
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 166
METHYL BROMIDE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr. R.F. Hertel and Dr. T. Kielhorn.
Fraunhofer Institute of Toxicology and Aerosol Research,
Hanover, Germany
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1995
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Methyl bromide.
(Environmental health criteria ; 166)
1.Hydrocarbons, Brominated - standards 2.Environmental exposure
I.Series
ISBN 92 4 157166 7 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
1. SUMMARY
1.1. Physical and chemical properties, and analytical
methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution, and
transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism
1.6. Effects on organisms in the environment
1.7. Effects on experimental animals
1.8. Effects on humans
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.2.1. Physical properties
2.2.2. Chemical properties
2.3. Conversion factors
2.4. Analytical methods
2.4.1. Methyl bromide in air
2.4.2. Methyl bromide in water
2.4.3. Determination of methyl bromide in soil
2.4.4. Methyl bromide in cereal grains and
other foods
2.4.5. Methyl bromide in serum, plasma and blood
and post-mortem tissue
2.4.6. Determination of inorganic bromide in air
2.4.7. Determination of inorganic bromide in water
2.4.8. Determination of inorganic bromide in soils
2.4.9. Determination of inorganic bromide in plant
material/food
2.4.10. Determination of inorganic bromide in
urine, blood/serum/plasma
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.1.1 Producers and world production
figures
3.2.1.2 Production processes
3.2.1.3 Losses to the environment during
normal production
3.2.1.4 Methods of transport
3.2.1.5 Accidental release or exposure
3.2.2. Uses
3.2.2.1 Soil fumigation
3.2.2.2 Quarantine and non-quarantine
commodity treatments
3.2.2.3 Structural fumigation
3.2.2.4 Industrial uses
3.2.3. Methyl bromide emission from motor
car exhausts
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Transport in air
4.1.2. Transport in water
4.1.3. Transport in soil
4.1.4. Vegetation and wildlife
4.1.5. Entry into the food chain
4.2. Biotransformation
4.2.1. Biodegradation
4.2.1.1 Soil
4.2.1.2 Stored product fumigation
4.2.2. Abiotic degradation
4.2.2.1 Hydrolysis
4.2.2.2 Light-assisted hydrolysis in water
4.2.2.3 Reaction with the hydroxyl radical
4.2.2.4 Photolysis in the atmosphere
4.2.3. Bioaccumulation
4.3. Interaction with other physical, chemical,
or biological factors
4.4. Ultimate fate following use
4.4.1. Methyl bromide and the ozone layer
4.4.2. Containment, recovery, recycling and disposal
options for methyl bromide
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.1.1 Global abundance
5.1.1.2 Measured oceanic and coastal air
levels of methyl bromide
5.1.1.3 Measured continental and urban
levels of methyl bromide
5.1.1.4 Vertical profiles of methyl bromide
in the atmosphere
5.1.1.5 Release of methyl bromide to
outside air from greenhouses
5.1.2. Water
5.1.2.1 Seawater
5.1.2.2 Inland waters
5.1.2.3 Waters around greenhouses
5.1.3. Soil
5.1.4. Food
5.1.4.1 After soil fumigation
5.1.4.2 After post-harvest fumigation
5.1.5. Animal feed
5.1.6. Other products
5.1.7. Terrestrial and aquatic organisms
5.2. General population exposure
5.2.1. Food
5.2.2. Drinking-water
5.2.3. Human breast milk
5.2.4. Sub-populations at special risk
5.3. Occupational exposure during manufacture,
formulation, or use
5.3.1. During manufacture
5.3.2. During fumigation
5.3.2.1 Structural fumigation
5.3.2.2 Soil fumigation
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Inhalation
6.1.1.1 Animal studies
6.1.1.2 Human studies
6.1.2. Dermal
6.1.3. Oral
6.1.4. Intraperitoneal injection
6.2. Distribution of methyl bromide and bromide
in tissues
6.2.1. Animal studies
6.2.2. Human studies
6.3. Metabolic transformation
6.3.1. Binding to proteins and lipids
6.3.2. Binding to DNA
6.3.3. The role of glutathione in methyl
bromide metabolism
6.3.3.1 Mammals
6.3.3.2 Insects
6.4. Elimination and excretion in expired air,
faeces, urine
6.5. Retention and turnover
6.6. Reaction with body components
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Soil microorganisms
7.2. Aquatic organisms
7.2.1. Effect of methyl bromide
7.2.2. Effect of bromide ion on aquatic organisms
7.3. Terrestrial organisms
7.3.1. Protozoa
7.3.2. Plants
7.3.2.1 Seed fumigation
7.3.2.2 Fumigation of plants or plant
products
7.3.2.3 The effects on plants of soil
fumigation
7.3.3. Soil invertebrates
7.3.4. Insects and arachnids
7.3.5. Gastropods
7.3.6. Birds
7.3.7. Other animals
7.4. Population and ecosystem effects
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.1.1. Oral
8.1.2. Inhalation
8.1.2.1 Guinea-pig and rabbit
8.1.2.2 Mouse
8.1.2.3 Rat
8.1.3. Dermal
8.1.4. Subcutaneous administration
8.2. Short-term exposure
8.2.1. Oral
8.2.2. Inhalation studies
8.2.2.1 Guinea-pig, rabbit, monkey
8.2.2.2 Mouse
8.2.2.3 Rat
8.2.3. Dermal
8.3. Skin and eye irritation
8.4. Long-term exposure
8.4.1. Oral
8.4.1.1 Rat
8.4.2. Inhalation studies
8.4.2.1 Mouse
8.4.2.2 Rat
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. Reproduction and embryotoxicity
8.5.2. Teratogenicity
8.6. Mutagenicity and related end-points
8.6.1. DNA damage
8.6.2. Mutation
8.6.3. Chromosomal effects
8.6.3.1 In vitro studies
8.6.3.2 In vivo studies
8.6.4. Cell transformation
8.7. Carcinogenicity and related end-points
8.7.1. Gavage studies
8.7.2. Inhalation studies
8.8. Special studies
8.8.1. Target organ effects
8.8.1.1 Inhalation studies
8.8.2. Neurotoxicity
8.8.3. Immunotoxicity
8.9. Factors modifying toxicity; toxicity of metabolites
8.10. Mechanisms of toxicity - mode of action
9. EFFECTS ON HUMANS
9.1. Clinical findings
9.1.1. Bromide levels in body tissues and fluids
9.1.2. Dermal exposure
9.1.3. Inhalation
9.2. General population exposure
9.2.1. Poisoning incidents
9.2.1.1 Poisoning associated with fire
extinguishers
9.2.1.2 Poisoning associated with bulk
or house fumigation
9.2.1.3 Poisoning associated with soil
fumigation
9.2.1.4 Miscellaneous incidents
9.3. Controlled human studies
9.4. Occupational exposure
9.4.1. Occupational exposure during manufacture
9.4.2. Occupational exposure due to methyl
bromide fumigation
9.4.2.1 Incidents involving bulk fumigation
9.4.2.2 Incidents involving soil fumigation
9.4.3. Studies measuring the levels of bromide
ion in biological fluids and tissues
9.4.3.1 Manufacturing
9.4.3.2 Fumigation
9.4.4. Haemoglobin adducts as a biological
index to methyl bromide exposure
9.4.5. Neurobehavioural and other studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Human exposure
10.1.1. Relevant animals studies
10.2. Environment
11. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
11.1. Human health protection
11.2. Environmental protection
11.3. Recommendations for further research
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12.1. FAO/WHO
12.1. IARC
12.3. UNEP
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR METHYL BROMIDE
Members
Dr I. Chahoud, Institute for Toxicology and Embryo-pharmacology,
Berlin, Germany
Mr B. Chakrabarti, Ministry of Agriculture, Fisheries and
Food, Slough, Berkshire, United Kingdom
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution,
London, United Kingdom (Chairman)
Dr S. Eustis, National Institute of Environmental Health and Safety,
Research Triangle Park, USA (Joint Rapporteur)
Dr K. Fujimori, National Institute of Health Sciences,
Tokyo, Japan
Dr L. Hansen, United States Environmental Protection
Agency, Washington DC, USA
Dr R.F. Hertel, Federal Health Office, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany (Joint Rapporteur)
Dr G. Rosner, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Dr S.A. Soliman, College of Agriculture and Veterinary
Medicine, King Saud University-Al-Qasseem, Bureidah,
Saudi Arabia (Vice-Chairman)
Dr M. Tasheva, National Center of Hygiene, Ecology and
Nutrition, Ministry of Health, Sofia, Bulgaria
Dr P.W. Wester, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
Prof. C. Zetzsch, Fraunhofer Institute of Toxicology and
Aerosol Research, Hanover, Germany
Observers
Dr W.K. Hayes, Ethyl Corporation, Baton Rouge, LA, USA
Dr M. Spiegelstein, Bromine Compounds Ltd., Beer Sheva,
Israel
Dr P. Montuschi, Catholic University of the Sacred Heart,
Rome, Italy (Representing the International Union of
Toxicology)
Secretariat
Dr D. McGregor, International Agency for Research on
Cancer, Lyon, France
Dr E. M. Smith, International Programme on Chemical
Safety, World Health Organization, Geneva, Switzerland.
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the environmental health
criteria monographs, readers are kindly requested to communicate any
errors that may have occurred to the Director of the International
Programme on Chemical Safety, World Health Organization, Geneva,
Switzerland, in order that they may be included in corrigenda, which
will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ESO2617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
ENVIRONMENTAL HEALTH CRITERIA FOR METHYL BROMIDE
A WHO Task Group on Environmental Health Criteria for Methyl
Bromide met at the Fraunhofer Institute for Toxicology and Aerosol
Research, Hanover, Germany, from 9 to 13 August 1993. Dr E.M. Smith,
IPCS, welcomed the participants on behalf of Dr M. Mercier, Director
of the IPCS, and the three IPCS cooperating organizations
(UNEP/ILO/WHO). The Group reviewed and revised the draft and made an
evaluation of the risks for human health and the environment from
exposure to methyl bromide.
The first draft of the EHC on methyl bromide was prepared by Dr
R. F. Hertel and Dr J. Kielhorn at the Fraunhofer Institute of
Toxicology and Aerosol Research in Hanover, Germany. Dr J. Kielhorn
assisted the IPCS Central Unit in the preparation of the second draft,
incorporating comments received following circulation of the first
draft to the IPCS contact points for Environmental Health Criteria
monographs.
Dr E.M. Smith of the IPCS Central Unit was responsible for the
scientific content of the monograph and Mrs M.O. Head, Oxford,
England, for the editing.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
1. SUMMARY
1.1 Physical and chemical properties, and analytical methods
Methyl bromide is a colourless gas at room temperature and
standard pressure with a boiling point of about 4 °C. It is heavier
than air and easily liquefied below its critical points. It is
odourless, except at high concentrations, when it has a
chloroform-like smell. It is non-flammable in air, except in the
concentration range of 10-16%, but burns in oxygen. Methyl bromide is
slightly soluble in water but freely soluble in other common solvents.
It can penetrate through many substances, such as concrete, leather,
rubber, and certain plastics.
Methyl bromide hydrolyses to methanol and hydrobromic acid in
aqueous solution, the rate of hydrolysis depending on pH. It is an
effective methylating agent that reacts with amines and
sulfur-containing compounds. Most metals are inert to pure, dry methyl
bromide, but surface reactions take place on zinc, tin, aluminium, and
magnesium in the presence of impurities or moisture. Explosive
reactions with aluminium and with dimethyl sulfoxide have been
reported.
Methyl bromide is commercially available as a liquefied gas.
Formulations for soil fumigation contain chloropicrin (2%) or amyl
acetate (0.3%) as warning agents. Other formulations include up to 70%
chloropicrin or other fumigants or hydrocarbons as inert diluents. For
commodity fumigation, 100% methyl bromide is used.
Analytical methods are described for the determination of methyl
bromide in air, water, soil, food, and animal feed. Direct methods for
determining methyl bromide in air, under field conditions, include
thermal conductivity gas analysers, colorimetric detector tubes,
infra-red analysers, and photo-ionization detectors. Gas
chromatography (GC) with electron capture detection (ECD) is
recommended for routine measurements with occasional mass
spectrometric (MS) confirmation in the laboratory.
Purge and trap techniques as well as headspace sampling are used
for the GC determination of methyl bromide in water. Extraction using
acetone/water followed by headspace capillary gas chromatography with
ECD is recommended for the routine determination of methyl bromide in
foods. As some of the methyl bromide is converted to bromide in soil,
foods, and biological materials, methods of bromide determination are
also discussed. Colorimetric methods, X-ray spectroscopy,
potentiometry, neutron activation analysis, gas chromatography, and
high-performance liquid chromatography (HPLC) are some of the methods
used for bromide determination in various matrices.
1.2 Sources of human and environmental exposure
Oceans are believed to be the major source of methyl bromide. The
main anthropogenic source of methyl bromide is the fumigation of soils
and indoor spaces. A small amount of methyl bromide is emitted from
motor vehicles using leaded petrol.
The world consumption of methyl bromide was over 67 million kg in
1990, an increase of 46% over 1984. It is commonly produced by the
interaction of methanol and hydrobromic acid, and, in some processes,
it is a coproduct together with tetrabromobisphenol A. Methyl bromide
is usually stored and transported as a liquefied gas, under pressure,
in steel containers.
About 77% of the methyl bromide produced is used for soil
fumigation, 12% for quarantine and commodity fumigation, 5% for
structural fumigation, and 6% for chemical intermediates.
The gas is used as a soil fumigant in either fields or
greenhouses for the control of pests. Methyl bromide is applied as a
liquid prior to planting, either by injection into the soil, or by
using evaporating jars under sheeting and allowing it to vaporize in
situ (cold method) or by heating (hot method). The methods permitted
in various countries differ. The type of plastic sheeting is also
important.
Doses of methyl bromide to be applied depend on the legal
standards of different countries, the plant parasite to be controlled
(type, extent of infestation), the following crop, type of soil, and
the plastic cover used (covering time and plastic type). Methyl
bromide is usually applied to soil at dosages of between 50 and 100
g/m2.
In space fumigation, methyl bromide is used for agricultural
commodity fumigation (e.g., foods, grains, nuts, etc.), termite
control, and rodent control. Dosages of 16-30 g methyl bromide/m3
are used for most goods stored in sealed rooms and silos and under
gas-proof sheets. A period of aeration must follow fumigation.
Fumigation is also important for fresh vegetables and fruits where
quarantine regulations have to be adhered to.
The industrial uses of methyl bromide include organic synthesis,
usually as a methylating agent, and as a low-boiling solvent, e.g.,
for extracting oils from nuts, seeds, and flowers. The uses of methyl
bromide as a refrigerant and as a general fire extinguishing agent are
now only of historical importance.
1.3 Environmental transport, distribution, and transformation
Methyl bromide is present naturally in the atmosphere.
Anthropogenic sources add to this. Although a small amount of methyl
bromide reacts with the hydroxyl radical in the troposphere, some
methyl bromide is transferred to the stratosphere by upward diffusion.
Here photolysis of methyl bromide becomes of increasing importance, it
being the most dominant loss mechanism in the lower stratosphere.
Active bromine species react with ozone in the stratosphere and are
thought to be partly responsible for the destruction of the ozone
layer.
In soil, methyl bromide is partially hydrolysed to bromide ion.
After fumigation using methyl bromide, soil can be leached with water
to prevent the bromide ions formed being taken up by plants
subsequently planted on the sterilized soil. This increase in bromide
levels may cause problems when surface water is used for leaching.
Methyl bromide may diffuse through polyethylene drinking-water pipes,
if the surrounding soil has been fumigated with methyl bromide.
In the soil, methyl bromide can diffuse to a depth of 0.8 m,
depending on the soil type, dosage, method of application, and length
of fumigation, the highest content of methyl bromide remaining in the
upper soil. The transport of the gas is caused by mass flow and
molecular diffusion, but it is also influenced by simultaneously
occurring sink processes, such as sorption and dissolution, and
irreversible sink processes, such as hydrolysis. The amount of methyl
bromide converted to bromide depends mainly on the organic matter
content of the soil. The bromide produced is largely water soluble and
can be taken up by plants or removed to lower soil levels by leaching
with water.
In plants, the amount of bromide accumulated depends on various
factors, such as dosage, exposure time, aeration rate, the physical
and chemical properties of the soil, the climatic trend (temperature
and rainfall), the plant species, and the type of plant tissue.
Especially leafy vegetables, such as lettuce and spinach, can take up
relatively large amounts of bromide ion without phytotoxic symptoms.
In contrast, other crops, such as carnations, citrus seedlings,
cotton, celery, peppers, and onions, are particularly sensitive to
methyl bromide fumigation.
Methyl bromide and its reaction products, of which only bromide
has been considered up to now, can enter the food chain in two ways;
through consumption of food grown in greenhouses or fields fumigated
before planting, or through eating food fumigated with methyl bromide
during storage. At certain levels, bromide may be hazardous for health
and tolerance levels are given for bromide in foodstuffs. Levels of
other reaction products have not been investigated.
Methyl bromide is degraded in soil by hydrolysis and microbial
degradation. The rate constant for hydrolysis varies with temperature
and pH and is enhanced by light.
The octanol/water partition coefficient (log Pow) of methyl
bromide is 1.19, suggesting a low bioaccumulation.
The methyl bromide that is not degraded during fumigation finds
its way into the troposphere and by upward diffusion into the
stratosphere. There does not seem to be a significant vertical
gradient for methyl bromide in the troposphere, but levels decrease
rapidly in the lower stratosphere where photolysis takes place.
1.4 Environmental levels and human exposure
Methyl bromide concentrations, measured in the air in unpopulated
areas, range from 40 to 100 ng/m3 (10 to 26pptv), readings in the
Northern hemisphere being higher than those in the Southern
hemisphere. Most readings are in the range of 9-15 pptv. Seasonal
differences have been found in some studies. In urban and industrial
areas, the levels are much higher, with average values of up to 800
ng/m3 and with some readings as high as 4 µg methyl bromide/m3. In
the proximity of fields and greenhouses, during fumigation and
aeration, the concentrations of methyl bromide are considerably
higher, values of 1-4 mg/m3 being measured in one study at distances
of up to 20 m from a greenhouse, a few hours after injection; a tenth
of this value was found 4 days later.
The methyl bromide concentration in a sample of surface seawater
has been given as 140 ng/litre. The average value of bromide ion
concentrations in samples of coastal water near the North Sea was 18.4
mg/litre; the level of bromide ion in inland rivers was much lower,
except in regions where fumigation with methyl bromide was practised,
or, in areas of industrial pollution. In drainage water from a
Netherlands greenhouse, levels of 9.3 mg methyl bromide/litre and 72
mg bromide ion/litre were reported. In water discharged from a Belgian
greenhouse, a value of 280 mg bromide/litre was recorded after
fumigation.
The natural bromide content of soil depends on the soil type, but
is usually less than 10 mg/kg. The residue of bromide in fumigated
soil depends on treatment, dosage, type of soil, amount of rain or
leaching water, and temperature.
Levels of methyl bromide or bromide may be elevated in foods that
have either grown on soil previously treated with methyl bromide or
have been fumigated post-harvest.
On rare occasions, bromide levels in fresh vegetables, grown on
soils previously fumigated with methyl bromide, have been observed to
exceed the permitted residue level. In some countries, it is not
permitted to grow vegetables on treated soils.
Methyl bromide is widely used for fumigating post-harvest
commodities, such as wheat and cereals, spices, nuts, dried and fresh
fruits, and tobacco. Methyl bromide concentrations usually decrease
rapidly after aeration and residues are not detectable after some
weeks. Some foods, such as nuts, seeds, and fatty foods like cheese,
tend to retain methyl bromide and inorganic bromide.
Individuals may be exposed to the fumigant and residues of
bromide ion. There could also be a risk of methyl bromide or increased
bromide contents in water in shallow wells near methyl bromide
fumigation operations.
People living in close proximity to fields, greenhouses, or
stores fumigated with methyl bromide, could be at risk of exposure to
the gas. Individuals can also be endangered if they accidentally, or
deliberately, enter private houses that have been fumigated to
eradicate pests before it is declared safe to do so.
Occupational exposure to methyl bromide is the most probable
hazard for operators during production, filling processes, and
fumigation operations. Because of strictly applied safety measures in
production facilities, only fumigators are now considered a high-risk
group. Fumigators engaged in structural fumigation may encounter
exposure much higher than the TLV after 24 h aeration (80-2000
mg/m3). However, properly trained operators will use appropriate
protective equipment. Field workers during soil fumigation may be
exposed for longer periods of time to transient doses of methyl
bromide. Because of the nature of greenhouse fumigation, operators may
also encounter higher concentrations (100-1200 mg/m3). However, risk
management developed for various aspects of fumigation requires strict
safety procedures and the use of protective equipment. Despite this,
individual cases of accidental overexposure still occur.
1.5 Kinetics and metabolism
Inhalation studies on rats, beagles, and humans have shown that
methyl bromide is rapidly absorbed through the lungs. It is also
rapidly absorbed in rats following oral administration.
After absorption, methyl bromide or metabolites are rapidly
distributed to many tissues including the lung, adrenal gland, kidney,
liver, nasal turbinates, brain, testis, and adipose tissue. In an
inhalation study on rats, the methyl bromide concentration in tissues
reached a maximum 1 h after exposure, but decreased rapidly, with no
traces 48 h later. The metabolism of inhaled methyl bromide has not
yet been elucidated, though glutathione may play a role.
Methylation of proteins and lipids has been observed in the
tissues of several species, including humans, exposed via inhalation.
Methylated DNA adducts have also been detected following the in vivo
and in vitro exposure of rodents or rodent cells.
In inhalation studies using [14C] labelled methyl bromide, the
exhalation of 14CO2 was the major route of elimination of 14C.
A lesser amount of 14C was excreted in the urine. Following oral
administration of methyl bromide, urinary excretion was the major
route of elimination of 14C.
The central nervous system is an important target for methyl
bromide. Changes in monoamine, amino acid contents and, possibly,
catecholamine contents may be factors involved in methyl
bromide-induced neurotoxicity.
1.6 Effects on organisms in the environment
Methyl bromide is used commercially to control nematodes, weeds,
and soil-borne fungi that cause diseases, such as damping off, crown
rot, root rot, and wilt.
There are few studies on the effects of methyl bromide on aquatic
organisms, as methyl bromide itself is only slightly soluble in water.
Values for LC50 range from a 4-h value of 17 mg/litre for Cyprinus
carpio L. to a 48-h value of 1.2 mg/litre for Poecilia reticulata.
At lethal concentrations, damage to the gills and oral epithelia was
the probable cause of death.
Bromide ion is formed from methyl bromide after fumigation and is
found in water after leaching. Bromide ions showed acute toxicity in
various freshwater organisms at concentrations ranging from 44 to 5800
mg Br-/litre; the no-observed-effect concentration (NOEC) in
long-term tests varied from 7.8 to 250 mg Br-/litre. Bromide ions
markedly impaired reproduction in both crustaceans and fish.
As a fumigant, methyl bromide can be applied directly to plant
seeds, plant cuttings, or harvested plant products, for disinfestation
during transportation and storage. Delay in germination or loss of
germinative capacity can occur if the moisture level or temperature is
too high.
Some crops, particularly leafy vegetables, are sensitive to
methyl bromide fumigation because of excess bromide in the soil, or,
indirectly because of effects on soil microflora. Sometimes, methyl
bromide has a positive effect on plants, increasing growth and crop
yields.
Methyl bromide fumigation eradicates not only target organisms
but also part of the soil flora, gastropods, arachnids, and
protozoans.
Methyl bromide is often used in preference to other insecticides
because of its ability to penetrate quickly and deeply into bulk
materials and soils. Dosages for methyl bromide as a storage fumigant
range mainly from 16 to 100 g/m3 for 2-3 days, the dosage depending
on temperature. A higher dosage is required to kill eggs and pupae
than adult insects. There is a variation in tolerance between
different insect species and stages and between different strains of
the same insect.
There are no data on the direct effects of methyl bromide on
birds and wild mammals.
1.7 Effects on experimental animals
Inhalation studies conducted on various mammalian species have
shown that there are clear species-related and sex-related differences
in susceptibility to methyl bromide. There was a steep dose-mortality
response in all animal species tested.
Neurological manifestations were the major clinical signs of
toxicity in rats and mice and, at higher concentrations, irritation of
the mucosal membranes was also observed.
Neurological manifestations included twitching and paralysis. At
lower dosages, changes in locomotor activity, dysfunction of the
peripheral nerve changes in circadian rhythm, and conditioned taste
aversion, have been reported by various authors.
Histopathological changes have been described in the brain,
kidney, nasal mucosa, heart, adrenal gland, liver, and testis of rats
and mice exposed to various levels of methyl bromide.
Olfactory sustentacular and mature sensory cells are damaged by
short-term exposure to methyl bromide, but there is rapid repair and
recovery.
Long-term inhalation studies (up to 2 years) on rats showed
lesions in the nasal mucosa and myocardium. In a similar long-term
study on mice, the primary toxic effects were observed in the brain,
heart, and nasal mucosa. Evidence of carcinogenicity was not observed
in either species.
Oral administration of 50 mg methyl bromide/kg body weight to
rats for up to 25 weeks produced inflammation and severe hyperplasia
of the forestomach epithelium. Following a post-exposure recovery
period, fibrosis of the forestomach was the principle lesion observed.
An early carcinoma of the forestomach was observed in the rat treated
daily for 25 weeks.
B6C3F mice and F344 rats exposed to up to 467 mg methyl
bromide/m3 for 13 weeks showed slight changes in sperm morphology
while the length of the estrous cycle was not affected.
Inhalation exposure to up to 350 mg methyl bromide/m3 did not
induce any noteworthy effects on the growth, reproductive processes,
and offspring of two consecutive generations of CD Sprague-Dawley
rats. The male and female fertility indices were reduced at the two
highest dose levels in the F1 generation F2B litter.
In studies on developmental toxicology with New Zealand White
rabbits, exposure to 311 mg methyl bromide/m3 (6 h/day; days 7-19 of
gestation) showed moderate to severe maternal toxicity. Developmental
effects, observed at the maternal toxic dose, consisted of decreased
fetal weights, an increase in the incidence of a minor skeletal
variation, and malformations (mostly missing gallbladder or missing
caudal lobe of the lung). However, at 272 mg/m3, maternal toxicity
was less marked and there were no embryotoxic effects.
No adverse maternal, embryonal, or fetal effects were observed in
rabbits exposed to 78 or 156 mg methyl bromide/m3. A
no-observed-effect level (NOEL) of 156 mg methyl bromide/m3 was
given for maternal and development toxicity in New Zealand White
rabbits.
Methyl bromide has been found to be mutagenic in several in
vitro and in vivo test systems. It induces sex-linked recessive
lethal mutations in Drosophila melanogaster and mutations in
cultured mammalian cells. It does not induce unscheduled DNA synthesis
or cell transformation in cultured mammalian cells. DNA methylation of
the liver and spleen was observed in mice administered methyl bromide
by various routes. Micronuclei were induced in bone-marrow and
peripheral blood cells of rats and mice.
The mechanism of methyl bromide toxicity is not known.
1.8 Effects on humans
Human exposure to methyl bromide may occur through inhalation of
the gas or contact with the liquid. Exposure through ingestion of
drinking-water contaminated with leaching water can also occur.
A controlled human study showed that uptake following inhalation
exposure was about 50% of the administered dose.
Methyl bromide is damaging to the nervous system, lung, nasal
mucosa, kidney, eye, and skin. Effects on the central nervous system
include blurred vision, mental confusion, numbness, tremor, and speech
defects. Topical exposure can cause skin irritation and burns, and eye
injury.
Exposure to high levels of methyl bromide causes pulmonary
oedema. Central nervous system depression with respiratory paralysis
and/or circulatory failure are often the immediate cause of death,
which is preceded by convulsions and coma.
Several different neuropsychiatric signs and symptoms have been
observed during acute and long-term methyl bromide poisonings.
Low-level short-term exposures to the vapour have produced a syndrome
of polyneuropathy without overt central manifestations.
Late sequelae include bronchopneumonia after severe pulmonary
lesions, and renal failure with anuria and severe weakness with, or
without, evidence of paralysis. Generally, these symptoms tend to
subside over a period of a few weeks or months. However, deficits
without recovery usually characterized by sensory disturbances,
weakness, disturbances of gait and blurred vision, have been observed.
Exposure to methyl bromide is accompanied by an increase in the
bromide level in the blood. In fumigators, there is a relationship
between the number of gas applications and the average plasma bromide
level.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL METHODS
2.1 Identity
2.1.1 Primary constituent
Chemical formula: CH3Br
Chemical structure:
H
'
H - C - Br
'
H
Relative molecular mass: 94.94
Common name: methyl bromide; bromomethane
CAS name: bromomethane
CAS registry number: 74-83-9
EEC No. 602-002-00-2
EINECS No. 200-813-2
Synonym: monobromomethane
2.1.2 Technical product
Methyl bromide is typically available as a liquefied gas
(Matheson Gas Data Book, 1980).
Purity: > 99.5%
Max. water content: 0.015%
Max. acidity (as HBr): 0.0010%
(Matheson Gas Data Book, 1980)
Impurities: traces of chloromethane
(Atochem, 1988)
Formulations include mixtures with other fumigants, most
frequently with chloropicrin or hydrocarbons, as inert diluents
(Stenger, 1978). Chloropicrin (2%) or amyl acetate (0.3%) are added to
methyl bromide to serve as a warning agent. Chloropicrin is a toxic
chemical with lacrimatory and irritating effects. However, it is
sensed at the 9 mg/m3 (1.3 ppm) level and a methyl bromide
concentration could be well above regulatory exposure limits by the
time the presence of chloropicrin is noticed.
Chemical, environmental, and toxicological data concerning
chloropicrin have been reviewed by Sassaman et al. (1986). For
commodity fumigation, 100 % methyl bromide should be used (Ethyl
Corporation, 1990).
Methyl bromide is marketed under several different trade names,
with formulations containing 30-100 % of the compound, e.g.,
Brom-o-gas, Desbrom, Haltox, MBR-2, Metabrom, Methybrom, Methyl
Bromide, Methyl-o-gas, Sobrom 9B, Terr-o-gas 100 (all 98-100% methyl
bromide); Bromopic, Sobrom 67, Terr-o-gas (80-30%, with decreasing
methyl bromide and increasing chloropicrin content).
2.2 Physical and chemical properties
2.2.1 Physical properties
Methyl bromide is a colourless gas at normal temperature and
pressure. Under increased pressure or below about 3 °C it is a clear,
colourless to straw-coloured liquid. It is odourless except in
relatively high concentrations, when it has a chloroform-like smell
(Matheson Gas Data Book, 1980). Individual odour thresholds range
between 80 mg/m3 and 4000 mg/m3 (Ruth, 1986).
The gas can penetrate many substances, including concrete,
leather, and rubber (Bond, 1984) as well as brick and wooden walls
(BBA, 1989). Methyl bromide did not permeate through certain plastics
(Herzel & Schmidt, 1984) or through metal or polyvinyl-chloride (PVC)
pipes, but permeation through low-density polyethylene (LDPE)
occurred. Permeation through LDPE pipes resulted in a concentration of
6% in the contained water after one week. This was independent of the
actual concentration outside the pipes. The methyl bromide seemed to
concentrate within the polymer. Permeation through high density
polyethylene (HDPE) was 5-8 times lower than through LDPE (Veenendahl
& Dibbets, 1981).
Liquid methyl bromide has a solvent action on many plastics and
organic materials. Natural rubber is attacked and acquires a strong
unpleasant smell (Thompson, 1966).
The physical properties of methyl bromide are summarized in Table
1.
Table 1. Physical properties of methyl bromide
Freezing point (1 atm): -93 °Ca,b
Boiling point (1 atm): 3.56 °Ca,b
Flash point: 194 °C, burns with difficultyc
Flammability: 13.5-14.5 % (by volume; flammable limits in air)a
10-16%d
Critical temperature 194 °Cc
Autoignition temperature: 536.7 °Ca
Vapour pressure (20 °C): 1893 kPa (1420 mmHg)b,e
Density (20 °C): 3.974b
(kg/m3) (0 °C): 1730a,b,c
Vapour density: 3.27c
(rel.; air=1) (20 °C)
Solubility in water: 18.5f (15.4 at 25 °C)f
(g/litre; 20 °C) 18.00g
16h
forms a voluminous crystalline hydrate
(CH3Br.2OH2O) below 4 °Cb
Solubility in other freely soluble in alcohol, chloroform, ether,
solvents: carbondisulfide, carbontetrachloride, and benzeneb
log n-octanol/water partition 1.19i,j
coefficient (log Pow):
Table 1. Con't
Henry's law constant: 0.533 (calculated using atmospheric
(kPa m3/mol) pressure)b
UV absorption: max. 202 nmk,l,m
a = Matheson Gas Data Book (1980); b = Windholz (1983);
c = Hommel (1984); d = NFPA (1984); e = Stenger (1978);
f = Wilhelm et al. (1977), g = Mackay & Shiu (1981); h = Atochem (1987);
i = Hansch & Leo (1979); j = Sangster (1989); k = Robbins (1976b);
l = Molina et al.(1982); m = Gillotay et al. (1989).
There are discrepancies in values for the solubility of methyl
bromide in water, some values in the literature being substantially
lower than those given in Table 1.
Methyl bromide is practically non-flammable in air, a narrow
range of 13.5-14.5 % by volume being quoted in the Matheson Gas Data
Book (1980), whereas a range of 16-20% is given in NFPA (1984). It
burns in oxygen (Windholz, 1983).
2.2.2 Chemical properties
Methyl bromide hydrolyses to methanol and hydrobromic acid. It is
a methylating agent reacting with amines, particularly the more basic
ones, to form methylammonium bromide derivatives. Methyl bromide also
reacts with sulfur compounds under alkaline conditions to give
mercaptans, thioethers, and disulfides. Most metals, other than
aluminium, are inert to pure, dry methyl bromide, but surface
reactions take place on zinc, tin, and magnesium, in the presence of
ethanol or moisture (Stenger, 1978). Explosions upon contact with
aluminium, as well as with dimethyl sulfoxide, have been reported
(NFPA, 1984). The liquid is corrosive to aluminium, magnesium and zinc
metals and their alloys.
Methyl bromide is not considered to be flammable. However, it
will burn in air in the presence of a high-energy source of ignition
and when within a narrow flammability range (see section 2.2.1).
Methyl bromide has no flash point. Thermal decomposition in a glass
vessel begins above 400 °C (Stenger, 1978). The products include HBr,
bromine, carbon oxybromide, as well as carbon dioxide and carbon
monoxide (von Oettingen, 1964).
2.3 Conversion factors
1 ppm = 3.89 mg/m3 at 25 °C, 1013 hPa
or 3.95 mg/m3 at 20 °C, 1013 hPa
1 mg/m3 = 0.257 ppm
1% methyl bromide = 10 000 ppm = 39.52 g/m3
at 20 °C and 101.3 kPa
2.4 Analytical methods
Methyl bromide residues have been determined indirectly as total
inorganic bromide. Methods are now available for the direct
determination of methyl bromide.
2.4.1 Methyl bromide in air
A summary of methods for the detection of methyl bromide in air
is given in Table 2.
The detection of methyl bromide in air is important at three
levels: control readings for warning fumigation workers; working place
(e.g. production/packing and sealing/transport) measurements; and the
measuring of levels of methyl bromide in the atmosphere.
In the first case, exposed fumigation workers must be warned
immediately of the presence of methyl bromide, as it is a toxic gas.
Many formulations, particularly those for commodity fumigation, do not
contain chloropicrin as a sensory warning.
Halide lamps cannot detect methyl bromide around occupational
exposure thresholds of 20 mg/m3 whereas electronic gas detectors,
though not specific for methyl bromide, are extremely sensitive.
Currently available gas detector tubes are also not specific for
methyl bromide but can be used to provide a reasonably precise
indication of methyl bromide level in a fumigation area before entry.
Direct reading colorimetric indicators are available (Saltzman,
1983; Leichnitz, 1985). However, Guillemin et al. (1990) noted that
several batches of these tubes produced unreliable results.
There is a direct-reading infrared analyser (MIRAN) that monitors
from 10 mg/m3 (2.3 ppm) methyl bromide (Foxboro, 1989). As this
instrument can measure methyl bromide below the threshold value, it
has been used to determine whether buildings are safe for occupation
after fumigation. However, Guillemin et al. (1990) reported that the
portable systems were mechanically and electrically unstable under
field conditions, and showed poor sensitivity and selectivity for
methyl bromide.
Table 2. Methods for the analysis of methyl bromide in aira
Sampling Analytical Detector Detection Comment Reference
method method limit
Gas collected by pump GC (30 m ECD used for ambient Harsch & Rasmussen
and pressurized capillary column) air (1977)
a ) isothermal runs 40 ng/m3 determinations
b ) temperature 2 ng/m3
programmed freeze-
out technique
Injection of 5 ml sample GC ECD 2 µg/m3 no common pollutants Pellizzari et al.
(3 m steel (scandium (upper interfere (1978)
column) tritide) limit with estimation
1 mg/m3)
Adsorb on charcoal; 100 m glass MS 14 ng/m3 Pellizari et al.
desorb (heat, purge with capillary column (21°C) (1978)
helium); dry (calcium
sulfate); readsorb
(Tenax GC); desorb as
before; trap liquid
nitrogen cooled;
vaporize onto GC
Adsorb (polymeric ECD 500 ng/m3 Krost et al. (1982)
beads); desorb (heat,
purge with helium);
trap directly on GC
column
Gas collected by pump GC ECD 40 µg/m3 Angerer (1982)
(2 m steel column)
Table 2 (continued)
Sampling Analytical Detector Detection Comment Reference
method method limit
Adsorb on charcoal; GC FID 1 mg/m3 Eller (1985),
desorb (carbon disulfide Peers (1985)
inject aliquot
Not given GC FID 2 ng for fumigation Dumas & Bond (1985)
control
Not given GC PID 10 pg for ambient air Dumas & Bond (1985)
sampling
Direct capillary GC ECD 50 ng/m3 methyl bromide and Kallio & Shibamoto
trapping with pump chloropicrin (1988)
detected
Charcoal air sampling GC ECD 50 ng designed to handle Woodrow et al. (1988)
tube/headspace sampler large numbers of
samples (45 samples
in 24 h); not specific
for methyl bromide
HBr-treated activated GC FID 1 mg/m3- personal monitoring Lefevre et al. (1989)
charcoal tubes/solvent ECD 1 g/m3 method
desorption
aAbbreviations:
ECD = electron capture detector; HECD = Hall electroconductivity detector;
FID = flame ionization detector; MS = mass spectrometry;
GC = gas chromatography; PID = photoionization detector.
Portable gas chromatographs measuring down to 0.04 mg/m3 (0.01
ppm) are also available for field work (Bond, 1984). Guillemin et al.
(1990) recommended for field conditions a photo-ionization detector
using a 10.2 eV source previously calibrated in the laboratory for
methyl bromide. The limitations were that readings were not specific
for methyl bromide and that sensitivity decreased with time.
Linenberg et al. (1991) used a portable GC with an argon
ionization detector (AID) to identify methyl bromide (0.12 mg/m3; 31
ppb) in the presence of other halohydrocarbon compounds for on-site
analysis.
In situ measurement of methyl bromide in indoor air using long
path Fourier transform infrared (FTIR) spectroscopy has been described
(Green et al., 1991). Quantitative determinations were made by
comparison with reference spectra of known concentration. Detection
limits were given as 0.14 mg/m3 (35 ppb), but conditions could be
optimized to obtain more sensitivity.
Methyl bromide is present in the atmosphere and its degradation
products may react with the ozone layer (see section 5.1.1).
Air samples can be collected using the following methods:
- cryogenesis using liquid nitrogen or helium,
- adsorption on (activated) charcoal,
- pumping into special containers,
- entry into already evacuated containers (BUA, 1987).
Plastic tubing or containers must not be used as they absorb
methyl bromide (Herzel & Schmidt, 1984).
Methods using electron capture detectors (ECD) are suitable for
routine measurements. GC/MS may be used for confirmation purposes.
In the monitoring of methyl bromide in air, stainless steel
canisters are recommended for collection with analysis using automated
cryogenic preconcentration followed by gas chromatography with a
selective detector - flame ionization (FID) and electron capture
detectors (ECD) connected in parallel (Jayanty, 1989).
2.4.2 Methyl bromide in water
Methods of determination of methyl bromide in water are
summarized in Table 3.
Purge and trap techniques, as well as headspace sampling, have
been used for the GC determination of methyl bromide in water. Details
of the collection, preservation, and handling of the water sample to
be analysed for methyl bromide are given in most references mentioned
in this section.
The headspace sampling technique can be used for analysis of
virtually any matrix.
Wylie (1988) compared headspace with purge and trap techniques
for the analysis of volatile priority pollutants. The headspace method
is more easily automated running 24 samples against only up to 10 with
a purge and trap unit with autosample. There is also less chance of
contamination from foaming or from high concentrations of a previous
analyte with headspace. Virtually any matrix can be used with
headspace, and glassware is disposable, which minimizes contamination.
Under some conditions, purge and trap is more sensitive than
headspace. US EPA recommended the purge and trap method for the
analysis of volatiles (EPA; 1984a).
An evaluation of methods for testing groundwater recommended in
US EPA Methods 8010 (GC/ECD) and 8240 (GC/MS) gave practical
quantification limits of 20 and 10 µg/litre, respectively, for methyl
bromide (Garman et al., 1987).
US EPA Methods 601 (GC/ECD), 602 (GC/MS) (Driscoll et al., 1987;
Duffy et al., 1988) and 624 (GC/MS) (Lopez-Avila et al., 1987) have
been updated for use with capillary column GC, to provide greater
sensitivity.
A sensitive headspace method for the gas-chromatographic
determination of methyl bromide in surface and drinking-waters was
reported by Cirilli & Borgioli (1986). This method is based on the
conversion of methyl bromide into methyl iodide by reaction with
sodium iodide.
Table 3. Determination of methyl bromide in watera
Sampling method Analytical Detector Detection Comment Reference
method limit
Headspace GC ECD 1 µg/litre Wegman et al. (1981)
Purge and trap GC ECD (n.d.)a US EPA (1982a)
(Method 8010)
Purge and trap GC MS 5 µg/litre US EPA (1982b)
(Method 8240)
Purge and trap GC MS (n.d.)a US EPA (1984a)
(packed column) (Method 624)
Purge and trap GC ECD 1.18 µg/ US EPA (1984b)
desorb as vapour litre (Method 601)
(heat to 180 °C,
backflush with inert
gas) on to GC column
Add internal standard GC MS 50 µg/litre US EPA (1984c)
(isotope labelled (Method 1624)
methyl bromide); purge,
trap and desorb as above
Purge (80 °C, nitrogen); GC ECD 0.05 µg/ Piet et al. (1985)
trap (Ambersorb or litre
Porapak N); desorb MS 0.05 µg/
(flash-heat) and trap litre
in "mini-trap"
(Ambersorb or Porapak N,
- 30°C); desorb (flash-
heat) on to GC column
Table 3 (continued)
Sampling method Analytical Detector Detection Comment Reference
method limit
Headspace capillary GC ECD 5 x 10-3 methyl bromide Cirilli & Borgioli
µg/litre converted quantitatively (1986)
to methyl iodide, which
is then determined
Purge and trap capillary GC ECD optimization of methods Driscoll et al. (1987)
PID 601, 602 to capillary Duffy et al. (1988)
column
Purge and trap capillary GC MS updating of methods; Lopez-Avila et al.
no separation of (1987)
bromomethane from
chloromethane
Headspace sampling capillary GC MS 20 µg/litre Gryder-Boutet &
Kennish (1988)
Samples purged for capillary GC FID 1 µg/litre Cochran (1988)
45 seconds directly
to a cryogenically
cooled, capillary
column
Purge and trap capillary GC ECD 1.1 µg/litre Ho (1989)
a For other abbreviations see Table 2.
n.d. = methyl bromide was not detected in the earlier determinations.
Singh et al. (1983) described the analysis of methyl bromide in
seawater samples. A 50-ml volume of seawater and an equal volume of
ultra-pure air were enclosed in all-glass syringes of 100-ml volume.
Once in the syringe, the equilibrium was allowed to reach completion
(enhanced by repeated shaking) in 15-30 min. This also allowed the
water to reach room temperature, which was carefully recorded. The air
in equilibrium with the 50-ml seawater was analysed for methyl bromide
using gas chromatography with ECD; the corresponding equilibrium
concentration of methyl bromide in seawater was determined from
solubility data at the measured room temperature, and the two were
added to obtain the methyl bromide concentrations in seawater. The
partition coefficient data and their temperature dependence for methyl
bromide were taken from Wilhelm et al. (1977) for pure water. The
salting-out coefficient of 1.2 was determined on the basis of
available data on the measured solubility of moderately soluble gases
in pure water and seawater.
2.4.3 Determination of methyl bromide in soil
Equipment and methods for sampling and analysing deep field soil
atmospheres have been described (Kolbezen & Abu-El-Haj, 1972). Soil
atmosphere samples were obtained from a vertical and horizontal grid
of sampling points placed into the soil before it was treated with
methyl bromide. The samples were withdrawn through fine stainless
steel tubing into syringes that could be transported to the laboratory
and directly applied to the gas chromatograph. A flame ionisation
detector (FID) was used (detection limit 40 mg/m3).
US EPA Methods 8010 and 8240 (Table 3) can also been used for the
determination of methyl bromide in solid waste and soils (US EPA,
1982a,b) with a detection limit of 1 µg/g. Extraction of non-aqueous
samples is carried out using methanol or polyethylene glycol.
2.4.4 Methyl bromide in cereal grains and other foods
Analytical methods are summarized in Table 4.
Table 4. Determination of methyl bromide in plant material and foodsa
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Flour, cold solvent extraction, GC FID 0.3 mg/kg 95% recovery Heuser & Scudamore
unground extraction time (1968; 1970),
wheat, increasing with food Scudamore (1987)
sultanas, particle size
peanuts,
maize,
ground-nuts
Whole wheat, extracted methyl GC ECD 0.01 mg/kg Fairall & Scudamore
flour, ground- bromide is reacted (1980)
nut, rapeseed, to form methyl iodide
dried milk
powder, cocoa
beans
Grain acetone/water GC (Carbo- ECD 0.05 mg/kg Greve & Hogendoorn
extraction; headspace wax-20 M) (1979)
analysis
Wheat flasks containing GC FID 0.3 µg/kg determination of Dumas (1982)
wheat flushed with (2 m Tenax) methyl bromide in
nitrogen and trap at wheat after
-78.5 °C fumigation
Grapefruit blended with water GC ECD 0.1 mg/kg King et al. (1981)
and vial sealed, 5 ml 2 µg/kg
headspace gas removed
with syringe and
injected
Table 4 (continued)
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Wheat, water added, GC ECD 0.4 µg/kg De Vries et al.
flour, equilibration at 30 °C (1985)
cocoa, headspace
peanuts
Cereal extract with acetone: GC ECD 150 µg/kg Scudamore (1985a)
grains water; add sodium
and chloride; separate
other layers; dry acetone
foods solution over
anhydrous calcium
chloride; inject
aliquot
extract with acetone: GC ECD 10 µg/kg Scudamore (1985b)
water, inject aliquot
of headspace vapour
Cherries headspace; adapted GC ECD 0.5 mg/kg determination of the Sell et al. (1988)
from King et al. rate of desorption
(1981) from fumigated
cherries
Apples headspace; adapted GC ECD 0.01 mg/kg Sell & Moffitt (1990)
from King et al.
(1981)
Table 4 (continued)
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Food extraction with 83% GC ECD, 55 µg/kg poor recovery and Daft (1987; 1988;
acetone (grains), 20% (packed HECD 20 µg/kg high coefficient 1989)
acetone (softer foods); column) of variation
residues partitioned
into isooctane by
shaking; fatty food
passed through micro-
Florisil columns
Nuts, comminuted food sample GC ECD dependent Page & Avon (1989)
food with sodium sulfate; (capillary) on lipid
aliquot to headspace; content of
cryogenic focusing at food 0.15-
-60°C and then elution by 0.65 µg/kg
temperature programming
Nuts extraction with sodium GC ECD, suitable for Daft (1992)
sulfate at 80 °C; purge (capillary) HECD screening nut
overnight samples at ng/g
levels; 40%
recovery; 29%
coefficient of
variation
Fish homogenization GC MS 200 µg/kg Easley et al. (1981)
purge and trap
a For abbreviations see Table 2.
Although bromide levels in food have been measured and documented
for several decades, the methods for the determination of methyl
bromide in foods are still being refined. The cold extraction or
soaking procedure was developed and optimum extraction times
determined for several foods, the extraction time increasing with food
particle size (Heuser & Scudamore, 1968, 1970). With several foods,
there was evidence of methyl bromide loss through reaction with food
components. The following extraction times for methyl bromide were
reported: flour (1 h), unground wheat (8 h), sultanas (8 h), peanuts
(8 h), maize (24 h), groundnuts (24 h), and cocoa beans (48 h). When
the procedure was reevaluated, it was found that the longer extraction
time required for unground grain, compared with flour, probably
reflected the migration of methyl bromide into the interior of the
grain (Scudamore, 1987).
An acetone/water extraction of grain followed by headspace
analysis was described by Greve & Hogendoorn (1979). The headspace
method has also been developed for sampling other selected foods,
e.g., grapefruit (King et al., 1981), flour, cocoa, unground wheat,
and peanuts (DeVries et al., 1985), cherries and apples (Sell et al.,
1988; Sell & Moffitt, 1990).
Headspace capillary gas chromatography with electron capture
detection was described by Page & Avon (1989). The difference between
this and other headspace procedures is the particle size reduction by
the blending or homogenization of the cold or frozen sample with ice
and cold water with only minimal loss of methyl bromide, resulting in
a rapid 1-h equilibrium in the headspace vial. An advantage of
headspace is that nonvolatile material is not introduced into the
chromatographic column or injector body, thus shortening the run. The
method is sensitive with detection limits of 0.15-0.65 µg/kg. These
different detection limits are due to an inverse relationship of
methyl bromide headspace response and food lipid content. Duplicate
samples from the same vial are not possible, and, for quantification,
a separate calibration curve is necessary for each food item.
Combining the methods of Page & Avon (1989) and Daft (1987, 1988,
1989), an improved method for the detection of methyl bromide in nuts
was developed using extraction with sodium sulfate solution at 80 °C
and purging overnight (Daft, 1992). A Hall electrolytic conductivity
detector, used in the determinative step, has been found to be about
3 times more sensitive to methyl bromide than ECD. Additionally, the
Hall detector is said to eliminate endogenous interference from the
nut samples. The recovery was 40% (coefficient of variation, 29%) and
the method can be used to screen assorted nut samples for ng/g levels
of incurred residues.
Siegwart (1987) suggested using the headspace method for
screening, but that with positive findings, the methyl bromide
concentration should be confirmed using mass spectography. In
addition, methyl bromide should then be converted to methyl iodide and
determined again. A detection limit of under 10 µg/kg, is given.
US EPA Method 624 (GC/MS) has been adapted for the determination
of methyl bromide in fish (Easley et al., 1981).
2.4.5 Methyl bromide in serum, plasma and blood, and post-mortem
tissue
Marraccini et al. (1983) used a purge and trap method followed by mass
spectroscopy to determine methyl bromide levels in post-mortem
tissues. Tissue levels lower than 1 mg/kg (1 ppm) were detectable.
Honma et al. (1985) detected methyl bromide in rat tissues using
GC/ECD. The tissues were extracted with toluene. The presence of
methyl bromide was confirmed by GC/MS. No detection limit was given
but the lowest values reported were 1 ng/g.
Headspace gas chromatography with split flame-ionization,
electron-capture detection has been used to detect volatile substances
including methyl bromide in biological fluids. The method offered
economy of time with a sensitivity equivalent to a packed column
(Streete et al., 1992).
2.4.6 Determination of inorganic bromide in air
Analytical methods for the determination of inorganic bromide in air
are not described here as the concentration of bromide is not
specifically related to the amount of methyl bromide in the
atmosphere.
2.4.7 Determination of inorganic bromide in water
Vanachter et al. (1981) carried out bromide determinations in
leaching water using the colorimetric method described by Malkomes
(1970), in which the sample is first heated to dryness, then phenol
red and chloramine-T (sodium p-toluenesulfochloramine) solution
added. After 5 min, the reaction is stopped with sodium thiosulfate.
The resulting blue colour is read on a spectrophotometer at 590 mµ.
The detection limit is 0.1 mg/litre (0.1 ppm).
In another method, water samples were evaporated to dryness at 90
°C. Sulfuric acid, ethylene oxide in diisopropylether, and
acetonitrile were added and the sample shaken. After 30 min, an
aliquot was removed and solid ammonium sulfate added and shaken. After
separation, the upper layer was removed and anhydrous sodium sulfate
added. An aliquot of the dried sample was analysed using GC/ECD
(detection limit 0.01 mg/litre) (Wegman et al., 1981, 1983).
Table 5. Inorganic bromide in plant material/fooda
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Grain grind samples, add acetonitrile, GLC ECD 0.07 mg/kg not suitable for Heuser & Scudamore
ethylene oxide, and sulfuric fresh vegetables (1970)
acid (4 h, 20 °C); separate
supernatant with ammonium
sulfate; extract with anhydrous
sulfate; supernatant analysed
Salad/ dry samples at 110 °C; grind; GLC ECD 0.1 mg/kg Roughan et al.
vegetables add NaOH, ethanol; evaporate (fresh mass) (1983)
to dryness; add to ulfuric
acid solution/slurry
acetonitrile and ethylene oxide;
analyse 2-bromoethanol
Vegetables extract sample with aqueous GC ECD 0.5 mg/kg interlaboratory Greve & Grevenstuk
ethanol; ash aliquot of (fresh mass) study (1979)
extract in the presence of
NaOH; treat extract with
ethylene oxide
Cereals, extraction of inorganic bromide GC ECD 1 mg/kg Thier & Zeumer
dried and conversion to 2-bromoethanol (fresh mass) (1987)
fruit, by suspension in aqueous 5 mg/kg
dried ethylene oxide and acidification (dried mass)
vegetablea by sulfuric acid; 2-
bromoethanol partitioned into
ethyl acetate and analysed
Table 5 (continued)
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Vegetables dried for 3 days and comminuted specific lowest value Basile &
aliquots soaked in alcoholic KOH ion given Lamberti (1981)
and mineralized overnight at electrode 0.1 mg/kg
600 °C; ash homogenized with
diluted NaNO3; supernatant
analysed
grind sample, shake with water potentiometric ECD 0.1 mg/kg Cova et al. (1986)
(6 h); centrifuge extract measurement
(50 ml) + NaNO3; evaporate with specific
residue, dissolve in water electrode
Peaches peaches blended with bromide- 0.2 mg/litre Austin & Phillips
NaNO3 crystals and selective (wet mass) (1985)
water; centrifugation electrode
supernatant
Cereals, ground/minced; dried X-ray 5 mg/kg Love et al. (1979)
nuts, 100 °C (18 h), ground fluorescence
spices, powdered sample with boric spectroscopy
fruit acid-sodium sulfate
Grain macerated grain refluxed thiosulfate lowest value Urga (1983)
in ethanol-ethanolamine; titration given
alkali digested; ashed (600°C); 4.5 mg/kg
water extraction; oxidized
with sodium hypochlorite
Table 5 (continued)
Medium Sampling method Analytical Detector Detection Comment Reference
method limit
Vegetables fresh sample homogenized and HPLC UV 4 mg/kg pH of the mobile Van Wees et al.
macerated with water then (205 nm) phase must be (1984)
homogenate centrifuged; adjusted to 5.0
supernatant filtered and the (at higher pH,
filtrate used for analysis e.g., 6.0-6.5, an
overlap between
Br- peak and
sample interferenaces
may occur)
a For abbreviations see Table 2.
2.4.8 Determination of inorganic bromide in soils
The colorimetric method of Malkomes (1970) (section 2.4.7) can
also be used for soil. The sample is first sieved, dry-ashed, boiled
in distilled water, and filtered. The filtrate is then analysed.
Brown et al. (1979) determined bromide in soil by extracting with
calcium nitrate solution (0.1 mol/litre) and using a bromide-specific
electrode for detection in the extract. No detection limit was given.
2.4.9 Determination of inorganic bromide in plant material/food
Various methods, such as X-ray spectroscopy, potentiometry,
thiosulfate titration, gas/liquid chromatography, and high-performance
liquid chromatography, have been used to determine bromide content
(section 5.1.4). A summary of methods is given in Table 5.
In the method described by Heuser & Scudamore (1970), bromide ion
is converted into 2-bromoethanol by reaction with ethylene oxide in
acetonitrile-diisopropyl ether, under acidic conditions. The
2-bromoethanol is then determined by gas-liquid chromatography with an
electron-capture detector (ECD). This procedure is suitable for wheat
and maize but is not ideal for salad crops (because of cleaning
procedures) where problems arise, such as severe tailing, lack of
resolution, and poor recovery (Roughan et al., 1983). These authors
varied some conditions, such as preparing the ethylene oxide in
acetonitrile and using Carbowax 20M TPA to prepare the GC column. The
samples (e.g., lettuce) were hydrolysed with alcoholic sodium
hydroxide overnight, ashed for 2 h at 500 °C (600 °C for oily
substances), and ground, prior to digestion with 0.6 N sulfuric acid
(Greve & Grevenstuk, 1976; 1979). Recoveries of 97 % were achieved and
the method was used to determine bromide down to 0.1 mg/kg of
substrate fresh mass (Roughan et al., 1983). A wide range of
vegetables and other crops have been analysed using this method
(section 5.1.4).
A similar procedure for cereals, dried fruit, and vegetables has
been described using GC/ECD (Thier & Zeumer, 1987). The finely ground
sample is suspended in an aqueous solution of ethylene oxide acidified
with sulfuric acid. The inorganic bromide is extracted simultaneously
and converted to 2-bromoethanol. This derivative is partitioned into
ethyl acetate and determined, without further clean up, by electron
capture gas chromatography.
Bromide concentration in plant material has also been determined
by X-ray fluoroscopy with a detection limit of around 5 mg/kg (Brown
et al., 1979; Love et al., 1979).
A specific ion electrode can be used for inorganic bromide
determination using a standard calibration curve with a detection
limit of around 0.1 mg/kg (Basile & Lamberti, 1981; Cova et al.,
1986). Austin & Phillips (1985) used a bromide-selective electrode to
detect levels of bromide ion in peaches; the detection limit for peach
extract was 0.2 mg/litre.
Urga (1983) used a thiosulfate titration method: the macerated
grain was refluxed in ethanol-ethanolamine mixture, and then ashed
(600 °C). The bromide ion was extracted with water and determined by
oxidizing with sodium hypochlorite solution. This was titrated with
sodium thiosulfate, using starch solution as indicator. The lowest
level measured was 4.5 mg/kg.
A quick screening method for inorganic bromide in vegetables,
using high-performance liquid chromatography (HPLC) with a detection
limit of around 4 mg/kg, was described by Van Wees et al. (1984).
2.4.10 Determination of inorganic bromide in urine, blood/
serum/plasma
Various methods for the determination of bromide in biological
fluids have been described: colorimetry (Kisser, 1967), X-ray
fluoroscopy (Rapaport et al., 1982; Shenberg et al., 1988), neutron
activation analysis (Heurtebise & Ross, 1971; Ohmori & Hirata, 1982),
ion-sensitive electrode (Angerer, 1977, 1980); and headspace GC with
FID (Yamano et al., 1987). Koga et al. (1991) compared headspace GC
and an ion chromatography coupled with a conductivity detector to
evaluate levels of bromide ion in urine. GC was more sensitive with a
detection limit of 0.04 mg/litre. Honma et al. (1985) used an GC/ECD
method for their studies on rats (section 6.2). A summary of methods
is given in Table 6.
In forensic science studies (overdose of bromide-containing
sleeping tablets as well as suspected methyl bromide poisoning),
colorimetric methods, such as that of Kisser (1967), have been
routinely used (Weller, 1982). For routine occupational studies, other
methods are more suitable.
Table 6. Determination of bromide in biological fluids and tissuesa
Medium Sampling method Analytical Detection Comment Reference
method limit
Urine/ add soda solution; evaporate + chloramine - - Kisser (1967)
blood and ash (550°C); ash + water T-solution, sodium
->filter filtrate->bromide thiosulfate
Urine alkali ashing (Kisser, 1967); ion-sensitive 1 mg/litre suitable for Angerer (1977,
with KMnO4, bromide->bromine; electrode occupational 1980)
bromine + sulfide soln->bromide exposure studies
Urine headspace; methylation GC 0.4 mg/litre 2.7% standard Koga et al. (1991)
with dimethylsulfate deviation
Urine ion chromatography 1.0 mg/litre 8.7% standard Koga et al. (1991)
deviation
Serum X-ray fluorescence 0.05 µg Rapaport et al., (1982);
Shenberg et al. (1988)
Urine, neutron activation not given Heurtebise & Ross
saliva, analysis (1971)
serum,
plasma
Serum/ neutron activation 120 µg/g; 4 µg/g occupational Ohmori & Hirata
hair analysis (estimated) studies (1982)
Plasma head space plasma + water + GC/FID 0.5 mg/litre Yamano et al. (1987)
dimethylsulfate (Br-->
methyl bromide)
a For abbreviations see Table 2.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural sources
The atmospheric levels of methyl bromide are controlled by the
amounts from natural and anthropogenic (man-made) sources and by the
atmospheric and surface removal processes. Observational data (UNEP,
1992) indicate that the current best estimate for the globally
averaged abundance of methyl bromide in the troposphere is between 9
and 13 pptv, which is equivalent to a total atmospheric loading of
150-220 million kg. If the atmospheric lifetime of methyl bromide is
two years, i.e., only tropospheric removal by reaction with OH - is
significant, then a total emission of about 75-110 million kg per year
is required to maintain the observed atmospheric level. However, if
the atmospheric lifetime is only one year (assuming surface removal
comparable in magnitude to the atmospheric removal), a global emission
of 150-220 million kg per year is required to maintain the atmospheric
methyl bromide at the same level (UNEP, 1992).
Khalil et al. (1993) have used similar input data (global
abundance of 10 pptv, lifetime of two years) to calculate a global
source of about 100 million kg/year. On the basis of their
measurements of ocean abundance and supersaturation (which differ
considerably from those of Singh et al. (1983)), they estimated an
ocean source of 35±5 million kg/year. They proposed that the
anthropogenic sources must be about 30 million kg/year, assuming that
the differences in calculated emissions for the northern and southern
hemispheres are solely due to man-made sources. This leaves about 35
million kg/year of emissions that cannot be categorized but are
believed to originate from the tropics.
From the surface water and air observations of methyl bromide
concentrations off the Pacific coasts of North and South America,
Singh et al. (1983) estimated the total natural emissions of methyl
bromide from the oceans to be 300 million kg/year. The total oceanic
emission quantified from the extrapolation of the limited data may not
be entirely justifiable. Using the currently accepted global
atmospheric loading of 150-220 million kg, a tropospheric lifetime of
6-9 months can be expected, meaning surface removal processes are even
more important than reaction with OH. It would also mean that
fumigation sources of methyl bromide are less than 10% of the total
global emission.
It is likely that the calibration standards of Singh et al.
(1983) were in error, leading to overestimation of methyl bromide
concentrations by a factor of about two. Corrections for this factor
would resolve part of the discrepancy between the estimates of Khalil
and Singh of the oceanic source. However, an unresolved difference in
supersaturation measurements (140% and 180% from two Khalil voyages
and 250% from Singh) leaves a conflict of about a factor of three that
cannot be resolved without more measurements.
In any event, the natural/anthropogenic balance of methyl bromide
emissions is very uncertain.
The major natural sources of methyl bromide are considered to be
oceanic biological processes (mainly algal), but the mechanism for the
production of methyl bromide in the marine environment, and its
oceanic distribution, are not well understood (Rogozen et al., 1987;
WMO, 1992).
Methyl bromide occurs naturally in coastal waters together with
methyl chloride and methyl iodide (Lovelock, 1975). This author
suggested that methyl iodide produced by large kelp, such as
Laminaria , reacts with the chloride and bromide ions in sea water
to produce methyl chloride and methyl bromide, respectively.
Harper (1985) reported the formation of methyl bromide from
cultures of a common wood-rotting fungus (Phellinus pomaceus) in the
presence of sodium bromide solution, with cellulose as the substrate.
3.2 Anthropogenic sources
Anthropogenic sources, primarily soil fumigation, add to the
amount of methyl bromide in the atmosphere. The amount released
depends greatly on the regulations, methods used, dosage, type of
plastic cover, length of covering, and precautions taken by the
fumigators. The portion released is a question of dispute. Daelemans
(1978) calculated that 70-90% of the applied amount of methyl bromide
(50-100 g/m2) disappeared into the atmosphere. Using a common
application method (15-25 cm injection with a 2-day cover), analysis
predicted emissions ranging from 45 to 53% (UNEP; 1992). In contrast,
Rolston & Glauz (1982) estimated that 70% of the applied methyl
bromide escaped into the atmosphere after fumigation using injection
chisels.
During structural fumigation, up to 90% of the applied methyl
bromide was estimated to escape into the environment (Reichmuth &
Noack, 1983). During storage fumigation, an estimated 30% of the
methyl bromide may escape from the fumigation chamber and enter the
environment, while the rest decomposes to organic bromine and
methylated derivatives of organic compounds (National Academy of
Science, 1978). Other estimates give an 80% loss of methyl bromide
used on perishable products (UNEP, 1992).
On the basis of the inventory of use and emissions coupled with
the analyses of Singh & Prather (UNEP, 1992), the current best
estimate for total anthropogenic emissions of methyl bromide is about
30 thousand tonnes per year, representing 25±10% of the total
emissions.
Methyl bromide is also emitted from motor vehicles using leaded
petrol (section 3.2.3).
Methyl bromide is listed as a controlled substance in the
"Montreal Protocol on Substances that Deplete the Ozone Layer".
3.2.1 Production levels and processes
3.2.1.1 Producers and world production figures
The total annual methyl bromide sales for the years 1984-90,
tabulated according to region, are shown in Table 7; production
figures for this period were almost identical. In Table 8, methyl
bromide sales are tabulated according to use. These figures were
provided by companies reporting to the Methyl Bromide Industry Panel,
Chemical Manufacturers Association in February, 1992.
Table 7. Methyl bromide sales (tonnes) according to region for 1984-90a,b
Year North South Europe North Afica Asia Australia Total sales
America America Africa
1984 19 659 1 389 11 364 183 1 595 10 678 704 45 572
1985 20 062 1 503 14 414 45 1 975 9 743 531 48 273
1986 20 410 1 775 13 870 380 2 205 11 278 538 50 445
1987 23 004 1 820 15 359 385 1 751 12 816 555 55 690
1988 24 848 2 058 17 478 277 1 582 3 555 812 60 610
1989 26 083 1 701 16 952 618 2 075 14 386 755 62 570
1990 28 101 1 621 19 119 432 1 838 14 605 928 66 641
Total 162 167 11 866 108 556 2 320 13 021 87 061 4,823 389 814
aCompiled by the Methyl Bromide Industry Panel, Chemical Manufacturers Association
(unpublished report, February 1992).
bThe 1990 production figures from other producing countries (e.g., India, China, former
USSR) is estimated to be about 2500 metric tonnes.
Table 8. Methyl bromide sales (tonnes) according to use category for 1984-90a
Year Pre-plant Post harvest Structural Residential/ Chemical Total sales
commercial intermediates
1984 30 408 9 001 1 285 881 3 997 45 572
1985 33 976 7 533 1 274 983 4 507 48 273
1986 36 090 8 332 1 030 999 4 004 50 455
1987 41 349 8 708 1 763 1 160 2 710 55 690
1988 45 131 8 028 1 910 1 737 3 804 60 610
1989 47 542 8 919 2 083 1 530 2 496 62 570
1990 51 306 8 411 1 740 1 494 3 693 66 644
Total 285 802 58 932 11 085 8 784 25 211 389 814
aCompiled by the Methyl Bromide Industry Panel, Chemical Manufacturers Association (unpublished
report, February 1992).
The following is a list of the companies, including any related
subsidiaries and/or joint ventures that reported production and
release data:
1. Association of Methyl Bromide Industry Japan (Japan)
(a) Sanko Kagaku Kogyo Co. Ltd
(b) Teijin Chemicals Ltd
(c) Nippon Chemicals Co. Ltd
(d) Dohkal Chemicals Co. Ltd
(e) Nippon Kayaku Co. Ltd
(f) Ichikawa Gohsei Chemical Co. Ltd
2. Atochem S.A. (France)
(a) Derivados Del Etilo, S.A. (Spain)
3. Dead Sea Bromine Group
(a) Dead Sea Bromine (US)
(b) Eurobrom B.V. (The Netherlands)
4. Ethyl Corporation (US)
(a) Ethyl S.A. (Belgium)
5. Great Lakes Chemical Company (US)
(a) Great Lakes Chemical (Europe) Ltd (UK)
6. Societa Azionaria Industria Bromo Italiano (Italy)
According to Eurobrom B.V. (personal communication), Atochem is
the sole producer of methyl bromide in Europe. Methyl bromide is also
imported into Europe from the USA and Israel (Ethyl Corporation and
Dead Sea Bromine Group).
The average rate of increase in total world sales between 1984
and 1990 was about 6% per year, more than 90% of these sales being in
the Northern Hemisphere. Of the 51.3 thousand tonnes used as a
pre-planting fumigant in 1990, about 80% was used in Europe and North
America.
3.2.1.2 Production processes
Methyl bromide is commonly produced by the interaction of
methanol (CH3OH) and hydrogen bromide (HBr). The hydrogen bromide
can be generated in situ from bromine and a reducing agent, such as
sulfur or hydrogen sulfide (Dagani et al., 1985). Methyl bromide is
distilled from the reactant mixture and the crude product purified by
further low-temperature fractional distillation (National Academy of
Science, 1978). Another method is to add sulfuric acid to a
concentrated sodium bromide and methanol solution (National Academy of
Sciences, 1978; Stenger, 1978).
Ethyl Corporation and Great Lakes Chemical Co. both use a
coproduction process that produces methyl bromide as a coproduct with
the production of tetrabromobisphenol A (TBBPA). In this process,
bisphenol A (BPA) is dissolved in methanol and then reacted with
bromine to yield TBBPA and hydrobromic acid. The hydrobromic acid
reacts with the methanol to yield methyl bromide (Ethyl Corporation,
Personal communication to the IPCS, 1990).
In the manufacturing process of a Japanese plant, bromine is
first mixed with methyl alcohol and heated at 60-80 °C in a boiler.
The methyl bromide produced is cooled, purified, and condensed. These
processes are mainly conducted in a closed system (Kishi et al.,
1991).
3.2.1.3 Losses to the environment during normal production
In 1973, the emission of methyl bromide from manufacturing
processes in the USA was estimated to be 100 000 kg compared with 11.3
million kg emitted when used as a fumigant (National Academy of
Science, 1978).
However, in 1990, in the USA, the total reported emission of
methyl bromide from industry was 1000 kg (US EPA Toxic Release Index,
1990). In general, because processes are enclosed, the amount of
methyl bromide lost during manufacture is negligible compared with the
amount released to the atmosphere when it is used as a fumigant.
3.2.1.4 Methods of transport
Methyl bromide is easily liquefied and is shipped in steel
cylinders as a liquefied gas under its own vapour pressure (Matheson
Gas Data Book, 1980). This may be augmented with nitrogen or carbon
dioxide before shipment to permit rapid ejection at low temperatures
(Stenger, 1978). Methyl bromide is also transported in cans and tanks.
An industrial code of practice for the handling and
transportation of methyl bromide in Europe has been recommended (EMBA,
1988).
3.2.1.5 Accidental release or exposure
Incidents of methyl bromide poisoning occur through accidental
exposure to the compound, particularly during soil or structural/space
fumigation and also during manufacture (section 9).
3.2.2 Uses
Methyl bromide is used as follows: soil (pre-planting) fumigation
(77%), quarantine and commodity fumigation (12%), structural
fumigation (5%), and chemical intermediates (6%) (UNEP, 1992) (Table
8).
The general use of methyl bromide in fire extinguishers has been
abandoned as it was the cause of a number of fatal accidents (see
section 9). However, it is still used for special-purpose fire
extinguishers (Matheson Gas Data Book, 1980).
Since 1960, methyl bromide has been used as a fumigant for a wide
range of stored, dry foodstuffs and other products, such as tobacco,
fresh fruit, and vegetables, in particular to comply with quarantine
regulations (Bond, 1984). It is used pre-harvest in glasshouses and in
the open as well as post-harvest in mills and warehouses. It is also
used to fumigate buildings, furniture, books, and archived material
(Alexeeff & Kilgore, 1983).
The techniques used for the different types of methyl bromide
fumigation are given in Table 9.
3.2.2.1 Soil fumigation
The gas is a soil fumigant for the control of weeds, weed seeds,
nematodes, insects, and soil-borne diseases (Meister, 1985). Methyl
bromide can be applied to soil under sheeting in a vaporized form
using either evaporating jars (cold method) or heating (hot method),
or injected as a liquid and allowed to vaporize in situ (Table 9).
Table 9. Outline of methyl bromide fumigation techniquesa
Examples of Fumigation Fumigation Application technique Ventilation of
Type application dosage period methyl bromide residues
1. Space Buildings 0.5-1% 2-3 days Sealing of all openings except one door with Natural ventilation (opening
fumigation (mills, in air plastic foil and adhesive tape; placement of of doors, windows) assisted
factories, (20-40 g/m3) methyl bromide cylinders at selected locations by mechanical exhaust
museums) inside building; opening of cylinders by team ventilation if available
of operators working backwards towards escape
door; sealing of escape door
2. Chamber Dried food 0.8-1% < 1 day Permanently installed delivery systems, Mechanical ventilation:
fumigation products, chamber operated from outside of chamber continuous dilution with fresh
wood volume air in atm. pressure chambers,
(32-40 g/m3) batch dilution cycles in
"vacuum" chambers
3. Fumigation Ducts, bins; 1-2% 1-3 days Sealing of goods/machines under plastic foil Removal of sheeting, natural
with stacked in air or tarpaulins; methyl bromide injection through ventilation
movable goods, pieces (40-80 g/m3) ports via flexible tubing, using (a) hot
delivery of machinery vapour systems (methyl bromide passed through
system heat exchanger in a water boiler), or (b) cold
vapour systems (pressurized cylinders on
trolleys)
4. Surface Soil, compost 50-100 g/m-2 2-5 days (a) Hot vapour application using perforated Removal of sheeting, latency
fumigation tubing prepared under plastic sheeting; period and/or watering before
(b) liquid methyl bromide injection, truck/ tillage
trailer with cylinders connected to injection
nozzles and reel unfolding plastic sheeting
behind truck; (c) methyl bromide cans place in
puncturing cups underneath sheeting, punched
open by operator walking on the sheeting
aFrom Guillemin et al. (1990).
The methods practised in various countries differ. In the USA,
methyl bromide is mainly applied by chisel application (injection).
Methods of soil disinfestation used in Belgium, for example, are given
in Table 10. In Israel, both soil fumigation in strips and blanket
(large area) fumigation are widely used (Klein, 1989). The methods
used are the hot gas method and injection method. Strip fumigation is
not as effective as blanket fumigation but, in some circumstances, is
more economical.
Table 10. Soil disinfestation methods and products used in Belgium and their relative importancea
Physical methods:
- steaming : - sheet steaming/steaming via drain pipes 7%
- vacuum steaming of rockwool substrates 2%
- solarization 0%
- microwave radiation 0%
- ozone 0%
Chemical methods:
- methyl bromide (MB) : fumigation (greenhouse/outdoor) 50%
+ injection (outdoor)
- chloropicrin (CP) : injection (greenhouse/outdoor) 10%
- MB + CP : injection (outdoor) 10%
- metham-sodium : injection (greenhouse/outdoor) 8%
- dazomet : soil mixing (greenhouse/outdoor) 3%
- dichloropropene : injection (greenhouse/outdoor) 8%
- others 2%
a From: Pauwels (1989).
Not only the method of application but also the type of plastic
sheeting used for covering is important for optimal fumigation
conditions as well as for the safety of the fumigators and reduction
of environmental pollution. Munnecke et al. (1978) showed that using
gas-tight films very high concentrations of methyl bromide reached the
soil, whereas, under low density polyethylene (LDPE) covers, these
concentrations rapidly dissipated. In the Netherlands where extensive
horticulture plays an important economic role, Wegman et al. (1981)
reported that 2 million kg of methyl bromide were being used in
glasshouses each year. De Heer et al. (1983) compared different
plastic films in trials in the main glasshouse district of the
Netherlands. They confirmed that the dose of methyl bromide could be
substantially reduced, without affecting the concentration-time
product in the soil, if gas-tight films were used instead of LDPE.
They emphasized that the reduction of methyl bromide losses depends
greatly on how the films are laid down and wetted and on how the
methyl bromide is distributed under the films. The use of methyl
bromide for soil fumigation was banned in the Netherlands in 1991.
Prior to this date, the use of LDPE sheeting was prohibited, the
mandatory cover time of the soil was extended to 10 days, and the dose
reduced to 20 g/m2 (De Heer et al., 1983). In 1983, this dose was
increased to 40 g/m2 but, in practice, doses of 60-80 g/m2 were
used up to the phase-out in 1991, because applying the protective
sheeting in a careful manner proved to be too time-consuming. In a
comparison of methyl bromide diffusion through different plastic films
40 µm thick, some showed excellent barrier properties to the gas
whereas 75% of the methyl bromide applied was lost through LDPE and
another type of plastic within 5 h (Van Wambeke et al., 1988).
Doses of methyl bromide to be applied depend on:
- the legal standards (regulations differing for each
country) and methods used;
- the pest to be controlled (type/degree of infestation/
infection);
- temperature;
- soil type;
- the plastic soil cover (covering time and type of
plastic).
Methyl bromide is particularly used in intensive horticulture,
where, because of specialization, only a few selected crops are grown
with a resulting increase in pests, microorganisms, and weeds that
could decrease the quality and quantity of the crop.
Table 11 gives examples of the use of methyl bromide as a soil
fumigant and the recommended dosage. Common rates of application of
methyl bromide to soil vary between 50 and 125 g/m2 (FAO, 1980).
Industry recommends that the dosage should not exceed 100 g/m3.
Table 11. Pest controlling dose rates of a 98% methyl bromide formulation in g/m2 according to soil type (in United Kingdom)a,b
Crops Dosage in g/m2 b Aeration Remarks
to control nematodes, to control damping- to control fungi (days)e
annual and perennial off fungi, e.g. causing rotsd and
weedsc and broomrape Rhizoctonia, Pythium wilts, e.g.,
spp., Thielaviopsis Scleroctiun
basicola, Phytophtora rolfsii, Pythium
spp. spp., Verticillium
spp., Fusarium
spp., Pyrenochaeta
spp.
Plant nurseries: 35-50 50 7-14 do not fumigate heavy
vegetables, soils to be used for
flowers celery nurseries
Vegetables: 35-50 75 75-100 7-14 for beta-alpha-type
cucurbits, tomatoes, cucumbers, soil
eggplants, peppers, leaching is obligatory
onions, radishes
Leafy vegetables: 35-50 75 75 7-21
celery, chicory,
cabbage, lettuce,
spinach
Strawberries 35-50 75 75 7
(nursery and field)
flowers: 35-50 75 75-100 7-14 even light soils must
annual, be leached before
perennial cut planting carnations
flowers,
ornamentals
Table 11 (contd.)
Crops Dosage in g/m2 b Aeration Remarks
to control nematodes, to control damping- to control fungi (days)e
annual and perennial off fungi, e.g. causing rotsd and
weedsc and broomrape Rhizoctonia, Pythium wilts, e.g.,
spp., Thielaviopsis Scleroctiun
basicola, Phytophtora rolfsii, Pythium
spp. spp., Verticillium
spp., Fusarium
spp., Pyrenochaeta
spp.
Bulbs and corms 35 50 75 7
(on light soils
only)
Citrus replanting 50 14
Deciduous 75-100 14
replanting
a From: Bromine & Chemicals Ltd. (1990).
b When a dose range is given, the smaller dose relates to light soil, the larger to medium and heavy soils.
c Purple nutsedge (nut grass) corms and seeds of horseweed, Erigeron (Conyza) , mallow (Malva) , and legumes are not
efficiently controlled.
d The dose rate to control Fusarium on all soil types is 100 g/m2.
e For light soils and/or high temperatures, the shorter aeration period is sufficient; for medium and heavy soils, and
all soil types at low temperatures, the longer aeration period is required; the long aeration period is also desirable for
direct seeded crops; if rain is expected during the aeration period, do not remove the plastic sheets entirely, but
allow for aeration while protecting the soil from direct rain.
In some cases, it is recommended that, after fumigation, the
ground should be thoroughly leached to remove the bromide salts that
are formed in the soil. Fumigation of peat soils before planting leafy
vegetables is not recommended, because of the resulting high bromide
residues. There are different national regulations concerning the
crops permitted to be grown after soil fumigation. In some countries,
the use of methyl bromide is severely restricted or prohibited.
3.2.2.2 Quarantine and non-quarantine commodity treatments
Methyl bromide is currently used for quarantine and
non-quarantine commodity treatment, because it is rapidly effective
(often less than 24 h) and can be used for pests on a wide range of
commodities at fumigation temperatures exceeding 4 °C. Imports of
products subject to infestation are often only permitted if the
product is fumigated in the country of origin or at the ports of
destination (UNEP, 1992).
Commodities fumigated with methyl bromide include durable food
commodities (such as cocoa and coffee beans, grains, dried fruit,
nuts), perishable food commodities (mainly fruits and vegetables), and
non-food commodities (forestry products, cut flowers, cotton, tobacco,
packaging, animal feed stuffs, artifacts, and other commodities).
Suggested dose rates for storage fumigation are given in Table 12 and
Table 13.
In special extermination problems, such as that of the
khapra-beetle (Trogoderma granarium Ev.) larvae in the transport of
bulk-loaded expeller (pressed remains from oil and fat seeds that are
used for making cattle feed), methyl bromide is used in combination
with hydrogen phosphide (Wohlgemuth et al., 1976).
Fumigation is followed by an aeration period when fresh air is
passed through the fumigation chamber to remove methyl bromide from
the air space. At least 2 h of aeration is required for fresh fruit
fumigated with methyl bromide. In addition, the aeration must continue
until the concentration of methyl bromide in the air vented from the
chamber is below an exposure limit value of 20 mg/m3 (usually within
4-12 h). This is to protect workers entering the fumigation chamber
immediately after the aeration period (Sell et al., 1988).
Table 12. Dosage rates and exposure times for space fumigation with methyl bromide
Commodity Vacuum chamber fumigation Fumigation at atmospheric Notes
pressurea
Dosage Exposure time Dosage Exposure time
rates (g/m3)b (h) rates (g/m3)b (h)
Foods:
coffee 32-55 3 16-40 16-24
cocoa beans 32-55 3 16-40 16-24
grains - - 20-38 24 Maximum moisture content
of 12%; lower doses for
upright storage and higher
doses for flat storage;
do not fumigate seed grain;
values are for 21-25 °C;
between 10-15 °C multiply
dosage by 1.5; between
16-20 °C multiply by 1.25
spices 40 3 16-24 16-24 At 20 °C and above only
cigarette 54-80 4 20-32 45-72
tobacco
Packing 40-58 3-4 24-48 16-24 Compressed bales should
materials: be fumigated under vacuum
at 55 g/m3, for 48 h at
15 °C and above
Factories - - 16-40 24 Use lower doses of range
and storage for spaces over 14 000 m3
premises
Table 12 (continued)
Commodity Vacuum chamber fumigation Fumigation at atmospheric Notes
pressurea
Dosage Exposure time Dosage Exposure time
rates (g/m3)b (h) rates (g/m3)b (h)
Transport - - 16-40 10-12 Multiply dose by 6
vessels, against Khapra beetle
freight
containers
a Including stacks under gas-proof sheets.
b Where a range is given, the fumigation dosage depends on temperature: the dosage rates are for a temperature
range of 4-32 °C; at lower temperatures higher doses and longer times should be used.
Table 13. Use of methyl bromide as a storage fumigant in Germanya
Area of use Dosage Length of
(g/m3) fumigation
(h)
Stored goods
- mills 16-30 24-48
- silos (elevators) 16-30 24-48
Expeller
- in barges, inland and coastal
motor boats
a ) in sacks 56-96 24
b ) as bulk material b 56-96 72
- in railway carriages
in sacks b 56-96 72
Stored goods (apart from
grain, expellers, tobacco)
- in vacuum chambers with gas
circulating apparatus 50 2
- in small silos with gas
circulating apparatus 70 16
- packed in gastight
sheeting 16-30 24
- in sufficiently gastight
rooms 16-30 24
a Adapted from: BUA (1987) and BBA (1989).
b Together with hydrogen phosphide.
The development of acceptable alternatives to methyl bromide for
certain commodities is complex (UNEP, 1992).
3.2.2.3 Structural fumigation
Methyl bromide is used extensively as a structural fumigant, and
this application currently accounts for about 5% of production. The
current use of methyl bromide as a structural fumigant is widespread
because of its efficacy, applicability for a wide variety of sites and
pests, suitability for use on accessible and inaccessible pests, short
fumigation period (about 1 day), lack of insect resistance, cost
effectiveness, and because it does not damage food, structures, or
equipment when used correctly. However, methyl bromide is toxic, must
be applied by skilled operators, and, in some instances, requires
multi-day aeration periods to reduce methyl bromide exposure to levels
safe for humans (UNEP, 1992).
In California and Florida, where homes are fumigated to eradicate
insect pests, poisoning incidents have occurred through unauthorized
access to buildings under fumigation (section 9.2.1.2). Fumigating
food in houses is mentioned in section 5.2.1.
3.2.2.4 Industrial uses
Methyl bromide is used in organic synthesis, principally as a
methylating agent (Torkelson & Rowe, 1981) and as a low-boiling
solvent, for example, for extracting oils from nuts, seeds, and
flowers (Windholz, 1983). Because of the toxicity of methyl bromide
(section 9), its use as a refrigerant and as a general fire
extinguishing agent is now of historical interest (likewise its use as
an anaesthetic in the 19th century). Methyl bromide may still be used
in fire extinguishers in special situations (Matheson Gas Data Book,
1980).
3.2.3 Methyl bromide emission from motor car exhausts
Harsch & Rasmussen (1977) reported the presence of methyl bromide
at sub-part per-billion concentration levels in urban areas (section
5.1.1). The major source of methyl bromide in urban areas is believed
to be automobile exhaust.
Engines operating on "leaded" petrol, containing ethylene
dibromide (EDB) as an additive, contribute a much larger amount of
methyl bromide to urban atmospheres than engines with catalytic
converters burning "unleaded" fuel. On the basis of an estimated
consumption of 12 million tonnes of leaded petrol per year, Bell
(1998) calculated that about 45 tonnes of methyl bromide is produced
from car exhaust in the United Kingdom annually. Methyl bromide
concentrations in the range of 90-190 µg/m3 have been measured in
the exhaust emissions of motor vehicles using leaded petrol with EDB
(Baumann & Heumann, 1987). According to these authors, the portion of
organobromine compounds is 22-44% of the total bromine that is emitted
in the exhaust gases, the concentration decreasing with increasing
engine temperature. Furthermore, the methyl bromide content of these
organic components varies between 64 and 82% (Baumann & Heumann,
1987). On the basis of an estimated global consumption of 50 000
tonnes of 1,2-dibromoethane as a petrol additive (Roskill, 1992), and,
from the above figures, and assuming that the bromide from the EDB is
emitted in full from the exhaust gases, it can be estimated that
between 7000 and 18 000 tonnes of methyl bromide could be emitted
annually from car exhaust. The increasing use of unleaded fuel should
result in a decrease in these levels.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Transport in air
In view of the destructive effects thatbromine compounds have on
the ozone layer, the release of methyl bromide into the atmosphere may
pose an environmental problem. Models indicate that inorganic bromine
can significantly affect ozone levels in the stratosphere (Wofsy et
al., 1975; Prather et al., 1984; Prather & Watson, 1990; UNEP, 1992).
The atmospheric budget of methyl bromide is controlled by the
magnitude of natural and anthropogenic sources (sections 3.1, 3.2, and
5.1.1) and by the atmospheric and surface removal processes.
The major natural source is oceanic and the major anthropogenic
source is the release into the atmosphere of methyl bromide during its
use as a fumigant and, to a lesser extent, from industrial and motor
vehicle emissions. As far as is known, removal processes are mainly
atmospheric. However, ocean and terrestrial surface removal could be
significant, but this remains to be quantified (UNEP, 1992).
There are three possible removal mechanisms for methyl bromide
within the atmosphere: (a) reaction with the hydroxyl radical and
other chemical species in the troposphere; (b) precipitation in the
troposphere; and (c) transport and subsequent photolytic and
chemical removal in the stratosphere (section 4.2.2). Removal by
precipitation is thought to be unimportant (UNEP, 1992). According to
the report, the most significant removal process is that of the
reaction of methyl bromide with the hydroxyl radical in the
troposphere (estimated removal time of 2±0.5 years). A minor removal
process is transport to the stratosphere followed by reaction with the
hydroxyl radical and photodissociation with an estimated lifetime of
about 30-40 years.
Between 20 and 25 km above sea level, photodissociation is of
equal importance to loss by diffusion and reaction with the hydroxyl
radical. Above this, photolysis plays the most important role
(Robbins, 1976a).
From the sparse data available (section 5.1.1), it appears that
methyl bromide has no significant vertical gradient in the
troposphere, but decreases rapidly in the high-latitude lower
stratosphere by at least a factor of 3-5 within 10 km of the
tropopause (UNEP, 1992). Further details are discussed in sections
4.2.2, 4.4, and 5.1.1.
4.1.2 Transport in water
The solubility of methyl bromide in water is between 16 and 18
g/litre at 20 °C (Table 1). In soil, it is partially hydrolysed to
bromide ion. After fumigation using methyl bromide, the soil may be
leached with water to prevent the uptake by plants, subsequently
planted on the sterilized soil, of the bromide ions formed. Between
300 and 600 mm of irrigation water is necessary to remove the bromide
ions effectively from the root zone of plants (Wegman et al., 1981).
In the Netherlands, this leaching caused problems because the water
was mostly withdrawn from surface waters and the drainage water after
leaching was discharged again into the surface waters; thus the
bromide ion concentration accumulated during leaching periods.
Vanachter et al. (1981) found a linearity in the inorganic
bromide amounts in the leaching water up to 200 mm. Between 200 and
400 mm, a decreased concentration was seen in two types of soil
tested, a sandy soil containing 2.15% organic matter and a loamy soil
containing 7.22 % organic matter. There was a direct correlation
between the initial bromide ion concentrations and the bromide ion
concentrations in the first fractions of leaching water. The average
half-life for methyl bromide in surface water, under field conditions,
was calculated to be 6.6 h at a water temperature of about 11 °C, the
decline being attributed to degradation and volatilization processes
(Wegman et al., 1981).
It has been found that methyl bromide is able to diffuse through,
and is adsorbed by, certain plastics, e.g., polyethylene.
Drinking-water pipes that are either free or in earth can thus be
contaminated within a few days, if surrounded by methyl bromide that
is being used for fumigation (Herzel & Schmidt, 1984).
4.1.3 Transport in soil
Methyl bromide is nearly four times heavier than air, and much of
that used as a soil fumigant diffuses throughout the surface to depths
of 60-240 cm, some of it being hydrolysed to bromide ion or decomposed
by microorganisms, the remainder (45-90%) (section 4.1.1) eventually
being dissipated into the atmosphere (Brown et al., 1979). Daelemans
(1978) found the rate of degradation of methyl bromide in soil was
about 6-14% per day at 20 °C.
Lepschy et al. (1979) carried out short- and long-term studies on
the effects of fumigation with methyl bromide on various soil types.
They found that methyl bromide could be detected up to 3 weeks after
fumigation in different soil types, the highest content being found in
the upper soil layers (0-40 cm). Traces of methyl bromide could be
measured down to a depth of 80 cm. The bromide derived from methyl
bromide was largely water soluble, the water solubility and total
bromide content reducing with time. Bromide levels were back to normal
after 1 year of cultivation.
During fumigation, the transport of methyl bromide gas through
soil (pores) is caused by mass flow and molecular diffusion, but its
transport is also influenced by simultaneously occurring sink
processes, such as sorption and dissolution, and irreversible sink
processes, such as hydrolysis (Brown & Rolston, 1980). Earlier
experiments by Chisholm & Koblitsky (1943) showed that the reversible
and irreversible methyl bromide sink capacities depended on soil
moisture content and decreased in the sequence peat, clay, sand. A
review of the mechanisms of breakdown of methyl bromide by Moje (1960)
indicated that unimolecular nucleophilic substitution should be the
major mechanism for the hydrolysis of methyl bromide in water. Maw &
Kempton (1973) suggested that the reactions of dissolved methyl
bromide with the soil organic matter involved the transference of the
methyl group to the carboxy groups and N- and S-containing groups of
the amino acids and proteins of soil organic matter. The reactions are
expected to be first order because of the excess of organic matter and
the production of bromide ion. Following fumigation by methyl bromide,
methylation is expected to be highly dependent upon the amount of
organic matter present.
Laboratory experiments by Brown & Rolston (1980) confirmed this
earlier work to a great extent, showing that rates of bromide
production were significantly influenced by soil type, being greatest
with muck, intermediate with loam, and least with sand. A first-order
kinetic model for the reversible sink term described effluent curves
more adequately than a linear, equilibrium model, though it appeared
that both models were inadequate in completely describing the
adsorption-desorption process. Irreversible sink processes had a
negligible effect on bromide ion production.
Methyl bromide is changed by the methylation of the organic
matter and to a smaller extent by hydrolysis to form bromide ion. In
contrast to naturally occurring bromide, the bromide formed from
methyl bromide is at first only slightly bound to soil particles and
is therefore free to move in the soil. Thus, it can be taken up by
plants or can be washed out by leaching the soil. It can be completely
leached out of sandy soils, but this is very difficult in clay soils
(CEC, 1985).
Arvieu & Cuany (1985) also described the dependence of the
adsorption and degradation of methyl bromide on the organic matter
content of the soil. Adsorption on to organic matter reduces the
available methyl bromide concentration in the aqueous and gaseous
phases of the soil. Irreversibly adsorbed molecules no longer have
biological activity and may persist as bound residues, if not
hydrolysed. Organic matter is also a main factor involved in the
degradation of methyl bromide in the soil. The reaction rate depends
on its nature, composition, and stage of decomposition.
Laboratory experiments carried out by Herzel & Schmidt (1984)
confirmed that the degradation of methyl bromide in soil depends
almost entirely, if not completely, on the organic composition of the
soil. In soil with much humus, the "half-life" of methyl bromide was
10 days, while, in a lighter soil, it was 30 days, and, in sand, about
100 days. The authors concluded that although methyl bromide is
degraded in shallow top soils, the fumigant is relatively persistent
in the underlying strata, where its diffusion into the atmosphere is
no longer possible. If there are unsuitable conditions, such as a high
water table, low temperatures, and a low density of the underlying
strata, then contamination of groundwater with methyl bromide after
fumigation cannot be ruled out.
Rolston & Glauz (1982) described a simulation model for the
transport of methyl bromide gas from injection chisels within the
field. The injected methyl bromide is assumed to form cylindrical,
parallel sources at the depth of injection. Transport of methyl
bromide is described by radial diffusion from the injection cylinders.
A theoretical model has been developed giving profiles of the
concentration of methyl bromide in the soil in both liquid and gas
phases, making it possible to judge the extent of the soil zone
treated and to forecast the behaviour of the substance (Mignard &
Benet, 1989).
4.1.4 Vegetation and wildlife
Bromide accumulation in plants depends on various factors, such
as the physical and chemical properties of the soil, the climatic
conditions (temperature and rainfall), the plant species, and the type
of plant tissue (Basile & Lamberti, 1981).
Wheat and soil/bromide dynamics have been studied in methyl
bromide-fumigated plots. The total crop bromine concentration was 0.5
g/m2 (only aerial parts) (Fransi et al., 1987). The bromine
concentration in the different parts of spring wheat decreased
throughout its development, indicating that the largest rates of
bromide uptake were in the first stages of growth. When the grain
filling started, there was an increase in bromide concentration,
except for the dried leaves fraction, coinciding with an increase in
ambient temperature. This increase was more marked in the senescent
leaves, which remained in the plant top and were subject to a higher
transpiration rate. Afterwards, throughout the grain-filling period,
the bromide concentration in all parts decreased sharply. The bromide
concentration in the ears was low, especially in the grains. There was
no scorching of plants. In another study, Brown & Jenkinson (1971)
reported scorching of wheat grown on soil fumigated with methyl
bromide by injection; the scorched plants contained up to 6.1%
bromide.
Leafy vegetables, such as lettuce and spinach, can take up
relatively large amounts of bromide ion without phytotoxic symptoms
(Wegman et al., 1981; see also section 5.1.4). In contrast, other
crops, such as carnations, citrus seedlings, cotton, celery, pepper,
and onions, are particularly sensitive to methyl bromide fumigation
(Bromine & Chemicals Ltd., 1990).
There are no data for accumulation in wildlife (see also section
4.2.3).
4.1.5 Entry into the food chain
The two main uses of methyl bromide connected with soil and food
fumigation, i.e., "sterilization" of soil prior to planting and
fumigation of foods after harvesting, must be considered in relation
to entry into the food chain. In the former case, the level of bromide
ion must be considered. In post-harvest fumigation, it is possible
that methyl bromide itself, as well as bromide and other possible
reaction products, may be found in food.
Most attention has been paid to bromide residues in foodstuffs,
as methyl bromide seems to be only transient (sections 5.1.4 and 6.3).
Possible methylation products are methionine sulfonium methyl bromide,
1-methyl histidine, S-methyl cysteine, S-methyl-glutathione, and
other minor methylated compounds. The composition and amount of each
residue depends on the type of foodstuff fumigated (Starratt & Bond,
1990a,b). These residues are also found in foodstuffs that have not
been fumigated.
4.2 Transformation
4.2.1 Biodegradation
4.2.1.1 Soil
Methyl bromide is degraded by three species of nitrifying
bacteria, the soil nitrifiers Nitrosomonas europaea and
Nitrosolobus multiformis , and the marine nitrifier Nitrococcus
oceanus (Rasche et al., 1990). Ammonia monooxygenase is thought to
be the enzyme that catalyses the degradation. Oxidation results in
dehalogenation with the release of bromide ions (Hyman & Wood, 1984).
4.2.1.2 Stored product fumigation
During stored product fumigation, most of the methyl bromide is
converted to inorganic bromide and other residues (section 6.3),
probably by reaction with amino or sulfhydryl groups in the products.
4.2.2 Abiotic degradation
4.2.2.1 Hydrolysis
Methyl bromide hydrolyses at neutral pH in laboratory light to
methanol, bromide, and hydrogen ion:
CH3Br + H2O -> CH3OH + H+ + Br-.
The rate constant of 3.0 x 10-7 s-1, at 25 °C, reported by
Moelwyn-Hughes (1938) has been confirmed by Castro & Belser (1981).
The influence of temperature (18 °C and 30 °C) on the hydrolysis
rate of methyl bromide was investigated using distilled water buffered
at pH 3, 5, 7, and 8 (Gentile et al., 1989). The results are given in
Table 14. The authors suggest two types of reaction. The dominant
mechanism in pH region 3-8 (where the OH- concentration is low) is
of the SN1 type. However, at higher pH values (>8), the faster SN2
type reaction is dominant.
H2O
CH3Br -> CH3+ + Br- -> CH3OH + H+ (SN1)
OH- + CH3Br -> [HO...CH3...Br]- -> CH3OH + Br- (SN2)
Table 14. Hydrolysis constant (K) and half-life of methyl bromide in distilled
water at different pH and at two different temperaturesa
pH 18°C Half- 30°C Half-
K(10-7s-1) life K(10-7s-1) life
(±SD) (days) (±SD) (days)
3.0 2.70(±0.11) 29.0 2.84(±0.19) 28.00
5.0 4.08(±0.10) 19.0 4.51(±0.22) 18.00
7.0 6.63(±0.34) 12.0 7.80(±0.25) 10.00
8.0 8.60(±0.44) 9.0 10.32(±0.15) 8.00
a From: Gentile et al. (1989). Distilled water was buffered at the given pH 3, 5,
7 and 8 using 0.2 mol/litre phosphate-citrate buffer. Methyl bromide was added
to obtain a final concentration of 50.5 µmol/litre.
The hydrolysis rate of methyl bromide in water taken from four
different wells was also measured at 18 °C and 30 °C (Table 15). The
authors could not explain the discrepancy between the results of the
two sets of experiments. The results from well water are comparable
with those given in Table 16 from other authors.
No significant variation in pH occurred with time in well water
after the addition, or during the hydrolysis, of methyl bromide
(Gentile et al., 1989). The authors explained these results for
natural waters by the low amounts of HBr produced by complete
hydrolysis of the fumigant and by the presence of natural buffering
systems, such as calcium bicarbonate.
Experiments by Herzel & Schmidt (1984) on the persistence of
methyl bromide in water confirmed the earlier work by Moelwyn-Hughes
(1938), which showed that hydrolysis depends mainly on temperature.
Whereas, at 40 °C, there was rapid degradation, at 22 °C, it took
several days before there was a rapid decrease in methyl bromide
concentrations. At 10 °C, there was an even slower rate of reaction.
Fig. 1 shows graphically the rate of hydrolysis of methyl bromide in
tap water at these three different temperatures.
The rate constant for methyl bromide hydrolysis in tap water
varies with temperature, not according to an equation of the Arrhenius
type, but according to the following formula:
log10k = 112.656 - 10236/T - 34.259 log10T
where T is the absolute temperature.
This formula agrees with experimental observations by
Moelwyn-Hughes (1938) carried out at temperatures of up to 100 °C.
Table 16 shows the dependence of the rate constant and half-life on
temperature.
Addition of soil to water greatly enhanced the degradation of
methyl bromide (Gentile et al., 1989).
Degradation by hydrolysis is the primary route of degradation of
methyl bromide in soils with a very low organic matter content. The
adsorption isotherms in these soils were found to be linear but slopes
were greatly reduced as moisture content increased (Arvieu, 1983). In
soils containing organic matter, two different reactions occurred,
adsorption and conversion through reaction with organic matter.
Table 15. Hydrolysis constant (K) and half-life of methyl bromide in well-water
at 18 °C and 30 °Ca
Site or pH 18 °C Half-life 30°C Half-life
well no. K(10-7s-1) (days) K(10-7s-1) (days)
(± SD) (± SD)
1 7.4 2.20 (±0.33) 36.0 5.28 (±0.24) 15.0
2 7.7 2.01 (±0.08) 40.0 4.62 (±0.34) 17.0
3 7.7 1.81 (±0.28) 43.0 4.27 (±0.34) 18.0
4 7.8 1.58 (±0.14) 50.0 3.95 (±0.53) 19.0
a From Gentile et al. (1989). Methyl bromide was added to well water to obtain
a concentration of 50.5 µmol/litre.
Table 16. Hydrolysis rate constant (k) and half-life of methyl
bromide in water at different temperatures
Temperature Observed Half-life
(°C) rate constant
(sec-1)
17 a1.07 x 10-7 75 days
25 b4.09 x 10-7 20 daysb
25 a3.57 x 10-7 21.3 days
35.7 a1.65 x 10-6 117 h
46.3 a6.71 x 10-6 28.6 h
100 a1.28 x 10-3 0.6 h
a Values from Moelwyn & Hughes (1938) using distilled water.
b Values from Mabey & Mill (1978) using freshwater, pH 7.
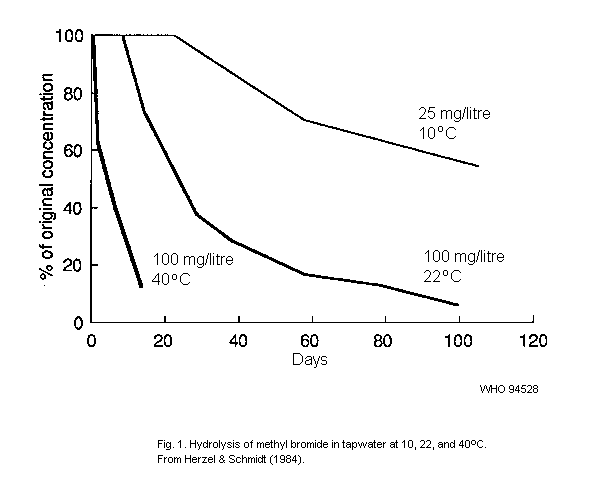 Degradation occurred by the methylation of carboxylic groups on
moist H-substituted peat. In soils containing other groups that can be
methylated, the mechanism and factors of methyl bromide degradation
are more complex.
Adsorption on to organic matter reduced the available
concentration of methyl bromide in the aqueous and gaseous phases of
soil. Organic matter was also the main factor involved in the
degradation of methyl bromide in the soil (Arvieu & Cuany, 1985).
4.2.2.2 Light-assisted hydrolysis in water
The UV-absorption cross sections for methyl bromide (174-262 nm)
have been confirmed by several authors (Robbins 1976b; Uthman et al.,
1978; Molina et al., 1982; Gillotay et al., 1989). The UV absorption
spectrum has a maximum of 202 nm with a steep decrease at longer
wavelengths reaching 0.2% of the maximum at 260 nm (Table 17). This is
much shorter than 290 nm, which is the shortest wavelength radiation
reaching the earth's surface from the sun.
It should be noted that the photoactivation of methyl bromide, as
well as its hydrolysis products in water or on soil surfaces, will
differ from the gas phase activities of these processes. The
hydrolysis products are in solution and differ energetically. Since
the species energy levels are changed from those of the isolated
molecule, critical absorption wavelengths would be shifted.
The influence of sunlight versus dark using natural waters in a
laboratory test showed little effect on the hydrolysis of methyl
bromide (Gentile et al., 1989, 1992).
Irradiation with a "pen-ray" low-pressure mercury lamp at 254 nm
gave a 6.6-fold increase in the rate constant. However, photolysis
does not alter the stoichiometry of the hydrolysis (Castro & Belser,
1981). The authors concluded that the almost exclusive path of decay
(>99.6 %) was the direct hydrolysis of photoactivated methyl bromide
with the formation of methanol, bromide ion, protons, and a trace of
methane (<0.4%).
4.2.2.3 Reaction with the hydroxyl radical
Reaction of methyl bromide with the hydroxyl radical is believed
to be the primary mechanism for the removal of methyl bromide from the
lower troposphere (Singh et al., 1981).
Methyl bromide reacts slowly with the hydroxyl radical:
CH3Br + OH- -> CH2Br- + H2O
with a reaction rate constant of about 3 x 10-14 cm3/molecule per
second at 298 °K (NASA, 1992).
Table 17. Absorption cross sections of methyl bromidea
lambda 1020 sigma lambda 1020 sigma
(nm) (cm2) (nm) (cm2)
190 44 230 15
192 53 232 12
194 62 234 9.9
196 69 236 7.6
198 76 238 5.9
200 79 240 4.5
202 80 242 3.3
204 79 244 2.5
206 77 246 1.8
208 73 248 1.3
210 67 250 0.96
212 61 252 0.69
214 56 254 0.49
216 49 256 0.34
218 44 258 0.23
220 38 260 0.16
222 32
224 28
226 23
228 19
a Values recommended by NASA (1992) and taken from
Gillotay et al. (1989). These authors measured the
cross sections down to 210 K; for <210 nm, the
temperature effect is negligible.
Table 18 shows values for hydroxyl radical rate constants.
Table 18. Atmospheric reactions of methyl bromide
Reaction Rate constant Height above Reference
sea level
(km)
CH3Br + hv 1 x 10-11/s 16 Robbins (1976a)
1 x 10-9/s 20
1 x 10-6/s 30
5 x 10-5/sec 50
CH3Br + OH- 3 x 10-14 cm3/s (298 °K) NASA (1992)a
k(T) = 3.6 x 10-12 exp [-(1430±150) K/T] cm3/s
a Profiles of OH- and temperature of the stratosphere are given in NASA (1992).
Recent laboratory data for the rate coefficient of the reaction
of methyl bromide (Mellouki et al., 1992a,b) with the hydroxyl radical
together with the estimated distribution of the hydroxyl radical (WMO,
1992), gave an estimated OH--removal lifetime for methyl bromide of
2±0.5 years (UNEP, 1992).
4.2.2.4 Photolysis in the atmosphere
In the upper stratosphere, above 25 km, the photodissociation of
methyl bromide is the most dominant loss mechanism. Below this, as
less UV radiation is able to penetrate through the atmosphere, the
role of photolysis decreases. Between 20 and 25 km above sea level,
photodissociation is equally as important as loss by diffusion and
reaction with OH-. Below 20 km, down to the troposphere,
photodissociation becomes negligible and losses by diffusion and
reaction with OH- are of approximately equal importance (Robbins,
1976a) (Table 18).
The end-products of photodissociation of methyl bromide and
reactions with hydroxyl radicals in the atmosphere are probably carbon
dioxide, carbon monoxide, and bromide species (BUA, 1987).
4.2.3 Bioaccumulation
There are no experimental data on bioaccumulation potential. The
octanol/water partition coefficient (log Pow) of methyl bromide has
been given as 1.19 (Hansch & Leo, 1979; Sangster, 1989), so it
probably does not have any significant tendency to bioaccumulate (see
also section 4.1.5).
4.3 Interaction with other physical, chemical, or biological factors
It has been found that methyl bromide is able to diffuse through
certain plastics (Herzel & Schmidt, 1984).
A reaction is possible between methyl bromide and the following
materials ( Bond, 1984):
- iodized salt, stabilized with sodium hyposulfite;
- certain baking sodas, salt blocks used for cattle licks, or
foods containing reactive sulfur compounds;
- full fat soy flour;
- sponge rubber;
- foam rubber as used in rug padding, pillows, cushions, and
mattresses;
- rubber stamps and similar forms of reclaimed rubber;
- furs, horsehair, and pillows (especially feather pillows);
- leather goods tanned using a sulfur process;
- woollens, especially angora; some adverse effects have been
noted on woollen socks, sweaters, and yarn;
- viscose rayons, made by a process that uses carbon
disulfide;
- cinder blocks or mixtures of mortar; mixed concrete
occasionally picks up odours;
- charcoal, which not only becomes contaminated but absorbs
great amounts of methyl bromide and, thus, reduces effective
fumigant concentrations;
- paper that has been cured by a sulfide process and silver
polishing papers;
- photographic chemicals, not including films;
- rug padding, vinyl, cellophane;
- any other materials that may contain reactive sulfur
compounds.
4.4 Ultimate fate following use
4.4.1 Methyl bromide and the ozone layer
Methyl bromide from natural and anthropogenic sources is released
into the atmosphere. Once organic bromine compounds, such as methyl
bromide and the halons (section 5.1.1), enter the stratosphere, they
decompose to release bromine atoms. Fig. 2 depicts the key
bromine-containing species in the stratosphere and shows the
interconversion between reactive (Br- and BrO-) and reservoir
(HBr, HOBr, BrCl, and BrONO2) species (UNEP, 1992).
Yung et al. (1980) proposed the following reactions for ozone
loss due to bromine:
Br- + O3 -> BrO- + O2
Cl- + O3 -> ClO- + O2
BrO- + ClO- -> Br- + Cl- + O2
Net 2O3 -> 3O2
Ozone removal by bromine is far more efficient on a per molecule
basis than that by chlorine. This bromine-catalysed ozone removal in
the lower stratosphere is thought to occur via the reaction between
BrO- and ClO- (WMO, 1992) whereby the efficiency of
bromine-induced ozone loss increases with increasing abundance of
stratospheric chlorine. Bromine catalysis is most efficient in the
lower stratosphere where the ozone concentration is largest.
Reactive bromine has been detected directly in the stratosphere
in the polar regions, as well as OClO-, for which the only known
source is the reaction of BrO- with ClO-. Analyses of these
measurements indicate that the BrO- + ClO- catalytic cycle is
responsible for roughly 25% of the observed total ozone loss in the
appearance of the Antarctic ozone hole (an event mainly restricted to
Antarctica and to the months of September-November, involving
heterogenous reactions on polar stratospheric clouds) (WMO, 1992).
Degradation occurred by the methylation of carboxylic groups on
moist H-substituted peat. In soils containing other groups that can be
methylated, the mechanism and factors of methyl bromide degradation
are more complex.
Adsorption on to organic matter reduced the available
concentration of methyl bromide in the aqueous and gaseous phases of
soil. Organic matter was also the main factor involved in the
degradation of methyl bromide in the soil (Arvieu & Cuany, 1985).
4.2.2.2 Light-assisted hydrolysis in water
The UV-absorption cross sections for methyl bromide (174-262 nm)
have been confirmed by several authors (Robbins 1976b; Uthman et al.,
1978; Molina et al., 1982; Gillotay et al., 1989). The UV absorption
spectrum has a maximum of 202 nm with a steep decrease at longer
wavelengths reaching 0.2% of the maximum at 260 nm (Table 17). This is
much shorter than 290 nm, which is the shortest wavelength radiation
reaching the earth's surface from the sun.
It should be noted that the photoactivation of methyl bromide, as
well as its hydrolysis products in water or on soil surfaces, will
differ from the gas phase activities of these processes. The
hydrolysis products are in solution and differ energetically. Since
the species energy levels are changed from those of the isolated
molecule, critical absorption wavelengths would be shifted.
The influence of sunlight versus dark using natural waters in a
laboratory test showed little effect on the hydrolysis of methyl
bromide (Gentile et al., 1989, 1992).
Irradiation with a "pen-ray" low-pressure mercury lamp at 254 nm
gave a 6.6-fold increase in the rate constant. However, photolysis
does not alter the stoichiometry of the hydrolysis (Castro & Belser,
1981). The authors concluded that the almost exclusive path of decay
(>99.6 %) was the direct hydrolysis of photoactivated methyl bromide
with the formation of methanol, bromide ion, protons, and a trace of
methane (<0.4%).
4.2.2.3 Reaction with the hydroxyl radical
Reaction of methyl bromide with the hydroxyl radical is believed
to be the primary mechanism for the removal of methyl bromide from the
lower troposphere (Singh et al., 1981).
Methyl bromide reacts slowly with the hydroxyl radical:
CH3Br + OH- -> CH2Br- + H2O
with a reaction rate constant of about 3 x 10-14 cm3/molecule per
second at 298 °K (NASA, 1992).
Table 17. Absorption cross sections of methyl bromidea
lambda 1020 sigma lambda 1020 sigma
(nm) (cm2) (nm) (cm2)
190 44 230 15
192 53 232 12
194 62 234 9.9
196 69 236 7.6
198 76 238 5.9
200 79 240 4.5
202 80 242 3.3
204 79 244 2.5
206 77 246 1.8
208 73 248 1.3
210 67 250 0.96
212 61 252 0.69
214 56 254 0.49
216 49 256 0.34
218 44 258 0.23
220 38 260 0.16
222 32
224 28
226 23
228 19
a Values recommended by NASA (1992) and taken from
Gillotay et al. (1989). These authors measured the
cross sections down to 210 K; for <210 nm, the
temperature effect is negligible.
Table 18 shows values for hydroxyl radical rate constants.
Table 18. Atmospheric reactions of methyl bromide
Reaction Rate constant Height above Reference
sea level
(km)
CH3Br + hv 1 x 10-11/s 16 Robbins (1976a)
1 x 10-9/s 20
1 x 10-6/s 30
5 x 10-5/sec 50
CH3Br + OH- 3 x 10-14 cm3/s (298 °K) NASA (1992)a
k(T) = 3.6 x 10-12 exp [-(1430±150) K/T] cm3/s
a Profiles of OH- and temperature of the stratosphere are given in NASA (1992).
Recent laboratory data for the rate coefficient of the reaction
of methyl bromide (Mellouki et al., 1992a,b) with the hydroxyl radical
together with the estimated distribution of the hydroxyl radical (WMO,
1992), gave an estimated OH--removal lifetime for methyl bromide of
2±0.5 years (UNEP, 1992).
4.2.2.4 Photolysis in the atmosphere
In the upper stratosphere, above 25 km, the photodissociation of
methyl bromide is the most dominant loss mechanism. Below this, as
less UV radiation is able to penetrate through the atmosphere, the
role of photolysis decreases. Between 20 and 25 km above sea level,
photodissociation is equally as important as loss by diffusion and
reaction with OH-. Below 20 km, down to the troposphere,
photodissociation becomes negligible and losses by diffusion and
reaction with OH- are of approximately equal importance (Robbins,
1976a) (Table 18).
The end-products of photodissociation of methyl bromide and
reactions with hydroxyl radicals in the atmosphere are probably carbon
dioxide, carbon monoxide, and bromide species (BUA, 1987).
4.2.3 Bioaccumulation
There are no experimental data on bioaccumulation potential. The
octanol/water partition coefficient (log Pow) of methyl bromide has
been given as 1.19 (Hansch & Leo, 1979; Sangster, 1989), so it
probably does not have any significant tendency to bioaccumulate (see
also section 4.1.5).
4.3 Interaction with other physical, chemical, or biological factors
It has been found that methyl bromide is able to diffuse through
certain plastics (Herzel & Schmidt, 1984).
A reaction is possible between methyl bromide and the following
materials ( Bond, 1984):
- iodized salt, stabilized with sodium hyposulfite;
- certain baking sodas, salt blocks used for cattle licks, or
foods containing reactive sulfur compounds;
- full fat soy flour;
- sponge rubber;
- foam rubber as used in rug padding, pillows, cushions, and
mattresses;
- rubber stamps and similar forms of reclaimed rubber;
- furs, horsehair, and pillows (especially feather pillows);
- leather goods tanned using a sulfur process;
- woollens, especially angora; some adverse effects have been
noted on woollen socks, sweaters, and yarn;
- viscose rayons, made by a process that uses carbon
disulfide;
- cinder blocks or mixtures of mortar; mixed concrete
occasionally picks up odours;
- charcoal, which not only becomes contaminated but absorbs
great amounts of methyl bromide and, thus, reduces effective
fumigant concentrations;
- paper that has been cured by a sulfide process and silver
polishing papers;
- photographic chemicals, not including films;
- rug padding, vinyl, cellophane;
- any other materials that may contain reactive sulfur
compounds.
4.4 Ultimate fate following use
4.4.1 Methyl bromide and the ozone layer
Methyl bromide from natural and anthropogenic sources is released
into the atmosphere. Once organic bromine compounds, such as methyl
bromide and the halons (section 5.1.1), enter the stratosphere, they
decompose to release bromine atoms. Fig. 2 depicts the key
bromine-containing species in the stratosphere and shows the
interconversion between reactive (Br- and BrO-) and reservoir
(HBr, HOBr, BrCl, and BrONO2) species (UNEP, 1992).
Yung et al. (1980) proposed the following reactions for ozone
loss due to bromine:
Br- + O3 -> BrO- + O2
Cl- + O3 -> ClO- + O2
BrO- + ClO- -> Br- + Cl- + O2
Net 2O3 -> 3O2
Ozone removal by bromine is far more efficient on a per molecule
basis than that by chlorine. This bromine-catalysed ozone removal in
the lower stratosphere is thought to occur via the reaction between
BrO- and ClO- (WMO, 1992) whereby the efficiency of
bromine-induced ozone loss increases with increasing abundance of
stratospheric chlorine. Bromine catalysis is most efficient in the
lower stratosphere where the ozone concentration is largest.
Reactive bromine has been detected directly in the stratosphere
in the polar regions, as well as OClO-, for which the only known
source is the reaction of BrO- with ClO-. Analyses of these
measurements indicate that the BrO- + ClO- catalytic cycle is
responsible for roughly 25% of the observed total ozone loss in the
appearance of the Antarctic ozone hole (an event mainly restricted to
Antarctica and to the months of September-November, involving
heterogenous reactions on polar stratospheric clouds) (WMO, 1992).
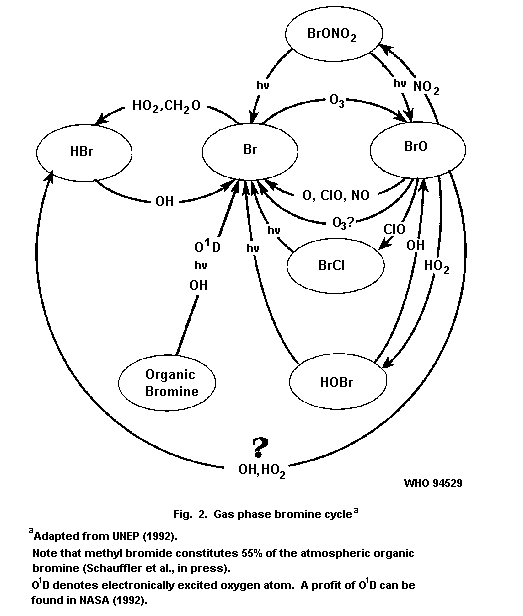 Another possible catalytic cycle is that between BrO- and HO2
(Poulet et al., 1992).
The Ozone Depletion Potential (ODP) represents the amount of
ozone destroyed by the emission of one kg of a chosen gas over a
particular time scale, compared with 1 kg of a reference molecule,
usually CFC-11 (Table 19). The amount of ozone depletion from methyl
bromide, and the ODP of the gas are dependent upon the atmospheric
abundance of chlorine. On the basis of current understanding, the
higher the abundance of chlorine, the higher the ODP of methyl bromide
and the amount of ozone depletion it causes.
Table 19. Lifetime and time-dependent depletion potential (ODP) for
methyl bromide (CH3Br) in comparison with chlorofluorcarbonsa
Speciesc Total Stratospheric Steady-state
atmospheric lifetime (year) empirical polar
lifetime (year) ODPb
CFC-11 55 55 1,0
(CFCl3)
CFC-113 110 110 1,10
(CFC12CF2Cl
CH3Br 2,0 35 0,7
a Values are relative to CFC-11 as the reference gas.
b ODP = ozone depletion potential.
c CFC = chlorofluorocarbon.
The key factors influencing the ODP of methyl bromide include:
(a) the atmospheric lifetime of the compound (relative to CFC-11);
(b) the release of reactive bromine from methyl bromide (relative to
CFC-11); and (c) the relative efficiency of reactive bromine for
ozone destruction compared with that of reactive chlorine (alpha
factor).
(a) Atmospheric lifetimes for methyl bromide have been
estimated at 1.6 years (WMO, 1992) and 2.0 years (UNEP,
1992). However, oceanic and terrestrial surface removal
processes have not been taken into account.
(b) As methyl bromide is a relatively short-lived molecule
within the stratosphere, it is expected that its release of
reactive bromine is faster than that of chlorine from
CFC-11.
(c) The current best estimate of alpha in the lower polar
stratosphere is about 40 (UNEP, 1992).
The current best estimate of the steady-state ODP for methyl
bromide from the semi-empirical approach and models including both
gas-phase and heterogeneous chemistry is 0.7 (UNEP, 1992). This
assumes its lifetime is solely determined by reaction with OH- in
the troposphere.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide is estimated to be about 3 pptv (UNEP,
1992). If anthropogenic methyl bromide is about 13-14% of total
atmospheric bromine (calculated by taking 25% of 11 pptv total methyl
bromide (UNEP, 1992) = 2.75% of total atmospheric bromine from
anthropogenic sources; and 55% of 20.8 pptv total organic bromine as
methyl bromide (Schauffler et al., in press) = 11%), the global ozone
loss due to anthropogenic methyl bromide is approximately 3%.
4.4.2 Containment, recovery, recycling, and disposal options for
methyl bromide
At present, after soil, commodity, and structural fumigation,
there is no special effort at containment of methyl bromide (see
section 3.2 for estimated emission rates from various fumigation
processes).
The methyl bromide industry is currently investigating practical
methods of recovering the gas by adsorption (using activated
charcoal), condensation, and scrubbing techniques. Most of these
methods are still in the research and development stage (UNEP, 1992).
When methyl bromide cylinders are refilled, instead of the present
practice of venting, it may be possible to recondense and recover the
gas. In this way, 1-2% of the total methyl bromide used could be
saved.
Methyl bromide is a toxic gas and the recommended waste disposal
method for large quantities is incineration by specialists. However,
incineration may be difficult to arrange safely unless an efficient
method of feeding the gas into the incinerator can be arranged.
Incineration requires dilution with additional fuel. If a suitably
designed combustion chamber is not available, return labelled
containers to supplier. Any release into the atmosphere should take
place in well-ventilated outdoor locations only.
All national and local regulations should be observed when
disposing of methyl bromide.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.1.1 Global abundance
The current best estimate for the global abundance of methyl
bromide in the troposphere is between 9 and 13 pptv (section 3.1 and
Tables 20 and 21) giving a total global burden of 150-220 million kg
(UNEP, 1992). From observations taken from the surface waters of the
Pacific, off North and South America, Singh et al. (1983) had
previously estimated the total natural emission of methyl bromide from
the oceans at 300 million kg/year with a residence time of 1.2 years.
This estimate is probably too high because of the difficulties with
absolute calibration.
The most abundant bromine source gas is methyl bromide, which
arises from both natural and anthropogenic sources (WMO, 1992; also
sections 3.1 and 3.2). The main natural sources of methyl bromide are
oceanic biological processes (mainly algal) where it is formed
together with bromoform (CHBr3), methylene bromide (CH2Br2),
CH2BrCl, and CHBrCl2 (WMO, 1992). Apart from methyl bromide, other
anthropogenic sources are the halons, CBrF3 (CFC-13B1; Halon 1301),
CBrClF2 (CFC-12B1; Halon 1211), and C2Br2F4 (Halon 2402),
which are used as special purpose fire extinguishers (Singh et al.,
1988; Schauffler et al., in press).
The atmospheric abundance of methyl bromide appears to be
1.3±0.15 greater in the Northern than in the Southern hemisphere
(Tables 20, 21) indicating an excess source in the Northern
hemisphere. The major source of methyl chloride is also thought to be
oceanic, but the atmospheric abundance of this gas appears to be
comparable in both hemispheres (UNEP, 1992). Some atmospheric models
compute the interhemispheric OH- concentration ratio
(OHN-/OHS-) to be about 0.8 (Tie et al., 1992). Assuming
reaction with OH- is the major tropospheric destructive process for
methyl bromide, this southern excess of OH- could largely explain
the lower southern atmospheric concentration of methyl bromide. In
contrast, Spivakovsky et al. (1990), using a model based on different
assumptions, calculated a higher OH- concentration in the northern
than in the southern hemisphere.
Ozone loss, catalysed by bromine, occurs mainly in the lower
stratosphere.The primary cause of the Antarctic ozone hole is most
certainly halogen chemistry (WMO, 1992). Bromine is now believed to
have a greater potential per molecule to destroy stratospheric ozone
than chlorine (section 4.4).
The upward mass transfer of air from the troposphere to the
stratosphere occurs mainly in the tropical latitudes between 30 °S and
30 °N (WMO, 1992). Schauffler et al. (in press) give the mixing ratios
of brominated compounds and total organic bromine from 12 samples
collected at the tropical tropopause (altitude about 16 km) from
January to March, 1992. The mean mixing ratio of total organic bromine
was 20.8±0.8 pptv. Methyl bromide was found to contribute 55% of the
total organic bromine, CH2Br2, about 7%, and the remaining 38% was
nearly evenly distributed between Halon 1302, Halon 1211, and Halon
2402.
It is against this background of the role of bromine in the ozone
depletion in the stratosphere that there has been recent concern about
levels of methyl bromide in the atmosphere and the potential effect of
the continuing use of this fumigant (see also sections 3.1, 3.2, 4.4).
5.1.1.2 Measured oceanic and coastal air levels of methyl bromide
Table 20 shows levels of methyl bromide in oceanic areas,
measured by ground-based, aircraft, and balloon techniques. The
differences between the individual readings do not seem to be due to
trends in abundance over the last decade or to seasonal variation, but
more likely to variations in calibration (UNEP, 1992). An abundance of
methyl bromide in the atmosphere of 9-13 pptv is equivalent to a total
global burden of 150-220 million kg.
Although there are differences in the values reported for the
absolute magnitudes of the observed methyl bromide abundances, ranging
from 10-26 pptv in the Northern hemisphere and 8-20 pptv in the
Southern hemisphere, the N/S ratio is almost constant at 1.3 (Table
20). The greater abundance of methyl bromide in the Northern than the
Southern hemisphere has been confirmed by all studies published up to
now. This is possible evidence for the anthropogenic addition of
methyl bromide to the atmosphere from its use as a fumigant (see also
sections 3.1, 3.2, and 4.4) as well as from motor vehicle exhaust.
Methyl bromide concentrations in the atmosphere are summarized in
Table 21, comparing oceanic and continental values with those in urban
areas.
Table 20. Atmospheric abundance of methyl bromide at, or near, ground level (pptv)a
Source Time Northern Southern N/S ratio Observational Reference
period hemisphere hemisphere platform
Singh 1981 26 20 1.3 Ship Singh et al. (1983)
Penkett 1982-83 15 11 1.4 Ship Penkett et al. (1985)
Rasmussen 1983-92 11 8 1.3 Coastal land MBSW (1992)
Cicerone 1985-87 12 10 1.2 Coastal land Cicerone, et al. (1988)
Rowland 1991 10 8 1.2 Coastal land MBSW (1992)
Heidt 1991 14 - - Aircraft MBSW (1992)
a From: UNEP (1992).
b MBSW = Methyl Bromide Science Workshop (1992).
Measurements by Singh et al. (1983) of air and seawater
concentrations of methyl halides in, and over, the Eastern Pacific
(40° N-32° S) gave average concentrations of 90 ng/m3 (23 pptv) in
air. However, there was a considerable difference between the readings
in the northern (101 ng/m3 [26 pptv]) compared with the southern (74
ng/m3 [19 pptv]) hemisphere. Because of the large scattering of
values, a seasonal trend was not identifiable. As mentioned in section
3.1, it is possible that calibration standards were in error, which
would explain the higher values compared with those of other authors.
Rasmussen & Khalil (1984) found that the highest concentrations
of methyl bromide in Arctic and Arctic haze were found in the summer
(average - 48.2 ng/n3 (12.4 pptv)) compared with the autumn and
winter (average - 39.7 ng/m3 (10.2 pptv)), and, that methyl bromide
did not show the same seasonal patterns as other bromine-containing
trace gases.
Penkett et al. (1985) measured four different bromine compounds,
including methyl bromide, over a large latitudinal range (40° N to 75°
S). They found that there was a clear reduction in concentrations
between the two hemispheres, the average concentration of methyl
bromide in the Northern Hemisphere being 60±7 ng/m3 (15.4±1.9 pptv)
compared with 41.2±3.5 ng/m3 (10.6±0.9 pptv) in the Southern
Hemisphere.
Table 21. Measured ambient concentrations of methyl bromide
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Oceanic and coastal air levels
Eastern Pacific 40°N 26 (101) Singh et al. (1983)
Ocean 32°S 19 (74)
Arctic 72°N 11.3 (44) Rasmussen & Khalil
(1984);
Berg et al. (1984)
90°S 7.5 Khalil & Rasmussen (1985)
Atlantic Ocean 40°N 15.4 (60) Penkett et al. (1985)
Southern Ocean 75°S 10.6 (41)
Alaska 71°N 11.16 (43) Cicerone et al. (1988)
Hawaii 20°N 10.75 (42)
Samoa 14°S 10.23 (40)
Tasmania 44°S 9.58 (37)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Alaska 71°N 14.1 Khalil et al. (1993)c
Oregon 45°N 12.6
Hawaii 19°N 11.4
Samoa 14°S 8.5
Tasmania 42°S 7.5
Antarctic 64.5°S 9.0
Continental air levels
USA
Pullman, Washington < 10-220 Harsch & Rasmussen
(< 40-870) (1977)
Los Angeles 244 (950) 894 (3480) Singh et al. (1981)
Oakland 55 (214) 108 (420)
Phoenix 67 (261) 190 (740)
Badger Pass 5 (19) 8 (31) Brodzinsky & Singh
Denver 120 (467) 190 (740) (1983)
Houston 100 (390) 170 (660)
Jetmore 5 (19) 5 (19)
Los Angelesb 150 (584) 580 (2256)
Menlo Park 16 (62) 16 (62)
Mill Valley 25 (97) 25 (97)
Oaklandb 55 (214) 78 (303)
Palm Springs 24 (93) 24 (93)
Phoenixb 67 (261) 120 (467) Brodzinsky & Singh
Point Arena 17 (66) 20 (78) (1983)
Point Reyes 93 (362) 93 (362)
Reese River 5 (19) 5 (19)
Riverside 250 (973) 560 (2180)
San Jose 31 (120) 31 (120)
St. Louis 81 (315) 100 (390)
San Jose (California, USA) Singh et al. (1992)
[4-16 April 1985] 400
(44-4661)
[12-24 August 1985] 121
(5-1067)
[13-21 December 1985] 2869
(239-15 424)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Downey, California, USA
[8-27 February 1984] 212
(18-815)
Houston, Texas, USA
[9-17 March 1984] 23
(11-48)
Denver, CO, USA
[24 March-1 April 1984] 22
(13-64)
The Netherlands
Delft, Vlaardingen 50-200 100-900 Guicherit & Schulting
and Terschellingd (195-780) (390-3500) (1985)
Japan
urban and suburban 15-31 JEA (1981)
(59-122)
a The converted values in brackets ( ) are only approximate.
b Includes substantial overlap with data reported in Singh et al. (1981).
c Values given from spring 1991. Fig. 3 gives averages over the last decade.
d About 350 samples per site during 1989.
Approximately 750 air samples from five surface sampling sites in
Alaska, Hawaii (2), Samoa, and New Zealand were analysed by Cicerone
et al. (1988) for methyl bromide between January 1985 and October 1987
(using GC/MS). Methyl bromide concentrations were typically 40-44
ng/m3 (10-11 pptv).
Khalil et al. (1993) presented a series of 1700 measurements
showing the latitudinal distribution of atmospheric methyl bromide
from 1983 to 1992 (Table 21 and Fig. 3). The levels of atmospheric
methyl bromide measured between 1988 and 1992 seemed to be higher than
those measured between 1983 and 1988.
Another possible catalytic cycle is that between BrO- and HO2
(Poulet et al., 1992).
The Ozone Depletion Potential (ODP) represents the amount of
ozone destroyed by the emission of one kg of a chosen gas over a
particular time scale, compared with 1 kg of a reference molecule,
usually CFC-11 (Table 19). The amount of ozone depletion from methyl
bromide, and the ODP of the gas are dependent upon the atmospheric
abundance of chlorine. On the basis of current understanding, the
higher the abundance of chlorine, the higher the ODP of methyl bromide
and the amount of ozone depletion it causes.
Table 19. Lifetime and time-dependent depletion potential (ODP) for
methyl bromide (CH3Br) in comparison with chlorofluorcarbonsa
Speciesc Total Stratospheric Steady-state
atmospheric lifetime (year) empirical polar
lifetime (year) ODPb
CFC-11 55 55 1,0
(CFCl3)
CFC-113 110 110 1,10
(CFC12CF2Cl
CH3Br 2,0 35 0,7
a Values are relative to CFC-11 as the reference gas.
b ODP = ozone depletion potential.
c CFC = chlorofluorocarbon.
The key factors influencing the ODP of methyl bromide include:
(a) the atmospheric lifetime of the compound (relative to CFC-11);
(b) the release of reactive bromine from methyl bromide (relative to
CFC-11); and (c) the relative efficiency of reactive bromine for
ozone destruction compared with that of reactive chlorine (alpha
factor).
(a) Atmospheric lifetimes for methyl bromide have been
estimated at 1.6 years (WMO, 1992) and 2.0 years (UNEP,
1992). However, oceanic and terrestrial surface removal
processes have not been taken into account.
(b) As methyl bromide is a relatively short-lived molecule
within the stratosphere, it is expected that its release of
reactive bromine is faster than that of chlorine from
CFC-11.
(c) The current best estimate of alpha in the lower polar
stratosphere is about 40 (UNEP, 1992).
The current best estimate of the steady-state ODP for methyl
bromide from the semi-empirical approach and models including both
gas-phase and heterogeneous chemistry is 0.7 (UNEP, 1992). This
assumes its lifetime is solely determined by reaction with OH- in
the troposphere.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide is estimated to be about 3 pptv (UNEP,
1992). If anthropogenic methyl bromide is about 13-14% of total
atmospheric bromine (calculated by taking 25% of 11 pptv total methyl
bromide (UNEP, 1992) = 2.75% of total atmospheric bromine from
anthropogenic sources; and 55% of 20.8 pptv total organic bromine as
methyl bromide (Schauffler et al., in press) = 11%), the global ozone
loss due to anthropogenic methyl bromide is approximately 3%.
4.4.2 Containment, recovery, recycling, and disposal options for
methyl bromide
At present, after soil, commodity, and structural fumigation,
there is no special effort at containment of methyl bromide (see
section 3.2 for estimated emission rates from various fumigation
processes).
The methyl bromide industry is currently investigating practical
methods of recovering the gas by adsorption (using activated
charcoal), condensation, and scrubbing techniques. Most of these
methods are still in the research and development stage (UNEP, 1992).
When methyl bromide cylinders are refilled, instead of the present
practice of venting, it may be possible to recondense and recover the
gas. In this way, 1-2% of the total methyl bromide used could be
saved.
Methyl bromide is a toxic gas and the recommended waste disposal
method for large quantities is incineration by specialists. However,
incineration may be difficult to arrange safely unless an efficient
method of feeding the gas into the incinerator can be arranged.
Incineration requires dilution with additional fuel. If a suitably
designed combustion chamber is not available, return labelled
containers to supplier. Any release into the atmosphere should take
place in well-ventilated outdoor locations only.
All national and local regulations should be observed when
disposing of methyl bromide.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.1.1 Global abundance
The current best estimate for the global abundance of methyl
bromide in the troposphere is between 9 and 13 pptv (section 3.1 and
Tables 20 and 21) giving a total global burden of 150-220 million kg
(UNEP, 1992). From observations taken from the surface waters of the
Pacific, off North and South America, Singh et al. (1983) had
previously estimated the total natural emission of methyl bromide from
the oceans at 300 million kg/year with a residence time of 1.2 years.
This estimate is probably too high because of the difficulties with
absolute calibration.
The most abundant bromine source gas is methyl bromide, which
arises from both natural and anthropogenic sources (WMO, 1992; also
sections 3.1 and 3.2). The main natural sources of methyl bromide are
oceanic biological processes (mainly algal) where it is formed
together with bromoform (CHBr3), methylene bromide (CH2Br2),
CH2BrCl, and CHBrCl2 (WMO, 1992). Apart from methyl bromide, other
anthropogenic sources are the halons, CBrF3 (CFC-13B1; Halon 1301),
CBrClF2 (CFC-12B1; Halon 1211), and C2Br2F4 (Halon 2402),
which are used as special purpose fire extinguishers (Singh et al.,
1988; Schauffler et al., in press).
The atmospheric abundance of methyl bromide appears to be
1.3±0.15 greater in the Northern than in the Southern hemisphere
(Tables 20, 21) indicating an excess source in the Northern
hemisphere. The major source of methyl chloride is also thought to be
oceanic, but the atmospheric abundance of this gas appears to be
comparable in both hemispheres (UNEP, 1992). Some atmospheric models
compute the interhemispheric OH- concentration ratio
(OHN-/OHS-) to be about 0.8 (Tie et al., 1992). Assuming
reaction with OH- is the major tropospheric destructive process for
methyl bromide, this southern excess of OH- could largely explain
the lower southern atmospheric concentration of methyl bromide. In
contrast, Spivakovsky et al. (1990), using a model based on different
assumptions, calculated a higher OH- concentration in the northern
than in the southern hemisphere.
Ozone loss, catalysed by bromine, occurs mainly in the lower
stratosphere.The primary cause of the Antarctic ozone hole is most
certainly halogen chemistry (WMO, 1992). Bromine is now believed to
have a greater potential per molecule to destroy stratospheric ozone
than chlorine (section 4.4).
The upward mass transfer of air from the troposphere to the
stratosphere occurs mainly in the tropical latitudes between 30 °S and
30 °N (WMO, 1992). Schauffler et al. (in press) give the mixing ratios
of brominated compounds and total organic bromine from 12 samples
collected at the tropical tropopause (altitude about 16 km) from
January to March, 1992. The mean mixing ratio of total organic bromine
was 20.8±0.8 pptv. Methyl bromide was found to contribute 55% of the
total organic bromine, CH2Br2, about 7%, and the remaining 38% was
nearly evenly distributed between Halon 1302, Halon 1211, and Halon
2402.
