may vary considerably, and it
is always going to be difficult to interpret any data dealing with the
turnover of total RNA or protein. However, with the availability of
newer separation methods for proteins (detergent gel electrophoresis
combined with chromatofocusing cellulose ion exchange chromatography,
etc.), soluble and bound polypeptides can be separated to study the
synthesis of individual proteins at the subcellular level, in specific
cell types and in localized brain regions. Large classes of RNA can be
analysed by hybridization procedures or specific mRNA molecules could
be targeted for investigation by using specific cDNA probes.
6.4 Lipids, Glycolipids, and Glycoproteins
Complex lipids, glycolipids, and glycoproteins have several
important functions in neural tissue (Zuber, 1978). They constitute
structural elements of the plasma membrane, act as components of ion
channels, comprise portions of neurotransmitter receptors, and are
major constituents of myelin. Complex lipids (e.g., phospholipids,
cerebrosides, sulfatides, and gangliosides) make up neuronal
membranes. Phosphatides (phosphatidylethanolamine, phosphatidyl-
choline, phosphatidylinositol, phosphatidylserine) are the most
important group of phospholipids. They consist of some of the most
metabolically active of the phospholipids (Prokhorova, 1974) and
participate in the movement of Na and K ions through the neural
membrane. A closely related group of phospholipids, the plasmalogens,
are concentrated in myelin and constitute 18-30% of total brain
phospholipids.
Ganliosides are localized primarily in the plasma membrane
(Karpova et al., 1978), but small amounts of monosialoganglioside are
detectable in myelin. Receptors of neurotransmitters, such as
serotonin, and other biologically-active compounds may contain
ganglioside (Avrona, 1971), and evidence suggests that gangliosides
may bind biologically-active compounds, as well as various
neurotoxins, through their terminal N-acetylneuraminic acid moiety
(Kryzhavosky, 1973). Glycosaminoglycans are also functional and
structural elements of the neuronal membrane and have been reported to
exhibit disturbed metabolism in various hereditary diseases of the
mucopolysaccharidosis type (Constantopoulos et al., 1976; Vasan &
Chase, 1976).
The most interesting of the functionally-active, minor components
of neuronal membranes are sialic acids, and particularly
N-acetylneuraminic acid. As sialo-glycoproteins and gangliosides,
N-acetylneuraminic acid participates in carrying out specific
neuronal functions such as the establishment of synaptic contact
neurotransmission, and axonal propagation (Partingron & Daly, 1979).
Gangliosides and sialo-glycoproteins bind Ca++ at their hydrophilic
ends and thus, by influencing the concentration of this ion, affect
depolarization and neurotransmitter release.
Myelin metabolism may be affected by a variety of toxic agents
(Cammer, 1980). After administration of these compounds, there is
often a delay before the appearance of neurotoxic signs and symptoms
such as the ataxia indicative of peripheral demyelination. Central
demyelination often occurs to a lesser extent. Compounds that cause
disturbances in myelin metabolism may exert their effect directly on
myelin-forming cells, or demyelination may be a secondary response to
a disturbance of neuronal metabolism.
Several compounds have been reported to alter complex lipids or
their metabolism. Neonatal exposure to lead has been reported to
decrease the total brain content of phospholipids, galactolipids,
plasmalogens, and cholesterol (Van Gelder, 1978). Hydrogen sulfide and
sulfur dioxide decrease total brain lipids and/or phospholipids
(Haider et al., 1980). Meta-SystoxR (phosphorothioic acid
O-[2-(ethylthio)ethyl] O,O-dimethyl ester mixture with
S-[2-(ethylthio)ethyl] O,O-dimethyl phosphorothioate)
(O,O-dimethyl-S-2 (ethylsulfinyl) ethylthiophosphate), an
organophosphorus pesticide, has been reported to decrease brain levels
of total lipids, phospholipids, cholesterol, esterfied fatty acids,
and gangliosides (Islam et al., 1983). Merkuryva et al. (1978) found
one of the early affects of carbon disulfide intoxication to be
changes in the enzyme substrate system of N-acetylneuraminic acid,
N-acetylneuraminate lyase. In the olfactory bulb of the rabbit,
carbon disulfide led to an accumulation of N-acetylneuraminic acid
because of a reduced degradation by ß-acetylneuraminate lyase. In some
cases, the carbon disulfide-induced disturbance of the metabolism of
cerebral glycoconjugates could be correlated with the changing of
physiological parameters (e.g., lowering of the amplitude of the
cortical evoked potential (Bokina et al., 1976)). Long-term exposure
to lead acetate in aging rats was reported to decrease
N-acetylneuraminic acid content in cerebral tissue and also to
decrease the activity of the degradative enzyme (Merkuryva &
Bushinskaya 1982). Long-term exposure to ethanol led to an
accumulation of N-acetylneuraminic acid in the subfornical region of
the hypothalamus and in the midbrain reticular formation (Merkuryva et
al., 1980).
A recently applied approach to the study of neurotoxicology
involves the investigation of lipid peroxidation. Peroxidation
involves the direct reaction of oxygen and lipids to form free radical
intermediates and semistable peroxides. Lipid peroxidation is damaging
because of the subsequent reactivity of these free radicals (Tappel,
1970). Biomembranes and subcellular organelles are the major cellular
components damaged by lipid peroxidation. Increased lipid peroxidation
has been reported after exposure to thallium, nickel, or cobalt (Hasan
& Ali, 1981). Since the estimate of lipid perioxidation is a
relatively simple technique, this method may prove to be an effective
tool for screening for neurotoxic compounds.
6.5 Neurotransmitters
Chemical synaptic transmission involves a complex series of events
(synthesis and storage of neurotransmitters, release of neurotrans-
mitters, re-uptake or degradation of transmitters, interaction of
transmitters with postsynaptic membrane), any or all of which could be
disturbed by neurotoxins. In addition to the classical neurotrans-
mitters (Table 5), a number of peptides have been discovered, which
may act as chemical messengers (Burgen et al., 1980). Most
toxicological studies have focused on the neurotransmitters listed in
Table 5, because considerable background information on these
neurotransmitters is already available. Nearly every class of neuron
contains some marker enzyme (Table 5), and these have been useful in
studies in which attempts have been made to identify neurotransmitter
specific cells and in the determination of the regional distribution
of neurotransmitter systems.
Table 5. Enzyme markers for neurotransmitter specific cells
Neurotransmitter Marker enzyme Receptor types
acetylcholine choline acetyltransferase mucarinic, nicotinic
dopamine tyrosine hydroxylase DA1 and DA2
noradrenaline dopamine-ß-hydroxylase alpha1, alpha2, ß1,
ß2 adrenoreceptors
GABA(gamma- glutamate decarboxylase GABAA and GABAB
aminobutyric acid) (strychnine-insensitive;
picrotoxin-sensitive)
glycine unknown strychnine-sensitive
serotonin 1-amino acid decarboxylase 5 HT1, 5 HT2
6.5.1 Synthesis/degradation
Many neurotoxic compounds have been reported to alter steady-state
levels of neurotransmitters, but: it is difficult to draw functional
inferences from such data since the steady-state levels of
neurotransmitters can be influenced via multiple mechanisms (e.g.,
rate of synthesis, rate of release, rate of degradation). More
informative data are obtained by examination of the turnover rates,
reflecting the metabolic half-life of the neurotransmitter (Costa,
1970). Turnover of 5-HT, after disulfiram treatment, was examined by
observing the accumulation of 5-HT following administration of
pargyline (Minegishi et al., 1979). It has been speculated that carbon
disulfide acts by altering brain catecholamine concentrations. Carbon
disulfide increased DA and decreased NE by inhibiting dopamine
ß-hydroxylase, the enzyme responsible for converting dopamine (DA)
into norepinephrine (NE) (McKenna & DiStefano, 1975). Manganese is
known to inhibit DA formation by blocking tyrosine hydroxylase in the
striatum (Bonilla, 1980) and many acute effects of organophosphorus
compounds are related to inhibition of AChE activity (O'Brien, 1976).
Soon after their introduction, the inhibition of AChE was accepted as
a mechanism for the acute toxic effects of organophosphorus
insecticides (DuBois et al., 1949). However, this mechanism does not
account for the delayed neurotoxicity seen after organophosphate
poisoning (Abou-Donia & Preissig, 1976; Abou-Donia, 1981).
Interference with axonal transport may also disrupt
neurotransmitter function by altering the availability of
neurotransmitter enzymes or by decreasing the transport of peptide
precursors. The transport of materials along axons is bidirectional:
anterograde and retrograde. Anterograde transport conveys materials
from cell body to axon and terminals at slow (1-3 mm per day) and fast
(410 mm per day) rates (Ochs, 1972; Hoffman & Lasek, 1975). The former
contains the bulk of axoplasmic proteins (notably of neurofilaments
and neurotubules) and the latter, membrane and other components.
Retrograde transport, a system that conveys materials at rapid rates
(300 mm per day) may also be affected early in toxic axonopathies.
Retrograde transport may be a critical factor in the toxicity
resulting from exposure to various compounds.
6.5.2 Transport/release
Neurotransmitters or their precursors can rapidly concentrate in
nerve endings through specific high-affinity uptake systems associated
with each transmitter-specific class of neurons (Iverson, 1971). The
high-affinity, Na+-dependent transport mechanisms are specific to
nerve cells and can be distinguished from the ubiquitous low-affinity
transport systems that concentrate a large variety of amino acids,
sugars, and nucleosides, or their precursors. High-affinity uptake
phenomena may be studied in brain homogenates, brain minces, or
synaptosomal preparations. Lead (Silbergeld & Goldberg, 1975), the
insecticide chlordecone (Chang-tsui & Ho, 1979), and erythrosin B
(Lafferman & Silbergeld, 1979) have been reported to interfere with
neurotransmitter uptake processes. However, a variety of nonspecific
events may influence apparent uptake, so caution must be exercised in
interpreting results of these studies. For example, the effects of
erythrosin B on uptake mechanisms were reported to be non-specific
(Mailman et al., 1980).
The release of neurotransmitters from synaptic endings occurs
through an exocytotic process that is triggered by an influx of
calcium ions on depolarization of the nerve endings (Cotman et al.,
1976). Neurotransmitter release can be measured by preloading nerve
endings with labelled neurotransmitters, exposing tissue slices or
synaptosomal fractions maintained on filter beds to calcium ions, and
measuring the appearance of labelled or unlabelled compounds in the
supernatant medium (Bondy & Harrington, 1979). Heavy metals such as
lead have been reported to interfere with calcium-dependent
neurotransmitter release (Bondy et al., 1979; Ramsay et al., 1980),
and manganese has been reported to block transmitter release (Kirpekar
et al., 1970; Balnave & Gage, 1973; Kostial et al., 1974), possibly by
blocking inward Ca2+ current (Baker et al., 1971). Again, caution
must be used in comparing the results from different assay systems.
6.5.3 Binding
Binding of neurotransmitters to specific membrane receptors is the
first step in a complex series of events initiated in the
post-synaptic cell. Such binding interactions are reversible,
stereospecific, nonenzymatic, have equilibria with low dissociation
contants, and involve configurational recognition (Yamamura et al,
1981). Studies on receptor binding have been made possible by the
availability of specific radioactive analogues of neurotransmitters.
In general, the most potent binding ligands are the pharmacological
antagonists or agonists of a given neurotransmitter, rather than the
transmitter itself. Kinetic characteristics of binding and receptor
density are estimated by incubating receptor-enriched membranes with
radioactive ligands. Excess ligands, not bound to membranes, can be
removed by filtration or centrifugation. Non-specific binding of the
labelled compound is estimated by repeating the incubation in the
presence of a high concentration (10-4 to 10-6 mol) of an
unlabelled analogue. The solubility of ligands either in lipids or in
water has to be considered when interpreting results of ligand-binding
studies. This method is relatively simple and has been suggested as a
useful screening procedure for the detection of neurotoxic compounds
(Damstra & Bondy, 1980, 1982). If properly employed, such analyses can
provide valuable information. However, interpretation of the data can
be complicated by the often low degree of specificity of the ligand
and the location of the receptor, which may be pre- or post-synaptic
or on non-neuronal glial elements. Furthermore, for many
neurotransmitters, several subtypes of receptors exist and may produce
different effects on the post-synaptic membrane. Functional
extrapolations from neurotransmitter binding studies, therefore,
should be made with caution.
Receptor binding techniques have only recently been applied to
neurotoxicological studies. However, in a short time, many compounds
have been reported to increase or decrease estimates of receptor
density. Acrylamide (Bondy et al., 1981), chlordecone (Seth et al.,
1981), lead and mercury (Bondy & Agrawal, 1980), and cadmium (Hedlund
et al., 1979) have all been reported to alter putative
neurotransmitter receptors.
6.5.4 Ion channels
Many neuronal functions are regulated by ion channels. The Na+
channel is responsible for depolarizing the membrane, and K+
channels are responsible for repolarizing the membrane. Maintenance of
appropriate Na+ and K+ concentrations requires the classical
Na+/K+ ATPase. Disturbed functioning of these ion channels or
disturbance of ATP availability can severely disturb neuronal
functioning. Several naturally-occurring toxins affect Na+ channels
(Hille, 1976; Ritchie, 1979; Catterall, 1977). Tetrodotoxin and
saxitoxin inactivate the channel at nanomolar concentrations and can
be used as probes for measuring channel density or isolation of
channel polypeptides (Agnew et al., 1978). Batrachotoxin and
veratridine bind the channels and produce persistent activation by
preventing channel inactivation (Albuquerque & Daly, 1976). Peptide
toxins, such as scorpion toxin (Couraud et al., 1978), exhibit either
voltage sensitive or insensitive binding to the extracellular surface
of the Na+ channel (Catterall, 1977). Several potassium channels
probably exist in neurons (Reichardt & Kelly, 1983), but purification
and characterization of these channels has lagged behind that of the
Na+ channel. However, a scorpion toxin that exibited K+ channel
affinity has been described (Carbone et al., 1982).
Na+/K+-ATPase consists of 2 polypeptide chains. The smaller
polypeptide is a glycoprotein (Carilli et al., 1982). The larger
polypeptide binds ATP internally and ouabain externally. Hormones and
neurotransmitters such as catecholamines regulate the ATPase (Clausen
& Flatman, 1977), and neurotoxicants could alter neuronal functioning
by disturbing this control of ionic gradients. Desaiah et al. (1980)
have reported inhibition of Na+/K+-ATPase and decreased ouabain
binding to synaptosomes after treatment of mice with the insecticide
chlordecone. The evaluation of ion balance and their control will
probably be used increasingly in neurotoxicology.
For the most part, transmitter release is regulated by Ca2+
entry through a Ca2+-selective channels. Depolarization,
neurotransmitters, and hormones regulate Ca2+-selective channel
(Reichardt & Kelly, 1983). Nerve terminals contain several Ca2+
calmodulin-dependent protein kinases and a Ca2+-phospholipid
activated kinase, each of which has a distinct set of protein
substrates. Cytoplasmic Ca2+ binds calmodulin and other Ca2+
binding proteins, which directly or indirectly activate other enzymes
via their Ca2+-dependent kinases. How such activation facilitates
exocytosis is not known, but presumably the phosphorylation state of
synapsin I, a phosphoprotein present primarily in nerve terminals and
associated with synaptic vesicles, is believed to regulate transmitter
vesicle release (Dolphin & Greengard, 1981).
Changes in Ca2+ concentration induced by neurotoxicants could
have significant consequences for neuronal function. Cytoplasmic
Ca2+ stimulates not only exocytosis, but also glucogenolysis and
mitochondrial respiration (Landowne & Ritchie, 1976), endocytosis
(Ceccarelli & Hurlbut, 1980), and neurotransmitter synthesis (Collier
& Ilson, 1977). Even though many of these events are homeostatic
responses to transmitter utilization, neurotoxic disruption of calcium
influx or sequestering could produce disturbances not restricted to
neurotransmitter release.
Few neurotoxic compounds have been investigated for their
influence on calcium channels. Manganese is reported to block
neurotransmitter release by blocking inward Ca2+ current (Kirpekar
et al., 1970), and other heavy metals have been reported to interfere
with calcium-mediated neurotransmitter release (Bondy et al., 1979;
Ramsay et al., 1980). Similar effects of heavy metals were also
observed for the Na+-Ca2+ exchange system. Ion channels and
Na+-Ca2+ probes may be a valuable screening approach for some
types of neurotoxic compounds.
6.5.5 Cyclic nucleotides
Nerve terminal function and the effects of neurotransmitters are
often regulated by cyclic nucleotides. Binding of agonists to
receptors on the nerve membrane can result in activation of second
messenger systems. Activation of different receptors results in
specific changes in cyclic AMP and cyclic GMP levels and consequent
alterations in protein kinases. It is thought that on phosphorylation,
conformational changes occur in membrane proteins that may change ion
permeabilities. Thus, the responsiveness of the nervous system to some
toxic agents can also be measured by determining changes in the
activities of adenylate cyclase, cyclic nucleotides, and protein
phosphorylation. For example, lead has been shown to inhibit
cerebellar adenylate cyclase (Nathanson & Bloom, 1975) and
dopamine-sensitive adenylate cyclase in the striatum (Wilson, 1982).
Various pesticides cause increases in the levels of cyclic nucleotides
in brain tissue (Aldridge et al., 1978). For example, tri-o-cresyl
phosphate enhanced Ca2+-calmodulin-dependent in vivo
phosphorylation of proteins in chicken brain (Patton et al., 1983).
6.5.6 Summary of nerve terminal function
The analysis of synaptic function and neurotransmitter metabolism
is reasonably complex, and it should be borne in mind that disturbance
at many levels of neuronal organization will ultimately alter synaptic
function. However, examination of the nerve terminal is an excellent
approach for the initial study of toxic chemicals. However, it is
important to recognize the dynamic nature of the nerve terminal and to
remain alert to the possibility of false positives.
6.6 Energy Metabolism
Nervous tissue, and brain tissue in particular, require
disproportionately large amounts of energy to sustain the
translocation of ions important for electrical activity and to
maintain the highly-active biosynthetic machinery of the tissue.
Since neural tissue has only limited stores of energy (e.g., glycogen
and creatine phosphate), glucose availability and enzymes critical for
energy production are vital to neuronal functioning. A number of
neurotoxic chemicals have been shown to interfere with glucose and
energy metabolism in both the central and peripheral nervous system.
These include methylmercury (Bull & Lutkenhoff, 1975), hexachlorophene
(Cammer & Moore, 1972), organotin compounds (Lock, 1976), and alcohol
(Merkuryva et al., 1980). Glycolytic enzymes have been shown to be
inhibited by alcohol (Merkuryva et al., 1980) and by chemicals known
to cause distal axonopathy, e.g., acrylamide (Howland et al., 1980)
carbon disulfide, and methyl-n-butyl ketone (Sabri et al., 1979).
Active brain areas have a higher rate of glucose consumption than
less active regions. The brain regional metabolic activity is
determined by the 14C-2-deoxyglucose method (2-DG) of Sokoloff et
al. (1977). This method is based on the use of 2-deoxy-D-14C-glucose
as a tracer for the exchange of glucose between the plasma and the
blood and its phosphorylation by hexokinase. The product,
2-deoxyglucose-6-phosphate (2-DG-P), is trapped in the tissue and its
accumulation in the various regions of the brain might be a measure of
the glucose use and neuronal activity. The method provides an index of
the in vivo glucose metabolic activity of brain regions and has been
used in experimental animals to determine which brain regions are
depressed or activated during acute Soman intoxication (McDonough et
al., 1983).
Metabolic compartmentalization in nerve tissue could provide an
additional tool in toxicological studies. It is well established that
neurons mainly use glucose in their energy metabolism, while glia use
substrates other than glucose (Hertz, 1981). Thus, it may be possible
to differentiate between the toxicological effects of chemical
substances on neuronal and glial populations in vitro or in vivo.
6.7 Biochemical Correlates of Axonal Degeneration
A relatively large class of neurotoxic agents is known to produce
axonal degeneration (Griffin & Price, 1980; Sabri & Spencer, 1980;
Thomas, 1980). Some, such as acrylamide and organophosphorus
compounds, produce the distal to proximal "dying back" pattern of
degeneration, while others, such as ß,ß-iminodipropionitrile, lead to
proximal to distal degeneration. Since many of these compounds produce
Wallerian degeneration, biochemical correlates of Wallerian
degeneration have been suggested as toxicological indices (Dewar &
Moffett, 1979). These authors tabulated the chemical changes in
peripheral nerves that occurred during degeneration and suggested that
ß-gluocorinadase and ß-galactosidase might be good indices of
toxicity. Acrylamide, methylmercury, and dimethyl sulfoxide were shown
to increase these lysosomal enzymes (Dewar & Moffett, 1979). The use
of lysosomal enzymes involves relatively simple procedures and might,
therefore, be appropriate for the initiation of a biochemical screen.
