
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 147
PROPACHLOR
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr L. Ivanova-Chemishanska,
Institute of Hygiene and Occupational Health,
Sofia, Bulgaria
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Propachlor.
(Environmental health criteria ; 147)
1.Acetanilides - adverse effects 2.Acetanilides - toxicity
3.Environmental exposure 4.Herbicides - adverse effects
5.Herbicides - toxicity I.Series
ISBN 92 4 157147 0 (NLM Classification: WA 249)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PROPACHLOR
1. SUMMARY AND EVALUATION
1.1. Identity, use pattern, physical and chemical properties,
analytical methods
1.2. Environmental transport, distribution and transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on laboratory animals and in vitro test systems
1.6. Effects on humans
1.7. Effects on organisms in the environment
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Production and uses
3.2. Methods and rates of application
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Soil
4.1.1.1 Abiotic factors
4.1.1.2 Biotic factors
4.1.1.3 Metabolites
4.1.1.4 Persistence
4.1.1.5 Environmental conditions affecting
distribution and breakdown
4.1.2. Water
4.1.3. Plants
4.2. Bioaccumulation and biomagnification
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Food
5.2. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Metabolic transformation
6.3. Elimination and excretion
6.4. Metabolism in laying hens
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Oral
7.1.2. Dermal
7.1.3. Inhalation
7.2. Short-term exposure
7.2.1. Oral
7.2.1.1 Dogs
7.2.1.2 Rodents
7.2.1.3 Mice
7.2.2. Dermal
7.3. Skin and eye irritation; sensitization
7.3.1. Skin irritation
7.3.2. Skin sensitization
7.3.3. Eye irritation
7.4. Reproduction, embryotoxicity and teratogenicity
7.4.1. Reproduction
7.4.1.1 Biochemical and histopathological studies
on gonads
7.4.1.2 Reproduction studies
7.4.2. Embryotoxicity and teratogenicity
7.4.2.1 Rats
7.4.2.2 Mice
7.4.2.3 Rabbits
7.5. Mutagenicity and related end-points
7.5.1. Bacterial test systems
7.5.2. Yeast assays
7.5.3. Plant assays
7.5.4. Cultured mammalian cell CHO/HGPRT assay
7.5.5. In vitro unscheduled DNA synthesis in primary rat
hepatocyte cultures
7.5.6. In vitro test for induction of chromosomal
aberrations using Chinese hamster ovary cells
7.5.7. In vivo rat bone marrow cytogenetic assay
7.5.8. Acute in vivo mouse bone marrow cytogenicity assay
7.5.9. In vivo/in vitro hepatocyte DNA repair assay
7.6. Long-term toxicity and oncogenicity studies
7.6.1. Rat
7.6.2. Mouse
7.6.3. Dog
7.7. Miscellaneous studies
8. EFFECTS ON HUMANS
8.1. Occupational exposure
8.2. General population exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.1.1. Soil
9.1.2. Water
9.2. Aquatic organisms
9.2.1. Aquatic invertebrates
9.2.2. Fish
9.3. Terrestrial organisms
9.3.1. Terrestrial invertebrates
9.3.2. Birds
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1. Conclusions
10.2. Recommendations for protection of human health
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET EVALUATION
RESUMEN Y EVALUACION
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR PROPACHLOR
Members
Dr L.A. Albert, Consultores Ambientales Asociados, Xalapa, Veracruz,
Mexico
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, Cambridgeshire,
United Kingdom
Dr L. Ivanova-Chemishanska, Institute of Hygiene and Occupational
Health, Sofia, Bulgaria ( Joint Rapporteur)
Dr J. Kangas, Kuopio Regional Institute of Occupational Health,
Kuopio, Finland
Dr S.K. Kashyap, National Institute of Occupational Health,
Meghaninagar, Ahmedabad, India
Professor A. Massoud, Department of Community, Environmental and
Occupational Medicine, Faculty of Medicine, Ain Shams University,
Cairo, Egypt ( Vice-Chairman)
Professor Wai-on Phoon, Department of Occupational Health, University
of Sydney, and Professional Education Program, National Institute
of Occupational Health and Safety, Worksafe Australia, Sydney,
Australia ( Chairman)
Professor L. Rosival, Institute of Preventive and Clinical Medicine,
Bratislava, Czechoslovakia
Dr K.C. Swentzel, Health Effects Division, US Environmental Protection
Agency, Washington, D.C., USA ( Joint Rapporteur)
Observers
Dr M. Carroll, Monsanto Services International, Brussels, Belgium
Dr B. Hammond, The Agricultural Group of Monsanto Company, St Louis,
Missouri, USA
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
* * *
The proprietary information contained in this monograph cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route, and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraph 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (FAO, 1982).
ENVIRONMENTAL HEALTH CRITERIA FOR PROPACHLOR
A WHO Task Group on Environmental Health Criteria for Propachlor
met at the World Health Organization, Geneva, from 4 to 8 November
1991. Dr K.W. Jager, IPCS, welcomed the participants on behalf of Dr
M. Mercier, Director of the IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Group reviewed and revised the draft
and made an evaluation of the risks for human health and the
environment from exposure to propachlor.
The first draft was prepared by Dr L. Ivanova-Chemishanska of the
Institute of Hygiene and Occupational Health, Sofia, Bulgaria, who
also assisted in the preparation of the second draft, incorporating
comments received following circulation of the first drafts to the
IPCS Contact Points for Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the monograph, and Dr P.G. Jenkins for the
technical editing.
The fact that Monsanto Agrochemical Company, St Louis, USA, made
available to the IPCS and the Task Group its proprietary toxicological
information on their product is gratefully acknowledged. This allowed
the Task Group to make its evaluation on a more complete data base.
The effort of all who helped in the preparation and finalization
of the monograph is gratefully acknowledged.
ABBREVIATIONS
a.i. active ingredient
AcP acid phosphatase
ATPase adenosine triphosphatase
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
GA gibberillic acid
GGPT = GGT gamma-glutamyltransferase
HGPRT hypoxanthine-guanine phosphoribosyltransferase
LAP leucine aminopeptidase
LDH lactate dehydrogenase
MAC maximum allowable concentration
MAP mercapturic acid pathway
mCi millicurie
MQL minimum quantifiable limit
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
OCT ornithine carbamoyltransferase
ppb parts per billion
ppm parts per million
SAP = AP alkaline phosphatase
SDH succinate dehydroganase
SGOT = AST aspartate aminotransferase
SGPT = ALT alanine aminotransferase
UDS unscheduled DNA synthesis
uv ultraviolet
WP wettable powder
1. SUMMARY AND EVALUATION
1.1 Identity, use pattern, physical and chemical properties,
analytical methods
Propachlor is a pre-emergence and early post-emergence herbicide
derived from acetanilide, and has been in use since 1965. The major
formulations are as wettable powder, liquid flowable (suspension
concentrate) and as granules. Its uses in agriculture include the
control of annual grasses and some broad-leaved weeds in several crops
including corn, sorghum, pumpkins, flax and flowers.
Propachlor is slightly soluble in water and readily soluble in
most organic solvents. It has a low volatility, is non-flammable and
is stable to ultraviolet radiation. The most practical method for
analysis is gas chromatography with electron capture detection after
suitable extraction and clean-up procedures.
1.2 Environmental transport, distribution and transformation
Propachlor is not known to photodegrade on soil surfaces.
Volatilization of the compound occurs under windy conditions while the
soil surface is still moist.
The adsorption of the compound to soil particles and organic
matter is only moderate. This leads to the potential for leaching
through the soil profile and into ground water. However, all studies
show that this potential is unlikely to be realised in practice. Very
high rainfall is required to move residues 30 cm down the soil
profile. Most authors report that the great majority of residues
remain within the upper 4 cm of soil. The characteristics of the soil
greatly influence movement of the compound. Most leaching occurs in
sandy soil with little organic matter.
Run-off of propachlor has been studied in both the laboratory and
field. The organic matter in the soil reduced run-off from 7% to 1% of
the applied herbicide in one study. Incorporation of propachlor into
the soil also reduced loss through run-off (from 3% to 0.8% in one
study).
By far the most significant factor in reducing propachlor levels
in soil and water is degradation by microorganisms. Both bacteria and
fungi have been shown to be involved in breakdown of the compound. Few
bacteria appear to be able to use propachlor as the sole carbon
source. Bacteria capable of utilizing soil metabolites of propachlor
have also been isolated.
The predominant metabolites formed in soil are water-soluble
oxanilic and sulfonic acids. A large number of other metabolites can
be formed, but these represent a small proportion of the total.
Propachlor disappears rapidly from soil, half-lives of up to 3
weeks having been reported. Most studies report almost complete
degradation within less than 6 months. Environmental conditions affect
the rate of degradation, which is favoured by high temperature and
soil moisture content. Those studies reporting longer persistence of
propachlor in soil were conducted under conditions of low temperature
or dry soil. Adequate nutrient levels in soil are also necessary for
degradation.
The conjugated N-isopropylaniline metabolite is much more
persistent than the parent compound. Residues of this metabolite have
been found up to 2 years after the application of propachlor
experimentally at higher rates than would normally be used in
agriculture.
Under normal conditions of use, propachlor is not expected to
leach through soil to ground water and will not persist in soil.
Exceptional conditions of low temperature or dryness will lead to
greater persistence of propachlor and its metabolites.
Under normal conditions, propachlor does not photodegrade
significantly in water. In the presence of photosensitizers,
photodegradation may take place. Propachlor is hydrolytically stable.
Volatilization from water is unlikely because of the high water
solubility and low vapour pressure of the compound.
As in soil, the major route of loss of propachlor from water is
biotic degradation. The rate of loss of propachlor from water is,
therefore, dependent on the microbial population. A study in water
with few bacteria present yielded a half-life of about 5 months. Ring
cleavage did not occur within six weeks in another study. Laboratory
model ecosystem studies showed almost complete degradation of
propachlor within 33 days.
In several studies on different plant species, propachlor was
shown to be rapidly metabolized in both intact plants and excized
plant tissues. The metabolic pathways were similar in all plants
studied, at least for the first 6 to 24 h, producing water-soluble
metabolites. No metabolic breakdown of the N-isopropylaniline moiety
was observed. Only a very small proportion (< 1% in one study) of the
metabolites was found in the fruit of the plants; the great majority
was in the roots and foliage. The major metabolites produced in plants
are identical with those produced in soil. Uptake of these metabolites
from soil is known to take place and it is uncertain in some studies
whether measured metabolites derive from the plant or the soil.
Although the octanol/water partition coefficient suggests a
moderate potential for bioaccumulation, studies show that propachlor
neither bioconcentrates nor biomagnifies in organisms.
1.3 Environmental levels and human exposure
Reported measurements of air concentrations of propachlor during
application are few and inadequate.
Concentrations in surface and ground water in the USA were
consistently low, the maxima being at 10 µg/litre in surface and 0.12
µg/litre in ground water. The highest water concentration recorded in
a run-off study was 46 µg/litre.
Propachlor residues in food are usually below the detection limit
of the analytical method (0.005 mg/kg). Experimental studies have
identified residues in the order of 0.05 mg/kg in tomatoes, peppers,
onions and cabbage.
Measurements of propachlor in the air of the working zone of
tractor drivers applying the compound ranged between 0.1 and 3.7
mg/m3.
1.4 Kinetics and metabolism
Propachlor can be absorbed into mammals through the respiratory
and gastrointestinal tract as well as through the skin. It does not
accumulate in the body. After 48 h it is not detectable in the
organism.
Most animal species (rats, pigs, chickens) metabolize propachlor
through the mercapturic acid pathway (MAP). Cysteine conjugates are
formed by glutathione conjugation and this conjugate has been proposed
as an intermediate in the metabolic formation of mercapturic acids.
Bacterial C-S lyase participates in the further metabolism of the
cysteine conjugate of propachlor and in the formation of the final
methylsulfonyl-containing metabolites, which are mainly excreted in
the urine (68% of the dose of propachlor), and insoluble residues,
which are excreted in the faeces (19%). The propachlor C-S lyase is
not active in germ-free rats.
Studies showed some differences in metabolism between the rat and
pig. The bile is the major route of elimination of MAP metabolites in
the rat, but it is has been proved that an extrabiliary route of
metabolism exists in the pig.
Metabolic studies on calves showed that they may be unable to
form mercapturic acids from glutathione conjugates, which may make
them more susceptible to poisoning.
1.5 Effects on laboratory animals and in vitro test systems
Propachlor is slightly toxic in acute oral exposure (the LD50
in rats ranges from 950 to 2176 mg/kg body weight). Signs of acute
intoxication are predominantly central nervous system effects
(excitement and convulsion followed by depression). The acute
inhalation toxicity in rodents is low (LC50 = 1.0 mg/litre).
Propachlor caused severe irritation effects on eyes and skin.
Propachlor has been tested in short- and long-term exposure
studies on rats, mice and dogs. The liver and kidneys are the target
organs. In dogs, the no-observed-adverse-effect level (NOAEL) was 45
mg/kg body weight in a 3-month dietary exposure study. In a one-year
study on dogs, the NOAEL was 9 mg/kg body weight (250 ppm in diet).
The no-observed-effect level (NOEL) in a 24-month dietary exposure
study on rats was 50 mg/kg diet (2.6 mg/kg body weight). In an
18-month dietary study in mice, the NOEL was 1.6 mg/kg body weight (10
ppm).
Propachlor was not found to be carcinogenic in mice and rats. It
showed a negative mutagenic response in most of the mammalian test
systems and positive results in a few assays. The experimental data
available provide insufficient evidence of the mutagenic potential.
When tested as a single dose (675 mg/kg) in rats and mice,
propachlor showed positive evidence of embryotoxicity. Embrytoxic
effects were also observed in repeated dose regimens (35.7-270 mg/kg).
However, in another rat study using a dose range of 20-200 mg/kg, no
embryotoxicity was observed.
At levels of 12 and 60 mg/kg body weight, propachlor (wettable
powder) resulted in a decrease in protein content and an increase in
ATPase and 5-nucleotidase activity in rat testis homogenate and
degenerative changes in the testes. In a two-generation reproduction
study there was no definite evidence of adverse effects.
1.6 Effects on humans
A few cases of contact and allergic dermatitis of farmers and
production workers exposed to propachlor (Ramrod and Satecid) have
been reported. Patch tests were carried out among some of them,
revealing a positive patch test reaction, irritation reaction or mono-
and bi-valent hypersensitivity.
There have been no reports of symptoms or diseases either among
occupationally exposed humans or the general population other than the
few reports of its effects on the skin of occupationally exposed
workers.
1.7 Effects on organisms in the environment
In studies on soil microorganisms, nitrifying bacteria were the
most sensitive group to the inhibitory effects of propachlor, their
numbers being reduced by a factor of 3 to 4 after the application of
8 to 10 kg propachlor/ha. Cellulose-decomposing bacteria were the
least sensitive. High adsorption to clay particles in soil and high
temperature both reduce the inhibitory effects.
A 96-h EC50 of 0.02 mg/litre for growth and a no-observed-
effect concentration (NOEC) of 0.01 mg/litre have been reported for
the alga Selenastrum capricornutum. A second study using a
formulation and conducted over 72 h suggested substantially less
hazard for the same organism.
LC50 values of 7.8 and 6.9 mg/litre have been reported for the
water flea Daphnia magna and a NOEC of < 5.6 mg/litre. The NOEC for
reproduction was 0.097 mg/litre. LC50 values of 0.79 and 1.8
mg/litre have been reported for two species of midge larvae.
The 96-h LC50 for rainbow trout is 0.17 mg/litre and the NOEC
in a 21-day study was 0.019 mg/litre.
Propachlor is considered to be moderately to highly toxic to
aquatic organisms.
Propachlor is not toxic to earthworms at exposure concentrations
in soil expected from normal use (the NOEC is 100 mg/kg soil). The
contact LD50 for honey bees (311 µg/bee) shows that propachlor will
not pose a hazard to these insects. Some beneficial parasitic insects
have been reported to be adversely affected by propachlor in
laboratory and field studies.
Propachlor is more toxic to birds when administered via the
stomach than when fed in the diet. Acute LD50 values range between
137 and 735 mg/kg body weight for different bird species. The LC50
values from dietary exposure exceed 5620 mg/kg diet in birds.
Propachlor does not pose a hazard to birds in the field, even
with the granular formulation.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Common name: Propachlor
Chemical structure
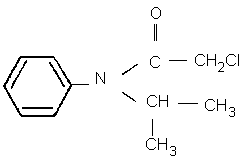 Chemical formula: C11H14ClNO
Common synonyms
and trade names: Ramrod, Acylide, Bexton (discontinued by Dow
Chemical Company), Niticid, Satecid
CAS chemical name: 2-chloro- N-(1-methylethyl)- N-phenyl acetamide
IUPAC name: 2-chloro- N-isopropylacetanilide (formerly
alpha-chloro- N-isopropylacetanilide)
CAS registry
number: 1918-16-7
RTECS registry
number: AE1575000
Propachlor is available as a technical material containing 93%
active ingredient for formulation of propachlor end-use products. It
is available in the form of granules (200 g active ingredient/kg),
wettable powder (WP, 650 g active ingredient/kg) and as a liquid
flowable formulation (suspension concentrate), among others. Mixtures
with other herbicides, e.g., atrazine and propazine are also used.
2.2 Physical and chemical properties
Some of the physical and chemical properties of propachlor are
given in Table 1.
Table 1. Some physical and chemical properties of propachlor
Physical state solid
Colour tan
Relative molecular mass 211.7
Melting point (°C) 77
Boiling point (°C) at 0.03 mmHg 110
Decomposition (°C) > 170
Vapour pressure (25 °C) 103 mPa
Solubility in water (20 °C) 580 mg/litre
(25 °C) 613 mg/litre
Solubility in organic solvents readily soluble in most organic
solvents except aliphatic
hydrocarbons:
acetone 448 g/kg
benzene 737 g/kg
chloroform 602 g/kg
ethanol 408 g/kg
xylene 239 g/kg
Log Kow 1.62-2.30
It is non-flammable and stable to ultraviolet radiation.
2.3 Conversion factors
At 25 °C 1 mg/m3 = 8.802 ppm
1 ppm = 0.1136 mg/m3
2.4 Analytical methods
The analytical methods described in the literature, which are
based on different types of determination of propachlor in different
media, are given in Table 2.
Table 2. Analytical methods for the determination of propachlor and metabolites
Sample type Method of detection Extraction and clean-up Detection limit Reference
Soil and plants thin-layer chromatography 2-h extraction with 0.02-0.04 mg/kg Kofman &
chloroform Nishko
(1984)
Soil and plants gas chromatography with extraction with benzene; clean-up 0.004-0.005 mg/kg Balinova
electron-capture detection by partition with hexane-acetonitrile (1981)
followed by column chromatography
(Florisil)
Soil gas chromatography with acetone extraction followed by alkane 0.01 mg/kg; re- Caverly &
electron-capture detection hydrolysis, steam distillation and coveries at residual Denney
concentration of anilines in toluene levels are generally (1978)
better than 80%
Soil gas liquid chromatography extraction with isopropanol and benzene; < 0.05 mg/kg Markus &
with flame-ionization detection column chromatography (Florisil) Puma (1973)
Immature plants gas-liquid chromatography extraction with isopropanol; column < 0.05 mg/kg Markus &
with flame-ionization detection chromatography (Florisil) Puma (1973)
Mature grain gas-liquid chromatography extraction with acetonitrile; column < 0.05 mg/kg Markus &
with flame-ionization detection chromatography (Florisil) Puma (1973)
Cabbage gas chromatography with NP extraction with acetone followed by 0.06 mg/kg (fresh Warholic et
detection alkaline hydrolysis to weight) al. (1983)
N-isopropylaniline; steam
distillation and extraction with
toluene
Table 2 (contd).
Sample type Method of detection Extraction and clean-up Detection limit Reference
Industrial and gas chromatography with extraction with methylene chloride; 1 ng/litre Pressley &
municipal waste electron-capture detection clean-up on a Florisil column Longbottom
water (1982)
Urine metabolites gas or liquid chromatography fractionation with lipophilic ion not given Sjovall et
of propachlor and mass spectrometry exchangers (Lipidex 1000, Lipidex DEAP al. (1983)
(glutathione SP-LH-20 and Sep pack C18)
conjugates)
High-pressure liquid chromatography with radioactive detection or
liquid scintillation has been used to purify the metabolites from
14C-labelled propachlor in several biological media, including egg
yolk, egg white, edible tissues and excreta of laying hens, after
extraction with organic solvents. The metabolites were characterized
by gas chromatography with radioactive detection mass spectrometry
(Bleeke et al., 1987). With liquid scintillation detection, the
minimum quantifiable limit (MQL) was 0.01 to 0005 ppm.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production and uses
Propachlor was developed by the Monsanto Chemical Company and
commercially introduced in 1965. Technical propachlor is produced in
the USA by Monsanto and in Germany by BASF. There are other
manufacturers of technical propachlor.
Propachlor is prepared by the reaction of chloracetyl chloride
and N-isopropylaniline.
It is a pre-emergence, pre-planting (incorporated) or early
post-emergence herbicide effective against annual grasses and some
broad-leaved weeds (Worthing & Hance, 1991). It is used on field corn,
hybrid seed corn, silage corn, grain sorghum (milo), green peas,
soybeans, flax, pumpkins and flowers. In 1971, 10 000 tonnes were
produced (US EPA, 1984a), but a more recent estimate of annual use in
the USA is 1800 tonnes.
3.2 Methods and rates of application
Application rates range from 4 to 6 kg active ingredient in
150-300 litres of water per ha (6-9 kg wettable powder formulation/ha)
in pre-emergence use. Some tests indicate that early post-emergence
applications are equally effective for weed control. The best response
occurred when broad-leaved weeds were between the cotyledonous stage
and the 2´-leaf stage and when grassy weeds were up to the one-leaf
stage.
Irrigation following application improves activity, particularly
under dry soil conditions. The duration of weed control ranges from 4
to 6 weeks, depending on the soil structure and organic content
(Humburg et al., 1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Soil
The fate and transport of propachlor in soil has been well
studied. The principal aim has been to determine the rate and products
of degradation, persistence, environmental factors and organisms
participating in the biodegradation, and the conditions under which
degradation takes place.
4.1.1.1 Abiotic factors
Beestman & Deming (1974) found that leaching did not contribute
to dissipation since no residues were found below the upper 4-cm
layer. The weak leaching ability was related to the high adsorption of
propachlor by soil. The ultraviolet absorption spectrum of propachlor
reveals no absorption at wavelengths longer than 280 nm. On the basis
of these data it was suggested that photodecomposition of soil-applied
propachlor would not be significant. Rapid herbicide volatilization
occurred under windy conditions during the period that exposed soil
surfaces remain moist.
In a study by Nesterova et al. (1980), propachlor was applied,
under dry weather conditions, at a rate of 7 kg/ha for 4 consecutive
years. The residues of propachlor in the soil were measured up to 4
weeks following the application. On the 5th day, residues were found
in the upper layers (0-10 cm), and on the 15th day, after abundant
rain (74 mm), they had reached 0-30 cm. There was rapid
biodegradation, mainly in the first 5 days, followed by reduced
degradation over the next 10 days. Between the 15th and 30th day, no
residues were detected.
The mobility of 14C-propachlor in the soil was investigated by
Brightwell et al. (1981) by determining its leaching as well as its
adsorption coefficient and desorption behaviour. Results were very
variable and depended on soil type. With a sandy loam, 89.5% of the
14C activity applied leached through laboratory soil columns,
whereas only 5.4% leached through silty clay loam. A high organic
matter content reduced the leachability of propachlor. Most of the
radioactivity represented the parent compound. Propachlor was adsorbed
only moderately, although the equivalent of 50 cm of rain was needed
to desorb 40 to 70% of the activity previously bound to soil to a
depth of 30 cm in the profile.
The mobility of propachlor in soil was the object of a study
carried out by Ritter et al. (1973). The diffusion coefficient of
propachlor in a silt loam soil was determined and was compared with
the coefficients of two other pesticides, atrazine and diazinone. It
was found that propachlor had the highest solubility and the largest
diffusion coefficient, which allowed it to move rapidly in the soil.
The movement increased with the temperature and moisture contents.
The run-off losses of propachlor have been studied by Baker &
Laflen (1979), Baker (1980) and Baker et al. (1982). Rainfall
simulation was used by Baker et al. (1982) to determine the effects of
corn residue on herbicide run-off losses from the soil. Propachlor was
applied to plots with 0, 375, 750 and 1500 kg corn residue per ha, and
a 2-h rainfall of 127 mm was simulated. For plots with no corn
residue, the average time to run-off was 11 min; run-off was 63 mm,
soil loss 11 tonnes/ha, and herbicide loss 7% of the amount applied.
Increased corn residue amounts increased time to run-off and decreased
run-off, erosion and herbicide losses. Time to run-off for the largest
corn residue amount was 30 min, run-off 18 mm, soil loss 1 tonne/ha,
and herbicide loss 1%. At least 84% of the herbicide losses were in
the dissolved phase. Herbicide placement had little or no effect on
the concentrations of herbicide in run-off water and sediment.
Herbicide concentrations in water and sediment were negatively
correlated with time to run-off.
The effects of incorporation and surface application on run-off
losses of propachlor were determined by measuring losses in water and
sediment from small plots during 122 mm of simulated rainfall (Baker
& Laflen, 1979). Losses of propachlor that was surface-applied to
plots were 3%, whereas losses from plots where the herbicide was
incorporated by disking were only 0.8%. Incorporation of herbicide has
the potential to decrease run-off losses and may be considered the
best application method.
In a laboratory study, propachlor was applied at rates of 0.4 and
1.8 kg/ha to corn ( Zea mays) residue, which in turn was subjected to
simulated rainfall (Martin et al., 1978). Initial concentrations in
wash-off water were high (9 mg/litre for the high application rate),
but this decreased rapidly with time. The mass balance showed that
most of the applied dose was washed off and little was retained by the
corn residue. Unexplained losses indicated the possibility of
volatilization occurring between application of herbicide and
application of wash-off water about 12 h later.
Gustafson (1989) described a method combining persistence and
mobility parameters to assess the potential for leaching and
contamination of ground water by propachlor. The author concluded that
propachlor was unlikely to leach through the soil into subsoil water
and ground water.
4.1.1.2 Biotic factors
Beestman & Deming (1974) carried out a large study to determine
the dissipation rate under laboratory and field conditions from Ray
silt and Wabash silty clay soils and to quantify the contributions of
microbial decomposition, chemical breakdown, volatilization and
leaching. Dissipation followed first-order kinetics with half-lives
ranging from 2 to 14 days. In moist Ray silt the half-life of
propachlor was 4.5 days. The important role of microbial degradation
was clearly established. Dissipation from sterilized soil was 50 times
slower (T´ = 141-151 days) than from unsterilized soil under
identical conditions.
Rankov & Velev (1977) conducted a model study over 120 days with
10 microscopic fungi from the genera Penicillium, Aspergillus,
Fusarium and Trichoderma concerning the degradation and
detoxication of propachlor in alluvial meadow soil at 28 °C and 65%
soil humidity. The results confirmed the important role of microscopic
fungi in increasing the rate of propachlor degradation and
detoxication.
Villarreal et al. (1991) enriched microbial cultures from a
pesticide disposal site to identify the range of metabolic capacity
for propachlor and its metabolites and the species involved in
breakdown of the compound. A single strain, corresponding most closely
to the genus Moraxella, could grow on propachlor as its sole carbon
source releasing a metabolite (2-chloro- N-isopropyl-acetamide) into
the medium. A second strain, corresponding to the genus Xanthobacter
grew on the metabolite. The Moraxella strain appears to use the
aromatic carbon atoms of the propachlor for growth since there was
induction of catechol 2,3-oxygenase activity in the cells and the
growth rate was sustainable only from this source.
Novick et al. (1986) showed that suspensions of soil treated in
the field with propachlor could mineralize 16-61% and 0.6-63% of
ring-labelled propachlor in 30 days at propachlor concentrations of
0.025 and 10 mg/litre, respectively. A mixture of two bacteria
mineralized 57.6% of propachlor within 52.5 h, producing
N-isopropylaniline as a metabolite.
4.1.1.3 Metabolites
N-isopropylaniline, N-isopropylacetanilide,
N-(1-hydroxyiso-propyl)-acetanilide and N-isopropyl-2-
acetoxyacetanilide are formed as metabolites of propachlor in soil
(Lee et al., 1982). Frank et al. (1977) demonstrated the existence of
a longer-lasting conjugated degradation product of propachlor in
onions and in organic soils following soil application. This was
conjugated N-isopropylaniline, which could be found in soil up to 2
years after application.
An extensive study on the environmental and metabolic fate of
propachlor was conducted by Brightwell et al. (1981). A variety of
soil metabolites was identified, the most significant resulting from
the modification of the C-2 carbon to yield water-soluble oxanilic and
sulfonic acids. The soil metabolism studies demonstrated the
predominant proportion of the water-soluble metabolites, i.e.
[(1-methylethyl) phenylamino] oxoacetic acid (IV), 2-[(1-methylethyl)
phenylamino]-2-oxoethanesulfonic acid (V) and {{[(methylethyl)
phenylamino] acetyl}sulfinyl}acetic acid (VI). These metabolites
accounted for 25, 17, and 6% of the applied 14C activity,
respectively, at different sampling points during the studies. There
was a decline in the level of these metabolites with time (Fig. 1).
In addition, several organic soluble metabolites were isolated
and identified; these included N-(1-methylethyl)-2-(methyl-
sulfinyl)- N-phenylacetamide (VII), N-(1-methylethyl)-2(methyl
sulfonyl)- N-phenylacetamide and 2-hydroxy- N-(1-methyl-ethyl)-
N-phenylacetamide (II). These accounted for no more than 6% of the
applied activity. The degradation products observed in the anaerobic
soil metabolism study were comparable to those observed under aerobic
conditions, but the rate of degradation in aerobic conditions was
higher.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
and the cysteine conjugate of propachlor in sandy loam soil. Both
compounds were metabolized at similar rates to three major products:
N-isopropyloxanilic acid, 2-sulfo- N-isopropyl-acetanilide and
2-(sulfinylmethylenecarboxy)- N-isopropyl-acetanilide.
Chemical formula: C11H14ClNO
Common synonyms
and trade names: Ramrod, Acylide, Bexton (discontinued by Dow
Chemical Company), Niticid, Satecid
CAS chemical name: 2-chloro- N-(1-methylethyl)- N-phenyl acetamide
IUPAC name: 2-chloro- N-isopropylacetanilide (formerly
alpha-chloro- N-isopropylacetanilide)
CAS registry
number: 1918-16-7
RTECS registry
number: AE1575000
Propachlor is available as a technical material containing 93%
active ingredient for formulation of propachlor end-use products. It
is available in the form of granules (200 g active ingredient/kg),
wettable powder (WP, 650 g active ingredient/kg) and as a liquid
flowable formulation (suspension concentrate), among others. Mixtures
with other herbicides, e.g., atrazine and propazine are also used.
2.2 Physical and chemical properties
Some of the physical and chemical properties of propachlor are
given in Table 1.
Table 1. Some physical and chemical properties of propachlor
Physical state solid
Colour tan
Relative molecular mass 211.7
Melting point (°C) 77
Boiling point (°C) at 0.03 mmHg 110
Decomposition (°C) > 170
Vapour pressure (25 °C) 103 mPa
Solubility in water (20 °C) 580 mg/litre
(25 °C) 613 mg/litre
Solubility in organic solvents readily soluble in most organic
solvents except aliphatic
hydrocarbons:
acetone 448 g/kg
benzene 737 g/kg
chloroform 602 g/kg
ethanol 408 g/kg
xylene 239 g/kg
Log Kow 1.62-2.30
It is non-flammable and stable to ultraviolet radiation.
2.3 Conversion factors
At 25 °C 1 mg/m3 = 8.802 ppm
1 ppm = 0.1136 mg/m3
2.4 Analytical methods
The analytical methods described in the literature, which are
based on different types of determination of propachlor in different
media, are given in Table 2.
Table 2. Analytical methods for the determination of propachlor and metabolites
Sample type Method of detection Extraction and clean-up Detection limit Reference
Soil and plants thin-layer chromatography 2-h extraction with 0.02-0.04 mg/kg Kofman &
chloroform Nishko
(1984)
Soil and plants gas chromatography with extraction with benzene; clean-up 0.004-0.005 mg/kg Balinova
electron-capture detection by partition with hexane-acetonitrile (1981)
followed by column chromatography
(Florisil)
Soil gas chromatography with acetone extraction followed by alkane 0.01 mg/kg; re- Caverly &
electron-capture detection hydrolysis, steam distillation and coveries at residual Denney
concentration of anilines in toluene levels are generally (1978)
better than 80%
Soil gas liquid chromatography extraction with isopropanol and benzene; < 0.05 mg/kg Markus &
with flame-ionization detection column chromatography (Florisil) Puma (1973)
Immature plants gas-liquid chromatography extraction with isopropanol; column < 0.05 mg/kg Markus &
with flame-ionization detection chromatography (Florisil) Puma (1973)
Mature grain gas-liquid chromatography extraction with acetonitrile; column < 0.05 mg/kg Markus &
with flame-ionization detection chromatography (Florisil) Puma (1973)
Cabbage gas chromatography with NP extraction with acetone followed by 0.06 mg/kg (fresh Warholic et
detection alkaline hydrolysis to weight) al. (1983)
N-isopropylaniline; steam
distillation and extraction with
toluene
Table 2 (contd).
Sample type Method of detection Extraction and clean-up Detection limit Reference
Industrial and gas chromatography with extraction with methylene chloride; 1 ng/litre Pressley &
municipal waste electron-capture detection clean-up on a Florisil column Longbottom
water (1982)
Urine metabolites gas or liquid chromatography fractionation with lipophilic ion not given Sjovall et
of propachlor and mass spectrometry exchangers (Lipidex 1000, Lipidex DEAP al. (1983)
(glutathione SP-LH-20 and Sep pack C18)
conjugates)
High-pressure liquid chromatography with radioactive detection or
liquid scintillation has been used to purify the metabolites from
14C-labelled propachlor in several biological media, including egg
yolk, egg white, edible tissues and excreta of laying hens, after
extraction with organic solvents. The metabolites were characterized
by gas chromatography with radioactive detection mass spectrometry
(Bleeke et al., 1987). With liquid scintillation detection, the
minimum quantifiable limit (MQL) was 0.01 to 0005 ppm.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Production and uses
Propachlor was developed by the Monsanto Chemical Company and
commercially introduced in 1965. Technical propachlor is produced in
the USA by Monsanto and in Germany by BASF. There are other
manufacturers of technical propachlor.
Propachlor is prepared by the reaction of chloracetyl chloride
and N-isopropylaniline.
It is a pre-emergence, pre-planting (incorporated) or early
post-emergence herbicide effective against annual grasses and some
broad-leaved weeds (Worthing & Hance, 1991). It is used on field corn,
hybrid seed corn, silage corn, grain sorghum (milo), green peas,
soybeans, flax, pumpkins and flowers. In 1971, 10 000 tonnes were
produced (US EPA, 1984a), but a more recent estimate of annual use in
the USA is 1800 tonnes.
3.2 Methods and rates of application
Application rates range from 4 to 6 kg active ingredient in
150-300 litres of water per ha (6-9 kg wettable powder formulation/ha)
in pre-emergence use. Some tests indicate that early post-emergence
applications are equally effective for weed control. The best response
occurred when broad-leaved weeds were between the cotyledonous stage
and the 2´-leaf stage and when grassy weeds were up to the one-leaf
stage.
Irrigation following application improves activity, particularly
under dry soil conditions. The duration of weed control ranges from 4
to 6 weeks, depending on the soil structure and organic content
(Humburg et al., 1989).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Soil
The fate and transport of propachlor in soil has been well
studied. The principal aim has been to determine the rate and products
of degradation, persistence, environmental factors and organisms
participating in the biodegradation, and the conditions under which
degradation takes place.
4.1.1.1 Abiotic factors
Beestman & Deming (1974) found that leaching did not contribute
to dissipation since no residues were found below the upper 4-cm
layer. The weak leaching ability was related to the high adsorption of
propachlor by soil. The ultraviolet absorption spectrum of propachlor
reveals no absorption at wavelengths longer than 280 nm. On the basis
of these data it was suggested that photodecomposition of soil-applied
propachlor would not be significant. Rapid herbicide volatilization
occurred under windy conditions during the period that exposed soil
surfaces remain moist.
In a study by Nesterova et al. (1980), propachlor was applied,
under dry weather conditions, at a rate of 7 kg/ha for 4 consecutive
years. The residues of propachlor in the soil were measured up to 4
weeks following the application. On the 5th day, residues were found
in the upper layers (0-10 cm), and on the 15th day, after abundant
rain (74 mm), they had reached 0-30 cm. There was rapid
biodegradation, mainly in the first 5 days, followed by reduced
degradation over the next 10 days. Between the 15th and 30th day, no
residues were detected.
The mobility of 14C-propachlor in the soil was investigated by
Brightwell et al. (1981) by determining its leaching as well as its
adsorption coefficient and desorption behaviour. Results were very
variable and depended on soil type. With a sandy loam, 89.5% of the
14C activity applied leached through laboratory soil columns,
whereas only 5.4% leached through silty clay loam. A high organic
matter content reduced the leachability of propachlor. Most of the
radioactivity represented the parent compound. Propachlor was adsorbed
only moderately, although the equivalent of 50 cm of rain was needed
to desorb 40 to 70% of the activity previously bound to soil to a
depth of 30 cm in the profile.
The mobility of propachlor in soil was the object of a study
carried out by Ritter et al. (1973). The diffusion coefficient of
propachlor in a silt loam soil was determined and was compared with
the coefficients of two other pesticides, atrazine and diazinone. It
was found that propachlor had the highest solubility and the largest
diffusion coefficient, which allowed it to move rapidly in the soil.
The movement increased with the temperature and moisture contents.
The run-off losses of propachlor have been studied by Baker &
Laflen (1979), Baker (1980) and Baker et al. (1982). Rainfall
simulation was used by Baker et al. (1982) to determine the effects of
corn residue on herbicide run-off losses from the soil. Propachlor was
applied to plots with 0, 375, 750 and 1500 kg corn residue per ha, and
a 2-h rainfall of 127 mm was simulated. For plots with no corn
residue, the average time to run-off was 11 min; run-off was 63 mm,
soil loss 11 tonnes/ha, and herbicide loss 7% of the amount applied.
Increased corn residue amounts increased time to run-off and decreased
run-off, erosion and herbicide losses. Time to run-off for the largest
corn residue amount was 30 min, run-off 18 mm, soil loss 1 tonne/ha,
and herbicide loss 1%. At least 84% of the herbicide losses were in
the dissolved phase. Herbicide placement had little or no effect on
the concentrations of herbicide in run-off water and sediment.
Herbicide concentrations in water and sediment were negatively
correlated with time to run-off.
The effects of incorporation and surface application on run-off
losses of propachlor were determined by measuring losses in water and
sediment from small plots during 122 mm of simulated rainfall (Baker
& Laflen, 1979). Losses of propachlor that was surface-applied to
plots were 3%, whereas losses from plots where the herbicide was
incorporated by disking were only 0.8%. Incorporation of herbicide has
the potential to decrease run-off losses and may be considered the
best application method.
In a laboratory study, propachlor was applied at rates of 0.4 and
1.8 kg/ha to corn ( Zea mays) residue, which in turn was subjected to
simulated rainfall (Martin et al., 1978). Initial concentrations in
wash-off water were high (9 mg/litre for the high application rate),
but this decreased rapidly with time. The mass balance showed that
most of the applied dose was washed off and little was retained by the
corn residue. Unexplained losses indicated the possibility of
volatilization occurring between application of herbicide and
application of wash-off water about 12 h later.
Gustafson (1989) described a method combining persistence and
mobility parameters to assess the potential for leaching and
contamination of ground water by propachlor. The author concluded that
propachlor was unlikely to leach through the soil into subsoil water
and ground water.
4.1.1.2 Biotic factors
Beestman & Deming (1974) carried out a large study to determine
the dissipation rate under laboratory and field conditions from Ray
silt and Wabash silty clay soils and to quantify the contributions of
microbial decomposition, chemical breakdown, volatilization and
leaching. Dissipation followed first-order kinetics with half-lives
ranging from 2 to 14 days. In moist Ray silt the half-life of
propachlor was 4.5 days. The important role of microbial degradation
was clearly established. Dissipation from sterilized soil was 50 times
slower (T´ = 141-151 days) than from unsterilized soil under
identical conditions.
Rankov & Velev (1977) conducted a model study over 120 days with
10 microscopic fungi from the genera Penicillium, Aspergillus,
Fusarium and Trichoderma concerning the degradation and
detoxication of propachlor in alluvial meadow soil at 28 °C and 65%
soil humidity. The results confirmed the important role of microscopic
fungi in increasing the rate of propachlor degradation and
detoxication.
Villarreal et al. (1991) enriched microbial cultures from a
pesticide disposal site to identify the range of metabolic capacity
for propachlor and its metabolites and the species involved in
breakdown of the compound. A single strain, corresponding most closely
to the genus Moraxella, could grow on propachlor as its sole carbon
source releasing a metabolite (2-chloro- N-isopropyl-acetamide) into
the medium. A second strain, corresponding to the genus Xanthobacter
grew on the metabolite. The Moraxella strain appears to use the
aromatic carbon atoms of the propachlor for growth since there was
induction of catechol 2,3-oxygenase activity in the cells and the
growth rate was sustainable only from this source.
Novick et al. (1986) showed that suspensions of soil treated in
the field with propachlor could mineralize 16-61% and 0.6-63% of
ring-labelled propachlor in 30 days at propachlor concentrations of
0.025 and 10 mg/litre, respectively. A mixture of two bacteria
mineralized 57.6% of propachlor within 52.5 h, producing
N-isopropylaniline as a metabolite.
4.1.1.3 Metabolites
N-isopropylaniline, N-isopropylacetanilide,
N-(1-hydroxyiso-propyl)-acetanilide and N-isopropyl-2-
acetoxyacetanilide are formed as metabolites of propachlor in soil
(Lee et al., 1982). Frank et al. (1977) demonstrated the existence of
a longer-lasting conjugated degradation product of propachlor in
onions and in organic soils following soil application. This was
conjugated N-isopropylaniline, which could be found in soil up to 2
years after application.
An extensive study on the environmental and metabolic fate of
propachlor was conducted by Brightwell et al. (1981). A variety of
soil metabolites was identified, the most significant resulting from
the modification of the C-2 carbon to yield water-soluble oxanilic and
sulfonic acids. The soil metabolism studies demonstrated the
predominant proportion of the water-soluble metabolites, i.e.
[(1-methylethyl) phenylamino] oxoacetic acid (IV), 2-[(1-methylethyl)
phenylamino]-2-oxoethanesulfonic acid (V) and {{[(methylethyl)
phenylamino] acetyl}sulfinyl}acetic acid (VI). These metabolites
accounted for 25, 17, and 6% of the applied 14C activity,
respectively, at different sampling points during the studies. There
was a decline in the level of these metabolites with time (Fig. 1).
In addition, several organic soluble metabolites were isolated
and identified; these included N-(1-methylethyl)-2-(methyl-
sulfinyl)- N-phenylacetamide (VII), N-(1-methylethyl)-2(methyl
sulfonyl)- N-phenylacetamide and 2-hydroxy- N-(1-methyl-ethyl)-
N-phenylacetamide (II). These accounted for no more than 6% of the
applied activity. The degradation products observed in the anaerobic
soil metabolism study were comparable to those observed under aerobic
conditions, but the rate of degradation in aerobic conditions was
higher.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
and the cysteine conjugate of propachlor in sandy loam soil. Both
compounds were metabolized at similar rates to three major products:
N-isopropyloxanilic acid, 2-sulfo- N-isopropyl-acetanilide and
2-(sulfinylmethylenecarboxy)- N-isopropyl-acetanilide.
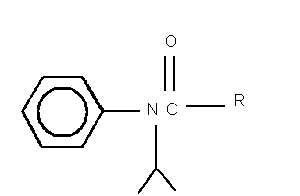 I. R = CH2Cl
2-chloro- N-(1-methylethyl)- N-phenylacetamide = propachlor
II. R = CH2OH
2-hydroxy- N-(1-methylethyl)- N-phenylacetamide
III. R = CH3
N-(1-methylethyl)- N-phenylacetamide
O
"
IV. R = COH
[(1-methylethyl)phenylamino]oxoacetic acid
V. R - CH2SO3H
2-[(1-methylethyl)phenylamino]-2-oxoethanesulfonic acid
O O
" "
VI. R = CH2SCH2COH
{{[(methylethyl)phenylamino]acetyl}sulfinyl}-acetic acid
O
"
VII. R = CH2SCH3
N-(1-methylethyl)-2-(methylsulfinyl)- N-phenylacetamide
Fig. 1. Structures of propachlor degradation products.
4.1.1.4 Persistence
Fletcher & Kirkwood (1982) reported a half-life of 2-3 weeks for
propachlor. Free propachlor disappeared rapidly in soil treated by
Ritter et al. (1973); in 21-28 days residues of free propachlor
declined 72-80%. In earlier laboratory studies and field bioassays
carried out by Menges & Tamez (1973), the soil persistence (> 90%
degradation) was found to be less than 6 months. According to Melnikov
et al. (1985) and Zhukova & Shirko (1979), the period of propachlor
degradation in soil to non-toxic products is about 2 months. The
presence of an alkyl group attached to the nitrogen atom in its
molecule prevents its decomposition to aniline or to azobenzolic
residues, which could later be transferred into tetrachlorazobenzene
(Panshina, 1985). Propachlor applied at 4-8 kg/ha was detoxified in
peat soil within 59-63 days (Vasilev, 1982). When it was applied at
6.5, 9 and 11 kg/ha, it was still detectable after 113 days in two
types of soil in the Voronezh and Krasnodar regions of the former USSR
(Kolesnikov et al., 1980). The longer persistence determined in this
study might be connected with the climatic conditions, particularly
the low temperatures. Roberts et al. (1978) and Balinova (1981)
confirmed that propachlor is rapidly decomposed in soil.
Field studies evaluating the degradation of propachlor in soil
showed that 70 days after application, propachlor was detectable in
insignificant quantities (0.04 mg/kg, i.e. 2% of the dose applied).
Degradation was slower in dry than humid weather (Zhukova & Shirko,
1979) (Table 3). The conclusions of the authors were that degradation
in soil is rapid and there is no possibility of propachlor
accumulation in crops.
Table 3. Dynamics of dissipation of propachlor in soil (average data
for 1974-1976)a
Dose application After 10th day On 30th day On 50th day On 70th day
(kg propachlor/ha) mg/kg %b mg/kg %b mg/kg %b mg/kg %b
6 1.73 86.4 0.95 42.8 0.10 5.2 0.04 2
8 2.44 92.6 1.55 58.3 0.20 7.8 0.04 2
10 3.14 94.2 2.53 76.0 0.40 12.0 0.04 2
a From: Zhukova & Shirko (1979)
b Percentage of dose applied
In a study by Frank et al. (1977), soil with high organic matter
content was treated with 19 kg propachlor/ha. The treatment times and
rates are given in Table 4. Soil samples were collected to a depth of
20 cm and analysed. Residues of the conjugated metabolite
N-isopropylaniline of up to 3.7 mg/kg soil were detected 2 years
after the application. They were released from soil by hydrolysis,
indicating the presence of active bonding sites for the metabolite in
the soil. The authors concluded that using propachlor in successive
years led to accumulation of long-lasting conjugated
N-isopropylaniline. Applications made once every 3 years, however,
did not lead to such accumulation and they recommended this type of
application.
Table 4. Residues of conjugated N-isopropylaniline in organic soil
treated with propachlor in 1971, 1972 and 1975a.
Year Time of application Rate of application N-isopropylaniline
(kg/ha)b residues in
oven-dried soil
(mg/kg)
1971 May and June 9 + 10 not analysed
1972 untreated none 3.67
May, July, August 6.7 + 3.4 + 3.4 7.70
May, July, August 6.7 + 6.7 + 6.7 9.47
1975 May and June 6.7 + 6.7 3.16
a From: Frank et al. (1977)
b Soil samples were collected in May 1973 and April 1976 and analysed
in January and April 1976, respectively.
US EPA (1984b) re-evaluated the existing data concerning some
environmental aspects of propachlor. It was concluded that microbes
were the primary factor in its breakdown in soil and that its loss
from photodecomposition and/or volatilization was low. Although this
earlier assessment suggested a potential for propachlor to contaminate
ground water, a later assessment (US EPA, 1988) concluded that "the
rapid degradation of low levels of propachlor in soil is expected to
result in a low potential for groundwater contamination by
propachlor".
4.1.1.5 Environmental conditions affecting distribution and breakdown
Walker & Brown (1982, 1985) carried out laboratory studies and
field trials in parallel. They found first-order kinetics dissipation
of propachlor and confirmed the results of Beestman & Deming (1974).
They also described a clear temperature dependence: an increase in
temperature of 10 °C reduced the half-life by a factor of between 1.9
and 2.5. Soil moisture also influenced degradation: slower rates of
loss were found in drier soil. The half-life in soil with a moisture
content of 6% was about twice as long as at 15% (Table 5). In general,
the time of persistence in the field was comparable to that measured
in laboratory studies. The half-life of propachlor varied from 4 to 22
days (Table 5).
Table 5. Half-life for propachlor degradation (days)a
Temperature (°C) 25 25 25 25 15 5
Moisture (%) 6 9 12 15 12 12
Propachlor 7.7 4.6 4.2 3.7 9.2 21.7
a From: Walker & Brown (1985)
The persistence of propachlor in soil as a result of all
microbial, chemical and physical processes has been studied by Zimdahl
& Clark (1982). They measured the half-life of propachlor in clay loam
and sandy loam soils in the laboratory, using different temperature
and moisture conditions (Table 6). An increase in temperature (from 10
to 30 °C) and moisture content (from 20 to 80%) shortened the
half-life.
Vasilev (1982) confirmed the importance of meterological
conditions, type of soil, and rate and period of application on the
degradation in soil. In dry weather, degradation took longer than in
humid conditions. Based on field experiments where soil was treated
with propachlor and carrots were then planted, the author reported the
following residues (measured at the moment of harvest in the soil
layer 0-20 cm) in soil: at an application rate of 4 kg/ha the
propachlor residues were 0.05 mg/kg; at 6 kg/ha they were 0.15 mg/kg;
and at 8 kg/ha they were 0.2 mg/kg.
Table 6. Half-lives of propachlor in clay loam and sandy loam as
determined by bioassaya
Storage conditions Half-life (days)
Temperature Soil moisture Sandy loam Clay loam
(°C) (%)
10 50 16.7 14.3
20 50 3.3 5.3
30 50 1.9 1
20 20 23.1 21.5
20 50 3.3 5.3
20 80 3.3 4.1
a From: Zimdahl & Clark (1982)
Shirko & Belova (1982) found that residues of propachlor in soil
and plants depended on the nitrogen and potassium content of the soil.
At the moment of harvesting, no residues of propachlor were detected
in soil after application at a rate of 4.5-6.5 kg/ha. According to the
authors, a better supply of plants with nutrients (in this case,
nitrogen and potassium fertilizers) leads to more intense
detoxification and degradation of the herbicide and, conversely, an
insufficiency delays the detoxification processes and leads to the
accumulation of residues.
4.1.2 Water
Photodegradation of propachlor in aqueous media was studied by
Tanaka et al. (1981) under laboratory conditions. They used a 10-ml
sample with propachlor concentrations ranging up to 100 mg/litre, and
the sample was irradiated for 135 min with a 300-nm sunlight lamp.
Very weak photolysis was registered; by the end of the study only 1%
of herbicide had been lost. Addition of a commercial surfactant (2%
heterogeneous non-ionic Tergitol TMN, acting as photosensitizer)
allowed 37% of propachlor to be photodegraded. It is difficult to make
conclusions on this study because of certain deficiencies. The use of
a commercial formulation in such studies should be avoided since some
of the constituents may cause indirect photochemical reactions. No
mention was made in the report of the purity of the compound studied.
The test should preferably be carried out at constant temperature. The
lack of data concerning the intensity of the irradiation source (US
EPA, 1984a) does not allow any extrapolation of these data to the
environment.
Rejtö et al. (1984) investigated the effects of ultraviolet
irradiation of propachlor solutions and found that 5 h of irradiation
led to 80% decomposition. The three photodecomposition products
identified were: N-isopropyloxindole, N-isopropyl-3-
hydroxyoxindole and a spiro compound. Irradiation of a solution of
propachlor with visible light for 12 h led to almost complete
decomposition in the presence of riboflavin as a photosensitizer. The
photodecomposition products after visible light irradiation were found
to be non-phytotoxic.
Monsanto (1987) demonstrated that propachlor is hydrolytically
stable.
Volatilization of propachlor from aqueous media is of limited
significance because of the high solubility in water and relatively
low vapour pressure of the compound. In agreement with Henry's
constant, the loss of propachlor by sorption and sedimentation in
water bodies does not appear to be very significant (US EPA, 1984a).
The role of microbial biodegradation appears to be of major
significance in water as well as in soil. Novick & Alexander (1985)
studied the metabolism of low concentrations (10 µg/litre) of
propachlor in sewage and lake water. They found that under aerobic
conditions microbial populations from sewage and lake waters were not
able to mineralize the carbon ring of propachlor in six weeks.
However, propachlor was extensively metabolized, the products obtained
were organic, and they were found to accumulate in the environment. In
a parallel study, it was found that aniline was readily cleaved under
similar conditions, indicating rapid mineralization of this compound.
It was concluded that structural characteristics of propachlor, other
than the ring, account for the mineralization of the compound. The
presence of the three substituents on the nitrogen atom in propachlor
may be the reason for its persistence. Steen & Collette (1989)
determined microbial transformation rate constants for seven amides in
natural pond water. A second order mathematical rate expression served
to describe propachlor degradation, and a value of 1.1 x 10-9
litres/organism per h was calculated. Brightwell et al. (1981)
presented data showing slow degradation of propachlor in lake water
under aerobic conditions. After 30 days, 84.5% of the propachlor
remained unchanged; under these conditions a half-life of about 5
months would be expected. The low rate of metabolism was due to a low
level of microorganisms. Yu et al. (1975) studied the degradation of
propachlor in water using a model ecosystem. An aquarium with
7-day-old sorghum plants was used with the addition of 50 µCi of 14C
ring-labelled propachlor. By the end of the 33-day experimental
period, 7 degradation products in water were determined by thin-layer
chromatography but were not identified. At that time only 0.4% of the
radioactivity of the dose applied remained in the water.
4.1.3 Plants
The metabolism of propachlor in corn seedlings and in excized
leaves of corn, sorghum, sugar cane and barley was studied by
Lamoureux et al. (1971). Metabolism was rapid and similar in all
mentioned plant species during the first 6-24 h following treatment.
At least 3 water-soluble metabolites were produced in each species
during this period. Two of these metabolites were isolated; the first
one was identified as the glutathione conjugate of propachlor
(compound I) and the second one appeared to be the gamma-
glutamylcysteine conjugate (compound II). The primary mode of
metabolism is a nucleophilic displacement of the alpha-chloro group of
propachlor by the sulfhydryl group of a peptide. The metabolic
reactions of propachlor proceed non-enzymatically in vitro, and the
in vivo reaction may be enzymatic and/or non-enzymatic. The high
percentage of propachlor converted to compounds I and II in corn
seedlings during the first 18 h following treatment indicates that the
reaction of propachlor with glutathione and/or gamma-glutamylcysteine
is quite specific. This would be expected if the reaction is
enzymatic. Some glutathione and gamma-glutamylcysteine conjugates in
plants may be end-products, but, in the case of propachlor, these
metabolites are transient intermediates. Further studies are needed to
establish the final steps (Lamoureux et al., 1971).
Pantano & Anderson (1987) studied the metabolism of propachlor in
sorghum (milo). Sorghum seeds were planted in soil treated with 14C
ring-labelled propachlor at a rate of 3.3 kg/ha and grown to maturity
in a greenhouse. Following senescence, plants were separated into
various anatomical parts, freeze-dried and analysed for activity. The
uptake of 14C in the foliage and grain was 9.5 and 0.5 mg/kg dry
weight, respectively. Four metabolites were identified in the foliage
extract; these represented 66.5% of the metabolites in foliage. The
four metabolites were [( N-iso-propyl) phenylamino]oxoacetic acid,
{{[( N-isopropyl)-phenyl-amino]acetyl}sulfinyl}lactic acid,
{{[( N-isopropyl)phenylamino] acetyl}sulfinyl}acetic acid and
2-[( N-isopropyl)phenylamino]-2-oxoethanesulfonic acid. In addition,
other metabolites found at low levels in the foliage extract were
characterized. Freeze-dried sorghum grain contained only 0.5 mg/kg of
14C activity and only one metabolite was identified (the first
compound mentioned above). This metabolite constituted at least 24.9%
of the activity in grain. In all of the major metabolites identified
in this study, no modification of the N-isopropylaniline moiety was
observed. On the basis of metabolites identified in this study and
known pathways for the metabolic transformations of related
chloroacetamides, a scheme for the metabolic fate of propachlor in
sorghum plants was postulated by the authors. The biotrans-formations
include: 1) displacement of the chlorine by an oxygen-containing
nucleophile followed by oxidation, and 2) conjugation with glutathione
followed by further metabolic modification of this conjugate.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
in soybean and found that it was rapidly metabolized to
homoglutathione conjugate in roots and foliage. This conjugate was
rapidly metabolized to the cysteine conjugate and then slowly
converted to a variety of other metabolites; four of these were
present up to 72 days after application of propachlor. These four were
malonylcysteine, malonylcysteine S-oxide, 3-sulfinyllactic acid and
O-malonyl glucoside conjugates of propachlor. Less than 1% of
metabolites was isolated from beans or pods, the great majority being
in roots and foliage. The major metabolites found in plants were the
same as those produced in soil. The authors suggested that it is
difficult to differentiate between metabolites formed in the plant and
those taken up from soil as to the relative importance of the two
sites of metabolic degradation.
4.2 Bioaccumulation and biomagnification
The two published values for the log octanol/water partition
coefficient (log Kow) of propachlor, i.e. 1.62 and 2.3 (US EPA,
1984b, 1988), indicate a moderate potential for bioaccumulation.
Barrows & Macek (1974) used bluegill sunfish exposed to
14C-labelled propachlor in a continuous-flow experiment in order to
assess the potential for the compound to bioconcentrate in aquatic
organisms. The fish were exposed for 35 days to a mean
14C-propachlor concentration of either 0.54 mg/litre (high level) or
0.012 mg/litre (low level) in the water. Analysis of the fish tissues
indicated bioconcentration factors (BCFs) in the edible tissue of 34
and 22 for the high and low levels, respectively, and in the
non-edible tissue of 22 and 20. When the fish were placed in clean
water the activity was rapidly eliminated from the non-edible portion
of the fish and at a somewhat slower rate from the edible tissue.
Because of the polar, water-soluble nature of the soil metabolites
(section 4.1.1), they would be less likely to bioconcentrate in
aquatic organisms. The results of a static study on catfish by Malik
(1982) showed that BCFs for both edible and non-edible tissues were
0.23 and that the low level of accumulated activity was eliminated
rapidly when the fish were placed in clean water.
Yu et al. (1975) studied 14C-propachlor in a model ecosystem
containing seven species. There was no indication of either
bioconcentration or biomagnification; total radioactivity declined
from 0.21 to 0.015 mg propachlor/kg through the seven stages of the
food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
The application of propachlor as a herbicide in plant protection
results in its presence in air, soil and water. It is taken up from
the soil into plants through the root system.
5.1.1 Air
Propachlor application resulted in its presence in the ambient
air 300 m away from the treated field at concentrations ranging from
0.02-0.6 mg/m3 (Panshina, 1976). No details on the analytical method
or quantity of propachlor sprayed were given in this report.
5.1.2 Water
Propachlor was found in 34 of 1690 surface water samples analysed
in the USA (US EPA, 1988). Samples of surface water were collected at
475 locations and groundwater samples at 94 locations. The compound
was detected in eight different states. The maximum concentration
found was 10 µg/litre in surface water and 0.12 µg/litre in
groundwater (the 85th percentile for all non-zero samples was 2
µg/litre in surface water and 0.12 µg/litre in ground water). Spalding
& Snow (1989) detected propachlor at a maximum concentration of 46
µg/litre in stream water receiving its flow from agricultural land
planted principally with corn. The monitoring was carried out during
a spring period of high water flow and run-off.
5.1.3 Food
The residues of propachlor in potato tubers and tomatoes were
lower than 0.005 mg/kg (the limit of detection) approximately 60-70
days after application of the recommended dose of 4-6 kg/ha (Balinova,
1981). Warholic et al. (1983) could not detect propachlor (detection
limit, 0.06 mg/kg fresh weight) in cabbages grown on soil treated with
propachlor at 0.6 kg a.i./ha (wettable powder).
Frank et al. (1977) reported the results of a study on the fate
of propachlor applied to onions. Planted in May, the onions were
harvested in September and analysed in January the following year.
Conjugated N-isopropylaniline was present in onion tissue after
harvest and after a normal storage period for the crop before sale.
Bearing in mind the reports of other authors (Lamoureux et al., 1971),
Frank et al. (1977) suggested that 2-chloro- N-isopro-pylacetanilide
could be hydrolysed to 2-hydroxy- N-isopropyl-acetanilide and bonded
to glutathione. It is probable that alkaline hydrolysis cleaved the
weaker N-carbonyl C linkage to given N-isopropylaniline rather
than the stronger C-S bond which would have resulted in 2-hydroxy- N-
isopropylaniline. Tissue residues of N-isopropylaniline increased as
the rate of application of propachlor was increased, and the later the
application was made the higher the tissue residues (see Table 7).
Table 7. Residues of conjugated N-isopropylanilinea
Year Time of application Rate of propachlor N-isopropylaniline
application (kg/ha) residues (mg/kg)
1974 untreated 0 0.03
pre-emergence 7.2 0.05
12.0 0.09
pre-emergence and early post-
emergence (21 May and 13 June, 7.2 + 7.2 0.17
respectively) 12.0 + 12.0 0.15
pre-emergence and late post-
emergence (21 May and 9 July, 7.2 + 7.2 0.28
respectively) 12.0 + 12 0.40
a From: Frank et al. (1977). Onions were planted 6 May 1974 and harvested
4 September 1974. Analyses were performed in January 1975.
Propachlor has been used for soil treatment (6 kg/ha) before
planting tomatoes and peppers. Sampling was performed at 15-day
intervals and the samples were analysed by gas chromatography
(detection limits, 0.005 mg/kg). Small quantities of propachlor (of
the order of 0.04 mg/kg) were found in tomatoes on the 70th and 85th
days after soil treatment. On the 73rd day, 0.07 mg/kg was found in
peppers, and on the 88th and 108th days the residues had decreased to
0.05 and 0.04 mg/kg, respectively. No herbicide residues were found in
the following years (Balinova & Konstantinov, 1975).
In a study where soil was treated (8 kg/ha) before the seedlings
were planted, samples of cabbages contained propachlor residues
0.6-0.8, 0.3-0.4 and 0.1-0.16 mg/kg after one, two and three months,
respectively (Medved, 1977). No residues were detected in cabbages 96
days after the application of propachlor, at harvesting (Kolesnikov et
al., 1979). A decrease in dose and application by conveyor belt
perceptibly reduced the residues (Table 8).
Table 8. Residues (mg/kg) of propachlor in cabbages (type Amager 611)
Propachlor Date of Sampling time (no. of days after application)
application rate application
13 33 64 96
8 kg/ha (before setting out) 31 May 0.6 0.3 0.1 No
8 kg/ha (after prior 31 May 0.8 0.4 0.16 No
application of 5 kg/ha)
4 kg/ha (conveyor-belt 7 June 0.4 0.2 0.03 No
application after setting
out)
In a study by Nesterova et al. (1980), a dry soil area planted
with cabbages was treated with propachlor (7 kg/ha) each year. In the
second year no residues were detectable two months after application.
In the third year, when the moisture content of the soil had
increased, propachlor degradation was more rapid and no residues were
detectable 15 days after the application. The soil was dry in the
fourth year, and residues were detectable in cabbages up to two months
after application.
Lottman & Cowell (1986) reported propachlor residues in sorghum
grains of between < 0.02 and 0.24 mg/kg after treatment of soil at
4.4 kg/ha. Lottman & Cowell (1987) found residues in corn grain of <
0.02 to 0.04 mg/kg after soil treatment with propachlor at 4.4 kg/ha
and of < 0.02 to 0.19 mg/kg after treatment at 6.6 kg/ha.
5.2 Occupational exposure
Only limited data are available on occupational exposure to
propachlor. Its application by a tractor-mounted sprayer to cabbages
resulted in its presence in the breathing zone of the tractor drivers
at levels of 0.8 to 2.1 mg/m3 (Panshina, 1977) and 0.1 to 3.7
mg/m3 (Panshina, 1976).
6. KINETICS AND METABOLISM
6.1 Absorption
Propachlor may be absorbed through the respiratory and
gastrointestinal tracts as well as through the skin. Following a
single oral administration in mammals, it is rapidly taken up into the
blood and internal organs, reaching its maximum blood concentration in
1 h. After 48 h it is no longer detectable in the organs (Panshina,
1985). An estimated 68% of a single 10 mg dose of ring-labelled
14C-propachlor administered to 12 rats was recovered in urine 56 h
later (Malik, 1986). These results are supported by those of other
studies in which 54-64% (Lamoureux & Davison, 1975) and 68.8% (Bakke
et al., 1980) of the administered dose was recovered in urine 24 h and
48 h after dose administration, respectively.
6.2 Metabolic transformation
Propachlor is rapidly metabolized. Its metabolism in animal
species has been studied by Bakke & Price (1979), Pekas et al. (1979),
Bakke et al. (1981a,b,c), Rafter et al. (1983a,b), Aschbacher &
Struble (1987) and Davison et al. (1988, 1990). Most of the animal
species studied metabolize propachlor through the mercapturic acid
pathway (MAP). The intestinal microflora is involved in the metabolism
of MAP intermediates (Bakke et al., 1981c). Metabolites of propachlor,
in which chlorine from the parent compound (2-chloro-
isopropylacetanilide) is removed by a nucleophilic displacement
(Rafter et al., 1983a) by a cysteine group or methylsulfonyl group
(CH3SO2), are present in the urine of rats dosed orally with
propachlor (Larsen & Bakke, 1979). It has been shown that a cysteine
conjugate of propachlor is the source of sulfur in methylsulfonyl-
containing metabolites, but that the carbon in the methylsulfonyl
group does not come from the cysteine moiety. Propachlor is conjugated
firstly with glutathione and the reaction is mediated by glutathione
transferases. The glutathione conjugation provides a means for
inactivation of reactive electrophiles. Glutathione conjugates have
the required physico-chemical properties for biliary excretion and
will generally be present, together with their catabolites
cysteinyl-glycine, cysteine and N-acetylcysteine-mercapturic acid,
in relatively high concentrations in the bile (Rafter et al., 1983a).
After excretion with the bile, they are metabolized in the intestine
where the C-S lyase present cleaves the cysteine conjugate, allowing
further metabolism of sulfur to a methylsulfonyl-containing moiety
(Larsen & Bakke, 1979).
The C-S lyase enzyme systems have been isolated in rat liver and
bacteria demonstrating that they are of bacterial origin. As a good
example, C-S lyase from Fusobacterium necrophorum, one of the pure
intestinal bacteria, has been isolated and characterized as a key
enzyme in mammalian metabolism (Larsen et al., 1983). This is
confirmed by the fact that in germ-free rats (Bakke et al., 1980)
(Fig. 2) and rats treated by antibiotics (Larsen & Bakke, 1981) 14C
was excreted from 14C-propachlor as MAP metabolites, but there were
no methylsulfonyl-containing metabolites in urine. Inextractable
residues were eliminated in the faeces. This shows that MAP
metabolites are available as substrates for the intestinal microflora.
Of the MAP metabolites studied, the glutathione and cysteine
conjugates are the best substrates both for production of
2-mercapto- N-isopropyl-acetanilide and for parallel formation of
insoluble 14C residues (Larsen & Bakke, 1983) which are excreted in
the faeces.
I. R = CH2Cl
2-chloro- N-(1-methylethyl)- N-phenylacetamide = propachlor
II. R = CH2OH
2-hydroxy- N-(1-methylethyl)- N-phenylacetamide
III. R = CH3
N-(1-methylethyl)- N-phenylacetamide
O
"
IV. R = COH
[(1-methylethyl)phenylamino]oxoacetic acid
V. R - CH2SO3H
2-[(1-methylethyl)phenylamino]-2-oxoethanesulfonic acid
O O
" "
VI. R = CH2SCH2COH
{{[(methylethyl)phenylamino]acetyl}sulfinyl}-acetic acid
O
"
VII. R = CH2SCH3
N-(1-methylethyl)-2-(methylsulfinyl)- N-phenylacetamide
Fig. 1. Structures of propachlor degradation products.
4.1.1.4 Persistence
Fletcher & Kirkwood (1982) reported a half-life of 2-3 weeks for
propachlor. Free propachlor disappeared rapidly in soil treated by
Ritter et al. (1973); in 21-28 days residues of free propachlor
declined 72-80%. In earlier laboratory studies and field bioassays
carried out by Menges & Tamez (1973), the soil persistence (> 90%
degradation) was found to be less than 6 months. According to Melnikov
et al. (1985) and Zhukova & Shirko (1979), the period of propachlor
degradation in soil to non-toxic products is about 2 months. The
presence of an alkyl group attached to the nitrogen atom in its
molecule prevents its decomposition to aniline or to azobenzolic
residues, which could later be transferred into tetrachlorazobenzene
(Panshina, 1985). Propachlor applied at 4-8 kg/ha was detoxified in
peat soil within 59-63 days (Vasilev, 1982). When it was applied at
6.5, 9 and 11 kg/ha, it was still detectable after 113 days in two
types of soil in the Voronezh and Krasnodar regions of the former USSR
(Kolesnikov et al., 1980). The longer persistence determined in this
study might be connected with the climatic conditions, particularly
the low temperatures. Roberts et al. (1978) and Balinova (1981)
confirmed that propachlor is rapidly decomposed in soil.
Field studies evaluating the degradation of propachlor in soil
showed that 70 days after application, propachlor was detectable in
insignificant quantities (0.04 mg/kg, i.e. 2% of the dose applied).
Degradation was slower in dry than humid weather (Zhukova & Shirko,
1979) (Table 3). The conclusions of the authors were that degradation
in soil is rapid and there is no possibility of propachlor
accumulation in crops.
Table 3. Dynamics of dissipation of propachlor in soil (average data
for 1974-1976)a
Dose application After 10th day On 30th day On 50th day On 70th day
(kg propachlor/ha) mg/kg %b mg/kg %b mg/kg %b mg/kg %b
6 1.73 86.4 0.95 42.8 0.10 5.2 0.04 2
8 2.44 92.6 1.55 58.3 0.20 7.8 0.04 2
10 3.14 94.2 2.53 76.0 0.40 12.0 0.04 2
a From: Zhukova & Shirko (1979)
b Percentage of dose applied
In a study by Frank et al. (1977), soil with high organic matter
content was treated with 19 kg propachlor/ha. The treatment times and
rates are given in Table 4. Soil samples were collected to a depth of
20 cm and analysed. Residues of the conjugated metabolite
N-isopropylaniline of up to 3.7 mg/kg soil were detected 2 years
after the application. They were released from soil by hydrolysis,
indicating the presence of active bonding sites for the metabolite in
the soil. The authors concluded that using propachlor in successive
years led to accumulation of long-lasting conjugated
N-isopropylaniline. Applications made once every 3 years, however,
did not lead to such accumulation and they recommended this type of
application.
Table 4. Residues of conjugated N-isopropylaniline in organic soil
treated with propachlor in 1971, 1972 and 1975a.
Year Time of application Rate of application N-isopropylaniline
(kg/ha)b residues in
oven-dried soil
(mg/kg)
1971 May and June 9 + 10 not analysed
1972 untreated none 3.67
May, July, August 6.7 + 3.4 + 3.4 7.70
May, July, August 6.7 + 6.7 + 6.7 9.47
1975 May and June 6.7 + 6.7 3.16
a From: Frank et al. (1977)
b Soil samples were collected in May 1973 and April 1976 and analysed
in January and April 1976, respectively.
US EPA (1984b) re-evaluated the existing data concerning some
environmental aspects of propachlor. It was concluded that microbes
were the primary factor in its breakdown in soil and that its loss
from photodecomposition and/or volatilization was low. Although this
earlier assessment suggested a potential for propachlor to contaminate
ground water, a later assessment (US EPA, 1988) concluded that "the
rapid degradation of low levels of propachlor in soil is expected to
result in a low potential for groundwater contamination by
propachlor".
4.1.1.5 Environmental conditions affecting distribution and breakdown
Walker & Brown (1982, 1985) carried out laboratory studies and
field trials in parallel. They found first-order kinetics dissipation
of propachlor and confirmed the results of Beestman & Deming (1974).
They also described a clear temperature dependence: an increase in
temperature of 10 °C reduced the half-life by a factor of between 1.9
and 2.5. Soil moisture also influenced degradation: slower rates of
loss were found in drier soil. The half-life in soil with a moisture
content of 6% was about twice as long as at 15% (Table 5). In general,
the time of persistence in the field was comparable to that measured
in laboratory studies. The half-life of propachlor varied from 4 to 22
days (Table 5).
Table 5. Half-life for propachlor degradation (days)a
Temperature (°C) 25 25 25 25 15 5
Moisture (%) 6 9 12 15 12 12
Propachlor 7.7 4.6 4.2 3.7 9.2 21.7
a From: Walker & Brown (1985)
The persistence of propachlor in soil as a result of all
microbial, chemical and physical processes has been studied by Zimdahl
& Clark (1982). They measured the half-life of propachlor in clay loam
and sandy loam soils in the laboratory, using different temperature
and moisture conditions (Table 6). An increase in temperature (from 10
to 30 °C) and moisture content (from 20 to 80%) shortened the
half-life.
Vasilev (1982) confirmed the importance of meterological
conditions, type of soil, and rate and period of application on the
degradation in soil. In dry weather, degradation took longer than in
humid conditions. Based on field experiments where soil was treated
with propachlor and carrots were then planted, the author reported the
following residues (measured at the moment of harvest in the soil
layer 0-20 cm) in soil: at an application rate of 4 kg/ha the
propachlor residues were 0.05 mg/kg; at 6 kg/ha they were 0.15 mg/kg;
and at 8 kg/ha they were 0.2 mg/kg.
Table 6. Half-lives of propachlor in clay loam and sandy loam as
determined by bioassaya
Storage conditions Half-life (days)
Temperature Soil moisture Sandy loam Clay loam
(°C) (%)
10 50 16.7 14.3
20 50 3.3 5.3
30 50 1.9 1
20 20 23.1 21.5
20 50 3.3 5.3
20 80 3.3 4.1
a From: Zimdahl & Clark (1982)
Shirko & Belova (1982) found that residues of propachlor in soil
and plants depended on the nitrogen and potassium content of the soil.
At the moment of harvesting, no residues of propachlor were detected
in soil after application at a rate of 4.5-6.5 kg/ha. According to the
authors, a better supply of plants with nutrients (in this case,
nitrogen and potassium fertilizers) leads to more intense
detoxification and degradation of the herbicide and, conversely, an
insufficiency delays the detoxification processes and leads to the
accumulation of residues.
4.1.2 Water
Photodegradation of propachlor in aqueous media was studied by
Tanaka et al. (1981) under laboratory conditions. They used a 10-ml
sample with propachlor concentrations ranging up to 100 mg/litre, and
the sample was irradiated for 135 min with a 300-nm sunlight lamp.
Very weak photolysis was registered; by the end of the study only 1%
of herbicide had been lost. Addition of a commercial surfactant (2%
heterogeneous non-ionic Tergitol TMN, acting as photosensitizer)
allowed 37% of propachlor to be photodegraded. It is difficult to make
conclusions on this study because of certain deficiencies. The use of
a commercial formulation in such studies should be avoided since some
of the constituents may cause indirect photochemical reactions. No
mention was made in the report of the purity of the compound studied.
The test should preferably be carried out at constant temperature. The
lack of data concerning the intensity of the irradiation source (US
EPA, 1984a) does not allow any extrapolation of these data to the
environment.
Rejtö et al. (1984) investigated the effects of ultraviolet
irradiation of propachlor solutions and found that 5 h of irradiation
led to 80% decomposition. The three photodecomposition products
identified were: N-isopropyloxindole, N-isopropyl-3-
hydroxyoxindole and a spiro compound. Irradiation of a solution of
propachlor with visible light for 12 h led to almost complete
decomposition in the presence of riboflavin as a photosensitizer. The
photodecomposition products after visible light irradiation were found
to be non-phytotoxic.
Monsanto (1987) demonstrated that propachlor is hydrolytically
stable.
Volatilization of propachlor from aqueous media is of limited
significance because of the high solubility in water and relatively
low vapour pressure of the compound. In agreement with Henry's
constant, the loss of propachlor by sorption and sedimentation in
water bodies does not appear to be very significant (US EPA, 1984a).
The role of microbial biodegradation appears to be of major
significance in water as well as in soil. Novick & Alexander (1985)
studied the metabolism of low concentrations (10 µg/litre) of
propachlor in sewage and lake water. They found that under aerobic
conditions microbial populations from sewage and lake waters were not
able to mineralize the carbon ring of propachlor in six weeks.
However, propachlor was extensively metabolized, the products obtained
were organic, and they were found to accumulate in the environment. In
a parallel study, it was found that aniline was readily cleaved under
similar conditions, indicating rapid mineralization of this compound.
It was concluded that structural characteristics of propachlor, other
than the ring, account for the mineralization of the compound. The
presence of the three substituents on the nitrogen atom in propachlor
may be the reason for its persistence. Steen & Collette (1989)
determined microbial transformation rate constants for seven amides in
natural pond water. A second order mathematical rate expression served
to describe propachlor degradation, and a value of 1.1 x 10-9
litres/organism per h was calculated. Brightwell et al. (1981)
presented data showing slow degradation of propachlor in lake water
under aerobic conditions. After 30 days, 84.5% of the propachlor
remained unchanged; under these conditions a half-life of about 5
months would be expected. The low rate of metabolism was due to a low
level of microorganisms. Yu et al. (1975) studied the degradation of
propachlor in water using a model ecosystem. An aquarium with
7-day-old sorghum plants was used with the addition of 50 µCi of 14C
ring-labelled propachlor. By the end of the 33-day experimental
period, 7 degradation products in water were determined by thin-layer
chromatography but were not identified. At that time only 0.4% of the
radioactivity of the dose applied remained in the water.
4.1.3 Plants
The metabolism of propachlor in corn seedlings and in excized
leaves of corn, sorghum, sugar cane and barley was studied by
Lamoureux et al. (1971). Metabolism was rapid and similar in all
mentioned plant species during the first 6-24 h following treatment.
At least 3 water-soluble metabolites were produced in each species
during this period. Two of these metabolites were isolated; the first
one was identified as the glutathione conjugate of propachlor
(compound I) and the second one appeared to be the gamma-
glutamylcysteine conjugate (compound II). The primary mode of
metabolism is a nucleophilic displacement of the alpha-chloro group of
propachlor by the sulfhydryl group of a peptide. The metabolic
reactions of propachlor proceed non-enzymatically in vitro, and the
in vivo reaction may be enzymatic and/or non-enzymatic. The high
percentage of propachlor converted to compounds I and II in corn
seedlings during the first 18 h following treatment indicates that the
reaction of propachlor with glutathione and/or gamma-glutamylcysteine
is quite specific. This would be expected if the reaction is
enzymatic. Some glutathione and gamma-glutamylcysteine conjugates in
plants may be end-products, but, in the case of propachlor, these
metabolites are transient intermediates. Further studies are needed to
establish the final steps (Lamoureux et al., 1971).
Pantano & Anderson (1987) studied the metabolism of propachlor in
sorghum (milo). Sorghum seeds were planted in soil treated with 14C
ring-labelled propachlor at a rate of 3.3 kg/ha and grown to maturity
in a greenhouse. Following senescence, plants were separated into
various anatomical parts, freeze-dried and analysed for activity. The
uptake of 14C in the foliage and grain was 9.5 and 0.5 mg/kg dry
weight, respectively. Four metabolites were identified in the foliage
extract; these represented 66.5% of the metabolites in foliage. The
four metabolites were [( N-iso-propyl) phenylamino]oxoacetic acid,
{{[( N-isopropyl)-phenyl-amino]acetyl}sulfinyl}lactic acid,
{{[( N-isopropyl)phenylamino] acetyl}sulfinyl}acetic acid and
2-[( N-isopropyl)phenylamino]-2-oxoethanesulfonic acid. In addition,
other metabolites found at low levels in the foliage extract were
characterized. Freeze-dried sorghum grain contained only 0.5 mg/kg of
14C activity and only one metabolite was identified (the first
compound mentioned above). This metabolite constituted at least 24.9%
of the activity in grain. In all of the major metabolites identified
in this study, no modification of the N-isopropylaniline moiety was
observed. On the basis of metabolites identified in this study and
known pathways for the metabolic transformations of related
chloroacetamides, a scheme for the metabolic fate of propachlor in
sorghum plants was postulated by the authors. The biotrans-formations
include: 1) displacement of the chlorine by an oxygen-containing
nucleophile followed by oxidation, and 2) conjugation with glutathione
followed by further metabolic modification of this conjugate.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
in soybean and found that it was rapidly metabolized to
homoglutathione conjugate in roots and foliage. This conjugate was
rapidly metabolized to the cysteine conjugate and then slowly
converted to a variety of other metabolites; four of these were
present up to 72 days after application of propachlor. These four were
malonylcysteine, malonylcysteine S-oxide, 3-sulfinyllactic acid and
O-malonyl glucoside conjugates of propachlor. Less than 1% of
metabolites was isolated from beans or pods, the great majority being
in roots and foliage. The major metabolites found in plants were the
same as those produced in soil. The authors suggested that it is
difficult to differentiate between metabolites formed in the plant and
those taken up from soil as to the relative importance of the two
sites of metabolic degradation.
