
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 164
Methylene Chloride
Second Edition)
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Methylene chloride.
(Environmental health criteria; 164)
1.Methylene chloride - adverse effects 2. Solvents
I.Series
ISBN 92 4 157164 0 (NLM Classification: QV 633)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
1. SUMMARY
1.1. Identity, physical and chemical properties, and analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on organisms in the environment
1.7. Effects on laboratory mammals and in vitro test systems
1.7.1. Single exposure
1.7.2. Short- and long-term exposure
1.7.3. Skin and eye irritation
1.7.4. Developmental and reproductive toxicity
1.7.5. Mutagenicity and related end-points
1.7.6. Chronic toxicity and carcinogenicity
1.8. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production
3.2.2. Uses
3.2.3. Consumer applications
3.2.4. Sources in the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Appraisal
4.1. Transport and distribution between media
4.1.1. Water/air
4.1.2. Soil/air
4.1.3. Water/soil
4.1.4. Multicompartment distribution
4.2. Abiotic degradation
4.2.1. Atmosphere
4.2.2. Water
4.2.3. Soil
4.3. Biotransformation
4.3.1. Aerobic
4.3.2. Anaerobic
4.3.3. Bioaccumulation
4.4. Interaction with other physical, chemical or biological
factors
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
5.1. Environmental levels
5.1.1. Atmosphere
5.1.1.1 Ambient air
5.1.1.2 Precipitation
5.1.2. Water
5.1.3. Aquatic organisms
5.1.4. Soil and sediment
5.2. Human exposure
5.2.1. General population
5.2.1.1 Indoor air
5.2.1.2 Drinking-water
5.2.1.3 Foodstuffs
5.2.1.4 Consumer exposure
5.2.2. Occupational exposure
5.2.2.1 Production
5.2.2.2 Paint stripping and related activities
5.2.2.3 Aerosol production and use
5.2.2.4 Use as a process solvent
5.2.2.5 Cleaning and degreasing
5.2.3. Occupational exposure limits
5.3. Human monitoring data
5.3.1. Body burden
5.3.2. Occupational exposure studies
5.3.3. Biological exposure indices
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Inhalation exposure
6.1.1.1 Human studies
6.1.1.2 Animal studies
6.1.2. Oral exposure
6.1.3. Dermal exposure
6.2. Distribution
6.2.1. Inhalation exposure
6.2.1.1 Human studies
6.2.1.2 Animal studies
6.2.2. Oral exposure
6.2.3. Dermal exposure
6.3. Metabolism
6.3.1. In vitro studies
6.3.2. In vivo studies
6.4. Elimination and excretion
6.4.1. Inhalation exposure
6.4.1.1 Human studies
6.4.1.2 Animal studies
6.4.2. Oral exposure
6.4.3. Dermal exposure
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.1.1. Bacteria
7.1.1.1 Aerobic bacteria
7.1.1.2 Anaerobic bacteria
7.1.2. Protozoa
7.1.3. Algae
7.2. Aquatic organisms
7.2.1. Plants
7.2.2. Invertebrates
7.2.2.1 Insects
7.2.2.2 Crustaceans
7.2.2.3 Molluscs
7.2.3. Fish
7.2.3.1 Acute toxicity
7.2.3.2 Chronic toxicity and reproduction
7.2.4. Amphibians
7.3. Terrestrial organisms
7.4. Population and ecosystem effects
7.4.1. Soil microorganisms
7.4.2. Sediment microorganisms
7.4.3. Microcosms and mesocosms
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.1.1. Acute toxicity data
8.1.2. Oral administration
8.1.3. Inhalation administration
8.1.3.1 Rat
8.1.3.2 Mouse
8.1.3.3 Other animals
8.1.4. Dermal administration
8.1.5. Intraperitoneal administration
8.1.6. Intravenous administration
8.1.7. Subcutaneous administration
8.1.8. Appraisal
8.2. Short-term exposure
8.2.1. Oral administration
8.2.2. Subcutaneous administration
8.2.3. Inhalation administration
8.2.3.1 Rat
8.2.3.2 Other animals
8.3. Long-term exposure
8.3.1. Rat
8.3.1.1 Inhalation exposure
8.3.1.2 Oral exposure
8.3.2. Mouse
8.3.2.1 Inhalation exposure
8.3.2.2 Oral exposure
8.3.3. Other animals
8.3.4. Appraisal
8.4. Skin and eye irritation; skin sensitization
8.4.1. Skin irritation
8.4.2. Eye irritation
8.4.3. Sensitization
8.4.4. Appraisal
8.5. Developmental and reproductive toxicity
8.5.1. Developmental toxicity
8.5.2. Reproductive toxicity
8.5.3. Appraisal
8.6. Mutagenicity and related end-points
8.6.1. In vitro
8.6.1.1 Bacteria
8.6.1.2 Fungi and yeasts
8.6.1.3 Mutation in mammalian cells
8.6.1.4 Chromosomal effects
8.6.1.5 DNA damage
8.6.1.6 DNA binding in vitro
8.6.1.7 Cell transformation
8.6.2. In vivo
8.6.2.1 Chromosome damage
8.6.2.2 Drosophila
8.6.2.3 DNA damage
8.6.2.4 DNA binding
8.6.2.5 Dominant lethal assay
8.6.2.6 Replicative DNA synthesis
8.6.3. Appraisal
8.7. Chronic toxicity and carcinogenicity
8.7.1. Inhalation exposure
8.7.1.1 Rat
8.7.1.2 Mouse
8.7.1.3 Hamster
8.7.2. Oral administration
8.7.2.1 Rat
8.7.2.2 Mouse
8.7.3. Appraisal
8.8. Mechanistic studies
8.8.1. In vitro metabolic studies
8.8.2. In vivo metabolic studies
8.8.3. Pulmonary effects
8.8.4. Studies on oncogene activation
8.8.5. The use of mechanistic studies in extrapolation
8.8.6. Mammary tumour promotion
8.8.7. Appraisal
8.9. Interspecies and dose extrapolations by kinetic modelling
9. EFFECTS ON HUMANS
9.1. General population exposure
9.1.1. Environmental exposure
9.1.2. Oral exposure
9.2. Occupational exposure
9.2.1. Short-term exposure
9.2.1.1 Case studies
9.2.1.2 Skin and eye effects
9.2.1.3 Laboratory studies
9.2.2. Long-term exposure
9.2.2.1 Case studies
9.3. Appraisal of human effects
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Chatelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new 14 partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As such,
they include and review studies that are of direct relevance for the
evaluation. However, they do not describe every study carried out.
Worldwide data are used and are quoted from original studies, not from
abstracts or reviews. Both published and unpublished reports are
considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant published
data are absent or when they are pivotal to the risk assessment. A
detailed policy statement is available that describes the procedures
used for unpublished proprietary data so that this information can be
used in the evaluation without compromising its confidential nature
(WHO (1990) Revised Guidelines for the Preparation of Environmental
Health Criteria Monographs. PCS/90.69, Geneva, World Health
Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in vitro
studies provide support and are used mainly to supply evidence missing
from human studies. It is mandatory that research on human subjects is
conducted in full accord with ethical principles, including the
provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation of
the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health and
the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the priority
list, the IPCS Secretariat consults with the Cooperating Organizations
and all the Participating Institutions before embarking on the
preparation of the monograph.
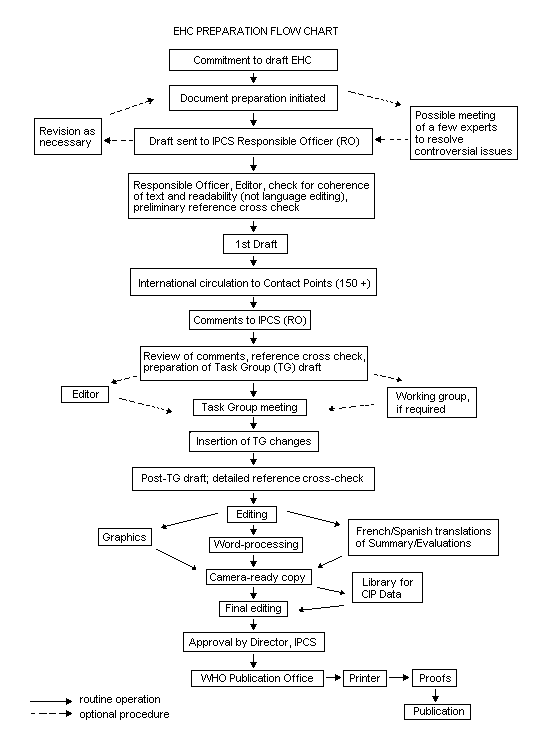
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for layout
and language. The first draft, prepared by consultants or, more
usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
Members
Dr L.A. Albert, Consultores Ambientales Associados, Xalapa, Veracruz,
Mexico
Mr D. Farrar, ICI Chemicals and Polymers, Runcorn, Cheshire, United
Kingdom (Rapporteur)
Dr R. Fransson-Steen, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden
Dr S. Henry, US Food and Drug Administration, Washington, DC, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Huntingdon, United Kingdom
Dr P. Standring, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr L. Stayner, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr T. G. Vermeire, Toxicology Advisory Centre, National Institute of
Public Health and Environmental Hygiene, Bilthoven, The
Netherlands (Chairman)
Dr Ruqiu Ye, National Environmental Protection Agency, Beijing, China
Observers
Dr C. De Rooij, Solvay & Cie S.A., Brussels, Belgium
Dr T. Green, ICI Chemicals & Polymers Ltd., Runcorn, Cheshire, United
Kingdom
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P. Demers, Unit of Analytical Epidemiology, International Agency
for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
A WHO Task Group on Environmental Health Criteria for Methylene
Chloride met at the Institute of Terrestrial Ecology, Monks Wood,
United Kingdom from 16 to 20 August 1993. Dr S. Dobson welcomed the
participants on behalf of the host institution, and Dr M. Gilbert
opened the meeting on behalf of the three cooperating organizations of
the IPCS (ILO/UNEP/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methylene chloride.
The first draft of this monograph was prepared by Mr D. Farrar,
ICI Chemicals and Polymers, Runcorn, United Kingdom.
Dr M. Gilbert, IPCS, was responsible for the overall scientific
content of this monograph. After his death in July 1994, this
responsibility was transferred to Dr P.G. Jenkins, IPCS, who also
dealt with the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ALT alanine aminotransferase
AST aspartate aminotransferase
BEI Biological Exposure Index
CO-Hb carboxyhaemoglobin
GST glutathione transferase
LEV local exhaust ventilation
MATC maximum acceptable toxicant concentration
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health (USA)
SCE sister-chromatid exchange
SGOT serum glutamic-oxaloacetic transaminase
SGPT serum glutamic-pyruvic transaminase
TT toxicity threshold
TWA time-weighted average
UDS unscheduled DNA synthesis
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical methods
Methylene chloride (dichloromethane) is a clear, highly volatile,
non-flammable liquid with a penetrating ether-like odour. The pure dry
compound is very stable. Methylene chloride hydrolyses slowly in the
presence of moisture, producing small quantities of hydrogen chloride.
Commercial methylene chloride normally contains small quantities of
stabilizers to prevent decomposition.
Analytical methods are available for the determination of
methylene chloride in biological media and environmental samples. All
methods involve gas chromatography in combination with a suitable
detector. In this way, very low detection limits have been reached
(e.g., in food: 7 ng/sample; water: 0.01 µg/litre; air: 1.76 µg/m3
(0.5 ppb); blood: 0.022 mg/litre).
1.2 Sources of human and environmental exposure
World production of methylene chloride is estimated to be
570 000 tonnes/year. Most applications are based on its solvent
capacity for grease, plastics and paint binding agents, in combination
with its volatility and stability. The worldwide usage pattern
comprises aerosols (20-25%), paint remover (25%), process solvent in
the chemical and pharmaceutical industry (35-40%), miscellaneous uses
(e.g., polyurethane foam manufacturing) and metal cleaning (10-15%).
The usage of methylene chloride is tending to decrease, at least in
western Europe.
More than 99% of the atmospheric releases of methylene chloride
result from its use as an end-product by various industries and the
use of paint removers and aerosol products at home.
1.3 Environmental transport, distribution and transformation
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where it
will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months.
Abiotic degradation in water is slow compared to evaporation.
Methylene chloride has been shown to disappear rapidly from soil and
ground water.
The aerobic and anaerobic degradation of methylene chloride has
been established by a variety of different test systems. Complete
biodegradation, especially by acclimated bacterial cultures under
aerobic conditions, is rapid (e.g., 49-66% mineralization in 50 h with
acclimated municipal sludge). In bioreactors, up to 10% degradation
per hour is achievable. There is no evidence of significant
bioaccumulation or biomagnification.
1.4 Environmental levels and human exposure
Methylene chloride has been detected in the ambient air of rural
and remote areas at concentrations of 0.07-0.29 µg/m3. In suburban
areas, the average concentration is < 2 µg/m3 and in urban areas
< 15 µg/m3. In the vicinity of hazardous waste sites up to
43 µg/m3 has been found. Precipitation may also contain methylene
chloride.
Methylene chloride enters the aquatic environment through waste
water discharge from various industries, and methylene chloride has
been found in surface water, ground water and sediment.
Exposure of members of the general public to methylene chloride
will occur from its use in consumer products such as paint removers,
which can result in relatively high levels being found in indoor air.
Occupational exposure during production arises primarily during
filling and packaging (manufacturing is in closed systems). Because of
its use in paint strippers, occupational exposure to methylene
chloride occurs during formulation of paint-remover, original
equipment manufacture, and in commercial furniture refinishing.
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products, in particular in the industries
mentioned in section 1.2.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor in regard to health risk, biological
monitoring based upon either analysis of carbon monoxide in exhaled
air or of carboxyhaemaglobin (CO-Hb) in blood is to be preferred.
However, this can only be used for non-smoking subjects. Sampling
should be done at about 0-2 h post-exposure, or after 16 h, i.e. on
the following morning.
Post-exposure CO-Hb levels 2 h after exposure ceases are not
expected to exceed 2-3%, and at 16 h 1%, in the case of an 8-h
exposure to less than 350 mg methylene chloride/m3 in non-smokers.
1.5 Kinetics and metabolism
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract, and dermal exposure results in absorption but
at a slower rate than via the other routes of exposure.
Methylene chloride is quite rapidly excreted, mostly via the lungs
in the exhaled air. It can cross the blood-brain barrier and be
transferred across the placenta, and small amounts can be excreted in
urine or in milk.
At high concentrations, most of the absorbed methylene chloride is
exhaled unchanged. The remainder is metabolized to carbon monoxide,
carbon dioxide and inorganic chloride. Metabolism occurs by either or
both of two pathways, whose relative contribution to the total
metabolism is markedly dependent on the dose and on the animal species
concerned. One pathway involves oxidative metabolism mediated by
cytochrome P-450 and leads to both carbon monoxide and carbon dioxide.
This pathway appears to operate similarly in all rodents studied and
in man. Whilst this is the predominant metabolic route at lower doses,
saturation occurs at a relatively low dose (around 1800 mg/m3).
Increasing the dose above the saturation level does not lead to extra
metabolism by this route.
The other pathway involves a glutathione transferase (GST), and
leads via formaldehyde and formate to carbon dioxide. This route seems
only to become important at doses above the saturation level of the
"preferred" oxidative pathway. In some species (e.g., the mouse) it
becomes the major metabolic pathway at sufficiently high doses. In
contrast, in other species (e.g., hamster, man) it seems to be used
very little at any dose.
Species difference in GST metabolism correlates well with the
observed species difference in carcinogenicity. The extent of
metabolism by this pathway in relevant species has been used as the
basis for a kinetic model to describe the metabolic behaviour of
methylene chloride in various species.
1.6 Effects on organisms in the environment
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria have been identified that are able to grow in
the presence of methylene chloride at much higher concentrations
including a saturated solution in water (section 4.2.4.1). Anaerobic
bacteria are more sensitive; growth inhibition has been observed at
1 mg/litre in anaerobic biological sludge.
In soil a concentration of 10 mg/kg strongly decreased the ATP
content of the biomass including fungi and aerobic bacteria, and
induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms methylene chloride is
moderately toxic (100-1000 µg/cm2) in the filter-paper contact
toxicity test. In sediment no toxic effects were observed even at very
high levels.
In higher plants no effects were found after exposure for 14 days
to 100 mg/m3.
Adult fish seem to be relatively insensitive to methylene chloride
even after prolonged exposure (14-day LC50 > 200 mg per litre). The
effect of methylene chloride on Daphnia is difficult to assess given
the large variation in the outcome of the studies performed. The
lowest reported EC50 was 12.5 mg/litre.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive with effects on hatching from
5.5 mg/litre.
1.7 Effects on laboratory mammals and in vitro test systems
1.7.1 Single exposures
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species are between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg were recorded. Acute effects after methylene chloride
administration by various routes of exposure are primarily associated
with the central nervous system (CNS) and the liver, and these
occurred at high doses. CNS disturbances were found at concentrations
of 14 100 mg/m3 or more, with slight changes in EEG at 1770 mg/m3.
Slight histological changes in the liver were found at 17 700 mg/m3
or more. Occasionally other organs were affected such as the kidney or
respiratory system. In mice, effects on the lungs were restricted to
the Clara cells after exposure to 7100 mg/m3. Cardiac sensitization
to adrenaline-induced arrhythmia has been reported. Cardiovascular
effects have been seen but the effects were inconsistent.
1.7.2 Short- and long-term exposure
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice from
17 700 mg/m3. Slight effects on the liver were noted in dogs
continuously exposed to 3500 mg/m3 for up to 100 days. After
intermittent exposure, effects on the liver were observed in rats at
3500 mg/m3 and in mice at 14 100 mg/m3.
Other target organs are the lungs and the kidneys.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations up to 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver from about 200 mg/kg per day.
1.7.3 Skin and eye irritation
Methylene chloride is moderately irritant to the skin and eyes of
experimental animals.
1.7.4 Developmental and reproductive toxicity
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3. No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
were observed in three animal studies. Small effects on either fetal
or maternal body weight were reported at 4400 mg/m3, and on
postnatal weight gain of male rats at 0.04% in the diet. A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations up to 5300 mg/m3, 6 h/day,
5 days/week for 17 weeks did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
1.7.5 Mutagenicity and related end-points
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (Salmonella or Escherichia coil). In eukaryotic systems
it gives either negative or, in one case, weakly positive results.
In vitro gene mutation assays and tests for unscheduled DNA
synthesis (UDS) in mammalian cells were uniformly negative. In vitro
assays for chromosomal aberrations using different cell types gave
positive results, whereas negative or equivocal results were obtained
in tests for sister chromatid exchange (SCE) induction.
The majority of the in vivo studies reported provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay, micronucleus test or UDS assay). Marginal increase
in frequencies of SCEs and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride.
There was no evidence of binding of methylene chloride to DNA or
DNA damage in rats or mice given high doses of methylene chloride.
These studies are potentially the most sensitive in vivo studies,
the best of which are capable of detecting one alkylation in 106
nucleotides.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride in
genotoxic in vivo.
1.7.6 Chronic toxicity and carcinogenicity
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following exposure to high concentrations (7100 and
14 100 mg/m3) of methylene chloride. The incidence of both lung and
liver tumours was increased in mice exposed to 7100 mg/m3 for 26
weeks and maintained for a further 78 weeks. There was no substantial
evidence of associated toxicity or hyperplasia in the target organs.
Syrian hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of tumours
in the region of the salivary gland was reported in female rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different cells, the statistical approach adopted
was inappropriate. Furthermore, it was reported that the rats in the
study had been infected with a common viral disease (sialoda-
cryoadenitis) early in the study, an infection that affects primarily
the salivary gland. It is likely that these tumours were not causally
related to exposure to methylene chloride but that the exposure had
exacerbated the response of the infection in the region of the
salivary gland. The response was not seen in a second study in which
rats were exposed to either 3500, 7100 or 14 100 mg/m3 for their
lifetime. A further inhalation study on rats exposed to methylene
chloride at concentrations up to 1770 mg/m3 for their lifetime
showed no evidence of carcinogenicity. Rats exposed to methylene
chloride via their drinking-water or by gavage similarly showed no
substantive evidence of carcinogenicity.
An increased incidence of benign mammary tumours in rats exposed
to methylene chloride has been reported in three studies, two
following exposure by inhalation and the third by gavage. There are no
reports of increases in mammary tumour incidence in hamsters or in
mice receiving methylene chloride at comparable dose levels. The
dependence of mammary tumours upon pituitary hormones in both male and
female rats has been established unequivocally. In the rat, prolactin
acts as both an initiator and promoter of mammary carcinogenesis.
There is good evidence that increased prolactin levels increase the
incidence of mammary tumours (e.g., the grafting of multiple pituitary
glands into Sprague-Dawley rats increases the incidence of mammary
tumours and there is a positive correlation between elevated blood
prolactin levels and mammary tumours in aged R-Amsterdam female rats).
Treatments that induce hyperprolactinaemia in female rats that have
received carcinogens produce a dramatic increase in tumour incidence.
These treatments include adrenalectomy, pituitary homografts and high
dietary fat.
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin. In common with the response to other agents which act via
hyperprolactinaemia, the methylene chloride-induced response is of
benign neoplasms only. There is no evidence for the binding of
methylene chloride to the DNA of other tissues and hence it seems
unlikely that it will bind to mammary tissue when the primary site of
metabolism is the liver. It seems most likely, therefore, that the
increased incidence of mammary adenomas is the result of an indirect
mechanism operating via hyperprolactinaemia.
In humans, there is conflicting evidence on whether or not mammary
tumours are as responsive to prolactin as is the case in the rat. The
rat has elevated levels of prolactin when fed ad libitum in
comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels. It
is the presence of all three factors that causes tubular-alveolar
growth of the mammary glands, which ultimately leads to tumour
development. Prolactin is not luteotrophic in primates. It is
unlikely, therefore, that this mechanism of tumour development is of
relevance to man.
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will occur only at doses of methylene
chloride which affect prolactin levels. There is no direct information
on prolactin levels in rats receiving low doses of methylene chloride,
but no increase in mammary adenomas has been observed following the
administration of low doses in either inhalation or drinking-water
studies (i.e. below 250 mg/kg body weight).
1.8 Effects on humans
Methylene chloride irritates the skin and eyes especially when
evaporation is prevented. In these circumstances, prolonged contact
may cause chemical burns. A case of serious pulmonary oedema has been
reported after excessive inhalation. Fatalities due to accidental
inhalation and skin contamination have been reported. The main toxic
effects of methylene chloride are reversible CNS depression and CO-Hb
formation. Liver and renal dysfunctions and effects on haematological
parameters have also been reported following exposure to methylene
chloride.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men with exposure for several years
to methylene chloride at concentrations ranging from 260 to
347 mg/m3. Similarly, a group of retired airplane strippers with a
long history of exposure to methylene chloride (22 years) at high but
unspecified levels performed a battery of neurophysiological and
psychological tests within the "normal" range, when compared with a
control group who had a history of either no or only low exposure to
methylene chloride.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to methylene
chloride. A causal relationship was not established because of
insufficiencies in the design of the study.
Several mortality studies in relevant cohorts show an inconsistent
pattern in the causes of death. Excesses in mortality from specific
diseases (e.g., pancreatic cancer, ischaemic heart disease) were not
consistently increased, but confined to single studies. These effects
cannot be attributed to exposure to methylene chloride.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Formula: CH2CI2
CI
'
Structure: CI - C - H
'
H
Relative molecular mass: 84.93
Common name: Methylene chloride
Synonyms: DCM; dichloromethane; methane
dichloride; methylene bichloride;
methylene dichloride; methylenum
chloratum
Tradenames: Aerothene MM; Freon 30; Narkotil;
Solaestin; Solmethine
CAS name (9 CI): Methane, dichloro-
CAS registry number: 75-09-2
EC registry number: 602-004-00-3
EINECS registry number: 200-838-9
RTECS registry number: PA 8050000
Purity of technical 99.9% (analytical grade)
product:
Impurities of technical Mostly C1- and C2-chlorinated
product hydrocarbons (up to 200 mg/kg)
(ECETOC, 1984)
Stabilizer: Typically 0.005-0.2% (w/w)
methanol, ethanol, amylene
(2-methyl-but-2-ene), cyclohexane
or tertiary butylamine (ECSA, 1989)
2.2 Physical and chemical properties
Methylene chloride is a clear, colourless, highly volatile, non-
flammable liquid with a penetrating ether-like odour. Pure dry
methylene chloride is a very stable compound and is non-corrosive. In
the presence of water, it undergoes very slow hydrolysis to produce
small quantities of hydrogen chloride, which can lead to corrosion,
e.g., to mild steel. This reaction is accelerated by elevated
temperatures and the presence of alkalis or metals. In the vapour
phase under abnormal conditions (elevated temperatures, high UV light
exposure, flame, sparks, red hot surfaces), methylene chloride may be
decomposed to give small amounts of hydrogen chloride, carbon monoxide
and phosgene (ECSA, 1989). Other physical and chemical properties are
given in Table 1.
Commercial methylene chloride is normally stabilized (section 2.1)
to prevent decomposition. Applications in aggressive conditions, such
as special metal cleaning operations may require more sophisticated
stabilizer technology. Poorly stabilized methylene chloride can react
violently with aluminium or other light metals.
2.3 Conversion factors
Conversion factor for methylene chloride concentrations in air,
calculated at 20°C and 1.013 hPa are:
1 mg/m3 = 0.28 ppm
1 ppm = 3.53 mg/m3
and for carbon monoxide:
1 mg/m3 = 0.86 ppm
1 ppm = 1.16 mg/m3
2.4 Analytical methods
Details of sampling and methods of analysis used in biological
media and environmental samples are given in Tables 2 and 3.
Table 1. Physical and chemical properties
Parameter, units Value Reference
Boiling temperature (°C at 1.013 hPa) 40 Weast et al. (1988)
Melting temperature (°C at 1.013 hPa) -95.1 Weast et al. (1988)
Relative density of liquid D (20) 1.3266 Weast et al. (1988)
(water at 4°C = 1 kg/m3) 4
Vapour pressure (hPa at 20°C) 470 ECSA (1989)
Saturation concentration in air 1.7 Calculated
(kg/m3 at 20°C)
Vapour density at 20°C (air = 1) 2.93 IPCS (1984)
Threshold odour concentration 743 Leonardos et al.
(mg/m3) (1969)
(odour: ether-like) 700-1060 DFG (1983)
880 Amoore & Hautala
(1983)
540-2160 Ruth (1986)
Solubility in water (g/kg at 20°C) 20 Verschueren (1983)
13.0 Horvath (1982)
Solubility in alcohol, ether, acetone Weast et al. (1988)
and benzene
Partition coefficients, at 20°C 1.25 IPCS (1984)
log Pow (octanol/water) 1.3 Hansch & Leo
(1979)
log Koc 0.89
calculated from Kow
(Karickhoff, 1981)
Henry's Law constant, Pa.m3/mol at 380
20°C Smith (1989)
Flash point, closed cup (°C) None ECSA (1989)
Explosion limits in aira (%) 13-22 ECSA (1989)
Auto-flammability, ignition temp. (°C) 605 ECSA (1989)
a This is with a high energy source; these conditions are unlikely
to arise in normal operations.
Table 2. Analytical methods for determining methylene chloride in biological monitoring (ATSDR, 1991)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Blood Heat sample, collect GC/FID 0.022 mg/litre 49.8±1.33 Di Vincenzo et al.
headspace vapour (1971)
Urine Heat sample, collect GC/FID No data 59±2.75 Di Vincenzo et al.
headspace vapour (1971)
Breath Heat sample, inject into gas GC/FID 0.706 ± 0.353 No data Di Vincenzo et al.
sample loop mg/m3 (1971)
(0.2 ± 0.1 ppm)
Adipose tissue Hydrolyse with acid, heat GC/FID 1.6 mg/kgb No data Engström & Bjurström
sample, collect headspace (1977)
vapour
Human milk Purge with helium, trap on GC/MS No data No data Pellizzari et al. (1982)
sorbent trap, desorb thermally
a FID = flame ionisation detector; GC = gas chromatography; MS = mass spectrometry
b Lowest reported concentration
Table 3. Analytical methods for determining methylene chloride in environmental samples (ATSDR, 1991)
Sample Preparation method Analytical Sample detection Percentage Reference
matrix methoda limit recovery
Air Adsorb on charcoal, desorb with GC/FID 88.25µg/m3 90-110c APHA (1977)
carbon disulfide (25 ppb)b
Air Adsorb on charcoal, desorb with GC/FID 0.01 mg 95.3 NIOSH (1987)
carbon disulfide
Air Adsorb on charcoal, desorb with GC/ECD approx. 1.76 µg/m3 No data Woodrow et al.
benzyl alcohol (approx. 0.5 ppb) (1988)
Water Purge with inert gas, trap on sorbent GC/HSD No data 85 US EPA (1989c)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent GC/ELCD 0.01 µg/litre 97-100 US EPA (1989)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent GC/MS 1.0 µg/litre 99 US EPA (1989b)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent HRGC/MS 0.03-0.09 µg/litre 95-97 US EPA (1989a)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent HRGC/ELCD 0.01-0.05 µg/litre 97±28 APHA (1989a)
trap, desorb thermally
Table 3 (Cont'd)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Water Purge with inert gas, trap on sorbent HRGC/MS 0.02-0.2 µg/litre 95±5 APHA (1989b)
trap, desorb thermally
Water Purge with helium, trap on sorbent GC/MS No data 99-105 Michael et al.
trap, desorb thermally (1988)
Waste Purge with inert gas, trap on sorbent GC/HSD 0.25 µg/litre 97.9±2.6 US EPA (1982a)
water trap, desorb thermally
Waste Purge with inert gas, trap on sorbent GC/MS 2.8 µg/litre 89±28 US EPA (1982b)
water trap, desorb thermally
Soil/solid Purge with inert gas, trap on sorbent GC/MS 5 µg/kg D-221 US EPA (1986a)
waste trap, desorb thermally
Soil/solid Purge with inert gas, trap on sorbent GC/HSD No data 25-162 US EPA (1986b)
waste trap, desorb thermally; or inject
directly into GC
Food Equilibrate in heated sodium sulfate GC/ELCD 0.05 ppm No data Page & Charbonneau
solution, collect headspace vapour (1984)
Food Isolate solvent by closed system GC/ELCD 7 ng 94 Page & Charbonneau
vacuum distillation with toluene as (1977)
carrier solvent
Table 3 (Cont'd)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Food Isolate solvent by closed system GC/ECD 7 ng 100 Page & Charbonneau
vacuum distillation with toluene as (1977)
carrier solvent
Food Purge with nitrogen, trap on sorbent GC/ELCD 1.2 mg/kgd 84-96 Heikes (1987)
trap, elute with hexane
Food Extract with acetone-water, back GC/ELCD 4 µg/kg 66 Daft (1987)
extract with iso-octane
a ECD = electron capture detector; ELCD = electrolytic conductivity detector; FID = flame ionisation detector; GC = gas chromatography;
HRGC = high resolution gas chromatography; HSD = halogen-specific detector; MS = mass spectrometry
b Lowest value for various compounds reported during collaborative testing of this method
c Estimated accuracy of the method when the personal sampling pump is calibrated with a charcoal tube in the line
d Lowest reported concentration
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Methylene chloride is not known to occur naturally in the
environment.
3.2 Anthropogenic sources
3.2.1 Production
Methylene chloride is produced almost exclusively by the Stauffer
process. Methyl chloride is first produced by the reaction of methanol
with hydrogen chloride and is then reacted with chlorine. Chloroform
and, to a lesser extent, carbon tetrachloride are also produced.
Historically the direct route to methylene chloride by chlorination of
methane was also used; this also produced the other three
chloromethanes in varying proportions depending on the conditions used
(CEC, 1986; ICI, personal communication to the IPCS).
World production of methylene chloride in 1980 was estimated to be
570 000 tonnes (Edwards et al., 1982); a similar figure is considered
to apply currently (ECSA, 1992). USA production was 229 000 tonnes in
1988, the demand being 207 000 tonnes. The total amount produced in
western Europe ranged from 331 500 tonnes in 1986 to 254 200 tonnes in
1991 (ECSA, 1992).
3.2.2 Uses
The usage of methylene chloride in Western Europe shows a decrease
from 200 000 tonnes/year in 1975-1985 (CEFIC, 1986) to
175 000 tonnes/year in 1989 and to 150 000 tonnes/year in 1992 (CEFIC,
1993).
Most of the applications of methylene chloride are based on its
considerable solvent capacity, especially for grease, plastics and
various paint-binding agents. Other important properties are its
volatility and stability; it is also non-flammable. Among its uses are
(CEFIC, 1983):
- a component of paint and varnish strippers, and adhesive
formulations
- a solvent in aerosol formulations
- an extractant in food and pharmaceutical industries
- a process solvent in cellulose ester production and fibre and
film forming
- a process solvent in polycarbonate production
- a blowing agent in flexible polyurethane foams
- the extraction of fats and paraffins
- plastics processing, and metal and textile treatment
- a vapour degreasing solvent in metal-working industries
An estimated breakdown of usage worldwide before 1985 is given in
Table 4.
Table 4. Estimated usage patterns (BUA, 1986)
USA (1985) Western Europe (1984)
Aerosols 25 10
Paint strippers 23 50
Degreasing agent 8 13
Film, electronics industries 7 15
Blowing agent 5
Others 35 12
It should be noted that these data apply to the situation
approximately 10 years ago and may have changed since. Reliable
reports on present trends are not available.
3.2.3 Consumer applications
The main use in consumer products is in paint strippers, where
methylene chloride is the main constituent (70-75%). The second
important use is in hairspray aerosols, where it acts as a solvent and
vapour pressure modifier. In the European Community (EC) it may be
used in such products at concentrations of up to 35% w/w (European
Council, 1982). The US Food and Drug Administration has banned the use
of methylene chloride in cosmetic products. It is also used in aerosol
paints. Other types of methylene chloride-containing products are
household cleaning products and lubricating, degreasing and automotive
products, some of which may be in aerosol form. Chemical products
containing methylene chloride were banned from sale or transfer to
consumers for their private use in 1993 according to the Swedish Code
of Statutes. Furthermore, it may not be used for working purposes
after 1st January 1996 (National Chemical Inspectorate, Sweden,
personal communication to the IPCS).
3.2.4 Sources in the environment
Most of the methylene chloride released to the environment results
from its use as an end-product by various industries, and the use of
paint removers and aerosol products in the home. Methylene chloride is
mainly released to the environment in air and, to a lesser extent, in
water and soil.
Methylene chloride is released to the atmosphere during its
production, storage and transport, but more than 99% of the
atmospheric releases result from industrial and consumer uses (US EPA,
1985). It has been estimated that 85% of the total amount of methylene
chloride produced in the USA is lost to the environment, of which 86%
is released to the atmosphere (US EPA, 1985). Data reported to the US
EPA for the 1988 Toxic Chemical Release Inventory indicate that
approximately 170 000 tonnes of the USA production volume for 1988
(230 000 tonnes) was lost to the atmosphere; of this, 60 000 tonnes
resulted from industrial methylene chloride emissions and 110 000
tonnes from the use of consumer products and from other sources such
as hazardous waste sites.
Estimates of annual global emissions of 500 000 tonnes have been
reported for methylene chloride (WMO, 1991). The short atmospheric
lifetime of methylene chloride (see section 4.2.1) implies that
emissions quantities given on a seasonal as well as on a regional
basis are more relevant for comparison with atmospheric measurements.
The total emission into the air in western Europe was estimated to be
173 000 tonnes for 1989 and 180 000 tonnes in 1991.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Appraisal
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where
it will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months. Given an intra-hemispheric
mixing time of approximately 1 month, transport can occur to regions
far removed from the emission source. The atmospheric lifetime is
fairly short relative to the inter-hemispheric transport time of 1 to
1.5 years, resulting in higher concentrations of methylene chloride
in the northern hemisphere, where most of the emissions occur at
present.
Methylene chloride is expected to have no significant impact on
stratospheric ozone depletion. It will not contribute significantly
to photochemical smog formation.
Hydrolysis and photolytically induced degradation in water are
slow compared to evaporation. Methylene chloride has been shown to
disappear rapidly from soil and ground water due to bio-
transformation.
The aerobic and anaerobic degradation of methylene chloride has
been proven by a variety of different test systems. Complete
biodegradation by acclimated bacterial cultures under aerobic
conditions is rapid. There is no evidence that significant
bioaccumulation or biomagnification of methylene chloride along the
food chain will occur.
4.1 Transport and distribution between media
4.1.1 Water/air
Methylene chloride enters the hydrosphere either directly, via
aqueous effluents, or indirectly from the atmosphere by dissolution in
sea water and in rain water. Due to its high volatility (Henry's Law
constant 380 Pa.m3/mol at 20°C) and low liquid-film transfer
coefficient (Kp = 0.005 m/h), methylene chloride is rapidly
transferred from the hydrosphere to the atmosphere.
Under laboratory conditions, the estimated half-life for
volatilization of methylene chloride from water at 25°C was 18-25 min
(when present at 1 mg/litre and stirred at 200 rpm). Removal of 90% of
the methylene chloride required 60-80 min. When stirring was minimal
(15 seconds every 5 min), the time required for 50% reduction in the
concentration was about 90 min. The presence of 3% sodium chloride (as
in sea water) decreased the evaporation rate by 10% (Dilling et al.,
1975; Dilling, 1977).
Various factors have been shown to affect the rate of
volatilization. For example, the half-life for volatilization of
methylene chloride from a depth of 1 m has been shown to be 3 h
(Lyman, 1982). The application of wind across the surface of the water
caused an increase of 17% in volatilization over a period of 20 min
compared to the presence of still conditions (Dilling et al., 1975). A
decrease in the water temperature decreased the rate of
volatilization. For example, over a period of 30 min, a 28% decrease
in rate was seen at 1-2°C compared to that at 25°C (Dilling et al.,
1975).
When measured under field conditions in experimental ponds, half-
lives for methylene chloride of 26-28 h have been reported (Merlin et
al., 1992). Its half-life for evaporation from the river Rhine has
been estimated to be 33-38 clays (Zoeteman et al., 1980). Further
estimates of the half-life for its evaporation are between 3 and 48 h
depending on wind and mixing conditions (Halbartschlager et al.,
1984). In a further study, methylene chloride was not detected at a
point 4-8 km from the point of release into an estuarine bay (Helz &
Hsu, 1978) or at 25 km below its discharge point in a river basin (De
Walle & Chain, 1978).
Rain-out is considered to be a limited process for removal of
methylene chloride from the troposphere. If it is assumed that its
aqueous-phase concentration is in equilibrium with the background
concentration in the northern hemisphere of about 123-134 ng/m3
(35-38 ppt) (Cox et al., 1976; WMO, 1991), the total amount of
methylene chloride rained out in the northern hemisphere will be
700 tonnes/year (assuming a rain fall of 2.5x1014 tonnes/year
containing 9.9 ng/m3 (2.8 ppt) at 10°C). The same calculation
performed at 20°C (Henry's constant is 1.57 times higher) would lead
to a value of 445 tonnes methylene chloride rained out annually in the
northern hemisphere. For the southern hemisphere, rainout quantities
of 390 and 248 tonnes methylene chloride can be calculated. The half-
life for removal by wet deposition is 550 years (Cupitt, 1980).
In 1978, it was estimated that 2.5% of releases at ground level
may reach the stratosphere (Derwent & Eggleton, 1978).
4.1.2 Soil/air
Methylene chloride present in the soil is predicted to evaporate
from the near-surface layer into the atmosphere because of its high
vapour pressure (470 hPa at 20°C).
4.1.3 Water/soil
The adsorption coefficient sediment/water for methylene chloride
is 8-10 (log Koc = 0.89-1.05). Methylene chloride has a low tendency
to adsorb to soil (adsorption coefficient 0.25 for a soil containing
1% organic carbon, Giger et al., 1983). Therefore there is a potential
for it to leach to ground water.
The amount of adsorption of methylene chloride to dry granular
bentonite clay added at a concentration of 375-750 mg/litre was found
to be 10-22% within 10-30 min. In the presence of 500 mg/litre peat
moss, about 40% of methylene chloride was absorbed after 10 min. Some
adsorption by dry-powdered dolomitic limestone was observed, but not
with silica sand (Dilling et al., 1975).
4.1.4 Multicompartment distribution
The regional distribution of methylene chloride over water, soil
and air compartments may be estimated by means of the fugacity model
developed by MacKay (Slooff & Ros, 1988). Application of this model
suggests that over 98% of the total emissions of the chemical will be
found in air, 1 to 2% in water and far less than 1% in soil and ground
water (BUA, 1986; Slooff & Ros, 1988).
4.2 Abiotic degradation
4.2.1 Atmosphere
The principal process by which methylene chloride is scavenged
from the atmosphere is the reaction with hydroxyl rate of methylene
chloride can be calculated from the rate constant for the initiating
breakdown reaction with HO. and the varying concentration of these
radicals in the troposphere. Determination of the rate constant for
the reaction of methylene chloride with hydroxyl radicals has been the
subject of various investigations. WMO (1991) recommends the following
value:
kOH = 5.8 × 10-12 exp(-1100/T) cm3 molecule-1 s-1
Other reactive species (e.g., ozone, oxygen atoms, chlorine atoms
and nitrate radicals) are not thought to contribute significantly to
the primary attack on methylene chloride (Table 5). As methylene
chloride does not absorb in the visible or near ultraviolet light
region (> 290 nm), direct homogeneous gas-phase photolysis in the
troposphere is of negligible importance.
Table 5. Primary tropospheric reactions of methylene chloride (other than with .OH)
Reaction k (at 25°C) Global average [X] Lifetime
with: (cm3 molecule-1 s-1) (molecule cm-3) (years)
.Cl 4.1 × 10-13 103 77
(IUPAC, 1992) (estimated) (estimated)
.NO3 <3.2 × 10-17 1.2 × 108 > 8.3
.O(3p) 6.44 × 10-16 2.5 × 104 approx. 2000
(Barassin & Cambourieu, 1973)
.O(1D) < 5 × 10-10 0.5 > 120
(estimated)
Carbon dioxide and hydrogen chloride are the major breakdown
products and minor quantities of carbon monoxide and phosgene are
formed (Sanhueza & Heicklen, 1975; Rayez et al., 1987). The breakdown
reaction can be described as follows:
CH2Cl2 + HO. --> .CHCl2 + H2O
.CHCl2 + O2 --> .CHCl2O2
.CHCl2O2 + NO --> .CHCl2O + NO2
.CHCl2O --> .Cl + HCOCl or
.CHCl2O + O2 --> COCl2 + HO2 (minor reaction)
Formyl chloride may be taken up by cloud droplets, hydrolysed to
formic acid and wet deposited as such, or dry deposited to the ocean
or land surfaces and then hydrolysed. The overall lifetime for wet or
dry deposition is unlikely to exceed a few months and may be much
shorter. On the other hand, degradation in the troposphere by
photolysis or reaction with HO. may possibly be a more rapid process.
The reaction products would be carbon oxides (CO, CO2) and HCl
(Libuda et al., 1990).
Phosgene is known to hydrolyse slowly in the gas phase, but
rapidly once dissolved in liquid water, to give CO2 and HCl.
HCl is removed from the troposphere by wet deposition (dissolution
in atmospheric water droplets and subsequent rain-out) or dry
deposition (direct uptake by the oceans, land surfaces, vegetation
etc.) with an average lifetime of about 1 week. The amount of chloride
deposited in this manner is completely negligible compared to the
natural atmospheric chloride flux of around 1010 tonnes/year
primarily from sea-salt aerosols (WMO, 1991).
In the stratosphere methylene chloride will rapidly degrade by
photolysis and reaction with chlorine radicals (Derwent et al., 1976).
4.2.2 Water
Sunlight absorption of water results in the formation of HO. and
hydrated electrons (e-aq). The near surface concentrations of HO.
and e-aq are 4 × 10-16 mol/litre and 5 × 10-17 mol/litre,
respectively, which corresponds to theoretical half-lives for
methylene chloride of 400 and 33 days. In water systems these
reactions are very limited, the reaction with hydroxyl radicals being
dominant. The total rate constant for the sunlight-induced
transformation in surface water (with a depth of 2.5 m, a DOC content
of 4 mg/litre, a chlorophyll a content of 10 µg/litre and a
suspended matter content of 40 mg/litre) was estimated to be
2.8 × 10-5 day-1 (half-life 68 years). The HO. causes 90% of this
transformation (Slooff & Ros, 1988). No direct photolysis of methylene
chloride was found after visible and UV irridiation for 5 days at 22°C
(Chodola et al., 1989).
The half-life of a 1 mg/litre aqueous solution of methylene
chloride was found to be about 1.5 years when measured in sealed glass
tubes in the dark at 25°C and pH 7 (Dilling et al., 1975). No
significant hydrolysis was found at 50°C and pH 4 or 9.2 after 7 days
in the dark (Chodola et al., 1989). Under acidic and basic conditions
in the temperature range of 80-150°C, the hydrolysis of methylene
chloride results in the formation of formaldehyde and HCl (Fells &
Moelwyn-Hughes, 1958). Extrapolation of these data to 25°C gives a
long half-life of about 680-704 years (Dilling et al., 1975; Radding
et al., 1977). As the activation energy for hydrolysis of methylene
chloride varies with temperature, the extrapolation of rate data from
80-150°C may not be valid.
No reductive dehalogenation of methylene chloride in water was
observed in the presence of sodium sulfide and haematein, a common
iron porphyrin (Klecka & Gonsior, 1984).
4.2.3 Soil
As is the case in aqueous systems, hydrolysis is probably not an
important process in the removal of methylene chloride from soil (see
section 4.2.2).
In a lysimeter experiment, a 90% decrease over 2.5 m soil column
was obtained (Nellor et al., 1985).
In the report of a spillage, the concentrations of methylene
chloride were up to 802 mg/m3 and 26 900 mg/m3 near the point of
leakage. In both cases, methylene chloride could not be detected some
hundred metres away from the points of contamination even in the
direction of the groundwater flow (ECSA, 1989). In the neighbourhood
of polluted areas, an increase of bacterial activity has been found.
In well-documented cases of accidental spills to soils, methylene
chloride disappeared rapidly from ground water, probably due to
(bio)degradation (Baldanf, 1981; Leitfaden für die Beurteilung, 1983).
4.3 Biotransformation
4.3.1 Aerobic
Negligible oxygen consumption was found in a biochemical oxygen
demand (BOD) test (Klecka, 1982), and methylene chloride was
considered to be degradation resistant in a degradation test following
the Japanese MITI standards (Kawasaki, 1980). However, complete
degradation occurred during a static-culture flask test (Tabak et al.,
1981).
In laboratory studies methylene chloride was almost completely
transformed within days by bacteria enriched from a primary sewage
sludge, municipal activated sludge (with or without acclimitization)
and industrial waste water (Rittmann & McCarty, 1980; Davis et al.,
1981; Klecka, 1982; Stover & Kincannon, 1983; Halbartschlager et al.,
1984).
In field studies it has been shown that methylene chloride is
efficiently removed from water treatment works (Namkung & Rittmann,
1987).
Certain strictly aerobic, facultative methylotrophic bacteria,
like Pseudomonas DMI and Hyphomicrobium DM2, both readily isolated
from contaminated soil and waste-water treatment plants, are capable
of using methylene chloride as a sole carbon source for growth
(Brunner et al., 1980; Stucki et al., 1981).
Secondary substrate utilisation of methylene chloride was
demonstrated by Pseudomonas sp. strain LP. This strain showed a
preference towards degrading methylene chloride over acetate, whether
it was the primary or the secondary substrate (Lapat-Polasko et al.,
1984).
In Hyphomicrobium DM2, a glutathione (GSH)-dependent, strongly
inducible enzyme (a glutathione S-transferase) was found to be
responsible for the degradation of methylene chloride. It converted
methylene chloride to formaldehyde via the nucleophilic displacement
of chloride and the formation of S-chloromethyl glutathione and
S-hydroxymethyl glutathione. This enzymic dehalogenation in extracts
of methylene-chloride-grown cells amounted to 1160 mg/g protein per h
under alkaline (pH 8-9) conditions (Stucki et al., 1981; Leisinger,
1983).
Eight other bacteria (mainly Pseudomonads ), capable of growing
on methylene chloride as their sole carbon source, were isolated from
enriched cultures. Maximum degradation rates for methylene chloride
(up to 860 mg/litre per h) were found for an initial saturated
solution of 14.5 g/litre in a pH-controlled fermenter (flow rate
10 ml/h). Further increases in degradation rate were limited by the
high salt concentration resulting from the neutralization of the
degradation products. In a fluidized bed reactor with bacteria
immobilized on silica, a degradation rate of methylene chloride of up
to 1600 mg/litre per h was observed (Gälli and Leisinger, 1985;
Stucki, 1990).
Ubiquitous soil- and water-dwelling nitrifying bacteria such as
Nitrosomonas europaea, which depends for growth on the oxidation of
ammonia, were able to degrade 1 mg methylene chloride/litre completely
within 24 h in the presence of ammonia and by 67% in the absence of
ammonia (Vannelli et al., 1990).
The removal of methylene chloride from aerobic soil was
significantly increased following exposure to methane (Henson et al.,
1988).
Flathman et al. (1992) described the remediation of ground water
contaminated with dichloromethane after a leak. Air stripping was used
initially on water pumped out from the contaminated site, and 97% of
the contamination was removed in this way. This was followed by the
first phase of bioremediation, in which contaminated water was
withdrawn from the site and added to a bioreactor containing bacteria
acclimated to DCM. The treated water was reinjected on the site
together with the bacteria. This phase decreased the concentration by
97% over a period of 40 days. A second phase of bioremediation
followed some 3 years later, dealing with a subsection of the original
site. In this case, the indigenous bacteria were used and nutrients
were added to the site. Concentrations before treatment were up to
5200 mg/litre; after 10 months these had reduced to < 2 mg/litre. At
this point active treatment ceased, but the levels of DCM continued to
decrease, falling below 10 µg/litre at all but one of the sampling
sites.
The biodegradation of methylene chloride in contaminated ground
water can be strongly inhibited in the presence of other contaminants
such as 1,2 dichloroethane, xylene and ethylbenzene (Scholz-Muramatsu
et al., 1988).
Aerobic biodegradation of methylene chloride was observed in a
variety of surface soils including sand, a sandy loam and a sandy clay
loam, as well as in subsurface clay soil. Degradation occurred over
concentrations ranging from approximately 0.1 to 5 mg/litre. The time
required for 50% disappearance of the parent compound varied between
1.3 and 191.4 days.
4.3.2 Anaerobic
Details of studies on the anaerobic biodegradation of methylene
chloride are given in Table 6.
Methylene chloride was degraded at a concentration of 200 µg/litre
in the aqueous phase of natural sediment. Degradation was observed to
proceed via methyl chloride, although accumulation was not observed
(Wood et al., 1981). After a varying acclimation period using
anaerobic digestion in waste water, 86-92% conversion to CO2 will
occur (Gossett, 1985). The half-life of methylene chloride in an
anaerobic water/sludge system is 11 days (Bayard et al., 1985).
Methylene chloride degradation was observed under anaerobic
conditions in sandy loam soil (Davis & Madsen, 1991).
4.3.3 Bioaccumulation
The n-octanol/water partition coefficient for methylene chloride
is 18 (log Pow = 1.25-1.3). As a consequence, its bioaccumulation is
not expected to be significant. Moreover, its high depuration and
degradation rate will reduce the probability of bioaccumulation.
No experimental bioconcentration factor (BCF) for methylene
chloride is available. Its theoretical BCF ranges between 0.91 and 7.9
(Veith et al., 1980; Lyman et al., 1982; Veith and Kosian, 1983;
Bayard et al., 1985). Further data indicative of bioaccumulation in
aquatic organisms and human breast milk can be found in sections 5.1.3
and 5.3.1, respectively.
There is no evidence of biomagnification.
4.4 Interaction with other physical, chemical or biological factors
The ozone-depletion potential (ODP) of methylene chloride, as
compared to the standard ODP of CFC11, can be estimated from the
numbers of chlorine atoms (2 as compared to 3 for CFC11) and the
atmospheric lifetime (0.7 years as compared to 60 years). This results
in an ODP for methylene chloride of 0.4% of that of CFC11.
Table 6. Aerobic biodegradation of methylene chloride
Test system Condition Duration Degradation Initial concentration Reference
Laboratory studies
Unknown aerobic, BOD 20 days none Klecka (1982)
Domestic waste aerobic 28 days none Kawasaki (1980)
water (MITI)
Domestic waste aerobic, static, 7 days for each 100% 5, 10 mg/litre, loss by Tabak et al. (1981)
water subcultures taken at days culture transformation volatilization 6.25%
14 and 21
Enriched primary aerobic, static, closed 24 h almost 25 mg/litre Rittmann & McCarty
sewage effluent complete (1980)
transformation
Industrial waste aerobic 6 h 92% 50 mg/litre Davis et al. (1981)
water, municipal transformation,
activated sludge no metabolites
Activated sludge aerobic, continuous-flow 2-6 days > 99% 180 mg/litre, loss by Stover & Kincannon
reactor volatilization 5% (1983)
Municipal activated aerobic 50 h 49-66% 1, 10, 100 mg/litre Klecka (1982)
sludge (9-11 days mineralization
acclimatization)
Activated sludge aerobic 20-28 mg//litre 264-1300 mg/litre Halbartschlager et al.
(6 weeks per hour (1984)
acclimatization) transformation
Table 6 (Cont'd)
Test system Condition Duration Degradation Initial concentration Reference
Field studies
Water treatment aerobic 30-55% removal 50-150 µg/litre Loehr (1987)
works
Conventional aerobic 5-6 h 96.0-96.3% Namkung & Rittmann
activated sludge transformation (1987)
plant
At the current estimated total emission rate of 500 000 tonnes per
year, the calculated tropospheric chlorine loading due to methylene
chloride is 35 ppt, i.e. approximately 1% of the total chlorine
loading of 3600 ppt (WMO, 1991).
As methylene chloride has a low photochemical ozone creation
potential in the troposphere (0.9), when compared with chemicals such
as ethanol (27) or ethylene (100), it will not contribute
significantly to photochemical smog formation (Derwent & Jenkin,
1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
As a consequence of release during its production and use,
methylene chloride is found in biota, water and air. Levels in water
and air tend to be higher in industrial and urban areas than in rural
areas. Improved control of emissions has led to lower environmental
levels of methylene chloride.
For the general population, air is the major source of exposure
to methylene chloride. In indoor air, higher levels may result from
the use of consumer products which contain methylene chloride. High
levels of methylene chloride may occur for short periods of time when
paint strippers and aerosols are used.
Exposure to methylene chloride can occur during its production
and use as a paint stripper. cleaner, degreaser, process solvent and
as an aerosol.
5.1 Environmental levels
Environmental levels measured before 1980 were summarized in
EHC 32: Methylene Chloride (IPCS, 1984). This monograph therefore
focuses on levels measured after 1980.
5.1.1 Atmosphere
5.1.1.1 Ambient air
In the ambient air of rural and remote areas, mean background
levels of methylene chloride are 0.07-0.29 µg/m3 (Table 7). The
average concentrations in suburban and urban areas, respectively, are
reported to be < 2 µg/m3 and < 15 µg/m3. In the vicinity of
hazardous waste sites, up to 43 µg/m3 have been round.
5.1.1.2 Precipitation
Rain water sampled in Koblenz (Germany) in 1982-1983 was found to
contain up to 4 µg methylene chloride/litre (Hellmann, 1984).
5.1.2 Water
Data on the levels of methylene chloride in water are presented in
Table 8.
Table 7. Methylene chloride levels in ambient air
Country/ Location Year of Concentration Reference
region measurement (µg/m3)
Germany urban area: Frankfurt 1980 2.1-4.2 Arendt et al. (1982)
Italy northern part 1983-1984 < 14 De Bortoli et al. (1986)
Netherlands Delft, Vlaardingen (urban 1980-1981 14.1 (max. annual mean) Guicherit & Schulting (1985)
area)
Isle of Terschelling (rural, 1980-1981 1.4 (max. annual mean) Guicherit & Schulting (1985)
suburban area)
mean concentration in 1980-1981 9 Guicherit & Schulting (1985)
the country
USA rural, suburban areas - 0.18-2.1 Shah & Heyerdahl (1988)
San Francisco Bay area 1984 3.2-9.1 Levaggi et al. (1988)
urban areas 1980 0.8-6.7 Shah & Heyerdahl (1988)
Shikiya et al. (1984)
1980-1981 1.35-6.76 Singh at al. (1982)
1981 0.8-2.5 Harkov (1984)
1982 2.4-4.2 Harkov (1984)
1987 0.95-1.64 Pleil & McClenny (1990)
1988 0.62-1.80 Pleil & McClenny (1990)
1989 0.48-1.68 Pleil & McClenny (1990)
hazardous waste sites 1983-1984 0.3-43 Harkov et al. (1985)
Arctic Spitzbergen July 1982 0.26±0.04 Hov et al, (1984)
March 1983 0.29±0.06 Hov et al. (1984)
Northern eastern Pacific 1981 0.12±0.15 Singh et al. (1983)
hemisphere
Southern eastern Pacific 1981 0.07 Singh et al, (1983)
hemisphere
Table 8. Methylene chloride levels in water
Country Location Year of Concentration Reference
measurement (µg/litre)
Ground water
Italy Milan 1983 4.5 CEFIC (1986)
USA Iowa 128 wells 1984-1985 1-5 (4 wells) Kelley (1985)
Surface water
Germany Mosel 1983 1.5-2.0 Hellmann (1984)
Neckar 1983 0.6-1.0 Hellmann (1984)
Elbe 1983 0.7-2.1 Hellmann (1984)
Elbe 1988 11 (max) LWA (1990)
Weser 1982-1983 < 0.5 Hellmann (1984)
Weser 1988 6 (max) LWA (1990)
Rhine at various sites 1981-1983 < 1 LWA (1981,1982,1983)
Rhine at Koblenz 1983 5.35-171 (monthly mean) Hellmann (1984)
Rhine at the Wesel 1983 < 2.0 Hellmann (1984)
Rhine at Duisburg 1984 1.5 (max) LWA (1984)
Rhine at various sites 1988 3.3 (max) LWA (1989)
Table 8 (Cont'd)
Country Location Year of Concentration Reference
measurement (µg/litre)
1989 1.0 (max) LWA (1990)
1990 1.1-3.9 (90th percentile) LWA (1991)
1991 < 0.1 (max) LWA (1992)
1986 0.1 (mean) BUA (1986)
Main 1985 ± 0.2 Van de Graaff (1986)
Emscher 1988 8.5 (max) LWA (1989)
1989 2.5 (max) LWA (1990)
1990 3.9 (max) LWA (1991)
1991 < 0.1 (max) LWA (1992)
Lippe 1988 5.5 (max) LWA (1989)
1989 < 1 (max) LWA (1990)
1990 2.4 (90th percentile) LWA (1991)
1991 < 0.1 (max) LWA (1992)
Wupper 1988 2.3 (max) LWA (1989)
1989 13.6 (90th percentile) LWA (1990)
1990 3.0 (90th percentile) LWA (1991)
Table 8 (Cont'd)
Country Location Year of Concentration Reference
measurement (µg/litre)
USA Susquehanna river, 1987 10 (mean) Smith (1989)
Columbia
Lancaster 1987 4.7 (mean) Smith (1989)
Ohio river basin (11 1980-1981 > 1 (238 samples) Howard et al. (1990)
stations, 4972 samples) > 10 (19 samples)
Sea and estuarine East Pacific Ocean 1981 0.002 (mean) Singh et al. (1983)
water (30 samples)
East Sea (German Coast) 1983 1.3-2.6 Hellmann (1984)
North Sea (German 1983 0.06-0.20 Hellmann (1984)
Coast)
In surface water, levels of methylene chloride have been reported
to vary from not detectable to 10 µg/litre. According to data recorded
in the US EPA STORET database, 30% of the samples showed methylene
chloride levels above the detection limits. A median concentration of
0.1 µg/litre was estimated (Staples et al., 1985).
Limited information concerning the contamination of sea water and
estuaries by methylene chloride is available. It appears that
methylene chloride can be found at up to 2.6 µg/litre in coastal
waters of the Baltic Sea. Levels of up to 0.20 µg/litre have been
found in North Sea coastal waters. Methylene chloride is generally not
detected in open oceans. A mean concentration of 2.2 ng/litre has been
reported in the South Pacific Ocean.
Methylene chloride enters the aquatic environment primarily
through waste water discharge. An estimated amount of 0.2% of the
total methylene chloride production is released in waste water
(Dequinze et al., 1984). The input from air rain-out has been
estimated for the northern and southern hemisphere (section 4.1.1).
Waste water from certain industries has been reported to contain
methylene chloride at average concentrations in excess of
1000 µg/litre, these being coal mining, aluminium forming,
photographic equipment and supplies, pharmaceutical manufacture,
organic chemical/plastics manufacture, paint and ink formulation,
rubber processing, foundries and laundries. The maximum concentration
measured was 210 mg/litre in waste water from the paint and ink
industry and the aluminium-forming industry (US EPA, 1981).
In the US EPA STORET database on industrial effluents, 38.8% of
the samples recorded contained methylene chloride with a median
concentration of 10 µg/litre (Staples et al., 1985).
Samples from the outfalls of four municipal treatment plants in
Southern California, USA, with both primary and secondary treatment,
contained < 10 to 400 µg methylene chloride/litre (Young et al.,
1983). In 30 Canadian water-treatment facilities, average
concentrations of methylene chloride in summer and winter were found
to be 10 µg/litre and 3 µg/litre, respectively (maximum, 50 µg/litre)
(Otson et al., 1982).
In leachate from industrial and municipal landfills, methylene
chloride concentrations were reported to range from 0.01 to
184 000 µg/litre (Sabel & Clark, 1984; Brown & Donnelly, 1988;
Sawhney, 1989).
Background data on ground water contamination by methylene
chloride are limited. It is the sixth most frequently detected organic
contaminant in ground water at hazardous waste disposal sites in the
CERCLA database (178 sites), the detection frequency being 19% (Plumb,
1987). In contaminated ground water in Minnesota, USA, up to
250 µg/litre has been detected (Sabel & Clark, 1984). Levels of up to
110 µg/litre were found in percolation water from a waste-disposal
site in Germany. However, methylene chloride was not found
(< 1 µg/litre) in the ground water below the site (Heil et al.,
1989).
5.1.3 Aquatic organisms
Concentrations of methylene chloride in freshwater organisms have
been reported for oyster and clams from Lake Ponchartrain, Louisiana,
USA. Levels ranging from 4.5 to 27 µg/kg (wet weight) could be
detected (Ferrario et al., 1985).
No methylene chloride was detected in fish taken from the River
Rhine in 1981 (Binnemann et al., 1983).
Levels of methylene chloride up to 700 µg/kg wet weight were found
in marine bottom fish taken from Commencement Bay in the state of
Washington, USA (Nicola et al., 1987).
Data on biota collected in the US EPA STORET data base show an
average level of 660 µg/kg in the 28% of the samples in which
methylene chloride was detected (Staples et al., 1985).
5.1.4 Soil and sediment
No data are available on the levels of methylene chloride in soil.
The levels of methylene chloride found in sediment from Lake
Pontchartrain, Louisiana ranged from not detectable to 3.2 µg/kg wet
weight (Ferrario et al., 1985).
Data recorded in the US EPA STORET database revealed a median
concentration of 13 µg/kg in 20% of 338 sediment sampling data
(Staples et al., 1985).
The levels of methylene chloride found in sediments from the river
Rhine in 1987-1988 varied from non-detectable to 30-40 µg/kg. At one
site maximum concentrations of 220-2200 µg/kg were measured (BUA,
1993, personal communication to the IPCS).
5.2 Human exposure
5.2.1 General population
5.2.1.1 Indoor air
In buildings where products containing methylene chloride are
used, air levels of methylene chloride much higher than outdoor levels
(< 15 µg/m3, see section 5.1.1.1) may be found (Table 7).
Relatively high levels (mean 670 µg/m3, peak level 5000 µg/m3)
have been found in the indoor air of residential houses (De Bortoli et
al., 1986).
5.2.1.2 Drinking-water
Methylene chloride has been detected in drinking-water supplies
(estimations made before 1980) in numerous cities in the USA (Dowty et
al., 1975; Coleman et al., 1976; Kopfler et al., 1977; Kool et al.,
1982), the mean concentrations reported being generally less than
1 µg/litre. An average of 3-10 µg/litre and a maximum of 50 µg/litre
were observed in a Canadian study of 30 drinkable water treatment
facilities (Otson et al., 1982).
Samples from 128 drinking-water wells in the USA showed that 3.1%
of them had methylene chloride levels of 1-5 µg/litre (Kelley, 1985).
Rodruigez Rojo et al. (1989) sampled the drinking-water of
Santiago de Compostela, Spain, in 1987. Methylene chloride was found
in 98.4% of the samples; the average concentration was 14.1 µg/litre,
with a range of 1.2-93.2 µg/litre. Other halomethanes were also found
and measured in the samples at average concentrations ranging from
9 to 25 µg/litre.
A wide sampling exercise involving 630 public community water
supplies (serving 6.9 million people in New Jersey, USA) was carried
out in 1984 and 1985 by McGeorge et al. (1987). The percentage of
positive results for methylene chloride ranged from 2.6 to 7.1%. The
median concentration ranged from 1.1 to 2.0 µg/litre and the range for
the whole sampling period was 0.5 to 39.6 µg/litre.
5.2.1.3 Foodstuffs
Although methylene chloride is used in food processing (solvent
extraction of coffee, spices, hops), there is little information on
its residual levels in food. In the USA, residues of methylene
chloride were found in decaffeinated coffee beans (0.32 to 0.42 mg/kg)
whilst a major coffee processor reported levels of 0.01 to 0.1 mg/kg
(ATSDR, 1992).
No methylene chloride was detected in ice-cream and yoghurt (BUA,
1986).
In seven types of decaffeinated ground coffee the methylene
chloride content ranged from < 0.05 to 4.04 mg/kg; in eight instant
coffee samples <0.05 to 0.91 mg/kg was found (Page & Charbonneau,
1984).
Heikes & Hopper (1986) analysed samples of grains and intermediate
grain-based foods for a range of fumigants using a purge-and-trap
method. Methylene chloride was not found in any of the grain samples,
nor in uncooked rice or dried lima beans. It was found in some of the
intermediate foods such as bleached flour (30 µg/kg), yellow corn meal
(4.7 µg/kg), lasagne noodles (5.4 µg/kg) and yellow cake mix
(4.6 µg/kg).
One of the authors (Heikes, 1987) investigated levels of methylene
chloride in table-ready foods, taken from the US Food and Drug
Administration's Total Diet Study. Of the 19 foods examined, eight
contained methylene chloride above the quantification limit (not
given). Detailed results for six of the foods are given in Table 9.
Table 9. Dichloromethane content of table ready foods
(Heikes, 1987)
Food Number of Number Range of
samples positive concentration
(µg/kg)
Butter 7 7 1.1-280
Margarine 7 7 1.2-81
Ready-to-eat cereal 11 10 1.6-300
Cheese 8 8 3.9-98
Peanut butter 7 4 26-49
Highly processed foodsa 12 10 5-310
a e.g., frozen chicken dinner, fish sticks, pot pie
5.2.1.4 Consumer exposure
Consumers are exposed to methylene chloride via the use of a
number of formulated products such as aerosols or paint strippers. A
USA survey found that 78% of paint removers and 66% of aerosol spray
paints sold as household products contained methylene chloride (US
EPA, 1987). Over 100 consumer products in Sweden contain methylene
chloride (National Chemical Inspectorate, Sweden, personal
communication to the IPCS). In Norway the number is around 140,
including 45 paint removers (AKZO, personal communication to the
IPCS).
Methylene chloride does not appear to be subject to widespread
volatile substance abuse. Statistics on deaths resulting from
substance abuse in the United Kingdom were collected over the period
1971-1991 and analysed by product type. Of the 1221 deaths recorded,
five were assigned to the group "paint thinners and paint strippers".
Methylene chloride is used only in the latter products, the former
containing solvents such as toluene and xylene which are known to be
substances of abuse (Flanagan et al., 1990).
A large do-it-yourself consumer population uses paint strippers
containing methylene chloride on furniture and woodwork. Formulations
are available mainly in liquid form, but also, occasionally, as an
aerosol. Exposures have been estimated on the basis of USA
investigations of household solvent products. The estimated levels
ranged from less than 35 mg/m3 to a few short-term exposures of 14
100 to 21 200 mg/m3. The majority of the concentration estimates
were below 1770 mg/m3 (US EPA, 1990).
Methylene chloride exposure was estimated while using a number of
formulations of paint stripper in a small room. Various ventilation
conditions were evaluated and a worst possible case was simulated,
with doors and windows closed. In one test, involving furniture
stripping in a room with through ventilation, the operator exposure
was found to be 289 mg/m3 on a 2-h TWA. Peaks of exposure were
observed during application (460 mg/m3) and during scraping-off
(710-1410 mg/m3) (ICl, 1988, personal communication to the IPCS).
A series of paint-stripping exercises were performed in a small
room. Various ventilation conditions were evaluated while using a
number of formulations of paint stripper. A worst possible case was
simulated with doors and windows closed. Concentrations of methylene
chloride in the room rose to 14.1-17.6 g/m3 (4000-5000 ppm),
although it is questionable whether anyone could work in such
conditions without breathing apparatus. Further exercises with the
door and windows open (as recommended by suppliers) reduced
atmospheric concentrations by more than a factor of 10. Exposure to
methylene choride resulted in an 8-h TWA of 187-226 mg/m3
(53-64 ppm). The actual stripping operations took between 1 and 1.5 h.
Maximum exposures occurred during initial application and scraping-
off. These exposures were of a few minutes duration and concentrations
never exceeded 3.53 g/m3 (1000 ppm) (ICI, 1990, personal
communication to the IPCS).
The effect of variation in the formulation was also investigated.
During paint stripping, background concentrations in the room varied
from 710-1410 mg/m3 to less than 350 mg/m3 depending on the
formulation. However, within 5-6 min of application the level of
methylene chloride fell to an equilibrium concentration of around
71 mg/m3, irrespective of the formulation used. The shortest time
before reaching equilibrium was about 2-2.5 min (ICI, 1990, personal
communication to the IPCS).
Studies in the Netherlands have measured peak concentrations in
salons of 21-106 mg/m3 (6-30 ppm), with an 8-h TWA of
3.53-17.65 mg/m3 (1-5 ppm) (CEC Scientific committee of
Cosmetology). The same study measured a peak concentration of
265 mg/m3 (75 ppm) arising from home use of a hairdressing aerosol
containing 35% methylene chloride. This equates to a TWA of
2.65 mg/m3 (0.75 ppm).
Studies in the United Kingdom simulating consumer exposure during
salon use yielded values well within the national Maxium Exposure
Limit (353 mg/m3 or 100 ppm for an 8-h TWA). The hairdresser
received an exposure of 77.7 mg/m3 (22 ppm, on an 8-h TWA) during
what is considered to be exceptionally heavy use (i.e. a 10-second
spray every 15 min for an 8-h period). The customer exposure was found
to be 106-265 mg/m3 (30-75 ppm) (10-min TWA) (ICI, 1990, personal
communication to the IPCS).
The same study simulated home use of personal-care aerosols
containing methylene chloride. Even adverse conditions (small room, no
ventilation) resulted in an exposure of 353 mg/m3 (100 ppm) (10-min
TWA), equating to 7.06 mg/m3 (2 ppm) on an 8-h TWA (ICI, 1990,
personal communication to the IPCS). This work was characterized by
low air changes, virtually no ventilation and a more frequent rate of
application than that determined by surveys of actual hairdressing
work in salons.
5.2.2 Occupational exposure
Exposure to methylene chloride can occur during its production and
use as a paint stripper, cleaner, degreaser, process solvent and as an
aerosol. Exposure concentrations that have been reported in various
industries are presented in Table 10. Below is a brief description of
exposure conditions in some of the reported industries.
5.2.2.1 Production
Production of methylene chloride is normally carried out in a
closed system, with a relatively small number of people being
involved. Exposure arises primarily during filling and packing
operations. Occupational exposures are listed in Table 10. Some
measured ranges, e.g., 35-81 mg/m3 and 85-244 mg/m3 (HSE, 1992),
indicate that engineering control techniques can bring 8-h TWA
exposures below 350 mg/m3.
5.2.2.2 Paint stripping and related activities
Workers in the formulation of paint removers are exposed while
transferring methylene chloride from storage tanks, during mixing
(blending) operations and while packaging. The extent of exposure will
depend on the control measures and work practices in force. Exposure
levels (8-h TWA) range from a low of 0-18 mg/m3 to over 1770 mg/m3
(US EPA, 1990).
Table 10. Occupational exposure to methylene chloride
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Production Production actvities 219-374 Maintenance activity with RPE; HSE (1987)
Results obtained at one plant
Aircraft Paint stripping 35-81 RPE provided HSE (1992)
Paint stripping 35-289 Submission to OSHA in 1987. RPE Air Transport Association
provided (USA)
Various industries Painting 21-299 See IARC (1986) Chrostek & Levine (1981)
Paint stripping 18-1765 US EPA (1990)
Used aerosol < 0-494 Some work areas were congested Fleeger & Lee (1988)
adhesives
Pharmaceuticals - 7.1-3749 NCI feasibility study Zahm et al. (1987)
Production work 0-18 Enclosed process HSE (1992)
-
Aerosol products Aerosol filling 95-628 ICI (UK) (1984)
Rubber products Fabrication 208-304 LEV HSE (1992)
Fibre glass Cleaning and mould 187-6693 intermittent exposure, RPE may be HSE (1992)
manufacture preparation worn; may not be representative
of the industry
Cleaning, mixing etc. 0-350 Small factory units Post et al. (1991)
Printing 3.5-558 NCI feasibility study Zahm et al. (1987)
Table 10 (Cont'd)
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Triacetate Production 180-350 ECSA (1989)
fibre/film 237-3442 NCI feasibility study Zahm et al. (1987)
manufacturing 180-2440 Products contain acetone Ott et al. (1983)
Furniture Paint stripping 25-3810 Many without adequate controls HSE (1992)
Paint stripping 201-1292 Variable degrees of control McCammon et al. (1991)
Washing/refinishing 53-780 Variable degrees of control McCammon et al, (1981)
Spraying adhesive 219-1490 Many without adequate controls HSE (1992)
General Cleaning, degreasing, 53-141 See IARC (1986) Ruhe et al. (1981)
manufacturing, etc. < 0-460 See IARC (1986) Ruhe et al. (1982)
cleaning and
degreasing
Foam industry Glue spraying 85-244 LEV HSE (1992)
Moulding 88-1090 High exposure due to HSE (1992)
insufficient/inadequate LEV
< 247 Jernelov & Antonsson
(1987)
Unknown 7.1-251 NCI feasibility study Zahm et al. (1987)
Various jobs 18-580 High exposure experienced by Boeniger (1991)
sprayers
Motor vehicle Paint spraying, 7.1-247 LEV and RPE HSE (1992)
manufacture stripping
Table 10 (Cont'd)
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Quarry Laboratory work, 71-1370 High exposures due to inadequate HSE (1992)
mineral processing control
Metal treatment 7.1-790 NCI feasibility study Zahm et al. (1987)
Nutrition Extraction < 0-106 See IARC (1986) Cohen et al. (1980)
a RPE = respiratory protection equipment; LEV = local exhaust ventilation
Paint strippers are widely used in a number of industries:
automotive, rubber products, furniture and fixtures, plastic, and
electronic industries. Exposure to methylene chloride takes place
during application, removal of the substrate soaked in methylene
chloride, and the disposal of the spent paint remover. Typical
exposures (8-h TWA) range from 18 mg/m3 to about 1770 mg/m3 (US
EPA, 1990).
Exposure of commercial furniture refinishers to methylene chloride
occurs when stripping involves either the dipping of furniture into a
tank containing a mixture of solvents including methylene chloride
(typically 65%) or coating it manually with a brush. Exposure levels
are highly variable and greatly influenced by the size of the
organization, engineering controls in place and work practices. Some
refinishers may operate on a part-time basis and from their homes. In
some instances where the worker was leaning over the tank or using a
brush to scrub the surface coating, concentrations of > 7100 mg/m3
have been recorded (McCammon et al., 1991; HSE, 1992). Better work
stations and work practices have helped to reduce exposures greatly.
5.2.2.3 Aerosol production and use
In the packaging of aerosol cans, exposure arises primarily during
filling and packing. Levels observed are generally below 180 mg/m3
(ECSA, 1989). Potential occupational exposure to methylene chloride as
a result of aerosol products varies according to the use and the work
undertaken.
Consumer exposure due to the use of cosmetic and paint spray
aerosols is discussed in section 5.2.1.4.
5.2.2.4 Use as a process solvent
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products. Most of the processes are
carried out in closed systems, with the exception of triacetate fibre
and film manufacture. Normally, exposure levels are low, but
occasionally high exposures (> 350 mg/m3; 10-min TWA) may occur in
such operations as filter changing, charging and discharging. Some
industrial processes involve somewhat higher exposure levels; in
particular, the manufacture of cellulose triacetate fibres and films
can involve exposure up to 350 mg/m3 (8-h TWA) even when good
engineering controls are installed (Zahm et al., 1987; ECSA, 1989). As
part of an epidemiological study of cellulose fibre production
workers, Ott et al. (1983) reported exposure levels ranging from 177
to 2436 mg/m3 in the processing area, and exposure ranging from 18
to 1341 mg/m3 in the preparation area based on sampling performed in
1978. The range for the entire plant was later reported to be from
below detectable limits to 6000 mg/m3 (Lanes et al., 1990). As part
of an epidemiological study of photographic film workers, Friedlander
et al. (1978) reported exposures ranging from 0 to 1236 mg/m3 based
on sampling carried out between 1959 and 1975. The highest mean
exposures at this plant were reported for group leaders (402 mg/m3)
(Hearne et al., 1987).
In the pharmaceutical industry, methylene chloride is used as a
solvent and extraction medium. Sealed processes, high recovery rates
and careful handling of discharges have helped to keep the exposure
levels below around 106 mg/m3 (Zahm et al., 1987; HSE, 1992).
Methylene chloride is also used as an extraction medium in the
nutrition industry, where the exposure levels are generally low when
the processes are adequately controlled (Cohen et al., 1980).
Methylene chloride is used in the foam industry for cleaning
process equipment, purging spray guns, and as an auxiliary blowing
agent. It is also used as a releasing agent in the moulding of
polyurethane products. Exposure levels ranging from a few mg/m3 to
short-term exposures of over 1770 mg/m3 have been reported (Jernelov
& Antonsson, 1987; Boeniger, 1991; HSE, 1992).
The use of methylene chloride as a solvent in adhesives can result
in occupational exposure, during the application of the adhesive, to
short-term levels in excess of 350 mg/m3 (Fleeger & Lee, 1988; HSE,
1992). Processes involving the formulation of adhesives are likely to
be well controlled.
Methylene chloride is also used as a solvent in the analysis of
bitumen samples. This work is normally carried out in small
laboratories and exposure levels will be high unless adequate control
measures are used.
5.2.2.5 Cleaning and degreasing
In the manufacture of metal products, cleaning (degreasing) is
required before painting, plating, plastic coating, etc. The degree of
exposure to methylene chloride will be influenced by many factors,
including the age of the equipment, type of engineering controls
available, their maintenance, handling, and drying methods. In
general, it is possible to reduce exposure levels to below 124 mg/m3
(Swedish National Board of Occupational Safety & Health, personal
communication to the IPCS).
5.2.3 Occupational exposure limits
A listing of some national occupational exposure limits is given
in Table 11.
Table 11. Occupational exposure limit valuesa
Country TWA STEL TWA STEL Remarks Reference
(mg/m3, 20°C)b (ppm) (ppm) (ppm)
Australia 350 - 100 - Suspected carcinogen ILO (1991)
Austria 360 1800c 100 500 Suspected of carcinogenic DFG (1991)
potential
Belgium 174 - 50 - Suspected human carcinogen ACGIH (1992)
Czechoslovakia 500 2500 - - ILO (1991)
Denmark 174 - 50 - Absorption through skin may be ILO (1991)
significant
Suspected carcinogen
Finland 350 870 100 250 ILO (1991)
France 360 1800 100 500 ILO (1991)
Germany 360 1800c 100 500 Suspected of carcinogenic DFG (1991)
potential
Italy 174 - 50 - Suspected human carcinogen ACGIH (1992)
Japan 350 - 100 - ILO (1991)
Netherlands 350 1750 100 500 Arbeidsinspectie
(1991)
Norway 125 - 35 - Carcinogen Arbeidstilsynet
(1990)
Table 11 (Cont'd)
Country TWA STEL TWA STEL Remarks Reference
(mg/m3, 20°C)b (ppm) (ppm) (ppm)
Portugal 174 - 50 - ILO (1991)
Sweden 120 250d 35 70 Classified as a low potent AFS (1990)
carcinogen
Switzerland 360 1800 100 500 Classification for teratogenic ILO (1991)
effects not possible; biological
monitoring required
United Kingdom 350 870 100 250 Maximum exposure limit
USA - ACGIH 174 50 Suspected human carcinogen ACGIH (1992)
a TWA = time-weighted average concentration (8-h working period); STEL = short-term exposure limit (15 min, unless specified)
b Official values; some countries use different conversion factors and/or other ambient temperature
c 30 min
d 15 min
5.3 Human monitoring data
5.3.1 Body burden
Methylene chloride was detected in all eight samples of human milk
from four urban areas (Pellizzari et al., 1982). Hardin & Manson
(1980) could still find methylene chloride in mother's milk 17 h after
the end of exposure.
In 12 male volunteers exposed to 2600 mg/m3, biopsies showed
adipose tissue levels of 10.2, 8.4 and 1.6 mg/kg at 1, 4, and 22 h,
respectively, after a 1-h exposure (Engström & Bjurström, 1977). When
Antoine et al. (1986) analysed whole blood from 250 individuals, the
mean concentration of methylene chloride was 0.7 µg/litre with a range
from not detected to 25 µg/litre.
BUA (1986) monitored saliva and tissues from people living in
industrialized areas of Beckum, Germany. They reported that no
methylene chloride was detected.
5.3.2 Occupational exposure studies
In a cohort of 14 furniture strippers exposed to methylene
chloride at concentrations of 53 to 1290 mg/m3, post-exposure breath
concentrations of methylene chloride ranged from 8.1 to 590 mg/m3
(McCammon et al., 1991).
Mother's milk in Soviet women manufacturing rubber articles
contained a mean of 74 µg/kg in 17 out of 28 samples approximately 5 h
after start of work, the level declining after termination of work
(Jensen, 1983).
A group of seven non-smoking workers, who had previously been
exposed to methylene chloride for several years, were exposed to a
mean concentration of 635 mg/m3 (in addition, there was exposure to
154 mg/m3 (mean) chloroform). The pre-exposure average carbon
monoxide level in alveolar air was 34 mg/m3, increasing to
58 mg/m3 during exposure; before the next exposure, the carbon
monoxide level was 27 mg/m3: this corresponded to carboxyhaemoglobin
(CO-Hb) levels of 4.9%, 8.3% and 3.9%, respectively. The biological
half-life of CO-Hb was 13 h (Rathey et al., 1974). Although methylene
chloride does not accumulate following repeated exposure, these data
clearly indicate that CO-Hb levels will be cumulative if the periods
between exposure are insufficient to allow the CO-Hb levels to return
to normal.
CO-Hb levels in a worker accidentally overcome by methylene
chloride vapour were found to have increased to 19%. A further worker
with a history of ischaemic heart disease, who had been exposed
concurrently with the first patient, had a CO-Hb level of 6% on the
day following the exposure (Benzon et al., 1978).
Methylene chloride levels in alveolar air and blood were measured
in 14 shoe-sole factory workers. The alveolar concentration, mean
blood levels, and CO-Hb levels, following exposure to 74±28 mg/m3
methylene chloride, were: 49 mg/m3, 0.41 mg/litre, and 4.0%,
respectively; upon exposure to 124+42 mg methylene chloride/m3:
71 mg/m3, 0.99 mg/litre, and 5.2%, respectively; and upon exposure
to 339±265 mg methylene chloride/m3: 229 mg/m3, 3.07 mg/litre, and
6.5%, respectively. In this factory, the methylene chloride exposure
was highly variable; the data are too limited to allow valid
extrapolation (Perbellini et al., 1977).
The relationship between low environmental levels of methylene
chloride, carbon monoxide in alveolar air and urinary excretion of
methylene chloride was studied in a group of 20 manufacturing workers
(12 smokers, 8 non-smokers). A good correlation was observed between
levels of methylene chloride and urinary excretion of methylene
chloride. The correlation between alveolar air and levels of methylene
chloride was poor except when the analysis was restricted to
nonsmokers (Ghittori et al., 1993)
5.3.3 Biological exposure indices
Biological Exposure Indices (BEIs) are reference values intended
for guidelines for the evaluation of potential health hazards in
industrial hygiene practice. The BEIs for methylene chloride at the
end of a working shift have been given as: CO-Hb level 5%, blood level
of methylene chloride 1 mg/litre.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor with respect to health risk,
biological monitoring based upon either analysis of carbon monoxide in
exhaled air or of CO-Hb in blood is to be preferred. However, this can
only be applied in non-smoking subjects. Sampling should be carried
out either about 0-2 h after exposure or 16 h later, i.e. on the
following morning.
In the case of an 8-h exposure to less than 350 mg methylene
chloride/m3 in non-smokers, CO-Hb levels 2-h after exposure ceases
are not expected to exceed 2-3%, and after 16 h to exceed 1%.
6. KINETICS AND METABOLISM
Appraisal
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract and dermal exposure results in absorption but
at a slower rate than that of the other exposure routes.
Distribution studies indicate that, via inhalation or dermal
exposure, methylene chloride distributes to all tissues. It can cross
the blood-brain barrier and it can be transferred across the
placenta. Concentrations of methylene chloride rise more slowly in
adipose tissue and longer exposures are required before these tissue
levels equal those of the blood. Data indicate that methylene
chloride and/or its metabolites do not accumulate in tissues.
Methylene chloride is metabolized to carbon monoxide, carbon
dioxide and inorganic chloride. Methylene chloride is eliminated from
the body primarily via the lungs in expired air. Urinary excretion
plays a minor role in its elimination. As exposure levels increase, a
large proportion of methylene chloride is exhaled unchanged.
Metabolism occurs by either or both of two pathways; their relative
contribution to the total metabolism is markedly dependent on the
exposure level and on the animal species concerned. One pathway
involves oxidative metabolism mediated by cytochrome P-450 and leads
to both carbon monoxide and carbon dioxide. This pathway appears to
operate similarly in a qualitative and quantitative sense in all
rodents studied and in humans. This is the predominant metabolic
route, but saturation occurs at around 1800 mg/m3. Increasing the
dose above the saturation level does not lead to extra metabolism by
this route.
The other pathway involves a glutathione transferase, and leads
via formaldehyde and formate to carbon dioxide. This route seems only
to become important at doses above the saturation level of the
"preferred" oxidative pathway. There are marked species and dose-
dependent differences in the contribution that this pathway makes to
the metabolism of methylene chloride.
6.1 Absorption
6.1.1 Inhalation exposure
6.1.1.1 Human studies
The principal route of human exposure to methylene chloride is
inhalation. Evaluation of pulmonary uptake indicated that 70-75% of
inhaled vapour was absorbed in human subjects exposed to 180, 350, 530
and 710 mg/m3. Initial absorption was rapid, as indicated by levels
of methylene chloride in the blood of approximately 0.6 mg/litre in
the first hour of exposure to levels of 350-710 mg/m3. At a level of
180 mg/m3, the increase in blood methylene chloride concentration
was 0.2 mg/litre for the first hour. There was a direct correlation
between the steady-state blood methylene chloride values and the
exposure concentration, both during the exposure and for the first 2 h
after the exposure in all groups. Steady-state blood levels appeared
to be reached after 4 h and remained constant until the end of
exposure. Once exposure ceased, methylene chloride was rapidly cleared
from the blood. Seven hours after exposure, less than 0.1 mg/litre was
detected following exposure to 180, 350 or 530 mg/m3. Only
1 mg/litre was detected in the highest group (710 mg/m3) 16 h after
exposure. In all other dose groups the blood levels had returned to
baseline concentrations (Di Vincenzo & Kaplan, 1981a,b).
In common with other lipophilic organic vapours, methylene
chloride absorption appears to be influenced by factors other than the
vapour concentration. Prior to reaching steady state, increased
physical activity increases the amount of methylene chloride absorbed
by the body, due to an increase in ventilation rate and cardiac
output, since these factors increase blood flow through the lungs and
promote absorption (Di Vincenzo et al., 1972; Astrand et al., 1975).
Uptake also increases with the body fat percentage, since
methylene chloride dissolves in fat to a greater extent than it
dissolves in aqueous media. Therefore, obese subjects will absorb and
retain more methylene chloride than lean subjects exposed to the same
vapour concentration. Under controlled conditions, there was a 30%
greater absorption and retention of methylene chloride by obese
subjects exposed to 2650 mg/m3 for 1 h as compared to lean subjects
(Engström & Bjurström, 1977).
Åstrand et al. (1975) reported that the amount of methylene
chloride taken up increases with physical workload, whereas the
retention decreases. With a 50-watt workload, the uptake was twice as
high, whereas the retention decreased from 55% to 45%. When exposure
was coupled with workload (physical exercise), the concentration in
alveolar air was increased during the whole post-exposure period
compared with exposure under rest conditions.
6.1.1.2 Animal studies
Studies of the relationship between inhalation exposures of
animals and their blood methylene chloride concentrations indicate
that absorption is proportional to the magnitude and duration of the
exposure over a methylene chloride concentration range of 350 to
28 200 mg/m3. This conclusion is based on the monitoring of blood
methylene chloride concentrations following inhalation exposure in
dogs and rats (Di Vincenzo et al., 1972; MacEwen et al., 1972; McKenna
et al., 1982). As is the case with humans, blood methylene chloride
levels reach a steady-state value which does not increase further as
the duration of exposure increases (McKenna et al., 1982).
The data from studies of blood methylene chloride values during a
6-h exposure of rats to between 180 and 5300 mg/m3 suggest that the
steady-state blood/air concentration ratio increases as the exposure
concentration increases. The ratio of the steady-state methylene
chloride concentrations in plasma to the exposure concentration was
0.001, 0.005 and 0.006 at levels of 180, 1800 and 5300 mg/m3,
respectively (McKenna et al., 1982). It is postulated that the
increased ratio at steady state results from saturation of metabolic
pathways as exposure increases, rather than from an increased
absorption coefficient.
Kim & Carlson (1986)conducted experiments to compare the effects
of a 12-h exposure schedule to those of an 8-h schedule on the CO-Hb
formation resulting from methylene chloride inhalation. Rats and mice
were exposed to 710, 1800 or 3500 mg/m3 8 h/day for 5 days, or
12 h/day for 4 days. No significant difference in carboxyhaemoglobin
levels was found. The peak blood methylene chloride level was found to
be dependent upon the methylene chloride exposure concentration, but
the half-life was independent of the duration of exposure or the
concentration of methylene chloride,
6.1.2 Oral exposure
No data are available on oral absorption of methylene chloride in
humans.
Treatment of mice and rats with methylene chloride in water or in
corn oil suggests that methylene chloride is easily absorbed from the
gastrointestinal tract. Methylene chloride levels were measured in gut
segments up to 40 min after rats were administered single oral doses
in water. The amounts measured were similar at both dose levels (50 or
200 mg/kg). Of the administered dose (200 mg/kg), 60% was recovered
from the upper gastrointestinal tract < 10 min after treatment (20%
recovery after 40 min). The amount of methylene chloride in the lower
gastrointestinal tract accounted for < 2% of the administered dose up
to the 40-min test interval (Angelo et al., 1986b).
In mice administered oral doses of non-radioactive methylene
chloride at 10 or 50 mg/kg in water, approximately 25% of the
administered dose was detected in the upper gastrointestinal tract
within < 20 min. Similarly, after treatment with methylene chloride
at 10, 50, or 1000 mg/kg in corn oil, approximately 55% of the
administered dose was detected in the upper gut segment and remained
there for 2 h (Angelo et al., 1986a).
6.1.3 Dermal exposure
Methylene chloride has been shown to be absorbed through human
skin (Stewart & Dodd, 1964). In this study, a volunteer immersed a
thumb in methylene chloride for 30 min under conditions which
precluded the inhalation of vapour. The subsequent alveolar air
concentration was 3.1 ml/m3; 2 h later it had fallen to
0.69 ml/m3.
Various studies on the rate of absorption through animal skin and
subsequent pharmacokinetics have been reported. Tissue concentrations
of methylene chloride were measured in various organs (lung, liver,
brain, kidney, heart and fat) of 128 white rats, using gas
chromatography, following immersion of two-thirds of their tails in
the solvent for 1, 2, 3 or 4 h. Small increases were seen in most
tissues after 1 or 2 h of exposure, and methylene chloride
concentrations in fatty tissues increased markedly after 3 h of
exposure. After 4 h of exposure, methylene chloride concentrations
remained elevated in fatty tissues and were increased in all other
tissues studied (Makisimov & Mamleyeva, 1977). The dermal absorption
rate for methylene chloride through mouse skin in vivo has been
measured to be 6.58 mg/h per cm2 (Tsuruta, 1975).
The dermal permeability constant for the absorption of methylene
chloride vapour through rat skin in vivo has been measured following
exposure to 106, 212 and 353 g/m3 for 4 h. Blood levels of methylene
chloride were shown to reach steady state levels after 1 h of exposure
to the two lower concentrations and after 3 h of exposure to
353 g/m3. The mean dermal permeability constant was calculated to be
0.28 mg/h per cm2 (McDougal et al., 1986).
6.2 Distribution
6.2.1 Inhalation exposure
6.2.1.1 Human studies
Engström & Bjurström (1977) exposed 12 male subjects (six slim and
six obese) to 2650 mg methylene chloride/m3 for 1 h. The total
uptake of methylene chloride in the slim group was 1116 ± 34 mg and in
the obese group 1446 ± 110 mg/kg. Estimation of methylene chloride in
needle biopsies showed that the adipose tissues contained
approximately 8 to 35% of the average total amount absorbed. The
amount or methylene chloride absorbed was highly correlated with the
degree of obesity and body weight. In the slim subjects, the
concentration in the adipose tissue during the 4-h period after
exposure was approximately twice that in the obese subjects. However,
despite a lower concentration, the total amount of methylene chloride
calculated to be in the body fat was greater in obese subjects.
A survey of the levels of methylene chloride in certain tissues
from pregnant or nursing women has been reported. The study was
conducted following observations of disturbances in the pattern of
pregnancy and lactation in female operatives in an industrial rubber
article manufacturing facility. The survey was conducted in an
unspecified number of women who had been exposed to several chemicals
during their work for at least 3 years. The chemicals included
gasoline, ethylene dichloride and methylene chloride. An estimate of
the average workplace concentration of methylene chloride was reported
to be 85.6 mg/m3. A control group (number unspecified) was
constituted from women working in the same facility but who had had no
direct contact with the chemicals. The tissues examined were the
blood, the fetal membranes and the fetus, all tissue samples being
obtained at the time of abortion of the fetus. The mean tissue
concentrations of methylene chloride (54 observations) were reported
to be 0.66 ± 0.21, 0.34 ± 0.10 and 1.15 ± 0.20 mg/kg for the blood,
fetal membranes and fetus, respectively, compared to 0.12 ± 0.07,
0.013 ± 0.01 and 0.016 ± 0.001 mg/kg in the controls. Methylene
chloride was also detected in 17 out of 28 specimens of breast milk
taken from exposed nursing women. An average concentration of
0.074 ± 0.04 µg/litre (n = 40) was found in the breast milk 5-7 h
after the start of the exposure; an insignificant quantity of
methylene chloride was reported 17 h after cessation of exposure
(Vosovaja et al., 1974).
6.2.1.2 Animal studies
Distribution studies in rats demonstrate that methylene chloride
(and/or its metabolites) is present in the liver, kidney, lungs,
brain, muscle and adipose tissues after inhalation exposures (Carlsson
& Hultengren, 1975; McKenna et al., 1982). One hour after exposure,
the highest concentration of radioactive material was found in the
white adipose tissue, followed by the liver. The concentration in the
kidney, adrenal and brain were less than half that in the liver.
Radioactivity in the fat deposits declined rapidly during the first
2 h after exposure (Carlsson & Hultengren, 1975). Concentrations in
the other tissues declined more slowly. Whole body autoradiography in
mice at one hour after inhalation of 10 µl 14C-methylene chloride
for 10 min showed a rapid and even distribution immediately after
exposure. A high uptake was noted in brain, body fat, blood, liver and
kidney. Evaporated sections showed a high retention of non-volatile
radioactivity, presumably representing metabolites, in the liver,
bronchi and kidneys. Thirty minutes after inhalation, radioactivity
started to appear in tissues with a high cell turnover such as bone
marrow, thymus and gastrointestinal mucosa, and in tissues with a high
rate of protein synthesis such as the spleen, exocrine pancreas and
salivary glands (Bergman, 1979).
On the other hand, after 5 days of exposure to 710 mg/m3 for
6 h/day, the concentration of methylene chloride in the perirenal fat
was 6-7 times greater than that in the blood and liver (Savolainen et
al., 1977). It has been suggested that methylene chloride first
saturates the blood and extravascular fluid compartment before
entering the fatty deposits (Di Vincenzo et al., 1972). Thus,
concentrations of methylene chloride will rise slowly in adipose
tissues, and longer exposures to methylene chloride will be required
before adipose tissue levels equal those in the blood. The animal data
are therefore consistent with the human adipose tissue data discussed
above.
Exposure of pregnant rats to methylene chloride leads to exposure
of the fetus to both methylene chloride and carbon monoxide (Anders &
Sunram, 1982).
6.2.2 Oral exposure
No studies are available regarding distribution of methylene
chloride in humans following oral exposure.
In animals, radioactivity from labelled methylene chloride was
detected in the liver, kidney, lung, brain, epididymal fat, muscle,
and testes after exposure of rats to a single gavage dose of 1 or
50 mg/kg. The tissue samples were taken 48 h after dosing. At that
time, the lowest concentration of radioactivity was found in the fat.
The highest concentrations were in the liver and kidney. This was true
for both doses (McKenna & Zempel, 1981).
Similar results were observed in rats administered methylene
chloride doses of 50-1000 mg/kg for 14 days. At each dose tested, and
in each tissue, the label was rapidly cleared during the 240 min
following each exposure (Angelo et al., 1986b).
6.2.3 Dermal exposure
No information is available regarding distribution in humans or
animals following dermal exposure to methylene chloride.
6.3 Metabolism
Species differences in metabolism and their relevance to
carcinogenicity are described in section 8.8.2.
6.3.1 In vitro studies
In vitro experiments using liver fractions, homogenates, slices
and hepatocytes, mainly from the rat, confirmed the presence of the
two metabolic pathways. The primary reaction, first described by Kubic
& Anders (1975), appears to be an oxidative dehalogenation giving
carbon monoxide and chloride ion. The reaction is catalysed by rat
liver microsomal fractions and is dependent upon NADPH and molecular
oxygen. The presence of a binding spectrum and inducers confirmed the
involvement of the cytochrome P-450 mixed function oxidase system.
More recent studies have identified the cytochrome P-450 isoenzyme as
cytochrome P-450 IIEl (Pankow et al., 1991; Guengerich et al., 1992;
Pankow & Jagielki, 1993). The highest activity was found in liver
microsomes, which were five times more active than lung microsomes and
thirty times more active than kidney microsomes. The proposed
metabolic route involves rearrangement of the primary hydroxylation
product to formyl chloride followed by decomposition to carbon
monoxide (Kubic & Anders, 1978). Although the transient intermediates
have not been isolated or identified, their formation is consistent
with the enzyme involved and the products formed.
The effect of pyrazole on methylene chloride metabolism in male
Wistar rats was investigated by Pankow et al. (1991). Rats received a
single methylene chloride close of 6.2 mmol/kg (0.4 ml/kg) orally.
Pyrazole was administered by intraperitoneal injection. The metabolism
of methylene chloride to carbon monoxide can be stimulated or
inhibited by pyrazole; the effect depends on the interval between
pyrazole and methylene chloride administration, and on the dose.
Stimulation of methylene chloride metabolism to carbon monoxide is due
to inducers of the isoenzyme cytochrome P-450 (CYP2El) such as
isoniazid, ethanol and other solvents. The inhibition was observed
following pre-treatment with high pyrazole doses or following
simultaneous administration of pyrazole and methylene chloride. The
inhibition may reflect the competition between pyrazole and methylene
chloride for oxidation by CYP2El as long as pyrazole is present in the
blood, or may also reflect the hepatotoxic effect of pyrazole.
Hepatic cytochrome P-450 levels were not increased in rats exposed
by inhalation to methylene chloride (5.29-10.59 g/m3 (1500 or
3000 ppm)) 6 h/day for 3 clays (Toftgard et al., 1982), nor in rats
exposed to 1.76 or 3.53 g/m3 (500 or 1000 ppm) 6 h/day for 2 weeks
(Kurppa & Vainio, 1981). Marginal increases were seen in a third study
(Norpoth et al., 1974) in which rats were exposed to 0.176-17.6 g/m3
(50-5000 ppm), 5 h/day for 10 days. In the study by Kurppa & Vainio
(1981) an increase in renal ethoxycoumarin de-ethylase activity was
reported.
The second metabolic pathway occurring in rat liver is localized
in the soluble (cytosolic) fraction (Ahmed & Anders, 1976, 1978). It
does not require oxygen but is dependent upon glutathione and a
glutathione- S-transferase enzyme, the products in vitro being
formaldehyde and chloride ion. The rapid and almost quantitative
conversion of formaldehyde to formic acid and then carbon dioxide
known to occur in vivo (Neely, 1964) is consistent with this pathway
being the source of carbon dioxide exhaled after exposure to methylene
chloride. The intermediates involved in the metabolism of methylene
chloride to formaldehyde are unknown, but the nature of the enzyme
involved and the dependence upon glutathione suggest that
S-chloromethyl-glutathione is formed and rapidly hydrolysed and
degraded to glutathione and formaldehyde (Ahmed & Anders, 1978). The
isoenzyme involved in the metabolism of methylene chloride has been
identified as a member of glutathione- S-transferase class theta
(Meyer et al., 1991).
The chemistry of the S-chloromethyl thioethers (Bohme et al.,
1949) and the lack of depletion of glutathione during this reaction
are consistent with these conjugates being extremely transient.
Formaldehyde, in addition to its metabolism to carbon dioxide, becomes
incorporated into the C-1 metabolic pool via formic acid. Therefore,
exposure to radiolabelled methylene chloride results in the
incorporation of radioactivity into macromolecules including nucleic
acids.
Hallier et al. (1993) described an apparent polymorphism in the
metabolism of methylene chloride in human blood. The metabolic
activity was reported to be localized in erythyrocytes (Thier et al.,
1991) and to be due to the presence of a glutathione- S-transferase
enzyme (Schroeder et. al., 1992). The work by Schroeder describes the
detection of enzyme activity in erythrocytes using methyl bromide as a
substrate, not methylene chloride. Furthermore, in experiments
investigating the influence of cofactors on enzyme activity,
glutathione could be replaced by L-cysteine, suggesting that this
enzyme is not a glutathione- S-transferase. The work by Thier et al.
(1991) identified metabolic activity in plasma and not, as reported by
Hallier et al. (1993), in erythrocytes.
6.3.2 In vivo studies
The metabolism of methylene chloride in various animal species and
in humans has been studied extensively (e.g., Fodor et al., 1973;
Kubic et al., 1974; Roth et al., 1975; Lee Rodkey & Collison, 1977;
Peterson, 1978; McKenna & Zempel, 1981; McKenna et al., 1982; Angelo
et al., 1986a,b).
Methylene chloride and the other dihalomethanes are unique in
being the only class of industrial chemicals known to be metabolized
to carbon monoxide. This metabolic pathway (dependent on cytochrome
P-450), first discovered in humans (Stewart et al., 1972), results in
elevated levels of CO-Hb and in increased levels of carbon monoxide in
expired air. Subsequent studies in experimental animals and in humans
established that this pathway is rate-limited by enzyme saturation, so
that at high doses the levels of CO-Hb become constant and independent
of dose (Lee Rodkey & Collison, 1977). Later experiments in animals
using radiolabelled methylene chloride identified carbon dioxide as
the other major metabolite (Di Vincenzo & Hamilton, 1975). Although
carbon dioxide is a known metabolite of carbon monoxide, the amount of
carbon dioxide formed from the monoxide was thought unlikely to
account for the levels found during exposure to methylene chloride.
This suggested the presence of a second pathway (dependent on
glutathione- S-transferase), which was subsequently confirmed in
experimental animals.
Two reports have described the effects of pretreatment or co-
administration of other organic solvents on the metabolism of
methylene chloride to carbon monoxide. Pankow et al. (1991) described
increases in CO-Hb levels in rats pretreated with a single gavage dose
of benzene, toluene or isomers of xylene, up to 32 h prior to a 6-h
exposure to methylene chloride. CO-Hb levels increased from 9.3%, in
rats exposed to methylene chloride alone, to 22.7% in rats pretreated
with m-xylene. Similar increases were seen in rats pretreated with a
single garage dose of methanol (Pankow & Jagielki, 1993). In both
studies, the levels of CO-Hb were reduced when the solvents were co-
administered with methylene chloride. The results of both studies were
considered to be consistent with the metabolism of methylene chloride
by cytochrome P-450 IIE1.
At first sight it might appear that the relative molar amounts of
carbon monoxide and carbon dioxide exhaled in vivo provide an index
of the activity of the two metabolic pathways. Studies using metabolic
inhibitors suggest that significant amounts of carbon dioxide are also
derived from the oxidative P-450 pathway (Gargas et al., 1986; Reitz
et al., 1986). Similar studies in mice using metabolic inhibitors have
confirmed these findings, leading to the conclusion of the authors
that the cytochrome P-450 pathway is the major route of metabolism of
methylene chloride within species (Ottenwalder et al., 1989). This
finding is consistent with either hydrolysis of formyl chloride to
formic acid or with formyl chloride reacting with glutathione to form
S-formyl glutathione. The rapid enzymatic and chemical breakdown of
this conjugate (Uotila & Koivusalo, 1974a,b) would yield formic acid
and hence carbon dioxide. Thus, a quantitative correlation between the
amount of carbon monoxide and carbon dioxide exhaled and the activity
of the two pathways no longer appears to be valid.
Levels of CO-Hb in the blood, following exposure to methylene
chloride, are both dose- and time-dependent. Human subjects exposed to
concentrations of 1770 mg/m3 or less for 1 h have CO-Hb levels of
1-4%. These levels rose to an average of 10% saturation within 1 h
after exposure to 3500 mg/m3 for 2 h (Stewart et al., 1972). Hake et
al. (1975) reported CO-Hb levels in excess of 8% following exposure to
880 mg/m3 for 7.5 h.
Human volunteers were exposed to 350 or 1240 mg/m3 for 6 h and
levels of methylene chloride in blood and exhaled air, CO-Hb and
exhaled CO were measured. At the end of the 6-h exposure, the CO-Hb
concentration of the group exposed to 1240 mg/m3 was 1.4 times
higher than that of the group exposed to the lower dose. Likewise, the
concentration of exhaled CO in the high-dose group was 2.1 times
higher than that of the low-dose group. The authors concluded that
their finding of non-linearity between administered dose and the CO-Hb
and CO levels is an indication of saturation of the metabolic pathway
(McKenna et al., 1980).