It is against this background of the role of bromine in the ozone
depletion in the stratosphere that there has been recent concern about
levels of methyl bromide in the atmosphere and the potential effect of
the continuing use of this fumigant (see also sections 3.1, 3.2, 4.4).
5.1.1.2 Measured oceanic and coastal air levels of methyl bromide
Table 20 shows levels of methyl bromide in oceanic areas,
measured by ground-based, aircraft, and balloon techniques. The
differences between the individual readings do not seem to be due to
trends in abundance over the last decade or to seasonal variation, but
more likely to variations in calibration (UNEP, 1992). An abundance of
methyl bromide in the atmosphere of 9-13 pptv is equivalent to a total
global burden of 150-220 million kg.
Although there are differences in the values reported for the
absolute magnitudes of the observed methyl bromide abundances, ranging
from 10-26 pptv in the Northern hemisphere and 8-20 pptv in the
Southern hemisphere, the N/S ratio is almost constant at 1.3 (Table
20). The greater abundance of methyl bromide in the Northern than the
Southern hemisphere has been confirmed by all studies published up to
now. This is possible evidence for the anthropogenic addition of
methyl bromide to the atmosphere from its use as a fumigant (see also
sections 3.1, 3.2, and 4.4) as well as from motor vehicle exhaust.
Methyl bromide concentrations in the atmosphere are summarized in
Table 21, comparing oceanic and continental values with those in urban
areas.
Table 20. Atmospheric abundance of methyl bromide at, or near, ground level (pptv)a
Source Time Northern Southern N/S ratio Observational Reference
period hemisphere hemisphere platform
Singh 1981 26 20 1.3 Ship Singh et al. (1983)
Penkett 1982-83 15 11 1.4 Ship Penkett et al. (1985)
Rasmussen 1983-92 11 8 1.3 Coastal land MBSW (1992)
Cicerone 1985-87 12 10 1.2 Coastal land Cicerone, et al. (1988)
Rowland 1991 10 8 1.2 Coastal land MBSW (1992)
Heidt 1991 14 - - Aircraft MBSW (1992)
a From: UNEP (1992).
b MBSW = Methyl Bromide Science Workshop (1992).
Measurements by Singh et al. (1983) of air and seawater
concentrations of methyl halides in, and over, the Eastern Pacific
(40° N-32° S) gave average concentrations of 90 ng/m3 (23 pptv) in
air. However, there was a considerable difference between the readings
in the northern (101 ng/m3 [26 pptv]) compared with the southern (74
ng/m3 [19 pptv]) hemisphere. Because of the large scattering of
values, a seasonal trend was not identifiable. As mentioned in section
3.1, it is possible that calibration standards were in error, which
would explain the higher values compared with those of other authors.
Rasmussen & Khalil (1984) found that the highest concentrations
of methyl bromide in Arctic and Arctic haze were found in the summer
(average - 48.2 ng/n3 (12.4 pptv)) compared with the autumn and
winter (average - 39.7 ng/m3 (10.2 pptv)), and, that methyl bromide
did not show the same seasonal patterns as other bromine-containing
trace gases.
Penkett et al. (1985) measured four different bromine compounds,
including methyl bromide, over a large latitudinal range (40° N to 75°
S). They found that there was a clear reduction in concentrations
between the two hemispheres, the average concentration of methyl
bromide in the Northern Hemisphere being 60±7 ng/m3 (15.4±1.9 pptv)
compared with 41.2±3.5 ng/m3 (10.6±0.9 pptv) in the Southern
Hemisphere.
Table 21. Measured ambient concentrations of methyl bromide
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Oceanic and coastal air levels
Eastern Pacific 40°N 26 (101) Singh et al. (1983)
Ocean 32°S 19 (74)
Arctic 72°N 11.3 (44) Rasmussen & Khalil
(1984);
Berg et al. (1984)
90°S 7.5 Khalil & Rasmussen (1985)
Atlantic Ocean 40°N 15.4 (60) Penkett et al. (1985)
Southern Ocean 75°S 10.6 (41)
Alaska 71°N 11.16 (43) Cicerone et al. (1988)
Hawaii 20°N 10.75 (42)
Samoa 14°S 10.23 (40)
Tasmania 44°S 9.58 (37)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Alaska 71°N 14.1 Khalil et al. (1993)c
Oregon 45°N 12.6
Hawaii 19°N 11.4
Samoa 14°S 8.5
Tasmania 42°S 7.5
Antarctic 64.5°S 9.0
Continental air levels
USA
Pullman, Washington < 10-220 Harsch & Rasmussen
(< 40-870) (1977)
Los Angeles 244 (950) 894 (3480) Singh et al. (1981)
Oakland 55 (214) 108 (420)
Phoenix 67 (261) 190 (740)
Badger Pass 5 (19) 8 (31) Brodzinsky & Singh
Denver 120 (467) 190 (740) (1983)
Houston 100 (390) 170 (660)
Jetmore 5 (19) 5 (19)
Los Angelesb 150 (584) 580 (2256)
Menlo Park 16 (62) 16 (62)
Mill Valley 25 (97) 25 (97)
Oaklandb 55 (214) 78 (303)
Palm Springs 24 (93) 24 (93)
Phoenixb 67 (261) 120 (467) Brodzinsky & Singh
Point Arena 17 (66) 20 (78) (1983)
Point Reyes 93 (362) 93 (362)
Reese River 5 (19) 5 (19)
Riverside 250 (973) 560 (2180)
San Jose 31 (120) 31 (120)
St. Louis 81 (315) 100 (390)
San Jose (California, USA) Singh et al. (1992)
[4-16 April 1985] 400
(44-4661)
[12-24 August 1985] 121
(5-1067)
[13-21 December 1985] 2869
(239-15 424)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Downey, California, USA
[8-27 February 1984] 212
(18-815)
Houston, Texas, USA
[9-17 March 1984] 23
(11-48)
Denver, CO, USA
[24 March-1 April 1984] 22
(13-64)
The Netherlands
Delft, Vlaardingen 50-200 100-900 Guicherit & Schulting
and Terschellingd (195-780) (390-3500) (1985)
Japan
urban and suburban 15-31 JEA (1981)
(59-122)
a The converted values in brackets ( ) are only approximate.
b Includes substantial overlap with data reported in Singh et al. (1981).
c Values given from spring 1991. Fig. 3 gives averages over the last decade.
d About 350 samples per site during 1989.
Approximately 750 air samples from five surface sampling sites in
Alaska, Hawaii (2), Samoa, and New Zealand were analysed by Cicerone
et al. (1988) for methyl bromide between January 1985 and October 1987
(using GC/MS). Methyl bromide concentrations were typically 40-44
ng/m3 (10-11 pptv).
Khalil et al. (1993) presented a series of 1700 measurements
showing the latitudinal distribution of atmospheric methyl bromide
from 1983 to 1992 (Table 21 and Fig. 3). The levels of atmospheric
methyl bromide measured between 1988 and 1992 seemed to be higher than
those measured between 1983 and 1988.
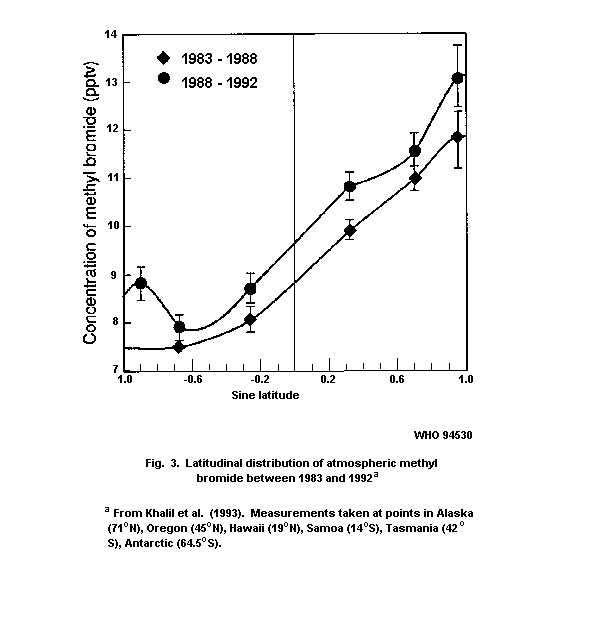 5.1.1.3 Measured continental and urban levels of methyl bromide
Data from seven cities in the USA showed significant elevations
of methyl bromide levels in urban areas with average concentrations of
159-1004 ng/m3 (41-259 pptv) (Singh et al., 1982). Concentrations as
high as 4000 ng/m3 were also reported. A further study of methyl
bromide levels at 16 sites in the USA confirmed these findings,
showing that the larger the city the higher the methyl bromide level
(Brodzinsky & Singh, 1983). Methyl bromide is emitted from motor
vehicles using leaded petrol. Since many of these urban studies were
made, unleaded petrol has been introduced, which could mean that
current urban levels of methyl bromide would be lower. Some background
levels of 20 ng/m3 (5 pptv) appear to be lower than oceanic values
(Singh et al., 1981).
The high values reported for 1984/85 by Singh (1992) in
California (Table 21) may have been influenced by nearby soil
fumigation.
In a 1980 environmental survey of methyl bromide levels in
Japanese urban and suburban areas, the gas was detected in 5 out of 27
samples with levels ranging from 59 to 122 ng/m3 (15 to 31 pptv)
(JEA, 1981). In the Netherlands, a study of the concentrations of
methyl bromide in ambient air was carried out by Guicherit & Schulting
(1985). Between 1979 and 1981, air samples were measured in three
locations: at the island of Terschelling in the north (little
pollution); at Delft, a small city in the densely populated western
part of The Netherlands, and in Vlaardingen, in a heavily
industrialized area near Rotterdam. The study gave an average of
195-778 ng/m3 (50-200 pptv) and a maximum 1-h concentration of
390-3500 ng/m3 (100-900 pptv). The estimated daily (10.0 µg) and
yearly (4.5 µg) average exposure values given were based on a total
respiratory volume of 20 m3 per day.
5.1.1.4 Vertical profiles of methyl bromide in the atmosphere
Data on vertical levels of methyl bromide lead to a better
understanding of the ultimate fate of methyl bromide in the atmosphere
and its role in ozone reduction in the lower stratosphere (section
4.4). Rasmussen & Khalil (1984) and Berg et al. (1984) showed no
significant decrease in methyl bromide levels up to 7 km. Penkett et
al. (1985) detected a methyl bromide level of 40 ng/m3 (10 pptv) in
the upper troposphere. As shown in Fig. 4, Fabian et al. (1981)
measured 1.2 pptv at 14.4 km, but could not detect any methyl bromide
at an altitude of 20 km. It has been suggested (UNEP, 1992) that there
is no significant vertical gradient in the troposphere, but that the
level of methyl bromide decreases rapidly in the lower stratosphere
(by at least a factor of 3-5 within 10 km above the tropopause). It is
here that ozone loss occurs. Schauffler et al. (in press) gave methyl
bromide levels in the mid to low troposphere of 13.6±1.4 pptv (37-45°
N; 69° W) and 12.7±1.1 (22-26° N; 94-97° W). In the tropical
tropopause (altitude 15.3-16.76 km; 24° N, 68-85° W), levels of
11.4±0.5 pptv (mean of 12 samples) were measured.
5.1.1.3 Measured continental and urban levels of methyl bromide
Data from seven cities in the USA showed significant elevations
of methyl bromide levels in urban areas with average concentrations of
159-1004 ng/m3 (41-259 pptv) (Singh et al., 1982). Concentrations as
high as 4000 ng/m3 were also reported. A further study of methyl
bromide levels at 16 sites in the USA confirmed these findings,
showing that the larger the city the higher the methyl bromide level
(Brodzinsky & Singh, 1983). Methyl bromide is emitted from motor
vehicles using leaded petrol. Since many of these urban studies were
made, unleaded petrol has been introduced, which could mean that
current urban levels of methyl bromide would be lower. Some background
levels of 20 ng/m3 (5 pptv) appear to be lower than oceanic values
(Singh et al., 1981).
The high values reported for 1984/85 by Singh (1992) in
California (Table 21) may have been influenced by nearby soil
fumigation.
In a 1980 environmental survey of methyl bromide levels in
Japanese urban and suburban areas, the gas was detected in 5 out of 27
samples with levels ranging from 59 to 122 ng/m3 (15 to 31 pptv)
(JEA, 1981). In the Netherlands, a study of the concentrations of
methyl bromide in ambient air was carried out by Guicherit & Schulting
(1985). Between 1979 and 1981, air samples were measured in three
locations: at the island of Terschelling in the north (little
pollution); at Delft, a small city in the densely populated western
part of The Netherlands, and in Vlaardingen, in a heavily
industrialized area near Rotterdam. The study gave an average of
195-778 ng/m3 (50-200 pptv) and a maximum 1-h concentration of
390-3500 ng/m3 (100-900 pptv). The estimated daily (10.0 µg) and
yearly (4.5 µg) average exposure values given were based on a total
respiratory volume of 20 m3 per day.
5.1.1.4 Vertical profiles of methyl bromide in the atmosphere
Data on vertical levels of methyl bromide lead to a better
understanding of the ultimate fate of methyl bromide in the atmosphere
and its role in ozone reduction in the lower stratosphere (section
4.4). Rasmussen & Khalil (1984) and Berg et al. (1984) showed no
significant decrease in methyl bromide levels up to 7 km. Penkett et
al. (1985) detected a methyl bromide level of 40 ng/m3 (10 pptv) in
the upper troposphere. As shown in Fig. 4, Fabian et al. (1981)
measured 1.2 pptv at 14.4 km, but could not detect any methyl bromide
at an altitude of 20 km. It has been suggested (UNEP, 1992) that there
is no significant vertical gradient in the troposphere, but that the
level of methyl bromide decreases rapidly in the lower stratosphere
(by at least a factor of 3-5 within 10 km above the tropopause). It is
here that ozone loss occurs. Schauffler et al. (in press) gave methyl
bromide levels in the mid to low troposphere of 13.6±1.4 pptv (37-45°
N; 69° W) and 12.7±1.1 (22-26° N; 94-97° W). In the tropical
tropopause (altitude 15.3-16.76 km; 24° N, 68-85° W), levels of
11.4±0.5 pptv (mean of 12 samples) were measured.
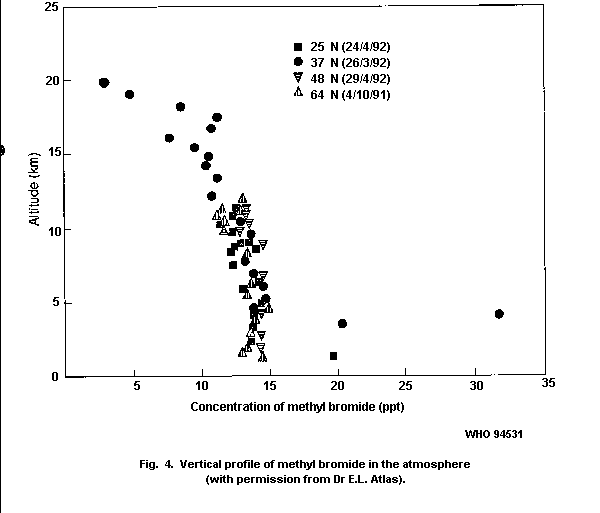 Fig. 4 shows the vertical profile for methyl bromide (Schauffler
et al., in press). Fig. 5 shows the vertical profile of methyl bromide
together with other atmospheric source gases in the middle Northern
hemisphere from air samples taken from the troposphere and
stratosphere (Fabian, 1984).
Fig. 4 shows the vertical profile for methyl bromide (Schauffler
et al., in press). Fig. 5 shows the vertical profile of methyl bromide
together with other atmospheric source gases in the middle Northern
hemisphere from air samples taken from the troposphere and
stratosphere (Fabian, 1984).
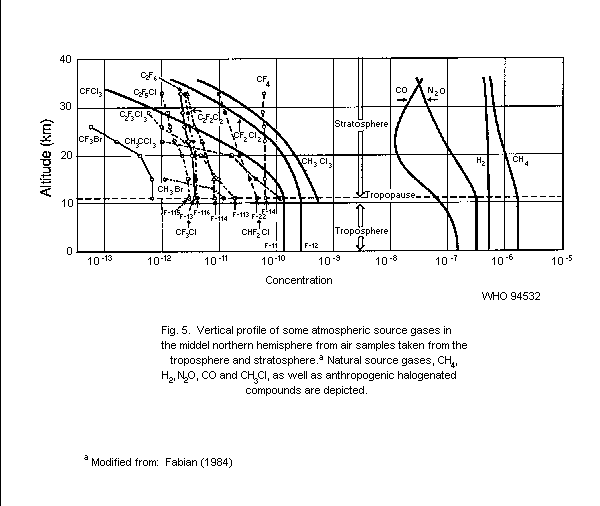 5.1.1.5 Release of methyl bromide to the outside air from greenhouses
During 1986, 400 000 kg methyl bromide were emitted into the air
in the Westland area of the Netherlands (Van Doorn et al., 1989).
Although soil disinfection is only allowed when the diluting capacity
of the atmosphere (wind force) is sufficient, this is very difficult
or impossible to control. Investigations by Netherlands Institutes
(TNO and RIVM) showed that the hourly average concentrations within a
20-m distance from the green-houses in 1981 (after the introduction of
gas-tight film) during the first hours was 5.9 mg/m3 after
fumigation with methyl bromide at a dose of 700 kg/ha. In 1982 and
1983, a few hours after injection and at a probable dosage of 400
kg/ha, concentrations measured ranged between 1 and 4 mg/m3 at
distances of 20 m. After the first few hours, the concentration of
methyl bromide decreased rapidly and, after 4 days, also at 20 m,
hourly averages of 0.2 mg/m3 were measured. Ten days after
fumigation, the covering foil was removed, after which the hourly
average concentration increased to a maximum of 0.4 mg/m3 (Van Doorn
et al., 1989).
5.1.2 Water
5.1.2.1 Seawater
In 1975, samples of seawater from near the shore at Dorset,
England were analysed for halomethanes and methyl bromide was detected
at levels ranging from 2.0 to 3.9 x 10-9 ml gas/ml water [apprx. 10
ng/litre] (Lovelock, 1975).
Average concentrations of 1.2 ng methyl bromide/litre were
measured in surface seawater in the Eastern Pacific Ocean (Singh et
al., 1983). The authors estimated that the oceans are supersaturated
with methyl bromide to 250%.
Khalil et al. (1993) measured methyl bromide concentrations in
ocean water in two open-ocean surveys, one covering latitudes from 45°
N to about 30° S, the other from 67° N to 50° S. On the first survey,
they found supersaturation of methyl bromide at 180% and, on the
second, 140%.
5.1.2.2 Inland waters
In 1988, the California Department of Food and Agriculture (CDFA)
reported the results of analyses of 43 056 well water samples taken
from 2977 wells in various Californian counties. Residues of 10
chemicals were detected. Methyl bromide detection was undertaken in 32
counties, but in all the wells tested only one sample showed the
presence of methyl bromide (CDFA, 1988).
5.1.2.3 Waters around greenhouses
In the Netherlands, where intensive horticulture in greenhouses
is practised, the soil level is lower than sea level in some areas,
with the result that water transport is very slow and the static water
volume is very high. There is a high density of greenhouses and all
holdings have to use the water from ditches as leaching water, thus,
this water is successively loaded with bromide. However, it should be
noted that, in 1992, methyl bromide was banned in the Netherlands for
soil fumigation purposes.
The concentrations of methyl bromide and bromide-ion were
measured in irrigation water, drainage water, and surface water during
the leaching periods in two Netherlands glasshouse soils after
fumigation with methyl bromide (Wegman et al., 1981). Maximum
concentrations in drainage water, determined within 24 h of the start
of leaching, were 9.3 mg/litre (methyl bromide) and 72 mg/litre
(Br-).
Further studies of bromide ion concentrations in precipitation,
surface water, and ground water in the polder district in the
Netherlands (a main horticultural area) in 1979-80 gave maximum values
of 0.98, 41, and 17 mg/litre respectively, the highest concentrations
being found during the main fumigation/leaching time in
September-October 1979 (Wegman et al., 1983). During these few weeks,
an estimated 1.78 million kg methyl bromide (about 1.5 million kg
bromide ion) were used for soil fumigation. From their measurements,
Wegman et al. (1983) calculated that 14% of the applied methyl bromide
was converted to bromide ion. This is in agreement with Daelemans
(1978), whose studies showed that 10-30% of methyl bromide is
converted to Br-. The fumigation method used was the "hot gas
method", whereby the fumigant was discharged under thin low-density
polyethylene (LDPE) sheeting. Subsequently, in June 1981, the use of
LDPE for this purpose was forbidden.
In 1985, bromide concentrations of 10-35 mg/litre were observed
in Westland (the Netherlands) surface water with maximum
concentrations in the Poel and Bosch polders of 38.7 and 31.7
mg/litre, respectively (Van Doorn et al., 1989). Private water
supplies from shallow pumps in the Netherlands near methyl bromide
soil operations were expected to have increased bromide contents (Van
Doorn et al., 1989). In 1986, in the Westland, 260 000 kg bromide
ended up in surface water, at an average dosage of 700 kg/ha.
Similar investigations were carried out by Vanachter et al.
(1981) in the glasshouse crop growing region of Malines - Antwerp
(Belgium) into bromide concentrations in surface water in periods of
intense soil fumigation and leaching (August-September) compared with
periods before and after this, when soil fumigation and leaching were
less frequent. Sampling was also carried out during leaching, near the
greenhouses, as well as in the ditches and draining water. For
comparison, samples of natural Br- concentrations in surface water
were taken from a region where methyl bromide fumigation was not
carried out.
The results showed that during the leaching period,
concentrations of Br- in the surface water in the greenhouse crop
growing area were significantly higher (maximum 9.6 mg/litre) but,
five weeks later, were either not detectable (<0.1 mg/litre) or very
low (1.59 mg/litre) in little brooks. The bromide concentration in
rivers was less than 0.8 mg/litre, except for one site, where either
sea water or the effluents from a local photographic plant resulted in
concentrations of up to 4.5 mg/litre. In the direct drainage area of
the glass houses, transient concentrations of up to 33 mg/litre were
measured, decreasing rapidly within a few days.
Guns (1989) measured groundwater levels in four greenhouses in
Belgium from September 1986 to August 1988. The bromide content of
groundwater depended, not only on the length of leaching, but also on
the type of soil. When the deeper soil layers contained more clay, the
concentration of the bromide ion in the groundwater, after fumigation
and leaching, was still relatively high (48 mg/litre before and 280
mg/litre after fumigation in one study).
5.1.3 Soil
The natural bromide present in unfumigated soil depends on the
soil type. Van Wambeke et al. (1974) gave values of 3.3 mg/litre for
fresh sandy loam soil and 2.5 mg/litre for peat soil. Hoffman &
Malkomes (1974) stated that the natural concentration of bromide in
the soil was less than 10 mg/kg, depending on the type of soil and
geographical situation.
Fallico & Ferrante (1991) measured bromide concentrations in
greenhouse soil before, and after, the application of methyl bromide
(80 g/m2). Before fumigation, bromide levels were about 5 mg/kg. Two
months after treatment, bromide levels of over 30 mg/kg were measured.
After a further 3 months, levels had decreased to less than 10 mg/kg.
Bromide ion concentrations following greenhouse and soil
fumigation depend on the dosage, exposure time, aeration period,
temperature, the type of soil, the amount of rain or leaching water,
and the type of covering (sealing conditions). A year after fumigation
with 70-80 g methyl bromide/m3, bromide values of 0.2-11.5 mg/kg
were recorded in the upper 30 cm of soil (Basile et al., 1987).
The bromide remaining in the soil after fumigation can affect
soil fertility (Rovira & Ridge, 1979). As wheat particularly tends to
concentrate bromine, soil and wheat dynamics were studied (1981-83) in
methyl bromide-fumigated plots in a Mediterranean climate (Italy).
Bromide residues ranged between 5 and 10 mg/kg in the fumigated soil
to a depth of 50-60 cm. The total amount of bromide in the soil was
5.8 g/m2 up to a depth of 1 m and remained almost constant during
the wheat-growing period (Fransi et al., 1987). The amount of bromide
residues was about 8 % of that applied (900 kg/ha) five months
previously, compared with the 20 % found by Van Wambeke et al. (1974)
in similar soils.
5.1.4 Food
When considering the published levels of methyl bromide and
inorganic bromide in various foods, the method of analysis is
important (section 2.4.9). Originally, bromide content only was
measured and there is very little information on the methyl bromide
content. Measurements of both entities are important as well as other
residual products.
5.1.4.1 After soil fumigation
Bromide accumulation in plants depends on various factors, such
as the physical and chemical properties of the soil, the climatic
condition, the plant species and particular tissues, and the cultural
practices (Basile & Lamberti, 1981). Moreover, it depends on dosage,
exposure time, and aeration.
A study was carried out in 1980 in Metaponto, in southern Italy,
where methyl bromide was used to control nematodes and other plant
pathogens in the soil (Basile & Lamberti, 1981). Bromide ion levels
were 20-51 mg/kg (tomatoes), 8-44 mg/kg (string beans), 25-149 mg/kg
(radishes), 18-60 mg/kg (aubergines), 6-165 mg/kg (cucumbers), 13-46
mg/kg (courgettes), and 3-27 mg/kg (peppers) on a fresh weight basis.
In experimental plot conditions, the methyl bromide concentration was
60 g/m2 under a plastic cover with the soil temperature 14-17 °C at
10 cm depth. The covering was removed after 2 days and the plot
rotavated at a depth of 20 cm to eliminate residual gases.
Roughan & Roughan (1984a,b) carried out surveys of bromide ion
residues in lettuces, cucumbers, tomatoes, and self-blanching celery
grown in soil fumigated with methyl bromide, and compared these values
with those in a range of home-produced and imported fruit and
vegetables. Lettuce grown on unfumigated soil contained less than 10
mg bromide ion /kg, while most lettuces harvested from methyl bromide
fumigated soil were found to contain considerably more, 30 %
containing over 500 mg/kg and 2 % even in excess of 2000 mg/kg
(Roughan & Roughan, 1984a).
Studies on vegetables grown under protective covers on soil
previously fumigated with methyl bromide showed levels ranging from 1
to 109 mg/kg (fresh weight) in 29 late-season cucumbers, 5 to 326
mg/kg in 242 tomato samples, and 2 to 521 mg/kg in 38 samples of
celery (Roughan & Roughan, 1984b); 65 % of the late season cucumbers
contained more than background of bromide levels up to 10 mg/kg. Crops
of tomatoes (summer 1981) grown on sites where methyl bromide
fumigation had taken place in 1980/1981, contained considerably higher
levels than those grown on a site fumigated in 1979. In a survey from
retail outlets (January-November 1979), tomatoes and cucumbers from
the Canary Islands and Spain generally contained less than 10 mg/kg
(fresh weight). Of those grown in the United Kingdom, levels exceeded
10 mg/kg in 35 %, and 100 mg/kg in 5 %, ranging up to 177 mg/kg. These
higher levels of bromide ion could be attributed to the plants having
been grown on soil fumigated with methyl bromide prior to planting
(Roughan & Roughan, 1984b). The total bromide ion contents of various
crops obtained from retail outlets in the United Kingdom during the
period June 1981 to July 1982 are summarized in Table 22. The survey
indicated that vegetables, such as tomatoes, celery, cucumbers,
lettuce, radishes, and aubergines, grown in United Kingdom and the
Netherlands contained higher levels than elsewhere. Other crops had
mean bromide levels of less than 10 mg/kg, similar to other figures
published for background bromide ion content.
Table 22. Total bromide ion content of various crops obtained from retail
outlets in United Kingdom during the period June 1981 to July 1982a
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
apple France 5 0.1-0.3 0.2
aubergine Netherlands 4 2-23 11
avocado pear not known 1 1
banana Windward Islands 2 2 2
bean, broad England 3 1-2 2
bean, french Guernsey 1 1
bean, runner England 2 1 1
bean, sprouts England 3 1 1
cabbage England 3 1-2 2
calebrese England 2 1 1
(broccoli)
carrot England 1 2
cauliflower England 3 0.3-1 1
Chinese England 3 1-2 1
leaves
celery England 12 1-178 28
Guernsey 1 9
Israel 4 7-14 10
Spain 16 2-8 4
USA 1 4
courgette England 3 1-3 2
cucumber Canary Islands 15 0.3-10 3
England 36 0.2-87 9
Spain 3 0.2-10 4
Netherlands 7 0.1-14 7
grapefruit
segments Cyprus/Israel,
S. Africa 4 0.1-0.4 0.3
green pepper Netherlands 7 0.4-5 2
ettuce Belgium 1 5
Cyprus 5 1 1
England 69 1-241 15
France 9 0.2-19 4
Israel 6 1-4 2
Spain 11 0.4-4 2
Netherlands 26 2-57 21
USA 12 0.1-2 1
Table 22 (continued)
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
marrow England 2 1-2 1
mushroom England 62 0.2-24 1
onions Israel,
Netherlands 3 0.4-1 1
onion, England 3 2-4 3
spring
orange S. Africa, Spain 6 <0.1-0.4 0.2
segments
pea England 4 1-3 2
potato Cyprus, England 3 1-2 1
radish England 18 0.2-3 1
Israel 2 5 5
Netherlands 12 0.1-48 13
USA 1 1
strawberry England 1 0.3
tomato Canary Islands 8 1-5 4
England 33 1-70 13
Spain 15 1-7 3
Netherlands 14 1-39 11
a From: Roughan & Roughan (1984b).
Brown et al. (1979) measured the bromide concentration in several
plant species in unfumigated and methyl bromide-fumigated plots in
California (Table 23). Many plants showed increased bromide
concentrations. Strawberries and grapevines absorbed relatively
little. As the interval increased between soil fumigation and
planting, there was a general decline in bromide levels, though the
interval could be as long as three years before the crops returned to
a level of about 10 mg/kg (Brown et al., 1979; Roughan & Roughan,
1984a). Table 24 shows bromide concentrations in plant material 1, 2,
3, and 4 years after fumigation with methyl bromide.
Fallico & Ferrante (1991) measured bromide levels in tomatoes in
crops grown in soil that had been fumigated with methyl bromide ten
days prior to planting. Bromide levels in tomatoes grown in the
treated soil and harvested after 60 days were about double (55 mg/kg)
those in tomatoes grown in untreated soil. The bromide levels
decreased with each successive harvest, but were still higher than
those in control plants after the fourth harvest.
Table 23. Bromide concentrations in several plant species in unfumigated and methyl bromide-fumigated plotsa,b
Plant species Treatment Range of Average bromide S.E. of
and part bromide concentration means
concentrations (mg/kg dry weight)
(mg/kg dry weight)
barley control 4 to 575 106 (19)c 38
(whole top) fumigated 120 to 5235 1788 (23) 373
bur clover control 1 to 407 96 (11) 40
(whole top) fumigated 196 to 2371 1334 (14) 155
filaree control 4 to 546 135 (14) 47
(whole top) fumigated 718 to 7380 2600 (10) 683
wild oats control 9 to 876 196 (14) 66
(whole top) fumigated 1233 to 5034 3364 (11) 441
spinach (leaves) fumigated 1772 to 3195 2521 (4) 387
ryegrass fumigated 1481 to 2790 2378 (4) 302
sweet potato
(leaves)
1st sampling fumigated 640 to 923 753 (4) 69
4th sampling fumigated 312 to 372 330 (4) 14
sweet potato
(root) fumigated 204 to 237 220 (2) 17
strawberry control 14 to 129 63 (9) 16
(leaves)d fumigated 3 to 372 88 (36) 13
grape (leaves)e control 1 to 101 28 (90) 3
fumigated 1 to 402 48 (278) 4
Table 23 (continued)
a From: Brown et al. (1979).
b These data represent samples collected the first year after fumigation with MeBr at rates of 34-68 g/m2,
except for grape leaves.
c Numbers in parentheses refer to the number of samples in the average.
d Strawberry leaves were collected after 1-6 annual fumigations with methyl bromide.
e Grape leaves were collected 2-4 years after fumigation with methyl bromide.
Table 24. Bromide concentrations in plant material (mg/kg dry
weight), 1, 2, 3, and 4 years after fumigation with methyl bromidea
County Untreated Years after fumigation
controls
1 2 3 4
Sonoma 94(19)b 3018(16) 360(15) 516(8) 12(3)
Napa 163(21) 1476(15) 742(10) 430(2)
a From: Brown et al. (1979).
b Numbers in parentheses = number of samples included in the
average.
Table 25. Inorganic bromide residues detected in samples of fruit and
vegetables in UK (1988 to 1989)a
Commodity Concentration Number of samples
range (mg/kg)c
Lettuces, produced n.d. 0
in United Kingdom
(proposed CAC 1-20 48
MRLb=100) 21-100 15
101-300 9
301-529 5
Lettuces, imported n.d. 0
1-20 21
21-45 3
Rice, imported n.d. 59
[MRL=50 1-10 37
(unprocessed 11-20 16
rice)] 21-50 14
50-100 10
121 and 124 2
Nuts (no MRL)
almonds n.d. 4
1-147 24
brazil nuts n.d. 0
3-140 22
Table 25 (continued)
Commodity Concentration Number of samples
range (mg/kg)c
cashew nuts n.d. 16
3-53 5
chestnuts n.d. 5
3-23 3
coconuts n.d. 0
2-6 5
hazel nuts n.d. 7
1-194 10
peanuts n.d. 4
2-109 18
pine nuts n.d. 4
46 1
Nuts (continued)
pistachio nuts n.d. 0
3-104 4
tiger nuts n.d. 0
17-20 4
walnuts n.d. 3
2-210 21
sesame seed n.d. 5
17-56 5
sunflower seed n.d. 3
2 6
3 1
Dried fruits
currants, imported
(CAC MRL = 100) n.d. 22
1-3 9
6-15 5
sultanas n.d. 20
(CAC MRL = 100) 1-6 6
6-12 7
Table 25 (continued)
a From: MAFF (1990).
b CAC = Codex Alimentarius Commission; MRL = maximum residue limit
(mg/kg) legally permitted in, or on, food commodities or animal feed.
c n.d. = not detected.
The results of a further survey (1988-89) in the United Kingdom
of inorganic bromide levels in fruit and vegetables are summarized in
Table 25. Fourteen samples of lettuce produced in the United Kingdom
exceeded the Codex Alimentarius Commission maximum residue limit (CAC
MRL) for inorganic bromide of 100 mg/kg (MAFF, 1990).
The effects of leaching following soil fumigation with methyl
bromide up to 100 g/m2 are shown in Table 26. At sites with low soil
bromide residues, the resulting bromide residues in lettuce were
nearly unaffected by leaching. At other sites, there was a positive,
but diminishing, response to increasing rates of leaching, very high
residues probably being due to high levels of organic matter (Smart,
1990).
Recent monitoring of samples of individual crops having a
likelihood of being grown on soil fumigated with methyl bromide showed
that only a small percentage contained residues above Codex
Alimentarius Commission recommended limits. The high levels were
mainly in lettuce. The report of Smart (1990) showed that the
proportion of samples having high residues had declined since the late
1970s, when, in some countries, residues in lettuce were as high as
500-1000 mg/kg. Leaching of treated soils, attention to timing of
application, and integrated pest control have all helped to reduce
such residue levels.
Bromide level tolerances for a variety of methyl bromide
fumigated raw agricultural commodities in the USA are shown in Table
27. However, according to the US EPA, because of its existing
toxicological data base and its environmental ubiquity, inorganic
bromide is not of toxicological concern. Requirements for residue data
to support existing inorganic bromide tolerances were waived by the
Agency (US EPA, 1989).
Table 26. Bromide residues in lettuce grown under protection of soil fumigated with bromomethane and leached with water
before planting in the United Kingdoma
Site Soil texture Soil organic Number of Interval between Bromide residues in lettuce (mg/kg fresh weight)
matter (%) applications planting and for the water application rates (mm)
in previous harvest
years (weeks) 0b 100 200 300 400
A fine sandy loam 4 2 16 81 102 92 150 142
B sandy loam 12 1 12 62 70 232 93 114
C sandy loam 13 2 13 307 215 98 157 117
D sandy loam 1 24 765 427 392 284 250
E sandy loam 8 4 21 676 335 328 164 134
Fc loamy peat 51 4 14 1958 1534 1001 - -
Mean sites A-E 378 230 228 170 151
a Summarized from Food and Agriculture Organization (1985) by Smart (1990).
b Residue figures for crops grown in soils not having any leaching.
c At F, the leaching treatments were applied after planting the lettuce crop.
Table 27. USA tolerancesa
Br in, or on, the following raw agricultural commodities,
which have been fumigated with the antimicrobial agent
and insecticide methyl bromide after harvest (with the
exception of strawberries) (mg/kg)
corn (pop) 240
almonds, brazil nuts, bush nuts, 200
butternuts, cashews, chestnuts,
cottonseed, filberts, hickory nuts,
peanuts, pecans, pistachio nuts,
soybeans, walnuts
asparagus, copra, cumin (seed), 100
ginger (roots), pomegranates
avocados, coffee beans, potatoes, 75
sweet potatoes
alfalfa (hay), barley, beans, 50
beans (green), benas (lima), beans
(snap), cabbage, cippolini (bulbs),
cocoa beans, corn, corn (sweet),
garlic, oats, peas, peas (blackeyed),
rice, rye, sorghum (grain), timothy
(hay), wheat
artichokes (Jerusalem), garden beets 30
(roots), sugar beets (roots), carrots,
citrus citron, cucumbers, grapefruit,
horseradish, kumquats, lemons, limes,
okra, oranges, parsnips (roots), peppers,
pimentos, radishes, rutabagas, salsify
(roots), squash (summer), tangerines,
turnips (roots)
apricots, blueberries, cantaloupes, 20
cherries, eggplant, grapes, honeydew
melons, mangoes, muskmelons, nectarines,
onions, papayas, peaches, pineapples,
plums, pumpkins, squash (winter), squash
(zucchini), tomatoes, watermelons
apples, pears, quinces 5
a From: US EPA (1988b; CFR 180.124).
5.1.4.2 After post-harvest fumigation
Methyl bromide is widely used as a post-harvest fumigant to kill,
or prevent, pest infestation. In 1976, around 100 000 metric tonnes of
food commodities were treated with methyl bromide in the United
Kingdom (Fairall & Scudamore, 1980).
Fairall & Scudamore (1980) measured methyl bromide residues in
dried milk, wheat, flour, rapeseed, and groundnut samples after store
fumigation (see Table 28). Products such as groundnuts and rapeseed
retained higher amounts of methyl bromide. No methyl bromide was
detected in any commodity after storage for 1 month (detection limit
10 µg/kg).
DeVries et al. (1985) measured the rate of decrease of methyl
bromide in wheat, flour, cocoa, and peanuts after fumigation with the
gas. Samples were analysed immediately and then after various time
intervals of exposure of the sample to air. The methyl bromide
concentration decreased very rapidly in all cases, no residual methyl
bromide being found in any of the samples after 2 weeks (detection
limit, 0.4 µg/kg).
(a) Wheat and cereals
Pesticide residues in home-grown and imported wheat were measured
in the United Kingdom by Osborne et al. (1989); 45 samples were
analysed for methyl bromide (method: Fairall & Scudamore, 1980) and
inorganic bromide. No methyl bromide was found in excess of the
detection limit (0.01 mg/kg); all samples contained inorganic bromide,
but at levels of 4 mg/kg or less, which is given as the level
naturally present in wheat as a result of uptake from the soil (Heuser
& Scudamore, 1970; Osborne et al., 1989).
Trials carried out on a commercial scale showed that CT
(concentration x time) products for methyl bromide generally lay in
the range of 50-2000 mg.h/litre (Scudamore, 1987). The higher values
are usually found in pockets of grain at the bottom of silos or bins.
Scudamore (1987) recommended careful monitoring, especially at
increased temperature and moisture content, when treating more
sorptive cereals, such as oats or maize, or those with which methyl
bromide reacts more readily. In three samples of wheat from different
parts of the United Kingdom, bromide levels increased between 2 and 3
times over a range of 11-16 % moisture content.
Table 28. Methyl bromide residue levels (mg kg-1) during storagea
Days
Commodity 0 0.04 0.25 1 2 4 7 11
dried milk 0.5 0.15 0.03 0.006 - - - -
wheat 0.8 0.42 0.33 0.08 0.04 n.d.b - -
flour 0.28 0.09 0.04 0.02 n.d. - - -
rapeseed 4.2 3.0 1.5 - 0.95 0.5 0.10 0.08
groundnuts 7.7 4.5 3.2 2.6 1.6 0.38 0.38 0.03
a From: Fairall & Scudamore (1980).
b n.d. = none detected.
Table 29. Bromide ion and methyl bromide concentrations (mg/kg) in
flours exposed to methyl bromide only in silos and in pastas obtained
from these fumigated and unfumigated floursa
Material Treatment Number of Bromide ion Methyl
samples (mean±S.D.) bromideb
flours unfumigated 3 1.26±0.03 n.d.
fumigated 3 1.60±0.11 n.d.
pasta from unfumigated 6 1.24±0.13 n.d.
(macaroni) flours
from fumigated 6 1.91±0.22 n.d.
flours
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than the detection limit of 0.01
mg/kg.
Stacks of bags containing stored grains and pulses (wheat,
lentils, maize, barley, chick-peas, peas, and sorghum) were covered
with PVC sheets and exposed to methyl bromide fumigation for 48 h
(Urga, 1983). Following aeration, residues of less than 50 mg/kg
bromide were measured, with the exception of 60 mg/kg after 24 h
aeration and 59 mg/kg after 36 h aeration. Cova et al. (1986)
investigated the effects of exposure to methyl bromide in flour, and
pastas made from it. Flour was exposed to methyl bromide in silos
(conditions: mean temperature - 18 °C, concentration - 24 g/m3,
duration of treatment - 68 h, duration of ventilation - 3 days). Table
29 shows levels of bromide ion before, and after, fumigation. Methyl
bromide could not be detected. In a second experiment (summarized in
Table 30) on pasta made from unfumigated flour, rice flour, and white
flour, Cova et al. (1986) examined the influence of the type of
packaging on the effects of fumigation. Every item was packed in two
different ways, i.e., a cardboard box or a transparent envelope made
of a double layer of polypropylene. The products were fumigated in a
closed room under the conditions given above, and the mean bromide ion
concentrations ranged between 2.03 mg/kg (pasta) and 46.23 mg/kg (egg
pasta), while methyl bromide was not detected.
Table 30. Bromide ion and methyl bromide concentrations (mg/kg) in unfumigated
and fumigated foodstuffs, treated in their retail packagingsa
Material Treatment Number of Bromide ion Methyl
samples (mean ± S.D.) bromideb
rice unfumigated 3 0.72 ± 0.06 n.d.
funfumigated 3 10.63 ± 0.67 n.d.
flour unfumigated 3 3.17 ± 0.09 n.d.
funfumigated 3 6.66 ± 0.01 n.d.
white flour unfumigated 3 1.22 ± 0.15 n.d.
funfumigated 3 4.19 ± 0.82 n.d.
pasta (macaroni) unfumigated 6 2.40 ± 0.26 n.d.
funfumigated 6 2.60 ± 0.27 n.d.
pasta (spaghetti) unfumigated 6 1.92 ± 0.17 n.d.
funfumigated 6 2.03 ± 0.07 n.d.
pasta with eggs unfumigated 6 4.13 ± 0.12 n.d.
funfumigated 6 46.23 ± 1.57 n.d.
pasta with eggs unfumigated 6 4.62 ± 0.31 n.d.
and spinach funfumigated 6 39.00 ± 0.01 n.d.
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than detection limit of 0.01 mg/kg.
(b) Spices, nuts, and dried fruits
In the USA in 1980, two warehouses containing imported spices
were fumigated to eradicate a khapra beetle infestation. Methyl
bromide and inorganic bromide residues were determined in the 52
spices before, and after, fumigation (using GC-ECD). In addition, an
ashing titration method for bromide ion residue was used, allowing a
comparison of the two analytical methods (Reeves et al., 1985). Before
fumigation, the highest methyl bromide residue was in parsley (14.9
mg/kg). Seventy-two hours after fumigation with 100 g/m3 for 12 h,
samples were collected and analysed. The highest methyl bromide
residue was found in sage (65.8 mg/kg). Levels of inorganic bromide
residues before fumigation (GC-ECD) were all lower than 200 mg/kg;
after fumigation, only two samples contained higher levels.
In a Canadian study, a number of spices, seeds, nuts, and dried
fruits and vegetables, including samples of celery, mustard, sesame,
coriander, pumpkin and sunflower seeds, cloves, peppercorns, dates,
figs, prunes, raisins, beans, minced onion, a vegetable mix, walnuts,
and peanuts were analysed for methyl bromide residues (Page & Avon,
1989). Of the 30 samples, only a sample of pumpkin seeds was found to
contain methyl bromide (3 µg/kg). Fifty-one chocolate and grain-based
products were also analysed and found not to contain any methyl
bromide.
In contrast, when samples of food known to have been treated with
methyl bromide were analysed, of the 60 samples, 16 contained residues
>1 ng/kg and 5 contained >100 ng/kg methyl bromide. These samples
included dried currants (2.9 mg methyl bromide/kg), chocolate-covered
nuts (0.66 and 0.19 mg/kg), and rice (2.3 mg/kg) and maize (0.21
mg/kg) flours. These findings were confirmed by mass spectrometry
(Page & Avon, 1989).
Bromide residues after methyl bromide fumigation were determined
in samples of dried fruits, cereals, nuts, and spices imported into
New Zealand in 1977 and early 1978 (Love et al., 1979). About one-half
of the nut and spice samples contained total bromide levels exceeding
50 mg/kg, and occasional high levels of bromide residues were found in
cereals.
Fairall & Scudamore (1980) showed that rapeseed and groundnut
samples retained higher amounts of methyl bromide than other
foodstuffs after store fumigation (Table 28). A survey of retail nuts,
seeds, and nut products in October 1984 (Table 31) showed that levels
of methyl bromide in some of these products were higher than the Codex
Alimentarius Commission guideline (MAFF, 1989).
Table 31. Residues of methyl bromide in samples of nuts, seeds, and nut productsa,b
Number of samples Residue concentrations
(mg/kg)
tested containing rangec mean
residues
almonds 5 2 n.d. to 0.06 < 0.02
brazil nuts 4 0 n.d. -
dried chestnuts 1 1 0.2 -
hazelnuts 4 0 n.d. -
mixed nuts 3 0 n.d. -
nuts and raisins 3 2 n.d. to 0.2 0.08
peanuts 12 9 n.d. to 0.3 0.06
walnuts 7 4 n.d. to 2.2 0.4
othersd 6 0 n.d. -
a From: MAFF (1989).
b 45 samples were obtained during October 1984. and analysed for
residues of methyl bromide. The CAC (1986) guideline level for
methyl bromide in nuts is 0.01 mg/kg.
Table 31 (continued)
c n.d. = not detected (the limit of determination was 0.02 mg/kg).
d One sample each of cashews, pecans, pine kernels, pistachios, sunflower seeds, and
tiger nuts.
(c) Fresh fruit
Methyl bromide residues determined in laboratory studies on fresh
fruits are summarized in Table 32. Studying the effects of fumigation
dose and length of the following aeration periods, Sell & Moffitt
(1990) and Sell et al. (1988) found that desorption of methyl bromide
from apples and cherries, respectively, followed pseudo-first-order
decay curves, the first component resulting from removal of the
pesticide from free air space in the chamber, and the second, from the
desorption from the fruits. Singh et al. (1982) found that methyl
bromide absorption in avocados depended on oil content rather than
skin thickness or protein content.
(d) Milk and cheese
Bromides may be present naturally in cows' milk in amounts up to
8 mg/litre. When cows were fed on grain fumigated post-harvest with
methyl bromide, higher levels of bromide were found in the milk, e.g.,
cows fed grain containing bromide at 220 mg/kg had bromide levels in
their milk of 10-20 mg/litre (Lynn et al., 1963).
Cheese fumigated with methyl bromide showed high bromide residues
(Laug, 1941).
5.1.5 Animal feed
Knight & Costner (1977) reported bromide residues of 6800-8400
mg/kg in hay that was harvested in the spring after the field had been
injected with methyl bromide. The resulting toxic effects on animals
fed with this hay are reported in section 7.3.7.
5.1.6 Other products
The chlorine and bromine contents in tobacco and tobacco smoke
were investigated by Häsänen et al. (1990). Smoke per cigarette
contained 1 µg bromine in the particulate phase and 5 µg bromine in
the gaseous phase. In the gaseous phase, methyl bromide accounted for
80% of the total bromine. Methyl bromide is used widely as a fumigant
in uncured tobacco storage and this can increase the bromine content
in tobacco considerably.
Methyl bromide has been used in growing tobacco seedlings (Ostrec
& Korunic, 1989).
5.1.7 Terrestrial and aquatic organisms
No data are available.
5.2 General population exposure
5.2.1 Food
The general population may be exposed to residues of methyl
bromide and inorganic bromide, and other possible metabolic products,
which may be present in food marketed for consumption. Levels of
methyl bromide and inorganic bromide in food are described in section
5.1.4.
Food commodities containing higher levels of oil and fat, such as
groundnut and rapeseed (Table 28), retain higher amounts of methyl
bromide residues after fumigation, which disappear after a storage
period of about one month (Fairall & Scudamore, 1980; DeVries et al.,
1985). However, higher levels of inorganic bromide residues may remain
in food commodities marketed after fumigation (section 5.1.4.2).
Scheffrahn et al. (1992) investigated the effects of methyl
bromide fumigation on foods in retail packages, in sealed and unsealed
plastic containers, with a view to simulating conditions in which
residues may be found in food products after fumigation of a
residential house in the USA. Fatty commodities in unsealed packages
contained higher residues (>1 mg/kg) than other foods. The fumigant
may have entered the containers by two routes, diffusion through the
air-spaces around the lids (opened peanut butter jar) or in porous
packaging (parmesan cheese in cardboard box), and by permeation into
polyurethane bagged foods. Factory sealed, polyethylene terephthalate
(PETE) containers gave better protection. Vacuum-packed foods in metal
(soup, coffee) or glass (sauce) containers yielded no residues.
Bromide residues in foodstuffs were discussed by Van Leeuwen &
Sangster (1987) in a review of the toxicity of the bromide ion. Total
diet surveys in the United Kingdom in 1978 and 1979 gave a daily
intake of 8.4 mg bromide per person. These values are consistent with
those of two Dutch surveys where average daily intakes of 9.4 and 7.7
mg bromide per person, respectively, were recorded, i.e.,
approximately 3 mg/kg diet (Van Leeuwen & Sangster, 1987).
Table 32. Methyl bromide residues in fresh fruits after fumigation
Fruit Fumigation time Storage Timea Residue Reference
and dose temperature (mg/kg)
(°C)
grapefruit 2 h, 64 mg/litre 24 1 h 26.9b King et al. (1981)
48 h 0.52
peaches 3.5 h, 32 g/m3 2.5 1 day 11.0 Austin & Phillips (1985)
7 days 4.0
mango N.D.c, 64 g/m3 N.D. 1 h < 15.0 Stein & Wolfenbarger (1989)
grapefruit 2h, 48 g/m3 15.6 5 days < 5 King & Benschoter (1991)
oranges, 2h, 48 g/m3 15.6 5 days < 10
mandarines,
tongors
avocado 2h, 32 g/m3 20 1 day up to 0.5d Singh et al. (1982)
2 days up to 0.1
a Time of analysis after end of fumigation period.
b Aeration period: 15 min.
c N.D. = no data given.
d Aeration period: 30 min.
The metabolites resulting from methyl bromide post-harvest
fumigation of a number of crops have been analysed (Starratt & Bond,
1990a,b). Both physically- and chemically-bound residues were
measured. In 7 out of the 9 commodities, over half of the total
residue was chemically bound 1 h after fumigation. These chemically
bound residues were stable for at least 6 months. Most reaction
products were O-, S-, and N-methylated proteins (section 6.3).
Methylation of purines and pyrimidines in the nucleic acids accounted
for 0.1-6 % of the chemically-bound residue, with N being the only
site of methylation. The methylation products identified in this study
are also known to occur naturally.
5.2.2 Drinking-water
In 1988, in California, only one sample out of 43 056 taken from
2977 wells, showed any presence of methyl bromide (detection limit -
1 µg/litre) (CDFA, 1988).
In areas, such as in the Netherlands, where private water
supplies are from shallow wells near methyl bromide soil operations,
there could be increased bromide contents in the water (Van Doorn et
al., 1989).
5.2.3 Human breast milk
There are no data available.
5.2.4 Sub-populations at special risk
People who live in close proximity to greenhouses or to fields or
storehouses that are being fumigated have a higher risk of exposure to
methyl bromide gas than the general population. Similarly, persons
inadvertently, or intentionally, entering buildings following, in
particular, structural fumigation, are at risk.
5.3 Occupational exposure during manufacture, formulation, or use
The number of incidents and fatalities (section 9) show that
occupational exposure, especially during fumigation, is potentially
hazardous.
5.3.1 During manufacture
In a methyl bromide plant in the USA, workplace air
concentrations of 78-116 mg/m3 (20-30 ppm) were recorded using
direct measurement (Evans, 1979).
In a methyl bromide-producing factory in Japan, methyl bromide
concentrations in the worker's breathing zone were usually under 4
mg/m3, but sometimes exceeded 20 mg/m3 (Kishi et al., 1988).
5.3.2 During fumigation
If safety procedures are not followed, workers may be exposed to
methyl bromide accidentally during, or after, fumigation operations.
Methyl bromide in low concentrations is odourless so that a toxic
atmosphere may not be apparent to the worker. Only at higher
concentrations (100 x the actual TLV(R) of 20 mg/m3 (5 ppm) does
it have a sweet smell (Van Den Oever et al., 1982). An odour threshold
of 65 mg/m3 has been reported for methyl bromide (Worthing & Walker,
1983). Therefore, it is usually marketed in the form of 98% methyl
bromide and 2% chloropicrin, as a lacrimatory agent. For post-harvest
fumigation, 100% methyl bromide is used.
The various fumigation techniques used together with methyl
bromide exposure values and methods of application are outlined in
Table 33 (Guillemin et al., 1990).
5.3.2.1 Structural fumigation
In carrying out the general fumigation of a building, sufficient
gas must be liberated into the free space to kill the insects and then
the toxic level maintained for a defined period of time. After the
treatment, the residual gas remaining in the building is dispersed to
the outside atmosphere. However, there are basic differences in
defining structures amongst various countries. In the USA, structural
fumigations mainly involve residential houses whereas, in Europe, they
generally refer to flour mills and food processing areas. The
fumigation procedures and the safety aspects in these circumstances
could be very different.
Methyl bromide exposure levels for structural fumigation workers
in California were measured by Anger et al. (1986). They described the
work involved. "Structural fumigation is conducted by a work crew of
2-4 men who cover the buildings to be fumigated with large vinyl
tarpaulins and connect them by spring clamps. Cylindrical tubes filled
with sand (sand snakes) are placed at the base of the structure to
hold down the tarpaulins, thus sealing the building. After this one to
two hour procedure, termed a closing, the fumigant is introduced into
the unoccupied house via a tube or hose, and the fumigators leave the
site. The fumigators wear self-contained breathing apparatus (SCBA).
The next day the work crew removes the tarpaulins, opens the windows,
and places fans in the house to clear the fumigant. It is in this 30-
to 45-min process, termed the opening, that worker exposures may
occur. Typically, each work crew, led by a State-licensed fumigator
(the "licensee"), conducts three openings and/or closings each day".
Table 33. Integrated samples of methyl bromide in air taken in a survey of methyl bromide
fumigation in Switzerlanda
Circumstances Exposure Sample Range of values
of sampling category No.
Minimum Maximum
mg/m3(ppm) mg/m3(ppm)
1. Space fumigation
- during fumigationb occupational 7 <0.8 (<0.2) 500 (128.4)
- during aerationb occupational 7 2.3 (0.6) 646 (166.0)
- resuming operation para-occupational 47 <0.8 (<0.2) 9 (2.3)
- during fumigation environmental 12 <0.8 (<0.2) 79 (20.4)
- during aeration environmental 12 <0.8 (<0.2) 105 (27.1)
2. Soil fumigation
- during fumigationb occupational 5 2 (0.5) 151 (39.0)
- removal of sheetingb occupational 3 34 (8.8) 144 (36.9)
- inside greenhousec para-occupational 5 12 (0.3) 9 (2.2)
3. Chamber fumigation
- outside chamber para-occupational 3 35 (8.9) 293 (75.3)
- removing contents para-occupational 4 5 (1.2) 17 (4.3)
a From: Guillemin et al. (1990).
b Fumigators generally wore respiratory protection during these operations.
c Greenhouse only partially fumigated; includes post-fumigation soil tillage.
Personal samples from fumigators taken when they entered houses
24 hours after fumigation with 23 000 to 31 000 mg methyl
bromide/m3, indicated that a house might contain 80-2000 mg methyl
bromide/m3 (Table 34). Personal samples taken on fumigators working
outside the houses when they were opened again showed concentrations
of between 0 and 8 mg/m3 in the half-hour periods during the cover
removal (Anger et al., 1986). Area samples taken within 3 and 6 m from
the buildings during the same period ranged from 0 to 31 and 0 to 10
mg/m3, respectively (Anger et al., 1986).
Concentrations of methyl bromide inside flour mills and in the
atmosphere around the mills during, and after, fumigation were
measured by Bond & Dumas (1987). Considerable variations in
concentration were found in buildings of different structure and under
varyious weather conditions. Concentrations ranging from trace amounts
up to 90 mg/m3 (23 ppm) were found in the air around the mills
during the aeration period.
Table 34. Methyl bromide exposure concentrations (mg/m3)
in residential fumigationa
State Fumigators Under Within 3 m of 3-6 m from
licensed working covers house house
fumigator outside house
when inside
house
1875.0 5.1 2.0 46.7 32.3 0 0
75.9 0 1.2 12.5 0 10.1
90.3 0 7.0 0 17.5 10.1
1042.1 3.9 3.1 0 0 0
204.2 8.6 0 0 0
Mean
657.4 3.1 46.7 7.0 3.9
a Adapted from: Anger et al. (1986).
Guillemin et al. (1990) conducted a survey in Switzerland on
exposure during space fumigation. The maximum exposure levels for 7
integrated samples was 646 mg/m3 (166 ppm) during aeration (see
Table 33). Fumigators wore respiratory protection during these
operations. Samples of transient air taken around the buildings not
from any specific spot (distance not given), showed methyl bromide
levels in the range of 0-105 mg/m3 (see Table 33).
5.3.2.2 Soil fumigation
The amount of methyl bromide released into the atmosphere during
soil fumigation depends on the methods used (Table 9), the type and
time of covering (section 3.2.2) and soil type.
(a) Field fumigation
A formulation of 75% methyl bromide was used to kill insects and
nematodes in a strawberry crop, with 25% chloropicrin as a fungicide.
The fumigant injected into the soil produced an equilibrium of 47
000-58 000 mg/m3 which, under the covers, resulted in personal
average exposures ranging from 0 to 24 mg/m3 for the fumigators
(Anger et al., 1986). Personal sample measurements of methyl bromide
in farm workers when removing the film ranged between 0 and 33
mg/m3. Other data showed that the personal exposures of fumigators
and farm workers, who covered the plastic film with earth were between
0 and 29 mg/m3 and 0 and 17 mg/m3, respectively. Exposure of soil
fumigators, about 8 h a day, was relatively constant during most of
the year, whereas farm workers received only occasional exposures when
fields were fumigated. Spot (area) samples, taken before and after
film removal, showed that the airborne concentration under the intact
film was 8950 mg/m3 before removal and 9.3 mg/m3 an hour after
removal.
(b) Greenhouse fumigation
Roosels et al. (1981) compared two methods of methyl bromide
fumigation in greenhouses, i.e., by injection into milled soil
followed by covering with a plastic cover, or, by surface fumigation
by means of plastic pipes under a plastic cover. Two different
formulations (70 % methyl bromide/30 % chloropicrin and 98 % methyl
bromide/1-2 % chloropicrin) were used and the concentrations of methyl
bromide in the air were measured by GC-FID. During injection into
soil, values of between 400 and 4000 mg/m3 (100 and 1000 ppm) were
found with peaks up to 12 000 mg/m3 (3000 ppm) and, in one case, up
to 40 000 mg/m3 (10 000 ppm). However, when preventive measures were
taken, values of 800 mg/m3 (200 ppm) were obtained. During
fumigation, concentrations ranged between 400 and 4000 mg/m3 (100
and 1000 ppm). Using the piped surface fumigation method,
concentrations around the treated area were between 320 and 3200
mg/m3 (80 and 800 ppm) with short-term exposures of the operators to
8000-12 000 mg/m3 (2000-3000 ppm) when the pipes were being
connected.
In another investigation, concentrations of 60-100 g methyl
bromide/m2 were applied under a polyethylene cover (Van Den Oever et
al., 1982). Depending on local ventilation, quite a lot of gas escaped
into the surrounding atmosphere. The concentration during application
varied from 117 to 11 700 mg/m3 (30 to 3000 ppm). Concentration in
the air declined with time to 16 mg methyl bromide/m3 (4 ppm) five
days after application. Removing the plastic sheet involved exposure
to peak values as high as 800 mg/m3 (200 ppm), for a few seconds. On
the ninth day after application, milling the soil exposed workers to
up to 60 mg/m3 (15 ppm); on the eleventh day, no methyl bromide was
detected in the air.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
6.1.1.1 Animal studies
Uptake of methyl bromide was investigated in male Fischer 344
rats by Andersen et al. (1980). Following whole body exposures to
recirculated atmospheres of 390-11 640 mg/m3 (100-3000 ppm), the
uptake (i.e., disappearance from the atmosphere) was rapid and
exhibited first-order kinetics without a saturable component, the rate
constant being 0.44 kg-1 h-1. This rate constant was later
recalculated as 0.55 kg-1 h-1 by Gargas & Andersen (1982).
Medinsky et al. (1985) carried out a nose-only inhalation of 50,
300, 5700, or 10 400 nmol (given as 6.2-1206 mg/m3) of [14C]
methyl bromide/litre of air for 6 h in male F344 rat (5 animals/
group). The results indicated that, at low concentrations (50-300
nmol/litre), about 50% of the inhaled material was absorbed. At 5700
nmol/litre, only 37% was absorbed and, at 10 400 nmol/litre, only 27%.
The same amount of methyl bromide (650 µmol/kg body weight) was
absorbed at the two higher exposure concentrations. At 10 400
nmol/litre, the total volume inhaled by the rats was reduced (Medinsky
et al., 1985).
Raabe (1986) found about 40% uptake of inhaled methyl bromide in
studies on beagle dogs. These results are compared in Fig. 6 with
those from rats (Medinsky et al., 1985) and those from human
volunteers (Raabe, 1988), described below.
6.1.1.2 Human studies
An inhalation study was carried out to determine systemic uptake
of low concentrations of methyl bromide from air during nasal or oral
breathing (Raabe, 1988). Two male and two female volunteers inhaled
about 0.1 mg 14C-labelled methyl bromide/m3 (25 ppb), once through
the nose and once through the mouth. The uptake (% of methyl bromide
inhaled) was 55.4% nasally and 52.1% orally.
6.1.2 Dermal
Exclusively dermal exposure has only been observed in human incidents
(section 9). There are no data dealing exclusively with dermal
exposure in animals.
6.1.3 Oral
Methyl bromide (75 or 100 mg/kg) was administered to rats in olive oil
by gavage (Miller & Haggard, 1943). The methyl bromide entered the
blood stream with only a moderate degree of hydrolysis in the
intestine.
5.1.1.5 Release of methyl bromide to the outside air from greenhouses
During 1986, 400 000 kg methyl bromide were emitted into the air
in the Westland area of the Netherlands (Van Doorn et al., 1989).
Although soil disinfection is only allowed when the diluting capacity
of the atmosphere (wind force) is sufficient, this is very difficult
or impossible to control. Investigations by Netherlands Institutes
(TNO and RIVM) showed that the hourly average concentrations within a
20-m distance from the green-houses in 1981 (after the introduction of
gas-tight film) during the first hours was 5.9 mg/m3 after
fumigation with methyl bromide at a dose of 700 kg/ha. In 1982 and
1983, a few hours after injection and at a probable dosage of 400
kg/ha, concentrations measured ranged between 1 and 4 mg/m3 at
distances of 20 m. After the first few hours, the concentration of
methyl bromide decreased rapidly and, after 4 days, also at 20 m,
hourly averages of 0.2 mg/m3 were measured. Ten days after
fumigation, the covering foil was removed, after which the hourly
average concentration increased to a maximum of 0.4 mg/m3 (Van Doorn
et al., 1989).
5.1.2 Water
5.1.2.1 Seawater
In 1975, samples of seawater from near the shore at Dorset,
England were analysed for halomethanes and methyl bromide was detected
at levels ranging from 2.0 to 3.9 x 10-9 ml gas/ml water [apprx. 10
ng/litre] (Lovelock, 1975).
Average concentrations of 1.2 ng methyl bromide/litre were
measured in surface seawater in the Eastern Pacific Ocean (Singh et
al., 1983). The authors estimated that the oceans are supersaturated
with methyl bromide to 250%.
Khalil et al. (1993) measured methyl bromide concentrations in
ocean water in two open-ocean surveys, one covering latitudes from 45°
N to about 30° S, the other from 67° N to 50° S. On the first survey,
they found supersaturation of methyl bromide at 180% and, on the
second, 140%.
5.1.2.2 Inland waters
In 1988, the California Department of Food and Agriculture (CDFA)
reported the results of analyses of 43 056 well water samples taken
from 2977 wells in various Californian counties. Residues of 10
chemicals were detected. Methyl bromide detection was undertaken in 32
counties, but in all the wells tested only one sample showed the
presence of methyl bromide (CDFA, 1988).
5.1.2.3 Waters around greenhouses
In the Netherlands, where intensive horticulture in greenhouses
is practised, the soil level is lower than sea level in some areas,
with the result that water transport is very slow and the static water
volume is very high. There is a high density of greenhouses and all
holdings have to use the water from ditches as leaching water, thus,
this water is successively loaded with bromide. However, it should be
noted that, in 1992, methyl bromide was banned in the Netherlands for
soil fumigation purposes.
The concentrations of methyl bromide and bromide-ion were
measured in irrigation water, drainage water, and surface water during
the leaching periods in two Netherlands glasshouse soils after
fumigation with methyl bromide (Wegman et al., 1981). Maximum
concentrations in drainage water, determined within 24 h of the start
of leaching, were 9.3 mg/litre (methyl bromide) and 72 mg/litre
(Br-).
Further studies of bromide ion concentrations in precipitation,
surface water, and ground water in the polder district in the
Netherlands (a main horticultural area) in 1979-80 gave maximum values
of 0.98, 41, and 17 mg/litre respectively, the highest concentrations
being found during the main fumigation/leaching time in
September-October 1979 (Wegman et al., 1983). During these few weeks,
an estimated 1.78 million kg methyl bromide (about 1.5 million kg
bromide ion) were used for soil fumigation. From their measurements,
Wegman et al. (1983) calculated that 14% of the applied methyl bromide
was converted to bromide ion. This is in agreement with Daelemans
(1978), whose studies showed that 10-30% of methyl bromide is
converted to Br-. The fumigation method used was the "hot gas
method", whereby the fumigant was discharged under thin low-density
polyethylene (LDPE) sheeting. Subsequently, in June 1981, the use of
LDPE for this purpose was forbidden.
In 1985, bromide concentrations of 10-35 mg/litre were observed
in Westland (the Netherlands) surface water with maximum
concentrations in the Poel and Bosch polders of 38.7 and 31.7
mg/litre, respectively (Van Doorn et al., 1989). Private water
supplies from shallow pumps in the Netherlands near methyl bromide
soil operations were expected to have increased bromide contents (Van
Doorn et al., 1989). In 1986, in the Westland, 260 000 kg bromide
ended up in surface water, at an average dosage of 700 kg/ha.
Similar investigations were carried out by Vanachter et al.
(1981) in the glasshouse crop growing region of Malines - Antwerp
(Belgium) into bromide concentrations in surface water in periods of
intense soil fumigation and leaching (August-September) compared with
periods before and after this, when soil fumigation and leaching were
less frequent. Sampling was also carried out during leaching, near the
greenhouses, as well as in the ditches and draining water. For
comparison, samples of natural Br- concentrations in surface water
were taken from a region where methyl bromide fumigation was not
carried out.
The results showed that during the leaching period,
concentrations of Br- in the surface water in the greenhouse crop
growing area were significantly higher (maximum 9.6 mg/litre) but,
five weeks later, were either not detectable (<0.1 mg/litre) or very
low (1.59 mg/litre) in little brooks. The bromide concentration in
rivers was less than 0.8 mg/litre, except for one site, where either
sea water or the effluents from a local photographic plant resulted in
concentrations of up to 4.5 mg/litre. In the direct drainage area of
the glass houses, transient concentrations of up to 33 mg/litre were
measured, decreasing rapidly within a few days.
Guns (1989) measured groundwater levels in four greenhouses in
Belgium from September 1986 to August 1988. The bromide content of
groundwater depended, not only on the length of leaching, but also on
the type of soil. When the deeper soil layers contained more clay, the
concentration of the bromide ion in the groundwater, after fumigation
and leaching, was still relatively high (48 mg/litre before and 280
mg/litre after fumigation in one study).
5.1.3 Soil
The natural bromide present in unfumigated soil depends on the
soil type. Van Wambeke et al. (1974) gave values of 3.3 mg/litre for
fresh sandy loam soil and 2.5 mg/litre for peat soil. Hoffman &
Malkomes (1974) stated that the natural concentration of bromide in
the soil was less than 10 mg/kg, depending on the type of soil and
geographical situation.
Fallico & Ferrante (1991) measured bromide concentrations in
greenhouse soil before, and after, the application of methyl bromide
(80 g/m2). Before fumigation, bromide levels were about 5 mg/kg. Two
months after treatment, bromide levels of over 30 mg/kg were measured.
After a further 3 months, levels had decreased to less than 10 mg/kg.
Bromide ion concentrations following greenhouse and soil
fumigation depend on the dosage, exposure time, aeration period,
temperature, the type of soil, the amount of rain or leaching water,
and the type of covering (sealing conditions). A year after fumigation
with 70-80 g methyl bromide/m3, bromide values of 0.2-11.5 mg/kg
were recorded in the upper 30 cm of soil (Basile et al., 1987).
The bromide remaining in the soil after fumigation can affect
soil fertility (Rovira & Ridge, 1979). As wheat particularly tends to
concentrate bromine, soil and wheat dynamics were studied (1981-83) in
methyl bromide-fumigated plots in a Mediterranean climate (Italy).
Bromide residues ranged between 5 and 10 mg/kg in the fumigated soil
to a depth of 50-60 cm. The total amount of bromide in the soil was
5.8 g/m2 up to a depth of 1 m and remained almost constant during
the wheat-growing period (Fransi et al., 1987). The amount of bromide
residues was about 8 % of that applied (900 kg/ha) five months
previously, compared with the 20 % found by Van Wambeke et al. (1974)
in similar soils.
5.1.4 Food
When considering the published levels of methyl bromide and
inorganic bromide in various foods, the method of analysis is
important (section 2.4.9). Originally, bromide content only was
measured and there is very little information on the methyl bromide
content. Measurements of both entities are important as well as other
residual products.
5.1.4.1 After soil fumigation
Bromide accumulation in plants depends on various factors, such
as the physical and chemical properties of the soil, the climatic
condition, the plant species and particular tissues, and the cultural
practices (Basile & Lamberti, 1981). Moreover, it depends on dosage,
exposure time, and aeration.
A study was carried out in 1980 in Metaponto, in southern Italy,
where methyl bromide was used to control nematodes and other plant
pathogens in the soil (Basile & Lamberti, 1981). Bromide ion levels
were 20-51 mg/kg (tomatoes), 8-44 mg/kg (string beans), 25-149 mg/kg
(radishes), 18-60 mg/kg (aubergines), 6-165 mg/kg (cucumbers), 13-46
mg/kg (courgettes), and 3-27 mg/kg (peppers) on a fresh weight basis.
In experimental plot conditions, the methyl bromide concentration was
60 g/m2 under a plastic cover with the soil temperature 14-17 °C at
10 cm depth. The covering was removed after 2 days and the plot
rotavated at a depth of 20 cm to eliminate residual gases.
Roughan & Roughan (1984a,b) carried out surveys of bromide ion
residues in lettuces, cucumbers, tomatoes, and self-blanching celery
grown in soil fumigated with methyl bromide, and compared these values
with those in a range of home-produced and imported fruit and
vegetables. Lettuce grown on unfumigated soil contained less than 10
mg bromide ion /kg, while most lettuces harvested from methyl bromide
fumigated soil were found to contain considerably more, 30 %
containing over 500 mg/kg and 2 % even in excess of 2000 mg/kg
(Roughan & Roughan, 1984a).
Studies on vegetables grown under protective covers on soil
previously fumigated with methyl bromide showed levels ranging from 1
to 109 mg/kg (fresh weight) in 29 late-season cucumbers, 5 to 326
mg/kg in 242 tomato samples, and 2 to 521 mg/kg in 38 samples of
celery (Roughan & Roughan, 1984b); 65 % of the late season cucumbers
contained more than background of bromide levels up to 10 mg/kg. Crops
of tomatoes (summer 1981) grown on sites where methyl bromide
fumigation had taken place in 1980/1981, contained considerably higher
levels than those grown on a site fumigated in 1979. In a survey from
retail outlets (January-November 1979), tomatoes and cucumbers from
the Canary Islands and Spain generally contained less than 10 mg/kg
(fresh weight). Of those grown in the United Kingdom, levels exceeded
10 mg/kg in 35 %, and 100 mg/kg in 5 %, ranging up to 177 mg/kg. These
higher levels of bromide ion could be attributed to the plants having
been grown on soil fumigated with methyl bromide prior to planting
(Roughan & Roughan, 1984b). The total bromide ion contents of various
crops obtained from retail outlets in the United Kingdom during the
period June 1981 to July 1982 are summarized in Table 22. The survey
indicated that vegetables, such as tomatoes, celery, cucumbers,
lettuce, radishes, and aubergines, grown in United Kingdom and the
Netherlands contained higher levels than elsewhere. Other crops had
mean bromide levels of less than 10 mg/kg, similar to other figures
published for background bromide ion content.
Table 22. Total bromide ion content of various crops obtained from retail
outlets in United Kingdom during the period June 1981 to July 1982a
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
apple France 5 0.1-0.3 0.2
aubergine Netherlands 4 2-23 11
avocado pear not known 1 1
banana Windward Islands 2 2 2
bean, broad England 3 1-2 2
bean, french Guernsey 1 1
bean, runner England 2 1 1
bean, sprouts England 3 1 1
cabbage England 3 1-2 2
calebrese England 2 1 1
(broccoli)
carrot England 1 2
cauliflower England 3 0.3-1 1
Chinese England 3 1-2 1
leaves
celery England 12 1-178 28
Guernsey 1 9
Israel 4 7-14 10
Spain 16 2-8 4
USA 1 4
courgette England 3 1-3 2
cucumber Canary Islands 15 0.3-10 3
England 36 0.2-87 9
Spain 3 0.2-10 4
Netherlands 7 0.1-14 7
grapefruit
segments Cyprus/Israel,
S. Africa 4 0.1-0.4 0.3
green pepper Netherlands 7 0.4-5 2
ettuce Belgium 1 5
Cyprus 5 1 1
England 69 1-241 15
France 9 0.2-19 4
Israel 6 1-4 2
Spain 11 0.4-4 2
Netherlands 26 2-57 21
USA 12 0.1-2 1
Table 22 (continued)
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
marrow England 2 1-2 1
mushroom England 62 0.2-24 1
onions Israel,
Netherlands 3 0.4-1 1
onion, England 3 2-4 3
spring
orange S. Africa, Spain 6 <0.1-0.4 0.2
segments
pea England 4 1-3 2
potato Cyprus, England 3 1-2 1
radish England 18 0.2-3 1
Israel 2 5 5
Netherlands 12 0.1-48 13
USA 1 1
strawberry England 1 0.3
tomato Canary Islands 8 1-5 4
England 33 1-70 13
Spain 15 1-7 3
Netherlands 14 1-39 11
a From: Roughan & Roughan (1984b).
Brown et al. (1979) measured the bromide concentration in several
plant species in unfumigated and methyl bromide-fumigated plots in
California (Table 23). Many plants showed increased bromide
concentrations. Strawberries and grapevines absorbed relatively
little. As the interval increased between soil fumigation and
planting, there was a general decline in bromide levels, though the
interval could be as long as three years before the crops returned to
a level of about 10 mg/kg (Brown et al., 1979; Roughan & Roughan,
1984a). Table 24 shows bromide concentrations in plant material 1, 2,
3, and 4 years after fumigation with methyl bromide.
Fallico & Ferrante (1991) measured bromide levels in tomatoes in
crops grown in soil that had been fumigated with methyl bromide ten
days prior to planting. Bromide levels in tomatoes grown in the
treated soil and harvested after 60 days were about double (55 mg/kg)
those in tomatoes grown in untreated soil. The bromide levels
decreased with each successive harvest, but were still higher than
those in control plants after the fourth harvest.
Table 23. Bromide concentrations in several plant species in unfumigated and methyl bromide-fumigated plotsa,b
Plant species Treatment Range of Average bromide S.E. of
and part bromide concentration means
concentrations (mg/kg dry weight)
(mg/kg dry weight)
barley control 4 to 575 106 (19)c 38
(whole top) fumigated 120 to 5235 1788 (23) 373
bur clover control 1 to 407 96 (11) 40
(whole top) fumigated 196 to 2371 1334 (14) 155
filaree control 4 to 546 135 (14) 47
(whole top) fumigated 718 to 7380 2600 (10) 683
wild oats control 9 to 876 196 (14) 66
(whole top) fumigated 1233 to 5034 3364 (11) 441
spinach (leaves) fumigated 1772 to 3195 2521 (4) 387
ryegrass fumigated 1481 to 2790 2378 (4) 302
sweet potato
(leaves)
1st sampling fumigated 640 to 923 753 (4) 69
4th sampling fumigated 312 to 372 330 (4) 14
sweet potato
(root) fumigated 204 to 237 220 (2) 17
strawberry control 14 to 129 63 (9) 16
(leaves)d fumigated 3 to 372 88 (36) 13
grape (leaves)e control 1 to 101 28 (90) 3
fumigated 1 to 402 48 (278) 4
Table 23 (continued)
a From: Brown et al. (1979).
b These data represent samples collected the first year after fumigation with MeBr at rates of 34-68 g/m2,
except for grape leaves.
c Numbers in parentheses refer to the number of samples in the average.
d Strawberry leaves were collected after 1-6 annual fumigations with methyl bromide.
e Grape leaves were collected 2-4 years after fumigation with methyl bromide.
Table 24. Bromide concentrations in plant material (mg/kg dry
weight), 1, 2, 3, and 4 years after fumigation with methyl bromidea
County Untreated Years after fumigation
controls
1 2 3 4
Sonoma 94(19)b 3018(16) 360(15) 516(8) 12(3)
Napa 163(21) 1476(15) 742(10) 430(2)
a From: Brown et al. (1979).
b Numbers in parentheses = number of samples included in the
average.
Table 25. Inorganic bromide residues detected in samples of fruit and
vegetables in UK (1988 to 1989)a
Commodity Concentration Number of samples
range (mg/kg)c
Lettuces, produced n.d. 0
in United Kingdom
(proposed CAC 1-20 48
MRLb=100) 21-100 15
101-300 9
301-529 5
Lettuces, imported n.d. 0
1-20 21
21-45 3
Rice, imported n.d. 59
[MRL=50 1-10 37
(unprocessed 11-20 16
rice)] 21-50 14
50-100 10
121 and 124 2
Nuts (no MRL)
almonds n.d. 4
1-147 24
brazil nuts n.d. 0
3-140 22
Table 25 (continued)
Commodity Concentration Number of samples
range (mg/kg)c
cashew nuts n.d. 16
3-53 5
chestnuts n.d. 5
3-23 3
coconuts n.d. 0
2-6 5
hazel nuts n.d. 7
1-194 10
peanuts n.d. 4
2-109 18
pine nuts n.d. 4
46 1
Nuts (continued)
pistachio nuts n.d. 0
3-104 4
tiger nuts n.d. 0
17-20 4
walnuts n.d. 3
2-210 21
sesame seed n.d. 5
17-56 5
sunflower seed n.d. 3
2 6
3 1
Dried fruits
currants, imported
(CAC MRL = 100) n.d. 22
1-3 9
6-15 5
sultanas n.d. 20
(CAC MRL = 100) 1-6 6
6-12 7
Table 25 (continued)
a From: MAFF (1990).
b CAC = Codex Alimentarius Commission; MRL = maximum residue limit
(mg/kg) legally permitted in, or on, food commodities or animal feed.
c n.d. = not detected.
The results of a further survey (1988-89) in the United Kingdom
of inorganic bromide levels in fruit and vegetables are summarized in
Table 25. Fourteen samples of lettuce produced in the United Kingdom
exceeded the Codex Alimentarius Commission maximum residue limit (CAC
MRL) for inorganic bromide of 100 mg/kg (MAFF, 1990).
The effects of leaching following soil fumigation with methyl
bromide up to 100 g/m2 are shown in Table 26. At sites with low soil
bromide residues, the resulting bromide residues in lettuce were
nearly unaffected by leaching. At other sites, there was a positive,
but diminishing, response to increasing rates of leaching, very high
residues probably being due to high levels of organic matter (Smart,
1990).
Recent monitoring of samples of individual crops having a
likelihood of being grown on soil fumigated with methyl bromide showed
that only a small percentage contained residues above Codex
Alimentarius Commission recommended limits. The high levels were
mainly in lettuce. The report of Smart (1990) showed that the
proportion of samples having high residues had declined since the late
1970s, when, in some countries, residues in lettuce were as high as
500-1000 mg/kg. Leaching of treated soils, attention to timing of
application, and integrated pest control have all helped to reduce
such residue levels.
Bromide level tolerances for a variety of methyl bromide
fumigated raw agricultural commodities in the USA are shown in Table
27. However, according to the US EPA, because of its existing
toxicological data base and its environmental ubiquity, inorganic
bromide is not of toxicological concern. Requirements for residue data
to support existing inorganic bromide tolerances were waived by the
Agency (US EPA, 1989).
Table 26. Bromide residues in lettuce grown under protection of soil fumigated with bromomethane and leached with water
before planting in the United Kingdoma
Site Soil texture Soil organic Number of Interval between Bromide residues in lettuce (mg/kg fresh weight)
matter (%) applications planting and for the water application rates (mm)
in previous harvest
years (weeks) 0b 100 200 300 400
A fine sandy loam 4 2 16 81 102 92 150 142
B sandy loam 12 1 12 62 70 232 93 114
C sandy loam 13 2 13 307 215 98 157 117
D sandy loam 1 24 765 427 392 284 250
E sandy loam 8 4 21 676 335 328 164 134
Fc loamy peat 51 4 14 1958 1534 1001 - -
Mean sites A-E 378 230 228 170 151
a Summarized from Food and Agriculture Organization (1985) by Smart (1990).
b Residue figures for crops grown in soils not having any leaching.
c At F, the leaching treatments were applied after planting the lettuce crop.
Table 27. USA tolerancesa
Br in, or on, the following raw agricultural commodities,
which have been fumigated with the antimicrobial agent
and insecticide methyl bromide after harvest (with the
exception of strawberries) (mg/kg)
corn (pop) 240
almonds, brazil nuts, bush nuts, 200
butternuts, cashews, chestnuts,
cottonseed, filberts, hickory nuts,
peanuts, pecans, pistachio nuts,
soybeans, walnuts
asparagus, copra, cumin (seed), 100
ginger (roots), pomegranates
avocados, coffee beans, potatoes, 75
sweet potatoes
alfalfa (hay), barley, beans, 50
beans (green), benas (lima), beans
(snap), cabbage, cippolini (bulbs),
cocoa beans, corn, corn (sweet),
garlic, oats, peas, peas (blackeyed),
rice, rye, sorghum (grain), timothy
(hay), wheat
artichokes (Jerusalem), garden beets 30
(roots), sugar beets (roots), carrots,
citrus citron, cucumbers, grapefruit,
horseradish, kumquats, lemons, limes,
okra, oranges, parsnips (roots), peppers,
pimentos, radishes, rutabagas, salsify
(roots), squash (summer), tangerines,
turnips (roots)
apricots, blueberries, cantaloupes, 20
cherries, eggplant, grapes, honeydew
melons, mangoes, muskmelons, nectarines,
onions, papayas, peaches, pineapples,
plums, pumpkins, squash (winter), squash
(zucchini), tomatoes, watermelons
apples, pears, quinces 5
a From: US EPA (1988b; CFR 180.124).
5.1.4.2 After post-harvest fumigation
Methyl bromide is widely used as a post-harvest fumigant to kill,
or prevent, pest infestation. In 1976, around 100 000 metric tonnes of
food commodities were treated with methyl bromide in the United
Kingdom (Fairall & Scudamore, 1980).
Fairall & Scudamore (1980) measured methyl bromide residues in
dried milk, wheat, flour, rapeseed, and groundnut samples after store
fumigation (see Table 28). Products such as groundnuts and rapeseed
retained higher amounts of methyl bromide. No methyl bromide was
detected in any commodity after storage for 1 month (detection limit
10 µg/kg).
DeVries et al. (1985) measured the rate of decrease of methyl
bromide in wheat, flour, cocoa, and peanuts after fumigation with the
gas. Samples were analysed immediately and then after various time
intervals of exposure of the sample to air. The methyl bromide
concentration decreased very rapidly in all cases, no residual methyl
bromide being found in any of the samples after 2 weeks (detection
limit, 0.4 µg/kg).
(a) Wheat and cereals
Pesticide residues in home-grown and imported wheat were measured
in the United Kingdom by Osborne et al. (1989); 45 samples were
analysed for methyl bromide (method: Fairall & Scudamore, 1980) and
inorganic bromide. No methyl bromide was found in excess of the
detection limit (0.01 mg/kg); all samples contained inorganic bromide,
but at levels of 4 mg/kg or less, which is given as the level
naturally present in wheat as a result of uptake from the soil (Heuser
& Scudamore, 1970; Osborne et al., 1989).
Trials carried out on a commercial scale showed that CT
(concentration x time) products for methyl bromide generally lay in
the range of 50-2000 mg.h/litre (Scudamore, 1987). The higher values
are usually found in pockets of grain at the bottom of silos or bins.
Scudamore (1987) recommended careful monitoring, especially at
increased temperature and moisture content, when treating more
sorptive cereals, such as oats or maize, or those with which methyl
bromide reacts more readily. In three samples of wheat from different
parts of the United Kingdom, bromide levels increased between 2 and 3
times over a range of 11-16 % moisture content.
Table 28. Methyl bromide residue levels (mg kg-1) during storagea
Days
Commodity 0 0.04 0.25 1 2 4 7 11
dried milk 0.5 0.15 0.03 0.006 - - - -
wheat 0.8 0.42 0.33 0.08 0.04 n.d.b - -
flour 0.28 0.09 0.04 0.02 n.d. - - -
rapeseed 4.2 3.0 1.5 - 0.95 0.5 0.10 0.08
groundnuts 7.7 4.5 3.2 2.6 1.6 0.38 0.38 0.03
a From: Fairall & Scudamore (1980).
b n.d. = none detected.
Table 29. Bromide ion and methyl bromide concentrations (mg/kg) in
flours exposed to methyl bromide only in silos and in pastas obtained
from these fumigated and unfumigated floursa
Material Treatment Number of Bromide ion Methyl
samples (mean±S.D.) bromideb
flours unfumigated 3 1.26±0.03 n.d.
fumigated 3 1.60±0.11 n.d.
pasta from unfumigated 6 1.24±0.13 n.d.
(macaroni) flours
from fumigated 6 1.91±0.22 n.d.
flours
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than the detection limit of 0.01
mg/kg.
Stacks of bags containing stored grains and pulses (wheat,
lentils, maize, barley, chick-peas, peas, and sorghum) were covered
with PVC sheets and exposed to methyl bromide fumigation for 48 h
(Urga, 1983). Following aeration, residues of less than 50 mg/kg
bromide were measured, with the exception of 60 mg/kg after 24 h
aeration and 59 mg/kg after 36 h aeration. Cova et al. (1986)
investigated the effects of exposure to methyl bromide in flour, and
pastas made from it. Flour was exposed to methyl bromide in silos
(conditions: mean temperature - 18 °C, concentration - 24 g/m3,
duration of treatment - 68 h, duration of ventilation - 3 days). Table
29 shows levels of bromide ion before, and after, fumigation. Methyl
bromide could not be detected. In a second experiment (summarized in
Table 30) on pasta made from unfumigated flour, rice flour, and white
flour, Cova et al. (1986) examined the influence of the type of
packaging on the effects of fumigation. Every item was packed in two
different ways, i.e., a cardboard box or a transparent envelope made
of a double layer of polypropylene. The products were fumigated in a
closed room under the conditions given above, and the mean bromide ion
concentrations ranged between 2.03 mg/kg (pasta) and 46.23 mg/kg (egg
pasta), while methyl bromide was not detected.
Table 30. Bromide ion and methyl bromide concentrations (mg/kg) in unfumigated
and fumigated foodstuffs, treated in their retail packagingsa
Material Treatment Number of Bromide ion Methyl
samples (mean ± S.D.) bromideb
rice unfumigated 3 0.72 ± 0.06 n.d.
funfumigated 3 10.63 ± 0.67 n.d.
flour unfumigated 3 3.17 ± 0.09 n.d.
funfumigated 3 6.66 ± 0.01 n.d.
white flour unfumigated 3 1.22 ± 0.15 n.d.
funfumigated 3 4.19 ± 0.82 n.d.
pasta (macaroni) unfumigated 6 2.40 ± 0.26 n.d.
funfumigated 6 2.60 ± 0.27 n.d.
pasta (spaghetti) unfumigated 6 1.92 ± 0.17 n.d.
funfumigated 6 2.03 ± 0.07 n.d.
pasta with eggs unfumigated 6 4.13 ± 0.12 n.d.
funfumigated 6 46.23 ± 1.57 n.d.
pasta with eggs unfumigated 6 4.62 ± 0.31 n.d.
and spinach funfumigated 6 39.00 ± 0.01 n.d.
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than detection limit of 0.01 mg/kg.
(b) Spices, nuts, and dried fruits
In the USA in 1980, two warehouses containing imported spices
were fumigated to eradicate a khapra beetle infestation. Methyl
bromide and inorganic bromide residues were determined in the 52
spices before, and after, fumigation (using GC-ECD). In addition, an
ashing titration method for bromide ion residue was used, allowing a
comparison of the two analytical methods (Reeves et al., 1985). Before
fumigation, the highest methyl bromide residue was in parsley (14.9
mg/kg). Seventy-two hours after fumigation with 100 g/m3 for 12 h,
samples were collected and analysed. The highest methyl bromide
residue was found in sage (65.8 mg/kg). Levels of inorganic bromide
residues before fumigation (GC-ECD) were all lower than 200 mg/kg;
after fumigation, only two samples contained higher levels.
In a Canadian study, a number of spices, seeds, nuts, and dried
fruits and vegetables, including samples of celery, mustard, sesame,
coriander, pumpkin and sunflower seeds, cloves, peppercorns, dates,
figs, prunes, raisins, beans, minced onion, a vegetable mix, walnuts,
and peanuts were analysed for methyl bromide residues (Page & Avon,
1989). Of the 30 samples, only a sample of pumpkin seeds was found to
contain methyl bromide (3 µg/kg). Fifty-one chocolate and grain-based
products were also analysed and found not to contain any methyl
bromide.
In contrast, when samples of food known to have been treated with
methyl bromide were analysed, of the 60 samples, 16 contained residues
>1 ng/kg and 5 contained >100 ng/kg methyl bromide. These samples
included dried currants (2.9 mg methyl bromide/kg), chocolate-covered
nuts (0.66 and 0.19 mg/kg), and rice (2.3 mg/kg) and maize (0.21
mg/kg) flours. These findings were confirmed by mass spectrometry
(Page & Avon, 1989).
Bromide residues after methyl bromide fumigation were determined
in samples of dried fruits, cereals, nuts, and spices imported into
New Zealand in 1977 and early 1978 (Love et al., 1979). About one-half
of the nut and spice samples contained total bromide levels exceeding
50 mg/kg, and occasional high levels of bromide residues were found in
cereals.
Fairall & Scudamore (1980) showed that rapeseed and groundnut
samples retained higher amounts of methyl bromide than other
foodstuffs after store fumigation (Table 28). A survey of retail nuts,
seeds, and nut products in October 1984 (Table 31) showed that levels
of methyl bromide in some of these products were higher than the Codex
Alimentarius Commission guideline (MAFF, 1989).
Table 31. Residues of methyl bromide in samples of nuts, seeds, and nut productsa,b
Number of samples Residue concentrations
(mg/kg)
tested containing rangec mean
residues
almonds 5 2 n.d. to 0.06 < 0.02
brazil nuts 4 0 n.d. -
dried chestnuts 1 1 0.2 -
hazelnuts 4 0 n.d. -
mixed nuts 3 0 n.d. -
nuts and raisins 3 2 n.d. to 0.2 0.08
peanuts 12 9 n.d. to 0.3 0.06
walnuts 7 4 n.d. to 2.2 0.4
othersd 6 0 n.d. -
a From: MAFF (1989).
b 45 samples were obtained during October 1984. and analysed for
residues of methyl bromide. The CAC (1986) guideline level for
methyl bromide in nuts is 0.01 mg/kg.
Table 31 (continued)
c n.d. = not detected (the limit of determination was 0.02 mg/kg).
d One sample each of cashews, pecans, pine kernels, pistachios, sunflower seeds, and
tiger nuts.
(c) Fresh fruit
Methyl bromide residues determined in laboratory studies on fresh
fruits are summarized in Table 32. Studying the effects of fumigation
dose and length of the following aeration periods, Sell & Moffitt
(1990) and Sell et al. (1988) found that desorption of methyl bromide
from apples and cherries, respectively, followed pseudo-first-order
decay curves, the first component resulting from removal of the
pesticide from free air space in the chamber, and the second, from the
desorption from the fruits. Singh et al. (1982) found that methyl
bromide absorption in avocados depended on oil content rather than
skin thickness or protein content.
(d) Milk and cheese
Bromides may be present naturally in cows' milk in amounts up to
8 mg/litre. When cows were fed on grain fumigated post-harvest with
methyl bromide, higher levels of bromide were found in the milk, e.g.,
cows fed grain containing bromide at 220 mg/kg had bromide levels in
their milk of 10-20 mg/litre (Lynn et al., 1963).
Cheese fumigated with methyl bromide showed high bromide residues
(Laug, 1941).
5.1.5 Animal feed
Knight & Costner (1977) reported bromide residues of 6800-8400
mg/kg in hay that was harvested in the spring after the field had been
injected with methyl bromide. The resulting toxic effects on animals
fed with this hay are reported in section 7.3.7.
5.1.6 Other products
The chlorine and bromine contents in tobacco and tobacco smoke
were investigated by Häsänen et al. (1990). Smoke per cigarette
contained 1 µg bromine in the particulate phase and 5 µg bromine in
the gaseous phase. In the gaseous phase, methyl bromide accounted for
80% of the total bromine. Methyl bromide is used widely as a fumigant
in uncured tobacco storage and this can increase the bromine content
in tobacco considerably.
Methyl bromide has been used in growing tobacco seedlings (Ostrec
& Korunic, 1989).
5.1.7 Terrestrial and aquatic organisms
No data are available.
5.2 General population exposure
5.2.1 Food
The general population may be exposed to residues of methyl
bromide and inorganic bromide, and other possible metabolic products,
which may be present in food marketed for consumption. Levels of
methyl bromide and inorganic bromide in food are described in section
5.1.4.
Food commodities containing higher levels of oil and fat, such as
groundnut and rapeseed (Table 28), retain higher amounts of methyl
bromide residues after fumigation, which disappear after a storage
period of about one month (Fairall & Scudamore, 1980; DeVries et al.,
1985). However, higher levels of inorganic bromide residues may remain
in food commodities marketed after fumigation (section 5.1.4.2).
Scheffrahn et al. (1992) investigated the effects of methyl
bromide fumigation on foods in retail packages, in sealed and unsealed
plastic containers, with a view to simulating conditions in which
residues may be found in food products after fumigation of a
residential house in the USA. Fatty commodities in unsealed packages
contained higher residues (>1 mg/kg) than other foods. The fumigant
may have entered the containers by two routes, diffusion through the
air-spaces around the lids (opened peanut butter jar) or in porous
packaging (parmesan cheese in cardboard box), and by permeation into
polyurethane bagged foods. Factory sealed, polyethylene terephthalate
(PETE) containers gave better protection. Vacuum-packed foods in metal
(soup, coffee) or glass (sauce) containers yielded no residues.
Bromide residues in foodstuffs were discussed by Van Leeuwen &
Sangster (1987) in a review of the toxicity of the bromide ion. Total
diet surveys in the United Kingdom in 1978 and 1979 gave a daily
intake of 8.4 mg bromide per person. These values are consistent with
those of two Dutch surveys where average daily intakes of 9.4 and 7.7
mg bromide per person, respectively, were recorded, i.e.,
approximately 3 mg/kg diet (Van Leeuwen & Sangster, 1987).
Table 32. Methyl bromide residues in fresh fruits after fumigation
Fruit Fumigation time Storage Timea Residue Reference
and dose temperature (mg/kg)
(°C)
grapefruit 2 h, 64 mg/litre 24 1 h 26.9b King et al. (1981)
48 h 0.52
peaches 3.5 h, 32 g/m3 2.5 1 day 11.0 Austin & Phillips (1985)
7 days 4.0
mango N.D.c, 64 g/m3 N.D. 1 h < 15.0 Stein & Wolfenbarger (1989)
grapefruit 2h, 48 g/m3 15.6 5 days < 5 King & Benschoter (1991)
oranges, 2h, 48 g/m3 15.6 5 days < 10
mandarines,
tongors
avocado 2h, 32 g/m3 20 1 day up to 0.5d Singh et al. (1982)
2 days up to 0.1
a Time of analysis after end of fumigation period.
b Aeration period: 15 min.
c N.D. = no data given.
d Aeration period: 30 min.
The metabolites resulting from methyl bromide post-harvest
fumigation of a number of crops have been analysed (Starratt & Bond,
1990a,b). Both physically- and chemically-bound residues were
measured. In 7 out of the 9 commodities, over half of the total
residue was chemically bound 1 h after fumigation. These chemically
bound residues were stable for at least 6 months. Most reaction
products were O-, S-, and N-methylated proteins (section 6.3).
Methylation of purines and pyrimidines in the nucleic acids accounted
for 0.1-6 % of the chemically-bound residue, with N being the only
site of methylation. The methylation products identified in this study
are also known to occur naturally.
5.2.2 Drinking-water
In 1988, in California, only one sample out of 43 056 taken from
2977 wells, showed any presence of methyl bromide (detection limit -
1 µg/litre) (CDFA, 1988).
In areas, such as in the Netherlands, where private water
supplies are from shallow wells near methyl bromide soil operations,
there could be increased bromide contents in the water (Van Doorn et
al., 1989).
5.2.3 Human breast milk
There are no data available.
5.2.4 Sub-populations at special risk
People who live in close proximity to greenhouses or to fields or
storehouses that are being fumigated have a higher risk of exposure to
methyl bromide gas than the general population. Similarly, persons
inadvertently, or intentionally, entering buildings following, in
particular, structural fumigation, are at risk.
5.3 Occupational exposure during manufacture, formulation, or use
The number of incidents and fatalities (section 9) show that
occupational exposure, especially during fumigation, is potentially
hazardous.
5.3.1 During manufacture
In a methyl bromide plant in the USA, workplace air
concentrations of 78-116 mg/m3 (20-30 ppm) were recorded using
direct measurement (Evans, 1979).
In a methyl bromide-producing factory in Japan, methyl bromide
concentrations in the worker's breathing zone were usually under 4
mg/m3, but sometimes exceeded 20 mg/m3 (Kishi et al., 1988).
5.3.2 During fumigation
If safety procedures are not followed, workers may be exposed to
methyl bromide accidentally during, or after, fumigation operations.
Methyl bromide in low concentrations is odourless so that a toxic
atmosphere may not be apparent to the worker. Only at higher
concentrations (100 x the actual TLV(R) of 20 mg/m3 (5 ppm) does
it have a sweet smell (Van Den Oever et al., 1982). An odour threshold
of 65 mg/m3 has been reported for methyl bromide (Worthing & Walker,
1983). Therefore, it is usually marketed in the form of 98% methyl
bromide and 2% chloropicrin, as a lacrimatory agent. For post-harvest
fumigation, 100% methyl bromide is used.
The various fumigation techniques used together with methyl
bromide exposure values and methods of application are outlined in
Table 33 (Guillemin et al., 1990).
5.3.2.1 Structural fumigation
In carrying out the general fumigation of a building, sufficient
gas must be liberated into the free space to kill the insects and then
the toxic level maintained for a defined period of time. After the
treatment, the residual gas remaining in the building is dispersed to
the outside atmosphere. However, there are basic differences in
defining structures amongst various countries. In the USA, structural
fumigations mainly involve residential houses whereas, in Europe, they
generally refer to flour mills and food processing areas. The
fumigation procedures and the safety aspects in these circumstances
could be very different.
Methyl bromide exposure levels for structural fumigation workers
in California were measured by Anger et al. (1986). They described the
work involved. "Structural fumigation is conducted by a work crew of
2-4 men who cover the buildings to be fumigated with large vinyl
tarpaulins and connect them by spring clamps. Cylindrical tubes filled
with sand (sand snakes) are placed at the base of the structure to
hold down the tarpaulins, thus sealing the building. After this one to
two hour procedure, termed a closing, the fumigant is introduced into
the unoccupied house via a tube or hose, and the fumigators leave the
site. The fumigators wear self-contained breathing apparatus (SCBA).
The next day the work crew removes the tarpaulins, opens the windows,
and places fans in the house to clear the fumigant. It is in this 30-
to 45-min process, termed the opening, that worker exposures may
occur. Typically, each work crew, led by a State-licensed fumigator
(the "licensee"), conducts three openings and/or closings each day".
Table 33. Integrated samples of methyl bromide in air taken in a survey of methyl bromide
fumigation in Switzerlanda
Circumstances Exposure Sample Range of values
of sampling category No.
Minimum Maximum
mg/m3(ppm) mg/m3(ppm)
1. Space fumigation
- during fumigationb occupational 7 <0.8 (<0.2) 500 (128.4)
- during aerationb occupational 7 2.3 (0.6) 646 (166.0)
- resuming operation para-occupational 47 <0.8 (<0.2) 9 (2.3)
- during fumigation environmental 12 <0.8 (<0.2) 79 (20.4)
- during aeration environmental 12 <0.8 (<0.2) 105 (27.1)
2. Soil fumigation
- during fumigationb occupational 5 2 (0.5) 151 (39.0)
- removal of sheetingb occupational 3 34 (8.8) 144 (36.9)
- inside greenhousec para-occupational 5 12 (0.3) 9 (2.2)
3. Chamber fumigation
- outside chamber para-occupational 3 35 (8.9) 293 (75.3)
- removing contents para-occupational 4 5 (1.2) 17 (4.3)
a From: Guillemin et al. (1990).
b Fumigators generally wore respiratory protection during these operations.
c Greenhouse only partially fumigated; includes post-fumigation soil tillage.
Personal samples from fumigators taken when they entered houses
24 hours after fumigation with 23 000 to 31 000 mg methyl
bromide/m3, indicated that a house might contain 80-2000 mg methyl
bromide/m3 (Table 34). Personal samples taken on fumigators working
outside the houses when they were opened again showed concentrations
of between 0 and 8 mg/m3 in the half-hour periods during the cover
removal (Anger et al., 1986). Area samples taken within 3 and 6 m from
the buildings during the same period ranged from 0 to 31 and 0 to 10
mg/m3, respectively (Anger et al., 1986).
Concentrations of methyl bromide inside flour mills and in the
atmosphere around the mills during, and after, fumigation were
measured by Bond & Dumas (1987). Considerable variations in
concentration were found in buildings of different structure and under
varyious weather conditions. Concentrations ranging from trace amounts
up to 90 mg/m3 (23 ppm) were found in the air around the mills
during the aeration period.
Table 34. Methyl bromide exposure concentrations (mg/m3)
in residential fumigationa
State Fumigators Under Within 3 m of 3-6 m from
licensed working covers house house
fumigator outside house
when inside
house
1875.0 5.1 2.0 46.7 32.3 0 0
75.9 0 1.2 12.5 0 10.1
90.3 0 7.0 0 17.5 10.1
1042.1 3.9 3.1 0 0 0
204.2 8.6 0 0 0
Mean
657.4 3.1 46.7 7.0 3.9
a Adapted from: Anger et al. (1986).
Guillemin et al. (1990) conducted a survey in Switzerland on
exposure during space fumigation. The maximum exposure levels for 7
integrated samples was 646 mg/m3 (166 ppm) during aeration (see
Table 33). Fumigators wore respiratory protection during these
operations. Samples of transient air taken around the buildings not
from any specific spot (distance not given), showed methyl bromide
levels in the range of 0-105 mg/m3 (see Table 33).
5.3.2.2 Soil fumigation
The amount of methyl bromide released into the atmosphere during
soil fumigation depends on the methods used (Table 9), the type and
time of covering (section 3.2.2) and soil type.
(a) Field fumigation
A formulation of 75% methyl bromide was used to kill insects and
nematodes in a strawberry crop, with 25% chloropicrin as a fungicide.
The fumigant injected into the soil produced an equilibrium of 47
000-58 000 mg/m3 which, under the covers, resulted in personal
average exposures ranging from 0 to 24 mg/m3 for the fumigators
(Anger et al., 1986). Personal sample measurements of methyl bromide
in farm workers when removing the film ranged between 0 and 33
mg/m3. Other data showed that the personal exposures of fumigators
and farm workers, who covered the plastic film with earth were between
0 and 29 mg/m3 and 0 and 17 mg/m3, respectively. Exposure of soil
fumigators, about 8 h a day, was relatively constant during most of
the year, whereas farm workers received only occasional exposures when
fields were fumigated. Spot (area) samples, taken before and after
film removal, showed that the airborne concentration under the intact
film was 8950 mg/m3 before removal and 9.3 mg/m3 an hour after
removal.
(b) Greenhouse fumigation
Roosels et al. (1981) compared two methods of methyl bromide
fumigation in greenhouses, i.e., by injection into milled soil
followed by covering with a plastic cover, or, by surface fumigation
by means of plastic pipes under a plastic cover. Two different
formulations (70 % methyl bromide/30 % chloropicrin and 98 % methyl
bromide/1-2 % chloropicrin) were used and the concentrations of methyl
bromide in the air were measured by GC-FID. During injection into
soil, values of between 400 and 4000 mg/m3 (100 and 1000 ppm) were
found with peaks up to 12 000 mg/m3 (3000 ppm) and, in one case, up
to 40 000 mg/m3 (10 000 ppm). However, when preventive measures were
taken, values of 800 mg/m3 (200 ppm) were obtained. During
fumigation, concentrations ranged between 400 and 4000 mg/m3 (100
and 1000 ppm). Using the piped surface fumigation method,
concentrations around the treated area were between 320 and 3200
mg/m3 (80 and 800 ppm) with short-term exposures of the operators to
8000-12 000 mg/m3 (2000-3000 ppm) when the pipes were being
connected.
In another investigation, concentrations of 60-100 g methyl
bromide/m2 were applied under a polyethylene cover (Van Den Oever et
al., 1982). Depending on local ventilation, quite a lot of gas escaped
into the surrounding atmosphere. The concentration during application
varied from 117 to 11 700 mg/m3 (30 to 3000 ppm). Concentration in
the air declined with time to 16 mg methyl bromide/m3 (4 ppm) five
days after application. Removing the plastic sheet involved exposure
to peak values as high as 800 mg/m3 (200 ppm), for a few seconds. On
the ninth day after application, milling the soil exposed workers to
up to 60 mg/m3 (15 ppm); on the eleventh day, no methyl bromide was
detected in the air.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
6.1.1.1 Animal studies
Uptake of methyl bromide was investigated in male Fischer 344
rats by Andersen et al. (1980). Following whole body exposures to
recirculated atmospheres of 390-11 640 mg/m3 (100-3000 ppm), the
uptake (i.e., disappearance from the atmosphere) was rapid and
exhibited first-order kinetics without a saturable component, the rate
constant being 0.44 kg-1 h-1. This rate constant was later
recalculated as 0.55 kg-1 h-1 by Gargas & Andersen (1982).
Medinsky et al. (1985) carried out a nose-only inhalation of 50,
300, 5700, or 10 400 nmol (given as 6.2-1206 mg/m3) of [14C]
methyl bromide/litre of air for 6 h in male F344 rat (5 animals/
group). The results indicated that, at low concentrations (50-300
nmol/litre), about 50% of the inhaled material was absorbed. At 5700
nmol/litre, only 37% was absorbed and, at 10 400 nmol/litre, only 27%.
The same amount of methyl bromide (650 µmol/kg body weight) was
absorbed at the two higher exposure concentrations. At 10 400
nmol/litre, the total volume inhaled by the rats was reduced (Medinsky
et al., 1985).
Raabe (1986) found about 40% uptake of inhaled methyl bromide in
studies on beagle dogs. These results are compared in Fig. 6 with
those from rats (Medinsky et al., 1985) and those from human
volunteers (Raabe, 1988), described below.
6.1.1.2 Human studies
An inhalation study was carried out to determine systemic uptake
of low concentrations of methyl bromide from air during nasal or oral
breathing (Raabe, 1988). Two male and two female volunteers inhaled
about 0.1 mg 14C-labelled methyl bromide/m3 (25 ppb), once through
the nose and once through the mouth. The uptake (% of methyl bromide
inhaled) was 55.4% nasally and 52.1% orally.
6.1.2 Dermal
Exclusively dermal exposure has only been observed in human incidents
(section 9). There are no data dealing exclusively with dermal
exposure in animals.
6.1.3 Oral
Methyl bromide (75 or 100 mg/kg) was administered to rats in olive oil
by gavage (Miller & Haggard, 1943). The methyl bromide entered the
blood stream with only a moderate degree of hydrolysis in the
intestine.
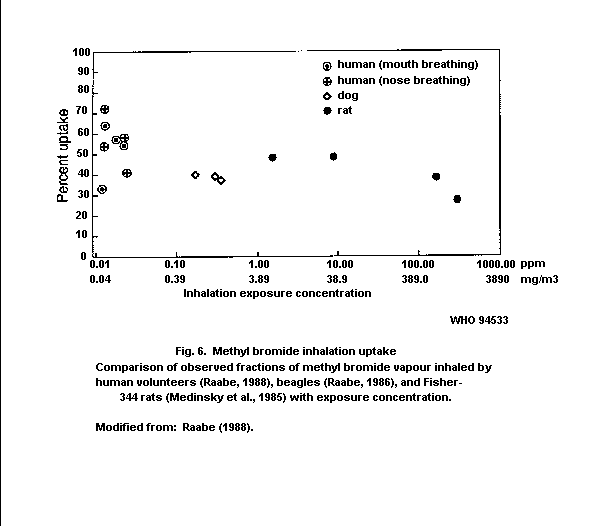 6.1.4 Intraperitoneal injection
Methyl bromide (120-180 mg/kg body weight) was administered i.p.
in hourly doses to rats (Miller & Haggard, 1943). The percentage of
methyl bromide eliminated was between 24 and 45%. Medinsky et al.
(1984) administered [14C] methyl bromide i.p., and reported that the
major route of elimination was exhalation of 14CO2 (46%) (section
6.4).
6.2 Distribution of methyl bromide and bromide in tissues
6.2.1 Animal studies
Methyl bromide is rapidly distributed to all tissues after
inhalation and rapidly metabolized. A small percentage is cleared
slowly and incorporated into metabolic pools (Jaskot et al., 1988).
At 72 h after oral or i.p. administration of 250 µmol of [14C]
methyl bromide/kg body weight, 14-17% of the 14C remained in the
rats, the liver and kidney being the major organs of retention
(Medinsky et al., 1984).
In rats exposed, nose only, to 337 nmol [14C] methyl
bromide/litre air, radioactivity was found widely distributed in
tissues immediately following exposures. The lung, adrenal gland,
kidney, liver, and nasal turbinates contained the highest
concentrations (250, 240, 180, 130, 110 nmol equivalents/g,
respectively) (see Table 35). Immediately after exposure,
radioactivity in the liver accounted for about 17% and all other
tissues about 10% of the absorbed methyl bromide (Bond et al., 1985).
Similarly, Jaskot et al. (1988) found that the liver, lung, and kidney
were the major organs of [14C] distribution in rats immediately
after exposure to [14C] methyl bromide at 214 mg/m3 (55 ppm) for
3 min.
Honma et al. (1985) measured methyl bromide levels in male
Sprague-Dawley rats exposed to 973 mg methyl bromide/m3 (250 ppm)
for 8 h and then sacrificed at successive time intervals. The
concentrations found in adipose tissue (maximum 1 µg methyl bromide/g
tissue) were much greater than those in blood (max. 0.1 µg/g) and
other tissues - brain, liver, muscle, and kidney (maximum about 0.01
µg/g; see Fig. 7). The methyl bromide in all tissues described reached
a maximum in 1 h after exposure commenced and maintained almost the
same concentrations during exposure. Honma et al. (1985) found peak
concentrations of bromine in blood at 4 h after cessation of methyl
bromide exposure, and in kidney and liver, 8 h after (Fig. 8).
Table 35. Concentration (nmol/g) of 14C in tissues from rats exposed for 6 h to 337 nmol
14C-methyl bromide/litre aira,b
0 hc 8 h 24 h 60 h
lung 250.4 ± 27.7 (3.6) 40.3 ± 5.5 (0.5) 19.5 ± 2.3 (0.2) 19.7 ± 2.3 (0.3)
adrenal 242.0 ± 16.8 (0.9) 25.8 ± 10.2 (0.1) 23.0 ± 1.4 (0.0) 19.5 ± 3.4 (0.0)
kidney 180.4 ± 4.6 (4.3) 76.1 ± 11.4 (1.5) 36.9 ± 2.6 (0.8) 35.1 ± 3.4 (0.7)
liver 129.9 ± 7.0(16.7) 119.6 ± 13.1(11.6) 82.4 ± 10.3 (9.0) 37.8 ± 7.5 (3.9)
turbinates 110.2 ± 6.5 (0.2) 35.2 ± 5.1 (0.1) 13.3 ± 1.4 (0.0) 18.9 ± 3.2 (0.0)
spleen 98.6 ± 3.4 (0.7) 28.8 ± 5.8 (0.2) 15.9 ± 2.1 (0.1) 15.8 ± 1.7 (0.1)
small intestine 96.5 ± 14.6 (2.0) 36.3 ± 0.8 (0.4) 19.9 ± 1.8 (0.2) 15.5 ± 3.2 (0.2)
trachea 84.3 ± 1.4 (0.1) 36.4 ± 6.3 (0.0) 15.5 ± 3.9 (0.0) 18.8 ± 4.3 (0.0)
stomach 80.0 ± 1.8 (1.3) 37.8 ± 5.3 (0.6) 31.9 ± 1.0 (0.5) 27.0 ± 8.3 (0.4)
large intestine 66.3 ± 9.2 (0.9) 43.6 ± 8.6 (0.6) 19.0 ± 2.3 (0.1) 17.8 ± 2.3 (0.2)
testes 65.4 ± 3.6 (2.5) 34.5 ± 3.9 (1.4) 17.1 ± 2.6 (0.6) 12.9 ± 2.3 (0.5)
larynx 61.1 ± 4.9 (0.0) 27.1 ± 5.9 (0.0) 10.5 ± 1.2 (0.0) 11.6 ± 2.0 (0.0)
brain 53.6 ± 9.5 (0.8) 35.8 ± 4.2 (0.6) 8.8 ± 1.1 (0.2) 7.4 ± 0.7 (0.1)
heart 51.7 ± 1.9 (0.6) 35.1 ± 5.3 (0.4) 16.8 ± 1.5 (0.2) 17.6 ± 2.9 (0.2)
thymus 48.4 ± 0.5 (0.2) 33.3 ± 6.7 (0.1) 19.9 ± 2.3 (0.1) 23.4 ± 3.1 (0.1)
urinary bladder 45.6 ± 6.9 (0.1) 27.7 ± 5.5 (0.0) 12.6 ± 1.9 (0.0) 11.8 ± 1.4 (0.0)
thyroid 28.7 ± 24.4 (0.0) 34.8 ± 6.1 (0.0) 16.1 ± 2.6 (0.0) 16.4 ± 1.4 (0.0)
a From: Bond et al. (1985).
b Values represent the x ± SE of 2-3 rats. Values in parentheses are percentages of the absorbed 14C-methyl bromide.
c Time after end of exposure.
6.1.4 Intraperitoneal injection
Methyl bromide (120-180 mg/kg body weight) was administered i.p.
in hourly doses to rats (Miller & Haggard, 1943). The percentage of
methyl bromide eliminated was between 24 and 45%. Medinsky et al.
(1984) administered [14C] methyl bromide i.p., and reported that the
major route of elimination was exhalation of 14CO2 (46%) (section
6.4).
6.2 Distribution of methyl bromide and bromide in tissues
6.2.1 Animal studies
Methyl bromide is rapidly distributed to all tissues after
inhalation and rapidly metabolized. A small percentage is cleared
slowly and incorporated into metabolic pools (Jaskot et al., 1988).
At 72 h after oral or i.p. administration of 250 µmol of [14C]
methyl bromide/kg body weight, 14-17% of the 14C remained in the
rats, the liver and kidney being the major organs of retention
(Medinsky et al., 1984).
In rats exposed, nose only, to 337 nmol [14C] methyl
bromide/litre air, radioactivity was found widely distributed in
tissues immediately following exposures. The lung, adrenal gland,
kidney, liver, and nasal turbinates contained the highest
concentrations (250, 240, 180, 130, 110 nmol equivalents/g,
respectively) (see Table 35). Immediately after exposure,
radioactivity in the liver accounted for about 17% and all other
tissues about 10% of the absorbed methyl bromide (Bond et al., 1985).
Similarly, Jaskot et al. (1988) found that the liver, lung, and kidney
were the major organs of [14C] distribution in rats immediately
after exposure to [14C] methyl bromide at 214 mg/m3 (55 ppm) for
3 min.
Honma et al. (1985) measured methyl bromide levels in male
Sprague-Dawley rats exposed to 973 mg methyl bromide/m3 (250 ppm)
for 8 h and then sacrificed at successive time intervals. The
concentrations found in adipose tissue (maximum 1 µg methyl bromide/g
tissue) were much greater than those in blood (max. 0.1 µg/g) and
other tissues - brain, liver, muscle, and kidney (maximum about 0.01
µg/g; see Fig. 7). The methyl bromide in all tissues described reached
a maximum in 1 h after exposure commenced and maintained almost the
same concentrations during exposure. Honma et al. (1985) found peak
concentrations of bromine in blood at 4 h after cessation of methyl
bromide exposure, and in kidney and liver, 8 h after (Fig. 8).
Table 35. Concentration (nmol/g) of 14C in tissues from rats exposed for 6 h to 337 nmol
14C-methyl bromide/litre aira,b
0 hc 8 h 24 h 60 h
lung 250.4 ± 27.7 (3.6) 40.3 ± 5.5 (0.5) 19.5 ± 2.3 (0.2) 19.7 ± 2.3 (0.3)
adrenal 242.0 ± 16.8 (0.9) 25.8 ± 10.2 (0.1) 23.0 ± 1.4 (0.0) 19.5 ± 3.4 (0.0)
kidney 180.4 ± 4.6 (4.3) 76.1 ± 11.4 (1.5) 36.9 ± 2.6 (0.8) 35.1 ± 3.4 (0.7)
liver 129.9 ± 7.0(16.7) 119.6 ± 13.1(11.6) 82.4 ± 10.3 (9.0) 37.8 ± 7.5 (3.9)
turbinates 110.2 ± 6.5 (0.2) 35.2 ± 5.1 (0.1) 13.3 ± 1.4 (0.0) 18.9 ± 3.2 (0.0)
spleen 98.6 ± 3.4 (0.7) 28.8 ± 5.8 (0.2) 15.9 ± 2.1 (0.1) 15.8 ± 1.7 (0.1)
small intestine 96.5 ± 14.6 (2.0) 36.3 ± 0.8 (0.4) 19.9 ± 1.8 (0.2) 15.5 ± 3.2 (0.2)
trachea 84.3 ± 1.4 (0.1) 36.4 ± 6.3 (0.0) 15.5 ± 3.9 (0.0) 18.8 ± 4.3 (0.0)
stomach 80.0 ± 1.8 (1.3) 37.8 ± 5.3 (0.6) 31.9 ± 1.0 (0.5) 27.0 ± 8.3 (0.4)
large intestine 66.3 ± 9.2 (0.9) 43.6 ± 8.6 (0.6) 19.0 ± 2.3 (0.1) 17.8 ± 2.3 (0.2)
testes 65.4 ± 3.6 (2.5) 34.5 ± 3.9 (1.4) 17.1 ± 2.6 (0.6) 12.9 ± 2.3 (0.5)
larynx 61.1 ± 4.9 (0.0) 27.1 ± 5.9 (0.0) 10.5 ± 1.2 (0.0) 11.6 ± 2.0 (0.0)
brain 53.6 ± 9.5 (0.8) 35.8 ± 4.2 (0.6) 8.8 ± 1.1 (0.2) 7.4 ± 0.7 (0.1)
heart 51.7 ± 1.9 (0.6) 35.1 ± 5.3 (0.4) 16.8 ± 1.5 (0.2) 17.6 ± 2.9 (0.2)
thymus 48.4 ± 0.5 (0.2) 33.3 ± 6.7 (0.1) 19.9 ± 2.3 (0.1) 23.4 ± 3.1 (0.1)
urinary bladder 45.6 ± 6.9 (0.1) 27.7 ± 5.5 (0.0) 12.6 ± 1.9 (0.0) 11.8 ± 1.4 (0.0)
thyroid 28.7 ± 24.4 (0.0) 34.8 ± 6.1 (0.0) 16.1 ± 2.6 (0.0) 16.4 ± 1.4 (0.0)
a From: Bond et al. (1985).
b Values represent the x ± SE of 2-3 rats. Values in parentheses are percentages of the absorbed 14C-methyl bromide.
c Time after end of exposure.
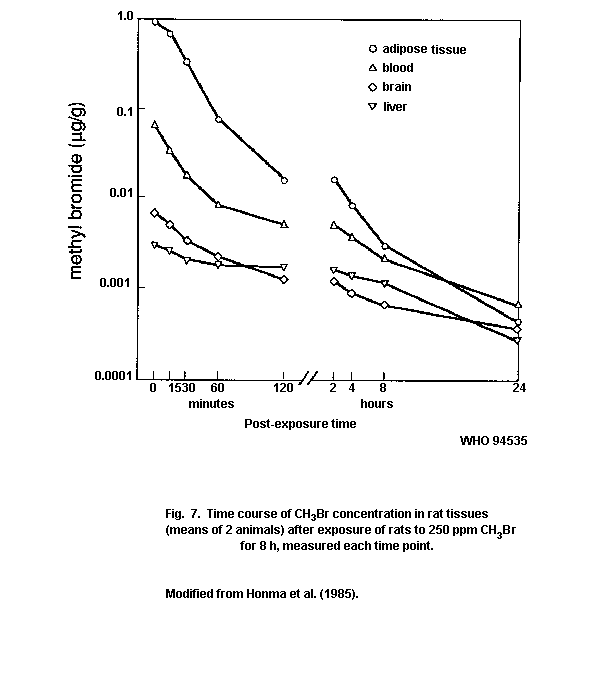 Calves fed for 49 days on a diet containing about 4650 mg
bromide/kg showed bromide concentrations in kidney, liver, and muscle
of 1808, 1015, and 465 mg/kg, respectively, on day 49. Serum and organ
bromide concentrations decreased markedly 14 days after the feeding of
this diet was discontinued (Knight & Reina-Guerra, 1977).
Calves fed for 49 days on a diet containing about 4650 mg
bromide/kg showed bromide concentrations in kidney, liver, and muscle
of 1808, 1015, and 465 mg/kg, respectively, on day 49. Serum and organ
bromide concentrations decreased markedly 14 days after the feeding of
this diet was discontinued (Knight & Reina-Guerra, 1977).
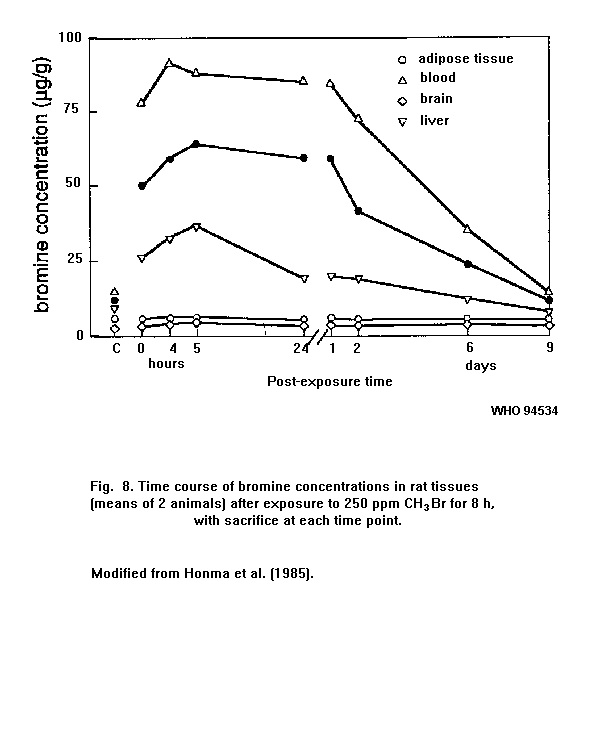 6.2.2 Human studies
Data on the concentrations of bromide in various human tissues after
methyl bromide poisoning are scarce. In an autopsy study of a methyl
bromide-exposed patient, Heimann (1944) reported the following bromide
values: lung (127 mg/kg), liver (187 mg/kg), brain (207 mg/kg), and,
in a composite sample of heart, kidney, and pancreas (107 mg/kg).
Traces of methyl alcohol and formaldehyde were also found in all the
tissues examined.
In four lethal cases of people exposed to methyl bromide,
Marraccini et al. (1983) found bromide ion concentrations in serum or
plasma ranging from 40 to 583 mg/litre. Methyl bromide was detected in
the brain of one patient (detection limit <1 mg/kg).
Values of 0.9 mg/kg in lymph nodes, 3.3 and 5.1 in ovaries and
testes, 7.5 in lung, and 8.2 mg/kg wet weight in kidney cortex have
been reported in autopsy samples (from accident victims) (Hamilton et
al., 1972/73). A study of the levels of bromide in adipose tissue from
human subjects in three countries showed the highest levels in the
United Kingdom, where 5.6% of the specimens contained levels ranging
from 4.0 to 4.5 mg/kg fat; the lowest levels were found in Germany
(0-0.9 mg/kg fat) whereas levels of 1-3.7 mg/kg were found in the
Netherlands samples (Crampton et al., 1971). Van Leeuwen & Sangster
(1987) stated that there was no evidence in humans of bromide
concentration in any particular organ that might indicate a specific
physiological function of this ion.
6.3 Metabolic transformation
The metabolism of methyl bromide has not been elucidated.
Bromide concentrations in blood (and target organs) were reported
to be increased in humans (Clarke et al., 1945; Rathus & Landy, 1961;
Hine, 1969) and in laboratory animals (Irish et al., 1940, 1941) after
exposure to methyl bromide. Miller & Haggard (1943) postulated that
methyl bromide is hydrolysed in the body with the formation of
inorganic bromide and methyl alcohol. In part, this hydrolysis may
occur intracellularly, resulting in a distribution of bromide that
differs from that for bromide given orally as sodium bromide. Sodium
bromide and methyl alcohol, given at the levels produced after methyl
bromide exposure, did not produce the same toxic and functional
response (Irish et al., 1940, 1941). This suggested that the toxicity
of methyl bromide was due to the reaction of the halide molecule with
the tissue and was not attributable to the hydrolytic products.
Hallier et al. (1990a) measured the cytosolic turnover rate of
methyl bromide in both liver and kidney from five different strains of
mice and rats, with in vitro incubation. The turnover rate in both
organs was consistently higher in tissues isolated from females. On
the basis of a similar effect with methyl chloride, this effect could
be attributed to a higher rate of glutathione conjugation in females.
The reaction products of methyl bromide in wheat were
characterized by Winteringham et al. (1955) using 14C- or
82Br-labelled methyl bromide. It was pointed out that, although most
attention is given to the bromide ion because it is the only part of
the residue that is easily determined, methyl methionine sulfonium
bromide, the methylated histidines, and possibly other residues of
methylation were also produced.
6.3.1 Binding to proteins and lipids
Methylation of cysteine- S and histidine- N residues of haemo-
globin in suspended mouse erythrocytes was found after in vitro
treatment with radiolabelled methyl bromide (Djalali-Behzad et al.,
1981). After inhalation of methyl bromide, alkylation of cysteine- S
residues was seen in mouse haemoglobin and liver proteins
(Djalali-Behzad et al., 1981).
Adducts result from reactions between toxic chemicals and amino
acids in haemoglobin or other proteins (or nucleosides in DNA - see
below). S-methyl-cysteine has been studied as a haemoglobin adduct
in mice (Iwasaki, 1988a,b) and rats (Xu et al., 1990) and as a serum
albumin adduct in human blood samples (Müller et al., 1991, 1992).
Both haemoglobin and serum albumin adducts have been studied in blood
samples of workers occupationally exposed to methyl bromide and these
have been proposed as suitable parameters for the biomonitoring of
exposure to the fumigant (Iwasaki et al., 1989; Müller et al., 1991,
1992) (see also section 9.4.4).
In the insect Triatoma infestans , Castro et al. (1976)
demonstrated that methyl bromide is irreversibly bound to lipids and
to proteins in both the nymph adult and eggs and that exposure to
methyl bromide significantly decreased the content of sulfhydryl
groups in nymph adult and egg proteins.
Studies on methylation by methyl bromide of wool, silk, collagen,
and gelatin (Blackburn & Phillips, 1944), wheat flour (Bridges, 1955;
Winteringham et al., 1955), and cocoa beans (Asante-Poku et al., 1974)
indicated that N-, O-, and S- methylation of proteins occurs
(Cova et al., 1986; Starratt & Bond, 1990b). The main site of
decomposition of methyl bromide in cocoa beans was shown to be in the
alcohol- insoluble proteins of the shell (Asante-Poku et al., 1974).
The methyl group of the fumigant became covalently bound to the
alpha-amino group of the various amino acids, the imidazole ring of
histidine, and the epsilon-amino group of lysine.
Winteringham et al. (1955) found that the gluten fraction of
whole-wheat flour exposed to [14C]methyl bromide was responsible for
80% of the decomposition of the absorbed fumigant with N-methyl,
dimethylsulfonium, and methoxyl and thiomethoxyl derivatives
accounting for 50, 30, and 20%, respectively, in this fraction.
Bridges (1955) reported that 1- N-methylhistidine,
3- N-methylhistidine and 1,3- N,N-dimethylhistidine accounted for
75% of the N-methyl derivatives, and that 10% was due to probably
epsilon- N-methyllysine.
Starratt & Bond (1990a,b) used [14C]methyl bromide to
distinguish naturally occurring residues from those formed during the
fumigation of a variety of commodities: maize, wheat, oatmeal,
peanuts, almonds, alfalfa, potatoes, oranges, and apples. In order to
get higher incorporation of the tracer, fumigation was carried out at
a level of 48 mg/litre, for 3 days. Methyl bromide was bound both
physically and chemically to the commodities. To measure the
physically-bound residue, a parallel test was run using unlabelled
fumigant. Methyl bromide levels were determined 1 h following
fumigation and then at 1, 2, 4, and 10 days. The levels in all the
commodities declined rapidly.
Extraction of the [14C]methyl bromide-fumigated commodities
with diethyl ether removed very little radioactivity, showing that
fats and other non-polar lipids were not methylated during treatment.
In maize, fractions corresponding to albumins, glutamines, zein, and
glutelin were all methylated.
Methylation of methionine is one of the main reactions forming
the relatively unstable methylmethionylsulfonium derivative.
Spontaneous decomposition yields dimethyl sulfide. Analysis of the
sites of methylation was uncertain as both acidic and basic hydrolysis
caused partial decomposition (Starratt & Bond, 1990a,b). The volatile
products included methanol, methyl mercaptan, and dimethyl sulfide.
Different commodities produced different residues (Starratt & Bond,
1990a,b). In potato and orange extracts, the main methylated
components were identified as S-methyl-glutathione,
gamma-glutamyl- S-methylcysteine and S-methyl cysteine. These
compounds were not found in maize. 1- N-methylhistidine and
3- N-methylhistidine were the major components from the fumigated
maize, almonds and other commodities. The highest level of histidine
methylation occurred in almonds, accounting for about 54% of the
chemically-bound residue.
6.3.2 Binding to DNA
Labelled 7-methylguanine was identified in DNA from liver and
spleen cells of mice exposed to [14C] methyl bromide (Djalali-Behzad
et al., 1981). In in vitro experiments with DNA solutions treated
with [14C] methyl bromide, predominantly [14C] 7-methylguanine was
identified (Starratt & Bond, 1988b).
Calf thymus DNA treated in the solid state with [14C] methyl
bromide, showed, on analysis, four major radiolabelled peaks with
retention times corresponding to 1-methyladenine, 7-methylguanine,
3-methyladenine, and 3- methylcytosine (Starratt & Bond, 1988b).
The DNA adducts, [14C]3-methyladenine, [14C]7-methylguanine,
and [14C]O6-methylguanine, have been found in the stomach and
forestomach of rats after both oral and inhalation exposure of
[14C]methyl bromide (Gansewendt et al., 1991).
In maize and wheat, 7-methylguanine and 1-methyladenine were
identified as major products after hydrolysis together with lesser
amounts of 3-methylcytosine and 3-methyladenine (Starratt & Bond,
1988a). Although the yields of DNA were low, Starratt & Bond (1990a)
also found evidence of radioactively-labelled 7-methylguanine and
1-methyladenine in [14C]methyl bromide-fumigated almonds and
potatoes.
6.3.3 The role of glutathione in methyl bromide metabolism
6.3.3.1 Mammals
Liver, kidney, lung, and brain from mice, exposed via inhalation
for 1 h to methyl bromide concentrations of from 870 to 5930 mg/m3,
were analysed for glutathione and bromide ion (Alexeeff et al., 1985).
The liver glutathione levels of the 4700 and 5930 mg/m3 exposure
groups were significantly lower than that of the controls. Bromide ion
levels were highest in the liver and kidney and lowest in the whole
blood. The lung and brain bromide levels were intermediate.
Methyl bromide has been shown in rats to increase the activity of
glutathione S-alkyl transferase and decrease the nonprotein
sulfhydryl content (Roycroft et al., 1981). A group of male
Sprague-Dawley rats were exposed to methyl bromide (117 mg/m3; 6
h/day; 10 days) and killed immediately after the last dose.
Biochemical analyses showed that glutathione S-alkyl transferase was
significantly increased in the lung (12%), liver (11.9%), and kidney
(6.9%). Non-protein sulfhydryl was significantly reduced by 11.4% in
the liver and 13.9% in the kidney. Glucose-6-phosphate dehydrogenase
was significantly increased in the kidney (8.5%), but not in the lung
or liver.
Studies on rats by Davenport et al. (1992) showed that
glutathione was depleted and regional brain
glutathione- S-transferase inhibited by methyl bromide inhalation
(584 mg methyl bromide/m3; 6 h/day; 5 days) [see sections 8.8.2 and
8.10].
In a preliminary report, Thomas & Morgan (1988) reported that
treatment of rats with buthionine sulfoximine (BSO) depleted
glutathione levels, prior to methyl bromide exposure, and increased
the toxicity of methyl bromide. This is in contrast to the findings of
Chellman et al. (1986) for methyl chloride in mice, who found that
glutathione depletion by BSO decreased methyl chloride toxicity in the
brain and kidney. However, the observation is consistent with those of
Tanaka et al. (1988), who showed that treatment of rats with
glutathione reduced the detrimental effects of methyl bromide on
sleep-wakefulness and its circadian rhythm and increased the LD50
value (section 8.8.2).
In whole body inhalation studies on rats exposed for 6 h/day, for
5 or 10 days, to 117 mg methyl bromide/m3 (30 ppm), glutathione
(GSH) S-transferase and glucose-6-phosphate dehydrogenase (G-6-PDH)
activities were increased in the lung. Decreases in GSH-reductase and
GSH- S-transferase activities were found in the liver (Jaskot et al.,
1988).
When human erythrocyte cytoplasm was incubated with methyl
bromide or methyl iodide in the presence of excess glutathione (GSH),
a spontaneous non-enzymatic conjugation was observed (Deutschmann et
al., 1989). This was verified in parallel experiments with boiled
cytoplasm and GSH added after boiling.
Enzymatic conjugation of methyl bromide with reduced glutathione
to produce S-methyl cysteine (the analysis) appears to be
isoenzyme-specific, since conjugation was observed in the erythrocyte
cytoplasm of a majority (13/20) of the population. The same
individuals also conjugated methyl chloride, a reaction that is
dependent on glutathione- S-transferase rho (GSTrho), a minor form of
the enzyme (Hallier et al., 1990b).
Later studies on the properties of the glutathione-
S-transferase (GST) responsible for methyl bromide conjugation with
GSH (measured by methyl bromide depletion) have separated the enzyme
GSTsigma from human erythrocytes; this new enzyme has not been found
in various non-human species (Schröder et al., 1992).
Measurement of methyl bromide disappearance in head-space vials
containing whole human blood cultures in an atmosphere of 19 460
mg/m3 (5000 ppm) at 37 °C indicated that the methyl bromide
concentration had fallen to zero within 1 h, in the presence of blood
from glutathione conjugators, whereas it had fallen to approximately
5836 mg/m3 (1500 ppm) at 1 h, in the presence of blood from
non-conjugators. Further reduction was slow, the methyl bromide
concentration being about 3890 mg/m3 (1000 ppm) after 6 h (Hallier
et al., 1993).
6.3.3.2 Insects
Glutathione is depleted and glutathione S-transferase can be
induced in the larvae of the khapra beetle (Trogoderma granarium) by
fumigation with methyl bromide at a lethal dose (Shivanandappa &
Rajendran, 1987).
Starratt & Bond (1981) demonstrated that, in the granary weevil
( Sitophilus granarius L.), the major pathway for the detoxication of
methyl bromide residues was by conjugation, primarily with
glutathione, and that increasing amounts of glutathione in the insects
resulted in increasing tolerance to methyl bromide exposure. Both
strains of the insect (methyl bromide sensitive and methyl bromide
resistant) metabolized methyl bromide primarily to substances that, on
thin layer chromatography, behaved consistently with their
identification as S-methyl glutathione.
6.4 Elimination and excretion in expired air, faeces, urine
The route of administration of methyl bromide affects the
pathways of excretion.
Miller & Haggard (1943) investigated the amount of methyl bromide
eliminated and the amount of bromide retained in the body after i.p.
administration of methyl bromide. Following a single i.p. injection of
60 mg methyl bromide/kg body weight, elimination continued for about
45 min and more than 90% was eliminated in the first 30 min. With
lethal i.p. doses of 120-180 mg methyl bromide/kg, 24- 45% of the
methyl bromide was eliminated; the amounts of fixed and nonvolatile
bromide being 94.9-126.6 mg/kg. In rats dosed orally (75-100 mg methyl
bromide/kg), similar bromide levels were found, but the methyl bromide
eliminated was given as only 2.4-4.6%.