4.2 Bioaccumulation and biomagnification
The two published values for the log octanol/water partition
coefficient (log Kow) of propachlor, i.e. 1.62 and 2.3 (US EPA,
1984b, 1988), indicate a moderate potential for bioaccumulation.
Barrows & Macek (1974) used bluegill sunfish exposed to
14C-labelled propachlor in a continuous-flow experiment in order to
assess the potential for the compound to bioconcentrate in aquatic
organisms. The fish were exposed for 35 days to a mean
14C-propachlor concentration of either 0.54 mg/litre (high level) or
0.012 mg/litre (low level) in the water. Analysis of the fish tissues
indicated bioconcentration factors (BCFs) in the edible tissue of 34
and 22 for the high and low levels, respectively, and in the
non-edible tissue of 22 and 20. When the fish were placed in clean
water the activity was rapidly eliminated from the non-edible portion
of the fish and at a somewhat slower rate from the edible tissue.
Because of the polar, water-soluble nature of the soil metabolites
(section 4.1.1), they would be less likely to bioconcentrate in
aquatic organisms. The results of a static study on catfish by Malik
(1982) showed that BCFs for both edible and non-edible tissues were
0.23 and that the low level of accumulated activity was eliminated
rapidly when the fish were placed in clean water.
Yu et al. (1975) studied 14C-propachlor in a model ecosystem
containing seven species. There was no indication of either
bioconcentration or biomagnification; total radioactivity declined
from 0.21 to 0.015 mg propachlor/kg through the seven stages of the
food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
The application of propachlor as a herbicide in plant protection
results in its presence in air, soil and water. It is taken up from
the soil into plants through the root system.
5.1.1 Air
Propachlor application resulted in its presence in the ambient
air 300 m away from the treated field at concentrations ranging from
0.02-0.6 mg/m3 (Panshina, 1976). No details on the analytical method
or quantity of propachlor sprayed were given in this report.
5.1.2 Water
Propachlor was found in 34 of 1690 surface water samples analysed
in the USA (US EPA, 1988). Samples of surface water were collected at
475 locations and groundwater samples at 94 locations. The compound
was detected in eight different states. The maximum concentration
found was 10 µg/litre in surface water and 0.12 µg/litre in
groundwater (the 85th percentile for all non-zero samples was 2
µg/litre in surface water and 0.12 µg/litre in ground water). Spalding
& Snow (1989) detected propachlor at a maximum concentration of 46
µg/litre in stream water receiving its flow from agricultural land
planted principally with corn. The monitoring was carried out during
a spring period of high water flow and run-off.
5.1.3 Food
The residues of propachlor in potato tubers and tomatoes were
lower than 0.005 mg/kg (the limit of detection) approximately 60-70
days after application of the recommended dose of 4-6 kg/ha (Balinova,
1981). Warholic et al. (1983) could not detect propachlor (detection
limit, 0.06 mg/kg fresh weight) in cabbages grown on soil treated with
propachlor at 0.6 kg a.i./ha (wettable powder).
Frank et al. (1977) reported the results of a study on the fate
of propachlor applied to onions. Planted in May, the onions were
harvested in September and analysed in January the following year.
Conjugated N-isopropylaniline was present in onion tissue after
harvest and after a normal storage period for the crop before sale.
Bearing in mind the reports of other authors (Lamoureux et al., 1971),
Frank et al. (1977) suggested that 2-chloro- N-isopro-pylacetanilide
could be hydrolysed to 2-hydroxy- N-isopropyl-acetanilide and bonded
to glutathione. It is probable that alkaline hydrolysis cleaved the
weaker N-carbonyl C linkage to given N-isopropylaniline rather
than the stronger C-S bond which would have resulted in 2-hydroxy- N-
isopropylaniline. Tissue residues of N-isopropylaniline increased as
the rate of application of propachlor was increased, and the later the
application was made the higher the tissue residues (see Table 7).
Table 7. Residues of conjugated N-isopropylanilinea
Year Time of application Rate of propachlor N-isopropylaniline
application (kg/ha) residues (mg/kg)
1974 untreated 0 0.03
pre-emergence 7.2 0.05
12.0 0.09
pre-emergence and early post-
emergence (21 May and 13 June, 7.2 + 7.2 0.17
respectively) 12.0 + 12.0 0.15
pre-emergence and late post-
emergence (21 May and 9 July, 7.2 + 7.2 0.28
respectively) 12.0 + 12 0.40
a From: Frank et al. (1977). Onions were planted 6 May 1974 and harvested
4 September 1974. Analyses were performed in January 1975.
Propachlor has been used for soil treatment (6 kg/ha) before
planting tomatoes and peppers. Sampling was performed at 15-day
intervals and the samples were analysed by gas chromatography
(detection limits, 0.005 mg/kg). Small quantities of propachlor (of
the order of 0.04 mg/kg) were found in tomatoes on the 70th and 85th
days after soil treatment. On the 73rd day, 0.07 mg/kg was found in
peppers, and on the 88th and 108th days the residues had decreased to
0.05 and 0.04 mg/kg, respectively. No herbicide residues were found in
the following years (Balinova & Konstantinov, 1975).
In a study where soil was treated (8 kg/ha) before the seedlings
were planted, samples of cabbages contained propachlor residues
0.6-0.8, 0.3-0.4 and 0.1-0.16 mg/kg after one, two and three months,
respectively (Medved, 1977). No residues were detected in cabbages 96
days after the application of propachlor, at harvesting (Kolesnikov et
al., 1979). A decrease in dose and application by conveyor belt
perceptibly reduced the residues (Table 8).
Table 8. Residues (mg/kg) of propachlor in cabbages (type Amager 611)
Propachlor Date of Sampling time (no. of days after application)
application rate application
13 33 64 96
8 kg/ha (before setting out) 31 May 0.6 0.3 0.1 No
8 kg/ha (after prior 31 May 0.8 0.4 0.16 No
application of 5 kg/ha)
4 kg/ha (conveyor-belt 7 June 0.4 0.2 0.03 No
application after setting
out)
In a study by Nesterova et al. (1980), a dry soil area planted
with cabbages was treated with propachlor (7 kg/ha) each year. In the
second year no residues were detectable two months after application.
In the third year, when the moisture content of the soil had
increased, propachlor degradation was more rapid and no residues were
detectable 15 days after the application. The soil was dry in the
fourth year, and residues were detectable in cabbages up to two months
after application.
Lottman & Cowell (1986) reported propachlor residues in sorghum
grains of between < 0.02 and 0.24 mg/kg after treatment of soil at
4.4 kg/ha. Lottman & Cowell (1987) found residues in corn grain of <
0.02 to 0.04 mg/kg after soil treatment with propachlor at 4.4 kg/ha
and of < 0.02 to 0.19 mg/kg after treatment at 6.6 kg/ha.
5.2 Occupational exposure
Only limited data are available on occupational exposure to
propachlor. Its application by a tractor-mounted sprayer to cabbages
resulted in its presence in the breathing zone of the tractor drivers
at levels of 0.8 to 2.1 mg/m3 (Panshina, 1977) and 0.1 to 3.7
mg/m3 (Panshina, 1976).
6. KINETICS AND METABOLISM
6.1 Absorption
Propachlor may be absorbed through the respiratory and
gastrointestinal tracts as well as through the skin. Following a
single oral administration in mammals, it is rapidly taken up into the
blood and internal organs, reaching its maximum blood concentration in
1 h. After 48 h it is no longer detectable in the organs (Panshina,
1985). An estimated 68% of a single 10 mg dose of ring-labelled
14C-propachlor administered to 12 rats was recovered in urine 56 h
later (Malik, 1986). These results are supported by those of other
studies in which 54-64% (Lamoureux & Davison, 1975) and 68.8% (Bakke
et al., 1980) of the administered dose was recovered in urine 24 h and
48 h after dose administration, respectively.
6.2 Metabolic transformation
Propachlor is rapidly metabolized. Its metabolism in animal
species has been studied by Bakke & Price (1979), Pekas et al. (1979),
Bakke et al. (1981a,b,c), Rafter et al. (1983a,b), Aschbacher &
Struble (1987) and Davison et al. (1988, 1990). Most of the animal
species studied metabolize propachlor through the mercapturic acid
pathway (MAP). The intestinal microflora is involved in the metabolism
of MAP intermediates (Bakke et al., 1981c). Metabolites of propachlor,
in which chlorine from the parent compound (2-chloro-
isopropylacetanilide) is removed by a nucleophilic displacement
(Rafter et al., 1983a) by a cysteine group or methylsulfonyl group
(CH3SO2), are present in the urine of rats dosed orally with
propachlor (Larsen & Bakke, 1979). It has been shown that a cysteine
conjugate of propachlor is the source of sulfur in methylsulfonyl-
containing metabolites, but that the carbon in the methylsulfonyl
group does not come from the cysteine moiety. Propachlor is conjugated
firstly with glutathione and the reaction is mediated by glutathione
transferases. The glutathione conjugation provides a means for
inactivation of reactive electrophiles. Glutathione conjugates have
the required physico-chemical properties for biliary excretion and
will generally be present, together with their catabolites
cysteinyl-glycine, cysteine and N-acetylcysteine-mercapturic acid,
in relatively high concentrations in the bile (Rafter et al., 1983a).
After excretion with the bile, they are metabolized in the intestine
where the C-S lyase present cleaves the cysteine conjugate, allowing
further metabolism of sulfur to a methylsulfonyl-containing moiety
(Larsen & Bakke, 1979).
The C-S lyase enzyme systems have been isolated in rat liver and
bacteria demonstrating that they are of bacterial origin. As a good
example, C-S lyase from Fusobacterium necrophorum, one of the pure
intestinal bacteria, has been isolated and characterized as a key
enzyme in mammalian metabolism (Larsen et al., 1983). This is
confirmed by the fact that in germ-free rats (Bakke et al., 1980)
(Fig. 2) and rats treated by antibiotics (Larsen & Bakke, 1981) 14C
was excreted from 14C-propachlor as MAP metabolites, but there were
no methylsulfonyl-containing metabolites in urine. Inextractable
residues were eliminated in the faeces. This shows that MAP
metabolites are available as substrates for the intestinal microflora.
Of the MAP metabolites studied, the glutathione and cysteine
conjugates are the best substrates both for production of
2-mercapto- N-isopropyl-acetanilide and for parallel formation of
insoluble 14C residues (Larsen & Bakke, 1983) which are excreted in
the faeces.
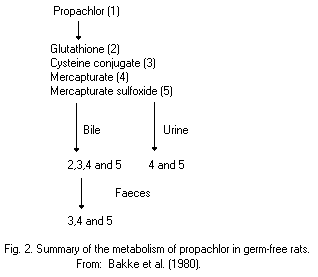 In normal rats dosed with propachlor, the above-mentioned final
metabolites were formed when MAP metabolites underwent a number of
reactions: carbon-sulfur bond cleavage by microflora, S-methylation,
S-oxidation, ring and alkyl-hydroxylation, glucuronide conjugation,
N-dealkylation and amide cleavage (Rafter et al., 1983b).
Fig. 3 shows the proposed metabolic pathway for the formation of
methylsulfonyl metabolites in rats and pigs (Aschbacher & Struble,
1987) and Fig. 4 the metabolism in normal rats treated with propachlor
proposed by Bakke et al. (1980).
The tissue in which propachlor enters the mercapturic acid
pathway has not been determined. The liver is an obvious site for
glutathione conjugation, but the intestine cannot be excluded (Bakke
et al., 1980). Organ perfusion studies have demonstrated that all
enzymes necessary for the formation of mercapturic acid conjugates are
present in the kidneys of both chickens and rats (Davison et al.,
1988, 1990) and in the livers of rats (Davison et al., 1990). Rat
caecal contents are similar to those of the pig with respect to C-S
lyase activity, which explains the general similarity of their
metabolic transformations (Larsen & Bakke, 1983).
When pig caecal contents were incubated with the glutathione
conjugate of propachlor, the formation of both insoluble residues and
the thiol increased with increase in incubation period (Table 9).
Digestive peptidases extracted during isolation of the metabolites
were thought to be the explanation for the presence of the cysteine
conjugate in the zero time samples, because no cysteine conjugate was
isolated from heat-treated caecal contents. A decrease in glutathione
concentration with increased incubation time was also evident and was
confirmation of a product-precursor relationship. This decrease in
glutathione conjugate concentration was assumed to be caused by
formation of cysteine conjugate (82%), due to cleavage by peptidase
activity, in the caecum (Larsen & Bakke, 1983).
In summary, three or more enterohepatic cycles for propachlor
metabolism in normal rats have been described. In the first,
propachlor is metabolized via the mercapturic acid pathway and the
conjugates are excreted in the bile. The second cycle is initiated
when the biliary mercapturic acid pathway metabolites are metabolized
by microbial/intestinal C-S lyase into reabsorbable metabolites
(possibly 2-mercapto- N-isopropylacetanilide). The reabsorbable
metabolites are further metabolized to glucuronides by glucuronidase
enzymes, and these are secreted with the bile. These biliary
glucuronides subsequently initiate the third cycle in the
enterohepatic circulation of propachlor metabolites.
No doubt the intestinal microorganisms complicate the metabolism
of propachlor (in comparison with the situation in germ-free and
antibiotic-treated rats) and create new non-polar compounds from the
products of the mercapturic acid pathway, which are reabsorbed into
the blood. These new compounds have to be converted again into polar
products in order to be excreted (Bakke et al., 1980).
In normal rats dosed with propachlor, the above-mentioned final
metabolites were formed when MAP metabolites underwent a number of
reactions: carbon-sulfur bond cleavage by microflora, S-methylation,
S-oxidation, ring and alkyl-hydroxylation, glucuronide conjugation,
N-dealkylation and amide cleavage (Rafter et al., 1983b).
Fig. 3 shows the proposed metabolic pathway for the formation of
methylsulfonyl metabolites in rats and pigs (Aschbacher & Struble,
1987) and Fig. 4 the metabolism in normal rats treated with propachlor
proposed by Bakke et al. (1980).
The tissue in which propachlor enters the mercapturic acid
pathway has not been determined. The liver is an obvious site for
glutathione conjugation, but the intestine cannot be excluded (Bakke
et al., 1980). Organ perfusion studies have demonstrated that all
enzymes necessary for the formation of mercapturic acid conjugates are
present in the kidneys of both chickens and rats (Davison et al.,
1988, 1990) and in the livers of rats (Davison et al., 1990). Rat
caecal contents are similar to those of the pig with respect to C-S
lyase activity, which explains the general similarity of their
metabolic transformations (Larsen & Bakke, 1983).
When pig caecal contents were incubated with the glutathione
conjugate of propachlor, the formation of both insoluble residues and
the thiol increased with increase in incubation period (Table 9).
Digestive peptidases extracted during isolation of the metabolites
were thought to be the explanation for the presence of the cysteine
conjugate in the zero time samples, because no cysteine conjugate was
isolated from heat-treated caecal contents. A decrease in glutathione
concentration with increased incubation time was also evident and was
confirmation of a product-precursor relationship. This decrease in
glutathione conjugate concentration was assumed to be caused by
formation of cysteine conjugate (82%), due to cleavage by peptidase
activity, in the caecum (Larsen & Bakke, 1983).
In summary, three or more enterohepatic cycles for propachlor
metabolism in normal rats have been described. In the first,
propachlor is metabolized via the mercapturic acid pathway and the
conjugates are excreted in the bile. The second cycle is initiated
when the biliary mercapturic acid pathway metabolites are metabolized
by microbial/intestinal C-S lyase into reabsorbable metabolites
(possibly 2-mercapto- N-isopropylacetanilide). The reabsorbable
metabolites are further metabolized to glucuronides by glucuronidase
enzymes, and these are secreted with the bile. These biliary
glucuronides subsequently initiate the third cycle in the
enterohepatic circulation of propachlor metabolites.
No doubt the intestinal microorganisms complicate the metabolism
of propachlor (in comparison with the situation in germ-free and
antibiotic-treated rats) and create new non-polar compounds from the
products of the mercapturic acid pathway, which are reabsorbed into
the blood. These new compounds have to be converted again into polar
products in order to be excreted (Bakke et al., 1980).
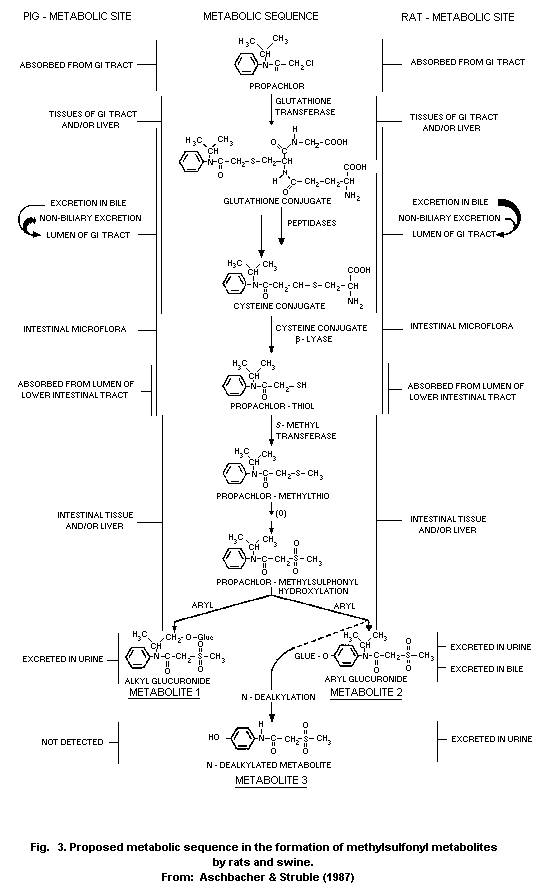
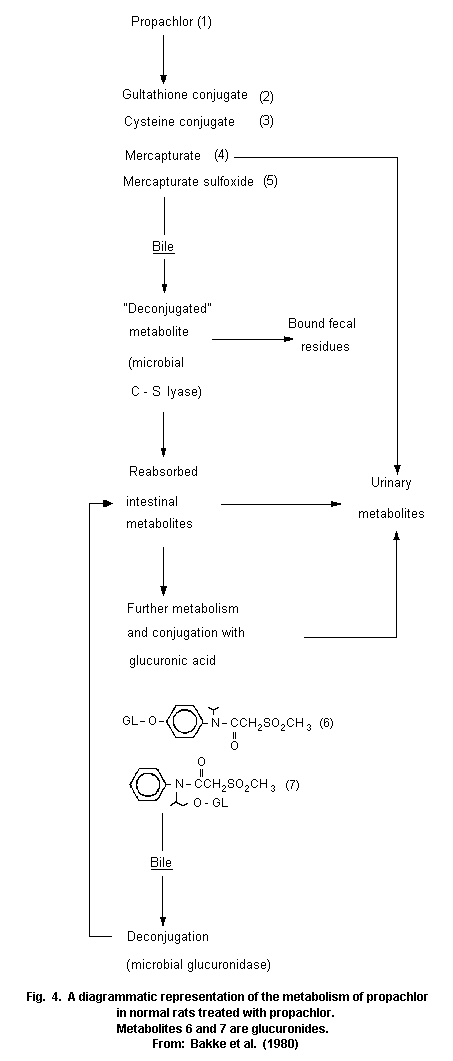 Table 9. Incubation of the glutathione conjugate of propachlor with pig caecal contents for various durationsa
Metabolites recovered Incubation time
0 20 min 40 min 1 h 2 h 4 h
2-Mercapto-N-isopropylacetanilide 7.7b 14.5b 23.0b 28.3b 34.7b 43.4c
(5.1-10.6) (5.5-22.8) (15.8-36.0) (22.9-38.7) (21.8-44.0) (38.7-49.2)
Glutathione conjugate 26.8 36.0 20.8 28.8 21.5 16.5
of propachlor (15.8-38.1) (18.1-51.6) (13.3-25.3) (16.6-42.3) (14.0-32.2) (14.8-19.2)
Cysteine conjugate of 47.5 32.5 35.1 20.9 11.9 3.8
propachlor (36.0-51.5) (21.3-43.7) (30.5-41.9) (12.0-27.9) (10.9-12.5) (2.2-5.6)
Non-extractable 14C 13.1 12.9 16.0 16.6 25.4 33.9
residues (13.0-13.4) (9.9-17.8) (13.5-20.5) (15.0-18.9) (22.8-28.2) (31.5-35.8)
a From: Larsen & Bakke (1983).
b Isolated as 2-carboxymethylthio- N-isopropylacetanilide
c Isolated as 2-(13C)-carboxymethylthio- N-isopropylacetanilide
Pigs were gilts. Results are shown as a percentage of the substrate. Values given in parentheses represent the range of values
obtained from 14C-recovery measurements.
More recent studies carried out by Aschbacher & Struble (1987) on
the metabolism of propachlor in pigs have proved the similarity, i.e.
the formation of methylsulfonyl-containing metabolites, with the
metabolism of rats, but have also revealed some differences.
A pig with a cannulated bile duct, which was dosed orally with
14C-propachlor, excreted 7.6% of the dose in the bile compared with
approximately 75% in the case of the rats. When enterohepatic
circulation was prevented in the bile of cannulated pigs,
CH3SO2-containing metabolites of propachlor were excreted in the
urine. As mentioned above, enterohepatic circulation is necessary for
the production of methylsulfonyl-containing metabolites in rats. In
experiments with germ-free pigs dosed orally with 14C-propachlor, it
was shown that they did not excrete urinary CH3SO2 metabolites, which
indicated involvement of the intestinal flora in the production of
these metabolites, as occurs in rats. Non-biliary excretion of
metabolites of propachlor into the lumen of the intestine probably
occurred. It is presumed that propachlor is absorbed by the mucosal
cells and conjugated with glutathione and that some of this conjugate
moves directly into the lumen of the intestinal tract by simple
diffusion, where it becomes the substrate of bacterial beta-lyase. The
presence of glutathione transferase has been demonstrated previously
in subcellular fractions of mucosal tissue homogenates.
In situ intestinal absorption of propachlor and non-biliary
excretion of metabolites into the intestinal tract of rats, pigs and
chickens was studied by Struble (1991). Propachlor was absorbed from
in situ intestinal loops of rats and pigs, the absorption half-times
being 7.5 and 16.5 min, respectively. Water-soluble 14C-labelled
metabolites that accumulated in the intestinal loops accounted for
31%, 53% and 25% of the initial 14C in rats, pigs and chickens,
respectively. Propachlor- S-cysteine was identified as the major
metabolite in the pig intestinal lumen (43% of the water-soluble
14C). It was concluded that the intestinal metabolism and intestinal
excretion of water-soluble metabolites of propachlor are important
physiological processes that occur in a variety of animal species.
These processes provide a route by which metabolites of xenobiotics
may reach the intestinal lumen in animals that are poor biliary
excretors. These studies demonstrated that although bile may be the
major route by which MAP metabolites are made available to the
intestinal microflora in the rat, an extrahepatic route exists in the
pig.
Davison (1991) conducted a study using six anaesthetized 2- to
21-day-old male Guernsey calves weighing 28 to 61 kg in which either
the left kidney was perfused (via the left renal artery) or the left
ureter was perfused with metabolites of propachlor. The glutathione
conjugate of propachlor (2- S-glutathionyl- N-acetyl-acetanilide)
was metabolized in both kidney and ureter to the cysteine conjugate.
When the mercapturic acid conjugate of propachlor was presented to the
kidney, it was eliminated in urine. First-pass metabolism and
elimination of the glutathione conjugate by the kidney was 16% of the
dose, whereas first-pass elimination of the mercapturic acid was 33%.
Absorption of the glutathione conjugate of propachlor or its
metabolites, or of glycine by the ureter was nil. The cattle may be
unable to form mercapturic acids from glutathione conjugates of some
xenobiotics, which may result in their being more easily poisoned by
these xenobiotics than chickens, pigs and rats.
The glutathione conjugate of 2-chloro- N-isopropyl[1-14C]acet-
anilide (14C-propachlor) was perfused through a calf kidney in situ
by Bakke et al. (1990). Twenty-three per cent of the dose was excreted
in the perfused kidney urine as the cysteine conjugate; no mercapturic
acid was detected. A 5-day-old calf dosed orally with 14C-propachlor
excreted 70% of the dose in the urine as the cysteine conjugate; again
no mercapturic acid was detected. Rumen microflora were established in
the calf when it was 5 weeks older and the experiment was repeated.
The same results were obtained.
6.3 Elimination and excretion
When 14C-propachlor was given to rats, 56-64% of the dose was
excreted in urine in the first 24 h and 5.7-7.0% in 24-48 h. In the
faeces, 8-13% and 2.2-7.7% were eliminated in 0-24 h and 24-48 h,
respectively; 0.4% of the 14C was eliminated as CO2 and 5-11% was in
the carcass. In total 80-97% was eliminated in 48 h (Lamoureux &
Davison, 1975).
According to Bakke et al. (1980), the metabolites of propachlor
formed in normal rats treated with propachlor are excreted mainly
through urine (68%) and faeces (19%). Eleven urinary metabolites were
isolated from rats given 14C-labelled propachlor orally. The major
metabolite was the mercapturate (17%), and six of the metabolites were
2-methylsulfonylacetanilides. Faecal residues (19%) of the
administered dose, insoluble in common solvents or by treatment with
diluted acid or base, were also determined.
Rats with cannulated bile ducts secreted 66% of an oral dose of
propachlor in the bile as the glutathione conjugate (2), cysteine
conjugate (3), mercapturate (4) and the mercapturate sulfoxide (5)
(see Table 10). Germ-free rats given orally 14C-labelled propachlor
excreted 98% of the dose in the urine and faeces within 48 h. Three
metabolites were isolated from the excreta and the faecal radioactive
metabolites were water soluble. The major metabolite was mercapturate
(4) and the other metabolites were the cysteine conjugate (3) (present
only in the faeces) and mercapturate sulfoxide (5) (Bakke et al.,
1980). Mercapturate sulfoxide was isolated from the excreta of
germ-free rats by Feil et al. (1981), who also demonstrated its
presence in the bile of rats and urine of chickens and pigs dosed with
propachlor. The metabolite was characterized by mass and nuclear
magnetic resonance spectro-metry on samples isolated from rats.
Table 10. Comparison of the excretion of single oral doses of 14C-propachlor
by control rats with fistulated bile ducts, and germ-free ratsa
Metabolite Recovery of 14C (% dose)b
Bile-fistulated
Control rats rats Germ-free rats
Urine Faeces Bile Urine Faeces
Glutathione 37
conjugate (2)b
Cysteine 13 19
conjugate (3)
Mercapturate (4) 17 12 63.1 3.7
Mercapturate 4 5.7 5.4
sulfoxide (5)
Non-extractable 19
residues
Other metabolites 51
Total 68 19 66 68.8 32.1
a From: Bakke et al. (1980)
b Metabolite designations are those used in Figs. 1 and 3.
As in the case of the glutathione and cysteine conjugates, the
sulfoxide is not excreted at detectable levels by normal rats and was
detected only in the bile (Bakke et al., 1981a). It may become a
substrate for the intestinal flora, but the ultimate in vivo fate of
this metabolite is unknown.
Bakke et al. (1981c) reported differences between some species
concerning the metabolism of propachlor in the MAP. Clear but
unexplained differences are that rats excrete no cysteine conjugate
and chickens form no methylsulfonyl-containing metabolites, whereas
sheep excrete large amounts of cysteine conjugate in urine.
Nadeau & Pantano (1986) carried out a study to determine the
rates and routes of excretion of orally administered synthetic
14C-labelled propachlor plant metabolites in lactating goats and to
quantify and identify the radioactive metabolites in the goat milk,
tissues, urine and faeces. The daily dose level for the three treated
goats was 15 mg/kg administered on 5 consecutive days (the actual dose
levels for the treated goats were 13, 14.5 and 13 mg/kg,
respectively). Each treated goat received a total of 130.5 mg of the
13C/14C-labelled propachlor plant metabolites mixture during the
dosing period. A control animal received placebo capsules. The
radioactivity eliminated in faeces accounted for 72.1, 64.5 and 57.9%
of the administered dose, respectively (average 64.8%), and that
excreted in urine accounted for 35.8, 28.1 and 32.3% (average 32.1%).
The percentage of the dose eliminated through faeces and urine
averaged 96.9%.
The radioactivity found in the milk accounted for only 0.084,
0.10 and 0.13% (average 0.10%) of the administered dose. These values
corresponded to metabolite concentrations in the milk of 11.9, 14.8
and 18.0 µg/litre (average 14.9 µg/litre). The metabolic
concentrations (µg/kg) in the tissues were: kidney 51.9, liver 30.4,
muscle 8.5, fat 19.5 and blood 50.1. The loss of radioactivity from
tissues, milk and excrement occurred rapidly, and after 5 days of
depuration the milk and tissue radioactivity levels were below the
limit of detection, except in the case of the liver (5.7 µg/kg).
6.4 Metabolism in laying hens
Since crops treated with propachlor are used in animal feed, it
is important to know the fate of propachlor in animal feed.
The purpose of the study by Bleeke et al. (1987) was to examine
the metabolic fate of propachlor plant metabolites in laying hens and
to determine whether they accumulate or persist in the eggs or edible
tissues. The compounds used for feeding were the three major
metabolites of propachlor found in sorghum, i.e. [( N-iso-propyl)-
phenylamino] oxacetic acid, sodium salt (I), {{[( N-iso-propyl)-
phenylamino]acetyl}sulfinyl} lactic acid, sodium salt (II) and
2-[( N-isopropyl)-phenylamino] 2-oxoethane sulfonic acid, sodium salt
(III).
The first study involved dosing chickens at a level of 5 mg/kg
diet for 6 consecutive days. Data for bioaccumulation and excretion of
plant metabolites were obtained from this study. A second group of
chickens, dosed at a level of 25 mg/kg diet (nominal dose) on 6
consecutive days, provided eggs and tissues with higher residues for
metabolic characterization. Each group consists of five hens. Control
groups received a single gelatine capsule per day. The total recovery
of 14C radioactivity was good in both studies.
An average of 87.4% of the total administered dose was recovered
from the chickens fed 5 mg/kg approximately 1 day after the last dose,
and 97.9% was recovered from the chickens fed 25 mg/kg, primarily in
the excreta. The eggs contained low residue levels; those from
chickens fed 5 mg/kg contained residues below the minimum quantifiable
limit (MQL) of 1.7 µg/kg. The level in the egg yolks reached an
average of 5.5 µg/kg by day 6 but by day 12 the residues in the yolk
had fallen below 1.6 µg/kg.
In the high-dose group, the residues in the egg white levelled
off at about 4 µg/kg on day 2, while those in the egg yolk increased
to a level of 27.3 µg/kg on day 6.
Tissues also contained low levels of residues. In the low-dose
group, the highest levels were found in the kidney and they averaged
only 6 µg/kg. The residue levels in the liver measured an average of
1.9 µg/kg and those in the fat 3.5 µg/kg. The residues in other fat
samples and organs were below the MQL (1.4-1.6 µg/kg). Residues in the
breast muscle were below the minimum detectable limit (7 µg/kg) and
those in the blood were 6-7 µg/kg.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity of propachlor for the rat, mouse and the
rabbit has been examined. According to the WHO hazard classification
of pesticides and as far as its acute oral toxicity to rats is
concerned, propachlor is slightly hazardous (Group III) (WHO, 1990).
LD50 values in various animal species reveal a higher susceptibility
for the mouse and the rabbit than for the rat (Table 11). The LD50
for rats ranges from 950 to 2176 mg/kg.
The clinical picture of acute oral intoxication by propachlor has
been described in three species of experimental animals: mice, rabbits
and rats (Panshina, 1973). When propachlor is given at lethal or toxic
doses, the main symptoms concern the central nervous system. In mice
and rabbits, a state of excitement, trembling and light convulsions is
observed 15 min after a toxic or lethal dose of propachlor. This
gradually increases, breathing becomes difficult and death follows in
fseveral hours. Intoxication in rats takes a different course: a state
of immobility and head and body tremors are accompanied by the sudden
onset of convulsions and death occurs within 24 h. This clinical
picture was confirmed by Strateva (1974,a,b).
Kronenberg (1988) reported clear signs of irritation to the
gastrointestinal tract and lungs that were evident at necropsy in
animals that died during the study. Histoenzymological changes
expressed as decreased enzyme activity were in full agreement with the
morphological picture (Strateva et al., 1974a,b).
7.1.2 Dermal
Results of studies in rabbits on the acute dermal toxicity of
propachlor and its formulations indicate that the dermal LD50 ranges
from 4000 mg/kg for a 65% WP formulation (Auletta & Rinehart, 1979) to
20 000 mg/kg for technical propachlor (94.5%) (Braun & Rinehart,
1978). Test animals exhibited moderate to severe erythema, severe
oedema and necrotic skin at the dermal application sites at a dose
level of 2800 mg/kg. Motor activity decrease and ataxia were noted at
dose levels of 2000 to 5600 mg/kg.
Table 11. Oral LD50 values for laboratory mammals
Species LD50 (mg/kg)a Reference
Rat 1056 (834-1278) Panshina (1973, 1977)
950 (860-1050) Strateva (1974b)
1340 Mirkova (1975)
Table 11 (contd).
Species LD50 (mg/kg)a Reference
2176 ± 220 Lehotzky et al. (1979)
Rat (Sprague-Dawley) 840 (419-1261) Blaszcak (1988)
(in corn oil)
1700 (1265-2135) Blaszcak (1988)
(in 1% methylcellulose)
Rat (Fischer-344) 550 (252-848) in corn oil Blaszcak (1988)
1359 (1009-1691) Blaszcak (1988)
(in 1% methylcellulose)
Rat (both sexes) 4000 Heenehan et al. (1979)
Rat (both sexes) 3269 Branch et al. (1982a)
Mouse 306 (275-337) Panshina (1973, 1977)
290 (240-350) Strateva (1974b)
a The concentration is based on the percentage active ingredient.
Figures in parentheses indicate the range of values.
Using groups of 16 Wistar rats, Baynova et al. (1977) studied the
acute dermal toxicity of a single application of propachlor 65 WP
(10-20% in aqueous suspension) with doses of 1500-4000 mg/kg body
weight (active ingredient). There was no mortality, and no signs of
intoxication were observed. No haematological or biochemical tests
were performed.
Propachlor and Satecid 65 WP caused severe dermatitis, ulceration
and necrosis in the skin of rabbit and ears of mice. None of the
compounds exhibited contact sensitization effects on guinea-pigs
(Lehotzky et al., 1979).
7.1.3 Inhalation
In a study by Bechtel (1991), technical propachlor (96.8%) was
dissolved in dimethylsulfoxide (DMSO) to generate an aerosol and
administered to five male and five female Sprague-Dawley rats in a
nose-only chamber at an analytically determined concentration of 1.2
mg/litre for 4 h. Control rats (five of each sex) were exposed to an
atmosphere of aerosolized DMSO for the same duration. Particle size
measurements on the propachlor/DMSO aerosol indicated a mass median
aerodynamic diameter of 3.5 µm, with 96% of the particles being less
than 10 µm and 1.8% less than 1 µm in diameter (Bechtel, 1991). No
treatment-related deaths occurred. Clinical signs included laboured
respiration and nasal discharge; all animals appeared normal by
post-exposure day 2. A transient weight loss was noted in both treated
and control animals during the first two days of the study, but normal
body weight was observed thereafter. No abnormalities were apparent
during postmortem examination of the animals.
In another acute inhalation study, three groups of Charles River
CD rats (five rats of each sex per group) were exposed to test
atmospheres of a propachlor formulation (44% active ingredient) for 4
h (Kaempfe, 1991). During the exposure period, animals were housed in
a 250-litre New York University style stainless steel chamber, and
were exposed to analytically confirmed concentrations of 0.18, 0.67
and 1.0 mg propachlor/litre in the breathing zone of the chamber. At
least 82% of the particles were less than 10 µm in diameter. No
animals died at the two lower exposure levels, whereas four out of ten
rats died at 1.0 mg/litre. Clinical observations during the
post-exposure period included ocular opacities, perinasal
encrustation, rapid or shallow respiration, perioral wetness and focal
loss of hair from animals in the highest exposure group. Fourteen days
after exposure, all surviving animals had body weights higher than the
pre-exposure (day 0) values with the exception of females in the
1.0-mg/litre group, which exhibited body weight depression. There were
no abnormal findings in rats that died during the test or were
sacrificed at 14 days. Based on the mortality observed, the LC50 was
slightly greater than 1.0 mg/litre.
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dogs
To assess potential subchronic toxicity, propachlor (96.1% pure)
was administered via the diet to five groups of two male and two
female beagle dogs for 4 weeks (Daly & Knezevich, 1984). Dietary
concentrations of propachlor were 0, 100, 500, 1000 and 1500 mg/kg
(equivalent to 0, 2.5, 12.5, 25, and 37.5 mg/kg body weight per day).
No mortality or clinical signs of toxicity related to treatment
occurred during this study. Food consumption was initially decreased,
but only in the females treated with 1000 and 1500 mg/kg (equal to 25
and 37.5 mg/kg body weight) and the males treated with 1000 mg/kg. The
consumption had returned to normal by the end of the study. Body
weight varied markedly. The decreased body weight and/or weight gain
noted in males fed 12.5 or 25 mg/kg body weight per day, and females
fed 37.5 mg/kg per day could have been treatment related.
Haematological examination showed slightly increased platelet counts
in high-dose males. No treatment-related gross pathological effects
were noted at sacrifice.
Following the 4-week pilot feeding study in beagle dogs, a 90-day
feeding study was undertaken (Naylor & Ruecker, 1986). Propachlor
(96.1% purity) was administered in the diet to groups of six dogs of
each sex per group for 90 days. Nominal dietary concentrations were 0,
100, 500 and 1500 mg/kg. There was no mortality or clinicopathological
or histopathological changes related to the treatment. The dose level
of 1500 mg/kg (45 mg/kg body weight) was a no-observed-adverse-effect
level (NOAEL).
7.2.1.2 Rodents
Propachlor (65% WP) was given orally by gavage to white rats at
daily doses of 21, 53 and 106 mg/kg body weight for 4 months, and its
cumulative effect was studied by Panshina (1973). Later, Strateva
(1974a, 1976) carried out a short-term study (45 days) at dose levels
of 50, 100 and 200 mg/kg body weight given orally by gavage to 104
Wistar rats (divided into four groups (three experimental and one
control) with equal numbers of both sexes), and a long-term oral study
(6 months) at dose levels of 0.05, 0.5, 5 and 20 mg/kg body weight
given to 220 Wistar rats divided into five groups (four experimental
and one control).
Strateva (1976) found a decrease in haemoglobin content and
number of erythrocytes, slight leucocytosis and an increased number of
neutrophils. The threshold dose for rats in long-term studies was 5
mg/kg body weight.
Panshina (1976) carried out a 10-month study on white rats given
propachlor by gavage at doses of 1, 3.5 and 10.6 mg/kg body weight.
Slight leucocytosis was found within 4-7 months. The first two doses
provoked a decreased activity of catalase and peroxidase as well as an
increase in nicotinamide adenine dinucleotide in brain and heart
tissue homogenates. No changes in haemoglobin content or number of
erythrocytes were noted. There were no alterations to the
pathomorphological picture.
Baynova et al. (1978a) compared the effects of continuous and
intermittent oral dosing of propachlor (65% WP) on white rats (equal
numbers of both sexes). The number of animals per group was not given.
The scheme of the experimental design is given in Table 12.
Table 12. Experimental design of the study by Baynova et al. (1978a)
Group Duration of Daily dose Dose (fraction
study (mg/kg) of the LD50)
Control 4 months - -
I (dosing every week) 4 months 70 1/20
II (dosing every second week) 4 months 140 1/10
III (dosing every second week) 8 months 70 1/20
Control 8 months - -
Continuous administration of propachlor led to more marked
changes in the main parenchymous organs than intermittent
administration. These changes were characterized by decreased activity
of oxido-reductive tissue enzymes. The hexabarbital sleeping time,
which characterized the detoxification function of the liver, showed
a statistically significant reduction. Propachlor administration at
120 mg/kg body weight for 6 consecutive days to male and female rats
increased the levels of both cytochrome and microsomal protein content
(Nenov & Baynova, 1978) as a result of the induction of mixed-function
oxidase in the liver (Baynova et al., 1978a).
A special study on the morphological changes in kidneys was
carried out. Propachlor (65% WP) was administered by gavage to male
white rats (10 animals per group and 1 control) at doses equivalent to
6, 12 and 60 mg/kg body weight for 3 months (Maleva & Zlateva, 1982).
Dose-dependent morphological changes were found in the proximal
convoluted renal tubules. The tubules were deformed and the epithelial
cells were vacuolized and dystrophic. A decreased cellular content of
ribonucleoproteins was also observed and pycnosis and caryolysis of
nuclei were seen. In the lumen, desquamated epithelia and hyalin
cylinders were found. The intestine was slightly swollen in certain
areas.
The subchronic toxicity of propachlor (96.1% purity) has also
been evaluated in Sprague-Dawley CD rats (30 of each sex per group)
fed diets containing 0, 300, 1500 or 7500 mg/kg for 90 days (Reyna &
Ribelin, 1984a). No animals died. A statistically significant
reduction in body weight was observed in animals fed 1500 mg/kg (8%
for males and females) and 7500 mg/kg (59% for males and 36% for
females). Food consumption was significantly depressed for animals at
the highest dose level for the first month of the study, but recovered
during the remainder of the study. After 6 weeks, reduced haemoglobin,
haematocrit, mean corpuscular haemoglobin and haematocrit, and an
increase in reticulocytes were observed in females at all dose levels
and in high-dose males. The anaemia was less evident in high-dose
males and was only present in high-dose females at study termination.
Significantly reduced lymphocyte counts were observed at week 6 for
high-dose males and mid- and high-dose females, and at study
termination for all groups. Levels of serum enzymes (SGPT, SAP, GGT),
cholesterol and total bilirubin were significantly increased for
high-dose animals at weeks 6 and 13 and there were significant
reductions in total protein, glucose, creatinine and albumin. No
histological changes were observed in the liver. Organ weights (with
the exception of female livers) were reduced, relative to controls,
for high-dose animals, and spleens were extremely small and
treatment-related in size in about 65% of the animals. No histological
changes were observed in the tissues examined, which included the
spleen.
7.2.1.3 Mice
The subchronic toxicity of propachlor (96.1% pure) was evaluated
in four groups of Charles River CD-1(R) mice (30 mice of each sex
per group) (Reyna & Ribelin, 1984b). Dietary levels were 0, 500, 1500
and 5000 mg/kg. No mortality or adverse clinical signs were observed
during the study. A statistically significant reduction (10%) in body
weight was observed in the mid- and high-dose males and females during
the study. Food consumption was also reduced during the first month
for mid- and high-dose animals. A dose-related statistically
significant reduction in the number of white blood cells was observed
in both sexes at all dose levels, except low-dose females, at the
7-week sampling period. At study termination, this reduction was
evident in mid- and high-dose animals but was statistically
significant only for high-dose males. Liver weights were increased for
males at all dietary levels and for mid- and high-dose females. There
was an accompanying statistically significant increase in the
incidence of centrilobular hepatocellular hypertrophy for mid- and
high-dose males, based on histological examination. No other
microscopic changes that could be considered treatment related were
evident in tissues. For males, the kidney to body weight ratio was
decreased at the high-dose level.
7.2.2 Dermal
A 21- and 90-day short- and longer-term dermal toxicity study on
Wistar rats (12 animals in each tested group plus 1 control) using
propachlor (65% wettable powder) administered 5 days per week was
carried out by Baynova et al. (1977). The doses applied were 50, 200
and 500 mg/kg in aqueous suspensions in the 21-day experiment, and 10,
25 and 50 mg/kg in the 90-day study. The dermal application was
performed uncovered (Draize, modification of Noakes). By the end of
day 21, anaemia was found at dose levels of 200 and 500 mg/kg, but
there was no methaemoglobinaemia. The same doses induced a
statistically significant decrease in the activity of certain enzymes
(SGOT, SGPT, OCT and AP). Dermal application of propachlor at dose
levels of 25 and 50 mg/kg to the skin of white rats (two groups of 12
animals) for 90 days did not provoke any changes in concentrations of
red blood cells and haemoglobin, but there were decreases in SGOT,
SGPT, LAP, AP and catalase. Decreased sulfur-containing enzymes were
found in tissue homogenates (liver and kidney). Decreases in the
levels of LDH, SDH, AcP and glucose-6-phosphate dehydrogenase were
determined histochemically. There was no evidence of clinical signs of
intoxication after repeated dermal application of the herbicide, but
the occurrence of the above-mentioned enzymatic changes demonstrated
the penetration of this herbicide through the dermal barrier. The
hexabarbital sleeping time was statistically significantly shortened
at the mid- and high-dose levels, thereby confirming the induction of
mixed-function oxidases reported by Nenov & Baynova (1978).
A threshold dermal dose of 25 mg/kg (1% aqueous suspension of
propachlor 65 WP) and a no-observed-effect level (dermal) of 10 mg/kg
(0.5% aqueous suspension) were determined in 90-day experiments by
Baynova et al. (1977).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Single application to Wistar rats (three rats of each sex per
group) of propachlor 65 WP at doses of 1500, 2000, 3000 and 4000 mg/kg
in 10 or 20% aqueous suspension, under the open modified method of
Noakes, did not cause any local irritative effects (Baynova et al.,
1977).
Panshina (1973) reported a strong irritative effect of propachlor
after single and repeated application. Single applications to rabbits
of 16% propachlor 65 WP in aqueous suspension at doses of 500, 700,
and 1000 mg/kg led to hyperaemia and even ulceration in the skin of
some animals. Ten applications of a 32% aqueous suspension of
propachlor at a level of 200 mg/kg had the same effect on the skin.
Seventeen applications of a 6.5% aqueous suspension at 100 mg/kg did
not result in mortality, and only slight hyperaemia was seen in the
treated skin area of the rabbits.
Primary irritation and contact sensitization effects of Satecid
65 WP containing 65% propachlor were investigated in rabbits, mice and
rats, and these were compared with the effects of technical grade
propachlor, propachlor with analytical purity, and the vehicle alone
(Lehotzky et al., 1979). Propachlor had strong to very strong
irritating effects on intact and scarified skin as well as on the eye
mucosa of rabbits and mice. The symptoms included erythema, oedema and
penetrating ulcer. Technical grade propachlor had the most potent
irritating effect.
Heise et al. (1983) conducted parallel experiments with two
propachlor preparations, one produced in the USA and the other in
Hungary, using an aqueous suspension applied under occlusion to the
skin of rabbits for 24 h. The irritant dose (ID50) for the USA
preparation was 2% and for the Hungarian preparation 0.6%.
Several dermal irritation studies have been carried out with
technical propachlor and its formulations, each of which involved the
use of six New Zealand white rabbits (2.5 to 3.5 kg). All application
sites were scored for erythema, eschar and oedema formation. For
technical propachlor (94.5% pure) a slight degree of dermal irritation
was observed (2.5/8.0 score) (Braun et al., 1979a); for a 20%
formulation of propachlor (again 94.5% pure) there was a slight degree
of dermal irritation (1.8/8.0 score) (Braun et al., 1979b); for a 65%
formulation a moderate degree of dermal irritation (3.4/8.0 score)
(Braun et al., 1979c); and for a 42% formulation corrosive effects
were observed (Branch et al., 1982b).
7.3.2 Skin sensitization
A modification of the maximalization test of Magnusson & Kligman
(1969) for identification of allergens was used by Heise et al.
(1983). Guinea-pigs (12 female and 12 male) were treated by
intramuscular injection of 0.2% propachlor aqueous suspension (using
preparations from both the USA and Hungary together with Freund
adjuvant), by subcutaneous injection of 0.05% aqueous suspension and
by epicutaneous application of a 1% water suspension of both
preparations for 6 weeks, both on healthy and scarified skin. A
challenge single application was given two weeks after the last
application using 0.2 ml of an 1% aqueous suspension of each
formulation. There was no significant difference between the reactions
to the two preparations. A challenge dose did not induce a reaction.
The dermal sensitization potential of propachlor was evaluated in
guinea-pigs using the Buehler procedure (Auletta, 1983). For the
induction phase, 0.2 ml of undiluted propachlor (95.7% purity) was
applied dermally using the closed patch technique to the shaved backs
of five male and five female Hartley albino guineapigs. The
applications were made for 6 h/day, 3 days/week, for 3 weeks. Two
weeks after the final dose, additional 0.2 ml doses of 25% propachlor
in ethanol (challenge dose) were applied to previously untreated areas
on those guinea-pigs that had received the induction doses and to six
others (three males and three females) which served as irritation
controls. Two positive control groups, treated with 1-chloro-2,4-
dinitrobenzene (DNCB) as positive control, were used. The negative
control group (five males and five females) was treated with saline
for the induction doses and challenged with acetone. All animals had
slight to moderate dermal irritation following the third induction
dose of propachlor, and some of them exhibited severe irritation,
including oedema, necrosis and exfoliation. Following application of
the challenge dose, one out of ten animals had an irritation score of
1 (slight), eight animals had a score of 2 (moderate) and one animal
had a score of 3 (severe) at 24 h. Nine of these animals exhibited
oedema. At 78 h, two animals had very low scores, six animals had
scores of 1, and two scores of 2. Six of these animals exhibited
oedema. Similar results were obtained when 20 and 40% propachlor
formulations were tested for sensitization in guinea-pigs (Auletta et
al., 1984a,b).
7.3.3 Eye irritation
Molnar & Paksy (1978) studied the effect of Satecid 65 WP on the
eye mucosa of CFY male albino rats using the Evans blue diffusion
technique. The aqueous suspension caused strong conjunctivitis at the
minimum concentration of 0.01%.
In a study by Auletta (1984), technical propachlor (96.1% pure)
was ground to a fine powder and introduced in the conjunctival sac of
the right eye of six (two males and four females) albino New Zealand
White rabbits. The left eye served as the control. The treated eyes
were rinsed 24 h later. Five of the animals exhibited severe
conjunctival irritation (redness, necrosis, chemosis and discharge),
five exhibited opacity and/or ulceration, and five had iridial damage.
Two animals exhibited neovascular-ization of the cornea and one
bulging of the cornea, indicative of increased intraocular pressure.
After 21 days of observation one animal continued to exhibit
conjunctival redness and one corneal opacity and bulging of the
cornea. The eyes of the remaining animals had minimal conjunctival
irritation.
In an eye irritation test on six New Zealand white rabbits using
technical propachlor (94.5%), 0.1 cm3 was instilled into one eye of
each rabbit and ocular reactions were observed on days 1, 2, 3, 4, 7,
10 and 14. According to Draize scores, all treated eyes were assigned
positive scores for corneal opacity and ulceration, conjunctival
redness, chemosis and necrosis. Two eyes were assigned positive scores
for iritis. Three eyes exhibited pannus, and corneal bulge was
observed in two eyes. Four eyes were clear of signs of irritation by
day 14 and two eyes showed signs of irritation at the termination of
the study (Braun, 1979).
US EPA (1984b) reviewed the existing data and concluded that
propachlor is corrosive to the rabbit eye, corneal opacity being
irreversible after 7 days.
7.4 Reproduction, embryotoxicity and teratogenicity
7.4.1 Reproduction
7.4.1.1 Biochemical and histopathological studies on gonads
The effect of propachlor 65 WP on protein content, activity of
5-nucleotidase and ATP in testis homogenate was evaluated by Maleva &
Stereva (1977), Stereva & Maleva (1977) and Zlateva & Maleva (1978,
1979). Each test group consisted of 20 male Wistar rats, administered
orally (by gavage) 12 or 60 mg propachlor/kg body weight per day for
6 months. A control group consisted of the same number of animals. At
the end of the experiment, there were dose-dependent statistically
significant changes in the biochemical indices studied (a decrease in
protein content and an increase in 5-nucleotidase and ATPase activity)
and these were accompanied by histomorphological changes, i.e.
disorganization of the seminiferous epithelium. After prolonged dosing
some dystrophic and degenerative changes in the gonad tissue and the
appearance of multinuclear giant cells with central chromatolysis were
observed. Adverse effects on the mitotic division of spermatogonia and
blockage of meiosis in the earlier phases were found (Zlateva &
Maleva, 1978, 1979).
In subchronic toxicity studies, there was no microscopic evidence
of testicular pathology in dogs administered the equivalent of 45 mg
propachlor/kg diet per day (Naylor & Ruecker, 1986), in rats fed the
equivalent dose of 250-310 mg/kg diet per day in the food (Reyna &
Ribelin, 1984a), or in mice fed the equivalent of 400 to 600 mg/kg
diet per day (Reyna & Ribelin, 1984b).
7.4.1.2 Reproduction studies
A two-generation rat reproduction study was undertaken to
evaluate the effects of propachlor (96.3% pure) (Rao, 1981). Four
groups of 30 male and 30 female 6-week-old Fischer-344 rats (F0
generation) were exposed to diets adjusted weekly to provide dose
levels of 0, 0.3, 3 and 30 mg propachlor/kg body weight per day for
100 days and were then allowed to mate to produce the F1 generation.
The F1 weanlings were similarly dosed for 120 days prior to
breeding. The F1 adults were mated twice to produce F2a and F2b
litters. At weaning of F1, F2a and F2b litters, 10 pups of each
sex at each dose level were sacrificed and selected organs were
examined histologically. No signs of toxicity in any animals were
found during the study. A significant decrease in food consumption and
body weight of adult F1 males dosed with 30 mg/kg per day was found.
No adverse effects in any of the treated groups over two generations
were noted with respect to gestation length, number of live pups
delivered, neonatal survival, litter weight and sex ratio. Decreased
pregnancy rates (fertility index) were observed in mid-dose (60%) and
high-dose (63%) F1 females following the first mating (F2a
litter), in comparison with 83% in the control rats. Test animals
continued to be treated with propachlor and were remated to produce a
second (F2b) litter. Pregnancy rates for the F2b litter were 83%
for controls and 83, 83 and 80% for low-, mid- and high-dose groups,
respectively.
No treatment-related gross necropsy findings were noted. Slight
increases in absolute and/or relative liver weights were noted in
high-dose F0 adults, mid- and high-dose F1 adults and high-dose
F2a weanlings. Hypertrophy of the centrolobular hepatocytes was
noted during microscopic examination of livers from the high-dose F0
and F1 adult females. There was no evidence of compound-related
microscopic changes in the testes and ovaries of test animals for
either generation.
7.4.2 Embryotoxicity and teratogenicity
7.4.2.1 Rats
a) Single dose treatment
Mirkova (1975) reported embryotoxic and teratogenic effects of
propachlor 65 WP in Wistar rats following single-dose treatment (675
mg/kg body weight) on each of the first 16 days of gestation.
Significantly increased lethality and frequency of haematomas,
predominantly in the head of the fetuses, and reduced cranio-caudal
size were observed when the treatment was performed from days 1 to 4
and on days 8, 10 and 12 of gestation (8 to 12 animals per test group
and 57 controls). The effect was more marked following treatment on
the first day of pregnancy. A weak teratogenic effect was observed
with a single dose, which included the induction of external
malformations (day 11, brachygnatia), abnormality of internal organs,
i.e. hydrocephalus (days 9, 11, 12 and 13 of gestation), defects in
skeletal ossification, i.e. non-ossification of sternum (day 9), and
delayed ossification of parietal bones (days 11, 12 and 13 of
gestation).
b) Repeated dose treatment
Wistar rats were treated daily with 67.5, 135 or 270 mg
propachlor/kg body weight on days 1-7 or 8-16 of gestation (six test
groups of 10 animals each). In a second set of experiments, a daily
treatment of 33.7, 67.5, 135 and 270 mg/kg body weight was given
throughout the gestation period (1-21 days) (Mirkova, 1975).
Propachlor induced embryotoxicity at all dose levels of repeated
treatment during the pre-implantation stages of embryogenesis (days
1-7 of gestation). This was expressed as raised lethality and
haemorrhages, predominantly in the head of the fetus, and reduced
weight and cranio-caudal size. The effects were significantly reduced
when administration was performed during the period of organogenesis
(8-16 days). Propachlor showed low teratogenic activity, producing
external malformations of the tail (short, curved tail), skeletal
abnormalities (non-ossified parietal and occipital bones) and
hydrocephalus, at all three dose levels during the pre-implantation
stage (days 1-7 of gestation). A slight degree of internal
hydrocephalus was observed with doses of 135 and 270 mg/kg on days
8-16 of gestation.
Using a similar treatment regimen, Ivanova-Chemishanska et al.
(1975) reported similar changes. No embryotoxic or teratogenic effect
was seen at a dose level of 10 mg/kg body weight given daily to rats
throughout the pregnancy (Medved, 1977; Panshina, 1985).
Teratogenic effects of technical propachlor were assessed in
pregnant Charles River COBS CD rats using a control and three
treatment groups consisting each of 25 females (Schardein & Wahlberg,
1982). Propachlor (96.5% pure) levels of 20, 60 and 200 mg/kg body
weight were administered orally by gavage as a single daily dose on
days 6-19 of gestation in a constant volume of 10 ml/kg corn oil. The
control group received the same dose of corn oil. Survival in the
control, low-dose and mid-dose groups was 100%. One female in the
high-dose group died on gestation day 18 due to an intubation error.
There were no differences in mean maternal body weight gain. No
changes were evident at gross necropsy that could be considered
treatment related. There were no differences between treated and
control groups with respect to the mean number of viable fetuses,
post-implantations loss, total implantations, corpora lutea, fetal
body weights, sex ratio distribution, and in the number of litters or
fetuses with external, visceral or skeletal malformations and/or
developmental and genetic variations.
7.4.2.2 Mice
When Balb/c mice (8-12 in each test group and 18 control pregnant
females) were treated with a single dose of 675 mg propachlor/kg body
weight on each of days 8-13 of gestation, and at dose levels of 33.7,
67.5, 135 and 270 mg/kg body weight per day from days 1 to 21, a
significant increase in post-implantational lethality and reduced
fetal weight and cranio-caudal size were observed at all dose
regimens. With both single and repeated doses, there was a
statistically significant increase in the frequency of internal
hydrocephalus, but only at a dose level of 675 mg/kg in a single
application on days 9 and 11 of gestation and on daily application at
270 mg/kg from days 1 to 18 of pregnancy (Mirkova, 1975).
7.4.2.3 Rabbits
Pregnant New Zealand White rabbits randomly assigned to a control
and three treatment groups (each consisting of 16 rabbits) were used
to determine the teratogenic potential of propachlor (95.6% pure)
(Schardein, 1982). Dose levels of 5, 15 and 50 mg/kg were administered
orally by gavage as a single daily dose on days 7-19 of gestation at
a constant volume (0.5 ml/kg dissolved in corn oil). The control group
received only 0.5 ml/kg corn oil. On gestation day 29, the number and
location of viable and non-viable fetuses, early and late resorptions,
number of total implantations and the number of corpora lutea were
recorded. All fetuses were examined for external, soft tissue and
skeletal malformations and for genetic or developmental variations.
Mortality (between 3 and 26 days of gestation) was recorded in
the control as well as the treated groups, i.e. two in the control
group, three at 5 mg/kg, three at 15 mg/kg and two at 50 mg/kg. Two
rabbits from the low-dose group and one from the mid-dose group
aborted on gestation days 22 to 25. Ante-mortem observations gave no
indication of a treatment-related effect at any dosage level.
Results of the reproductive parameters revealed slight to
moderate increases in the number of post-implantation losses and the
number of early resorptions in the mid- and high-dose groups when
compared with controls (Table 13). Single animals with resorptions
only were also found at the two highest dose levels. This resulted in
a subsequent decrease in the average number of viable fetuses in the
mid- and high-dose groups when compared with controls. Although the
number of post-implantation losses fell within the range of historical
control frequencies, all the above parameters were considered to imply
a mild embryotoxic response. No effects were observed in the number of
late resorptions, total implantations, number of corpora lutea, fetal
sex distribution or the mean fetal body weights. Examination of the
fetuses revealed no external, skeletal or soft tissue malformations,
or genetic and developmental variations that could be considered to be
related to treatment. These abnormalities observed were low in
frequency and within the range of historical control values. A
statistically significant increase in the total number of litters with
malformations was observed in the mid-dose group when compared to the
control group. This trend, however, was not found in the high-dose
group and the malformations in the group given 15 mg/kg per day were
considered to be incidental to treatment.
Table 13. Mean number of resorptions (late and early),
post-implantation losses and total implantations in
New Zealand rabbits (Schardein, 1982)
Dose Fetuses Resorptions Post-implantation Total
(mg/kg) viable non-viable late early losses implantation
0 8.0 0.0 0.3 0.3 0.6 8.6
5 7.9 0.0 0.0 0.4 0.4 8.3
15 5.2a 0.1 0.0 1.4 1.4 6.6
50 5.9 0.0 0.2 0.9 1.1 7.0
a P < 0.05
7.5 Mutagenicity and related end-points
Appraisal
Many in vitro and in vivo studies on the genotoxicity of
propachlor have been reported. It has been found to be clastogenic
in in vivo lower test systems and plant assays. It is cytotoxic
and also shows a weakly positive clastogenic response in in vitro
mammalian tests, inducing chromosomal aberrations in Chinese hamster
ovary cells. Variable results do not allow any definite conclusions
to be drawn. However, on the basis of negative results in many in
vivo assays in mammalian test systems, the available experimental
data provide insufficient evidence of a mutagenic potential. Data
on mutagenicity testing are summarized in Table 14.
7.5.1 Bacterial test systems
Salmonella mutation assays using standard tester strains
(TA1535; TA1538; TA98 and TA100), both with and without metabolic
activation with rat liver S9, were negative for formulations and
technical grades of propachlor (Plewa et al., 1984; Flowers, 1984).
No mutagenicity was observed with extracts of plants treated with
propachlor.
Negatives results for propachlor in spot tests using Salmonella
typhimurium test strains were also reported by Njagi & Gopalan
(1980) and Eisenbeis et al. (1981).
Table 14. Summary of mutagenicity testing
Test materiala Test system Resultc Reference
-S9 +S9 1S
Gene mutation in Prokaryotes
Propachlor U Ames spot test - Njagi & Gopalan (1980)
Propachlor U Ames spot test - - Eisenbeis et al. (1981)
Propachlor T Ames plate test - - Flowers (1984)
Propachlor T Ames plate test - - -
Propachlor + Cyanazineb Ames plate test + Plewa et al. (1984)
Gene mutation in plants
Propachlor C maize Wx locus - Plewa et al. (1984)
Gene mutation in mammals ( in vitro)
Propachlor T CHO/HGPRT - - Flowers (1985)
Chromosome effects in plants
Propachlor U Vicia faba cytogenetics + Njagi & Gopalan (1981)
Propachlor U maize cytogenetics + Lapina et al. (1984)
Propachlor U maize chlorophyll mutations + Lapina et al. (1984)
Chromosome effects in mammals ( in vitro)
Propachlor T CHO cytogenetics - + Li & Meyers (1987)
Chromosome effects in mammals ( in vivo)
Propachlor U in vivo mouse bone marrow cyt. + Pilinskaya et al. (1980)
Propachlor T in vivo rat bone marrow cyt. - Ernst & Blazak (1985)
Table 14 (contd).
Test materiala Test system Resultc Reference
-S9 +S9 1S
DNA damage/recombination in yeast
Propachlor T gene conversion - + Gentile et al. (1977)
Propachlor T gene conversion - - + Plewa et al. (1984)
Propachlor C gene conversion - - + Plewa et al. (1984)
Propachlor + Cyanazine gene conversion - Plewa et al. (1984)
DNA damage in mammals ( in vitro)
Propachlor T in vitro hepatocyte UDS - Steinmetz & Mirsalis (1984)
Propachlor T in vivo/in vitro hepatocyte UDS - Steinmetz & Mirsalis (1986)
a T = technical grade; C = commercial grade; U = unspecified grade
b Propachlor and cyanazine were both of commercial grade.
c +S9 and -S9 = with or without rat liver S9 activation mixture;
1S = with maize 1S fraction
7.5.2 Yeast assays
Propachlor was not recombinogenic when tested at 1000 mg/litre in
Saccharomyces cerevisae strain D4 (ade 2-1, ade 2-2; trp 5-12, trp
5-27) either with or without a liver microsomal activation system
(Gentille et al., 1977).
Extracts of plants treated with propachlor cause a weak
recombinogenic activity (Gentille et al., 1977). Maize extracts
treated with 25 ppm propachlor increased the rate of mitotic gene
conversion at the ade locus in S. cerevisae strain D4 approximately
4-fold over the control levels. Extracts of plants treated with a
technical grade of propachlor (1.306 x 10-4 mol/litre) and
formulated grade (1.306 x 10-3 mol/litre) induced a significant
increase in gene conversion at the ade locus and a lower increase at
the trp locus in S. cerevisae D4. Results for both formulated and
technical grades of propachlor were negative after mammalian S9
activation (Plewa et al., 1984).
7.5.3 Plant assays
Njagi & Gopalan (1981) studied the cytogenic and cytological
effects of propachlor at a wide range of concentrations (10, 50, 100,
500, 1000, 5000 and 10 000 ppm) in root-tip meristematic cells of
Vicia faba. At a concentration of 100 ppm, propachlor induced,
within 2 h, chromosomal aberrations, such as anaphase bridge
formation, micronuclei as well as inhibition of mitosis, formation of
highly pycnotic nuclei and premature chromosome condensation. The
mutagenic properties of propachlor were not detectable in the field
experiments using reverse mutations at the wx-C locus in maize as the
genetic end-point. Lapina et al. (1984) also reported that propachlor
induced mitotic chromosomal aberrations in the root-tip meristematic
cells of an inbred F2 strain of maize. At a concentration of 10-3
mol/litre and within 2 h of treatment of maize grains, propachlor
increased the rate of mitotic chromosomal aberrations more than 4-fold
over the control levels. The main types of cytogenetic damage included
single bridges (72%) and fragments (28%). Propachlor did not show
evidence of being significantly cytotoxic.
7.5.4 Cultured mammalian cell CHO/HGPRT assay
Propachlor (96.1%) was tested in a gene mutation assay in
cultured mammalian cells (CHO/HGPRT assay), both in the presence and
absence of mammalian metabolic activation, at doses ranging from 10-60
mg/litre. It was found to be cytotoxic at some of the higher
concentrations tested, but no mutagenicity was observed (Flowers,
1985).
7.5.5 In vitro unscheduled DNA synthesis in primary rat hepatocyte
cultures
Steinmetz & Mirsalis (1986) studied the potential of propachlor
to induce unscheduled DNA synthesis (UDS) in primary rat hepatocyte
cultures. They used ten concentrations of propachlor (95.8% pure)
ranging from 0.1 to 5000 mg/litre and, in another assay, five
concentrations ranging from 0.1 to 50 mg/litre. Results of this study
showed a cytotoxic effect of propachlor at concentrations of 50
mg/litre or more, but there was no evidence of UDS induction.
7.5.6 In vitro test for induction of chromosomal aberrations using
Chinese hamster ovary cells
Propachlor was tested for its potential to induce chromosomal
aberrations in cultured Chinese hamster ovary (CHO) cells treated with
propachlor (97.4% pure) at concentrations of 5, 10 or 15 mg/litre for
5 h both in the presence and absence of exogenous activation (Li &
Meyers, 1987). As indicated both by decrease in mitotic index and
lengthening of average cell generation times, the highest treatment
level was cytotoxic both in the presence and absence of activation. In
the non-activated study, no statistically significant increases in the
percentage of cells with structural aberrations or in average
structural aberrations per cell were observed at any treatment level.
In the study with S9 activation (15 mg propachlor/litre), the average
number of aberrations per cell was statistically elevated at 12 h and
24 h (P < 0.05 test). Chromatid and chromosome gaps were not
included. The percentage of cells with structural aberrations
following treatment at 15 mg/litre was statistically elevated at 12 h
but not at 24 h. No apparent dose-response relationship was observed
at either harvest time. Under these conditions, propachlor was
considered to be negative in the assay without exogenous metabolic
activation and a weak clastogen with activation.
7.5.7 In vivo rat bone marrow cytogenetic assay
Ernst & Blazak (1985) conducted an in vivo rat bone marrow
cytogenetic assay using technical grade propachlor (95.6% pure) at
dose levels of 0, 0.05, 0.2 and 1 mg/kg body weight. The results were
evaluated for mitotic index (based on at least 1000 cells per animal)
and 60 cells per animal were examined for chromosomal aberrations.
Chromatid and isochromatid gaps were recorded for each cell, but these
were not considered chromosomal aberrations. The results showed that
propachlor does not induce chromosomal damage in male or female
Fischer-344 rats under the conditions used in this study.
7.5.8 Acute in vivo mouse bone marrow cytogenicity assay
Pilinskaya et al. (1980) studied the cytogenetic effect of
propachlor on the bone marrow of mice treated orally with 10, 50 and
100 mg/kg body weight. The metaphase frequency of aberration at the
lowest dose applied was 2.67 ± 0.66 compared with 0.7 ± 0.19 in the
control. At this dose level an approximately 4-fold increase in the
frequency of chromosome aberrations was observed. No statistically
significant changes were found at a dose level of 1 mg/kg.
7.5.9 In vivo/in vitro hepatocyte DNA repair assay
Steinmetz & Mirsalis (1986) presented data on the in vivo/in
vitro hepatocyte DNA repair assay. Propachlor (97.7% pure) was
suspended in corn oil and administered by gavage to five groups of
male Fischer-344 rats at dose levels of 25, 250, 300, 400 and 1000
mg/kg. A positive control group received 2-acetyl aminofluorene
(2-AAF) (50 mg/kg) while a negative one was given corn oil. The
results were negative.
7.6 Long-term toxicity and oncogenicity studies
7.6.1 Rat
Propachlor (96% pure) was administered via the diet to four
groups of 60 male and 60 female Charles River CD SDBR rats at
concentrations of 0, 10, 50 and 500 mg/kg (equivalent to 0, 0.5, 2.6
and 27 mg/kg body weight) (Hamada, 1987a). A complete battery of
haematological, clinical chemistry and urinalysis tests was carried
out every 6 months for 2 years. No treatment-related effects were
noted on survival, incidence of clinical and ophthalmoscopic
observations, body weight gain or food consumption. Haematological and
clinical chemistry parameters and urinalysis data of treated animals
were comparable to control values. Thyroid and liver weights were
slightly increased in high-dose males at the 12-month interim
sacrifice but not at the end of the study. An increased incidence of
liver changes (centrilobular hypertrophy, clear cell cytoplasmic
alteration and eosinophilic cytoplasmic alteration) was observed in
high-dose males and females. A slightly higher incidence of thyroid
c-cell tumours (adenomas and carcinomas) noted in high-dose animals
was within the testing laboratories' historical range for such
tumours. All other organ weight changes and gross and microscopic
pathological findings were comparable to controls. An increase in
benign granulosa/theca cell tumours was observed for high-dose females
(4/60) compared to controls (0/60). The historical control data for
this tumour ranged from 0/80 to 4/96. On the basis of these results,
50 mg/kg diet (2.6 mg/kg body weight) can be considered the
no-observed-effect level (NOEL) for rats.
7.6.2 Mouse
When propachlor (96.1% pure) was administered in the diet to male
and female CD1 mice at dose levels of 10, 50 and 500 mg/kg (equivalent
to 1.6, 8.1 and 81.3 mg/kg for males and 2.0, 10.5 and 104.9 mg/kg for
females) for 18 months, no histological changes were noted (Hamada et
al., 1987b). The survival of all treated male groups was similar to
that of controls. Female survival was lower than that of controls in
all treated groups. There was a significant increase in segmented
neutrophilic leucocytes in the 500-mg/kg group at 12 months, but this
change was not evident at study termination. At the end of the study,
ratio of liver (with gall bladder) to body weight was increased in the
50- and 500-mg/kg group females, and the ratio of kidney to body
weight was decreased in males of the highest-dose group. The NOEL was
considered to be 10 mg/kg (1.6 mg/kg per day).
7.6.3 Dog
In a study by Naylor & Ruecker (1986), propachlor was
administered daily via the diet to four groups of six male and six
female beagle dogs for 12 months (0, 25, 250 and 1000 mg/kg diet). No
mortality occurred during the study. Absolute body weights were lower
than those of controls at study termination (approximately 14% for
males and 8% for females in the high-dose group and 5% for males in
the mid-dose group. A slight increase in emesis and/or stool change
was noted in treated dogs. No other changes in clinical and
pathological parameters were observed that could be related to
treatment. Based on the body weight depression at the high dose, a
dietary level of 250 mg/kg (9 mg/kg body weight) was considered a
no-observed-adverse-effect level (NOAEL) in this chronic study.
7.7 Miscellaneous studies
Propachlor has a strong inhibitory effect on the proliferation of
L1210 mouse leukaemia cells in vitro (the ID50 for cell
proliferation is < 3 x 10-7 mol/litre). Propachlor also inhibits
significantly the uptake of leucine, thymidine and uridine. The
inhibitory effect of propachlor is largely reversible, i.e. cells
grown in propachlor and then washed free of the compound return to an
almost normal rate of proliferation (Zilkah et al., 1981).
It has been demonstrated that propachlor treatment also causes
L1210 cells to accumulate in the G1 phase. This effect is dose
dependent; a concentration of 10 µmol/litre causes more than 90% of
cells to accumulate in the G1 phase (Zilkah et al., 1985).
The combined effect of propachlor and vibration has been studied.
Propachlor was given orally to Wistar rats (20 animals in each group)
at doses equivalent to 6, 12 and 60 mg/kg body weight per day for 3
months, and the animals were exposed to low-frequency vibration (15
Hz) during the last 30 days of the study. The results were compared
with those obtained from groups of rats subjected to the effect of 15
Hz vibration daily for 30 days, as well as with those of groups of
rats dosed only with propachlor. It was found that the combined
exposure (with 12 or 60 mg propachlor/kg) caused more severe
haemodynamic alterations and degenerative (even necrotic) changes in
the epithelial cells of the renal tubules, either in the proximal or
distal convoluted tubules (Maleva & Zlateva, 1982).
In a study by Baynova et al. (1978b), groups of 20 white rats
were pretreated with oral doses of 4 ml/kg 40% ethanol (one tenth of
the LD50), 6 days per week, for 4 months. Propachlor was then given
daily at a dose level of 140 mg/kg (one tenth of the LD50), 6 days
per week, every other week for a further 4-month period. The controls
were subjected to 8 months of treatment with ethanol and 4 months of
intermittent (every second week) treatment with propachlor using the
same doses as in the combined experiment. Both experiments had
untreated control groups. The combined treatment led to a reduction of
the hepatotoxic effect. This was probably due to induction of
mixed-function oxidases and to a more rapid biotransformation in the
hepatocytes. It has also been found that propachlor (140 mg/kg for 6
days) reduces the hexabarbital sleeping time by speeding up its
metabolism in the hepatic endoplasmic reticulum as a result of
induction of the mixed-function oxidase system in the liver cell
microsomes (Nenov & Baynova, 1978).
8. EFFECTS ON HUMANS
8.1 Occupational exposure
There have been few reports dealing with the effects of
propachlor on humans.
Von Schubert (1979) reported a case of contact eczema on the
palms, wrists and forearms of a 29-year-old agricultural worker who
had been in contact with propachlor for 8 days. Once this contact
ceased, the skin lesions disappeared.
Iden & Schroeter (1977) patch-tested 17 patients, predomi-nantly
farmers, who had been exposed simultaneously to many types of
herbicides. Seven showed a positive patch test reaction and five
others had an irritant reaction to propachlor. One of these farmers,
who was highly sensitive to propachlor, used to develop generalized
airborne contact dermatitis each spring when his neighbours used this
product. Farkasdy et al. (1976) and Dombay & Farkasdy (1978) examined
dermatologically 79 workers manufacturing Satecid 65 WP (65%
propachlor). Of these, 19% showed contact dermatitis attributed to
propachlor exposure. Patch testing was carried out on 67 workers of
this group, 12 of whom showed monovalent and bivalent hypersensitivity
reaction to propachlor. Photosensitization tests were negative in 28
of the cases tested. Three years later, the same authors examined 108
workers who were continuously exposed to Satecid in the same factory
without finding any sign of sensitization.
Jung et al. (1989) patch-tested 19 allergic cases and one
irritative case with occupational contact eczema, who were exposed to
different types of herbicides. Two of them showed positive testing to
Satecid 65 WP. One of these two was only exposed to the substance for
10 days and the other for 6 months.
8.2 General population exposure
No reports on general population exposure are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Soil
The effects of propachlor with regard to changes in numbers of
four types of soil bacteria (aerobic nitrogen-fixing, aerobic
cellulose-decomposing, ammonifying and nitrifying) was studied by
Helmeczi (1977). The studies were carried out using pseudomycelial
chernozem soil with maize plants. For the purpose of the microbial
examinations, samples from the field were taken three times a year
(after spraying propachlor in spring, summer, and autumn at a rate of
8-10 kg/ha) from the upper 20-cm layer of the soil. Sampling was
carried out under sterile conditions and samples were collected in
sterile vessels. The nitrifying bacteria had the greatest sensitivity
to propachlor, their number decreasing considerably from 2720 to 610
per g soil in 1974 and from 4510 to 1920 per g soil in 1975. The
aerobic cellulose-decomposing bacteria were the least sensitive, their
numbers, with respect to the control bacteria, increasing to a small
extent.
Long-term studies in smolnista and alluvial soils have been
carried out to establish the effects of propachlor on useful soil
microorganisms and the processes of nitrification (Bakalivanov &
Kostov, 1981). These studies were conducted under laboratory
conditions at 20 °C and 60-70% soil moisture for a period of 10 days.
The concentration of propachlor used was 80 mg/kg soil. The results
showed an inhibitory effect on nitrification and weaker effect on soil
microorganisms (bacteria, actinomycetes and microscopic fungi). High
adsorption of propachlor to clay minerals reduced the toxic effect on
microorganisms.
Rankov & Velev (1976) studied the effect of propachlor on the
biological activity of soil microorganisms and showed that inhibition
was greater at a temperature of 2 to 4 °C than at 20 to 22 °C.
9.1.2 Water
The acute toxicity of propachlor to Selenastrum capricornutum
Printz has been assessed in two studies. In the first (Richards &
Kaiser, 1984), the 96-h EC50 for growth in algae was calculated to
be 0.029 mg/litre with 95% confidence intervals of 0.021-0.038
mg/litre. The no-observed-effect concentration (NOEC) was calculated
to be 0.01 mg/litre. In the second study (Zschaler & Jonas, 1990), the
72-h EC50 for growth was 5.3 mg/litre and the NOEC 1.9 mg/litre for
a propachlor formulation containing 65% active ingredient. The
differences in toxicity observed between the two studies may be either
because the 65% formulation is less toxic than technical propachlor or
because the duration of the second study was 24 h shorter.
9.2 Aquatic organisms
9.2.1 Aquatic invertebrates
Thompson & Forbes (1979a) determined a 24-h LC50 of 11 mg/litre
and a 48-h LC50 of 7.8 mg/litre for the water flea Daphnia magna.
The NOEC in this study was < 5.6 mg/litre. A 48-h LC50 of 6.9
mg/litre in the same species was reported by Mayer & Ellersieck
(1986). The same authors reported a 48-h LC50 of 0.79 mg/litre for
the midge larva Chironomus plumosus. Another midge larva Chironomus
riparius was reported to show a 24-h LC50 of 5.2 mg/litre and a
48-h LC50 of 1.8 mg/litre (Buhl & Faerber, 1989).
The effects of propachlor on daphnia reproduction was assessed in
a 21-day life-cycle study (Thun, 1990a). In this study, there was
decreased reproductive performance at concentrations from 0.29 to 2.6
mg/litre; the NOEC for reproduction was considered to be 0.097
mg/litre.
9.2.2 Fish
Thompson & Forbes (1979b) determined 24-h, 48-h and 96-h LC50
values for rainbow trout ( Onchorynchus mykiss) of 0.75, 0.28 and
0.17 mg/litre, respectively. Thun (1990b) conducted a 21-day study on
rainbow trout and found that fish died at concentrations between 0.11
and 0.3 mg/litre. No deaths related to exposure occurred at
concentrations between 0.009 and 0.075 mg/litre. The NOEC was
considered to be 0.019 mg/litre. Mayer & Ellersieck (1986) reported a
96-h LC50 of 0.23 mg/litre for the channel catfish (Channa
punctatus).
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The toxicity of propachlor to earthworms was assessed in a 14-day
study (Thun et al., 1991). Propachlor (97.8% pure) was added to the
soil at five concentrations ranging from 100 to 1000 mg/kg, and 40
worms were added to the soil at each concentration. Worms were removed
from the soil at 7 and 14 days and examined for any adverse effects.
Body weights were measured at the beginning and end of the test. The
LC100 was 560 mg/kg and the LC0 218 mg/kg; the NOEC was 100 mg/kg.
The contact LD50 of propachlor for honey bees was reported to
be 311 µg/bee (Kenaga, 1979).
Under laboratory conditions, Tanke & Franz (1978) studied the
side effects of propachlor on some beneficial insects by measuring the
reduction of the beneficial capacity of three enthomophagous insects.
The egg parasite Trichogramma cacoeciae March reacted very strongly
in laboratory as well as in field conditions to the effects of the
herbicide. Contact toxicity tested using residues of propachlor on
glass plates (residue level not stated) caused a reduction of the
degree of parasitization leading to total mortality of the population
in contaminated cages. In a study on the contact toxicity of the spray
deposit on single leaves as well as on whole plants, the
parasitization of Trichogramma was reduced by 100 and 83%,
respectively. The direct toxic effect should be distinguished from a
repellent effect, which is probably more important in the field. In
addition to this direct contact effect of spray deposits, systemic
application also caused an effect after transport of the herbicide
through the soil and the plant. This was demonstrated by a reduction
of the degree of parasitization compared with untreated controls.