Physical exercise performed during exposure to methylene chloride
will produce higher blood CO-Hb levels than those found in sedentary
workers (Åstrand et al., 1975; Di Vincenzo & Kaplan, 1981b). Under a
moderate workload, an exposure to 350 mg/m3 for 7.5 h may cause a
CO-Hb saturation of about 5% at the end of the exposure period (Di
Vincenzo & Kaplan, 1981b). Other factors, including smoking and
exposure to combustion and automobile exhaust, will increase CO-Hb
levels.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
6.4.1.1 Human studies
Methylene chloride is removed from the body mainly in expired air
and urine. In four human subjects exposed to methylene chloride
(350 mg/m3) for 2 h, an average of 22.6 µg methylene chloride was
excreted in the urine within 24 h after the exposure. In seven
subjects exposed to 710 mg/m3 for 2 h, the corresponding value was
81.5 µg (Di Vincenzo et al., 1972). These data show that the amount
excreted in the urine is insignificant. Methylene chloride excretion
in expired air was most evident during the first 30 min after
exposure. Initial post-exposure concentrations of methylene chloride
in expired breath following 2-and 4-h exposure periods were about
71 mg/m3 and fell to about 18 mg/m3 at the end of 30 min. Small
amounts of methylene chloride remained in the expired air at 2.5 h.
A detailed study of the relationship between the measurements of
methylene chloride in expired air or blood, carbon monoxide in expired
air and CO-Hb in blood was undertaken by Di Vincenzo & Kaplan
(1981a,b). At the end of exposure of non-smoking, sedentary volunteers
for 7.5 h to methylene chloride vapour concentrations of
180-710 mg/m3, the mean concentration of the solvent in alveolar air
and in blood, and the percent CO-Hb saturation were measured, as shown
in Table 12.
By 7 h after exposure to any concentration, the expired air
contained less than 3.5 mg/m3 methylene chloride; at 16 h, only
negligible levels were detected (Di Vincenzo & Kaplan, 1981a). These
data suggest that, due to its rapid elimination, measurements of
methylene chloride in expired air are unsuitable for use as a marker
of occupational exposure.
Table 12. Methylene chloride in expired air and blood, and carboxyhaemoglobin
(CO-Hb) levels of human volunteers following 7.5 h exposure
(from Di Vincenzo & Kaplan, 1981a)
Methylene Methylene choride Methylene chloride CO-Hb
chloride exposure in expired air in blood levels
(mg/m3) (mg/m3) (mg/litre)
180 53 0.3 1.9%
350 124 0.8 3.4%
530 194 1.2 5.3%
710 282 1.8 6.8%
Di Vincenzo & Kaplan (1981a) reported that, in a human volunteer
study, exposure to 180, 350, 530 or 710 mg/m3 for 7.5 h/day (for 5
days) resulted in peak CO-Hb levels of 1.9, 3.4, 5.3 and 6.8%,
respectively (Table 12). It was estimated that an 8-h exposure to
about 530 mg methylene chloride/m3 is equivalent to an 8-h exposure
to 124 mg carbon monoxide/m3, in as much as either exposure under
sedentary conditions will increase blood CO-Hb levels to about 5% of
saturation by the end of the exposure.
Di Vincenzo & Kaplan (1981b) also investigated the effects of
exercise and cigarette smoking on the uptake, metabolism and excretion
of methylene chloride. The effects of smoking and methylene chloride
exposure on CO-Hb saturation levels were found to be additive.
Exercise was found to increase the absorption of methylene chloride
and CO-Hb levels. However, the effects of exercise on CO-Hb were not
observed to increase with heavy workloads beyond the level achieved
with moderate work-loads, suggesting a saturation of this effect (see
also section 5.3.2).
Engström & Bjurström (1977) found that, during the first 2 h after
exposure, the concentration in alveolar air tended to be lower and
declined more rapidly in obese subjects than in slim ones. After this,
the concentration dropped more slowly in the obese group. During the
late phase of elimination, the obese subjects tended to have a higher
concentration in expired air.
6.4.1.2 Animal studies
In rats, methylene chloride was excreted in the expired air,
urine, and faeces following a single 6-h exposure to 180, 1800 or
53 000 mg methylene chloride/m3 (McKenna et al., 1982). At
180 mg/m3, only 5% of the exhaled label was found as methylene
chloride. The remainder was exhaled as CO and CO2. As the exposures
increased, so did the exhalation of non-metabolized methylene
chloride. Methylene chloride accounted for 30% of the label from the
1800 mg/m3 dose and 55% of the label for the 53 000 mg/m3 dose. A
combination of exhaled methylene chloride, CO2 and CO accounted for
58%, 71% and 79% of the inhaled methylene chloride dose for the 180,
1800 and 53 000 mg/m3 doses, respectively. Urinary excretion
accounted for 7.2-8.9% of the dose and 1.9-2.3% of the dose was in the
faeces.
6.4.2 Oral exposure
Expired air accounted for 78-90% of the excreted dose in rats in
the 48-h period following a 1 or 50 mg/kg dose of methylene chloride
in aqueous solution (McKenna & Zempel, 1981). The radiolabel was
present in the exhaled air as CO and CO2, as well as in expired
methylene chloride. The amount of methylene chloride in the expired
air increased from 12% to 72% as the dose was increased from 1 to
50 mg/kg. Radiolabel in the urine accounted for 2-5% of the dose under
the above exposure conditions, while 1% or less of the dose was found
in the faeces. These data indicate that the lungs are the major organ
of methylene chloride excretion even under oral exposure conditions.
Mice excreted 40% of the administered dose (100 mg/kg) unchanged in
expired air within 96 h (Yesair et al., 1977).
6.4.3 Dermal exposure
No information is available regarding excretion and elimination in
humans or animals following dermal exposure to methylene chloride.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Appraisal
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria which are able to grow in the presence of
methylene chloride at much higher concentrations (including a
saturated solution in water) have been identified. Anaerobic bacteria
are more sensitive; growth inhibition has been observed at 3 mg/litre
in anaerobic biological sludge.
In the aquatic environment, fish and amphibian embryos are the
most sensitive with effects on hatching from 5.5 mg/litre.
Adult fish are relatively insensitive to methylene chloride. even
after prolonged exposure (14-day LC50 > 200 mg/litre). The effect
of methylene chloride on Daphnia is variable; the lowest reported
EC50 was 135 mg/litre in a closed system.
In soil, 10 mg/kg strongly decreased the ATP content of the
biomass, adversely affected the growth of fungi and aerobic bacteria.
and induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms, LC50 values were in the
range of 300 to more than 1000 µg/cm2 ). In sediment, no toxic
effects were observed even at very high levels.
In higher plants, no effects were found after exposure for 14
days to 100 mg/m3.
7.1 Microorganisms
7.1.1 Bacteria
7.1.1.1 Aerobic bacteria
No inhibition of growth was observed at 19.6-19 600 mg/litre
methylene chloride in Bacillus subtilis, Pseudomonas cepacia and
Aeromonas hydrophylia (Schubert, 1979). Inhibition of
bioluminescence of Photobacterium phosphoreum by 50% occurred after
a 15-min exposure to 2880 mg/litre (Hermens et al., 1985).
In a standard 16-h growth-inhibition test with Pseudomonas
putida, a threshold of 500 mg/litre for methylene chloride was
determined (Bringmann & Kühn, 1977b). The glycolysis of Pseudomonas
putida was inhibited after a 16-h exposure to 1000 mg/litre
(Bringmann & Meinck, 1964).
Nenzda & Seydel (1988) report minimum inhibitory methylene
chloride concentrations for the bacteria Escherichia coli and
M. smegmatis of 1049 and 1468 mg/litre, respectively.
For heterotrophs, 50% inhibition of oxygen consumption occurred at
320 mg/litre after 24 h (Blum & Speece, 1991).
With other bacteria (Acinetobacter, Alcaligenes, Flavobacterium,
Pseudomonas cepacia, Aeromonas hydrophila), stimulation of growth
was observed at 200 mg/litre (Davis et al., 1981).
The IC50 for inhibition of multiplication of Escherichia coli
was 37.2 mg/litre (Nendza & Seydel, 1988).
In the OECD activated sludge respiration-inhibition test (method
209) using sealed vessels, the EC50 value for methylene chloride was
more than 1000 mg/litre after 30 min (Volskay & Grady, 1988).
Concentrations up to 1000 mg/litre had no effect on the oxygen
consumption or glucose metabolism of activated sludge acclimated to
methylene chloride for 3 days (Klecka, 1982).
In methylene chloride-utilizing bacteria, e.g., Hypho-microbium,
up to 1700 mg/litre did not interfere with growth (Stucki et al.,
1981).
Blum & Speece (1991) found that the IC50 for reduction of
ammonia was 1.2 mg/litre after 24 h for Nitrosomonas.
7.1.1.2 Anaerobic bacteria
Anaerobic bacteria are more sensitive than aerobic bacteria.
Methanogenesis of mixed rumen microflora was inhibited from
136 mg/litre (Bauchop, 1967). At 93 mg/litre, the growth of a mixed
bacterial population from an anaerobic digester was inhibited by 50%
(Thiel, 1969). Addition of methylene chloride to anaerobic sludge from
an operating municipal digester showed, after 48 h, a 20% inhibition
of gas production at 3 mg/litre and a 50% inhibition at 50 mg/litre
(Hayes & Bailey, 1977). Addition of methylene chloride to the feed of
a mixed anaerobic culture, developed in the laboratory from seed from
a sewage treatment plant, decreased the gas production to such an
extent that at 3.3 mg/litre it had virtually ceased after 5 days,
compared with 15 days in controls (Vargas & Ahlert, 1987).
Blum & Speece (1991) determined the toxicity of methylene chloride
to methanogenic bacteria and found an IC50 for inhibition of gas
production of 7.2 mg/litre.
Stuckey et al. (1980) used a batch system in which methylene
chloride was added in ethanol to sludge from a laboratory digester in
a Warburg apparatus. Some inhibition was noted at the lowest
concentration tested (3.16 mg/litre); the concentration for 50%
inhibition over 60 h was estimated to be 14 mg/litre.
7.1.2. Protozoa
The bacteriovorous ciliated protozoan Uronema parduczi Chatton-
Lwoff was not affected after a 20-h exposure to 16 000 mg/litre (EC5
inhibition cell proliferation) (Bringmann & Kühn, 1980). Also, no
effects were observed in Microregma heterostoma after a 28-h
exposure to 1000 mg/litre (Bringmann & Meinck, 1964).
7.1.3 Algae
In several freshwater green algae (Selenastrum capricornutum,
Scenedesmus subspicatus. Scenedesmus quadricauda, Chlorella vulgaris,
Chlamydomonas angulosa), photosynthesis (chlorophyll a content,
CO2 uptake) and cell number were only affected by methylene chloride
from 1450 mg/litre (Bringmann & Kühn, 1978; Hutchinson et al., 1978;
US EPA, 1980). The threshold (7-day EC3) for effects in the
cyanobacterium (blue-green alga) Microcystis aeruginosa was
550 mg/litre (Bringmann & Kühn, 1978).
In the marine diatom Skeletonema costatum methylene chloride
exposure had no effect on chlorophyll a content or cell number at
662 mg/litre (US EPA, 1980).
7.2 Aquatic organisms
The volatility of methylene chloride presents difficulties in
aquatic toxicity testing. Therefore, care should be taken when
interpreting results based on nominal concentrations and open static
systems. Flow-through systems or closed static systems are necessary
to conduct adequate toxicity studies on volatile substances. However,
these systems were not always used (Tables 14 and 15).
7.2.1 Plants
The EC50 for Lemna minor growth was 2000 mg/litre, whilst both
growth and photosynthesis of the plant Groenlandia densa were
totally inhibited at this concentration after 7 days (Merlin et al.,
1992).
7.2.2 Invertebrates
7.2.2.1 Insects
The toxicity of methylene chloride for insects was investigated in
adult Tribolium confusum and grain weevil (Calandra granaria). The
LC50 for a 5-h exposure in fumigation vessels was 82 and
380 mg/litre, respectively (Ferguson & Pirie, 1948; Negherbon, 1959).
Table 13. Acute aquatic toxicity of methylene chloride to algae
Organism Description Method Parametera Concentration Reference
(mg/litre)
Diatom Skeletonema costatum Chlorophyll a content, 96-h EC50 > 662 US EPA (1980)
(salt water) cell number
Green alga Selenastrum Chlorophyll a content, 96-h EC50 > 662 US EPA (1980)
capricornutum cell number
Green alga Scenedesmus Cell number 8-day TT 1450 Bringmann & Kühn
quadricauda (1978)
Green alga Chlorella vulgaris CO2 uptake 3-h EC50 2292 Hutchinson et al.
(1978)
Green alga Chlamydomonas CO2 uptake 3-h EC50 1477 Hutchinson et al.
angulosa (1978)
Cyanobacterium Microcystis aeruginosa Cell number 8-day TT 550 Bringmann & Kühn
(blue-green alga) (1978)
a TT = toxicity threshold (i.e. the concentration at which cell multiplication was inhibited by more than 3%)
Table 14. Acute aquatic toxicity of methylene chloride in fresh water
pH/dissolved Hardness
Organism Temperature oxygen (mg CaCO3 Flow/ Parameter Concentration References
(°C) (mg/litre) per litre) Static (mg/litre) and remarks
Crustacean
Water flea 22 7.4-9.4/ 173 Static 48-h LC50 220 Le Blanc (1980)
(Daphnia magna) 6.5-9.1 48-h NOEC 68 (nominal concentration
Water flea 20-22 7.6-7.7 Unknown Static 24-h EC50 2100-2270 Bringmann & Kühn
(Daphnia magna) 24-h NOEC 1550-1707 (1977a, 1982)
(nominal concentration)
Water flea Unknown Unknown Unknown 48-h LC50 1250 Bringmann & Meinck
(Daphnia magna) (1964)
Water flea Unknown Unknown Unknown Static 24-h EC50 1959 Kühn et al, (1989)
(Daphnia magna) 48-h EC50 1682 (closed system)
Water flea Unknown Unknown Unknown Static 48-h EC50 135 Abernethy et al. (1986)
(Daphnia magna) (closed system)
Water flea 18-20 8/8.7-8.8 11.7 Unknown 48-h LC50 480 RIVM (1986)
(Daphnia magna) 48-h NOEC 100 (nominal concentration
closed system)
Fish
Goldfish Unknown Unknown Unknown Static 24-h LC50 420 Jenson (1978)
(Carrassius (nominal concentration)
auratus)
Table 14 (Cont'd)
pH/dissolved Hardness
Organism Temperature oxygen (mg CaCO3 Flow/ Parameter Concentration References
(°C) (mg/litre) per litre) Static (mg/litre) and remarks
Fathead minnow 12 7.8-8.0/ 67 Static 98-h LC50 310 Alexander et al, (1978)
(Pimephales > 5 Flow 96-h LC50 193 (static test nominal, flow-
promelas) (adult) 96-h NOEC 66.3 through measured
concentrations;
aquarium covered with
plastic film for the first
24-h)
Fathead minnow 25 Unknown 73-82 Flow 96-h LC50 502 Dill et al. (1987)
(Pimephales (analysed concentration)
promelas)
(juvenile)
Bluegill, 21-23 6.5-7.9/ 32-48 Static 96-h LC50 220 Buccafusco et al. (1981)
(Lepomis unknown (nominal concentration,
macrochirus) aquarium not capped)
Table 15. Acute aquatic toxicity of methylene chloride in salt water
Temperature pH/dissolved Hardness Flow/ References
Organism (°C) oxygen (mg CaCO3 Stat Parameter Concentration and remarks
(mg/litre) per litre) (mg/litre)
Crustacean
Mysid shrimp Unknown Unknown Unknown Static 96-h LC50 260 US EPA (1980) (nominal
(Mysidopsis bahia) concentration)
Grass shrimp 20±2 6.1-8.0 8-12 Static 48-h LC50 108.5 Burton & Fischer (1990)
(Palaemonetes pugia) > 4
Fish
Golden orfe Unknown Unknown Unknown Static 48-h LC50 521-528 Juhnke & Lüdemann (1978)
(Leuciscus idus)
Killifish (juvenile) 20±2 6.1-8.0/ Unknown Static 48-h LC50 97.0 Burton & Fischer (1990)
(Fundulus > 4
heteroclitus)a
Sheepshead minnow 25-31 Unknown 10-31 Static 98-h LC50 330 Heitmuller et al. (1981)
(Cyprinodon 98-h NOEC 130 (nominal concentration)
variegatus)
a fish died within 1 hour; the measured 1-h LC50 was 135 mg/litre. The 48-h value was the average of the initial and final concentrations.
7.2.2.2 Crustaceans
Data on the toxicity of methylene chloride to crustaceans are
presented in Table 14. Daniels et al. (1985) and Knie (1988) reported
a 48-h LC50 of 27 mg/litre and a 24-h LC50 of 12.5 mg/litre; such
values are lower than those presented in Table 14 by almost one order
of magnitude. However, no experimental details were given and,
therefore, the validity of the data cannot be assessed.
7.2.2.3 Molluscs
In seawater, metamorphosis was induced in up to 63% of the larvae
of the nudibranch mollusc (Phestilla sibogae) when exposed to
8.5-25.5 mg/litre (Pennington & Hadfield, 1989).
7.2.3 Fish
7.2.3.1 Acute toxicity
Data on the acute toxicity of methylene chloride to fish are
presented in Table 14.
The acute toxicity of methylene chloride to adult fathead minnows
(Pimephales promelas) has been studied both in a static and a flow-
though system. The observed effects (loss of equilibrium,
melanization, narcosis and swollen, haemorrhaging gills) were
reversible at a sublethal level (Alexander et al., 1978).
7.2.3.2 Chronic toxicity and reproduction
Data on the chronic and embryo-larval toxicity of methylene
chloride to fish are summarized in Table 16.
In a 32-day embryo-larval test with fathead minnow (Pimephales
promelas), the larval survival and weight was affected from 209 and
142 mg/litre, respectively. The maximum acceptable toxicant
concentration (MATC) based on body weight was calculated to be
108 mg/litre. The ratio between the acute 8-day LC50 value and the
32-day embryo-larval MATC is 4.6, indicating a small difference
between acute and chronic effects of methylene chloride (Dill et al.,
1987).
7.2.4 Amphibians
In closed flow-through systems, short-term embryo-larval tests
were carried out, from 2 to 6 h post-spawning to 4 days post-hatch, on
amphibian eggs of Rana catesbeiana. R. palustris and Bufo fowleri
(hatching times ranged from 3 to 4 days). After combining frequencies
for lethality and teratogenesis, the analytically determined post-
hatching LC50s were > 32 mg/litre for the pickerel frog
Table 16. Chronic and embryo-larval toxicity of methylene chloride to fish
Dissolved Hardness Flow/ References and
Description Temperature pH oxygen (mg CaCO3 Static Parameter Concentration remarks
(°C) (mg/litre) per litre) (mg/litre)
Chronic toxicity
Guppy 22±1 ? > 5 25 daily LC50, 14 days 295 Könemann
(Poecilia reticulata) renewal (1981) (covered
with glass,
nominal)
Fathead minnow 25±1 6.8-8.6 > 9 73-82 flow LC50, 8 days 471 Dill et al.
(Pimephales promelas) NOEC, (1987)
(juvenile) 8 days 357 (analytical
concentration)
Embryo-larva toxicity
Fathead minnow 20.4±0.6 7.8 6.5 95 flow LC50a 34 Black et al.
(Pimephales promelas) (1982)
(embryo-larva)
Fathead minnow 25±1 6.8-8.6 > 9 73-82 flow LOEC, 32 days 209 Dill et al.
(Pimephales promelas) (survival) (1987)
(embryo-larva) LOEC, 32 days 142 (analytical
(weight) concentration)
Rainbow trout 13.3±0.3 7.8 9.4 106 flow LC50a,b 13.1 Black et al.
(Salmo gairdneri) (1982)
(embryo)
Table 16 (Cont'd)
Dissolved Hardness Flow/ References and
Description Temperature pH oxygen (mg CaCO3 Static Parameter Concentration remarks
(°C) (mg/litre) per litre) (mg/litre)
Rice fish 25±1 7.6-8.4 4.5-8.8 11.7 renewal LC50, 3 weeks 106 RIVM (1986)
(Oryzias latipes) 23±2 3 times/ NOEC, 3 weeks 75 (analytical
(egg-larva) week concentration)
a Eggs were exposed from 30 min after fertilization to 4 days post-hatch
b Teratogenic effects were observed at 5.5 mg/litre
(R. palustris) and Fowler's toad (Bufo fowleri) and 17.78 mg/litre
for the bullfrog (R. catesbeiana). In the latter, anomalous larvae
and 16% decreased hatching were observed at 6.73 mg/litre. For the
pickerel frog and Fowler's toad, hatching was decreased by 14 and 20%
at 10 and 32 mg/litre, respectively. In the hatched populations
slightly higher incidences of teratogenic effects were observed (Birge
et al., 1980).
Black et al. (1982) exposed several amphibian species to methylene
chloride from 30 min after fertilization to 4 days post-hatch. Post-
hatching LC50 values ranging from 16.9 to > 48 mg per litre were
found for the European common frog (Rana temporaria), Northwestern
salamander (Arabystoma gracile), African clawed frog (Xenopus
laevis) and the Leopard frog (R. pipiens). The European common
frog and the Northwestern salamander were the most sensitive to
methylene chloride.
7.3 Terrestrial organisms
The toxicity of methylene chloride to higher plants (Phaseolus
vulgaris, Raphanus sativus radicula, Lepidum sativum, Trifolium
pratense, Saintpaula ionatha, Petunia hybrida) was evaluated, using
the LIS (Landesanstalt für Immissionsschutz, Essen) test; no effect
was observed at 100 mg/m3 exposure over 14 days (Van Haut & Prinz,
1979).
In leaves of alfalfa (Medicago sativa), the effect of methylene
chloride vapour on the photosynthetic fixation of 14CO2 was
tested; photosynthesis appeared to be reduced from 388 000 mg/m3
(Lehman & Paech, 1972).
In a 48-h filter-paper contact toxicity test on the earthworm
Eisenia fetida, the LC50 was 304 µg/cm2 in one study and
> 1000 µg/cm2 in another. Therefore, methylene chloride was
classified as moderately toxic (100-1000 µg/cm2) (Roberts & Dorough,
1984; Neuhauser et al., 1985).
In embryos of White leghorn chicken, the LD50 for injection of
methylene chloride in the yolk sac is 14 mg/egg (Verrett et al.,
1980).
7.4 Population and ecosystem effects
7.4.1 Soil microorganisms
When added to brown soil at 10 mg/kg (dry weight), methylene
chloride decreased the ATP content of the soil biomass by 80-85%,
compared to controls, and adversely affected the growth of soil fungi
and aerobic bacteria after 3 days. A slight recovery was observed by
the end of the 56-day experiment. Anaerobic bacteria were hardly
influenced and, in the case of the obligate anaerobic Clostridium
sp., the growth was even increased. The replacement of oxygen
probably explained the stimulation of growth in the latter case
(Kanazawa & Filip, 1987).
Incubation of soil for 2 months with 1-10 mg/kg (dry weight)
methylene chloride reduced the activity of ß-glucosidase,
ß-acetylglucosaminidase and proteinase during the first 28 days, with
recovery after 2 months; no effect was observed at 0.1 mg/kg (Kanazawa
& Filip, 1986).
7.4.2 Sediment microorganisms
In sediment from a freshwater stream, methylene chloride did not
significantly affect the electron transport system (ETS) activity
during a 1-h enzymatic assay at 66 500 mg/kg. When assayed over an
11-day period, 1330-66 500 mg/kg caused a fluctuating stimulation of
ETS activity, which may indicate a marked alteration of the stability
of the biological activity in the sediment. Microbial respiration,
measured by CO2 evolution, was inhibited (EC50) after 7 days
at 15 500 mg/kg. However, when measured by oxygen uptake, it was
stimulated at up to 26 500 mg/kg (Trevors, 1985).
7.4.3 Microcosms and mesocosms
Microcosms composed of water plants (Elodea canadensis, Lemna
minor), algae (Scenedesmus subspicatus) and snails (Physa sp.)
were exposed to 500 or 1000 mg/litre for 21 days. At 1000 mg/litre a
decrease in oxygen content of the water was observed, together with
mortality in snails and algae, as well as necrosis on fronds of Lemna
minor. The photosynthesis of the plants was inhibited. These effects
were less at 500 mg/litre, but this concentration was still lethal to
snails. In outdoor mesocosms, containing a large diversity of species,
initial concentrations of 137-156 mg/litre did not induce any toxicity
(Merlin et al., 1992).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity data
See Table 17.
8.1.2 Oral administration
Musculoskeletal disturbances were found in Sprague-Dawley rats at
doses of 530 mg/kg or more. Hypotension, hypothermia and haematuria
were also noted (dose threshold not reported). The gastrointestinal
tract was found to be congested with micro-haemorrhages or partial
destruction from doses of 530 mg/kg or more. Blood CO-Hb increased in
a dose-related manner (Laham, 1978).
Various effects have been reported following the acute
administration of large oral doses of methylene chloride. The effects
include a decreased cytochrome P-450 content in liver microsomes of
Sprague-Dawley rats receiving 1000 mg/kg (Moody et al., 1981), CNS
effects and evidence of pathological changes in the liver and kidney
of Wistar rats receiving 2000 mg/kg (Janssen & Pott, 1988a), evidence
of liver necrosis and increased glucose-6-phosphatase activity in male
rats (strain unspecified) receiving 2210 mg/kg (Reynolds & Yee, 1967),
and decreased hepatic secretion of triglycerides followed by an
increased hepatic triglyceride content in male mice receiving
2700 mg/kg (Selan & Evans, 1982).
No liver toxicity was found in male Wistar rats receiving up to
4400 mg/kg by oral administration (Danni et al., 1981).
Liver damage was investigated in Sprague-Dawley rats exposed to up
to 1275 mg/kg given orally. Serum ALT activity was unaffected, as was
liver cytochrome P-450 and glutathione content. However, increased
ornithine decarboxylase activity and DNA damage were found in the
liver (Kitchin & Brown, 1989).
8.1.3 Inhalation administration
8.1.3.1 Rat
Behavioural changes and CNS disturbances were found in several
studies. Decreased running activity was found in rats exposed to
17 700 mg/m3 for 1.5 h (Heppel & Neal, 1944); hypothermia,
hypotension and convulsion in Sprague-Dawley rats from 28 200 mg/m3
(6-h exposure) (Laham, 1978); CNS depression in Alderley-Park rats at
31 800 mg/m3 (10-min EC50) (Clark & Tinston, 1982) and in rats at
40 000 mg/m3 (2-h exposure) (Ulanova & Yonovskayo, 1959); and
dyspnoea and anaesthesia in rats from 53 000 mg/m3 (30-min exposure)
(Schumacher & Grandjean, 1960; Kashin et al., 1980).
Table 17. Acute toxicity of methylene chloride
Species Route Vehicle Parameter Concentration Reference
Rat (Wistar, male) oral none LD50 1710-2250 mg/kg Klimmer (1988)
Rat CDF (F-344) oral none LD50 1530-2524 mg/kg Carreon (1981)
Rat (Sprague-Dawley) oral none LD50 2120 mg/kg Kimura et al, (1971)
young male
Rat (Sprague-Dawley)
- male oral none LD50 2280 mg/kg Laham (1978)
- female oral none LD50 1410 mg/kg
Mouse (CF-1, male) oral unknown LD50 1987 mg/kg Aviado et al. (1977a,b)
Dog oral mucillage of LD50 3000 mg/kg Barsoum & Saad
acacia (1934)
Rat (Alderley Park) inhalation 15-min LC50 197 790 mg/m3 Clark & Tinston (1982)
Rat (Sprague-Dawley) inhalation 6-h LC50 52 000 mg/m3 Bonnet et al. (1980)
male
Rat (Sprague-Dawley) inhalation 6-h LC50 > 28 000 mg/m3 Landry et al, (1981)
Mouse (CF-1, male) inhalation 20-min LC50 92 680 mg/m3 Aviado et al. (1977a,b)
Mouse (LF1, female) inhalation 6-h LC50 49 100 mg/m3 Gradiski et al, (1978)
Mouse (ICR, male) inhalation 6-h LC50 55 870 mg/m3 Scott et al. (1979)
Guinea-pig inhalation 6-h LC50 40 200 mg/m3 Balmer et al. (1976)
Table 17 (Cont'd)
Species Route Vehicle Parameter Concentration Reference
Rat (Sprague-Dawley) intratracheal ALDa 350 mg/kg McCarty et al. (1992)
male
Mouse (CF-1, male) intraperitoneal unknown LD50 448 mg/kg Aviado et at. (1977a,b)
Mouse intraperitoneal unknown LD50 500 mg/kg Schumacher &
Grandjean (1960)
Mouse (Swiss-Webster, intraperitoneal corn oil LD50 1990 mg/kg Klaassen & Plaa (1966)
male)
Dog intraperitoneal corn oil LD50 1260 mg/kg Klaassen & Plaa (1967)
Mouse subcutaneous olive oil LD50 6500 mg/kg Kutob & Plaa (1962)
a "ALD = Approximate Lethal Dose, the lowest dose causing death within 3 days
Studies on sleeping patterns in Wistar rats by measuring
electroencephalographic (EEG) and electromyographic (EMG) activity
have shown a dose-related increase in total sleeping time and
intervals between rapid eye movement (REM) sleep when the rats were
exposed to 1770, 3500 or 10 600 mg/m3 (500, 1000 or 3000 ppm)
methylene chloride for 3 h. During exposure to 3500 or 10 600 mg/m3,
the percentage of sleep defined as REM decreased by nearly 20% (Fodor
& Winneke, 1971; Fodor et al., 1973). Studies using similar techniques
on the narcotic effects of methylene chloride in rats (strain
unspecified) have been reported by Berger & Fodor (1968). Rats were
exposed to a range of concentrations of methylene chloride from 9900
to 99 000 mg/m3 (2800 to 28000 ppm) for unspecified periods of time.
There was an initial period of excitation followed by deep narcosis
with a decrease in muscle tone and EEG activity and subsequent
breathing difficulties. Cessation of electrical activity was noted
after a 1.5-h exposure to 88 000 or 99 000 mg/m3 (25 000 or
28 000 ppm) and after a 6-h exposure to 54 000 or 64 000 mg/m3
(16000 or 18000 ppm). Following exposure to 17 700 to 31 800 mg/m3
(5000 to 9000 ppm) methylene chloride, long periods of sleep occurred
without desynchronization phases. Following exposure to concentrations
of methylene chloride below 17 700 mg/m3 (5000 ppm), there were no
measurable effects on either EEG or EMG activity (Berger & Fodor,
1968).
Exposure of F-344 rats to 7100 mg/m3 for 2.5 h caused
statistically significant changes in somatosensory evoked responses
and EEG. The lack of effect of 157 mg carbon monoxide/m3 (which
induces a CO-Hb level of 10%, comparable with that produced by
exposure to 7100 mg methylene chloride/m3) on evoked responses,
indicated that the effects were probably due to methylene chloride
itself and not to its principal metabolite carbon monoxide (Mattsson
et al., 1988). Alterations in somatosensory evoked potentials were
also observed after a 1-h exposure of F-344 rats to dose levels of
17 700 mg/m3 or more (Rebert et al., 1989).
No macroscopic lesions were found in rats at the 6-h LC50 of
53 000 mg/m3 (Bonnet et al., 1980). Congestion of various organs, as
well as oedema of the brain, heart, lungs and hip region, was noted
following exposure to 71 000 mg/m3 (6-h exposure) in Sprague-Dawley
rats (Laham, 1978).
Increased CO-Hb levels were found from 1770 mg/m3 in various
strains of rats (MacEwen et al, 1972; Laham, 1978; Dill et al., 1978;
Kurppa et al., 1981). Ascorbic acid content of the liver was increased
in rats exposed to 40 000 mg/m3 for 2 h (Ulanova & Yonovskayo,
1959), but no effect on cytochrome P-450 or specific liver enzymes was
noted in Wistar rats after a 3-h exposure to 3500 mg/m3 or in
Sprague-Dawley rats after a 5-min (repeated 5 times) exposure to
350 mg/m3, except for increased microsomal and decreased lysosomal
ß-glucuronidase activity (Kurppa et al., 1981).
Intratracheal administration of methylene chloride in Sprague-
Dawley rats showed lethal levels at 350 mg/kg (corresponding to 17.5%
of the oral LD50), death occurring in a few seconds. This result
emphasises that aspiration of methylene chloride may present more of a
hazard than oral ingestion (McCarty et al., 1992).
8.1.3.2 Mouse
CNS depression resulting in reversible narcosis was reported in
mice exposed to between 14 100 and 52 200 mg/m3 methylene chloride
for 2-6 h (Flury & Zernik, 1931) or to 47 700 mg/m3 for 128 min
(EC50) (Kashin et al., 1980). This effect was also noted in CF-1
mice exposed to 35 300 mg/m3 for 20 min (Aviado et al., 1977a,b) or
in Swiss mice exposed to 45 900 mg/m3 for 7 h (Svirbely et al.,
1947). Exposure at 35 000 mg/m3 for 2 h was the minimal CNS
effective concentration found in the mouse (Lazarew, 1929).
Ability to learn a simple passive avoidance task, 1-4 days after
exposure, was reduced in Swiss-Webster mice exposed to 168 000 mg/m3
for 20 seconds (Alexeef & Kiglore, 1983).
Fatty changes were noted in the liver and, less frequently, in the
kidney from 56 500 mg/m3 after a 7-h exposure (Svirbely et al.,
1947). Microscopic lesions in the liver, kidneys, lungs and adrenal
were found (no dose-response relationship) in mice exposed to lethal
doses for 6 h (Gradiski et al., 1978).
Cardiac sensitization to the effects of adrenaline was reported in
Swiss mice exposed to 710 000 mg/m3 for 6 min) (Aviado & Belej,
1974).
Male NMRI mice were exposed to methylene chloride by inhalation in
a study of tolerance, i.e. decreased responsiveness to a chemical that
arises as a result of previous exposure to the same chemical
(Kjellstrand et al., 1990). Motor activity measured with Doppler radar
units was used to monitor the behavioural reactions of the animals.
Motor activity increased on exposure to methylene chloride and
decreased on constant exposure. Termination of exposure was followed
by hypoactivity.
8.1.3.3 Other animals
After 6 h of exposure to 17 700 mg/m3, the concentration of
triglycerides was increased in the liver of guinea-pigs, and reduced
in the serum (Bulmer et al., 1976; Morris et al., 1979).
Histopathological liver changes, consisting of the appearance of lipid
droplets, were first seen in guinea-pigs at 17 700 mg/m3 (Morris et
al., 1979). Slight to moderate vacuolation in the liver of guinea-pigs
was seen after a 6-h exposure to 38 800 mg/m3. In addition, lungs
showed congestion and haemorrhage. Behavioural changes were also noted
(Balmer et al., 1976).
No effect on blood pressure, heart rate or EEG activity was found
in rabbits exposed to 90 000 mg/m3 for 2 h (Truhaut et al, 1972).
Only serum AST was significantly increased.
In Beagle dogs, ECG changes as well as decreased blood pressure,
heart and respiratory rate were found at 141 200 mg/m3 (7-h
exposure). Behavioural effects were already noted after a 1-h exposure
to 53 000 mg/m3 (reduced reflexes) (Von Oettingen et al., 1950).
Increased CO-Hb values, but no change in haematocrit, haemoglobin
concentration or red cell count, were reported in dogs and rhesus
monkeys exposed for 24 h to 17 700 mg/m3. Slight pathological
changes in the liver (fatty changes, vacuolization) could be
attributed to the treatment (MacEwen et al., 1972).
Cardiac effects such as arrhythmia, tachycardia and hypotension
were found in monkeys, dogs and rabbits exposed for 1-5 min to levels
of methylene chloride exceeding 35 300 mg/m3 (Belej et al., 1974;
Adams & Erickson, 1976; Taylor et al., 1976; Aviado et al., 1977a,b;
Aviado, 1978).
One study on rabbits showed allergic reactions after inhalation
(Shmuter & Kashin, 1978). However, the experimental protocol of this
study is questionable and the result has not been confirmed.
8.1.4 Dermal administration
No effect was noted on rats receiving a dermal application of
methylene chloride of 710 mg/kg for 0.5 to 4 h, except for an increase
in CO-Hb levels (Makisimov & Mamleyeva, 1977). Only slight behavioural
effects and macroscopic changes in the liver (swelling) were found in
Wistar rats receiving 2000 mg/kg under an occlusive dressing for 24 h
(Janssen & Pot, 1988b). Haemoglobinuria was observed in rats when
abdominal skin was immersed in methylene chloride for 2-20 min
(Schutz, 1960).
8.1.5 Intraperitoneal administration
A single intraperitoneal injection of 510 mg methylene chloride/kg
in rats slowed down the sciatic motor conduction velocity by 11% and
gave rise to a CO-Hb level of 6.8% (Pankow et al., 1979).
Signs of CNS depression were found in Wistar rats and CFI mice
receiving, respectively, 1060 mg/kg (with phenobarbital pretreatment)
and 114 mg/kg (Aviado et al., 1977; Masuda et al., 1980).
Various biological investigations in rats and mice showed a dose-
related increase in serum AST and/or ALT activity at > 660 mg/kg.
No effect was observed on glucose-6-phosphatase activity, cytochrome
P-450 content or BSP retention (Klaassen & Plaa, 1966; Cornish et al.,
1973; Masuda et al, 1980; Corsi et al., 1983). Increased ALT activity
was reported in mongrel dogs receiving 800 mg/kg in corn oil (Klaassen
& Plaa, 1967). No histological changes in the liver could be found in
rats exposed to < 1300 mg/kg (Cornish et al., 1973; Corsi et al.,
1983). However, mild hepatic inflammation was noted in Swiss Webster
mice receiving lethal doses of methylene chloride (2000 mg/kg)
(Klaassen & Plaa, 1966). Renal tubular changes were noted in F-344
rats administered intrapertinoneally with 1330 mg/kg in corn oil
(Kluwe et al., 1982) and in Swiss-Webster mice receiving 2000 mg/kg in
corn oil (Klaassen & Plaa, 1966). However, no renal histological
change could be found in Swiss mice receiving 1300 mg/kg in corn oil
(Plaa & Larson, 1965). Slight histological changes were noted in the
liver and kidneys of mongrel dogs receiving 1300 mg/kg (Klaassen &
Plaa, 1967).
8.1.6 Intravenous administration
The minimum lethal concentration in anaesthetized dogs was found
to be 200 mg/kg in olive oil after intravenous administration (Barsoum
& Saad, 1934).
Methylene chloride shortened the duration of nystagmus induced by
rotation in Sprague-Dawley rats during intravenous perfusion of
5.1 mg/kg per min for 60 min (Tham et al., 1984).
Following a single intravenous injection of 3.1, 6.2 or
12.4 mmol/kg, methylene chloride was found to sensitize the myocardium
of rats to arrhythmia development in response to catecholamines. The
release by methylene chloride of endogenous catecholamines is possibly
a cause of these modified cardiovascular actions (Mueller et al.,
1991).
8.1.7 Subcutaneous administration
The minimum lethal concentration in the rabbit was found to be
2700 mg/kg (Barsoum & Saad, 1934). Prolongation of phenobarbital-
induced sleeping time occurred at 1700 mg/kg in Swiss mice. No effect
on liver function or histology was observed at up to 5000 mg/kg (Kutob
& Plaa, 1962).
8.1.8 Appraisal
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species lie between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg have been recorded. Acute effects after methylene
chloride administration by various routes of exposure are primarily
associated with the central nervous system (CNS) and the liver. CNS
disturbances were found at 14 100 mg/m3 or more and slight changes
in EEG at 1770 mg/m3. Slight histological changes in the liver
were found at concentrations of 17 700 mg/m3 or more. In the mouse,
but not in the rat, effects on the lungs restricted to the Clara
cells were observed after exposure to 7100 mg/m3. Occasionally the
kidney is affected. Cardiac sensitization to adrenaline-induced
arrhythmias has been reported, and cardiovascular effects were seen
at concentrations above 35 000 mg/m3. However, the effects were
inconsistent.
8.2 Short-term exposure
8.2.1 Oral administration
CD1 mice given doses of up to 665 mg/kg per day of methylene
chloride in corn oil by gavage for 14 days did not show any effect on
liver enzymes. Microscopic examination revealed slight vacuolation in
the liver from 333 mg/kg. No damage to the kidneys was reported
(Condie et al., 1983).
8.2.2 Subcutaneous administration
Reduction of the systolic blood pressure of hypertensive Sprague-
Dawley rats was reported after subcutaneous exposure to 2000 mg/kg
twice a week for 17 weeks. No effect was found in normotensive rats.
Very slight histopathological changes in the liver and lungs of
hypertensive rats were described (Loyke, 1973).
8.2.3 Inhalation administration
8.2.3.1 Rat
Changes in Sprague-Dawley rats were reported after exposure to
methylene chloride (3500 mg/m3) 2 h/day for 15 days; decreased body
weight, increased hepatic lipid peroxidation, and high concentrations
of methylene chloride in the brain, kidney and blood immediately after
inhalation were observed (Ito et al., 1990). Similar hepatic effects
(hypertrophic hepatocytes, and increased lipid peroxidation and
glutathione peroxidase activity) were reported in male Wistar rats
exposed to 3500 mg/m3 2 h/day for 20 consecutive days (Takashita et
al., 1991). Biochemical tests were performed on the serum and brain of
groups of six male Sprague-Dawley rats which were exposed to 250, 1100
or 3500 mg/m3 6 h/day for 3 days and killed 16-18 h later. A
selective reduction in dopamine concentration, with changes in
dopamine turnover in some forebrain dopamine nerve terminal systems,
was reported. A dose-dependent increase in noradrenaline turnover in
the anterior periventricular hypothalamic area and dose-dependent
decreased noradrenaline concentration in the posterior periventricular
area were observed. No significant changes were reported in the
secretion of anterior pituitary hormones (Fuxe et al., 1984).
Groups of five male and five female F-344 rats were exposed to
methylene chloride at concentrations of 5740, 11 500, 22 900, 45 900
or 56 500 mg/m3, 6 h/day for 19 days. Intermittent scratching,
ataxia and hyperactivity were seen in all rats exposed to
22 900 mg/m3 or more. Dyspnoea and anaesthesia were observed in
animals exposed to 45 900 mg/m3 or more. Some deaths were also
observed at these concentrations (NTP, 1986).
No, or limited, lesions were found in the liver and lungs of F-344
rats, exposed to 7100 or 14 100 mg/m3, 6 h/day for 10 days, after
light and electron microscopic examination (Hext et al., 1986). No
effect on the liver was found in rats (strain unspecified) after
inhalation of 880 mg/m3, for 5 h/day for 28 days (Norpoth et al.,
1974)
Inhalation exposure of Sprague-Dawley rats to methylene chloride
concentrations of 12 800 mg/m3 (5 h/day, 5 days/week) for 4 weeks
revealed an inflammatory response and cell damage in the lungs as
demonstrated by the increase biochemical response (enzymatic and non-
enzymatic) observed in cell-free lavage effluents from the lungs (Sahn
& Lowther, 1981).
8.2.3.2 Other animals
Increased liver weight and increased mitotic activity in
hepatocytes were observed in male B6C3F1 mice after 2 weeks of
repeated exposure to 14 100 mg/m3 6 h/day for up to 3 weeks
(Eisenbrandt & Reitz, 1986). Necrosis of occasional epithelial cells
in the bronchi and bronchioles, together with reactive hyperplasia of
adjacent lymphoid tissue, was observed in a few animals.
Groups of five male and five female B6C3F1 mice were exposed to
methylene chloride at concentrations of 5740, 11 500, 22 900, 45 900
or 56 500 mg/m3, 6 h/day for 19 days. Hyperactivity (dose-related)
was seen in exposed mice, but no exposure-related pathological
findings were observed. Some deaths occurred in mice exposed to
45 900 mg/m3 or more (NTP, 1986).
CD-1 mice, Golden Syrian hamsters, Sprague-Dawley and CDF (F-344)
rats were exposed to 0, 8800, 17 700 or 28 200 mg/m3, 6 h/day,
5 days/week, for a total of 19, 18, 19 or 7 exposures, respectively,
over a period of 21 to 28 days. Animals exposed to 28 000 mg/m3
showed anaesthetic effects and a decrease in the body weight of rats.
At 18 000 mg/m3 there was slight anaesthesia, decreased body weight
in male rats, increased aspartate aminotransferase (ASAT) in female
mice and Sprague-Dawley rats, and increased liver weights in female
mice, hamsters and rats. At 8800 mg/m3, the animals exhibited more
scratching activity than the controls, but showed no other effects
attributable to exposure (Nitschke et al., 1981)
Following exposure to 17 700 mg/m3, a reduction in body weight
of mice was observed, and relative liver weights were increased up to
the end of the 7-days of continuous exposure. Fatty infiltration, an
increase in the triglyceride concentration and hydropic degeneration
of the endoplasmic reticulum gradually disappeared. Protein synthesis
was depressed. Necrosis was observed in a few hepatocytes (Weinstein
et al., 1972).
Carboxyhaemoglobin levels were raised after continuous exposure of
monkeys to 88.25 mg/m3 (25 ppm) for 28 days (MacEwen & Vernot, 1972;
Haun et al., 1972).
A group of NMRI mice was continuously exposed to 130-1059 mg/m3
(37-300 ppm) for 30 days, while another was intermittently exposed for
1-12 h/day to 2118-25 416 mg/m3 (600-7200 ppm) for 30 days,
corresponding to an average exposure (24-h mean value) of 1059 mg/m3
(300 ppm). In addition, groups of mice were continuously exposed to
1059 mg/m3 for 4, 8, 15 and 90 days. The blood level of
butyrylcholinesterase was significantly increased from 265 mg/m3
(75 ppm) in continuously exposed male mice and after intermittent
exposure in male rats. Moreover, liver weight was significantly
increased in a dose-related manner from 265 mg/m3 (75 ppm) and
330 mg/m3 (150 ppm) in male and female mice, respectively. Finally,
fatty accumulation was found in both sexes at 265 mg/m3 (75 ppm) or
more. All effects were reversible (Kjellstrand et al., 1986). CO-Hb
levels were raised after continuous exposure of monkeys to
88.25 mg/m3 (25 ppm) for 28 days (MacEwen et al., 1972, Haun et al.,
1972).
8.3 Long-term exposure
8.3.1 Rat
8.3.1.1 Inhalation exposure
In a study by Leuschner et al. 1984, 20 male and 20 female
Sprague-Dawley rats were exposed to 35 g/m3, 6 h/day for 90 days.
Haematological, clinical chemistry and urinary parameters were
measured and histological examinations were performed. A slight
redness of the conjunctiva lasting for 1-10 h was observed after each
exposure. No other treatment-related signs of toxicity were reported.
Groups of 10 male and 10 female F-344 rats were exposed to
methylene chloride at concentrations of 1850, 3700, 7400, 14 800 and
29 700 mg/m3 for 6 h/day, 5 days/week for 13 weeks. One male and one
female rat exposed to 29 700 mg/m3 died before the end of the study,
whereas none of the control rats died. Foreign body pneumonia was
observed in some rats exposed to > 7410 mg/m3. The mean body
weight in males and females exposed to 29 700 mg/m3 was lower than
in controls. Liver lipid to liver weight ratios were statistically
significantly reduced in both males and females exposed to
29 700 mg/m3 and in females exposed to 14 800 mg/m3 when compared
to controls (NTP, 1986).
Male and female F-344 rats were exposed to 177, 710 or
7100 mg/m3 6 h/day, 5 days/week for 13 weeks. No treatment-related
alterations in sensory evoked potentials (flash, auditory brainstem,
somatosensory or caudal nerve) or neuropathology were observed at any
of the exposure levels (Mattsson et al., 1990).
CNS depression was found in rats during each daily session of
repeated exposure to 35 000 mg/m3 7 h/day, 5 days/week for 6 months
(Heppel et al., 1944).
Rats (sex and strain unspecified) were continuously exposed to
either 88 or 350 mg/m3 for 100 days. Slight cytoplasmatic
vacuolization with positive fat stains in the liver and tubular
degeneration in the kidney were observed (Haun et al., 1972).
8.3.1.2 Oral exposure
Rats receiving methylene chloride in the drinking-water at a
concentration of 125 mg/litre for 13 weeks did not show any effects on
behaviour, body weight, haematology, urinalysis, blood glucose level,
plasma free fatty acids, or the oestrous cycle (Bornmann & Loeser,
1967).
Groups of 20 male and 20 female Fischer-344 rats were given 0.15,
0.45, and 1.50% methylene chloride in drinking-water for 3 months,
equivalent to 166, 420 and 1200 mg/kg per day, respectively, for males
and 209, 607, and 1469 mg/kg per day for females. Slightly decreased
body weights were observed in mid-dose males and high-dose females
throughout the study. There were no differences between treated and
control animals with regard to mortality, physical observations, food
consumption or gross necropsy results. There were no exposure-related
effects observed following the histopathological evaluation of rat
tissues from the 1 month interim necropsies. However, hepatocellular
changes were observed following treatment for 3 months; central
lobular necrosis, granulomatous foci, ceroid or lipofuscin
accumulation, and cytoplasmic eosinophilic bodies were observed in
high-dose males and females and in some mid-dose females. A dose-
dependent increased incidence of hepatocyte vacuolation was also
observed, many of the vacuoles containing lipid which was generalized
or concentrated in the central lobular region (Kirschman et al.,
1986).
8.3.2 Mouse
8.3.2.1 Inhalation exposure
Groups of 10 male and 10 female B6C3F, mice were exposed to
methylene chloride at concentrations of 1850, 3700, 7400, 14 800 or
29 700 mg/m3 for 6 h/day, 5 days/week for 13 weeks. Exposure-related
deaths were seen in some mice exposed to 29 700 mg/m3. Hepatic
centrilobular hydropic degeneration was observed in males and females
exposed to 29 700 mg/m3 and in females exposed to 14 800 mg/m3.
Both mean body weights and liver lipid to liver weight ratios were
reduced in males and females exposed to 29 700 mg/m3 when compared
to controls (NTP, 1986).
Mice (strain and sex unspecified) were continuously exposed to
either 88 or 350 mg/m3 for 100 days. Slight cytoplasmatic
vacuolization was found at both dose levels, and a decrease in the
microsomal cytochrome P-450 content was found in the liver of mice
exposed to methylene chloride at 350 mg/m3 (Haun et al., 1972).
Female ICR mice were continuously exposed to 350 mg/m3 for 10
weeks. Fatty infiltration, vacuolization and enlarged hepatocyte
nuclei persisted up to the end of the exposure period. A reversible
increase in plasma triglycerides was also observed (Weinstein &
Diamond, 1972).
8.3.2.2 Oral exposure
Groups of 20 male and 20 female B6C3F1 mice were given 0.15,
0.45 and 1.50% methylene chloride in drinking-water for 3 months,
equivalent to 226, 587 and 1911 mg/kg per day, respectively, for males
and 231, 586 and 2030 mg/kg per day for females. Slightly lower body
weights were observed in mid-dose and high-dose males and in females
from week 6 to the end of the study. Treated and control animals did
not differ with respect to physical and ophthalmological observations
or food consumption. There were no exposure-related effects observed
following the histopathological evaluation of mice following a 1-month
exposure. However, after 3 months of exposure, subtle centrilobular
fatty changes in the liver were observed, these being most prominent
in mice receiving either 587 or 1911 mg/kg per day. No other exposure-
related changes were reported (Kirschmann et al., 1986).
8.3.3 Other animals
Heppel et al. (1944) did not find organ lesions related to
exposure at 17 700 mg/m3 (7 h/day, 5 days/week for 6 months) in
studies on dogs, monkeys, rats, rabbits and guinea-pigs, with the
exception of moderate centrilobular fatty degeneration of the liver
and pneumonia in 3 out of 14 guinea-pigs. CNS depression was found in
all species following exposure to 35 000 mg/m3; all animals became
inactive, sometimes after initial excitement. At 35 000 mg/m3, dogs
also showed fatty degeneration of the liver.
Hepatic changes (slight cytoplasmatic vacuolation) and vacuolar
changes in the renal tubules were found in dogs exposed continuously
to 3500 mg/m3 for up to 100 days. Abnormal haematology and increased
activity of serum enzymes were reported after 4 weeks. Oedema of the
brain was observed at a concentration of 17 350 mg/m3 (Haun et al.,
1972).
Three male and three female beagle dogs were exposed to
17 700 mg/m3, 6 h/day for 90 days. Haematology, clinical chemistry
and urinary parameters were measured and ECG and circulatory functions
were examined. At the end of the study, histological examinations were
performed. Slight sedation was induced throughout the exposure period
and all dogs had slight erythema, lasting up to 10 h after exposure.
No deaths and no other signs of toxicity were observed (Leuschner et
al., 1984).
Decreased levels of neurotransmitter amino acids were observed in
gerbil brains after continuous inhalation exposure to methylene
chloride (340 mg/m3) for 3 months (Briving et al., 1986). In gerbils
exposed by inhalation to 1240 mg/m3 for 3 months, followed by a 4-
month solvent-free period, increased brain concentrations of two
astroglial proteins and decreased levels of DNA in the hippocampus and
cerebellum were observed (Rosengren et al., 1986). Decreased
hippocampal DNA levels were also observed in gerbils exposed to
740 mg/m3 (Rosengren et al., 1986; Karlsson et al., 1987). It was
suggested by the authors that this effect may have been the result of
the loss of nerve cells.
8.3.4 Appraisal
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3 ) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice at
> 17 700 mg/m3 . After intermittent exposure, effects on the liver
were observed in rats at 3500 mg/m3 and in mice at 14 100 mg/nz3 .
After continuous exposure, slight cytoplasmatic vacuolization in the
liver of both rats and mice were found at 88 and 350 mg/m3.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations of < 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver with a no-observed-effect level of 125 mg/m3.
8.4 Skin and eye irritation; sensitization
8.4.1 Skin irritation
Application of 0.5 ml methylene chloride to rabbits for 24 h,
under a semi-occlusive patch on abraded or intact skin, caused severe
erythema and oedema with necrosis and acanthosis (Duprat et al.,
1976). Rabbits exposed to 0.5 ml methylene chloride for 4 h under
occlusive patch test condition, either with or without simultaneous
exposure to other chlorinated solvents, showed moderate skin
irritation but no corrosive effect (Van Beek, 1990).
8.4.2 Eye irritation
Duprat et al. (1976) and Ballantyne et al. (1976) exposed rabbits
once to 0.1 ml methylene chloride by ocular instillation. Moderate to
severe changes were seen in the conjunctiva, together with increased
corneal thickness and intra-ocular tension. All effects were
reversible. Vapour exposure of the eyes to 17 700 mg/m3 caused
slight increases in corneal thickness and intra-ocular tension.
8.4.3 Sensitization
No data are available.
8.4.4 Appraisal
Liquid methylene chloride is moderately irritant to the skin and
eyes in experimental animals.
8.5 Developmental and reproductive toxicity
8.5.1 Developmental toxicity
When groups of Sprague-Dawley rats and Swiss-Webster mice were
exposed to methylene chloride at a concentration of 4400 mg/m3 on
days 6-15 of pregnancy for 7 h/day, maternal body weight in the mice
was increased and the dams of both rats and mice had CO-Hb levels as
high as 12.5% during exposure. In both species, an increased incidence
of minor skeletal anomalies was observed, i.e. dilated renal pelvis in
rats and extra sternebrae in mice (Schwetz et al., 1975). No
significant teratogenic or fetotoxic effects were observed in either
species.
Groups of 18 rats were exposed before and/or during 17 days of
pregnancy to a methylene chloride concentration of 16 250 mg/m3 for
6 h/day. The exposed dams exhibited increased blood CO-Hb levels,
ranging from 7.1 to 10.1%, and increased relative and absolute liver
weights. Fetal body weight was decreased, but no increase in the
incidences of dead fetuses and/or resorptions nor any skeletal and/or
visceral malformations were observed (Hardin & Manson, 1980). After
exposure to methylene chloride using the same experimental conditions,
litters from four groups of 10 rats were used for behavioural testing.
Body weight gain, food and water consumption, wheel running activity
and avoidance learning were all unaffected by the exposure. However,
changes in the general activity of pups were found in both sexes
starting at the age of 10 days, and were still present in male
offspring at the age of 150 days. The effects cannot be definitely and
directly attributed to methylene chloride, however, since elevated
maternal CO-Hb- or methylene chloride-induced changes in maternal-
litter interactions could have been contributing factors (Bornschein
et al., 1980).
Groups of seven female Wistar rats were fed methylene chloride at
levels of 0.04, 0.4 and 4.0% in their diet from days 0-20 of
pregnancy. Fetuses were examined on day 20 and neonatal growth was
measured for 8 weeks after birth. Maternal body weight was
significantly reduced in the 4.0% group. Although a reduction in the
fetal weight of the females in the 0.4% group was observed, there were
no differences in any group in the number of implantations and
resorptions. No external malformations were observed by fetal,
skeletal and visceral examination, and no differences were observed in
any group in the frequency of delayed ossifications or in the dilation
of the renal pelvis. A decrease in postnatal weight gain and in
absolute liver weight was found in the 0.04% group males at the 8th
week after birth (Nishio et al., 1984).
Groups of F-344 rats (number not specified) received methylene
chloride by gavage (dose level not specified) in corn oil on gestation
days 6- 15. The compound was tested with at least two dose levels plus
a concurrent control group, the high dose (not specified) being
selected to cause maternal toxicity. The dams were allowed to deliver
and their litters were examined post-natally. Although a small change
in maternal weight was found, no effects on litters were reported
(Narotsky et al., 1992).
8.5.2 Reproductive toxicity
When rats received methylene chloride in the drinking-water at a
level of 125 mg/litre during a period of 13 weeks before mating, no
effects were found on the female fertility index, litter size,
survival of pups at 4 weeks or the number of resorptions (Bornmann &
Loeser, 1967).
A two-generation inhalation study was conducted to evaluate the
effects of inhaled methylene chloride on the reproductive capability,
neonatal growth and survival of rats (Nitschke et al., 1988b). Groups
of 30 male and 30 female 6-week-old F-344 rats (F0) were exposed to
0, 350, 1770 or 5300 mg/m3 (6 h/day, 5 days/week for 14 weeks).
After this exposure, F0 animals were allowed to mate using one male
and one female of the respective treatment groups to produce the F1
litters. After weaning, 30 males and 30 females (4 weeks old) from
each treatment group were randomly selected and assigned to the
respective exposure groups. After exposure to the relevant
concentration of methylene chloride (6 h/day, 5 days/week for 17
weeks), the F1 adults were allowed to mate to produce F2 litters.
Reproductive parameters examined included fertility, litter size and
neonatal growth and survival. All adults and selected weanlings were
examined for grossly visible lesions. No adverse effects on
reproductive parameters, neonatal survival or neonatal growth were
noted in animals exposed to methylene chloride in either the F0 or
F1 generations. Similarly, there were no treatment-related gross
pathological changes in F0 and F1 adults or F1 and F2
weanlings; histopathological examination of tissues did not reveal any
lesions in F1 and F2 weanlings attributable to exposure to
methylene chloride. Therefore, the results of this study indicate that
exposure to concentrations as high as 5300 mg/m3 does not affect the
normal reproductive function of rats (Nitschke et al., 1988b).
8.5.3 Appraisal
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3 . No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
was observed in three animal studies. Small effects on either fetal
or maternal body weights were reported at 4400 mg/m3 . A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations of up to 5300 mg/m3 ,
6 h/day, 5 days/week, did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
8.6 Mutagenicity and related end-points
Studies on the mutagenic potential of methylene chloride have been
performed on bacteria, fungi and cultured mammalian cells. Results
from in vivo studies on mice and rats have also been reported.
8.6.1 In vitro
8.6.1.1 Bacteria
Methylene chloride is mutagenic when tested using the Ames
protocol in Salmonella typhimurium TA98, TA100 and TA 1535
(Table 18). The number of revertants increased 3- to 7-fold in a dose-
related manner when plates were exposed to vapour of methylene
chloride of undisclosed purity at levels ranging from 20 100 up to
201 000 mg/m3. Metabolic activation by either induced rat liver S9
fraction, cytosol fraction, or microsomal fraction increased the
mutagenicity of methylene chloride (Simmon et al., 1977; Jongen et
al., 1978, 1982; McGregor, 1979; Kirwin et al., 1980; Barber et al.,
1980; Nestmann et al., 1980, 1981; Gocke et al., 1981; Dillon et al.,
1990). In this respect the cytosolic fraction was more active than the
microsomal fraction (Green, 1983). A positive result was reported in
strains TA98 and TA100 with and without 30% hamster liver S9 (Zeiger
et al., 1990) using the vapour phase (desiccator procedure) protocol.
Negative mutagenicity results were obtained in studies not using
vapour phase exposure (Rapson et al., 1980; Nestmann et al., 1980). No
mutagenic activity was found when methylene chloride was tested in
Salmonella typhimurium strains TA100, TA1535, TA1537, TA97 and TA98
with or without the addition of 10% or 30% rat/hamster liver S9, using
the preincubation protocol (Zeiger et al., 1990).
Metabolic studies of methylene chloride (see section 6.3) indicate
that the conjugation of methylene chloride with glutathione (GSH),
catalysed by cytosolic glutathione- S-transferase, may play a role in
the observed mutagenicity of methylene chloride in Salmonella.
However, the direct reaction of glutathione with methylene chloride
only produced a very small increase in mutagenicity (Jongen et al.,
1982). In another study, Salmonella typhimurium TA100 and the GSH-
deficient strain TA100 gsh were exposed to 0-5% methylene chloride
using a vapour phase protocol. The mutagenic response, with and
without Aroclor-induced rat-liver S9, microsomes or cytosol, was
marginally higher at the highest methylene chloride concentrations.
Salmonella typhimurium TA100 gsh was slightly less responsive than
TA100 at high doses in the absence of S9. This difference was not seen
in the presence of S9. The addition of exogenous GSH had only a small
effect on the mutagenic response in TA100 or TA100 gsh in the absence
or presence of S9. According to the authors, these data suggest that
if the interaction between methylene chloride and GSH is responsible
for the observed mutagenicity, it occurs at extremely low levels of
intracellular GSH and is not significantly affected by exogenous GSH
(Dillon et al., 1992).
The mutagenicity of methylene chloride has also been studied in a
variety of other microbial systems using a vapour phase protocol.
Mutagenic effects were observed in E. coli WP2uvrA and pKM101 which
were exposed to 0-5% methylene chloride using a vapour phase protocol
(Dillon et al., 1992). These strains of E. coli are deficient in
glutathione, containing approximately 25% of the level of glutathione
present in the strain TA100.
Table 18. In vitro mutagenicity assays
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100, ± 10% 100-10 000 -ve Preincubation protocol Zeiger et al.
typhimurium TA1535, or 30% µg//plate (1990)
TA1537, rat or
TA97, TA98 hamster
liver S9
Salmonella TA100, TA98 ± 30% 0.1-1.0 +ve Vapour protocol-dessicator Zeiger et al.
typhimurium rat or ml/chamber procedure (1990)
hamster
liver S9
Salmonella TA100, ±S9 -ve Standard plate-incorporation protocol Nestmann et al.
typhimurium TA1535, (1980)
TA1537,
TA98, TA1538
Salmonella TA100, +ve 0.5µl in open glass dish within a Nestmann et al.
typhimurium TA1535 desiccator; revertants: 2-fold (1980)
increase in TA1535; 6-fold in TA100
Salmonella TA1535, ± S9 up to 750/µl in +ve Active in strains TA98 and TA100 Gocke et al.
typhimurium TA100, a 9 litre (1981)
TA1538, desiccator
TA98, TA1537
Salmonella TA100 ±S9 0-1.4% +ve Significant increases in mutagenic Jongen et al.
typhimurium activity by addition of rat liver (1982)
cytosol fraction; marginal increases
by addition of microsomal fraction
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100, ±S9 0-5% for 2, 4, 6 +ve Vapour phase protocol. Data Dillon et al.
typhimurium 100gsh or 48 h suggest interaction between (1992)
(glutathione methylene chloride and GSH
deficient) responsible for the mutagenic
activity
Salmonella TA100 S9 2.8 v/v +ve Green (1983)
typhimurium
Salmonella TA100 -S9 0, 50-800/µl +ve Vapour phase protocol; dose-related Simmon et al.
typhimurium per 9 litre increase in the number of revertants, (1977)
desiccator with a mutation rate over 7-fold
(approx 18-318 higher than controls at 320 mg/m3;
mg/m3) for 7 h two experiments were conducted
Salmonella TA98, TA100 ±S9 0, 20, 100 to +ve Vapour phase protocol; S9 prepared Jongen et al.
typhimurium 201 000 mg per m3 from the livers of phenobarbital (1978)
(5 concentrations) pretreated rats; a dose-related
for 48 h increase of up to 5-8 fold (mean
value for 3 experiments) was seen,
slightly higher in the presence of
S9; toxicity was noted at the
highest dose level
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100 Not stated Vapour phase protocol; time-course Jongen (1984)
typhimurium study to evaluate the most
appropriate exposure time for
maximum differentiation of the
methylene chloride induced
reversion rates in the presence and
absence of a metabolic activation
system; the maximum differentation
was obtained following 4-6 h
exposure, and, consequently, an
exposure time of 6 h was used by
these workers in the study of Jongen
et al. (1982)
Salmonella TA100 ±S9 0 or 1 ml/9 litre +ve Study summarized in review, original Simmon &
typhimurium desiccator data were not available; vapour Kauhanen (1978)
(approx 390 mg phase control; S9) from livers of
per m3) for 6.5 Aroclor-pretreated rats; addition of
or 8 h S9 increased the mutation rate 1.5
fold; no further details are available
Salmonella TA98, TA100 ±S9 0,125, 250, 500 +ve Vapour phase control; S9 from livers Rapson et al.
typhimurium or 750/µl per of Aroclor pretreated rats; dose- (1980)
9 litre desiccator related increase in the mutation rate,
(0-293 mg/m3) with an approximate 10-fold increase
for 8 h for both strains at the highest dose
used; the presence of S9 resulted in
a slightly higher mutation rate
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA1535, Not stated +ve Same study, but no data were given
typhimurium TA1537, for the strains studied; there is no
TA1538 evidence for an independent
confirmatory experiment
Salmonella TA1535, 0.5 ml/desiccator -ve Vapour phase protocol; result Nestmann et al.
typhimurium TA1537, (volume unknown) doubling of revertants for strain (1980)
TA1538, TA1535 and a 6-fold increase for
TA98, TA100 strain TA100 when 0.5 ml methylene
chloride was added directly to the
culture rather than a seperate dish
Salmonella TA100 ±S9 0, 98 800, +ve Vapour phase control; S9 from livers Green (1983)
typhimurium 177 000, or of Aroclor pretreated rats; a dose-
297 000 mg/m3 related increase in the mutation rate
for 3 days was observed
0 or 98 800 +ve Vapour phase protocol; the presence
mg/m3 for 3 of S9 enhanced the mutation rate
days 1.19 fold; on dividing the S9 material
into microsomal and high-speed
supernatant (cytosolic) fractions, only
the high-speed supernatant enhanced
(1.27 fold) the mutation rate;
a small isotope effect was observed
when 2H-methylene chloride was
substituted for 1H-methylene chloride
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Direct examination of the metabolism
of methylene chloride by the
bacterium indicated that radio-
labelled carbon dioxide and trace
amounts of radiolabelled carbon
monoxide were formed, together
with considerable incorporation of
radioactivity into endogenous
materials
Salmonella TA 1535 0, 24 700, +ve Vapour phase protocol; data McGregor (1979)
typhimurium 42 400, 81 200, available in summary form only;
162 400 or dose-response relationship; TA100
331 800 mg per m3; was reported to give a more marked
condensation response
of methylene
chloride onto
agar plates
occurred
Salmonella TA1535, ±S9 0, 38, 76, 96 or +ve Vapour phase protocol data available Barber et al.
typhimurium TA98, TA100 115 µmol/plate in summary form only; dose-response (1980)
(0-38 500 mg relationship (source of S9 not
per m3 vapour) stated) (negative results were
in gas-tight obtained in studies not employing
chambers gas-tight jars)
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA1535, +S9 Concentration +ve Vapour phase protocol; study Longstaff et al.
typhimurium TA100 range not reported only briefly, methylene (1984)
stated; exposure chloride being used as a positive
for 72 h control; S9 prepared from livers of
Aroclor pretreated rats. Mutation rate
increased 6-fold for TA1535 (with a
50% methylene chloride-air mixture)
and 2.4 fold for TA100 (with a 1%
methylene chloride-air mixture); the
increased mutation rate was
reproducible; apparently, one strain
at least showed a positive dose
response
Salmonella TA100 0.1-1000µg -ve Plate-incorporation assays; Rapson et al.
typhimurium per plate (5 insufficient information was given to (1980)
concentrations), evaluate the result
one plate per
concentration
Salmonella TA1535, ± S9 Up to 26 -ve Plate-incorporation assays; S9 from Nestmann et al.
typhimurium TA1537, mg/plate, livers of Aroclor-induced rats; tested (1980)
TA1538, dissolved in to limit of toxicity; positive results
TA98, TA100 dimethyl-sulfoxide for 3 pro-mutagens were obtained using
the same batch of S9, but not
necessarily in parallel incubations; a
second, independent assay was
conducted over a limited concentration
range; this was not a satisfactory
demonstration of a negative
response
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Escherichia WP2uvrA, ±S9 0-5% for 2, 4, 6 +ve Vapour phase protocol; data suggest Dillon et al.
coli pKM101 or 48 h interaction between methylene (1992)
chloride and GSH responsible for the
mutagenic activity
Microscreen E. coli WP2S ±S9 0.78-100 +ve Rossman et al.
( ) µl/well (1991)
Saccharomyces D7 0-209 mM +ve Induced mitiotic gene convertants Callen et al.
cerevisiae and recombinants, and, to a lesser (1980)
extent, gene revertants
Aspergillus Diploid P1 0-0.8 v/v +ve Crebelli et al.
nidulans (1988, 1992)
Sister Human +ve Thilagar et al.
chromatid peripheral (1984a,b)
exchange lymphocytes,
CHO cells,
mouse
lymphoma
L5178Y cells
Sister Chinese ±S9 0-5000 µg/ml -ve Standard (25-29 h after treatment) Anderson et al.
chromatid hamster harvest time (1990)
exchange ovary (CHO)
cells
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Sister CHO cells ±S9 0-15 µl/ml -ve Thilagar &
chromatid Kumaroo (1983)
exchange
Sister Chinese 0-4% ± Marginal (< 2-fold), reproducible Jongen et al.
chromatid hamster V79 increases in frequency; not dose- (1981)
exchange cells related
Chromosome Human Not stated +ve Thilagar et al.
aberration peripheral (1984a,b)
lymphocytes,
CHO; mouse
lymphoma
L5178Y cells
Chromosome CHO cells ±S9 0-15 µl/ml +ve Dose-dependent increase Thilagar &
aberration Kumaroo (1983)
Chromosome CHO cells ±S9 0-5000 µg/ml -ve Standard (10-14 h after treatment) Anderson et al.
aberration harvest time (1990)
Cells in vitro Chinese 0.5-5% v/v -ve Forward mutation to 6-thioguanine Jongen et al.
hamster cells resistance; mutation rate was (1981)
corrected for survival
Cells in vitro Epithelial -S9 0, 35 (300- -ve Varying the expression time was Jongen et al.
cells (V79) 141 000 mg/m3 reported to have no effect; cell (1981)
(4 concentrations survival was reduced by about 20%
for 1 h); expression at 141 000 mg/m3
time of 6 days
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Cell mutation L5178Y Not stated -ve Thilagar et al.
mouse (1984a,b)
lymphoma
Cell mutation L5178Y ±S9 0-3000 µl/ml ± Overall questionable evaluation of Myhr et al.
mouse activity (1990)
lymphoma
Cell Primary 0.5 ml/4.6 litre +ve Enhanced transformation by SA7 Hatch et al.
transformation Syrian chamber adenovirus (1983)
hamster
embyro cells
HGPRT- Chinese 0-4% -ve Jongen et al.
deficient hamster V79 (1981)
cells
Micronucleus Chinese Not stated -ve Gu & Wang
hamster V79 (1988)
cells
Unscheduled Primary rat Not stated -ve Trueman et al.
DNA synthesis hepatocytes (1987)
Unscheduled Primary rat Not stated ± Thilagar et al.
DNA synthesis hepatocytes (1984a)
Cell BALB/C-3T3 0.01% -ve Price et al.
transformation mouse (1978)
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Cell C3H-10T1/2 Not stated -ve Thilagar et al.
transformation CL8 mouse (1984a)
Unscheduled Primary rat ± A "marginal" positive result reported Thilagar et al.
DNA synthesis hepatocytes (1984a)
Unscheduled Human ±S9 2.5-10.0 µl/ml -ve Perocco & Prodi
DNA synthesis lymphocytes (1981)
Unscheduled Chinese 0-5% -ve Jongen et al.
DNA synthesis hamster V79 (1981)
cells
DNA repair Primary rat 0.7-16.0 mM -ve Andrae & Wolff
synthesis hepatocytes (1983)
a +ve = positive; -ve = negative; ± = equivocal or inconclusive
A recent study demonstrated that glutathione- S-transferase 5-5
expression in Salmonella typhimurium increases mutation rates caused
by methylene chloride. The plasmid pKK233-2 containing rat
glutathione- S-transferase 5-5 cDNA, either in the correct or reverse
direction, was transfected into TA1535. The resulting sense-
transformed TA1535 (RSJ 100) expressed the enzyme and enhanced base-
pair revertants as compared to the anti-sense strain (TPT 100).
Mutagenicity was not seen when GSH, purified glutathione- S-
transferase and a methylene dihalide such as methylene bromide were
added to the pre-incubation mixture with TA1535. Formaldehyde did not
produce mutations in any of the three strains (Thier et al., 1993).
The nature and distribution of forward mutations in the N-terminal
region of the loc I gene of excision repair-proficient (Uvr+) and
excision repair-defective (Uvr B-) strains of E. coli have been
described by Zielenska et al. (1993). A total of 116 locI-d
mutations were characterized.
8.6.1.2 Fungi and yeasts
A dose-related increase in the frequency of gene conversions,
mitotic recombinations, and reversions was found for cultures of
Saccharomyces cerevisiae strain D7, but not for strains D4 and D3,
exposed to methylene chloride of undisclosed purity. However, the
mutagenic results in D7 occurred at toxic doses (1270 g/m3) in which
survival of the yeast cells was reduced to 42% (Cullen et al., 1980).
When assayed for the induction of mitotic segregation in
Aspergillus nidulans P1, methylene chloride significantly increased
the frequency of morphologically abnormal colonies, which produced
euploid whole-chromosome segregants (Crebelli et al., 1988; Crebelli
et al., 1992).
8.6.1.3 Mutation in mammalian cells
Methylene chloride was not mutagenic in several tests in which
mammalian somatic or human cells were used (Gocke et al., 1981; Jongen
et al., 1981; Perocco & Prodi, 1981; Andrue & Wolff, 1983; Burek et
al., 1984).
Both negative (Thilagar et al., 1984a) and questionable results
(Myhr et al., 1990) were reported when methylene chloride was tested
for gene mutations in a L5178Y mouse lymphoma assay at the thymidine
kinuse locus. No increase in micronuclei was found when methylene
chloride was tested in Chinese hamster V79 cells (Gu & Wang, 1988).
8.6.1.4 Chromosomal effects
Studies on chromosome morphology in cultured mammalian cells
indicate that methylene chloride is clastogenic. Chromosomni
alterations (chromatid damage, chromosomni exchanges, but no increase
in sister chromatid exchanges) were observed in CHO cells (Thilagar &
Kumaroo, 1983), human lymphocytes and L5178Y cells (Thilagar et al.,
1984a,b), both with and without metabolic activation. A small increase
in sister-chromatid exchanges (SCEs), without clear evidence of a
dose-response relationship, was found in V79 cells when exposed to
gaseous methylene chloride at concentrations up to 5% (Jongen et al.,
1981 ). A dose-related increase in SCEs was observed in CHO cells
after a 24-h exposure to methylene chloride. The results were
statistically significant only at the highest concentration (7%) and
exposures of shorter duration (2, 4 or 6 h) were without effect
(McCaroll et al., 1983). In a more recent study, Anderson et al.
(1990) reported no increase in chromosomal aberrations or SCEs in CHO
cells exposed to up to 5 mg/ml.
Hallier et al. (1993) described an apparent polymorphism in human
blood samples used to measure SCEs in lymphocytes. Those blood samples
possessing metabolic activity (conjugators) were inactive in the
assay, whereas those samples which were metabolically inactive (non-
conjugators) produced significant increases in SCEs (see also section
6.3.1).
8.6.1.5 DNA damage
Concentrations of up to 16 mM methylene chloride failed to induce
unscheduled DNA synthesis (UDS) in cultured rat hepatocyte, although
some reduction in replicative DNA synthesis occurred at the higher
doses (Andrae & Wolff, 1983). The absence of evidence of methylene
chloride-induced UDS in primary rat hepatocytes was also reported by
Trueman et al. (1987). Thilagar et al. (1984a) reported a "marginal"
positive result in a primary rat hepatocytes UDS assay, but details
were not available. In experiments using no exogenous activation
systems, exposure of hamster V79 cells or human fibroblasts (AH cells)
to methylene chloride concentrations of between 0.5 and 5% did not
induce UDS (Jongen et al., 1981). A non-specific but reversible
inhibition of replicative DNA synthesis was observed in both cell
lines, probably due to a metabolic block of synthesis. Methylene
chloride in doses of 2.5, 5 or 10 µ/ml did not induce UDS in human
lymphocytes in either the presence or absence of rat liver S9 (Perocco
& Prodi, 1981).
8.6.1.6 DNA binding in vitro
Several studies have investigated the potential of methylene
chloride and its metabolites to bind covalently to DNA. Incubation of
14C-labelled methylene chloride failed to detect any DNA binding,
but binding to proteins and lipids was observed (Cunningham et al.,
1981). After incubation of calf thymus DNA in vitro with 14C-
labelled methylene chloride (0.8 µmol/ml), together with hepatic
microsomes and a NADPH-generating system, there was no evidence of any
DNA alkylation (Di Renzo et al., 1982). These studies confirm those of
an earlier in vitro study which failed to detect any DNA binding of
methylene chloride or its metabolites (Anders et al., 1977).
8.6.1.7 Cell transformation
Methylene chloride has been tested for its ability to induce
transformation in a variety of cell systems. Negative results were
obtained in C3H-10T1/2 CL8 mouse cells at 10 µl/ml (Thilagar et al.,
1984a) and in Balb/C-3T3 mouse cells at 0.01% (Price et al., 1978).
Methylene chloride significantly enhanced the frequency of
transformation by SA7 virus in a dose-related manner (Hatch et al.,
1983).
8.6.2 In vivo
8.6.2.1 Chromosome damage
Large doses of methylene chloride (425, 850 and 1700 mg/kg) given
twice intraperitoneally to NMRI mice did not increase micronuclei in
the bone marrow micronucleus test (Gocke et al., 1981). Doses of up to
4 g/kg body weight (the maximum tolerated dose) administered by gavage
to C57BL/6J/Alpk mice also failed to induce any increase in bone
marrow micronuclei (Sheldon et al., 1987). The results of in vivo
mutagenicity assays are presented in Table 19.
Intraperitoneal injections of methylene chloride (100, 1000, 1500
or 2000 mg/kg) did not increase the frequencies of either SCEs or
chromosome aberrations in bone marrow cells of male C57BL/6J mice
(Wesforook-Collins et al., 1988; Wesforook-Collins et al., 1990).
No increase in the frequency of either SCEs or chromosome
aberrations was observed in bone marrow cells of female B6C3F1 mice
after a single subcutaneous injection of methylene chloride (2500 or
5000 mg/kg) (Westbrook-Collins et al., 1989; Allen et al., 1990).
Inhalation exposure of female B6C3F1 mice to 14 000 or
28 000 mg/m3 for 10 days (6 h/day, 5 days/week) resulted in slight
increases in the frequency of SCEs in lung cells and peripheral blood
lymphocytes, in chromosome aberrations in lung and bone marrow cells,
and in micronuclei in peripheral blood erythrocytes. The results were
statistically significant at 28 200 mg/m3 for all end-points. At
14 100 mg/m3, statistical significance was reached only for the SCE
frequency in lung cells. A marginal increase in lung cell SCEs and
micronuclei in peripheral blood erythrocytes was observed following a
3-month inhalation exposure to 7100 mg/m3 (Westbrook-Collins et al.,
Table 19. In vivo mutagenicity assays
Assay Strain/type Resulta Observations Reference
Chromosome Male C57BL/6 mouse bone -ve i.p., 0-2000 mg/kg Westbrook-Collins et al. (1988);
aberration marrow Westbrook-Collins et al. (1990)
Chromosome Female B6C3F1 mouse bone -ve s.c., 2500 or 5000 mg/kg Westbrook-Collins et al. (1989);
aberration marrow Allen et al. (1990)
Chromosome Female B6C3F1 mouse bone ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
aberration marrow 10 days Allen et al. (1990)
Chromosome Rat bone marrow -ve inhalation, 1770, 3500 or 12 400 mg/m3 Burek et al. (1984)
aberration 6 h/day, 5 days/week for 6 months
Sister-chromatid Male C57BL/6 mouse bone -ve i.p. 0-2000 mg/kg Westbrook-Collins et al. (1988);
exchange marrow Westbrook-Collins et al. (1990)
Sister-chromatid Female B6C3F1 mouse bone -ve s.c., 2500 or 5000 mg/kg Westbrook-Collins et al. (1989);
exchange marrow Allen et al. (1990)
Sister-chromatid Female B6C3F1 mouse lung ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
exchange cells, peripheral blood 10 days Allen et al, (1990)
lymphocytes
Sister-chromatid Female B6C3F1 mouse lung ± inhalation, 7100 mg/m3 for 3 months; Westbrook-Collins et al. (1989);
exchange cells small increase Allen et al. (1990)
Synaptonemal Male C57BL/6 mouse ± i.p., 0-1500 mg/kg Westbrook-Collins et al. (1988)
complex
(damage at
meiotic
prophase)
Table 19 (Cont'd)
Assay Strain/type Resulta Observations Reference
Micronucleus Female B6C3F1 mouse ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
peripheral blood 10 days Allen et al. (1990)
erythrocytes
Micronucleus Female B6C3F1 mouse ± inhalation, 7100 mg/m3 for 3 months Westbrook-Collins et al. (1988);
peripheral blood Allen et al. (1990)
erythrocytes
Micronucleus C57BL/6 mouse bone -ve oral in corn oil, up to 4 g/kg Sheldon et al. (1987)
marrow
Unscheduled Male Alpk: AP rat -ve oral gavage, 100, 500 or 1000 mg/kg; Trueman et al. (1987)
DNA synthesis hepatocytes autoradiography 4 and 12 h after
treatment
Unscheduled Male B6C3F1 mouse -ve oral gavage, 100 mg/kg in corn oil Lefevre & Ashby (1989)
DNA synthesis hepatocytes
Unscheduled Male Fischer-344 rat, and -ve inhalation, 7100 or 14 100 mg/m3 for 2 Trueman & Ashby (1987)
DNA synthesis male B6C3F1 mouse or 6 h
hepatocytes
Unscheduled Male B6C3F1 mouse -ve inhalation, 14 100 mg/m3 for 2 h Lefevre & Ashby (1989)
DNA synthesis hepatocytes
Dominant lethal Male Swiss-Webster mouse -ve s.c., 5 ml/kg, 5% or 10% v/v in corn oil, Raje et al. (1988)
3/week for 4 weeks
inhalation at 350, 530 or 710 mg/m3, Basso et al., (1987);
2 h/day, 5 days/week for 6 weeks Raje et al. (1988)
no microscopic lesions of testes or brain
Table 19 (Cont'd)
Assay Strain/type Resulta Observations Reference
Drosphila Berlin K, Basc -ve 0 to 14 260 mg/m3 for 6 h, 1 week or 2 Kramers et al. (1991)
melanogaster weeks; sex-linked recessive lethal assay,
and somatic mutation and recombination
test
Drosphila Berlin K, Basc +ve 125 or 620 mM; increased frequency of Gocke et al. (1981)
melanogaster recessive lethals in Basc test
a +ve = positive; -ve = negative; ± = equivocal or inconclusive
1989; Allen et al., 1990). Background data and positive control
results were not available. Chromosome aberration data (excluding
gaps) were not reported, and so the increases might not be
significant. Increases in SCE and micronuclei were small (up to 2-fold
at 28 000 mg/m3).
No increase in chromosomal aberrations was observed in bone marrow
cells of Sprague-Dawley rats (5 of each sex per group) following
inhalation exposure to 1770, 3500 or 12 400 mg/m3 (6 h/day,
5 days/week) for 6 months (Burek et al., 1984).
Inconclusive results were reported when methylene chloride was
tested in C57B1/6 mice for its ability to induce damage in the
synaptosomal complex, an experimental end-point which can reveal
induced damage at the meiotic prophase, following intraperitoneal
injection of 1500 mg/kg (Westbrook-Collins et al., 1988).
8.6.2.2 Drosophila
No mutagenicity was detected in the recessive lethal test on
Drosophila melanogaster fed, or injected with, 1-2% methylene
chloride (Abrahamson & Valencia, 1980). A marginal increase in the
number of recessive deaths was found after feeding 125 or 650 nmol
methylene chloride in 2% dimethylsulfoxide to Drosophila melanogaster
(Gocke et al., 1981). This study may not be reliable because control
values from different solvent treatments were pooled, and because the
increases seen were significant only when results from the two dose
levels were combined.
Methylene chloride was not active in the sex-linked recessive
lethal assay or the somatic mutation and recombination test carried
out with Drosophila melanogaster using inhalation exposure up to
14 260 mg/m3 (Kramers et al., 1991).
8.6.2.3 DNA damage
Methylene chloride has been evaluated for its ability to initiate
unscheduled DNA synthesis (UDS) in the livers of male mice and rats
in vivo. Alpk:AP rats were exposed by oral garage to 100, 500 or
1000 mg/kg body weight, and hepatocytes were assessed for UDS via
autoradiography 4 and 12 h later (Trueman & Ashby, 1987). In a second
study, F-344 rats or B6C3F1 mice were exposed by inhalation to
either 7100 or 14 100 mg/m3 for 2 or 6 h, and hepatocytes were
assessed for UDS immediately after exposure. In both studies,
methylene chloride failed to induce UDS. Similar results were reported
by Lefevre & Ashby (1989) following exposure of male B6C3F1 mice
either by oral gavage in corn oil (1000 mg/kg) or by inhalation of an
atmosphere containing 14 100 mg/m3 for 2 h.
Slightly increased DNA damage, measured by alkaline elution of DNA
from hepatocytes of rats orally exposed to methylene chloride
(2.55 g/kg) in corn oil for 24 h, has been reported (Kitchin & Brown,
1989).
DNA single strand breaks were found in mouse hepatocytes after in
vivo exposure to 14 120 mg/m3 for 3 or 6 h but not in hepatocytes
of similarly exposed rats. It was further observed in vitro that the
lowest concentration of methylene chloride needed to induce DNA single
strand breaks in mouse hepatocytes was 75 times below that in rat
hepatocytes. This DNA damage was not accompanied by cytotoxicity. The
relation of these findings to the mechanism for carcinogenic effects
is discussed in section 8.8.1.
8.6.2.4 DNA binding
Several studies have investigated the potential of methylene
chloride to bind covalently to DNA after in vivo exposure. DNA has
been isolated from the livers and lungs of mice and rats exposed to
14 100 mg/m3 (Green et al., 1987a,b,c). In both studies the DNA was
hydrolysed and analysed by chromatography to distinguish between
alkylation of DNA and incorporation of radioactivity though the C-1
pool. No evidence of alkylation was found, both studies having the
power to detect one alkylation per 106 nucleotides.
No alkylation of DNA was reported by Ottenwalder & Peter (1989),
following a DNA binding assay of methylene chloride in rats and mice.
Male mice and hamsters were exposed to 14 100 mg/m3, 6 h/day for
2 days, followed on the third day by a 6-h exposure to a decreasing
concentration (15 900 to 8800 mg/m3) of 14C-labelled methylene
chloride (Casanova et al., 1992). DNA-protein cross-links (DPX) were
detected in mouse liver, but not in mouse lung or hamster liver or
lung. The failure to detect DPX in mouse lung did not exclude possible
formation in a sub-population of lung cells. These results demonstrate
that formaldehyde derived from methylene chloride can form DNA-protein
cross-links in the liver of B6C3F1 mice, the formation of DPX being
dependent on the activity of the GST pathway.
8.6.2.5 Dominant lethal assay
Groups of 20 Swiss-Webster male mice were injected subcutaneously
3 times/week for 4 weeks with 5 ml/kg of 5% v/v or 10% v/v methylene
chloride in corn oil (Raje et al., 1988). Other groups of Swiss-
Webster male mice were exposed to 350, 530 or 710 mg/m3 (2 h/day, 5
days/week for 6 weeks) (Basso et al., 1987; Raje et al., 1988). Mating
was started 1 week later for the injection group and 2 days later for
the inhalation group, each male mouse being mated with a virgin adult
female. The mating continued for 2 weeks. The fetuses were examined on
day 17 of gestation. No significant differences in any of the
mutagenicity parameters were found between control and treated groups.
No microscopic lesions were found in the testes (Basso et al., 1987;
Raje et al., 1988) or brain (Basso et al., 1987) of the treated males.
8.6.2.6 Replicative DNA synthesis
A number of studies have evaluated the ability of methylene
chloride to induce replicative DNA synthesis (S-phase) in the livers
and lungs of B6C3F1 mice and in the livers of Sprague-Dawley rats. A
small, but statistically significant, increase in DNA synthesis was
observed in the livers of mice exposed to 13 800 mg/m3 for 2 h, but
not following a single oral dose of 1000 mg/kg (Lefevre & Ashby,
1989). The biological significance of these increases is unclear due
to similar increases being seen in some control groups.
There were no sustained increases in DNA synthesis in the livers
of female mice exposed by inhalation to 3.53, 7.06, 14.12 or
28.24 g/m3 (1000, 2000, 4000 or 8000 ppm) for up to 4 weeks, nor in
female mice exposed to 7.06 g/m3 for up to 2 years (Foley et al.,
1993).
Increases in DNA synthesis were not seen in rats exposed to
1770 mg/m3 for 6 or 12 months (Nitschke et al., 1988a,b).
Replicative DNA synthesis was measured in the lungs of female mice
exposed to 7.06 or 28.24 g/m3 for 1, 2, 3 or 4 weeks and in mice
exposed to 7.06 g/m3 for 13 and 26 weeks. Small decreases in the
labelling indices were reported at all these points (Kanno et al.,
1993). In contrast, Foster et al. (1992) reported significant
increases in the number of cells in S-phase in both the bronchiolar
and alveolar epithelium of male mice exposed to 14.12 g/m3 for
13 weeks.
8.6.3 Appraisal
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (S. typhimurium or E. coli). In eukaryotic systems it
gives either negative or, in one case, weakly positive results. In
vitro gene mutation assays and tests for UDS in mammalian cells were
uniformly negative. In vitro assays for chromosomal aberrations
using different cell types gave positive results, whereas negative or
equivocal results were obtained in tests for SCE induction.
The majority of the in vivo studies reported have provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay,micronucleus test or UDS assay). Where positive
responses have been seen, they are restricted to tests using B6C3F1
mice. Marginal increases in the frequencies of SCEs, chromosomal
aberrations and micronuclei in mice have been reported following
inhalation of high concentrations of methylene chloride. Increases in
hepatic DNA single strand breaks and DNA-protein crosslinks were seen
in mice, but not in rats, exposed to 14 100 mg/m3.
Within the limitations of the short-term tests currently
available, in vivo genetic activity has only been detected in tests
using B6C3F1 mice.
8.7 Chronic toxicity and carcinogenicity
8.7.1 Inhalation exposure
8.7.1.1 Rat
Groups of 95 male and 95 female Sprague-Dawley rats (8 weeks old)
were exposed by inhalation to 0, 1770, 5300 or 12 400 mg/m3
methylene chloride (99% pure) 6 h/day, 5 days per week for 2 years
(Table 20). Overall survival in the study, including that of controls,
was poor. Mortality among high-dose females was statistically
increased from the 18th month when compared to controls. Increases in
CO-Hb were found in treated groups from 6 months, but not in a dose-
related manner. From 12 months, non-neoplastic pathological effects on
the liver (increased hepatocellular vacuolization consistent with
fatty change) were observed in both males and females at all exposure
levels in a dose-related fashion. In males (5300 and 12 400 mg/m3)
and females (12 400 mg/m3), a decrease in the incidence (females)
and severity (males) of age-associated chronic progressive
glomerulonephrotoxicity was observed. The only reported increases in
tumour incidence occurred in benign mammary gland tumours in males and
females, and tumours in the mid-cervical region close to the salivary
gland in males. There was no significant increase in the proportion of
animals with benign or malignant mammary tumours; however, the total
number of benign mammary tumours showed a marginally significant dose-
related increase in males (controls, 8/95; low-dose, 6/95; mid-dose
11/95; high-dose, 17/97; p = 0.046) and a dose-related increase in the
total number of benign mammary tumours was observed in females
(165/96; 218/95; 245/95; 287/97; p < 0.001). There was no indication
of an increase in the number or incidence of malignant mammary tumours
in either males or females. The background historical incidence for
Sprague-Dawley rats in the laboratory normally exceeds 80% in females
and is about 10% in males (Burek et al., 1984).
Table 20. Carcinogenicity studies using the inhalation routes
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Rat Sprague-Dawley, Inhalation, 6 h/day, 5 0, 1770, Increase in various types of sarcomas in the Burek et al.
male and female days/week for 2 years 5300, mid-cervical area in the region of the (1984)
95 animals/sex/group 12 400 salivary gland in mid- and high-dose males
(1/92, 0/95, 5/95, 11/97). Slight dose-
related increase in total number of benign
mammary tumours in males (8/95, 6/95,
11/95, 17/97; p =0.046); dose-related
increase in total number of benign
mammary tumours in females (165/96,
218/95, 245/95, 287/97; p<O.001)
Rat Sprague-Dawley, Inhalation, 6 h/day, 5 O, 177, 710, No increase in incidence of benign Nitschke et al.
male and female days/week for 20 1770 mammary tumours in males or in females (1988a)
months (males), 24 exposed to 177 or 710 mg/m3; increased
months (females); 90 incidence of benign mammary tumours in
males/group females at 1770 mg/m3; no increase in the
198 females/group number of any malignant tumours; NOAEL
710 mg/m3
Rat Fischer-344/N, Inhalation, 6 h/day, 5 O, 3500, Dose-dependent increase in benign NTP (1986)
male and female days/week for 102 7100, mammary tumours; male: 0/50, 0/50, 2/50, Mennear et al.
weeks; 50 animals/sex 14 100 5/50; female: 5/50, 11/50, 13/50, 23/50 (1988)
group
Table 20 (Cont'd)
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Rat Sprague-Dawley, Inhalation, 4 h/day, 5 350 No effect on percentage of animals bearing Maltoni et al.
females, during days/week for 7 benign and/or malignant tumours, in (1988)
and after weeks, then 7 h/day, 5 maternal or offspring animals; no
pregnancy days per week for 97 statistically significant increase in total
weeks; malignant tumours
54 females exposed
60 female controls
Rat Sprague-Dawley, inhalation, protocol as 350 As above Maltoni et al.
male and female above for 15 and 104 (1988)
offspring from weeks; 60-70 males or
12th day in utero females/group, 158
male and 149 female
controls
Mouse B6C3F1 inhalation, 6 h/day, 5 7100 Mice exposed for more than one year Kari et al. (1992)
days/week for various showed an excess of lung and liver tumours
periods up to 104
weeks; killed at end of
exposure; 364 females
exposed, 364 female
controls
Table 20 (Cont'd)
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Mouse B6C3F1 Inhalation to 6 h/day, 7100 All exposed groups showed excesses of Kari et al. (1992)
5 days/week for either lung and liver tumours
26, 52, 28 or 104
weeks; all mice
maintained for 104
weeks; 68 females
exposed, 68 female
controls
Mouse B6C3F1, male Inhalation, 6 h/day, 5 0, 7100, Dose-dependent increases in (1) NTP (1986);
and female days/week for 102 14 100 alveolar/bronchiolar adenomas Mennear et al.
weeks; 50 males: 3/50, 19/50, 24/50 (1988)
animals/sex/group females: 2/50, 23/48, 28/48
(2) alveolar/bronchiolar carcinomas
males: 2/50, 10/50, 28/50
females: 1/50, 13/48, 29/48
(3) hepatocellular adenoma and carcinoma -
combined
males: 22/50, 24/49, 33/49
females: 3/50, 16/48, 40/48
Hamster Syrian Golden, Inhalation, 6 h/day, 5 0, 1770, No significant increase in incidence of Burek et al.
male and female days/week for 2 years; 5300, benign tumours (1984)
95 animals/sex/group 12 400
Males exposed to 5300 or 12 400 mg/ma showed an increase in the
number of sarcomas in the mid-cervical area in the region of the
salivary glands (1 subcutaneous sarcoma in controls, n = 92, 2
subcutaneous sarcomas and 3 salivary gland schwannoma at 5300 mg/m3,
n = 95; and 5 subcutaneous fibrosarcomas and 2 subcutaneous
mesofibrosarcomas as well as 2 salivary gland sarcomas at
12 400 mg/m3, n = 97). A number of uncertainties affect the
toxicological significance of these observations. As stated by the
investigators, all but two of these tumours were large and appeared to
invade all adjacent tissues in the neck region. Histologically all
were sarcomas. Some tumours morphologically resembled the overall
type, fibrosarcoma while others resembled another, neurofibrosarcoma,
and still others were undifferentiated or pleomorphic. Only two
tumours were small enough to be localized in the salivary gland,
appearing to arise in interstitial and capsular tissue. Based on these
findings the author suggested that all probably arose from the
salivary gland, although appearing to arise from mesenchymal tissue
they represent a variety of tumour types. There is no explanation for
the sex difference observed. Both male and female rats in the study
had a viral disease (siabclacryoudenitis, which affects the salivary
glands) during the first 2 months of the study. The infection did not
increase mortality and all exposure groups appeared to be affected to
the same degree (Burek et al., 1984).
A later study in the same laboratory where the highest dose level
was 1770 mg/m3 revealed salivary gland sarcomas in only two animals,
a male at 1770 mg/m3 and a female at 177 mg/m3. The historical
incidence in this laboratory was reported to range from 0 to 2% in
control groups of Sprague-Dawley rats (Nitschke et al., 1988a).
Groups of 90 male and 108 female Sprague-Dawley rats (6-8 weeks
old) were exposed to 0, 177, 710 or 1770 mg/m3 6 h/day, 5 days/week
for 20 and 24 months, respectively. Exposure-related increases in
CO-Hb levels were found. Exposure-related histo-pathological changes
were limited to the liver of male and female rats and mammary glands
of female rats exposed to 1770 mg/m3. An increased incidence of
hepatocellular vacuolization was found in males and females exposed to
1770 mg/m3. Females exposed to this dose level also had an increased
incidence of multinucleated hepatocytes. The incidence of benign
mammary tumours in female rats exposed to 177, 710 and 1770 mg/m3
was comparable to historical control values (79-82%), but the number
(2.2) of benign mammary tumours per tumour-bearing female rat exposed
to 1770 mg/m3 was greater than that of controls (1.8) (p < 0.05).
There were no increases in the incidence of malignant tumours at any
site in female rats, nor of benign or malignant tumours in male rats,
exposed to methylene chloride. The no-observed-adverse-effect level
for chronic inhalation exposure of Sprague-Dawley rats was judged to
be 710 mg/m3 (Nitschke et al., 1988a).
A group of 54 pregnant Sprague-Dawley rats was exposed to
350 mg/m3 4 h/day, 5 days/week for 7 weeks and subsequently for
7 h/day, 5 days/week for a further 97 weeks. A further group of 60
rats served as controls. In addition, groups of 60-70 males or females
were exposed to 212 mg/m3 from the 12th day in utero for a total
of either 15 or 104 weeks; there were 158 male and 149 female
controls. In exposed maternal or offspring rats, methylene chloride
did not affect the percentage of animals bearing benign and/or
malignant tumours. Although there was a slight increase in total
malignant rumours in rats exposed to 350 mg/m3 for 104 weeks, this
was not deemed to be statistically significant (Maltoni et al., 1988).
The Task Group noted the absence of relevant data on the design of the
experiment such as the statistical methods, housing conditions and
histopathological techniques.
When groups of 50 male and 50 female F-344/N rats, 7-8 weeks old,
were exposed by inhalation to 0, 3500, 7100 or 14 100 mg/m3
methylene chloride (99% pure) 6 h/day, 5 days per week, for 102 weeks
(NTP, 1986), body weight gains for both exposed males and females were
comparable to those of the control group. The survival of exposed male
rats was also comparable to that of the controls, although the
survival of all groups of males at the end of the study was low.
Similarly, the survival of female rats was comparable with that of the
controls, with the exception of the high-dose group. Non-neoplastic
treatment-related increases in the incidence of renal tubular cell
degeneration, splenic fibrosis and (females only) nasal cavity
squamous metaplasia were observed.
Increased incidences of benign tumours of the mammary gland (all
fibroadenomas, except for one adenoma in the high-dose group) were
observed in treated females (control, 5/50; low-dose, 11/50; mid-dose,
13/50; high-dose, 23/50; p < 0.001). There was a positive trend in
the incidence of benign tumours of the mammary gland in males (0/50,
0/50, 2/50, 5/50; p < 0.01). The range of the historical control
incidence of mammary gland fibroadenomas in this laboratory for the
male rat was 0/50 to 6/49 and for the female rat was 5/50 to 24/49.
There were no other exposure-related increases in tumour incidence
(NTP, 1986; Mennear et al., 1988).
8.7.1.2 Mouse
Groups of 50 male and 50 female B6C3F1 mice, 8-9 weeks old, were
exposed to 0, 7100 or 14 100 mg/m3 (> 99% pure) 6 h/day,
5 days/week for 102 weeks and killed after 104 weeks of study.
Survival to the end of the study period in males was: control, 39/50;
low-dose, 24/50; and high-dose, 11/50; that in females was: 25/50,
25/49 and 8/49. This reduced survival may have been due to the
chemically induced development of liver and lung neoplasia in both
sexes, significant dose-related increases in lung and liver tumours
having been found. The incidences of alveolar/bronchiolar adenomas
were: males - 3/50, 19/50 and 24/50 (p < 0.001); and females - 2/50,
23/48 and 28/48 (p < 0.001). Those for alveolar/bronchiolar
carcinomas were: males - 2/50, 10/50 and 28/50 (p < 0.001); and
females - 1/50, 13/48 and 29/48 (p < 0.001). Incidence of
hepatocellular adenomas or carcinomas (combined) were increased in
high-dose males and dosed females (males: 22/50, 24/49 and 33/49;
females: 3/50, 16/48 and 40/48). Dose-related increases were observed
in the incidences of testicular atrophy in male mice and uterine and
ovarian atrophy in female mice; these effects were considered to be
secondary responses to neoplasia (NTP, 1986; Mennear et al., 1988).
Studies have been conducted on the B6C3F1 mouse to study the
time-dependency of exposure to methylene chloride leading to tumour
formation. Groups of 364 female B6C3F1 mice were exposed to either
air or 7100 mg/m3 methylene chloride for 6 h/day, 5 days/week for
104 weeks. Following exposure for either 462, 494, 515, 529, 571, 597,
618, 639 or 660 days, 10 exposed and 5 control mice were randomly
selected and sacrificed for the purposes of following the progression
of neoplasia in the lung and the liver. Increased incidences of
alveolar/bronchiolar adenomas and carcinomas and of hepatocellular
adenomas and carcinomas in mice exposed for 104 weeks were confirmed.
In mice sacrificed following less than one year's exposure (368 days),
there were no lung lesions observed and only minimal indications of
proliferative lesions in the liver. Mice exposed for longer than one
year showed a progressive increase in the incidence of both
alveolar/bronchiolar adenomas and carcinomas. The incidence of liver
tumours in mice exposed for longer than one year was relatively
constant (40-60%), although a time-dependant increase in the total
liver tumour burden per animal was reported (Kari et al., 1993).
Groups of 68 female B6C3F1 mice were exposed to 100 mg/m3
6 h/day, 5 days/week for either 26, 52 or 78 weeks. All groups of mice
were maintained for 104 weeks, at which time they were sacrificed. The
exposures were split into two phases, i.e. exposure to either air or
to methylene chloride for the specified period at the beginning or the
end of the total exposure period. Further groups of 68 female mice
were exposed either to air or to 7100 mg/m3 methylene chloride for
the full 104 weeks. The percentage incidences of lung adenomas or
adenocarcinomas or liver adenomas or adenocarcinomas are given in
Table 21. The percentage incidence of both lung and liver tumours in
mice exposed to methylene chloride during the first phase increased
with the duration of exposure. In mice exposed to methylene chloride
during the second phase, significant increases in the percentage
incidence of lung and liver rumours were only observed following 78
weeks of exposure to methylene chloride. The study also showed that
lung rumours appeared earlier in methylene chloride-exposed animals
than did liver tumours. Comparison of the results of this study with
those presented in the previous paragraph suggested that the
percentage incidence of lung tumours but not liver tumours increased
following withdrawal from exposure to methylene chloride, particularly
when the mutagenicity of tumours was taken into account (Kari et al.,
1993).
Table 21. The percentage incidence of lung and liver tumours in female mice exposed to
methylene chloride for different time periods
Exposurea % incidence of tumours
(adenomas or carcinomas)
Phase I Phase II Lung Liver
air 104 weeks 7.5 27
methylene chloride 26 weeks air 78 weeks 31b 40
methylene chloride 52 weeks air 52 weeks 63b 44b
methylene chloride 78 weeks air 26 weeks 56b 62b
methylene chloride 104 weeks 63b 69b
air 26 weeks methylene chloride 78 weeks 19b 48b
air 52 weeks methylene chloride 52 weeks 15 31
air 78 weeks methylene chloride 26 weeks 4 34
a exposure to methylene chloride was 7100 mg/m3, 6 h/day, 5 days/week
b statistically significant p < 0.05
8.7.1.3 Hamster
When groups of 95 male and 95 female Syrian golden hamsters
(8 weeks old) were exposed by inhalation to 0, 1770, 5300 or
12 400 mg/m3 6 h/day, 5 days/week for 2 years, no exposure-related
changes were observed in mean body weight and mean organ weights.
CO-Hb levels were increased but this effect was not dose-related. The
numbers of animals surviving to the end of the study were 16, 20, 11
and 14 for males and 0, 4, 10 and 9 for females, respectively. The
incidence of lymphosarcomas was slightly higher in treated females
than in controls (control, 1/91; low-dose, 6/92; mid-dose, 3/91; high-
dose, 7/91; p = 0.032). The increased incidence of benign tumours was
considered to be related to the higher survival of the exposed
hamsters and not a direct result of exposure to methylene chloride
(Burek et al., 1984).