Disturbance of axoplasmic transport has been suggested as an
alternative approach to detecting compounds producing axonal
degeneration. Several substances, e.g., organophosphates (Reichart &
About-Donia, 1980) and acrylamide (Pleasure et al., 1969) disrupt
axonal transport. However, these methods are more difficult, more
expensive, and more time-consuming than the lysosomal enzyme assays.
6.8 Neuroendocrine Assessments
The number of toxic compounds being recognized for their
neuroendocrine actions is increasing. Hormonal balance results from
the integrated action of the hypothalamus, pituitary, and endocrine
target organ. Each site is susceptible to disruption by environmental
toxic agents. This disruption may result from the direct interaction
of the toxic agent with the endocrine organ, pituitary, or
hypothalamus. Alternatively, neuroendocrine dysfunction may occur
because of a disturbance in the regulation and/or modulatory elements
of the complex neuroendocrine feedback systems. Any such disturbances
would ultimately modify anterior pituitary secretions. Thus, the
analysis of blood levels is the most appropriate starting point
(section 6.8.2.1).
6.8.1 Anterior pituitary hormones
The main effector organ in the neuroendocrine system is the
pituitary. This "master gland" consists of an anterior
adenohypophysis, and a posterior neurohypophysis. Based on
histological, immunocytochemical, and electron microscopic examination,
the following cell types are differentiated in the anterior pituitary
(Junqueira et al., 1977); follicular cells, which do not contain any
secretory granules but form the support stroma of the glandular cells;
chromophobe cells, which are undifferentiated, nonsecretory and
secretory cells without discernible granules in the light microscope;
somatotropic cells, which are acidophilic with immunocytochemically-
detectable growth hormone and contain granules of about 350 nm
diameter; prolactin cells, which are acidophilic and contain prolactin
granules of 600-900 nm diameter; gonadotropic cells, which consist of
2 basophilic subgroups: follicle-stimulating hormone secreting and
luteinizing-hormone secreting; follicle-stimulating hormone secreting
cells, which are large round cells with dense 200 nm diameter
granules; luteinizing hormone-containing cells, which are small and
contain 200-250 nm diameter granules; thyrotropic cells, which are
basophilic with thyroid-stimulating hormone granules of 120-200 nm
diameter; and finally, cortico cells, which are the least abundant of
the cell types and contain basophilic granules 100-200 nm in diameter.
The glandular cells of the anterior pituitary secrete seven
endocrine hormones, of which four act directly on other endocrine
glands. Follicle-stimulating hormone (FSH) promotes spermatogenesis in
the male testes and facilitates follicular maturation and estrogen
secretion in the female ovary. Lutenizing hormone (LH) acts on the
male testes to facilitate testosterone secretion from the interstitial
cells of Leydig and is a key hormone that promotes follicular rupture
(ovulation) in the female ovary. Thyroid-stimulating hormone (TSH)
triggers the secretion of thyroxine from the thyroid gland, and
adrenocorticotropic hormone (ACTH) causes the adrenal cortex to
secrete its products, especially glucocorticoids. The secretion of
these tropic, anterior pituitary hormones is in turn regulated by the
hormones of the endocrine gland. In most cases, this is a negative
feedback regulation. Estrogen decreases the output of pituitary FSH
and LH; thyroxine decreases the secretion of TSH; and glucocorticoids
reduce the output of ACTH.
The three other anterior pituitary hormones are prolactin (PRL),
melanocyte-stimulating hormone (MSH), and growth hormone (GH). A major
target for prolactin is the mammary gland, where prolactin promotes
milk production. PRL also plays a role in maintaining the ovarian
corpus luteum after ovulation. The entire body is a target for GH,
which promotes growth and maintenance of cellular integrity. GH
facilitates growth, in part, because it increases the transport of
amino acids into the cell, where they can be used for protein
synthesis. MSH increases the production of melanin by the melanocytes
of the skin.
Hypothalamic control of anterior pituitary secretions occurs
through the release of hypothalamic-hypophysiotropic hormones (HHH).
The hypothalmus secretes these HHH into vessels of the hypothalamic-
hypophyseal portal system, by which they are transported to the
anterior pituitary. One of the major advances of the last two decades
has been the isolation and characterization of these HHH.
6.8.2 Disruption of neuroendocrine function
Disruption of neuroendocrine function can occur at any one or all
of the levels of hypothalamic-pituitary-target organ integration.
Alternatively, neuroendocrine effects may result from modification of
"higher" levels of neuronal processing or from alterations in
peripheral metabolism. In the following discussion, approaches will be
indicated for the study of each of these possible sites of neurotoxic
action.
6.8.2.1 Direct pituitary effects
At the most dramatic level, massive changes in the weight or size
of the pituitary, such as occur in the adenohypophysis after large
doses of estrogen (Schreiber & Pribyl, 1980) might be seen. However,
by far the most accessible method for studying pituitary function
relies on the examination of blood levels of pituitary hormones by
radioimmunoassay. Furthermore, the analysis of blood-hormone levels is
one of the few techniques applicable for screening human populations.
A large number of toxic substances have been reported to modify
adenohypophyseal secretions. Methallibure and allied substances
inhibit thyrotropin (Tulloch et al., 1963), and prolactin (Benson &
Zagni, 1965) secretion. Modifications of pituitary hormone secretions
have been reported for acrylamide (Uphouse et al., 1982), alcohol
(Mendelson et al., 1977), 1,4-DDT (Gellert et al., 1972), and
chlordecone (Uphouse et al., 1984). Dimethylsulfoxide may influence
adrenal glucocorticoids by elevating pituitary secretions (Allen &
Allen, 1975). In spite of the fact that changes in the blood levels of
pituitary hormones may be the most sensitive index of neuroendocrine
modification by toxic compounds, they have been examined for
relatively few compounds. The high sensitivity of this approach
results from the fact that the blood levels represent the final
consequence of the complex neuroendocrine integration. Regardless of
the actual site of modification, neuroendocrine disturbances will
usually be revealed in modified levels of circulating hormones.
Therefore, such changes, alone, cannot be regarded as evidence of
direct neuroendocrine disruption. The major disadvantage of serum-
hormone measurements is the responsiveness of the hypothalamic-
pituitary axis to a variety of environmental and chemical stimuli. It
can be difficult to identify the toxic agent as the causal agent.
Because hormones are secreted in a pulsatile manner, single point
analyses of serum hormones must be cautiously interpreted. However,
even with these limitations, analysis of blood levels of pituitary
hormones remains the most appropriate starting point for evaluating
potential neuroendocrine toxicity.
Similar analytical methods may be used to investigate the
pituitary content of respective pituitary secretions. The pituitary is
removed, homogenized, and extracted for use with the appropriate RIA.
For most pituitary secretions, neurotoxicologists have not yet studied
pituitary tissue directly. However, pituitary endorphins has been
reported to be modified by chlordecone (Hong & All, 1982).
6.8.2.2 Peripheral target effects
Most anterior pituitary hormones are subject to negative feedback
control by peripheral endocrine glands. If the neurotoxic agent
modifies peripheral secretions, neuroendocrine changes can result from
this altered feedback. Modifications in the functioning of these
endocrine secretions could occur after toxic exposure. Such approaches
have been widely applied in the experimental and clinical literature
and have shown that a number of compounds alter blood levels of
glucocorticoids, thyroxine, estrogen, and testosterones (Chapman,
1983).
Target-tissue effects can also be evaluated by a variety of
additional techniques. One of the simplest assessments is the change
in the weight, morphology, or biochemistry of the target organ.
Various environmental contaminants have been reported to produce such
changes. Lesions of the thyroid have been reported after exposure to
carbon disulfide (Cavalleri, 1975) and strong magnetic fields
(Persinger et al., 1978). Marked changes occur in the adrenals during
antimony and lead poisoning (Minkins et al., 1973) and in response to
very long chain saturated fatty acids (Powers et al., 1980).
Chlordecone causes adrenal, cortical, and medullary hypertrophy
(Eroschenko & Wilson, 1975) and alters the epinephrine and
norephinephrine content of the adrenal medulla (Baggett et al., 1980).
DDT (McBlain et al., 1976) and other chlorinated hydrocarbons
(Dicksith & Datta, 1972; Fellegiova et al., 1977), carbon disulfide
(Rosewickyi et al., 1973), and cadmium salts (Parizek, 1956;
Zylber-Haran et al., 1982) have all been described as having toxic
effects on the gonads. Although such changes are not necessarily due
to direct neuroendocrine effects, target organ changes can often be a
first indication of neuroendocrine changes. In some cases, even
relatively gross target organ events have been a first clue towards
the mode of action of a neurotoxic agent. For example, the chlorinated
pesticides, DDT and chlordecone, modify gonads in a manner reminiscent
of the natural steroid, estrogen (Gellert et al., 1972; Eroschenko &
Palmiter, 1980; Kupfer & Bulger, 1980). Uterine and/or oviduct weight
changes in the immature animal were the first evidence that the
pesticides might exert estrogenic action.
6.8.2.3 Disruption of hypothalamic control of pituitary secretions
Hypothalamic control of anterior pituitary secretions includes
direct regulation through hypothalamic hypophysiotropic hormones and
modulation through neurotransmitters and neurally-active peptides.
Evaluation of the effect of a neurotoxic agent on HHH is directly
measureable only for the HHH for which antisera are available. For
these compounds, it is possible (though difficult) to measure release
into the portal blood supply, to evaluate the effects of toxic agents
on either amounts or release, and to identify pituitary responsiveness
to the hypothalamic factors. Where identified hormones are not
available, hypothalamic extracts may still be used with in vitro
pituitary responsiveness as a bioassay. Details of such methods can be
found in several sources (Burgus & Guillemin, 1970; Oliver et al.,
1974). However, the value of such approaches for neurotoxicology has
yet to, be tested. Such methods will be of greatest value in testing
hypotheses regarding the mechanism of action of known neuroendocrine
toxic agents. They are not recommended for initial screens.
Biochemical changes in the hypothalamus can also be used as
indices of potential neuroendocrine disruption. Such hypothalamic
effects of neurotoxic compounds have received much less attention than
brain areas such as the striatum, cortex, or cerebellum. Methods for
studying the hypothalamus are the same as those used for other brain
areas. However, the hypothalamic neurons that regulate pituitary
function receive numerous synaptic inputs, both conventional and from
putative neurotransmitters. Consequently, the neuroendocrine
significance of changes in hypothalamic neurotransmitters and
neuropeptides is usually only inferential. However, any disruption of
hypothalamic biochemistry has the potential to alter neuroendocrine
function. When combined with more direct measurements of
neuroendocrine function, such studies are very important. There have
been a few reports of biochemical changes in the hypothalamus and/or
pituitary, correlated with neuroendocrine toxicity. One approach has
been to examine the effects of toxic compounds on hormone-mediated
changes. Administration of estrogens is followed by hypothalamic
ascorbic acid depletion (Schreiber et al., 1982) and by increases in
polyphenol oxidase (ceruloplasmin) activity in the hypothalamus and
the blood (Schreiber & Pribyl, 1980). This can be inhibited by the
simultaneous administration of silver nitrate. Disulfiram
(tetraethylthiuram disulfide) inhibits the reaction of the
adenohypophysis (Schreiber et al., 1979). Disulfiram also inhibits
dopamine-ß-hydroxylase (Szmigielski, 1975) and lowers the
blood-prolactin level (Cavalleri et al., 1978). The effect of
disulfirams on prolactin may be due to a "sparing of dopamine" through
inhibition of dopamine-ß-hydroxylase.
Hypothalamic peptides have been studied most extensively after
chlordecone treatment. Long-term exposure to the pesticide reduced
hypothalamic ß-endorphin levels under conditions where substance P,
neurotensin, and met-enkephalin were unchanged (Ali et al., 1982).
6.8.2.4 Other sites of action
For many neurotoxic agents, there may be no, identifiable effects
on neuroendocrine function, but it may be altered through the indirect
effects of the toxic compound. Such interruption could occur via
modification of neural integration leading to an altered response of
the organism to environmental challenge. Detection of such integrative
action and its relevance to neuroendocrine function might include
treatment with the toxic agent followed by environmental,
pharmacological, or hormonal challenges known to produce a
neuroendocrine response. Several toxic agents have been reported to
produce behavioural changes, such as stress-induced analgesia, and
these undoubtedly involve some aspect of neuroendocrine function. CNS
opiate systems are important components of the CNS response to stress
and studies of peripheral and brain endorphins and encephalins are
potentially relevant to such disturbances. For example, neonatal
treatment with chlordecone dissolved in dimethyl sulfoxide produces an
elevated corticosterone response to footshock (Rosecrans et al.,
1982), and long-term exposure of female rats to chlordecone has been
reported to decrease hypothalamic ß-endorphin levels (Ali et al.,
1982).
A toxic agent could also indirectly affect neuroendocrine function
by altering the peripheral metabolism of endocrine secretions. Such
metabolic differences could indirectly influence CNS function.
However, hormone transformation and the formation of active
metabolites occurs in the CNS so that metabolic disturbances may even
have a direct effect on CNS functioning. Many metabolic variables have
been reported to change after treatment with a toxic agent.
Aromatization of testosterone to estrogen, an important step in the
metabolism of estrogen, took place in the brain of animals (Gallard et
al., 1978). Its inhibition by aminoglutethimide or other blockers of
aromatization (Morali et al., 1977) markedly altered the sexual
behaviour of experimental animals. Aminoglutethimide also inhibited
total adrenal steroidogenesis as well as gonadal steroidogenesis and
thyroid function (Schreiber et al., 1969). The transformation of
thyroxine to the more active metabolite triiodothyronine was inhibited
by a series of toxic substances such as ethanol (Shimizu et al.,
1978), propylthiouracil (Leonard & Rosenberg, 1980), iopanoic acid
(Kaplan, 1980), and sodium salicylate (Chopra et al., 1980). Since the
monodeiodination of thryoxine to triiodothyronine also takes place in
the adenohypophysis, these factors may be an important component of
neuroendocrine reactions to neurotoxic substances. There is evidence
that demonstrates that the direct dopaminergic effect of estrogen on
the pituitary may require conversion of estrogen to catechol estrogen
(Paul et al., 1980). Such conversion occurs not only in the pituitary,
but also in the hypothalamus and cerebral cortex and may mediate some
of the effects of estrogen on the CNS. Study of this enzyme promises
to be important for future research, especially for the investigation
of estrogen-like toxic agents.
A final way in which the toxic agent might disrupt neuroendocrine
function is by altering the peripheral metabolism of the endocrine
secretions within liver tissue. Although beyond the scope of this
publication, several neurotoxic agents (chlordecone, dieldrin,
heptachlor, lindane, p,p'-DDE, and toxaphene) stimulate the
metabolism of estrone by liver microsomal enzymes (Welch et al.,
1971). Biochemical approaches for identifying these metabolic
disturbances are the same as those discussed in other sections.
However, the precise metabolic end-point should be carefully chosen,
when inferences are to be made about neuroendocrine function.
6.8.3 Sex differences
Because neurotoxic agents may produce changes in neuroendocrine
function and, since males and females differ with regard to a variety
of metabolic variables, a valuable approach to any neurotoxicological
investigation is the comparison of the toxic response in the sexes.
Whenever neuroendocrine effects are suspected, sex differences should
be a routine aspect of the overall investigation. Sex-related
differences have been observed for many environmental compounds.
Often, these result from the influence of gonadal hormones on the
metabolism of the compound. Hormonal influences on the
biotransformation and toxicity of DDT (Durham et al., 1956) and several
organophosphates (Murphy & DuBois, 1958; DuBois & Puchala, 1961) have
been demonstrated. Parathion is an organophosphate insecticide that
exerts its toxicity through its active metabolite paraoxon phosphate.
Agrawal et al. (1982) have recently shown that the sex difference in
AChE inhibition after parathion treatment was not evident with
paraoxon treatment. This suggests that the sex difference was in the
rate of metabolism to the toxic product. The female's increased
sensitivity to amobarbital (Castro & Gillette, 1967) may also be due
to sex differences in hepatic metabolism. Sex differences have also
been reported for the rate of disposition of chlordiazepoxide
(Greenblatt et al., 1977; Roberts et al., 1979) and for the toxic
effects of ethylmorphine, aniline, p-nitroanisole (Nicholas &
Barron, 1932; Holck et al., 1937; Quinn et al., 1958) and
polychlorinated hydrocarbons (Lamartiniere et al., 1979).
6.9 Recommendations for Future Research
Several biochemical and neuroendocrine approaches are available
that have not yet been applied to the study of neurotoxic compounds.
These have the advantage of increasing the sensitivity of the
biochemical approach and furthering understanding of the ways in which
compounds disrupt nervous system function. For example, some enzymes
involved in neurotransmitter synthesis have been purified and used for
the production of specific antibodies (John et al., 1973). It is now
possible to use immunological titration to determine whether changes
in enzyme activity result from activity alterations or variations in
the amount of enzyme protein.
Identification of direct pituitary modification can be
accomplished by preparation of anterior pituitary cell cultures and
the measurement of hormone release in response to hypothalamic
releasing factors, neurotransmitters, or suspect toxic compounds. The
pituitary cells are removed, dispersed, and agitated with collagenase.
After resuspension in medium, the cells are plated and used for
examination. Under such culture conditions, pituitary cells respond to
releasing factors and neurotransmitter regulation (Enjalbert et al.,
1978; Drouin & Labrie, 1981). Thus, it is possible to determine
whether the toxic agent modifies the responsiveness of the pituitary
to hypothalamic control. By comparing the effects of the toxic agent
in vivo with those in vitro, direct versus indirect effects of the
compound can be identified.
Furthermore, for suspect steroid-like compounds, direct evaluation
of receptor interactions is possible. Steroid receptors modify their
target tissue by binding to intracellular receptors. Measurement of
intracellular steroid receptors involves the preparation of a high
speed cytosol (180 000 g) fraction from the appropriate target tissue.
Identification of binding is accomplished by incubating the cytosol
in vitro in the presence of radioactively-labelled hormone. Bound
label is removed from unbound by a variety of techniques distinct for
the particular receptor of interest. Specific binding is assessed by
incubating the labelled hormone in the presence of excess unlabelled
hormone as competitor. Specific binding refers to bound molecules that
are removed in the presence of this unlabelled competitor. When a
neurotoxic agent is suspected of interacting with hormone receptors,
the toxic agent may be substituted for the unlabelled competitor and
its ability to compete for binding determined. This procedure has
demonstrated competition by both chlordecone (Palmiter & Mulvihill,
1978) and o,p'-DDT (Kupfer & Bulger, 1976) for the estradiol
cytosol receptor in the uterus. Using an estradiol exchange method,
investigators have shown that these pesticides produce translocation
of the estradiol receptor to the nucleus (Hammond et al., 1979).
Employment of the exchange method necessitates the in vivo treatment
of the organism with the toxic agent. Nuclei and cytosol are prepared
from the target tissue and incubated in vitro with the
radioactively-labelled hormone. Since a major movement of cytosol
receptors into the nucleus occurs only after binding to the hormone,
the presence of the receptor in the nucleus after exposure to the
toxic agent suggests direct binding of the toxic agent to the steroid
cytosol receptor.
Modifications of the procedures described for neurotransmitter
receptor binding are used in the measurement of membrane receptors.
However, no neurotoxic agents have yet been tested for their ability
to modify these hormone receptors. For both intracellular and
extracellular hormone receptors, most studies have been on peripheral
tissues. However, the brain and the pituitary contain receptors for a
variety of steroid and peptide hormones, and these offer a number of
targets for disruption by environmental compounds. Such analyses have
great potential for future studies of neurotoxic compounds. However,
they will usually be applied for the investigation of mechanisms of
action rather than as screens for neurotoxic compounds.
Finally, hypothalamic regulation of pituitary function should
receive further emphasis. Neurotransmitters also regulate pituitary
secretions via neurotransmitter receptors of the pituitary gland. The
potential for a direct modification of these receptor interactions by
neurotoxic compounds is only just being realized. The most thoroughly
described pituitary neurotransmitter receptor is that of dopamine,
which regulates the inhibition of prolactin release by dopamine.
Steroid hormones, such as estrogen, by conversion to catechol
estrogens, increase prolactin secretion by direct interaction with the
pituitary dopamine receptors (Gudelsky et al., 1981; Fishman, 1982).