Propachlor does not seem to have any influence on Chysopa carnea
Steph larvae. No effect was visible either in tests of contact
toxicity or contaminated sandy soil, in choice studies on a repellent
action of the residue or after topical application of the preparation.
After oral application through an artificial food chain, no influence
could be demonstrated. Only overdosage increased the mortality rate of
the test larvae. No repellent effect of the herbicide on adults was
demonstrated (Tanke & Franz, 1978).
The syrphid Epistrophe balteata DeG was more sensitive. Both in
contact toxicity studies and in studies for a possible repellent
effect, an influence of propachlor on the larval stages was shown.
Oral intake through an artificial food chain did not have an effect on
the larvae. When the herbicide was sprayed directly on the plant, an
increase in larval mortality was observed. Adults of this syrphid also
showed reactions to herbicides; during egg deposition, females avoided
surfaces previously treated (Tanke & Franz, 1978).
The potential toxicity of a 65% formulation of propachlor to
Aleochara bilineta Gyll was assessed by Pietrzik (1991). The imagos
were dug into moist sand and the test substance was added at
recommended use rates (8 kg in 400 litres/ha water). For the
assessment of parasitical capacity, pupae of Delia antiqua were dug
into the sand. At the termination of the test, the sum of hatched
Aleochara larvae in the pupae was determined. These were compared to
the number of control organisms treated with tap water. The parasitic
capacity of the test beetles was decreased by 11.4% when compared to
controls.
9.3.2 Birds
Palazzolo (1964) reported data for pheasants indicating an acute
oral LD50 of 735 mg/kg body weight. Increased respiration, loss of
reflexes, mydriasis, salivation and intermittent tonic/clonic
convulsions were found among birds receiving dose levels of 900 and
1350 mg/kg. The onset of symptoms occurred approximately 15 min
following dosing and persisted until death 3-5 h later. Kenaga (1979)
reported an LD50 of 512 mg/kg for Mallard duck and Beavers & Fink
(1979) reported an LD50 for Bobwhite quail of 137 mg/kg body weight
for a 65% propachlor formulation.
When propachlor was administered in the diet to quail or Mallard
ducks for five consecutive days, the LC50 for both species of birds
was more than 5620 mg/kg diet (Beavers & Fink, 1983a,b). For the
quail, a dietary level of 5620 mg/kg is approximately equivalent to a
dose of 1400 mg/kg per day. The data presented above indicate that the
quail is more susceptible to exposure to propachlor via stomach tube
than via the diet.
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1 Conclusions
* Under conditions of normal use, the general population is not
likely to be exposed to propachlor.
* Those occupationally exposed to propachlor should take adequate
safety and hygienic precautions in order to protect the skin, eye
and respiratory tract.
* Propachlor is rapidly degraded in the environment under most
conditions. It persists longer in cold dry environments. The
conjugated N-isopropylaniline metabolite persists longer than
the parent compound. Propachlor does not bioconcentrate or
biomagnify.
* Propachlor is highly toxic to some aquatic organisms. Exposure of
aquatic organisms under conditions of normal use is low, the
maximum expected concentrations being several orders of magnitude
lower than the no-observed-effect concentrations. Direct
contamination of water courses will kill aquatic organisms and
should be avoided. Propachlor poses a low hazard to birds,
earthworms and honey-bees.
10.2 Recommendations for protection of human health
Workers should be educated about the hazards of propachlor and
systematically trained to practice safety and personal hygiene and to
use protective equipment.
11. FURTHER RESEARCH
* The results of the existing animal studies on mutagenicity are
inconclusive and more research is needed.
* Studies should be performed in laboratory animals to determine
the potential neurotoxic effects of propachlor.
* Only validated analytical methods for residues of propachlor
should be used.
* Epidemiological studies on occupationally exposed workers are
needed.
* There is a need to develop methods of biological monitoring for
evaluating human exposure to propachlor.
* Research is needed to clarify the exposure of workers in the
production and agricultural use of propachlor. Studies should
also include examination of health effects at the measured
exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO recommended classification of pesticides by hazard,
technical propachlor is classified in Class III as slightly hazardous
in normal use (WHO, 1990). A data sheet on propachlor has been issued
(WHO/FAO, 1989).
Neither the FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
nor the International Agency for Research on Cancer (IARC) has so far
evaluated propachlor.
REFERENCES
Aschbacher PW & Struble CB (1987) Evidence for involvement of
non-biliary excretion into the intestine of the formation of
methyl-sulphonyl containing metabolites of 2-chloro- N-
isopropylacetanilide (propachlor) by swine and rats. Xenobiotica,
13(2): 115-126.
Auletta CS (1983) A propachlor dermal sensitization study in
guinea-pigs (Project No. 4236-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS (1984) Eye irritation study with propachlor in rabbits
(Project No. 5050-84). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Auletta CS & Rinehart WE (1979) Acute dermal toxicity study in rabbits
(Project No. 4892-77). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
Agricultural Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984a) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4946-84). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984b) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4540-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Bakalivanov D & Kostov O (1981) Soil microbiological assessment of
toxicity of alkanoate, amide, and other herbicides. Acta microb Acad
Sci Hung, 28: 141-146.
Baker JL (1980) Agricultural areas as non-point sources of pollution.
In: Overcash MR & Davidson JM ed. Environmental impact of non-point
source pollution. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 275-310.
Baker JL & Laflen JM (1979) Run-off losses of surface applied
herbicides as affected by wheel tracks and incorporation. J Environ
Qual, 8(4): 602-607.
Baker JL, Laflen JM, & Hartwig RO (1982) Effects of corn residue and
herbicide placement on herbicide run-off losses. Trans Am Soc Anal
Eng, 25(2): 340-343.
Bakke JE, Gustafsson JA, & Gustafsson BE (1980) Metabolism of
propachlor by the germ-free rat. Science, 210: 433-435.
Bakke JE & Price CE (1979) Metabolism of 2-chloro-N-
isopropylacetanilide (propachlor) in the rat. J Environ Sci Health,
B14(4): 427-441.
Bakke JE, Davison KL, & Larsen GL (1990) Evidence for the absence of
cysteine S-conjugate N-acetyltransferase activity in the metabolism of
propachlor, naphthalene, and dichlobenil in calves. Xenobiotica, 20:
801-807.
Bakke JE, Rafter J, Larsen GL, Gustafsson JA, & Gustafsson BE (1981a)
Enterohepatic circulation of the mercapturic acid and cysteine
conjugates of propachlor. Drug Metab Dispos, 9(6): 525-528.
Bakke JE, Rafter J, Lindeskog P, Feil V, Gustafsson JA, & Gustafsson
BE (1981b) Metabolism of 2-acetamido-4-(chlormethyl) thioazole in
germ-free and conventional rats. Biochem Pharmacol, 30(13): 1831-1844.
Bakke JE, Larsen GL, Aschbacher PW, Rafter J, & Gustafsson JA (1981c)
Role of gut microflora in metabolism of glutathione conjugates of
xenobiotics. In: Rosen JD, Magee PhS, & Casida JE ed. Sulfur in
pesticide action and metabolism. Washington, DC, American Chemical
Society, pp 165-178 (ACS Symposium Series No. 158).
Balinova AM (1981) Investigations in the residues of some herbicides
in plant production in view of its hygienic evaluation. Sofia,
University of Sofia (Dissertation).
Balinova A & Konstantinov K (1975) Investigations on residues of
several herbicides in vegetables. In: Proceedings of the COMECOM
Symposium on Residues of Herbicides in Plants and Soils, Vrozlav,
1973, pp 111-116.
Barrows ME & Macek KJ (1974) Exposure of fish to 14C-RamrodTM:
Accumulation, distribution and elimination of 14C-residues. Wareham,
Massachusetts, Bionomics, E.G. & G. Inc., 18 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Baynova A, Burkova T, & Michailova A (1977) [Determination of dermal
toxicity of Ramrod.] Gig Zdraveopazvane, 20(3): 234-240 (in Russian).
Baynova A, Burkova T, & Michailova A (1978a) [Comparative study on
intermittent and monotonous effects of the herbicide Ramrod.] Gig
Zdraveopazvane, 21: 568-573 (in Russian).
Baynova A, Krastev L, Dikova M, & Michailova JA (1978b) Effect of
alcohol on acute and chronic intoxication with the herbicide Ramrod.
Acta med Bulg, 2: 36-41.
Beavers JB & Fink R (1979) Acute oral LD50-Bobwhite Quail.
Propachlor report No. WL-79-026. Easton, Maryland, Wildlife
International Ltd (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beavers JB & Fink R (1983a) Eight-day dietary LC50 - Bobwhite Quail
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-90, submitted to WHO by Monsanto
International Services, Brussels).
Beavers JB & Fink (1983b) Eight-day dietary LC50 - Mallard Duck
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-89, submitted to WHO by Monsanto
International Services, Brussels).
Bechtel CL (1991) Acute inhalation study of propachlor Technical
(Project No. ML-91-2). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beestman GB & Deming JM (1974) Dissipation of acetanilide herbicides
from soils. Agron J, 66: 306-311.
Blaszcak DL (1988) A comparative acute oral toxicity study in
Sprague-Dawley and Fischer rats (Project No. 4359-87). East Millstone,
New Jersey, Bio/dynamics Inc., 37 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Bleeke MS, Carr KH, & Elliot RC (1987) Propachlor metabolism in the
laying hen (Project No. MSL-6217). St. Louis, Missouri, Monsanto
Agricultural Company, 134 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982a) Acute oral toxicity of
Ramrod(R) 4L flowable herbicide for rats (Project No. ML-81-182).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982b) Primary skin irritation of
Ramrod(R) 4L flowable herbicide to rabbits (Project No. 810096).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Braun WG (1979) Rabbit eye irritation study (Project No. 4889-77).
East Millstone, New Jersey, Bio/dynamics Inc., 4 pp (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
Braun WG & Rinehart E (1978) Acute dermal toxicity study in rabbits
(Project No. 4888-77). East Millstone, New Jersey, Bio/dynamics Inc.,
4 pp (Unpublished proprietary report, submitted to WHO by Monsanto
International Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979a) Primary dermal irritation
study in rabbits (Project No. 4890-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979b) Primary dermal irritation
study in rabbits (Project No. 4898-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979c) Primary dermal irritation
study in rabbits (Project No. 4894-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Brightwell BB & Rueppel ML (1976) Lasso(R) and Ramrod(R): physical
properties and their relationship to the environment. St. Louis,
Missouri, Monsanto Agricultural Company (Unpublished report No. 443,
submitted to WHO by Monsanto International Services, Brussels).
Brightwell BB, Malik JM, & Wong SC (1981) The environmental fate of
propachlor [2-chloro-N (1-methyl)-N-phenyl-acetamide] in soil and
water. St. Louis, Missouri, Monsanto Research Laboratory, 1300 pp
(Unpublished proprietary report No. MSL-1900, submitted to WHO by
Monsanto International Services, Brussels).
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides and
surfactants to larvae of the midge Chironomus riparius. Arch Environ
Contam Toxicol, 18: 530-536.
Caverly DJ & Denney RC (1978) Determination of substituted ureas and
some related herbicide residues in soils by gas chromatography.
Analyst, 103: 368-374.
Daly I & Knezevich A (1984) Four-week toxicity study in dogs
(Bio/dynamics Inc. Project No. 84-2798). Unpublished proprietary
report of Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels.
Davison KL (1991) Propachlor-s-glutathione metabolism by kidneys and
ureters of calves. J Anim Sci, 69: 1116-1121.
Davison KL, Bakke JE, & Larsen GL (1988) A kidney perfusion method for
metabolism. Studies with chickens using propachlor as a model.
Xenobiotica, 18(3): 323-329.
Davison KL, Bakke JE, & Larsen GL (1990) Metabolism of the glutathione
conjugate of propachlor by in situ perfused kidneys and livers of
rats. Xenobiotica, 20(4): 375-383.
Dombay Z & Farkasdy J (1978) [Problems of safety technique and of
occupational safety as related to propachlor and its formulations.]
In: [Conference on Safety Technology in the Application of
Agricultural Chemicals], Budapest, Technoinform, pp. 49-58 (in
German).
Eisenbeis SJ, Lynch DL, & Hampel AE (1981) The Ames mutagen assay
tested against herbicides and herbicide combinations. Soil Sci,
131(1): 44-46.
Ernst L & Blazak F (1985) An assessment of the mutagenic potential of
propachlor utilizing the acute in vivo rat bone marrow cytogenetics
assay (Project No. SR-84-180). Menlo Park, California, Stanford
Research Institute, 39 pp. (Unpublished proprietary report, submitted
to WHO by Monsanto International Services, Brussels).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
Farkasdy J, Horvath A, & Meszleri E (1976) [Occupational dermatitis
caused by the selective herbicide Satecid 65WP.] Borgyogy venerol Sz,
52: 112-118 (in German).
Feil VJ, Bakke JE, & Larsen GL (1981) A new metabolite of propachlor
isolated from germ-free rat excreta. Biomed Mass Spectrom, 8(1): 1-4.
Fletcher WW & Kirkwood RC (1982) Herbicide and plant growth
regulators. London, Toronto, Sydney, New York, Granada.
Flowers LJ (1984) Salmonella mutagenicity assay of propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 18 pp
(Unpublished proprietary report No. MSL-1694, submitted to WHO by
Monsanto International Services, Brussels).
Flowers LJ (1985) CHO/HGPRT gene mutation assay with propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 9 pp
(Unpublished proprietary report No. ML-84-237, submitted to WHO by
Monsanto International Services, Brussels).
Frank R, Sirons GJ, Paik NJ, & Valk M (1977) Fate of propachlor
applied to onions and organic soils. Can J Plant Sci, 57: 473-477.
Gentille JM, Wagner ED, & Plewa MJ (1977) The detection of weak
recombinogenic activities in the herbicides alachlor and propachlor
using a plant activation bioassay. Mutat Res, 48: 113-116.
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 8: 339-357.
Hamada NN (1987a) Combined chronic toxicity and oncogenicity study in
rats with propachlor (Study No. HL-83-350/241-169). Vienna, Virginia,
Hazleton Laboratories America Inc., 2452 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Hamada NN (1987b) Oncogenicity study with propachlor in mice (Study
No. HL-83-349/241-159). Vienna, Virginia, Hazleton Laboratories
America Inc., 520 pp (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Heenehan BS, Rinehart WE, & Braun WG (1979) Acute oral toxicity study
in rats (Project No. 1895-77) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Heise H, Mattheus A, & Pushkeiler TH (1983) [The irritant and allergic
effect of the herbicidal agent propachlor.] Z Gesamte Hyg, 29(11):
675-677 (in German).
Helmeczi B (1977) The effect of herbicides on soil bacteria belonging
to certain physiological groups. Acta phytopathol Acad Sci Hung,
12(1-2): 41-49.
Humburg NE, Colby SR, Lym RG, Prasad R, Hill ER, Kitchen LM, & McAvoy
WJ (1989) Herbicide handbook, 6th ed. Champaign, Illinois, The Weed
Science Society of America.
Iden DL & Schroeter AL (1977) Allergic contact dermatitis to
herbicides. Arch Dermatol, 113(7): 983.
Ivanova-Chemishanska L, Vergieva T, & Mirkova E (1975) Embryotoxic and
teratogenic effects of some pesticides. Exp Med Morfol, 14(1): 29-33.
Jung HD, Honemann W, Kloth C, Lubbe D, & Pambor M (1989) Contact
eczema caused by pesticides in East Germany. Dermatol Monatschrift,
175: 203-214.
Kaempfe BS (1991) Acute inhalation study of MON 15606 (Project No.
ML-91-48). Newstead, St Louis, Monsanto Company Environmental Health
Laboratory. (Unpublished proprietary report of Monsanto Agricultural
Company, submitted to WHO by Monsanto International Services,
Brussels).
Kenaga EE (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down Earth, 35(2): 25-31.
Kofman IS & Nishko P (1984) Determination of micro-quantities of
herbicides from a group of acetylanilides by thin-layer
chromatography. Physiol Biochem Cult Plant, 16(3): 290-292.
Kolesnikov VA, Abramova KA, & Grip OK (1979) Use of herbicides on
white cabbage. Khim Sel'skom Khoz, 17(10): 27-31.
Kolesnikov VA, Karetskaia NA, & Petuchova VI (1980) Efficacy of
herbicides in garlic crop. Khim Sel'skom Khoz, 18(8): 38-40.
Lamoureux GL & Davidson KL (1975) Mercapturic acid formation in the
metabolism of propachlor, CDAA and Flurodifen in the rat. Pestic
Biochem Physiol, 5: 497-506.
Lamoureux GL & Rusness DG (1989) Propachlor metabolism in soybean
plants, excised soybean tissues, and soil. Pestic Biochem Physiol, 34:
187-204.
Lamoureux GL, Stafford LE, & Tanaka FS (1971) Metabolism of
2-chloror- N-isopropyl-acetanilide (propachlor) in the leaves of
corn, sorghum, sugar cane, and barley. J Agric Food Chem, 19(2):
346-350.
Lapina TV, Grigorenko NV, Morgun V, Logvinenko VF, & Merjinskii JG
(1984) Study of the mutagenic effect of the herbicides Treflan,
Ramrod, and Banvel on corn. Tsitol i Genet, 18(2): 119-122.
Larsen GL & Bakke JE (1979) Studies on the origin of the
methylsulfonyl-containing metabolites from propachlor. J Environ Sci
Health, B14: 427.
Larsen GL & Bakke JE (1981) Enterohepatic circulation in formation of
propachlor (2-chloro-N-N-isopropylacetanilide) metabolites in the rat.
Xenobiotica, 11(7): 473-480.
Larsen GL & Bakke JE (1983) Metabolism of mercapturic acid-pathway
metabolites of 2-chloro- N-isopropylacetanilide (propachlor) by
gastrointestinal bacteria. Xenobiotica, 13(2): 115-126.
Larsen GL, Larsen JD, & Gustafson JA (1983) Cysteine conjugate
beta-lyase in the gastrointestinal bacterium Fusobacterium
necrophorum. Xenobiotica, 13(11): 689-700.
Lee JK, Minard RD, & Bollag JM (1982) Microbial metabolism of
propachlor in soil suspension. J Korean Agric Chem Saf, 25(1): 44-53.
Lehotzky K, Szeberenyl JMS, & Tatrai E (1979) On the local and
systematic toxic effects of the herbicide propachlor (Satecid 65WP) in
experimental animals. Egeszegtudomany, 23: 89-96.
Li AP & Meyers CA (1987) In vitro cytogenetics study of propachlor
(Study No. MSL-6939), 24 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Lottman CM & Cowell JE (1986) Propachlor residues in processed sorghum
grain applications following pre-emergent applications of Ramrod 4L
herbicide (Project No. MSL 6091) (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Lottman CM & Cowell JE (1987) Propachlor residues in processed corn
grain fractions following pre-emergent applications at Ramrod 4 h
herbicide (Study No. MSL-6260) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Magnusson B & Kligman AM (1969) The identification of allergens by
animal assay. The guinea-pig maximalization test. J Invest Dermatol,
52: 268-276.
Maleva E & Stereva M (1977) [Biochemical investigation on changes of
gonads of male white rats under combined action of low frequency
vibrations and intoxication with Ramrod. I. Changes in the content of
protein.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part 2], pp. 306-313 (in Bulgarian).
Maleva E & Zlateva M (1982) Histomorphological changes in kidneys of
experimental animals under the combined effect of low-frequency
vibration and intoxication with Ramrod herbicide. Hig Zdraveopazvane,
25(3): 244-249.
Malik JM (1982) Bioconcentration of propachlor in channel catfish
under static conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, 144 pp (Unpublished proprietary report No. 24242,
submitted to WHO by Monsanto International Services, Brussels).
Malik JM (1986) Metabolism of propachlor in rats (Project No. 7815).
St. Louis, Missouri, Monsanto Agricultural Company, Research
Department (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Markus JR & Puma B (1973) Pesticide analytical manual. Volume II:
Methods for individual pesticide residues. Springfield, Virginia, US
Department of Commerce, National Technical Information Service, pp
1-7.
Martin CD, Baker JL, Erbach DC, & Johnson HP (1978) Wash-off of
herbicides applied to corn residue. Trans Am Soc Anal Eng, 21(6):
1164-1168.
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Medved LI (1977) [Manual of pesticides.] Kiev, K. Urojai (in Russian).
Melnikov NN, Novojilov KV, Belen SP, & Pilova TN (1985) [Manual of
pesticides.] Moscow, M. Khimia (in Russian).
Menges RM & Tamez S (1973) Effect of soil incorporation on
selectivity, movement, and persistence of herbicides in onion
plantings. J Am Soc Hortic Sci, 98(4): 390-393.
Mirkova E (1975) Embryotoxic and teratogenic action of pesticides
Basfungin and Ramrod. Sofia, University of Sofia (Dissertation).
Molnar J & Paksy KA (1978) Acute inflammation of rat membranes evoked
by eye-instillation and inhalation of pesticide formulations
containing propachlor and TMTD. Egeszsegtudomany, 22: 393-397.
Nadeau RG & Pantano LK (1986) Metabolism study of synthetic
13C/14C-labelled plant metabolites of propachlor in lactating
goats. Part II: Identification characterization and quantification of
metabolites (Project No. MSL-5909), 119 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Naylor MW & Ruecker RW (1986) One-year dog study with propachlor
(Study No. ML-84-379). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Nenov P & Baynova A (1978) Ramrod (propachlor) effect on the activity
of rat hepatic microsomal enzymosystems. Acta med Bulg, 5(2): 44-52.
Nesterova OA, Popova VV, & Kolesnikova OP (1980) [Accumulation of
semeron, mesoranil, and Ramrod in the soil and cabbage during
long-term use.] Sibirski Vestn S-Kh Nauki, 10(4): 19-21 (in Russian).
Njagi GDE & Gopalan HNB (1980) Mutagenicity testing of some selected
food preservatives, herbicides, and insecticides. II. Ames test,
Bangladesh. J Bot, 9(2): 141-146.
Njagi GDE & Gopalan HNB (1981) Mutagenicity testing of herbicides,
fungicides, and insecticides. I. Chromosome aberrations in Vicia
faba. Cytologia, 46: 169-172.
Novick NJ & Alexander M (1985) Co-metabolism of low concentrations of
propachlor, alachlor, and cycloate in sewage and lake water. Appl
Environ Microbiol, April: 737-743.
Novick NJ, Mukherjee R, & Alexander M (1986) Metabolism of alachlor
and propachlor in suspensions of pretreated soil and in samples from
ground water aquifers. J Agric Food Chem, 34: 721-725.
Palazzolo RJ (1964) Acute oral toxicity study on CP 31393 in Golden
Ring-necked pheasants. Northbrook, Illinois, Industrial Biotest
Laboratories (Unpublished proprietary report No. BLT-64-3, submitted
to WHO by Monsanto International Services, Brussels).
Panshina TN (1973) [Materials about toxicology of new herbicide
Ramrod.] Gig Primen toksikol pestic klin otravlenni, 1973: 301-303 (in
Russian).
Panshina TN (1976) [Hygienic aspects of the use of certain herbicides
in agriculture and toxicology.] Gig i Sanit, 7: 38-41 (in Russian).
Panshina TN (1977) Establishment of maximum permissible concentrations
and approximately safe levels of action exerted by herbicides of
haloid-substituted carboxylic acid anilides in the air of a production
sector. Gig Tr Prof Zabol, 12: 30-33 (in Russian).
Panshina TN (1985) IRPTC scientific reviews of Soviet literature on
toxicity and hazards of chemicals No. 83: Propachlor (Ramrod). Moscow,
Centre of International Projects, GKNT, 19 pp.
Pantano LK & Anderson KS (1987) The metabolic fate of propachlor
[2-chloro-N-(1-methylethyl)-N-Phenylacetamide] in sorghum foliage and
grain (Project No. MSL-5412). St. Louis, Missouri, Monsanto
Agricultural Company (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Pekas JV, Larsen GL, & Feil VJ (1979) Propachlor detoxication in the
small intestine: cysteine conjugation. J Toxicol environ Health, 5:
653-662.
Pietrzik J (1991) [Laboratory determination of side effects of Ramrod
65WP on the short-winged beetle Aleochara bilineata Gyll (Project
No. 112/01-AB).] Niefern-Öchselbronn, Austria, GAB Biotechnologie GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels) (in
German).
Pilinskaya M, Kurinny AI, & Ivova TS (1980) [The primary evaluation of
cytogenetic activity and potential mutagenic hazard of 22 pesticides.]
Cytol Genet, 14(6): 41-46 (in Russian).
Plewa MJ, Wagner ED, Glenda G, & Gentille JM (1984) An evaluation of
the genotoxic properties of herbicides following plant and animal
activation. Mutat Res, 136: 233-245.
Pressley TA & Longbottom JE (1982) The determination of organochlorine
pesticides in industrial and municipal waste water. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental Monitoring and
Support Laboratory, Office of Research and Development.
Rafter JJ, Bakke J, Larsen G, Gustafson B, & Gustafson JA (1983a) Role
of the intestinal microflora in the formation of sulfur-containing
conjugates of xenobiotics. Rev Biochem Toxicol, 5: 387-408.
Rafter JJ, Gustafson JA, Bakke J, Larsen G, Norin KE, & Gustafson BE
(1983b) Studies on the re-establishment of the intestinal microflora
in germ-free rats with special reference to the metabolism of
N-isopropyl-alpha-chloracetanilide (propachlor). Xenobiotica, 13(3):
171-178.
Rankov V & Velev B (1976) Effect of temperature on the interaction
between the metribuzin and propachlor herbicides and soil
microorganisms. Agrokhimiya, 11(5): 100-105.
Rankov V & Velev B (1977) Detoxication of the herbicide propachlor by
certain microscopic fungi. Acta phytopathol Acad Sci Hung, 12(1-2):
67-72.
Rao KS (1981) Two-generation dietary reproduction study with
propachlor in rats (Study No. HET-K-077810(8)). Midland, Michigan, Dow
Chemical Company, Health and Environmental Science, Toxicology
Research Laboratory, 171 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Rejtö M, Saltzman S, & Acher AJ (1984) Photodecomposition of
propachlor. J Agric Food Chem, 32: 226-230.
Reyna MS & Ribelin WE (1984a) Three-month feeding study of propachlor
to albino rats (Study No. 83037). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Reyna MS & Ribelin WE (1984b) Three-month feeding study of propachlor
to albino mice (Study No. 820061). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Richards C & Kaiser FE (1984) Acute toxicity of propachlor (AB 83-367)
to Selenastrum capricornutum Printz. Columbia, Missouri, Analytical
Biochemistry Laboratories, 39 pp (Unpublished proprietary report No.
31348, submitted to WHO by Monsanto International Services, Brussels).
Ritter WP, Johnson HP, & Lovely WG (1973) Diffusion of atrazine,
propachlor, and diazinon in silt loam soil. Weed Sci, 5: 381-384.
Roberts HA, Walker A, & Bond W (1978) Persistence of chlorthaldimethyl
activity in soil. In: Proceedings of the British Crop Protection
Conference: Weeds. Croydon, British Crop Protection Council, pp 87-92.
Schardein JW (1982) Teratology study in rabbits. Mattawan, Michigan,
International Research and Development Corporation, 47 pp (Unpublished
proprietary report No. IR 81-224, submitted to WHO by Monsanto
International Services, Brussels).
Schardein JW & Wahlberg A (1982) Teratology study in rats. Mattawan,
Michigan, International Research and Development Corporation, 81 pp
(Unpublished proprietary report No. IR 81-264, submitted to WHO by
Monsanto International Services, Brussels).
Shirko ST & Belova VL (1982) [Level of Ramrod, semeron, and mesoranil
in the soil and cabbage plants in relation of dose of nitrogen and
potassium.] Khim Sel'skom Khoz, 20(I): 51-53 (in Russian).
Sjovall J, Rafter J, Larsen G, & Egestad B (1983) Lipophilic ion
exchange for group separation of conjugated metabolites of
xenobiotics. J Chromatogr, 276: 150-156.
Spalding RF & Snow DD (1989) Stream levels of agrochemicals during a
spring discharge event. Chemosphere, 19(8/9): 1129-1140.
Steen WC & Collette TW (1989) Microbial degradation of seven amides by
suspended bacterial populations. Appl Environ Microbiol, 55(10):
2545-2549.
Steinmetz L & Mirsalis C (1984) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the primary rat
hepatocyte cultures (Project No. LSC-7538). Menlo Park, California,
Stanford Research Institute, 8 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Steinmetz L & Mirsalis C (1986) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the in vivo- in
vitro rat hepatocyte DNA repair assay (Project No. LSC-2021). Menlo
Park, California, Stanford Research Institute, 37 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Stereva M & Maleva E (1977) [Biochemical studies on changes in gonads
of male white rats under combined action of low frequency vibrations
and intoxication with Ramrod. II. Dynamic in changes of 5-nucleotidase
activity.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part I], pp 133-139 (in Bulgarian).
Strateva A (1974a) [Hygienic-toxicologic study on Ramrod aiming its
hygienic standardization in water.] Sofia, University of Sofia
(Dissertation) (in Bulgarian).
Strateva A (1974b) [Amid preparations with herbicide action.
Experimental data on the acute oral toxicity of propachlor (Ramrod).]
Exp Med Morphol, 13(2): 123-129 (in Russian).
Strateva A (1975) [On some toxicologic properties of Ramrod and their
significance on its hygienic standardization in waters.] Hyg
Zdraveopazvane, 18(4): 401-405 (in Russian).
Strateva A (1976) [The experimental determination of MAC of Ramrod in
water.] Gig i Sanit, 4: 85-87 (in Russian).
Strateva A, Velisarov A, & Georgiev A (1974) [Morphological and
enzymohistochemical investigations on some internal organs of
experimental animals under the effect of Ramrod in acute experiment.]
Hyg Zdraveopazvane, 17(3): 283-286 (in Russian).
Struble CB (1991) In situ intestinal absorption of
2-chloro-N-isopropylacetanilide (propachlor) and non-biliary excretion
of metabolites into the intestinal tract of rats, pigs and chickens.
Xenobiotica, 21: 85-95.
Tanaka FS, Wien RG, & Mansager ER (1981) Survey for surfactant effects
on the photodegradation of herbicides in aqueous media. J Agric Food
Chem, 29: 227-230.
Tanke W & Franz JM (1978) [Side effects of herbicides on economically
useful insects.] Entomophaga, 23(3): 275-280 (in German).
Thompson CM & Forbes AD (1979a) Acute toxicity of propachlor to
Daphnia magna. Columbia, Missouri, ABC-Laboratories Inc., 40 pp
(Unpublished proprietary report No. 24708, submitted to WHO by
Monsanto International Services, Brussels).
Thompson CM & Forbes AD (1979b) Acute toxicity of propachlor to
Rainbow trout Salmo gairdneri. Columbia, Missouri, ABC-Laboratories
Inc., 40 pp (Unpublished proprietary report No. 24019, submitted to
WHO by Monsanto International Services, Brussels).
Thun S (1990a) Propachlor 21-day reproduction test in Daphnia
(Project Number XX-89-126). Walsrode, Germany, IBR Forschung GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Thun S (1990b) Prolonged fish toxicity test in the rainbow trout
( Salmo gairdneri) (Project Number XX-89-163). Walsrode, Germany, IBR
Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Thun S, Lemke G, & Fulst S (1991) Acute toxicity in earthworms
according to OECD 207. (Project Number IB-90-566). Walsrode, Germany,
IBR Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
US EPA (1984a) Guidance for the registration of pesticide products
containing propachlor (019101) (EPA Case number 17). Washington, DC,
US Environmental Protection Agency, Office of Pesticide Programme.
US EPA (1984b) Health and environmental effects profile for
propachlor. Cincinnati, Ohio, US Environmental Protection Agency
(600/X-84/335).
US EPA (1988) Propachlor. Washington, DC, US Environmental Protection
Agency, Health Advisory Office of Drinking-Water.
Vasilev NV (1982) [Residual quantity of Ramrod in soil and fodder
rurabages.] Khim Sel'skom Khoz, 20(6): 51-53 (in Russian).
Villarreal DT, Turco RF, & Konopka A (1991) Propachlor degradation by
a soil bacterial community. Appl Environ Microbiol, 57(8): 2135-2140.
Von Schubert H (1979) [Allergic contact dermatitis caused by
propachlor.] Dermatol Monatsschr, 165: 495-498 (in German).
Walker A & Brown PA (1982) Effects of soil on the activity and
persistence of propachlor. In: Proceedings of the British Crop
Protection Conference: Weeds. Croydon, British Crop Protection
Council, pp 171-177.
Walker A & Brown PA (1985) The relative persistence in soil of five
acetanilide herbicides. Bull Environ Contam Toxicol, 34: 143-149.
Warholic DT, Gutenmann WH, & Lisk DJ (1983) Propachlor herbicide
residue studies in cabbage using modified analytical procedure. Bull
Environ Contam Toxicol, 31: 585-587.
WHO/FAO (1989) Data sheets on pesticides No. 78: Propachlor. Geneva,
World Health Organization, 7 pp (WHO/VBC/DS/87.78).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-91. Geneva, World Health
Organization, 39 pp (WHO/PCS/90.1).
Worthing CR & Hance RJ (1991) The pesticide manual, 9th ed. Croydon,
British Crop Protection Council.
Yu Ching Chien, Booth GM, Hansen DJ, & Larsen JR (1975) Fate of
alachlor and propachlor in a model ecosystem. J Agric Food Chem,
23(5): 877-878.
Zhukova PS & Shirko TS (1979) [Herbicide residues in soil and
vegetables.] Khim Sel'skom Khoz, 17(6): 46-50 (in Russian).
Zilkah S, Osband ME, & Mccaffrey RP (1981) Characterization of the
inhibitory effects of the phytotoxic agent propachlor on L1210 cell
proliferation. Cancer Lett. (Irel), 13(3): 241-248.
Zilkah S, Osband ME, Mccaffrey RP, & Shapiro H (1985) The effect of
the plant cell inhibitor propachlor on the cell cycle of LI210 cells
as evaluated by flow cytometry. Life Sci, 6: 2111-2115.
Zimdahl RL & Clark SK (1982) Degradation of three acetanilide
herbicides in soil. Weed Sci, 30: 545-548.
Zlateva M & Maleva E (1978) [Late changes in the gonads of male white
rats upon combined treatment with low frequency vibrations and Ramrod
intoxication.] Med Fizkult, pp 85-91 (in Bulgarian).
Zlateva M & Maleva E (1979) Disorders in the processes of division and
differentiation of seminiferous epithelium in case of chronic
intoxication with Ramrod. Exp Med Morphol, 18(1): 35-39.
Zlateva M, Maleva E, & Stareva M (1978) Changes of adenosine
triphosphatase activity in the testes of albino rats under combined
influence of low-frequency vibration and intoxication with the
herbicide Ramrod. Rev Toxicol Eur Res, 6(78): 375-378.
Zschaler R & Jonas W (1990) Growth inhibition test with algae (Project
No. NC-90-592). Hamburg, Natec Institut für
Naturwissenschaftlich-technische Dienste GmbH. (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
RESUME ET EVALUATION
1. Identité, modalités d'utilisation, propriétés physiques et
chimiques, méthodes d'analyse
Le propachlor est un herbicide dérivé de l'acétanilide qu'on
utilise depuis 1965 en traitement de pré-levée et en traitement
précoce de post-levée. Il est principalement formulé en poudre
mouillable, liquide (concentré en suspension) et granulés. On
l'utilise en agriculture pour détruire les graminées annuelles et
certaines adventices à feuilles larges dans des cultures comme le
maïs, le sorgho, les potirons, le lin et les fleurs.
Le propachlor est légèrement soluble dans l'eau et facilement
soluble dans la plupart des solvants organiques. Il est peu volatil,
non inflammable et stable au rayonnement ultra-violet. La méthode
d'analyse la plus pratique est la chromatographie en phase gazeuse
avec détection par capture d'électrons après extraction et
purification par des méthodes appropriées.
2. Transport, distribution et transformation dans l'environnement
Le propachlor ne semble pas subir de dégradation photochimique à
la surface du sol. Il se volatilise en présence de vent lorsque la
surface du sol est encore humide.
Il n'est que modérément adsorbé aux particules du sol et aux
matières organiques, d'où un risque de lessivage à travers le sol
jusqu'aux eaux souterraines. Toutefois toutes les études montrent que
ce risque est faible en pratique. Il faut des précipitations très
importantes pour que les résidus se déplacent de 30 cm à l'intérieur
du sol. Selon la plupart des auteurs, la majorité des résidus
demeurent à moins de 4 cm de profondeur. Les caractéristiques du sol
influent beaucoup sur la mobilité du composé. Le lessivage se produit
surtout dans des sols sableux pauvres en matières organiques.
On a étudié en laboratoire et sur le terrain l'entraînement du
propachlor par ruissellement. Une des études a montré que la présence
de matières organiques dans le sol réduisait de 7 à 1 % de la quantité
appliquée, la concentration de l'herbicide dans l'eau de
ruissellement. L'incorporation de propachlor dans le sol réduit
également les pertes par ruissellement (de 3 à 0,8 % selon une étude).
En ce qui concerne la réduction de la concentration de propachlor
dans le sol et dans l'eau, le facteur de loin le plus important est sa
dégradation par les micro-organismes. On a montré que bactéries et
champignons étaient responsables de la dégradation de ce composé. Peu
de bactéries sont apparemment capables d'utiliser le propachlor comme
seule source de carbone. On a également isolé des bactéries qui sont
en mesure d'utiliser des métabolites telluriques du propachlor.
Les principaux métabolites qui se forment dans le sol sont des
acides oxaniliques et sulfoniques solubles dans l'eau. Il peut se
former un grand nombre d'autres métabolites mais ils ne représentent
qu'une faible proportion du total.
Le propachlor disparaît rapidement du sol et on a fait état de
demi-vies allant jusqu'à trois semaines. La plupart des études
indiquent une dégradation pratiquement complète en moins de six mois.
Les conditions écologiques influent sur la vitesse de dégradation qui
est favorisée par une température élevée et une forte humidité du sol.
Les résultats qui indiquent une persistance élevée du propachlor dans
le sol ont été obtenus à basse température et dans des sols secs. La
présence de nutriments en quantité suffisante dans le sol est
également nécessaire à la dégradation du propachlor.
Un des métabolites, la N-isopropylaniline conjuguée est
beaucoup plus persistante que le composé initial. On a retrouvé des
résidus de ce métabolite jusqu'à deux ans après l'épandage de
propachlor à titre expérimental à des doses plus élevées que celles
que l'on utilise normalement en agriculture.
Dans les conditions normales d'utilisation, le propachlor ne
devrait pas être entraîné à travers le sol par lessivage jusqu'aux
nappes souterraines et ne demeure pas dans le sol. Des conditions
exceptionnelles (température très basse ou sécheresse) peuvent
prolonger la persistance du propachlor et de ses métabolites.
Dans les conditions normales, le propachlor ne subit pas de
dégradation photochimique importante dans l'eau. Cependant, en
présence de photosensibilisateurs, cette dégradation photochimique
peut se produire. Le propachlor est stable à l'hydrolyse. Il est peu
probable qu'il se volatilise à partir de l'eau du fait de sa forte
solubilité dans l'eau et de sa faible tension de vapeur.
Comme dans le sol, c'est principalement par dégradation
biologique que le propachlor s'élimine de l'eau. Sa vitesse
d'élimination est donc liée à la population microbienne. Une étude
effectuée sur de l'eau contenant peu de bactéries a permis d'obtenir
une demi-vie d'environ cinq mois. Une autre étude a montré qu'au bout
de six semaines, il n'y avait toujours pas d'ouverture du cycle. Des
études de laboratoire portant sur des modèles d'écosystèmes ont montré
que le propachlor était presque complètement dégradé en l'espace de 33
jours.
Plusieurs études portant sur différentes espèces végétales ont
montré que le propachlor était rapidement métabolisé par les plantes
intactes ainsi que par des tissus végétaux excisés. Les voies
métaboliques se sont révélées analogues chez tous les végétaux
étudiés, tout au moins pendant les six à 24 premières heures,
conduisant à des métabolites hydrosolubles. On n'a pas observé de
décomposition métabolique du reste N-isopro-pylaniline. Seule une
très faible proportion (< 1 % selon une étude) des métabolites a été
retrouvée dans les fruits de ces végétaux; ils étaient concentrés en
grande majorité dans les racines et les feuilles. Les principaux
métabolites produits par les plantes sont identiques à ceux qui
prennent naissance dans le sol. On sait que ces métabolites peuvent
être fixés à partir du sol et un certain nombre d'études ne permettent
pas de déterminer avec certitude si les métabolites analysés
proviennent de la plantes ou du sol.
Bien que, d'après le coefficient de partage octanol/eau, ce
composé ait une tendance modérée à la bioaccumulation, on a montré
qu'il n'y avait ni bioconcentration ni bioamplification chez les êtres
vivants.
3. Concentrations dans l'environnement et exposition humaine
Les rapports d'analyse du propachlor dans l'air au cours de
l'épandage sont rares et insuffisants.
Aux Etats-Unis d'Amérique, la concentration du propachlor dans
les eaux de surface et les eaux souterraines est toujours faible, les
maxima atteignant 10 µg/litre dans les eaux de surface et 0,12
µg/litre dans les eaux souterraines. Le concentration la plus forte
enregistrée lors d'une étude sur les eaux de ruissellement était de 46
µg/litre.
Les résidus de propachlor dans les denrées alimentaires sont
généralement inférieurs à la limite de détection de la méthode
d'analyse (0,005 mg/kg). L'expérimentation a permis de retrouver des
résidus de l'ordre de 0,005 mg/kg dans des tomates, des poivrons, des
oignons et des choux.
Le dosage du propachlor dans l'atmosphère de la zone de travail
de conducteurs de tracteur pendant l'épandage du composé a donné des
résultats allant de 0,1 à 3,7 mg/m3.
4. Cinétique et métabolisme
Chez les mammifères, le propachlor peut être absorbé au niveau
des voies respiratoires, des voies digestives et de la peau. Il ne
s'accumule pas dans l'organisme et devient indétectable au bout de 48
heures.
La plupart des espèces animales (rats, porcs, poulets)
métabolisent le propachlor par la voie de l'acide mercapturique (MAP).
Il se forme des conjugués de cystéine par conjugaison avec le
glutathion et on a avancé que ces conjugués jouaient le rôle
d'intermédiaires dans la formation métabolique des acides
mercapturiques. La métabolisation du conjugué cystéinique du
propachlor se poursuit, notamment sous l'action de la C-S lyase
bactérienne qui intervient également dans la formation des métabolites
terminaux contenant le groupement méthylsulfonyle, métabolites qui
sont principalement excrétés dans l'urine (68 % de la dose initiale de
propachlor) et, sous forme de résidus insolubles, dans les matières
fécales (19 %). La C-S lyase du propachlor est sans action chez les
rats axéniques.
On a montré qu'il y avait un certain nombre de différences dans
le métabolisme du propachlor chez le rat et le porc. La bile est la
principale voie d'élimination des métabolites mercapturiques chez le
rat, mais on a montré qu'il existait une voie métabolique
extra-biliaire chez le porc.
Des études de métabolisme effectués sur des veaux ont montré que
ces animaux pourraient être incapables de former de l'acide
mercapturique à partir des conjugués du glutathion, ce qui les
rendrait plus sensibles à l'intoxication par le propachlor.
5. Effets sur les animaux d'expérience et les systèmes d'épreuves in
vitro
Le propachlor est légèrement toxique en cas d'exposition aiguë
par voie orale (la DL50 pour le rat varie de 950 à 2176 mg/kg de
poids corporel). Les signes d'intoxication aiguë proviennent
principalement d'effets sur le système nerveux central (excitation et
convulsions suivies d'une dépression). Chez des rongeurs, la toxicité
aiguë par inhalation est faible (CL50 = 1,0 mg/litre). Le propachlor
provoque de graves irritations des yeux et de la peau.
Des rats, des souris et des chiens ont été soumis à une
exposition de longue ou de courte durée au propachlor. Les organes
cibles sont le foie et les reins. Chez le chien, la dose sans effet
nocif observable a été de 45 mg/kg de poids corporel lors d'une étude
de trois mois où l'animal était exposé par la voie alimentaire. Lors
d'une étude d'une année sur des chiens, la dose sans effet nocif
observable a été estimée à 9 mg/kg de poids corporel (200 ppm dans la
nourriture). Lors d'une étude sur des rats exposés de la même manière
pendant 24 mois au propachlor, la dose sans effet observable a été
évaluée à 50 mg/kg de nourriture (2,6 mg/kg de poids corporel). Lors
d'une étude semblable effectuée sur des souris pendant 18 mois, on a
évalué à 1,6 mg/kg de poids corporel (10 ppm) la dose sans effet
observable.
Le propachlor ne s'est révélé cancérogène ni pour la souris ni
pour le rat. Dans la plupart des systèmes d'épreuve mammaliens, sa
réponse mutagène est négative tout en étant positive dans quelques
autres cas. Les données expérimentales disponibles de fournissent pas
une preuve suffisante de son pouvoir mutagène.
Administré en dose unique (675 mg/kg) à des rats et à des souris,
le propachlor a donné lieu à des signes d'embryotoxicité. On a
également observé des effets embryotoxiques lors d'études où du
propachlor avait été administré à plusieurs reprises (35,7 à 270
mg/kg). Toutefois, lors d'une autre étude sur des rats, où les doses
variaient de 20 à 200 mg/kg, aucun effet embryotoxique n'a été
observé.
Aux doses respectives de 12 et 60 mg/kg de poids corporel, le
propachlor (en poudre mouillable) a entraîné une réduction de la
teneur en protéines et un accroissement de l'activité de l'ATPase et
de la 5-nucléotidase dans des homogénats de testicules de rats,
provoquant également une dégénérescence du tissu testiculaire. Lors
d'une étude de reproduction portant sur deux générations, on n'a pas
véritablement obtenu la preuve d'effets indésirables.
6. Effets sur l'homme
On a signalé quelques dermatites de contact et dermatites
allergiques chez des agriculteurs et des ouvriers de production
exposés au propachlor (Ramrod et Satecid). Des tests cutanés ont été
effectués parmi un certain nombre d'entre eux, avec un résultat
positif, qui révèle une réaction d'irritation et une hypersensibilité
mono- et bivalente.
On n'a pas eu connaissance de symptômes ou de maladies, soit chez
des personnes exposées de par leur profession, soit dans la population
générale - à part les quelques cas d'effets cutanés chez les ouvriers
professionnellement exposés.
7. Effets sur les êtres vivant dans leur milieu naturel
Des études portant sur la microflore terricole ont montré que les
bactéries nitrifiantes constituaient le groupe le plus sensible aux
effets inhibiteurs du propachlor, leur nombre étant réduit d'un
facteur 3 à 4 après épandage de 8 à 10 kg de propachlor par hectare.
Ce sont les bactéries décomposant la cellulose qui étaient les moins
sensibles. La forte adsorption du propachlor aux particules d'argile
présentes dans le sol et une température élevée sont deux facteurs qui
réduisent les effets inhibiteurs.
Chez l'algue Selenastrum capricornutum, on a fait état d'une
CE50 à 96 heures de 0,02 mg/litre (pour la croissance) et d'une
concentration sans effet observable de 0,01 mg/litre. D'après une
deuxième étude portant sur une formulation de propachlor et menée
pendant 72 heures, le risque pour cette même algue serait nettement
moindre.
Pour la daphnie Daphnia magna, on donne des CL50 de 7,8 ou
6,9 mg/litre et une concentration sans effet observable inférieure à
5,6 mg/litre. La concentration sans effet observable sur la
reproduction était de 0,097 mg/litre. Pour des larves de deux espèces
de moucherons, on a obtenu pour la CL50 des valeurs respectives de
0,79 et 1,8 mg/litre.
Chez la truite arc-en-ciel la CL50 à 96 heures est de 0,17
mg/litre et la concentration sans effet observable sur 21 jours, de
0,019 mg/litre.
Le propachlor est considéré comme modérément à fortement toxique
pour les organismes aquatiques.
Le propachlor n'est pas toxique pour les lombrics aux
concentrations présentes dans le sol par suite d'un usage normal (la
concentration sans effet observable est de 100 mg/kg de terre). La
DL50 par contact pour les abeilles (311 µg/abeille) montre que le
propachlor ne constitue pas un danger pour ces insectes. En revanche,
des études menées en laboratoire et sur le terrain montrent que des
parasites utiles peuvent souffrir des effets du propachlor.
Le propachlor est plus toxique pour les oiseaux lorsqu'on
l'introduit directement dans l'estomac que lorsqu'on l'administre dans
la nourriture. La DL50 aiguë varie de 137 à 735 mg/kg de poids
corporel pour différentes espèces. Quant à la CL50 lors d'une
exposition par voie alimentaire, elle dépasse 5620 mg/kg de nourriture
chez les oiseaux.
Le propachlor ne constitue pas un danger pour les oiseaux dans la
nature, même sous forme de granulés.
RESUMEN Y EVALUACION
1. Identidad, modalidades de uso, propiedades físicas y químicas y
método analíticos
El propacloro es un herbicida derivado de la acetanilida
utilizado desde 1965 que actúa en las plantas antes de nacer o poco
después. Las principales formulaciones son de polvo humectable,
líquido fluido (concentrado en suspensión) y gránulos. Se aplica en la
agricultura a la lucha contra las gramíneas anuales y algunas malas
hierbas de hoja ancha en varios cultivos, como los de maíz, sorgo,
calabaza, lino y flores ornamentales.
El propacloro es ligeramente soluble en agua y se disuelve
fácilmente en la mayoría de los disolventes orgánicos. Su volatilidad
es escasa, no es inflamable y es estable a la radiación ultravioleta.
El método más práctico de análisis es la cromatografía de gases con
detección por captura de electrones, después de aplicar procedimientos
apropiados de extracción y purificación.
2. Transporte, distribución y transformación en el medio ambiente
Según la información disponible, el propacloro no se fotodegrada
sobre la superficie del suelo. El producto se volatiliza cuando hay
viento y la superficie del suelo está todavía mojada.
La adsorción sobre las partículas del suelo y la materia orgánica
es sólo moderada. Debido a esto, es posible la lixiviación a través
del perfil del suelo hacia el agua subterránea. Sin embargo, todos los
estudios indican que en la práctica es poco probable que esto ocurra.
Se requiere una precipitación muy abundante para hacer descender los
residuos 30 cm en el perfil del suelo. La mayoría de los autores
señalan que la mayor parte de los residuos se mantienen en una capa
superficial del suelo de 4 cm. Las características del suelo influyen
mucho en el desplazamiento del producto. La lixiviación se produce en
su mayor parte en suelo arenoso con poca materia orgánica.
Se ha estudiado el arrastre del propacloro por el agua tanto en
el laboratorio como sobre el terreno. En un estudio, la materia
orgánica del suelo redujo el arrastre del herbicida aplicado del 7% al
1%. Su incorporación al suelo también redujo la pérdida por arrastre
del agua (del 3% al 0,8% en un estudio).
El factor que más contribuye, con diferencia, a reducir los
niveles de propacloro en el suelo y el agua es la degradación por los
microorganismos. Se ha comprobado que en la degradación de la
sustancia intervienen tanto bacterias como hongos. Son pocas las
bacterias que parecen tener capacidad para usar el propacloro como
única fuente de carbono. También se han aislado bacterias que pueden
utilizar los metabolitos del propacloro presentes en el suelo.
Los metabolitos predominantes entre los que se forman en el suelo
son los ácidos oxanílico y sulfónico, solubles en agua. Pueden
formarse otros muchos metabolitos, pero representan una proporción
pequeña del total.
El propacloro desaparece con rapidez del suelo, habiéndose
registrado semividas de hasta tres semanas. En la mayoría de los
estudios se señala que la degradación es casi completa en menos de
seis meses. Las condiciones del medio ambiente influyen en la
velocidad de degradación, que se ve favorecida por los valores
elevados de la temperatura y el contenido de humedad del suelo. Los
estudios en los que se describía una permanencia más prolongada del
propacloro en el suelo se realizaron en condiciones de baja
temperatura o suelo seco. También es necesaria una cantidad suficiente
de nutrientes en el suelo para la degradación.
El metabolito conjugado N-isopropilanilina es mucho más
persistente que el producto del que procede. Se han encontrado
residuos de este metabolito hasta dos años después de la aplicación
experimental de dosis de propacloro más altas de las que se utilizan
normalmente en la agricultura.
Con el uso normal no es previsible que el propacloro llegue por
lixiviación a través del suelo hasta el agua subterránea, y no se
mantiene mucho tiempo en el suelo. En condiciones excepcionales de
baja temperatura o sequedad, el propacloro y sus metabolitos pueden
permanecer más tiempo en él. En condiciones normales, el propacloro
no sufre una fotodegradación significativa en el agua. En presencia de
fotosensibilizadores puede fotodegradarse. El propacloro es estable
desde el punto de vista hidrolítico. No es probable la volatilización
a partir del agua, debido a la elevada solubilidad en ella y la baja
tensión de vapor del producto.
Al igual que en el suelo, la principal ruta de pérdida de
propacloro del agua es la degradación biótica. La velocidad de
desaparición del propacloro del agua depende, pues, de la población
microbiana. En un estudio realizado con un pequeño número de bacterias
presentes en el agua se obtuvo una semivida de unos cinco meses. En
otro estudio, a las seis semanas no se había roto el anillo. En
estudios de laboratorio con modelos de ecosistemas se observó una
degradación casi completa en un período de 33 días.
En varios estudios con distintas especies vegetales se vio que el
propacloro se metabolizaba con rapidez tanto en las plantas intactas
como en tejidos vegetales extirpados. Las rutas metabólicas eran
análogas en todas las plantas estudiadas, por lo menos durante las
primeras 6-24 horas, produciendo metabolitos hidrosolubles. No se
observó degradación metabólica del fragmento de la
N-isopropilanilina. En los frutos de las plantas solamente se
encontró una proporción muy pequeña (< 1% en un estudio) de los
metabolitos; en su mayor parte estaban en las raíces y el follaje. Los
principales metabolitos producidos en las plantas son idénticos a los
que se forman en el suelo. Se sabe que las plantas absorben esos
metabolitos del suelo, y en algunos estudios había dudas acerca de si
los metabolitos medidos procedían de la planta o del suelo.
Aunque del coeficiente de reparto en octanol y agua parece
deducirse un potencial moderado de bioacumulación, los estudios
realizados indican que no hay ni bioconcentración ni bioamplificación
en los organismos.
3. Niveles medioambientales y exposición humana
Las mediciones descritas de la concentración del propacloro en el
aire durante la aplicación son pocas e inadecuadas.
Las concentraciones en el agua superficial y subterránea en los
Estados Unidos fueron siempre bajas, con un máximo de 10 µg/litro en
la superficial y de 0,12 µg/litro en la subterránea. La mayor
concentración registrada en el agua en un estudio del arrastre fue de
46 µg/litro.
Los residuos de propacloro en los alimentos suelen ser inferiores
al límite de detección del método analítico (0,005 mg/kg). En estudios
experimentales se han identificado concentraciones de residuos del
orden de 0,05 mg/kg en tomates, pimientos, cebollas y coles.
Las concentraciones de propacloro en el aire de la zona de
trabajo de los conductores de tractores que aplicaban la sustancia
oscilaban entre 0,1 y 3,7 mg/m3.
4. Cinética y metabolismo
Los mamíferos pueden absorber el propacloro por los tractos
respiratorio y gastrointestinal, así como a través de la piel. El
producto no se acumula en el organismo, en el que no es detectable a
las 48 horas.
La mayoría de las especies animales (ratas, cerdos, pollos)
metabolizan el propacloro siguiendo la vía de los ácidos
mercaptúricos. Mediante conjugación con el glutatión se forman
productos conjugados de cisteína, que se han señalado como posibles
intermediarios en la formación metabólica de ácidos mercaptúricos. La
C-S liasa bacteriana participa en el ulterior metabolismo del sistema
conjugado del propacloro con la cisteína y en la formación de los
metabolitos finales con metilsulfonilo, que se excretan sobre todo en
la orina (68% de la dosis de propacloro), y de residuos insolubles,
que se excretan en las heces (19%). La propacloro C-S liasa es
inactiva en ratas sin microorganismos.
En los estudios realizados se observaron algunas diferencias en
cuanto al metabolismo entre las ratas y los cerdos. La bilis es el
principal camino de eliminación de los metabolitos de la vía de los
ácidos mercaptúricos en las ratas, pero se ha demostrado que en los
cerdos existe una vía metabólica extrabiliar.
En estudios metabólicos con terneros se observó que pueden ser
incapaces de formar ácidos mercaptúricos a partir de los productos
conjugados del glutatión, por lo que pueden ser más susceptibles a la
intoxicación.
5. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
El propacloro es ligeramente tóxico en la exposición oral aguda
(la DL50 en ratas va de 950 a 2176 mg/kg de peso corporal). Los
signos de intoxicación aguda son predominantemente efectos sobre el
sistema nervioso central (excitación y convulsiones, seguidas de
depresión). La toxicidad aguda por inhalación en roedores es baja
(CL50 = 1,0 mg/litro). El propacloro produjo efectos graves de
irritación en los ojos y la piel.
El propacloro se ha ensayado en estudios de exposición de corta
y larga duración en ratas, ratones y perros. Los órganos sobre los que
actuaba eran el hígado y los riñones. En un estudio de administración
con los alimentos a perros durante tres meses, el nivel sin efectos
adversos observados (NOAEL) fue de 45 mg/kg de peso corporal. En otro
estudio de un año, también en perros, el NOAEL fue de 9 mg/kg de peso
corporal (250 ppm en la dieta). El nivel sin efectos observados (NOEL)
en un estudio de alimentación de ratas durante 24 meses fue de 50
mg/kg de la dieta (2,6 mg/kg de peso corporal). En un estudio en el
que administró el producto a ratones con los alimentos durante 18
meses, el NOEL fue de 1,6 mg/kg de peso corporal (10 ppm).
No se encontraron efectos carcinogénicos del propacloro en
ratones y ratas. En la mayoría de los sistemas de prueba de mamíferos
la respuesta mutagénica fue negativa, obteniéndose resultados
positivos en un pequeño número de ensayos. Los datos experimentales
disponibles no aportan suficientes pruebas de su potencial mutagénico.
En pruebas de dosis única (675 mg/kg) con ratones y ratas se
demostró la embriotoxicidad del propacloro. También se detectaron
efectos embriotóxicos en tratamientos con dosis repetidas (35,7-270
mg/kg). Sin embargo, en otro estudio en ratas, con una gama de dosis
de 20-200 mg/kg, no se observó embriotoxicidad.
Con niveles de 12 y 60 mg/kg de peso corporal, el propacloro
(polvo humectable) provocó una disminución del contenido de proteínas
y un aumento de la actividad de la ATPasa y la 5-nucleotidasa en un
homogeneizado de testículo de rata y cambios degenerativos en los
testículos. En un estudio de reproducción de dos generaciones no se
obtuvieron pruebas definitivas de efectos adversos.
6. Efectos en la especie humana
Se ha descrito un pequeño número de casos de dermatitis de
contacto y alérgica en agricultores y trabajadores expuestos al
propacloro durante la producción (Ramrod y Satecid). En algunos de
ellos se efectuaron pruebas del parche y la reacción fue positiva, con
irritación e hipersensibilidad monovalente y bivalente.
No se han notificado casos de síntomas o enfermedades entre las
personas expuestas profesionalmente o en la población general, salvo
un pequeño número de informes de sus efectos cutáneos en trabajadores
expuestos profesionalmente.
7. Efectos en los seres vivos del medio ambiente
En varios estudios sobre los microorganismos del suelo, las
bacterias nitrificantes fueron el grupo más sensible a los efectos
inhibidores del propacloro, reduciéndose su número a la tercera o
cuarta parte tras la aplicación de 8-10 kg/ha de propacloro. Las menos
sensibles fueron las bacterias celulolíticas. La adsorción elevada
sobre las partículas de arcilla del suelo y la temperatura alta
reducen los efectos inhibidores.
En el alga Selenastrum capricornutum se ha registrado una CE50
a las 96 horas de 0,02 mg/litro para el crecimiento y una
concentración sin efectos observados (NOEC) de 0,01 mg/litro. De un
segundo estudio de 72 horas realizado con una formulación, parece
desprenderse que el peligro es mucho menor para este mismo organismo.
Se ha informado de unos valores de la CL50 para Daphnia magna
de 7,8 y 6,9 mg/litro y una NOEC de < 5,6 mg/litro. En las larvas de
dos especies de moscas enanas, los valores registrados de la CL50
fueron de 0,79 y 1,8 mg/litro.
La CL50 para la trucha arcoiris a las 96 horas es de 0,17
mg/litro, y la NOEC en un estudio de 26 días fue de 0,019 mg/litro.
Se considera que el propacloro tiene una toxicidad entre moderada
e intensa para los organismos acuáticos.
La exposición a las concentraciones de propacloro que cabe prever
en el suelo no es tóxica para las lombrices de tierra si la aplicación
es normal (la NOEC es de 100 mg/kg de suelo). La DL50 por contacto
para las abejas (311 µg/abeja) demuestra que el propacloro no
representa un peligro para estos insectos. En estudios de laboratorio
y de campo se han detectado efectos adversos del propacloro en algunos
insectos parasitarios beneficiosos.
El propacloro es más tóxico para las aves cuando se administra
mediante sonda gástrica que cuando se incorpora a la dieta. Los
valores de la DL50 aguda para distintas especies de aves oscilan
entre 137 y 735 mg/kg de peso corporal. Los valores de la CL50 en la
administración con los alimentos fueron superiores a los 5620 mg/kg de
la dieta.
El propacloro no representa un peligro para las aves en el campo,
ni siquiera con la formulación granulada.
Table 9. Incubation of the glutathione conjugate of propachlor with pig caecal contents for various durationsa
Metabolites recovered Incubation time
0 20 min 40 min 1 h 2 h 4 h
2-Mercapto-N-isopropylacetanilide 7.7b 14.5b 23.0b 28.3b 34.7b 43.4c
(5.1-10.6) (5.5-22.8) (15.8-36.0) (22.9-38.7) (21.8-44.0) (38.7-49.2)
Glutathione conjugate 26.8 36.0 20.8 28.8 21.5 16.5
of propachlor (15.8-38.1) (18.1-51.6) (13.3-25.3) (16.6-42.3) (14.0-32.2) (14.8-19.2)
Cysteine conjugate of 47.5 32.5 35.1 20.9 11.9 3.8
propachlor (36.0-51.5) (21.3-43.7) (30.5-41.9) (12.0-27.9) (10.9-12.5) (2.2-5.6)
Non-extractable 14C 13.1 12.9 16.0 16.6 25.4 33.9
residues (13.0-13.4) (9.9-17.8) (13.5-20.5) (15.0-18.9) (22.8-28.2) (31.5-35.8)
a From: Larsen & Bakke (1983).
b Isolated as 2-carboxymethylthio- N-isopropylacetanilide
c Isolated as 2-(13C)-carboxymethylthio- N-isopropylacetanilide
Pigs were gilts. Results are shown as a percentage of the substrate. Values given in parentheses represent the range of values
obtained from 14C-recovery measurements.
More recent studies carried out by Aschbacher & Struble (1987) on
the metabolism of propachlor in pigs have proved the similarity, i.e.
the formation of methylsulfonyl-containing metabolites, with the
metabolism of rats, but have also revealed some differences.
A pig with a cannulated bile duct, which was dosed orally with
14C-propachlor, excreted 7.6% of the dose in the bile compared with
approximately 75% in the case of the rats. When enterohepatic
circulation was prevented in the bile of cannulated pigs,
CH3SO2-containing metabolites of propachlor were excreted in the
urine. As mentioned above, enterohepatic circulation is necessary for
the production of methylsulfonyl-containing metabolites in rats. In
experiments with germ-free pigs dosed orally with 14C-propachlor, it
was shown that they did not excrete urinary CH3SO2 metabolites, which
indicated involvement of the intestinal flora in the production of
these metabolites, as occurs in rats. Non-biliary excretion of
metabolites of propachlor into the lumen of the intestine probably
occurred. It is presumed that propachlor is absorbed by the mucosal
cells and conjugated with glutathione and that some of this conjugate
moves directly into the lumen of the intestinal tract by simple
diffusion, where it becomes the substrate of bacterial beta-lyase. The
presence of glutathione transferase has been demonstrated previously
in subcellular fractions of mucosal tissue homogenates.
In situ intestinal absorption of propachlor and non-biliary
excretion of metabolites into the intestinal tract of rats, pigs and
chickens was studied by Struble (1991). Propachlor was absorbed from
in situ intestinal loops of rats and pigs, the absorption half-times
being 7.5 and 16.5 min, respectively. Water-soluble 14C-labelled
metabolites that accumulated in the intestinal loops accounted for
31%, 53% and 25% of the initial 14C in rats, pigs and chickens,
respectively. Propachlor- S-cysteine was identified as the major
metabolite in the pig intestinal lumen (43% of the water-soluble
14C). It was concluded that the intestinal metabolism and intestinal
excretion of water-soluble metabolites of propachlor are important
physiological processes that occur in a variety of animal species.
These processes provide a route by which metabolites of xenobiotics
may reach the intestinal lumen in animals that are poor biliary
excretors. These studies demonstrated that although bile may be the
major route by which MAP metabolites are made available to the
intestinal microflora in the rat, an extrahepatic route exists in the
pig.
Davison (1991) conducted a study using six anaesthetized 2- to
21-day-old male Guernsey calves weighing 28 to 61 kg in which either
the left kidney was perfused (via the left renal artery) or the left
ureter was perfused with metabolites of propachlor. The glutathione
conjugate of propachlor (2- S-glutathionyl- N-acetyl-acetanilide)
was metabolized in both kidney and ureter to the cysteine conjugate.
When the mercapturic acid conjugate of propachlor was presented to the
kidney, it was eliminated in urine. First-pass metabolism and
elimination of the glutathione conjugate by the kidney was 16% of the
dose, whereas first-pass elimination of the mercapturic acid was 33%.
Absorption of the glutathione conjugate of propachlor or its
metabolites, or of glycine by the ureter was nil. The cattle may be
unable to form mercapturic acids from glutathione conjugates of some
xenobiotics, which may result in their being more easily poisoned by
these xenobiotics than chickens, pigs and rats.
The glutathione conjugate of 2-chloro- N-isopropyl[1-14C]acet-
anilide (14C-propachlor) was perfused through a calf kidney in situ
by Bakke et al. (1990). Twenty-three per cent of the dose was excreted
in the perfused kidney urine as the cysteine conjugate; no mercapturic
acid was detected. A 5-day-old calf dosed orally with 14C-propachlor
excreted 70% of the dose in the urine as the cysteine conjugate; again
no mercapturic acid was detected. Rumen microflora were established in
the calf when it was 5 weeks older and the experiment was repeated.
The same results were obtained.
6.3 Elimination and excretion
When 14C-propachlor was given to rats, 56-64% of the dose was
excreted in urine in the first 24 h and 5.7-7.0% in 24-48 h. In the
faeces, 8-13% and 2.2-7.7% were eliminated in 0-24 h and 24-48 h,
respectively; 0.4% of the 14C was eliminated as CO2 and 5-11% was in
the carcass. In total 80-97% was eliminated in 48 h (Lamoureux &
Davison, 1975).
According to Bakke et al. (1980), the metabolites of propachlor
formed in normal rats treated with propachlor are excreted mainly
through urine (68%) and faeces (19%). Eleven urinary metabolites were
isolated from rats given 14C-labelled propachlor orally. The major
metabolite was the mercapturate (17%), and six of the metabolites were
2-methylsulfonylacetanilides. Faecal residues (19%) of the
administered dose, insoluble in common solvents or by treatment with
diluted acid or base, were also determined.
Rats with cannulated bile ducts secreted 66% of an oral dose of
propachlor in the bile as the glutathione conjugate (2), cysteine
conjugate (3), mercapturate (4) and the mercapturate sulfoxide (5)
(see Table 10). Germ-free rats given orally 14C-labelled propachlor
excreted 98% of the dose in the urine and faeces within 48 h. Three
metabolites were isolated from the excreta and the faecal radioactive
metabolites were water soluble. The major metabolite was mercapturate
(4) and the other metabolites were the cysteine conjugate (3) (present
only in the faeces) and mercapturate sulfoxide (5) (Bakke et al.,
1980). Mercapturate sulfoxide was isolated from the excreta of
germ-free rats by Feil et al. (1981), who also demonstrated its
presence in the bile of rats and urine of chickens and pigs dosed with
propachlor. The metabolite was characterized by mass and nuclear
magnetic resonance spectro-metry on samples isolated from rats.
Table 10. Comparison of the excretion of single oral doses of 14C-propachlor
by control rats with fistulated bile ducts, and germ-free ratsa
Metabolite Recovery of 14C (% dose)b
Bile-fistulated
Control rats rats Germ-free rats
Urine Faeces Bile Urine Faeces
Glutathione 37
conjugate (2)b
Cysteine 13 19
conjugate (3)
Mercapturate (4) 17 12 63.1 3.7
Mercapturate 4 5.7 5.4
sulfoxide (5)
Non-extractable 19
residues
Other metabolites 51
Total 68 19 66 68.8 32.1
a From: Bakke et al. (1980)
b Metabolite designations are those used in Figs. 1 and 3.
As in the case of the glutathione and cysteine conjugates, the
sulfoxide is not excreted at detectable levels by normal rats and was
detected only in the bile (Bakke et al., 1981a). It may become a
substrate for the intestinal flora, but the ultimate in vivo fate of
this metabolite is unknown.
Bakke et al. (1981c) reported differences between some species
concerning the metabolism of propachlor in the MAP. Clear but
unexplained differences are that rats excrete no cysteine conjugate
and chickens form no methylsulfonyl-containing metabolites, whereas
sheep excrete large amounts of cysteine conjugate in urine.
Nadeau & Pantano (1986) carried out a study to determine the
rates and routes of excretion of orally administered synthetic
14C-labelled propachlor plant metabolites in lactating goats and to
quantify and identify the radioactive metabolites in the goat milk,
tissues, urine and faeces. The daily dose level for the three treated
goats was 15 mg/kg administered on 5 consecutive days (the actual dose
levels for the treated goats were 13, 14.5 and 13 mg/kg,
respectively). Each treated goat received a total of 130.5 mg of the
13C/14C-labelled propachlor plant metabolites mixture during the
dosing period. A control animal received placebo capsules. The
radioactivity eliminated in faeces accounted for 72.1, 64.5 and 57.9%
of the administered dose, respectively (average 64.8%), and that
excreted in urine accounted for 35.8, 28.1 and 32.3% (average 32.1%).
The percentage of the dose eliminated through faeces and urine
averaged 96.9%.
The radioactivity found in the milk accounted for only 0.084,
0.10 and 0.13% (average 0.10%) of the administered dose. These values
corresponded to metabolite concentrations in the milk of 11.9, 14.8
and 18.0 µg/litre (average 14.9 µg/litre). The metabolic
concentrations (µg/kg) in the tissues were: kidney 51.9, liver 30.4,
muscle 8.5, fat 19.5 and blood 50.1. The loss of radioactivity from
tissues, milk and excrement occurred rapidly, and after 5 days of
depuration the milk and tissue radioactivity levels were below the
limit of detection, except in the case of the liver (5.7 µg/kg).
6.4 Metabolism in laying hens
Since crops treated with propachlor are used in animal feed, it
is important to know the fate of propachlor in animal feed.
The purpose of the study by Bleeke et al. (1987) was to examine
the metabolic fate of propachlor plant metabolites in laying hens and
to determine whether they accumulate or persist in the eggs or edible
tissues. The compounds used for feeding were the three major
metabolites of propachlor found in sorghum, i.e. [( N-iso-propyl)-
phenylamino] oxacetic acid, sodium salt (I), {{[( N-iso-propyl)-
phenylamino]acetyl}sulfinyl} lactic acid, sodium salt (II) and
2-[( N-isopropyl)-phenylamino] 2-oxoethane sulfonic acid, sodium salt
(III).
The first study involved dosing chickens at a level of 5 mg/kg
diet for 6 consecutive days. Data for bioaccumulation and excretion of
plant metabolites were obtained from this study. A second group of
chickens, dosed at a level of 25 mg/kg diet (nominal dose) on 6
consecutive days, provided eggs and tissues with higher residues for
metabolic characterization. Each group consists of five hens. Control
groups received a single gelatine capsule per day. The total recovery
of 14C radioactivity was good in both studies.
An average of 87.4% of the total administered dose was recovered
from the chickens fed 5 mg/kg approximately 1 day after the last dose,
and 97.9% was recovered from the chickens fed 25 mg/kg, primarily in
the excreta. The eggs contained low residue levels; those from
chickens fed 5 mg/kg contained residues below the minimum quantifiable
limit (MQL) of 1.7 µg/kg. The level in the egg yolks reached an
average of 5.5 µg/kg by day 6 but by day 12 the residues in the yolk
had fallen below 1.6 µg/kg.
In the high-dose group, the residues in the egg white levelled
off at about 4 µg/kg on day 2, while those in the egg yolk increased
to a level of 27.3 µg/kg on day 6.
Tissues also contained low levels of residues. In the low-dose
group, the highest levels were found in the kidney and they averaged
only 6 µg/kg. The residue levels in the liver measured an average of
1.9 µg/kg and those in the fat 3.5 µg/kg. The residues in other fat
samples and organs were below the MQL (1.4-1.6 µg/kg). Residues in the
breast muscle were below the minimum detectable limit (7 µg/kg) and
those in the blood were 6-7 µg/kg.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity of propachlor for the rat, mouse and the
rabbit has been examined. According to the WHO hazard classification
of pesticides and as far as its acute oral toxicity to rats is
concerned, propachlor is slightly hazardous (Group III) (WHO, 1990).
LD50 values in various animal species reveal a higher susceptibility
for the mouse and the rabbit than for the rat (Table 11). The LD50
for rats ranges from 950 to 2176 mg/kg.
The clinical picture of acute oral intoxication by propachlor has
been described in three species of experimental animals: mice, rabbits
and rats (Panshina, 1973). When propachlor is given at lethal or toxic
doses, the main symptoms concern the central nervous system. In mice
and rabbits, a state of excitement, trembling and light convulsions is
observed 15 min after a toxic or lethal dose of propachlor. This
gradually increases, breathing becomes difficult and death follows in
fseveral hours. Intoxication in rats takes a different course: a state
of immobility and head and body tremors are accompanied by the sudden
onset of convulsions and death occurs within 24 h. This clinical
picture was confirmed by Strateva (1974,a,b).
Kronenberg (1988) reported clear signs of irritation to the
gastrointestinal tract and lungs that were evident at necropsy in
animals that died during the study. Histoenzymological changes
expressed as decreased enzyme activity were in full agreement with the
morphological picture (Strateva et al., 1974a,b).
7.1.2 Dermal
Results of studies in rabbits on the acute dermal toxicity of
propachlor and its formulations indicate that the dermal LD50 ranges
from 4000 mg/kg for a 65% WP formulation (Auletta & Rinehart, 1979) to
20 000 mg/kg for technical propachlor (94.5%) (Braun & Rinehart,
1978). Test animals exhibited moderate to severe erythema, severe
oedema and necrotic skin at the dermal application sites at a dose
level of 2800 mg/kg. Motor activity decrease and ataxia were noted at
dose levels of 2000 to 5600 mg/kg.
Table 11. Oral LD50 values for laboratory mammals
Species LD50 (mg/kg)a Reference
Rat 1056 (834-1278) Panshina (1973, 1977)
950 (860-1050) Strateva (1974b)
1340 Mirkova (1975)
Table 11 (contd).
Species LD50 (mg/kg)a Reference
2176 ± 220 Lehotzky et al. (1979)
Rat (Sprague-Dawley) 840 (419-1261) Blaszcak (1988)
(in corn oil)
1700 (1265-2135) Blaszcak (1988)
(in 1% methylcellulose)
Rat (Fischer-344) 550 (252-848) in corn oil Blaszcak (1988)
1359 (1009-1691) Blaszcak (1988)
(in 1% methylcellulose)
Rat (both sexes) 4000 Heenehan et al. (1979)
Rat (both sexes) 3269 Branch et al. (1982a)
Mouse 306 (275-337) Panshina (1973, 1977)
290 (240-350) Strateva (1974b)
a The concentration is based on the percentage active ingredient.
Figures in parentheses indicate the range of values.
Using groups of 16 Wistar rats, Baynova et al. (1977) studied the
acute dermal toxicity of a single application of propachlor 65 WP
(10-20% in aqueous suspension) with doses of 1500-4000 mg/kg body
weight (active ingredient). There was no mortality, and no signs of
intoxication were observed. No haematological or biochemical tests
were performed.
Propachlor and Satecid 65 WP caused severe dermatitis, ulceration
and necrosis in the skin of rabbit and ears of mice. None of the
compounds exhibited contact sensitization effects on guinea-pigs
(Lehotzky et al., 1979).
7.1.3 Inhalation
In a study by Bechtel (1991), technical propachlor (96.8%) was
dissolved in dimethylsulfoxide (DMSO) to generate an aerosol and
administered to five male and five female Sprague-Dawley rats in a
nose-only chamber at an analytically determined concentration of 1.2
mg/litre for 4 h. Control rats (five of each sex) were exposed to an
atmosphere of aerosolized DMSO for the same duration. Particle size
measurements on the propachlor/DMSO aerosol indicated a mass median
aerodynamic diameter of 3.5 µm, with 96% of the particles being less
than 10 µm and 1.8% less than 1 µm in diameter (Bechtel, 1991). No
treatment-related deaths occurred. Clinical signs included laboured
respiration and nasal discharge; all animals appeared normal by
post-exposure day 2. A transient weight loss was noted in both treated
and control animals during the first two days of the study, but normal
body weight was observed thereafter. No abnormalities were apparent
during postmortem examination of the animals.
In another acute inhalation study, three groups of Charles River
CD rats (five rats of each sex per group) were exposed to test
atmospheres of a propachlor formulation (44% active ingredient) for 4
h (Kaempfe, 1991). During the exposure period, animals were housed in
a 250-litre New York University style stainless steel chamber, and
were exposed to analytically confirmed concentrations of 0.18, 0.67
and 1.0 mg propachlor/litre in the breathing zone of the chamber. At
least 82% of the particles were less than 10 µm in diameter. No
animals died at the two lower exposure levels, whereas four out of ten
rats died at 1.0 mg/litre. Clinical observations during the
post-exposure period included ocular opacities, perinasal
encrustation, rapid or shallow respiration, perioral wetness and focal
loss of hair from animals in the highest exposure group. Fourteen days
after exposure, all surviving animals had body weights higher than the
pre-exposure (day 0) values with the exception of females in the
1.0-mg/litre group, which exhibited body weight depression. There were
no abnormal findings in rats that died during the test or were
sacrificed at 14 days. Based on the mortality observed, the LC50 was
slightly greater than 1.0 mg/litre.
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dogs
To assess potential subchronic toxicity, propachlor (96.1% pure)
was administered via the diet to five groups of two male and two
female beagle dogs for 4 weeks (Daly & Knezevich, 1984). Dietary
concentrations of propachlor were 0, 100, 500, 1000 and 1500 mg/kg
(equivalent to 0, 2.5, 12.5, 25, and 37.5 mg/kg body weight per day).
No mortality or clinical signs of toxicity related to treatment
occurred during this study. Food consumption was initially decreased,
but only in the females treated with 1000 and 1500 mg/kg (equal to 25
and 37.5 mg/kg body weight) and the males treated with 1000 mg/kg. The
consumption had returned to normal by the end of the study. Body
weight varied markedly. The decreased body weight and/or weight gain
noted in males fed 12.5 or 25 mg/kg body weight per day, and females
fed 37.5 mg/kg per day could have been treatment related.
Haematological examination showed slightly increased platelet counts
in high-dose males. No treatment-related gross pathological effects
were noted at sacrifice.
Following the 4-week pilot feeding study in beagle dogs, a 90-day
feeding study was undertaken (Naylor & Ruecker, 1986). Propachlor
(96.1% purity) was administered in the diet to groups of six dogs of
each sex per group for 90 days. Nominal dietary concentrations were 0,
100, 500 and 1500 mg/kg. There was no mortality or clinicopathological
or histopathological changes related to the treatment. The dose level
of 1500 mg/kg (45 mg/kg body weight) was a no-observed-adverse-effect
level (NOAEL).
7.2.1.2 Rodents
Propachlor (65% WP) was given orally by gavage to white rats at
daily doses of 21, 53 and 106 mg/kg body weight for 4 months, and its
cumulative effect was studied by Panshina (1973). Later, Strateva
(1974a, 1976) carried out a short-term study (45 days) at dose levels
of 50, 100 and 200 mg/kg body weight given orally by gavage to 104
Wistar rats (divided into four groups (three experimental and one
control) with equal numbers of both sexes), and a long-term oral study
(6 months) at dose levels of 0.05, 0.5, 5 and 20 mg/kg body weight
given to 220 Wistar rats divided into five groups (four experimental
and one control).
Strateva (1976) found a decrease in haemoglobin content and
number of erythrocytes, slight leucocytosis and an increased number of
neutrophils. The threshold dose for rats in long-term studies was 5
mg/kg body weight.
Panshina (1976) carried out a 10-month study on white rats given
propachlor by gavage at doses of 1, 3.5 and 10.6 mg/kg body weight.
Slight leucocytosis was found within 4-7 months. The first two doses
provoked a decreased activity of catalase and peroxidase as well as an
increase in nicotinamide adenine dinucleotide in brain and heart
tissue homogenates. No changes in haemoglobin content or number of
erythrocytes were noted. There were no alterations to the
pathomorphological picture.
Baynova et al. (1978a) compared the effects of continuous and
intermittent oral dosing of propachlor (65% WP) on white rats (equal
numbers of both sexes). The number of animals per group was not given.
The scheme of the experimental design is given in Table 12.