These results have been confirmed by studies using radioactively
labelled methyl bromide (Medinsky et al., 1984). In rats dosed
intraperitoneally with [14C] methyl bromide, the major route of
elimination was the exhalation of 14CO2 (46%). In contrast,
urinary excretion of [14C] was the major route of elimination (43%
of the dose), when methyl bromide was given orally. Very little
appeared in the faeces (<3% of the dose), regardless of the route of
administration. In rats with bile duct cannulations, 46% of an oral
dose appeared in the bile over a 24-h period (Medinsky et al., 1984).
The authors suggested that reabsorption of biliary metabolites from
the gut played a significant role in the disposition of [14C] methyl
bromide.
In inhalation studies on rats exposed for 6 h to 337 nmol [14C]
methyl bromide/litre air, Bond et al. (1985) showed that excretion of
[14C] as 14CO2 was the major route of elimination, about 47%
(3900 nmol/rat) of the total [14C] methyl bromide absorbed being
excreted by this route. CO2 excretion exhibited a biphasic
elimination pattern with 85% of the 14CO2 being excreted with a
half-time of 3.9 h and 15% excreted with a half-time of 11.2 h.
Half-times for the elimination of 14C in urine and faeces were 9.6
and 16.1 h, respectively; 65 h after exposure, about 75% of the
initial radioactivity had been excreted with 25% remaining in the body
(Bond et al., 1985). Elimination half-times of [14C] from tissues
were 1.5-8 h. In all tissues examined, over 90% of the 14C in the
tissues were methyl bromide metabolites (Bond et al., 1985). The data
from this study indicate that, after inhalation, methyl bromide is
rapidly metabolized in tissues and readily eliminated.
Male CD rats were exposed (nose only) to [14C]-methyl bromide
at 214 mg/m3 (55 ppm) for 3 min. The liver, lung, and kidney were
the major organs of [14C] distribution, immediately after exposure.
Up to 32 h after exposure, the major routes of excretion were
pulmonary and renal with elimination of 43% and 21% of the total
inhaled label, respectively (Jaskot et al., 1988).
Studies into the time course of methyl bromide and bromide
elimination in rat tissue have been described by Honma et al. (1985).
Male Sprague-Dawley rats were exposed to 973 mg methyl bromide/m3
(250 ppm) for 8 h and methyl bromide and bromine concentrations
measured at successive time intervals (see also section 6.2). The
results are shown in Fig. 7 (bromine) and Fig. 8 (methyl bromide). The
methyl bromide levels in all tissues described reached a maximum in 1
h following exposure and remained at almost the same levels during
exposure. Methyl bromide levels decreased rapidly after exposure;
after 30 min only half of the methyl bromide concentrations in adipose
tissue and blood were still present (Fig. 7). The methyl bromide
levels in the brain and liver were very low, but the elimination of
methyl bromide from these organs was slower. Forty-eight hours after
exposure, methyl bromide could not be detected in any tissue examined
(Honma et al., 1985). In contrast to the methyl bromide study, peak
concentrations of bromine in blood, kidneys, and liver occurred 4-8 h
after bromide exposure, and the half-life in these tissues was about
5 days.
Honma et al. (1985) carried out a regression analysis of methyl
bromide and bromine concentrations in blood, kidney, liver, brain and
adipose in male rats after a 2-h exposure to 0, 973, 1946, 2918, or
3890 mg methyl bromide/m3 (0, 250, 500, 750, or 1000 ppm). Linear
relationships were obtained between exposure concentrations and the
tissue methyl bromide or bromine values.
Over 95% of the bromide ion in mice exposed to methyl bromide was
eliminated within 2.5 days, bringing the concentration close to the
control levels and the limit of detection (Alexeeff et al., 1985).
Lactating cows fed a methyl bromide fumigated grain ration (220
mg bromide/kg food) compared with unfumigated diets secreted increased
levels of bromide in the milk, i.e., 10-20 mg/litre instead of about
5 mg/litre (Lynn et al., 1963).
6.5 Retention and turnover
The biological half-life of bromide ions in human blood was found
to be about 12 days (Söremark, 1960).
The half-lives of bromide in the blood and brain of rats after
i.p. injection of methyl bromide were approximately 8.7 and 4.3 days,
respectively (Tanaka et al., 1988). In inhalation studies, a half-life
of bromide of about 5 days was reported in the blood, kidneys, and
liver (Honma et al., 1985). In contrast, methyl bromide concentrations
in the blood and adipose tissue were reduced by half after 30 min,
though elimination from the brain and liver was much slower (Honma et
al., 1985).
6.6 Reaction with body components
Honma et al. (1987) suggested that the main target of methyl
bromide in the body was the central nervous system. The nor-
epinephrine content of the hypothalamus and cortex with hippo-campus
was reduced on exposure to methyl bromide (Honma et al., 1982) and
changes in the amino acid content and metabolism in the brain were
noted (Honma et al., 1983). The results of further studies suggested
that alterations in catecholamine metabolism might be a factor in
methyl bromide-induced neurotoxicity (Honma et al., 1987) (see section
8.8). Kato et al. (1986) described histopathological changes in the
brain.
Eustis et al, (1986, 1988) found clear species- and sex- related
differences in the susceptibility of specific organs and tissues to
methyl bromide effects (section 8.8.1). In rats, neuronal necrosis
occurred primarily in the cerebral cortex, hippocampus, and thalamus
of the brain, whereas in mice, necrosis of the internal granular layer
of the cerebellar folia was more frequently observed. Nephrosis
occurred in all treated mice. Myocardial degeneration was observed in
male and female rats more frequently than male mice. Atrophy of the
adrenal cortex and testis and necrosis of the olfactory epithelium
were described. Similar findings were described by Hurtt et al.
(1987). Degeneration and regeneration of the olfactory epithelium were
also described by Hurtt et al. (1988) and Hastings et al. (1989);
Hastings (1990).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Soil microorganisms
Methyl bromide is used commercially to control soil- borne fungi
that cause:
- damping off: Rhizoctonia solani, Pythium spp., Sclerotium
bataticola (Macrophomina phaseolina), Phytophtora spp.,
and Thielaviopsis basicola.
- crown rot: Sclerotium rolfsii and Sclerotinia spp.
- root rot: Pythium spp., Stromatinia, Fusarium spp.,
Sclerotium bataticola, (Macrophomina phaseolina),
Rhizoctonia solani, Pyrenochaeta spp. (corky root, pink
root), Armillaria and Phytophthora spp.
- wilt: Fusarium spp., and Verticillium spp.
Generally, concentration-time products of methyl bromide required
to kill fungi are much higher than those needed to control insect and
nematode pests, susceptibility increasing with temperature. To control
fungi, methyl bromide is generally used at rates of 40-100 g/m2
(Davis et al., 1977).
Filip & Roth (1977) demonstrated the efficacy of methyl bromide
against Armillaria root rot in the stumps of ponderosa pine ( Pinus
ponderosa Laws). The survival of young pine trees in areas infected
with such fungi was increased after the elimination of the fungus,
using the fumigant.
Heungens & Roos (1982) recovered non-pathogenic fungi
(Penicillium and Mucor) following the application of methyl
bromide (300 g/m2) to pine litter, but no pathogenic fungi were
recovered.
After an initial reduction, the numbers of bacteria and fungi in
fumigated soil remained high and low, respectively, in comparison with
those in untreated soil (Sivasithamparam et al., 1987). In the
fumigated soil, Trichoderma species rapidly recolonized the soil,
becoming the dominant fungus within 15 days. In a study on the
microflora in the rhizosphere of wheat, the same authors found that,
though there was no difference in the total number of bacteria,
actinomycetes, and fungi, before and after fumigation with methyl
bromide, there were some fungal species differences with Fusarium
merismoides, T. koningii , and T. viride present in significantly
higher numbers, other fungi being less abundant.
Kelley & Rodriguez-Kabana (1979) reported that methyl bromide did
not cause any permanent changes in soil enzyme activities or adversely
affect the mycorrhizal root development of pine seedlings.
Methyl bromide is used for controlling fungal infections in the
poultry industry (Harry et al., 1972; Davis et al., 1977).
Methyl bromide is much less frequently used as a bactericide than
as an insecticide (Davis et al., 1977). It is used to control certain
soil-borne bacteria, e.g., bacterial canker (Corynebacterium
michiganese) , and bacterial wilt (Pseudomonas solanacerum)
(Bromine & Chemicals Ltd.,1990). A summary of the acute toxic effects
of methyl bromide on bacteria and viruses is given in Table 36.
Methyl bromide, used at a concentration-time product of 800
mg.h/litre at 25 °C, with a relative humidity of 70%, eliminated
salmonellae from artificially contaminated poultry foodstuffs (Tucker
et al., 1974).
Although there have been many studies on the effectiveness of
methyl bromide in reducing diseases produced by pathogenic
micro-organisms, there are fewer data on its effects on soil microbes.
Matta & Porta-Puglia (1968) tested methyl bromide on several
morphologically and functionally different groups of soil microbes. In
isolated soil samples, treated and maintained under constant
temperature (22-23 °C) and humidity (16-18%), microorganisms were
counted at 2, 21, 54, and 87 days following fumigation with 300 g
methyl bromide/m3. After 2 days, most bacteria were dead; after 87
days, there were very low counts of fungi, aerobic nitrogen-fixing,
nitrifying, and cellulolytic bacteria, whereas denitrifying,
proteolytic, amylolytic, and ammonifying bacteria showed a marked
resurgence in recolonization (Matta & Porta-Puglia, 1968). The
selective-action effects of methyl bromide fumigation on a given
microbe population in soil appear to be more significant than the
effects on microbe number.
Table 36. Effects of methyl bromide fumigation on bacteria and virusesa
Organism Dosage Conditions Reference
Vibrio cholera, Shigella 33 g/m3 10-h exposure LD100 Saiki (1952)
dysenteriae, Salmonella
typhi, Salmonella paratyphi A,
Salmonella paratyphi B
Corynebacterium 10 % bromomethane, 18-h exposure LD100 Richardson & Monro (1962)
sepedonicum 5 % ethylene oxide 85 % CO2
Arabis mosaic virus 0.32 kg/m3 controlled virus on strawberry Harrison et al. (1963)
plants
Tobacco mosaic virus, 640 g/m3 inactivated virus Inouye et al. (1967)
Cucumber green mottle virus 110 g/m3 inactivated at 27°C
320 g/m3 inactivated at 14-16°C
Escherichia coli 1257 1000 g/m3 40°C and 90 % relative humidity Prishchep &Nikiforova
provided control (1969)
Bacillus larvae, Bacillus 5000 g/m3 5-day exposure controlled Smirnov (1970)
paraalvei, Streptococcus bacteria in bee honeycombs
apis, Streptococcus pluton,
Pseudomonas apisepticus
Tobacco mosaic virus 200 g/m3 inactivated virus in 3 kg soil Doraiswamy et al. (1972)
in which tomatoes were grown
Salmonella typhimurium 800 mg-h/litre 25 °C and 70% relative humidity Tucker et al. (1974)
provided control
Xanthomonas begoniae 0.32 kg/m3 24-h exposure eliminated Strider (1975)
bacterial blight from Rieger
begonia
a Adapted from: Davis et al. (1977).
In a study on bacterial flora involved in the nitrogen cycle, hot
fumigation with methyl bromide at concentrations of 80 g/m3 was
carried out in greenhouses at 6 different sites (Turtura et al.,
1988). Seven months after treatment, the total aerobic mesophile
bacteria count, aerobic nitrogen-fixing, ammonifying,
ammonia-oxidizing, and nitrite-oxidizing bacteria, always showed
higher values in fumigated than in unfumigated control soils.
Recolonization was more marked in the upper 0-30 cm soil samples in
which the development of ammonifying and nitrifying bacteria was
highly significant.
Rovira & Ridge (1979) found no long-term effects on aerobic soil
bacteria or actinomycetes with application of 22 g methyl bromide/m2.
Yeates et al. (1991) described the recolonization of soils
sterilized in the laboratory and returned to their original pasture
and forest sites, under four different types of field conditions.
Sampling took place over 166 days (midsummer to midwinter) with two of
the sites having a moderate, and two a high, rainfall. Both microbial
biomass and dehydrogenase activity recovered rapidly but remained
consistently lower in the fumigated than in the untreated samples in
all four sites. Bacterial numbers also recovered rapidly. Fungal
hyphal lengths were 25% lower in the fumigated soil. Fumigation showed
no detectable effects on the subsequent rates of nitrogen
mineralization and little effect on nitrification rates. Protozoa were
almost completely eliminated by fumigation, numbers recovering most
rapidly in moist forest soil and slowly in dry pasture soil. Nematodes
were eliminated by fumigation; recolonization was first detected on
day 26. Numbers (10 and 62/g, respectively) and species (10 and 31,
respectively) remained much lower in fumigated, compared with
untreated, soil.
After sterilization of greenhouse soil with methyl bromide (75
g/m2), there were profound qualitative and quantitative disturbances
up to a soil depth of 30 cm (Bourbos & Skoudridakis, 1991); 7-9
species of soil mycoflora were isolated from the fumigated soil
compared with 107 from control soils. The 31-40 cm soil layer was not
affected by disinfestation. After two months, recolonization had taken
place of only 35-40% in species and 60-63% in density of the primary
microflora.
The use of methyl bromide as an effective fumigant for
greenhouses has been questioned (Bourbos & Skoudridakis, 1991).
Certain saprophytic fungi have developed a degree of tolerance.
Disinfested soil was quickly contaminated by certain pathogens
( Fusarium and Pythium spp.) and there was also a possibility of
reinfection from the lower layer of soil not reached by the fumigant.
Some fungi controlled by methyl bromide are listed in Table 37.
7.2 Aquatic organisms
7.2.1 Effect of methyl bromide
LC50 (96-h) values of 11 mg methyl bromide/litre and 12 mg
methyl bromide/litre for bluegill sunfish, Lepomis macrochirus
(freshwater) and tidewater silversides, Menidia beryllina
(saltwater), respectively, have been determined following exposure to
methyl bromide (Dawson et al., 1975/77).
The acute toxicity of methyl bromide for carp ( Cyprinus carpio
L.) was determined in studies with a 4-h exposure period (Segers et
al., 1984). The 4-h LC50 was calculated to be approximately 17
mg/litre. Damage to the gill epithelium was the most pronounced
morphological damage, which probably caused the death of the fish by
suffocation.
In a short-term study, triplicate groups of 10 fish ( P.
reticulata (guppy) and Oryzias latipes (medaka)) were exposed to
0.56, 1.0, or 1.8 mg methyl bromide/litre for 4 days. All methyl
bromide-exposed fish displayed abnormal behaviour (reduced activity).
Limited mortality was noted in P. reticulata exposed to 1.0 and 1.8
mg/litre and O. latipes exposed to 1.8 mg/litre, but there was no
increase in mortality in the next lower concentration groups (Wester
et al., 1988).
In a long-term study, P. reticulata and O. latipes were
exposed for 1 and 3 months to 0.032-3.2 mg methyl bromide/litre
(Wester et al., 1988). All guppies died in the 3.2 mg/litre group
within 3 days, and, in the 1.0 mg/litre group, within 3 weeks. The
NOLC (no observed lethal concentration) and NOEC (no observed effect
concentration: behaviour, appearance) values were 0.32 and 0.1
mg/litre, respectively. A significant decrease in weight was noted in
both sexes in the 0.32 mg/litre group.
All medaka embryos exposed to 1.8 or 3.2 mg/litre and most of the
1.0 mg/litre group died before hatching. The NOLC after 3 months was
0.32 mg/litre. The NOEC values, on the basis of behaviour and
appearance, were 0.56 mg/litre and 0.32 mg/litre after 1 and 3 months,
respectively (Wester et al., 1988).
A short-term study with (lethal) concentrations of 0.56, 1.0, or
1.8 mg methyl bromide/litre showed (using scanning electron
microscopy) major degenerative and regenerative changes in the
superficial epithelia, especially of the gills and oral mucosa,
caused, apparently, by the local irritating action of this compound.
Necrotic changes were also seen in the thymic cortex and the testis
(Wester et al., 1988). In a long-term study on guppies and medakas
exposed to the highest concentrations for 1 month and 3 months,
respectively, no significant organ or tissue changes could be detected
in routine histopathology.
Table 37. Some fungi controlled by methyl bromide
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Alternaria sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Armillaria melea 4-98 g/m2 citrus/ Munnecke et al. (1969)
grape Kissler et al. (1973)
Aspergillus sp. 1.6-5 g/m2 pecan/ Wells & Payne (1975)
honeycomb Smirnov (1970)
Byssochalamy fuloa 60-120 mg/kg starch Ito et al. (1972)
Cladosporium sp. 1.6-3.3 g/m2 pecan Wells & Payne (1975)
Eumargodes laivgi 24 g/m2 not stated Hitchcock (1968)
Fusarium sp. 45-100 g/m3 tomato Westeijn (1973)
Weihing et al. (1971)
Wells & Payne (1975)
Perrotta (1968)
Vanachter (1974)
Monochaeta sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Penicillium sp. 1.6-3.3 g/m3 pecan
Pestalotia sp. 1.6-3.3 g/m3 pecan
Phoma sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Phytophora parasitica 49 g/m2 citrus Grimm & Alexander (1971)
Phytophora capsici 40 g/m2 green peppers Alfaro Moreno & Vehg (1971)
Table 37 (continued)
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Plasmodiophora brassicae 50-150 g/m2 cabbage Winstead & Garriss (1960)
Plasmosisphora brassicae 48 g/m2 cabbage Wimalajewa (1975)
Pyrenochaeta lycopersici 125 g/m3 soil Vanachter (1974)
Rhizoctania solani 50-150 g/m2 soil Winstead & Garriss (1960)
Sclerotium rolfsii 50 g/m2 iris Kiewnick (1968)
Sclerotina sclerotiorum 50 g/m2 tobacco Hartill & Campbell (1973)
Thielaviopsis basicola 50 g/m3 tobacco Mounat & Hitier (1959)
Verticillium sp. 45-70 g/m2 tomato Perrotta (1968)
Table 38. Effects on aquatic organisms of short-term exposure to sodium bromidea
Results (g Br-/litre) at:
Test species Parameter 24 h 48 h 72 h 96 h
Scenedesmus EC50 (growth) 5.8 7.8 8.5 10
pannonicus NOEC (growth) 2.5 2.5 2.5 2.5
(alga)
Daphnia magna LC50 (mortality) 11 11 - -
(crustacea) EC50 (mort./abn. behaviour) 5.8 5.8 - -
NOLC (mortality) 7.8 7.8 - -
NOEC (mort./abn. behaviour) 4.3 4.3 - -
Poecilia LC50 (mortality) 16 16 16 16
reticulata EC50 (mort./abn. behaviour) 0.44 0.14 0.044 0.044
(fish) NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.078 0.025 0.025
Oryzias latipes LC50 (mortality) 26 25 24 24
(fish) EC50 (mort./abn. behaviour) 0.44 0.44 0.44 0.44
NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.25 0.25 0.25
a From: Canton et al. (1983).
b NOE(L)C = no observed (specified) effect concentration.
7.2.2 Effect of bromide ion on aquatic organisms
The main degradation product of methyl bromide is inorganic
bromide.
To evaluate the potential impact of pollution with the bromide
ion, Canton et al.(1983) investigated the short-term effects of sodium
bromide on various freshwater organisms, using algae (Scenedesmus
pannonicus) , crustaceans (Daphnia magna), and fish (P. reticulata
and O. latipes) (Table 38). Depending on the species tested, acute
toxic effects were seen at concentrations ranging from 44 to 5800 mg
Br-/litre and, in long-term tests, the NOEC varied from 7.8 to 250
mg Br-/litre. Bromide ion markedly impaired reproduction in both
crustaceans and fish.
Further tests were performed on P. reticulata (guppy) and O.
latipes (medaka) following sodium bromide exposure for 1 and 3
months at concentration ranges of 10-32 000 mg/litre (guppy) and
180-56 000 mg/litre (medaka). NOLC values for guppies were 10 000
mg/litre and 1000 mg/litre after 1 and 3 months, respectively. The
NOEC value was 32 mg/litre in both studies. Histopathological changes
were observed at concentrations of 100 mg/litre or more (Wester et
al., 1988). For medakas, the NOLC values were 5600 and 3200 mg/litre
after 3 weeks and 3 months, respectively, whereas the NOEC value
(behaviour) for both periods was 320 mg/litre (Wester et al., 1988).
The relative susceptibility to sodium bromide of 11 taxonomically
different freshwater species was determined in medium-term toxicity
tests by Slooff & Canton (1983). The data are summarized in Table 39.
In semi-static, long-term toxicity tests on Daphnia magna, EC50
and EC10 values of 27 and 18 mg/litre, respectively, were determined
(Van Leeuwen et al., 1986).
7.3 Terrestrial organisms
7.3.1 Protozoa
Long et al. (1972) studied the effect of methyl bromide on
protozoa (Eimeria tenella and E. acervulina) at a dosage of 5
g/m3 for 20 h at 25 °C; 100 % control (destruction of oocysts) was
achieved.
7.3.2 Plants
Methyl bromide is often applied, as a fumigant, directly to plant
seeds, plant cuttings, or harvested plant products to disinfect
before, and during, transportation or storage (Davis et al., 1977).
Additionally, methyl bromide is used as a soil fumigant to control
certain plant pathogens and weed seeds in areas to be planted.
Table 39. Summary of the results of medium-term toxicity tests using
sodium bromide on 11 different freshwater test speciesa
Test species Exposure Criteria NOL(E)C
time valuesb
(days) (mg/litre)
Pseudomonas 0.3 specific growth rate 3200
fluorescens
(bacterium)
Microcytis aeruginosa 4 specific growth rate 3200
(cyanobacterium)
Scenedesmus 4 growth (biomass) 3200
pannonicus
(alga)
Lemna minor (plant) 7 specific growth rate 3200
Daphnia magna 21 mortality 3200
(crustacea) reproduction 10
Culex pipiens 25 mortality 100
(insect) development 100
Hydra oligactis 21 specific growth rate 1000
(hydrozoan)
Lymnaea stagnalis 40 mortality 3200
(mollusc) reproduction 10
hatching 3200
Poecilia recticulata 28 mortality 100
(viviparous fish) mortality and behaviour 32
growth 320
Oryzias latipes 40 mortality 3200
(viviparous fish) mortality and behaviour 320
hatching growth 10 000
Xenopus laevis 100 mortality 32
(amphibian) development 320
development 320
a Adapted from: Slooff & Canton (1983).
b NOL(E)C = no observed (specified) effect concentration.
As chloropicrin is phytotoxic, methyl bromide formulations that
contain it as a warning agent are not used on nursery stock or other
living plants (Bond, 1984).
7.3.2.1 Seed fumigation
As shown in Table 40, fumigation with methyl bromide can result
in delay in the germination of seeds and some loss of total
germinative capacity, depending on the variety, moisture content, and
the extent of exposure to the gas (Davis et al., 1977). This was
confirmed by Sittisuang & Nakakita (1985) who compared the effects of
methyl bromide on the germination of rice seeds ( Oryza sativa L.,
Japicona type) and corn (maize) seeds ( Zea mays L.). No detrimental
effect of methyl bromide up to 4 mg/litre was observed in rice seeds
at a moisture content of 11%, but as the moisture content and
temperature increased, methyl bromide had an increasing effect on
germination. Maize seeds were much more tolerant to methyl bromide.
Exposure to 5 mg methyl bromide/litre, which caused heavy damage to
rice seeds in most cases, did not generally produce any harmful effect
on the germination of maize seeds, regardless of the moisture content
and temperature. At concentrations higher than 10 mg/litre, the
viability of maize seeds declined in a similar way to that of rice
seeds. Rice seeds were found to absorb more methyl bromide than corn
seeds. Seeds with a higher moisture content absorbed more methyl
bromide and seeds with the same moisture content absorbed more methyl
bromide at higher temperatures than at lower temperatures. The authors
suggested that changes in certain proteins and enzymes were major
factors in seed viability.
The effects of methyl bromide fumigation on the germination of
different cultivars of wheat seed have been investigated. The
germination of all cultivars was reduced following fumigation at a
dose of 16 mg/litre for 24 h (11% moisture, temperature not given).
The optimal conditions were a moisture content of 9% and a temperature
of 18 °C. With increasing moisture or temperature, the percentage
germination decreased. At higher levels of moisture, the concentration
of methyl bromide appeared to be a more important factor than exposure
time at a constant concentration x time product (CTP) of 768
mg.h/litre (Khanna & Yadav, 1987).
Hanson et al. (1987) found that fumigation with methyl bromide
not only caused a delay in germination and loss of germinative
capacity but also that certain varieties of seed barley were damaged,
exhibiting symptoms of albinism and stunted growth. The authors
suggested that great care should be taken in the selection of stored
barley intended for seed and, in particular, in the fumigation of
samples in standard reference collections. A CTP of 200 mg.h/litre is
used commercially, but higher concentrations may be attained if the
distribution during fumigation is poor.
Table 40. Effects on germination of seeds fumigated with methyl bromidea
Seed Fumigation conditions Germination results Reference
hemp 70-140 g/m3 5-23 % reduction Tkalich (1974)
onion 42 g/m3 for 24 h 95 % reduction in laboratory Powell (1975)
11.5% reduction in cool soil
peanuts 32 mg/litre (24 h, 27°C, 80% reduction of: Leesch et al. (1974)
paper container relative humidity), applied 21.7%
burlap bags under cover, aerated 72 h 11.4%
oat, wheat, rye, 0, 600, or 1200 g.h/m3 at 8, 11at 18% moisture content: Blackith & Lubatti (1965)
barley 14, or 18% moisture content - no germination after 6 years storage
at 8% moisture content:
- 90% germination after 6 years storageb
Picea abies, Picea seeds at various moisture germination normal after storage Jones (1968)
glauca, Pinus mugo content; 48 g/m3, 24°C, 2-5 h, only if seeds aerated 24 h before
mughus, Pinus then aerated 1-25 h and stored storage; all but P. sylvestris
sylvestris (seeds) in sealed containers at 7°C for required drying to 5% moisture content
1 year before storage
tobacco seed 16-32 g/m3 or germination satisfactory at <10 % seed Guthrie & Kincaid (1957)
32-48 g/m3 moisture content: germination dcreased at
seed moisture contents above 10%
barley, corn, grain 32 g/m3 (< 24 h, 26°C); unimpaired germination seed Whitney et al. (1958)
sorghum, oats, wheat moisture content less than 12%
a From: Davis et al. (1977).
b Except rye, germinated well only up to 3 years storage.
7.3.2.2 Fumigation of plants or plant products
Direct fumigation of plants or plant products is used to retard,
or prevent, pest infestations and to overcome quarantine barriers.
Post-harvest fumigation is discussed in section 5.1.4.2.
7.3.2.3 The effects on plants of soil fumigation
Methyl bromide can have adverse as well as positive effects on
plants.
The phytotoxic effects of methyl bromide as a soil sterilant can
be caused by:
(1) the action on plants of methyl bromide itself;
(2) the action of inorganic bromide formed by the breakdown of
methyl bromide in the soil;
(3) indirect action through effects of either methyl bromide or
inorganic bromide on soil microflora, soil structure, or
composition (Maw & Kempton, 1973).
Where the crops are affected by lack of mycorrhizae, the plants
are stunted. Experiments have proved that this problem can be
rectified by fertilizing with phosphoric acid in the irrigation water,
using a trickle system (Bromine & Chemicals Ltd., 1990).
The phytotoxicity of methyl bromide is thought to be due mainly
to the high level of bromide ion. Drosihn et al. (1968) showed that
the degree of susceptibility of carnations to methyl bromide
fumigation of the soil depends on the intensity of subsequent leaching
of the soil. Similar findings were described by Kempton & Maw (1974).
In contrast to this, tomato plants were relatively insensitive to
bromide; growing tomatoes tolerated up to 0.1 mg bromide/g soil
without signs of injury or growth retardation (Maw & Kempton, 1973).
These authors found lettuce to be particularly resistant to inorganic
bromide, with some varieties growing in the presence of as much as 5
mg Br-/g soil.
Reichmuth & Noack (1983) determined the threshold concentration
of methyl bromide in air that should not be exceeded in the vicinity
of fumigated buildings, in order to protect plants. The test plants
( Lactuca sativa capitata (lettuce) and Nasturtium officinale
(water cress)) were exposed to concentrations of between 4 and 1400
mg methyl bromide/m3 for 72 h. At 400 mg/m3, yellowing of lettuce
leaves became apparent, while no visible effects were observed on
water cress up to the highest concentration.
(a) Cultivated plants
Only a limited number of genera, species, or varieties of plants
are susceptible to methyl bromide. Of the 441 species of glasshouse
plants tested by Latta & Cowgill (1941), 414 (93.9%) were not affected
and only 27 sustained various levels of damage; of these, five species
were severely burned. For example, roses showed no pronounced toxic
effects, when planted in soils aerated for four days after methyl
bromide fumigation, however, carnations were extremely sensitive to
both residual methyl bromide gas and inorganic bromide in the soil
(Kempton & Maw, 1974). Other crops, such as cotton, celery, pepper,
and onion, do not reach adequate growth, when grown in fumigated soil
(Bromine & Chemicals Ltd., 1990). Plants, actively growing, are more
likely to sustain injury than dormant plants (Bond, 1984).
(b) Weeds
The phytotoxic effects of methyl bromide on weeds are important
in soil fumigation.
Table 11 shows that the recommended dose rates to eradicate weeds
is 35-50 g/m2, though purple nutsedge (nut grass), corms and seeds
of horseweed Erigeron (Conyza) , mallow (Malva) , and legumes are
not efficiently controlled at this dose (Bromine & Chemicals Ltd.,
1990).
Methyl bromide (40-80 g/m2) is mentioned as being the best soil
fumigant against yellow nutsedge ( Cyperus esculentus L.), but the
weed was not completely eradicated because dormant tubers below the
tillage depth survived (Rotteveel & Naber, 1987).
7.3.3 Soil invertebrates
Soil fumigation with methyl bromide (and chloropicrin) results
generally in toxic effects in both target and non-target organisms.
The concentrations used are sufficiently high to eradicate populations
of a wide variety of organisms. Fumigants, including methyl bromide
and chloropicrin, were all strongly nematocidal. Methyl bromide killed
virtually all soil arthropods, including mites; Collembola were almost
completely eradicated. Methyl bromide was very toxic for symphylids
and millipedes (details of dose not given) (Edwards & Thompson, 1973).
Methyl bromide (concentration not given) and chloropicrin were very
toxic for earthworms, even those that lived in deep burrows (Van Rhee,
1977). Chloropicrin was repellent to most arthropods in soil (Edwards
& Thompson, 1973). To control nematodes, methyl bromide is generally
used at rates of up to 80 g/m2 (Table 41).
7.3.4 Insects and arachnids
Methyl bromide is used as a fumigant to control insect pests.
Although it is not as toxic for insect species as some other
fumigants, such as HCN, acrylonitrile, and ethylene dibromide, its
ability to penetrate quickly and deeply into sorptive materials makes
it an effective and versatile fumigant (Davis et al., 1977; Sassaman
et al., 1986). The commercial dosage for methyl bromide as a storage
fumigant ranges from 16 to 100 g/m3 for up to 3 days (Tables 12 and
13).
The dosage required depends also on the temperature. The
threshold concentration levels identified at 15 and 25 °C differed by
a factor of two or three. These investigations by Bell (1988) were
carried out on the adult beetle. The dosage of methyl bromide required
to kill eggs and pupae is greater than that required to kill all
adults. Pupae and older larvae of Tribolium spp., for example,
required CTPs of up to 180 mg.h/litre for control (Hole, 1981). For
other species and exposure conditions see Table 42.
In an FAO study, it was found that there was a variation in
tolerance to methyl bromide in different strains of eight species of
stored product beetles collected from different parts of the world
(Hole, 1981). Although resistant strains have been identified in the
laboratory, there have been no reports of resistance to methyl bromide
in practice (Bell, 1988).
The effects of lethal concentrations of methyl bromide (48 g/m3
for 2 h) on embryos of the codling moth ( Cydia pomonella L.) were
assessed using light microscopy and transmission electron microscopy
(Cheetham, 1990). Cell division stopped within one hour in nearly all
embryos, a small number of terata being produced.
Methyl bromide is used to eradicate various wood and household
pests, particularly in the warmer climates of southern and western
USA. The primary targets are drywood termites (Kalotermitidiae).
Scheffrahn & Su (1992) have assessed the toxicity of methyl bromide at
27 °C against pseudergates, nymphs, or alates of several species.
Estimates of lethal accumulated doses for 50 and 99% mortality ranged,
respectively, from 11.4 and 16.5 for R. hesperus pseudergates to
45.9 and 75.0 mg h/litre for C. cavifrons pseudergates and nymphs.
Alates were more susceptible to methyl bromide than pseudergates or
nymphs. Boczek et al. (1975) reported three periods in the development
of Acarus siro that have increased sensitivity to methyl bromide:
(a) before the beginning of gastrulation movements in the germ band;
(b) during the formation of the nervous system; and (c) the period
preceding dorsal closure. For this species, Burkholder (1966) found
LD100 values ranging from 3.4 to 16.8 g/m3 at various exposure
times at 16 °C and 85% relative humidity whilst achieving the same CTP
(65-83 g.h/m3).
Table 41. Effects of methyl bromide on nematodes
Nematode Effective Exposure Reference
control Conditions
concentration
Anguina agrostis CTP 600-800 12% moisture Hague (1963)
mg.h/litre
Belonolaimus 98 g/m2 98% methyl bromide Darby et al. (1962)
longicaudans 2% chloropicrin: covered 48 h
Ditylenchus dipsaci CTP 850 10-14% moisture Hague & Clark (1959)
mg.h/litre
Dorylaimus sp. 2.3 g/m3 40 h Van Gundy et al. (1972)
Hemicyclophora parvana 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Heterodera rostochiensis Ct 500-1000 treatment with water before fumigation Hague (1959)
mg.h/litre enhanced penetration of methyl bromide
Heterodera rostochiensis 111 g/m2 covered 16 days: 98 % methyl bromide Whitehead et al.
and 2% chloropicrin (1972)
Heterodera schachtii 0.5-30 g/m3 1-21 days Abdalla & Lear (1975)
Hoplolaimus columbus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Hoplolaimus tylenchiformis 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Meloidogyne sp. 50 g/m2 manure applied prior to fumigation Scotto La Massese
decreased nematocidal effect & Mars (1975)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Meloidogyne incognita 2.3 g/m3 38 h Van Gundy et al. (1972)
Meloidogyne incognita 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Meloidogyne incognita 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne incognita 0.6-2.5 g/m3 1-21 days Abdalla & Lear (1975)
Meloidogyne incognita 17-22 g/m2 chisel applicator, covered or rolled Sher et al. (1958)
acrita
Meloidogyne javanica 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne javanica 22-34 g/m2 chisel application, covered Thomason (1959)
Meloidogyne javanica 56-112 g/m2 98% methyl bromide Milne (1962)
and 2% chloropicrin
Pratylenchus sp. 45-67 g/m2 covered Raski et al. (1975)
Pratylenchus sp. 4.9-9.7 g/m3 1-3 days Abdalla & Lear (1975)
Pratylenchus brachynrus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Pratylenchus brachyurus 25-51 g/m3 24 hours; 25°C Minton & Gillenwater
(1973)
Pratylenchus penetrans 50 g/m2 not stated Chen et al. (1962)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Pratylenchus thornei 49 g/m2 covered after application Van Gundy et al. (1974)
for unspecified time
Pratylenchus zeae 100 g/m2 covered for 48 h Oakes et al. (1956)
following fumigation
Trichoderus christiei 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin:
covered 48 h
Xiphinema americanum 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 2.3 g/m3 28 h Van Gundy et al. (1972)
Xiphinema index 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 0.2-2.0 g/m3 1-21 days Abdalla & Lear (1975)
Table 42. Some insects controlled by methyl bromidea
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Antagenus picus 32 Pence & Morganroth
(black carpet beetle) (1962)
Anthonomus grandis 16-80 Roth & Kennedy (1972)
(cotton boll weevil)
Anthrenus flavipes 32 Pence & Morgenroth
(furniture carpet beetle) (1962)
Anthrenus verbasci 32
(varied carpet beetle)
Araecerus fasciculatus 6.2 (eggs) Majumder et al. (1961)
3.4 (larvae)
7.4 (pupae)
4.5 (adults)
Blatta orientalis 64 Hickin (1961)
(cockroach)
Blatella germanica 64
(cockroach)
Bruchus rufimanus 28 Roth & Richardson
(broad bean weevil) (1974)
Cadra cautella 32 Leesch et al. (1974)
(almond moth)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Callosobruchus 0.85 (eggs) 1.25 (eggs) Adu & Muthu (1985)
chinensis (L) 2.2 (instar 2.72 (instar
(cowpea weevil) larvae) larvae)
0.89 (pupae) 3.98 (pupae)
1.17 (adult) 1.4 (adult)
Chilo agamemnon 20 (larvae) Isa et al. (1970)
(corn borer)
Corcyra cephalonica 1.66-1.78 (eggs) El-Buzz et al. (1974)
(rice moth) 1.10-1.68 (instar
larvae)
2.79 (pupae)
Curcilio caryae 32-112 Leesch & Gillenwater
(pecan weevil) (1976)
Ephestia kuehniella 2.02-2.46 Mostafa et al. (1972)
(mediterranean flour moth)
Gnorimoschema operculella 11.74 (larvae) Pradhan et al. (1960)
(potato tuber moth)
Gryllotalpa 70-100 g/m2 Dzidzariya (1972)
(mole cricket)
Hemp leaf roller 40-45 Tkalich (1972)
Laspeyresia pomonella 32 Morgan et al. (1974)
(codling moth) 32 Anthon et al. (1975)
Megastigmus acuelatus 50 Vodolagin (1971)
(dog rose weevil)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Musca domestica 64 Hickin (1961)
(housefly)
Onychuirus hortensis 89 g/m2 Edwards (1962)
(springtail)
Oryzaephilus mercator 200 Joshi (1974)
(merchant grain beetle) 32 Leesch et al. (1974)
Ostrinia nubilalis 20 (larvae) Isa et al. (1970)
(corn borer)
Periplaneta americana 64 Hickin (1961)
(cockroach)
Plodia interpunctella 5.5 (normal larvae) Sardesai (1972)
(indian meal moth) 10.2 (diapausing larvae)
32 Leesch et al. (1974)
Sitophilus oryzae 5.45-6.19
(rice weevil)
Sitotroga cerealella 1.85-2.21
(angoumois grain moth)
Tenebroides mauritanicus 16 23 Bond (1956)
(cadelle) 25.5-43.3 Monro et al. (1966)
Isoptera
(termites; 5 species) 64 Hickin (1961)
Tribolium castaneum 0.009 (adults)
(red flour beetle) 3.06-6.19 Mostafa et al. (1972)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Trilobium confusum 3.6 - 91 4.64-145.14 Kenaga (1961)
(confused flour beetle) 15b
21.5-23.7 Monro et al. (1966)
Trogoderma granaria 0.038 (larvae) Pradhan & Govindan
(grain beetle) (1954)
Trogoderma variable 32-40 (eggs) Vincent & Lindgren
(warehouse beetle) (1975)
16-56 (instar larvae)
32-72 (pupae)
24-36 (adults)
a A range of LD values reflects effects of time, temperature, or pressure.
b Preceded by gamma radiation (50 krad).
LD100 values have been determined for Rhipicephalus sanguineus
(brown dog tick) ranging from 6 to 96 g/m3 (3.5 h, 22 °C and 6 h, 11
°C, respectively) (Roth, 1973).
7.3.5 Gastropods
The effects of methyl bromide on various gastropods (slugs,
snails, limpets) have been studied. Roth & Kennedy (1973) found an
LD100 for Helidella candidula and H. conspurcata, exposed for 24
h at a dosage of 240 g/m3 . Similarly, for Cochicella barbara (72
h, 13 °C) and Theba pisana (10 h, 13 °C) a dosage of 128 g/m3 was
lethal (Richardson & Roth, 1965).
7.3.6 Birds
Rhode Island Red female hens were fed, from hatching, on diets
that had been fumigated with methyl bromide at the concentration
recommended for the elimination of salmonellae (800 mg.h/litre) or at
1´ times this value (Cooper et al., 1978). Body weight, egg weight,
and egg number were not significantly affected by treatments, but
sexual maturity may have been slightly delayed. The egg flavour was
adversely affected. The same group had previously shown that the taste
of meat from broiler chickens was similarly tainted (Griffiths et al.,
1978).
No adverse effects on either the fertility or hatchability of
hens' eggs, previously fumigated with methyl bromide at 32 g/m3 for
24 h, were observed (Devaney & Beerwinkle, 1982).
7.3.7 Other animals
Data are not available on the direct environmental exposure to
methyl bromide of other animals. Effects on test animals are given in
section 8.
Bromide intoxication was reported by Knight & Costner (1977)
after horses, goats, and cattle were accidentally fed oat hay that had
been cut from a field treated with methyl bromide the previous autumn.
The bromide content of the hay ranged from 6800 to 8400 mg/kg so that
the estimated mean daily intake was 9, 49, and 70 g of bromide ion in
goats, horses, and cattle, respectively. Signs of intoxication
reported included lethargy, weakness, and ataxia. Similar symptoms
were noticed between the 7th and 9th days in animals fed this hay on
an experimental basis. Signs of in-coordination (between 10th and 12th
days) were correlated with serum bromide concentrations of 30
mEq/litre (2.4 g/litre) or more (Knight & Reina-Guerra, 1977). Serum
bromide concentrations and the associated neurological signs subsided
markedly 14 days after feeding discontinued (Knight & Reina-Guerra,
1977). Methyl bromide is not approved for use prior to the planting of
forage crops.
7.4 Population and ecosystem effects
Application of methyl bromide as a soil fumigant resulted in the
almost complete eradication of populations of a wide variety of
microflora and fauna, as well as other soil organisms, thus altering,
at least temporarily, the trophic structure of the soil environment
(Sassaman et al., 1986).
Treatment with 100% methyl bromide and other methyl
bromide/chloropicrin formulations reduced populations of Fusarium,
Pythium, and Rhizooctonia species in soil. Nine weeks after
application, populations were still significantly lower. Seedlings
grown in treated plots had the least amount of damping off and root
rot (Enebak et al., 1988).
Methyl bromide dosed under plastic sheeting at a rate of 300
g/m3 (for 30 cm depth 100 g/m2) killed all insects, though small
numbers of soil nematodes and mites were collected during subsequent
sampling (Heungens & Roos, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
A summary of acute oral toxicity data is given in Table 43. Very
few studies have been carried out, mainly because methyl bromide is a
gas at temperatures above 4 °C. The minimum lethal oral dose of methyl
bromide for rabbits was found to be 60-65 mg/kg body weight (Dudley et
al., 1940; Dudley & Neal, 1942). Miller & Haggard (1943) found that
all rats given a single oral dose of 100 mg/kg body weight in olive
oil died in 5-7 h.
8.1.2 Inhalation
8.1.2.1 Guinea-pig and rabbit
Single exposure toxicity tests conducted on various mammalian
species have shown that methyl bromide is highly toxic. A summary of
the acute toxicity data is presented in Table 44.
Studies on guinea-pigs (Sayers et al., 1929) and rabbits (Irish
et al., 1940) were carried out. Rabbits were exposed to concentrations
of 420, 852, 1000, 2000, 10 000, 20 000, and 50 000 mg methyl
bromide/m3. Table 45 shows the exposure times giving 100% survival
and 100% mortality. Concentrations of methyl bromide above 10 000
mg/m3 sometimes caused the rabbits to close their eyes; otherwise
they appeared normal until they became too weak to hold up their heads
(Irish et al., 1940). Rabbits that survived 1000 mg methyl
bromide/m3 for 2 days after exposure usually became paralysed (Irish
et al., 1940).
Toxicity is a function of the concentration levels and the
exposure times (see also Table 45). The steep dose-mortality response
to methyl bromide found by many authors can be seen in Fig. 9.
8.1.2.2 Mouse
The results of single exposure inhalation studies on mice,
carried out by Alexeeff et al. (1985), Yamano (1991), and the Japanese
Ministry of Labour (1992), are given in Tables 46 and 47. The sharp
onset of lethal toxicity was shown in all cases. Alexeeff et al.
(1985) exposed male mice to methyl bromide (870-5930 mg/m3) for 1 h
and observed that clinical signs and mortality were dose related, with
the possibility of delayed effects in target organs, such as the
kidney.
Table 43. Acute and short-term oral (gavage) toxicity
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rabbit n.d. single 56-71 all rabbits given an oral dose of Dudley et al. (1940)
63.9 mg/kg died; one rabbit receiving Dudley & Neal (1942)
56.3 mg/kg died; all rabbits given
56.1 mg/kg or less survived;
destruction of superficial layers of
stomach and duodenum with accompanying
haemorrhage and hyperaemia; minimal lethal
dose: 60-65 mg/kg body weight
rat n.d. single 100 all died in 5-7 h Miller & Haggard (1943)
rat n.d; single 190-239 LD50, 214 mg/kg Danse et al. (1984)
rat n.d. 4 weeks; 50 epithelial hyperplasia, hyperkeratosis
7 days/week and ulceration of the forestomach
rat 10 (male) 13 weeks; 0 10 and 50 mg/kg: proliferative
(Wistar) 10 (female) 5 days/week 0.4 alterations of forestomach mucosa;
2 50 mg/kg: haematological changes
10 13/20 squamous cell carcinomas of
50 forestomach
rat 15 13-25 weeks; 0 treated group: week 13: forestomach Boorman et al.(1986)
12 weeks 50 acanthosis, fibrosis, pseudoepitheliomatous
recovery for hyperplasia; week 25: hyperplastic lesions
some groups of forestomach
recovery group: regression of stomach lesions,
but adhesions, fibrosis, and mild acanthosis
remained; evidence of malignancy in one rat
Table 43 (continued)
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rat n.d. 4,8,13, and 0 treated groups: forestomach ulceration Hubbs & Hartington (1986)
(n.d.) 17 weeks; 25 pseudoepitheliomatous hyperplasia
5 days/week 50
n.d. 13 weeks; 0 recovery period: marked but incomplete
5 days/week 25 regression of lesions;
recovery for 50 no evidence of malignancy
4-8 weeks
a n.d.= no details given.
Table 44. Single exposure inhalation studies of methyl bromide on mammalsa
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
mouse 94 950 25 100 % died within 6 h Bachem (1927)
mouse 700 n.d. 100 % survived Bachem (1927)
rat 20 000 6 100 % survived Irish et al. (1940)
24 100 % died
rat 43 000 3 survived Clarke et al. (1945)
rat 50 000 3 100 % survived Irish et al. (1940)
6 100 % died
rabbit 70 40 change in motor Balander & Polyak
reflex behaviour (1962)
rabbit 19 000 25 deep (fatigued) Beyne & Goett
breathing (1934)
rabbit 20 000 36 100 % survived Irish et al. (1940)
rabbit 20 000 84 100 % died
rabbit 25 000 30 died Beyne & Goett
(1934)
rabbit 31 600 5 died after 8-10 h Duvoir et al. (1937)
rabbit 36 000 25 died Beyne & Goett
(1934)
rabbit 50 000 12 100 % survived Irish et al. (1940)
30 100 % died
dog 10 000 5-6 h died Beyne & Goett
(1934)
dog 17 000 n.d. died Merzbach (1928)
dog 19 000 n.d. died Beyne & Goett
(1934)
dog 34 000 60 died Merzbach (1928)
Table 44 (continued)
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
dog 48 000 40 died Duvoir et al. (1937)
dog 50 000 45 died Merzbach (1928)
a Adapted from Henschler (1990).
b n.d. = no details given.
6.2.2 Human studies
Data on the concentrations of bromide in various human tissues after
methyl bromide poisoning are scarce. In an autopsy study of a methyl
bromide-exposed patient, Heimann (1944) reported the following bromide
values: lung (127 mg/kg), liver (187 mg/kg), brain (207 mg/kg), and,
in a composite sample of heart, kidney, and pancreas (107 mg/kg).
Traces of methyl alcohol and formaldehyde were also found in all the
tissues examined.
In four lethal cases of people exposed to methyl bromide,
Marraccini et al. (1983) found bromide ion concentrations in serum or
plasma ranging from 40 to 583 mg/litre. Methyl bromide was detected in
the brain of one patient (detection limit <1 mg/kg).
Values of 0.9 mg/kg in lymph nodes, 3.3 and 5.1 in ovaries and
testes, 7.5 in lung, and 8.2 mg/kg wet weight in kidney cortex have
been reported in autopsy samples (from accident victims) (Hamilton et
al., 1972/73). A study of the levels of bromide in adipose tissue from
human subjects in three countries showed the highest levels in the
United Kingdom, where 5.6% of the specimens contained levels ranging
from 4.0 to 4.5 mg/kg fat; the lowest levels were found in Germany
(0-0.9 mg/kg fat) whereas levels of 1-3.7 mg/kg were found in the
Netherlands samples (Crampton et al., 1971). Van Leeuwen & Sangster
(1987) stated that there was no evidence in humans of bromide
concentration in any particular organ that might indicate a specific
physiological function of this ion.
6.3 Metabolic transformation
The metabolism of methyl bromide has not been elucidated.
Bromide concentrations in blood (and target organs) were reported
to be increased in humans (Clarke et al., 1945; Rathus & Landy, 1961;
Hine, 1969) and in laboratory animals (Irish et al., 1940, 1941) after
exposure to methyl bromide. Miller & Haggard (1943) postulated that
methyl bromide is hydrolysed in the body with the formation of
inorganic bromide and methyl alcohol. In part, this hydrolysis may
occur intracellularly, resulting in a distribution of bromide that
differs from that for bromide given orally as sodium bromide. Sodium
bromide and methyl alcohol, given at the levels produced after methyl
bromide exposure, did not produce the same toxic and functional
response (Irish et al., 1940, 1941). This suggested that the toxicity
of methyl bromide was due to the reaction of the halide molecule with
the tissue and was not attributable to the hydrolytic products.
Hallier et al. (1990a) measured the cytosolic turnover rate of
methyl bromide in both liver and kidney from five different strains of
mice and rats, with in vitro incubation. The turnover rate in both
organs was consistently higher in tissues isolated from females. On
the basis of a similar effect with methyl chloride, this effect could
be attributed to a higher rate of glutathione conjugation in females.
The reaction products of methyl bromide in wheat were
characterized by Winteringham et al. (1955) using 14C- or
82Br-labelled methyl bromide. It was pointed out that, although most
attention is given to the bromide ion because it is the only part of
the residue that is easily determined, methyl methionine sulfonium
bromide, the methylated histidines, and possibly other residues of
methylation were also produced.
6.3.1 Binding to proteins and lipids
Methylation of cysteine- S and histidine- N residues of haemo-
globin in suspended mouse erythrocytes was found after in vitro
treatment with radiolabelled methyl bromide (Djalali-Behzad et al.,
1981). After inhalation of methyl bromide, alkylation of cysteine- S
residues was seen in mouse haemoglobin and liver proteins
(Djalali-Behzad et al., 1981).
Adducts result from reactions between toxic chemicals and amino
acids in haemoglobin or other proteins (or nucleosides in DNA - see
below). S-methyl-cysteine has been studied as a haemoglobin adduct
in mice (Iwasaki, 1988a,b) and rats (Xu et al., 1990) and as a serum
albumin adduct in human blood samples (Müller et al., 1991, 1992).
Both haemoglobin and serum albumin adducts have been studied in blood
samples of workers occupationally exposed to methyl bromide and these
have been proposed as suitable parameters for the biomonitoring of
exposure to the fumigant (Iwasaki et al., 1989; Müller et al., 1991,
1992) (see also section 9.4.4).
In the insect Triatoma infestans , Castro et al. (1976)
demonstrated that methyl bromide is irreversibly bound to lipids and
to proteins in both the nymph adult and eggs and that exposure to
methyl bromide significantly decreased the content of sulfhydryl
groups in nymph adult and egg proteins.
Studies on methylation by methyl bromide of wool, silk, collagen,
and gelatin (Blackburn & Phillips, 1944), wheat flour (Bridges, 1955;
Winteringham et al., 1955), and cocoa beans (Asante-Poku et al., 1974)
indicated that N-, O-, and S- methylation of proteins occurs
(Cova et al., 1986; Starratt & Bond, 1990b). The main site of
decomposition of methyl bromide in cocoa beans was shown to be in the
alcohol- insoluble proteins of the shell (Asante-Poku et al., 1974).
The methyl group of the fumigant became covalently bound to the
alpha-amino group of the various amino acids, the imidazole ring of
histidine, and the epsilon-amino group of lysine.
Winteringham et al. (1955) found that the gluten fraction of
whole-wheat flour exposed to [14C]methyl bromide was responsible for
80% of the decomposition of the absorbed fumigant with N-methyl,
dimethylsulfonium, and methoxyl and thiomethoxyl derivatives
accounting for 50, 30, and 20%, respectively, in this fraction.
Bridges (1955) reported that 1- N-methylhistidine,
3- N-methylhistidine and 1,3- N,N-dimethylhistidine accounted for
75% of the N-methyl derivatives, and that 10% was due to probably
epsilon- N-methyllysine.
Starratt & Bond (1990a,b) used [14C]methyl bromide to
distinguish naturally occurring residues from those formed during the
fumigation of a variety of commodities: maize, wheat, oatmeal,
peanuts, almonds, alfalfa, potatoes, oranges, and apples. In order to
get higher incorporation of the tracer, fumigation was carried out at
a level of 48 mg/litre, for 3 days. Methyl bromide was bound both
physically and chemically to the commodities. To measure the
physically-bound residue, a parallel test was run using unlabelled
fumigant. Methyl bromide levels were determined 1 h following
fumigation and then at 1, 2, 4, and 10 days. The levels in all the
commodities declined rapidly.
Extraction of the [14C]methyl bromide-fumigated commodities
with diethyl ether removed very little radioactivity, showing that
fats and other non-polar lipids were not methylated during treatment.
In maize, fractions corresponding to albumins, glutamines, zein, and
glutelin were all methylated.
Methylation of methionine is one of the main reactions forming
the relatively unstable methylmethionylsulfonium derivative.
Spontaneous decomposition yields dimethyl sulfide. Analysis of the
sites of methylation was uncertain as both acidic and basic hydrolysis
caused partial decomposition (Starratt & Bond, 1990a,b). The volatile
products included methanol, methyl mercaptan, and dimethyl sulfide.
Different commodities produced different residues (Starratt & Bond,
1990a,b). In potato and orange extracts, the main methylated
components were identified as S-methyl-glutathione,
gamma-glutamyl- S-methylcysteine and S-methyl cysteine. These
compounds were not found in maize. 1- N-methylhistidine and
3- N-methylhistidine were the major components from the fumigated
maize, almonds and other commodities. The highest level of histidine
methylation occurred in almonds, accounting for about 54% of the
chemically-bound residue.
6.3.2 Binding to DNA
Labelled 7-methylguanine was identified in DNA from liver and
spleen cells of mice exposed to [14C] methyl bromide (Djalali-Behzad
et al., 1981). In in vitro experiments with DNA solutions treated
with [14C] methyl bromide, predominantly [14C] 7-methylguanine was
identified (Starratt & Bond, 1988b).
Calf thymus DNA treated in the solid state with [14C] methyl
bromide, showed, on analysis, four major radiolabelled peaks with
retention times corresponding to 1-methyladenine, 7-methylguanine,
3-methyladenine, and 3- methylcytosine (Starratt & Bond, 1988b).
The DNA adducts, [14C]3-methyladenine, [14C]7-methylguanine,
and [14C]O6-methylguanine, have been found in the stomach and
forestomach of rats after both oral and inhalation exposure of
[14C]methyl bromide (Gansewendt et al., 1991).
In maize and wheat, 7-methylguanine and 1-methyladenine were
identified as major products after hydrolysis together with lesser
amounts of 3-methylcytosine and 3-methyladenine (Starratt & Bond,
1988a). Although the yields of DNA were low, Starratt & Bond (1990a)
also found evidence of radioactively-labelled 7-methylguanine and
1-methyladenine in [14C]methyl bromide-fumigated almonds and
potatoes.
6.3.3 The role of glutathione in methyl bromide metabolism
6.3.3.1 Mammals
Liver, kidney, lung, and brain from mice, exposed via inhalation
for 1 h to methyl bromide concentrations of from 870 to 5930 mg/m3,
were analysed for glutathione and bromide ion (Alexeeff et al., 1985).
The liver glutathione levels of the 4700 and 5930 mg/m3 exposure
groups were significantly lower than that of the controls. Bromide ion
levels were highest in the liver and kidney and lowest in the whole
blood. The lung and brain bromide levels were intermediate.
Methyl bromide has been shown in rats to increase the activity of
glutathione S-alkyl transferase and decrease the nonprotein
sulfhydryl content (Roycroft et al., 1981). A group of male
Sprague-Dawley rats were exposed to methyl bromide (117 mg/m3; 6
h/day; 10 days) and killed immediately after the last dose.
Biochemical analyses showed that glutathione S-alkyl transferase was
significantly increased in the lung (12%), liver (11.9%), and kidney
(6.9%). Non-protein sulfhydryl was significantly reduced by 11.4% in
the liver and 13.9% in the kidney. Glucose-6-phosphate dehydrogenase
was significantly increased in the kidney (8.5%), but not in the lung
or liver.
Studies on rats by Davenport et al. (1992) showed that
glutathione was depleted and regional brain
glutathione- S-transferase inhibited by methyl bromide inhalation
(584 mg methyl bromide/m3; 6 h/day; 5 days) [see sections 8.8.2 and
8.10].
In a preliminary report, Thomas & Morgan (1988) reported that
treatment of rats with buthionine sulfoximine (BSO) depleted
glutathione levels, prior to methyl bromide exposure, and increased
the toxicity of methyl bromide. This is in contrast to the findings of
Chellman et al. (1986) for methyl chloride in mice, who found that
glutathione depletion by BSO decreased methyl chloride toxicity in the
brain and kidney. However, the observation is consistent with those of
Tanaka et al. (1988), who showed that treatment of rats with
glutathione reduced the detrimental effects of methyl bromide on
sleep-wakefulness and its circadian rhythm and increased the LD50
value (section 8.8.2).
In whole body inhalation studies on rats exposed for 6 h/day, for
5 or 10 days, to 117 mg methyl bromide/m3 (30 ppm), glutathione
(GSH) S-transferase and glucose-6-phosphate dehydrogenase (G-6-PDH)
activities were increased in the lung. Decreases in GSH-reductase and
GSH- S-transferase activities were found in the liver (Jaskot et al.,
1988).
When human erythrocyte cytoplasm was incubated with methyl
bromide or methyl iodide in the presence of excess glutathione (GSH),
a spontaneous non-enzymatic conjugation was observed (Deutschmann et
al., 1989). This was verified in parallel experiments with boiled
cytoplasm and GSH added after boiling.
Enzymatic conjugation of methyl bromide with reduced glutathione
to produce S-methyl cysteine (the analysis) appears to be
isoenzyme-specific, since conjugation was observed in the erythrocyte
cytoplasm of a majority (13/20) of the population. The same
individuals also conjugated methyl chloride, a reaction that is
dependent on glutathione- S-transferase rho (GSTrho), a minor form of
the enzyme (Hallier et al., 1990b).
Later studies on the properties of the glutathione-
S-transferase (GST) responsible for methyl bromide conjugation with
GSH (measured by methyl bromide depletion) have separated the enzyme
GSTsigma from human erythrocytes; this new enzyme has not been found
in various non-human species (Schröder et al., 1992).
Measurement of methyl bromide disappearance in head-space vials
containing whole human blood cultures in an atmosphere of 19 460
mg/m3 (5000 ppm) at 37 °C indicated that the methyl bromide
concentration had fallen to zero within 1 h, in the presence of blood
from glutathione conjugators, whereas it had fallen to approximately
5836 mg/m3 (1500 ppm) at 1 h, in the presence of blood from
non-conjugators. Further reduction was slow, the methyl bromide
concentration being about 3890 mg/m3 (1000 ppm) after 6 h (Hallier
et al., 1993).
6.3.3.2 Insects
Glutathione is depleted and glutathione S-transferase can be
induced in the larvae of the khapra beetle (Trogoderma granarium) by
fumigation with methyl bromide at a lethal dose (Shivanandappa &
Rajendran, 1987).
Starratt & Bond (1981) demonstrated that, in the granary weevil
( Sitophilus granarius L.), the major pathway for the detoxication of
methyl bromide residues was by conjugation, primarily with
glutathione, and that increasing amounts of glutathione in the insects
resulted in increasing tolerance to methyl bromide exposure. Both
strains of the insect (methyl bromide sensitive and methyl bromide
resistant) metabolized methyl bromide primarily to substances that, on
thin layer chromatography, behaved consistently with their
identification as S-methyl glutathione.
6.4 Elimination and excretion in expired air, faeces, urine
The route of administration of methyl bromide affects the
pathways of excretion.
Miller & Haggard (1943) investigated the amount of methyl bromide
eliminated and the amount of bromide retained in the body after i.p.
administration of methyl bromide. Following a single i.p. injection of
60 mg methyl bromide/kg body weight, elimination continued for about
45 min and more than 90% was eliminated in the first 30 min. With
lethal i.p. doses of 120-180 mg methyl bromide/kg, 24- 45% of the
methyl bromide was eliminated; the amounts of fixed and nonvolatile
bromide being 94.9-126.6 mg/kg. In rats dosed orally (75-100 mg methyl
bromide/kg), similar bromide levels were found, but the methyl bromide
eliminated was given as only 2.4-4.6%.
These results have been confirmed by studies using radioactively
labelled methyl bromide (Medinsky et al., 1984). In rats dosed
intraperitoneally with [14C] methyl bromide, the major route of
elimination was the exhalation of 14CO2 (46%). In contrast,
urinary excretion of [14C] was the major route of elimination (43%
of the dose), when methyl bromide was given orally. Very little
appeared in the faeces (<3% of the dose), regardless of the route of
administration. In rats with bile duct cannulations, 46% of an oral
dose appeared in the bile over a 24-h period (Medinsky et al., 1984).
The authors suggested that reabsorption of biliary metabolites from
the gut played a significant role in the disposition of [14C] methyl
bromide.
In inhalation studies on rats exposed for 6 h to 337 nmol [14C]
methyl bromide/litre air, Bond et al. (1985) showed that excretion of
[14C] as 14CO2 was the major route of elimination, about 47%
(3900 nmol/rat) of the total [14C] methyl bromide absorbed being
excreted by this route. CO2 excretion exhibited a biphasic
elimination pattern with 85% of the 14CO2 being excreted with a
half-time of 3.9 h and 15% excreted with a half-time of 11.2 h.
Half-times for the elimination of 14C in urine and faeces were 9.6
and 16.1 h, respectively; 65 h after exposure, about 75% of the
initial radioactivity had been excreted with 25% remaining in the body
(Bond et al., 1985). Elimination half-times of [14C] from tissues
were 1.5-8 h. In all tissues examined, over 90% of the 14C in the
tissues were methyl bromide metabolites (Bond et al., 1985). The data
from this study indicate that, after inhalation, methyl bromide is
rapidly metabolized in tissues and readily eliminated.
Male CD rats were exposed (nose only) to [14C]-methyl bromide
at 214 mg/m3 (55 ppm) for 3 min. The liver, lung, and kidney were
the major organs of [14C] distribution, immediately after exposure.
Up to 32 h after exposure, the major routes of excretion were
pulmonary and renal with elimination of 43% and 21% of the total
inhaled label, respectively (Jaskot et al., 1988).
Studies into the time course of methyl bromide and bromide
elimination in rat tissue have been described by Honma et al. (1985).
Male Sprague-Dawley rats were exposed to 973 mg methyl bromide/m3
(250 ppm) for 8 h and methyl bromide and bromine concentrations
measured at successive time intervals (see also section 6.2). The
results are shown in Fig. 7 (bromine) and Fig. 8 (methyl bromide). The
methyl bromide levels in all tissues described reached a maximum in 1
h following exposure and remained at almost the same levels during
exposure. Methyl bromide levels decreased rapidly after exposure;
after 30 min only half of the methyl bromide concentrations in adipose
tissue and blood were still present (Fig. 7). The methyl bromide
levels in the brain and liver were very low, but the elimination of
methyl bromide from these organs was slower. Forty-eight hours after
exposure, methyl bromide could not be detected in any tissue examined
(Honma et al., 1985). In contrast to the methyl bromide study, peak
concentrations of bromine in blood, kidneys, and liver occurred 4-8 h
after bromide exposure, and the half-life in these tissues was about
5 days.
Honma et al. (1985) carried out a regression analysis of methyl
bromide and bromine concentrations in blood, kidney, liver, brain and
adipose in male rats after a 2-h exposure to 0, 973, 1946, 2918, or
3890 mg methyl bromide/m3 (0, 250, 500, 750, or 1000 ppm). Linear
relationships were obtained between exposure concentrations and the
tissue methyl bromide or bromine values.
Over 95% of the bromide ion in mice exposed to methyl bromide was
eliminated within 2.5 days, bringing the concentration close to the
control levels and the limit of detection (Alexeeff et al., 1985).
Lactating cows fed a methyl bromide fumigated grain ration (220
mg bromide/kg food) compared with unfumigated diets secreted increased
levels of bromide in the milk, i.e., 10-20 mg/litre instead of about
5 mg/litre (Lynn et al., 1963).
6.5 Retention and turnover
The biological half-life of bromide ions in human blood was found
to be about 12 days (Söremark, 1960).
The half-lives of bromide in the blood and brain of rats after
i.p. injection of methyl bromide were approximately 8.7 and 4.3 days,
respectively (Tanaka et al., 1988). In inhalation studies, a half-life
of bromide of about 5 days was reported in the blood, kidneys, and
liver (Honma et al., 1985). In contrast, methyl bromide concentrations
in the blood and adipose tissue were reduced by half after 30 min,
though elimination from the brain and liver was much slower (Honma et
al., 1985).
6.6 Reaction with body components
Honma et al. (1987) suggested that the main target of methyl
bromide in the body was the central nervous system. The nor-
epinephrine content of the hypothalamus and cortex with hippo-campus
was reduced on exposure to methyl bromide (Honma et al., 1982) and
changes in the amino acid content and metabolism in the brain were
noted (Honma et al., 1983). The results of further studies suggested
that alterations in catecholamine metabolism might be a factor in
methyl bromide-induced neurotoxicity (Honma et al., 1987) (see section
8.8). Kato et al. (1986) described histopathological changes in the
brain.
Eustis et al, (1986, 1988) found clear species- and sex- related
differences in the susceptibility of specific organs and tissues to
methyl bromide effects (section 8.8.1). In rats, neuronal necrosis
occurred primarily in the cerebral cortex, hippocampus, and thalamus
of the brain, whereas in mice, necrosis of the internal granular layer
of the cerebellar folia was more frequently observed. Nephrosis
occurred in all treated mice. Myocardial degeneration was observed in
male and female rats more frequently than male mice. Atrophy of the
adrenal cortex and testis and necrosis of the olfactory epithelium
were described. Similar findings were described by Hurtt et al.
(1987). Degeneration and regeneration of the olfactory epithelium were
also described by Hurtt et al. (1988) and Hastings et al. (1989);
Hastings (1990).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Soil microorganisms
Methyl bromide is used commercially to control soil- borne fungi
that cause:
- damping off: Rhizoctonia solani, Pythium spp., Sclerotium
bataticola (Macrophomina phaseolina), Phytophtora spp.,
and Thielaviopsis basicola.
- crown rot: Sclerotium rolfsii and Sclerotinia spp.
- root rot: Pythium spp., Stromatinia, Fusarium spp.,
Sclerotium bataticola, (Macrophomina phaseolina),
Rhizoctonia solani, Pyrenochaeta spp. (corky root, pink
root), Armillaria and Phytophthora spp.
- wilt: Fusarium spp., and Verticillium spp.
Generally, concentration-time products of methyl bromide required
to kill fungi are much higher than those needed to control insect and
nematode pests, susceptibility increasing with temperature. To control
fungi, methyl bromide is generally used at rates of 40-100 g/m2
(Davis et al., 1977).
Filip & Roth (1977) demonstrated the efficacy of methyl bromide
against Armillaria root rot in the stumps of ponderosa pine ( Pinus
ponderosa Laws). The survival of young pine trees in areas infected
with such fungi was increased after the elimination of the fungus,
using the fumigant.
Heungens & Roos (1982) recovered non-pathogenic fungi
(Penicillium and Mucor) following the application of methyl
bromide (300 g/m2) to pine litter, but no pathogenic fungi were
recovered.
After an initial reduction, the numbers of bacteria and fungi in
fumigated soil remained high and low, respectively, in comparison with
those in untreated soil (Sivasithamparam et al., 1987). In the
fumigated soil, Trichoderma species rapidly recolonized the soil,
becoming the dominant fungus within 15 days. In a study on the
microflora in the rhizosphere of wheat, the same authors found that,
though there was no difference in the total number of bacteria,
actinomycetes, and fungi, before and after fumigation with methyl
bromide, there were some fungal species differences with Fusarium
merismoides, T. koningii , and T. viride present in significantly
higher numbers, other fungi being less abundant.
Kelley & Rodriguez-Kabana (1979) reported that methyl bromide did
not cause any permanent changes in soil enzyme activities or adversely
affect the mycorrhizal root development of pine seedlings.
Methyl bromide is used for controlling fungal infections in the
poultry industry (Harry et al., 1972; Davis et al., 1977).
Methyl bromide is much less frequently used as a bactericide than
as an insecticide (Davis et al., 1977). It is used to control certain
soil-borne bacteria, e.g., bacterial canker (Corynebacterium
michiganese) , and bacterial wilt (Pseudomonas solanacerum)
(Bromine & Chemicals Ltd.,1990). A summary of the acute toxic effects
of methyl bromide on bacteria and viruses is given in Table 36.
Methyl bromide, used at a concentration-time product of 800
mg.h/litre at 25 °C, with a relative humidity of 70%, eliminated
salmonellae from artificially contaminated poultry foodstuffs (Tucker
et al., 1974).
Although there have been many studies on the effectiveness of
methyl bromide in reducing diseases produced by pathogenic
micro-organisms, there are fewer data on its effects on soil microbes.
Matta & Porta-Puglia (1968) tested methyl bromide on several
morphologically and functionally different groups of soil microbes. In
isolated soil samples, treated and maintained under constant
temperature (22-23 °C) and humidity (16-18%), microorganisms were
counted at 2, 21, 54, and 87 days following fumigation with 300 g
methyl bromide/m3. After 2 days, most bacteria were dead; after 87
days, there were very low counts of fungi, aerobic nitrogen-fixing,
nitrifying, and cellulolytic bacteria, whereas denitrifying,
proteolytic, amylolytic, and ammonifying bacteria showed a marked
resurgence in recolonization (Matta & Porta-Puglia, 1968). The
selective-action effects of methyl bromide fumigation on a given
microbe population in soil appear to be more significant than the
effects on microbe number.
Table 36. Effects of methyl bromide fumigation on bacteria and virusesa
Organism Dosage Conditions Reference
Vibrio cholera, Shigella 33 g/m3 10-h exposure LD100 Saiki (1952)
dysenteriae, Salmonella
typhi, Salmonella paratyphi A,
Salmonella paratyphi B
Corynebacterium 10 % bromomethane, 18-h exposure LD100 Richardson & Monro (1962)
sepedonicum 5 % ethylene oxide 85 % CO2
Arabis mosaic virus 0.32 kg/m3 controlled virus on strawberry Harrison et al. (1963)
plants
Tobacco mosaic virus, 640 g/m3 inactivated virus Inouye et al. (1967)
Cucumber green mottle virus 110 g/m3 inactivated at 27°C
320 g/m3 inactivated at 14-16°C
Escherichia coli 1257 1000 g/m3 40°C and 90 % relative humidity Prishchep &Nikiforova
provided control (1969)
Bacillus larvae, Bacillus 5000 g/m3 5-day exposure controlled Smirnov (1970)
paraalvei, Streptococcus bacteria in bee honeycombs
apis, Streptococcus pluton,
Pseudomonas apisepticus
Tobacco mosaic virus 200 g/m3 inactivated virus in 3 kg soil Doraiswamy et al. (1972)
in which tomatoes were grown
Salmonella typhimurium 800 mg-h/litre 25 °C and 70% relative humidity Tucker et al. (1974)
provided control
Xanthomonas begoniae 0.32 kg/m3 24-h exposure eliminated Strider (1975)
bacterial blight from Rieger
begonia
a Adapted from: Davis et al. (1977).
In a study on bacterial flora involved in the nitrogen cycle, hot
fumigation with methyl bromide at concentrations of 80 g/m3 was
carried out in greenhouses at 6 different sites (Turtura et al.,
1988). Seven months after treatment, the total aerobic mesophile
bacteria count, aerobic nitrogen-fixing, ammonifying,
ammonia-oxidizing, and nitrite-oxidizing bacteria, always showed
higher values in fumigated than in unfumigated control soils.
Recolonization was more marked in the upper 0-30 cm soil samples in
which the development of ammonifying and nitrifying bacteria was
highly significant.
Rovira & Ridge (1979) found no long-term effects on aerobic soil
bacteria or actinomycetes with application of 22 g methyl bromide/m2.
Yeates et al. (1991) described the recolonization of soils
sterilized in the laboratory and returned to their original pasture
and forest sites, under four different types of field conditions.
Sampling took place over 166 days (midsummer to midwinter) with two of
the sites having a moderate, and two a high, rainfall. Both microbial
biomass and dehydrogenase activity recovered rapidly but remained
consistently lower in the fumigated than in the untreated samples in
all four sites. Bacterial numbers also recovered rapidly. Fungal
hyphal lengths were 25% lower in the fumigated soil. Fumigation showed
no detectable effects on the subsequent rates of nitrogen
mineralization and little effect on nitrification rates. Protozoa were
almost completely eliminated by fumigation, numbers recovering most
rapidly in moist forest soil and slowly in dry pasture soil. Nematodes
were eliminated by fumigation; recolonization was first detected on
day 26. Numbers (10 and 62/g, respectively) and species (10 and 31,
respectively) remained much lower in fumigated, compared with
untreated, soil.
After sterilization of greenhouse soil with methyl bromide (75
g/m2), there were profound qualitative and quantitative disturbances
up to a soil depth of 30 cm (Bourbos & Skoudridakis, 1991); 7-9
species of soil mycoflora were isolated from the fumigated soil
compared with 107 from control soils. The 31-40 cm soil layer was not
affected by disinfestation. After two months, recolonization had taken
place of only 35-40% in species and 60-63% in density of the primary
microflora.
The use of methyl bromide as an effective fumigant for
greenhouses has been questioned (Bourbos & Skoudridakis, 1991).
Certain saprophytic fungi have developed a degree of tolerance.
Disinfested soil was quickly contaminated by certain pathogens
( Fusarium and Pythium spp.) and there was also a possibility of
reinfection from the lower layer of soil not reached by the fumigant.
Some fungi controlled by methyl bromide are listed in Table 37.
7.2 Aquatic organisms
7.2.1 Effect of methyl bromide
LC50 (96-h) values of 11 mg methyl bromide/litre and 12 mg
methyl bromide/litre for bluegill sunfish, Lepomis macrochirus
(freshwater) and tidewater silversides, Menidia beryllina
(saltwater), respectively, have been determined following exposure to
methyl bromide (Dawson et al., 1975/77).
The acute toxicity of methyl bromide for carp ( Cyprinus carpio
L.) was determined in studies with a 4-h exposure period (Segers et
al., 1984). The 4-h LC50 was calculated to be approximately 17
mg/litre. Damage to the gill epithelium was the most pronounced
morphological damage, which probably caused the death of the fish by
suffocation.
In a short-term study, triplicate groups of 10 fish ( P.
reticulata (guppy) and Oryzias latipes (medaka)) were exposed to
0.56, 1.0, or 1.8 mg methyl bromide/litre for 4 days. All methyl
bromide-exposed fish displayed abnormal behaviour (reduced activity).
Limited mortality was noted in P. reticulata exposed to 1.0 and 1.8
mg/litre and O. latipes exposed to 1.8 mg/litre, but there was no
increase in mortality in the next lower concentration groups (Wester
et al., 1988).
In a long-term study, P. reticulata and O. latipes were
exposed for 1 and 3 months to 0.032-3.2 mg methyl bromide/litre
(Wester et al., 1988). All guppies died in the 3.2 mg/litre group
within 3 days, and, in the 1.0 mg/litre group, within 3 weeks. The
NOLC (no observed lethal concentration) and NOEC (no observed effect
concentration: behaviour, appearance) values were 0.32 and 0.1
mg/litre, respectively. A significant decrease in weight was noted in
both sexes in the 0.32 mg/litre group.
All medaka embryos exposed to 1.8 or 3.2 mg/litre and most of the
1.0 mg/litre group died before hatching. The NOLC after 3 months was
0.32 mg/litre. The NOEC values, on the basis of behaviour and
appearance, were 0.56 mg/litre and 0.32 mg/litre after 1 and 3 months,
respectively (Wester et al., 1988).
A short-term study with (lethal) concentrations of 0.56, 1.0, or
1.8 mg methyl bromide/litre showed (using scanning electron
microscopy) major degenerative and regenerative changes in the
superficial epithelia, especially of the gills and oral mucosa,
caused, apparently, by the local irritating action of this compound.
Necrotic changes were also seen in the thymic cortex and the testis
(Wester et al., 1988). In a long-term study on guppies and medakas
exposed to the highest concentrations for 1 month and 3 months,
respectively, no significant organ or tissue changes could be detected
in routine histopathology.
Table 37. Some fungi controlled by methyl bromide
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Alternaria sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Armillaria melea 4-98 g/m2 citrus/ Munnecke et al. (1969)
grape Kissler et al. (1973)
Aspergillus sp. 1.6-5 g/m2 pecan/ Wells & Payne (1975)
honeycomb Smirnov (1970)
Byssochalamy fuloa 60-120 mg/kg starch Ito et al. (1972)
Cladosporium sp. 1.6-3.3 g/m2 pecan Wells & Payne (1975)
Eumargodes laivgi 24 g/m2 not stated Hitchcock (1968)
Fusarium sp. 45-100 g/m3 tomato Westeijn (1973)
Weihing et al. (1971)
Wells & Payne (1975)
Perrotta (1968)
Vanachter (1974)
Monochaeta sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Penicillium sp. 1.6-3.3 g/m3 pecan
Pestalotia sp. 1.6-3.3 g/m3 pecan
Phoma sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Phytophora parasitica 49 g/m2 citrus Grimm & Alexander (1971)
Phytophora capsici 40 g/m2 green peppers Alfaro Moreno & Vehg (1971)
Table 37 (continued)
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Plasmodiophora brassicae 50-150 g/m2 cabbage Winstead & Garriss (1960)
Plasmosisphora brassicae 48 g/m2 cabbage Wimalajewa (1975)
Pyrenochaeta lycopersici 125 g/m3 soil Vanachter (1974)
Rhizoctania solani 50-150 g/m2 soil Winstead & Garriss (1960)
Sclerotium rolfsii 50 g/m2 iris Kiewnick (1968)
Sclerotina sclerotiorum 50 g/m2 tobacco Hartill & Campbell (1973)
Thielaviopsis basicola 50 g/m3 tobacco Mounat & Hitier (1959)
Verticillium sp. 45-70 g/m2 tomato Perrotta (1968)
Table 38. Effects on aquatic organisms of short-term exposure to sodium bromidea
Results (g Br-/litre) at:
Test species Parameter 24 h 48 h 72 h 96 h
Scenedesmus EC50 (growth) 5.8 7.8 8.5 10
pannonicus NOEC (growth) 2.5 2.5 2.5 2.5
(alga)
Daphnia magna LC50 (mortality) 11 11 - -
(crustacea) EC50 (mort./abn. behaviour) 5.8 5.8 - -
NOLC (mortality) 7.8 7.8 - -
NOEC (mort./abn. behaviour) 4.3 4.3 - -
Poecilia LC50 (mortality) 16 16 16 16
reticulata EC50 (mort./abn. behaviour) 0.44 0.14 0.044 0.044
(fish) NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.078 0.025 0.025
Oryzias latipes LC50 (mortality) 26 25 24 24
(fish) EC50 (mort./abn. behaviour) 0.44 0.44 0.44 0.44
NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.25 0.25 0.25
a From: Canton et al. (1983).
b NOE(L)C = no observed (specified) effect concentration.
7.2.2 Effect of bromide ion on aquatic organisms
The main degradation product of methyl bromide is inorganic
bromide.
To evaluate the potential impact of pollution with the bromide
ion, Canton et al.(1983) investigated the short-term effects of sodium
bromide on various freshwater organisms, using algae (Scenedesmus
pannonicus) , crustaceans (Daphnia magna), and fish (P. reticulata
and O. latipes) (Table 38). Depending on the species tested, acute
toxic effects were seen at concentrations ranging from 44 to 5800 mg
Br-/litre and, in long-term tests, the NOEC varied from 7.8 to 250
mg Br-/litre. Bromide ion markedly impaired reproduction in both
crustaceans and fish.
Further tests were performed on P. reticulata (guppy) and O.
latipes (medaka) following sodium bromide exposure for 1 and 3
months at concentration ranges of 10-32 000 mg/litre (guppy) and
180-56 000 mg/litre (medaka). NOLC values for guppies were 10 000
mg/litre and 1000 mg/litre after 1 and 3 months, respectively. The
NOEC value was 32 mg/litre in both studies. Histopathological changes
were observed at concentrations of 100 mg/litre or more (Wester et
al., 1988). For medakas, the NOLC values were 5600 and 3200 mg/litre
after 3 weeks and 3 months, respectively, whereas the NOEC value
(behaviour) for both periods was 320 mg/litre (Wester et al., 1988).
The relative susceptibility to sodium bromide of 11 taxonomically
different freshwater species was determined in medium-term toxicity
tests by Slooff & Canton (1983). The data are summarized in Table 39.
In semi-static, long-term toxicity tests on Daphnia magna, EC50
and EC10 values of 27 and 18 mg/litre, respectively, were determined
(Van Leeuwen et al., 1986).
7.3 Terrestrial organisms
7.3.1 Protozoa
Long et al. (1972) studied the effect of methyl bromide on
protozoa (Eimeria tenella and E. acervulina) at a dosage of 5
g/m3 for 20 h at 25 °C; 100 % control (destruction of oocysts) was
achieved.
7.3.2 Plants
Methyl bromide is often applied, as a fumigant, directly to plant
seeds, plant cuttings, or harvested plant products to disinfect
before, and during, transportation or storage (Davis et al., 1977).
Additionally, methyl bromide is used as a soil fumigant to control
certain plant pathogens and weed seeds in areas to be planted.
Table 39. Summary of the results of medium-term toxicity tests using
sodium bromide on 11 different freshwater test speciesa
Test species Exposure Criteria NOL(E)C
time valuesb
(days) (mg/litre)
Pseudomonas 0.3 specific growth rate 3200
fluorescens
(bacterium)
Microcytis aeruginosa 4 specific growth rate 3200
(cyanobacterium)
Scenedesmus 4 growth (biomass) 3200
pannonicus
(alga)
Lemna minor (plant) 7 specific growth rate 3200
Daphnia magna 21 mortality 3200
(crustacea) reproduction 10
Culex pipiens 25 mortality 100
(insect) development 100
Hydra oligactis 21 specific growth rate 1000
(hydrozoan)
Lymnaea stagnalis 40 mortality 3200
(mollusc) reproduction 10
hatching 3200
Poecilia recticulata 28 mortality 100
(viviparous fish) mortality and behaviour 32
growth 320
Oryzias latipes 40 mortality 3200
(viviparous fish) mortality and behaviour 320
hatching growth 10 000
Xenopus laevis 100 mortality 32
(amphibian) development 320
development 320
a Adapted from: Slooff & Canton (1983).
b NOL(E)C = no observed (specified) effect concentration.
As chloropicrin is phytotoxic, methyl bromide formulations that
contain it as a warning agent are not used on nursery stock or other
living plants (Bond, 1984).
7.3.2.1 Seed fumigation
As shown in Table 40, fumigation with methyl bromide can result
in delay in the germination of seeds and some loss of total
germinative capacity, depending on the variety, moisture content, and
the extent of exposure to the gas (Davis et al., 1977). This was
confirmed by Sittisuang & Nakakita (1985) who compared the effects of
methyl bromide on the germination of rice seeds ( Oryza sativa L.,
Japicona type) and corn (maize) seeds ( Zea mays L.). No detrimental
effect of methyl bromide up to 4 mg/litre was observed in rice seeds
at a moisture content of 11%, but as the moisture content and
temperature increased, methyl bromide had an increasing effect on
germination. Maize seeds were much more tolerant to methyl bromide.
Exposure to 5 mg methyl bromide/litre, which caused heavy damage to
rice seeds in most cases, did not generally produce any harmful effect
on the germination of maize seeds, regardless of the moisture content
and temperature. At concentrations higher than 10 mg/litre, the
viability of maize seeds declined in a similar way to that of rice
seeds. Rice seeds were found to absorb more methyl bromide than corn
seeds. Seeds with a higher moisture content absorbed more methyl
bromide and seeds with the same moisture content absorbed more methyl
bromide at higher temperatures than at lower temperatures. The authors
suggested that changes in certain proteins and enzymes were major
factors in seed viability.
The effects of methyl bromide fumigation on the germination of
different cultivars of wheat seed have been investigated. The
germination of all cultivars was reduced following fumigation at a
dose of 16 mg/litre for 24 h (11% moisture, temperature not given).
The optimal conditions were a moisture content of 9% and a temperature
of 18 °C. With increasing moisture or temperature, the percentage
germination decreased. At higher levels of moisture, the concentration
of methyl bromide appeared to be a more important factor than exposure
time at a constant concentration x time product (CTP) of 768
mg.h/litre (Khanna & Yadav, 1987).
Hanson et al. (1987) found that fumigation with methyl bromide
not only caused a delay in germination and loss of germinative
capacity but also that certain varieties of seed barley were damaged,
exhibiting symptoms of albinism and stunted growth. The authors
suggested that great care should be taken in the selection of stored
barley intended for seed and, in particular, in the fumigation of
samples in standard reference collections. A CTP of 200 mg.h/litre is
used commercially, but higher concentrations may be attained if the
distribution during fumigation is poor.
Table 40. Effects on germination of seeds fumigated with methyl bromidea
Seed Fumigation conditions Germination results Reference
hemp 70-140 g/m3 5-23 % reduction Tkalich (1974)
onion 42 g/m3 for 24 h 95 % reduction in laboratory Powell (1975)
11.5% reduction in cool soil
peanuts 32 mg/litre (24 h, 27°C, 80% reduction of: Leesch et al. (1974)
paper container relative humidity), applied 21.7%
burlap bags under cover, aerated 72 h 11.4%
oat, wheat, rye, 0, 600, or 1200 g.h/m3 at 8, 11at 18% moisture content: Blackith & Lubatti (1965)
barley 14, or 18% moisture content - no germination after 6 years storage
at 8% moisture content:
- 90% germination after 6 years storageb
Picea abies, Picea seeds at various moisture germination normal after storage Jones (1968)
glauca, Pinus mugo content; 48 g/m3, 24°C, 2-5 h, only if seeds aerated 24 h before
mughus, Pinus then aerated 1-25 h and stored storage; all but P. sylvestris
sylvestris (seeds) in sealed containers at 7°C for required drying to 5% moisture content
1 year before storage
tobacco seed 16-32 g/m3 or germination satisfactory at <10 % seed Guthrie & Kincaid (1957)
32-48 g/m3 moisture content: germination dcreased at
seed moisture contents above 10%
barley, corn, grain 32 g/m3 (< 24 h, 26°C); unimpaired germination seed Whitney et al. (1958)
sorghum, oats, wheat moisture content less than 12%
a From: Davis et al. (1977).
b Except rye, germinated well only up to 3 years storage.
7.3.2.2 Fumigation of plants or plant products
Direct fumigation of plants or plant products is used to retard,
or prevent, pest infestations and to overcome quarantine barriers.
Post-harvest fumigation is discussed in section 5.1.4.2.
7.3.2.3 The effects on plants of soil fumigation
Methyl bromide can have adverse as well as positive effects on
plants.
The phytotoxic effects of methyl bromide as a soil sterilant can
be caused by:
(1) the action on plants of methyl bromide itself;
(2) the action of inorganic bromide formed by the breakdown of
methyl bromide in the soil;
(3) indirect action through effects of either methyl bromide or
inorganic bromide on soil microflora, soil structure, or
composition (Maw & Kempton, 1973).
Where the crops are affected by lack of mycorrhizae, the plants
are stunted. Experiments have proved that this problem can be
rectified by fertilizing with phosphoric acid in the irrigation water,
using a trickle system (Bromine & Chemicals Ltd., 1990).
The phytotoxicity of methyl bromide is thought to be due mainly
to the high level of bromide ion. Drosihn et al. (1968) showed that
the degree of susceptibility of carnations to methyl bromide
fumigation of the soil depends on the intensity of subsequent leaching
of the soil. Similar findings were described by Kempton & Maw (1974).
In contrast to this, tomato plants were relatively insensitive to
bromide; growing tomatoes tolerated up to 0.1 mg bromide/g soil
without signs of injury or growth retardation (Maw & Kempton, 1973).
These authors found lettuce to be particularly resistant to inorganic
bromide, with some varieties growing in the presence of as much as 5
mg Br-/g soil.
Reichmuth & Noack (1983) determined the threshold concentration
of methyl bromide in air that should not be exceeded in the vicinity
of fumigated buildings, in order to protect plants. The test plants
( Lactuca sativa capitata (lettuce) and Nasturtium officinale
(water cress)) were exposed to concentrations of between 4 and 1400
mg methyl bromide/m3 for 72 h. At 400 mg/m3, yellowing of lettuce
leaves became apparent, while no visible effects were observed on
water cress up to the highest concentration.
(a) Cultivated plants
Only a limited number of genera, species, or varieties of plants
are susceptible to methyl bromide. Of the 441 species of glasshouse
plants tested by Latta & Cowgill (1941), 414 (93.9%) were not affected
and only 27 sustained various levels of damage; of these, five species
were severely burned. For example, roses showed no pronounced toxic
effects, when planted in soils aerated for four days after methyl
bromide fumigation, however, carnations were extremely sensitive to
both residual methyl bromide gas and inorganic bromide in the soil
(Kempton & Maw, 1974). Other crops, such as cotton, celery, pepper,
and onion, do not reach adequate growth, when grown in fumigated soil
(Bromine & Chemicals Ltd., 1990). Plants, actively growing, are more
likely to sustain injury than dormant plants (Bond, 1984).
(b) Weeds
The phytotoxic effects of methyl bromide on weeds are important
in soil fumigation.
Table 11 shows that the recommended dose rates to eradicate weeds
is 35-50 g/m2, though purple nutsedge (nut grass), corms and seeds
of horseweed Erigeron (Conyza) , mallow (Malva) , and legumes are
not efficiently controlled at this dose (Bromine & Chemicals Ltd.,
1990).
Methyl bromide (40-80 g/m2) is mentioned as being the best soil
fumigant against yellow nutsedge ( Cyperus esculentus L.), but the
weed was not completely eradicated because dormant tubers below the
tillage depth survived (Rotteveel & Naber, 1987).
7.3.3 Soil invertebrates
Soil fumigation with methyl bromide (and chloropicrin) results
generally in toxic effects in both target and non-target organisms.
The concentrations used are sufficiently high to eradicate populations
of a wide variety of organisms. Fumigants, including methyl bromide
and chloropicrin, were all strongly nematocidal. Methyl bromide killed
virtually all soil arthropods, including mites; Collembola were almost
completely eradicated. Methyl bromide was very toxic for symphylids
and millipedes (details of dose not given) (Edwards & Thompson, 1973).
Methyl bromide (concentration not given) and chloropicrin were very
toxic for earthworms, even those that lived in deep burrows (Van Rhee,
1977). Chloropicrin was repellent to most arthropods in soil (Edwards
& Thompson, 1973). To control nematodes, methyl bromide is generally
used at rates of up to 80 g/m2 (Table 41).
7.3.4 Insects and arachnids
Methyl bromide is used as a fumigant to control insect pests.
Although it is not as toxic for insect species as some other
fumigants, such as HCN, acrylonitrile, and ethylene dibromide, its
ability to penetrate quickly and deeply into sorptive materials makes
it an effective and versatile fumigant (Davis et al., 1977; Sassaman
et al., 1986). The commercial dosage for methyl bromide as a storage
fumigant ranges from 16 to 100 g/m3 for up to 3 days (Tables 12 and
13).
The dosage required depends also on the temperature. The
threshold concentration levels identified at 15 and 25 °C differed by
a factor of two or three. These investigations by Bell (1988) were
carried out on the adult beetle. The dosage of methyl bromide required
to kill eggs and pupae is greater than that required to kill all
adults. Pupae and older larvae of Tribolium spp., for example,
required CTPs of up to 180 mg.h/litre for control (Hole, 1981). For
other species and exposure conditions see Table 42.
In an FAO study, it was found that there was a variation in
tolerance to methyl bromide in different strains of eight species of
stored product beetles collected from different parts of the world
(Hole, 1981). Although resistant strains have been identified in the
laboratory, there have been no reports of resistance to methyl bromide
in practice (Bell, 1988).
The effects of lethal concentrations of methyl bromide (48 g/m3
for 2 h) on embryos of the codling moth ( Cydia pomonella L.) were
assessed using light microscopy and transmission electron microscopy
(Cheetham, 1990). Cell division stopped within one hour in nearly all
embryos, a small number of terata being produced.
Methyl bromide is used to eradicate various wood and household
pests, particularly in the warmer climates of southern and western
USA. The primary targets are drywood termites (Kalotermitidiae).
Scheffrahn & Su (1992) have assessed the toxicity of methyl bromide at
27 °C against pseudergates, nymphs, or alates of several species.
Estimates of lethal accumulated doses for 50 and 99% mortality ranged,
respectively, from 11.4 and 16.5 for R. hesperus pseudergates to
45.9 and 75.0 mg h/litre for C. cavifrons pseudergates and nymphs.
Alates were more susceptible to methyl bromide than pseudergates or
nymphs. Boczek et al. (1975) reported three periods in the development
of Acarus siro that have increased sensitivity to methyl bromide:
(a) before the beginning of gastrulation movements in the germ band;
(b) during the formation of the nervous system; and (c) the period
preceding dorsal closure. For this species, Burkholder (1966) found
LD100 values ranging from 3.4 to 16.8 g/m3 at various exposure
times at 16 °C and 85% relative humidity whilst achieving the same CTP
(65-83 g.h/m3).
Table 41. Effects of methyl bromide on nematodes
Nematode Effective Exposure Reference
control Conditions
concentration
Anguina agrostis CTP 600-800 12% moisture Hague (1963)
mg.h/litre
Belonolaimus 98 g/m2 98% methyl bromide Darby et al. (1962)
longicaudans 2% chloropicrin: covered 48 h
Ditylenchus dipsaci CTP 850 10-14% moisture Hague & Clark (1959)
mg.h/litre
Dorylaimus sp. 2.3 g/m3 40 h Van Gundy et al. (1972)
Hemicyclophora parvana 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Heterodera rostochiensis Ct 500-1000 treatment with water before fumigation Hague (1959)
mg.h/litre enhanced penetration of methyl bromide
Heterodera rostochiensis 111 g/m2 covered 16 days: 98 % methyl bromide Whitehead et al.
and 2% chloropicrin (1972)
Heterodera schachtii 0.5-30 g/m3 1-21 days Abdalla & Lear (1975)
Hoplolaimus columbus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Hoplolaimus tylenchiformis 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Meloidogyne sp. 50 g/m2 manure applied prior to fumigation Scotto La Massese
decreased nematocidal effect & Mars (1975)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Meloidogyne incognita 2.3 g/m3 38 h Van Gundy et al. (1972)
Meloidogyne incognita 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Meloidogyne incognita 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne incognita 0.6-2.5 g/m3 1-21 days Abdalla & Lear (1975)
Meloidogyne incognita 17-22 g/m2 chisel applicator, covered or rolled Sher et al. (1958)
acrita
Meloidogyne javanica 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne javanica 22-34 g/m2 chisel application, covered Thomason (1959)
Meloidogyne javanica 56-112 g/m2 98% methyl bromide Milne (1962)
and 2% chloropicrin
Pratylenchus sp. 45-67 g/m2 covered Raski et al. (1975)
Pratylenchus sp. 4.9-9.7 g/m3 1-3 days Abdalla & Lear (1975)
Pratylenchus brachynrus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Pratylenchus brachyurus 25-51 g/m3 24 hours; 25°C Minton & Gillenwater
(1973)
Pratylenchus penetrans 50 g/m2 not stated Chen et al. (1962)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Pratylenchus thornei 49 g/m2 covered after application Van Gundy et al. (1974)
for unspecified time
Pratylenchus zeae 100 g/m2 covered for 48 h Oakes et al. (1956)
following fumigation
Trichoderus christiei 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin:
covered 48 h
Xiphinema americanum 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 2.3 g/m3 28 h Van Gundy et al. (1972)
Xiphinema index 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 0.2-2.0 g/m3 1-21 days Abdalla & Lear (1975)
Table 42. Some insects controlled by methyl bromidea
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Antagenus picus 32 Pence & Morganroth
(black carpet beetle) (1962)
Anthonomus grandis 16-80 Roth & Kennedy (1972)
(cotton boll weevil)
Anthrenus flavipes 32 Pence & Morgenroth
(furniture carpet beetle) (1962)
Anthrenus verbasci 32
(varied carpet beetle)
Araecerus fasciculatus 6.2 (eggs) Majumder et al. (1961)
3.4 (larvae)
7.4 (pupae)
4.5 (adults)
Blatta orientalis 64 Hickin (1961)
(cockroach)
Blatella germanica 64
(cockroach)
Bruchus rufimanus 28 Roth & Richardson
(broad bean weevil) (1974)
Cadra cautella 32 Leesch et al. (1974)
(almond moth)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Callosobruchus 0.85 (eggs) 1.25 (eggs) Adu & Muthu (1985)
chinensis (L) 2.2 (instar 2.72 (instar
(cowpea weevil) larvae) larvae)
0.89 (pupae) 3.98 (pupae)
1.17 (adult) 1.4 (adult)
Chilo agamemnon 20 (larvae) Isa et al. (1970)
(corn borer)
Corcyra cephalonica 1.66-1.78 (eggs) El-Buzz et al. (1974)
(rice moth) 1.10-1.68 (instar
larvae)
2.79 (pupae)
Curcilio caryae 32-112 Leesch & Gillenwater
(pecan weevil) (1976)
Ephestia kuehniella 2.02-2.46 Mostafa et al. (1972)
(mediterranean flour moth)
Gnorimoschema operculella 11.74 (larvae) Pradhan et al. (1960)
(potato tuber moth)
Gryllotalpa 70-100 g/m2 Dzidzariya (1972)
(mole cricket)
Hemp leaf roller 40-45 Tkalich (1972)
Laspeyresia pomonella 32 Morgan et al. (1974)
(codling moth) 32 Anthon et al. (1975)
Megastigmus acuelatus 50 Vodolagin (1971)
(dog rose weevil)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Musca domestica 64 Hickin (1961)
(housefly)
Onychuirus hortensis 89 g/m2 Edwards (1962)
(springtail)
Oryzaephilus mercator 200 Joshi (1974)
(merchant grain beetle) 32 Leesch et al. (1974)
Ostrinia nubilalis 20 (larvae) Isa et al. (1970)
(corn borer)
Periplaneta americana 64 Hickin (1961)
(cockroach)
Plodia interpunctella 5.5 (normal larvae) Sardesai (1972)
(indian meal moth) 10.2 (diapausing larvae)
32 Leesch et al. (1974)
Sitophilus oryzae 5.45-6.19
(rice weevil)
Sitotroga cerealella 1.85-2.21
(angoumois grain moth)
Tenebroides mauritanicus 16 23 Bond (1956)
(cadelle) 25.5-43.3 Monro et al. (1966)
Isoptera
(termites; 5 species) 64 Hickin (1961)
Tribolium castaneum 0.009 (adults)
(red flour beetle) 3.06-6.19 Mostafa et al. (1972)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Trilobium confusum 3.6 - 91 4.64-145.14 Kenaga (1961)
(confused flour beetle) 15b
21.5-23.7 Monro et al. (1966)
Trogoderma granaria 0.038 (larvae) Pradhan & Govindan
(grain beetle) (1954)
Trogoderma variable 32-40 (eggs) Vincent & Lindgren
(warehouse beetle) (1975)
16-56 (instar larvae)
32-72 (pupae)
24-36 (adults)
a A range of LD values reflects effects of time, temperature, or pressure.
b Preceded by gamma radiation (50 krad).
LD100 values have been determined for Rhipicephalus sanguineus
(brown dog tick) ranging from 6 to 96 g/m3 (3.5 h, 22 °C and 6 h, 11
°C, respectively) (Roth, 1973).
7.3.5 Gastropods
The effects of methyl bromide on various gastropods (slugs,
snails, limpets) have been studied. Roth & Kennedy (1973) found an
LD100 for Helidella candidula and H. conspurcata, exposed for 24
h at a dosage of 240 g/m3 . Similarly, for Cochicella barbara (72
h, 13 °C) and Theba pisana (10 h, 13 °C) a dosage of 128 g/m3 was
lethal (Richardson & Roth, 1965).
7.3.6 Birds
Rhode Island Red female hens were fed, from hatching, on diets
that had been fumigated with methyl bromide at the concentration
recommended for the elimination of salmonellae (800 mg.h/litre) or at
1´ times this value (Cooper et al., 1978). Body weight, egg weight,
and egg number were not significantly affected by treatments, but
sexual maturity may have been slightly delayed. The egg flavour was
adversely affected. The same group had previously shown that the taste
of meat from broiler chickens was similarly tainted (Griffiths et al.,
1978).
No adverse effects on either the fertility or hatchability of
hens' eggs, previously fumigated with methyl bromide at 32 g/m3 for
24 h, were observed (Devaney & Beerwinkle, 1982).
7.3.7 Other animals
Data are not available on the direct environmental exposure to
methyl bromide of other animals. Effects on test animals are given in
section 8.
Bromide intoxication was reported by Knight & Costner (1977)
after horses, goats, and cattle were accidentally fed oat hay that had
been cut from a field treated with methyl bromide the previous autumn.
The bromide content of the hay ranged from 6800 to 8400 mg/kg so that
the estimated mean daily intake was 9, 49, and 70 g of bromide ion in
goats, horses, and cattle, respectively. Signs of intoxication
reported included lethargy, weakness, and ataxia. Similar symptoms
were noticed between the 7th and 9th days in animals fed this hay on
an experimental basis. Signs of in-coordination (between 10th and 12th
days) were correlated with serum bromide concentrations of 30
mEq/litre (2.4 g/litre) or more (Knight & Reina-Guerra, 1977). Serum
bromide concentrations and the associated neurological signs subsided
markedly 14 days after feeding discontinued (Knight & Reina-Guerra,
1977). Methyl bromide is not approved for use prior to the planting of
forage crops.
7.4 Population and ecosystem effects
Application of methyl bromide as a soil fumigant resulted in the
almost complete eradication of populations of a wide variety of
microflora and fauna, as well as other soil organisms, thus altering,
at least temporarily, the trophic structure of the soil environment
(Sassaman et al., 1986).
Treatment with 100% methyl bromide and other methyl
bromide/chloropicrin formulations reduced populations of Fusarium,
Pythium, and Rhizooctonia species in soil. Nine weeks after
application, populations were still significantly lower. Seedlings
grown in treated plots had the least amount of damping off and root
rot (Enebak et al., 1988).
Methyl bromide dosed under plastic sheeting at a rate of 300
g/m3 (for 30 cm depth 100 g/m2) killed all insects, though small
numbers of soil nematodes and mites were collected during subsequent
sampling (Heungens & Roos, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
A summary of acute oral toxicity data is given in Table 43. Very
few studies have been carried out, mainly because methyl bromide is a
gas at temperatures above 4 °C. The minimum lethal oral dose of methyl
bromide for rabbits was found to be 60-65 mg/kg body weight (Dudley et
al., 1940; Dudley & Neal, 1942). Miller & Haggard (1943) found that
all rats given a single oral dose of 100 mg/kg body weight in olive
oil died in 5-7 h.
8.1.2 Inhalation
8.1.2.1 Guinea-pig and rabbit
Single exposure toxicity tests conducted on various mammalian
species have shown that methyl bromide is highly toxic. A summary of
the acute toxicity data is presented in Table 44.
Studies on guinea-pigs (Sayers et al., 1929) and rabbits (Irish
et al., 1940) were carried out. Rabbits were exposed to concentrations
of 420, 852, 1000, 2000, 10 000, 20 000, and 50 000 mg methyl
bromide/m3. Table 45 shows the exposure times giving 100% survival
and 100% mortality. Concentrations of methyl bromide above 10 000
mg/m3 sometimes caused the rabbits to close their eyes; otherwise
they appeared normal until they became too weak to hold up their heads
(Irish et al., 1940). Rabbits that survived 1000 mg methyl
bromide/m3 for 2 days after exposure usually became paralysed (Irish
et al., 1940).
Toxicity is a function of the concentration levels and the
exposure times (see also Table 45). The steep dose-mortality response
to methyl bromide found by many authors can be seen in Fig. 9.
8.1.2.2 Mouse
The results of single exposure inhalation studies on mice,
carried out by Alexeeff et al. (1985), Yamano (1991), and the Japanese
Ministry of Labour (1992), are given in Tables 46 and 47. The sharp
onset of lethal toxicity was shown in all cases. Alexeeff et al.
(1985) exposed male mice to methyl bromide (870-5930 mg/m3) for 1 h
and observed that clinical signs and mortality were dose related, with
the possibility of delayed effects in target organs, such as the
kidney.
Table 43. Acute and short-term oral (gavage) toxicity
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rabbit n.d. single 56-71 all rabbits given an oral dose of Dudley et al. (1940)
63.9 mg/kg died; one rabbit receiving Dudley & Neal (1942)
56.3 mg/kg died; all rabbits given
56.1 mg/kg or less survived;
destruction of superficial layers of
stomach and duodenum with accompanying
haemorrhage and hyperaemia; minimal lethal
dose: 60-65 mg/kg body weight
rat n.d. single 100 all died in 5-7 h Miller & Haggard (1943)
rat n.d; single 190-239 LD50, 214 mg/kg Danse et al. (1984)
rat n.d. 4 weeks; 50 epithelial hyperplasia, hyperkeratosis
7 days/week and ulceration of the forestomach
rat 10 (male) 13 weeks; 0 10 and 50 mg/kg: proliferative
(Wistar) 10 (female) 5 days/week 0.4 alterations of forestomach mucosa;
2 50 mg/kg: haematological changes
10 13/20 squamous cell carcinomas of
50 forestomach
rat 15 13-25 weeks; 0 treated group: week 13: forestomach Boorman et al.(1986)
12 weeks 50 acanthosis, fibrosis, pseudoepitheliomatous
recovery for hyperplasia; week 25: hyperplastic lesions
some groups of forestomach
recovery group: regression of stomach lesions,
but adhesions, fibrosis, and mild acanthosis
remained; evidence of malignancy in one rat
Table 43 (continued)
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rat n.d. 4,8,13, and 0 treated groups: forestomach ulceration Hubbs & Hartington (1986)
(n.d.) 17 weeks; 25 pseudoepitheliomatous hyperplasia
5 days/week 50
n.d. 13 weeks; 0 recovery period: marked but incomplete
5 days/week 25 regression of lesions;
recovery for 50 no evidence of malignancy
4-8 weeks
a n.d.= no details given.
Table 44. Single exposure inhalation studies of methyl bromide on mammalsa
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
mouse 94 950 25 100 % died within 6 h Bachem (1927)
mouse 700 n.d. 100 % survived Bachem (1927)
rat 20 000 6 100 % survived Irish et al. (1940)
24 100 % died
rat 43 000 3 survived Clarke et al. (1945)
rat 50 000 3 100 % survived Irish et al. (1940)
6 100 % died
rabbit 70 40 change in motor Balander & Polyak
reflex behaviour (1962)
rabbit 19 000 25 deep (fatigued) Beyne & Goett
breathing (1934)
rabbit 20 000 36 100 % survived Irish et al. (1940)
rabbit 20 000 84 100 % died
rabbit 25 000 30 died Beyne & Goett
(1934)
rabbit 31 600 5 died after 8-10 h Duvoir et al. (1937)
rabbit 36 000 25 died Beyne & Goett
(1934)
rabbit 50 000 12 100 % survived Irish et al. (1940)
30 100 % died
dog 10 000 5-6 h died Beyne & Goett
(1934)
dog 17 000 n.d. died Merzbach (1928)
dog 19 000 n.d. died Beyne & Goett
(1934)
dog 34 000 60 died Merzbach (1928)
Table 44 (continued)
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
dog 48 000 40 died Duvoir et al. (1937)
dog 50 000 45 died Merzbach (1928)
a Adapted from Henschler (1990).
b n.d. = no details given.
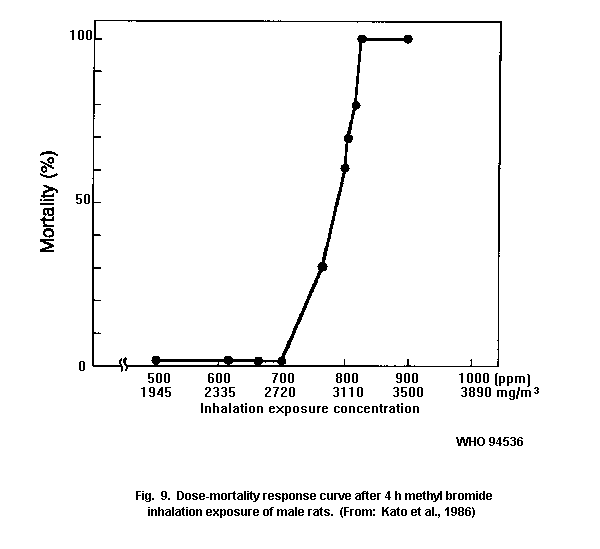 Table 45. Acute inhalation toxicity of methyl bromide for rats and rabbitsa
Concentration Exposure time in hours
(mg/m3) Rats Rabbits
100 % fatality 100 % survival 100 % fatality 100 % survival
50 000 0.1 0.03 0.5 0.2
20 000 0.4 0.1 1.4 0.6
10 000 0.7 0.4 2.2 1.0
2 000 6 2 11 6
1 000 22 8 24 15
852 26 12 32 20
420 -b 22 -b -b
a From: Irish et al. (1940).
b No data.
Table 46. LC50 values for methyl bromide
Species Concentration Exposure Reference
(mg/m3) time
mouse 6 600 30 min Bakhishev (1973)
mouse 4 680 1 h Alexeeff et al. (1985)
mouse 1 540 2 h Balander & Polyak (1962)
mouse 1 575 4 h Yamano (1991)
rat 11 000 30 min Bakhishev (1973)
rat 7 300 1 h Zwart (1988); Zwart et al.
(1992)
rat 3 034 4 h Kato et al.(1986)
rat 1 175 8 h Honma et al. (1985)
Further details of target organ studies and biochemical findings
from the series of mouse studies (Alexeeff et al., 1985) are given in
sections 8.8 and 6.3, respectively.
An LC50 of 1575 mg methyl bromide/m3 (405 ppm ± 20) was
determined after a 4 h exposure (Yamano, 1991). In a further study,
the author exposed mice to 1945 mg methyl bromide/m3 (500 ppm).
After 2 h of exposure, there were no deaths, but, after a further 30
min, there was 85% mortality. Mice treated prior to exposure with
glutathione (500 mg/kg i.p.) showed only 5.3% mortality after this
time.
Table 47. Some single exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 6 (male) (nose only) 0 Alexeeff et al.
(Swiss-Webster) 1 h, surviving 870 (+); no toxic response (1985)
mice sacrificed 1720 (+); no toxic response
one week 2200 (+); significantly decreased lung and
later 2720 liver weights
3500 (+); additionally enlarged, pale kidneys
and kidney lesions
3820 (-); additionally abnormal clinical
signs, weight loss and mortality;
cerebral haemorrhage
4700 (-); additionally liver lesion, liver
congestion and haemorrhage
5770 (-); additionally decreased motor
coordination; cerebral congestion;
colonic haemorrhage; congested
kidneys
5930 (-); all effects mentioned above
(1 h-LC50 of 4680 mg/m3 determined)
a (+) = Able to recall a single task passive avoidance test.
b (-) = Not able to recall a single task passive avoidance test.
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 10 (male) 4 h 389 0% mortality Japanese
(Crj: BDF1) 10 (female) 584 0% mortality Ministry of
873 0% mortality Labour (1992)
1315 0% mortality
Pathology: respiratory metaplasia of the
olfactory epithelium of the nasal cavity
(female)
1970 80% mortality (male) and 100% mortality
(female)
Clinical signs: decrease in locomotor
movement, tremor, convulsion, diarrhoea,
bradypnoea, dyspnoea (dead)
Pathology:
Dead: congestion of the lung, necrosis
and degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis of the
thymus and lymph node, necrosis of the
olfactory epithelium of the nasal cavity
Survived: tubular necrosis and regeneration
of the kidney, necrosis and respiratory
metaplasia of the olfactory epithelium of the
nasal cavity
2950 100% mortality
clinical sign and pathology: same as
1970 mg/m3 dead animals
mouse 1 h 45 min 1945 0% mortality Yamano (1991)
2 h 0% mortality
2 h 10 min 11% mortality
2 h 20 min 15% mortality
2 h 30 min 85% mortality
3 h 90% mortality
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 10(male) 4 h 584 0% mortality Japanese
(F344/DuCrj) 10(female) 875 0% mortality Ministry of
Pathology: disarrangement and Labour (1992)
respiratory metaplasia of the olfactory
epithelium of the nasal cavity
1315 0% mortality; Pathology: same as 875 mg/m3
1970 0% mortality; Pathology: same as 875 mg/m3
2956 100% mortality
Clinical signs: closed eyelid, decrease in
locomotor movement, ataxic gait, serous
discharge of nose, lacrimation, diarrhoea,
irregular breathing and bradypnoea
Pathology: congestion of the lung,
degeneration of the liver, tubular necrosis
of the kidney, haemorrhage of heart,
haemorrhage or necrosis of the adrenal glands,
necrosis of the olfactory epithelium of the
nasal cavity, congestion of the thymus
4435 100% mortality
Clinical signs and pathology: same as 2956 mg/m3
rat 2 (male) 5.2-86 min 7500- 1-h LC50 was 7300 mg/m3. Zwart (1988)
(SPF-Wistar) 57 000 Rangec: 3.5-min LC50 = 75 700 mg/m3 Zwart et al.
480-min LC50 = 1300 mg/m3 (1992)
rat 5 (male) 8 h 1042- LC50 = 1175 mg/m3 Honma et al.
(Sprague-Dawley) 2085 (1985)
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 8 (male) 8 h 245- locomotor activity decreased at
(Sprague-Dawley) 972 731 mg/m3; rectal temperature fell
2°C at 486 mg/m3; decrease in feed
consumption and body weight gain at
486 mg/m3; all animals lost righting
reflex at 245 mg/m3
rat 8 (male) 6 h 778 extensive destruction of the Hurtt et
(F-344) olfactory epithelium al. (1988)
c Total of 23 combinations of time and dosage; the animals were observed for up to 2 weeks.
Groups (10 male+10 female) of BDF1 mice were exposed to methyl
bromide (99.9% pure) concentrations of 389, 584, 873, 1315, 1970, or
2950 mg/m3 (100, 150, 225, 338, 506, or 760 ppm) for 4 h (Japanese
Ministry of Labour, 1992). Mice exposed to concentrations of 1970 and
2950 mg/m3 showed decreased locomotor activity, tremor, convulsions,
diarrhoea, dyspnoea, and bradypnoea. In the 2950 mg/m3 group, all
the mice died; at 1970 mg/m3, 2 males survived. Mice exposed to
concentrations of between 389 and 1315 mg/m3 did not exhibit any
abnormal clinical signs.
Pathology in a female mouse exposed to 1315 mg/m3 showed
metaplasia of the olfactory epithelium. In the 2 male mice surviving
exposure to 1970 mg/m3, there was renal tubular necrosis and
regeneration, and necrosis and metaplasia of the olfactory epithelium.
In the other mice exposed to 1970 and 2950 mg/m3, there was
pulmonary congestion, hepatic degeneration and necrosis, renal tubular
necrosis, karyorrhexis of the thymus and lymph nodes, and necrosis of
the olfactory epithelium.
8.1.2.3 Rat
Irish et al. (1940) exposed rats to concentrations of 420, 852,
1000, 2000, 10 000, 20 000, or 50 000 mg methyl bromide/m3. Table 45
shows the exposure time in hours resulting in 100 % survival and 100
% fatality. Rats exposed to concentrations below 10 000 mg/m3 showed
roughening of the fur, hunching of the back, drowsiness, heavy
breathing, and sometimes lacrimation. At higher concentrations, the
first signs were nose irritation and lacrimation followed by the
reactions already mentioned. Those exposed for 20 h to 1000 mg methyl
bromide/m3 often became hyperactive until exhausted.
As well as neurological manifestations of toxicity in rats,
methyl bromide at concentrations of 1000-20 000 mg/m3 caused
irritation of the lungs, producing acute congestion and oedema (Irish
et al., 1940).
Groups (10 male + 10 female) of F344 rats were exposed to methyl
bromide (99.9% pure) concentrations of 584, 875, 1315, 1970, 2956, or
4435 mg/m3 (150, 225, 338, 506, 760, or 1140 ppm) for 4 h in a
chamber (Japanese Ministry of Labour, 1992). At concentrations of 1315
mg/m3 and above, there was decreased locomotor activity, ataxia,
nasal discharge, lacrimation, diarrhoea, and irregular breathing and
bradypnoea. In the 2956 and 4435 mg/m3 exposure groups, all the rats
died. Pathology of these groups showed pulmonary congestion, hepatic
degeneration, renal necrosis, myocardial haemorrhages, haemorrhage and
necrosis of the adrenal glands, and congestion of the thymus. In rats
exposed to 875, 1315, or 1970 mg/m3, there was metaplasia of the
olfactory epithelium and, in those exposed to the two highest doses,
also necrosis of the olfactory epithelium.
A single 6-h exposure of rats to 780 mg methyl bromide/m3
caused extensive destruction of the olfactory mucosal epithelium
(Hurtt et al., 1988).
Kato et al. (1986) determined a 4-h LC50 for methyl bromide in
male Sprague-Dawley rats (Fig. 9). Groups of 5 rats were exposed for
4 h to methyl bromide at concentrations of 1952, 2420, 3108, or 3485
mg/m3 (502, 622, 799, or 896 ppm), and approximate values of 100%
survival and 100% lethal concentration were determined (2529 and 3501
mg/m3, respectively). In a further test, 10 rats each were exposed
to 2727, 2984, 3143, 3178, or 3236 mg/m3 (701, 767, 808, 817, or 832
ppm). An LC50 value of 3034 mg/m3 (780 ppm) was calculated from
mortality at one week after exposure (Kato et al., 1986).
The dependence of methyl bromide toxicity on time and
concentration was demonstrated in studies performed by Zwart (1988)
and Zwart et al. (1992). Male SPF-Wistar rats were exposed to a total
of 23 combinations (2 rats each) of time and concentration and LC50
values were determined at seven time points ranging from 3.5 to 480
min. LC50s ranged from 75 700 mg/m3 at 3.5 min to 1300 mg/m3 at
480 min. The 1-h LC50 was 7300 mg/m3. Most animals showed some
incoordination, decreased response to stimuli, and had lame limbs,
directly after exposure. All mortalities occurred during the first
week and, on examination, red discoloured lungs and red/black spots in
the thymus were found in most dead rats. After two weeks, the
surviving animals were sacrificed. Some of these rats showed clear or
light red stained fluid in the lungs (Zwart, 1988).
Honma et al. (1985) carried out various investigations into the
effects of a single, 8-h exposure to methyl bromide on male
Sprague-Dawley rats. An acute toxicity study was carried out with five
groups of five animals exposed to 1042, 1303, 1564, 1824, or 2085
mg/m3 (268, 335, 402, 469, or 536 ppm), respectively. An 8-h LC50
of 1175 mg/m3 (302 ppm) with 95% confidence limits of 1040-1323
mg/m3 (267-340 ppm) was determined.
Body temperature was measured in four groups of five rats each
exposed to 245, 486, or 972 mg methyl bromide/m3 (63, 125, or 250
ppm). Exposure to 245 mg/m3 did not effect rectal temperature, while
8-h exposure at 486 or 972 mg/m3 decreased body temperature by about
2°C; however, this normalized within one day (Honma et al., 1985).
The effects of methyl bromide on body weight gain were
investigated. Food deprivation (feeding only twice a day) was started
at least 2 weeks before exposure. Rats were exposed to 245, 486, or
972 mg methyl bromide/m3 (63, 125, or 250 ppm) for 8 h and feed was
provided immediately afterwards. Decrease in food consumption and
depression in body weight gain were observed in groups exposed to 486
and 972 mg methyl bromide/m3, but not in groups exposed to 245 mg
methyl bromide/m3. The control group gained 15 g/day whereas with
exposure to 972 mg methyl bromide/m3, weight gain was almost fully
suppressed and was still partially depressed (+10 g) the following day
(Honma et al., 1985).
8.1.3 Dermal
Toxicity studies concerning the dermal route of exposure in
animals have not been reported.
8.1.4 Subcutaneous administration
For a single subcutaneous administration in Sprague-Dawley male
rats (9 rats/group) an LD50 for methyl bromide was found to be 135
mg/kg body weight (range 75- 250 mg/kg body weight) (Tanaka et al.,
1988).
8.2 Short-term exposure
8.2.1 Oral
A summary of studies concerned with oral exposure to methyl
bromide by gavage is given in Table 43.
A group of 12 rabbits was fed a mixed diet that had been
fumigated with methyl bromide for 24 h. The rabbits were fed
immediately after the fumigation was completed and the content of
methyl bromide in the feed was 3865 mg/kg. The first animal died 3
days after feeding was begun and the last in 13 days. All were
paralysed prior to death and all showed pulmonary damage. No changes
in the gastrointestinal tract were found (Dudley et al., 1940).
Studies on rats (8-week preliminary test, 16-week, and 20-week
test) and rabbits (52 weeks) were carried out by Dudley & Neal (1942).
Results from the rat study showed that, when high (5290-6200 mg Br/kg
food) amounts of organic and inorganic bromides were present in food
after fumigation with methyl bromide, mortality increased, body weight
gain and activity were reduced, and general health and reproductivity
were adversely affected. When feed containing 240-300 mg Br/kg,
following fumigation with 58 g methyl bromide/m3 for 24 h, was fed,
or when fumigated fruits and vegetables were fed, few or no
deleterious effects were noted (Dudley & Neal, 1942). Activity,
general condition, body weight gain, and reproductivity were normal.
Dudley & Neal (1942) carried out similar studies on rabbits. All 12
rabbits died within 2 weeks of being fed a diet containing about 3000
mg Br/kg. However, the 12 rabbits fed a diet of 60-100 mg Br/kg for 52
weeks showed few or no deleterious effects.
No apparent effects on appearance and general behaviour were
observed in Wistar rats (male and female) given doses, by gavage, of
up to 50 mg methyl bromide/kg body weight in a 90-day study (Danse et
al., 1984). Body weight gain in the male rats was significantly less
than that of controls, though this was not the case for females. There
were slight haematological changes and, in the two higher dosage
groups, several animals showed proliferative alterations of the
forestomach mucosa, characterized by hyperkeratosis and papilloma
(section 8.7).
A study by Boorman et al. (1986), based on a study design by
Danse et al. (1984), included dose groups with a recovery period, in
order to study the progression or regression of lesions. Details are
given in section 8.7 and Table 43. Boorman et al. (1986) found
forestomach lesions similar to those described by Danse et al. (1984),
but these lesions regressed in the 60-day recovery period.
Similar findings were reported by Hubbs & Harrington (1986). They
administered methyl bromide in peanut oil to rats at doses of 0, 25,
or 50 mg/kg body weight per day for up to 120 days. In a regression
study, some of the rats were treated for 90 days and then allowed to
recover for 30-60 days (Table 43 and section 8.7).
Three groups of four beagle dogs (3 male, 1 female) were fed
methyl bromide-fumigated food for 6-8 weeks in doses equivalent to an
average daily ingestion of 35, 75, or 150 mg/kg body weight of bromide
ion, respectively (Rosenblum et al., 1960). A further group of 4 dogs
received 128 mg sodium bromide/kg per day (equivalent to 100 mg
bromide ion/kg per day). A control group of 6 dogs (3 male and 3
female) received only dog chow. After one year of observation and
monthly blood and urine tests, the remaining dogs were killed, the
organs weighed, and histological studies carried out. No evidence of
toxicity that could be attributed to bromide was observed in animals
that received 35 or 75 mg bromide/kg per day. Dogs in the group
receiving 150 mg/kg per day became lethargic and had occasional
episodes of salivation and diarrhoea. No significant effects on blood
chemistry, haematology, or urinary values were reported, nor were
treatment-related deaths or histological lesions noted.
8.2.2 Inhalation studies
8.2.2.1 Guinea-pig, rabbit, monkey
A summary of short-term exposure studies is given in Table 48.
Irish et al. (1940) carried out extensive studies into the
long-term exposure of animals to methyl bromide. A total number of 135
rats, 98 guinea-pigs, 104 rabbits, and 13 monkeys were exposed to 65,
130, 250, 420, or 850 mg methyl bromide/m3, 7-8 h/day, 5 days/week
for 6 months, or, until the majority had either died or shown a severe
reaction (Table 49).
8.2.2.2 Mouse
In the short-term studies on male and female B6C3F1 mice, exposed
6 h/day for 10 days over 14 days (778 mg methyl bromide/m3),
described by NTP (1992), five mice/dose group per sex were evaluated
for haematology, serum pseudocholinesterase activity, and pathology.
Necropsied animals from the two highest dosage groups were examined
histopathologically. The results are summarized in Table 48.
Eustis et al. (1988) carried out a special target organ study on
B6C3F1 mice (and F344/N rats). Male and female B6C3F1 mice were
exposed to either 622 mg methyl bromide/m3 (160 ppm) or air for 6
h/day, 5 days/week. The animals were scheduled for sacrifice after 3,
10, or 30 exposures. When 50% mortality was observed in any group, the
surviving animals in that group were sacrificed. Mice were evaluated
for body weight, mortality, organ weights, haematology, and
histopathology. In addition to these end-points, urine chemistry and
plasma enzymes were assessed in the rats. Significantly different
mortality rates were observed between the two species, with the mice
demonstrating a higher sensitivity to 622 mg methyl bromide/m3 than
rats. Body weight differences were exposure-related (Eustis et al.,
1988). The results are summarized in Table 48. Mortality exceeded 50%
after 8 and 6 exposures in male and female mice, respectively. The
remaining male mice were killed after 10 exposures and the females
after 8 exposures. There were significant reductions in body weight
and corresponding reductions in organ weights in both sexes, whereas
there were sex differences in the haematological parameters. The
responses to exposure were minimal in males, but marked in females, in
which there were large and significant reductions in RBC, haemoglobin,
haematocrit values, and mean corpuscular haemoglobin concentrations,
and increases in WBC and mean corpuscular volume (Eustis et al.,
1988). Histopathological changes in target organs are described in
section 8.8.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). Survival was reduced at all
exposure concentrations and none of the mice exposed to 1315 or 1712
mg methyl bromide/m3 survived. At all exposure concentrations, mice
exhibited decreased locomotor activity, piloerection, lacrimation,
ataxia, and tremor.
Table 48. Short-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 10 (male) 6 h/day; 5 days/week; 477 78 mg/m3 group: 9 male, 6 female died; NTP (1992)
(B6C3F1) 10 (female) 2 weeks 97 All groups: no body weight changes; bloody
195 urine; trembling, jumpiness, paralysis in
389 all groups, but most pronounced in highest
778 dosage groups; haematology parameters/
pseudo cholinesterase activity - no
consistent dose-related effects
1 female mouse showed minimal
hyperaemia of lungs, liver, kidneys
Mouse 20 (male) 6 h/day; 5 days/week; 622 50% mortality (male) after 8 exp. days, Eustis et
(B6C3F1) 20 (female) 10 exp. days 50% mortality (female) after 6 exp. days; al. (1988)
for males and exposure-related lesions were seen in
8 exp. days the brain, heart, kidneys, thymus, and
for females spleen of both sexes; in the testes,
nose, and lungs of males, and in the
adrenal glands of females
Mouse 15 (male) 6 h/day; 5 days/week; 0 Eustis et al. (1988)
(B6C3F1) 15 (female) 6 weeks 622 lethargy; curling and crossing
(continued) of hind-limbs, forelimb twitching
and tremors; decrease in body weight
gain after 5 days; decrease in organ
weight (lung, heart, thymus, brain,
liver) neuronal necrosis; nephrosis;
atrophy of inner zone of adrenal
cortex; testicular degeneration;
decrease in RBC, increase in WBC
(females only)
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 18-30 (males) 5 days/week; 6 h/day; 0 All dose groups: no significant organ NTP (1992)
(B6C3F1) 18-30 (female) 13 weeks 39 weight effects;
78
156 decrease in Hb and MCV, increase in RBC (males);
311 decrease in Hb and MCV, increase in RBC (males);
467 17 % mortality in males; decrease in body weight,
additionally severe curling and crossing
of hindlimbs and twitching of forelimbs
(male > female)
Mouse 10 (male) 6 h/day; 5 days/week 0 Japanese
(Crj:BDF1) 10 (female) 13 weeks 29 no toxic effects; Ministry of
58 no toxic effects; Labour
117 no toxic effects; (1992)
234 depression of body weight gain;
Haematology: increase in MCV in females;
Urinalysis: increased protein in females
Mouse 10 (male) 6 h/day; 5 days/week 599 10% mortality (male) and 0% (female) Japanese
(Crj:BDF1) 10 (female) 2 weeks depression of body weight gain; Ministry of
(continued) Clinical signs: Labour (1992)
Dead mice: decrease in locomotor activity,
piloerection, lacrimation, bradypnoea,
opacity of eye, diarrhoea
Surviving mice: decrease in locomotor activity,
bradypnoea, ataxic gait, sub-normal
temperature, tremor, lacrimation, soiled,
pallor, hunched posture, piloerection
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology:
Dead mice: tubular necrosis of the kidney,
ulcer of the stomach, testicular
atrophy, atrophy of the spleen, atrophy
and karyorrhexis of the lymph node,
myocardial necrosis
Surviving mice: degeneration of the granular
layer of the cerebellum, tubular necrosis and
regeneration of the kidney, necrosis and
respiratory metaplasia of the olfactory
epithelium
Mouse 778 50% mortality of males and 80% mortality of Japanese Ministry
(Crj:BDF1) females, depression of body weight gain; of Labour (1992)
(continued) Clinical signs: same as 599 mg/m3 group
Pathology: Dead mice: degeneration of the
granular layer of the cerebellum, tubular
necrosis and regeneration of the kidney,
extramedullary haematopoiesis and atrophy
of the spleen, karyorrhexis of the thymus,
myocardial necrosis, necrosis of the
olfactory epithelium
1011 90% mortality (male and female)
depression of body weight gain;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
1315 100% mortality (male and female);
Clinical signs: same as mice dying
in 599 mg/m3 group
Pathology: congestion of the lung,
degeneration of the liver, hyaline droplet
and tubular necrosis of the kidney,
karyorrhexis of the thymus and spleen,
myocardial necrosis, necrosis of the olfactory
epithelium.
Mouse 1712 100% mortality (male and female); Japanese Ministry
(Crj:BDF1) Clinical signs: same as mice dying of Labour
(continued) in the 599 mg/m3 group (1992)
Pathology: congestion of the lung,
degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis
of the thymus and spleen.
467 mortality, 10% in males and 90% in females;
depression of body weight gain;
Clinical signs: ataxic gait;
Haematology: increased MCV in males;
Urinalysis: same as 234 mg/m3 group;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, congestion of the lung, karyorrhexis
and atrophy of the thymus, tubular necrosis
of the kidney, necrosis of the heart,
vacuolic change of the adrenal glands
Rat 6 (male) 6 h/day; 5 days 0 NTP (1992)
(SPF Wistar) (week 1) 150 brain weight depression 4-5%
6 h/day; 3 days 375 (dose related) at 750 mg/m3;
(week 2) 750 additionally, marked growth retardation;
tremors, motor incoordination; liver
weights decreased by 26%; no distinct
microscopic changes in eight organs (but
lungs of three high-dose rats were
hyperaemic)
Rat 6 (male) 6 h/day; 5 days/week 0 no toxic effects; NTP (1992)
(SPF Wistar) 6 (female) (week 1,2,3) 70 no toxic effects (marginal no-effect level); (Dutch
6 h/day; 7 days/week 200 decrease in feed consumption, decrease Study)
(week 4) in body weight gain;
disturbed gait and tremors;
600 histopathological changes in heart
and lungs, 8 rats died before end
of study
Rat 10 (male) 6 h/day; 5 day/week; 0 no deaths; no clinical findings; no Wilmer et
(Wistar) 10 (female) 13 weeks 4 change in body weight al. (1983)
25
166 minimal changes in liver
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 10 (male) 6 h/day; 5 days/week; 599 depression of body weight gain Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
Pathology: disarrangement and
respiratory metaplasia of the
olfactory epithelium
778 depression of body weight gain;
Pathology: disarrangement and respiratory
metaplasia of the olfactory epithelium
of the nasal cavity, cellular
vacuolization in the adrenal glands
1011 depression of body weight gain;
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
Rat 10 (male) 6 h/day; 5 days/week; 1315 70% mortality in males and 10% mortality Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
(continued) Dead mice: depression of body weight gain; (continued)
Clinical signs: decrease in locomotor
movement, soiled, piloerection, lacrimation,
serous or bloody nose discharge, diarrhoea,
pallor, irregular breathing;
Dead and surviving mice: decrease in
locomotor movement, hunched posture, soiled,
piloerection, haemorrhagic discharge of nose,
lacrimation
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: interstitial pneumonia,
karryorrhexis of of the thymus,
myocardial damage, cellular vacuolization
of the adrenal glands, disarrangement and
respiratory metaplasia of the olfactory
epithelium
1712 100% mortality;
Clinical signs: same as 338 ppm group dead
rats;
Pathology: congestion and haemorrhage of
the lung, congestion, necrosis, and fatty
changes in the liver, tubular necrosis of
the kidney, myocardial necrosis, haemorrhage,
necrosis and cellular vacuolization of the
adrenal glands, necrosis and
respiratory metaplasia of the olfactory
epithelium, congestion of the thymus,
inflammation of the bone marrow
Rat 10-12 (male) 4 h/day; 6 weeks 584 decrease in body weight, Kato et
(Sprague- no clinical changes; al. (1986)
Dawley) 778 decrease in body weight,
no clinical changes;
1167 3/12: paralysis of hindlimbs;
1556 5/10: ataxia after 2 weeks, paralysis
after 3 weeks, 1/10: died after 4 weeks,
3/10: died after 5 weeks;
Haematology: no change in RBC, Hb, Hct, WBC
1167 mg/m3 group: increase in serum enzyme
activities;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Organ weights: decrease in all groups, but
no clear dose dependency;
Residual bromide: increase in all groups
584 mg/m3: spleen>kidney>liver;
higher dosage groups: kidney>spleen>liver;
Histopathological changes: in brain, heart
and testes
Rat 5 (male) 3 weeks 4 biochemical changes Sato et
(Sprague- 20 al. (1985)
Dawley) 39
Rat 10 (male) 6 h/day; 5 days 350 no observable effects; Hurtt et
(F-344) 680 dose-dependent vacuolar degeneration al. (1987)
of zona fasciculata (adrenal gland),
cerebellar granular cell degeneration
and olfactory sensory cell degeneration;
973 as above, plus: diarrhoea, haemoglobinuria,
1264 some gait disturbances and convulsions;
hepatocellular degeneration (at 1264
mg/m3 only) - cerebral cortical degeneation
ation and minor alterations in testicular
histology
Rat total 84 6 h/day; 5 days 778 increase in mean body weight; Hurtt et al. (1988)
(F-344) (male) degeneration and regeneration
of olfactory epithelium
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 40 (male); 6 h/day; 5 days 778 decrease in plasma testosterone Hurtt & Working
(F-344) 10 (male) concentration and nonprotein sulfhydryl (1988)
killed on contents of liver and testis
each of day
1,3,5 and 8
35 (male); 6 h/day; 5 days
5 killed on
each of day 6,
10, 17, 24, 38,
52, 73
Rat 5 (male) 6 h/day (3 days) 622 50 % mortality in males after 14 exp. Eustis et
(F-344) 5 (female) days; remaining males sacrificed; al. (1988)
females killed after 6 weeks;clear
5 + 10 (male) 6 h/day, 5 days/week sex-related differences in susceptibility
5 (female) (2 weeks) of specific organs to CH3Br:brain, kidney,
nasal cavity, heart, adrenal, liver, and
10 (female) 6 h/day, 5 days/week testis; neuronal necrosis in cerebral cortex,
(6 weeks) hippocampus and thalamus of brain; necrosis of
olfactory epithelium; myocardial degeneration;
testicular degeneration
Rat 18 6 h/day; 5 day/week; 0 All dose groups: no deaths or clinical Haber et al.
(F-344/N) 18 (female) 13 weeks 117 signs; no consistent organ weight effects; (1985) [abstract]
234 * body weight (females) NTP (1990)
467 * body weight (both sexes);
minor neurobehavioural changes; (females)
* Hct, * Hb, * RBC;
olfactory epithelial dysplasia and cysts
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 36 (male) 6 h/day; 5 and 117 GSH and G-6-PDH activities: increase in Jaskot et
(CD) 10 days lung and decrease in liver; al. (1988)
serum: decrease in cholinesterase, BUN,
uric acid, cholesterol,
increase in leucine amino-peptidase
Rat total 30 4 h/day; 4 days/week, 778 damage of olfactory epithelium; Hastings
(Long-Evans) (15 control) 2 weeks repair by day 4; impaired nasal (1990)
function recovered after 4 days
Rat 10 (male) 6 h/day; 5 day/week; 0 Japanese Ministry
10 (female) 13 weeks 29 no toxic effects of Labour
73 Blood biochemistry: * K (male), ** total (1992)
cholesterol (female)
183 Blood biochemistry: * in potassium (male)
** in total cholesterol, GOT, GPT (female)
455 * of body weight gain
Haematology: ** Hct, ** MCV, ** platelet
(male) ** MCV (female)
Blood biochemistry: * K (male), ** total
** total cholesterol, GOT, GPT * glucose,
creatinine (female)
Rat 1140 100% mortality; Japanese
(continued) Clinical signs: * locomotor activity, Ministry of Labour
hunchback position, piloerection, (1992) (continued)
soiled, ataxic gait, tremor, convulsion,
diarrhoea, loose stool or cyanosis,
haematuria, serous or haemorrhagic
discharge of nose, haemorrhagic discharge of
eye, lacrimation, irregular breathing
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, karyorrhexis, haemorrhage and atrophy
of the thymus, tubular necrosis of the kidney,
atrophy of the testis, foamy cell accumulation
and interstitial pneumonia, myocardial damage,
cellular vacuolization of the adrenal glands,
pigmentation of the Harderian glands,
necrosis, disarrangement and respiratory
metaplasia of the olfactory epithelium
GSH = glutathione S-transferase; G-6-PDH = glucose-6-phosphate dehydrogenase; BUN = blood urea nitrogen;
RBC = red blood cell count; exp. = exposure; p.c. = post copulation.
* = decrease; ** = increase
Histopathology showed degenerative cerebellar changes, renal
tubular necrosis and regeneration, and metaplasia and necrosis of
olfactory epithelium in all exposed groups. In male mice exposed to
599 mg/m3, there were also stomach ulceration, testicular atrophy,
atrophy of the spleen, and atrophy and karyorrhexis in the lymph
nodes. At concentrations above 778 mg/m3, there were, also karyor-
rhexis of the thymus, and myocardial necrosis. Hepatic degeneration
and pulmonary congestion were found at 1315 and 1712 mg methyl
bromide/m3. For further details see Table 48.