8.7.2 Oral administration
8.7.2.1 Rat
Groups of 85 male and 85 female F-344 rats (7 weeks old) were
administered methylene chloride in the drinking-water at
concentrations of 0, 5, 50, 125 and 250 mg/kg per day for 104 weeks
(Table 22). Interim sacrifices were carried out at 26, 52 and 78
weeks, such that 50 males and 50 females per group received the
treatment for 104 weeks. Additional groups of 50 male and 50 female
F-344 rats received methylene chloride at a concentration of 250 mg/kg
per day for 78 weeks, with further groups of 50 male or female rats
serving as controls. Small changes in mean body weight and food/water
consumption were seen in rats receiving either 125 or 250 mg/kg per
day. Dose-related increases were noted in mean haematocrit,
haemoglobin levels and red blood cell counts at the three highest
doses. Decreases in serum alkaline phosphatase activity and in
creatinine, blood urea nitrogen, serum protein and cholesterol levels
in both sexes were found at 250 mg/kg per day. Treatment-related
histopathological changes were seen in the liver of rats receiving
250 mg/kg per day. There were no increases in the incidence of any
tumour in treated rats when compared to controls (Serota et al.,
1986a).
When groups of 50 male and 50 female Sprague-Dawley rats were
given 0, 100 or 500 mg/kg methylene chloride (purity 99.97%) by gavage
in olive oil, 4-5 days/week for 64 weeks, excess mortality was
observed in rats of each sex receiving 500 mg/kg. A slightly higher
incidence of adenocarcinomas of the mammary gland was observed in
females receiving 500 mg/kg (4/50, 3/50, and 9/50 in the controls,
low-dose and high-dose, respectively). There was no effect on the
total tumour incidence in the exposed groups (Maltoni et al., 1988).
The Task Group noted the short length of the exposure period as well
as the absence of relevant data on the design of the experiment, such
as the statistical methods applied, housing conditions,
histopathological techniques and pathology schedule.
Table 22. Carcinogenicity studies by oral route
Species Strain/type Route of Doses Observations Reference
administration/protocol/
group/size
Rat Fischer-344, Drinking-water ad libitum for 0, 5, 50, 125 No increase in incidence of neoplasms; Serota et al.
mate and female 104 weeks; 85 animals/sex or 250 mg/kg survival and other findings not affected (1986a)
per dose; scheduled kills: 5 at per day by methylene chloride; significant
26 weeks, 10 at 52 weeks, 20 decreases in body weight gain at 125
at 78 weeks; also additional and 250 mg/kg per day and evidence of
groups of controls and liver damage at doses above 50 mg/kg
250 mg/kg (50/sex) which per day
received methylene chloride
for 78 weeks only
Rat Sprague-Dawley, Gavage in olive oil for 64 0, 100 or 500 No effect on total tumour incidence in Maltoni et
male and female weeks; 50 animals/sex/dose; mg/kg per exposed rats. Higher incidence, not al. (1988)
additional control group (not day statistically significant, of malignant
dosed) of 20 males and 26 mammary tumours in high-dose
females females; survival decreased in high dose
males and females
Mouse B6C3F1, male Drinking water ad libitum for 0, 60, 125, 185 No increase in incidence of neoplasms; Serota et al.
and female 104 weeks; group size; or 250 mg/kg evidence of slight liver damage at 250 (1986b)
125 m, 100 f (controls) per day mg/kg per day
200 m, 100 f (60 mg/kg)
100 m, 50 f (125 mg/kg)
100 m, 50 f (185 mg/kg)
125 m, 50 f (250 mg/kg)
Table 22 (Cont'd)
Species Strain/type Route of Doses Observations Reference
administration/protocol/
group/size
Mouse Swiss, male and Gavage in olive oil for 64 0, 100 or Decrease in body weight in exposed Maltoni et
female weeks 500 mg/kg males and females after 36-40 weeks; al, (1988)
50 animals/sex/dose per day dose-related increase in pulmonary
60 animals/sex/controls tumours in males - not significant
without considering mortality rate;
significant (p<0.05) taking into account
the mortality rate; no treatment-related
increase in the percentage of animals
bearing benign and malignant tumours,
or of animals bearing malignant
tumours, or of the number of total
malignant tumours per 100 animals
8.7.2.2 Mouse
Groups of male and female B6C3F1 mice (7 weeks old) received
methylene chloride in the drinking-water for 104 weeks at levels of 0
(control groups 60/65 males, 50/50 females), 60 (200 males, 100
females), 125 (100 males, 50 females), 185 (100 males, 50 females) or
250 (125 males, 50 females) mg/kg per day. Histopathological changes
were observed in the liver of mice receiving 250 mg/kg per day. There
was no increase in the incidence of tumours in any of the exposed
groups, when compared to controls (Serota et al., 1986b).
Groups of 50 male and 50 female Swiss mice received either 100 or
500 mg/kg methylene chloride (purity 99.97%) in olive oil by gavage on
4-5 days/week for 64 weeks. Groups of 60 males and 60 females served
as controls and received only olive oil. In male mice dying between 52
and 78 weeks, an increase in the incidence of pulmonary adenomas was
observed (0/27 in controls, 4/41 low dose level, 7/33 high dose level)
although the effect was not statistically significant in exposed male
mice when mortality was not taken into account. There was no increase
in the total tumour burden in exposed mice. A decrease in body weight
was observed in exposed males and females, compared to controls after
weeks 36-40. No other exposure-related findings were reported (Maltoni
et al., 1988)
8.7.3 Appraisal
Methylene chloride is carcinogenic in the mouse, causing both
lung and liver tumours, following exposure to high concentrations
(7100 and 14 100 mg/m3 ). The incidence of both lung and liver
tumours was increased in mice exposed to 7100 mg/m3 for 26 weeks
and maintained for a further 78 weeks. Associated toxicity or
hyperplasia in the target organs was not observed.
Hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of
tumours in the region of the salivary gland was reported in male rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different tissues, the statistical approach of
combining tumours was inappropriate. The response was not seen in a
second study in which rats were exposed to either 3500, 7100 or
14 100 mg/m3 throughout their lifetime. A further inhalation study
in rats exposed to methylene chloride at concentrations up to
1770 mg/m3 throughout their lifetime showed no evidence of
carcinogenicity. These studies, taken together with the absence of
effect on the salivary gland in all other inhalation studies, raise
doubts regarding their biological and toxicological significance.
Rats exposed to methylene chloride via the drinking-water or by
gavage similarly showed no substantive evidence of carcinogenicity.
An increase in either the incidence or the multiplicity of benign
mammary tumours (fibroadenomas) in rats exposed to methylene chloride
via inhalation has been reported in three studies. Increases in
multiplicity were dose-related in male and female Sprague-Dawley rats
(historical incidence 10% males and 80% females). The increase in
multiplicity was observed only in females of the highest dose
(1770 mg/m3 ) group. No effects were observed in males or the other
dose groups of females. A dose-related increased incidence in benign
mammary tumours was observed in female F-344/N rats, although the
incidences were in the range of historical incidence (10 to 25%). An
increased incidence in high dose (14 100 mg/m3 ) males was also
within the historical incidence range of 0 to 11%.
In one study, a slight increase in the incidence of
adenocarcinomas in the mammary gland was observed in female Sprague-
Dawley rats receiving 500 mg/kg by the oral route. A study in
Fischer-344 rats with dose levels up to 250 mg/kg by the oral route
(drinking-water) showed no carcinogenic effects.
No increases in mammary tumours were observed in the mouse or
hamster by inhalation or oral administration.
8.8 Mechanistic studies
8.8.1 In vitro metabolic studies
There are three transient reactive intermediates in the metabolism
of methylene chloride. Two of them, formyl chloride and
S-chloromethyl-glutathione, are assumed to be present on the basis
of knowledge of the metabolic pathways; the third, formaldehyde, has
been identified in vitro. All three have the reactivity necessary to
bind covalently to macromolecules. Of these S-chloromethyl-
glutathione is potentially the most potent alkylating agent, a
conclusion based on the known reactivity of the halothioethers (Bohme
et al., 1949), structural similarities to the mutagenic glutathione
conjugates of the 1,2-dihaloethanes, and on the outcome of several
studies using different liver fractions in the Salmonella mutation
assay (Jongen et al., 1982; Green, 1983). Formyl chloride is highly
unstable, existing chemically only at low temperatures (-80°C) in
inert solvents (Staab & Datta, 1964). Formaldehyde is a common
metabolic product in vivo which is efficiently metabolized in the
liver to formic acid. The endogenous formation and metabolism of
formaldehyde occurs at a high rate, and the additional formaldehyde
derived from methylene would be metabolized by the same efficient
pathways.
Comparisons of the rates of metabolism of methylene chloride by
each pathway in liver fractions from rats, mice, hamsters and man have
been carried out (Green et al., 1986b,c; Reitz et al., 1989). These
experiments demonstrated that the rates of metabolism in these
pathways in vitro had similar differences to those seen in vivo in
rats and mice, and enabled a comparison to be made with those species
(hamster and man) where in vivo data was not available.
Rates of metabolism in human liver have been measured for both
glutathione- S-transferase (Green et al., 1987a,b,c; Reitz et al.,
1989; Bogaards et al., 1993) and cytochrome P-450 (Green et al.,
1987a,b,c; Reitz et al., 1989) pathways of methylene chloride
metabolism. A total of 33 human liver samples have been assayed for
glutathione- S-transferase activity and a range of activities
reported. In the work by Bogaards et al. (1993), 3 samples had no
activity, a further group of 11 had activity in the range of 0.20-0.41
(mean 0.31 ± 0.08) nmol/min per mg protein, and 8 samples had activity
in the range 0.82-1.23 (1.03 ± 0.14) nmol/min per mg protein. The
rates measured by Green et al. (1987a,b,c) were within the range found
by Bogaards et al., (1993) (0.05-0.93 nmol/min per mg protein; mean
0.42 ± 0.32; n = 7) and those found by Reitz et al. (1989) were
slightly higher (range 0-3.03 nmol/min per mg; mean 2.09 ± 1.40). The
activity in all the samples assayed was at least 1.4 lower than that
in rat liver cytosol. Two human lung samples were assayed and found to
be lacking in glutathione- S-transferase activity (Reitz et al.,
1989). However, enzyme activities of this type are significantly lower
in the lung than the liver, and any such activity may not be detected
by the currently available assays.
The 10-fold difference in glutathione- S-transferase activity
measured in vivo in mice and rats was also found in vitro. There
is an excellent correlation between glutathione- S-transferase
metabolism and the outcome of the 2-year cancer studies in the three
animal species. More support for this correlation was obtained in DNA
damage tests (section 8.6.2.3.) No such correlation exists for the
cytochrome P-450 pathway where, for example, the metabolic rate in the
hamster is very similar to that in the mouse. Cytochrome P-450-
catalysed metabolism of methylene chloride could be detected in lung
tissue from all three animal species, the relative activities being
similar to those in the livers. Glutathione- S-transferase activity
was detectable only in mouse lung fractions.
The low rates of metabolism of methylene chloride by the
glutathione- S-transferase pathway in human liver samples has been
attributed to a deficiency in the transferase isoenzyme responsible.
The same liver samples had similar activity to rat liver when assayed
with an alternative substrate for these enzymes (Green et al.,
1986b,c).
8.8.2 In vivo metabolic studies
A comparative kinetic profile of methylene chloride and its
metabolites was determined in B6C3F1 mice and F-344 rats both during
and after a 6-h exposure to atmospheres containing various
concentrations from 350 to 14 100 mg/m3 (Green et al., 1986b,
1987a,b,c, 1988). Blood levels of methylene chloride and CO-Hb and the
rates of elimination of methylene chloride, carbon monoxide and carbon
dioxide in exhaled air were measured. Stable isotopes were used to
quantify the amount of carbon dioxide from each pathway at dose levels
of 350, 1770 and 14 100 mg/m3, but only in the mouse. The steady-
state blood levels of methylene chloride during exposure were up to
5 times higher in rats than in mice at the higher dose levels. A
comparison of the CO-Hb levels in blood and carbon monoxide levels in
expired air showed that rate of metabolism by the cytochrome P-450
pathway was similar in both rats and mice. The pathway was saturated
in both species at exposures of less that 1770 mg/m3, resulting in
maximal CO-Hb levels of 16% (Green et al., 1987a,b,c).
Saturation of the cytochrome P-450 pathway in mice was also
clearly shown by a 5-10 fold increase in the blood levels of methylene
chloride when the inhaled concentration was doubled from 1770 to
3500 mg/m3 (Green et al., 1987a,b,c).
The stable isotope studies demonstrated that the cytochrome P-450
pathway was the major source of carbon dioxide at low exposure levels
(350 mg/m3) whereas at high levels ( 14 100 mg/m3) the
glutathione- S-transferase pathway was the principal source of carbon
dioxide. A comparison of the rate of elimination of the carbon dioxide
by rats and mice at the top dose level showed the glutathione- S-
transferase pathway to be 10-12 times more active in mice than rats.
The higher rate of metabolic conversion of methylene chloride by mice
when compared to rats largely accounts for the low blood levels of the
parent chemical in this species.
In summary, the in vitro and in vivo studies have provided
evidence for the following:
1. The cytochrome P-450 pathway is saturated at 1770 mg/m3 and is
quantitatively similar in rats and mice in vivo and in rat,
mouse, hamster and human livers in vitro.
2. The glutathione- S-transferase pathway is a major pathway only in
mice, its activity at the 14 100 mg/m3 dose level being an order
of magnitude greater than in rats.
3. In all, 33 human liver samples have been assayed for glutathione-
S-transferase activity. In all cases the levels of activity were
lower than those measured in rat liver.
4. Methylene chloride metabolism is dose-dependent. The utilization
of the two pathways is significantly different at the dose levels
used in the carcinogenicity studies than at low dose levels.
5. These studies provided the metabolic rate constants used in the
physiologically based pharmacokinetic models described in section
8.9.
8.8.3 Pulmonary effects
A number of studies have examined the effects of methylene
chloride on the mouse and rat lung (Eisenbrandt & Reitz, 1986; Hext et
al., 1986; Green et al., 1987a, Foster et al., 1992; Kanno et al.,
1993). Following a single exposure at concentrations of 7100 mg/m3
or more, a specific lesion characterized by marked vacuolization of
Clara cells were seen in the mouse, but not the rat. No other cell
types were affected in the mouse (Green et al., 1987a; Foster et al.,
1992). The morphological damage in Clara cells recovered after 5 days
of repeated 6-h exposures (Foster et al., 1992). The damage to Clara
cells was accompanied by a change in the ability to metabolize
methylene chloride by the two pathways. Cytochrome P-450 metabolism
was suppressed while glutathione- S-transferase remained unchanged.
In a thirteen week study (Foster et al., 1992), damage to the Clara
cells was seen following the first exposure of each week of the study.
Between days 2 and 9 of this study, a significant increase in the
number of cells in S-phase was observed in both bronchiolar and
alveolar epithelium. A similar study (Kanno et al., 1993) failed to
detect an increase in the number of cells in S-phase.
Significant pulmonary lesions were observed in male B6C3F1 mice
1 day after a single 6-h exposure to 14 100 mg/m3 (Eisenbrandt &
Reitz, 1986). Necrosis of the epithelial cells in the bronchi and
bronchioles were observed. Non-ciliated (Clara) cells were swollen and
vacuolated.
8.8.4 Studies on oncogene activation
Further studies have been conducted on the B6C3F1 mouse into the
role of oncogene activation as a potential mechanism of action of the
carcinogenic effect of methylene chloride. A group of 145 female
B6C3F1 mice was exposed to 7100 mg/m3 for 6 h/day, 5 days/week for
up to 27 months. Another group of 235 females acted as controls. These
mice were used for the purpose of providing spontaneous and methylene-
chloride-induced tumour tissue from both the liver and the lung for
the analysis of proto-oncogene activation and tumour suppressor gene
inactivation. The DNA recovered from 54 methylene-chloride-induced
B6C3F1 lung tumours and from 7 spontaneous B6C3F1 lung tumours was
analysed by the direct sequencing of PCR (polymerase chain reaction)
amplified DNA fragments of the K-ras gene for first and second exon
mutations. Twelve mutations were identified in the tumours from
exposed mice, 5 in exon one and 7 in exon two. There was no difference
in the frequency of K-ras activation in tumour tissue derived from
both exposed mice when compared to controls. DNA was isolated from 49
spontaneous and 50 methylene-chloride-induced liver tumours and
screened by the oligonucleotide hybridization of PCR (polymerase chain
reaction) amplified H-ras gene fragments for codon 61 mutations. The
mutation profile of the H-ras gene was similar in the tumour tissue
derived from both control and treated mice (Devereux et al., 1993).
Mutations of the p53 rumour suppresser gene were examined in lung
tumours from female mice exposed to 7100 mg/m3 (2000 ppm) methylene
chloride for 2 years (Hegi et al., 1993). The limited number of p53
mutations identified in this study and the small number of spontaneous
tumours precluded any conclusions concerning the mutagenic spectrum or
possible genotoxicity of methylene chloride.
8.8.5 The use of mechanistic studies in extrapolation
Studies using liver fractions from rats, mice, hamsters and humans
have confirmed the existence of two pathways for the metabolism of
methylene chloride (the cytochrome P-450 pathway and the glutathione-
S-transferase pathway) and have established substantial differences
between species in the utilization of these pathways. The rates of
metabolism of methylene chloride by the two enzymes in the liver
fractions from a few species have been established. The activities of
the cytochrome P-450 pathway in the mouse and hamster were similar,
whereas those in the rat and human were lower. In marked contrast, the
activity of the glutathione- S-transferase in the mouse was very high
when compared with the other species; there being a 10-fold difference
in activity between the mouse and the rat. Rates of metabolism by this
pathway in hamsters and humans were even lower than in rats.
The results of a full pharmacokinetic analysis of the behaviour of
methylene chloride and its metabolites in vivo were consistent with
the species differences observed in vitro. Saturation of the
cytochrome P-450 pathway occurred in rats and mice at dose levels of
less than 1770 mg/m3 and resulted in maximal CO-Hb levels of 16% in
both species. Comparisons of the glutathion- S-transferase pathway
based on expired carbon dioxide levels at high exposure concentrations
found the same 10- to 12-fold difference between mice and rats that
had been observed in vitro. The higher metabolic rates in mice
accounted for the lower blood levels seen in this species compared to
the rat.
Exposure of mice to atmospheric concentrations of 7100 mg/m3 or
more led to recurrent cytotoxicity, increases in DNA synthesis
( S-phase) and changes in the metabolic complement of mouse lung
Clara cells. All the effects were specific to the mouse and to the
Clara cells. Several of the changes (cytotoxicity and S-phase) were
frequently associated with the development of tumours. However the
significance of these findings with respect to the development of lung
tumours in mice exposed to methylene chloride remains to be
established. Effects on chromosomes have also been reported in the
mouse lung.
Studies on the potential role of activation of Ras oncogenes in
the development of methylene-chloride-induced lung and liver tumours
have been unable to distinguish between the tumours seen in methylene-
chloride-treated animals and those occurring spontaneously in control
animals.
Studies in which mice were exposed for different intervals of a
2-year carcinogenicity study established that lung tumours developed
quicker and after shorter exposures than liver tumours. Whether this
indicates a different mechanism in the lung from the liver or reflects
the cytotoxicity and cell division seen in the lungs of mice is
unknown at the present time.
The more recent studies in bacteria using glutathione-deficient
strains and strains in which mammalian transferase enzymes have been
expressed have established the role of this pathway in mutagenesis.
Consistent with this are findings of DNA single strand breaks and DNA-
protein cross-links in the livers of mice, but not rats or hamsters,
exposed in vivo to 14 000 mg/m3. Both the single strand beaks and
cross-links have been shown to be derived from metabolites of the
glutathione- S-transferase pathway. At the present time, these
effects have not been demonstrated in mouse lung.
There is a consistency between the bacterial mutagenicity assays,
the pharmacokinetic data and the studies of DNA single strand breaks
and DNA protein cross-links which leads to the conclusion that the
liver tumours seen in mice are derived from an interaction between
metabolites of the glutathione- S-transferase pathway and DNA. The
same level of detail is not available for the mouse lung. However the
responses seen in the Clara cells and in mouse lung chromosomes are
not inconsistent with this mechanism.
The studies of the comparative metabolism and pharmaco-kinetics of
methylene chloride in the rat, mouse and hamster also provided a
plausible explanation for the species differences in the carcino-
genicity of this chemical. The differing metabolic rates by the
glutathione- S-transferase pathway are consistent with the outcome of
the cancer studies whereas the blood levels of the parent chemical and
the metabolic rates by the cytochrome P-450 pathway are not. These
results are also consistent with the different responses seen in the
three mouse cancer bioassays (NTP, 1986; Serota et al. 1986b; Maltoni
et al., 1986). At the high dose levels used in the NTP study, the
glutathione- S-transferase pathway would have been the major
metabolic pathway and high tumour incidences were observed. At the
lower dose levels used by Serota et al. (1986b) and Maltoni et al.
(1986), methylene chloride would have been metabolized mainly by
cytochrome P-450, and glutathione- S-transferase metabolism would
have been minimal. Consequently there were no significant increases in
either lung or liver tumours in these studies.
8.8.6 Mammary tumour promotion
The dependence of mammary tumours upon pituitary hormones in both
male and female rats has been established unequivocally (Welsch &
Nagasawa, 1977; Welsch 1985). In the rat, prolactin acts as a promoter
of mammary carcinogenesis. There is good evidence that increased
prolactin levels increase the incidence of mammary tumours (Welsch et
al., 1970), and there is a positive correlation between elevated blood
prolactin levels and mammary rumours in aged R-Amsterdam female rats
(Kwa et al., 1974).
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin (Breslin & Landry, 1986). When male and female Sprague-
Dawley rats were exposed to 10 600 mg/m3 (3000 ppm) methylene
chloride for 15 to 19 consecutive days, a significant increase (2.3 x)
in basal serum prolactin levels was observed in female rats. No
significant effect was observed in male rats (Breslin & Landry, 1986).
In humans, there is conflicting evidence on whether or not mammary
rumours are as responsive to prolactin as in the case of rats (Sinha,
1981). The rat has elevated levels of prolactin when fed ad libitum
in comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so easily responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels.
The presence of all three factors that causes tubular-alveolar growth
of the mammary glands may ultimately lead to tumour development.
Prolactin is not luteotrophic in primates (Neumann, 1991).
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will probably occur only at doses of
methylene chloride which affect prolactin levels. There is no direct
information on prolactin levels in rats receiving low doses of
methylene chloride, but no increase in mammary adenomas has been
observed following the administration of low doses in either
inhalation or drinking-water studies (i.e., below 250 mg/kg body
weight or 1770 mg/m3).
8.8.7 Appraisal
In vitro and in vivo metabolism and biochemical studies and
mutagenicity assays in bacteria and B6C3F1 mice have provided a
plausible explanation for the mechanism of action and the species
differences in the carcinogenicity of methylene chloride to the lung
and liver. This explanation is based on the existence of an isoenzyme
of glutathione-S- transferase which specifically metabolizes
methylene chloride to the reactive intermediates responsible for
tumour induction in the mouse. Markedly lower levels of this enzyme
in rats and hamsters are consistent with the fact that these tumours
do not appear in these species. The levels of the enzyme are lower in
human liver than those of the rat or hamster.
Mutagenicity studies on methylene chloride in bacteria and in the
B6C3F1 mouse, which shows a very high level of activity of the
isoenzyme, reveal positive effects, whereas mutagenicity has not been
demonstrated in standard in vivo mutagenicity assays using other
systems. These observations are consistent with the above hypothesis
and provide a mechanistic basis for the induction of tumours in the
mouse.
The role of the glutathione-S- transferase isoenzyme in the
mediation of the demonstrated mutagenic effects, and the correlation
between the activity of this pathway and the species differences in
carcinogenic response in lung and liver, has led to its use as the
dose surrogate in physiologically based pharmacokinetic models used
for human health risk assessment.
The pharmacokinetics of methylene chloride and the response seen
in B6C3F1 mice suggest that this species is a poor model on which
to base human hazard assessment to methylene chloride.
The mechanism of mammary tumour formation in the rat is probably
related to the effect of methylene chloride on prolactin levels in
this species.
8.9 Interspecies and dose extrapolations by kinetic modelling
Two physiologically based pharmacokinetic (PB-PK) models (Andersen
et al., 1987; ECETOC, 1988) have been developed and provide
quantitative estimates of the levels of methylene chloride metabolites
in four mammalian species (mice, rats, hamsters and humans) following
inhalation exposure. The models use information on various
physiological parameters and metabolic constants for the cytochrome
P-450 and glutathione- S-transferase pathways in the lung and liver,
measured in vitro for four species and measured in vivo for the
mouse, with values for rats, hamsters and humans scaled from the mouse
data. The metabolic constants for these models were obtained from the
data described in section 8.8 and from gas uptake studies described by
Andersen et al. (1987). The models were validated against other human
experimental data such as the elimination of carbon monoxide and blood
levels of methylene chloride. Time-course concentration data from the
model were compared to experimental results in F-344 rats, Syrian
Golden hamsters, B6C3F1 mice and human volunteers. The predicted
values for each of the four species were in agreement with the
experimental data. The models were also shown to predict the
appearance and elimination of methylene chloride metabolites. A
similar model was used by Andersen et al. (1991) to predict the time
course of the disappearance of CO-Hb after exposure to methylene
chloride. This model was also shown to predict CO-Hb levels in rats
and humans exposed to methylene chloride.
Several authors have discussed the assumptions, range and
variability of the data used to construct these models. While these
kinetic models have been extremely useful in improving the
characterization of human exposure and potential risk, it should be
recognized that they are based on a set of assumptions with varying
degrees of certainty. Portier & Kaplan (1989) investigated the impact
of varying the intra-population values of the biological parameters
used in the model developed by Andersen et al. (1987) using Monte
Carlo and resampling statistical methods. The results from this
analysis indicated that the estimates of "effective" doses in humans
may vary widely if variability of the parameters is taken into account
in the PBPK model.
Dankovic & Baiter (1993) investigated the impact of exercise and
human inter-subject variability on the estimates of dose derived from
the PBPK model. The model developed by Andersen et al. (1987) and
Reitz et al. (1989) assumed resting values for the parameters
governing cardiac output, alveolar ventilation and blood flow to the
tissues. The authors modified these parameters to reflect light
working conditions. The metabolic parameters for humans used in the
Andersen et al. (1987) model were based on the average of four
individual liver samples. The authors examined the impact of using the
individual values rather than the average value in the PBPK model.
Modifying the physiological parameters to reflect light work
conditions increased the glutathion- S-transferase pathway metabolic
contribution by a factor of 2.9 for the liver and 2.4 for the lung.
When the model was also modified to reflect metabolic inter-individual
(n = 4) variability in humans, the glutathione- S-transferase pathway
contribution estimates were increased by as much as 5.4-fold for the
liver and 3.6-fold for the lung. More recent data have become
available on human metabolic parameters, and are summarized in section
8.8.1. Based on 33 individual liver samples, they suggest that the
Portier & Kaplan estimates which assume 200% variation for metabolic
variability may be exaggerated. Since Dankovik & Bailer's calculations
were based on the four actual values, their estimates would not
change; however, three of their values represent the three highest
values observed for human glutathione- S-transferase activity.
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Environmental exposure
Bell et al. (1991) conducted a study to examine the relationship
between birth weight in Monroe County and exposure to emissions of
methylene chloride from manufacturing processes of the Eastman Kodak
Company in Rochester, New York, USA. County census tracts were
categorized as high, moderate, low or no methylene chloride exposure,
based on the Kodak Air Monitoring Program. Birth weight and
information on variables known to influence birth weight were obtained
from 91 302 birth certificates of white, single births to Monroe
County residents from 1976 to 1987. At the level of methylene chloride
exposure (highest predicted average concentration, 50 µg/m3), no
significant adverse effect of exposure on birth weight was found,
although several problems in the method of estimation of exposure were
identified.
9.1.2 Oral exposure
A 56-year-old woman was found deeply unconscious and cyanosed
after ingesting approximately 300 ml of a paint remover containing
mainly methylene chloride and methanol. Approximately one hour after
ingestion the CO-Hb level measured was found to be 9%. This level
varied between 2.5-12% over the following 2 days and dropped below 1%
thereafter. The woman regained consciousness after 14 h, but over the
following 3 weeks her condition was complicated by progressive renal
failure, pneumonia, pancreatitis, on-going gastrointestinal
haemorrhage and sepsis, which eventually led to death some 25 days
following ingestion. It was considered that the corrosive properties
of the formulation rather than the formation of CO-Hb were responsible
for the lethal outcome (Hughes & Tracey, 1993).
In an earlier poisoning case with the same paint remover
formulation, there was recovery after ingestion of 0.5-1 litre
(Roberts & Marshall, 1976).
9.2 Occupational exposure
9.2.1 Short-term exposure
9.2.1.1 Case studies
A number of case-reports have been published regarding short-term
exposure to methylene chloride in the occupational environment.
Hall & Rumack (1990) described four cases of serious methylene
chloride poisoning, including two fatalities, in small-scale
furniture-stripping shops in Denver, Colorado, USA. In the three
patients discovered while still alive, cardiac irregularities were
recorded. Corneal burns with first- and/or second-degree burns were
reported in areas having direct contact with the methylene chloride-
based paint-stripping compound, and measured CO-Hb levels did not
exceed 8.6%. In each case, no respiratory protection was worn and
ventilation was inadequate, but exposure levels were not known. The
authors concluded that the toxic effects were due to the anaesthetic
properties of methylene chloride.
A 67-year-old male who had been using a paint stripper in a poorly
ventilated location was brought to a hospital emergency room
complaining of headache and chest pain; he was also confused,
disorientated, had a progressive loss of mental alertness, increased
fatigue and lethargy, slurred speech, little recall of either recent
or past events, and was disorientated to time (ATSDR, 1993).
These and other case studies of methylene chloride poisoning
during paint-stripping operations have demonstrated that inhalation
can be fatal to humans (Hall & Rumack, 1990; Novak & Hain, 1990;
Leikin et al., 1990; Manno et al., 1992). In the majority of cases
reported, quantitative estimates of exposure levels have not been
reported, although methylene chloride was detected in various tissues.
In one case (Manno et al., 1989), air samples collected a few hours
later from a well in which two men were found dead, were analysed by
gas chromatography/mass spectrometry and were found to contain up to
583 mg methylene chloride/litre and much lower or trace amounts of
other solvents; blood levels collected at necropsy contained 571 and
601 mg methylene chloride/litre and only trace to a few mg/litre of
other solvents. The CO-Hb levels were about 30% in blood taken 24 h
after death.
In most cases, the cause of death was not clarified. However, in
the report of five victims, including two deaths, described by Leikin
et al. (1990), the authors concluded that the cause of death was due
to solvent-induced narcosis and not carbon monoxide poisoning.
Signs of CNS depression, narcosis, irritation of the eyes and
respiratory tract, lung oedema and sometimes death were found after
accidental exposures to methylene chloride or paint remover containing
this compound (Moskowitz & Shapiro, 1952; Hughes, 1954; Bonventre et
al., 1977; Fagin et al., 1980). Three myocardial infarctions in one
subject were reported to have followed three exposures to a paint
remover containing methylene chloride over a period of approximately 8
months. The subject was exposed in a poorly ventilated room, and
concentrations were up to 4511 mg/m3 in the breathing zones (Stewart
et al., 1976). Electrocardiographic changes resembling those after
carbon monoxide poisoning were found in an exposed man with a history
suggesting ischaemic heart disease (Benzon et al., 1978). Three
probable cases of phosgene poisoning occurred after the use of
methylene-chloride-based paint remover near a source of heat
(Gerritsen & Buschmann, 1960; English, 1964).
A serious case of pulmonary oedema with bilateral exudative
pleural effusions was reported in a 34-year-old man who presented with
respiratory distress. Buie et al. (1986) speculated that hydrochloric
acid, a product of dichloromethane under warm, moist conditions, may
have played a role in this patient's parenchymal abnormalities.
Recovery was complete with the exception of neuropsychiatric
abnormalities thought to be related to exposure to methylene chloride.
Miller et al. (1985) reported the case of a 19-year-old man using
a tile remover, again in a poorly ventilated room. This patient
presented with an array of signs and symptoms ranging from liver
enzyme elevations to poorly localized abdominal pain. Renal studies
and biopsy confirmed the diagnosis of acute tubular necrosis.
Histological studies demonstrated plasma membrane changes in addition
to mitochondrial effects suggestive of anoxic damage. Serum enzyme
changes noted during the patient's stay in hospital suggested that
hepatocellular injury accompanied the nephrotoxic sequelae.
Another case of chemically induced hepatitis resulting from
accidental exposure to methylene chloride alone has been described by
Cordes et al. (1988). The liver was palpable but not enlarged or
tender. The results of initial tests were normal except for a
leukocyte count of 4900 µl with a left shift, and elevated serum
enzyme levels of alkaline phosphatase 142, lactic dehydrogenase and
serum aspartate aminotransferase (ALAT). Five days after admission,
the patient was discharged from hospital. Laboratory tests for
hepatitis A and B antibodies were negative.
Further evidence for hepatic effects of methylene chloride were
reported by Puurunen & Sotaniemi (1985). One week after a brief but
extensive body exposure to methylene chloride, serum ALAT was elevated
three-fold in a 24-year-old male chemical factory worker. The serum
ALAT returned to normal within 2 weeks.
9.2.1.2 Skin and eye effects
The irritating action of methylene chloride on the eyes and skin
has been shown in several cases (see section 9.2.1.1).
Slight erythema was found when methylene-chloride-containing
aerosol-spray deodorants were used twice a day for 12 weeks by 75 men
and women (Meltzer et al., 1977). On direct contact, methylene
chloride caused a burning sensation and pain (Stewart & Dodd, 1964).
Weber et al. (1990) reported a case of an individual who fell into
a vat containing methylene chloride and methanol. After being immersed
for about 15 min, the subject suffered extensive lesions, including
skin burns of superficial and deep severe epidermal damage and a
severe kerato-conjunctivitis.
Wells & Waldron (1984) briefly reported on a young employee who
climbed into a small open vessel with a bucket of about two litres of
methylene chloride in order to clean the walls. The concentration of
methylene chloride vapour within the vessel built up and he became
unconscious, overturning the bucket as he slumped into the bottom of
the vessel. After about 30 min, the man was rescued. During the time
that he was in the vessel, the man sustained second and third degree
burns to both legs, the areas affected being those which were bearing
the weight of his body while he was unconscious. On his discharge from
hospital, these areas were dry and required no skin grafting (Wells &
Waldron, 1984).
9.2.1.3 Laboratory studies
Neurobehavioural changes were observed at low exposure level after
volunteers were exposed to 694 mg/m3 for 1.5-3 h. Vigilance
disturbance and impaired combined tracking monitoring performance were
found (Putz et al., 1976). The critical flicker frequency, one of the
measures for visual function, was reduced after 95 min of exposure to
1040 mg/m3 (Fodor & Winneke, 1971). Visually evoked responses (one
of the surrogate methods of measuring visual functions) were altered
after 1 h of exposure to 2400 mg/m3, while exposed subjects
experienced lightheadedness. Blood and urine variables, except CO-Hb
levels, were normal in this study after 1-2 h of exposure to levels of
methylene chloride between 739 and 3420 mg/m3. No eye, nose, or
throat irritation was observed (Stewart et al., 1972). Most
neurobehavioural effects observed were less pronounced or absent, with
carbon monoxide exposures resulting in comparable CO-Hb levels (Putz
et al., 1976).
In a double-blind laboratory experiment, a short inhalation
exposure to 2.5 mg methylene chloride/litre did not impair vigilance
performance in human volunteers (time of exposure and number of
subjects not stated) (Kozena et al., 1990).
A clinical laboratory evaluation of 266 exposed volunteer workers
and 251 reference volunteer workers from two cellulose di- and
tri-acetate plants in the USA, which took into account smoking habits,
race, sex, age, intensity of exposure, and time of venepuncture,
revealed increases in red cell counts, haemoglobin levels and
haematocrit among white women exposed to a methylene chloride level of
approximately 1650 mg/m3. CO-Hb levels were elevated in all exposed
groups at all exposure levels (section 5.3). A dose-related increase
was observed in serum bilirubin for exposed subjects of both sexes. A
group of 24 exposed male volunteers and 26 reference male volunteers
from the above two industries was also selected for 24-h electro-
cardiographic monitoring. Three exposed and 8 reference workers had
reported a history of heart disease. Neither increased ventricular or
supraventricular ectopic activity nor increased episodic ST-segment
depression was found to be associated with methylene chloride exposure
(Ott et al., 1983).
9.2.2 Long-term exposure
9.2.2.1 Case studies
Irreversible damage to the central nervous system with acoustic
and optical illusions and hallucinations was diagnosed in one man who
had been exposed for 5 years to methylene chloride at levels ranging
from 2290 to 12 500 mg/m3 (Weiss, 1967). Another man, exposed for
3 years to levels of methylene chloride ranging from 1735 to
3470 mg/m3 showed a bilateral temporal lobe degeneration
(Barrowcliff & Knell, 1979). A case of delirium and seizures was
reported in a man who was exposed to methylene chloride for 4 years.
The man reported a 12-month history of intermittent headache, nausea,
blurred vision, shortness of breath, and transient memory
disturbances. Neuropsychological and EEG examinations revealed a
dysfunction of the right hemisphere. All symptoms and signs cleared
with removal from the workplace (Tariot, 1983).
Between December 1984 and June 1986, 34 men with occupational
exposure to methylene chloride were evaluated at the Greater
Cincinnati Occupational Health Centre. The mean value of the exposure
was reported to be 240 mg/m3, ranging from 11 to 544 mg/m3.
Although the primary complaint of these employees involved problems
associated with central nervous dysfunction, 8 of the 34 complained of
testicular, epididymal or lower abdominal pain, and had clinical
histories relating to infertility. Low sperm counts were reported in
workers who used methylene chloride in bonding operations which also
resulted in possible skin exposure. It is uncertain whether the effect
was due to methylene chloride since the workers were also exposed to
other chemicals (Kelly, 1988).
a) Morbidity studies
The few reports available deal with small groups of occupationally
exposed subjects.
Workers exposed occupationally to a time-weighted average of
114 mg/m3 had CO-Hb levels of between 0.8 and 2.5%. No effects were
found on clinical chemistry, haematology or electrocardio-grams (Di
Vincenzo & Kaplan, 1981a). Cherry et al. (1981) did not find any
exposure-related, long-term damage in 29 subjects as shown by
subjective symptoms, neurobehavioural tests, motor nerve conduction
velocity, electrocardiograms and clinical examinations. The men had
been exposed for several years to levels of methylene chloride ranging
from 260 to 347 mg/m3. Age-matched controls were used. In a study
without a control group, neurasthenic disorders and irritation of the
eyes and respiratory passages were experienced by half of the 33
workers exposed to methylene chloride for an average of 2 years.
Digestive disorders were reported by one-third of these workers.
Formic acid was found in the urine. No other deviations were found
during the internal, nervous system, eye and laboratory examinations.
The methylene chloride concentrations measured varied between 100 and
17 000 mg/m3 (Kuzelova & Vlasak, 1966).
A group of 1758 retired airline maintenance workers was surveyed
by mail and telephone to identify a cohort of workers with more than
22 years of methylene chloride exposure following the stripping of
paint from airplanes. A cohort of 25 exposed and 21 non-exposed
retirees met the criteria and were tested extensively (Becker & Lash,
1990; Lash et al., 1991). Following a specially prepared battery of
neuropsychological and neurophysio-logical tests performed by
professionals without prior knowledge of exposure status of the
employees, exposed and control outcome measures were all within the
"normal" range. No statistically significant difference was found
between exposed and control groups, although subtle differences in
attention and memory were detected.
In 46 subjects exposed to methylene chloride concentrations of
6-34 mg/m3 for several years, an excess (not significant) of
digestive disorders and hypotonia was found over controls, while
symptoms of gall bladder pathology and swollen liver were frequent. No
details were given concerning drinking or smoking habits (Kashin et
al., 1980).
A case-control study on 44 women who had a spontaneous abortion
was performed within a cohort of female workers employed in Finnish
pharmaceutical factories during 1973 or 1975 to 1980. Three controls
matched for age at conception within 2.5 years were chosen for every
case except two. Information about pregnancy outcome was collected
from hospital data, and data on exposures from health personnel at the
factories. The odds ratio for methylene chloride exposure, based on 11
exposed cases, was of borderline significance (2.3 with a 95%
confidence interval, 1.0-5.7; p = 0.06). Odds ratios were also
increased for exposures to many other solvents. For those exposed to
methylene chloride less than once a week the odds ratio was 2.0 (95%
CI = 0.6-6.6); whereas for those exposed more than once a week the
odds ratio was 2.8 (95% CI = 0.8-9.5) (Taskinen et al., 1986).
A group of active workers (n = 150) who had worked for at least 10
years in an area where average exposures were 1677 mg/m3 were
compared to an unexposed group of workers (n = 260) with regards to
symptoms and blood chemistry. The methylene chloride workers were also
exposed to acetone and methanol (900 ppm and 100 ppm 8-h TWAs,
respectively). Health history and blood samples had been collected as
part of a company-sponsored health monitoring programme in which both
exposed and unexposed workers were participants. No remarkable or
statistically significant differences were observed in the selected
symptoms (including irregular heartbeat, dizziness or loss of memory)
or in SGPT, bilirubin or haematocrit. The only noticeable difference
was in SGOT, where the non-exposed group had higher levels than the
exposed group (means of 28.2 versus 25.1, p = 0.06). A limitation to
this study is that both groups consisted of active healthy workers.
The age and sex distribution of the two groups was reported to be
similar, but was not given Soden, 1993).
b) Mortality studies
Several studies have evaluated the effects of long-term exposure
to methylene chloride on mortality of workers. The first study was by
Friedlander et al. (1978), who performed both a retrospective cohort
mortality, i.e. standardized mortality ratio (SMR) study, and a
proportionate mortality ratio (PMR) study of men exposed to methylene
chloride at a Kodak photographic film production facility in
Rochester, New York. The PMR study included 334 deaths that occurred
between 1956 and 1976 among former workers who were exposed to
methylene chloride at the facility. The retrospective cohort mortality
study included 751 workers employed during 1964 and involved follow-up
of this cohort up to 1976. Hearne & Friedlander (1981) extended the
follow-up of this cohort to 1980 and subsequently expanded the study
population to include all workers (n = 1013) who were exposed for at
least one year between 1964 and 1970 (Hearne et al., 1987). In a more
recent publication Hearne et al. (1990) extended follow-up of this
expanded cohort to 1988.
The Friedlander et al. (1978) study was initially conducted to
test the hypothesis that exposure to methylene chloride increases the
risk of ischaemic heart disease. This hypothesis was based on the fact
that methylene chloride is metabolized to carbon monoxide and induces
the formation of CO-Hb in humans (Stewart et al., 1972). Increases in
CO-Hb as low as 2% (Allred et al., 1989) have been shown to induce
electrocardiographic changes in exercising patients with pre-existing
coronary artery disease. An excess of ischaemic heart disease
mortality has also been reported in a cohort of tunnel workers exposed
to carbon monoxide (Stern et al., 1988). Liver and lung cancer were
also considered a priori hypotheses in the subsequent articles by
Hearne et al., based on the results from the animal bioassay data
described in chapter 8.
Comparisons in both studies (Friedlander et al., 1978 and Hearne
et al., 1990) were made with mortality rates (or proportions) from New
York State and from an internal unexposed cohort from the Kodak
facility. Extensive industrial hygiene measurements were available for
this cohort from after 1980, which indicated that 8-h TWA exposure
concentrations for different occupational classifications ranged from
approximately 35.3-402 mg/m3 (10-114 ppm) and the mean exposure was
91.8 mg/m3 (26 ppm) (Hearne et al., 1987). An exposure-response
analysis, which was presented by Hearne et al. (1987), failed to
demonstrate an increasing risk for these causes of death with
increasing methylene chloride exposure. Hearne et al. (1987) observed
an excess of pancreatic cancer mortality (8 observed, SMR = 2.58, 95%
confidence interval (CI) = 1.11-5.08). Mirer et al. (1988) published a
letter suggesting that the excess of pancreatic cancer mortality
increased with time since first exposure (latency) and was greatest
among workers in the highest exposure (750 ppm-years) and latency
(> 30 years) categories (4 observed, SMR = 4.49, 95%
CI = 1.22-11.49). No new pancreatic cancer cases were identified with
additional follow-up, and with the additional data the excess was not
statistically significant (SMR = 1.90, 95% CI = 0.82-3.75) (Hearne et
al., 1990). The study by Friedlander et al. (1978) and the subsequent
studies by Hearne et al. (1987,1990) failed to detect a significantly
increased risk of ischaemic heart disease, lung cancer, liver cancer
or other cancers among methylene-chloride-exposed workers. It also
important to recognize that workers at the Kodak facility (T. Hearne,
personal communication to the IPCS) were not permitted to smoke at
their workstations and that this fact may have induced a negative bias
in these studies, particularly with respect to lung cancer or
cardiovascular disease. Unfortunately detailed information on
cigarette smoking was not available for this cohort and thus
adjustments for this potential bias could not be made.
Ott et al. (1983) evaluated a cohort of workers exposed to
methylene chloride in the production of triacetate fibre at a
manufacturing plant in Rock Hill, South Carolina, USA. This cohort
included 1271 males and female workers who were employed for at least
3 months, sometime between 1954 and 1977, Workers from another textile
facility that were not exposed to methylene chloride, but met the same
inclusion criteria as the exposed cohort, were also included for
comparison purposes. All workers (both exposed and unexposed) were
followed for vital status ascertainment up to June 1977. Eight-hour
TWA methylene chloride exposures in this cohort were estimated to
range from 494 to 1677 mg/m3 (140 to 475 ppm) from a survey
conducted in 1977 and 1978. Workers in this study were also reported
to have been exposed to methanol and acetone. The mortality experience
of the exposed cohort was compared with the mortality of the USA
population using a modified life-table approach (SMRs). Direct
comparisons were also made between the mortality of the exposed and
unexposed cohorts. Mortality from cardiovascular disease or any other
cause was not found to be significantly increased relative to the USA
population. However, the authors did observe a significant increase in
the risk of ischaemic heart disease (RR = 3.1, p < 0.05) among white
men in the analysis when the mortality rates of the exposed and
unexposed cohorts were compared. It was also noted that 8 of the 14
ischaemic heart disease deaths among exposed white men occurred among
workers who were actively employed. Although Ott et al. (1983) did not
report any increase in cancer mortality, this study was not designed
to evaluate cancer and only included seven malignant neoplasms.
The follow-up of the exposed cohort (but not the unexposed one)
studied by Ott et al. (1983) was subsequently extended to 1986 by
Lanes et al. (1990). The analyses presented in this paper were solely
based upon comparisons with the USA population and did not include any
direct comparisons with the unexposed cohort as did the study by Ott
et al. (1983). This study failed to detect an excess of cardiovascular
or ischaemic heart disease. A significant excess of cancers of the
biliary passages and liver (SMR = 5.75, 95% CI = 1.82-13.78) was
observed. Three of the cancers were cholangiocarcinomas of various
biliary sites while the fourth was a liver adenocarcinoma. The SMR for
biliary cancer was estimated using mortality rates from 1973 to 1977.
The SMR for biliary cancer alone was 20 (95% CI = 5.2-56). Three of
the four liver and biliary cancer deaths observed in this study were
thought to have occurred among workers with 10 or more years of
employment and at least 20 years since first employment (0.35
expected, SMR = 11.43), a pattern consistent with a potential
occupational etiology. One of the cases had only been exposed to
methylene chloride for one year. Lanes et al. (1993) recently extended
the follow-up of the cohort for an additional 4 years. Although no
additional cases of liver or biliary cancer were observed, an excess
from the previous study persisted (SMR = 2.98, 95% CI = 0.81-7.63].
This latest report did not include an analysis for biliary cancer
alone.
Another retrospective cohort mortality study of workers from a
Hoechst-Celanese cellulose acetate fibre plant in Cumberland,
Maryland, USA was reported by Gibbs (1992). This study included 3211
cellulose fibre workers employed in or after 1970 and followed until
1989. The cohort was divided into three groups: high (> 1235 mg/m3,
> 350 ppm) methylene chloride exposure, low exposure (176-350 mg/m3,
50-100 ppm), and no exposure. Comparisons were made with USA, Maryland
and county mortality rates. Estimates of exposure levels for this
population were based on industrial hygiene measurements from the plant
studied by Ott et al. (1983), which used similar production methods.
Cancer of the prostate was significantly elevated among men, and
particularly among those with long latency and with high levels of
methylene chloride exposure. A significant excess of cervical cancer
was observed among women in the low exposure group, based on Maryland
rates (SMR = 4.75 based upon 5 observed), but there was no evidence of
a dose-response relationship. Two cases (exp = 1.40) of biliary cancer
were observed among the combined high and low exposure groups. An
excess of ischaemic heart disease mortality was observed among workers
in all three groups when comparisons were based upon Maryland rates,
but not when local county rates were used. Mortality from lung,
pancreatic, liver/biliary and other cancers was not observed to be
significantly elevated in this study. As with the Kodak study, workers
at the Hoechst-Celanese facilities were not permitted to smoke at
their workstations. Again this fact may have induced a negative bias
in these studies, particularly with respect to lung cancer or
cardiovascular disease.
A cohort study of chemical workers included a sub-cohort of 226
men employed for at least one year in chlorinated methanes production
(Ott et al., 1983). Methylene chloride is principally produced by a
method involving the hydrochlorination of methanol which also results
in the production of chloroform and carbon tetrachloride (IARC, 1986).
The men had been employed between 1940 and 1969 and were followed for
mortality until 1979. In all, 42 deaths were observed and no excesses
of respiratory cancer (SMR = 0.70, based on 3 observed) or circulatory
disease (SMR = 0.68, based on 18 observed) were seen. The results for
liver and biliary cancer were not reported, but three cases of
pancreatic cancer were observed (0.9 expected). All three persons had
worked in chlorinated methanes production between 1942 and 1946; two
had been employed for less than 5 years, the third for 6 years. No
further information on exposure for the individuals or the sub-cohort
was given and the mixed exposure to methylene chloride, chloroform,
and carbon tetrachloride limits the interpretation of the results with
respect to methylene chloride.
Finally, Heineman et al. (1994) reported the results of a case-
control study of astrocytic brain cancer and occupational exposure to
chlorinated aliphatic hydrocarbons. The study included 300 cases with
a hospital diagnosis of astrocytic brain cancer and 320 controls
matched on age, year of death and geographical area. A job-exposure
matrix was used to classify cases and controls in terms of potential
exposure to chlorinated aliphatic hydrocarbons including methylene
chloride (Gomez et al., 1994); 119 cases and 108 controls were
classified as being potentially exposed to methylene chloride in this
study. The risk was reported to increase with the probability of
exposure (Odds Ratio (OR) - 2.4 for high probability, 95%
CI = 0.9-6.4) and duration of employment (OR = 1.9 for > 20 years,
95% CI = 0.7-5.2) in jobs considered to be exposed to methylene
chloride after adjustment for other solvent exposures. The exposure
information used in this study is weaker than that generally used in
the retrospective cohort mortality studies described above and the
results should therefore be viewed more cautiously.
9.3 Appraisal of human effects
The main toxic effects of methylene chloride are reversible CNS
depression and CO-Hb formation. Liver and renal dysfunctions and
effects on haematological parameters have also been reported
following exposure to methylene chloride. Methylene chloride will
irritate the skin and eyes especially when evaporation is prevented.
Prolonged contact may cause chemical burns.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men exposed to methylene chloride
for several years at concentrations ranging from 260 to 347 mg/m3.
Similarly, the performance of a group of retired airplane strippers,
with a long history of exposure to methylene chloride (22 years) at
high but unspecified levels, in a battery of neurophysiological and
psychological tests was within the "normal" range when compared with
a control group who had a history of either no or only low exposure
to methylene chloride.
Fatalities due to excessive oral exposure to methylene chloride
have been reported. A case of serious pulmonary oedema has been
reported after excessive inhalation.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to
methylene chloride. This isolated finding from a limited study makes
it difficult to interpret the significance of the observations.
Five mortality studies on methylene chloride have been conducted
and evaluated specifically with regard to cancer and cardiovascular
disease. None of the studies demonstrated a relationship between
exposure to methylene chloride and lung or liver cancer mortality.
With regard to the lung cancers, the lack of smoking histories
hampers the interpretability of the results. An excess of mortality
from biliary cancer was reported in one study, but this was not
corroborated by other studies. Two studies showed an excess in
mortality from pancreatic cancer. In one of the studies no new
pancreatic cancer cases were identified with additional follow-up;
with the additional data the excess was not statistically
significant. It should be noted that the size of these studies
resulted in very low statistical power to detect an excess
particularly for rare cancers such as liver and biliary tract
tumours.
Associations between exposure to methylene chloride and prostate
and cervical cancers have been reported in studies, each of which had
its limitations. An association between the potential for exposure to
methylene chloride and other organic solvents and brain cancer was
found in a case-control study which classified exposure to methylene
chloride using a job exposure matrix. This finding should be viewed
with caution.
The results from these studies have been contradictory with
respect to mortality from ischaemic heart disease. A role for
methylene chloride in the induction of ischaemic heart disease is
plausible based on the fact that methylene chloride is metabolized to
carbon monoxide and induces the formation of carboxyhaemoglobin in
humans. An excess of cardiovascular disease was reported in one of
the mortality studies. The fact that further studies did not provide
any compelling evidence of an increased risk of cardiovascular
disease might be attributable to their reliance on comparisons with
the general population as the referent group. The use of general
mortality rates in occupational cohort mortality studies may bias the
results towards the null (i.e. no effect) due to the "healthy worker
effect" which is particularly strong for cardiovascular diseases.
This bias may have been further exacerbated by the fact that workers
were not permitted to smoke at their workstations.
The currently available epidemiological studies are inadequate
for drawing any firm conclusions with regard to either cancer or
cardiovascular disease risk.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human exposure to methylene chloride is mainly by inhalation of
the vapour. Exposure of the general population to methylene chloride
depends strongly on the indoor air concentration. Owing to the use of
products containing methylene chloride, peak concentrations of up to
4000 µg/m3 have been reported. However, 24-h average exposures are
in general below 50 µg/m3.
Methylene chloride is rapidly absorbed through the lung and also
from the gastrointestinal tract. It is absorbed via the skin, but at a
much slower rate than by the other routes. Once absorbed, methylene
chloride is distributed throughout the body and will cross both the
placenta and the blood-brain barrier. It is rapidly excreted, the
majority being exhaled unchanged via the lungs. The remainder is
metabolized to carbon monoxide, carbon dioxide and inorganic chloride.
Two metabolic pathways have been identified, one involving cytochrome
P-450 and the second involving glutathione- S-transferase. Clear
species differences exist in the relative contributions of these two
pathways. These differences have been used as a basis for a
physiologically based pharmaco-kinetic (PB-PK) model for methylene
chloride, which allows interspecies comparison of the concentrations
of active metabolites at the target tissues, thus enhancing the value
of results from animal studies in human health risk assessment. This
approach has been used in assessing the human cancer risk associated
with exposure to methylene chloride.
The acute toxicity of methylene chloride is low. The predominant
effects in human beings are CNS depression and elevated blood
carboxyhaemoglobin (CO-Hb) levels. These effects are reversible. Other
target organs can be the liver and, occasionally, the kidney. The
odour threshold concentration of methylene chloride is reported to be
540 mg/m3 or more. Mild CNS effects have been reported following
exposure to concentrations as low as 694 mg/m3 for 1.5-3 h
(behavioural disturbances). More significant effects occur at
concentrations in excess of 2000 mg/m3. Narcosis has been reported
to occur following a 0.5-h exposure to 69 000 mg/m3. Metabolism to
carbon monoxide leads to increases in blood CO-Hb levels following
acute exposure to the vapour, a process which becomes saturable
following exposures to high levels of methylene chloride. Exposure to
either 100 or 530 mg/m3 for 7.5 h leads to CO-Hb levels of 3.4% and
5.3%, respectively, in human volunteers. This effect forms the basis
of most, if not all, published occupational exposure limits, where a
level of 5.0% is judged to be acceptable.
The predominant effects following repeated or long-term exposure
to methylene chloride are the same as for acute exposure. Reversible
symptoms of CNS depression are seen in several species, including
humans. The lowest-observed-effect level (LOEL) for this effect in all
animal species is 7100 mg/m3 by inhalation. No evidence of
irreversible neurological damage was seen in rats exposed to methylene
chloride by inhalation at concentrations up to 7100 mg/m3 for
13 weeks. Additional target organs reported in various species
chronically exposed to methylene chloride include the liver and,
occasionally, the kidney. The no-observed-adverse-effect level (NOAEL)
for chronic intermittent inhalation exposure was judged to be
710 mg/m3 in rats. After continuous exposure, slight cytoplasmic
vacuolization in the liver of both mice and rats was observed at
88-350 mg/m3.
A single study has reported the presence of methylene chloride in
the placenta, fetus and breast milk of women following occupational
exposure. The teratogenic potential of methylene chloride has been
assessed in three animal studies. Small effects on either fetal or
maternal body weights were reported, but no evidence of an effect on
the incidence of skeletal malformations or other developmental effects
was seen. A well-conducted two-generation reproductive toxicity study
in rats exposed to methylene chloride by inhalation at concentrations
up to 5300 mg/m3, 6 h/day, for 5 days/week showed no evidence of an
adverse effect on any reproductive parameter, neonatal survival or
neonatal growth in either the F0 or F1 generations.
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms (Salmonella or E. coli)
with or without metabolic activation. In eukaryotic systems it gave
either negative or, in one case, weakly positive results. In vitro
gene mutation assays and tests for unscheduled DNA synthesis (UDS) in
mammalian cells were uniformly negative. In vitro assays for
chromosomal aberrations using different cell types gave positive
results, whereas negative or equivocal results were obtained in tests
for sister chromatid exchange (SCE) induction. The majority of the in
vivo studies reported provided no evidence of mutagenicity of
methylene chloride (e.g., chromosome aberration assay, micronucleus
test or UDS assay). A very marginal increase in frequencies of SCEs,
chromosomal aberrations and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride. The significance of these results is questionable due to
methodological deficiencies in the statistical analysis. There was no
evidence of binding of methylene chloride to DNA or DNA damage in rats
or mice given high doses.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride is
genotoxic in vivo.
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following lifetime exposure to high concentrations
(7100 and 14 100 mg/m3). These tumours were not seen in the rat or
the hamster.
Increased incidence of benign mammary tumours in female rats was
observed in one study, and increased incidence and multiplicity were
observed in two other rat studies. The increased incidence of these
tumours was within the historical control range; nevertheless there
was a dose-response relationship within one study. It is considered
that an increase in a tumour type, which occurs with high and variable
incidences in control animals, which does not progress to malignancy,
and which may be related to changes in prolactin levels, is of little
importance in human hazard assessment.
In vitro and in vivo metabolism and biochemical studies, and
mutagenicity assays in bacteria and B6C3F1 mice have provided a
plausible explanation for the mechanism of action and the species
differences in the carcinogenicity of methylene chloride to the lung
and liver. This explanation is based on the existence of an isoenzyme
of glutathione- S-transferase which specifically metabolizes
methylene chloride to the reactive intermediates responsible for
tumour induction in the mouse. Markedly lower levels of this enzyme in
rats and hamsters are consistent with the fact that these tumours do
not appear in these species. The levels of the enzyme in the liver are
lower in humans than in rats or hamsters. The variability of the
current estimates of this enzyme activity in human liver is low, but
the possibility of wider variation existing in subpopulations cannot
be discounted. Although the currently available information on enzyme
activity in human lung is limited, it is expected to be lower than in
human liver. The carcinogenic potency of methylene chloride in man is
expected to be low.
Mutagenicity studies on methylene chloride in bacteria and in the
B6C3F1 mouse, which shows a very high level of activity of the
isoenzyme, reveal positive effects, whereas mutagenicity has not been
demonstrated in standard in vivo mutagenicity assays using other
systems. These observations are consistent with the above hypothesis
and provide a mechanistic basis for the induction of tumours in the
mouse.
The role of the glutathione- S-transferase isoenzyme in the
mediation of the demonstrated mutagenic effects, together with the
correlation between the activity of this pathway and the species
differences in carcinogenic response, has led to its use as the dose
surrogate in physiologically based pharmacokinetic models used for
human health risk assessment.
Overall, animal inhalation studies have shown effects on the liver
from 710 mg/m3 and on other organs from 1700 mg/m3. However, these
effects have not been observed in epidemiological studies. Effects on
the CNS have been observed in both animals and humans and a threshold
in humans has been defined, based on the level of the metabolite
carbon monoxide in the blood, leading to exposure limits of the order
of 177 mg/m3.
10.2 Evaluation of effects on the environment
Due to its high volatility methylene chloride released to the
environment will end up in the atmosphere where it can be transported
to regions far removed from the emission source. Methylene chloride is
degraded in the troposphere by reaction with hydroxyl radicals giving
carbon dioxide and hydrogen chloride as major breakdown products.
Based on a lifetime in the troposphere of about 6 months it may be
assumed that only a few percent, if any, of methylene chloride will
reach the stratosphere. No significant impact on stratospheric ozone
depletion is expected. Methylene chloride will also not contribute
significantly to photochemical smog formation. In ambient air in rural
and remote areas, background levels of 0.07-0.29 µg/m3 have been
measured. In suburban and urban areas levels up to 2 and 15 µg/m3
have been found.
Concentrations of methylene chloride in the surface water of
rivers in industrialized areas stay generally below 10 µg/litre. In
industrial effluents, outfalls of municipal water treatment plants and
leachates of landfills, concentrations of methylene chloride of up to
200 mg/litre have been measured.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive to methylene chloride, with effects on
hatching from 5.5 mg/litre; adult aquatic organisms are relatively
insensitive even after prolonged exposure. There is no evidence to
suggest that methylene chloride and/or its metabolites bioaccumulate
in the environment. Given the concentrations observed in surface water
(< 10 µg/litre) and those in contaminated effluents
(< 200 mg/litre), no significant impact on the aquatic environment is
expected.
Localized contamination of soils will not significantly disperse
despite the mobility of methylene chloride; in groundwaters and soils,
biological degradation processes have been identified capable of
mineralizing methylene chloride in a few days. From the limited
information on soil organisms, it may be assumed that contamination of
soil has only a local and transient effect.
Apart from accidental spills, it is concluded that the present use
of methylene chloride has no significant impact on the environment.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & MacKay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: the key role of organism- water partitioning.
Aquatic Toxicology 8:163-174.
Abrahamson S & Valencia R (1980) Evaluation of substances of interest
for genetic damage using Drosophila melanogaster. In: Mutagenicity
of methylene chloride. Oakridge, Tennessee, National Toxicology
Programme.
ACGIH (1992) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, US American
Conference of Governmental Industrial Hygienists.
Adams JD & Erickson HH (1976) The effects of repeated exposure to
methylene chloride vapour. Preprint from the Annual Scientific Meeting
of the Aerospace Medical Association. Washington, DC, Aerospace
Medical Association, pp 61-62.
AFS (1990) [Labour Protection Board Statute Book. Hygiene threshold
values.] Stockholm, 13 pp (in Swedish).
Agency for Toxic Substances and Disease Registry (1993) Methylene
chloride toxicity. Am Fam Phys, 47(5): 1159-1166.
Ahmed AE & Anders MW (1978) Metabolism of dihalomethanes to
formaldehyde and inorganic chloride II. Studies on the mechanism of
the reaction. Blochem Pharmacol, 27: 2021-2025.
Ahmed AE & Anders MW (1976) Metabolism of dihalomethanes to
formaldehyde and inorganic chloride. Drug Metab Dispos, 4: 357-361.
Alexander HC, McCarty WM, & Bartlett EA (1978) Toxicity of
perchloroethylene and methylene chloride to fathead minnows. Bull
Environ Contam Toxicol, 20: 344-352.
Alexeef G & Kiglore W (1983) Learning impairment in mice following
acute exposure to dichloromethane and carbon tetrachlorlde. J Toxicol
Environ Health, 11: 569-581.
Allen J, Kligerman A, Campbell J, Westbrook-Collins B, Erexson G, Kari
F, & Zeiger E (1990) Cytogenetic analysis of mice exposed to
dichloromethane. Environ Mol Mutagen, 15: 221-228.
Allred EN, Bleecker ER, Chaitman BR, Dalims TE, Gotlieb SO, Hackney
JD, Hayes D, Pagano M, & Selvester RH (1989) Acute effects of carbon
monoxide exposure on individuals with coronary artery disease. Health
Effects Institute, 98 pp (Research Report No. 25).
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Anders MW & Sunram JM (1982) Transplacental passage of dichloromethane
and carbon monoxide. Toxicol Lett, 12: 231-244.
Anders MW, Kubic VL, & Ahmed ME (1977) Metabolism of halogenated
methanes and macromolecular binding. J Environ Pathol Toxicol,
1: 117-121.
Andersen ME, Clewell III H J, Gargas ML, Smith FA, & Reitz RH (1987)
Physiologically based pharmacokinetics and the risk assessment process
for methylene chloride. Toxicol Appl Pharmacol, 87: 185-205.
Andersen CHJ, Gargas ML, MacNaughton MG, Reitz RH, Nolen RJ, & McKenna
MJ (1991) Physiologically based pharmacokinetic modelling with
dichloromethane, its metabolite, carbon monoxide, and blood
carboxyhaemoglobin in rats and humans. Toxicol Appl Pharmacol,
108: 14-27.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16: 55-137.
Andrae U & Wolff T (1983) Dichloromethane is not genotoxic in isolated
rat hepatocytes. Arch Toxicol, 52: 287-290.
Angelo MJ, Pritchard AB, Hawkins DR, Waller AR, & Roberts A (1986a)
The pharmacokinetics of dichloromethane, I. Disposition in B6C3F1
mice following intravenous and oral administration. Food Chem Toxicol,
24: 965-974.
Angelo MJ, Pritchard AB, Hawkins DR, Waller AR, & Roberts A (1986b)
The pharmacokinetics of dichloromethane, II. Disposition in Fischer
344 rats following intravenous and oral administration. Food Chem
Toxicol, 24: 975-980.
Antoine SR, de Leon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
APHA (1977) Methods of sampling and analysis. 2nd ed. Washington, DC:
American Public Health Association, pp 894-902.
APHA (1989a) Purge and trap capillary-column gas chromatographic
method. In: Standard methods for the examination of water and
wastewater, 17th ed. Washington, DC, American Public Health
Association.
APHA (1989b) Purge and trap capillary-column gas chromatographic/mass
spectrometric method In: Standard methods for the examination of water
and wastewater, 17th ed. Washington, DC, American Public Health
Association.
Arbeidsinspectie (1991) Labour Inspectorate (1991) [The national MAC
list 1991.] Directorate of Labour, Ministry for Social Affairs and
Employment (in Dutch).
Arbeidstilsynet (1990) Labour Inspectorate (1991) [Administrative
standards for atmospheric pollution in the workplace 1990.]
Directorate for the Supervision of Labour (in Norwegian).
Arendt G, Haag F, & Puggmayer D (1982) [Determination of air pollution
with organic compounds in conurbations. Research report of the Federal
Office of the Environment.] (UFOPLAN 104 02 510). Frankfurt/Main,
Batelle Institut (in German).
Åstrand I, Övrum P, & Carlsson A (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during rest
and exercise and its metabolism. Scand. J Work Environ Health,
1: 78-94.
ATSDR (1992) Toxicological Profile for Methylene Chloride. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-SZ-13).
Aviado DM (1978) Effects of fluorocarbons, chlorinated solvents and
inosine on the cardiopulmonary system. Environ Health Perspect,
26: 207-215.
Aviado DM & Belej MA (1974) Toxicity of aerosol propellants on the
respiratory and circulatory systems. 1. Cardiac arrythmia in the
mouse. Toxicology, 2: 31-42.
Aviado DM, Zakhari S, & Watanabe T (1977a) Methylene chloride. In:
Goldberg L ed. Non-fluorinated propellants and solvents for aerosols.
Cleveland, Ohio, CRC Press, pp 19-36.
Aviado DM, Zakhari S, & Watanabe T (1977b) Interactions among
hydrocarbon propellants, methylene chloride and ethanol. In: Goldberg
L ed. Non-fluorinated propellants and solvents for aerosols.
Cleveland, Ohio, CRC Press, pp 83-89.
Baldanf G (1981) The case of Grenzach - example of groundwater
pollution by environmentally relevant substances. DVGW Schr.reihe
Wasset, 29: 53-69.
Ballantyne B, Gazzard MF, & Swanston DW (1976) The ophthalmic
toxicology of dichloromethane. Toxicology, 6: 173-187.
Balmer MF, Smith FA, Leach LJ, & Yuile CL (1976) Effects in the liver
of methylene chloride inhaled alone and with ethyl alcohol. Am Ind Hyg
Assoc J, 37: 345-352.
Barassin J & Combourieu J (1973) Etude cinétique des reactions entre
l'oxygène atomique et les derivés chlorés du méthane. 1. Réaction
CH2Cl2+O. Bull Soc Chim France, 7-8: 2173-2177.
Barber ED, Donish WH, & Mueller KR (1980) Quantitative measurement of
the mutagenicity of volatile liquids in the Ames Salmonella/microsome
assay. Environ Mutagen, 2(2): 307 (Abstract P39).
Barrowcliff DF & Knell AJ (1979) Cerebral damage due to endogenous
chronic carbon monoxide poisoning caused by exposure to methylene
chloride. J Soc Occup Med, 29: 12-14.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Basso M, Raje R, & Greening M (1987) Evaluation of in vivo
mutagenicity of methylene chloride following inhalation exposure in
mice by dominant lethal test [abstract]. Toxicologist, 7: 1034.
Bauchop T (1967) Inhibition of rumen methanogenesis by methane
analogues. J Bacteriol, 94: 171-175.
Bayard SP, Bayliss DL, Davidson IWF, Fowle III Jr, Greenberg M,
Haberman BH, Kotchmar D, Benignus V, Parker JC, & Singh D (1985)
Health assessment document for dichloromethane (methylene chloride).
Final report. Washington, DC, US Environmental Protection Agency
(EPA-600/8-82-004B; NTIS PB85 191559).
Becker CE & Lash A (1990) Study of neurological effects of chronic
methylene chloride exposure in airline maintenance mechanics [abstract
7]. Vet Hum Toxicol, 32: 342.
Belej MA, Smith GA, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory system. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bell BP, Franks P, Hildreth N, & Melius J (1991) Methylene chloride
exposure and birthweight in Monroe County, New York. Environ Res,
55: 31-39.
Benzon HT, Claybon L, & Brunner EA (1978) Elevated carbon monoxide
levels from exposure to methylene chloride. J Am Med Assoc, 239: 2341.
Berger M & Fodor GG (1968) CNS disorders under the influence of air
mixtures containing dichloromethane. Zent.bl Bakteriol, 215: 517.
Bergman K (1979) Whole body radiography and allied tracer techniques
in distribution and elimination studies of some organic solvents:
benzene, toluene, xylene, styrene, methylene chloride. Scand J Work
Environ Health 5(1): 1-263.
Binnemann PH, Sandmeyer U, & Schmuk E (1983) [Contents of heavy
metals, organochlorine pesticides, PCB and volatile organohalogen
compounds in fish in the Upper Rhine and Lake Constance.] Z
Lebensm.unters Forsch, 176: 253-261 (in German).
Birge WJ, Black JA, & Kuehne RA (1980) Effects of organic compounds on
amphibian reproduction. Lexington. Kentucky, Kentucky University,
Water Resources Research Institute (Research Report No. 121; NTIS
PB80-147523).
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, Kentucky
University, Water Resources Research Institute (Research Report
No. 133: NTIS PB82-224601).
Blum DJW & Speece RE (1991) Quantitative structure-activity
relationships for chemicals toxicity to environmental bacteria.
Ecotoxicol Environ Saf, 22: 198-224.
Boeniger MF (1991) Nonisocyanate exposures in 3 flexible polyurethane
manufacturing facilities. Appl Occup Hyg, 6: 945-952.
Bogaards JJP, Van Ommen B, & Bladeten PJ (1993) Individual differences
in the in vitro conjugation of methylene chloride with glutathione
by cytosolic glutathione S-transferase in 22 human liver samples.
Blochem Pharmacol, 45(10): 2166-2169.
Bohme H, Fischer H, & Frank R (1949) Preparation and properties of the
x-halogenated thioethers. Ann Chem, 563: 54-72.
Bonnet P, Francin JM, Gradiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof Med,
41: 317-321.
Bonventre J, Brennan O, Jason D, Henderson A, & Bastos ML (1977) Two
deaths following accidental inhalation of dichloromethane and
1,1,1-trichloroethane. J Anal Toxicol, 1: 158-160.
Bornmann G & Loeser A (1967) [The question of the chronic toxic action
of dichloromethane.] Z Lebensm. unters Forsch, 136: 14-18 (in German).
Bornschein RL, Hastings L, & Manson JM (1980) Behavioral toxicity in
the offspring of rats following maternal exposure to dichloromethane.
Toxicol Appl Pharmacol, 52: 29-37.
Breslin WJ & Landry TD (1986) Methylene chloride: Effects on estrous
cycling and serum prolactin in Sprague-Dawley rats. Midland, Michigan,
Dow Chemical USA (Internal report).
Bringmann G & Kühn R (1977a) [Findings on the harmful effects of water
pollutants on Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1977b) Limiting values for damaging action of
water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test. Z Wasset Abwasser Forsch, 10: 87-98.
Bringmann G & Kühn R (1978) [Threshold values for the harmful effects
of water pollutants on blue algae (Microcystis aeruginosa) and green
algae (Scenedemus quadricauda) in the cell reproduction inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) Determination of the harmful biological
effect of water pollutants on protozoa. II. Bacteriovorous ciliates.
Z Wasser Abwasser Forsch, 13(1): 26-31.
Bringmann G & Kühn R (1982) [Findings on the harmful effect of water
pollutants on Daphnia magna in a further developed standardized test
procedure.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G & Meinck F (1964) [Water toxicological assessment of
industrial waste waters.] Gesundheits-Ingenieur, 85: 229-236 (in
German).
Briving C, Hamberger A, Kjellstrand P, Rosengren L, Karlsson JE, &
Haglid KG (1986) Chronic effects of dichloromethane on amino acids,
glutathione and phosphoethanolamine in gerbil brain. Scand J Work
Environ Health, 12: 216-220.
Brown KW & Donnelly KC (1988) An estimation of the risk associated
with the organic constituents of hazardous and municipal waste
landfill leachates. Hazard Waste Hazard Mater, 5: 1-30.
Brunner W, Staub D, & Leisinger TH (1980) Bacterial degradation of
dichloromethane. Appl Environ Microbiol, 40: 950-958.
BUA (Advisory body on wastes with environmental implications) (1986)
[Dichlormethane.] BUA-Substance Report 6. Weinheim, VCH (BUA Report
No. 6) (in German).
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Buie SE, Pratt DS, & May JJ (1986) Diffuse pulmonary injury following
paint remover exposure. Am J Med, 81: 702-704.
Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE,
Dittenber DA, Rampy LW, & McKenna MJ (1980) Methylene chloride: A two-
year inhalation toxicity and oncogenicity study in rats and hamsters.
Final report, Toxicology Research Laboratory, Health and Environmental
Sciences, Midland, Michigan, Dow Chemical USA.
Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE,
Dittenber DA, Rampy LW, & McKenna MJ (1984) Methylene chloride: A two-
year inhalation toxicity and oncogenicity study in rats and hamsters.
Fundam Appl Toxicol, 4: 30-47.
Burton DT & Fischer DJ (1990) Acute toxicity of cadmium, copper, zinc,
ammonia, 3,3'-dichlorobenzidine, 2,6-dichloro-4-nitroaniline,
methylene chloride, and 2,4,6-trichlorophenol to juvenile grass shrimp
and killifish. Bull Environ Contam Toxicol, 44: 776-783.
Callen DF, Wolf CR, & Philpot RM (1980) Cytochrome P-450 mediated
genetic activity and cytotoxicity of seven halogenated aliphatic
hydrocarbons in Saccharomyces cerevisiae. Mutat Res, 77: 55-63.
Carlsson A & Hultengren M (1975) Exposure to methylene chloride, III.
Metabolism of 14C-labelled methylene chloride in rat. Scand J Work
Environ Health, 1: 104-108.
Carreon R (1981) Methylene chloride: Acute oral toxicity. Midland,
Michigan, Dow Chemical Company (Internal report).
Casanova M, Deyo DF, & Heck Hd'A (1992) Dichloromethane (methylene
chloride): Metabolism to formaldehyde and formation of DNA-protein
cross-links in B6C3F1 mice and Syrian golden hamsters. Toxicol Appl
Pharmacol, 114: 162-165.
CEC (1980) Carcinogenicity volume II: Summary reviews of scientific
evidence. Luxembourg, European Commission, Health and Safety
Directorate, Directorate-General for Employment, Industrial Relations
and Social Affairs, pp 67-74.
CEC (1982) Multiannual programme of the Joint Research Centre
1980-1983, 1982 Annual Status Report - Protection of the environment.
Luxembourg, European Commission (Report EUR 8527-EN).
CEC (1986) Organochlorine solvents, health risks to workers.
Luxembourg, European Commission (Report EUR-10531-EN).
CEFIC (1986) The occurrence of chlorinated solvents in the
environment. European Chemical Industry Council. Chem Ind,
15: 861-869.
CEFIC (1993) Methylene chloride: Use in industrial applications.
Brussels, European Chemical Industry Council.
Cherry N, Venables H, Waldron HA, & Wells GG (1981) Some observations
on workers exposed to methylene chloride. Br J Ind Med, 38: 351-355.
Chodola GR, Biswas N, Bewtra JK, St Pierre CC, & Zytner RG (1989) Fate
of selected volatile organic substances in aqueous environment. Water
Pollut Res J Can, 24: 119-142.
Chrostek WJ & Levine MS (1981) Health Hazard Evaluation Report No.
HHE-80-154-1207: Bechtel Powder Corporation, Betwick, Pennsylvania,
24 pp.
Clark DG & Tinston DJ (1982) Acute inhalation toxicity of some
halogenated and non-halogenated hydrocarbons. Hum Toxicol, 1: 239-247.
Cohen JM, Dawson R, & Koketsu M (1980) Extent-of-exposure survey of
methylene chloride. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 53 pp (Document No. 80-131).
Coleman WE, Lingg RD, Melton RG, & Kopfler FC (1976) The occurrence of
volatile organics in five drinking water supplies using gas
chromatography/mass spectrometry. In: Keith LH, ed. Identification and
analysis of organic pollutants in water. Ann Arbor, Michigan, Ann
Arbor Science, pp 305-327.
Condie LW, Smallwood CL, & Laurie RD (1983) Comparative renal and
hepatotoxicity of halomethanes: bromodichloromethane, bromoform,
chloroform, dibromochloromethane and methylene chloride. Drug Chem
Toxicol, 6: 563-578.
Cordes DH, Brown WD, & Quinn KM (1988) Chemically induced hepatitis
after inhaling organic solvents. West J Med, 148: 458-460.
Cornish HH, Ling BP, & Barth ML (1973) Phenobarbital and organic
solvent toxicity. Am Ind Hyg Assoc J, 34: 487-492.
Corsi GC, Valentini F, & Bertazzon A (1983) Effects of subtoxic
amounts of furan, acetylfuran and methylene chloride on some serum
enzymes of rat. Boll Soc Ital Biol Sper, 59: 1049-1052.
Cox RA, Derwent RG, & Eggleton AE (1976) Photochemical oxidation of
halocarbons in the troposphere. Atmos Environ, 10: 305-308.
Crebelli R, Andreoli C, Carere A, Conti G, Conti L, Cotta Ramusino M,
& Benigni R (1992) The induction of mitotic chromosome malsegregation
in Aspergillus nidulans. Quantitative structure activity
relationship (QSAR) analysis with chlorinated aliphatic hydrocarbons.
Mutat Res, 266: 117-134.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: unspecific or specific mechanism? Murat
Res, 201: 401-411.
Cunningham ML, Gandolfi AJ, Brendel K, & Sipes IG (1981) Covalent
binding of halogenated volatile solvents to subcellular macromolecules
in hepatocytes. Life Sci, 29: 1207-1212.
Cuppit LT (1980) Fate of toxic and hazardous materials in the air
environment. Washington, DC, US Environmental Protection (NTIS
PB80-221948).
Daft JL (1987) Determining multifumigants in whole grain and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daniels SL, Hoerger FD, & Moolenaer RJ (1985) Environmental exposure
assessment: experience under the toxic substances control act. Environ
Toxicol Chem, 4: 107-117.
Dankovic DA & Baiter AJ (1994) The impact of exercise and intersubject
variability on dose estimates for dichloromethane derived from a
physiologically based pharmacokinetic model. Fundam Appl Toxicol,
22: 20-25.
Danni O, Brossa O, Burdino E, Milillo P, & Ugazio G (1981) Toxicity of
halogenated hydrocarbons in pretreated rats - an experimental model
for the study of integrated permissible limits of environmental
poisons. Int Arch Occup Health, 49: 164-176.
Davis JW & Madsen SS (1991) The biodegradation of methylene chloride
in soils. Environ Toxicol Chem, 10: 463-474.
Davis EM, Murray HE, Liehr JG, & Powers EL (1981) Basic microbial
rates and chemical byproducts of selected organic compounds. Water
Res, 15: 1125-1127.
De Walle FB & Chain ESK (1978) Presence of trace organics in the
Delaware river and their discharge by municipal and industrial
sources. Proc Ind Waste Conf, 32: 908-919.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in Northern Italy. Environ Int,
12: 343-350.
Dequinze J, Scimar C, & Edeline F (1984) Identification of the
substances and their derived products on the list of 129 substances
(list 1 of the Directive 76/464/EEC), present in the refuse of
chlorine derived organic chemistry industry CEBEDEAU (Final report
No. 83/205).
Derwent RG & Eggleton AEJ (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos Environ, 12: 1261-1269.
Derwent RG & Jenkin ME (1991) Hydrocarbons and the long-range
transport of ozone and PAN across Europe. Atmos Environ,
25A: 1661-1678.
Devereux TR, Foley JF, Maronpot RR, Kari F, & Anderson MW (1993) RAS
proto-oncogene activation in liver and lung tumors from B6C3F1 mice
exposed chronically to methylene chloride. Carcinogenesis,
14(5): 795-801.
DFG (Deutsche Forschungsgemeinschaft) (1983) In: Henschler D ed.
[Maximum workplace concentration and biological tolerance values for
working materials.] Verlage Chemie, Weinheim, Germany (in German).
DFG (Deutsche Forschungsgemeinschaft) (1991), Senate Committee for the
Testing of Working Materials Endangering Health (1991) Maximum
workplace concentrations and biological tolerance values for working
materials. Weinheim, Germany, VCH Publishers (in German).
Di Renzo AB, Gandolt AJ, & Sipes IG (1982) Microsomal and covalent
binding of aliphatic halides to DNA. Toxicol Lett, 11: 243-252.
Di Vincenzo GD & Kaplan CJ (1981a) Uptake, metabolism and elimination
of methylene chloride vapor by humans. Toxicol Appl Pharmacol,
59: 130-140.
Di Vincenzo GD & Kaplan CJ (1981b) Effect of exercise or smoking on
the uptake, metabolism, and excretion of methylene chloride vapor.
Toxicol Appl Pharmacol, 59: 141-148.
Di Vincenzo GD & Hamilton ML (1975) Fate and disposition of 14C-
methylene chloride in the rat. Toxicol Appl Pharmacol, 32: 385-393.
Di Vincenzo GD, Yanno FJ, & Astill BD (1971) The gas chromatographic
analysis of methylene chloride in breath, blood and urine. Am Ind Hyg
Assoc J, 32: 387-391.
Di Vincenzo GD, Yanno F J, & Astill BD (1972) Human and canine
exposure to methylene chloride vapor. Am Ind Hyg Assoo J, 33: 125-135.
Dill DC, Watanabe PG, & Norris JM (1978) Effect of methylene chloride
on the oxyhemoglobin dissociation curve of rat and human blood.
Toxicol Appl Pharmacol, 46(1): 125-129.
Dill DC, Murphy PG, & Mayes MA (1987) Toxicity of methylene chloride
to life stages of fathead minnow, Pimephales promelas Rafinesque.
Bull Environ Toxicol, 39: 869-876.
Dilling WL, Tefertiller NB, & Kallos GJ (1975) Evaporation rates of
methyl chloride, chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute aqueous
solutions. Environ Sci Technol, 9: 833-838.
Dilling WL (1977) Interphase transfer processes. II. Evaporation rates
of chloromethanes, ethanes, ethylenes, propanes, and propylenes from
dilute aqueous solutions; Comparisons with theoretical predictions.
Environ Sci Technol, 4: 405-409.
Dillon DM, Combos RD, McConville M, & Zeiger E (1990) The role of
metabolism and glutathione in the mutagenicity of vapour phase
dichloromethane in bacteria. Environ Mol Mutagen, 15 (Suppl 17):
Abstr 52.
Dillon DM, Edwards IR, Combos RD, McConville M, & Zeiger E (1992) The
role of glutathione in the bacterial mutagenicity of vapor phase
dichloromethane. Environ Mol Mutagen, 20: 211-217.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Technol, 9: 762-765.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvents chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. J Eur J Toxicol, 9(3): 171-177.
ECETOC (1984) Joint assessment of commodity chemicals No. 4, Methylene
chloride. Brussels, European Centre for Ecotoxicology and Toxicology
of Chemicals.
ECETOC (1988) Methylene chloride (dichloromethane): human risk
assessment using experimental animal data. Brussels, European Centre
for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 32).
ECETOC (1989) Methylene chloride (dichloromethane): an overview of
experimental work investigating species, differences in
carcinogenicity and their relevance to man. Brussels, European Centre
for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 34).
ECSA (European Chlorinated Solvents Association) (1989) Methylene
chloride, its properties, uses, occurrence in the environment,
toxicology and safe handling. Brussels, European Chemical Industry
Council.
ECSA (European Chlorinated Solvents Association) (1992) ECSA
production figures for methylene chloride. Brussels, European Chemical
Industry Council.
Edwards PR, Campbell I, & Milne GS (1982) The impact of chloromethanes
on the environment. Part 2. Methyl chloride and methylene chloride.
Chem Ind, 619-622.
Eisenbrandt DL & Reitz RH (1986) Acute toxicity of methylene chloride:
Tumorigenic implications for B6C3F1 mice. Toxicologist, 6: 662.
English JM (1964) A case of probable phosgene poisoning. Br Med J,
1: 38.
Engström J & Bjurström R (1977) Exposure to methylene chloride:
Content in subcutaneous adipose tissue. Scand J Work Environ Health,
3: 215-224.
European Council (1982) Council Directive of 17 May 1982 amending for
the second time Directive 76/768/EEC on the approximation of the laws
of the Member States relating to cosmetic products (82/368/EEC). Off J
Eur Communities, L167: 1-8.
Fagin J, Bradley J, & Williams D (1980) Carbon monoxide poisoning
secondary to inhaling methylene chloride. Br Med J, 281: 1461.
Fells I & Moelwyn-Hughes EA (1958) The kinetics of the hydrolysis of
metylene chloride. J Chem Soc, 1326-1333.
Ferguson J & Pirie H (1948) The toxicity of vapours to the grain
weevil. Ann Appl Biol, 35: 532-550.
Ferrario JB, Lawler GC, DeLeon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Flanagan R J, Ruprah M, Meredith T J, & Ramsey JD (1990) An
introduction to the clinical toxicology of volatiles substances. Drug
Saf, 5(5): 359-383.
Flathman PA, Jerger DE, & Woodhall PM (1992) Remediation of
dichloromethane (DCM)-contaminated ground water. Environ Prog,
11(3): 202-209.
Fleeger AK & Lee JS (1988) Characterization of worker exposures to
methylene chloride resulting from application of aerosol glue in the
asbestos abatement industry. Appl Ind Hyg, 3: 245-250.
Flury F & Zernik F (1931) [Harmful gases, yapours, mists, smokes, and
dusts]. Berlin, Julius Springer, pp 311-312 (in German).
Foder GG, Prajsnar D, & Schlipkoter KW (1973) Endogenous conformation
by incorporated halogenated hydrocarbons of the methane series.
Staubreinhalt Luft, 33: 260-261.
Fodor GR & Winneke (1971) Nervous system disturbances in men and
animals experimentally exposed to industrial solvent vapors. In:
England HM ed. Proceedings of the 2nd International Clean Air
Congress. New York, Academic Press, pp 238-243.
Fodor GG, Schlipköter HW, & Zimmermann M (1973) The objective study of
sleeping behaviour in animals as a test of behavioural toxicity.
In: Horvath M (ed.) Adverse effects of environmental chemicals and
psychoretapic drugs: quantitative interpretation of functional tests.
London, Elsevier, pp 115-123.
Foley JF, Tuck PD, Ton TVT, Frost M, Kari F, & Anderson MW (1993)
Inhalation to a hepatocarcinogenic concentration of methylene chloride
does not induce sustained replicative DNA synthesis in hepatocytes of
female B6C3F1 mice. Carcinogenesis, 14(5): 811-817.
Foster JR, Green T, Smith LL, Lewis RW, Hext PM, & Wyatt I (1992)
Methylene chloride - an inhalation study to investigate pathological
and biochemical events occurring in the lungs of mice over an exposure
period of 90 days. Fundam Appl Toxicol, 18: 376-388.
Friedlander BR, Hearne T, & Hall S (1978) Epidemiologic investigation
of employees chronically exposed to methylene chloride. J Occup Med,
20: 657-666.
Fuxe K, Andersson K, Hansson T, Agnati LF, Eneroth P, & Gustafsson JA
(1984) Central catecholamine neurons and exposure to dichloromethane.
Selective changes in amine levels and turnover in tel- and
diencephalic DA and NE nerve terminal systems and in secretion of
anterior pituitary hormones in the male rat. Toxicology, 29: 293-305.
Gälli R & Leisinger T (1985) Specialized bacterial strains for the
removal of dichloromethane from industrial waste. Conserv Recycl,
8: 91-100.
Gargas ML, Clewell III HJ, & Anderson ME (1986) Metabolism of inhaled
dihalomethanes in vivo: differentiation of kinetic constants for two
independent pathways. Toxicol Appl Pharmacol, 82: 211-223.
Gerritsen WB & Buschmann CH (1960) Phosgene poisoning caused by the
use of chemical paint removers containing methylene chloride in ill-
ventilated rooms heated by kerosene stoves. Br J Ind Med, 17: 187-189.
Ghittori S, Marraccini P, Franco G, & Imbirani M (1993) Methylene
chloride exposure in industrial workers. Am Ind Hyg Assoc J,
54(1): 27-31.
Gibbs GW (1992) The mortality of workers employed at a cellulose
acetate and triacetate fibers plant in Cumberland Maryland, a "1970"
cohort followed 1970-1989. Final report by Safety Health Environmental
International Consultants, Winterburn, Alberta (TO). Somerville, New
Jersey, Hoechst Celanese.
Giger W, Schwarzenbach RP, Hoehn E, Schellenberg K, Schneider JK,
Wasmer HR, Westall J, & Zobrist J (1983) [Das Verhalten organischer
Wasser-inhaltstoffe bei der Grundwasserbildung und im Grundwasser.]
Gaz-Eaux-usées, 63: 517-531 (in German).
Gocke E, King M-T, Eckhardt K, & Wild D (1981) Mutagenicity of
cosmetic ingredients licensed by the European Communities. Murat Res,
90: 91-109.
Gomez MR, Cocco P, Dfosemeci M, & Stewart PA (1994) Occupational
exposure to chlorinated aliphatic hydrocarbons: Job exposure matrix.
Am J Ind Med, 26(2): 171-183.
Gossett JM (1985) Anaerobic degradation of C1 and C2 chlorinated
hydrocarbons. Air Force Engineering Service Centre, Engineering
Service Laboratory, 153 p (ESL-TR-85-38).
Gradiski D, Bonnet P, Raoult G, Magadur JL, & Francin JM (1978)
Toxicité aiguë comparée par inhalation de principaux solvants
alphatiques chlorés. Arch Mal Prof Méd Trav Sécur Soc, 39: 249-257.
Green T (1983) The metabolic activation of dichloromethane in a
bacterial assay using Salmonella typhimurium. Mutat Res,
118: 277-288.
Green T, Provan WM, Collinge DC, & Guest AE (1986a) Methylene chloride
(dichloromethane): Interaction with rat and mouse liver and lung DNA
in vivo. Alderley Park, Macclesfield (Cheshire), ICI (Technical Report
CTL/R/851).
Green T, Provan WM, Nash JA, & Gowans N (1986b) Methylene chloride
(dichloromethane): In vivo inhalation pharmacokinetics and metabolism
in F344 rats and B6C3F1 mice. Alderley Park, Macclesfield (Cheshire),
ICI (Technical Report CTL/R/880).
Green T, Nash JA, & Mainwaring G (1986c) Methylene chloride
(dichloromethane): In vitro metabolism in rat, mouse and hamster liver
and lung fractions and in human liver fractions. Alderley Park,
Macclesfield (Cheshire), ICI (Technical Report CTL/R/879).
Green T, Provan WM, & Gowans N (1987a) Methylene chloride
(dichloromethane): In vivo inhalation pharmacokinetics in B6C3F1 mice
(using stable isotopes) and F344 rats. AlderIcy Park, Macclesfield
(Cheshire), ICI (Technical Report CTL/R/931).
Green T, Nash JA, & Hill SJ (1987b) Methylene chloride
(dichloromethane): Glutathione- S-transferase metabolism in vitro in
rat, mouse, hamster, and human liver cytosol fractions. Alderley Park,
Macclesfield (Cheshire), ICI (Technical Report CTL/R/934).
Green T, Nash JA, Hill SJ, & Foster JR (1987c) Methylene chloride
(Dichloromethane): The effects of exposure to 4000 ppm on mouse lung
enzymes. Alderley Park, Macclesfield (Cheshire), ICI (Report
CTL/R/935).
Green T, Provan WM, Collinge DC, & Guest AE (1988) Molecular
interactions of inhaled methylene chloride in rats and mice. Toxicol
Appl Pharmacol, 93(1): 1-10.
Gu Z & Wang Y (1988) [Evaluation of genotoxic effect of 16 chemicals
using the micronucleus assay in vitro]. Weisheng Dulixue Zazhi,
2: 1-4 (in Chinese).
Guengerich FP, Shimada T, Raney KD, Yun CH, Meyer DJ, Ketterer B,
Harris TM, Groopman JD, & Kadlubar FF (1992) Elucidation of catalytic
specificites of human cytochrome P450 and glutathione-S-transferase
enzymes and relevance to molecular epidemiology. Environ Health
Perspect, 98: 75-80.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of The Netherlands. Sci Total Environ, 43: 193-219.
Guidelines on the Evaluation and Treatment of Groundwater Pollution
with Readily Volatile chlorohydrocarbons (1983) Published by the
Ministry for Nutrition, Agriculture and Environment, Baden-
Württemberg, Heft, 13 August.
Hake CL, Stewart RD, Wu A, & Graff SA (1975) Carboxyhaemoglobin levels
of humans exposed to methylene chloride. 14th Annual Meeting of the
Society of Toxicity, Williamsbergh, Virginia. Toxicol Appl Pharmacol,
33(1): 145 (Abstract 59).
Halbartschlager J, Kohler H, Swerinski H, & Bardtke D (1984) [Studies
on the biological decomposition of chlorohydrocarbons, using the
example of dichlormethane (methylene chloride).] GWF-Wasser/Abwasser,
125: 380-386 (in German).
Hall AH & Rumack BH (1990) Methylene chloride exposure in furniture-
stripping shops: Ventilation and respirator use practices. J Occup
Med, 32: 33-37.
Hallier E, Laughof T, Dannappel D, Leutbecher M, Schroeder K, Goergeus
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methylbromide, ethylene oxide and dichloromethane in human blood:
Influence of the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol 67: 173-178.
Hansch C & Leo A (1979) Substituent Constants for Correlation Analysis
in Chemistry and Biology. New York, Chichester, Brisbane, Toronto,
John Wiley and Sons.
Hardin BD & Manson JM (1980) Absence of dichloromethane teratogenicity
with inhalation exposure in rats. Toxicol Appl Pharmacol, 52: 22-28.
Harkov R (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci Total
Environ, 38: 259-274.
Harkov R, Giante SJ, Bozelli JW, & LaRegina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20: 491-501.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnoro S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Haun CC, Vernot EH, Darmer KI, & Diamond SS (1972) Continuous animal
exposure to low levels of dichloromethane. In: Proceedings of the 3rd
Annual Conference on Environmental Toxicology. Dayton, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
pp. 199-208 (Paper No. 12; AMRL-TR-130).
Hayes WC & Bailey RE (1977) The inhibition of anaerobic sludge gas
production by 1,1,1-trichloroethane, methylene chloride,
trichloroethylene and perchloroethylene. Report dated 27 Jan 1977.
Midland, Michigan, USA, Dow Chemical.
Hearne FT, Grose F, Pifer WJ, Friedlander BR, & Raleigh RL (1987)
Methylene chloride mortality study: Dose-response characterization and
animal model comparison. J Occup Med, 29: 217-228.
Hearne FT & Friedlander BR (1981) Follow-up of methylene chloride
study. J Occup Med, 23: 660.
Hearne FT, Pifer JW, & Grose F (1990) Absence of adverse mortality
effects in workers exposed to methylene chloride. An update. J Occup
Med, 32(3): 234-240.
Hegi ME, Soderkvist P, Foley JE, Schronhoven R, Swenberg JA, Kari F,
Maronpot RR, Anderson MW, & Wiseman RW (1993) Characterization of p53
mutations in methylene chloride-induced lung tumors from B6C3F1 mice.
Carcinogenesis, 14(5): 803-810.
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products and intermediate
grain based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heikes DL (1987) Purge and trap method for determination of volatile
hydrocarbons and carbon disulphide in table ready foods. J Assoc Off
Anal Chem, 70(2): 215-226.
Heil H, Eikman T, Einbrodt HJ, König H, Lahl U, & Zeschmar-Lahl B
(1989) [Consequences of the Bielefeld-brake Atlas case.] Vom Wasser,
72: 321-348 (in German).
Heineman EF, Cocco P, Gomez MR, Dosemeci M, Stewart PA, Hayes RB,
Zahim SH, Thomas TL, & Blair A (1994) Occupational exposure to
chlorinated aliphatic hydrocarbons and risk of astrocytic brain
cancer. Am J Ind Med, 26(2): 155-169.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity to 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellmann H (1984) [Readily volatile chlorohydrocarbons in the inland
waters of the Federal Republic of Germany - occurrence and
quantities.] Gesundheits-Ingenieur, 105: 269-278 (in German).
Helz GR & Hsu RY (1978) Volatile chloro- and bromocarbons in coastal
waters. Limnol Oceanogr, 23: 858-869.
Henson JM, Yates MV, Cochran JW, & Shackleford DL (1988) Microbial
removal of halogenated methanes, ethanes, and ethylenes in an aerobic
soil exposed to methane. FEMS Microbiol Ecol, 53: 193-201.
Heppel LA, Neal PA, Perrin TL, Orr ML, & Porterfield VT (1944)
Toxicology of dichloromethane (methylene chloride). I. Studies on
effects of daily inhalation. J Ind Hyg Toxicol, 26: 8-16.
Heppel LA & Neal PA (1944) Toxicology of dichloromethane (methylene
chloride). II. Its effect upon running activity in the male rat. J Ind
Hyg Toxicol, 26: 17-21.
Hermens J, Busser F, Leeuwangh P, & Musch A (1985) Quantitative
structure-activity relationships and mixture toxicity of organic
chemicals in Photobacterium phosphoreum: the microtox test.
Ecotoxicol Environ Saf, 9: 17-25.
Hext PM, Foster J, & Millward SW (1986) Methylene chloride
(Dichloromethane): 10-day inhalation toxicity study to investigate the
effects on rat and mouse liver and lungs. ICI Report No. CTL/P/1432.
Horvath AL (1982) Halogenated hydrocarbons: solubility-miscibility
with water. New York, Marcel Dekker, Inc.
Hov O, Penkett SA, lsaksen ISA, & Semb A (1984) Organic gases in the
Norwegian Arctic. Geophys Res Left, 11: 425-428.
Howard PH (1990) Dichloromethane. In: Howard PH ed. Handbook of
environmental fate and exposure data for organic chemicals. Chelsea,
Michigan, Lewis Publishers, Inc., pp 176-183.
Howard PH, Sage GW, & Jarvis WF ed. (1990) Handbook on Environmental
Fate and Exposure Data for Organic Chemicals. Chelsea, Michigan, Lewis
Publishers Inc., pp 176-183.
HSE (UK Health and Safety Executive) (1987) ACTS review:
dichloromethane. London, Her Majesty's Stationery Office.
HSE (UK Health and Safety Executive) (1992) National exposure data
base. Bootle (Merseyside), United Kingdom. Health and Safety
Executive.
Hughes JP (1954) Hazardous exposure to some so-called safe solvents. J
Am Med AssPc, 156: 234-237.
Hughes NJ & Tracey JA (1993) A case of methylene chloride (nitremans)
poisoning, effects on carboxyhaemoglobin levels. Hum Exp Toxicol,
12(2) 159-160.
Hutchinson TC, Hellebust JA, Tam D, MacKay D, Mascarenhas RA, & Shiu
WY (1978) The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1986) Some halogenated hydrocarbons and pesticide exposure.
Lyon, International Agency for Research on Cancer, 43-85 (IARC
Monograph on the Evaluation of Carcinogenic Risk of Chemicals to
Humans, Volume 41).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office (Occupational Safety and
Health Series No. 37).
IPCS (International Programme on Chemical Safety) (1984) Environmental
health criteria 32: methylene chloride. Geneva, World Health
Organization.
Ito A, Kawata F, Takeshita T, & Ito M (1990) [Experimental studies of
effects of methylene chloride on living body (1)]. Hochudoku, 8: 64-65
(in Japanese).
Janssen PJM & Pot TE (1988a) Acute oral toxicity study with
dichloromethane in rats. Weesp, The Netherlands, Solvay Duphar
(Unpublished document 56645/33/88).
Janssen PJM & Pot TE (1988b) Acute dermal toxicity study with
dichloromethane in rats. Weesp, The Netherlands, Solvay Duphar
(Unpublished document 55645/24/88).
Jensen AA (1983) Chemical contaminants in human milk. Residue Rev,
89: 1-108.
Jenson RA (1978) A simplified bioassay using finfish for estimating
potential spill damage. In: Proceedings of the Meeting on Control of
Hazardous Material Spills, Rochville, Maryland, pp 104-108.
Jernelov M & Antonsson AB (1987) [Exposure to solvents when pouring
polyurethane into moulds.] Oslo, IVL (Report No. B869) (in Norwegian).
Jongen WMF, Alink GM, & Koeman JH (1978) Mutagenic effect of
dichloromethane on Salmonella typhimurium. Mutat Res, 56: 245-248.
Jongen WMF, Lohman PHM, Kottenhagen M J, Alink GM, Betends F, & Koeman
JH (1981) Mutagenicity testing of dichloroethane in short-term
mammalian test systems. Mutat Res, 81(2): 203-213.
Jongen WMF, Harmsen EGM, Alink GM, & Koeman JH (1982) The effect of
glutathione conjugation and microsomal oxidation on the mutagenicity
of dichloromethane in Salmonella typhimurium. Mutat Res,
95: 183-189.
Jongen WMF (1984) Relationship between exposure time and metabolic
activation of dichloromethane in Salmonella typhimurium. Mutat Res,
136(2): 107-108.
Juhnke I & Ltidemann D (1978) [Results of the testing of 200 chemical
compounds for acute toxicity for fish in the golden orfe test.] Z
Wasser Abwasser Forsch, 11: 161-164 (in German).
Kanazawa S & Filip Z (1987) Effects of trichloroethylene and
dichloromethane on soil biomass and microbial counts. Zent.bl
Bakteriol Hyg, 184; 24-33.
Kanazawa S & Filip Z (1986) Effect of trichloroethylene,
tetrachloroethylene and dichloromethane on enzymatic activities in
soil. Appl Microbiol Biotechnol, 25: 76-81.
Kanno J, Foley JF, Kari F, Anderson MW, & Maronport RR (1993) Effect
of methylene chloride inhalation on replicative DNA synthesis in the
lungs of female B6C3F1 mice. Environ Health Perspect, 101 (Suppl 5):
271-286.
Kari FW, Maronpot RR, & Anderson MW (1992) Testimony for the OSHA
hearing on the proposed occupational standard for methylene chloride.
Research Triangle Park, North Carolina, National Institute of
Environmental Health Sciences.
Kari FW, Foley JF, Seilkop SK, Maronpot RR, & Anderson MW (1993)
Effect of varying exposure regimens on methylene chloride induced lung
and liver tumors in female B6C3F1 mice. Carcinogenesis,
14(5): 819-826.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-847.
Karlsson J-E, Rosengren LE, Kjellstrand P, & Haglid KG (1987) Effects
of low-dose inhalation of three chlorinated aliphatic organic solvents
on deoxyribonucleic acid in gerbil brain. Scand J Work Environ Health,
13: 453-458.
Kashin LM, Makotchenko VM, Malinina-Putsenko VP, Mikhailovskaja LF, &
Shmuter LM (1980) [Experimental and clinico-hygienic investigations of
methylene chloride toxicity.] Vrach Delo, 1: 100-103 (in Russian).
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
Kelley RD (1985) Synthetic organic compound sampling survey of public
water supplies. Washington, DC, Environmental Protection Agency (NTIS
PB85-214427).
Kelly M (1988) Case reports of individuals with oligospermia and
methylene chloride exposures. Reprod Toxicol, 2: 13-17.
Kim YC & Carlson GP (1986) The effect of an unusual workshift on
chemical toxicity, I. Studies on the exposure of rats and mice to
dichloromethane. Fundam Appl Toxicol, 6: 162-171.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kirschman JC, Brown NM, Coots RH, & Morgareidge K (1986) Review of
investigations of dichloromethane metabolism and subchronic oral
toxicity study as the basis for the design of chronic oral studies in
rats and mice. Food Chem Toxicol, 24: 943-949.
Kirwin CJ, Thomas WC, & Simmon VF (1980) In vitro microbiological
mutagenicity studies of hydrocarbon propellants. J Soc Cosmet Chem,
31(7): 367-370.
Kitchin KT & Brown JL (1989) Biochemical effects of three carcinogenic
chlorinated methanes in rat livers. Teratogen Carcinogen Mutagen,
9: 61-69.
Kjellstrand P, Bjerkemp M, Adler-Maihofer M, & Holmquist B (1986)
Effects of methylene chloride on body and organ weight and plasma
butyrylcholinesterase activity in mice. Acta Pharmacol Toxicol,
59: 73-79.
Kjellstrand P, Mansson L, Holmquist B, & Jonsson I (1990) Tolerance
during inhalation of organic solvents. Pharmacol Toxicol, 66: 409-414.
Klaassen CD & Plaa GL (1966) Relative effects of various chlorinated
hydrocarbons on liver and kidney function in mice. Toxicol Appl
Pharmacol, 9: 139-151.
Klaassen CD & Plaa GL (1967) Relative effects of various chlorinated
hydrocarbons on liver and kidney function in dogs. Toxicol Appl
Pharmacol, 10: 119-131.
Klecka GM & Gonsior SJ (1984) Nonenzymatic reductive dechlorination of
chlorinated methanes and ethanes in aqueous solution. Chemosphere,
13: 391-402.
Klecka GM (1982) Fate and effects of methylene chloride in activated
sludge. Appl Environ Microbiol, 44: 701-707.
Klimmer OR (1968) Working paper on dichloromethane. Presented to the
Committee on Foreign Substances in Food of the German Research
Council, Bad Godesberg, 5 July 1968. Bonn, German Research Council (in
German).