Neuroleptic drugs elevate prolactin secretion by antagonistic action
on pituitary dopamine receptors (Horowski & Graf, 1979; Besser et al.,
1980).
7. CONCLUSIONS AND RECOMMENDATIONS
There is ample evidence of real and potential hazards of
environmental chemicals for nervous system function. Changes or
disturbances in central nervous function, many times manifest by vague
complaints and alterations in behaviour, reflect on the quality of
life; however, they have not yet received attention.
Neurotoxicological assessment is therefore an important area for
toxicological research.
It has become evident, particularly in the last decade, that
low-level exposure to certain toxic agents can produce deleterious
neural effects that may be discovered only when appropriate procedures
are used. While there are still episodes of large-scale poisoning,
concern has shifted to the more subtle deficits that reduce
functioning of the nervous system in less obvious, but still important
ways, so that intelligence, memory, emotion, and other complex neural
functions are affected.
Information on neurobehaviour, neurochemistry, neurophysiology,
neuroendocrinology, and neuropathology is vital for understanding the
mechanisms of neurotoxicity. One of the major objectives of a
multifaceted approach to toxicological studies is to understand
effects across all levels of neural organization. Such a multifaceted
approach is necessary for confirmation that the nervous system is the
target organ for the effect. Interdisciplinary studies are also
necessary to understand the significance of any behavioural changes
observed and thus, to aid in extrapolation to human beings by
providing specific neurotoxic profiles. Concomitant measurements at
different levels of neural organization can improve the validity of
results.
The following recommendations are made on the basis of the
information contained in this book:
1. Health personnel should take into account the possible role of
exposure to chemicals whenever a patient presents with any
neurobehavioural complaint.
2. Neurotoxicological testing should be an essential consideration in
any profile developed by the agencies responsible for the control
of toxic chemicals.
3. In the determination of the potential of a chemical to produce
neurotoxic effects, a multidisciplinary strategy should be used.
4. Priority should be given to obtaining clinical and epidemiological
data when exposure occurs to chemicals suspected of being
neurotoxic.
5. Test development in preclinical neurotoxicology has evolved to the
point where interlaboratory validation of procedures using
prototypic neurotoxic agents could be attempted.
REFERENCES
ABOU-DONIA, M.B. (1981) Organophosphorus ester-induced delayed
neurotoxicity. Ann. Rev. Pharmacol. Toxicol., 21 511-548.
ABOU-DONIA, M.B. & PREISSIG, S.H. (1976) Delayed neurotoxicity of
leptophos: toxic effects on the nervous system of hens. Toxicol.
appl. Pharmacol., 35 269-282.
ABOU-DONIA, M.G., JENSEN, D.N., & LAPADULA, D.M. (1983) Neurologic
manifestations of tri-o-cresyl phosphate delayed neurotoxicity in
cats. Neurobehav. Toxicol. Teratol., 5: 431-442.
AGNEW, W.S., LEVINSON, S.R., BRABSON, J.S., & RAFTERY, M.A. (1978)
Purification of the tetrodotoxin-binding component associated with the
voltage-sensitive sodium channel from Electrophorus electricus
electroplax membranes. Proc. Natl Acad. Sci. (USA), 75: 2606-2610.
AGRAWAL, A.K., SQUIBB, R.E., & BONDY, S,C. (1981) The effects of
acrylamide treatment upon the dopamine receptor. Toxicol. appl.
Pharmacol., 58 89-99.
AGRAWAL, D.K., MISRA, D., AGARWAL, S,, SETH, P.K., & KOHLI, J.D.
(1982) Influence of sex hormones on parathion toxicity in rats;
antiacetylcholinesterase activity of parathion and paraoxon in plasma,
erythrocytes, and brain. J. Toxicol. environ. Health, 9: 451-459.
ALBURQUERQUE, E.X. & DALY, J.W. (1976) Steroidal alkaloid toxins and
ion transport in electrogenic membranes. In: Cuatracasas, P., ed. The
specificity of animal, bacterial, and plant toxins, London, Chapman
and Hall, pp. 297-338.
ALDER, S. & ZBINDEN, G. (1977) Methods for the evaluation of physical,
neuromuscular, and behavioral development in rats in early postnatal
life. In: Newbert, D., Merber, H.J., & Kwasigroch, T.E., ed. Methods
in prenatal toxicology, Stuttgart, George Thiene, pp. 175-185.
ALDRIDGE, W.M., CLOTHIER, B., FORSHAW, P., JOHNSON, M.K., PARKER,
V.H., PRICE, R.J., SKILLETER, D.N., VERSCHOYLE, P.D., & STEVENS, C.
(1978) The effect of DDT and pyrethroids cismethrin and decamethrin on
the acetylcholine and cyclic nucliotide content of rat brain.
Biochem. Pharmacol., 27: 1703-1706.
ALEU, F.P., KATZMAN, R., & TERRY, R.D. (1963) Fine structures and
electrolyte analyses of cerebral oedema induced by alkyl tin
intoxication. J. Neuropathol. exp. Neurol., 22: 403-413.
ALEXANDER, B.K. BEYERSTEIN, B.L., HADAWAY, P.F., & COAMBS, R.B.
(1981) Effect of early and later colony housing on oral ingestion of
morphine in rats. Pharmacol. Biochem. Behav., 15: 571-576.
ALFANO, D.P. & PETIT, T.L. (1981) Behavioral effects of postnatal lead
exposure. Possible relationship to hippocampal dysfunction. Behav.
neural Biol., 32: 319-333.
ALI, S.F., HONG, J.S., WILSON, W.E., LAMB, J.C., MOORE, J.A., MASON,
G.A., & BONDY, S.C. (1982) Subchronic dietary exposure of rats to
chlordecone (kepone) modifies levels of hypothalamic beta-endorphin.
Neurotoxicology, 3: 119-124.
ALLEN, J.P. & ALLEN, C.F. (1975) The effect of dimethyl sulfoxide on
hypothalamic-pituitary-adrenal functions in the rat. Ann. NY Acad.
Sci., 243: 325-336.
ALTMAN, J. & SUDARSHAN, K. (1975) Postnatal development of locomotion
in the laboratory rat. Anim. Behav., 23: 896-920.
AMIN-ZAKI, L., MAJEED, M.A., ELHASSANI, S.B., CLARKSON, T.W.,
GREENWOOD, M.R. & DOHERTY, R.A. (1979) Prenatal methylmercury
poisoning: clinical observations over five years. J. Dis. Child,
133: 172-177.
ANDRIANOV, V.V., ZAKHAROV, N.C., & FADEEV, JU.A. (1977) Microiono-
phoretic analysis of chemical properties of cortical units in freely
moving animals. Physiol. J. (USSR), 63(4): 602-605.
ANGELL, F. & WEISS, B. (1982) Operant behavior of rats exposed to lead
before or after weaning. Toxicol. appl. Pharmacol., 63: 62-71.
ANGER, W.K. (1984) Neurobehavioral testing of chemicals: impact on
recommended standards. Neurobehav. Toxicol. Teratol., 6(2): 147-153.
ANGER, W.K. & JOHNSON, B.L. (1985) Chemicals affecting behavior. In:
O'Donoghue, J.L., ed. Neurotoxicity of industrial and commercial
chemicals, Boca Raton, Florida, CRC Press.
ANGER, W.K. & SETYES, J.V. (1979) Effects of oral and intramuscular
carbaryl administration on repeated chain acquisition in monkeys.
J. Toxicol. environ. Health, 5: 793-808.
ANNAU, W. (1975) The comparative effects of carbon monoxide and
hypoxia on behaviour. In: Weiss, B. & Laties, V.G., ed. Behavioural
toxicology, New York, Plenum Press, pp. 105-127.
ANOKHIN, P.K. (1935) [Problem of center and periphery in contemporary
physiology of nervous system.] In: Anokhin, P.K., ed. [Problem of
centre and periphery in physiology of nervous activity], Gorkiy,
Gorkiy Publishing House, pp. 9-71 (in Russian).
ANOKHIN, P.K. (1964) [Comparative electrophysical analysis of
alterations of laility of the cortical potential components in
postnatal ontogenesis.] Fiziol. Zh. SSSR, 50: 773-778 (in Russian).
ANOKHIN, P.K. (1968) [Biology and neurophysiology of the conditioned
reflex,] Moscow, Meditsina, pp. 547 (in Russian).
ANOKHIN, P.K. (1974) The functional system as a basis of the
physiological architecture of the behavioural act. In: Anokhin, P.K.,
ed. Biology and neurophysiology of the conditioned reflex and its
role in adaptive behaviour, New York, Pergamon Press, pp. 190-254.
APFELBACH, R. & DELGADO, J.M.R. (1974) Social hierarchy in monkeys
(Macaca mulatta) modified by chlordiazepoxide hydrochloride.
Neuropharmacology, 13: 11-20.
APPEL, S.H. & DAY, E.D. (1976) Cellular and subcellular fractionation.
In: Seigel, G.L., Albers, R.W., Katzman, R., & Agranoff, B.W., ed.
Basic neurochemistry, Boston, Toronto, Little, Brown and Company,
pp. 34-57.
ARBUKHANAOVA, M.S. (1981) Metabolism of glutamic acid in the cerebral
mitochondria during cooling disease. In: Mitochondria: the
mechanisms of conjugation and regulation, Puschino, p. 76.
ASABAYEV, CH., BONCHKOVSKAYA, T.YU., & ZHEGALLO, I.G. (1972) [A study
of the reaction of the animal nervous system to the effect of
low-strength electromagnetic fields.] In: [Labour hygiene and the
biological effect of radio frequency electromagnetic fields,]
Moscow, pp. 48-49 (in Russian).
AVROVA, N.F. (1971) Brain ganglioside patterns of vertebrates.
J. Neurochem., 18: 667-674.
BAENNINGER, L.P. (1966) The reliability of dominance orders in rats.
Anim. Behav., 14: 367-371.
BAGGETT, J.MCC., THURESON-KLEIN, A., & KLEIN, R.L. (1980) Effects of
chlordecone on the adrenal medulla of the rat. Toxicol. appl.
Pharmacol., 52: 313-322.
BAKER, P.F., HODGKIN, A.L., & RIDGWAY, E.B. (1971) Depolarization and
calcium entry in giant squid axons. J. Physiol., 218: 709-775.
BAKIR, F., DAMLUJI, S.F., AMIN-ZAKI, L., MURTADHA, M., KHALIDI, A.
AL-RAWL, N.Y. TIKRITI, S. DHARIR, H.I., CLARKSON, T.W., SMITH, J.C.,
& DOHERTY, R.A. (1973) Methylmercury poisoning in Iraq: an
inter-university report. Science, 181: 230-241.
BALNAVE, R.J. & GAGE, P.W. (1973) The inhibitory effect of manganese
on transmitter release at the neuromuscular junctions of the toad.
Br. J. Pharmacol., 47: 339-352.
BANCROFT, J.D. & STEVENS, A. (1977) Theory and practice of
histological techniques, New York, Churchill Livingston.
BARKER, J.L. & MCKELVY, J.F., ed. (1983) Current methods in cellular
neurobiology: electrophysiological and optical recording techniques,
New York, John Wiley and Sons, Vol. 3.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1973) A behavioral and
pharmacological analysis of variables mediating active avoidance
behaviour in rats. J. comp. Physiol. Psychol., 82: 489-500.
BARRETT, R.J., LEITH, N.J., & RAY, O.S. (1974) An analysis of the
facilitation of avoidance acquisition produced by delta-amphetamine
and scopolamine. Behav. Biol., 11: 189-203.
BASAR, E. (1980) EEG-brain dynamics. In: Relation between EEG and
brain-evoked potentials, Amsterdam, Oxford, New York, Elsevier
Science Publishers.
BAUMGARTNER, G. GAWEL, M.J. KAESER, H.E., PALLIS, C.A., CLIFFORD,
F.R., SCHAUMBURG, H.H., THOMAS, P.K., & WADIA, N.H. (1979)
Neurotoxicity of halogenated hydroxyquinolines: clinical analysis of
cases reported outside Japan. J. Neurol. Neurosurg. Psychiatr.,
42: 1073-1083.
BEARD, R.R. & GRANDSTAFF, N. (1975) Carbon monoxide and human
function. In: Weiss, B. & Laties, V., ed. Behavioural toxicology,
New York, Plenum Press, pp. 1-26.
BECK, E.C., DUSTMAN, R.E., & LEWIS, E.G. (1975) The use of the
averaged evoked potential in the evaluation of central nervous system
disorders. Int. J. Neurol., 9: 211-232.
BENESOVA, O., HORVATH, M., & MIKISKA, A. (1956) [Assessment of the
depth and duration of narcosis according to the electrophysiological
characteristics of the cerebral cortex.] Physiol. bohemoslov.,
5: 188-194 (in German).
BENNETT, S. & BOWERS, D. (1976) An introduction to multivariate
techniques for social and behavioral sciences, New York, Halsted
Press.
BENSON, C.K. & ZAGNI, P.A. (1965) The effect of 1-alpha-
methylallylthiocarbamyl-2-methylthiocarbamylhydrazine (ICI 33828) on
lactation in the rat. J. Endocr., 32: 275-285.
BERGER, H. (1929) [On the electroencephalogram in man.] Arch.
Psychiat. Nervenkr., 87: 527-550 (in German).
BERNARD, C. (1927) An introduction to the study of experimental
medicine, New York, MacMillan Inc., p. 127.
BESSER, G.M., DELITALA, G., GROSSMAN, A., STUBBS, W.A., & YEO, T.
(1980) Chlorpromazine, haloperidol, metoclopramide, and domperidone
release prolactin through dopamine antagonism at low concentrations
but paradoxically inhibit prolactin release at high concentrations.
Br. J. Pharmacol., 71: 569-573.
BHAGAT, B. & WHEELER, M. (1973) Effect of amphetamine on the swimming
endurance of rats. Neuropharmacology, 12: 1161-1165.
BIGNAMI, G., ROSIC, N., MICHALEK, H., MILOSEVIC, M., & GATTI, G.L.
(1975) Behavioral toxicity of anticholinesterase agents:
methodological, neurochemical and neuropsychological aspects. In:
Weiss, B. & Laties, V.G., ed. Behavioral toxicology, New York,
Plenum Press, pp. 155-215.
BLACKWOOD, W., MCMENEMEY, W.H., MEYER, A., NORMAN, R.M., & RUSSELL,
D.S. (1971) Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd.
BLOOM, A.S., STOOTZ, C.G., & DIERINGER, T. (1983) Pyrethroid effects
on operant responding and feeding. Neurobehav. Toxicol. Teratol.,
5: 321-324.
BOER, G.J. (1974) A microassay for arylamidase activity. Anal.
Biochem., 59: 410-411.
BOKINA, A.I., EKSLER, N.D., SEMENENKO, A.D., & MERKURYVA, R.V. (1976)
Investigation of the mechanism of action of atmospheric pollutants on
the central nervous system and comparative evaluation of methods of
study. Environ. Health Perspect., 13: 37-42.
BOKINS, A.I., KAN, T.M., BEREZINA, O.V., & PINIGINA, I.A. (1979)
[Study of complex forms of animal behavior in a hygenic evaluation of
toxic substances.] Gig. i Sanit., 7: 49-51 (in Russian).
BONASHEVSKAYA, T.I., SHAMARIN, A.A., KESAREV, V.S., & YURASOVA, O.I.
(1983) [Morphological criteria and a system of indexes for the
evaluation of neurotoxic effects produced by environmental chemical
factors (on the basis of a carbon monoxide model).] Gig. i Sanit.,
10: 31-33 (in Russian, with English summary).
BONDY, S.C. & AGRAWAL, A.K. (1980) The inhibition of cerebral high
affinity receptor sites by lead and mercury compounds. Arch.
Toxicol., 46: 249-256.
BONDY, S.C. & HARRINGTON, M.L. (1979) Calcium-dependent release of
putative neurotransmitters in the chick visual system. Neuroscience,
4: 1521-1527.
BONDY, S.C., HARRINGTON, M.L., ANDERSON, C.L., & PRASAD, K.N. (1979)
Low concentration of an organic lead compound alters transport and
release of putative neurotransmitters. Toxicol. Lett., 3: 35-41.
BONDY, S.C., TILSON, H.A., & AGRAWAL, A.K. (1981) Neurotransmitter
receptors in brains of acrylamide-treated rats. II. Effects of
extended exposure to acrylamide. Pharmacol. Biochem. Behav.,
14: 533-537.
BONILLA, E. (1980) L-tyrosine hydroxylase activity in the rat brain
after chronic oral administration of manganese chloride. Neurobehav.
Toxicol., 2: 37-41.
BONILLA, E. SALAZAR, E. VILLASMIL, J.J., & VILLALOBOS, R. (1982) The
regional distribution of manganese in the normal human brain.
Neurochem. Res., 7: 221-227.
BOYES, W.K., JENKINS, D.E., & DYER, R.S. (1985) Chlordimeform produces
contrast-dependent changes in visual-evoked potentials in hooded rats.
Exp. Neurol., 89: 391-407.
BRACELAND, F.J. (1942) Mental symptoms following carbon disulfide
absorption and intoxication. Ann. intern. Med., 16: 246-261.
BRADLEY, P.B. (1958) The central action of certain drugs in relation
to the reticular formation of the brain. In: Jasper, H.H., Proctor,
L.D., Knighton, R.S., Noshay, W.C., & Costello, R.T., ed. Reticular
formation of the brain, Boston, Toronto, Little, Brown and Company,
pp. 123-149.
BRADY, K., HERRERA, Y., & ZENICK, H. (1976) Influence of parental lead
exposure on subsequent learning ability of offspring. Pharmacol.
Biochem. Behav., 3: 561-565.
BRIERLEY, J.B. (1976) Cerebral hypoxia. In: Blackwood, W. & Corsellis,
J., ed. Greenfield's neuropathology, London, Edward Arnold
Publishing, Ltd., pp. 43-85.
BROWN, A.W., ALDRIDGE, W.N., STREET, B.W., & VERSCHOYLE, R.D. (1979)
The behavioural and neuropathological sequelae of intoxication by
trimethyltin compounds in the rat. Am. J. Pathol., 97: 59-82.
BUELKE-SAM, J. & KIMMEL, C.A. (1979) Development and standardization
of screening methods for behavioral teratology. Teratology,
20: 17-29.
BULL, R.J. & LUTKENHOFF, S.D. (1975) Changes in the metabolic
responses of brain tissue to stimulation, in vitro, produced by in
vivo administration of methylmercury. Neuropharmacology,
14: 351-359.
BURES, J. & BURESOVA, O. (1979) New methods in memory research. In:
Proceedings of the 21st Annual Meeting of the Czechoslovak
Psychopharmacological Society, Jesenik Spa, 9-14 January, 1979,
pp. 7-12.
BURESOVA, O. & BURES, J. (1982) Capacity of working memory in rats as
determined by performance on a radial maze. Behav. Processes,
7: 63-72.
BURGEN, A.S.V., KOSTERLITZ, H.W., & IVERSEN, L.L., ed. (1980)
Neuroactive peptides, London, Royal Society.
BURGUS, R. & GUILLEMIN, R. (1970) Chemistry of thyrotropin releasing
factor (TRF). In: Meites, J., ed. Hypophysiotropic hormones of the
hypothalamus: assays and chemistry, Baltimore, Maryland, Williams
and Wilkins Company, pp. 227-241.
BUSHNELL, P.J. & BOWMAN, R.E. (1979) Effects of chronic lead ingestion
on social development in infant rhesus monkeys. Neurobehav.
Toxicol., 1: 207-219.
BUTCHER, R.E. (1976) Behavioral testing as a method for assessing
risk. Environ. Health Perspect., 18: 75-81.
BUTCHER, R.E. & VORHEES, C.V. (1979) A preliminary test battery for
the investigation of the behavioral teratology of selected
psychotropic drugs. Neurobehav. Toxicol., 1(Suppl. 1): 207-212.
CABE, P.A. & TILSON, H.A. (1978) Hind limb extensor response: a method
for assessing motor dysfunction in rats. Pharmacol. Biochem. Behav.,
9: 133-136.
CAMMER, W. (1980) Toxic demyelination: biochemical studies and
hypothetical mechanisms. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 239-256.
CAMMER, W. & MOORE, C.L. (1972) The effect of hexachlorophene on the
respiration of brain and liver mitochondria. Biochem. biophys. Res.
Comm., 46: 1887-1894.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W., HAYES, C.,
STRAUB, W.E., LANDRIGAM, P.J., & LIDDLE, J.A. (1978) Epidemic kepone
poisoning in chemical workers. Am. J. Epidemiol., 17: 529.