B6C3F1 mice were exposed to methyl bromide concentrations of 0,
39, 78, 156, 312, or 468 mg/m3 for 6 h per day on five days a week
for 13 weeks (NTP, 1992). There were reductions in survival and body
weight gain among male mice exposed to 468 mg/m3. No reduction was
observed among males in the other groups or in female mice in any
group. Clinical findings in the high-dose group were severe curling
and crossing of the hindlimbs and twitching of the forelimbs. These
signs were more severe among male than among female mice. Mild
behavioural test response deviations reached a maximum after about 6
weeks with no further increase in severity in the later 7 weeks of the
study. Plasma cholinesterase activity was unaffected by treatment. No
exposure-related histopathological changes were described.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 58, 117, 234, or 467 mg/m3 (0,
7.5, 15, 30, 60, or 120 ppm) for 13 weeks, 6 h/day, and 5 days/week
(Japanese Ministry of Labour, 1992). At 467 mg/m3, there was a
mortality rate of 10% (males) and 90% (females). Ataxia was noted in
mice exposed to 467 mg/m3. There were no abnormal clinical signs at
concentrations of between 29 and 234 mg/m3. Slight increases in mean
corpuscular volume (MCV) in female mice exposed to 234 mg/m3 and in
male mice exposed to 467 mg/m3 were not accompanied by other
haematological effects. Blood biochemistry showed no abnormalities. No
treatment-related, histopathological effects were observed in the 29
and 234 mg/m3 groups. In the mice that did not survive exposure to
467 mg/m3, there were cerebellar degeneration and brain necrosis,
pulmonary congestion, thymic atrophy, myocardial damage, renal tubular
necrosis, and vacuolization of the adrenal glands (Table 49).
Table 49. Long-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
mouse 86 (male) 5 days/week; 0 All dose groups: no biologically significant NTP (1992)
(B6C3F1) 86 (female) 6 h/day;2 year 39 haematology values; no carcinogenic
[planned; (interim 128 effects; increased incidence of
because of high sacrifice at 6 389 nonneoplastic lesions in brain, bone,
mortality, and 15 months) heart, and nose
regime altered] 389 mg/m3 dosage group: high mortality
(40/86 males and 9/86 females) after 20
weeks; decrease in body weight and
in thymus weight;
tremors, abnormal posture and limb paralysis;
significant behavioural differences at 3
months (males), less so in females
mouse 50 (male) 6 h/day; 5 days/ 0 Japanese
(Crj: 50 (female) week; 16 no toxic effects; Ministry of
BDF1) 2 years 62 no toxic effects; Labour (1992)
250 no mortality change;
depression of body weight gain
Blood biochemistry: increase in CPK, inorganic
phosphorus (male), chloride; decrease
in albumin (female);
Pathology: atrophy of the granular layer of
the cerebellum no increase in neoplastic change
was considered
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 90 (male) 6 h/day; 5 days/ 0 All dose groups: degenerative and Dreef-van der
(Wistar) 80 (female) week; 29 months 12 hyperplastic lesions in nasal mucosa; no Meulen et al.
117 tumours induced by methyl bromide; (1989)
350 350 mg/m3 group: * body weight gain; Reuzel et al.
* absolute brain weight; hyperkeratosis in (1991)
oesophagus and forestomach; ** incidence
of haemothorax, myocardial degeneration;
thrombi in the heart; and increased
mortality
rat 50 (male) 6 h/day; 5 days/ 16 Pathology: ** incidence and severity of Japanese
(F344/ 50 (female) week; 2 years inflammation of the nasal cavity (male); Ministry of
DuCrj) 78 Urinalysis: * in protein (male); Labour (1992)
Pathology: same as 16 mg/m3
389 no mortality change, * body weight gain;
Haematology: ** RBC, Hb, Hct (male)
** Hb, Hct (female);
Blood biochemistry: * LDH, CPK, Na+, K+,
Cl- (male), * Glu, CPK, Ca2+, ** LAP (female);
rat Urinalysis: * protein
(F344/DuCrj) Pathology: ** incidence of necrosis and
(continued) respiratory metaplasia of the olfactory
epithelium; ** incidence and severity of
inflammation of the nasal cavity (male);
marginal ** necrosis of the olfactory
epithelium and inflammation of the nasal
cavity (female);
no increase in neoplasms observed
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 8 6 h/day; 5 days/ 0 no effect on nerve conduction velocity, Anger et al.
(Sprague- 32 (n.g.)b week; 36 weeks 214 open field activity, or coordination (1981)
Dawley) over 12 months
a RBC = red blood count; Hb = haemoglobin; Hct = haematocrit; WBC = white blood count; MCV = mean cell volume.
b n.g.= sex not given; * = decrease; ** = increase.
8.2.2.3 Rat
F344-rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). All rats in the 1712 mg/m3
group and 7 males and one female in the 1315 mg/m3 group died before
the end of the study. Piloerection and haemorrhagic nasal discharge
were reported at exposure concentrations of 1011, 1315, and 1712
mg/m3. In addition, decreased locomotor activity, a hunched position
and lacrimation were observed in rats that survived exposure to 1315
mg/m3. Diarrhoea and irregular breathing were noted in the rats that
died following exposure to 1315 and 1712 mg/m3.
Pathology showed metaplasia of the olfactory epithelium at methyl
bromide concentrations of 599 and 778 mg/m3, plus necrosis at
concentrations between 1011 and 1712 mg/m3. Vacuolization of the
adrenal glands was found at concentrations of 778, 1011, and 1315
mg/m3 with necrosis at 1712 mg/m3. Myocardial damage was reported
at concentrations between 1011 and 1712 mg/m3. In the rats exposed
to 1315 and 1712 mg/m3, there was also pulmonary congestion and
haemorrhage, renal tubular necrosis and congestion of the thymus; bone
marrow inflammation was reported at 1712 mg/m3 (Table 48).
Two short-term range finding studies were conducted in SPF Wistar
rats (NTP, 1992). In the first study, groups of six male rats were
exposed to up to 750 mg methyl bromide/m3 for 6 h/day for 2 weeks
(see Table 48). In the second range-finding study, groups of six male
and six female rats were exposed to up to 600 mg methyl bromide/m3
for 4 weeks. Five male and three female rats in the high-dose group
died before the end of the study. The most important histopathological
changes occurred in the heart and lungs of animals in the high-dose
group. Diffuse fatty vacuolization and diffuse myocardial fibre
degeneration appeared. The lungs showed hyperaemic and dilated
alveoli; in some animals, interstitial pneumonia was noted (NTP,
1992).
Male Sprague-Dawley rats were exposed to up to 1556 mg methyl
bromide/m3 for 4 h/day for 6 weeks (Kato et al., 1986). Changes in
body weight, general condition, haematology parameters, organ weight,
tissue bromide ion concentration, and the histopathology of several
organs were determined. Suppression of body weight gain, abnormal
clinical signs, and severe weakness were observed at 1556 mg methyl
bromide/m3. Bromide ion accumulation was seen, especially in the
kidney and spleen, without significant dose-related change. Pronounced
histopathological changes were noted in the brain (section 8.8) and
multiple small necrotic foci in the heart.
Other short-term, inhalation exposure studies concentrated on one
aspect/target organ of methyl bromide exposure.
Sato et al. (1985) described biochemical findings in rats exposed
to 4, 20, or 39 mg methyl bromide/m3, continuously, for 3 weeks.
After sacrifice, the organs were weighed and biochemical examinations
were performed on the blood and a homogenate of heart, liver, and
lungs. The results showed no differences between the 4 mg/m3 and the
control group. In the 20 mg/m3 group, several changes were observed;
serum creatine phosphokinase (CPK), phospholipids (PL), and blood
glucose levels, and thymus weight decreased, while blood haemoglobin
(Hb), reduced glutathione (GSH), serum total protein, and lung
gamma-glutamate-pyruvate transaminase (GTP) levels increased after
exposure. In the 39 mg/m3 group, increases were observed in serum
glutamate oxaloacetate transaminase (GOT), lactate dehydrogenase
(LDH), alpha-hydroxy-butyrate dehydrogenase (alpha-HBDH), total
protein, blood Hb, GSH, lung acid phosphatase (AcP), gamma-GTP, LDH
total, liver PL, and triglycerides (TriG), while decreases were noted
in serum cholinesterase (ChE), CPK, PL, TriG, lung alkaline
phosphatase (AlP), free-cholesterol (f-Chol), lactate, blood glucose,
heart lactate, glucose, lung AlP, liver free fatty acids (FFA), and
glycogen. Pulmonary haemorrhage was observed in almost all animals in
this group.
The effects of short-term inhalation exposure to 1264 mg methyl
bromide/m3 (6 h/day for 5 days) including those on the target organ
histopathology of male F-344 rats were studied by Hurtt et al. (1987).
Clinical changes (noted only in the 973 and 1264 mg/m3 groups) were
diarrhoea, haemoglobinuria, and, in a few cases, gait disturbances and
convulsions. A dose-dependent vacuolar degeneration of the zona
fasciculata of the adrenal glands, cerebellar granule cell
degeneration, and olfactory sensory cell degeneration were seen in the
680, 973, and 1264 mg/m3 groups. Cerebral cortical degeneration and
minor alterations in testicular histology were seen only in the 1264
mg/m3 group whereas hepatocellular degeneration was also found in
the 973 mg/m3 group. No changes were found in the kidneys or
epididymides.
In a further study, the degeneration and regeneration of the
olfactory epithelium were examined following exposure of a total of 84
rats to 778 mg methyl bromide/m3 for 6 h/day for 5 days (Hurtt et
al., 1988). Groups of five rats were sacrificed after days 1, 3, and
5 and after exposure weeks 1, 2, 3, 5, and 10. Cell replication rate
and histopathology were used to assess the kinetics of repair. In
addition, olfactory function was assessed using a buried food test.
Extensive damage to the olfactory epithelium was evident in animals
killed directly after 6 h of exposure. The specific site of damage
appeared to be in the olfactory sustentacular cells and mature sensory
cells, the basal cells generally being unaffected. By day 3, despite
continuous exposure, there was replacement of the olfactory epithelium
by a squamous cell layer that increased in thickness and basophilic
staining over the next 2 days. One week after exposure, the epithelial
region was covered by a layer of polyhedral, basophilic cells and from
2 to 10 weeks exposure, the epithelium exhibited progressive
reorganization to restore the original olfactory epithelium pattern;
75-80% of the olfactory epithelium appeared morphologically normal by
week 10.
In the same study, the ability of the rats to locate feed was not
affected by exposure to 350 mg methyl bromide/m3, but treatment with
778 mg methyl bromide/m3 rendered all animals temporarily incapable
of locating buried pellets, though they demonstrated searching
activity. Four to six days after treatment the animals recovered
sufficient olfactory function to find food pellets.
Similar results were reported by Hastings et al. (1989) and
Hastings (1990). Studies on Long-Evans rats showed that, after 4 h
exposure to methyl bromide (778 mg/m3), recovery of buried food was
greatly impaired but, even with continuous exposure, recovery occurred
until, by day 4 of exposure, olfactory function was essentially
normal. Extensive damage to the olfactory epithelium was evident after
day 1 of exposure; repair of the epithelium was in progress by day 4.
The specific site of damage appeared to be in the olfactory
sustentacular cell population while the respiratory epithelium was
largely spared (Hastings et al.,1989; Hastings, 1990). Bolon et al.
(1990) suggested that prior exposure to methyl bromide as well as
caging conditions (e.g., inhalation of ammonia from soiled bedding)
could influence the olfactory epithelial response.
Evans & Hastings (1992) reported that methyl bromide induced an
olfactory function deficit in rats. The olfactory threshold to ethyl
acetate was measured in six rats using a conditioned suppression
behavioural protocol. Three out of the six rats showed an increase in
absolute threshold or threshold response variability after a single,
6-h exposure to 778 mg methyl bromide/m3.
F-344 rats (20 male + 20 female) were exposed (6 h/day; 5
days/week) to 0 or 622 mg methyl bromide/m3 (0 or 160 ppm) for 3,
10, or 30 days (Eustis et al., 1988). Toxicological end-points
assessed included clinical observations, mortality, body and organ
weights, haematology, clinical chemistry, urinalysis, gross pathology,
and histopathology. There were no apparent treatment- related changes
in any of the clinical chemistry and urinalysis analytes measured.
Treatment-related effects in rats included neuronal necrosis in the
brain, myocardial degeneration, olfactory epithelial degeneration and
atrophy, and testicular degeneration and atrophy.
A study by Eustis et al. (1988) confirmed the very steep
concentration-response curve for methyl bromide in rats (and mice).
The authors also found clear species and sex differences in
sensitivity to methyl bromide toxicity. When rats were compared with
mice, the order of susceptibility was female mice>male mice>male
rats> female rats. Species and sex-related differences in the
histopathology of certain organs were also observed.
Kato et al. (1986) exposed Sprague-Dawley rats for 11 weeks, 4
h/day, to 584 mg methyl bromide/m3. No abnormal clinical signs were
reported. Small necrotic foci and fibrosis were observed in the heart
muscle.
F-344 rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 73, 183, 455, or 1140 mg/m3 (0,
7.5, 18.8, 46.9, 117, or 293 ppm) for 13 weeks for 6 h/day, 5
days/week (Japanese Ministry of Labour, 1992). At 1140 mg/m3, there
was 100% mortality. At this concentration of methyl bromide, rats
exhibited decreased locomotor activity, piloerection, ataxia, tremor,
cyanosis, haematuria, and nasal and ocular discharge. No clinical
signs were reported at lower doses.
Haematological studies showed increased MCV in male and female
rats exposed to 455 mg/m3; there was also an increased number of
platelets in the male rats. Serum potassium was decreased in male rats
exposed to concentrations of 73 mg/m3 or more. Glutamic oxalacetic
transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were
significantly increased in female rats exposed to concentrations of
183, 455, and 1140 mg/m3. Serum glucose and creatinine levels were
significantly decreased in female rats at 455 mg/m3.
There were no abnormal histopathological findings in rats exposed
to methyl bromide concentrations of between 29 and 455 mg/m3. Rats
exposed to 1140 mg/m3 exhibited cerebellar degeneration and brain
necrosis, thymic haemorrhages and atrophy, renal tubular necrosis,
pneumonitis, myocardial damage, adrenal vacuolization, Harderian gland
pigmentation and metaplasia, and necrosis of the olfactory epithelium
(Table 49).
Thirteen-week inhalation toxicity studies were conducted in which
groups of ten male and ten female Wistar rats were exposed to methyl
bromide at target concentrations of up to 166 mg/m3 for 6 h/day, 5
days/week (Wilmer et al., 1983). No deaths occurred, and no abnormal
clinical findings were observed. Body weight gain was not affected in
any of the exposed groups. Leukocyte counts were 22% higher in high-
dose males than in male control animals. Plasma alkaline phosphatase
activity was lower in both high-dose males (32%) and females (53%)
than in controls, and the plasma albumin concentration was 10% higher
in high-dose females than in controls. The absolute and relative liver
weights were up to 16% lower for high-dose males and females compared
with controls. The only exposure-related histopathological change,
which occurred in the liver of high-dose male and female rats, was
characterized by small hepatocytes with homogeneous eosinophilic
cytoplasm. This alteration varied in extent from slight to severe and
was seen in 6/10 males and 7/10 females. The no-adverse-effect level
for these 13-week inhalation toxicity studies was considered to be 25
mg/m3.
Groups of 18 rats of each sex were exposed to up to 467 mg/m3
for 6 h/day and 5 days/week over 13 weeks (NTP, 1992). All rats
survived to the end of the studies, few or no clinical effects of
exposure to methyl bromide could be seen. Significant decreases in
body weight gain were noted in both sexes at 467 mg/m3 and in
females at 234 mg/m3. No consistent organ weight effects were
observed. Minor neurobehavioural changes were noted in both sexes in
the highest dosage group. Females, but not males, of this group had
significantly lower haematocrit, haemoglobin levels, and erythrocyte
counts compared with those of controls. Olfactory epithelial dysplasia
and cysts, characterized by irregularity in mucosal thickness and
focal cavitated spaces, respectively, were seen in both sexes at 467
mg/m3.
8.2.3 Dermal
There are no reports of studies on short-term dermal exposure.
8.3 Skin and eye irritation
Irish et al. (1940) noticed lacrimation in rats after inhalation
of methyl bromide levels above 10 000 mg/m3. Irritation of the eye
membranes in mice at concentrations of 3200 mg methyl bromide/m3 was
described by Balander & Polyak (1962). There are no reports on skin
effects in animals.
8.4 Long-term exposure
8.4.1 Oral
8.4.1.1 Rat
Rats were fed for 7-8 months on wheat grain or peanuts fumigated
with methyl bromide having residual bromide levels of 20 and 22-46
mg/kg, respectively. There were no effects on weight gain of the
animals, haemoglobin content, or red or white blood cell numbers.
However, a decrease in iodine and calcium levels in the blood and
abnormal changes in the thyroid and parathyroid glands were reported
(Vitte et al., 1970).
A two-year, oral, long-term toxicity and carcinogenicity study
was carried out on 60 male and 60 female F-344 rats per group fed
diets fumigated with methyl bromide (Mitsumori et al., 1990). Diets
containing 80, 200, or 500 mg total bromide/kg food (methyl bromide
concentration <20 mg/kg food) were fed, the controls receiving
commercial basal diet (containing 30 mg bromide/kg) and a diet
containing 500 mg potassium bromide/kg. No effects were observed on
the behaviour of the rats in any of the groups fed the fumigated or
KBr- containing diets. In rats fed the diets fumigated with methyl
bromide, there were no marked toxic changes, except for a slight
depression in body weight from week 60 onwards in males in the 500
mg/kg group. Tumour incidence was unaffected. Rats given a diet
containing KBr did not show any treatment-related changes. The
no-effect level was 200 mg/kg (equivalent to 6.77 mg total bromine/kg
body weight per day) in males. No effects were observed in females at
the doses studied.
8.4.2 Inhalation studies
Long-term exposure inhalation studies are summarized in Table 49.
8.4.2.1 Mouse
In a carcinogenicity study, 86 B6C3F1 mice/sex were exposed to
39, 128, or 389 mg methyl bromide/m3 for 6 h/day, 5 days/week, for
2 years with scheduled interim sacrifices at 6 months and 15 months
(NTP, 1992). Because of the high mortality early in this study in the
389 mg/m3 group (27/86 males, 7/86 females), the exposure to methyl
bromide at this level was stopped at 20 weeks and the surviving mice
were exposed to air for the rest of the study.
Terminal survival rates (Kaplan-Meier determinations) in the
control, 39 mg/m3, 128 mg/m3, and 389 mg/m3 groups were: males
82%, 74%, 80%, and 23%; and females, 71%, 82%, 90%, and 65%,
respectively. Significantly lower mean body weights of the highest
dosage group compared with controls appeared by week 11 and persisted
after termination of dosing at week 20 until the end of the studies.
The only biologically significant change appeared to be reduced
absolute and relative thymus weights in both sexes. Neurological signs
were also observed, mostly in the highest dosage group, including
tremors, abnormal posture (lateral curvature of the spine), and limb
paralysis. These symptoms generally persisted (NTP, 1992).
Exposure-related histological lesions occurred primarily in the 389
mg/m3 exposure group and included degeneration in the cerebellum,
cerebrum and heart, chronic cardiomyopathy, sternal dysplasia, and
either necrosis or metaplasia of the olfactory epithelium. There were
no neoplasms attributed to exposure to methyl bromide.
Quantitative neurobehavioural testing showed significant
differences in the behaviour of the high-dose males at 3 months. The
animals were less active and demonstrated a reduction in startle
response latency. Because of the high early mortality in high-dose
males, only males in the controls and two lower dosage groups were
tested after 3 months. After 6 months of exposure, the 389 mg/m3
females had significantly lower activity scores than females in other
groups, but their higher startle responses had disappeared. Although
at 9 months of exposure no behavioural differences were apparent,
lower activity and heightened startle response were again noted at the
2-year testing time (NTP, 1992).
Toxicity and carcinogenicity studies were conducted by inhalation
exposure of groups of 50 male and 50 female Crj:BDF1 mice (6 h/day, 5
days/week) to methyl bromide (99.9% pure) for 104 weeks (Japanese
Ministry of Labour, 1992). Methyl bromide concentrations of 0, 16, 62,
and 250 mg/m3 were used.
Body weight gains in male and female mice exposed to 250 mg/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of atrophy (slight) of the granular layer of
the cerebellum were observed in male and female mice exposed to 250
mg/m3. There were no treatment-related neoplasms in male or female
mice.
8.4.2.2 Rat
Toxicity and carcinogenicity studies were also conducted by
inhalation exposure to methyl bromide (99.9% pure) of groups of 50
male and 50 female F344/DuCrj rats (6 h/day, 5 days/week) for 104
weeks (Japanese Ministry of Labour, 1992). Methyl bromide
concentrations of 0, 16, 78, and 389 mg/m3 were used. Body weight
gains in males and female rats exposed to 389 mg methyl bromide/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of necrosis and respiratory metaplasia of
the olfactory epithelium of the nasal cavity were observed in male
rats exposed to 389 mg methyl bromide/m3, and increased incidence
and severity of inflammation of the nasal cavity were observed in male
rats exposed at all concentrations used. Necrosis of the olfactory
epithelium and inflammation of the nasal cavity were marginally
increased in female rats exposed to 389 mg methyl bromide/m3. There
were no exposure-related increased incidences of neoplasms in male and
female rats.
In a long-term inhalation study, a total of 360 male and 360
female rats (Wistar) were exposed to concentrations of up to 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 29 months (Dreef-van
der Meulen et al., 1989; Reuzel et al., 1991). Non-neoplastic and
neoplastic lesions were scored in all control and high-level animals
of the main group, and the nose examined histopathologically at all
exposure levels. Mortality was higher in males and females at the 350
mg/m3 level than in controls from the end of the second year
onwards. Body weights in the 350 mg/m3 group (both sexes) were
slightly lower than controls from week 4 onwards. No essential
differences between groups were observed in haematology, clinical and
blood chemistry, and urine analysis at three months and one year. The
absolute brain weight was decreased in females at 350 mg/m3. A
higher incidence of haemothorax was seen in males and females at 350
mg/m3 than in controls. In the heart there was an increased
incidence of thrombi and myocardial degeneration in rats exposed to
350 mg/m3. There was hyperkeratosis of the oesophagus and
forestomach. The incidences of neoplastic lesions did not differ
significantly among the groups.
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction and embryotoxicity
McGregor (1981) conducted a sperm abnormality assay exposing
groups of 10 male B6C3F1 mice to air containing 0, 78, or 272 mg
methyl bromide/m3 (0, 20, or 70 ppm) for 7 h/day, for 5 days. No
sperm abnormalities were found at these concentrations.
Atrophy of seminal epithelium, incomplete spermatogenesis, and
giant cells in the seminal tubules, were detected unilaterally in one
rat (group of 10) after inhalation of 778 mg methyl bromide/m3 (200
ppm) (4 h/day; 5 days/week for 6 weeks) and in six rats (group of 10)
after inhalation of 1167 mg methyl bromide/m3 (same length of
exposure). In the tubules of the epididymis adjacent to the atrophied
testis, necrotic spermatocytes had accumulated in seminal fluid, but
spermatozoa were not seen (Kato et al., 1986).
Spermatogenesis and sperm quality were evaluated in the rat
(F-344/N) following exposure to 778 mg methyl bromide/m3, 6 h/day
for 5 days (Hurtt & Working, 1988). Although methyl bromide caused a
transient decrease in plasma testosterone and testicular nonprotein
sulfhydryl concentrations during acute exposure, no other reproductive
indices, including testis weight, daily sperm production, cauda
epididymal sperm count, sperm morphology, percentage motile sperm,
linear sperm velocity, and epididymal and testicular histology, were
affected by methyl bromide exposure.
Testicular degeneration and atrophy occurred in several rats and
mice following repeated (6 h/day, 5 days/week for up to 6 weeks)
inhalation exposure to 622 mg methyl bromide/m3 (Eustis et al.,
1988). In rats, degeneration included separation and sloughing of
spermatocytes and late stage spermatids and/or formation of
intratubular multinucleate giant cells. Atrophy was characterized by
variable loss of all components of the spermatogenic epithelium. In
exposed mice, degeneration of testes occurred frequently, but it was
not always severe.
Thirteen-week inhalation studies on sperm morphology and vaginal
cytology examinations (SMVCEs) in B6C3F1 mice and F-344 rats were
carried out by Morrissey et al. (1988). Results from mouse studies
(inhalation of 39, 156, or 467 mg methyl bromide/m3) showed a
decrease in terminal body weight, and a relative increase in the
weight of epididymis and testis. A decrease in sperm density and an
increase in the percentage of abnormal sperm were also noted. In rats
that inhaled 117, 233, or 467 mg methyl bromide/m3, a decrease in
terminal body weight as well as a decrease in the weight of the cauda
epididymis, and a relative increase in the weight of the testis were
noted. A decrease in sperm motility was also observed. In females,
exposure to methyl bromide did not affect the length of the estrous
cycle (Morrissey et al., 1988).
Female rats exposed to 272 mg methyl bromide/m3 (70 ppm) for up
to 40 days survived and reproduced without impairment (Hardin et al.,
1981; Sikov et al., 1981).
In a dominant lethal assay carried out in rats (McGregor, 1981),
groups of 10 male, adult CD rats were exposed to 0, 78, or 272 mg
methyl bromide/m3 for 7 h/day on five consecutive days, then
serially mated at weekly intervals for 10 weeks with untreated virgin
females in the ratio one male: two females. When the females were
sacrificed, their ovaries were examined for corpora lutea graviditatis
and the uteri were opened and examined for live implantations, late
deaths, and early deaths. The frequency of pregnancy was determined by
(a) females with corpora lutea graviditatis and (b) females with
implantations. With either method, methyl bromide did not cause a
significant decrease in the frequency of pregnancy, and the number of
corpora lutea per pregnancy and the frequency of early deaths were
unaffected.
The effects of inhalation exposure to 0, 12, 117, or 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week, for around 8 months, on
the growth, reproduction, and offspring in two consecutive generations
of CD Sprague-Dawley rats were investigated (American Biogenics
Corporation, 1986). Body weight depressions were noted in the males in
the highest concentration group at 5 of the 10 pre-mating collection
intervals and at final sacrifice. The pre-mating and total weight
gains were also less than those of the untreated control males. In the
F1 generation, parental body weight was comparable in exposed and
control animals. In the F2a litter, a slight body weight depression
was noted in gestating and lactating dams in the highest concentration
group compared with controls. Otherwise, maternal body weights were
comparable with those of the controls.
There was a marginal reduction in female fertility index in the
two top dose groups of the F2a litter. The mean numbers of pups
delivered viable were comparable with controls. Survival of the pups
was reduced in the 350 mg/m3 dose group in late lactation in the F1a
generation. Body weights of pups were reduced in both the 350 and 117
mg methyl bromide/m3 dose groups of the F1a, F2a and F2b
generations, though no consistent changes were observed in the F1b
generation.
No anomalies were noted in the treated progeny that were
attributed to methyl bromide exposure.
Gross pathological examination of parent animals from both
generations and randomly selected F1b and F2b progeny did not reveal
any treatment-related lesions. A statistically significant decrease in
the mean brain weight in the F0 males and an increased liver to body
weight ratio in both sexes were found in the upper concentration
group. In the F1 generation, both male and female brain weights were
decreased at 350 mg methyl bromide/m3.
Statistical analyses of the F1b progeny final body weight and
organ weight data revealed no significant differences. Statistical
reductions were found in the final body weight data obtained for the
F2b males (350 mg methyl bromide/m3) and F2b females (117 and 350 mg
methyl bromide/m3) compared with untreated control progeny. Analysis
of F2b progeny organ weight data revealed significant decreases,
compared with controls, for the female brain, heart, kidney, and liver
weights (350 mg methyl bromide/m3) and female liver weight (117 mg
methyl bromide/m3). In these two upper concentration groups, the
females brain to body weight ratio was increased and the liver to
brain ratio, decreased. Microscopic examination of the reproductive
organs and abnormal tissues did not reveal any treatment-related
lesions.
8.5.2 Teratogenicity
Female Wistar rats were exposed for 7 h/day, 5 days/week for 3
weeks to methyl bromide concentrations of 78 or 272 mg/m3. After
this time, they were mated. There was a total of 7 different exposure
groups (Table 48). Female rats whose vaginal smears showed evidence of
sperm were exposed for 7 h daily from day 1 (=day of finding sperm in
vaginal lavage) to 19 of gestation. The day before term (gestation day
21), they were sacrificed and necropsied. The results showed no
effects of the exposure on the female rats, nor were embryotoxic or
other teratogenic effects found (Sikov et al., 1981).
In a further series of studies by Sikov et al. (1981), groups of
24 female New Zealand white rabbits were exposed for 7 h daily to 78
or 272 mg methyl bromide/m3 (20 or 70 ppm) from the day of
artificial insemination. The 78 mg/m3 group was exposed up to day 24
of gestation; ecause of toxic effects, exposure had to be stopped on
day 15 for the 272 mg/m3 group, in which all but one pregnant rabbit
died. The remaining rabbits from the control and 78 mg/m3 exposure
group were sacrificed on the 30th day of gestation. The low exposure
group showed no fetotoxic or eratogenic effects. An evaluation of the
fetotoxicity or teratogenic results from the high dose group was not
possible because of the high mortality rate in the pregnant rabbits
(Hardin et al., 1981; Sikov et al., 1981).
In a preliminary teratology study (Breslin et al., 1990),
pregnant New Zealand White rabbits were exposed via inhalation for 6
h/day to 0, 39, 117, 195, 272, or 545 mg methyl bromide/m3 (0, 10,
30, 50, 70, or 140 ppm). Toxicity was observed in rabbits exposed to
545 mg methyl bromide/m3 and these were sacrificed before the end of
the study. No apparent embryotoxic effects were observed at any
exposure level (Breslin et al., 1990).
A subsequent study by the same investigators was conducted in two
parts. In Part I, groups of 26 inseminated New Zealand White rabbits
were exposed via inhalation to 0, 78, 156, or 311 mg methyl
bromide/m3 (0, 20, 40, or 80 ppm) on days 7-19 of gestation. In Part
II, groups of 17 inseminated rabbits were exposed for 6 h/day to 0 or
311 mg methyl bromide/m3 (0 or 80 ppm) on days 7-19 of gestation. An
additional group of 16 females, inseminated by a single male, acted as
controls. On day 28 of gestation, all surviving animals were
necropsied. Maternal liver, kidney, lung, brain weights, gravid
uterine weights, and the number of corpora lutea, implantations,
resorptions, and live/dead fetuses were noted. The fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
alterations.
Rabbits exposed to 311 mg methyl bromide/m3 showed moderate to
severe maternal toxicity. Decreased body weight and/or body weight
gain were noted. Clinical alterations included lethargy, right-sided
head tilt, ataxia, and lateral recumbency. The last three signs were
associated with significant histopathological lesions of the brain
(multifocal areas of inflammation of the meninges and/or bilaterally
symmetrical necrosis or spongiosis in the midbrain) in the
above-mentioned probe study with 545 mg methyl bromide/m3. At an
exposure level of 311 mg methyl bromide/m3, developmental effects
were observed consisting of decreased fetal weights, an increase in
the incidence of fused sternebrae, and an increase in the incidence of
malformations [18/23] (mostly missing gallbladder or missing caudal
lobe of the lung).
No adverse maternal, embryonal, or fetal effects were observed in
rabbits exposed to 78 or 156 mg methyl bromide/m3 (Breslin et al.,
1990).
Methyl bromide at 0, 0.5, 5, 25, or 50 mg/kg body weight was
administered in peanut oil, by gavage, to pregnant rats on days 5-20
of gestation (Peters et al., 1982). Signs of maternal toxicity were
evident in the two highest dose groups. Total resorption of embryos
was observed in the highest dose group and was considered to be the
result of the poor health of the pregnant rats and not a primary toxic
effect. In the control and 25 mg/kg groups, no effects on the skeleton
or internal organs were reported. In this study, methyl bromide was
not considered teratogenic and adverse effects on prenatal development
only occurred when maternal toxicity was present.
8.6 Mutagenicity and related end-points
Table 50 summarizes the results of tests for the genotoxic
activity of methyl bromide.
8.6.1 DNA damage
DNA adducts have been demonstrated in F344 rats, following oral
or inhalation administration 14C-methyl bromide. The adducts were
isolated from liver, lung, stomach, and forestomach and identified as
3-methylguanine, 7-methylguanine, and 0 6-methylguanine. Following
exposure by either route, the levels of adducts were higher in female
than in male rats and the guanine adducts were particularly prominent
in the stomach and forestomach (Gansewendt et al., 1991).
Alkylation of guanine-N-7 in the DNA of liver and spleen was
observed after treatment of male CBA mice with 14C-labelled methyl
bromide (4.9-5.0 mCi/mmol) by inhalation (340 mCi/kg body weight for
4 h) or i.p. injection (4.4 µmol/kg body weight) (Djalali-Behzad et
al., 1981). They noted that the extent of DNA alkylation in the liver
and spleen in vivo was 200 and 20 times lower, respectively, than
expected from the extent of the alkylation of haemoglobin and from the
relative reactivities of DNA and haemoglobin towards methyl bromide
in vitro.
Table 50. Mutagenicity tests with methyl bromide
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Reverse mutation Salmonella 0.02-0.2% +/- positive, Simmon et al.
typhimurium (in desiccators) TA 100 (1977)
TA 100, TA 98,
TA 1535, TA 1537,2 h
TA 1538
Reverse mutation Salmonella 500-5000 mg/m3, +/- positive, Moriya et al.
typhimurium 2 days TA 100, (1983)
TA 100, 1535
TA 98, 1537, 1538 TA 1535
Escherichia coli positive
WP 2 hcr
Reverse mutation Salmonella 500-50 000 mg/m3 +/- positive at Kramers et al.
typhimurium TA 100 (plate), 5 days 1900 mg/m3 (1985a)
TA 98 negative
SOS-umu (modified Salmonella 1.5 litre/min for positive Ong et al. (1987)
Ames test) typhimurium TA 1535/ 30 min
pSK 1002
Forward mutations Klebsiella pneumoniae 950-19 000 mg/m3, positive at Kramers et al.
streptomycin ur- pro- 20 h 4750 mg/m3 (1985a)
resistance
Forward mutation Escherichia coli 0.5-6 mmol/litre, 1 mutation/108 Djalali-Behzad et
Sd 4 1 h surviving al. (1981)
bacteria/mmol*h
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Forward mutation L5178Y mouse 0.03-30 mg/litre, positive Kramers et al.
24 h (1985a)
Sex-linked Drosophila 78 or 272 mg/m3, negative McGregor (1981)
recessive lethal melanogaster 5 h
Sex-linked Drosophila 750 mg/m3 (6 h) negative Kramers et al.
recessive melanogaster 375 mg/m3 (5 x 6 h) positive (1985a,b)
200 mg/m3 (15 x 6 h) positive
Sister chromatid Phytohaemagglutinin- 4.3% increased SCE Tucker et al.
exchanges (SCE) stimulated frequency from (1985)
human whole blood 10-16.84/cell
after 100-second
exposures
SCE cultured human 10-4-10-6 +/- positive Garry et al.
lymphocytes mol/litre (1990)
SCE bone marrow cells 47-778 mg/m3 dose response NTP (1992)
of exposed B6C3F1 6 h/day; 5 days/ observed at 14 days
mice week; 14 days and only; higher in
12 weeks females than males
same dose range negative
and exposure
Unscheduled DNA human diploid up to 70% +/- negative McGregor (1981)
synthesis fibroblasts 3 h
(human embryonic
intestinal cells)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Unscheduled DNA SPF male Wistar 10-30 mg/litre negative Kramers et al.
synthesis rats, primary liver (1985a)
Cell Syrian hamster 3890-31 120 mg/m3 negative Hatch et al.
transformation embryo cells 2-20 h in sealed (1983)
chambers
Chromosomal male and female CD 78 or 272 mg/m3 negative McGregor (1981)
aberrations (ex Sprague-Dawley) single 7 h or
rat bone marrow 7 h/day; 5 days
cells
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
BDF1 mice 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
(778 mg/m3) and
6-fold in females
(600 mg/m3); in
peripheral blood
increased 32-fold
in males (778 mg/m3)
and 3-fold in females
(600 mg/m3)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
F344 rats 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
and 3-fold in females
at 1314 mg/m3
Micronucleus in peripheral 47-778 mg/m3 elevated (only at NTP (1992)
erythrocytes of 6 h/day; 5 days/ 14 days) responses
B6C3F1 mice week; 14 days over entire dose
range with greatest
response at 389 and
778 mg/m3 in
females; males less
responsive
same dose range and negative
exposure routine;
13 weeks
Dominant lethal rats, male CD 78 or 272 mg/m3 negative McGregor
(Sprague-Dawley) 7 h/day; 5 days (1981)
Starratt & Bond (1988a) found that methylation of DNA in maize
and wheat took place during fumigation using 14C- labelled methyl
bromide (48 mg/litre for 72 h). They identified 7-methylguanine and
1-methyladenine as major products and lesser amounts of
3-methyl-cytosine and 3- methyladenine and 7-methyladenine; 0.5-1% of
the guanine residues in the DNA were methylated. Methylation of solid
samples of calf thymus DNA and salmon testes DNA gave similar results,
except that the quantity of 1-methyladenine exceeded that of
7-methylguanine (Starratt & Bond, 1988b).
In contrast, a different pattern of methylated bases was found
when solutions of DNA were treated with 14C-labelled methyl bromide.
Here, predominantly 7-methylguanine with a small amount of
3-methyladenine and only traces of 1-methyladenine and
3-methyl-cytosine were identified (Starratt & Bond, 1988b). The
authors noted that these results agreed better with the in vivo
studies of Djalali-Behzad et al. (1981).
An in vitro assay for unscheduled DNA synthesis (UDS) was
carried out in human diploid fibroblasts with exposures of 3 h and
concentrations up to 70% in air. No increase in UDS was found
(McGregor, 1981).
As measured by autoradiography, methyl bromide (tested at 10-30
mg/litre) did not induce unscheduled DNA synthesis in primary cultures
of rat hepatocytes treated in air-tight bottles (Kramers et al.,
1985a).
8.6.2 Mutation
Methyl bromide has been tested in various in vitro and in vivo
test systems (Table 50).
Methyl bromide was mutagenic to Salmonella typhimurium TA 100
when tested at concentrations of 0.02-0.2%, in desiccators, in the
absence of an exogenous metabolizing system (Simmon et al., 1977).
Positive results were also obtained in strain TA 100 in a liquid
assay (tested at 10-1000 mg/litre) and a plate assay (tested in closed
containers at concentrations of 500-50 000 mg/m3). Methyl bromide
was mutagenic at concentrations of 1900 mg/m3 and higher (plate
tests), and concentrations of 285 mg/litre (medium) and higher
(suspension test). The activity in the plate assay was unaffected by
the presence of liver homogenates from Aroclor(R)-induced rats
(Kramers et al., 1985a).
Methyl bromide (tested at 0.5-5 g/m3 in a closed container) was
mutagenic to S. typhimurium TA 1535 and TA 100 and to E. coli WP2
her, in the absence of an exogenous metabolic system (Moriya et al.,
1983).
An aqueous solution of methyl bromide (tested at 0.5-6 µmol/
litre) induced mutations to streptomycin independence in E. coli
Sd-4 (Djalali-Behzad et al., 1981).
Methyl bromide showed no mutagenic activity in a modified Ames
test using the impingement (in situ) test system, but, with the SOS
umu-test, this compound induced a significant SOS response, even
with only 30 min impingement (Ong et al., 1987). The SOS function
induced by genotoxic agents was detected by a colorimetric measurement
of beta-galactosidase activity encoded by the 1acZ gene, which is
regulated by the Umu operon.
Mutations to streptomycin resistance were induced in the
fluctuation test with Klebsiella pneumoniae at concentrations of
4750 mg methyl bromide/m3 and higher (tested at 950- 19 000 mg/m3)
(Kramers et al., 1985a).
In barley, a few mutations were induced after treatment of
kernels with 1.4 mmol methyl bromide/litre for 24 h, in closed vessels
(Ehrenberg et al., 1974).
A sex-related recessive lethal assay was carried out on
Drosophila melanogaster (McGregor, 1981). Male strain Oregon K
Drosophila were exposed to 78 or 272 mg methyl bromide/m3 for 5 h
and were mated on days 1,3, or 8 following exposure. The F1 progeny
from these matings were then mated brother to sister, 1-4 days after
emergence from pupae and the F2 generation was ex-amined for the
absence of wild-type males. At 78 mg methyl bromide/m3, the
frequencies of lethal mutations in the F2 generations from one of the
stocks were elevated, but this was not thought by the authors to be
compound-related as these were higher than those at the higher
concentration range.
In a sex-linked recessive lethal test on Drosophila
melanogaster , flies of the Berlin K strain were exposed to methyl
bromide at concentrations of 70-750 mg/m3 for increasing periods;
mutation frequencies were significantly increased at the highest
nontoxic concentrations. At a concentration of 600 mg/m3, all flies
died within a short time during the fourth day of exposure (Kramers et
al., 1985a). Prolongation of the exposure time permitted lower
concentrations to be detected as mutagenic; 487 mg/m3 for 5 x 6 h
and 200 mg/m3 for 15 x 6 h were effective exposures whereas
treatment with up to 750 mg/m3 for 6 h was not sufficient to produce
significantly increased mutation frequencies. The mutagenic effect of
methyl bromide was most pronounced in postmeiotic germ cell stages
(Kramers et al., 1985b).
Treatment of L5178Y mouse lymphoma cells with 0.03-30 mg methyl
bromide/litre, in air-tight bottles, resulted in a dose-related
increase in 6-thioguanine- and bromodeoxyuridine-resistant mutants
(Kramers et al., 1985a).
8.6.3 Chromosomal effects
8.6.3.1 In vitro studies
Exposure of human lymphocyte cultures to 4.3% methyl bromide for 100
seconds increased the frequency of sister chromatid exchanges (SCEs)
from 10.0 to 16.8 per cell (Tucker et al., 1985, 1986).
Human G0 lymphocytes were treated with methyl bromide (0-24
µg/ml) for 30 min with, and without, addition of rat liver homogenate.
After culture, the prepared slides were studied and dose-related
sister chromatid exchanges (SCEs) and chromosome aberrations (CAs)
were found. Methyl bromide significantly induced chromosome
aberrations in the presence of S-9 (Garry et al., 1990).
Inhalation of methyl bromide gas induced mitotic recombination in
somatic cells (somatic wing-spot assay) of Drosophila melanogaster
(Katz, 1985, 1987). Third instar larvae trans-dihybrid for two
recessive wing-hair mutations were exposed via inhalation to methyl
bromide (0-20 000 mg/m3) for 1 h. Wings of surviving adults were
scored for the presence of clones of cells possessing malformed wing-
hairs. Small and large, single (indicating a variety of genetic
alterations) as well as twin spots (from mitotic recom bination) were
found.
8.6.3.2 In vivo studies
The results of in vivo mammalian tests for chromosomal
aberrations in rat bone marrow were negative (McGregor, 1981).
Micronuclei formation was studied on F-344 rats and BDF1 mice
exposed to 0, 600, 778, 1011, 1314, or 1712 mg methyl bromide/m3 (0,
154, 200, 260, 338, or 440 ppm) for 6 h/day and 5 days/week for 14
days (Ikawa et al., 1986). In the surviving mice, poly-chromatic
erythrocytes with micronuclei in the bone marrow increased 10-fold in
males (778 mg/m3) and 6-fold in females (600 mg/m3) and those in
peripheral blood increased 32-fold in males (778 mg/m3) and 3-fold
in females (600 mg/m3). In rats, poly-chromatic erythrocytes
containing micronuclei in the bone marrow increased 10-fold in males
and 3-fold in females (both 1314 mg/m3).
Increases in SCEs and micronuclei were observed in the bone
marrow cells of male and female B6C3F1 mice exposed via inhalation to
a concentration of 778 mg methyl bromide/m3 (200 ppm) for 14 days (6
h/day, 5 days/week), the increases were more pronounced in female
mice. In contrast, no significant increases in either SCEs or
micronuclei were observed in male or female mice exposed via
inhalation to a concentration of 467 mg methyl bromide/m3 (120 ppm)
for 13 weeks (NTP, 1992).
8.6.4 Cell transformation
Transformation in Syrian hamster embryo cells by SA/adenovirus
was not enhanced by exposure to 4000-16 000 mg methyl bromide/m3
(1000-4000 ppm) for 2 or 20 h, in sealed chambers (Hatch et al.,
1983).
8.7 Carcinogenicity and related end-points
8.7.1 Gavage studies
Danse et al. (1984) administered methyl bromide, by gavage, to
groups (10 male + 10 female) of weanling Wistar rats for 90 days at
doses of 0.4, 2, 10, or 50 mg/kg body weight. At the highest dose
level of 50 mg/kg, squamous cell carcinomas of the forestomach
developed in 13 out of 20 animals. A marked diffuse hyperplasia of the
epithelium of the forestomach was seen in all animals in this group.
However, from subsequent examination of the slides from this study it
was concluded that the forestomach lesions reported at 50 mg
represented inflammation and hyperplasia rather than malignant lesions
(Pesticide & Toxic Chemical News, 1984).
Boorman et al. (1986) administered methyl bromide in peanut oil
(50 mg/kg body weight), by gavage, to groups of 15 male Wistar rats
for 13 weeks (five times a week) with a 12 week recovery period; other
groups were exposed for 17, 21, or 25 weeks to methyl bromide;
necropsies were performed at 13, 17, 21, and 25 weeks. At week 13,
inflammation, acanthosis, fibrosis, and pseudo- epitheliomatous
hyperplasia in the forestomach were observed microscopically in dosed
animals. At week 25, all rats had hyperplastic lesions of the
forestomach that were more severe than those at 13 weeks. Evidence of
malignancy, seen in one rat, was considered to be a very early
carcinoma. In the stop treatment group that had received methyl
bromide for 13 weeks, there was regression of the stomach lesions, but
at the 12-week final sacrifice, adhesions, fibrosis, and mild
acanthosis remained (Table 43).
Hubbs & Harrington (1986) reported similar results. Gross and
microscopic alterations were most pronounced in the non-glandular part
of the stomach of the treated rats. Histological change in the
squamous epithelial portion of the stomach included ulceration and
pseudoepitheliomatous hyperplasia characterized by hyperkeratosis,
acanthosis, and epithelial peg formation. Fibrosis, foreign material
(hair) and subacute to chronic inflammation were found in the
muscularis mucosa, submucosa, and tunica muscularis of some rats. A
30-60 day recovery period was accompanied by marked, but incomplete,
regression of lesions. No evidence of malignancy was seen in the
stomachs of treated rats (Hubbs & Harrington, 1986).
A two-year, oral, carcinogenicity study was carried out on 60
male and 60 female F-344 rats fed on diets fumigated with methyl
bromide (Mitsumori et al., 1990). Diets contained 80, 200, or 500 mg
total bromide/kg, respectively, (methyl bromide concentration being
<20 mg/kg), the controls were fed commercial basal diet (containing
30 mg bromide/kg) and a diet containing 500 mg potassium bromide
(KBr)/kg. No carcinogenic effects were observed (see also section
8.4.1.2).
8.7.2 Inhalation studies
In lifetime, inhalation, carcinogenicity studies (Table 49),
groups of 90 male and 80 female Wistar rats were exposed to methyl
bromide concentrations of up to 350 mg/m3 for 6 h/day, 5 days/ week,
for up to 130 weeks (Dreef-van der Meulen et al., 1989; Reuzel et al.,
1991). Methyl bromide was a mild nasal irritant at all exposure
concentrations. There was no increased incidence of neoplasms.
In a 2-year inhalation study (NTP, 1992) on male and female
B6C3F1 mice exposed to 39, 128, or 389 mg methyl bromide/m3 (Table
49), there was no evidence of carcinogenic activity in either male or
female mice.
8.8 Special studies
8.8.1 Target organ effects
8.8.1.1 Inhalation studies
Studies on laboratory animals have shown that, following
inhalation exposure, the primary target organs are the brain, kidney,
nasal cavity, heart, adrenal gland, lung, liver, and testis (Irish et
al., 1940; Hurtt et al., 1987; Eustis et al., 1988) and lung (Bond et
al., 1985; Kato et al., 1986; Jaskot et al., 1988) (Table 48).
Extensive target organ studies have been carried out by Eustis et
al. (1988) on B6C3F1 mice and F-344 rats after inhalation of 622 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 6 weeks. The
histopathological changes observed were as follows:
Brain: neuronal necrosis in the internal granular layer of the
cerebellar folia (mice); neurosis and loss of neurons in the cerebral
cortex, hippocampus, and thalamus (rats).
Kidney: nephrosis, which occurred in all treated mice, was
charac-terized by degeneration, necrosis, and sloughing of the
epithelium of convoluted tubules in the renal cortex. In rats, there
was dilatation of tubules with atrophy of the epithelium, hyaline and
granular casts in the tubules, and increased cytoplasmic basophilia,
indicative of epithelial regeneration. There was minimal nephrosis in
one female rat.
Testes: degeneration of the testes occurred frequently in exposed
mice and mild bilateral atrophy was present in two. Degeneration and
atrophy of seminiferous tubules of the testes was noted in several
exposed rats.
Nasal cavity: exposed male mice had various levels of degeneration
and atrophy of the nasal olfactory epithelium. Minimal degeneration of
the olfactory epithelium was seen in only one female mouse. In male
and female rats killed after three exposures to methyl bromide, there
was moderate to marked degeneration of the olfactory epithelium of the
ethmoturbinates and posterior dorsal nasal septum. There was no
inflammatory response. In rats, killed or dying after 10 or more
exposures, actual degeneration of the olfactory epithelium was minimal
or mild, but there was focal or multifocal loss of olfactory sensory
cells.
Heart: degeneration of the myocardium primarily occurred in treated
males with minimal myocardial degeneration in two female mice.
Degeneration of the myocardium occurred more frequently and with
greater severity in treated than in control male and female rats.
There were increased numbers of mononuclear and fusiform nuclei that
may represent interstitial cells, the foci showing a relative increase
in fine reticular fibres, and scattered clear vacuoles.
Adrenal gland: exposed female mice showed diminished cellularity of
the x-zone of the adrenal cortex and the affected cells contained less
cytoplasm and smaller, more hyperchromatic nuclei than normal cells.
Minimal to mild cytoplasmic vacuolation occurred in the adrenal cortex
of exposed rats.
Liver: individual cell necrosis was noted in the liver of several
treated rats and was generally more severe in affected males than
females. In three males and one female, an inflammatory reaction
occurred. There were no data for mice.
Thymus and spleen: thymic atrophy and lymphoid depletion of the
spleen was noted in mice and rats of both sexes.
8.8.2 Neurotoxicity
Irish et al. (1940) examined the effects of a wide range of
inhalation exposure conditions in rats, rabbits, guinea- pigs, and
monkeys, using visual inspection to assess behavioural deficits. They
observed that nearly 60% (34 out of 58) of the rabbits surviving
exposure to 128 mg/m3 (33 ppm) developed hindlimb paralysis during
the 6-month exposure period. This was the lowest concentration at
which definite neurobehavioural effects could be detected in the
rabbit, the other species being unaffected at this concentration.
Anger et al. (1981) studied the neurobehavioural effects of long-
and short-term methyl bromide inhalation exposure on Sprague-Dawley
rats and New Zealand White rabbits using more sensitive tests than
those available in 1940 when the study of Irish et al., was performed.
Exposure to 252 mg/m3 (65 ppm) for weeks caused sig-nificantly
reduced eye blink responses and nerve conduction velocity in rabbits,
but not in rats. No effect on nerve conduction velocity, open-field
activity, or coordination in rats could be seen after extended
exposure at 214 mg/m3 (55 ppm) for 36 weeks. In further studies
(Russo et al., 1984), rabbits did not show any neurobehavioural
effects after inhalation exposure to 105 mg methyl bromide/m3 (27
ppm) for 7.5 h per day, 4 days per week over 8 months. A separate
group of rabbits exposed to 252 mg/m3 exhibited severe neuromuscular
effects followed by partial recovery, 6-8 weeks after exposure ceased.
Behavioural effects in rats following repeated exposure to methyl
bromide at concentrations of 778 or 1167 mg/m3 (200 or 300 ppm) for
3 weeks were investigated by Ikeda et al. (1980). Relatively prolonged
(12 days) motor incoordination (rotarod) was observed.
Conditioned taste aversion induced by inhalation exposure to
methyl bromide in rats has been reported (Miyagawa, 1982). Rats kept
under a water restriction schedule for 7 days, were permitted access
to 0.3% (w/v) sodium saccharin, and were exposed to 0, 97, 195, or 389
mg methyl bromide/m3 (0, 25, 50, or 100 ppm) for 4 h. Three days
after exposure, saccharin preference tests were carried out, revealing
dose-dependent saccharin aversion in the exposure group (Miyagawa,
1982).
Male Swiss-Webster mice exposed to methyl bromide concentrations
up to 3500 mg/m3 showed no effects on single-task passive avoidance
but had significantly lower rotarod performance (Alexeeff et al.,
1985).
Effects of methyl bromide on locomotor activity were observed in
five groups of three rats after exposure to 245, 486, 731, or 972
mg/m3 (63, 125, 188, or 250 ppm) for 8 h. The locomotor activity was
measured in an activity cage before, and immediately after, the
exposure, shown as an average count per hour in 12-h blocks (day -2
(before exposure) and day 1) or 4-h blocks (day 0). In the 245 mg/m3
group, the activity was the same as that in the control group. The
activity was decreased by 731 mg/m3 and in-hibited strongly by 972
mg methyl bromide/m3 for up to 6 h after exposure (Honma et al.,
1985).
The effects of methyl bromide exposure on the thiopental-induced
reduction of righting reflex of rats was investigated. Three groups of
eight rats were exposed to 0, 245, or 486 mg/m3 (63 or 125 ppm) and
then were injected i.p. with 60 mg thiopental/kg to induce a loss of
righting reflex. The time of loss of righting reflex was measured and
group means were calculated. In the control group, two out of eight
rats lost the righting reflex, while in the 245 and 486 mg methyl
bromide/m3-exposure groups, the loss of righting reflex was observed
in all rats and was considerably more prolonged (Honma et al., 1985).
Kato et al. (1986) described neurological signs in rats, such as
paralysis and ataxia, after exposure to 1167 or 1556 mg methyl
bromide/m3, for 4 h/day over 6 weeks (section 8.4).
The acute effects of methyl bromide on electroencephalographic
activity and on sleep-wakefulness and circadian rhythms have been
investigated in rats (Tanaka et al., 1988). Slowing of the EEG
frequency in the wakefulness (W) stage and spike-wave activity
appeared at a single subcutaneous dose of 135 mg methyl bromide/kg
body weight. These abnormal EEG activities did not occur at lower dose
levels. Administration of methyl bromide at doses of 45, 15, or 5
mg/kg produced dose-related changes in amounts of W, non-REM sleep,
and REM sleep and in their circadian rhythms. Pretreatment with
glutathione effectively lessened the detrimental effects of methyl
bromide on sleep-wakefulness and its circadian rhythms and increased
the LD50 (Tanaka et al., 1988).
The effects were studied on regional brain glutathione- S-
transferase (GST) activity and the concentrations of glutathione
(GSH), monoamines, and amino acids in F-344 rats exposed to methyl
bromide (Davenport et al., 1992). Exposure to 584 mg/m3 was for 6
h/day for five days. While no histological evidence of brain lesions
was noted, there was GSH depletion and GST inhibition in the frontal
cortex, caudate nucleus, hippocampus (examined for GSH only), brain
stem, and cerebellum. No significant changes were observed in the
concentrations of monoamines (only measured in males), but there were
increases in the concentrations of aspartic acid and glycine in the
frontal cortex and of aspartic acid only in the cerebellum (only
measured in males).
Honma et al. (1982) exposed groups of 5-6 SD rats to methyl
bromide concentrations of 39-467 mg/m3 for 24 h or to 4-40 mg/m3
for 3 weeks. After sacrifice (microwave), the brain was dissected and
analysed for monoamines. A reduction in the norepinephrine (NE)
contents of the hypothalamus and cortex + hippocampus was found at
concentrations of 390 mg/m3 and above (24-h study) or 39 mg/m3
(3-week study), whereas levels of dopamine (DA), sero-tonin (5HT), and
acetylcholine (ACh), were only slightly affected by exposure. In more
detailed studies, rats were exposed to 120, 240, 486, or 973 mg/m3
for 8 h (Honma, 1987; Honma et al., 1987). Decreases in DA and NE and
increases in the metabolites homovanillic acid (HVA) and
3-methoxy-4-hydroxyphenylglycol (MHPG) were observed in various
regions of the CNS in rats exposed to 319 mg/m3. The maximal effects
were obtained 0 or 2 h after exposure with the largest changes in DA
(-24%) and NE (-34%) seen in the striatum. HVA and MHPG levels were
increased primarily in the frontal cortex (81%) and striatum (38%).
The tyrosine hydroxylase (TH) activity in the striatum, hypothalamus,
frontal cortex, midbrain, and medulla oblongata was measured in brain
homogenates from rats exposed to 62-973 mg/m3 (16-250 ppm) for 8 h
(Honma et al., 1991). The rats were sacrificed serially (decapitation)
between 0 and 16 h after exposure. TH was also measured in vivo
following administration of decarboxylase inhibitor. Exposure to
methyl bromide dose-dependently inhibited both TH activity in vitro
and in vivo . TH activity in the hypothalamus was most sensitive to
methyl bromide compared with the other brain areas. The authors
suggested that methyl bromide reduces catecholaminergic neuronal
activity in the brain via inhibition of TH activity.
The direct effects of methyl bromide on the rat brain have been
investigated using a two-probe (right striatum and left ventricle)
microdialysis method (Honma, 1992). Methyl bromide (1 µg or 0.5
µg/µlitre) dissolved in 10% ethanol/90% artificial cerebrospinal fluid
was perfused through the ventricle probe at a rate of 2.5 µlitre/min
and changes in 3,4-dihydroxyphenylacetic acid (DOPAC), HVA, and
5-hydroxyindoleacetic acid, a serotonin metabolite, (5HIAA) content in
the striatum perfusate were measured. DOPAC and HVA increased
following the initiation of ventricle perfusion but the 5HIAA content
of the striatum perfusate was reduced. The increases in DOPAC and HVA
persisted after perfusion and the decreased 5HIAA level returned to
normal. A concentration of 0.1 µg methyl bromide/µlitre had no effects
on DOPAC and HVA levels, though the 5HIAA level was reduced. These
findings support the findings of an increase in DOPAC and HVA in the
brain homogenate of rats exposed to methyl bromide (Honma, 1987; Honma
et al., 1987) indicating that dopamine metabolites in the
extracellular, and, probably, intracellular, regions of dopamine nerve
terminals were increased by methyl bromide itself. The extracellular
level of the 5HIAA in microdialized perfusate was reduced by
intraventricularly administered methyl bromide (Honma, 1992).
Systematic neuropathological studies of their central and
peripheral nervous systems were carried out on male Wistar rats
exposed to methyl bromide (1128 or 1945 mg/m3) for 6 h/day, 3
days/week for 3-8 weeks (Furuta et al. 1993). Among the rats exposed
to 1945 mg methyl bromide/m3 for 10-18 days, the following
neuropathological changes were noted: axonal degeneration of
myelinated fibres at the cervical level of the fasciculus gracilis and
the necrosis and atrophy of neurons in the caudate-putamen, thalamus,
and cingulate cortex. Rats exposed to a concentration of 1128 mg
methyl bromide/m3 for 8 weeks did not show any abnormalities.
8.8.3 Immunotoxicity
No data on the immunotoxicity of methyl bromide are available.
8.9 Factors modifying toxicity; toxicity of metabolites
Cysteine has been shown to reduce the toxicity of methyl bromide,
when administered to rats, mice, and rabbits, orally or s.c., 30 min
before, or s.c. within 5 min following, acute poisoning (Mizyukova &
Bakhishev, 1971). Cysteine treatment prevented the death, paralysis,
paresis, and spasms that developed on the 3rd-4th days after methyl
bromide inhalation in other animals.
8.10 Mechanisms of toxicity - mode of action
The mode of action of methyl bromide is still not understood.
Proposed mechanisms of toxicity include the direct cytotoxic effect of
the intact methyl bromide molecule or toxicity due to one of its
metabolites. The bromide ion concentrations are insufficient to
explain methyl bromide toxicity, but it may be related to its
alkylating ability.
Honma et al. (1985) concluded that the CNS toxicity seems to be
due to the methyl bromide molecule itself or the methyl moiety
incorporated into the tissue and does not appear to be attributable to
inorganic bromide or methyl alcohol (Honma et al., 1985). In humans
showing medium or severe toxic symptoms of the CNS, methanol
concentrations in the blood ranged between 200 and 3000 mg/litre
(Swartz et al., 1981).
The direct cytotoxic effect of methyl bromide on HeLa cells in
vitro has been shown, whereas bromide appeared not to cause cell
damage (Nishimura et al., 1980).
Methyl bromide reacted in vitro with a number of SH enzymes and
caused progressive and irreversible inhibition (Lewis, 1948). DNA and
protein alkylation by methyl bromide have previously been discussed
(section 6.3).
The role of glutathione (GSH) in reducing toxicity is also not
clear. Studies on the possible role of glutathione are given in
section 6.3. One hypothesis for the toxicity of methyl bromide
proposes the formation of a reactive species through a methyl
bromide-glutathione conjugation process, but it is more probable that
glutathione acts as a detoxifying agent.
9. EFFECTS ON HUMANS
Humans can be exposed to methyl bromide via inhalation, by skin
contact with the compound, or through residues in food that has been
fumigated with the gas to control pests. Exposure is also possible
through drinking- water from wells contaminated with leaching water.
In this section, only direct exposure to gaseous or liquid methyl
bromide is considered.
Since the first case reported by Schuler in 1899, there have been
hundreds of cases of methyl bromide poisoning involving fatalities,
systemic poisoning, skin and eye injuries, and damage to the central
nervous system (von Oettingen, 1946, 1964; Hine, 1969; Torkelson &
Rowe, 1981; Weller, 1982; Alexeeff & Kilgore, 1983).
Table 51 shows acute cases up to 1983 reported throughout the
world. Up to 1955, most cases were from chemical manufacture or fire
extinguisher incidents. Since this date, cases of poisoning from its
use as a fumigant have predominated.
9.1 Clinical findings
It is important to note that the manifestations of methyl bromide
poisoning may be delayed. The latent period may vary from 2 to 48 h
(Holling & Clarke, 1944).
The acute and long-term effects of methyl bromide can be divided
broadly into two categories: neurological and non-neurological.
The principal non-neurological symptoms reported after acute
inhalation of methyl bromide are associated with the respiratory
system. Chest pain or difficulty in breathing have been reported,
which is consistent with pathological findings at autopsy including
pulmonary oedema, bronchopneumonia, congestion, and haemorrhage
(Holling & Clarke, 1944; Hine, 1969).
In fatal poisoning, the early symptoms and signs are headache,
visual disturbances, nausea and vomiting, smarting of the eyes,
itching of the skin, listlessness, vertigo, and tremor. Progression is
usually rapid, with the development of convulsions, often with a
Jacksonian-type of progress, fever, tachypnoea associated with signs
of severe pulmonary oedema, cyanosis, pallor, and death. Several
neuropsychiatric signs and symptoms, such as mental confusion, mania,
muscular twitches, and slurring of speech, may precede death (Wyers,
1945; Sax et al., 1984; Gosselin et al., 1984).
Table 51. Methyl bromide poisoning incidents up to 1955 and between 1955 and 1983a
Resulting from: Fatalities Systemic Poisoning Skin Injuries Eye Injuries Other or unspecified injuries
up to 1955- up to 1955- up to 1955- up to 1955- up to 1955-
1955 1983 1955 1983 1955 1983 1955 1983 1955 1983
Chemical manufacture, 16 1 108 45 26 4 0b 0b
filling, storage,
transportation, or
disposal
Use or leaking fire 24 11 38 28 10 0 0b 0b
extinguishers
Uses as a fumigant 44 16 3 298 0 145 0b 57 0b 78
a Adapted from: Alexeeff & Kilgore (1983). Data up to 1955 were taken from von Oettingen (1955).
b No cases known.
The clinical picture in non-fatal poisoning is extremely
variable. Fatigue, blurred or double vision, nausea, and vomiting are
frequent; incoordination, tremors, convulsions, exaggeration of the
patellar reflexes, and a positive Babinski's sign may develop. Nearly
every type of nervous disturbance has been reported. The pulmonary
symptoms are comparatively slight (Sax et al., 1984). Low-level,
short-term exposures to the vapour have produced a syndrome of
polyneuropathy without overt central manifestations, characterized by
persistent numbness in the hands and legs, impaired superficial
sensation, muscle weakness, unsteadiness of gait, and absent or
hypoactive distal tendon reflexes (Kantarjian & Shaheen, 1963). Victor
& Adams (1980) stated that the signs and symptoms of chronic bromide
poisoning intoxication consisted of dizziness, drowsiness,
irritability, emotional lability, impairment of thought or memory,
and, in severe cases, delirium and mania or stupor or coma.
Locally, methyl bromide is an intense vesicant on human skin. The
blisters produced by methyl bromide are enormous, but rarely deep
enough to destroy the entire skin layer, even though it may produce
severe burns (Watrous, 1942; Butler et al., 1945; Wyers, 1945).
Both central and peripheral neurological deficits may persist;
organic brain syndrome has occurred (Greenberg, 1971; Goulon et al.,
1975). Profound psychological depression can occur during prolonged
convalescence (Hine, 1969). Late sequelae include bronchopneumonia
after severe pulmonary lesions, renal failure, and severe weakness
with, or without, evidence of paralysis. Renal failure due to tubular
necrosis may be a late sequela, but this is uncommon and usually of
mild proportions (Benatt & Courtney, 1948; Prain & Smith, 1952). There
have been occasional reports of jaundice, elevations of liver enzyme
activity in serum, and abnormal liver function tests suggestive of
mild hepatotoxicity after exposure to methyl bromide (Verberk et al.,
1979). In a 6- year-old boy, signs of liver involvement in conjunction
with encephalopathy after exposure to methyl bromide vapour were at
first mistaken for Reye's syndrome (Shield et al., 1977).
Recovery is frequently prolonged and there may be permanent
injury, commonly characterized by sensory disturbances, weakness,
disturbances of gait, irritability, and blurred vision.
The effects of methyl bromide on the nervous system, based on
case histories, showed that dysfunction affected the peripheral and
optic nerves, cerebellar connections, and certain spinal cord tracts
(Carter, 1945). Brain stem damage was described in a man who died
after 30 days of unconsciousness following methyl bromide exposure
(Cavanagh, 1992; Squier et al., 1992). Serum bromide levels, 2 days
after admission, were 160 mg/litre (normal 3-4 mg/litre). At
post-mortem examination, the brain showed mild generalized swelling,
normal ventricles, and well- defined symmetrical lesions, including
loss of neurones, in the mammilary bodies and inferior colliculi. The
cerebellar dentate nuclei had occasional foci of neuronal loss; other
brain stem regions were normal. The spinal cord was normal, but dorsal
root ganglia had scattered nodules of neuronal loss. Nerve roots and
thoracic peripheral nerve showed sparse chronic inflammatory exudate
and patchy axonal and myelin loss. The thyroid gland was densely
infiltrated with lymphocytes, plasma cells were rare, and there was no
significant fibrosis. The lungs were slightly oedematous and
congested. In another case of methyl bromide poisoning, a woman
remained in a coma for 4 years and 8 months before death. At
post-mortem, there was necrosis of both colliculi, with gliosis in the
upper brainstem, reticular formation, and moderate changes in the
dentate and pontine nuclei (Goulon et al., 1975).
Various visual disturbances have been reported following acute
methyl bromide poisoning. These include blurring of vision, diplopia,
lacrimation from eye irritation, accommodative disturbance, and
central scotomata for shades of green (Grant, 1974). Ocular findings
in a case of chronic methyl bromide poisoning included persistent
bilateral decrease in vision, temporal optic nerve head pallor, marked
attenuation of visual evoked response amplitude with normal latencies,
normal electro-retinogram but abnormal electro-oculogram, and
deuteranomalous (green) defect (Chavez et al., 1985).
Electrophysiological studies have been performed on patients as
part of a clinical investigation of the neurological effects of methyl
bromide poisoning (Goulon et al., 1975; Verberk et al., 1979; Audry et
al., 1985; Lopez et al., 1986a, b; Uncini et al., 1990; Mazzini et
al., 1992; Hustinx et al., 1993). Abnormal findings included
epileptiform patterns in electroencephalograms, enlarged and
asymmetric somatosensory evoked potentials, and evidence of axonal
neuropathy in peripheral nerves.
9.1.1 Bromide levels in body tissues and fluids
In cases of methyl bromide poisoning, methyl bromide itself has
been detected in human tissue on only one occcasion. This may be due
to the absence of methyl bromide or, more probably, to its very short
half-life in tissues, as well as difficulties in analysis. There is an
inconsistency in the data as to whether there is a correlation between
bromide levels and the symptoms of methyl bromide poisoning. Some
authors have suggested a direct correlation between blood bromide
levels and the degree of intoxication (Rathus & Landy, 1961; Hine,
1969): 400 mg/litre (ppm) - severe disability and death in some cases;
250 mg/litre (ppm) - convulsive seizure and sometimes death; 176
mg/litre (ppm) - slight residual ataxia; 135 mg/litre (ppm) - moderate
disability; 100 mg/litre (ppm) or less -recovery, but symptoms of
poisoning have been reported at bromide levels as low as 28 mg/litre
blood and severe symptoms at blood bromide levels of 120 mg/litre
(Rathus & Landy, 1961). Brenner (1978) and Bowers & Onoreski (1990)
considered that the toxic threshold level for bromide in serum in
humans was 500 mg/kg, though effects were observed in patients with
lower levels.
However, Weller (1982) disputed a correlation between the bromide
levels in serum and the severity of poisoning. In workers in a methyl
bromide manufacturing plant, no definite association was found between
symptoms and urine bromine concentrations (Kishi et al., 1991). A
direct association between serum bromine concentrations and the
severity of neurological symptoms was not found in a poisoning
incident involving greenhouse workers (Hustinx et al., 1993).
9.1.2 Dermal exposure
Dermal exposure can result from direct contact with liquid methyl
bromide, e.g., from accidental splashing, or through contact with
contaminated boots, clothing, bandages, or gloves (Alexeeff & Kilgore,
1983). These articles are often made of rubber, which can absorb
methyl bromide (Watrous, 1942). Direct eye injury or irritation can
also occur. Dermal exposure to gaseous methyl bromide can also cause
poisoning.
When liquid methyl bromide is spilled on the skin, it evaporates
rapidly producing a cool, but not a cold, or burning sensation
(Watrous, 1942). Repeated application of the liquid caused a burning
or tingling sensation in the skin, which, in more severe cases, led to
a sense of numbness, followed later by aching. In the early stages,
the skin appeared red and slightly swollen, with blisters and
second-degree burns appearing after 2-12 h in severe cases. In less
severe cases, an itching dermatitis may develop after a latent period
of about seven days (Watrous, 1942).
Cases of skin burns have been reported during fumigation (Bruhin,
1942) and from methyl bromide fire extinguishers (Butler et al.,
1945). Bruhin (1942) reported three cases of dermal exposure during
bulk fumigation of a mill. Methyl bromide poisoning occurred due to
percutaneous absorption; the men were using breathing apparatus. All
three men were exposed to the same conditions, they suffered first or
second degree skin burns and one died as a consequence of the
exposure.
Skin lesions occurred in 6 patients who were exposed to methyl
bromide during the fumigation of a castle (Zwaveling et al., 1987;
Hezemans-Boer et al., 1988). They had adequate airway protection, so
poisoning was by dermal exposure and not by inhalation. Exposure to
high concentrations of methyl bromide (about 40 g/m3) for 40 min led
to redness and blistering of the skin. Standard protective clothing
(overalls - 35% cotton/65% polyester), worn over normal clothing, PVC
gloves, and working shoes did not prevent this. Redness, oedema, and
blistering of the skin were limited to areas where perspiration is
relatively high, i.e., armpits, groin, genitals, and the skin under
the waistbelt. The authors suggested that methyl bromide absorption
might be increased in this partly lipophilic (sebaceous glands),
partly hydrophilic (sweat glands) environment, perhaps leading to
increased absorption through the skin as well. The skin in these parts
is also thinner, favouring absorption. Plasma bromide levels were
highest about 13 h after exposure (mean 9.0± 1.4 mg/litre) and fell in
subsequent hours (mean 6.8±2.3 mg/litre; 25 h after exposure),
suggesting absorption of methyl bromide through the skin. Since the
published half-life of bromide is about 100 h, the falls in plasma
levels must be due almost exclusively to the distribution of bromide
in tissues. No systemic effects were noted.
9.1.3 Inhalation
Inhalation is the primary route of exposure. Poisoning has
occurred following acute, short-, and long-term exposures. Acute cases
have occurred involving spilling or leaks while handling methyl
bromide or where the persons were unaware of its presence (Alexeeff &
Kilgore, 1983).
In spite of stronger regulations for the safe and licensed use of
methyl bromide, cases of poisoning still occur. Cases have been
reported in California (Edmiston & Maddy, 1987; Maddy et al., 1990)
and incidents of poisoning have been reported with occupational
exposure (Cantineau et al., 1988; Herzstein & Cullen, 1990), in
members of the general public (Goldman et al., 1987; Polkowski et al.,
1990), and in burglars entering fumigated premises (Marraccini et al.,
1983; O'Neal, 1987).
9.2 General population exposure
9.2.1 Poisoning incidents
Earlier poisoning incidents involving the general public were
mainly from the methyl bromide in fire extinguishers. More poisoning
incidents have involved unauthorized entry into fumigated buildings or
persons living near fumigated buildings, greenhouses, or fields being
fumigated with methyl bromide.
9.2.1.1 Poisoning associated with fire extinguishers
Incidents involving fire extinguishers filled with methyl bromide
often involved exposure to the gas by inhalation and severe burns when
the liquid was inadvertently squirted on the feet or clothing. Butler
et al. (1945) described two cases where drivers suffered burns after
extinguishing a fire in an armoured car. Both recovered after 2 and 8
weeks, respectively. Twenty-two cases of methyl bromide poisoning in
incidents involving fire extinguishers in ships, including 6
fatalities, were reported between 1939 and 1945 (Holling & Clarke,
1944; Clarke et al., 1945). In another incident, 8 boys, 6 of whom
died, were exposed accidentally to methyl bromide from a fire
extinguisher (Prain & Harvey Smith, 1952). Longley & Jones (1965)
described an accident during methyl bromide filling where there was
massive skin contamination. Although the victim removed the
contaminated clothing and washed himself thoroughly, symptoms and
signs of poisoning, commencing with severe nausea appeared within 2 h,
and CNS effects 5 h after the exposure. The CNS effects persisted and
6 months later there was still some disability. There were no dermal
effects apart from oedema and itching of the eyelids with subsequent
peeling of the skin and no evidence of pulmonary or renal involvement.
Goulon et al. (1975) reported a detailed study of three cases
where one patient died after 5 years in a stuporous state with
myoclonus. Two of these were females who had slept in bedrooms
containing fire extinguishers filled with methyl bromide; one of these
patients died, still in coma, 4 years and 8 months later. The other
case was a male lorry driver, who was found in a coma after spending
a night in his lorry cab, which contained a leaking fire extinguisher;
4 years later he showed only partial recovery with persisting lack of
coordination, ataxia, and dysarthria. Behrens & Dukes (1986) described
the case of a scrap-dealer who was poisoned by methyl bromide from
fire extinguishers in obsolete aircraft engines.
A case of poisoning and the resulting death of a man (and his
dog) from methyl bromide leaking from an old corroded fire
extinguisher has been reported (Cavanagh, 1992; Squier et al., 1992).
Since methyl bromide is no longer in general use for fire
extinguishers, the occurrence of such cases is now extremely rare.
9.2.1.2 Poisoning associated with bulk or house fumigation
In May 1978, severe methyl bromide poisoning occurred in 4 people
living above a warehouse in Japan (Ishizu et al., 1988). The
warehouse, containing herbs, was accidentally fumigated with a greater
quantity of methyl bromide than normal giving an estimated
concentration of 38 900-58 350 mg/m3 (10 000-15 000 ppm). Three days
later, one member of the family, a girl aged 12 years, developed
severe convulsions and then 2 others had severe convulsions and the
fourth had marked mental confusion. The serum or plasma bromide ion
levels ranged from 280 to 600 mg/litre. Clinical laboratory tests
showed that GOT exceeded the normal level in 3 out of 4 cases.
Moreover, the LDH activity was above the normal range in three cases
and CPK activity was increased in all the cases (Ishizu et al., 1988).
In California, the most frequent cause of death from
non-occupational exposure, in recent years, has been unauthorized
entry into structures under fumigation with methyl bromide. Most often
the entry was by burglars, transients, or intoxicated persons who
broke into buildings that were covered with gas resistant sheeting,
locked, and posted with warning signs. During 1982-87 there were 13
such fatalities (Maddy et al., 1990).
In Florida, where homes are fumigated to destroy termites by
placing a huge coloured tent over the house, the methyl bromide being
released by trained technicians, 27 non-fatal cases and four
fatalities following unauthorized entry were reported between 1957 and
1982 (Marraccini et al., 1983). The intervals between exposure and
death ranged between 2.5 and 36 h. Three of these deaths together with
three additional hospitalizations followed burglaries of tented houses
during a nine-month period.
O'Neal (1987) reported that, at one hospital in Florida, 15-25
patients a year, including fatalities, were treated for methyl bromide
poisoning. Proper diagnosis and therapy were hampered by the latency
period (4-48 h before onset of symptoms) and the fact that the toxic
state also mimics other diseases.
Even after apartments have been cleared for habitation, deaths
due to methyl bromide poisoning have been reported. The fumigant can
be absorbed by furniture, water beds, and walls, and can sequester in
enclosed spaces, with subsequent desorption (Dempsey et al., 1992).
Prockop & Smith (1986) reported a case in which a house fumigator
wearing a faulty protective face mask was exposed briefly on one day.
This was followed by malaise, although he continued to work, and then
again, ten days later, when tremor and increasing malaise resulted in
admission to hospital. On the next day, he was comatose with
generalized major motor convulsions, and myoclonic limb movements
between convulsions. On admission, the serum bromide level was 350
mg/litre (falling to 30 mg/litre 29 days later). Five years later, the
patient still exhibited dysarthria, impaired arm coordination, and
disturbance of gait.
A fatal case of methyl bromide poisoning was reported after
fumigation of a restaurant. Although the methyl bromide levels were
checked, for some reason the apparatus showed a reading of "no
detectable levels" and, without putting the ventilation system on, the
premises were declared safe. A restaurant employee, who then entered
the building, was found dead 2.5 h later; the post-mortem inorganic
bromide level in serum was 48 mg/litre. Four other workers who were in
the building for 45 min had blood bromide concentrations ranging from
40 to 51 mg/litre. Another worker who was in the restaurant for 1 h 15
min, had a blood bromide concentration of 101 mg /litre. These 5
workers had immediate symptoms of malaise and fatigue, but no chronic
symptoms (Fuortes, 1992).
A 13-year-old girl accidentally exposed to excessive methyl
bromide concentrations after a warehouse fumigation had early symptoms
of headache, dizziness, and nausea, followed a few hours later by
unconsciousness, from which she recovered three days later. At that
time, myoclonic jerks developed, the intensity of which progressed and
was unresponsive to treatment over a two-month period. In a two-year
follow-up, treatment with clonazepam and phenobarbitol decreased the
intensity of myoclonus and she was able to return to school.
Electrophysiological findings suggested that the methyl bromide
exposure had induced a type of cortical reflex myoclonus (Uncini et
al., 1990).
9.2.1.3 Poisoning associated with soil fumigation
Goldman et al. (1987) reported four episodes of community
exposure to methyl bromide and chloropicrin soil fumigation in
California in 1973, 1980, and two in 1984. A total of over sixty cases
were involved, and, in all episodes, either evacuation of homes or
cessation of fumigation was the outcome. In one episode, a strawberry
field was fumigated preplanting with a methyl bromide/ chloropicrin
formulation (dosage and % composition not given). Local weather
conditions included a temperature of 30 °C with an inversion; 32
adults and 4 children were treated with incident-related symptoms,
such as eye irritation, sore throat, headache, shortness of breath,
and cough. One child was hallucinating. Fumigant-related symptoms
dropped off sharply with distance, 30% within 1 km compared with 4.5%
more than 2 km from the field. It should be noted that these symptoms
could be attributed to either methyl bromide or chloropicrin.
A nurseryman was poisoned after glasshouses near his house had
been fumigated with methyl bromide (Bishop, 1992). The symptoms were
delayed. By day 3 after the fumigation, he had convulsions and became
comatose. There was a long recovery period and persistence of
neurological signs and symptoms.
9.2.1.4 Miscellaneous incidents
Inhalation of methyl bromide from leaking canisters caused acute
methyl bromide poisoning. Two grandparents and a child showed various
levels of poisoning. The man showed predominantly mental disturbances,
with only mild neurological signs and no convulsions; initial euphoria
and lack of concern progressed to a florid psychosis. His mental state
was normal 6 months later. The woman was severely affected and
remained in status epilepticus for 7 days. The boy had an upper
respiratory illness 24 h before methyl bromide poisoning, which might
have increased his susceptibility to the toxic agent. He developed an
acute encephalopathy with fluctuating conscious state, hypotonia,
araflexia, and extensor plantar responses. Before methyl bromide was
identified as the cause of the illness, Reye's syndrome had been
diagnosed (Shield et al., 1977).
A tractor trailer-truck overturned and a cylinder (max. capacity
680 kg) was punctured. Although people were warned of the risk, ten
were admitted to hospital. The symptoms included nausea, vomiting,
breathing difficulty, headache, dizziness, burning throat, coughing,
and chest tightness. The primary route of exposure was inhalation for
seven patients, dermal for two, and both dermal and inhalation for
one. All patients recovered without neurological sequelae (Polkowski
et al., 1990).
Leakage from a compressed gas cylinder caused unconsciousness and
status epilepticus in a man (Mazzini et al., 1992). After awaking, he
suffered from severe myoclonic jerks and major epileptic convulsions.
Six months later, he still had severe action myoclonus of the limbs.
A standardized neuropsychological evaluation revealed severe cognitive
impairment.
9.3 Controlled human studies
Raabe (1988) carried out an inhalation study using [14C]-
labelled methyl bromide up to 0.1 mg/m3 to determine the percentage
of methyl bromide absorbed by the human body from air containing
ambient concentrations of the gas. The uptake was 55.4% nasal and
52.1% oral (section 6.1.1.2 and Fig. 6).
9.4 Occupational exposure
Up to 1955, the majority of methyl bromide poisoning incidents
resulted from chemical manufacture and filling operations, followed by
fire extinguishers and fumigation (von Oettingen, 1955). Since 1955,
fumigation has become the major source of fatalities (Alexeeff &
Kilgore, 1983).
9.4.1 Occupational exposure during manufacture
Incidents of methyl bromide poisoning in industry have been
described by Watrous (1942) and Wyers (1945). The main incidents were
dermatological cases occurring principally among those who filled the
small cylinders for distribution from the larger ones. Other sources
of danger were defects in the plant, such as ill-fitting joints and
bursting of cylinders by an undue rise in temperature. Carelessness
with handling was also a cause.
Kishi et al. (1991) carried out a questionnaire survey to
determine the symptoms of workers exposed to methyl bromide in the
manufacturing process. Seventy five male workers (exposure length:
1-25 years) were compared with a reference group of railway workers.
The questionnaire covered acute symptoms during workshift (16
questions), and general symptoms (61 questions). Acute and general
symptoms were statistically higher in frequency among the exposed
workers (sign test of pairwise comparison). Methyl bromide
concentrations in the air in the workers' breathing zone were measured
every 6 months, and were normally less than 4 mg/m3, but, during
some accidental events, they exceeded 20 mg/m3. The mean bromide ion
concentration in the urine of men working in the manufacture of methyl
bromide was 18.9 mg/litre± 10.4 (range 3.2-54.0 mg/litre), with no
correlation with the symptoms reported.
Previous studies had shown that urinary bromide concentrations
were an index of relatively acute exposure, correlating with the air
concentration of the working site. The concentration in the work-place
air sometimes accidently exceeded 58 mg/m3. There were some cases of
sub-acute poisoning with lethargy, ataxia, and retrobulbar optic
neuritis. In one incident, the mean urine concentration of 20 workers
exposed to methyl bromide was 277 mg/litre. On that occasion, 14 of
the 21 exposed workers had abnormally accelerated tendon reflexes, 8
had paraesthesia, and 4 showed disturbance of the convergence reflex
of the eyes (Kishi et al., 1991).
9.4.2 Occupational exposure due to methyl bromide fumigation
In many countries, the use of methyl bromide is restricted to
trained and licensed personnel.
Occupational exposure to methyl bromide can occur during the
manufacturing, filling, and packaging processes, as well as during its
use as a fumigant. As described in section 3.2.2, methyl bromide is
used mainly for soil fumigation as well as for the fumigation of
buildings and commodities, to prevent pest infestation. The various
occupations involved are given in section 5.3.
Methyl bromide was used as a fumigant in the USA by an estimated
105 000 workers between 1972 and 1974 (NIOSH, 1984) and 75 000 workers
in 1980 (Anger et al., 1981).
9.4.2.1 Incidents involving bulk fumigation
A poisoning incident in a mill where three fumigators were
poisoned through dermal exposure to gaseous methyl bromide has been
described in section 9.1.2 (Bruhin, 1942).
Johnstone (1945) and Ingram (1951) reported incidents in
data-processing factories where over 200 employees were taken ill,
with over 50 cases of frank methyl bromide poisoning, including two
workers suspected of insanity. All 34 packers, described by Johnstone
(1945), had visual disturbances and there was a high incidence of
speech difficulties, mental confusion, hallucinations, and
paraesthesia. Four workers claimed permanent and six temporary
disabilities. The main factor in these poisonings was that the workers
ignored the necessary precautionary measures (Johnstone, 1945).
Kantarjian & Shaheen (1963) described 8 cases of polyneuropathy in
date factory workers who suffered repeated exposure to the vapour due
to faulty working techniques over a period of three months. Within 6
months all had recovered. Only 8 out of 14 employees developed
symptoms, suggesting a variation in susceptibility in different
individuals.
Seven workers employed in the bulk fumigation of houses were
poisoned by methyl bromide (Rathus & Landy (1961). The cause was the
insufficient absorption properties of the canisters in their
respirators. Three of the men were burned on the area of the face
covered by the respirators.
Drawneek et al. (1964) described a case of irreversible brain
damage in a methyl bromide fumigator. Seven other workers, without any
recognisable illness, were found to have serum bromide levels of over
50 mg/litre, at which level mild euphoria may develop and lead to
carelessness in the handling of methyl bromide, and, therefore, to
possible acute exposure.
Hine (1969) reported two acute and eight chronic cases of
fumigation-related poisoning in California from 1957 to 1966. Four of
the patients died. The most common symptoms were malaise, weakness,
and dyspnoea. The deaths were preceded by convulsions and coma. All
cases could be traced to a failure in preventative procedures.
A few hours after exposure, during the fumigation of cocoa beans,
a dock worker developed status epilepticus, coma, and pulmonary oedema
(Greenberg, 1971).
Ten cases of methyl bromide poisoning occurred in the hold of a
ship whilst a rice cargo was being fumigated in port (Brodniewicz,
1967). The accident was the result of several circumstances: sealing
of the crew's quarters was forgotten, some of the crew remained on the
ship during, and after, fumigation, and the port fumigating team
lacked experience and proper qualifications. One victim died following
convulsions, acute pulmonary oedema, and heart failure. In the other
cases, the main complaints were dizziness and nausea.
In a fatal case of methyl bromide poisoning of a ship's boy who
slept in the crew's quarters, the levels of methyl bromide measured
after fumigation had not exceeded 40 mg/m3, but the boy was
poison-ed by a high concentration of the gas leaking from the
fumigated commodity, because of large temperature differences (Weller,
1982).
An employee habitually not wearing a mask in a fumigating plant
spraying fruits and vegetables was initially treated for psychosis, as
the early symptoms of methyl bromide poisoning are similar (Zatuchni
& Hong, 1981).
Cases of dermal exposure occurring during the fumigation of a
castle have been reported (Zwaveling et al., 1987; Hezemans-Boer et
al., 1988). Details are given in section 9.1.2.
Systemic and neuro-ophthalmic manifestations of methyl bromide
poisoning including increased serum bromide level (66 mg/litre),
paraesthesia and burning dysesthesia on hands and feet, and visual
impairment, were described in an assistant fumigator who had been
exposed acutely, twice, during the year previous to examination
(Chavez et al., 1985).
9.4.2.2 Incidents involving soil fumigation
(a) Field workers
Table 52 shows occupational poisoning incidents, reported in
California, due to methyl bromide exposure in the years 1950, 1986,
and 1987, and analysed according to work activity (Edmiston & Maddy,
1987; Maddy et al., 1990).
Seven deaths through occupational exposure were reported in
California between 1951 and 1965. The distribution of cases was: 1952
(1), 1956 (2), 1958 (1), 1959 (2), and 1965 (1) (Maddy et al., 1990).
Methyl bromide poisoning of four field workers occurred during
the removal of soil fumigation sheets under cool weather conditions
(Herzstein & Cullen, 1990). Ten days after injection of methyl bromide
into the soil, the polyethylene sheets covering the soil were removed.
The 4 field workers developed fatigue and light-headedness and 3 had
progressive respiratory, gastrointestinal, and neurological symptoms.
The acute systemic symptoms improved over several days, but
neuropsychiatric symptoms that developed later persisted for several
weeks. The 2% chloropicrin was not enough to warn the workers of the
presence of methyl bromide.
(b) Greenhouse workers
Methyl bromide has been used extensively in the USA since the
1950s, and, in Western Europe, since the 1960s, in the fumigation of
soils in greenhouses. This can be hazardous to the health of the
workers because of the enclosed area in which methyl bromide is
applied (Roosels et al., 1981). Poisoning incidents have occurred in
operators and other people entering fumigated areas. An analysis of
eight severe cases was made by Van den Oever et al. (1978). The signs
and symptoms followed the usual pattern. Nearly all incidents were
associated with professional fumigation workers using the injection
method.
In a case of acute occupational methyl bromide intoxication, a
27-year-old man, using methyl bromide in a greenhouse, suffered
continuous epileptic convulsions for 3 weeks, followed by repeated
generalized convulsions. Myoclony of the face and the fingers
accompanied by a complete motor deficiency persisted; there was also
bilateral deafness (Cantineau et al., 1988).
In an accident involving nine greenhouse workers (2 women, 7 men;
aged 21-40 years), exposed to an inadvertent spread of methyl bromide
during fumigation, two patients needed intensive care for several
weeks because of severe reactive myoclonus and tonic-clonic
generalized convulsions (Hustinx et al., 1993).
Table 52. Summary of occupational cases of methyl bromide poisoning in California
as reported by physicians, listed according to work activity and type of illness/injury a
Year Work activity Systemic Eye Skin Eye/ Total
skin cases
1950 all occupational 3 0 0 0 3
exposures
1986 chamber fumigator 2 0 1 0 3
1987 chamber fumigator 2 0 0 0 2
1986 field fumigator 0 1 5 0 6
1987 field fumigator 1 1 4 0 6
1987b field fumigator 6 2 1 0 9
1986 "tarp" cover fumigator 4 0 1 1 6
1987 "tarp" cover fumigator 1 0 1 0 2
1987b "tarp" cover fumigator 1 0 0 0 1
1986 coincidental exposure 0 1 0 0 1
1987 coincidental exposure 4 0 0 0 4
1987b coincidental exposure 2 0 0 0 2
1987 emergency response 2 1 0 0 3
1987b personnel 1 0 0 1 1
a 1950 and 1987 figures taken from Maddy et al. (1990);
1986 figures taken from Edmiston & Maddy (1987)
b A methyl bromide/chloropicrin formulation was used.
9.4.3 Studies measuring the levels of bromide ion in biological
fluids and tissues
There have been a number of studies to investigate whether there
is a correlation between methyl bromide exposure levels and bromide
ion concentrations, but the results are conflicting.
9.4.3.1 Manufacturing
Ohmori & Hirata (1982), using radioactivation analysis, reported
mean bromine concentrations in serum (66 µg/g) and hair (11 µg/g) from
14 methyl bromide workers, and mean bromine concentrations in serum
(40 µg/g) and hair (4 µg/g) in controls. A worker suspected of methyl
bromide poisoning had a level of 412 µg/g serum, 13 days after
exposure.
Average bromide ion concentrations detected in 36 urine samples
of workers exposed to methyl bromide were 13.3±7.7 mg/litre compared
with 7.1±2.1 mg/litre in a non-exposed group (Koga et al., 1991). The
atmospheric methyl bromide concentrations of exposed workers were
monitored with passive samplers during their work shifts (8 h). No
significant correlation was found between these methyl bromide
readings and the bromide ion concentrations in urine.
9.4.3.2 Fumigation
Routine monitoring of bromide levels in the blood of a group of
fumigators, undertaking both bulk and soil fumigation, was carried out
in England between 1971 and 1979 (Cornwell, 1979). It was found that
the plasma bromide levels between January and the end of the year were
correlated with the number of gas applications, irrespective of the
type of fumigation work undertaken (Fig. 10).
The plasma bromide level was thought to rise as a result of repeated
exposure to low concentrations of methyl bromide with small amounts
accumulating in the body faster than the rate of elimination. During
the Christmas period, when all the men took a break at the same time,
the plasma bromide levels were substantially reduced. Results from
October 1974 to January 1978 indicated that average plasma levels
increased from 15 mg/litre in January to 25 mg/litre in October. Some
fumigators carried out disproportionally more treatments of stacks,
containers, and lighters, and reached plasma bromide levels of 30-60
mg/litre. A relationship between plasma bromide levels and the number
of fumigation applications was found (Fig. 11).
Table 45. Acute inhalation toxicity of methyl bromide for rats and rabbitsa
Concentration Exposure time in hours
(mg/m3) Rats Rabbits
100 % fatality 100 % survival 100 % fatality 100 % survival
50 000 0.1 0.03 0.5 0.2
20 000 0.4 0.1 1.4 0.6
10 000 0.7 0.4 2.2 1.0
2 000 6 2 11 6
1 000 22 8 24 15
852 26 12 32 20
420 -b 22 -b -b
a From: Irish et al. (1940).
b No data.
Table 46. LC50 values for methyl bromide
Species Concentration Exposure Reference
(mg/m3) time
mouse 6 600 30 min Bakhishev (1973)
mouse 4 680 1 h Alexeeff et al. (1985)
mouse 1 540 2 h Balander & Polyak (1962)
mouse 1 575 4 h Yamano (1991)
rat 11 000 30 min Bakhishev (1973)
rat 7 300 1 h Zwart (1988); Zwart et al.
(1992)
rat 3 034 4 h Kato et al.(1986)
rat 1 175 8 h Honma et al. (1985)
Further details of target organ studies and biochemical findings
from the series of mouse studies (Alexeeff et al., 1985) are given in
sections 8.8 and 6.3, respectively.
An LC50 of 1575 mg methyl bromide/m3 (405 ppm ± 20) was
determined after a 4 h exposure (Yamano, 1991). In a further study,
the author exposed mice to 1945 mg methyl bromide/m3 (500 ppm).
After 2 h of exposure, there were no deaths, but, after a further 30
min, there was 85% mortality. Mice treated prior to exposure with
glutathione (500 mg/kg i.p.) showed only 5.3% mortality after this
time.
Table 47. Some single exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 6 (male) (nose only) 0 Alexeeff et al.
(Swiss-Webster) 1 h, surviving 870 (+); no toxic response (1985)
mice sacrificed 1720 (+); no toxic response
one week 2200 (+); significantly decreased lung and
later 2720 liver weights
3500 (+); additionally enlarged, pale kidneys
and kidney lesions
3820 (-); additionally abnormal clinical
signs, weight loss and mortality;
cerebral haemorrhage
4700 (-); additionally liver lesion, liver
congestion and haemorrhage
5770 (-); additionally decreased motor
coordination; cerebral congestion;
colonic haemorrhage; congested
kidneys
5930 (-); all effects mentioned above
(1 h-LC50 of 4680 mg/m3 determined)
a (+) = Able to recall a single task passive avoidance test.
b (-) = Not able to recall a single task passive avoidance test.
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 10 (male) 4 h 389 0% mortality Japanese
(Crj: BDF1) 10 (female) 584 0% mortality Ministry of
873 0% mortality Labour (1992)
1315 0% mortality
Pathology: respiratory metaplasia of the
olfactory epithelium of the nasal cavity
(female)
1970 80% mortality (male) and 100% mortality
(female)
Clinical signs: decrease in locomotor
movement, tremor, convulsion, diarrhoea,
bradypnoea, dyspnoea (dead)
Pathology:
Dead: congestion of the lung, necrosis
and degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis of the
thymus and lymph node, necrosis of the
olfactory epithelium of the nasal cavity
Survived: tubular necrosis and regeneration
of the kidney, necrosis and respiratory
metaplasia of the olfactory epithelium of the
nasal cavity
2950 100% mortality
clinical sign and pathology: same as
1970 mg/m3 dead animals
mouse 1 h 45 min 1945 0% mortality Yamano (1991)
2 h 0% mortality
2 h 10 min 11% mortality
2 h 20 min 15% mortality
2 h 30 min 85% mortality
3 h 90% mortality
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 10(male) 4 h 584 0% mortality Japanese
(F344/DuCrj) 10(female) 875 0% mortality Ministry of
Pathology: disarrangement and Labour (1992)
respiratory metaplasia of the olfactory
epithelium of the nasal cavity
1315 0% mortality; Pathology: same as 875 mg/m3
1970 0% mortality; Pathology: same as 875 mg/m3
2956 100% mortality
Clinical signs: closed eyelid, decrease in
locomotor movement, ataxic gait, serous
discharge of nose, lacrimation, diarrhoea,
irregular breathing and bradypnoea
Pathology: congestion of the lung,
degeneration of the liver, tubular necrosis
of the kidney, haemorrhage of heart,
haemorrhage or necrosis of the adrenal glands,
necrosis of the olfactory epithelium of the
nasal cavity, congestion of the thymus
4435 100% mortality
Clinical signs and pathology: same as 2956 mg/m3
rat 2 (male) 5.2-86 min 7500- 1-h LC50 was 7300 mg/m3. Zwart (1988)
(SPF-Wistar) 57 000 Rangec: 3.5-min LC50 = 75 700 mg/m3 Zwart et al.
480-min LC50 = 1300 mg/m3 (1992)
rat 5 (male) 8 h 1042- LC50 = 1175 mg/m3 Honma et al.
(Sprague-Dawley) 2085 (1985)
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 8 (male) 8 h 245- locomotor activity decreased at
(Sprague-Dawley) 972 731 mg/m3; rectal temperature fell
2°C at 486 mg/m3; decrease in feed
consumption and body weight gain at
486 mg/m3; all animals lost righting
reflex at 245 mg/m3
rat 8 (male) 6 h 778 extensive destruction of the Hurtt et
(F-344) olfactory epithelium al. (1988)
c Total of 23 combinations of time and dosage; the animals were observed for up to 2 weeks.
Groups (10 male+10 female) of BDF1 mice were exposed to methyl
bromide (99.9% pure) concentrations of 389, 584, 873, 1315, 1970, or
2950 mg/m3 (100, 150, 225, 338, 506, or 760 ppm) for 4 h (Japanese
Ministry of Labour, 1992). Mice exposed to concentrations of 1970 and
2950 mg/m3 showed decreased locomotor activity, tremor, convulsions,
diarrhoea, dyspnoea, and bradypnoea. In the 2950 mg/m3 group, all
the mice died; at 1970 mg/m3, 2 males survived. Mice exposed to
concentrations of between 389 and 1315 mg/m3 did not exhibit any
abnormal clinical signs.
Pathology in a female mouse exposed to 1315 mg/m3 showed
metaplasia of the olfactory epithelium. In the 2 male mice surviving
exposure to 1970 mg/m3, there was renal tubular necrosis and
regeneration, and necrosis and metaplasia of the olfactory epithelium.
In the other mice exposed to 1970 and 2950 mg/m3, there was
pulmonary congestion, hepatic degeneration and necrosis, renal tubular
necrosis, karyorrhexis of the thymus and lymph nodes, and necrosis of
the olfactory epithelium.
8.1.2.3 Rat
Irish et al. (1940) exposed rats to concentrations of 420, 852,
1000, 2000, 10 000, 20 000, or 50 000 mg methyl bromide/m3. Table 45
shows the exposure time in hours resulting in 100 % survival and 100
% fatality. Rats exposed to concentrations below 10 000 mg/m3 showed
roughening of the fur, hunching of the back, drowsiness, heavy
breathing, and sometimes lacrimation. At higher concentrations, the
first signs were nose irritation and lacrimation followed by the
reactions already mentioned. Those exposed for 20 h to 1000 mg methyl
bromide/m3 often became hyperactive until exhausted.
As well as neurological manifestations of toxicity in rats,
methyl bromide at concentrations of 1000-20 000 mg/m3 caused
irritation of the lungs, producing acute congestion and oedema (Irish
et al., 1940).
Groups (10 male + 10 female) of F344 rats were exposed to methyl
bromide (99.9% pure) concentrations of 584, 875, 1315, 1970, 2956, or
4435 mg/m3 (150, 225, 338, 506, 760, or 1140 ppm) for 4 h in a
chamber (Japanese Ministry of Labour, 1992). At concentrations of 1315
mg/m3 and above, there was decreased locomotor activity, ataxia,
nasal discharge, lacrimation, diarrhoea, and irregular breathing and
bradypnoea. In the 2956 and 4435 mg/m3 exposure groups, all the rats
died. Pathology of these groups showed pulmonary congestion, hepatic
degeneration, renal necrosis, myocardial haemorrhages, haemorrhage and
necrosis of the adrenal glands, and congestion of the thymus. In rats
exposed to 875, 1315, or 1970 mg/m3, there was metaplasia of the
olfactory epithelium and, in those exposed to the two highest doses,
also necrosis of the olfactory epithelium.
A single 6-h exposure of rats to 780 mg methyl bromide/m3
caused extensive destruction of the olfactory mucosal epithelium
(Hurtt et al., 1988).
Kato et al. (1986) determined a 4-h LC50 for methyl bromide in
male Sprague-Dawley rats (Fig. 9). Groups of 5 rats were exposed for
4 h to methyl bromide at concentrations of 1952, 2420, 3108, or 3485
mg/m3 (502, 622, 799, or 896 ppm), and approximate values of 100%
survival and 100% lethal concentration were determined (2529 and 3501
mg/m3, respectively). In a further test, 10 rats each were exposed
to 2727, 2984, 3143, 3178, or 3236 mg/m3 (701, 767, 808, 817, or 832
ppm). An LC50 value of 3034 mg/m3 (780 ppm) was calculated from
mortality at one week after exposure (Kato et al., 1986).
The dependence of methyl bromide toxicity on time and
concentration was demonstrated in studies performed by Zwart (1988)
and Zwart et al. (1992). Male SPF-Wistar rats were exposed to a total
of 23 combinations (2 rats each) of time and concentration and LC50
values were determined at seven time points ranging from 3.5 to 480
min. LC50s ranged from 75 700 mg/m3 at 3.5 min to 1300 mg/m3 at
480 min. The 1-h LC50 was 7300 mg/m3. Most animals showed some
incoordination, decreased response to stimuli, and had lame limbs,
directly after exposure. All mortalities occurred during the first
week and, on examination, red discoloured lungs and red/black spots in
the thymus were found in most dead rats. After two weeks, the
surviving animals were sacrificed. Some of these rats showed clear or
light red stained fluid in the lungs (Zwart, 1988).
Honma et al. (1985) carried out various investigations into the
effects of a single, 8-h exposure to methyl bromide on male
Sprague-Dawley rats. An acute toxicity study was carried out with five
groups of five animals exposed to 1042, 1303, 1564, 1824, or 2085
mg/m3 (268, 335, 402, 469, or 536 ppm), respectively. An 8-h LC50
of 1175 mg/m3 (302 ppm) with 95% confidence limits of 1040-1323
mg/m3 (267-340 ppm) was determined.
Body temperature was measured in four groups of five rats each
exposed to 245, 486, or 972 mg methyl bromide/m3 (63, 125, or 250
ppm). Exposure to 245 mg/m3 did not effect rectal temperature, while
8-h exposure at 486 or 972 mg/m3 decreased body temperature by about
2°C; however, this normalized within one day (Honma et al., 1985).
The effects of methyl bromide on body weight gain were
investigated. Food deprivation (feeding only twice a day) was started
at least 2 weeks before exposure. Rats were exposed to 245, 486, or
972 mg methyl bromide/m3 (63, 125, or 250 ppm) for 8 h and feed was
provided immediately afterwards. Decrease in food consumption and
depression in body weight gain were observed in groups exposed to 486
and 972 mg methyl bromide/m3, but not in groups exposed to 245 mg
methyl bromide/m3. The control group gained 15 g/day whereas with
exposure to 972 mg methyl bromide/m3, weight gain was almost fully
suppressed and was still partially depressed (+10 g) the following day
(Honma et al., 1985).
8.1.3 Dermal
Toxicity studies concerning the dermal route of exposure in
animals have not been reported.
8.1.4 Subcutaneous administration
For a single subcutaneous administration in Sprague-Dawley male
rats (9 rats/group) an LD50 for methyl bromide was found to be 135
mg/kg body weight (range 75- 250 mg/kg body weight) (Tanaka et al.,
1988).
8.2 Short-term exposure
8.2.1 Oral
A summary of studies concerned with oral exposure to methyl
bromide by gavage is given in Table 43.
A group of 12 rabbits was fed a mixed diet that had been
fumigated with methyl bromide for 24 h. The rabbits were fed
immediately after the fumigation was completed and the content of
methyl bromide in the feed was 3865 mg/kg. The first animal died 3
days after feeding was begun and the last in 13 days. All were
paralysed prior to death and all showed pulmonary damage. No changes
in the gastrointestinal tract were found (Dudley et al., 1940).
Studies on rats (8-week preliminary test, 16-week, and 20-week
test) and rabbits (52 weeks) were carried out by Dudley & Neal (1942).
Results from the rat study showed that, when high (5290-6200 mg Br/kg
food) amounts of organic and inorganic bromides were present in food
after fumigation with methyl bromide, mortality increased, body weight
gain and activity were reduced, and general health and reproductivity
were adversely affected. When feed containing 240-300 mg Br/kg,
following fumigation with 58 g methyl bromide/m3 for 24 h, was fed,
or when fumigated fruits and vegetables were fed, few or no
deleterious effects were noted (Dudley & Neal, 1942). Activity,
general condition, body weight gain, and reproductivity were normal.
Dudley & Neal (1942) carried out similar studies on rabbits. All 12
rabbits died within 2 weeks of being fed a diet containing about 3000
mg Br/kg. However, the 12 rabbits fed a diet of 60-100 mg Br/kg for 52
weeks showed few or no deleterious effects.
No apparent effects on appearance and general behaviour were
observed in Wistar rats (male and female) given doses, by gavage, of
up to 50 mg methyl bromide/kg body weight in a 90-day study (Danse et
al., 1984). Body weight gain in the male rats was significantly less
than that of controls, though this was not the case for females. There
were slight haematological changes and, in the two higher dosage
groups, several animals showed proliferative alterations of the
forestomach mucosa, characterized by hyperkeratosis and papilloma
(section 8.7).
A study by Boorman et al. (1986), based on a study design by
Danse et al. (1984), included dose groups with a recovery period, in
order to study the progression or regression of lesions. Details are
given in section 8.7 and Table 43. Boorman et al. (1986) found
forestomach lesions similar to those described by Danse et al. (1984),
but these lesions regressed in the 60-day recovery period.
Similar findings were reported by Hubbs & Harrington (1986). They
administered methyl bromide in peanut oil to rats at doses of 0, 25,
or 50 mg/kg body weight per day for up to 120 days. In a regression
study, some of the rats were treated for 90 days and then allowed to
recover for 30-60 days (Table 43 and section 8.7).
Three groups of four beagle dogs (3 male, 1 female) were fed
methyl bromide-fumigated food for 6-8 weeks in doses equivalent to an
average daily ingestion of 35, 75, or 150 mg/kg body weight of bromide
ion, respectively (Rosenblum et al., 1960). A further group of 4 dogs
received 128 mg sodium bromide/kg per day (equivalent to 100 mg
bromide ion/kg per day). A control group of 6 dogs (3 male and 3
female) received only dog chow. After one year of observation and
monthly blood and urine tests, the remaining dogs were killed, the
organs weighed, and histological studies carried out. No evidence of
toxicity that could be attributed to bromide was observed in animals
that received 35 or 75 mg bromide/kg per day. Dogs in the group
receiving 150 mg/kg per day became lethargic and had occasional
episodes of salivation and diarrhoea. No significant effects on blood
chemistry, haematology, or urinary values were reported, nor were
treatment-related deaths or histological lesions noted.
8.2.2 Inhalation studies
8.2.2.1 Guinea-pig, rabbit, monkey
A summary of short-term exposure studies is given in Table 48.
Irish et al. (1940) carried out extensive studies into the
long-term exposure of animals to methyl bromide. A total number of 135
rats, 98 guinea-pigs, 104 rabbits, and 13 monkeys were exposed to 65,
130, 250, 420, or 850 mg methyl bromide/m3, 7-8 h/day, 5 days/week
for 6 months, or, until the majority had either died or shown a severe
reaction (Table 49).
8.2.2.2 Mouse
In the short-term studies on male and female B6C3F1 mice, exposed
6 h/day for 10 days over 14 days (778 mg methyl bromide/m3),
described by NTP (1992), five mice/dose group per sex were evaluated
for haematology, serum pseudocholinesterase activity, and pathology.
Necropsied animals from the two highest dosage groups were examined
histopathologically. The results are summarized in Table 48.
Eustis et al. (1988) carried out a special target organ study on
B6C3F1 mice (and F344/N rats). Male and female B6C3F1 mice were
exposed to either 622 mg methyl bromide/m3 (160 ppm) or air for 6
h/day, 5 days/week. The animals were scheduled for sacrifice after 3,
10, or 30 exposures. When 50% mortality was observed in any group, the
surviving animals in that group were sacrificed. Mice were evaluated
for body weight, mortality, organ weights, haematology, and
histopathology. In addition to these end-points, urine chemistry and
plasma enzymes were assessed in the rats. Significantly different
mortality rates were observed between the two species, with the mice
demonstrating a higher sensitivity to 622 mg methyl bromide/m3 than
rats. Body weight differences were exposure-related (Eustis et al.,
1988). The results are summarized in Table 48. Mortality exceeded 50%
after 8 and 6 exposures in male and female mice, respectively. The
remaining male mice were killed after 10 exposures and the females
after 8 exposures. There were significant reductions in body weight
and corresponding reductions in organ weights in both sexes, whereas
there were sex differences in the haematological parameters. The
responses to exposure were minimal in males, but marked in females, in
which there were large and significant reductions in RBC, haemoglobin,
haematocrit values, and mean corpuscular haemoglobin concentrations,
and increases in WBC and mean corpuscular volume (Eustis et al.,
1988). Histopathological changes in target organs are described in
section 8.8.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). Survival was reduced at all
exposure concentrations and none of the mice exposed to 1315 or 1712
mg methyl bromide/m3 survived. At all exposure concentrations, mice
exhibited decreased locomotor activity, piloerection, lacrimation,
ataxia, and tremor.
Table 48. Short-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 10 (male) 6 h/day; 5 days/week; 477 78 mg/m3 group: 9 male, 6 female died; NTP (1992)
(B6C3F1) 10 (female) 2 weeks 97 All groups: no body weight changes; bloody
195 urine; trembling, jumpiness, paralysis in
389 all groups, but most pronounced in highest
778 dosage groups; haematology parameters/
pseudo cholinesterase activity - no
consistent dose-related effects
1 female mouse showed minimal
hyperaemia of lungs, liver, kidneys
Mouse 20 (male) 6 h/day; 5 days/week; 622 50% mortality (male) after 8 exp. days, Eustis et
(B6C3F1) 20 (female) 10 exp. days 50% mortality (female) after 6 exp. days; al. (1988)
for males and exposure-related lesions were seen in
8 exp. days the brain, heart, kidneys, thymus, and
for females spleen of both sexes; in the testes,
nose, and lungs of males, and in the
adrenal glands of females
Mouse 15 (male) 6 h/day; 5 days/week; 0 Eustis et al. (1988)
(B6C3F1) 15 (female) 6 weeks 622 lethargy; curling and crossing
(continued) of hind-limbs, forelimb twitching
and tremors; decrease in body weight
gain after 5 days; decrease in organ
weight (lung, heart, thymus, brain,
liver) neuronal necrosis; nephrosis;
atrophy of inner zone of adrenal
cortex; testicular degeneration;
decrease in RBC, increase in WBC
(females only)
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 18-30 (males) 5 days/week; 6 h/day; 0 All dose groups: no significant organ NTP (1992)
(B6C3F1) 18-30 (female) 13 weeks 39 weight effects;
78
156 decrease in Hb and MCV, increase in RBC (males);
311 decrease in Hb and MCV, increase in RBC (males);
467 17 % mortality in males; decrease in body weight,
additionally severe curling and crossing
of hindlimbs and twitching of forelimbs
(male > female)
Mouse 10 (male) 6 h/day; 5 days/week 0 Japanese
(Crj:BDF1) 10 (female) 13 weeks 29 no toxic effects; Ministry of
58 no toxic effects; Labour
117 no toxic effects; (1992)
234 depression of body weight gain;
Haematology: increase in MCV in females;
Urinalysis: increased protein in females
Mouse 10 (male) 6 h/day; 5 days/week 599 10% mortality (male) and 0% (female) Japanese
(Crj:BDF1) 10 (female) 2 weeks depression of body weight gain; Ministry of
(continued) Clinical signs: Labour (1992)
Dead mice: decrease in locomotor activity,
piloerection, lacrimation, bradypnoea,
opacity of eye, diarrhoea
Surviving mice: decrease in locomotor activity,
bradypnoea, ataxic gait, sub-normal
temperature, tremor, lacrimation, soiled,
pallor, hunched posture, piloerection
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology:
Dead mice: tubular necrosis of the kidney,
ulcer of the stomach, testicular
atrophy, atrophy of the spleen, atrophy
and karyorrhexis of the lymph node,
myocardial necrosis
Surviving mice: degeneration of the granular
layer of the cerebellum, tubular necrosis and
regeneration of the kidney, necrosis and
respiratory metaplasia of the olfactory
epithelium
Mouse 778 50% mortality of males and 80% mortality of Japanese Ministry
(Crj:BDF1) females, depression of body weight gain; of Labour (1992)
(continued) Clinical signs: same as 599 mg/m3 group
Pathology: Dead mice: degeneration of the
granular layer of the cerebellum, tubular
necrosis and regeneration of the kidney,
extramedullary haematopoiesis and atrophy
of the spleen, karyorrhexis of the thymus,
myocardial necrosis, necrosis of the
olfactory epithelium
1011 90% mortality (male and female)
depression of body weight gain;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
1315 100% mortality (male and female);
Clinical signs: same as mice dying
in 599 mg/m3 group
Pathology: congestion of the lung,
degeneration of the liver, hyaline droplet
and tubular necrosis of the kidney,
karyorrhexis of the thymus and spleen,
myocardial necrosis, necrosis of the olfactory
epithelium.
Mouse 1712 100% mortality (male and female); Japanese Ministry
(Crj:BDF1) Clinical signs: same as mice dying of Labour
(continued) in the 599 mg/m3 group (1992)
Pathology: congestion of the lung,
degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis
of the thymus and spleen.
467 mortality, 10% in males and 90% in females;
depression of body weight gain;
Clinical signs: ataxic gait;
Haematology: increased MCV in males;
Urinalysis: same as 234 mg/m3 group;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, congestion of the lung, karyorrhexis
and atrophy of the thymus, tubular necrosis
of the kidney, necrosis of the heart,
vacuolic change of the adrenal glands
Rat 6 (male) 6 h/day; 5 days 0 NTP (1992)
(SPF Wistar) (week 1) 150 brain weight depression 4-5%
6 h/day; 3 days 375 (dose related) at 750 mg/m3;
(week 2) 750 additionally, marked growth retardation;
tremors, motor incoordination; liver
weights decreased by 26%; no distinct
microscopic changes in eight organs (but
lungs of three high-dose rats were
hyperaemic)
Rat 6 (male) 6 h/day; 5 days/week 0 no toxic effects; NTP (1992)
(SPF Wistar) 6 (female) (week 1,2,3) 70 no toxic effects (marginal no-effect level); (Dutch
6 h/day; 7 days/week 200 decrease in feed consumption, decrease Study)
(week 4) in body weight gain;
disturbed gait and tremors;
600 histopathological changes in heart
and lungs, 8 rats died before end
of study
Rat 10 (male) 6 h/day; 5 day/week; 0 no deaths; no clinical findings; no Wilmer et
(Wistar) 10 (female) 13 weeks 4 change in body weight al. (1983)
25
166 minimal changes in liver
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 10 (male) 6 h/day; 5 days/week; 599 depression of body weight gain Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
Pathology: disarrangement and
respiratory metaplasia of the
olfactory epithelium
778 depression of body weight gain;
Pathology: disarrangement and respiratory
metaplasia of the olfactory epithelium
of the nasal cavity, cellular
vacuolization in the adrenal glands
1011 depression of body weight gain;
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
Rat 10 (male) 6 h/day; 5 days/week; 1315 70% mortality in males and 10% mortality Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
(continued) Dead mice: depression of body weight gain; (continued)
Clinical signs: decrease in locomotor
movement, soiled, piloerection, lacrimation,
serous or bloody nose discharge, diarrhoea,
pallor, irregular breathing;
Dead and surviving mice: decrease in
locomotor movement, hunched posture, soiled,
piloerection, haemorrhagic discharge of nose,
lacrimation
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: interstitial pneumonia,
karryorrhexis of of the thymus,
myocardial damage, cellular vacuolization
of the adrenal glands, disarrangement and
respiratory metaplasia of the olfactory
epithelium
1712 100% mortality;
Clinical signs: same as 338 ppm group dead
rats;
Pathology: congestion and haemorrhage of
the lung, congestion, necrosis, and fatty
changes in the liver, tubular necrosis of
the kidney, myocardial necrosis, haemorrhage,
necrosis and cellular vacuolization of the
adrenal glands, necrosis and
respiratory metaplasia of the olfactory
epithelium, congestion of the thymus,
inflammation of the bone marrow
Rat 10-12 (male) 4 h/day; 6 weeks 584 decrease in body weight, Kato et
(Sprague- no clinical changes; al. (1986)
Dawley) 778 decrease in body weight,
no clinical changes;
1167 3/12: paralysis of hindlimbs;
1556 5/10: ataxia after 2 weeks, paralysis
after 3 weeks, 1/10: died after 4 weeks,
3/10: died after 5 weeks;
Haematology: no change in RBC, Hb, Hct, WBC
1167 mg/m3 group: increase in serum enzyme
activities;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Organ weights: decrease in all groups, but
no clear dose dependency;
Residual bromide: increase in all groups
584 mg/m3: spleen>kidney>liver;
higher dosage groups: kidney>spleen>liver;
Histopathological changes: in brain, heart
and testes
Rat 5 (male) 3 weeks 4 biochemical changes Sato et
(Sprague- 20 al. (1985)
Dawley) 39
Rat 10 (male) 6 h/day; 5 days 350 no observable effects; Hurtt et
(F-344) 680 dose-dependent vacuolar degeneration al. (1987)
of zona fasciculata (adrenal gland),
cerebellar granular cell degeneration
and olfactory sensory cell degeneration;
973 as above, plus: diarrhoea, haemoglobinuria,
1264 some gait disturbances and convulsions;
hepatocellular degeneration (at 1264
mg/m3 only) - cerebral cortical degeneation
ation and minor alterations in testicular
histology
Rat total 84 6 h/day; 5 days 778 increase in mean body weight; Hurtt et al. (1988)
(F-344) (male) degeneration and regeneration
of olfactory epithelium
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 40 (male); 6 h/day; 5 days 778 decrease in plasma testosterone Hurtt & Working
(F-344) 10 (male) concentration and nonprotein sulfhydryl (1988)
killed on contents of liver and testis
each of day
1,3,5 and 8
35 (male); 6 h/day; 5 days
5 killed on
each of day 6,
10, 17, 24, 38,
52, 73
Rat 5 (male) 6 h/day (3 days) 622 50 % mortality in males after 14 exp. Eustis et
(F-344) 5 (female) days; remaining males sacrificed; al. (1988)
females killed after 6 weeks;clear
5 + 10 (male) 6 h/day, 5 days/week sex-related differences in susceptibility
5 (female) (2 weeks) of specific organs to CH3Br:brain, kidney,
nasal cavity, heart, adrenal, liver, and
10 (female) 6 h/day, 5 days/week testis; neuronal necrosis in cerebral cortex,
(6 weeks) hippocampus and thalamus of brain; necrosis of
olfactory epithelium; myocardial degeneration;
testicular degeneration
Rat 18 6 h/day; 5 day/week; 0 All dose groups: no deaths or clinical Haber et al.
(F-344/N) 18 (female) 13 weeks 117 signs; no consistent organ weight effects; (1985) [abstract]
234 * body weight (females) NTP (1990)
467 * body weight (both sexes);
minor neurobehavioural changes; (females)
* Hct, * Hb, * RBC;
olfactory epithelial dysplasia and cysts
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 36 (male) 6 h/day; 5 and 117 GSH and G-6-PDH activities: increase in Jaskot et
(CD) 10 days lung and decrease in liver; al. (1988)
serum: decrease in cholinesterase, BUN,
uric acid, cholesterol,
increase in leucine amino-peptidase
Rat total 30 4 h/day; 4 days/week, 778 damage of olfactory epithelium; Hastings
(Long-Evans) (15 control) 2 weeks repair by day 4; impaired nasal (1990)
function recovered after 4 days
Rat 10 (male) 6 h/day; 5 day/week; 0 Japanese Ministry
10 (female) 13 weeks 29 no toxic effects of Labour
73 Blood biochemistry: * K (male), ** total (1992)
cholesterol (female)
183 Blood biochemistry: * in potassium (male)
** in total cholesterol, GOT, GPT (female)
455 * of body weight gain
Haematology: ** Hct, ** MCV, ** platelet
(male) ** MCV (female)
Blood biochemistry: * K (male), ** total
** total cholesterol, GOT, GPT * glucose,
creatinine (female)
Rat 1140 100% mortality; Japanese
(continued) Clinical signs: * locomotor activity, Ministry of Labour
hunchback position, piloerection, (1992) (continued)
soiled, ataxic gait, tremor, convulsion,
diarrhoea, loose stool or cyanosis,
haematuria, serous or haemorrhagic
discharge of nose, haemorrhagic discharge of
eye, lacrimation, irregular breathing
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, karyorrhexis, haemorrhage and atrophy
of the thymus, tubular necrosis of the kidney,
atrophy of the testis, foamy cell accumulation
and interstitial pneumonia, myocardial damage,
cellular vacuolization of the adrenal glands,
pigmentation of the Harderian glands,
necrosis, disarrangement and respiratory
metaplasia of the olfactory epithelium
GSH = glutathione S-transferase; G-6-PDH = glucose-6-phosphate dehydrogenase; BUN = blood urea nitrogen;
RBC = red blood cell count; exp. = exposure; p.c. = post copulation.
* = decrease; ** = increase
Histopathology showed degenerative cerebellar changes, renal
tubular necrosis and regeneration, and metaplasia and necrosis of
olfactory epithelium in all exposed groups. In male mice exposed to
599 mg/m3, there were also stomach ulceration, testicular atrophy,
atrophy of the spleen, and atrophy and karyorrhexis in the lymph
nodes. At concentrations above 778 mg/m3, there were, also karyor-
rhexis of the thymus, and myocardial necrosis. Hepatic degeneration
and pulmonary congestion were found at 1315 and 1712 mg methyl
bromide/m3. For further details see Table 48.
B6C3F1 mice were exposed to methyl bromide concentrations of 0,
39, 78, 156, 312, or 468 mg/m3 for 6 h per day on five days a week
for 13 weeks (NTP, 1992). There were reductions in survival and body
weight gain among male mice exposed to 468 mg/m3. No reduction was
observed among males in the other groups or in female mice in any
group. Clinical findings in the high-dose group were severe curling
and crossing of the hindlimbs and twitching of the forelimbs. These
signs were more severe among male than among female mice. Mild
behavioural test response deviations reached a maximum after about 6
weeks with no further increase in severity in the later 7 weeks of the
study. Plasma cholinesterase activity was unaffected by treatment. No
exposure-related histopathological changes were described.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 58, 117, 234, or 467 mg/m3 (0,
7.5, 15, 30, 60, or 120 ppm) for 13 weeks, 6 h/day, and 5 days/week
(Japanese Ministry of Labour, 1992). At 467 mg/m3, there was a
mortality rate of 10% (males) and 90% (females). Ataxia was noted in
mice exposed to 467 mg/m3. There were no abnormal clinical signs at
concentrations of between 29 and 234 mg/m3. Slight increases in mean
corpuscular volume (MCV) in female mice exposed to 234 mg/m3 and in
male mice exposed to 467 mg/m3 were not accompanied by other
haematological effects. Blood biochemistry showed no abnormalities. No
treatment-related, histopathological effects were observed in the 29
and 234 mg/m3 groups. In the mice that did not survive exposure to
467 mg/m3, there were cerebellar degeneration and brain necrosis,
pulmonary congestion, thymic atrophy, myocardial damage, renal tubular
necrosis, and vacuolization of the adrenal glands (Table 49).
Table 49. Long-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
mouse 86 (male) 5 days/week; 0 All dose groups: no biologically significant NTP (1992)
(B6C3F1) 86 (female) 6 h/day;2 year 39 haematology values; no carcinogenic
[planned; (interim 128 effects; increased incidence of
because of high sacrifice at 6 389 nonneoplastic lesions in brain, bone,
mortality, and 15 months) heart, and nose
regime altered] 389 mg/m3 dosage group: high mortality
(40/86 males and 9/86 females) after 20
weeks; decrease in body weight and
in thymus weight;
tremors, abnormal posture and limb paralysis;
significant behavioural differences at 3
months (males), less so in females
mouse 50 (male) 6 h/day; 5 days/ 0 Japanese
(Crj: 50 (female) week; 16 no toxic effects; Ministry of
BDF1) 2 years 62 no toxic effects; Labour (1992)
250 no mortality change;
depression of body weight gain
Blood biochemistry: increase in CPK, inorganic
phosphorus (male), chloride; decrease
in albumin (female);
Pathology: atrophy of the granular layer of
the cerebellum no increase in neoplastic change
was considered
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 90 (male) 6 h/day; 5 days/ 0 All dose groups: degenerative and Dreef-van der
(Wistar) 80 (female) week; 29 months 12 hyperplastic lesions in nasal mucosa; no Meulen et al.
117 tumours induced by methyl bromide; (1989)
350 350 mg/m3 group: * body weight gain; Reuzel et al.
* absolute brain weight; hyperkeratosis in (1991)
oesophagus and forestomach; ** incidence
of haemothorax, myocardial degeneration;
thrombi in the heart; and increased
mortality
rat 50 (male) 6 h/day; 5 days/ 16 Pathology: ** incidence and severity of Japanese
(F344/ 50 (female) week; 2 years inflammation of the nasal cavity (male); Ministry of
DuCrj) 78 Urinalysis: * in protein (male); Labour (1992)
Pathology: same as 16 mg/m3
389 no mortality change, * body weight gain;
Haematology: ** RBC, Hb, Hct (male)
** Hb, Hct (female);
Blood biochemistry: * LDH, CPK, Na+, K+,
Cl- (male), * Glu, CPK, Ca2+, ** LAP (female);
rat Urinalysis: * protein
(F344/DuCrj) Pathology: ** incidence of necrosis and
(continued) respiratory metaplasia of the olfactory
epithelium; ** incidence and severity of
inflammation of the nasal cavity (male);
marginal ** necrosis of the olfactory
epithelium and inflammation of the nasal
cavity (female);
no increase in neoplasms observed
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 8 6 h/day; 5 days/ 0 no effect on nerve conduction velocity, Anger et al.
(Sprague- 32 (n.g.)b week; 36 weeks 214 open field activity, or coordination (1981)
Dawley) over 12 months
a RBC = red blood count; Hb = haemoglobin; Hct = haematocrit; WBC = white blood count; MCV = mean cell volume.
b n.g.= sex not given; * = decrease; ** = increase.
8.2.2.3 Rat
F344-rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). All rats in the 1712 mg/m3
group and 7 males and one female in the 1315 mg/m3 group died before
the end of the study. Piloerection and haemorrhagic nasal discharge
were reported at exposure concentrations of 1011, 1315, and 1712
mg/m3. In addition, decreased locomotor activity, a hunched position
and lacrimation were observed in rats that survived exposure to 1315
mg/m3. Diarrhoea and irregular breathing were noted in the rats that
died following exposure to 1315 and 1712 mg/m3.
Pathology showed metaplasia of the olfactory epithelium at methyl
bromide concentrations of 599 and 778 mg/m3, plus necrosis at
concentrations between 1011 and 1712 mg/m3. Vacuolization of the
adrenal glands was found at concentrations of 778, 1011, and 1315
mg/m3 with necrosis at 1712 mg/m3. Myocardial damage was reported
at concentrations between 1011 and 1712 mg/m3. In the rats exposed
to 1315 and 1712 mg/m3, there was also pulmonary congestion and
haemorrhage, renal tubular necrosis and congestion of the thymus; bone
marrow inflammation was reported at 1712 mg/m3 (Table 48).
Two short-term range finding studies were conducted in SPF Wistar
rats (NTP, 1992). In the first study, groups of six male rats were
exposed to up to 750 mg methyl bromide/m3 for 6 h/day for 2 weeks
(see Table 48). In the second range-finding study, groups of six male
and six female rats were exposed to up to 600 mg methyl bromide/m3
for 4 weeks. Five male and three female rats in the high-dose group
died before the end of the study. The most important histopathological
changes occurred in the heart and lungs of animals in the high-dose
group. Diffuse fatty vacuolization and diffuse myocardial fibre
degeneration appeared. The lungs showed hyperaemic and dilated
alveoli; in some animals, interstitial pneumonia was noted (NTP,
1992).
Male Sprague-Dawley rats were exposed to up to 1556 mg methyl
bromide/m3 for 4 h/day for 6 weeks (Kato et al., 1986). Changes in
body weight, general condition, haematology parameters, organ weight,
tissue bromide ion concentration, and the histopathology of several
organs were determined. Suppression of body weight gain, abnormal
clinical signs, and severe weakness were observed at 1556 mg methyl
bromide/m3. Bromide ion accumulation was seen, especially in the
kidney and spleen, without significant dose-related change. Pronounced
histopathological changes were noted in the brain (section 8.8) and
multiple small necrotic foci in the heart.
Other short-term, inhalation exposure studies concentrated on one
aspect/target organ of methyl bromide exposure.
Sato et al. (1985) described biochemical findings in rats exposed
to 4, 20, or 39 mg methyl bromide/m3, continuously, for 3 weeks.
After sacrifice, the organs were weighed and biochemical examinations
were performed on the blood and a homogenate of heart, liver, and
lungs. The results showed no differences between the 4 mg/m3 and the
control group. In the 20 mg/m3 group, several changes were observed;
serum creatine phosphokinase (CPK), phospholipids (PL), and blood
glucose levels, and thymus weight decreased, while blood haemoglobin
(Hb), reduced glutathione (GSH), serum total protein, and lung
gamma-glutamate-pyruvate transaminase (GTP) levels increased after
exposure. In the 39 mg/m3 group, increases were observed in serum
glutamate oxaloacetate transaminase (GOT), lactate dehydrogenase
(LDH), alpha-hydroxy-butyrate dehydrogenase (alpha-HBDH), total
protein, blood Hb, GSH, lung acid phosphatase (AcP), gamma-GTP, LDH
total, liver PL, and triglycerides (TriG), while decreases were noted
in serum cholinesterase (ChE), CPK, PL, TriG, lung alkaline
phosphatase (AlP), free-cholesterol (f-Chol), lactate, blood glucose,
heart lactate, glucose, lung AlP, liver free fatty acids (FFA), and
glycogen. Pulmonary haemorrhage was observed in almost all animals in
this group.
The effects of short-term inhalation exposure to 1264 mg methyl
bromide/m3 (6 h/day for 5 days) including those on the target organ
histopathology of male F-344 rats were studied by Hurtt et al. (1987).
Clinical changes (noted only in the 973 and 1264 mg/m3 groups) were
diarrhoea, haemoglobinuria, and, in a few cases, gait disturbances and
convulsions. A dose-dependent vacuolar degeneration of the zona
fasciculata of the adrenal glands, cerebellar granule cell
degeneration, and olfactory sensory cell degeneration were seen in the
680, 973, and 1264 mg/m3 groups. Cerebral cortical degeneration and
minor alterations in testicular histology were seen only in the 1264
mg/m3 group whereas hepatocellular degeneration was also found in
the 973 mg/m3 group. No changes were found in the kidneys or
epididymides.
In a further study, the degeneration and regeneration of the
olfactory epithelium were examined following exposure of a total of 84
rats to 778 mg methyl bromide/m3 for 6 h/day for 5 days (Hurtt et
al., 1988). Groups of five rats were sacrificed after days 1, 3, and
5 and after exposure weeks 1, 2, 3, 5, and 10. Cell replication rate
and histopathology were used to assess the kinetics of repair. In
addition, olfactory function was assessed using a buried food test.
Extensive damage to the olfactory epithelium was evident in animals
killed directly after 6 h of exposure. The specific site of damage
appeared to be in the olfactory sustentacular cells and mature sensory
cells, the basal cells generally being unaffected. By day 3, despite
continuous exposure, there was replacement of the olfactory epithelium
by a squamous cell layer that increased in thickness and basophilic
staining over the next 2 days. One week after exposure, the epithelial
region was covered by a layer of polyhedral, basophilic cells and from
2 to 10 weeks exposure, the epithelium exhibited progressive
reorganization to restore the original olfactory epithelium pattern;
75-80% of the olfactory epithelium appeared morphologically normal by
week 10.
In the same study, the ability of the rats to locate feed was not
affected by exposure to 350 mg methyl bromide/m3, but treatment with
778 mg methyl bromide/m3 rendered all animals temporarily incapable
of locating buried pellets, though they demonstrated searching
activity. Four to six days after treatment the animals recovered
sufficient olfactory function to find food pellets.
Similar results were reported by Hastings et al. (1989) and
Hastings (1990). Studies on Long-Evans rats showed that, after 4 h
exposure to methyl bromide (778 mg/m3), recovery of buried food was
greatly impaired but, even with continuous exposure, recovery occurred
until, by day 4 of exposure, olfactory function was essentially
normal. Extensive damage to the olfactory epithelium was evident after
day 1 of exposure; repair of the epithelium was in progress by day 4.
The specific site of damage appeared to be in the olfactory
sustentacular cell population while the respiratory epithelium was
largely spared (Hastings et al.,1989; Hastings, 1990). Bolon et al.
(1990) suggested that prior exposure to methyl bromide as well as
caging conditions (e.g., inhalation of ammonia from soiled bedding)
could influence the olfactory epithelial response.
Evans & Hastings (1992) reported that methyl bromide induced an
olfactory function deficit in rats. The olfactory threshold to ethyl
acetate was measured in six rats using a conditioned suppression
behavioural protocol. Three out of the six rats showed an increase in
absolute threshold or threshold response variability after a single,
6-h exposure to 778 mg methyl bromide/m3.
F-344 rats (20 male + 20 female) were exposed (6 h/day; 5
days/week) to 0 or 622 mg methyl bromide/m3 (0 or 160 ppm) for 3,
10, or 30 days (Eustis et al., 1988). Toxicological end-points
assessed included clinical observations, mortality, body and organ
weights, haematology, clinical chemistry, urinalysis, gross pathology,
and histopathology. There were no apparent treatment- related changes
in any of the clinical chemistry and urinalysis analytes measured.
Treatment-related effects in rats included neuronal necrosis in the
brain, myocardial degeneration, olfactory epithelial degeneration and
atrophy, and testicular degeneration and atrophy.
A study by Eustis et al. (1988) confirmed the very steep
concentration-response curve for methyl bromide in rats (and mice).
The authors also found clear species and sex differences in
sensitivity to methyl bromide toxicity. When rats were compared with
mice, the order of susceptibility was female mice>male mice>male
rats> female rats. Species and sex-related differences in the
histopathology of certain organs were also observed.
Kato et al. (1986) exposed Sprague-Dawley rats for 11 weeks, 4
h/day, to 584 mg methyl bromide/m3. No abnormal clinical signs were
reported. Small necrotic foci and fibrosis were observed in the heart
muscle.
F-344 rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 73, 183, 455, or 1140 mg/m3 (0,
7.5, 18.8, 46.9, 117, or 293 ppm) for 13 weeks for 6 h/day, 5
days/week (Japanese Ministry of Labour, 1992). At 1140 mg/m3, there
was 100% mortality. At this concentration of methyl bromide, rats
exhibited decreased locomotor activity, piloerection, ataxia, tremor,
cyanosis, haematuria, and nasal and ocular discharge. No clinical
signs were reported at lower doses.
Haematological studies showed increased MCV in male and female
rats exposed to 455 mg/m3; there was also an increased number of
platelets in the male rats. Serum potassium was decreased in male rats
exposed to concentrations of 73 mg/m3 or more. Glutamic oxalacetic
transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were
significantly increased in female rats exposed to concentrations of
183, 455, and 1140 mg/m3. Serum glucose and creatinine levels were
significantly decreased in female rats at 455 mg/m3.
There were no abnormal histopathological findings in rats exposed
to methyl bromide concentrations of between 29 and 455 mg/m3. Rats
exposed to 1140 mg/m3 exhibited cerebellar degeneration and brain
necrosis, thymic haemorrhages and atrophy, renal tubular necrosis,
pneumonitis, myocardial damage, adrenal vacuolization, Harderian gland
pigmentation and metaplasia, and necrosis of the olfactory epithelium
(Table 49).
Thirteen-week inhalation toxicity studies were conducted in which
groups of ten male and ten female Wistar rats were exposed to methyl
bromide at target concentrations of up to 166 mg/m3 for 6 h/day, 5
days/week (Wilmer et al., 1983). No deaths occurred, and no abnormal
clinical findings were observed. Body weight gain was not affected in
any of the exposed groups. Leukocyte counts were 22% higher in high-
dose males than in male control animals. Plasma alkaline phosphatase
activity was lower in both high-dose males (32%) and females (53%)
than in controls, and the plasma albumin concentration was 10% higher
in high-dose females than in controls. The absolute and relative liver
weights were up to 16% lower for high-dose males and females compared
with controls. The only exposure-related histopathological change,
which occurred in the liver of high-dose male and female rats, was
characterized by small hepatocytes with homogeneous eosinophilic
cytoplasm. This alteration varied in extent from slight to severe and
was seen in 6/10 males and 7/10 females. The no-adverse-effect level
for these 13-week inhalation toxicity studies was considered to be 25
mg/m3.
Groups of 18 rats of each sex were exposed to up to 467 mg/m3
for 6 h/day and 5 days/week over 13 weeks (NTP, 1992). All rats
survived to the end of the studies, few or no clinical effects of
exposure to methyl bromide could be seen. Significant decreases in
body weight gain were noted in both sexes at 467 mg/m3 and in
females at 234 mg/m3. No consistent organ weight effects were
observed. Minor neurobehavioural changes were noted in both sexes in
the highest dosage group. Females, but not males, of this group had
significantly lower haematocrit, haemoglobin levels, and erythrocyte
counts compared with those of controls. Olfactory epithelial dysplasia
and cysts, characterized by irregularity in mucosal thickness and
focal cavitated spaces, respectively, were seen in both sexes at 467
mg/m3.
8.2.3 Dermal
There are no reports of studies on short-term dermal exposure.
8.3 Skin and eye irritation
Irish et al. (1940) noticed lacrimation in rats after inhalation
of methyl bromide levels above 10 000 mg/m3. Irritation of the eye
membranes in mice at concentrations of 3200 mg methyl bromide/m3 was
described by Balander & Polyak (1962). There are no reports on skin
effects in animals.