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and in vitro.
J Pharmacol Exp Ther, 220: 597-603.
KNIE (1988) The dynamic daphnia test - practical experience in the
monitoring of inland waters. Gewasserschutz-Wasser-Abwasser,
102: 341-357 (in German).
Koch M, Dolfing J, Whurmann K, & Zehnder AJB (1983) Pathways of
propionate degradation by enriched methanogenic cultures. Appl Environ
Microbiol, 45: 1411-1414.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies, part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-211.
Kool HJ, Van Kreijl CF, & Zoeteman BCJ (1982) Toxicology assessment of
organic compounds in drinking water. CRC Crit Rev Environ Control,
12: 307-350.
Kopfler FC, Melton RG, Lingg RD, & Coleman WE (1977) Human exposure to
water pollutants. Adv Environ Sci Technol, 8: 419-433.
Kozena L, Frantik E, & Vodickova A (1990) Methylene chloride does not
impair vigilance performance at blood levels simulating limit
exposure. Acta Nerv Super, 32: 35-37.
Kramers PGN, Mout HCA, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252:17-33.
Kubic VL & Andera MW (1978) Metabolism of the dichloromethane to
carbon monoxide III. Studies on the mechanism of the reaction. Blochem
Pharmacol, 27: 2349-2355.
Kubic VL, Anders MW, Engel RR, Bertow CH, & Caughey WS (1974)
Metabolism of dichloromethanes to carbon monoxide I. In vivo
studies. Drug Metab Dispos, 2: 53-57.
Kubic VL & Anders MW (1975) Metabolism of dihalomethanes to carbon
monoxide. II. In vitro studies. Drug Metab Dispos, 3: 104-112.
Kühn R, Pattard M, Pernak KD, & Winter A (1989) Results of the harmful
effects of selected water pollutants (anilines, aliphatic compounds)
to Daphnia magna. Water Res, 23: 495-499.
Kühn R (1979) Results of ecotoxicological testing of about 200
selected compounds. Paris, Organisation for Economic Co-operation and
Development, Chemicals Testing Programme (ECO 22).
Kurppa K, Kivisto H, & Vainio H (1981) Dichloromethane and carbon
monoxide inhalation: carboxyhaemoglobin addition and drug metabolising
enzymes in rat. Int Arch Occup Environ Health, 49: 83-87.
Kurppa K & Vainio H (1981) Effects of intermittent dichloromethane
inhalation on blood carboxyhaemoglobin concentration and drug
metabolizing enzymes in rat. Res Commun Chem Pathol Pharmacol,
32: 535-544.
Kutob SD & Plaa GL (1962) A procedure for estimating the hepatoxic
potential of certain industrial solvents. Toxicol Appl Pharmacol,
4: 354-361.
Kuzelova M & Vlasak R (1966) [The effect of methylene chloride on the
health of workers in production of film-foils and investigation of
formic acid as a methylene-dichloride metabolite]. Prac Lek,
18: 167-170 (in Czech).
Kwa HG, Van der Gugten AA, & Verhofstad F (1974) Radioimmunoassay of
rat prolactin. Prolactin levels of rats with spontaneous pituitary
tumours, primary oestrogen-induced pituitary tumours or pituitary
transplants. Eur J Cancer, 5: 571-579.
Laham S (1978) Toxicological studies on dichloromethane, a solvent
simulating carbon monomide poisoning. Toxicol Eur Res, 1: 63-73.
Landry TD, Burek JD, Bell TJ, & Wolfe EL (1981) Methylene chloride: An
acute inhalation toxicity study in rats. Midland, Michigan, Dow
Chemical Company (Internal report).
Lanes SF, Cohen A, Rothman KJ, Dreyer NA, & Soden KJ (1990) Mortality
of cellulose fiber production workers. Scand J Work Environ Health,
16: 247-251.
Lanes SF, Rothman KG, Dreyer NA, & Soden KJ (1993) Mortality update of
cellulose fiber production workers. Scand J Work Environ Health,
19(6): 426-428.
Lapat-Polasko LT, McCarty PL, & Zehnder AJB (1984) Secondary substrate
utilisation of methylene chloride by an isolated strain of
Pseudonomas sp. Appl Environ Microbiol, 47: 825-830.
Lash AA, Becker CE, So Y, & Shore M (1991) Neurotoxic effects of
methylene chloride: Are they long lasting in humans? Br J Ind Med,
48: 418-426.
Lazarew NW (1929) The narcotic effect of vpours from the chlorine
derivatives of methane, ethane and ethylene. Arch Exp Pathol
Pharmakol, 141: 19-24.
Le Blanc GA (1984) Interspecies relationships of priority pollutants
to water flea (Daphnia magna). Bull Environ Contam Toxicol,
24: 684-691.
Le Blanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee Rodkey F & Collison HH (1977) Biological oxidation of [14C]
methylene chloride to carbon monoxide and carbon chloride by the rat.
Toxicol Appl Pharmacol, 40: 33-38.
Lefevre PA & Ashby J (1989) Evaluation of dichloromethane as an
indicator of DNA synthesis in the B6C3F1 mouse liver. Carcinogenesis,
10: 1067-1072.
Lehman J & Paech C (1972) [Einfluss einiger lipophiler Lösungsmittel
in gasförmigem Zustand auf die CO2-Fixierung durch Luzerne.]
Experientia (Basel), 28: 1415-1416.
Leikin JB, Kaufman D, Lipscomb JW, Burda AM, & Hryhorczuk DO (1990)
Methylene chloride: Report of five exposures and two deaths. Am J
Emerg Med, 8: 534-537.
Leisinger T (1983) Microorganisms and xenobiotic compounds.
Experientia (Basel), 39: 1183-1191.
Leonardos G, Kendall D, & Barnard N (1969) Odor threshold
determination of 53 odorant chemicals. J Air Pollut Control Assoc,
19: 91-95.
Leuschner F, Neumann BW, & Hubscher F (1984) Report on subacute
toxicological studies with dichloromethane in rats and dogs by
inhalation. Arzneimittel forschung, 34: 1772-1774.
Levaggi DA, Siu W, Zerrudo RV, & La Voie JW (1988) Experiences in
gaseous toxic monitoring in the San Francisco Bay area. Proc APCA
Annual Meet, 81: 88-95A.
Libuda HG, Zabel F, Fink EH, & Becker KH (1990) Formyl chloride: UV
absorption cross sections and rate constants for the reactions of Cl
and OH. J Phys Chem, 94: 5860-5865.
Longstaff E, Robinson M, Bradbrook C, Styles JA, & Purchase IFH (1984)
Genotoxicity and carcinogenicity of fluorocarbons assessment by short-
term in vitro tests and chronic exposure in rats. Toxicol Appl
Pharmacol, 72(1): 15-31.
Loyke HF (1973) Methylene chloride and chronic renal hypertension.
Arch Pathol, 95(2): 130-131.
LWA (1980) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1981) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1982) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1983) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1984) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1989) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1990) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1991) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1992) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
Lyman WJ, Reehl WF, & Rosenblatt DH ed. (1982) Handbook of Chemical
Property Estimation Methods: Environmental Behavior of Organic
Compounds. New York, McGraw-Hill Book Co.
MacEwen JD & Vernot EM (1972) Toxic hazards research unit annual
technical report. Dayton, Ohio, Wright Patterson Air Force Base.
Aerospace Medical Research Laboratory (AMRL-TR-72-62).
MacEwen JD, Vernot EH, & Haun CC (1972) Continuous animal exposure to
dichloromethane. Dayton, Ohio, Wright Patterson Air Force Base.
Aerospace Medical Research Laboratory (AMRL-TR-72-28).
Makisimov GG & Mamleyeva NK (1977) [An assessment of the hazard
presented by methylene chloride entering the organism percutaneously.]
Absorbtion of industrial poisons through the skin, and prevention
thereof.] 83-88 (in Russian).
Maltoni C, Cotti G, & Perino G (1986) Experimental research on
methylene chloride carcinogenesis. In: Maltoni C & Mehlman MA (eds.)
Archives Research Industrial Carcinogenesis, Volume 4, Princeton, New
Jersey, Princeton Scientific Publishing Co.
Maltoni C, Cotti G, & Perino G (1988) Long-term carcinogenicity
bioassays administered by ingestion to Sprague-Dawley rats and Swiss
mice and by inhalation to Sprague-Dawley rats. Ann NY Acad Sci,
534: 352-366.
Manno M, Chirillo R, Danlotti G, Cocheo V, & Albrizio F (1989)
Carboxyhaemoglobin and fatal methylene chloride poisoning. Lancet,
2(8657): 274.
Manno M, Rugge M, & Cockeo V (1992) Double fatal inhalation of
dichloromethane. Hum Exp Toxicol, 11(6): 540-545.
Masuda Y, Yano I, & Murano T (1980) Comparative studies on the
hepatoxic actions of chloroform and related halogenomethanes in normal
and phenobarbital-pretreated animals. J Pharm Dyn, 3: 53-64.
Mattsson JL, Albee RR, & Streeter CM (1988) Evaluation of the acute
neuropharmacologic effects of dichloromethane in rats. Midland,
Michigan, Dow Chemical Company (Internal Report).
Mattsson JL, Albee RR, & Eisenbrandt DL (1990) Neurotoxicologic
evaluation of rats after 13 weeks of inhalation exposure to
dichloromethane or carbon monoxide. Pharmacol Blochem Behav,
36: 671-681.
McCammon CS, Glaser RA, Wells VE, Plupps FC, & Halperin WE (1991)
Exposure of workers engaged in furniture stripping to methylene
chloride as determined by environmental biological monitoring. Appl
Occup Environ Hyg, 6: 371-379.
McCarroll NE, Cortina TA, Zito MJ, & Farrow MG (1983) Evaluation of
methylene chloride and vinylidene chloride in mutational assays.
Environ Mutagen, 5(3): 426-427.
McCarty WM (1979) Toxicity of methylene chloride to Daphnids. Midland,
Michigan, Dow Chemical (DR 001-5849-099-005).
McCarty LP, Flannagan DC, Randall SA, & Johnson KA (1992) Acute
toxicity in rats of chlorinated hydrocarbons given via the intracheal
route. Hum Exp Toxicol, 11: 173-177.
McDougal JN, Jepsono GW, Clewell III HJ, MacNaughton MG, & Andersen ME
(1986) A physiological pharmacokinetic model for dermal absorption of
vapors in the rat. Toxicol Appl Pharmacol, 85: 286-294.
McGeorge L, Krietzman S, Bukowski G, & Hamill B (1987) Implementation
and results of a mandatory state-wide program for organic contaminant
analysis of delivered water. In: Proceedings of the Water Quality
Technology Conference, Vol 15, pp 71-102.
McGregor DB (1979) Practical experience in testing unknowns in vitro.
In: Pagel GE (ed) Mutagenesis in Submammalian Systems, Status and
Significance. MTP Press, pp 53-71.
McKenna MJ, Saunders JH, & Boeckler WH (1980) The pharmacokinetics of
inhaled methylene chloride in human volunteers. Toxicol Appl
Pharmacol, Abstr 59.
McKenna MJ & Zempel JA (1981) The dose-dependent metabolism of
14C-methylene chloride following oral administration to rat. Food
Cosmet Toxlcol, 19: 73-78.
McKenna MJ, Zempel JA, & Braun WH (1982) The pharmacokinetics of
inhaled methylene chloride in rats. Toxicol Appl pharmacol, 65: 1-10.
Meltzer N, Rampy L, Bielinski P, Garofalo M, & Sayad R (1977) Skin
irritation, inhalation toxicity studies of aerosols using methylene
chloride. Drug Cosmet Ind, 120: 38-40.
Mennear JH, McConnell EE, Huff JE, Renne RA, & Giddens E (1988)
Inhalation toxicology and carcinogenesis studies of methylene chloride
(dichloromethane) in F344/N rats and B6C3F1 mice. Ann NY Acad Sci,
534: 343-351.
Merlin G, Thiebaud H, Blake G, Sembiring S, & Alary J (1992) Mesocosms
and microcosms utilization for ecotoxicity evaluation of
dichloromethane, a chlorinated solvent. Chemosphere, 24: 37-50.
Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, & Kitterer B
(1991) Theta, a new class of glutathione transferases purified from
rat and man. Biochem J, 274: 409-414.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol 22: 565-570.
Miller L, Pateras V, Friederici H, & Engel G (1985) Acute tubular
necrosis after inhalation exposure to methylene chloride. Report of a
case. Arch Intern Med, 145: 145-146.
Mirer FE, Silverstein M, & Park R (1988) Methylene chloride and cancer
of the pancreas [letter]. J Occup Med, 30: 475-476.
Moody DE, James JL, Clawson GA, & Smuckler EA (1981) Correlations
among the changes in hepatic microsomal components after intoxication
with alkyl halides and other hepatoxins. Mol Pharmacol, 20: 685-693.
Morris JB, Smith FA, & Garman RH (1979) Studies on methylene chloride-
induced fatty liver. Exp Mol Pathol, 30: 386-393.
Moskowitz S & Shapiro H (1952) Fatal exposure to methylene chloride
vapor. Am J Ind Hyg Occup Med, 5: 116-123.
Mueller S, Weise M, Krug T, & Hoffmann P (1991) Adrenergic
cardiovascular actions in rats as affected by dichloromethane
exposure. Biomed Blochim Acta, 50: 307-311.
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martini R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16
(Suppl 18): 138-167.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J WPCF, 59: 670-678.
Narotsky MG, Hamby BT, Mitchell DS, & Kavlock RJ (1992) Full-litter
resorptions caused by low-molecular weight hydrocarbons in F-344 rats
[abstract 67]. Teratology, 45: 472.
Neely WB (1964) Metabolic fate of formaldehyde-14C intraperitoneally
administered to the rat. Biochem Pharmacol, 13: 1137-1142.
Negherbon WO (1959) Methylene chloride. In: Negherborn WO (ed.)
Handbook of toxicology III: insecticides, a compendium. London,
Saunders, pp 485-486.
Nellor MH, Baird RD, & Smyth JR (1985) Health effects of indirect
potable water reuse. J Am Water Works Assoc, 77(7): 88-96.
Nendza M & Seydel JK (1988) Multivariate data analysis of various
biological test systems used for the quantification of ecotoxic
compounds. Quant Struct-Act Relatsh, 7: 165-174.
Nestmann ER, Otson R, Williams DT, & Kowbel DJ (1981) Mutagenicity of
paint removers containing dichloromethane. Cancer Lett, 11: 295-302.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DR, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14: 383-388.
Neumann F (1991 ) Early indicators for carcinogenesis in sex-hormone-
sensitive organs. Mutat Res, 248: 341-356.
Nicola RM, Branchflower R, & Pierce D (1987) Chemical contaminants in
bottomfish. J Environ Health, 49: 342-347.
NIOSH (1976) Criteria for a Recommended Standard ... Occupational
Exposure to Methylene Chloride. Cincinnati, Ohio, National Institute
of Occupational Safety and Health (DHEW Publication No. 76-138).
NIOSH (1987) Method No. 1005, Revision 1. NIOSH Manual of Analytical
Methods, 3rd ed. Cincinnati, Ohio, National Institute for Occupational
Safety and Health.
Nishio A, Yajema S, Yahogi M, Sasaki Y, Sawano Y, & Miyao N (1984)
[Studies on the teratogenicity of dichloromethane in rats]. Gakujutsu
Hikoku-Kagoshima Daigaku Nogakubu, 34: 95-103 (in Japanese).
Nitschke KD, Stevens GA, Kociba RJ, Keyes DG, & Rampy LW (1981)
Methylene chloride: a four week inhalation toxicity study in rats,
hamsters and mice. Midland, Michigan, Dow Chemical Company (Internal
report).
Nitschke KD, Burek JD, Bell TJ, Kociba RJ, Rampy LW, & McKenna MJ
(1988a) Methylene chloride: A 2-year inhalation toxicity and
oncogenicity study in rats. Fundam Appl Toxicol, 11: 48-59.
Nitschke KD, Eisenbrandt DL, Lomax LG, & Rao KS (1988b) Methylene
chloride: Two-generation inhalation reproductive study in rats. Fundam
Appl Toxicol, 11: 60-67.
Norpoth K, Witting U, & Springorum M (1974) Induction of microsomal
enzymes in the rat liver by inhalation of hydrocarbon solvents. Int
Arch Arbeitsmed, 33: 315-321.
Novak JJ & Hain JR (1990) Furniture stripping vapor inhalation
fatalities: two case studies. Appl Occup Environ Hyg, 5: 843-847.
Novakova V, Musil J, Buckiova D, Taborsky O, Sollova H, & Vyborny P
(1981) Effect of tetrachloromethane and other chlorinated hydrocarbons
on the hepatic metabolism in the isolated perfused rat liver. J Hyg
Epidemiol Microbiol Immunol, 25: 369-383.
NTP (US National Toxicology Program) (1986) Toxicology and
carcinogenesis studies of dichloromethane (methylene chloride) (CAS
No. 75-09-2) in F344/N rats and B6C3F1 mice (inhalation studies).
Research Triangle Park, North Carolina, National Toxicology Programme
(Technical Report No. 306; NIH Publication No. 86-2562).
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1375.
Ott MG, Skory LK, Holder BB, Bronson JM, & Williams PR (1983) Health
evaluation of employees occupationally exposed to methylene chloride.
Scand J Work Environ Health, 9 (Suppl 1): 1-38.
Ottenwalder H & Peter H (1989) DNA binding assay of methylene chloride
in rats and mice [letter]. Arch Toxicol, 63: 162-163.
Ottenwalder H, Jager R, Thier R, & Bolt HM (1989) Influence of
cytochrome P-450 inhibitors on the inhalative uptake of methyl
chloride and methylene chloride in male B6C3F1 mice. Arch Toxicol, 13
(Suppl): 258-261.
Page BD & Charbonneau CF (1977) Gas chromatographic determination of
residual methylene chloride and trichloroethylene in decaffeinated
instant and ground coffee with electrolytic conductivity and electron
capture detection. J Assoc Off Anal Chem, 60: 710-715.
Page BD & Charbonneau CF (1984) Headspace gas chromatographic
determination of residual methylene chloride in decaffeinated tea and
coffee with electronic conductivity detection. J Assoc Off Anal Chem,
67: 757-761.
Pankow D, Gutewort R, Glatzel W, & Tieze K (1979) Effect of
dichloromethane on the sciatic motor conduction velocity of rats.
Experientia (Basel), 35: 373-374.
Pankow D, Dretschmer S, & Weise M (1991) Effect of pyrazole on
dichloromethane metabolism to carbon monoxide. Recent Developments in
Toxicology: Trends, Methods and Problems. Arch Toxicol, 14 (Suppl):
246-248.
Pankow D & Jagielki S (1993) Effect of methanol or modifications of
the hepatic glutathione concentration on the metabolism of
dichloromethane to carbon monoxide in rats. Hum Exp Toxicol,
12(3): 227-231.
Pellizzari ED, Hartwell TD, Harris BS, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pennington JT & Hadfield MG (1989) Larvae of nudibranch mollusc
(Phestilla sibogae) metamorphose when exposed to common organic
solvents. Biol Bull, 177: 350-355.
Penverne Y & Montiel A (1985) Etude des organohalogénés volatils dans
les eaux souterraines du departement du Val-de-Marne (France). Trib
Cebedeau, 505: 23-30.
Perbellini L, Brugnone F, Grigolini L, Cunegatti P, & Tacconi A (1977)
Alveolar air and blood dichloromethane concentration in shoe sole
factory workers. Int Arch Occup Environ Health, 40(4): 241-247.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Peterson JE (1978) Modeling the uptake, metabolism, and excretion of
dichloromethane by man. Am Ind Hyg Assoc J, 39: 41-47.
Plaa GL & Larson RE (1965) Relative nephrotoxic properties of
chlorinated methane, ethane, and ethylene derivatives in mice. Toxicol
Appl Pharmacol, 7: 37-44.
Pleil JD & McClenny WA (1990) Canister-based sampling and subsequent
GC/MS analysis for measurement of trace-level volatile organohalogen
compounds. In: Proceedings of the 10th International Meeting Dioxin
90-Organohalogen compounds, Vol 2, pp 411-414.
Plumb RH Jr (1987) A comparison of ground water monitoring from CERCLA
and RCRA sites. Ground Water Monit Rev, Fall 1987: 94-100.
Poplawski-Tabarelli S & Uehleke H (1982) Inhibition of microsomal drug
oxidations by aliphatic hydrocarbons: correlation with vapour
pressure. Xenobiotica, 12: 55-61.
Portier CJ & Kaplan NL (1989) Variability of safe dose estimates when
using complicated models of the carcinogenic process. Fundam Appl
Toxicol, 13: 533-544.
Post W, Kromhout H, Heederik D, Noy D, & Duijzentkunst RS (1991)
Semiquantitative estimates of exposure to methylene chloride and
styrene: The influence of quantitative exposure data. Appl Occup
Environ Hyg, 6(3): 197-204.
Price P J, Hassett CM, & Mansield JI (1978) Transforming activities of
trichloroethylene and proposed industrial alternatives. In Vitro,
14: 290.
Putz VR, Johnson BL, & Setzer JV (1976) A comparative study of the
effects of carbon monoxide and methylene chloride on human
performance. J Environ Pathol Toxicol, 2: 97-112.
Puurunen J & Sotaniemi E (1985) Usefulness of follow-up liver-function
tests after dichloromethane exposure. Lancet, 1: 822.
Radding SB, Liv DH, Johnson HL, & Mill T (1977) Review of the
environmental fate of selected chemicals. Washington, DC, US
Environmental Protection Agency (EPA-560/5-77-003).
Raje R, Basso M, Tolen T, & Greening M (1988) Evaluation of in vivo
mutagenicity of low-dose methylene chloride in mice. J Am Coll
Toxicol, 7: 699-703.
Ranna R, Rugge R, & Cocheo V (1992) Double fatal inhalation of
dichloromethane. Hum Exp Toxicol, 11: 540-545.
Rapson WH, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24(4): 590-596.
Ratney RS, Wegman DH, & Elkins HB (1974) In vivo conversion of
methylene chloride to carbon monoxide. Arch Environ Health,
28: 223-226.
Rayez JC, Rayez MT, Halvick P, Duguay B, & Lesclaux R (1987) A
theorical study of the decomposition of halogenated alkoxy radicals.
I. Hydrogen and chlorine extrusions. Chem Phys, 116: 203-213.
Rebert CS, Matteuci M J, & Pryor GT(1989) Acute effects of inhaled
dichloromethane on the EEG and sensory-evoked potentials of Fischer-
344 rats. Pharmacol Biochem Behav, 34: 619-629.
Reitz RH, Smith FA, & Andersen ME (1986) In vivo metabolism of
14C-methylene chloride [abstract]. Toxicologist 6, A 1048.
Reitz RH, Mendrala AL, & Guengerich FP (1989) In vitro metabolism of
methylene chloride in human and animal tissues: use of physiologically
based pharmacokinetic models. Toxicol Appl Pharmacol, 97: 230-246.
Reynolds ES & Yee AG (1967) Liver parenchymal cell injury. V.
Relationships between patterns of chloromethane-C14 incorporation
into constituents of liver in vivo and cellular injury. Lab Invest,
16: 591-603.
Rittmann BE & McCarty PL (1980) Utilization of dichloromethane by
suspended and fixed-film bacteria. Appl Environ Microbiol,
39: 1225-1226.
RIVM (Rijksinstituut voor Volksgezondheid en Milieuhygiene) (1986)
Methylene chloride; 48 hour IC50/EC50 Daphnia magna (86/HO63) and
embryotoxicity for Oryzia latipes (86/HO65) (Project No. 840820)
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Roberts CYC & Marshall FPP (1976) Recovery after "lethal" quantity of
paint remover. Br Med J, 1: 20-21.
Rodruigez Rojo A, Freiria Gandara M J, Alvarez Devesa A, Lorenzo
Ferreira RA, & Bermejo Martinez F (1989) Determination of halogenated
hydrocarbons in the water supply of Santiago de Compostela (Spain).
Environ Technol Lett, 10(8): 717-724.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of dichloromethane on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Br J Ind Med, 43: 291-299.
Rossman TG, Molina M, Meyer L, Boone P, Klein CB, Wang Z, Li F, Lin
WC, & Kinney PL (1991) Performance of 133 compounds in the lambda
prophage induction endpoint of the Microscreen assay and a comparison
with S. typhimurium mutagenicity and rodent carcinogenicity assays.
Murat Res, 260: 349-367.
Roth RP, Drew RT, Lo RJ, & Fouts JR (1975) Dichloromethane inhalation,
carboxyhaemoglobin concentrations and drug metabolizing enzymes in
rabbits. Toxicol Appl Pharmacol, 33: 427-437.
Ruhe RL, Watanabe A, & Stein G (1981) Health Hazard Evaluation Report
No. HHE-80-49-808: Superior Tube Company, Collegeville, PA,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
Ruhe RL, Singal M, & Hervin RL (1982) Health Hazard Evaluation Report
No. HETA-80-79-1189.: Rexall Drug Company, St Louis, MO, Cincinnati,
Ohio, National Institute of Occupational Safety and Health.
Ruth H (1986) Odor thresholds and initiation levels of several
chemical substances. A review. Am Ind Hyg Asspc J, 47: A141-A151.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sahn SC & Lowther DK (1981) Pulmonary reactions to inhalation of
methylene chloride: Effects on lipid peroxidation in rats. Toxicol
Lett, 8(4-5): 253-256.
Sanhueza E & Heicklen J (1975) Chlorine-atom sensitized oxidation of
dichloromethane and chloromethane. J Phys Chem, 79: 7-11.
Savolainen H, Pfäffli P, Tengen M, & Vainio H (1977) Biochemical and
behavioral effects of inhalation exposure to tetrachloroethylene and
dichloromethane. J Neuropathol Exp Neurol, 36: 941-949.
Sawhney BL (1989) Movement of organic chemicals through landfill and
hazardous waste disposal sites. Soil Science Society of America and
American Society of Agronomy, pp 447-474.
Scholz-Muramatsu H, Schneider V, Gaiser S, & Bardtke D (1988)
Biological elimination of dichloromethane from contaminated
groundwater-interference by components of the groundwater. Wat Sci
Tech, 20: 393-397.
Schubert R (1979) Toxicity of organohalogen compounds towards bacteria
and their degradability. Spez Ber Kernforschungsaulage, 1979: 211-218.
Schumacher H & Grandjean E (1960) Comparative investigations on the
anaesthetic effect and acute toxicity of nine solvents. Arch
Gewerbepathol Gewerbehyg, 18: 109-119.
Schutz E (1960) Effects of organic liquids on the skin.
Arzeimittelforschung, 10: 1027-1029.
Schroeder KR, Hallier E, Peter N, & Bolt HM (1992) Dissociation of a
new glutathione-S-transferase activity in human erythrocytes. Blochem
Pharmacol, 43: 1671-1674.
Schwetz BA, Leong BKJ, & Gehring PJ (1975) The effect of maternally
inhaled trichloroethylene, perchloroethylene, methyl chloroform, and
methylene chloride on embryonal and fetal development in mice and
rats. Toxicol Appl Pharmacol, 32: 84-96.
Scott JB, Smith FA, & Garman RH (1979) Exposure of mice to CH2Cl2
and CH3OH alone and in combination [abstract]. Toxicol Appl
Pharmacol, 48: A 105.
Selan FM & Evans MA (1982) Role of hepatic microtubule system in
chlorinated hydrocarbon induced hepatic steatosis. Toxicologist,
2: 134 [abstract].
Selenka F & Bauer U (1978) [Survey of organochlorine compounds in
water.] Forsch Ber, A27: 187-188 (in German).
Serota DG, Thakur AK, Ulland BM, Kirschman JC, Brown NM, Cotts RG, &
Morgareidge K (1986a) A two-year drinking-water study of
dichloromethane in rodents I. Rats. Food Chem Toxicol, 24: 951-958.
Serota DG, Thakur AK, Ulland BM, Kirschman JC, Brown NM, Cotts RG, &
Morgareidge K (1986b) A two-year drinking-water study of
dichloromethane in rodents. II. Mice. Food Chem Toxicol, 24: 959-963.
Shah JJ & Heyerdahl EK (1988) National ambient volatile organic
compounds (VOCs): data base update. Rep. by Nero and Associated,
Portland (OR), EPA/600/3-88/010a. Research Triangle Park, North
Carolina, Atmospheric Sciences Research Laboratory.
Sheldon T, Richardson CR, & Elliott BM (1987) Inactivity of methylene
chloride in the mouse bone marrow micronucleus assay. Mutagenesis,
2: 57-59.
Shikiya J, Tsou G, Kowalski J, & Leh F (1984) Ambient monitoring of
selected halogenated hydrocarbons and benzene in the California South
Coast Air Basin. Proceeding of 77th Annual Meeting of the Air
Pollution Control Association 24-29 June.
Shmuter LM & Kashin LM (1978) Experimental study of the sensitising
effect of some chlorinated aliphatic hydrocarbons. Gig Tr Prof Zabol,
3: 57-59 (in Russian).
Simmon VF, Kauhanen K, & Tardill RG (1977) Mutagenic activity of
chemicals identified in drinking water. Dev Toxicol Environ Sci,
2: 249-258.
Singh HB, Salas LJ, & Stiles RE (1983) Selected man-made halogenated
chemicals in the air and oceanic environment. J Geophys Res,
88: 3675-3683.
Sinha YN (1981) Plasma prolactin analysis as a potential predictor of
murine mammary tumorigenesis. In: McPike PK, Sitteri, & Welsch CW ed.
Hormones and breast cancer. Cold Spring Harbor, New York, Cold Spring
Laboratory (Banbury Report No. 8).
Slooff W & Ros JPM (1988) Integrated criteria document:
Dichloromethane. Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (Report No. 758473009).
Smith RL (1989) A computer assisted, risk-based screening of a mixture
of drinking water chemicals. Trace Subst Environ Health, 22: 215-232.
Soden KJ (1993) An evaluation of chronic metylene chloride exposure.
J Occup Med, 35(3): 282-286.
Staab HA & Datta AP (1964) Formyl chloride. Angew Chem Int ed, 3: 132.
Staples CA, Frances Werner A, & Hoogheem TJ (1985) Assessment of
priority pollutant concentrations in the United States using STORET
database. Environ Toxicol Chem, 4: 131-142.
Stern FB, Halperin WE, Hornung RW, Ringenburg VL, & McCammon CS (1988)
Heart disease mortality among bridge and tunnel officers exposed to
carbon monoxide. Am J Epidemiol, 128(6): 1276-1288.
Stevenson MF, Chemoweth MB, & Cooper GL (1978) Effect on
carboxyheamoglobin of exposure to aerosol spray paints with methylene
chloride. Clin Toxicol, 12: 551-561.
Stewart RD & Dodd HC (1964) Absorption of carbon tetrachloride,
trichloroethylene, tetrachloroethylene, methylene chloride, and
1,1,1-trichloroethane through the human skin. Am Ind Hyg Assoc J,
25: 439-446.
Stewart RD, Hake CL, & Wu A (1976) Use of breath analysis to monitor
methylene chloride exposure. Scand J Work Environ Health, 2: 57-70.
Stewart RD, Fisher TN, Hosko MJ, Peterson JE, Baretta ED, & Dodd HC
(1972) Experimental human exposure to methylene chloride. Arch Environ
Health, 25: 342-348.
Stover EL & Kincannon DF (1983) Biological treatability of specific
organic compounds found in chemical industry wastewaters. J Water
Pollut Control Fed, 55: 97-109.
Stuckey DC, Owen WF, & McCarty PL (1980) Anaerobic toxicity evaluation
by batch and semi-continuous assays. J Water Pollu Control Fed,
52(4): 720-729.
Stucki G, Gälli R, Ebersold H-R, & Leisinger TH (1981) Dehalogenation
of dichloromethane by cell extracts of Hyphomicrobium DM2. Arch
Microbiol, 130: 366-371.
Stucki G (1990) Biological decomposition of dichloromethane from a
chemical process effluent. Biodegradation, 1: 221-228.
Svirbely JL, Highman B, Alford we, & Von Oettingen WF (1947) The
toxicity and narcotic action of mono-chloro-mono-bromo-methane with
special reference to inorganic and volatile bromide in blood, urine
and brain. J Ind Hyg Toxlcol, 29: 382-389.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Takashita T, lto A, Kawata F, Ito M, & lto K (1991) [Experimental
studies of effects of methylene chloride on living bodies (2)].
Hochudoku, 9: 100-101 (in Japanese).
Tariot PM (1983) Delirium resulting from methylene chloride exposure:
Case report. J Clin Psychiatry, 44: 340-342.
Taskinen H, Lindbohm M-L, & Hemminki K (1986) Spontaneous abortions
among women working in the pharmaceutical industry. Br J Ind Med,
43: 199-205.
Taylor G J, Drew R J, Lores EM, & Clemmer TA (1976) Cardiac depression
by haloalkane propellants, solvents, and inhalation anesthetics in
rabbits. Toxicol Appl Pharmacol, 38: 379-387.
Tham R, Bunnfors I, Erikkson B, Larsby B, Lindgren S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thiel PG (1969) The effect of methane analogues on methanogenesis in
anaerobic digestion. Water Res, 3: 215-223.
Thier R, Forst U, Deutschmann S, Schroeder KR, Westphal G, Hallier E,
& Peter H (1991) Distribution of CH2Cl2 in human blood. Recent
developments in toxicology: trends, methods and problems. Arch
Toxicol, 14(Suppl): 254-258.
Thief R, Pemble SM, Taylor JB, Humphreys WG, & Ketterer B (1993)
Glutathione S-transf erases 5-5 expression in Salmonella typhimurium
increases mutation rate caused by methylene dihalides. Pharmacol
Toxicol, 73(Suppl 11): 42 (Abstract FCZ/12).
Thilagar AK, Back AM, Kirby PE, Kumaroo PV, Pant KJ, Clarke JJ, Knight
R, & Haworth SR (1984a) Evaluation of dichloromethane in short-term
in vitro genetic toxicity assays. Environ Mutagen, 6: 418-419.
Thilagar AK, Kumaroo PV, Clarke JJ, Kott S, Back AM, & Kirby PE
(1984b) Induction of chromosome damage by dichloromethane in cultured
human peripheral lymphocytes, CHO cells and mouse lymphoma L5178Y
cells. Environ Mutagen, 6: 422.
Thilagar AK & Kumaroo PV (1983) Induction of chromosome damage by
methylene chloride in CHO cells. Mutat Res, 116: 361-367.
Toftgard R, Nilsen OG, & Gustafsson J-A (1982) Dose dependent
induction of rat liver microsomal P-450 and microsomal enzymatic
activities after inhalation of toluene and dichloromethane. Acta
Pharmacol Toxicol, 51: 108-114.
Trevors JT (1985) Effect on methylene chloride on respiration and
electron transport system (ETS) activity in freshwater sediment. Bull
Environ Contam Toxicol, 34: 239-245.
Trueman RW & Ashby J (1987) Lack of UDS activity in the livers of mice
and rats exposed to dichloromethane. Environ Mol Mutagen, 10: 189-195.
Trueman RW, Burlinson B, Lefevre PA, & Ashby J (1987) Inactivity of
methylene chloride as a UDS-initiating agent in mouse and rat
hepatocytes exposed in vivo and in vitro. Mutat Res, 181: 346
[abstract].
Truhaut R, Boudene C, Jounany JM, & Bouant A (1972) The application of
the physiogram to the investigation of the acute toxicity of
chlorinated solvents. Eur J Toxicol, 5(5): 284-292.
Tsuruta H (1975) Percutaneous absorption of organic solvents. 1.
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Ulanova IP & Yonovskayo BI (1959) Changes in the ascorbic acid content
of the internal organs of white rats in response to the action of
chlorinated hydrocarbons II. Effect of methylene chloride. Biull Eksp
Biol Med, 48: 846.
Uotila L & Koivusalo M (1974a) Formaldehyde dehydrogenase from human
liver. J Biol Chem, 249: 7653-7663.
Uotila L & Koivusalo M (1974b) Distribution and properties of
S-formylglutathione hydrolase from human liver. J Biol Chem,
249: 7664-7672.
US EPA (1980) Ambient water quality criteria for halomethanes.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-051;
NTIS PB81-117 624).
US EPA (1981) Environmental risk assessment of dichloromethane.
Washington, DC, US Environmental Protection Agency (Draft report).
US EPA (1982a) Purgeable halocarbons-method 601. In: Methods for
organic chemical analysis of municipal and industrial wastewater.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Monitoring and Support Laboratory (EPA-600/4-82-057).
US EPA (1982b) Purgeables method 624. in: Methods for organic chemical
analysis of municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Monitoring and Support
Laboratory (EPA-600/4-82-057).
US EPA (1985) Health assessment document for dichloromethane
(Methylene chloride). Washington, DC, US Environmental Protection
Agency (EPA/600/8-82/OO4F).
US EPA (1986a) Gas chromatography/mass spectrometry for volatile
organics-method 8240. In: Test methods for evaluating solid waste, 3rd
ed. Washington, DC, US Environmental Protection Agency, Office of
Solid Waste and Emergency Response (SW-846).
US EPA (1986b) Halogenated volatile organics-method 8010. In: Test
methods for evaluating solid waste, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and Emergency
Response (SW-846).
US EPA (1987) Household solvent products: A national usage survey.
Washington, DC, US Environmental Protection Agency.
US EPA (1989a) Measurement of purgeable organic compounds in water by
capillary column gas chromatography/mass spectrometry-Method 524.2.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1989b) Measurement of purgeable organic compounds in water by
packed column gas chromatography/mass spectrometry-Method 524.1.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1989c) Volatile halogenated organic compounds in water by
purge and trap gas chromatography-method 502.1. In: Methods for the
determination of organic compounds in drinking water. Cincinnati,
Ohio, US Environmental Protection Agency, Environmental Monitoring
Systems Laboratory (EPA/600/4-88/039).
US EPA (1989d) Volatile organic compounds in water by purge and trap
capillary column gas chromatography with protoionization and
electrolytic conductivity detectors in series-method 502.2.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1990) Paint stripping, options selection paper. Washington,
DC, US Environmental Protection Agency.
Van Beck L (1990) Investigation of a possibility to reduce the use of
rabbits in skin irritation tests; experiments with dichloromethane,
trichloroethylene, tetrachloroethylene and 1,1,1-trichloroethane.
Doc. 56645/34/90, rep. V 89.265. Zeist, The Netherlands, TNO-CIVO
Institutes.
Van Haut H & Prinz B (1979) [Evaluation of the relative harmfulness to
plants of organic air pollutants in the LIS short-term test.] Staub-
Reinhalt Luft, 39: 408-414 (in German).
Van de Graaff S (1986) [Estimation of harmful effects of
environmentally relevant compounds in river water.] Münch Beitr
Abwasser-Fisch-Flussbiol, 40: 556-572 (in German).
Vannelli T, Logan M, Arciero DM, & Hooper AB (1990) Degradation of
halogenated aliphatic compounds by the ammonia oxidzing bacterium
Nitrosomas europaea. Appl Environ Microbiol, 56: 1169-1171.
Vargas C & Ahlert RC (1987) Anaerobic degradation of chlorinated
solvents. J Water Pollut Control Fed, 59(11): 964-968.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM-STP 707).
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. Physical behaviour of PCBs in
Great Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 269-282.
Verrett M J, Scott WF, Reynaldo EF, Alterman EK, & Thomas CA (1980)
Toxicity and teratogenicity of food additive chemicals in the
developing chicken embryo. Toxicol Appl Pharmacol, 56: 265-273.
Verschueren K (1983) Handbook of Environmental data on Organic
Chemicals, 2nd ed. New York, Van Nostrand Reinhold Publishers,
pp 848-849.
Verschuuren HG & Wilmer JW (1983) Neurotoxicity of 1,1,1-
trichloroethane questioned. Scand J Work Environ Health, 16: 144-146.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Oettingen WF, Powell CC, Sharpless NE, Alford WC, & Pecora LJ
(1950) Comparative studies on the toxicity and pharmacodynamic action
of chlorinated methanes with special reference to their physical and
chemical characteristics. Arch Int Pharmacodyn Ther, 81: 17-34.
Vosovaja MA, Maljorowa LR, & Yenikeyera KM (1974) Levels of methylene
chloride in biological fluids of pregnant or lactating workers in an
industrial rubber products company. Gig Tr Prof Zabol, 4: 42-43.
Weast RC, Astle M J, & Beyer WH (eds) (1988) CRC Handbook of chemistry
and physics. 69th ed. (1988-1989). Boca Raton, Florida, CRC Press,
pp C-161., D-212.
Weber M, Martin A, Bollaert P-E, Bauer Ph, Leroy F, Meley M, Mur J-M,
Carry C, & Lambert H (1990) Intoxication aiguä par chlorure de
méthylène et méthanol par voie percutanée. Arch Mal Prof, 51: 103-106.
Weinstein RS, Boyd D, & Back KC (1972) Effects of continuous
inhalation of dichloromethane in the mouse: morphologic and functional
observations. Toxicol Appl Pharmacol, 23: 660-679.
Weinstein RS & Diamond SS (1972) Hepatotoxicity of dichloromethane
(methylene chloride) with continuous exposure at a low dose level.
In: Proceedings of the 3rd Annual Conference on Environmental
Toxicology. Dayton, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TR-72-130, 209-220).
Weiss G (1967) Toxic encephalosis in occupational contact with
methylene chloride. Zent.bl Arbeitsmed Arbeitsschutz, 17(9): 282-285.
Wells GG & Waldron HA (1984) Methylene chloride burns. Br J Ind Med,
41: 420.
Welsch CW (1985) Host-factors affecting the growth of carcinogen-
induced rat mammary carcinomas: a review and tribute to Charles
Brenton Huggins. Cancer Res, 45: 3415-3443.
Welsch CW, Jenkins JW, & Mertes J (1970) Increased incidence of
mammary tumors in female rat grafted with multiple pituitaries. Cancer
Res, 30: 1024-1029.
Welsch CW & Nagasawa H (1977) Prolactin and murine mammary
tumorigenesis: a review. Cancer Res, 37: 951-963.
Westbrook-Collins B, Allen JW, Sharief Y, & Campbell J (1990) Further
evidence that dichloromethane does not induce chromosome damage. J
Appl Toxicol, 10: 79-81.
Westbrook-Collins B, Allen JW, Kligerman A, Campbell JA, Erexson GL,
Kari F, & Zeiger E (1989) Dichloromethane-induced cytogenetic damage
in mice. Environ Mol Mutagen, 14(Suppl 15): [abstact 630].
Wesforook-Collins B, Campbell JA, Poorman PA, Sharief Y, & Allen JW
(1988) SCE, chromosome aberration, and synaptonemal complex analyses
in mice exposed to dichloromethane. Environ Mol Mutagen, 11(Suppl 11):
112 [abstract 2741.
WMO (World Meteorological Organization) (1991) Scientific assessment
of ozone depletion: 1991. Report No 25, 8.8. WMO, Geneva, p 8.8.
Wood PR, Parsons FZ, DeMarco J, Harween HJ, Lang RF, Payan IL, & Ruiz
MC (1981) Introductory study of the biodegradation of the chlorinated
methane, ethane, and ethene compounds. Presented at the American Water
Works Association Meeting, June 1981.
Woodrow JE, McChesney MM, & Seiber JN (1988) Determination of methyl
bromide in air samples by headspace gas chromatography. Anal Chem,
60: 509-512.
Yagafarova AB, Ivanova TS, & Khabirova FY (1981) The toxic allergic
action of hydrocarbons on the eyes. Kazanski Med Zh, 67: 72-74.
Yesair DW, Jacques D, Schepspis P, & Liss RH (1977) Dose-related
pharmacokinetics of 14C methylene chloride in mice. Fed Proc,
36: 998.
Young DR, Gossett RW, Baird RB, Brown DA, Taylor PA, & Mille MJ (1983)
Waste water inputs and marine bioaccumulation of priority pollutant
organics off Southern California, USA. In: Jolley RL ed. Proceedings
of the 4th Conference on Water Chlorination. Volume 4: Environmental
Impact and Health Effect - Part 2: Environmental health and risk. Ann
Arbor, Michigan, Ann Arbor Science Publishers.
Zahm SH, Stewart ZP, & Blair A (1987) A study of mortality among
workers exposed to methylene chloride. Feasibility report. Bethesda,
Maryland, US National Cancer Institute.
Zeiger E, Haseman JK, Shelby MD, Margolin BH, & Tennant RW (1990)
Evaluation of four in vitro genetic toxicity tests for predicting
rodent carcinogenicity: Confirmation of earlier results with 41
additional chemicals. Environ Mol Mutagen, 16: 1-14.
Zielenska M, Ahmed A, Pienkowska M, Anderson M, & Glickman BW (1993)
Mutational specificlties of environmental carcinogens in the LACL gene
of Escherichia coli. VI. Analysis of methylene chloride induced
mutational distribution in UVR + UVRB-strains. Carcinogenesis,
14(5): 789-794.
Zoeteman BC, Harmsen K, Linders JB, Morra CF, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of the
Netherlands. Chemosphere, 9: 231-249.
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le chlorure de méthylène (dichlorométhane) est un liquide limpide,
ininflammable et extrêmement volatil qui possède une puissante odeur
éthérée. Lorsqu'il est pur et anhydre, ce composé est très stable. Le
chlorure de méthylène s'hydrolyse lentement en présence d'humidité,
pour donner une petite quantité de chlorure d'hydrogène. Le chlorure
de méthylène du commerce est généralement additionné de petites
quantités de stabilisants afin d'en éviter la décomposition.
Il existe des méthodes d'analyse pour le dosage du chlorure de
méthylène dans les milieux biologiques et les échantillons prélevés
dans l'environnement. Dans tous les cas, on fait appel à la
chromatographie en phase gazeuse avec un détecteur convenable. On
obtient ainsi des limites de détection très basses (par exemple dans
les denrées alimentaires 7 ng/échantillon; dans l'eau 0,01/µg/litre;
dans l'air 1,76/µg/m3 (0,5 ppb); dans le sang 0,022 mg/litre).
2. Sources d'exposition humaine et environnementale
On estime à 570 000 tonnes la production annuelle mondiale de
chlorure de méthylène. On l'utilise la plupart du temps comme solvant
des graisses, des matières plastiques et des liants pour peinture, en
particulier à cause de sa volatilité et de sa stabilité. Dans
l'ensemble du monde, il est utilisé à 20-25% dans des aérosols, à 25%
comme décapant des peintures, à 35-40% comme solvant au cours des
différentes opérations de l'industrie chimique et pharmaceutique, et
enfin à 10-15% dans diverses applications allant de la fabrication de
mousse de polyuréthane au décapage des métaux. Son utilisation tend à
augmenter, tout au moins en Europe de l'Ouest.
Les émissions atmosphériques de chlorure de méthylène proviennent
à plus de 99% de son utilisation comme produit final par diverses
industries ou encore comme décapant des surfaces peintes et comme
constituant des bombes aérosols à usage domestique.
3. Transport, distribution et transformation dans l'environnement
En raison de sa forte volatilité, la majeure partie du chlorure de
méthylène libéré dans l'environnement se répartit dans l'atmosphère où
il est décomposé en l'espace de six mois par réaction avec des
radicaux hydroxyles d'origine photochimique.
Dans l'eau, il est décomposé par voie abiotique beaucoup plus
lentement qu'il ne s'évapore. On a montré que le chlorure de méthylène
disparaissait rapidement du sol et des eaux souterraines.
Grâce à divers systèmes d'épreuve on a pu établir les modalités de
la décomposition aérobic et anaérobie du chlorure de méthylène. Sa
biodécomposition complète est rapide, notamment en aérobiose, sous
l'action de cultures bactériennes acclimatées (par exemple 49 à 66% de
minéralisation en 50 heures en présence de boues d'effluents
municipaux acclimatées). Dans les réacteurs biologiques, la
décomposition peut atteindre 10% à l'heure. Rien n'indique qu'il y ait
une bioaccumulation ou une bioamplification importante.
4. Concentrations dans l'environnement et exposition humaine
On a décelé la présence de chlorure de méthylène dans l'air
ambiant de régions rurales et de zones écartées, à la concentration de
0,07-0,29/µg/m3. Dans les zones de banlieue, la concentration
moyenne est inférieure à 2 µ/m3 et dans les zones urbaines, elle est
inférieure à 15 µg/m3. A proximité de décharges jugées dangereuses,
on a trouvé des concentrations allant jusqu'à 43 µg/m3. Les
précipitations peuvent également contenir du chlorure de méthylène.
Le chlorure de méthylène pénètre dans l'environnement aquatique
par suite de la décharge d'eaux résiduaires provenant des diverses
industries et on en a retrouvé dans les eaux superficielles et
souterraines ainsi que dans les sédiments.
S'il y a exposition au chlorure de méthylène de personnes
appartenant à la population générale, c'est par suite de son
utilisation dans certains produits de consommation tels que les
décapants pour peinture, dont l'emploi peut entraîner la présence de
teneurs relativement importantes dans l'air intérieur. En ce qui
concerne l'exposition professionnelle au cours de la production de
chlorure de méthylène, elle se produit essentiellement au cours du
remplissage et du conditionnement (la fabrication s'effectue en
circuit fermé). Du fait de l'utilisation de ce composé comme décapant
pour peinture, il peut également y avoir exposition professionnelle
lors de la préparation de ces décapants, lors de la fabrication de
certains équipements et également lorsqu'on procède au décapage du
mobilier. Le chlorure de méthylène est largement utilisé comme solvant
lors de la préparation de différents produits, en particulier dans les
industries citées à la section 1.2.
La surveillance biologique de l'exposition au chlorure de
méthylène peut s'effectuer par dosage du solvant lui-même dans l'air
expiré ou dans le sang. Toutefois, étant donné que la production de
monoxyde de carbone constitue le facteur limitant du risque en cas
d'exposition supérieure à 3 ou 4 heures par jour, il vaut mieux que la
surveillance biologique s'effectue soit par dosage du monoxyde de
carbone dans l'air expiré, soit par dosage de la carboxyhémoglobine
(CO-Hb) dans le sang. Toutefois cette méthode ne vaut que pour les
sujets non fumeurs. Les prélèvements doivent s'effectuer environ 0 à 2
heures après l'exposition, ou au bout de 16 heures, c'est-à-dire le
matin suivant.
Les taux de CO-Hb, 2 heures après cessation de l'exposition, ne
devraient pas dépasser 2 à 3%, et au bout de 16 heures 1%, dans le cas
d'une exposition de 8 heures à moins de 350 mg de chlorure de
méthylène par m3 chez un non fumeur.
5. Cinétique et métabolisme chez les animaux de laboratoire et l'homme
Le chlorure de méthylène est rapidement absorbé au niveau des
alvéoles pulmonaires et pénètre dans le courant sanguin. Il est
également absorbé dans les voies digestives et aussi par voie
percutanée, mais cette voie est la plus lente de toutes.
Le chlorure de méthylène est très rapidement excrété, en majeure
partie dans l'air expiré. Il peut traverser la barrière hémato-
encéphalique ainsi que le placenta et on peut le retrouver en petites
quantités dans les urines ou le lait.
A fortes concentrations, la majeure partie du chlorure de
méthylène absorbée est expirée tel quel. Le reste est métabolisé en
monoxyde, dioxyde de carbone et chlorures minéraux. La métabolisation
s'effectue selon l'une ou l'autre de ces deux voies ou les deux à la
fois, et la prédominance de l'une ou de l'autre dépend largement de la
dose et de l'espèce animale en cause. Une de ces voies comporte un
processus oxydatif, par l'intermédiaire du cytochrome P-450, et alle
conduit à la production de monoxyde et de dioxyde de carbone. Il
semble que cette voie soit identique chez tous les rongeurs étudiés et
chez l'homme. Il s'agit de la voie prédominante aux faibles doses,
mais il y a saturation à des doses relativement modérées (autour de
1800 mg/m3). Même si la dose dépasse la valeur de saturation, il n'y
pas accroissement de la métabolisation par cette voie.
Dans l'autre voie métabolique intervient une glutathion-
transférase (GST) qui conduit à la formation de dioxyde de carbone par
l'intermédiaire du formaldéhyde et du formiate. Il semble que cette
voie ne prenne de l'importance que lorsque les doses dépassent la
valeur de saturation de la voie oxydative "préférentielle". Chez
certaines espèces (par exemple la souris), elle devient la voie
principale lorsque la dose est suffisamment élevée. En revanche, chez
d'autres espèces (par exemple le hamster et l'homme), elle semble
n'être que peu utilisée, qu'elle que soit la dose.
Les différentes interspécifiques touchant le métabolisme par la
voie de la GST sont en bonne corrélation avec les différences
observées selon les espèces, notamment en ce qui concerne la
cancérogénicité du chlorure de méthylène. Le taux de métabolisation
selon cette voie chez les espèces concernées est utilisé comme modèle
cinétique pour la description du comportement métabolique du chlorure
de méthylène chez diverses espèces.
6. Effets sur les êtres vivants dans leur milieu naturel
Aux concentrations inférieures à 500 mg/litre, il n'y a aucune
inhibition de la croissance des algues et des bactéries aérobies. Il
existe des bactéries qui sont capables de croître en présence de
chlorure de méthylène à des concentrations beaucoup plus élevées et
notamment en solution aqueuse (section 4.2.4.1 ). Les bactéries
anaérobies sont beaucoup plus sensibles; ainsi on a observé un blocage
de la croissance à la dose de 1 mg/litre, dans des boues biologiques
anaérobies.
A la concentration de 10 mg/kg de terre, on a constaté une forte
diminution de la teneur en ATP de la biomasse, notamment des
champignons et des bactéries aérobies, et une inhibition transitoire
de l'activité enzymatique. La dose sans effets observables était dans
ce cas de 0,1 mg/kg. Le chlorure de méthylène est modérément toxique
pour les lombrics (100 à 1000 µg/cm2) soumis au test de toxicité par
contact sur papier filtre. Dans les sédiments, on n'a pas observé
d'effets toxiques, même à des doses très élevées.
Chez les plantes supérieures, on n'a pas constaté d'effets après
une exposition de 14 jours à la dose de 100 mg/m3.
Les poissons adultes semblent être relativement insensibles au
chlorure de méthylène, même après une exposition prolongée (la CL50
à 14 jours est supérieure à 200 mg/litre). Il est difficile
d'apprécier l'effet du chlorure de méthylène sur les daphnies en
raison de la très grande dispersion des résultats fournis par les
différentes études. La CE50 la plus basse qui ait été rapportée
était égale à 12,5 mg/litre.
En milieu aquatique, c'est chez les embryons de poissons et
d'amphibiens que l'on a constaté la sensibilité la plus élevée avec
des effets sur l'éclosion â partir de 5,5 mg/litre.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Lorsqu'il est absorbé par inhalation ou par voie orale, le
chlorure de méthylène présente une faible toxicité aiguë. Ainsi, les
valeurs de la CL50 à 6 heures pour l'ensemble des espèces étudiées,
se situent entre 40 200 et 55 870 mg/m3. En ce qui concerne la
DL50 par voie orale, on a obtenu des valeurs allant de
1410-3000 mg/kg. Les effets aigus observés après administration de
chlorure de méthylène par diverses voies d'exposition affectent
essentiellement le système nerveux central (SNC) et le foie, et tous
les effets observés l'ont été à des doses élevées. Des troubles
neurologiques ont été observés à des concentrations supérieures ou
égaies à 14 100 mg/m3, avec de légères altérations du tracé électro-
encéphalographique à la dose de 1770 mg/m3. De légères modifications
histologiques ont été également constatées dans le foie aux
concentrations supérieures ou égaies à 17 700 mg/m3. Il est
également arrivé que d'autres organes soient touchés, comme les reins
ou le système respiratoire. Chez la souris, les effets au niveau
pulmonaire étaient limités, après exposition à 7100 mg/m3, aux
cellules de Clara. On a également fait état d'une sensibilisation
cardiaque à l'arythmie induite par l'adrénaline. D'autres effets
cardio-vasculaires ont également été observés, mais pas de façon
systématique.
7.2 Exposition à court et à long terme
Une exposition prolongée à de fortes concentrations de chlorure de
méthylène (> 17 700 mg/m3) a causé des effets réversibles sur le
SNC, une légère irritation oculaire et une certaine mortalité chez
diverses espèces d'animaux de laboratoire. Chez le rat on a observé à
3500 mg/ma une réduction du poids corporel, cet effet étant
également observé chez la souris à partir de 17 700 mg/m3. De légers
effets hépatiques ont été également observés chez des chiens exposés
de manière continue à des doses de 3500 mg/m3 pendant des périodes
allant jusqu'à 100 jours. Après exposition intermittente, des effets
ont également été observés au niveau du foie chez des rats à la dose
de 3500 mg/m3 et chez des souris à 14 100 mg/m3.
Les autres organes cibles sont les poumons et les reins.
Chez des rats exposés à du chlorure de méthylène pendant 13
semaines par voie respiratoire à des concentrations allant jusqu'à
7100 mg/m3, on a observé aucun signe de lésion neurologique
irréversible.
L'administration de chlorure de méthylène par voie orale à des
rats a entraîné des effets hépatiques à partir d'une dose journalière
d'environ 200 mg/kg.
7.3 Irritation cutanée et oculaire
Le chlorure de méthylène se révèle modérément irritant pour la
peau et les yeux chez les animaux de laboratoire.
7.4 Effets toxiques sur le développement et la reproduction
Le chlorure de méthylène n'est pas tératogène chez le rat ou la
souris à des concentrations allant jusqu'à 16 250 mg/m3. Trois
études effectuées sur ces animaux n'ont pas permis de relever d'effets
se traduisant par l'apparition de malformations squelettiques ou
autres anomalies du développement. Un léger effet, se manifestant par
une modification du poids foetal ou maternel, a été relevé à la dose
de 4400 mg/m3, et l'on a constaté qu'après la naissance, le gain de
poids des rats mâles était également affecté à la dose de 0,04% dans
la nourriture. Une étude toxicologique portant sur deux générations a
été effectuée sur des rats qui ont été exposés par la voie
respiratoire à du chlorure de méthylène, à des concentrations allant
jusqu'à 5300 mg/m3, et cela pendant 17 semaines, 6 heures par jour
et 5 jours par semaine. Aucun effet nocif, de quelque nature ce que
soit, n'a été relevé sur la reproduction, ni sur la survie ou la
croissance des ratons nouveaux-nés appartenant à la génération F0 ou
F1
7.5 Mutagénicité et points d'aboutissement correspondants
Dans certaines conditions d'exposition, le chlorure de méthylène
se révèle mutagène pour les microorganismes procaryotes, avec ou sans
activation métabolique (Salmonella ou Escherichia coli) Dans les
systèmes eucaryotes, il est sans effet mais, dans un cas, il a donné
des résultats légèrement positifs. On a également obtenu des résultats
uniformément négatifs lors des tests de mutation génique in vitro et
des tests de recherche d'une synthèse non programmée de l'ADN sur
cellules mammaliennes. La recherche d'aberrations chromosomiques in
vitro sur différents types de cellules a donné des résultats positif
s; en revanche, des tests visant à mettre en évidence l'induction
d'échanges entre chromatides soeurs ont été soit négatif s, soit
ambigus.
Dans leur majorité, les études in vivo publiées n'ont pas fourni
d'éléments en faveur d'une mutagénicité du chlorure de méthylène (par
exemple recherche d'aberrations chromosomiques, recherche de
micronoyaux ou synthèse non programmée de l'ADN). Après avoir fait
inhaler à des souris de fortes concentrations de chlorure de
méthylène, on a observé un accroissement minime de la fréquence des
échanges entre chromatides soeurs et du nombre de micronoyaux.
Après administration à des rats et à des souris de fortes doses de
chlorure de méthylène, on n'a pas constaté de liaison de ce composé à
FADN ni de lésions de l'ADN. Ces méthodes pourraient être les plus
sensibles in vivo et les meilleures d'entre elles sont capables de
déceler un site d'alkylation sur 106 nucléotides.
Dans les limites des épreuves à court terme actuelles, rien ne
permet de conclure que le chlorure de méthylène soit génotoxique
in vivo.
7.6 Toxicité chronique et cancérogénicité
Le chlorure de méthylène est cancérogène pour la souris et il
provoque l'apparition de rumeurs du poumon et du foie après exposition
à des concentrations élevées (7100 et 14 100 mg/m3). Chez les souris
qui avaient été exposées 26 semaines à la dose de 7100 mg/m3, on a
constaté que l'incidence des tumeurs pulmonaires et hépatiques
augmentait lorsqu'on poursuivait l'exposition pendant 78 semaines
supplémentaires. Rien n'indique la présence d'effets toxiques
concomitants ou d'une hyperplasie au niveau des organes cibles.
Des hamsters dorés exposés pendant deux ans à du chlorure de
méthylène, à des concentrations allant jusqu'à 12 400 mg/m3, n'ont
présenté aucun signe d'effets cancérogènes qui soient attribuables à
ce composé.
Chez des rats exposés par différentes voies à du chlorure de
méthylène, on a constaté un accroissement de l'incidence tumorale à
certaines localisations. Ainsi, chez des rattes exposées pendant deux
ans à des doses égaies soit à 5300, soit à 12 400 mg/m3, on a
constaté un excès de la fréquence tumorale au niveau des glandes
salivaires. Cet excès n'apparaissait que lorsque les rumeurs, toutes
d'origine mésenchymateuse, étaient regroupées en vue de l'analyse
statistique. Etant donné que ces rumeurs trouvent leur origine dans
des cellules de types différents, l'analyse statistique utilisée s'est
révélée inappropriée. En outre, il a été précisé que les rats utilisés
pour cette étude avaient contracté une virose commune
(sialodacryoadénite) au début de l'expérience, affection qui concerne
essentiellement les glandes salivaires. Il est donc probable qu'il n'y
a pas de lien causal entre ces tumeurs et l'exposition au chlorure de
méthylène, mais que l'exposition à ce composé a exacerbé la réaction à
l'infection au niveau de la glande salivaire. D'ailleurs cette
réaction n'a pas été observée lors d'une deuxième étude au cours de
laquelle des rats ont été exposés pendant toute la durée de leur vie à
des doses respectivement égaies à 3500, 7100 et 14 100 mg/m3. Une
autre étude au cours de laquelle des rats ont été également exposés à
du chlorure de méthylène par la voie respiratoire et durant toute leur
vie, à des concentrations allant jusqu'à 1770 mg/m3, n'a pas révélé
de signe de cancérogénicité. Aucun signe notable de cancérogénicité
n'a été non plus observé chez des rats à qui l'on avait administré du
chlorure de méthylène, soit par gavage, soit par mélange à leur eau de
boisson.
Trois études font état d'une augmentation de l'incidence des
tumeurs mammaires bénignes chez des rats exposés à du chlorure de
méthylène; dans une des études le composé a été administré par garage
tandis que dans les deux autres l'exposition a eu lieu par la voie
respiratoire. Il n'y a aucune publication faisant état d'un
accroissement des tumeurs mammaires chez des hamsters ou des souris
qui avaient reçu du chlorure de méthylène à des doses comparables. On
a établi sans aucun doute possible que les tumeurs mammaires étaient
liées aux hormones hypophysaires tant chez les rats mâles que chez les
femelles. Chez le rat, la prolactine se comporte à la fois comme un
initiateur et comme un promoteur des cancers mammaires. On a de bonnes
raisons de penser qu'un accroissement du taux de prolactine augmente
l'incidence des tumeurs mamamaires (par exemple la greffe de plusieurs
hypophyses à des rats Sprague-Dawley augmente l'incidence des tumeurs
mammaires chez ces animaux et on a relevé l'existence d'une
corrélation positive entre un taux sanguin élevé de prolactine et la
présence de tumeurs mammaires chez des rattes âgées de souche
R-Amsterdam). Après administration de composés cancérogènes à des
rattes, une hyperprolactinémie provoquée chez ces animaux entraîne un
accroissement spectaculaire de l'incidence tumorale. On peut notamment
provoquer l'hyperprolactinémie par surrénalectomie, homogreffe
d'hypophyse et régime alimentaire hyperlipidique.
Il est important pour l'évaluation du risque chez l'homme, de
connaître le mécanisme par lequel le chlorure de méthylène provoque
l'apparition d'adénomes mammaires chez le rat. Les rattes Sprague-
Dawley à qui l'on a administré du chlorure de méthylène présentent un
taux sanguin élevé de prolactine. De même qu'avec les autres composés
qui agissent par l'intermédiaire d'une hyperprolactinémie, la réaction
au chlorure de méthylène se traduit uniquement par l'apparition de
néoformations à caractère bénin. Rien n'indique que le chlorure de
méthylène se lie à l'ADN d'autres tissus et par conséquent il paraît
improbable qu'il se lie à l'ADN des tissus mammaires alors qu'il est
principalement métabolisé dans le foie. Il paraît donc probable que
l'accroissement de l'incidence des adénomes mammaires résulte d'un
mécanisme indirect agissant par l'intermédiaire d'une
hyperprolactinémie.
Chez l'homme, les faits relatifs à la question de savoir si les
tumeurs mammaires sont sous la dépendance de la prolactine comme chez
le rat, apparaissent contradictoires. Le rat présente un taux élevé de
prolactine lorsqu'on le laisse s'alimenter ad libitum plutôt que de
le soumettre à un régime strict et cela pourrait expliquer pourquoi
l'incidence des tumeurs mammaires est si clépendante des divers effets
environnementaux ou autres. Chez le rat toutefois, la prolactine a un
caractère lutéotrope. L'augmentation du taux de prolactine dans le
sang circulant conduit à une augmentation du taux de progestérone et
d'oestrogène exogènes. C'est la présence de l'ensemble de ces trois
facteurs qui provoque la croissance tubulo-alvéolaire des glandes
mammaires et qui finit par déboucher sur la formation de tumeurs. Ce
mécanisme de formation tumorale n'a donc vraisemblablement pas à être
pris en considération chez l'homme.
Le mécanisme de formation de tumeurs mammaires chez le rat par
l'intermédiaire d'une hyperprolactinémie n'intervient qu'à des doses
où le chlorure de méthylène agit sur les taux de prolactine. On ne
dispose pas de données de première main sur les taux de prolactine
chez des rats soumis à de faibles doses de chlorure de méthylène, mais
l'administration de faibles doses de ce composé, soit par inhalation,
soit par mélange à l'eau de boisson (doses inférieures à 250 mg/kg de
poids corporel) ne conduit pas, selon les études effectuées, à un
accroissement des adénomes mammaires.
8. Effets sur l'homme
Le chlorure de méthylène irrite la peau et les yeux, en
particulier lorsqu'il ne peut pas s'évaporer. Dans ces conditions, un
contact prolongé peut entraîner des brûlures chimiques. On a signalé
un cas grave d'oedème pulmonaire consécutif à une inhalation excessive
de chlorure de méthylène. On a également signalé des cas de décès par
suite de l'inhalation accidentelle de chlorure de méthylène ou d'un
contact cutané avec ce composé. Les principaux effets toxiques du
chlorure de méthylène consistent dans une dépression réversible du
système nerveux central et dans la formation de carboxyhémoglobine. On
a également rapporté des cas d'insuffisance hépatique et rénale avec
anomalies hématologiques, à la suite d'une exposition à du chlorure de
méthylène.
Chez des volontaires humains exposés pendant une heure et demie à
trois heures à du chlorure de méthylène à la concentration de
694 mg/m3, on a observé des troubles neurophysiologiques et neuro-
comportementaux. En revanche, aucun signe d'effet neurologique n'a été
observé chez des hommes exposés plusieurs années à du chlorure de
méthylène à des concentrations allant de 260 à 347 mg/m3. De même,
on a soumis à une batterie de tests neurophysiologiques et
psychologiques un groupe d'anciens décapeurs d'aéronefs qui avaient
été longtemps exposés à du chlorure de méthylène (22 ans), à des doses
élevées mais non précisées; comparés à ceux d'un groupe témoin qui
n'avait jamais été exposé à du chlorure de méthylène ou du moins
uniquement à de faibles doses, les résultats obtenus par les ouvriers
se sont révélés "normaux".
On a attribué à une exposition au chlorure de méthylène une
augmentation du taux d'avortements spontanés constatée chez des
employées des industries pharmaceutiques finlandaises. L'étude en
question présentait cependant des défauts de conception qui n'ont pas
permis d'établir une relation causale.
Plusieurs éludes de mortalité sur des cohortes exposées au
chlorure de méthylène font ressortir une absence d'uniformité dans les
causes de décès. La surmortalité due à certaines maladies (par exemple
cancer du pancréas, cardiopathies ischémiques) ne se manifeste pas de
façon uniforme, mais seulement dans certaines éludes. Ces effets ne
peuvent être attribués à l'exposition au chlorure de méthylène.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El cloruro de metileno (diclorometano) es un liquido claro,
altamente volátil y no inflamable, con un olor penetrante parecido al
del éter. El compuesto puro en polvo es muy estable. El cloruro de
metileno se hidroliza lentamente en presencia de humedad, dando lugar
a pequeñas cantidades de ácido clorhídrico. Al compuesto comercial se
agregan pequeñas cantidades de estabilizadores para prevenir su
descomposición.
Existen métodos analíticos para determinar el cloruro de metileno
en medios biológicos y muestras ambientales; en todos ellos se utiliza
cromatografía de gases y un detector apropiado. De esta manera se han
alcanzado limites de detección muy bajos (p. ej., alimentos:
7 ng/muestra; agua: 0,01 µg/litro; aire: 1,76 µg/m3 (0,5 ppb);
sangre: 0,022 mg/litro).
2. Fuentes de exposición humana y ambiental
Se estima que la producción mundial de cloruro de metileno
asciende a 570 000 toneladas/año. La mayoría sus aplicaciones se basan
en su capacidad para disolver grasas, plásticos y agentes aglutinantes
de pintura, así como en su volatilidad y estabilidad. Su uso a nivel
mundial se reparte del siguiente modo: aerosoles (20%-25%),
quitapinturas (25%), disolvente en la industria química y farmacéutica
(35%-40%), usos varios (p. ej., la fabricación de espuma de
poliuretano) y limpieza de metales (10%-15%). El uso de cloruro de
metileno tiende a disminuir, al menos en Europa occidental.
Más del 99% del cloruro de metileno liberado a la atmósfera
procede de diversas industrias que lo emiten como producto fina], o es
el resultado del uso doméstico de quitapinturas y aerosoles.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta volatilidad, la mayor parte del cloruro de
metileno liberado al medio pasa a la atmósfera, donde se degrada
reaccionando con radicales hidroxilo de origen fotoquímico; su tiempo
de permanencia es de seis meses.
La degradación abiótica del compuesto en agua es lenta en
comparación con la evaporación. Se ha comprobado que el cloruro de
metileno desaparece rápidamente del suelo y de las aguas subterráneas.
Se han utilizado diversos sistemas de ensayo para determinar la
degradación aerobia y anaerobia del cloruro de metileno. La
biodegradación completa, sobre todo en cultivos bacterianos tratados y
en condiciones aerobias, es rápida (p. ej., mineralización del 49%-66%
en 50 horas en fangos urbanos tratados). En los biorreactores se puede
alcanzar una degradación de hasta un 10% por hora. No hay indicios de
una bioacumulación o biomagnificación importantes.
4. Niveles medioambientales y exposición humana
Se ha detectado cloruro de metileno en el aire ambiente de zonas
rurales y remotas a concentraciones comprendidas entre 0,07 y
0,29 µg/m3. En zonas suburbanas la concentración promedio es
<2 µg/m3, y en zonas urbanas, <15 µg/m3. En las proximidades de
vertederos de desechos peligrosos se han hallado hasta 43 µg/m3. Las
precipitaciones también contienen a veces cloruro de metileno.
El cloruro de metileno penetra en el medio acuático a través de
las descargas de aguas residuales de diversas industrias, habiéndose
detectado su presencia en aguas superficiales, aguas subterráneas y
sedimentos.
La población general se expone al cloruro de metileno cuando
utiliza productos de consumo tales como los quitapinturas, cuyo empleo
puede acompañarse de la presencia de niveles relativamente altos en el
aire del interior de los domicilios. La exposición ocupacional durante
la producción tiene lugar sobre todo durante el llenado y envasado (la
fabricación se lleva a cabo en sistemas cerrados). Tratándose de un
compuesto usado en los quitapinturas, la exposición laboral al cloruro
de metileno se produce durante la elaboración de quitapinturas, la
fabricación de material para ordenadores y el acabado comercial de
muebles. El cloruro de metileno es ampliamente empleado como
disolvente industrial en la elaboración de diversos productos, sobre
todo en las industrias que se mencionan en la sección 1.2.
La vigilancia biológica de la exposición al cloruro de metileno
puede basarse en la medición del propio disolvente en el aire espirado
o en la sangre. No obstante, dado que la producción de monóxido de
carbono con una exposición de más de 3-4 horas/día parece ser el
factor limitante en lo que respecta a los riesgos para la salud, es
preferible basar la vigilancia biológica en el análisis bien del
monóxido de carbono presente en el aire espirado, o bien de la
carboxihemoglobina (CO-Hb) en sangre. Así y todo, esto sólo se puede
aplicar a las personas no fumadoras. Deben tomarse muestras antes de
transcurridas aproximadamente dos horas tras la exposición, o bien al
cabo de 16 horas, esto es, a la mañana siguiente.
Los niveles postexposición de CO-Hb a las dos horas de interrumpir
la exposición no deben sobrepasar el 2%-3%, y a las 16 horas el 1%, en
los no fumadores expuestos durante ocho horas a menos de 350 mg/m3
de cloruro de metileno.
5. Cinética y metabolismo
El cloruro de metileno es absorbido rápidamente por los alvéolos
pulmonares, a través de los cuales llega a la circulación sistémica.
Es absorbido también por el tracto gastrointestinal, así como por vía
cutánea, si bien en este último caso la velocidad de absorción es
menor que por otras vías de exposición.
El cloruro de metileno se excreta con considerable rapidez,
fundamentalmente a través del aire espirado por los pulmones. Puede
atravesar la barrera hematoencefálica, así como la placenta, y se
excreta también en pequeñas cantidades por la orina y la leche.
A altas concentraciones la mayoría del cloruro de metileno
absorbido se espira inalterado. El resto es metabolizado en monóxido
de carbono, dióxido de carbono y cloruro inorgánico. Hay dos vías
posibles de metabolización, cuya contribución relativa al metabolismo
total depende en gran medida de la dosis y de la especie animal
considerada. Una vía consiste en un proceso de metabolismo oxidativo
mediado por el citocromo P-450, que conduce a la producción tanto de
monóxido de carbono como de dióxido de carbono. Esta vía funciona de
manera parecida en todos los roedores estudiados y en el hombre. Si
bien es la vía metabólica predominante a dosis bajas, se satura
también a dosis relativamente bajas (en torno a 1800 mg/m3).
Aumentar la dosis por encima de ese nivel de saturación no conlleva un
mayor metabolismo a través de esa vía.
La otra vía está mediada por una glutatión-transferasa (GTF) y
conduce, previa producción de formaldehído y de formato, a la
formación de dióxido de carbono. Al parecer esta vía sólo adquiere
importancia a dosis superiores al nivel de saturación de la vía
oxidativa «preferente». En algunas especies (p. ej., el ratón)
constituye la principal vía metabólica a dosis suficientemente altas.
Por el contrario, en otras especies (p. ej., el hámster o el hombre)
esta vía apenas es utilizada, cualquiera que sea la dosis.
Las diferencias interespecies del metabolismo mediado por la GTF
guardan una clara relación con las diferencias interespecies
observadas en lo que respecta a la carcinogenicidad. Analizando la
intensidad del metabolismo mediado por esta vía en determinadas
especies, se ha elaborado un modelo cinético del metabolismo del
cloruro de metileno en diversas especies.
6. Efectos en organismos presentes en el medio ambiente
Por debajo de 500 mg/litro no se observa inhibición del
crecimiento de algas y de bacterias aerobias. Se han descubierto
bacterias capaces de crecer en presencia de cloruro de metileno a
concentraciones mucho mayores, incluida una solución saturada en agua
(sección 4.2.4.1 ). Las bacterias anaerobias son más sensibles; se ha
observado inhibición del crecimiento a una concentración de 1 mg/litro
en fangos biológicos anaerobios.
En el suelo, se observó que una concentración de 10 mg/kg reducía
considerablemente el contenido de ATP de la biomasa, incluidos hongos
y bacterias aerobias, e inducía una inhibición transitoria de la
actividad enzimática. El nivel sin efectos observados fue de
0,1 mg/kg. En las lombrices de tierra el cloruro de metileno tiene un
efecto moderadamente tóxico (100-1000 µg/cm2), como demuestra la
prueba de toxicidad de contacto con papel de filtro. En sedimentos no
se observaron efectos tóxicos ni siquiera a concentraciones muy altas.
En plantas superiores no se observaron efectos al cabo de 14 días
de exposición a 100 mg/m3.
Los peces adultos parecen relativamente insensibles al cloruro de
metileno, incluso después de una exposición prolongada (14 días,
CL50 > 200 mg/litro). El efecto del cloruro de metileno en Daphnia
resulta difícil de evaluar porque hay grandes diferencias entre los
resultados de los estudios realizados. La CE50 más baja notificada
es de 12,5 mg/litro.
En cuanto al entorno acuático, se ha demostrado que los embriones
de peces y anfibios son los más sensibles, observándose efectos sobre
la incubación a partir de 5,5 mg/litro.
7. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
7.1 Exposiciones aisladas
La toxicidad aguda del cloruro de metileno por vía respiratoria y
por vía oral es baja. La CL50-6h por inhalación está comprendida en
todas las especies entre 40 200 y 55 870 mg/m3. Se han registrado
DL50 orales de 1410-3000 mg/kg. Los efectos agudos de la
administración de cloruro de metileno por diversas vías de exposición
se manifiestan fundamentalmente en el sistema nervioso central (SNC) y
en el hígado, y se producen a dosis altas. Se han observado trastornos
del SNC a concentraciones de 14 100 mg/m3 o más, con ligeras
variaciones del EEG a 1770 mg/m3. A concentraciones de
17 700 mg/m3 o más se observaron leves cambios histológicos en el
hígado. Ocasionalmente se vieron afectados otros órganos, tales como
el riñón o el sistema respiratorio. En el ratón, los efectos sobre los
pulmones se limitaron a las células de Clara después de una exposición
a 7100 mg/m3. Se ha notificado la aparición de sensibilización
cardiaca a la arritmia inducida por adrenalina. Se han observado
efectos cardiovasculares, si bien de manera irregular.
7.2 Exposición a corto y a largo plazo
La exposición prolongada a concentraciones altas de cloruro de
metileno (>17 700 mg/m3) causó efectos reversibles sobre el SNC,
ligera irritación ocular y mortalidad en varias especies de
laboratorio. Se observó una reducción del peso corporal en ratas a
3500 mg/m3, y en ratones a partir de 17 700 mg/m3. El hígado de
perros expuestos continuamente a 3500 mg/m3 por espacio de hasta 100
días se vio ligeramente afectado. Se observaron asimismo efectos en el
hígado tras la exposición intermitente a 3500 mg/m3 en la rata, y a
14 100 mg/m3 en el ratón.
Otros órganos diana son los pulmones y los riñones.
No se hallaron indicios de daño neurológico irreversible en ratas
expuestas por inhalación a concentraciones de hasta 7100 mg/m3
durante 13 semanas.
La administración oral de cloruro de metileno a ratas causó
efectos hepáticos a partir de 200 mg/kg al día.
7.3 Irritación cutánea y ocular
El cloruro de metileno es moderadamente irritante para la piel y
los ojos de animales experimentales.
7.4 Toxicidad para el desarrollo y la reproducción
El cloruro de metileno no es teratógeno en la rata o el ratón a
concentraciones de hasta 16 250 mg/m3. En tres estudios realizados
con animales no se observaron indicios de variación de la incidencia
de malformaciones esqueléticas ni otros efectos sobre el desarrollo.
Se notificaron efectos leves sobre el peso corporal fetal o materno a
una concentración de 4400 mg/m3, así como sobre el aumento de peso
postnatal de ratas macho a una concentración del 0,04% en la dieta. Un
estudio de toxicidad reproductiva llevado a cabo en dos generaciones
de ratas expuestas a cloruro de metileno por inhalación a
concentraciones de hasta 5300 mg/m3, 6 h/día, 5 días/semana durante
17 semanas no puso de manifiesto ningún efecto adverso en lo tocante a
los parámetros reproductivos, la supervivencia neonatal o el
crecimiento neonatal en ninguna de las generaciones, F0 o F1.
7.5 Mutagenicidad y criterios de evaluación relacionados
En condiciones de exposición adecuadas el cloruro de metileno
tiene efectos mutágenos en microorganismos procariotas, con o sin
activación metabólica (Salmonella o Escherichia coli). En los
sistemas eucariotas los resultados son negativos, salvo en un caso en
que fueron débilmente positivos. Los ensayos y pruebas de mutación
genética in vitro basados en la síntesis no programada de ADN (UDS)
en células de mamífero fueron siempre negativos. Los ensayos in vitro
realizados para detectar aberraciones cromosómicas en diferentes tipos
de células dieron resultados positivos, mientras que en las pruebas de
inducción de intercambio de cromátides hermanas (SCE) se obtuvieron
resultados negativos o ambiguos.
La mayoría de los estudios in vivo publicados no han aportado
ningún dato indicativo de mutagenicidad del cloruro de metileno
(determinada por ejemplo, mediante la prueba de aberración
cromosómica, la prueba de los micronúcleos o el ensayo UDS). Se ha
notificado un aumento mínimo de la frecuencia de SCE y de micronúcleos
en el ratón tras la exposición por inhalación a altas concentraciones
de cloruro de metileno.
En ratas o ratones a los que se administraron dosis altas de
cloruro de metileno no se observaron indicios de unión del cloruro de
metileno al ADN ni de lesiones de éste. Son éstos los estudios
in vivo potencialmente más sensibles, el mejor de los cuales permite
detectar una alquilación por cada 106 nucleótidos.
Dentro de las limitaciones de las pruebas a corto plazo
actualmente disponibles, no hay pruebas concluyentes de que el cloruro
de metileno sea genotóxico in vivo.
7.6 Toxicidad crónica y carcinogenicidad
El cloruro de metileno es carcinógeno en el ratón, en el que la
exposición a altas concentraciones (7100 y 14 100 mg/m3) es causa de
tumores tanto pulmonares como hepáticos. La incidencia de esos dos
tipos de tumores aumentó en ratones expuestos a 7100 mg/m3 durante
26 semanas y estudiados durante 78 semanas más. No se observaron
signos claros de toxicidad o hiperplasia asociadas en los órganos
diana.
La exposición de hámsters sirios a cloruro de metileno por
inhalación a concentraciones de hasta 12 400 mg/m3 durante dos años
no tuvo efectos carcinógenos.
Se ha observado que las ratas expuestas al cloruro de metileno por
diversas vías sufren una mayor incidencia de tumores en determinados
lugares. Se ha notificado un exceso de tumores en la región de las
glándulas salivales en ratas hembra expuestas a 5300 ó 12 400 mg/m3
durante dos años. Ese exceso sólo se hizo patente cuando se procedió a
agrupar los tumores, todos ellos de origen mesenquimatoso, con fines
estadísticos. El método estadístico utilizado era inapropiado dado que
los tumores procedían de células de diverso tipo. Además, se señaló
que las ratas utilizadas se habían visto infectadas al principio del
estudio por un virus causante de una enfermedad común, la
sialodacrioadenitis, que afecta sobre todo a la glándula salival.
Probablemente los tumores no estaban relacionados causalmente con la
exposición al cloruro de metileno, y la exposición se limitó a
exacerbar la respuesta a la infección en la región de la glándula
salival. El efecto no se reprodujo en un segundo estudio realizado con
ratas expuestas a 3500, 7100 ó 14 100 mg/m3 durante su ciclo de
vida. Un estudio ulterior realizado con ratas expuestas por inhalación
a concentraciones de hasta 1770 mg/m3 de cloruro de metileno durante
todo su ciclo de vida no reveló indicios de carcinogenicidad. En ratas
expuestas al cloruro de metileno a través del agua que consumían o de
alimentos administrados con sonda tampoco se observaron indicios
significativos de carcinogenicidad.
Tres estudios han puesto de manifiesto un aumento de la incidencia
de tumores mamarios benignos en ratas expuestas a cloruro de metileno,
en dos de los casos por inhalación y en el tercero por administración
forzada. No se ha notificado ningún aumento de la incidencia de
tumores mamarios en hámsters o en ratones sometidos a dosis
comparables de cloruro de metileno. La dependencia de los tumores
mamarios de las hormonas hipofisarias en la rata, tanto macho como
hembra, es un dato incontrovertible. En la rata, la prolactina actúa
como iniciador y como promotor de la carcinogénesis mamaria. Hay datos
convincentes de que el aumento de los niveles de prolactina incrementa
la incidencia de tumores mamarios (p. ej., el injerto de varias
hipófisis en ratas Sprague-Dawley aumenta la incidencia de tumores
mamarios, y se ha observado además una correlación positiva entre la
existencia de niveles elevados de prolactina en sangre y la incidencia
de tumores mamarios en ratas hembra R-Amsterdam viejas). En las ratas
hembra que han recibido carcinógenos, los tratamientos inductores de
hiperprolactinemia dan lugar a un aumento espectacular de la
incidencia de tumores. Entre esos tratamientos cabe citar la
adrenalectomía, los homoinjertos hipofisarios y el consumo de
alimentos ricos en grasas.
El conocimiento de los mecanismos de inducción de adenomas
mamarios por el cloruro de metileno en la rata es importante para
poder evaluar los riesgos para el hombre. Las ratas Sprague-Dawley
hembras sometidas a cloruro de metileno presentan una elevada
concentración de prolactina en sangre. Al igual que la respuesta a
otros agentes cuya acción está mediada por una hiperprolactinemia, la
respuesta inducida por el cloruro de metileno se limita a la aparición
de neoplasias benignas. No hay datos indicativos de una unión del
cloruro de metileno al ADN de otros tejidos, por lo que parece
improbable que pueda unirse al tejido mamario, tanto más cuanto que su
metabolismo se produce fundamentalmente en el hígado. Es más probable,
por tanto, que el aumento de la incidencia de adenomas mamarios se
deba a un mecanismo indirecto en el que intervenga la hiperprolac-
tinemia.
En cuanto al hombre, hay datos contradictorios respecto a si los
tumores mamarios son tan sensibles a la prolactina como en la rata.
Este animal presenta niveles elevados de prolactina cuando es
alimentado ad libitum en lugar de sometido a una dieta restringida,
lo cual explica quizá la gran sensibilidad de la incidencia de tumores
mamarios a diversos efectos ambientales y de otro tipo. En la rata, no
obstante, la prolactina es luteotrófica. Un aumento de la prolactina
circulante da lugar a un aumento de los niveles de progesterona y de
estrógenos exógenos. Es la coincidencia de estos tres factores lo que
causa el crecimiento túbulo-alveolar de las glándulas mamarias y,
finalmente, el desarrollo del tumor. La prolactina no es luteotrófica
en los primates; es improbable, por tanto, que este mecanismo de
desarrollo tumoral pueda tener importancia en el hombre.
En la rata, el mecanismo de desarrollo de tumores mamarios mediado
por la hiperprolactinemia sólo entra en juego a las dosis de cloruro
de metileno que alteran los niveles de prolactina. No se dispone de
información directa sobre los niveles de prolactina en ratas sometidas
a dosis bajas de cloruro de metileno, pero no se ha observado ningún
aumento de la incidencia de adenomas mamarios tras la administración
de dosis bajas por inhalación o a través del agua de bebida (p. ej.,
dosis inferiores a 250 mg/kg peso corporal).
8. Efectos en el hombre
El cloruro de metileno es irritante para la piel y para los ojos,
sobre todo cuando se impide su evaporación. En estas condiciones, el
contacto prolongado puede causar quemaduras químicas. Se ha notificado
un caso de edema pulmonar grave por inhalación excesiva. Se han
producido también defunciones en casos de inhalación o contaminación
cutánea accidentales. Los principales efectos tóxicos del cloruro de
metileno son la depresión reversible del SNC y la formación de CO-Hb.
Se ha señalado también la aparición de disfunciones hepáticas y
renales y de trastornos hematológicos tras la exposición al producto.
Se han observado problemas neurofisiológicos y neurocomporta-
mentales en voluntarios humanos expuestos a concentraciones de cloruro
de metileno de 694 mg/m3 durante 1,5-3,0 horas. No se han observado
efectos neurológicos en hombres expuestos durante varios años a
concentraciones del producto comprendidas entre 260 y 347 mg/m3. De
forma parecida, un grupo de raspadores de pintura de aviones ya
jubilados con antecedentes de una larga (22 años) exposición a
concentraciones altas, si bien no especificadas, de cloruro de
metileno obtuvieron resultados "normales" en una batería de pruebas
neurofisiológicas y psicológicas en comparación con un grupo testigo
sin antecedentes de exposición, o en todo caso con antecedentes de una
baja exposición al compuesto.
Un aumento de la tasa de abortos espontáneos entre empleadas de la
industria farmacéutica finlandesa se ha atribuido a la exposición a
cloruro de metileno. Sin embargo, el diseño incorrecto del estudio ha
impedido establecer una relación causal.
Varios estudios de mortalidad realizados en cohortes pertinentes
muestran resultados dispares en cuanto a las causas de defunción. Se
ha observado un aumento de la mortalidad por enfermedades especificas
(como por ejemplo el cáncer pancreático o la cardiopatía isquémica),
pero de forma irregular y sólo en determinados estudios. Estos efectos
no se pueden atribuir a la exposición al cloruro de metileno.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
Members
Dr L.A. Albert, Consultores Ambientales Associados, Xalapa, Veracruz,
Mexico
Mr D. Farrar, ICI Chemicals and Polymers, Runcorn, Cheshire, United
Kingdom (Rapporteur)
Dr R. Fransson-Steen, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden
Dr S. Henry, US Food and Drug Administration, Washington, DC, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Huntingdon, United Kingdom
Dr P. Standring, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr L. Stayner, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr T. G. Vermeire, Toxicology Advisory Centre, National Institute of
Public Health and Environmental Hygiene, Bilthoven, The
Netherlands (Chairman)
Dr Ruqiu Ye, National Environmental Protection Agency, Beijing, China
Observers
Dr C. De Rooij, Solvay & Cie S.A., Brussels, Belgium
Dr T. Green, ICI Chemicals & Polymers Ltd., Runcorn, Cheshire, United
Kingdom
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P. Demers, Unit of Analytical Epidemiology, International Agency
for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
A WHO Task Group on Environmental Health Criteria for Methylene
Chloride met at the Institute of Terrestrial Ecology, Monks Wood,
United Kingdom from 16 to 20 August 1993. Dr S. Dobson welcomed the
participants on behalf of the host institution, and Dr M. Gilbert
opened the meeting on behalf of the three cooperating organizations of
the IPCS (ILO/UNEP/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methylene chloride.
The first draft of this monograph was prepared by Mr D. Farrar,
ICI Chemicals and Polymers, Runcorn, United Kingdom.
Dr M. Gilbert, IPCS, was responsible for the overall scientific
content of this monograph. After his death in July 1994, this
responsibility was transferred to Dr P.G. Jenkins, IPCS, who also
dealt with the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ALT alanine aminotransferase
AST aspartate aminotransferase
BEI Biological Exposure Index
CO-Hb carboxyhaemoglobin
GST glutathione transferase
LEV local exhaust ventilation
MATC maximum acceptable toxicant concentration
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health (USA)
SCE sister-chromatid exchange
SGOT serum glutamic-oxaloacetic transaminase
SGPT serum glutamic-pyruvic transaminase
TT toxicity threshold
TWA time-weighted average
UDS unscheduled DNA synthesis
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical methods
Methylene chloride (dichloromethane) is a clear, highly volatile,
non-flammable liquid with a penetrating ether-like odour. The pure dry
compound is very stable. Methylene chloride hydrolyses slowly in the
presence of moisture, producing small quantities of hydrogen chloride.
Commercial methylene chloride normally contains small quantities of
stabilizers to prevent decomposition.
Analytical methods are available for the determination of
methylene chloride in biological media and environmental samples. All
methods involve gas chromatography in combination with a suitable
detector. In this way, very low detection limits have been reached
(e.g., in food: 7 ng/sample; water: 0.01 µg/litre; air: 1.76 µg/m3
(0.5 ppb); blood: 0.022 mg/litre).
1.2 Sources of human and environmental exposure
World production of methylene chloride is estimated to be
570 000 tonnes/year. Most applications are based on its solvent
capacity for grease, plastics and paint binding agents, in combination
with its volatility and stability. The worldwide usage pattern
comprises aerosols (20-25%), paint remover (25%), process solvent in
the chemical and pharmaceutical industry (35-40%), miscellaneous uses
(e.g., polyurethane foam manufacturing) and metal cleaning (10-15%).
The usage of methylene chloride is tending to decrease, at least in
western Europe.
More than 99% of the atmospheric releases of methylene chloride
result from its use as an end-product by various industries and the
use of paint removers and aerosol products at home.
1.3 Environmental transport, distribution and transformation
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where it
will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months.
Abiotic degradation in water is slow compared to evaporation.
Methylene chloride has been shown to disappear rapidly from soil and
ground water.
The aerobic and anaerobic degradation of methylene chloride has
been established by a variety of different test systems. Complete
biodegradation, especially by acclimated bacterial cultures under
aerobic conditions, is rapid (e.g., 49-66% mineralization in 50 h with
acclimated municipal sludge). In bioreactors, up to 10% degradation
per hour is achievable. There is no evidence of significant
bioaccumulation or biomagnification.
1.4 Environmental levels and human exposure
Methylene chloride has been detected in the ambient air of rural
and remote areas at concentrations of 0.07-0.29 µg/m3. In suburban
areas, the average concentration is < 2 µg/m3 and in urban areas
< 15 µg/m3. In the vicinity of hazardous waste sites up to
43 µg/m3 has been found. Precipitation may also contain methylene
chloride.
Methylene chloride enters the aquatic environment through waste
water discharge from various industries, and methylene chloride has
been found in surface water, ground water and sediment.
Exposure of members of the general public to methylene chloride
will occur from its use in consumer products such as paint removers,
which can result in relatively high levels being found in indoor air.
Occupational exposure during production arises primarily during
filling and packaging (manufacturing is in closed systems). Because of
its use in paint strippers, occupational exposure to methylene
chloride occurs during formulation of paint-remover, original
equipment manufacture, and in commercial furniture refinishing.
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products, in particular in the industries
mentioned in section 1.2.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor in regard to health risk, biological
monitoring based upon either analysis of carbon monoxide in exhaled
air or of carboxyhaemaglobin (CO-Hb) in blood is to be preferred.
However, this can only be used for non-smoking subjects. Sampling
should be done at about 0-2 h post-exposure, or after 16 h, i.e. on
the following morning.
Post-exposure CO-Hb levels 2 h after exposure ceases are not
expected to exceed 2-3%, and at 16 h 1%, in the case of an 8-h
exposure to less than 350 mg methylene chloride/m3 in non-smokers.
1.5 Kinetics and metabolism
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract, and dermal exposure results in absorption but
at a slower rate than via the other routes of exposure.
Methylene chloride is quite rapidly excreted, mostly via the lungs
in the exhaled air. It can cross the blood-brain barrier and be
transferred across the placenta, and small amounts can be excreted in
urine or in milk.
At high concentrations, most of the absorbed methylene chloride is
exhaled unchanged. The remainder is metabolized to carbon monoxide,
carbon dioxide and inorganic chloride. Metabolism occurs by either or
both of two pathways, whose relative contribution to the total
metabolism is markedly dependent on the dose and on the animal species
concerned. One pathway involves oxidative metabolism mediated by
cytochrome P-450 and leads to both carbon monoxide and carbon dioxide.
This pathway appears to operate similarly in all rodents studied and
in man. Whilst this is the predominant metabolic route at lower doses,
saturation occurs at a relatively low dose (around 1800 mg/m3).
Increasing the dose above the saturation level does not lead to extra
metabolism by this route.
The other pathway involves a glutathione transferase (GST), and
leads via formaldehyde and formate to carbon dioxide. This route seems
only to become important at doses above the saturation level of the
"preferred" oxidative pathway. In some species (e.g., the mouse) it
becomes the major metabolic pathway at sufficiently high doses. In
contrast, in other species (e.g., hamster, man) it seems to be used
very little at any dose.
Species difference in GST metabolism correlates well with the
observed species difference in carcinogenicity. The extent of
metabolism by this pathway in relevant species has been used as the
basis for a kinetic model to describe the metabolic behaviour of
methylene chloride in various species.
1.6 Effects on organisms in the environment
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria have been identified that are able to grow in
the presence of methylene chloride at much higher concentrations
including a saturated solution in water (section 4.2.4.1). Anaerobic
bacteria are more sensitive; growth inhibition has been observed at
1 mg/litre in anaerobic biological sludge.
In soil a concentration of 10 mg/kg strongly decreased the ATP
content of the biomass including fungi and aerobic bacteria, and
induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms methylene chloride is
moderately toxic (100-1000 µg/cm2) in the filter-paper contact
toxicity test. In sediment no toxic effects were observed even at very
high levels.
In higher plants no effects were found after exposure for 14 days
to 100 mg/m3.
Adult fish seem to be relatively insensitive to methylene chloride
even after prolonged exposure (14-day LC50 > 200 mg per litre). The
effect of methylene chloride on Daphnia is difficult to assess given
the large variation in the outcome of the studies performed. The
lowest reported EC50 was 12.5 mg/litre.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive with effects on hatching from
5.5 mg/litre.
1.7 Effects on laboratory mammals and in vitro test systems
1.7.1 Single exposures
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species are between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg were recorded. Acute effects after methylene chloride
administration by various routes of exposure are primarily associated
with the central nervous system (CNS) and the liver, and these
occurred at high doses. CNS disturbances were found at concentrations
of 14 100 mg/m3 or more, with slight changes in EEG at 1770 mg/m3.
Slight histological changes in the liver were found at 17 700 mg/m3
or more. Occasionally other organs were affected such as the kidney or
respiratory system. In mice, effects on the lungs were restricted to
the Clara cells after exposure to 7100 mg/m3. Cardiac sensitization
to adrenaline-induced arrhythmia has been reported. Cardiovascular
effects have been seen but the effects were inconsistent.
1.7.2 Short- and long-term exposure
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice from
17 700 mg/m3. Slight effects on the liver were noted in dogs
continuously exposed to 3500 mg/m3 for up to 100 days. After
intermittent exposure, effects on the liver were observed in rats at
3500 mg/m3 and in mice at 14 100 mg/m3.
Other target organs are the lungs and the kidneys.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations up to 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver from about 200 mg/kg per day.
1.7.3 Skin and eye irritation
Methylene chloride is moderately irritant to the skin and eyes of
experimental animals.
1.7.4 Developmental and reproductive toxicity
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3. No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
were observed in three animal studies. Small effects on either fetal
or maternal body weight were reported at 4400 mg/m3, and on
postnatal weight gain of male rats at 0.04% in the diet. A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations up to 5300 mg/m3, 6 h/day,
5 days/week for 17 weeks did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
1.7.5 Mutagenicity and related end-points
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (Salmonella or Escherichia coil). In eukaryotic systems
it gives either negative or, in one case, weakly positive results.
In vitro gene mutation assays and tests for unscheduled DNA
synthesis (UDS) in mammalian cells were uniformly negative. In vitro
assays for chromosomal aberrations using different cell types gave
positive results, whereas negative or equivocal results were obtained
in tests for sister chromatid exchange (SCE) induction.
The majority of the in vivo studies reported provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay, micronucleus test or UDS assay). Marginal increase
in frequencies of SCEs and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride.
There was no evidence of binding of methylene chloride to DNA or
DNA damage in rats or mice given high doses of methylene chloride.
These studies are potentially the most sensitive in vivo studies,
the best of which are capable of detecting one alkylation in 106
nucleotides.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride in
genotoxic in vivo.
1.7.6 Chronic toxicity and carcinogenicity
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following exposure to high concentrations (7100 and
14 100 mg/m3) of methylene chloride. The incidence of both lung and
liver tumours was increased in mice exposed to 7100 mg/m3 for 26
weeks and maintained for a further 78 weeks. There was no substantial
evidence of associated toxicity or hyperplasia in the target organs.
Syrian hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of tumours
in the region of the salivary gland was reported in female rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different cells, the statistical approach adopted
was inappropriate. Furthermore, it was reported that the rats in the
study had been infected with a common viral disease (sialoda-
cryoadenitis) early in the study, an infection that affects primarily
the salivary gland. It is likely that these tumours were not causally
related to exposure to methylene chloride but that the exposure had
exacerbated the response of the infection in the region of the
salivary gland. The response was not seen in a second study in which
rats were exposed to either 3500, 7100 or 14 100 mg/m3 for their
lifetime. A further inhalation study on rats exposed to methylene
chloride at concentrations up to 1770 mg/m3 for their lifetime
showed no evidence of carcinogenicity. Rats exposed to methylene
chloride via their drinking-water or by gavage similarly showed no
substantive evidence of carcinogenicity.
An increased incidence of benign mammary tumours in rats exposed
to methylene chloride has been reported in three studies, two
following exposure by inhalation and the third by gavage. There are no
reports of increases in mammary tumour incidence in hamsters or in
mice receiving methylene chloride at comparable dose levels. The
dependence of mammary tumours upon pituitary hormones in both male and
female rats has been established unequivocally. In the rat, prolactin
acts as both an initiator and promoter of mammary carcinogenesis.
There is good evidence that increased prolactin levels increase the
incidence of mammary tumours (e.g., the grafting of multiple pituitary
glands into Sprague-Dawley rats increases the incidence of mammary
tumours and there is a positive correlation between elevated blood
prolactin levels and mammary tumours in aged R-Amsterdam female rats).
Treatments that induce hyperprolactinaemia in female rats that have
received carcinogens produce a dramatic increase in tumour incidence.
These treatments include adrenalectomy, pituitary homografts and high
dietary fat.
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin. In common with the response to other agents which act via
hyperprolactinaemia, the methylene chloride-induced response is of
benign neoplasms only. There is no evidence for the binding of
methylene chloride to the DNA of other tissues and hence it seems
unlikely that it will bind to mammary tissue when the primary site of
metabolism is the liver. It seems most likely, therefore, that the
increased incidence of mammary adenomas is the result of an indirect
mechanism operating via hyperprolactinaemia.
In humans, there is conflicting evidence on whether or not mammary
tumours are as responsive to prolactin as is the case in the rat. The
rat has elevated levels of prolactin when fed ad libitum in
comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels. It
is the presence of all three factors that causes tubular-alveolar
growth of the mammary glands, which ultimately leads to tumour
development. Prolactin is not luteotrophic in primates. It is
unlikely, therefore, that this mechanism of tumour development is of
relevance to man.
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will occur only at doses of methylene
chloride which affect prolactin levels. There is no direct information
on prolactin levels in rats receiving low doses of methylene chloride,
but no increase in mammary adenomas has been observed following the
administration of low doses in either inhalation or drinking-water
studies (i.e. below 250 mg/kg body weight).
1.8 Effects on humans
Methylene chloride irritates the skin and eyes especially when
evaporation is prevented. In these circumstances, prolonged contact
may cause chemical burns. A case of serious pulmonary oedema has been
reported after excessive inhalation. Fatalities due to accidental
inhalation and skin contamination have been reported. The main toxic
effects of methylene chloride are reversible CNS depression and CO-Hb
formation. Liver and renal dysfunctions and effects on haematological
parameters have also been reported following exposure to methylene
chloride.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men with exposure for several years
to methylene chloride at concentrations ranging from 260 to
347 mg/m3. Similarly, a group of retired airplane strippers with a
long history of exposure to methylene chloride (22 years) at high but
unspecified levels performed a battery of neurophysiological and
psychological tests within the "normal" range, when compared with a
control group who had a history of either no or only low exposure to
methylene chloride.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to methylene
chloride. A causal relationship was not established because of
insufficiencies in the design of the study.
Several mortality studies in relevant cohorts show an inconsistent
pattern in the causes of death. Excesses in mortality from specific
diseases (e.g., pancreatic cancer, ischaemic heart disease) were not
consistently increased, but confined to single studies. These effects
cannot be attributed to exposure to methylene chloride.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Formula: CH2CI2
CI
'
Structure: CI - C - H
'
H
Relative molecular mass: 84.93
Common name: Methylene chloride
Synonyms: DCM; dichloromethane; methane
dichloride; methylene bichloride;
methylene dichloride; methylenum
chloratum
Tradenames: Aerothene MM; Freon 30; Narkotil;
Solaestin; Solmethine
CAS name (9 CI): Methane, dichloro-
CAS registry number: 75-09-2
EC registry number: 602-004-00-3
EINECS registry number: 200-838-9
RTECS registry number: PA 8050000
Purity of technical 99.9% (analytical grade)
product:
Impurities of technical Mostly C1- and C2-chlorinated
product hydrocarbons (up to 200 mg/kg)
(ECETOC, 1984)
Stabilizer: Typically 0.005-0.2% (w/w)
methanol, ethanol, amylene
(2-methyl-but-2-ene), cyclohexane
or tertiary butylamine (ECSA, 1989)
2.2 Physical and chemical properties
Methylene chloride is a clear, colourless, highly volatile, non-
flammable liquid with a penetrating ether-like odour. Pure dry
methylene chloride is a very stable compound and is non-corrosive. In
the presence of water, it undergoes very slow hydrolysis to produce
small quantities of hydrogen chloride, which can lead to corrosion,
e.g., to mild steel. This reaction is accelerated by elevated
temperatures and the presence of alkalis or metals. In the vapour
phase under abnormal conditions (elevated temperatures, high UV light
exposure, flame, sparks, red hot surfaces), methylene chloride may be
decomposed to give small amounts of hydrogen chloride, carbon monoxide
and phosgene (ECSA, 1989). Other physical and chemical properties are
given in Table 1.
Commercial methylene chloride is normally stabilized (section 2.1)
to prevent decomposition. Applications in aggressive conditions, such
as special metal cleaning operations may require more sophisticated
stabilizer technology. Poorly stabilized methylene chloride can react
violently with aluminium or other light metals.
2.3 Conversion factors
Conversion factor for methylene chloride concentrations in air,
calculated at 20°C and 1.013 hPa are:
1 mg/m3 = 0.28 ppm
1 ppm = 3.53 mg/m3
and for carbon monoxide:
1 mg/m3 = 0.86 ppm
1 ppm = 1.16 mg/m3
2.4 Analytical methods
Details of sampling and methods of analysis used in biological
media and environmental samples are given in Tables 2 and 3.
Table 1. Physical and chemical properties
Parameter, units Value Reference
Boiling temperature (°C at 1.013 hPa) 40 Weast et al. (1988)
Melting temperature (°C at 1.013 hPa) -95.1 Weast et al. (1988)
Relative density of liquid D (20) 1.3266 Weast et al. (1988)
(water at 4°C = 1 kg/m3) 4
Vapour pressure (hPa at 20°C) 470 ECSA (1989)
Saturation concentration in air 1.7 Calculated
(kg/m3 at 20°C)
Vapour density at 20°C (air = 1) 2.93 IPCS (1984)
Threshold odour concentration 743 Leonardos et al.