CARBONE, E., WANKE, E., PRESTIPINO, G., POSSANI, L.D. & MAELICKE, A.
(1982) Selective blockage of voltage-dependent K+ channels by a
novel scorpion toxin. Nature (Lond.), 296: 90-91.
CARILLI, C.T., FARLEY, R.A., PERLMAN, D.M., & GANTHEY, L.C. (1982)
The active site structure of Na+- and K+- stimulated ATPase. J.
biol. Chem., 257: 5601-5606.
CASARETT, L.J. (1975) Toxicologic evaluation. In: Casarett, L.J. &
Doull, J., ed. Toxicology; the basic science of poisons, New York,
MacMillan Inc., pp. 11-25.
CASTRO, J.A. & GILLETTE, J.R. (1967) Species and sex differences in
the kinetic constants for the N-demethylation of ethyl-morphine by
liver microsomes. Biochem. biophys. Res. Comm., 28: 426-430.
CATTERALL, W.A. (1977) Activation of the action potential Na+
ionophore by neurotoxins. J. biol. Chem., 252: 8669-8676.
CAVALLERI, A. (1975) Serum thyroxine in the early diagnosis of carbon
disulfide poisoning. Arch. environ. Health, 30: 85-87.
CAVALLERI, A., POLATTI, F., & BOLIS, P.F. (1978) Acute effects of
tetramethylthiuram disulfide on serum levels of hypophyseal hormones
in humans. Scand. Work environ. Health, 4: 66-72.
CAVANAGH, J.B. (1964) The significance of the "dying-back" process in
experimental and human neurological disease. Int. Rev. exp. Pathol.,
3: 219-267.
CAVANAGH, J.B. & CHEN, F.C.-K. (1971) The effects of methyl-mercury-
dicyandiamide on the peripheral nerves and spinal cord of rats. Acta
neuropathol., 19: 208-215.
CAVANAGH, J.B. & JACOBS, J.M. (1954) Some quantitative aspects of
diphtheritic neuropathy. Br. J. exp. Pathol., 45: 309-327.
CAVANAGH, J.B., PASSINGHAM, R.J., & VOGT, J.A. (1964) Staining of
sensory and motor nerves in muscles with Sudan Black B. J. Pathol.
Bacteriol., 88: 89-92.
CECCARELLI, B. & HURLBUT, W.P. (1980) Ca-dependent recycling of
synaptic vesicles at the frog neuromuscular junction. J. cell Biol.,
87: 297-303
CHANCE, M.R.A. (1946) Aggregation as a factor influencing the toxicity
of sympathomimetic amines in mice. J. Pharmacol. exp. Ther.,
87: 214-219.
CHANG, L. (1977) Neurotoxic effects of mercury -- a review. Environ.
Res., 14: 329-373.
CHANG, L. (1980) Mercury. In: Spencer, P.S. and Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 508-526.
CHANG-TSUI, Y.H. & HO, I.K. (1979) Effects of kepone (chlordecone) on
synaptosomal gamma-aminobutryic acid uptake in the mouse.
Neurotoxicology, 1: 357-361.
CHAPMAN, R.M. (1983) Gonadal injury resulting from chemotherapy. Am.
J. ind. Med., 4: 149-161.
CHIBA, S. & ANDO, K. (1976) Effects of chronic administration of
kanamycin on conditioned suppression to auditory stimulus in rats.
Jpn. J. Pharmacol., 26: 419-426.
CHO, E.S., SPENCER, P.S., JORTNER, B.S., & SCHAUMBURG, H.H. (1980) A
single intravenous injection of doxorubicin (AdriamycinR) induces
sensory neuropathy in rats. Neurotoxicology, 1: 583-591.
CHOPRA, I.J., SOLOMON, D.J., TECO, G.N.C., & NGUYEN, A.H. (1980)
Inhibition of hepatic outer ring monodeiodination of thyroxine and
3,3',5'-triiodothyronine by sodium salicylate. Endocrinology,
106: 1728-1734.
CIOMS (1983) Safety requirements for the first use of new drugs and
diagnostic agents in man, Geneva, Council for International
Organizations of Medical Sciences.
CITOVIC, I.S. (1930) [The procedure of studying the effects of oil
products on the organism of animals.] Inst. Work Saf., 1: 92
(in Russian).
CLAUSEN, T. & FLATMAN, J.A. (1977) The effects of catecholamines on
Na-K transport and membrane potential in rat soleus muscle.
J. Physiol., 270: 383-414.
COLLIER, B. & ILSON, D. (1977) The effect of preganglionic nerve
stimulation on the accumulation of certain analogues of choline by a
sympathetic ganglion. J. Physiol., 264: 489-509.
COLLINS, R.D. (1982) Illustrated manual of neurologic diagnosis, 2nd
ed., Philadelphia, Pennsylvania, J.B. Lippincott Company.
COLOTLA, V.A. (1981) Adjunctive polydipsia as a model of alcoholism.
Neurosci. Biobehav. Rev., 5: 335-342.
COLOTLA, A., BAUTISTA, M., LORENZANA-JIMENEZ, M., & RODRIGUEZ, R.
(1979) Effects of solvents on schedule-controlled behavior.
Neurobehav. Toxicol., 1(Suppl. 1): 113-118.
CONSTANTOPOULOS, G., MCCOMB, R.D., & DECABAN, A. (1976) Neurochemistry
of the mucopolysaccharidoses: brain glycosaminoglycans in normal and
four types of mucopolysaccharidoses. J. Neurochem., 26: 901-908.
CORY-SLECHTA, D.A. & THOMPSON, T. (1979) Behavioral toxicity of
chronic postweaning lead exposure in the rat. Toxicol. appl.
Pharmacol., 47: 151-159.
CORY-SLECHTA, D.A., BISSEN, T., YOUNG, M., & THOMPSON, T. (1981)
Chronic postweaning lead exposure and response duration performance.
Toxicol. appl. Pharmacol., 60: 78-84.
COSTA, A. & MURAD, J.E. (1969) Study of the association between
chlorpromazine and analgesics through the method of electrical
stimulation of the rabbit dental pulp. Rev. Bras de Pesgnisa Med.
Biol., 2: 235-240.
COSTA, E. (1970) Simple neuronal models to estimate turnover rate of
noradrenergic transmitters in vivo. In: Costa, E. & Giabocini, E.,
ed. Advances in biochemical psychopharmacology. II. Biochemistry of
simple neuronal models, New York, Raven Press.
COTMAN, C.W. & BARKER, G.A. (1974) The making of a synapse. Rev.
Neurosci., 1: 1-62.
COTMAN, C.W., HAYCOCK, J.W., & WHITE, W.F. (1976) Stimulus secretion
coupling processes in brain: analysis of noradrenaline and gamma-
aminobutyric acid release. J. Physiol., 254: 475-505.
COURAUD, F.C., ROCHAT, H., & LISSITSKY, S. (1978) Binding of scorpion
and sea anemone neurotoxins to a common site related to the action
potential Na+ ionophore in neuroblastoma cells. Biochem. biophys.
Res. Comm., 83: 1525-1530.
CRANE, A.M. & GOLDMAN, P.S. (1979) An improved method for embedding
brain tissue in albumin-gelatin. Stain Technol., 54: 71-75.
CRAPPER, D.R. & DE BONI, U. (1980) Aluminum. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 326-335.
CUTLER, M.G. (1977) Effects of exposure to lead on social behaviour in
the laboratory mouse. Psychopharmacology, 52: 279-282.
DAMSTRA, T. (1978) Environmental chemicals and nervous system
dysfunction. Yale J. Biol. Med., 51: 457-468.
DAMSTRA, T. & BONDY, S.C. (1980) The current status and future of
biochemical assays for neurotoxicity. In: Spencer, P.S. & Schaumburg,
H.H., ed. Experimental and clinical neurotoxicology, Baltimore,
Maryland, Williams and Wilkins Company, pp. 820-833.
DAMSTRA, T. & BONDY, S.C. (1982) Neurochemical approaches to the
detection of neurotoxicity. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 349-373.
DAUBE, J. (1980) Nerve conduction studies. In: Aminoff, J., ed.
Electrodiagnosis in clinical neurology, New York, Churchill
Livingstone, pp. 229-264.
DAVIS, M. (1980) Neurochemical modulation of sensory-motor reactivity:
acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev.,
4: 241-263.
DE JESUS, C.P.V., TOCOFIGHI, J., & SNYDER, D.R. (1978) Sural nerve
conduction study in the rat: a new technique for studying experimental
neuropathies. Muscle Nerve, 1: 162-167.
DESAIAH, D., GILLILAND, T., HO, I.K., & MEHENDALE, H.M. (1980)
Inhibition of mouse brain synaptosomal ATPases and ouabain binding by
chlordecone. Toxicol. Lett., 6: 275-285.
DESCLIN, J.C. (1974) Histological evidence supporting the inferior
olive as the major source of cerebellar climbing fibres in the rat.
Brain Res., 77: 365-384.
DESI, I. (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav. Toxicol. Teratol., 5: 503-515.
DESI, I. & SOS, J. (1962) Central nervous injury by a chemical
herbicide. Acta Med. Acad. Sci. Hung., 18: 429-433.
DESI, I. & SOS, J. (1963) Experimental lesions of the central nervous
system induced by triorthocresyl phosphate. Acta Physiol. Acad. Sci.
Hung., 23: 63-68.
DESI, I., GONCZI, L., SIMON, G., FARKAS, I., & KNEFFEL, Z. (1974)
Neurotoxicologic studies of two carbamate pesticides in subacute
animal experiments. Toxicol. appl. Pharmacol., 27: 465-476.
DEWAR, A.J. & MOFFETT, B.J. (1979) Biochemical methods for detecting
neurotoxicity. A short review. Pharmacol. Ther., 5: 545-562.
DEWAR, A.J., MOFFETT, B.J., & SITTON, M.F. (1977) The effect of
anti-inflammatory drugs on retinal dystrophy in the rat. Toxicol.
appl. Pharmacol., 42: 65-74.
DEWS, P.B. (1982) Epistemology of screening for behavioral toxicity.
In: Mitchell, C.L., ed. Nervous system toxicology, New York, Raven
Press, pp. 229-236.
DEWS, P.B. & WENGER, G.R. (1979) Testing for behavioral effects of
agents. Neurobehav. Toxicol., 1(Suppl. 1): 119-127.
DIAMOND, S.S. & SLEIGHT, S.D. (1972) Acute and subchronic
methylmercury toxicosis in the rat. Toxicol. appl. Pharmacol.,
23: 197-207.
DICKSITH, T.S.S. & DATTA, K.K. (1972) Effect of intratesticular
injection of lindane and endrine on the testes of rats. Acta
pharmacol., 31: 1-10.
DIETZ, D.D., MCMILLAN, D.E., GRANT, L.D., & KIMMEL, C.A. (1978)
Effects of lead on temporally-spaced responding in rats. Drug chem.
Toxicol., 1(4): 401-419.
DINGLEDINE, R., ed. (1984) Brain slices, New York, Plenum Press,
442 pp.
DIXON, R.L. (1976) Problems in extrapolating toxicity data for
laboratory animals to man. Environ. Health Perspect., 13: 43-50.
DOLPHIN, A.C. & GREENGARD, P. (1981) Neurotransmitter- and
neuromodulator-independent alterations in phosphorylation of protein I
in slices of rat facial nucleus. J. Neurosci., 1: 192-203.
DRAPER, W.A. (1967) A behavioural study of the home-cage activity of
the white rat. Behaviour, 28: 280-306.
DRENTH, H.J., ENSBERG, I.F.G., ROBERTS, D.V., & WILSON, A. (1972)
Neuromuscular function in agriculture workers using pesticides. Arch.
environ. Health, 25: 395-398.
DROUIN, J. & LABRIE, F. (1981) Interactions between 17 beta-estradiol
and progesterone in the control of luteinizing hormone and follicle
stimulating hormone release in rat pituitary cells in culture.
Endocrinology, 108: 52-57.
DUBOIS, K.P. & PUCHALA, E. (1961) Studies on the sex differences in
the toxicity of cholinergic phosphorothioate (26791). Proc. Soc. Exp.
Biol. Med., 107: 908-911.
DUBOIS, K.P., DOULL, J., SALERNO, P.R., & COON, J.M. (1949) Studies on
the toxicity and mechanisms of action of p-nitro-phenyl diethyl-
thionosphosphate (parathion). J. Pharmacol. exp. Ther., 95: 79-91.
DUBOWITZ, V. & BROOKE, M.H. (1973) Muscle biopsy: a modern approach,
London, W.B. Saunders.
DUNLOP, D.S., VAN ELDEN, W., & LAJTHA, A. (1975) Optimal conditions
for protein synthesis in incubated slices of rat brain. Brain Res.,
99: 303-318.
DUNN, A.J. (1975) Intercerebral injections inhibit amino acid
incorporation into brain protein. Brain Res., 99: 405-409.
DUNN, A.J. (1977) Measurements of the rate of brain protein synthesis.
In: Roberts, S., Lajtha, A., & Gispen, W.H., ed. Mechanisms,
regulation, and special function of protein synthesis in the brain,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 97-105.
DURHAM, W.F., CUETO, C., Jr, & HAYES, W.J., Jr (1956) Hormonal
influence on DDT metabolism in the white rat. Am. J. Physiol.,
187: 373-377.
DYCK, P.J., THOMAS, P.K., LAMBERT, E.H., & BUNGE, R. (1984)
Peripheral neuropathy, 2nd ed., Philadelphia, Pennsylvania, W.B.
Saunders, Vol. 1.
DYER, R.S. (1980) Effects of prenatal and postnatal exposure to carbon
monoxide on visually-evoked responses in rats. In: Weiss, B. &
Merigan, W., ed. Neurotoxicity of the visual system, New York, Raven
Press, pp. 17-33.
DYER, R.S. (1985a) The use of sensory-evoked potentials in toxicology.
Fundam. appl. Toxicol., 5: 24-40.
DYER, R.S. (1985b) Neurophysiological measures following exposure to
toxic substances. In: Annau, Z., ed. Behavioral toxicology,
Baltimore, Maryland, Johns Hopkins.
DYER, R.S. & BOYES, W.K. (1983) Use of neurophysiological challenges
for the detection of neurotoxicity. Fed. Proc., 42: 3201-3206.
DYER, R.S., ECCLES, C.U., & ANNAU, Z. (1978) Evoked potential
alterations following prenatal methylmercury exposure. Pharmacol.
Biochem. Behav., 8: 137-141.
DYER, R.S., SWARTZWELDER, H.S., ECCLES, C.W., & ANNAU, Z. (1979)
Hippocampal after discharges and their post-ictal sequelae in rats: a
potential tool for assessment of CNS neurotoxicity. Neurobehav.
Toxicol., 1: 5-19.
ECKERMAN, D.A., GORDON, W.A., EDWARDS, J.D., MACPHAIL, R.C., & GAGE,
M.I. (1980) Effects of scopolamine, pentabarbital, and amphetamine on
radial arm maze performance in the rat. Pharmacol. Biochem. Behav.,
12: 595-602.
EDWARDS, P.M. & PARKER, V.H. (1977) A simple, sensitive, and objective
method for early assessment of acrylamide neuropathy in rats.
Toxicol. appl. Pharmacol., 40: 589-591.
EGAN, G.F., LEWIS, S.C., & SCALA, R.A. (1980) Experimental design for
animal toxicity studies. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams & Wilkins Company, pp. 708-725.
EICHELMAN, B., HEGSTRAND, L.R., MCMURRAY, T., & KANTAX, K.M. (1981)
Cyclic nucleotides and aggressive behaviour. Psychopharmacology of
aggression and social behaviour. Pharmacol. Biochem. Behav.,
14(Suppl. 1): 7-12.
ELSNER, J., LOOSER, R., & ZBINDEN, G. (1979) Quantitative analysis of
rat behaviour patterns in a residential maze. Neurobehav. Toxicol.,
1(Suppl. 1): 163-174.
EMSON, P.C. (1983) Chemical neuroanatomy, New York, Raven Press.
ENJALBERT, A., RUBERG, M., & KORDON, C. (1978) Neuroendocrine control
of prolactin secretion. In: Robyn, C. & Harter, M., ed. Progress in
prolactin physiology and pathology, Amsterdam, Oxford, New York,
Elsevier Science Publishers, pp. 83-94.
EROSCHENKO, V.P. & PALMITER, R.D. (1980) Estrogenicity of kepone in
birds and mammals. In: McLachlan, J., ed. Estrogens in the
environment, Amsterdam, Oxford, New York, Elsevier Science
Publishers, pp. 305-325.
EROSCHENKO, V.O. & WILSON, W.O. (1975) Cellular changes in the gonads,
liver, and adrenal glands of Japanese quail as affected by the
insecticide kepone. Toxicol. appl. Pharmacol., 31: 491-504.
EVANS, H.L. (1978) Behavioral assessment of visual toxicity. Environ.
Health Perspect., 26: 53-58.
EVANS, H.L. & WEISS, B. (1978) Behavioral toxicology. In: Blackman,
D.E. & Sanger, D.J., ed. Contemporary research in behavioral
pharmacology, New York, Plenum Press, pp. 449-487.
EVANS, H.L., LATIES, V.G., & WEISS, B. (1975) Behavioral effects of
mercury and methylmercury. Fed. Proc., 34: 1858-1867.
EVANS, H.L., GARMAN, R.H., & WEISS, B. (1977) Methylmercury: exposure
duration and regional distribution as determinants of neurotoxicity in
non-human primates. Toxicol. appl. Pharmacol., 41: 15-33.
EVANS, W.O. (1962) A comparison of the analgetic potency of some
analgesics as measured by the "flinch-jump" procedure.
Psychopharmacologia, 3: 51-54.
FADEEV, JU.A. (1980) [Unit activity of the brain cortex in realization
of goal-directed behaviour.] Usp. fiziol. nauk., 11(3): 12-46 (in
Russian).
FADEEV, JU.A. & ANDRIANOV, V.V. (1971) Methods of registration of
neuronal activity in freely-moving animals. Physiol. J. (USSR),
57(8): 1217-1219.
FALCK, B., HILLARP, N.A., THIEME, G., & TORP, A. (1962) Fluorescence
of catecholamines and related compounds condensed with formaldehyde.
J. Histochem. Cytochem., 10: 348-354.
FALK, J.L. (1970) Readings in behavioral pharmacology, New York,
Appleton-Century-Crofts.
FELLEGIOVA, M., ADAMEC, O., & DAHKOVA, A. (1977) [The effect of
lindane on the metabolism of testosterone in the rat.] Cs. Hyg.,
22: 115-120 (in Slovak).
FERSTER, C.B. & SKINNER, B.F. (1957) Schedules of reinforcement, New
York, Appleton-Century-Crofts.
FESTING, M.F. (1979) Properties of inbred strains and outbred stocks,
with special reference to toxicity testing. J. environ. Health
Toxicol., 5: 53-68.
FILE, S. (1980) The use of social interaction as a method for
detecting anxiolytic activity of chlordiarepoxide-like drugs.
J. Neurosci. Methods, 2: 219-238.
FINK, G. (1979) Feedback actions of target hormones on hypothalamus
and pituitary with special reference to gonadal steroids. Ann. Rev.
Physiol., 41: 571-585.
FISHMAN, J. (1982) Catechol estrogens and pituitary function. In:
Muldoon, T.G., Mahesh, V.B., & Perez-Ballester, B., ed. Recent
advances in fertility research. Part A. Developments in reproductive
endocrinology, New York, Alan R. Lyss, pp. 183-189.
FISHMAN, W.H. & BERNFELD, P. (1955) Glucuronidases. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 262.
FITZGERALD, G.J., KAUFMAN, E.E., SOKOLOFF, L., & SHEIN, H.M. (1974)
D(-)-beta-hydroxybutyrote dehydrogenase activity in closed cell lines
of glial and neuronal origin. J. Neurochem., 22: 1163-1166.
FODOR, G.G., SCHLIPKOTER, H.W., & ZIMMERMAN, M. (1973) The objective
study of sleeping behaviour in animals as a test of behaviour
toxicity. In: Horvath, M. & Frantik, M., ed. Adverse effects of
environmental chemicals and psychotropic drugs, Amsterdam, London,
New York, Elsevier Science Publishers, Vol. 1, pp. 115-122.
FOLCH, J., ASCOLI, I., LEES, M., MEATH, J.A., & LEBARON, F.N. (1951)
Preparation of lipid extracts from brain tissue. J. biol. Chem.,
191: 833-841.
FOWLER, S.C. & PRICE, A.W. (1978) Some effects of chlordiaz-epoxide
and delta-amphetamine on response force during punished responding in
rats. Psychopharmacology, 56: 211-215.