8.4 Long-term exposure
8.4.1 Oral
8.4.1.1 Rat
Rats were fed for 7-8 months on wheat grain or peanuts fumigated
with methyl bromide having residual bromide levels of 20 and 22-46
mg/kg, respectively. There were no effects on weight gain of the
animals, haemoglobin content, or red or white blood cell numbers.
However, a decrease in iodine and calcium levels in the blood and
abnormal changes in the thyroid and parathyroid glands were reported
(Vitte et al., 1970).
A two-year, oral, long-term toxicity and carcinogenicity study
was carried out on 60 male and 60 female F-344 rats per group fed
diets fumigated with methyl bromide (Mitsumori et al., 1990). Diets
containing 80, 200, or 500 mg total bromide/kg food (methyl bromide
concentration <20 mg/kg food) were fed, the controls receiving
commercial basal diet (containing 30 mg bromide/kg) and a diet
containing 500 mg potassium bromide/kg. No effects were observed on
the behaviour of the rats in any of the groups fed the fumigated or
KBr- containing diets. In rats fed the diets fumigated with methyl
bromide, there were no marked toxic changes, except for a slight
depression in body weight from week 60 onwards in males in the 500
mg/kg group. Tumour incidence was unaffected. Rats given a diet
containing KBr did not show any treatment-related changes. The
no-effect level was 200 mg/kg (equivalent to 6.77 mg total bromine/kg
body weight per day) in males. No effects were observed in females at
the doses studied.
8.4.2 Inhalation studies
Long-term exposure inhalation studies are summarized in Table 49.
8.4.2.1 Mouse
In a carcinogenicity study, 86 B6C3F1 mice/sex were exposed to
39, 128, or 389 mg methyl bromide/m3 for 6 h/day, 5 days/week, for
2 years with scheduled interim sacrifices at 6 months and 15 months
(NTP, 1992). Because of the high mortality early in this study in the
389 mg/m3 group (27/86 males, 7/86 females), the exposure to methyl
bromide at this level was stopped at 20 weeks and the surviving mice
were exposed to air for the rest of the study.
Terminal survival rates (Kaplan-Meier determinations) in the
control, 39 mg/m3, 128 mg/m3, and 389 mg/m3 groups were: males
82%, 74%, 80%, and 23%; and females, 71%, 82%, 90%, and 65%,
respectively. Significantly lower mean body weights of the highest
dosage group compared with controls appeared by week 11 and persisted
after termination of dosing at week 20 until the end of the studies.
The only biologically significant change appeared to be reduced
absolute and relative thymus weights in both sexes. Neurological signs
were also observed, mostly in the highest dosage group, including
tremors, abnormal posture (lateral curvature of the spine), and limb
paralysis. These symptoms generally persisted (NTP, 1992).
Exposure-related histological lesions occurred primarily in the 389
mg/m3 exposure group and included degeneration in the cerebellum,
cerebrum and heart, chronic cardiomyopathy, sternal dysplasia, and
either necrosis or metaplasia of the olfactory epithelium. There were
no neoplasms attributed to exposure to methyl bromide.
Quantitative neurobehavioural testing showed significant
differences in the behaviour of the high-dose males at 3 months. The
animals were less active and demonstrated a reduction in startle
response latency. Because of the high early mortality in high-dose
males, only males in the controls and two lower dosage groups were
tested after 3 months. After 6 months of exposure, the 389 mg/m3
females had significantly lower activity scores than females in other
groups, but their higher startle responses had disappeared. Although
at 9 months of exposure no behavioural differences were apparent,
lower activity and heightened startle response were again noted at the
2-year testing time (NTP, 1992).
Toxicity and carcinogenicity studies were conducted by inhalation
exposure of groups of 50 male and 50 female Crj:BDF1 mice (6 h/day, 5
days/week) to methyl bromide (99.9% pure) for 104 weeks (Japanese
Ministry of Labour, 1992). Methyl bromide concentrations of 0, 16, 62,
and 250 mg/m3 were used.
Body weight gains in male and female mice exposed to 250 mg/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of atrophy (slight) of the granular layer of
the cerebellum were observed in male and female mice exposed to 250
mg/m3. There were no treatment-related neoplasms in male or female
mice.
8.4.2.2 Rat
Toxicity and carcinogenicity studies were also conducted by
inhalation exposure to methyl bromide (99.9% pure) of groups of 50
male and 50 female F344/DuCrj rats (6 h/day, 5 days/week) for 104
weeks (Japanese Ministry of Labour, 1992). Methyl bromide
concentrations of 0, 16, 78, and 389 mg/m3 were used. Body weight
gains in males and female rats exposed to 389 mg methyl bromide/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of necrosis and respiratory metaplasia of
the olfactory epithelium of the nasal cavity were observed in male
rats exposed to 389 mg methyl bromide/m3, and increased incidence
and severity of inflammation of the nasal cavity were observed in male
rats exposed at all concentrations used. Necrosis of the olfactory
epithelium and inflammation of the nasal cavity were marginally
increased in female rats exposed to 389 mg methyl bromide/m3. There
were no exposure-related increased incidences of neoplasms in male and
female rats.
In a long-term inhalation study, a total of 360 male and 360
female rats (Wistar) were exposed to concentrations of up to 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 29 months (Dreef-van
der Meulen et al., 1989; Reuzel et al., 1991). Non-neoplastic and
neoplastic lesions were scored in all control and high-level animals
of the main group, and the nose examined histopathologically at all
exposure levels. Mortality was higher in males and females at the 350
mg/m3 level than in controls from the end of the second year
onwards. Body weights in the 350 mg/m3 group (both sexes) were
slightly lower than controls from week 4 onwards. No essential
differences between groups were observed in haematology, clinical and
blood chemistry, and urine analysis at three months and one year. The
absolute brain weight was decreased in females at 350 mg/m3. A
higher incidence of haemothorax was seen in males and females at 350
mg/m3 than in controls. In the heart there was an increased
incidence of thrombi and myocardial degeneration in rats exposed to
350 mg/m3. There was hyperkeratosis of the oesophagus and
forestomach. The incidences of neoplastic lesions did not differ
significantly among the groups.
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction and embryotoxicity
McGregor (1981) conducted a sperm abnormality assay exposing
groups of 10 male B6C3F1 mice to air containing 0, 78, or 272 mg
methyl bromide/m3 (0, 20, or 70 ppm) for 7 h/day, for 5 days. No
sperm abnormalities were found at these concentrations.
Atrophy of seminal epithelium, incomplete spermatogenesis, and
giant cells in the seminal tubules, were detected unilaterally in one
rat (group of 10) after inhalation of 778 mg methyl bromide/m3 (200
ppm) (4 h/day; 5 days/week for 6 weeks) and in six rats (group of 10)
after inhalation of 1167 mg methyl bromide/m3 (same length of
exposure). In the tubules of the epididymis adjacent to the atrophied
testis, necrotic spermatocytes had accumulated in seminal fluid, but
spermatozoa were not seen (Kato et al., 1986).
Spermatogenesis and sperm quality were evaluated in the rat
(F-344/N) following exposure to 778 mg methyl bromide/m3, 6 h/day
for 5 days (Hurtt & Working, 1988). Although methyl bromide caused a
transient decrease in plasma testosterone and testicular nonprotein
sulfhydryl concentrations during acute exposure, no other reproductive
indices, including testis weight, daily sperm production, cauda
epididymal sperm count, sperm morphology, percentage motile sperm,
linear sperm velocity, and epididymal and testicular histology, were
affected by methyl bromide exposure.
Testicular degeneration and atrophy occurred in several rats and
mice following repeated (6 h/day, 5 days/week for up to 6 weeks)
inhalation exposure to 622 mg methyl bromide/m3 (Eustis et al.,
1988). In rats, degeneration included separation and sloughing of
spermatocytes and late stage spermatids and/or formation of
intratubular multinucleate giant cells. Atrophy was characterized by
variable loss of all components of the spermatogenic epithelium. In
exposed mice, degeneration of testes occurred frequently, but it was
not always severe.
Thirteen-week inhalation studies on sperm morphology and vaginal
cytology examinations (SMVCEs) in B6C3F1 mice and F-344 rats were
carried out by Morrissey et al. (1988). Results from mouse studies
(inhalation of 39, 156, or 467 mg methyl bromide/m3) showed a
decrease in terminal body weight, and a relative increase in the
weight of epididymis and testis. A decrease in sperm density and an
increase in the percentage of abnormal sperm were also noted. In rats
that inhaled 117, 233, or 467 mg methyl bromide/m3, a decrease in
terminal body weight as well as a decrease in the weight of the cauda
epididymis, and a relative increase in the weight of the testis were
noted. A decrease in sperm motility was also observed. In females,
exposure to methyl bromide did not affect the length of the estrous
cycle (Morrissey et al., 1988).
Female rats exposed to 272 mg methyl bromide/m3 (70 ppm) for up
to 40 days survived and reproduced without impairment (Hardin et al.,
1981; Sikov et al., 1981).
In a dominant lethal assay carried out in rats (McGregor, 1981),
groups of 10 male, adult CD rats were exposed to 0, 78, or 272 mg
methyl bromide/m3 for 7 h/day on five consecutive days, then
serially mated at weekly intervals for 10 weeks with untreated virgin
females in the ratio one male: two females. When the females were
sacrificed, their ovaries were examined for corpora lutea graviditatis
and the uteri were opened and examined for live implantations, late
deaths, and early deaths. The frequency of pregnancy was determined by
(a) females with corpora lutea graviditatis and (b) females with
implantations. With either method, methyl bromide did not cause a
significant decrease in the frequency of pregnancy, and the number of
corpora lutea per pregnancy and the frequency of early deaths were
unaffected.
The effects of inhalation exposure to 0, 12, 117, or 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week, for around 8 months, on
the growth, reproduction, and offspring in two consecutive generations
of CD Sprague-Dawley rats were investigated (American Biogenics
Corporation, 1986). Body weight depressions were noted in the males in
the highest concentration group at 5 of the 10 pre-mating collection
intervals and at final sacrifice. The pre-mating and total weight
gains were also less than those of the untreated control males. In the
F1 generation, parental body weight was comparable in exposed and
control animals. In the F2a litter, a slight body weight depression
was noted in gestating and lactating dams in the highest concentration
group compared with controls. Otherwise, maternal body weights were
comparable with those of the controls.
There was a marginal reduction in female fertility index in the
two top dose groups of the F2a litter. The mean numbers of pups
delivered viable were comparable with controls. Survival of the pups
was reduced in the 350 mg/m3 dose group in late lactation in the F1a
generation. Body weights of pups were reduced in both the 350 and 117
mg methyl bromide/m3 dose groups of the F1a, F2a and F2b
generations, though no consistent changes were observed in the F1b
generation.
No anomalies were noted in the treated progeny that were
attributed to methyl bromide exposure.
Gross pathological examination of parent animals from both
generations and randomly selected F1b and F2b progeny did not reveal
any treatment-related lesions. A statistically significant decrease in
the mean brain weight in the F0 males and an increased liver to body
weight ratio in both sexes were found in the upper concentration
group. In the F1 generation, both male and female brain weights were
decreased at 350 mg methyl bromide/m3.
Statistical analyses of the F1b progeny final body weight and
organ weight data revealed no significant differences. Statistical
reductions were found in the final body weight data obtained for the
F2b males (350 mg methyl bromide/m3) and F2b females (117 and 350 mg
methyl bromide/m3) compared with untreated control progeny. Analysis
of F2b progeny organ weight data revealed significant decreases,
compared with controls, for the female brain, heart, kidney, and liver
weights (350 mg methyl bromide/m3) and female liver weight (117 mg
methyl bromide/m3). In these two upper concentration groups, the
females brain to body weight ratio was increased and the liver to
brain ratio, decreased. Microscopic examination of the reproductive
organs and abnormal tissues did not reveal any treatment-related
lesions.
8.5.2 Teratogenicity
Female Wistar rats were exposed for 7 h/day, 5 days/week for 3
weeks to methyl bromide concentrations of 78 or 272 mg/m3. After
this time, they were mated. There was a total of 7 different exposure
groups (Table 48). Female rats whose vaginal smears showed evidence of
sperm were exposed for 7 h daily from day 1 (=day of finding sperm in
vaginal lavage) to 19 of gestation. The day before term (gestation day
21), they were sacrificed and necropsied. The results showed no
effects of the exposure on the female rats, nor were embryotoxic or
other teratogenic effects found (Sikov et al., 1981).
In a further series of studies by Sikov et al. (1981), groups of
24 female New Zealand white rabbits were exposed for 7 h daily to 78
or 272 mg methyl bromide/m3 (20 or 70 ppm) from the day of
artificial insemination. The 78 mg/m3 group was exposed up to day 24
of gestation; ecause of toxic effects, exposure had to be stopped on
day 15 for the 272 mg/m3 group, in which all but one pregnant rabbit
died. The remaining rabbits from the control and 78 mg/m3 exposure
group were sacrificed on the 30th day of gestation. The low exposure
group showed no fetotoxic or eratogenic effects. An evaluation of the
fetotoxicity or teratogenic results from the high dose group was not
possible because of the high mortality rate in the pregnant rabbits
(Hardin et al., 1981; Sikov et al., 1981).
In a preliminary teratology study (Breslin et al., 1990),
pregnant New Zealand White rabbits were exposed via inhalation for 6
h/day to 0, 39, 117, 195, 272, or 545 mg methyl bromide/m3 (0, 10,
30, 50, 70, or 140 ppm). Toxicity was observed in rabbits exposed to
545 mg methyl bromide/m3 and these were sacrificed before the end of
the study. No apparent embryotoxic effects were observed at any
exposure level (Breslin et al., 1990).
A subsequent study by the same investigators was conducted in two
parts. In Part I, groups of 26 inseminated New Zealand White rabbits
were exposed via inhalation to 0, 78, 156, or 311 mg methyl
bromide/m3 (0, 20, 40, or 80 ppm) on days 7-19 of gestation. In Part
II, groups of 17 inseminated rabbits were exposed for 6 h/day to 0 or
311 mg methyl bromide/m3 (0 or 80 ppm) on days 7-19 of gestation. An
additional group of 16 females, inseminated by a single male, acted as
controls. On day 28 of gestation, all surviving animals were
necropsied. Maternal liver, kidney, lung, brain weights, gravid
uterine weights, and the number of corpora lutea, implantations,
resorptions, and live/dead fetuses were noted. The fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
alterations.
Rabbits exposed to 311 mg methyl bromide/m3 showed moderate to
severe maternal toxicity. Decreased body weight and/or body weight
gain were noted. Clinical alterations included lethargy, right-sided
head tilt, ataxia, and lateral recumbency. The last three signs were
associated with significant histopathological lesions of the brain
(multifocal areas of inflammation of the meninges and/or bilaterally
symmetrical necrosis or spongiosis in the midbrain) in the
above-mentioned probe study with 545 mg methyl bromide/m3. At an
exposure level of 311 mg methyl bromide/m3, developmental effects
were observed consisting of decreased fetal weights, an increase in
the incidence of fused sternebrae, and an increase in the incidence of
malformations [18/23] (mostly missing gallbladder or missing caudal
lobe of the lung).
No adverse maternal, embryonal, or fetal effects were observed in
rabbits exposed to 78 or 156 mg methyl bromide/m3 (Breslin et al.,
1990).
Methyl bromide at 0, 0.5, 5, 25, or 50 mg/kg body weight was
administered in peanut oil, by gavage, to pregnant rats on days 5-20
of gestation (Peters et al., 1982). Signs of maternal toxicity were
evident in the two highest dose groups. Total resorption of embryos
was observed in the highest dose group and was considered to be the
result of the poor health of the pregnant rats and not a primary toxic
effect. In the control and 25 mg/kg groups, no effects on the skeleton
or internal organs were reported. In this study, methyl bromide was
not considered teratogenic and adverse effects on prenatal development
only occurred when maternal toxicity was present.
8.6 Mutagenicity and related end-points
Table 50 summarizes the results of tests for the genotoxic
activity of methyl bromide.
8.6.1 DNA damage
DNA adducts have been demonstrated in F344 rats, following oral
or inhalation administration 14C-methyl bromide. The adducts were
isolated from liver, lung, stomach, and forestomach and identified as
3-methylguanine, 7-methylguanine, and 0 6-methylguanine. Following
exposure by either route, the levels of adducts were higher in female
than in male rats and the guanine adducts were particularly prominent
in the stomach and forestomach (Gansewendt et al., 1991).
Alkylation of guanine-N-7 in the DNA of liver and spleen was
observed after treatment of male CBA mice with 14C-labelled methyl
bromide (4.9-5.0 mCi/mmol) by inhalation (340 mCi/kg body weight for
4 h) or i.p. injection (4.4 µmol/kg body weight) (Djalali-Behzad et
al., 1981). They noted that the extent of DNA alkylation in the liver
and spleen in vivo was 200 and 20 times lower, respectively, than
expected from the extent of the alkylation of haemoglobin and from the
relative reactivities of DNA and haemoglobin towards methyl bromide
in vitro.
Table 50. Mutagenicity tests with methyl bromide
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Reverse mutation Salmonella 0.02-0.2% +/- positive, Simmon et al.
typhimurium (in desiccators) TA 100 (1977)
TA 100, TA 98,
TA 1535, TA 1537,2 h
TA 1538
Reverse mutation Salmonella 500-5000 mg/m3, +/- positive, Moriya et al.
typhimurium 2 days TA 100, (1983)
TA 100, 1535
TA 98, 1537, 1538 TA 1535
Escherichia coli positive
WP 2 hcr
Reverse mutation Salmonella 500-50 000 mg/m3 +/- positive at Kramers et al.
typhimurium TA 100 (plate), 5 days 1900 mg/m3 (1985a)
TA 98 negative
SOS-umu (modified Salmonella 1.5 litre/min for positive Ong et al. (1987)
Ames test) typhimurium TA 1535/ 30 min
pSK 1002
Forward mutations Klebsiella pneumoniae 950-19 000 mg/m3, positive at Kramers et al.
streptomycin ur- pro- 20 h 4750 mg/m3 (1985a)
resistance
Forward mutation Escherichia coli 0.5-6 mmol/litre, 1 mutation/108 Djalali-Behzad et
Sd 4 1 h surviving al. (1981)
bacteria/mmol*h
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Forward mutation L5178Y mouse 0.03-30 mg/litre, positive Kramers et al.
24 h (1985a)
Sex-linked Drosophila 78 or 272 mg/m3, negative McGregor (1981)
recessive lethal melanogaster 5 h
Sex-linked Drosophila 750 mg/m3 (6 h) negative Kramers et al.
recessive melanogaster 375 mg/m3 (5 x 6 h) positive (1985a,b)
200 mg/m3 (15 x 6 h) positive
Sister chromatid Phytohaemagglutinin- 4.3% increased SCE Tucker et al.
exchanges (SCE) stimulated frequency from (1985)
human whole blood 10-16.84/cell
after 100-second
exposures
SCE cultured human 10-4-10-6 +/- positive Garry et al.
lymphocytes mol/litre (1990)
SCE bone marrow cells 47-778 mg/m3 dose response NTP (1992)
of exposed B6C3F1 6 h/day; 5 days/ observed at 14 days
mice week; 14 days and only; higher in
12 weeks females than males
same dose range negative
and exposure
Unscheduled DNA human diploid up to 70% +/- negative McGregor (1981)
synthesis fibroblasts 3 h
(human embryonic
intestinal cells)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Unscheduled DNA SPF male Wistar 10-30 mg/litre negative Kramers et al.
synthesis rats, primary liver (1985a)
Cell Syrian hamster 3890-31 120 mg/m3 negative Hatch et al.
transformation embryo cells 2-20 h in sealed (1983)
chambers
Chromosomal male and female CD 78 or 272 mg/m3 negative McGregor (1981)
aberrations (ex Sprague-Dawley) single 7 h or
rat bone marrow 7 h/day; 5 days
cells
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
BDF1 mice 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
(778 mg/m3) and
6-fold in females
(600 mg/m3); in
peripheral blood
increased 32-fold
in males (778 mg/m3)
and 3-fold in females
(600 mg/m3)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
F344 rats 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
and 3-fold in females
at 1314 mg/m3
Micronucleus in peripheral 47-778 mg/m3 elevated (only at NTP (1992)
erythrocytes of 6 h/day; 5 days/ 14 days) responses
B6C3F1 mice week; 14 days over entire dose
range with greatest
response at 389 and
778 mg/m3 in
females; males less
responsive
same dose range and negative
exposure routine;
13 weeks
Dominant lethal rats, male CD 78 or 272 mg/m3 negative McGregor
(Sprague-Dawley) 7 h/day; 5 days (1981)
Starratt & Bond (1988a) found that methylation of DNA in maize
and wheat took place during fumigation using 14C- labelled methyl
bromide (48 mg/litre for 72 h). They identified 7-methylguanine and
1-methyladenine as major products and lesser amounts of
3-methyl-cytosine and 3- methyladenine and 7-methyladenine; 0.5-1% of
the guanine residues in the DNA were methylated. Methylation of solid
samples of calf thymus DNA and salmon testes DNA gave similar results,
except that the quantity of 1-methyladenine exceeded that of
7-methylguanine (Starratt & Bond, 1988b).
In contrast, a different pattern of methylated bases was found
when solutions of DNA were treated with 14C-labelled methyl bromide.
Here, predominantly 7-methylguanine with a small amount of
3-methyladenine and only traces of 1-methyladenine and
3-methyl-cytosine were identified (Starratt & Bond, 1988b). The
authors noted that these results agreed better with the in vivo
studies of Djalali-Behzad et al. (1981).
An in vitro assay for unscheduled DNA synthesis (UDS) was
carried out in human diploid fibroblasts with exposures of 3 h and
concentrations up to 70% in air. No increase in UDS was found
(McGregor, 1981).
As measured by autoradiography, methyl bromide (tested at 10-30
mg/litre) did not induce unscheduled DNA synthesis in primary cultures
of rat hepatocytes treated in air-tight bottles (Kramers et al.,
1985a).
8.6.2 Mutation
Methyl bromide has been tested in various in vitro and in vivo
test systems (Table 50).
Methyl bromide was mutagenic to Salmonella typhimurium TA 100
when tested at concentrations of 0.02-0.2%, in desiccators, in the
absence of an exogenous metabolizing system (Simmon et al., 1977).
Positive results were also obtained in strain TA 100 in a liquid
assay (tested at 10-1000 mg/litre) and a plate assay (tested in closed
containers at concentrations of 500-50 000 mg/m3). Methyl bromide
was mutagenic at concentrations of 1900 mg/m3 and higher (plate
tests), and concentrations of 285 mg/litre (medium) and higher
(suspension test). The activity in the plate assay was unaffected by
the presence of liver homogenates from Aroclor(R)-induced rats
(Kramers et al., 1985a).
Methyl bromide (tested at 0.5-5 g/m3 in a closed container) was
mutagenic to S. typhimurium TA 1535 and TA 100 and to E. coli WP2
her, in the absence of an exogenous metabolic system (Moriya et al.,
1983).
An aqueous solution of methyl bromide (tested at 0.5-6 µmol/
litre) induced mutations to streptomycin independence in E. coli
Sd-4 (Djalali-Behzad et al., 1981).
Methyl bromide showed no mutagenic activity in a modified Ames
test using the impingement (in situ) test system, but, with the SOS
umu-test, this compound induced a significant SOS response, even
with only 30 min impingement (Ong et al., 1987). The SOS function
induced by genotoxic agents was detected by a colorimetric measurement
of beta-galactosidase activity encoded by the 1acZ gene, which is
regulated by the Umu operon.
Mutations to streptomycin resistance were induced in the
fluctuation test with Klebsiella pneumoniae at concentrations of
4750 mg methyl bromide/m3 and higher (tested at 950- 19 000 mg/m3)
(Kramers et al., 1985a).
In barley, a few mutations were induced after treatment of
kernels with 1.4 mmol methyl bromide/litre for 24 h, in closed vessels
(Ehrenberg et al., 1974).
A sex-related recessive lethal assay was carried out on
Drosophila melanogaster (McGregor, 1981). Male strain Oregon K
Drosophila were exposed to 78 or 272 mg methyl bromide/m3 for 5 h
and were mated on days 1,3, or 8 following exposure. The F1 progeny
from these matings were then mated brother to sister, 1-4 days after
emergence from pupae and the F2 generation was ex-amined for the
absence of wild-type males. At 78 mg methyl bromide/m3, the
frequencies of lethal mutations in the F2 generations from one of the
stocks were elevated, but this was not thought by the authors to be
compound-related as these were higher than those at the higher
concentration range.
In a sex-linked recessive lethal test on Drosophila
melanogaster , flies of the Berlin K strain were exposed to methyl
bromide at concentrations of 70-750 mg/m3 for increasing periods;
mutation frequencies were significantly increased at the highest
nontoxic concentrations. At a concentration of 600 mg/m3, all flies
died within a short time during the fourth day of exposure (Kramers et
al., 1985a). Prolongation of the exposure time permitted lower
concentrations to be detected as mutagenic; 487 mg/m3 for 5 x 6 h
and 200 mg/m3 for 15 x 6 h were effective exposures whereas
treatment with up to 750 mg/m3 for 6 h was not sufficient to produce
significantly increased mutation frequencies. The mutagenic effect of
methyl bromide was most pronounced in postmeiotic germ cell stages
(Kramers et al., 1985b).
Treatment of L5178Y mouse lymphoma cells with 0.03-30 mg methyl
bromide/litre, in air-tight bottles, resulted in a dose-related
increase in 6-thioguanine- and bromodeoxyuridine-resistant mutants
(Kramers et al., 1985a).
8.6.3 Chromosomal effects
8.6.3.1 In vitro studies
Exposure of human lymphocyte cultures to 4.3% methyl bromide for 100
seconds increased the frequency of sister chromatid exchanges (SCEs)
from 10.0 to 16.8 per cell (Tucker et al., 1985, 1986).
Human G0 lymphocytes were treated with methyl bromide (0-24
µg/ml) for 30 min with, and without, addition of rat liver homogenate.
After culture, the prepared slides were studied and dose-related
sister chromatid exchanges (SCEs) and chromosome aberrations (CAs)
were found. Methyl bromide significantly induced chromosome
aberrations in the presence of S-9 (Garry et al., 1990).
Inhalation of methyl bromide gas induced mitotic recombination in
somatic cells (somatic wing-spot assay) of Drosophila melanogaster
(Katz, 1985, 1987). Third instar larvae trans-dihybrid for two
recessive wing-hair mutations were exposed via inhalation to methyl
bromide (0-20 000 mg/m3) for 1 h. Wings of surviving adults were
scored for the presence of clones of cells possessing malformed wing-
hairs. Small and large, single (indicating a variety of genetic
alterations) as well as twin spots (from mitotic recom bination) were
found.
8.6.3.2 In vivo studies
The results of in vivo mammalian tests for chromosomal
aberrations in rat bone marrow were negative (McGregor, 1981).
Micronuclei formation was studied on F-344 rats and BDF1 mice
exposed to 0, 600, 778, 1011, 1314, or 1712 mg methyl bromide/m3 (0,
154, 200, 260, 338, or 440 ppm) for 6 h/day and 5 days/week for 14
days (Ikawa et al., 1986). In the surviving mice, poly-chromatic
erythrocytes with micronuclei in the bone marrow increased 10-fold in
males (778 mg/m3) and 6-fold in females (600 mg/m3) and those in
peripheral blood increased 32-fold in males (778 mg/m3) and 3-fold
in females (600 mg/m3). In rats, poly-chromatic erythrocytes
containing micronuclei in the bone marrow increased 10-fold in males
and 3-fold in females (both 1314 mg/m3).
Increases in SCEs and micronuclei were observed in the bone
marrow cells of male and female B6C3F1 mice exposed via inhalation to
a concentration of 778 mg methyl bromide/m3 (200 ppm) for 14 days (6
h/day, 5 days/week), the increases were more pronounced in female
mice. In contrast, no significant increases in either SCEs or
micronuclei were observed in male or female mice exposed via
inhalation to a concentration of 467 mg methyl bromide/m3 (120 ppm)
for 13 weeks (NTP, 1992).
8.6.4 Cell transformation
Transformation in Syrian hamster embryo cells by SA/adenovirus
was not enhanced by exposure to 4000-16 000 mg methyl bromide/m3
(1000-4000 ppm) for 2 or 20 h, in sealed chambers (Hatch et al.,
1983).
8.7 Carcinogenicity and related end-points
8.7.1 Gavage studies
Danse et al. (1984) administered methyl bromide, by gavage, to
groups (10 male + 10 female) of weanling Wistar rats for 90 days at
doses of 0.4, 2, 10, or 50 mg/kg body weight. At the highest dose
level of 50 mg/kg, squamous cell carcinomas of the forestomach
developed in 13 out of 20 animals. A marked diffuse hyperplasia of the
epithelium of the forestomach was seen in all animals in this group.
However, from subsequent examination of the slides from this study it
was concluded that the forestomach lesions reported at 50 mg
represented inflammation and hyperplasia rather than malignant lesions
(Pesticide & Toxic Chemical News, 1984).
Boorman et al. (1986) administered methyl bromide in peanut oil
(50 mg/kg body weight), by gavage, to groups of 15 male Wistar rats
for 13 weeks (five times a week) with a 12 week recovery period; other
groups were exposed for 17, 21, or 25 weeks to methyl bromide;
necropsies were performed at 13, 17, 21, and 25 weeks. At week 13,
inflammation, acanthosis, fibrosis, and pseudo- epitheliomatous
hyperplasia in the forestomach were observed microscopically in dosed
animals. At week 25, all rats had hyperplastic lesions of the
forestomach that were more severe than those at 13 weeks. Evidence of
malignancy, seen in one rat, was considered to be a very early
carcinoma. In the stop treatment group that had received methyl
bromide for 13 weeks, there was regression of the stomach lesions, but
at the 12-week final sacrifice, adhesions, fibrosis, and mild
acanthosis remained (Table 43).
Hubbs & Harrington (1986) reported similar results. Gross and
microscopic alterations were most pronounced in the non-glandular part
of the stomach of the treated rats. Histological change in the
squamous epithelial portion of the stomach included ulceration and
pseudoepitheliomatous hyperplasia characterized by hyperkeratosis,
acanthosis, and epithelial peg formation. Fibrosis, foreign material
(hair) and subacute to chronic inflammation were found in the
muscularis mucosa, submucosa, and tunica muscularis of some rats. A
30-60 day recovery period was accompanied by marked, but incomplete,
regression of lesions. No evidence of malignancy was seen in the
stomachs of treated rats (Hubbs & Harrington, 1986).
A two-year, oral, carcinogenicity study was carried out on 60
male and 60 female F-344 rats fed on diets fumigated with methyl
bromide (Mitsumori et al., 1990). Diets contained 80, 200, or 500 mg
total bromide/kg, respectively, (methyl bromide concentration being
<20 mg/kg), the controls were fed commercial basal diet (containing
30 mg bromide/kg) and a diet containing 500 mg potassium bromide
(KBr)/kg. No carcinogenic effects were observed (see also section
8.4.1.2).
8.7.2 Inhalation studies
In lifetime, inhalation, carcinogenicity studies (Table 49),
groups of 90 male and 80 female Wistar rats were exposed to methyl
bromide concentrations of up to 350 mg/m3 for 6 h/day, 5 days/ week,
for up to 130 weeks (Dreef-van der Meulen et al., 1989; Reuzel et al.,
1991). Methyl bromide was a mild nasal irritant at all exposure
concentrations. There was no increased incidence of neoplasms.
In a 2-year inhalation study (NTP, 1992) on male and female
B6C3F1 mice exposed to 39, 128, or 389 mg methyl bromide/m3 (Table
49), there was no evidence of carcinogenic activity in either male or
female mice.
8.8 Special studies
8.8.1 Target organ effects
8.8.1.1 Inhalation studies
Studies on laboratory animals have shown that, following
inhalation exposure, the primary target organs are the brain, kidney,
nasal cavity, heart, adrenal gland, lung, liver, and testis (Irish et
al., 1940; Hurtt et al., 1987; Eustis et al., 1988) and lung (Bond et
al., 1985; Kato et al., 1986; Jaskot et al., 1988) (Table 48).
Extensive target organ studies have been carried out by Eustis et
al. (1988) on B6C3F1 mice and F-344 rats after inhalation of 622 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 6 weeks. The
histopathological changes observed were as follows:
Brain: neuronal necrosis in the internal granular layer of the
cerebellar folia (mice); neurosis and loss of neurons in the cerebral
cortex, hippocampus, and thalamus (rats).
Kidney: nephrosis, which occurred in all treated mice, was
charac-terized by degeneration, necrosis, and sloughing of the
epithelium of convoluted tubules in the renal cortex. In rats, there
was dilatation of tubules with atrophy of the epithelium, hyaline and
granular casts in the tubules, and increased cytoplasmic basophilia,
indicative of epithelial regeneration. There was minimal nephrosis in
one female rat.
Testes: degeneration of the testes occurred frequently in exposed
mice and mild bilateral atrophy was present in two. Degeneration and
atrophy of seminiferous tubules of the testes was noted in several
exposed rats.
Nasal cavity: exposed male mice had various levels of degeneration
and atrophy of the nasal olfactory epithelium. Minimal degeneration of
the olfactory epithelium was seen in only one female mouse. In male
and female rats killed after three exposures to methyl bromide, there
was moderate to marked degeneration of the olfactory epithelium of the
ethmoturbinates and posterior dorsal nasal septum. There was no
inflammatory response. In rats, killed or dying after 10 or more
exposures, actual degeneration of the olfactory epithelium was minimal
or mild, but there was focal or multifocal loss of olfactory sensory
cells.
Heart: degeneration of the myocardium primarily occurred in treated
males with minimal myocardial degeneration in two female mice.
Degeneration of the myocardium occurred more frequently and with
greater severity in treated than in control male and female rats.
There were increased numbers of mononuclear and fusiform nuclei that
may represent interstitial cells, the foci showing a relative increase
in fine reticular fibres, and scattered clear vacuoles.
Adrenal gland: exposed female mice showed diminished cellularity of
the x-zone of the adrenal cortex and the affected cells contained less
cytoplasm and smaller, more hyperchromatic nuclei than normal cells.
Minimal to mild cytoplasmic vacuolation occurred in the adrenal cortex
of exposed rats.
Liver: individual cell necrosis was noted in the liver of several
treated rats and was generally more severe in affected males than
females. In three males and one female, an inflammatory reaction
occurred. There were no data for mice.
Thymus and spleen: thymic atrophy and lymphoid depletion of the
spleen was noted in mice and rats of both sexes.
8.8.2 Neurotoxicity
Irish et al. (1940) examined the effects of a wide range of
inhalation exposure conditions in rats, rabbits, guinea- pigs, and
monkeys, using visual inspection to assess behavioural deficits. They
observed that nearly 60% (34 out of 58) of the rabbits surviving
exposure to 128 mg/m3 (33 ppm) developed hindlimb paralysis during
the 6-month exposure period. This was the lowest concentration at
which definite neurobehavioural effects could be detected in the
rabbit, the other species being unaffected at this concentration.
Anger et al. (1981) studied the neurobehavioural effects of long-
and short-term methyl bromide inhalation exposure on Sprague-Dawley
rats and New Zealand White rabbits using more sensitive tests than
those available in 1940 when the study of Irish et al., was performed.
Exposure to 252 mg/m3 (65 ppm) for weeks caused sig-nificantly
reduced eye blink responses and nerve conduction velocity in rabbits,
but not in rats. No effect on nerve conduction velocity, open-field
activity, or coordination in rats could be seen after extended
exposure at 214 mg/m3 (55 ppm) for 36 weeks. In further studies
(Russo et al., 1984), rabbits did not show any neurobehavioural
effects after inhalation exposure to 105 mg methyl bromide/m3 (27
ppm) for 7.5 h per day, 4 days per week over 8 months. A separate
group of rabbits exposed to 252 mg/m3 exhibited severe neuromuscular
effects followed by partial recovery, 6-8 weeks after exposure ceased.
Behavioural effects in rats following repeated exposure to methyl
bromide at concentrations of 778 or 1167 mg/m3 (200 or 300 ppm) for
3 weeks were investigated by Ikeda et al. (1980). Relatively prolonged
(12 days) motor incoordination (rotarod) was observed.
Conditioned taste aversion induced by inhalation exposure to
methyl bromide in rats has been reported (Miyagawa, 1982). Rats kept
under a water restriction schedule for 7 days, were permitted access
to 0.3% (w/v) sodium saccharin, and were exposed to 0, 97, 195, or 389
mg methyl bromide/m3 (0, 25, 50, or 100 ppm) for 4 h. Three days
after exposure, saccharin preference tests were carried out, revealing
dose-dependent saccharin aversion in the exposure group (Miyagawa,
1982).
Male Swiss-Webster mice exposed to methyl bromide concentrations
up to 3500 mg/m3 showed no effects on single-task passive avoidance
but had significantly lower rotarod performance (Alexeeff et al.,
1985).
Effects of methyl bromide on locomotor activity were observed in
five groups of three rats after exposure to 245, 486, 731, or 972
mg/m3 (63, 125, 188, or 250 ppm) for 8 h. The locomotor activity was
measured in an activity cage before, and immediately after, the
exposure, shown as an average count per hour in 12-h blocks (day -2
(before exposure) and day 1) or 4-h blocks (day 0). In the 245 mg/m3
group, the activity was the same as that in the control group. The
activity was decreased by 731 mg/m3 and in-hibited strongly by 972
mg methyl bromide/m3 for up to 6 h after exposure (Honma et al.,
1985).
The effects of methyl bromide exposure on the thiopental-induced
reduction of righting reflex of rats was investigated. Three groups of
eight rats were exposed to 0, 245, or 486 mg/m3 (63 or 125 ppm) and
then were injected i.p. with 60 mg thiopental/kg to induce a loss of
righting reflex. The time of loss of righting reflex was measured and
group means were calculated. In the control group, two out of eight
rats lost the righting reflex, while in the 245 and 486 mg methyl
bromide/m3-exposure groups, the loss of righting reflex was observed
in all rats and was considerably more prolonged (Honma et al., 1985).
Kato et al. (1986) described neurological signs in rats, such as
paralysis and ataxia, after exposure to 1167 or 1556 mg methyl
bromide/m3, for 4 h/day over 6 weeks (section 8.4).
The acute effects of methyl bromide on electroencephalographic
activity and on sleep-wakefulness and circadian rhythms have been
investigated in rats (Tanaka et al., 1988). Slowing of the EEG
frequency in the wakefulness (W) stage and spike-wave activity
appeared at a single subcutaneous dose of 135 mg methyl bromide/kg
body weight. These abnormal EEG activities did not occur at lower dose
levels. Administration of methyl bromide at doses of 45, 15, or 5
mg/kg produced dose-related changes in amounts of W, non-REM sleep,
and REM sleep and in their circadian rhythms. Pretreatment with
glutathione effectively lessened the detrimental effects of methyl
bromide on sleep-wakefulness and its circadian rhythms and increased
the LD50 (Tanaka et al., 1988).
The effects were studied on regional brain glutathione- S-
transferase (GST) activity and the concentrations of glutathione
(GSH), monoamines, and amino acids in F-344 rats exposed to methyl
bromide (Davenport et al., 1992). Exposure to 584 mg/m3 was for 6
h/day for five days. While no histological evidence of brain lesions
was noted, there was GSH depletion and GST inhibition in the frontal
cortex, caudate nucleus, hippocampus (examined for GSH only), brain
stem, and cerebellum. No significant changes were observed in the
concentrations of monoamines (only measured in males), but there were
increases in the concentrations of aspartic acid and glycine in the
frontal cortex and of aspartic acid only in the cerebellum (only
measured in males).
Honma et al. (1982) exposed groups of 5-6 SD rats to methyl
bromide concentrations of 39-467 mg/m3 for 24 h or to 4-40 mg/m3
for 3 weeks. After sacrifice (microwave), the brain was dissected and
analysed for monoamines. A reduction in the norepinephrine (NE)
contents of the hypothalamus and cortex + hippocampus was found at
concentrations of 390 mg/m3 and above (24-h study) or 39 mg/m3
(3-week study), whereas levels of dopamine (DA), sero-tonin (5HT), and
acetylcholine (ACh), were only slightly affected by exposure. In more
detailed studies, rats were exposed to 120, 240, 486, or 973 mg/m3
for 8 h (Honma, 1987; Honma et al., 1987). Decreases in DA and NE and
increases in the metabolites homovanillic acid (HVA) and
3-methoxy-4-hydroxyphenylglycol (MHPG) were observed in various
regions of the CNS in rats exposed to 319 mg/m3. The maximal effects
were obtained 0 or 2 h after exposure with the largest changes in DA
(-24%) and NE (-34%) seen in the striatum. HVA and MHPG levels were
increased primarily in the frontal cortex (81%) and striatum (38%).
The tyrosine hydroxylase (TH) activity in the striatum, hypothalamus,
frontal cortex, midbrain, and medulla oblongata was measured in brain
homogenates from rats exposed to 62-973 mg/m3 (16-250 ppm) for 8 h
(Honma et al., 1991). The rats were sacrificed serially (decapitation)
between 0 and 16 h after exposure. TH was also measured in vivo
following administration of decarboxylase inhibitor. Exposure to
methyl bromide dose-dependently inhibited both TH activity in vitro
and in vivo . TH activity in the hypothalamus was most sensitive to
methyl bromide compared with the other brain areas. The authors
suggested that methyl bromide reduces catecholaminergic neuronal
activity in the brain via inhibition of TH activity.
The direct effects of methyl bromide on the rat brain have been
investigated using a two-probe (right striatum and left ventricle)
microdialysis method (Honma, 1992). Methyl bromide (1 µg or 0.5
µg/µlitre) dissolved in 10% ethanol/90% artificial cerebrospinal fluid
was perfused through the ventricle probe at a rate of 2.5 µlitre/min
and changes in 3,4-dihydroxyphenylacetic acid (DOPAC), HVA, and
5-hydroxyindoleacetic acid, a serotonin metabolite, (5HIAA) content in
the striatum perfusate were measured. DOPAC and HVA increased
following the initiation of ventricle perfusion but the 5HIAA content
of the striatum perfusate was reduced. The increases in DOPAC and HVA
persisted after perfusion and the decreased 5HIAA level returned to
normal. A concentration of 0.1 µg methyl bromide/µlitre had no effects
on DOPAC and HVA levels, though the 5HIAA level was reduced. These
findings support the findings of an increase in DOPAC and HVA in the
brain homogenate of rats exposed to methyl bromide (Honma, 1987; Honma
et al., 1987) indicating that dopamine metabolites in the
extracellular, and, probably, intracellular, regions of dopamine nerve
terminals were increased by methyl bromide itself. The extracellular
level of the 5HIAA in microdialized perfusate was reduced by
intraventricularly administered methyl bromide (Honma, 1992).
Systematic neuropathological studies of their central and
peripheral nervous systems were carried out on male Wistar rats
exposed to methyl bromide (1128 or 1945 mg/m3) for 6 h/day, 3
days/week for 3-8 weeks (Furuta et al. 1993). Among the rats exposed
to 1945 mg methyl bromide/m3 for 10-18 days, the following
neuropathological changes were noted: axonal degeneration of
myelinated fibres at the cervical level of the fasciculus gracilis and
the necrosis and atrophy of neurons in the caudate-putamen, thalamus,
and cingulate cortex. Rats exposed to a concentration of 1128 mg
methyl bromide/m3 for 8 weeks did not show any abnormalities.
8.8.3 Immunotoxicity
No data on the immunotoxicity of methyl bromide are available.
8.9 Factors modifying toxicity; toxicity of metabolites
Cysteine has been shown to reduce the toxicity of methyl bromide,
when administered to rats, mice, and rabbits, orally or s.c., 30 min
before, or s.c. within 5 min following, acute poisoning (Mizyukova &
Bakhishev, 1971). Cysteine treatment prevented the death, paralysis,
paresis, and spasms that developed on the 3rd-4th days after methyl
bromide inhalation in other animals.
8.10 Mechanisms of toxicity - mode of action
The mode of action of methyl bromide is still not understood.
Proposed mechanisms of toxicity include the direct cytotoxic effect of
the intact methyl bromide molecule or toxicity due to one of its
metabolites. The bromide ion concentrations are insufficient to
explain methyl bromide toxicity, but it may be related to its
alkylating ability.
Honma et al. (1985) concluded that the CNS toxicity seems to be
due to the methyl bromide molecule itself or the methyl moiety
incorporated into the tissue and does not appear to be attributable to
inorganic bromide or methyl alcohol (Honma et al., 1985). In humans
showing medium or severe toxic symptoms of the CNS, methanol
concentrations in the blood ranged between 200 and 3000 mg/litre
(Swartz et al., 1981).
The direct cytotoxic effect of methyl bromide on HeLa cells in
vitro has been shown, whereas bromide appeared not to cause cell
damage (Nishimura et al., 1980).
Methyl bromide reacted in vitro with a number of SH enzymes and
caused progressive and irreversible inhibition (Lewis, 1948). DNA and
protein alkylation by methyl bromide have previously been discussed
(section 6.3).
The role of glutathione (GSH) in reducing toxicity is also not
clear. Studies on the possible role of glutathione are given in
section 6.3. One hypothesis for the toxicity of methyl bromide
proposes the formation of a reactive species through a methyl
bromide-glutathione conjugation process, but it is more probable that
glutathione acts as a detoxifying agent.
9. EFFECTS ON HUMANS
Humans can be exposed to methyl bromide via inhalation, by skin
contact with the compound, or through residues in food that has been
fumigated with the gas to control pests. Exposure is also possible
through drinking- water from wells contaminated with leaching water.
In this section, only direct exposure to gaseous or liquid methyl
bromide is considered.
Since the first case reported by Schuler in 1899, there have been
hundreds of cases of methyl bromide poisoning involving fatalities,
systemic poisoning, skin and eye injuries, and damage to the central
nervous system (von Oettingen, 1946, 1964; Hine, 1969; Torkelson &
Rowe, 1981; Weller, 1982; Alexeeff & Kilgore, 1983).
Table 51 shows acute cases up to 1983 reported throughout the
world. Up to 1955, most cases were from chemical manufacture or fire
extinguisher incidents. Since this date, cases of poisoning from its
use as a fumigant have predominated.
9.1 Clinical findings
It is important to note that the manifestations of methyl bromide
poisoning may be delayed. The latent period may vary from 2 to 48 h
(Holling & Clarke, 1944).
The acute and long-term effects of methyl bromide can be divided
broadly into two categories: neurological and non-neurological.
The principal non-neurological symptoms reported after acute
inhalation of methyl bromide are associated with the respiratory
system. Chest pain or difficulty in breathing have been reported,
which is consistent with pathological findings at autopsy including
pulmonary oedema, bronchopneumonia, congestion, and haemorrhage
(Holling & Clarke, 1944; Hine, 1969).
In fatal poisoning, the early symptoms and signs are headache,
visual disturbances, nausea and vomiting, smarting of the eyes,
itching of the skin, listlessness, vertigo, and tremor. Progression is
usually rapid, with the development of convulsions, often with a
Jacksonian-type of progress, fever, tachypnoea associated with signs
of severe pulmonary oedema, cyanosis, pallor, and death. Several
neuropsychiatric signs and symptoms, such as mental confusion, mania,
muscular twitches, and slurring of speech, may precede death (Wyers,
1945; Sax et al., 1984; Gosselin et al., 1984).
Table 51. Methyl bromide poisoning incidents up to 1955 and between 1955 and 1983a
Resulting from: Fatalities Systemic Poisoning Skin Injuries Eye Injuries Other or unspecified injuries
up to 1955- up to 1955- up to 1955- up to 1955- up to 1955-
1955 1983 1955 1983 1955 1983 1955 1983 1955 1983
Chemical manufacture, 16 1 108 45 26 4 0b 0b
filling, storage,
transportation, or
disposal
Use or leaking fire 24 11 38 28 10 0 0b 0b
extinguishers
Uses as a fumigant 44 16 3 298 0 145 0b 57 0b 78
a Adapted from: Alexeeff & Kilgore (1983). Data up to 1955 were taken from von Oettingen (1955).
b No cases known.
The clinical picture in non-fatal poisoning is extremely
variable. Fatigue, blurred or double vision, nausea, and vomiting are
frequent; incoordination, tremors, convulsions, exaggeration of the
patellar reflexes, and a positive Babinski's sign may develop. Nearly
every type of nervous disturbance has been reported. The pulmonary
symptoms are comparatively slight (Sax et al., 1984). Low-level,
short-term exposures to the vapour have produced a syndrome of
polyneuropathy without overt central manifestations, characterized by
persistent numbness in the hands and legs, impaired superficial
sensation, muscle weakness, unsteadiness of gait, and absent or
hypoactive distal tendon reflexes (Kantarjian & Shaheen, 1963). Victor
& Adams (1980) stated that the signs and symptoms of chronic bromide
poisoning intoxication consisted of dizziness, drowsiness,
irritability, emotional lability, impairment of thought or memory,
and, in severe cases, delirium and mania or stupor or coma.
Locally, methyl bromide is an intense vesicant on human skin. The
blisters produced by methyl bromide are enormous, but rarely deep
enough to destroy the entire skin layer, even though it may produce
severe burns (Watrous, 1942; Butler et al., 1945; Wyers, 1945).
Both central and peripheral neurological deficits may persist;
organic brain syndrome has occurred (Greenberg, 1971; Goulon et al.,
1975). Profound psychological depression can occur during prolonged
convalescence (Hine, 1969). Late sequelae include bronchopneumonia
after severe pulmonary lesions, renal failure, and severe weakness
with, or without, evidence of paralysis. Renal failure due to tubular
necrosis may be a late sequela, but this is uncommon and usually of
mild proportions (Benatt & Courtney, 1948; Prain & Smith, 1952). There
have been occasional reports of jaundice, elevations of liver enzyme
activity in serum, and abnormal liver function tests suggestive of
mild hepatotoxicity after exposure to methyl bromide (Verberk et al.,
1979). In a 6- year-old boy, signs of liver involvement in conjunction
with encephalopathy after exposure to methyl bromide vapour were at
first mistaken for Reye's syndrome (Shield et al., 1977).
Recovery is frequently prolonged and there may be permanent
injury, commonly characterized by sensory disturbances, weakness,
disturbances of gait, irritability, and blurred vision.
The effects of methyl bromide on the nervous system, based on
case histories, showed that dysfunction affected the peripheral and
optic nerves, cerebellar connections, and certain spinal cord tracts
(Carter, 1945). Brain stem damage was described in a man who died
after 30 days of unconsciousness following methyl bromide exposure
(Cavanagh, 1992; Squier et al., 1992). Serum bromide levels, 2 days
after admission, were 160 mg/litre (normal 3-4 mg/litre). At
post-mortem examination, the brain showed mild generalized swelling,
normal ventricles, and well- defined symmetrical lesions, including
loss of neurones, in the mammilary bodies and inferior colliculi. The
cerebellar dentate nuclei had occasional foci of neuronal loss; other
brain stem regions were normal. The spinal cord was normal, but dorsal
root ganglia had scattered nodules of neuronal loss. Nerve roots and
thoracic peripheral nerve showed sparse chronic inflammatory exudate
and patchy axonal and myelin loss. The thyroid gland was densely
infiltrated with lymphocytes, plasma cells were rare, and there was no
significant fibrosis. The lungs were slightly oedematous and
congested. In another case of methyl bromide poisoning, a woman
remained in a coma for 4 years and 8 months before death. At
post-mortem, there was necrosis of both colliculi, with gliosis in the
upper brainstem, reticular formation, and moderate changes in the
dentate and pontine nuclei (Goulon et al., 1975).
Various visual disturbances have been reported following acute
methyl bromide poisoning. These include blurring of vision, diplopia,
lacrimation from eye irritation, accommodative disturbance, and
central scotomata for shades of green (Grant, 1974). Ocular findings
in a case of chronic methyl bromide poisoning included persistent
bilateral decrease in vision, temporal optic nerve head pallor, marked
attenuation of visual evoked response amplitude with normal latencies,
normal electro-retinogram but abnormal electro-oculogram, and
deuteranomalous (green) defect (Chavez et al., 1985).
Electrophysiological studies have been performed on patients as
part of a clinical investigation of the neurological effects of methyl
bromide poisoning (Goulon et al., 1975; Verberk et al., 1979; Audry et
al., 1985; Lopez et al., 1986a, b; Uncini et al., 1990; Mazzini et
al., 1992; Hustinx et al., 1993). Abnormal findings included
epileptiform patterns in electroencephalograms, enlarged and
asymmetric somatosensory evoked potentials, and evidence of axonal
neuropathy in peripheral nerves.
9.1.1 Bromide levels in body tissues and fluids
In cases of methyl bromide poisoning, methyl bromide itself has
been detected in human tissue on only one occcasion. This may be due
to the absence of methyl bromide or, more probably, to its very short
half-life in tissues, as well as difficulties in analysis. There is an
inconsistency in the data as to whether there is a correlation between
bromide levels and the symptoms of methyl bromide poisoning. Some
authors have suggested a direct correlation between blood bromide
levels and the degree of intoxication (Rathus & Landy, 1961; Hine,
1969): 400 mg/litre (ppm) - severe disability and death in some cases;
250 mg/litre (ppm) - convulsive seizure and sometimes death; 176
mg/litre (ppm) - slight residual ataxia; 135 mg/litre (ppm) - moderate
disability; 100 mg/litre (ppm) or less -recovery, but symptoms of
poisoning have been reported at bromide levels as low as 28 mg/litre
blood and severe symptoms at blood bromide levels of 120 mg/litre
(Rathus & Landy, 1961). Brenner (1978) and Bowers & Onoreski (1990)
considered that the toxic threshold level for bromide in serum in
humans was 500 mg/kg, though effects were observed in patients with
lower levels.
However, Weller (1982) disputed a correlation between the bromide
levels in serum and the severity of poisoning. In workers in a methyl
bromide manufacturing plant, no definite association was found between
symptoms and urine bromine concentrations (Kishi et al., 1991). A
direct association between serum bromine concentrations and the
severity of neurological symptoms was not found in a poisoning
incident involving greenhouse workers (Hustinx et al., 1993).
9.1.2 Dermal exposure
Dermal exposure can result from direct contact with liquid methyl
bromide, e.g., from accidental splashing, or through contact with
contaminated boots, clothing, bandages, or gloves (Alexeeff & Kilgore,
1983). These articles are often made of rubber, which can absorb
methyl bromide (Watrous, 1942). Direct eye injury or irritation can
also occur. Dermal exposure to gaseous methyl bromide can also cause
poisoning.
When liquid methyl bromide is spilled on the skin, it evaporates
rapidly producing a cool, but not a cold, or burning sensation
(Watrous, 1942). Repeated application of the liquid caused a burning
or tingling sensation in the skin, which, in more severe cases, led to
a sense of numbness, followed later by aching. In the early stages,
the skin appeared red and slightly swollen, with blisters and
second-degree burns appearing after 2-12 h in severe cases. In less
severe cases, an itching dermatitis may develop after a latent period
of about seven days (Watrous, 1942).
Cases of skin burns have been reported during fumigation (Bruhin,
1942) and from methyl bromide fire extinguishers (Butler et al.,
1945). Bruhin (1942) reported three cases of dermal exposure during
bulk fumigation of a mill. Methyl bromide poisoning occurred due to
percutaneous absorption; the men were using breathing apparatus. All
three men were exposed to the same conditions, they suffered first or
second degree skin burns and one died as a consequence of the
exposure.
Skin lesions occurred in 6 patients who were exposed to methyl
bromide during the fumigation of a castle (Zwaveling et al., 1987;
Hezemans-Boer et al., 1988). They had adequate airway protection, so
poisoning was by dermal exposure and not by inhalation. Exposure to
high concentrations of methyl bromide (about 40 g/m3) for 40 min led
to redness and blistering of the skin. Standard protective clothing
(overalls - 35% cotton/65% polyester), worn over normal clothing, PVC
gloves, and working shoes did not prevent this. Redness, oedema, and
blistering of the skin were limited to areas where perspiration is
relatively high, i.e., armpits, groin, genitals, and the skin under
the waistbelt. The authors suggested that methyl bromide absorption
might be increased in this partly lipophilic (sebaceous glands),
partly hydrophilic (sweat glands) environment, perhaps leading to
increased absorption through the skin as well. The skin in these parts
is also thinner, favouring absorption. Plasma bromide levels were
highest about 13 h after exposure (mean 9.0± 1.4 mg/litre) and fell in
subsequent hours (mean 6.8±2.3 mg/litre; 25 h after exposure),
suggesting absorption of methyl bromide through the skin. Since the
published half-life of bromide is about 100 h, the falls in plasma
levels must be due almost exclusively to the distribution of bromide
in tissues. No systemic effects were noted.
9.1.3 Inhalation
Inhalation is the primary route of exposure. Poisoning has
occurred following acute, short-, and long-term exposures. Acute cases
have occurred involving spilling or leaks while handling methyl
bromide or where the persons were unaware of its presence (Alexeeff &
Kilgore, 1983).
In spite of stronger regulations for the safe and licensed use of
methyl bromide, cases of poisoning still occur. Cases have been
reported in California (Edmiston & Maddy, 1987; Maddy et al., 1990)
and incidents of poisoning have been reported with occupational
exposure (Cantineau et al., 1988; Herzstein & Cullen, 1990), in
members of the general public (Goldman et al., 1987; Polkowski et al.,
1990), and in burglars entering fumigated premises (Marraccini et al.,
1983; O'Neal, 1987).
9.2 General population exposure
9.2.1 Poisoning incidents
Earlier poisoning incidents involving the general public were
mainly from the methyl bromide in fire extinguishers. More poisoning
incidents have involved unauthorized entry into fumigated buildings or
persons living near fumigated buildings, greenhouses, or fields being
fumigated with methyl bromide.
9.2.1.1 Poisoning associated with fire extinguishers
Incidents involving fire extinguishers filled with methyl bromide
often involved exposure to the gas by inhalation and severe burns when
the liquid was inadvertently squirted on the feet or clothing. Butler
et al. (1945) described two cases where drivers suffered burns after
extinguishing a fire in an armoured car. Both recovered after 2 and 8
weeks, respectively. Twenty-two cases of methyl bromide poisoning in
incidents involving fire extinguishers in ships, including 6
fatalities, were reported between 1939 and 1945 (Holling & Clarke,
1944; Clarke et al., 1945). In another incident, 8 boys, 6 of whom
died, were exposed accidentally to methyl bromide from a fire
extinguisher (Prain & Harvey Smith, 1952). Longley & Jones (1965)
described an accident during methyl bromide filling where there was
massive skin contamination. Although the victim removed the
contaminated clothing and washed himself thoroughly, symptoms and
signs of poisoning, commencing with severe nausea appeared within 2 h,
and CNS effects 5 h after the exposure. The CNS effects persisted and
6 months later there was still some disability. There were no dermal
effects apart from oedema and itching of the eyelids with subsequent
peeling of the skin and no evidence of pulmonary or renal involvement.
Goulon et al. (1975) reported a detailed study of three cases
where one patient died after 5 years in a stuporous state with
myoclonus. Two of these were females who had slept in bedrooms
containing fire extinguishers filled with methyl bromide; one of these
patients died, still in coma, 4 years and 8 months later. The other
case was a male lorry driver, who was found in a coma after spending
a night in his lorry cab, which contained a leaking fire extinguisher;
4 years later he showed only partial recovery with persisting lack of
coordination, ataxia, and dysarthria. Behrens & Dukes (1986) described
the case of a scrap-dealer who was poisoned by methyl bromide from
fire extinguishers in obsolete aircraft engines.
A case of poisoning and the resulting death of a man (and his
dog) from methyl bromide leaking from an old corroded fire
extinguisher has been reported (Cavanagh, 1992; Squier et al., 1992).
Since methyl bromide is no longer in general use for fire
extinguishers, the occurrence of such cases is now extremely rare.
9.2.1.2 Poisoning associated with bulk or house fumigation
In May 1978, severe methyl bromide poisoning occurred in 4 people
living above a warehouse in Japan (Ishizu et al., 1988). The
warehouse, containing herbs, was accidentally fumigated with a greater
quantity of methyl bromide than normal giving an estimated
concentration of 38 900-58 350 mg/m3 (10 000-15 000 ppm). Three days
later, one member of the family, a girl aged 12 years, developed
severe convulsions and then 2 others had severe convulsions and the
fourth had marked mental confusion. The serum or plasma bromide ion
levels ranged from 280 to 600 mg/litre. Clinical laboratory tests
showed that GOT exceeded the normal level in 3 out of 4 cases.
Moreover, the LDH activity was above the normal range in three cases
and CPK activity was increased in all the cases (Ishizu et al., 1988).
In California, the most frequent cause of death from
non-occupational exposure, in recent years, has been unauthorized
entry into structures under fumigation with methyl bromide. Most often
the entry was by burglars, transients, or intoxicated persons who
broke into buildings that were covered with gas resistant sheeting,
locked, and posted with warning signs. During 1982-87 there were 13
such fatalities (Maddy et al., 1990).
In Florida, where homes are fumigated to destroy termites by
placing a huge coloured tent over the house, the methyl bromide being
released by trained technicians, 27 non-fatal cases and four
fatalities following unauthorized entry were reported between 1957 and
1982 (Marraccini et al., 1983). The intervals between exposure and
death ranged between 2.5 and 36 h. Three of these deaths together with
three additional hospitalizations followed burglaries of tented houses
during a nine-month period.
O'Neal (1987) reported that, at one hospital in Florida, 15-25
patients a year, including fatalities, were treated for methyl bromide
poisoning. Proper diagnosis and therapy were hampered by the latency
period (4-48 h before onset of symptoms) and the fact that the toxic
state also mimics other diseases.
Even after apartments have been cleared for habitation, deaths
due to methyl bromide poisoning have been reported. The fumigant can
be absorbed by furniture, water beds, and walls, and can sequester in
enclosed spaces, with subsequent desorption (Dempsey et al., 1992).
Prockop & Smith (1986) reported a case in which a house fumigator
wearing a faulty protective face mask was exposed briefly on one day.
This was followed by malaise, although he continued to work, and then
again, ten days later, when tremor and increasing malaise resulted in
admission to hospital. On the next day, he was comatose with
generalized major motor convulsions, and myoclonic limb movements
between convulsions. On admission, the serum bromide level was 350
mg/litre (falling to 30 mg/litre 29 days later). Five years later, the
patient still exhibited dysarthria, impaired arm coordination, and
disturbance of gait.
A fatal case of methyl bromide poisoning was reported after
fumigation of a restaurant. Although the methyl bromide levels were
checked, for some reason the apparatus showed a reading of "no
detectable levels" and, without putting the ventilation system on, the
premises were declared safe. A restaurant employee, who then entered
the building, was found dead 2.5 h later; the post-mortem inorganic
bromide level in serum was 48 mg/litre. Four other workers who were in
the building for 45 min had blood bromide concentrations ranging from
40 to 51 mg/litre. Another worker who was in the restaurant for 1 h 15
min, had a blood bromide concentration of 101 mg /litre. These 5
workers had immediate symptoms of malaise and fatigue, but no chronic
symptoms (Fuortes, 1992).
A 13-year-old girl accidentally exposed to excessive methyl
bromide concentrations after a warehouse fumigation had early symptoms
of headache, dizziness, and nausea, followed a few hours later by
unconsciousness, from which she recovered three days later. At that
time, myoclonic jerks developed, the intensity of which progressed and
was unresponsive to treatment over a two-month period. In a two-year
follow-up, treatment with clonazepam and phenobarbitol decreased the
intensity of myoclonus and she was able to return to school.
Electrophysiological findings suggested that the methyl bromide
exposure had induced a type of cortical reflex myoclonus (Uncini et
al., 1990).
9.2.1.3 Poisoning associated with soil fumigation
Goldman et al. (1987) reported four episodes of community
exposure to methyl bromide and chloropicrin soil fumigation in
California in 1973, 1980, and two in 1984. A total of over sixty cases
were involved, and, in all episodes, either evacuation of homes or
cessation of fumigation was the outcome. In one episode, a strawberry
field was fumigated preplanting with a methyl bromide/ chloropicrin
formulation (dosage and % composition not given). Local weather
conditions included a temperature of 30 °C with an inversion; 32
adults and 4 children were treated with incident-related symptoms,
such as eye irritation, sore throat, headache, shortness of breath,
and cough. One child was hallucinating. Fumigant-related symptoms
dropped off sharply with distance, 30% within 1 km compared with 4.5%
more than 2 km from the field. It should be noted that these symptoms
could be attributed to either methyl bromide or chloropicrin.
A nurseryman was poisoned after glasshouses near his house had
been fumigated with methyl bromide (Bishop, 1992). The symptoms were
delayed. By day 3 after the fumigation, he had convulsions and became
comatose. There was a long recovery period and persistence of
neurological signs and symptoms.
9.2.1.4 Miscellaneous incidents
Inhalation of methyl bromide from leaking canisters caused acute
methyl bromide poisoning. Two grandparents and a child showed various
levels of poisoning. The man showed predominantly mental disturbances,
with only mild neurological signs and no convulsions; initial euphoria
and lack of concern progressed to a florid psychosis. His mental state
was normal 6 months later. The woman was severely affected and
remained in status epilepticus for 7 days. The boy had an upper
respiratory illness 24 h before methyl bromide poisoning, which might
have increased his susceptibility to the toxic agent. He developed an
acute encephalopathy with fluctuating conscious state, hypotonia,
araflexia, and extensor plantar responses. Before methyl bromide was
identified as the cause of the illness, Reye's syndrome had been
diagnosed (Shield et al., 1977).
A tractor trailer-truck overturned and a cylinder (max. capacity
680 kg) was punctured. Although people were warned of the risk, ten
were admitted to hospital. The symptoms included nausea, vomiting,
breathing difficulty, headache, dizziness, burning throat, coughing,
and chest tightness. The primary route of exposure was inhalation for
seven patients, dermal for two, and both dermal and inhalation for
one. All patients recovered without neurological sequelae (Polkowski
et al., 1990).
Leakage from a compressed gas cylinder caused unconsciousness and
status epilepticus in a man (Mazzini et al., 1992). After awaking, he
suffered from severe myoclonic jerks and major epileptic convulsions.
Six months later, he still had severe action myoclonus of the limbs.
A standardized neuropsychological evaluation revealed severe cognitive
impairment.
9.3 Controlled human studies
Raabe (1988) carried out an inhalation study using [14C]-
labelled methyl bromide up to 0.1 mg/m3 to determine the percentage
of methyl bromide absorbed by the human body from air containing
ambient concentrations of the gas. The uptake was 55.4% nasal and
52.1% oral (section 6.1.1.2 and Fig. 6).
9.4 Occupational exposure
Up to 1955, the majority of methyl bromide poisoning incidents
resulted from chemical manufacture and filling operations, followed by
fire extinguishers and fumigation (von Oettingen, 1955). Since 1955,
fumigation has become the major source of fatalities (Alexeeff &
Kilgore, 1983).
9.4.1 Occupational exposure during manufacture
Incidents of methyl bromide poisoning in industry have been
described by Watrous (1942) and Wyers (1945). The main incidents were
dermatological cases occurring principally among those who filled the
small cylinders for distribution from the larger ones. Other sources
of danger were defects in the plant, such as ill-fitting joints and
bursting of cylinders by an undue rise in temperature. Carelessness
with handling was also a cause.
Kishi et al. (1991) carried out a questionnaire survey to
determine the symptoms of workers exposed to methyl bromide in the
manufacturing process. Seventy five male workers (exposure length:
1-25 years) were compared with a reference group of railway workers.
The questionnaire covered acute symptoms during workshift (16
questions), and general symptoms (61 questions). Acute and general
symptoms were statistically higher in frequency among the exposed
workers (sign test of pairwise comparison). Methyl bromide
concentrations in the air in the workers' breathing zone were measured
every 6 months, and were normally less than 4 mg/m3, but, during
some accidental events, they exceeded 20 mg/m3. The mean bromide ion
concentration in the urine of men working in the manufacture of methyl
bromide was 18.9 mg/litre± 10.4 (range 3.2-54.0 mg/litre), with no
correlation with the symptoms reported.
Previous studies had shown that urinary bromide concentrations
were an index of relatively acute exposure, correlating with the air
concentration of the working site. The concentration in the work-place
air sometimes accidently exceeded 58 mg/m3. There were some cases of
sub-acute poisoning with lethargy, ataxia, and retrobulbar optic
neuritis. In one incident, the mean urine concentration of 20 workers
exposed to methyl bromide was 277 mg/litre. On that occasion, 14 of
the 21 exposed workers had abnormally accelerated tendon reflexes, 8
had paraesthesia, and 4 showed disturbance of the convergence reflex
of the eyes (Kishi et al., 1991).
9.4.2 Occupational exposure due to methyl bromide fumigation
In many countries, the use of methyl bromide is restricted to
trained and licensed personnel.
Occupational exposure to methyl bromide can occur during the
manufacturing, filling, and packaging processes, as well as during its
use as a fumigant. As described in section 3.2.2, methyl bromide is
used mainly for soil fumigation as well as for the fumigation of
buildings and commodities, to prevent pest infestation. The various
occupations involved are given in section 5.3.
Methyl bromide was used as a fumigant in the USA by an estimated
105 000 workers between 1972 and 1974 (NIOSH, 1984) and 75 000 workers
in 1980 (Anger et al., 1981).
9.4.2.1 Incidents involving bulk fumigation
A poisoning incident in a mill where three fumigators were
poisoned through dermal exposure to gaseous methyl bromide has been
described in section 9.1.2 (Bruhin, 1942).
Johnstone (1945) and Ingram (1951) reported incidents in
data-processing factories where over 200 employees were taken ill,
with over 50 cases of frank methyl bromide poisoning, including two
workers suspected of insanity. All 34 packers, described by Johnstone
(1945), had visual disturbances and there was a high incidence of
speech difficulties, mental confusion, hallucinations, and
paraesthesia. Four workers claimed permanent and six temporary
disabilities. The main factor in these poisonings was that the workers
ignored the necessary precautionary measures (Johnstone, 1945).
Kantarjian & Shaheen (1963) described 8 cases of polyneuropathy in
date factory workers who suffered repeated exposure to the vapour due
to faulty working techniques over a period of three months. Within 6
months all had recovered. Only 8 out of 14 employees developed
symptoms, suggesting a variation in susceptibility in different
individuals.
Seven workers employed in the bulk fumigation of houses were
poisoned by methyl bromide (Rathus & Landy (1961). The cause was the
insufficient absorption properties of the canisters in their
respirators. Three of the men were burned on the area of the face
covered by the respirators.
Drawneek et al. (1964) described a case of irreversible brain
damage in a methyl bromide fumigator. Seven other workers, without any
recognisable illness, were found to have serum bromide levels of over
50 mg/litre, at which level mild euphoria may develop and lead to
carelessness in the handling of methyl bromide, and, therefore, to
possible acute exposure.
Hine (1969) reported two acute and eight chronic cases of
fumigation-related poisoning in California from 1957 to 1966. Four of
the patients died. The most common symptoms were malaise, weakness,
and dyspnoea. The deaths were preceded by convulsions and coma. All
cases could be traced to a failure in preventative procedures.
A few hours after exposure, during the fumigation of cocoa beans,
a dock worker developed status epilepticus, coma, and pulmonary oedema
(Greenberg, 1971).
Ten cases of methyl bromide poisoning occurred in the hold of a
ship whilst a rice cargo was being fumigated in port (Brodniewicz,
1967). The accident was the result of several circumstances: sealing
of the crew's quarters was forgotten, some of the crew remained on the
ship during, and after, fumigation, and the port fumigating team
lacked experience and proper qualifications. One victim died following
convulsions, acute pulmonary oedema, and heart failure. In the other
cases, the main complaints were dizziness and nausea.
In a fatal case of methyl bromide poisoning of a ship's boy who
slept in the crew's quarters, the levels of methyl bromide measured
after fumigation had not exceeded 40 mg/m3, but the boy was
poison-ed by a high concentration of the gas leaking from the
fumigated commodity, because of large temperature differences (Weller,
1982).
An employee habitually not wearing a mask in a fumigating plant
spraying fruits and vegetables was initially treated for psychosis, as
the early symptoms of methyl bromide poisoning are similar (Zatuchni
& Hong, 1981).
Cases of dermal exposure occurring during the fumigation of a
castle have been reported (Zwaveling et al., 1987; Hezemans-Boer et
al., 1988). Details are given in section 9.1.2.
Systemic and neuro-ophthalmic manifestations of methyl bromide
poisoning including increased serum bromide level (66 mg/litre),
paraesthesia and burning dysesthesia on hands and feet, and visual
impairment, were described in an assistant fumigator who had been
exposed acutely, twice, during the year previous to examination
(Chavez et al., 1985).
9.4.2.2 Incidents involving soil fumigation
(a) Field workers
Table 52 shows occupational poisoning incidents, reported in
California, due to methyl bromide exposure in the years 1950, 1986,
and 1987, and analysed according to work activity (Edmiston & Maddy,
1987; Maddy et al., 1990).
Seven deaths through occupational exposure were reported in
California between 1951 and 1965. The distribution of cases was: 1952
(1), 1956 (2), 1958 (1), 1959 (2), and 1965 (1) (Maddy et al., 1990).
Methyl bromide poisoning of four field workers occurred during
the removal of soil fumigation sheets under cool weather conditions
(Herzstein & Cullen, 1990). Ten days after injection of methyl bromide
into the soil, the polyethylene sheets covering the soil were removed.
The 4 field workers developed fatigue and light-headedness and 3 had
progressive respiratory, gastrointestinal, and neurological symptoms.
The acute systemic symptoms improved over several days, but
neuropsychiatric symptoms that developed later persisted for several
weeks. The 2% chloropicrin was not enough to warn the workers of the
presence of methyl bromide.
(b) Greenhouse workers
Methyl bromide has been used extensively in the USA since the
1950s, and, in Western Europe, since the 1960s, in the fumigation of
soils in greenhouses. This can be hazardous to the health of the
workers because of the enclosed area in which methyl bromide is
applied (Roosels et al., 1981). Poisoning incidents have occurred in
operators and other people entering fumigated areas. An analysis of
eight severe cases was made by Van den Oever et al. (1978). The signs
and symptoms followed the usual pattern. Nearly all incidents were
associated with professional fumigation workers using the injection
method.
In a case of acute occupational methyl bromide intoxication, a
27-year-old man, using methyl bromide in a greenhouse, suffered
continuous epileptic convulsions for 3 weeks, followed by repeated
generalized convulsions. Myoclony of the face and the fingers
accompanied by a complete motor deficiency persisted; there was also
bilateral deafness (Cantineau et al., 1988).
In an accident involving nine greenhouse workers (2 women, 7 men;
aged 21-40 years), exposed to an inadvertent spread of methyl bromide
during fumigation, two patients needed intensive care for several
weeks because of severe reactive myoclonus and tonic-clonic
generalized convulsions (Hustinx et al., 1993).
Table 52. Summary of occupational cases of methyl bromide poisoning in California
as reported by physicians, listed according to work activity and type of illness/injury a
Year Work activity Systemic Eye Skin Eye/ Total
skin cases
1950 all occupational 3 0 0 0 3
exposures
1986 chamber fumigator 2 0 1 0 3
1987 chamber fumigator 2 0 0 0 2
1986 field fumigator 0 1 5 0 6
1987 field fumigator 1 1 4 0 6
1987b field fumigator 6 2 1 0 9
1986 "tarp" cover fumigator 4 0 1 1 6
1987 "tarp" cover fumigator 1 0 1 0 2
1987b "tarp" cover fumigator 1 0 0 0 1
1986 coincidental exposure 0 1 0 0 1
1987 coincidental exposure 4 0 0 0 4
1987b coincidental exposure 2 0 0 0 2
1987 emergency response 2 1 0 0 3
1987b personnel 1 0 0 1 1
a 1950 and 1987 figures taken from Maddy et al. (1990);
1986 figures taken from Edmiston & Maddy (1987)
b A methyl bromide/chloropicrin formulation was used.
9.4.3 Studies measuring the levels of bromide ion in biological
fluids and tissues
There have been a number of studies to investigate whether there
is a correlation between methyl bromide exposure levels and bromide
ion concentrations, but the results are conflicting.
9.4.3.1 Manufacturing
Ohmori & Hirata (1982), using radioactivation analysis, reported
mean bromine concentrations in serum (66 µg/g) and hair (11 µg/g) from
14 methyl bromide workers, and mean bromine concentrations in serum
(40 µg/g) and hair (4 µg/g) in controls. A worker suspected of methyl
bromide poisoning had a level of 412 µg/g serum, 13 days after
exposure.
Average bromide ion concentrations detected in 36 urine samples
of workers exposed to methyl bromide were 13.3±7.7 mg/litre compared
with 7.1±2.1 mg/litre in a non-exposed group (Koga et al., 1991). The
atmospheric methyl bromide concentrations of exposed workers were
monitored with passive samplers during their work shifts (8 h). No
significant correlation was found between these methyl bromide
readings and the bromide ion concentrations in urine.
9.4.3.2 Fumigation
Routine monitoring of bromide levels in the blood of a group of
fumigators, undertaking both bulk and soil fumigation, was carried out
in England between 1971 and 1979 (Cornwell, 1979). It was found that
the plasma bromide levels between January and the end of the year were
correlated with the number of gas applications, irrespective of the
type of fumigation work undertaken (Fig. 10).
The plasma bromide level was thought to rise as a result of repeated
exposure to low concentrations of methyl bromide with small amounts
accumulating in the body faster than the rate of elimination. During
the Christmas period, when all the men took a break at the same time,
the plasma bromide levels were substantially reduced. Results from
October 1974 to January 1978 indicated that average plasma levels
increased from 15 mg/litre in January to 25 mg/litre in October. Some
fumigators carried out disproportionally more treatments of stacks,
containers, and lighters, and reached plasma bromide levels of 30-60
mg/litre. A relationship between plasma bromide levels and the number
of fumigation applications was found (Fig. 11).
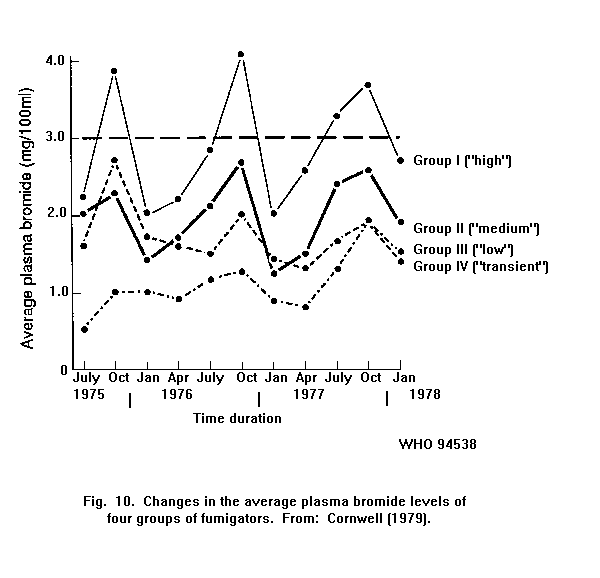 Blood bromide levels, EEGs, and transaminases were measured in 33
methyl bromide workers engaged in soil disinfection inside
greenhouses. Blood bromide varied between 4 and 23 mg/litre (Verberk
et al., 1979).
In a study of the bromide ion concentration in the plasma of 39
methyl bromide workers, a mean level of 6.9 mg/litre was measured
compared with controls (100 workers) who had a mean of 3.7 mg/ litre
(Yamano et al., 1987).
Blood bromide levels, EEGs, and transaminases were measured in 33
methyl bromide workers engaged in soil disinfection inside
greenhouses. Blood bromide varied between 4 and 23 mg/litre (Verberk
et al., 1979).
In a study of the bromide ion concentration in the plasma of 39
methyl bromide workers, a mean level of 6.9 mg/litre was measured
compared with controls (100 workers) who had a mean of 3.7 mg/ litre
(Yamano et al., 1987).
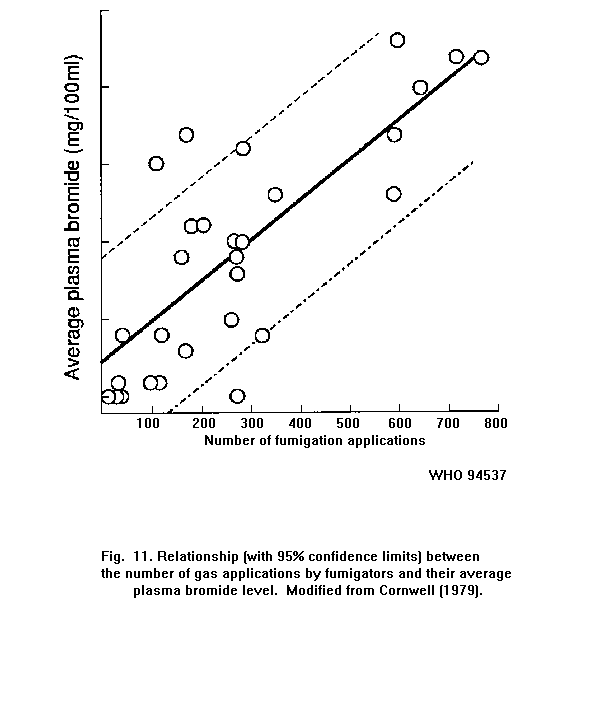 Tanaka et al. (1991) measured ambient methyl bromide
concen-trations and urinary bromine concentrations at various plant
quarantine fumigation sites in Japan. The geometric mean for exposed
workers was 9.0±1.85 mg/litre while the arithmetic mean for a matched
control population was 6.3±2.5 mg/litre. The authors found a
statistically significant positive correlation between the ambient
methyl bromide concentrations collected with a personal sampling
device and the urinary concentrations.
9.4.4 Haemoglobin adducts as a biological index to methyl bromide
exposure
Iwasaki et al. (1989) determined the haemoglobin adduct
(haemoglobin MeCys) in methyl bromide manufacturing workers, and
examined its effectiveness as a biological index of exposure to methyl
bromide. Methyl bromide reacts with cysteine to form
S-methylcysteine (MeCys) in haemoglobin (Djalali-Behzad et al.,
1981). It was suggested that determination of MeCys in haemoglobin
could detect previous methyl bromide exposure levels so low that they
were not detected by a special medical check-up (Iwasaki et al.,
1989). Haemoglobin adducts have a life span of about 2 months, so
workers only intermittently exposed to methyl bromide would also be
detected in such a survey. Previous studies by the same authors showed
individual differences in mice in the levels of MeCys within each dose
and time series group (Iwasaki, 1988a,b). These individual differences
may be caused by the differential susceptibility of haemoglobin in red
blood cells or individual differences in methyl bromide metabolism.
These differences were found to be minor in the human study (Iwasaki
et al., 1989).
Results from animal studies (Xu et al., 1990) supported the
suggestion of Iwasaki et al. (1989) that the Hb adduct of methyl
bromide might be a useful parameter in the biological exposure
monitoring of methyl bromide workers.
9.4.5 Neurobehavioural and other studies
Anger et al. (1986) carried out a neurobehavioural study on soil
fumigators who were exposed to 9 mg methyl bromide/m3 over an 8-h
day and structural fumigators who were exposed to 0-9 mg/m3 for 1.5
h per day. The fumigators, who reported a significantly higher
prevalence of 18 symptoms, consistent with methyl bromide toxicity,
than the controls, did not perform so well on 23 out of 27 behavioural
tests, and were significantly lower on one test of finger sensitivity
and one of cognitive performance.
EEGs and transaminases were measured in 33 methyl bromide workers
engaged in soil disinfection inside greenhouses in the Netherlands.
Symptoms of methyl bromide intoxication were determined by means of a
specially designed questionnaire. A general neurological examination
was performed. No differences compared with controls were found, with
the exception of slight electro-encephalographic changes in 10 workers
involving diffuse increase of beta and theta activity. Workers with
abnormal EEGs had higher blood bromide levels (geometric mean 10.9
mg/litre compared with a mean of 8.2 mg/litre in the other workers;
the difference was statistically significant ( P <0.05) (Verberk et
al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human exposure
Human exposure to methyl bromide occurs predominantly as an
occupational hazard, particularly during soil or bulk fumigation, but
also during manufacture. Individuals in the vicinity of fumigated
fields or buildings may also be exposed. Methyl bromide has widespread
use for fumigating post-harvest foods, such as cereals, spices,
dried-fruits, and nuts, as well as fresh fruits and vegetables. The
determination of levels of methyl bromide and inorganic bromide in the
fumigated food is important for the assessment of possible risks for
human and animal health. Methyl bromide levels in food commodities
usually decrease rapidly after aeration, but may be detected for some
weeks after treatment. Fumigation with methyl bromide results in
increased levels of inorganic bromide in food commodities and,
equally, in produce grown on fumigated soils. Fumigant dosage
standards should be adhered to, so that the bromide levels do not
exceed the recommended limits.
The major health concern is from acute exposure.
Delayed onset of symptoms may occur. Fatal poisoning has resulted
from exposures to relatively high concentrations (from 33 000 mg/m3
or 8600 ppm onwards) of methyl bromide vapours. Non-fatal poisoning
has resulted from exposure to concentrations as low as 390-1950
mg/m3 (100- 500 ppm). Organs affected by exposure include the
nervous system, lung, nasal mucosa, kidney, eye, and skin. There are
no epidemiological data on reproductive toxicity and carcinogenicity
in humans. There are no data on any human health effects of methyl
bromide residues in food or drinking-water.
10.1.1 Relevant animal studies
Methyl bromide is very toxic for all animal species by all routes
of administration studied. Deaths from exposure follow a steep
dose-response curve. LC50 (1-h) values for mice and rats are 4680
and 7300 mg/m3, respectively.
Deaths and neurotoxicity occur within hours or days after a
single inhalation exposure at high concentrations. Studies on mice
show that more prolonged exposure at low concentrations (6 h/day; 389
mg/m3) may also produce neurotoxicity or deaths, appearing with a
delayed onset of several months.
Absorption and distribution to various tissues is rapid, as is
elimination. The metabolic pathways and mode of action are unknown.
The principal toxic effects associated with lethality occur in
the brain and kidney. Histopathology of the brain shows necrosis of
granular cells of the cerebellum in mice and rats and neuronal
necrosis in the cerebral cortex, hippocampus, and thalamus, in rats.
The lowest doses at which these lesions were observed were 250
mg/m3, 6 h/day, for 2 years, in mice, and, 622 mg/m3, 6 h/day, for
5 weeks, in rats. In the kidney, necrosis of the convoluted tubule
epithelium occurs at doses of 599 mg/m3, 6 h/day, for 2 weeks, in
mice, and 1712 mg/m3, 6 h/day, for 2 weeks, in rats. Other effects
are observed in the heart (degeneration of focal necrosis), nose
(necrosis of olfactory epithelium), testis (degeneration of
seminiferous tubules), and other organs. In non-lethal exposure, nasal
irritation and neuro-toxicity (including histopathological lesions)
are the major toxic effects.
No teratogenic effects have been observed in rats or rabbits.
Embryotoxicity occurred in rats and rabbits only at doses that were
also maternally toxic. In a rat multigeneration study, there were
reductions in the fertility index in the second generation, when the
animals were exposed to 117 or 350 mg/m3 (30 or 90 ppm) for 6 h/
day, but effects were not observed following exposure to 12 mg/m3 (3
ppm) for 6 h/day.
Methyl bromide was mutagenic in several in vivo and in vitro
assays.
Long-term inhalation studies on rats and mice did not reveal any
evidence of carcinogenicity. Lesions originally interpreted as
carcinomas of the forestomach in rats, following gavage
administration, were shown in a subsequent study to regress after
termination of treatment, and were considered not relevant for human
risk assessment.
10.2 Environment
Methyl bromide is predominantly a naturally occurring compound.
Oceans are believed to be a major natural source of methyl bromide.
Another source(s) may exist in the tropics, which is yet to be
explained. Anthropogenic sources from fumigation and, to a much lesser
extent, motor vehicles (from the combustion of organic bromine
additives in leaded petrol) add to these. Present data indicate that
the globally averaged atmospheric abundance of methyl bromide is
between 9 and 13 pptv, equivalent to a total atmospheric loading of
150-220 thousand tonnes. If the atmospheric lifetime is two years
(assuming only atmospheric removal processes are significant),
anthropogenic sources of methyl bromide represent about 25% (±10%) of
the total emissions. The world production of methyl bromide in 1990
was 69 000 tonnes, having increased at a rate of 6% per year from 1984
to 1990. About 50% of the methyl bromide produced is released into the
atmosphere during, or after, use. Although methyl bromide reacts with
the hydroxyl radical in the troposphere, some methyl bromide is
transferred by upward diffusion to the stratosphere, where it
photolyses. Active bromine species react with ozone in the lower
stratosphere and are partly responsible for the depletion of the ozone
layer. It is estimated that anthropogenic releases of methyl bromide
cause about 3% of the present total stratospheric ozone loss.
Methyl bromide is used for soil fumigation (about 77%), for
quarantine, commodity fumigation (12%), for structural fumigation
(5%), and for chemical intermediates (6%).
In soil, methyl bromide is degraded by hydrolysis and microbial
activity. The remainder (about 50%) eventually dissipates into the
atmosphere. The degradation product, principally as inorganic bromide,
remains as a residue in soil.
Methyl bromide has herbicidal properties. In the vicinity of
greenhouses and fumigated structures, phytotoxic effects may occur.
Visible damage to the leaves of lettuce (a sensitive test plant) was
noticed at 400 mg/m3.
Soil fumigation using methyl bromide (with 2% chloropicrin)
affects both target and non-target organisms: various soil microflora
and fauna are adversely affected, at least temporarily, by fumigation.
High mortality of non-target insects has been noticed from fumigation
under plastic sheeting. Methyl bromide could be detected in different
soil types up to 3 weeks after the treatments, the highest levels
being found in the upper layers (0-40 cm) of the soil.
Methyl bromide is highly toxic for aquatic organisms, though it
is generally of no risk to the aquatic environment. The lowest EC50
or LC50 values reported are 2.8 mg/litre for algae, 1.7 mg/litre for
daphnids, and 0.3 mg/litre for fish. NOEC levels, derived from
long-term studies, as low as 0.06 mg/litre have been reported for
daphnids and fish. Toxic concentrations are not expected to be reached
under normal circumstances, because most of the methyl bromide applied
on soil is degraded or lost, due to evaporation, before it reaches
surface water via run-off. Only in very special situations (leaching
of greenhouse soils to reduce inorganic bromide residues), can levels
of methyl bromide in the mg/litre range occur in water. Concentrations
of up to 9.3 mg methyl bromide/litre were measured in drainage water.
Similarly, relatively high levels of bromide (up to 72 mg/litre)
that could adversely affect aquatic organisms were measured in
drainage water from greenhouses. Long-term exposure to bromide ion
resulted in an EC50 value of 27 mg bromide/litre for daphnia and the
lowest NOEC for different fish species was 25 mg bromide/litre.
11. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
11.1 Human health protection
In most countries, methyl bromide is strictly regulated for
application as a fumigant for soil, commodities, and structural
purposes. Adherence to good practices and guidelines should ensure no
adverse effects on persons exposed occupationally. Considerable care
should be taken by manufacturers in the production process of methyl
bromide and by suppliers in its transfer and use.
Methyl bromide does not pose a significant risk for the general
population. It is, however, a very toxic substance and precautions
have to be taken: exposure of the general public should be avoided
through adequate precautions in fumigated buildings, greenhouses, and
stores used for commodity fumigation. Fumigated premises should be
clearly marked, in order to prevent accidental exposure of
individuals.
11.2 Environmental protection
Emission of methyl bromide from anthropogenic sources should be
reduced as far as possible. It is, therefore, desirable to reduce
emissions from soil, commodity, and structural fumigation.
Improvements should be sought in these areas:
(1) improved injection methods;
(2) better barrier films;
(3) better measurement of the efficacy of methyl bromide, with
the goal of lower dosage rates where possible.
For commodity and, possibly, for structural fumigation,
techniques should be developed to:
(1) seal fumigation chambers more tightly;
(2) capture and recycle fumigation gas.
11.3 Recommendations for further research
There is a need for:
- study of the metabolism and toxic mechanisms of methyl
bromide;
- a postnatal behavioural study;
- a human epidemiological study including exposure
assessment;
- development of a treatment protocol for cases of human
poisoning.
Effects of methyl bromide on the depletion of the ozone layer are
not yet entirely understood, indicating a need for:
- studies quantifying the distribution sources of methyl
bromide and other organic bromine compounds.
There is also a need for:
- a study of the impact of sea spray on the formation of
organic bromine compounds;
- a study on the degradation products of methyl bromide in the
troposphere;
- further study of the rate constant for the reaction with
OH-;
- studies on soil fumigation with the aim of reducing
emissions while keeping efficacy.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12.1 FAO/WHO
The toxicology of methyl bromide was evaluated by the FAO/WHO
Joint Meeting on Pesticide Residues in 1965 and 1966 (FAO/ WHO, 1965
a,b; WHO, 1967, a,b). In 1965, no acceptable daily intake (ADI) was
allocated, but, in 1966, an ADI of 1 mg/kg body weight, as bromide
ion, was established. In 1988, the JMPR evaluated the toxicology of
the bromide ion (FAO/WHO 1988a,b) and concluded that the level causing
no toxicological effect was:
Rat: 240 ppm, equivalent to 12 mg bromide/kg body weight
per day
Human: 9 mg bromide/kg body weight per day
The acceptable daily intake (ADI) of 1 mg/kg body weight was
confirmed.
12.2 IARC
The carcinogenic risks for humans were evaluated by an
International Agency for Research on Cancer ad hoc expert group in
1986. The evaluation was updated in 1987 and it was concluded that
there was inadequate evidence for carcinogenicity to humans and
limited evidence for carcinogenicity to animals; the overall
evaluation of carcinogenicity to humans was "not classifiable" [group
3] (IARC, 1986, 1987).
12.3 UNEP
In 1992, the United Nations Environment Programme evaluated methyl
bromide on behalf of the Contracting Parties to the Montreal Protocol
(UNEP, 1992). The executive summary of the evaluation reads:
"The report covers the current understanding of the impact
of methyl bromide on the ozone layer and on the uses of, and
alternatives to, methyl bromide."
It was requested by the United Nations Environment Programme on behalf
of the Parties to the Montreal Protocol. The report includes
information presented and discussed at the International Methyl
Bromide Science Workshop held in Washington, DC, on 2-3 June 1992, and
at the International Workshop on Alternatives and Substitutes to
Methyl Bromide held in Washington, DC, on 16-18 June 1992.
The current state of scientific knowledge concerning bromine
compounds in the atmosphere is considerably less developed than the
corresponding understanding of chlorine compounds. In addition, the
evaluation of the alternatives and substitutes to methyl bromide as a
fumigant is in an earlier stage than that for chlorofluorocarbons
(CFCs), methylchloroform, carbon tetrachloride, and halons.
Methyl bromide is used as a fumigant for soils, commodities, and
structures. It is currently vital for the economic viability of
certain agricultural production (especially strawberries, tomatoes,
peppers, tobacco, eggplants, nursery stock, vines, and turf) and for
the quarantine treatment of certain products in international trade.
Many developing countries are particularly dependent on the export of
products currently fumigated using methyl bromide, before shipment and
at ports of entry.
Total annual production and sales of methyl bromide for
fumigation have increased from about 42 000 tonnes to about 63 000
tonnes between 1984 and 1990. Combining the approximate methyl bromide
use-pattern data with the currently estimated fraction that escapes to
the atmosphere from each use (soils: 80% of use, 50 % emitted;
commodities: 15 % of use, <80 % emitted; and structural: 5 % of use,
80% emitted) indicates that about half of the methyl bromide used as
a fumigant is emitted into the atmosphere. Based on current
understanding, this implies an anthropogenic emission from fumigation
applications of about 30 thousand tonnes in 1990, which represents
25±10 % of the total (natural and anthropogenic) emissions.
Ozone destruction by bromine is more efficient on a per molecule
basis than destruction by chlorine by a factor of 30-60. Therefore, 1
part per trillion by volume (pptv) of bromine is equivalent to
0.03-0.06 parts per billion by volume (ppbv) of stratospheric
chlorine.
The current best estimate of the steady-state value of the Ozone
Depletion Potential (ODP) for methyl bromide is 0.7. Because of the
short atmospheric lifetime of methyl bromide, its relative impact on
ozone is expected to be much greater over the next decade (when
chlorine abundances and ozone losses are predicted to reach their
maximum) than is indicated by its steady-state ODP.
There are significant uncertainties in the atmospheric budget and
ODP of methyl bromide, especially the quantification of possible
oceanic and terrestrial surface removal processes and the rate of
formation of unreactive bromine in the stratosphere.
Model calculations suggest that anthropogenic emissions of methyl
bromide used for fumigation applications could have accounted for
about one-twentieth to one-tenth of the current observed global ozone
loss of 4-6% and could grow to about one-sixth of the predicted ozone
loss by the year 2000, if methyl bromide emissions continue to
increase at the present rate of about 5-6% per year.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide from fumigation applications is about 3
pptv, which is equivalent to 0.09-0.18 ppbv of stratospheric chlorine.
An advance of the CFC and carbon tetrachloride phaseout schedule by
three years would reduce the peak chlorine loading by 0.18 ppbv.
Therefore, elimination of methyl bromide used as a fumigant could
provide an ozone-layer protection equivalent to that of an advance of
the CFC and carbon tetrachloride phaseout schedule by about 1.5-3
years.
There is no single alternative to methyl bromide in the broad
spectrum of applications for which it is currently used. There are,
however, many alternative chemicals and procedures for specific
applications. The introduction of some chemical alternatives may
require government approval, which could be a lengthy process.
Rough estimates indicate that a significant fraction (from as low
as 30% to as high as 90%), albeit uncertain, of methyl bromide used
for soil fumigation could be replaced by chemical substitutes during
the 1990s; that a substantial proportion of emissions from fumigation
chambers could be captured and recycled or destroyed; that a small
fraction (1-2%) of methyl bromide emissions could be eliminated by
better procedures during tank filling; and that significant reductions
in emissions could be made using other alternatives or techniques,
alone, or in combination. However, there are some applications for
which there are limited or no alternatives, including some
agricultural situations that have developed a dependence on methyl
bromide, quarantine treatments, and some structural fumigation uses.
REFERENCES
Abdalla N & Lear B (1975) Lethal dosages of methyl bromide for four
plant-parasitic nematodes and the effect of soil temperature on its
nematicidal activity. Plant Dis Rep, 59: 224-228.
Adu OO & Muthu M (1985) The relative toxicity of seven fumigants to
life cycle stages of Callosobruchus chinensis (L). Insect Sci Appl,
6: 75-78.
Alexeeff GV & Kilgore WW (1983) Methyl bromide. In: Gunther FA &
Gunther JD ed. Residue reviews. Residues of pesticides and other
contaminants in the total environment, Vol. 88. Berlin, Heidelberg,
New York, Springer-Verlag, pp 102-153.
Alexeeff GV, Kilgore WW, Munoz P, & Watt D (1985) Determination of
acute toxic effects in mice following exposure to methyl bromide. J
Toxicol Environ Health, 15: 109-123.
Alfaro Moreno A & Vegh I (1971) [Green pepper diseases produced by
Phytophthora capsici.] An Inst Nac Invest Agr Ser Prot Veg, 1: 9-42
(in Spanish).
American Biogenics Corporation (1986) Two-generation reproduction
study in albino rats with methyl bromide - results of both generations
(Study No. 4500-1525) (Unpublished final report).
Andersen ME, Gargas ML, Jones RA, & Jenkins LJ (1980) Determination of
the kinetic constants for metabolism of inhaled toxicants in vivo
using gas uptake measurements. Toxicol Appl Pharmacol, 54: 100-116.
Anger WK, Setzer JV, Russo JM, Brightwell WS, Wait RG, & Johnson BL
(1981) Neurobehavioral effects of methyl bromide inhalation exposures.
Scand J Work Environ Health, 7: 40-47.
Anger WK, Moody L, Burg J, Brightwell WS, Taylor BJ, Russo JM,
Dickerson N, Setzer JV, Johnson BL, & Hicks K (1986) Neurobehavioral
evaluation of soil and structural fumigators using methyl bromide and
sulfuryl fluoride. Neurotoxicology, 7: 137-156.
Angerer J (1977) [A method for determination of bromide in urine with
help of a ionsensitive electrode.] J Clin Chem Clin Biochem, 15:
201-204 (in German).
Angerer J (1980) [Bromide. Determination in urine.] In: Henschler D
ed. [Analytical methods for the investigation of work materials
detrimental to health. Analysis in biological materials.] Weinheim,
Verlag Chemie, vol 2/2, pp D1-D6, 1-9 (in German).
Angerer J (1982) [Methyl bromide. Air analysis]. In: Henschler D ed.
[Analytical methods for the investigation of work materials
detrimental to health. Air analysis.] Weinheim, Verlag Chemie, vol
1/2, D1-D2, 1-8 (in German).
Anthon EW, Moffit HR, Couey HM, & Smith LO (1975) Control of codling
moth in harvested sweet cherries with methyl bromide and effects upon
quality and taste of treated fruit. J Econ Entomol, 68: 524-526.
Arvieu JC (1983) Some physico-chemical aspects of methyl bromide
behaviour in soil. Acta Hortic, 152: 267-275.
Arvieu JC & Cuany A (1985) Effets de la matière organique sur la
bioactivité et la dégradation du bromure de méthyle dans le sol. EPPO
Bull, 15: 87-96.
Asante-Poku S, Aston WP, & Schmidt DE Jr (1974) Site of decomposition
of methyl bromide in cocoa beans. J Sci Food Agric, 25: 285-291.
Atochem (1987) Fumyl-o-gas. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 4 pp.
Atochem (1988) BM 98 SOBROM. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 2 pp.
Audry D, Soichot P, Giard MH, & Mauguiere F (1985) Une cause rare de
myoclonies avec PES "Géants": l'intoxication par le bromure de
méthyle. A propos d'une observation à prédominance unilaterale. Rev
EEG Neurophysiol, 15: 45-52.
Austin RK & Phillips DJ (1985) Use of a standard-addition
bromide-selective electrode technique to determine bromide and trace
its migration in peaches. J Agric Food Chem, 33: 1165-1168.
Bachem C (1927) [Contribution to the toxicology of the alkyl
halogens.] Arch Exp Pathol Pharmakol, 122: 69-76 (in German).
Bakhishev GN (1973) [Relative toxicity of aliphatic halohydrocarbons
to rats.] Farmakol Toksikol, 8: 140-142 (in Russian).
Balander PA & Polyak MG (1962) [Toxicological characteristics of
methyl bromide.] Gig i Toksikol, 60: 412-419 (in Russian).
Basile M & Lamberti F (1981) Bromide residues in edible organs of
plants grown in soil treated with methyl bromide. Meded Fac
Landbouwwet Rijksuniv Gent, 46: 337-341.
Basile M, Lamberti F, & Basile AC (1987) [Inorganic bromide
concentrations in soil after fumigation with methyl bromide.] Essenze
Deriv Agrum, 57: 116-120 (in Italian).
Baumann H & Heumann KG (1987) Analysis of organobromine compounds and
hydrogen bromide in motor car exhaust gases with a GC/microwave plasma
system. Fresenius Z Anal Chem, 327: 186-192.
BBA (Biologische Bundesanstalt für Land- und Forstwirtschaft) (1989)
[Plant protective agent register, 1989/90, Part 5.] 37th ed.
Braunschweig, Federal Institute for Agriculture and Forestry, 66 pp
(in German).
Behrens RH & Dukes DCD (1986) Fatal methyl bromide poisoning. Br J Ind
Med, 43: 561-562.
Bell CH (1988) Minimum concentration levels of methyl bromide required
for full efficacy against seven species of stored-product beetle at
two temperatures. Pestic Sci, 24: 97-109.
Benatt AJ & Courtney TRB (1948) Uraemia in methyl bromide poisoning:
A case report. Br J Ind Med, 5: 21-25.
Berg WW, Heidt LE, Pollock W, Sperry PD, Cicerone RJ, & Gladney ES
(1984) Brominated organic species in the arctic atmosphere. Geophys
Res Lett, 11: 429-432.
Beyne M & Goett C (1934) Toxicité de certains appareils extincteurs
d'incendie et précautions qu'ils comportent dans leur emploi. Arch Méd
Pharm Nav, 124: 409.
Bird GW, Rich JR, & Glover SU (1974) Increased endomycorrhizae of
cotton roots in soil treated with nematicides. Phytopathology, 64:
48-51.
Bishop CM (1992) A case of methyl bromide poisoning. J Occup Med, 42:
107-109.
Blackburn S & Philips H (1944) Experiments on the methylation and
acetylation of wool, silk fibroin, collagen and gelatin. Biochem J,
38: 171-178.
Blackith RE & Lubatti OF (1965) Fumigation of agricultural products.
XX. Prolonged storage of cereals fumigated with methyl bromide. J Sci
Food Agric, 16: 455-457.
Boczek J, Klag J, & Komorowska B (1975) Effect of methyl bromide on
the embryonic development of Acarus siro (Acarina, Acaridae). J
Stored Prod Res, 11: 41-46.
Bolon B, Bonnefoi M, & Morgan KT (1990) Effect of olfactory epithelial
regeneration and cage environment on the sensitivity of the rat
olfactory epithelium to methyl bromide. Chem Senses, 15: 554-555.
Bond EJ (1956) The effect of methyl bromide on the respiration of the
cadelle Tenebroides mauritanicus L. (Coleoptera: Ostomidae). Can J
Zool, 34: 405-415.
Bond EJ (1984) Manual of fumigation for insect control. 6. Chemicals
used as fumigants. Rome, Food and Agriculture Organization of the
United Nations, pp 71-96 (Plant Production and Protection Paper 54).
Bond EJ & Dumas T (1987) Concentrations of methyl bromide inside flour
mills and in the atmosphere around the mills during and after
fumigation. Proc Entomol Soc Ont, 118: 1-6.
Bond JA, Dutcher JS, Medinsky MA, Henderson RF, & Birnbaum LS (1985)
Disposition of [14C]methyl bromide in rats after inhalation. Toxicol
Appl Pharmacol, 78: 259-267.
Boorman GA, Hong HL, Jamieson CW, Yoshitomi K, & Maronpot RR (1986)
Regression of methyl bromide induced forestomach lesions in the rat.
Toxicol Appl Pharmacol, 86: 131-139.
Bourbos VA & Skoudridakis MT (1991) Effect of methyl bromide and
chloropicrin on the soil mycoflora in greenhouse tomato. Plant Pathol
Lab, 56: 569-575.
Bowers GN Jr & Onoreski M (1990) Hyperchloremia and the incidence of
bromism in 1990. Clin Chem, 36: 1399-1403.
Brenner I (1978) Bromism: alive in well. Am J Psychiatry, 7: 857-858.
Breslin WJ, Zablotny CL, Bradley GF, & Lomax LG (1990) Methyl bromide
inhalation teratology study in New Zealand white rabbits. Midland,
Michigan, The Dow Chemical Company (Unpublished final report).
Bridges RG (1955) The fate of labelled insecticide residues in food
products. III. N-methylation as a result of fumigating wheat with
methyl bromide. J Sci Food Agric, 6: 261-268.
Brodniewicz A (1967) Poisoning of seamen with methyl bromide due to
fumigation of a Polish cargo ship in Haiphong (Vietnam). Arh Hig Rada,
18: 241-246 (in Polish with English translation).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: an assessment of available data (Contract-No. 68-02-3452).
Menlo Park, California, SRI International (EPA/DF-83/005a).
Bromine and Chemicals Ltd (1990) Methylbromide soil fumigation - use
and application instructions. London, Bromine and Chemicals Ltd, pp
1-36.
Brown G & Jenkinson DS (1971) Bromine in wheat grown on soil fumigated
with methyl bromide. Soil Sci Plant Anal, 2: 45-54.
Brown D & Rolston DE (1980) Transport and transformation of methyl
bromide in soils. Soil Sci, 130: 68-75.
Brown AL, Burau RG, Meyer RD, Raski DJ, Wilhelm S, & Quick J (1979)
Plant uptake of bromide following soil fumigation with methyl bromide.
Calif Agric, 33: 11-13.
Bruhin J (1942) [Percutaneous poisoning with methyl bromide during
pest control.] University of Zurich, Medical Faculty, 31 pp (M.D.
Thesis) (in German).
BUA (1987) [BUA Chemical Report 14: Methyl bromide.] Weinheim,
Advisory Committee for Environmental Priority Pollutants (BUA) of the
German Chemists Association, 64 pp (in German).
Burkholder WE (1966) Toxicity of methyl bromide to Acarus siro , a
cheese-infesting mite. J Econ Ent, 59: 110-112.
Butler ECB, Perry KMA, & Williams JRF (1945) Methyl bromide burns. Br
J Ind Med, 2: 30-31.
CAC (1986) Guide to Codex Alimentarius recommendations on pesticide
residues. Part 3. Guideline levels for pesticide residues. Rome, Food
and Agriculture Organization (CAC/PR 3-1986).
Cantineau A, Thomas R, Breurec JY, Bousser J, Baert A, Bonny JS,
Bouget J, Dubois C, Venisse T, & Curtes JP (1988) Acute methylbromide
poisoning. J Toxicol Clin Exp, 8: 83-87.
Canton JH, Wester PW, & Matthijssen-Spiekman, EAM (1983) Study on the
toxicity of sodium bromide to different freshwater organisms. Food
Chem Toxicol, 21: 369-378.
Carter B (1945) Methyl bromide poisoning. Effects on the nervous
system. Br Med J, 1: 43-45.
Castro CE & Belser NO (1981) Photohydrolysis of methyl bromide and
chloropicrin. J Agric Food Chem, 29: 1005-1008.
Castro JA, Zerba EN, De Licastro SA, Picollo MI, Wood EJ, Ruveda MA,
De Moutier Aldao EM, & Libertella R (1976) Toxicity of methyl bromide
and other gaseous insecticides to Triatoma infestans. Acta Physiol
Lat Am, 26: 106-114.
Cavanagh JB (1992) Methyl bromide intoxication and acute energy
deprivation syndromes. Neuropathol Appl Neurobiol, 18: 573-578.
CDFA (1988) Sampling for pesticide residues in California well water:
1988 update; well inventory data base. Sacramento, California,
Department of Food and Agriculture, Division of Pest Management,
Environmental Protection and Worker Safety, Environmental Monitoring
and Pest Management Branch, 66 pp (Report EH 88-10).
CEC (1985) Report of the Scientific Committee for Pesticides on the
use of methyl bromide as a fumigant for plant culture media.
Luxembourg, Commission of the European Communities, pp 15-33 (EUR
10211).
Chavez CT, Hepler RS, & Straatsma BR (1985) Methyl bromide optic
atrophy. Am J Ophthalmol, 99: 715-719.
Cheetham T (1990) Pathological alterations in embryos of the codling
moth (Lepidoptera: Tortricidae) induced by methyl bromide. Ann.
Entomol. Soc. Am., 83: 59-67.
Chellman GJ, White RD, Norton RM, & Bus JS (1986) Inhibition of the
acute toxicity of methyl chloride in male B6C3F1 mice by glutathione
depletion. Toxicol Appl Pharmacol, 86: 93-104.
Chen T, Kilpatrick RA, & Rich AE (1962) Stylet-bearing nematodes
associated with white clovers in New Hampshire. Plant Dis Rep, 46:
346-347.
Chisholm RD & Koblitsky L (1943) Sorption of methyl bromide by soil in
a fumigation chamber. J Econ Entomol, 36: 549-551.
Cicerone RJ, Heidt LE, & Pollock WH (1988) Measurements of atmospheric
methyl bromide and bromoform. J Geophys Res, 93: 3745-3749.
Cirilli L & Borgioli A (1986) Methyl bromide in surface drinking
waters. Gas-chromatographic determination by the headspace technique.
Water Res, 20: 273-275.
Clarke CA, Roworth CG, & Holling HE (1945) Methyl bromide poisoning.
Br J Ind Med, 2: 17-23.
Cochran JW (1988) A rapid, sensitive method for the analysis of
halogenated gases in water. J High Resol Chromatogr Chromatogr Commun,
11: 663-665.
Cooper DM, Griffiths NM, Hobson-Frohock A, Land DG, & Rowell JG (1978)
Fumigation of poultry food with methyl bromide: effects on egg
flavour, number and weight. Br Poult Sci, 19: 537-542.
Cornwell PB (1979) Health monitoring experience of fumigators using
methylbromide. Proceedings of the Fifth British Pest Control
Conference, Stratford-on-Avon, England, 26-29 September 1979. London,
British Pest Control Association (Sixth Session; Paper No. 17).
Cova D, Molinari GP, & Rossini L (1986) Residues after fumigation with
methyl bromide: bromide ion and methyl bromide in middlings and final
cereal foodstuffs. Food Addit Contam, 3: 235-240.
Crampton RF, Elias PS, & Gangolli SD (1971) The bromide content of
human tissue. Br J Nutr, 25: 317-322.
Daelemans A (1978) Uptake of methyl bromide by plantss and uptake of
bromide from decontaminated soils. Leuwen, Belgium, Catholic
University (Dissertation) (in Flemish).
Daft J (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71: 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1992) Headspace method for rapid determination of methyl
bromide in assorted nut samples. J Assoc Off Anal Chem, 75: 701-706.
Dagani MJ, Barda HJ, & Sanders DC (1985) Bromine compounds. Weinheim,
Verlag Chemie, pp 405-420.
Danse LHJC, Van elsen FL, & Van der Heijden CA (1984) Methylbromide:
carcinogenic effects in the rat forestomach. Toxicol Appl Pharmacol,
72: 262-271.
Darby JF, Dieter CE, & Rau GJ (1962) Evaluation of treatments for
control of soil-borne pests in celery seedbeds. Plant Dis Rep, 46:
441-443.
Davenport CJ, Ali SF, Miller FJ, Lipe GW, Morgan KT, & Bonnefoi MS
(1992) Effect of methyl bromide on regional brain glutathione,
glutathione- S-transferases, monoamines, and amino acids in F344
rats. Toxicol Appl Pharmacol, 112: 120-127.
Davis LN, Strange JR, Hoecker JE, Howard PH, & Santodonato J (1977)
Investigation of selected potential environmental contaminants:
monohalomethanes. Washington, DC, US Environmental Protection Agency
(EPA-560/2-77-007).
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1: 303-318.
De Heer H, Tuinstra LGMPH, Hamaker PH, & Van der burg AMM (1983) Use
of gas-tight plastic films during fumigation of glasshouse soils with
methylbromide. I. Significance of permeation and leakage for the
emission into the outside air. Acta Hortic, 152: 109-126.
Dempsey DA, Becker CE & Mihm FC (1992) Death after entering an
apartment building fumigated with methyl bromide and cleared for
habitation. Vet Hum Toxicol, 34: 356.
Deutschmann S, Reichel C, & Kettschau F (1989) Metabolism of methyl
bromide and methyl iodide in erythrocytes compared with methyl
chloride. Naunyn-Schmiedeberg's Arch Pharmacol, 339: R12.
Deutschmann S, Peter H, Reichel C, Bolt M, & Hallier E (1989)
[Kinetics of metabolism of methyl bromide and methyl iodide in
erythrocytes of humans and related species.] Verh Dtsch Gesundh
Arbeitsmed, 29: 517-519 (in German).
Devaney JA & Beerwinkle KR (1982) Control of the northern fowl mite on
inanimate objects by fumigation: field studies. Poult Sci, 62: 43-46.
Devries JW, Borge JM, Schroeder JP, Bowers RH, Larson PA, & Burns NM
(1985) Headspace gas chromatographic method for determination of
methyl bromide in food ingredients. J Assoc Off Anal Chem, 68:
1112-1116.
Djalali-Behzad G, Hussain S, Ostermann-Golker S, & Segerbaeck D (1981)
Estimation of genetic risks of alkylating agents. VI. Exposure of mice
and bacteria to methyl bromide. Mutat Res, 84: 1-9.
Doraiswamy S, Van Winckel A, & Van Assche C (1972) Effect in vitro of
soil fumigants on soil microbial population in relation to degradation
of tobacco mosaic virus. Meded Fac Landbouwwet Rijksuniv Gent, 37:
492-499.
Drawneek W, O'Brien MJ, Goldsmith HJ, & Bourdillon RE (1964)
Industrial methyl bromide poisoning in fumigators. Lancet, October 17:
855-856.
Dreef-Van der Meulen HC, Reuzel PGJ, Kuper CF, Hollanders VMH, & Feron
VJ (1989) Chronic inhalation toxicity of methyl bromide in rats. Third
International Symposium on Soil Disinfestation, Leuwen, Belgium,
September 26-30, 1988. Acta Hortic, 255: 313-315.
Driscoll JN, Duffy M, Pappas S, & Webb M (1987) Analysis for purgeable
organics in water by capillary GC/PID-ELCD. J chromatogr sci, 25:
369-375.
Drosihn UG, Stephan BR, & Hoffmann GM (1968) [Investigations into soil
contamination with methyl bromide.] Z Pflanzenkr Pflanzenschutz, 75:
272-287 (in German).
Dudley HC & Neal PA (1942) Methyl bromide as a fumigant for foods.
Food Res, 7: 412-429.
Dudley HC, Miller JW, Neal PA, & Sayers RR (1940) Studies on
foodstuffs fumigated with methyl bromide. Public Health Rep, 55:
2251-2282.
Duffy M, Driscoll JN, Pappas S, & Sanford W (1988) Analysis of ppb
levels of organics in water by means of purge-and-trap, capillary gas
chromatography and selective detectors. J Chromatogr, 441: 73-80.
Dumas T (1982) Trapping low levels of methyl bromide in air or as
residues at ambient and lower temperatures for gas chromatography. J
Assoc Off Anal Chem, 65: 913-915.
Dumas T & Bond EJ (1985) Analysis of methyl bromide at ultra low
concentration levels. J Agric Food Chem, 33: 276-278.
Duvoir M, Fabre R, & Layani F (1937). L'intoxication par le bromure de
méthyle. Bull Soc Méd Paris, 53: 1540.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards CA (1962) Springtail damage to bean seedlings. Plant Pathol,
11: 67-68.
Edwards CA & Thompson AR (1973) Pesticides and the soil fauna. In:
Gunther FA & Gunter JD, ed. Residue reviews. Residues of pesticides
and other contaminants in the total environment. Berlin, Heidelberg,
New York, Springer-Verlag, vol 45, pp 1-79.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
beta-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
El Buzz HK, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1974) Effect of
diet on the susceptibility of the different developmental stages of
Corcyra cephalonica staint. to carbon bisulphide and methyl bromide.
Agric Res Rev, 52: 21-29.
Eller PM (1985) NIOSH manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 2520/1-2520/-4 (NIOSH Publication No. 84-100).
EMBA (1988) Handling and transportation of methyl bromide. Code of
practice. Brussels, European Methyl Bromide Association, pp 1-8.
Enebak SA, Palmer MA, & Blanchette RA (1988) Effect of soil-borne
pathogen control methods on the production of white pine nursery
seedlings. Phytopathology, 78: 1532-1533.
Ethyl Corporation (1990a) Methyl bromide applications manual. Baton
Rouge, Louisiana, Ethyl Corporation, 3 pp.
Eustis SL, Haber SB, Drew RT, & Yang RSH (1988) Toxicology and
pathology of methyl bromide in F344 rats and B6C3F1 mice following
repeated inhalation exposure. Fundam Appl Toxicol, 11: 594-610.
Evans JE & Hastings L (1992) The effect of methyl bromide on measures
of olfactory function. Toxicologist, 12: 270.
Fabian P (1984) Atmosphere and environment. Berlin, Heidelberg, New
York, Springer-Verlag, p 41.
Fabian P, Borchers R, Penkett SA & Prosser NJD (1981) Halocarbons in
the stratosphere. Nature (Lond), 294: 733-735.
Fairall RJ & Scudamore KA (1980) Determination of residual methyl
bromide in fumigated commodities using derivative gas-liquid
chromatography. Analyst, 105: 251-256.
Fallico R & Ferrante M (1991) Exposure to inorganic bromides from
greenhouse crops where methyl bromide was applied for soil fumigation.
Zent.bl Hyg Umweltmed, 191: 555-562.
FAO (1980) Pesticide residues in food. Methyl bromide. Rome, Food and
Agriculture Organization of the United Nations, pp 89-97 (FAO Plant
Production and Protection Paper 20 Sup).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Group on Pesticide
Residues. Rome, Food and Agriculture Organization of the United
Nations; Geneva, World Health Organization (FAO Meeting Report No.
PL/1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1965b) Evaluation of hazards to consumers resulting from the
use of fumigants in the protection of food. Rome, Food and Agriculture
Organization of the United Nations; Geneva, World Health Organization
(FAO Meeting Report No. PL/1965/10/2; WHO/Food Add./28.65).
FAO/WHO (1988a) Pesticide residues in food - 1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environmental and the WHO Expert Group on Pesticide Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 92).
FAO/WHO (1988b) Pesticide residues in food - 1988 evaluations. Part II
-Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 93/2).
Filip GM & Roth LF (1977) Stump injections with soil fumigant to
eradicate Armillariella mellea from young-growth ponderosa pine
killed by root rot. Can J For Res, 7: 226-231.
Flick EW (1985) Industrial solvents handbook, 3rd ed. Park Ridge, New
Jersey, Noyes Data Corporation, 4 pp.
Foxboro (1989) Ambient air instrumentation sheet. East Bridgewater,
Maryland, The Foxboro Company.
Fransi A, Pons R, Sala A, Vallejo VR, & Bertran C (1987) Wheat and
soil bromide dynamics after fumigation with methyl bromide in a
mediterranean climate. Plant Soil, 98: 417-424.
Fuortes LJ (1992) A case of fatal methyl bromide poisoning. Vet Hum
Toxicol, 34: 240-241.
Furuta A, Hyakudo T, Ohnishi A, Hori H & Tanaka E (1993) Neurotoxicity
of methyl bromide - neuropathologic evaluation, preliminary study.
Sangyo Ika Daigaku Zasshi, 15: 21-27.
Gansewendt B, Foest U, Xu D, Hallier E, Bolt HM, & Peter H (1991)
Formation of DNA adducts in F-344 rats after oral administration or
inhalation of [14C]methyl bromide. Food Chem Toxicol, 29: 557-563.
Gargas ML & Andersen ME (1982) Metabolism of inhaled brominated
hydrocarbons: validation of gas uptake results by determination of a
stable metabolite. Toxicol Appl Pharmacol, 66: 55-68.
Garman JR, Freund T, & Lawless EW (1987) Testing for groundwater
contamination at hazardous waste sites. J Chromatogr Sci, 25: 328-337.
Garry VF, Nelson RL, Griffith J, & Harkins M (1990) Preparation for
human study of pesticide applicators: sister chromatid exchanges and
chromosome aberrations in cultured human lymphocytes exposed to
selected fumigants. Teratog Carcinog Mutagen, 10: 21-29.
Gentile IA, Ferraris L, Crespi S, & Belligno A (1989) The degradation
of methyl bromide in some natural fresh waters. Influence of
temperature, pH and light. Pestic Sci, 25: 261-272.
Gentile IA, Ferraris L, Sanguinetti M, Tiprigan M, & Fisichella G
(1992) Methyl bromide in natural fresh waters: hydrolysis and
volatilisation. Pestic Sci, 34: 297-301.
Gillotay D, Simon PC & Dierickx L (1989) Temperature dependence of
ultraviolet absorption cross-sections of brominated methanes an
ethanes. In: Bojkov RD & Fabian P, ed. Ozone in the atmosphere.
Proceedings of the Quadrennial Ozone Symposium 1988 and Tropospheric
Ozone Workshop, Göttingen, Germany, 4-13 August 1988. Hampton,
Virginia, Deepak Publishing, pp 706-709.
Goldman LR, Mengle D, Epstein DM, Feson D, Kelly K, & Jackson RJ
(1987) Acute symptoms in persons residing near a field treated with
the soil fumigants methyl bromide and chloropicrin. West J Med, 147:
95-98.
Gosselin RE, Smith RP, Hodge HC, & Braddock JE (1984) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams &
Wilkins Company, pp III/280-III/284.
Goulon M, Nouailhat F, Escourolle R, Zarranz-Imirizaldu JJ, Grosbuis
S, & Levy-Alcover MA (1975) Intoxication par le bromure de méthyle.
Trois observations, dont une mortelle. Etude neuro-pathologique d'un
cas de stupeur avec myoclonies, suivi pendant cinq ans. Rev. Neurol.,
131: 445-468.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Charles C. Thomas, pp 680-685.
Green M, Seiber JN, & Biermann HW (1991) in situ measurement of methyl
bromide in indoor air using long-path ftir spectroscopy. In: Schiff
HI, ed. Measurement of atmospheric gases. Proceedings spie - the
international society for optical engineering. Los angeles,
California, 2-3 January 1991 (Volume 1433). Washington, DC, The
International Society for Optical Engineering, pp 270-274.
Greenberg JO (1971) The neurological effects of methyl bromide
poisoning. Ind Med, 40: 27-29.
Greve PA & Grevenstuk WBF (1976) Optimization studies on the
determination of bromide residues in lettuce. Meded Fac Landbouwwet
Rijksuniv Gent, 41: 1371-1381.
Greve PA & Grevenstuk WBF (1979) Gas-liquid chromatographic
determination of bromide ion in lettuce: interlaboratory studies. J
Assoc Off Anal Chem, 62: 1155-1159.
Greve PA & Hogendoorn EA (1979) Determination of fumigant residues in
grain. Meded Fac Landbouwwet Rijksuniv Gent, 44: 877-884.
Griffiths NM, Hobson-Frohock A, Land DG, Levett JM, Cooper DM, &
Rowell JG (1978) Fumigation of poultry food with methyl bromide:
effects on flavour and acceptability of broiler meat. Br Poult Sci,
19: 529-535.
Grimm GR & Alexander AR (1971) Fumigation of phytophthora in sandy
soil by surface application of methyl bromide and methyl
bromide-chloropicrin. Plant Dis Rep, 55: 929-931.
Gryder-Boutet DE & Kennish JM (1988) Using headspace sampling with
capillary column GC-MS to analyze trace volatile organics in water and
wastewater. Am Water Works Assoc J, 80: 52-55.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Guillemin MP, Hillier RS, & Bernhard CA (1990) Occupational and
environmental hygiene assessment of fumigations with methyl bromide.
Ann Occup Hyg, 34: 591-607.
Guns MF (1989) Monitoring of the groundwater bromine content and plant
residues in methyl bromide fumigated glasshouses. Third International
Symposium on Soil Disinfestation, Leuven, Belgium, September 26-30,
1988. Acta Hortic, 255: 337-346.
Haber SB, Drew RT, Eustis S, & Yang RSH (1985) Methyl bromide
toxicity: a target organ? Toxicologist, 5: 130 (Abstract No. 518).
Haesanen E, Manninen PKG, Himberg K, & Väätäinen V (1990) Chlorine and
bromine contents in tobacco and tobacco smoke. J Radioanal Nucl Chem,
144: 367-372.
Hague NGM (1963) Fumigation of agricultural products. XVIII. Effect of
methyl bromide on the bent grass nematode, Anguina agrostis,
steinbuch 1799 , filipjev 1936, and on the germination of bent
grass, Agrostis tenuis. J Sci Food Agric, 14: 577-579.
Hague NGM & Clark WC (1959) Fumigation with methyl bromide and
chloropicrin to control seed-borne infestations of the stem eelworm.
Meded Landbouwhogesch Opzoekingasstn Gent, 24: 628-636.
Hallier E, Jaeger R, Deutschmann S, Bolt HM, & Peter H (1990a)
Glutathione conjugation and cytochrome p-450 metabolism of methyl
chloride in vitro. Toxic in vitro , 4: 513-517.
Hallier E, Deutschmann S, Reichel C, Bolt HM, & Peter H (1990b) A
comparative investigation of the metabolism of methyl bromide and
methyl iodide in human erythrocytes. Int Arch Occup Environ Health,
62: 221-225.
Hallier E, Langhof T, Dannappel D, Leutbecher M, Schröder K, Goergens
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methyl bromide, ethylene oxide and dichloromethane in human blood:
influence on the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol, 67: 173-178.
Hamilton I, Minski MJ, Milton EI, & Cleary JJ, (1972/73) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study. Sci
Total Environ, 1: 341-374.
Hansch C & Leo A (1979) Substituent constants for correlation in
chemistry and biology. New York, Chichester, Brisbane, Toronto, John
Wiley & Sons, p 173.
Hanson PR, Wainman HE, & Chakrabarti B (1987) The effects of methyl
bromide on the seed of some varieties of barley. Seed Sci Technol, 15:
155-162.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Belilies RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7: 66-75.
Harper DB (1985) Halomethane from halide ion - a highly efficient
fungal conversion of environmental significance. Nature (Lond), 315:
55-57.
Harrison BO, Peachey JE, & Winslow RO (1963) The use of nematicides to
control the spread of arabis mosaic virus by Xiphinema
diversicaudatum (Micol.). Ann Appl Biol, 52: 243-255.
Harry EG, Brown WB, & Goodship G (1972) The disinfecting activity of
methyl bromide on various microbes and infected material under
controlled conditions. J Appl Bacteriol, 35: 485-491.
Harsch DE & Rasmussen RA (1977) Identification of methyl bromide in
urban air. Anal Lett, 10: 1041-1047.
Hartill WFT & Campbell JM (1973) Control of sclerotinia in tobacco
seedbeds. Plant Dis Rep, 57: 932-934.
Hastings L (1990) Sensory neurotoxicology: use of the olfactory system
in the assessment of toxicity. Neurotoxicol Teratol, 12: 455-459.
Hastings L, Miller M, Minnema D, & Evans J (1989) Effects of methyl
bromide on rat olfactory system. Chem Senses, 14: 708.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res, 43:
1945-1950.
Heimann H (1944) Poisoning as result of industrial exposure to methyl
bromide. NY State Ind Bull, 23: 103-105.
Henschler D (1990) Chemicals harmful to health: toxicological-medical
establishment of MAK-values, 16th ed. Weinheim, VCH
Verlagsgesellschaft mbH.
Heungens A & Roos A (1982) L'influence du dazomet et du bromure de
méthyle sur la faune et la flore de la litière de pin. Rev Agric,
35(2): 2005-2050.
Heurtebise M & Ross WJ (1971) Application of 80m Br to the
semi-automated analysis of bromine in biological fluids. J Radioanal
Chem, 8: 5-12.
Heuser SG & Scudamore KA (1968) Fumigant residues in wheat and flour:
solvent extraction and gas-chromatographic determination of free
methyl bromide and ethylene oxide. Analyst, 93: 252-258.
Heuser SG & Scudamore KA (1970) Selective determination of ionised
bromide and organic bromides in foodstuffs by gas-liquid
chromatography with special reference to fumigant residues. Pestic
Sci, 1: 244-249.
Hezemans-Boer M, Toonstra J, Meulenbelt J, Zwaveling JH, Sangster B,
& Van Vloten WA (1988) Skin lesions due to exposure to methyl bromide.
Arch Dermatol, 124: 917-921.
Hicken NE (1961) Termite fumigation with methyl bromide. Chem Prod,
24: 205-207.
Hine CH (1969) Methyl bromide poisoning - a review of ten cases. J
Occup Med, 11: 1-10.
Hitchcock BE (1968) Progress of earth pearl studies in Queensland.
Proc Int Soc Sugar-Cane Technol, 13: 1382-1388.
Ho JSY (1989) A sequential analysis for volatile organics in water by
purge-and-trap capillary column gas chromatography with
photoionization and electrolytic conductivity detectors in series. J
Chromatogr Sci, 27: 91-98.
Hoffman GM & Malkomes HP (1974) Bromide residues in vegetable crops
after soil fumigation with methyl bromide. Agric Environ, 1: 321-328.
Hole BD (1981) Variation in tolerance of seven species of stored
product coleoptera to methyl bromide and phosphine in strains from
twenty-nine countries. Bull Entomol Res, 71: 299-306.
Holling HE & Clarke CA (1944) Methyl bromide intoxication. J R Navy
Med Serv, 30: 218-224.
Hommel G (1984) [Handbook of dangerous goods (Explanatory leaflet
127).] Berlin, Heidelberg, New York, Springer-Verlag (in German).
Honma T (1987) Alteration of catecholamine metabolism in rat brain
produced by inhalation exposure to methyl bromide. Jpn J Ind Health,
29: 218-219.
Honma T (1992) Brain microdialysis study of the effects of hazardous
chemicals on the central nervous system. 1. Changes in monoamine
metabolites induced by cerebral methyl bromide administration measured
by two-probe microdialysis (TPMD) method. Ind Health, 30: 47-60.
Honma T, Sudo A, Miyagawa M, & Sato M (1982) Significant changes in
monoamines in rat brain induced by exposure to methyl bromide.
Neurobehav Toxicol Teratol, 4: 521-524.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Changes in
free amino acid contents of rat brain induced by exposure to methyl
bromide. Toxicol Lett, 15: 317-321.
Honma T, Miyagawa M, Sato M, & Hasegawa H (1985) Neurotoxicity and
metabolism of methyl bromide in rats. Toxicol Appl Pharmacol, 81:
183-191.
Honma T, Muneyuki M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain. Neurotoxicol
Teratol, 9: 369-375.
Honma T, Miyagawa M, & Sato M (1991) Inhibition of tyrosine
hydroxylase activity by methyl bromide exposure. Neurotoxicol Teratol,
13: 1-4.
Hubbs AF & Harrington DD (1986) Further evaluation of the potential
gastric carcinogenic effects of subchronic methyl bromide
administration. In: Proceedings of the 36th Meeting of the American
College of Veterinary Pathology and the Annual Meeting of the American
Society for Veterinary and Clinical Pathology, Denver, Colorado,
December 1985. Denver, Colorado, American Society for Veterinary and
Clinical Pathology, p 92.
Hurtt ME & Working PK (1988) Evaluation of spermatogenesis and sperm
quality in the rat following acute inhalation exposure to methyl
bromide. Fundam Appl Toxicol, 10: 490-498.
Hurtt ME, Morgan KT, & Working PK (1987) Histopathology of acute toxic
responses in selected tissues from rats exposed by inhalation to
methyl bromide. Fundam Appl Toxicol, 9: 352-365.
Hurtt ME, Thomas DA, Working PK, Monticello TM, & Morgan KT (1988)
Degeneration and regeneration of the olfactory epithelium following
inhalation exposure to methyl bromide: pathology, cell kinetics, and
olfactory function. Toxicol Appl Pharmacol, 94: 311-328.
Hustinx WNM, Van de Laar RTH, Van Huffelen AC, Vrey JC, Meulenbelt J,
& Savelkoul TJF (1993) Systemic effects of inhalation methyl bromide
poisoning: a study of nine cases occupationally exposed due to
inadvertent spread during fumigation. Br J Ind Med, 50: 155-159.
Hyman MR & Wood PM (1984) Bromocarbon oxidations by Nitrosomonas
europaea. In: Crawford RL & Hanson RS ed. Microbial growth on C1
compounds. Washington, DC, American Society for Microbiology, pp
49-52.
IARC (1986) Methyl bromide. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 187-212 (IARC Monograph on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1-42. Lyon, International Agency for Research
on Cancer, p 245 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Ikawa N, Araki A, Nozaki K, & Matsushima T (1986) Micronucleus test of
methyl bromide by the inhalation method. Mutat Res, 164: 269
(Abstract).
Ikeda T, Kishi R, Yamamura K, Miyake H, & Sato M (1980) Behavioural
effects in rats following repeated exposure to methyl bromide. Toxicol
Lett, 6: 317-321.
Ingram FR (1951) Methyl bromide fumigation control in the date-packing
industry. Arch Ind Hyg Occup Med, 4: 193-198.
Inouye T, Inouye N, Asatani M, & Mitsuhata K (1967) Cucumber green
mottle mosaic virus in Japan. III. Inactivation of cucumber green
mottle mosaic virus in infected plant tissue buried in soil treated
with methyl bromide. Nogaku Kenkyu, 51: 199-207.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1940) The response
attending exposure of laboratory animals to vapors of methyl bromide.
J Ind Hyg Toxicol, 22: 218-230.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1941) Chemical changes of
methyl bromide in the animal body in relation to its physiological
effects. J Ind Hyg Toxicol, 23: 408-411.
Isa AL, Fam EZ, Kamel AH, & Awadallah WH (1970) On the effect of
certain fumigants on the overwintering corn borers larvae. Agric Res
Rev, 48: 43-47.
Ishizu S, Kato N, Morinobu S, Nagao N, Yamano Y, & Ito I (1988) [Cases
of severe methyl bromide poisoning residing above a warehouse.] Jpn J
Ind Health, 30: 54-60 (in Japanese with English abstract).
Ito KA, Seeger ML, & Lee WH (1972) Destruction of Byssochlamys fulva
asci by low concentrations of gaseous methyl bromide and by aqueous
solutions of chlorine, an iodophor, and peracetic acid. J Appl
Bacteriol, 35: 479-483.
Iwasaki K (1988a) Determination of S-methylcysteine in mouse
hemoglobin following exposure to methyl bromide. Ind Health, 26:
187-190.
Iwasaki K (1988b) Individual differences in the formation of
hemoglobin adducts following exposure to methyl bromide. Ind Health,
26: 257-262.
Iwasaki K, Ito I, & Kagawa J (1989) Biological exposure monitoring of
methyl bromide workers by determination of hemoglobin adducts. Ind
Health, 27: 181-183.
Japanese Ministry of Labour (1992) Toxicology and carcinogenesis
studies of methyl bromide in F344 rats and BDF mice (inhalation
studies). Tokyo, Industrial Safety and Health Association, Japanese
Bioassay Laboratory, 197 pp (Unpublished report).
Jaskot RH, Grose EC, Most BM, Menache MG, Williams TB, &
Roycroft JH (1988) The distribution and toxicological effects of
inhaled methyl bromide in the rat. J Am Coll Toxicol, 7: 631-642.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 3: 777-782.
JEA (Japanese Environmental Agency) (1981) Chemicals in the
environment. Tokyo, Japanese Environmental Agency, Office of Health
Studies, Environmental Health Department, pp 92-93.
Johnstone RT (1945) Methyl bromide intoxication of a large group of
workers. Ind Med, 14: 495-497.
Jones L (1968) effect of methyl bromide treatments on several species
of conifer seed. J For, 8: 858-860.
Joshi GP (1974) Toxicity of certain chemicals on Oryzaephilus
mercator F. (Coleoptra: Cucujidae). Appl Entomol Zool, 9: 280-281.
Kallio H & Shibamoto T (1988) Direct capillary trapping and gas
chromatographic analysis of bromomethane and other highly volatile air
pollutants. J Chromatogr, 454: 392-397.
Kantarjian AD & Shaheen AS (1963) Methyl bromide poisoning with
nervous system manifestations resembling polyneuropathy. Neurology,
13: 1054-1058.
Kato N, Morinobu S, & Ishizu S (1986) Subacute inhalation experiment
for methyl bromide in rats. Ind Health, 24: 87-103.
Katz AJ (1985) Genotoxicity of methyl bromide in somatic cells of
Drosophila larvae. Environ Mutagen, 7: 13 (Abstract).
Katz AJ (1987) Inhalation of methyl bromide gas induces mitotic
recombination in somatic cells of Drosophila melanogaster. Mutat Res,
192: 131-135.
Kelley WD & Rodriguez-Kabana R (1979) Effects of sodium azide and
methyl bromide on soil bacterial populations, enzymic activities and
other biological variables. Pestic Sci, 10: 207-215.
Kempton RJ & Maw GA (1974) Soil fumigation with methyl bromide: the
phytotoxicity of inorganic bromide to carnation plants. Ann Appl Biol,
76: 217-229.
Kenaga EE (1961) Time, temperature and dosage relationships of several
insecticidal fumigants. J Econ Entomol, 54: 537-542.
Khalil MAK & Rasmussen RA (1985) The trend of bromodifluoromethane
(CBrCIF2) and the concentration of other bromine containing gases at
the South Pole. Antarct J US, 21: 206-207.
Khalil MAK, Rasmussen RA, & Gunawardena R (1993) Atmospheric methyl
bromide: Trends and global mass balance. J Geophys Res, 98: 2887-2896.
Khanna SC & Yadav TD (1987) Effect of methyl bromide fumigation on the
germinability of wheat seed. Seed Res, 15: 183-186.
Kiewnick L (1968) Occurrence and control of sclerotium rolfsii.
Meded Fac Landbouwwet Rijksuniv Gent, 33: 987-995.