Table 12. Experimental design of the study by Baynova et al. (1978a)
Group Duration of Daily dose Dose (fraction
study (mg/kg) of the LD50)
Control 4 months - -
I (dosing every week) 4 months 70 1/20
II (dosing every second week) 4 months 140 1/10
III (dosing every second week) 8 months 70 1/20
Control 8 months - -
Continuous administration of propachlor led to more marked
changes in the main parenchymous organs than intermittent
administration. These changes were characterized by decreased activity
of oxido-reductive tissue enzymes. The hexabarbital sleeping time,
which characterized the detoxification function of the liver, showed
a statistically significant reduction. Propachlor administration at
120 mg/kg body weight for 6 consecutive days to male and female rats
increased the levels of both cytochrome and microsomal protein content
(Nenov & Baynova, 1978) as a result of the induction of mixed-function
oxidase in the liver (Baynova et al., 1978a).
A special study on the morphological changes in kidneys was
carried out. Propachlor (65% WP) was administered by gavage to male
white rats (10 animals per group and 1 control) at doses equivalent to
6, 12 and 60 mg/kg body weight for 3 months (Maleva & Zlateva, 1982).
Dose-dependent morphological changes were found in the proximal
convoluted renal tubules. The tubules were deformed and the epithelial
cells were vacuolized and dystrophic. A decreased cellular content of
ribonucleoproteins was also observed and pycnosis and caryolysis of
nuclei were seen. In the lumen, desquamated epithelia and hyalin
cylinders were found. The intestine was slightly swollen in certain
areas.
The subchronic toxicity of propachlor (96.1% purity) has also
been evaluated in Sprague-Dawley CD rats (30 of each sex per group)
fed diets containing 0, 300, 1500 or 7500 mg/kg for 90 days (Reyna &
Ribelin, 1984a). No animals died. A statistically significant
reduction in body weight was observed in animals fed 1500 mg/kg (8%
for males and females) and 7500 mg/kg (59% for males and 36% for
females). Food consumption was significantly depressed for animals at
the highest dose level for the first month of the study, but recovered
during the remainder of the study. After 6 weeks, reduced haemoglobin,
haematocrit, mean corpuscular haemoglobin and haematocrit, and an
increase in reticulocytes were observed in females at all dose levels
and in high-dose males. The anaemia was less evident in high-dose
males and was only present in high-dose females at study termination.
Significantly reduced lymphocyte counts were observed at week 6 for
high-dose males and mid- and high-dose females, and at study
termination for all groups. Levels of serum enzymes (SGPT, SAP, GGT),
cholesterol and total bilirubin were significantly increased for
high-dose animals at weeks 6 and 13 and there were significant
reductions in total protein, glucose, creatinine and albumin. No
histological changes were observed in the liver. Organ weights (with
the exception of female livers) were reduced, relative to controls,
for high-dose animals, and spleens were extremely small and
treatment-related in size in about 65% of the animals. No histological
changes were observed in the tissues examined, which included the
spleen.
7.2.1.3 Mice
The subchronic toxicity of propachlor (96.1% pure) was evaluated
in four groups of Charles River CD-1(R) mice (30 mice of each sex
per group) (Reyna & Ribelin, 1984b). Dietary levels were 0, 500, 1500
and 5000 mg/kg. No mortality or adverse clinical signs were observed
during the study. A statistically significant reduction (10%) in body
weight was observed in the mid- and high-dose males and females during
the study. Food consumption was also reduced during the first month
for mid- and high-dose animals. A dose-related statistically
significant reduction in the number of white blood cells was observed
in both sexes at all dose levels, except low-dose females, at the
7-week sampling period. At study termination, this reduction was
evident in mid- and high-dose animals but was statistically
significant only for high-dose males. Liver weights were increased for
males at all dietary levels and for mid- and high-dose females. There
was an accompanying statistically significant increase in the
incidence of centrilobular hepatocellular hypertrophy for mid- and
high-dose males, based on histological examination. No other
microscopic changes that could be considered treatment related were
evident in tissues. For males, the kidney to body weight ratio was
decreased at the high-dose level.
7.2.2 Dermal
A 21- and 90-day short- and longer-term dermal toxicity study on
Wistar rats (12 animals in each tested group plus 1 control) using
propachlor (65% wettable powder) administered 5 days per week was
carried out by Baynova et al. (1977). The doses applied were 50, 200
and 500 mg/kg in aqueous suspensions in the 21-day experiment, and 10,
25 and 50 mg/kg in the 90-day study. The dermal application was
performed uncovered (Draize, modification of Noakes). By the end of
day 21, anaemia was found at dose levels of 200 and 500 mg/kg, but
there was no methaemoglobinaemia. The same doses induced a
statistically significant decrease in the activity of certain enzymes
(SGOT, SGPT, OCT and AP). Dermal application of propachlor at dose
levels of 25 and 50 mg/kg to the skin of white rats (two groups of 12
animals) for 90 days did not provoke any changes in concentrations of
red blood cells and haemoglobin, but there were decreases in SGOT,
SGPT, LAP, AP and catalase. Decreased sulfur-containing enzymes were
found in tissue homogenates (liver and kidney). Decreases in the
levels of LDH, SDH, AcP and glucose-6-phosphate dehydrogenase were
determined histochemically. There was no evidence of clinical signs of
intoxication after repeated dermal application of the herbicide, but
the occurrence of the above-mentioned enzymatic changes demonstrated
the penetration of this herbicide through the dermal barrier. The
hexabarbital sleeping time was statistically significantly shortened
at the mid- and high-dose levels, thereby confirming the induction of
mixed-function oxidases reported by Nenov & Baynova (1978).
A threshold dermal dose of 25 mg/kg (1% aqueous suspension of
propachlor 65 WP) and a no-observed-effect level (dermal) of 10 mg/kg
(0.5% aqueous suspension) were determined in 90-day experiments by
Baynova et al. (1977).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Single application to Wistar rats (three rats of each sex per
group) of propachlor 65 WP at doses of 1500, 2000, 3000 and 4000 mg/kg
in 10 or 20% aqueous suspension, under the open modified method of
Noakes, did not cause any local irritative effects (Baynova et al.,
1977).
Panshina (1973) reported a strong irritative effect of propachlor
after single and repeated application. Single applications to rabbits
of 16% propachlor 65 WP in aqueous suspension at doses of 500, 700,
and 1000 mg/kg led to hyperaemia and even ulceration in the skin of
some animals. Ten applications of a 32% aqueous suspension of
propachlor at a level of 200 mg/kg had the same effect on the skin.
Seventeen applications of a 6.5% aqueous suspension at 100 mg/kg did
not result in mortality, and only slight hyperaemia was seen in the
treated skin area of the rabbits.
Primary irritation and contact sensitization effects of Satecid
65 WP containing 65% propachlor were investigated in rabbits, mice and
rats, and these were compared with the effects of technical grade
propachlor, propachlor with analytical purity, and the vehicle alone
(Lehotzky et al., 1979). Propachlor had strong to very strong
irritating effects on intact and scarified skin as well as on the eye
mucosa of rabbits and mice. The symptoms included erythema, oedema and
penetrating ulcer. Technical grade propachlor had the most potent
irritating effect.
Heise et al. (1983) conducted parallel experiments with two
propachlor preparations, one produced in the USA and the other in
Hungary, using an aqueous suspension applied under occlusion to the
skin of rabbits for 24 h. The irritant dose (ID50) for the USA
preparation was 2% and for the Hungarian preparation 0.6%.
Several dermal irritation studies have been carried out with
technical propachlor and its formulations, each of which involved the
use of six New Zealand white rabbits (2.5 to 3.5 kg). All application
sites were scored for erythema, eschar and oedema formation. For
technical propachlor (94.5% pure) a slight degree of dermal irritation
was observed (2.5/8.0 score) (Braun et al., 1979a); for a 20%
formulation of propachlor (again 94.5% pure) there was a slight degree
of dermal irritation (1.8/8.0 score) (Braun et al., 1979b); for a 65%
formulation a moderate degree of dermal irritation (3.4/8.0 score)
(Braun et al., 1979c); and for a 42% formulation corrosive effects
were observed (Branch et al., 1982b).
7.3.2 Skin sensitization
A modification of the maximalization test of Magnusson & Kligman
(1969) for identification of allergens was used by Heise et al.
(1983). Guinea-pigs (12 female and 12 male) were treated by
intramuscular injection of 0.2% propachlor aqueous suspension (using
preparations from both the USA and Hungary together with Freund
adjuvant), by subcutaneous injection of 0.05% aqueous suspension and
by epicutaneous application of a 1% water suspension of both
preparations for 6 weeks, both on healthy and scarified skin. A
challenge single application was given two weeks after the last
application using 0.2 ml of an 1% aqueous suspension of each
formulation. There was no significant difference between the reactions
to the two preparations. A challenge dose did not induce a reaction.
The dermal sensitization potential of propachlor was evaluated in
guinea-pigs using the Buehler procedure (Auletta, 1983). For the
induction phase, 0.2 ml of undiluted propachlor (95.7% purity) was
applied dermally using the closed patch technique to the shaved backs
of five male and five female Hartley albino guineapigs. The
applications were made for 6 h/day, 3 days/week, for 3 weeks. Two
weeks after the final dose, additional 0.2 ml doses of 25% propachlor
in ethanol (challenge dose) were applied to previously untreated areas
on those guinea-pigs that had received the induction doses and to six
others (three males and three females) which served as irritation
controls. Two positive control groups, treated with 1-chloro-2,4-
dinitrobenzene (DNCB) as positive control, were used. The negative
control group (five males and five females) was treated with saline
for the induction doses and challenged with acetone. All animals had
slight to moderate dermal irritation following the third induction
dose of propachlor, and some of them exhibited severe irritation,
including oedema, necrosis and exfoliation. Following application of
the challenge dose, one out of ten animals had an irritation score of
1 (slight), eight animals had a score of 2 (moderate) and one animal
had a score of 3 (severe) at 24 h. Nine of these animals exhibited
oedema. At 78 h, two animals had very low scores, six animals had
scores of 1, and two scores of 2. Six of these animals exhibited
oedema. Similar results were obtained when 20 and 40% propachlor
formulations were tested for sensitization in guinea-pigs (Auletta et
al., 1984a,b).
7.3.3 Eye irritation
Molnar & Paksy (1978) studied the effect of Satecid 65 WP on the
eye mucosa of CFY male albino rats using the Evans blue diffusion
technique. The aqueous suspension caused strong conjunctivitis at the
minimum concentration of 0.01%.
In a study by Auletta (1984), technical propachlor (96.1% pure)
was ground to a fine powder and introduced in the conjunctival sac of
the right eye of six (two males and four females) albino New Zealand
White rabbits. The left eye served as the control. The treated eyes
were rinsed 24 h later. Five of the animals exhibited severe
conjunctival irritation (redness, necrosis, chemosis and discharge),
five exhibited opacity and/or ulceration, and five had iridial damage.
Two animals exhibited neovascular-ization of the cornea and one
bulging of the cornea, indicative of increased intraocular pressure.
After 21 days of observation one animal continued to exhibit
conjunctival redness and one corneal opacity and bulging of the
cornea. The eyes of the remaining animals had minimal conjunctival
irritation.
In an eye irritation test on six New Zealand white rabbits using
technical propachlor (94.5%), 0.1 cm3 was instilled into one eye of
each rabbit and ocular reactions were observed on days 1, 2, 3, 4, 7,
10 and 14. According to Draize scores, all treated eyes were assigned
positive scores for corneal opacity and ulceration, conjunctival
redness, chemosis and necrosis. Two eyes were assigned positive scores
for iritis. Three eyes exhibited pannus, and corneal bulge was
observed in two eyes. Four eyes were clear of signs of irritation by
day 14 and two eyes showed signs of irritation at the termination of
the study (Braun, 1979).
US EPA (1984b) reviewed the existing data and concluded that
propachlor is corrosive to the rabbit eye, corneal opacity being
irreversible after 7 days.
7.4 Reproduction, embryotoxicity and teratogenicity
7.4.1 Reproduction
7.4.1.1 Biochemical and histopathological studies on gonads
The effect of propachlor 65 WP on protein content, activity of
5-nucleotidase and ATP in testis homogenate was evaluated by Maleva &
Stereva (1977), Stereva & Maleva (1977) and Zlateva & Maleva (1978,
1979). Each test group consisted of 20 male Wistar rats, administered
orally (by gavage) 12 or 60 mg propachlor/kg body weight per day for
6 months. A control group consisted of the same number of animals. At
the end of the experiment, there were dose-dependent statistically
significant changes in the biochemical indices studied (a decrease in
protein content and an increase in 5-nucleotidase and ATPase activity)
and these were accompanied by histomorphological changes, i.e.
disorganization of the seminiferous epithelium. After prolonged dosing
some dystrophic and degenerative changes in the gonad tissue and the
appearance of multinuclear giant cells with central chromatolysis were
observed. Adverse effects on the mitotic division of spermatogonia and
blockage of meiosis in the earlier phases were found (Zlateva &
Maleva, 1978, 1979).
In subchronic toxicity studies, there was no microscopic evidence
of testicular pathology in dogs administered the equivalent of 45 mg
propachlor/kg diet per day (Naylor & Ruecker, 1986), in rats fed the
equivalent dose of 250-310 mg/kg diet per day in the food (Reyna &
Ribelin, 1984a), or in mice fed the equivalent of 400 to 600 mg/kg
diet per day (Reyna & Ribelin, 1984b).
7.4.1.2 Reproduction studies
A two-generation rat reproduction study was undertaken to
evaluate the effects of propachlor (96.3% pure) (Rao, 1981). Four
groups of 30 male and 30 female 6-week-old Fischer-344 rats (F0
generation) were exposed to diets adjusted weekly to provide dose
levels of 0, 0.3, 3 and 30 mg propachlor/kg body weight per day for
100 days and were then allowed to mate to produce the F1 generation.
The F1 weanlings were similarly dosed for 120 days prior to
breeding. The F1 adults were mated twice to produce F2a and F2b
litters. At weaning of F1, F2a and F2b litters, 10 pups of each
sex at each dose level were sacrificed and selected organs were
examined histologically. No signs of toxicity in any animals were
found during the study. A significant decrease in food consumption and
body weight of adult F1 males dosed with 30 mg/kg per day was found.
No adverse effects in any of the treated groups over two generations
were noted with respect to gestation length, number of live pups
delivered, neonatal survival, litter weight and sex ratio. Decreased
pregnancy rates (fertility index) were observed in mid-dose (60%) and
high-dose (63%) F1 females following the first mating (F2a
litter), in comparison with 83% in the control rats. Test animals
continued to be treated with propachlor and were remated to produce a
second (F2b) litter. Pregnancy rates for the F2b litter were 83%
for controls and 83, 83 and 80% for low-, mid- and high-dose groups,
respectively.
No treatment-related gross necropsy findings were noted. Slight
increases in absolute and/or relative liver weights were noted in
high-dose F0 adults, mid- and high-dose F1 adults and high-dose
F2a weanlings. Hypertrophy of the centrolobular hepatocytes was
noted during microscopic examination of livers from the high-dose F0
and F1 adult females. There was no evidence of compound-related
microscopic changes in the testes and ovaries of test animals for
either generation.
7.4.2 Embryotoxicity and teratogenicity
7.4.2.1 Rats
a) Single dose treatment
Mirkova (1975) reported embryotoxic and teratogenic effects of
propachlor 65 WP in Wistar rats following single-dose treatment (675
mg/kg body weight) on each of the first 16 days of gestation.
Significantly increased lethality and frequency of haematomas,
predominantly in the head of the fetuses, and reduced cranio-caudal
size were observed when the treatment was performed from days 1 to 4
and on days 8, 10 and 12 of gestation (8 to 12 animals per test group
and 57 controls). The effect was more marked following treatment on
the first day of pregnancy. A weak teratogenic effect was observed
with a single dose, which included the induction of external
malformations (day 11, brachygnatia), abnormality of internal organs,
i.e. hydrocephalus (days 9, 11, 12 and 13 of gestation), defects in
skeletal ossification, i.e. non-ossification of sternum (day 9), and
delayed ossification of parietal bones (days 11, 12 and 13 of
gestation).
b) Repeated dose treatment
Wistar rats were treated daily with 67.5, 135 or 270 mg
propachlor/kg body weight on days 1-7 or 8-16 of gestation (six test
groups of 10 animals each). In a second set of experiments, a daily
treatment of 33.7, 67.5, 135 and 270 mg/kg body weight was given
throughout the gestation period (1-21 days) (Mirkova, 1975).
Propachlor induced embryotoxicity at all dose levels of repeated
treatment during the pre-implantation stages of embryogenesis (days
1-7 of gestation). This was expressed as raised lethality and
haemorrhages, predominantly in the head of the fetus, and reduced
weight and cranio-caudal size. The effects were significantly reduced
when administration was performed during the period of organogenesis
(8-16 days). Propachlor showed low teratogenic activity, producing
external malformations of the tail (short, curved tail), skeletal
abnormalities (non-ossified parietal and occipital bones) and
hydrocephalus, at all three dose levels during the pre-implantation
stage (days 1-7 of gestation). A slight degree of internal
hydrocephalus was observed with doses of 135 and 270 mg/kg on days
8-16 of gestation.
Using a similar treatment regimen, Ivanova-Chemishanska et al.
(1975) reported similar changes. No embryotoxic or teratogenic effect
was seen at a dose level of 10 mg/kg body weight given daily to rats
throughout the pregnancy (Medved, 1977; Panshina, 1985).
Teratogenic effects of technical propachlor were assessed in
pregnant Charles River COBS CD rats using a control and three
treatment groups consisting each of 25 females (Schardein & Wahlberg,
1982). Propachlor (96.5% pure) levels of 20, 60 and 200 mg/kg body
weight were administered orally by gavage as a single daily dose on
days 6-19 of gestation in a constant volume of 10 ml/kg corn oil. The
control group received the same dose of corn oil. Survival in the
control, low-dose and mid-dose groups was 100%. One female in the
high-dose group died on gestation day 18 due to an intubation error.
There were no differences in mean maternal body weight gain. No
changes were evident at gross necropsy that could be considered
treatment related. There were no differences between treated and
control groups with respect to the mean number of viable fetuses,
post-implantations loss, total implantations, corpora lutea, fetal
body weights, sex ratio distribution, and in the number of litters or
fetuses with external, visceral or skeletal malformations and/or
developmental and genetic variations.
7.4.2.2 Mice
When Balb/c mice (8-12 in each test group and 18 control pregnant
females) were treated with a single dose of 675 mg propachlor/kg body
weight on each of days 8-13 of gestation, and at dose levels of 33.7,
67.5, 135 and 270 mg/kg body weight per day from days 1 to 21, a
significant increase in post-implantational lethality and reduced
fetal weight and cranio-caudal size were observed at all dose
regimens. With both single and repeated doses, there was a
statistically significant increase in the frequency of internal
hydrocephalus, but only at a dose level of 675 mg/kg in a single
application on days 9 and 11 of gestation and on daily application at
270 mg/kg from days 1 to 18 of pregnancy (Mirkova, 1975).
7.4.2.3 Rabbits
Pregnant New Zealand White rabbits randomly assigned to a control
and three treatment groups (each consisting of 16 rabbits) were used
to determine the teratogenic potential of propachlor (95.6% pure)
(Schardein, 1982). Dose levels of 5, 15 and 50 mg/kg were administered
orally by gavage as a single daily dose on days 7-19 of gestation at
a constant volume (0.5 ml/kg dissolved in corn oil). The control group
received only 0.5 ml/kg corn oil. On gestation day 29, the number and
location of viable and non-viable fetuses, early and late resorptions,
number of total implantations and the number of corpora lutea were
recorded. All fetuses were examined for external, soft tissue and
skeletal malformations and for genetic or developmental variations.
Mortality (between 3 and 26 days of gestation) was recorded in
the control as well as the treated groups, i.e. two in the control
group, three at 5 mg/kg, three at 15 mg/kg and two at 50 mg/kg. Two
rabbits from the low-dose group and one from the mid-dose group
aborted on gestation days 22 to 25. Ante-mortem observations gave no
indication of a treatment-related effect at any dosage level.
Results of the reproductive parameters revealed slight to
moderate increases in the number of post-implantation losses and the
number of early resorptions in the mid- and high-dose groups when
compared with controls (Table 13). Single animals with resorptions
only were also found at the two highest dose levels. This resulted in
a subsequent decrease in the average number of viable fetuses in the
mid- and high-dose groups when compared with controls. Although the
number of post-implantation losses fell within the range of historical
control frequencies, all the above parameters were considered to imply
a mild embryotoxic response. No effects were observed in the number of
late resorptions, total implantations, number of corpora lutea, fetal
sex distribution or the mean fetal body weights. Examination of the
fetuses revealed no external, skeletal or soft tissue malformations,
or genetic and developmental variations that could be considered to be
related to treatment. These abnormalities observed were low in
frequency and within the range of historical control values. A
statistically significant increase in the total number of litters with
malformations was observed in the mid-dose group when compared to the
control group. This trend, however, was not found in the high-dose
group and the malformations in the group given 15 mg/kg per day were
considered to be incidental to treatment.
Table 13. Mean number of resorptions (late and early),
post-implantation losses and total implantations in
New Zealand rabbits (Schardein, 1982)
Dose Fetuses Resorptions Post-implantation Total
(mg/kg) viable non-viable late early losses implantation
0 8.0 0.0 0.3 0.3 0.6 8.6
5 7.9 0.0 0.0 0.4 0.4 8.3
15 5.2a 0.1 0.0 1.4 1.4 6.6
50 5.9 0.0 0.2 0.9 1.1 7.0
a P < 0.05
7.5 Mutagenicity and related end-points
Appraisal
Many in vitro and in vivo studies on the genotoxicity of
propachlor have been reported. It has been found to be clastogenic
in in vivo lower test systems and plant assays. It is cytotoxic
and also shows a weakly positive clastogenic response in in vitro
mammalian tests, inducing chromosomal aberrations in Chinese hamster
ovary cells. Variable results do not allow any definite conclusions
to be drawn. However, on the basis of negative results in many in
vivo assays in mammalian test systems, the available experimental
data provide insufficient evidence of a mutagenic potential. Data
on mutagenicity testing are summarized in Table 14.
7.5.1 Bacterial test systems
Salmonella mutation assays using standard tester strains
(TA1535; TA1538; TA98 and TA100), both with and without metabolic
activation with rat liver S9, were negative for formulations and
technical grades of propachlor (Plewa et al., 1984; Flowers, 1984).
No mutagenicity was observed with extracts of plants treated with
propachlor.
Negatives results for propachlor in spot tests using Salmonella
typhimurium test strains were also reported by Njagi & Gopalan
(1980) and Eisenbeis et al. (1981).
Table 14. Summary of mutagenicity testing
Test materiala Test system Resultc Reference
-S9 +S9 1S
Gene mutation in Prokaryotes
Propachlor U Ames spot test - Njagi & Gopalan (1980)
Propachlor U Ames spot test - - Eisenbeis et al. (1981)
Propachlor T Ames plate test - - Flowers (1984)
Propachlor T Ames plate test - - -
Propachlor + Cyanazineb Ames plate test + Plewa et al. (1984)
Gene mutation in plants
Propachlor C maize Wx locus - Plewa et al. (1984)
Gene mutation in mammals ( in vitro)
Propachlor T CHO/HGPRT - - Flowers (1985)
Chromosome effects in plants
Propachlor U Vicia faba cytogenetics + Njagi & Gopalan (1981)
Propachlor U maize cytogenetics + Lapina et al. (1984)
Propachlor U maize chlorophyll mutations + Lapina et al. (1984)
Chromosome effects in mammals ( in vitro)
Propachlor T CHO cytogenetics - + Li & Meyers (1987)
Chromosome effects in mammals ( in vivo)
Propachlor U in vivo mouse bone marrow cyt. + Pilinskaya et al. (1980)
Propachlor T in vivo rat bone marrow cyt. - Ernst & Blazak (1985)
Table 14 (contd).
Test materiala Test system Resultc Reference
-S9 +S9 1S
DNA damage/recombination in yeast
Propachlor T gene conversion - + Gentile et al. (1977)
Propachlor T gene conversion - - + Plewa et al. (1984)
Propachlor C gene conversion - - + Plewa et al. (1984)
Propachlor + Cyanazine gene conversion - Plewa et al. (1984)
DNA damage in mammals ( in vitro)
Propachlor T in vitro hepatocyte UDS - Steinmetz & Mirsalis (1984)
Propachlor T in vivo/in vitro hepatocyte UDS - Steinmetz & Mirsalis (1986)
a T = technical grade; C = commercial grade; U = unspecified grade
b Propachlor and cyanazine were both of commercial grade.
c +S9 and -S9 = with or without rat liver S9 activation mixture;
1S = with maize 1S fraction
7.5.2 Yeast assays
Propachlor was not recombinogenic when tested at 1000 mg/litre in
Saccharomyces cerevisae strain D4 (ade 2-1, ade 2-2; trp 5-12, trp
5-27) either with or without a liver microsomal activation system
(Gentille et al., 1977).
Extracts of plants treated with propachlor cause a weak
recombinogenic activity (Gentille et al., 1977). Maize extracts
treated with 25 ppm propachlor increased the rate of mitotic gene
conversion at the ade locus in S. cerevisae strain D4 approximately
4-fold over the control levels. Extracts of plants treated with a
technical grade of propachlor (1.306 x 10-4 mol/litre) and
formulated grade (1.306 x 10-3 mol/litre) induced a significant
increase in gene conversion at the ade locus and a lower increase at
the trp locus in S. cerevisae D4. Results for both formulated and
technical grades of propachlor were negative after mammalian S9
activation (Plewa et al., 1984).
7.5.3 Plant assays
Njagi & Gopalan (1981) studied the cytogenic and cytological
effects of propachlor at a wide range of concentrations (10, 50, 100,
500, 1000, 5000 and 10 000 ppm) in root-tip meristematic cells of
Vicia faba. At a concentration of 100 ppm, propachlor induced,
within 2 h, chromosomal aberrations, such as anaphase bridge
formation, micronuclei as well as inhibition of mitosis, formation of
highly pycnotic nuclei and premature chromosome condensation. The
mutagenic properties of propachlor were not detectable in the field
experiments using reverse mutations at the wx-C locus in maize as the
genetic end-point. Lapina et al. (1984) also reported that propachlor
induced mitotic chromosomal aberrations in the root-tip meristematic
cells of an inbred F2 strain of maize. At a concentration of 10-3
mol/litre and within 2 h of treatment of maize grains, propachlor
increased the rate of mitotic chromosomal aberrations more than 4-fold
over the control levels. The main types of cytogenetic damage included
single bridges (72%) and fragments (28%). Propachlor did not show
evidence of being significantly cytotoxic.
7.5.4 Cultured mammalian cell CHO/HGPRT assay
Propachlor (96.1%) was tested in a gene mutation assay in
cultured mammalian cells (CHO/HGPRT assay), both in the presence and
absence of mammalian metabolic activation, at doses ranging from 10-60
mg/litre. It was found to be cytotoxic at some of the higher
concentrations tested, but no mutagenicity was observed (Flowers,
1985).
7.5.5 In vitro unscheduled DNA synthesis in primary rat hepatocyte
cultures
Steinmetz & Mirsalis (1986) studied the potential of propachlor
to induce unscheduled DNA synthesis (UDS) in primary rat hepatocyte
cultures. They used ten concentrations of propachlor (95.8% pure)
ranging from 0.1 to 5000 mg/litre and, in another assay, five
concentrations ranging from 0.1 to 50 mg/litre. Results of this study
showed a cytotoxic effect of propachlor at concentrations of 50
mg/litre or more, but there was no evidence of UDS induction.
7.5.6 In vitro test for induction of chromosomal aberrations using
Chinese hamster ovary cells
Propachlor was tested for its potential to induce chromosomal
aberrations in cultured Chinese hamster ovary (CHO) cells treated with
propachlor (97.4% pure) at concentrations of 5, 10 or 15 mg/litre for
5 h both in the presence and absence of exogenous activation (Li &
Meyers, 1987). As indicated both by decrease in mitotic index and
lengthening of average cell generation times, the highest treatment
level was cytotoxic both in the presence and absence of activation. In
the non-activated study, no statistically significant increases in the
percentage of cells with structural aberrations or in average
structural aberrations per cell were observed at any treatment level.
In the study with S9 activation (15 mg propachlor/litre), the average
number of aberrations per cell was statistically elevated at 12 h and
24 h (P < 0.05 test). Chromatid and chromosome gaps were not
included. The percentage of cells with structural aberrations
following treatment at 15 mg/litre was statistically elevated at 12 h
but not at 24 h. No apparent dose-response relationship was observed
at either harvest time. Under these conditions, propachlor was
considered to be negative in the assay without exogenous metabolic
activation and a weak clastogen with activation.
7.5.7 In vivo rat bone marrow cytogenetic assay
Ernst & Blazak (1985) conducted an in vivo rat bone marrow
cytogenetic assay using technical grade propachlor (95.6% pure) at
dose levels of 0, 0.05, 0.2 and 1 mg/kg body weight. The results were
evaluated for mitotic index (based on at least 1000 cells per animal)
and 60 cells per animal were examined for chromosomal aberrations.
Chromatid and isochromatid gaps were recorded for each cell, but these
were not considered chromosomal aberrations. The results showed that
propachlor does not induce chromosomal damage in male or female
Fischer-344 rats under the conditions used in this study.
7.5.8 Acute in vivo mouse bone marrow cytogenicity assay
Pilinskaya et al. (1980) studied the cytogenetic effect of
propachlor on the bone marrow of mice treated orally with 10, 50 and
100 mg/kg body weight. The metaphase frequency of aberration at the
lowest dose applied was 2.67 ± 0.66 compared with 0.7 ± 0.19 in the
control. At this dose level an approximately 4-fold increase in the
frequency of chromosome aberrations was observed. No statistically
significant changes were found at a dose level of 1 mg/kg.
7.5.9 In vivo/in vitro hepatocyte DNA repair assay
Steinmetz & Mirsalis (1986) presented data on the in vivo/in
vitro hepatocyte DNA repair assay. Propachlor (97.7% pure) was
suspended in corn oil and administered by gavage to five groups of
male Fischer-344 rats at dose levels of 25, 250, 300, 400 and 1000
mg/kg. A positive control group received 2-acetyl aminofluorene
(2-AAF) (50 mg/kg) while a negative one was given corn oil. The
results were negative.
7.6 Long-term toxicity and oncogenicity studies
7.6.1 Rat
Propachlor (96% pure) was administered via the diet to four
groups of 60 male and 60 female Charles River CD SDBR rats at
concentrations of 0, 10, 50 and 500 mg/kg (equivalent to 0, 0.5, 2.6
and 27 mg/kg body weight) (Hamada, 1987a). A complete battery of
haematological, clinical chemistry and urinalysis tests was carried
out every 6 months for 2 years. No treatment-related effects were
noted on survival, incidence of clinical and ophthalmoscopic
observations, body weight gain or food consumption. Haematological and
clinical chemistry parameters and urinalysis data of treated animals
were comparable to control values. Thyroid and liver weights were
slightly increased in high-dose males at the 12-month interim
sacrifice but not at the end of the study. An increased incidence of
liver changes (centrilobular hypertrophy, clear cell cytoplasmic
alteration and eosinophilic cytoplasmic alteration) was observed in
high-dose males and females. A slightly higher incidence of thyroid
c-cell tumours (adenomas and carcinomas) noted in high-dose animals
was within the testing laboratories' historical range for such
tumours. All other organ weight changes and gross and microscopic
pathological findings were comparable to controls. An increase in
benign granulosa/theca cell tumours was observed for high-dose females
(4/60) compared to controls (0/60). The historical control data for
this tumour ranged from 0/80 to 4/96. On the basis of these results,
50 mg/kg diet (2.6 mg/kg body weight) can be considered the
no-observed-effect level (NOEL) for rats.
7.6.2 Mouse
When propachlor (96.1% pure) was administered in the diet to male
and female CD1 mice at dose levels of 10, 50 and 500 mg/kg (equivalent
to 1.6, 8.1 and 81.3 mg/kg for males and 2.0, 10.5 and 104.9 mg/kg for
females) for 18 months, no histological changes were noted (Hamada et
al., 1987b). The survival of all treated male groups was similar to
that of controls. Female survival was lower than that of controls in
all treated groups. There was a significant increase in segmented
neutrophilic leucocytes in the 500-mg/kg group at 12 months, but this
change was not evident at study termination. At the end of the study,
ratio of liver (with gall bladder) to body weight was increased in the
50- and 500-mg/kg group females, and the ratio of kidney to body
weight was decreased in males of the highest-dose group. The NOEL was
considered to be 10 mg/kg (1.6 mg/kg per day).
7.6.3 Dog
In a study by Naylor & Ruecker (1986), propachlor was
administered daily via the diet to four groups of six male and six
female beagle dogs for 12 months (0, 25, 250 and 1000 mg/kg diet). No
mortality occurred during the study. Absolute body weights were lower
than those of controls at study termination (approximately 14% for
males and 8% for females in the high-dose group and 5% for males in
the mid-dose group. A slight increase in emesis and/or stool change
was noted in treated dogs. No other changes in clinical and
pathological parameters were observed that could be related to
treatment. Based on the body weight depression at the high dose, a
dietary level of 250 mg/kg (9 mg/kg body weight) was considered a
no-observed-adverse-effect level (NOAEL) in this chronic study.
7.7 Miscellaneous studies
Propachlor has a strong inhibitory effect on the proliferation of
L1210 mouse leukaemia cells in vitro (the ID50 for cell
proliferation is < 3 x 10-7 mol/litre). Propachlor also inhibits
significantly the uptake of leucine, thymidine and uridine. The
inhibitory effect of propachlor is largely reversible, i.e. cells
grown in propachlor and then washed free of the compound return to an
almost normal rate of proliferation (Zilkah et al., 1981).
It has been demonstrated that propachlor treatment also causes
L1210 cells to accumulate in the G1 phase. This effect is dose
dependent; a concentration of 10 µmol/litre causes more than 90% of
cells to accumulate in the G1 phase (Zilkah et al., 1985).
The combined effect of propachlor and vibration has been studied.
Propachlor was given orally to Wistar rats (20 animals in each group)
at doses equivalent to 6, 12 and 60 mg/kg body weight per day for 3
months, and the animals were exposed to low-frequency vibration (15
Hz) during the last 30 days of the study. The results were compared
with those obtained from groups of rats subjected to the effect of 15
Hz vibration daily for 30 days, as well as with those of groups of
rats dosed only with propachlor. It was found that the combined
exposure (with 12 or 60 mg propachlor/kg) caused more severe
haemodynamic alterations and degenerative (even necrotic) changes in
the epithelial cells of the renal tubules, either in the proximal or
distal convoluted tubules (Maleva & Zlateva, 1982).
In a study by Baynova et al. (1978b), groups of 20 white rats
were pretreated with oral doses of 4 ml/kg 40% ethanol (one tenth of
the LD50), 6 days per week, for 4 months. Propachlor was then given
daily at a dose level of 140 mg/kg (one tenth of the LD50), 6 days
per week, every other week for a further 4-month period. The controls
were subjected to 8 months of treatment with ethanol and 4 months of
intermittent (every second week) treatment with propachlor using the
same doses as in the combined experiment. Both experiments had
untreated control groups. The combined treatment led to a reduction of
the hepatotoxic effect. This was probably due to induction of
mixed-function oxidases and to a more rapid biotransformation in the
hepatocytes. It has also been found that propachlor (140 mg/kg for 6
days) reduces the hexabarbital sleeping time by speeding up its
metabolism in the hepatic endoplasmic reticulum as a result of
induction of the mixed-function oxidase system in the liver cell
microsomes (Nenov & Baynova, 1978).
8. EFFECTS ON HUMANS
8.1 Occupational exposure
There have been few reports dealing with the effects of
propachlor on humans.
Von Schubert (1979) reported a case of contact eczema on the
palms, wrists and forearms of a 29-year-old agricultural worker who
had been in contact with propachlor for 8 days. Once this contact
ceased, the skin lesions disappeared.
Iden & Schroeter (1977) patch-tested 17 patients, predomi-nantly
farmers, who had been exposed simultaneously to many types of
herbicides. Seven showed a positive patch test reaction and five
others had an irritant reaction to propachlor. One of these farmers,
who was highly sensitive to propachlor, used to develop generalized
airborne contact dermatitis each spring when his neighbours used this
product. Farkasdy et al. (1976) and Dombay & Farkasdy (1978) examined
dermatologically 79 workers manufacturing Satecid 65 WP (65%
propachlor). Of these, 19% showed contact dermatitis attributed to
propachlor exposure. Patch testing was carried out on 67 workers of
this group, 12 of whom showed monovalent and bivalent hypersensitivity
reaction to propachlor. Photosensitization tests were negative in 28
of the cases tested. Three years later, the same authors examined 108
workers who were continuously exposed to Satecid in the same factory
without finding any sign of sensitization.
Jung et al. (1989) patch-tested 19 allergic cases and one
irritative case with occupational contact eczema, who were exposed to
different types of herbicides. Two of them showed positive testing to
Satecid 65 WP. One of these two was only exposed to the substance for
10 days and the other for 6 months.
8.2 General population exposure
No reports on general population exposure are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Soil
The effects of propachlor with regard to changes in numbers of
four types of soil bacteria (aerobic nitrogen-fixing, aerobic
cellulose-decomposing, ammonifying and nitrifying) was studied by
Helmeczi (1977). The studies were carried out using pseudomycelial
chernozem soil with maize plants. For the purpose of the microbial
examinations, samples from the field were taken three times a year
(after spraying propachlor in spring, summer, and autumn at a rate of
8-10 kg/ha) from the upper 20-cm layer of the soil. Sampling was
carried out under sterile conditions and samples were collected in
sterile vessels. The nitrifying bacteria had the greatest sensitivity
to propachlor, their number decreasing considerably from 2720 to 610
per g soil in 1974 and from 4510 to 1920 per g soil in 1975. The
aerobic cellulose-decomposing bacteria were the least sensitive, their
numbers, with respect to the control bacteria, increasing to a small
extent.
Long-term studies in smolnista and alluvial soils have been
carried out to establish the effects of propachlor on useful soil
microorganisms and the processes of nitrification (Bakalivanov &
Kostov, 1981). These studies were conducted under laboratory
conditions at 20 °C and 60-70% soil moisture for a period of 10 days.
The concentration of propachlor used was 80 mg/kg soil. The results
showed an inhibitory effect on nitrification and weaker effect on soil
microorganisms (bacteria, actinomycetes and microscopic fungi). High
adsorption of propachlor to clay minerals reduced the toxic effect on
microorganisms.
Rankov & Velev (1976) studied the effect of propachlor on the
biological activity of soil microorganisms and showed that inhibition
was greater at a temperature of 2 to 4 °C than at 20 to 22 °C.
9.1.2 Water
The acute toxicity of propachlor to Selenastrum capricornutum
Printz has been assessed in two studies. In the first (Richards &
Kaiser, 1984), the 96-h EC50 for growth in algae was calculated to
be 0.029 mg/litre with 95% confidence intervals of 0.021-0.038
mg/litre. The no-observed-effect concentration (NOEC) was calculated
to be 0.01 mg/litre. In the second study (Zschaler & Jonas, 1990), the
72-h EC50 for growth was 5.3 mg/litre and the NOEC 1.9 mg/litre for
a propachlor formulation containing 65% active ingredient. The
differences in toxicity observed between the two studies may be either
because the 65% formulation is less toxic than technical propachlor or
because the duration of the second study was 24 h shorter.
9.2 Aquatic organisms
9.2.1 Aquatic invertebrates
Thompson & Forbes (1979a) determined a 24-h LC50 of 11 mg/litre
and a 48-h LC50 of 7.8 mg/litre for the water flea Daphnia magna.
The NOEC in this study was < 5.6 mg/litre. A 48-h LC50 of 6.9
mg/litre in the same species was reported by Mayer & Ellersieck
(1986). The same authors reported a 48-h LC50 of 0.79 mg/litre for
the midge larva Chironomus plumosus. Another midge larva Chironomus
riparius was reported to show a 24-h LC50 of 5.2 mg/litre and a
48-h LC50 of 1.8 mg/litre (Buhl & Faerber, 1989).
The effects of propachlor on daphnia reproduction was assessed in
a 21-day life-cycle study (Thun, 1990a). In this study, there was
decreased reproductive performance at concentrations from 0.29 to 2.6
mg/litre; the NOEC for reproduction was considered to be 0.097
mg/litre.
9.2.2 Fish
Thompson & Forbes (1979b) determined 24-h, 48-h and 96-h LC50
values for rainbow trout ( Onchorynchus mykiss) of 0.75, 0.28 and
0.17 mg/litre, respectively. Thun (1990b) conducted a 21-day study on
rainbow trout and found that fish died at concentrations between 0.11
and 0.3 mg/litre. No deaths related to exposure occurred at
concentrations between 0.009 and 0.075 mg/litre. The NOEC was
considered to be 0.019 mg/litre. Mayer & Ellersieck (1986) reported a
96-h LC50 of 0.23 mg/litre for the channel catfish (Channa
punctatus).
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The toxicity of propachlor to earthworms was assessed in a 14-day
study (Thun et al., 1991). Propachlor (97.8% pure) was added to the
soil at five concentrations ranging from 100 to 1000 mg/kg, and 40
worms were added to the soil at each concentration. Worms were removed
from the soil at 7 and 14 days and examined for any adverse effects.
Body weights were measured at the beginning and end of the test. The
LC100 was 560 mg/kg and the LC0 218 mg/kg; the NOEC was 100 mg/kg.
The contact LD50 of propachlor for honey bees was reported to
be 311 µg/bee (Kenaga, 1979).
Under laboratory conditions, Tanke & Franz (1978) studied the
side effects of propachlor on some beneficial insects by measuring the
reduction of the beneficial capacity of three enthomophagous insects.
The egg parasite Trichogramma cacoeciae March reacted very strongly
in laboratory as well as in field conditions to the effects of the
herbicide. Contact toxicity tested using residues of propachlor on
glass plates (residue level not stated) caused a reduction of the
degree of parasitization leading to total mortality of the population
in contaminated cages. In a study on the contact toxicity of the spray
deposit on single leaves as well as on whole plants, the
parasitization of Trichogramma was reduced by 100 and 83%,
respectively. The direct toxic effect should be distinguished from a
repellent effect, which is probably more important in the field. In
addition to this direct contact effect of spray deposits, systemic
application also caused an effect after transport of the herbicide
through the soil and the plant. This was demonstrated by a reduction
of the degree of parasitization compared with untreated controls.
Propachlor does not seem to have any influence on Chysopa carnea
Steph larvae. No effect was visible either in tests of contact
toxicity or contaminated sandy soil, in choice studies on a repellent
action of the residue or after topical application of the preparation.
After oral application through an artificial food chain, no influence
could be demonstrated. Only overdosage increased the mortality rate of
the test larvae. No repellent effect of the herbicide on adults was
demonstrated (Tanke & Franz, 1978).
The syrphid Epistrophe balteata DeG was more sensitive. Both in
contact toxicity studies and in studies for a possible repellent
effect, an influence of propachlor on the larval stages was shown.
Oral intake through an artificial food chain did not have an effect on
the larvae. When the herbicide was sprayed directly on the plant, an
increase in larval mortality was observed. Adults of this syrphid also
showed reactions to herbicides; during egg deposition, females avoided
surfaces previously treated (Tanke & Franz, 1978).
The potential toxicity of a 65% formulation of propachlor to
Aleochara bilineta Gyll was assessed by Pietrzik (1991). The imagos
were dug into moist sand and the test substance was added at
recommended use rates (8 kg in 400 litres/ha water). For the
assessment of parasitical capacity, pupae of Delia antiqua were dug
into the sand. At the termination of the test, the sum of hatched
Aleochara larvae in the pupae was determined. These were compared to
the number of control organisms treated with tap water. The parasitic
capacity of the test beetles was decreased by 11.4% when compared to
controls.
9.3.2 Birds
Palazzolo (1964) reported data for pheasants indicating an acute
oral LD50 of 735 mg/kg body weight. Increased respiration, loss of
reflexes, mydriasis, salivation and intermittent tonic/clonic
convulsions were found among birds receiving dose levels of 900 and
1350 mg/kg. The onset of symptoms occurred approximately 15 min
following dosing and persisted until death 3-5 h later. Kenaga (1979)
reported an LD50 of 512 mg/kg for Mallard duck and Beavers & Fink
(1979) reported an LD50 for Bobwhite quail of 137 mg/kg body weight
for a 65% propachlor formulation.
When propachlor was administered in the diet to quail or Mallard
ducks for five consecutive days, the LC50 for both species of birds
was more than 5620 mg/kg diet (Beavers & Fink, 1983a,b). For the
quail, a dietary level of 5620 mg/kg is approximately equivalent to a
dose of 1400 mg/kg per day. The data presented above indicate that the
quail is more susceptible to exposure to propachlor via stomach tube
than via the diet.
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1 Conclusions
* Under conditions of normal use, the general population is not
likely to be exposed to propachlor.
* Those occupationally exposed to propachlor should take adequate
safety and hygienic precautions in order to protect the skin, eye
and respiratory tract.
* Propachlor is rapidly degraded in the environment under most
conditions. It persists longer in cold dry environments. The
conjugated N-isopropylaniline metabolite persists longer than
the parent compound. Propachlor does not bioconcentrate or
biomagnify.
* Propachlor is highly toxic to some aquatic organisms. Exposure of
aquatic organisms under conditions of normal use is low, the
maximum expected concentrations being several orders of magnitude
lower than the no-observed-effect concentrations. Direct
contamination of water courses will kill aquatic organisms and
should be avoided. Propachlor poses a low hazard to birds,
earthworms and honey-bees.
10.2 Recommendations for protection of human health
Workers should be educated about the hazards of propachlor and
systematically trained to practice safety and personal hygiene and to
use protective equipment.
11. FURTHER RESEARCH
* The results of the existing animal studies on mutagenicity are
inconclusive and more research is needed.
* Studies should be performed in laboratory animals to determine
the potential neurotoxic effects of propachlor.
* Only validated analytical methods for residues of propachlor
should be used.
* Epidemiological studies on occupationally exposed workers are
needed.
* There is a need to develop methods of biological monitoring for
evaluating human exposure to propachlor.
* Research is needed to clarify the exposure of workers in the
production and agricultural use of propachlor. Studies should
also include examination of health effects at the measured
exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO recommended classification of pesticides by hazard,
technical propachlor is classified in Class III as slightly hazardous
in normal use (WHO, 1990). A data sheet on propachlor has been issued
(WHO/FAO, 1989).
Neither the FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
nor the International Agency for Research on Cancer (IARC) has so far
evaluated propachlor.
REFERENCES
Aschbacher PW & Struble CB (1987) Evidence for involvement of
non-biliary excretion into the intestine of the formation of
methyl-sulphonyl containing metabolites of 2-chloro- N-
isopropylacetanilide (propachlor) by swine and rats. Xenobiotica,
13(2): 115-126.
Auletta CS (1983) A propachlor dermal sensitization study in
guinea-pigs (Project No. 4236-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS (1984) Eye irritation study with propachlor in rabbits
(Project No. 5050-84). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Auletta CS & Rinehart WE (1979) Acute dermal toxicity study in rabbits
(Project No. 4892-77). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
Agricultural Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984a) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4946-84). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984b) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4540-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Bakalivanov D & Kostov O (1981) Soil microbiological assessment of
toxicity of alkanoate, amide, and other herbicides. Acta microb Acad
Sci Hung, 28: 141-146.
Baker JL (1980) Agricultural areas as non-point sources of pollution.
In: Overcash MR & Davidson JM ed. Environmental impact of non-point
source pollution. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 275-310.
Baker JL & Laflen JM (1979) Run-off losses of surface applied
herbicides as affected by wheel tracks and incorporation. J Environ
Qual, 8(4): 602-607.
Baker JL, Laflen JM, & Hartwig RO (1982) Effects of corn residue and
herbicide placement on herbicide run-off losses. Trans Am Soc Anal
Eng, 25(2): 340-343.
Bakke JE, Gustafsson JA, & Gustafsson BE (1980) Metabolism of
propachlor by the germ-free rat. Science, 210: 433-435.
Bakke JE & Price CE (1979) Metabolism of 2-chloro-N-
isopropylacetanilide (propachlor) in the rat. J Environ Sci Health,
B14(4): 427-441.
Bakke JE, Davison KL, & Larsen GL (1990) Evidence for the absence of
cysteine S-conjugate N-acetyltransferase activity in the metabolism of
propachlor, naphthalene, and dichlobenil in calves. Xenobiotica, 20:
801-807.
Bakke JE, Rafter J, Larsen GL, Gustafsson JA, & Gustafsson BE (1981a)
Enterohepatic circulation of the mercapturic acid and cysteine
conjugates of propachlor. Drug Metab Dispos, 9(6): 525-528.
Bakke JE, Rafter J, Lindeskog P, Feil V, Gustafsson JA, & Gustafsson
BE (1981b) Metabolism of 2-acetamido-4-(chlormethyl) thioazole in
germ-free and conventional rats. Biochem Pharmacol, 30(13): 1831-1844.
Bakke JE, Larsen GL, Aschbacher PW, Rafter J, & Gustafsson JA (1981c)
Role of gut microflora in metabolism of glutathione conjugates of
xenobiotics. In: Rosen JD, Magee PhS, & Casida JE ed. Sulfur in
pesticide action and metabolism. Washington, DC, American Chemical
Society, pp 165-178 (ACS Symposium Series No. 158).
Balinova AM (1981) Investigations in the residues of some herbicides
in plant production in view of its hygienic evaluation. Sofia,
University of Sofia (Dissertation).
Balinova A & Konstantinov K (1975) Investigations on residues of
several herbicides in vegetables. In: Proceedings of the COMECOM
Symposium on Residues of Herbicides in Plants and Soils, Vrozlav,
1973, pp 111-116.
Barrows ME & Macek KJ (1974) Exposure of fish to 14C-RamrodTM:
Accumulation, distribution and elimination of 14C-residues. Wareham,
Massachusetts, Bionomics, E.G. & G. Inc., 18 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Baynova A, Burkova T, & Michailova A (1977) [Determination of dermal
toxicity of Ramrod.] Gig Zdraveopazvane, 20(3): 234-240 (in Russian).
Baynova A, Burkova T, & Michailova A (1978a) [Comparative study on
intermittent and monotonous effects of the herbicide Ramrod.] Gig
Zdraveopazvane, 21: 568-573 (in Russian).
Baynova A, Krastev L, Dikova M, & Michailova JA (1978b) Effect of
alcohol on acute and chronic intoxication with the herbicide Ramrod.
Acta med Bulg, 2: 36-41.
Beavers JB & Fink R (1979) Acute oral LD50-Bobwhite Quail.
Propachlor report No. WL-79-026. Easton, Maryland, Wildlife
International Ltd (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beavers JB & Fink R (1983a) Eight-day dietary LC50 - Bobwhite Quail
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-90, submitted to WHO by Monsanto
International Services, Brussels).
Beavers JB & Fink (1983b) Eight-day dietary LC50 - Mallard Duck
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-89, submitted to WHO by Monsanto
International Services, Brussels).
Bechtel CL (1991) Acute inhalation study of propachlor Technical
(Project No. ML-91-2). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beestman GB & Deming JM (1974) Dissipation of acetanilide herbicides
from soils. Agron J, 66: 306-311.
Blaszcak DL (1988) A comparative acute oral toxicity study in
Sprague-Dawley and Fischer rats (Project No. 4359-87). East Millstone,
New Jersey, Bio/dynamics Inc., 37 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Bleeke MS, Carr KH, & Elliot RC (1987) Propachlor metabolism in the
laying hen (Project No. MSL-6217). St. Louis, Missouri, Monsanto
Agricultural Company, 134 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982a) Acute oral toxicity of
Ramrod(R) 4L flowable herbicide for rats (Project No. ML-81-182).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982b) Primary skin irritation of
Ramrod(R) 4L flowable herbicide to rabbits (Project No. 810096).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Braun WG (1979) Rabbit eye irritation study (Project No. 4889-77).
East Millstone, New Jersey, Bio/dynamics Inc., 4 pp (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
Braun WG & Rinehart E (1978) Acute dermal toxicity study in rabbits
(Project No. 4888-77). East Millstone, New Jersey, Bio/dynamics Inc.,
4 pp (Unpublished proprietary report, submitted to WHO by Monsanto
International Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979a) Primary dermal irritation
study in rabbits (Project No. 4890-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979b) Primary dermal irritation
study in rabbits (Project No. 4898-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979c) Primary dermal irritation
study in rabbits (Project No. 4894-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Brightwell BB & Rueppel ML (1976) Lasso(R) and Ramrod(R): physical
properties and their relationship to the environment. St. Louis,
Missouri, Monsanto Agricultural Company (Unpublished report No. 443,
submitted to WHO by Monsanto International Services, Brussels).
Brightwell BB, Malik JM, & Wong SC (1981) The environmental fate of
propachlor [2-chloro-N (1-methyl)-N-phenyl-acetamide] in soil and
water. St. Louis, Missouri, Monsanto Research Laboratory, 1300 pp
(Unpublished proprietary report No. MSL-1900, submitted to WHO by
Monsanto International Services, Brussels).
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides and
surfactants to larvae of the midge Chironomus riparius. Arch Environ
Contam Toxicol, 18: 530-536.
Caverly DJ & Denney RC (1978) Determination of substituted ureas and
some related herbicide residues in soils by gas chromatography.
Analyst, 103: 368-374.
Daly I & Knezevich A (1984) Four-week toxicity study in dogs
(Bio/dynamics Inc. Project No. 84-2798). Unpublished proprietary
report of Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels.
Davison KL (1991) Propachlor-s-glutathione metabolism by kidneys and
ureters of calves. J Anim Sci, 69: 1116-1121.
Davison KL, Bakke JE, & Larsen GL (1988) A kidney perfusion method for
metabolism. Studies with chickens using propachlor as a model.
Xenobiotica, 18(3): 323-329.
Davison KL, Bakke JE, & Larsen GL (1990) Metabolism of the glutathione
conjugate of propachlor by in situ perfused kidneys and livers of
rats. Xenobiotica, 20(4): 375-383.
Dombay Z & Farkasdy J (1978) [Problems of safety technique and of
occupational safety as related to propachlor and its formulations.]
In: [Conference on Safety Technology in the Application of
Agricultural Chemicals], Budapest, Technoinform, pp. 49-58 (in
German).
Eisenbeis SJ, Lynch DL, & Hampel AE (1981) The Ames mutagen assay
tested against herbicides and herbicide combinations. Soil Sci,
131(1): 44-46.
Ernst L & Blazak F (1985) An assessment of the mutagenic potential of
propachlor utilizing the acute in vivo rat bone marrow cytogenetics
assay (Project No. SR-84-180). Menlo Park, California, Stanford
Research Institute, 39 pp. (Unpublished proprietary report, submitted
to WHO by Monsanto International Services, Brussels).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
Farkasdy J, Horvath A, & Meszleri E (1976) [Occupational dermatitis
caused by the selective herbicide Satecid 65WP.] Borgyogy venerol Sz,
52: 112-118 (in German).
Feil VJ, Bakke JE, & Larsen GL (1981) A new metabolite of propachlor
isolated from germ-free rat excreta. Biomed Mass Spectrom, 8(1): 1-4.
Fletcher WW & Kirkwood RC (1982) Herbicide and plant growth
regulators. London, Toronto, Sydney, New York, Granada.
Flowers LJ (1984) Salmonella mutagenicity assay of propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 18 pp
(Unpublished proprietary report No. MSL-1694, submitted to WHO by
Monsanto International Services, Brussels).
Flowers LJ (1985) CHO/HGPRT gene mutation assay with propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 9 pp
(Unpublished proprietary report No. ML-84-237, submitted to WHO by
Monsanto International Services, Brussels).
Frank R, Sirons GJ, Paik NJ, & Valk M (1977) Fate of propachlor
applied to onions and organic soils. Can J Plant Sci, 57: 473-477.
Gentille JM, Wagner ED, & Plewa MJ (1977) The detection of weak
recombinogenic activities in the herbicides alachlor and propachlor
using a plant activation bioassay. Mutat Res, 48: 113-116.
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 8: 339-357.
Hamada NN (1987a) Combined chronic toxicity and oncogenicity study in
rats with propachlor (Study No. HL-83-350/241-169). Vienna, Virginia,
Hazleton Laboratories America Inc., 2452 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Hamada NN (1987b) Oncogenicity study with propachlor in mice (Study
No. HL-83-349/241-159). Vienna, Virginia, Hazleton Laboratories
America Inc., 520 pp (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Heenehan BS, Rinehart WE, & Braun WG (1979) Acute oral toxicity study
in rats (Project No. 1895-77) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Heise H, Mattheus A, & Pushkeiler TH (1983) [The irritant and allergic
effect of the herbicidal agent propachlor.] Z Gesamte Hyg, 29(11):
675-677 (in German).
Helmeczi B (1977) The effect of herbicides on soil bacteria belonging
to certain physiological groups. Acta phytopathol Acad Sci Hung,
12(1-2): 41-49.
Humburg NE, Colby SR, Lym RG, Prasad R, Hill ER, Kitchen LM, & McAvoy
WJ (1989) Herbicide handbook, 6th ed. Champaign, Illinois, The Weed
Science Society of America.
Iden DL & Schroeter AL (1977) Allergic contact dermatitis to
herbicides. Arch Dermatol, 113(7): 983.
Ivanova-Chemishanska L, Vergieva T, & Mirkova E (1975) Embryotoxic and
teratogenic effects of some pesticides. Exp Med Morfol, 14(1): 29-33.
Jung HD, Honemann W, Kloth C, Lubbe D, & Pambor M (1989) Contact
eczema caused by pesticides in East Germany. Dermatol Monatschrift,
175: 203-214.
Kaempfe BS (1991) Acute inhalation study of MON 15606 (Project No.
ML-91-48). Newstead, St Louis, Monsanto Company Environmental Health
Laboratory. (Unpublished proprietary report of Monsanto Agricultural
Company, submitted to WHO by Monsanto International Services,
Brussels).
Kenaga EE (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down Earth, 35(2): 25-31.
Kofman IS & Nishko P (1984) Determination of micro-quantities of
herbicides from a group of acetylanilides by thin-layer
chromatography. Physiol Biochem Cult Plant, 16(3): 290-292.
Kolesnikov VA, Abramova KA, & Grip OK (1979) Use of herbicides on
white cabbage. Khim Sel'skom Khoz, 17(10): 27-31.
Kolesnikov VA, Karetskaia NA, & Petuchova VI (1980) Efficacy of
herbicides in garlic crop. Khim Sel'skom Khoz, 18(8): 38-40.
Lamoureux GL & Davidson KL (1975) Mercapturic acid formation in the
metabolism of propachlor, CDAA and Flurodifen in the rat. Pestic
Biochem Physiol, 5: 497-506.
Lamoureux GL & Rusness DG (1989) Propachlor metabolism in soybean
plants, excised soybean tissues, and soil. Pestic Biochem Physiol, 34:
187-204.
Lamoureux GL, Stafford LE, & Tanaka FS (1971) Metabolism of
2-chloror- N-isopropyl-acetanilide (propachlor) in the leaves of
corn, sorghum, sugar cane, and barley. J Agric Food Chem, 19(2):
346-350.
Lapina TV, Grigorenko NV, Morgun V, Logvinenko VF, & Merjinskii JG
(1984) Study of the mutagenic effect of the herbicides Treflan,
Ramrod, and Banvel on corn. Tsitol i Genet, 18(2): 119-122.
Larsen GL & Bakke JE (1979) Studies on the origin of the
methylsulfonyl-containing metabolites from propachlor. J Environ Sci
Health, B14: 427.
Larsen GL & Bakke JE (1981) Enterohepatic circulation in formation of
propachlor (2-chloro-N-N-isopropylacetanilide) metabolites in the rat.
Xenobiotica, 11(7): 473-480.
Larsen GL & Bakke JE (1983) Metabolism of mercapturic acid-pathway
metabolites of 2-chloro- N-isopropylacetanilide (propachlor) by
gastrointestinal bacteria. Xenobiotica, 13(2): 115-126.
Larsen GL, Larsen JD, & Gustafson JA (1983) Cysteine conjugate
beta-lyase in the gastrointestinal bacterium Fusobacterium
necrophorum. Xenobiotica, 13(11): 689-700.
Lee JK, Minard RD, & Bollag JM (1982) Microbial metabolism of
propachlor in soil suspension. J Korean Agric Chem Saf, 25(1): 44-53.
Lehotzky K, Szeberenyl JMS, & Tatrai E (1979) On the local and
systematic toxic effects of the herbicide propachlor (Satecid 65WP) in
experimental animals. Egeszegtudomany, 23: 89-96.
Li AP & Meyers CA (1987) In vitro cytogenetics study of propachlor
(Study No. MSL-6939), 24 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Lottman CM & Cowell JE (1986) Propachlor residues in processed sorghum
grain applications following pre-emergent applications of Ramrod 4L
herbicide (Project No. MSL 6091) (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Lottman CM & Cowell JE (1987) Propachlor residues in processed corn
grain fractions following pre-emergent applications at Ramrod 4 h
herbicide (Study No. MSL-6260) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Magnusson B & Kligman AM (1969) The identification of allergens by
animal assay. The guinea-pig maximalization test. J Invest Dermatol,
52: 268-276.
Maleva E & Stereva M (1977) [Biochemical investigation on changes of
gonads of male white rats under combined action of low frequency
vibrations and intoxication with Ramrod. I. Changes in the content of
protein.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part 2], pp. 306-313 (in Bulgarian).
Maleva E & Zlateva M (1982) Histomorphological changes in kidneys of
experimental animals under the combined effect of low-frequency
vibration and intoxication with Ramrod herbicide. Hig Zdraveopazvane,
25(3): 244-249.
Malik JM (1982) Bioconcentration of propachlor in channel catfish
under static conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, 144 pp (Unpublished proprietary report No. 24242,
submitted to WHO by Monsanto International Services, Brussels).
Malik JM (1986) Metabolism of propachlor in rats (Project No. 7815).
St. Louis, Missouri, Monsanto Agricultural Company, Research
Department (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Markus JR & Puma B (1973) Pesticide analytical manual. Volume II:
Methods for individual pesticide residues. Springfield, Virginia, US
Department of Commerce, National Technical Information Service, pp
1-7.
Martin CD, Baker JL, Erbach DC, & Johnson HP (1978) Wash-off of
herbicides applied to corn residue. Trans Am Soc Anal Eng, 21(6):
1164-1168.
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Medved LI (1977) [Manual of pesticides.] Kiev, K. Urojai (in Russian).
Melnikov NN, Novojilov KV, Belen SP, & Pilova TN (1985) [Manual of
pesticides.] Moscow, M. Khimia (in Russian).
Menges RM & Tamez S (1973) Effect of soil incorporation on
selectivity, movement, and persistence of herbicides in onion
plantings. J Am Soc Hortic Sci, 98(4): 390-393.
Mirkova E (1975) Embryotoxic and teratogenic action of pesticides
Basfungin and Ramrod. Sofia, University of Sofia (Dissertation).
Molnar J & Paksy KA (1978) Acute inflammation of rat membranes evoked
by eye-instillation and inhalation of pesticide formulations
containing propachlor and TMTD. Egeszsegtudomany, 22: 393-397.
Nadeau RG & Pantano LK (1986) Metabolism study of synthetic
13C/14C-labelled plant metabolites of propachlor in lactating
goats. Part II: Identification characterization and quantification of
metabolites (Project No. MSL-5909), 119 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Naylor MW & Ruecker RW (1986) One-year dog study with propachlor
(Study No. ML-84-379). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Nenov P & Baynova A (1978) Ramrod (propachlor) effect on the activity
of rat hepatic microsomal enzymosystems. Acta med Bulg, 5(2): 44-52.
Nesterova OA, Popova VV, & Kolesnikova OP (1980) [Accumulation of
semeron, mesoranil, and Ramrod in the soil and cabbage during
long-term use.] Sibirski Vestn S-Kh Nauki, 10(4): 19-21 (in Russian).
Njagi GDE & Gopalan HNB (1980) Mutagenicity testing of some selected
food preservatives, herbicides, and insecticides. II. Ames test,
Bangladesh. J Bot, 9(2): 141-146.
Njagi GDE & Gopalan HNB (1981) Mutagenicity testing of herbicides,
fungicides, and insecticides. I. Chromosome aberrations in Vicia
faba. Cytologia, 46: 169-172.
Novick NJ & Alexander M (1985) Co-metabolism of low concentrations of
propachlor, alachlor, and cycloate in sewage and lake water. Appl
Environ Microbiol, April: 737-743.
Novick NJ, Mukherjee R, & Alexander M (1986) Metabolism of alachlor
and propachlor in suspensions of pretreated soil and in samples from
ground water aquifers. J Agric Food Chem, 34: 721-725.
Palazzolo RJ (1964) Acute oral toxicity study on CP 31393 in Golden
Ring-necked pheasants. Northbrook, Illinois, Industrial Biotest
Laboratories (Unpublished proprietary report No. BLT-64-3, submitted
to WHO by Monsanto International Services, Brussels).
Panshina TN (1973) [Materials about toxicology of new herbicide
Ramrod.] Gig Primen toksikol pestic klin otravlenni, 1973: 301-303 (in
Russian).
Panshina TN (1976) [Hygienic aspects of the use of certain herbicides
in agriculture and toxicology.] Gig i Sanit, 7: 38-41 (in Russian).
Panshina TN (1977) Establishment of maximum permissible concentrations
and approximately safe levels of action exerted by herbicides of
haloid-substituted carboxylic acid anilides in the air of a production
sector. Gig Tr Prof Zabol, 12: 30-33 (in Russian).
Panshina TN (1985) IRPTC scientific reviews of Soviet literature on
toxicity and hazards of chemicals No. 83: Propachlor (Ramrod). Moscow,
Centre of International Projects, GKNT, 19 pp.
Pantano LK & Anderson KS (1987) The metabolic fate of propachlor
[2-chloro-N-(1-methylethyl)-N-Phenylacetamide] in sorghum foliage and
grain (Project No. MSL-5412). St. Louis, Missouri, Monsanto
Agricultural Company (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Pekas JV, Larsen GL, & Feil VJ (1979) Propachlor detoxication in the
small intestine: cysteine conjugation. J Toxicol environ Health, 5:
653-662.
Pietrzik J (1991) [Laboratory determination of side effects of Ramrod
65WP on the short-winged beetle Aleochara bilineata Gyll (Project
No. 112/01-AB).] Niefern-Öchselbronn, Austria, GAB Biotechnologie GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels) (in
German).
Pilinskaya M, Kurinny AI, & Ivova TS (1980) [The primary evaluation of
cytogenetic activity and potential mutagenic hazard of 22 pesticides.]
Cytol Genet, 14(6): 41-46 (in Russian).
Plewa MJ, Wagner ED, Glenda G, & Gentille JM (1984) An evaluation of
the genotoxic properties of herbicides following plant and animal
activation. Mutat Res, 136: 233-245.
Pressley TA & Longbottom JE (1982) The determination of organochlorine
pesticides in industrial and municipal waste water. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental Monitoring and
Support Laboratory, Office of Research and Development.
Rafter JJ, Bakke J, Larsen G, Gustafson B, & Gustafson JA (1983a) Role
of the intestinal microflora in the formation of sulfur-containing
conjugates of xenobiotics. Rev Biochem Toxicol, 5: 387-408.
Rafter JJ, Gustafson JA, Bakke J, Larsen G, Norin KE, & Gustafson BE
(1983b) Studies on the re-establishment of the intestinal microflora
in germ-free rats with special reference to the metabolism of
N-isopropyl-alpha-chloracetanilide (propachlor). Xenobiotica, 13(3):
171-178.
Rankov V & Velev B (1976) Effect of temperature on the interaction
between the metribuzin and propachlor herbicides and soil
microorganisms. Agrokhimiya, 11(5): 100-105.
Rankov V & Velev B (1977) Detoxication of the herbicide propachlor by
certain microscopic fungi. Acta phytopathol Acad Sci Hung, 12(1-2):
67-72.
Rao KS (1981) Two-generation dietary reproduction study with
propachlor in rats (Study No. HET-K-077810(8)). Midland, Michigan, Dow
Chemical Company, Health and Environmental Science, Toxicology
Research Laboratory, 171 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Rejtö M, Saltzman S, & Acher AJ (1984) Photodecomposition of
propachlor. J Agric Food Chem, 32: 226-230.
Reyna MS & Ribelin WE (1984a) Three-month feeding study of propachlor
to albino rats (Study No. 83037). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Reyna MS & Ribelin WE (1984b) Three-month feeding study of propachlor
to albino mice (Study No. 820061). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Richards C & Kaiser FE (1984) Acute toxicity of propachlor (AB 83-367)
to Selenastrum capricornutum Printz. Columbia, Missouri, Analytical
Biochemistry Laboratories, 39 pp (Unpublished proprietary report No.
31348, submitted to WHO by Monsanto International Services, Brussels).
Ritter WP, Johnson HP, & Lovely WG (1973) Diffusion of atrazine,
propachlor, and diazinon in silt loam soil. Weed Sci, 5: 381-384.
Roberts HA, Walker A, & Bond W (1978) Persistence of chlorthaldimethyl
activity in soil. In: Proceedings of the British Crop Protection
Conference: Weeds. Croydon, British Crop Protection Council, pp 87-92.
Schardein JW (1982) Teratology study in rabbits. Mattawan, Michigan,
International Research and Development Corporation, 47 pp (Unpublished
proprietary report No. IR 81-224, submitted to WHO by Monsanto
International Services, Brussels).
Schardein JW & Wahlberg A (1982) Teratology study in rats. Mattawan,
Michigan, International Research and Development Corporation, 81 pp
(Unpublished proprietary report No. IR 81-264, submitted to WHO by
Monsanto International Services, Brussels).
Shirko ST & Belova VL (1982) [Level of Ramrod, semeron, and mesoranil
in the soil and cabbage plants in relation of dose of nitrogen and
potassium.] Khim Sel'skom Khoz, 20(I): 51-53 (in Russian).
Sjovall J, Rafter J, Larsen G, & Egestad B (1983) Lipophilic ion
exchange for group separation of conjugated metabolites of
xenobiotics. J Chromatogr, 276: 150-156.
Spalding RF & Snow DD (1989) Stream levels of agrochemicals during a
spring discharge event. Chemosphere, 19(8/9): 1129-1140.
Steen WC & Collette TW (1989) Microbial degradation of seven amides by
suspended bacterial populations. Appl Environ Microbiol, 55(10):
2545-2549.
Steinmetz L & Mirsalis C (1984) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the primary rat
hepatocyte cultures (Project No. LSC-7538). Menlo Park, California,
Stanford Research Institute, 8 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Steinmetz L & Mirsalis C (1986) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the in vivo- in
vitro rat hepatocyte DNA repair assay (Project No. LSC-2021). Menlo
Park, California, Stanford Research Institute, 37 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Stereva M & Maleva E (1977) [Biochemical studies on changes in gonads
of male white rats under combined action of low frequency vibrations
and intoxication with Ramrod. II. Dynamic in changes of 5-nucleotidase
activity.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part I], pp 133-139 (in Bulgarian).
Strateva A (1974a) [Hygienic-toxicologic study on Ramrod aiming its
hygienic standardization in water.] Sofia, University of Sofia
(Dissertation) (in Bulgarian).
Strateva A (1974b) [Amid preparations with herbicide action.
Experimental data on the acute oral toxicity of propachlor (Ramrod).]
Exp Med Morphol, 13(2): 123-129 (in Russian).
Strateva A (1975) [On some toxicologic properties of Ramrod and their
significance on its hygienic standardization in waters.] Hyg
Zdraveopazvane, 18(4): 401-405 (in Russian).
Strateva A (1976) [The experimental determination of MAC of Ramrod in
water.] Gig i Sanit, 4: 85-87 (in Russian).
Strateva A, Velisarov A, & Georgiev A (1974) [Morphological and
enzymohistochemical investigations on some internal organs of
experimental animals under the effect of Ramrod in acute experiment.]
Hyg Zdraveopazvane, 17(3): 283-286 (in Russian).
Struble CB (1991) In situ intestinal absorption of
2-chloro-N-isopropylacetanilide (propachlor) and non-biliary excretion
of metabolites into the intestinal tract of rats, pigs and chickens.
Xenobiotica, 21: 85-95.
Tanaka FS, Wien RG, & Mansager ER (1981) Survey for surfactant effects
on the photodegradation of herbicides in aqueous media. J Agric Food
Chem, 29: 227-230.
Tanke W & Franz JM (1978) [Side effects of herbicides on economically
useful insects.] Entomophaga, 23(3): 275-280 (in German).
Thompson CM & Forbes AD (1979a) Acute toxicity of propachlor to
Daphnia magna. Columbia, Missouri, ABC-Laboratories Inc., 40 pp
(Unpublished proprietary report No. 24708, submitted to WHO by
Monsanto International Services, Brussels).
Thompson CM & Forbes AD (1979b) Acute toxicity of propachlor to
Rainbow trout Salmo gairdneri. Columbia, Missouri, ABC-Laboratories
Inc., 40 pp (Unpublished proprietary report No. 24019, submitted to
WHO by Monsanto International Services, Brussels).
Thun S (1990a) Propachlor 21-day reproduction test in Daphnia
(Project Number XX-89-126). Walsrode, Germany, IBR Forschung GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Thun S (1990b) Prolonged fish toxicity test in the rainbow trout
( Salmo gairdneri) (Project Number XX-89-163). Walsrode, Germany, IBR
Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Thun S, Lemke G, & Fulst S (1991) Acute toxicity in earthworms
according to OECD 207. (Project Number IB-90-566). Walsrode, Germany,
IBR Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
US EPA (1984a) Guidance for the registration of pesticide products
containing propachlor (019101) (EPA Case number 17). Washington, DC,
US Environmental Protection Agency, Office of Pesticide Programme.
US EPA (1984b) Health and environmental effects profile for
propachlor. Cincinnati, Ohio, US Environmental Protection Agency
(600/X-84/335).
US EPA (1988) Propachlor. Washington, DC, US Environmental Protection
Agency, Health Advisory Office of Drinking-Water.
Vasilev NV (1982) [Residual quantity of Ramrod in soil and fodder
rurabages.] Khim Sel'skom Khoz, 20(6): 51-53 (in Russian).
Villarreal DT, Turco RF, & Konopka A (1991) Propachlor degradation by
a soil bacterial community. Appl Environ Microbiol, 57(8): 2135-2140.
Von Schubert H (1979) [Allergic contact dermatitis caused by
propachlor.] Dermatol Monatsschr, 165: 495-498 (in German).
Walker A & Brown PA (1982) Effects of soil on the activity and
persistence of propachlor. In: Proceedings of the British Crop
Protection Conference: Weeds. Croydon, British Crop Protection
Council, pp 171-177.
Walker A & Brown PA (1985) The relative persistence in soil of five
acetanilide herbicides. Bull Environ Contam Toxicol, 34: 143-149.
Warholic DT, Gutenmann WH, & Lisk DJ (1983) Propachlor herbicide
residue studies in cabbage using modified analytical procedure. Bull
Environ Contam Toxicol, 31: 585-587.
WHO/FAO (1989) Data sheets on pesticides No. 78: Propachlor. Geneva,
World Health Organization, 7 pp (WHO/VBC/DS/87.78).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-91. Geneva, World Health
Organization, 39 pp (WHO/PCS/90.1).
Worthing CR & Hance RJ (1991) The pesticide manual, 9th ed. Croydon,
British Crop Protection Council.
Yu Ching Chien, Booth GM, Hansen DJ, & Larsen JR (1975) Fate of
alachlor and propachlor in a model ecosystem. J Agric Food Chem,
23(5): 877-878.
Zhukova PS & Shirko TS (1979) [Herbicide residues in soil and
vegetables.] Khim Sel'skom Khoz, 17(6): 46-50 (in Russian).
Zilkah S, Osband ME, & Mccaffrey RP (1981) Characterization of the
inhibitory effects of the phytotoxic agent propachlor on L1210 cell
proliferation. Cancer Lett. (Irel), 13(3): 241-248.
Zilkah S, Osband ME, Mccaffrey RP, & Shapiro H (1985) The effect of
the plant cell inhibitor propachlor on the cell cycle of LI210 cells
as evaluated by flow cytometry. Life Sci, 6: 2111-2115.
Zimdahl RL & Clark SK (1982) Degradation of three acetanilide
herbicides in soil. Weed Sci, 30: 545-548.
Zlateva M & Maleva E (1978) [Late changes in the gonads of male white
rats upon combined treatment with low frequency vibrations and Ramrod
intoxication.] Med Fizkult, pp 85-91 (in Bulgarian).
Zlateva M & Maleva E (1979) Disorders in the processes of division and
differentiation of seminiferous epithelium in case of chronic
intoxication with Ramrod. Exp Med Morphol, 18(1): 35-39.
Zlateva M, Maleva E, & Stareva M (1978) Changes of adenosine
triphosphatase activity in the testes of albino rats under combined
influence of low-frequency vibration and intoxication with the
herbicide Ramrod. Rev Toxicol Eur Res, 6(78): 375-378.
Zschaler R & Jonas W (1990) Growth inhibition test with algae (Project
No. NC-90-592). Hamburg, Natec Institut für
Naturwissenschaftlich-technische Dienste GmbH. (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
RESUME ET EVALUATION
1. Identité, modalités d'utilisation, propriétés physiques et
chimiques, méthodes d'analyse
Le propachlor est un herbicide dérivé de l'acétanilide qu'on
utilise depuis 1965 en traitement de pré-levée et en traitement
précoce de post-levée. Il est principalement formulé en poudre
mouillable, liquide (concentré en suspension) et granulés. On
l'utilise en agriculture pour détruire les graminées annuelles et
certaines adventices à feuilles larges dans des cultures comme le
maïs, le sorgho, les potirons, le lin et les fleurs.
Le propachlor est légèrement soluble dans l'eau et facilement
soluble dans la plupart des solvants organiques. Il est peu volatil,
non inflammable et stable au rayonnement ultra-violet. La méthode
d'analyse la plus pratique est la chromatographie en phase gazeuse
avec détection par capture d'électrons après extraction et
purification par des méthodes appropriées.
2. Transport, distribution et transformation dans l'environnement
Le propachlor ne semble pas subir de dégradation photochimique à
la surface du sol. Il se volatilise en présence de vent lorsque la
surface du sol est encore humide.
Il n'est que modérément adsorbé aux particules du sol et aux
matières organiques, d'où un risque de lessivage à travers le sol
jusqu'aux eaux souterraines. Toutefois toutes les études montrent que
ce risque est faible en pratique. Il faut des précipitations très
importantes pour que les résidus se déplacent de 30 cm à l'intérieur
du sol. Selon la plupart des auteurs, la majorité des résidus
demeurent à moins de 4 cm de profondeur. Les caractéristiques du sol
influent beaucoup sur la mobilité du composé. Le lessivage se produit
surtout dans des sols sableux pauvres en matières organiques.
On a étudié en laboratoire et sur le terrain l'entraînement du
propachlor par ruissellement. Une des études a montré que la présence
de matières organiques dans le sol réduisait de 7 à 1 % de la quantité
appliquée, la concentration de l'herbicide dans l'eau de
ruissellement. L'incorporation de propachlor dans le sol réduit
également les pertes par ruissellement (de 3 à 0,8 % selon une étude).
En ce qui concerne la réduction de la concentration de propachlor
dans le sol et dans l'eau, le facteur de loin le plus important est sa
dégradation par les micro-organismes. On a montré que bactéries et
champignons étaient responsables de la dégradation de ce composé. Peu
de bactéries sont apparemment capables d'utiliser le propachlor comme
seule source de carbone. On a également isolé des bactéries qui sont
en mesure d'utiliser des métabolites telluriques du propachlor.
Les principaux métabolites qui se forment dans le sol sont des
acides oxaniliques et sulfoniques solubles dans l'eau. Il peut se
former un grand nombre d'autres métabolites mais ils ne représentent
qu'une faible proportion du total.
Le propachlor disparaît rapidement du sol et on a fait état de
demi-vies allant jusqu'à trois semaines. La plupart des études
indiquent une dégradation pratiquement complète en moins de six mois.
Les conditions écologiques influent sur la vitesse de dégradation qui
est favorisée par une température élevée et une forte humidité du sol.
Les résultats qui indiquent une persistance élevée du propachlor dans
le sol ont été obtenus à basse température et dans des sols secs. La
présence de nutriments en quantité suffisante dans le sol est
également nécessaire à la dégradation du propachlor.
Un des métabolites, la N-isopropylaniline conjuguée est
beaucoup plus persistante que le composé initial. On a retrouvé des
résidus de ce métabolite jusqu'à deux ans après l'épandage de
propachlor à titre expérimental à des doses plus élevées que celles
que l'on utilise normalement en agriculture.
Dans les conditions normales d'utilisation, le propachlor ne
devrait pas être entraîné à travers le sol par lessivage jusqu'aux
nappes souterraines et ne demeure pas dans le sol. Des conditions
exceptionnelles (température très basse ou sécheresse) peuvent
prolonger la persistance du propachlor et de ses métabolites.
Dans les conditions normales, le propachlor ne subit pas de
dégradation photochimique importante dans l'eau. Cependant, en
présence de photosensibilisateurs, cette dégradation photochimique
peut se produire. Le propachlor est stable à l'hydrolyse. Il est peu
probable qu'il se volatilise à partir de l'eau du fait de sa forte
solubilité dans l'eau et de sa faible tension de vapeur.
Comme dans le sol, c'est principalement par dégradation
biologique que le propachlor s'élimine de l'eau. Sa vitesse
d'élimination est donc liée à la population microbienne. Une étude
effectuée sur de l'eau contenant peu de bactéries a permis d'obtenir
une demi-vie d'environ cinq mois. Une autre étude a montré qu'au bout
de six semaines, il n'y avait toujours pas d'ouverture du cycle. Des
études de laboratoire portant sur des modèles d'écosystèmes ont montré
que le propachlor était presque complètement dégradé en l'espace de 33
jours.