FOX, D.A., LOWNDES, H.E., & BIERKAMPER, G.G. (1982) Electro-
physiological techniques in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 299-335.
FOX, W.M. (1965) Reflex-ontogeny and behavioral development of the
mouse. Anim. Behav., 13: 234-241.
FRANKOVA, S. (1970) Behavioral responses of rats to early
overnutrition. Nutr. Metab., 12: 228-230.
FRANKOVA, S. (1971) Relationship between nutrition during lactation
and maternal behavior of rats. Act. Nerv. Super. (Praha), 13: 1-8.
FRANKOVA, S. (1977) Drug-induced changes in the maternal behavior of
rats. Psychopharmacology, 3: 83-87.
FRANTIK, E. (1970) The development of motor disturbances in
experimental chronic carbon disulfide intoxication. Med. Lav.,
61: 309-313.
FRANTIK, E. & BENES, V. (1984) Central nervous effect and blood level
regressions on exposure time paralleled in solvents (toluene, carbon
tetrachloride, and chloroform). Act. Nerv. super. (Praha), 26: 131.
FUJIMORI, K., BENET, H., MEHENDALE, H.M., & HO, I.K. (1982) Comparison
of brain discrete area distribution of chlordecone and mirex in the
mouse. Neurotoxicology, 3: 125-130.
FULLERTON, P.M. (1966) Chronic peripheral neuropathy produced by lead
poisoning in guinea-pigs. J. Neuropathol. exp. Neurol., 25: 214-236.
GABE, M. (1976) Histological techniques, New York, Paris,
Springer-Verlag, Masson.
GAD, S.C. (1982) Statistical analysis of behavioral toxicology data
and studies. Arch. Toxicol., Suppl. 5: 256-266.
GAD, S.C. & WEIL, C.S. (1982) Statistics for toxicologists. In: Hayes,
A.W., ed. Principles and methods of toxicology, New York, Raven
Press, pp. 270-320.
GALLARD, G.V., PETRO, Z., & RYAN, K.J. (1978) Phylogenetic
distribution of aromatase and other androgen-converting enzymes in the
central nervous system. Endocrinology, 10: 2283-2290.
GELLER, I., MENDEZ, V., HAMILTON, M., HARTMANN, R.J., & GAUSE, E.
(1979) Effects of carbon monoxide on operant behavior of laboratory
rats and baboons. Neurobehav. Toxicol., 1(Suppl. 1): 179-184.
GELLERT, R.J., HEINRICHS, W.L., & SWERDLOFF, R.S. (1972) DDT
homologues: estrogen-like effects on the vagina, uterus, and pituitary
of the rat. Endocrinology, 91: 1095-1100.
GERHART, J.M., HONG. J.S., UPHOUSE, L.L., & TILSON, H.A. (1982)
Chlordecone-induced tremor: quantification and pharmacological
analysis. Toxicol. appl. Pharmacol., 66(2): 234-243.
GILLIATT, R.W. (1973) Recent advances in the pathophysiology of nerve
conduction. In: Desmedt, J.E., ed. New developments in
electromyography and clinical neurophysiology, Basel, Karger,
Vol. 2, pp. 2-18.
GLATT, A.F., TALAAT, H.N., & KOELLA, W.P. (1979) Testing of peripheral
nerve function in chronic experiments with rats. Pharmacol. Ther.,
5: 539-543.
GLAUSERT, A.M. (1980) Fixation, dehydration, and embedding of
biological specimens, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
GLOWINSKI, J. & IVERSEN, L.L. (1966) Regional studies of
catecholamines in the rat brain. I. The disposition of 3H-norephine-
phrine, 3H-dopamine, and 3H-DOPA in various regions of the brain. J.
Neurochem., 13: 655-699.
GOLDSTEIN, B.D., LOWNDES, H.E., & CHO, E.S. (1981) Neurotoxicology of
vincristine in the cat: electrophysiological studies. Arch.
Toxicol., 48: 253-264.
GONCHARUK, V.D. (1981) Alteration of activity of glutamate
dehydrogenase in ganglionated neurons of rabbits under acute
experimental emotional stress. Bull. exp. Biol. Med., 10: 494-496.
GOOD, P. (1979) Detection of a treatment effect when not all
experimental subjects will respond to treatment. Biometrics,
35: 483-489
GORIDIS, C., MASSARELLI, R., SERSENBRENNER, M., & MANDEL, P. (1974)
Guanyl cyclase in chick embryo brain cell cultures: evidence of
neuronal localization. J. Neurochem., 23: 135-139.
GOYER, R.A. & RHYNE, B.C. (1973) Pathological effects of lead. Int.
Rev. exp. Pathol., 12: 1-7.
GRANT, L.D. (1976) Research strategies for behavioral toxicology
studies. Environ. Health Perspect., 18: 85-94.
GRAY, E.G. & WHITTAKER, V.P. (1962) The isolation of nerve endings
from brain: an electron microscopic study of the cell fragments of
homogenation and centrifugation. J. Anat., 96: 79-87.
GREENBLATT, D.J., SHADER, R.I., FRANKE, K.K., MACLAUGHLIN, E.D.,
RONSIL, G.J., & KOCH-WESER, J. (1977) Kinetics of intravenous
chlordiazepoxide: sex differences in drug metabolism. Clin.
Pharmacol. Ther., 22: 893-903.
GREENGARD, P. (1979) Cyclic nucleotides, phosphorylated proteins and
the neuronal system. Fed. Proc., 38: 2208-2217.
GREGORY, E.H. & PFAFF, D.W. (1971) Development of olfactory-guided
behavior in infant rats. Physiol. Behav., 6: 573-576.
GRIFFIN, J.W. & PRICE, D.L. (1980) Proximal axonopathies induced by
toxic chemicals. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicology, Baltimore, Maryland,
Williams and Wilkins Company, pp. 161-178.
GRIFFIN, J.W., PRICE, D.L., KLUTHE, D.O., & GOLDBERG, M.A. (1980)
Neurotoxicity of misonidazole in rats. Neuropathol. appl.
Neurobiol., 6: 656-666.
GROSS-SELBECK, E. & GROSS-SELBECK, M. (1981) Changes in operant
behavior of rats exposed to lead at the accepted no-effect level.
Clin. Toxicol., 18: 1247-1256.
GROTA, L.S. & ADER, R. (1969) Continuous recording of maternal
behavior in the rat. Anim. Behav., 17: 72-729.
GUDELSKY, G.A., NANSEL, D.D., & PORTER, J.C. (1981) Role of estrogen
in the dopaminergic control of prolactin secretion. Endocrinology,
108: 440-444.
HAIDER, S.S., HANSAN, M., & ISLAM, F. (1980) Effect of air pollutant
hydrogen sulfide on the levels of total lipids, phospholipids, and
cholesterol in different regions of the guinea-pig brain. Ind. J.
exp. Biol., 18: 418-420.
HAMMOND, B., KATZENELLENBOGEN, B.S., KRAUTHAMMER, N., & MCCONNEL, J.
(1979) Estrogenic activity of the insecticide chlordecone (kepone) and
interaction with uterine estrogen receptors. Proc. Natl Acad. Sci.
(USA), 76: 6641-6645.
HARPUR, E.S. & D'ARCY, P.F. (1975) The quantification of kanamycin
ototoxicity in the rat using conditioned tone discrimination.
J. Pharm. Pharmacol., 27: 907-913.
HARRY, G.J. & TILSON, H.A. (1982) Postpartum exposure to triethyltin
produces long-term alterations in responsiveness to apomorphine.
Neurotoxicology, 3: 64-71.
HASAN, M. & ALI, S.F. (1981) Effects of thallium, nickel, and cobalt
administration on the lipid peroxidation in different regions of the
rat brain. Toxicol. appl. Pharmacol., 57: 8-13.
HAY, A. (1978) Neurotoxins may go unrecognized. Nature (Lond.),
274: 206.
HAYAT, M.A. (1973) Principles and techniques of electron microscopy,
New York, Van Rostrand Reinhold Company, Vols. 1 and 2.
HEDLUND, B., GAMARA, M., & BARTFAI, I. (1979) Inhibition of striatal
muscarinic receptors in vivo by cadium. Brain Res., 168: 216-218.
HEISE, G.A. (1983) Toward a behavioural toxicology of learning and
memory. In: Zbinden, G., Cuomo, V., Racagni, G., & Weiss, B., ed.
Application of behaviour pharmacology and toxicology, New York,
Raven Press, pp. 27-38.
HEISE, G.A. & MILAR, C. (1984) Drugs and stimulus control. In:
Iverson, S.D. & Snyder, S.H., ed. Handbook of psychopharmacology,
New York, Plenum Press, pp. 129-190.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930.
HERMAN, S.P., KLEIN, R., TALLEY, F.A., & KRIGMAN, M. (1973) An
ultrastructural study of methylmercury-induced primary sensory
neuropathy in the rat. Lab. Inv., 28: 104-118.
HERTZ, L. (1981) Gamma amino butyric-acid glutamate pathway in glia.
5th European Neuroscience Congress, Liege, Belgium, 14-18 September,
1981. Neurosci. Lett, Suppl. 7: 172.
HETZLER, B.E., HEILBRONNER, R.L., GRIFFIN, J., & GRIFFIN, G. (1981)
Acute effects of alcohol on evoked potentials in visual cortex and
superior colliculus of the rat. Electroencephalogr. clin.
Neurophysiol., 51 69-79.
HILLE, B. (1976) Gating in sodium channels of nerve. Ann. Rev.
Physiol., 38 139-152.
HINDE, R. (1974) Biological basis of human social behaviour, New
York, McGraw Hill, Inc.
HOFFMAN, H.S. & FLESHLER, M. (1963) Startle reaction: modification by
background acoustical stimulation. Science, 141: 928-930
HOFFMAN, P.N. & LASEK, R.J. (1975) The slow component of axonal
transport. Identification of major structural polypeptides of the axon
and their generality among mammalian neurons. J. cell Biol.,
66: 351.
HOLCK, H.G.O., MUNIR, A.K., MILLS, L.M., & SMITH, E.L. (1937) Studies
upon the sex differences in rats in tolerance to certain barbiturates
and to nicotine. J. Pharmacol. exp. Ther., 60: 323-346.
HOLMSTEDT, B. (1959) Pharmacology of organophosphorus cholinesterase
inhibitors. Pharmacol. Rev., 11: 567-688.
HONG, J.S. & ALI, S.F. (1982) Chlordecone (kepone) exposure in the
neonate selectively alters brain and pituitary endorphin levels in
prepuberal and adult rats. Neurotoxicology, 3: 111-118.
HOPF, H.C. & EYSHOLDT, M. (1978) Impaired refractory periods of
peripheral sensory nerves in multiple sclerosis. Ann. Neurol.,
4: 499-501.
HOROWSKI, D. & GRAF, K.J. (1979) Neuroendocrine effects of
neuropsychotropic drugs and their possible influence on toxic
reactions in animals and man: the role of dopamine-prolactin system.
Arch. Toxicol. Suppl. 2: 93-104.
HORVATH, M., ed. (1973) Adverse effects of environmental chemicals
and psychotropic drugs. Quantitative interpretation of functional
tests, Amsterdam, Oxford, New York, Elsevier Science Publishers,
Vol. 1, 292 pp.
HORVATH, M. & FRANTIK, E. (1973) Quantitative interpretation of
experimental toxicological data: the use of reference substances. In:
Horvath, M., ed. Adverse effects of environmental chemicals and
psychotropic drugs, Amsterdam, Oxford, New York, Elsevier Scientific
Publishing Co., Vol. 1, pp. 11-21.
HORVATH, M. & FRANTIK, M., ed. (1976) Adverse effects of
environmental chemicals and psychotropic drugs: neurophysiological
and behavioural tests, Amsterdam, New York, Oxford, Elsevier,
Vol. 2, 334 pp.
HORVATH, M. & FRANTIK, E. (1979) Industrial chemicals and drugs
lowering central nervous systems activation level: quantitative
assessment of hazard in man and animals. Act. Nerv. super. (Praha),
21: 269-272.
HORVATH, M. & MICHALOVA, C. (1956) Changes of EEG in experimental
exposure to carbon disulfide in the light of Vvedenskys theory of
parabiosis. Arch. Gewerbepathol., 15: 131-136.
HORVATH, M., FRANTIK, E., & KREKULE, P. (1981) Diazepam impairs
alertness and potentiates a similar effect of toluene. Act. Nerv.
super. (Praha), 22: 17-19.
HOWLAND, R.D., VYAS, I.L., LOWNDES, H.E., & ARGENTIERI, T.M. (1980)
The etiology of toxic peripheral neuropathies; in vitro effects of
acrylamide and 2,5-hexanedione on brain enolase and other glycolytic
enzymes. Brain Res., 202: 131-142.
HUGHES, J.A. & SPARBER, S.B. (1978) Delta-amphetamine unmasks
postnatal consequences of exposure to methylmercury in utero:
methods for studying behavioral teratogenesis. Pharmacol. Biochem.
Behav., 8: 365-375.
HUNT, V.R., SMITH, M.K., & WORK, D., ed. (1982) Banbury Report II -
Environmental factors in human growth and development, New York,
Cold Spring Harbor Laboratory.
HUNTER, D. & RUSSELL, D.S. (1954) Focal cerebral and cerebellar
atrophy in human subjects due to organic mercury compounds.
J. Neurol. Neurosurg. Psychiatr., 17: 235-241.
HUNTER, D., BORNFORD, R., & RUSSELL, D. (1940) Poisoning by
methylmercury compounds. Q. S. Med., 9: 193-213.
HUTCHINGS, D.E. (1978) Behavioral teratology: embryopathic and
behavioural effects of drugs during pregnancy. In: Gottlieb, G., ed.
Studies on the development of behaviour and nervous system: early
influences, New York, Academic Press, pp. 7-34.
IRWIN, S. (1968) Comprehensive observational assessment: a systematic
quantitative procedure for assessing the behavioral and psychologic
state of the mouse. Psychopharmacologia, 13: 222-257.
ISLAM, F., TAYYABA, K., & HASAN, M. (1983) Organophosphate metasystox-
induced increment of lipase activity and lipid peroxidation in
cerebral hemisphere: diminution of lipids in discrete areas of the
brain. Acta pharmacol. toxicol., 53: 121-124.
IVERSEN, L.L. (1971) Role of transmitter uptake mechanisms in synaptic
neurotransmission. Br. J. Pharmacol., 44: 571-591.
JACOBWITZ, D.M. (1974) Removal of discrete fresh regions of the rat
brain. Brain Res., 80: 111-115.
JOHN, T.H., GEGHMAN, C., & REIS, D.J. (1973) Immunochemical
demonstration of increased accumulation of tyrosine hydroxylase
protein in sympathetic ganglia and adrenal medulla elicited by
reserpine. Proc. Natl Acad. Sci. (USA), 70: 2767-2771.
JOHNSON, B.L. (1980) Electrophysiological methods in neurotoxicity
testing. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental and
clinical neurotoxicology, Baltimore, Maryland, Williams and Wilkins
Company, pp. 726-742.
JOHNSON, B.L. & ANGER, W.K. (1983) Behavioral toxicology. In: Rom,
W.N., ed. Environmental and occupational medicine, Boston, Little
Brown and Co., pp. 329-350.
JOHNSON, B.L., ANGER, W.K., SETZIER, J.V., & XINTARAS, C. (1975) The
application of a computer-controlled time discrimination performance
to problems in behavioral toxicology. In: Weiss, B. & Laties, V.G.,
ed. Behavioral toxicology, New York, London, Plenum Press,
pp. 129-153.
JOHNSON, E.W. (1980) Practical electromyography, Baltimore,
Maryland, Williams and Wilkins Company.
JOHNSON, J.E., Jr (1981) Aging and cell structure, New York, Plenum
Press, Vol. 1.
JOY, R.M. (1982) Chlorinated hydrocarbon insecticides. In: Ecobichon,
D.J. & Joy, R.M., ed. Pesticides and neurological diseases, Boca
Raton, Florida, CRC Press, pp. 91-150.
JOY, R.M. (1985) Kindling as a tool in neurotoxicology. Fund. appl.
Pharmacol., 5: 41-65.
JOY, R.M., STARK, L.G., PETERSON, S.L., BOWYER, J.T., & ALBERTSON,
T.E. (1980) The kindled seizure; production of and modification by
dieldrin in rats. Neurobehav. Toxicol., 2: 117-124.
JUNQUEIRA, L.C., CARNEIRO, J., & CONTOPOULOS, A. (1977) Basic
Histology, 2nd ed., Los Altos, Lange.
KAPLAN, K.L. (1980) Thyroxine 5'-monodeiodination in rat pituitary
homogenates. Endocrinology, 106: 567-576.
KARPOVA, O.B., OBEHOVA, E.L., AVIOVA, N.F., & SCHVARTS, E.G. (1978)
[Content and composition of cerebral gangliosides during Down's
Syndrome.] Vopros. med. Xhim., 4: 524-527 (in Russian).
KELLEHER, R.T. & MORSE, W.H. (1968) Determinations of the specificity
of behavioral effects of drugs. Ergeb. Physiol., 60: 1-56.
KHOLODOV, Ju.A. & SOLOV'EVA, G.R. (1971) [Occasional publication:
news on medical equipment manufacture,] fasc. 3, pp. 69-72
(in Russian).
KIMURA, J. (1976) Collision techniques: physiological block of nerve
impulses in studies of motor nerve conduction velocity. Neurology,
26: 680-682.
KING, J.A. & VESTAL, B.M. (1974) Visual acuity of peromyscus.
J. Mammal., 55: 238-243.
KIRPEKAR, S.M., DIXON, W., & PRATT, J.C. (1970) Inhibitory effect of
manganese on norephinephrine release from the splenic nerves of cat.
J. Pharmacol. exp. Ther., 174: 72-76.
KLEIN, R., HERMAN, S., BRUBAKER, P.E., & LUCIER, G.W. (1972) A model
of acute methylmercury intoxication in rats. Arch. Path.,
93: 404-418.
KODAMA, J., FUKUSHIMA, M., & SAKATA, T. (1978) Impaired taste
discrimination against quinine following chronic administration of
theophylline in rats. Physiol. Behav., 20: 151-155.
KOHLER, W. (1976) The mentality of ages, New York, Liveright,
336 pp.
KONAT, G. & CLAUSEN, J. (1977) Triethyl lead-induced intoxication as
an experimental model of hypomyelination. Proc. Int. Soc.
Neurochem., 6: 215.
KORNBERG, A. (1955) Lactic dehydrogenase in muscle. In: Colowick, S.P.
& Kaplan, N.O., ed. Methods in enzymology, New York, Academic Press,
Vol. 1, p. 441.
KOSTIAL, K., LANDEKA, M., & SLAT, B. (1974) Manganese ions and
synaptic transmission in the superior cervical ganglion of the cat.
Br. J. Pharmacol., 51: 231-235.
KOTLIAREVSKI, L.I. (1951) [Methodology for studying motor conditioned
reflexes in some small animals (white rats and guinea-pigs.]
Zh. Vyssh. nerv. deyat. imeni, 1(5): 753-761 (in Russian).
KOTLIAREVSKI, L.I. (1957) [Procedure for the investigation of motor-
food conditioned reflexes in small animals.] Tr. Inst. Vyssh. nerv.
deyat. AN SSR, 3: 23-28 (in Russian).
KRIGMAN, M.R. & HOGAN, E.L. (1974) Effect of lead intoxication on
the postnatal growth of the rat nervous system. Environ. Health
Perspect., 7: 187-199.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., SUTER, Y., THOMANN, G., &
HESS, R. (1981) Pyridoxine megavitaminosis produces degeneration of
peripheral sensory neurons (sensory neuronopathy) in the dog.
Neurotoxicology, 2: 13-24.
KRINKE, G., SCHAUMBURG, H.H., SPENCER, P.S., THOMANA, P., & HESS, R.
(1979) Clinoquinol and 2,5-hexanedione induce different types of
distal axonopathy in the dog. Acta neuropathol., 47: 213.
KRSIAK, M. (1979) Effects of drugs on behavior of aggressive mice.
Br. J. Pharmacol., 65: 525-533.
KRUSIUS, T. & FINNE, J. (1977) Structural features of tissue
glycoproteins. Fractionation and methylation analysis of gylcopeptides
derived from rat brain, kidney, and liver. Eur. J. Biochem.,
78: 369-379.
KRYZHANOVSKY, G.N. (1973) Mechanism of action of tetanus toxin. Effect
on synaptic processes and some particular features of toxin binding by
the nervous tissue. Jaunyn-Schmiedeberg's Arch., 276: 247-270.