King JR & Benschoter CA (1991) Comparative methyl bromide residues in
Florida citrus: A basis for proposing quarantine treatments against
caribbean fruit fly. J Agric Food Chem, 39: 1307-1309.
King JR, Benschoter CA, & Burditt AK, Jr (1981) Residues of methyl
bromide in fumigated grapefruit determined by a rapid, headspace
assay. J Agric Food Chem, 29: 1003-1005.
Kishi R, Ishizu I, Ito I, Katoh N, Miyake H, & Harabuch I (1988)
Health research of methyl bromide manufacturing workers. Part 1.
Symptoms of long-term exposure. Occupational health in the chemical
industry. XXII ICOH (International Commission on Occupational Health)
Congress, Sydney, New South Wales, Australia, 27 September-2 October
1987. Copenhagen, World Health Organization Regional Office for
Europe, pp 120-134.
Kishi R, Itoh I, Ishizu S, Harabuchi I, & Miyake H (1991) Symptoms
among workers with long-term exposure to methyl bromide. An
epidemiological study. Jpn J Ind Health, 33: 241-250.
Kisser W (1967) [The detection and quantitative determination of
brominated urea derivatives in toxicology.] Arch Toxicol, 22: 404-409
(in German).
Kissler JJ, Lider JV, Raabe RD, Raski DJ, Schmitt RV, & Hurlimann JH
(1973) Soil fumigation for control of nematodes and oak root fungus in
vineyard replants. Plant Dis Rep, 57: 115-119.
Klein L (1989) Methyl bromide strip fumigation - problems encountered
and ways to combat them. Third International Symposium on Soil
Disinfestation, Leuven, Belgium, September 26-30, 1988. Acta Hortic,
255: 213-225.
Knight HD & Costner GC (1977) Bromide intoxication of horses, goats,
and cattle. J Am Vet Med Assoc, 171: 446-448.
Knight HD & Reina-Guerra M (1977) Intoxication of cattle with sodium
bromide-contaminated feed. Am J Vet Res, 38: 407-409.
Koga M, Hara K, Hori H, Kodama Y, & Okubo T (1991) [Determination of
bromide ion concentration in urine using a head-space gas
chromatography and an ion chromatography. Biological monitoring for
methyl bromide exposure.] J Univ Occup Environ Health, 13: 19-24 (in
Japanese with English summary).
Kolbezen MJ & Abu-El-Haj FJ (1972) Fumigation with methyl bromide. II.
Equipment and methods for sampling and analysing deep field soil
atmospheres. Pestic Sci, 3: 73-80.
Kramers PGN, Bissumbhar B, & Mout HCA (1985b) Studies with gaseous
mutagens in Drosophila melanogaster. In: Waters MD, Sandhu SS, Lewtas
J, Claxton L, Straus G, & Nesnow S, ed. Short-term bioassays in the
analysis of complex environmental mixtures IV., New York, London,
Plenum Press, pp 65-73.
Kramers PGN, Voogd CE, Knaap AGAC, & Van der Heijden CA (1985a)
Mutagenicity of methyl bromide in a series of short-term tests. Mutat
Res, 155: 41-47.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Latta R & Cowgill H (1941) Methyl bromide fumigation of greenhouse
plants at U.S. Plant Introduction Garden, Glenn Dale, Md. (Abstract
S.8189, Bureau of Entomology and Plant Quarantine). Washington, DC, US
Department of Agriculture (E-526).
Laug EP (1941) Bromide residues in foodstuffs. Volatile and
nonvolatile residues following experimental exposure to methyl
bromide. Ind Eng Chem, 33: 803-805.
Leesch JG, Gillenwater HB, & Woodward JO (1974) Methyl bromide
fumigation of shelled peanuts in bulk containers. J Econ Entomol, 67:
769-771.
Lefevre C, Ferrari P, Günier JP, & Muller J (1989) Sampling and
analysis of airborne methylbromide. Chromatographia, 27: 37-43.
Leichnitz K (1985) [Dräger-tubes methyl bromide 3/a. Detector-tubes
pocket book 6.] Lübeck, Dragerwerk (in German).
Lepschy J, Stark H, & Sub A (1979) [Behaviour of methyl bromide
(Terabol) in soil.] Gartenbauwissenschaften, 44: 84-89 (in German).
Lewis SE (1948) Inhibition of SH proteins by methyl bromide. Nature
(Lond), 161: 692-693.
Linenberg A, Davis R, & Robinson D (1991) Hazardous waste site
measurements of low levels of chlorinated hydrocarbons using a
portable gas chromatography. Hazard Mater Control, 4: 42-46.
Long PL, Brown WB, & Goodship G (1972) Effect of methyl bromide on
coccidial oocysts determined under controlled conditions. Vet Rec, 90:
562-566.
Longley EO & Jones AT (1965) Methyl bromide poisoning in man. Ind Med
Surg, 34: 499-502.
Lopez VC, Fernandez JAM, Yanez LP, & Vazquez MS (1986a) [Methyl
bromide poisoning: I. Some historical, clinical, legal and
neuropsychiatric aspects.] Arch Neurobiol, 49: 279-290 (in Spanish).
Lopez CV, Fernandez MJA, Yanez PL, & Vazquez SM (1986b) [Methyl
bromide poisoning. II. Contribution of 2 cases.] Arch Neurobiol, 49:
311-328 (in Spanish).
Lopez-Avila V, Wood R, Flanagan M, & Scott R (1987) Determination of
volatile priority pollutants in water by purge and trap and capillary
column gas chromatography/mass spectrometry. J Chromatogr Sci, 25:
286-291.
Love JL, Winchester RV, & Keating DL (1979) Fumigant and contact
insecticide residues on dried fruits, cereals, nuts, and spices
imported into New Zealand. N Z J Sci, 22: 95-98.
Lovelock JE (1975) Natural halocarbons in the air and in the sea.
Nature (Lond), 256: 193-194.
Lynn GE, Shrader SA, Hammer OH, & Lassiter CA (1963) Occurrence of
bromides in the milk of cows fed sodium bromide and grain fumigated
with methyl bromide. J Agric Food Chem, 11: 87-91.
Mabey W & Mill T (1978) Critical review of organic compounds in water
under environmental conditions. J Phys Chem Ref Data, 7: 383-415.
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds, Report No. 32 - Individual compound report: methyl bromide.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, 190 pp (PB83-130211).
Mackay D & Shiu WK (1981) A critical review of Henry's Law Constants
for chemicals of environmental interest. J Phys Chem Ref Data, 10:
1175-1199.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. In: Gunter FA
ed. Reviews of environmental contamination and toxicology. Berlin,
Heidelberg, New York, Springer-Verlag, vol 14, pp 57-123.
MAFF (1990) Report of the working party on pesticide residues:
1988-89. Supplement to issue No. 8 (1990) of the pesticides register.
London, Ministry of Agriculture, Fisheries and Food, Health and Safety
Executive, 11 pp.
MAFF (1989) Report of the working party on pesticide residues:
1985-88. The twenty-fifth report of the steering group on food
surveillance. London, Ministry of Agriculture, Fisheries and Food, 3
pp (Food Surveillance Paper No. 25).
Malkomes H-P (1970) [Investigations into the degradation of methyl
bromide during substrate fumigation and its influence on the following
crops.] Hanover, Technical University, pp 18-35 (Dissertation) (in
German).
Marraccini JV, Thomas GE, Ongley JP, Pfaffenberger D, Davis JH, &
Bednarczyk LR (1983) Death and injury caused by methyl bromide, an
insecticide fumigant. J Forensic Sci Soc, 28: 601-607.
Matheson gas data book (1980) The Matheson gas data book. East
Rutherford, New Jersey, Matheson Gas products (A Division of Will
Ross), vol 6, pp 361-363.
Matta A & Porta-Puglia A (1968) [Sensitivity to methyl bromide and
vapam and secondary growth in fumigated soil of distinct functional
groups of microorganisms.] Ann Fac Agrar, 4: 261-274 (in Italian).
Maw GA & Kempton RJ (1973) Methyl bromide as a soil fumigant. Soils
Fertil, 36: 41-47.
Mazzini L, Galante M, Rezzonico M, & Kokodoko A (1992) Methylbromide
intoxication: A case report. Schweiz Arch Neurol Psychiatr, 143:
75-80.
MBSW (1992) Proceedings of the Methyl Bromide Science Workshop,
Washington, DC, 2-3 June 1992. Washington, DC, Atmospheric
Environmental Research, Inc., pp 90-97.
Medinsky MA, Bond JA, Dutcher JS, & Birnbaum LS (1984) Disposition of
[14C]methyl bromide in Fischer-344 rats after oral or
intraperitoneal administration. Toxicology, 32: 187-196.
Medinsky MA, Dutcher JS, Bond JA, Henderson RF, Mauderly JL, Snipes
MB, Mewhinney JA, Cheng YS, & Birnbaum LS (1985) Uptake and excretion
of [14C]methyl bromide as influenced by exposure concentration.
Toxicol Appl Pharmacol, 78: 215-225.
Meister RT (1985) In: Meister RT ed. Farm chemicals handbook 85.
Pesticide dictionary -Section C. Willoughby, Ohio, Meister Publishing
Co., 154 pp.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992a) Atmospheric lifetimes and ozone
depletition potentials of methyl bromide (CH3Br) and dibromomethane
(CH2Br2). Geophys Res Lett, 19: 2059-2062.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992b) Correction to "atmospheric lifetimes and
ozone depletion potentials of methylbromide (CH3Br) and
dibromomethane (CH2Br2)". Geophys Res Lett, 19: 2279-2280.
Merzbach L (1928) [The pharmacology of methyl bromide and some related
compounds.] Z. Ges. Exp. Med., 63: 383-392 (in German).
Mignard E & Benet JC (1989) Diffusion of methyl bromide in soil. J
Soil Sci, 40: 151-165.
Miller DP & Haggard HW (1943) Intracellular penetration of bromide as
a feature in the toxicity of alkyl bromides. J Ind Hyg Toxicol, 25:
423-433.
Minton NA & Gillenwater HB (1973) Methyl bromide fumigation of
Pratylenchus brachyurus in peatnut shells. J Nematol, 5: 147-149.
Mitsumori K, Maita K, Kosaka T, Miyaoka T, & Shirasu Y (1990) Two-year
oral chronic toxicity and carcinogenicity study in rats of diets
fumigated with methyl bromide. Food Chem Toxicol, 28: 109-119.
Miyagawa M (1982) Conditioned taste aversion induced by inhalation
exposure to methyl bromide in rats. Toxicol Lett, 10: 411-416.
Mizyukova IG & Bakhishev CN (1971) [Specific treatment of acute
poisoning with methyl bromide.] Vrach Delo, 7: 128-131 (in Russian
with English abstract).
Moelwyn-Hughes EA (1938) The hydrolysis of the methyl halides. Proc R
Soc, A164: 295-306.
Moje W (1960) The chemistry and nematocidal activity of organic
halides. In: Metcalf RL, ed. Volume III. Advances in pest control
research. New York, Intersience Publishers, pp 181-217.
Molina LT, Molina MJ, & Rowland FS (1982) Ultraviolet absorption cross
sections of several brominated methanes and ethanes of atmospheric
interest. J Phys Chem, 86: 2672-2676.
Monro HAU, Dumas T, & Ruckland CT (1966) The influence of vapour
pressure of different fumigants on the mortality of two stored-product
insects in vacuum fumigation. J Stored Prod Res, 1: 207-222.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrissey RE, Schwetz BA, Lamb JCIV, Ross MD, Teague JL, & Morris RW
(1988) Evaluation of rodent sperm, vaginal cytology, and reproductive
organ weight data from National Toxicology Program 13-week studies.
Fundam Appl Toxicol, 11: 343-358.
Mostafa SAS, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1972) Toxicity
of carbon bisulphide and methyl bromide to the eggs of four stored
product insects. J Stored Prod Res, 8: 193-198.
Mounat A & Hitier H (1959) Chemical disinfection of the soil of
tobacco seed beds. Ann Inst Exp Tabac Bergerac, 3: 287-298.
Mueller A, Jorritsma U, Hindermeier U, & Gansewendt B (1991) Human
serum albumin adducts as a parameter for biomonitoring of exposure to
methyl bromide. Naunyn-Schmiedeberg's Arch Pharmacol, 344: R121.
Mueller A, Jorritsma U, & Gansewendt B (1992) Different binding of
methyl bromide to blood macromolecules due to conjugator status.
Naunyn-Schmiedeberg's Arch Pharmacol, 345: R 42.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol, 17:
405-429.
Munnecke DE, Kolbezen MJ, & Stolzy LH (1969) Factors affecting field
fumigation of citrus soils for control of Armillaria mellea.
Proceedings of the First International Citrus Symposium. Lancaster,
Pennsylvania, Society of the Plastics Industry, vol 3, pp 1273-1277.
Munnecke DE, Kolbezen MJ, & Wilbur WD (1978) Types and thickness of
plastic films in relation to methyl bromide fumigation. Proceedings of
the Agricultural Plastic Congress, San Diego, pp 482-487.
National Academy of Science (1978) Chloroform, carbon tetrachloride,
and other halomethanes: an environmental assessment. Washington, DC,
National Academy of Sciences, 294 pp.
NASA (1992) Chemical kinetics and photochemical data for use in
stratospheric modeling. Evaluation No. 10: NASA Panel for Data
Evaluation. Pasadena, California Institute of Toxicology, Jet
Propulsion Laboratory, National Aeronautics and Space Administration.
NFPA (1984) Fire protection guide on hazardous materials, 9th ed.
Quincy, Maryland, National Fire Protection Agency, 5 pp (NFPA No.
SPP-1E).
NIOSH (1984) Monohalomethanes: methyl chloride CH3Cl, methyl bromide
CH3Br, methyl iodide CH3I. Curr Intell Bull, 42: 1-22.
Nishimura M, Umeda M, Ishizu S, & Sato M (1980) Effect of methyl
bromide on cultured mammalian cells. J Toxicol Sci, 5: 321-330.
NTP (1992) Toxicology and carcinogenesis studies of methyl bromide
(CAS No. 74-83-9) in B6C3F1 mice (inhalation studies). Research
Triangle Park, North Carolina, National Toxicology Program, 212 pp
(Technical Report Series No. 385).
Oakes JY, Bollich CN, Melville DR, Fielding MJ, & Hollis JP (1956)
Soil fumigation for control of parasitic nematodes on corn at Curtis,
Louisiana. Plant Dis Rep, 40: 853-854.
Ohmori S & Hirata M (1982) [Determination of bromine contents in blood
and hair of workers exposed to methyl by radioactivation analysis
method bromide]. Jpn J Ind Health, 24: 119-125 (in Japanese).
O'Neal L (1987) Acute methyl bromide toxicity. J. Emerg Nurs, 13:
96-98.
Ong JM, Stewart J, Wen Y, & Whong W (1987) Application of SOS umu-test
for the detection of genotoxic volatile chemicals and air pollutants.
Environ Mutagen, 9: 171-176.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
Ostrec L & Korunic Z (1989) [Some new knowledge on the applying of
methylbromide in the tobacco nurseries.] Zast Bilja, 40: 375-385 (in
Yugoslavian with English abstract).
Page BD & Avon RJ (1989) Determination of methyl bromide in foods by
headspace capillary gas chromatography with electron capture
detection. J Assoc Off Anal Chem, 72: 815-822.
Pauwels F (1989) Soil disinfestation in the Belgian horticulture: a
practice view. Third International Symposium on Soil Disinfestation,
Leuven, Belgium, September 26-30, 1988. Acta Hortic, 255: 31-36.
Peers AMK (1985) The determination of methyl bromide in air. In:
Fishbein L & O`Neill IK ed. Environmental carcinogens selected methods
of analysis. Volume 7 - Some volatile halogenated hydrocarbons. Lyon,
International Agency for Research on Cancer, pp 227-233 (IARC
Scientific Publications No. 68).
Pellizzari ED, Zweidinger RA, & Erickson MD (1978) Environmental
monitoring near industrial sites: brominated chemicals part II:
appendix. Washington, DC, US Environmental Protection Agency
(EPA-560/6-78-002A; US NTIS PB-286483).
Pence RJ & Morganroth J (1962) Field effects of methyl bromide on
carpet beetle eggs. Pest Control, 30: 20, 22, 24.
Penkett SA, Jones BMR, Rycroft MJ, & Simmons DA (1985) An
interhemispheric comparison of the concentrations of bromine compounds
in the atmosphere. Nature (Lond), 318: 550-553.
Perrotta G (1968) [Combating `Tracheomycosis' (tomato wilt disease) in
greenhouse tomatoes.] Not Mal Piante, 78-79: 135-140 (in Italian).
Pesticide & Toxic Chemical News (1984) No evidence of methyl bromide
carcinogenicity found by NTP-panel. Pestic Toxic Chem News, 13: 9-10.
Peters PWJ, Verhoef A, De Liefde A, Van Velsen FL, Van Soolingen J, De
Geus D, Danse LHJ, & Van Logten MJ (1982) [Teratogenicity study of
methyl bromide by oral administration.] Bilthoven, National Institute
for Public Health and Environmental Protection (RIVM Report No. 618102
002) (in Dutch).
Piet GJ, Luijten WCMM, & Van Noort PCM (1985) 'Dynamic' head-space
determination of volatile organic halogen compounds in water. In:
Fishbein L & O`Neill IK, ed. Environmental carcinogens selected
methods of analysis. Volume 7 - Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Scientific Publications No. 68).
Polkowski J, Crowley MS, & Moore AM (1990) Unintentional methyl
bromide gas release- Florida, 1988. Morbid Mortal Wkly Rep, 38:
880-882.
Poulet G, Pirre M, Maguin F, Ramaroson R, & Le Bras G (1992) "Role of
the BrO + HO2 reaction in the stratospheric chemistry of bromine".
Geophys Res Lett, 19: 2305-2308.
Powell DF (1975) The effect of methyl bromide fumigation on the
germination of onion seed and vigor of the seedlings. Plant Pathol,
24: 237-241.
Prain JH & Harvey Smith G (1952) A clinical-pathological report of
eight cases of methyl bromide poisoning. Br J Ind Med, 9: 44-49.
Prather MJ & Watson RT (1990) Stratospheric ozone depletion and future
levels of atmospheric chlorine and bromine. Nature (Lond), 344:
729-734.
Prather MJ, McElroy MB, & Wofsy SC (1984) Reductions in ozone at high
concentrations of stratosperic halogens. Nature (Lond), 312: 227-231.
Prishchep AG & Nikiforova EN (1969) [Bactericidal action of methyl
bromide on Escherichia coli group bacteria.] Sci Trans Inst Vet Sanit,
32: 279-281 (in Russian).
Prockop LD & Smith AO (1986) Seizures and action myoclonus after
occupational exposure to methyl bromide. J Fla Med Assoc, 73: 690-692.
Raabe OG (1986) Inhalation uptake of selected chemical vapors at trace
levels. Final report to the California Air Resources Board Control
(No. A3-132-33). Springfield, Virginia, National Technical Information
Service, 290 pp (NTIS PB 86-209863).
Raabe OG (1988) Inhalation uptake of xenobiotic vapors by people
(Final report August 1986-March 1988). Davis, University of
California, Laboratory for Energy-related Health Research, 94 pp (ISS
ARB-R-88/338; PB 88-202726).
Rasche E, Hyman MR, & Arp DJ (1990) Biodegradation of halogenated
hydrocarbon fumigants by nitrifying bacteria. Appl Environ Microbiol,
56: 2568-2571.
Raski DJ, Jones NO, Kissler JJ, & Luvisi DA (1975) Further results
from deep-placement fumigation for control of nematodes in vineyards.
Plant Dis Rep, 59: 345-349.
Rasmussen RA & Khalil MAK (1984) Gaseous bromine in the arctic and
arctic haze. Geophys Res Lett, 11: 433-436.
Rathus EM & Landy PJ (1961) Methyl bromide poisoning. Br J Ind Med,
18: 53-57.
Reeves RG, McDaniel CA, & Ford JH (1985) Organic and inorganic bromide
residues in spices fumigated with methyl bromide. J Agric Food Chem,
33: 780-783.
Reichmuth C & Noack S (1983) [Environmental effects of the fumigation
of commodities.] Technol Z Getreide Mehl Backwaren, 37: 139-144 (in
German).
Reuzel PGJ, Dreef-Van der Meulen HC, Hollanders VMH, Kuper CF, Feron
VJ & Van der Heijden CA (1991) Chronic inhalation toxicity and
carcinogenicity study of methyl bromide in Wistar rats. Food Chem
Toxicol, 29: 31-39.
Richardson HH & Monro HAU (1962) Fumigation of jute bags with ethylene
oxide and methyl bromide to eradicate potato ring rot bacteria. Appl
Microbiol, 10: 448-451.
Richardson HH & Roth H (1965) Methyl bromide, sulfuryl fluoride, and
other fumigants against quarantinable Cochlicella and Theba snails. J
Econ Entomol, 58: 690-693.
Robbins DE (1976a) Photodissociation of methyl chloride and methyl
bromide in the atmosphere. Geophys Res Lett, 3: 213-216.
Robbins DE (1976b) UV absorption cross sections for methyl bromide and
methyl chloride. Geophys Res Lett, 3: 757-758.
Rogozen MB, Rich HE, Guttman MA, & Grosjean D (1987) Evaluation of
potential toxic air contaminants. Phase 1. Final report (ISS
SAIC-76/1035, ARB-R-88/3339. Manhattan Beach, California, Science
Applications International Corporation, 582 pp (PB 88-183330/GAR)
Rolston DE & Glauz RD (1982) Comparisons of simulated with measured
transport and transformation of methyl bromide gas in soils. Pestic
Sci, 13: 653-664.
Roosels D, Van den Oever R, & Lahaye D (1981) Dangerous concentrations
of methyl bromide used as a fumigant in Belgian greenhouses. Int Arch
Occup Environ Health, 48: 243-250.
Rosenblum I, Stein AA, & Eisinger G (1960) Chronic ingestion by dogs
of methyl bromide-fumigated food. Arch Environ Health, 1: 316-323.
Roskill (1992) Report on the economics of bromine, 6th ed. London,
Roskill Information Service, p 58.
Roth H (1973) Fumigants for quarantine control of the adult brown dog
tick: laboratory studies. J Econ Entomol, 66: 1283-1286.
Roth H & Kennedy JW (1972) Methyl bromide and aluminium phosphide as
fumigants for control of adult boll weevils: laboratory studies. J
Econ Entomol, 65: 1650-1651.
Roth H & Kennedy JW (1973) Helicella snails infesting rosemary seeds.
Methyl bromide and other fumigants for quarantine control. J Econ
Entomol, 66: 935-936.
Roth H & Richardson HH (1974) Broadbeen weevil: methyl bromide
fumigation of infested faba beans. J Econ Entomol, 67: 799-803.
Rotteveel AJW & Naber H (1987) The use of soil fumigation against
yellow nutsedge. Meded Fac Landbouwwet Rijksuniv Gent, 52: 1207-1212.
Roughan JA & Roughan PA (1984a) Pesticide residues in foodstuffs in
England and Wales: Part I. Inorganic bromide ion in lettuce grown in
soil fumigated with bromomethane. Pestic Sci, 15: 431-438.
Roughan JA & Roughan PA (1984b) Pesticide residues in foodstuffs in
England and Wales. Part II: Inorganic bromide ion in cucumber, tomato
and self-blanching celery grown in soil fumigated with bromomethane,
and the "natural" bromide ion content in a range of fresh fruit and
vegetables. Pestic Sci, 15: 630-636.
Roughan JA, Roughan PA, & Wilkins JPG (1983) Modified gas-liquid
chromatographic method for determining bromide/total bromine in
foodstuffs and soils. Analyst, 108: 742-747.
Rovira AD & Ridge EH (1979) The effect of methyl bromide and
chloropicrin on some chemical and biological properties of soil and on
the growth and nutrition of wheat. Dev Agr Managed For Ecol, 6:
231-250.
Roycroft JH, Jascot RH, Grose EC, & Gardner DE (1981) The effects of
inhalation exposure of methyl bromide in rats. Toxicologist, 1: 79.
Russo JM, Anger WK, Setzer JV, & Brightwell WS (1984) Neurobehavioural
assessment of chronic low-level methyl-bromide exposure in the rabbit.
J. Toxicol Environ Health, 14: 247-255.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A/142-A/151.
Saiki E (1952) Studies on the disinfecting effect of chloropicrin,
methyl bromide, prussic acid and formalin on bacteria. Nagoya J Med
Sci, 15: 270.
Saltzman BE (1983) Direct reading colorimetric indicators. In: Lioy PJ
& Lioy MJY ed. Air sampling instruments for evaluation of atmospheric
contaminations, 6th ed. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 29 pp.
Sangster J (1989) Octanol-water partition coefficients of simple
organic compounds. J Phys Chem Ref Data, 18: 1185.
Sardesai JB (1972) Response of diapausing and nondiapausing larvae of
Plodia interpunctella to hydrogen cyanide and methyl bromide. J Econ
Entomol, 65: 1562-1565.
Sassaman JF, Jacobs MM, Chin PH, Hsia S, Pienta RJ, & Kelly JM (1986)
Pesticide background statements: methyl bromide and chloropicin. In:
Volume II. Fungicides and fumigants. Washington, DC, US Department of
Agriculture, Forest Service (MB/C-1-156) (Agriculture Handbook No.
661).
Sato M, Miyagawa M, Honma T, & Hasegawa H (1985) Subacute effects of
methyl bromide dosed by inhalation exposure to rats. Ind Health, 23:
235-238.
Sax NI, Feiner B, Fitzgerald J, Haley TJ & Weisburger EK (1984)
Dangerous properties of industrial materials, 6th ed. New York, Van
Nostrand Reinhold Company, p 531.
Sayers RR, Yant WP, Thomas BGH, & Berger LB (1929) Physiological
response attending exposure to vapors of methyl bromide, methyl
chloride, ethyl bromide and ethyl chloride. Public Health Bull, 185:
1-56.
Schauffler SM, Heidt LE, Pollock WH, Gilpin TM, Vedder J, Solomon S,
Lueb RA, & Atlas EL (in press) Measurements of halogenated organic
compounds near the tropical tropopause. Geophys Res Lett.
Scheffrahn RH, Bodalbhai L, & Su NY (1992) Residues of methyl bromide
and sulfuryl fluoride in manufacturer-packaged household foods
following fumigation. Bull Environ Contam Toxicol, 48: 821-827.
Scheffrahn RH & Su NY (1992) Comparative toxicity of methyl bromide
against ten nearctic termite species (Isoptera: Termopsidae,
Kalotermitidae, Rhinotermitidae). J Econ Entomol, 85: 845-847.
Schroeder KR, Hallier E, Peter H, & Bolt H (1992) Dissociation of a
new glutathione S-transferase activity in human erythrocytes. Biochem
Pharmacol, 43: 1671-1674.
Scotto La Massese C & Mars S (1975) Etude préliminaire des résidus de
brome dans les cultures faites dans des sols ayant reçu des
applications de brome minéral KBr et organique CH3Br. Phytiatr
Phytopharm, 24: 57-66.
Scudamore KA (1985) Multi-residue gas chromatographic method for
determination of fumigant residues in cereal grains and other foods.
In: Fishbein L & O`Neill IK ed. Environmental carcinogens selected
methods of analysis. Volume 7 - Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
351-359 (IARC Scientific Publications No. 68).
Scudamore KA (1985) Determination of methyl bromide in grain using
head-space analysis. In: Fishbein L & O`Neill IK ed. Environmental
carcinogens selected methods of analysis. Volume 7 - Some volatile
halogenated hydrocarbons. Lyon, International Agency for Research on
Cancer, pp 375-380 (IARC Scientific Publications No. 68).
Scudamore KA (1987) Fumigant residues in wheat and other cereal grains
resulting from the use of 1,1,1-trichloroethane and bromomethane as a
liquid fumigant mixture. Pestic Sci, 20: 1-18.
Segers JHL, Temmink JHM, Van den Berg JHJ, & Wegman RCC (1984)
Morphological changes in the gill of carp ( Cyprinus carpio L.)
exposed to acutely toxic concentrations of methyl bromide. Water Res,
18: 1437-1441.
Sell CR & Moffitt HR (1990) Non-destructive method for estimating
methyl bromide residues in apples during aeration following
fumigation. Pestic Sci, 29: 19-28.
Sell CR, Klag NG, & Burditt AK, Jr (1988) Methyl bromide residues in
fresh cherries: effects of parameters in fumigation. Pestic Sci, 23:
41-50.
Sher SA, Thomason IJ, & McCaslin RL (1958) Chisel application of
methyl bromide for root-knot nematode control. Plant Dis Rep, 42:
288-290.
Shield LK, Coleman TL, & Markesbery WR (1977) Methyl bromide
intoxication: Neurologic features, including simulation of Reyes
syndrome. Neurology, 27: 959-962.
Shivanandappa T & Rajendran S (1987) Induction of glutathione
S-transferase by fumigants in larvae of the khapra beetle,
Trogoderma granarium (E.). Pestic Biochem Physiol, 28: 121-126.
Siegwart Y (1987) [The implementation of food quality control in
Switzerland in the year 1986.] Mitt Geb Lebensm.unters Hyg, 78:
237-256 (in German).
Sikov MR, Cannon WC, Carr DB, Miller RA, Montgomery LF, & Phelps DW
(1981) Teratologic assessment of buthylene oxide, styrene oxide and
methyl bromide (Contract-No. 210-78-0025). Cincinnati, Ohio, US
Department of Health and Human Services, 84 pp.
Simmon VF, Kauhanen K, & Tardiff RG (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH, ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North-Holland Biomedical Press, pp 249-258.
Singh HB, Salas LJ, Smith SJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1983) Methyl halides in and over the
Eastern Pacific (40°N-32°S). J Geophys Res, 88: 3684-3690.
Singh ON, Borchers R, Fabian P, Lal S, & Subbaraya BH (1988)
Measurements of atmospheric BrOx radicals in the tropical and
mid-latitude atmosphere. Nature (Lond), 334: 593-595.
Singh HB, Salas L, Viezee W, Sitton B, & Ferek R (1992) Measurement of
volatile organic chemicals at selected sites in California. Atmos
Environ, 26A: 2929-2946.
Sittisuang P & Nakakita H (1985) The effect of phosphine and methyl
bromide on germination of rice and corn seeds. J Pestic Sci, 10:
461-468.
Sivasithamparam K, MacNish GC, Fang CS, & Parker CA (1987) Microflora
of soil and wheat rhizosphere in a field following fumigation. Aust J
Soil Res, 25: 491-498.
Slooff W & Canton JH (1983) Comparison of the susceptibility of 11
freshwater species to 8 chemical compounds. II. (Semi)chronic toxicity
tests. Aquat Toxicol, 4: 271-282.
Smart NA (1990) Residues in foodstuffs from bromomethane soil
fumigator. Adv Environ Sci Technol, 23: 227-255.
Smirnov AM (1970) [Disinfection of combs containing beebread by gases
in contagious bee diseases.] Sci Trans Inst Vet Sanit, 37: 267-280 (in
Russian).
Soremark R (1960) The biological half-life of bromide ions in human
blood. Acta Physiol Scand, 50: 119-123.
Spivakovsky CM, Yerich R, Logan JA, Wofsy SC, McElroy, & Prather MJ
(1990) Tropospheric OH in a three-dimensional chemicaltracer model: An
assessment based on observations of CH3 CC/3. J Geophys Res, 95:
18,441-18,471.
Squier MV, Thompson J, & Rajgopalan B (1992) Case report:
Neuropathology of methyl bromide intoxication. Neuropathol Appl
Neurobiol, 18: 579-584.
Starratt AN & Bond EJ (1981) Metabolism of methyl bromide by
susceptible and resistant strains of the granary weevil, Sitophilus
granarius (L.). Pestic Biochem Physiol, 15: 275-281.
Starratt AN & Bond EJ (1988a) Methylation of DNA of maize and wheat
grains during fumigation with methyl bromide. J Agric Food Chem, 36:
1035-1039.
Starratt AN & Bond EJ (1988b) In vitro methylation of DNA by the
fumigant methyl bromide. J. Environ. Health., B23: 513-525.
Starratt AN & Bond EJ (1990a) Residues of methyl bromide in fumigated
commodities. Study submitted by Methyl Bromide Panel. London, Ontario,
Research Centre, Agriculture Canada, 238 pp.
Starratt AN & Bond EJ (1990b) Methylation of food commodities during
fumigation with methyl bromide. In: Studies of the magnitude and
nature of pesticide residues in stored products using radiotracer
techniques. London, Ontario, Research Centre, Agriculture Canada, pp
97-109.
Stein ER & Wolfenbarger DA (1989) Methyl bromide residues in fumigated
mangos. J Agric Food Chem, 37: 1507-1509.
Stenger VA (1978) Bromine compounds. In: Kirk-Othmer encyclopedia of
chemical technology, 3rd ed. New York, Chichester, Brisbane, Toronto,
John Wiley and Sons, vol 4, pp 243-263.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid the diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Strider DL (1975) Chemical control of bacterial blight of Rieger
elatior begonias caused by Xanthomonas begoniae. Plant Dis Rep, 59:
66-70.
Swartz RD, Millman RP, Billi JE, Bondar NP, Migdal SD, Simonian SK,
Monforte JR, McDonald FD, Harness JK, & Cole KL (1981) Epidemic
methanol poisoning: clinical and biochemical analysis of a recent
episode. Medicine, 60: 373-382.
Tanaka S, Arito H, Abuku S, & Imamiya S (1988) Acute effects of methyl
bromide on electroencephalographic activity and sleep-wakefulness in
rats. Ind Health, 26: 101-114.
Tanaka S, Abuku SI, Seki Y, & Imamiya SI (1991) Evaluation of methyl
bromide exposure on the plant quarantine fumigators by environmental
and biological monitoring. Ind Health, 29: 11-22.
Thier P & Zeumer H (1987) Manual of pesticide residue analysis. Volume
1. Bromine-containing fumigants (as total inorganic bromide).
Weinheim, Verlag Chemie, pp 377-381.
Thomas DA & Morgan KT (1988) Olfactory toxicity: studies of methyl
bromide. Chem Ind Inst Toxicol (CIIT) Act, 8: 1-7.
Thomason IJ (1959) Chisel application of methyl bromide for control of
root-knot nematode and fusarium wilt. Plant Dis Rep, 43: 580-583.
Thompson RH (1966) A review of the properties and usage of methyl
bromide as a fumigant. J Stored Prod Res, 1: 353-376.
Tie X, Ko, C-Y, Mroz EJ, Cicerone RJ, Alyea FN, & Cunnold DM (1992)
Three-dimensional simulation of atmospheric methyl chloroform: Effect
of an ocean sink. J Geophys Res, 97: 20,751-20,769.
Tkalich PP (1972) [Effectiveness of methyl bromide used against the
hemp leaf roller.] Khim Sel'sk Khoz, 10: 350-352 (in Russian).
Tkalich PP (1974) [Toxicological evaluation of fumigated hemp seeds.]
Tr Nauch Issled Inst Lub Kul't, 35: 97-101 (in Russian).
Torkelson TR & Rowe VK (1981) Methyl bromide. In: Patty's industrial
hygiene and toxicology, 3rd rev. edn. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 3442-3446.
Tucker JF, Brown BW, & Goodship G (1974) Fumigation with methyl
bromide of poultry foods artificially contaminated with Salmonella. Br
Poult Sci, 15: 587-595.
Tucker JD, Xu J, Stewart J, & Ong T (1985) Development of a method to
detect volatile genotoxins using sister chromatid exchanges. Environ
Mutagen, 7: 48 (Abstract).
Tucker JD, Xu J, Stewart J, Baciu PC, & Ong T (1986) Detection of
sister chromatid exchanges induced by volatile genotoxicants. Teratog
carcinog mutagen, 6: 14-21.
Tunney J (1972) The use of methyl bromide in disease control. Mushroom
Sci, 8: 755-762.
Turtura GC, Massa S, & Zani G (1988) Effects of methyl bromide on some
bacterial groups in greenhouse soils. Ann Microbiol Enzimol, 38:
93-98.
Uncini A, Basciani M, Di Muzio A, Antonini D, & Onofrj M (1990) Methyl
bromide myoclonus: an electro physiological study. Acta Neurol Scand,
81: 159-164.
UNEP (1992) Methyl bromide and the ozone layer: a summary of current
understanding; Montreal Protocol Assessment Supplement; Synthesis
report of the methyl bromide interim scientific assessment and methyl
bromide interim technology and economic assessment requested by the
United Nations Environment Programme on behalf of the Contracting
Parties to the Montreal Protocol, June 1992. Nairobi, United Nations
Environment Programme, pp 33.
Urga K (1983) Methyl bromide and phosphine residues in cereal grains
and pulses. Ethiop Med, 21: 79-83.
US EPA (1982a) Method 8010. Halogenated volatile organics, 2nd ed.
Washington, DC, US Environmental Protection Agency, pp 278-289 (EPA
No. SW-846; PB 87-120-291).
US EPA (1982b) Method 8240. GS/MS method for volatile organics, 2nd
ed. Washington, DC, US Environmental Protection Agency, pp 433-454
(EPA No. SW-846; PB 87-120-291).
US EPA (1984a) Method 624. Guidelines establishing test procedures for
the analysis of pollutants under the Clean Water Act (40 CFR 136
Purgeables). Fed Reg, 49: 43373-43384.
US EPA (1984b) Appendix A to part 136 - Methods for organic chemical
analysis of principal and industrial wastewater. Method 601. Purgeable
halocarbons. Fed Reg, 49: 43261-43271.
US EPA (1984c) Method 1624. Revision B. Guidelines establishing test
procedures for the analysis of pollutants under the Clean Water Act
(40 CFR 136). Volatile organic compounds by isotope dilution GC/MS.
Fed Reg, 49: 43407-43415.
US EPA (1988a) Pesticide fact handbook. Park Ridge, New Jersey, Noyes
Data Corporation.
US EPA (1988b) Pesticide tolerances for inorganic bromides resulting
from fumigation with methyl bromide; correction. Fed Reg, 53:
30053-30054.
Uthman AP, Demlein PJ, Allston TD, Withiam MC, McClements MJ, & Takacs
GA (1978) Photoabsorption spectra of gaseous methyl bromide, ethylene
dibromide, nitrosyl bromide, thionyl chloride and sulfuryl chloride.
J Phys Chem, 82: 2252-2257.
Vanachter A (1974) Soil disinfestation in cauliflower, tomato, and
witloof crops in Belgium. Agric Environ, 1: 265-276.
Vanachter A, Feyaerts J, Van Wambeke E, & Van Assche C (1981) Bromide
concentrations in water after methyl bromide soil disinfestation. II.
Relation between leaching of methyl bromide fumigated greenhouse soils
and bromide concentrations in the surrounding surface waters. Meded
Fac Landbouwwet Rijksuniv Gent, 46: 351-358.
Van den Oever R, Van de Mierop L, & Lahaye D (1978) [Intoxication of
professionally exposed methyl bromide workers.] Belg Arch Soc Gen, 36:
353-369 (in Flemish).
Van den Oever R, Roosels D, & Lahaye D (1982) Actual hazard of methyl
bromide fumigation in soil disinfection. Br J Ind Med, 39: 140-144.
Van Doorn IAM, Feron VJ, Van Genderen H, & Zoeteman BCJ (1989) [Report
of the Netherlands Committee concerning the use of methyl bromide as
a soil disinfectant in agriculture.] The Hague, Ministry of
Agriculture and Fisheries, pp 3-23 (in Dutch).
Van Gundy SD, Munnecke D, Bricker J, & Minteer R (1972) Response of
Meloidogyne incognita, Xiphinema index , and Dorylaimus species to
methyl bromide fumigation. Phytopathology, 62: 191-192.
Van Gundy SD, Perez JG, Stolzy LH, & Thomason IJ (1974) A pest
management approach to the control of Pratylenchus thornei on wheat
in Mexico. J Nematol, 6: 107-116.
Van Leeuwen CJ, Rijkeboer M, & Niebeek G (1986) Population dynamics of
Daphnia magna and modified by chronic bromide stress. Hydrobiologia,
133: 277-285.
Van Leeuwen FXR & Sangster B (1987) The toxicology of bromide ion. CRC
Crit Rev Toxicol, 18: 189-213.
Van Rhee JA (1977) Effects of soil pollution on earthworms.
Pedobiologia, 17: 201-208.
Van Wambeke E, Vanachter A, & Van Assche C (1974) Some factors
affecting bromide residues in soil and plant. Meded Fac Landbouwwet
Rijksuniv Gent, 39: 1311-1324.
Van Wambeke E, Vanachter A, & Van Assche C (1988) Measures for
improving soil fumigation. Brighton Crop Protection Conference on
Pests and Diseases, Brighton, England, 21-24 November 1988. London,
The British Crop Protection Council, pp 199-204.
Van Wees AMP, Rijk MAH, Rijnaars MW, & De Vos RH (1984)
Chromatographic methods for the determination of inorganic bromide in
vegetables. In: Frigerio A & Milon H, ed. Chromatography and mass
spectrometry in nutrition science and food safety. Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp 19-25.
Veenendahl G & Dibbets G (1981) [Permeation of methyl bromide into
different types of drinking waterpipes.] Kiwa, Inspection Institute
for Water Pipeline Materials (KIWA), pp 1-35 (in Dutch with English
Summary).
Verberk MM, Rooyakkers-Beemster T, De Vlieger M, & Van Vliet AGM
(1979) Bromine in blood, EEG and transaminases in methyl bromide
workers. Br J Ind Med, 36: 59-62.
Victor M & Adams RD (1980) Nonbarbiturate sedative-hypnotics;
psychtherapeutic, stimulant, and psychotogenic drugs. In: Isselbacher
KJ, Adams RD, Braunwald E, Petersdorf RG, & Wilson, JD ed. Harrison's
principles of internal medicine, 9th ed. New York, McGraw-Hill Book
Company (Abstract 229).
Vincent LE & Lindgren DL (1975) Toxicity of phosphine and methyl
bromide at various temperatures and exposure periods to the four
metamorphic stages of Trogoderma variabile. J Econ Entomol, 68:
53-56.
Vitte VI, Sorokina LV, & Subbotina SB (1970) [Residual amounts of
bromides in plant food products fumigated with methyl bromide and
characteristics of their biological action.] Gig Primen Toksikol
Pestic Klin Otrav, 8: 386-392 (in Russian).
Vodolagin VD (1971) Biology of the dog rose-eating weevil
(Megastigmus aculeatus) and measures for controlling it. Sci Trans
Efirnomaslich Cult Inst, 3: 115-117.
Von Oettingen WF (1946) The toxicity and potential dangers of methyl
bromide with special reference to its use in the chemical industry, in
fire extinguishers and in fumigation. Washington, DC, US Government
Printing Office, pp 1-41.
Von Oettingen WF (1955) The halogenated aliphatic, olefinic, cyclic,
aromatic, and aliphatic-aromatic hydrocarbons including the
halogenated insecticides, their toxicity and potential dangers.
Washington, DC, US Public Health Service, pp 15-30 (Publication No.
414).
Von Oettingen WF (1964) Methyl bromide. The halogenated hydrocarbons
of industrial and toxicological importance. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 25-55.
Watrous RM (1942) Methyl bromide-local and mild systemic effects. Ind
Med, 11: 575-579.
Wegman RCC, Greve PA, De Heer H, & Hamaker PH (1981) Methyl bromide
and bromide-ion in drainage water after leaching of glasshouse soils.
Water Air Soil Pollut, 16: 3-11.
Wegman RCC, Hamaker, PH, & De Heer H (1983) Bromide-ion balance of a
polder district with large-scale use of methyl bromide for soil
fumigation. Food Chem Toxicol, 21: 361-367.
Weihing JL, Schuster ML, Riesselman JH, & Cook JA (1971) Control of
fusarium wilt of petunia with three soil fumigants. Plant Dis Rep, 55:
580-582.
Weller D (1982) [Methyl bromide: Toxicology and therapy.] Frankfurt am
Main, Degesch GmbH, pp 21-42 (in German).
Wells JM & Payne JA (1975) Mycoflora of pecans treated with heat, low
temperatures, or methyl bromide for control of the pecan weevil.
Phytopathology, 65: 1393-1395.
Wester PW, Canton JH, & Dormans JAMA (1988) Pathological effects in
freshwater fish Poecilia reticulata (guppy) and Oryzias latipes
(medaka) following methyl bromide and sodium bromide exposure. Aquat
Toxicol, 12: 323-343.
Weststeijn G (1973) Soil sterilization and glasshouse disinfection to
control Fusarium oxysporum f. lycopersici in tomatoes in the
Netherlands. Neth J Plant Pathol, 79: 36-40.
Whitehead AG, Fraser JE, & Storey G (1972) Chemical control of potato
cyst-nematode in sandy clay soil. Ann Appl Biol, 72: 81-88.
Whitney WK, Jantz OK, & Bulger CS (1958) Effects of methyl bromide
fumigation on the viability of barley, corn, grain sorghum, oats, and
wheat seeds. J Econ Entomol, 51: 847-861.
Wilhelm E, Battino R, & Wilcock RJ (1977) Low pressure solubility of
gases in liquid water. Chem Rev, 77: 219-262.
Wilmer JWGM, Reuzel PGJ, & Dreef van der Meulen HC (1983) Subchronic
(13 week) inhalation toxicity study of methyl bromide in rats. Zeist,
The Netherlands, CIVO Institutes, TNO, 46 pp (CIVO Report No. V
82.378).
Windholz M (1983) The Merck index, 10th ed. Rahway, New Jersey, Merck
& Co., Inc., p 865.
Winstead NN & Garriss HR (1960) Control of cabbage clubroot in North
Carolina. Plant Dis Rep, 44: 14-18.
Winteringham FPW, Harrison A, Bridges RG, & Bridges PM (1955) The fate
of labelled insecticide residues in food products. II. The nature of
methyl bromide residues in fumigated wheat. J Sci Food Agric, 6:
269-275.
WMO (1992) Scientific assessment of ozone depletion, 1991. Geneva,
World Meteorological Organization, 330 pp (Report No. 25).
Wofsy SC, McElroy MB, & Yung YL (1975) The chemistry of atmospheric
bromide. Geophys Res Lett, 2: 215-218.
Wohlgemuth R, Drosihn J, & El-Lakwah F (1976) [Experiments to fumigate
piled expeller lying in quarantine against the Khapra beetle.] Berlin,
Federal Biological Institute for Agriculture and Forestry, 29 pp (in
German).
Woodrow JE, McChesney MM, & Seiber JN (1988) Determination of methyl
bromide in air samples by headspace gas chromatography. Anal Chem, 60:
509-512.
Wyers H (1945) Methyl bromide intoxication. Br J Ind Med, 2: 24-29.
Wylie PL (1988) Comparing headspace with purge and trap for analysis
of volatile priority pollutants. Am Water Works Assoc J, 80: 65-72.
Xu D, Peter H, Hallier E, & Bolt HM (1990) Hemoglobin adducts of
monohalomethanes. Ind Health, 28: 121-123.
Yamano Y (1991) [Experimental study on methyl bromide poisoning in
mice. Acute inhalation study and the effect of glutathione as an
antidote.] Jpn J Ind Health, 33: 23-30 (in Japanese).
Yamano Y, Ito I, Nagao N, & Ishizu S (1987) [A simple determination
method of bromide ion in plasma of methyl bromide workers by
head-space gas chromatography.] Jpn J Ind Health, 29: 196-201 (in
Japanese with English abstract).
Yeates GW, Bamforth SS, Ross DJ, Tate KR, & Sparling GP (1991)
Recolonization of methyl bromide sterilized soils under four different
field conditions. Biol Fertil Soils, 11: 181-189.
Yung YL, Pinto JP, Watson RT, & Sander SP (1980) Atmospheric bromine
and ozone pertubations in the lower stratosphere. J Atmos Sci, 37:
339-353.
Zatuchni J & Hong K (1981) Methyl bromide poisoning seen initially as
psychosis. Arch Neurol, 38: 529-530.
Zwart A, Arts JHE, Ten Berge WF, & Appelman LM (1992) Alternative
acute inhalation toxicity testing by determination of the
concentration-time-mortality relationship: experimental comparison
with standard LC50 testing. Regul Toxicol Pharmacol, 15: 278-290.
Zwart A (1988) Acute inhalation study of methylbromide in rats. Zeist,
The Netherlands, CIVO Institutes, TNO, 17 pp (CIVO Report-No. V88.
127/27).
Zwaveling JH, De Kort Wlam, Meulenbelt J, Hezemans-Boer M, Van Vloten
WA, & Sangster B (1987) Exposure of the skin to methyl bromide: a
study of six cases occupationally exposed to high concentrations
during fumigation. Hum Toxicol, 6: 491-495.
RESUME
1 Propriétés physiques et chimiques, méthodes d'analyse
A la température ambiante et sous la pression normale, le bromure
de méthyle est un gaz incolore dont le point d'ébullition est
d'environ 4 °C. Il est plus lourd que l'air et on peut facilement le
liquéfier en-dessous du point critique. Il est inodore sauf à fortes
concentrations auquel cas il dégage une odeur chloroformique. Il est
ininflammable à l'air, sauf aux concentrations comprises entre 10 et
16%, mais brûle dans l'oxygène. Le bromure de méthyle n'est que
légèrement soluble dans l'eau mais facilement soluble dans les autres
solvants courants. Il peut pénétrer dans de nombreuses substances
comme le béton, le cuir, le caoutchouc et certaines matières
plastiques.
Le bromure de méthyle s'hydrolyse en méthanol et acide brom-
hydrique en solution aqueuse, la vitesse d'hydrolyse dépendant du pH.
C'est un agent de méthylation efficace qui réagit sur les amines et
les composés soufrés. La plupart des métaux sont inertes au bromure de
méthyle pur et sec mais des réactions de surface peuvent avoir lieu
sur le zinc, l'étain, l'aluminium et le magnésium en présence
d'impuretés ou d'humidité. Des réactions explosives ont été observées
avec l'aluminium et le diméthylsulfoxyde.
Le bromure de méthyle existe dans le commerce sous forme de gaz
liquéfié. Les formulations destinées à la fumigation des sols
contiennent de la chloropicrine (2%) ou de l'acétate d'amyle (0,3%)
comme agents d'alerte. D'autres formulations peuvent contenir jusqu'à
70% de chloropicrine ainsi que d'autres fumigants ou hydrocarbures
comme diluants. Pour la fumigation des marchandises, on utilise du
bromure de méthyle à 100%.
Il existe des méthodes d'analyse pour le dosage du bromure de
méthyle dans l'air, l'eau, le sol, les produits alimentaires et la
nourriture pour animaux. Parmi les méthodes directes de dosage du
bromure de méthyle dans l'air sur le terrain, on peu citer les
analyseurs de gaz par conductivité thermique, les tubes de détection
colorimétrique, les analyseurs à infrarouge, et les détecteurs par
photo-ionisation. Pour les dosages de routine, on recommande la
chromatographie en phase gazeuse avec détection par capture
d'électrons, éventuellement couplée à la spectrométrie de masse pour
la confirmation au laboratoire.
Pour le dosage par chromatographie en phase gazeuse du bromure de
méthyle dans l'eau, on peut utiliser diverses techniques d'injection
(purge, piégeage, espace de tête par exemple). Pour le dosage en
routine du bromure de méthyle dans les produits alimentaires, on
recommande de procéder à une extraction par un mélange d'acétone et
d'eau puis à une chromatographie en phase gazeuse sur colonne
capillaire selon la technique de l'espace de tête avec détection par
capture d'électrons. Etant donné que le bromure de méthyle se
transforme en partie en bromure dans le sol, les denrées alimentaires
et les produits biologiques, l'ouvrage étudie également les méthodes
de recherche et de dosage des bromures. Parmi les méthodes utilisées
pour le dosage des bromures dans diverses matrices, on peut citer les
méthodes colorimétriques, la spectroscopie de rayons X, la
potentiométrie, l'analyse par activation neutronique, la
chromatographie en phase gazeuse ainsi que la chromatographie en phase
liquide à haute performance (HLPC).
2 Sources d'exposition humaine et environnementale
On pense que les océans sont la source essentielle de bromure de
méthyle. La principale source d'origine humaine en est la fumigation
des sols et de l'intérieur des habitations. Les véhicules à moteur qui
utilisent de l'essence au plomb émettent également une petite quantité
de bromure de méthyle.
En 1990, la consommation mondiale de bromure de méthyle a dépassé
67 millions de kg soit une augmentation de 46% depuis 1984. On produit
généralement le bromure de méthyle par action du méthanol sur l'acide
bromhydrique et, dans certains procédés, on l'obtient à côté du
tétrabromobisphénol A. Le bromure de méthyle est généralement stocké
et transporté à l'état liquide sous pression dans des récipients
d'acier.
Le bromure de méthyle est utilisé à environ 77% pour la
fumigation des sols, à 12% pour la fumigation des marchandises et
notamment des marchandises soumises à quarantaine, à 5% pour la
fumigation des construction et à 6% comme intermédiaire dans
l'industrie chimique.
Sous forme gazeuse, il est utilisé comme fumigant des sols, soit
en plein champ, soit sous serre pour la destruction des ravageurs.
Sous forme liquide, on l'applique avant la plantation, soit par
injection dans le sol, soit en plaçant des jattes pleines de bromure
de méthyle sous une couverture en plastique et en laissant le produit
s'évaporer sur place (méthode à froid) ou par chauffage (méthode à
chaud). Les méthodes autorisées varient selon le pays. Le type de
matière plastique utilisé est également important.
Les doses de bromure de méthyle à appliquer dépendent égale-ment
des normes légales en vigueur dans les différents pays, de l'espèce de
ravageur à détruire (type, ampleur de l'infestation), de la récolte
suivante, du type de sol et de la couverture plastique utilisée (durée
de couverture et nature de la matière plastique). Le bromure de
méthyle est généralement épandu sur le sol à des doses comprises entre
50 et 100 g/m2.
En fumigation spatiale, le bromure de méthyle est utilisé sur les
produits agricoles (par exemple fourrage, céréales, noix, etc.) ainsi
que pour la destruction des termites et des rongeurs. Pour la plupart
des denrées stockées dans des pièces et des silos fermés ou sous une
couverture étanche au gaz, on utilise des doses de 16 à 30 g de
bromure de méthyle par m3. Après la fumigation, il faut aérer les
locaux pendant un certain temps. Cette fumigation est également
importante dans le cas des légumes et des fruits frais soumis à
quarantaine.
Parmi les utilisations industrielles du bromure de méthyle, on
l'emploie en synthèse organique, généralement en tant qu'agent de
méthylation, ou comme solvant à bas point d'ébullition, par exemple
pour l'extraction de l'huile de noix et de différentes graines ou
celle des huiles essentielles. L'emploi du bromure de méthyle comme
réfrigérant ou comme produit extincteur à usage général, n'a plus
qu'un intérêt historique.
3 Transport, distribution et transformation dans l'environnement
Le bromure de méthyle est naturellement présent dans l'atmosphère
et les quantités qui résultent de l'activité humaine viennent s'y
ajouter. Le bromure de méthyle réagit en petites quantités sur les
radicaux hydroxyles de la troposphère mais il en passe une certaine
proportion dans la stratosphère par diffusion verticale. Là, c'est la
photolyse qui gagne en importance, puisqu'elle devient le mécanisme
dominant d'élimination du bromure de méthyle dans la partie basse de
la stratosphère. Il en résulte la formation de brome actif qui réagit
sur l'ozone stratosphérique et pourrait être en partie responsable de
la destruction de la couche d'ozone.
Dans le sol, le bromure de méthyle est partiellement hydrolysé en
ion bromure. Après fumigation au bromure de méthyle, on peut lessiver
le sol à l'eau afin d'éviter que les ions bromure formés ne soient
fixés par les végétaux plantés après stérilisation du sol. Cet
accroissement de la concentration en bromure peut poser des problèmes
si l'on utilise pour cela des eaux de surface. Le bromure de méthyle
peut diffuser à travers les canalisations d'eau potable en
polyéthylène si le sol environnant a subi une fumigation avec ce
produit.
Dans le sol, le bromure de méthyle peut diffuser jusqu'à une
profondeur de 0,8 m selon le type de sol, la dose d'emploi, le mode
d'épandage et la durée de fumigation, la couche supérieure du sol en
retenant la majeure partie. Le transport du gaz s'effectue par
écoulement ou diffusion moléculaire, mais il est également influencé
par le présence de processus simultanés de piégeage comme une sorption
ou une dissolution, et qui peuvent être irréversibles comme
l'hydrolyse. La quantité de bromure de méthyle transformée en bromure
dépend principalement de la teneur du sol en matières organiques. Les
bromures ainsi produits sont largement solubles dans l'eau et peuvent
être fixés par les plantes ou descendre plus profondément dans le sol
par lessivage.
La quantité de bromure qui s'accumule dans les plantes dépend de
divers facteurs comme la dose d'emploi, la durée d'exposition, le
débit d'aération, les propriétés physiques et chimiques du sol, la
climatologie (température et précipitations), l'espèce végétale et la
nature du tissu végétal. En particulier, les légumes-feuilles comme la
laitue et les épinards, peuvent fixer des quantités relativement
importantes d'ions bromure sans présenter de symptômes de
phyto-toxicité. En revanche, d'autres plantes cultivées comme les
oeillets, les plants d'agrumes, le coton, le céleri, les poivrons ou
les oignons sont particulièrement sensibles à la fumigation par le
bromure de méthyle.
Le bromure de méthyle et ses produits de réaction, dont on n'a
considéré jusqu'ici que les bromures, peuvent pénétrer dans la chaîne
alimentaire de deux manières; soit par consommation de plantes
cultivées dans des serres ou dans des champs traités par fumigation
avant la plantation, soit par consommation de produits alimentaires
traités au bromure de méthyle pendant l'entreposage. A partir d'une
certaine concentration, les bromures peuvent être dangereux pour la
santé et il existe un seuil de tolérance pour le bromure présent dans
les denrées alimentaires. La concentration des autres produits de
réaction n'a pas été étudiée.
Dans le sol, le bromure de méthyle subit une dégradation par
hydrolyse et décomposition microbienne. La constante de vitesse
d'hydrolyse varie avec la température et le pH et augmente avec
l'intensité de la lumière.
Le coefficient de partage octanol/eau (log Pow) du bromure de
méthyle est égal à 1,19, ce qui indique que sa bioaccumulation est
faible.
Le bromure de méthyle qui n'est pas décomposé lors de la
fumigation pénètre dans la troposphère et gagne la stratosphère par
diffusion verticale. Il ne semble pas qu'il existe un important
gradient de concentration vertical du bromure de méthyle dans la
troposphère, mais sa concentration diminue rapidement dans la basse
stratosphère où il subit une photolyse.
4 Concentrations dans l'environnement et exposition humaine
La concentration du bromure de méthyle mesurée dans l'air de
zones inhabitées, varie de 40 à 100 ng/m3 (10 à 26 pptv), les
valeurs pour l'hémisphère nord étant supérieures à celles à celles que
l'on trouve dans l'hémisphère sud. La plupart des valeurs vont de 9 à
15 pptv. Des différences saisonnières ont été constatées à l'occasion
d'un certain nombre d'études. Dans les zones urbanisées et
industrialisées, les concentrations sont beaucoup plus élevées avec
des valeurs moyennes allant jusqu'à 800 ng/m3 voire jusqu'à 4 µg de
bromure de méthyle par m3. A proximité des champs et des serres, on
constate, lors de la fumigation et de l'aération, des concentrations
de bromure de méthyle beaucoup plus élevées, des valeurs de 1 à 4
mg/m3 ayant été mesurées dans une étude à des distances allant
jusqu'à 20 m d'une serre, quelques heures après l'injection du
produit; quatre jours plus tard, la concentration était tombée au
dixième de cette valeur.
Dans un échantillon d'eau de mer prélevé en surface, on a trouvé
une concentration de bromure de méthyle de 140 ng/litre. Dans des
échantillons d'eau littorales de la Mer du Nord, la concentration
moyenne en ions bromure était de 18,4 mg/litre; dans les cours d'eau
de l'arrière pays, cette concentration était beaucoup plus faible,
sauf là où l'on pratiquait la fumigation par le bromure de méthyle ou
dans les zones polluées par les rejets industriels. Dans les eaux de
drainage d'une serre hollandaise, on a signalé des concentrations de
9,3 mg de bromure de méthyle par litre et de 72 mg d'ions bromure par
litre. Dans les eaux rejetées par une serre belge, on a trouvé après
fumigation une concentration de 280 mg de bromure par litre.
La teneur naturelle du sol en bromure dépend du type de ce sol
mais elle est généralement inférieure à 10 mg/kg. La teneur en résidus
de bromure dans les sols traités dépend du traitement, de la dose
d'emploi, du type de sol, des précipitations, du lessivage par l'eau
et de la température.
Il peut y avoir de fortes concentrations de bromure de méthyle ou
d'ions bromure dans les cultures vivrières poussant sur des sols
préalablement traités au bromure de méthyle ou qui ont subi une
fumigation après récolte.
Plus rarement, on a constaté que les concentrations de bromure
dans les légumes frais cultivés sur des sols qui avaient traité avec
du bromure de méthyle, dépassaient les teneurs en résidus autorisées.
Dans certains pays, il est interdit de cultiver des légumes sur des
sols traités de cette manière.
On utilise largement le bromure de méthyle pour la fumigation des
denrées alimentaires après récolte, par exemple les céréales et
notamment le froment, les épices, les noix, les fruits secs ou frais
et le tabac. La concentration en bromure de méthyle diminue en général
rapidement après aération et au bout de quelques semaines on ne peut
plus déceler de résidus. Certains produits comme les noix et les
graines ou des aliments gras comme le fromage ont tendance à retenir
le bromure de méthyle et le brome minéral.
Il peut y avoir exposition humaine au fumigant lui-même comme aux
résidus d'ions bromure. Il pourrait également y avoir un risque
d'accroissement de la concentration du bromure de méthyle ou des
bromures dans l'eau des puits peu profonds situés à proximité de
terrains où l'on pratique la fumigation par le bromure de méthyle.
Les personnes qui vivent à proximité immédiate des champs, des
serres ou des entrepôts traités au bromure de méthyle pourraient
courir le risque d'une exposition au gaz. Il existe également un
risque individuel pour les personnes qui pénètrent accidentellement ou
délibérément dans une maison traitée contre la vermine avant que l'on
ait annoncé qu'il n'y a plus de danger.
L'exposition professionnelle au bromure de méthyle est le risque
le plus probable pour les travailleurs lors de la production, du
remplissage des récipients et des opérations de fumigation. Seuls les
fumigateurs sont désormais considérés comme constituant un groupe à
haut risque étant donné que, sur les lieux de production, des mesures
de sécurité très strictes sont appliquées. Ceux qui sont employés au
traitement des constructions peuvent être exposés à des concentrations
beaucoup plus élevées que les valeurs-seuil après 24 heures d'aération
(80 à 2000 mg/m3). Cependant, un ouvrier expérimenté utilisera un
équipement protecteur approprié. Les travailleurs agricoles qui
procèdent à la fumigation du sol peuvent être exposés pendant de
longues durées à des doses transitoires élevées de bromure de méthyle.
De par la nature même du processus, le traitement d'une serre peut
exposer les travailleurs à d'importantes concentrations de bromure de
méthyle (100-1200 mg/m3). Il est vrai cependant, que pour combattre
des risques inhérents aux diverses opérations de fumigation, on a
établi des règles de sécurité très strictes qui impliquent notamment
le port d'un équipement protecteur. Cela n'empêche pas des accidents
de se produire par suite d'une exposition excessive.
5 Cinétique et métabolisme
Des études au cours desquelles on a fait inhaler du bromure de
méthyle à des rats, des chiens beagle et des volontaires humains ont
révélé que le produit était rapidement résorbé au niveau pulmonaire.
La résorption est également rapide chez le rat après administration
par voie orale.
Une fois résorbé, le bromure de méthyle ou ses métabolites se
répartissent rapidement dans de nombreux tissus, notamment les
poumons, les surrénales, les reins, le foie, les fosses nasales, le
cerveau, les testicules et les tissus adipeux. Lors d'une étude par
inhalation sur des rats, on a constaté que la concentration tissulaire
du bromure de méthyle passait par un maximum une heure après
l'exposition puis diminuait rapidement, aucune trace n'étant plus
décelable au bout de 48 heures. On n'a pas encore élucidé le
métabolisme du bromure de méthyle inhalé mais le glutathion pourrait
jouer un rôle.
Dans les tissus de plusieurs espèces, notamment l'homme, on a
observé une méthylation des protéines et des lipides après exposition
au bromure de méthyle par voie respiratoire. On a également mis en
évidence la présence d'adduits méthylés de l'ADN après exposition de
rongeurs ou de cellules de rongeurs in vivo ou in vitro.
Lors d'études d'inhalation avec du bromure de méthyle marqué par
du [14C], on a observé que la principale voie d'élimination du
radiocarbone était l'exhalation de 14CO2. La quantité de
radiocarbone retrouvée dans le urines a été moindre. Après
administration par voie orale de bromure de méthyle, on a constaté que
c'était par la voie urinaire que le 14C était principalement
excrété.
Le système nerveux central est également est un organe cible
important du bromure de méthyle. La neurotoxicité de ce composé est
sans doute liée à une modification de la teneur en monoamines, en
acides aminés et éventuellement en catécholamines.
6 Effets sur les êtres vivants dans leur milieu naturel
L'une des utilisations commerciales du bromure de méthyle
consiste dans la destruction des nématodes, des mauvaises herbes et
des champignons terricoles qui causent des maladies chez les plantes
comme la fonte des semis, et divers types de pourritures et de
verticillioses.
Peu d'études ont été consacrées aux effets du bromure de méthyle
sur les organismes aquatiques car ce composé n'est lui-même que peu
soluble dans l'eau. Les valeurs de la CL50 vont de 17 mg/litre pour
Cyprinus carpio L. sur 4 heures, à 1,2 mg/litre pour Poecilia
reticulata sur 48 heures. Aux concentrations mortelles, il est
probable que la mort est causée par des lésions au niveau des
branchies et de l'épithélium buccal.
Après fumigation par le bromure de méthyle, il se forme des ions
bromure que l'on retrouve dans l'eau après lessivage. Ces ions bromure
ont été à l'origine d'intoxications aiguës chez différents organismes
dulçaquicoles à des concentrations allant de 44 à 5800 mg de
Br-/litre; la concentration sans effets observables lors d'épreuves
à long terme allant de 7,8 à 250 mg de Br-/litre. Les ions bromure
ont également un effet perturbateur marqué sur la reproduction des
crustacés et des poissons.
En fumigation, on peut appliquer du bromure de méthyle
directement sur les semis, sur les boutures ou sur les plantes après
récolte, pour les débarrasser des parasites pendant le transport et
l'entreposage. Si le taux d'humidité ou la température sont trop
élevés, il peut y avoir retard à la germination, voire perte de la
capacité germinative.
Certains végétaux, en particulier les légumes-feuilles, sont
sensibles à la fumigation par le bromure de méthyle, soit par excès de
bromure dans le sol, soit indirectement en raison des effets de ce
composé sur la microflore terricole. Quelquefois, le bromure de
méthyle a au contraire un effet positif sur les végétaux car il peut
stimuler leur croissance et améliorer le rendement des cultures.
La fumigation par le bromure de méthyle détruit non seulement les
parasites visés mais également une partie de la flore, des
gastéropodes, des arachnides et des protozoaires terricoles.
On l'utilise de préférence à d'autres insecticides en raison de
son aptitude à pénétrer rapidement et profondément dans la masse des
produits traités et dans les sols. Les doses d'emploi pour traiter les
marchandises entreposées vont essentiellement de 16 à 100 g/m3
pendant deux à trois jours, selon la température. La dose nécessaire
pour la destruction des oeufs et des pupes est plus élevée que pour
les insectes adultes. La tolérance varie selon les diverses espèces
d'insectes, selon leurs divers stades de développement et chez une
même espèce, en fonction de la souche.
On ne dispose d'aucune donnée relative aux effets directs du
bromure de méthyle sur les oiseaux et les mammifères sauvages.
7 Effets sur les animaux d'expérience
Des études d'inhalation effectuées sur divers espèces de
mammifères montrent que la sensibilité au bromure de méthyle varie
nettement selon l'espèce et le sexe. La droite dose-mortalité présente
une pente accentuée chez toutes les espèces animales étudiées.
Chez le rat et la souris, les principaux signes cliniques
d'intoxication sont des manifestations neurologiques avec, à fortes
concentrations, une irritation des muqueuses.
Ces manifestations neurologiques consistent notamment en
fasciculations et paralysie. Divers auteurs ont également fait état à
doses plus faibles, de modifications dans l'activité locomotrice,
d'anomalies de la fonction nerveuse périphérique, de modifications du
rythme circadien et d'aversion gustative conditionnée.
On a également décrit des altérations histo- pathologiques au
niveau du cerveau, des reins, des muqueuses nasales, du coeur, des
surrénales, du foie et des testicules chez des rats et des souris
exposés à diverses concentrations de bromure de méthyle.
Une exposition de brève durée au bromure de méthyle provoque des
lésions au niveau des cellules de soutien de l'épithélium olfactif et
des cellules sensorielles matures mais la réparation et la
récupération sont rapides.
Les études d'inhalation de longue durée (jusqu'à deux ans)
effectuées sur des rats, ont révélé la présence de lésions de la
muqueuse nasale et du myocarde. Lors d'une étude à long terme analogue
chez la souris, les lésions primitives ont été observées au niveau du
cerveau, du coeur et de la muqueuse nasale. Aucun signe de
cancérogénicité n'a été observé chez l'une ou l'autre espèce.
L'administration par voie orale de 50 mg de bromure de méthyle
par kg de poids corporel à des rats pendant des périodes allant
jusqu'à 25 semaines a entraîné une inflammation et une hyperplasie
grave au niveau de l'épithélium de la portion cardiaque de l'estomac.
Après une période de récupération, qui a suivi l'exposition au bromure
de méthyle, la principale lésion observée était une fibrose de la
portion cardiaque de l'estomac. Chez des rats traités quotidiennement
pendant 25 semaines, on a également constaté la présence d'un cancer
précoce à ce niveau.
Des souris B6C3F et des rats F344 exposés à des concentrations de
bromure de méthyle allant jusqu'à 467 mg/m3 pendant 13 semaines, ont
présenté de légères anomalies morphologiques des spermatozoïdes, la
durée du cycle oestral n'étant pas affectée.
Deux générations consécutives de rats CD Sprague- Dawley ont été
soumis par la voie respiratoire à des concentrations de bromure de
méthyle allant jusqu'à 350 mg/m3 sans que l'on constate d'effets
notables sur leur croissance, les divers processus de la reproduction
ni sur leur descendance. Il y avait diminution des indices de
fécondité des mâles et des femelles aux deux doses les plus élevées,
chez les rats de la génération F1 et de la génération F2b.
On a étudié les effets toxiques du bromure de méthyle sur le
développement de lapins blancs de Nouvelle-Zélande, exposés à 311 mg
de bromure de méthyle par m3 (6 heures/jour, du 7ième au 19ième jour
de la gestation). Les effets toxiques relevés chez les mères étaient
modérés à graves. Les effets sur le développement observés aux doses
toxiques pour les mères, consistaient en une réduction du poids du
foetus, une augmentation de la fréquence des modifications
squelettiques mineures ainsi qu'en malformations (essentiellement
absence de la vésicule biliaire ou du lobe inférieur du poumon).
Toutefois à la dose de 272 mg/m3, la toxicité maternelle était moins
marquée et il n'y avait plus d'effets embryotoxiques.
Aucun effet nocif maternel, embryonnaire ou foetal n'a été
observé chez des lapins exposés à des doses de bromure de méthyle
égales à 78 ou 156 mg/m3. Pour les lapins blancs de
Nouvelle-Zélande, on a fixé à 156 mg/m3 la dose sans effets
observables pour la toxicité maternelle et les effets toxiques sur le
développement.
Le bromure de méthyle s'est révélé mutagène dans plusieurs
systèmes d'épreuve in vitro et in vivo. Il provoque des mutations
létales récessives liées au sexe chez Drosophila melanogaster ainsi
que des mutations dans des cellules mammaliennes en culture. Il ne
provoque pas de synthèse anarchique de l'ADN (non programmée) ni de
transformation cellulaire dans les cultures de cellules mammaliennes.
Chez des souris à qui on avait administré du bromure de méthyle par
diverses voies, on a observé une méthylation de l'ADN au niveau des
cellules hépatiques et spléniques. Chez des rats et des souris, on a
observé la formation de micro-noyaux dans les cellules de la moelle
osseuse et les leucocytes périphériques. On ignore le mécanisme de la
toxicité du bromure de méthyle.
8 Effets sur l'homme
Il peut y avoir exposition humaine au bromure de méthyle, soit
par inhalation du gaz, soit par contact avec le liquide. L'exposition
peut également se produire par la voie digestive à la suite de
l'ingestion d'eau de boisson contaminée par des eaux de lessivage
polluées.
Une étude contrôlée sur l'homme a révélé que, après inhalation,
environ 50% de la dose administrée étaient absorbés.
Le bromure de méthyle provoque des lésions au niveau du système
nerveux, des poumons, des muqueuses nasales, du rein, de l'oeil et de
la peau. Les effets sur le système nerveux central consistent en
troubles visuels, confusion mentale, engourdissement, tremblements, et
troubles de l'élocution. Une contamination topique peut entraîner une
irritation et des brûlures cutanées ainsi que des lésions oculaires.
L'exposition à de fortes concentrations de bromure de méthyle
provoque un oedème pulmonaire. Dans les cas d'intoxication par le
bromure de méthyle, la cause immédiate de la mort est généralement une
dépression du système nerveux central entraînant une paralysie
respiratoire et/ou une défaillance circulatoire, précédées par des
convulsions et un coma.
Lors d'intoxications aiguës ou chroniques par le bromure de
méthyle, on a observé différents symptômes neuro- psychiatriques. Une
exposition de brève durée à de faibles concentrations de vapeurs de
bromure de méthyle peut produire un syndrome polyneuropathique sans
manifestations centrales évidentes.
Parmi les séquelles tardives de l'intoxication on peut noter une
broncho-pneumonie consécutive à de graves lésions pulmonaires, une
insuffisance rénale avec anurie et une très importante faiblesse avec
ou sans signes de paralysie. Généralement ces symptômes tendent à
disparaître en quelques semaines à quelques mois. Toutefois, on a
observé des déficits permanents généralement caractérisés par des
troubles sensoriels, de la faiblesse, une baisse de l'acuité visuelle
et des troubles de la démarche.
L'exposition au bromure de méthyle s'accompagne d'une
augmentation du taux sanguin de bromure. Chez les fumigateurs, il
existe une relation entre le nombre d'opérations auxquelles ils ont
participé et le taux plasmatique moyen de bromure.
RESUMEN
1 Propiedades físicas y químicas y métodos analíticos
El bromuro metílico es un gas incoloro a la temperatura ambiente
y a la presión atmosférica normal, con un punto de ebullición de 4 oC
aproximadamente. Es más pesado que el aire y se licúa con facilidad
por debajo de sus puntos críticos. Es inodoro, excepto en
concentraciones altas, en las que tiene un olor parecido al
cloroformo. No es inflamable en el aire, excepto en la gama de
concentraciones del 10-16%, pero arde en oxígeno. El bromuro metílico
es ligeramente soluble en agua, pero fácilmente soluble en otros
disolventes corrientes. Puede penetrar a través de numerosas
sustancias, como cemento, cuero, caucho y ciertos plásticos.
El bromuro metílico se hidroliza dando metanol y ácido
bromhídrico en solución acuosa, con una velocidad de hidrólisis que
depende del pH. Es un agente metilante eficaz que reacciona con las
aminas y con los productos que contienen azufre. La mayoría de los
metales son inertes ante el bromuro metílico seco y puro, pero se
producen reacciones de superficie sobre el zinc, el estaño, el
aluminio y el magnesio en presencia de impurezas o humedad. Se han
señalado reacciones explosivas con el aluminio y el sulfóxido
dimetílico.
El bromuro metílico se comercializa en forma de gas licuado. Las
formulaciones par la fumigación del suelo contienen cloropicrina (2%)
o acetato amílico (0,3%) como agentes de aviso. Otras formulaciones
incluyen hasta el 70% de cloropicrina o de otros fumigantes o
hidrocarburos como diluyentes inertes. Para la fumigación de
mercancías se utiliza bromuro metílico al 100%.
Se han descrito métodos analíticos para la determinación del
bromuro metílico en el aire, el agua, el suelo, los alimentos y los
piensos. Entre los aparatos para la determinación directa del bromuro
metílico en el aire, en condiciones prácticas, figuran los
analizadores de gases por conductividad térmica, los tubos de
detección colorimétrica, los analizadores en infrarrojos y los
detectores por fotoionización. Se recomienda la cromatografía de gases
(CG) con detección por captura de electrones (DCE) para las mediciones
corrientes, seguida a veces de la confirmación por espectrometría de
masa (EM) en el laboratorio.
Para la determinación por CG del bromuro metílico en el agua se
utilizan técnicas de purga y captura, así como de muestreo en el
espacio superior. Se recomienda la extracción con acetona y agua
seguida de la cromatografía capilar de gas del espacio superior con
DCE para la determinación ordinaria del bromuro metílico en los
alimentos. Teniendo en cuenta que una parte del bromuro metílico se
convierte en bromuro en el suelo, los alimentos y los productos
biológicos, se examinan también los métodos de determinación del
bromuro. Entre los utilizados para esa determinación en distintas
matrices figuran métodos colorimétricos, la espectroscopia de rayos X,
la potenciometría, el análisis por activación neutrónica, la
cromatografía de gases y la cromatografía de líquidos de alto
rendimiento.
2 Fuentes de exposición humana y ambiental
Se estima que los océanos son la fuente más importante de bromuro
metílico. La principal fuente antropogénica de bromuro metílico es la
fumigación de suelos y locales. Los vehículos de motor que utilizan
gasolina con plomo emiten una pequeña cantidad de bromuro metílico.
En 1990, el consumo mundial de bromuro metílico fue superior a
67 millones de kg, con un aumento del 46% respecto a 1984. Se fabrica
corrientemente por reacción entre el metanol y el ácido bromhídrico y
en algunos procedimientos es un coproducto que acompaña al
tetrabromobisfenol A. El bromuro metílico se almacena y transporta
habitualmente como gas licuado a presión en recipientes de acero.
El 77% aproximadamente del bromuro metílico fabricado se emplea
para la fumigación del suelo, el 12% para la fumigación de cuarentena
y de mercancías, el 5% para la fumigación de edificios y el 6% para
obtener intermediarios químicos.
El gas se emplea como fumigante del suelo en los campos o los
invernaderos en la lucha contra las plagas. Se aplica en forma de
líquido antes de la plantación, por inyección en el suelo o por
evaporación en recipientes colocados bajo las cubiertas de plástico,
dejando que el producto se evapore in situ (método frío) o por
calentamiento (método caliente). Hay diferencias en los métodos
utilizados en los distintos países. También es importante el tipo de
cubierta de plástico empleada.
Las dosis de bromuro metílico que se han de aplicar dependen de
las normas reglamentarias de los distintos países, el parásito vegetal
que se ha de eliminar (tipo, amplitud de la infestación), el cultivo
siguiente, el tipo de suelo y la cubierta de plástico empleada (tiempo
de recubrimiento y tipo de plástico). El bromuro metílico se aplica
habitualmente al suelo en concentraciones comprendidas entre 50 y 100
g/m2.
En la fumigación espacial se emplea el bromuro metílico para el
tratamiento de productos agrícolas (por ej., alimentos, cereales,
nueces, etc.) y la lucha contra las termitas y los roedores. Se
emplean concentraciones de 16-30 g de bromuro metílico por m3 para
la mayor parte de los productos almacenados en naves y silos cerrados
herméticamente y bajo cubiertas impermeables a los gases. La
fumigación debe ir seguida de un periodo de aireación. También es
importante la fumigación de hortalizas y frutas frescas en donde han
de observarse reglamentos de cuarentena.
Entre los usos industriales del bromuro metílico figuran la
síntesis orgánica, habitualmente como agente metilante, y el empleo
como disolvente de baja temperatura de ebullición, por ejemplo, para
la extracción de aceites de nueces, semillas y flores. La utilización
del bromuro metílico como refrigerante y como agente general de
extinción de incendios sólo tiene ahora impor-tancia histórica.
3 Transporte, distribución y transformación en el medio ambiente
El bromuro metílico se halla presente de modo natural en la
atmósfera. Se suman a esa presencia las fuentes antropogénicas. Aunque
una pequeña cantidad del bromuro metílico reacciona con el radical
hidroxilo en la troposfera, parte del bromuro metílico pasa a la
estratosfera por difusión ascendente. En esa capa adquiere importancia
creciente la fotólisis del bromuro metílico, siendo el mecanismo
predominante de desaparición en la estratosfera baja. El bromo activo
reacciona con el ozono en la estratosfera y se cree que es en parte
responsable de la destrucción de la capa de ozono.
En el suelo, el bromuro metílico se hidroliza parcialmente para
dar ion bromuro. Después de la fumigación con bromuro metílico, el
suelo puede ser lixiviado con agua para evitar que los iones bromuro
formados sean captados por los vegetales plantados después en el suelo
esterilizado. Este aumento de las concen- traciones de bromuro puede
producir problemas cuando se utilizan aguas superficiales para la
lixiviación. El bromuro metílico puede difundirse a través de las
tuberías de polietileno de conducción de agua potable, si el suelo que
las rodea ha sido fumigado con bromuro metílico.
En el suelo, el bromuro metílico puede difundirse hasta una
profundidad de 0,8 m, en función del tipo de suelo, la dosis, el
método de aplicación y la duración de la fumigación; la mayor
concentración de bromuro metílico se alcanza en la parte superior del
suelo. El transporte del gas se produce por flujo de masas y difusión
molecular, pero también influyen los procesos de desa-parición que se
produzcan simultáneamente, como la absorción y la disolución, y los
procesos de desaparición irreversible, como la hidrólisis. La cantidad
de bromuro metílico convertido en bromuro depende principalmente del
contenido en materias orgánicas del suelo. El bromuro producido es
principalmente hidrosoluble y puede ser captado por las plantas o
desplazado a niveles inferiores del suelo por lixiviación con agua.
En las plantas, la cantidad de bromuro acumulado depende de
distintos factores, como la concentración, el tiempo de exposición, la
tasa de aireación, las propiedades físicas y químicas del suelo, las
tendencias climáticas (temperatura y pluviosidad), las especies
vegetales y el tipo de tejido de las plantas. En particular las
hortalizas de hoja, como la lechuga y la espinaca, pueden captar
cantidades relativamente altas de ion bromuro sin síntomas
fitotóxicos. Por el contrario, otros cultivos, como los claveles, los
planteles de cítricos, el algodón, el apio, los pimientos y las
cebollas, son especialmente sensibles a la fumigación con bromuro
metílico.
El bromuro metílico y sus productos de reacción, entre los cuales
sólo se ha considerado hasta ahora el bromuro, pueden entrar en la
cadena alimentaria de dos modos: consumo de alimentos cultivados en
invernaderos o en campos fumigados antes de la plantación o
alimentación con productos fumigados con bromuro metílico en el curso
del almacenamiento. En determinadas concentraciones, el bromuro puede
ser peligroso para la salud; se han indicado niveles de tolerancia
para el bromuro contenido en los alimentos. No se han investigado los
niveles de otros productos de reacción.
El bromuro metílico se degrada en el suelo por hidrólisis y
descomposición microbiana. La constante de hidrólisis varía con la
temperatura y el pH, y aumenta con la luz.
El coeficiente de partición octanol/agua (log Pow) del bromuro
metílico es de 1,19, lo que sugiere la existencia de una
bio-acumulación baja.
El bromuro metílico que no se ha degradado en el curso de la
fumigación pasa a la troposfera y por difusión ascendente a la
estratosfera. No parece que haya un gradiente vertical importante del
bromuro metílico en la troposfera, pero las concentraciones disminuyen
con rapidez en la estratosfera baja por acción de la fotólisis.
4 Niveles ambientales y exposición humana
Las concentraciones de bromuro metílico, medidas en el aire en
zonas sin habitar, varían entre 40 y 100 ng/m3 (10 a 26 pptv),
siendo los valores en el hemisferio Norte superiores a los del
hemisferio Sur. La mayoría de las concentraciones se hallan en la gama
de 9-15 pptv. En algunos estudios se han observado diferencias
estacionales. En las zonas urbanas e industriales, las concentraciones
son mucho mayores, con valores medios de hasta 800 ng/m3, llegando
a veces hasta 4 µg de bromuro metílico por m3. En el curso de la
fumigación y la aireación, las concentraciones de bromuro metílico son
apreciablemente más altas cerca de los campos y los invernaderos,
habiéndose medido valores de 1-4 mg/m3 en un estudio a distancias de
hasta 20 m de un invernadero, algunas horas después de la inyección en
el suelo; cuatro días más tarde se observó la décima parte de ese
valor.
La concentración de bromuro metílico en una muestra de agua del
mar de superficie fue de 140 ng/litro. En muestras de agua costera
cerca del mar del Norte, el valor medio de las concentraciones de ion
bromuro fue de 18,4 mg/litro; la concentración de ion bromuro en los
ríos de tierra adentro fue mucho más baja, excepto en las regiones
donde se practicaba la fumigación con bromuro metílico o en las zonas
de contaminación industrial. En el agua de drenaje de un invernadero
de los Países Bajos se señalaron concentraciones de 9,3 mg de bromuro
metílico/litro y de 72 mg de ion bromuro/litro. En el agua evacuada de
un invernadero belga se registró un valor de 280 mg de bromuro/litro
después de la fumigación.
El contenido de bromuro natural del suelo depende del tipo de
suelo, pero suele ser inferior a 10 mg/kg. La presencia de restos de
bromuro en el suelo fumigado depende del tratamiento, la dosis, el
tipo de suelo, la cantidad de lluvia o de agua de lixiviación y la
temperatura.
Las concentraciones de bromuro metílico o bromuro pueden ser
altas en los alimentos que se han cultivado en suelos tratados
previamente con bromuro metílico o que se han fumigado después de la
recolección.
En hortalizas frescas cultivadas en suelos previamente fumigados
con bromuro metílico se han observado excepcional-mente
concentraciones de bromuro que rebasaban el nivel autori-zado de
residuos. En algunos países no se permite cultivar hortalizas en los
suelos tratados.
El bromuro metílico se utiliza ampliamente para la fumigación de
productos alimenticios después de la recolección, como trigo y
cereales, especias, nueces, frutas frescas y desecadas, y tabaco. Las
concentraciones de bromuro metílico suelen descender con rapidez
después de la aireación y no se detectan residuos al cabo de unas
semanas. Algunos alimentos, como las nueces, las semillas y productos
grasos como el queso, tienden a retener el bromuro metílico y el
bromuro inorgánico.
Las personas pueden estar expuestas al fumigante y a restos de
ion bromuro. También existe el riesgo de que haya bromuro metílico o
un aumento del contenido de bromuro en el agua de pozos situados cerca
de lugares en donde se ha fumigado bromuro metílico.
Las personas que viven cerca de campos, invernaderos o almacenes
fumigados con bromuro metílico pueden estar expuestos al gas. Los
seres humanos pueden también correr peligro si accidental o
deliberadamente penetran en locales que han sido fumigados para
erradicar plagas antes de declararlos seguros.
La exposición profesional al bromuro metílico es el riesgo más
probable de los operarios en el curso de la fabricación, el llenado y
la fumigación. Dadas las medidas de seguridad aplicadas estrictamente
en las fábricas, sólo se considera actualmente como grupo de alto
riesgo a los fumigadores. Los fumigadores que realizan el tratamiento
de edificios pueden tener una exposición muy superior al valor umbral
límite (VUL) después de 24 horas de aireación (80-2000 mg/m3). Sin
embargo, los operarios convenientemente capacitados utilizarán equipo
protector apropiado. Los obreros que trabajan en el campo durante la
fumigación del suelo pueden estar expuestos durante periodos más
prolongados a dosis pasajeras de bromuro metílico. Dada la naturaleza
de la fumigación de los invernaderos, los operarios pueden también
encontrar concentraciones más altas (100-1200 mg/m3). Así pues, la
gestión del riesgo provocado por los distintos aspectos de la
fumigación exige medidas de seguridad estrictas y el empleo de equipo
protector. Pese a ello se producirán todavía casos aislados de
sobreexposición accidental.
5 Cinética y metabolismo
Los estudios de inhalación efectuados en ratas, perros sabuesos
y seres humanos han mostrado la absorción rápida del bromuro de metilo
por los pulmones. También se absorbe con rapidez en las ratas después
de la administración oral.
Tras la absorción, el bromuro metílico o sus metabolitos se
distribuyen con rapidez en numerosos tejidos, comprendidos los
pulmones, las glándulas suprarrenales, los riñones, el hígado, los
cornetes nasales, el cerebro, los testículos y el tejido adiposo. En
un estudio de inhalación efectuado en ratas, la concentración tisular
de bromuro metílico alcanzó el valor máximo una hora después de la
exposición, pero descendió con rapidez, no encontrándose indicios 48
horas más tarde. Todavía no se ha esclarecido el metabolismo del
bromuro metílico inhalado, pero parece que interviene el glutatión.
Se ha observado la metilación de proteínas y lípidos en los
tejidos de varias especies, incluidos los seres humanos, expuestos a
través de la inhalación. También se han detectado aductos de ADN
metilado después de la exposición in vivo e in vitro de roedores
o células de roedores.
En los estudios de inhalación con bromuro metílico marcado con
[14C], la expiración de 14CO2 fue la principal vía de
eliminación de 14C. Por la orina se eliminó una cantidad menor de
14C. Tras la administración oral de bromuro metílico, la excreción
urinaria fue la principal vía de eliminación del 14C.
El sistema nervioso central es un importante destinatario del
bromuro metílico. En la neurotoxicidad provocada por el bromuro
metílico pueden intervenir modificaciones del contenido de monoaminas
y aminoácidos y tal vez de catecolaminas.
6 Efectos en los seres vivos del medio ambiente
El bromuro metílico se utiliza comercialmente en la lucha contra
los nematodos, las malas hierbas y los hongos transmitidos por el
suelo que provocan trastornos tales como el resecamiento, la
putrefacción de la copa o las raíces y el marchitado.
Se han realizado escasos estudios sobre los efectos del bromuro
metílico en los seres acuáticos, pues el propio bromuro metílico sólo
es ligeramente soluble en agua. Los valores de la CL50 van de un
valor a las cuatro horas de 17 mg/litro para Cyprinus carpio L. a
otro a las 48 horas de 1,2 mg/litro para Poecilia reticulata. En
concentraciones letales, las lesiones de las agallas y el epitelio
oral son la causa probable de la muerte.
El ion bromuro se forma a partir del bromuro metílico después de
la fumigación y se halla en el agua tras la lixiviación. Se observó
una toxicidad aguda por iones bromuro en distintos seres de agua dulce
en concentraciones comprendidas entre 44 y 5800 mg de Br-/litro; la
concentración de efecto no observado (NOEC) en las pruebas de larga
duración varió entre 7,8 y 250 mg de Br-/litro. Los iones bromuro
produjeron una marcada alteración de la reproducción de crustáceos y
peces.
El bromuro metílico puede aplicarse directamente como fumigante
a las semillas o los esquejes de las plantas o a los productos
alimenticios después de la recolección para la desin-festación en el
curso del transporte y el almacenamiento. Puede producirse el retraso
de la germinación o la pérdida de la capacidad germinativa si la
humedad o la temperatura son demasiado altas.
Algunos cultivos, en particular las hortalizas de hoja, son
sensibles a la fumigación con bromuro metílico debido a la presencia
de bromuro en exceso en el suelo o indirectamente por los efectos en
la microflora del suelo. El bromuro metílico tiene a veces un efecto
positivo sobre las plantas, favoreciendo su crecimiento y el
rendimiento de los cultivos.
La fumigación con bromuro metílico erradica no sólo los seres
vivos a los que se aplica sino también una parte de la flora del
suelo, los gastrópodos, los arácnidos y los protozoos.
El bromuro metílico se utiliza a menudo de preferencia a otros
insecticidas por su capacidad para penetrar con rapidez y profundidad
en productos no envasados y en los suelos. Las dosis de bromuro de
metilo utilizado como fumigante en almacenes se sitúan sobre todo
entre 16 y 100 g/m3 durante 2-3 días, dependiendo la dosis de la
temperatura. Para matar los huevos y las pupas se necesita una dosis
más alta que en el caso de los insectos adultos. Existen variaciones
en la tolerancia de las distintas especies y fases de insectos y entre
las distintas estirpes del mismo insecto.
No existen datos sobre los efectos directos del bromuro metílico
en las aves y los mamíferos silvestres.
7 Efectos en los animales de experimentación
Los estudios de inhalación realizados en varias especies de
mamíferos han mostrado que existen claras diferencias relacionadas con
la especie y el sexo en lo que se refiere a la susceptibilidad al
bromuro metílico. No se observó una respuesta dosis-mortalidad muy
marcada en ninguna de las especies animales ensayadas.
Las manifestaciones neurológicas son los principales signos
clínicos de toxicidad en las ratas y los ratones y, en concentraciones
más altas, se ha observado también la irritación de las mucosas.
Entre las manifestaciones neurológicas destacan los espasmos y la
parálisis. Con dosis más altas varios autores han señalado
modificaciones de la actividad locomotriz, disfunción de los medios
periféricos, cambios del ritmo circadiano y aversión gustativa
condicionada.
Se han descrito lesiones histopatológicas en el cerebro, el
riñón, la mucosa nasal, el corazón, las glándulas suprarrenales, el
hígado y los testículos de ratas y ratones expuestos a distintas
concentraciones de bromuro metílico.
Las células de soporte olfativas y las sensoriales maduras sufren
lesiones por la exposición a corto plazo al bromuro metílico, pero la
reparación y la recuperación son rápidas.
Los estudios de inhalación de larga duración (hasta 2 años) en
ratas mostraron lesiones de la mucosa nasal y el miocardio. En un
estudio análogo de larga duración en ratones se observaron los efectos
tóxicos primarios en el cerebro, el corazón y la mucosa nasal. En
ninguna de ambas especies se registraron signos de carcinogenicidad.
La administración oral de 50 mg de bromuro metílico/kg de peso
corporal a ratas durante un periodo de hasta 25 semanas produjo
inflamación e hiperplasia intensa del epitelio del antro cardial. Tras
un periodo de recuperación postexposición, la principal lesión
observada fue la fibrosis del antro cardial. En la rata tratada
diariamente durante 25 semanas se observó un carcinoma inicial del
antro cardial.
Los ratones B6C3F y las ratas F344 expuestos a dosis de hasta 467
mg de bromuro metílico/m3 durante 13 semanas mostraron cambios
ligeros de la morfología del esperma sin que se afectara la duración
del ciclo estral.
La exposición por inhalación a dosis de hasta 350 mg de bromuro
metílico/m3 no produjo ningún efecto digno de mención sobre el
crecimiento, los procesos reproductivos y las crías de dos
generaciones consecutivas de ratas CD Sprague-Dawley. Los índices de
fecundidad de machos y hembras se redujeron con dos niveles máximos de
concentraciones en la camada F2B de la generación F1.
En los estudios sobre la toxicología del desarrollo efectuados en
conejos blancos de Nueva Zelandia, la exposición a 311 mg de bromuro
metílico/m3 (6 h/día; días 7-19 de la gestación) mostró una
toxicidad materna moderada a intensa. Los efectos en el desarrollo
observados con dosis tóxicas para la madre consistieron en
dis-minución del peso del feto, aumento de la incidencia de
variaciones óseas ligeras y presencia de malformaciones
(principalmente ausencia de la vesícula biliar o del lóbulo caudal del
pulmón). Sin embargo, con dosis de 272 mg/m3 la toxicidad materna
fue menos marcada y no se produjeron efectos embriotóxicos.
No se observaron efectos maternos, embrionarios o fetales
adversos en conejos expuestos a 78 ó 156 mg de bromuro metílico/m3.
En conejos blancos de Nueva Zelandia se indicó un nivel sin efectos
observados (NOEL) de 156 mg de bromuro metílico/m3 en lo que
respecta a la toxicidad materna y del desarrollo.
Se ha observado que el bromuro metílico es mutagénico en varios
sistemas de ensayo in vitro e in vivo. Provoca mutaciones letales
recesivas ligadas al sexo en Drosophila melanogaster y mutaciones en
células de mamífero cultivadas. No induce la síntesis no programada
del ADN ni la transformación celular en células de mamífero
cultivadas. En ratones a los que se administró bromuro metílico por
distintas vías se observó la metilación del ADN en el hígado y el
bazo. Se indujo la formación de micronúcleos en las células de la
médula ósea y de la sangre periférica de ratones y ratas.
Se desconoce el mecanismo de la toxicidad del bromuro metílico.
8 Efectos en la especie humana
La exposición humana al bromuro metílico puede producirse por
inhalación del gas o por contacto con el líquido. También se produce
exposición por ingestión de agua de bebida contaminada con agua de
lixiviación.
Un estudio en la especie humana con testigos mostró que la
captación del producto después de la exposición por inhalación es del
50% aproximadamente de la dosis administrada.
El bromuro metílico es nocivo para el sistema nervioso, los
pulmones, la mucosa nasal, los riñones, los ojos y la piel. Entre los
efectos en el sistema nervioso central figuran la visión enturbiada,
la confusión mental, la pérdida de sensibilidad, el temblor y los
defectos del habla. La exposición tópica puede provocar irritación
cutánea, quemaduras y lesiones oculares.
La exposición a altas concentraciones de bromuro metílico causa
edema pulmonar. La depresión del sistema nervioso central con
parálisis respiratoria e insuficiencia respiratoria es a menudo la
causa inmediata de la muerte, que va precedida de convulsiones y coma.
Se han observado distintos signos y síntomas neuropsiquiátricos
en el curso de las intoxicaciones agudas y prolongadas producidas por
bromuro metílico. Las exposiciones de corta duración a dosis bajas de
vapores han producido un síndrome de polineuropatía con
manifestaciones centrales patentes.
Entre las secuelas tardías figuran la bronconeumonía consecutiva
a lesiones pulmonares graves, la insuficiencia renal con anuria y la
debilidad extrema, con o sin signos de parálisis. Por lo general esos
síntomas tienden a remitir después de un periodo de unas semanas o
meses. Sin embargo, se han observado deficiencias sin recuperación,
caracterizadas habitualmente por trastornos sensoriales, debilidad,
alteraciones del carácter y enturbiamiento de la visión.
La exposición al bromuro metílico va acompañada de un aumento de
la concentración de bromuro en la sangre. En los fumigadores se
observa una relación entre el número de aplicaciones del gas y la
concentración plasmática media de bromuro.
Tanaka et al. (1991) measured ambient methyl bromide
concen-trations and urinary bromine concentrations at various plant
quarantine fumigation sites in Japan. The geometric mean for exposed
workers was 9.0±1.85 mg/litre while the arithmetic mean for a matched
control population was 6.3±2.5 mg/litre. The authors found a
statistically significant positive correlation between the ambient
methyl bromide concentrations collected with a personal sampling
device and the urinary concentrations.
9.4.4 Haemoglobin adducts as a biological index to methyl bromide
exposure
Iwasaki et al. (1989) determined the haemoglobin adduct
(haemoglobin MeCys) in methyl bromide manufacturing workers, and
examined its effectiveness as a biological index of exposure to methyl
bromide. Methyl bromide reacts with cysteine to form
S-methylcysteine (MeCys) in haemoglobin (Djalali-Behzad et al.,
1981). It was suggested that determination of MeCys in haemoglobin
could detect previous methyl bromide exposure levels so low that they
were not detected by a special medical check-up (Iwasaki et al.,
1989). Haemoglobin adducts have a life span of about 2 months, so
workers only intermittently exposed to methyl bromide would also be
detected in such a survey. Previous studies by the same authors showed
individual differences in mice in the levels of MeCys within each dose
and time series group (Iwasaki, 1988a,b). These individual differences
may be caused by the differential susceptibility of haemoglobin in red
blood cells or individual differences in methyl bromide metabolism.
These differences were found to be minor in the human study (Iwasaki
et al., 1989).
Results from animal studies (Xu et al., 1990) supported the
suggestion of Iwasaki et al. (1989) that the Hb adduct of methyl
bromide might be a useful parameter in the biological exposure
monitoring of methyl bromide workers.
9.4.5 Neurobehavioural and other studies
Anger et al. (1986) carried out a neurobehavioural study on soil
fumigators who were exposed to 9 mg methyl bromide/m3 over an 8-h
day and structural fumigators who were exposed to 0-9 mg/m3 for 1.5
h per day. The fumigators, who reported a significantly higher
prevalence of 18 symptoms, consistent with methyl bromide toxicity,
than the controls, did not perform so well on 23 out of 27 behavioural
tests, and were significantly lower on one test of finger sensitivity
and one of cognitive performance.
EEGs and transaminases were measured in 33 methyl bromide workers
engaged in soil disinfection inside greenhouses in the Netherlands.
Symptoms of methyl bromide intoxication were determined by means of a
specially designed questionnaire. A general neurological examination
was performed. No differences compared with controls were found, with
the exception of slight electro-encephalographic changes in 10 workers
involving diffuse increase of beta and theta activity. Workers with
abnormal EEGs had higher blood bromide levels (geometric mean 10.9
mg/litre compared with a mean of 8.2 mg/litre in the other workers;
the difference was statistically significant ( P <0.05) (Verberk et
al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human exposure
Human exposure to methyl bromide occurs predominantly as an
occupational hazard, particularly during soil or bulk fumigation, but
also during manufacture. Individuals in the vicinity of fumigated
fields or buildings may also be exposed. Methyl bromide has widespread
use for fumigating post-harvest foods, such as cereals, spices,
dried-fruits, and nuts, as well as fresh fruits and vegetables. The
determination of levels of methyl bromide and inorganic bromide in the
fumigated food is important for the assessment of possible risks for
human and animal health. Methyl bromide levels in food commodities
usually decrease rapidly after aeration, but may be detected for some
weeks after treatment. Fumigation with methyl bromide results in
increased levels of inorganic bromide in food commodities and,
equally, in produce grown on fumigated soils. Fumigant dosage
standards should be adhered to, so that the bromide levels do not
exceed the recommended limits.
The major health concern is from acute exposure.
Delayed onset of symptoms may occur. Fatal poisoning has resulted
from exposures to relatively high concentrations (from 33 000 mg/m3
or 8600 ppm onwards) of methyl bromide vapours. Non-fatal poisoning
has resulted from exposure to concentrations as low as 390-1950
mg/m3 (100- 500 ppm). Organs affected by exposure include the
nervous system, lung, nasal mucosa, kidney, eye, and skin. There are
no epidemiological data on reproductive toxicity and carcinogenicity
in humans. There are no data on any human health effects of methyl
bromide residues in food or drinking-water.
10.1.1 Relevant animal studies
Methyl bromide is very toxic for all animal species by all routes
of administration studied. Deaths from exposure follow a steep
dose-response curve. LC50 (1-h) values for mice and rats are 4680
and 7300 mg/m3, respectively.
Deaths and neurotoxicity occur within hours or days after a
single inhalation exposure at high concentrations. Studies on mice
show that more prolonged exposure at low concentrations (6 h/day; 389
mg/m3) may also produce neurotoxicity or deaths, appearing with a
delayed onset of several months.
Absorption and distribution to various tissues is rapid, as is
elimination. The metabolic pathways and mode of action are unknown.
The principal toxic effects associated with lethality occur in
the brain and kidney. Histopathology of the brain shows necrosis of
granular cells of the cerebellum in mice and rats and neuronal
necrosis in the cerebral cortex, hippocampus, and thalamus, in rats.
The lowest doses at which these lesions were observed were 250
mg/m3, 6 h/day, for 2 years, in mice, and, 622 mg/m3, 6 h/day, for
5 weeks, in rats. In the kidney, necrosis of the convoluted tubule
epithelium occurs at doses of 599 mg/m3, 6 h/day, for 2 weeks, in
mice, and 1712 mg/m3, 6 h/day, for 2 weeks, in rats. Other effects
are observed in the heart (degeneration of focal necrosis), nose
(necrosis of olfactory epithelium), testis (degeneration of
seminiferous tubules), and other organs. In non-lethal exposure, nasal
irritation and neuro-toxicity (including histopathological lesions)
are the major toxic effects.
No teratogenic effects have been observed in rats or rabbits.
Embryotoxicity occurred in rats and rabbits only at doses that were
also maternally toxic. In a rat multigeneration study, there were
reductions in the fertility index in the second generation, when the
animals were exposed to 117 or 350 mg/m3 (30 or 90 ppm) for 6 h/
day, but effects were not observed following exposure to 12 mg/m3 (3
ppm) for 6 h/day.
Methyl bromide was mutagenic in several in vivo and in vitro
assays.
Long-term inhalation studies on rats and mice did not reveal any
evidence of carcinogenicity. Lesions originally interpreted as
carcinomas of the forestomach in rats, following gavage
administration, were shown in a subsequent study to regress after
termination of treatment, and were considered not relevant for human
risk assessment.
10.2 Environment
Methyl bromide is predominantly a naturally occurring compound.
Oceans are believed to be a major natural source of methyl bromide.
Another source(s) may exist in the tropics, which is yet to be
explained. Anthropogenic sources from fumigation and, to a much lesser
extent, motor vehicles (from the combustion of organic bromine
additives in leaded petrol) add to these. Present data indicate that
the globally averaged atmospheric abundance of methyl bromide is
between 9 and 13 pptv, equivalent to a total atmospheric loading of
150-220 thousand tonnes. If the atmospheric lifetime is two years
(assuming only atmospheric removal processes are significant),
anthropogenic sources of methyl bromide represent about 25% (±10%) of
the total emissions. The world production of methyl bromide in 1990
was 69 000 tonnes, having increased at a rate of 6% per year from 1984
to 1990. About 50% of the methyl bromide produced is released into the
atmosphere during, or after, use. Although methyl bromide reacts with
the hydroxyl radical in the troposphere, some methyl bromide is
transferred by upward diffusion to the stratosphere, where it
photolyses. Active bromine species react with ozone in the lower
stratosphere and are partly responsible for the depletion of the ozone
layer. It is estimated that anthropogenic releases of methyl bromide
cause about 3% of the present total stratospheric ozone loss.
Methyl bromide is used for soil fumigation (about 77%), for
quarantine, commodity fumigation (12%), for structural fumigation
(5%), and for chemical intermediates (6%).
In soil, methyl bromide is degraded by hydrolysis and microbial
activity. The remainder (about 50%) eventually dissipates into the
atmosphere. The degradation product, principally as inorganic bromide,
remains as a residue in soil.
Methyl bromide has herbicidal properties. In the vicinity of
greenhouses and fumigated structures, phytotoxic effects may occur.
Visible damage to the leaves of lettuce (a sensitive test plant) was
noticed at 400 mg/m3.
Soil fumigation using methyl bromide (with 2% chloropicrin)
affects both target and non-target organisms: various soil microflora
and fauna are adversely affected, at least temporarily, by fumigation.
High mortality of non-target insects has been noticed from fumigation
under plastic sheeting. Methyl bromide could be detected in different
soil types up to 3 weeks after the treatments, the highest levels
being found in the upper layers (0-40 cm) of the soil.
Methyl bromide is highly toxic for aquatic organisms, though it
is generally of no risk to the aquatic environment. The lowest EC50
or LC50 values reported are 2.8 mg/litre for algae, 1.7 mg/litre for
daphnids, and 0.3 mg/litre for fish. NOEC levels, derived from
long-term studies, as low as 0.06 mg/litre have been reported for
daphnids and fish. Toxic concentrations are not expected to be reached
under normal circumstances, because most of the methyl bromide applied
on soil is degraded or lost, due to evaporation, before it reaches
surface water via run-off. Only in very special situations (leaching
of greenhouse soils to reduce inorganic bromide residues), can levels
of methyl bromide in the mg/litre range occur in water. Concentrations
of up to 9.3 mg methyl bromide/litre were measured in drainage water.
Similarly, relatively high levels of bromide (up to 72 mg/litre)
that could adversely affect aquatic organisms were measured in
drainage water from greenhouses. Long-term exposure to bromide ion
resulted in an EC50 value of 27 mg bromide/litre for daphnia and the
lowest NOEC for different fish species was 25 mg bromide/litre.
11. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
11.1 Human health protection
In most countries, methyl bromide is strictly regulated for
application as a fumigant for soil, commodities, and structural
purposes. Adherence to good practices and guidelines should ensure no
adverse effects on persons exposed occupationally. Considerable care
should be taken by manufacturers in the production process of methyl
bromide and by suppliers in its transfer and use.
Methyl bromide does not pose a significant risk for the general
population. It is, however, a very toxic substance and precautions
have to be taken: exposure of the general public should be avoided
through adequate precautions in fumigated buildings, greenhouses, and
stores used for commodity fumigation. Fumigated premises should be
clearly marked, in order to prevent accidental exposure of
individuals.
11.2 Environmental protection
Emission of methyl bromide from anthropogenic sources should be
reduced as far as possible. It is, therefore, desirable to reduce
emissions from soil, commodity, and structural fumigation.
Improvements should be sought in these areas:
(1) improved injection methods;
(2) better barrier films;
(3) better measurement of the efficacy of methyl bromide, with
the goal of lower dosage rates where possible.
For commodity and, possibly, for structural fumigation,
techniques should be developed to:
(1) seal fumigation chambers more tightly;
(2) capture and recycle fumigation gas.
11.3 Recommendations for further research
There is a need for:
- study of the metabolism and toxic mechanisms of methyl
bromide;
- a postnatal behavioural study;
- a human epidemiological study including exposure
assessment;
- development of a treatment protocol for cases of human
poisoning.
Effects of methyl bromide on the depletion of the ozone layer are
not yet entirely understood, indicating a need for:
- studies quantifying the distribution sources of methyl
bromide and other organic bromine compounds.
There is also a need for:
- a study of the impact of sea spray on the formation of
organic bromine compounds;
- a study on the degradation products of methyl bromide in the
troposphere;
- further study of the rate constant for the reaction with
OH-;
- studies on soil fumigation with the aim of reducing
emissions while keeping efficacy.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12.1 FAO/WHO
The toxicology of methyl bromide was evaluated by the FAO/WHO
Joint Meeting on Pesticide Residues in 1965 and 1966 (FAO/ WHO, 1965
a,b; WHO, 1967, a,b). In 1965, no acceptable daily intake (ADI) was
allocated, but, in 1966, an ADI of 1 mg/kg body weight, as bromide
ion, was established. In 1988, the JMPR evaluated the toxicology of
the bromide ion (FAO/WHO 1988a,b) and concluded that the level causing
no toxicological effect was:
Rat: 240 ppm, equivalent to 12 mg bromide/kg body weight
per day
Human: 9 mg bromide/kg body weight per day
The acceptable daily intake (ADI) of 1 mg/kg body weight was
confirmed.
12.2 IARC
The carcinogenic risks for humans were evaluated by an
International Agency for Research on Cancer ad hoc expert group in
1986. The evaluation was updated in 1987 and it was concluded that
there was inadequate evidence for carcinogenicity to humans and
limited evidence for carcinogenicity to animals; the overall
evaluation of carcinogenicity to humans was "not classifiable" [group
3] (IARC, 1986, 1987).
12.3 UNEP
In 1992, the United Nations Environment Programme evaluated methyl
bromide on behalf of the Contracting Parties to the Montreal Protocol
(UNEP, 1992). The executive summary of the evaluation reads:
"The report covers the current understanding of the impact
of methyl bromide on the ozone layer and on the uses of, and
alternatives to, methyl bromide."
It was requested by the United Nations Environment Programme on behalf
of the Parties to the Montreal Protocol. The report includes
information presented and discussed at the International Methyl
Bromide Science Workshop held in Washington, DC, on 2-3 June 1992, and
at the International Workshop on Alternatives and Substitutes to
Methyl Bromide held in Washington, DC, on 16-18 June 1992.
The current state of scientific knowledge concerning bromine
compounds in the atmosphere is considerably less developed than the
corresponding understanding of chlorine compounds. In addition, the
evaluation of the alternatives and substitutes to methyl bromide as a
fumigant is in an earlier stage than that for chlorofluorocarbons
(CFCs), methylchloroform, carbon tetrachloride, and halons.
Methyl bromide is used as a fumigant for soils, commodities, and
structures. It is currently vital for the economic viability of
certain agricultural production (especially strawberries, tomatoes,
peppers, tobacco, eggplants, nursery stock, vines, and turf) and for
the quarantine treatment of certain products in international trade.
Many developing countries are particularly dependent on the export of
products currently fumigated using methyl bromide, before shipment and
at ports of entry.
Total annual production and sales of methyl bromide for
fumigation have increased from about 42 000 tonnes to about 63 000
tonnes between 1984 and 1990. Combining the approximate methyl bromide
use-pattern data with the currently estimated fraction that escapes to
the atmosphere from each use (soils: 80% of use, 50 % emitted;
commodities: 15 % of use, <80 % emitted; and structural: 5 % of use,
80% emitted) indicates that about half of the methyl bromide used as
a fumigant is emitted into the atmosphere. Based on current
understanding, this implies an anthropogenic emission from fumigation
applications of about 30 thousand tonnes in 1990, which represents
25±10 % of the total (natural and anthropogenic) emissions.
Ozone destruction by bromine is more efficient on a per molecule
basis than destruction by chlorine by a factor of 30-60. Therefore, 1
part per trillion by volume (pptv) of bromine is equivalent to
0.03-0.06 parts per billion by volume (ppbv) of stratospheric
chlorine.
The current best estimate of the steady-state value of the Ozone
Depletion Potential (ODP) for methyl bromide is 0.7. Because of the
short atmospheric lifetime of methyl bromide, its relative impact on
ozone is expected to be much greater over the next decade (when
chlorine abundances and ozone losses are predicted to reach their
maximum) than is indicated by its steady-state ODP.
There are significant uncertainties in the atmospheric budget and
ODP of methyl bromide, especially the quantification of possible
oceanic and terrestrial surface removal processes and the rate of
formation of unreactive bromine in the stratosphere.
Model calculations suggest that anthropogenic emissions of methyl
bromide used for fumigation applications could have accounted for
about one-twentieth to one-tenth of the current observed global ozone
loss of 4-6% and could grow to about one-sixth of the predicted ozone
loss by the year 2000, if methyl bromide emissions continue to
increase at the present rate of about 5-6% per year.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide from fumigation applications is about 3
pptv, which is equivalent to 0.09-0.18 ppbv of stratospheric chlorine.
An advance of the CFC and carbon tetrachloride phaseout schedule by
three years would reduce the peak chlorine loading by 0.18 ppbv.
Therefore, elimination of methyl bromide used as a fumigant could
provide an ozone-layer protection equivalent to that of an advance of
the CFC and carbon tetrachloride phaseout schedule by about 1.5-3
years.
There is no single alternative to methyl bromide in the broad
spectrum of applications for which it is currently used. There are,
however, many alternative chemicals and procedures for specific
applications. The introduction of some chemical alternatives may
require government approval, which could be a lengthy process.
Rough estimates indicate that a significant fraction (from as low
as 30% to as high as 90%), albeit uncertain, of methyl bromide used
for soil fumigation could be replaced by chemical substitutes during
the 1990s; that a substantial proportion of emissions from fumigation
chambers could be captured and recycled or destroyed; that a small
fraction (1-2%) of methyl bromide emissions could be eliminated by
better procedures during tank filling; and that significant reductions
in emissions could be made using other alternatives or techniques,
alone, or in combination. However, there are some applications for
which there are limited or no alternatives, including some
agricultural situations that have developed a dependence on methyl
bromide, quarantine treatments, and some structural fumigation uses.
REFERENCES
Abdalla N & Lear B (1975) Lethal dosages of methyl bromide for four
plant-parasitic nematodes and the effect of soil temperature on its
nematicidal activity. Plant Dis Rep, 59: 224-228.
Adu OO & Muthu M (1985) The relative toxicity of seven fumigants to
life cycle stages of Callosobruchus chinensis (L). Insect Sci Appl,
6: 75-78.
Alexeeff GV & Kilgore WW (1983) Methyl bromide. In: Gunther FA &
Gunther JD ed. Residue reviews. Residues of pesticides and other
contaminants in the total environment, Vol. 88. Berlin, Heidelberg,
New York, Springer-Verlag, pp 102-153.
Alexeeff GV, Kilgore WW, Munoz P, & Watt D (1985) Determination of
acute toxic effects in mice following exposure to methyl bromide. J
Toxicol Environ Health, 15: 109-123.
Alfaro Moreno A & Vegh I (1971) [Green pepper diseases produced by
Phytophthora capsici.] An Inst Nac Invest Agr Ser Prot Veg, 1: 9-42
(in Spanish).
American Biogenics Corporation (1986) Two-generation reproduction
study in albino rats with methyl bromide - results of both generations
(Study No. 4500-1525) (Unpublished final report).
Andersen ME, Gargas ML, Jones RA, & Jenkins LJ (1980) Determination of
the kinetic constants for metabolism of inhaled toxicants in vivo
using gas uptake measurements. Toxicol Appl Pharmacol, 54: 100-116.
Anger WK, Setzer JV, Russo JM, Brightwell WS, Wait RG, & Johnson BL
(1981) Neurobehavioral effects of methyl bromide inhalation exposures.
Scand J Work Environ Health, 7: 40-47.
Anger WK, Moody L, Burg J, Brightwell WS, Taylor BJ, Russo JM,
Dickerson N, Setzer JV, Johnson BL, & Hicks K (1986) Neurobehavioral
evaluation of soil and structural fumigators using methyl bromide and
sulfuryl fluoride. Neurotoxicology, 7: 137-156.
Angerer J (1977) [A method for determination of bromide in urine with
help of a ionsensitive electrode.] J Clin Chem Clin Biochem, 15:
201-204 (in German).
Angerer J (1980) [Bromide. Determination in urine.] In: Henschler D
ed. [Analytical methods for the investigation of work materials
detrimental to health. Analysis in biological materials.] Weinheim,
Verlag Chemie, vol 2/2, pp D1-D6, 1-9 (in German).
Angerer J (1982) [Methyl bromide. Air analysis]. In: Henschler D ed.
[Analytical methods for the investigation of work materials
detrimental to health. Air analysis.] Weinheim, Verlag Chemie, vol
1/2, D1-D2, 1-8 (in German).
Anthon EW, Moffit HR, Couey HM, & Smith LO (1975) Control of codling
moth in harvested sweet cherries with methyl bromide and effects upon
quality and taste of treated fruit. J Econ Entomol, 68: 524-526.
Arvieu JC (1983) Some physico-chemical aspects of methyl bromide
behaviour in soil. Acta Hortic, 152: 267-275.
Arvieu JC & Cuany A (1985) Effets de la matière organique sur la
bioactivité et la dégradation du bromure de méthyle dans le sol. EPPO
Bull, 15: 87-96.
Asante-Poku S, Aston WP, & Schmidt DE Jr (1974) Site of decomposition
of methyl bromide in cocoa beans. J Sci Food Agric, 25: 285-291.
Atochem (1987) Fumyl-o-gas. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 4 pp.
Atochem (1988) BM 98 SOBROM. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 2 pp.
Audry D, Soichot P, Giard MH, & Mauguiere F (1985) Une cause rare de
myoclonies avec PES "Géants": l'intoxication par le bromure de
méthyle. A propos d'une observation à prédominance unilaterale. Rev
EEG Neurophysiol, 15: 45-52.
Austin RK & Phillips DJ (1985) Use of a standard-addition
bromide-selective electrode technique to determine bromide and trace
its migration in peaches. J Agric Food Chem, 33: 1165-1168.
Bachem C (1927) [Contribution to the toxicology of the alkyl
halogens.] Arch Exp Pathol Pharmakol, 122: 69-76 (in German).
Bakhishev GN (1973) [Relative toxicity of aliphatic halohydrocarbons
to rats.] Farmakol Toksikol, 8: 140-142 (in Russian).
Balander PA & Polyak MG (1962) [Toxicological characteristics of
methyl bromide.] Gig i Toksikol, 60: 412-419 (in Russian).
Basile M & Lamberti F (1981) Bromide residues in edible organs of
plants grown in soil treated with methyl bromide. Meded Fac
Landbouwwet Rijksuniv Gent, 46: 337-341.
Basile M, Lamberti F, & Basile AC (1987) [Inorganic bromide
concentrations in soil after fumigation with methyl bromide.] Essenze
Deriv Agrum, 57: 116-120 (in Italian).
Baumann H & Heumann KG (1987) Analysis of organobromine compounds and
hydrogen bromide in motor car exhaust gases with a GC/microwave plasma
system. Fresenius Z Anal Chem, 327: 186-192.
BBA (Biologische Bundesanstalt für Land- und Forstwirtschaft) (1989)
[Plant protective agent register, 1989/90, Part 5.] 37th ed.
Braunschweig, Federal Institute for Agriculture and Forestry, 66 pp
(in German).
Behrens RH & Dukes DCD (1986) Fatal methyl bromide poisoning. Br J Ind
Med, 43: 561-562.
Bell CH (1988) Minimum concentration levels of methyl bromide required
for full efficacy against seven species of stored-product beetle at
two temperatures. Pestic Sci, 24: 97-109.
Benatt AJ & Courtney TRB (1948) Uraemia in methyl bromide poisoning:
A case report. Br J Ind Med, 5: 21-25.
Berg WW, Heidt LE, Pollock W, Sperry PD, Cicerone RJ, & Gladney ES
(1984) Brominated organic species in the arctic atmosphere. Geophys
Res Lett, 11: 429-432.
Beyne M & Goett C (1934) Toxicité de certains appareils extincteurs
d'incendie et précautions qu'ils comportent dans leur emploi. Arch Méd
Pharm Nav, 124: 409.
Bird GW, Rich JR, & Glover SU (1974) Increased endomycorrhizae of
cotton roots in soil treated with nematicides. Phytopathology, 64:
48-51.
Bishop CM (1992) A case of methyl bromide poisoning. J Occup Med, 42:
107-109.
Blackburn S & Philips H (1944) Experiments on the methylation and
acetylation of wool, silk fibroin, collagen and gelatin. Biochem J,
38: 171-178.
Blackith RE & Lubatti OF (1965) Fumigation of agricultural products.
XX. Prolonged storage of cereals fumigated with methyl bromide. J Sci
Food Agric, 16: 455-457.
Boczek J, Klag J, & Komorowska B (1975) Effect of methyl bromide on
the embryonic development of Acarus siro (Acarina, Acaridae). J
Stored Prod Res, 11: 41-46.
Bolon B, Bonnefoi M, & Morgan KT (1990) Effect of olfactory epithelial
regeneration and cage environment on the sensitivity of the rat
olfactory epithelium to methyl bromide. Chem Senses, 15: 554-555.
Bond EJ (1956) The effect of methyl bromide on the respiration of the
cadelle Tenebroides mauritanicus L. (Coleoptera: Ostomidae). Can J
Zool, 34: 405-415.
Bond EJ (1984) Manual of fumigation for insect control. 6. Chemicals
used as fumigants. Rome, Food and Agriculture Organization of the
United Nations, pp 71-96 (Plant Production and Protection Paper 54).
Bond EJ & Dumas T (1987) Concentrations of methyl bromide inside flour
mills and in the atmosphere around the mills during and after
fumigation. Proc Entomol Soc Ont, 118: 1-6.
Bond JA, Dutcher JS, Medinsky MA, Henderson RF, & Birnbaum LS (1985)
Disposition of [14C]methyl bromide in rats after inhalation. Toxicol
Appl Pharmacol, 78: 259-267.
Boorman GA, Hong HL, Jamieson CW, Yoshitomi K, & Maronpot RR (1986)
Regression of methyl bromide induced forestomach lesions in the rat.
Toxicol Appl Pharmacol, 86: 131-139.
Bourbos VA & Skoudridakis MT (1991) Effect of methyl bromide and
chloropicrin on the soil mycoflora in greenhouse tomato. Plant Pathol
Lab, 56: 569-575.
Bowers GN Jr & Onoreski M (1990) Hyperchloremia and the incidence of
bromism in 1990. Clin Chem, 36: 1399-1403.
Brenner I (1978) Bromism: alive in well. Am J Psychiatry, 7: 857-858.
Breslin WJ, Zablotny CL, Bradley GF, & Lomax LG (1990) Methyl bromide
inhalation teratology study in New Zealand white rabbits. Midland,
Michigan, The Dow Chemical Company (Unpublished final report).
Bridges RG (1955) The fate of labelled insecticide residues in food
products. III. N-methylation as a result of fumigating wheat with
methyl bromide. J Sci Food Agric, 6: 261-268.
Brodniewicz A (1967) Poisoning of seamen with methyl bromide due to
fumigation of a Polish cargo ship in Haiphong (Vietnam). Arh Hig Rada,
18: 241-246 (in Polish with English translation).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: an assessment of available data (Contract-No. 68-02-3452).
Menlo Park, California, SRI International (EPA/DF-83/005a).
Bromine and Chemicals Ltd (1990) Methylbromide soil fumigation - use
and application instructions. London, Bromine and Chemicals Ltd, pp
1-36.
Brown G & Jenkinson DS (1971) Bromine in wheat grown on soil fumigated
with methyl bromide. Soil Sci Plant Anal, 2: 45-54.
Brown D & Rolston DE (1980) Transport and transformation of methyl
bromide in soils. Soil Sci, 130: 68-75.
Brown AL, Burau RG, Meyer RD, Raski DJ, Wilhelm S, & Quick J (1979)
Plant uptake of bromide following soil fumigation with methyl bromide.
Calif Agric, 33: 11-13.
Bruhin J (1942) [Percutaneous poisoning with methyl bromide during
pest control.] University of Zurich, Medical Faculty, 31 pp (M.D.
Thesis) (in German).
BUA (1987) [BUA Chemical Report 14: Methyl bromide.] Weinheim,
Advisory Committee for Environmental Priority Pollutants (BUA) of the
German Chemists Association, 64 pp (in German).
Burkholder WE (1966) Toxicity of methyl bromide to Acarus siro , a
cheese-infesting mite. J Econ Ent, 59: 110-112.
Butler ECB, Perry KMA, & Williams JRF (1945) Methyl bromide burns. Br
J Ind Med, 2: 30-31.
CAC (1986) Guide to Codex Alimentarius recommendations on pesticide
residues. Part 3. Guideline levels for pesticide residues. Rome, Food
and Agriculture Organization (CAC/PR 3-1986).
Cantineau A, Thomas R, Breurec JY, Bousser J, Baert A, Bonny JS,
Bouget J, Dubois C, Venisse T, & Curtes JP (1988) Acute methylbromide
poisoning. J Toxicol Clin Exp, 8: 83-87.
Canton JH, Wester PW, & Matthijssen-Spiekman, EAM (1983) Study on the
toxicity of sodium bromide to different freshwater organisms. Food
Chem Toxicol, 21: 369-378.
Carter B (1945) Methyl bromide poisoning. Effects on the nervous
system. Br Med J, 1: 43-45.
Castro CE & Belser NO (1981) Photohydrolysis of methyl bromide and
chloropicrin. J Agric Food Chem, 29: 1005-1008.
Castro JA, Zerba EN, De Licastro SA, Picollo MI, Wood EJ, Ruveda MA,
De Moutier Aldao EM, & Libertella R (1976) Toxicity of methyl bromide
and other gaseous insecticides to Triatoma infestans. Acta Physiol
Lat Am, 26: 106-114.
Cavanagh JB (1992) Methyl bromide intoxication and acute energy
deprivation syndromes. Neuropathol Appl Neurobiol, 18: 573-578.
CDFA (1988) Sampling for pesticide residues in California well water:
1988 update; well inventory data base. Sacramento, California,
Department of Food and Agriculture, Division of Pest Management,
Environmental Protection and Worker Safety, Environmental Monitoring
and Pest Management Branch, 66 pp (Report EH 88-10).
CEC (1985) Report of the Scientific Committee for Pesticides on the
use of methyl bromide as a fumigant for plant culture media.
Luxembourg, Commission of the European Communities, pp 15-33 (EUR
10211).
Chavez CT, Hepler RS, & Straatsma BR (1985) Methyl bromide optic
atrophy. Am J Ophthalmol, 99: 715-719.
Cheetham T (1990) Pathological alterations in embryos of the codling
moth (Lepidoptera: Tortricidae) induced by methyl bromide. Ann.
Entomol. Soc. Am., 83: 59-67.
Chellman GJ, White RD, Norton RM, & Bus JS (1986) Inhibition of the
acute toxicity of methyl chloride in male B6C3F1 mice by glutathione
depletion. Toxicol Appl Pharmacol, 86: 93-104.
Chen T, Kilpatrick RA, & Rich AE (1962) Stylet-bearing nematodes
associated with white clovers in New Hampshire. Plant Dis Rep, 46:
346-347.
Chisholm RD & Koblitsky L (1943) Sorption of methyl bromide by soil in
a fumigation chamber. J Econ Entomol, 36: 549-551.
Cicerone RJ, Heidt LE, & Pollock WH (1988) Measurements of atmospheric
methyl bromide and bromoform. J Geophys Res, 93: 3745-3749.
Cirilli L & Borgioli A (1986) Methyl bromide in surface drinking
waters. Gas-chromatographic determination by the headspace technique.
Water Res, 20: 273-275.
Clarke CA, Roworth CG, & Holling HE (1945) Methyl bromide poisoning.
Br J Ind Med, 2: 17-23.
Cochran JW (1988) A rapid, sensitive method for the analysis of
halogenated gases in water. J High Resol Chromatogr Chromatogr Commun,
11: 663-665.
Cooper DM, Griffiths NM, Hobson-Frohock A, Land DG, & Rowell JG (1978)
Fumigation of poultry food with methyl bromide: effects on egg
flavour, number and weight. Br Poult Sci, 19: 537-542.
Cornwell PB (1979) Health monitoring experience of fumigators using
methylbromide. Proceedings of the Fifth British Pest Control
Conference, Stratford-on-Avon, England, 26-29 September 1979. London,
British Pest Control Association (Sixth Session; Paper No. 17).
Cova D, Molinari GP, & Rossini L (1986) Residues after fumigation with
methyl bromide: bromide ion and methyl bromide in middlings and final
cereal foodstuffs. Food Addit Contam, 3: 235-240.
Crampton RF, Elias PS, & Gangolli SD (1971) The bromide content of
human tissue. Br J Nutr, 25: 317-322.
Daelemans A (1978) Uptake of methyl bromide by plantss and uptake of
bromide from decontaminated soils. Leuwen, Belgium, Catholic
University (Dissertation) (in Flemish).
Daft J (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71: 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1992) Headspace method for rapid determination of methyl
bromide in assorted nut samples. J Assoc Off Anal Chem, 75: 701-706.
Dagani MJ, Barda HJ, & Sanders DC (1985) Bromine compounds. Weinheim,
Verlag Chemie, pp 405-420.
Danse LHJC, Van elsen FL, & Van der Heijden CA (1984) Methylbromide:
carcinogenic effects in the rat forestomach. Toxicol Appl Pharmacol,
72: 262-271.
Darby JF, Dieter CE, & Rau GJ (1962) Evaluation of treatments for
control of soil-borne pests in celery seedbeds. Plant Dis Rep, 46:
441-443.
Davenport CJ, Ali SF, Miller FJ, Lipe GW, Morgan KT, & Bonnefoi MS
(1992) Effect of methyl bromide on regional brain glutathione,
glutathione- S-transferases, monoamines, and amino acids in F344
rats. Toxicol Appl Pharmacol, 112: 120-127.
Davis LN, Strange JR, Hoecker JE, Howard PH, & Santodonato J (1977)
Investigation of selected potential environmental contaminants:
monohalomethanes. Washington, DC, US Environmental Protection Agency
(EPA-560/2-77-007).
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1: 303-318.
De Heer H, Tuinstra LGMPH, Hamaker PH, & Van der burg AMM (1983) Use
of gas-tight plastic films during fumigation of glasshouse soils with
methylbromide. I. Significance of permeation and leakage for the
emission into the outside air. Acta Hortic, 152: 109-126.
Dempsey DA, Becker CE & Mihm FC (1992) Death after entering an
apartment building fumigated with methyl bromide and cleared for
habitation. Vet Hum Toxicol, 34: 356.
Deutschmann S, Reichel C, & Kettschau F (1989) Metabolism of methyl
bromide and methyl iodide in erythrocytes compared with methyl
chloride. Naunyn-Schmiedeberg's Arch Pharmacol, 339: R12.
Deutschmann S, Peter H, Reichel C, Bolt M, & Hallier E (1989)
[Kinetics of metabolism of methyl bromide and methyl iodide in
erythrocytes of humans and related species.] Verh Dtsch Gesundh
Arbeitsmed, 29: 517-519 (in German).
Devaney JA & Beerwinkle KR (1982) Control of the northern fowl mite on
inanimate objects by fumigation: field studies. Poult Sci, 62: 43-46.
Devries JW, Borge JM, Schroeder JP, Bowers RH, Larson PA, & Burns NM
(1985) Headspace gas chromatographic method for determination of
methyl bromide in food ingredients. J Assoc Off Anal Chem, 68:
1112-1116.
Djalali-Behzad G, Hussain S, Ostermann-Golker S, & Segerbaeck D (1981)
Estimation of genetic risks of alkylating agents. VI. Exposure of mice
and bacteria to methyl bromide. Mutat Res, 84: 1-9.
Doraiswamy S, Van Winckel A, & Van Assche C (1972) Effect in vitro of
soil fumigants on soil microbial population in relation to degradation
of tobacco mosaic virus. Meded Fac Landbouwwet Rijksuniv Gent, 37:
492-499.
Drawneek W, O'Brien MJ, Goldsmith HJ, & Bourdillon RE (1964)
Industrial methyl bromide poisoning in fumigators. Lancet, October 17:
855-856.
Dreef-Van der Meulen HC, Reuzel PGJ, Kuper CF, Hollanders VMH, & Feron
VJ (1989) Chronic inhalation toxicity of methyl bromide in rats. Third
International Symposium on Soil Disinfestation, Leuwen, Belgium,
September 26-30, 1988. Acta Hortic, 255: 313-315.
Driscoll JN, Duffy M, Pappas S, & Webb M (1987) Analysis for purgeable
organics in water by capillary GC/PID-ELCD. J chromatogr sci, 25:
369-375.
Drosihn UG, Stephan BR, & Hoffmann GM (1968) [Investigations into soil
contamination with methyl bromide.] Z Pflanzenkr Pflanzenschutz, 75:
272-287 (in German).
Dudley HC & Neal PA (1942) Methyl bromide as a fumigant for foods.
Food Res, 7: 412-429.
Dudley HC, Miller JW, Neal PA, & Sayers RR (1940) Studies on
foodstuffs fumigated with methyl bromide. Public Health Rep, 55:
2251-2282.
Duffy M, Driscoll JN, Pappas S, & Sanford W (1988) Analysis of ppb
levels of organics in water by means of purge-and-trap, capillary gas
chromatography and selective detectors. J Chromatogr, 441: 73-80.
Dumas T (1982) Trapping low levels of methyl bromide in air or as
residues at ambient and lower temperatures for gas chromatography. J
Assoc Off Anal Chem, 65: 913-915.
Dumas T & Bond EJ (1985) Analysis of methyl bromide at ultra low
concentration levels. J Agric Food Chem, 33: 276-278.
Duvoir M, Fabre R, & Layani F (1937). L'intoxication par le bromure de
méthyle. Bull Soc Méd Paris, 53: 1540.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards CA (1962) Springtail damage to bean seedlings. Plant Pathol,
11: 67-68.
Edwards CA & Thompson AR (1973) Pesticides and the soil fauna. In:
Gunther FA & Gunter JD, ed. Residue reviews. Residues of pesticides
and other contaminants in the total environment. Berlin, Heidelberg,
New York, Springer-Verlag, vol 45, pp 1-79.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
beta-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
El Buzz HK, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1974) Effect of
diet on the susceptibility of the different developmental stages of
Corcyra cephalonica staint. to carbon bisulphide and methyl bromide.
Agric Res Rev, 52: 21-29.
Eller PM (1985) NIOSH manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 2520/1-2520/-4 (NIOSH Publication No. 84-100).
EMBA (1988) Handling and transportation of methyl bromide. Code of
practice. Brussels, European Methyl Bromide Association, pp 1-8.
Enebak SA, Palmer MA, & Blanchette RA (1988) Effect of soil-borne
pathogen control methods on the production of white pine nursery
seedlings. Phytopathology, 78: 1532-1533.
Ethyl Corporation (1990a) Methyl bromide applications manual. Baton
Rouge, Louisiana, Ethyl Corporation, 3 pp.
Eustis SL, Haber SB, Drew RT, & Yang RSH (1988) Toxicology and
pathology of methyl bromide in F344 rats and B6C3F1 mice following
repeated inhalation exposure. Fundam Appl Toxicol, 11: 594-610.
Evans JE & Hastings L (1992) The effect of methyl bromide on measures
of olfactory function. Toxicologist, 12: 270.
Fabian P (1984) Atmosphere and environment. Berlin, Heidelberg, New
York, Springer-Verlag, p 41.
Fabian P, Borchers R, Penkett SA & Prosser NJD (1981) Halocarbons in
the stratosphere. Nature (Lond), 294: 733-735.
Fairall RJ & Scudamore KA (1980) Determination of residual methyl
bromide in fumigated commodities using derivative gas-liquid
chromatography. Analyst, 105: 251-256.
Fallico R & Ferrante M (1991) Exposure to inorganic bromides from
greenhouse crops where methyl bromide was applied for soil fumigation.
Zent.bl Hyg Umweltmed, 191: 555-562.
FAO (1980) Pesticide residues in food. Methyl bromide. Rome, Food and
Agriculture Organization of the United Nations, pp 89-97 (FAO Plant
Production and Protection Paper 20 Sup).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Group on Pesticide
Residues. Rome, Food and Agriculture Organization of the United
Nations; Geneva, World Health Organization (FAO Meeting Report No.
PL/1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1965b) Evaluation of hazards to consumers resulting from the
use of fumigants in the protection of food. Rome, Food and Agriculture
Organization of the United Nations; Geneva, World Health Organization
(FAO Meeting Report No. PL/1965/10/2; WHO/Food Add./28.65).
FAO/WHO (1988a) Pesticide residues in food - 1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environmental and the WHO Expert Group on Pesticide Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 92).
FAO/WHO (1988b) Pesticide residues in food - 1988 evaluations. Part II
-Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 93/2).
Filip GM & Roth LF (1977) Stump injections with soil fumigant to
eradicate Armillariella mellea from young-growth ponderosa pine
killed by root rot. Can J For Res, 7: 226-231.
Flick EW (1985) Industrial solvents handbook, 3rd ed. Park Ridge, New
Jersey, Noyes Data Corporation, 4 pp.
Foxboro (1989) Ambient air instrumentation sheet. East Bridgewater,
Maryland, The Foxboro Company.
Fransi A, Pons R, Sala A, Vallejo VR, & Bertran C (1987) Wheat and
soil bromide dynamics after fumigation with methyl bromide in a
mediterranean climate. Plant Soil, 98: 417-424.
Fuortes LJ (1992) A case of fatal methyl bromide poisoning. Vet Hum
Toxicol, 34: 240-241.
Furuta A, Hyakudo T, Ohnishi A, Hori H & Tanaka E (1993) Neurotoxicity
of methyl bromide - neuropathologic evaluation, preliminary study.
Sangyo Ika Daigaku Zasshi, 15: 21-27.
Gansewendt B, Foest U, Xu D, Hallier E, Bolt HM, & Peter H (1991)
Formation of DNA adducts in F-344 rats after oral administration or
inhalation of [14C]methyl bromide. Food Chem Toxicol, 29: 557-563.
Gargas ML & Andersen ME (1982) Metabolism of inhaled brominated
hydrocarbons: validation of gas uptake results by determination of a
stable metabolite. Toxicol Appl Pharmacol, 66: 55-68.
Garman JR, Freund T, & Lawless EW (1987) Testing for groundwater
contamination at hazardous waste sites. J Chromatogr Sci, 25: 328-337.
Garry VF, Nelson RL, Griffith J, & Harkins M (1990) Preparation for
human study of pesticide applicators: sister chromatid exchanges and
chromosome aberrations in cultured human lymphocytes exposed to
selected fumigants. Teratog Carcinog Mutagen, 10: 21-29.