Plusieurs études portant sur différentes espèces végétales ont
montré que le propachlor était rapidement métabolisé par les plantes
intactes ainsi que par des tissus végétaux excisés. Les voies
métaboliques se sont révélées analogues chez tous les végétaux
étudiés, tout au moins pendant les six à 24 premières heures,
conduisant à des métabolites hydrosolubles. On n'a pas observé de
décomposition métabolique du reste N-isopro-pylaniline. Seule une
très faible proportion (< 1 % selon une étude) des métabolites a été
retrouvée dans les fruits de ces végétaux; ils étaient concentrés en
grande majorité dans les racines et les feuilles. Les principaux
métabolites produits par les plantes sont identiques à ceux qui
prennent naissance dans le sol. On sait que ces métabolites peuvent
être fixés à partir du sol et un certain nombre d'études ne permettent
pas de déterminer avec certitude si les métabolites analysés
proviennent de la plantes ou du sol.
Bien que, d'après le coefficient de partage octanol/eau, ce
composé ait une tendance modérée à la bioaccumulation, on a montré
qu'il n'y avait ni bioconcentration ni bioamplification chez les êtres
vivants.
3. Concentrations dans l'environnement et exposition humaine
Les rapports d'analyse du propachlor dans l'air au cours de
l'épandage sont rares et insuffisants.
Aux Etats-Unis d'Amérique, la concentration du propachlor dans
les eaux de surface et les eaux souterraines est toujours faible, les
maxima atteignant 10 µg/litre dans les eaux de surface et 0,12
µg/litre dans les eaux souterraines. Le concentration la plus forte
enregistrée lors d'une étude sur les eaux de ruissellement était de 46
µg/litre.
Les résidus de propachlor dans les denrées alimentaires sont
généralement inférieurs à la limite de détection de la méthode
d'analyse (0,005 mg/kg). L'expérimentation a permis de retrouver des
résidus de l'ordre de 0,005 mg/kg dans des tomates, des poivrons, des
oignons et des choux.
Le dosage du propachlor dans l'atmosphère de la zone de travail
de conducteurs de tracteur pendant l'épandage du composé a donné des
résultats allant de 0,1 à 3,7 mg/m3.
4. Cinétique et métabolisme
Chez les mammifères, le propachlor peut être absorbé au niveau
des voies respiratoires, des voies digestives et de la peau. Il ne
s'accumule pas dans l'organisme et devient indétectable au bout de 48
heures.
La plupart des espèces animales (rats, porcs, poulets)
métabolisent le propachlor par la voie de l'acide mercapturique (MAP).
Il se forme des conjugués de cystéine par conjugaison avec le
glutathion et on a avancé que ces conjugués jouaient le rôle
d'intermédiaires dans la formation métabolique des acides
mercapturiques. La métabolisation du conjugué cystéinique du
propachlor se poursuit, notamment sous l'action de la C-S lyase
bactérienne qui intervient également dans la formation des métabolites
terminaux contenant le groupement méthylsulfonyle, métabolites qui
sont principalement excrétés dans l'urine (68 % de la dose initiale de
propachlor) et, sous forme de résidus insolubles, dans les matières
fécales (19 %). La C-S lyase du propachlor est sans action chez les
rats axéniques.
On a montré qu'il y avait un certain nombre de différences dans
le métabolisme du propachlor chez le rat et le porc. La bile est la
principale voie d'élimination des métabolites mercapturiques chez le
rat, mais on a montré qu'il existait une voie métabolique
extra-biliaire chez le porc.
Des études de métabolisme effectués sur des veaux ont montré que
ces animaux pourraient être incapables de former de l'acide
mercapturique à partir des conjugués du glutathion, ce qui les
rendrait plus sensibles à l'intoxication par le propachlor.
5. Effets sur les animaux d'expérience et les systèmes d'épreuves in
vitro
Le propachlor est légèrement toxique en cas d'exposition aiguë
par voie orale (la DL50 pour le rat varie de 950 à 2176 mg/kg de
poids corporel). Les signes d'intoxication aiguë proviennent
principalement d'effets sur le système nerveux central (excitation et
convulsions suivies d'une dépression). Chez des rongeurs, la toxicité
aiguë par inhalation est faible (CL50 = 1,0 mg/litre). Le propachlor
provoque de graves irritations des yeux et de la peau.
Des rats, des souris et des chiens ont été soumis à une
exposition de longue ou de courte durée au propachlor. Les organes
cibles sont le foie et les reins. Chez le chien, la dose sans effet
nocif observable a été de 45 mg/kg de poids corporel lors d'une étude
de trois mois où l'animal était exposé par la voie alimentaire. Lors
d'une étude d'une année sur des chiens, la dose sans effet nocif
observable a été estimée à 9 mg/kg de poids corporel (200 ppm dans la
nourriture). Lors d'une étude sur des rats exposés de la même manière
pendant 24 mois au propachlor, la dose sans effet observable a été
évaluée à 50 mg/kg de nourriture (2,6 mg/kg de poids corporel). Lors
d'une étude semblable effectuée sur des souris pendant 18 mois, on a
évalué à 1,6 mg/kg de poids corporel (10 ppm) la dose sans effet
observable.
Le propachlor ne s'est révélé cancérogène ni pour la souris ni
pour le rat. Dans la plupart des systèmes d'épreuve mammaliens, sa
réponse mutagène est négative tout en étant positive dans quelques
autres cas. Les données expérimentales disponibles de fournissent pas
une preuve suffisante de son pouvoir mutagène.
Administré en dose unique (675 mg/kg) à des rats et à des souris,
le propachlor a donné lieu à des signes d'embryotoxicité. On a
également observé des effets embryotoxiques lors d'études où du
propachlor avait été administré à plusieurs reprises (35,7 à 270
mg/kg). Toutefois, lors d'une autre étude sur des rats, où les doses
variaient de 20 à 200 mg/kg, aucun effet embryotoxique n'a été
observé.
Aux doses respectives de 12 et 60 mg/kg de poids corporel, le
propachlor (en poudre mouillable) a entraîné une réduction de la
teneur en protéines et un accroissement de l'activité de l'ATPase et
de la 5-nucléotidase dans des homogénats de testicules de rats,
provoquant également une dégénérescence du tissu testiculaire. Lors
d'une étude de reproduction portant sur deux générations, on n'a pas
véritablement obtenu la preuve d'effets indésirables.
6. Effets sur l'homme
On a signalé quelques dermatites de contact et dermatites
allergiques chez des agriculteurs et des ouvriers de production
exposés au propachlor (Ramrod et Satecid). Des tests cutanés ont été
effectués parmi un certain nombre d'entre eux, avec un résultat
positif, qui révèle une réaction d'irritation et une hypersensibilité
mono- et bivalente.
On n'a pas eu connaissance de symptômes ou de maladies, soit chez
des personnes exposées de par leur profession, soit dans la population
générale - à part les quelques cas d'effets cutanés chez les ouvriers
professionnellement exposés.
7. Effets sur les êtres vivant dans leur milieu naturel
Des études portant sur la microflore terricole ont montré que les
bactéries nitrifiantes constituaient le groupe le plus sensible aux
effets inhibiteurs du propachlor, leur nombre étant réduit d'un
facteur 3 à 4 après épandage de 8 à 10 kg de propachlor par hectare.
Ce sont les bactéries décomposant la cellulose qui étaient les moins
sensibles. La forte adsorption du propachlor aux particules d'argile
présentes dans le sol et une température élevée sont deux facteurs qui
réduisent les effets inhibiteurs.
Chez l'algue Selenastrum capricornutum, on a fait état d'une
CE50 à 96 heures de 0,02 mg/litre (pour la croissance) et d'une
concentration sans effet observable de 0,01 mg/litre. D'après une
deuxième étude portant sur une formulation de propachlor et menée
pendant 72 heures, le risque pour cette même algue serait nettement
moindre.
Pour la daphnie Daphnia magna, on donne des CL50 de 7,8 ou
6,9 mg/litre et une concentration sans effet observable inférieure à
5,6 mg/litre. La concentration sans effet observable sur la
reproduction était de 0,097 mg/litre. Pour des larves de deux espèces
de moucherons, on a obtenu pour la CL50 des valeurs respectives de
0,79 et 1,8 mg/litre.
Chez la truite arc-en-ciel la CL50 à 96 heures est de 0,17
mg/litre et la concentration sans effet observable sur 21 jours, de
0,019 mg/litre.
Le propachlor est considéré comme modérément à fortement toxique
pour les organismes aquatiques.
Le propachlor n'est pas toxique pour les lombrics aux
concentrations présentes dans le sol par suite d'un usage normal (la
concentration sans effet observable est de 100 mg/kg de terre). La
DL50 par contact pour les abeilles (311 µg/abeille) montre que le
propachlor ne constitue pas un danger pour ces insectes. En revanche,
des études menées en laboratoire et sur le terrain montrent que des
parasites utiles peuvent souffrir des effets du propachlor.
Le propachlor est plus toxique pour les oiseaux lorsqu'on
l'introduit directement dans l'estomac que lorsqu'on l'administre dans
la nourriture. La DL50 aiguë varie de 137 à 735 mg/kg de poids
corporel pour différentes espèces. Quant à la CL50 lors d'une
exposition par voie alimentaire, elle dépasse 5620 mg/kg de nourriture
chez les oiseaux.
Le propachlor ne constitue pas un danger pour les oiseaux dans la
nature, même sous forme de granulés.
RESUMEN Y EVALUACION
1. Identidad, modalidades de uso, propiedades físicas y químicas y
método analíticos
El propacloro es un herbicida derivado de la acetanilida
utilizado desde 1965 que actúa en las plantas antes de nacer o poco
después. Las principales formulaciones son de polvo humectable,
líquido fluido (concentrado en suspensión) y gránulos. Se aplica en la
agricultura a la lucha contra las gramíneas anuales y algunas malas
hierbas de hoja ancha en varios cultivos, como los de maíz, sorgo,
calabaza, lino y flores ornamentales.
El propacloro es ligeramente soluble en agua y se disuelve
fácilmente en la mayoría de los disolventes orgánicos. Su volatilidad
es escasa, no es inflamable y es estable a la radiación ultravioleta.
El método más práctico de análisis es la cromatografía de gases con
detección por captura de electrones, después de aplicar procedimientos
apropiados de extracción y purificación.
2. Transporte, distribución y transformación en el medio ambiente
Según la información disponible, el propacloro no se fotodegrada
sobre la superficie del suelo. El producto se volatiliza cuando hay
viento y la superficie del suelo está todavía mojada.
La adsorción sobre las partículas del suelo y la materia orgánica
es sólo moderada. Debido a esto, es posible la lixiviación a través
del perfil del suelo hacia el agua subterránea. Sin embargo, todos los
estudios indican que en la práctica es poco probable que esto ocurra.
Se requiere una precipitación muy abundante para hacer descender los
residuos 30 cm en el perfil del suelo. La mayoría de los autores
señalan que la mayor parte de los residuos se mantienen en una capa
superficial del suelo de 4 cm. Las características del suelo influyen
mucho en el desplazamiento del producto. La lixiviación se produce en
su mayor parte en suelo arenoso con poca materia orgánica.
Se ha estudiado el arrastre del propacloro por el agua tanto en
el laboratorio como sobre el terreno. En un estudio, la materia
orgánica del suelo redujo el arrastre del herbicida aplicado del 7% al
1%. Su incorporación al suelo también redujo la pérdida por arrastre
del agua (del 3% al 0,8% en un estudio).
El factor que más contribuye, con diferencia, a reducir los
niveles de propacloro en el suelo y el agua es la degradación por los
microorganismos. Se ha comprobado que en la degradación de la
sustancia intervienen tanto bacterias como hongos. Son pocas las
bacterias que parecen tener capacidad para usar el propacloro como
única fuente de carbono. También se han aislado bacterias que pueden
utilizar los metabolitos del propacloro presentes en el suelo.
Los metabolitos predominantes entre los que se forman en el suelo
son los ácidos oxanílico y sulfónico, solubles en agua. Pueden
formarse otros muchos metabolitos, pero representan una proporción
pequeña del total.
El propacloro desaparece con rapidez del suelo, habiéndose
registrado semividas de hasta tres semanas. En la mayoría de los
estudios se señala que la degradación es casi completa en menos de
seis meses. Las condiciones del medio ambiente influyen en la
velocidad de degradación, que se ve favorecida por los valores
elevados de la temperatura y el contenido de humedad del suelo. Los
estudios en los que se describía una permanencia más prolongada del
propacloro en el suelo se realizaron en condiciones de baja
temperatura o suelo seco. También es necesaria una cantidad suficiente
de nutrientes en el suelo para la degradación.
El metabolito conjugado N-isopropilanilina es mucho más
persistente que el producto del que procede. Se han encontrado
residuos de este metabolito hasta dos años después de la aplicación
experimental de dosis de propacloro más altas de las que se utilizan
normalmente en la agricultura.
Con el uso normal no es previsible que el propacloro llegue por
lixiviación a través del suelo hasta el agua subterránea, y no se
mantiene mucho tiempo en el suelo. En condiciones excepcionales de
baja temperatura o sequedad, el propacloro y sus metabolitos pueden
permanecer más tiempo en él. En condiciones normales, el propacloro
no sufre una fotodegradación significativa en el agua. En presencia de
fotosensibilizadores puede fotodegradarse. El propacloro es estable
desde el punto de vista hidrolítico. No es probable la volatilización
a partir del agua, debido a la elevada solubilidad en ella y la baja
tensión de vapor del producto.
Al igual que en el suelo, la principal ruta de pérdida de
propacloro del agua es la degradación biótica. La velocidad de
desaparición del propacloro del agua depende, pues, de la población
microbiana. En un estudio realizado con un pequeño número de bacterias
presentes en el agua se obtuvo una semivida de unos cinco meses. En
otro estudio, a las seis semanas no se había roto el anillo. En
estudios de laboratorio con modelos de ecosistemas se observó una
degradación casi completa en un período de 33 días.
En varios estudios con distintas especies vegetales se vio que el
propacloro se metabolizaba con rapidez tanto en las plantas intactas
como en tejidos vegetales extirpados. Las rutas metabólicas eran
análogas en todas las plantas estudiadas, por lo menos durante las
primeras 6-24 horas, produciendo metabolitos hidrosolubles. No se
observó degradación metabólica del fragmento de la
N-isopropilanilina. En los frutos de las plantas solamente se
encontró una proporción muy pequeña (< 1% en un estudio) de los
metabolitos; en su mayor parte estaban en las raíces y el follaje. Los
principales metabolitos producidos en las plantas son idénticos a los
que se forman en el suelo. Se sabe que las plantas absorben esos
metabolitos del suelo, y en algunos estudios había dudas acerca de si
los metabolitos medidos procedían de la planta o del suelo.
Aunque del coeficiente de reparto en octanol y agua parece
deducirse un potencial moderado de bioacumulación, los estudios
realizados indican que no hay ni bioconcentración ni bioamplificación
en los organismos.
3. Niveles medioambientales y exposición humana
Las mediciones descritas de la concentración del propacloro en el
aire durante la aplicación son pocas e inadecuadas.
Las concentraciones en el agua superficial y subterránea en los
Estados Unidos fueron siempre bajas, con un máximo de 10 µg/litro en
la superficial y de 0,12 µg/litro en la subterránea. La mayor
concentración registrada en el agua en un estudio del arrastre fue de
46 µg/litro.
Los residuos de propacloro en los alimentos suelen ser inferiores
al límite de detección del método analítico (0,005 mg/kg). En estudios
experimentales se han identificado concentraciones de residuos del
orden de 0,05 mg/kg en tomates, pimientos, cebollas y coles.
Las concentraciones de propacloro en el aire de la zona de
trabajo de los conductores de tractores que aplicaban la sustancia
oscilaban entre 0,1 y 3,7 mg/m3.
4. Cinética y metabolismo
Los mamíferos pueden absorber el propacloro por los tractos
respiratorio y gastrointestinal, así como a través de la piel. El
producto no se acumula en el organismo, en el que no es detectable a
las 48 horas.
La mayoría de las especies animales (ratas, cerdos, pollos)
metabolizan el propacloro siguiendo la vía de los ácidos
mercaptúricos. Mediante conjugación con el glutatión se forman
productos conjugados de cisteína, que se han señalado como posibles
intermediarios en la formación metabólica de ácidos mercaptúricos. La
C-S liasa bacteriana participa en el ulterior metabolismo del sistema
conjugado del propacloro con la cisteína y en la formación de los
metabolitos finales con metilsulfonilo, que se excretan sobre todo en
la orina (68% de la dosis de propacloro), y de residuos insolubles,
que se excretan en las heces (19%). La propacloro C-S liasa es
inactiva en ratas sin microorganismos.
En los estudios realizados se observaron algunas diferencias en
cuanto al metabolismo entre las ratas y los cerdos. La bilis es el
principal camino de eliminación de los metabolitos de la vía de los
ácidos mercaptúricos en las ratas, pero se ha demostrado que en los
cerdos existe una vía metabólica extrabiliar.
En estudios metabólicos con terneros se observó que pueden ser
incapaces de formar ácidos mercaptúricos a partir de los productos
conjugados del glutatión, por lo que pueden ser más susceptibles a la
intoxicación.
5. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
El propacloro es ligeramente tóxico en la exposición oral aguda
(la DL50 en ratas va de 950 a 2176 mg/kg de peso corporal). Los
signos de intoxicación aguda son predominantemente efectos sobre el
sistema nervioso central (excitación y convulsiones, seguidas de
depresión). La toxicidad aguda por inhalación en roedores es baja
(CL50 = 1,0 mg/litro). El propacloro produjo efectos graves de
irritación en los ojos y la piel.
El propacloro se ha ensayado en estudios de exposición de corta
y larga duración en ratas, ratones y perros. Los órganos sobre los que
actuaba eran el hígado y los riñones. En un estudio de administración
con los alimentos a perros durante tres meses, el nivel sin efectos
adversos observados (NOAEL) fue de 45 mg/kg de peso corporal. En otro
estudio de un año, también en perros, el NOAEL fue de 9 mg/kg de peso
corporal (250 ppm en la dieta). El nivel sin efectos observados (NOEL)
en un estudio de alimentación de ratas durante 24 meses fue de 50
mg/kg de la dieta (2,6 mg/kg de peso corporal). En un estudio en el
que administró el producto a ratones con los alimentos durante 18
meses, el NOEL fue de 1,6 mg/kg de peso corporal (10 ppm).
No se encontraron efectos carcinogénicos del propacloro en
ratones y ratas. En la mayoría de los sistemas de prueba de mamíferos
la respuesta mutagénica fue negativa, obteniéndose resultados
positivos en un pequeño número de ensayos. Los datos experimentales
disponibles no aportan suficientes pruebas de su potencial mutagénico.
En pruebas de dosis única (675 mg/kg) con ratones y ratas se
demostró la embriotoxicidad del propacloro. También se detectaron
efectos embriotóxicos en tratamientos con dosis repetidas (35,7-270
mg/kg). Sin embargo, en otro estudio en ratas, con una gama de dosis
de 20-200 mg/kg, no se observó embriotoxicidad.
Con niveles de 12 y 60 mg/kg de peso corporal, el propacloro
(polvo humectable) provocó una disminución del contenido de proteínas
y un aumento de la actividad de la ATPasa y la 5-nucleotidasa en un
homogeneizado de testículo de rata y cambios degenerativos en los
testículos. En un estudio de reproducción de dos generaciones no se
obtuvieron pruebas definitivas de efectos adversos.
6. Efectos en la especie humana
Se ha descrito un pequeño número de casos de dermatitis de
contacto y alérgica en agricultores y trabajadores expuestos al
propacloro durante la producción (Ramrod y Satecid). En algunos de
ellos se efectuaron pruebas del parche y la reacción fue positiva, con
irritación e hipersensibilidad monovalente y bivalente.
No se han notificado casos de síntomas o enfermedades entre las
personas expuestas profesionalmente o en la población general, salvo
un pequeño número de informes de sus efectos cutáneos en trabajadores
expuestos profesionalmente.
7. Efectos en los seres vivos del medio ambiente
En varios estudios sobre los microorganismos del suelo, las
bacterias nitrificantes fueron el grupo más sensible a los efectos
inhibidores del propacloro, reduciéndose su número a la tercera o
cuarta parte tras la aplicación de 8-10 kg/ha de propacloro. Las menos
sensibles fueron las bacterias celulolíticas. La adsorción elevada
sobre las partículas de arcilla del suelo y la temperatura alta
reducen los efectos inhibidores.
En el alga Selenastrum capricornutum se ha registrado una CE50
a las 96 horas de 0,02 mg/litro para el crecimiento y una
concentración sin efectos observados (NOEC) de 0,01 mg/litro. De un
segundo estudio de 72 horas realizado con una formulación, parece
desprenderse que el peligro es mucho menor para este mismo organismo.
Se ha informado de unos valores de la CL50 para Daphnia magna
de 7,8 y 6,9 mg/litro y una NOEC de < 5,6 mg/litro. En las larvas de
dos especies de moscas enanas, los valores registrados de la CL50
fueron de 0,79 y 1,8 mg/litro.
La CL50 para la trucha arcoiris a las 96 horas es de 0,17
mg/litro, y la NOEC en un estudio de 26 días fue de 0,019 mg/litro.
Se considera que el propacloro tiene una toxicidad entre moderada
e intensa para los organismos acuáticos.
La exposición a las concentraciones de propacloro que cabe prever
en el suelo no es tóxica para las lombrices de tierra si la aplicación
es normal (la NOEC es de 100 mg/kg de suelo). La DL50 por contacto
para las abejas (311 µg/abeja) demuestra que el propacloro no
representa un peligro para estos insectos. En estudios de laboratorio
y de campo se han detectado efectos adversos del propacloro en algunos
insectos parasitarios beneficiosos.
El propacloro es más tóxico para las aves cuando se administra
mediante sonda gástrica que cuando se incorpora a la dieta. Los
valores de la DL50 aguda para distintas especies de aves oscilan
entre 137 y 735 mg/kg de peso corporal. Los valores de la CL50 en la
administración con los alimentos fueron superiores a los 5620 mg/kg de
la dieta.
El propacloro no representa un peligro para las aves en el campo,
ni siquiera con la formulación granulada.
 Chemical formula: C11H14ClNO
Common synonyms
and trade names: Ramrod, Acylide, Bexton (discontinued by Dow
Chemical Company), Niticid, Satecid
CAS chemical name: 2-chloro- N-(1-methylethyl)- N-phenyl acetamide
IUPAC name: 2-chloro- N-isopropylacetanilide (formerly
alpha-chloro- N-isopropylacetanilide)
CAS registry
number:
Chemical formula: C11H14ClNO
Common synonyms
and trade names: Ramrod, Acylide, Bexton (discontinued by Dow
Chemical Company), Niticid, Satecid
CAS chemical name: 2-chloro- N-(1-methylethyl)- N-phenyl acetamide
IUPAC name: 2-chloro- N-isopropylacetanilide (formerly
alpha-chloro- N-isopropylacetanilide)
CAS registry
number:  I. R = CH2Cl
2-chloro- N-(1-methylethyl)- N-phenylacetamide = propachlor
II. R = CH2OH
2-hydroxy- N-(1-methylethyl)- N-phenylacetamide
III. R = CH3
N-(1-methylethyl)- N-phenylacetamide
O
"
IV. R = COH
[(1-methylethyl)phenylamino]oxoacetic acid
V. R - CH2SO3H
2-[(1-methylethyl)phenylamino]-2-oxoethanesulfonic acid
O O
" "
VI. R = CH2SCH2COH
{{[(methylethyl)phenylamino]acetyl}sulfinyl}-acetic acid
O
"
VII. R = CH2SCH3
N-(1-methylethyl)-2-(methylsulfinyl)- N-phenylacetamide
Fig. 1. Structures of propachlor degradation products.
4.1.1.4 Persistence
Fletcher & Kirkwood (1982) reported a half-life of 2-3 weeks for
propachlor. Free propachlor disappeared rapidly in soil treated by
Ritter et al. (1973); in 21-28 days residues of free propachlor
declined 72-80%. In earlier laboratory studies and field bioassays
carried out by Menges & Tamez (1973), the soil persistence (> 90%
degradation) was found to be less than 6 months. According to Melnikov
et al. (1985) and Zhukova & Shirko (1979), the period of propachlor
degradation in soil to non-toxic products is about 2 months. The
presence of an alkyl group attached to the nitrogen atom in its
molecule prevents its decomposition to aniline or to azobenzolic
residues, which could later be transferred into tetrachlorazobenzene
(Panshina, 1985). Propachlor applied at 4-8 kg/ha was detoxified in
peat soil within 59-63 days (Vasilev, 1982). When it was applied at
6.5, 9 and 11 kg/ha, it was still detectable after 113 days in two
types of soil in the Voronezh and Krasnodar regions of the former USSR
(Kolesnikov et al., 1980). The longer persistence determined in this
study might be connected with the climatic conditions, particularly
the low temperatures. Roberts et al. (1978) and Balinova (1981)
confirmed that propachlor is rapidly decomposed in soil.
Field studies evaluating the degradation of propachlor in soil
showed that 70 days after application, propachlor was detectable in
insignificant quantities (0.04 mg/kg, i.e. 2% of the dose applied).
Degradation was slower in dry than humid weather (Zhukova & Shirko,
1979) (Table 3). The conclusions of the authors were that degradation
in soil is rapid and there is no possibility of propachlor
accumulation in crops.
Table 3. Dynamics of dissipation of propachlor in soil (average data
for 1974-1976)a
Dose application After 10th day On 30th day On 50th day On 70th day
(kg propachlor/ha) mg/kg %b mg/kg %b mg/kg %b mg/kg %b
6 1.73 86.4 0.95 42.8 0.10 5.2 0.04 2
8 2.44 92.6 1.55 58.3 0.20 7.8 0.04 2
10 3.14 94.2 2.53 76.0 0.40 12.0 0.04 2
a From: Zhukova & Shirko (1979)
b Percentage of dose applied
In a study by Frank et al. (1977), soil with high organic matter
content was treated with 19 kg propachlor/ha. The treatment times and
rates are given in Table 4. Soil samples were collected to a depth of
20 cm and analysed. Residues of the conjugated metabolite
N-isopropylaniline of up to 3.7 mg/kg soil were detected 2 years
after the application. They were released from soil by hydrolysis,
indicating the presence of active bonding sites for the metabolite in
the soil. The authors concluded that using propachlor in successive
years led to accumulation of long-lasting conjugated
N-isopropylaniline. Applications made once every 3 years, however,
did not lead to such accumulation and they recommended this type of
application.
Table 4. Residues of conjugated N-isopropylaniline in organic soil
treated with propachlor in 1971, 1972 and 1975a.
Year Time of application Rate of application N-isopropylaniline
(kg/ha)b residues in
oven-dried soil
(mg/kg)
1971 May and June 9 + 10 not analysed
1972 untreated none 3.67
May, July, August 6.7 + 3.4 + 3.4 7.70
May, July, August 6.7 + 6.7 + 6.7 9.47
1975 May and June 6.7 + 6.7 3.16
a From: Frank et al. (1977)
b Soil samples were collected in May 1973 and April 1976 and analysed
in January and April 1976, respectively.
US EPA (1984b) re-evaluated the existing data concerning some
environmental aspects of propachlor. It was concluded that microbes
were the primary factor in its breakdown in soil and that its loss
from photodecomposition and/or volatilization was low. Although this
earlier assessment suggested a potential for propachlor to contaminate
ground water, a later assessment (US EPA, 1988) concluded that "the
rapid degradation of low levels of propachlor in soil is expected to
result in a low potential for groundwater contamination by
propachlor".
4.1.1.5 Environmental conditions affecting distribution and breakdown
Walker & Brown (1982, 1985) carried out laboratory studies and
field trials in parallel. They found first-order kinetics dissipation
of propachlor and confirmed the results of Beestman & Deming (1974).
They also described a clear temperature dependence: an increase in
temperature of 10 °C reduced the half-life by a factor of between 1.9
and 2.5. Soil moisture also influenced degradation: slower rates of
loss were found in drier soil. The half-life in soil with a moisture
content of 6% was about twice as long as at 15% (Table 5). In general,
the time of persistence in the field was comparable to that measured
in laboratory studies. The half-life of propachlor varied from 4 to 22
days (Table 5).
Table 5. Half-life for propachlor degradation (days)a
Temperature (°C) 25 25 25 25 15 5
Moisture (%) 6 9 12 15 12 12
Propachlor 7.7 4.6 4.2 3.7 9.2 21.7
a From: Walker & Brown (1985)
The persistence of propachlor in soil as a result of all
microbial, chemical and physical processes has been studied by Zimdahl
& Clark (1982). They measured the half-life of propachlor in clay loam
and sandy loam soils in the laboratory, using different temperature
and moisture conditions (Table 6). An increase in temperature (from 10
to 30 °C) and moisture content (from 20 to 80%) shortened the
half-life.
Vasilev (1982) confirmed the importance of meterological
conditions, type of soil, and rate and period of application on the
degradation in soil. In dry weather, degradation took longer than in
humid conditions. Based on field experiments where soil was treated
with propachlor and carrots were then planted, the author reported the
following residues (measured at the moment of harvest in the soil
layer 0-20 cm) in soil: at an application rate of 4 kg/ha the
propachlor residues were 0.05 mg/kg; at 6 kg/ha they were 0.15 mg/kg;
and at 8 kg/ha they were 0.2 mg/kg.
Table 6. Half-lives of propachlor in clay loam and sandy loam as
determined by bioassaya
Storage conditions Half-life (days)
Temperature Soil moisture Sandy loam Clay loam
(°C) (%)
10 50 16.7 14.3
20 50 3.3 5.3
30 50 1.9 1
20 20 23.1 21.5
20 50 3.3 5.3
20 80 3.3 4.1
a From: Zimdahl & Clark (1982)
Shirko & Belova (1982) found that residues of propachlor in soil
and plants depended on the nitrogen and potassium content of the soil.
At the moment of harvesting, no residues of propachlor were detected
in soil after application at a rate of 4.5-6.5 kg/ha. According to the
authors, a better supply of plants with nutrients (in this case,
nitrogen and potassium fertilizers) leads to more intense
detoxification and degradation of the herbicide and, conversely, an
insufficiency delays the detoxification processes and leads to the
accumulation of residues.
4.1.2 Water
Photodegradation of propachlor in aqueous media was studied by
Tanaka et al. (1981) under laboratory conditions. They used a 10-ml
sample with propachlor concentrations ranging up to 100 mg/litre, and
the sample was irradiated for 135 min with a 300-nm sunlight lamp.
Very weak photolysis was registered; by the end of the study only 1%
of herbicide had been lost. Addition of a commercial surfactant (2%
heterogeneous non-ionic Tergitol TMN, acting as photosensitizer)
allowed 37% of propachlor to be photodegraded. It is difficult to make
conclusions on this study because of certain deficiencies. The use of
a commercial formulation in such studies should be avoided since some
of the constituents may cause indirect photochemical reactions. No
mention was made in the report of the purity of the compound studied.
The test should preferably be carried out at constant temperature. The
lack of data concerning the intensity of the irradiation source (US
EPA, 1984a) does not allow any extrapolation of these data to the
environment.
Rejtö et al. (1984) investigated the effects of ultraviolet
irradiation of propachlor solutions and found that 5 h of irradiation
led to 80% decomposition. The three photodecomposition products
identified were: N-isopropyloxindole, N-isopropyl-3-
hydroxyoxindole and a spiro compound. Irradiation of a solution of
propachlor with visible light for 12 h led to almost complete
decomposition in the presence of riboflavin as a photosensitizer. The
photodecomposition products after visible light irradiation were found
to be non-phytotoxic.
Monsanto (1987) demonstrated that propachlor is hydrolytically
stable.
Volatilization of propachlor from aqueous media is of limited
significance because of the high solubility in water and relatively
low vapour pressure of the compound. In agreement with Henry's
constant, the loss of propachlor by sorption and sedimentation in
water bodies does not appear to be very significant (US EPA, 1984a).
The role of microbial biodegradation appears to be of major
significance in water as well as in soil. Novick & Alexander (1985)
studied the metabolism of low concentrations (10 µg/litre) of
propachlor in sewage and lake water. They found that under aerobic
conditions microbial populations from sewage and lake waters were not
able to mineralize the carbon ring of propachlor in six weeks.
However, propachlor was extensively metabolized, the products obtained
were organic, and they were found to accumulate in the environment. In
a parallel study, it was found that aniline was readily cleaved under
similar conditions, indicating rapid mineralization of this compound.
It was concluded that structural characteristics of propachlor, other
than the ring, account for the mineralization of the compound. The
presence of the three substituents on the nitrogen atom in propachlor
may be the reason for its persistence. Steen & Collette (1989)
determined microbial transformation rate constants for seven amides in
natural pond water. A second order mathematical rate expression served
to describe propachlor degradation, and a value of 1.1 x 10-9
litres/organism per h was calculated. Brightwell et al. (1981)
presented data showing slow degradation of propachlor in lake water
under aerobic conditions. After 30 days, 84.5% of the propachlor
remained unchanged; under these conditions a half-life of about 5
months would be expected. The low rate of metabolism was due to a low
level of microorganisms. Yu et al. (1975) studied the degradation of
propachlor in water using a model ecosystem. An aquarium with
7-day-old sorghum plants was used with the addition of 50 µCi of 14C
ring-labelled propachlor. By the end of the 33-day experimental
period, 7 degradation products in water were determined by thin-layer
chromatography but were not identified. At that time only 0.4% of the
radioactivity of the dose applied remained in the water.
4.1.3 Plants
The metabolism of propachlor in corn seedlings and in excized
leaves of corn, sorghum, sugar cane and barley was studied by
Lamoureux et al. (1971). Metabolism was rapid and similar in all
mentioned plant species during the first 6-24 h following treatment.
At least 3 water-soluble metabolites were produced in each species
during this period. Two of these metabolites were isolated; the first
one was identified as the glutathione conjugate of propachlor
(compound I) and the second one appeared to be the gamma-
glutamylcysteine conjugate (compound II). The primary mode of
metabolism is a nucleophilic displacement of the alpha-chloro group of
propachlor by the sulfhydryl group of a peptide. The metabolic
reactions of propachlor proceed non-enzymatically in vitro, and the
in vivo reaction may be enzymatic and/or non-enzymatic. The high
percentage of propachlor converted to compounds I and II in corn
seedlings during the first 18 h following treatment indicates that the
reaction of propachlor with glutathione and/or gamma-glutamylcysteine
is quite specific. This would be expected if the reaction is
enzymatic. Some glutathione and gamma-glutamylcysteine conjugates in
plants may be end-products, but, in the case of propachlor, these
metabolites are transient intermediates. Further studies are needed to
establish the final steps (Lamoureux et al., 1971).
Pantano & Anderson (1987) studied the metabolism of propachlor in
sorghum (milo). Sorghum seeds were planted in soil treated with 14C
ring-labelled propachlor at a rate of 3.3 kg/ha and grown to maturity
in a greenhouse. Following senescence, plants were separated into
various anatomical parts, freeze-dried and analysed for activity. The
uptake of 14C in the foliage and grain was 9.5 and 0.5 mg/kg dry
weight, respectively. Four metabolites were identified in the foliage
extract; these represented 66.5% of the metabolites in foliage. The
four metabolites were [( N-iso-propyl) phenylamino]oxoacetic acid,
{{[( N-isopropyl)-phenyl-amino]acetyl}sulfinyl}lactic acid,
{{[( N-isopropyl)phenylamino] acetyl}sulfinyl}acetic acid and
2-[( N-isopropyl)phenylamino]-2-oxoethanesulfonic acid. In addition,
other metabolites found at low levels in the foliage extract were
characterized. Freeze-dried sorghum grain contained only 0.5 mg/kg of
14C activity and only one metabolite was identified (the first
compound mentioned above). This metabolite constituted at least 24.9%
of the activity in grain. In all of the major metabolites identified
in this study, no modification of the N-isopropylaniline moiety was
observed. On the basis of metabolites identified in this study and
known pathways for the metabolic transformations of related
chloroacetamides, a scheme for the metabolic fate of propachlor in
sorghum plants was postulated by the authors. The biotrans-formations
include: 1) displacement of the chlorine by an oxygen-containing
nucleophile followed by oxidation, and 2) conjugation with glutathione
followed by further metabolic modification of this conjugate.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
in soybean and found that it was rapidly metabolized to
homoglutathione conjugate in roots and foliage. This conjugate was
rapidly metabolized to the cysteine conjugate and then slowly
converted to a variety of other metabolites; four of these were
present up to 72 days after application of propachlor. These four were
malonylcysteine, malonylcysteine S-oxide, 3-sulfinyllactic acid and
O-malonyl glucoside conjugates of propachlor. Less than 1% of
metabolites was isolated from beans or pods, the great majority being
in roots and foliage. The major metabolites found in plants were the
same as those produced in soil. The authors suggested that it is
difficult to differentiate between metabolites formed in the plant and
those taken up from soil as to the relative importance of the two
sites of metabolic degradation.
4.2 Bioaccumulation and biomagnification
The two published values for the log octanol/water partition
coefficient (log Kow) of propachlor, i.e. 1.62 and 2.3 (US EPA,
1984b, 1988), indicate a moderate potential for bioaccumulation.
Barrows & Macek (1974) used bluegill sunfish exposed to
14C-labelled propachlor in a continuous-flow experiment in order to
assess the potential for the compound to bioconcentrate in aquatic
organisms. The fish were exposed for 35 days to a mean
14C-propachlor concentration of either 0.54 mg/litre (high level) or
0.012 mg/litre (low level) in the water. Analysis of the fish tissues
indicated bioconcentration factors (BCFs) in the edible tissue of 34
and 22 for the high and low levels, respectively, and in the
non-edible tissue of 22 and 20. When the fish were placed in clean
water the activity was rapidly eliminated from the non-edible portion
of the fish and at a somewhat slower rate from the edible tissue.
Because of the polar, water-soluble nature of the soil metabolites
(section 4.1.1), they would be less likely to bioconcentrate in
aquatic organisms. The results of a static study on catfish by Malik
(1982) showed that BCFs for both edible and non-edible tissues were
0.23 and that the low level of accumulated activity was eliminated
rapidly when the fish were placed in clean water.
Yu et al. (1975) studied 14C-propachlor in a model ecosystem
containing seven species. There was no indication of either
bioconcentration or biomagnification; total radioactivity declined
from 0.21 to 0.015 mg propachlor/kg through the seven stages of the
food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
The application of propachlor as a herbicide in plant protection
results in its presence in air, soil and water. It is taken up from
the soil into plants through the root system.
5.1.1 Air
Propachlor application resulted in its presence in the ambient
air 300 m away from the treated field at concentrations ranging from
0.02-0.6 mg/m3 (Panshina, 1976). No details on the analytical method
or quantity of propachlor sprayed were given in this report.
5.1.2 Water
Propachlor was found in 34 of 1690 surface water samples analysed
in the USA (US EPA, 1988). Samples of surface water were collected at
475 locations and groundwater samples at 94 locations. The compound
was detected in eight different states. The maximum concentration
found was 10 µg/litre in surface water and 0.12 µg/litre in
groundwater (the 85th percentile for all non-zero samples was 2
µg/litre in surface water and 0.12 µg/litre in ground water). Spalding
& Snow (1989) detected propachlor at a maximum concentration of 46
µg/litre in stream water receiving its flow from agricultural land
planted principally with corn. The monitoring was carried out during
a spring period of high water flow and run-off.
5.1.3 Food
The residues of propachlor in potato tubers and tomatoes were
lower than 0.005 mg/kg (the limit of detection) approximately 60-70
days after application of the recommended dose of 4-6 kg/ha (Balinova,
1981). Warholic et al. (1983) could not detect propachlor (detection
limit, 0.06 mg/kg fresh weight) in cabbages grown on soil treated with
propachlor at 0.6 kg a.i./ha (wettable powder).
Frank et al. (1977) reported the results of a study on the fate
of propachlor applied to onions. Planted in May, the onions were
harvested in September and analysed in January the following year.
Conjugated N-isopropylaniline was present in onion tissue after
harvest and after a normal storage period for the crop before sale.
Bearing in mind the reports of other authors (Lamoureux et al., 1971),
Frank et al. (1977) suggested that 2-chloro- N-isopro-pylacetanilide
could be hydrolysed to 2-hydroxy- N-isopropyl-acetanilide and bonded
to glutathione. It is probable that alkaline hydrolysis cleaved the
weaker N-carbonyl C linkage to given N-isopropylaniline rather
than the stronger C-S bond which would have resulted in 2-hydroxy- N-
isopropylaniline. Tissue residues of N-isopropylaniline increased as
the rate of application of propachlor was increased, and the later the
application was made the higher the tissue residues (see Table 7).
Table 7. Residues of conjugated N-isopropylanilinea
Year Time of application Rate of propachlor N-isopropylaniline
application (kg/ha) residues (mg/kg)
1974 untreated 0 0.03
pre-emergence 7.2 0.05
12.0 0.09
pre-emergence and early post-
emergence (21 May and 13 June, 7.2 + 7.2 0.17
respectively) 12.0 + 12.0 0.15
pre-emergence and late post-
emergence (21 May and 9 July, 7.2 + 7.2 0.28
respectively) 12.0 + 12 0.40
a From: Frank et al. (1977). Onions were planted 6 May 1974 and harvested
4 September 1974. Analyses were performed in January 1975.
Propachlor has been used for soil treatment (6 kg/ha) before
planting tomatoes and peppers. Sampling was performed at 15-day
intervals and the samples were analysed by gas chromatography
(detection limits, 0.005 mg/kg). Small quantities of propachlor (of
the order of 0.04 mg/kg) were found in tomatoes on the 70th and 85th
days after soil treatment. On the 73rd day, 0.07 mg/kg was found in
peppers, and on the 88th and 108th days the residues had decreased to
0.05 and 0.04 mg/kg, respectively. No herbicide residues were found in
the following years (Balinova & Konstantinov, 1975).
In a study where soil was treated (8 kg/ha) before the seedlings
were planted, samples of cabbages contained propachlor residues
0.6-0.8, 0.3-0.4 and 0.1-0.16 mg/kg after one, two and three months,
respectively (Medved, 1977). No residues were detected in cabbages 96
days after the application of propachlor, at harvesting (Kolesnikov et
al., 1979). A decrease in dose and application by conveyor belt
perceptibly reduced the residues (Table 8).
Table 8. Residues (mg/kg) of propachlor in cabbages (type Amager 611)
Propachlor Date of Sampling time (no. of days after application)
application rate application
13 33 64 96
8 kg/ha (before setting out) 31 May 0.6 0.3 0.1 No
8 kg/ha (after prior 31 May 0.8 0.4 0.16 No
application of 5 kg/ha)
4 kg/ha (conveyor-belt 7 June 0.4 0.2 0.03 No
application after setting
out)
In a study by Nesterova et al. (1980), a dry soil area planted
with cabbages was treated with propachlor (7 kg/ha) each year. In the
second year no residues were detectable two months after application.
In the third year, when the moisture content of the soil had
increased, propachlor degradation was more rapid and no residues were
detectable 15 days after the application. The soil was dry in the
fourth year, and residues were detectable in cabbages up to two months
after application.
Lottman & Cowell (1986) reported propachlor residues in sorghum
grains of between < 0.02 and 0.24 mg/kg after treatment of soil at
4.4 kg/ha. Lottman & Cowell (1987) found residues in corn grain of <
0.02 to 0.04 mg/kg after soil treatment with propachlor at 4.4 kg/ha
and of < 0.02 to 0.19 mg/kg after treatment at 6.6 kg/ha.
5.2 Occupational exposure
Only limited data are available on occupational exposure to
propachlor. Its application by a tractor-mounted sprayer to cabbages
resulted in its presence in the breathing zone of the tractor drivers
at levels of 0.8 to 2.1 mg/m3 (Panshina, 1977) and 0.1 to 3.7
mg/m3 (Panshina, 1976).
6. KINETICS AND METABOLISM
6.1 Absorption
Propachlor may be absorbed through the respiratory and
gastrointestinal tracts as well as through the skin. Following a
single oral administration in mammals, it is rapidly taken up into the
blood and internal organs, reaching its maximum blood concentration in
1 h. After 48 h it is no longer detectable in the organs (Panshina,
1985). An estimated 68% of a single 10 mg dose of ring-labelled
14C-propachlor administered to 12 rats was recovered in urine 56 h
later (Malik, 1986). These results are supported by those of other
studies in which 54-64% (Lamoureux & Davison, 1975) and 68.8% (Bakke
et al., 1980) of the administered dose was recovered in urine 24 h and
48 h after dose administration, respectively.
6.2 Metabolic transformation
Propachlor is rapidly metabolized. Its metabolism in animal
species has been studied by Bakke & Price (1979), Pekas et al. (1979),
Bakke et al. (1981a,b,c), Rafter et al. (1983a,b), Aschbacher &
Struble (1987) and Davison et al. (1988, 1990). Most of the animal
species studied metabolize propachlor through the mercapturic acid
pathway (MAP). The intestinal microflora is involved in the metabolism
of MAP intermediates (Bakke et al., 1981c). Metabolites of propachlor,
in which chlorine from the parent compound (2-chloro-
isopropylacetanilide) is removed by a nucleophilic displacement
(Rafter et al., 1983a) by a cysteine group or methylsulfonyl group
(CH3SO2), are present in the urine of rats dosed orally with
propachlor (Larsen & Bakke, 1979). It has been shown that a cysteine
conjugate of propachlor is the source of sulfur in methylsulfonyl-
containing metabolites, but that the carbon in the methylsulfonyl
group does not come from the cysteine moiety. Propachlor is conjugated
firstly with glutathione and the reaction is mediated by glutathione
transferases. The glutathione conjugation provides a means for
inactivation of reactive electrophiles. Glutathione conjugates have
the required physico-chemical properties for biliary excretion and
will generally be present, together with their catabolites
cysteinyl-glycine, cysteine and N-acetylcysteine-mercapturic acid,
in relatively high concentrations in the bile (Rafter et al., 1983a).
After excretion with the bile, they are metabolized in the intestine
where the C-S lyase present cleaves the cysteine conjugate, allowing
further metabolism of sulfur to a methylsulfonyl-containing moiety
(Larsen & Bakke, 1979).
The C-S lyase enzyme systems have been isolated in rat liver and
bacteria demonstrating that they are of bacterial origin. As a good
example, C-S lyase from Fusobacterium necrophorum, one of the pure
intestinal bacteria, has been isolated and characterized as a key
enzyme in mammalian metabolism (Larsen et al., 1983). This is
confirmed by the fact that in germ-free rats (Bakke et al., 1980)
(Fig. 2) and rats treated by antibiotics (Larsen & Bakke, 1981) 14C
was excreted from 14C-propachlor as MAP metabolites, but there were
no methylsulfonyl-containing metabolites in urine. Inextractable
residues were eliminated in the faeces. This shows that MAP
metabolites are available as substrates for the intestinal microflora.
Of the MAP metabolites studied, the glutathione and cysteine
conjugates are the best substrates both for production of
2-mercapto- N-isopropyl-acetanilide and for parallel formation of
insoluble 14C residues (Larsen & Bakke, 1983) which are excreted in
the faeces.
I. R = CH2Cl
2-chloro- N-(1-methylethyl)- N-phenylacetamide = propachlor
II. R = CH2OH
2-hydroxy- N-(1-methylethyl)- N-phenylacetamide
III. R = CH3
N-(1-methylethyl)- N-phenylacetamide
O
"
IV. R = COH
[(1-methylethyl)phenylamino]oxoacetic acid
V. R - CH2SO3H
2-[(1-methylethyl)phenylamino]-2-oxoethanesulfonic acid
O O
" "
VI. R = CH2SCH2COH
{{[(methylethyl)phenylamino]acetyl}sulfinyl}-acetic acid
O
"
VII. R = CH2SCH3
N-(1-methylethyl)-2-(methylsulfinyl)- N-phenylacetamide
Fig. 1. Structures of propachlor degradation products.
4.1.1.4 Persistence
Fletcher & Kirkwood (1982) reported a half-life of 2-3 weeks for
propachlor. Free propachlor disappeared rapidly in soil treated by
Ritter et al. (1973); in 21-28 days residues of free propachlor
declined 72-80%. In earlier laboratory studies and field bioassays
carried out by Menges & Tamez (1973), the soil persistence (> 90%
degradation) was found to be less than 6 months. According to Melnikov
et al. (1985) and Zhukova & Shirko (1979), the period of propachlor
degradation in soil to non-toxic products is about 2 months. The
presence of an alkyl group attached to the nitrogen atom in its
molecule prevents its decomposition to aniline or to azobenzolic
residues, which could later be transferred into tetrachlorazobenzene
(Panshina, 1985). Propachlor applied at 4-8 kg/ha was detoxified in
peat soil within 59-63 days (Vasilev, 1982). When it was applied at
6.5, 9 and 11 kg/ha, it was still detectable after 113 days in two
types of soil in the Voronezh and Krasnodar regions of the former USSR
(Kolesnikov et al., 1980). The longer persistence determined in this
study might be connected with the climatic conditions, particularly
the low temperatures. Roberts et al. (1978) and Balinova (1981)
confirmed that propachlor is rapidly decomposed in soil.
Field studies evaluating the degradation of propachlor in soil
showed that 70 days after application, propachlor was detectable in
insignificant quantities (0.04 mg/kg, i.e. 2% of the dose applied).
Degradation was slower in dry than humid weather (Zhukova & Shirko,
1979) (Table 3). The conclusions of the authors were that degradation
in soil is rapid and there is no possibility of propachlor
accumulation in crops.
Table 3. Dynamics of dissipation of propachlor in soil (average data
for 1974-1976)a
Dose application After 10th day On 30th day On 50th day On 70th day
(kg propachlor/ha) mg/kg %b mg/kg %b mg/kg %b mg/kg %b
6 1.73 86.4 0.95 42.8 0.10 5.2 0.04 2
8 2.44 92.6 1.55 58.3 0.20 7.8 0.04 2
10 3.14 94.2 2.53 76.0 0.40 12.0 0.04 2
a From: Zhukova & Shirko (1979)
b Percentage of dose applied
In a study by Frank et al. (1977), soil with high organic matter
content was treated with 19 kg propachlor/ha. The treatment times and
rates are given in Table 4. Soil samples were collected to a depth of
20 cm and analysed. Residues of the conjugated metabolite
N-isopropylaniline of up to 3.7 mg/kg soil were detected 2 years
after the application. They were released from soil by hydrolysis,
indicating the presence of active bonding sites for the metabolite in
the soil. The authors concluded that using propachlor in successive
years led to accumulation of long-lasting conjugated
N-isopropylaniline. Applications made once every 3 years, however,
did not lead to such accumulation and they recommended this type of
application.
Table 4. Residues of conjugated N-isopropylaniline in organic soil
treated with propachlor in 1971, 1972 and 1975a.
Year Time of application Rate of application N-isopropylaniline
(kg/ha)b residues in
oven-dried soil
(mg/kg)
1971 May and June 9 + 10 not analysed
1972 untreated none 3.67
May, July, August 6.7 + 3.4 + 3.4 7.70
May, July, August 6.7 + 6.7 + 6.7 9.47
1975 May and June 6.7 + 6.7 3.16
a From: Frank et al. (1977)
b Soil samples were collected in May 1973 and April 1976 and analysed
in January and April 1976, respectively.
US EPA (1984b) re-evaluated the existing data concerning some
environmental aspects of propachlor. It was concluded that microbes
were the primary factor in its breakdown in soil and that its loss
from photodecomposition and/or volatilization was low. Although this
earlier assessment suggested a potential for propachlor to contaminate
ground water, a later assessment (US EPA, 1988) concluded that "the
rapid degradation of low levels of propachlor in soil is expected to
result in a low potential for groundwater contamination by
propachlor".
4.1.1.5 Environmental conditions affecting distribution and breakdown
Walker & Brown (1982, 1985) carried out laboratory studies and
field trials in parallel. They found first-order kinetics dissipation
of propachlor and confirmed the results of Beestman & Deming (1974).
They also described a clear temperature dependence: an increase in
temperature of 10 °C reduced the half-life by a factor of between 1.9
and 2.5. Soil moisture also influenced degradation: slower rates of
loss were found in drier soil. The half-life in soil with a moisture
content of 6% was about twice as long as at 15% (Table 5). In general,
the time of persistence in the field was comparable to that measured
in laboratory studies. The half-life of propachlor varied from 4 to 22
days (Table 5).
Table 5. Half-life for propachlor degradation (days)a
Temperature (°C) 25 25 25 25 15 5
Moisture (%) 6 9 12 15 12 12
Propachlor 7.7 4.6 4.2 3.7 9.2 21.7
a From: Walker & Brown (1985)
The persistence of propachlor in soil as a result of all
microbial, chemical and physical processes has been studied by Zimdahl
& Clark (1982). They measured the half-life of propachlor in clay loam
and sandy loam soils in the laboratory, using different temperature
and moisture conditions (Table 6). An increase in temperature (from 10
to 30 °C) and moisture content (from 20 to 80%) shortened the
half-life.
Vasilev (1982) confirmed the importance of meterological
conditions, type of soil, and rate and period of application on the
degradation in soil. In dry weather, degradation took longer than in
humid conditions. Based on field experiments where soil was treated
with propachlor and carrots were then planted, the author reported the
following residues (measured at the moment of harvest in the soil
layer 0-20 cm) in soil: at an application rate of 4 kg/ha the
propachlor residues were 0.05 mg/kg; at 6 kg/ha they were 0.15 mg/kg;
and at 8 kg/ha they were 0.2 mg/kg.
Table 6. Half-lives of propachlor in clay loam and sandy loam as
determined by bioassaya
Storage conditions Half-life (days)
Temperature Soil moisture Sandy loam Clay loam
(°C) (%)
10 50 16.7 14.3
20 50 3.3 5.3
30 50 1.9 1
20 20 23.1 21.5
20 50 3.3 5.3
20 80 3.3 4.1
a From: Zimdahl & Clark (1982)
Shirko & Belova (1982) found that residues of propachlor in soil
and plants depended on the nitrogen and potassium content of the soil.
At the moment of harvesting, no residues of propachlor were detected
in soil after application at a rate of 4.5-6.5 kg/ha. According to the
authors, a better supply of plants with nutrients (in this case,
nitrogen and potassium fertilizers) leads to more intense
detoxification and degradation of the herbicide and, conversely, an
insufficiency delays the detoxification processes and leads to the
accumulation of residues.
4.1.2 Water
Photodegradation of propachlor in aqueous media was studied by
Tanaka et al. (1981) under laboratory conditions. They used a 10-ml
sample with propachlor concentrations ranging up to 100 mg/litre, and
the sample was irradiated for 135 min with a 300-nm sunlight lamp.
Very weak photolysis was registered; by the end of the study only 1%
of herbicide had been lost. Addition of a commercial surfactant (2%
heterogeneous non-ionic Tergitol TMN, acting as photosensitizer)
allowed 37% of propachlor to be photodegraded. It is difficult to make
conclusions on this study because of certain deficiencies. The use of
a commercial formulation in such studies should be avoided since some
of the constituents may cause indirect photochemical reactions. No
mention was made in the report of the purity of the compound studied.
The test should preferably be carried out at constant temperature. The
lack of data concerning the intensity of the irradiation source (US
EPA, 1984a) does not allow any extrapolation of these data to the
environment.
Rejtö et al. (1984) investigated the effects of ultraviolet
irradiation of propachlor solutions and found that 5 h of irradiation
led to 80% decomposition. The three photodecomposition products
identified were: N-isopropyloxindole, N-isopropyl-3-
hydroxyoxindole and a spiro compound. Irradiation of a solution of
propachlor with visible light for 12 h led to almost complete
decomposition in the presence of riboflavin as a photosensitizer. The
photodecomposition products after visible light irradiation were found
to be non-phytotoxic.
Monsanto (1987) demonstrated that propachlor is hydrolytically
stable.
Volatilization of propachlor from aqueous media is of limited
significance because of the high solubility in water and relatively
low vapour pressure of the compound. In agreement with Henry's
constant, the loss of propachlor by sorption and sedimentation in
water bodies does not appear to be very significant (US EPA, 1984a).
The role of microbial biodegradation appears to be of major
significance in water as well as in soil. Novick & Alexander (1985)
studied the metabolism of low concentrations (10 µg/litre) of
propachlor in sewage and lake water. They found that under aerobic
conditions microbial populations from sewage and lake waters were not
able to mineralize the carbon ring of propachlor in six weeks.
However, propachlor was extensively metabolized, the products obtained
were organic, and they were found to accumulate in the environment. In
a parallel study, it was found that aniline was readily cleaved under
similar conditions, indicating rapid mineralization of this compound.
It was concluded that structural characteristics of propachlor, other
than the ring, account for the mineralization of the compound. The
presence of the three substituents on the nitrogen atom in propachlor
may be the reason for its persistence. Steen & Collette (1989)
determined microbial transformation rate constants for seven amides in
natural pond water. A second order mathematical rate expression served
to describe propachlor degradation, and a value of 1.1 x 10-9
litres/organism per h was calculated. Brightwell et al. (1981)
presented data showing slow degradation of propachlor in lake water
under aerobic conditions. After 30 days, 84.5% of the propachlor
remained unchanged; under these conditions a half-life of about 5
months would be expected. The low rate of metabolism was due to a low
level of microorganisms. Yu et al. (1975) studied the degradation of
propachlor in water using a model ecosystem. An aquarium with
7-day-old sorghum plants was used with the addition of 50 µCi of 14C
ring-labelled propachlor. By the end of the 33-day experimental
period, 7 degradation products in water were determined by thin-layer
chromatography but were not identified. At that time only 0.4% of the
radioactivity of the dose applied remained in the water.
4.1.3 Plants
The metabolism of propachlor in corn seedlings and in excized
leaves of corn, sorghum, sugar cane and barley was studied by
Lamoureux et al. (1971). Metabolism was rapid and similar in all
mentioned plant species during the first 6-24 h following treatment.
At least 3 water-soluble metabolites were produced in each species
during this period. Two of these metabolites were isolated; the first
one was identified as the glutathione conjugate of propachlor
(compound I) and the second one appeared to be the gamma-
glutamylcysteine conjugate (compound II). The primary mode of
metabolism is a nucleophilic displacement of the alpha-chloro group of
propachlor by the sulfhydryl group of a peptide. The metabolic
reactions of propachlor proceed non-enzymatically in vitro, and the
in vivo reaction may be enzymatic and/or non-enzymatic. The high
percentage of propachlor converted to compounds I and II in corn
seedlings during the first 18 h following treatment indicates that the
reaction of propachlor with glutathione and/or gamma-glutamylcysteine
is quite specific. This would be expected if the reaction is
enzymatic. Some glutathione and gamma-glutamylcysteine conjugates in
plants may be end-products, but, in the case of propachlor, these
metabolites are transient intermediates. Further studies are needed to
establish the final steps (Lamoureux et al., 1971).
Pantano & Anderson (1987) studied the metabolism of propachlor in
sorghum (milo). Sorghum seeds were planted in soil treated with 14C
ring-labelled propachlor at a rate of 3.3 kg/ha and grown to maturity
in a greenhouse. Following senescence, plants were separated into
various anatomical parts, freeze-dried and analysed for activity. The
uptake of 14C in the foliage and grain was 9.5 and 0.5 mg/kg dry
weight, respectively. Four metabolites were identified in the foliage
extract; these represented 66.5% of the metabolites in foliage. The
four metabolites were [( N-iso-propyl) phenylamino]oxoacetic acid,
{{[( N-isopropyl)-phenyl-amino]acetyl}sulfinyl}lactic acid,
{{[( N-isopropyl)phenylamino] acetyl}sulfinyl}acetic acid and
2-[( N-isopropyl)phenylamino]-2-oxoethanesulfonic acid. In addition,
other metabolites found at low levels in the foliage extract were
characterized. Freeze-dried sorghum grain contained only 0.5 mg/kg of
14C activity and only one metabolite was identified (the first
compound mentioned above). This metabolite constituted at least 24.9%
of the activity in grain. In all of the major metabolites identified
in this study, no modification of the N-isopropylaniline moiety was
observed. On the basis of metabolites identified in this study and
known pathways for the metabolic transformations of related
chloroacetamides, a scheme for the metabolic fate of propachlor in
sorghum plants was postulated by the authors. The biotrans-formations
include: 1) displacement of the chlorine by an oxygen-containing
nucleophile followed by oxidation, and 2) conjugation with glutathione
followed by further metabolic modification of this conjugate.
Lamoureux & Rusness (1989) studied the metabolism of propachlor
in soybean and found that it was rapidly metabolized to
homoglutathione conjugate in roots and foliage. This conjugate was
rapidly metabolized to the cysteine conjugate and then slowly
converted to a variety of other metabolites; four of these were
present up to 72 days after application of propachlor. These four were
malonylcysteine, malonylcysteine S-oxide, 3-sulfinyllactic acid and
O-malonyl glucoside conjugates of propachlor. Less than 1% of
metabolites was isolated from beans or pods, the great majority being
in roots and foliage. The major metabolites found in plants were the
same as those produced in soil. The authors suggested that it is
difficult to differentiate between metabolites formed in the plant and
those taken up from soil as to the relative importance of the two
sites of metabolic degradation.
4.2 Bioaccumulation and biomagnification
The two published values for the log octanol/water partition
coefficient (log Kow) of propachlor, i.e. 1.62 and 2.3 (US EPA,
1984b, 1988), indicate a moderate potential for bioaccumulation.
Barrows & Macek (1974) used bluegill sunfish exposed to
14C-labelled propachlor in a continuous-flow experiment in order to
assess the potential for the compound to bioconcentrate in aquatic
organisms. The fish were exposed for 35 days to a mean
14C-propachlor concentration of either 0.54 mg/litre (high level) or
0.012 mg/litre (low level) in the water. Analysis of the fish tissues
indicated bioconcentration factors (BCFs) in the edible tissue of 34
and 22 for the high and low levels, respectively, and in the
non-edible tissue of 22 and 20. When the fish were placed in clean
water the activity was rapidly eliminated from the non-edible portion
of the fish and at a somewhat slower rate from the edible tissue.
Because of the polar, water-soluble nature of the soil metabolites
(section 4.1.1), they would be less likely to bioconcentrate in
aquatic organisms. The results of a static study on catfish by Malik
(1982) showed that BCFs for both edible and non-edible tissues were
0.23 and that the low level of accumulated activity was eliminated
rapidly when the fish were placed in clean water.
Yu et al. (1975) studied 14C-propachlor in a model ecosystem
containing seven species. There was no indication of either
bioconcentration or biomagnification; total radioactivity declined
from 0.21 to 0.015 mg propachlor/kg through the seven stages of the
food chain.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
The application of propachlor as a herbicide in plant protection
results in its presence in air, soil and water. It is taken up from
the soil into plants through the root system.
5.1.1 Air
Propachlor application resulted in its presence in the ambient
air 300 m away from the treated field at concentrations ranging from
0.02-0.6 mg/m3 (Panshina, 1976). No details on the analytical method
or quantity of propachlor sprayed were given in this report.
5.1.2 Water
Propachlor was found in 34 of 1690 surface water samples analysed
in the USA (US EPA, 1988). Samples of surface water were collected at
475 locations and groundwater samples at 94 locations. The compound
was detected in eight different states. The maximum concentration
found was 10 µg/litre in surface water and 0.12 µg/litre in
groundwater (the 85th percentile for all non-zero samples was 2
µg/litre in surface water and 0.12 µg/litre in ground water). Spalding
& Snow (1989) detected propachlor at a maximum concentration of 46
µg/litre in stream water receiving its flow from agricultural land
planted principally with corn. The monitoring was carried out during
a spring period of high water flow and run-off.
5.1.3 Food
The residues of propachlor in potato tubers and tomatoes were
lower than 0.005 mg/kg (the limit of detection) approximately 60-70
days after application of the recommended dose of 4-6 kg/ha (Balinova,
1981). Warholic et al. (1983) could not detect propachlor (detection
limit, 0.06 mg/kg fresh weight) in cabbages grown on soil treated with
propachlor at 0.6 kg a.i./ha (wettable powder).
Frank et al. (1977) reported the results of a study on the fate
of propachlor applied to onions. Planted in May, the onions were
harvested in September and analysed in January the following year.
Conjugated N-isopropylaniline was present in onion tissue after
harvest and after a normal storage period for the crop before sale.
Bearing in mind the reports of other authors (Lamoureux et al., 1971),
Frank et al. (1977) suggested that 2-chloro- N-isopro-pylacetanilide
could be hydrolysed to 2-hydroxy- N-isopropyl-acetanilide and bonded
to glutathione. It is probable that alkaline hydrolysis cleaved the
weaker N-carbonyl C linkage to given N-isopropylaniline rather
than the stronger C-S bond which would have resulted in 2-hydroxy- N-
isopropylaniline. Tissue residues of N-isopropylaniline increased as
the rate of application of propachlor was increased, and the later the
application was made the higher the tissue residues (see Table 7).
Table 7. Residues of conjugated N-isopropylanilinea
Year Time of application Rate of propachlor N-isopropylaniline
application (kg/ha) residues (mg/kg)
1974 untreated 0 0.03
pre-emergence 7.2 0.05
12.0 0.09
pre-emergence and early post-
emergence (21 May and 13 June, 7.2 + 7.2 0.17
respectively) 12.0 + 12.0 0.15
pre-emergence and late post-
emergence (21 May and 9 July, 7.2 + 7.2 0.28
respectively) 12.0 + 12 0.40
a From: Frank et al. (1977). Onions were planted 6 May 1974 and harvested
4 September 1974. Analyses were performed in January 1975.
Propachlor has been used for soil treatment (6 kg/ha) before
planting tomatoes and peppers. Sampling was performed at 15-day
intervals and the samples were analysed by gas chromatography
(detection limits, 0.005 mg/kg). Small quantities of propachlor (of
the order of 0.04 mg/kg) were found in tomatoes on the 70th and 85th
days after soil treatment. On the 73rd day, 0.07 mg/kg was found in
peppers, and on the 88th and 108th days the residues had decreased to
0.05 and 0.04 mg/kg, respectively. No herbicide residues were found in
the following years (Balinova & Konstantinov, 1975).
In a study where soil was treated (8 kg/ha) before the seedlings
were planted, samples of cabbages contained propachlor residues
0.6-0.8, 0.3-0.4 and 0.1-0.16 mg/kg after one, two and three months,
respectively (Medved, 1977). No residues were detected in cabbages 96
days after the application of propachlor, at harvesting (Kolesnikov et
al., 1979). A decrease in dose and application by conveyor belt
perceptibly reduced the residues (Table 8).
Table 8. Residues (mg/kg) of propachlor in cabbages (type Amager 611)
Propachlor Date of Sampling time (no. of days after application)
application rate application
13 33 64 96
8 kg/ha (before setting out) 31 May 0.6 0.3 0.1 No
8 kg/ha (after prior 31 May 0.8 0.4 0.16 No
application of 5 kg/ha)
4 kg/ha (conveyor-belt 7 June 0.4 0.2 0.03 No
application after setting
out)
In a study by Nesterova et al. (1980), a dry soil area planted
with cabbages was treated with propachlor (7 kg/ha) each year. In the
second year no residues were detectable two months after application.
In the third year, when the moisture content of the soil had
increased, propachlor degradation was more rapid and no residues were
detectable 15 days after the application. The soil was dry in the
fourth year, and residues were detectable in cabbages up to two months
after application.
Lottman & Cowell (1986) reported propachlor residues in sorghum
grains of between < 0.02 and 0.24 mg/kg after treatment of soil at
4.4 kg/ha. Lottman & Cowell (1987) found residues in corn grain of <
0.02 to 0.04 mg/kg after soil treatment with propachlor at 4.4 kg/ha
and of < 0.02 to 0.19 mg/kg after treatment at 6.6 kg/ha.
5.2 Occupational exposure
Only limited data are available on occupational exposure to
propachlor. Its application by a tractor-mounted sprayer to cabbages
resulted in its presence in the breathing zone of the tractor drivers
at levels of 0.8 to 2.1 mg/m3 (Panshina, 1977) and 0.1 to 3.7
mg/m3 (Panshina, 1976).
6. KINETICS AND METABOLISM
6.1 Absorption
Propachlor may be absorbed through the respiratory and
gastrointestinal tracts as well as through the skin. Following a
single oral administration in mammals, it is rapidly taken up into the
blood and internal organs, reaching its maximum blood concentration in
1 h. After 48 h it is no longer detectable in the organs (Panshina,
1985). An estimated 68% of a single 10 mg dose of ring-labelled
14C-propachlor administered to 12 rats was recovered in urine 56 h
later (Malik, 1986). These results are supported by those of other
studies in which 54-64% (Lamoureux & Davison, 1975) and 68.8% (Bakke
et al., 1980) of the administered dose was recovered in urine 24 h and
48 h after dose administration, respectively.
6.2 Metabolic transformation
Propachlor is rapidly metabolized. Its metabolism in animal
species has been studied by Bakke & Price (1979), Pekas et al. (1979),
Bakke et al. (1981a,b,c), Rafter et al. (1983a,b), Aschbacher &
Struble (1987) and Davison et al. (1988, 1990). Most of the animal
species studied metabolize propachlor through the mercapturic acid
pathway (MAP). The intestinal microflora is involved in the metabolism
of MAP intermediates (Bakke et al., 1981c). Metabolites of propachlor,
in which chlorine from the parent compound (2-chloro-
isopropylacetanilide) is removed by a nucleophilic displacement
(Rafter et al., 1983a) by a cysteine group or methylsulfonyl group
(CH3SO2), are present in the urine of rats dosed orally with
propachlor (Larsen & Bakke, 1979). It has been shown that a cysteine
conjugate of propachlor is the source of sulfur in methylsulfonyl-
containing metabolites, but that the carbon in the methylsulfonyl
group does not come from the cysteine moiety. Propachlor is conjugated
firstly with glutathione and the reaction is mediated by glutathione
transferases. The glutathione conjugation provides a means for
inactivation of reactive electrophiles. Glutathione conjugates have
the required physico-chemical properties for biliary excretion and
will generally be present, together with their catabolites
cysteinyl-glycine, cysteine and N-acetylcysteine-mercapturic acid,
in relatively high concentrations in the bile (Rafter et al., 1983a).
After excretion with the bile, they are metabolized in the intestine
where the C-S lyase present cleaves the cysteine conjugate, allowing
further metabolism of sulfur to a methylsulfonyl-containing moiety
(Larsen & Bakke, 1979).
The C-S lyase enzyme systems have been isolated in rat liver and
bacteria demonstrating that they are of bacterial origin. As a good
example, C-S lyase from Fusobacterium necrophorum, one of the pure
intestinal bacteria, has been isolated and characterized as a key
enzyme in mammalian metabolism (Larsen et al., 1983). This is
confirmed by the fact that in germ-free rats (Bakke et al., 1980)
(Fig. 2) and rats treated by antibiotics (Larsen & Bakke, 1981) 14C
was excreted from 14C-propachlor as MAP metabolites, but there were
no methylsulfonyl-containing metabolites in urine. Inextractable
residues were eliminated in the faeces. This shows that MAP
metabolites are available as substrates for the intestinal microflora.
Of the MAP metabolites studied, the glutathione and cysteine
conjugates are the best substrates both for production of
2-mercapto- N-isopropyl-acetanilide and for parallel formation of
insoluble 14C residues (Larsen & Bakke, 1983) which are excreted in
the faeces.
 In normal rats dosed with propachlor, the above-mentioned final
metabolites were formed when MAP metabolites underwent a number of
reactions: carbon-sulfur bond cleavage by microflora, S-methylation,
S-oxidation, ring and alkyl-hydroxylation, glucuronide conjugation,
N-dealkylation and amide cleavage (Rafter et al., 1983b).
Fig. 3 shows the proposed metabolic pathway for the formation of
methylsulfonyl metabolites in rats and pigs (Aschbacher & Struble,
1987) and Fig. 4 the metabolism in normal rats treated with propachlor
proposed by Bakke et al. (1980).
The tissue in which propachlor enters the mercapturic acid
pathway has not been determined. The liver is an obvious site for
glutathione conjugation, but the intestine cannot be excluded (Bakke
et al., 1980). Organ perfusion studies have demonstrated that all
enzymes necessary for the formation of mercapturic acid conjugates are
present in the kidneys of both chickens and rats (Davison et al.,
1988, 1990) and in the livers of rats (Davison et al., 1990). Rat
caecal contents are similar to those of the pig with respect to C-S
lyase activity, which explains the general similarity of their
metabolic transformations (Larsen & Bakke, 1983).
When pig caecal contents were incubated with the glutathione
conjugate of propachlor, the formation of both insoluble residues and
the thiol increased with increase in incubation period (Table 9).
Digestive peptidases extracted during isolation of the metabolites
were thought to be the explanation for the presence of the cysteine
conjugate in the zero time samples, because no cysteine conjugate was
isolated from heat-treated caecal contents. A decrease in glutathione
concentration with increased incubation time was also evident and was
confirmation of a product-precursor relationship. This decrease in
glutathione conjugate concentration was assumed to be caused by
formation of cysteine conjugate (82%), due to cleavage by peptidase
activity, in the caecum (Larsen & Bakke, 1983).
In summary, three or more enterohepatic cycles for propachlor
metabolism in normal rats have been described. In the first,
propachlor is metabolized via the mercapturic acid pathway and the
conjugates are excreted in the bile. The second cycle is initiated
when the biliary mercapturic acid pathway metabolites are metabolized
by microbial/intestinal C-S lyase into reabsorbable metabolites
(possibly 2-mercapto- N-isopropylacetanilide). The reabsorbable
metabolites are further metabolized to glucuronides by glucuronidase
enzymes, and these are secreted with the bile. These biliary
glucuronides subsequently initiate the third cycle in the
enterohepatic circulation of propachlor metabolites.
No doubt the intestinal microorganisms complicate the metabolism
of propachlor (in comparison with the situation in germ-free and
antibiotic-treated rats) and create new non-polar compounds from the
products of the mercapturic acid pathway, which are reabsorbed into
the blood. These new compounds have to be converted again into polar
products in order to be excreted (Bakke et al., 1980).
In normal rats dosed with propachlor, the above-mentioned final
metabolites were formed when MAP metabolites underwent a number of
reactions: carbon-sulfur bond cleavage by microflora, S-methylation,
S-oxidation, ring and alkyl-hydroxylation, glucuronide conjugation,
N-dealkylation and amide cleavage (Rafter et al., 1983b).
Fig. 3 shows the proposed metabolic pathway for the formation of
methylsulfonyl metabolites in rats and pigs (Aschbacher & Struble,
1987) and Fig. 4 the metabolism in normal rats treated with propachlor
proposed by Bakke et al. (1980).
The tissue in which propachlor enters the mercapturic acid
pathway has not been determined. The liver is an obvious site for
glutathione conjugation, but the intestine cannot be excluded (Bakke
et al., 1980). Organ perfusion studies have demonstrated that all
enzymes necessary for the formation of mercapturic acid conjugates are
present in the kidneys of both chickens and rats (Davison et al.,
1988, 1990) and in the livers of rats (Davison et al., 1990). Rat
caecal contents are similar to those of the pig with respect to C-S
lyase activity, which explains the general similarity of their
metabolic transformations (Larsen & Bakke, 1983).
When pig caecal contents were incubated with the glutathione
conjugate of propachlor, the formation of both insoluble residues and
the thiol increased with increase in incubation period (Table 9).
Digestive peptidases extracted during isolation of the metabolites
were thought to be the explanation for the presence of the cysteine
conjugate in the zero time samples, because no cysteine conjugate was
isolated from heat-treated caecal contents. A decrease in glutathione
concentration with increased incubation time was also evident and was
confirmation of a product-precursor relationship. This decrease in
glutathione conjugate concentration was assumed to be caused by
formation of cysteine conjugate (82%), due to cleavage by peptidase
activity, in the caecum (Larsen & Bakke, 1983).
In summary, three or more enterohepatic cycles for propachlor
metabolism in normal rats have been described. In the first,
propachlor is metabolized via the mercapturic acid pathway and the
conjugates are excreted in the bile. The second cycle is initiated
when the biliary mercapturic acid pathway metabolites are metabolized
by microbial/intestinal C-S lyase into reabsorbable metabolites
(possibly 2-mercapto- N-isopropylacetanilide). The reabsorbable
metabolites are further metabolized to glucuronides by glucuronidase
enzymes, and these are secreted with the bile. These biliary
glucuronides subsequently initiate the third cycle in the
enterohepatic circulation of propachlor metabolites.
No doubt the intestinal microorganisms complicate the metabolism
of propachlor (in comparison with the situation in germ-free and
antibiotic-treated rats) and create new non-polar compounds from the
products of the mercapturic acid pathway, which are reabsorbed into
the blood. These new compounds have to be converted again into polar
products in order to be excreted (Bakke et al., 1980).

 Table 9. Incubation of the glutathione conjugate of propachlor with pig caecal contents for various durationsa
Metabolites recovered Incubation time
0 20 min 40 min 1 h 2 h 4 h
2-Mercapto-N-isopropylacetanilide 7.7b 14.5b 23.0b 28.3b 34.7b 43.4c
(5.1-10.6) (5.5-22.8) (15.8-36.0) (22.9-38.7) (21.8-44.0) (38.7-49.2)
Glutathione conjugate 26.8 36.0 20.8 28.8 21.5 16.5
of propachlor (15.8-38.1) (18.1-51.6) (13.3-25.3) (16.6-42.3) (14.0-32.2) (14.8-19.2)
Cysteine conjugate of 47.5 32.5 35.1 20.9 11.9 3.8
propachlor (36.0-51.5) (21.3-43.7) (30.5-41.9) (12.0-27.9) (10.9-12.5) (2.2-5.6)
Non-extractable 14C 13.1 12.9 16.0 16.6 25.4 33.9
residues (13.0-13.4) (9.9-17.8) (13.5-20.5) (15.0-18.9) (22.8-28.2) (31.5-35.8)
a From: Larsen & Bakke (1983).
b Isolated as 2-carboxymethylthio- N-isopropylacetanilide
c Isolated as 2-(13C)-carboxymethylthio- N-isopropylacetanilide
Pigs were gilts. Results are shown as a percentage of the substrate. Values given in parentheses represent the range of values
obtained from 14C-recovery measurements.
More recent studies carried out by Aschbacher & Struble (1987) on
the metabolism of propachlor in pigs have proved the similarity, i.e.
the formation of methylsulfonyl-containing metabolites, with the
metabolism of rats, but have also revealed some differences.
A pig with a cannulated bile duct, which was dosed orally with
14C-propachlor, excreted 7.6% of the dose in the bile compared with
approximately 75% in the case of the rats. When enterohepatic
circulation was prevented in the bile of cannulated pigs,
CH3SO2-containing metabolites of propachlor were excreted in the
urine. As mentioned above, enterohepatic circulation is necessary for
the production of methylsulfonyl-containing metabolites in rats. In
experiments with germ-free pigs dosed orally with 14C-propachlor, it
was shown that they did not excrete urinary CH3SO2 metabolites, which
indicated involvement of the intestinal flora in the production of
these metabolites, as occurs in rats. Non-biliary excretion of
metabolites of propachlor into the lumen of the intestine probably
occurred. It is presumed that propachlor is absorbed by the mucosal
cells and conjugated with glutathione and that some of this conjugate
moves directly into the lumen of the intestinal tract by simple
diffusion, where it becomes the substrate of bacterial beta-lyase. The
presence of glutathione transferase has been demonstrated previously
in subcellular fractions of mucosal tissue homogenates.
In situ intestinal absorption of propachlor and non-biliary
excretion of metabolites into the intestinal tract of rats, pigs and
chickens was studied by Struble (1991). Propachlor was absorbed from
in situ intestinal loops of rats and pigs, the absorption half-times
being 7.5 and 16.5 min, respectively. Water-soluble 14C-labelled
metabolites that accumulated in the intestinal loops accounted for
31%, 53% and 25% of the initial 14C in rats, pigs and chickens,
respectively. Propachlor- S-cysteine was identified as the major
metabolite in the pig intestinal lumen (43% of the water-soluble
14C). It was concluded that the intestinal metabolism and intestinal
excretion of water-soluble metabolites of propachlor are important
physiological processes that occur in a variety of animal species.
These processes provide a route by which metabolites of xenobiotics
may reach the intestinal lumen in animals that are poor biliary
excretors. These studies demonstrated that although bile may be the
major route by which MAP metabolites are made available to the
intestinal microflora in the rat, an extrahepatic route exists in the
pig.
Davison (1991) conducted a study using six anaesthetized 2- to
21-day-old male Guernsey calves weighing 28 to 61 kg in which either
the left kidney was perfused (via the left renal artery) or the left
ureter was perfused with metabolites of propachlor. The glutathione
conjugate of propachlor (2- S-glutathionyl- N-acetyl-acetanilide)
was metabolized in both kidney and ureter to the cysteine conjugate.
When the mercapturic acid conjugate of propachlor was presented to the
kidney, it was eliminated in urine. First-pass metabolism and
elimination of the glutathione conjugate by the kidney was 16% of the
dose, whereas first-pass elimination of the mercapturic acid was 33%.
Absorption of the glutathione conjugate of propachlor or its
metabolites, or of glycine by the ureter was nil. The cattle may be
unable to form mercapturic acids from glutathione conjugates of some
xenobiotics, which may result in their being more easily poisoned by
these xenobiotics than chickens, pigs and rats.
The glutathione conjugate of 2-chloro- N-isopropyl[1-14C]acet-
anilide (14C-propachlor) was perfused through a calf kidney in situ
by Bakke et al. (1990). Twenty-three per cent of the dose was excreted
in the perfused kidney urine as the cysteine conjugate; no mercapturic
acid was detected. A 5-day-old calf dosed orally with 14C-propachlor
excreted 70% of the dose in the urine as the cysteine conjugate; again
no mercapturic acid was detected. Rumen microflora were established in
the calf when it was 5 weeks older and the experiment was repeated.
The same results were obtained.