(mg/m3) (1969)
(odour: ether-like) 700-1060 DFG (1983)
880 Amoore & Hautala
(1983)
540-2160 Ruth (1986)
Solubility in water (g/kg at 20°C) 20 Verschueren (1983)
13.0 Horvath (1982)
Solubility in alcohol, ether, acetone Weast et al. (1988)
and benzene
Partition coefficients, at 20°C 1.25 IPCS (1984)
log Pow (octanol/water) 1.3 Hansch & Leo
(1979)
log Koc 0.89
calculated from Kow
(Karickhoff, 1981)
Henry's Law constant, Pa.m3/mol at 380
20°C Smith (1989)
Flash point, closed cup (°C) None ECSA (1989)
Explosion limits in aira (%) 13-22 ECSA (1989)
Auto-flammability, ignition temp. (°C) 605 ECSA (1989)
a This is with a high energy source; these conditions are unlikely
to arise in normal operations.
Table 2. Analytical methods for determining methylene chloride in biological monitoring (ATSDR, 1991)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Blood Heat sample, collect GC/FID 0.022 mg/litre 49.8±1.33 Di Vincenzo et al.
headspace vapour (1971)
Urine Heat sample, collect GC/FID No data 59±2.75 Di Vincenzo et al.
headspace vapour (1971)
Breath Heat sample, inject into gas GC/FID 0.706 ± 0.353 No data Di Vincenzo et al.
sample loop mg/m3 (1971)
(0.2 ± 0.1 ppm)
Adipose tissue Hydrolyse with acid, heat GC/FID 1.6 mg/kgb No data Engström & Bjurström
sample, collect headspace (1977)
vapour
Human milk Purge with helium, trap on GC/MS No data No data Pellizzari et al. (1982)
sorbent trap, desorb thermally
a FID = flame ionisation detector; GC = gas chromatography; MS = mass spectrometry
b Lowest reported concentration
Table 3. Analytical methods for determining methylene chloride in environmental samples (ATSDR, 1991)
Sample Preparation method Analytical Sample detection Percentage Reference
matrix methoda limit recovery
Air Adsorb on charcoal, desorb with GC/FID 88.25µg/m3 90-110c APHA (1977)
carbon disulfide (25 ppb)b
Air Adsorb on charcoal, desorb with GC/FID 0.01 mg 95.3 NIOSH (1987)
carbon disulfide
Air Adsorb on charcoal, desorb with GC/ECD approx. 1.76 µg/m3 No data Woodrow et al.
benzyl alcohol (approx. 0.5 ppb) (1988)
Water Purge with inert gas, trap on sorbent GC/HSD No data 85 US EPA (1989c)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent GC/ELCD 0.01 µg/litre 97-100 US EPA (1989)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent GC/MS 1.0 µg/litre 99 US EPA (1989b)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent HRGC/MS 0.03-0.09 µg/litre 95-97 US EPA (1989a)
trap, desorb thermally
Water Purge with inert gas, trap on sorbent HRGC/ELCD 0.01-0.05 µg/litre 97±28 APHA (1989a)
trap, desorb thermally
Table 3 (Cont'd)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Water Purge with inert gas, trap on sorbent HRGC/MS 0.02-0.2 µg/litre 95±5 APHA (1989b)
trap, desorb thermally
Water Purge with helium, trap on sorbent GC/MS No data 99-105 Michael et al.
trap, desorb thermally (1988)
Waste Purge with inert gas, trap on sorbent GC/HSD 0.25 µg/litre 97.9±2.6 US EPA (1982a)
water trap, desorb thermally
Waste Purge with inert gas, trap on sorbent GC/MS 2.8 µg/litre 89±28 US EPA (1982b)
water trap, desorb thermally
Soil/solid Purge with inert gas, trap on sorbent GC/MS 5 µg/kg D-221 US EPA (1986a)
waste trap, desorb thermally
Soil/solid Purge with inert gas, trap on sorbent GC/HSD No data 25-162 US EPA (1986b)
waste trap, desorb thermally; or inject
directly into GC
Food Equilibrate in heated sodium sulfate GC/ELCD 0.05 ppm No data Page & Charbonneau
solution, collect headspace vapour (1984)
Food Isolate solvent by closed system GC/ELCD 7 ng 94 Page & Charbonneau
vacuum distillation with toluene as (1977)
carrier solvent
Table 3 (Cont'd)
Sample matrix Preparation method Analytical Sample detection Percentage Reference
methoda limit recovery
Food Isolate solvent by closed system GC/ECD 7 ng 100 Page & Charbonneau
vacuum distillation with toluene as (1977)
carrier solvent
Food Purge with nitrogen, trap on sorbent GC/ELCD 1.2 mg/kgd 84-96 Heikes (1987)
trap, elute with hexane
Food Extract with acetone-water, back GC/ELCD 4 µg/kg 66 Daft (1987)
extract with iso-octane
a ECD = electron capture detector; ELCD = electrolytic conductivity detector; FID = flame ionisation detector; GC = gas chromatography;
HRGC = high resolution gas chromatography; HSD = halogen-specific detector; MS = mass spectrometry
b Lowest value for various compounds reported during collaborative testing of this method
c Estimated accuracy of the method when the personal sampling pump is calibrated with a charcoal tube in the line
d Lowest reported concentration
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Methylene chloride is not known to occur naturally in the
environment.
3.2 Anthropogenic sources
3.2.1 Production
Methylene chloride is produced almost exclusively by the Stauffer
process. Methyl chloride is first produced by the reaction of methanol
with hydrogen chloride and is then reacted with chlorine. Chloroform
and, to a lesser extent, carbon tetrachloride are also produced.
Historically the direct route to methylene chloride by chlorination of
methane was also used; this also produced the other three
chloromethanes in varying proportions depending on the conditions used
(CEC, 1986; ICI, personal communication to the IPCS).
World production of methylene chloride in 1980 was estimated to be
570 000 tonnes (Edwards et al., 1982); a similar figure is considered
to apply currently (ECSA, 1992). USA production was 229 000 tonnes in
1988, the demand being 207 000 tonnes. The total amount produced in
western Europe ranged from 331 500 tonnes in 1986 to 254 200 tonnes in
1991 (ECSA, 1992).
3.2.2 Uses
The usage of methylene chloride in Western Europe shows a decrease
from 200 000 tonnes/year in 1975-1985 (CEFIC, 1986) to
175 000 tonnes/year in 1989 and to 150 000 tonnes/year in 1992 (CEFIC,
1993).
Most of the applications of methylene chloride are based on its
considerable solvent capacity, especially for grease, plastics and
various paint-binding agents. Other important properties are its
volatility and stability; it is also non-flammable. Among its uses are
(CEFIC, 1983):
- a component of paint and varnish strippers, and adhesive
formulations
- a solvent in aerosol formulations
- an extractant in food and pharmaceutical industries
- a process solvent in cellulose ester production and fibre and
film forming
- a process solvent in polycarbonate production
- a blowing agent in flexible polyurethane foams
- the extraction of fats and paraffins
- plastics processing, and metal and textile treatment
- a vapour degreasing solvent in metal-working industries
An estimated breakdown of usage worldwide before 1985 is given in
Table 4.
Table 4. Estimated usage patterns (BUA, 1986)
USA (1985) Western Europe (1984)
Aerosols 25 10
Paint strippers 23 50
Degreasing agent 8 13
Film, electronics industries 7 15
Blowing agent 5
Others 35 12
It should be noted that these data apply to the situation
approximately 10 years ago and may have changed since. Reliable
reports on present trends are not available.
3.2.3 Consumer applications
The main use in consumer products is in paint strippers, where
methylene chloride is the main constituent (70-75%). The second
important use is in hairspray aerosols, where it acts as a solvent and
vapour pressure modifier. In the European Community (EC) it may be
used in such products at concentrations of up to 35% w/w (European
Council, 1982). The US Food and Drug Administration has banned the use
of methylene chloride in cosmetic products. It is also used in aerosol
paints. Other types of methylene chloride-containing products are
household cleaning products and lubricating, degreasing and automotive
products, some of which may be in aerosol form. Chemical products
containing methylene chloride were banned from sale or transfer to
consumers for their private use in 1993 according to the Swedish Code
of Statutes. Furthermore, it may not be used for working purposes
after 1st January 1996 (National Chemical Inspectorate, Sweden,
personal communication to the IPCS).
3.2.4 Sources in the environment
Most of the methylene chloride released to the environment results
from its use as an end-product by various industries, and the use of
paint removers and aerosol products in the home. Methylene chloride is
mainly released to the environment in air and, to a lesser extent, in
water and soil.
Methylene chloride is released to the atmosphere during its
production, storage and transport, but more than 99% of the
atmospheric releases result from industrial and consumer uses (US EPA,
1985). It has been estimated that 85% of the total amount of methylene
chloride produced in the USA is lost to the environment, of which 86%
is released to the atmosphere (US EPA, 1985). Data reported to the US
EPA for the 1988 Toxic Chemical Release Inventory indicate that
approximately 170 000 tonnes of the USA production volume for 1988
(230 000 tonnes) was lost to the atmosphere; of this, 60 000 tonnes
resulted from industrial methylene chloride emissions and 110 000
tonnes from the use of consumer products and from other sources such
as hazardous waste sites.
Estimates of annual global emissions of 500 000 tonnes have been
reported for methylene chloride (WMO, 1991). The short atmospheric
lifetime of methylene chloride (see section 4.2.1) implies that
emissions quantities given on a seasonal as well as on a regional
basis are more relevant for comparison with atmospheric measurements.
The total emission into the air in western Europe was estimated to be
173 000 tonnes for 1989 and 180 000 tonnes in 1991.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
Appraisal
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where
it will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months. Given an intra-hemispheric
mixing time of approximately 1 month, transport can occur to regions
far removed from the emission source. The atmospheric lifetime is
fairly short relative to the inter-hemispheric transport time of 1 to
1.5 years, resulting in higher concentrations of methylene chloride
in the northern hemisphere, where most of the emissions occur at
present.
Methylene chloride is expected to have no significant impact on
stratospheric ozone depletion. It will not contribute significantly
to photochemical smog formation.
Hydrolysis and photolytically induced degradation in water are
slow compared to evaporation. Methylene chloride has been shown to
disappear rapidly from soil and ground water due to bio-
transformation.
The aerobic and anaerobic degradation of methylene chloride has
been proven by a variety of different test systems. Complete
biodegradation by acclimated bacterial cultures under aerobic
conditions is rapid. There is no evidence that significant
bioaccumulation or biomagnification of methylene chloride along the
food chain will occur.
4.1 Transport and distribution between media
4.1.1 Water/air
Methylene chloride enters the hydrosphere either directly, via
aqueous effluents, or indirectly from the atmosphere by dissolution in
sea water and in rain water. Due to its high volatility (Henry's Law
constant 380 Pa.m3/mol at 20°C) and low liquid-film transfer
coefficient (Kp = 0.005 m/h), methylene chloride is rapidly
transferred from the hydrosphere to the atmosphere.
Under laboratory conditions, the estimated half-life for
volatilization of methylene chloride from water at 25°C was 18-25 min
(when present at 1 mg/litre and stirred at 200 rpm). Removal of 90% of
the methylene chloride required 60-80 min. When stirring was minimal
(15 seconds every 5 min), the time required for 50% reduction in the
concentration was about 90 min. The presence of 3% sodium chloride (as
in sea water) decreased the evaporation rate by 10% (Dilling et al.,
1975; Dilling, 1977).
Various factors have been shown to affect the rate of
volatilization. For example, the half-life for volatilization of
methylene chloride from a depth of 1 m has been shown to be 3 h
(Lyman, 1982). The application of wind across the surface of the water
caused an increase of 17% in volatilization over a period of 20 min
compared to the presence of still conditions (Dilling et al., 1975). A
decrease in the water temperature decreased the rate of
volatilization. For example, over a period of 30 min, a 28% decrease
in rate was seen at 1-2°C compared to that at 25°C (Dilling et al.,
1975).
When measured under field conditions in experimental ponds, half-
lives for methylene chloride of 26-28 h have been reported (Merlin et
al., 1992). Its half-life for evaporation from the river Rhine has
been estimated to be 33-38 clays (Zoeteman et al., 1980). Further
estimates of the half-life for its evaporation are between 3 and 48 h
depending on wind and mixing conditions (Halbartschlager et al.,
1984). In a further study, methylene chloride was not detected at a
point 4-8 km from the point of release into an estuarine bay (Helz &
Hsu, 1978) or at 25 km below its discharge point in a river basin (De
Walle & Chain, 1978).
Rain-out is considered to be a limited process for removal of
methylene chloride from the troposphere. If it is assumed that its
aqueous-phase concentration is in equilibrium with the background
concentration in the northern hemisphere of about 123-134 ng/m3
(35-38 ppt) (Cox et al., 1976; WMO, 1991), the total amount of
methylene chloride rained out in the northern hemisphere will be
700 tonnes/year (assuming a rain fall of 2.5x1014 tonnes/year
containing 9.9 ng/m3 (2.8 ppt) at 10°C). The same calculation
performed at 20°C (Henry's constant is 1.57 times higher) would lead
to a value of 445 tonnes methylene chloride rained out annually in the
northern hemisphere. For the southern hemisphere, rainout quantities
of 390 and 248 tonnes methylene chloride can be calculated. The half-
life for removal by wet deposition is 550 years (Cupitt, 1980).
In 1978, it was estimated that 2.5% of releases at ground level
may reach the stratosphere (Derwent & Eggleton, 1978).
4.1.2 Soil/air
Methylene chloride present in the soil is predicted to evaporate
from the near-surface layer into the atmosphere because of its high
vapour pressure (470 hPa at 20°C).
4.1.3 Water/soil
The adsorption coefficient sediment/water for methylene chloride
is 8-10 (log Koc = 0.89-1.05). Methylene chloride has a low tendency
to adsorb to soil (adsorption coefficient 0.25 for a soil containing
1% organic carbon, Giger et al., 1983). Therefore there is a potential
for it to leach to ground water.
The amount of adsorption of methylene chloride to dry granular
bentonite clay added at a concentration of 375-750 mg/litre was found
to be 10-22% within 10-30 min. In the presence of 500 mg/litre peat
moss, about 40% of methylene chloride was absorbed after 10 min. Some
adsorption by dry-powdered dolomitic limestone was observed, but not
with silica sand (Dilling et al., 1975).
4.1.4 Multicompartment distribution
The regional distribution of methylene chloride over water, soil
and air compartments may be estimated by means of the fugacity model
developed by MacKay (Slooff & Ros, 1988). Application of this model
suggests that over 98% of the total emissions of the chemical will be
found in air, 1 to 2% in water and far less than 1% in soil and ground
water (BUA, 1986; Slooff & Ros, 1988).
4.2 Abiotic degradation
4.2.1 Atmosphere
The principal process by which methylene chloride is scavenged
from the atmosphere is the reaction with hydroxyl rate of methylene
chloride can be calculated from the rate constant for the initiating
breakdown reaction with HO. and the varying concentration of these
radicals in the troposphere. Determination of the rate constant for
the reaction of methylene chloride with hydroxyl radicals has been the
subject of various investigations. WMO (1991) recommends the following
value:
kOH = 5.8 × 10-12 exp(-1100/T) cm3 molecule-1 s-1
Other reactive species (e.g., ozone, oxygen atoms, chlorine atoms
and nitrate radicals) are not thought to contribute significantly to
the primary attack on methylene chloride (Table 5). As methylene
chloride does not absorb in the visible or near ultraviolet light
region (> 290 nm), direct homogeneous gas-phase photolysis in the
troposphere is of negligible importance.
Table 5. Primary tropospheric reactions of methylene chloride (other than with .OH)
Reaction k (at 25°C) Global average [X] Lifetime
with: (cm3 molecule-1 s-1) (molecule cm-3) (years)
.Cl 4.1 × 10-13 103 77
(IUPAC, 1992) (estimated) (estimated)
.NO3 <3.2 × 10-17 1.2 × 108 > 8.3
.O(3p) 6.44 × 10-16 2.5 × 104 approx. 2000
(Barassin & Cambourieu, 1973)
.O(1D) < 5 × 10-10 0.5 > 120
(estimated)
Carbon dioxide and hydrogen chloride are the major breakdown
products and minor quantities of carbon monoxide and phosgene are
formed (Sanhueza & Heicklen, 1975; Rayez et al., 1987). The breakdown
reaction can be described as follows:
CH2Cl2 + HO. --> .CHCl2 + H2O
.CHCl2 + O2 --> .CHCl2O2
.CHCl2O2 + NO --> .CHCl2O + NO2
.CHCl2O --> .Cl + HCOCl or
.CHCl2O + O2 --> COCl2 + HO2 (minor reaction)
Formyl chloride may be taken up by cloud droplets, hydrolysed to
formic acid and wet deposited as such, or dry deposited to the ocean
or land surfaces and then hydrolysed. The overall lifetime for wet or
dry deposition is unlikely to exceed a few months and may be much
shorter. On the other hand, degradation in the troposphere by
photolysis or reaction with HO. may possibly be a more rapid process.
The reaction products would be carbon oxides (CO, CO2) and HCl
(Libuda et al., 1990).
Phosgene is known to hydrolyse slowly in the gas phase, but
rapidly once dissolved in liquid water, to give CO2 and HCl.
HCl is removed from the troposphere by wet deposition (dissolution
in atmospheric water droplets and subsequent rain-out) or dry
deposition (direct uptake by the oceans, land surfaces, vegetation
etc.) with an average lifetime of about 1 week. The amount of chloride
deposited in this manner is completely negligible compared to the
natural atmospheric chloride flux of around 1010 tonnes/year
primarily from sea-salt aerosols (WMO, 1991).
In the stratosphere methylene chloride will rapidly degrade by
photolysis and reaction with chlorine radicals (Derwent et al., 1976).
4.2.2 Water
Sunlight absorption of water results in the formation of HO. and
hydrated electrons (e-aq). The near surface concentrations of HO.
and e-aq are 4 × 10-16 mol/litre and 5 × 10-17 mol/litre,
respectively, which corresponds to theoretical half-lives for
methylene chloride of 400 and 33 days. In water systems these
reactions are very limited, the reaction with hydroxyl radicals being
dominant. The total rate constant for the sunlight-induced
transformation in surface water (with a depth of 2.5 m, a DOC content
of 4 mg/litre, a chlorophyll a content of 10 µg/litre and a
suspended matter content of 40 mg/litre) was estimated to be
2.8 × 10-5 day-1 (half-life 68 years). The HO. causes 90% of this
transformation (Slooff & Ros, 1988). No direct photolysis of methylene
chloride was found after visible and UV irridiation for 5 days at 22°C
(Chodola et al., 1989).
The half-life of a 1 mg/litre aqueous solution of methylene
chloride was found to be about 1.5 years when measured in sealed glass
tubes in the dark at 25°C and pH 7 (Dilling et al., 1975). No
significant hydrolysis was found at 50°C and pH 4 or 9.2 after 7 days
in the dark (Chodola et al., 1989). Under acidic and basic conditions
in the temperature range of 80-150°C, the hydrolysis of methylene
chloride results in the formation of formaldehyde and HCl (Fells &
Moelwyn-Hughes, 1958). Extrapolation of these data to 25°C gives a
long half-life of about 680-704 years (Dilling et al., 1975; Radding
et al., 1977). As the activation energy for hydrolysis of methylene
chloride varies with temperature, the extrapolation of rate data from
80-150°C may not be valid.
No reductive dehalogenation of methylene chloride in water was
observed in the presence of sodium sulfide and haematein, a common
iron porphyrin (Klecka & Gonsior, 1984).
4.2.3 Soil
As is the case in aqueous systems, hydrolysis is probably not an
important process in the removal of methylene chloride from soil (see
section 4.2.2).
In a lysimeter experiment, a 90% decrease over 2.5 m soil column
was obtained (Nellor et al., 1985).
In the report of a spillage, the concentrations of methylene
chloride were up to 802 mg/m3 and 26 900 mg/m3 near the point of
leakage. In both cases, methylene chloride could not be detected some
hundred metres away from the points of contamination even in the
direction of the groundwater flow (ECSA, 1989). In the neighbourhood
of polluted areas, an increase of bacterial activity has been found.
In well-documented cases of accidental spills to soils, methylene
chloride disappeared rapidly from ground water, probably due to
(bio)degradation (Baldanf, 1981; Leitfaden für die Beurteilung, 1983).
4.3 Biotransformation
4.3.1 Aerobic
Negligible oxygen consumption was found in a biochemical oxygen
demand (BOD) test (Klecka, 1982), and methylene chloride was
considered to be degradation resistant in a degradation test following
the Japanese MITI standards (Kawasaki, 1980). However, complete
degradation occurred during a static-culture flask test (Tabak et al.,
1981).
In laboratory studies methylene chloride was almost completely
transformed within days by bacteria enriched from a primary sewage
sludge, municipal activated sludge (with or without acclimitization)
and industrial waste water (Rittmann & McCarty, 1980; Davis et al.,
1981; Klecka, 1982; Stover & Kincannon, 1983; Halbartschlager et al.,
1984).
In field studies it has been shown that methylene chloride is
efficiently removed from water treatment works (Namkung & Rittmann,
1987).
Certain strictly aerobic, facultative methylotrophic bacteria,
like Pseudomonas DMI and Hyphomicrobium DM2, both readily isolated
from contaminated soil and waste-water treatment plants, are capable
of using methylene chloride as a sole carbon source for growth
(Brunner et al., 1980; Stucki et al., 1981).
Secondary substrate utilisation of methylene chloride was
demonstrated by Pseudomonas sp. strain LP. This strain showed a
preference towards degrading methylene chloride over acetate, whether
it was the primary or the secondary substrate (Lapat-Polasko et al.,
1984).
In Hyphomicrobium DM2, a glutathione (GSH)-dependent, strongly
inducible enzyme (a glutathione S-transferase) was found to be
responsible for the degradation of methylene chloride. It converted
methylene chloride to formaldehyde via the nucleophilic displacement
of chloride and the formation of S-chloromethyl glutathione and
S-hydroxymethyl glutathione. This enzymic dehalogenation in extracts
of methylene-chloride-grown cells amounted to 1160 mg/g protein per h
under alkaline (pH 8-9) conditions (Stucki et al., 1981; Leisinger,
1983).
Eight other bacteria (mainly Pseudomonads ), capable of growing
on methylene chloride as their sole carbon source, were isolated from
enriched cultures. Maximum degradation rates for methylene chloride
(up to 860 mg/litre per h) were found for an initial saturated
solution of 14.5 g/litre in a pH-controlled fermenter (flow rate
10 ml/h). Further increases in degradation rate were limited by the
high salt concentration resulting from the neutralization of the
degradation products. In a fluidized bed reactor with bacteria
immobilized on silica, a degradation rate of methylene chloride of up
to 1600 mg/litre per h was observed (Gälli and Leisinger, 1985;
Stucki, 1990).
Ubiquitous soil- and water-dwelling nitrifying bacteria such as
Nitrosomonas europaea, which depends for growth on the oxidation of
ammonia, were able to degrade 1 mg methylene chloride/litre completely
within 24 h in the presence of ammonia and by 67% in the absence of
ammonia (Vannelli et al., 1990).
The removal of methylene chloride from aerobic soil was
significantly increased following exposure to methane (Henson et al.,
1988).
Flathman et al. (1992) described the remediation of ground water
contaminated with dichloromethane after a leak. Air stripping was used
initially on water pumped out from the contaminated site, and 97% of
the contamination was removed in this way. This was followed by the
first phase of bioremediation, in which contaminated water was
withdrawn from the site and added to a bioreactor containing bacteria
acclimated to DCM. The treated water was reinjected on the site
together with the bacteria. This phase decreased the concentration by
97% over a period of 40 days. A second phase of bioremediation
followed some 3 years later, dealing with a subsection of the original
site. In this case, the indigenous bacteria were used and nutrients
were added to the site. Concentrations before treatment were up to
5200 mg/litre; after 10 months these had reduced to < 2 mg/litre. At
this point active treatment ceased, but the levels of DCM continued to
decrease, falling below 10 µg/litre at all but one of the sampling
sites.
The biodegradation of methylene chloride in contaminated ground
water can be strongly inhibited in the presence of other contaminants
such as 1,2 dichloroethane, xylene and ethylbenzene (Scholz-Muramatsu
et al., 1988).
Aerobic biodegradation of methylene chloride was observed in a
variety of surface soils including sand, a sandy loam and a sandy clay
loam, as well as in subsurface clay soil. Degradation occurred over
concentrations ranging from approximately 0.1 to 5 mg/litre. The time
required for 50% disappearance of the parent compound varied between
1.3 and 191.4 days.
4.3.2 Anaerobic
Details of studies on the anaerobic biodegradation of methylene
chloride are given in Table 6.
Methylene chloride was degraded at a concentration of 200 µg/litre
in the aqueous phase of natural sediment. Degradation was observed to
proceed via methyl chloride, although accumulation was not observed
(Wood et al., 1981). After a varying acclimation period using
anaerobic digestion in waste water, 86-92% conversion to CO2 will
occur (Gossett, 1985). The half-life of methylene chloride in an
anaerobic water/sludge system is 11 days (Bayard et al., 1985).
Methylene chloride degradation was observed under anaerobic
conditions in sandy loam soil (Davis & Madsen, 1991).
4.3.3 Bioaccumulation
The n-octanol/water partition coefficient for methylene chloride
is 18 (log Pow = 1.25-1.3). As a consequence, its bioaccumulation is
not expected to be significant. Moreover, its high depuration and
degradation rate will reduce the probability of bioaccumulation.
No experimental bioconcentration factor (BCF) for methylene
chloride is available. Its theoretical BCF ranges between 0.91 and 7.9
(Veith et al., 1980; Lyman et al., 1982; Veith and Kosian, 1983;
Bayard et al., 1985). Further data indicative of bioaccumulation in
aquatic organisms and human breast milk can be found in sections 5.1.3
and 5.3.1, respectively.
There is no evidence of biomagnification.
4.4 Interaction with other physical, chemical or biological factors
The ozone-depletion potential (ODP) of methylene chloride, as
compared to the standard ODP of CFC11, can be estimated from the
numbers of chlorine atoms (2 as compared to 3 for CFC11) and the
atmospheric lifetime (0.7 years as compared to 60 years). This results
in an ODP for methylene chloride of 0.4% of that of CFC11.
Table 6. Aerobic biodegradation of methylene chloride
Test system Condition Duration Degradation Initial concentration Reference
Laboratory studies
Unknown aerobic, BOD 20 days none Klecka (1982)
Domestic waste aerobic 28 days none Kawasaki (1980)
water (MITI)
Domestic waste aerobic, static, 7 days for each 100% 5, 10 mg/litre, loss by Tabak et al. (1981)
water subcultures taken at days culture transformation volatilization 6.25%
14 and 21
Enriched primary aerobic, static, closed 24 h almost 25 mg/litre Rittmann & McCarty
sewage effluent complete (1980)
transformation
Industrial waste aerobic 6 h 92% 50 mg/litre Davis et al. (1981)
water, municipal transformation,
activated sludge no metabolites
Activated sludge aerobic, continuous-flow 2-6 days > 99% 180 mg/litre, loss by Stover & Kincannon
reactor volatilization 5% (1983)
Municipal activated aerobic 50 h 49-66% 1, 10, 100 mg/litre Klecka (1982)
sludge (9-11 days mineralization
acclimatization)
Activated sludge aerobic 20-28 mg//litre 264-1300 mg/litre Halbartschlager et al.
(6 weeks per hour (1984)
acclimatization) transformation
Table 6 (Cont'd)
Test system Condition Duration Degradation Initial concentration Reference
Field studies
Water treatment aerobic 30-55% removal 50-150 µg/litre Loehr (1987)
works
Conventional aerobic 5-6 h 96.0-96.3% Namkung & Rittmann
activated sludge transformation (1987)
plant
At the current estimated total emission rate of 500 000 tonnes per
year, the calculated tropospheric chlorine loading due to methylene
chloride is 35 ppt, i.e. approximately 1% of the total chlorine
loading of 3600 ppt (WMO, 1991).
As methylene chloride has a low photochemical ozone creation
potential in the troposphere (0.9), when compared with chemicals such
as ethanol (27) or ethylene (100), it will not contribute
significantly to photochemical smog formation (Derwent & Jenkin,
1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
As a consequence of release during its production and use,
methylene chloride is found in biota, water and air. Levels in water
and air tend to be higher in industrial and urban areas than in rural
areas. Improved control of emissions has led to lower environmental
levels of methylene chloride.
For the general population, air is the major source of exposure
to methylene chloride. In indoor air, higher levels may result from
the use of consumer products which contain methylene chloride. High
levels of methylene chloride may occur for short periods of time when
paint strippers and aerosols are used.
Exposure to methylene chloride can occur during its production
and use as a paint stripper. cleaner, degreaser, process solvent and
as an aerosol.
5.1 Environmental levels
Environmental levels measured before 1980 were summarized in
EHC 32: Methylene Chloride (IPCS, 1984). This monograph therefore
focuses on levels measured after 1980.
5.1.1 Atmosphere
5.1.1.1 Ambient air
In the ambient air of rural and remote areas, mean background
levels of methylene chloride are 0.07-0.29 µg/m3 (Table 7). The
average concentrations in suburban and urban areas, respectively, are
reported to be < 2 µg/m3 and < 15 µg/m3. In the vicinity of
hazardous waste sites, up to 43 µg/m3 have been round.
5.1.1.2 Precipitation
Rain water sampled in Koblenz (Germany) in 1982-1983 was found to
contain up to 4 µg methylene chloride/litre (Hellmann, 1984).
5.1.2 Water
Data on the levels of methylene chloride in water are presented in
Table 8.
Table 7. Methylene chloride levels in ambient air
Country/ Location Year of Concentration Reference
region measurement (µg/m3)
Germany urban area: Frankfurt 1980 2.1-4.2 Arendt et al. (1982)
Italy northern part 1983-1984 < 14 De Bortoli et al. (1986)
Netherlands Delft, Vlaardingen (urban 1980-1981 14.1 (max. annual mean) Guicherit & Schulting (1985)
area)
Isle of Terschelling (rural, 1980-1981 1.4 (max. annual mean) Guicherit & Schulting (1985)
suburban area)
mean concentration in 1980-1981 9 Guicherit & Schulting (1985)
the country
USA rural, suburban areas - 0.18-2.1 Shah & Heyerdahl (1988)
San Francisco Bay area 1984 3.2-9.1 Levaggi et al. (1988)
urban areas 1980 0.8-6.7 Shah & Heyerdahl (1988)
Shikiya et al. (1984)
1980-1981 1.35-6.76 Singh at al. (1982)
1981 0.8-2.5 Harkov (1984)
1982 2.4-4.2 Harkov (1984)
1987 0.95-1.64 Pleil & McClenny (1990)
1988 0.62-1.80 Pleil & McClenny (1990)
1989 0.48-1.68 Pleil & McClenny (1990)
hazardous waste sites 1983-1984 0.3-43 Harkov et al. (1985)
Arctic Spitzbergen July 1982 0.26±0.04 Hov et al, (1984)
March 1983 0.29±0.06 Hov et al. (1984)
Northern eastern Pacific 1981 0.12±0.15 Singh et al. (1983)
hemisphere
Southern eastern Pacific 1981 0.07 Singh et al, (1983)
hemisphere
Table 8. Methylene chloride levels in water
Country Location Year of Concentration Reference
measurement (µg/litre)
Ground water
Italy Milan 1983 4.5 CEFIC (1986)
USA Iowa 128 wells 1984-1985 1-5 (4 wells) Kelley (1985)
Surface water
Germany Mosel 1983 1.5-2.0 Hellmann (1984)
Neckar 1983 0.6-1.0 Hellmann (1984)
Elbe 1983 0.7-2.1 Hellmann (1984)
Elbe 1988 11 (max) LWA (1990)
Weser 1982-1983 < 0.5 Hellmann (1984)
Weser 1988 6 (max) LWA (1990)
Rhine at various sites 1981-1983 < 1 LWA (1981,1982,1983)
Rhine at Koblenz 1983 5.35-171 (monthly mean) Hellmann (1984)
Rhine at the Wesel 1983 < 2.0 Hellmann (1984)
Rhine at Duisburg 1984 1.5 (max) LWA (1984)
Rhine at various sites 1988 3.3 (max) LWA (1989)
Table 8 (Cont'd)
Country Location Year of Concentration Reference
measurement (µg/litre)
1989 1.0 (max) LWA (1990)
1990 1.1-3.9 (90th percentile) LWA (1991)
1991 < 0.1 (max) LWA (1992)
1986 0.1 (mean) BUA (1986)
Main 1985 ± 0.2 Van de Graaff (1986)
Emscher 1988 8.5 (max) LWA (1989)
1989 2.5 (max) LWA (1990)
1990 3.9 (max) LWA (1991)
1991 < 0.1 (max) LWA (1992)
Lippe 1988 5.5 (max) LWA (1989)
1989 < 1 (max) LWA (1990)
1990 2.4 (90th percentile) LWA (1991)
1991 < 0.1 (max) LWA (1992)
Wupper 1988 2.3 (max) LWA (1989)
1989 13.6 (90th percentile) LWA (1990)
1990 3.0 (90th percentile) LWA (1991)
Table 8 (Cont'd)
Country Location Year of Concentration Reference
measurement (µg/litre)
USA Susquehanna river, 1987 10 (mean) Smith (1989)
Columbia
Lancaster 1987 4.7 (mean) Smith (1989)
Ohio river basin (11 1980-1981 > 1 (238 samples) Howard et al. (1990)
stations, 4972 samples) > 10 (19 samples)
Sea and estuarine East Pacific Ocean 1981 0.002 (mean) Singh et al. (1983)
water (30 samples)
East Sea (German Coast) 1983 1.3-2.6 Hellmann (1984)
North Sea (German 1983 0.06-0.20 Hellmann (1984)
Coast)
In surface water, levels of methylene chloride have been reported
to vary from not detectable to 10 µg/litre. According to data recorded
in the US EPA STORET database, 30% of the samples showed methylene
chloride levels above the detection limits. A median concentration of
0.1 µg/litre was estimated (Staples et al., 1985).
Limited information concerning the contamination of sea water and
estuaries by methylene chloride is available. It appears that
methylene chloride can be found at up to 2.6 µg/litre in coastal
waters of the Baltic Sea. Levels of up to 0.20 µg/litre have been
found in North Sea coastal waters. Methylene chloride is generally not
detected in open oceans. A mean concentration of 2.2 ng/litre has been
reported in the South Pacific Ocean.
Methylene chloride enters the aquatic environment primarily
through waste water discharge. An estimated amount of 0.2% of the
total methylene chloride production is released in waste water
(Dequinze et al., 1984). The input from air rain-out has been
estimated for the northern and southern hemisphere (section 4.1.1).
Waste water from certain industries has been reported to contain
methylene chloride at average concentrations in excess of
1000 µg/litre, these being coal mining, aluminium forming,
photographic equipment and supplies, pharmaceutical manufacture,
organic chemical/plastics manufacture, paint and ink formulation,
rubber processing, foundries and laundries. The maximum concentration
measured was 210 mg/litre in waste water from the paint and ink
industry and the aluminium-forming industry (US EPA, 1981).
In the US EPA STORET database on industrial effluents, 38.8% of
the samples recorded contained methylene chloride with a median
concentration of 10 µg/litre (Staples et al., 1985).
Samples from the outfalls of four municipal treatment plants in
Southern California, USA, with both primary and secondary treatment,
contained < 10 to 400 µg methylene chloride/litre (Young et al.,
1983). In 30 Canadian water-treatment facilities, average
concentrations of methylene chloride in summer and winter were found
to be 10 µg/litre and 3 µg/litre, respectively (maximum, 50 µg/litre)
(Otson et al., 1982).
In leachate from industrial and municipal landfills, methylene
chloride concentrations were reported to range from 0.01 to
184 000 µg/litre (Sabel & Clark, 1984; Brown & Donnelly, 1988;
Sawhney, 1989).
Background data on ground water contamination by methylene
chloride are limited. It is the sixth most frequently detected organic
contaminant in ground water at hazardous waste disposal sites in the
CERCLA database (178 sites), the detection frequency being 19% (Plumb,
1987). In contaminated ground water in Minnesota, USA, up to
250 µg/litre has been detected (Sabel & Clark, 1984). Levels of up to
110 µg/litre were found in percolation water from a waste-disposal
site in Germany. However, methylene chloride was not found
(< 1 µg/litre) in the ground water below the site (Heil et al.,
1989).
5.1.3 Aquatic organisms
Concentrations of methylene chloride in freshwater organisms have
been reported for oyster and clams from Lake Ponchartrain, Louisiana,
USA. Levels ranging from 4.5 to 27 µg/kg (wet weight) could be
detected (Ferrario et al., 1985).
No methylene chloride was detected in fish taken from the River
Rhine in 1981 (Binnemann et al., 1983).
Levels of methylene chloride up to 700 µg/kg wet weight were found
in marine bottom fish taken from Commencement Bay in the state of
Washington, USA (Nicola et al., 1987).
Data on biota collected in the US EPA STORET data base show an
average level of 660 µg/kg in the 28% of the samples in which
methylene chloride was detected (Staples et al., 1985).
5.1.4 Soil and sediment
No data are available on the levels of methylene chloride in soil.
The levels of methylene chloride found in sediment from Lake
Pontchartrain, Louisiana ranged from not detectable to 3.2 µg/kg wet
weight (Ferrario et al., 1985).
Data recorded in the US EPA STORET database revealed a median
concentration of 13 µg/kg in 20% of 338 sediment sampling data
(Staples et al., 1985).
The levels of methylene chloride found in sediments from the river
Rhine in 1987-1988 varied from non-detectable to 30-40 µg/kg. At one
site maximum concentrations of 220-2200 µg/kg were measured (BUA,
1993, personal communication to the IPCS).
5.2 Human exposure
5.2.1 General population
5.2.1.1 Indoor air
In buildings where products containing methylene chloride are
used, air levels of methylene chloride much higher than outdoor levels
(< 15 µg/m3, see section 5.1.1.1) may be found (Table 7).
Relatively high levels (mean 670 µg/m3, peak level 5000 µg/m3)
have been found in the indoor air of residential houses (De Bortoli et
al., 1986).
5.2.1.2 Drinking-water
Methylene chloride has been detected in drinking-water supplies
(estimations made before 1980) in numerous cities in the USA (Dowty et
al., 1975; Coleman et al., 1976; Kopfler et al., 1977; Kool et al.,
1982), the mean concentrations reported being generally less than
1 µg/litre. An average of 3-10 µg/litre and a maximum of 50 µg/litre
were observed in a Canadian study of 30 drinkable water treatment
facilities (Otson et al., 1982).
Samples from 128 drinking-water wells in the USA showed that 3.1%
of them had methylene chloride levels of 1-5 µg/litre (Kelley, 1985).
Rodruigez Rojo et al. (1989) sampled the drinking-water of
Santiago de Compostela, Spain, in 1987. Methylene chloride was found
in 98.4% of the samples; the average concentration was 14.1 µg/litre,
with a range of 1.2-93.2 µg/litre. Other halomethanes were also found
and measured in the samples at average concentrations ranging from
9 to 25 µg/litre.
A wide sampling exercise involving 630 public community water
supplies (serving 6.9 million people in New Jersey, USA) was carried
out in 1984 and 1985 by McGeorge et al. (1987). The percentage of
positive results for methylene chloride ranged from 2.6 to 7.1%. The
median concentration ranged from 1.1 to 2.0 µg/litre and the range for
the whole sampling period was 0.5 to 39.6 µg/litre.
5.2.1.3 Foodstuffs
Although methylene chloride is used in food processing (solvent
extraction of coffee, spices, hops), there is little information on
its residual levels in food. In the USA, residues of methylene
chloride were found in decaffeinated coffee beans (0.32 to 0.42 mg/kg)
whilst a major coffee processor reported levels of 0.01 to 0.1 mg/kg
(ATSDR, 1992).
No methylene chloride was detected in ice-cream and yoghurt (BUA,
1986).
In seven types of decaffeinated ground coffee the methylene
chloride content ranged from < 0.05 to 4.04 mg/kg; in eight instant
coffee samples <0.05 to 0.91 mg/kg was found (Page & Charbonneau,
1984).
Heikes & Hopper (1986) analysed samples of grains and intermediate
grain-based foods for a range of fumigants using a purge-and-trap
method. Methylene chloride was not found in any of the grain samples,
nor in uncooked rice or dried lima beans. It was found in some of the
intermediate foods such as bleached flour (30 µg/kg), yellow corn meal
(4.7 µg/kg), lasagne noodles (5.4 µg/kg) and yellow cake mix
(4.6 µg/kg).
One of the authors (Heikes, 1987) investigated levels of methylene
chloride in table-ready foods, taken from the US Food and Drug
Administration's Total Diet Study. Of the 19 foods examined, eight
contained methylene chloride above the quantification limit (not
given). Detailed results for six of the foods are given in Table 9.
Table 9. Dichloromethane content of table ready foods
(Heikes, 1987)
Food Number of Number Range of
samples positive concentration
(µg/kg)
Butter 7 7 1.1-280
Margarine 7 7 1.2-81
Ready-to-eat cereal 11 10 1.6-300
Cheese 8 8 3.9-98
Peanut butter 7 4 26-49
Highly processed foodsa 12 10 5-310
a e.g., frozen chicken dinner, fish sticks, pot pie
5.2.1.4 Consumer exposure
Consumers are exposed to methylene chloride via the use of a
number of formulated products such as aerosols or paint strippers. A
USA survey found that 78% of paint removers and 66% of aerosol spray
paints sold as household products contained methylene chloride (US
EPA, 1987). Over 100 consumer products in Sweden contain methylene
chloride (National Chemical Inspectorate, Sweden, personal
communication to the IPCS). In Norway the number is around 140,
including 45 paint removers (AKZO, personal communication to the
IPCS).
Methylene chloride does not appear to be subject to widespread
volatile substance abuse. Statistics on deaths resulting from
substance abuse in the United Kingdom were collected over the period
1971-1991 and analysed by product type. Of the 1221 deaths recorded,
five were assigned to the group "paint thinners and paint strippers".
Methylene chloride is used only in the latter products, the former
containing solvents such as toluene and xylene which are known to be
substances of abuse (Flanagan et al., 1990).
A large do-it-yourself consumer population uses paint strippers
containing methylene chloride on furniture and woodwork. Formulations
are available mainly in liquid form, but also, occasionally, as an
aerosol. Exposures have been estimated on the basis of USA
investigations of household solvent products. The estimated levels
ranged from less than 35 mg/m3 to a few short-term exposures of 14
100 to 21 200 mg/m3. The majority of the concentration estimates
were below 1770 mg/m3 (US EPA, 1990).
Methylene chloride exposure was estimated while using a number of
formulations of paint stripper in a small room. Various ventilation
conditions were evaluated and a worst possible case was simulated,
with doors and windows closed. In one test, involving furniture
stripping in a room with through ventilation, the operator exposure
was found to be 289 mg/m3 on a 2-h TWA. Peaks of exposure were
observed during application (460 mg/m3) and during scraping-off
(710-1410 mg/m3) (ICl, 1988, personal communication to the IPCS).
A series of paint-stripping exercises were performed in a small
room. Various ventilation conditions were evaluated while using a
number of formulations of paint stripper. A worst possible case was
simulated with doors and windows closed. Concentrations of methylene
chloride in the room rose to 14.1-17.6 g/m3 (4000-5000 ppm),
although it is questionable whether anyone could work in such
conditions without breathing apparatus. Further exercises with the
door and windows open (as recommended by suppliers) reduced
atmospheric concentrations by more than a factor of 10. Exposure to
methylene choride resulted in an 8-h TWA of 187-226 mg/m3
(53-64 ppm). The actual stripping operations took between 1 and 1.5 h.
Maximum exposures occurred during initial application and scraping-
off. These exposures were of a few minutes duration and concentrations
never exceeded 3.53 g/m3 (1000 ppm) (ICI, 1990, personal
communication to the IPCS).
The effect of variation in the formulation was also investigated.
During paint stripping, background concentrations in the room varied
from 710-1410 mg/m3 to less than 350 mg/m3 depending on the
formulation. However, within 5-6 min of application the level of
methylene chloride fell to an equilibrium concentration of around
71 mg/m3, irrespective of the formulation used. The shortest time
before reaching equilibrium was about 2-2.5 min (ICI, 1990, personal
communication to the IPCS).
Studies in the Netherlands have measured peak concentrations in
salons of 21-106 mg/m3 (6-30 ppm), with an 8-h TWA of
3.53-17.65 mg/m3 (1-5 ppm) (CEC Scientific committee of
Cosmetology). The same study measured a peak concentration of
265 mg/m3 (75 ppm) arising from home use of a hairdressing aerosol
containing 35% methylene chloride. This equates to a TWA of
2.65 mg/m3 (0.75 ppm).
Studies in the United Kingdom simulating consumer exposure during
salon use yielded values well within the national Maxium Exposure
Limit (353 mg/m3 or 100 ppm for an 8-h TWA). The hairdresser
received an exposure of 77.7 mg/m3 (22 ppm, on an 8-h TWA) during
what is considered to be exceptionally heavy use (i.e. a 10-second
spray every 15 min for an 8-h period). The customer exposure was found
to be 106-265 mg/m3 (30-75 ppm) (10-min TWA) (ICI, 1990, personal
communication to the IPCS).
The same study simulated home use of personal-care aerosols
containing methylene chloride. Even adverse conditions (small room, no
ventilation) resulted in an exposure of 353 mg/m3 (100 ppm) (10-min
TWA), equating to 7.06 mg/m3 (2 ppm) on an 8-h TWA (ICI, 1990,
personal communication to the IPCS). This work was characterized by
low air changes, virtually no ventilation and a more frequent rate of
application than that determined by surveys of actual hairdressing
work in salons.
5.2.2 Occupational exposure
Exposure to methylene chloride can occur during its production and
use as a paint stripper, cleaner, degreaser, process solvent and as an
aerosol. Exposure concentrations that have been reported in various
industries are presented in Table 10. Below is a brief description of
exposure conditions in some of the reported industries.
5.2.2.1 Production
Production of methylene chloride is normally carried out in a
closed system, with a relatively small number of people being
involved. Exposure arises primarily during filling and packing
operations. Occupational exposures are listed in Table 10. Some
measured ranges, e.g., 35-81 mg/m3 and 85-244 mg/m3 (HSE, 1992),
indicate that engineering control techniques can bring 8-h TWA
exposures below 350 mg/m3.
5.2.2.2 Paint stripping and related activities
Workers in the formulation of paint removers are exposed while
transferring methylene chloride from storage tanks, during mixing
(blending) operations and while packaging. The extent of exposure will
depend on the control measures and work practices in force. Exposure
levels (8-h TWA) range from a low of 0-18 mg/m3 to over 1770 mg/m3
(US EPA, 1990).
Table 10. Occupational exposure to methylene chloride
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Production Production actvities 219-374 Maintenance activity with RPE; HSE (1987)
Results obtained at one plant
Aircraft Paint stripping 35-81 RPE provided HSE (1992)
Paint stripping 35-289 Submission to OSHA in 1987. RPE Air Transport Association
provided (USA)
Various industries Painting 21-299 See IARC (1986) Chrostek & Levine (1981)
Paint stripping 18-1765 US EPA (1990)
Used aerosol < 0-494 Some work areas were congested Fleeger & Lee (1988)
adhesives
Pharmaceuticals - 7.1-3749 NCI feasibility study Zahm et al. (1987)
Production work 0-18 Enclosed process HSE (1992)
-
Aerosol products Aerosol filling 95-628 ICI (UK) (1984)
Rubber products Fabrication 208-304 LEV HSE (1992)
Fibre glass Cleaning and mould 187-6693 intermittent exposure, RPE may be HSE (1992)
manufacture preparation worn; may not be representative
of the industry
Cleaning, mixing etc. 0-350 Small factory units Post et al. (1991)
Printing 3.5-558 NCI feasibility study Zahm et al. (1987)
Table 10 (Cont'd)
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Triacetate Production 180-350 ECSA (1989)
fibre/film 237-3442 NCI feasibility study Zahm et al. (1987)
manufacturing 180-2440 Products contain acetone Ott et al. (1983)
Furniture Paint stripping 25-3810 Many without adequate controls HSE (1992)
Paint stripping 201-1292 Variable degrees of control McCammon et al. (1991)
Washing/refinishing 53-780 Variable degrees of control McCammon et al, (1981)
Spraying adhesive 219-1490 Many without adequate controls HSE (1992)
General Cleaning, degreasing, 53-141 See IARC (1986) Ruhe et al. (1981)
manufacturing, etc. < 0-460 See IARC (1986) Ruhe et al. (1982)
cleaning and
degreasing
Foam industry Glue spraying 85-244 LEV HSE (1992)
Moulding 88-1090 High exposure due to HSE (1992)
insufficient/inadequate LEV
< 247 Jernelov & Antonsson
(1987)
Unknown 7.1-251 NCI feasibility study Zahm et al. (1987)
Various jobs 18-580 High exposure experienced by Boeniger (1991)
sprayers
Motor vehicle Paint spraying, 7.1-247 LEV and RPE HSE (1992)
manufacture stripping
Table 10 (Cont'd)
Industry Activity Exposure range Commentsa Reference
(8-h TWA) (mg/m3)
Quarry Laboratory work, 71-1370 High exposures due to inadequate HSE (1992)
mineral processing control
Metal treatment 7.1-790 NCI feasibility study Zahm et al. (1987)
Nutrition Extraction < 0-106 See IARC (1986) Cohen et al. (1980)
a RPE = respiratory protection equipment; LEV = local exhaust ventilation
Paint strippers are widely used in a number of industries:
automotive, rubber products, furniture and fixtures, plastic, and
electronic industries. Exposure to methylene chloride takes place
during application, removal of the substrate soaked in methylene
chloride, and the disposal of the spent paint remover. Typical
exposures (8-h TWA) range from 18 mg/m3 to about 1770 mg/m3 (US
EPA, 1990).
Exposure of commercial furniture refinishers to methylene chloride
occurs when stripping involves either the dipping of furniture into a
tank containing a mixture of solvents including methylene chloride
(typically 65%) or coating it manually with a brush. Exposure levels
are highly variable and greatly influenced by the size of the
organization, engineering controls in place and work practices. Some
refinishers may operate on a part-time basis and from their homes. In
some instances where the worker was leaning over the tank or using a
brush to scrub the surface coating, concentrations of > 7100 mg/m3
have been recorded (McCammon et al., 1991; HSE, 1992). Better work
stations and work practices have helped to reduce exposures greatly.
5.2.2.3 Aerosol production and use
In the packaging of aerosol cans, exposure arises primarily during
filling and packing. Levels observed are generally below 180 mg/m3
(ECSA, 1989). Potential occupational exposure to methylene chloride as
a result of aerosol products varies according to the use and the work
undertaken.
Consumer exposure due to the use of cosmetic and paint spray
aerosols is discussed in section 5.2.1.4.
5.2.2.4 Use as a process solvent
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products. Most of the processes are
carried out in closed systems, with the exception of triacetate fibre
and film manufacture. Normally, exposure levels are low, but
occasionally high exposures (> 350 mg/m3; 10-min TWA) may occur in
such operations as filter changing, charging and discharging. Some
industrial processes involve somewhat higher exposure levels; in
particular, the manufacture of cellulose triacetate fibres and films
can involve exposure up to 350 mg/m3 (8-h TWA) even when good
engineering controls are installed (Zahm et al., 1987; ECSA, 1989). As
part of an epidemiological study of cellulose fibre production
workers, Ott et al. (1983) reported exposure levels ranging from 177
to 2436 mg/m3 in the processing area, and exposure ranging from 18
to 1341 mg/m3 in the preparation area based on sampling performed in
1978. The range for the entire plant was later reported to be from
below detectable limits to 6000 mg/m3 (Lanes et al., 1990). As part
of an epidemiological study of photographic film workers, Friedlander
et al. (1978) reported exposures ranging from 0 to 1236 mg/m3 based
on sampling carried out between 1959 and 1975. The highest mean
exposures at this plant were reported for group leaders (402 mg/m3)
(Hearne et al., 1987).
In the pharmaceutical industry, methylene chloride is used as a
solvent and extraction medium. Sealed processes, high recovery rates
and careful handling of discharges have helped to keep the exposure
levels below around 106 mg/m3 (Zahm et al., 1987; HSE, 1992).
Methylene chloride is also used as an extraction medium in the
nutrition industry, where the exposure levels are generally low when
the processes are adequately controlled (Cohen et al., 1980).
Methylene chloride is used in the foam industry for cleaning
process equipment, purging spray guns, and as an auxiliary blowing
agent. It is also used as a releasing agent in the moulding of
polyurethane products. Exposure levels ranging from a few mg/m3 to
short-term exposures of over 1770 mg/m3 have been reported (Jernelov
& Antonsson, 1987; Boeniger, 1991; HSE, 1992).
The use of methylene chloride as a solvent in adhesives can result
in occupational exposure, during the application of the adhesive, to
short-term levels in excess of 350 mg/m3 (Fleeger & Lee, 1988; HSE,
1992). Processes involving the formulation of adhesives are likely to
be well controlled.
Methylene chloride is also used as a solvent in the analysis of
bitumen samples. This work is normally carried out in small
laboratories and exposure levels will be high unless adequate control
measures are used.
5.2.2.5 Cleaning and degreasing
In the manufacture of metal products, cleaning (degreasing) is
required before painting, plating, plastic coating, etc. The degree of
exposure to methylene chloride will be influenced by many factors,
including the age of the equipment, type of engineering controls
available, their maintenance, handling, and drying methods. In
general, it is possible to reduce exposure levels to below 124 mg/m3
(Swedish National Board of Occupational Safety & Health, personal
communication to the IPCS).
5.2.3 Occupational exposure limits
A listing of some national occupational exposure limits is given
in Table 11.
Table 11. Occupational exposure limit valuesa
Country TWA STEL TWA STEL Remarks Reference
(mg/m3, 20°C)b (ppm) (ppm) (ppm)
Australia 350 - 100 - Suspected carcinogen ILO (1991)
Austria 360 1800c 100 500 Suspected of carcinogenic DFG (1991)
potential
Belgium 174 - 50 - Suspected human carcinogen ACGIH (1992)
Czechoslovakia 500 2500 - - ILO (1991)
Denmark 174 - 50 - Absorption through skin may be ILO (1991)
significant
Suspected carcinogen
Finland 350 870 100 250 ILO (1991)
France 360 1800 100 500 ILO (1991)
Germany 360 1800c 100 500 Suspected of carcinogenic DFG (1991)
potential
Italy 174 - 50 - Suspected human carcinogen ACGIH (1992)
Japan 350 - 100 - ILO (1991)
Netherlands 350 1750 100 500 Arbeidsinspectie
(1991)
Norway 125 - 35 - Carcinogen Arbeidstilsynet
(1990)
Table 11 (Cont'd)
Country TWA STEL TWA STEL Remarks Reference
(mg/m3, 20°C)b (ppm) (ppm) (ppm)
Portugal 174 - 50 - ILO (1991)
Sweden 120 250d 35 70 Classified as a low potent AFS (1990)
carcinogen
Switzerland 360 1800 100 500 Classification for teratogenic ILO (1991)
effects not possible; biological
monitoring required
United Kingdom 350 870 100 250 Maximum exposure limit
USA - ACGIH 174 50 Suspected human carcinogen ACGIH (1992)
a TWA = time-weighted average concentration (8-h working period); STEL = short-term exposure limit (15 min, unless specified)
b Official values; some countries use different conversion factors and/or other ambient temperature
c 30 min
d 15 min
5.3 Human monitoring data
5.3.1 Body burden
Methylene chloride was detected in all eight samples of human milk
from four urban areas (Pellizzari et al., 1982). Hardin & Manson
(1980) could still find methylene chloride in mother's milk 17 h after
the end of exposure.
In 12 male volunteers exposed to 2600 mg/m3, biopsies showed
adipose tissue levels of 10.2, 8.4 and 1.6 mg/kg at 1, 4, and 22 h,
respectively, after a 1-h exposure (Engström & Bjurström, 1977). When
Antoine et al. (1986) analysed whole blood from 250 individuals, the
mean concentration of methylene chloride was 0.7 µg/litre with a range
from not detected to 25 µg/litre.
BUA (1986) monitored saliva and tissues from people living in
industrialized areas of Beckum, Germany. They reported that no
methylene chloride was detected.
5.3.2 Occupational exposure studies
In a cohort of 14 furniture strippers exposed to methylene
chloride at concentrations of 53 to 1290 mg/m3, post-exposure breath
concentrations of methylene chloride ranged from 8.1 to 590 mg/m3
(McCammon et al., 1991).
Mother's milk in Soviet women manufacturing rubber articles
contained a mean of 74 µg/kg in 17 out of 28 samples approximately 5 h
after start of work, the level declining after termination of work
(Jensen, 1983).
A group of seven non-smoking workers, who had previously been
exposed to methylene chloride for several years, were exposed to a
mean concentration of 635 mg/m3 (in addition, there was exposure to
154 mg/m3 (mean) chloroform). The pre-exposure average carbon
monoxide level in alveolar air was 34 mg/m3, increasing to
58 mg/m3 during exposure; before the next exposure, the carbon
monoxide level was 27 mg/m3: this corresponded to carboxyhaemoglobin
(CO-Hb) levels of 4.9%, 8.3% and 3.9%, respectively. The biological
half-life of CO-Hb was 13 h (Rathey et al., 1974). Although methylene
chloride does not accumulate following repeated exposure, these data
clearly indicate that CO-Hb levels will be cumulative if the periods
between exposure are insufficient to allow the CO-Hb levels to return
to normal.
CO-Hb levels in a worker accidentally overcome by methylene
chloride vapour were found to have increased to 19%. A further worker
with a history of ischaemic heart disease, who had been exposed
concurrently with the first patient, had a CO-Hb level of 6% on the
day following the exposure (Benzon et al., 1978).
Methylene chloride levels in alveolar air and blood were measured
in 14 shoe-sole factory workers. The alveolar concentration, mean
blood levels, and CO-Hb levels, following exposure to 74±28 mg/m3
methylene chloride, were: 49 mg/m3, 0.41 mg/litre, and 4.0%,
respectively; upon exposure to 124+42 mg methylene chloride/m3:
71 mg/m3, 0.99 mg/litre, and 5.2%, respectively; and upon exposure
to 339±265 mg methylene chloride/m3: 229 mg/m3, 3.07 mg/litre, and
6.5%, respectively. In this factory, the methylene chloride exposure
was highly variable; the data are too limited to allow valid
extrapolation (Perbellini et al., 1977).
The relationship between low environmental levels of methylene
chloride, carbon monoxide in alveolar air and urinary excretion of
methylene chloride was studied in a group of 20 manufacturing workers
(12 smokers, 8 non-smokers). A good correlation was observed between
levels of methylene chloride and urinary excretion of methylene
chloride. The correlation between alveolar air and levels of methylene
chloride was poor except when the analysis was restricted to
nonsmokers (Ghittori et al., 1993)
5.3.3 Biological exposure indices
Biological Exposure Indices (BEIs) are reference values intended
for guidelines for the evaluation of potential health hazards in
industrial hygiene practice. The BEIs for methylene chloride at the
end of a working shift have been given as: CO-Hb level 5%, blood level
of methylene chloride 1 mg/litre.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor with respect to health risk,
biological monitoring based upon either analysis of carbon monoxide in
exhaled air or of CO-Hb in blood is to be preferred. However, this can
only be applied in non-smoking subjects. Sampling should be carried
out either about 0-2 h after exposure or 16 h later, i.e. on the
following morning.
In the case of an 8-h exposure to less than 350 mg methylene
chloride/m3 in non-smokers, CO-Hb levels 2-h after exposure ceases
are not expected to exceed 2-3%, and after 16 h to exceed 1%.
6. KINETICS AND METABOLISM
Appraisal
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract and dermal exposure results in absorption but
at a slower rate than that of the other exposure routes.
Distribution studies indicate that, via inhalation or dermal
exposure, methylene chloride distributes to all tissues. It can cross
the blood-brain barrier and it can be transferred across the
placenta. Concentrations of methylene chloride rise more slowly in
adipose tissue and longer exposures are required before these tissue
levels equal those of the blood. Data indicate that methylene
chloride and/or its metabolites do not accumulate in tissues.
Methylene chloride is metabolized to carbon monoxide, carbon
dioxide and inorganic chloride. Methylene chloride is eliminated from
the body primarily via the lungs in expired air. Urinary excretion
plays a minor role in its elimination. As exposure levels increase, a
large proportion of methylene chloride is exhaled unchanged.
Metabolism occurs by either or both of two pathways; their relative
contribution to the total metabolism is markedly dependent on the
exposure level and on the animal species concerned. One pathway
involves oxidative metabolism mediated by cytochrome P-450 and leads
to both carbon monoxide and carbon dioxide. This pathway appears to
operate similarly in a qualitative and quantitative sense in all
rodents studied and in humans. This is the predominant metabolic
route, but saturation occurs at around 1800 mg/m3. Increasing the
dose above the saturation level does not lead to extra metabolism by
this route.
The other pathway involves a glutathione transferase, and leads
via formaldehyde and formate to carbon dioxide. This route seems only
to become important at doses above the saturation level of the
"preferred" oxidative pathway. There are marked species and dose-
dependent differences in the contribution that this pathway makes to
the metabolism of methylene chloride.
6.1 Absorption
6.1.1 Inhalation exposure
6.1.1.1 Human studies
The principal route of human exposure to methylene chloride is
inhalation. Evaluation of pulmonary uptake indicated that 70-75% of
inhaled vapour was absorbed in human subjects exposed to 180, 350, 530
and 710 mg/m3. Initial absorption was rapid, as indicated by levels
of methylene chloride in the blood of approximately 0.6 mg/litre in
the first hour of exposure to levels of 350-710 mg/m3. At a level of
180 mg/m3, the increase in blood methylene chloride concentration
was 0.2 mg/litre for the first hour. There was a direct correlation
between the steady-state blood methylene chloride values and the
exposure concentration, both during the exposure and for the first 2 h
after the exposure in all groups. Steady-state blood levels appeared
to be reached after 4 h and remained constant until the end of
exposure. Once exposure ceased, methylene chloride was rapidly cleared
from the blood. Seven hours after exposure, less than 0.1 mg/litre was
detected following exposure to 180, 350 or 530 mg/m3. Only
1 mg/litre was detected in the highest group (710 mg/m3) 16 h after
exposure. In all other dose groups the blood levels had returned to
baseline concentrations (Di Vincenzo & Kaplan, 1981a,b).
In common with other lipophilic organic vapours, methylene
chloride absorption appears to be influenced by factors other than the
vapour concentration. Prior to reaching steady state, increased
physical activity increases the amount of methylene chloride absorbed
by the body, due to an increase in ventilation rate and cardiac
output, since these factors increase blood flow through the lungs and
promote absorption (Di Vincenzo et al., 1972; Astrand et al., 1975).
Uptake also increases with the body fat percentage, since
methylene chloride dissolves in fat to a greater extent than it
dissolves in aqueous media. Therefore, obese subjects will absorb and
retain more methylene chloride than lean subjects exposed to the same
vapour concentration. Under controlled conditions, there was a 30%
greater absorption and retention of methylene chloride by obese
subjects exposed to 2650 mg/m3 for 1 h as compared to lean subjects
(Engström & Bjurström, 1977).
Åstrand et al. (1975) reported that the amount of methylene
chloride taken up increases with physical workload, whereas the
retention decreases. With a 50-watt workload, the uptake was twice as
high, whereas the retention decreased from 55% to 45%. When exposure
was coupled with workload (physical exercise), the concentration in
alveolar air was increased during the whole post-exposure period
compared with exposure under rest conditions.
6.1.1.2 Animal studies
Studies of the relationship between inhalation exposures of
animals and their blood methylene chloride concentrations indicate
that absorption is proportional to the magnitude and duration of the
exposure over a methylene chloride concentration range of 350 to
28 200 mg/m3. This conclusion is based on the monitoring of blood
methylene chloride concentrations following inhalation exposure in
dogs and rats (Di Vincenzo et al., 1972; MacEwen et al., 1972; McKenna
et al., 1982). As is the case with humans, blood methylene chloride
levels reach a steady-state value which does not increase further as
the duration of exposure increases (McKenna et al., 1982).
The data from studies of blood methylene chloride values during a
6-h exposure of rats to between 180 and 5300 mg/m3 suggest that the
steady-state blood/air concentration ratio increases as the exposure
concentration increases. The ratio of the steady-state methylene
chloride concentrations in plasma to the exposure concentration was
0.001, 0.005 and 0.006 at levels of 180, 1800 and 5300 mg/m3,
respectively (McKenna et al., 1982). It is postulated that the
increased ratio at steady state results from saturation of metabolic
pathways as exposure increases, rather than from an increased
absorption coefficient.
Kim & Carlson (1986)conducted experiments to compare the effects
of a 12-h exposure schedule to those of an 8-h schedule on the CO-Hb
formation resulting from methylene chloride inhalation. Rats and mice
were exposed to 710, 1800 or 3500 mg/m3 8 h/day for 5 days, or
12 h/day for 4 days. No significant difference in carboxyhaemoglobin
levels was found. The peak blood methylene chloride level was found to
be dependent upon the methylene chloride exposure concentration, but
the half-life was independent of the duration of exposure or the
concentration of methylene chloride,
6.1.2 Oral exposure
No data are available on oral absorption of methylene chloride in
humans.
Treatment of mice and rats with methylene chloride in water or in
corn oil suggests that methylene chloride is easily absorbed from the
gastrointestinal tract. Methylene chloride levels were measured in gut
segments up to 40 min after rats were administered single oral doses
in water. The amounts measured were similar at both dose levels (50 or
200 mg/kg). Of the administered dose (200 mg/kg), 60% was recovered
from the upper gastrointestinal tract < 10 min after treatment (20%
recovery after 40 min). The amount of methylene chloride in the lower
gastrointestinal tract accounted for < 2% of the administered dose up
to the 40-min test interval (Angelo et al., 1986b).
In mice administered oral doses of non-radioactive methylene
chloride at 10 or 50 mg/kg in water, approximately 25% of the
administered dose was detected in the upper gastrointestinal tract
within < 20 min. Similarly, after treatment with methylene chloride
at 10, 50, or 1000 mg/kg in corn oil, approximately 55% of the
administered dose was detected in the upper gut segment and remained
there for 2 h (Angelo et al., 1986a).
6.1.3 Dermal exposure
Methylene chloride has been shown to be absorbed through human
skin (Stewart & Dodd, 1964). In this study, a volunteer immersed a
thumb in methylene chloride for 30 min under conditions which
precluded the inhalation of vapour. The subsequent alveolar air
concentration was 3.1 ml/m3; 2 h later it had fallen to
0.69 ml/m3.
Various studies on the rate of absorption through animal skin and
subsequent pharmacokinetics have been reported. Tissue concentrations
of methylene chloride were measured in various organs (lung, liver,
brain, kidney, heart and fat) of 128 white rats, using gas
chromatography, following immersion of two-thirds of their tails in
the solvent for 1, 2, 3 or 4 h. Small increases were seen in most
tissues after 1 or 2 h of exposure, and methylene chloride
concentrations in fatty tissues increased markedly after 3 h of
exposure. After 4 h of exposure, methylene chloride concentrations
remained elevated in fatty tissues and were increased in all other
tissues studied (Makisimov & Mamleyeva, 1977). The dermal absorption
rate for methylene chloride through mouse skin in vivo has been
measured to be 6.58 mg/h per cm2 (Tsuruta, 1975).
The dermal permeability constant for the absorption of methylene
chloride vapour through rat skin in vivo has been measured following
exposure to 106, 212 and 353 g/m3 for 4 h. Blood levels of methylene
chloride were shown to reach steady state levels after 1 h of exposure
to the two lower concentrations and after 3 h of exposure to
353 g/m3. The mean dermal permeability constant was calculated to be
0.28 mg/h per cm2 (McDougal et al., 1986).
6.2 Distribution
6.2.1 Inhalation exposure
6.2.1.1 Human studies
Engström & Bjurström (1977) exposed 12 male subjects (six slim and
six obese) to 2650 mg methylene chloride/m3 for 1 h. The total
uptake of methylene chloride in the slim group was 1116 ± 34 mg and in
the obese group 1446 ± 110 mg/kg. Estimation of methylene chloride in
needle biopsies showed that the adipose tissues contained
approximately 8 to 35% of the average total amount absorbed. The
amount or methylene chloride absorbed was highly correlated with the
degree of obesity and body weight. In the slim subjects, the
concentration in the adipose tissue during the 4-h period after
exposure was approximately twice that in the obese subjects. However,
despite a lower concentration, the total amount of methylene chloride
calculated to be in the body fat was greater in obese subjects.
A survey of the levels of methylene chloride in certain tissues
from pregnant or nursing women has been reported. The study was
conducted following observations of disturbances in the pattern of
pregnancy and lactation in female operatives in an industrial rubber
article manufacturing facility. The survey was conducted in an
unspecified number of women who had been exposed to several chemicals
during their work for at least 3 years. The chemicals included
gasoline, ethylene dichloride and methylene chloride. An estimate of
the average workplace concentration of methylene chloride was reported
to be 85.6 mg/m3. A control group (number unspecified) was
constituted from women working in the same facility but who had had no
direct contact with the chemicals. The tissues examined were the
blood, the fetal membranes and the fetus, all tissue samples being
obtained at the time of abortion of the fetus. The mean tissue
concentrations of methylene chloride (54 observations) were reported
to be 0.66 ± 0.21, 0.34 ± 0.10 and 1.15 ± 0.20 mg/kg for the blood,
fetal membranes and fetus, respectively, compared to 0.12 ± 0.07,
0.013 ± 0.01 and 0.016 ± 0.001 mg/kg in the controls. Methylene
chloride was also detected in 17 out of 28 specimens of breast milk
taken from exposed nursing women. An average concentration of
0.074 ± 0.04 µg/litre (n = 40) was found in the breast milk 5-7 h
after the start of the exposure; an insignificant quantity of
methylene chloride was reported 17 h after cessation of exposure
(Vosovaja et al., 1974).
6.2.1.2 Animal studies
Distribution studies in rats demonstrate that methylene chloride
(and/or its metabolites) is present in the liver, kidney, lungs,
brain, muscle and adipose tissues after inhalation exposures (Carlsson
& Hultengren, 1975; McKenna et al., 1982). One hour after exposure,
the highest concentration of radioactive material was found in the
white adipose tissue, followed by the liver. The concentration in the
kidney, adrenal and brain were less than half that in the liver.
Radioactivity in the fat deposits declined rapidly during the first
2 h after exposure (Carlsson & Hultengren, 1975). Concentrations in
the other tissues declined more slowly. Whole body autoradiography in
mice at one hour after inhalation of 10 µl 14C-methylene chloride
for 10 min showed a rapid and even distribution immediately after
exposure. A high uptake was noted in brain, body fat, blood, liver and
kidney. Evaporated sections showed a high retention of non-volatile
radioactivity, presumably representing metabolites, in the liver,
bronchi and kidneys. Thirty minutes after inhalation, radioactivity
started to appear in tissues with a high cell turnover such as bone
marrow, thymus and gastrointestinal mucosa, and in tissues with a high
rate of protein synthesis such as the spleen, exocrine pancreas and
salivary glands (Bergman, 1979).
On the other hand, after 5 days of exposure to 710 mg/m3 for
6 h/day, the concentration of methylene chloride in the perirenal fat
was 6-7 times greater than that in the blood and liver (Savolainen et
al., 1977). It has been suggested that methylene chloride first
saturates the blood and extravascular fluid compartment before
entering the fatty deposits (Di Vincenzo et al., 1972). Thus,
concentrations of methylene chloride will rise slowly in adipose
tissues, and longer exposures to methylene chloride will be required
before adipose tissue levels equal those in the blood. The animal data
are therefore consistent with the human adipose tissue data discussed
above.
Exposure of pregnant rats to methylene chloride leads to exposure
of the fetus to both methylene chloride and carbon monoxide (Anders &
Sunram, 1982).
6.2.2 Oral exposure
No studies are available regarding distribution of methylene
chloride in humans following oral exposure.
In animals, radioactivity from labelled methylene chloride was
detected in the liver, kidney, lung, brain, epididymal fat, muscle,
and testes after exposure of rats to a single gavage dose of 1 or
50 mg/kg. The tissue samples were taken 48 h after dosing. At that
time, the lowest concentration of radioactivity was found in the fat.
The highest concentrations were in the liver and kidney. This was true
for both doses (McKenna & Zempel, 1981).
Similar results were observed in rats administered methylene
chloride doses of 50-1000 mg/kg for 14 days. At each dose tested, and
in each tissue, the label was rapidly cleared during the 240 min
following each exposure (Angelo et al., 1986b).
6.2.3 Dermal exposure
No information is available regarding distribution in humans or
animals following dermal exposure to methylene chloride.
6.3 Metabolism
Species differences in metabolism and their relevance to
carcinogenicity are described in section 8.8.2.
6.3.1 In vitro studies
In vitro experiments using liver fractions, homogenates, slices
and hepatocytes, mainly from the rat, confirmed the presence of the
two metabolic pathways. The primary reaction, first described by Kubic
& Anders (1975), appears to be an oxidative dehalogenation giving
carbon monoxide and chloride ion. The reaction is catalysed by rat
liver microsomal fractions and is dependent upon NADPH and molecular
oxygen. The presence of a binding spectrum and inducers confirmed the
involvement of the cytochrome P-450 mixed function oxidase system.
More recent studies have identified the cytochrome P-450 isoenzyme as
cytochrome P-450 IIEl (Pankow et al., 1991; Guengerich et al., 1992;
Pankow & Jagielki, 1993). The highest activity was found in liver
microsomes, which were five times more active than lung microsomes and
thirty times more active than kidney microsomes. The proposed
metabolic route involves rearrangement of the primary hydroxylation
product to formyl chloride followed by decomposition to carbon
monoxide (Kubic & Anders, 1978). Although the transient intermediates
have not been isolated or identified, their formation is consistent
with the enzyme involved and the products formed.
The effect of pyrazole on methylene chloride metabolism in male
Wistar rats was investigated by Pankow et al. (1991). Rats received a
single methylene chloride close of 6.2 mmol/kg (0.4 ml/kg) orally.
Pyrazole was administered by intraperitoneal injection. The metabolism
of methylene chloride to carbon monoxide can be stimulated or
inhibited by pyrazole; the effect depends on the interval between
pyrazole and methylene chloride administration, and on the dose.
Stimulation of methylene chloride metabolism to carbon monoxide is due
to inducers of the isoenzyme cytochrome P-450 (CYP2El) such as
isoniazid, ethanol and other solvents. The inhibition was observed
following pre-treatment with high pyrazole doses or following
simultaneous administration of pyrazole and methylene chloride. The
inhibition may reflect the competition between pyrazole and methylene
chloride for oxidation by CYP2El as long as pyrazole is present in the
blood, or may also reflect the hepatotoxic effect of pyrazole.
Hepatic cytochrome P-450 levels were not increased in rats exposed
by inhalation to methylene chloride (5.29-10.59 g/m3 (1500 or
3000 ppm)) 6 h/day for 3 clays (Toftgard et al., 1982), nor in rats
exposed to 1.76 or 3.53 g/m3 (500 or 1000 ppm) 6 h/day for 2 weeks
(Kurppa & Vainio, 1981). Marginal increases were seen in a third study
(Norpoth et al., 1974) in which rats were exposed to 0.176-17.6 g/m3
(50-5000 ppm), 5 h/day for 10 days. In the study by Kurppa & Vainio
(1981) an increase in renal ethoxycoumarin de-ethylase activity was
reported.
The second metabolic pathway occurring in rat liver is localized
in the soluble (cytosolic) fraction (Ahmed & Anders, 1976, 1978). It
does not require oxygen but is dependent upon glutathione and a
glutathione- S-transferase enzyme, the products in vitro being
formaldehyde and chloride ion. The rapid and almost quantitative
conversion of formaldehyde to formic acid and then carbon dioxide
known to occur in vivo (Neely, 1964) is consistent with this pathway
being the source of carbon dioxide exhaled after exposure to methylene
chloride. The intermediates involved in the metabolism of methylene
chloride to formaldehyde are unknown, but the nature of the enzyme
involved and the dependence upon glutathione suggest that
S-chloromethyl-glutathione is formed and rapidly hydrolysed and
degraded to glutathione and formaldehyde (Ahmed & Anders, 1978). The
isoenzyme involved in the metabolism of methylene chloride has been
identified as a member of glutathione- S-transferase class theta
(Meyer et al., 1991).
The chemistry of the S-chloromethyl thioethers (Bohme et al.,
1949) and the lack of depletion of glutathione during this reaction
are consistent with these conjugates being extremely transient.
Formaldehyde, in addition to its metabolism to carbon dioxide, becomes
incorporated into the C-1 metabolic pool via formic acid. Therefore,
exposure to radiolabelled methylene chloride results in the
incorporation of radioactivity into macromolecules including nucleic
acids.
Hallier et al. (1993) described an apparent polymorphism in the
metabolism of methylene chloride in human blood. The metabolic
activity was reported to be localized in erythyrocytes (Thier et al.,
1991) and to be due to the presence of a glutathione- S-transferase
enzyme (Schroeder et. al., 1992). The work by Schroeder describes the
detection of enzyme activity in erythrocytes using methyl bromide as a
substrate, not methylene chloride. Furthermore, in experiments
investigating the influence of cofactors on enzyme activity,
glutathione could be replaced by L-cysteine, suggesting that this
enzyme is not a glutathione- S-transferase. The work by Thier et al.
(1991) identified metabolic activity in plasma and not, as reported by
Hallier et al. (1993), in erythrocytes.
6.3.2 In vivo studies
The metabolism of methylene chloride in various animal species and
in humans has been studied extensively (e.g., Fodor et al., 1973;
Kubic et al., 1974; Roth et al., 1975; Lee Rodkey & Collison, 1977;
Peterson, 1978; McKenna & Zempel, 1981; McKenna et al., 1982; Angelo
et al., 1986a,b).
Methylene chloride and the other dihalomethanes are unique in
being the only class of industrial chemicals known to be metabolized
to carbon monoxide. This metabolic pathway (dependent on cytochrome
P-450), first discovered in humans (Stewart et al., 1972), results in
elevated levels of CO-Hb and in increased levels of carbon monoxide in
expired air. Subsequent studies in experimental animals and in humans
established that this pathway is rate-limited by enzyme saturation, so
that at high doses the levels of CO-Hb become constant and independent
of dose (Lee Rodkey & Collison, 1977). Later experiments in animals
using radiolabelled methylene chloride identified carbon dioxide as
the other major metabolite (Di Vincenzo & Hamilton, 1975). Although
carbon dioxide is a known metabolite of carbon monoxide, the amount of
carbon dioxide formed from the monoxide was thought unlikely to
account for the levels found during exposure to methylene chloride.
This suggested the presence of a second pathway (dependent on
glutathione- S-transferase), which was subsequently confirmed in
experimental animals.
Two reports have described the effects of pretreatment or co-
administration of other organic solvents on the metabolism of
methylene chloride to carbon monoxide. Pankow et al. (1991) described
increases in CO-Hb levels in rats pretreated with a single gavage dose
of benzene, toluene or isomers of xylene, up to 32 h prior to a 6-h
exposure to methylene chloride. CO-Hb levels increased from 9.3%, in
rats exposed to methylene chloride alone, to 22.7% in rats pretreated
with m-xylene. Similar increases were seen in rats pretreated with a
single garage dose of methanol (Pankow & Jagielki, 1993). In both
studies, the levels of CO-Hb were reduced when the solvents were co-
administered with methylene chloride. The results of both studies were
considered to be consistent with the metabolism of methylene chloride
by cytochrome P-450 IIE1.
At first sight it might appear that the relative molar amounts of
carbon monoxide and carbon dioxide exhaled in vivo provide an index
of the activity of the two metabolic pathways. Studies using metabolic
inhibitors suggest that significant amounts of carbon dioxide are also
derived from the oxidative P-450 pathway (Gargas et al., 1986; Reitz
et al., 1986). Similar studies in mice using metabolic inhibitors have
confirmed these findings, leading to the conclusion of the authors
that the cytochrome P-450 pathway is the major route of metabolism of
methylene chloride within species (Ottenwalder et al., 1989). This
finding is consistent with either hydrolysis of formyl chloride to
formic acid or with formyl chloride reacting with glutathione to form
S-formyl glutathione. The rapid enzymatic and chemical breakdown of
this conjugate (Uotila & Koivusalo, 1974a,b) would yield formic acid
and hence carbon dioxide. Thus, a quantitative correlation between the
amount of carbon monoxide and carbon dioxide exhaled and the activity
of the two pathways no longer appears to be valid.
Levels of CO-Hb in the blood, following exposure to methylene
chloride, are both dose- and time-dependent. Human subjects exposed to
concentrations of 1770 mg/m3 or less for 1 h have CO-Hb levels of
1-4%. These levels rose to an average of 10% saturation within 1 h
after exposure to 3500 mg/m3 for 2 h (Stewart et al., 1972). Hake et
al. (1975) reported CO-Hb levels in excess of 8% following exposure to
880 mg/m3 for 7.5 h.
Human volunteers were exposed to 350 or 1240 mg/m3 for 6 h and
levels of methylene chloride in blood and exhaled air, CO-Hb and
exhaled CO were measured. At the end of the 6-h exposure, the CO-Hb
concentration of the group exposed to 1240 mg/m3 was 1.4 times
higher than that of the group exposed to the lower dose. Likewise, the
concentration of exhaled CO in the high-dose group was 2.1 times
higher than that of the low-dose group. The authors concluded that
their finding of non-linearity between administered dose and the CO-Hb
and CO levels is an indication of saturation of the metabolic pathway
(McKenna et al., 1980).
Physical exercise performed during exposure to methylene chloride
will produce higher blood CO-Hb levels than those found in sedentary
workers (Åstrand et al., 1975; Di Vincenzo & Kaplan, 1981b). Under a
moderate workload, an exposure to 350 mg/m3 for 7.5 h may cause a
CO-Hb saturation of about 5% at the end of the exposure period (Di
Vincenzo & Kaplan, 1981b). Other factors, including smoking and
exposure to combustion and automobile exhaust, will increase CO-Hb
levels.
6.4 Elimination and excretion
6.4.1 Inhalation exposure
6.4.1.1 Human studies
Methylene chloride is removed from the body mainly in expired air
and urine. In four human subjects exposed to methylene chloride
(350 mg/m3) for 2 h, an average of 22.6 µg methylene chloride was
excreted in the urine within 24 h after the exposure. In seven
subjects exposed to 710 mg/m3 for 2 h, the corresponding value was
81.5 µg (Di Vincenzo et al., 1972). These data show that the amount
excreted in the urine is insignificant. Methylene chloride excretion
in expired air was most evident during the first 30 min after
exposure. Initial post-exposure concentrations of methylene chloride
in expired breath following 2-and 4-h exposure periods were about
71 mg/m3 and fell to about 18 mg/m3 at the end of 30 min. Small
amounts of methylene chloride remained in the expired air at 2.5 h.
A detailed study of the relationship between the measurements of
methylene chloride in expired air or blood, carbon monoxide in expired
air and CO-Hb in blood was undertaken by Di Vincenzo & Kaplan
(1981a,b). At the end of exposure of non-smoking, sedentary volunteers
for 7.5 h to methylene chloride vapour concentrations of
180-710 mg/m3, the mean concentration of the solvent in alveolar air
and in blood, and the percent CO-Hb saturation were measured, as shown
in Table 12.
By 7 h after exposure to any concentration, the expired air
contained less than 3.5 mg/m3 methylene chloride; at 16 h, only
negligible levels were detected (Di Vincenzo & Kaplan, 1981a). These
data suggest that, due to its rapid elimination, measurements of
methylene chloride in expired air are unsuitable for use as a marker
of occupational exposure.
Table 12. Methylene chloride in expired air and blood, and carboxyhaemoglobin
(CO-Hb) levels of human volunteers following 7.5 h exposure
(from Di Vincenzo & Kaplan, 1981a)
Methylene Methylene choride Methylene chloride CO-Hb
chloride exposure in expired air in blood levels
(mg/m3) (mg/m3) (mg/litre)
180 53 0.3 1.9%
350 124 0.8 3.4%
530 194 1.2 5.3%
710 282 1.8 6.8%
Di Vincenzo & Kaplan (1981a) reported that, in a human volunteer
study, exposure to 180, 350, 530 or 710 mg/m3 for 7.5 h/day (for 5
days) resulted in peak CO-Hb levels of 1.9, 3.4, 5.3 and 6.8%,
respectively (Table 12). It was estimated that an 8-h exposure to
about 530 mg methylene chloride/m3 is equivalent to an 8-h exposure
to 124 mg carbon monoxide/m3, in as much as either exposure under
sedentary conditions will increase blood CO-Hb levels to about 5% of
saturation by the end of the exposure.
Di Vincenzo & Kaplan (1981b) also investigated the effects of
exercise and cigarette smoking on the uptake, metabolism and excretion
of methylene chloride. The effects of smoking and methylene chloride
exposure on CO-Hb saturation levels were found to be additive.
Exercise was found to increase the absorption of methylene chloride
and CO-Hb levels. However, the effects of exercise on CO-Hb were not
observed to increase with heavy workloads beyond the level achieved
with moderate work-loads, suggesting a saturation of this effect (see
also section 5.3.2).
Engström & Bjurström (1977) found that, during the first 2 h after
exposure, the concentration in alveolar air tended to be lower and
declined more rapidly in obese subjects than in slim ones. After this,
the concentration dropped more slowly in the obese group. During the
late phase of elimination, the obese subjects tended to have a higher
concentration in expired air.
6.4.1.2 Animal studies
In rats, methylene chloride was excreted in the expired air,
urine, and faeces following a single 6-h exposure to 180, 1800 or
53 000 mg methylene chloride/m3 (McKenna et al., 1982). At
180 mg/m3, only 5% of the exhaled label was found as methylene
chloride. The remainder was exhaled as CO and CO2. As the exposures
increased, so did the exhalation of non-metabolized methylene
chloride. Methylene chloride accounted for 30% of the label from the
1800 mg/m3 dose and 55% of the label for the 53 000 mg/m3 dose. A
combination of exhaled methylene chloride, CO2 and CO accounted for
58%, 71% and 79% of the inhaled methylene chloride dose for the 180,
1800 and 53 000 mg/m3 doses, respectively. Urinary excretion
accounted for 7.2-8.9% of the dose and 1.9-2.3% of the dose was in the
faeces.
6.4.2 Oral exposure
Expired air accounted for 78-90% of the excreted dose in rats in
the 48-h period following a 1 or 50 mg/kg dose of methylene chloride
in aqueous solution (McKenna & Zempel, 1981). The radiolabel was
present in the exhaled air as CO and CO2, as well as in expired
methylene chloride. The amount of methylene chloride in the expired
air increased from 12% to 72% as the dose was increased from 1 to
50 mg/kg. Radiolabel in the urine accounted for 2-5% of the dose under
the above exposure conditions, while 1% or less of the dose was found
in the faeces. These data indicate that the lungs are the major organ
of methylene chloride excretion even under oral exposure conditions.
Mice excreted 40% of the administered dose (100 mg/kg) unchanged in
expired air within 96 h (Yesair et al., 1977).
6.4.3 Dermal exposure
No information is available regarding excretion and elimination in
humans or animals following dermal exposure to methylene chloride.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Appraisal
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria which are able to grow in the presence of
methylene chloride at much higher concentrations (including a
saturated solution in water) have been identified. Anaerobic bacteria
are more sensitive; growth inhibition has been observed at 3 mg/litre
in anaerobic biological sludge.
In the aquatic environment, fish and amphibian embryos are the
most sensitive with effects on hatching from 5.5 mg/litre.
Adult fish are relatively insensitive to methylene chloride. even
after prolonged exposure (14-day LC50 > 200 mg/litre). The effect
of methylene chloride on Daphnia is variable; the lowest reported
EC50 was 135 mg/litre in a closed system.
In soil, 10 mg/kg strongly decreased the ATP content of the
biomass, adversely affected the growth of fungi and aerobic bacteria.
and induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms, LC50 values were in the
range of 300 to more than 1000 µg/cm2 ). In sediment, no toxic
effects were observed even at very high levels.
In higher plants, no effects were found after exposure for 14
days to 100 mg/m3.
7.1 Microorganisms
7.1.1 Bacteria
7.1.1.1 Aerobic bacteria
No inhibition of growth was observed at 19.6-19 600 mg/litre
methylene chloride in Bacillus subtilis, Pseudomonas cepacia and
Aeromonas hydrophylia (Schubert, 1979). Inhibition of
bioluminescence of Photobacterium phosphoreum by 50% occurred after
a 15-min exposure to 2880 mg/litre (Hermens et al., 1985).
In a standard 16-h growth-inhibition test with Pseudomonas
putida, a threshold of 500 mg/litre for methylene chloride was
determined (Bringmann & Kühn, 1977b). The glycolysis of Pseudomonas
putida was inhibited after a 16-h exposure to 1000 mg/litre
(Bringmann & Meinck, 1964).
Nenzda & Seydel (1988) report minimum inhibitory methylene
chloride concentrations for the bacteria Escherichia coli and
M. smegmatis of 1049 and 1468 mg/litre, respectively.
For heterotrophs, 50% inhibition of oxygen consumption occurred at
320 mg/litre after 24 h (Blum & Speece, 1991).
With other bacteria (Acinetobacter, Alcaligenes, Flavobacterium,
Pseudomonas cepacia, Aeromonas hydrophila), stimulation of growth
was observed at 200 mg/litre (Davis et al., 1981).
The IC50 for inhibition of multiplication of Escherichia coli
was 37.2 mg/litre (Nendza & Seydel, 1988).
In the OECD activated sludge respiration-inhibition test (method
209) using sealed vessels, the EC50 value for methylene chloride was
more than 1000 mg/litre after 30 min (Volskay & Grady, 1988).
Concentrations up to 1000 mg/litre had no effect on the oxygen
consumption or glucose metabolism of activated sludge acclimated to
methylene chloride for 3 days (Klecka, 1982).
In methylene chloride-utilizing bacteria, e.g., Hypho-microbium,
up to 1700 mg/litre did not interfere with growth (Stucki et al.,
1981).
Blum & Speece (1991) found that the IC50 for reduction of
ammonia was 1.2 mg/litre after 24 h for Nitrosomonas.
7.1.1.2 Anaerobic bacteria
Anaerobic bacteria are more sensitive than aerobic bacteria.
Methanogenesis of mixed rumen microflora was inhibited from
136 mg/litre (Bauchop, 1967). At 93 mg/litre, the growth of a mixed
bacterial population from an anaerobic digester was inhibited by 50%
(Thiel, 1969). Addition of methylene chloride to anaerobic sludge from
an operating municipal digester showed, after 48 h, a 20% inhibition
of gas production at 3 mg/litre and a 50% inhibition at 50 mg/litre
(Hayes & Bailey, 1977). Addition of methylene chloride to the feed of
a mixed anaerobic culture, developed in the laboratory from seed from
a sewage treatment plant, decreased the gas production to such an
extent that at 3.3 mg/litre it had virtually ceased after 5 days,
compared with 15 days in controls (Vargas & Ahlert, 1987).
Blum & Speece (1991) determined the toxicity of methylene chloride
to methanogenic bacteria and found an IC50 for inhibition of gas
production of 7.2 mg/litre.
Stuckey et al. (1980) used a batch system in which methylene
chloride was added in ethanol to sludge from a laboratory digester in
a Warburg apparatus. Some inhibition was noted at the lowest
concentration tested (3.16 mg/litre); the concentration for 50%
inhibition over 60 h was estimated to be 14 mg/litre.
7.1.2. Protozoa
The bacteriovorous ciliated protozoan Uronema parduczi Chatton-
Lwoff was not affected after a 20-h exposure to 16 000 mg/litre (EC5
inhibition cell proliferation) (Bringmann & Kühn, 1980). Also, no
effects were observed in Microregma heterostoma after a 28-h
exposure to 1000 mg/litre (Bringmann & Meinck, 1964).
7.1.3 Algae
In several freshwater green algae (Selenastrum capricornutum,
Scenedesmus subspicatus. Scenedesmus quadricauda, Chlorella vulgaris,
Chlamydomonas angulosa), photosynthesis (chlorophyll a content,
CO2 uptake) and cell number were only affected by methylene chloride
from 1450 mg/litre (Bringmann & Kühn, 1978; Hutchinson et al., 1978;
US EPA, 1980). The threshold (7-day EC3) for effects in the
cyanobacterium (blue-green alga) Microcystis aeruginosa was
550 mg/litre (Bringmann & Kühn, 1978).
In the marine diatom Skeletonema costatum methylene chloride
exposure had no effect on chlorophyll a content or cell number at
662 mg/litre (US EPA, 1980).
7.2 Aquatic organisms
The volatility of methylene chloride presents difficulties in
aquatic toxicity testing. Therefore, care should be taken when
interpreting results based on nominal concentrations and open static
systems. Flow-through systems or closed static systems are necessary
to conduct adequate toxicity studies on volatile substances. However,
these systems were not always used (Tables 14 and 15).
7.2.1 Plants
The EC50 for Lemna minor growth was 2000 mg/litre, whilst both
growth and photosynthesis of the plant Groenlandia densa were
totally inhibited at this concentration after 7 days (Merlin et al.,
1992).
7.2.2 Invertebrates
7.2.2.1 Insects
The toxicity of methylene chloride for insects was investigated in
adult Tribolium confusum and grain weevil (Calandra granaria). The
LC50 for a 5-h exposure in fumigation vessels was 82 and
380 mg/litre, respectively (Ferguson & Pirie, 1948; Negherbon, 1959).
Table 13. Acute aquatic toxicity of methylene chloride to algae
Organism Description Method Parametera Concentration Reference
(mg/litre)
Diatom Skeletonema costatum Chlorophyll a content, 96-h EC50 > 662 US EPA (1980)
(salt water) cell number
Green alga Selenastrum Chlorophyll a content, 96-h EC50 > 662 US EPA (1980)
capricornutum cell number
Green alga Scenedesmus Cell number 8-day TT 1450 Bringmann & Kühn
quadricauda (1978)
Green alga Chlorella vulgaris CO2 uptake 3-h EC50 2292 Hutchinson et al.
(1978)
Green alga Chlamydomonas CO2 uptake 3-h EC50 1477 Hutchinson et al.
angulosa (1978)
Cyanobacterium Microcystis aeruginosa Cell number 8-day TT 550 Bringmann & Kühn
(blue-green alga) (1978)
a TT = toxicity threshold (i.e. the concentration at which cell multiplication was inhibited by more than 3%)
Table 14. Acute aquatic toxicity of methylene chloride in fresh water
pH/dissolved Hardness
Organism Temperature oxygen (mg CaCO3 Flow/ Parameter Concentration References
(°C) (mg/litre) per litre) Static (mg/litre) and remarks
Crustacean
Water flea 22 7.4-9.4/ 173 Static 48-h LC50 220 Le Blanc (1980)
(Daphnia magna) 6.5-9.1 48-h NOEC 68 (nominal concentration
Water flea 20-22 7.6-7.7 Unknown Static 24-h EC50 2100-2270 Bringmann & Kühn
(Daphnia magna) 24-h NOEC 1550-1707 (1977a, 1982)
(nominal concentration)
Water flea Unknown Unknown Unknown 48-h LC50 1250 Bringmann & Meinck
(Daphnia magna) (1964)
Water flea Unknown Unknown Unknown Static 24-h EC50 1959 Kühn et al, (1989)
(Daphnia magna) 48-h EC50 1682 (closed system)
Water flea Unknown Unknown Unknown Static 48-h EC50 135 Abernethy et al. (1986)
(Daphnia magna) (closed system)
Water flea 18-20 8/8.7-8.8 11.7 Unknown 48-h LC50 480 RIVM (1986)
(Daphnia magna) 48-h NOEC 100 (nominal concentration
closed system)
Fish
Goldfish Unknown Unknown Unknown Static 24-h LC50 420 Jenson (1978)
(Carrassius (nominal concentration)
auratus)
Table 14 (Cont'd)
pH/dissolved Hardness
Organism Temperature oxygen (mg CaCO3 Flow/ Parameter Concentration References
(°C) (mg/litre) per litre) Static (mg/litre) and remarks
Fathead minnow 12 7.8-8.0/ 67 Static 98-h LC50 310 Alexander et al, (1978)
(Pimephales > 5 Flow 96-h LC50 193 (static test nominal, flow-
promelas) (adult) 96-h NOEC 66.3 through measured
concentrations;
aquarium covered with
plastic film for the first
24-h)
Fathead minnow 25 Unknown 73-82 Flow 96-h LC50 502 Dill et al. (1987)
(Pimephales (analysed concentration)
promelas)
(juvenile)
Bluegill, 21-23 6.5-7.9/ 32-48 Static 96-h LC50 220 Buccafusco et al. (1981)
(Lepomis unknown (nominal concentration,
macrochirus) aquarium not capped)
Table 15. Acute aquatic toxicity of methylene chloride in salt water
Temperature pH/dissolved Hardness Flow/ References
Organism (°C) oxygen (mg CaCO3 Stat Parameter Concentration and remarks
(mg/litre) per litre) (mg/litre)
Crustacean
Mysid shrimp Unknown Unknown Unknown Static 96-h LC50 260 US EPA (1980) (nominal
(Mysidopsis bahia) concentration)
Grass shrimp 20±2 6.1-8.0 8-12 Static 48-h LC50 108.5 Burton & Fischer (1990)
(Palaemonetes pugia) > 4
Fish
Golden orfe Unknown Unknown Unknown Static 48-h LC50 521-528 Juhnke & Lüdemann (1978)
(Leuciscus idus)
Killifish (juvenile) 20±2 6.1-8.0/ Unknown Static 48-h LC50 97.0 Burton & Fischer (1990)
(Fundulus > 4
heteroclitus)a
Sheepshead minnow 25-31 Unknown 10-31 Static 98-h LC50 330 Heitmuller et al. (1981)
(Cyprinodon 98-h NOEC 130 (nominal concentration)
variegatus)
a fish died within 1 hour; the measured 1-h LC50 was 135 mg/litre. The 48-h value was the average of the initial and final concentrations.
7.2.2.2 Crustaceans
Data on the toxicity of methylene chloride to crustaceans are
presented in Table 14. Daniels et al. (1985) and Knie (1988) reported
a 48-h LC50 of 27 mg/litre and a 24-h LC50 of 12.5 mg/litre; such
values are lower than those presented in Table 14 by almost one order
of magnitude. However, no experimental details were given and,
therefore, the validity of the data cannot be assessed.
7.2.2.3 Molluscs
In seawater, metamorphosis was induced in up to 63% of the larvae
of the nudibranch mollusc (Phestilla sibogae) when exposed to
8.5-25.5 mg/litre (Pennington & Hadfield, 1989).
7.2.3 Fish
7.2.3.1 Acute toxicity
Data on the acute toxicity of methylene chloride to fish are
presented in Table 14.
The acute toxicity of methylene chloride to adult fathead minnows
(Pimephales promelas) has been studied both in a static and a flow-
though system. The observed effects (loss of equilibrium,
melanization, narcosis and swollen, haemorrhaging gills) were
reversible at a sublethal level (Alexander et al., 1978).
7.2.3.2 Chronic toxicity and reproduction
Data on the chronic and embryo-larval toxicity of methylene
chloride to fish are summarized in Table 16.
In a 32-day embryo-larval test with fathead minnow (Pimephales
promelas), the larval survival and weight was affected from 209 and
142 mg/litre, respectively. The maximum acceptable toxicant
concentration (MATC) based on body weight was calculated to be
108 mg/litre. The ratio between the acute 8-day LC50 value and the
32-day embryo-larval MATC is 4.6, indicating a small difference
between acute and chronic effects of methylene chloride (Dill et al.,
1987).
7.2.4 Amphibians
In closed flow-through systems, short-term embryo-larval tests
were carried out, from 2 to 6 h post-spawning to 4 days post-hatch, on
amphibian eggs of Rana catesbeiana. R. palustris and Bufo fowleri
(hatching times ranged from 3 to 4 days). After combining frequencies
for lethality and teratogenesis, the analytically determined post-
hatching LC50s were > 32 mg/litre for the pickerel frog
Table 16. Chronic and embryo-larval toxicity of methylene chloride to fish
Dissolved Hardness Flow/ References and
Description Temperature pH oxygen (mg CaCO3 Static Parameter Concentration remarks
(°C) (mg/litre) per litre) (mg/litre)
Chronic toxicity
Guppy 22±1 ? > 5 25 daily LC50, 14 days 295 Könemann
(Poecilia reticulata) renewal (1981) (covered
with glass,
nominal)
Fathead minnow 25±1 6.8-8.6 > 9 73-82 flow LC50, 8 days 471 Dill et al.
(Pimephales promelas) NOEC, (1987)
(juvenile) 8 days 357 (analytical
concentration)
Embryo-larva toxicity
Fathead minnow 20.4±0.6 7.8 6.5 95 flow LC50a 34 Black et al.
(Pimephales promelas) (1982)
(embryo-larva)
Fathead minnow 25±1 6.8-8.6 > 9 73-82 flow LOEC, 32 days 209 Dill et al.
(Pimephales promelas) (survival) (1987)
(embryo-larva) LOEC, 32 days 142 (analytical
(weight) concentration)
Rainbow trout 13.3±0.3 7.8 9.4 106 flow LC50a,b 13.1 Black et al.
(Salmo gairdneri) (1982)
(embryo)
Table 16 (Cont'd)
Dissolved Hardness Flow/ References and
Description Temperature pH oxygen (mg CaCO3 Static Parameter Concentration remarks
(°C) (mg/litre) per litre) (mg/litre)
Rice fish 25±1 7.6-8.4 4.5-8.8 11.7 renewal LC50, 3 weeks 106 RIVM (1986)
(Oryzias latipes) 23±2 3 times/ NOEC, 3 weeks 75 (analytical
(egg-larva) week concentration)
a Eggs were exposed from 30 min after fertilization to 4 days post-hatch
b Teratogenic effects were observed at 5.5 mg/litre
(R. palustris) and Fowler's toad (Bufo fowleri) and 17.78 mg/litre
for the bullfrog (R. catesbeiana). In the latter, anomalous larvae
and 16% decreased hatching were observed at 6.73 mg/litre. For the
pickerel frog and Fowler's toad, hatching was decreased by 14 and 20%
at 10 and 32 mg/litre, respectively. In the hatched populations
slightly higher incidences of teratogenic effects were observed (Birge
et al., 1980).
Black et al. (1982) exposed several amphibian species to methylene
chloride from 30 min after fertilization to 4 days post-hatch. Post-
hatching LC50 values ranging from 16.9 to > 48 mg per litre were
found for the European common frog (Rana temporaria), Northwestern
salamander (Arabystoma gracile), African clawed frog (Xenopus
laevis) and the Leopard frog (R. pipiens). The European common
frog and the Northwestern salamander were the most sensitive to
methylene chloride.
7.3 Terrestrial organisms
The toxicity of methylene chloride to higher plants (Phaseolus
vulgaris, Raphanus sativus radicula, Lepidum sativum, Trifolium
pratense, Saintpaula ionatha, Petunia hybrida) was evaluated, using
the LIS (Landesanstalt für Immissionsschutz, Essen) test; no effect
was observed at 100 mg/m3 exposure over 14 days (Van Haut & Prinz,
1979).
In leaves of alfalfa (Medicago sativa), the effect of methylene
chloride vapour on the photosynthetic fixation of 14CO2 was
tested; photosynthesis appeared to be reduced from 388 000 mg/m3
(Lehman & Paech, 1972).
In a 48-h filter-paper contact toxicity test on the earthworm
Eisenia fetida, the LC50 was 304 µg/cm2 in one study and
> 1000 µg/cm2 in another. Therefore, methylene chloride was
classified as moderately toxic (100-1000 µg/cm2) (Roberts & Dorough,
1984; Neuhauser et al., 1985).
In embryos of White leghorn chicken, the LD50 for injection of
methylene chloride in the yolk sac is 14 mg/egg (Verrett et al.,
1980).
7.4 Population and ecosystem effects
7.4.1 Soil microorganisms
When added to brown soil at 10 mg/kg (dry weight), methylene
chloride decreased the ATP content of the soil biomass by 80-85%,
compared to controls, and adversely affected the growth of soil fungi
and aerobic bacteria after 3 days. A slight recovery was observed by
the end of the 56-day experiment. Anaerobic bacteria were hardly
influenced and, in the case of the obligate anaerobic Clostridium
sp., the growth was even increased. The replacement of oxygen
probably explained the stimulation of growth in the latter case
(Kanazawa & Filip, 1987).
Incubation of soil for 2 months with 1-10 mg/kg (dry weight)
methylene chloride reduced the activity of ß-glucosidase,
ß-acetylglucosaminidase and proteinase during the first 28 days, with
recovery after 2 months; no effect was observed at 0.1 mg/kg (Kanazawa
& Filip, 1986).
7.4.2 Sediment microorganisms
In sediment from a freshwater stream, methylene chloride did not
significantly affect the electron transport system (ETS) activity
during a 1-h enzymatic assay at 66 500 mg/kg. When assayed over an
11-day period, 1330-66 500 mg/kg caused a fluctuating stimulation of
ETS activity, which may indicate a marked alteration of the stability
of the biological activity in the sediment. Microbial respiration,
measured by CO2 evolution, was inhibited (EC50) after 7 days
at 15 500 mg/kg. However, when measured by oxygen uptake, it was
stimulated at up to 26 500 mg/kg (Trevors, 1985).
7.4.3 Microcosms and mesocosms
Microcosms composed of water plants (Elodea canadensis, Lemna
minor), algae (Scenedesmus subspicatus) and snails (Physa sp.)
were exposed to 500 or 1000 mg/litre for 21 days. At 1000 mg/litre a
decrease in oxygen content of the water was observed, together with
mortality in snails and algae, as well as necrosis on fronds of Lemna
minor. The photosynthesis of the plants was inhibited. These effects
were less at 500 mg/litre, but this concentration was still lethal to
snails. In outdoor mesocosms, containing a large diversity of species,
initial concentrations of 137-156 mg/litre did not induce any toxicity
(Merlin et al., 1992).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Acute toxicity data
See Table 17.
8.1.2 Oral administration
Musculoskeletal disturbances were found in Sprague-Dawley rats at
doses of 530 mg/kg or more. Hypotension, hypothermia and haematuria
were also noted (dose threshold not reported). The gastrointestinal
tract was found to be congested with micro-haemorrhages or partial
destruction from doses of 530 mg/kg or more. Blood CO-Hb increased in
a dose-related manner (Laham, 1978).
Various effects have been reported following the acute
administration of large oral doses of methylene chloride. The effects
include a decreased cytochrome P-450 content in liver microsomes of
Sprague-Dawley rats receiving 1000 mg/kg (Moody et al., 1981), CNS
effects and evidence of pathological changes in the liver and kidney
of Wistar rats receiving 2000 mg/kg (Janssen & Pott, 1988a), evidence
of liver necrosis and increased glucose-6-phosphatase activity in male
rats (strain unspecified) receiving 2210 mg/kg (Reynolds & Yee, 1967),
and decreased hepatic secretion of triglycerides followed by an
increased hepatic triglyceride content in male mice receiving
2700 mg/kg (Selan & Evans, 1982).
No liver toxicity was found in male Wistar rats receiving up to
4400 mg/kg by oral administration (Danni et al., 1981).
Liver damage was investigated in Sprague-Dawley rats exposed to up
to 1275 mg/kg given orally. Serum ALT activity was unaffected, as was
liver cytochrome P-450 and glutathione content. However, increased
ornithine decarboxylase activity and DNA damage were found in the
liver (Kitchin & Brown, 1989).