Gentile IA, Ferraris L, Crespi S, & Belligno A (1989) The degradation
of methyl bromide in some natural fresh waters. Influence of
temperature, pH and light. Pestic Sci, 25: 261-272.
Gentile IA, Ferraris L, Sanguinetti M, Tiprigan M, & Fisichella G
(1992) Methyl bromide in natural fresh waters: hydrolysis and
volatilisation. Pestic Sci, 34: 297-301.
Gillotay D, Simon PC & Dierickx L (1989) Temperature dependence of
ultraviolet absorption cross-sections of brominated methanes an
ethanes. In: Bojkov RD & Fabian P, ed. Ozone in the atmosphere.
Proceedings of the Quadrennial Ozone Symposium 1988 and Tropospheric
Ozone Workshop, Göttingen, Germany, 4-13 August 1988. Hampton,
Virginia, Deepak Publishing, pp 706-709.
Goldman LR, Mengle D, Epstein DM, Feson D, Kelly K, & Jackson RJ
(1987) Acute symptoms in persons residing near a field treated with
the soil fumigants methyl bromide and chloropicrin. West J Med, 147:
95-98.
Gosselin RE, Smith RP, Hodge HC, & Braddock JE (1984) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams &
Wilkins Company, pp III/280-III/284.
Goulon M, Nouailhat F, Escourolle R, Zarranz-Imirizaldu JJ, Grosbuis
S, & Levy-Alcover MA (1975) Intoxication par le bromure de méthyle.
Trois observations, dont une mortelle. Etude neuro-pathologique d'un
cas de stupeur avec myoclonies, suivi pendant cinq ans. Rev. Neurol.,
131: 445-468.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Charles C. Thomas, pp 680-685.
Green M, Seiber JN, & Biermann HW (1991) in situ measurement of methyl
bromide in indoor air using long-path ftir spectroscopy. In: Schiff
HI, ed. Measurement of atmospheric gases. Proceedings spie - the
international society for optical engineering. Los angeles,
California, 2-3 January 1991 (Volume 1433). Washington, DC, The
International Society for Optical Engineering, pp 270-274.
Greenberg JO (1971) The neurological effects of methyl bromide
poisoning. Ind Med, 40: 27-29.
Greve PA & Grevenstuk WBF (1976) Optimization studies on the
determination of bromide residues in lettuce. Meded Fac Landbouwwet
Rijksuniv Gent, 41: 1371-1381.
Greve PA & Grevenstuk WBF (1979) Gas-liquid chromatographic
determination of bromide ion in lettuce: interlaboratory studies. J
Assoc Off Anal Chem, 62: 1155-1159.
Greve PA & Hogendoorn EA (1979) Determination of fumigant residues in
grain. Meded Fac Landbouwwet Rijksuniv Gent, 44: 877-884.
Griffiths NM, Hobson-Frohock A, Land DG, Levett JM, Cooper DM, &
Rowell JG (1978) Fumigation of poultry food with methyl bromide:
effects on flavour and acceptability of broiler meat. Br Poult Sci,
19: 529-535.
Grimm GR & Alexander AR (1971) Fumigation of phytophthora in sandy
soil by surface application of methyl bromide and methyl
bromide-chloropicrin. Plant Dis Rep, 55: 929-931.
Gryder-Boutet DE & Kennish JM (1988) Using headspace sampling with
capillary column GC-MS to analyze trace volatile organics in water and
wastewater. Am Water Works Assoc J, 80: 52-55.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Guillemin MP, Hillier RS, & Bernhard CA (1990) Occupational and
environmental hygiene assessment of fumigations with methyl bromide.
Ann Occup Hyg, 34: 591-607.
Guns MF (1989) Monitoring of the groundwater bromine content and plant
residues in methyl bromide fumigated glasshouses. Third International
Symposium on Soil Disinfestation, Leuven, Belgium, September 26-30,
1988. Acta Hortic, 255: 337-346.
Haber SB, Drew RT, Eustis S, & Yang RSH (1985) Methyl bromide
toxicity: a target organ? Toxicologist, 5: 130 (Abstract No. 518).
Haesanen E, Manninen PKG, Himberg K, & Väätäinen V (1990) Chlorine and
bromine contents in tobacco and tobacco smoke. J Radioanal Nucl Chem,
144: 367-372.
Hague NGM (1963) Fumigation of agricultural products. XVIII. Effect of
methyl bromide on the bent grass nematode, Anguina agrostis,
steinbuch 1799 , filipjev 1936, and on the germination of bent
grass, Agrostis tenuis. J Sci Food Agric, 14: 577-579.
Hague NGM & Clark WC (1959) Fumigation with methyl bromide and
chloropicrin to control seed-borne infestations of the stem eelworm.
Meded Landbouwhogesch Opzoekingasstn Gent, 24: 628-636.
Hallier E, Jaeger R, Deutschmann S, Bolt HM, & Peter H (1990a)
Glutathione conjugation and cytochrome p-450 metabolism of methyl
chloride in vitro. Toxic in vitro , 4: 513-517.
Hallier E, Deutschmann S, Reichel C, Bolt HM, & Peter H (1990b) A
comparative investigation of the metabolism of methyl bromide and
methyl iodide in human erythrocytes. Int Arch Occup Environ Health,
62: 221-225.
Hallier E, Langhof T, Dannappel D, Leutbecher M, Schröder K, Goergens
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methyl bromide, ethylene oxide and dichloromethane in human blood:
influence on the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol, 67: 173-178.
Hamilton I, Minski MJ, Milton EI, & Cleary JJ, (1972/73) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study. Sci
Total Environ, 1: 341-374.
Hansch C & Leo A (1979) Substituent constants for correlation in
chemistry and biology. New York, Chichester, Brisbane, Toronto, John
Wiley & Sons, p 173.
Hanson PR, Wainman HE, & Chakrabarti B (1987) The effects of methyl
bromide on the seed of some varieties of barley. Seed Sci Technol, 15:
155-162.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Belilies RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7: 66-75.
Harper DB (1985) Halomethane from halide ion - a highly efficient
fungal conversion of environmental significance. Nature (Lond), 315:
55-57.
Harrison BO, Peachey JE, & Winslow RO (1963) The use of nematicides to
control the spread of arabis mosaic virus by Xiphinema
diversicaudatum (Micol.). Ann Appl Biol, 52: 243-255.
Harry EG, Brown WB, & Goodship G (1972) The disinfecting activity of
methyl bromide on various microbes and infected material under
controlled conditions. J Appl Bacteriol, 35: 485-491.
Harsch DE & Rasmussen RA (1977) Identification of methyl bromide in
urban air. Anal Lett, 10: 1041-1047.
Hartill WFT & Campbell JM (1973) Control of sclerotinia in tobacco
seedbeds. Plant Dis Rep, 57: 932-934.
Hastings L (1990) Sensory neurotoxicology: use of the olfactory system
in the assessment of toxicity. Neurotoxicol Teratol, 12: 455-459.
Hastings L, Miller M, Minnema D, & Evans J (1989) Effects of methyl
bromide on rat olfactory system. Chem Senses, 14: 708.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res, 43:
1945-1950.
Heimann H (1944) Poisoning as result of industrial exposure to methyl
bromide. NY State Ind Bull, 23: 103-105.
Henschler D (1990) Chemicals harmful to health: toxicological-medical
establishment of MAK-values, 16th ed. Weinheim, VCH
Verlagsgesellschaft mbH.
Heungens A & Roos A (1982) L'influence du dazomet et du bromure de
méthyle sur la faune et la flore de la litière de pin. Rev Agric,
35(2): 2005-2050.
Heurtebise M & Ross WJ (1971) Application of 80m Br to the
semi-automated analysis of bromine in biological fluids. J Radioanal
Chem, 8: 5-12.
Heuser SG & Scudamore KA (1968) Fumigant residues in wheat and flour:
solvent extraction and gas-chromatographic determination of free
methyl bromide and ethylene oxide. Analyst, 93: 252-258.
Heuser SG & Scudamore KA (1970) Selective determination of ionised
bromide and organic bromides in foodstuffs by gas-liquid
chromatography with special reference to fumigant residues. Pestic
Sci, 1: 244-249.
Hezemans-Boer M, Toonstra J, Meulenbelt J, Zwaveling JH, Sangster B,
& Van Vloten WA (1988) Skin lesions due to exposure to methyl bromide.
Arch Dermatol, 124: 917-921.
Hicken NE (1961) Termite fumigation with methyl bromide. Chem Prod,
24: 205-207.
Hine CH (1969) Methyl bromide poisoning - a review of ten cases. J
Occup Med, 11: 1-10.
Hitchcock BE (1968) Progress of earth pearl studies in Queensland.
Proc Int Soc Sugar-Cane Technol, 13: 1382-1388.
Ho JSY (1989) A sequential analysis for volatile organics in water by
purge-and-trap capillary column gas chromatography with
photoionization and electrolytic conductivity detectors in series. J
Chromatogr Sci, 27: 91-98.
Hoffman GM & Malkomes HP (1974) Bromide residues in vegetable crops
after soil fumigation with methyl bromide. Agric Environ, 1: 321-328.
Hole BD (1981) Variation in tolerance of seven species of stored
product coleoptera to methyl bromide and phosphine in strains from
twenty-nine countries. Bull Entomol Res, 71: 299-306.
Holling HE & Clarke CA (1944) Methyl bromide intoxication. J R Navy
Med Serv, 30: 218-224.
Hommel G (1984) [Handbook of dangerous goods (Explanatory leaflet
127).] Berlin, Heidelberg, New York, Springer-Verlag (in German).
Honma T (1987) Alteration of catecholamine metabolism in rat brain
produced by inhalation exposure to methyl bromide. Jpn J Ind Health,
29: 218-219.
Honma T (1992) Brain microdialysis study of the effects of hazardous
chemicals on the central nervous system. 1. Changes in monoamine
metabolites induced by cerebral methyl bromide administration measured
by two-probe microdialysis (TPMD) method. Ind Health, 30: 47-60.
Honma T, Sudo A, Miyagawa M, & Sato M (1982) Significant changes in
monoamines in rat brain induced by exposure to methyl bromide.
Neurobehav Toxicol Teratol, 4: 521-524.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Changes in
free amino acid contents of rat brain induced by exposure to methyl
bromide. Toxicol Lett, 15: 317-321.
Honma T, Miyagawa M, Sato M, & Hasegawa H (1985) Neurotoxicity and
metabolism of methyl bromide in rats. Toxicol Appl Pharmacol, 81:
183-191.
Honma T, Muneyuki M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain. Neurotoxicol
Teratol, 9: 369-375.
Honma T, Miyagawa M, & Sato M (1991) Inhibition of tyrosine
hydroxylase activity by methyl bromide exposure. Neurotoxicol Teratol,
13: 1-4.
Hubbs AF & Harrington DD (1986) Further evaluation of the potential
gastric carcinogenic effects of subchronic methyl bromide
administration. In: Proceedings of the 36th Meeting of the American
College of Veterinary Pathology and the Annual Meeting of the American
Society for Veterinary and Clinical Pathology, Denver, Colorado,
December 1985. Denver, Colorado, American Society for Veterinary and
Clinical Pathology, p 92.
Hurtt ME & Working PK (1988) Evaluation of spermatogenesis and sperm
quality in the rat following acute inhalation exposure to methyl
bromide. Fundam Appl Toxicol, 10: 490-498.
Hurtt ME, Morgan KT, & Working PK (1987) Histopathology of acute toxic
responses in selected tissues from rats exposed by inhalation to
methyl bromide. Fundam Appl Toxicol, 9: 352-365.
Hurtt ME, Thomas DA, Working PK, Monticello TM, & Morgan KT (1988)
Degeneration and regeneration of the olfactory epithelium following
inhalation exposure to methyl bromide: pathology, cell kinetics, and
olfactory function. Toxicol Appl Pharmacol, 94: 311-328.
Hustinx WNM, Van de Laar RTH, Van Huffelen AC, Vrey JC, Meulenbelt J,
& Savelkoul TJF (1993) Systemic effects of inhalation methyl bromide
poisoning: a study of nine cases occupationally exposed due to
inadvertent spread during fumigation. Br J Ind Med, 50: 155-159.
Hyman MR & Wood PM (1984) Bromocarbon oxidations by Nitrosomonas
europaea. In: Crawford RL & Hanson RS ed. Microbial growth on C1
compounds. Washington, DC, American Society for Microbiology, pp
49-52.
IARC (1986) Methyl bromide. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 187-212 (IARC Monograph on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1-42. Lyon, International Agency for Research
on Cancer, p 245 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Ikawa N, Araki A, Nozaki K, & Matsushima T (1986) Micronucleus test of
methyl bromide by the inhalation method. Mutat Res, 164: 269
(Abstract).
Ikeda T, Kishi R, Yamamura K, Miyake H, & Sato M (1980) Behavioural
effects in rats following repeated exposure to methyl bromide. Toxicol
Lett, 6: 317-321.
Ingram FR (1951) Methyl bromide fumigation control in the date-packing
industry. Arch Ind Hyg Occup Med, 4: 193-198.
Inouye T, Inouye N, Asatani M, & Mitsuhata K (1967) Cucumber green
mottle mosaic virus in Japan. III. Inactivation of cucumber green
mottle mosaic virus in infected plant tissue buried in soil treated
with methyl bromide. Nogaku Kenkyu, 51: 199-207.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1940) The response
attending exposure of laboratory animals to vapors of methyl bromide.
J Ind Hyg Toxicol, 22: 218-230.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1941) Chemical changes of
methyl bromide in the animal body in relation to its physiological
effects. J Ind Hyg Toxicol, 23: 408-411.
Isa AL, Fam EZ, Kamel AH, & Awadallah WH (1970) On the effect of
certain fumigants on the overwintering corn borers larvae. Agric Res
Rev, 48: 43-47.
Ishizu S, Kato N, Morinobu S, Nagao N, Yamano Y, & Ito I (1988) [Cases
of severe methyl bromide poisoning residing above a warehouse.] Jpn J
Ind Health, 30: 54-60 (in Japanese with English abstract).
Ito KA, Seeger ML, & Lee WH (1972) Destruction of Byssochlamys fulva
asci by low concentrations of gaseous methyl bromide and by aqueous
solutions of chlorine, an iodophor, and peracetic acid. J Appl
Bacteriol, 35: 479-483.
Iwasaki K (1988a) Determination of S-methylcysteine in mouse
hemoglobin following exposure to methyl bromide. Ind Health, 26:
187-190.
Iwasaki K (1988b) Individual differences in the formation of
hemoglobin adducts following exposure to methyl bromide. Ind Health,
26: 257-262.
Iwasaki K, Ito I, & Kagawa J (1989) Biological exposure monitoring of
methyl bromide workers by determination of hemoglobin adducts. Ind
Health, 27: 181-183.
Japanese Ministry of Labour (1992) Toxicology and carcinogenesis
studies of methyl bromide in F344 rats and BDF mice (inhalation
studies). Tokyo, Industrial Safety and Health Association, Japanese
Bioassay Laboratory, 197 pp (Unpublished report).
Jaskot RH, Grose EC, Most BM, Menache MG, Williams TB, &
Roycroft JH (1988) The distribution and toxicological effects of
inhaled methyl bromide in the rat. J Am Coll Toxicol, 7: 631-642.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 3: 777-782.
JEA (Japanese Environmental Agency) (1981) Chemicals in the
environment. Tokyo, Japanese Environmental Agency, Office of Health
Studies, Environmental Health Department, pp 92-93.
Johnstone RT (1945) Methyl bromide intoxication of a large group of
workers. Ind Med, 14: 495-497.
Jones L (1968) effect of methyl bromide treatments on several species
of conifer seed. J For, 8: 858-860.
Joshi GP (1974) Toxicity of certain chemicals on Oryzaephilus
mercator F. (Coleoptra: Cucujidae). Appl Entomol Zool, 9: 280-281.
Kallio H & Shibamoto T (1988) Direct capillary trapping and gas
chromatographic analysis of bromomethane and other highly volatile air
pollutants. J Chromatogr, 454: 392-397.
Kantarjian AD & Shaheen AS (1963) Methyl bromide poisoning with
nervous system manifestations resembling polyneuropathy. Neurology,
13: 1054-1058.
Kato N, Morinobu S, & Ishizu S (1986) Subacute inhalation experiment
for methyl bromide in rats. Ind Health, 24: 87-103.
Katz AJ (1985) Genotoxicity of methyl bromide in somatic cells of
Drosophila larvae. Environ Mutagen, 7: 13 (Abstract).
Katz AJ (1987) Inhalation of methyl bromide gas induces mitotic
recombination in somatic cells of Drosophila melanogaster. Mutat Res,
192: 131-135.
Kelley WD & Rodriguez-Kabana R (1979) Effects of sodium azide and
methyl bromide on soil bacterial populations, enzymic activities and
other biological variables. Pestic Sci, 10: 207-215.
Kempton RJ & Maw GA (1974) Soil fumigation with methyl bromide: the
phytotoxicity of inorganic bromide to carnation plants. Ann Appl Biol,
76: 217-229.
Kenaga EE (1961) Time, temperature and dosage relationships of several
insecticidal fumigants. J Econ Entomol, 54: 537-542.
Khalil MAK & Rasmussen RA (1985) The trend of bromodifluoromethane
(CBrCIF2) and the concentration of other bromine containing gases at
the South Pole. Antarct J US, 21: 206-207.
Khalil MAK, Rasmussen RA, & Gunawardena R (1993) Atmospheric methyl
bromide: Trends and global mass balance. J Geophys Res, 98: 2887-2896.
Khanna SC & Yadav TD (1987) Effect of methyl bromide fumigation on the
germinability of wheat seed. Seed Res, 15: 183-186.
Kiewnick L (1968) Occurrence and control of sclerotium rolfsii.
Meded Fac Landbouwwet Rijksuniv Gent, 33: 987-995.
King JR & Benschoter CA (1991) Comparative methyl bromide residues in
Florida citrus: A basis for proposing quarantine treatments against
caribbean fruit fly. J Agric Food Chem, 39: 1307-1309.
King JR, Benschoter CA, & Burditt AK, Jr (1981) Residues of methyl
bromide in fumigated grapefruit determined by a rapid, headspace
assay. J Agric Food Chem, 29: 1003-1005.
Kishi R, Ishizu I, Ito I, Katoh N, Miyake H, & Harabuch I (1988)
Health research of methyl bromide manufacturing workers. Part 1.
Symptoms of long-term exposure. Occupational health in the chemical
industry. XXII ICOH (International Commission on Occupational Health)
Congress, Sydney, New South Wales, Australia, 27 September-2 October
1987. Copenhagen, World Health Organization Regional Office for
Europe, pp 120-134.
Kishi R, Itoh I, Ishizu S, Harabuchi I, & Miyake H (1991) Symptoms
among workers with long-term exposure to methyl bromide. An
epidemiological study. Jpn J Ind Health, 33: 241-250.
Kisser W (1967) [The detection and quantitative determination of
brominated urea derivatives in toxicology.] Arch Toxicol, 22: 404-409
(in German).
Kissler JJ, Lider JV, Raabe RD, Raski DJ, Schmitt RV, & Hurlimann JH
(1973) Soil fumigation for control of nematodes and oak root fungus in
vineyard replants. Plant Dis Rep, 57: 115-119.
Klein L (1989) Methyl bromide strip fumigation - problems encountered
and ways to combat them. Third International Symposium on Soil
Disinfestation, Leuven, Belgium, September 26-30, 1988. Acta Hortic,
255: 213-225.
Knight HD & Costner GC (1977) Bromide intoxication of horses, goats,
and cattle. J Am Vet Med Assoc, 171: 446-448.
Knight HD & Reina-Guerra M (1977) Intoxication of cattle with sodium
bromide-contaminated feed. Am J Vet Res, 38: 407-409.
Koga M, Hara K, Hori H, Kodama Y, & Okubo T (1991) [Determination of
bromide ion concentration in urine using a head-space gas
chromatography and an ion chromatography. Biological monitoring for
methyl bromide exposure.] J Univ Occup Environ Health, 13: 19-24 (in
Japanese with English summary).
Kolbezen MJ & Abu-El-Haj FJ (1972) Fumigation with methyl bromide. II.
Equipment and methods for sampling and analysing deep field soil
atmospheres. Pestic Sci, 3: 73-80.
Kramers PGN, Bissumbhar B, & Mout HCA (1985b) Studies with gaseous
mutagens in Drosophila melanogaster. In: Waters MD, Sandhu SS, Lewtas
J, Claxton L, Straus G, & Nesnow S, ed. Short-term bioassays in the
analysis of complex environmental mixtures IV., New York, London,
Plenum Press, pp 65-73.
Kramers PGN, Voogd CE, Knaap AGAC, & Van der Heijden CA (1985a)
Mutagenicity of methyl bromide in a series of short-term tests. Mutat
Res, 155: 41-47.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Latta R & Cowgill H (1941) Methyl bromide fumigation of greenhouse
plants at U.S. Plant Introduction Garden, Glenn Dale, Md. (Abstract
S.8189, Bureau of Entomology and Plant Quarantine). Washington, DC, US
Department of Agriculture (E-526).
Laug EP (1941) Bromide residues in foodstuffs. Volatile and
nonvolatile residues following experimental exposure to methyl
bromide. Ind Eng Chem, 33: 803-805.
Leesch JG, Gillenwater HB, & Woodward JO (1974) Methyl bromide
fumigation of shelled peanuts in bulk containers. J Econ Entomol, 67:
769-771.
Lefevre C, Ferrari P, Günier JP, & Muller J (1989) Sampling and
analysis of airborne methylbromide. Chromatographia, 27: 37-43.
Leichnitz K (1985) [Dräger-tubes methyl bromide 3/a. Detector-tubes
pocket book 6.] Lübeck, Dragerwerk (in German).
Lepschy J, Stark H, & Sub A (1979) [Behaviour of methyl bromide
(Terabol) in soil.] Gartenbauwissenschaften, 44: 84-89 (in German).
Lewis SE (1948) Inhibition of SH proteins by methyl bromide. Nature
(Lond), 161: 692-693.
Linenberg A, Davis R, & Robinson D (1991) Hazardous waste site
measurements of low levels of chlorinated hydrocarbons using a
portable gas chromatography. Hazard Mater Control, 4: 42-46.
Long PL, Brown WB, & Goodship G (1972) Effect of methyl bromide on
coccidial oocysts determined under controlled conditions. Vet Rec, 90:
562-566.
Longley EO & Jones AT (1965) Methyl bromide poisoning in man. Ind Med
Surg, 34: 499-502.
Lopez VC, Fernandez JAM, Yanez LP, & Vazquez MS (1986a) [Methyl
bromide poisoning: I. Some historical, clinical, legal and
neuropsychiatric aspects.] Arch Neurobiol, 49: 279-290 (in Spanish).
Lopez CV, Fernandez MJA, Yanez PL, & Vazquez SM (1986b) [Methyl
bromide poisoning. II. Contribution of 2 cases.] Arch Neurobiol, 49:
311-328 (in Spanish).
Lopez-Avila V, Wood R, Flanagan M, & Scott R (1987) Determination of
volatile priority pollutants in water by purge and trap and capillary
column gas chromatography/mass spectrometry. J Chromatogr Sci, 25:
286-291.
Love JL, Winchester RV, & Keating DL (1979) Fumigant and contact
insecticide residues on dried fruits, cereals, nuts, and spices
imported into New Zealand. N Z J Sci, 22: 95-98.
Lovelock JE (1975) Natural halocarbons in the air and in the sea.
Nature (Lond), 256: 193-194.
Lynn GE, Shrader SA, Hammer OH, & Lassiter CA (1963) Occurrence of
bromides in the milk of cows fed sodium bromide and grain fumigated
with methyl bromide. J Agric Food Chem, 11: 87-91.
Mabey W & Mill T (1978) Critical review of organic compounds in water
under environmental conditions. J Phys Chem Ref Data, 7: 383-415.
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds, Report No. 32 - Individual compound report: methyl bromide.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, 190 pp (PB83-130211).
Mackay D & Shiu WK (1981) A critical review of Henry's Law Constants
for chemicals of environmental interest. J Phys Chem Ref Data, 10:
1175-1199.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. In: Gunter FA
ed. Reviews of environmental contamination and toxicology. Berlin,
Heidelberg, New York, Springer-Verlag, vol 14, pp 57-123.
MAFF (1990) Report of the working party on pesticide residues:
1988-89. Supplement to issue No. 8 (1990) of the pesticides register.
London, Ministry of Agriculture, Fisheries and Food, Health and Safety
Executive, 11 pp.
MAFF (1989) Report of the working party on pesticide residues:
1985-88. The twenty-fifth report of the steering group on food
surveillance. London, Ministry of Agriculture, Fisheries and Food, 3
pp (Food Surveillance Paper No. 25).
Malkomes H-P (1970) [Investigations into the degradation of methyl
bromide during substrate fumigation and its influence on the following
crops.] Hanover, Technical University, pp 18-35 (Dissertation) (in
German).
Marraccini JV, Thomas GE, Ongley JP, Pfaffenberger D, Davis JH, &
Bednarczyk LR (1983) Death and injury caused by methyl bromide, an
insecticide fumigant. J Forensic Sci Soc, 28: 601-607.
Matheson gas data book (1980) The Matheson gas data book. East
Rutherford, New Jersey, Matheson Gas products (A Division of Will
Ross), vol 6, pp 361-363.
Matta A & Porta-Puglia A (1968) [Sensitivity to methyl bromide and
vapam and secondary growth in fumigated soil of distinct functional
groups of microorganisms.] Ann Fac Agrar, 4: 261-274 (in Italian).
Maw GA & Kempton RJ (1973) Methyl bromide as a soil fumigant. Soils
Fertil, 36: 41-47.
Mazzini L, Galante M, Rezzonico M, & Kokodoko A (1992) Methylbromide
intoxication: A case report. Schweiz Arch Neurol Psychiatr, 143:
75-80.
MBSW (1992) Proceedings of the Methyl Bromide Science Workshop,
Washington, DC, 2-3 June 1992. Washington, DC, Atmospheric
Environmental Research, Inc., pp 90-97.
Medinsky MA, Bond JA, Dutcher JS, & Birnbaum LS (1984) Disposition of
[14C]methyl bromide in Fischer-344 rats after oral or
intraperitoneal administration. Toxicology, 32: 187-196.
Medinsky MA, Dutcher JS, Bond JA, Henderson RF, Mauderly JL, Snipes
MB, Mewhinney JA, Cheng YS, & Birnbaum LS (1985) Uptake and excretion
of [14C]methyl bromide as influenced by exposure concentration.
Toxicol Appl Pharmacol, 78: 215-225.
Meister RT (1985) In: Meister RT ed. Farm chemicals handbook 85.
Pesticide dictionary -Section C. Willoughby, Ohio, Meister Publishing
Co., 154 pp.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992a) Atmospheric lifetimes and ozone
depletition potentials of methyl bromide (CH3Br) and dibromomethane
(CH2Br2). Geophys Res Lett, 19: 2059-2062.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992b) Correction to "atmospheric lifetimes and
ozone depletion potentials of methylbromide (CH3Br) and
dibromomethane (CH2Br2)". Geophys Res Lett, 19: 2279-2280.
Merzbach L (1928) [The pharmacology of methyl bromide and some related
compounds.] Z. Ges. Exp. Med., 63: 383-392 (in German).
Mignard E & Benet JC (1989) Diffusion of methyl bromide in soil. J
Soil Sci, 40: 151-165.
Miller DP & Haggard HW (1943) Intracellular penetration of bromide as
a feature in the toxicity of alkyl bromides. J Ind Hyg Toxicol, 25:
423-433.
Minton NA & Gillenwater HB (1973) Methyl bromide fumigation of
Pratylenchus brachyurus in peatnut shells. J Nematol, 5: 147-149.
Mitsumori K, Maita K, Kosaka T, Miyaoka T, & Shirasu Y (1990) Two-year
oral chronic toxicity and carcinogenicity study in rats of diets
fumigated with methyl bromide. Food Chem Toxicol, 28: 109-119.
Miyagawa M (1982) Conditioned taste aversion induced by inhalation
exposure to methyl bromide in rats. Toxicol Lett, 10: 411-416.
Mizyukova IG & Bakhishev CN (1971) [Specific treatment of acute
poisoning with methyl bromide.] Vrach Delo, 7: 128-131 (in Russian
with English abstract).
Moelwyn-Hughes EA (1938) The hydrolysis of the methyl halides. Proc R
Soc, A164: 295-306.
Moje W (1960) The chemistry and nematocidal activity of organic
halides. In: Metcalf RL, ed. Volume III. Advances in pest control
research. New York, Intersience Publishers, pp 181-217.
Molina LT, Molina MJ, & Rowland FS (1982) Ultraviolet absorption cross
sections of several brominated methanes and ethanes of atmospheric
interest. J Phys Chem, 86: 2672-2676.
Monro HAU, Dumas T, & Ruckland CT (1966) The influence of vapour
pressure of different fumigants on the mortality of two stored-product
insects in vacuum fumigation. J Stored Prod Res, 1: 207-222.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrissey RE, Schwetz BA, Lamb JCIV, Ross MD, Teague JL, & Morris RW
(1988) Evaluation of rodent sperm, vaginal cytology, and reproductive
organ weight data from National Toxicology Program 13-week studies.
Fundam Appl Toxicol, 11: 343-358.
Mostafa SAS, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1972) Toxicity
of carbon bisulphide and methyl bromide to the eggs of four stored
product insects. J Stored Prod Res, 8: 193-198.
Mounat A & Hitier H (1959) Chemical disinfection of the soil of
tobacco seed beds. Ann Inst Exp Tabac Bergerac, 3: 287-298.
Mueller A, Jorritsma U, Hindermeier U, & Gansewendt B (1991) Human
serum albumin adducts as a parameter for biomonitoring of exposure to
methyl bromide. Naunyn-Schmiedeberg's Arch Pharmacol, 344: R121.
Mueller A, Jorritsma U, & Gansewendt B (1992) Different binding of
methyl bromide to blood macromolecules due to conjugator status.
Naunyn-Schmiedeberg's Arch Pharmacol, 345: R 42.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol, 17:
405-429.
Munnecke DE, Kolbezen MJ, & Stolzy LH (1969) Factors affecting field
fumigation of citrus soils for control of Armillaria mellea.
Proceedings of the First International Citrus Symposium. Lancaster,
Pennsylvania, Society of the Plastics Industry, vol 3, pp 1273-1277.
Munnecke DE, Kolbezen MJ, & Wilbur WD (1978) Types and thickness of
plastic films in relation to methyl bromide fumigation. Proceedings of
the Agricultural Plastic Congress, San Diego, pp 482-487.
National Academy of Science (1978) Chloroform, carbon tetrachloride,
and other halomethanes: an environmental assessment. Washington, DC,
National Academy of Sciences, 294 pp.
NASA (1992) Chemical kinetics and photochemical data for use in
stratospheric modeling. Evaluation No. 10: NASA Panel for Data
Evaluation. Pasadena, California Institute of Toxicology, Jet
Propulsion Laboratory, National Aeronautics and Space Administration.
NFPA (1984) Fire protection guide on hazardous materials, 9th ed.
Quincy, Maryland, National Fire Protection Agency, 5 pp (NFPA No.
SPP-1E).
NIOSH (1984) Monohalomethanes: methyl chloride CH3Cl, methyl bromide
CH3Br, methyl iodide CH3I. Curr Intell Bull, 42: 1-22.
Nishimura M, Umeda M, Ishizu S, & Sato M (1980) Effect of methyl
bromide on cultured mammalian cells. J Toxicol Sci, 5: 321-330.
NTP (1992) Toxicology and carcinogenesis studies of methyl bromide
(CAS No. 74-83-9) in B6C3F1 mice (inhalation studies). Research
Triangle Park, North Carolina, National Toxicology Program, 212 pp
(Technical Report Series No. 385).
Oakes JY, Bollich CN, Melville DR, Fielding MJ, & Hollis JP (1956)
Soil fumigation for control of parasitic nematodes on corn at Curtis,
Louisiana. Plant Dis Rep, 40: 853-854.
Ohmori S & Hirata M (1982) [Determination of bromine contents in blood
and hair of workers exposed to methyl by radioactivation analysis
method bromide]. Jpn J Ind Health, 24: 119-125 (in Japanese).
O'Neal L (1987) Acute methyl bromide toxicity. J. Emerg Nurs, 13:
96-98.
Ong JM, Stewart J, Wen Y, & Whong W (1987) Application of SOS umu-test
for the detection of genotoxic volatile chemicals and air pollutants.
Environ Mutagen, 9: 171-176.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported wheat.
Pestic Sci, 27: 103-109.
Ostrec L & Korunic Z (1989) [Some new knowledge on the applying of
methylbromide in the tobacco nurseries.] Zast Bilja, 40: 375-385 (in
Yugoslavian with English abstract).
Page BD & Avon RJ (1989) Determination of methyl bromide in foods by
headspace capillary gas chromatography with electron capture
detection. J Assoc Off Anal Chem, 72: 815-822.
Pauwels F (1989) Soil disinfestation in the Belgian horticulture: a
practice view. Third International Symposium on Soil Disinfestation,
Leuven, Belgium, September 26-30, 1988. Acta Hortic, 255: 31-36.
Peers AMK (1985) The determination of methyl bromide in air. In:
Fishbein L & O`Neill IK ed. Environmental carcinogens selected methods
of analysis. Volume 7 - Some volatile halogenated hydrocarbons. Lyon,
International Agency for Research on Cancer, pp 227-233 (IARC
Scientific Publications No. 68).
Pellizzari ED, Zweidinger RA, & Erickson MD (1978) Environmental
monitoring near industrial sites: brominated chemicals part II:
appendix. Washington, DC, US Environmental Protection Agency
(EPA-560/6-78-002A; US NTIS PB-286483).
Pence RJ & Morganroth J (1962) Field effects of methyl bromide on
carpet beetle eggs. Pest Control, 30: 20, 22, 24.
Penkett SA, Jones BMR, Rycroft MJ, & Simmons DA (1985) An
interhemispheric comparison of the concentrations of bromine compounds
in the atmosphere. Nature (Lond), 318: 550-553.
Perrotta G (1968) [Combating `Tracheomycosis' (tomato wilt disease) in
greenhouse tomatoes.] Not Mal Piante, 78-79: 135-140 (in Italian).
Pesticide & Toxic Chemical News (1984) No evidence of methyl bromide
carcinogenicity found by NTP-panel. Pestic Toxic Chem News, 13: 9-10.
Peters PWJ, Verhoef A, De Liefde A, Van Velsen FL, Van Soolingen J, De
Geus D, Danse LHJ, & Van Logten MJ (1982) [Teratogenicity study of
methyl bromide by oral administration.] Bilthoven, National Institute
for Public Health and Environmental Protection (RIVM Report No. 618102
002) (in Dutch).
Piet GJ, Luijten WCMM, & Van Noort PCM (1985) 'Dynamic' head-space
determination of volatile organic halogen compounds in water. In:
Fishbein L & O`Neill IK, ed. Environmental carcinogens selected
methods of analysis. Volume 7 - Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
331-343 (IARC Scientific Publications No. 68).
Polkowski J, Crowley MS, & Moore AM (1990) Unintentional methyl
bromide gas release- Florida, 1988. Morbid Mortal Wkly Rep, 38:
880-882.
Poulet G, Pirre M, Maguin F, Ramaroson R, & Le Bras G (1992) "Role of
the BrO + HO2 reaction in the stratospheric chemistry of bromine".
Geophys Res Lett, 19: 2305-2308.
Powell DF (1975) The effect of methyl bromide fumigation on the
germination of onion seed and vigor of the seedlings. Plant Pathol,
24: 237-241.
Prain JH & Harvey Smith G (1952) A clinical-pathological report of
eight cases of methyl bromide poisoning. Br J Ind Med, 9: 44-49.
Prather MJ & Watson RT (1990) Stratospheric ozone depletion and future
levels of atmospheric chlorine and bromine. Nature (Lond), 344:
729-734.
Prather MJ, McElroy MB, & Wofsy SC (1984) Reductions in ozone at high
concentrations of stratosperic halogens. Nature (Lond), 312: 227-231.
Prishchep AG & Nikiforova EN (1969) [Bactericidal action of methyl
bromide on Escherichia coli group bacteria.] Sci Trans Inst Vet Sanit,
32: 279-281 (in Russian).
Prockop LD & Smith AO (1986) Seizures and action myoclonus after
occupational exposure to methyl bromide. J Fla Med Assoc, 73: 690-692.
Raabe OG (1986) Inhalation uptake of selected chemical vapors at trace
levels. Final report to the California Air Resources Board Control
(No. A3-132-33). Springfield, Virginia, National Technical Information
Service, 290 pp (NTIS PB 86-209863).
Raabe OG (1988) Inhalation uptake of xenobiotic vapors by people
(Final report August 1986-March 1988). Davis, University of
California, Laboratory for Energy-related Health Research, 94 pp (ISS
ARB-R-88/338; PB 88-202726).
Rasche E, Hyman MR, & Arp DJ (1990) Biodegradation of halogenated
hydrocarbon fumigants by nitrifying bacteria. Appl Environ Microbiol,
56: 2568-2571.
Raski DJ, Jones NO, Kissler JJ, & Luvisi DA (1975) Further results
from deep-placement fumigation for control of nematodes in vineyards.
Plant Dis Rep, 59: 345-349.
Rasmussen RA & Khalil MAK (1984) Gaseous bromine in the arctic and
arctic haze. Geophys Res Lett, 11: 433-436.
Rathus EM & Landy PJ (1961) Methyl bromide poisoning. Br J Ind Med,
18: 53-57.
Reeves RG, McDaniel CA, & Ford JH (1985) Organic and inorganic bromide
residues in spices fumigated with methyl bromide. J Agric Food Chem,
33: 780-783.
Reichmuth C & Noack S (1983) [Environmental effects of the fumigation
of commodities.] Technol Z Getreide Mehl Backwaren, 37: 139-144 (in
German).
Reuzel PGJ, Dreef-Van der Meulen HC, Hollanders VMH, Kuper CF, Feron
VJ & Van der Heijden CA (1991) Chronic inhalation toxicity and
carcinogenicity study of methyl bromide in Wistar rats. Food Chem
Toxicol, 29: 31-39.
Richardson HH & Monro HAU (1962) Fumigation of jute bags with ethylene
oxide and methyl bromide to eradicate potato ring rot bacteria. Appl
Microbiol, 10: 448-451.
Richardson HH & Roth H (1965) Methyl bromide, sulfuryl fluoride, and
other fumigants against quarantinable Cochlicella and Theba snails. J
Econ Entomol, 58: 690-693.
Robbins DE (1976a) Photodissociation of methyl chloride and methyl
bromide in the atmosphere. Geophys Res Lett, 3: 213-216.
Robbins DE (1976b) UV absorption cross sections for methyl bromide and
methyl chloride. Geophys Res Lett, 3: 757-758.
Rogozen MB, Rich HE, Guttman MA, & Grosjean D (1987) Evaluation of
potential toxic air contaminants. Phase 1. Final report (ISS
SAIC-76/1035, ARB-R-88/3339. Manhattan Beach, California, Science
Applications International Corporation, 582 pp (PB 88-183330/GAR)
Rolston DE & Glauz RD (1982) Comparisons of simulated with measured
transport and transformation of methyl bromide gas in soils. Pestic
Sci, 13: 653-664.
Roosels D, Van den Oever R, & Lahaye D (1981) Dangerous concentrations
of methyl bromide used as a fumigant in Belgian greenhouses. Int Arch
Occup Environ Health, 48: 243-250.
Rosenblum I, Stein AA, & Eisinger G (1960) Chronic ingestion by dogs
of methyl bromide-fumigated food. Arch Environ Health, 1: 316-323.
Roskill (1992) Report on the economics of bromine, 6th ed. London,
Roskill Information Service, p 58.
Roth H (1973) Fumigants for quarantine control of the adult brown dog
tick: laboratory studies. J Econ Entomol, 66: 1283-1286.
Roth H & Kennedy JW (1972) Methyl bromide and aluminium phosphide as
fumigants for control of adult boll weevils: laboratory studies. J
Econ Entomol, 65: 1650-1651.
Roth H & Kennedy JW (1973) Helicella snails infesting rosemary seeds.
Methyl bromide and other fumigants for quarantine control. J Econ
Entomol, 66: 935-936.
Roth H & Richardson HH (1974) Broadbeen weevil: methyl bromide
fumigation of infested faba beans. J Econ Entomol, 67: 799-803.
Rotteveel AJW & Naber H (1987) The use of soil fumigation against
yellow nutsedge. Meded Fac Landbouwwet Rijksuniv Gent, 52: 1207-1212.
Roughan JA & Roughan PA (1984a) Pesticide residues in foodstuffs in
England and Wales: Part I. Inorganic bromide ion in lettuce grown in
soil fumigated with bromomethane. Pestic Sci, 15: 431-438.
Roughan JA & Roughan PA (1984b) Pesticide residues in foodstuffs in
England and Wales. Part II: Inorganic bromide ion in cucumber, tomato
and self-blanching celery grown in soil fumigated with bromomethane,
and the "natural" bromide ion content in a range of fresh fruit and
vegetables. Pestic Sci, 15: 630-636.
Roughan JA, Roughan PA, & Wilkins JPG (1983) Modified gas-liquid
chromatographic method for determining bromide/total bromine in
foodstuffs and soils. Analyst, 108: 742-747.
Rovira AD & Ridge EH (1979) The effect of methyl bromide and
chloropicrin on some chemical and biological properties of soil and on
the growth and nutrition of wheat. Dev Agr Managed For Ecol, 6:
231-250.
Roycroft JH, Jascot RH, Grose EC, & Gardner DE (1981) The effects of
inhalation exposure of methyl bromide in rats. Toxicologist, 1: 79.
Russo JM, Anger WK, Setzer JV, & Brightwell WS (1984) Neurobehavioural
assessment of chronic low-level methyl-bromide exposure in the rabbit.
J. Toxicol Environ Health, 14: 247-255.
Ruth JH (1986) Odor thresholds and irritation levels of several
chemical substances: a review. Am Ind Hyg Assoc J, 47: A/142-A/151.
Saiki E (1952) Studies on the disinfecting effect of chloropicrin,
methyl bromide, prussic acid and formalin on bacteria. Nagoya J Med
Sci, 15: 270.
Saltzman BE (1983) Direct reading colorimetric indicators. In: Lioy PJ
& Lioy MJY ed. Air sampling instruments for evaluation of atmospheric
contaminations, 6th ed. Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 29 pp.
Sangster J (1989) Octanol-water partition coefficients of simple
organic compounds. J Phys Chem Ref Data, 18: 1185.
Sardesai JB (1972) Response of diapausing and nondiapausing larvae of
Plodia interpunctella to hydrogen cyanide and methyl bromide. J Econ
Entomol, 65: 1562-1565.
Sassaman JF, Jacobs MM, Chin PH, Hsia S, Pienta RJ, & Kelly JM (1986)
Pesticide background statements: methyl bromide and chloropicin. In:
Volume II. Fungicides and fumigants. Washington, DC, US Department of
Agriculture, Forest Service (MB/C-1-156) (Agriculture Handbook No.
661).
Sato M, Miyagawa M, Honma T, & Hasegawa H (1985) Subacute effects of
methyl bromide dosed by inhalation exposure to rats. Ind Health, 23:
235-238.
Sax NI, Feiner B, Fitzgerald J, Haley TJ & Weisburger EK (1984)
Dangerous properties of industrial materials, 6th ed. New York, Van
Nostrand Reinhold Company, p 531.
Sayers RR, Yant WP, Thomas BGH, & Berger LB (1929) Physiological
response attending exposure to vapors of methyl bromide, methyl
chloride, ethyl bromide and ethyl chloride. Public Health Bull, 185:
1-56.
Schauffler SM, Heidt LE, Pollock WH, Gilpin TM, Vedder J, Solomon S,
Lueb RA, & Atlas EL (in press) Measurements of halogenated organic
compounds near the tropical tropopause. Geophys Res Lett.
Scheffrahn RH, Bodalbhai L, & Su NY (1992) Residues of methyl bromide
and sulfuryl fluoride in manufacturer-packaged household foods
following fumigation. Bull Environ Contam Toxicol, 48: 821-827.
Scheffrahn RH & Su NY (1992) Comparative toxicity of methyl bromide
against ten nearctic termite species (Isoptera: Termopsidae,
Kalotermitidae, Rhinotermitidae). J Econ Entomol, 85: 845-847.
Schroeder KR, Hallier E, Peter H, & Bolt H (1992) Dissociation of a
new glutathione S-transferase activity in human erythrocytes. Biochem
Pharmacol, 43: 1671-1674.
Scotto La Massese C & Mars S (1975) Etude préliminaire des résidus de
brome dans les cultures faites dans des sols ayant reçu des
applications de brome minéral KBr et organique CH3Br. Phytiatr
Phytopharm, 24: 57-66.
Scudamore KA (1985) Multi-residue gas chromatographic method for
determination of fumigant residues in cereal grains and other foods.
In: Fishbein L & O`Neill IK ed. Environmental carcinogens selected
methods of analysis. Volume 7 - Some volatile halogenated
hydrocarbons. Lyon, International Agency for Research on Cancer, pp
351-359 (IARC Scientific Publications No. 68).
Scudamore KA (1985) Determination of methyl bromide in grain using
head-space analysis. In: Fishbein L & O`Neill IK ed. Environmental
carcinogens selected methods of analysis. Volume 7 - Some volatile
halogenated hydrocarbons. Lyon, International Agency for Research on
Cancer, pp 375-380 (IARC Scientific Publications No. 68).
Scudamore KA (1987) Fumigant residues in wheat and other cereal grains
resulting from the use of 1,1,1-trichloroethane and bromomethane as a
liquid fumigant mixture. Pestic Sci, 20: 1-18.
Segers JHL, Temmink JHM, Van den Berg JHJ, & Wegman RCC (1984)
Morphological changes in the gill of carp ( Cyprinus carpio L.)
exposed to acutely toxic concentrations of methyl bromide. Water Res,
18: 1437-1441.
Sell CR & Moffitt HR (1990) Non-destructive method for estimating
methyl bromide residues in apples during aeration following
fumigation. Pestic Sci, 29: 19-28.
Sell CR, Klag NG, & Burditt AK, Jr (1988) Methyl bromide residues in
fresh cherries: effects of parameters in fumigation. Pestic Sci, 23:
41-50.
Sher SA, Thomason IJ, & McCaslin RL (1958) Chisel application of
methyl bromide for root-knot nematode control. Plant Dis Rep, 42:
288-290.
Shield LK, Coleman TL, & Markesbery WR (1977) Methyl bromide
intoxication: Neurologic features, including simulation of Reyes
syndrome. Neurology, 27: 959-962.
Shivanandappa T & Rajendran S (1987) Induction of glutathione
S-transferase by fumigants in larvae of the khapra beetle,
Trogoderma granarium (E.). Pestic Biochem Physiol, 28: 121-126.
Siegwart Y (1987) [The implementation of food quality control in
Switzerland in the year 1986.] Mitt Geb Lebensm.unters Hyg, 78:
237-256 (in German).
Sikov MR, Cannon WC, Carr DB, Miller RA, Montgomery LF, & Phelps DW
(1981) Teratologic assessment of buthylene oxide, styrene oxide and
methyl bromide (Contract-No. 210-78-0025). Cincinnati, Ohio, US
Department of Health and Human Services, 84 pp.
Simmon VF, Kauhanen K, & Tardiff RG (1977) Mutagenic activity of
chemicals identified in drinking water. In: Scott D, Bridges BA, &
Sobels FH, ed. Progress in genetic toxicology. Amsterdam,
Elsevier/North-Holland Biomedical Press, pp 249-258.
Singh HB, Salas LJ, Smith SJ, & Shigeishi H (1981) Measurements of
some potentially hazardous organic chemicals in urban environments.
Atmos Environ, 15: 601-612.
Singh HB, Salas LJ, & Stiles RE (1983) Methyl halides in and over the
Eastern Pacific (40°N-32°S). J Geophys Res, 88: 3684-3690.
Singh ON, Borchers R, Fabian P, Lal S, & Subbaraya BH (1988)
Measurements of atmospheric BrOx radicals in the tropical and
mid-latitude atmosphere. Nature (Lond), 334: 593-595.
Singh HB, Salas L, Viezee W, Sitton B, & Ferek R (1992) Measurement of
volatile organic chemicals at selected sites in California. Atmos
Environ, 26A: 2929-2946.
Sittisuang P & Nakakita H (1985) The effect of phosphine and methyl
bromide on germination of rice and corn seeds. J Pestic Sci, 10:
461-468.
Sivasithamparam K, MacNish GC, Fang CS, & Parker CA (1987) Microflora
of soil and wheat rhizosphere in a field following fumigation. Aust J
Soil Res, 25: 491-498.
Slooff W & Canton JH (1983) Comparison of the susceptibility of 11
freshwater species to 8 chemical compounds. II. (Semi)chronic toxicity
tests. Aquat Toxicol, 4: 271-282.
Smart NA (1990) Residues in foodstuffs from bromomethane soil
fumigator. Adv Environ Sci Technol, 23: 227-255.
Smirnov AM (1970) [Disinfection of combs containing beebread by gases
in contagious bee diseases.] Sci Trans Inst Vet Sanit, 37: 267-280 (in
Russian).
Soremark R (1960) The biological half-life of bromide ions in human
blood. Acta Physiol Scand, 50: 119-123.
Spivakovsky CM, Yerich R, Logan JA, Wofsy SC, McElroy, & Prather MJ
(1990) Tropospheric OH in a three-dimensional chemicaltracer model: An
assessment based on observations of CH3 CC/3. J Geophys Res, 95:
18,441-18,471.
Squier MV, Thompson J, & Rajgopalan B (1992) Case report:
Neuropathology of methyl bromide intoxication. Neuropathol Appl
Neurobiol, 18: 579-584.
Starratt AN & Bond EJ (1981) Metabolism of methyl bromide by
susceptible and resistant strains of the granary weevil, Sitophilus
granarius (L.). Pestic Biochem Physiol, 15: 275-281.
Starratt AN & Bond EJ (1988a) Methylation of DNA of maize and wheat
grains during fumigation with methyl bromide. J Agric Food Chem, 36:
1035-1039.
Starratt AN & Bond EJ (1988b) In vitro methylation of DNA by the
fumigant methyl bromide. J. Environ. Health., B23: 513-525.
Starratt AN & Bond EJ (1990a) Residues of methyl bromide in fumigated
commodities. Study submitted by Methyl Bromide Panel. London, Ontario,
Research Centre, Agriculture Canada, 238 pp.
Starratt AN & Bond EJ (1990b) Methylation of food commodities during
fumigation with methyl bromide. In: Studies of the magnitude and
nature of pesticide residues in stored products using radiotracer
techniques. London, Ontario, Research Centre, Agriculture Canada, pp
97-109.
Stein ER & Wolfenbarger DA (1989) Methyl bromide residues in fumigated
mangos. J Agric Food Chem, 37: 1507-1509.
Stenger VA (1978) Bromine compounds. In: Kirk-Othmer encyclopedia of
chemical technology, 3rd ed. New York, Chichester, Brisbane, Toronto,
John Wiley and Sons, vol 4, pp 243-263.
Streete PJ, Ruprah M, Ramsey JD, & Flanagan RJ (1992) Detection and
identification of volatile substances by headspace capillary gas
chromatography to aid the diagnosis of acute poisoning. Analyst, 117:
1111-1127.
Strider DL (1975) Chemical control of bacterial blight of Rieger
elatior begonias caused by Xanthomonas begoniae. Plant Dis Rep, 59:
66-70.
Swartz RD, Millman RP, Billi JE, Bondar NP, Migdal SD, Simonian SK,
Monforte JR, McDonald FD, Harness JK, & Cole KL (1981) Epidemic
methanol poisoning: clinical and biochemical analysis of a recent
episode. Medicine, 60: 373-382.
Tanaka S, Arito H, Abuku S, & Imamiya S (1988) Acute effects of methyl
bromide on electroencephalographic activity and sleep-wakefulness in
rats. Ind Health, 26: 101-114.
Tanaka S, Abuku SI, Seki Y, & Imamiya SI (1991) Evaluation of methyl
bromide exposure on the plant quarantine fumigators by environmental
and biological monitoring. Ind Health, 29: 11-22.
Thier P & Zeumer H (1987) Manual of pesticide residue analysis. Volume
1. Bromine-containing fumigants (as total inorganic bromide).
Weinheim, Verlag Chemie, pp 377-381.
Thomas DA & Morgan KT (1988) Olfactory toxicity: studies of methyl
bromide. Chem Ind Inst Toxicol (CIIT) Act, 8: 1-7.
Thomason IJ (1959) Chisel application of methyl bromide for control of
root-knot nematode and fusarium wilt. Plant Dis Rep, 43: 580-583.
Thompson RH (1966) A review of the properties and usage of methyl
bromide as a fumigant. J Stored Prod Res, 1: 353-376.
Tie X, Ko, C-Y, Mroz EJ, Cicerone RJ, Alyea FN, & Cunnold DM (1992)
Three-dimensional simulation of atmospheric methyl chloroform: Effect
of an ocean sink. J Geophys Res, 97: 20,751-20,769.
Tkalich PP (1972) [Effectiveness of methyl bromide used against the
hemp leaf roller.] Khim Sel'sk Khoz, 10: 350-352 (in Russian).
Tkalich PP (1974) [Toxicological evaluation of fumigated hemp seeds.]
Tr Nauch Issled Inst Lub Kul't, 35: 97-101 (in Russian).
Torkelson TR & Rowe VK (1981) Methyl bromide. In: Patty's industrial
hygiene and toxicology, 3rd rev. edn. New York, Chichester, Brisbane,
Toronto, John Wiley and Sons, pp 3442-3446.
Tucker JF, Brown BW, & Goodship G (1974) Fumigation with methyl
bromide of poultry foods artificially contaminated with Salmonella. Br
Poult Sci, 15: 587-595.
Tucker JD, Xu J, Stewart J, & Ong T (1985) Development of a method to
detect volatile genotoxins using sister chromatid exchanges. Environ
Mutagen, 7: 48 (Abstract).
Tucker JD, Xu J, Stewart J, Baciu PC, & Ong T (1986) Detection of
sister chromatid exchanges induced by volatile genotoxicants. Teratog
carcinog mutagen, 6: 14-21.
Tunney J (1972) The use of methyl bromide in disease control. Mushroom
Sci, 8: 755-762.
Turtura GC, Massa S, & Zani G (1988) Effects of methyl bromide on some
bacterial groups in greenhouse soils. Ann Microbiol Enzimol, 38:
93-98.
Uncini A, Basciani M, Di Muzio A, Antonini D, & Onofrj M (1990) Methyl
bromide myoclonus: an electro physiological study. Acta Neurol Scand,
81: 159-164.
UNEP (1992) Methyl bromide and the ozone layer: a summary of current
understanding; Montreal Protocol Assessment Supplement; Synthesis
report of the methyl bromide interim scientific assessment and methyl
bromide interim technology and economic assessment requested by the
United Nations Environment Programme on behalf of the Contracting
Parties to the Montreal Protocol, June 1992. Nairobi, United Nations
Environment Programme, pp 33.
Urga K (1983) Methyl bromide and phosphine residues in cereal grains
and pulses. Ethiop Med, 21: 79-83.
US EPA (1982a) Method 8010. Halogenated volatile organics, 2nd ed.
Washington, DC, US Environmental Protection Agency, pp 278-289 (EPA
No. SW-846; PB 87-120-291).
US EPA (1982b) Method 8240. GS/MS method for volatile organics, 2nd
ed. Washington, DC, US Environmental Protection Agency, pp 433-454
(EPA No. SW-846; PB 87-120-291).
US EPA (1984a) Method 624. Guidelines establishing test procedures for
the analysis of pollutants under the Clean Water Act (40 CFR 136
Purgeables). Fed Reg, 49: 43373-43384.
US EPA (1984b) Appendix A to part 136 - Methods for organic chemical
analysis of principal and industrial wastewater. Method 601. Purgeable
halocarbons. Fed Reg, 49: 43261-43271.
US EPA (1984c) Method 1624. Revision B. Guidelines establishing test
procedures for the analysis of pollutants under the Clean Water Act
(40 CFR 136). Volatile organic compounds by isotope dilution GC/MS.
Fed Reg, 49: 43407-43415.
US EPA (1988a) Pesticide fact handbook. Park Ridge, New Jersey, Noyes
Data Corporation.
US EPA (1988b) Pesticide tolerances for inorganic bromides resulting
from fumigation with methyl bromide; correction. Fed Reg, 53:
30053-30054.
Uthman AP, Demlein PJ, Allston TD, Withiam MC, McClements MJ, & Takacs
GA (1978) Photoabsorption spectra of gaseous methyl bromide, ethylene
dibromide, nitrosyl bromide, thionyl chloride and sulfuryl chloride.
J Phys Chem, 82: 2252-2257.
Vanachter A (1974) Soil disinfestation in cauliflower, tomato, and
witloof crops in Belgium. Agric Environ, 1: 265-276.
Vanachter A, Feyaerts J, Van Wambeke E, & Van Assche C (1981) Bromide
concentrations in water after methyl bromide soil disinfestation. II.
Relation between leaching of methyl bromide fumigated greenhouse soils
and bromide concentrations in the surrounding surface waters. Meded
Fac Landbouwwet Rijksuniv Gent, 46: 351-358.
Van den Oever R, Van de Mierop L, & Lahaye D (1978) [Intoxication of
professionally exposed methyl bromide workers.] Belg Arch Soc Gen, 36:
353-369 (in Flemish).
Van den Oever R, Roosels D, & Lahaye D (1982) Actual hazard of methyl
bromide fumigation in soil disinfection. Br J Ind Med, 39: 140-144.
Van Doorn IAM, Feron VJ, Van Genderen H, & Zoeteman BCJ (1989) [Report
of the Netherlands Committee concerning the use of methyl bromide as
a soil disinfectant in agriculture.] The Hague, Ministry of
Agriculture and Fisheries, pp 3-23 (in Dutch).
Van Gundy SD, Munnecke D, Bricker J, & Minteer R (1972) Response of
Meloidogyne incognita, Xiphinema index , and Dorylaimus species to
methyl bromide fumigation. Phytopathology, 62: 191-192.
Van Gundy SD, Perez JG, Stolzy LH, & Thomason IJ (1974) A pest
management approach to the control of Pratylenchus thornei on wheat
in Mexico. J Nematol, 6: 107-116.
Van Leeuwen CJ, Rijkeboer M, & Niebeek G (1986) Population dynamics of
Daphnia magna and modified by chronic bromide stress. Hydrobiologia,
133: 277-285.
Van Leeuwen FXR & Sangster B (1987) The toxicology of bromide ion. CRC
Crit Rev Toxicol, 18: 189-213.
Van Rhee JA (1977) Effects of soil pollution on earthworms.
Pedobiologia, 17: 201-208.
Van Wambeke E, Vanachter A, & Van Assche C (1974) Some factors
affecting bromide residues in soil and plant. Meded Fac Landbouwwet
Rijksuniv Gent, 39: 1311-1324.
Van Wambeke E, Vanachter A, & Van Assche C (1988) Measures for
improving soil fumigation. Brighton Crop Protection Conference on
Pests and Diseases, Brighton, England, 21-24 November 1988. London,
The British Crop Protection Council, pp 199-204.
Van Wees AMP, Rijk MAH, Rijnaars MW, & De Vos RH (1984)
Chromatographic methods for the determination of inorganic bromide in
vegetables. In: Frigerio A & Milon H, ed. Chromatography and mass
spectrometry in nutrition science and food safety. Amsterdam, Oxford,
New York, Elsevier Science Publishers, pp 19-25.
Veenendahl G & Dibbets G (1981) [Permeation of methyl bromide into
different types of drinking waterpipes.] Kiwa, Inspection Institute
for Water Pipeline Materials (KIWA), pp 1-35 (in Dutch with English
Summary).
Verberk MM, Rooyakkers-Beemster T, De Vlieger M, & Van Vliet AGM
(1979) Bromine in blood, EEG and transaminases in methyl bromide
workers. Br J Ind Med, 36: 59-62.
Victor M & Adams RD (1980) Nonbarbiturate sedative-hypnotics;
psychtherapeutic, stimulant, and psychotogenic drugs. In: Isselbacher
KJ, Adams RD, Braunwald E, Petersdorf RG, & Wilson, JD ed. Harrison's
principles of internal medicine, 9th ed. New York, McGraw-Hill Book
Company (Abstract 229).
Vincent LE & Lindgren DL (1975) Toxicity of phosphine and methyl
bromide at various temperatures and exposure periods to the four
metamorphic stages of Trogoderma variabile. J Econ Entomol, 68:
53-56.
Vitte VI, Sorokina LV, & Subbotina SB (1970) [Residual amounts of
bromides in plant food products fumigated with methyl bromide and
characteristics of their biological action.] Gig Primen Toksikol
Pestic Klin Otrav, 8: 386-392 (in Russian).
Vodolagin VD (1971) Biology of the dog rose-eating weevil
(Megastigmus aculeatus) and measures for controlling it. Sci Trans
Efirnomaslich Cult Inst, 3: 115-117.
Von Oettingen WF (1946) The toxicity and potential dangers of methyl
bromide with special reference to its use in the chemical industry, in
fire extinguishers and in fumigation. Washington, DC, US Government
Printing Office, pp 1-41.
Von Oettingen WF (1955) The halogenated aliphatic, olefinic, cyclic,
aromatic, and aliphatic-aromatic hydrocarbons including the
halogenated insecticides, their toxicity and potential dangers.
Washington, DC, US Public Health Service, pp 15-30 (Publication No.
414).
Von Oettingen WF (1964) Methyl bromide. The halogenated hydrocarbons
of industrial and toxicological importance. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 25-55.
Watrous RM (1942) Methyl bromide-local and mild systemic effects. Ind
Med, 11: 575-579.
Wegman RCC, Greve PA, De Heer H, & Hamaker PH (1981) Methyl bromide
and bromide-ion in drainage water after leaching of glasshouse soils.
Water Air Soil Pollut, 16: 3-11.
Wegman RCC, Hamaker, PH, & De Heer H (1983) Bromide-ion balance of a
polder district with large-scale use of methyl bromide for soil
fumigation. Food Chem Toxicol, 21: 361-367.
Weihing JL, Schuster ML, Riesselman JH, & Cook JA (1971) Control of
fusarium wilt of petunia with three soil fumigants. Plant Dis Rep, 55:
580-582.
Weller D (1982) [Methyl bromide: Toxicology and therapy.] Frankfurt am
Main, Degesch GmbH, pp 21-42 (in German).
Wells JM & Payne JA (1975) Mycoflora of pecans treated with heat, low
temperatures, or methyl bromide for control of the pecan weevil.
Phytopathology, 65: 1393-1395.
Wester PW, Canton JH, & Dormans JAMA (1988) Pathological effects in
freshwater fish Poecilia reticulata (guppy) and Oryzias latipes
(medaka) following methyl bromide and sodium bromide exposure. Aquat
Toxicol, 12: 323-343.
Weststeijn G (1973) Soil sterilization and glasshouse disinfection to
control Fusarium oxysporum f. lycopersici in tomatoes in the
Netherlands. Neth J Plant Pathol, 79: 36-40.
Whitehead AG, Fraser JE, & Storey G (1972) Chemical control of potato
cyst-nematode in sandy clay soil. Ann Appl Biol, 72: 81-88.
Whitney WK, Jantz OK, & Bulger CS (1958) Effects of methyl bromide
fumigation on the viability of barley, corn, grain sorghum, oats, and
wheat seeds. J Econ Entomol, 51: 847-861.
Wilhelm E, Battino R, & Wilcock RJ (1977) Low pressure solubility of
gases in liquid water. Chem Rev, 77: 219-262.
Wilmer JWGM, Reuzel PGJ, & Dreef van der Meulen HC (1983) Subchronic
(13 week) inhalation toxicity study of methyl bromide in rats. Zeist,
The Netherlands, CIVO Institutes, TNO, 46 pp (CIVO Report No. V
82.378).
Windholz M (1983) The Merck index, 10th ed. Rahway, New Jersey, Merck
& Co., Inc., p 865.
Winstead NN & Garriss HR (1960) Control of cabbage clubroot in North
Carolina. Plant Dis Rep, 44: 14-18.
Winteringham FPW, Harrison A, Bridges RG, & Bridges PM (1955) The fate
of labelled insecticide residues in food products. II. The nature of
methyl bromide residues in fumigated wheat. J Sci Food Agric, 6:
269-275.
WMO (1992) Scientific assessment of ozone depletion, 1991. Geneva,
World Meteorological Organization, 330 pp (Report No. 25).
Wofsy SC, McElroy MB, & Yung YL (1975) The chemistry of atmospheric
bromide. Geophys Res Lett, 2: 215-218.
Wohlgemuth R, Drosihn J, & El-Lakwah F (1976) [Experiments to fumigate
piled expeller lying in quarantine against the Khapra beetle.] Berlin,
Federal Biological Institute for Agriculture and Forestry, 29 pp (in
German).
Woodrow JE, McChesney MM, & Seiber JN (1988) Determination of methyl
bromide in air samples by headspace gas chromatography. Anal Chem, 60:
509-512.
Wyers H (1945) Methyl bromide intoxication. Br J Ind Med, 2: 24-29.
Wylie PL (1988) Comparing headspace with purge and trap for analysis
of volatile priority pollutants. Am Water Works Assoc J, 80: 65-72.
Xu D, Peter H, Hallier E, & Bolt HM (1990) Hemoglobin adducts of
monohalomethanes. Ind Health, 28: 121-123.
Yamano Y (1991) [Experimental study on methyl bromide poisoning in
mice. Acute inhalation study and the effect of glutathione as an
antidote.] Jpn J Ind Health, 33: 23-30 (in Japanese).
Yamano Y, Ito I, Nagao N, & Ishizu S (1987) [A simple determination
method of bromide ion in plasma of methyl bromide workers by
head-space gas chromatography.] Jpn J Ind Health, 29: 196-201 (in
Japanese with English abstract).
Yeates GW, Bamforth SS, Ross DJ, Tate KR, & Sparling GP (1991)
Recolonization of methyl bromide sterilized soils under four different
field conditions. Biol Fertil Soils, 11: 181-189.
Yung YL, Pinto JP, Watson RT, & Sander SP (1980) Atmospheric bromine
and ozone pertubations in the lower stratosphere. J Atmos Sci, 37:
339-353.
Zatuchni J & Hong K (1981) Methyl bromide poisoning seen initially as
psychosis. Arch Neurol, 38: 529-530.
Zwart A, Arts JHE, Ten Berge WF, & Appelman LM (1992) Alternative
acute inhalation toxicity testing by determination of the
concentration-time-mortality relationship: experimental comparison
with standard LC50 testing. Regul Toxicol Pharmacol, 15: 278-290.
Zwart A (1988) Acute inhalation study of methylbromide in rats. Zeist,
The Netherlands, CIVO Institutes, TNO, 17 pp (CIVO Report-No. V88.
127/27).
Zwaveling JH, De Kort Wlam, Meulenbelt J, Hezemans-Boer M, Van Vloten
WA, & Sangster B (1987) Exposure of the skin to methyl bromide: a
study of six cases occupationally exposed to high concentrations
during fumigation. Hum Toxicol, 6: 491-495.
RESUME
1 Propriétés physiques et chimiques, méthodes d'analyse
A la température ambiante et sous la pression normale, le bromure
de méthyle est un gaz incolore dont le point d'ébullition est
d'environ 4 °C. Il est plus lourd que l'air et on peut facilement le
liquéfier en-dessous du point critique. Il est inodore sauf à fortes
concentrations auquel cas il dégage une odeur chloroformique. Il est
ininflammable à l'air, sauf aux concentrations comprises entre 10 et
16%, mais brûle dans l'oxygène. Le bromure de méthyle n'est que
légèrement soluble dans l'eau mais facilement soluble dans les autres
solvants courants. Il peut pénétrer dans de nombreuses substances
comme le béton, le cuir, le caoutchouc et certaines matières
plastiques.
Le bromure de méthyle s'hydrolyse en méthanol et acide brom-
hydrique en solution aqueuse, la vitesse d'hydrolyse dépendant du pH.
C'est un agent de méthylation efficace qui réagit sur les amines et
les composés soufrés. La plupart des métaux sont inertes au bromure de
méthyle pur et sec mais des réactions de surface peuvent avoir lieu
sur le zinc, l'étain, l'aluminium et le magnésium en présence
d'impuretés ou d'humidité. Des réactions explosives ont été observées
avec l'aluminium et le diméthylsulfoxyde.
Le bromure de méthyle existe dans le commerce sous forme de gaz
liquéfié. Les formulations destinées à la fumigation des sols
contiennent de la chloropicrine (2%) ou de l'acétate d'amyle (0,3%)
comme agents d'alerte. D'autres formulations peuvent contenir jusqu'à
70% de chloropicrine ainsi que d'autres fumigants ou hydrocarbures
comme diluants. Pour la fumigation des marchandises, on utilise du
bromure de méthyle à 100%.
Il existe des méthodes d'analyse pour le dosage du bromure de
méthyle dans l'air, l'eau, le sol, les produits alimentaires et la
nourriture pour animaux. Parmi les méthodes directes de dosage du
bromure de méthyle dans l'air sur le terrain, on peu citer les
analyseurs de gaz par conductivité thermique, les tubes de détection
colorimétrique, les analyseurs à infrarouge, et les détecteurs par
photo-ionisation. Pour les dosages de routine, on recommande la
chromatographie en phase gazeuse avec détection par capture
d'électrons, éventuellement couplée à la spectrométrie de masse pour
la confirmation au laboratoire.
Pour le dosage par chromatographie en phase gazeuse du bromure de
méthyle dans l'eau, on peut utiliser diverses techniques d'injection
(purge, piégeage, espace de tête par exemple). Pour le dosage en
routine du bromure de méthyle dans les produits alimentaires, on
recommande de procéder à une extraction par un mélange d'acétone et
d'eau puis à une chromatographie en phase gazeuse sur colonne
capillaire selon la technique de l'espace de tête avec détection par
capture d'électrons. Etant donné que le bromure de méthyle se
transforme en partie en bromure dans le sol, les denrées alimentaires
et les produits biologiques, l'ouvrage étudie également les méthodes
de recherche et de dosage des bromures. Parmi les méthodes utilisées
pour le dosage des bromures dans diverses matrices, on peut citer les
méthodes colorimétriques, la spectroscopie de rayons X, la
potentiométrie, l'analyse par activation neutronique, la
chromatographie en phase gazeuse ainsi que la chromatographie en phase
liquide à haute performance (HLPC).
2 Sources d'exposition humaine et environnementale
On pense que les océans sont la source essentielle de bromure de
méthyle. La principale source d'origine humaine en est la fumigation
des sols et de l'intérieur des habitations. Les véhicules à moteur qui
utilisent de l'essence au plomb émettent également une petite quantité
de bromure de méthyle.
En 1990, la consommation mondiale de bromure de méthyle a dépassé
67 millions de kg soit une augmentation de 46% depuis 1984. On produit
généralement le bromure de méthyle par action du méthanol sur l'acide
bromhydrique et, dans certains procédés, on l'obtient à côté du
tétrabromobisphénol A. Le bromure de méthyle est généralement stocké
et transporté à l'état liquide sous pression dans des récipients
d'acier.
Le bromure de méthyle est utilisé à environ 77% pour la
fumigation des sols, à 12% pour la fumigation des marchandises et
notamment des marchandises soumises à quarantaine, à 5% pour la
fumigation des construction et à 6% comme intermédiaire dans
l'industrie chimique.
Sous forme gazeuse, il est utilisé comme fumigant des sols, soit
en plein champ, soit sous serre pour la destruction des ravageurs.
Sous forme liquide, on l'applique avant la plantation, soit par
injection dans le sol, soit en plaçant des jattes pleines de bromure
de méthyle sous une couverture en plastique et en laissant le produit
s'évaporer sur place (méthode à froid) ou par chauffage (méthode à
chaud). Les méthodes autorisées varient selon le pays. Le type de
matière plastique utilisé est également important.
Les doses de bromure de méthyle à appliquer dépendent égale-ment
des normes légales en vigueur dans les différents pays, de l'espèce de
ravageur à détruire (type, ampleur de l'infestation), de la récolte
suivante, du type de sol et de la couverture plastique utilisée (durée
de couverture et nature de la matière plastique). Le bromure de
méthyle est généralement épandu sur le sol à des doses comprises entre
50 et 100 g/m2.
En fumigation spatiale, le bromure de méthyle est utilisé sur les
produits agricoles (par exemple fourrage, céréales, noix, etc.) ainsi
que pour la destruction des termites et des rongeurs. Pour la plupart
des denrées stockées dans des pièces et des silos fermés ou sous une
couverture étanche au gaz, on utilise des doses de 16 à 30 g de
bromure de méthyle par m3. Après la fumigation, il faut aérer les
locaux pendant un certain temps. Cette fumigation est également
importante dans le cas des légumes et des fruits frais soumis à
quarantaine.
Parmi les utilisations industrielles du bromure de méthyle, on
l'emploie en synthèse organique, généralement en tant qu'agent de
méthylation, ou comme solvant à bas point d'ébullition, par exemple
pour l'extraction de l'huile de noix et de différentes graines ou
celle des huiles essentielles. L'emploi du bromure de méthyle comme
réfrigérant ou comme produit extincteur à usage général, n'a plus
qu'un intérêt historique.
3 Transport, distribution et transformation dans l'environnement
Le bromure de méthyle est naturellement présent dans l'atmosphère
et les quantités qui résultent de l'activité humaine viennent s'y
ajouter. Le bromure de méthyle réagit en petites quantités sur les
radicaux hydroxyles de la troposphère mais il en passe une certaine
proportion dans la stratosphère par diffusion verticale. Là, c'est la
photolyse qui gagne en importance, puisqu'elle devient le mécanisme
dominant d'élimination du bromure de méthyle dans la partie basse de
la stratosphère. Il en résulte la formation de brome actif qui réagit
sur l'ozone stratosphérique et pourrait être en partie responsable de
la destruction de la couche d'ozone.
Dans le sol, le bromure de méthyle est partiellement hydrolysé en
ion bromure. Après fumigation au bromure de méthyle, on peut lessiver
le sol à l'eau afin d'éviter que les ions bromure formés ne soient
fixés par les végétaux plantés après stérilisation du sol. Cet
accroissement de la concentration en bromure peut poser des problèmes
si l'on utilise pour cela des eaux de surface. Le bromure de méthyle
peut diffuser à travers les canalisations d'eau potable en
polyéthylène si le sol environnant a subi une fumigation avec ce
produit.
Dans le sol, le bromure de méthyle peut diffuser jusqu'à une
profondeur de 0,8 m selon le type de sol, la dose d'emploi, le mode
d'épandage et la durée de fumigation, la couche supérieure du sol en
retenant la majeure partie. Le transport du gaz s'effectue par
écoulement ou diffusion moléculaire, mais il est également influencé
par le présence de processus simultanés de piégeage comme une sorption
ou une dissolution, et qui peuvent être irréversibles comme
l'hydrolyse. La quantité de bromure de méthyle transformée en bromure
dépend principalement de la teneur du sol en matières organiques. Les
bromures ainsi produits sont largement solubles dans l'eau et peuvent
être fixés par les plantes ou descendre plus profondément dans le sol
par lessivage.
La quantité de bromure qui s'accumule dans les plantes dépend de
divers facteurs comme la dose d'emploi, la durée d'exposition, le
débit d'aération, les propriétés physiques et chimiques du sol, la
climatologie (température et précipitations), l'espèce végétale et la
nature du tissu végétal. En particulier, les légumes-feuilles comme la
laitue et les épinards, peuvent fixer des quantités relativement
importantes d'ions bromure sans présenter de symptômes de
phyto-toxicité. En revanche, d'autres plantes cultivées comme les
oeillets, les plants d'agrumes, le coton, le céleri, les poivrons ou
les oignons sont particulièrement sensibles à la fumigation par le
bromure de méthyle.
Le bromure de méthyle et ses produits de réaction, dont on n'a
considéré jusqu'ici que les bromures, peuvent pénétrer dans la chaîne
alimentaire de deux manières; soit par consommation de plantes
cultivées dans des serres ou dans des champs traités par fumigation
avant la plantation, soit par consommation de produits alimentaires
traités au bromure de méthyle pendant l'entreposage. A partir d'une
certaine concentration, les bromures peuvent être dangereux pour la
santé et il existe un seuil de tolérance pour le bromure présent dans
les denrées alimentaires. La concentration des autres produits de
réaction n'a pas été étudiée.
Dans le sol, le bromure de méthyle subit une dégradation par
hydrolyse et décomposition microbienne. La constante de vitesse
d'hydrolyse varie avec la température et le pH et augmente avec
l'intensité de la lumière.
Le coefficient de partage octanol/eau (log Pow) du bromure de
méthyle est égal à 1,19, ce qui indique que sa bioaccumulation est
faible.
Le bromure de méthyle qui n'est pas décomposé lors de la
fumigation pénètre dans la troposphère et gagne la stratosphère par
diffusion verticale. Il ne semble pas qu'il existe un important
gradient de concentration vertical du bromure de méthyle dans la
troposphère, mais sa concentration diminue rapidement dans la basse
stratosphère où il subit une photolyse.
4 Concentrations dans l'environnement et exposition humaine
La concentration du bromure de méthyle mesurée dans l'air de
zones inhabitées, varie de 40 à 100 ng/m3 (10 à 26 pptv), les
valeurs pour l'hémisphère nord étant supérieures à celles à celles que
l'on trouve dans l'hémisphère sud. La plupart des valeurs vont de 9 à
15 pptv. Des différences saisonnières ont été constatées à l'occasion
d'un certain nombre d'études. Dans les zones urbanisées et
industrialisées, les concentrations sont beaucoup plus élevées avec
des valeurs moyennes allant jusqu'à 800 ng/m3 voire jusqu'à 4 µg de
bromure de méthyle par m3. A proximité des champs et des serres, on
constate, lors de la fumigation et de l'aération, des concentrations
de bromure de méthyle beaucoup plus élevées, des valeurs de 1 à 4
mg/m3 ayant été mesurées dans une étude à des distances allant
jusqu'à 20 m d'une serre, quelques heures après l'injection du
produit; quatre jours plus tard, la concentration était tombée au
dixième de cette valeur.
Dans un échantillon d'eau de mer prélevé en surface, on a trouvé
une concentration de bromure de méthyle de 140 ng/litre. Dans des
échantillons d'eau littorales de la Mer du Nord, la concentration
moyenne en ions bromure était de 18,4 mg/litre; dans les cours d'eau
de l'arrière pays, cette concentration était beaucoup plus faible,
sauf là où l'on pratiquait la fumigation par le bromure de méthyle ou
dans les zones polluées par les rejets industriels. Dans les eaux de
drainage d'une serre hollandaise, on a signalé des concentrations de
9,3 mg de bromure de méthyle par litre et de 72 mg d'ions bromure par
litre. Dans les eaux rejetées par une serre belge, on a trouvé après
fumigation une concentration de 280 mg de bromure par litre.
La teneur naturelle du sol en bromure dépend du type de ce sol
mais elle est généralement inférieure à 10 mg/kg. La teneur en résidus
de bromure dans les sols traités dépend du traitement, de la dose
d'emploi, du type de sol, des précipitations, du lessivage par l'eau
et de la température.
Il peut y avoir de fortes concentrations de bromure de méthyle ou
d'ions bromure dans les cultures vivrières poussant sur des sols
préalablement traités au bromure de méthyle ou qui ont subi une
fumigation après récolte.
Plus rarement, on a constaté que les concentrations de bromure
dans les légumes frais cultivés sur des sols qui avaient traité avec
du bromure de méthyle, dépassaient les teneurs en résidus autorisées.
Dans certains pays, il est interdit de cultiver des légumes sur des
sols traités de cette manière.
On utilise largement le bromure de méthyle pour la fumigation des
denrées alimentaires après récolte, par exemple les céréales et
notamment le froment, les épices, les noix, les fruits secs ou frais
et le tabac. La concentration en bromure de méthyle diminue en général
rapidement après aération et au bout de quelques semaines on ne peut
plus déceler de résidus. Certains produits comme les noix et les
graines ou des aliments gras comme le fromage ont tendance à retenir
le bromure de méthyle et le brome minéral.
Il peut y avoir exposition humaine au fumigant lui-même comme aux
résidus d'ions bromure. Il pourrait également y avoir un risque
d'accroissement de la concentration du bromure de méthyle ou des
bromures dans l'eau des puits peu profonds situés à proximité de
terrains où l'on pratique la fumigation par le bromure de méthyle.
Les personnes qui vivent à proximité immédiate des champs, des
serres ou des entrepôts traités au bromure de méthyle pourraient
courir le risque d'une exposition au gaz. Il existe également un
risque individuel pour les personnes qui pénètrent accidentellement ou
délibérément dans une maison traitée contre la vermine avant que l'on
ait annoncé qu'il n'y a plus de danger.
L'exposition professionnelle au bromure de méthyle est le risque
le plus probable pour les travailleurs lors de la production, du
remplissage des récipients et des opérations de fumigation. Seuls les
fumigateurs sont désormais considérés comme constituant un groupe à
haut risque étant donné que, sur les lieux de production, des mesures
de sécurité très strictes sont appliquées. Ceux qui sont employés au
traitement des constructions peuvent être exposés à des concentrations
beaucoup plus élevées que les valeurs-seuil après 24 heures d'aération
(80 à 2000 mg/m3). Cependant, un ouvrier expérimenté utilisera un
équipement protecteur approprié. Les travailleurs agricoles qui
procèdent à la fumigation du sol peuvent être exposés pendant de
longues durées à des doses transitoires élevées de bromure de méthyle.
De par la nature même du processus, le traitement d'une serre peut
exposer les travailleurs à d'importantes concentrations de bromure de
méthyle (100-1200 mg/m3). Il est vrai cependant, que pour combattre
des risques inhérents aux diverses opérations de fumigation, on a
établi des règles de sécurité très strictes qui impliquent notamment
le port d'un équipement protecteur. Cela n'empêche pas des accidents
de se produire par suite d'une exposition excessive.
5 Cinétique et métabolisme
Des études au cours desquelles on a fait inhaler du bromure de
méthyle à des rats, des chiens beagle et des volontaires humains ont
révélé que le produit était rapidement résorbé au niveau pulmonaire.
La résorption est également rapide chez le rat après administration
par voie orale.
Une fois résorbé, le bromure de méthyle ou ses métabolites se
répartissent rapidement dans de nombreux tissus, notamment les
poumons, les surrénales, les reins, le foie, les fosses nasales, le
cerveau, les testicules et les tissus adipeux. Lors d'une étude par
inhalation sur des rats, on a constaté que la concentration tissulaire
du bromure de méthyle passait par un maximum une heure après
l'exposition puis diminuait rapidement, aucune trace n'étant plus
décelable au bout de 48 heures. On n'a pas encore élucidé le
métabolisme du bromure de méthyle inhalé mais le glutathion pourrait
jouer un rôle.
Dans les tissus de plusieurs espèces, notamment l'homme, on a
observé une méthylation des protéines et des lipides après exposition
au bromure de méthyle par voie respiratoire. On a également mis en
évidence la présence d'adduits méthylés de l'ADN après exposition de
rongeurs ou de cellules de rongeurs in vivo ou in vitro.
Lors d'études d'inhalation avec du bromure de méthyle marqué par
du [14C], on a observé que la principale voie d'élimination du
radiocarbone était l'exhalation de 14CO2. La quantité de
radiocarbone retrouvée dans le urines a été moindre. Après
administration par voie orale de bromure de méthyle, on a constaté que
c'était par la voie urinaire que le 14C était principalement
excrété.
Le système nerveux central est également est un organe cible
important du bromure de méthyle. La neurotoxicité de ce composé est
sans doute liée à une modification de la teneur en monoamines, en
acides aminés et éventuellement en catécholamines.
6 Effets sur les êtres vivants dans leur milieu naturel
L'une des utilisations commerciales du bromure de méthyle
consiste dans la destruction des nématodes, des mauvaises herbes et
des champignons terricoles qui causent des maladies chez les plantes
comme la fonte des semis, et divers types de pourritures et de
verticillioses.
Peu d'études ont été consacrées aux effets du bromure de méthyle
sur les organismes aquatiques car ce composé n'est lui-même que peu
soluble dans l'eau. Les valeurs de la CL50 vont de 17 mg/litre pour
Cyprinus carpio L. sur 4 heures, à 1,2 mg/litre pour Poecilia
reticulata sur 48 heures. Aux concentrations mortelles, il est
probable que la mort est causée par des lésions au niveau des
branchies et de l'épithélium buccal.
Après fumigation par le bromure de méthyle, il se forme des ions
bromure que l'on retrouve dans l'eau après lessivage. Ces ions bromure
ont été à l'origine d'intoxications aiguës chez différents organismes
dulçaquicoles à des concentrations allant de 44 à 5800 mg de
Br-/litre; la concentration sans effets observables lors d'épreuves
à long terme allant de 7,8 à 250 mg de Br-/litre. Les ions bromure
ont également un effet perturbateur marqué sur la reproduction des
crustacés et des poissons.
En fumigation, on peut appliquer du bromure de méthyle
directement sur les semis, sur les boutures ou sur les plantes après
récolte, pour les débarrasser des parasites pendant le transport et
l'entreposage. Si le taux d'humidité ou la température sont trop
élevés, il peut y avoir retard à la germination, voire perte de la
capacité germinative.
Certains végétaux, en particulier les légumes-feuilles, sont
sensibles à la fumigation par le bromure de méthyle, soit par excès de
bromure dans le sol, soit indirectement en raison des effets de ce
composé sur la microflore terricole. Quelquefois, le bromure de
méthyle a au contraire un effet positif sur les végétaux car il peut
stimuler leur croissance et améliorer le rendement des cultures.
La fumigation par le bromure de méthyle détruit non seulement les
parasites visés mais également une partie de la flore, des
gastéropodes, des arachnides et des protozoaires terricoles.
On l'utilise de préférence à d'autres insecticides en raison de
son aptitude à pénétrer rapidement et profondément dans la masse des
produits traités et dans les sols. Les doses d'emploi pour traiter les
marchandises entreposées vont essentiellement de 16 à 100 g/m3
pendant deux à trois jours, selon la température. La dose nécessaire
pour la destruction des oeufs et des pupes est plus élevée que pour
les insectes adultes. La tolérance varie selon les diverses espèces
d'insectes, selon leurs divers stades de développement et chez une
même espèce, en fonction de la souche.
On ne dispose d'aucune donnée relative aux effets directs du
bromure de méthyle sur les oiseaux et les mammifères sauvages.
7 Effets sur les animaux d'expérience
Des études d'inhalation effectuées sur divers espèces de
mammifères montrent que la sensibilité au bromure de méthyle varie
nettement selon l'espèce et le sexe. La droite dose-mortalité présente
une pente accentuée chez toutes les espèces animales étudiées.
Chez le rat et la souris, les principaux signes cliniques
d'intoxication sont des manifestations neurologiques avec, à fortes
concentrations, une irritation des muqueuses.
Ces manifestations neurologiques consistent notamment en
fasciculations et paralysie. Divers auteurs ont également fait état à
doses plus faibles, de modifications dans l'activité locomotrice,
d'anomalies de la fonction nerveuse périphérique, de modifications du
rythme circadien et d'aversion gustative conditionnée.
On a également décrit des altérations histo- pathologiques au
niveau du cerveau, des reins, des muqueuses nasales, du coeur, des
surrénales, du foie et des testicules chez des rats et des souris
exposés à diverses concentrations de bromure de méthyle.
Une exposition de brève durée au bromure de méthyle provoque des
lésions au niveau des cellules de soutien de l'épithélium olfactif et
des cellules sensorielles matures mais la réparation et la
récupération sont rapides.
Les études d'inhalation de longue durée (jusqu'à deux ans)
effectuées sur des rats, ont révélé la présence de lésions de la
muqueuse nasale et du myocarde. Lors d'une étude à long terme analogue
chez la souris, les lésions primitives ont été observées au niveau du
cerveau, du coeur et de la muqueuse nasale. Aucun signe de
cancérogénicité n'a été observé chez l'une ou l'autre espèce.
L'administration par voie orale de 50 mg de bromure de méthyle
par kg de poids corporel à des rats pendant des périodes allant
jusqu'à 25 semaines a entraîné une inflammation et une hyperplasie
grave au niveau de l'épithélium de la portion cardiaque de l'estomac.
Après une période de récupération, qui a suivi l'exposition au bromure
de méthyle, la principale lésion observée était une fibrose de la
portion cardiaque de l'estomac. Chez des rats traités quotidiennement
pendant 25 semaines, on a également constaté la présence d'un cancer
précoce à ce niveau.
Des souris B6C3F et des rats F344 exposés à des concentrations de
bromure de méthyle allant jusqu'à 467 mg/m3 pendant 13 semaines, ont
présenté de légères anomalies morphologiques des spermatozoïdes, la
durée du cycle oestral n'étant pas affectée.
Deux générations consécutives de rats CD Sprague- Dawley ont été
soumis par la voie respiratoire à des concentrations de bromure de
méthyle allant jusqu'à 350 mg/m3 sans que l'on constate d'effets
notables sur leur croissance, les divers processus de la reproduction
ni sur leur descendance. Il y avait diminution des indices de
fécondité des mâles et des femelles aux deux doses les plus élevées,
chez les rats de la génération F1 et de la génération F2b.
On a étudié les effets toxiques du bromure de méthyle sur le
développement de lapins blancs de Nouvelle-Zélande, exposés à 311 mg
de bromure de méthyle par m3 (6 heures/jour, du 7ième au 19ième jour
de la gestation). Les effets toxiques relevés chez les mères étaient
modérés à graves. Les effets sur le développement observés aux doses
toxiques pour les mères, consistaient en une réduction du poids du
foetus, une augmentation de la fréquence des modifications
squelettiques mineures ainsi qu'en malformations (essentiellement
absence de la vésicule biliaire ou du lobe inférieur du poumon).
Toutefois à la dose de 272 mg/m3, la toxicité maternelle était moins
marquée et il n'y avait plus d'effets embryotoxiques.
Aucun effet nocif maternel, embryonnaire ou foetal n'a été
observé chez des lapins exposés à des doses de bromure de méthyle
égales à 78 ou 156 mg/m3. Pour les lapins blancs de
Nouvelle-Zélande, on a fixé à 156 mg/m3 la dose sans effets
observables pour la toxicité maternelle et les effets toxiques sur le
développement.
Le bromure de méthyle s'est révélé mutagène dans plusieurs
systèmes d'épreuve in vitro et in vivo. Il provoque des mutations
létales récessives liées au sexe chez Drosophila melanogaster ainsi
que des mutations dans des cellules mammaliennes en culture. Il ne
provoque pas de synthèse anarchique de l'ADN (non programmée) ni de
transformation cellulaire dans les cultures de cellules mammaliennes.
Chez des souris à qui on avait administré du bromure de méthyle par
diverses voies, on a observé une méthylation de l'ADN au niveau des
cellules hépatiques et spléniques. Chez des rats et des souris, on a
observé la formation de micro-noyaux dans les cellules de la moelle
osseuse et les leucocytes périphériques. On ignore le mécanisme de la
toxicité du bromure de méthyle.
8 Effets sur l'homme
Il peut y avoir exposition humaine au bromure de méthyle, soit
par inhalation du gaz, soit par contact avec le liquide. L'exposition
peut également se produire par la voie digestive à la suite de
l'ingestion d'eau de boisson contaminée par des eaux de lessivage
polluées.
Une étude contrôlée sur l'homme a révélé que, après inhalation,
environ 50% de la dose administrée étaient absorbés.
Le bromure de méthyle provoque des lésions au niveau du système
nerveux, des poumons, des muqueuses nasales, du rein, de l'oeil et de
la peau. Les effets sur le système nerveux central consistent en
troubles visuels, confusion mentale, engourdissement, tremblements, et
troubles de l'élocution. Une contamination topique peut entraîner une
irritation et des brûlures cutanées ainsi que des lésions oculaires.
L'exposition à de fortes concentrations de bromure de méthyle
provoque un oedème pulmonaire. Dans les cas d'intoxication par le
bromure de méthyle, la cause immédiate de la mort est généralement une
dépression du système nerveux central entraînant une paralysie
respiratoire et/ou une défaillance circulatoire, précédées par des
convulsions et un coma.
Lors d'intoxications aiguës ou chroniques par le bromure de
méthyle, on a observé différents symptômes neuro- psychiatriques. Une
exposition de brève durée à de faibles concentrations de vapeurs de
bromure de méthyle peut produire un syndrome polyneuropathique sans
manifestations centrales évidentes.
Parmi les séquelles tardives de l'intoxication on peut noter une
broncho-pneumonie consécutive à de graves lésions pulmonaires, une
insuffisance rénale avec anurie et une très importante faiblesse avec
ou sans signes de paralysie. Généralement ces symptômes tendent à
disparaître en quelques semaines à quelques mois. Toutefois, on a
observé des déficits permanents généralement caractérisés par des
troubles sensoriels, de la faiblesse, une baisse de l'acuité visuelle
et des troubles de la démarche.
L'exposition au bromure de méthyle s'accompagne d'une
augmentation du taux sanguin de bromure. Chez les fumigateurs, il
existe une relation entre le nombre d'opérations auxquelles ils ont
participé et le taux plasmatique moyen de bromure.
RESUMEN
1 Propiedades físicas y químicas y métodos analíticos
El bromuro metílico es un gas incoloro a la temperatura ambiente
y a la presión atmosférica normal, con un punto de ebullición de 4 oC
aproximadamente. Es más pesado que el aire y se licúa con facilidad
por debajo de sus puntos críticos. Es inodoro, excepto en
concentraciones altas, en las que tiene un olor parecido al
cloroformo. No es inflamable en el aire, excepto en la gama de
concentraciones del 10-16%, pero arde en oxígeno. El bromuro metílico
es ligeramente soluble en agua, pero fácilmente soluble en otros
disolventes corrientes. Puede penetrar a través de numerosas
sustancias, como cemento, cuero, caucho y ciertos plásticos.
El bromuro metílico se hidroliza dando metanol y ácido
bromhídrico en solución acuosa, con una velocidad de hidrólisis que
depende del pH. Es un agente metilante eficaz que reacciona con las
aminas y con los productos que contienen azufre. La mayoría de los
metales son inertes ante el bromuro metílico seco y puro, pero se
producen reacciones de superficie sobre el zinc, el estaño, el
aluminio y el magnesio en presencia de impurezas o humedad. Se han
señalado reacciones explosivas con el aluminio y el sulfóxido
dimetílico.
El bromuro metílico se comercializa en forma de gas licuado. Las
formulaciones par la fumigación del suelo contienen cloropicrina (2%)
o acetato amílico (0,3%) como agentes de aviso. Otras formulaciones
incluyen hasta el 70% de cloropicrina o de otros fumigantes o
hidrocarburos como diluyentes inertes. Para la fumigación de
mercancías se utiliza bromuro metílico al 100%.
Se han descrito métodos analíticos para la determinación del
bromuro metílico en el aire, el agua, el suelo, los alimentos y los
piensos. Entre los aparatos para la determinación directa del bromuro
metílico en el aire, en condiciones prácticas, figuran los
analizadores de gases por conductividad térmica, los tubos de
detección colorimétrica, los analizadores en infrarrojos y los
detectores por fotoionización. Se recomienda la cromatografía de gases
(CG) con detección por captura de electrones (DCE) para las mediciones
corrientes, seguida a veces de la confirmación por espectrometría de
masa (EM) en el laboratorio.
Para la determinación por CG del bromuro metílico en el agua se
utilizan técnicas de purga y captura, así como de muestreo en el
espacio superior. Se recomienda la extracción con acetona y agua
seguida de la cromatografía capilar de gas del espacio superior con
DCE para la determinación ordinaria del bromuro metílico en los
alimentos. Teniendo en cuenta que una parte del bromuro metílico se
convierte en bromuro en el suelo, los alimentos y los productos
biológicos, se examinan también los métodos de determinación del
bromuro. Entre los utilizados para esa determinación en distintas
matrices figuran métodos colorimétricos, la espectroscopia de rayos X,
la potenciometría, el análisis por activación neutrónica, la
cromatografía de gases y la cromatografía de líquidos de alto
rendimiento.
2 Fuentes de exposición humana y ambiental
Se estima que los océanos son la fuente más importante de bromuro
metílico. La principal fuente antropogénica de bromuro metílico es la
fumigación de suelos y locales. Los vehículos de motor que utilizan
gasolina con plomo emiten una pequeña cantidad de bromuro metílico.
En 1990, el consumo mundial de bromuro metílico fue superior a
67 millones de kg, con un aumento del 46% respecto a 1984. Se fabrica
corrientemente por reacción entre el metanol y el ácido bromhídrico y
en algunos procedimientos es un coproducto que acompaña al
tetrabromobisfenol A. El bromuro metílico se almacena y transporta
habitualmente como gas licuado a presión en recipientes de acero.
El 77% aproximadamente del bromuro metílico fabricado se emplea
para la fumigación del suelo, el 12% para la fumigación de cuarentena
y de mercancías, el 5% para la fumigación de edificios y el 6% para
obtener intermediarios químicos.
El gas se emplea como fumigante del suelo en los campos o los
invernaderos en la lucha contra las plagas. Se aplica en forma de
líquido antes de la plantación, por inyección en el suelo o por
evaporación en recipientes colocados bajo las cubiertas de plástico,
dejando que el producto se evapore in situ (método frío) o por
calentamiento (método caliente). Hay diferencias en los métodos
utilizados en los distintos países. También es importante el tipo de
cubierta de plástico empleada.
Las dosis de bromuro metílico que se han de aplicar dependen de
las normas reglamentarias de los distintos países, el parásito vegetal
que se ha de eliminar (tipo, amplitud de la infestación), el cultivo
siguiente, el tipo de suelo y la cubierta de plástico empleada (tiempo
de recubrimiento y tipo de plástico). El bromuro metílico se aplica
habitualmente al suelo en concentraciones comprendidas entre 50 y 100
g/m2.
En la fumigación espacial se emplea el bromuro metílico para el
tratamiento de productos agrícolas (por ej., alimentos, cereales,
nueces, etc.) y la lucha contra las termitas y los roedores. Se
emplean concentraciones de 16-30 g de bromuro metílico por m3 para
la mayor parte de los productos almacenados en naves y silos cerrados
herméticamente y bajo cubiertas impermeables a los gases. La
fumigación debe ir seguida de un periodo de aireación. También es
importante la fumigación de hortalizas y frutas frescas en donde han
de observarse reglamentos de cuarentena.
Entre los usos industriales del bromuro metílico figuran la
síntesis orgánica, habitualmente como agente metilante, y el empleo
como disolvente de baja temperatura de ebullición, por ejemplo, para
la extracción de aceites de nueces, semillas y flores. La utilización
del bromuro metílico como refrigerante y como agente general de
extinción de incendios sólo tiene ahora impor-tancia histórica.
3 Transporte, distribución y transformación en el medio ambiente
El bromuro metílico se halla presente de modo natural en la
atmósfera. Se suman a esa presencia las fuentes antropogénicas. Aunque
una pequeña cantidad del bromuro metílico reacciona con el radical
hidroxilo en la troposfera, parte del bromuro metílico pasa a la
estratosfera por difusión ascendente. En esa capa adquiere importancia
creciente la fotólisis del bromuro metílico, siendo el mecanismo
predominante de desaparición en la estratosfera baja. El bromo activo
reacciona con el ozono en la estratosfera y se cree que es en parte
responsable de la destrucción de la capa de ozono.
En el suelo, el bromuro metílico se hidroliza parcialmente para
dar ion bromuro. Después de la fumigación con bromuro metílico, el
suelo puede ser lixiviado con agua para evitar que los iones bromuro
formados sean captados por los vegetales plantados después en el suelo
esterilizado. Este aumento de las concen- traciones de bromuro puede
producir problemas cuando se utilizan aguas superficiales para la
lixiviación. El bromuro metílico puede difundirse a través de las
tuberías de polietileno de conducción de agua potable, si el suelo que
las rodea ha sido fumigado con bromuro metílico.
En el suelo, el bromuro metílico puede difundirse hasta una
profundidad de 0,8 m, en función del tipo de suelo, la dosis, el
método de aplicación y la duración de la fumigación; la mayor
concentración de bromuro metílico se alcanza en la parte superior del
suelo. El transporte del gas se produce por flujo de masas y difusión
molecular, pero también influyen los procesos de desa-parición que se
produzcan simultáneamente, como la absorción y la disolución, y los
procesos de desaparición irreversible, como la hidrólisis. La cantidad
de bromuro metílico convertido en bromuro depende principalmente del
contenido en materias orgánicas del suelo. El bromuro producido es
principalmente hidrosoluble y puede ser captado por las plantas o
desplazado a niveles inferiores del suelo por lixiviación con agua.
En las plantas, la cantidad de bromuro acumulado depende de
distintos factores, como la concentración, el tiempo de exposición, la
tasa de aireación, las propiedades físicas y químicas del suelo, las
tendencias climáticas (temperatura y pluviosidad), las especies
vegetales y el tipo de tejido de las plantas. En particular las
hortalizas de hoja, como la lechuga y la espinaca, pueden captar
cantidades relativamente altas de ion bromuro sin síntomas
fitotóxicos. Por el contrario, otros cultivos, como los claveles, los
planteles de cítricos, el algodón, el apio, los pimientos y las
cebollas, son especialmente sensibles a la fumigación con bromuro
metílico.
El bromuro metílico y sus productos de reacción, entre los cuales
sólo se ha considerado hasta ahora el bromuro, pueden entrar en la
cadena alimentaria de dos modos: consumo de alimentos cultivados en
invernaderos o en campos fumigados antes de la plantación o
alimentación con productos fumigados con bromuro metílico en el curso
del almacenamiento. En determinadas concentraciones, el bromuro puede
ser peligroso para la salud; se han indicado niveles de tolerancia
para el bromuro contenido en los alimentos. No se han investigado los
niveles de otros productos de reacción.
El bromuro metílico se degrada en el suelo por hidrólisis y
descomposición microbiana. La constante de hidrólisis varía con la
temperatura y el pH, y aumenta con la luz.
El coeficiente de partición octanol/agua (log Pow) del bromuro
metílico es de 1,19, lo que sugiere la existencia de una
bio-acumulación baja.
El bromuro metílico que no se ha degradado en el curso de la
fumigación pasa a la troposfera y por difusión ascendente a la
estratosfera. No parece que haya un gradiente vertical importante del
bromuro metílico en la troposfera, pero las concentraciones disminuyen
con rapidez en la estratosfera baja por acción de la fotólisis.
4 Niveles ambientales y exposición humana
Las concentraciones de bromuro metílico, medidas en el aire en
zonas sin habitar, varían entre 40 y 100 ng/m3 (10 a 26 pptv),
siendo los valores en el hemisferio Norte superiores a los del
hemisferio Sur. La mayoría de las concentraciones se hallan en la gama
de 9-15 pptv. En algunos estudios se han observado diferencias
estacionales. En las zonas urbanas e industriales, las concentraciones
son mucho mayores, con valores medios de hasta 800 ng/m3, llegando
a veces hasta 4 µg de bromuro metílico por m3. En el curso de la
fumigación y la aireación, las concentraciones de bromuro metílico son
apreciablemente más altas cerca de los campos y los invernaderos,
habiéndose medido valores de 1-4 mg/m3 en un estudio a distancias de
hasta 20 m de un invernadero, algunas horas después de la inyección en
el suelo; cuatro días más tarde se observó la décima parte de ese
valor.
La concentración de bromuro metílico en una muestra de agua del
mar de superficie fue de 140 ng/litro. En muestras de agua costera
cerca del mar del Norte, el valor medio de las concentraciones de ion
bromuro fue de 18,4 mg/litro; la concentración de ion bromuro en los
ríos de tierra adentro fue mucho más baja, excepto en las regiones
donde se practicaba la fumigación con bromuro metílico o en las zonas
de contaminación industrial. En el agua de drenaje de un invernadero
de los Países Bajos se señalaron concentraciones de 9,3 mg de bromuro
metílico/litro y de 72 mg de ion bromuro/litro. En el agua evacuada de
un invernadero belga se registró un valor de 280 mg de bromuro/litro
después de la fumigación.
El contenido de bromuro natural del suelo depende del tipo de
suelo, pero suele ser inferior a 10 mg/kg. La presencia de restos de
bromuro en el suelo fumigado depende del tratamiento, la dosis, el
tipo de suelo, la cantidad de lluvia o de agua de lixiviación y la
temperatura.
Las concentraciones de bromuro metílico o bromuro pueden ser
altas en los alimentos que se han cultivado en suelos tratados
previamente con bromuro metílico o que se han fumigado después de la
recolección.
En hortalizas frescas cultivadas en suelos previamente fumigados
con bromuro metílico se han observado excepcional-mente
concentraciones de bromuro que rebasaban el nivel autori-zado de
residuos. En algunos países no se permite cultivar hortalizas en los
suelos tratados.
El bromuro metílico se utiliza ampliamente para la fumigación de
productos alimenticios después de la recolección, como trigo y
cereales, especias, nueces, frutas frescas y desecadas, y tabaco. Las
concentraciones de bromuro metílico suelen descender con rapidez
después de la aireación y no se detectan residuos al cabo de unas
semanas. Algunos alimentos, como las nueces, las semillas y productos
grasos como el queso, tienden a retener el bromuro metílico y el
bromuro inorgánico.
Las personas pueden estar expuestas al fumigante y a restos de
ion bromuro. También existe el riesgo de que haya bromuro metílico o
un aumento del contenido de bromuro en el agua de pozos situados cerca
de lugares en donde se ha fumigado bromuro metílico.
Las personas que viven cerca de campos, invernaderos o almacenes
fumigados con bromuro metílico pueden estar expuestos al gas. Los
seres humanos pueden también correr peligro si accidental o
deliberadamente penetran en locales que han sido fumigados para
erradicar plagas antes de declararlos seguros.
La exposición profesional al bromuro metílico es el riesgo más
probable de los operarios en el curso de la fabricación, el llenado y
la fumigación. Dadas las medidas de seguridad aplicadas estrictamente
en las fábricas, sólo se considera actualmente como grupo de alto
riesgo a los fumigadores. Los fumigadores que realizan el tratamiento
de edificios pueden tener una exposición muy superior al valor umbral
límite (VUL) después de 24 horas de aireación (80-2000 mg/m3). Sin
embargo, los operarios convenientemente capacitados utilizarán equipo
protector apropiado. Los obreros que trabajan en el campo durante la
fumigación del suelo pueden estar expuestos durante periodos más
prolongados a dosis pasajeras de bromuro metílico. Dada la naturaleza
de la fumigación de los invernaderos, los operarios pueden también
encontrar concentraciones más altas (100-1200 mg/m3). Así pues, la
gestión del riesgo provocado por los distintos aspectos de la
fumigación exige medidas de seguridad estrictas y el empleo de equipo
protector. Pese a ello se producirán todavía casos aislados de
sobreexposición accidental.
5 Cinética y metabolismo
Los estudios de inhalación efectuados en ratas, perros sabuesos
y seres humanos han mostrado la absorción rápida del bromuro de metilo
por los pulmones. También se absorbe con rapidez en las ratas después
de la administración oral.
Tras la absorción, el bromuro metílico o sus metabolitos se
distribuyen con rapidez en numerosos tejidos, comprendidos los
pulmones, las glándulas suprarrenales, los riñones, el hígado, los
cornetes nasales, el cerebro, los testículos y el tejido adiposo. En
un estudio de inhalación efectuado en ratas, la concentración tisular
de bromuro metílico alcanzó el valor máximo una hora después de la
exposición, pero descendió con rapidez, no encontrándose indicios 48
horas más tarde. Todavía no se ha esclarecido el metabolismo del
bromuro metílico inhalado, pero parece que interviene el glutatión.
Se ha observado la metilación de proteínas y lípidos en los
tejidos de varias especies, incluidos los seres humanos, expuestos a
través de la inhalación. También se han detectado aductos de ADN
metilado después de la exposición in vivo e in vitro de roedores
o células de roedores.
En los estudios de inhalación con bromuro metílico marcado con
[14C], la expiración de 14CO2 fue la principal vía de
eliminación de 14C. Por la orina se eliminó una cantidad menor de
14C. Tras la administración oral de bromuro metílico, la excreción
urinaria fue la principal vía de eliminación del 14C.
El sistema nervioso central es un importante destinatario del
bromuro metílico. En la neurotoxicidad provocada por el bromuro
metílico pueden intervenir modificaciones del contenido de monoaminas
y aminoácidos y tal vez de catecolaminas.
6 Efectos en los seres vivos del medio ambiente
El bromuro metílico se utiliza comercialmente en la lucha contra
los nematodos, las malas hierbas y los hongos transmitidos por el
suelo que provocan trastornos tales como el resecamiento, la
putrefacción de la copa o las raíces y el marchitado.
Se han realizado escasos estudios sobre los efectos del bromuro
metílico en los seres acuáticos, pues el propio bromuro metílico sólo
es ligeramente soluble en agua. Los valores de la CL50 van de un
valor a las cuatro horas de 17 mg/litro para Cyprinus carpio L. a
otro a las 48 horas de 1,2 mg/litro para Poecilia reticulata. En
concentraciones letales, las lesiones de las agallas y el epitelio
oral son la causa probable de la muerte.
El ion bromuro se forma a partir del bromuro metílico después de
la fumigación y se halla en el agua tras la lixiviación. Se observó
una toxicidad aguda por iones bromuro en distintos seres de agua dulce
en concentraciones comprendidas entre 44 y 5800 mg de Br-/litro; la
concentración de efecto no observado (NOEC) en las pruebas de larga
duración varió entre 7,8 y 250 mg de Br-/litro. Los iones bromuro
produjeron una marcada alteración de la reproducción de crustáceos y
peces.
El bromuro metílico puede aplicarse directamente como fumigante
a las semillas o los esquejes de las plantas o a los productos
alimenticios después de la recolección para la desin-festación en el
curso del transporte y el almacenamiento. Puede producirse el retraso
de la germinación o la pérdida de la capacidad germinativa si la
humedad o la temperatura son demasiado altas.
Algunos cultivos, en particular las hortalizas de hoja, son
sensibles a la fumigación con bromuro metílico debido a la presencia
de bromuro en exceso en el suelo o indirectamente por los efectos en
la microflora del suelo. El bromuro metílico tiene a veces un efecto
positivo sobre las plantas, favoreciendo su crecimiento y el
rendimiento de los cultivos.
La fumigación con bromuro metílico erradica no sólo los seres
vivos a los que se aplica sino también una parte de la flora del
suelo, los gastrópodos, los arácnidos y los protozoos.
El bromuro metílico se utiliza a menudo de preferencia a otros
insecticidas por su capacidad para penetrar con rapidez y profundidad
en productos no envasados y en los suelos. Las dosis de bromuro de
metilo utilizado como fumigante en almacenes se sitúan sobre todo
entre 16 y 100 g/m3 durante 2-3 días, dependiendo la dosis de la
temperatura. Para matar los huevos y las pupas se necesita una dosis
más alta que en el caso de los insectos adultos. Existen variaciones
en la tolerancia de las distintas especies y fases de insectos y entre
las distintas estirpes del mismo insecto.
No existen datos sobre los efectos directos del bromuro metílico
en las aves y los mamíferos silvestres.
7 Efectos en los animales de experimentación
Los estudios de inhalación realizados en varias especies de
mamíferos han mostrado que existen claras diferencias relacionadas con
la especie y el sexo en lo que se refiere a la susceptibilidad al
bromuro metílico. No se observó una respuesta dosis-mortalidad muy
marcada en ninguna de las especies animales ensayadas.
Las manifestaciones neurológicas son los principales signos
clínicos de toxicidad en las ratas y los ratones y, en concentraciones
más altas, se ha observado también la irritación de las mucosas.
Entre las manifestaciones neurológicas destacan los espasmos y la
parálisis. Con dosis más altas varios autores han señalado
modificaciones de la actividad locomotriz, disfunción de los medios
periféricos, cambios del ritmo circadiano y aversión gustativa
condicionada.
Se han descrito lesiones histopatológicas en el cerebro, el
riñón, la mucosa nasal, el corazón, las glándulas suprarrenales, el
hígado y los testículos de ratas y ratones expuestos a distintas
concentraciones de bromuro metílico.
Las células de soporte olfativas y las sensoriales maduras sufren
lesiones por la exposición a corto plazo al bromuro metílico, pero la
reparación y la recuperación son rápidas.
Los estudios de inhalación de larga duración (hasta 2 años) en
ratas mostraron lesiones de la mucosa nasal y el miocardio. En un
estudio análogo de larga duración en ratones se observaron los efectos
tóxicos primarios en el cerebro, el corazón y la mucosa nasal. En
ninguna de ambas especies se registraron signos de carcinogenicidad.
La administración oral de 50 mg de bromuro metílico/kg de peso
corporal a ratas durante un periodo de hasta 25 semanas produjo
inflamación e hiperplasia intensa del epitelio del antro cardial. Tras
un periodo de recuperación postexposición, la principal lesión
observada fue la fibrosis del antro cardial. En la rata tratada
diariamente durante 25 semanas se observó un carcinoma inicial del
antro cardial.
Los ratones B6C3F y las ratas F344 expuestos a dosis de hasta 467
mg de bromuro metílico/m3 durante 13 semanas mostraron cambios
ligeros de la morfología del esperma sin que se afectara la duración
del ciclo estral.
La exposición por inhalación a dosis de hasta 350 mg de bromuro
metílico/m3 no produjo ningún efecto digno de mención sobre el
crecimiento, los procesos reproductivos y las crías de dos
generaciones consecutivas de ratas CD Sprague-Dawley. Los índices de
fecundidad de machos y hembras se redujeron con dos niveles máximos de
concentraciones en la camada F2B de la generación F1.
En los estudios sobre la toxicología del desarrollo efectuados en
conejos blancos de Nueva Zelandia, la exposición a 311 mg de bromuro
metílico/m3 (6 h/día; días 7-19 de la gestación) mostró una
toxicidad materna moderada a intensa. Los efectos en el desarrollo
observados con dosis tóxicas para la madre consistieron en
dis-minución del peso del feto, aumento de la incidencia de
variaciones óseas ligeras y presencia de malformaciones
(principalmente ausencia de la vesícula biliar o del lóbulo caudal del
pulmón). Sin embargo, con dosis de 272 mg/m3 la toxicidad materna
fue menos marcada y no se produjeron efectos embriotóxicos.
No se observaron efectos maternos, embrionarios o fetales
adversos en conejos expuestos a 78 ó 156 mg de bromuro metílico/m3.
En conejos blancos de Nueva Zelandia se indicó un nivel sin efectos
observados (NOEL) de 156 mg de bromuro metílico/m3 en lo que
respecta a la toxicidad materna y del desarrollo.
Se ha observado que el bromuro metílico es mutagénico en varios
sistemas de ensayo in vitro e in vivo. Provoca mutaciones letales
recesivas ligadas al sexo en Drosophila melanogaster y mutaciones en
células de mamífero cultivadas. No induce la síntesis no programada
del ADN ni la transformación celular en células de mamífero
cultivadas. En ratones a los que se administró bromuro metílico por
distintas vías se observó la metilación del ADN en el hígado y el
bazo. Se indujo la formación de micronúcleos en las células de la
médula ósea y de la sangre periférica de ratones y ratas.
Se desconoce el mecanismo de la toxicidad del bromuro metílico.
8 Efectos en la especie humana
La exposición humana al bromuro metílico puede producirse por
inhalación del gas o por contacto con el líquido. También se produce
exposición por ingestión de agua de bebida contaminada con agua de
lixiviación.
Un estudio en la especie humana con testigos mostró que la
captación del producto después de la exposición por inhalación es del
50% aproximadamente de la dosis administrada.
El bromuro metílico es nocivo para el sistema nervioso, los
pulmones, la mucosa nasal, los riñones, los ojos y la piel. Entre los
efectos en el sistema nervioso central figuran la visión enturbiada,
la confusión mental, la pérdida de sensibilidad, el temblor y los
defectos del habla. La exposición tópica puede provocar irritación
cutánea, quemaduras y lesiones oculares.
La exposición a altas concentraciones de bromuro metílico causa
edema pulmonar. La depresión del sistema nervioso central con
parálisis respiratoria e insuficiencia respiratoria es a menudo la
causa inmediata de la muerte, que va precedida de convulsiones y coma.
Se han observado distintos signos y síntomas neuropsiquiátricos
en el curso de las intoxicaciones agudas y prolongadas producidas por
bromuro metílico. Las exposiciones de corta duración a dosis bajas de
vapores han producido un síndrome de polineuropatía con
manifestaciones centrales patentes.
Entre las secuelas tardías figuran la bronconeumonía consecutiva
a lesiones pulmonares graves, la insuficiencia renal con anuria y la
debilidad extrema, con o sin signos de parálisis. Por lo general esos
síntomas tienden a remitir después de un periodo de unas semanas o
meses. Sin embargo, se han observado deficiencias sin recuperación,
caracterizadas habitualmente por trastornos sensoriales, debilidad,
alteraciones del carácter y enturbiamiento de la visión.
La exposición al bromuro metílico va acompañada de un aumento de
la concentración de bromuro en la sangre. En los fumigadores se
observa una relación entre el número de aplicaciones del gas y la
concentración plasmática media de bromuro.
 Degradation occurred by the methylation of carboxylic groups on
moist H-substituted peat. In soils containing other groups that can be
methylated, the mechanism and factors of methyl bromide degradation
are more complex.
Adsorption on to organic matter reduced the available
concentration of methyl bromide in the aqueous and gaseous phases of
soil. Organic matter was also the main factor involved in the
degradation of methyl bromide in the soil (Arvieu & Cuany, 1985).
4.2.2.2 Light-assisted hydrolysis in water
The UV-absorption cross sections for methyl bromide (174-262 nm)
have been confirmed by several authors (Robbins 1976b; Uthman et al.,
1978; Molina et al., 1982; Gillotay et al., 1989). The UV absorption
spectrum has a maximum of 202 nm with a steep decrease at longer
wavelengths reaching 0.2% of the maximum at 260 nm (Table 17). This is
much shorter than 290 nm, which is the shortest wavelength radiation
reaching the earth's surface from the sun.
It should be noted that the photoactivation of methyl bromide, as
well as its hydrolysis products in water or on soil surfaces, will
differ from the gas phase activities of these processes. The
hydrolysis products are in solution and differ energetically. Since
the species energy levels are changed from those of the isolated
molecule, critical absorption wavelengths would be shifted.
The influence of sunlight versus dark using natural waters in a
laboratory test showed little effect on the hydrolysis of methyl
bromide (Gentile et al., 1989, 1992).
Irradiation with a "pen-ray" low-pressure mercury lamp at 254 nm
gave a 6.6-fold increase in the rate constant. However, photolysis
does not alter the stoichiometry of the hydrolysis (Castro & Belser,
1981). The authors concluded that the almost exclusive path of decay
(>99.6 %) was the direct hydrolysis of photoactivated methyl bromide
with the formation of methanol, bromide ion, protons, and a trace of
methane (<0.4%).
4.2.2.3 Reaction with the hydroxyl radical
Reaction of methyl bromide with the hydroxyl radical is believed
to be the primary mechanism for the removal of methyl bromide from the
lower troposphere (Singh et al., 1981).
Methyl bromide reacts slowly with the hydroxyl radical:
CH3Br + OH- -> CH2Br- + H2O
with a reaction rate constant of about 3 x 10-14 cm3/molecule per
second at 298 °K (NASA, 1992).
Table 17. Absorption cross sections of methyl bromidea
lambda 1020 sigma lambda 1020 sigma
(nm) (cm2) (nm) (cm2)
190 44 230 15
192 53 232 12
194 62 234 9.9
196 69 236 7.6
198 76 238 5.9
200 79 240 4.5
202 80 242 3.3
204 79 244 2.5
206 77 246 1.8
208 73 248 1.3
210 67 250 0.96
212 61 252 0.69
214 56 254 0.49
216 49 256 0.34
218 44 258 0.23
220 38 260 0.16
222 32
224 28
226 23
228 19
a Values recommended by NASA (1992) and taken from
Gillotay et al. (1989). These authors measured the
cross sections down to 210 K; for <210 nm, the
temperature effect is negligible.
Table 18 shows values for hydroxyl radical rate constants.
Table 18. Atmospheric reactions of methyl bromide
Reaction Rate constant Height above Reference
sea level
(km)
CH3Br + hv 1 x 10-11/s 16 Robbins (1976a)
1 x 10-9/s 20
1 x 10-6/s 30
5 x 10-5/sec 50
CH3Br + OH- 3 x 10-14 cm3/s (298 °K) NASA (1992)a
k(T) = 3.6 x 10-12 exp [-(1430±150) K/T] cm3/s
a Profiles of OH- and temperature of the stratosphere are given in NASA (1992).
Recent laboratory data for the rate coefficient of the reaction
of methyl bromide (Mellouki et al., 1992a,b) with the hydroxyl radical
together with the estimated distribution of the hydroxyl radical (WMO,
1992), gave an estimated OH--removal lifetime for methyl bromide of
2±0.5 years (UNEP, 1992).
4.2.2.4 Photolysis in the atmosphere
In the upper stratosphere, above 25 km, the photodissociation of
methyl bromide is the most dominant loss mechanism. Below this, as
less UV radiation is able to penetrate through the atmosphere, the
role of photolysis decreases. Between 20 and 25 km above sea level,
photodissociation is equally as important as loss by diffusion and
reaction with OH-. Below 20 km, down to the troposphere,
photodissociation becomes negligible and losses by diffusion and
reaction with OH- are of approximately equal importance (Robbins,
1976a) (Table 18).
The end-products of photodissociation of methyl bromide and
reactions with hydroxyl radicals in the atmosphere are probably carbon
dioxide, carbon monoxide, and bromide species (BUA, 1987).
4.2.3 Bioaccumulation
There are no experimental data on bioaccumulation potential. The
octanol/water partition coefficient (log Pow) of methyl bromide has
been given as 1.19 (Hansch & Leo, 1979; Sangster, 1989), so it
probably does not have any significant tendency to bioaccumulate (see
also section 4.1.5).
4.3 Interaction with other physical, chemical, or biological factors
It has been found that methyl bromide is able to diffuse through
certain plastics (Herzel & Schmidt, 1984).
A reaction is possible between methyl bromide and the following
materials ( Bond, 1984):
- iodized salt, stabilized with sodium hyposulfite;
- certain baking sodas, salt blocks used for cattle licks, or
foods containing reactive sulfur compounds;
- full fat soy flour;
- sponge rubber;
- foam rubber as used in rug padding, pillows, cushions, and
mattresses;
- rubber stamps and similar forms of reclaimed rubber;
- furs, horsehair, and pillows (especially feather pillows);
- leather goods tanned using a sulfur process;
- woollens, especially angora; some adverse effects have been
noted on woollen socks, sweaters, and yarn;
- viscose rayons, made by a process that uses carbon
disulfide;
- cinder blocks or mixtures of mortar; mixed concrete
occasionally picks up odours;
- charcoal, which not only becomes contaminated but absorbs
great amounts of methyl bromide and, thus, reduces effective
fumigant concentrations;
- paper that has been cured by a sulfide process and silver
polishing papers;
- photographic chemicals, not including films;
- rug padding, vinyl, cellophane;
- any other materials that may contain reactive sulfur
compounds.
4.4 Ultimate fate following use
4.4.1 Methyl bromide and the ozone layer
Methyl bromide from natural and anthropogenic sources is released
into the atmosphere. Once organic bromine compounds, such as methyl
bromide and the halons (section 5.1.1), enter the stratosphere, they
decompose to release bromine atoms. Fig. 2 depicts the key
bromine-containing species in the stratosphere and shows the
interconversion between reactive (Br- and BrO-) and reservoir
(HBr, HOBr, BrCl, and BrONO2) species (UNEP, 1992).
Yung et al. (1980) proposed the following reactions for ozone
loss due to bromine:
Br- + O3 -> BrO- + O2
Cl- + O3 -> ClO- + O2
BrO- + ClO- -> Br- + Cl- + O2
Net 2O3 -> 3O2
Ozone removal by bromine is far more efficient on a per molecule
basis than that by chlorine. This bromine-catalysed ozone removal in
the lower stratosphere is thought to occur via the reaction between
BrO- and ClO- (WMO, 1992) whereby the efficiency of
bromine-induced ozone loss increases with increasing abundance of
stratospheric chlorine. Bromine catalysis is most efficient in the
lower stratosphere where the ozone concentration is largest.
Reactive bromine has been detected directly in the stratosphere
in the polar regions, as well as OClO-, for which the only known
source is the reaction of BrO- with ClO-. Analyses of these
measurements indicate that the BrO- + ClO- catalytic cycle is
responsible for roughly 25% of the observed total ozone loss in the
appearance of the Antarctic ozone hole (an event mainly restricted to
Antarctica and to the months of September-November, involving
heterogenous reactions on polar stratospheric clouds) (WMO, 1992).
Degradation occurred by the methylation of carboxylic groups on
moist H-substituted peat. In soils containing other groups that can be
methylated, the mechanism and factors of methyl bromide degradation
are more complex.
Adsorption on to organic matter reduced the available
concentration of methyl bromide in the aqueous and gaseous phases of
soil. Organic matter was also the main factor involved in the
degradation of methyl bromide in the soil (Arvieu & Cuany, 1985).
4.2.2.2 Light-assisted hydrolysis in water
The UV-absorption cross sections for methyl bromide (174-262 nm)
have been confirmed by several authors (Robbins 1976b; Uthman et al.,
1978; Molina et al., 1982; Gillotay et al., 1989). The UV absorption
spectrum has a maximum of 202 nm with a steep decrease at longer
wavelengths reaching 0.2% of the maximum at 260 nm (Table 17). This is
much shorter than 290 nm, which is the shortest wavelength radiation
reaching the earth's surface from the sun.
It should be noted that the photoactivation of methyl bromide, as
well as its hydrolysis products in water or on soil surfaces, will
differ from the gas phase activities of these processes. The
hydrolysis products are in solution and differ energetically. Since
the species energy levels are changed from those of the isolated
molecule, critical absorption wavelengths would be shifted.
The influence of sunlight versus dark using natural waters in a
laboratory test showed little effect on the hydrolysis of methyl
bromide (Gentile et al., 1989, 1992).
Irradiation with a "pen-ray" low-pressure mercury lamp at 254 nm
gave a 6.6-fold increase in the rate constant. However, photolysis
does not alter the stoichiometry of the hydrolysis (Castro & Belser,
1981). The authors concluded that the almost exclusive path of decay
(>99.6 %) was the direct hydrolysis of photoactivated methyl bromide
with the formation of methanol, bromide ion, protons, and a trace of
methane (<0.4%).
4.2.2.3 Reaction with the hydroxyl radical
Reaction of methyl bromide with the hydroxyl radical is believed
to be the primary mechanism for the removal of methyl bromide from the
lower troposphere (Singh et al., 1981).
Methyl bromide reacts slowly with the hydroxyl radical:
CH3Br + OH- -> CH2Br- + H2O
with a reaction rate constant of about 3 x 10-14 cm3/molecule per
second at 298 °K (NASA, 1992).
Table 17. Absorption cross sections of methyl bromidea
lambda 1020 sigma lambda 1020 sigma
(nm) (cm2) (nm) (cm2)
190 44 230 15
192 53 232 12
194 62 234 9.9
196 69 236 7.6
198 76 238 5.9
200 79 240 4.5
202 80 242 3.3
204 79 244 2.5
206 77 246 1.8
208 73 248 1.3
210 67 250 0.96
212 61 252 0.69
214 56 254 0.49
216 49 256 0.34
218 44 258 0.23
220 38 260 0.16
222 32
224 28
226 23
228 19
a Values recommended by NASA (1992) and taken from
Gillotay et al. (1989). These authors measured the
cross sections down to 210 K; for <210 nm, the
temperature effect is negligible.
Table 18 shows values for hydroxyl radical rate constants.
Table 18. Atmospheric reactions of methyl bromide
Reaction Rate constant Height above Reference
sea level
(km)
CH3Br + hv 1 x 10-11/s 16 Robbins (1976a)
1 x 10-9/s 20
1 x 10-6/s 30
5 x 10-5/sec 50
CH3Br + OH- 3 x 10-14 cm3/s (298 °K) NASA (1992)a
k(T) = 3.6 x 10-12 exp [-(1430±150) K/T] cm3/s
a Profiles of OH- and temperature of the stratosphere are given in NASA (1992).
Recent laboratory data for the rate coefficient of the reaction
of methyl bromide (Mellouki et al., 1992a,b) with the hydroxyl radical
together with the estimated distribution of the hydroxyl radical (WMO,
1992), gave an estimated OH--removal lifetime for methyl bromide of
2±0.5 years (UNEP, 1992).
4.2.2.4 Photolysis in the atmosphere
In the upper stratosphere, above 25 km, the photodissociation of
methyl bromide is the most dominant loss mechanism. Below this, as
less UV radiation is able to penetrate through the atmosphere, the
role of photolysis decreases. Between 20 and 25 km above sea level,
photodissociation is equally as important as loss by diffusion and
reaction with OH-. Below 20 km, down to the troposphere,
photodissociation becomes negligible and losses by diffusion and
reaction with OH- are of approximately equal importance (Robbins,
1976a) (Table 18).
The end-products of photodissociation of methyl bromide and
reactions with hydroxyl radicals in the atmosphere are probably carbon
dioxide, carbon monoxide, and bromide species (BUA, 1987).
4.2.3 Bioaccumulation
There are no experimental data on bioaccumulation potential. The
octanol/water partition coefficient (log Pow) of methyl bromide has
been given as 1.19 (Hansch & Leo, 1979; Sangster, 1989), so it
probably does not have any significant tendency to bioaccumulate (see
also section 4.1.5).
4.3 Interaction with other physical, chemical, or biological factors
It has been found that methyl bromide is able to diffuse through
certain plastics (Herzel & Schmidt, 1984).
A reaction is possible between methyl bromide and the following
materials ( Bond, 1984):
- iodized salt, stabilized with sodium hyposulfite;
- certain baking sodas, salt blocks used for cattle licks, or
foods containing reactive sulfur compounds;
- full fat soy flour;
- sponge rubber;
- foam rubber as used in rug padding, pillows, cushions, and
mattresses;
- rubber stamps and similar forms of reclaimed rubber;
- furs, horsehair, and pillows (especially feather pillows);
- leather goods tanned using a sulfur process;
- woollens, especially angora; some adverse effects have been
noted on woollen socks, sweaters, and yarn;
- viscose rayons, made by a process that uses carbon
disulfide;
- cinder blocks or mixtures of mortar; mixed concrete
occasionally picks up odours;
- charcoal, which not only becomes contaminated but absorbs
great amounts of methyl bromide and, thus, reduces effective
fumigant concentrations;
- paper that has been cured by a sulfide process and silver
polishing papers;
- photographic chemicals, not including films;
- rug padding, vinyl, cellophane;
- any other materials that may contain reactive sulfur
compounds.
4.4 Ultimate fate following use
4.4.1 Methyl bromide and the ozone layer
Methyl bromide from natural and anthropogenic sources is released
into the atmosphere. Once organic bromine compounds, such as methyl
bromide and the halons (section 5.1.1), enter the stratosphere, they
decompose to release bromine atoms. Fig. 2 depicts the key
bromine-containing species in the stratosphere and shows the
interconversion between reactive (Br- and BrO-) and reservoir
(HBr, HOBr, BrCl, and BrONO2) species (UNEP, 1992).
Yung et al. (1980) proposed the following reactions for ozone
loss due to bromine:
Br- + O3 -> BrO- + O2
Cl- + O3 -> ClO- + O2
BrO- + ClO- -> Br- + Cl- + O2
Net 2O3 -> 3O2
Ozone removal by bromine is far more efficient on a per molecule
basis than that by chlorine. This bromine-catalysed ozone removal in
the lower stratosphere is thought to occur via the reaction between
BrO- and ClO- (WMO, 1992) whereby the efficiency of
bromine-induced ozone loss increases with increasing abundance of
stratospheric chlorine. Bromine catalysis is most efficient in the
lower stratosphere where the ozone concentration is largest.
Reactive bromine has been detected directly in the stratosphere
in the polar regions, as well as OClO-, for which the only known
source is the reaction of BrO- with ClO-. Analyses of these
measurements indicate that the BrO- + ClO- catalytic cycle is
responsible for roughly 25% of the observed total ozone loss in the
appearance of the Antarctic ozone hole (an event mainly restricted to
Antarctica and to the months of September-November, involving
heterogenous reactions on polar stratospheric clouds) (WMO, 1992).
 Another possible catalytic cycle is that between BrO- and HO2
(Poulet et al., 1992).
The Ozone Depletion Potential (ODP) represents the amount of
ozone destroyed by the emission of one kg of a chosen gas over a
particular time scale, compared with 1 kg of a reference molecule,
usually CFC-11 (Table 19). The amount of ozone depletion from methyl
bromide, and the ODP of the gas are dependent upon the atmospheric
abundance of chlorine. On the basis of current understanding, the
higher the abundance of chlorine, the higher the ODP of methyl bromide
and the amount of ozone depletion it causes.
Table 19. Lifetime and time-dependent depletion potential (ODP) for
methyl bromide (CH3Br) in comparison with chlorofluorcarbonsa
Speciesc Total Stratospheric Steady-state
atmospheric lifetime (year) empirical polar
lifetime (year) ODPb
CFC-11 55 55 1,0
(CFCl3)
CFC-113 110 110 1,10
(CFC12CF2Cl
CH3Br 2,0 35 0,7
a Values are relative to CFC-11 as the reference gas.
b ODP = ozone depletion potential.
c CFC = chlorofluorocarbon.
The key factors influencing the ODP of methyl bromide include:
(a) the atmospheric lifetime of the compound (relative to CFC-11);
(b) the release of reactive bromine from methyl bromide (relative to
CFC-11); and (c) the relative efficiency of reactive bromine for
ozone destruction compared with that of reactive chlorine (alpha
factor).
(a) Atmospheric lifetimes for methyl bromide have been
estimated at 1.6 years (WMO, 1992) and 2.0 years (UNEP,
1992). However, oceanic and terrestrial surface removal
processes have not been taken into account.
(b) As methyl bromide is a relatively short-lived molecule
within the stratosphere, it is expected that its release of
reactive bromine is faster than that of chlorine from
CFC-11.
(c) The current best estimate of alpha in the lower polar
stratosphere is about 40 (UNEP, 1992).
The current best estimate of the steady-state ODP for methyl
bromide from the semi-empirical approach and models including both
gas-phase and heterogeneous chemistry is 0.7 (UNEP, 1992). This
assumes its lifetime is solely determined by reaction with OH- in
the troposphere.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide is estimated to be about 3 pptv (UNEP,
1992). If anthropogenic methyl bromide is about 13-14% of total
atmospheric bromine (calculated by taking 25% of 11 pptv total methyl
bromide (UNEP, 1992) = 2.75% of total atmospheric bromine from
anthropogenic sources; and 55% of 20.8 pptv total organic bromine as
methyl bromide (Schauffler et al., in press) = 11%), the global ozone
loss due to anthropogenic methyl bromide is approximately 3%.
4.4.2 Containment, recovery, recycling, and disposal options for
methyl bromide
At present, after soil, commodity, and structural fumigation,
there is no special effort at containment of methyl bromide (see
section 3.2 for estimated emission rates from various fumigation
processes).
The methyl bromide industry is currently investigating practical
methods of recovering the gas by adsorption (using activated
charcoal), condensation, and scrubbing techniques. Most of these
methods are still in the research and development stage (UNEP, 1992).
When methyl bromide cylinders are refilled, instead of the present
practice of venting, it may be possible to recondense and recover the
gas. In this way, 1-2% of the total methyl bromide used could be
saved.
Methyl bromide is a toxic gas and the recommended waste disposal
method for large quantities is incineration by specialists. However,
incineration may be difficult to arrange safely unless an efficient
method of feeding the gas into the incinerator can be arranged.
Incineration requires dilution with additional fuel. If a suitably
designed combustion chamber is not available, return labelled
containers to supplier. Any release into the atmosphere should take
place in well-ventilated outdoor locations only.
All national and local regulations should be observed when
disposing of methyl bromide.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.1.1 Global abundance
The current best estimate for the global abundance of methyl
bromide in the troposphere is between 9 and 13 pptv (section 3.1 and
Tables 20 and 21) giving a total global burden of 150-220 million kg
(UNEP, 1992). From observations taken from the surface waters of the
Pacific, off North and South America, Singh et al. (1983) had
previously estimated the total natural emission of methyl bromide from
the oceans at 300 million kg/year with a residence time of 1.2 years.
This estimate is probably too high because of the difficulties with
absolute calibration.
The most abundant bromine source gas is methyl bromide, which
arises from both natural and anthropogenic sources (WMO, 1992; also
sections 3.1 and 3.2). The main natural sources of methyl bromide are
oceanic biological processes (mainly algal) where it is formed
together with bromoform (CHBr3), methylene bromide (CH2Br2),
CH2BrCl, and CHBrCl2 (WMO, 1992). Apart from methyl bromide, other
anthropogenic sources are the halons, CBrF3 (CFC-13B1; Halon 1301),
CBrClF2 (CFC-12B1; Halon 1211), and C2Br2F4 (Halon 2402),
which are used as special purpose fire extinguishers (Singh et al.,
1988; Schauffler et al., in press).
The atmospheric abundance of methyl bromide appears to be
1.3±0.15 greater in the Northern than in the Southern hemisphere
(Tables 20, 21) indicating an excess source in the Northern
hemisphere. The major source of methyl chloride is also thought to be
oceanic, but the atmospheric abundance of this gas appears to be
comparable in both hemispheres (UNEP, 1992). Some atmospheric models
compute the interhemispheric OH- concentration ratio
(OHN-/OHS-) to be about 0.8 (Tie et al., 1992). Assuming
reaction with OH- is the major tropospheric destructive process for
methyl bromide, this southern excess of OH- could largely explain
the lower southern atmospheric concentration of methyl bromide. In
contrast, Spivakovsky et al. (1990), using a model based on different
assumptions, calculated a higher OH- concentration in the northern
than in the southern hemisphere.
Ozone loss, catalysed by bromine, occurs mainly in the lower
stratosphere.The primary cause of the Antarctic ozone hole is most
certainly halogen chemistry (WMO, 1992). Bromine is now believed to
have a greater potential per molecule to destroy stratospheric ozone
than chlorine (section 4.4).
The upward mass transfer of air from the troposphere to the
stratosphere occurs mainly in the tropical latitudes between 30 °S and
30 °N (WMO, 1992). Schauffler et al. (in press) give the mixing ratios
of brominated compounds and total organic bromine from 12 samples
collected at the tropical tropopause (altitude about 16 km) from
January to March, 1992. The mean mixing ratio of total organic bromine
was 20.8±0.8 pptv. Methyl bromide was found to contribute 55% of the
total organic bromine, CH2Br2, about 7%, and the remaining 38% was
nearly evenly distributed between Halon 1302, Halon 1211, and Halon
2402.
It is against this background of the role of bromine in the ozone
depletion in the stratosphere that there has been recent concern about
levels of methyl bromide in the atmosphere and the potential effect of
the continuing use of this fumigant (see also sections 3.1, 3.2, 4.4).
5.1.1.2 Measured oceanic and coastal air levels of methyl bromide
Table 20 shows levels of methyl bromide in oceanic areas,
measured by ground-based, aircraft, and balloon techniques. The
differences between the individual readings do not seem to be due to
trends in abundance over the last decade or to seasonal variation, but
more likely to variations in calibration (UNEP, 1992). An abundance of
methyl bromide in the atmosphere of 9-13 pptv is equivalent to a total
global burden of 150-220 million kg.
Although there are differences in the values reported for the
absolute magnitudes of the observed methyl bromide abundances, ranging
from 10-26 pptv in the Northern hemisphere and 8-20 pptv in the
Southern hemisphere, the N/S ratio is almost constant at 1.3 (Table
20). The greater abundance of methyl bromide in the Northern than the
Southern hemisphere has been confirmed by all studies published up to
now. This is possible evidence for the anthropogenic addition of
methyl bromide to the atmosphere from its use as a fumigant (see also
sections 3.1, 3.2, and 4.4) as well as from motor vehicle exhaust.
Methyl bromide concentrations in the atmosphere are summarized in
Table 21, comparing oceanic and continental values with those in urban
areas.