KUCZENSKI, R.T. & MANDELL, A.J. (1972) Regulatory properties of
soluble and particulate rat brain tyrosine hydroxylase. J. biol.
Chem., 247: 3114-3116.
KULKOSKY, P.J., ZELLNER, D.A., HYSON, R.L., & RILEY, A.L. (1980)
Ethanol consumption of rats in individual, group, and colonial housing
conditions. Physiol. Psychol., 8: 56-60.
KUPFER, D. & BULGER, W.H. (1976) Studies on the mechanism of
estrogenic actions of o-p'-DDT; interactions with the estrogen
receptor. Pestic. Biochem. Physiol., 6: 561-570.
KUPFER, D. & BULGER, W.H. (1980) Estrogenic properties of DDT and its
analogues. In: McLachlan, J., ed. Estrogens in the environment,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 239-263.
LAFFERMAN, J.A. & SILBERGELD, E.K. (1979) Erythrosin B inhibition of
neurotransmitter accumulation by rat brain homogenate. Science,
206: 363-365.
LAJTHA, A. & MARKS, N. (1971) Protein turnover. In: Lajtha, A., ed.
Handbook Neurochemistry, New York, Plenum Press, Vol. 5, pp. 551-629
LAMARTINIERE, C.A., DIERINGER, C.S., & LUCIER, G.W. (1979) Altered
ontogeny of glutathione S-transferases by 2,4,5-2',4',5'-
hexachlorobiphenyl. Toxicol. appl. Pharmacol., 51: 233-238.
LANDOWNE, D. & RITCHIE, J.M. (1976) On the control of glycogenolysis
in mammalian nervous tissue by calcium. J. Physiol., 212: 503-517.
LATIES, V.G. (1982) Contributions of operant conditioning to
behavioral toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 67-80.
LAZAROVA, M., BENDOTTI, C., & SAMANIN, R. (1984) Evidence that the
dorsal raphe area is involved in the effect of clonidine against
pentylenetetrazol-induced seizures in rats. Naunyn-Schmiedebergs
Arch. Pharmacol., 325: 12-16.
LEANDER, J.D. & MACPHAIL, R.C. (1980) Effect of chlordimeform
(a formamidine pesticide) on schedule-controlled responding of
pigeons. Neurobehav. Toxicol., 2: 315-321.
LEANDER, J.D., MCMILLAN, D.E., & BARLOW, T.S. (1977) Chronic mercuric
chloride: behavioural effects in pigeons. Environ. Res.,
14: 424-435.
LEHMANN, H.J. & TACKMANN, E. (1974) Neurographic analysis of trains of
frequent electrical stimuli in the diagnosis of peripheral nerve
disease. Eur. Neurol., 12: 293-308.
LEHRER, G.M. (1974) Measurement of minimal brain dysfunction. In:
Xintaras, C., Johnson, B.L., & DeGroot, I., ed. Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, pp. 450-454 (US DREW (NIOSH) Publication No. 74-126).
LEONARD, J.L. & ROSENBERG, L.H. (1980) Characterization of essential
enzyme sulfhydryl groups of thryoxine 5'deiodinase from rat kidney.
Endocrinology, 108: 444-451.
LEVINE, T.E. (1976) Effects of carbon disulfide and FLA-63 on operant
behavior in pigeons. J. Pharmacol. exp. Ther., 199: 669-678.
LEWEY, F.H. (1941) Experimental chronic carbon disulfide poisoning in
dogs. J. ind. Hyg. Toxicol., 23: 415-535.
LILIENTHAL, H., WINNEKE, G., BROCKHAUS, A., MOLIK, B., & SCHLIPKOTER,
H.-W. (1983) Learning set-formation in rhesus monkeys pre- and
postnatally exposed to lead. In: Proceedings of the International
Conference on Heavy metals in the Environment, Heidelberg, September,
1983, Edinburgh, CEP Consultants, p. 901.
LINDSLEY, D.B. & WICKE, J.D. (1974) The electroencephalogram:
autonomous electrical activity in man and animals. In: Thompson, R. &
Patterson, M., ed. Bioelectric recording techniques, Part B, New
York, Academic Press.
LIPP, J.A. (1968) Cerebral electrical activity following soman
administration. Arch. int. Pharmacodyn., 175: 161-169.
LOCK, E.A. (1976) Increase in cerebral fluids in rats after treatment
with hexachlorophene or triethyltin. Biochem. Pharmacol.,
25: 1455-1458.
LORENZO, A.V. & GERWITZ, M. (1977) Inhibition of 14C-tryptophane
transport into brain of lead-exposed neonatal rabbits. Brain Res.,
132: 386-392.
LOWITZSCH, K., GOHRING, U., HECKING, E., & KOHLER, H. (1981)
Refractory period, sensory conduction velocity, and visual-evoked
potentials before and after haemodialysis. J. Neurol. Neurosurg.
Psychiatr., 44: 121-128.
LOWNDES, H.E. & BAKER, T. (1976) Studies on drug-induced neuropathies.
III. Motor nerve deficit in cats with acrylamide neuropathy. Eur. J.
Pharmacol., 35: 177-184.
LOWNDES, H.E., BAKER, T., CHO, E.S., & JORTNER, B.S. (1978a) Position
sensitivity of deafferented muscle spindles in experimental acrylamide
neuropathy. J. Pharmacol. exp. Ther., 205: 40-48.
LOWNDES, H.E., BAKER, T., MICHAELSON, L.P., & VINCENT-ABLAZEY, M.
(1978b) Attenuated dynamic responses of primary endings of muscle
spindles; a basis for depressed tendon responses in acrylamide
neuropathy. Ann. Neurol., 3: 435-437.
LUKAS, E. (1970) Stimulation electrohypography in experimental
toxicology (carbon disulfide neuropathy in rats). Med. Lav.,
61: 302-308.
MCBLAIN, W.A., LEWIN, V., & WOLFE, F.H. (1976) Differing estrogenic
activities for the enatiomers of o,p'-DDT in immature female rat.
Can. J. Physiol., 54: 629-632.
MACDONALD, J.S. & HARBISON, R.D. (1977) Methylmercury induced
encephalopathy in mice. Toxicol. appl. Pharmacol., 39: 195-205.
MCDONALD, W.I. (1963) The effects of experimental demyelination on
conduction in peripheral nerve. II. Electrophysical observations.
Brain, 86: 501-524.
MCDONOUGH, J.H., Jr, HACKLEY, B.E., Jr, CROSS, R., SAMSON, F., &
NELSON, S. (1983) Brain regional glucose use during soman-induced
seizures. Neurotoxicology, 4: 203-210.
MCKENNA, M.J. & DESTEFANO, V. (1975) A proposed mechanism of action of
carbon disulfide on carbon disulfide on dopamine-beta-hydroxylase.
Toxicol. appl. Pharmacol., 33: 137.
MCMILLAM, D.E. (1982) Effects of chronic administration of pesticides
on schedule-controlled responding by rats and pigeons. In: Chambers,
J.E. & Yarborough, J.D., ed. Effects of chronic exposures to
pesticides on animal systems, New York, Raven Press, pp. 211-226.
MACPHAIL, R.C. (1982) Studies on the flavour aversions induced by
trialkyltin compounds. Neurobehav. Toxicol. Teratol., 4: 225-230.
MACPHAIL, R.C. & LEANDER, J.D. (1980) Flavour aversions induced by
chlordimeform. Neurobehav. Toxicol., 2: 363-365.
MACPHAIL, R.C., CROFTON, K.M., & REITER, L.W. (1983) Use of
environmental challenges in behavioral toxicology. Fed. Proc.,
42: 3196-3200.
MACTUTUS, C.F., UNGER, K.L., & TILSON, H.A. (1982) Neonatal
chlordecone exposure impairs early learning and memory in the rat on a
multiple measure passive avoidance task. Neurotoxicology, 3: 24-44.
MAGATA, Y. & TSUKADA, W. (1978) Bulk separation of neuronal cell
bodies and glial cells from mammalian brain and some of their
biochemical properties. Rev. Neurosci., 3: 18-27.
MAILMAN, R.B., FERRIS, R.M., VOGEL, R.A., KILTS, C.D., LIPTOM, M.A.,
TANG, F.L., SMITH, D.A., MUELLER, R.A., & BREESE, G.R. (1980)
Non-specificity of the biochemical actions of erythrosine (Red No. 3):
what relation to behavioural changes. Science, 207: 535-537.
MARES, P. & VELISEK, L. (1983) Influence of ethosuximide on metrazol-
induced seizures during ontogenesis in rats. Act. Nerv. super
(Praha), 25: 292-298.
MARSHALL, J.F. (1975) Increased orientation to sensory stimuli
following lateral hypothalamic damage in rats. Brain Res.,
86: 373-387.
MARSHALL, J.F. & TEITELBAUM, P. (1974) Further analysis of sensory
inattention following lateral hypothalamic damage in rats. J. comp.
physiol. Psychol., 86: 375-395.
MARSHALL, J.F., TURNER, D.H., & TEITELBAUM, P. (1971) Sensory neglect
produced by lateral hypothaLamic damage. Science, 174: 523-525.
MARSHALL, J.F., RICHARDSON, J.S., & TEITELBAUM, P. (1974)
Nigrostriatal bundle damage and the lateral hypothalamic syndrome.
J. comp. Physiol. Psychol., 87: 808-830.
MARSLAND, T.A., GLEES, P., & ERIKSON, L.B. (1954) Modification of
Glee's silver impregnation for paraffin sections. J. Neuropathol.
exp. Neurol., 13: 587-591.
MASTEROV, A.V. (1974) [Features of the interaction of rats in an
electromagnetic field following extinction of conditioned food
reactions.] In: [Problems of clinical and experimental medicine,]
Moscow, pp. 61-63 (in Russian).
MAURISSEN, J.P.J. (1979) Effects of toxicants on the somatosensory
system. Neurobehav. Toxicol., 1(Suppl. 1): 23-32.
MEDVEDEV, B.M. (1975) [The effect of a static electric field on
conditioned reflex activity.] Fiziol. Zh., 3: 320-325 (in Russian).
MELLO, N.K. (1975) Behavioral toxicology; a developing discipline.
Fed. Proc., 34: 1832-1834.
MENDELL, J.R., SAIDA, K., GANANSIA, M.F., JACKSON, D.B., WEISS, H.,
GARDIER, R.S., CHRISMAN, C., ALLEN, N., COURI, D., O'NEILL, J., MARKS,
B., & HETLAND, L. (1974) Toxic polyneuropathy produced by methyl
n-butyl ketone. Science, 185: 787-789.
MENDELSON, J.H., MELLO, N.K., & ELLINGBOE, J. (1977) Effects of acute
alcohol intake on pituitary-gonadal hormones in normal human males.
J. Pharmacol. exp. Ther., 202: 675-682.
MERIGAN, W.H. (1979) Effects of toxicants on visual systems.
Neurobehav. Toxicol., 1: 15-22.
MERIGAN, W.H. (1980) Visual fields and flicker thresholds in
methylmercury poisoned monkeys. In; Merigan, W.H. & Weiss, B., ed.
Neurotoxicity of the visual system, New York, Raven Press,
pp. 149-163.
MERKURYVA, R.V. & BUSHINSKAYA, L.I. (1982) [Metabolism of
sialo-containing glycoproteins and lysosomal enzyme activity under
neurotropic effect of chemical environmental factors.] Ukranian
Biochem. J., 53: 31-35 (in Russian).
MERKURYVA, R.V. & TSAPKOVA, N.N. (1982) Hygienic significance of a
system of biochemical criteria for the evaluation of hepatotoxic and
neurotoxic effects of some chemical factors in the environment.
J. Hyg. Epidemiol. Microbiol. Immunol., 26: 336-341.
MERKURYVA, R.V., BUSHINSKAIA, L.J., AULIKA, B.V., SHATERNIKOVA, J.S.,
PROTSENKO, S.J., KULYGINA, A.A., & EKSLER, N.D. (1978) The activity of
lyosomal and cytoplasmic enzymes under the experimental influence of
bisulfide of carbon. Vop. Med. Xhim., 2: 151-156.
MERKURYVA, R.V., KOTOV, A.V., SILOV, V.G., BUSHINSKAIA, L.J.,
MUCHAMBETOVA, L.KH., & TSAPKOVA, N.N. (1980) Experimental study of
biochemical and physiological mechanisms of cerebral activity
influenced by alcohol, Nauka, Minsk Publishing House, pp. 93-94.
MEYER, O.A., TILSON, H.A., BYRD, W.C., & RILEY, H.T. (1979) A method
for the routine assessment of fore- and hindlimb grip strength of rats
and mice. Neurobehav. Toxicol., 1: 233-236.
MICZEK, K.A. & BARRY, H. (1976) Pharmacology of sex and aggression.
In: Glick, S.D. & Goldforb, J., ed. Behavioral pharmacology,
St. Louis, Missouri, C.V. Mosby Co., pp. 176-257.
MIKISKA, A. (1960) [Determination of the susceptibility to electrical
activity of the cerebral motor cortex, and its pharmacological and
toxicological applications. I. Methodology, controlled trials and
physiological basis.] Arch. Gewerbepathol. Gewerbehyg., 18: 286-299
(in German).
MIKISKOVA, H. & MIKISKA, A. (1962) [A contribution to the application
of defensive skin stimulation in the toxicity of substances acting on
the CNS. II. Measuring stimulus threshold intensity in guinea-pigs.]
Int. Arch. Gewerbepathol. Gewerbehyg., 19: 68-75 (in German).
MIKISKOVA, H. & MIKISKA, A. (1966) Trichloroethanol in
trichloroethylene poisoning. Br. J. ind. Med., 23: 116-125.
MIKISKOVA, H. & MIKISKA, A. (1968) Some electrophysiological methods
for studying the action of narcotic agents in animals, with special
reference to industrial solvents: a review. Br. J. ind. Med.,
25: 81-105.
MILLER, D.B. (1983) Neurotoxicity of the pesticidal carbamates.
Neurobehav. Toxicol. Teratol., 4: 779-788.
MILLER, E.K. & DAWSON, R.M.C. (1972) Can mitochondria and synaptosomes
of guinea-pig brain synthesize phospholipids? Biochem. J.,
126: 805-807.
MINEGISHI, A., FUJUMORI, R., SATOH, T., KILAGAWA, H., & YANAURA, S.
(1979) Changes in serotonin turnover and the brain sensitivity to
barbituates by disulfiram treatment in rats. Res. Comm. Chem. Pathol.
Pharmacol., 24: 273-285.
MINKINS, C., CHEKUNOVA, M.P., & LEVINA, E.N. (1973) [Effect of
antimony and lead on the adrenal glands and biogenic amines.]
Gig. Tr. Prof. Zabol., 17: 21-24 (in Russian).
MITCHELL, C.L. & TILSON, H.A. (1982) Behavioral toxicology in risk
assessment: problems and research needs, Boca Raton, Florida, CRC
Press, pp. 265-274 (CRC Critical Reviews in Toxicology 10).
MITCHELL, C.L., TILSON, H.A., & CABE, P.A. (1982) Screening for
neurobehavioral toxicity: factors to consider. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 237-245.
MIYOSHI, T. & GOTO, I. (1973) Serial in vivo determinations of nerve
conduction velocity in rat tails: physiological and pathological
changes. Electroencephal. clin. Neurophysiol., 35: 125-131.
MOLINENGO, L. & ORSETTI, M. (1976) Drug action on the "grasping"
reflex and on swimming endurance: an attempt to characterize
experimentally anti-depressant drugs. Neuropharmacology,
15: 257-260.
MORALI, G., LARSSON, K., & BEYER, C. (1977) Inhibition of
testosterone-induced sexual behaviour in the castrated male rat by
aromatization blockers. Horm. Behav., 9: 203-213.
MORGANTI, J.B., LOWNE, B.A., SALVATERRA, P., & MASSARO, E.J. (1976)
Effects of open-field behaviour of mice exposed to multiple doses of
methylmercury. Gen. Pharmacol., 7(1): 41-44.
MUNRO, A.J., JACKSON, R.J., & KORNER, A. (1964) Disaggregation of
brain polysomes. Biochem. J., 92: 289-294.
MURPHY, S.D. & DUBOIS, K.P. (1958) The influence of various factors on
the enzymatic conversion of organic thiophosphates to
anticholinesterase agents. J. Pharmacol. exp. Ther., 124: 194-202.
NARAHASHI, T. (1982) Cellular and molecular mechanisms of action of
insecticides: neurophysiological approach. Neurobehav. Toxicol.
Teratol., 4: 753-758.
NARAHASHI, T. & HAAS, H.G. (1967) DDT: interaction with nerve membrane
conductance changes. Science, 157: 1438-1440.
NARAHASHI, T. & HAAS, H.G. (1968) Interaction with DDT with the
components of lobster nerve membrane conductance. J. gen. Physiol.,
51: 177-198.
NATHANSON, J.A. & BLOOM, F.E. (1975) Lead-induced inhibition of brain
adenylcyclase. Nature (Lond.), 255: 419-420.
NICHOLAS, G.S. & BARRON, D.H. (1932) The use of sodium amytal in the
production of anaesthesia in the rat. J. Pharmacol. exp. Ther., 46;
125-129.
NIEDERMEYER, E. & LOPES DA SILVA, F. (1982) Electroencephalography:
basic principles, clinical applications, and related fields,
Baltimore, Maryland, Munich, Urban Schwartzenberg.
NIOKESARYISKY, A.A. & TUROVSKY, V.S. (1980) N-acetylneuraminic acid
content in water-soluble and membranous tissue fractions of various
analysers of cerebral cortex. Biochimia, 45: 1829-1932.
NORTON, S. (1973) Amphetamine as a model for hyperactivity in the rat.
Physiol. Behav., 11: 181-186.
O'BRIEN, R.D. (1976) Acetylcholinesterase and its inhibition. In:
Wilkerson, C.F., ed. Insecticide biochemistry and physiology, New
York, Plenum Press, pp. 271-296.
O'CALLAGHAN, J.P., LAVIN, K.L., CHESS, Q., & CLOUET, D.H. (1983) A
method for dissection of discrete regions of rat brain following
microwave irradiation. Brain Res., 11: 31-42.
O'DONOGHUE, J.L., ed. (1985) Neurotoxicity of industrial and
commercial chemicals, Boca Raton, Florida, CRC Press.
OCHOA, J. (1972) Ultrathin longitudinal sections of single myelinated
fibres for electron microscopy. J. neurol. Sci., 17: 103.
OCHS, S. (1972) Fast transport of materials in mammalian nerve fibres.
Science, 176: 252.
OCHS, S. (1982) Axoplasmic transport and its relation to other nerve
functions, New York, John Wiley and Sons.
OHI, G., NISHIGAKI, S., SEKI, H., TAMURA, Y., MIZOGUCHI, I., YAGYA,
H., & NAGASHIMA, K. (1978) Tail rotation, an early neurological sign
of methylmercury poisoning in the rat. Environ. Res., 16: 353-359.
OLIVER, C., ESKAY, R.L., BEN-JONATHAN, N., & PORTER, J.C. (1974)
Distribution and concentration of TRH in rat brain. Endocrinology,
96: 540-546.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1979) Hippocampus
space, and memory. Behav. Brain Sci., 2: 313-365.
OLTON, D.S., BECKER, J.T., & HANDELMANN, G.E. (1980) Hippocampal
function: working memory or cognitive mapping? Physiol. Psychol.,
8: 239-246.
OTTO, D.A. (1977) Neurobehavioral toxicology: problems and methods in
human research. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 10/1-10/31
(EPA 600/9-77-042).
OTTO, D.A. (1978) Multidisciplinary perspectives in event-related
brain potential research. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Washington
DC, US Environmental Protection Agency (EPA 600/9-77-043).
OTTO, D.A., BENIGNUS, V.A., MULLER, K.E., & BARTON, C.N. (1981)
Effects of age and body lead burden on CNS function in young children.
I. Slow cortical potentials. Electroencephalogr. clin.
Neurophysiol., 52: 229-239.
OVERMANN, S.R. (1977) Behavioral effects of asymptomatic lead exposure
during neonatal development in rats. Toxicol. appl. Pharmacol.,
41: 459-471.
PADICH, R. & ZENICK, M. (1977) The effects of developmental and/or
direct lead exposure on FR behavior in the rat. Pharmacol. Biochem.
Behav., 6: 371-375.
PALKOVITS, M. (1973) Isolated removal of hypothalamic or other brain
nuclei of the rat. Brain Res., 59: 449-450.
PALMITER, R.D. & MULVIHILL, E.D. (1978) Estrogenic activity of the
insecticide kepone on the chick oviduct. Science, 201: 356-358.