8.1.3 Inhalation administration
8.1.3.1 Rat
Behavioural changes and CNS disturbances were found in several
studies. Decreased running activity was found in rats exposed to
17 700 mg/m3 for 1.5 h (Heppel & Neal, 1944); hypothermia,
hypotension and convulsion in Sprague-Dawley rats from 28 200 mg/m3
(6-h exposure) (Laham, 1978); CNS depression in Alderley-Park rats at
31 800 mg/m3 (10-min EC50) (Clark & Tinston, 1982) and in rats at
40 000 mg/m3 (2-h exposure) (Ulanova & Yonovskayo, 1959); and
dyspnoea and anaesthesia in rats from 53 000 mg/m3 (30-min exposure)
(Schumacher & Grandjean, 1960; Kashin et al., 1980).
Table 17. Acute toxicity of methylene chloride
Species Route Vehicle Parameter Concentration Reference
Rat (Wistar, male) oral none LD50 1710-2250 mg/kg Klimmer (1988)
Rat CDF (F-344) oral none LD50 1530-2524 mg/kg Carreon (1981)
Rat (Sprague-Dawley) oral none LD50 2120 mg/kg Kimura et al, (1971)
young male
Rat (Sprague-Dawley)
- male oral none LD50 2280 mg/kg Laham (1978)
- female oral none LD50 1410 mg/kg
Mouse (CF-1, male) oral unknown LD50 1987 mg/kg Aviado et al. (1977a,b)
Dog oral mucillage of LD50 3000 mg/kg Barsoum & Saad
acacia (1934)
Rat (Alderley Park) inhalation 15-min LC50 197 790 mg/m3 Clark & Tinston (1982)
Rat (Sprague-Dawley) inhalation 6-h LC50 52 000 mg/m3 Bonnet et al. (1980)
male
Rat (Sprague-Dawley) inhalation 6-h LC50 > 28 000 mg/m3 Landry et al, (1981)
Mouse (CF-1, male) inhalation 20-min LC50 92 680 mg/m3 Aviado et al. (1977a,b)
Mouse (LF1, female) inhalation 6-h LC50 49 100 mg/m3 Gradiski et al, (1978)
Mouse (ICR, male) inhalation 6-h LC50 55 870 mg/m3 Scott et al. (1979)
Guinea-pig inhalation 6-h LC50 40 200 mg/m3 Balmer et al. (1976)
Table 17 (Cont'd)
Species Route Vehicle Parameter Concentration Reference
Rat (Sprague-Dawley) intratracheal ALDa 350 mg/kg McCarty et al. (1992)
male
Mouse (CF-1, male) intraperitoneal unknown LD50 448 mg/kg Aviado et at. (1977a,b)
Mouse intraperitoneal unknown LD50 500 mg/kg Schumacher &
Grandjean (1960)
Mouse (Swiss-Webster, intraperitoneal corn oil LD50 1990 mg/kg Klaassen & Plaa (1966)
male)
Dog intraperitoneal corn oil LD50 1260 mg/kg Klaassen & Plaa (1967)
Mouse subcutaneous olive oil LD50 6500 mg/kg Kutob & Plaa (1962)
a "ALD = Approximate Lethal Dose, the lowest dose causing death within 3 days
Studies on sleeping patterns in Wistar rats by measuring
electroencephalographic (EEG) and electromyographic (EMG) activity
have shown a dose-related increase in total sleeping time and
intervals between rapid eye movement (REM) sleep when the rats were
exposed to 1770, 3500 or 10 600 mg/m3 (500, 1000 or 3000 ppm)
methylene chloride for 3 h. During exposure to 3500 or 10 600 mg/m3,
the percentage of sleep defined as REM decreased by nearly 20% (Fodor
& Winneke, 1971; Fodor et al., 1973). Studies using similar techniques
on the narcotic effects of methylene chloride in rats (strain
unspecified) have been reported by Berger & Fodor (1968). Rats were
exposed to a range of concentrations of methylene chloride from 9900
to 99 000 mg/m3 (2800 to 28000 ppm) for unspecified periods of time.
There was an initial period of excitation followed by deep narcosis
with a decrease in muscle tone and EEG activity and subsequent
breathing difficulties. Cessation of electrical activity was noted
after a 1.5-h exposure to 88 000 or 99 000 mg/m3 (25 000 or
28 000 ppm) and after a 6-h exposure to 54 000 or 64 000 mg/m3
(16000 or 18000 ppm). Following exposure to 17 700 to 31 800 mg/m3
(5000 to 9000 ppm) methylene chloride, long periods of sleep occurred
without desynchronization phases. Following exposure to concentrations
of methylene chloride below 17 700 mg/m3 (5000 ppm), there were no
measurable effects on either EEG or EMG activity (Berger & Fodor,
1968).
Exposure of F-344 rats to 7100 mg/m3 for 2.5 h caused
statistically significant changes in somatosensory evoked responses
and EEG. The lack of effect of 157 mg carbon monoxide/m3 (which
induces a CO-Hb level of 10%, comparable with that produced by
exposure to 7100 mg methylene chloride/m3) on evoked responses,
indicated that the effects were probably due to methylene chloride
itself and not to its principal metabolite carbon monoxide (Mattsson
et al., 1988). Alterations in somatosensory evoked potentials were
also observed after a 1-h exposure of F-344 rats to dose levels of
17 700 mg/m3 or more (Rebert et al., 1989).
No macroscopic lesions were found in rats at the 6-h LC50 of
53 000 mg/m3 (Bonnet et al., 1980). Congestion of various organs, as
well as oedema of the brain, heart, lungs and hip region, was noted
following exposure to 71 000 mg/m3 (6-h exposure) in Sprague-Dawley
rats (Laham, 1978).
Increased CO-Hb levels were found from 1770 mg/m3 in various
strains of rats (MacEwen et al, 1972; Laham, 1978; Dill et al., 1978;
Kurppa et al., 1981). Ascorbic acid content of the liver was increased
in rats exposed to 40 000 mg/m3 for 2 h (Ulanova & Yonovskayo,
1959), but no effect on cytochrome P-450 or specific liver enzymes was
noted in Wistar rats after a 3-h exposure to 3500 mg/m3 or in
Sprague-Dawley rats after a 5-min (repeated 5 times) exposure to
350 mg/m3, except for increased microsomal and decreased lysosomal
ß-glucuronidase activity (Kurppa et al., 1981).
Intratracheal administration of methylene chloride in Sprague-
Dawley rats showed lethal levels at 350 mg/kg (corresponding to 17.5%
of the oral LD50), death occurring in a few seconds. This result
emphasises that aspiration of methylene chloride may present more of a
hazard than oral ingestion (McCarty et al., 1992).
8.1.3.2 Mouse
CNS depression resulting in reversible narcosis was reported in
mice exposed to between 14 100 and 52 200 mg/m3 methylene chloride
for 2-6 h (Flury & Zernik, 1931) or to 47 700 mg/m3 for 128 min
(EC50) (Kashin et al., 1980). This effect was also noted in CF-1
mice exposed to 35 300 mg/m3 for 20 min (Aviado et al., 1977a,b) or
in Swiss mice exposed to 45 900 mg/m3 for 7 h (Svirbely et al.,
1947). Exposure at 35 000 mg/m3 for 2 h was the minimal CNS
effective concentration found in the mouse (Lazarew, 1929).
Ability to learn a simple passive avoidance task, 1-4 days after
exposure, was reduced in Swiss-Webster mice exposed to 168 000 mg/m3
for 20 seconds (Alexeef & Kiglore, 1983).
Fatty changes were noted in the liver and, less frequently, in the
kidney from 56 500 mg/m3 after a 7-h exposure (Svirbely et al.,
1947). Microscopic lesions in the liver, kidneys, lungs and adrenal
were found (no dose-response relationship) in mice exposed to lethal
doses for 6 h (Gradiski et al., 1978).
Cardiac sensitization to the effects of adrenaline was reported in
Swiss mice exposed to 710 000 mg/m3 for 6 min) (Aviado & Belej,
1974).
Male NMRI mice were exposed to methylene chloride by inhalation in
a study of tolerance, i.e. decreased responsiveness to a chemical that
arises as a result of previous exposure to the same chemical
(Kjellstrand et al., 1990). Motor activity measured with Doppler radar
units was used to monitor the behavioural reactions of the animals.
Motor activity increased on exposure to methylene chloride and
decreased on constant exposure. Termination of exposure was followed
by hypoactivity.
8.1.3.3 Other animals
After 6 h of exposure to 17 700 mg/m3, the concentration of
triglycerides was increased in the liver of guinea-pigs, and reduced
in the serum (Bulmer et al., 1976; Morris et al., 1979).
Histopathological liver changes, consisting of the appearance of lipid
droplets, were first seen in guinea-pigs at 17 700 mg/m3 (Morris et
al., 1979). Slight to moderate vacuolation in the liver of guinea-pigs
was seen after a 6-h exposure to 38 800 mg/m3. In addition, lungs
showed congestion and haemorrhage. Behavioural changes were also noted
(Balmer et al., 1976).
No effect on blood pressure, heart rate or EEG activity was found
in rabbits exposed to 90 000 mg/m3 for 2 h (Truhaut et al, 1972).
Only serum AST was significantly increased.
In Beagle dogs, ECG changes as well as decreased blood pressure,
heart and respiratory rate were found at 141 200 mg/m3 (7-h
exposure). Behavioural effects were already noted after a 1-h exposure
to 53 000 mg/m3 (reduced reflexes) (Von Oettingen et al., 1950).
Increased CO-Hb values, but no change in haematocrit, haemoglobin
concentration or red cell count, were reported in dogs and rhesus
monkeys exposed for 24 h to 17 700 mg/m3. Slight pathological
changes in the liver (fatty changes, vacuolization) could be
attributed to the treatment (MacEwen et al., 1972).
Cardiac effects such as arrhythmia, tachycardia and hypotension
were found in monkeys, dogs and rabbits exposed for 1-5 min to levels
of methylene chloride exceeding 35 300 mg/m3 (Belej et al., 1974;
Adams & Erickson, 1976; Taylor et al., 1976; Aviado et al., 1977a,b;
Aviado, 1978).
One study on rabbits showed allergic reactions after inhalation
(Shmuter & Kashin, 1978). However, the experimental protocol of this
study is questionable and the result has not been confirmed.
8.1.4 Dermal administration
No effect was noted on rats receiving a dermal application of
methylene chloride of 710 mg/kg for 0.5 to 4 h, except for an increase
in CO-Hb levels (Makisimov & Mamleyeva, 1977). Only slight behavioural
effects and macroscopic changes in the liver (swelling) were found in
Wistar rats receiving 2000 mg/kg under an occlusive dressing for 24 h
(Janssen & Pot, 1988b). Haemoglobinuria was observed in rats when
abdominal skin was immersed in methylene chloride for 2-20 min
(Schutz, 1960).
8.1.5 Intraperitoneal administration
A single intraperitoneal injection of 510 mg methylene chloride/kg
in rats slowed down the sciatic motor conduction velocity by 11% and
gave rise to a CO-Hb level of 6.8% (Pankow et al., 1979).
Signs of CNS depression were found in Wistar rats and CFI mice
receiving, respectively, 1060 mg/kg (with phenobarbital pretreatment)
and 114 mg/kg (Aviado et al., 1977; Masuda et al., 1980).
Various biological investigations in rats and mice showed a dose-
related increase in serum AST and/or ALT activity at > 660 mg/kg.
No effect was observed on glucose-6-phosphatase activity, cytochrome
P-450 content or BSP retention (Klaassen & Plaa, 1966; Cornish et al.,
1973; Masuda et al, 1980; Corsi et al., 1983). Increased ALT activity
was reported in mongrel dogs receiving 800 mg/kg in corn oil (Klaassen
& Plaa, 1967). No histological changes in the liver could be found in
rats exposed to < 1300 mg/kg (Cornish et al., 1973; Corsi et al.,
1983). However, mild hepatic inflammation was noted in Swiss Webster
mice receiving lethal doses of methylene chloride (2000 mg/kg)
(Klaassen & Plaa, 1966). Renal tubular changes were noted in F-344
rats administered intrapertinoneally with 1330 mg/kg in corn oil
(Kluwe et al., 1982) and in Swiss-Webster mice receiving 2000 mg/kg in
corn oil (Klaassen & Plaa, 1966). However, no renal histological
change could be found in Swiss mice receiving 1300 mg/kg in corn oil
(Plaa & Larson, 1965). Slight histological changes were noted in the
liver and kidneys of mongrel dogs receiving 1300 mg/kg (Klaassen &
Plaa, 1967).
8.1.6 Intravenous administration
The minimum lethal concentration in anaesthetized dogs was found
to be 200 mg/kg in olive oil after intravenous administration (Barsoum
& Saad, 1934).
Methylene chloride shortened the duration of nystagmus induced by
rotation in Sprague-Dawley rats during intravenous perfusion of
5.1 mg/kg per min for 60 min (Tham et al., 1984).
Following a single intravenous injection of 3.1, 6.2 or
12.4 mmol/kg, methylene chloride was found to sensitize the myocardium
of rats to arrhythmia development in response to catecholamines. The
release by methylene chloride of endogenous catecholamines is possibly
a cause of these modified cardiovascular actions (Mueller et al.,
1991).
8.1.7 Subcutaneous administration
The minimum lethal concentration in the rabbit was found to be
2700 mg/kg (Barsoum & Saad, 1934). Prolongation of phenobarbital-
induced sleeping time occurred at 1700 mg/kg in Swiss mice. No effect
on liver function or histology was observed at up to 5000 mg/kg (Kutob
& Plaa, 1962).
8.1.8 Appraisal
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species lie between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg have been recorded. Acute effects after methylene
chloride administration by various routes of exposure are primarily
associated with the central nervous system (CNS) and the liver. CNS
disturbances were found at 14 100 mg/m3 or more and slight changes
in EEG at 1770 mg/m3. Slight histological changes in the liver
were found at concentrations of 17 700 mg/m3 or more. In the mouse,
but not in the rat, effects on the lungs restricted to the Clara
cells were observed after exposure to 7100 mg/m3. Occasionally the
kidney is affected. Cardiac sensitization to adrenaline-induced
arrhythmias has been reported, and cardiovascular effects were seen
at concentrations above 35 000 mg/m3. However, the effects were
inconsistent.
8.2 Short-term exposure
8.2.1 Oral administration
CD1 mice given doses of up to 665 mg/kg per day of methylene
chloride in corn oil by gavage for 14 days did not show any effect on
liver enzymes. Microscopic examination revealed slight vacuolation in
the liver from 333 mg/kg. No damage to the kidneys was reported
(Condie et al., 1983).
8.2.2 Subcutaneous administration
Reduction of the systolic blood pressure of hypertensive Sprague-
Dawley rats was reported after subcutaneous exposure to 2000 mg/kg
twice a week for 17 weeks. No effect was found in normotensive rats.
Very slight histopathological changes in the liver and lungs of
hypertensive rats were described (Loyke, 1973).
8.2.3 Inhalation administration
8.2.3.1 Rat
Changes in Sprague-Dawley rats were reported after exposure to
methylene chloride (3500 mg/m3) 2 h/day for 15 days; decreased body
weight, increased hepatic lipid peroxidation, and high concentrations
of methylene chloride in the brain, kidney and blood immediately after
inhalation were observed (Ito et al., 1990). Similar hepatic effects
(hypertrophic hepatocytes, and increased lipid peroxidation and
glutathione peroxidase activity) were reported in male Wistar rats
exposed to 3500 mg/m3 2 h/day for 20 consecutive days (Takashita et
al., 1991). Biochemical tests were performed on the serum and brain of
groups of six male Sprague-Dawley rats which were exposed to 250, 1100
or 3500 mg/m3 6 h/day for 3 days and killed 16-18 h later. A
selective reduction in dopamine concentration, with changes in
dopamine turnover in some forebrain dopamine nerve terminal systems,
was reported. A dose-dependent increase in noradrenaline turnover in
the anterior periventricular hypothalamic area and dose-dependent
decreased noradrenaline concentration in the posterior periventricular
area were observed. No significant changes were reported in the
secretion of anterior pituitary hormones (Fuxe et al., 1984).
Groups of five male and five female F-344 rats were exposed to
methylene chloride at concentrations of 5740, 11 500, 22 900, 45 900
or 56 500 mg/m3, 6 h/day for 19 days. Intermittent scratching,
ataxia and hyperactivity were seen in all rats exposed to
22 900 mg/m3 or more. Dyspnoea and anaesthesia were observed in
animals exposed to 45 900 mg/m3 or more. Some deaths were also
observed at these concentrations (NTP, 1986).
No, or limited, lesions were found in the liver and lungs of F-344
rats, exposed to 7100 or 14 100 mg/m3, 6 h/day for 10 days, after
light and electron microscopic examination (Hext et al., 1986). No
effect on the liver was found in rats (strain unspecified) after
inhalation of 880 mg/m3, for 5 h/day for 28 days (Norpoth et al.,
1974)
Inhalation exposure of Sprague-Dawley rats to methylene chloride
concentrations of 12 800 mg/m3 (5 h/day, 5 days/week) for 4 weeks
revealed an inflammatory response and cell damage in the lungs as
demonstrated by the increase biochemical response (enzymatic and non-
enzymatic) observed in cell-free lavage effluents from the lungs (Sahn
& Lowther, 1981).
8.2.3.2 Other animals
Increased liver weight and increased mitotic activity in
hepatocytes were observed in male B6C3F1 mice after 2 weeks of
repeated exposure to 14 100 mg/m3 6 h/day for up to 3 weeks
(Eisenbrandt & Reitz, 1986). Necrosis of occasional epithelial cells
in the bronchi and bronchioles, together with reactive hyperplasia of
adjacent lymphoid tissue, was observed in a few animals.
Groups of five male and five female B6C3F1 mice were exposed to
methylene chloride at concentrations of 5740, 11 500, 22 900, 45 900
or 56 500 mg/m3, 6 h/day for 19 days. Hyperactivity (dose-related)
was seen in exposed mice, but no exposure-related pathological
findings were observed. Some deaths occurred in mice exposed to
45 900 mg/m3 or more (NTP, 1986).
CD-1 mice, Golden Syrian hamsters, Sprague-Dawley and CDF (F-344)
rats were exposed to 0, 8800, 17 700 or 28 200 mg/m3, 6 h/day,
5 days/week, for a total of 19, 18, 19 or 7 exposures, respectively,
over a period of 21 to 28 days. Animals exposed to 28 000 mg/m3
showed anaesthetic effects and a decrease in the body weight of rats.
At 18 000 mg/m3 there was slight anaesthesia, decreased body weight
in male rats, increased aspartate aminotransferase (ASAT) in female
mice and Sprague-Dawley rats, and increased liver weights in female
mice, hamsters and rats. At 8800 mg/m3, the animals exhibited more
scratching activity than the controls, but showed no other effects
attributable to exposure (Nitschke et al., 1981)
Following exposure to 17 700 mg/m3, a reduction in body weight
of mice was observed, and relative liver weights were increased up to
the end of the 7-days of continuous exposure. Fatty infiltration, an
increase in the triglyceride concentration and hydropic degeneration
of the endoplasmic reticulum gradually disappeared. Protein synthesis
was depressed. Necrosis was observed in a few hepatocytes (Weinstein
et al., 1972).
Carboxyhaemoglobin levels were raised after continuous exposure of
monkeys to 88.25 mg/m3 (25 ppm) for 28 days (MacEwen & Vernot, 1972;
Haun et al., 1972).
A group of NMRI mice was continuously exposed to 130-1059 mg/m3
(37-300 ppm) for 30 days, while another was intermittently exposed for
1-12 h/day to 2118-25 416 mg/m3 (600-7200 ppm) for 30 days,
corresponding to an average exposure (24-h mean value) of 1059 mg/m3
(300 ppm). In addition, groups of mice were continuously exposed to
1059 mg/m3 for 4, 8, 15 and 90 days. The blood level of
butyrylcholinesterase was significantly increased from 265 mg/m3
(75 ppm) in continuously exposed male mice and after intermittent
exposure in male rats. Moreover, liver weight was significantly
increased in a dose-related manner from 265 mg/m3 (75 ppm) and
330 mg/m3 (150 ppm) in male and female mice, respectively. Finally,
fatty accumulation was found in both sexes at 265 mg/m3 (75 ppm) or
more. All effects were reversible (Kjellstrand et al., 1986). CO-Hb
levels were raised after continuous exposure of monkeys to
88.25 mg/m3 (25 ppm) for 28 days (MacEwen et al., 1972, Haun et al.,
1972).
8.3 Long-term exposure
8.3.1 Rat
8.3.1.1 Inhalation exposure
In a study by Leuschner et al. 1984, 20 male and 20 female
Sprague-Dawley rats were exposed to 35 g/m3, 6 h/day for 90 days.
Haematological, clinical chemistry and urinary parameters were
measured and histological examinations were performed. A slight
redness of the conjunctiva lasting for 1-10 h was observed after each
exposure. No other treatment-related signs of toxicity were reported.
Groups of 10 male and 10 female F-344 rats were exposed to
methylene chloride at concentrations of 1850, 3700, 7400, 14 800 and
29 700 mg/m3 for 6 h/day, 5 days/week for 13 weeks. One male and one
female rat exposed to 29 700 mg/m3 died before the end of the study,
whereas none of the control rats died. Foreign body pneumonia was
observed in some rats exposed to > 7410 mg/m3. The mean body
weight in males and females exposed to 29 700 mg/m3 was lower than
in controls. Liver lipid to liver weight ratios were statistically
significantly reduced in both males and females exposed to
29 700 mg/m3 and in females exposed to 14 800 mg/m3 when compared
to controls (NTP, 1986).
Male and female F-344 rats were exposed to 177, 710 or
7100 mg/m3 6 h/day, 5 days/week for 13 weeks. No treatment-related
alterations in sensory evoked potentials (flash, auditory brainstem,
somatosensory or caudal nerve) or neuropathology were observed at any
of the exposure levels (Mattsson et al., 1990).
CNS depression was found in rats during each daily session of
repeated exposure to 35 000 mg/m3 7 h/day, 5 days/week for 6 months
(Heppel et al., 1944).
Rats (sex and strain unspecified) were continuously exposed to
either 88 or 350 mg/m3 for 100 days. Slight cytoplasmatic
vacuolization with positive fat stains in the liver and tubular
degeneration in the kidney were observed (Haun et al., 1972).
8.3.1.2 Oral exposure
Rats receiving methylene chloride in the drinking-water at a
concentration of 125 mg/litre for 13 weeks did not show any effects on
behaviour, body weight, haematology, urinalysis, blood glucose level,
plasma free fatty acids, or the oestrous cycle (Bornmann & Loeser,
1967).
Groups of 20 male and 20 female Fischer-344 rats were given 0.15,
0.45, and 1.50% methylene chloride in drinking-water for 3 months,
equivalent to 166, 420 and 1200 mg/kg per day, respectively, for males
and 209, 607, and 1469 mg/kg per day for females. Slightly decreased
body weights were observed in mid-dose males and high-dose females
throughout the study. There were no differences between treated and
control animals with regard to mortality, physical observations, food
consumption or gross necropsy results. There were no exposure-related
effects observed following the histopathological evaluation of rat
tissues from the 1 month interim necropsies. However, hepatocellular
changes were observed following treatment for 3 months; central
lobular necrosis, granulomatous foci, ceroid or lipofuscin
accumulation, and cytoplasmic eosinophilic bodies were observed in
high-dose males and females and in some mid-dose females. A dose-
dependent increased incidence of hepatocyte vacuolation was also
observed, many of the vacuoles containing lipid which was generalized
or concentrated in the central lobular region (Kirschman et al.,
1986).
8.3.2 Mouse
8.3.2.1 Inhalation exposure
Groups of 10 male and 10 female B6C3F, mice were exposed to
methylene chloride at concentrations of 1850, 3700, 7400, 14 800 or
29 700 mg/m3 for 6 h/day, 5 days/week for 13 weeks. Exposure-related
deaths were seen in some mice exposed to 29 700 mg/m3. Hepatic
centrilobular hydropic degeneration was observed in males and females
exposed to 29 700 mg/m3 and in females exposed to 14 800 mg/m3.
Both mean body weights and liver lipid to liver weight ratios were
reduced in males and females exposed to 29 700 mg/m3 when compared
to controls (NTP, 1986).
Mice (strain and sex unspecified) were continuously exposed to
either 88 or 350 mg/m3 for 100 days. Slight cytoplasmatic
vacuolization was found at both dose levels, and a decrease in the
microsomal cytochrome P-450 content was found in the liver of mice
exposed to methylene chloride at 350 mg/m3 (Haun et al., 1972).
Female ICR mice were continuously exposed to 350 mg/m3 for 10
weeks. Fatty infiltration, vacuolization and enlarged hepatocyte
nuclei persisted up to the end of the exposure period. A reversible
increase in plasma triglycerides was also observed (Weinstein &
Diamond, 1972).
8.3.2.2 Oral exposure
Groups of 20 male and 20 female B6C3F1 mice were given 0.15,
0.45 and 1.50% methylene chloride in drinking-water for 3 months,
equivalent to 226, 587 and 1911 mg/kg per day, respectively, for males
and 231, 586 and 2030 mg/kg per day for females. Slightly lower body
weights were observed in mid-dose and high-dose males and in females
from week 6 to the end of the study. Treated and control animals did
not differ with respect to physical and ophthalmological observations
or food consumption. There were no exposure-related effects observed
following the histopathological evaluation of mice following a 1-month
exposure. However, after 3 months of exposure, subtle centrilobular
fatty changes in the liver were observed, these being most prominent
in mice receiving either 587 or 1911 mg/kg per day. No other exposure-
related changes were reported (Kirschmann et al., 1986).
8.3.3 Other animals
Heppel et al. (1944) did not find organ lesions related to
exposure at 17 700 mg/m3 (7 h/day, 5 days/week for 6 months) in
studies on dogs, monkeys, rats, rabbits and guinea-pigs, with the
exception of moderate centrilobular fatty degeneration of the liver
and pneumonia in 3 out of 14 guinea-pigs. CNS depression was found in
all species following exposure to 35 000 mg/m3; all animals became
inactive, sometimes after initial excitement. At 35 000 mg/m3, dogs
also showed fatty degeneration of the liver.
Hepatic changes (slight cytoplasmatic vacuolation) and vacuolar
changes in the renal tubules were found in dogs exposed continuously
to 3500 mg/m3 for up to 100 days. Abnormal haematology and increased
activity of serum enzymes were reported after 4 weeks. Oedema of the
brain was observed at a concentration of 17 350 mg/m3 (Haun et al.,
1972).
Three male and three female beagle dogs were exposed to
17 700 mg/m3, 6 h/day for 90 days. Haematology, clinical chemistry
and urinary parameters were measured and ECG and circulatory functions
were examined. At the end of the study, histological examinations were
performed. Slight sedation was induced throughout the exposure period
and all dogs had slight erythema, lasting up to 10 h after exposure.
No deaths and no other signs of toxicity were observed (Leuschner et
al., 1984).
Decreased levels of neurotransmitter amino acids were observed in
gerbil brains after continuous inhalation exposure to methylene
chloride (340 mg/m3) for 3 months (Briving et al., 1986). In gerbils
exposed by inhalation to 1240 mg/m3 for 3 months, followed by a 4-
month solvent-free period, increased brain concentrations of two
astroglial proteins and decreased levels of DNA in the hippocampus and
cerebellum were observed (Rosengren et al., 1986). Decreased
hippocampal DNA levels were also observed in gerbils exposed to
740 mg/m3 (Rosengren et al., 1986; Karlsson et al., 1987). It was
suggested by the authors that this effect may have been the result of
the loss of nerve cells.
8.3.4 Appraisal
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3 ) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice at
> 17 700 mg/m3 . After intermittent exposure, effects on the liver
were observed in rats at 3500 mg/m3 and in mice at 14 100 mg/nz3 .
After continuous exposure, slight cytoplasmatic vacuolization in the
liver of both rats and mice were found at 88 and 350 mg/m3.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations of < 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver with a no-observed-effect level of 125 mg/m3.
8.4 Skin and eye irritation; sensitization
8.4.1 Skin irritation
Application of 0.5 ml methylene chloride to rabbits for 24 h,
under a semi-occlusive patch on abraded or intact skin, caused severe
erythema and oedema with necrosis and acanthosis (Duprat et al.,
1976). Rabbits exposed to 0.5 ml methylene chloride for 4 h under
occlusive patch test condition, either with or without simultaneous
exposure to other chlorinated solvents, showed moderate skin
irritation but no corrosive effect (Van Beek, 1990).
8.4.2 Eye irritation
Duprat et al. (1976) and Ballantyne et al. (1976) exposed rabbits
once to 0.1 ml methylene chloride by ocular instillation. Moderate to
severe changes were seen in the conjunctiva, together with increased
corneal thickness and intra-ocular tension. All effects were
reversible. Vapour exposure of the eyes to 17 700 mg/m3 caused
slight increases in corneal thickness and intra-ocular tension.
8.4.3 Sensitization
No data are available.
8.4.4 Appraisal
Liquid methylene chloride is moderately irritant to the skin and
eyes in experimental animals.
8.5 Developmental and reproductive toxicity
8.5.1 Developmental toxicity
When groups of Sprague-Dawley rats and Swiss-Webster mice were
exposed to methylene chloride at a concentration of 4400 mg/m3 on
days 6-15 of pregnancy for 7 h/day, maternal body weight in the mice
was increased and the dams of both rats and mice had CO-Hb levels as
high as 12.5% during exposure. In both species, an increased incidence
of minor skeletal anomalies was observed, i.e. dilated renal pelvis in
rats and extra sternebrae in mice (Schwetz et al., 1975). No
significant teratogenic or fetotoxic effects were observed in either
species.
Groups of 18 rats were exposed before and/or during 17 days of
pregnancy to a methylene chloride concentration of 16 250 mg/m3 for
6 h/day. The exposed dams exhibited increased blood CO-Hb levels,
ranging from 7.1 to 10.1%, and increased relative and absolute liver
weights. Fetal body weight was decreased, but no increase in the
incidences of dead fetuses and/or resorptions nor any skeletal and/or
visceral malformations were observed (Hardin & Manson, 1980). After
exposure to methylene chloride using the same experimental conditions,
litters from four groups of 10 rats were used for behavioural testing.
Body weight gain, food and water consumption, wheel running activity
and avoidance learning were all unaffected by the exposure. However,
changes in the general activity of pups were found in both sexes
starting at the age of 10 days, and were still present in male
offspring at the age of 150 days. The effects cannot be definitely and
directly attributed to methylene chloride, however, since elevated
maternal CO-Hb- or methylene chloride-induced changes in maternal-
litter interactions could have been contributing factors (Bornschein
et al., 1980).
Groups of seven female Wistar rats were fed methylene chloride at
levels of 0.04, 0.4 and 4.0% in their diet from days 0-20 of
pregnancy. Fetuses were examined on day 20 and neonatal growth was
measured for 8 weeks after birth. Maternal body weight was
significantly reduced in the 4.0% group. Although a reduction in the
fetal weight of the females in the 0.4% group was observed, there were
no differences in any group in the number of implantations and
resorptions. No external malformations were observed by fetal,
skeletal and visceral examination, and no differences were observed in
any group in the frequency of delayed ossifications or in the dilation
of the renal pelvis. A decrease in postnatal weight gain and in
absolute liver weight was found in the 0.04% group males at the 8th
week after birth (Nishio et al., 1984).
Groups of F-344 rats (number not specified) received methylene
chloride by gavage (dose level not specified) in corn oil on gestation
days 6- 15. The compound was tested with at least two dose levels plus
a concurrent control group, the high dose (not specified) being
selected to cause maternal toxicity. The dams were allowed to deliver
and their litters were examined post-natally. Although a small change
in maternal weight was found, no effects on litters were reported
(Narotsky et al., 1992).
8.5.2 Reproductive toxicity
When rats received methylene chloride in the drinking-water at a
level of 125 mg/litre during a period of 13 weeks before mating, no
effects were found on the female fertility index, litter size,
survival of pups at 4 weeks or the number of resorptions (Bornmann &
Loeser, 1967).
A two-generation inhalation study was conducted to evaluate the
effects of inhaled methylene chloride on the reproductive capability,
neonatal growth and survival of rats (Nitschke et al., 1988b). Groups
of 30 male and 30 female 6-week-old F-344 rats (F0) were exposed to
0, 350, 1770 or 5300 mg/m3 (6 h/day, 5 days/week for 14 weeks).
After this exposure, F0 animals were allowed to mate using one male
and one female of the respective treatment groups to produce the F1
litters. After weaning, 30 males and 30 females (4 weeks old) from
each treatment group were randomly selected and assigned to the
respective exposure groups. After exposure to the relevant
concentration of methylene chloride (6 h/day, 5 days/week for 17
weeks), the F1 adults were allowed to mate to produce F2 litters.
Reproductive parameters examined included fertility, litter size and
neonatal growth and survival. All adults and selected weanlings were
examined for grossly visible lesions. No adverse effects on
reproductive parameters, neonatal survival or neonatal growth were
noted in animals exposed to methylene chloride in either the F0 or
F1 generations. Similarly, there were no treatment-related gross
pathological changes in F0 and F1 adults or F1 and F2
weanlings; histopathological examination of tissues did not reveal any
lesions in F1 and F2 weanlings attributable to exposure to
methylene chloride. Therefore, the results of this study indicate that
exposure to concentrations as high as 5300 mg/m3 does not affect the
normal reproductive function of rats (Nitschke et al., 1988b).
8.5.3 Appraisal
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3 . No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
was observed in three animal studies. Small effects on either fetal
or maternal body weights were reported at 4400 mg/m3 . A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations of up to 5300 mg/m3 ,
6 h/day, 5 days/week, did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
8.6 Mutagenicity and related end-points
Studies on the mutagenic potential of methylene chloride have been
performed on bacteria, fungi and cultured mammalian cells. Results
from in vivo studies on mice and rats have also been reported.
8.6.1 In vitro
8.6.1.1 Bacteria
Methylene chloride is mutagenic when tested using the Ames
protocol in Salmonella typhimurium TA98, TA100 and TA 1535
(Table 18). The number of revertants increased 3- to 7-fold in a dose-
related manner when plates were exposed to vapour of methylene
chloride of undisclosed purity at levels ranging from 20 100 up to
201 000 mg/m3. Metabolic activation by either induced rat liver S9
fraction, cytosol fraction, or microsomal fraction increased the
mutagenicity of methylene chloride (Simmon et al., 1977; Jongen et
al., 1978, 1982; McGregor, 1979; Kirwin et al., 1980; Barber et al.,
1980; Nestmann et al., 1980, 1981; Gocke et al., 1981; Dillon et al.,
1990). In this respect the cytosolic fraction was more active than the
microsomal fraction (Green, 1983). A positive result was reported in
strains TA98 and TA100 with and without 30% hamster liver S9 (Zeiger
et al., 1990) using the vapour phase (desiccator procedure) protocol.
Negative mutagenicity results were obtained in studies not using
vapour phase exposure (Rapson et al., 1980; Nestmann et al., 1980). No
mutagenic activity was found when methylene chloride was tested in
Salmonella typhimurium strains TA100, TA1535, TA1537, TA97 and TA98
with or without the addition of 10% or 30% rat/hamster liver S9, using
the preincubation protocol (Zeiger et al., 1990).
Metabolic studies of methylene chloride (see section 6.3) indicate
that the conjugation of methylene chloride with glutathione (GSH),
catalysed by cytosolic glutathione- S-transferase, may play a role in
the observed mutagenicity of methylene chloride in Salmonella.
However, the direct reaction of glutathione with methylene chloride
only produced a very small increase in mutagenicity (Jongen et al.,
1982). In another study, Salmonella typhimurium TA100 and the GSH-
deficient strain TA100 gsh were exposed to 0-5% methylene chloride
using a vapour phase protocol. The mutagenic response, with and
without Aroclor-induced rat-liver S9, microsomes or cytosol, was
marginally higher at the highest methylene chloride concentrations.
Salmonella typhimurium TA100 gsh was slightly less responsive than
TA100 at high doses in the absence of S9. This difference was not seen
in the presence of S9. The addition of exogenous GSH had only a small
effect on the mutagenic response in TA100 or TA100 gsh in the absence
or presence of S9. According to the authors, these data suggest that
if the interaction between methylene chloride and GSH is responsible
for the observed mutagenicity, it occurs at extremely low levels of
intracellular GSH and is not significantly affected by exogenous GSH
(Dillon et al., 1992).
The mutagenicity of methylene chloride has also been studied in a
variety of other microbial systems using a vapour phase protocol.
Mutagenic effects were observed in E. coli WP2uvrA and pKM101 which
were exposed to 0-5% methylene chloride using a vapour phase protocol
(Dillon et al., 1992). These strains of E. coli are deficient in
glutathione, containing approximately 25% of the level of glutathione
present in the strain TA100.
Table 18. In vitro mutagenicity assays
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100, ± 10% 100-10 000 -ve Preincubation protocol Zeiger et al.
typhimurium TA1535, or 30% µg//plate (1990)
TA1537, rat or
TA97, TA98 hamster
liver S9
Salmonella TA100, TA98 ± 30% 0.1-1.0 +ve Vapour protocol-dessicator Zeiger et al.
typhimurium rat or ml/chamber procedure (1990)
hamster
liver S9
Salmonella TA100, ±S9 -ve Standard plate-incorporation protocol Nestmann et al.
typhimurium TA1535, (1980)
TA1537,
TA98, TA1538
Salmonella TA100, +ve 0.5µl in open glass dish within a Nestmann et al.
typhimurium TA1535 desiccator; revertants: 2-fold (1980)
increase in TA1535; 6-fold in TA100
Salmonella TA1535, ± S9 up to 750/µl in +ve Active in strains TA98 and TA100 Gocke et al.
typhimurium TA100, a 9 litre (1981)
TA1538, desiccator
TA98, TA1537
Salmonella TA100 ±S9 0-1.4% +ve Significant increases in mutagenic Jongen et al.
typhimurium activity by addition of rat liver (1982)
cytosol fraction; marginal increases
by addition of microsomal fraction
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100, ±S9 0-5% for 2, 4, 6 +ve Vapour phase protocol. Data Dillon et al.
typhimurium 100gsh or 48 h suggest interaction between (1992)
(glutathione methylene chloride and GSH
deficient) responsible for the mutagenic
activity
Salmonella TA100 S9 2.8 v/v +ve Green (1983)
typhimurium
Salmonella TA100 -S9 0, 50-800/µl +ve Vapour phase protocol; dose-related Simmon et al.
typhimurium per 9 litre increase in the number of revertants, (1977)
desiccator with a mutation rate over 7-fold
(approx 18-318 higher than controls at 320 mg/m3;
mg/m3) for 7 h two experiments were conducted
Salmonella TA98, TA100 ±S9 0, 20, 100 to +ve Vapour phase protocol; S9 prepared Jongen et al.
typhimurium 201 000 mg per m3 from the livers of phenobarbital (1978)
(5 concentrations) pretreated rats; a dose-related
for 48 h increase of up to 5-8 fold (mean
value for 3 experiments) was seen,
slightly higher in the presence of
S9; toxicity was noted at the
highest dose level
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA100 Not stated Vapour phase protocol; time-course Jongen (1984)
typhimurium study to evaluate the most
appropriate exposure time for
maximum differentiation of the
methylene chloride induced
reversion rates in the presence and
absence of a metabolic activation
system; the maximum differentation
was obtained following 4-6 h
exposure, and, consequently, an
exposure time of 6 h was used by
these workers in the study of Jongen
et al. (1982)
Salmonella TA100 ±S9 0 or 1 ml/9 litre +ve Study summarized in review, original Simmon &
typhimurium desiccator data were not available; vapour Kauhanen (1978)
(approx 390 mg phase control; S9) from livers of
per m3) for 6.5 Aroclor-pretreated rats; addition of
or 8 h S9 increased the mutation rate 1.5
fold; no further details are available
Salmonella TA98, TA100 ±S9 0,125, 250, 500 +ve Vapour phase control; S9 from livers Rapson et al.
typhimurium or 750/µl per of Aroclor pretreated rats; dose- (1980)
9 litre desiccator related increase in the mutation rate,
(0-293 mg/m3) with an approximate 10-fold increase
for 8 h for both strains at the highest dose
used; the presence of S9 resulted in
a slightly higher mutation rate
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA1535, Not stated +ve Same study, but no data were given
typhimurium TA1537, for the strains studied; there is no
TA1538 evidence for an independent
confirmatory experiment
Salmonella TA1535, 0.5 ml/desiccator -ve Vapour phase protocol; result Nestmann et al.
typhimurium TA1537, (volume unknown) doubling of revertants for strain (1980)
TA1538, TA1535 and a 6-fold increase for
TA98, TA100 strain TA100 when 0.5 ml methylene
chloride was added directly to the
culture rather than a seperate dish
Salmonella TA100 ±S9 0, 98 800, +ve Vapour phase control; S9 from livers Green (1983)
typhimurium 177 000, or of Aroclor pretreated rats; a dose-
297 000 mg/m3 related increase in the mutation rate
for 3 days was observed
0 or 98 800 +ve Vapour phase protocol; the presence
mg/m3 for 3 of S9 enhanced the mutation rate
days 1.19 fold; on dividing the S9 material
into microsomal and high-speed
supernatant (cytosolic) fractions, only
the high-speed supernatant enhanced
(1.27 fold) the mutation rate;
a small isotope effect was observed
when 2H-methylene chloride was
substituted for 1H-methylene chloride
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Direct examination of the metabolism
of methylene chloride by the
bacterium indicated that radio-
labelled carbon dioxide and trace
amounts of radiolabelled carbon
monoxide were formed, together
with considerable incorporation of
radioactivity into endogenous
materials
Salmonella TA 1535 0, 24 700, +ve Vapour phase protocol; data McGregor (1979)
typhimurium 42 400, 81 200, available in summary form only;
162 400 or dose-response relationship; TA100
331 800 mg per m3; was reported to give a more marked
condensation response
of methylene
chloride onto
agar plates
occurred
Salmonella TA1535, ±S9 0, 38, 76, 96 or +ve Vapour phase protocol data available Barber et al.
typhimurium TA98, TA100 115 µmol/plate in summary form only; dose-response (1980)
(0-38 500 mg relationship (source of S9 not
per m3 vapour) stated) (negative results were
in gas-tight obtained in studies not employing
chambers gas-tight jars)
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Salmonella TA1535, +S9 Concentration +ve Vapour phase protocol; study Longstaff et al.
typhimurium TA100 range not reported only briefly, methylene (1984)
stated; exposure chloride being used as a positive
for 72 h control; S9 prepared from livers of
Aroclor pretreated rats. Mutation rate
increased 6-fold for TA1535 (with a
50% methylene chloride-air mixture)
and 2.4 fold for TA100 (with a 1%
methylene chloride-air mixture); the
increased mutation rate was
reproducible; apparently, one strain
at least showed a positive dose
response
Salmonella TA100 0.1-1000µg -ve Plate-incorporation assays; Rapson et al.
typhimurium per plate (5 insufficient information was given to (1980)
concentrations), evaluate the result
one plate per
concentration
Salmonella TA1535, ± S9 Up to 26 -ve Plate-incorporation assays; S9 from Nestmann et al.
typhimurium TA1537, mg/plate, livers of Aroclor-induced rats; tested (1980)
TA1538, dissolved in to limit of toxicity; positive results
TA98, TA100 dimethyl-sulfoxide for 3 pro-mutagens were obtained using
the same batch of S9, but not
necessarily in parallel incubations; a
second, independent assay was
conducted over a limited concentration
range; this was not a satisfactory
demonstration of a negative
response
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Escherichia WP2uvrA, ±S9 0-5% for 2, 4, 6 +ve Vapour phase protocol; data suggest Dillon et al.
coli pKM101 or 48 h interaction between methylene (1992)
chloride and GSH responsible for the
mutagenic activity
Microscreen E. coli WP2S ±S9 0.78-100 +ve Rossman et al.
( ) µl/well (1991)
Saccharomyces D7 0-209 mM +ve Induced mitiotic gene convertants Callen et al.
cerevisiae and recombinants, and, to a lesser (1980)
extent, gene revertants
Aspergillus Diploid P1 0-0.8 v/v +ve Crebelli et al.
nidulans (1988, 1992)
Sister Human +ve Thilagar et al.
chromatid peripheral (1984a,b)
exchange lymphocytes,
CHO cells,
mouse
lymphoma
L5178Y cells
Sister Chinese ±S9 0-5000 µg/ml -ve Standard (25-29 h after treatment) Anderson et al.
chromatid hamster harvest time (1990)
exchange ovary (CHO)
cells
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Sister CHO cells ±S9 0-15 µl/ml -ve Thilagar &
chromatid Kumaroo (1983)
exchange
Sister Chinese 0-4% ± Marginal (< 2-fold), reproducible Jongen et al.
chromatid hamster V79 increases in frequency; not dose- (1981)
exchange cells related
Chromosome Human Not stated +ve Thilagar et al.
aberration peripheral (1984a,b)
lymphocytes,
CHO; mouse
lymphoma
L5178Y cells
Chromosome CHO cells ±S9 0-15 µl/ml +ve Dose-dependent increase Thilagar &
aberration Kumaroo (1983)
Chromosome CHO cells ±S9 0-5000 µg/ml -ve Standard (10-14 h after treatment) Anderson et al.
aberration harvest time (1990)
Cells in vitro Chinese 0.5-5% v/v -ve Forward mutation to 6-thioguanine Jongen et al.
hamster cells resistance; mutation rate was (1981)
corrected for survival
Cells in vitro Epithelial -S9 0, 35 (300- -ve Varying the expression time was Jongen et al.
cells (V79) 141 000 mg/m3 reported to have no effect; cell (1981)
(4 concentrations survival was reduced by about 20%
for 1 h); expression at 141 000 mg/m3
time of 6 days
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Cell mutation L5178Y Not stated -ve Thilagar et al.
mouse (1984a,b)
lymphoma
Cell mutation L5178Y ±S9 0-3000 µl/ml ± Overall questionable evaluation of Myhr et al.
mouse activity (1990)
lymphoma
Cell Primary 0.5 ml/4.6 litre +ve Enhanced transformation by SA7 Hatch et al.
transformation Syrian chamber adenovirus (1983)
hamster
embyro cells
HGPRT- Chinese 0-4% -ve Jongen et al.
deficient hamster V79 (1981)
cells
Micronucleus Chinese Not stated -ve Gu & Wang
hamster V79 (1988)
cells
Unscheduled Primary rat Not stated -ve Trueman et al.
DNA synthesis hepatocytes (1987)
Unscheduled Primary rat Not stated ± Thilagar et al.
DNA synthesis hepatocytes (1984a)
Cell BALB/C-3T3 0.01% -ve Price et al.
transformation mouse (1978)
Table 18 (Cont'd)
Assay Strain/type S9 Dose Resulta Observations Reference
activation
Cell C3H-10T1/2 Not stated -ve Thilagar et al.
transformation CL8 mouse (1984a)
Unscheduled Primary rat ± A "marginal" positive result reported Thilagar et al.
DNA synthesis hepatocytes (1984a)
Unscheduled Human ±S9 2.5-10.0 µl/ml -ve Perocco & Prodi
DNA synthesis lymphocytes (1981)
Unscheduled Chinese 0-5% -ve Jongen et al.
DNA synthesis hamster V79 (1981)
cells
DNA repair Primary rat 0.7-16.0 mM -ve Andrae & Wolff
synthesis hepatocytes (1983)
a +ve = positive; -ve = negative; ± = equivocal or inconclusive
A recent study demonstrated that glutathione- S-transferase 5-5
expression in Salmonella typhimurium increases mutation rates caused
by methylene chloride. The plasmid pKK233-2 containing rat
glutathione- S-transferase 5-5 cDNA, either in the correct or reverse
direction, was transfected into TA1535. The resulting sense-
transformed TA1535 (RSJ 100) expressed the enzyme and enhanced base-
pair revertants as compared to the anti-sense strain (TPT 100).
Mutagenicity was not seen when GSH, purified glutathione- S-
transferase and a methylene dihalide such as methylene bromide were
added to the pre-incubation mixture with TA1535. Formaldehyde did not
produce mutations in any of the three strains (Thier et al., 1993).
The nature and distribution of forward mutations in the N-terminal
region of the loc I gene of excision repair-proficient (Uvr+) and
excision repair-defective (Uvr B-) strains of E. coli have been
described by Zielenska et al. (1993). A total of 116 locI-d
mutations were characterized.
8.6.1.2 Fungi and yeasts
A dose-related increase in the frequency of gene conversions,
mitotic recombinations, and reversions was found for cultures of
Saccharomyces cerevisiae strain D7, but not for strains D4 and D3,
exposed to methylene chloride of undisclosed purity. However, the
mutagenic results in D7 occurred at toxic doses (1270 g/m3) in which
survival of the yeast cells was reduced to 42% (Cullen et al., 1980).
When assayed for the induction of mitotic segregation in
Aspergillus nidulans P1, methylene chloride significantly increased
the frequency of morphologically abnormal colonies, which produced
euploid whole-chromosome segregants (Crebelli et al., 1988; Crebelli
et al., 1992).
8.6.1.3 Mutation in mammalian cells
Methylene chloride was not mutagenic in several tests in which
mammalian somatic or human cells were used (Gocke et al., 1981; Jongen
et al., 1981; Perocco & Prodi, 1981; Andrue & Wolff, 1983; Burek et
al., 1984).
Both negative (Thilagar et al., 1984a) and questionable results
(Myhr et al., 1990) were reported when methylene chloride was tested
for gene mutations in a L5178Y mouse lymphoma assay at the thymidine
kinuse locus. No increase in micronuclei was found when methylene
chloride was tested in Chinese hamster V79 cells (Gu & Wang, 1988).
8.6.1.4 Chromosomal effects
Studies on chromosome morphology in cultured mammalian cells
indicate that methylene chloride is clastogenic. Chromosomni
alterations (chromatid damage, chromosomni exchanges, but no increase
in sister chromatid exchanges) were observed in CHO cells (Thilagar &
Kumaroo, 1983), human lymphocytes and L5178Y cells (Thilagar et al.,
1984a,b), both with and without metabolic activation. A small increase
in sister-chromatid exchanges (SCEs), without clear evidence of a
dose-response relationship, was found in V79 cells when exposed to
gaseous methylene chloride at concentrations up to 5% (Jongen et al.,
1981 ). A dose-related increase in SCEs was observed in CHO cells
after a 24-h exposure to methylene chloride. The results were
statistically significant only at the highest concentration (7%) and
exposures of shorter duration (2, 4 or 6 h) were without effect
(McCaroll et al., 1983). In a more recent study, Anderson et al.
(1990) reported no increase in chromosomal aberrations or SCEs in CHO
cells exposed to up to 5 mg/ml.
Hallier et al. (1993) described an apparent polymorphism in human
blood samples used to measure SCEs in lymphocytes. Those blood samples
possessing metabolic activity (conjugators) were inactive in the
assay, whereas those samples which were metabolically inactive (non-
conjugators) produced significant increases in SCEs (see also section
6.3.1).
8.6.1.5 DNA damage
Concentrations of up to 16 mM methylene chloride failed to induce
unscheduled DNA synthesis (UDS) in cultured rat hepatocyte, although
some reduction in replicative DNA synthesis occurred at the higher
doses (Andrae & Wolff, 1983). The absence of evidence of methylene
chloride-induced UDS in primary rat hepatocytes was also reported by
Trueman et al. (1987). Thilagar et al. (1984a) reported a "marginal"
positive result in a primary rat hepatocytes UDS assay, but details
were not available. In experiments using no exogenous activation
systems, exposure of hamster V79 cells or human fibroblasts (AH cells)
to methylene chloride concentrations of between 0.5 and 5% did not
induce UDS (Jongen et al., 1981). A non-specific but reversible
inhibition of replicative DNA synthesis was observed in both cell
lines, probably due to a metabolic block of synthesis. Methylene
chloride in doses of 2.5, 5 or 10 µ/ml did not induce UDS in human
lymphocytes in either the presence or absence of rat liver S9 (Perocco
& Prodi, 1981).
8.6.1.6 DNA binding in vitro
Several studies have investigated the potential of methylene
chloride and its metabolites to bind covalently to DNA. Incubation of
14C-labelled methylene chloride failed to detect any DNA binding,
but binding to proteins and lipids was observed (Cunningham et al.,
1981). After incubation of calf thymus DNA in vitro with 14C-
labelled methylene chloride (0.8 µmol/ml), together with hepatic
microsomes and a NADPH-generating system, there was no evidence of any
DNA alkylation (Di Renzo et al., 1982). These studies confirm those of
an earlier in vitro study which failed to detect any DNA binding of
methylene chloride or its metabolites (Anders et al., 1977).
8.6.1.7 Cell transformation
Methylene chloride has been tested for its ability to induce
transformation in a variety of cell systems. Negative results were
obtained in C3H-10T1/2 CL8 mouse cells at 10 µl/ml (Thilagar et al.,
1984a) and in Balb/C-3T3 mouse cells at 0.01% (Price et al., 1978).
Methylene chloride significantly enhanced the frequency of
transformation by SA7 virus in a dose-related manner (Hatch et al.,
1983).
8.6.2 In vivo
8.6.2.1 Chromosome damage
Large doses of methylene chloride (425, 850 and 1700 mg/kg) given
twice intraperitoneally to NMRI mice did not increase micronuclei in
the bone marrow micronucleus test (Gocke et al., 1981). Doses of up to
4 g/kg body weight (the maximum tolerated dose) administered by gavage
to C57BL/6J/Alpk mice also failed to induce any increase in bone
marrow micronuclei (Sheldon et al., 1987). The results of in vivo
mutagenicity assays are presented in Table 19.
Intraperitoneal injections of methylene chloride (100, 1000, 1500
or 2000 mg/kg) did not increase the frequencies of either SCEs or
chromosome aberrations in bone marrow cells of male C57BL/6J mice
(Wesforook-Collins et al., 1988; Wesforook-Collins et al., 1990).
No increase in the frequency of either SCEs or chromosome
aberrations was observed in bone marrow cells of female B6C3F1 mice
after a single subcutaneous injection of methylene chloride (2500 or
5000 mg/kg) (Westbrook-Collins et al., 1989; Allen et al., 1990).
Inhalation exposure of female B6C3F1 mice to 14 000 or
28 000 mg/m3 for 10 days (6 h/day, 5 days/week) resulted in slight
increases in the frequency of SCEs in lung cells and peripheral blood
lymphocytes, in chromosome aberrations in lung and bone marrow cells,
and in micronuclei in peripheral blood erythrocytes. The results were
statistically significant at 28 200 mg/m3 for all end-points. At
14 100 mg/m3, statistical significance was reached only for the SCE
frequency in lung cells. A marginal increase in lung cell SCEs and
micronuclei in peripheral blood erythrocytes was observed following a
3-month inhalation exposure to 7100 mg/m3 (Westbrook-Collins et al.,
Table 19. In vivo mutagenicity assays
Assay Strain/type Resulta Observations Reference
Chromosome Male C57BL/6 mouse bone -ve i.p., 0-2000 mg/kg Westbrook-Collins et al. (1988);
aberration marrow Westbrook-Collins et al. (1990)
Chromosome Female B6C3F1 mouse bone -ve s.c., 2500 or 5000 mg/kg Westbrook-Collins et al. (1989);
aberration marrow Allen et al. (1990)
Chromosome Female B6C3F1 mouse bone ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
aberration marrow 10 days Allen et al. (1990)
Chromosome Rat bone marrow -ve inhalation, 1770, 3500 or 12 400 mg/m3 Burek et al. (1984)
aberration 6 h/day, 5 days/week for 6 months
Sister-chromatid Male C57BL/6 mouse bone -ve i.p. 0-2000 mg/kg Westbrook-Collins et al. (1988);
exchange marrow Westbrook-Collins et al. (1990)
Sister-chromatid Female B6C3F1 mouse bone -ve s.c., 2500 or 5000 mg/kg Westbrook-Collins et al. (1989);
exchange marrow Allen et al. (1990)
Sister-chromatid Female B6C3F1 mouse lung ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
exchange cells, peripheral blood 10 days Allen et al, (1990)
lymphocytes
Sister-chromatid Female B6C3F1 mouse lung ± inhalation, 7100 mg/m3 for 3 months; Westbrook-Collins et al. (1989);
exchange cells small increase Allen et al. (1990)
Synaptonemal Male C57BL/6 mouse ± i.p., 0-1500 mg/kg Westbrook-Collins et al. (1988)
complex
(damage at
meiotic
prophase)
Table 19 (Cont'd)
Assay Strain/type Resulta Observations Reference
Micronucleus Female B6C3F1 mouse ± inhalation, 14 100 or 28 200 mg/m3 for Westbrook-Collins et al. (1989);
peripheral blood 10 days Allen et al. (1990)
erythrocytes
Micronucleus Female B6C3F1 mouse ± inhalation, 7100 mg/m3 for 3 months Westbrook-Collins et al. (1988);
peripheral blood Allen et al. (1990)
erythrocytes
Micronucleus C57BL/6 mouse bone -ve oral in corn oil, up to 4 g/kg Sheldon et al. (1987)
marrow
Unscheduled Male Alpk: AP rat -ve oral gavage, 100, 500 or 1000 mg/kg; Trueman et al. (1987)
DNA synthesis hepatocytes autoradiography 4 and 12 h after
treatment
Unscheduled Male B6C3F1 mouse -ve oral gavage, 100 mg/kg in corn oil Lefevre & Ashby (1989)
DNA synthesis hepatocytes
Unscheduled Male Fischer-344 rat, and -ve inhalation, 7100 or 14 100 mg/m3 for 2 Trueman & Ashby (1987)
DNA synthesis male B6C3F1 mouse or 6 h
hepatocytes
Unscheduled Male B6C3F1 mouse -ve inhalation, 14 100 mg/m3 for 2 h Lefevre & Ashby (1989)
DNA synthesis hepatocytes
Dominant lethal Male Swiss-Webster mouse -ve s.c., 5 ml/kg, 5% or 10% v/v in corn oil, Raje et al. (1988)
3/week for 4 weeks
inhalation at 350, 530 or 710 mg/m3, Basso et al., (1987);
2 h/day, 5 days/week for 6 weeks Raje et al. (1988)
no microscopic lesions of testes or brain
Table 19 (Cont'd)
Assay Strain/type Resulta Observations Reference
Drosphila Berlin K, Basc -ve 0 to 14 260 mg/m3 for 6 h, 1 week or 2 Kramers et al. (1991)
melanogaster weeks; sex-linked recessive lethal assay,
and somatic mutation and recombination
test
Drosphila Berlin K, Basc +ve 125 or 620 mM; increased frequency of Gocke et al. (1981)
melanogaster recessive lethals in Basc test
a +ve = positive; -ve = negative; ± = equivocal or inconclusive
1989; Allen et al., 1990). Background data and positive control
results were not available. Chromosome aberration data (excluding
gaps) were not reported, and so the increases might not be
significant. Increases in SCE and micronuclei were small (up to 2-fold
at 28 000 mg/m3).
No increase in chromosomal aberrations was observed in bone marrow
cells of Sprague-Dawley rats (5 of each sex per group) following
inhalation exposure to 1770, 3500 or 12 400 mg/m3 (6 h/day,
5 days/week) for 6 months (Burek et al., 1984).
Inconclusive results were reported when methylene chloride was
tested in C57B1/6 mice for its ability to induce damage in the
synaptosomal complex, an experimental end-point which can reveal
induced damage at the meiotic prophase, following intraperitoneal
injection of 1500 mg/kg (Westbrook-Collins et al., 1988).
8.6.2.2 Drosophila
No mutagenicity was detected in the recessive lethal test on
Drosophila melanogaster fed, or injected with, 1-2% methylene
chloride (Abrahamson & Valencia, 1980). A marginal increase in the
number of recessive deaths was found after feeding 125 or 650 nmol
methylene chloride in 2% dimethylsulfoxide to Drosophila melanogaster
(Gocke et al., 1981). This study may not be reliable because control
values from different solvent treatments were pooled, and because the
increases seen were significant only when results from the two dose
levels were combined.
Methylene chloride was not active in the sex-linked recessive
lethal assay or the somatic mutation and recombination test carried
out with Drosophila melanogaster using inhalation exposure up to
14 260 mg/m3 (Kramers et al., 1991).
8.6.2.3 DNA damage
Methylene chloride has been evaluated for its ability to initiate
unscheduled DNA synthesis (UDS) in the livers of male mice and rats
in vivo. Alpk:AP rats were exposed by oral garage to 100, 500 or
1000 mg/kg body weight, and hepatocytes were assessed for UDS via
autoradiography 4 and 12 h later (Trueman & Ashby, 1987). In a second
study, F-344 rats or B6C3F1 mice were exposed by inhalation to
either 7100 or 14 100 mg/m3 for 2 or 6 h, and hepatocytes were
assessed for UDS immediately after exposure. In both studies,
methylene chloride failed to induce UDS. Similar results were reported
by Lefevre & Ashby (1989) following exposure of male B6C3F1 mice
either by oral gavage in corn oil (1000 mg/kg) or by inhalation of an
atmosphere containing 14 100 mg/m3 for 2 h.
Slightly increased DNA damage, measured by alkaline elution of DNA
from hepatocytes of rats orally exposed to methylene chloride
(2.55 g/kg) in corn oil for 24 h, has been reported (Kitchin & Brown,
1989).
DNA single strand breaks were found in mouse hepatocytes after in
vivo exposure to 14 120 mg/m3 for 3 or 6 h but not in hepatocytes
of similarly exposed rats. It was further observed in vitro that the
lowest concentration of methylene chloride needed to induce DNA single
strand breaks in mouse hepatocytes was 75 times below that in rat
hepatocytes. This DNA damage was not accompanied by cytotoxicity. The
relation of these findings to the mechanism for carcinogenic effects
is discussed in section 8.8.1.
8.6.2.4 DNA binding
Several studies have investigated the potential of methylene
chloride to bind covalently to DNA after in vivo exposure. DNA has
been isolated from the livers and lungs of mice and rats exposed to
14 100 mg/m3 (Green et al., 1987a,b,c). In both studies the DNA was
hydrolysed and analysed by chromatography to distinguish between
alkylation of DNA and incorporation of radioactivity though the C-1
pool. No evidence of alkylation was found, both studies having the
power to detect one alkylation per 106 nucleotides.
No alkylation of DNA was reported by Ottenwalder & Peter (1989),
following a DNA binding assay of methylene chloride in rats and mice.
Male mice and hamsters were exposed to 14 100 mg/m3, 6 h/day for
2 days, followed on the third day by a 6-h exposure to a decreasing
concentration (15 900 to 8800 mg/m3) of 14C-labelled methylene
chloride (Casanova et al., 1992). DNA-protein cross-links (DPX) were
detected in mouse liver, but not in mouse lung or hamster liver or
lung. The failure to detect DPX in mouse lung did not exclude possible
formation in a sub-population of lung cells. These results demonstrate
that formaldehyde derived from methylene chloride can form DNA-protein
cross-links in the liver of B6C3F1 mice, the formation of DPX being
dependent on the activity of the GST pathway.
8.6.2.5 Dominant lethal assay
Groups of 20 Swiss-Webster male mice were injected subcutaneously
3 times/week for 4 weeks with 5 ml/kg of 5% v/v or 10% v/v methylene
chloride in corn oil (Raje et al., 1988). Other groups of Swiss-
Webster male mice were exposed to 350, 530 or 710 mg/m3 (2 h/day, 5
days/week for 6 weeks) (Basso et al., 1987; Raje et al., 1988). Mating
was started 1 week later for the injection group and 2 days later for
the inhalation group, each male mouse being mated with a virgin adult
female. The mating continued for 2 weeks. The fetuses were examined on
day 17 of gestation. No significant differences in any of the
mutagenicity parameters were found between control and treated groups.
No microscopic lesions were found in the testes (Basso et al., 1987;
Raje et al., 1988) or brain (Basso et al., 1987) of the treated males.
8.6.2.6 Replicative DNA synthesis
A number of studies have evaluated the ability of methylene
chloride to induce replicative DNA synthesis (S-phase) in the livers
and lungs of B6C3F1 mice and in the livers of Sprague-Dawley rats. A
small, but statistically significant, increase in DNA synthesis was
observed in the livers of mice exposed to 13 800 mg/m3 for 2 h, but
not following a single oral dose of 1000 mg/kg (Lefevre & Ashby,
1989). The biological significance of these increases is unclear due
to similar increases being seen in some control groups.
There were no sustained increases in DNA synthesis in the livers
of female mice exposed by inhalation to 3.53, 7.06, 14.12 or
28.24 g/m3 (1000, 2000, 4000 or 8000 ppm) for up to 4 weeks, nor in
female mice exposed to 7.06 g/m3 for up to 2 years (Foley et al.,
1993).
Increases in DNA synthesis were not seen in rats exposed to
1770 mg/m3 for 6 or 12 months (Nitschke et al., 1988a,b).
Replicative DNA synthesis was measured in the lungs of female mice
exposed to 7.06 or 28.24 g/m3 for 1, 2, 3 or 4 weeks and in mice
exposed to 7.06 g/m3 for 13 and 26 weeks. Small decreases in the
labelling indices were reported at all these points (Kanno et al.,
1993). In contrast, Foster et al. (1992) reported significant
increases in the number of cells in S-phase in both the bronchiolar
and alveolar epithelium of male mice exposed to 14.12 g/m3 for
13 weeks.
8.6.3 Appraisal
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (S. typhimurium or E. coli). In eukaryotic systems it
gives either negative or, in one case, weakly positive results. In
vitro gene mutation assays and tests for UDS in mammalian cells were
uniformly negative. In vitro assays for chromosomal aberrations
using different cell types gave positive results, whereas negative or
equivocal results were obtained in tests for SCE induction.
The majority of the in vivo studies reported have provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay,micronucleus test or UDS assay). Where positive
responses have been seen, they are restricted to tests using B6C3F1
mice. Marginal increases in the frequencies of SCEs, chromosomal
aberrations and micronuclei in mice have been reported following
inhalation of high concentrations of methylene chloride. Increases in
hepatic DNA single strand breaks and DNA-protein crosslinks were seen
in mice, but not in rats, exposed to 14 100 mg/m3.
Within the limitations of the short-term tests currently
available, in vivo genetic activity has only been detected in tests
using B6C3F1 mice.
8.7 Chronic toxicity and carcinogenicity
8.7.1 Inhalation exposure
8.7.1.1 Rat
Groups of 95 male and 95 female Sprague-Dawley rats (8 weeks old)
were exposed by inhalation to 0, 1770, 5300 or 12 400 mg/m3
methylene chloride (99% pure) 6 h/day, 5 days per week for 2 years
(Table 20). Overall survival in the study, including that of controls,
was poor. Mortality among high-dose females was statistically
increased from the 18th month when compared to controls. Increases in
CO-Hb were found in treated groups from 6 months, but not in a dose-
related manner. From 12 months, non-neoplastic pathological effects on
the liver (increased hepatocellular vacuolization consistent with
fatty change) were observed in both males and females at all exposure
levels in a dose-related fashion. In males (5300 and 12 400 mg/m3)
and females (12 400 mg/m3), a decrease in the incidence (females)
and severity (males) of age-associated chronic progressive
glomerulonephrotoxicity was observed. The only reported increases in
tumour incidence occurred in benign mammary gland tumours in males and
females, and tumours in the mid-cervical region close to the salivary
gland in males. There was no significant increase in the proportion of
animals with benign or malignant mammary tumours; however, the total
number of benign mammary tumours showed a marginally significant dose-
related increase in males (controls, 8/95; low-dose, 6/95; mid-dose
11/95; high-dose, 17/97; p = 0.046) and a dose-related increase in the
total number of benign mammary tumours was observed in females
(165/96; 218/95; 245/95; 287/97; p < 0.001). There was no indication
of an increase in the number or incidence of malignant mammary tumours
in either males or females. The background historical incidence for
Sprague-Dawley rats in the laboratory normally exceeds 80% in females
and is about 10% in males (Burek et al., 1984).
Table 20. Carcinogenicity studies using the inhalation routes
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Rat Sprague-Dawley, Inhalation, 6 h/day, 5 0, 1770, Increase in various types of sarcomas in the Burek et al.
male and female days/week for 2 years 5300, mid-cervical area in the region of the (1984)
95 animals/sex/group 12 400 salivary gland in mid- and high-dose males
(1/92, 0/95, 5/95, 11/97). Slight dose-
related increase in total number of benign
mammary tumours in males (8/95, 6/95,
11/95, 17/97; p =0.046); dose-related
increase in total number of benign
mammary tumours in females (165/96,
218/95, 245/95, 287/97; p<O.001)
Rat Sprague-Dawley, Inhalation, 6 h/day, 5 O, 177, 710, No increase in incidence of benign Nitschke et al.
male and female days/week for 20 1770 mammary tumours in males or in females (1988a)
months (males), 24 exposed to 177 or 710 mg/m3; increased
months (females); 90 incidence of benign mammary tumours in
males/group females at 1770 mg/m3; no increase in the
198 females/group number of any malignant tumours; NOAEL
710 mg/m3
Rat Fischer-344/N, Inhalation, 6 h/day, 5 O, 3500, Dose-dependent increase in benign NTP (1986)
male and female days/week for 102 7100, mammary tumours; male: 0/50, 0/50, 2/50, Mennear et al.
weeks; 50 animals/sex 14 100 5/50; female: 5/50, 11/50, 13/50, 23/50 (1988)
group
Table 20 (Cont'd)
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Rat Sprague-Dawley, Inhalation, 4 h/day, 5 350 No effect on percentage of animals bearing Maltoni et al.
females, during days/week for 7 benign and/or malignant tumours, in (1988)
and after weeks, then 7 h/day, 5 maternal or offspring animals; no
pregnancy days per week for 97 statistically significant increase in total
weeks; malignant tumours
54 females exposed
60 female controls
Rat Sprague-Dawley, inhalation, protocol as 350 As above Maltoni et al.
male and female above for 15 and 104 (1988)
offspring from weeks; 60-70 males or
12th day in utero females/group, 158
male and 149 female
controls
Mouse B6C3F1 inhalation, 6 h/day, 5 7100 Mice exposed for more than one year Kari et al. (1992)
days/week for various showed an excess of lung and liver tumours
periods up to 104
weeks; killed at end of
exposure; 364 females
exposed, 364 female
controls
Table 20 (Cont'd)
Species Strain/sex Route of Doses Observations Reference
administration/protocol/ (mg/m3)
group size
Mouse B6C3F1 Inhalation to 6 h/day, 7100 All exposed groups showed excesses of Kari et al. (1992)
5 days/week for either lung and liver tumours
26, 52, 28 or 104
weeks; all mice
maintained for 104
weeks; 68 females
exposed, 68 female
controls
Mouse B6C3F1, male Inhalation, 6 h/day, 5 0, 7100, Dose-dependent increases in (1) NTP (1986);
and female days/week for 102 14 100 alveolar/bronchiolar adenomas Mennear et al.
weeks; 50 males: 3/50, 19/50, 24/50 (1988)
animals/sex/group females: 2/50, 23/48, 28/48
(2) alveolar/bronchiolar carcinomas
males: 2/50, 10/50, 28/50
females: 1/50, 13/48, 29/48
(3) hepatocellular adenoma and carcinoma -
combined
males: 22/50, 24/49, 33/49
females: 3/50, 16/48, 40/48
Hamster Syrian Golden, Inhalation, 6 h/day, 5 0, 1770, No significant increase in incidence of Burek et al.
male and female days/week for 2 years; 5300, benign tumours (1984)
95 animals/sex/group 12 400
Males exposed to 5300 or 12 400 mg/ma showed an increase in the
number of sarcomas in the mid-cervical area in the region of the
salivary glands (1 subcutaneous sarcoma in controls, n = 92, 2
subcutaneous sarcomas and 3 salivary gland schwannoma at 5300 mg/m3,
n = 95; and 5 subcutaneous fibrosarcomas and 2 subcutaneous
mesofibrosarcomas as well as 2 salivary gland sarcomas at
12 400 mg/m3, n = 97). A number of uncertainties affect the
toxicological significance of these observations. As stated by the
investigators, all but two of these tumours were large and appeared to
invade all adjacent tissues in the neck region. Histologically all
were sarcomas. Some tumours morphologically resembled the overall
type, fibrosarcoma while others resembled another, neurofibrosarcoma,
and still others were undifferentiated or pleomorphic. Only two
tumours were small enough to be localized in the salivary gland,
appearing to arise in interstitial and capsular tissue. Based on these
findings the author suggested that all probably arose from the
salivary gland, although appearing to arise from mesenchymal tissue
they represent a variety of tumour types. There is no explanation for
the sex difference observed. Both male and female rats in the study
had a viral disease (siabclacryoudenitis, which affects the salivary
glands) during the first 2 months of the study. The infection did not
increase mortality and all exposure groups appeared to be affected to
the same degree (Burek et al., 1984).
A later study in the same laboratory where the highest dose level
was 1770 mg/m3 revealed salivary gland sarcomas in only two animals,
a male at 1770 mg/m3 and a female at 177 mg/m3. The historical
incidence in this laboratory was reported to range from 0 to 2% in
control groups of Sprague-Dawley rats (Nitschke et al., 1988a).
Groups of 90 male and 108 female Sprague-Dawley rats (6-8 weeks
old) were exposed to 0, 177, 710 or 1770 mg/m3 6 h/day, 5 days/week
for 20 and 24 months, respectively. Exposure-related increases in
CO-Hb levels were found. Exposure-related histo-pathological changes
were limited to the liver of male and female rats and mammary glands
of female rats exposed to 1770 mg/m3. An increased incidence of
hepatocellular vacuolization was found in males and females exposed to
1770 mg/m3. Females exposed to this dose level also had an increased
incidence of multinucleated hepatocytes. The incidence of benign
mammary tumours in female rats exposed to 177, 710 and 1770 mg/m3
was comparable to historical control values (79-82%), but the number
(2.2) of benign mammary tumours per tumour-bearing female rat exposed
to 1770 mg/m3 was greater than that of controls (1.8) (p < 0.05).
There were no increases in the incidence of malignant tumours at any
site in female rats, nor of benign or malignant tumours in male rats,
exposed to methylene chloride. The no-observed-adverse-effect level
for chronic inhalation exposure of Sprague-Dawley rats was judged to
be 710 mg/m3 (Nitschke et al., 1988a).
A group of 54 pregnant Sprague-Dawley rats was exposed to
350 mg/m3 4 h/day, 5 days/week for 7 weeks and subsequently for
7 h/day, 5 days/week for a further 97 weeks. A further group of 60
rats served as controls. In addition, groups of 60-70 males or females
were exposed to 212 mg/m3 from the 12th day in utero for a total
of either 15 or 104 weeks; there were 158 male and 149 female
controls. In exposed maternal or offspring rats, methylene chloride
did not affect the percentage of animals bearing benign and/or
malignant tumours. Although there was a slight increase in total
malignant rumours in rats exposed to 350 mg/m3 for 104 weeks, this
was not deemed to be statistically significant (Maltoni et al., 1988).
The Task Group noted the absence of relevant data on the design of the
experiment such as the statistical methods, housing conditions and
histopathological techniques.
When groups of 50 male and 50 female F-344/N rats, 7-8 weeks old,
were exposed by inhalation to 0, 3500, 7100 or 14 100 mg/m3
methylene chloride (99% pure) 6 h/day, 5 days per week, for 102 weeks
(NTP, 1986), body weight gains for both exposed males and females were
comparable to those of the control group. The survival of exposed male
rats was also comparable to that of the controls, although the
survival of all groups of males at the end of the study was low.
Similarly, the survival of female rats was comparable with that of the
controls, with the exception of the high-dose group. Non-neoplastic
treatment-related increases in the incidence of renal tubular cell
degeneration, splenic fibrosis and (females only) nasal cavity
squamous metaplasia were observed.
Increased incidences of benign tumours of the mammary gland (all
fibroadenomas, except for one adenoma in the high-dose group) were
observed in treated females (control, 5/50; low-dose, 11/50; mid-dose,
13/50; high-dose, 23/50; p < 0.001). There was a positive trend in
the incidence of benign tumours of the mammary gland in males (0/50,
0/50, 2/50, 5/50; p < 0.01). The range of the historical control
incidence of mammary gland fibroadenomas in this laboratory for the
male rat was 0/50 to 6/49 and for the female rat was 5/50 to 24/49.
There were no other exposure-related increases in tumour incidence
(NTP, 1986; Mennear et al., 1988).
8.7.1.2 Mouse
Groups of 50 male and 50 female B6C3F1 mice, 8-9 weeks old, were
exposed to 0, 7100 or 14 100 mg/m3 (> 99% pure) 6 h/day,
5 days/week for 102 weeks and killed after 104 weeks of study.
Survival to the end of the study period in males was: control, 39/50;
low-dose, 24/50; and high-dose, 11/50; that in females was: 25/50,
25/49 and 8/49. This reduced survival may have been due to the
chemically induced development of liver and lung neoplasia in both
sexes, significant dose-related increases in lung and liver tumours
having been found. The incidences of alveolar/bronchiolar adenomas
were: males - 3/50, 19/50 and 24/50 (p < 0.001); and females - 2/50,
23/48 and 28/48 (p < 0.001). Those for alveolar/bronchiolar
carcinomas were: males - 2/50, 10/50 and 28/50 (p < 0.001); and
females - 1/50, 13/48 and 29/48 (p < 0.001). Incidence of
hepatocellular adenomas or carcinomas (combined) were increased in
high-dose males and dosed females (males: 22/50, 24/49 and 33/49;
females: 3/50, 16/48 and 40/48). Dose-related increases were observed
in the incidences of testicular atrophy in male mice and uterine and
ovarian atrophy in female mice; these effects were considered to be
secondary responses to neoplasia (NTP, 1986; Mennear et al., 1988).
Studies have been conducted on the B6C3F1 mouse to study the
time-dependency of exposure to methylene chloride leading to tumour
formation. Groups of 364 female B6C3F1 mice were exposed to either
air or 7100 mg/m3 methylene chloride for 6 h/day, 5 days/week for
104 weeks. Following exposure for either 462, 494, 515, 529, 571, 597,
618, 639 or 660 days, 10 exposed and 5 control mice were randomly
selected and sacrificed for the purposes of following the progression
of neoplasia in the lung and the liver. Increased incidences of
alveolar/bronchiolar adenomas and carcinomas and of hepatocellular
adenomas and carcinomas in mice exposed for 104 weeks were confirmed.
In mice sacrificed following less than one year's exposure (368 days),
there were no lung lesions observed and only minimal indications of
proliferative lesions in the liver. Mice exposed for longer than one
year showed a progressive increase in the incidence of both
alveolar/bronchiolar adenomas and carcinomas. The incidence of liver
tumours in mice exposed for longer than one year was relatively
constant (40-60%), although a time-dependant increase in the total
liver tumour burden per animal was reported (Kari et al., 1993).
Groups of 68 female B6C3F1 mice were exposed to 100 mg/m3
6 h/day, 5 days/week for either 26, 52 or 78 weeks. All groups of mice
were maintained for 104 weeks, at which time they were sacrificed. The
exposures were split into two phases, i.e. exposure to either air or
to methylene chloride for the specified period at the beginning or the
end of the total exposure period. Further groups of 68 female mice
were exposed either to air or to 7100 mg/m3 methylene chloride for
the full 104 weeks. The percentage incidences of lung adenomas or
adenocarcinomas or liver adenomas or adenocarcinomas are given in
Table 21. The percentage incidence of both lung and liver tumours in
mice exposed to methylene chloride during the first phase increased
with the duration of exposure. In mice exposed to methylene chloride
during the second phase, significant increases in the percentage
incidence of lung and liver rumours were only observed following 78
weeks of exposure to methylene chloride. The study also showed that
lung rumours appeared earlier in methylene chloride-exposed animals
than did liver tumours. Comparison of the results of this study with
those presented in the previous paragraph suggested that the
percentage incidence of lung tumours but not liver tumours increased
following withdrawal from exposure to methylene chloride, particularly
when the mutagenicity of tumours was taken into account (Kari et al.,
1993).
Table 21. The percentage incidence of lung and liver tumours in female mice exposed to
methylene chloride for different time periods
Exposurea % incidence of tumours
(adenomas or carcinomas)
Phase I Phase II Lung Liver
air 104 weeks 7.5 27
methylene chloride 26 weeks air 78 weeks 31b 40
methylene chloride 52 weeks air 52 weeks 63b 44b
methylene chloride 78 weeks air 26 weeks 56b 62b
methylene chloride 104 weeks 63b 69b
air 26 weeks methylene chloride 78 weeks 19b 48b
air 52 weeks methylene chloride 52 weeks 15 31
air 78 weeks methylene chloride 26 weeks 4 34
a exposure to methylene chloride was 7100 mg/m3, 6 h/day, 5 days/week
b statistically significant p < 0.05
8.7.1.3 Hamster
When groups of 95 male and 95 female Syrian golden hamsters
(8 weeks old) were exposed by inhalation to 0, 1770, 5300 or
12 400 mg/m3 6 h/day, 5 days/week for 2 years, no exposure-related
changes were observed in mean body weight and mean organ weights.
CO-Hb levels were increased but this effect was not dose-related. The
numbers of animals surviving to the end of the study were 16, 20, 11
and 14 for males and 0, 4, 10 and 9 for females, respectively. The
incidence of lymphosarcomas was slightly higher in treated females
than in controls (control, 1/91; low-dose, 6/92; mid-dose, 3/91; high-
dose, 7/91; p = 0.032). The increased incidence of benign tumours was
considered to be related to the higher survival of the exposed
hamsters and not a direct result of exposure to methylene chloride
(Burek et al., 1984).
8.7.2 Oral administration
8.7.2.1 Rat
Groups of 85 male and 85 female F-344 rats (7 weeks old) were
administered methylene chloride in the drinking-water at
concentrations of 0, 5, 50, 125 and 250 mg/kg per day for 104 weeks
(Table 22). Interim sacrifices were carried out at 26, 52 and 78
weeks, such that 50 males and 50 females per group received the
treatment for 104 weeks. Additional groups of 50 male and 50 female
F-344 rats received methylene chloride at a concentration of 250 mg/kg
per day for 78 weeks, with further groups of 50 male or female rats
serving as controls. Small changes in mean body weight and food/water
consumption were seen in rats receiving either 125 or 250 mg/kg per
day. Dose-related increases were noted in mean haematocrit,
haemoglobin levels and red blood cell counts at the three highest
doses. Decreases in serum alkaline phosphatase activity and in
creatinine, blood urea nitrogen, serum protein and cholesterol levels
in both sexes were found at 250 mg/kg per day. Treatment-related
histopathological changes were seen in the liver of rats receiving
250 mg/kg per day. There were no increases in the incidence of any
tumour in treated rats when compared to controls (Serota et al.,
1986a).
When groups of 50 male and 50 female Sprague-Dawley rats were
given 0, 100 or 500 mg/kg methylene chloride (purity 99.97%) by gavage
in olive oil, 4-5 days/week for 64 weeks, excess mortality was
observed in rats of each sex receiving 500 mg/kg. A slightly higher
incidence of adenocarcinomas of the mammary gland was observed in
females receiving 500 mg/kg (4/50, 3/50, and 9/50 in the controls,
low-dose and high-dose, respectively). There was no effect on the
total tumour incidence in the exposed groups (Maltoni et al., 1988).
The Task Group noted the short length of the exposure period as well
as the absence of relevant data on the design of the experiment, such
as the statistical methods applied, housing conditions,
histopathological techniques and pathology schedule.
Table 22. Carcinogenicity studies by oral route
Species Strain/type Route of Doses Observations Reference
administration/protocol/
group/size
Rat Fischer-344, Drinking-water ad libitum for 0, 5, 50, 125 No increase in incidence of neoplasms; Serota et al.
mate and female 104 weeks; 85 animals/sex or 250 mg/kg survival and other findings not affected (1986a)
per dose; scheduled kills: 5 at per day by methylene chloride; significant
26 weeks, 10 at 52 weeks, 20 decreases in body weight gain at 125
at 78 weeks; also additional and 250 mg/kg per day and evidence of
groups of controls and liver damage at doses above 50 mg/kg
250 mg/kg (50/sex) which per day
received methylene chloride
for 78 weeks only
Rat Sprague-Dawley, Gavage in olive oil for 64 0, 100 or 500 No effect on total tumour incidence in Maltoni et
male and female weeks; 50 animals/sex/dose; mg/kg per exposed rats. Higher incidence, not al. (1988)
additional control group (not day statistically significant, of malignant
dosed) of 20 males and 26 mammary tumours in high-dose
females females; survival decreased in high dose
males and females
Mouse B6C3F1, male Drinking water ad libitum for 0, 60, 125, 185 No increase in incidence of neoplasms; Serota et al.
and female 104 weeks; group size; or 250 mg/kg evidence of slight liver damage at 250 (1986b)
125 m, 100 f (controls) per day mg/kg per day
200 m, 100 f (60 mg/kg)
100 m, 50 f (125 mg/kg)
100 m, 50 f (185 mg/kg)
125 m, 50 f (250 mg/kg)
Table 22 (Cont'd)
Species Strain/type Route of Doses Observations Reference
administration/protocol/
group/size
Mouse Swiss, male and Gavage in olive oil for 64 0, 100 or Decrease in body weight in exposed Maltoni et
female weeks 500 mg/kg males and females after 36-40 weeks; al, (1988)
50 animals/sex/dose per day dose-related increase in pulmonary
60 animals/sex/controls tumours in males - not significant
without considering mortality rate;
significant (p<0.05) taking into account
the mortality rate; no treatment-related
increase in the percentage of animals
bearing benign and malignant tumours,
or of animals bearing malignant
tumours, or of the number of total
malignant tumours per 100 animals
8.7.2.2 Mouse
Groups of male and female B6C3F1 mice (7 weeks old) received
methylene chloride in the drinking-water for 104 weeks at levels of 0
(control groups 60/65 males, 50/50 females), 60 (200 males, 100
females), 125 (100 males, 50 females), 185 (100 males, 50 females) or
250 (125 males, 50 females) mg/kg per day. Histopathological changes
were observed in the liver of mice receiving 250 mg/kg per day. There
was no increase in the incidence of tumours in any of the exposed
groups, when compared to controls (Serota et al., 1986b).
Groups of 50 male and 50 female Swiss mice received either 100 or
500 mg/kg methylene chloride (purity 99.97%) in olive oil by gavage on
4-5 days/week for 64 weeks. Groups of 60 males and 60 females served
as controls and received only olive oil. In male mice dying between 52
and 78 weeks, an increase in the incidence of pulmonary adenomas was
observed (0/27 in controls, 4/41 low dose level, 7/33 high dose level)
although the effect was not statistically significant in exposed male
mice when mortality was not taken into account. There was no increase
in the total tumour burden in exposed mice. A decrease in body weight
was observed in exposed males and females, compared to controls after
weeks 36-40. No other exposure-related findings were reported (Maltoni
et al., 1988)
8.7.3 Appraisal
Methylene chloride is carcinogenic in the mouse, causing both
lung and liver tumours, following exposure to high concentrations
(7100 and 14 100 mg/m3 ). The incidence of both lung and liver
tumours was increased in mice exposed to 7100 mg/m3 for 26 weeks
and maintained for a further 78 weeks. Associated toxicity or
hyperplasia in the target organs was not observed.
Hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of
tumours in the region of the salivary gland was reported in male rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different tissues, the statistical approach of
combining tumours was inappropriate. The response was not seen in a
second study in which rats were exposed to either 3500, 7100 or
14 100 mg/m3 throughout their lifetime. A further inhalation study
in rats exposed to methylene chloride at concentrations up to
1770 mg/m3 throughout their lifetime showed no evidence of
carcinogenicity. These studies, taken together with the absence of
effect on the salivary gland in all other inhalation studies, raise
doubts regarding their biological and toxicological significance.
Rats exposed to methylene chloride via the drinking-water or by
gavage similarly showed no substantive evidence of carcinogenicity.
An increase in either the incidence or the multiplicity of benign
mammary tumours (fibroadenomas) in rats exposed to methylene chloride
via inhalation has been reported in three studies. Increases in
multiplicity were dose-related in male and female Sprague-Dawley rats
(historical incidence 10% males and 80% females). The increase in
multiplicity was observed only in females of the highest dose
(1770 mg/m3 ) group. No effects were observed in males or the other
dose groups of females. A dose-related increased incidence in benign
mammary tumours was observed in female F-344/N rats, although the
incidences were in the range of historical incidence (10 to 25%). An
increased incidence in high dose (14 100 mg/m3 ) males was also
within the historical incidence range of 0 to 11%.
In one study, a slight increase in the incidence of
adenocarcinomas in the mammary gland was observed in female Sprague-
Dawley rats receiving 500 mg/kg by the oral route. A study in
Fischer-344 rats with dose levels up to 250 mg/kg by the oral route
(drinking-water) showed no carcinogenic effects.
No increases in mammary tumours were observed in the mouse or
hamster by inhalation or oral administration.
8.8 Mechanistic studies
8.8.1 In vitro metabolic studies
There are three transient reactive intermediates in the metabolism
of methylene chloride. Two of them, formyl chloride and
S-chloromethyl-glutathione, are assumed to be present on the basis
of knowledge of the metabolic pathways; the third, formaldehyde, has
been identified in vitro. All three have the reactivity necessary to
bind covalently to macromolecules. Of these S-chloromethyl-
glutathione is potentially the most potent alkylating agent, a
conclusion based on the known reactivity of the halothioethers (Bohme
et al., 1949), structural similarities to the mutagenic glutathione
conjugates of the 1,2-dihaloethanes, and on the outcome of several
studies using different liver fractions in the Salmonella mutation
assay (Jongen et al., 1982; Green, 1983). Formyl chloride is highly
unstable, existing chemically only at low temperatures (-80°C) in
inert solvents (Staab & Datta, 1964). Formaldehyde is a common
metabolic product in vivo which is efficiently metabolized in the
liver to formic acid. The endogenous formation and metabolism of
formaldehyde occurs at a high rate, and the additional formaldehyde
derived from methylene would be metabolized by the same efficient
pathways.
Comparisons of the rates of metabolism of methylene chloride by
each pathway in liver fractions from rats, mice, hamsters and man have
been carried out (Green et al., 1986b,c; Reitz et al., 1989). These
experiments demonstrated that the rates of metabolism in these
pathways in vitro had similar differences to those seen in vivo in
rats and mice, and enabled a comparison to be made with those species
(hamster and man) where in vivo data was not available.
Rates of metabolism in human liver have been measured for both
glutathione- S-transferase (Green et al., 1987a,b,c; Reitz et al.,
1989; Bogaards et al., 1993) and cytochrome P-450 (Green et al.,
1987a,b,c; Reitz et al., 1989) pathways of methylene chloride
metabolism. A total of 33 human liver samples have been assayed for
glutathione- S-transferase activity and a range of activities
reported. In the work by Bogaards et al. (1993), 3 samples had no
activity, a further group of 11 had activity in the range of 0.20-0.41
(mean 0.31 ± 0.08) nmol/min per mg protein, and 8 samples had activity
in the range 0.82-1.23 (1.03 ± 0.14) nmol/min per mg protein. The
rates measured by Green et al. (1987a,b,c) were within the range found
by Bogaards et al., (1993) (0.05-0.93 nmol/min per mg protein; mean
0.42 ± 0.32; n = 7) and those found by Reitz et al. (1989) were
slightly higher (range 0-3.03 nmol/min per mg; mean 2.09 ± 1.40). The
activity in all the samples assayed was at least 1.4 lower than that
in rat liver cytosol. Two human lung samples were assayed and found to
be lacking in glutathione- S-transferase activity (Reitz et al.,
1989). However, enzyme activities of this type are significantly lower
in the lung than the liver, and any such activity may not be detected
by the currently available assays.
The 10-fold difference in glutathione- S-transferase activity
measured in vivo in mice and rats was also found in vitro. There
is an excellent correlation between glutathione- S-transferase
metabolism and the outcome of the 2-year cancer studies in the three
animal species. More support for this correlation was obtained in DNA
damage tests (section 8.6.2.3.) No such correlation exists for the
cytochrome P-450 pathway where, for example, the metabolic rate in the
hamster is very similar to that in the mouse. Cytochrome P-450-
catalysed metabolism of methylene chloride could be detected in lung
tissue from all three animal species, the relative activities being
similar to those in the livers. Glutathione- S-transferase activity
was detectable only in mouse lung fractions.
The low rates of metabolism of methylene chloride by the
glutathione- S-transferase pathway in human liver samples has been
attributed to a deficiency in the transferase isoenzyme responsible.
The same liver samples had similar activity to rat liver when assayed
with an alternative substrate for these enzymes (Green et al.,
1986b,c).
8.8.2 In vivo metabolic studies
A comparative kinetic profile of methylene chloride and its
metabolites was determined in B6C3F1 mice and F-344 rats both during
and after a 6-h exposure to atmospheres containing various
concentrations from 350 to 14 100 mg/m3 (Green et al., 1986b,
1987a,b,c, 1988). Blood levels of methylene chloride and CO-Hb and the
rates of elimination of methylene chloride, carbon monoxide and carbon
dioxide in exhaled air were measured. Stable isotopes were used to
quantify the amount of carbon dioxide from each pathway at dose levels
of 350, 1770 and 14 100 mg/m3, but only in the mouse. The steady-
state blood levels of methylene chloride during exposure were up to
5 times higher in rats than in mice at the higher dose levels. A
comparison of the CO-Hb levels in blood and carbon monoxide levels in
expired air showed that rate of metabolism by the cytochrome P-450
pathway was similar in both rats and mice. The pathway was saturated
in both species at exposures of less that 1770 mg/m3, resulting in
maximal CO-Hb levels of 16% (Green et al., 1987a,b,c).
Saturation of the cytochrome P-450 pathway in mice was also
clearly shown by a 5-10 fold increase in the blood levels of methylene
chloride when the inhaled concentration was doubled from 1770 to
3500 mg/m3 (Green et al., 1987a,b,c).
The stable isotope studies demonstrated that the cytochrome P-450
pathway was the major source of carbon dioxide at low exposure levels
(350 mg/m3) whereas at high levels ( 14 100 mg/m3) the
glutathione- S-transferase pathway was the principal source of carbon
dioxide. A comparison of the rate of elimination of the carbon dioxide
by rats and mice at the top dose level showed the glutathione- S-
transferase pathway to be 10-12 times more active in mice than rats.
The higher rate of metabolic conversion of methylene chloride by mice
when compared to rats largely accounts for the low blood levels of the
parent chemical in this species.
In summary, the in vitro and in vivo studies have provided
evidence for the following:
1. The cytochrome P-450 pathway is saturated at 1770 mg/m3 and is
quantitatively similar in rats and mice in vivo and in rat,
mouse, hamster and human livers in vitro.
2. The glutathione- S-transferase pathway is a major pathway only in
mice, its activity at the 14 100 mg/m3 dose level being an order
of magnitude greater than in rats.
3. In all, 33 human liver samples have been assayed for glutathione-
S-transferase activity. In all cases the levels of activity were
lower than those measured in rat liver.
4. Methylene chloride metabolism is dose-dependent. The utilization
of the two pathways is significantly different at the dose levels
used in the carcinogenicity studies than at low dose levels.
5. These studies provided the metabolic rate constants used in the
physiologically based pharmacokinetic models described in section
8.9.
8.8.3 Pulmonary effects
A number of studies have examined the effects of methylene
chloride on the mouse and rat lung (Eisenbrandt & Reitz, 1986; Hext et
al., 1986; Green et al., 1987a, Foster et al., 1992; Kanno et al.,
1993). Following a single exposure at concentrations of 7100 mg/m3
or more, a specific lesion characterized by marked vacuolization of
Clara cells were seen in the mouse, but not the rat. No other cell
types were affected in the mouse (Green et al., 1987a; Foster et al.,
1992). The morphological damage in Clara cells recovered after 5 days
of repeated 6-h exposures (Foster et al., 1992). The damage to Clara
cells was accompanied by a change in the ability to metabolize
methylene chloride by the two pathways. Cytochrome P-450 metabolism
was suppressed while glutathione- S-transferase remained unchanged.
In a thirteen week study (Foster et al., 1992), damage to the Clara
cells was seen following the first exposure of each week of the study.
Between days 2 and 9 of this study, a significant increase in the
number of cells in S-phase was observed in both bronchiolar and
alveolar epithelium. A similar study (Kanno et al., 1993) failed to
detect an increase in the number of cells in S-phase.
Significant pulmonary lesions were observed in male B6C3F1 mice
1 day after a single 6-h exposure to 14 100 mg/m3 (Eisenbrandt &
Reitz, 1986). Necrosis of the epithelial cells in the bronchi and
bronchioles were observed. Non-ciliated (Clara) cells were swollen and
vacuolated.
8.8.4 Studies on oncogene activation
Further studies have been conducted on the B6C3F1 mouse into the
role of oncogene activation as a potential mechanism of action of the
carcinogenic effect of methylene chloride. A group of 145 female
B6C3F1 mice was exposed to 7100 mg/m3 for 6 h/day, 5 days/week for
up to 27 months. Another group of 235 females acted as controls. These
mice were used for the purpose of providing spontaneous and methylene-
chloride-induced tumour tissue from both the liver and the lung for
the analysis of proto-oncogene activation and tumour suppressor gene
inactivation. The DNA recovered from 54 methylene-chloride-induced
B6C3F1 lung tumours and from 7 spontaneous B6C3F1 lung tumours was
analysed by the direct sequencing of PCR (polymerase chain reaction)
amplified DNA fragments of the K-ras gene for first and second exon
mutations. Twelve mutations were identified in the tumours from
exposed mice, 5 in exon one and 7 in exon two. There was no difference
in the frequency of K-ras activation in tumour tissue derived from
both exposed mice when compared to controls. DNA was isolated from 49
spontaneous and 50 methylene-chloride-induced liver tumours and
screened by the oligonucleotide hybridization of PCR (polymerase chain
reaction) amplified H-ras gene fragments for codon 61 mutations. The
mutation profile of the H-ras gene was similar in the tumour tissue
derived from both control and treated mice (Devereux et al., 1993).
Mutations of the p53 rumour suppresser gene were examined in lung
tumours from female mice exposed to 7100 mg/m3 (2000 ppm) methylene
chloride for 2 years (Hegi et al., 1993). The limited number of p53
mutations identified in this study and the small number of spontaneous
tumours precluded any conclusions concerning the mutagenic spectrum or
possible genotoxicity of methylene chloride.
8.8.5 The use of mechanistic studies in extrapolation
Studies using liver fractions from rats, mice, hamsters and humans
have confirmed the existence of two pathways for the metabolism of
methylene chloride (the cytochrome P-450 pathway and the glutathione-
S-transferase pathway) and have established substantial differences
between species in the utilization of these pathways. The rates of
metabolism of methylene chloride by the two enzymes in the liver
fractions from a few species have been established. The activities of
the cytochrome P-450 pathway in the mouse and hamster were similar,
whereas those in the rat and human were lower. In marked contrast, the
activity of the glutathione- S-transferase in the mouse was very high
when compared with the other species; there being a 10-fold difference
in activity between the mouse and the rat. Rates of metabolism by this
pathway in hamsters and humans were even lower than in rats.
The results of a full pharmacokinetic analysis of the behaviour of
methylene chloride and its metabolites in vivo were consistent with
the species differences observed in vitro. Saturation of the
cytochrome P-450 pathway occurred in rats and mice at dose levels of
less than 1770 mg/m3 and resulted in maximal CO-Hb levels of 16% in
both species. Comparisons of the glutathion- S-transferase pathway
based on expired carbon dioxide levels at high exposure concentrations
found the same 10- to 12-fold difference between mice and rats that
had been observed in vitro. The higher metabolic rates in mice
accounted for the lower blood levels seen in this species compared to
the rat.
Exposure of mice to atmospheric concentrations of 7100 mg/m3 or
more led to recurrent cytotoxicity, increases in DNA synthesis
( S-phase) and changes in the metabolic complement of mouse lung
Clara cells. All the effects were specific to the mouse and to the
Clara cells. Several of the changes (cytotoxicity and S-phase) were
frequently associated with the development of tumours. However the
significance of these findings with respect to the development of lung
tumours in mice exposed to methylene chloride remains to be
established. Effects on chromosomes have also been reported in the
mouse lung.
Studies on the potential role of activation of Ras oncogenes in
the development of methylene-chloride-induced lung and liver tumours
have been unable to distinguish between the tumours seen in methylene-
chloride-treated animals and those occurring spontaneously in control
animals.
Studies in which mice were exposed for different intervals of a
2-year carcinogenicity study established that lung tumours developed
quicker and after shorter exposures than liver tumours. Whether this
indicates a different mechanism in the lung from the liver or reflects
the cytotoxicity and cell division seen in the lungs of mice is
unknown at the present time.
The more recent studies in bacteria using glutathione-deficient
strains and strains in which mammalian transferase enzymes have been
expressed have established the role of this pathway in mutagenesis.
Consistent with this are findings of DNA single strand breaks and DNA-
protein cross-links in the livers of mice, but not rats or hamsters,
exposed in vivo to 14 000 mg/m3. Both the single strand beaks and
cross-links have been shown to be derived from metabolites of the
glutathione- S-transferase pathway. At the present time, these
effects have not been demonstrated in mouse lung.
There is a consistency between the bacterial mutagenicity assays,
the pharmacokinetic data and the studies of DNA single strand breaks
and DNA protein cross-links which leads to the conclusion that the
liver tumours seen in mice are derived from an interaction between
metabolites of the glutathione- S-transferase pathway and DNA. The
same level of detail is not available for the mouse lung. However the
responses seen in the Clara cells and in mouse lung chromosomes are
not inconsistent with this mechanism.
The studies of the comparative metabolism and pharmaco-kinetics of
methylene chloride in the rat, mouse and hamster also provided a
plausible explanation for the species differences in the carcino-
genicity of this chemical. The differing metabolic rates by the
glutathione- S-transferase pathway are consistent with the outcome of
the cancer studies whereas the blood levels of the parent chemical and
the metabolic rates by the cytochrome P-450 pathway are not. These
results are also consistent with the different responses seen in the
three mouse cancer bioassays (NTP, 1986; Serota et al. 1986b; Maltoni
et al., 1986). At the high dose levels used in the NTP study, the
glutathione- S-transferase pathway would have been the major
metabolic pathway and high tumour incidences were observed. At the
lower dose levels used by Serota et al. (1986b) and Maltoni et al.
(1986), methylene chloride would have been metabolized mainly by
cytochrome P-450, and glutathione- S-transferase metabolism would
have been minimal. Consequently there were no significant increases in
either lung or liver tumours in these studies.
8.8.6 Mammary tumour promotion
The dependence of mammary tumours upon pituitary hormones in both
male and female rats has been established unequivocally (Welsch &
Nagasawa, 1977; Welsch 1985). In the rat, prolactin acts as a promoter
of mammary carcinogenesis. There is good evidence that increased
prolactin levels increase the incidence of mammary tumours (Welsch et
al., 1970), and there is a positive correlation between elevated blood
prolactin levels and mammary rumours in aged R-Amsterdam female rats
(Kwa et al., 1974).
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin (Breslin & Landry, 1986). When male and female Sprague-
Dawley rats were exposed to 10 600 mg/m3 (3000 ppm) methylene
chloride for 15 to 19 consecutive days, a significant increase (2.3 x)
in basal serum prolactin levels was observed in female rats. No
significant effect was observed in male rats (Breslin & Landry, 1986).
In humans, there is conflicting evidence on whether or not mammary
rumours are as responsive to prolactin as in the case of rats (Sinha,
1981). The rat has elevated levels of prolactin when fed ad libitum
in comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so easily responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels.
The presence of all three factors that causes tubular-alveolar growth
of the mammary glands may ultimately lead to tumour development.
Prolactin is not luteotrophic in primates (Neumann, 1991).
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will probably occur only at doses of
methylene chloride which affect prolactin levels. There is no direct
information on prolactin levels in rats receiving low doses of
methylene chloride, but no increase in mammary adenomas has been
observed following the administration of low doses in either
inhalation or drinking-water studies (i.e., below 250 mg/kg body
weight or 1770 mg/m3).
8.8.7 Appraisal
In vitro and in vivo metabolism and biochemical studies and
mutagenicity assays in bacteria and B6C3F1 mice have provided a
plausible explanation for the mechanism of action and the species
differences in the carcinogenicity of methylene chloride to the lung
and liver. This explanation is based on the existence of an isoenzyme
of glutathione-S- transferase which specifically metabolizes
methylene chloride to the reactive intermediates responsible for
tumour induction in the mouse. Markedly lower levels of this enzyme
in rats and hamsters are consistent with the fact that these tumours
do not appear in these species. The levels of the enzyme are lower in
human liver than those of the rat or hamster.
Mutagenicity studies on methylene chloride in bacteria and in the
B6C3F1 mouse, which shows a very high level of activity of the
isoenzyme, reveal positive effects, whereas mutagenicity has not been
demonstrated in standard in vivo mutagenicity assays using other
systems. These observations are consistent with the above hypothesis
and provide a mechanistic basis for the induction of tumours in the
mouse.
The role of the glutathione-S- transferase isoenzyme in the
mediation of the demonstrated mutagenic effects, and the correlation
between the activity of this pathway and the species differences in
carcinogenic response in lung and liver, has led to its use as the
dose surrogate in physiologically based pharmacokinetic models used
for human health risk assessment.
The pharmacokinetics of methylene chloride and the response seen
in B6C3F1 mice suggest that this species is a poor model on which
to base human hazard assessment to methylene chloride.
The mechanism of mammary tumour formation in the rat is probably
related to the effect of methylene chloride on prolactin levels in
this species.
8.9 Interspecies and dose extrapolations by kinetic modelling
Two physiologically based pharmacokinetic (PB-PK) models (Andersen
et al., 1987; ECETOC, 1988) have been developed and provide
quantitative estimates of the levels of methylene chloride metabolites
in four mammalian species (mice, rats, hamsters and humans) following
inhalation exposure. The models use information on various
physiological parameters and metabolic constants for the cytochrome
P-450 and glutathione- S-transferase pathways in the lung and liver,
measured in vitro for four species and measured in vivo for the
mouse, with values for rats, hamsters and humans scaled from the mouse
data. The metabolic constants for these models were obtained from the
data described in section 8.8 and from gas uptake studies described by
Andersen et al. (1987). The models were validated against other human
experimental data such as the elimination of carbon monoxide and blood
levels of methylene chloride. Time-course concentration data from the
model were compared to experimental results in F-344 rats, Syrian
Golden hamsters, B6C3F1 mice and human volunteers. The predicted
values for each of the four species were in agreement with the
experimental data. The models were also shown to predict the
appearance and elimination of methylene chloride metabolites. A
similar model was used by Andersen et al. (1991) to predict the time
course of the disappearance of CO-Hb after exposure to methylene
chloride. This model was also shown to predict CO-Hb levels in rats
and humans exposed to methylene chloride.
Several authors have discussed the assumptions, range and
variability of the data used to construct these models. While these
kinetic models have been extremely useful in improving the
characterization of human exposure and potential risk, it should be
recognized that they are based on a set of assumptions with varying
degrees of certainty. Portier & Kaplan (1989) investigated the impact
of varying the intra-population values of the biological parameters
used in the model developed by Andersen et al. (1987) using Monte
Carlo and resampling statistical methods. The results from this
analysis indicated that the estimates of "effective" doses in humans
may vary widely if variability of the parameters is taken into account
in the PBPK model.
Dankovic & Baiter (1993) investigated the impact of exercise and
human inter-subject variability on the estimates of dose derived from
the PBPK model. The model developed by Andersen et al. (1987) and
Reitz et al. (1989) assumed resting values for the parameters
governing cardiac output, alveolar ventilation and blood flow to the
tissues. The authors modified these parameters to reflect light
working conditions. The metabolic parameters for humans used in the
Andersen et al. (1987) model were based on the average of four
individual liver samples. The authors examined the impact of using the
individual values rather than the average value in the PBPK model.