6.3 Elimination and excretion
When 14C-propachlor was given to rats, 56-64% of the dose was
excreted in urine in the first 24 h and 5.7-7.0% in 24-48 h. In the
faeces, 8-13% and 2.2-7.7% were eliminated in 0-24 h and 24-48 h,
respectively; 0.4% of the 14C was eliminated as CO2 and 5-11% was in
the carcass. In total 80-97% was eliminated in 48 h (Lamoureux &
Davison, 1975).
According to Bakke et al. (1980), the metabolites of propachlor
formed in normal rats treated with propachlor are excreted mainly
through urine (68%) and faeces (19%). Eleven urinary metabolites were
isolated from rats given 14C-labelled propachlor orally. The major
metabolite was the mercapturate (17%), and six of the metabolites were
2-methylsulfonylacetanilides. Faecal residues (19%) of the
administered dose, insoluble in common solvents or by treatment with
diluted acid or base, were also determined.
Rats with cannulated bile ducts secreted 66% of an oral dose of
propachlor in the bile as the glutathione conjugate (2), cysteine
conjugate (3), mercapturate (4) and the mercapturate sulfoxide (5)
(see Table 10). Germ-free rats given orally 14C-labelled propachlor
excreted 98% of the dose in the urine and faeces within 48 h. Three
metabolites were isolated from the excreta and the faecal radioactive
metabolites were water soluble. The major metabolite was mercapturate
(4) and the other metabolites were the cysteine conjugate (3) (present
only in the faeces) and mercapturate sulfoxide (5) (Bakke et al.,
1980). Mercapturate sulfoxide was isolated from the excreta of
germ-free rats by Feil et al. (1981), who also demonstrated its
presence in the bile of rats and urine of chickens and pigs dosed with
propachlor. The metabolite was characterized by mass and nuclear
magnetic resonance spectro-metry on samples isolated from rats.
Table 10. Comparison of the excretion of single oral doses of 14C-propachlor
by control rats with fistulated bile ducts, and germ-free ratsa
Metabolite Recovery of 14C (% dose)b
Bile-fistulated
Control rats rats Germ-free rats
Urine Faeces Bile Urine Faeces
Glutathione 37
conjugate (2)b
Cysteine 13 19
conjugate (3)
Mercapturate (4) 17 12 63.1 3.7
Mercapturate 4 5.7 5.4
sulfoxide (5)
Non-extractable 19
residues
Other metabolites 51
Total 68 19 66 68.8 32.1
a From: Bakke et al. (1980)
b Metabolite designations are those used in Figs. 1 and 3.
As in the case of the glutathione and cysteine conjugates, the
sulfoxide is not excreted at detectable levels by normal rats and was
detected only in the bile (Bakke et al., 1981a). It may become a
substrate for the intestinal flora, but the ultimate in vivo fate of
this metabolite is unknown.
Bakke et al. (1981c) reported differences between some species
concerning the metabolism of propachlor in the MAP. Clear but
unexplained differences are that rats excrete no cysteine conjugate
and chickens form no methylsulfonyl-containing metabolites, whereas
sheep excrete large amounts of cysteine conjugate in urine.
Nadeau & Pantano (1986) carried out a study to determine the
rates and routes of excretion of orally administered synthetic
14C-labelled propachlor plant metabolites in lactating goats and to
quantify and identify the radioactive metabolites in the goat milk,
tissues, urine and faeces. The daily dose level for the three treated
goats was 15 mg/kg administered on 5 consecutive days (the actual dose
levels for the treated goats were 13, 14.5 and 13 mg/kg,
respectively). Each treated goat received a total of 130.5 mg of the
13C/14C-labelled propachlor plant metabolites mixture during the
dosing period. A control animal received placebo capsules. The
radioactivity eliminated in faeces accounted for 72.1, 64.5 and 57.9%
of the administered dose, respectively (average 64.8%), and that
excreted in urine accounted for 35.8, 28.1 and 32.3% (average 32.1%).
The percentage of the dose eliminated through faeces and urine
averaged 96.9%.
The radioactivity found in the milk accounted for only 0.084,
0.10 and 0.13% (average 0.10%) of the administered dose. These values
corresponded to metabolite concentrations in the milk of 11.9, 14.8
and 18.0 µg/litre (average 14.9 µg/litre). The metabolic
concentrations (µg/kg) in the tissues were: kidney 51.9, liver 30.4,
muscle 8.5, fat 19.5 and blood 50.1. The loss of radioactivity from
tissues, milk and excrement occurred rapidly, and after 5 days of
depuration the milk and tissue radioactivity levels were below the
limit of detection, except in the case of the liver (5.7 µg/kg).
6.4 Metabolism in laying hens
Since crops treated with propachlor are used in animal feed, it
is important to know the fate of propachlor in animal feed.
The purpose of the study by Bleeke et al. (1987) was to examine
the metabolic fate of propachlor plant metabolites in laying hens and
to determine whether they accumulate or persist in the eggs or edible
tissues. The compounds used for feeding were the three major
metabolites of propachlor found in sorghum, i.e. [( N-iso-propyl)-
phenylamino] oxacetic acid, sodium salt (I), {{[( N-iso-propyl)-
phenylamino]acetyl}sulfinyl} lactic acid, sodium salt (II) and
2-[( N-isopropyl)-phenylamino] 2-oxoethane sulfonic acid, sodium salt
(III).
The first study involved dosing chickens at a level of 5 mg/kg
diet for 6 consecutive days. Data for bioaccumulation and excretion of
plant metabolites were obtained from this study. A second group of
chickens, dosed at a level of 25 mg/kg diet (nominal dose) on 6
consecutive days, provided eggs and tissues with higher residues for
metabolic characterization. Each group consists of five hens. Control
groups received a single gelatine capsule per day. The total recovery
of 14C radioactivity was good in both studies.
An average of 87.4% of the total administered dose was recovered
from the chickens fed 5 mg/kg approximately 1 day after the last dose,
and 97.9% was recovered from the chickens fed 25 mg/kg, primarily in
the excreta. The eggs contained low residue levels; those from
chickens fed 5 mg/kg contained residues below the minimum quantifiable
limit (MQL) of 1.7 µg/kg. The level in the egg yolks reached an
average of 5.5 µg/kg by day 6 but by day 12 the residues in the yolk
had fallen below 1.6 µg/kg.
In the high-dose group, the residues in the egg white levelled
off at about 4 µg/kg on day 2, while those in the egg yolk increased
to a level of 27.3 µg/kg on day 6.
Tissues also contained low levels of residues. In the low-dose
group, the highest levels were found in the kidney and they averaged
only 6 µg/kg. The residue levels in the liver measured an average of
1.9 µg/kg and those in the fat 3.5 µg/kg. The residues in other fat
samples and organs were below the MQL (1.4-1.6 µg/kg). Residues in the
breast muscle were below the minimum detectable limit (7 µg/kg) and
those in the blood were 6-7 µg/kg.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity of propachlor for the rat, mouse and the
rabbit has been examined. According to the WHO hazard classification
of pesticides and as far as its acute oral toxicity to rats is
concerned, propachlor is slightly hazardous (Group III) (WHO, 1990).
LD50 values in various animal species reveal a higher susceptibility
for the mouse and the rabbit than for the rat (Table 11). The LD50
for rats ranges from 950 to 2176 mg/kg.
The clinical picture of acute oral intoxication by propachlor has
been described in three species of experimental animals: mice, rabbits
and rats (Panshina, 1973). When propachlor is given at lethal or toxic
doses, the main symptoms concern the central nervous system. In mice
and rabbits, a state of excitement, trembling and light convulsions is
observed 15 min after a toxic or lethal dose of propachlor. This
gradually increases, breathing becomes difficult and death follows in
fseveral hours. Intoxication in rats takes a different course: a state
of immobility and head and body tremors are accompanied by the sudden
onset of convulsions and death occurs within 24 h. This clinical
picture was confirmed by Strateva (1974,a,b).
Kronenberg (1988) reported clear signs of irritation to the
gastrointestinal tract and lungs that were evident at necropsy in
animals that died during the study. Histoenzymological changes
expressed as decreased enzyme activity were in full agreement with the
morphological picture (Strateva et al., 1974a,b).
7.1.2 Dermal
Results of studies in rabbits on the acute dermal toxicity of
propachlor and its formulations indicate that the dermal LD50 ranges
from 4000 mg/kg for a 65% WP formulation (Auletta & Rinehart, 1979) to
20 000 mg/kg for technical propachlor (94.5%) (Braun & Rinehart,
1978). Test animals exhibited moderate to severe erythema, severe
oedema and necrotic skin at the dermal application sites at a dose
level of 2800 mg/kg. Motor activity decrease and ataxia were noted at
dose levels of 2000 to 5600 mg/kg.
Table 11. Oral LD50 values for laboratory mammals
Species LD50 (mg/kg)a Reference
Rat 1056 (834-1278) Panshina (1973, 1977)
950 (860-1050) Strateva (1974b)
1340 Mirkova (1975)
Table 11 (contd).
Species LD50 (mg/kg)a Reference
2176 ± 220 Lehotzky et al. (1979)
Rat (Sprague-Dawley) 840 (419-1261) Blaszcak (1988)
(in corn oil)
1700 (1265-2135) Blaszcak (1988)
(in 1% methylcellulose)
Rat (Fischer-344) 550 (252-848) in corn oil Blaszcak (1988)
1359 (1009-1691) Blaszcak (1988)
(in 1% methylcellulose)
Rat (both sexes) 4000 Heenehan et al. (1979)
Rat (both sexes) 3269 Branch et al. (1982a)
Mouse 306 (275-337) Panshina (1973, 1977)
290 (240-350) Strateva (1974b)
a The concentration is based on the percentage active ingredient.
Figures in parentheses indicate the range of values.
Using groups of 16 Wistar rats, Baynova et al. (1977) studied the
acute dermal toxicity of a single application of propachlor 65 WP
(10-20% in aqueous suspension) with doses of 1500-4000 mg/kg body
weight (active ingredient). There was no mortality, and no signs of
intoxication were observed. No haematological or biochemical tests
were performed.
Propachlor and Satecid 65 WP caused severe dermatitis, ulceration
and necrosis in the skin of rabbit and ears of mice. None of the
compounds exhibited contact sensitization effects on guinea-pigs
(Lehotzky et al., 1979).
7.1.3 Inhalation
In a study by Bechtel (1991), technical propachlor (96.8%) was
dissolved in dimethylsulfoxide (DMSO) to generate an aerosol and
administered to five male and five female Sprague-Dawley rats in a
nose-only chamber at an analytically determined concentration of 1.2
mg/litre for 4 h. Control rats (five of each sex) were exposed to an
atmosphere of aerosolized DMSO for the same duration. Particle size
measurements on the propachlor/DMSO aerosol indicated a mass median
aerodynamic diameter of 3.5 µm, with 96% of the particles being less
than 10 µm and 1.8% less than 1 µm in diameter (Bechtel, 1991). No
treatment-related deaths occurred. Clinical signs included laboured
respiration and nasal discharge; all animals appeared normal by
post-exposure day 2. A transient weight loss was noted in both treated
and control animals during the first two days of the study, but normal
body weight was observed thereafter. No abnormalities were apparent
during postmortem examination of the animals.
In another acute inhalation study, three groups of Charles River
CD rats (five rats of each sex per group) were exposed to test
atmospheres of a propachlor formulation (44% active ingredient) for 4
h (Kaempfe, 1991). During the exposure period, animals were housed in
a 250-litre New York University style stainless steel chamber, and
were exposed to analytically confirmed concentrations of 0.18, 0.67
and 1.0 mg propachlor/litre in the breathing zone of the chamber. At
least 82% of the particles were less than 10 µm in diameter. No
animals died at the two lower exposure levels, whereas four out of ten
rats died at 1.0 mg/litre. Clinical observations during the
post-exposure period included ocular opacities, perinasal
encrustation, rapid or shallow respiration, perioral wetness and focal
loss of hair from animals in the highest exposure group. Fourteen days
after exposure, all surviving animals had body weights higher than the
pre-exposure (day 0) values with the exception of females in the
1.0-mg/litre group, which exhibited body weight depression. There were
no abnormal findings in rats that died during the test or were
sacrificed at 14 days. Based on the mortality observed, the LC50 was
slightly greater than 1.0 mg/litre.
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dogs
To assess potential subchronic toxicity, propachlor (96.1% pure)
was administered via the diet to five groups of two male and two
female beagle dogs for 4 weeks (Daly & Knezevich, 1984). Dietary
concentrations of propachlor were 0, 100, 500, 1000 and 1500 mg/kg
(equivalent to 0, 2.5, 12.5, 25, and 37.5 mg/kg body weight per day).
No mortality or clinical signs of toxicity related to treatment
occurred during this study. Food consumption was initially decreased,
but only in the females treated with 1000 and 1500 mg/kg (equal to 25
and 37.5 mg/kg body weight) and the males treated with 1000 mg/kg. The
consumption had returned to normal by the end of the study. Body
weight varied markedly. The decreased body weight and/or weight gain
noted in males fed 12.5 or 25 mg/kg body weight per day, and females
fed 37.5 mg/kg per day could have been treatment related.
Haematological examination showed slightly increased platelet counts
in high-dose males. No treatment-related gross pathological effects
were noted at sacrifice.
Following the 4-week pilot feeding study in beagle dogs, a 90-day
feeding study was undertaken (Naylor & Ruecker, 1986). Propachlor
(96.1% purity) was administered in the diet to groups of six dogs of
each sex per group for 90 days. Nominal dietary concentrations were 0,
100, 500 and 1500 mg/kg. There was no mortality or clinicopathological
or histopathological changes related to the treatment. The dose level
of 1500 mg/kg (45 mg/kg body weight) was a no-observed-adverse-effect
level (NOAEL).
7.2.1.2 Rodents
Propachlor (65% WP) was given orally by gavage to white rats at
daily doses of 21, 53 and 106 mg/kg body weight for 4 months, and its
cumulative effect was studied by Panshina (1973). Later, Strateva
(1974a, 1976) carried out a short-term study (45 days) at dose levels
of 50, 100 and 200 mg/kg body weight given orally by gavage to 104
Wistar rats (divided into four groups (three experimental and one
control) with equal numbers of both sexes), and a long-term oral study
(6 months) at dose levels of 0.05, 0.5, 5 and 20 mg/kg body weight
given to 220 Wistar rats divided into five groups (four experimental
and one control).
Strateva (1976) found a decrease in haemoglobin content and
number of erythrocytes, slight leucocytosis and an increased number of
neutrophils. The threshold dose for rats in long-term studies was 5
mg/kg body weight.
Panshina (1976) carried out a 10-month study on white rats given
propachlor by gavage at doses of 1, 3.5 and 10.6 mg/kg body weight.
Slight leucocytosis was found within 4-7 months. The first two doses
provoked a decreased activity of catalase and peroxidase as well as an
increase in nicotinamide adenine dinucleotide in brain and heart
tissue homogenates. No changes in haemoglobin content or number of
erythrocytes were noted. There were no alterations to the
pathomorphological picture.
Baynova et al. (1978a) compared the effects of continuous and
intermittent oral dosing of propachlor (65% WP) on white rats (equal
numbers of both sexes). The number of animals per group was not given.
The scheme of the experimental design is given in Table 12.
Table 12. Experimental design of the study by Baynova et al. (1978a)
Group Duration of Daily dose Dose (fraction
study (mg/kg) of the LD50)
Control 4 months - -
I (dosing every week) 4 months 70 1/20
II (dosing every second week) 4 months 140 1/10
III (dosing every second week) 8 months 70 1/20
Control 8 months - -
Continuous administration of propachlor led to more marked
changes in the main parenchymous organs than intermittent
administration. These changes were characterized by decreased activity
of oxido-reductive tissue enzymes. The hexabarbital sleeping time,
which characterized the detoxification function of the liver, showed
a statistically significant reduction. Propachlor administration at
120 mg/kg body weight for 6 consecutive days to male and female rats
increased the levels of both cytochrome and microsomal protein content
(Nenov & Baynova, 1978) as a result of the induction of mixed-function
oxidase in the liver (Baynova et al., 1978a).
A special study on the morphological changes in kidneys was
carried out. Propachlor (65% WP) was administered by gavage to male
white rats (10 animals per group and 1 control) at doses equivalent to
6, 12 and 60 mg/kg body weight for 3 months (Maleva & Zlateva, 1982).
Dose-dependent morphological changes were found in the proximal
convoluted renal tubules. The tubules were deformed and the epithelial
cells were vacuolized and dystrophic. A decreased cellular content of
ribonucleoproteins was also observed and pycnosis and caryolysis of
nuclei were seen. In the lumen, desquamated epithelia and hyalin
cylinders were found. The intestine was slightly swollen in certain
areas.
The subchronic toxicity of propachlor (96.1% purity) has also
been evaluated in Sprague-Dawley CD rats (30 of each sex per group)
fed diets containing 0, 300, 1500 or 7500 mg/kg for 90 days (Reyna &
Ribelin, 1984a). No animals died. A statistically significant
reduction in body weight was observed in animals fed 1500 mg/kg (8%
for males and females) and 7500 mg/kg (59% for males and 36% for
females). Food consumption was significantly depressed for animals at
the highest dose level for the first month of the study, but recovered
during the remainder of the study. After 6 weeks, reduced haemoglobin,
haematocrit, mean corpuscular haemoglobin and haematocrit, and an
increase in reticulocytes were observed in females at all dose levels
and in high-dose males. The anaemia was less evident in high-dose
males and was only present in high-dose females at study termination.
Significantly reduced lymphocyte counts were observed at week 6 for
high-dose males and mid- and high-dose females, and at study
termination for all groups. Levels of serum enzymes (SGPT, SAP, GGT),
cholesterol and total bilirubin were significantly increased for
high-dose animals at weeks 6 and 13 and there were significant
reductions in total protein, glucose, creatinine and albumin. No
histological changes were observed in the liver. Organ weights (with
the exception of female livers) were reduced, relative to controls,
for high-dose animals, and spleens were extremely small and
treatment-related in size in about 65% of the animals. No histological
changes were observed in the tissues examined, which included the
spleen.
7.2.1.3 Mice
The subchronic toxicity of propachlor (96.1% pure) was evaluated
in four groups of Charles River CD-1(R) mice (30 mice of each sex
per group) (Reyna & Ribelin, 1984b). Dietary levels were 0, 500, 1500
and 5000 mg/kg. No mortality or adverse clinical signs were observed
during the study. A statistically significant reduction (10%) in body
weight was observed in the mid- and high-dose males and females during
the study. Food consumption was also reduced during the first month
for mid- and high-dose animals. A dose-related statistically
significant reduction in the number of white blood cells was observed
in both sexes at all dose levels, except low-dose females, at the
7-week sampling period. At study termination, this reduction was
evident in mid- and high-dose animals but was statistically
significant only for high-dose males. Liver weights were increased for
males at all dietary levels and for mid- and high-dose females. There
was an accompanying statistically significant increase in the
incidence of centrilobular hepatocellular hypertrophy for mid- and
high-dose males, based on histological examination. No other
microscopic changes that could be considered treatment related were
evident in tissues. For males, the kidney to body weight ratio was
decreased at the high-dose level.
7.2.2 Dermal
A 21- and 90-day short- and longer-term dermal toxicity study on
Wistar rats (12 animals in each tested group plus 1 control) using
propachlor (65% wettable powder) administered 5 days per week was
carried out by Baynova et al. (1977). The doses applied were 50, 200
and 500 mg/kg in aqueous suspensions in the 21-day experiment, and 10,
25 and 50 mg/kg in the 90-day study. The dermal application was
performed uncovered (Draize, modification of Noakes). By the end of
day 21, anaemia was found at dose levels of 200 and 500 mg/kg, but
there was no methaemoglobinaemia. The same doses induced a
statistically significant decrease in the activity of certain enzymes
(SGOT, SGPT, OCT and AP). Dermal application of propachlor at dose
levels of 25 and 50 mg/kg to the skin of white rats (two groups of 12
animals) for 90 days did not provoke any changes in concentrations of
red blood cells and haemoglobin, but there were decreases in SGOT,
SGPT, LAP, AP and catalase. Decreased sulfur-containing enzymes were
found in tissue homogenates (liver and kidney). Decreases in the
levels of LDH, SDH, AcP and glucose-6-phosphate dehydrogenase were
determined histochemically. There was no evidence of clinical signs of
intoxication after repeated dermal application of the herbicide, but
the occurrence of the above-mentioned enzymatic changes demonstrated
the penetration of this herbicide through the dermal barrier. The
hexabarbital sleeping time was statistically significantly shortened
at the mid- and high-dose levels, thereby confirming the induction of
mixed-function oxidases reported by Nenov & Baynova (1978).
A threshold dermal dose of 25 mg/kg (1% aqueous suspension of
propachlor 65 WP) and a no-observed-effect level (dermal) of 10 mg/kg
(0.5% aqueous suspension) were determined in 90-day experiments by
Baynova et al. (1977).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Single application to Wistar rats (three rats of each sex per
group) of propachlor 65 WP at doses of 1500, 2000, 3000 and 4000 mg/kg
in 10 or 20% aqueous suspension, under the open modified method of
Noakes, did not cause any local irritative effects (Baynova et al.,
1977).
Panshina (1973) reported a strong irritative effect of propachlor
after single and repeated application. Single applications to rabbits
of 16% propachlor 65 WP in aqueous suspension at doses of 500, 700,
and 1000 mg/kg led to hyperaemia and even ulceration in the skin of
some animals. Ten applications of a 32% aqueous suspension of
propachlor at a level of 200 mg/kg had the same effect on the skin.
Seventeen applications of a 6.5% aqueous suspension at 100 mg/kg did
not result in mortality, and only slight hyperaemia was seen in the
treated skin area of the rabbits.
Primary irritation and contact sensitization effects of Satecid
65 WP containing 65% propachlor were investigated in rabbits, mice and
rats, and these were compared with the effects of technical grade
propachlor, propachlor with analytical purity, and the vehicle alone
(Lehotzky et al., 1979). Propachlor had strong to very strong
irritating effects on intact and scarified skin as well as on the eye
mucosa of rabbits and mice. The symptoms included erythema, oedema and
penetrating ulcer. Technical grade propachlor had the most potent
irritating effect.
Heise et al. (1983) conducted parallel experiments with two
propachlor preparations, one produced in the USA and the other in
Hungary, using an aqueous suspension applied under occlusion to the
skin of rabbits for 24 h. The irritant dose (ID50) for the USA
preparation was 2% and for the Hungarian preparation 0.6%.
Several dermal irritation studies have been carried out with
technical propachlor and its formulations, each of which involved the
use of six New Zealand white rabbits (2.5 to 3.5 kg). All application
sites were scored for erythema, eschar and oedema formation. For
technical propachlor (94.5% pure) a slight degree of dermal irritation
was observed (2.5/8.0 score) (Braun et al., 1979a); for a 20%
formulation of propachlor (again 94.5% pure) there was a slight degree
of dermal irritation (1.8/8.0 score) (Braun et al., 1979b); for a 65%
formulation a moderate degree of dermal irritation (3.4/8.0 score)
(Braun et al., 1979c); and for a 42% formulation corrosive effects
were observed (Branch et al., 1982b).
7.3.2 Skin sensitization
A modification of the maximalization test of Magnusson & Kligman
(1969) for identification of allergens was used by Heise et al.
(1983). Guinea-pigs (12 female and 12 male) were treated by
intramuscular injection of 0.2% propachlor aqueous suspension (using
preparations from both the USA and Hungary together with Freund
adjuvant), by subcutaneous injection of 0.05% aqueous suspension and
by epicutaneous application of a 1% water suspension of both
preparations for 6 weeks, both on healthy and scarified skin. A
challenge single application was given two weeks after the last
application using 0.2 ml of an 1% aqueous suspension of each
formulation. There was no significant difference between the reactions
to the two preparations. A challenge dose did not induce a reaction.
The dermal sensitization potential of propachlor was evaluated in
guinea-pigs using the Buehler procedure (Auletta, 1983). For the
induction phase, 0.2 ml of undiluted propachlor (95.7% purity) was
applied dermally using the closed patch technique to the shaved backs
of five male and five female Hartley albino guineapigs. The
applications were made for 6 h/day, 3 days/week, for 3 weeks. Two
weeks after the final dose, additional 0.2 ml doses of 25% propachlor
in ethanol (challenge dose) were applied to previously untreated areas
on those guinea-pigs that had received the induction doses and to six
others (three males and three females) which served as irritation
controls. Two positive control groups, treated with 1-chloro-2,4-
dinitrobenzene (DNCB) as positive control, were used. The negative
control group (five males and five females) was treated with saline
for the induction doses and challenged with acetone. All animals had
slight to moderate dermal irritation following the third induction
dose of propachlor, and some of them exhibited severe irritation,
including oedema, necrosis and exfoliation. Following application of
the challenge dose, one out of ten animals had an irritation score of
1 (slight), eight animals had a score of 2 (moderate) and one animal
had a score of 3 (severe) at 24 h. Nine of these animals exhibited
oedema. At 78 h, two animals had very low scores, six animals had
scores of 1, and two scores of 2. Six of these animals exhibited
oedema. Similar results were obtained when 20 and 40% propachlor
formulations were tested for sensitization in guinea-pigs (Auletta et
al., 1984a,b).
7.3.3 Eye irritation
Molnar & Paksy (1978) studied the effect of Satecid 65 WP on the
eye mucosa of CFY male albino rats using the Evans blue diffusion
technique. The aqueous suspension caused strong conjunctivitis at the
minimum concentration of 0.01%.
In a study by Auletta (1984), technical propachlor (96.1% pure)
was ground to a fine powder and introduced in the conjunctival sac of
the right eye of six (two males and four females) albino New Zealand
White rabbits. The left eye served as the control. The treated eyes
were rinsed 24 h later. Five of the animals exhibited severe
conjunctival irritation (redness, necrosis, chemosis and discharge),
five exhibited opacity and/or ulceration, and five had iridial damage.
Two animals exhibited neovascular-ization of the cornea and one
bulging of the cornea, indicative of increased intraocular pressure.
After 21 days of observation one animal continued to exhibit
conjunctival redness and one corneal opacity and bulging of the
cornea. The eyes of the remaining animals had minimal conjunctival
irritation.
In an eye irritation test on six New Zealand white rabbits using
technical propachlor (94.5%), 0.1 cm3 was instilled into one eye of
each rabbit and ocular reactions were observed on days 1, 2, 3, 4, 7,
10 and 14. According to Draize scores, all treated eyes were assigned
positive scores for corneal opacity and ulceration, conjunctival
redness, chemosis and necrosis. Two eyes were assigned positive scores
for iritis. Three eyes exhibited pannus, and corneal bulge was
observed in two eyes. Four eyes were clear of signs of irritation by
day 14 and two eyes showed signs of irritation at the termination of
the study (Braun, 1979).
US EPA (1984b) reviewed the existing data and concluded that
propachlor is corrosive to the rabbit eye, corneal opacity being
irreversible after 7 days.
7.4 Reproduction, embryotoxicity and teratogenicity
7.4.1 Reproduction
7.4.1.1 Biochemical and histopathological studies on gonads
The effect of propachlor 65 WP on protein content, activity of
5-nucleotidase and ATP in testis homogenate was evaluated by Maleva &
Stereva (1977), Stereva & Maleva (1977) and Zlateva & Maleva (1978,
1979). Each test group consisted of 20 male Wistar rats, administered
orally (by gavage) 12 or 60 mg propachlor/kg body weight per day for
6 months. A control group consisted of the same number of animals. At
the end of the experiment, there were dose-dependent statistically
significant changes in the biochemical indices studied (a decrease in
protein content and an increase in 5-nucleotidase and ATPase activity)
and these were accompanied by histomorphological changes, i.e.
disorganization of the seminiferous epithelium. After prolonged dosing
some dystrophic and degenerative changes in the gonad tissue and the
appearance of multinuclear giant cells with central chromatolysis were
observed. Adverse effects on the mitotic division of spermatogonia and
blockage of meiosis in the earlier phases were found (Zlateva &
Maleva, 1978, 1979).
In subchronic toxicity studies, there was no microscopic evidence
of testicular pathology in dogs administered the equivalent of 45 mg
propachlor/kg diet per day (Naylor & Ruecker, 1986), in rats fed the
equivalent dose of 250-310 mg/kg diet per day in the food (Reyna &
Ribelin, 1984a), or in mice fed the equivalent of 400 to 600 mg/kg
diet per day (Reyna & Ribelin, 1984b).
7.4.1.2 Reproduction studies
A two-generation rat reproduction study was undertaken to
evaluate the effects of propachlor (96.3% pure) (Rao, 1981). Four
groups of 30 male and 30 female 6-week-old Fischer-344 rats (F0
generation) were exposed to diets adjusted weekly to provide dose
levels of 0, 0.3, 3 and 30 mg propachlor/kg body weight per day for
100 days and were then allowed to mate to produce the F1 generation.
The F1 weanlings were similarly dosed for 120 days prior to
breeding. The F1 adults were mated twice to produce F2a and F2b
litters. At weaning of F1, F2a and F2b litters, 10 pups of each
sex at each dose level were sacrificed and selected organs were
examined histologically. No signs of toxicity in any animals were
found during the study. A significant decrease in food consumption and
body weight of adult F1 males dosed with 30 mg/kg per day was found.
No adverse effects in any of the treated groups over two generations
were noted with respect to gestation length, number of live pups
delivered, neonatal survival, litter weight and sex ratio. Decreased
pregnancy rates (fertility index) were observed in mid-dose (60%) and
high-dose (63%) F1 females following the first mating (F2a
litter), in comparison with 83% in the control rats. Test animals
continued to be treated with propachlor and were remated to produce a
second (F2b) litter. Pregnancy rates for the F2b litter were 83%
for controls and 83, 83 and 80% for low-, mid- and high-dose groups,
respectively.
No treatment-related gross necropsy findings were noted. Slight
increases in absolute and/or relative liver weights were noted in
high-dose F0 adults, mid- and high-dose F1 adults and high-dose
F2a weanlings. Hypertrophy of the centrolobular hepatocytes was
noted during microscopic examination of livers from the high-dose F0
and F1 adult females. There was no evidence of compound-related
microscopic changes in the testes and ovaries of test animals for
either generation.
7.4.2 Embryotoxicity and teratogenicity
7.4.2.1 Rats
a) Single dose treatment
Mirkova (1975) reported embryotoxic and teratogenic effects of
propachlor 65 WP in Wistar rats following single-dose treatment (675
mg/kg body weight) on each of the first 16 days of gestation.
Significantly increased lethality and frequency of haematomas,
predominantly in the head of the fetuses, and reduced cranio-caudal
size were observed when the treatment was performed from days 1 to 4
and on days 8, 10 and 12 of gestation (8 to 12 animals per test group
and 57 controls). The effect was more marked following treatment on
the first day of pregnancy. A weak teratogenic effect was observed
with a single dose, which included the induction of external
malformations (day 11, brachygnatia), abnormality of internal organs,
i.e. hydrocephalus (days 9, 11, 12 and 13 of gestation), defects in
skeletal ossification, i.e. non-ossification of sternum (day 9), and
delayed ossification of parietal bones (days 11, 12 and 13 of
gestation).
b) Repeated dose treatment
Wistar rats were treated daily with 67.5, 135 or 270 mg
propachlor/kg body weight on days 1-7 or 8-16 of gestation (six test
groups of 10 animals each). In a second set of experiments, a daily
treatment of 33.7, 67.5, 135 and 270 mg/kg body weight was given
throughout the gestation period (1-21 days) (Mirkova, 1975).
Propachlor induced embryotoxicity at all dose levels of repeated
treatment during the pre-implantation stages of embryogenesis (days
1-7 of gestation). This was expressed as raised lethality and
haemorrhages, predominantly in the head of the fetus, and reduced
weight and cranio-caudal size. The effects were significantly reduced
when administration was performed during the period of organogenesis
(8-16 days). Propachlor showed low teratogenic activity, producing
external malformations of the tail (short, curved tail), skeletal
abnormalities (non-ossified parietal and occipital bones) and
hydrocephalus, at all three dose levels during the pre-implantation
stage (days 1-7 of gestation). A slight degree of internal
hydrocephalus was observed with doses of 135 and 270 mg/kg on days
8-16 of gestation.
Using a similar treatment regimen, Ivanova-Chemishanska et al.
(1975) reported similar changes. No embryotoxic or teratogenic effect
was seen at a dose level of 10 mg/kg body weight given daily to rats
throughout the pregnancy (Medved, 1977; Panshina, 1985).
Teratogenic effects of technical propachlor were assessed in
pregnant Charles River COBS CD rats using a control and three
treatment groups consisting each of 25 females (Schardein & Wahlberg,
1982). Propachlor (96.5% pure) levels of 20, 60 and 200 mg/kg body
weight were administered orally by gavage as a single daily dose on
days 6-19 of gestation in a constant volume of 10 ml/kg corn oil. The
control group received the same dose of corn oil. Survival in the
control, low-dose and mid-dose groups was 100%. One female in the
high-dose group died on gestation day 18 due to an intubation error.
There were no differences in mean maternal body weight gain. No
changes were evident at gross necropsy that could be considered
treatment related. There were no differences between treated and
control groups with respect to the mean number of viable fetuses,
post-implantations loss, total implantations, corpora lutea, fetal
body weights, sex ratio distribution, and in the number of litters or
fetuses with external, visceral or skeletal malformations and/or
developmental and genetic variations.
7.4.2.2 Mice
When Balb/c mice (8-12 in each test group and 18 control pregnant
females) were treated with a single dose of 675 mg propachlor/kg body
weight on each of days 8-13 of gestation, and at dose levels of 33.7,
67.5, 135 and 270 mg/kg body weight per day from days 1 to 21, a
significant increase in post-implantational lethality and reduced
fetal weight and cranio-caudal size were observed at all dose
regimens. With both single and repeated doses, there was a
statistically significant increase in the frequency of internal
hydrocephalus, but only at a dose level of 675 mg/kg in a single
application on days 9 and 11 of gestation and on daily application at
270 mg/kg from days 1 to 18 of pregnancy (Mirkova, 1975).
7.4.2.3 Rabbits
Pregnant New Zealand White rabbits randomly assigned to a control
and three treatment groups (each consisting of 16 rabbits) were used
to determine the teratogenic potential of propachlor (95.6% pure)
(Schardein, 1982). Dose levels of 5, 15 and 50 mg/kg were administered
orally by gavage as a single daily dose on days 7-19 of gestation at
a constant volume (0.5 ml/kg dissolved in corn oil). The control group
received only 0.5 ml/kg corn oil. On gestation day 29, the number and
location of viable and non-viable fetuses, early and late resorptions,
number of total implantations and the number of corpora lutea were
recorded. All fetuses were examined for external, soft tissue and
skeletal malformations and for genetic or developmental variations.
Mortality (between 3 and 26 days of gestation) was recorded in
the control as well as the treated groups, i.e. two in the control
group, three at 5 mg/kg, three at 15 mg/kg and two at 50 mg/kg. Two
rabbits from the low-dose group and one from the mid-dose group
aborted on gestation days 22 to 25. Ante-mortem observations gave no
indication of a treatment-related effect at any dosage level.
Results of the reproductive parameters revealed slight to
moderate increases in the number of post-implantation losses and the
number of early resorptions in the mid- and high-dose groups when
compared with controls (Table 13). Single animals with resorptions
only were also found at the two highest dose levels. This resulted in
a subsequent decrease in the average number of viable fetuses in the
mid- and high-dose groups when compared with controls. Although the
number of post-implantation losses fell within the range of historical
control frequencies, all the above parameters were considered to imply
a mild embryotoxic response. No effects were observed in the number of
late resorptions, total implantations, number of corpora lutea, fetal
sex distribution or the mean fetal body weights. Examination of the
fetuses revealed no external, skeletal or soft tissue malformations,
or genetic and developmental variations that could be considered to be
related to treatment. These abnormalities observed were low in
frequency and within the range of historical control values. A
statistically significant increase in the total number of litters with
malformations was observed in the mid-dose group when compared to the
control group. This trend, however, was not found in the high-dose
group and the malformations in the group given 15 mg/kg per day were
considered to be incidental to treatment.
Table 13. Mean number of resorptions (late and early),
post-implantation losses and total implantations in
New Zealand rabbits (Schardein, 1982)
Dose Fetuses Resorptions Post-implantation Total
(mg/kg) viable non-viable late early losses implantation
0 8.0 0.0 0.3 0.3 0.6 8.6
5 7.9 0.0 0.0 0.4 0.4 8.3
15 5.2a 0.1 0.0 1.4 1.4 6.6
50 5.9 0.0 0.2 0.9 1.1 7.0
a P < 0.05
7.5 Mutagenicity and related end-points
Appraisal
Many in vitro and in vivo studies on the genotoxicity of
propachlor have been reported. It has been found to be clastogenic
in in vivo lower test systems and plant assays. It is cytotoxic
and also shows a weakly positive clastogenic response in in vitro
mammalian tests, inducing chromosomal aberrations in Chinese hamster
ovary cells. Variable results do not allow any definite conclusions
to be drawn. However, on the basis of negative results in many in
vivo assays in mammalian test systems, the available experimental
data provide insufficient evidence of a mutagenic potential. Data
on mutagenicity testing are summarized in Table 14.
7.5.1 Bacterial test systems
Salmonella mutation assays using standard tester strains
(TA1535; TA1538; TA98 and TA100), both with and without metabolic
activation with rat liver S9, were negative for formulations and
technical grades of propachlor (Plewa et al., 1984; Flowers, 1984).
No mutagenicity was observed with extracts of plants treated with
propachlor.
Negatives results for propachlor in spot tests using Salmonella
typhimurium test strains were also reported by Njagi & Gopalan
(1980) and Eisenbeis et al. (1981).
Table 14. Summary of mutagenicity testing
Test materiala Test system Resultc Reference
-S9 +S9 1S
Gene mutation in Prokaryotes
Propachlor U Ames spot test - Njagi & Gopalan (1980)
Propachlor U Ames spot test - - Eisenbeis et al. (1981)
Propachlor T Ames plate test - - Flowers (1984)
Propachlor T Ames plate test - - -
Propachlor + Cyanazineb Ames plate test + Plewa et al. (1984)
Gene mutation in plants
Propachlor C maize Wx locus - Plewa et al. (1984)
Gene mutation in mammals ( in vitro)
Propachlor T CHO/HGPRT - - Flowers (1985)
Chromosome effects in plants
Propachlor U Vicia faba cytogenetics + Njagi & Gopalan (1981)
Propachlor U maize cytogenetics + Lapina et al. (1984)
Propachlor U maize chlorophyll mutations + Lapina et al. (1984)
Chromosome effects in mammals ( in vitro)
Propachlor T CHO cytogenetics - + Li & Meyers (1987)
Chromosome effects in mammals ( in vivo)
Propachlor U in vivo mouse bone marrow cyt. + Pilinskaya et al. (1980)
Propachlor T in vivo rat bone marrow cyt. - Ernst & Blazak (1985)
Table 14 (contd).
Test materiala Test system Resultc Reference
-S9 +S9 1S
DNA damage/recombination in yeast
Propachlor T gene conversion - + Gentile et al. (1977)
Propachlor T gene conversion - - + Plewa et al. (1984)
Propachlor C gene conversion - - + Plewa et al. (1984)
Propachlor + Cyanazine gene conversion - Plewa et al. (1984)
DNA damage in mammals ( in vitro)
Propachlor T in vitro hepatocyte UDS - Steinmetz & Mirsalis (1984)
Propachlor T in vivo/in vitro hepatocyte UDS - Steinmetz & Mirsalis (1986)
a T = technical grade; C = commercial grade; U = unspecified grade
b Propachlor and cyanazine were both of commercial grade.
c +S9 and -S9 = with or without rat liver S9 activation mixture;
1S = with maize 1S fraction
7.5.2 Yeast assays
Propachlor was not recombinogenic when tested at 1000 mg/litre in
Saccharomyces cerevisae strain D4 (ade 2-1, ade 2-2; trp 5-12, trp
5-27) either with or without a liver microsomal activation system
(Gentille et al., 1977).
Extracts of plants treated with propachlor cause a weak
recombinogenic activity (Gentille et al., 1977). Maize extracts
treated with 25 ppm propachlor increased the rate of mitotic gene
conversion at the ade locus in S. cerevisae strain D4 approximately
4-fold over the control levels. Extracts of plants treated with a
technical grade of propachlor (1.306 x 10-4 mol/litre) and
formulated grade (1.306 x 10-3 mol/litre) induced a significant
increase in gene conversion at the ade locus and a lower increase at
the trp locus in S. cerevisae D4. Results for both formulated and
technical grades of propachlor were negative after mammalian S9
activation (Plewa et al., 1984).
7.5.3 Plant assays
Njagi & Gopalan (1981) studied the cytogenic and cytological
effects of propachlor at a wide range of concentrations (10, 50, 100,
500, 1000, 5000 and 10 000 ppm) in root-tip meristematic cells of
Vicia faba. At a concentration of 100 ppm, propachlor induced,
within 2 h, chromosomal aberrations, such as anaphase bridge
formation, micronuclei as well as inhibition of mitosis, formation of
highly pycnotic nuclei and premature chromosome condensation. The
mutagenic properties of propachlor were not detectable in the field
experiments using reverse mutations at the wx-C locus in maize as the
genetic end-point. Lapina et al. (1984) also reported that propachlor
induced mitotic chromosomal aberrations in the root-tip meristematic
cells of an inbred F2 strain of maize. At a concentration of 10-3
mol/litre and within 2 h of treatment of maize grains, propachlor
increased the rate of mitotic chromosomal aberrations more than 4-fold
over the control levels. The main types of cytogenetic damage included
single bridges (72%) and fragments (28%). Propachlor did not show
evidence of being significantly cytotoxic.
7.5.4 Cultured mammalian cell CHO/HGPRT assay
Propachlor (96.1%) was tested in a gene mutation assay in
cultured mammalian cells (CHO/HGPRT assay), both in the presence and
absence of mammalian metabolic activation, at doses ranging from 10-60
mg/litre. It was found to be cytotoxic at some of the higher
concentrations tested, but no mutagenicity was observed (Flowers,
1985).
7.5.5 In vitro unscheduled DNA synthesis in primary rat hepatocyte
cultures
Steinmetz & Mirsalis (1986) studied the potential of propachlor
to induce unscheduled DNA synthesis (UDS) in primary rat hepatocyte
cultures. They used ten concentrations of propachlor (95.8% pure)
ranging from 0.1 to 5000 mg/litre and, in another assay, five
concentrations ranging from 0.1 to 50 mg/litre. Results of this study
showed a cytotoxic effect of propachlor at concentrations of 50
mg/litre or more, but there was no evidence of UDS induction.
7.5.6 In vitro test for induction of chromosomal aberrations using
Chinese hamster ovary cells
Propachlor was tested for its potential to induce chromosomal
aberrations in cultured Chinese hamster ovary (CHO) cells treated with
propachlor (97.4% pure) at concentrations of 5, 10 or 15 mg/litre for
5 h both in the presence and absence of exogenous activation (Li &
Meyers, 1987). As indicated both by decrease in mitotic index and
lengthening of average cell generation times, the highest treatment
level was cytotoxic both in the presence and absence of activation. In
the non-activated study, no statistically significant increases in the
percentage of cells with structural aberrations or in average
structural aberrations per cell were observed at any treatment level.
In the study with S9 activation (15 mg propachlor/litre), the average
number of aberrations per cell was statistically elevated at 12 h and
24 h (P < 0.05 test). Chromatid and chromosome gaps were not
included. The percentage of cells with structural aberrations
following treatment at 15 mg/litre was statistically elevated at 12 h
but not at 24 h. No apparent dose-response relationship was observed
at either harvest time. Under these conditions, propachlor was
considered to be negative in the assay without exogenous metabolic
activation and a weak clastogen with activation.
7.5.7 In vivo rat bone marrow cytogenetic assay
Ernst & Blazak (1985) conducted an in vivo rat bone marrow
cytogenetic assay using technical grade propachlor (95.6% pure) at
dose levels of 0, 0.05, 0.2 and 1 mg/kg body weight. The results were
evaluated for mitotic index (based on at least 1000 cells per animal)
and 60 cells per animal were examined for chromosomal aberrations.
Chromatid and isochromatid gaps were recorded for each cell, but these
were not considered chromosomal aberrations. The results showed that
propachlor does not induce chromosomal damage in male or female
Fischer-344 rats under the conditions used in this study.
7.5.8 Acute in vivo mouse bone marrow cytogenicity assay
Pilinskaya et al. (1980) studied the cytogenetic effect of
propachlor on the bone marrow of mice treated orally with 10, 50 and
100 mg/kg body weight. The metaphase frequency of aberration at the
lowest dose applied was 2.67 ± 0.66 compared with 0.7 ± 0.19 in the
control. At this dose level an approximately 4-fold increase in the
frequency of chromosome aberrations was observed. No statistically
significant changes were found at a dose level of 1 mg/kg.
7.5.9 In vivo/in vitro hepatocyte DNA repair assay
Steinmetz & Mirsalis (1986) presented data on the in vivo/in
vitro hepatocyte DNA repair assay. Propachlor (97.7% pure) was
suspended in corn oil and administered by gavage to five groups of
male Fischer-344 rats at dose levels of 25, 250, 300, 400 and 1000
mg/kg. A positive control group received 2-acetyl aminofluorene
(2-AAF) (50 mg/kg) while a negative one was given corn oil. The
results were negative.
7.6 Long-term toxicity and oncogenicity studies
7.6.1 Rat
Propachlor (96% pure) was administered via the diet to four
groups of 60 male and 60 female Charles River CD SDBR rats at
concentrations of 0, 10, 50 and 500 mg/kg (equivalent to 0, 0.5, 2.6
and 27 mg/kg body weight) (Hamada, 1987a). A complete battery of
haematological, clinical chemistry and urinalysis tests was carried
out every 6 months for 2 years. No treatment-related effects were
noted on survival, incidence of clinical and ophthalmoscopic
observations, body weight gain or food consumption. Haematological and
clinical chemistry parameters and urinalysis data of treated animals
were comparable to control values. Thyroid and liver weights were
slightly increased in high-dose males at the 12-month interim
sacrifice but not at the end of the study. An increased incidence of
liver changes (centrilobular hypertrophy, clear cell cytoplasmic
alteration and eosinophilic cytoplasmic alteration) was observed in
high-dose males and females. A slightly higher incidence of thyroid
c-cell tumours (adenomas and carcinomas) noted in high-dose animals
was within the testing laboratories' historical range for such
tumours. All other organ weight changes and gross and microscopic
pathological findings were comparable to controls. An increase in
benign granulosa/theca cell tumours was observed for high-dose females
(4/60) compared to controls (0/60). The historical control data for
this tumour ranged from 0/80 to 4/96. On the basis of these results,
50 mg/kg diet (2.6 mg/kg body weight) can be considered the
no-observed-effect level (NOEL) for rats.
7.6.2 Mouse
When propachlor (96.1% pure) was administered in the diet to male
and female CD1 mice at dose levels of 10, 50 and 500 mg/kg (equivalent
to 1.6, 8.1 and 81.3 mg/kg for males and 2.0, 10.5 and 104.9 mg/kg for
females) for 18 months, no histological changes were noted (Hamada et
al., 1987b). The survival of all treated male groups was similar to
that of controls. Female survival was lower than that of controls in
all treated groups. There was a significant increase in segmented
neutrophilic leucocytes in the 500-mg/kg group at 12 months, but this
change was not evident at study termination. At the end of the study,
ratio of liver (with gall bladder) to body weight was increased in the
50- and 500-mg/kg group females, and the ratio of kidney to body
weight was decreased in males of the highest-dose group. The NOEL was
considered to be 10 mg/kg (1.6 mg/kg per day).
7.6.3 Dog
In a study by Naylor & Ruecker (1986), propachlor was
administered daily via the diet to four groups of six male and six
female beagle dogs for 12 months (0, 25, 250 and 1000 mg/kg diet). No
mortality occurred during the study. Absolute body weights were lower
than those of controls at study termination (approximately 14% for
males and 8% for females in the high-dose group and 5% for males in
the mid-dose group. A slight increase in emesis and/or stool change
was noted in treated dogs. No other changes in clinical and
pathological parameters were observed that could be related to
treatment. Based on the body weight depression at the high dose, a
dietary level of 250 mg/kg (9 mg/kg body weight) was considered a
no-observed-adverse-effect level (NOAEL) in this chronic study.
7.7 Miscellaneous studies
Propachlor has a strong inhibitory effect on the proliferation of
L1210 mouse leukaemia cells in vitro (the ID50 for cell
proliferation is < 3 x 10-7 mol/litre). Propachlor also inhibits
significantly the uptake of leucine, thymidine and uridine. The
inhibitory effect of propachlor is largely reversible, i.e. cells
grown in propachlor and then washed free of the compound return to an
almost normal rate of proliferation (Zilkah et al., 1981).
It has been demonstrated that propachlor treatment also causes
L1210 cells to accumulate in the G1 phase. This effect is dose
dependent; a concentration of 10 µmol/litre causes more than 90% of
cells to accumulate in the G1 phase (Zilkah et al., 1985).
The combined effect of propachlor and vibration has been studied.
Propachlor was given orally to Wistar rats (20 animals in each group)
at doses equivalent to 6, 12 and 60 mg/kg body weight per day for 3
months, and the animals were exposed to low-frequency vibration (15
Hz) during the last 30 days of the study. The results were compared
with those obtained from groups of rats subjected to the effect of 15
Hz vibration daily for 30 days, as well as with those of groups of
rats dosed only with propachlor. It was found that the combined
exposure (with 12 or 60 mg propachlor/kg) caused more severe
haemodynamic alterations and degenerative (even necrotic) changes in
the epithelial cells of the renal tubules, either in the proximal or
distal convoluted tubules (Maleva & Zlateva, 1982).
In a study by Baynova et al. (1978b), groups of 20 white rats
were pretreated with oral doses of 4 ml/kg 40% ethanol (one tenth of
the LD50), 6 days per week, for 4 months. Propachlor was then given
daily at a dose level of 140 mg/kg (one tenth of the LD50), 6 days
per week, every other week for a further 4-month period. The controls
were subjected to 8 months of treatment with ethanol and 4 months of
intermittent (every second week) treatment with propachlor using the
same doses as in the combined experiment. Both experiments had
untreated control groups. The combined treatment led to a reduction of
the hepatotoxic effect. This was probably due to induction of
mixed-function oxidases and to a more rapid biotransformation in the
hepatocytes. It has also been found that propachlor (140 mg/kg for 6
days) reduces the hexabarbital sleeping time by speeding up its
metabolism in the hepatic endoplasmic reticulum as a result of
induction of the mixed-function oxidase system in the liver cell
microsomes (Nenov & Baynova, 1978).
8. EFFECTS ON HUMANS
8.1 Occupational exposure
There have been few reports dealing with the effects of
propachlor on humans.
Von Schubert (1979) reported a case of contact eczema on the
palms, wrists and forearms of a 29-year-old agricultural worker who
had been in contact with propachlor for 8 days. Once this contact
ceased, the skin lesions disappeared.
Iden & Schroeter (1977) patch-tested 17 patients, predomi-nantly
farmers, who had been exposed simultaneously to many types of
herbicides. Seven showed a positive patch test reaction and five
others had an irritant reaction to propachlor. One of these farmers,
who was highly sensitive to propachlor, used to develop generalized
airborne contact dermatitis each spring when his neighbours used this
product. Farkasdy et al. (1976) and Dombay & Farkasdy (1978) examined
dermatologically 79 workers manufacturing Satecid 65 WP (65%
propachlor). Of these, 19% showed contact dermatitis attributed to
propachlor exposure. Patch testing was carried out on 67 workers of
this group, 12 of whom showed monovalent and bivalent hypersensitivity
reaction to propachlor. Photosensitization tests were negative in 28
of the cases tested. Three years later, the same authors examined 108
workers who were continuously exposed to Satecid in the same factory
without finding any sign of sensitization.
Jung et al. (1989) patch-tested 19 allergic cases and one
irritative case with occupational contact eczema, who were exposed to
different types of herbicides. Two of them showed positive testing to
Satecid 65 WP. One of these two was only exposed to the substance for
10 days and the other for 6 months.
8.2 General population exposure
No reports on general population exposure are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Soil
The effects of propachlor with regard to changes in numbers of
four types of soil bacteria (aerobic nitrogen-fixing, aerobic
cellulose-decomposing, ammonifying and nitrifying) was studied by
Helmeczi (1977). The studies were carried out using pseudomycelial
chernozem soil with maize plants. For the purpose of the microbial
examinations, samples from the field were taken three times a year
(after spraying propachlor in spring, summer, and autumn at a rate of
8-10 kg/ha) from the upper 20-cm layer of the soil. Sampling was
carried out under sterile conditions and samples were collected in
sterile vessels. The nitrifying bacteria had the greatest sensitivity
to propachlor, their number decreasing considerably from 2720 to 610
per g soil in 1974 and from 4510 to 1920 per g soil in 1975. The
aerobic cellulose-decomposing bacteria were the least sensitive, their
numbers, with respect to the control bacteria, increasing to a small
extent.
Long-term studies in smolnista and alluvial soils have been
carried out to establish the effects of propachlor on useful soil
microorganisms and the processes of nitrification (Bakalivanov &
Kostov, 1981). These studies were conducted under laboratory
conditions at 20 °C and 60-70% soil moisture for a period of 10 days.
The concentration of propachlor used was 80 mg/kg soil. The results
showed an inhibitory effect on nitrification and weaker effect on soil
microorganisms (bacteria, actinomycetes and microscopic fungi). High
adsorption of propachlor to clay minerals reduced the toxic effect on
microorganisms.
Rankov & Velev (1976) studied the effect of propachlor on the
biological activity of soil microorganisms and showed that inhibition
was greater at a temperature of 2 to 4 °C than at 20 to 22 °C.
9.1.2 Water
The acute toxicity of propachlor to Selenastrum capricornutum
Printz has been assessed in two studies. In the first (Richards &
Kaiser, 1984), the 96-h EC50 for growth in algae was calculated to
be 0.029 mg/litre with 95% confidence intervals of 0.021-0.038
mg/litre. The no-observed-effect concentration (NOEC) was calculated
to be 0.01 mg/litre. In the second study (Zschaler & Jonas, 1990), the
72-h EC50 for growth was 5.3 mg/litre and the NOEC 1.9 mg/litre for
a propachlor formulation containing 65% active ingredient. The
differences in toxicity observed between the two studies may be either
because the 65% formulation is less toxic than technical propachlor or
because the duration of the second study was 24 h shorter.
9.2 Aquatic organisms
9.2.1 Aquatic invertebrates
Thompson & Forbes (1979a) determined a 24-h LC50 of 11 mg/litre
and a 48-h LC50 of 7.8 mg/litre for the water flea Daphnia magna.
The NOEC in this study was < 5.6 mg/litre. A 48-h LC50 of 6.9
mg/litre in the same species was reported by Mayer & Ellersieck
(1986). The same authors reported a 48-h LC50 of 0.79 mg/litre for
the midge larva Chironomus plumosus. Another midge larva Chironomus
riparius was reported to show a 24-h LC50 of 5.2 mg/litre and a
48-h LC50 of 1.8 mg/litre (Buhl & Faerber, 1989).
The effects of propachlor on daphnia reproduction was assessed in
a 21-day life-cycle study (Thun, 1990a). In this study, there was
decreased reproductive performance at concentrations from 0.29 to 2.6
mg/litre; the NOEC for reproduction was considered to be 0.097
mg/litre.
9.2.2 Fish
Thompson & Forbes (1979b) determined 24-h, 48-h and 96-h LC50
values for rainbow trout ( Onchorynchus mykiss) of 0.75, 0.28 and
0.17 mg/litre, respectively. Thun (1990b) conducted a 21-day study on
rainbow trout and found that fish died at concentrations between 0.11
and 0.3 mg/litre. No deaths related to exposure occurred at
concentrations between 0.009 and 0.075 mg/litre. The NOEC was
considered to be 0.019 mg/litre. Mayer & Ellersieck (1986) reported a
96-h LC50 of 0.23 mg/litre for the channel catfish (Channa
punctatus).
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The toxicity of propachlor to earthworms was assessed in a 14-day
study (Thun et al., 1991). Propachlor (97.8% pure) was added to the
soil at five concentrations ranging from 100 to 1000 mg/kg, and 40
worms were added to the soil at each concentration. Worms were removed
from the soil at 7 and 14 days and examined for any adverse effects.
Body weights were measured at the beginning and end of the test. The
LC100 was 560 mg/kg and the LC0 218 mg/kg; the NOEC was 100 mg/kg.
The contact LD50 of propachlor for honey bees was reported to
be 311 µg/bee (Kenaga, 1979).
Under laboratory conditions, Tanke & Franz (1978) studied the
side effects of propachlor on some beneficial insects by measuring the
reduction of the beneficial capacity of three enthomophagous insects.
The egg parasite Trichogramma cacoeciae March reacted very strongly
in laboratory as well as in field conditions to the effects of the
herbicide. Contact toxicity tested using residues of propachlor on
glass plates (residue level not stated) caused a reduction of the
degree of parasitization leading to total mortality of the population
in contaminated cages. In a study on the contact toxicity of the spray
deposit on single leaves as well as on whole plants, the
parasitization of Trichogramma was reduced by 100 and 83%,
respectively. The direct toxic effect should be distinguished from a
repellent effect, which is probably more important in the field. In
addition to this direct contact effect of spray deposits, systemic
application also caused an effect after transport of the herbicide
through the soil and the plant. This was demonstrated by a reduction
of the degree of parasitization compared with untreated controls.
Propachlor does not seem to have any influence on Chysopa carnea
Steph larvae. No effect was visible either in tests of contact
toxicity or contaminated sandy soil, in choice studies on a repellent
action of the residue or after topical application of the preparation.
After oral application through an artificial food chain, no influence
could be demonstrated. Only overdosage increased the mortality rate of
the test larvae. No repellent effect of the herbicide on adults was
demonstrated (Tanke & Franz, 1978).
The syrphid Epistrophe balteata DeG was more sensitive. Both in
contact toxicity studies and in studies for a possible repellent
effect, an influence of propachlor on the larval stages was shown.
Oral intake through an artificial food chain did not have an effect on
the larvae. When the herbicide was sprayed directly on the plant, an
increase in larval mortality was observed. Adults of this syrphid also
showed reactions to herbicides; during egg deposition, females avoided
surfaces previously treated (Tanke & Franz, 1978).
The potential toxicity of a 65% formulation of propachlor to
Aleochara bilineta Gyll was assessed by Pietrzik (1991). The imagos
were dug into moist sand and the test substance was added at
recommended use rates (8 kg in 400 litres/ha water). For the
assessment of parasitical capacity, pupae of Delia antiqua were dug
into the sand. At the termination of the test, the sum of hatched
Aleochara larvae in the pupae was determined. These were compared to
the number of control organisms treated with tap water. The parasitic
capacity of the test beetles was decreased by 11.4% when compared to
controls.
9.3.2 Birds
Palazzolo (1964) reported data for pheasants indicating an acute
oral LD50 of 735 mg/kg body weight. Increased respiration, loss of
reflexes, mydriasis, salivation and intermittent tonic/clonic
convulsions were found among birds receiving dose levels of 900 and
1350 mg/kg. The onset of symptoms occurred approximately 15 min
following dosing and persisted until death 3-5 h later. Kenaga (1979)
reported an LD50 of 512 mg/kg for Mallard duck and Beavers & Fink
(1979) reported an LD50 for Bobwhite quail of 137 mg/kg body weight
for a 65% propachlor formulation.
When propachlor was administered in the diet to quail or Mallard
ducks for five consecutive days, the LC50 for both species of birds
was more than 5620 mg/kg diet (Beavers & Fink, 1983a,b). For the
quail, a dietary level of 5620 mg/kg is approximately equivalent to a
dose of 1400 mg/kg per day. The data presented above indicate that the
quail is more susceptible to exposure to propachlor via stomach tube
than via the diet.
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1 Conclusions
* Under conditions of normal use, the general population is not
likely to be exposed to propachlor.
* Those occupationally exposed to propachlor should take adequate
safety and hygienic precautions in order to protect the skin, eye
and respiratory tract.
* Propachlor is rapidly degraded in the environment under most
conditions. It persists longer in cold dry environments. The
conjugated N-isopropylaniline metabolite persists longer than
the parent compound. Propachlor does not bioconcentrate or
biomagnify.
* Propachlor is highly toxic to some aquatic organisms. Exposure of
aquatic organisms under conditions of normal use is low, the
maximum expected concentrations being several orders of magnitude
lower than the no-observed-effect concentrations. Direct
contamination of water courses will kill aquatic organisms and
should be avoided. Propachlor poses a low hazard to birds,
earthworms and honey-bees.
10.2 Recommendations for protection of human health
Workers should be educated about the hazards of propachlor and
systematically trained to practice safety and personal hygiene and to
use protective equipment.
11. FURTHER RESEARCH
* The results of the existing animal studies on mutagenicity are
inconclusive and more research is needed.
* Studies should be performed in laboratory animals to determine
the potential neurotoxic effects of propachlor.
* Only validated analytical methods for residues of propachlor
should be used.
* Epidemiological studies on occupationally exposed workers are
needed.
* There is a need to develop methods of biological monitoring for
evaluating human exposure to propachlor.
* Research is needed to clarify the exposure of workers in the
production and agricultural use of propachlor. Studies should
also include examination of health effects at the measured
exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO recommended classification of pesticides by hazard,
technical propachlor is classified in Class III as slightly hazardous
in normal use (WHO, 1990). A data sheet on propachlor has been issued
(WHO/FAO, 1989).
Neither the FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
nor the International Agency for Research on Cancer (IARC) has so far
evaluated propachlor.
REFERENCES
Aschbacher PW & Struble CB (1987) Evidence for involvement of
non-biliary excretion into the intestine of the formation of
methyl-sulphonyl containing metabolites of 2-chloro- N-
isopropylacetanilide (propachlor) by swine and rats. Xenobiotica,
13(2): 115-126.
Auletta CS (1983) A propachlor dermal sensitization study in
guinea-pigs (Project No. 4236-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS (1984) Eye irritation study with propachlor in rabbits
(Project No. 5050-84). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Auletta CS & Rinehart WE (1979) Acute dermal toxicity study in rabbits
(Project No. 4892-77). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
Agricultural Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984a) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4946-84). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984b) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4540-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Bakalivanov D & Kostov O (1981) Soil microbiological assessment of
toxicity of alkanoate, amide, and other herbicides. Acta microb Acad
Sci Hung, 28: 141-146.
Baker JL (1980) Agricultural areas as non-point sources of pollution.
In: Overcash MR & Davidson JM ed. Environmental impact of non-point
source pollution. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 275-310.
Baker JL & Laflen JM (1979) Run-off losses of surface applied
herbicides as affected by wheel tracks and incorporation. J Environ
Qual, 8(4): 602-607.
Baker JL, Laflen JM, & Hartwig RO (1982) Effects of corn residue and
herbicide placement on herbicide run-off losses. Trans Am Soc Anal
Eng, 25(2): 340-343.
Bakke JE, Gustafsson JA, & Gustafsson BE (1980) Metabolism of
propachlor by the germ-free rat. Science, 210: 433-435.
Bakke JE & Price CE (1979) Metabolism of 2-chloro-N-
isopropylacetanilide (propachlor) in the rat. J Environ Sci Health,
B14(4): 427-441.
Bakke JE, Davison KL, & Larsen GL (1990) Evidence for the absence of
cysteine S-conjugate N-acetyltransferase activity in the metabolism of
propachlor, naphthalene, and dichlobenil in calves. Xenobiotica, 20:
801-807.
Bakke JE, Rafter J, Larsen GL, Gustafsson JA, & Gustafsson BE (1981a)
Enterohepatic circulation of the mercapturic acid and cysteine
conjugates of propachlor. Drug Metab Dispos, 9(6): 525-528.
Bakke JE, Rafter J, Lindeskog P, Feil V, Gustafsson JA, & Gustafsson
BE (1981b) Metabolism of 2-acetamido-4-(chlormethyl) thioazole in
germ-free and conventional rats. Biochem Pharmacol, 30(13): 1831-1844.
Bakke JE, Larsen GL, Aschbacher PW, Rafter J, & Gustafsson JA (1981c)
Role of gut microflora in metabolism of glutathione conjugates of
xenobiotics. In: Rosen JD, Magee PhS, & Casida JE ed. Sulfur in
pesticide action and metabolism. Washington, DC, American Chemical
Society, pp 165-178 (ACS Symposium Series No. 158).
Balinova AM (1981) Investigations in the residues of some herbicides
in plant production in view of its hygienic evaluation. Sofia,
University of Sofia (Dissertation).
Balinova A & Konstantinov K (1975) Investigations on residues of
several herbicides in vegetables. In: Proceedings of the COMECOM
Symposium on Residues of Herbicides in Plants and Soils, Vrozlav,
1973, pp 111-116.
Barrows ME & Macek KJ (1974) Exposure of fish to 14C-RamrodTM:
Accumulation, distribution and elimination of 14C-residues. Wareham,
Massachusetts, Bionomics, E.G. & G. Inc., 18 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Baynova A, Burkova T, & Michailova A (1977) [Determination of dermal
toxicity of Ramrod.] Gig Zdraveopazvane, 20(3): 234-240 (in Russian).
Baynova A, Burkova T, & Michailova A (1978a) [Comparative study on
intermittent and monotonous effects of the herbicide Ramrod.] Gig
Zdraveopazvane, 21: 568-573 (in Russian).
Baynova A, Krastev L, Dikova M, & Michailova JA (1978b) Effect of
alcohol on acute and chronic intoxication with the herbicide Ramrod.
Acta med Bulg, 2: 36-41.
Beavers JB & Fink R (1979) Acute oral LD50-Bobwhite Quail.
Propachlor report No. WL-79-026. Easton, Maryland, Wildlife
International Ltd (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beavers JB & Fink R (1983a) Eight-day dietary LC50 - Bobwhite Quail
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-90, submitted to WHO by Monsanto
International Services, Brussels).
Beavers JB & Fink (1983b) Eight-day dietary LC50 - Mallard Duck
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-89, submitted to WHO by Monsanto
International Services, Brussels).
Bechtel CL (1991) Acute inhalation study of propachlor Technical
(Project No. ML-91-2). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beestman GB & Deming JM (1974) Dissipation of acetanilide herbicides
from soils. Agron J, 66: 306-311.
Blaszcak DL (1988) A comparative acute oral toxicity study in
Sprague-Dawley and Fischer rats (Project No. 4359-87). East Millstone,
New Jersey, Bio/dynamics Inc., 37 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Bleeke MS, Carr KH, & Elliot RC (1987) Propachlor metabolism in the
laying hen (Project No. MSL-6217). St. Louis, Missouri, Monsanto
Agricultural Company, 134 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982a) Acute oral toxicity of
Ramrod(R) 4L flowable herbicide for rats (Project No. ML-81-182).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982b) Primary skin irritation of
Ramrod(R) 4L flowable herbicide to rabbits (Project No. 810096).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Braun WG (1979) Rabbit eye irritation study (Project No. 4889-77).
East Millstone, New Jersey, Bio/dynamics Inc., 4 pp (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
Braun WG & Rinehart E (1978) Acute dermal toxicity study in rabbits
(Project No. 4888-77). East Millstone, New Jersey, Bio/dynamics Inc.,
4 pp (Unpublished proprietary report, submitted to WHO by Monsanto
International Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979a) Primary dermal irritation
study in rabbits (Project No. 4890-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979b) Primary dermal irritation
study in rabbits (Project No. 4898-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979c) Primary dermal irritation
study in rabbits (Project No. 4894-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Brightwell BB & Rueppel ML (1976) Lasso(R) and Ramrod(R): physical
properties and their relationship to the environment. St. Louis,
Missouri, Monsanto Agricultural Company (Unpublished report No. 443,
submitted to WHO by Monsanto International Services, Brussels).
Brightwell BB, Malik JM, & Wong SC (1981) The environmental fate of
propachlor [2-chloro-N (1-methyl)-N-phenyl-acetamide] in soil and
water. St. Louis, Missouri, Monsanto Research Laboratory, 1300 pp
(Unpublished proprietary report No. MSL-1900, submitted to WHO by
Monsanto International Services, Brussels).
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides and
surfactants to larvae of the midge Chironomus riparius. Arch Environ
Contam Toxicol, 18: 530-536.
Caverly DJ & Denney RC (1978) Determination of substituted ureas and
some related herbicide residues in soils by gas chromatography.
Analyst, 103: 368-374.
Daly I & Knezevich A (1984) Four-week toxicity study in dogs
(Bio/dynamics Inc. Project No. 84-2798). Unpublished proprietary
report of Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels.
Davison KL (1991) Propachlor-s-glutathione metabolism by kidneys and
ureters of calves. J Anim Sci, 69: 1116-1121.
Davison KL, Bakke JE, & Larsen GL (1988) A kidney perfusion method for
metabolism. Studies with chickens using propachlor as a model.
Xenobiotica, 18(3): 323-329.
Davison KL, Bakke JE, & Larsen GL (1990) Metabolism of the glutathione
conjugate of propachlor by in situ perfused kidneys and livers of
rats. Xenobiotica, 20(4): 375-383.
Dombay Z & Farkasdy J (1978) [Problems of safety technique and of
occupational safety as related to propachlor and its formulations.]
In: [Conference on Safety Technology in the Application of
Agricultural Chemicals], Budapest, Technoinform, pp. 49-58 (in
German).
Eisenbeis SJ, Lynch DL, & Hampel AE (1981) The Ames mutagen assay
tested against herbicides and herbicide combinations. Soil Sci,
131(1): 44-46.
Ernst L & Blazak F (1985) An assessment of the mutagenic potential of
propachlor utilizing the acute in vivo rat bone marrow cytogenetics
assay (Project No. SR-84-180). Menlo Park, California, Stanford
Research Institute, 39 pp. (Unpublished proprietary report, submitted
to WHO by Monsanto International Services, Brussels).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
Farkasdy J, Horvath A, & Meszleri E (1976) [Occupational dermatitis
caused by the selective herbicide Satecid 65WP.] Borgyogy venerol Sz,
52: 112-118 (in German).
Feil VJ, Bakke JE, & Larsen GL (1981) A new metabolite of propachlor
isolated from germ-free rat excreta. Biomed Mass Spectrom, 8(1): 1-4.
Fletcher WW & Kirkwood RC (1982) Herbicide and plant growth
regulators. London, Toronto, Sydney, New York, Granada.
Flowers LJ (1984) Salmonella mutagenicity assay of propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 18 pp
(Unpublished proprietary report No. MSL-1694, submitted to WHO by
Monsanto International Services, Brussels).
Flowers LJ (1985) CHO/HGPRT gene mutation assay with propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 9 pp
(Unpublished proprietary report No. ML-84-237, submitted to WHO by
Monsanto International Services, Brussels).
Frank R, Sirons GJ, Paik NJ, & Valk M (1977) Fate of propachlor
applied to onions and organic soils. Can J Plant Sci, 57: 473-477.
Gentille JM, Wagner ED, & Plewa MJ (1977) The detection of weak
recombinogenic activities in the herbicides alachlor and propachlor
using a plant activation bioassay. Mutat Res, 48: 113-116.
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 8: 339-357.
Hamada NN (1987a) Combined chronic toxicity and oncogenicity study in
rats with propachlor (Study No. HL-83-350/241-169). Vienna, Virginia,
Hazleton Laboratories America Inc., 2452 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Hamada NN (1987b) Oncogenicity study with propachlor in mice (Study
No. HL-83-349/241-159). Vienna, Virginia, Hazleton Laboratories
America Inc., 520 pp (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Heenehan BS, Rinehart WE, & Braun WG (1979) Acute oral toxicity study
in rats (Project No. 1895-77) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Heise H, Mattheus A, & Pushkeiler TH (1983) [The irritant and allergic
effect of the herbicidal agent propachlor.] Z Gesamte Hyg, 29(11):
675-677 (in German).
Helmeczi B (1977) The effect of herbicides on soil bacteria belonging
to certain physiological groups. Acta phytopathol Acad Sci Hung,
12(1-2): 41-49.
Humburg NE, Colby SR, Lym RG, Prasad R, Hill ER, Kitchen LM, & McAvoy
WJ (1989) Herbicide handbook, 6th ed. Champaign, Illinois, The Weed
Science Society of America.
Iden DL & Schroeter AL (1977) Allergic contact dermatitis to
herbicides. Arch Dermatol, 113(7): 983.
Ivanova-Chemishanska L, Vergieva T, & Mirkova E (1975) Embryotoxic and
teratogenic effects of some pesticides. Exp Med Morfol, 14(1): 29-33.
Jung HD, Honemann W, Kloth C, Lubbe D, & Pambor M (1989) Contact
eczema caused by pesticides in East Germany. Dermatol Monatschrift,
175: 203-214.
Kaempfe BS (1991) Acute inhalation study of MON 15606 (Project No.
ML-91-48). Newstead, St Louis, Monsanto Company Environmental Health
Laboratory. (Unpublished proprietary report of Monsanto Agricultural
Company, submitted to WHO by Monsanto International Services,
Brussels).
Kenaga EE (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down Earth, 35(2): 25-31.
Kofman IS & Nishko P (1984) Determination of micro-quantities of
herbicides from a group of acetylanilides by thin-layer
chromatography. Physiol Biochem Cult Plant, 16(3): 290-292.
Kolesnikov VA, Abramova KA, & Grip OK (1979) Use of herbicides on
white cabbage. Khim Sel'skom Khoz, 17(10): 27-31.
Kolesnikov VA, Karetskaia NA, & Petuchova VI (1980) Efficacy of
herbicides in garlic crop. Khim Sel'skom Khoz, 18(8): 38-40.
Lamoureux GL & Davidson KL (1975) Mercapturic acid formation in the
metabolism of propachlor, CDAA and Flurodifen in the rat. Pestic
Biochem Physiol, 5: 497-506.
Lamoureux GL & Rusness DG (1989) Propachlor metabolism in soybean
plants, excised soybean tissues, and soil. Pestic Biochem Physiol, 34:
187-204.
Lamoureux GL, Stafford LE, & Tanaka FS (1971) Metabolism of
2-chloror- N-isopropyl-acetanilide (propachlor) in the leaves of
corn, sorghum, sugar cane, and barley. J Agric Food Chem, 19(2):
346-350.
Lapina TV, Grigorenko NV, Morgun V, Logvinenko VF, & Merjinskii JG
(1984) Study of the mutagenic effect of the herbicides Treflan,
Ramrod, and Banvel on corn. Tsitol i Genet, 18(2): 119-122.
Larsen GL & Bakke JE (1979) Studies on the origin of the
methylsulfonyl-containing metabolites from propachlor. J Environ Sci
Health, B14: 427.
Larsen GL & Bakke JE (1981) Enterohepatic circulation in formation of
propachlor (2-chloro-N-N-isopropylacetanilide) metabolites in the rat.
Xenobiotica, 11(7): 473-480.
Larsen GL & Bakke JE (1983) Metabolism of mercapturic acid-pathway
metabolites of 2-chloro- N-isopropylacetanilide (propachlor) by
gastrointestinal bacteria. Xenobiotica, 13(2): 115-126.
Larsen GL, Larsen JD, & Gustafson JA (1983) Cysteine conjugate
beta-lyase in the gastrointestinal bacterium Fusobacterium
necrophorum. Xenobiotica, 13(11): 689-700.
Lee JK, Minard RD, & Bollag JM (1982) Microbial metabolism of
propachlor in soil suspension. J Korean Agric Chem Saf, 25(1): 44-53.
Lehotzky K, Szeberenyl JMS, & Tatrai E (1979) On the local and
systematic toxic effects of the herbicide propachlor (Satecid 65WP) in
experimental animals. Egeszegtudomany, 23: 89-96.
Li AP & Meyers CA (1987) In vitro cytogenetics study of propachlor
(Study No. MSL-6939), 24 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Lottman CM & Cowell JE (1986) Propachlor residues in processed sorghum
grain applications following pre-emergent applications of Ramrod 4L
herbicide (Project No. MSL 6091) (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Lottman CM & Cowell JE (1987) Propachlor residues in processed corn
grain fractions following pre-emergent applications at Ramrod 4 h
herbicide (Study No. MSL-6260) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Magnusson B & Kligman AM (1969) The identification of allergens by
animal assay. The guinea-pig maximalization test. J Invest Dermatol,
52: 268-276.
Maleva E & Stereva M (1977) [Biochemical investigation on changes of
gonads of male white rats under combined action of low frequency
vibrations and intoxication with Ramrod. I. Changes in the content of
protein.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part 2], pp. 306-313 (in Bulgarian).
Maleva E & Zlateva M (1982) Histomorphological changes in kidneys of
experimental animals under the combined effect of low-frequency
vibration and intoxication with Ramrod herbicide. Hig Zdraveopazvane,
25(3): 244-249.
Malik JM (1982) Bioconcentration of propachlor in channel catfish
under static conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, 144 pp (Unpublished proprietary report No. 24242,
submitted to WHO by Monsanto International Services, Brussels).
Malik JM (1986) Metabolism of propachlor in rats (Project No. 7815).
St. Louis, Missouri, Monsanto Agricultural Company, Research
Department (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Markus JR & Puma B (1973) Pesticide analytical manual. Volume II:
Methods for individual pesticide residues. Springfield, Virginia, US
Department of Commerce, National Technical Information Service, pp
1-7.
Martin CD, Baker JL, Erbach DC, & Johnson HP (1978) Wash-off of
herbicides applied to corn residue. Trans Am Soc Anal Eng, 21(6):
1164-1168.
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Medved LI (1977) [Manual of pesticides.] Kiev, K. Urojai (in Russian).
Melnikov NN, Novojilov KV, Belen SP, & Pilova TN (1985) [Manual of
pesticides.] Moscow, M. Khimia (in Russian).
Menges RM & Tamez S (1973) Effect of soil incorporation on
selectivity, movement, and persistence of herbicides in onion
plantings. J Am Soc Hortic Sci, 98(4): 390-393.
Mirkova E (1975) Embryotoxic and teratogenic action of pesticides
Basfungin and Ramrod. Sofia, University of Sofia (Dissertation).
Molnar J & Paksy KA (1978) Acute inflammation of rat membranes evoked
by eye-instillation and inhalation of pesticide formulations
containing propachlor and TMTD. Egeszsegtudomany, 22: 393-397.
Nadeau RG & Pantano LK (1986) Metabolism study of synthetic
13C/14C-labelled plant metabolites of propachlor in lactating
goats. Part II: Identification characterization and quantification of
metabolites (Project No. MSL-5909), 119 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Naylor MW & Ruecker RW (1986) One-year dog study with propachlor
(Study No. ML-84-379). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Nenov P & Baynova A (1978) Ramrod (propachlor) effect on the activity
of rat hepatic microsomal enzymosystems. Acta med Bulg, 5(2): 44-52.
Nesterova OA, Popova VV, & Kolesnikova OP (1980) [Accumulation of
semeron, mesoranil, and Ramrod in the soil and cabbage during
long-term use.] Sibirski Vestn S-Kh Nauki, 10(4): 19-21 (in Russian).
Njagi GDE & Gopalan HNB (1980) Mutagenicity testing of some selected
food preservatives, herbicides, and insecticides. II. Ames test,
Bangladesh. J Bot, 9(2): 141-146.
Njagi GDE & Gopalan HNB (1981) Mutagenicity testing of herbicides,
fungicides, and insecticides. I. Chromosome aberrations in Vicia
faba. Cytologia, 46: 169-172.
Novick NJ & Alexander M (1985) Co-metabolism of low concentrations of
propachlor, alachlor, and cycloate in sewage and lake water. Appl
Environ Microbiol, April: 737-743.
Novick NJ, Mukherjee R, & Alexander M (1986) Metabolism of alachlor
and propachlor in suspensions of pretreated soil and in samples from
ground water aquifers. J Agric Food Chem, 34: 721-725.
Palazzolo RJ (1964) Acute oral toxicity study on CP 31393 in Golden
Ring-necked pheasants. Northbrook, Illinois, Industrial Biotest
Laboratories (Unpublished proprietary report No. BLT-64-3, submitted
to WHO by Monsanto International Services, Brussels).
Panshina TN (1973) [Materials about toxicology of new herbicide
Ramrod.] Gig Primen toksikol pestic klin otravlenni, 1973: 301-303 (in
Russian).
Panshina TN (1976) [Hygienic aspects of the use of certain herbicides
in agriculture and toxicology.] Gig i Sanit, 7: 38-41 (in Russian).
Panshina TN (1977) Establishment of maximum permissible concentrations
and approximately safe levels of action exerted by herbicides of
haloid-substituted carboxylic acid anilides in the air of a production
sector. Gig Tr Prof Zabol, 12: 30-33 (in Russian).
Panshina TN (1985) IRPTC scientific reviews of Soviet literature on
toxicity and hazards of chemicals No. 83: Propachlor (Ramrod). Moscow,
Centre of International Projects, GKNT, 19 pp.
Pantano LK & Anderson KS (1987) The metabolic fate of propachlor
[2-chloro-N-(1-methylethyl)-N-Phenylacetamide] in sorghum foliage and
grain (Project No. MSL-5412). St. Louis, Missouri, Monsanto
Agricultural Company (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Pekas JV, Larsen GL, & Feil VJ (1979) Propachlor detoxication in the
small intestine: cysteine conjugation. J Toxicol environ Health, 5:
653-662.
Pietrzik J (1991) [Laboratory determination of side effects of Ramrod
65WP on the short-winged beetle Aleochara bilineata Gyll (Project
No. 112/01-AB).] Niefern-Öchselbronn, Austria, GAB Biotechnologie GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels) (in
German).
Pilinskaya M, Kurinny AI, & Ivova TS (1980) [The primary evaluation of
cytogenetic activity and potential mutagenic hazard of 22 pesticides.]
Cytol Genet, 14(6): 41-46 (in Russian).
Plewa MJ, Wagner ED, Glenda G, & Gentille JM (1984) An evaluation of
the genotoxic properties of herbicides following plant and animal
activation. Mutat Res, 136: 233-245.