PANCHENKO, L.F., DUDCHENKO, A.M., & SHPAKOV, A.A. (1975) The influence
of acute hypoxic hypoxia on the activity of rats cerebral cortex:
mitochondria enzymes possessing different sensibility to oxygen
deficiency. In: Gorky, ed. Biochemistry of hypoxia, pp. 59-64.
PANSKY, B. & ALLEN, D.J. (1980) Review of neuroscience, New York,
McMillan Inc.
PARIZEK, J. (1956) Effects of cadmium salts on testicular tissue.
Nature (Lond.), 177: 1036-1037.
PARTINGRON, C.R. & DALY, J.W. (1979) Effect of gangliosides on
adenylate cyclase activity in rat cerebral cortical membranes. Mol.
Pharmacol., 15: 484-491.
PARTRIDGE, W.M. (1976) Inorganic mercury: selective effects on
blood-brain barrier transport systems. J. Neurochem., 27: 333-335.
PATTON, S.E., O'CALLAGHAN, J.P., MILLER, D.B., & ABOU-DONIA, M.B.
(1983) Effect of oral adminstration of tri-o-cresyl phosphate on in
vitro phosphorylation of membrane and cytosolic proteins from
chicken brain. J. Neurochem., 41: 897-901.
PAUL, S.M., HOFFMAN, A.R., & AXELROD, A. (1980) Catechol estrogens:
synthesis and metabolism in brain and other endocrine tissues. In:
Martini, L. & Ganong, W.F., ed. Frontiers in neuroendocrinology, New
York, Raven Press, pp. 203-217.
PAVLENKO, S.M. (1975) Methods for the study of the central nervous
system in toxicological tests. In: Methods used in the USSR for
establishing biologically safe levels of toxic substances, Geneva,
World Health Organization, pp. 86-108.
PAVLOV, I.P (1927) Conditioned reflexes, Oxford, Oxford University
Press.
PEARSE, A.G.E. (1968) Histochemistry: theoretical and applied, 3rd
ed., Baltimore, Maryland, Williams and Wilkins Company.
PEARSON, C.M. & MOSTOFI, F.K. (1973) The striated muscle, Baltimore,
Maryland, Williams and Wilkins Company.
PEASE, D.E. (1964) Histological techniques for electron microscopy,
2nd ed., New York, Academic Press.
PERSINGER, M.A., LAFREINIERE, G.P., & CARREY, N.V. (1978) Thyroid
morphology and wet organ weight changes in rats exposed to different
low intensity 0.5-Hz magnetic fields and pre-experimental caging
conditions. Biometeorology, 22: 67-73.
PILCHER, C.W.T. & JONES, S.M. (1981) Social crowding enhances
aversiveness of naloxone in rats. Pharmacol. Biochem. Behav.,
14: 299-303.
PIRCH, J.H. & RECH, R.H. (1968) Effect of isolation on alpha-
methyltyrosine-induced behavioral depression. Life Sci., 7: 173-182.
PLEASURE, D.E., MISHLER, K.C., & ENGEL, W.K. (1969) Axonal transport
of proteins in experimental neuropathies. Science, 166: 524-525.
POST, E.M., YANG, M.G., KING, J.A., & SANGER, V.L. (1973) Behavioral
changes of young rats force-fed methylmercury chloride. Proc. Soc.
Exp. Biol. Med., 143: 1113-1116.
POWERS, J.M., SCHAUMBURG, H.H., JOHNSON, A.E., & RAINE, C.S. (1980) A
correlative study of the adrenal cortex in adrenoleukodystrophy:
evidence for a fatal intoxication with very long-chain saturated fatty
acids. Invest. cell Pathol., 3: 353-376.
PRABHU, V.G. (1972) Amphetamine aggregation effect in mice under
conditions of altered microsomal enzymes. Arch. int. Pharmacodyn.
Ther., 195: 81-86.
PROKHOROVA, M.J. (1974) Structure and function of phosphornositides
and gangliosides of the brain. In: Progress of neurochemistry,
Leningrad, Nauka, pp. 61-73.
PRYOR, G.T., UYENO, E.T., TILSON, H.A., & MITCHELL, C.L. (1983)
Assessment of chemicals using a battery of neurobehavioral tests: a
comparative study. Neurobehav. Toxicol. Teratol., 5: 91-117.
PURPURA, D.P., PENRY, J.K, TOWER, P., WOODBURY, D.M., & WALTER, R.
(1972) Experimental models of epilepsy, New York, Raven Press.
QUEVEDO, L., CONCHA, J., MARCHANT, Z., ESKUCHE, W., & MADARIAGA, J.
(1980) Nerve accommodation in chronic alcoholic subjects.
Pharmacology, 21: 229-232.
QUINN, G.P., AXELROD, J., & BRODIE, B.B. (1958) Species, strain, and
sex differences in metabolism of hexobarbitone, amidopyrine,
antipyrine, and aniline. Biochem. Pharmacol., 1: 152-159.
RADIN, N.S., BRENHERT, A., ARORA, R.C., SELLINGER, O.Z., & FLANGAS,
A.L. (1972) Glial and neuronal localization of cerebroside-
metabolizing enzymes. Brain Res., 39: 163-166.
RAFALES, L.S., BORNSCHEIN, R.L., MICHAELSON, A., LOCH, R.K., & BARKER,
G.F. (1979) Drug-induced activity in lead-exposed mice. Pharmacol.
Biochem. Behav., 10: 95-104.
RAMSAY, R.B., KRIGMAN, M.R., & MORELL, P. (1980) Developmental studies
of the uptake of choline, GABA, and dopamine by crude synaptosomal
preparations after in vivo or in vitro lead treatment. Brain
Res., 187: 383-387.
RAY, O.S. & BARRETT, R.J. (1975) Behavioral, pharmacological, and
biochemical analysis of genetic differences in rats. Behav. Biol.,
15: 391-417.
REICHARDT, L.F. & KELLY, R.B. (1983) A molecular description of nerve
terminal function. Ann. Rev. Biochem., 52: 871-926.
REICHERT, B.L. & ABOU-DONIA, M.B. (1980) Inhibition of fast axoplasmic
transport by delayed neurotoxic organophosphorus esters: a possible
mode of action. Mol. Pharmacol., 17: 56-60.
REITER, L.W. (1978) Use of activity measures in behavioral toxicology.
Environ. Health Perspect., 26: 9-20.
REITER, L.W. (1980) Neurotoxicology -- meet the real world.
Neurobehav. Toxicol., 2: 73-74.
REITER, L.W. (1983) Chemical exposure and animal activity: utility of
the Figure-Eight Maze. In: Hayes, A.H., Schnell, R.C., & Miya, T.S.,
ed. Developments in the science and practice of toxicology,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 78-84.
REITER, L.W. & MACPHAIL, R.C. (1979) Motor activity: a survey of
methods with potential use in toxicity testing. Neurobehav.
Toxicol., 1(Suppl. 1): 53-66.
REITER, L.W. & MACPHAIL, R.C. (1982) Factors influencing motor
activity measurements in neurotoxicology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 45-65.
REITER, L.W., KIDD, K., HEAVNER, G., & RUPPERT, P. (1980) Behavioral
toxicity of acute and subacute exposure to triethyltin in the rat.
Neurotoxicology, 2: 97-112.
REUHL, K. & CHANG, L. (1979) Effects of methylmercury on the
development of the nervous system: a review. Neurotoxicology,
1: 21-55.
REYNOLDS, G.S. (1958) A primer of operant conditioning, Glenview,
Illinois, Scott, Foresman and Co.
RICE, D.C. & GILBERT, S.G. (1982) Early chronic low-level
methylmercury poisoning in monkeys impairs spatial vision. Science,
216: 759-761.
RICE, D.C. & WILLES, R.F. (1979) Neonatal low-level lead exposure in
monkeys (Macaca fascicularis): effects on two choice non-spatial
form discrimination. J. environ. Pathol. Toxicol., 2: 1195-1203.
RICE, D.C., GILBERT, S.G., & WILLES, R.F. (1979) Neonatal low-level
lead exposure in monkeys: locomotor activity, schedule-control
behavior, and the affects of amphetamine. Toxicol. appl. Pharmacol.,
51: 503-513.
RICE, D.F. (1985) Central nervous system effects of perinatal exposure
to lead or methylmercury in the monkey. In: Clarkson, T.W. & Nordberg,
G., ed. Reproductive developmental toxicity of metals, New York,
Plenum Press.
RITCHIE, J.M. (1979) A pharmacological approach to the structure of
sodium channels in myelinated axons. Ann. Rev. Neurosci.,
2: 341-362.
ROBERTS, D.V. (1977) A longitudinal electromyographic study of six men
occupationally exposed to organophosphorus compounds. Int. Arch.
occup. environ. Health, 38: 221-229.
ROBERTS, R.K., DESMOND, P.V., WILKINSON, G.R., & SCHENKER, S. (1979)
Disposition of chlordiazepoxide; sex differences and effects of oral
contraceptives. Clin. Pharmacol. Ther., 25: 826-831.
ROBINS, E., HIRSCH, H.E., & EMMONS, S.S. (1968) Glycosidases in the
nervous system. I. Assay, some properties, and distribution of
beta-galactosidase, beta-glucoronidase, and beta-glucosidase.
J. biol. Chem., 243: 4246-4249.
RODIER, P. (1978) Behavioral teratology. In: Wilson, J.G. & Fraser,
F.C., ed. Handbook of teratology, New York, Plenum Press, Vol. 4,
pp. 397-428.
ROEDER, R.J. & RUTTER, W.J. (1969) Multiple forms of DNA-dependent RNA
polymerase in eukaryotic organisms. Nature (Lond.), 224: 234-236.
ROSE, S.P.R. & SINHA, A.K. (1970) Separation of neuronal and neuropil
cell fractions: a modified procedure. Life Sci., 9: 907-912.
ROSECRANS, J.A., HONG, J.S., SQUIBB, R.E., JOHNSON, J.H., WILSON,
W.E., & TILSON, H.A. (1982) Effects of perinatal exposure to
chlordecone (kepone) on neuroendocrine and neurochemical
responsiveness of rats to environmental challenges. Neurotoxicology,
3: 131-142.
ROSEWICKYI, S., STANOSZ, S., & SAMOCHOWIEC, L. (1973) [The effect of
protracted exposure to carbon disulfide on the hormonal activity of
the ovaries in rats.] Med. Pracy., 24: 133-136 (in Polish).
ROZIER, L., SHIRAKI, H., & GRCEVIC, N., ed. (1977) Neurotoxicology,
New York, Raven Press, Vol. 1, 658 pp.
RUDNEV, M., BOKINA, A., ESKLER, N., & NAVAKATIKYAN, M. (1978) The use
of evoked potential and behavioural measures in the assessment of
environmental insult. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency, pp. 444-447 (EPA 600/9-77-0443).
RUSSELL, R.W., WARBURTON, D.M., & SEGAL, D.S. (1969) Behavioral
tolerance during chronic changes in the cholinergic system. Comm.
Behav. Biol., 4: 121-128.
RUSAK, B. & ZUCKER, I. (1975) Biological rhythms and animal behavior.
Ann. Rev. Psychol., 27: 137-171.
SABRI, M.I. & SPENCER, P.S. (1980) Toxic distal axonopathy:
biochemical studies and hypothetical mechanisms. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 206-219.
SABRI, M.I., MOORE, C.L., & SPENCER, P.S. (1979) Studies on the
biochemical basis of distal axonopathies. Inhibition of glycolysis
produced by neurotoxic hexacarbon compounds. J. Neurochem.,
32: 683-690.
SAKMANN, B. & NEHER, E. (1983) Single channel recording, New York,
Plenum Press, 503 pp.
SALVATERRA, P., LOWN, B., MORGANTI, J., & MARSARO, E.J. (1973)
Alterations in neurochemical and behavioural parameters in the mouse
induced by low doses of methylmercury. Acta pharmacol. toxicol.,
33(3): 177-190.
SANTINI, M. (1975) Golgi Centennial Symposium: Perspectives in
Neurobiology, New York, Raven Press.
SAVOLAINEN, H. (1983) Trends and prospects in experimental
neurotoxicology. Scand. J. Work environ. Health, 9: 214-218.
SAVOLAINEN, H. & JARVISALO, J. (1977) Effects of acute CS2
intoxication on protein metabolism in rat brain. Chem.-biol.
Interact., 17: 51-59.
SAVOLAINEN, H., PFAFFLI, P., TENGEN, M., & VAINO, H. (1977)
Biochemical and behavioural effects of inhalation exposure to
tetrachloroethylene and dichloromethane. J. Neuropathol. exp.
Neurol., 36: 941-949.
SCHALLERT, T., WHISHAW, I.Q., RAMIREZ, V.D., & TEITELBAUM, P. (1978)
Compulsive, abnormal walking caused by anticholinergies in akinetic, 6
hydroxydopamine-treated rats. Science, 199: 1461-1463.
SCHAUMBURG, H.H. (1982) Pyridoxine megavitaminosis produces sensory
neuropathy in humans. Ann. Neurol., 12: 107-108.
SCHAUMBURG, H.H., SPENCER, P.S., & THOMAS, P.K. (1983) Disorders of
peripheral nerves, Philadelphia, Pennsylvania, F.A. Davis.
SCHAUMBURG, H.H., WISNIEWSKI, H., & SPENCER, P.S. (1974)
Ultrastructural studies of the dying-back process. I. Peripheral nerve
terminal and axon degeneration in systemic acrylamide intoxication.
J. Neuropathol. exp. Neurol., 33: 260-284.
SCHECHTER, M.D. & WINTER, J.C. (1971) Effect of mescaline and lysergic
acid diethylamide on flicker discrimination in the rat. J. Pharmacol.
exp. Ther., 177: 461-467
SCHEUHAMMER, A.M. & CHERIAN, M.G. (1982) The regional distribution of
lead in normal rat brain. Neurotoxicology, 3: 85-92.
SCHEVING, L.E., HALBERG, F., & PAULY, J.E., ed. (1974)
Chronobiology, Tokyo, Igaku Shoin Ltd.
SCHNAITMAN, C., ERWIN, V.G., & GREENSWALT, J.W. (1967) The
submitochondrial localization of monoamine oxidase. An enzymatic
marker for the outer membrane of rat liver mitochondria. J. cell
Biol., 32: 719-720.
SCHOENFELD, W.N., ed., (1970) The theory of reinforcement schedules,
New York, Appleton-Century-Crofts.
SCHOTMAN, P., GIPON, L., JENNEKENS, F.G.I., & GISPEN, W.H. (1978)
Polyneuropathies and CNS protein metabolism. III. Changes in protein
synthesis induced by acrylamide intoxication. J. Neuropathol. exp.
Neurol., 37: 820-837.
SCHREIBER, V. & PRIBYL, T. (1980) Possible role of ceruloplasmin in
endocrine regulation. In: Ishü, S., Hirano, T., & Wade, M., ed.
Hormones, adaptation, and evolution, Tokyo, Japan Science Society
Press/Berlin, Springer-Verlag.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1982) Inhibition by an
ergoline derivative (dopaminergic agonist) of estradiol-induced
adenohypophyseal growth and of the decrease in the hypothalamic
ascorbic acid (HA) concentration in rats. Physiol. Bohemoslov.,
31: 97-100.
SCHREIBER, V., PRIBYL, T., & JAHODOVA, J. (1979) Effect of a dopamine
p-hydroxylase inhibitor (disulfiram) on the response of
adenohypophysis, serum ceruloplasmin, and hypothalamic ascorbic acid
to estradiol treatment. Endoc. Exper. (Bratislava), 13: 131-138.
SCHREIBER, V., ZBUZEK, V., & ZBUZKOVA-KMENTOVA, V. (1969) Reactions of
the rat and hamster endocrine system to aminoglutethimide. Physiol.
Bohemoslov., 18: 249-261.
SEPPALAINEN, A.M. & LINNOILA, I. (1975) Electrophysiological studies
on rabbits in long-term exposure to carbon disulphide. Scand. J. Work
environ. Health, 1: 178-183.
SEPPALAINEN, A.M., TOLONEN, M., KARLI, P., HANNINEN, H., & HEINBERG,
S. (1972) Neurophysiological findings in chronic carbon disulfide
poisoning: a descriptive study. Work Environ. Health, 9: 71-75.
SETH, P.K., AGRAWAL, A.K., & BONDY, S.C. (1981) Biochemical changes in
the brain consequent to dietary exposure of developing and mature rats
to chlordecone (kepone). Toxicol. appl. Pharmacol., 26: 262-267.
SHANDALA, M.G., RUDNEV, M.I., & NAVAKATIKYAN, M.A. (1980) [Behavioural
reactions in experimental hygienic research.] Gig. i Sanit.,
6: 43-48 (in Russian).
SHAW, C.M., MOTTET, N.K., & CHEN, W.J. (1980) Effects of methylmercury
on the visual system of rhesus macaque (Macaca mulatta). II.
Neuropathological findings (with emphasis on vascular lesions in the
brain). In: Merigan, W.H. & Weiss, B., ed. Neurotoxicity of the
visual system, New York, Raven Press, pp. 123-134.
SHAW, C.M., MOTTET, N.K., BODY, R.L., & LUSCHEI, E.S. (1975)
Variability of neuropathological lesions in experimental methylmercury
encephalopathy in primates. Am. J. Pathol., 80: 451-470.
SHIBUSAWA, K., YAMAMOTO, T., NISHI, K., ABE, C., & TOMIE, S. (1959)
Urinary secretion of TRF in various functional states of the thyroid.
Endocrinol. Jpn., 6: 131-136.
SHILJAGINA, N.N. (1971) [Evoked potentials in the projection of sign
stimulus during conditional reflex formation in ontogenesis.]
Zh. Vyssh. nerv. deyat. imeni, 21: 492-500 (in Russian).
SHIMIZU, T., TAKAISHI, M., & SHISHIBE, Y. (1978) Effect of ethanol on
the peripheral metabolism of thyroxine. Endocrinol. Jpn,
25: 201-204.
SHUGALOV, N.P. (1969) Primary and associative evoked responses to
optic irritants during various stages of conditioned reflex formation
in cats. In: Summary of Reports of the 22nd Meeting on the Problems
of Higher Nervous Activity, Ryazan, USSR, p. 268.
SHUMILINA, A.I. (1971) Characteristics of summary electric activity
and evoked potentials under motivating stimulation of various
biological significance. In: Proceedings of the Sixth All-Union
Conference on Electrophysiology of the Central Nervous System,
pp. 301-302.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974a) Hyperactivity: a
lead-induced behavior disorder. Environ. Health Perspect.,
7: 227-232.
SILBERGELD, E.K. & GOLDBERG, A.M. (1974b) Lead-induced behavioral
dysfunction: an animal model of hyperactivity. Exp. Neurol.,
42: 146-157.
SILBERGELD, E.K. & GOLDBERG, A.M. (1975) Pharmacological and
neurochemical investigations of lead-induced hyperactivity.
Neuropharmacology, 14: 431-444.
SILBERGELD, E.K., HRUSKA, R.E., MILLER, L.P., & ENG, N. (1980) Effects
of lead in vivo and in vitro on GABA energic neurochemistry.
J. Neurochem., 34: 1711-1718.
SILVERMAN, A.P. (1965) Ethological and statistical analysis of drug
effects on the social behaviour of laboratory rats. Br. J.
Pharmacol., 24: 579-590.
SLOANE, S.A., SHEA, S.L., PROCTER, M.M., & DEWSBURY, D. (1978) Visual
cliff performance in 10 species of muroid rodents. Anim. Learn.
Behav., 6: 244-248.
SNOWDON, C.T. (1973) Learning deficits in lead injected rats.
Pharmacol. Biochem. Behav., 1: 599-603.
SNYDER, D.R. & BRAUN, J.J. (1977) Dissociation between behavioral and
physiological indices of organomercurial ingestion. Toxicol. appl.
Pharmacol., 41: 277-284.
SNYDER, S.H. & INMS, R.B. (1979) Peptide neurotransmitters. Ann. Rev.
Biochem., 48: 755-782.
SOBOTKA, T.J., BRODIE, R.E., & COOK, M.P. (1975) Psychophysiologic
effects of early lead exposure. Toxicology, 5: 175-191.
SOKOLOFF, L., REIVICH, M., KENNEDY, C., DES ROSIERS, M.H., PATLAK,
C.S., PETTIGREW, K.D., SAKURADA, O., & SHINOHARA, M. (1977) The
14C-deoxyglucose method for the measurement of local cerebral glucose
utilization: theory, procedure, and normal values in the conscious and
anaesthetized albino rat. J. Neurochem., 28: 897-916.