Modifying the physiological parameters to reflect light work
conditions increased the glutathion- S-transferase pathway metabolic
contribution by a factor of 2.9 for the liver and 2.4 for the lung.
When the model was also modified to reflect metabolic inter-individual
(n = 4) variability in humans, the glutathione- S-transferase pathway
contribution estimates were increased by as much as 5.4-fold for the
liver and 3.6-fold for the lung. More recent data have become
available on human metabolic parameters, and are summarized in section
8.8.1. Based on 33 individual liver samples, they suggest that the
Portier & Kaplan estimates which assume 200% variation for metabolic
variability may be exaggerated. Since Dankovik & Bailer's calculations
were based on the four actual values, their estimates would not
change; however, three of their values represent the three highest
values observed for human glutathione- S-transferase activity.
9. EFFECTS ON HUMANS
9.1 General population exposure
9.1.1 Environmental exposure
Bell et al. (1991) conducted a study to examine the relationship
between birth weight in Monroe County and exposure to emissions of
methylene chloride from manufacturing processes of the Eastman Kodak
Company in Rochester, New York, USA. County census tracts were
categorized as high, moderate, low or no methylene chloride exposure,
based on the Kodak Air Monitoring Program. Birth weight and
information on variables known to influence birth weight were obtained
from 91 302 birth certificates of white, single births to Monroe
County residents from 1976 to 1987. At the level of methylene chloride
exposure (highest predicted average concentration, 50 µg/m3), no
significant adverse effect of exposure on birth weight was found,
although several problems in the method of estimation of exposure were
identified.
9.1.2 Oral exposure
A 56-year-old woman was found deeply unconscious and cyanosed
after ingesting approximately 300 ml of a paint remover containing
mainly methylene chloride and methanol. Approximately one hour after
ingestion the CO-Hb level measured was found to be 9%. This level
varied between 2.5-12% over the following 2 days and dropped below 1%
thereafter. The woman regained consciousness after 14 h, but over the
following 3 weeks her condition was complicated by progressive renal
failure, pneumonia, pancreatitis, on-going gastrointestinal
haemorrhage and sepsis, which eventually led to death some 25 days
following ingestion. It was considered that the corrosive properties
of the formulation rather than the formation of CO-Hb were responsible
for the lethal outcome (Hughes & Tracey, 1993).
In an earlier poisoning case with the same paint remover
formulation, there was recovery after ingestion of 0.5-1 litre
(Roberts & Marshall, 1976).
9.2 Occupational exposure
9.2.1 Short-term exposure
9.2.1.1 Case studies
A number of case-reports have been published regarding short-term
exposure to methylene chloride in the occupational environment.
Hall & Rumack (1990) described four cases of serious methylene
chloride poisoning, including two fatalities, in small-scale
furniture-stripping shops in Denver, Colorado, USA. In the three
patients discovered while still alive, cardiac irregularities were
recorded. Corneal burns with first- and/or second-degree burns were
reported in areas having direct contact with the methylene chloride-
based paint-stripping compound, and measured CO-Hb levels did not
exceed 8.6%. In each case, no respiratory protection was worn and
ventilation was inadequate, but exposure levels were not known. The
authors concluded that the toxic effects were due to the anaesthetic
properties of methylene chloride.
A 67-year-old male who had been using a paint stripper in a poorly
ventilated location was brought to a hospital emergency room
complaining of headache and chest pain; he was also confused,
disorientated, had a progressive loss of mental alertness, increased
fatigue and lethargy, slurred speech, little recall of either recent
or past events, and was disorientated to time (ATSDR, 1993).
These and other case studies of methylene chloride poisoning
during paint-stripping operations have demonstrated that inhalation
can be fatal to humans (Hall & Rumack, 1990; Novak & Hain, 1990;
Leikin et al., 1990; Manno et al., 1992). In the majority of cases
reported, quantitative estimates of exposure levels have not been
reported, although methylene chloride was detected in various tissues.
In one case (Manno et al., 1989), air samples collected a few hours
later from a well in which two men were found dead, were analysed by
gas chromatography/mass spectrometry and were found to contain up to
583 mg methylene chloride/litre and much lower or trace amounts of
other solvents; blood levels collected at necropsy contained 571 and
601 mg methylene chloride/litre and only trace to a few mg/litre of
other solvents. The CO-Hb levels were about 30% in blood taken 24 h
after death.
In most cases, the cause of death was not clarified. However, in
the report of five victims, including two deaths, described by Leikin
et al. (1990), the authors concluded that the cause of death was due
to solvent-induced narcosis and not carbon monoxide poisoning.
Signs of CNS depression, narcosis, irritation of the eyes and
respiratory tract, lung oedema and sometimes death were found after
accidental exposures to methylene chloride or paint remover containing
this compound (Moskowitz & Shapiro, 1952; Hughes, 1954; Bonventre et
al., 1977; Fagin et al., 1980). Three myocardial infarctions in one
subject were reported to have followed three exposures to a paint
remover containing methylene chloride over a period of approximately 8
months. The subject was exposed in a poorly ventilated room, and
concentrations were up to 4511 mg/m3 in the breathing zones (Stewart
et al., 1976). Electrocardiographic changes resembling those after
carbon monoxide poisoning were found in an exposed man with a history
suggesting ischaemic heart disease (Benzon et al., 1978). Three
probable cases of phosgene poisoning occurred after the use of
methylene-chloride-based paint remover near a source of heat
(Gerritsen & Buschmann, 1960; English, 1964).
A serious case of pulmonary oedema with bilateral exudative
pleural effusions was reported in a 34-year-old man who presented with
respiratory distress. Buie et al. (1986) speculated that hydrochloric
acid, a product of dichloromethane under warm, moist conditions, may
have played a role in this patient's parenchymal abnormalities.
Recovery was complete with the exception of neuropsychiatric
abnormalities thought to be related to exposure to methylene chloride.
Miller et al. (1985) reported the case of a 19-year-old man using
a tile remover, again in a poorly ventilated room. This patient
presented with an array of signs and symptoms ranging from liver
enzyme elevations to poorly localized abdominal pain. Renal studies
and biopsy confirmed the diagnosis of acute tubular necrosis.
Histological studies demonstrated plasma membrane changes in addition
to mitochondrial effects suggestive of anoxic damage. Serum enzyme
changes noted during the patient's stay in hospital suggested that
hepatocellular injury accompanied the nephrotoxic sequelae.
Another case of chemically induced hepatitis resulting from
accidental exposure to methylene chloride alone has been described by
Cordes et al. (1988). The liver was palpable but not enlarged or
tender. The results of initial tests were normal except for a
leukocyte count of 4900 µl with a left shift, and elevated serum
enzyme levels of alkaline phosphatase 142, lactic dehydrogenase and
serum aspartate aminotransferase (ALAT). Five days after admission,
the patient was discharged from hospital. Laboratory tests for
hepatitis A and B antibodies were negative.
Further evidence for hepatic effects of methylene chloride were
reported by Puurunen & Sotaniemi (1985). One week after a brief but
extensive body exposure to methylene chloride, serum ALAT was elevated
three-fold in a 24-year-old male chemical factory worker. The serum
ALAT returned to normal within 2 weeks.
9.2.1.2 Skin and eye effects
The irritating action of methylene chloride on the eyes and skin
has been shown in several cases (see section 9.2.1.1).
Slight erythema was found when methylene-chloride-containing
aerosol-spray deodorants were used twice a day for 12 weeks by 75 men
and women (Meltzer et al., 1977). On direct contact, methylene
chloride caused a burning sensation and pain (Stewart & Dodd, 1964).
Weber et al. (1990) reported a case of an individual who fell into
a vat containing methylene chloride and methanol. After being immersed
for about 15 min, the subject suffered extensive lesions, including
skin burns of superficial and deep severe epidermal damage and a
severe kerato-conjunctivitis.
Wells & Waldron (1984) briefly reported on a young employee who
climbed into a small open vessel with a bucket of about two litres of
methylene chloride in order to clean the walls. The concentration of
methylene chloride vapour within the vessel built up and he became
unconscious, overturning the bucket as he slumped into the bottom of
the vessel. After about 30 min, the man was rescued. During the time
that he was in the vessel, the man sustained second and third degree
burns to both legs, the areas affected being those which were bearing
the weight of his body while he was unconscious. On his discharge from
hospital, these areas were dry and required no skin grafting (Wells &
Waldron, 1984).
9.2.1.3 Laboratory studies
Neurobehavioural changes were observed at low exposure level after
volunteers were exposed to 694 mg/m3 for 1.5-3 h. Vigilance
disturbance and impaired combined tracking monitoring performance were
found (Putz et al., 1976). The critical flicker frequency, one of the
measures for visual function, was reduced after 95 min of exposure to
1040 mg/m3 (Fodor & Winneke, 1971). Visually evoked responses (one
of the surrogate methods of measuring visual functions) were altered
after 1 h of exposure to 2400 mg/m3, while exposed subjects
experienced lightheadedness. Blood and urine variables, except CO-Hb
levels, were normal in this study after 1-2 h of exposure to levels of
methylene chloride between 739 and 3420 mg/m3. No eye, nose, or
throat irritation was observed (Stewart et al., 1972). Most
neurobehavioural effects observed were less pronounced or absent, with
carbon monoxide exposures resulting in comparable CO-Hb levels (Putz
et al., 1976).
In a double-blind laboratory experiment, a short inhalation
exposure to 2.5 mg methylene chloride/litre did not impair vigilance
performance in human volunteers (time of exposure and number of
subjects not stated) (Kozena et al., 1990).
A clinical laboratory evaluation of 266 exposed volunteer workers
and 251 reference volunteer workers from two cellulose di- and
tri-acetate plants in the USA, which took into account smoking habits,
race, sex, age, intensity of exposure, and time of venepuncture,
revealed increases in red cell counts, haemoglobin levels and
haematocrit among white women exposed to a methylene chloride level of
approximately 1650 mg/m3. CO-Hb levels were elevated in all exposed
groups at all exposure levels (section 5.3). A dose-related increase
was observed in serum bilirubin for exposed subjects of both sexes. A
group of 24 exposed male volunteers and 26 reference male volunteers
from the above two industries was also selected for 24-h electro-
cardiographic monitoring. Three exposed and 8 reference workers had
reported a history of heart disease. Neither increased ventricular or
supraventricular ectopic activity nor increased episodic ST-segment
depression was found to be associated with methylene chloride exposure
(Ott et al., 1983).
9.2.2 Long-term exposure
9.2.2.1 Case studies
Irreversible damage to the central nervous system with acoustic
and optical illusions and hallucinations was diagnosed in one man who
had been exposed for 5 years to methylene chloride at levels ranging
from 2290 to 12 500 mg/m3 (Weiss, 1967). Another man, exposed for
3 years to levels of methylene chloride ranging from 1735 to
3470 mg/m3 showed a bilateral temporal lobe degeneration
(Barrowcliff & Knell, 1979). A case of delirium and seizures was
reported in a man who was exposed to methylene chloride for 4 years.
The man reported a 12-month history of intermittent headache, nausea,
blurred vision, shortness of breath, and transient memory
disturbances. Neuropsychological and EEG examinations revealed a
dysfunction of the right hemisphere. All symptoms and signs cleared
with removal from the workplace (Tariot, 1983).
Between December 1984 and June 1986, 34 men with occupational
exposure to methylene chloride were evaluated at the Greater
Cincinnati Occupational Health Centre. The mean value of the exposure
was reported to be 240 mg/m3, ranging from 11 to 544 mg/m3.
Although the primary complaint of these employees involved problems
associated with central nervous dysfunction, 8 of the 34 complained of
testicular, epididymal or lower abdominal pain, and had clinical
histories relating to infertility. Low sperm counts were reported in
workers who used methylene chloride in bonding operations which also
resulted in possible skin exposure. It is uncertain whether the effect
was due to methylene chloride since the workers were also exposed to
other chemicals (Kelly, 1988).
a) Morbidity studies
The few reports available deal with small groups of occupationally
exposed subjects.
Workers exposed occupationally to a time-weighted average of
114 mg/m3 had CO-Hb levels of between 0.8 and 2.5%. No effects were
found on clinical chemistry, haematology or electrocardio-grams (Di
Vincenzo & Kaplan, 1981a). Cherry et al. (1981) did not find any
exposure-related, long-term damage in 29 subjects as shown by
subjective symptoms, neurobehavioural tests, motor nerve conduction
velocity, electrocardiograms and clinical examinations. The men had
been exposed for several years to levels of methylene chloride ranging
from 260 to 347 mg/m3. Age-matched controls were used. In a study
without a control group, neurasthenic disorders and irritation of the
eyes and respiratory passages were experienced by half of the 33
workers exposed to methylene chloride for an average of 2 years.
Digestive disorders were reported by one-third of these workers.
Formic acid was found in the urine. No other deviations were found
during the internal, nervous system, eye and laboratory examinations.
The methylene chloride concentrations measured varied between 100 and
17 000 mg/m3 (Kuzelova & Vlasak, 1966).
A group of 1758 retired airline maintenance workers was surveyed
by mail and telephone to identify a cohort of workers with more than
22 years of methylene chloride exposure following the stripping of
paint from airplanes. A cohort of 25 exposed and 21 non-exposed
retirees met the criteria and were tested extensively (Becker & Lash,
1990; Lash et al., 1991). Following a specially prepared battery of
neuropsychological and neurophysio-logical tests performed by
professionals without prior knowledge of exposure status of the
employees, exposed and control outcome measures were all within the
"normal" range. No statistically significant difference was found
between exposed and control groups, although subtle differences in
attention and memory were detected.
In 46 subjects exposed to methylene chloride concentrations of
6-34 mg/m3 for several years, an excess (not significant) of
digestive disorders and hypotonia was found over controls, while
symptoms of gall bladder pathology and swollen liver were frequent. No
details were given concerning drinking or smoking habits (Kashin et
al., 1980).
A case-control study on 44 women who had a spontaneous abortion
was performed within a cohort of female workers employed in Finnish
pharmaceutical factories during 1973 or 1975 to 1980. Three controls
matched for age at conception within 2.5 years were chosen for every
case except two. Information about pregnancy outcome was collected
from hospital data, and data on exposures from health personnel at the
factories. The odds ratio for methylene chloride exposure, based on 11
exposed cases, was of borderline significance (2.3 with a 95%
confidence interval, 1.0-5.7; p = 0.06). Odds ratios were also
increased for exposures to many other solvents. For those exposed to
methylene chloride less than once a week the odds ratio was 2.0 (95%
CI = 0.6-6.6); whereas for those exposed more than once a week the
odds ratio was 2.8 (95% CI = 0.8-9.5) (Taskinen et al., 1986).
A group of active workers (n = 150) who had worked for at least 10
years in an area where average exposures were 1677 mg/m3 were
compared to an unexposed group of workers (n = 260) with regards to
symptoms and blood chemistry. The methylene chloride workers were also
exposed to acetone and methanol (900 ppm and 100 ppm 8-h TWAs,
respectively). Health history and blood samples had been collected as
part of a company-sponsored health monitoring programme in which both
exposed and unexposed workers were participants. No remarkable or
statistically significant differences were observed in the selected
symptoms (including irregular heartbeat, dizziness or loss of memory)
or in SGPT, bilirubin or haematocrit. The only noticeable difference
was in SGOT, where the non-exposed group had higher levels than the
exposed group (means of 28.2 versus 25.1, p = 0.06). A limitation to
this study is that both groups consisted of active healthy workers.
The age and sex distribution of the two groups was reported to be
similar, but was not given Soden, 1993).
b) Mortality studies
Several studies have evaluated the effects of long-term exposure
to methylene chloride on mortality of workers. The first study was by
Friedlander et al. (1978), who performed both a retrospective cohort
mortality, i.e. standardized mortality ratio (SMR) study, and a
proportionate mortality ratio (PMR) study of men exposed to methylene
chloride at a Kodak photographic film production facility in
Rochester, New York. The PMR study included 334 deaths that occurred
between 1956 and 1976 among former workers who were exposed to
methylene chloride at the facility. The retrospective cohort mortality
study included 751 workers employed during 1964 and involved follow-up
of this cohort up to 1976. Hearne & Friedlander (1981) extended the
follow-up of this cohort to 1980 and subsequently expanded the study
population to include all workers (n = 1013) who were exposed for at
least one year between 1964 and 1970 (Hearne et al., 1987). In a more
recent publication Hearne et al. (1990) extended follow-up of this
expanded cohort to 1988.
The Friedlander et al. (1978) study was initially conducted to
test the hypothesis that exposure to methylene chloride increases the
risk of ischaemic heart disease. This hypothesis was based on the fact
that methylene chloride is metabolized to carbon monoxide and induces
the formation of CO-Hb in humans (Stewart et al., 1972). Increases in
CO-Hb as low as 2% (Allred et al., 1989) have been shown to induce
electrocardiographic changes in exercising patients with pre-existing
coronary artery disease. An excess of ischaemic heart disease
mortality has also been reported in a cohort of tunnel workers exposed
to carbon monoxide (Stern et al., 1988). Liver and lung cancer were
also considered a priori hypotheses in the subsequent articles by
Hearne et al., based on the results from the animal bioassay data
described in chapter 8.
Comparisons in both studies (Friedlander et al., 1978 and Hearne
et al., 1990) were made with mortality rates (or proportions) from New
York State and from an internal unexposed cohort from the Kodak
facility. Extensive industrial hygiene measurements were available for
this cohort from after 1980, which indicated that 8-h TWA exposure
concentrations for different occupational classifications ranged from
approximately 35.3-402 mg/m3 (10-114 ppm) and the mean exposure was
91.8 mg/m3 (26 ppm) (Hearne et al., 1987). An exposure-response
analysis, which was presented by Hearne et al. (1987), failed to
demonstrate an increasing risk for these causes of death with
increasing methylene chloride exposure. Hearne et al. (1987) observed
an excess of pancreatic cancer mortality (8 observed, SMR = 2.58, 95%
confidence interval (CI) = 1.11-5.08). Mirer et al. (1988) published a
letter suggesting that the excess of pancreatic cancer mortality
increased with time since first exposure (latency) and was greatest
among workers in the highest exposure (750 ppm-years) and latency
(> 30 years) categories (4 observed, SMR = 4.49, 95%
CI = 1.22-11.49). No new pancreatic cancer cases were identified with
additional follow-up, and with the additional data the excess was not
statistically significant (SMR = 1.90, 95% CI = 0.82-3.75) (Hearne et
al., 1990). The study by Friedlander et al. (1978) and the subsequent
studies by Hearne et al. (1987,1990) failed to detect a significantly
increased risk of ischaemic heart disease, lung cancer, liver cancer
or other cancers among methylene-chloride-exposed workers. It also
important to recognize that workers at the Kodak facility (T. Hearne,
personal communication to the IPCS) were not permitted to smoke at
their workstations and that this fact may have induced a negative bias
in these studies, particularly with respect to lung cancer or
cardiovascular disease. Unfortunately detailed information on
cigarette smoking was not available for this cohort and thus
adjustments for this potential bias could not be made.
Ott et al. (1983) evaluated a cohort of workers exposed to
methylene chloride in the production of triacetate fibre at a
manufacturing plant in Rock Hill, South Carolina, USA. This cohort
included 1271 males and female workers who were employed for at least
3 months, sometime between 1954 and 1977, Workers from another textile
facility that were not exposed to methylene chloride, but met the same
inclusion criteria as the exposed cohort, were also included for
comparison purposes. All workers (both exposed and unexposed) were
followed for vital status ascertainment up to June 1977. Eight-hour
TWA methylene chloride exposures in this cohort were estimated to
range from 494 to 1677 mg/m3 (140 to 475 ppm) from a survey
conducted in 1977 and 1978. Workers in this study were also reported
to have been exposed to methanol and acetone. The mortality experience
of the exposed cohort was compared with the mortality of the USA
population using a modified life-table approach (SMRs). Direct
comparisons were also made between the mortality of the exposed and
unexposed cohorts. Mortality from cardiovascular disease or any other
cause was not found to be significantly increased relative to the USA
population. However, the authors did observe a significant increase in
the risk of ischaemic heart disease (RR = 3.1, p < 0.05) among white
men in the analysis when the mortality rates of the exposed and
unexposed cohorts were compared. It was also noted that 8 of the 14
ischaemic heart disease deaths among exposed white men occurred among
workers who were actively employed. Although Ott et al. (1983) did not
report any increase in cancer mortality, this study was not designed
to evaluate cancer and only included seven malignant neoplasms.
The follow-up of the exposed cohort (but not the unexposed one)
studied by Ott et al. (1983) was subsequently extended to 1986 by
Lanes et al. (1990). The analyses presented in this paper were solely
based upon comparisons with the USA population and did not include any
direct comparisons with the unexposed cohort as did the study by Ott
et al. (1983). This study failed to detect an excess of cardiovascular
or ischaemic heart disease. A significant excess of cancers of the
biliary passages and liver (SMR = 5.75, 95% CI = 1.82-13.78) was
observed. Three of the cancers were cholangiocarcinomas of various
biliary sites while the fourth was a liver adenocarcinoma. The SMR for
biliary cancer was estimated using mortality rates from 1973 to 1977.
The SMR for biliary cancer alone was 20 (95% CI = 5.2-56). Three of
the four liver and biliary cancer deaths observed in this study were
thought to have occurred among workers with 10 or more years of
employment and at least 20 years since first employment (0.35
expected, SMR = 11.43), a pattern consistent with a potential
occupational etiology. One of the cases had only been exposed to
methylene chloride for one year. Lanes et al. (1993) recently extended
the follow-up of the cohort for an additional 4 years. Although no
additional cases of liver or biliary cancer were observed, an excess
from the previous study persisted (SMR = 2.98, 95% CI = 0.81-7.63].
This latest report did not include an analysis for biliary cancer
alone.
Another retrospective cohort mortality study of workers from a
Hoechst-Celanese cellulose acetate fibre plant in Cumberland,
Maryland, USA was reported by Gibbs (1992). This study included 3211
cellulose fibre workers employed in or after 1970 and followed until
1989. The cohort was divided into three groups: high (> 1235 mg/m3,
> 350 ppm) methylene chloride exposure, low exposure (176-350 mg/m3,
50-100 ppm), and no exposure. Comparisons were made with USA, Maryland
and county mortality rates. Estimates of exposure levels for this
population were based on industrial hygiene measurements from the plant
studied by Ott et al. (1983), which used similar production methods.
Cancer of the prostate was significantly elevated among men, and
particularly among those with long latency and with high levels of
methylene chloride exposure. A significant excess of cervical cancer
was observed among women in the low exposure group, based on Maryland
rates (SMR = 4.75 based upon 5 observed), but there was no evidence of
a dose-response relationship. Two cases (exp = 1.40) of biliary cancer
were observed among the combined high and low exposure groups. An
excess of ischaemic heart disease mortality was observed among workers
in all three groups when comparisons were based upon Maryland rates,
but not when local county rates were used. Mortality from lung,
pancreatic, liver/biliary and other cancers was not observed to be
significantly elevated in this study. As with the Kodak study, workers
at the Hoechst-Celanese facilities were not permitted to smoke at
their workstations. Again this fact may have induced a negative bias
in these studies, particularly with respect to lung cancer or
cardiovascular disease.
A cohort study of chemical workers included a sub-cohort of 226
men employed for at least one year in chlorinated methanes production
(Ott et al., 1983). Methylene chloride is principally produced by a
method involving the hydrochlorination of methanol which also results
in the production of chloroform and carbon tetrachloride (IARC, 1986).
The men had been employed between 1940 and 1969 and were followed for
mortality until 1979. In all, 42 deaths were observed and no excesses
of respiratory cancer (SMR = 0.70, based on 3 observed) or circulatory
disease (SMR = 0.68, based on 18 observed) were seen. The results for
liver and biliary cancer were not reported, but three cases of
pancreatic cancer were observed (0.9 expected). All three persons had
worked in chlorinated methanes production between 1942 and 1946; two
had been employed for less than 5 years, the third for 6 years. No
further information on exposure for the individuals or the sub-cohort
was given and the mixed exposure to methylene chloride, chloroform,
and carbon tetrachloride limits the interpretation of the results with
respect to methylene chloride.
Finally, Heineman et al. (1994) reported the results of a case-
control study of astrocytic brain cancer and occupational exposure to
chlorinated aliphatic hydrocarbons. The study included 300 cases with
a hospital diagnosis of astrocytic brain cancer and 320 controls
matched on age, year of death and geographical area. A job-exposure
matrix was used to classify cases and controls in terms of potential
exposure to chlorinated aliphatic hydrocarbons including methylene
chloride (Gomez et al., 1994); 119 cases and 108 controls were
classified as being potentially exposed to methylene chloride in this
study. The risk was reported to increase with the probability of
exposure (Odds Ratio (OR) - 2.4 for high probability, 95%
CI = 0.9-6.4) and duration of employment (OR = 1.9 for > 20 years,
95% CI = 0.7-5.2) in jobs considered to be exposed to methylene
chloride after adjustment for other solvent exposures. The exposure
information used in this study is weaker than that generally used in
the retrospective cohort mortality studies described above and the
results should therefore be viewed more cautiously.
9.3 Appraisal of human effects
The main toxic effects of methylene chloride are reversible CNS
depression and CO-Hb formation. Liver and renal dysfunctions and
effects on haematological parameters have also been reported
following exposure to methylene chloride. Methylene chloride will
irritate the skin and eyes especially when evaporation is prevented.
Prolonged contact may cause chemical burns.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men exposed to methylene chloride
for several years at concentrations ranging from 260 to 347 mg/m3.
Similarly, the performance of a group of retired airplane strippers,
with a long history of exposure to methylene chloride (22 years) at
high but unspecified levels, in a battery of neurophysiological and
psychological tests was within the "normal" range when compared with
a control group who had a history of either no or only low exposure
to methylene chloride.
Fatalities due to excessive oral exposure to methylene chloride
have been reported. A case of serious pulmonary oedema has been
reported after excessive inhalation.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to
methylene chloride. This isolated finding from a limited study makes
it difficult to interpret the significance of the observations.
Five mortality studies on methylene chloride have been conducted
and evaluated specifically with regard to cancer and cardiovascular
disease. None of the studies demonstrated a relationship between
exposure to methylene chloride and lung or liver cancer mortality.
With regard to the lung cancers, the lack of smoking histories
hampers the interpretability of the results. An excess of mortality
from biliary cancer was reported in one study, but this was not
corroborated by other studies. Two studies showed an excess in
mortality from pancreatic cancer. In one of the studies no new
pancreatic cancer cases were identified with additional follow-up;
with the additional data the excess was not statistically
significant. It should be noted that the size of these studies
resulted in very low statistical power to detect an excess
particularly for rare cancers such as liver and biliary tract
tumours.
Associations between exposure to methylene chloride and prostate
and cervical cancers have been reported in studies, each of which had
its limitations. An association between the potential for exposure to
methylene chloride and other organic solvents and brain cancer was
found in a case-control study which classified exposure to methylene
chloride using a job exposure matrix. This finding should be viewed
with caution.
The results from these studies have been contradictory with
respect to mortality from ischaemic heart disease. A role for
methylene chloride in the induction of ischaemic heart disease is
plausible based on the fact that methylene chloride is metabolized to
carbon monoxide and induces the formation of carboxyhaemoglobin in
humans. An excess of cardiovascular disease was reported in one of
the mortality studies. The fact that further studies did not provide
any compelling evidence of an increased risk of cardiovascular
disease might be attributable to their reliance on comparisons with
the general population as the referent group. The use of general
mortality rates in occupational cohort mortality studies may bias the
results towards the null (i.e. no effect) due to the "healthy worker
effect" which is particularly strong for cardiovascular diseases.
This bias may have been further exacerbated by the fact that workers
were not permitted to smoke at their workstations.
The currently available epidemiological studies are inadequate
for drawing any firm conclusions with regard to either cancer or
cardiovascular disease risk.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Evaluation of human health risks
Human exposure to methylene chloride is mainly by inhalation of
the vapour. Exposure of the general population to methylene chloride
depends strongly on the indoor air concentration. Owing to the use of
products containing methylene chloride, peak concentrations of up to
4000 µg/m3 have been reported. However, 24-h average exposures are
in general below 50 µg/m3.
Methylene chloride is rapidly absorbed through the lung and also
from the gastrointestinal tract. It is absorbed via the skin, but at a
much slower rate than by the other routes. Once absorbed, methylene
chloride is distributed throughout the body and will cross both the
placenta and the blood-brain barrier. It is rapidly excreted, the
majority being exhaled unchanged via the lungs. The remainder is
metabolized to carbon monoxide, carbon dioxide and inorganic chloride.
Two metabolic pathways have been identified, one involving cytochrome
P-450 and the second involving glutathione- S-transferase. Clear
species differences exist in the relative contributions of these two
pathways. These differences have been used as a basis for a
physiologically based pharmaco-kinetic (PB-PK) model for methylene
chloride, which allows interspecies comparison of the concentrations
of active metabolites at the target tissues, thus enhancing the value
of results from animal studies in human health risk assessment. This
approach has been used in assessing the human cancer risk associated
with exposure to methylene chloride.
The acute toxicity of methylene chloride is low. The predominant
effects in human beings are CNS depression and elevated blood
carboxyhaemoglobin (CO-Hb) levels. These effects are reversible. Other
target organs can be the liver and, occasionally, the kidney. The
odour threshold concentration of methylene chloride is reported to be
540 mg/m3 or more. Mild CNS effects have been reported following
exposure to concentrations as low as 694 mg/m3 for 1.5-3 h
(behavioural disturbances). More significant effects occur at
concentrations in excess of 2000 mg/m3. Narcosis has been reported
to occur following a 0.5-h exposure to 69 000 mg/m3. Metabolism to
carbon monoxide leads to increases in blood CO-Hb levels following
acute exposure to the vapour, a process which becomes saturable
following exposures to high levels of methylene chloride. Exposure to
either 100 or 530 mg/m3 for 7.5 h leads to CO-Hb levels of 3.4% and
5.3%, respectively, in human volunteers. This effect forms the basis
of most, if not all, published occupational exposure limits, where a
level of 5.0% is judged to be acceptable.
The predominant effects following repeated or long-term exposure
to methylene chloride are the same as for acute exposure. Reversible
symptoms of CNS depression are seen in several species, including
humans. The lowest-observed-effect level (LOEL) for this effect in all
animal species is 7100 mg/m3 by inhalation. No evidence of
irreversible neurological damage was seen in rats exposed to methylene
chloride by inhalation at concentrations up to 7100 mg/m3 for
13 weeks. Additional target organs reported in various species
chronically exposed to methylene chloride include the liver and,
occasionally, the kidney. The no-observed-adverse-effect level (NOAEL)
for chronic intermittent inhalation exposure was judged to be
710 mg/m3 in rats. After continuous exposure, slight cytoplasmic
vacuolization in the liver of both mice and rats was observed at
88-350 mg/m3.
A single study has reported the presence of methylene chloride in
the placenta, fetus and breast milk of women following occupational
exposure. The teratogenic potential of methylene chloride has been
assessed in three animal studies. Small effects on either fetal or
maternal body weights were reported, but no evidence of an effect on
the incidence of skeletal malformations or other developmental effects
was seen. A well-conducted two-generation reproductive toxicity study
in rats exposed to methylene chloride by inhalation at concentrations
up to 5300 mg/m3, 6 h/day, for 5 days/week showed no evidence of an
adverse effect on any reproductive parameter, neonatal survival or
neonatal growth in either the F0 or F1 generations.
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms (Salmonella or E. coli)
with or without metabolic activation. In eukaryotic systems it gave
either negative or, in one case, weakly positive results. In vitro
gene mutation assays and tests for unscheduled DNA synthesis (UDS) in
mammalian cells were uniformly negative. In vitro assays for
chromosomal aberrations using different cell types gave positive
results, whereas negative or equivocal results were obtained in tests
for sister chromatid exchange (SCE) induction. The majority of the in
vivo studies reported provided no evidence of mutagenicity of
methylene chloride (e.g., chromosome aberration assay, micronucleus
test or UDS assay). A very marginal increase in frequencies of SCEs,
chromosomal aberrations and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride. The significance of these results is questionable due to
methodological deficiencies in the statistical analysis. There was no
evidence of binding of methylene chloride to DNA or DNA damage in rats
or mice given high doses.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride is
genotoxic in vivo.
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following lifetime exposure to high concentrations
(7100 and 14 100 mg/m3). These tumours were not seen in the rat or
the hamster.
Increased incidence of benign mammary tumours in female rats was
observed in one study, and increased incidence and multiplicity were
observed in two other rat studies. The increased incidence of these
tumours was within the historical control range; nevertheless there
was a dose-response relationship within one study. It is considered
that an increase in a tumour type, which occurs with high and variable
incidences in control animals, which does not progress to malignancy,
and which may be related to changes in prolactin levels, is of little
importance in human hazard assessment.
In vitro and in vivo metabolism and biochemical studies, and
mutagenicity assays in bacteria and B6C3F1 mice have provided a
plausible explanation for the mechanism of action and the species
differences in the carcinogenicity of methylene chloride to the lung
and liver. This explanation is based on the existence of an isoenzyme
of glutathione- S-transferase which specifically metabolizes
methylene chloride to the reactive intermediates responsible for
tumour induction in the mouse. Markedly lower levels of this enzyme in
rats and hamsters are consistent with the fact that these tumours do
not appear in these species. The levels of the enzyme in the liver are
lower in humans than in rats or hamsters. The variability of the
current estimates of this enzyme activity in human liver is low, but
the possibility of wider variation existing in subpopulations cannot
be discounted. Although the currently available information on enzyme
activity in human lung is limited, it is expected to be lower than in
human liver. The carcinogenic potency of methylene chloride in man is
expected to be low.
Mutagenicity studies on methylene chloride in bacteria and in the
B6C3F1 mouse, which shows a very high level of activity of the
isoenzyme, reveal positive effects, whereas mutagenicity has not been
demonstrated in standard in vivo mutagenicity assays using other
systems. These observations are consistent with the above hypothesis
and provide a mechanistic basis for the induction of tumours in the
mouse.
The role of the glutathione- S-transferase isoenzyme in the
mediation of the demonstrated mutagenic effects, together with the
correlation between the activity of this pathway and the species
differences in carcinogenic response, has led to its use as the dose
surrogate in physiologically based pharmacokinetic models used for
human health risk assessment.
Overall, animal inhalation studies have shown effects on the liver
from 710 mg/m3 and on other organs from 1700 mg/m3. However, these
effects have not been observed in epidemiological studies. Effects on
the CNS have been observed in both animals and humans and a threshold
in humans has been defined, based on the level of the metabolite
carbon monoxide in the blood, leading to exposure limits of the order
of 177 mg/m3.
10.2 Evaluation of effects on the environment
Due to its high volatility methylene chloride released to the
environment will end up in the atmosphere where it can be transported
to regions far removed from the emission source. Methylene chloride is
degraded in the troposphere by reaction with hydroxyl radicals giving
carbon dioxide and hydrogen chloride as major breakdown products.
Based on a lifetime in the troposphere of about 6 months it may be
assumed that only a few percent, if any, of methylene chloride will
reach the stratosphere. No significant impact on stratospheric ozone
depletion is expected. Methylene chloride will also not contribute
significantly to photochemical smog formation. In ambient air in rural
and remote areas, background levels of 0.07-0.29 µg/m3 have been
measured. In suburban and urban areas levels up to 2 and 15 µg/m3
have been found.
Concentrations of methylene chloride in the surface water of
rivers in industrialized areas stay generally below 10 µg/litre. In
industrial effluents, outfalls of municipal water treatment plants and
leachates of landfills, concentrations of methylene chloride of up to
200 mg/litre have been measured.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive to methylene chloride, with effects on
hatching from 5.5 mg/litre; adult aquatic organisms are relatively
insensitive even after prolonged exposure. There is no evidence to
suggest that methylene chloride and/or its metabolites bioaccumulate
in the environment. Given the concentrations observed in surface water
(< 10 µg/litre) and those in contaminated effluents
(< 200 mg/litre), no significant impact on the aquatic environment is
expected.
Localized contamination of soils will not significantly disperse
despite the mobility of methylene chloride; in groundwaters and soils,
biological degradation processes have been identified capable of
mineralizing methylene chloride in a few days. From the limited
information on soil organisms, it may be assumed that contamination of
soil has only a local and transient effect.
Apart from accidental spills, it is concluded that the present use
of methylene chloride has no significant impact on the environment.
REFERENCES
Abernethy S, Bobra AM, Shiu WY, Wells PG, & MacKay D (1986) Acute
lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two
planktonic crustaceans: the key role of organism- water partitioning.
Aquatic Toxicology 8:163-174.
Abrahamson S & Valencia R (1980) Evaluation of substances of interest
for genetic damage using Drosophila melanogaster. In: Mutagenicity
of methylene chloride. Oakridge, Tennessee, National Toxicology
Programme.
ACGIH (1992) Documentation of the threshold limit values and
biological exposure indices, 6th ed. Cincinnati, Ohio, US American
Conference of Governmental Industrial Hygienists.
Adams JD & Erickson HH (1976) The effects of repeated exposure to
methylene chloride vapour. Preprint from the Annual Scientific Meeting
of the Aerospace Medical Association. Washington, DC, Aerospace
Medical Association, pp 61-62.
AFS (1990) [Labour Protection Board Statute Book. Hygiene threshold
values.] Stockholm, 13 pp (in Swedish).
Agency for Toxic Substances and Disease Registry (1993) Methylene
chloride toxicity. Am Fam Phys, 47(5): 1159-1166.
Ahmed AE & Anders MW (1978) Metabolism of dihalomethanes to
formaldehyde and inorganic chloride II. Studies on the mechanism of
the reaction. Blochem Pharmacol, 27: 2021-2025.
Ahmed AE & Anders MW (1976) Metabolism of dihalomethanes to
formaldehyde and inorganic chloride. Drug Metab Dispos, 4: 357-361.
Alexander HC, McCarty WM, & Bartlett EA (1978) Toxicity of
perchloroethylene and methylene chloride to fathead minnows. Bull
Environ Contam Toxicol, 20: 344-352.
Alexeef G & Kiglore W (1983) Learning impairment in mice following
acute exposure to dichloromethane and carbon tetrachlorlde. J Toxicol
Environ Health, 11: 569-581.
Allen J, Kligerman A, Campbell J, Westbrook-Collins B, Erexson G, Kari
F, & Zeiger E (1990) Cytogenetic analysis of mice exposed to
dichloromethane. Environ Mol Mutagen, 15: 221-228.
Allred EN, Bleecker ER, Chaitman BR, Dalims TE, Gotlieb SO, Hackney
JD, Hayes D, Pagano M, & Selvester RH (1989) Acute effects of carbon
monoxide exposure on individuals with coronary artery disease. Health
Effects Institute, 98 pp (Research Report No. 25).
Amoore JE & Hautala E (1983) Odor as an aid to chemical safety: odor
thresholds compared with threshold limit values and volatilities for
214 industrial chemicals in air and water dilution. J Appl Toxicol,
3: 272-290.
Anders MW & Sunram JM (1982) Transplacental passage of dichloromethane
and carbon monoxide. Toxicol Lett, 12: 231-244.
Anders MW, Kubic VL, & Ahmed ME (1977) Metabolism of halogenated
methanes and macromolecular binding. J Environ Pathol Toxicol,
1: 117-121.
Andersen ME, Clewell III H J, Gargas ML, Smith FA, & Reitz RH (1987)
Physiologically based pharmacokinetics and the risk assessment process
for methylene chloride. Toxicol Appl Pharmacol, 87: 185-205.
Andersen CHJ, Gargas ML, MacNaughton MG, Reitz RH, Nolen RJ, & McKenna
MJ (1991) Physiologically based pharmacokinetic modelling with
dichloromethane, its metabolite, carbon monoxide, and blood
carboxyhaemoglobin in rats and humans. Toxicol Appl Pharmacol,
108: 14-27.
Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL, &
Loveday KS (1990) Chromosome aberration and sister chromatid exchange
test results with 42 chemicals. Environ Mol Mutagen, 16: 55-137.
Andrae U & Wolff T (1983) Dichloromethane is not genotoxic in isolated
rat hepatocytes. Arch Toxicol, 52: 287-290.
Angelo MJ, Pritchard AB, Hawkins DR, Waller AR, & Roberts A (1986a)
The pharmacokinetics of dichloromethane, I. Disposition in B6C3F1
mice following intravenous and oral administration. Food Chem Toxicol,
24: 965-974.
Angelo MJ, Pritchard AB, Hawkins DR, Waller AR, & Roberts A (1986b)
The pharmacokinetics of dichloromethane, II. Disposition in Fischer
344 rats following intravenous and oral administration. Food Chem
Toxicol, 24: 975-980.
Antoine SR, de Leon IR, & O'Dell-Smith RM (1986) Environmentally
significant volatile organic pollutants in human blood. Bull Environ
Contam Toxicol, 36(3): 364-371.
APHA (1977) Methods of sampling and analysis. 2nd ed. Washington, DC:
American Public Health Association, pp 894-902.
APHA (1989a) Purge and trap capillary-column gas chromatographic
method. In: Standard methods for the examination of water and
wastewater, 17th ed. Washington, DC, American Public Health
Association.
APHA (1989b) Purge and trap capillary-column gas chromatographic/mass
spectrometric method In: Standard methods for the examination of water
and wastewater, 17th ed. Washington, DC, American Public Health
Association.
Arbeidsinspectie (1991) Labour Inspectorate (1991) [The national MAC
list 1991.] Directorate of Labour, Ministry for Social Affairs and
Employment (in Dutch).
Arbeidstilsynet (1990) Labour Inspectorate (1991) [Administrative
standards for atmospheric pollution in the workplace 1990.]
Directorate for the Supervision of Labour (in Norwegian).
Arendt G, Haag F, & Puggmayer D (1982) [Determination of air pollution
with organic compounds in conurbations. Research report of the Federal
Office of the Environment.] (UFOPLAN 104 02 510). Frankfurt/Main,
Batelle Institut (in German).
Åstrand I, Övrum P, & Carlsson A (1975) Exposure to methylene
chloride. I. Its concentration in alveolar air and blood during rest
and exercise and its metabolism. Scand. J Work Environ Health,
1: 78-94.
ATSDR (1992) Toxicological Profile for Methylene Chloride. Atlanta,
Georgia, Agency for Toxic Substances and Disease Registry (TP-SZ-13).
Aviado DM (1978) Effects of fluorocarbons, chlorinated solvents and
inosine on the cardiopulmonary system. Environ Health Perspect,
26: 207-215.
Aviado DM & Belej MA (1974) Toxicity of aerosol propellants on the
respiratory and circulatory systems. 1. Cardiac arrythmia in the
mouse. Toxicology, 2: 31-42.
Aviado DM, Zakhari S, & Watanabe T (1977a) Methylene chloride. In:
Goldberg L ed. Non-fluorinated propellants and solvents for aerosols.
Cleveland, Ohio, CRC Press, pp 19-36.
Aviado DM, Zakhari S, & Watanabe T (1977b) Interactions among
hydrocarbon propellants, methylene chloride and ethanol. In: Goldberg
L ed. Non-fluorinated propellants and solvents for aerosols.
Cleveland, Ohio, CRC Press, pp 83-89.
Baldanf G (1981) The case of Grenzach - example of groundwater
pollution by environmentally relevant substances. DVGW Schr.reihe
Wasset, 29: 53-69.
Ballantyne B, Gazzard MF, & Swanston DW (1976) The ophthalmic
toxicology of dichloromethane. Toxicology, 6: 173-187.
Balmer MF, Smith FA, Leach LJ, & Yuile CL (1976) Effects in the liver
of methylene chloride inhaled alone and with ethyl alcohol. Am Ind Hyg
Assoc J, 37: 345-352.
Barassin J & Combourieu J (1973) Etude cinétique des reactions entre
l'oxygène atomique et les derivés chlorés du méthane. 1. Réaction
CH2Cl2+O. Bull Soc Chim France, 7-8: 2173-2177.
Barber ED, Donish WH, & Mueller KR (1980) Quantitative measurement of
the mutagenicity of volatile liquids in the Ames Salmonella/microsome
assay. Environ Mutagen, 2(2): 307 (Abstract P39).
Barrowcliff DF & Knell AJ (1979) Cerebral damage due to endogenous
chronic carbon monoxide poisoning caused by exposure to methylene
chloride. J Soc Occup Med, 29: 12-14.
Barsoum GS & Saad K (1934) Relative toxicity of certain chlorine
derivatives of the aliphatic series. Q J Pharm Pharmacol, 7: 205-214.
Basso M, Raje R, & Greening M (1987) Evaluation of in vivo
mutagenicity of methylene chloride following inhalation exposure in
mice by dominant lethal test [abstract]. Toxicologist, 7: 1034.
Bauchop T (1967) Inhibition of rumen methanogenesis by methane
analogues. J Bacteriol, 94: 171-175.
Bayard SP, Bayliss DL, Davidson IWF, Fowle III Jr, Greenberg M,
Haberman BH, Kotchmar D, Benignus V, Parker JC, & Singh D (1985)
Health assessment document for dichloromethane (methylene chloride).
Final report. Washington, DC, US Environmental Protection Agency
(EPA-600/8-82-004B; NTIS PB85 191559).
Becker CE & Lash A (1990) Study of neurological effects of chronic
methylene chloride exposure in airline maintenance mechanics [abstract
7]. Vet Hum Toxicol, 32: 342.
Belej MA, Smith GA, & Aviado DM (1974) Toxicity of aerosol propellants
in the respiratory and circulatory system. IV. Cardiotoxicity in the
monkey. Toxicology, 2: 381-395.
Bell BP, Franks P, Hildreth N, & Melius J (1991) Methylene chloride
exposure and birthweight in Monroe County, New York. Environ Res,
55: 31-39.
Benzon HT, Claybon L, & Brunner EA (1978) Elevated carbon monoxide
levels from exposure to methylene chloride. J Am Med Assoc, 239: 2341.
Berger M & Fodor GG (1968) CNS disorders under the influence of air
mixtures containing dichloromethane. Zent.bl Bakteriol, 215: 517.
Bergman K (1979) Whole body radiography and allied tracer techniques
in distribution and elimination studies of some organic solvents:
benzene, toluene, xylene, styrene, methylene chloride. Scand J Work
Environ Health 5(1): 1-263.
Binnemann PH, Sandmeyer U, & Schmuk E (1983) [Contents of heavy
metals, organochlorine pesticides, PCB and volatile organohalogen
compounds in fish in the Upper Rhine and Lake Constance.] Z
Lebensm.unters Forsch, 176: 253-261 (in German).
Birge WJ, Black JA, & Kuehne RA (1980) Effects of organic compounds on
amphibian reproduction. Lexington. Kentucky, Kentucky University,
Water Resources Research Institute (Research Report No. 121; NTIS
PB80-147523).
Black JA, Birge WJ, McDonnell WE, Westerman AG, Ramey BA, & Bruser DM
(1982) The aquatic toxicity of organic compounds to embryo-larval
stages of fish and amphibians. Lexington, Kentucky, Kentucky
University, Water Resources Research Institute (Research Report
No. 133: NTIS PB82-224601).
Blum DJW & Speece RE (1991) Quantitative structure-activity
relationships for chemicals toxicity to environmental bacteria.
Ecotoxicol Environ Saf, 22: 198-224.
Boeniger MF (1991) Nonisocyanate exposures in 3 flexible polyurethane
manufacturing facilities. Appl Occup Hyg, 6: 945-952.
Bogaards JJP, Van Ommen B, & Bladeten PJ (1993) Individual differences
in the in vitro conjugation of methylene chloride with glutathione
by cytosolic glutathione S-transferase in 22 human liver samples.
Blochem Pharmacol, 45(10): 2166-2169.
Bohme H, Fischer H, & Frank R (1949) Preparation and properties of the
x-halogenated thioethers. Ann Chem, 563: 54-72.
Bonnet P, Francin JM, Gradiski D, Raoult G, & Zissu D (1980)
Détermination de la concentration léthale50 des principaux
hydrocarbures aliphatiques chlorés chez le rat. Arch Mal Prof Med,
41: 317-321.
Bonventre J, Brennan O, Jason D, Henderson A, & Bastos ML (1977) Two
deaths following accidental inhalation of dichloromethane and
1,1,1-trichloroethane. J Anal Toxicol, 1: 158-160.
Bornmann G & Loeser A (1967) [The question of the chronic toxic action
of dichloromethane.] Z Lebensm. unters Forsch, 136: 14-18 (in German).
Bornschein RL, Hastings L, & Manson JM (1980) Behavioral toxicity in
the offspring of rats following maternal exposure to dichloromethane.
Toxicol Appl Pharmacol, 52: 29-37.
Breslin WJ & Landry TD (1986) Methylene chloride: Effects on estrous
cycling and serum prolactin in Sprague-Dawley rats. Midland, Michigan,
Dow Chemical USA (Internal report).
Bringmann G & Kühn R (1977a) [Findings on the harmful effects of water
pollutants on Daphnia magna.] Z Wasser Abwasser Forsch, 10: 161-166
(in German).
Bringmann G & Kühn R (1977b) Limiting values for damaging action of
water pollutants to bacteria (Pseudomonas putida) and green algae
(Scenedesmus quadricauda) in the cell multiplication inhibition
test. Z Wasset Abwasser Forsch, 10: 87-98.
Bringmann G & Kühn R (1978) [Threshold values for the harmful effects
of water pollutants on blue algae (Microcystis aeruginosa) and green
algae (Scenedemus quadricauda) in the cell reproduction inhibition
test.] Vom Wasser, 50: 45-60 (in German).
Bringmann G & Kühn R (1980) Determination of the harmful biological
effect of water pollutants on protozoa. II. Bacteriovorous ciliates.
Z Wasser Abwasser Forsch, 13(1): 26-31.
Bringmann G & Kühn R (1982) [Findings on the harmful effect of water
pollutants on Daphnia magna in a further developed standardized test
procedure.] Z Wasser Abwasser Forsch, 15: 1-6 (in German).
Bringmann G & Meinck F (1964) [Water toxicological assessment of
industrial waste waters.] Gesundheits-Ingenieur, 85: 229-236 (in
German).
Briving C, Hamberger A, Kjellstrand P, Rosengren L, Karlsson JE, &
Haglid KG (1986) Chronic effects of dichloromethane on amino acids,
glutathione and phosphoethanolamine in gerbil brain. Scand J Work
Environ Health, 12: 216-220.
Brown KW & Donnelly KC (1988) An estimation of the risk associated
with the organic constituents of hazardous and municipal waste
landfill leachates. Hazard Waste Hazard Mater, 5: 1-30.
Brunner W, Staub D, & Leisinger TH (1980) Bacterial degradation of
dichloromethane. Appl Environ Microbiol, 40: 950-958.
BUA (Advisory body on wastes with environmental implications) (1986)
[Dichlormethane.] BUA-Substance Report 6. Weinheim, VCH (BUA Report
No. 6) (in German).
Buccafusco RJ, Ells SJ, & Leblanc GA (1981) Acute toxicity of priority
pollutants to bluegill (Lepomis macrochirus). Bull Environ Contam
Toxicol, 26: 446-452.
Buie SE, Pratt DS, & May JJ (1986) Diffuse pulmonary injury following
paint remover exposure. Am J Med, 81: 702-704.
Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE,
Dittenber DA, Rampy LW, & McKenna MJ (1980) Methylene chloride: A two-
year inhalation toxicity and oncogenicity study in rats and hamsters.
Final report, Toxicology Research Laboratory, Health and Environmental
Sciences, Midland, Michigan, Dow Chemical USA.
Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE,
Dittenber DA, Rampy LW, & McKenna MJ (1984) Methylene chloride: A two-
year inhalation toxicity and oncogenicity study in rats and hamsters.
Fundam Appl Toxicol, 4: 30-47.
Burton DT & Fischer DJ (1990) Acute toxicity of cadmium, copper, zinc,
ammonia, 3,3'-dichlorobenzidine, 2,6-dichloro-4-nitroaniline,
methylene chloride, and 2,4,6-trichlorophenol to juvenile grass shrimp
and killifish. Bull Environ Contam Toxicol, 44: 776-783.
Callen DF, Wolf CR, & Philpot RM (1980) Cytochrome P-450 mediated
genetic activity and cytotoxicity of seven halogenated aliphatic
hydrocarbons in Saccharomyces cerevisiae. Mutat Res, 77: 55-63.
Carlsson A & Hultengren M (1975) Exposure to methylene chloride, III.
Metabolism of 14C-labelled methylene chloride in rat. Scand J Work
Environ Health, 1: 104-108.
Carreon R (1981) Methylene chloride: Acute oral toxicity. Midland,
Michigan, Dow Chemical Company (Internal report).
Casanova M, Deyo DF, & Heck Hd'A (1992) Dichloromethane (methylene
chloride): Metabolism to formaldehyde and formation of DNA-protein
cross-links in B6C3F1 mice and Syrian golden hamsters. Toxicol Appl
Pharmacol, 114: 162-165.
CEC (1980) Carcinogenicity volume II: Summary reviews of scientific
evidence. Luxembourg, European Commission, Health and Safety
Directorate, Directorate-General for Employment, Industrial Relations
and Social Affairs, pp 67-74.
CEC (1982) Multiannual programme of the Joint Research Centre
1980-1983, 1982 Annual Status Report - Protection of the environment.
Luxembourg, European Commission (Report EUR 8527-EN).
CEC (1986) Organochlorine solvents, health risks to workers.
Luxembourg, European Commission (Report EUR-10531-EN).
CEFIC (1986) The occurrence of chlorinated solvents in the
environment. European Chemical Industry Council. Chem Ind,
15: 861-869.
CEFIC (1993) Methylene chloride: Use in industrial applications.
Brussels, European Chemical Industry Council.
Cherry N, Venables H, Waldron HA, & Wells GG (1981) Some observations
on workers exposed to methylene chloride. Br J Ind Med, 38: 351-355.
Chodola GR, Biswas N, Bewtra JK, St Pierre CC, & Zytner RG (1989) Fate
of selected volatile organic substances in aqueous environment. Water
Pollut Res J Can, 24: 119-142.
Chrostek WJ & Levine MS (1981) Health Hazard Evaluation Report No.
HHE-80-154-1207: Bechtel Powder Corporation, Betwick, Pennsylvania,
24 pp.
Clark DG & Tinston DJ (1982) Acute inhalation toxicity of some
halogenated and non-halogenated hydrocarbons. Hum Toxicol, 1: 239-247.
Cohen JM, Dawson R, & Koketsu M (1980) Extent-of-exposure survey of
methylene chloride. Cincinnati, Ohio, National Institute of
Occupational Safety and Health, 53 pp (Document No. 80-131).
Coleman WE, Lingg RD, Melton RG, & Kopfler FC (1976) The occurrence of
volatile organics in five drinking water supplies using gas
chromatography/mass spectrometry. In: Keith LH, ed. Identification and
analysis of organic pollutants in water. Ann Arbor, Michigan, Ann
Arbor Science, pp 305-327.
Condie LW, Smallwood CL, & Laurie RD (1983) Comparative renal and
hepatotoxicity of halomethanes: bromodichloromethane, bromoform,
chloroform, dibromochloromethane and methylene chloride. Drug Chem
Toxicol, 6: 563-578.
Cordes DH, Brown WD, & Quinn KM (1988) Chemically induced hepatitis
after inhaling organic solvents. West J Med, 148: 458-460.
Cornish HH, Ling BP, & Barth ML (1973) Phenobarbital and organic
solvent toxicity. Am Ind Hyg Assoc J, 34: 487-492.
Corsi GC, Valentini F, & Bertazzon A (1983) Effects of subtoxic
amounts of furan, acetylfuran and methylene chloride on some serum
enzymes of rat. Boll Soc Ital Biol Sper, 59: 1049-1052.
Cox RA, Derwent RG, & Eggleton AE (1976) Photochemical oxidation of
halocarbons in the troposphere. Atmos Environ, 10: 305-308.
Crebelli R, Andreoli C, Carere A, Conti G, Conti L, Cotta Ramusino M,
& Benigni R (1992) The induction of mitotic chromosome malsegregation
in Aspergillus nidulans. Quantitative structure activity
relationship (QSAR) analysis with chlorinated aliphatic hydrocarbons.
Mutat Res, 266: 117-134.
Crebelli R, Benigni R, Franekic J, Conti G, Conti L, & Carere A (1988)
Induction of chromosome malsegregation by halogenated organic solvents
in Aspergillus nidulans: unspecific or specific mechanism? Murat
Res, 201: 401-411.
Cunningham ML, Gandolfi AJ, Brendel K, & Sipes IG (1981) Covalent
binding of halogenated volatile solvents to subcellular macromolecules
in hepatocytes. Life Sci, 29: 1207-1212.
Cuppit LT (1980) Fate of toxic and hazardous materials in the air
environment. Washington, DC, US Environmental Protection (NTIS
PB80-221948).
Daft JL (1987) Determining multifumigants in whole grain and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daniels SL, Hoerger FD, & Moolenaer RJ (1985) Environmental exposure
assessment: experience under the toxic substances control act. Environ
Toxicol Chem, 4: 107-117.
Dankovic DA & Baiter AJ (1994) The impact of exercise and intersubject
variability on dose estimates for dichloromethane derived from a
physiologically based pharmacokinetic model. Fundam Appl Toxicol,
22: 20-25.
Danni O, Brossa O, Burdino E, Milillo P, & Ugazio G (1981) Toxicity of
halogenated hydrocarbons in pretreated rats - an experimental model
for the study of integrated permissible limits of environmental
poisons. Int Arch Occup Health, 49: 164-176.
Davis JW & Madsen SS (1991) The biodegradation of methylene chloride
in soils. Environ Toxicol Chem, 10: 463-474.
Davis EM, Murray HE, Liehr JG, & Powers EL (1981) Basic microbial
rates and chemical byproducts of selected organic compounds. Water
Res, 15: 1125-1127.
De Walle FB & Chain ESK (1978) Presence of trace organics in the
Delaware river and their discharge by municipal and industrial
sources. Proc Ind Waste Conf, 32: 908-919.
De Bortoli M, Knöppel H, Pecchio E, Peil A, Rogora L, Schauenburg H,
Schlitt H, & Vissers H (1986) Concentrations of selected organic
pollutants in indoor and outdoor air in Northern Italy. Environ Int,
12: 343-350.
Dequinze J, Scimar C, & Edeline F (1984) Identification of the
substances and their derived products on the list of 129 substances
(list 1 of the Directive 76/464/EEC), present in the refuse of
chlorine derived organic chemistry industry CEBEDEAU (Final report
No. 83/205).
Derwent RG & Eggleton AEJ (1978) Halocarbon lifetimes and
concentration distributions calculated using a two-dimensional
tropospheric model. Atmos Environ, 12: 1261-1269.
Derwent RG & Jenkin ME (1991) Hydrocarbons and the long-range
transport of ozone and PAN across Europe. Atmos Environ,
25A: 1661-1678.
Devereux TR, Foley JF, Maronpot RR, Kari F, & Anderson MW (1993) RAS
proto-oncogene activation in liver and lung tumors from B6C3F1 mice
exposed chronically to methylene chloride. Carcinogenesis,
14(5): 795-801.
DFG (Deutsche Forschungsgemeinschaft) (1983) In: Henschler D ed.
[Maximum workplace concentration and biological tolerance values for
working materials.] Verlage Chemie, Weinheim, Germany (in German).
DFG (Deutsche Forschungsgemeinschaft) (1991), Senate Committee for the
Testing of Working Materials Endangering Health (1991) Maximum
workplace concentrations and biological tolerance values for working
materials. Weinheim, Germany, VCH Publishers (in German).
Di Renzo AB, Gandolt AJ, & Sipes IG (1982) Microsomal and covalent
binding of aliphatic halides to DNA. Toxicol Lett, 11: 243-252.
Di Vincenzo GD & Kaplan CJ (1981a) Uptake, metabolism and elimination
of methylene chloride vapor by humans. Toxicol Appl Pharmacol,
59: 130-140.
Di Vincenzo GD & Kaplan CJ (1981b) Effect of exercise or smoking on
the uptake, metabolism, and excretion of methylene chloride vapor.
Toxicol Appl Pharmacol, 59: 141-148.
Di Vincenzo GD & Hamilton ML (1975) Fate and disposition of 14C-
methylene chloride in the rat. Toxicol Appl Pharmacol, 32: 385-393.
Di Vincenzo GD, Yanno FJ, & Astill BD (1971) The gas chromatographic
analysis of methylene chloride in breath, blood and urine. Am Ind Hyg
Assoc J, 32: 387-391.
Di Vincenzo GD, Yanno F J, & Astill BD (1972) Human and canine
exposure to methylene chloride vapor. Am Ind Hyg Assoo J, 33: 125-135.
Dill DC, Watanabe PG, & Norris JM (1978) Effect of methylene chloride
on the oxyhemoglobin dissociation curve of rat and human blood.
Toxicol Appl Pharmacol, 46(1): 125-129.
Dill DC, Murphy PG, & Mayes MA (1987) Toxicity of methylene chloride
to life stages of fathead minnow, Pimephales promelas Rafinesque.
Bull Environ Toxicol, 39: 869-876.
Dilling WL, Tefertiller NB, & Kallos GJ (1975) Evaporation rates of
methyl chloride, chloroform, 1,1,1-trichloroethane, trichloroethylene,
tetrachloroethylene, and other chlorinated compounds in dilute aqueous
solutions. Environ Sci Technol, 9: 833-838.
Dilling WL (1977) Interphase transfer processes. II. Evaporation rates
of chloromethanes, ethanes, ethylenes, propanes, and propylenes from
dilute aqueous solutions; Comparisons with theoretical predictions.
Environ Sci Technol, 4: 405-409.
Dillon DM, Combos RD, McConville M, & Zeiger E (1990) The role of
metabolism and glutathione in the mutagenicity of vapour phase
dichloromethane in bacteria. Environ Mol Mutagen, 15 (Suppl 17):
Abstr 52.
Dillon DM, Edwards IR, Combos RD, McConville M, & Zeiger E (1992) The
role of glutathione in the bacterial mutagenicity of vapor phase
dichloromethane. Environ Mol Mutagen, 20: 211-217.
Dowty BJ, Carlisle DR, & Laseter JL (1975) New Orleans drinking water
sources tested by gas chromatography-mass spectrometry. Environ Sci
Technol, 9: 762-765.
Duprat P, Delsaut L, & Gradiski D (1976) Pouvoir irritant des
principaux solvents chlorés aliphatiques sur la peau et les muqueuses
oculaires du lapin. J Eur J Toxicol, 9(3): 171-177.
ECETOC (1984) Joint assessment of commodity chemicals No. 4, Methylene
chloride. Brussels, European Centre for Ecotoxicology and Toxicology
of Chemicals.
ECETOC (1988) Methylene chloride (dichloromethane): human risk
assessment using experimental animal data. Brussels, European Centre
for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 32).
ECETOC (1989) Methylene chloride (dichloromethane): an overview of
experimental work investigating species, differences in
carcinogenicity and their relevance to man. Brussels, European Centre
for Ecotoxicology and Toxicology of Chemicals (Technical Report
No. 34).
ECSA (European Chlorinated Solvents Association) (1989) Methylene
chloride, its properties, uses, occurrence in the environment,
toxicology and safe handling. Brussels, European Chemical Industry
Council.
ECSA (European Chlorinated Solvents Association) (1992) ECSA
production figures for methylene chloride. Brussels, European Chemical
Industry Council.
Edwards PR, Campbell I, & Milne GS (1982) The impact of chloromethanes
on the environment. Part 2. Methyl chloride and methylene chloride.
Chem Ind, 619-622.
Eisenbrandt DL & Reitz RH (1986) Acute toxicity of methylene chloride:
Tumorigenic implications for B6C3F1 mice. Toxicologist, 6: 662.
English JM (1964) A case of probable phosgene poisoning. Br Med J,
1: 38.
Engström J & Bjurström R (1977) Exposure to methylene chloride:
Content in subcutaneous adipose tissue. Scand J Work Environ Health,
3: 215-224.
European Council (1982) Council Directive of 17 May 1982 amending for
the second time Directive 76/768/EEC on the approximation of the laws
of the Member States relating to cosmetic products (82/368/EEC). Off J
Eur Communities, L167: 1-8.
Fagin J, Bradley J, & Williams D (1980) Carbon monoxide poisoning
secondary to inhaling methylene chloride. Br Med J, 281: 1461.
Fells I & Moelwyn-Hughes EA (1958) The kinetics of the hydrolysis of
metylene chloride. J Chem Soc, 1326-1333.
Ferguson J & Pirie H (1948) The toxicity of vapours to the grain
weevil. Ann Appl Biol, 35: 532-550.
Ferrario JB, Lawler GC, DeLeon IR, & Laseter JL (1985) Volatile
organic pollutants in biota and sediments of Lake Pontchartrain. Bull
Environ Contam Toxicol, 34: 246-255.
Flanagan R J, Ruprah M, Meredith T J, & Ramsey JD (1990) An
introduction to the clinical toxicology of volatiles substances. Drug
Saf, 5(5): 359-383.
Flathman PA, Jerger DE, & Woodhall PM (1992) Remediation of
dichloromethane (DCM)-contaminated ground water. Environ Prog,
11(3): 202-209.
Fleeger AK & Lee JS (1988) Characterization of worker exposures to
methylene chloride resulting from application of aerosol glue in the
asbestos abatement industry. Appl Ind Hyg, 3: 245-250.
Flury F & Zernik F (1931) [Harmful gases, yapours, mists, smokes, and
dusts]. Berlin, Julius Springer, pp 311-312 (in German).
Foder GG, Prajsnar D, & Schlipkoter KW (1973) Endogenous conformation
by incorporated halogenated hydrocarbons of the methane series.
Staubreinhalt Luft, 33: 260-261.
Fodor GR & Winneke (1971) Nervous system disturbances in men and
animals experimentally exposed to industrial solvent vapors. In:
England HM ed. Proceedings of the 2nd International Clean Air
Congress. New York, Academic Press, pp 238-243.
Fodor GG, Schlipköter HW, & Zimmermann M (1973) The objective study of
sleeping behaviour in animals as a test of behavioural toxicity.
In: Horvath M (ed.) Adverse effects of environmental chemicals and
psychoretapic drugs: quantitative interpretation of functional tests.
London, Elsevier, pp 115-123.
Foley JF, Tuck PD, Ton TVT, Frost M, Kari F, & Anderson MW (1993)
Inhalation to a hepatocarcinogenic concentration of methylene chloride
does not induce sustained replicative DNA synthesis in hepatocytes of
female B6C3F1 mice. Carcinogenesis, 14(5): 811-817.
Foster JR, Green T, Smith LL, Lewis RW, Hext PM, & Wyatt I (1992)
Methylene chloride - an inhalation study to investigate pathological
and biochemical events occurring in the lungs of mice over an exposure
period of 90 days. Fundam Appl Toxicol, 18: 376-388.
Friedlander BR, Hearne T, & Hall S (1978) Epidemiologic investigation
of employees chronically exposed to methylene chloride. J Occup Med,
20: 657-666.
Fuxe K, Andersson K, Hansson T, Agnati LF, Eneroth P, & Gustafsson JA
(1984) Central catecholamine neurons and exposure to dichloromethane.
Selective changes in amine levels and turnover in tel- and
diencephalic DA and NE nerve terminal systems and in secretion of
anterior pituitary hormones in the male rat. Toxicology, 29: 293-305.
Gälli R & Leisinger T (1985) Specialized bacterial strains for the
removal of dichloromethane from industrial waste. Conserv Recycl,
8: 91-100.
Gargas ML, Clewell III HJ, & Anderson ME (1986) Metabolism of inhaled
dihalomethanes in vivo: differentiation of kinetic constants for two
independent pathways. Toxicol Appl Pharmacol, 82: 211-223.
Gerritsen WB & Buschmann CH (1960) Phosgene poisoning caused by the
use of chemical paint removers containing methylene chloride in ill-
ventilated rooms heated by kerosene stoves. Br J Ind Med, 17: 187-189.
Ghittori S, Marraccini P, Franco G, & Imbirani M (1993) Methylene
chloride exposure in industrial workers. Am Ind Hyg Assoc J,
54(1): 27-31.
Gibbs GW (1992) The mortality of workers employed at a cellulose
acetate and triacetate fibers plant in Cumberland Maryland, a "1970"
cohort followed 1970-1989. Final report by Safety Health Environmental
International Consultants, Winterburn, Alberta (TO). Somerville, New
Jersey, Hoechst Celanese.
Giger W, Schwarzenbach RP, Hoehn E, Schellenberg K, Schneider JK,
Wasmer HR, Westall J, & Zobrist J (1983) [Das Verhalten organischer
Wasser-inhaltstoffe bei der Grundwasserbildung und im Grundwasser.]
Gaz-Eaux-usées, 63: 517-531 (in German).
Gocke E, King M-T, Eckhardt K, & Wild D (1981) Mutagenicity of
cosmetic ingredients licensed by the European Communities. Murat Res,
90: 91-109.
Gomez MR, Cocco P, Dfosemeci M, & Stewart PA (1994) Occupational
exposure to chlorinated aliphatic hydrocarbons: Job exposure matrix.
Am J Ind Med, 26(2): 171-183.
Gossett JM (1985) Anaerobic degradation of C1 and C2 chlorinated
hydrocarbons. Air Force Engineering Service Centre, Engineering
Service Laboratory, 153 p (ESL-TR-85-38).
Gradiski D, Bonnet P, Raoult G, Magadur JL, & Francin JM (1978)
Toxicité aiguë comparée par inhalation de principaux solvants
alphatiques chlorés. Arch Mal Prof Méd Trav Sécur Soc, 39: 249-257.
Green T (1983) The metabolic activation of dichloromethane in a
bacterial assay using Salmonella typhimurium. Mutat Res,
118: 277-288.
Green T, Provan WM, Collinge DC, & Guest AE (1986a) Methylene chloride
(dichloromethane): Interaction with rat and mouse liver and lung DNA
in vivo. Alderley Park, Macclesfield (Cheshire), ICI (Technical Report
CTL/R/851).
Green T, Provan WM, Nash JA, & Gowans N (1986b) Methylene chloride
(dichloromethane): In vivo inhalation pharmacokinetics and metabolism
in F344 rats and B6C3F1 mice. Alderley Park, Macclesfield (Cheshire),
ICI (Technical Report CTL/R/880).
Green T, Nash JA, & Mainwaring G (1986c) Methylene chloride
(dichloromethane): In vitro metabolism in rat, mouse and hamster liver
and lung fractions and in human liver fractions. Alderley Park,
Macclesfield (Cheshire), ICI (Technical Report CTL/R/879).
Green T, Provan WM, & Gowans N (1987a) Methylene chloride
(dichloromethane): In vivo inhalation pharmacokinetics in B6C3F1 mice
(using stable isotopes) and F344 rats. AlderIcy Park, Macclesfield
(Cheshire), ICI (Technical Report CTL/R/931).
Green T, Nash JA, & Hill SJ (1987b) Methylene chloride
(dichloromethane): Glutathione- S-transferase metabolism in vitro in
rat, mouse, hamster, and human liver cytosol fractions. Alderley Park,
Macclesfield (Cheshire), ICI (Technical Report CTL/R/934).
Green T, Nash JA, Hill SJ, & Foster JR (1987c) Methylene chloride
(Dichloromethane): The effects of exposure to 4000 ppm on mouse lung
enzymes. Alderley Park, Macclesfield (Cheshire), ICI (Report
CTL/R/935).
Green T, Provan WM, Collinge DC, & Guest AE (1988) Molecular
interactions of inhaled methylene chloride in rats and mice. Toxicol
Appl Pharmacol, 93(1): 1-10.
Gu Z & Wang Y (1988) [Evaluation of genotoxic effect of 16 chemicals
using the micronucleus assay in vitro]. Weisheng Dulixue Zazhi,
2: 1-4 (in Chinese).
Guengerich FP, Shimada T, Raney KD, Yun CH, Meyer DJ, Ketterer B,
Harris TM, Groopman JD, & Kadlubar FF (1992) Elucidation of catalytic
specificites of human cytochrome P450 and glutathione-S-transferase
enzymes and relevance to molecular epidemiology. Environ Health
Perspect, 98: 75-80.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of The Netherlands. Sci Total Environ, 43: 193-219.
Guidelines on the Evaluation and Treatment of Groundwater Pollution
with Readily Volatile chlorohydrocarbons (1983) Published by the
Ministry for Nutrition, Agriculture and Environment, Baden-
Württemberg, Heft, 13 August.
Hake CL, Stewart RD, Wu A, & Graff SA (1975) Carboxyhaemoglobin levels
of humans exposed to methylene chloride. 14th Annual Meeting of the
Society of Toxicity, Williamsbergh, Virginia. Toxicol Appl Pharmacol,
33(1): 145 (Abstract 59).
Halbartschlager J, Kohler H, Swerinski H, & Bardtke D (1984) [Studies
on the biological decomposition of chlorohydrocarbons, using the
example of dichlormethane (methylene chloride).] GWF-Wasser/Abwasser,
125: 380-386 (in German).
Hall AH & Rumack BH (1990) Methylene chloride exposure in furniture-
stripping shops: Ventilation and respirator use practices. J Occup
Med, 32: 33-37.
Hallier E, Laughof T, Dannappel D, Leutbecher M, Schroeder K, Goergeus
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methylbromide, ethylene oxide and dichloromethane in human blood:
Influence of the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol 67: 173-178.
Hansch C & Leo A (1979) Substituent Constants for Correlation Analysis
in Chemistry and Biology. New York, Chichester, Brisbane, Toronto,
John Wiley and Sons.
Hardin BD & Manson JM (1980) Absence of dichloromethane teratogenicity
with inhalation exposure in rats. Toxicol Appl Pharmacol, 52: 22-28.
Harkov R (1984) Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci Total
Environ, 38: 259-274.
Harkov R, Giante SJ, Bozelli JW, & LaRegina JE (1985) Monitoring
volatile organic compounds at hazardous and sanitary landfills in New
Jersey. J Environ Sci Health, A20: 491-501.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnoro S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous volatile chlorinated methanes and ethanes. Cancer Res,
43: 1945-1950.
Haun CC, Vernot EH, Darmer KI, & Diamond SS (1972) Continuous animal
exposure to low levels of dichloromethane. In: Proceedings of the 3rd
Annual Conference on Environmental Toxicology. Dayton, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
pp. 199-208 (Paper No. 12; AMRL-TR-130).
Hayes WC & Bailey RE (1977) The inhibition of anaerobic sludge gas
production by 1,1,1-trichloroethane, methylene chloride,
trichloroethylene and perchloroethylene. Report dated 27 Jan 1977.
Midland, Michigan, USA, Dow Chemical.
Hearne FT, Grose F, Pifer WJ, Friedlander BR, & Raleigh RL (1987)
Methylene chloride mortality study: Dose-response characterization and
animal model comparison. J Occup Med, 29: 217-228.
Hearne FT & Friedlander BR (1981) Follow-up of methylene chloride
study. J Occup Med, 23: 660.
Hearne FT, Pifer JW, & Grose F (1990) Absence of adverse mortality
effects in workers exposed to methylene chloride. An update. J Occup
Med, 32(3): 234-240.
Hegi ME, Soderkvist P, Foley JE, Schronhoven R, Swenberg JA, Kari F,
Maronpot RR, Anderson MW, & Wiseman RW (1993) Characterization of p53
mutations in methylene chloride-induced lung tumors from B6C3F1 mice.
Carcinogenesis, 14(5): 803-810.
Heikes DL & Hopper ML (1986) Purge and trap method for determination
of fumigants in whole grains, milled grain products and intermediate
grain based foods. J Assoc Off Anal Chem, 69(6): 990-998.
Heikes DL (1987) Purge and trap method for determination of volatile
hydrocarbons and carbon disulphide in table ready foods. J Assoc Off
Anal Chem, 70(2): 215-226.
Heil H, Eikman T, Einbrodt HJ, König H, Lahl U, & Zeschmar-Lahl B
(1989) [Consequences of the Bielefeld-brake Atlas case.] Vom Wasser,
72: 321-348 (in German).
Heineman EF, Cocco P, Gomez MR, Dosemeci M, Stewart PA, Hayes RB,
Zahim SH, Thomas TL, & Blair A (1994) Occupational exposure to
chlorinated aliphatic hydrocarbons and risk of astrocytic brain
cancer. Am J Ind Med, 26(2): 155-169.
Heitmuller PT, Hollister TA, & Parrish PR (1981) Acute toxicity to 54
industrial chemicals to sheepshead minnows (Cyprinodon variegatus).
Bull Environ Contam Toxicol, 27: 596-604.
Hellmann H (1984) [Readily volatile chlorohydrocarbons in the inland
waters of the Federal Republic of Germany - occurrence and
quantities.] Gesundheits-Ingenieur, 105: 269-278 (in German).
Helz GR & Hsu RY (1978) Volatile chloro- and bromocarbons in coastal
waters. Limnol Oceanogr, 23: 858-869.
Henson JM, Yates MV, Cochran JW, & Shackleford DL (1988) Microbial
removal of halogenated methanes, ethanes, and ethylenes in an aerobic
soil exposed to methane. FEMS Microbiol Ecol, 53: 193-201.
Heppel LA, Neal PA, Perrin TL, Orr ML, & Porterfield VT (1944)
Toxicology of dichloromethane (methylene chloride). I. Studies on
effects of daily inhalation. J Ind Hyg Toxicol, 26: 8-16.
Heppel LA & Neal PA (1944) Toxicology of dichloromethane (methylene
chloride). II. Its effect upon running activity in the male rat. J Ind
Hyg Toxicol, 26: 17-21.
Hermens J, Busser F, Leeuwangh P, & Musch A (1985) Quantitative
structure-activity relationships and mixture toxicity of organic
chemicals in Photobacterium phosphoreum: the microtox test.
Ecotoxicol Environ Saf, 9: 17-25.
Hext PM, Foster J, & Millward SW (1986) Methylene chloride
(Dichloromethane): 10-day inhalation toxicity study to investigate the
effects on rat and mouse liver and lungs. ICI Report No. CTL/P/1432.
Horvath AL (1982) Halogenated hydrocarbons: solubility-miscibility
with water. New York, Marcel Dekker, Inc.
Hov O, Penkett SA, lsaksen ISA, & Semb A (1984) Organic gases in the
Norwegian Arctic. Geophys Res Left, 11: 425-428.
Howard PH (1990) Dichloromethane. In: Howard PH ed. Handbook of
environmental fate and exposure data for organic chemicals. Chelsea,
Michigan, Lewis Publishers, Inc., pp 176-183.
Howard PH, Sage GW, & Jarvis WF ed. (1990) Handbook on Environmental
Fate and Exposure Data for Organic Chemicals. Chelsea, Michigan, Lewis
Publishers Inc., pp 176-183.
HSE (UK Health and Safety Executive) (1987) ACTS review:
dichloromethane. London, Her Majesty's Stationery Office.
HSE (UK Health and Safety Executive) (1992) National exposure data
base. Bootle (Merseyside), United Kingdom. Health and Safety
Executive.
Hughes JP (1954) Hazardous exposure to some so-called safe solvents. J
Am Med AssPc, 156: 234-237.
Hughes NJ & Tracey JA (1993) A case of methylene chloride (nitremans)
poisoning, effects on carboxyhaemoglobin levels. Hum Exp Toxicol,
12(2) 159-160.
Hutchinson TC, Hellebust JA, Tam D, MacKay D, Mascarenhas RA, & Shiu
WY (1978) The correlation of the toxicity to algae of hydrocarbons and
halogenated hydrocarbons with their physical-chemical properties.
Environ Sci Res, 16: 577-586.
IARC (1986) Some halogenated hydrocarbons and pesticide exposure.
Lyon, International Agency for Research on Cancer, 43-85 (IARC
Monograph on the Evaluation of Carcinogenic Risk of Chemicals to
Humans, Volume 41).
ILO (1991) Occupational exposure limits for airborne toxic substances,
3rd ed. Geneva, International Labour Office (Occupational Safety and
Health Series No. 37).
IPCS (International Programme on Chemical Safety) (1984) Environmental
health criteria 32: methylene chloride. Geneva, World Health
Organization.
Ito A, Kawata F, Takeshita T, & Ito M (1990) [Experimental studies of
effects of methylene chloride on living body (1)]. Hochudoku, 8: 64-65
(in Japanese).
Janssen PJM & Pot TE (1988a) Acute oral toxicity study with
dichloromethane in rats. Weesp, The Netherlands, Solvay Duphar
(Unpublished document 56645/33/88).
Janssen PJM & Pot TE (1988b) Acute dermal toxicity study with
dichloromethane in rats. Weesp, The Netherlands, Solvay Duphar
(Unpublished document 55645/24/88).
Jensen AA (1983) Chemical contaminants in human milk. Residue Rev,
89: 1-108.
Jenson RA (1978) A simplified bioassay using finfish for estimating
potential spill damage. In: Proceedings of the Meeting on Control of
Hazardous Material Spills, Rochville, Maryland, pp 104-108.
Jernelov M & Antonsson AB (1987) [Exposure to solvents when pouring
polyurethane into moulds.] Oslo, IVL (Report No. B869) (in Norwegian).
Jongen WMF, Alink GM, & Koeman JH (1978) Mutagenic effect of
dichloromethane on Salmonella typhimurium. Mutat Res, 56: 245-248.
Jongen WMF, Lohman PHM, Kottenhagen M J, Alink GM, Betends F, & Koeman
JH (1981) Mutagenicity testing of dichloroethane in short-term
mammalian test systems. Mutat Res, 81(2): 203-213.
Jongen WMF, Harmsen EGM, Alink GM, & Koeman JH (1982) The effect of
glutathione conjugation and microsomal oxidation on the mutagenicity
of dichloromethane in Salmonella typhimurium. Mutat Res,
95: 183-189.
Jongen WMF (1984) Relationship between exposure time and metabolic
activation of dichloromethane in Salmonella typhimurium. Mutat Res,
136(2): 107-108.
Juhnke I & Ltidemann D (1978) [Results of the testing of 200 chemical
compounds for acute toxicity for fish in the golden orfe test.] Z
Wasser Abwasser Forsch, 11: 161-164 (in German).
Kanazawa S & Filip Z (1987) Effects of trichloroethylene and
dichloromethane on soil biomass and microbial counts. Zent.bl
Bakteriol Hyg, 184; 24-33.
Kanazawa S & Filip Z (1986) Effect of trichloroethylene,
tetrachloroethylene and dichloromethane on enzymatic activities in
soil. Appl Microbiol Biotechnol, 25: 76-81.
Kanno J, Foley JF, Kari F, Anderson MW, & Maronport RR (1993) Effect
of methylene chloride inhalation on replicative DNA synthesis in the
lungs of female B6C3F1 mice. Environ Health Perspect, 101 (Suppl 5):
271-286.
Kari FW, Maronpot RR, & Anderson MW (1992) Testimony for the OSHA
hearing on the proposed occupational standard for methylene chloride.
Research Triangle Park, North Carolina, National Institute of
Environmental Health Sciences.
Kari FW, Foley JF, Seilkop SK, Maronpot RR, & Anderson MW (1993)
Effect of varying exposure regimens on methylene chloride induced lung
and liver tumors in female B6C3F1 mice. Carcinogenesis,
14(5): 819-826.
Karickhoff SW (1981) Semi-empirical estimation of sorption of
hydrophobic pollutants on natural sediments and soils. Chemosphere,
10(8): 833-847.
Karlsson J-E, Rosengren LE, Kjellstrand P, & Haglid KG (1987) Effects
of low-dose inhalation of three chlorinated aliphatic organic solvents
on deoxyribonucleic acid in gerbil brain. Scand J Work Environ Health,
13: 453-458.
Kashin LM, Makotchenko VM, Malinina-Putsenko VP, Mikhailovskaja LF, &
Shmuter LM (1980) [Experimental and clinico-hygienic investigations of
methylene chloride toxicity.] Vrach Delo, 1: 100-103 (in Russian).
Kawasaki M (1980) Experiences with the test scheme under the chemical
control law of Japan: An approach to structure-activity correlations.
Ecotoxicol Environ Saf, 4: 444-454.
Kelley RD (1985) Synthetic organic compound sampling survey of public
water supplies. Washington, DC, Environmental Protection Agency (NTIS
PB85-214427).
Kelly M (1988) Case reports of individuals with oligospermia and
methylene chloride exposures. Reprod Toxicol, 2: 13-17.
Kim YC & Carlson GP (1986) The effect of an unusual workshift on
chemical toxicity, I. Studies on the exposure of rats and mice to
dichloromethane. Fundam Appl Toxicol, 6: 162-171.
Kimura ET, Ebert DM, & Dodge PW (1971) Acute toxicity and limits of
solvent residue for sixteen organic solvents. Toxicol Appl Pharmacol,
19: 699-704.
Kirschman JC, Brown NM, Coots RH, & Morgareidge K (1986) Review of
investigations of dichloromethane metabolism and subchronic oral
toxicity study as the basis for the design of chronic oral studies in
rats and mice. Food Chem Toxicol, 24: 943-949.
Kirwin CJ, Thomas WC, & Simmon VF (1980) In vitro microbiological
mutagenicity studies of hydrocarbon propellants. J Soc Cosmet Chem,
31(7): 367-370.
Kitchin KT & Brown JL (1989) Biochemical effects of three carcinogenic
chlorinated methanes in rat livers. Teratogen Carcinogen Mutagen,
9: 61-69.
Kjellstrand P, Bjerkemp M, Adler-Maihofer M, & Holmquist B (1986)
Effects of methylene chloride on body and organ weight and plasma
butyrylcholinesterase activity in mice. Acta Pharmacol Toxicol,
59: 73-79.
Kjellstrand P, Mansson L, Holmquist B, & Jonsson I (1990) Tolerance
during inhalation of organic solvents. Pharmacol Toxicol, 66: 409-414.
Klaassen CD & Plaa GL (1966) Relative effects of various chlorinated
hydrocarbons on liver and kidney function in mice. Toxicol Appl
Pharmacol, 9: 139-151.
Klaassen CD & Plaa GL (1967) Relative effects of various chlorinated
hydrocarbons on liver and kidney function in dogs. Toxicol Appl
Pharmacol, 10: 119-131.
Klecka GM & Gonsior SJ (1984) Nonenzymatic reductive dechlorination of
chlorinated methanes and ethanes in aqueous solution. Chemosphere,
13: 391-402.
Klecka GM (1982) Fate and effects of methylene chloride in activated
sludge. Appl Environ Microbiol, 44: 701-707.
Klimmer OR (1968) Working paper on dichloromethane. Presented to the
Committee on Foreign Substances in Food of the German Research
Council, Bad Godesberg, 5 July 1968. Bonn, German Research Council (in
German).
Kluwe WM, Harrington FW, & Cooper SE (1982) Toxic effects of
organohalide compounds on renal tubular cells in vivo and in vitro.
J Pharmacol Exp Ther, 220: 597-603.
KNIE (1988) The dynamic daphnia test - practical experience in the
monitoring of inland waters. Gewasserschutz-Wasser-Abwasser,
102: 341-357 (in German).
Koch M, Dolfing J, Whurmann K, & Zehnder AJB (1983) Pathways of
propionate degradation by enriched methanogenic cultures. Appl Environ
Microbiol, 45: 1411-1414.
Könemann H (1981) Quantitative structure-activity relationships in
fish toxicity studies, part 1: Relationship for 50 industrial
pollutants. Toxicology, 19: 209-211.
Kool HJ, Van Kreijl CF, & Zoeteman BCJ (1982) Toxicology assessment of
organic compounds in drinking water. CRC Crit Rev Environ Control,
12: 307-350.
Kopfler FC, Melton RG, Lingg RD, & Coleman WE (1977) Human exposure to
water pollutants. Adv Environ Sci Technol, 8: 419-433.
Kozena L, Frantik E, & Vodickova A (1990) Methylene chloride does not
impair vigilance performance at blood levels simulating limit
exposure. Acta Nerv Super, 32: 35-37.
Kramers PGN, Mout HCA, Bissumbhar B, & Mulder CR (1991) Inhalation
exposure in Drosophila mutagenesis assays: experiments with aliphatic
halogenated hydrocarbons, with emphasis on the genetic activity
profile of 1,2-dichloroethane. Mutat Res, 252:17-33.
Kubic VL & Andera MW (1978) Metabolism of the dichloromethane to
carbon monoxide III. Studies on the mechanism of the reaction. Blochem
Pharmacol, 27: 2349-2355.
Kubic VL, Anders MW, Engel RR, Bertow CH, & Caughey WS (1974)
Metabolism of dichloromethanes to carbon monoxide I. In vivo
studies. Drug Metab Dispos, 2: 53-57.
Kubic VL & Anders MW (1975) Metabolism of dihalomethanes to carbon
monoxide. II. In vitro studies. Drug Metab Dispos, 3: 104-112.
Kühn R, Pattard M, Pernak KD, & Winter A (1989) Results of the harmful
effects of selected water pollutants (anilines, aliphatic compounds)
to Daphnia magna. Water Res, 23: 495-499.
Kühn R (1979) Results of ecotoxicological testing of about 200
selected compounds. Paris, Organisation for Economic Co-operation and
Development, Chemicals Testing Programme (ECO 22).
Kurppa K, Kivisto H, & Vainio H (1981) Dichloromethane and carbon
monoxide inhalation: carboxyhaemoglobin addition and drug metabolising
enzymes in rat. Int Arch Occup Environ Health, 49: 83-87.
Kurppa K & Vainio H (1981) Effects of intermittent dichloromethane
inhalation on blood carboxyhaemoglobin concentration and drug
metabolizing enzymes in rat. Res Commun Chem Pathol Pharmacol,
32: 535-544.
Kutob SD & Plaa GL (1962) A procedure for estimating the hepatoxic
potential of certain industrial solvents. Toxicol Appl Pharmacol,
4: 354-361.
Kuzelova M & Vlasak R (1966) [The effect of methylene chloride on the
health of workers in production of film-foils and investigation of
formic acid as a methylene-dichloride metabolite]. Prac Lek,
18: 167-170 (in Czech).
Kwa HG, Van der Gugten AA, & Verhofstad F (1974) Radioimmunoassay of
rat prolactin. Prolactin levels of rats with spontaneous pituitary
tumours, primary oestrogen-induced pituitary tumours or pituitary
transplants. Eur J Cancer, 5: 571-579.
Laham S (1978) Toxicological studies on dichloromethane, a solvent
simulating carbon monomide poisoning. Toxicol Eur Res, 1: 63-73.
Landry TD, Burek JD, Bell TJ, & Wolfe EL (1981) Methylene chloride: An
acute inhalation toxicity study in rats. Midland, Michigan, Dow
Chemical Company (Internal report).
Lanes SF, Cohen A, Rothman KJ, Dreyer NA, & Soden KJ (1990) Mortality
of cellulose fiber production workers. Scand J Work Environ Health,
16: 247-251.
Lanes SF, Rothman KG, Dreyer NA, & Soden KJ (1993) Mortality update of
cellulose fiber production workers. Scand J Work Environ Health,
19(6): 426-428.
Lapat-Polasko LT, McCarty PL, & Zehnder AJB (1984) Secondary substrate
utilisation of methylene chloride by an isolated strain of
Pseudonomas sp. Appl Environ Microbiol, 47: 825-830.
Lash AA, Becker CE, So Y, & Shore M (1991) Neurotoxic effects of
methylene chloride: Are they long lasting in humans? Br J Ind Med,
48: 418-426.
Lazarew NW (1929) The narcotic effect of vpours from the chlorine
derivatives of methane, ethane and ethylene. Arch Exp Pathol
Pharmakol, 141: 19-24.
Le Blanc GA (1984) Interspecies relationships of priority pollutants
to water flea (Daphnia magna). Bull Environ Contam Toxicol,
24: 684-691.
Le Blanc GA (1980) Acute toxicity of priority pollutants to water flea
(Daphnia magna). Bull Environ Contam Toxicol, 24: 684-691.
Lee Rodkey F & Collison HH (1977) Biological oxidation of [14C]
methylene chloride to carbon monoxide and carbon chloride by the rat.
Toxicol Appl Pharmacol, 40: 33-38.
Lefevre PA & Ashby J (1989) Evaluation of dichloromethane as an
indicator of DNA synthesis in the B6C3F1 mouse liver. Carcinogenesis,
10: 1067-1072.
Lehman J & Paech C (1972) [Einfluss einiger lipophiler Lösungsmittel
in gasförmigem Zustand auf die CO2-Fixierung durch Luzerne.]
Experientia (Basel), 28: 1415-1416.
Leikin JB, Kaufman D, Lipscomb JW, Burda AM, & Hryhorczuk DO (1990)
Methylene chloride: Report of five exposures and two deaths. Am J
Emerg Med, 8: 534-537.
Leisinger T (1983) Microorganisms and xenobiotic compounds.
Experientia (Basel), 39: 1183-1191.
Leonardos G, Kendall D, & Barnard N (1969) Odor threshold
determination of 53 odorant chemicals. J Air Pollut Control Assoc,
19: 91-95.
Leuschner F, Neumann BW, & Hubscher F (1984) Report on subacute
toxicological studies with dichloromethane in rats and dogs by
inhalation. Arzneimittel forschung, 34: 1772-1774.
Levaggi DA, Siu W, Zerrudo RV, & La Voie JW (1988) Experiences in
gaseous toxic monitoring in the San Francisco Bay area. Proc APCA
Annual Meet, 81: 88-95A.
Libuda HG, Zabel F, Fink EH, & Becker KH (1990) Formyl chloride: UV
absorption cross sections and rate constants for the reactions of Cl
and OH. J Phys Chem, 94: 5860-5865.
Longstaff E, Robinson M, Bradbrook C, Styles JA, & Purchase IFH (1984)
Genotoxicity and carcinogenicity of fluorocarbons assessment by short-
term in vitro tests and chronic exposure in rats. Toxicol Appl
Pharmacol, 72(1): 15-31.
Loyke HF (1973) Methylene chloride and chronic renal hypertension.
Arch Pathol, 95(2): 130-131.
LWA (1980) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1981) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1982) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1983) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1984) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1989) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1990) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1991) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
LWA (1992) [Water quality report.] Düsseldorf, Germany, State Board
for Water and Waste of North Rhein-Westphalia (in German).
Lyman WJ, Reehl WF, & Rosenblatt DH ed. (1982) Handbook of Chemical
Property Estimation Methods: Environmental Behavior of Organic
Compounds. New York, McGraw-Hill Book Co.
MacEwen JD & Vernot EM (1972) Toxic hazards research unit annual
technical report. Dayton, Ohio, Wright Patterson Air Force Base.
Aerospace Medical Research Laboratory (AMRL-TR-72-62).
MacEwen JD, Vernot EH, & Haun CC (1972) Continuous animal exposure to
dichloromethane. Dayton, Ohio, Wright Patterson Air Force Base.
Aerospace Medical Research Laboratory (AMRL-TR-72-28).
Makisimov GG & Mamleyeva NK (1977) [An assessment of the hazard
presented by methylene chloride entering the organism percutaneously.]
Absorbtion of industrial poisons through the skin, and prevention
thereof.] 83-88 (in Russian).
Maltoni C, Cotti G, & Perino G (1986) Experimental research on
methylene chloride carcinogenesis. In: Maltoni C & Mehlman MA (eds.)
Archives Research Industrial Carcinogenesis, Volume 4, Princeton, New
Jersey, Princeton Scientific Publishing Co.
Maltoni C, Cotti G, & Perino G (1988) Long-term carcinogenicity
bioassays administered by ingestion to Sprague-Dawley rats and Swiss
mice and by inhalation to Sprague-Dawley rats. Ann NY Acad Sci,
534: 352-366.
Manno M, Chirillo R, Danlotti G, Cocheo V, & Albrizio F (1989)
Carboxyhaemoglobin and fatal methylene chloride poisoning. Lancet,
2(8657): 274.
Manno M, Rugge M, & Cockeo V (1992) Double fatal inhalation of
dichloromethane. Hum Exp Toxicol, 11(6): 540-545.
Masuda Y, Yano I, & Murano T (1980) Comparative studies on the
hepatoxic actions of chloroform and related halogenomethanes in normal
and phenobarbital-pretreated animals. J Pharm Dyn, 3: 53-64.
Mattsson JL, Albee RR, & Streeter CM (1988) Evaluation of the acute
neuropharmacologic effects of dichloromethane in rats. Midland,
Michigan, Dow Chemical Company (Internal Report).
Mattsson JL, Albee RR, & Eisenbrandt DL (1990) Neurotoxicologic
evaluation of rats after 13 weeks of inhalation exposure to
dichloromethane or carbon monoxide. Pharmacol Blochem Behav,
36: 671-681.
McCammon CS, Glaser RA, Wells VE, Plupps FC, & Halperin WE (1991)
Exposure of workers engaged in furniture stripping to methylene
chloride as determined by environmental biological monitoring. Appl
Occup Environ Hyg, 6: 371-379.
McCarroll NE, Cortina TA, Zito MJ, & Farrow MG (1983) Evaluation of
methylene chloride and vinylidene chloride in mutational assays.
Environ Mutagen, 5(3): 426-427.
McCarty WM (1979) Toxicity of methylene chloride to Daphnids. Midland,
Michigan, Dow Chemical (DR 001-5849-099-005).
McCarty LP, Flannagan DC, Randall SA, & Johnson KA (1992) Acute
toxicity in rats of chlorinated hydrocarbons given via the intracheal
route. Hum Exp Toxicol, 11: 173-177.
McDougal JN, Jepsono GW, Clewell III HJ, MacNaughton MG, & Andersen ME
(1986) A physiological pharmacokinetic model for dermal absorption of
vapors in the rat. Toxicol Appl Pharmacol, 85: 286-294.
McGeorge L, Krietzman S, Bukowski G, & Hamill B (1987) Implementation
and results of a mandatory state-wide program for organic contaminant
analysis of delivered water. In: Proceedings of the Water Quality
Technology Conference, Vol 15, pp 71-102.
McGregor DB (1979) Practical experience in testing unknowns in vitro.
In: Pagel GE (ed) Mutagenesis in Submammalian Systems, Status and
Significance. MTP Press, pp 53-71.
McKenna MJ, Saunders JH, & Boeckler WH (1980) The pharmacokinetics of
inhaled methylene chloride in human volunteers. Toxicol Appl
Pharmacol, Abstr 59.
McKenna MJ & Zempel JA (1981) The dose-dependent metabolism of
14C-methylene chloride following oral administration to rat. Food
Cosmet Toxlcol, 19: 73-78.
McKenna MJ, Zempel JA, & Braun WH (1982) The pharmacokinetics of
inhaled methylene chloride in rats. Toxicol Appl pharmacol, 65: 1-10.
Meltzer N, Rampy L, Bielinski P, Garofalo M, & Sayad R (1977) Skin
irritation, inhalation toxicity studies of aerosols using methylene
chloride. Drug Cosmet Ind, 120: 38-40.
Mennear JH, McConnell EE, Huff JE, Renne RA, & Giddens E (1988)
Inhalation toxicology and carcinogenesis studies of methylene chloride
(dichloromethane) in F344/N rats and B6C3F1 mice. Ann NY Acad Sci,
534: 343-351.
Merlin G, Thiebaud H, Blake G, Sembiring S, & Alary J (1992) Mesocosms
and microcosms utilization for ecotoxicity evaluation of
dichloromethane, a chlorinated solvent. Chemosphere, 24: 37-50.
Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, & Kitterer B
(1991) Theta, a new class of glutathione transferases purified from
rat and man. Biochem J, 274: 409-414.
Michael LC, Pellizzari ED, & Wiseman RW (1988) Development and
evaluation of a procedure for determining volatile organics in water.
Environ Sci Technol 22: 565-570.
Miller L, Pateras V, Friederici H, & Engel G (1985) Acute tubular
necrosis after inhalation exposure to methylene chloride. Report of a
case. Arch Intern Med, 145: 145-146.
Mirer FE, Silverstein M, & Park R (1988) Methylene chloride and cancer
of the pancreas [letter]. J Occup Med, 30: 475-476.
Moody DE, James JL, Clawson GA, & Smuckler EA (1981) Correlations
among the changes in hepatic microsomal components after intoxication
with alkyl halides and other hepatoxins. Mol Pharmacol, 20: 685-693.
Morris JB, Smith FA, & Garman RH (1979) Studies on methylene chloride-
induced fatty liver. Exp Mol Pathol, 30: 386-393.
Moskowitz S & Shapiro H (1952) Fatal exposure to methylene chloride
vapor. Am J Ind Hyg Occup Med, 5: 116-123.
Mueller S, Weise M, Krug T, & Hoffmann P (1991) Adrenergic
cardiovascular actions in rats as affected by dichloromethane
exposure. Biomed Blochim Acta, 50: 307-311.
Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D,
Martini R, & Caspary WJ (1990) L5178Y mouse lymphoma cell mutation
assay results with 41 compounds. Environ Mol Mutagen, 16
(Suppl 18): 138-167.
Namkung E & Rittmann BE (1987) Estimating volatile organic compound
emissions from publicly owned treatment works. J WPCF, 59: 670-678.
Narotsky MG, Hamby BT, Mitchell DS, & Kavlock RJ (1992) Full-litter
resorptions caused by low-molecular weight hydrocarbons in F-344 rats
[abstract 67]. Teratology, 45: 472.
Neely WB (1964) Metabolic fate of formaldehyde-14C intraperitoneally
administered to the rat. Biochem Pharmacol, 13: 1137-1142.
Negherbon WO (1959) Methylene chloride. In: Negherborn WO (ed.)
Handbook of toxicology III: insecticides, a compendium. London,
Saunders, pp 485-486.
Nellor MH, Baird RD, & Smyth JR (1985) Health effects of indirect
potable water reuse. J Am Water Works Assoc, 77(7): 88-96.
Nendza M & Seydel JK (1988) Multivariate data analysis of various
biological test systems used for the quantification of ecotoxic
compounds. Quant Struct-Act Relatsh, 7: 165-174.
Nestmann ER, Otson R, Williams DT, & Kowbel DJ (1981) Mutagenicity of
paint removers containing dichloromethane. Cancer Lett, 11: 295-302.
Nestmann ER, Lee EGH, Matula TI, Douglas GR, & Mueller JC (1980)
Mutagenicity of constituents identified in pulp and paper mill
effluents using the Salmonella/mammalian-microsome assay. Mutat Res,
79: 203-212.
Neuhauser EF, Loehr RC, Malecki MR, Milligan DR, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14: 383-388.
Neumann F (1991 ) Early indicators for carcinogenesis in sex-hormone-
sensitive organs. Mutat Res, 248: 341-356.
Nicola RM, Branchflower R, & Pierce D (1987) Chemical contaminants in
bottomfish. J Environ Health, 49: 342-347.
NIOSH (1976) Criteria for a Recommended Standard ... Occupational
Exposure to Methylene Chloride. Cincinnati, Ohio, National Institute
of Occupational Safety and Health (DHEW Publication No. 76-138).
NIOSH (1987) Method No. 1005, Revision 1. NIOSH Manual of Analytical
Methods, 3rd ed. Cincinnati, Ohio, National Institute for Occupational
Safety and Health.
Nishio A, Yajema S, Yahogi M, Sasaki Y, Sawano Y, & Miyao N (1984)
[Studies on the teratogenicity of dichloromethane in rats]. Gakujutsu
Hikoku-Kagoshima Daigaku Nogakubu, 34: 95-103 (in Japanese).
Nitschke KD, Stevens GA, Kociba RJ, Keyes DG, & Rampy LW (1981)
Methylene chloride: a four week inhalation toxicity study in rats,
hamsters and mice. Midland, Michigan, Dow Chemical Company (Internal
report).
Nitschke KD, Burek JD, Bell TJ, Kociba RJ, Rampy LW, & McKenna MJ
(1988a) Methylene chloride: A 2-year inhalation toxicity and
oncogenicity study in rats. Fundam Appl Toxicol, 11: 48-59.
Nitschke KD, Eisenbrandt DL, Lomax LG, & Rao KS (1988b) Methylene
chloride: Two-generation inhalation reproductive study in rats. Fundam
Appl Toxicol, 11: 60-67.
Norpoth K, Witting U, & Springorum M (1974) Induction of microsomal
enzymes in the rat liver by inhalation of hydrocarbon solvents. Int
Arch Arbeitsmed, 33: 315-321.
Novak JJ & Hain JR (1990) Furniture stripping vapor inhalation
fatalities: two case studies. Appl Occup Environ Hyg, 5: 843-847.
Novakova V, Musil J, Buckiova D, Taborsky O, Sollova H, & Vyborny P
(1981) Effect of tetrachloromethane and other chlorinated hydrocarbons
on the hepatic metabolism in the isolated perfused rat liver. J Hyg
Epidemiol Microbiol Immunol, 25: 369-383.
NTP (US National Toxicology Program) (1986) Toxicology and
carcinogenesis studies of dichloromethane (methylene chloride) (CAS
No. 75-09-2) in F344/N rats and B6C3F1 mice (inhalation studies).
Research Triangle Park, North Carolina, National Toxicology Programme
(Technical Report No. 306; NIH Publication No. 86-2562).
Otson R, Williams DT, & Bothwell PD (1982) Volatile organic compounds
in water at thirty Canadian potable water treatment facilities. J
Assoc Off Anal Chem, 65: 1370-1375.
Ott MG, Skory LK, Holder BB, Bronson JM, & Williams PR (1983) Health
evaluation of employees occupationally exposed to methylene chloride.
Scand J Work Environ Health, 9 (Suppl 1): 1-38.
Ottenwalder H & Peter H (1989) DNA binding assay of methylene chloride
in rats and mice [letter]. Arch Toxicol, 63: 162-163.
Ottenwalder H, Jager R, Thier R, & Bolt HM (1989) Influence of
cytochrome P-450 inhibitors on the inhalative uptake of methyl
chloride and methylene chloride in male B6C3F1 mice. Arch Toxicol, 13
(Suppl): 258-261.
Page BD & Charbonneau CF (1977) Gas chromatographic determination of
residual methylene chloride and trichloroethylene in decaffeinated
instant and ground coffee with electrolytic conductivity and electron
capture detection. J Assoc Off Anal Chem, 60: 710-715.
Page BD & Charbonneau CF (1984) Headspace gas chromatographic
determination of residual methylene chloride in decaffeinated tea and
coffee with electronic conductivity detection. J Assoc Off Anal Chem,
67: 757-761.
Pankow D, Gutewort R, Glatzel W, & Tieze K (1979) Effect of
dichloromethane on the sciatic motor conduction velocity of rats.
Experientia (Basel), 35: 373-374.
Pankow D, Dretschmer S, & Weise M (1991) Effect of pyrazole on
dichloromethane metabolism to carbon monoxide. Recent Developments in
Toxicology: Trends, Methods and Problems. Arch Toxicol, 14 (Suppl):
246-248.
Pankow D & Jagielki S (1993) Effect of methanol or modifications of
the hepatic glutathione concentration on the metabolism of
dichloromethane to carbon monoxide in rats. Hum Exp Toxicol,
12(3): 227-231.
Pellizzari ED, Hartwell TD, Harris BS, Waddell RD, Whitaker DA, &
Erickson MD (1982) Purgeable organic compounds in mother's milk. Bull
Environ Contam Toxicol, 28: 322-328.
Pennington JT & Hadfield MG (1989) Larvae of nudibranch mollusc
(Phestilla sibogae) metamorphose when exposed to common organic
solvents. Biol Bull, 177: 350-355.
Penverne Y & Montiel A (1985) Etude des organohalogénés volatils dans
les eaux souterraines du departement du Val-de-Marne (France). Trib
Cebedeau, 505: 23-30.