Pressley TA & Longbottom JE (1982) The determination of organochlorine
pesticides in industrial and municipal waste water. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental Monitoring and
Support Laboratory, Office of Research and Development.
Rafter JJ, Bakke J, Larsen G, Gustafson B, & Gustafson JA (1983a) Role
of the intestinal microflora in the formation of sulfur-containing
conjugates of xenobiotics. Rev Biochem Toxicol, 5: 387-408.
Rafter JJ, Gustafson JA, Bakke J, Larsen G, Norin KE, & Gustafson BE
(1983b) Studies on the re-establishment of the intestinal microflora
in germ-free rats with special reference to the metabolism of
N-isopropyl-alpha-chloracetanilide (propachlor). Xenobiotica, 13(3):
171-178.
Rankov V & Velev B (1976) Effect of temperature on the interaction
between the metribuzin and propachlor herbicides and soil
microorganisms. Agrokhimiya, 11(5): 100-105.
Rankov V & Velev B (1977) Detoxication of the herbicide propachlor by
certain microscopic fungi. Acta phytopathol Acad Sci Hung, 12(1-2):
67-72.
Rao KS (1981) Two-generation dietary reproduction study with
propachlor in rats (Study No. HET-K-077810(8)). Midland, Michigan, Dow
Chemical Company, Health and Environmental Science, Toxicology
Research Laboratory, 171 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Rejtö M, Saltzman S, & Acher AJ (1984) Photodecomposition of
propachlor. J Agric Food Chem, 32: 226-230.
Reyna MS & Ribelin WE (1984a) Three-month feeding study of propachlor
to albino rats (Study No. 83037). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Reyna MS & Ribelin WE (1984b) Three-month feeding study of propachlor
to albino mice (Study No. 820061). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Richards C & Kaiser FE (1984) Acute toxicity of propachlor (AB 83-367)
to Selenastrum capricornutum Printz. Columbia, Missouri, Analytical
Biochemistry Laboratories, 39 pp (Unpublished proprietary report No.
31348, submitted to WHO by Monsanto International Services, Brussels).
Ritter WP, Johnson HP, & Lovely WG (1973) Diffusion of atrazine,
propachlor, and diazinon in silt loam soil. Weed Sci, 5: 381-384.
Roberts HA, Walker A, & Bond W (1978) Persistence of chlorthaldimethyl
activity in soil. In: Proceedings of the British Crop Protection
Conference: Weeds. Croydon, British Crop Protection Council, pp 87-92.
Schardein JW (1982) Teratology study in rabbits. Mattawan, Michigan,
International Research and Development Corporation, 47 pp (Unpublished
proprietary report No. IR 81-224, submitted to WHO by Monsanto
International Services, Brussels).
Schardein JW & Wahlberg A (1982) Teratology study in rats. Mattawan,
Michigan, International Research and Development Corporation, 81 pp
(Unpublished proprietary report No. IR 81-264, submitted to WHO by
Monsanto International Services, Brussels).
Shirko ST & Belova VL (1982) [Level of Ramrod, semeron, and mesoranil
in the soil and cabbage plants in relation of dose of nitrogen and
potassium.] Khim Sel'skom Khoz, 20(I): 51-53 (in Russian).
Sjovall J, Rafter J, Larsen G, & Egestad B (1983) Lipophilic ion
exchange for group separation of conjugated metabolites of
xenobiotics. J Chromatogr, 276: 150-156.
Spalding RF & Snow DD (1989) Stream levels of agrochemicals during a
spring discharge event. Chemosphere, 19(8/9): 1129-1140.
Steen WC & Collette TW (1989) Microbial degradation of seven amides by
suspended bacterial populations. Appl Environ Microbiol, 55(10):
2545-2549.
Steinmetz L & Mirsalis C (1984) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the primary rat
hepatocyte cultures (Project No. LSC-7538). Menlo Park, California,
Stanford Research Institute, 8 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Steinmetz L & Mirsalis C (1986) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the in vivo- in
vitro rat hepatocyte DNA repair assay (Project No. LSC-2021). Menlo
Park, California, Stanford Research Institute, 37 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Stereva M & Maleva E (1977) [Biochemical studies on changes in gonads
of male white rats under combined action of low frequency vibrations
and intoxication with Ramrod. II. Dynamic in changes of 5-nucleotidase
activity.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part I], pp 133-139 (in Bulgarian).
Strateva A (1974a) [Hygienic-toxicologic study on Ramrod aiming its
hygienic standardization in water.] Sofia, University of Sofia
(Dissertation) (in Bulgarian).
Strateva A (1974b) [Amid preparations with herbicide action.
Experimental data on the acute oral toxicity of propachlor (Ramrod).]
Exp Med Morphol, 13(2): 123-129 (in Russian).
Strateva A (1975) [On some toxicologic properties of Ramrod and their
significance on its hygienic standardization in waters.] Hyg
Zdraveopazvane, 18(4): 401-405 (in Russian).
Strateva A (1976) [The experimental determination of MAC of Ramrod in
water.] Gig i Sanit, 4: 85-87 (in Russian).
Strateva A, Velisarov A, & Georgiev A (1974) [Morphological and
enzymohistochemical investigations on some internal organs of
experimental animals under the effect of Ramrod in acute experiment.]
Hyg Zdraveopazvane, 17(3): 283-286 (in Russian).
Struble CB (1991) In situ intestinal absorption of
2-chloro-N-isopropylacetanilide (propachlor) and non-biliary excretion
of metabolites into the intestinal tract of rats, pigs and chickens.
Xenobiotica, 21: 85-95.
Tanaka FS, Wien RG, & Mansager ER (1981) Survey for surfactant effects
on the photodegradation of herbicides in aqueous media. J Agric Food
Chem, 29: 227-230.
Tanke W & Franz JM (1978) [Side effects of herbicides on economically
useful insects.] Entomophaga, 23(3): 275-280 (in German).
Thompson CM & Forbes AD (1979a) Acute toxicity of propachlor to
Daphnia magna. Columbia, Missouri, ABC-Laboratories Inc., 40 pp
(Unpublished proprietary report No. 24708, submitted to WHO by
Monsanto International Services, Brussels).
Thompson CM & Forbes AD (1979b) Acute toxicity of propachlor to
Rainbow trout Salmo gairdneri. Columbia, Missouri, ABC-Laboratories
Inc., 40 pp (Unpublished proprietary report No. 24019, submitted to
WHO by Monsanto International Services, Brussels).
Thun S (1990a) Propachlor 21-day reproduction test in Daphnia
(Project Number XX-89-126). Walsrode, Germany, IBR Forschung GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Thun S (1990b) Prolonged fish toxicity test in the rainbow trout
( Salmo gairdneri) (Project Number XX-89-163). Walsrode, Germany, IBR
Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Thun S, Lemke G, & Fulst S (1991) Acute toxicity in earthworms
according to OECD 207. (Project Number IB-90-566). Walsrode, Germany,
IBR Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
US EPA (1984a) Guidance for the registration of pesticide products
containing propachlor (019101) (EPA Case number 17). Washington, DC,
US Environmental Protection Agency, Office of Pesticide Programme.
US EPA (1984b) Health and environmental effects profile for
propachlor. Cincinnati, Ohio, US Environmental Protection Agency
(600/X-84/335).
US EPA (1988) Propachlor. Washington, DC, US Environmental Protection
Agency, Health Advisory Office of Drinking-Water.
Vasilev NV (1982) [Residual quantity of Ramrod in soil and fodder
rurabages.] Khim Sel'skom Khoz, 20(6): 51-53 (in Russian).
Villarreal DT, Turco RF, & Konopka A (1991) Propachlor degradation by
a soil bacterial community. Appl Environ Microbiol, 57(8): 2135-2140.
Von Schubert H (1979) [Allergic contact dermatitis caused by
propachlor.] Dermatol Monatsschr, 165: 495-498 (in German).
Walker A & Brown PA (1982) Effects of soil on the activity and
persistence of propachlor. In: Proceedings of the British Crop
Protection Conference: Weeds. Croydon, British Crop Protection
Council, pp 171-177.
Walker A & Brown PA (1985) The relative persistence in soil of five
acetanilide herbicides. Bull Environ Contam Toxicol, 34: 143-149.
Warholic DT, Gutenmann WH, & Lisk DJ (1983) Propachlor herbicide
residue studies in cabbage using modified analytical procedure. Bull
Environ Contam Toxicol, 31: 585-587.
WHO/FAO (1989) Data sheets on pesticides No. 78: Propachlor. Geneva,
World Health Organization, 7 pp (WHO/VBC/DS/87.78).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-91. Geneva, World Health
Organization, 39 pp (WHO/PCS/90.1).
Worthing CR & Hance RJ (1991) The pesticide manual, 9th ed. Croydon,
British Crop Protection Council.
Yu Ching Chien, Booth GM, Hansen DJ, & Larsen JR (1975) Fate of
alachlor and propachlor in a model ecosystem. J Agric Food Chem,
23(5): 877-878.
Zhukova PS & Shirko TS (1979) [Herbicide residues in soil and
vegetables.] Khim Sel'skom Khoz, 17(6): 46-50 (in Russian).
Zilkah S, Osband ME, & Mccaffrey RP (1981) Characterization of the
inhibitory effects of the phytotoxic agent propachlor on L1210 cell
proliferation. Cancer Lett. (Irel), 13(3): 241-248.
Zilkah S, Osband ME, Mccaffrey RP, & Shapiro H (1985) The effect of
the plant cell inhibitor propachlor on the cell cycle of LI210 cells
as evaluated by flow cytometry. Life Sci, 6: 2111-2115.
Zimdahl RL & Clark SK (1982) Degradation of three acetanilide
herbicides in soil. Weed Sci, 30: 545-548.
Zlateva M & Maleva E (1978) [Late changes in the gonads of male white
rats upon combined treatment with low frequency vibrations and Ramrod
intoxication.] Med Fizkult, pp 85-91 (in Bulgarian).
Zlateva M & Maleva E (1979) Disorders in the processes of division and
differentiation of seminiferous epithelium in case of chronic
intoxication with Ramrod. Exp Med Morphol, 18(1): 35-39.
Zlateva M, Maleva E, & Stareva M (1978) Changes of adenosine
triphosphatase activity in the testes of albino rats under combined
influence of low-frequency vibration and intoxication with the
herbicide Ramrod. Rev Toxicol Eur Res, 6(78): 375-378.
Zschaler R & Jonas W (1990) Growth inhibition test with algae (Project
No. NC-90-592). Hamburg, Natec Institut für
Naturwissenschaftlich-technische Dienste GmbH. (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
RESUME ET EVALUATION
1. Identité, modalités d'utilisation, propriétés physiques et
chimiques, méthodes d'analyse
Le propachlor est un herbicide dérivé de l'acétanilide qu'on
utilise depuis 1965 en traitement de pré-levée et en traitement
précoce de post-levée. Il est principalement formulé en poudre
mouillable, liquide (concentré en suspension) et granulés. On
l'utilise en agriculture pour détruire les graminées annuelles et
certaines adventices à feuilles larges dans des cultures comme le
maïs, le sorgho, les potirons, le lin et les fleurs.
Le propachlor est légèrement soluble dans l'eau et facilement
soluble dans la plupart des solvants organiques. Il est peu volatil,
non inflammable et stable au rayonnement ultra-violet. La méthode
d'analyse la plus pratique est la chromatographie en phase gazeuse
avec détection par capture d'électrons après extraction et
purification par des méthodes appropriées.
2. Transport, distribution et transformation dans l'environnement
Le propachlor ne semble pas subir de dégradation photochimique à
la surface du sol. Il se volatilise en présence de vent lorsque la
surface du sol est encore humide.
Il n'est que modérément adsorbé aux particules du sol et aux
matières organiques, d'où un risque de lessivage à travers le sol
jusqu'aux eaux souterraines. Toutefois toutes les études montrent que
ce risque est faible en pratique. Il faut des précipitations très
importantes pour que les résidus se déplacent de 30 cm à l'intérieur
du sol. Selon la plupart des auteurs, la majorité des résidus
demeurent à moins de 4 cm de profondeur. Les caractéristiques du sol
influent beaucoup sur la mobilité du composé. Le lessivage se produit
surtout dans des sols sableux pauvres en matières organiques.
On a étudié en laboratoire et sur le terrain l'entraînement du
propachlor par ruissellement. Une des études a montré que la présence
de matières organiques dans le sol réduisait de 7 à 1 % de la quantité
appliquée, la concentration de l'herbicide dans l'eau de
ruissellement. L'incorporation de propachlor dans le sol réduit
également les pertes par ruissellement (de 3 à 0,8 % selon une étude).
En ce qui concerne la réduction de la concentration de propachlor
dans le sol et dans l'eau, le facteur de loin le plus important est sa
dégradation par les micro-organismes. On a montré que bactéries et
champignons étaient responsables de la dégradation de ce composé. Peu
de bactéries sont apparemment capables d'utiliser le propachlor comme
seule source de carbone. On a également isolé des bactéries qui sont
en mesure d'utiliser des métabolites telluriques du propachlor.
Les principaux métabolites qui se forment dans le sol sont des
acides oxaniliques et sulfoniques solubles dans l'eau. Il peut se
former un grand nombre d'autres métabolites mais ils ne représentent
qu'une faible proportion du total.
Le propachlor disparaît rapidement du sol et on a fait état de
demi-vies allant jusqu'à trois semaines. La plupart des études
indiquent une dégradation pratiquement complète en moins de six mois.
Les conditions écologiques influent sur la vitesse de dégradation qui
est favorisée par une température élevée et une forte humidité du sol.
Les résultats qui indiquent une persistance élevée du propachlor dans
le sol ont été obtenus à basse température et dans des sols secs. La
présence de nutriments en quantité suffisante dans le sol est
également nécessaire à la dégradation du propachlor.
Un des métabolites, la N-isopropylaniline conjuguée est
beaucoup plus persistante que le composé initial. On a retrouvé des
résidus de ce métabolite jusqu'à deux ans après l'épandage de
propachlor à titre expérimental à des doses plus élevées que celles
que l'on utilise normalement en agriculture.
Dans les conditions normales d'utilisation, le propachlor ne
devrait pas être entraîné à travers le sol par lessivage jusqu'aux
nappes souterraines et ne demeure pas dans le sol. Des conditions
exceptionnelles (température très basse ou sécheresse) peuvent
prolonger la persistance du propachlor et de ses métabolites.
Dans les conditions normales, le propachlor ne subit pas de
dégradation photochimique importante dans l'eau. Cependant, en
présence de photosensibilisateurs, cette dégradation photochimique
peut se produire. Le propachlor est stable à l'hydrolyse. Il est peu
probable qu'il se volatilise à partir de l'eau du fait de sa forte
solubilité dans l'eau et de sa faible tension de vapeur.
Comme dans le sol, c'est principalement par dégradation
biologique que le propachlor s'élimine de l'eau. Sa vitesse
d'élimination est donc liée à la population microbienne. Une étude
effectuée sur de l'eau contenant peu de bactéries a permis d'obtenir
une demi-vie d'environ cinq mois. Une autre étude a montré qu'au bout
de six semaines, il n'y avait toujours pas d'ouverture du cycle. Des
études de laboratoire portant sur des modèles d'écosystèmes ont montré
que le propachlor était presque complètement dégradé en l'espace de 33
jours.
Plusieurs études portant sur différentes espèces végétales ont
montré que le propachlor était rapidement métabolisé par les plantes
intactes ainsi que par des tissus végétaux excisés. Les voies
métaboliques se sont révélées analogues chez tous les végétaux
étudiés, tout au moins pendant les six à 24 premières heures,
conduisant à des métabolites hydrosolubles. On n'a pas observé de
décomposition métabolique du reste N-isopro-pylaniline. Seule une
très faible proportion (< 1 % selon une étude) des métabolites a été
retrouvée dans les fruits de ces végétaux; ils étaient concentrés en
grande majorité dans les racines et les feuilles. Les principaux
métabolites produits par les plantes sont identiques à ceux qui
prennent naissance dans le sol. On sait que ces métabolites peuvent
être fixés à partir du sol et un certain nombre d'études ne permettent
pas de déterminer avec certitude si les métabolites analysés
proviennent de la plantes ou du sol.
Bien que, d'après le coefficient de partage octanol/eau, ce
composé ait une tendance modérée à la bioaccumulation, on a montré
qu'il n'y avait ni bioconcentration ni bioamplification chez les êtres
vivants.
3. Concentrations dans l'environnement et exposition humaine
Les rapports d'analyse du propachlor dans l'air au cours de
l'épandage sont rares et insuffisants.
Aux Etats-Unis d'Amérique, la concentration du propachlor dans
les eaux de surface et les eaux souterraines est toujours faible, les
maxima atteignant 10 µg/litre dans les eaux de surface et 0,12
µg/litre dans les eaux souterraines. Le concentration la plus forte
enregistrée lors d'une étude sur les eaux de ruissellement était de 46
µg/litre.
Les résidus de propachlor dans les denrées alimentaires sont
généralement inférieurs à la limite de détection de la méthode
d'analyse (0,005 mg/kg). L'expérimentation a permis de retrouver des
résidus de l'ordre de 0,005 mg/kg dans des tomates, des poivrons, des
oignons et des choux.
Le dosage du propachlor dans l'atmosphère de la zone de travail
de conducteurs de tracteur pendant l'épandage du composé a donné des
résultats allant de 0,1 à 3,7 mg/m3.
4. Cinétique et métabolisme
Chez les mammifères, le propachlor peut être absorbé au niveau
des voies respiratoires, des voies digestives et de la peau. Il ne
s'accumule pas dans l'organisme et devient indétectable au bout de 48
heures.
La plupart des espèces animales (rats, porcs, poulets)
métabolisent le propachlor par la voie de l'acide mercapturique (MAP).
Il se forme des conjugués de cystéine par conjugaison avec le
glutathion et on a avancé que ces conjugués jouaient le rôle
d'intermédiaires dans la formation métabolique des acides
mercapturiques. La métabolisation du conjugué cystéinique du
propachlor se poursuit, notamment sous l'action de la C-S lyase
bactérienne qui intervient également dans la formation des métabolites
terminaux contenant le groupement méthylsulfonyle, métabolites qui
sont principalement excrétés dans l'urine (68 % de la dose initiale de
propachlor) et, sous forme de résidus insolubles, dans les matières
fécales (19 %). La C-S lyase du propachlor est sans action chez les
rats axéniques.
On a montré qu'il y avait un certain nombre de différences dans
le métabolisme du propachlor chez le rat et le porc. La bile est la
principale voie d'élimination des métabolites mercapturiques chez le
rat, mais on a montré qu'il existait une voie métabolique
extra-biliaire chez le porc.
Des études de métabolisme effectués sur des veaux ont montré que
ces animaux pourraient être incapables de former de l'acide
mercapturique à partir des conjugués du glutathion, ce qui les
rendrait plus sensibles à l'intoxication par le propachlor.
5. Effets sur les animaux d'expérience et les systèmes d'épreuves in
vitro
Le propachlor est légèrement toxique en cas d'exposition aiguë
par voie orale (la DL50 pour le rat varie de 950 à 2176 mg/kg de
poids corporel). Les signes d'intoxication aiguë proviennent
principalement d'effets sur le système nerveux central (excitation et
convulsions suivies d'une dépression). Chez des rongeurs, la toxicité
aiguë par inhalation est faible (CL50 = 1,0 mg/litre). Le propachlor
provoque de graves irritations des yeux et de la peau.
Des rats, des souris et des chiens ont été soumis à une
exposition de longue ou de courte durée au propachlor. Les organes
cibles sont le foie et les reins. Chez le chien, la dose sans effet
nocif observable a été de 45 mg/kg de poids corporel lors d'une étude
de trois mois où l'animal était exposé par la voie alimentaire. Lors
d'une étude d'une année sur des chiens, la dose sans effet nocif
observable a été estimée à 9 mg/kg de poids corporel (200 ppm dans la
nourriture). Lors d'une étude sur des rats exposés de la même manière
pendant 24 mois au propachlor, la dose sans effet observable a été
évaluée à 50 mg/kg de nourriture (2,6 mg/kg de poids corporel). Lors
d'une étude semblable effectuée sur des souris pendant 18 mois, on a
évalué à 1,6 mg/kg de poids corporel (10 ppm) la dose sans effet
observable.
Le propachlor ne s'est révélé cancérogène ni pour la souris ni
pour le rat. Dans la plupart des systèmes d'épreuve mammaliens, sa
réponse mutagène est négative tout en étant positive dans quelques
autres cas. Les données expérimentales disponibles de fournissent pas
une preuve suffisante de son pouvoir mutagène.
Administré en dose unique (675 mg/kg) à des rats et à des souris,
le propachlor a donné lieu à des signes d'embryotoxicité. On a
également observé des effets embryotoxiques lors d'études où du
propachlor avait été administré à plusieurs reprises (35,7 à 270
mg/kg). Toutefois, lors d'une autre étude sur des rats, où les doses
variaient de 20 à 200 mg/kg, aucun effet embryotoxique n'a été
observé.
Aux doses respectives de 12 et 60 mg/kg de poids corporel, le
propachlor (en poudre mouillable) a entraîné une réduction de la
teneur en protéines et un accroissement de l'activité de l'ATPase et
de la 5-nucléotidase dans des homogénats de testicules de rats,
provoquant également une dégénérescence du tissu testiculaire. Lors
d'une étude de reproduction portant sur deux générations, on n'a pas
véritablement obtenu la preuve d'effets indésirables.
6. Effets sur l'homme
On a signalé quelques dermatites de contact et dermatites
allergiques chez des agriculteurs et des ouvriers de production
exposés au propachlor (Ramrod et Satecid). Des tests cutanés ont été
effectués parmi un certain nombre d'entre eux, avec un résultat
positif, qui révèle une réaction d'irritation et une hypersensibilité
mono- et bivalente.
On n'a pas eu connaissance de symptômes ou de maladies, soit chez
des personnes exposées de par leur profession, soit dans la population
générale - à part les quelques cas d'effets cutanés chez les ouvriers
professionnellement exposés.
7. Effets sur les êtres vivant dans leur milieu naturel
Des études portant sur la microflore terricole ont montré que les
bactéries nitrifiantes constituaient le groupe le plus sensible aux
effets inhibiteurs du propachlor, leur nombre étant réduit d'un
facteur 3 à 4 après épandage de 8 à 10 kg de propachlor par hectare.
Ce sont les bactéries décomposant la cellulose qui étaient les moins
sensibles. La forte adsorption du propachlor aux particules d'argile
présentes dans le sol et une température élevée sont deux facteurs qui
réduisent les effets inhibiteurs.
Chez l'algue Selenastrum capricornutum, on a fait état d'une
CE50 à 96 heures de 0,02 mg/litre (pour la croissance) et d'une
concentration sans effet observable de 0,01 mg/litre. D'après une
deuxième étude portant sur une formulation de propachlor et menée
pendant 72 heures, le risque pour cette même algue serait nettement
moindre.
Pour la daphnie Daphnia magna, on donne des CL50 de 7,8 ou
6,9 mg/litre et une concentration sans effet observable inférieure à
5,6 mg/litre. La concentration sans effet observable sur la
reproduction était de 0,097 mg/litre. Pour des larves de deux espèces
de moucherons, on a obtenu pour la CL50 des valeurs respectives de
0,79 et 1,8 mg/litre.
Chez la truite arc-en-ciel la CL50 à 96 heures est de 0,17
mg/litre et la concentration sans effet observable sur 21 jours, de
0,019 mg/litre.
Le propachlor est considéré comme modérément à fortement toxique
pour les organismes aquatiques.
Le propachlor n'est pas toxique pour les lombrics aux
concentrations présentes dans le sol par suite d'un usage normal (la
concentration sans effet observable est de 100 mg/kg de terre). La
DL50 par contact pour les abeilles (311 µg/abeille) montre que le
propachlor ne constitue pas un danger pour ces insectes. En revanche,
des études menées en laboratoire et sur le terrain montrent que des
parasites utiles peuvent souffrir des effets du propachlor.
Le propachlor est plus toxique pour les oiseaux lorsqu'on
l'introduit directement dans l'estomac que lorsqu'on l'administre dans
la nourriture. La DL50 aiguë varie de 137 à 735 mg/kg de poids
corporel pour différentes espèces. Quant à la CL50 lors d'une
exposition par voie alimentaire, elle dépasse 5620 mg/kg de nourriture
chez les oiseaux.
Le propachlor ne constitue pas un danger pour les oiseaux dans la
nature, même sous forme de granulés.
RESUMEN Y EVALUACION
1. Identidad, modalidades de uso, propiedades físicas y químicas y
método analíticos
El propacloro es un herbicida derivado de la acetanilida
utilizado desde 1965 que actúa en las plantas antes de nacer o poco
después. Las principales formulaciones son de polvo humectable,
líquido fluido (concentrado en suspensión) y gránulos. Se aplica en la
agricultura a la lucha contra las gramíneas anuales y algunas malas
hierbas de hoja ancha en varios cultivos, como los de maíz, sorgo,
calabaza, lino y flores ornamentales.
El propacloro es ligeramente soluble en agua y se disuelve
fácilmente en la mayoría de los disolventes orgánicos. Su volatilidad
es escasa, no es inflamable y es estable a la radiación ultravioleta.
El método más práctico de análisis es la cromatografía de gases con
detección por captura de electrones, después de aplicar procedimientos
apropiados de extracción y purificación.
2. Transporte, distribución y transformación en el medio ambiente
Según la información disponible, el propacloro no se fotodegrada
sobre la superficie del suelo. El producto se volatiliza cuando hay
viento y la superficie del suelo está todavía mojada.
La adsorción sobre las partículas del suelo y la materia orgánica
es sólo moderada. Debido a esto, es posible la lixiviación a través
del perfil del suelo hacia el agua subterránea. Sin embargo, todos los
estudios indican que en la práctica es poco probable que esto ocurra.
Se requiere una precipitación muy abundante para hacer descender los
residuos 30 cm en el perfil del suelo. La mayoría de los autores
señalan que la mayor parte de los residuos se mantienen en una capa
superficial del suelo de 4 cm. Las características del suelo influyen
mucho en el desplazamiento del producto. La lixiviación se produce en
su mayor parte en suelo arenoso con poca materia orgánica.
Se ha estudiado el arrastre del propacloro por el agua tanto en
el laboratorio como sobre el terreno. En un estudio, la materia
orgánica del suelo redujo el arrastre del herbicida aplicado del 7% al
1%. Su incorporación al suelo también redujo la pérdida por arrastre
del agua (del 3% al 0,8% en un estudio).
El factor que más contribuye, con diferencia, a reducir los
niveles de propacloro en el suelo y el agua es la degradación por los
microorganismos. Se ha comprobado que en la degradación de la
sustancia intervienen tanto bacterias como hongos. Son pocas las
bacterias que parecen tener capacidad para usar el propacloro como
única fuente de carbono. También se han aislado bacterias que pueden
utilizar los metabolitos del propacloro presentes en el suelo.
Los metabolitos predominantes entre los que se forman en el suelo
son los ácidos oxanílico y sulfónico, solubles en agua. Pueden
formarse otros muchos metabolitos, pero representan una proporción
pequeña del total.
El propacloro desaparece con rapidez del suelo, habiéndose
registrado semividas de hasta tres semanas. En la mayoría de los
estudios se señala que la degradación es casi completa en menos de
seis meses. Las condiciones del medio ambiente influyen en la
velocidad de degradación, que se ve favorecida por los valores
elevados de la temperatura y el contenido de humedad del suelo. Los
estudios en los que se describía una permanencia más prolongada del
propacloro en el suelo se realizaron en condiciones de baja
temperatura o suelo seco. También es necesaria una cantidad suficiente
de nutrientes en el suelo para la degradación.
El metabolito conjugado N-isopropilanilina es mucho más
persistente que el producto del que procede. Se han encontrado
residuos de este metabolito hasta dos años después de la aplicación
experimental de dosis de propacloro más altas de las que se utilizan
normalmente en la agricultura.
Con el uso normal no es previsible que el propacloro llegue por
lixiviación a través del suelo hasta el agua subterránea, y no se
mantiene mucho tiempo en el suelo. En condiciones excepcionales de
baja temperatura o sequedad, el propacloro y sus metabolitos pueden
permanecer más tiempo en él. En condiciones normales, el propacloro
no sufre una fotodegradación significativa en el agua. En presencia de
fotosensibilizadores puede fotodegradarse. El propacloro es estable
desde el punto de vista hidrolítico. No es probable la volatilización
a partir del agua, debido a la elevada solubilidad en ella y la baja
tensión de vapor del producto.
Al igual que en el suelo, la principal ruta de pérdida de
propacloro del agua es la degradación biótica. La velocidad de
desaparición del propacloro del agua depende, pues, de la población
microbiana. En un estudio realizado con un pequeño número de bacterias
presentes en el agua se obtuvo una semivida de unos cinco meses. En
otro estudio, a las seis semanas no se había roto el anillo. En
estudios de laboratorio con modelos de ecosistemas se observó una
degradación casi completa en un período de 33 días.
En varios estudios con distintas especies vegetales se vio que el
propacloro se metabolizaba con rapidez tanto en las plantas intactas
como en tejidos vegetales extirpados. Las rutas metabólicas eran
análogas en todas las plantas estudiadas, por lo menos durante las
primeras 6-24 horas, produciendo metabolitos hidrosolubles. No se
observó degradación metabólica del fragmento de la
N-isopropilanilina. En los frutos de las plantas solamente se
encontró una proporción muy pequeña (< 1% en un estudio) de los
metabolitos; en su mayor parte estaban en las raíces y el follaje. Los
principales metabolitos producidos en las plantas son idénticos a los
que se forman en el suelo. Se sabe que las plantas absorben esos
metabolitos del suelo, y en algunos estudios había dudas acerca de si
los metabolitos medidos procedían de la planta o del suelo.
Aunque del coeficiente de reparto en octanol y agua parece
deducirse un potencial moderado de bioacumulación, los estudios
realizados indican que no hay ni bioconcentración ni bioamplificación
en los organismos.
3. Niveles medioambientales y exposición humana
Las mediciones descritas de la concentración del propacloro en el
aire durante la aplicación son pocas e inadecuadas.
Las concentraciones en el agua superficial y subterránea en los
Estados Unidos fueron siempre bajas, con un máximo de 10 µg/litro en
la superficial y de 0,12 µg/litro en la subterránea. La mayor
concentración registrada en el agua en un estudio del arrastre fue de
46 µg/litro.
Los residuos de propacloro en los alimentos suelen ser inferiores
al límite de detección del método analítico (0,005 mg/kg). En estudios
experimentales se han identificado concentraciones de residuos del
orden de 0,05 mg/kg en tomates, pimientos, cebollas y coles.
Las concentraciones de propacloro en el aire de la zona de
trabajo de los conductores de tractores que aplicaban la sustancia
oscilaban entre 0,1 y 3,7 mg/m3.
4. Cinética y metabolismo
Los mamíferos pueden absorber el propacloro por los tractos
respiratorio y gastrointestinal, así como a través de la piel. El
producto no se acumula en el organismo, en el que no es detectable a
las 48 horas.
La mayoría de las especies animales (ratas, cerdos, pollos)
metabolizan el propacloro siguiendo la vía de los ácidos
mercaptúricos. Mediante conjugación con el glutatión se forman
productos conjugados de cisteína, que se han señalado como posibles
intermediarios en la formación metabólica de ácidos mercaptúricos. La
C-S liasa bacteriana participa en el ulterior metabolismo del sistema
conjugado del propacloro con la cisteína y en la formación de los
metabolitos finales con metilsulfonilo, que se excretan sobre todo en
la orina (68% de la dosis de propacloro), y de residuos insolubles,
que se excretan en las heces (19%). La propacloro C-S liasa es
inactiva en ratas sin microorganismos.
En los estudios realizados se observaron algunas diferencias en
cuanto al metabolismo entre las ratas y los cerdos. La bilis es el
principal camino de eliminación de los metabolitos de la vía de los
ácidos mercaptúricos en las ratas, pero se ha demostrado que en los
cerdos existe una vía metabólica extrabiliar.
En estudios metabólicos con terneros se observó que pueden ser
incapaces de formar ácidos mercaptúricos a partir de los productos
conjugados del glutatión, por lo que pueden ser más susceptibles a la
intoxicación.
5. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
El propacloro es ligeramente tóxico en la exposición oral aguda
(la DL50 en ratas va de 950 a 2176 mg/kg de peso corporal). Los
signos de intoxicación aguda son predominantemente efectos sobre el
sistema nervioso central (excitación y convulsiones, seguidas de
depresión). La toxicidad aguda por inhalación en roedores es baja
(CL50 = 1,0 mg/litro). El propacloro produjo efectos graves de
irritación en los ojos y la piel.
El propacloro se ha ensayado en estudios de exposición de corta
y larga duración en ratas, ratones y perros. Los órganos sobre los que
actuaba eran el hígado y los riñones. En un estudio de administración
con los alimentos a perros durante tres meses, el nivel sin efectos
adversos observados (NOAEL) fue de 45 mg/kg de peso corporal. En otro
estudio de un año, también en perros, el NOAEL fue de 9 mg/kg de peso
corporal (250 ppm en la dieta). El nivel sin efectos observados (NOEL)
en un estudio de alimentación de ratas durante 24 meses fue de 50
mg/kg de la dieta (2,6 mg/kg de peso corporal). En un estudio en el
que administró el producto a ratones con los alimentos durante 18
meses, el NOEL fue de 1,6 mg/kg de peso corporal (10 ppm).
No se encontraron efectos carcinogénicos del propacloro en
ratones y ratas. En la mayoría de los sistemas de prueba de mamíferos
la respuesta mutagénica fue negativa, obteniéndose resultados
positivos en un pequeño número de ensayos. Los datos experimentales
disponibles no aportan suficientes pruebas de su potencial mutagénico.
En pruebas de dosis única (675 mg/kg) con ratones y ratas se
demostró la embriotoxicidad del propacloro. También se detectaron
efectos embriotóxicos en tratamientos con dosis repetidas (35,7-270
mg/kg). Sin embargo, en otro estudio en ratas, con una gama de dosis
de 20-200 mg/kg, no se observó embriotoxicidad.
Con niveles de 12 y 60 mg/kg de peso corporal, el propacloro
(polvo humectable) provocó una disminución del contenido de proteínas
y un aumento de la actividad de la ATPasa y la 5-nucleotidasa en un
homogeneizado de testículo de rata y cambios degenerativos en los
testículos. En un estudio de reproducción de dos generaciones no se
obtuvieron pruebas definitivas de efectos adversos.
6. Efectos en la especie humana
Se ha descrito un pequeño número de casos de dermatitis de
contacto y alérgica en agricultores y trabajadores expuestos al
propacloro durante la producción (Ramrod y Satecid). En algunos de
ellos se efectuaron pruebas del parche y la reacción fue positiva, con
irritación e hipersensibilidad monovalente y bivalente.
No se han notificado casos de síntomas o enfermedades entre las
personas expuestas profesionalmente o en la población general, salvo
un pequeño número de informes de sus efectos cutáneos en trabajadores
expuestos profesionalmente.
7. Efectos en los seres vivos del medio ambiente
En varios estudios sobre los microorganismos del suelo, las
bacterias nitrificantes fueron el grupo más sensible a los efectos
inhibidores del propacloro, reduciéndose su número a la tercera o
cuarta parte tras la aplicación de 8-10 kg/ha de propacloro. Las menos
sensibles fueron las bacterias celulolíticas. La adsorción elevada
sobre las partículas de arcilla del suelo y la temperatura alta
reducen los efectos inhibidores.
En el alga Selenastrum capricornutum se ha registrado una CE50
a las 96 horas de 0,02 mg/litro para el crecimiento y una
concentración sin efectos observados (NOEC) de 0,01 mg/litro. De un
segundo estudio de 72 horas realizado con una formulación, parece
desprenderse que el peligro es mucho menor para este mismo organismo.
Se ha informado de unos valores de la CL50 para Daphnia magna
de 7,8 y 6,9 mg/litro y una NOEC de < 5,6 mg/litro. En las larvas de
dos especies de moscas enanas, los valores registrados de la CL50
fueron de 0,79 y 1,8 mg/litro.
La CL50 para la trucha arcoiris a las 96 horas es de 0,17
mg/litro, y la NOEC en un estudio de 26 días fue de 0,019 mg/litro.
Se considera que el propacloro tiene una toxicidad entre moderada
e intensa para los organismos acuáticos.
La exposición a las concentraciones de propacloro que cabe prever
en el suelo no es tóxica para las lombrices de tierra si la aplicación
es normal (la NOEC es de 100 mg/kg de suelo). La DL50 por contacto
para las abejas (311 µg/abeja) demuestra que el propacloro no
representa un peligro para estos insectos. En estudios de laboratorio
y de campo se han detectado efectos adversos del propacloro en algunos
insectos parasitarios beneficiosos.
El propacloro es más tóxico para las aves cuando se administra
mediante sonda gástrica que cuando se incorpora a la dieta. Los
valores de la DL50 aguda para distintas especies de aves oscilan
entre 137 y 735 mg/kg de peso corporal. Los valores de la CL50 en la
administración con los alimentos fueron superiores a los 5620 mg/kg de
la dieta.
El propacloro no representa un peligro para las aves en el campo,
ni siquiera con la formulación granulada.
Table 9. Incubation of the glutathione conjugate of propachlor with pig caecal contents for various durationsa
Metabolites recovered Incubation time
0 20 min 40 min 1 h 2 h 4 h
2-Mercapto-N-isopropylacetanilide 7.7b 14.5b 23.0b 28.3b 34.7b 43.4c
(5.1-10.6) (5.5-22.8) (15.8-36.0) (22.9-38.7) (21.8-44.0) (38.7-49.2)
Glutathione conjugate 26.8 36.0 20.8 28.8 21.5 16.5
of propachlor (15.8-38.1) (18.1-51.6) (13.3-25.3) (16.6-42.3) (14.0-32.2) (14.8-19.2)
Cysteine conjugate of 47.5 32.5 35.1 20.9 11.9 3.8
propachlor (36.0-51.5) (21.3-43.7) (30.5-41.9) (12.0-27.9) (10.9-12.5) (2.2-5.6)
Non-extractable 14C 13.1 12.9 16.0 16.6 25.4 33.9
residues (13.0-13.4) (9.9-17.8) (13.5-20.5) (15.0-18.9) (22.8-28.2) (31.5-35.8)
a From: Larsen & Bakke (1983).
b Isolated as 2-carboxymethylthio- N-isopropylacetanilide
c Isolated as 2-(13C)-carboxymethylthio- N-isopropylacetanilide
Pigs were gilts. Results are shown as a percentage of the substrate. Values given in parentheses represent the range of values
obtained from 14C-recovery measurements.
More recent studies carried out by Aschbacher & Struble (1987) on
the metabolism of propachlor in pigs have proved the similarity, i.e.
the formation of methylsulfonyl-containing metabolites, with the
metabolism of rats, but have also revealed some differences.
A pig with a cannulated bile duct, which was dosed orally with
14C-propachlor, excreted 7.6% of the dose in the bile compared with
approximately 75% in the case of the rats. When enterohepatic
circulation was prevented in the bile of cannulated pigs,
CH3SO2-containing metabolites of propachlor were excreted in the
urine. As mentioned above, enterohepatic circulation is necessary for
the production of methylsulfonyl-containing metabolites in rats. In
experiments with germ-free pigs dosed orally with 14C-propachlor, it
was shown that they did not excrete urinary CH3SO2 metabolites, which
indicated involvement of the intestinal flora in the production of
these metabolites, as occurs in rats. Non-biliary excretion of
metabolites of propachlor into the lumen of the intestine probably
occurred. It is presumed that propachlor is absorbed by the mucosal
cells and conjugated with glutathione and that some of this conjugate
moves directly into the lumen of the intestinal tract by simple
diffusion, where it becomes the substrate of bacterial beta-lyase. The
presence of glutathione transferase has been demonstrated previously
in subcellular fractions of mucosal tissue homogenates.
In situ intestinal absorption of propachlor and non-biliary
excretion of metabolites into the intestinal tract of rats, pigs and
chickens was studied by Struble (1991). Propachlor was absorbed from
in situ intestinal loops of rats and pigs, the absorption half-times
being 7.5 and 16.5 min, respectively. Water-soluble 14C-labelled
metabolites that accumulated in the intestinal loops accounted for
31%, 53% and 25% of the initial 14C in rats, pigs and chickens,
respectively. Propachlor- S-cysteine was identified as the major
metabolite in the pig intestinal lumen (43% of the water-soluble
14C). It was concluded that the intestinal metabolism and intestinal
excretion of water-soluble metabolites of propachlor are important
physiological processes that occur in a variety of animal species.
These processes provide a route by which metabolites of xenobiotics
may reach the intestinal lumen in animals that are poor biliary
excretors. These studies demonstrated that although bile may be the
major route by which MAP metabolites are made available to the
intestinal microflora in the rat, an extrahepatic route exists in the
pig.
Davison (1991) conducted a study using six anaesthetized 2- to
21-day-old male Guernsey calves weighing 28 to 61 kg in which either
the left kidney was perfused (via the left renal artery) or the left
ureter was perfused with metabolites of propachlor. The glutathione
conjugate of propachlor (2- S-glutathionyl- N-acetyl-acetanilide)
was metabolized in both kidney and ureter to the cysteine conjugate.
When the mercapturic acid conjugate of propachlor was presented to the
kidney, it was eliminated in urine. First-pass metabolism and
elimination of the glutathione conjugate by the kidney was 16% of the
dose, whereas first-pass elimination of the mercapturic acid was 33%.
Absorption of the glutathione conjugate of propachlor or its
metabolites, or of glycine by the ureter was nil. The cattle may be
unable to form mercapturic acids from glutathione conjugates of some
xenobiotics, which may result in their being more easily poisoned by
these xenobiotics than chickens, pigs and rats.
The glutathione conjugate of 2-chloro- N-isopropyl[1-14C]acet-
anilide (14C-propachlor) was perfused through a calf kidney in situ
by Bakke et al. (1990). Twenty-three per cent of the dose was excreted
in the perfused kidney urine as the cysteine conjugate; no mercapturic
acid was detected. A 5-day-old calf dosed orally with 14C-propachlor
excreted 70% of the dose in the urine as the cysteine conjugate; again
no mercapturic acid was detected. Rumen microflora were established in
the calf when it was 5 weeks older and the experiment was repeated.
The same results were obtained.
6.3 Elimination and excretion
When 14C-propachlor was given to rats, 56-64% of the dose was
excreted in urine in the first 24 h and 5.7-7.0% in 24-48 h. In the
faeces, 8-13% and 2.2-7.7% were eliminated in 0-24 h and 24-48 h,
respectively; 0.4% of the 14C was eliminated as CO2 and 5-11% was in
the carcass. In total 80-97% was eliminated in 48 h (Lamoureux &
Davison, 1975).
According to Bakke et al. (1980), the metabolites of propachlor
formed in normal rats treated with propachlor are excreted mainly
through urine (68%) and faeces (19%). Eleven urinary metabolites were
isolated from rats given 14C-labelled propachlor orally. The major
metabolite was the mercapturate (17%), and six of the metabolites were
2-methylsulfonylacetanilides. Faecal residues (19%) of the
administered dose, insoluble in common solvents or by treatment with
diluted acid or base, were also determined.
Rats with cannulated bile ducts secreted 66% of an oral dose of
propachlor in the bile as the glutathione conjugate (2), cysteine
conjugate (3), mercapturate (4) and the mercapturate sulfoxide (5)
(see Table 10). Germ-free rats given orally 14C-labelled propachlor
excreted 98% of the dose in the urine and faeces within 48 h. Three
metabolites were isolated from the excreta and the faecal radioactive
metabolites were water soluble. The major metabolite was mercapturate
(4) and the other metabolites were the cysteine conjugate (3) (present
only in the faeces) and mercapturate sulfoxide (5) (Bakke et al.,
1980). Mercapturate sulfoxide was isolated from the excreta of
germ-free rats by Feil et al. (1981), who also demonstrated its
presence in the bile of rats and urine of chickens and pigs dosed with
propachlor. The metabolite was characterized by mass and nuclear
magnetic resonance spectro-metry on samples isolated from rats.
Table 10. Comparison of the excretion of single oral doses of 14C-propachlor
by control rats with fistulated bile ducts, and germ-free ratsa
Metabolite Recovery of 14C (% dose)b
Bile-fistulated
Control rats rats Germ-free rats
Urine Faeces Bile Urine Faeces
Glutathione 37
conjugate (2)b
Cysteine 13 19
conjugate (3)
Mercapturate (4) 17 12 63.1 3.7
Mercapturate 4 5.7 5.4
sulfoxide (5)
Non-extractable 19
residues
Other metabolites 51
Total 68 19 66 68.8 32.1
a From: Bakke et al. (1980)
b Metabolite designations are those used in Figs. 1 and 3.
As in the case of the glutathione and cysteine conjugates, the
sulfoxide is not excreted at detectable levels by normal rats and was
detected only in the bile (Bakke et al., 1981a). It may become a
substrate for the intestinal flora, but the ultimate in vivo fate of
this metabolite is unknown.
Bakke et al. (1981c) reported differences between some species
concerning the metabolism of propachlor in the MAP. Clear but
unexplained differences are that rats excrete no cysteine conjugate
and chickens form no methylsulfonyl-containing metabolites, whereas
sheep excrete large amounts of cysteine conjugate in urine.
Nadeau & Pantano (1986) carried out a study to determine the
rates and routes of excretion of orally administered synthetic
14C-labelled propachlor plant metabolites in lactating goats and to
quantify and identify the radioactive metabolites in the goat milk,
tissues, urine and faeces. The daily dose level for the three treated
goats was 15 mg/kg administered on 5 consecutive days (the actual dose
levels for the treated goats were 13, 14.5 and 13 mg/kg,
respectively). Each treated goat received a total of 130.5 mg of the
13C/14C-labelled propachlor plant metabolites mixture during the
dosing period. A control animal received placebo capsules. The
radioactivity eliminated in faeces accounted for 72.1, 64.5 and 57.9%
of the administered dose, respectively (average 64.8%), and that
excreted in urine accounted for 35.8, 28.1 and 32.3% (average 32.1%).
The percentage of the dose eliminated through faeces and urine
averaged 96.9%.
The radioactivity found in the milk accounted for only 0.084,
0.10 and 0.13% (average 0.10%) of the administered dose. These values
corresponded to metabolite concentrations in the milk of 11.9, 14.8
and 18.0 µg/litre (average 14.9 µg/litre). The metabolic
concentrations (µg/kg) in the tissues were: kidney 51.9, liver 30.4,
muscle 8.5, fat 19.5 and blood 50.1. The loss of radioactivity from
tissues, milk and excrement occurred rapidly, and after 5 days of
depuration the milk and tissue radioactivity levels were below the
limit of detection, except in the case of the liver (5.7 µg/kg).
6.4 Metabolism in laying hens
Since crops treated with propachlor are used in animal feed, it
is important to know the fate of propachlor in animal feed.
The purpose of the study by Bleeke et al. (1987) was to examine
the metabolic fate of propachlor plant metabolites in laying hens and
to determine whether they accumulate or persist in the eggs or edible
tissues. The compounds used for feeding were the three major
metabolites of propachlor found in sorghum, i.e. [( N-iso-propyl)-
phenylamino] oxacetic acid, sodium salt (I), {{[( N-iso-propyl)-
phenylamino]acetyl}sulfinyl} lactic acid, sodium salt (II) and
2-[( N-isopropyl)-phenylamino] 2-oxoethane sulfonic acid, sodium salt
(III).
The first study involved dosing chickens at a level of 5 mg/kg
diet for 6 consecutive days. Data for bioaccumulation and excretion of
plant metabolites were obtained from this study. A second group of
chickens, dosed at a level of 25 mg/kg diet (nominal dose) on 6
consecutive days, provided eggs and tissues with higher residues for
metabolic characterization. Each group consists of five hens. Control
groups received a single gelatine capsule per day. The total recovery
of 14C radioactivity was good in both studies.
An average of 87.4% of the total administered dose was recovered
from the chickens fed 5 mg/kg approximately 1 day after the last dose,
and 97.9% was recovered from the chickens fed 25 mg/kg, primarily in
the excreta. The eggs contained low residue levels; those from
chickens fed 5 mg/kg contained residues below the minimum quantifiable
limit (MQL) of 1.7 µg/kg. The level in the egg yolks reached an
average of 5.5 µg/kg by day 6 but by day 12 the residues in the yolk
had fallen below 1.6 µg/kg.
In the high-dose group, the residues in the egg white levelled
off at about 4 µg/kg on day 2, while those in the egg yolk increased
to a level of 27.3 µg/kg on day 6.
Tissues also contained low levels of residues. In the low-dose
group, the highest levels were found in the kidney and they averaged
only 6 µg/kg. The residue levels in the liver measured an average of
1.9 µg/kg and those in the fat 3.5 µg/kg. The residues in other fat
samples and organs were below the MQL (1.4-1.6 µg/kg). Residues in the
breast muscle were below the minimum detectable limit (7 µg/kg) and
those in the blood were 6-7 µg/kg.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Oral
The acute oral toxicity of propachlor for the rat, mouse and the
rabbit has been examined. According to the WHO hazard classification
of pesticides and as far as its acute oral toxicity to rats is
concerned, propachlor is slightly hazardous (Group III) (WHO, 1990).
LD50 values in various animal species reveal a higher susceptibility
for the mouse and the rabbit than for the rat (Table 11). The LD50
for rats ranges from 950 to 2176 mg/kg.
The clinical picture of acute oral intoxication by propachlor has
been described in three species of experimental animals: mice, rabbits
and rats (Panshina, 1973). When propachlor is given at lethal or toxic
doses, the main symptoms concern the central nervous system. In mice
and rabbits, a state of excitement, trembling and light convulsions is
observed 15 min after a toxic or lethal dose of propachlor. This
gradually increases, breathing becomes difficult and death follows in
fseveral hours. Intoxication in rats takes a different course: a state
of immobility and head and body tremors are accompanied by the sudden
onset of convulsions and death occurs within 24 h. This clinical
picture was confirmed by Strateva (1974,a,b).
Kronenberg (1988) reported clear signs of irritation to the
gastrointestinal tract and lungs that were evident at necropsy in
animals that died during the study. Histoenzymological changes
expressed as decreased enzyme activity were in full agreement with the
morphological picture (Strateva et al., 1974a,b).
7.1.2 Dermal
Results of studies in rabbits on the acute dermal toxicity of
propachlor and its formulations indicate that the dermal LD50 ranges
from 4000 mg/kg for a 65% WP formulation (Auletta & Rinehart, 1979) to
20 000 mg/kg for technical propachlor (94.5%) (Braun & Rinehart,
1978). Test animals exhibited moderate to severe erythema, severe
oedema and necrotic skin at the dermal application sites at a dose
level of 2800 mg/kg. Motor activity decrease and ataxia were noted at
dose levels of 2000 to 5600 mg/kg.
Table 11. Oral LD50 values for laboratory mammals
Species LD50 (mg/kg)a Reference
Rat 1056 (834-1278) Panshina (1973, 1977)
950 (860-1050) Strateva (1974b)
1340 Mirkova (1975)
Table 11 (contd).
Species LD50 (mg/kg)a Reference
2176 ± 220 Lehotzky et al. (1979)
Rat (Sprague-Dawley) 840 (419-1261) Blaszcak (1988)
(in corn oil)
1700 (1265-2135) Blaszcak (1988)
(in 1% methylcellulose)
Rat (Fischer-344) 550 (252-848) in corn oil Blaszcak (1988)
1359 (1009-1691) Blaszcak (1988)
(in 1% methylcellulose)
Rat (both sexes) 4000 Heenehan et al. (1979)
Rat (both sexes) 3269 Branch et al. (1982a)
Mouse 306 (275-337) Panshina (1973, 1977)
290 (240-350) Strateva (1974b)
a The concentration is based on the percentage active ingredient.
Figures in parentheses indicate the range of values.
Using groups of 16 Wistar rats, Baynova et al. (1977) studied the
acute dermal toxicity of a single application of propachlor 65 WP
(10-20% in aqueous suspension) with doses of 1500-4000 mg/kg body
weight (active ingredient). There was no mortality, and no signs of
intoxication were observed. No haematological or biochemical tests
were performed.
Propachlor and Satecid 65 WP caused severe dermatitis, ulceration
and necrosis in the skin of rabbit and ears of mice. None of the
compounds exhibited contact sensitization effects on guinea-pigs
(Lehotzky et al., 1979).
7.1.3 Inhalation
In a study by Bechtel (1991), technical propachlor (96.8%) was
dissolved in dimethylsulfoxide (DMSO) to generate an aerosol and
administered to five male and five female Sprague-Dawley rats in a
nose-only chamber at an analytically determined concentration of 1.2
mg/litre for 4 h. Control rats (five of each sex) were exposed to an
atmosphere of aerosolized DMSO for the same duration. Particle size
measurements on the propachlor/DMSO aerosol indicated a mass median
aerodynamic diameter of 3.5 µm, with 96% of the particles being less
than 10 µm and 1.8% less than 1 µm in diameter (Bechtel, 1991). No
treatment-related deaths occurred. Clinical signs included laboured
respiration and nasal discharge; all animals appeared normal by
post-exposure day 2. A transient weight loss was noted in both treated
and control animals during the first two days of the study, but normal
body weight was observed thereafter. No abnormalities were apparent
during postmortem examination of the animals.
In another acute inhalation study, three groups of Charles River
CD rats (five rats of each sex per group) were exposed to test
atmospheres of a propachlor formulation (44% active ingredient) for 4
h (Kaempfe, 1991). During the exposure period, animals were housed in
a 250-litre New York University style stainless steel chamber, and
were exposed to analytically confirmed concentrations of 0.18, 0.67
and 1.0 mg propachlor/litre in the breathing zone of the chamber. At
least 82% of the particles were less than 10 µm in diameter. No
animals died at the two lower exposure levels, whereas four out of ten
rats died at 1.0 mg/litre. Clinical observations during the
post-exposure period included ocular opacities, perinasal
encrustation, rapid or shallow respiration, perioral wetness and focal
loss of hair from animals in the highest exposure group. Fourteen days
after exposure, all surviving animals had body weights higher than the
pre-exposure (day 0) values with the exception of females in the
1.0-mg/litre group, which exhibited body weight depression. There were
no abnormal findings in rats that died during the test or were
sacrificed at 14 days. Based on the mortality observed, the LC50 was
slightly greater than 1.0 mg/litre.
7.2 Short-term exposure
7.2.1 Oral
7.2.1.1 Dogs
To assess potential subchronic toxicity, propachlor (96.1% pure)
was administered via the diet to five groups of two male and two
female beagle dogs for 4 weeks (Daly & Knezevich, 1984). Dietary
concentrations of propachlor were 0, 100, 500, 1000 and 1500 mg/kg
(equivalent to 0, 2.5, 12.5, 25, and 37.5 mg/kg body weight per day).
No mortality or clinical signs of toxicity related to treatment
occurred during this study. Food consumption was initially decreased,
but only in the females treated with 1000 and 1500 mg/kg (equal to 25
and 37.5 mg/kg body weight) and the males treated with 1000 mg/kg. The
consumption had returned to normal by the end of the study. Body
weight varied markedly. The decreased body weight and/or weight gain
noted in males fed 12.5 or 25 mg/kg body weight per day, and females
fed 37.5 mg/kg per day could have been treatment related.
Haematological examination showed slightly increased platelet counts
in high-dose males. No treatment-related gross pathological effects
were noted at sacrifice.
Following the 4-week pilot feeding study in beagle dogs, a 90-day
feeding study was undertaken (Naylor & Ruecker, 1986). Propachlor
(96.1% purity) was administered in the diet to groups of six dogs of
each sex per group for 90 days. Nominal dietary concentrations were 0,
100, 500 and 1500 mg/kg. There was no mortality or clinicopathological
or histopathological changes related to the treatment. The dose level
of 1500 mg/kg (45 mg/kg body weight) was a no-observed-adverse-effect
level (NOAEL).
7.2.1.2 Rodents
Propachlor (65% WP) was given orally by gavage to white rats at
daily doses of 21, 53 and 106 mg/kg body weight for 4 months, and its
cumulative effect was studied by Panshina (1973). Later, Strateva
(1974a, 1976) carried out a short-term study (45 days) at dose levels
of 50, 100 and 200 mg/kg body weight given orally by gavage to 104
Wistar rats (divided into four groups (three experimental and one
control) with equal numbers of both sexes), and a long-term oral study
(6 months) at dose levels of 0.05, 0.5, 5 and 20 mg/kg body weight
given to 220 Wistar rats divided into five groups (four experimental
and one control).
Strateva (1976) found a decrease in haemoglobin content and
number of erythrocytes, slight leucocytosis and an increased number of
neutrophils. The threshold dose for rats in long-term studies was 5
mg/kg body weight.
Panshina (1976) carried out a 10-month study on white rats given
propachlor by gavage at doses of 1, 3.5 and 10.6 mg/kg body weight.
Slight leucocytosis was found within 4-7 months. The first two doses
provoked a decreased activity of catalase and peroxidase as well as an
increase in nicotinamide adenine dinucleotide in brain and heart
tissue homogenates. No changes in haemoglobin content or number of
erythrocytes were noted. There were no alterations to the
pathomorphological picture.
Baynova et al. (1978a) compared the effects of continuous and
intermittent oral dosing of propachlor (65% WP) on white rats (equal
numbers of both sexes). The number of animals per group was not given.
The scheme of the experimental design is given in Table 12.
Table 12. Experimental design of the study by Baynova et al. (1978a)
Group Duration of Daily dose Dose (fraction
study (mg/kg) of the LD50)
Control 4 months - -
I (dosing every week) 4 months 70 1/20
II (dosing every second week) 4 months 140 1/10
III (dosing every second week) 8 months 70 1/20
Control 8 months - -
Continuous administration of propachlor led to more marked
changes in the main parenchymous organs than intermittent
administration. These changes were characterized by decreased activity
of oxido-reductive tissue enzymes. The hexabarbital sleeping time,
which characterized the detoxification function of the liver, showed
a statistically significant reduction. Propachlor administration at
120 mg/kg body weight for 6 consecutive days to male and female rats
increased the levels of both cytochrome and microsomal protein content
(Nenov & Baynova, 1978) as a result of the induction of mixed-function
oxidase in the liver (Baynova et al., 1978a).
A special study on the morphological changes in kidneys was
carried out. Propachlor (65% WP) was administered by gavage to male
white rats (10 animals per group and 1 control) at doses equivalent to
6, 12 and 60 mg/kg body weight for 3 months (Maleva & Zlateva, 1982).
Dose-dependent morphological changes were found in the proximal
convoluted renal tubules. The tubules were deformed and the epithelial
cells were vacuolized and dystrophic. A decreased cellular content of
ribonucleoproteins was also observed and pycnosis and caryolysis of
nuclei were seen. In the lumen, desquamated epithelia and hyalin
cylinders were found. The intestine was slightly swollen in certain
areas.
The subchronic toxicity of propachlor (96.1% purity) has also
been evaluated in Sprague-Dawley CD rats (30 of each sex per group)
fed diets containing 0, 300, 1500 or 7500 mg/kg for 90 days (Reyna &
Ribelin, 1984a). No animals died. A statistically significant
reduction in body weight was observed in animals fed 1500 mg/kg (8%
for males and females) and 7500 mg/kg (59% for males and 36% for
females). Food consumption was significantly depressed for animals at
the highest dose level for the first month of the study, but recovered
during the remainder of the study. After 6 weeks, reduced haemoglobin,
haematocrit, mean corpuscular haemoglobin and haematocrit, and an
increase in reticulocytes were observed in females at all dose levels
and in high-dose males. The anaemia was less evident in high-dose
males and was only present in high-dose females at study termination.
Significantly reduced lymphocyte counts were observed at week 6 for
high-dose males and mid- and high-dose females, and at study
termination for all groups. Levels of serum enzymes (SGPT, SAP, GGT),
cholesterol and total bilirubin were significantly increased for
high-dose animals at weeks 6 and 13 and there were significant
reductions in total protein, glucose, creatinine and albumin. No
histological changes were observed in the liver. Organ weights (with
the exception of female livers) were reduced, relative to controls,
for high-dose animals, and spleens were extremely small and
treatment-related in size in about 65% of the animals. No histological
changes were observed in the tissues examined, which included the
spleen.
7.2.1.3 Mice
The subchronic toxicity of propachlor (96.1% pure) was evaluated
in four groups of Charles River CD-1(R) mice (30 mice of each sex
per group) (Reyna & Ribelin, 1984b). Dietary levels were 0, 500, 1500
and 5000 mg/kg. No mortality or adverse clinical signs were observed
during the study. A statistically significant reduction (10%) in body
weight was observed in the mid- and high-dose males and females during
the study. Food consumption was also reduced during the first month
for mid- and high-dose animals. A dose-related statistically
significant reduction in the number of white blood cells was observed
in both sexes at all dose levels, except low-dose females, at the
7-week sampling period. At study termination, this reduction was
evident in mid- and high-dose animals but was statistically
significant only for high-dose males. Liver weights were increased for
males at all dietary levels and for mid- and high-dose females. There
was an accompanying statistically significant increase in the
incidence of centrilobular hepatocellular hypertrophy for mid- and
high-dose males, based on histological examination. No other
microscopic changes that could be considered treatment related were
evident in tissues. For males, the kidney to body weight ratio was
decreased at the high-dose level.
7.2.2 Dermal
A 21- and 90-day short- and longer-term dermal toxicity study on
Wistar rats (12 animals in each tested group plus 1 control) using
propachlor (65% wettable powder) administered 5 days per week was
carried out by Baynova et al. (1977). The doses applied were 50, 200
and 500 mg/kg in aqueous suspensions in the 21-day experiment, and 10,
25 and 50 mg/kg in the 90-day study. The dermal application was
performed uncovered (Draize, modification of Noakes). By the end of
day 21, anaemia was found at dose levels of 200 and 500 mg/kg, but
there was no methaemoglobinaemia. The same doses induced a
statistically significant decrease in the activity of certain enzymes
(SGOT, SGPT, OCT and AP). Dermal application of propachlor at dose
levels of 25 and 50 mg/kg to the skin of white rats (two groups of 12
animals) for 90 days did not provoke any changes in concentrations of
red blood cells and haemoglobin, but there were decreases in SGOT,
SGPT, LAP, AP and catalase. Decreased sulfur-containing enzymes were
found in tissue homogenates (liver and kidney). Decreases in the
levels of LDH, SDH, AcP and glucose-6-phosphate dehydrogenase were
determined histochemically. There was no evidence of clinical signs of
intoxication after repeated dermal application of the herbicide, but
the occurrence of the above-mentioned enzymatic changes demonstrated
the penetration of this herbicide through the dermal barrier. The
hexabarbital sleeping time was statistically significantly shortened
at the mid- and high-dose levels, thereby confirming the induction of
mixed-function oxidases reported by Nenov & Baynova (1978).
A threshold dermal dose of 25 mg/kg (1% aqueous suspension of
propachlor 65 WP) and a no-observed-effect level (dermal) of 10 mg/kg
(0.5% aqueous suspension) were determined in 90-day experiments by
Baynova et al. (1977).
7.3 Skin and eye irritation; sensitization
7.3.1 Skin irritation
Single application to Wistar rats (three rats of each sex per
group) of propachlor 65 WP at doses of 1500, 2000, 3000 and 4000 mg/kg
in 10 or 20% aqueous suspension, under the open modified method of
Noakes, did not cause any local irritative effects (Baynova et al.,
1977).
Panshina (1973) reported a strong irritative effect of propachlor
after single and repeated application. Single applications to rabbits
of 16% propachlor 65 WP in aqueous suspension at doses of 500, 700,
and 1000 mg/kg led to hyperaemia and even ulceration in the skin of
some animals. Ten applications of a 32% aqueous suspension of
propachlor at a level of 200 mg/kg had the same effect on the skin.
Seventeen applications of a 6.5% aqueous suspension at 100 mg/kg did
not result in mortality, and only slight hyperaemia was seen in the
treated skin area of the rabbits.
Primary irritation and contact sensitization effects of Satecid
65 WP containing 65% propachlor were investigated in rabbits, mice and
rats, and these were compared with the effects of technical grade
propachlor, propachlor with analytical purity, and the vehicle alone
(Lehotzky et al., 1979). Propachlor had strong to very strong
irritating effects on intact and scarified skin as well as on the eye
mucosa of rabbits and mice. The symptoms included erythema, oedema and
penetrating ulcer. Technical grade propachlor had the most potent
irritating effect.
Heise et al. (1983) conducted parallel experiments with two
propachlor preparations, one produced in the USA and the other in
Hungary, using an aqueous suspension applied under occlusion to the
skin of rabbits for 24 h. The irritant dose (ID50) for the USA
preparation was 2% and for the Hungarian preparation 0.6%.
Several dermal irritation studies have been carried out with
technical propachlor and its formulations, each of which involved the
use of six New Zealand white rabbits (2.5 to 3.5 kg). All application
sites were scored for erythema, eschar and oedema formation. For
technical propachlor (94.5% pure) a slight degree of dermal irritation
was observed (2.5/8.0 score) (Braun et al., 1979a); for a 20%
formulation of propachlor (again 94.5% pure) there was a slight degree
of dermal irritation (1.8/8.0 score) (Braun et al., 1979b); for a 65%
formulation a moderate degree of dermal irritation (3.4/8.0 score)
(Braun et al., 1979c); and for a 42% formulation corrosive effects
were observed (Branch et al., 1982b).
7.3.2 Skin sensitization
A modification of the maximalization test of Magnusson & Kligman
(1969) for identification of allergens was used by Heise et al.
(1983). Guinea-pigs (12 female and 12 male) were treated by
intramuscular injection of 0.2% propachlor aqueous suspension (using
preparations from both the USA and Hungary together with Freund
adjuvant), by subcutaneous injection of 0.05% aqueous suspension and
by epicutaneous application of a 1% water suspension of both
preparations for 6 weeks, both on healthy and scarified skin. A
challenge single application was given two weeks after the last
application using 0.2 ml of an 1% aqueous suspension of each
formulation. There was no significant difference between the reactions
to the two preparations. A challenge dose did not induce a reaction.
The dermal sensitization potential of propachlor was evaluated in
guinea-pigs using the Buehler procedure (Auletta, 1983). For the
induction phase, 0.2 ml of undiluted propachlor (95.7% purity) was
applied dermally using the closed patch technique to the shaved backs
of five male and five female Hartley albino guineapigs. The
applications were made for 6 h/day, 3 days/week, for 3 weeks. Two
weeks after the final dose, additional 0.2 ml doses of 25% propachlor
in ethanol (challenge dose) were applied to previously untreated areas
on those guinea-pigs that had received the induction doses and to six
others (three males and three females) which served as irritation
controls. Two positive control groups, treated with 1-chloro-2,4-
dinitrobenzene (DNCB) as positive control, were used. The negative
control group (five males and five females) was treated with saline
for the induction doses and challenged with acetone. All animals had
slight to moderate dermal irritation following the third induction
dose of propachlor, and some of them exhibited severe irritation,
including oedema, necrosis and exfoliation. Following application of
the challenge dose, one out of ten animals had an irritation score of
1 (slight), eight animals had a score of 2 (moderate) and one animal
had a score of 3 (severe) at 24 h. Nine of these animals exhibited
oedema. At 78 h, two animals had very low scores, six animals had
scores of 1, and two scores of 2. Six of these animals exhibited
oedema. Similar results were obtained when 20 and 40% propachlor
formulations were tested for sensitization in guinea-pigs (Auletta et
al., 1984a,b).
7.3.3 Eye irritation
Molnar & Paksy (1978) studied the effect of Satecid 65 WP on the
eye mucosa of CFY male albino rats using the Evans blue diffusion
technique. The aqueous suspension caused strong conjunctivitis at the
minimum concentration of 0.01%.
In a study by Auletta (1984), technical propachlor (96.1% pure)
was ground to a fine powder and introduced in the conjunctival sac of
the right eye of six (two males and four females) albino New Zealand
White rabbits. The left eye served as the control. The treated eyes
were rinsed 24 h later. Five of the animals exhibited severe
conjunctival irritation (redness, necrosis, chemosis and discharge),
five exhibited opacity and/or ulceration, and five had iridial damage.
Two animals exhibited neovascular-ization of the cornea and one
bulging of the cornea, indicative of increased intraocular pressure.
After 21 days of observation one animal continued to exhibit
conjunctival redness and one corneal opacity and bulging of the
cornea. The eyes of the remaining animals had minimal conjunctival
irritation.
In an eye irritation test on six New Zealand white rabbits using
technical propachlor (94.5%), 0.1 cm3 was instilled into one eye of
each rabbit and ocular reactions were observed on days 1, 2, 3, 4, 7,
10 and 14. According to Draize scores, all treated eyes were assigned
positive scores for corneal opacity and ulceration, conjunctival
redness, chemosis and necrosis. Two eyes were assigned positive scores
for iritis. Three eyes exhibited pannus, and corneal bulge was
observed in two eyes. Four eyes were clear of signs of irritation by
day 14 and two eyes showed signs of irritation at the termination of
the study (Braun, 1979).
US EPA (1984b) reviewed the existing data and concluded that
propachlor is corrosive to the rabbit eye, corneal opacity being
irreversible after 7 days.
7.4 Reproduction, embryotoxicity and teratogenicity
7.4.1 Reproduction
7.4.1.1 Biochemical and histopathological studies on gonads
The effect of propachlor 65 WP on protein content, activity of
5-nucleotidase and ATP in testis homogenate was evaluated by Maleva &
Stereva (1977), Stereva & Maleva (1977) and Zlateva & Maleva (1978,
1979). Each test group consisted of 20 male Wistar rats, administered
orally (by gavage) 12 or 60 mg propachlor/kg body weight per day for
6 months. A control group consisted of the same number of animals. At
the end of the experiment, there were dose-dependent statistically
significant changes in the biochemical indices studied (a decrease in
protein content and an increase in 5-nucleotidase and ATPase activity)
and these were accompanied by histomorphological changes, i.e.
disorganization of the seminiferous epithelium. After prolonged dosing
some dystrophic and degenerative changes in the gonad tissue and the
appearance of multinuclear giant cells with central chromatolysis were
observed. Adverse effects on the mitotic division of spermatogonia and
blockage of meiosis in the earlier phases were found (Zlateva &
Maleva, 1978, 1979).
In subchronic toxicity studies, there was no microscopic evidence
of testicular pathology in dogs administered the equivalent of 45 mg
propachlor/kg diet per day (Naylor & Ruecker, 1986), in rats fed the
equivalent dose of 250-310 mg/kg diet per day in the food (Reyna &
Ribelin, 1984a), or in mice fed the equivalent of 400 to 600 mg/kg
diet per day (Reyna & Ribelin, 1984b).
7.4.1.2 Reproduction studies
A two-generation rat reproduction study was undertaken to
evaluate the effects of propachlor (96.3% pure) (Rao, 1981). Four
groups of 30 male and 30 female 6-week-old Fischer-344 rats (F0
generation) were exposed to diets adjusted weekly to provide dose
levels of 0, 0.3, 3 and 30 mg propachlor/kg body weight per day for
100 days and were then allowed to mate to produce the F1 generation.
The F1 weanlings were similarly dosed for 120 days prior to
breeding. The F1 adults were mated twice to produce F2a and F2b
litters. At weaning of F1, F2a and F2b litters, 10 pups of each
sex at each dose level were sacrificed and selected organs were
examined histologically. No signs of toxicity in any animals were
found during the study. A significant decrease in food consumption and
body weight of adult F1 males dosed with 30 mg/kg per day was found.
No adverse effects in any of the treated groups over two generations
were noted with respect to gestation length, number of live pups
delivered, neonatal survival, litter weight and sex ratio. Decreased
pregnancy rates (fertility index) were observed in mid-dose (60%) and
high-dose (63%) F1 females following the first mating (F2a
litter), in comparison with 83% in the control rats. Test animals
continued to be treated with propachlor and were remated to produce a
second (F2b) litter. Pregnancy rates for the F2b litter were 83%
for controls and 83, 83 and 80% for low-, mid- and high-dose groups,
respectively.
No treatment-related gross necropsy findings were noted. Slight
increases in absolute and/or relative liver weights were noted in
high-dose F0 adults, mid- and high-dose F1 adults and high-dose
F2a weanlings. Hypertrophy of the centrolobular hepatocytes was
noted during microscopic examination of livers from the high-dose F0
and F1 adult females. There was no evidence of compound-related
microscopic changes in the testes and ovaries of test animals for
either generation.
7.4.2 Embryotoxicity and teratogenicity
7.4.2.1 Rats
a) Single dose treatment
Mirkova (1975) reported embryotoxic and teratogenic effects of
propachlor 65 WP in Wistar rats following single-dose treatment (675
mg/kg body weight) on each of the first 16 days of gestation.
Significantly increased lethality and frequency of haematomas,
predominantly in the head of the fetuses, and reduced cranio-caudal
size were observed when the treatment was performed from days 1 to 4
and on days 8, 10 and 12 of gestation (8 to 12 animals per test group
and 57 controls). The effect was more marked following treatment on
the first day of pregnancy. A weak teratogenic effect was observed
with a single dose, which included the induction of external
malformations (day 11, brachygnatia), abnormality of internal organs,
i.e. hydrocephalus (days 9, 11, 12 and 13 of gestation), defects in
skeletal ossification, i.e. non-ossification of sternum (day 9), and
delayed ossification of parietal bones (days 11, 12 and 13 of
gestation).
b) Repeated dose treatment
Wistar rats were treated daily with 67.5, 135 or 270 mg
propachlor/kg body weight on days 1-7 or 8-16 of gestation (six test
groups of 10 animals each). In a second set of experiments, a daily
treatment of 33.7, 67.5, 135 and 270 mg/kg body weight was given
throughout the gestation period (1-21 days) (Mirkova, 1975).
Propachlor induced embryotoxicity at all dose levels of repeated
treatment during the pre-implantation stages of embryogenesis (days
1-7 of gestation). This was expressed as raised lethality and
haemorrhages, predominantly in the head of the fetus, and reduced
weight and cranio-caudal size. The effects were significantly reduced
when administration was performed during the period of organogenesis
(8-16 days). Propachlor showed low teratogenic activity, producing
external malformations of the tail (short, curved tail), skeletal
abnormalities (non-ossified parietal and occipital bones) and
hydrocephalus, at all three dose levels during the pre-implantation
stage (days 1-7 of gestation). A slight degree of internal
hydrocephalus was observed with doses of 135 and 270 mg/kg on days
8-16 of gestation.
Using a similar treatment regimen, Ivanova-Chemishanska et al.
(1975) reported similar changes. No embryotoxic or teratogenic effect
was seen at a dose level of 10 mg/kg body weight given daily to rats
throughout the pregnancy (Medved, 1977; Panshina, 1985).
Teratogenic effects of technical propachlor were assessed in
pregnant Charles River COBS CD rats using a control and three
treatment groups consisting each of 25 females (Schardein & Wahlberg,
1982). Propachlor (96.5% pure) levels of 20, 60 and 200 mg/kg body
weight were administered orally by gavage as a single daily dose on
days 6-19 of gestation in a constant volume of 10 ml/kg corn oil. The
control group received the same dose of corn oil. Survival in the
control, low-dose and mid-dose groups was 100%. One female in the
high-dose group died on gestation day 18 due to an intubation error.
There were no differences in mean maternal body weight gain. No
changes were evident at gross necropsy that could be considered
treatment related. There were no differences between treated and
control groups with respect to the mean number of viable fetuses,
post-implantations loss, total implantations, corpora lutea, fetal
body weights, sex ratio distribution, and in the number of litters or
fetuses with external, visceral or skeletal malformations and/or
developmental and genetic variations.
7.4.2.2 Mice
When Balb/c mice (8-12 in each test group and 18 control pregnant
females) were treated with a single dose of 675 mg propachlor/kg body
weight on each of days 8-13 of gestation, and at dose levels of 33.7,
67.5, 135 and 270 mg/kg body weight per day from days 1 to 21, a
significant increase in post-implantational lethality and reduced
fetal weight and cranio-caudal size were observed at all dose
regimens. With both single and repeated doses, there was a
statistically significant increase in the frequency of internal
hydrocephalus, but only at a dose level of 675 mg/kg in a single
application on days 9 and 11 of gestation and on daily application at
270 mg/kg from days 1 to 18 of pregnancy (Mirkova, 1975).
7.4.2.3 Rabbits
Pregnant New Zealand White rabbits randomly assigned to a control
and three treatment groups (each consisting of 16 rabbits) were used
to determine the teratogenic potential of propachlor (95.6% pure)
(Schardein, 1982). Dose levels of 5, 15 and 50 mg/kg were administered
orally by gavage as a single daily dose on days 7-19 of gestation at
a constant volume (0.5 ml/kg dissolved in corn oil). The control group
received only 0.5 ml/kg corn oil. On gestation day 29, the number and
location of viable and non-viable fetuses, early and late resorptions,
number of total implantations and the number of corpora lutea were
recorded. All fetuses were examined for external, soft tissue and
skeletal malformations and for genetic or developmental variations.
Mortality (between 3 and 26 days of gestation) was recorded in
the control as well as the treated groups, i.e. two in the control
group, three at 5 mg/kg, three at 15 mg/kg and two at 50 mg/kg. Two
rabbits from the low-dose group and one from the mid-dose group
aborted on gestation days 22 to 25. Ante-mortem observations gave no
indication of a treatment-related effect at any dosage level.
Results of the reproductive parameters revealed slight to
moderate increases in the number of post-implantation losses and the
number of early resorptions in the mid- and high-dose groups when
compared with controls (Table 13). Single animals with resorptions
only were also found at the two highest dose levels. This resulted in
a subsequent decrease in the average number of viable fetuses in the
mid- and high-dose groups when compared with controls. Although the
number of post-implantation losses fell within the range of historical
control frequencies, all the above parameters were considered to imply
a mild embryotoxic response. No effects were observed in the number of
late resorptions, total implantations, number of corpora lutea, fetal
sex distribution or the mean fetal body weights. Examination of the
fetuses revealed no external, skeletal or soft tissue malformations,
or genetic and developmental variations that could be considered to be
related to treatment. These abnormalities observed were low in
frequency and within the range of historical control values. A
statistically significant increase in the total number of litters with
malformations was observed in the mid-dose group when compared to the
control group. This trend, however, was not found in the high-dose
group and the malformations in the group given 15 mg/kg per day were
considered to be incidental to treatment.
Table 13. Mean number of resorptions (late and early),
post-implantation losses and total implantations in
New Zealand rabbits (Schardein, 1982)
Dose Fetuses Resorptions Post-implantation Total
(mg/kg) viable non-viable late early losses implantation
0 8.0 0.0 0.3 0.3 0.6 8.6
5 7.9 0.0 0.0 0.4 0.4 8.3
15 5.2a 0.1 0.0 1.4 1.4 6.6
50 5.9 0.0 0.2 0.9 1.1 7.0
a P < 0.05
7.5 Mutagenicity and related end-points
Appraisal
Many in vitro and in vivo studies on the genotoxicity of
propachlor have been reported. It has been found to be clastogenic
in in vivo lower test systems and plant assays. It is cytotoxic
and also shows a weakly positive clastogenic response in in vitro
mammalian tests, inducing chromosomal aberrations in Chinese hamster
ovary cells. Variable results do not allow any definite conclusions
to be drawn. However, on the basis of negative results in many in
vivo assays in mammalian test systems, the available experimental
data provide insufficient evidence of a mutagenic potential. Data
on mutagenicity testing are summarized in Table 14.
7.5.1 Bacterial test systems
Salmonella mutation assays using standard tester strains
(TA1535; TA1538; TA98 and TA100), both with and without metabolic
activation with rat liver S9, were negative for formulations and
technical grades of propachlor (Plewa et al., 1984; Flowers, 1984).
No mutagenicity was observed with extracts of plants treated with
propachlor.
Negatives results for propachlor in spot tests using Salmonella
typhimurium test strains were also reported by Njagi & Gopalan
(1980) and Eisenbeis et al. (1981).
Table 14. Summary of mutagenicity testing
Test materiala Test system Resultc Reference
-S9 +S9 1S
Gene mutation in Prokaryotes
Propachlor U Ames spot test - Njagi & Gopalan (1980)
Propachlor U Ames spot test - - Eisenbeis et al. (1981)
Propachlor T Ames plate test - - Flowers (1984)
Propachlor T Ames plate test - - -
Propachlor + Cyanazineb Ames plate test + Plewa et al. (1984)
Gene mutation in plants
Propachlor C maize Wx locus - Plewa et al. (1984)
Gene mutation in mammals ( in vitro)
Propachlor T CHO/HGPRT - - Flowers (1985)
Chromosome effects in plants
Propachlor U Vicia faba cytogenetics + Njagi & Gopalan (1981)
Propachlor U maize cytogenetics + Lapina et al. (1984)
Propachlor U maize chlorophyll mutations + Lapina et al. (1984)
Chromosome effects in mammals ( in vitro)
Propachlor T CHO cytogenetics - + Li & Meyers (1987)
Chromosome effects in mammals ( in vivo)
Propachlor U in vivo mouse bone marrow cyt. + Pilinskaya et al. (1980)
Propachlor T in vivo rat bone marrow cyt. - Ernst & Blazak (1985)
Table 14 (contd).
Test materiala Test system Resultc Reference
-S9 +S9 1S
DNA damage/recombination in yeast
Propachlor T gene conversion - + Gentile et al. (1977)
Propachlor T gene conversion - - + Plewa et al. (1984)
Propachlor C gene conversion - - + Plewa et al. (1984)
Propachlor + Cyanazine gene conversion - Plewa et al. (1984)
DNA damage in mammals ( in vitro)
Propachlor T in vitro hepatocyte UDS - Steinmetz & Mirsalis (1984)
Propachlor T in vivo/in vitro hepatocyte UDS - Steinmetz & Mirsalis (1986)
a T = technical grade; C = commercial grade; U = unspecified grade
b Propachlor and cyanazine were both of commercial grade.
c +S9 and -S9 = with or without rat liver S9 activation mixture;
1S = with maize 1S fraction
7.5.2 Yeast assays
Propachlor was not recombinogenic when tested at 1000 mg/litre in
Saccharomyces cerevisae strain D4 (ade 2-1, ade 2-2; trp 5-12, trp
5-27) either with or without a liver microsomal activation system
(Gentille et al., 1977).