SPENCER, P.S. & BISCHOFF, M.C. (1982) Contemporary neuropathological
methods in toxicology. In: Mitchell, C.L., ed. Nervous system
toxicology, New York, Raven Press, pp. 259-275.
SPENCER, P.S. & LIEBERMAN, A.R. (1971) Scanning electron microscopy of
isolated peripheral nerve fibres. Normal surface structure and
alterations proximal to neuromas. Z. Zellforsch. Mikrosk. Anat.,
119: 534-551.
SPENCER, P.S. & SCHAUMBURG, H.H. (1980) Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
929 pp.
SPENCER, P.S. & THOMAS, P.K. (1970) The examination of isolated nerve
fibres by light and electron microscopy, with observations on
demyelination proximal to neuromas. Acta neuropathol., 16: 177-186.
SPENCER, P.S., BISCHOFF, M., & SCHAUMBURG, H.H. (1980)
Neuropathological methods for the detection of neurotoxic disease. In:
Spencer, P.S. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 743-757.
SPERANSKIJ, S.V. (1965) [Advantages incident to the use of mounting
current in analysing the ability of Albino mice to summate subliminal
impulses.] Farmakol. i Toksikol., 1: 123-124 (in Russian).
SPYKER, J. (1975) Behavioral teratology and toxicology. In: Weiss, B.
& Laties, V.G., ed. Behavioral toxicology, New York, Plenum Press,
pp. 311-349.
SQUIBB, R.E. & TILSON, H.A. (1982) Neurobehavioural changes in adult
Fisher 344 rats exposed to dietary levels of chlordecone (KeponeR):
a 90-day chronic dosing study. Neurotoxicology, 3: 59-66.
STEBBINS, W.C. (1970) Animal psychophysics: the design and conduct of
animal experiments, New York, Appleton-Century-Crofts.
STEEL, R.G.D. & TORRIE, J.H. (1960) Principles and procedures of
statistics, New York, McGraw-Hill, pp. 83-347.
STEELE, W.J. & BUSCH, H. (1963) Studies on acidic nuclear proteins of
the Walker tumour and liver. Cancer Res., 23: 1153-1157.
STOCKINGER, H.E. (1974) Behavioral toxicology in the development of
threshold limit values. In: Xintaras, C., Johnson, B.L., & DeGroot,
I., ed. Behavioral toxicology, Cincinnati, Ohio, US Department of
Health, Education and Welfare, pp. 18-19 (US DHEW (NIOSH) Publication
No. 74-126).
SUDAKOV, K.V. (1982) Functional system theory in physiology.
Agressologie, 23: 167-176.
SZMIGIELSKI, A. (1975) Effect of disulfiram and sodium
diethyldithiocarbamate on thyroxine and dopamine hydroxylases in rat
brain and breast. Pol. J. Pharmacol. Pharm., 27: 153-159.
TAKEUCHI, T. (1968) Pathology of Minamata disease. In: Katsuma, M.,
ed. Minamata disease. Study Group of Minamata Disease, Japan,
Kumamoto University, pp. 141-228.
TAKEUCHI, T., ETO, N., & ETO, K. (1979) Neuropathology of childhood
cases of methylmercury poisoning (Minamata disease) with prolonged
symptoms with particular reference to the decortication syndrome.
Neurotoxicology, 1: 1-20.
TALKE, T., HIRABAGASHI, J., KONDO, R., MATSUMOTO, M., & KOJIMA, K.
(1979) Effect of butyrate on glycolipid metabolism of two cell types
of rat ascites hepatomes with different ganglioside biosynthesis.
J. Biochem., 96: 1139-1402.
TAPPEL, A.L. (1970) Lipid peroxidation damage to cell components.
Fed. Proc., 29: 239.
TAYLOR, J.R., SELHORST, J.B., & CALABRESE, V.P. (1980) Chlordecone.
In: Spencer, P. & Schaumburg, H.H., ed. Experimental and clinical
neurotoxicology, Baltimore, Maryland, Williams and Wilkins Company,
pp. 407-421.
THIESSEN, D.D. (1964) Population density and behavior: a review of
theoretical and physiological contributions. Texas Rep. Biol. Med.,
22: 266-314.
THOMAS, P.K. (1970) The quantitation of nerve biopsy findings.
J. neurol. Sci., 11: 285-295.
THOMAS, P.K. (1980) The peripheral nervous system as a target for
toxic substances. In: Spencer, P.S. & Schaumburg, H.H., ed.
Experimental and clinical neurotoxicity, Baltimore, Maryland,
Williams and Wilkins Company, pp. 35-47.
THOMPSON, S.W. (1963) Selected histochemical and histopathological
methods, Springfield, Illinois, Charles C. Thomas.
THORNDIKE, E.L. (1932) Reward and punishment in animal learning.
Comp. Psychol. Monogr., 39: 8.
TILSON, H.A. & CABE, P.A. (1978) Strategy for the assessment of
neurobehavioral consequences of environmental factors. Environ.
Health Perspect., 26: 287-299.
TILSON, H.A. & HARRY, G.J. (1982) Behavioral principles for use in
behavioral toxicology and pharmacology. In: Mitchell, C.L., ed.
Nervous system toxicology, New York, Raven Press, pp. 1-27.
TILSON, H.A. & MITCHELL, C.L. (1984) Neurobehavioral techniques to
assess the effects of chemicals on the nervous system. Ann. Rev.
Pharmacol. Toxicol., 24: 425-450.
TILSON, H.A. & SQUIBB, R.D. (1982) The effects of acrylamide on the
behavioral suppression produced by psychoactive agents.
Neurotoxicology, 3: 113-120.
TILSON, H.A., BYRD, N., & RILEY, M. (1979a) Neurobehavioral effects of
exposing rats to kepone via the diet. Environ. Health Perspect.,
33: 321.
TILSON, H.A., CABE, P.A., ELLINWOOD, E.H., Jr, & GONZALEZ, L.P.
(1979b) Effects of carbon disulfide on motor function and
responsiveness to delta-amphetamine in rats. Neurobehav. Toxicol.,
1: 57-63.
TILSON, H.A., MITCHELL, C.L., & CABE, P.A. (1979c) Screening for
neurobehavioral toxicity: the need for and examples of validation of
testing procedures. Neurobehav. Toxicol., 1(Suppl. 1): 137-148.
TILSON, H.A., CABE, P.A., & BURNE, T.A. (1980) Behavioral procedures
used in the assessment of neurotoxicity. In: Spencer, P.S. &
Schaumburg, H.H., ed. Experimental and clinical neurotoxicology,
Baltimore, Maryland, Williams and Wilkins Company, pp. 758-766.
TILSON, H.A., MACTUTUS, C.F., MCLAMB, R.L., & BURNE, T.A. (1982a)
Characterization of triethyl lead chloride neurotoxicity in adult
rats. Neurobehav. Toxicol. Teratol., 4: 671-681.
TILSON, H.A., SQUIBB, R.E., & BURNE, T.A. (1982b) Neurobehavioral
effects following a single dose of chlordecone (kepone) administered
neonatally to rats. Neurotoxicology, 3: 45-52.
TOLMAN, E.C. (1948) Cognitive maps in rats and men. Psychol. Rev.,
55: 189-208.
TOWFIGHI, J., GONATAS, N.K., & MCCREE, L. (1975) Hexachlorophene
retinopathy in rats. Lab. Invest., 32: 330-338.
TROPKINA, J.K., SELZNEVA, S.N., & POGODAEV, K.V. (1981) Prenatal
influence of hypoxia on metabolic structure of cerebral mitochondria
in the oncogenesis with epileptic superactivity. In: Mitochondria:
mechanisms of conjugation and regulation, Puscino, p. 39.
TRUEX, R.C. & CARPENTER, M.B. (1983) Human neuroanatomy, 8th ed.,
Baltimore, Maryland, Williams and Wilkins Company.
TSUJI, S. & FUJIHSIMA H. (1975) Paraplegias: clinical statistics,
Fukuoka, Kyushu Rosai Hospital, Department of Orthopedics and
Neurology.
TULLOCH, M.L., CROOKS, J., & BROWN, P.S. (1963]) Inhibition of thyroid
function by a dithiocarbamoylhydrazine. Nature (Lond.),
199: 288-289.
ULRICH, C.E., RINEHART, W., & BRANDT, M. (1979) Evaluation of the
chronic inhalation toxicity of a manganese oxide aerosol. III.
Pulmonary function, electromyograms, limb tremor, and tissue manganese
data. Am. Ind. Hyg. Assoc. J., 40: 349-353.
UPHOUSE, L., NEMEROFF, C.B., MASON, G., PRANGE, A.J., & BONDY, S.C.
(1982) Interactions between "handling" and acrylamide on endocrine
responses in rats. Neurotoxicology, 3: 121-125.
UPHOUSE, L., MASON, G., & HUNTER, V. (1984) Persistent vaginal estrus
and serum hormones after chlordecone (kepone) treatment of adult rats.
Toxicol. appl. Pharmacol., 72: 177-186.
US FDA (1978) Nonclinical laboratory studies. Good laboratory
practice regulation, Washington DC, US Food and Drug Administration,
Department of Health, Education and Welfare (Federal Register 43:
59986-60025).
US NAS (1975) Principles for evaluating chemicals in the
environment, Washington DC, National Academy of Science (A Report of
the Committee for the Working Conference on Principles of Protocols
for Evaluating Chemicals in the Environment).
US NAS/NRC (1970) Evaluating the safety of food chemicals,
Washington DC, National Academy of Sciences, National Research
Council, Food Protection Committee, Food Nutrition Branch, Division of
Biology and Agriculture.
VAN GELDER, G.A. (1978) Lead and the nervous system. In: Oehme, F.W.,
ed. Toxicity of heavy metals in the environment, Part 1, Basel, New
York, Marcel Dekker, Inc., pp. 101-121.
VANDERWOLF, C.H. & LEUNG, L.-W.S. (1983) Hippocampal rhythmic slow
activity: a brief history and the effects of entorhinal lesions and
phencyclidine. In: Seifert, W., ed. Neurobiology of the hippocampus,
New York, Academic Press, pp. 275-302.
VASAN, N.S. & CHASE, H.P. (1976) Brain glucosaminoglycans
(mucopolysaccharides) following intrauterine growth retardation.
Biol. Neonate, 28: 196-206.
VEKSLER, M.S. & ARBUKHANOVA, M.S. (1981) Cerebral and spinal cord
mitochondria under strenuous physical work. In: Mitochondria:
mechanism of conjugation and regulation, Puscino, p. 81.
VERGIEVA, T. & ZAIKOV, H.R. (1981) Behavioral changes in rats with
inhalation of styrene. Hig. Zdraveopazvane, 24: 242-247.
VERITY, M.A. (1972) Cation modulation of synaptosomal respiration.
J. Neurochem., 19: 1305-1309.
VERITY, M.A., BROWN, W.J., CHEUNG, M., & DZER, G. (1977) Methylmercury
inhibition of synaptosomes and brain slices protein synthesis: in
vivo and in vitro studies. J. Neurochem., 29: 673-679.
VILA, J. & COLOTLA, V.A. (1981) Some stimulus properties of inhalants:
preliminary findings. Neurobehav. Toxicol., 3: 447-480.
VINAR, O., FRANTIK, E., & HORVATH, M. (1981) Subjectively-perceived
effects of diazepam, toluene, and their combination. Act. Nerv.
super. (Praha), 23: 179-182.
VORHEES, C.V. (1974) Some behavioral effects of maternal
hypervitaminosis A in rats. Teratology, 17: 269-273.
VORHEES, C.V. & BUTCHER, R.E. (1982) Behavioral teratogenicity. In:
Snell, L., ed. Developmental toxicology, London, Croom Helm Press,
pp. 247-298.
VORHEES, C.V., BRUNNER, R.L., MCDANIEL, C.R., & BUTCHER, R.E. (1978)
The relationship of gestational age to vitamin A induced postnatal
dysfunction. Teratology, 17: 271-276.
WALLMAN, J. (1975) A simple technique using an optomotor response for
visual psychophysical measurements in animals. Vision Res., 15: 3-8.
WALSH, J.M., CURLEY, M.D., BURCH, L.S., & KURLANSIK, L. (1982) The
behavioral toxicity of a tributyltin ester in the rat. Neurobehav.
Toxicol. Teratol., 4: 241-246.
WALSH, R.N. (1980) On the necessity for a shift in emphasis from
means-oriented to problems-oriented research in developmental
psychobiology. Dev. Psychobiol., 13: 229-231.
WALSH, T.J., MILLER, D.B., & DYER, R.S. (1982b) Trimethyltin, a
selective limbic system neurotoxicant, impairs radial-arm maze
performance. Neurobehav. Toxicol. Teratol., 4: 177-184.
WALTON, J.N. (1974) Disorders of voluntary muscle, 3rd ed.,
Edinburg, London, Churchill Livingston.
WANG, G.H. (1932) Relation between spontaneous activity and oestrous
cycle in the white rat. Comp. Psychol. Monogr., 6: 1-32.
WEIBEL, E.R. (1979) Stereological methods. In: Practical methods for
biological morphometry, New York, Academic Press, Vol. 1.
WEISS, B., FERRIN, J., MERIGAN, M., & COX, C. (1981) Modification of
rat operant behaviour by ozone exposure. Toxicol. appl. Pharmacol.,
58: 244-251.
WEISS, B. & LATIES, V. (1961) Changes in pain tolerance and other
behavior produced by salicylates. J. Pharmacol. exp. Ther.,
131: 120-129.
WEISS, B. & LATIES, V. (1969) Behavioral pharmacology and toxicology.
Ann. Rev. Pharmacol., 9: 297-326.
WEISS, B. & LATIES, V.G., ed. (1975) Behavioral toxicology, New
York, Plenum Press, 469 pp.
WEISS, B. & LATIES, V.G. (1979) Assays fox behavioural toxicity: a
strategy for the Environmental Protection Agency. Neurobehav.
Toxicol., 1(Suppl. 1): 213-215.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, J., CONNEY, A.H.
(1971) Effect of halogenated hydrocarbon insecticides on the
metabolism and uterotropic action of estrogens in rats and mice.
Toxicol. appl. Pharmacol., 19: 234-246.
WELLS, G.A.H., HOWELL, J.M., & GOPINATH, C. (1976) Experimental lead
encephalopathy of calves: histological observations on the nature and
distribution of the lesions. Neuropathol. appl. Neurobiol.,
2: 175-190.
WHEATLEY, B., BARBEAU, A., CLARKSON, T.W., & LAPHAM, L.W. (1979)
Methylmercury poisoning in Canadian indians -- the elusive diagnosis.
Can. J. neurol. Sci., 6: 417-422.
WHO (1975) Methods used in the USSR for establishing biologically
safe levels of toxic substances, Geneva, World Health Organization,
171 pp.
WHO (1978) Principles and methods of evaluating the toxicity of
chemicals. Part 1, Geneva, World Health Organization (Environmental
Health Criteria Series No. 6, Part 1).
WHO (1984) Principles for evaluating health risks to progeny
associated with exposure to chemicals during pregnancy, Geneva,
World Health Organization (Environmental Health Criteria Series
No. 30).
WHO (in preparation) Principles and methods of evaluating the
toxicity of chemicals. Part 2, Geneva, World Health Organization
(Environmental Health Criteria Series No. 6, Part 2).
WILLIAMS, P.L. & WARWICK, R. (1975) Functional neuroanatomy of man,
Philadelphia, Pennsylvania, W.B. Saunders Company.
WILSON, W.E. (1982) Dopamine-sensitive adenylate cyclase inactivation
by organolead compounds. Neurotoxicology, 3: 100-107.
WINNEKE, G. (1979) Modification of visual evoked potentials in rats
after long-term blood lead elevation. Act. Nerv. super. (Praha),
21: 282-284.
WINNEKE, G., BROCKHAUS, A., & BALTISSEN, R. (1977) Neurobehavioral and
systematic effects of longterm blood lead-elevation in rats. I.
Discrimination learning and open field behavior. Arch. Toxicol.,
37: 247-263.
WINNEKE, G., FODOR, G., & SCHLIPKOTER, H. (1978) Carbon monoxide,
trichloroethylene, and alcohol: reliability and validity of
neurobehavioural effects. Multidisciplinary perspectives in event-
related brain potentials. In: Proceedings of the Fourth International
Congress on Event-Related Slow Potentials of the Brain, Henderson,
North Carolina, 4-10 April, 1976 (EPIC IV), Washington DC, US
Environmental Protection Agency (EPA 600/9-77-0443).
WINNEKE, G., LILIENTHAL, H., & ZIMMERMANN, U. (1984) Neurobehavioural
effects of lead and cadmium. In: Proceedings of the Third
International Congress on Toxicology, San Diego, 28 August-2
September 1983, Amsterdam, Oxford, New York, Elsevier Science
Publishers.
WOLF, D.L. (1976) Rotating rod, spontaneous activity, and passive
avoidance conditioning: their suitability as functional tests in
industrial toxicology. In: Horvath, M. & Frantik, E., ed. Adverse
effects of environmental chemicals and psycho- tropic drugs,
Amsterdam, Oxford, New York, Elsevier Science Publishers, pp. 293-303.
WOOD, R.W. (1978) Stimulus properties of inhaled substances. Environ.
Health Perspect., 26: 69-76.
WOOD, R.W. (1979) Reinforcing properties of inhaled substances.
Neurobehav. Toxicol., 1(Suppl. 1): 67-72.
WOOD, R.W. (1981) Neurobehavioral toxicity of carbon disulfide.
Neurobehav. Toxicol. Teratol., 3: 397-405.
WOOLLEY, D.E. (1977) Electrophysiological techniques in
neurotoxicology. In: Zenick, H. & Reiter, L.W., ed. Behavioral
toxicology: an emerging discipline, Research Triangle Park, North
Carolina, US Environmental Protection Agency, pp. 9-1 -- 9-25 (EPA
600/9-77-042).
XINTARAS, C. & BURG, J.R. (1981) Neurotoxicity studies of workers
exposed to carbon disulfide. G. Ital. Med. Lav., 3: 83-86.
XINTARAS, C., JOHNSON, B.L., & DEGROOT, I., ed. (1974) Behavioral
toxicology, Cincinnati, Ohio, US Department of Health, Education and
Welfare, 507 pp (US DHEW (NIOSH) Publication No. 74-126).
YAMAMURA, H.I., ENNA, S.J., & KUHAR, M.J. (1981) Neurotransmitter
receptor binding, New York, Raven Press.
YONESAWA, T., BORNSTEIN, M.B., & PETERSON, E.R. (1980) Organotypic
cultures of nerve tissue as a model for neurotoxicity investigation
and screening. In: Spencer, P.S. & Schaumburg, H.H., ed. Experimental
and clinical neurotoxicology, Baltimore, Maryland, Williams and
Wilkins Company, pp. 788-802.
YOUNG, J.S. & FECHTER, L.D. (1983) Reflex inhibition procedures for
animal audiometry: a technique for assessing ototoxicity. J. Acoust.
Soc. Am., 73: 1686-1693.
ZAKUSOV, V.V. (1936) [Estimation of the reflex time under the
influence of some narcotic substances.] Fiziol. Zh. USSR,
23: 276-278 (in Russian).
ZAKUSOV, V.V. (1953) [Pharmacology of the nervous system,]
Leningrad, Medgiz, 258 pp (in Russian).
ZBINDEN, G. (1981) Experimental methods in behavioral teratology.
Arch. Toxicol., 48: 69-88.
ZEMAN, W. & INNES, J.R.M. (1963) Craigie's neuroanatomy of the rat,
New York, Academic Press.
ZENICK, H., RODRIQUEZ, W., WARD, J., & ELKINGTON, B. (1979) Deficits
in fixed-interval performance following prenatal and postnatal lead
exposure. Dev. Psychobiol., 12(5): 509-514.
ZILOV, V.G., ROGACHEVA, S.K., & IVANOVA, L.I. (1983)
[Electroencephalographic analysis of limbic-reticular interrelations
in various motivational reactions of rabbits under ethanol.] Bull.
exp. Biol. Med., 11: 74-77 (in Russian).
ZUBER, V.L. (1978) Intensity of metabolism of various fractions of
cerebral phospholipids under hyperphenyl-alaninaemia. Vop. med.
Xhim., 2: 163-166.
ZYLBER-HARAN, E.A., GERSHMAN, H., ROSENMANN, E., & SPITZ, I.M. (1982)
Gonadotrophin, testosterone, and prolactin interrelationships in
cadmium-treated rats. J. Endocrinol., 92: 123-130.