Perbellini L, Brugnone F, Grigolini L, Cunegatti P, & Tacconi A (1977)
Alveolar air and blood dichloromethane concentration in shoe sole
factory workers. Int Arch Occup Environ Health, 40(4): 241-247.
Perocco P & Prodi G (1981) DNA damage by haloalkanes in human
lymphocytes cultured in vitro. Cancer Lett, 13: 213-218.
Peterson JE (1978) Modeling the uptake, metabolism, and excretion of
dichloromethane by man. Am Ind Hyg Assoc J, 39: 41-47.
Plaa GL & Larson RE (1965) Relative nephrotoxic properties of
chlorinated methane, ethane, and ethylene derivatives in mice. Toxicol
Appl Pharmacol, 7: 37-44.
Pleil JD & McClenny WA (1990) Canister-based sampling and subsequent
GC/MS analysis for measurement of trace-level volatile organohalogen
compounds. In: Proceedings of the 10th International Meeting Dioxin
90-Organohalogen compounds, Vol 2, pp 411-414.
Plumb RH Jr (1987) A comparison of ground water monitoring from CERCLA
and RCRA sites. Ground Water Monit Rev, Fall 1987: 94-100.
Poplawski-Tabarelli S & Uehleke H (1982) Inhibition of microsomal drug
oxidations by aliphatic hydrocarbons: correlation with vapour
pressure. Xenobiotica, 12: 55-61.
Portier CJ & Kaplan NL (1989) Variability of safe dose estimates when
using complicated models of the carcinogenic process. Fundam Appl
Toxicol, 13: 533-544.
Post W, Kromhout H, Heederik D, Noy D, & Duijzentkunst RS (1991)
Semiquantitative estimates of exposure to methylene chloride and
styrene: The influence of quantitative exposure data. Appl Occup
Environ Hyg, 6(3): 197-204.
Price P J, Hassett CM, & Mansield JI (1978) Transforming activities of
trichloroethylene and proposed industrial alternatives. In Vitro,
14: 290.
Putz VR, Johnson BL, & Setzer JV (1976) A comparative study of the
effects of carbon monoxide and methylene chloride on human
performance. J Environ Pathol Toxicol, 2: 97-112.
Puurunen J & Sotaniemi E (1985) Usefulness of follow-up liver-function
tests after dichloromethane exposure. Lancet, 1: 822.
Radding SB, Liv DH, Johnson HL, & Mill T (1977) Review of the
environmental fate of selected chemicals. Washington, DC, US
Environmental Protection Agency (EPA-560/5-77-003).
Raje R, Basso M, Tolen T, & Greening M (1988) Evaluation of in vivo
mutagenicity of low-dose methylene chloride in mice. J Am Coll
Toxicol, 7: 699-703.
Ranna R, Rugge R, & Cocheo V (1992) Double fatal inhalation of
dichloromethane. Hum Exp Toxicol, 11: 540-545.
Rapson WH, Nazar MA, & Butsky VV (1980) Mutagenicity produced by
aqueous chlorination of organic compounds. Bull Environ Contam
Toxicol, 24(4): 590-596.
Ratney RS, Wegman DH, & Elkins HB (1974) In vivo conversion of
methylene chloride to carbon monoxide. Arch Environ Health,
28: 223-226.
Rayez JC, Rayez MT, Halvick P, Duguay B, & Lesclaux R (1987) A
theorical study of the decomposition of halogenated alkoxy radicals.
I. Hydrogen and chlorine extrusions. Chem Phys, 116: 203-213.
Rebert CS, Matteuci M J, & Pryor GT(1989) Acute effects of inhaled
dichloromethane on the EEG and sensory-evoked potentials of Fischer-
344 rats. Pharmacol Biochem Behav, 34: 619-629.
Reitz RH, Smith FA, & Andersen ME (1986) In vivo metabolism of
14C-methylene chloride [abstract]. Toxicologist 6, A 1048.
Reitz RH, Mendrala AL, & Guengerich FP (1989) In vitro metabolism of
methylene chloride in human and animal tissues: use of physiologically
based pharmacokinetic models. Toxicol Appl Pharmacol, 97: 230-246.
Reynolds ES & Yee AG (1967) Liver parenchymal cell injury. V.
Relationships between patterns of chloromethane-C14 incorporation
into constituents of liver in vivo and cellular injury. Lab Invest,
16: 591-603.
Rittmann BE & McCarty PL (1980) Utilization of dichloromethane by
suspended and fixed-film bacteria. Appl Environ Microbiol,
39: 1225-1226.
RIVM (Rijksinstituut voor Volksgezondheid en Milieuhygiene) (1986)
Methylene chloride; 48 hour IC50/EC50 Daphnia magna (86/HO63) and
embryotoxicity for Oryzia latipes (86/HO65) (Project No. 840820)
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Roberts CYC & Marshall FPP (1976) Recovery after "lethal" quantity of
paint remover. Br Med J, 1: 20-21.
Rodruigez Rojo A, Freiria Gandara M J, Alvarez Devesa A, Lorenzo
Ferreira RA, & Bermejo Martinez F (1989) Determination of halogenated
hydrocarbons in the water supply of Santiago de Compostela (Spain).
Environ Technol Lett, 10(8): 717-724.
Rosengren LE, Kjellstrand P, Aurell A, & Haglid KG (1986) Irreversible
effects of dichloromethane on the brain after long term exposure: A
quantitative study of DNA and the glial cell marker proteins S-100 and
GFA. Br J Ind Med, 43: 291-299.
Rossman TG, Molina M, Meyer L, Boone P, Klein CB, Wang Z, Li F, Lin
WC, & Kinney PL (1991) Performance of 133 compounds in the lambda
prophage induction endpoint of the Microscreen assay and a comparison
with S. typhimurium mutagenicity and rodent carcinogenicity assays.
Murat Res, 260: 349-367.
Roth RP, Drew RT, Lo RJ, & Fouts JR (1975) Dichloromethane inhalation,
carboxyhaemoglobin concentrations and drug metabolizing enzymes in
rabbits. Toxicol Appl Pharmacol, 33: 427-437.
Ruhe RL, Watanabe A, & Stein G (1981) Health Hazard Evaluation Report
No. HHE-80-49-808: Superior Tube Company, Collegeville, PA,
Cincinnati, Ohio, National Institute of Occupational Safety and
Health.
Ruhe RL, Singal M, & Hervin RL (1982) Health Hazard Evaluation Report
No. HETA-80-79-1189.: Rexall Drug Company, St Louis, MO, Cincinnati,
Ohio, National Institute of Occupational Safety and Health.
Ruth H (1986) Odor thresholds and initiation levels of several
chemical substances. A review. Am Ind Hyg Asspc J, 47: A141-A151.
Sabel GV & Clark TP (1984) Volatile organic compounds as indicators of
municipal solid waste leachate contamination. Waste Manage Res,
2: 119-130.
Sahn SC & Lowther DK (1981) Pulmonary reactions to inhalation of
methylene chloride: Effects on lipid peroxidation in rats. Toxicol
Lett, 8(4-5): 253-256.
Sanhueza E & Heicklen J (1975) Chlorine-atom sensitized oxidation of
dichloromethane and chloromethane. J Phys Chem, 79: 7-11.
Savolainen H, Pfäffli P, Tengen M, & Vainio H (1977) Biochemical and
behavioral effects of inhalation exposure to tetrachloroethylene and
dichloromethane. J Neuropathol Exp Neurol, 36: 941-949.
Sawhney BL (1989) Movement of organic chemicals through landfill and
hazardous waste disposal sites. Soil Science Society of America and
American Society of Agronomy, pp 447-474.
Scholz-Muramatsu H, Schneider V, Gaiser S, & Bardtke D (1988)
Biological elimination of dichloromethane from contaminated
groundwater-interference by components of the groundwater. Wat Sci
Tech, 20: 393-397.
Schubert R (1979) Toxicity of organohalogen compounds towards bacteria
and their degradability. Spez Ber Kernforschungsaulage, 1979: 211-218.
Schumacher H & Grandjean E (1960) Comparative investigations on the
anaesthetic effect and acute toxicity of nine solvents. Arch
Gewerbepathol Gewerbehyg, 18: 109-119.
Schutz E (1960) Effects of organic liquids on the skin.
Arzeimittelforschung, 10: 1027-1029.
Schroeder KR, Hallier E, Peter N, & Bolt HM (1992) Dissociation of a
new glutathione-S-transferase activity in human erythrocytes. Blochem
Pharmacol, 43: 1671-1674.
Schwetz BA, Leong BKJ, & Gehring PJ (1975) The effect of maternally
inhaled trichloroethylene, perchloroethylene, methyl chloroform, and
methylene chloride on embryonal and fetal development in mice and
rats. Toxicol Appl Pharmacol, 32: 84-96.
Scott JB, Smith FA, & Garman RH (1979) Exposure of mice to CH2Cl2
and CH3OH alone and in combination [abstract]. Toxicol Appl
Pharmacol, 48: A 105.
Selan FM & Evans MA (1982) Role of hepatic microtubule system in
chlorinated hydrocarbon induced hepatic steatosis. Toxicologist,
2: 134 [abstract].
Selenka F & Bauer U (1978) [Survey of organochlorine compounds in
water.] Forsch Ber, A27: 187-188 (in German).
Serota DG, Thakur AK, Ulland BM, Kirschman JC, Brown NM, Cotts RG, &
Morgareidge K (1986a) A two-year drinking-water study of
dichloromethane in rodents I. Rats. Food Chem Toxicol, 24: 951-958.
Serota DG, Thakur AK, Ulland BM, Kirschman JC, Brown NM, Cotts RG, &
Morgareidge K (1986b) A two-year drinking-water study of
dichloromethane in rodents. II. Mice. Food Chem Toxicol, 24: 959-963.
Shah JJ & Heyerdahl EK (1988) National ambient volatile organic
compounds (VOCs): data base update. Rep. by Nero and Associated,
Portland (OR), EPA/600/3-88/010a. Research Triangle Park, North
Carolina, Atmospheric Sciences Research Laboratory.
Sheldon T, Richardson CR, & Elliott BM (1987) Inactivity of methylene
chloride in the mouse bone marrow micronucleus assay. Mutagenesis,
2: 57-59.
Shikiya J, Tsou G, Kowalski J, & Leh F (1984) Ambient monitoring of
selected halogenated hydrocarbons and benzene in the California South
Coast Air Basin. Proceeding of 77th Annual Meeting of the Air
Pollution Control Association 24-29 June.
Shmuter LM & Kashin LM (1978) Experimental study of the sensitising
effect of some chlorinated aliphatic hydrocarbons. Gig Tr Prof Zabol,
3: 57-59 (in Russian).
Simmon VF, Kauhanen K, & Tardill RG (1977) Mutagenic activity of
chemicals identified in drinking water. Dev Toxicol Environ Sci,
2: 249-258.
Singh HB, Salas LJ, & Stiles RE (1983) Selected man-made halogenated
chemicals in the air and oceanic environment. J Geophys Res,
88: 3675-3683.
Sinha YN (1981) Plasma prolactin analysis as a potential predictor of
murine mammary tumorigenesis. In: McPike PK, Sitteri, & Welsch CW ed.
Hormones and breast cancer. Cold Spring Harbor, New York, Cold Spring
Laboratory (Banbury Report No. 8).
Slooff W & Ros JPM (1988) Integrated criteria document:
Dichloromethane. Bilthoven, The Netherlands, National Institute of
Public Health and Environmental Protection (Report No. 758473009).
Smith RL (1989) A computer assisted, risk-based screening of a mixture
of drinking water chemicals. Trace Subst Environ Health, 22: 215-232.
Soden KJ (1993) An evaluation of chronic metylene chloride exposure.
J Occup Med, 35(3): 282-286.
Staab HA & Datta AP (1964) Formyl chloride. Angew Chem Int ed, 3: 132.
Staples CA, Frances Werner A, & Hoogheem TJ (1985) Assessment of
priority pollutant concentrations in the United States using STORET
database. Environ Toxicol Chem, 4: 131-142.
Stern FB, Halperin WE, Hornung RW, Ringenburg VL, & McCammon CS (1988)
Heart disease mortality among bridge and tunnel officers exposed to
carbon monoxide. Am J Epidemiol, 128(6): 1276-1288.
Stevenson MF, Chemoweth MB, & Cooper GL (1978) Effect on
carboxyheamoglobin of exposure to aerosol spray paints with methylene
chloride. Clin Toxicol, 12: 551-561.
Stewart RD & Dodd HC (1964) Absorption of carbon tetrachloride,
trichloroethylene, tetrachloroethylene, methylene chloride, and
1,1,1-trichloroethane through the human skin. Am Ind Hyg Assoc J,
25: 439-446.
Stewart RD, Hake CL, & Wu A (1976) Use of breath analysis to monitor
methylene chloride exposure. Scand J Work Environ Health, 2: 57-70.
Stewart RD, Fisher TN, Hosko MJ, Peterson JE, Baretta ED, & Dodd HC
(1972) Experimental human exposure to methylene chloride. Arch Environ
Health, 25: 342-348.
Stover EL & Kincannon DF (1983) Biological treatability of specific
organic compounds found in chemical industry wastewaters. J Water
Pollut Control Fed, 55: 97-109.
Stuckey DC, Owen WF, & McCarty PL (1980) Anaerobic toxicity evaluation
by batch and semi-continuous assays. J Water Pollu Control Fed,
52(4): 720-729.
Stucki G, Gälli R, Ebersold H-R, & Leisinger TH (1981) Dehalogenation
of dichloromethane by cell extracts of Hyphomicrobium DM2. Arch
Microbiol, 130: 366-371.
Stucki G (1990) Biological decomposition of dichloromethane from a
chemical process effluent. Biodegradation, 1: 221-228.
Svirbely JL, Highman B, Alford we, & Von Oettingen WF (1947) The
toxicity and narcotic action of mono-chloro-mono-bromo-methane with
special reference to inorganic and volatile bromide in blood, urine
and brain. J Ind Hyg Toxlcol, 29: 382-389.
Tabak HH, Quave SA, Mashni CI, & Barth EF (1981) Biodegradability
studies with organic priority pollutant compounds. J Water Pollut
Control Fed, 53: 1503-1518.
Takashita T, lto A, Kawata F, Ito M, & lto K (1991) [Experimental
studies of effects of methylene chloride on living bodies (2)].
Hochudoku, 9: 100-101 (in Japanese).
Tariot PM (1983) Delirium resulting from methylene chloride exposure:
Case report. J Clin Psychiatry, 44: 340-342.
Taskinen H, Lindbohm M-L, & Hemminki K (1986) Spontaneous abortions
among women working in the pharmaceutical industry. Br J Ind Med,
43: 199-205.
Taylor G J, Drew R J, Lores EM, & Clemmer TA (1976) Cardiac depression
by haloalkane propellants, solvents, and inhalation anesthetics in
rabbits. Toxicol Appl Pharmacol, 38: 379-387.
Tham R, Bunnfors I, Erikkson B, Larsby B, Lindgren S, & Odkvist LM
(1984) Vestibulo-ocular disturbances in rats exposed to organic
solvents. Acta Pharmacol Toxicol, 54: 58-63.
Thiel PG (1969) The effect of methane analogues on methanogenesis in
anaerobic digestion. Water Res, 3: 215-223.
Thier R, Forst U, Deutschmann S, Schroeder KR, Westphal G, Hallier E,
& Peter H (1991) Distribution of CH2Cl2 in human blood. Recent
developments in toxicology: trends, methods and problems. Arch
Toxicol, 14(Suppl): 254-258.
Thief R, Pemble SM, Taylor JB, Humphreys WG, & Ketterer B (1993)
Glutathione S-transf erases 5-5 expression in Salmonella typhimurium
increases mutation rate caused by methylene dihalides. Pharmacol
Toxicol, 73(Suppl 11): 42 (Abstract FCZ/12).
Thilagar AK, Back AM, Kirby PE, Kumaroo PV, Pant KJ, Clarke JJ, Knight
R, & Haworth SR (1984a) Evaluation of dichloromethane in short-term
in vitro genetic toxicity assays. Environ Mutagen, 6: 418-419.
Thilagar AK, Kumaroo PV, Clarke JJ, Kott S, Back AM, & Kirby PE
(1984b) Induction of chromosome damage by dichloromethane in cultured
human peripheral lymphocytes, CHO cells and mouse lymphoma L5178Y
cells. Environ Mutagen, 6: 422.
Thilagar AK & Kumaroo PV (1983) Induction of chromosome damage by
methylene chloride in CHO cells. Mutat Res, 116: 361-367.
Toftgard R, Nilsen OG, & Gustafsson J-A (1982) Dose dependent
induction of rat liver microsomal P-450 and microsomal enzymatic
activities after inhalation of toluene and dichloromethane. Acta
Pharmacol Toxicol, 51: 108-114.
Trevors JT (1985) Effect on methylene chloride on respiration and
electron transport system (ETS) activity in freshwater sediment. Bull
Environ Contam Toxicol, 34: 239-245.
Trueman RW & Ashby J (1987) Lack of UDS activity in the livers of mice
and rats exposed to dichloromethane. Environ Mol Mutagen, 10: 189-195.
Trueman RW, Burlinson B, Lefevre PA, & Ashby J (1987) Inactivity of
methylene chloride as a UDS-initiating agent in mouse and rat
hepatocytes exposed in vivo and in vitro. Mutat Res, 181: 346
[abstract].
Truhaut R, Boudene C, Jounany JM, & Bouant A (1972) The application of
the physiogram to the investigation of the acute toxicity of
chlorinated solvents. Eur J Toxicol, 5(5): 284-292.
Tsuruta H (1975) Percutaneous absorption of organic solvents. 1.
Comparative study of the in vivo percutaneous absorption of
chlorinated solvents in mice. Ind Health, 13: 227-236.
Ulanova IP & Yonovskayo BI (1959) Changes in the ascorbic acid content
of the internal organs of white rats in response to the action of
chlorinated hydrocarbons II. Effect of methylene chloride. Biull Eksp
Biol Med, 48: 846.
Uotila L & Koivusalo M (1974a) Formaldehyde dehydrogenase from human
liver. J Biol Chem, 249: 7653-7663.
Uotila L & Koivusalo M (1974b) Distribution and properties of
S-formylglutathione hydrolase from human liver. J Biol Chem,
249: 7664-7672.
US EPA (1980) Ambient water quality criteria for halomethanes.
Washington, DC, US Environmental Protection Agency (EPA 440/5-80-051;
NTIS PB81-117 624).
US EPA (1981) Environmental risk assessment of dichloromethane.
Washington, DC, US Environmental Protection Agency (Draft report).
US EPA (1982a) Purgeable halocarbons-method 601. In: Methods for
organic chemical analysis of municipal and industrial wastewater.
Cincinnati, Ohio, US Environmental Protection Agency, Environmental
Monitoring and Support Laboratory (EPA-600/4-82-057).
US EPA (1982b) Purgeables method 624. in: Methods for organic chemical
analysis of municipal and industrial wastewater. Cincinnati, Ohio, US
Environmental Protection Agency, Environmental Monitoring and Support
Laboratory (EPA-600/4-82-057).
US EPA (1985) Health assessment document for dichloromethane
(Methylene chloride). Washington, DC, US Environmental Protection
Agency (EPA/600/8-82/OO4F).
US EPA (1986a) Gas chromatography/mass spectrometry for volatile
organics-method 8240. In: Test methods for evaluating solid waste, 3rd
ed. Washington, DC, US Environmental Protection Agency, Office of
Solid Waste and Emergency Response (SW-846).
US EPA (1986b) Halogenated volatile organics-method 8010. In: Test
methods for evaluating solid waste, 3rd ed. Washington, DC, US
Environmental Protection Agency, Office of Solid Waste and Emergency
Response (SW-846).
US EPA (1987) Household solvent products: A national usage survey.
Washington, DC, US Environmental Protection Agency.
US EPA (1989a) Measurement of purgeable organic compounds in water by
capillary column gas chromatography/mass spectrometry-Method 524.2.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1989b) Measurement of purgeable organic compounds in water by
packed column gas chromatography/mass spectrometry-Method 524.1.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1989c) Volatile halogenated organic compounds in water by
purge and trap gas chromatography-method 502.1. In: Methods for the
determination of organic compounds in drinking water. Cincinnati,
Ohio, US Environmental Protection Agency, Environmental Monitoring
Systems Laboratory (EPA/600/4-88/039).
US EPA (1989d) Volatile organic compounds in water by purge and trap
capillary column gas chromatography with protoionization and
electrolytic conductivity detectors in series-method 502.2.
In: Methods for the determination of organic compounds in drinking
water. Cincinnati, Ohio, US Environmental Protection Agency,
Environmental Monitoring Systems Laboratory (EPA/600/4-88/039).
US EPA (1990) Paint stripping, options selection paper. Washington,
DC, US Environmental Protection Agency.
Van Beck L (1990) Investigation of a possibility to reduce the use of
rabbits in skin irritation tests; experiments with dichloromethane,
trichloroethylene, tetrachloroethylene and 1,1,1-trichloroethane.
Doc. 56645/34/90, rep. V 89.265. Zeist, The Netherlands, TNO-CIVO
Institutes.
Van Haut H & Prinz B (1979) [Evaluation of the relative harmfulness to
plants of organic air pollutants in the LIS short-term test.] Staub-
Reinhalt Luft, 39: 408-414 (in German).
Van de Graaff S (1986) [Estimation of harmful effects of
environmentally relevant compounds in river water.] Münch Beitr
Abwasser-Fisch-Flussbiol, 40: 556-572 (in German).
Vannelli T, Logan M, Arciero DM, & Hooper AB (1990) Degradation of
halogenated aliphatic compounds by the ammonia oxidzing bacterium
Nitrosomas europaea. Appl Environ Microbiol, 56: 1169-1171.
Vargas C & Ahlert RC (1987) Anaerobic degradation of chlorinated
solvents. J Water Pollut Control Fed, 59(11): 964-968.
Veith GD, Macek KJ, Petrocelli SR, & Carroll J (1980) An evaluation of
using partition coefficients and water solubility to estimate
bioconcentration factors for organic chemicals in fish. Philadelphia,
Pennsylvania, American Society for Testing and Materials, pp 116-129
(ASTM-STP 707).
Veith GD & Kosian P (1983) Estimating bioconcentration potential from
octanol/water partition coefficients. Physical behaviour of PCBs in
Great Lakes. Ann Arbor, Michigan, Ann Arbor Science Publishers,
pp 269-282.
Verrett M J, Scott WF, Reynaldo EF, Alterman EK, & Thomas CA (1980)
Toxicity and teratogenicity of food additive chemicals in the
developing chicken embryo. Toxicol Appl Pharmacol, 56: 265-273.
Verschueren K (1983) Handbook of Environmental data on Organic
Chemicals, 2nd ed. New York, Van Nostrand Reinhold Publishers,
pp 848-849.
Verschuuren HG & Wilmer JW (1983) Neurotoxicity of 1,1,1-
trichloroethane questioned. Scand J Work Environ Health, 16: 144-146.
Volskay VT & Grady CPL (1988) Toxicity of selected RCRA compounds to
activated sludge microorganisms. J Water Pollut Control Fed,
60: 1850-1856.
Von Oettingen WF, Powell CC, Sharpless NE, Alford WC, & Pecora LJ
(1950) Comparative studies on the toxicity and pharmacodynamic action
of chlorinated methanes with special reference to their physical and
chemical characteristics. Arch Int Pharmacodyn Ther, 81: 17-34.
Vosovaja MA, Maljorowa LR, & Yenikeyera KM (1974) Levels of methylene
chloride in biological fluids of pregnant or lactating workers in an
industrial rubber products company. Gig Tr Prof Zabol, 4: 42-43.
Weast RC, Astle M J, & Beyer WH (eds) (1988) CRC Handbook of chemistry
and physics. 69th ed. (1988-1989). Boca Raton, Florida, CRC Press,
pp C-161., D-212.
Weber M, Martin A, Bollaert P-E, Bauer Ph, Leroy F, Meley M, Mur J-M,
Carry C, & Lambert H (1990) Intoxication aiguä par chlorure de
méthylène et méthanol par voie percutanée. Arch Mal Prof, 51: 103-106.
Weinstein RS, Boyd D, & Back KC (1972) Effects of continuous
inhalation of dichloromethane in the mouse: morphologic and functional
observations. Toxicol Appl Pharmacol, 23: 660-679.
Weinstein RS & Diamond SS (1972) Hepatotoxicity of dichloromethane
(methylene chloride) with continuous exposure at a low dose level.
In: Proceedings of the 3rd Annual Conference on Environmental
Toxicology. Dayton, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TR-72-130, 209-220).
Weiss G (1967) Toxic encephalosis in occupational contact with
methylene chloride. Zent.bl Arbeitsmed Arbeitsschutz, 17(9): 282-285.
Wells GG & Waldron HA (1984) Methylene chloride burns. Br J Ind Med,
41: 420.
Welsch CW (1985) Host-factors affecting the growth of carcinogen-
induced rat mammary carcinomas: a review and tribute to Charles
Brenton Huggins. Cancer Res, 45: 3415-3443.
Welsch CW, Jenkins JW, & Mertes J (1970) Increased incidence of
mammary tumors in female rat grafted with multiple pituitaries. Cancer
Res, 30: 1024-1029.
Welsch CW & Nagasawa H (1977) Prolactin and murine mammary
tumorigenesis: a review. Cancer Res, 37: 951-963.
Westbrook-Collins B, Allen JW, Sharief Y, & Campbell J (1990) Further
evidence that dichloromethane does not induce chromosome damage. J
Appl Toxicol, 10: 79-81.
Westbrook-Collins B, Allen JW, Kligerman A, Campbell JA, Erexson GL,
Kari F, & Zeiger E (1989) Dichloromethane-induced cytogenetic damage
in mice. Environ Mol Mutagen, 14(Suppl 15): [abstact 630].
Wesforook-Collins B, Campbell JA, Poorman PA, Sharief Y, & Allen JW
(1988) SCE, chromosome aberration, and synaptonemal complex analyses
in mice exposed to dichloromethane. Environ Mol Mutagen, 11(Suppl 11):
112 [abstract 2741.
WMO (World Meteorological Organization) (1991) Scientific assessment
of ozone depletion: 1991. Report No 25, 8.8. WMO, Geneva, p 8.8.
Wood PR, Parsons FZ, DeMarco J, Harween HJ, Lang RF, Payan IL, & Ruiz
MC (1981) Introductory study of the biodegradation of the chlorinated
methane, ethane, and ethene compounds. Presented at the American Water
Works Association Meeting, June 1981.
Woodrow JE, McChesney MM, & Seiber JN (1988) Determination of methyl
bromide in air samples by headspace gas chromatography. Anal Chem,
60: 509-512.
Yagafarova AB, Ivanova TS, & Khabirova FY (1981) The toxic allergic
action of hydrocarbons on the eyes. Kazanski Med Zh, 67: 72-74.
Yesair DW, Jacques D, Schepspis P, & Liss RH (1977) Dose-related
pharmacokinetics of 14C methylene chloride in mice. Fed Proc,
36: 998.
Young DR, Gossett RW, Baird RB, Brown DA, Taylor PA, & Mille MJ (1983)
Waste water inputs and marine bioaccumulation of priority pollutant
organics off Southern California, USA. In: Jolley RL ed. Proceedings
of the 4th Conference on Water Chlorination. Volume 4: Environmental
Impact and Health Effect - Part 2: Environmental health and risk. Ann
Arbor, Michigan, Ann Arbor Science Publishers.
Zahm SH, Stewart ZP, & Blair A (1987) A study of mortality among
workers exposed to methylene chloride. Feasibility report. Bethesda,
Maryland, US National Cancer Institute.
Zeiger E, Haseman JK, Shelby MD, Margolin BH, & Tennant RW (1990)
Evaluation of four in vitro genetic toxicity tests for predicting
rodent carcinogenicity: Confirmation of earlier results with 41
additional chemicals. Environ Mol Mutagen, 16: 1-14.
Zielenska M, Ahmed A, Pienkowska M, Anderson M, & Glickman BW (1993)
Mutational specificlties of environmental carcinogens in the LACL gene
of Escherichia coli. VI. Analysis of methylene chloride induced
mutational distribution in UVR + UVRB-strains. Carcinogenesis,
14(5): 789-794.
Zoeteman BC, Harmsen K, Linders JB, Morra CF, & Slooff W (1980)
Persistent organic pollutants in river water and ground water of the
Netherlands. Chemosphere, 9: 231-249.
RESUME
1. Identité, propriétés physiques et chimiques, et méthodes d'analyse
Le chlorure de méthylène (dichlorométhane) est un liquide limpide,
ininflammable et extrêmement volatil qui possède une puissante odeur
éthérée. Lorsqu'il est pur et anhydre, ce composé est très stable. Le
chlorure de méthylène s'hydrolyse lentement en présence d'humidité,
pour donner une petite quantité de chlorure d'hydrogène. Le chlorure
de méthylène du commerce est généralement additionné de petites
quantités de stabilisants afin d'en éviter la décomposition.
Il existe des méthodes d'analyse pour le dosage du chlorure de
méthylène dans les milieux biologiques et les échantillons prélevés
dans l'environnement. Dans tous les cas, on fait appel à la
chromatographie en phase gazeuse avec un détecteur convenable. On
obtient ainsi des limites de détection très basses (par exemple dans
les denrées alimentaires 7 ng/échantillon; dans l'eau 0,01/µg/litre;
dans l'air 1,76/µg/m3 (0,5 ppb); dans le sang 0,022 mg/litre).
2. Sources d'exposition humaine et environnementale
On estime à 570 000 tonnes la production annuelle mondiale de
chlorure de méthylène. On l'utilise la plupart du temps comme solvant
des graisses, des matières plastiques et des liants pour peinture, en
particulier à cause de sa volatilité et de sa stabilité. Dans
l'ensemble du monde, il est utilisé à 20-25% dans des aérosols, à 25%
comme décapant des peintures, à 35-40% comme solvant au cours des
différentes opérations de l'industrie chimique et pharmaceutique, et
enfin à 10-15% dans diverses applications allant de la fabrication de
mousse de polyuréthane au décapage des métaux. Son utilisation tend à
augmenter, tout au moins en Europe de l'Ouest.
Les émissions atmosphériques de chlorure de méthylène proviennent
à plus de 99% de son utilisation comme produit final par diverses
industries ou encore comme décapant des surfaces peintes et comme
constituant des bombes aérosols à usage domestique.
3. Transport, distribution et transformation dans l'environnement
En raison de sa forte volatilité, la majeure partie du chlorure de
méthylène libéré dans l'environnement se répartit dans l'atmosphère où
il est décomposé en l'espace de six mois par réaction avec des
radicaux hydroxyles d'origine photochimique.
Dans l'eau, il est décomposé par voie abiotique beaucoup plus
lentement qu'il ne s'évapore. On a montré que le chlorure de méthylène
disparaissait rapidement du sol et des eaux souterraines.
Grâce à divers systèmes d'épreuve on a pu établir les modalités de
la décomposition aérobic et anaérobie du chlorure de méthylène. Sa
biodécomposition complète est rapide, notamment en aérobiose, sous
l'action de cultures bactériennes acclimatées (par exemple 49 à 66% de
minéralisation en 50 heures en présence de boues d'effluents
municipaux acclimatées). Dans les réacteurs biologiques, la
décomposition peut atteindre 10% à l'heure. Rien n'indique qu'il y ait
une bioaccumulation ou une bioamplification importante.
4. Concentrations dans l'environnement et exposition humaine
On a décelé la présence de chlorure de méthylène dans l'air
ambiant de régions rurales et de zones écartées, à la concentration de
0,07-0,29/µg/m3. Dans les zones de banlieue, la concentration
moyenne est inférieure à 2 µ/m3 et dans les zones urbaines, elle est
inférieure à 15 µg/m3. A proximité de décharges jugées dangereuses,
on a trouvé des concentrations allant jusqu'à 43 µg/m3. Les
précipitations peuvent également contenir du chlorure de méthylène.
Le chlorure de méthylène pénètre dans l'environnement aquatique
par suite de la décharge d'eaux résiduaires provenant des diverses
industries et on en a retrouvé dans les eaux superficielles et
souterraines ainsi que dans les sédiments.
S'il y a exposition au chlorure de méthylène de personnes
appartenant à la population générale, c'est par suite de son
utilisation dans certains produits de consommation tels que les
décapants pour peinture, dont l'emploi peut entraîner la présence de
teneurs relativement importantes dans l'air intérieur. En ce qui
concerne l'exposition professionnelle au cours de la production de
chlorure de méthylène, elle se produit essentiellement au cours du
remplissage et du conditionnement (la fabrication s'effectue en
circuit fermé). Du fait de l'utilisation de ce composé comme décapant
pour peinture, il peut également y avoir exposition professionnelle
lors de la préparation de ces décapants, lors de la fabrication de
certains équipements et également lorsqu'on procède au décapage du
mobilier. Le chlorure de méthylène est largement utilisé comme solvant
lors de la préparation de différents produits, en particulier dans les
industries citées à la section 1.2.
La surveillance biologique de l'exposition au chlorure de
méthylène peut s'effectuer par dosage du solvant lui-même dans l'air
expiré ou dans le sang. Toutefois, étant donné que la production de
monoxyde de carbone constitue le facteur limitant du risque en cas
d'exposition supérieure à 3 ou 4 heures par jour, il vaut mieux que la
surveillance biologique s'effectue soit par dosage du monoxyde de
carbone dans l'air expiré, soit par dosage de la carboxyhémoglobine
(CO-Hb) dans le sang. Toutefois cette méthode ne vaut que pour les
sujets non fumeurs. Les prélèvements doivent s'effectuer environ 0 à 2
heures après l'exposition, ou au bout de 16 heures, c'est-à-dire le
matin suivant.
Les taux de CO-Hb, 2 heures après cessation de l'exposition, ne
devraient pas dépasser 2 à 3%, et au bout de 16 heures 1%, dans le cas
d'une exposition de 8 heures à moins de 350 mg de chlorure de
méthylène par m3 chez un non fumeur.
5. Cinétique et métabolisme chez les animaux de laboratoire et l'homme
Le chlorure de méthylène est rapidement absorbé au niveau des
alvéoles pulmonaires et pénètre dans le courant sanguin. Il est
également absorbé dans les voies digestives et aussi par voie
percutanée, mais cette voie est la plus lente de toutes.
Le chlorure de méthylène est très rapidement excrété, en majeure
partie dans l'air expiré. Il peut traverser la barrière hémato-
encéphalique ainsi que le placenta et on peut le retrouver en petites
quantités dans les urines ou le lait.
A fortes concentrations, la majeure partie du chlorure de
méthylène absorbée est expirée tel quel. Le reste est métabolisé en
monoxyde, dioxyde de carbone et chlorures minéraux. La métabolisation
s'effectue selon l'une ou l'autre de ces deux voies ou les deux à la
fois, et la prédominance de l'une ou de l'autre dépend largement de la
dose et de l'espèce animale en cause. Une de ces voies comporte un
processus oxydatif, par l'intermédiaire du cytochrome P-450, et alle
conduit à la production de monoxyde et de dioxyde de carbone. Il
semble que cette voie soit identique chez tous les rongeurs étudiés et
chez l'homme. Il s'agit de la voie prédominante aux faibles doses,
mais il y a saturation à des doses relativement modérées (autour de
1800 mg/m3). Même si la dose dépasse la valeur de saturation, il n'y
pas accroissement de la métabolisation par cette voie.
Dans l'autre voie métabolique intervient une glutathion-
transférase (GST) qui conduit à la formation de dioxyde de carbone par
l'intermédiaire du formaldéhyde et du formiate. Il semble que cette
voie ne prenne de l'importance que lorsque les doses dépassent la
valeur de saturation de la voie oxydative "préférentielle". Chez
certaines espèces (par exemple la souris), elle devient la voie
principale lorsque la dose est suffisamment élevée. En revanche, chez
d'autres espèces (par exemple le hamster et l'homme), elle semble
n'être que peu utilisée, qu'elle que soit la dose.
Les différentes interspécifiques touchant le métabolisme par la
voie de la GST sont en bonne corrélation avec les différences
observées selon les espèces, notamment en ce qui concerne la
cancérogénicité du chlorure de méthylène. Le taux de métabolisation
selon cette voie chez les espèces concernées est utilisé comme modèle
cinétique pour la description du comportement métabolique du chlorure
de méthylène chez diverses espèces.
6. Effets sur les êtres vivants dans leur milieu naturel
Aux concentrations inférieures à 500 mg/litre, il n'y a aucune
inhibition de la croissance des algues et des bactéries aérobies. Il
existe des bactéries qui sont capables de croître en présence de
chlorure de méthylène à des concentrations beaucoup plus élevées et
notamment en solution aqueuse (section 4.2.4.1 ). Les bactéries
anaérobies sont beaucoup plus sensibles; ainsi on a observé un blocage
de la croissance à la dose de 1 mg/litre, dans des boues biologiques
anaérobies.
A la concentration de 10 mg/kg de terre, on a constaté une forte
diminution de la teneur en ATP de la biomasse, notamment des
champignons et des bactéries aérobies, et une inhibition transitoire
de l'activité enzymatique. La dose sans effets observables était dans
ce cas de 0,1 mg/kg. Le chlorure de méthylène est modérément toxique
pour les lombrics (100 à 1000 µg/cm2) soumis au test de toxicité par
contact sur papier filtre. Dans les sédiments, on n'a pas observé
d'effets toxiques, même à des doses très élevées.
Chez les plantes supérieures, on n'a pas constaté d'effets après
une exposition de 14 jours à la dose de 100 mg/m3.
Les poissons adultes semblent être relativement insensibles au
chlorure de méthylène, même après une exposition prolongée (la CL50
à 14 jours est supérieure à 200 mg/litre). Il est difficile
d'apprécier l'effet du chlorure de méthylène sur les daphnies en
raison de la très grande dispersion des résultats fournis par les
différentes études. La CE50 la plus basse qui ait été rapportée
était égale à 12,5 mg/litre.
En milieu aquatique, c'est chez les embryons de poissons et
d'amphibiens que l'on a constaté la sensibilité la plus élevée avec
des effets sur l'éclosion â partir de 5,5 mg/litre.
7. Effets sur les mammifères de laboratoire et les systèmes d'épreuve
in vitro
7.1 Exposition unique
Lorsqu'il est absorbé par inhalation ou par voie orale, le
chlorure de méthylène présente une faible toxicité aiguë. Ainsi, les
valeurs de la CL50 à 6 heures pour l'ensemble des espèces étudiées,
se situent entre 40 200 et 55 870 mg/m3. En ce qui concerne la
DL50 par voie orale, on a obtenu des valeurs allant de
1410-3000 mg/kg. Les effets aigus observés après administration de
chlorure de méthylène par diverses voies d'exposition affectent
essentiellement le système nerveux central (SNC) et le foie, et tous
les effets observés l'ont été à des doses élevées. Des troubles
neurologiques ont été observés à des concentrations supérieures ou
égaies à 14 100 mg/m3, avec de légères altérations du tracé électro-
encéphalographique à la dose de 1770 mg/m3. De légères modifications
histologiques ont été également constatées dans le foie aux
concentrations supérieures ou égaies à 17 700 mg/m3. Il est
également arrivé que d'autres organes soient touchés, comme les reins
ou le système respiratoire. Chez la souris, les effets au niveau
pulmonaire étaient limités, après exposition à 7100 mg/m3, aux
cellules de Clara. On a également fait état d'une sensibilisation
cardiaque à l'arythmie induite par l'adrénaline. D'autres effets
cardio-vasculaires ont également été observés, mais pas de façon
systématique.
7.2 Exposition à court et à long terme
Une exposition prolongée à de fortes concentrations de chlorure de
méthylène (> 17 700 mg/m3) a causé des effets réversibles sur le
SNC, une légère irritation oculaire et une certaine mortalité chez
diverses espèces d'animaux de laboratoire. Chez le rat on a observé à
3500 mg/ma une réduction du poids corporel, cet effet étant
également observé chez la souris à partir de 17 700 mg/m3. De légers
effets hépatiques ont été également observés chez des chiens exposés
de manière continue à des doses de 3500 mg/m3 pendant des périodes
allant jusqu'à 100 jours. Après exposition intermittente, des effets
ont également été observés au niveau du foie chez des rats à la dose
de 3500 mg/m3 et chez des souris à 14 100 mg/m3.
Les autres organes cibles sont les poumons et les reins.
Chez des rats exposés à du chlorure de méthylène pendant 13
semaines par voie respiratoire à des concentrations allant jusqu'à
7100 mg/m3, on a observé aucun signe de lésion neurologique
irréversible.
L'administration de chlorure de méthylène par voie orale à des
rats a entraîné des effets hépatiques à partir d'une dose journalière
d'environ 200 mg/kg.
7.3 Irritation cutanée et oculaire
Le chlorure de méthylène se révèle modérément irritant pour la
peau et les yeux chez les animaux de laboratoire.
7.4 Effets toxiques sur le développement et la reproduction
Le chlorure de méthylène n'est pas tératogène chez le rat ou la
souris à des concentrations allant jusqu'à 16 250 mg/m3. Trois
études effectuées sur ces animaux n'ont pas permis de relever d'effets
se traduisant par l'apparition de malformations squelettiques ou
autres anomalies du développement. Un léger effet, se manifestant par
une modification du poids foetal ou maternel, a été relevé à la dose
de 4400 mg/m3, et l'on a constaté qu'après la naissance, le gain de
poids des rats mâles était également affecté à la dose de 0,04% dans
la nourriture. Une étude toxicologique portant sur deux générations a
été effectuée sur des rats qui ont été exposés par la voie
respiratoire à du chlorure de méthylène, à des concentrations allant
jusqu'à 5300 mg/m3, et cela pendant 17 semaines, 6 heures par jour
et 5 jours par semaine. Aucun effet nocif, de quelque nature ce que
soit, n'a été relevé sur la reproduction, ni sur la survie ou la
croissance des ratons nouveaux-nés appartenant à la génération F0 ou
F1
7.5 Mutagénicité et points d'aboutissement correspondants
Dans certaines conditions d'exposition, le chlorure de méthylène
se révèle mutagène pour les microorganismes procaryotes, avec ou sans
activation métabolique (Salmonella ou Escherichia coli) Dans les
systèmes eucaryotes, il est sans effet mais, dans un cas, il a donné
des résultats légèrement positifs. On a également obtenu des résultats
uniformément négatifs lors des tests de mutation génique in vitro et
des tests de recherche d'une synthèse non programmée de l'ADN sur
cellules mammaliennes. La recherche d'aberrations chromosomiques in
vitro sur différents types de cellules a donné des résultats positif
s; en revanche, des tests visant à mettre en évidence l'induction
d'échanges entre chromatides soeurs ont été soit négatif s, soit
ambigus.
Dans leur majorité, les études in vivo publiées n'ont pas fourni
d'éléments en faveur d'une mutagénicité du chlorure de méthylène (par
exemple recherche d'aberrations chromosomiques, recherche de
micronoyaux ou synthèse non programmée de l'ADN). Après avoir fait
inhaler à des souris de fortes concentrations de chlorure de
méthylène, on a observé un accroissement minime de la fréquence des
échanges entre chromatides soeurs et du nombre de micronoyaux.
Après administration à des rats et à des souris de fortes doses de
chlorure de méthylène, on n'a pas constaté de liaison de ce composé à
FADN ni de lésions de l'ADN. Ces méthodes pourraient être les plus
sensibles in vivo et les meilleures d'entre elles sont capables de
déceler un site d'alkylation sur 106 nucléotides.
Dans les limites des épreuves à court terme actuelles, rien ne
permet de conclure que le chlorure de méthylène soit génotoxique
in vivo.
7.6 Toxicité chronique et cancérogénicité
Le chlorure de méthylène est cancérogène pour la souris et il
provoque l'apparition de rumeurs du poumon et du foie après exposition
à des concentrations élevées (7100 et 14 100 mg/m3). Chez les souris
qui avaient été exposées 26 semaines à la dose de 7100 mg/m3, on a
constaté que l'incidence des tumeurs pulmonaires et hépatiques
augmentait lorsqu'on poursuivait l'exposition pendant 78 semaines
supplémentaires. Rien n'indique la présence d'effets toxiques
concomitants ou d'une hyperplasie au niveau des organes cibles.
Des hamsters dorés exposés pendant deux ans à du chlorure de
méthylène, à des concentrations allant jusqu'à 12 400 mg/m3, n'ont
présenté aucun signe d'effets cancérogènes qui soient attribuables à
ce composé.
Chez des rats exposés par différentes voies à du chlorure de
méthylène, on a constaté un accroissement de l'incidence tumorale à
certaines localisations. Ainsi, chez des rattes exposées pendant deux
ans à des doses égaies soit à 5300, soit à 12 400 mg/m3, on a
constaté un excès de la fréquence tumorale au niveau des glandes
salivaires. Cet excès n'apparaissait que lorsque les rumeurs, toutes
d'origine mésenchymateuse, étaient regroupées en vue de l'analyse
statistique. Etant donné que ces rumeurs trouvent leur origine dans
des cellules de types différents, l'analyse statistique utilisée s'est
révélée inappropriée. En outre, il a été précisé que les rats utilisés
pour cette étude avaient contracté une virose commune
(sialodacryoadénite) au début de l'expérience, affection qui concerne
essentiellement les glandes salivaires. Il est donc probable qu'il n'y
a pas de lien causal entre ces tumeurs et l'exposition au chlorure de
méthylène, mais que l'exposition à ce composé a exacerbé la réaction à
l'infection au niveau de la glande salivaire. D'ailleurs cette
réaction n'a pas été observée lors d'une deuxième étude au cours de
laquelle des rats ont été exposés pendant toute la durée de leur vie à
des doses respectivement égaies à 3500, 7100 et 14 100 mg/m3. Une
autre étude au cours de laquelle des rats ont été également exposés à
du chlorure de méthylène par la voie respiratoire et durant toute leur
vie, à des concentrations allant jusqu'à 1770 mg/m3, n'a pas révélé
de signe de cancérogénicité. Aucun signe notable de cancérogénicité
n'a été non plus observé chez des rats à qui l'on avait administré du
chlorure de méthylène, soit par gavage, soit par mélange à leur eau de
boisson.
Trois études font état d'une augmentation de l'incidence des
tumeurs mammaires bénignes chez des rats exposés à du chlorure de
méthylène; dans une des études le composé a été administré par garage
tandis que dans les deux autres l'exposition a eu lieu par la voie
respiratoire. Il n'y a aucune publication faisant état d'un
accroissement des tumeurs mammaires chez des hamsters ou des souris
qui avaient reçu du chlorure de méthylène à des doses comparables. On
a établi sans aucun doute possible que les tumeurs mammaires étaient
liées aux hormones hypophysaires tant chez les rats mâles que chez les
femelles. Chez le rat, la prolactine se comporte à la fois comme un
initiateur et comme un promoteur des cancers mammaires. On a de bonnes
raisons de penser qu'un accroissement du taux de prolactine augmente
l'incidence des tumeurs mamamaires (par exemple la greffe de plusieurs
hypophyses à des rats Sprague-Dawley augmente l'incidence des tumeurs
mammaires chez ces animaux et on a relevé l'existence d'une
corrélation positive entre un taux sanguin élevé de prolactine et la
présence de tumeurs mammaires chez des rattes âgées de souche
R-Amsterdam). Après administration de composés cancérogènes à des
rattes, une hyperprolactinémie provoquée chez ces animaux entraîne un
accroissement spectaculaire de l'incidence tumorale. On peut notamment
provoquer l'hyperprolactinémie par surrénalectomie, homogreffe
d'hypophyse et régime alimentaire hyperlipidique.
Il est important pour l'évaluation du risque chez l'homme, de
connaître le mécanisme par lequel le chlorure de méthylène provoque
l'apparition d'adénomes mammaires chez le rat. Les rattes Sprague-
Dawley à qui l'on a administré du chlorure de méthylène présentent un
taux sanguin élevé de prolactine. De même qu'avec les autres composés
qui agissent par l'intermédiaire d'une hyperprolactinémie, la réaction
au chlorure de méthylène se traduit uniquement par l'apparition de
néoformations à caractère bénin. Rien n'indique que le chlorure de
méthylène se lie à l'ADN d'autres tissus et par conséquent il paraît
improbable qu'il se lie à l'ADN des tissus mammaires alors qu'il est
principalement métabolisé dans le foie. Il paraît donc probable que
l'accroissement de l'incidence des adénomes mammaires résulte d'un
mécanisme indirect agissant par l'intermédiaire d'une
hyperprolactinémie.
Chez l'homme, les faits relatifs à la question de savoir si les
tumeurs mammaires sont sous la dépendance de la prolactine comme chez
le rat, apparaissent contradictoires. Le rat présente un taux élevé de
prolactine lorsqu'on le laisse s'alimenter ad libitum plutôt que de
le soumettre à un régime strict et cela pourrait expliquer pourquoi
l'incidence des tumeurs mammaires est si clépendante des divers effets
environnementaux ou autres. Chez le rat toutefois, la prolactine a un
caractère lutéotrope. L'augmentation du taux de prolactine dans le
sang circulant conduit à une augmentation du taux de progestérone et
d'oestrogène exogènes. C'est la présence de l'ensemble de ces trois
facteurs qui provoque la croissance tubulo-alvéolaire des glandes
mammaires et qui finit par déboucher sur la formation de tumeurs. Ce
mécanisme de formation tumorale n'a donc vraisemblablement pas à être
pris en considération chez l'homme.
Le mécanisme de formation de tumeurs mammaires chez le rat par
l'intermédiaire d'une hyperprolactinémie n'intervient qu'à des doses
où le chlorure de méthylène agit sur les taux de prolactine. On ne
dispose pas de données de première main sur les taux de prolactine
chez des rats soumis à de faibles doses de chlorure de méthylène, mais
l'administration de faibles doses de ce composé, soit par inhalation,
soit par mélange à l'eau de boisson (doses inférieures à 250 mg/kg de
poids corporel) ne conduit pas, selon les études effectuées, à un
accroissement des adénomes mammaires.
8. Effets sur l'homme
Le chlorure de méthylène irrite la peau et les yeux, en
particulier lorsqu'il ne peut pas s'évaporer. Dans ces conditions, un
contact prolongé peut entraîner des brûlures chimiques. On a signalé
un cas grave d'oedème pulmonaire consécutif à une inhalation excessive
de chlorure de méthylène. On a également signalé des cas de décès par
suite de l'inhalation accidentelle de chlorure de méthylène ou d'un
contact cutané avec ce composé. Les principaux effets toxiques du
chlorure de méthylène consistent dans une dépression réversible du
système nerveux central et dans la formation de carboxyhémoglobine. On
a également rapporté des cas d'insuffisance hépatique et rénale avec
anomalies hématologiques, à la suite d'une exposition à du chlorure de
méthylène.
Chez des volontaires humains exposés pendant une heure et demie à
trois heures à du chlorure de méthylène à la concentration de
694 mg/m3, on a observé des troubles neurophysiologiques et neuro-
comportementaux. En revanche, aucun signe d'effet neurologique n'a été
observé chez des hommes exposés plusieurs années à du chlorure de
méthylène à des concentrations allant de 260 à 347 mg/m3. De même,
on a soumis à une batterie de tests neurophysiologiques et
psychologiques un groupe d'anciens décapeurs d'aéronefs qui avaient
été longtemps exposés à du chlorure de méthylène (22 ans), à des doses
élevées mais non précisées; comparés à ceux d'un groupe témoin qui
n'avait jamais été exposé à du chlorure de méthylène ou du moins
uniquement à de faibles doses, les résultats obtenus par les ouvriers
se sont révélés "normaux".
On a attribué à une exposition au chlorure de méthylène une
augmentation du taux d'avortements spontanés constatée chez des
employées des industries pharmaceutiques finlandaises. L'étude en
question présentait cependant des défauts de conception qui n'ont pas
permis d'établir une relation causale.
Plusieurs éludes de mortalité sur des cohortes exposées au
chlorure de méthylène font ressortir une absence d'uniformité dans les
causes de décès. La surmortalité due à certaines maladies (par exemple
cancer du pancréas, cardiopathies ischémiques) ne se manifeste pas de
façon uniforme, mais seulement dans certaines éludes. Ces effets ne
peuvent être attribués à l'exposition au chlorure de méthylène.
RESUMEN
1. Identidad, propiedades físicas y químicas, y métodos analíticos
El cloruro de metileno (diclorometano) es un liquido claro,
altamente volátil y no inflamable, con un olor penetrante parecido al
del éter. El compuesto puro en polvo es muy estable. El cloruro de
metileno se hidroliza lentamente en presencia de humedad, dando lugar
a pequeñas cantidades de ácido clorhídrico. Al compuesto comercial se
agregan pequeñas cantidades de estabilizadores para prevenir su
descomposición.
Existen métodos analíticos para determinar el cloruro de metileno
en medios biológicos y muestras ambientales; en todos ellos se utiliza
cromatografía de gases y un detector apropiado. De esta manera se han
alcanzado limites de detección muy bajos (p. ej., alimentos:
7 ng/muestra; agua: 0,01 µg/litro; aire: 1,76 µg/m3 (0,5 ppb);
sangre: 0,022 mg/litro).
2. Fuentes de exposición humana y ambiental
Se estima que la producción mundial de cloruro de metileno
asciende a 570 000 toneladas/año. La mayoría sus aplicaciones se basan
en su capacidad para disolver grasas, plásticos y agentes aglutinantes
de pintura, así como en su volatilidad y estabilidad. Su uso a nivel
mundial se reparte del siguiente modo: aerosoles (20%-25%),
quitapinturas (25%), disolvente en la industria química y farmacéutica
(35%-40%), usos varios (p. ej., la fabricación de espuma de
poliuretano) y limpieza de metales (10%-15%). El uso de cloruro de
metileno tiende a disminuir, al menos en Europa occidental.
Más del 99% del cloruro de metileno liberado a la atmósfera
procede de diversas industrias que lo emiten como producto fina], o es
el resultado del uso doméstico de quitapinturas y aerosoles.
3. Transporte, distribución y transformación en el medio ambiente
Debido a su alta volatilidad, la mayor parte del cloruro de
metileno liberado al medio pasa a la atmósfera, donde se degrada
reaccionando con radicales hidroxilo de origen fotoquímico; su tiempo
de permanencia es de seis meses.
La degradación abiótica del compuesto en agua es lenta en
comparación con la evaporación. Se ha comprobado que el cloruro de
metileno desaparece rápidamente del suelo y de las aguas subterráneas.
Se han utilizado diversos sistemas de ensayo para determinar la
degradación aerobia y anaerobia del cloruro de metileno. La
biodegradación completa, sobre todo en cultivos bacterianos tratados y
en condiciones aerobias, es rápida (p. ej., mineralización del 49%-66%
en 50 horas en fangos urbanos tratados). En los biorreactores se puede
alcanzar una degradación de hasta un 10% por hora. No hay indicios de
una bioacumulación o biomagnificación importantes.
4. Niveles medioambientales y exposición humana
Se ha detectado cloruro de metileno en el aire ambiente de zonas
rurales y remotas a concentraciones comprendidas entre 0,07 y
0,29 µg/m3. En zonas suburbanas la concentración promedio es
<2 µg/m3, y en zonas urbanas, <15 µg/m3. En las proximidades de
vertederos de desechos peligrosos se han hallado hasta 43 µg/m3. Las
precipitaciones también contienen a veces cloruro de metileno.
El cloruro de metileno penetra en el medio acuático a través de
las descargas de aguas residuales de diversas industrias, habiéndose
detectado su presencia en aguas superficiales, aguas subterráneas y
sedimentos.
La población general se expone al cloruro de metileno cuando
utiliza productos de consumo tales como los quitapinturas, cuyo empleo
puede acompañarse de la presencia de niveles relativamente altos en el
aire del interior de los domicilios. La exposición ocupacional durante
la producción tiene lugar sobre todo durante el llenado y envasado (la
fabricación se lleva a cabo en sistemas cerrados). Tratándose de un
compuesto usado en los quitapinturas, la exposición laboral al cloruro
de metileno se produce durante la elaboración de quitapinturas, la
fabricación de material para ordenadores y el acabado comercial de
muebles. El cloruro de metileno es ampliamente empleado como
disolvente industrial en la elaboración de diversos productos, sobre
todo en las industrias que se mencionan en la sección 1.2.
La vigilancia biológica de la exposición al cloruro de metileno
puede basarse en la medición del propio disolvente en el aire espirado
o en la sangre. No obstante, dado que la producción de monóxido de
carbono con una exposición de más de 3-4 horas/día parece ser el
factor limitante en lo que respecta a los riesgos para la salud, es
preferible basar la vigilancia biológica en el análisis bien del
monóxido de carbono presente en el aire espirado, o bien de la
carboxihemoglobina (CO-Hb) en sangre. Así y todo, esto sólo se puede
aplicar a las personas no fumadoras. Deben tomarse muestras antes de
transcurridas aproximadamente dos horas tras la exposición, o bien al
cabo de 16 horas, esto es, a la mañana siguiente.
Los niveles postexposición de CO-Hb a las dos horas de interrumpir
la exposición no deben sobrepasar el 2%-3%, y a las 16 horas el 1%, en
los no fumadores expuestos durante ocho horas a menos de 350 mg/m3
de cloruro de metileno.
5. Cinética y metabolismo
El cloruro de metileno es absorbido rápidamente por los alvéolos
pulmonares, a través de los cuales llega a la circulación sistémica.
Es absorbido también por el tracto gastrointestinal, así como por vía
cutánea, si bien en este último caso la velocidad de absorción es
menor que por otras vías de exposición.
El cloruro de metileno se excreta con considerable rapidez,
fundamentalmente a través del aire espirado por los pulmones. Puede
atravesar la barrera hematoencefálica, así como la placenta, y se
excreta también en pequeñas cantidades por la orina y la leche.
A altas concentraciones la mayoría del cloruro de metileno
absorbido se espira inalterado. El resto es metabolizado en monóxido
de carbono, dióxido de carbono y cloruro inorgánico. Hay dos vías
posibles de metabolización, cuya contribución relativa al metabolismo
total depende en gran medida de la dosis y de la especie animal
considerada. Una vía consiste en un proceso de metabolismo oxidativo
mediado por el citocromo P-450, que conduce a la producción tanto de
monóxido de carbono como de dióxido de carbono. Esta vía funciona de
manera parecida en todos los roedores estudiados y en el hombre. Si
bien es la vía metabólica predominante a dosis bajas, se satura
también a dosis relativamente bajas (en torno a 1800 mg/m3).
Aumentar la dosis por encima de ese nivel de saturación no conlleva un
mayor metabolismo a través de esa vía.
La otra vía está mediada por una glutatión-transferasa (GTF) y
conduce, previa producción de formaldehído y de formato, a la
formación de dióxido de carbono. Al parecer esta vía sólo adquiere
importancia a dosis superiores al nivel de saturación de la vía
oxidativa «preferente». En algunas especies (p. ej., el ratón)
constituye la principal vía metabólica a dosis suficientemente altas.
Por el contrario, en otras especies (p. ej., el hámster o el hombre)
esta vía apenas es utilizada, cualquiera que sea la dosis.
Las diferencias interespecies del metabolismo mediado por la GTF
guardan una clara relación con las diferencias interespecies
observadas en lo que respecta a la carcinogenicidad. Analizando la
intensidad del metabolismo mediado por esta vía en determinadas
especies, se ha elaborado un modelo cinético del metabolismo del
cloruro de metileno en diversas especies.
6. Efectos en organismos presentes en el medio ambiente
Por debajo de 500 mg/litro no se observa inhibición del
crecimiento de algas y de bacterias aerobias. Se han descubierto
bacterias capaces de crecer en presencia de cloruro de metileno a
concentraciones mucho mayores, incluida una solución saturada en agua
(sección 4.2.4.1 ). Las bacterias anaerobias son más sensibles; se ha
observado inhibición del crecimiento a una concentración de 1 mg/litro
en fangos biológicos anaerobios.
En el suelo, se observó que una concentración de 10 mg/kg reducía
considerablemente el contenido de ATP de la biomasa, incluidos hongos
y bacterias aerobias, e inducía una inhibición transitoria de la
actividad enzimática. El nivel sin efectos observados fue de
0,1 mg/kg. En las lombrices de tierra el cloruro de metileno tiene un
efecto moderadamente tóxico (100-1000 µg/cm2), como demuestra la
prueba de toxicidad de contacto con papel de filtro. En sedimentos no
se observaron efectos tóxicos ni siquiera a concentraciones muy altas.
En plantas superiores no se observaron efectos al cabo de 14 días
de exposición a 100 mg/m3.
Los peces adultos parecen relativamente insensibles al cloruro de
metileno, incluso después de una exposición prolongada (14 días,
CL50 > 200 mg/litro). El efecto del cloruro de metileno en Daphnia
resulta difícil de evaluar porque hay grandes diferencias entre los
resultados de los estudios realizados. La CE50 más baja notificada
es de 12,5 mg/litro.
En cuanto al entorno acuático, se ha demostrado que los embriones
de peces y anfibios son los más sensibles, observándose efectos sobre
la incubación a partir de 5,5 mg/litro.
7. Efectos en mamíferos de laboratorio y en sistemas de prueba
in vitro
7.1 Exposiciones aisladas
La toxicidad aguda del cloruro de metileno por vía respiratoria y
por vía oral es baja. La CL50-6h por inhalación está comprendida en
todas las especies entre 40 200 y 55 870 mg/m3. Se han registrado
DL50 orales de 1410-3000 mg/kg. Los efectos agudos de la
administración de cloruro de metileno por diversas vías de exposición
se manifiestan fundamentalmente en el sistema nervioso central (SNC) y
en el hígado, y se producen a dosis altas. Se han observado trastornos
del SNC a concentraciones de 14 100 mg/m3 o más, con ligeras
variaciones del EEG a 1770 mg/m3. A concentraciones de
17 700 mg/m3 o más se observaron leves cambios histológicos en el
hígado. Ocasionalmente se vieron afectados otros órganos, tales como
el riñón o el sistema respiratorio. En el ratón, los efectos sobre los
pulmones se limitaron a las células de Clara después de una exposición
a 7100 mg/m3. Se ha notificado la aparición de sensibilización
cardiaca a la arritmia inducida por adrenalina. Se han observado
efectos cardiovasculares, si bien de manera irregular.
7.2 Exposición a corto y a largo plazo
La exposición prolongada a concentraciones altas de cloruro de
metileno (>17 700 mg/m3) causó efectos reversibles sobre el SNC,
ligera irritación ocular y mortalidad en varias especies de
laboratorio. Se observó una reducción del peso corporal en ratas a
3500 mg/m3, y en ratones a partir de 17 700 mg/m3. El hígado de
perros expuestos continuamente a 3500 mg/m3 por espacio de hasta 100
días se vio ligeramente afectado. Se observaron asimismo efectos en el
hígado tras la exposición intermitente a 3500 mg/m3 en la rata, y a
14 100 mg/m3 en el ratón.
Otros órganos diana son los pulmones y los riñones.
No se hallaron indicios de daño neurológico irreversible en ratas
expuestas por inhalación a concentraciones de hasta 7100 mg/m3
durante 13 semanas.
La administración oral de cloruro de metileno a ratas causó
efectos hepáticos a partir de 200 mg/kg al día.
7.3 Irritación cutánea y ocular
El cloruro de metileno es moderadamente irritante para la piel y
los ojos de animales experimentales.
7.4 Toxicidad para el desarrollo y la reproducción
El cloruro de metileno no es teratógeno en la rata o el ratón a
concentraciones de hasta 16 250 mg/m3. En tres estudios realizados
con animales no se observaron indicios de variación de la incidencia
de malformaciones esqueléticas ni otros efectos sobre el desarrollo.
Se notificaron efectos leves sobre el peso corporal fetal o materno a
una concentración de 4400 mg/m3, así como sobre el aumento de peso
postnatal de ratas macho a una concentración del 0,04% en la dieta. Un
estudio de toxicidad reproductiva llevado a cabo en dos generaciones
de ratas expuestas a cloruro de metileno por inhalación a
concentraciones de hasta 5300 mg/m3, 6 h/día, 5 días/semana durante
17 semanas no puso de manifiesto ningún efecto adverso en lo tocante a
los parámetros reproductivos, la supervivencia neonatal o el
crecimiento neonatal en ninguna de las generaciones, F0 o F1.
7.5 Mutagenicidad y criterios de evaluación relacionados
En condiciones de exposición adecuadas el cloruro de metileno
tiene efectos mutágenos en microorganismos procariotas, con o sin
activación metabólica (Salmonella o Escherichia coli). En los
sistemas eucariotas los resultados son negativos, salvo en un caso en
que fueron débilmente positivos. Los ensayos y pruebas de mutación
genética in vitro basados en la síntesis no programada de ADN (UDS)
en células de mamífero fueron siempre negativos. Los ensayos in vitro
realizados para detectar aberraciones cromosómicas en diferentes tipos
de células dieron resultados positivos, mientras que en las pruebas de
inducción de intercambio de cromátides hermanas (SCE) se obtuvieron
resultados negativos o ambiguos.
La mayoría de los estudios in vivo publicados no han aportado
ningún dato indicativo de mutagenicidad del cloruro de metileno
(determinada por ejemplo, mediante la prueba de aberración
cromosómica, la prueba de los micronúcleos o el ensayo UDS). Se ha
notificado un aumento mínimo de la frecuencia de SCE y de micronúcleos
en el ratón tras la exposición por inhalación a altas concentraciones
de cloruro de metileno.
En ratas o ratones a los que se administraron dosis altas de
cloruro de metileno no se observaron indicios de unión del cloruro de
metileno al ADN ni de lesiones de éste. Son éstos los estudios
in vivo potencialmente más sensibles, el mejor de los cuales permite
detectar una alquilación por cada 106 nucleótidos.
Dentro de las limitaciones de las pruebas a corto plazo
actualmente disponibles, no hay pruebas concluyentes de que el cloruro
de metileno sea genotóxico in vivo.
7.6 Toxicidad crónica y carcinogenicidad
El cloruro de metileno es carcinógeno en el ratón, en el que la
exposición a altas concentraciones (7100 y 14 100 mg/m3) es causa de
tumores tanto pulmonares como hepáticos. La incidencia de esos dos
tipos de tumores aumentó en ratones expuestos a 7100 mg/m3 durante
26 semanas y estudiados durante 78 semanas más. No se observaron
signos claros de toxicidad o hiperplasia asociadas en los órganos
diana.
La exposición de hámsters sirios a cloruro de metileno por
inhalación a concentraciones de hasta 12 400 mg/m3 durante dos años
no tuvo efectos carcinógenos.
Se ha observado que las ratas expuestas al cloruro de metileno por
diversas vías sufren una mayor incidencia de tumores en determinados
lugares. Se ha notificado un exceso de tumores en la región de las
glándulas salivales en ratas hembra expuestas a 5300 ó 12 400 mg/m3
durante dos años. Ese exceso sólo se hizo patente cuando se procedió a
agrupar los tumores, todos ellos de origen mesenquimatoso, con fines
estadísticos. El método estadístico utilizado era inapropiado dado que
los tumores procedían de células de diverso tipo. Además, se señaló
que las ratas utilizadas se habían visto infectadas al principio del
estudio por un virus causante de una enfermedad común, la
sialodacrioadenitis, que afecta sobre todo a la glándula salival.
Probablemente los tumores no estaban relacionados causalmente con la
exposición al cloruro de metileno, y la exposición se limitó a
exacerbar la respuesta a la infección en la región de la glándula
salival. El efecto no se reprodujo en un segundo estudio realizado con
ratas expuestas a 3500, 7100 ó 14 100 mg/m3 durante su ciclo de
vida. Un estudio ulterior realizado con ratas expuestas por inhalación
a concentraciones de hasta 1770 mg/m3 de cloruro de metileno durante
todo su ciclo de vida no reveló indicios de carcinogenicidad. En ratas
expuestas al cloruro de metileno a través del agua que consumían o de
alimentos administrados con sonda tampoco se observaron indicios
significativos de carcinogenicidad.
Tres estudios han puesto de manifiesto un aumento de la incidencia
de tumores mamarios benignos en ratas expuestas a cloruro de metileno,
en dos de los casos por inhalación y en el tercero por administración
forzada. No se ha notificado ningún aumento de la incidencia de
tumores mamarios en hámsters o en ratones sometidos a dosis
comparables de cloruro de metileno. La dependencia de los tumores
mamarios de las hormonas hipofisarias en la rata, tanto macho como
hembra, es un dato incontrovertible. En la rata, la prolactina actúa
como iniciador y como promotor de la carcinogénesis mamaria. Hay datos
convincentes de que el aumento de los niveles de prolactina incrementa
la incidencia de tumores mamarios (p. ej., el injerto de varias
hipófisis en ratas Sprague-Dawley aumenta la incidencia de tumores
mamarios, y se ha observado además una correlación positiva entre la
existencia de niveles elevados de prolactina en sangre y la incidencia
de tumores mamarios en ratas hembra R-Amsterdam viejas). En las ratas
hembra que han recibido carcinógenos, los tratamientos inductores de
hiperprolactinemia dan lugar a un aumento espectacular de la
incidencia de tumores. Entre esos tratamientos cabe citar la
adrenalectomía, los homoinjertos hipofisarios y el consumo de
alimentos ricos en grasas.
El conocimiento de los mecanismos de inducción de adenomas
mamarios por el cloruro de metileno en la rata es importante para
poder evaluar los riesgos para el hombre. Las ratas Sprague-Dawley
hembras sometidas a cloruro de metileno presentan una elevada
concentración de prolactina en sangre. Al igual que la respuesta a
otros agentes cuya acción está mediada por una hiperprolactinemia, la
respuesta inducida por el cloruro de metileno se limita a la aparición
de neoplasias benignas. No hay datos indicativos de una unión del
cloruro de metileno al ADN de otros tejidos, por lo que parece
improbable que pueda unirse al tejido mamario, tanto más cuanto que su
metabolismo se produce fundamentalmente en el hígado. Es más probable,
por tanto, que el aumento de la incidencia de adenomas mamarios se
deba a un mecanismo indirecto en el que intervenga la hiperprolac-
tinemia.
En cuanto al hombre, hay datos contradictorios respecto a si los
tumores mamarios son tan sensibles a la prolactina como en la rata.
Este animal presenta niveles elevados de prolactina cuando es
alimentado ad libitum en lugar de sometido a una dieta restringida,
lo cual explica quizá la gran sensibilidad de la incidencia de tumores
mamarios a diversos efectos ambientales y de otro tipo. En la rata, no
obstante, la prolactina es luteotrófica. Un aumento de la prolactina
circulante da lugar a un aumento de los niveles de progesterona y de
estrógenos exógenos. Es la coincidencia de estos tres factores lo que
causa el crecimiento túbulo-alveolar de las glándulas mamarias y,
finalmente, el desarrollo del tumor. La prolactina no es luteotrófica
en los primates; es improbable, por tanto, que este mecanismo de
desarrollo tumoral pueda tener importancia en el hombre.
En la rata, el mecanismo de desarrollo de tumores mamarios mediado
por la hiperprolactinemia sólo entra en juego a las dosis de cloruro
de metileno que alteran los niveles de prolactina. No se dispone de
información directa sobre los niveles de prolactina en ratas sometidas
a dosis bajas de cloruro de metileno, pero no se ha observado ningún
aumento de la incidencia de adenomas mamarios tras la administración
de dosis bajas por inhalación o a través del agua de bebida (p. ej.,
dosis inferiores a 250 mg/kg peso corporal).
8. Efectos en el hombre
El cloruro de metileno es irritante para la piel y para los ojos,
sobre todo cuando se impide su evaporación. En estas condiciones, el
contacto prolongado puede causar quemaduras químicas. Se ha notificado
un caso de edema pulmonar grave por inhalación excesiva. Se han
producido también defunciones en casos de inhalación o contaminación
cutánea accidentales. Los principales efectos tóxicos del cloruro de
metileno son la depresión reversible del SNC y la formación de CO-Hb.
Se ha señalado también la aparición de disfunciones hepáticas y
renales y de trastornos hematológicos tras la exposición al producto.
Se han observado problemas neurofisiológicos y neurocomporta-
mentales en voluntarios humanos expuestos a concentraciones de cloruro
de metileno de 694 mg/m3 durante 1,5-3,0 horas. No se han observado
efectos neurológicos en hombres expuestos durante varios años a
concentraciones del producto comprendidas entre 260 y 347 mg/m3. De
forma parecida, un grupo de raspadores de pintura de aviones ya
jubilados con antecedentes de una larga (22 años) exposición a
concentraciones altas, si bien no especificadas, de cloruro de
metileno obtuvieron resultados "normales" en una batería de pruebas
neurofisiológicas y psicológicas en comparación con un grupo testigo
sin antecedentes de exposición, o en todo caso con antecedentes de una
baja exposición al compuesto.
Un aumento de la tasa de abortos espontáneos entre empleadas de la
industria farmacéutica finlandesa se ha atribuido a la exposición a
cloruro de metileno. Sin embargo, el diseño incorrecto del estudio ha
impedido establecer una relación causal.
Varios estudios de mortalidad realizados en cohortes pertinentes
muestran resultados dispares en cuanto a las causas de defunción. Se
ha observado un aumento de la mortalidad por enfermedades especificas
(como por ejemplo el cáncer pancreático o la cardiopatía isquémica),
pero de forma irregular y sólo en determinados estudios. Estos efectos
no se pueden atribuir a la exposición al cloruro de metileno.
See Also:
Toxicological Abbreviations
Methylene chloride (EHC 32, 1984, 1st edition)
Methylene chloride (HSG 6, 1987)
Methylene chloride (PIM 343)
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
Members
Dr L.A. Albert, Consultores Ambientales Associados, Xalapa, Veracruz,
Mexico
Mr D. Farrar, ICI Chemicals and Polymers, Runcorn, Cheshire, United
Kingdom (Rapporteur)
Dr R. Fransson-Steen, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden
Dr S. Henry, US Food and Drug Administration, Washington, DC, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Huntingdon, United Kingdom
Dr P. Standring, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr L. Stayner, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr T. G. Vermeire, Toxicology Advisory Centre, National Institute of
Public Health and Environmental Hygiene, Bilthoven, The
Netherlands (Chairman)
Dr Ruqiu Ye, National Environmental Protection Agency, Beijing, China
Observers
Dr C. De Rooij, Solvay & Cie S.A., Brussels, Belgium
Dr T. Green, ICI Chemicals & Polymers Ltd., Runcorn, Cheshire, United
Kingdom
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P. Demers, Unit of Analytical Epidemiology, International Agency
for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
A WHO Task Group on Environmental Health Criteria for Methylene
Chloride met at the Institute of Terrestrial Ecology, Monks Wood,
United Kingdom from 16 to 20 August 1993. Dr S. Dobson welcomed the
participants on behalf of the host institution, and Dr M. Gilbert
opened the meeting on behalf of the three cooperating organizations of
the IPCS (ILO/UNEP/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methylene chloride.
The first draft of this monograph was prepared by Mr D. Farrar,
ICI Chemicals and Polymers, Runcorn, United Kingdom.
Dr M. Gilbert, IPCS, was responsible for the overall scientific
content of this monograph. After his death in July 1994, this
responsibility was transferred to Dr P.G. Jenkins, IPCS, who also
dealt with the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ALT alanine aminotransferase
AST aspartate aminotransferase
BEI Biological Exposure Index
CO-Hb carboxyhaemoglobin
GST glutathione transferase
LEV local exhaust ventilation
MATC maximum acceptable toxicant concentration
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health (USA)
SCE sister-chromatid exchange
SGOT serum glutamic-oxaloacetic transaminase
SGPT serum glutamic-pyruvic transaminase
TT toxicity threshold
TWA time-weighted average
UDS unscheduled DNA synthesis
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical methods
Methylene chloride (dichloromethane) is a clear, highly volatile,
non-flammable liquid with a penetrating ether-like odour. The pure dry
compound is very stable. Methylene chloride hydrolyses slowly in the
presence of moisture, producing small quantities of hydrogen chloride.
Commercial methylene chloride normally contains small quantities of
stabilizers to prevent decomposition.
Analytical methods are available for the determination of
methylene chloride in biological media and environmental samples. All
methods involve gas chromatography in combination with a suitable
detector. In this way, very low detection limits have been reached
(e.g., in food: 7 ng/sample; water: 0.01 µg/litre; air: 1.76 µg/m3
(0.5 ppb); blood: 0.022 mg/litre).
1.2 Sources of human and environmental exposure
World production of methylene chloride is estimated to be
570 000 tonnes/year. Most applications are based on its solvent
capacity for grease, plastics and paint binding agents, in combination
with its volatility and stability. The worldwide usage pattern
comprises aerosols (20-25%), paint remover (25%), process solvent in
the chemical and pharmaceutical industry (35-40%), miscellaneous uses
(e.g., polyurethane foam manufacturing) and metal cleaning (10-15%).
The usage of methylene chloride is tending to decrease, at least in
western Europe.
More than 99% of the atmospheric releases of methylene chloride
result from its use as an end-product by various industries and the
use of paint removers and aerosol products at home.
1.3 Environmental transport, distribution and transformation
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where it
will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months.
Abiotic degradation in water is slow compared to evaporation.
Methylene chloride has been shown to disappear rapidly from soil and
ground water.
The aerobic and anaerobic degradation of methylene chloride has
been established by a variety of different test systems. Complete
biodegradation, especially by acclimated bacterial cultures under
aerobic conditions, is rapid (e.g., 49-66% mineralization in 50 h with
acclimated municipal sludge). In bioreactors, up to 10% degradation
per hour is achievable. There is no evidence of significant
bioaccumulation or biomagnification.
1.4 Environmental levels and human exposure
Methylene chloride has been detected in the ambient air of rural
and remote areas at concentrations of 0.07-0.29 µg/m3. In suburban
areas, the average concentration is < 2 µg/m3 and in urban areas
< 15 µg/m3. In the vicinity of hazardous waste sites up to
43 µg/m3 has been found. Precipitation may also contain methylene
chloride.
Methylene chloride enters the aquatic environment through waste
water discharge from various industries, and methylene chloride has
been found in surface water, ground water and sediment.
Exposure of members of the general public to methylene chloride
will occur from its use in consumer products such as paint removers,
which can result in relatively high levels being found in indoor air.
Occupational exposure during production arises primarily during
filling and packaging (manufacturing is in closed systems). Because of
its use in paint strippers, occupational exposure to methylene
chloride occurs during formulation of paint-remover, original
equipment manufacture, and in commercial furniture refinishing.
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products, in particular in the industries
mentioned in section 1.2.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor in regard to health risk, biological
monitoring based upon either analysis of carbon monoxide in exhaled
air or of carboxyhaemaglobin (CO-Hb) in blood is to be preferred.
However, this can only be used for non-smoking subjects. Sampling
should be done at about 0-2 h post-exposure, or after 16 h, i.e. on
the following morning.
Post-exposure CO-Hb levels 2 h after exposure ceases are not
expected to exceed 2-3%, and at 16 h 1%, in the case of an 8-h
exposure to less than 350 mg methylene chloride/m3 in non-smokers.
1.5 Kinetics and metabolism
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract, and dermal exposure results in absorption but
at a slower rate than via the other routes of exposure.
Methylene chloride is quite rapidly excreted, mostly via the lungs
in the exhaled air. It can cross the blood-brain barrier and be
transferred across the placenta, and small amounts can be excreted in
urine or in milk.
At high concentrations, most of the absorbed methylene chloride is
exhaled unchanged. The remainder is metabolized to carbon monoxide,
carbon dioxide and inorganic chloride. Metabolism occurs by either or
both of two pathways, whose relative contribution to the total
metabolism is markedly dependent on the dose and on the animal species
concerned. One pathway involves oxidative metabolism mediated by
cytochrome P-450 and leads to both carbon monoxide and carbon dioxide.
This pathway appears to operate similarly in all rodents studied and
in man. Whilst this is the predominant metabolic route at lower doses,
saturation occurs at a relatively low dose (around 1800 mg/m3).
Increasing the dose above the saturation level does not lead to extra
metabolism by this route.
The other pathway involves a glutathione transferase (GST), and
leads via formaldehyde and formate to carbon dioxide. This route seems
only to become important at doses above the saturation level of the
"preferred" oxidative pathway. In some species (e.g., the mouse) it
becomes the major metabolic pathway at sufficiently high doses. In
contrast, in other species (e.g., hamster, man) it seems to be used
very little at any dose.
Species difference in GST metabolism correlates well with the
observed species difference in carcinogenicity. The extent of
metabolism by this pathway in relevant species has been used as the
basis for a kinetic model to describe the metabolic behaviour of
methylene chloride in various species.
1.6 Effects on organisms in the environment
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria have been identified that are able to grow in
the presence of methylene chloride at much higher concentrations
including a saturated solution in water (section 4.2.4.1). Anaerobic
bacteria are more sensitive; growth inhibition has been observed at
1 mg/litre in anaerobic biological sludge.
In soil a concentration of 10 mg/kg strongly decreased the ATP
content of the biomass including fungi and aerobic bacteria, and
induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms methylene chloride is
moderately toxic (100-1000 µg/cm2) in the filter-paper contact
toxicity test. In sediment no toxic effects were observed even at very
high levels.
In higher plants no effects were found after exposure for 14 days
to 100 mg/m3.
Adult fish seem to be relatively insensitive to methylene chloride
even after prolonged exposure (14-day LC50 > 200 mg per litre). The
effect of methylene chloride on Daphnia is difficult to assess given
the large variation in the outcome of the studies performed. The
lowest reported EC50 was 12.5 mg/litre.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive with effects on hatching from
5.5 mg/litre.
1.7 Effects on laboratory mammals and in vitro test systems
1.7.1 Single exposures
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species are between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg were recorded. Acute effects after methylene chloride
administration by various routes of exposure are primarily associated
with the central nervous system (CNS) and the liver, and these
occurred at high doses. CNS disturbances were found at concentrations
of 14 100 mg/m3 or more, with slight changes in EEG at 1770 mg/m3.
Slight histological changes in the liver were found at 17 700 mg/m3
or more. Occasionally other organs were affected such as the kidney or
respiratory system. In mice, effects on the lungs were restricted to
the Clara cells after exposure to 7100 mg/m3. Cardiac sensitization
to adrenaline-induced arrhythmia has been reported. Cardiovascular
effects have been seen but the effects were inconsistent.
1.7.2 Short- and long-term exposure
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice from
17 700 mg/m3. Slight effects on the liver were noted in dogs
continuously exposed to 3500 mg/m3 for up to 100 days. After
intermittent exposure, effects on the liver were observed in rats at
3500 mg/m3 and in mice at 14 100 mg/m3.
Other target organs are the lungs and the kidneys.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations up to 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver from about 200 mg/kg per day.
1.7.3 Skin and eye irritation
Methylene chloride is moderately irritant to the skin and eyes of
experimental animals.
1.7.4 Developmental and reproductive toxicity
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3. No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
were observed in three animal studies. Small effects on either fetal
or maternal body weight were reported at 4400 mg/m3, and on
postnatal weight gain of male rats at 0.04% in the diet. A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations up to 5300 mg/m3, 6 h/day,
5 days/week for 17 weeks did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
1.7.5 Mutagenicity and related end-points
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (Salmonella or Escherichia coil). In eukaryotic systems
it gives either negative or, in one case, weakly positive results.
In vitro gene mutation assays and tests for unscheduled DNA
synthesis (UDS) in mammalian cells were uniformly negative. In vitro
assays for chromosomal aberrations using different cell types gave
positive results, whereas negative or equivocal results were obtained
in tests for sister chromatid exchange (SCE) induction.
The majority of the in vivo studies reported provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay, micronucleus test or UDS assay). Marginal increase
in frequencies of SCEs and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride.
There was no evidence of binding of methylene chloride to DNA or
DNA damage in rats or mice given high doses of methylene chloride.
These studies are potentially the most sensitive in vivo studies,
the best of which are capable of detecting one alkylation in 106
nucleotides.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride in
genotoxic in vivo.
1.7.6 Chronic toxicity and carcinogenicity
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following exposure to high concentrations (7100 and
14 100 mg/m3) of methylene chloride. The incidence of both lung and
liver tumours was increased in mice exposed to 7100 mg/m3 for 26
weeks and maintained for a further 78 weeks. There was no substantial
evidence of associated toxicity or hyperplasia in the target organs.
Syrian hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of tumours
in the region of the salivary gland was reported in female rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different cells, the statistical approach adopted
was inappropriate. Furthermore, it was reported that the rats in the
study had been infected with a common viral disease (sialoda-
cryoadenitis) early in the study, an infection that affects primarily
the salivary gland. It is likely that these tumours were not causally
related to exposure to methylene chloride but that the exposure had
exacerbated the response of the infection in the region of the
salivary gland. The response was not seen in a second study in which
rats were exposed to either 3500, 7100 or 14 100 mg/m3 for their
lifetime. A further inhalation study on rats exposed to methylene
chloride at concentrations up to 1770 mg/m3 for their lifetime
showed no evidence of carcinogenicity. Rats exposed to methylene
chloride via their drinking-water or by gavage similarly showed no
substantive evidence of carcinogenicity.
An increased incidence of benign mammary tumours in rats exposed
to methylene chloride has been reported in three studies, two
following exposure by inhalation and the third by gavage. There are no
reports of increases in mammary tumour incidence in hamsters or in
mice receiving methylene chloride at comparable dose levels. The
dependence of mammary tumours upon pituitary hormones in both male and
female rats has been established unequivocally. In the rat, prolactin
acts as both an initiator and promoter of mammary carcinogenesis.
There is good evidence that increased prolactin levels increase the
incidence of mammary tumours (e.g., the grafting of multiple pituitary
glands into Sprague-Dawley rats increases the incidence of mammary
tumours and there is a positive correlation between elevated blood
prolactin levels and mammary tumours in aged R-Amsterdam female rats).
Treatments that induce hyperprolactinaemia in female rats that have
received carcinogens produce a dramatic increase in tumour incidence.
These treatments include adrenalectomy, pituitary homografts and high
dietary fat.
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin. In common with the response to other agents which act via
hyperprolactinaemia, the methylene chloride-induced response is of
benign neoplasms only. There is no evidence for the binding of
methylene chloride to the DNA of other tissues and hence it seems
unlikely that it will bind to mammary tissue when the primary site of
metabolism is the liver. It seems most likely, therefore, that the
increased incidence of mammary adenomas is the result of an indirect
mechanism operating via hyperprolactinaemia.
In humans, there is conflicting evidence on whether or not mammary
tumours are as responsive to prolactin as is the case in the rat. The
rat has elevated levels of prolactin when fed ad libitum in
comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels. It
is the presence of all three factors that causes tubular-alveolar
growth of the mammary glands, which ultimately leads to tumour
development. Prolactin is not luteotrophic in primates. It is
unlikely, therefore, that this mechanism of tumour development is of
relevance to man.
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will occur only at doses of methylene
chloride which affect prolactin levels. There is no direct information
on prolactin levels in rats receiving low doses of methylene chloride,
but no increase in mammary adenomas has been observed following the
administration of low doses in either inhalation or drinking-water
studies (i.e. below 250 mg/kg body weight).
1.8 Effects on humans
Methylene chloride irritates the skin and eyes especially when
evaporation is prevented. In these circumstances, prolonged contact
may cause chemical burns. A case of serious pulmonary oedema has been
reported after excessive inhalation. Fatalities due to accidental
inhalation and skin contamination have been reported. The main toxic
effects of methylene chloride are reversible CNS depression and CO-Hb
formation. Liver and renal dysfunctions and effects on haematological
parameters have also been reported following exposure to methylene
chloride.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men with exposure for several years
to methylene chloride at concentrations ranging from 260 to
347 mg/m3. Similarly, a group of retired airplane strippers with a
long history of exposure to methylene chloride (22 years) at high but
unspecified levels performed a battery of neurophysiological and
psychological tests within the "normal" range, when compared with a
control group who had a history of either no or only low exposure to
methylene chloride.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to methylene
chloride. A causal relationship was not established because of
insufficiencies in the design of the study.
Several mortality studies in relevant cohorts show an inconsistent
pattern in the causes of death. Excesses in mortality from specific
diseases (e.g., pancreatic cancer, ischaemic heart disease) were not
consistently increased, but confined to single studies. These effects
cannot be attributed to exposure to methylene chloride.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Formula: CH2CI2
CI
'
Structure: CI - C - H
'
H
Relative molecular mass: 84.93
Common name: Methylene chloride
Synonyms: DCM; dichloromethane; methane
dichloride; methylene bichloride;
methylene dichloride; methylenum
chloratum
Tradenames: Aerothene MM; Freon 30; Narkotil;
Solaestin; Solmethine
CAS name (9 CI): Methane, dichloro-
CAS registry number:
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
Members
Dr L.A. Albert, Consultores Ambientales Associados, Xalapa, Veracruz,
Mexico
Mr D. Farrar, ICI Chemicals and Polymers, Runcorn, Cheshire, United
Kingdom (Rapporteur)
Dr R. Fransson-Steen, Institute of Environmental Medicine, Karolinska
Institute, Stockholm, Sweden
Dr S. Henry, US Food and Drug Administration, Washington, DC, USA
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Huntingdon, United Kingdom
Dr P. Standring, Health and Safety Executive, Bootle, Merseyside,
United Kingdom
Dr L. Stayner, Division of Standards Development and Technology
Transfer, National Institute for Occupational Safety and Health,
Cincinnati, Ohio, USA
Dr T. G. Vermeire, Toxicology Advisory Centre, National Institute of
Public Health and Environmental Hygiene, Bilthoven, The
Netherlands (Chairman)
Dr Ruqiu Ye, National Environmental Protection Agency, Beijing, China
Observers
Dr C. De Rooij, Solvay & Cie S.A., Brussels, Belgium
Dr T. Green, ICI Chemicals & Polymers Ltd., Runcorn, Cheshire, United
Kingdom
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr P. Demers, Unit of Analytical Epidemiology, International Agency
for Research on Cancer, Lyon, France
ENVIRONMENTAL HEALTH CRITERIA FOR METHYLENE CHLORIDE
A WHO Task Group on Environmental Health Criteria for Methylene
Chloride met at the Institute of Terrestrial Ecology, Monks Wood,
United Kingdom from 16 to 20 August 1993. Dr S. Dobson welcomed the
participants on behalf of the host institution, and Dr M. Gilbert
opened the meeting on behalf of the three cooperating organizations of
the IPCS (ILO/UNEP/WHO). The Task Group reviewed and revised the draft
monograph and made an evaluation of the risks for human health and the
environment from exposure to methylene chloride.
The first draft of this monograph was prepared by Mr D. Farrar,
ICI Chemicals and Polymers, Runcorn, United Kingdom.
Dr M. Gilbert, IPCS, was responsible for the overall scientific
content of this monograph. After his death in July 1994, this
responsibility was transferred to Dr P.G. Jenkins, IPCS, who also
dealt with the technical editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ALT alanine aminotransferase
AST aspartate aminotransferase
BEI Biological Exposure Index
CO-Hb carboxyhaemoglobin
GST glutathione transferase
LEV local exhaust ventilation
MATC maximum acceptable toxicant concentration
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health (USA)
SCE sister-chromatid exchange
SGOT serum glutamic-oxaloacetic transaminase
SGPT serum glutamic-pyruvic transaminase
TT toxicity threshold
TWA time-weighted average
UDS unscheduled DNA synthesis
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical methods
Methylene chloride (dichloromethane) is a clear, highly volatile,
non-flammable liquid with a penetrating ether-like odour. The pure dry
compound is very stable. Methylene chloride hydrolyses slowly in the
presence of moisture, producing small quantities of hydrogen chloride.
Commercial methylene chloride normally contains small quantities of
stabilizers to prevent decomposition.
Analytical methods are available for the determination of
methylene chloride in biological media and environmental samples. All
methods involve gas chromatography in combination with a suitable
detector. In this way, very low detection limits have been reached
(e.g., in food: 7 ng/sample; water: 0.01 µg/litre; air: 1.76 µg/m3
(0.5 ppb); blood: 0.022 mg/litre).
1.2 Sources of human and environmental exposure
World production of methylene chloride is estimated to be
570 000 tonnes/year. Most applications are based on its solvent
capacity for grease, plastics and paint binding agents, in combination
with its volatility and stability. The worldwide usage pattern
comprises aerosols (20-25%), paint remover (25%), process solvent in
the chemical and pharmaceutical industry (35-40%), miscellaneous uses
(e.g., polyurethane foam manufacturing) and metal cleaning (10-15%).
The usage of methylene chloride is tending to decrease, at least in
western Europe.
More than 99% of the atmospheric releases of methylene chloride
result from its use as an end-product by various industries and the
use of paint removers and aerosol products at home.
1.3 Environmental transport, distribution and transformation
Due to its high volatility, most of the methylene chloride
released to the environment will partition to the atmosphere, where it
will degrade by reaction with photochemically produced hydroxyl
radicals with a lifetime of 6 months.
Abiotic degradation in water is slow compared to evaporation.
Methylene chloride has been shown to disappear rapidly from soil and
ground water.
The aerobic and anaerobic degradation of methylene chloride has
been established by a variety of different test systems. Complete
biodegradation, especially by acclimated bacterial cultures under
aerobic conditions, is rapid (e.g., 49-66% mineralization in 50 h with
acclimated municipal sludge). In bioreactors, up to 10% degradation
per hour is achievable. There is no evidence of significant
bioaccumulation or biomagnification.
1.4 Environmental levels and human exposure
Methylene chloride has been detected in the ambient air of rural
and remote areas at concentrations of 0.07-0.29 µg/m3. In suburban
areas, the average concentration is < 2 µg/m3 and in urban areas
< 15 µg/m3. In the vicinity of hazardous waste sites up to
43 µg/m3 has been found. Precipitation may also contain methylene
chloride.
Methylene chloride enters the aquatic environment through waste
water discharge from various industries, and methylene chloride has
been found in surface water, ground water and sediment.
Exposure of members of the general public to methylene chloride
will occur from its use in consumer products such as paint removers,
which can result in relatively high levels being found in indoor air.
Occupational exposure during production arises primarily during
filling and packaging (manufacturing is in closed systems). Because of
its use in paint strippers, occupational exposure to methylene
chloride occurs during formulation of paint-remover, original
equipment manufacture, and in commercial furniture refinishing.
Methylene chloride is widely used as a process solvent in the
manufacture of a variety of products, in particular in the industries
mentioned in section 1.2.
Biological monitoring of methylene chloride exposure can be based
on measurement of the solvent itself in exhaled air or blood. However,
as production of carbon monoxide with exposure for more than 3-4 h/day
appears to be the limiting factor in regard to health risk, biological
monitoring based upon either analysis of carbon monoxide in exhaled
air or of carboxyhaemaglobin (CO-Hb) in blood is to be preferred.
However, this can only be used for non-smoking subjects. Sampling
should be done at about 0-2 h post-exposure, or after 16 h, i.e. on
the following morning.
Post-exposure CO-Hb levels 2 h after exposure ceases are not
expected to exceed 2-3%, and at 16 h 1%, in the case of an 8-h
exposure to less than 350 mg methylene chloride/m3 in non-smokers.
1.5 Kinetics and metabolism
Methylene chloride is rapidly absorbed though the alveoli of the
lungs into the systemic circulation. It is also absorbed from the
gastrointestinal tract, and dermal exposure results in absorption but
at a slower rate than via the other routes of exposure.
Methylene chloride is quite rapidly excreted, mostly via the lungs
in the exhaled air. It can cross the blood-brain barrier and be
transferred across the placenta, and small amounts can be excreted in
urine or in milk.
At high concentrations, most of the absorbed methylene chloride is
exhaled unchanged. The remainder is metabolized to carbon monoxide,
carbon dioxide and inorganic chloride. Metabolism occurs by either or
both of two pathways, whose relative contribution to the total
metabolism is markedly dependent on the dose and on the animal species
concerned. One pathway involves oxidative metabolism mediated by
cytochrome P-450 and leads to both carbon monoxide and carbon dioxide.
This pathway appears to operate similarly in all rodents studied and
in man. Whilst this is the predominant metabolic route at lower doses,
saturation occurs at a relatively low dose (around 1800 mg/m3).
Increasing the dose above the saturation level does not lead to extra
metabolism by this route.
The other pathway involves a glutathione transferase (GST), and
leads via formaldehyde and formate to carbon dioxide. This route seems
only to become important at doses above the saturation level of the
"preferred" oxidative pathway. In some species (e.g., the mouse) it
becomes the major metabolic pathway at sufficiently high doses. In
contrast, in other species (e.g., hamster, man) it seems to be used
very little at any dose.
Species difference in GST metabolism correlates well with the
observed species difference in carcinogenicity. The extent of
metabolism by this pathway in relevant species has been used as the
basis for a kinetic model to describe the metabolic behaviour of
methylene chloride in various species.
1.6 Effects on organisms in the environment
Algae and aerobic bacteria show no inhibition of growth below
500 mg/litre. Bacteria have been identified that are able to grow in
the presence of methylene chloride at much higher concentrations
including a saturated solution in water (section 4.2.4.1). Anaerobic
bacteria are more sensitive; growth inhibition has been observed at
1 mg/litre in anaerobic biological sludge.
In soil a concentration of 10 mg/kg strongly decreased the ATP
content of the biomass including fungi and aerobic bacteria, and
induced transient inhibition of enzyme activity. The no-observed-
effect level was 0.1 mg/kg. In earthworms methylene chloride is
moderately toxic (100-1000 µg/cm2) in the filter-paper contact
toxicity test. In sediment no toxic effects were observed even at very
high levels.
In higher plants no effects were found after exposure for 14 days
to 100 mg/m3.
Adult fish seem to be relatively insensitive to methylene chloride
even after prolonged exposure (14-day LC50 > 200 mg per litre). The
effect of methylene chloride on Daphnia is difficult to assess given
the large variation in the outcome of the studies performed. The
lowest reported EC50 was 12.5 mg/litre.
In the aquatic environment, fish and amphibian embryos have been
shown to be the most sensitive with effects on hatching from
5.5 mg/litre.
1.7 Effects on laboratory mammals and in vitro test systems
1.7.1 Single exposures
The acute toxicity of methylene chloride by inhalation and oral
administration is low. The inhalation 6-h LC50 values for all
species are between 40 200 and 55 870 mg/m3. Oral LD50 values of
1410-3000 mg/kg were recorded. Acute effects after methylene chloride
administration by various routes of exposure are primarily associated
with the central nervous system (CNS) and the liver, and these
occurred at high doses. CNS disturbances were found at concentrations
of 14 100 mg/m3 or more, with slight changes in EEG at 1770 mg/m3.
Slight histological changes in the liver were found at 17 700 mg/m3
or more. Occasionally other organs were affected such as the kidney or
respiratory system. In mice, effects on the lungs were restricted to
the Clara cells after exposure to 7100 mg/m3. Cardiac sensitization
to adrenaline-induced arrhythmia has been reported. Cardiovascular
effects have been seen but the effects were inconsistent.
1.7.2 Short- and long-term exposure
Prolonged exposure to high concentrations of methylene chloride
(> 17 700 mg/m3) caused reversible CNS effects, slight eye
irritation and mortality in several laboratory species. Body weight
reduction was observed in rats at 3500 mg/m3 and in mice from
17 700 mg/m3. Slight effects on the liver were noted in dogs
continuously exposed to 3500 mg/m3 for up to 100 days. After
intermittent exposure, effects on the liver were observed in rats at
3500 mg/m3 and in mice at 14 100 mg/m3.
Other target organs are the lungs and the kidneys.
No evidence of irreversible neurological damage was seen in rats
exposed by inhalation to concentrations up to 7100 mg/m3 for 13
weeks.
Oral administration of methylene chloride to rats caused effects
on the liver from about 200 mg/kg per day.
1.7.3 Skin and eye irritation
Methylene chloride is moderately irritant to the skin and eyes of
experimental animals.
1.7.4 Developmental and reproductive toxicity
Methylene chloride is not teratogenic in rats or mice at
concentrations up to 16 250 mg/m3. No evidence of an effect on the
incidence of skeletal malformations or other developmental effects
were observed in three animal studies. Small effects on either fetal
or maternal body weight were reported at 4400 mg/m3, and on
postnatal weight gain of male rats at 0.04% in the diet. A two-
generation reproductive toxicity study in rats exposed to methylene
chloride by inhalation at concentrations up to 5300 mg/m3, 6 h/day,
5 days/week for 17 weeks did not show evidence of an adverse effect on
any reproductive parameter, neonatal survival or neonatal growth in
either the F0 or F1 generation.
1.7.5 Mutagenicity and related end-points
Under appropriate exposure conditions, methylene chloride is
mutagenic in prokaryotic microorganisms with or without metabolic
activation (Salmonella or Escherichia coil). In eukaryotic systems
it gives either negative or, in one case, weakly positive results.
In vitro gene mutation assays and tests for unscheduled DNA
synthesis (UDS) in mammalian cells were uniformly negative. In vitro
assays for chromosomal aberrations using different cell types gave
positive results, whereas negative or equivocal results were obtained
in tests for sister chromatid exchange (SCE) induction.
The majority of the in vivo studies reported provided no
evidence of mutagenicity of methylene chloride (e.g., chromosome
aberration assay, micronucleus test or UDS assay). Marginal increase
in frequencies of SCEs and micronuclei in mice has been reported
following inhalation exposure to high concentrations of methylene
chloride.
There was no evidence of binding of methylene chloride to DNA or
DNA damage in rats or mice given high doses of methylene chloride.
These studies are potentially the most sensitive in vivo studies,
the best of which are capable of detecting one alkylation in 106
nucleotides.
Within the limitations of the short-term tests currently
available, there is no conclusive evidence that methylene chloride in
genotoxic in vivo.
1.7.6 Chronic toxicity and carcinogenicity
Methylene chloride is carcinogenic in the mouse, causing both lung
and liver tumours, following exposure to high concentrations (7100 and
14 100 mg/m3) of methylene chloride. The incidence of both lung and
liver tumours was increased in mice exposed to 7100 mg/m3 for 26
weeks and maintained for a further 78 weeks. There was no substantial
evidence of associated toxicity or hyperplasia in the target organs.
Syrian hamsters exposed to methylene chloride by inhalation at
concentrations up to 12 400 mg/m3 for 2 years showed no evidence of
a carcinogenic effect related to exposure to methylene chloride.
Rats exposed to methylene chloride via various routes have shown
increased incidences of tumours at certain sites. An excess of tumours
in the region of the salivary gland was reported in female rats
exposed to either 5300 or 12 400 mg/m3 for 2 years. This excess was
only evident when the tumours, which were all of mesenchymal origin,
were grouped together for statistical analysis. As the tumours arose
from a variety of different cells, the statistical approach adopted
was inappropriate. Furthermore, it was reported that the rats in the
study had been infected with a common viral disease (sialoda-
cryoadenitis) early in the study, an infection that affects primarily
the salivary gland. It is likely that these tumours were not causally
related to exposure to methylene chloride but that the exposure had
exacerbated the response of the infection in the region of the
salivary gland. The response was not seen in a second study in which
rats were exposed to either 3500, 7100 or 14 100 mg/m3 for their
lifetime. A further inhalation study on rats exposed to methylene
chloride at concentrations up to 1770 mg/m3 for their lifetime
showed no evidence of carcinogenicity. Rats exposed to methylene
chloride via their drinking-water or by gavage similarly showed no
substantive evidence of carcinogenicity.
An increased incidence of benign mammary tumours in rats exposed
to methylene chloride has been reported in three studies, two
following exposure by inhalation and the third by gavage. There are no
reports of increases in mammary tumour incidence in hamsters or in
mice receiving methylene chloride at comparable dose levels. The
dependence of mammary tumours upon pituitary hormones in both male and
female rats has been established unequivocally. In the rat, prolactin
acts as both an initiator and promoter of mammary carcinogenesis.
There is good evidence that increased prolactin levels increase the
incidence of mammary tumours (e.g., the grafting of multiple pituitary
glands into Sprague-Dawley rats increases the incidence of mammary
tumours and there is a positive correlation between elevated blood
prolactin levels and mammary tumours in aged R-Amsterdam female rats).
Treatments that induce hyperprolactinaemia in female rats that have
received carcinogens produce a dramatic increase in tumour incidence.
These treatments include adrenalectomy, pituitary homografts and high
dietary fat.
The mechanism by which methylene chloride induces mammary adenomas
in the rat is important for human hazard assessment. Female Sprague-
Dawley rats receiving methylene chloride have a high blood level of
prolactin. In common with the response to other agents which act via
hyperprolactinaemia, the methylene chloride-induced response is of
benign neoplasms only. There is no evidence for the binding of
methylene chloride to the DNA of other tissues and hence it seems
unlikely that it will bind to mammary tissue when the primary site of
metabolism is the liver. It seems most likely, therefore, that the
increased incidence of mammary adenomas is the result of an indirect
mechanism operating via hyperprolactinaemia.
In humans, there is conflicting evidence on whether or not mammary
tumours are as responsive to prolactin as is the case in the rat. The
rat has elevated levels of prolactin when fed ad libitum in
comparison to a restricted dietary regimen and this may explain why
the mammary tumour incidence is so responsive to a variety of
environmental and other effects. In the rat, however, prolactin is
luteotrophic. An increase in the circulating levels of prolactin will
lead to an increase in progesterone and exogenous oestrogen levels. It
is the presence of all three factors that causes tubular-alveolar
growth of the mammary glands, which ultimately leads to tumour
development. Prolactin is not luteotrophic in primates. It is
unlikely, therefore, that this mechanism of tumour development is of
relevance to man.
The mechanism of production of mammary tumours in the rat
involving hyperprolactinaemia will occur only at doses of methylene
chloride which affect prolactin levels. There is no direct information
on prolactin levels in rats receiving low doses of methylene chloride,
but no increase in mammary adenomas has been observed following the
administration of low doses in either inhalation or drinking-water
studies (i.e. below 250 mg/kg body weight).
1.8 Effects on humans
Methylene chloride irritates the skin and eyes especially when
evaporation is prevented. In these circumstances, prolonged contact
may cause chemical burns. A case of serious pulmonary oedema has been
reported after excessive inhalation. Fatalities due to accidental
inhalation and skin contamination have been reported. The main toxic
effects of methylene chloride are reversible CNS depression and CO-Hb
formation. Liver and renal dysfunctions and effects on haematological
parameters have also been reported following exposure to methylene
chloride.
Neurophysiological and neurobehavioural disturbances have been
observed in human volunteers exposed to methylene chloride at
concentrations of 694 mg/m3 for 1.5-3.0 h. No evidence of
neurological effects was seen in men with exposure for several years
to methylene chloride at concentrations ranging from 260 to
347 mg/m3. Similarly, a group of retired airplane strippers with a
long history of exposure to methylene chloride (22 years) at high but
unspecified levels performed a battery of neurophysiological and
psychological tests within the "normal" range, when compared with a
control group who had a history of either no or only low exposure to
methylene chloride.
An increased rate of spontaneous abortion in employees in Finnish
pharmaceutical industries has been attributed to exposure to methylene
chloride. A causal relationship was not established because of
insufficiencies in the design of the study.
Several mortality studies in relevant cohorts show an inconsistent
pattern in the causes of death. Excesses in mortality from specific
diseases (e.g., pancreatic cancer, ischaemic heart disease) were not
consistently increased, but confined to single studies. These effects
cannot be attributed to exposure to methylene chloride.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
Formula: CH2CI2
CI
'
Structure: CI - C - H
'
H
Relative molecular mass: 84.93
Common name: Methylene chloride
Synonyms: DCM; dichloromethane; methane
dichloride; methylene bichloride;
methylene dichloride; methylenum
chloratum
Tradenames: Aerothene MM; Freon 30; Narkotil;
Solaestin; Solmethine
CAS name (9 CI): Methane, dichloro-
CAS registry number: 