Extracts of plants treated with propachlor cause a weak
recombinogenic activity (Gentille et al., 1977). Maize extracts
treated with 25 ppm propachlor increased the rate of mitotic gene
conversion at the ade locus in S. cerevisae strain D4 approximately
4-fold over the control levels. Extracts of plants treated with a
technical grade of propachlor (1.306 x 10-4 mol/litre) and
formulated grade (1.306 x 10-3 mol/litre) induced a significant
increase in gene conversion at the ade locus and a lower increase at
the trp locus in S. cerevisae D4. Results for both formulated and
technical grades of propachlor were negative after mammalian S9
activation (Plewa et al., 1984).
7.5.3 Plant assays
Njagi & Gopalan (1981) studied the cytogenic and cytological
effects of propachlor at a wide range of concentrations (10, 50, 100,
500, 1000, 5000 and 10 000 ppm) in root-tip meristematic cells of
Vicia faba. At a concentration of 100 ppm, propachlor induced,
within 2 h, chromosomal aberrations, such as anaphase bridge
formation, micronuclei as well as inhibition of mitosis, formation of
highly pycnotic nuclei and premature chromosome condensation. The
mutagenic properties of propachlor were not detectable in the field
experiments using reverse mutations at the wx-C locus in maize as the
genetic end-point. Lapina et al. (1984) also reported that propachlor
induced mitotic chromosomal aberrations in the root-tip meristematic
cells of an inbred F2 strain of maize. At a concentration of 10-3
mol/litre and within 2 h of treatment of maize grains, propachlor
increased the rate of mitotic chromosomal aberrations more than 4-fold
over the control levels. The main types of cytogenetic damage included
single bridges (72%) and fragments (28%). Propachlor did not show
evidence of being significantly cytotoxic.
7.5.4 Cultured mammalian cell CHO/HGPRT assay
Propachlor (96.1%) was tested in a gene mutation assay in
cultured mammalian cells (CHO/HGPRT assay), both in the presence and
absence of mammalian metabolic activation, at doses ranging from 10-60
mg/litre. It was found to be cytotoxic at some of the higher
concentrations tested, but no mutagenicity was observed (Flowers,
1985).
7.5.5 In vitro unscheduled DNA synthesis in primary rat hepatocyte
cultures
Steinmetz & Mirsalis (1986) studied the potential of propachlor
to induce unscheduled DNA synthesis (UDS) in primary rat hepatocyte
cultures. They used ten concentrations of propachlor (95.8% pure)
ranging from 0.1 to 5000 mg/litre and, in another assay, five
concentrations ranging from 0.1 to 50 mg/litre. Results of this study
showed a cytotoxic effect of propachlor at concentrations of 50
mg/litre or more, but there was no evidence of UDS induction.
7.5.6 In vitro test for induction of chromosomal aberrations using
Chinese hamster ovary cells
Propachlor was tested for its potential to induce chromosomal
aberrations in cultured Chinese hamster ovary (CHO) cells treated with
propachlor (97.4% pure) at concentrations of 5, 10 or 15 mg/litre for
5 h both in the presence and absence of exogenous activation (Li &
Meyers, 1987). As indicated both by decrease in mitotic index and
lengthening of average cell generation times, the highest treatment
level was cytotoxic both in the presence and absence of activation. In
the non-activated study, no statistically significant increases in the
percentage of cells with structural aberrations or in average
structural aberrations per cell were observed at any treatment level.
In the study with S9 activation (15 mg propachlor/litre), the average
number of aberrations per cell was statistically elevated at 12 h and
24 h (P < 0.05 test). Chromatid and chromosome gaps were not
included. The percentage of cells with structural aberrations
following treatment at 15 mg/litre was statistically elevated at 12 h
but not at 24 h. No apparent dose-response relationship was observed
at either harvest time. Under these conditions, propachlor was
considered to be negative in the assay without exogenous metabolic
activation and a weak clastogen with activation.
7.5.7 In vivo rat bone marrow cytogenetic assay
Ernst & Blazak (1985) conducted an in vivo rat bone marrow
cytogenetic assay using technical grade propachlor (95.6% pure) at
dose levels of 0, 0.05, 0.2 and 1 mg/kg body weight. The results were
evaluated for mitotic index (based on at least 1000 cells per animal)
and 60 cells per animal were examined for chromosomal aberrations.
Chromatid and isochromatid gaps were recorded for each cell, but these
were not considered chromosomal aberrations. The results showed that
propachlor does not induce chromosomal damage in male or female
Fischer-344 rats under the conditions used in this study.
7.5.8 Acute in vivo mouse bone marrow cytogenicity assay
Pilinskaya et al. (1980) studied the cytogenetic effect of
propachlor on the bone marrow of mice treated orally with 10, 50 and
100 mg/kg body weight. The metaphase frequency of aberration at the
lowest dose applied was 2.67 ± 0.66 compared with 0.7 ± 0.19 in the
control. At this dose level an approximately 4-fold increase in the
frequency of chromosome aberrations was observed. No statistically
significant changes were found at a dose level of 1 mg/kg.
7.5.9 In vivo/in vitro hepatocyte DNA repair assay
Steinmetz & Mirsalis (1986) presented data on the in vivo/in
vitro hepatocyte DNA repair assay. Propachlor (97.7% pure) was
suspended in corn oil and administered by gavage to five groups of
male Fischer-344 rats at dose levels of 25, 250, 300, 400 and 1000
mg/kg. A positive control group received 2-acetyl aminofluorene
(2-AAF) (50 mg/kg) while a negative one was given corn oil. The
results were negative.
7.6 Long-term toxicity and oncogenicity studies
7.6.1 Rat
Propachlor (96% pure) was administered via the diet to four
groups of 60 male and 60 female Charles River CD SDBR rats at
concentrations of 0, 10, 50 and 500 mg/kg (equivalent to 0, 0.5, 2.6
and 27 mg/kg body weight) (Hamada, 1987a). A complete battery of
haematological, clinical chemistry and urinalysis tests was carried
out every 6 months for 2 years. No treatment-related effects were
noted on survival, incidence of clinical and ophthalmoscopic
observations, body weight gain or food consumption. Haematological and
clinical chemistry parameters and urinalysis data of treated animals
were comparable to control values. Thyroid and liver weights were
slightly increased in high-dose males at the 12-month interim
sacrifice but not at the end of the study. An increased incidence of
liver changes (centrilobular hypertrophy, clear cell cytoplasmic
alteration and eosinophilic cytoplasmic alteration) was observed in
high-dose males and females. A slightly higher incidence of thyroid
c-cell tumours (adenomas and carcinomas) noted in high-dose animals
was within the testing laboratories' historical range for such
tumours. All other organ weight changes and gross and microscopic
pathological findings were comparable to controls. An increase in
benign granulosa/theca cell tumours was observed for high-dose females
(4/60) compared to controls (0/60). The historical control data for
this tumour ranged from 0/80 to 4/96. On the basis of these results,
50 mg/kg diet (2.6 mg/kg body weight) can be considered the
no-observed-effect level (NOEL) for rats.
7.6.2 Mouse
When propachlor (96.1% pure) was administered in the diet to male
and female CD1 mice at dose levels of 10, 50 and 500 mg/kg (equivalent
to 1.6, 8.1 and 81.3 mg/kg for males and 2.0, 10.5 and 104.9 mg/kg for
females) for 18 months, no histological changes were noted (Hamada et
al., 1987b). The survival of all treated male groups was similar to
that of controls. Female survival was lower than that of controls in
all treated groups. There was a significant increase in segmented
neutrophilic leucocytes in the 500-mg/kg group at 12 months, but this
change was not evident at study termination. At the end of the study,
ratio of liver (with gall bladder) to body weight was increased in the
50- and 500-mg/kg group females, and the ratio of kidney to body
weight was decreased in males of the highest-dose group. The NOEL was
considered to be 10 mg/kg (1.6 mg/kg per day).
7.6.3 Dog
In a study by Naylor & Ruecker (1986), propachlor was
administered daily via the diet to four groups of six male and six
female beagle dogs for 12 months (0, 25, 250 and 1000 mg/kg diet). No
mortality occurred during the study. Absolute body weights were lower
than those of controls at study termination (approximately 14% for
males and 8% for females in the high-dose group and 5% for males in
the mid-dose group. A slight increase in emesis and/or stool change
was noted in treated dogs. No other changes in clinical and
pathological parameters were observed that could be related to
treatment. Based on the body weight depression at the high dose, a
dietary level of 250 mg/kg (9 mg/kg body weight) was considered a
no-observed-adverse-effect level (NOAEL) in this chronic study.
7.7 Miscellaneous studies
Propachlor has a strong inhibitory effect on the proliferation of
L1210 mouse leukaemia cells in vitro (the ID50 for cell
proliferation is < 3 x 10-7 mol/litre). Propachlor also inhibits
significantly the uptake of leucine, thymidine and uridine. The
inhibitory effect of propachlor is largely reversible, i.e. cells
grown in propachlor and then washed free of the compound return to an
almost normal rate of proliferation (Zilkah et al., 1981).
It has been demonstrated that propachlor treatment also causes
L1210 cells to accumulate in the G1 phase. This effect is dose
dependent; a concentration of 10 µmol/litre causes more than 90% of
cells to accumulate in the G1 phase (Zilkah et al., 1985).
The combined effect of propachlor and vibration has been studied.
Propachlor was given orally to Wistar rats (20 animals in each group)
at doses equivalent to 6, 12 and 60 mg/kg body weight per day for 3
months, and the animals were exposed to low-frequency vibration (15
Hz) during the last 30 days of the study. The results were compared
with those obtained from groups of rats subjected to the effect of 15
Hz vibration daily for 30 days, as well as with those of groups of
rats dosed only with propachlor. It was found that the combined
exposure (with 12 or 60 mg propachlor/kg) caused more severe
haemodynamic alterations and degenerative (even necrotic) changes in
the epithelial cells of the renal tubules, either in the proximal or
distal convoluted tubules (Maleva & Zlateva, 1982).
In a study by Baynova et al. (1978b), groups of 20 white rats
were pretreated with oral doses of 4 ml/kg 40% ethanol (one tenth of
the LD50), 6 days per week, for 4 months. Propachlor was then given
daily at a dose level of 140 mg/kg (one tenth of the LD50), 6 days
per week, every other week for a further 4-month period. The controls
were subjected to 8 months of treatment with ethanol and 4 months of
intermittent (every second week) treatment with propachlor using the
same doses as in the combined experiment. Both experiments had
untreated control groups. The combined treatment led to a reduction of
the hepatotoxic effect. This was probably due to induction of
mixed-function oxidases and to a more rapid biotransformation in the
hepatocytes. It has also been found that propachlor (140 mg/kg for 6
days) reduces the hexabarbital sleeping time by speeding up its
metabolism in the hepatic endoplasmic reticulum as a result of
induction of the mixed-function oxidase system in the liver cell
microsomes (Nenov & Baynova, 1978).
8. EFFECTS ON HUMANS
8.1 Occupational exposure
There have been few reports dealing with the effects of
propachlor on humans.
Von Schubert (1979) reported a case of contact eczema on the
palms, wrists and forearms of a 29-year-old agricultural worker who
had been in contact with propachlor for 8 days. Once this contact
ceased, the skin lesions disappeared.
Iden & Schroeter (1977) patch-tested 17 patients, predomi-nantly
farmers, who had been exposed simultaneously to many types of
herbicides. Seven showed a positive patch test reaction and five
others had an irritant reaction to propachlor. One of these farmers,
who was highly sensitive to propachlor, used to develop generalized
airborne contact dermatitis each spring when his neighbours used this
product. Farkasdy et al. (1976) and Dombay & Farkasdy (1978) examined
dermatologically 79 workers manufacturing Satecid 65 WP (65%
propachlor). Of these, 19% showed contact dermatitis attributed to
propachlor exposure. Patch testing was carried out on 67 workers of
this group, 12 of whom showed monovalent and bivalent hypersensitivity
reaction to propachlor. Photosensitization tests were negative in 28
of the cases tested. Three years later, the same authors examined 108
workers who were continuously exposed to Satecid in the same factory
without finding any sign of sensitization.
Jung et al. (1989) patch-tested 19 allergic cases and one
irritative case with occupational contact eczema, who were exposed to
different types of herbicides. Two of them showed positive testing to
Satecid 65 WP. One of these two was only exposed to the substance for
10 days and the other for 6 months.
8.2 General population exposure
No reports on general population exposure are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
9.1.1 Soil
The effects of propachlor with regard to changes in numbers of
four types of soil bacteria (aerobic nitrogen-fixing, aerobic
cellulose-decomposing, ammonifying and nitrifying) was studied by
Helmeczi (1977). The studies were carried out using pseudomycelial
chernozem soil with maize plants. For the purpose of the microbial
examinations, samples from the field were taken three times a year
(after spraying propachlor in spring, summer, and autumn at a rate of
8-10 kg/ha) from the upper 20-cm layer of the soil. Sampling was
carried out under sterile conditions and samples were collected in
sterile vessels. The nitrifying bacteria had the greatest sensitivity
to propachlor, their number decreasing considerably from 2720 to 610
per g soil in 1974 and from 4510 to 1920 per g soil in 1975. The
aerobic cellulose-decomposing bacteria were the least sensitive, their
numbers, with respect to the control bacteria, increasing to a small
extent.
Long-term studies in smolnista and alluvial soils have been
carried out to establish the effects of propachlor on useful soil
microorganisms and the processes of nitrification (Bakalivanov &
Kostov, 1981). These studies were conducted under laboratory
conditions at 20 °C and 60-70% soil moisture for a period of 10 days.
The concentration of propachlor used was 80 mg/kg soil. The results
showed an inhibitory effect on nitrification and weaker effect on soil
microorganisms (bacteria, actinomycetes and microscopic fungi). High
adsorption of propachlor to clay minerals reduced the toxic effect on
microorganisms.
Rankov & Velev (1976) studied the effect of propachlor on the
biological activity of soil microorganisms and showed that inhibition
was greater at a temperature of 2 to 4 °C than at 20 to 22 °C.
9.1.2 Water
The acute toxicity of propachlor to Selenastrum capricornutum
Printz has been assessed in two studies. In the first (Richards &
Kaiser, 1984), the 96-h EC50 for growth in algae was calculated to
be 0.029 mg/litre with 95% confidence intervals of 0.021-0.038
mg/litre. The no-observed-effect concentration (NOEC) was calculated
to be 0.01 mg/litre. In the second study (Zschaler & Jonas, 1990), the
72-h EC50 for growth was 5.3 mg/litre and the NOEC 1.9 mg/litre for
a propachlor formulation containing 65% active ingredient. The
differences in toxicity observed between the two studies may be either
because the 65% formulation is less toxic than technical propachlor or
because the duration of the second study was 24 h shorter.
9.2 Aquatic organisms
9.2.1 Aquatic invertebrates
Thompson & Forbes (1979a) determined a 24-h LC50 of 11 mg/litre
and a 48-h LC50 of 7.8 mg/litre for the water flea Daphnia magna.
The NOEC in this study was < 5.6 mg/litre. A 48-h LC50 of 6.9
mg/litre in the same species was reported by Mayer & Ellersieck
(1986). The same authors reported a 48-h LC50 of 0.79 mg/litre for
the midge larva Chironomus plumosus. Another midge larva Chironomus
riparius was reported to show a 24-h LC50 of 5.2 mg/litre and a
48-h LC50 of 1.8 mg/litre (Buhl & Faerber, 1989).
The effects of propachlor on daphnia reproduction was assessed in
a 21-day life-cycle study (Thun, 1990a). In this study, there was
decreased reproductive performance at concentrations from 0.29 to 2.6
mg/litre; the NOEC for reproduction was considered to be 0.097
mg/litre.
9.2.2 Fish
Thompson & Forbes (1979b) determined 24-h, 48-h and 96-h LC50
values for rainbow trout ( Onchorynchus mykiss) of 0.75, 0.28 and
0.17 mg/litre, respectively. Thun (1990b) conducted a 21-day study on
rainbow trout and found that fish died at concentrations between 0.11
and 0.3 mg/litre. No deaths related to exposure occurred at
concentrations between 0.009 and 0.075 mg/litre. The NOEC was
considered to be 0.019 mg/litre. Mayer & Ellersieck (1986) reported a
96-h LC50 of 0.23 mg/litre for the channel catfish (Channa
punctatus).
9.3 Terrestrial organisms
9.3.1 Terrestrial invertebrates
The toxicity of propachlor to earthworms was assessed in a 14-day
study (Thun et al., 1991). Propachlor (97.8% pure) was added to the
soil at five concentrations ranging from 100 to 1000 mg/kg, and 40
worms were added to the soil at each concentration. Worms were removed
from the soil at 7 and 14 days and examined for any adverse effects.
Body weights were measured at the beginning and end of the test. The
LC100 was 560 mg/kg and the LC0 218 mg/kg; the NOEC was 100 mg/kg.
The contact LD50 of propachlor for honey bees was reported to
be 311 µg/bee (Kenaga, 1979).
Under laboratory conditions, Tanke & Franz (1978) studied the
side effects of propachlor on some beneficial insects by measuring the
reduction of the beneficial capacity of three enthomophagous insects.
The egg parasite Trichogramma cacoeciae March reacted very strongly
in laboratory as well as in field conditions to the effects of the
herbicide. Contact toxicity tested using residues of propachlor on
glass plates (residue level not stated) caused a reduction of the
degree of parasitization leading to total mortality of the population
in contaminated cages. In a study on the contact toxicity of the spray
deposit on single leaves as well as on whole plants, the
parasitization of Trichogramma was reduced by 100 and 83%,
respectively. The direct toxic effect should be distinguished from a
repellent effect, which is probably more important in the field. In
addition to this direct contact effect of spray deposits, systemic
application also caused an effect after transport of the herbicide
through the soil and the plant. This was demonstrated by a reduction
of the degree of parasitization compared with untreated controls.
Propachlor does not seem to have any influence on Chysopa carnea
Steph larvae. No effect was visible either in tests of contact
toxicity or contaminated sandy soil, in choice studies on a repellent
action of the residue or after topical application of the preparation.
After oral application through an artificial food chain, no influence
could be demonstrated. Only overdosage increased the mortality rate of
the test larvae. No repellent effect of the herbicide on adults was
demonstrated (Tanke & Franz, 1978).
The syrphid Epistrophe balteata DeG was more sensitive. Both in
contact toxicity studies and in studies for a possible repellent
effect, an influence of propachlor on the larval stages was shown.
Oral intake through an artificial food chain did not have an effect on
the larvae. When the herbicide was sprayed directly on the plant, an
increase in larval mortality was observed. Adults of this syrphid also
showed reactions to herbicides; during egg deposition, females avoided
surfaces previously treated (Tanke & Franz, 1978).
The potential toxicity of a 65% formulation of propachlor to
Aleochara bilineta Gyll was assessed by Pietrzik (1991). The imagos
were dug into moist sand and the test substance was added at
recommended use rates (8 kg in 400 litres/ha water). For the
assessment of parasitical capacity, pupae of Delia antiqua were dug
into the sand. At the termination of the test, the sum of hatched
Aleochara larvae in the pupae was determined. These were compared to
the number of control organisms treated with tap water. The parasitic
capacity of the test beetles was decreased by 11.4% when compared to
controls.
9.3.2 Birds
Palazzolo (1964) reported data for pheasants indicating an acute
oral LD50 of 735 mg/kg body weight. Increased respiration, loss of
reflexes, mydriasis, salivation and intermittent tonic/clonic
convulsions were found among birds receiving dose levels of 900 and
1350 mg/kg. The onset of symptoms occurred approximately 15 min
following dosing and persisted until death 3-5 h later. Kenaga (1979)
reported an LD50 of 512 mg/kg for Mallard duck and Beavers & Fink
(1979) reported an LD50 for Bobwhite quail of 137 mg/kg body weight
for a 65% propachlor formulation.
When propachlor was administered in the diet to quail or Mallard
ducks for five consecutive days, the LC50 for both species of birds
was more than 5620 mg/kg diet (Beavers & Fink, 1983a,b). For the
quail, a dietary level of 5620 mg/kg is approximately equivalent to a
dose of 1400 mg/kg per day. The data presented above indicate that the
quail is more susceptible to exposure to propachlor via stomach tube
than via the diet.
10. CONCLUSIONS AND RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
10.1 Conclusions
* Under conditions of normal use, the general population is not
likely to be exposed to propachlor.
* Those occupationally exposed to propachlor should take adequate
safety and hygienic precautions in order to protect the skin, eye
and respiratory tract.
* Propachlor is rapidly degraded in the environment under most
conditions. It persists longer in cold dry environments. The
conjugated N-isopropylaniline metabolite persists longer than
the parent compound. Propachlor does not bioconcentrate or
biomagnify.
* Propachlor is highly toxic to some aquatic organisms. Exposure of
aquatic organisms under conditions of normal use is low, the
maximum expected concentrations being several orders of magnitude
lower than the no-observed-effect concentrations. Direct
contamination of water courses will kill aquatic organisms and
should be avoided. Propachlor poses a low hazard to birds,
earthworms and honey-bees.
10.2 Recommendations for protection of human health
Workers should be educated about the hazards of propachlor and
systematically trained to practice safety and personal hygiene and to
use protective equipment.
11. FURTHER RESEARCH
* The results of the existing animal studies on mutagenicity are
inconclusive and more research is needed.
* Studies should be performed in laboratory animals to determine
the potential neurotoxic effects of propachlor.
* Only validated analytical methods for residues of propachlor
should be used.
* Epidemiological studies on occupationally exposed workers are
needed.
* There is a need to develop methods of biological monitoring for
evaluating human exposure to propachlor.
* Research is needed to clarify the exposure of workers in the
production and agricultural use of propachlor. Studies should
also include examination of health effects at the measured
exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
In the WHO recommended classification of pesticides by hazard,
technical propachlor is classified in Class III as slightly hazardous
in normal use (WHO, 1990). A data sheet on propachlor has been issued
(WHO/FAO, 1989).
Neither the FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
nor the International Agency for Research on Cancer (IARC) has so far
evaluated propachlor.
REFERENCES
Aschbacher PW & Struble CB (1987) Evidence for involvement of
non-biliary excretion into the intestine of the formation of
methyl-sulphonyl containing metabolites of 2-chloro- N-
isopropylacetanilide (propachlor) by swine and rats. Xenobiotica,
13(2): 115-126.
Auletta CS (1983) A propachlor dermal sensitization study in
guinea-pigs (Project No. 4236-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS (1984) Eye irritation study with propachlor in rabbits
(Project No. 5050-84). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Auletta CS & Rinehart WE (1979) Acute dermal toxicity study in rabbits
(Project No. 4892-77). East Millstone, New Jersey, Bio/dynamics Inc.
(Unpublished proprietary report submitted to WHO by Monsanto
Agricultural Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984a) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4946-84). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Auletta CS, Erickson J, & Webb M (1984b) A closed-patch repeated
insult dermal sensitization study in guinea-pigs (modified Buehler
method) (Project No. 4540-83). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Bakalivanov D & Kostov O (1981) Soil microbiological assessment of
toxicity of alkanoate, amide, and other herbicides. Acta microb Acad
Sci Hung, 28: 141-146.
Baker JL (1980) Agricultural areas as non-point sources of pollution.
In: Overcash MR & Davidson JM ed. Environmental impact of non-point
source pollution. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 275-310.
Baker JL & Laflen JM (1979) Run-off losses of surface applied
herbicides as affected by wheel tracks and incorporation. J Environ
Qual, 8(4): 602-607.
Baker JL, Laflen JM, & Hartwig RO (1982) Effects of corn residue and
herbicide placement on herbicide run-off losses. Trans Am Soc Anal
Eng, 25(2): 340-343.
Bakke JE, Gustafsson JA, & Gustafsson BE (1980) Metabolism of
propachlor by the germ-free rat. Science, 210: 433-435.
Bakke JE & Price CE (1979) Metabolism of 2-chloro-N-
isopropylacetanilide (propachlor) in the rat. J Environ Sci Health,
B14(4): 427-441.
Bakke JE, Davison KL, & Larsen GL (1990) Evidence for the absence of
cysteine S-conjugate N-acetyltransferase activity in the metabolism of
propachlor, naphthalene, and dichlobenil in calves. Xenobiotica, 20:
801-807.
Bakke JE, Rafter J, Larsen GL, Gustafsson JA, & Gustafsson BE (1981a)
Enterohepatic circulation of the mercapturic acid and cysteine
conjugates of propachlor. Drug Metab Dispos, 9(6): 525-528.
Bakke JE, Rafter J, Lindeskog P, Feil V, Gustafsson JA, & Gustafsson
BE (1981b) Metabolism of 2-acetamido-4-(chlormethyl) thioazole in
germ-free and conventional rats. Biochem Pharmacol, 30(13): 1831-1844.
Bakke JE, Larsen GL, Aschbacher PW, Rafter J, & Gustafsson JA (1981c)
Role of gut microflora in metabolism of glutathione conjugates of
xenobiotics. In: Rosen JD, Magee PhS, & Casida JE ed. Sulfur in
pesticide action and metabolism. Washington, DC, American Chemical
Society, pp 165-178 (ACS Symposium Series No. 158).
Balinova AM (1981) Investigations in the residues of some herbicides
in plant production in view of its hygienic evaluation. Sofia,
University of Sofia (Dissertation).
Balinova A & Konstantinov K (1975) Investigations on residues of
several herbicides in vegetables. In: Proceedings of the COMECOM
Symposium on Residues of Herbicides in Plants and Soils, Vrozlav,
1973, pp 111-116.
Barrows ME & Macek KJ (1974) Exposure of fish to 14C-RamrodTM:
Accumulation, distribution and elimination of 14C-residues. Wareham,
Massachusetts, Bionomics, E.G. & G. Inc., 18 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Baynova A, Burkova T, & Michailova A (1977) [Determination of dermal
toxicity of Ramrod.] Gig Zdraveopazvane, 20(3): 234-240 (in Russian).
Baynova A, Burkova T, & Michailova A (1978a) [Comparative study on
intermittent and monotonous effects of the herbicide Ramrod.] Gig
Zdraveopazvane, 21: 568-573 (in Russian).
Baynova A, Krastev L, Dikova M, & Michailova JA (1978b) Effect of
alcohol on acute and chronic intoxication with the herbicide Ramrod.
Acta med Bulg, 2: 36-41.
Beavers JB & Fink R (1979) Acute oral LD50-Bobwhite Quail.
Propachlor report No. WL-79-026. Easton, Maryland, Wildlife
International Ltd (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beavers JB & Fink R (1983a) Eight-day dietary LC50 - Bobwhite Quail
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-90, submitted to WHO by Monsanto
International Services, Brussels).
Beavers JB & Fink (1983b) Eight-day dietary LC50 - Mallard Duck
propachlor. Easton, Maryland, Wildlife International Ltd (Unpublished
proprietary report No. WL-83-89, submitted to WHO by Monsanto
International Services, Brussels).
Bechtel CL (1991) Acute inhalation study of propachlor Technical
(Project No. ML-91-2). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Beestman GB & Deming JM (1974) Dissipation of acetanilide herbicides
from soils. Agron J, 66: 306-311.
Blaszcak DL (1988) A comparative acute oral toxicity study in
Sprague-Dawley and Fischer rats (Project No. 4359-87). East Millstone,
New Jersey, Bio/dynamics Inc., 37 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Bleeke MS, Carr KH, & Elliot RC (1987) Propachlor metabolism in the
laying hen (Project No. MSL-6217). St. Louis, Missouri, Monsanto
Agricultural Company, 134 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982a) Acute oral toxicity of
Ramrod(R) 4L flowable herbicide for rats (Project No. ML-81-182).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report submitted to WHO by Monsanto
International Services, Brussels).
Branch DK, Stout LD, & Folk RN (1982b) Primary skin irritation of
Ramrod(R) 4L flowable herbicide to rabbits (Project No. 810096).
Newstead, St. Louis, Monsanto Environmental Health Laboratory
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Braun WG (1979) Rabbit eye irritation study (Project No. 4889-77).
East Millstone, New Jersey, Bio/dynamics Inc., 4 pp (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
Braun WG & Rinehart E (1978) Acute dermal toxicity study in rabbits
(Project No. 4888-77). East Millstone, New Jersey, Bio/dynamics Inc.,
4 pp (Unpublished proprietary report, submitted to WHO by Monsanto
International Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979a) Primary dermal irritation
study in rabbits (Project No. 4890-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979b) Primary dermal irritation
study in rabbits (Project No. 4898-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Braun WG, Heenehan PR, & Rinehart WE (1979c) Primary dermal irritation
study in rabbits (Project No. 4894-77). East Millstone, New Jersey,
Bio/dynamics Inc. (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Brightwell BB & Rueppel ML (1976) Lasso(R) and Ramrod(R): physical
properties and their relationship to the environment. St. Louis,
Missouri, Monsanto Agricultural Company (Unpublished report No. 443,
submitted to WHO by Monsanto International Services, Brussels).
Brightwell BB, Malik JM, & Wong SC (1981) The environmental fate of
propachlor [2-chloro-N (1-methyl)-N-phenyl-acetamide] in soil and
water. St. Louis, Missouri, Monsanto Research Laboratory, 1300 pp
(Unpublished proprietary report No. MSL-1900, submitted to WHO by
Monsanto International Services, Brussels).
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides and
surfactants to larvae of the midge Chironomus riparius. Arch Environ
Contam Toxicol, 18: 530-536.
Caverly DJ & Denney RC (1978) Determination of substituted ureas and
some related herbicide residues in soils by gas chromatography.
Analyst, 103: 368-374.
Daly I & Knezevich A (1984) Four-week toxicity study in dogs
(Bio/dynamics Inc. Project No. 84-2798). Unpublished proprietary
report of Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels.
Davison KL (1991) Propachlor-s-glutathione metabolism by kidneys and
ureters of calves. J Anim Sci, 69: 1116-1121.
Davison KL, Bakke JE, & Larsen GL (1988) A kidney perfusion method for
metabolism. Studies with chickens using propachlor as a model.
Xenobiotica, 18(3): 323-329.
Davison KL, Bakke JE, & Larsen GL (1990) Metabolism of the glutathione
conjugate of propachlor by in situ perfused kidneys and livers of
rats. Xenobiotica, 20(4): 375-383.
Dombay Z & Farkasdy J (1978) [Problems of safety technique and of
occupational safety as related to propachlor and its formulations.]
In: [Conference on Safety Technology in the Application of
Agricultural Chemicals], Budapest, Technoinform, pp. 49-58 (in
German).
Eisenbeis SJ, Lynch DL, & Hampel AE (1981) The Ames mutagen assay
tested against herbicides and herbicide combinations. Soil Sci,
131(1): 44-46.
Ernst L & Blazak F (1985) An assessment of the mutagenic potential of
propachlor utilizing the acute in vivo rat bone marrow cytogenetics
assay (Project No. SR-84-180). Menlo Park, California, Stanford
Research Institute, 39 pp. (Unpublished proprietary report, submitted
to WHO by Monsanto International Services, Brussels).
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
Farkasdy J, Horvath A, & Meszleri E (1976) [Occupational dermatitis
caused by the selective herbicide Satecid 65WP.] Borgyogy venerol Sz,
52: 112-118 (in German).
Feil VJ, Bakke JE, & Larsen GL (1981) A new metabolite of propachlor
isolated from germ-free rat excreta. Biomed Mass Spectrom, 8(1): 1-4.
Fletcher WW & Kirkwood RC (1982) Herbicide and plant growth
regulators. London, Toronto, Sydney, New York, Granada.
Flowers LJ (1984) Salmonella mutagenicity assay of propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 18 pp
(Unpublished proprietary report No. MSL-1694, submitted to WHO by
Monsanto International Services, Brussels).
Flowers LJ (1985) CHO/HGPRT gene mutation assay with propachlor.
Newstead, St. Louis, Monsanto Environmental Health Laboratory, 9 pp
(Unpublished proprietary report No. ML-84-237, submitted to WHO by
Monsanto International Services, Brussels).
Frank R, Sirons GJ, Paik NJ, & Valk M (1977) Fate of propachlor
applied to onions and organic soils. Can J Plant Sci, 57: 473-477.
Gentille JM, Wagner ED, & Plewa MJ (1977) The detection of weak
recombinogenic activities in the herbicides alachlor and propachlor
using a plant activation bioassay. Mutat Res, 48: 113-116.
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 8: 339-357.
Hamada NN (1987a) Combined chronic toxicity and oncogenicity study in
rats with propachlor (Study No. HL-83-350/241-169). Vienna, Virginia,
Hazleton Laboratories America Inc., 2452 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Hamada NN (1987b) Oncogenicity study with propachlor in mice (Study
No. HL-83-349/241-159). Vienna, Virginia, Hazleton Laboratories
America Inc., 520 pp (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Heenehan BS, Rinehart WE, & Braun WG (1979) Acute oral toxicity study
in rats (Project No. 1895-77) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Heise H, Mattheus A, & Pushkeiler TH (1983) [The irritant and allergic
effect of the herbicidal agent propachlor.] Z Gesamte Hyg, 29(11):
675-677 (in German).
Helmeczi B (1977) The effect of herbicides on soil bacteria belonging
to certain physiological groups. Acta phytopathol Acad Sci Hung,
12(1-2): 41-49.
Humburg NE, Colby SR, Lym RG, Prasad R, Hill ER, Kitchen LM, & McAvoy
WJ (1989) Herbicide handbook, 6th ed. Champaign, Illinois, The Weed
Science Society of America.
Iden DL & Schroeter AL (1977) Allergic contact dermatitis to
herbicides. Arch Dermatol, 113(7): 983.
Ivanova-Chemishanska L, Vergieva T, & Mirkova E (1975) Embryotoxic and
teratogenic effects of some pesticides. Exp Med Morfol, 14(1): 29-33.
Jung HD, Honemann W, Kloth C, Lubbe D, & Pambor M (1989) Contact
eczema caused by pesticides in East Germany. Dermatol Monatschrift,
175: 203-214.
Kaempfe BS (1991) Acute inhalation study of MON 15606 (Project No.
ML-91-48). Newstead, St Louis, Monsanto Company Environmental Health
Laboratory. (Unpublished proprietary report of Monsanto Agricultural
Company, submitted to WHO by Monsanto International Services,
Brussels).
Kenaga EE (1979) Acute and chronic toxicity of 75 pesticides to
various animal species. Down Earth, 35(2): 25-31.
Kofman IS & Nishko P (1984) Determination of micro-quantities of
herbicides from a group of acetylanilides by thin-layer
chromatography. Physiol Biochem Cult Plant, 16(3): 290-292.
Kolesnikov VA, Abramova KA, & Grip OK (1979) Use of herbicides on
white cabbage. Khim Sel'skom Khoz, 17(10): 27-31.
Kolesnikov VA, Karetskaia NA, & Petuchova VI (1980) Efficacy of
herbicides in garlic crop. Khim Sel'skom Khoz, 18(8): 38-40.
Lamoureux GL & Davidson KL (1975) Mercapturic acid formation in the
metabolism of propachlor, CDAA and Flurodifen in the rat. Pestic
Biochem Physiol, 5: 497-506.
Lamoureux GL & Rusness DG (1989) Propachlor metabolism in soybean
plants, excised soybean tissues, and soil. Pestic Biochem Physiol, 34:
187-204.
Lamoureux GL, Stafford LE, & Tanaka FS (1971) Metabolism of
2-chloror- N-isopropyl-acetanilide (propachlor) in the leaves of
corn, sorghum, sugar cane, and barley. J Agric Food Chem, 19(2):
346-350.
Lapina TV, Grigorenko NV, Morgun V, Logvinenko VF, & Merjinskii JG
(1984) Study of the mutagenic effect of the herbicides Treflan,
Ramrod, and Banvel on corn. Tsitol i Genet, 18(2): 119-122.
Larsen GL & Bakke JE (1979) Studies on the origin of the
methylsulfonyl-containing metabolites from propachlor. J Environ Sci
Health, B14: 427.
Larsen GL & Bakke JE (1981) Enterohepatic circulation in formation of
propachlor (2-chloro-N-N-isopropylacetanilide) metabolites in the rat.
Xenobiotica, 11(7): 473-480.
Larsen GL & Bakke JE (1983) Metabolism of mercapturic acid-pathway
metabolites of 2-chloro- N-isopropylacetanilide (propachlor) by
gastrointestinal bacteria. Xenobiotica, 13(2): 115-126.
Larsen GL, Larsen JD, & Gustafson JA (1983) Cysteine conjugate
beta-lyase in the gastrointestinal bacterium Fusobacterium
necrophorum. Xenobiotica, 13(11): 689-700.
Lee JK, Minard RD, & Bollag JM (1982) Microbial metabolism of
propachlor in soil suspension. J Korean Agric Chem Saf, 25(1): 44-53.
Lehotzky K, Szeberenyl JMS, & Tatrai E (1979) On the local and
systematic toxic effects of the herbicide propachlor (Satecid 65WP) in
experimental animals. Egeszegtudomany, 23: 89-96.
Li AP & Meyers CA (1987) In vitro cytogenetics study of propachlor
(Study No. MSL-6939), 24 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Lottman CM & Cowell JE (1986) Propachlor residues in processed sorghum
grain applications following pre-emergent applications of Ramrod 4L
herbicide (Project No. MSL 6091) (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Lottman CM & Cowell JE (1987) Propachlor residues in processed corn
grain fractions following pre-emergent applications at Ramrod 4 h
herbicide (Study No. MSL-6260) (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Magnusson B & Kligman AM (1969) The identification of allergens by
animal assay. The guinea-pig maximalization test. J Invest Dermatol,
52: 268-276.
Maleva E & Stereva M (1977) [Biochemical investigation on changes of
gonads of male white rats under combined action of low frequency
vibrations and intoxication with Ramrod. I. Changes in the content of
protein.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part 2], pp. 306-313 (in Bulgarian).
Maleva E & Zlateva M (1982) Histomorphological changes in kidneys of
experimental animals under the combined effect of low-frequency
vibration and intoxication with Ramrod herbicide. Hig Zdraveopazvane,
25(3): 244-249.
Malik JM (1982) Bioconcentration of propachlor in channel catfish
under static conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, 144 pp (Unpublished proprietary report No. 24242,
submitted to WHO by Monsanto International Services, Brussels).
Malik JM (1986) Metabolism of propachlor in rats (Project No. 7815).
St. Louis, Missouri, Monsanto Agricultural Company, Research
Department (Unpublished proprietary report submitted to WHO by
Monsanto International Services, Brussels).
Markus JR & Puma B (1973) Pesticide analytical manual. Volume II:
Methods for individual pesticide residues. Springfield, Virginia, US
Department of Commerce, National Technical Information Service, pp
1-7.
Martin CD, Baker JL, Erbach DC, & Johnson HP (1978) Wash-off of
herbicides applied to corn residue. Trans Am Soc Anal Eng, 21(6):
1164-1168.
Mayer FL Jr & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Medved LI (1977) [Manual of pesticides.] Kiev, K. Urojai (in Russian).
Melnikov NN, Novojilov KV, Belen SP, & Pilova TN (1985) [Manual of
pesticides.] Moscow, M. Khimia (in Russian).
Menges RM & Tamez S (1973) Effect of soil incorporation on
selectivity, movement, and persistence of herbicides in onion
plantings. J Am Soc Hortic Sci, 98(4): 390-393.
Mirkova E (1975) Embryotoxic and teratogenic action of pesticides
Basfungin and Ramrod. Sofia, University of Sofia (Dissertation).
Molnar J & Paksy KA (1978) Acute inflammation of rat membranes evoked
by eye-instillation and inhalation of pesticide formulations
containing propachlor and TMTD. Egeszsegtudomany, 22: 393-397.
Nadeau RG & Pantano LK (1986) Metabolism study of synthetic
13C/14C-labelled plant metabolites of propachlor in lactating
goats. Part II: Identification characterization and quantification of
metabolites (Project No. MSL-5909), 119 pp (Unpublished proprietary
report submitted to WHO by Monsanto International Services, Brussels).
Naylor MW & Ruecker RW (1986) One-year dog study with propachlor
(Study No. ML-84-379). Newstead, St. Louis, Monsanto Environmental
Health Laboratory (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Nenov P & Baynova A (1978) Ramrod (propachlor) effect on the activity
of rat hepatic microsomal enzymosystems. Acta med Bulg, 5(2): 44-52.
Nesterova OA, Popova VV, & Kolesnikova OP (1980) [Accumulation of
semeron, mesoranil, and Ramrod in the soil and cabbage during
long-term use.] Sibirski Vestn S-Kh Nauki, 10(4): 19-21 (in Russian).
Njagi GDE & Gopalan HNB (1980) Mutagenicity testing of some selected
food preservatives, herbicides, and insecticides. II. Ames test,
Bangladesh. J Bot, 9(2): 141-146.
Njagi GDE & Gopalan HNB (1981) Mutagenicity testing of herbicides,
fungicides, and insecticides. I. Chromosome aberrations in Vicia
faba. Cytologia, 46: 169-172.
Novick NJ & Alexander M (1985) Co-metabolism of low concentrations of
propachlor, alachlor, and cycloate in sewage and lake water. Appl
Environ Microbiol, April: 737-743.
Novick NJ, Mukherjee R, & Alexander M (1986) Metabolism of alachlor
and propachlor in suspensions of pretreated soil and in samples from
ground water aquifers. J Agric Food Chem, 34: 721-725.
Palazzolo RJ (1964) Acute oral toxicity study on CP 31393 in Golden
Ring-necked pheasants. Northbrook, Illinois, Industrial Biotest
Laboratories (Unpublished proprietary report No. BLT-64-3, submitted
to WHO by Monsanto International Services, Brussels).
Panshina TN (1973) [Materials about toxicology of new herbicide
Ramrod.] Gig Primen toksikol pestic klin otravlenni, 1973: 301-303 (in
Russian).
Panshina TN (1976) [Hygienic aspects of the use of certain herbicides
in agriculture and toxicology.] Gig i Sanit, 7: 38-41 (in Russian).
Panshina TN (1977) Establishment of maximum permissible concentrations
and approximately safe levels of action exerted by herbicides of
haloid-substituted carboxylic acid anilides in the air of a production
sector. Gig Tr Prof Zabol, 12: 30-33 (in Russian).
Panshina TN (1985) IRPTC scientific reviews of Soviet literature on
toxicity and hazards of chemicals No. 83: Propachlor (Ramrod). Moscow,
Centre of International Projects, GKNT, 19 pp.
Pantano LK & Anderson KS (1987) The metabolic fate of propachlor
[2-chloro-N-(1-methylethyl)-N-Phenylacetamide] in sorghum foliage and
grain (Project No. MSL-5412). St. Louis, Missouri, Monsanto
Agricultural Company (Unpublished proprietary report submitted to WHO
by Monsanto International Services, Brussels).
Pekas JV, Larsen GL, & Feil VJ (1979) Propachlor detoxication in the
small intestine: cysteine conjugation. J Toxicol environ Health, 5:
653-662.
Pietrzik J (1991) [Laboratory determination of side effects of Ramrod
65WP on the short-winged beetle Aleochara bilineata Gyll (Project
No. 112/01-AB).] Niefern-Öchselbronn, Austria, GAB Biotechnologie GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels) (in
German).
Pilinskaya M, Kurinny AI, & Ivova TS (1980) [The primary evaluation of
cytogenetic activity and potential mutagenic hazard of 22 pesticides.]
Cytol Genet, 14(6): 41-46 (in Russian).
Plewa MJ, Wagner ED, Glenda G, & Gentille JM (1984) An evaluation of
the genotoxic properties of herbicides following plant and animal
activation. Mutat Res, 136: 233-245.
Pressley TA & Longbottom JE (1982) The determination of organochlorine
pesticides in industrial and municipal waste water. Cincinnati, Ohio,
US Environmental Protection Agency, Environmental Monitoring and
Support Laboratory, Office of Research and Development.
Rafter JJ, Bakke J, Larsen G, Gustafson B, & Gustafson JA (1983a) Role
of the intestinal microflora in the formation of sulfur-containing
conjugates of xenobiotics. Rev Biochem Toxicol, 5: 387-408.
Rafter JJ, Gustafson JA, Bakke J, Larsen G, Norin KE, & Gustafson BE
(1983b) Studies on the re-establishment of the intestinal microflora
in germ-free rats with special reference to the metabolism of
N-isopropyl-alpha-chloracetanilide (propachlor). Xenobiotica, 13(3):
171-178.
Rankov V & Velev B (1976) Effect of temperature on the interaction
between the metribuzin and propachlor herbicides and soil
microorganisms. Agrokhimiya, 11(5): 100-105.
Rankov V & Velev B (1977) Detoxication of the herbicide propachlor by
certain microscopic fungi. Acta phytopathol Acad Sci Hung, 12(1-2):
67-72.
Rao KS (1981) Two-generation dietary reproduction study with
propachlor in rats (Study No. HET-K-077810(8)). Midland, Michigan, Dow
Chemical Company, Health and Environmental Science, Toxicology
Research Laboratory, 171 pp (Unpublished proprietary report submitted
to WHO by Monsanto International Services, Brussels).
Rejtö M, Saltzman S, & Acher AJ (1984) Photodecomposition of
propachlor. J Agric Food Chem, 32: 226-230.
Reyna MS & Ribelin WE (1984a) Three-month feeding study of propachlor
to albino rats (Study No. 83037). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Reyna MS & Ribelin WE (1984b) Three-month feeding study of propachlor
to albino mice (Study No. 820061). Newstead, St. Louis, Monsanto
Environmental Health Laboratory (Unpublished proprietary report of
Monsanto Agricultural Company, submitted to WHO by Monsanto
International Services, Brussels).
Richards C & Kaiser FE (1984) Acute toxicity of propachlor (AB 83-367)
to Selenastrum capricornutum Printz. Columbia, Missouri, Analytical
Biochemistry Laboratories, 39 pp (Unpublished proprietary report No.
31348, submitted to WHO by Monsanto International Services, Brussels).
Ritter WP, Johnson HP, & Lovely WG (1973) Diffusion of atrazine,
propachlor, and diazinon in silt loam soil. Weed Sci, 5: 381-384.
Roberts HA, Walker A, & Bond W (1978) Persistence of chlorthaldimethyl
activity in soil. In: Proceedings of the British Crop Protection
Conference: Weeds. Croydon, British Crop Protection Council, pp 87-92.
Schardein JW (1982) Teratology study in rabbits. Mattawan, Michigan,
International Research and Development Corporation, 47 pp (Unpublished
proprietary report No. IR 81-224, submitted to WHO by Monsanto
International Services, Brussels).
Schardein JW & Wahlberg A (1982) Teratology study in rats. Mattawan,
Michigan, International Research and Development Corporation, 81 pp
(Unpublished proprietary report No. IR 81-264, submitted to WHO by
Monsanto International Services, Brussels).
Shirko ST & Belova VL (1982) [Level of Ramrod, semeron, and mesoranil
in the soil and cabbage plants in relation of dose of nitrogen and
potassium.] Khim Sel'skom Khoz, 20(I): 51-53 (in Russian).
Sjovall J, Rafter J, Larsen G, & Egestad B (1983) Lipophilic ion
exchange for group separation of conjugated metabolites of
xenobiotics. J Chromatogr, 276: 150-156.
Spalding RF & Snow DD (1989) Stream levels of agrochemicals during a
spring discharge event. Chemosphere, 19(8/9): 1129-1140.
Steen WC & Collette TW (1989) Microbial degradation of seven amides by
suspended bacterial populations. Appl Environ Microbiol, 55(10):
2545-2549.
Steinmetz L & Mirsalis C (1984) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the primary rat
hepatocyte cultures (Project No. LSC-7538). Menlo Park, California,
Stanford Research Institute, 8 pp (Unpublished proprietary report
submitted to WHO by Monsanto International Services, Brussels).
Steinmetz L & Mirsalis C (1986) Evaluation of the potential of
propachlor to induce unscheduled DNA synthesis in the in vivo- in
vitro rat hepatocyte DNA repair assay (Project No. LSC-2021). Menlo
Park, California, Stanford Research Institute, 37 pp (Unpublished
proprietary report submitted to WHO by Monsanto International
Services, Brussels).
Stereva M & Maleva E (1977) [Biochemical studies on changes in gonads
of male white rats under combined action of low frequency vibrations
and intoxication with Ramrod. II. Dynamic in changes of 5-nucleotidase
activity.] In: [Proceedings of the National Scientific Session of
Chemists with Medical Direction, Part I], pp 133-139 (in Bulgarian).
Strateva A (1974a) [Hygienic-toxicologic study on Ramrod aiming its
hygienic standardization in water.] Sofia, University of Sofia
(Dissertation) (in Bulgarian).
Strateva A (1974b) [Amid preparations with herbicide action.
Experimental data on the acute oral toxicity of propachlor (Ramrod).]
Exp Med Morphol, 13(2): 123-129 (in Russian).
Strateva A (1975) [On some toxicologic properties of Ramrod and their
significance on its hygienic standardization in waters.] Hyg
Zdraveopazvane, 18(4): 401-405 (in Russian).
Strateva A (1976) [The experimental determination of MAC of Ramrod in
water.] Gig i Sanit, 4: 85-87 (in Russian).
Strateva A, Velisarov A, & Georgiev A (1974) [Morphological and
enzymohistochemical investigations on some internal organs of
experimental animals under the effect of Ramrod in acute experiment.]
Hyg Zdraveopazvane, 17(3): 283-286 (in Russian).
Struble CB (1991) In situ intestinal absorption of
2-chloro-N-isopropylacetanilide (propachlor) and non-biliary excretion
of metabolites into the intestinal tract of rats, pigs and chickens.
Xenobiotica, 21: 85-95.
Tanaka FS, Wien RG, & Mansager ER (1981) Survey for surfactant effects
on the photodegradation of herbicides in aqueous media. J Agric Food
Chem, 29: 227-230.
Tanke W & Franz JM (1978) [Side effects of herbicides on economically
useful insects.] Entomophaga, 23(3): 275-280 (in German).
Thompson CM & Forbes AD (1979a) Acute toxicity of propachlor to
Daphnia magna. Columbia, Missouri, ABC-Laboratories Inc., 40 pp
(Unpublished proprietary report No. 24708, submitted to WHO by
Monsanto International Services, Brussels).
Thompson CM & Forbes AD (1979b) Acute toxicity of propachlor to
Rainbow trout Salmo gairdneri. Columbia, Missouri, ABC-Laboratories
Inc., 40 pp (Unpublished proprietary report No. 24019, submitted to
WHO by Monsanto International Services, Brussels).
Thun S (1990a) Propachlor 21-day reproduction test in Daphnia
(Project Number XX-89-126). Walsrode, Germany, IBR Forschung GmbH
(Unpublished proprietary report of Monsanto Agricultural Company,
submitted to WHO by Monsanto International Services, Brussels).
Thun S (1990b) Prolonged fish toxicity test in the rainbow trout
( Salmo gairdneri) (Project Number XX-89-163). Walsrode, Germany, IBR
Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
Thun S, Lemke G, & Fulst S (1991) Acute toxicity in earthworms
according to OECD 207. (Project Number IB-90-566). Walsrode, Germany,
IBR Forschung GmbH (Unpublished proprietary report of Monsanto
Agricultural Company, submitted to WHO by Monsanto International
Services, Brussels).
US EPA (1984a) Guidance for the registration of pesticide products
containing propachlor (019101) (EPA Case number 17). Washington, DC,
US Environmental Protection Agency, Office of Pesticide Programme.
US EPA (1984b) Health and environmental effects profile for
propachlor. Cincinnati, Ohio, US Environmental Protection Agency
(600/X-84/335).
US EPA (1988) Propachlor. Washington, DC, US Environmental Protection
Agency, Health Advisory Office of Drinking-Water.
Vasilev NV (1982) [Residual quantity of Ramrod in soil and fodder
rurabages.] Khim Sel'skom Khoz, 20(6): 51-53 (in Russian).
Villarreal DT, Turco RF, & Konopka A (1991) Propachlor degradation by
a soil bacterial community. Appl Environ Microbiol, 57(8): 2135-2140.
Von Schubert H (1979) [Allergic contact dermatitis caused by
propachlor.] Dermatol Monatsschr, 165: 495-498 (in German).
Walker A & Brown PA (1982) Effects of soil on the activity and
persistence of propachlor. In: Proceedings of the British Crop
Protection Conference: Weeds. Croydon, British Crop Protection
Council, pp 171-177.
Walker A & Brown PA (1985) The relative persistence in soil of five
acetanilide herbicides. Bull Environ Contam Toxicol, 34: 143-149.
Warholic DT, Gutenmann WH, & Lisk DJ (1983) Propachlor herbicide
residue studies in cabbage using modified analytical procedure. Bull
Environ Contam Toxicol, 31: 585-587.
WHO/FAO (1989) Data sheets on pesticides No. 78: Propachlor. Geneva,
World Health Organization, 7 pp (WHO/VBC/DS/87.78).
WHO (1990) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1990-91. Geneva, World Health
Organization, 39 pp (WHO/PCS/90.1).
Worthing CR & Hance RJ (1991) The pesticide manual, 9th ed. Croydon,
British Crop Protection Council.
Yu Ching Chien, Booth GM, Hansen DJ, & Larsen JR (1975) Fate of
alachlor and propachlor in a model ecosystem. J Agric Food Chem,
23(5): 877-878.
Zhukova PS & Shirko TS (1979) [Herbicide residues in soil and
vegetables.] Khim Sel'skom Khoz, 17(6): 46-50 (in Russian).
Zilkah S, Osband ME, & Mccaffrey RP (1981) Characterization of the
inhibitory effects of the phytotoxic agent propachlor on L1210 cell
proliferation. Cancer Lett. (Irel), 13(3): 241-248.
Zilkah S, Osband ME, Mccaffrey RP, & Shapiro H (1985) The effect of
the plant cell inhibitor propachlor on the cell cycle of LI210 cells
as evaluated by flow cytometry. Life Sci, 6: 2111-2115.
Zimdahl RL & Clark SK (1982) Degradation of three acetanilide
herbicides in soil. Weed Sci, 30: 545-548.
Zlateva M & Maleva E (1978) [Late changes in the gonads of male white
rats upon combined treatment with low frequency vibrations and Ramrod
intoxication.] Med Fizkult, pp 85-91 (in Bulgarian).
Zlateva M & Maleva E (1979) Disorders in the processes of division and
differentiation of seminiferous epithelium in case of chronic
intoxication with Ramrod. Exp Med Morphol, 18(1): 35-39.
Zlateva M, Maleva E, & Stareva M (1978) Changes of adenosine
triphosphatase activity in the testes of albino rats under combined
influence of low-frequency vibration and intoxication with the
herbicide Ramrod. Rev Toxicol Eur Res, 6(78): 375-378.
Zschaler R & Jonas W (1990) Growth inhibition test with algae (Project
No. NC-90-592). Hamburg, Natec Institut für
Naturwissenschaftlich-technische Dienste GmbH. (Unpublished
proprietary report of Monsanto Agricultural Company, submitted to WHO
by Monsanto International Services, Brussels).
RESUME ET EVALUATION
1. Identité, modalités d'utilisation, propriétés physiques et
chimiques, méthodes d'analyse
Le propachlor est un herbicide dérivé de l'acétanilide qu'on
utilise depuis 1965 en traitement de pré-levée et en traitement
précoce de post-levée. Il est principalement formulé en poudre
mouillable, liquide (concentré en suspension) et granulés. On
l'utilise en agriculture pour détruire les graminées annuelles et
certaines adventices à feuilles larges dans des cultures comme le
maïs, le sorgho, les potirons, le lin et les fleurs.
Le propachlor est légèrement soluble dans l'eau et facilement
soluble dans la plupart des solvants organiques. Il est peu volatil,
non inflammable et stable au rayonnement ultra-violet. La méthode
d'analyse la plus pratique est la chromatographie en phase gazeuse
avec détection par capture d'électrons après extraction et
purification par des méthodes appropriées.
2. Transport, distribution et transformation dans l'environnement
Le propachlor ne semble pas subir de dégradation photochimique à
la surface du sol. Il se volatilise en présence de vent lorsque la
surface du sol est encore humide.
Il n'est que modérément adsorbé aux particules du sol et aux
matières organiques, d'où un risque de lessivage à travers le sol
jusqu'aux eaux souterraines. Toutefois toutes les études montrent que
ce risque est faible en pratique. Il faut des précipitations très
importantes pour que les résidus se déplacent de 30 cm à l'intérieur
du sol. Selon la plupart des auteurs, la majorité des résidus
demeurent à moins de 4 cm de profondeur. Les caractéristiques du sol
influent beaucoup sur la mobilité du composé. Le lessivage se produit
surtout dans des sols sableux pauvres en matières organiques.
On a étudié en laboratoire et sur le terrain l'entraînement du
propachlor par ruissellement. Une des études a montré que la présence
de matières organiques dans le sol réduisait de 7 à 1 % de la quantité
appliquée, la concentration de l'herbicide dans l'eau de
ruissellement. L'incorporation de propachlor dans le sol réduit
également les pertes par ruissellement (de 3 à 0,8 % selon une étude).
En ce qui concerne la réduction de la concentration de propachlor
dans le sol et dans l'eau, le facteur de loin le plus important est sa
dégradation par les micro-organismes. On a montré que bactéries et
champignons étaient responsables de la dégradation de ce composé. Peu
de bactéries sont apparemment capables d'utiliser le propachlor comme
seule source de carbone. On a également isolé des bactéries qui sont
en mesure d'utiliser des métabolites telluriques du propachlor.
Les principaux métabolites qui se forment dans le sol sont des
acides oxaniliques et sulfoniques solubles dans l'eau. Il peut se
former un grand nombre d'autres métabolites mais ils ne représentent
qu'une faible proportion du total.
Le propachlor disparaît rapidement du sol et on a fait état de
demi-vies allant jusqu'à trois semaines. La plupart des études
indiquent une dégradation pratiquement complète en moins de six mois.
Les conditions écologiques influent sur la vitesse de dégradation qui
est favorisée par une température élevée et une forte humidité du sol.
Les résultats qui indiquent une persistance élevée du propachlor dans
le sol ont été obtenus à basse température et dans des sols secs. La
présence de nutriments en quantité suffisante dans le sol est
également nécessaire à la dégradation du propachlor.
Un des métabolites, la N-isopropylaniline conjuguée est
beaucoup plus persistante que le composé initial. On a retrouvé des
résidus de ce métabolite jusqu'à deux ans après l'épandage de
propachlor à titre expérimental à des doses plus élevées que celles
que l'on utilise normalement en agriculture.
Dans les conditions normales d'utilisation, le propachlor ne
devrait pas être entraîné à travers le sol par lessivage jusqu'aux
nappes souterraines et ne demeure pas dans le sol. Des conditions
exceptionnelles (température très basse ou sécheresse) peuvent
prolonger la persistance du propachlor et de ses métabolites.
Dans les conditions normales, le propachlor ne subit pas de
dégradation photochimique importante dans l'eau. Cependant, en
présence de photosensibilisateurs, cette dégradation photochimique
peut se produire. Le propachlor est stable à l'hydrolyse. Il est peu
probable qu'il se volatilise à partir de l'eau du fait de sa forte
solubilité dans l'eau et de sa faible tension de vapeur.
Comme dans le sol, c'est principalement par dégradation
biologique que le propachlor s'élimine de l'eau. Sa vitesse
d'élimination est donc liée à la population microbienne. Une étude
effectuée sur de l'eau contenant peu de bactéries a permis d'obtenir
une demi-vie d'environ cinq mois. Une autre étude a montré qu'au bout
de six semaines, il n'y avait toujours pas d'ouverture du cycle. Des
études de laboratoire portant sur des modèles d'écosystèmes ont montré
que le propachlor était presque complètement dégradé en l'espace de 33
jours.
Plusieurs études portant sur différentes espèces végétales ont
montré que le propachlor était rapidement métabolisé par les plantes
intactes ainsi que par des tissus végétaux excisés. Les voies
métaboliques se sont révélées analogues chez tous les végétaux
étudiés, tout au moins pendant les six à 24 premières heures,
conduisant à des métabolites hydrosolubles. On n'a pas observé de
décomposition métabolique du reste N-isopro-pylaniline. Seule une
très faible proportion (< 1 % selon une étude) des métabolites a été
retrouvée dans les fruits de ces végétaux; ils étaient concentrés en
grande majorité dans les racines et les feuilles. Les principaux
métabolites produits par les plantes sont identiques à ceux qui
prennent naissance dans le sol. On sait que ces métabolites peuvent
être fixés à partir du sol et un certain nombre d'études ne permettent
pas de déterminer avec certitude si les métabolites analysés
proviennent de la plantes ou du sol.
Bien que, d'après le coefficient de partage octanol/eau, ce
composé ait une tendance modérée à la bioaccumulation, on a montré
qu'il n'y avait ni bioconcentration ni bioamplification chez les êtres
vivants.
3. Concentrations dans l'environnement et exposition humaine
Les rapports d'analyse du propachlor dans l'air au cours de
l'épandage sont rares et insuffisants.
Aux Etats-Unis d'Amérique, la concentration du propachlor dans
les eaux de surface et les eaux souterraines est toujours faible, les
maxima atteignant 10 µg/litre dans les eaux de surface et 0,12
µg/litre dans les eaux souterraines. Le concentration la plus forte
enregistrée lors d'une étude sur les eaux de ruissellement était de 46
µg/litre.
Les résidus de propachlor dans les denrées alimentaires sont
généralement inférieurs à la limite de détection de la méthode
d'analyse (0,005 mg/kg). L'expérimentation a permis de retrouver des
résidus de l'ordre de 0,005 mg/kg dans des tomates, des poivrons, des
oignons et des choux.
Le dosage du propachlor dans l'atmosphère de la zone de travail
de conducteurs de tracteur pendant l'épandage du composé a donné des
résultats allant de 0,1 à 3,7 mg/m3.
4. Cinétique et métabolisme
Chez les mammifères, le propachlor peut être absorbé au niveau
des voies respiratoires, des voies digestives et de la peau. Il ne
s'accumule pas dans l'organisme et devient indétectable au bout de 48
heures.
La plupart des espèces animales (rats, porcs, poulets)
métabolisent le propachlor par la voie de l'acide mercapturique (MAP).
Il se forme des conjugués de cystéine par conjugaison avec le
glutathion et on a avancé que ces conjugués jouaient le rôle
d'intermédiaires dans la formation métabolique des acides
mercapturiques. La métabolisation du conjugué cystéinique du
propachlor se poursuit, notamment sous l'action de la C-S lyase
bactérienne qui intervient également dans la formation des métabolites
terminaux contenant le groupement méthylsulfonyle, métabolites qui
sont principalement excrétés dans l'urine (68 % de la dose initiale de
propachlor) et, sous forme de résidus insolubles, dans les matières
fécales (19 %). La C-S lyase du propachlor est sans action chez les
rats axéniques.
On a montré qu'il y avait un certain nombre de différences dans
le métabolisme du propachlor chez le rat et le porc. La bile est la
principale voie d'élimination des métabolites mercapturiques chez le
rat, mais on a montré qu'il existait une voie métabolique
extra-biliaire chez le porc.
Des études de métabolisme effectués sur des veaux ont montré que
ces animaux pourraient être incapables de former de l'acide
mercapturique à partir des conjugués du glutathion, ce qui les
rendrait plus sensibles à l'intoxication par le propachlor.
5. Effets sur les animaux d'expérience et les systèmes d'épreuves in
vitro
Le propachlor est légèrement toxique en cas d'exposition aiguë
par voie orale (la DL50 pour le rat varie de 950 à 2176 mg/kg de
poids corporel). Les signes d'intoxication aiguë proviennent
principalement d'effets sur le système nerveux central (excitation et
convulsions suivies d'une dépression). Chez des rongeurs, la toxicité
aiguë par inhalation est faible (CL50 = 1,0 mg/litre). Le propachlor
provoque de graves irritations des yeux et de la peau.
Des rats, des souris et des chiens ont été soumis à une
exposition de longue ou de courte durée au propachlor. Les organes
cibles sont le foie et les reins. Chez le chien, la dose sans effet
nocif observable a été de 45 mg/kg de poids corporel lors d'une étude
de trois mois où l'animal était exposé par la voie alimentaire. Lors
d'une étude d'une année sur des chiens, la dose sans effet nocif
observable a été estimée à 9 mg/kg de poids corporel (200 ppm dans la
nourriture). Lors d'une étude sur des rats exposés de la même manière
pendant 24 mois au propachlor, la dose sans effet observable a été
évaluée à 50 mg/kg de nourriture (2,6 mg/kg de poids corporel). Lors
d'une étude semblable effectuée sur des souris pendant 18 mois, on a
évalué à 1,6 mg/kg de poids corporel (10 ppm) la dose sans effet
observable.
Le propachlor ne s'est révélé cancérogène ni pour la souris ni
pour le rat. Dans la plupart des systèmes d'épreuve mammaliens, sa
réponse mutagène est négative tout en étant positive dans quelques
autres cas. Les données expérimentales disponibles de fournissent pas
une preuve suffisante de son pouvoir mutagène.
Administré en dose unique (675 mg/kg) à des rats et à des souris,
le propachlor a donné lieu à des signes d'embryotoxicité. On a
également observé des effets embryotoxiques lors d'études où du
propachlor avait été administré à plusieurs reprises (35,7 à 270
mg/kg). Toutefois, lors d'une autre étude sur des rats, où les doses
variaient de 20 à 200 mg/kg, aucun effet embryotoxique n'a été
observé.
Aux doses respectives de 12 et 60 mg/kg de poids corporel, le
propachlor (en poudre mouillable) a entraîné une réduction de la
teneur en protéines et un accroissement de l'activité de l'ATPase et
de la 5-nucléotidase dans des homogénats de testicules de rats,
provoquant également une dégénérescence du tissu testiculaire. Lors
d'une étude de reproduction portant sur deux générations, on n'a pas
véritablement obtenu la preuve d'effets indésirables.
6. Effets sur l'homme
On a signalé quelques dermatites de contact et dermatites
allergiques chez des agriculteurs et des ouvriers de production
exposés au propachlor (Ramrod et Satecid). Des tests cutanés ont été
effectués parmi un certain nombre d'entre eux, avec un résultat
positif, qui révèle une réaction d'irritation et une hypersensibilité
mono- et bivalente.
On n'a pas eu connaissance de symptômes ou de maladies, soit chez
des personnes exposées de par leur profession, soit dans la population
générale - à part les quelques cas d'effets cutanés chez les ouvriers
professionnellement exposés.
7. Effets sur les êtres vivant dans leur milieu naturel
Des études portant sur la microflore terricole ont montré que les
bactéries nitrifiantes constituaient le groupe le plus sensible aux
effets inhibiteurs du propachlor, leur nombre étant réduit d'un
facteur 3 à 4 après épandage de 8 à 10 kg de propachlor par hectare.
Ce sont les bactéries décomposant la cellulose qui étaient les moins
sensibles. La forte adsorption du propachlor aux particules d'argile
présentes dans le sol et une température élevée sont deux facteurs qui
réduisent les effets inhibiteurs.
Chez l'algue Selenastrum capricornutum, on a fait état d'une
CE50 à 96 heures de 0,02 mg/litre (pour la croissance) et d'une
concentration sans effet observable de 0,01 mg/litre. D'après une
deuxième étude portant sur une formulation de propachlor et menée
pendant 72 heures, le risque pour cette même algue serait nettement
moindre.
Pour la daphnie Daphnia magna, on donne des CL50 de 7,8 ou
6,9 mg/litre et une concentration sans effet observable inférieure à
5,6 mg/litre. La concentration sans effet observable sur la
reproduction était de 0,097 mg/litre. Pour des larves de deux espèces
de moucherons, on a obtenu pour la CL50 des valeurs respectives de
0,79 et 1,8 mg/litre.
Chez la truite arc-en-ciel la CL50 à 96 heures est de 0,17
mg/litre et la concentration sans effet observable sur 21 jours, de
0,019 mg/litre.
Le propachlor est considéré comme modérément à fortement toxique
pour les organismes aquatiques.
Le propachlor n'est pas toxique pour les lombrics aux
concentrations présentes dans le sol par suite d'un usage normal (la
concentration sans effet observable est de 100 mg/kg de terre). La
DL50 par contact pour les abeilles (311 µg/abeille) montre que le
propachlor ne constitue pas un danger pour ces insectes. En revanche,
des études menées en laboratoire et sur le terrain montrent que des
parasites utiles peuvent souffrir des effets du propachlor.
Le propachlor est plus toxique pour les oiseaux lorsqu'on
l'introduit directement dans l'estomac que lorsqu'on l'administre dans
la nourriture. La DL50 aiguë varie de 137 à 735 mg/kg de poids
corporel pour différentes espèces. Quant à la CL50 lors d'une
exposition par voie alimentaire, elle dépasse 5620 mg/kg de nourriture
chez les oiseaux.
Le propachlor ne constitue pas un danger pour les oiseaux dans la
nature, même sous forme de granulés.
RESUMEN Y EVALUACION
1. Identidad, modalidades de uso, propiedades físicas y químicas y
método analíticos
El propacloro es un herbicida derivado de la acetanilida
utilizado desde 1965 que actúa en las plantas antes de nacer o poco
después. Las principales formulaciones son de polvo humectable,
líquido fluido (concentrado en suspensión) y gránulos. Se aplica en la
agricultura a la lucha contra las gramíneas anuales y algunas malas
hierbas de hoja ancha en varios cultivos, como los de maíz, sorgo,
calabaza, lino y flores ornamentales.
El propacloro es ligeramente soluble en agua y se disuelve
fácilmente en la mayoría de los disolventes orgánicos. Su volatilidad
es escasa, no es inflamable y es estable a la radiación ultravioleta.
El método más práctico de análisis es la cromatografía de gases con
detección por captura de electrones, después de aplicar procedimientos
apropiados de extracción y purificación.
2. Transporte, distribución y transformación en el medio ambiente
Según la información disponible, el propacloro no se fotodegrada
sobre la superficie del suelo. El producto se volatiliza cuando hay
viento y la superficie del suelo está todavía mojada.
La adsorción sobre las partículas del suelo y la materia orgánica
es sólo moderada. Debido a esto, es posible la lixiviación a través
del perfil del suelo hacia el agua subterránea. Sin embargo, todos los
estudios indican que en la práctica es poco probable que esto ocurra.
Se requiere una precipitación muy abundante para hacer descender los
residuos 30 cm en el perfil del suelo. La mayoría de los autores
señalan que la mayor parte de los residuos se mantienen en una capa
superficial del suelo de 4 cm. Las características del suelo influyen
mucho en el desplazamiento del producto. La lixiviación se produce en
su mayor parte en suelo arenoso con poca materia orgánica.
Se ha estudiado el arrastre del propacloro por el agua tanto en
el laboratorio como sobre el terreno. En un estudio, la materia
orgánica del suelo redujo el arrastre del herbicida aplicado del 7% al
1%. Su incorporación al suelo también redujo la pérdida por arrastre
del agua (del 3% al 0,8% en un estudio).
El factor que más contribuye, con diferencia, a reducir los
niveles de propacloro en el suelo y el agua es la degradación por los
microorganismos. Se ha comprobado que en la degradación de la
sustancia intervienen tanto bacterias como hongos. Son pocas las
bacterias que parecen tener capacidad para usar el propacloro como
única fuente de carbono. También se han aislado bacterias que pueden
utilizar los metabolitos del propacloro presentes en el suelo.
Los metabolitos predominantes entre los que se forman en el suelo
son los ácidos oxanílico y sulfónico, solubles en agua. Pueden
formarse otros muchos metabolitos, pero representan una proporción
pequeña del total.
El propacloro desaparece con rapidez del suelo, habiéndose
registrado semividas de hasta tres semanas. En la mayoría de los
estudios se señala que la degradación es casi completa en menos de
seis meses. Las condiciones del medio ambiente influyen en la
velocidad de degradación, que se ve favorecida por los valores
elevados de la temperatura y el contenido de humedad del suelo. Los
estudios en los que se describía una permanencia más prolongada del
propacloro en el suelo se realizaron en condiciones de baja
temperatura o suelo seco. También es necesaria una cantidad suficiente
de nutrientes en el suelo para la degradación.
El metabolito conjugado N-isopropilanilina es mucho más
persistente que el producto del que procede. Se han encontrado
residuos de este metabolito hasta dos años después de la aplicación
experimental de dosis de propacloro más altas de las que se utilizan
normalmente en la agricultura.
Con el uso normal no es previsible que el propacloro llegue por
lixiviación a través del suelo hasta el agua subterránea, y no se
mantiene mucho tiempo en el suelo. En condiciones excepcionales de
baja temperatura o sequedad, el propacloro y sus metabolitos pueden
permanecer más tiempo en él. En condiciones normales, el propacloro
no sufre una fotodegradación significativa en el agua. En presencia de
fotosensibilizadores puede fotodegradarse. El propacloro es estable
desde el punto de vista hidrolítico. No es probable la volatilización
a partir del agua, debido a la elevada solubilidad en ella y la baja
tensión de vapor del producto.
Al igual que en el suelo, la principal ruta de pérdida de
propacloro del agua es la degradación biótica. La velocidad de
desaparición del propacloro del agua depende, pues, de la población
microbiana. En un estudio realizado con un pequeño número de bacterias
presentes en el agua se obtuvo una semivida de unos cinco meses. En
otro estudio, a las seis semanas no se había roto el anillo. En
estudios de laboratorio con modelos de ecosistemas se observó una
degradación casi completa en un período de 33 días.
En varios estudios con distintas especies vegetales se vio que el
propacloro se metabolizaba con rapidez tanto en las plantas intactas
como en tejidos vegetales extirpados. Las rutas metabólicas eran
análogas en todas las plantas estudiadas, por lo menos durante las
primeras 6-24 horas, produciendo metabolitos hidrosolubles. No se
observó degradación metabólica del fragmento de la
N-isopropilanilina. En los frutos de las plantas solamente se
encontró una proporción muy pequeña (< 1% en un estudio) de los
metabolitos; en su mayor parte estaban en las raíces y el follaje. Los
principales metabolitos producidos en las plantas son idénticos a los
que se forman en el suelo. Se sabe que las plantas absorben esos
metabolitos del suelo, y en algunos estudios había dudas acerca de si
los metabolitos medidos procedían de la planta o del suelo.
Aunque del coeficiente de reparto en octanol y agua parece
deducirse un potencial moderado de bioacumulación, los estudios
realizados indican que no hay ni bioconcentración ni bioamplificación
en los organismos.
3. Niveles medioambientales y exposición humana
Las mediciones descritas de la concentración del propacloro en el
aire durante la aplicación son pocas e inadecuadas.
Las concentraciones en el agua superficial y subterránea en los
Estados Unidos fueron siempre bajas, con un máximo de 10 µg/litro en
la superficial y de 0,12 µg/litro en la subterránea. La mayor
concentración registrada en el agua en un estudio del arrastre fue de
46 µg/litro.
Los residuos de propacloro en los alimentos suelen ser inferiores
al límite de detección del método analítico (0,005 mg/kg). En estudios
experimentales se han identificado concentraciones de residuos del
orden de 0,05 mg/kg en tomates, pimientos, cebollas y coles.
Las concentraciones de propacloro en el aire de la zona de
trabajo de los conductores de tractores que aplicaban la sustancia
oscilaban entre 0,1 y 3,7 mg/m3.
4. Cinética y metabolismo
Los mamíferos pueden absorber el propacloro por los tractos
respiratorio y gastrointestinal, así como a través de la piel. El
producto no se acumula en el organismo, en el que no es detectable a
las 48 horas.
La mayoría de las especies animales (ratas, cerdos, pollos)
metabolizan el propacloro siguiendo la vía de los ácidos
mercaptúricos. Mediante conjugación con el glutatión se forman
productos conjugados de cisteína, que se han señalado como posibles
intermediarios en la formación metabólica de ácidos mercaptúricos. La
C-S liasa bacteriana participa en el ulterior metabolismo del sistema
conjugado del propacloro con la cisteína y en la formación de los
metabolitos finales con metilsulfonilo, que se excretan sobre todo en
la orina (68% de la dosis de propacloro), y de residuos insolubles,
que se excretan en las heces (19%). La propacloro C-S liasa es
inactiva en ratas sin microorganismos.
En los estudios realizados se observaron algunas diferencias en
cuanto al metabolismo entre las ratas y los cerdos. La bilis es el
principal camino de eliminación de los metabolitos de la vía de los
ácidos mercaptúricos en las ratas, pero se ha demostrado que en los
cerdos existe una vía metabólica extrabiliar.
En estudios metabólicos con terneros se observó que pueden ser
incapaces de formar ácidos mercaptúricos a partir de los productos
conjugados del glutatión, por lo que pueden ser más susceptibles a la
intoxicación.
5. Efectos en los animales de experimentación y en sistemas de prueba
in vitro
El propacloro es ligeramente tóxico en la exposición oral aguda
(la DL50 en ratas va de 950 a 2176 mg/kg de peso corporal). Los
signos de intoxicación aguda son predominantemente efectos sobre el
sistema nervioso central (excitación y convulsiones, seguidas de
depresión). La toxicidad aguda por inhalación en roedores es baja
(CL50 = 1,0 mg/litro). El propacloro produjo efectos graves de
irritación en los ojos y la piel.
El propacloro se ha ensayado en estudios de exposición de corta
y larga duración en ratas, ratones y perros. Los órganos sobre los que
actuaba eran el hígado y los riñones. En un estudio de administración
con los alimentos a perros durante tres meses, el nivel sin efectos
adversos observados (NOAEL) fue de 45 mg/kg de peso corporal. En otro
estudio de un año, también en perros, el NOAEL fue de 9 mg/kg de peso
corporal (250 ppm en la dieta). El nivel sin efectos observados (NOEL)
en un estudio de alimentación de ratas durante 24 meses fue de 50
mg/kg de la dieta (2,6 mg/kg de peso corporal). En un estudio en el
que administró el producto a ratones con los alimentos durante 18
meses, el NOEL fue de 1,6 mg/kg de peso corporal (10 ppm).
No se encontraron efectos carcinogénicos del propacloro en
ratones y ratas. En la mayoría de los sistemas de prueba de mamíferos
la respuesta mutagénica fue negativa, obteniéndose resultados
positivos en un pequeño número de ensayos. Los datos experimentales
disponibles no aportan suficientes pruebas de su potencial mutagénico.
En pruebas de dosis única (675 mg/kg) con ratones y ratas se
demostró la embriotoxicidad del propacloro. También se detectaron
efectos embriotóxicos en tratamientos con dosis repetidas (35,7-270
mg/kg). Sin embargo, en otro estudio en ratas, con una gama de dosis
de 20-200 mg/kg, no se observó embriotoxicidad.
Con niveles de 12 y 60 mg/kg de peso corporal, el propacloro
(polvo humectable) provocó una disminución del contenido de proteínas
y un aumento de la actividad de la ATPasa y la 5-nucleotidasa en un
homogeneizado de testículo de rata y cambios degenerativos en los
testículos. En un estudio de reproducción de dos generaciones no se
obtuvieron pruebas definitivas de efectos adversos.
6. Efectos en la especie humana
Se ha descrito un pequeño número de casos de dermatitis de
contacto y alérgica en agricultores y trabajadores expuestos al
propacloro durante la producción (Ramrod y Satecid). En algunos de
ellos se efectuaron pruebas del parche y la reacción fue positiva, con
irritación e hipersensibilidad monovalente y bivalente.
No se han notificado casos de síntomas o enfermedades entre las
personas expuestas profesionalmente o en la población general, salvo
un pequeño número de informes de sus efectos cutáneos en trabajadores
expuestos profesionalmente.
7. Efectos en los seres vivos del medio ambiente
En varios estudios sobre los microorganismos del suelo, las
bacterias nitrificantes fueron el grupo más sensible a los efectos
inhibidores del propacloro, reduciéndose su número a la tercera o
cuarta parte tras la aplicación de 8-10 kg/ha de propacloro. Las menos
sensibles fueron las bacterias celulolíticas. La adsorción elevada
sobre las partículas de arcilla del suelo y la temperatura alta
reducen los efectos inhibidores.
En el alga Selenastrum capricornutum se ha registrado una CE50
a las 96 horas de 0,02 mg/litro para el crecimiento y una
concentración sin efectos observados (NOEC) de 0,01 mg/litro. De un
segundo estudio de 72 horas realizado con una formulación, parece
desprenderse que el peligro es mucho menor para este mismo organismo.
Se ha informado de unos valores de la CL50 para Daphnia magna
de 7,8 y 6,9 mg/litro y una NOEC de < 5,6 mg/litro. En las larvas de
dos especies de moscas enanas, los valores registrados de la CL50
fueron de 0,79 y 1,8 mg/litro.
La CL50 para la trucha arcoiris a las 96 horas es de 0,17
mg/litro, y la NOEC en un estudio de 26 días fue de 0,019 mg/litro.
Se considera que el propacloro tiene una toxicidad entre moderada
e intensa para los organismos acuáticos.
La exposición a las concentraciones de propacloro que cabe prever
en el suelo no es tóxica para las lombrices de tierra si la aplicación
es normal (la NOEC es de 100 mg/kg de suelo). La DL50 por contacto
para las abejas (311 µg/abeja) demuestra que el propacloro no
representa un peligro para estos insectos. En estudios de laboratorio
y de campo se han detectado efectos adversos del propacloro en algunos
insectos parasitarios beneficiosos.
El propacloro es más tóxico para las aves cuando se administra
mediante sonda gástrica que cuando se incorpora a la dieta. Los
valores de la DL50 aguda para distintas especies de aves oscilan
entre 137 y 735 mg/kg de peso corporal. Los valores de la CL50 en la
administración con los alimentos fueron superiores a los 5620 mg/kg de
la dieta.
El propacloro no representa un peligro para las aves en el campo,
ni siquiera con la formulación granulada.