Table 20. Atmospheric abundance of methyl bromide at, or near, ground level (pptv)a
Source Time Northern Southern N/S ratio Observational Reference
period hemisphere hemisphere platform
Singh 1981 26 20 1.3 Ship Singh et al. (1983)
Penkett 1982-83 15 11 1.4 Ship Penkett et al. (1985)
Rasmussen 1983-92 11 8 1.3 Coastal land MBSW (1992)
Cicerone 1985-87 12 10 1.2 Coastal land Cicerone, et al. (1988)
Rowland 1991 10 8 1.2 Coastal land MBSW (1992)
Heidt 1991 14 - - Aircraft MBSW (1992)
a From: UNEP (1992).
b MBSW = Methyl Bromide Science Workshop (1992).
Measurements by Singh et al. (1983) of air and seawater
concentrations of methyl halides in, and over, the Eastern Pacific
(40° N-32° S) gave average concentrations of 90 ng/m3 (23 pptv) in
air. However, there was a considerable difference between the readings
in the northern (101 ng/m3 [26 pptv]) compared with the southern (74
ng/m3 [19 pptv]) hemisphere. Because of the large scattering of
values, a seasonal trend was not identifiable. As mentioned in section
3.1, it is possible that calibration standards were in error, which
would explain the higher values compared with those of other authors.
Rasmussen & Khalil (1984) found that the highest concentrations
of methyl bromide in Arctic and Arctic haze were found in the summer
(average - 48.2 ng/n3 (12.4 pptv)) compared with the autumn and
winter (average - 39.7 ng/m3 (10.2 pptv)), and, that methyl bromide
did not show the same seasonal patterns as other bromine-containing
trace gases.
Penkett et al. (1985) measured four different bromine compounds,
including methyl bromide, over a large latitudinal range (40° N to 75°
S). They found that there was a clear reduction in concentrations
between the two hemispheres, the average concentration of methyl
bromide in the Northern Hemisphere being 60±7 ng/m3 (15.4±1.9 pptv)
compared with 41.2±3.5 ng/m3 (10.6±0.9 pptv) in the Southern
Hemisphere.
Table 21. Measured ambient concentrations of methyl bromide
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Oceanic and coastal air levels
Eastern Pacific 40°N 26 (101) Singh et al. (1983)
Ocean 32°S 19 (74)
Arctic 72°N 11.3 (44) Rasmussen & Khalil
(1984);
Berg et al. (1984)
90°S 7.5 Khalil & Rasmussen (1985)
Atlantic Ocean 40°N 15.4 (60) Penkett et al. (1985)
Southern Ocean 75°S 10.6 (41)
Alaska 71°N 11.16 (43) Cicerone et al. (1988)
Hawaii 20°N 10.75 (42)
Samoa 14°S 10.23 (40)
Tasmania 44°S 9.58 (37)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Alaska 71°N 14.1 Khalil et al. (1993)c
Oregon 45°N 12.6
Hawaii 19°N 11.4
Samoa 14°S 8.5
Tasmania 42°S 7.5
Antarctic 64.5°S 9.0
Continental air levels
USA
Pullman, Washington < 10-220 Harsch & Rasmussen
(< 40-870) (1977)
Los Angeles 244 (950) 894 (3480) Singh et al. (1981)
Oakland 55 (214) 108 (420)
Phoenix 67 (261) 190 (740)
Badger Pass 5 (19) 8 (31) Brodzinsky & Singh
Denver 120 (467) 190 (740) (1983)
Houston 100 (390) 170 (660)
Jetmore 5 (19) 5 (19)
Los Angelesb 150 (584) 580 (2256)
Menlo Park 16 (62) 16 (62)
Mill Valley 25 (97) 25 (97)
Oaklandb 55 (214) 78 (303)
Palm Springs 24 (93) 24 (93)
Phoenixb 67 (261) 120 (467) Brodzinsky & Singh
Point Arena 17 (66) 20 (78) (1983)
Point Reyes 93 (362) 93 (362)
Reese River 5 (19) 5 (19)
Riverside 250 (973) 560 (2180)
San Jose 31 (120) 31 (120)
St. Louis 81 (315) 100 (390)
San Jose (California, USA) Singh et al. (1992)
[4-16 April 1985] 400
(44-4661)
[12-24 August 1985] 121
(5-1067)
[13-21 December 1985] 2869
(239-15 424)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Downey, California, USA
[8-27 February 1984] 212
(18-815)
Houston, Texas, USA
[9-17 March 1984] 23
(11-48)
Denver, CO, USA
[24 March-1 April 1984] 22
(13-64)
The Netherlands
Delft, Vlaardingen 50-200 100-900 Guicherit & Schulting
and Terschellingd (195-780) (390-3500) (1985)
Japan
urban and suburban 15-31 JEA (1981)
(59-122)
a The converted values in brackets ( ) are only approximate.
b Includes substantial overlap with data reported in Singh et al. (1981).
c Values given from spring 1991. Fig. 3 gives averages over the last decade.
d About 350 samples per site during 1989.
Approximately 750 air samples from five surface sampling sites in
Alaska, Hawaii (2), Samoa, and New Zealand were analysed by Cicerone
et al. (1988) for methyl bromide between January 1985 and October 1987
(using GC/MS). Methyl bromide concentrations were typically 40-44
ng/m3 (10-11 pptv).
Khalil et al. (1993) presented a series of 1700 measurements
showing the latitudinal distribution of atmospheric methyl bromide
from 1983 to 1992 (Table 21 and Fig. 3). The levels of atmospheric
methyl bromide measured between 1988 and 1992 seemed to be higher than
those measured between 1983 and 1988.
Another possible catalytic cycle is that between BrO- and HO2
(Poulet et al., 1992).
The Ozone Depletion Potential (ODP) represents the amount of
ozone destroyed by the emission of one kg of a chosen gas over a
particular time scale, compared with 1 kg of a reference molecule,
usually CFC-11 (Table 19). The amount of ozone depletion from methyl
bromide, and the ODP of the gas are dependent upon the atmospheric
abundance of chlorine. On the basis of current understanding, the
higher the abundance of chlorine, the higher the ODP of methyl bromide
and the amount of ozone depletion it causes.
Table 19. Lifetime and time-dependent depletion potential (ODP) for
methyl bromide (CH3Br) in comparison with chlorofluorcarbonsa
Speciesc Total Stratospheric Steady-state
atmospheric lifetime (year) empirical polar
lifetime (year) ODPb
CFC-11 55 55 1,0
(CFCl3)
CFC-113 110 110 1,10
(CFC12CF2Cl
CH3Br 2,0 35 0,7
a Values are relative to CFC-11 as the reference gas.
b ODP = ozone depletion potential.
c CFC = chlorofluorocarbon.
The key factors influencing the ODP of methyl bromide include:
(a) the atmospheric lifetime of the compound (relative to CFC-11);
(b) the release of reactive bromine from methyl bromide (relative to
CFC-11); and (c) the relative efficiency of reactive bromine for
ozone destruction compared with that of reactive chlorine (alpha
factor).
(a) Atmospheric lifetimes for methyl bromide have been
estimated at 1.6 years (WMO, 1992) and 2.0 years (UNEP,
1992). However, oceanic and terrestrial surface removal
processes have not been taken into account.
(b) As methyl bromide is a relatively short-lived molecule
within the stratosphere, it is expected that its release of
reactive bromine is faster than that of chlorine from
CFC-11.
(c) The current best estimate of alpha in the lower polar
stratosphere is about 40 (UNEP, 1992).
The current best estimate of the steady-state ODP for methyl
bromide from the semi-empirical approach and models including both
gas-phase and heterogeneous chemistry is 0.7 (UNEP, 1992). This
assumes its lifetime is solely determined by reaction with OH- in
the troposphere.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide is estimated to be about 3 pptv (UNEP,
1992). If anthropogenic methyl bromide is about 13-14% of total
atmospheric bromine (calculated by taking 25% of 11 pptv total methyl
bromide (UNEP, 1992) = 2.75% of total atmospheric bromine from
anthropogenic sources; and 55% of 20.8 pptv total organic bromine as
methyl bromide (Schauffler et al., in press) = 11%), the global ozone
loss due to anthropogenic methyl bromide is approximately 3%.
4.4.2 Containment, recovery, recycling, and disposal options for
methyl bromide
At present, after soil, commodity, and structural fumigation,
there is no special effort at containment of methyl bromide (see
section 3.2 for estimated emission rates from various fumigation
processes).
The methyl bromide industry is currently investigating practical
methods of recovering the gas by adsorption (using activated
charcoal), condensation, and scrubbing techniques. Most of these
methods are still in the research and development stage (UNEP, 1992).
When methyl bromide cylinders are refilled, instead of the present
practice of venting, it may be possible to recondense and recover the
gas. In this way, 1-2% of the total methyl bromide used could be
saved.
Methyl bromide is a toxic gas and the recommended waste disposal
method for large quantities is incineration by specialists. However,
incineration may be difficult to arrange safely unless an efficient
method of feeding the gas into the incinerator can be arranged.
Incineration requires dilution with additional fuel. If a suitably
designed combustion chamber is not available, return labelled
containers to supplier. Any release into the atmosphere should take
place in well-ventilated outdoor locations only.
All national and local regulations should be observed when
disposing of methyl bromide.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
5.1.1.1 Global abundance
The current best estimate for the global abundance of methyl
bromide in the troposphere is between 9 and 13 pptv (section 3.1 and
Tables 20 and 21) giving a total global burden of 150-220 million kg
(UNEP, 1992). From observations taken from the surface waters of the
Pacific, off North and South America, Singh et al. (1983) had
previously estimated the total natural emission of methyl bromide from
the oceans at 300 million kg/year with a residence time of 1.2 years.
This estimate is probably too high because of the difficulties with
absolute calibration.
The most abundant bromine source gas is methyl bromide, which
arises from both natural and anthropogenic sources (WMO, 1992; also
sections 3.1 and 3.2). The main natural sources of methyl bromide are
oceanic biological processes (mainly algal) where it is formed
together with bromoform (CHBr3), methylene bromide (CH2Br2),
CH2BrCl, and CHBrCl2 (WMO, 1992). Apart from methyl bromide, other
anthropogenic sources are the halons, CBrF3 (CFC-13B1; Halon 1301),
CBrClF2 (CFC-12B1; Halon 1211), and C2Br2F4 (Halon 2402),
which are used as special purpose fire extinguishers (Singh et al.,
1988; Schauffler et al., in press).
The atmospheric abundance of methyl bromide appears to be
1.3±0.15 greater in the Northern than in the Southern hemisphere
(Tables 20, 21) indicating an excess source in the Northern
hemisphere. The major source of methyl chloride is also thought to be
oceanic, but the atmospheric abundance of this gas appears to be
comparable in both hemispheres (UNEP, 1992). Some atmospheric models
compute the interhemispheric OH- concentration ratio
(OHN-/OHS-) to be about 0.8 (Tie et al., 1992). Assuming
reaction with OH- is the major tropospheric destructive process for
methyl bromide, this southern excess of OH- could largely explain
the lower southern atmospheric concentration of methyl bromide. In
contrast, Spivakovsky et al. (1990), using a model based on different
assumptions, calculated a higher OH- concentration in the northern
than in the southern hemisphere.
Ozone loss, catalysed by bromine, occurs mainly in the lower
stratosphere.The primary cause of the Antarctic ozone hole is most
certainly halogen chemistry (WMO, 1992). Bromine is now believed to
have a greater potential per molecule to destroy stratospheric ozone
than chlorine (section 4.4).
The upward mass transfer of air from the troposphere to the
stratosphere occurs mainly in the tropical latitudes between 30 °S and
30 °N (WMO, 1992). Schauffler et al. (in press) give the mixing ratios
of brominated compounds and total organic bromine from 12 samples
collected at the tropical tropopause (altitude about 16 km) from
January to March, 1992. The mean mixing ratio of total organic bromine
was 20.8±0.8 pptv. Methyl bromide was found to contribute 55% of the
total organic bromine, CH2Br2, about 7%, and the remaining 38% was
nearly evenly distributed between Halon 1302, Halon 1211, and Halon
2402.
It is against this background of the role of bromine in the ozone
depletion in the stratosphere that there has been recent concern about
levels of methyl bromide in the atmosphere and the potential effect of
the continuing use of this fumigant (see also sections 3.1, 3.2, 4.4).
5.1.1.2 Measured oceanic and coastal air levels of methyl bromide
Table 20 shows levels of methyl bromide in oceanic areas,
measured by ground-based, aircraft, and balloon techniques. The
differences between the individual readings do not seem to be due to
trends in abundance over the last decade or to seasonal variation, but
more likely to variations in calibration (UNEP, 1992). An abundance of
methyl bromide in the atmosphere of 9-13 pptv is equivalent to a total
global burden of 150-220 million kg.
Although there are differences in the values reported for the
absolute magnitudes of the observed methyl bromide abundances, ranging
from 10-26 pptv in the Northern hemisphere and 8-20 pptv in the
Southern hemisphere, the N/S ratio is almost constant at 1.3 (Table
20). The greater abundance of methyl bromide in the Northern than the
Southern hemisphere has been confirmed by all studies published up to
now. This is possible evidence for the anthropogenic addition of
methyl bromide to the atmosphere from its use as a fumigant (see also
sections 3.1, 3.2, and 4.4) as well as from motor vehicle exhaust.
Methyl bromide concentrations in the atmosphere are summarized in
Table 21, comparing oceanic and continental values with those in urban
areas.
Table 20. Atmospheric abundance of methyl bromide at, or near, ground level (pptv)a
Source Time Northern Southern N/S ratio Observational Reference
period hemisphere hemisphere platform
Singh 1981 26 20 1.3 Ship Singh et al. (1983)
Penkett 1982-83 15 11 1.4 Ship Penkett et al. (1985)
Rasmussen 1983-92 11 8 1.3 Coastal land MBSW (1992)
Cicerone 1985-87 12 10 1.2 Coastal land Cicerone, et al. (1988)
Rowland 1991 10 8 1.2 Coastal land MBSW (1992)
Heidt 1991 14 - - Aircraft MBSW (1992)
a From: UNEP (1992).
b MBSW = Methyl Bromide Science Workshop (1992).
Measurements by Singh et al. (1983) of air and seawater
concentrations of methyl halides in, and over, the Eastern Pacific
(40° N-32° S) gave average concentrations of 90 ng/m3 (23 pptv) in
air. However, there was a considerable difference between the readings
in the northern (101 ng/m3 [26 pptv]) compared with the southern (74
ng/m3 [19 pptv]) hemisphere. Because of the large scattering of
values, a seasonal trend was not identifiable. As mentioned in section
3.1, it is possible that calibration standards were in error, which
would explain the higher values compared with those of other authors.
Rasmussen & Khalil (1984) found that the highest concentrations
of methyl bromide in Arctic and Arctic haze were found in the summer
(average - 48.2 ng/n3 (12.4 pptv)) compared with the autumn and
winter (average - 39.7 ng/m3 (10.2 pptv)), and, that methyl bromide
did not show the same seasonal patterns as other bromine-containing
trace gases.
Penkett et al. (1985) measured four different bromine compounds,
including methyl bromide, over a large latitudinal range (40° N to 75°
S). They found that there was a clear reduction in concentrations
between the two hemispheres, the average concentration of methyl
bromide in the Northern Hemisphere being 60±7 ng/m3 (15.4±1.9 pptv)
compared with 41.2±3.5 ng/m3 (10.6±0.9 pptv) in the Southern
Hemisphere.
Table 21. Measured ambient concentrations of methyl bromide
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Oceanic and coastal air levels
Eastern Pacific 40°N 26 (101) Singh et al. (1983)
Ocean 32°S 19 (74)
Arctic 72°N 11.3 (44) Rasmussen & Khalil
(1984);
Berg et al. (1984)
90°S 7.5 Khalil & Rasmussen (1985)
Atlantic Ocean 40°N 15.4 (60) Penkett et al. (1985)
Southern Ocean 75°S 10.6 (41)
Alaska 71°N 11.16 (43) Cicerone et al. (1988)
Hawaii 20°N 10.75 (42)
Samoa 14°S 10.23 (40)
Tasmania 44°S 9.58 (37)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Alaska 71°N 14.1 Khalil et al. (1993)c
Oregon 45°N 12.6
Hawaii 19°N 11.4
Samoa 14°S 8.5
Tasmania 42°S 7.5
Antarctic 64.5°S 9.0
Continental air levels
USA
Pullman, Washington < 10-220 Harsch & Rasmussen
(< 40-870) (1977)
Los Angeles 244 (950) 894 (3480) Singh et al. (1981)
Oakland 55 (214) 108 (420)
Phoenix 67 (261) 190 (740)
Badger Pass 5 (19) 8 (31) Brodzinsky & Singh
Denver 120 (467) 190 (740) (1983)
Houston 100 (390) 170 (660)
Jetmore 5 (19) 5 (19)
Los Angelesb 150 (584) 580 (2256)
Menlo Park 16 (62) 16 (62)
Mill Valley 25 (97) 25 (97)
Oaklandb 55 (214) 78 (303)
Palm Springs 24 (93) 24 (93)
Phoenixb 67 (261) 120 (467) Brodzinsky & Singh
Point Arena 17 (66) 20 (78) (1983)
Point Reyes 93 (362) 93 (362)
Reese River 5 (19) 5 (19)
Riverside 250 (973) 560 (2180)
San Jose 31 (120) 31 (120)
St. Louis 81 (315) 100 (390)
San Jose (California, USA) Singh et al. (1992)
[4-16 April 1985] 400
(44-4661)
[12-24 August 1985] 121
(5-1067)
[13-21 December 1985] 2869
(239-15 424)
Table 21 (continued)
Location Concentration pptv (ng/m3)a Reference
mean max
or range
Downey, California, USA
[8-27 February 1984] 212
(18-815)
Houston, Texas, USA
[9-17 March 1984] 23
(11-48)
Denver, CO, USA
[24 March-1 April 1984] 22
(13-64)
The Netherlands
Delft, Vlaardingen 50-200 100-900 Guicherit & Schulting
and Terschellingd (195-780) (390-3500) (1985)
Japan
urban and suburban 15-31 JEA (1981)
(59-122)
a The converted values in brackets ( ) are only approximate.
b Includes substantial overlap with data reported in Singh et al. (1981).
c Values given from spring 1991. Fig. 3 gives averages over the last decade.
d About 350 samples per site during 1989.
Approximately 750 air samples from five surface sampling sites in
Alaska, Hawaii (2), Samoa, and New Zealand were analysed by Cicerone
et al. (1988) for methyl bromide between January 1985 and October 1987
(using GC/MS). Methyl bromide concentrations were typically 40-44
ng/m3 (10-11 pptv).
Khalil et al. (1993) presented a series of 1700 measurements
showing the latitudinal distribution of atmospheric methyl bromide
from 1983 to 1992 (Table 21 and Fig. 3). The levels of atmospheric
methyl bromide measured between 1988 and 1992 seemed to be higher than
those measured between 1983 and 1988.
 5.1.1.3 Measured continental and urban levels of methyl bromide
Data from seven cities in the USA showed significant elevations
of methyl bromide levels in urban areas with average concentrations of
159-1004 ng/m3 (41-259 pptv) (Singh et al., 1982). Concentrations as
high as 4000 ng/m3 were also reported. A further study of methyl
bromide levels at 16 sites in the USA confirmed these findings,
showing that the larger the city the higher the methyl bromide level
(Brodzinsky & Singh, 1983). Methyl bromide is emitted from motor
vehicles using leaded petrol. Since many of these urban studies were
made, unleaded petrol has been introduced, which could mean that
current urban levels of methyl bromide would be lower. Some background
levels of 20 ng/m3 (5 pptv) appear to be lower than oceanic values
(Singh et al., 1981).
The high values reported for 1984/85 by Singh (1992) in
California (Table 21) may have been influenced by nearby soil
fumigation.
In a 1980 environmental survey of methyl bromide levels in
Japanese urban and suburban areas, the gas was detected in 5 out of 27
samples with levels ranging from 59 to 122 ng/m3 (15 to 31 pptv)
(JEA, 1981). In the Netherlands, a study of the concentrations of
methyl bromide in ambient air was carried out by Guicherit & Schulting
(1985). Between 1979 and 1981, air samples were measured in three
locations: at the island of Terschelling in the north (little
pollution); at Delft, a small city in the densely populated western
part of The Netherlands, and in Vlaardingen, in a heavily
industrialized area near Rotterdam. The study gave an average of
195-778 ng/m3 (50-200 pptv) and a maximum 1-h concentration of
390-3500 ng/m3 (100-900 pptv). The estimated daily (10.0 µg) and
yearly (4.5 µg) average exposure values given were based on a total
respiratory volume of 20 m3 per day.
5.1.1.4 Vertical profiles of methyl bromide in the atmosphere
Data on vertical levels of methyl bromide lead to a better
understanding of the ultimate fate of methyl bromide in the atmosphere
and its role in ozone reduction in the lower stratosphere (section
4.4). Rasmussen & Khalil (1984) and Berg et al. (1984) showed no
significant decrease in methyl bromide levels up to 7 km. Penkett et
al. (1985) detected a methyl bromide level of 40 ng/m3 (10 pptv) in
the upper troposphere. As shown in Fig. 4, Fabian et al. (1981)
measured 1.2 pptv at 14.4 km, but could not detect any methyl bromide
at an altitude of 20 km. It has been suggested (UNEP, 1992) that there
is no significant vertical gradient in the troposphere, but that the
level of methyl bromide decreases rapidly in the lower stratosphere
(by at least a factor of 3-5 within 10 km above the tropopause). It is
here that ozone loss occurs. Schauffler et al. (in press) gave methyl
bromide levels in the mid to low troposphere of 13.6±1.4 pptv (37-45°
N; 69° W) and 12.7±1.1 (22-26° N; 94-97° W). In the tropical
tropopause (altitude 15.3-16.76 km; 24° N, 68-85° W), levels of
11.4±0.5 pptv (mean of 12 samples) were measured.
5.1.1.3 Measured continental and urban levels of methyl bromide
Data from seven cities in the USA showed significant elevations
of methyl bromide levels in urban areas with average concentrations of
159-1004 ng/m3 (41-259 pptv) (Singh et al., 1982). Concentrations as
high as 4000 ng/m3 were also reported. A further study of methyl
bromide levels at 16 sites in the USA confirmed these findings,
showing that the larger the city the higher the methyl bromide level
(Brodzinsky & Singh, 1983). Methyl bromide is emitted from motor
vehicles using leaded petrol. Since many of these urban studies were
made, unleaded petrol has been introduced, which could mean that
current urban levels of methyl bromide would be lower. Some background
levels of 20 ng/m3 (5 pptv) appear to be lower than oceanic values
(Singh et al., 1981).
The high values reported for 1984/85 by Singh (1992) in
California (Table 21) may have been influenced by nearby soil
fumigation.
In a 1980 environmental survey of methyl bromide levels in
Japanese urban and suburban areas, the gas was detected in 5 out of 27
samples with levels ranging from 59 to 122 ng/m3 (15 to 31 pptv)
(JEA, 1981). In the Netherlands, a study of the concentrations of
methyl bromide in ambient air was carried out by Guicherit & Schulting
(1985). Between 1979 and 1981, air samples were measured in three
locations: at the island of Terschelling in the north (little
pollution); at Delft, a small city in the densely populated western
part of The Netherlands, and in Vlaardingen, in a heavily
industrialized area near Rotterdam. The study gave an average of
195-778 ng/m3 (50-200 pptv) and a maximum 1-h concentration of
390-3500 ng/m3 (100-900 pptv). The estimated daily (10.0 µg) and
yearly (4.5 µg) average exposure values given were based on a total
respiratory volume of 20 m3 per day.
5.1.1.4 Vertical profiles of methyl bromide in the atmosphere
Data on vertical levels of methyl bromide lead to a better
understanding of the ultimate fate of methyl bromide in the atmosphere
and its role in ozone reduction in the lower stratosphere (section
4.4). Rasmussen & Khalil (1984) and Berg et al. (1984) showed no
significant decrease in methyl bromide levels up to 7 km. Penkett et
al. (1985) detected a methyl bromide level of 40 ng/m3 (10 pptv) in
the upper troposphere. As shown in Fig. 4, Fabian et al. (1981)
measured 1.2 pptv at 14.4 km, but could not detect any methyl bromide
at an altitude of 20 km. It has been suggested (UNEP, 1992) that there
is no significant vertical gradient in the troposphere, but that the
level of methyl bromide decreases rapidly in the lower stratosphere
(by at least a factor of 3-5 within 10 km above the tropopause). It is
here that ozone loss occurs. Schauffler et al. (in press) gave methyl
bromide levels in the mid to low troposphere of 13.6±1.4 pptv (37-45°
N; 69° W) and 12.7±1.1 (22-26° N; 94-97° W). In the tropical
tropopause (altitude 15.3-16.76 km; 24° N, 68-85° W), levels of
11.4±0.5 pptv (mean of 12 samples) were measured.
 Fig. 4 shows the vertical profile for methyl bromide (Schauffler
et al., in press). Fig. 5 shows the vertical profile of methyl bromide
together with other atmospheric source gases in the middle Northern
hemisphere from air samples taken from the troposphere and
stratosphere (Fabian, 1984).
Fig. 4 shows the vertical profile for methyl bromide (Schauffler
et al., in press). Fig. 5 shows the vertical profile of methyl bromide
together with other atmospheric source gases in the middle Northern
hemisphere from air samples taken from the troposphere and
stratosphere (Fabian, 1984).
 5.1.1.5 Release of methyl bromide to the outside air from greenhouses
During 1986, 400 000 kg methyl bromide were emitted into the air
in the Westland area of the Netherlands (Van Doorn et al., 1989).
Although soil disinfection is only allowed when the diluting capacity
of the atmosphere (wind force) is sufficient, this is very difficult
or impossible to control. Investigations by Netherlands Institutes
(TNO and RIVM) showed that the hourly average concentrations within a
20-m distance from the green-houses in 1981 (after the introduction of
gas-tight film) during the first hours was 5.9 mg/m3 after
fumigation with methyl bromide at a dose of 700 kg/ha. In 1982 and
1983, a few hours after injection and at a probable dosage of 400
kg/ha, concentrations measured ranged between 1 and 4 mg/m3 at
distances of 20 m. After the first few hours, the concentration of
methyl bromide decreased rapidly and, after 4 days, also at 20 m,
hourly averages of 0.2 mg/m3 were measured. Ten days after
fumigation, the covering foil was removed, after which the hourly
average concentration increased to a maximum of 0.4 mg/m3 (Van Doorn
et al., 1989).
5.1.2 Water
5.1.2.1 Seawater
In 1975, samples of seawater from near the shore at Dorset,
England were analysed for halomethanes and methyl bromide was detected
at levels ranging from 2.0 to 3.9 x 10-9 ml gas/ml water [apprx. 10
ng/litre] (Lovelock, 1975).
Average concentrations of 1.2 ng methyl bromide/litre were
measured in surface seawater in the Eastern Pacific Ocean (Singh et
al., 1983). The authors estimated that the oceans are supersaturated
with methyl bromide to 250%.
Khalil et al. (1993) measured methyl bromide concentrations in
ocean water in two open-ocean surveys, one covering latitudes from 45°
N to about 30° S, the other from 67° N to 50° S. On the first survey,
they found supersaturation of methyl bromide at 180% and, on the
second, 140%.
5.1.2.2 Inland waters
In 1988, the California Department of Food and Agriculture (CDFA)
reported the results of analyses of 43 056 well water samples taken
from 2977 wells in various Californian counties. Residues of 10
chemicals were detected. Methyl bromide detection was undertaken in 32
counties, but in all the wells tested only one sample showed the
presence of methyl bromide (CDFA, 1988).
5.1.2.3 Waters around greenhouses
In the Netherlands, where intensive horticulture in greenhouses
is practised, the soil level is lower than sea level in some areas,
with the result that water transport is very slow and the static water
volume is very high. There is a high density of greenhouses and all
holdings have to use the water from ditches as leaching water, thus,
this water is successively loaded with bromide. However, it should be
noted that, in 1992, methyl bromide was banned in the Netherlands for
soil fumigation purposes.
The concentrations of methyl bromide and bromide-ion were
measured in irrigation water, drainage water, and surface water during
the leaching periods in two Netherlands glasshouse soils after
fumigation with methyl bromide (Wegman et al., 1981). Maximum
concentrations in drainage water, determined within 24 h of the start
of leaching, were 9.3 mg/litre (methyl bromide) and 72 mg/litre
(Br-).
Further studies of bromide ion concentrations in precipitation,
surface water, and ground water in the polder district in the
Netherlands (a main horticultural area) in 1979-80 gave maximum values
of 0.98, 41, and 17 mg/litre respectively, the highest concentrations
being found during the main fumigation/leaching time in
September-October 1979 (Wegman et al., 1983). During these few weeks,
an estimated 1.78 million kg methyl bromide (about 1.5 million kg
bromide ion) were used for soil fumigation. From their measurements,
Wegman et al. (1983) calculated that 14% of the applied methyl bromide
was converted to bromide ion. This is in agreement with Daelemans
(1978), whose studies showed that 10-30% of methyl bromide is
converted to Br-. The fumigation method used was the "hot gas
method", whereby the fumigant was discharged under thin low-density
polyethylene (LDPE) sheeting. Subsequently, in June 1981, the use of
LDPE for this purpose was forbidden.
In 1985, bromide concentrations of 10-35 mg/litre were observed
in Westland (the Netherlands) surface water with maximum
concentrations in the Poel and Bosch polders of 38.7 and 31.7
mg/litre, respectively (Van Doorn et al., 1989). Private water
supplies from shallow pumps in the Netherlands near methyl bromide
soil operations were expected to have increased bromide contents (Van
Doorn et al., 1989). In 1986, in the Westland, 260 000 kg bromide
ended up in surface water, at an average dosage of 700 kg/ha.
Similar investigations were carried out by Vanachter et al.
(1981) in the glasshouse crop growing region of Malines - Antwerp
(Belgium) into bromide concentrations in surface water in periods of
intense soil fumigation and leaching (August-September) compared with
periods before and after this, when soil fumigation and leaching were
less frequent. Sampling was also carried out during leaching, near the
greenhouses, as well as in the ditches and draining water. For
comparison, samples of natural Br- concentrations in surface water
were taken from a region where methyl bromide fumigation was not
carried out.
The results showed that during the leaching period,
concentrations of Br- in the surface water in the greenhouse crop
growing area were significantly higher (maximum 9.6 mg/litre) but,
five weeks later, were either not detectable (<0.1 mg/litre) or very
low (1.59 mg/litre) in little brooks. The bromide concentration in
rivers was less than 0.8 mg/litre, except for one site, where either
sea water or the effluents from a local photographic plant resulted in
concentrations of up to 4.5 mg/litre. In the direct drainage area of
the glass houses, transient concentrations of up to 33 mg/litre were
measured, decreasing rapidly within a few days.
Guns (1989) measured groundwater levels in four greenhouses in
Belgium from September 1986 to August 1988. The bromide content of
groundwater depended, not only on the length of leaching, but also on
the type of soil. When the deeper soil layers contained more clay, the
concentration of the bromide ion in the groundwater, after fumigation
and leaching, was still relatively high (48 mg/litre before and 280
mg/litre after fumigation in one study).
5.1.3 Soil
The natural bromide present in unfumigated soil depends on the
soil type. Van Wambeke et al. (1974) gave values of 3.3 mg/litre for
fresh sandy loam soil and 2.5 mg/litre for peat soil. Hoffman &
Malkomes (1974) stated that the natural concentration of bromide in
the soil was less than 10 mg/kg, depending on the type of soil and
geographical situation.
Fallico & Ferrante (1991) measured bromide concentrations in
greenhouse soil before, and after, the application of methyl bromide
(80 g/m2). Before fumigation, bromide levels were about 5 mg/kg. Two
months after treatment, bromide levels of over 30 mg/kg were measured.
After a further 3 months, levels had decreased to less than 10 mg/kg.
Bromide ion concentrations following greenhouse and soil
fumigation depend on the dosage, exposure time, aeration period,
temperature, the type of soil, the amount of rain or leaching water,
and the type of covering (sealing conditions). A year after fumigation
with 70-80 g methyl bromide/m3, bromide values of 0.2-11.5 mg/kg
were recorded in the upper 30 cm of soil (Basile et al., 1987).
The bromide remaining in the soil after fumigation can affect
soil fertility (Rovira & Ridge, 1979). As wheat particularly tends to
concentrate bromine, soil and wheat dynamics were studied (1981-83) in
methyl bromide-fumigated plots in a Mediterranean climate (Italy).
Bromide residues ranged between 5 and 10 mg/kg in the fumigated soil
to a depth of 50-60 cm. The total amount of bromide in the soil was
5.8 g/m2 up to a depth of 1 m and remained almost constant during
the wheat-growing period (Fransi et al., 1987). The amount of bromide
residues was about 8 % of that applied (900 kg/ha) five months
previously, compared with the 20 % found by Van Wambeke et al. (1974)
in similar soils.
5.1.4 Food
When considering the published levels of methyl bromide and
inorganic bromide in various foods, the method of analysis is
important (section 2.4.9). Originally, bromide content only was
measured and there is very little information on the methyl bromide
content. Measurements of both entities are important as well as other
residual products.
5.1.4.1 After soil fumigation
Bromide accumulation in plants depends on various factors, such
as the physical and chemical properties of the soil, the climatic
condition, the plant species and particular tissues, and the cultural
practices (Basile & Lamberti, 1981). Moreover, it depends on dosage,
exposure time, and aeration.
A study was carried out in 1980 in Metaponto, in southern Italy,
where methyl bromide was used to control nematodes and other plant
pathogens in the soil (Basile & Lamberti, 1981). Bromide ion levels
were 20-51 mg/kg (tomatoes), 8-44 mg/kg (string beans), 25-149 mg/kg
(radishes), 18-60 mg/kg (aubergines), 6-165 mg/kg (cucumbers), 13-46
mg/kg (courgettes), and 3-27 mg/kg (peppers) on a fresh weight basis.
In experimental plot conditions, the methyl bromide concentration was
60 g/m2 under a plastic cover with the soil temperature 14-17 °C at
10 cm depth. The covering was removed after 2 days and the plot
rotavated at a depth of 20 cm to eliminate residual gases.
Roughan & Roughan (1984a,b) carried out surveys of bromide ion
residues in lettuces, cucumbers, tomatoes, and self-blanching celery
grown in soil fumigated with methyl bromide, and compared these values
with those in a range of home-produced and imported fruit and
vegetables. Lettuce grown on unfumigated soil contained less than 10
mg bromide ion /kg, while most lettuces harvested from methyl bromide
fumigated soil were found to contain considerably more, 30 %
containing over 500 mg/kg and 2 % even in excess of 2000 mg/kg
(Roughan & Roughan, 1984a).
Studies on vegetables grown under protective covers on soil
previously fumigated with methyl bromide showed levels ranging from 1
to 109 mg/kg (fresh weight) in 29 late-season cucumbers, 5 to 326
mg/kg in 242 tomato samples, and 2 to 521 mg/kg in 38 samples of
celery (Roughan & Roughan, 1984b); 65 % of the late season cucumbers
contained more than background of bromide levels up to 10 mg/kg. Crops
of tomatoes (summer 1981) grown on sites where methyl bromide
fumigation had taken place in 1980/1981, contained considerably higher
levels than those grown on a site fumigated in 1979. In a survey from
retail outlets (January-November 1979), tomatoes and cucumbers from
the Canary Islands and Spain generally contained less than 10 mg/kg
(fresh weight). Of those grown in the United Kingdom, levels exceeded
10 mg/kg in 35 %, and 100 mg/kg in 5 %, ranging up to 177 mg/kg. These
higher levels of bromide ion could be attributed to the plants having
been grown on soil fumigated with methyl bromide prior to planting
(Roughan & Roughan, 1984b). The total bromide ion contents of various
crops obtained from retail outlets in the United Kingdom during the
period June 1981 to July 1982 are summarized in Table 22. The survey
indicated that vegetables, such as tomatoes, celery, cucumbers,
lettuce, radishes, and aubergines, grown in United Kingdom and the
Netherlands contained higher levels than elsewhere. Other crops had
mean bromide levels of less than 10 mg/kg, similar to other figures
published for background bromide ion content.
Table 22. Total bromide ion content of various crops obtained from retail
outlets in United Kingdom during the period June 1981 to July 1982a
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
apple France 5 0.1-0.3 0.2
aubergine Netherlands 4 2-23 11
avocado pear not known 1 1
banana Windward Islands 2 2 2
bean, broad England 3 1-2 2
bean, french Guernsey 1 1
bean, runner England 2 1 1
bean, sprouts England 3 1 1
cabbage England 3 1-2 2
calebrese England 2 1 1
(broccoli)
carrot England 1 2
cauliflower England 3 0.3-1 1
Chinese England 3 1-2 1
leaves
celery England 12 1-178 28
Guernsey 1 9
Israel 4 7-14 10
Spain 16 2-8 4
USA 1 4
courgette England 3 1-3 2
cucumber Canary Islands 15 0.3-10 3
England 36 0.2-87 9
Spain 3 0.2-10 4
Netherlands 7 0.1-14 7
grapefruit
segments Cyprus/Israel,
S. Africa 4 0.1-0.4 0.3
green pepper Netherlands 7 0.4-5 2
ettuce Belgium 1 5
Cyprus 5 1 1
England 69 1-241 15
France 9 0.2-19 4
Israel 6 1-4 2
Spain 11 0.4-4 2
Netherlands 26 2-57 21
USA 12 0.1-2 1
Table 22 (continued)
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
marrow England 2 1-2 1
mushroom England 62 0.2-24 1
onions Israel,
Netherlands 3 0.4-1 1
onion, England 3 2-4 3
spring
orange S. Africa, Spain 6 <0.1-0.4 0.2
segments
pea England 4 1-3 2
potato Cyprus, England 3 1-2 1
radish England 18 0.2-3 1
Israel 2 5 5
Netherlands 12 0.1-48 13
USA 1 1
strawberry England 1 0.3
tomato Canary Islands 8 1-5 4
England 33 1-70 13
Spain 15 1-7 3
Netherlands 14 1-39 11
a From: Roughan & Roughan (1984b).
Brown et al. (1979) measured the bromide concentration in several
plant species in unfumigated and methyl bromide-fumigated plots in
California (Table 23). Many plants showed increased bromide
concentrations. Strawberries and grapevines absorbed relatively
little. As the interval increased between soil fumigation and
planting, there was a general decline in bromide levels, though the
interval could be as long as three years before the crops returned to
a level of about 10 mg/kg (Brown et al., 1979; Roughan & Roughan,
1984a). Table 24 shows bromide concentrations in plant material 1, 2,
3, and 4 years after fumigation with methyl bromide.
Fallico & Ferrante (1991) measured bromide levels in tomatoes in
crops grown in soil that had been fumigated with methyl bromide ten
days prior to planting. Bromide levels in tomatoes grown in the
treated soil and harvested after 60 days were about double (55 mg/kg)
those in tomatoes grown in untreated soil. The bromide levels
decreased with each successive harvest, but were still higher than
those in control plants after the fourth harvest.
Table 23. Bromide concentrations in several plant species in unfumigated and methyl bromide-fumigated plotsa,b
Plant species Treatment Range of Average bromide S.E. of
and part bromide concentration means
concentrations (mg/kg dry weight)
(mg/kg dry weight)
barley control 4 to 575 106 (19)c 38
(whole top) fumigated 120 to 5235 1788 (23) 373
bur clover control 1 to 407 96 (11) 40
(whole top) fumigated 196 to 2371 1334 (14) 155
filaree control 4 to 546 135 (14) 47
(whole top) fumigated 718 to 7380 2600 (10) 683
wild oats control 9 to 876 196 (14) 66
(whole top) fumigated 1233 to 5034 3364 (11) 441
spinach (leaves) fumigated 1772 to 3195 2521 (4) 387
ryegrass fumigated 1481 to 2790 2378 (4) 302
sweet potato
(leaves)
1st sampling fumigated 640 to 923 753 (4) 69
4th sampling fumigated 312 to 372 330 (4) 14
sweet potato
(root) fumigated 204 to 237 220 (2) 17
strawberry control 14 to 129 63 (9) 16
(leaves)d fumigated 3 to 372 88 (36) 13
grape (leaves)e control 1 to 101 28 (90) 3
fumigated 1 to 402 48 (278) 4
Table 23 (continued)
a From: Brown et al. (1979).
b These data represent samples collected the first year after fumigation with MeBr at rates of 34-68 g/m2,
except for grape leaves.
c Numbers in parentheses refer to the number of samples in the average.
d Strawberry leaves were collected after 1-6 annual fumigations with methyl bromide.
e Grape leaves were collected 2-4 years after fumigation with methyl bromide.
Table 24. Bromide concentrations in plant material (mg/kg dry
weight), 1, 2, 3, and 4 years after fumigation with methyl bromidea
County Untreated Years after fumigation
controls
1 2 3 4
Sonoma 94(19)b 3018(16) 360(15) 516(8) 12(3)
Napa 163(21) 1476(15) 742(10) 430(2)
a From: Brown et al. (1979).
b Numbers in parentheses = number of samples included in the
average.
Table 25. Inorganic bromide residues detected in samples of fruit and
vegetables in UK (1988 to 1989)a
Commodity Concentration Number of samples
range (mg/kg)c
Lettuces, produced n.d. 0
in United Kingdom
(proposed CAC 1-20 48
MRLb=100) 21-100 15
101-300 9
301-529 5
Lettuces, imported n.d. 0
1-20 21
21-45 3
Rice, imported n.d. 59
[MRL=50 1-10 37
(unprocessed 11-20 16
rice)] 21-50 14
50-100 10
121 and 124 2
Nuts (no MRL)
almonds n.d. 4
1-147 24
brazil nuts n.d. 0
3-140 22
Table 25 (continued)
Commodity Concentration Number of samples
range (mg/kg)c
cashew nuts n.d. 16
3-53 5
chestnuts n.d. 5
3-23 3
coconuts n.d. 0
2-6 5
hazel nuts n.d. 7
1-194 10
peanuts n.d. 4
2-109 18
pine nuts n.d. 4
46 1
Nuts (continued)
pistachio nuts n.d. 0
3-104 4
tiger nuts n.d. 0
17-20 4
walnuts n.d. 3
2-210 21
sesame seed n.d. 5
17-56 5
sunflower seed n.d. 3
2 6
3 1
Dried fruits
currants, imported
(CAC MRL = 100) n.d. 22
1-3 9
6-15 5
sultanas n.d. 20
(CAC MRL = 100) 1-6 6
6-12 7
Table 25 (continued)
a From: MAFF (1990).
b CAC = Codex Alimentarius Commission; MRL = maximum residue limit
(mg/kg) legally permitted in, or on, food commodities or animal feed.
c n.d. = not detected.
The results of a further survey (1988-89) in the United Kingdom
of inorganic bromide levels in fruit and vegetables are summarized in
Table 25. Fourteen samples of lettuce produced in the United Kingdom
exceeded the Codex Alimentarius Commission maximum residue limit (CAC
MRL) for inorganic bromide of 100 mg/kg (MAFF, 1990).
The effects of leaching following soil fumigation with methyl
bromide up to 100 g/m2 are shown in Table 26. At sites with low soil
bromide residues, the resulting bromide residues in lettuce were
nearly unaffected by leaching. At other sites, there was a positive,
but diminishing, response to increasing rates of leaching, very high
residues probably being due to high levels of organic matter (Smart,
1990).
Recent monitoring of samples of individual crops having a
likelihood of being grown on soil fumigated with methyl bromide showed
that only a small percentage contained residues above Codex
Alimentarius Commission recommended limits. The high levels were
mainly in lettuce. The report of Smart (1990) showed that the
proportion of samples having high residues had declined since the late
1970s, when, in some countries, residues in lettuce were as high as
500-1000 mg/kg. Leaching of treated soils, attention to timing of
application, and integrated pest control have all helped to reduce
such residue levels.
Bromide level tolerances for a variety of methyl bromide
fumigated raw agricultural commodities in the USA are shown in Table
27. However, according to the US EPA, because of its existing
toxicological data base and its environmental ubiquity, inorganic
bromide is not of toxicological concern. Requirements for residue data
to support existing inorganic bromide tolerances were waived by the
Agency (US EPA, 1989).
Table 26. Bromide residues in lettuce grown under protection of soil fumigated with bromomethane and leached with water
before planting in the United Kingdoma
Site Soil texture Soil organic Number of Interval between Bromide residues in lettuce (mg/kg fresh weight)
matter (%) applications planting and for the water application rates (mm)
in previous harvest
years (weeks) 0b 100 200 300 400
A fine sandy loam 4 2 16 81 102 92 150 142
B sandy loam 12 1 12 62 70 232 93 114
C sandy loam 13 2 13 307 215 98 157 117
D sandy loam 1 24 765 427 392 284 250
E sandy loam 8 4 21 676 335 328 164 134
Fc loamy peat 51 4 14 1958 1534 1001 - -
Mean sites A-E 378 230 228 170 151
a Summarized from Food and Agriculture Organization (1985) by Smart (1990).
b Residue figures for crops grown in soils not having any leaching.
c At F, the leaching treatments were applied after planting the lettuce crop.
Table 27. USA tolerancesa
Br in, or on, the following raw agricultural commodities,
which have been fumigated with the antimicrobial agent
and insecticide methyl bromide after harvest (with the
exception of strawberries) (mg/kg)
corn (pop) 240
almonds, brazil nuts, bush nuts, 200
butternuts, cashews, chestnuts,
cottonseed, filberts, hickory nuts,
peanuts, pecans, pistachio nuts,
soybeans, walnuts
asparagus, copra, cumin (seed), 100
ginger (roots), pomegranates
avocados, coffee beans, potatoes, 75
sweet potatoes
alfalfa (hay), barley, beans, 50
beans (green), benas (lima), beans
(snap), cabbage, cippolini (bulbs),
cocoa beans, corn, corn (sweet),
garlic, oats, peas, peas (blackeyed),
rice, rye, sorghum (grain), timothy
(hay), wheat
artichokes (Jerusalem), garden beets 30
(roots), sugar beets (roots), carrots,
citrus citron, cucumbers, grapefruit,
horseradish, kumquats, lemons, limes,
okra, oranges, parsnips (roots), peppers,
pimentos, radishes, rutabagas, salsify
(roots), squash (summer), tangerines,
turnips (roots)
apricots, blueberries, cantaloupes, 20
cherries, eggplant, grapes, honeydew
melons, mangoes, muskmelons, nectarines,
onions, papayas, peaches, pineapples,
plums, pumpkins, squash (winter), squash
(zucchini), tomatoes, watermelons
apples, pears, quinces 5
a From: US EPA (1988b; CFR 180.124).
5.1.4.2 After post-harvest fumigation
Methyl bromide is widely used as a post-harvest fumigant to kill,
or prevent, pest infestation. In 1976, around 100 000 metric tonnes of
food commodities were treated with methyl bromide in the United
Kingdom (Fairall & Scudamore, 1980).
Fairall & Scudamore (1980) measured methyl bromide residues in
dried milk, wheat, flour, rapeseed, and groundnut samples after store
fumigation (see Table 28). Products such as groundnuts and rapeseed
retained higher amounts of methyl bromide. No methyl bromide was
detected in any commodity after storage for 1 month (detection limit
10 µg/kg).
DeVries et al. (1985) measured the rate of decrease of methyl
bromide in wheat, flour, cocoa, and peanuts after fumigation with the
gas. Samples were analysed immediately and then after various time
intervals of exposure of the sample to air. The methyl bromide
concentration decreased very rapidly in all cases, no residual methyl
bromide being found in any of the samples after 2 weeks (detection
limit, 0.4 µg/kg).
(a) Wheat and cereals
Pesticide residues in home-grown and imported wheat were measured
in the United Kingdom by Osborne et al. (1989); 45 samples were
analysed for methyl bromide (method: Fairall & Scudamore, 1980) and
inorganic bromide. No methyl bromide was found in excess of the
detection limit (0.01 mg/kg); all samples contained inorganic bromide,
but at levels of 4 mg/kg or less, which is given as the level
naturally present in wheat as a result of uptake from the soil (Heuser
& Scudamore, 1970; Osborne et al., 1989).
Trials carried out on a commercial scale showed that CT
(concentration x time) products for methyl bromide generally lay in
the range of 50-2000 mg.h/litre (Scudamore, 1987). The higher values
are usually found in pockets of grain at the bottom of silos or bins.
Scudamore (1987) recommended careful monitoring, especially at
increased temperature and moisture content, when treating more
sorptive cereals, such as oats or maize, or those with which methyl
bromide reacts more readily. In three samples of wheat from different
parts of the United Kingdom, bromide levels increased between 2 and 3
times over a range of 11-16 % moisture content.
Table 28. Methyl bromide residue levels (mg kg-1) during storagea
Days
Commodity 0 0.04 0.25 1 2 4 7 11
dried milk 0.5 0.15 0.03 0.006 - - - -
wheat 0.8 0.42 0.33 0.08 0.04 n.d.b - -
flour 0.28 0.09 0.04 0.02 n.d. - - -
rapeseed 4.2 3.0 1.5 - 0.95 0.5 0.10 0.08
groundnuts 7.7 4.5 3.2 2.6 1.6 0.38 0.38 0.03
a From: Fairall & Scudamore (1980).
b n.d. = none detected.
Table 29. Bromide ion and methyl bromide concentrations (mg/kg) in
flours exposed to methyl bromide only in silos and in pastas obtained
from these fumigated and unfumigated floursa
Material Treatment Number of Bromide ion Methyl
samples (mean±S.D.) bromideb
flours unfumigated 3 1.26±0.03 n.d.
fumigated 3 1.60±0.11 n.d.
pasta from unfumigated 6 1.24±0.13 n.d.
(macaroni) flours
from fumigated 6 1.91±0.22 n.d.
flours
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than the detection limit of 0.01
mg/kg.
Stacks of bags containing stored grains and pulses (wheat,
lentils, maize, barley, chick-peas, peas, and sorghum) were covered
with PVC sheets and exposed to methyl bromide fumigation for 48 h
(Urga, 1983). Following aeration, residues of less than 50 mg/kg
bromide were measured, with the exception of 60 mg/kg after 24 h
aeration and 59 mg/kg after 36 h aeration. Cova et al. (1986)
investigated the effects of exposure to methyl bromide in flour, and
pastas made from it. Flour was exposed to methyl bromide in silos
(conditions: mean temperature - 18 °C, concentration - 24 g/m3,
duration of treatment - 68 h, duration of ventilation - 3 days). Table
29 shows levels of bromide ion before, and after, fumigation. Methyl
bromide could not be detected. In a second experiment (summarized in
Table 30) on pasta made from unfumigated flour, rice flour, and white
flour, Cova et al. (1986) examined the influence of the type of
packaging on the effects of fumigation. Every item was packed in two
different ways, i.e., a cardboard box or a transparent envelope made
of a double layer of polypropylene. The products were fumigated in a
closed room under the conditions given above, and the mean bromide ion
concentrations ranged between 2.03 mg/kg (pasta) and 46.23 mg/kg (egg
pasta), while methyl bromide was not detected.
Table 30. Bromide ion and methyl bromide concentrations (mg/kg) in unfumigated
and fumigated foodstuffs, treated in their retail packagingsa
Material Treatment Number of Bromide ion Methyl
samples (mean ± S.D.) bromideb
rice unfumigated 3 0.72 ± 0.06 n.d.
funfumigated 3 10.63 ± 0.67 n.d.
flour unfumigated 3 3.17 ± 0.09 n.d.
funfumigated 3 6.66 ± 0.01 n.d.
white flour unfumigated 3 1.22 ± 0.15 n.d.
funfumigated 3 4.19 ± 0.82 n.d.
pasta (macaroni) unfumigated 6 2.40 ± 0.26 n.d.
funfumigated 6 2.60 ± 0.27 n.d.
pasta (spaghetti) unfumigated 6 1.92 ± 0.17 n.d.
funfumigated 6 2.03 ± 0.07 n.d.
pasta with eggs unfumigated 6 4.13 ± 0.12 n.d.
funfumigated 6 46.23 ± 1.57 n.d.
pasta with eggs unfumigated 6 4.62 ± 0.31 n.d.
and spinach funfumigated 6 39.00 ± 0.01 n.d.
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than detection limit of 0.01 mg/kg.
(b) Spices, nuts, and dried fruits
In the USA in 1980, two warehouses containing imported spices
were fumigated to eradicate a khapra beetle infestation. Methyl
bromide and inorganic bromide residues were determined in the 52
spices before, and after, fumigation (using GC-ECD). In addition, an
ashing titration method for bromide ion residue was used, allowing a
comparison of the two analytical methods (Reeves et al., 1985). Before
fumigation, the highest methyl bromide residue was in parsley (14.9
mg/kg). Seventy-two hours after fumigation with 100 g/m3 for 12 h,
samples were collected and analysed. The highest methyl bromide
residue was found in sage (65.8 mg/kg). Levels of inorganic bromide
residues before fumigation (GC-ECD) were all lower than 200 mg/kg;
after fumigation, only two samples contained higher levels.
In a Canadian study, a number of spices, seeds, nuts, and dried
fruits and vegetables, including samples of celery, mustard, sesame,
coriander, pumpkin and sunflower seeds, cloves, peppercorns, dates,
figs, prunes, raisins, beans, minced onion, a vegetable mix, walnuts,
and peanuts were analysed for methyl bromide residues (Page & Avon,
1989). Of the 30 samples, only a sample of pumpkin seeds was found to
contain methyl bromide (3 µg/kg). Fifty-one chocolate and grain-based
products were also analysed and found not to contain any methyl
bromide.
In contrast, when samples of food known to have been treated with
methyl bromide were analysed, of the 60 samples, 16 contained residues
>1 ng/kg and 5 contained >100 ng/kg methyl bromide. These samples
included dried currants (2.9 mg methyl bromide/kg), chocolate-covered
nuts (0.66 and 0.19 mg/kg), and rice (2.3 mg/kg) and maize (0.21
mg/kg) flours. These findings were confirmed by mass spectrometry
(Page & Avon, 1989).
Bromide residues after methyl bromide fumigation were determined
in samples of dried fruits, cereals, nuts, and spices imported into
New Zealand in 1977 and early 1978 (Love et al., 1979). About one-half
of the nut and spice samples contained total bromide levels exceeding
50 mg/kg, and occasional high levels of bromide residues were found in
cereals.
Fairall & Scudamore (1980) showed that rapeseed and groundnut
samples retained higher amounts of methyl bromide than other
foodstuffs after store fumigation (Table 28). A survey of retail nuts,
seeds, and nut products in October 1984 (Table 31) showed that levels
of methyl bromide in some of these products were higher than the Codex
Alimentarius Commission guideline (MAFF, 1989).
Table 31. Residues of methyl bromide in samples of nuts, seeds, and nut productsa,b
Number of samples Residue concentrations
(mg/kg)
tested containing rangec mean
residues
almonds 5 2 n.d. to 0.06 < 0.02
brazil nuts 4 0 n.d. -
dried chestnuts 1 1 0.2 -
hazelnuts 4 0 n.d. -
mixed nuts 3 0 n.d. -
nuts and raisins 3 2 n.d. to 0.2 0.08
peanuts 12 9 n.d. to 0.3 0.06
walnuts 7 4 n.d. to 2.2 0.4
othersd 6 0 n.d. -
a From: MAFF (1989).
b 45 samples were obtained during October 1984. and analysed for
residues of methyl bromide. The CAC (1986) guideline level for
methyl bromide in nuts is 0.01 mg/kg.
Table 31 (continued)
c n.d. = not detected (the limit of determination was 0.02 mg/kg).
d One sample each of cashews, pecans, pine kernels, pistachios, sunflower seeds, and
tiger nuts.
(c) Fresh fruit
Methyl bromide residues determined in laboratory studies on fresh
fruits are summarized in Table 32. Studying the effects of fumigation
dose and length of the following aeration periods, Sell & Moffitt
(1990) and Sell et al. (1988) found that desorption of methyl bromide
from apples and cherries, respectively, followed pseudo-first-order
decay curves, the first component resulting from removal of the
pesticide from free air space in the chamber, and the second, from the
desorption from the fruits. Singh et al. (1982) found that methyl
bromide absorption in avocados depended on oil content rather than
skin thickness or protein content.
(d) Milk and cheese
Bromides may be present naturally in cows' milk in amounts up to
8 mg/litre. When cows were fed on grain fumigated post-harvest with
methyl bromide, higher levels of bromide were found in the milk, e.g.,
cows fed grain containing bromide at 220 mg/kg had bromide levels in
their milk of 10-20 mg/litre (Lynn et al., 1963).
Cheese fumigated with methyl bromide showed high bromide residues
(Laug, 1941).
5.1.5 Animal feed
Knight & Costner (1977) reported bromide residues of 6800-8400
mg/kg in hay that was harvested in the spring after the field had been
injected with methyl bromide. The resulting toxic effects on animals
fed with this hay are reported in section 7.3.7.
5.1.6 Other products
The chlorine and bromine contents in tobacco and tobacco smoke
were investigated by Häsänen et al. (1990). Smoke per cigarette
contained 1 µg bromine in the particulate phase and 5 µg bromine in
the gaseous phase. In the gaseous phase, methyl bromide accounted for
80% of the total bromine. Methyl bromide is used widely as a fumigant
in uncured tobacco storage and this can increase the bromine content
in tobacco considerably.
Methyl bromide has been used in growing tobacco seedlings (Ostrec
& Korunic, 1989).
5.1.7 Terrestrial and aquatic organisms
No data are available.
5.2 General population exposure
5.2.1 Food
The general population may be exposed to residues of methyl
bromide and inorganic bromide, and other possible metabolic products,
which may be present in food marketed for consumption. Levels of
methyl bromide and inorganic bromide in food are described in section
5.1.4.
Food commodities containing higher levels of oil and fat, such as
groundnut and rapeseed (Table 28), retain higher amounts of methyl
bromide residues after fumigation, which disappear after a storage
period of about one month (Fairall & Scudamore, 1980; DeVries et al.,
1985). However, higher levels of inorganic bromide residues may remain
in food commodities marketed after fumigation (section 5.1.4.2).
Scheffrahn et al. (1992) investigated the effects of methyl
bromide fumigation on foods in retail packages, in sealed and unsealed
plastic containers, with a view to simulating conditions in which
residues may be found in food products after fumigation of a
residential house in the USA. Fatty commodities in unsealed packages
contained higher residues (>1 mg/kg) than other foods. The fumigant
may have entered the containers by two routes, diffusion through the
air-spaces around the lids (opened peanut butter jar) or in porous
packaging (parmesan cheese in cardboard box), and by permeation into
polyurethane bagged foods. Factory sealed, polyethylene terephthalate
(PETE) containers gave better protection. Vacuum-packed foods in metal
(soup, coffee) or glass (sauce) containers yielded no residues.
Bromide residues in foodstuffs were discussed by Van Leeuwen &
Sangster (1987) in a review of the toxicity of the bromide ion. Total
diet surveys in the United Kingdom in 1978 and 1979 gave a daily
intake of 8.4 mg bromide per person. These values are consistent with
those of two Dutch surveys where average daily intakes of 9.4 and 7.7
mg bromide per person, respectively, were recorded, i.e.,
approximately 3 mg/kg diet (Van Leeuwen & Sangster, 1987).
Table 32. Methyl bromide residues in fresh fruits after fumigation
Fruit Fumigation time Storage Timea Residue Reference
and dose temperature (mg/kg)
(°C)
grapefruit 2 h, 64 mg/litre 24 1 h 26.9b King et al. (1981)
48 h 0.52
peaches 3.5 h, 32 g/m3 2.5 1 day 11.0 Austin & Phillips (1985)
7 days 4.0
mango N.D.c, 64 g/m3 N.D. 1 h < 15.0 Stein & Wolfenbarger (1989)
grapefruit 2h, 48 g/m3 15.6 5 days < 5 King & Benschoter (1991)
oranges, 2h, 48 g/m3 15.6 5 days < 10
mandarines,
tongors
avocado 2h, 32 g/m3 20 1 day up to 0.5d Singh et al. (1982)
2 days up to 0.1
a Time of analysis after end of fumigation period.
b Aeration period: 15 min.
c N.D. = no data given.
d Aeration period: 30 min.
The metabolites resulting from methyl bromide post-harvest
fumigation of a number of crops have been analysed (Starratt & Bond,
1990a,b). Both physically- and chemically-bound residues were
measured. In 7 out of the 9 commodities, over half of the total
residue was chemically bound 1 h after fumigation. These chemically
bound residues were stable for at least 6 months. Most reaction
products were O-, S-, and N-methylated proteins (section 6.3).
Methylation of purines and pyrimidines in the nucleic acids accounted
for 0.1-6 % of the chemically-bound residue, with N being the only
site of methylation. The methylation products identified in this study
are also known to occur naturally.
5.2.2 Drinking-water
In 1988, in California, only one sample out of 43 056 taken from
2977 wells, showed any presence of methyl bromide (detection limit -
1 µg/litre) (CDFA, 1988).
In areas, such as in the Netherlands, where private water
supplies are from shallow wells near methyl bromide soil operations,
there could be increased bromide contents in the water (Van Doorn et
al., 1989).
5.2.3 Human breast milk
There are no data available.
5.2.4 Sub-populations at special risk
People who live in close proximity to greenhouses or to fields or
storehouses that are being fumigated have a higher risk of exposure to
methyl bromide gas than the general population. Similarly, persons
inadvertently, or intentionally, entering buildings following, in
particular, structural fumigation, are at risk.
5.3 Occupational exposure during manufacture, formulation, or use
The number of incidents and fatalities (section 9) show that
occupational exposure, especially during fumigation, is potentially
hazardous.
5.3.1 During manufacture
In a methyl bromide plant in the USA, workplace air
concentrations of 78-116 mg/m3 (20-30 ppm) were recorded using
direct measurement (Evans, 1979).
In a methyl bromide-producing factory in Japan, methyl bromide
concentrations in the worker's breathing zone were usually under 4
mg/m3, but sometimes exceeded 20 mg/m3 (Kishi et al., 1988).
5.3.2 During fumigation
If safety procedures are not followed, workers may be exposed to
methyl bromide accidentally during, or after, fumigation operations.
Methyl bromide in low concentrations is odourless so that a toxic
atmosphere may not be apparent to the worker. Only at higher
concentrations (100 x the actual TLV(R) of 20 mg/m3 (5 ppm) does
it have a sweet smell (Van Den Oever et al., 1982). An odour threshold
of 65 mg/m3 has been reported for methyl bromide (Worthing & Walker,
1983). Therefore, it is usually marketed in the form of 98% methyl
bromide and 2% chloropicrin, as a lacrimatory agent. For post-harvest
fumigation, 100% methyl bromide is used.
The various fumigation techniques used together with methyl
bromide exposure values and methods of application are outlined in
Table 33 (Guillemin et al., 1990).
5.3.2.1 Structural fumigation
In carrying out the general fumigation of a building, sufficient
gas must be liberated into the free space to kill the insects and then
the toxic level maintained for a defined period of time. After the
treatment, the residual gas remaining in the building is dispersed to
the outside atmosphere. However, there are basic differences in
defining structures amongst various countries. In the USA, structural
fumigations mainly involve residential houses whereas, in Europe, they
generally refer to flour mills and food processing areas. The
fumigation procedures and the safety aspects in these circumstances
could be very different.
Methyl bromide exposure levels for structural fumigation workers
in California were measured by Anger et al. (1986). They described the
work involved. "Structural fumigation is conducted by a work crew of
2-4 men who cover the buildings to be fumigated with large vinyl
tarpaulins and connect them by spring clamps. Cylindrical tubes filled
with sand (sand snakes) are placed at the base of the structure to
hold down the tarpaulins, thus sealing the building. After this one to
two hour procedure, termed a closing, the fumigant is introduced into
the unoccupied house via a tube or hose, and the fumigators leave the
site. The fumigators wear self-contained breathing apparatus (SCBA).
The next day the work crew removes the tarpaulins, opens the windows,
and places fans in the house to clear the fumigant. It is in this 30-
to 45-min process, termed the opening, that worker exposures may
occur. Typically, each work crew, led by a State-licensed fumigator
(the "licensee"), conducts three openings and/or closings each day".
Table 33. Integrated samples of methyl bromide in air taken in a survey of methyl bromide
fumigation in Switzerlanda
Circumstances Exposure Sample Range of values
of sampling category No.
Minimum Maximum
mg/m3(ppm) mg/m3(ppm)
1. Space fumigation
- during fumigationb occupational 7 <0.8 (<0.2) 500 (128.4)
- during aerationb occupational 7 2.3 (0.6) 646 (166.0)
- resuming operation para-occupational 47 <0.8 (<0.2) 9 (2.3)
- during fumigation environmental 12 <0.8 (<0.2) 79 (20.4)
- during aeration environmental 12 <0.8 (<0.2) 105 (27.1)
2. Soil fumigation
- during fumigationb occupational 5 2 (0.5) 151 (39.0)
- removal of sheetingb occupational 3 34 (8.8) 144 (36.9)
- inside greenhousec para-occupational 5 12 (0.3) 9 (2.2)
3. Chamber fumigation
- outside chamber para-occupational 3 35 (8.9) 293 (75.3)
- removing contents para-occupational 4 5 (1.2) 17 (4.3)
a From: Guillemin et al. (1990).
b Fumigators generally wore respiratory protection during these operations.
c Greenhouse only partially fumigated; includes post-fumigation soil tillage.
Personal samples from fumigators taken when they entered houses
24 hours after fumigation with 23 000 to 31 000 mg methyl
bromide/m3, indicated that a house might contain 80-2000 mg methyl
bromide/m3 (Table 34). Personal samples taken on fumigators working
outside the houses when they were opened again showed concentrations
of between 0 and 8 mg/m3 in the half-hour periods during the cover
removal (Anger et al., 1986). Area samples taken within 3 and 6 m from
the buildings during the same period ranged from 0 to 31 and 0 to 10
mg/m3, respectively (Anger et al., 1986).
Concentrations of methyl bromide inside flour mills and in the
atmosphere around the mills during, and after, fumigation were
measured by Bond & Dumas (1987). Considerable variations in
concentration were found in buildings of different structure and under
varyious weather conditions. Concentrations ranging from trace amounts
up to 90 mg/m3 (23 ppm) were found in the air around the mills
during the aeration period.
Table 34. Methyl bromide exposure concentrations (mg/m3)
in residential fumigationa
State Fumigators Under Within 3 m of 3-6 m from
licensed working covers house house
fumigator outside house
when inside
house
1875.0 5.1 2.0 46.7 32.3 0 0
75.9 0 1.2 12.5 0 10.1
90.3 0 7.0 0 17.5 10.1
1042.1 3.9 3.1 0 0 0
204.2 8.6 0 0 0
Mean
657.4 3.1 46.7 7.0 3.9
a Adapted from: Anger et al. (1986).
Guillemin et al. (1990) conducted a survey in Switzerland on
exposure during space fumigation. The maximum exposure levels for 7
integrated samples was 646 mg/m3 (166 ppm) during aeration (see
Table 33). Fumigators wore respiratory protection during these
operations. Samples of transient air taken around the buildings not
from any specific spot (distance not given), showed methyl bromide
levels in the range of 0-105 mg/m3 (see Table 33).
5.3.2.2 Soil fumigation
The amount of methyl bromide released into the atmosphere during
soil fumigation depends on the methods used (Table 9), the type and
time of covering (section 3.2.2) and soil type.
(a) Field fumigation
A formulation of 75% methyl bromide was used to kill insects and
nematodes in a strawberry crop, with 25% chloropicrin as a fungicide.
The fumigant injected into the soil produced an equilibrium of 47
000-58 000 mg/m3 which, under the covers, resulted in personal
average exposures ranging from 0 to 24 mg/m3 for the fumigators
(Anger et al., 1986). Personal sample measurements of methyl bromide
in farm workers when removing the film ranged between 0 and 33
mg/m3. Other data showed that the personal exposures of fumigators
and farm workers, who covered the plastic film with earth were between
0 and 29 mg/m3 and 0 and 17 mg/m3, respectively. Exposure of soil
fumigators, about 8 h a day, was relatively constant during most of
the year, whereas farm workers received only occasional exposures when
fields were fumigated. Spot (area) samples, taken before and after
film removal, showed that the airborne concentration under the intact
film was 8950 mg/m3 before removal and 9.3 mg/m3 an hour after
removal.
(b) Greenhouse fumigation
Roosels et al. (1981) compared two methods of methyl bromide
fumigation in greenhouses, i.e., by injection into milled soil
followed by covering with a plastic cover, or, by surface fumigation
by means of plastic pipes under a plastic cover. Two different
formulations (70 % methyl bromide/30 % chloropicrin and 98 % methyl
bromide/1-2 % chloropicrin) were used and the concentrations of methyl
bromide in the air were measured by GC-FID. During injection into
soil, values of between 400 and 4000 mg/m3 (100 and 1000 ppm) were
found with peaks up to 12 000 mg/m3 (3000 ppm) and, in one case, up
to 40 000 mg/m3 (10 000 ppm). However, when preventive measures were
taken, values of 800 mg/m3 (200 ppm) were obtained. During
fumigation, concentrations ranged between 400 and 4000 mg/m3 (100
and 1000 ppm). Using the piped surface fumigation method,
concentrations around the treated area were between 320 and 3200
mg/m3 (80 and 800 ppm) with short-term exposures of the operators to
8000-12 000 mg/m3 (2000-3000 ppm) when the pipes were being
connected.
In another investigation, concentrations of 60-100 g methyl
bromide/m2 were applied under a polyethylene cover (Van Den Oever et
al., 1982). Depending on local ventilation, quite a lot of gas escaped
into the surrounding atmosphere. The concentration during application
varied from 117 to 11 700 mg/m3 (30 to 3000 ppm). Concentration in
the air declined with time to 16 mg methyl bromide/m3 (4 ppm) five
days after application. Removing the plastic sheet involved exposure
to peak values as high as 800 mg/m3 (200 ppm), for a few seconds. On
the ninth day after application, milling the soil exposed workers to
up to 60 mg/m3 (15 ppm); on the eleventh day, no methyl bromide was
detected in the air.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
6.1.1.1 Animal studies
Uptake of methyl bromide was investigated in male Fischer 344
rats by Andersen et al. (1980). Following whole body exposures to
recirculated atmospheres of 390-11 640 mg/m3 (100-3000 ppm), the
uptake (i.e., disappearance from the atmosphere) was rapid and
exhibited first-order kinetics without a saturable component, the rate
constant being 0.44 kg-1 h-1. This rate constant was later
recalculated as 0.55 kg-1 h-1 by Gargas & Andersen (1982).
Medinsky et al. (1985) carried out a nose-only inhalation of 50,
300, 5700, or 10 400 nmol (given as 6.2-1206 mg/m3) of [14C]
methyl bromide/litre of air for 6 h in male F344 rat (5 animals/
group). The results indicated that, at low concentrations (50-300
nmol/litre), about 50% of the inhaled material was absorbed. At 5700
nmol/litre, only 37% was absorbed and, at 10 400 nmol/litre, only 27%.
The same amount of methyl bromide (650 µmol/kg body weight) was
absorbed at the two higher exposure concentrations. At 10 400
nmol/litre, the total volume inhaled by the rats was reduced (Medinsky
et al., 1985).
Raabe (1986) found about 40% uptake of inhaled methyl bromide in
studies on beagle dogs. These results are compared in Fig. 6 with
those from rats (Medinsky et al., 1985) and those from human
volunteers (Raabe, 1988), described below.
6.1.1.2 Human studies
An inhalation study was carried out to determine systemic uptake
of low concentrations of methyl bromide from air during nasal or oral
breathing (Raabe, 1988). Two male and two female volunteers inhaled
about 0.1 mg 14C-labelled methyl bromide/m3 (25 ppb), once through
the nose and once through the mouth. The uptake (% of methyl bromide
inhaled) was 55.4% nasally and 52.1% orally.
6.1.2 Dermal
Exclusively dermal exposure has only been observed in human incidents
(section 9). There are no data dealing exclusively with dermal
exposure in animals.
6.1.3 Oral
Methyl bromide (75 or 100 mg/kg) was administered to rats in olive oil
by gavage (Miller & Haggard, 1943). The methyl bromide entered the
blood stream with only a moderate degree of hydrolysis in the
intestine.
5.1.1.5 Release of methyl bromide to the outside air from greenhouses
During 1986, 400 000 kg methyl bromide were emitted into the air
in the Westland area of the Netherlands (Van Doorn et al., 1989).
Although soil disinfection is only allowed when the diluting capacity
of the atmosphere (wind force) is sufficient, this is very difficult
or impossible to control. Investigations by Netherlands Institutes
(TNO and RIVM) showed that the hourly average concentrations within a
20-m distance from the green-houses in 1981 (after the introduction of
gas-tight film) during the first hours was 5.9 mg/m3 after
fumigation with methyl bromide at a dose of 700 kg/ha. In 1982 and
1983, a few hours after injection and at a probable dosage of 400
kg/ha, concentrations measured ranged between 1 and 4 mg/m3 at
distances of 20 m. After the first few hours, the concentration of
methyl bromide decreased rapidly and, after 4 days, also at 20 m,
hourly averages of 0.2 mg/m3 were measured. Ten days after
fumigation, the covering foil was removed, after which the hourly
average concentration increased to a maximum of 0.4 mg/m3 (Van Doorn
et al., 1989).
5.1.2 Water
5.1.2.1 Seawater
In 1975, samples of seawater from near the shore at Dorset,
England were analysed for halomethanes and methyl bromide was detected
at levels ranging from 2.0 to 3.9 x 10-9 ml gas/ml water [apprx. 10
ng/litre] (Lovelock, 1975).
Average concentrations of 1.2 ng methyl bromide/litre were
measured in surface seawater in the Eastern Pacific Ocean (Singh et
al., 1983). The authors estimated that the oceans are supersaturated
with methyl bromide to 250%.
Khalil et al. (1993) measured methyl bromide concentrations in
ocean water in two open-ocean surveys, one covering latitudes from 45°
N to about 30° S, the other from 67° N to 50° S. On the first survey,
they found supersaturation of methyl bromide at 180% and, on the
second, 140%.
5.1.2.2 Inland waters
In 1988, the California Department of Food and Agriculture (CDFA)
reported the results of analyses of 43 056 well water samples taken
from 2977 wells in various Californian counties. Residues of 10
chemicals were detected. Methyl bromide detection was undertaken in 32
counties, but in all the wells tested only one sample showed the
presence of methyl bromide (CDFA, 1988).
5.1.2.3 Waters around greenhouses
In the Netherlands, where intensive horticulture in greenhouses
is practised, the soil level is lower than sea level in some areas,
with the result that water transport is very slow and the static water
volume is very high. There is a high density of greenhouses and all
holdings have to use the water from ditches as leaching water, thus,
this water is successively loaded with bromide. However, it should be
noted that, in 1992, methyl bromide was banned in the Netherlands for
soil fumigation purposes.
The concentrations of methyl bromide and bromide-ion were
measured in irrigation water, drainage water, and surface water during
the leaching periods in two Netherlands glasshouse soils after
fumigation with methyl bromide (Wegman et al., 1981). Maximum
concentrations in drainage water, determined within 24 h of the start
of leaching, were 9.3 mg/litre (methyl bromide) and 72 mg/litre
(Br-).
Further studies of bromide ion concentrations in precipitation,
surface water, and ground water in the polder district in the
Netherlands (a main horticultural area) in 1979-80 gave maximum values
of 0.98, 41, and 17 mg/litre respectively, the highest concentrations
being found during the main fumigation/leaching time in
September-October 1979 (Wegman et al., 1983). During these few weeks,
an estimated 1.78 million kg methyl bromide (about 1.5 million kg
bromide ion) were used for soil fumigation. From their measurements,
Wegman et al. (1983) calculated that 14% of the applied methyl bromide
was converted to bromide ion. This is in agreement with Daelemans
(1978), whose studies showed that 10-30% of methyl bromide is
converted to Br-. The fumigation method used was the "hot gas
method", whereby the fumigant was discharged under thin low-density
polyethylene (LDPE) sheeting. Subsequently, in June 1981, the use of
LDPE for this purpose was forbidden.
In 1985, bromide concentrations of 10-35 mg/litre were observed
in Westland (the Netherlands) surface water with maximum
concentrations in the Poel and Bosch polders of 38.7 and 31.7
mg/litre, respectively (Van Doorn et al., 1989). Private water
supplies from shallow pumps in the Netherlands near methyl bromide
soil operations were expected to have increased bromide contents (Van
Doorn et al., 1989). In 1986, in the Westland, 260 000 kg bromide
ended up in surface water, at an average dosage of 700 kg/ha.
Similar investigations were carried out by Vanachter et al.
(1981) in the glasshouse crop growing region of Malines - Antwerp
(Belgium) into bromide concentrations in surface water in periods of
intense soil fumigation and leaching (August-September) compared with
periods before and after this, when soil fumigation and leaching were
less frequent. Sampling was also carried out during leaching, near the
greenhouses, as well as in the ditches and draining water. For
comparison, samples of natural Br- concentrations in surface water
were taken from a region where methyl bromide fumigation was not
carried out.
The results showed that during the leaching period,
concentrations of Br- in the surface water in the greenhouse crop
growing area were significantly higher (maximum 9.6 mg/litre) but,
five weeks later, were either not detectable (<0.1 mg/litre) or very
low (1.59 mg/litre) in little brooks. The bromide concentration in
rivers was less than 0.8 mg/litre, except for one site, where either
sea water or the effluents from a local photographic plant resulted in
concentrations of up to 4.5 mg/litre. In the direct drainage area of
the glass houses, transient concentrations of up to 33 mg/litre were
measured, decreasing rapidly within a few days.
Guns (1989) measured groundwater levels in four greenhouses in
Belgium from September 1986 to August 1988. The bromide content of
groundwater depended, not only on the length of leaching, but also on
the type of soil. When the deeper soil layers contained more clay, the
concentration of the bromide ion in the groundwater, after fumigation
and leaching, was still relatively high (48 mg/litre before and 280
mg/litre after fumigation in one study).
5.1.3 Soil
The natural bromide present in unfumigated soil depends on the
soil type. Van Wambeke et al. (1974) gave values of 3.3 mg/litre for
fresh sandy loam soil and 2.5 mg/litre for peat soil. Hoffman &
Malkomes (1974) stated that the natural concentration of bromide in
the soil was less than 10 mg/kg, depending on the type of soil and
geographical situation.
Fallico & Ferrante (1991) measured bromide concentrations in
greenhouse soil before, and after, the application of methyl bromide
(80 g/m2). Before fumigation, bromide levels were about 5 mg/kg. Two
months after treatment, bromide levels of over 30 mg/kg were measured.
After a further 3 months, levels had decreased to less than 10 mg/kg.
Bromide ion concentrations following greenhouse and soil
fumigation depend on the dosage, exposure time, aeration period,
temperature, the type of soil, the amount of rain or leaching water,
and the type of covering (sealing conditions). A year after fumigation
with 70-80 g methyl bromide/m3, bromide values of 0.2-11.5 mg/kg
were recorded in the upper 30 cm of soil (Basile et al., 1987).
The bromide remaining in the soil after fumigation can affect
soil fertility (Rovira & Ridge, 1979). As wheat particularly tends to
concentrate bromine, soil and wheat dynamics were studied (1981-83) in
methyl bromide-fumigated plots in a Mediterranean climate (Italy).
Bromide residues ranged between 5 and 10 mg/kg in the fumigated soil
to a depth of 50-60 cm. The total amount of bromide in the soil was
5.8 g/m2 up to a depth of 1 m and remained almost constant during
the wheat-growing period (Fransi et al., 1987). The amount of bromide
residues was about 8 % of that applied (900 kg/ha) five months
previously, compared with the 20 % found by Van Wambeke et al. (1974)
in similar soils.
5.1.4 Food
When considering the published levels of methyl bromide and
inorganic bromide in various foods, the method of analysis is
important (section 2.4.9). Originally, bromide content only was
measured and there is very little information on the methyl bromide
content. Measurements of both entities are important as well as other
residual products.
5.1.4.1 After soil fumigation
Bromide accumulation in plants depends on various factors, such
as the physical and chemical properties of the soil, the climatic
condition, the plant species and particular tissues, and the cultural
practices (Basile & Lamberti, 1981). Moreover, it depends on dosage,
exposure time, and aeration.
A study was carried out in 1980 in Metaponto, in southern Italy,
where methyl bromide was used to control nematodes and other plant
pathogens in the soil (Basile & Lamberti, 1981). Bromide ion levels
were 20-51 mg/kg (tomatoes), 8-44 mg/kg (string beans), 25-149 mg/kg
(radishes), 18-60 mg/kg (aubergines), 6-165 mg/kg (cucumbers), 13-46
mg/kg (courgettes), and 3-27 mg/kg (peppers) on a fresh weight basis.
In experimental plot conditions, the methyl bromide concentration was
60 g/m2 under a plastic cover with the soil temperature 14-17 °C at
10 cm depth. The covering was removed after 2 days and the plot
rotavated at a depth of 20 cm to eliminate residual gases.
Roughan & Roughan (1984a,b) carried out surveys of bromide ion
residues in lettuces, cucumbers, tomatoes, and self-blanching celery
grown in soil fumigated with methyl bromide, and compared these values
with those in a range of home-produced and imported fruit and
vegetables. Lettuce grown on unfumigated soil contained less than 10
mg bromide ion /kg, while most lettuces harvested from methyl bromide
fumigated soil were found to contain considerably more, 30 %
containing over 500 mg/kg and 2 % even in excess of 2000 mg/kg
(Roughan & Roughan, 1984a).
Studies on vegetables grown under protective covers on soil
previously fumigated with methyl bromide showed levels ranging from 1
to 109 mg/kg (fresh weight) in 29 late-season cucumbers, 5 to 326
mg/kg in 242 tomato samples, and 2 to 521 mg/kg in 38 samples of
celery (Roughan & Roughan, 1984b); 65 % of the late season cucumbers
contained more than background of bromide levels up to 10 mg/kg. Crops
of tomatoes (summer 1981) grown on sites where methyl bromide
fumigation had taken place in 1980/1981, contained considerably higher
levels than those grown on a site fumigated in 1979. In a survey from
retail outlets (January-November 1979), tomatoes and cucumbers from
the Canary Islands and Spain generally contained less than 10 mg/kg
(fresh weight). Of those grown in the United Kingdom, levels exceeded
10 mg/kg in 35 %, and 100 mg/kg in 5 %, ranging up to 177 mg/kg. These
higher levels of bromide ion could be attributed to the plants having
been grown on soil fumigated with methyl bromide prior to planting
(Roughan & Roughan, 1984b). The total bromide ion contents of various
crops obtained from retail outlets in the United Kingdom during the
period June 1981 to July 1982 are summarized in Table 22. The survey
indicated that vegetables, such as tomatoes, celery, cucumbers,
lettuce, radishes, and aubergines, grown in United Kingdom and the
Netherlands contained higher levels than elsewhere. Other crops had
mean bromide levels of less than 10 mg/kg, similar to other figures
published for background bromide ion content.
Table 22. Total bromide ion content of various crops obtained from retail
outlets in United Kingdom during the period June 1981 to July 1982a
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
apple France 5 0.1-0.3 0.2
aubergine Netherlands 4 2-23 11
avocado pear not known 1 1
banana Windward Islands 2 2 2
bean, broad England 3 1-2 2
bean, french Guernsey 1 1
bean, runner England 2 1 1
bean, sprouts England 3 1 1
cabbage England 3 1-2 2
calebrese England 2 1 1
(broccoli)
carrot England 1 2
cauliflower England 3 0.3-1 1
Chinese England 3 1-2 1
leaves
celery England 12 1-178 28
Guernsey 1 9
Israel 4 7-14 10
Spain 16 2-8 4
USA 1 4
courgette England 3 1-3 2
cucumber Canary Islands 15 0.3-10 3
England 36 0.2-87 9
Spain 3 0.2-10 4
Netherlands 7 0.1-14 7
grapefruit
segments Cyprus/Israel,
S. Africa 4 0.1-0.4 0.3
green pepper Netherlands 7 0.4-5 2
ettuce Belgium 1 5
Cyprus 5 1 1
England 69 1-241 15
France 9 0.2-19 4
Israel 6 1-4 2
Spain 11 0.4-4 2
Netherlands 26 2-57 21
USA 12 0.1-2 1
Table 22 (continued)
Crop Country of Total No. Bromide ion
origin of samples (mg/kg fresh weight)
Range Mean
marrow England 2 1-2 1
mushroom England 62 0.2-24 1
onions Israel,
Netherlands 3 0.4-1 1
onion, England 3 2-4 3
spring
orange S. Africa, Spain 6 <0.1-0.4 0.2
segments
pea England 4 1-3 2
potato Cyprus, England 3 1-2 1
radish England 18 0.2-3 1
Israel 2 5 5
Netherlands 12 0.1-48 13
USA 1 1
strawberry England 1 0.3
tomato Canary Islands 8 1-5 4
England 33 1-70 13
Spain 15 1-7 3
Netherlands 14 1-39 11
a From: Roughan & Roughan (1984b).
Brown et al. (1979) measured the bromide concentration in several
plant species in unfumigated and methyl bromide-fumigated plots in
California (Table 23). Many plants showed increased bromide
concentrations. Strawberries and grapevines absorbed relatively
little. As the interval increased between soil fumigation and
planting, there was a general decline in bromide levels, though the
interval could be as long as three years before the crops returned to
a level of about 10 mg/kg (Brown et al., 1979; Roughan & Roughan,
1984a). Table 24 shows bromide concentrations in plant material 1, 2,
3, and 4 years after fumigation with methyl bromide.
Fallico & Ferrante (1991) measured bromide levels in tomatoes in
crops grown in soil that had been fumigated with methyl bromide ten
days prior to planting. Bromide levels in tomatoes grown in the
treated soil and harvested after 60 days were about double (55 mg/kg)
those in tomatoes grown in untreated soil. The bromide levels
decreased with each successive harvest, but were still higher than
those in control plants after the fourth harvest.
Table 23. Bromide concentrations in several plant species in unfumigated and methyl bromide-fumigated plotsa,b
Plant species Treatment Range of Average bromide S.E. of
and part bromide concentration means
concentrations (mg/kg dry weight)
(mg/kg dry weight)
barley control 4 to 575 106 (19)c 38
(whole top) fumigated 120 to 5235 1788 (23) 373
bur clover control 1 to 407 96 (11) 40
(whole top) fumigated 196 to 2371 1334 (14) 155
filaree control 4 to 546 135 (14) 47
(whole top) fumigated 718 to 7380 2600 (10) 683
wild oats control 9 to 876 196 (14) 66
(whole top) fumigated 1233 to 5034 3364 (11) 441
spinach (leaves) fumigated 1772 to 3195 2521 (4) 387
ryegrass fumigated 1481 to 2790 2378 (4) 302
sweet potato
(leaves)
1st sampling fumigated 640 to 923 753 (4) 69
4th sampling fumigated 312 to 372 330 (4) 14
sweet potato
(root) fumigated 204 to 237 220 (2) 17
strawberry control 14 to 129 63 (9) 16
(leaves)d fumigated 3 to 372 88 (36) 13
grape (leaves)e control 1 to 101 28 (90) 3
fumigated 1 to 402 48 (278) 4
Table 23 (continued)
a From: Brown et al. (1979).
b These data represent samples collected the first year after fumigation with MeBr at rates of 34-68 g/m2,
except for grape leaves.
c Numbers in parentheses refer to the number of samples in the average.
d Strawberry leaves were collected after 1-6 annual fumigations with methyl bromide.
e Grape leaves were collected 2-4 years after fumigation with methyl bromide.
Table 24. Bromide concentrations in plant material (mg/kg dry
weight), 1, 2, 3, and 4 years after fumigation with methyl bromidea
County Untreated Years after fumigation
controls
1 2 3 4
Sonoma 94(19)b 3018(16) 360(15) 516(8) 12(3)
Napa 163(21) 1476(15) 742(10) 430(2)
a From: Brown et al. (1979).
b Numbers in parentheses = number of samples included in the
average.
Table 25. Inorganic bromide residues detected in samples of fruit and
vegetables in UK (1988 to 1989)a
Commodity Concentration Number of samples
range (mg/kg)c
Lettuces, produced n.d. 0
in United Kingdom
(proposed CAC 1-20 48
MRLb=100) 21-100 15
101-300 9
301-529 5
Lettuces, imported n.d. 0
1-20 21
21-45 3
Rice, imported n.d. 59
[MRL=50 1-10 37
(unprocessed 11-20 16
rice)] 21-50 14
50-100 10
121 and 124 2
Nuts (no MRL)
almonds n.d. 4
1-147 24
brazil nuts n.d. 0
3-140 22
Table 25 (continued)
Commodity Concentration Number of samples
range (mg/kg)c
cashew nuts n.d. 16
3-53 5
chestnuts n.d. 5
3-23 3
coconuts n.d. 0
2-6 5
hazel nuts n.d. 7
1-194 10
peanuts n.d. 4
2-109 18
pine nuts n.d. 4
46 1
Nuts (continued)
pistachio nuts n.d. 0
3-104 4
tiger nuts n.d. 0
17-20 4
walnuts n.d. 3
2-210 21
sesame seed n.d. 5
17-56 5
sunflower seed n.d. 3
2 6
3 1
Dried fruits
currants, imported
(CAC MRL = 100) n.d. 22
1-3 9
6-15 5
sultanas n.d. 20
(CAC MRL = 100) 1-6 6
6-12 7
Table 25 (continued)
a From: MAFF (1990).
b CAC = Codex Alimentarius Commission; MRL = maximum residue limit
(mg/kg) legally permitted in, or on, food commodities or animal feed.
c n.d. = not detected.
The results of a further survey (1988-89) in the United Kingdom
of inorganic bromide levels in fruit and vegetables are summarized in
Table 25. Fourteen samples of lettuce produced in the United Kingdom
exceeded the Codex Alimentarius Commission maximum residue limit (CAC
MRL) for inorganic bromide of 100 mg/kg (MAFF, 1990).
The effects of leaching following soil fumigation with methyl
bromide up to 100 g/m2 are shown in Table 26. At sites with low soil
bromide residues, the resulting bromide residues in lettuce were
nearly unaffected by leaching. At other sites, there was a positive,
but diminishing, response to increasing rates of leaching, very high
residues probably being due to high levels of organic matter (Smart,
1990).
Recent monitoring of samples of individual crops having a
likelihood of being grown on soil fumigated with methyl bromide showed
that only a small percentage contained residues above Codex
Alimentarius Commission recommended limits. The high levels were
mainly in lettuce. The report of Smart (1990) showed that the
proportion of samples having high residues had declined since the late
1970s, when, in some countries, residues in lettuce were as high as
500-1000 mg/kg. Leaching of treated soils, attention to timing of
application, and integrated pest control have all helped to reduce
such residue levels.
Bromide level tolerances for a variety of methyl bromide
fumigated raw agricultural commodities in the USA are shown in Table
27. However, according to the US EPA, because of its existing
toxicological data base and its environmental ubiquity, inorganic
bromide is not of toxicological concern. Requirements for residue data
to support existing inorganic bromide tolerances were waived by the
Agency (US EPA, 1989).
Table 26. Bromide residues in lettuce grown under protection of soil fumigated with bromomethane and leached with water
before planting in the United Kingdoma
Site Soil texture Soil organic Number of Interval between Bromide residues in lettuce (mg/kg fresh weight)
matter (%) applications planting and for the water application rates (mm)
in previous harvest
years (weeks) 0b 100 200 300 400
A fine sandy loam 4 2 16 81 102 92 150 142
B sandy loam 12 1 12 62 70 232 93 114
C sandy loam 13 2 13 307 215 98 157 117
D sandy loam 1 24 765 427 392 284 250
E sandy loam 8 4 21 676 335 328 164 134
Fc loamy peat 51 4 14 1958 1534 1001 - -
Mean sites A-E 378 230 228 170 151
a Summarized from Food and Agriculture Organization (1985) by Smart (1990).
b Residue figures for crops grown in soils not having any leaching.
c At F, the leaching treatments were applied after planting the lettuce crop.
Table 27. USA tolerancesa
Br in, or on, the following raw agricultural commodities,
which have been fumigated with the antimicrobial agent
and insecticide methyl bromide after harvest (with the
exception of strawberries) (mg/kg)
corn (pop) 240
almonds, brazil nuts, bush nuts, 200
butternuts, cashews, chestnuts,
cottonseed, filberts, hickory nuts,
peanuts, pecans, pistachio nuts,
soybeans, walnuts
asparagus, copra, cumin (seed), 100
ginger (roots), pomegranates
avocados, coffee beans, potatoes, 75
sweet potatoes
alfalfa (hay), barley, beans, 50
beans (green), benas (lima), beans
(snap), cabbage, cippolini (bulbs),
cocoa beans, corn, corn (sweet),
garlic, oats, peas, peas (blackeyed),
rice, rye, sorghum (grain), timothy
(hay), wheat
artichokes (Jerusalem), garden beets 30
(roots), sugar beets (roots), carrots,
citrus citron, cucumbers, grapefruit,
horseradish, kumquats, lemons, limes,
okra, oranges, parsnips (roots), peppers,
pimentos, radishes, rutabagas, salsify
(roots), squash (summer), tangerines,
turnips (roots)
apricots, blueberries, cantaloupes, 20
cherries, eggplant, grapes, honeydew
melons, mangoes, muskmelons, nectarines,
onions, papayas, peaches, pineapples,
plums, pumpkins, squash (winter), squash
(zucchini), tomatoes, watermelons
apples, pears, quinces 5
a From: US EPA (1988b; CFR 180.124).
5.1.4.2 After post-harvest fumigation
Methyl bromide is widely used as a post-harvest fumigant to kill,
or prevent, pest infestation. In 1976, around 100 000 metric tonnes of
food commodities were treated with methyl bromide in the United
Kingdom (Fairall & Scudamore, 1980).
Fairall & Scudamore (1980) measured methyl bromide residues in
dried milk, wheat, flour, rapeseed, and groundnut samples after store
fumigation (see Table 28). Products such as groundnuts and rapeseed
retained higher amounts of methyl bromide. No methyl bromide was
detected in any commodity after storage for 1 month (detection limit
10 µg/kg).
DeVries et al. (1985) measured the rate of decrease of methyl
bromide in wheat, flour, cocoa, and peanuts after fumigation with the
gas. Samples were analysed immediately and then after various time
intervals of exposure of the sample to air. The methyl bromide
concentration decreased very rapidly in all cases, no residual methyl
bromide being found in any of the samples after 2 weeks (detection
limit, 0.4 µg/kg).
(a) Wheat and cereals
Pesticide residues in home-grown and imported wheat were measured
in the United Kingdom by Osborne et al. (1989); 45 samples were
analysed for methyl bromide (method: Fairall & Scudamore, 1980) and
inorganic bromide. No methyl bromide was found in excess of the
detection limit (0.01 mg/kg); all samples contained inorganic bromide,
but at levels of 4 mg/kg or less, which is given as the level
naturally present in wheat as a result of uptake from the soil (Heuser
& Scudamore, 1970; Osborne et al., 1989).
Trials carried out on a commercial scale showed that CT
(concentration x time) products for methyl bromide generally lay in
the range of 50-2000 mg.h/litre (Scudamore, 1987). The higher values
are usually found in pockets of grain at the bottom of silos or bins.
Scudamore (1987) recommended careful monitoring, especially at
increased temperature and moisture content, when treating more
sorptive cereals, such as oats or maize, or those with which methyl
bromide reacts more readily. In three samples of wheat from different
parts of the United Kingdom, bromide levels increased between 2 and 3
times over a range of 11-16 % moisture content.
Table 28. Methyl bromide residue levels (mg kg-1) during storagea
Days
Commodity 0 0.04 0.25 1 2 4 7 11
dried milk 0.5 0.15 0.03 0.006 - - - -
wheat 0.8 0.42 0.33 0.08 0.04 n.d.b - -
flour 0.28 0.09 0.04 0.02 n.d. - - -
rapeseed 4.2 3.0 1.5 - 0.95 0.5 0.10 0.08
groundnuts 7.7 4.5 3.2 2.6 1.6 0.38 0.38 0.03
a From: Fairall & Scudamore (1980).
b n.d. = none detected.
Table 29. Bromide ion and methyl bromide concentrations (mg/kg) in
flours exposed to methyl bromide only in silos and in pastas obtained
from these fumigated and unfumigated floursa
Material Treatment Number of Bromide ion Methyl
samples (mean±S.D.) bromideb
flours unfumigated 3 1.26±0.03 n.d.
fumigated 3 1.60±0.11 n.d.
pasta from unfumigated 6 1.24±0.13 n.d.
(macaroni) flours
from fumigated 6 1.91±0.22 n.d.
flours
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than the detection limit of 0.01
mg/kg.
Stacks of bags containing stored grains and pulses (wheat,
lentils, maize, barley, chick-peas, peas, and sorghum) were covered
with PVC sheets and exposed to methyl bromide fumigation for 48 h
(Urga, 1983). Following aeration, residues of less than 50 mg/kg
bromide were measured, with the exception of 60 mg/kg after 24 h
aeration and 59 mg/kg after 36 h aeration. Cova et al. (1986)
investigated the effects of exposure to methyl bromide in flour, and
pastas made from it. Flour was exposed to methyl bromide in silos
(conditions: mean temperature - 18 °C, concentration - 24 g/m3,
duration of treatment - 68 h, duration of ventilation - 3 days). Table
29 shows levels of bromide ion before, and after, fumigation. Methyl
bromide could not be detected. In a second experiment (summarized in
Table 30) on pasta made from unfumigated flour, rice flour, and white
flour, Cova et al. (1986) examined the influence of the type of
packaging on the effects of fumigation. Every item was packed in two
different ways, i.e., a cardboard box or a transparent envelope made
of a double layer of polypropylene. The products were fumigated in a
closed room under the conditions given above, and the mean bromide ion
concentrations ranged between 2.03 mg/kg (pasta) and 46.23 mg/kg (egg
pasta), while methyl bromide was not detected.
Table 30. Bromide ion and methyl bromide concentrations (mg/kg) in unfumigated
and fumigated foodstuffs, treated in their retail packagingsa
Material Treatment Number of Bromide ion Methyl
samples (mean ± S.D.) bromideb
rice unfumigated 3 0.72 ± 0.06 n.d.
funfumigated 3 10.63 ± 0.67 n.d.
flour unfumigated 3 3.17 ± 0.09 n.d.
funfumigated 3 6.66 ± 0.01 n.d.
white flour unfumigated 3 1.22 ± 0.15 n.d.
funfumigated 3 4.19 ± 0.82 n.d.
pasta (macaroni) unfumigated 6 2.40 ± 0.26 n.d.
funfumigated 6 2.60 ± 0.27 n.d.
pasta (spaghetti) unfumigated 6 1.92 ± 0.17 n.d.
funfumigated 6 2.03 ± 0.07 n.d.
pasta with eggs unfumigated 6 4.13 ± 0.12 n.d.
funfumigated 6 46.23 ± 1.57 n.d.
pasta with eggs unfumigated 6 4.62 ± 0.31 n.d.
and spinach funfumigated 6 39.00 ± 0.01 n.d.
a From: Cova et al. (1986).
b n.d. = not detected, i.e., lower than detection limit of 0.01 mg/kg.
(b) Spices, nuts, and dried fruits
In the USA in 1980, two warehouses containing imported spices
were fumigated to eradicate a khapra beetle infestation. Methyl
bromide and inorganic bromide residues were determined in the 52
spices before, and after, fumigation (using GC-ECD). In addition, an
ashing titration method for bromide ion residue was used, allowing a
comparison of the two analytical methods (Reeves et al., 1985). Before
fumigation, the highest methyl bromide residue was in parsley (14.9
mg/kg). Seventy-two hours after fumigation with 100 g/m3 for 12 h,
samples were collected and analysed. The highest methyl bromide
residue was found in sage (65.8 mg/kg). Levels of inorganic bromide
residues before fumigation (GC-ECD) were all lower than 200 mg/kg;
after fumigation, only two samples contained higher levels.
In a Canadian study, a number of spices, seeds, nuts, and dried
fruits and vegetables, including samples of celery, mustard, sesame,
coriander, pumpkin and sunflower seeds, cloves, peppercorns, dates,
figs, prunes, raisins, beans, minced onion, a vegetable mix, walnuts,
and peanuts were analysed for methyl bromide residues (Page & Avon,
1989). Of the 30 samples, only a sample of pumpkin seeds was found to
contain methyl bromide (3 µg/kg). Fifty-one chocolate and grain-based
products were also analysed and found not to contain any methyl
bromide.
In contrast, when samples of food known to have been treated with
methyl bromide were analysed, of the 60 samples, 16 contained residues
>1 ng/kg and 5 contained >100 ng/kg methyl bromide. These samples
included dried currants (2.9 mg methyl bromide/kg), chocolate-covered
nuts (0.66 and 0.19 mg/kg), and rice (2.3 mg/kg) and maize (0.21
mg/kg) flours. These findings were confirmed by mass spectrometry
(Page & Avon, 1989).
Bromide residues after methyl bromide fumigation were determined
in samples of dried fruits, cereals, nuts, and spices imported into
New Zealand in 1977 and early 1978 (Love et al., 1979). About one-half
of the nut and spice samples contained total bromide levels exceeding
50 mg/kg, and occasional high levels of bromide residues were found in
cereals.
Fairall & Scudamore (1980) showed that rapeseed and groundnut
samples retained higher amounts of methyl bromide than other
foodstuffs after store fumigation (Table 28). A survey of retail nuts,
seeds, and nut products in October 1984 (Table 31) showed that levels
of methyl bromide in some of these products were higher than the Codex
Alimentarius Commission guideline (MAFF, 1989).
Table 31. Residues of methyl bromide in samples of nuts, seeds, and nut productsa,b
Number of samples Residue concentrations
(mg/kg)
tested containing rangec mean
residues
almonds 5 2 n.d. to 0.06 < 0.02
brazil nuts 4 0 n.d. -
dried chestnuts 1 1 0.2 -
hazelnuts 4 0 n.d. -
mixed nuts 3 0 n.d. -
nuts and raisins 3 2 n.d. to 0.2 0.08
peanuts 12 9 n.d. to 0.3 0.06
walnuts 7 4 n.d. to 2.2 0.4
othersd 6 0 n.d. -
a From: MAFF (1989).
b 45 samples were obtained during October 1984. and analysed for
residues of methyl bromide. The CAC (1986) guideline level for
methyl bromide in nuts is 0.01 mg/kg.
Table 31 (continued)
c n.d. = not detected (the limit of determination was 0.02 mg/kg).
d One sample each of cashews, pecans, pine kernels, pistachios, sunflower seeds, and
tiger nuts.
(c) Fresh fruit
Methyl bromide residues determined in laboratory studies on fresh
fruits are summarized in Table 32. Studying the effects of fumigation
dose and length of the following aeration periods, Sell & Moffitt
(1990) and Sell et al. (1988) found that desorption of methyl bromide
from apples and cherries, respectively, followed pseudo-first-order
decay curves, the first component resulting from removal of the
pesticide from free air space in the chamber, and the second, from the
desorption from the fruits. Singh et al. (1982) found that methyl
bromide absorption in avocados depended on oil content rather than
skin thickness or protein content.
(d) Milk and cheese
Bromides may be present naturally in cows' milk in amounts up to
8 mg/litre. When cows were fed on grain fumigated post-harvest with
methyl bromide, higher levels of bromide were found in the milk, e.g.,
cows fed grain containing bromide at 220 mg/kg had bromide levels in
their milk of 10-20 mg/litre (Lynn et al., 1963).
Cheese fumigated with methyl bromide showed high bromide residues
(Laug, 1941).
5.1.5 Animal feed
Knight & Costner (1977) reported bromide residues of 6800-8400
mg/kg in hay that was harvested in the spring after the field had been
injected with methyl bromide. The resulting toxic effects on animals
fed with this hay are reported in section 7.3.7.
5.1.6 Other products
The chlorine and bromine contents in tobacco and tobacco smoke
were investigated by Häsänen et al. (1990). Smoke per cigarette
contained 1 µg bromine in the particulate phase and 5 µg bromine in
the gaseous phase. In the gaseous phase, methyl bromide accounted for
80% of the total bromine. Methyl bromide is used widely as a fumigant
in uncured tobacco storage and this can increase the bromine content
in tobacco considerably.
Methyl bromide has been used in growing tobacco seedlings (Ostrec
& Korunic, 1989).
5.1.7 Terrestrial and aquatic organisms
No data are available.
5.2 General population exposure
5.2.1 Food
The general population may be exposed to residues of methyl
bromide and inorganic bromide, and other possible metabolic products,
which may be present in food marketed for consumption. Levels of
methyl bromide and inorganic bromide in food are described in section
5.1.4.
Food commodities containing higher levels of oil and fat, such as
groundnut and rapeseed (Table 28), retain higher amounts of methyl
bromide residues after fumigation, which disappear after a storage
period of about one month (Fairall & Scudamore, 1980; DeVries et al.,
1985). However, higher levels of inorganic bromide residues may remain
in food commodities marketed after fumigation (section 5.1.4.2).
Scheffrahn et al. (1992) investigated the effects of methyl
bromide fumigation on foods in retail packages, in sealed and unsealed
plastic containers, with a view to simulating conditions in which
residues may be found in food products after fumigation of a
residential house in the USA. Fatty commodities in unsealed packages
contained higher residues (>1 mg/kg) than other foods. The fumigant
may have entered the containers by two routes, diffusion through the
air-spaces around the lids (opened peanut butter jar) or in porous
packaging (parmesan cheese in cardboard box), and by permeation into
polyurethane bagged foods. Factory sealed, polyethylene terephthalate
(PETE) containers gave better protection. Vacuum-packed foods in metal
(soup, coffee) or glass (sauce) containers yielded no residues.
Bromide residues in foodstuffs were discussed by Van Leeuwen &
Sangster (1987) in a review of the toxicity of the bromide ion. Total
diet surveys in the United Kingdom in 1978 and 1979 gave a daily
intake of 8.4 mg bromide per person. These values are consistent with
those of two Dutch surveys where average daily intakes of 9.4 and 7.7
mg bromide per person, respectively, were recorded, i.e.,
approximately 3 mg/kg diet (Van Leeuwen & Sangster, 1987).
Table 32. Methyl bromide residues in fresh fruits after fumigation
Fruit Fumigation time Storage Timea Residue Reference
and dose temperature (mg/kg)
(°C)
grapefruit 2 h, 64 mg/litre 24 1 h 26.9b King et al. (1981)
48 h 0.52
peaches 3.5 h, 32 g/m3 2.5 1 day 11.0 Austin & Phillips (1985)
7 days 4.0
mango N.D.c, 64 g/m3 N.D. 1 h < 15.0 Stein & Wolfenbarger (1989)
grapefruit 2h, 48 g/m3 15.6 5 days < 5 King & Benschoter (1991)
oranges, 2h, 48 g/m3 15.6 5 days < 10
mandarines,
tongors
avocado 2h, 32 g/m3 20 1 day up to 0.5d Singh et al. (1982)
2 days up to 0.1
a Time of analysis after end of fumigation period.
b Aeration period: 15 min.
c N.D. = no data given.
d Aeration period: 30 min.
The metabolites resulting from methyl bromide post-harvest
fumigation of a number of crops have been analysed (Starratt & Bond,
1990a,b). Both physically- and chemically-bound residues were
measured. In 7 out of the 9 commodities, over half of the total
residue was chemically bound 1 h after fumigation. These chemically
bound residues were stable for at least 6 months. Most reaction
products were O-, S-, and N-methylated proteins (section 6.3).
Methylation of purines and pyrimidines in the nucleic acids accounted
for 0.1-6 % of the chemically-bound residue, with N being the only
site of methylation. The methylation products identified in this study
are also known to occur naturally.
5.2.2 Drinking-water
In 1988, in California, only one sample out of 43 056 taken from
2977 wells, showed any presence of methyl bromide (detection limit -
1 µg/litre) (CDFA, 1988).
In areas, such as in the Netherlands, where private water
supplies are from shallow wells near methyl bromide soil operations,
there could be increased bromide contents in the water (Van Doorn et
al., 1989).
5.2.3 Human breast milk
There are no data available.
5.2.4 Sub-populations at special risk
People who live in close proximity to greenhouses or to fields or
storehouses that are being fumigated have a higher risk of exposure to
methyl bromide gas than the general population. Similarly, persons
inadvertently, or intentionally, entering buildings following, in
particular, structural fumigation, are at risk.
5.3 Occupational exposure during manufacture, formulation, or use
The number of incidents and fatalities (section 9) show that
occupational exposure, especially during fumigation, is potentially
hazardous.
5.3.1 During manufacture
In a methyl bromide plant in the USA, workplace air
concentrations of 78-116 mg/m3 (20-30 ppm) were recorded using
direct measurement (Evans, 1979).
In a methyl bromide-producing factory in Japan, methyl bromide
concentrations in the worker's breathing zone were usually under 4
mg/m3, but sometimes exceeded 20 mg/m3 (Kishi et al., 1988).
5.3.2 During fumigation
If safety procedures are not followed, workers may be exposed to
methyl bromide accidentally during, or after, fumigation operations.
Methyl bromide in low concentrations is odourless so that a toxic
atmosphere may not be apparent to the worker. Only at higher
concentrations (100 x the actual TLV(R) of 20 mg/m3 (5 ppm) does
it have a sweet smell (Van Den Oever et al., 1982). An odour threshold
of 65 mg/m3 has been reported for methyl bromide (Worthing & Walker,
1983). Therefore, it is usually marketed in the form of 98% methyl
bromide and 2% chloropicrin, as a lacrimatory agent. For post-harvest
fumigation, 100% methyl bromide is used.
The various fumigation techniques used together with methyl
bromide exposure values and methods of application are outlined in
Table 33 (Guillemin et al., 1990).
5.3.2.1 Structural fumigation
In carrying out the general fumigation of a building, sufficient
gas must be liberated into the free space to kill the insects and then
the toxic level maintained for a defined period of time. After the
treatment, the residual gas remaining in the building is dispersed to
the outside atmosphere. However, there are basic differences in
defining structures amongst various countries. In the USA, structural
fumigations mainly involve residential houses whereas, in Europe, they
generally refer to flour mills and food processing areas. The
fumigation procedures and the safety aspects in these circumstances
could be very different.
Methyl bromide exposure levels for structural fumigation workers
in California were measured by Anger et al. (1986). They described the
work involved. "Structural fumigation is conducted by a work crew of
2-4 men who cover the buildings to be fumigated with large vinyl
tarpaulins and connect them by spring clamps. Cylindrical tubes filled
with sand (sand snakes) are placed at the base of the structure to
hold down the tarpaulins, thus sealing the building. After this one to
two hour procedure, termed a closing, the fumigant is introduced into
the unoccupied house via a tube or hose, and the fumigators leave the
site. The fumigators wear self-contained breathing apparatus (SCBA).
The next day the work crew removes the tarpaulins, opens the windows,
and places fans in the house to clear the fumigant. It is in this 30-
to 45-min process, termed the opening, that worker exposures may
occur. Typically, each work crew, led by a State-licensed fumigator
(the "licensee"), conducts three openings and/or closings each day".
Table 33. Integrated samples of methyl bromide in air taken in a survey of methyl bromide
fumigation in Switzerlanda
Circumstances Exposure Sample Range of values
of sampling category No.
Minimum Maximum
mg/m3(ppm) mg/m3(ppm)
1. Space fumigation
- during fumigationb occupational 7 <0.8 (<0.2) 500 (128.4)
- during aerationb occupational 7 2.3 (0.6) 646 (166.0)
- resuming operation para-occupational 47 <0.8 (<0.2) 9 (2.3)
- during fumigation environmental 12 <0.8 (<0.2) 79 (20.4)
- during aeration environmental 12 <0.8 (<0.2) 105 (27.1)
2. Soil fumigation
- during fumigationb occupational 5 2 (0.5) 151 (39.0)
- removal of sheetingb occupational 3 34 (8.8) 144 (36.9)
- inside greenhousec para-occupational 5 12 (0.3) 9 (2.2)
3. Chamber fumigation
- outside chamber para-occupational 3 35 (8.9) 293 (75.3)
- removing contents para-occupational 4 5 (1.2) 17 (4.3)
a From: Guillemin et al. (1990).
b Fumigators generally wore respiratory protection during these operations.
c Greenhouse only partially fumigated; includes post-fumigation soil tillage.
Personal samples from fumigators taken when they entered houses
24 hours after fumigation with 23 000 to 31 000 mg methyl
bromide/m3, indicated that a house might contain 80-2000 mg methyl
bromide/m3 (Table 34). Personal samples taken on fumigators working
outside the houses when they were opened again showed concentrations
of between 0 and 8 mg/m3 in the half-hour periods during the cover
removal (Anger et al., 1986). Area samples taken within 3 and 6 m from
the buildings during the same period ranged from 0 to 31 and 0 to 10
mg/m3, respectively (Anger et al., 1986).
Concentrations of methyl bromide inside flour mills and in the
atmosphere around the mills during, and after, fumigation were
measured by Bond & Dumas (1987). Considerable variations in
concentration were found in buildings of different structure and under
varyious weather conditions. Concentrations ranging from trace amounts
up to 90 mg/m3 (23 ppm) were found in the air around the mills
during the aeration period.
Table 34. Methyl bromide exposure concentrations (mg/m3)
in residential fumigationa
State Fumigators Under Within 3 m of 3-6 m from
licensed working covers house house
fumigator outside house
when inside
house
1875.0 5.1 2.0 46.7 32.3 0 0
75.9 0 1.2 12.5 0 10.1
90.3 0 7.0 0 17.5 10.1
1042.1 3.9 3.1 0 0 0
204.2 8.6 0 0 0
Mean
657.4 3.1 46.7 7.0 3.9
a Adapted from: Anger et al. (1986).
Guillemin et al. (1990) conducted a survey in Switzerland on
exposure during space fumigation. The maximum exposure levels for 7
integrated samples was 646 mg/m3 (166 ppm) during aeration (see
Table 33). Fumigators wore respiratory protection during these
operations. Samples of transient air taken around the buildings not
from any specific spot (distance not given), showed methyl bromide
levels in the range of 0-105 mg/m3 (see Table 33).
5.3.2.2 Soil fumigation
The amount of methyl bromide released into the atmosphere during
soil fumigation depends on the methods used (Table 9), the type and
time of covering (section 3.2.2) and soil type.
(a) Field fumigation
A formulation of 75% methyl bromide was used to kill insects and
nematodes in a strawberry crop, with 25% chloropicrin as a fungicide.
The fumigant injected into the soil produced an equilibrium of 47
000-58 000 mg/m3 which, under the covers, resulted in personal
average exposures ranging from 0 to 24 mg/m3 for the fumigators
(Anger et al., 1986). Personal sample measurements of methyl bromide
in farm workers when removing the film ranged between 0 and 33
mg/m3. Other data showed that the personal exposures of fumigators
and farm workers, who covered the plastic film with earth were between
0 and 29 mg/m3 and 0 and 17 mg/m3, respectively. Exposure of soil
fumigators, about 8 h a day, was relatively constant during most of
the year, whereas farm workers received only occasional exposures when
fields were fumigated. Spot (area) samples, taken before and after
film removal, showed that the airborne concentration under the intact
film was 8950 mg/m3 before removal and 9.3 mg/m3 an hour after
removal.
(b) Greenhouse fumigation
Roosels et al. (1981) compared two methods of methyl bromide
fumigation in greenhouses, i.e., by injection into milled soil
followed by covering with a plastic cover, or, by surface fumigation
by means of plastic pipes under a plastic cover. Two different
formulations (70 % methyl bromide/30 % chloropicrin and 98 % methyl
bromide/1-2 % chloropicrin) were used and the concentrations of methyl
bromide in the air were measured by GC-FID. During injection into
soil, values of between 400 and 4000 mg/m3 (100 and 1000 ppm) were
found with peaks up to 12 000 mg/m3 (3000 ppm) and, in one case, up
to 40 000 mg/m3 (10 000 ppm). However, when preventive measures were
taken, values of 800 mg/m3 (200 ppm) were obtained. During
fumigation, concentrations ranged between 400 and 4000 mg/m3 (100
and 1000 ppm). Using the piped surface fumigation method,
concentrations around the treated area were between 320 and 3200
mg/m3 (80 and 800 ppm) with short-term exposures of the operators to
8000-12 000 mg/m3 (2000-3000 ppm) when the pipes were being
connected.
In another investigation, concentrations of 60-100 g methyl
bromide/m2 were applied under a polyethylene cover (Van Den Oever et
al., 1982). Depending on local ventilation, quite a lot of gas escaped
into the surrounding atmosphere. The concentration during application
varied from 117 to 11 700 mg/m3 (30 to 3000 ppm). Concentration in
the air declined with time to 16 mg methyl bromide/m3 (4 ppm) five
days after application. Removing the plastic sheet involved exposure
to peak values as high as 800 mg/m3 (200 ppm), for a few seconds. On
the ninth day after application, milling the soil exposed workers to
up to 60 mg/m3 (15 ppm); on the eleventh day, no methyl bromide was
detected in the air.
6. KINETICS AND METABOLISM
6.1 Absorption
6.1.1 Inhalation
6.1.1.1 Animal studies
Uptake of methyl bromide was investigated in male Fischer 344
rats by Andersen et al. (1980). Following whole body exposures to
recirculated atmospheres of 390-11 640 mg/m3 (100-3000 ppm), the
uptake (i.e., disappearance from the atmosphere) was rapid and
exhibited first-order kinetics without a saturable component, the rate
constant being 0.44 kg-1 h-1. This rate constant was later
recalculated as 0.55 kg-1 h-1 by Gargas & Andersen (1982).
Medinsky et al. (1985) carried out a nose-only inhalation of 50,
300, 5700, or 10 400 nmol (given as 6.2-1206 mg/m3) of [14C]
methyl bromide/litre of air for 6 h in male F344 rat (5 animals/
group). The results indicated that, at low concentrations (50-300
nmol/litre), about 50% of the inhaled material was absorbed. At 5700
nmol/litre, only 37% was absorbed and, at 10 400 nmol/litre, only 27%.
The same amount of methyl bromide (650 µmol/kg body weight) was
absorbed at the two higher exposure concentrations. At 10 400
nmol/litre, the total volume inhaled by the rats was reduced (Medinsky
et al., 1985).
Raabe (1986) found about 40% uptake of inhaled methyl bromide in
studies on beagle dogs. These results are compared in Fig. 6 with
those from rats (Medinsky et al., 1985) and those from human
volunteers (Raabe, 1988), described below.
6.1.1.2 Human studies
An inhalation study was carried out to determine systemic uptake
of low concentrations of methyl bromide from air during nasal or oral
breathing (Raabe, 1988). Two male and two female volunteers inhaled
about 0.1 mg 14C-labelled methyl bromide/m3 (25 ppb), once through
the nose and once through the mouth. The uptake (% of methyl bromide
inhaled) was 55.4% nasally and 52.1% orally.
6.1.2 Dermal
Exclusively dermal exposure has only been observed in human incidents
(section 9). There are no data dealing exclusively with dermal
exposure in animals.
6.1.3 Oral
Methyl bromide (75 or 100 mg/kg) was administered to rats in olive oil
by gavage (Miller & Haggard, 1943). The methyl bromide entered the
blood stream with only a moderate degree of hydrolysis in the
intestine.
 6.1.4 Intraperitoneal injection
Methyl bromide (120-180 mg/kg body weight) was administered i.p.
in hourly doses to rats (Miller & Haggard, 1943). The percentage of
methyl bromide eliminated was between 24 and 45%. Medinsky et al.
(1984) administered [14C] methyl bromide i.p., and reported that the
major route of elimination was exhalation of 14CO2 (46%) (section
6.4).
6.2 Distribution of methyl bromide and bromide in tissues
6.2.1 Animal studies
Methyl bromide is rapidly distributed to all tissues after
inhalation and rapidly metabolized. A small percentage is cleared
slowly and incorporated into metabolic pools (Jaskot et al., 1988).
At 72 h after oral or i.p. administration of 250 µmol of [14C]
methyl bromide/kg body weight, 14-17% of the 14C remained in the
rats, the liver and kidney being the major organs of retention
(Medinsky et al., 1984).
In rats exposed, nose only, to 337 nmol [14C] methyl
bromide/litre air, radioactivity was found widely distributed in
tissues immediately following exposures. The lung, adrenal gland,
kidney, liver, and nasal turbinates contained the highest
concentrations (250, 240, 180, 130, 110 nmol equivalents/g,
respectively) (see Table 35). Immediately after exposure,
radioactivity in the liver accounted for about 17% and all other
tissues about 10% of the absorbed methyl bromide (Bond et al., 1985).
Similarly, Jaskot et al. (1988) found that the liver, lung, and kidney
were the major organs of [14C] distribution in rats immediately
after exposure to [14C] methyl bromide at 214 mg/m3 (55 ppm) for
3 min.
Honma et al. (1985) measured methyl bromide levels in male
Sprague-Dawley rats exposed to 973 mg methyl bromide/m3 (250 ppm)
for 8 h and then sacrificed at successive time intervals. The
concentrations found in adipose tissue (maximum 1 µg methyl bromide/g
tissue) were much greater than those in blood (max. 0.1 µg/g) and
other tissues - brain, liver, muscle, and kidney (maximum about 0.01
µg/g; see Fig. 7). The methyl bromide in all tissues described reached
a maximum in 1 h after exposure commenced and maintained almost the
same concentrations during exposure. Honma et al. (1985) found peak
concentrations of bromine in blood at 4 h after cessation of methyl
bromide exposure, and in kidney and liver, 8 h after (Fig. 8).
Table 35. Concentration (nmol/g) of 14C in tissues from rats exposed for 6 h to 337 nmol
14C-methyl bromide/litre aira,b
0 hc 8 h 24 h 60 h
lung 250.4 ± 27.7 (3.6) 40.3 ± 5.5 (0.5) 19.5 ± 2.3 (0.2) 19.7 ± 2.3 (0.3)
adrenal 242.0 ± 16.8 (0.9) 25.8 ± 10.2 (0.1) 23.0 ± 1.4 (0.0) 19.5 ± 3.4 (0.0)
kidney 180.4 ± 4.6 (4.3) 76.1 ± 11.4 (1.5) 36.9 ± 2.6 (0.8) 35.1 ± 3.4 (0.7)
liver 129.9 ± 7.0(16.7) 119.6 ± 13.1(11.6) 82.4 ± 10.3 (9.0) 37.8 ± 7.5 (3.9)
turbinates 110.2 ± 6.5 (0.2) 35.2 ± 5.1 (0.1) 13.3 ± 1.4 (0.0) 18.9 ± 3.2 (0.0)
spleen 98.6 ± 3.4 (0.7) 28.8 ± 5.8 (0.2) 15.9 ± 2.1 (0.1) 15.8 ± 1.7 (0.1)
small intestine 96.5 ± 14.6 (2.0) 36.3 ± 0.8 (0.4) 19.9 ± 1.8 (0.2) 15.5 ± 3.2 (0.2)
trachea 84.3 ± 1.4 (0.1) 36.4 ± 6.3 (0.0) 15.5 ± 3.9 (0.0) 18.8 ± 4.3 (0.0)
stomach 80.0 ± 1.8 (1.3) 37.8 ± 5.3 (0.6) 31.9 ± 1.0 (0.5) 27.0 ± 8.3 (0.4)
large intestine 66.3 ± 9.2 (0.9) 43.6 ± 8.6 (0.6) 19.0 ± 2.3 (0.1) 17.8 ± 2.3 (0.2)
testes 65.4 ± 3.6 (2.5) 34.5 ± 3.9 (1.4) 17.1 ± 2.6 (0.6) 12.9 ± 2.3 (0.5)
larynx 61.1 ± 4.9 (0.0) 27.1 ± 5.9 (0.0) 10.5 ± 1.2 (0.0) 11.6 ± 2.0 (0.0)
brain 53.6 ± 9.5 (0.8) 35.8 ± 4.2 (0.6) 8.8 ± 1.1 (0.2) 7.4 ± 0.7 (0.1)
heart 51.7 ± 1.9 (0.6) 35.1 ± 5.3 (0.4) 16.8 ± 1.5 (0.2) 17.6 ± 2.9 (0.2)
thymus 48.4 ± 0.5 (0.2) 33.3 ± 6.7 (0.1) 19.9 ± 2.3 (0.1) 23.4 ± 3.1 (0.1)
urinary bladder 45.6 ± 6.9 (0.1) 27.7 ± 5.5 (0.0) 12.6 ± 1.9 (0.0) 11.8 ± 1.4 (0.0)
thyroid 28.7 ± 24.4 (0.0) 34.8 ± 6.1 (0.0) 16.1 ± 2.6 (0.0) 16.4 ± 1.4 (0.0)
a From: Bond et al. (1985).
b Values represent the x ± SE of 2-3 rats. Values in parentheses are percentages of the absorbed 14C-methyl bromide.
c Time after end of exposure.
6.1.4 Intraperitoneal injection
Methyl bromide (120-180 mg/kg body weight) was administered i.p.
in hourly doses to rats (Miller & Haggard, 1943). The percentage of
methyl bromide eliminated was between 24 and 45%. Medinsky et al.
(1984) administered [14C] methyl bromide i.p., and reported that the
major route of elimination was exhalation of 14CO2 (46%) (section
6.4).
6.2 Distribution of methyl bromide and bromide in tissues
6.2.1 Animal studies
Methyl bromide is rapidly distributed to all tissues after
inhalation and rapidly metabolized. A small percentage is cleared
slowly and incorporated into metabolic pools (Jaskot et al., 1988).
At 72 h after oral or i.p. administration of 250 µmol of [14C]
methyl bromide/kg body weight, 14-17% of the 14C remained in the
rats, the liver and kidney being the major organs of retention
(Medinsky et al., 1984).
In rats exposed, nose only, to 337 nmol [14C] methyl
bromide/litre air, radioactivity was found widely distributed in
tissues immediately following exposures. The lung, adrenal gland,
kidney, liver, and nasal turbinates contained the highest
concentrations (250, 240, 180, 130, 110 nmol equivalents/g,
respectively) (see Table 35). Immediately after exposure,
radioactivity in the liver accounted for about 17% and all other
tissues about 10% of the absorbed methyl bromide (Bond et al., 1985).
Similarly, Jaskot et al. (1988) found that the liver, lung, and kidney
were the major organs of [14C] distribution in rats immediately
after exposure to [14C] methyl bromide at 214 mg/m3 (55 ppm) for
3 min.
Honma et al. (1985) measured methyl bromide levels in male
Sprague-Dawley rats exposed to 973 mg methyl bromide/m3 (250 ppm)
for 8 h and then sacrificed at successive time intervals. The
concentrations found in adipose tissue (maximum 1 µg methyl bromide/g
tissue) were much greater than those in blood (max. 0.1 µg/g) and
other tissues - brain, liver, muscle, and kidney (maximum about 0.01
µg/g; see Fig. 7). The methyl bromide in all tissues described reached
a maximum in 1 h after exposure commenced and maintained almost the
same concentrations during exposure. Honma et al. (1985) found peak
concentrations of bromine in blood at 4 h after cessation of methyl
bromide exposure, and in kidney and liver, 8 h after (Fig. 8).
Table 35. Concentration (nmol/g) of 14C in tissues from rats exposed for 6 h to 337 nmol
14C-methyl bromide/litre aira,b
0 hc 8 h 24 h 60 h
lung 250.4 ± 27.7 (3.6) 40.3 ± 5.5 (0.5) 19.5 ± 2.3 (0.2) 19.7 ± 2.3 (0.3)
adrenal 242.0 ± 16.8 (0.9) 25.8 ± 10.2 (0.1) 23.0 ± 1.4 (0.0) 19.5 ± 3.4 (0.0)
kidney 180.4 ± 4.6 (4.3) 76.1 ± 11.4 (1.5) 36.9 ± 2.6 (0.8) 35.1 ± 3.4 (0.7)
liver 129.9 ± 7.0(16.7) 119.6 ± 13.1(11.6) 82.4 ± 10.3 (9.0) 37.8 ± 7.5 (3.9)
turbinates 110.2 ± 6.5 (0.2) 35.2 ± 5.1 (0.1) 13.3 ± 1.4 (0.0) 18.9 ± 3.2 (0.0)
spleen 98.6 ± 3.4 (0.7) 28.8 ± 5.8 (0.2) 15.9 ± 2.1 (0.1) 15.8 ± 1.7 (0.1)
small intestine 96.5 ± 14.6 (2.0) 36.3 ± 0.8 (0.4) 19.9 ± 1.8 (0.2) 15.5 ± 3.2 (0.2)
trachea 84.3 ± 1.4 (0.1) 36.4 ± 6.3 (0.0) 15.5 ± 3.9 (0.0) 18.8 ± 4.3 (0.0)
stomach 80.0 ± 1.8 (1.3) 37.8 ± 5.3 (0.6) 31.9 ± 1.0 (0.5) 27.0 ± 8.3 (0.4)
large intestine 66.3 ± 9.2 (0.9) 43.6 ± 8.6 (0.6) 19.0 ± 2.3 (0.1) 17.8 ± 2.3 (0.2)
testes 65.4 ± 3.6 (2.5) 34.5 ± 3.9 (1.4) 17.1 ± 2.6 (0.6) 12.9 ± 2.3 (0.5)
larynx 61.1 ± 4.9 (0.0) 27.1 ± 5.9 (0.0) 10.5 ± 1.2 (0.0) 11.6 ± 2.0 (0.0)
brain 53.6 ± 9.5 (0.8) 35.8 ± 4.2 (0.6) 8.8 ± 1.1 (0.2) 7.4 ± 0.7 (0.1)
heart 51.7 ± 1.9 (0.6) 35.1 ± 5.3 (0.4) 16.8 ± 1.5 (0.2) 17.6 ± 2.9 (0.2)
thymus 48.4 ± 0.5 (0.2) 33.3 ± 6.7 (0.1) 19.9 ± 2.3 (0.1) 23.4 ± 3.1 (0.1)
urinary bladder 45.6 ± 6.9 (0.1) 27.7 ± 5.5 (0.0) 12.6 ± 1.9 (0.0) 11.8 ± 1.4 (0.0)
thyroid 28.7 ± 24.4 (0.0) 34.8 ± 6.1 (0.0) 16.1 ± 2.6 (0.0) 16.4 ± 1.4 (0.0)
a From: Bond et al. (1985).
b Values represent the x ± SE of 2-3 rats. Values in parentheses are percentages of the absorbed 14C-methyl bromide.
c Time after end of exposure.
 Calves fed for 49 days on a diet containing about 4650 mg
bromide/kg showed bromide concentrations in kidney, liver, and muscle
of 1808, 1015, and 465 mg/kg, respectively, on day 49. Serum and organ
bromide concentrations decreased markedly 14 days after the feeding of
this diet was discontinued (Knight & Reina-Guerra, 1977).
Calves fed for 49 days on a diet containing about 4650 mg
bromide/kg showed bromide concentrations in kidney, liver, and muscle
of 1808, 1015, and 465 mg/kg, respectively, on day 49. Serum and organ
bromide concentrations decreased markedly 14 days after the feeding of
this diet was discontinued (Knight & Reina-Guerra, 1977).
 6.2.2 Human studies
Data on the concentrations of bromide in various human tissues after
methyl bromide poisoning are scarce. In an autopsy study of a methyl
bromide-exposed patient, Heimann (1944) reported the following bromide
values: lung (127 mg/kg), liver (187 mg/kg), brain (207 mg/kg), and,
in a composite sample of heart, kidney, and pancreas (107 mg/kg).
Traces of methyl alcohol and formaldehyde were also found in all the
tissues examined.
In four lethal cases of people exposed to methyl bromide,
Marraccini et al. (1983) found bromide ion concentrations in serum or
plasma ranging from 40 to 583 mg/litre. Methyl bromide was detected in
the brain of one patient (detection limit <1 mg/kg).
Values of 0.9 mg/kg in lymph nodes, 3.3 and 5.1 in ovaries and
testes, 7.5 in lung, and 8.2 mg/kg wet weight in kidney cortex have
been reported in autopsy samples (from accident victims) (Hamilton et
al., 1972/73). A study of the levels of bromide in adipose tissue from
human subjects in three countries showed the highest levels in the
United Kingdom, where 5.6% of the specimens contained levels ranging
from 4.0 to 4.5 mg/kg fat; the lowest levels were found in Germany
(0-0.9 mg/kg fat) whereas levels of 1-3.7 mg/kg were found in the
Netherlands samples (Crampton et al., 1971). Van Leeuwen & Sangster
(1987) stated that there was no evidence in humans of bromide
concentration in any particular organ that might indicate a specific
physiological function of this ion.
6.3 Metabolic transformation
The metabolism of methyl bromide has not been elucidated.
Bromide concentrations in blood (and target organs) were reported
to be increased in humans (Clarke et al., 1945; Rathus & Landy, 1961;
Hine, 1969) and in laboratory animals (Irish et al., 1940, 1941) after
exposure to methyl bromide. Miller & Haggard (1943) postulated that
methyl bromide is hydrolysed in the body with the formation of
inorganic bromide and methyl alcohol. In part, this hydrolysis may
occur intracellularly, resulting in a distribution of bromide that
differs from that for bromide given orally as sodium bromide. Sodium
bromide and methyl alcohol, given at the levels produced after methyl
bromide exposure, did not produce the same toxic and functional
response (Irish et al., 1940, 1941). This suggested that the toxicity
of methyl bromide was due to the reaction of the halide molecule with
the tissue and was not attributable to the hydrolytic products.
Hallier et al. (1990a) measured the cytosolic turnover rate of
methyl bromide in both liver and kidney from five different strains of
mice and rats, with in vitro incubation. The turnover rate in both
organs was consistently higher in tissues isolated from females. On
the basis of a similar effect with methyl chloride, this effect could
be attributed to a higher rate of glutathione conjugation in females.
The reaction products of methyl bromide in wheat were
characterized by Winteringham et al. (1955) using 14C- or
82Br-labelled methyl bromide. It was pointed out that, although most
attention is given to the bromide ion because it is the only part of
the residue that is easily determined, methyl methionine sulfonium
bromide, the methylated histidines, and possibly other residues of
methylation were also produced.
6.3.1 Binding to proteins and lipids
Methylation of cysteine- S and histidine- N residues of haemo-
globin in suspended mouse erythrocytes was found after in vitro
treatment with radiolabelled methyl bromide (Djalali-Behzad et al.,
1981). After inhalation of methyl bromide, alkylation of cysteine- S
residues was seen in mouse haemoglobin and liver proteins
(Djalali-Behzad et al., 1981).
Adducts result from reactions between toxic chemicals and amino
acids in haemoglobin or other proteins (or nucleosides in DNA - see
below). S-methyl-cysteine has been studied as a haemoglobin adduct
in mice (Iwasaki, 1988a,b) and rats (Xu et al., 1990) and as a serum
albumin adduct in human blood samples (Müller et al., 1991, 1992).
Both haemoglobin and serum albumin adducts have been studied in blood
samples of workers occupationally exposed to methyl bromide and these
have been proposed as suitable parameters for the biomonitoring of
exposure to the fumigant (Iwasaki et al., 1989; Müller et al., 1991,
1992) (see also section 9.4.4).
In the insect Triatoma infestans , Castro et al. (1976)
demonstrated that methyl bromide is irreversibly bound to lipids and
to proteins in both the nymph adult and eggs and that exposure to
methyl bromide significantly decreased the content of sulfhydryl
groups in nymph adult and egg proteins.
Studies on methylation by methyl bromide of wool, silk, collagen,
and gelatin (Blackburn & Phillips, 1944), wheat flour (Bridges, 1955;
Winteringham et al., 1955), and cocoa beans (Asante-Poku et al., 1974)
indicated that N-, O-, and S- methylation of proteins occurs
(Cova et al., 1986; Starratt & Bond, 1990b). The main site of
decomposition of methyl bromide in cocoa beans was shown to be in the
alcohol- insoluble proteins of the shell (Asante-Poku et al., 1974).
The methyl group of the fumigant became covalently bound to the
alpha-amino group of the various amino acids, the imidazole ring of
histidine, and the epsilon-amino group of lysine.
Winteringham et al. (1955) found that the gluten fraction of
whole-wheat flour exposed to [14C]methyl bromide was responsible for
80% of the decomposition of the absorbed fumigant with N-methyl,
dimethylsulfonium, and methoxyl and thiomethoxyl derivatives
accounting for 50, 30, and 20%, respectively, in this fraction.
Bridges (1955) reported that 1- N-methylhistidine,
3- N-methylhistidine and 1,3- N,N-dimethylhistidine accounted for
75% of the N-methyl derivatives, and that 10% was due to probably
epsilon- N-methyllysine.
Starratt & Bond (1990a,b) used [14C]methyl bromide to
distinguish naturally occurring residues from those formed during the
fumigation of a variety of commodities: maize, wheat, oatmeal,
peanuts, almonds, alfalfa, potatoes, oranges, and apples. In order to
get higher incorporation of the tracer, fumigation was carried out at
a level of 48 mg/litre, for 3 days. Methyl bromide was bound both
physically and chemically to the commodities. To measure the
physically-bound residue, a parallel test was run using unlabelled
fumigant. Methyl bromide levels were determined 1 h following
fumigation and then at 1, 2, 4, and 10 days. The levels in all the
commodities declined rapidly.
Extraction of the [14C]methyl bromide-fumigated commodities
with diethyl ether removed very little radioactivity, showing that
fats and other non-polar lipids were not methylated during treatment.
In maize, fractions corresponding to albumins, glutamines, zein, and
glutelin were all methylated.
Methylation of methionine is one of the main reactions forming
the relatively unstable methylmethionylsulfonium derivative.
Spontaneous decomposition yields dimethyl sulfide. Analysis of the
sites of methylation was uncertain as both acidic and basic hydrolysis
caused partial decomposition (Starratt & Bond, 1990a,b). The volatile
products included methanol, methyl mercaptan, and dimethyl sulfide.
Different commodities produced different residues (Starratt & Bond,
1990a,b). In potato and orange extracts, the main methylated
components were identified as S-methyl-glutathione,
gamma-glutamyl- S-methylcysteine and S-methyl cysteine. These
compounds were not found in maize. 1- N-methylhistidine and
3- N-methylhistidine were the major components from the fumigated
maize, almonds and other commodities. The highest level of histidine
methylation occurred in almonds, accounting for about 54% of the
chemically-bound residue.
6.3.2 Binding to DNA
Labelled 7-methylguanine was identified in DNA from liver and
spleen cells of mice exposed to [14C] methyl bromide (Djalali-Behzad
et al., 1981). In in vitro experiments with DNA solutions treated
with [14C] methyl bromide, predominantly [14C] 7-methylguanine was
identified (Starratt & Bond, 1988b).
Calf thymus DNA treated in the solid state with [14C] methyl
bromide, showed, on analysis, four major radiolabelled peaks with
retention times corresponding to 1-methyladenine, 7-methylguanine,
3-methyladenine, and 3- methylcytosine (Starratt & Bond, 1988b).
The DNA adducts, [14C]3-methyladenine, [14C]7-methylguanine,
and [14C]O6-methylguanine, have been found in the stomach and
forestomach of rats after both oral and inhalation exposure of
[14C]methyl bromide (Gansewendt et al., 1991).
In maize and wheat, 7-methylguanine and 1-methyladenine were
identified as major products after hydrolysis together with lesser
amounts of 3-methylcytosine and 3-methyladenine (Starratt & Bond,
1988a). Although the yields of DNA were low, Starratt & Bond (1990a)
also found evidence of radioactively-labelled 7-methylguanine and
1-methyladenine in [14C]methyl bromide-fumigated almonds and
potatoes.
6.3.3 The role of glutathione in methyl bromide metabolism
6.3.3.1 Mammals
Liver, kidney, lung, and brain from mice, exposed via inhalation
for 1 h to methyl bromide concentrations of from 870 to 5930 mg/m3,
were analysed for glutathione and bromide ion (Alexeeff et al., 1985).
The liver glutathione levels of the 4700 and 5930 mg/m3 exposure
groups were significantly lower than that of the controls. Bromide ion
levels were highest in the liver and kidney and lowest in the whole
blood. The lung and brain bromide levels were intermediate.
Methyl bromide has been shown in rats to increase the activity of
glutathione S-alkyl transferase and decrease the nonprotein
sulfhydryl content (Roycroft et al., 1981). A group of male
Sprague-Dawley rats were exposed to methyl bromide (117 mg/m3; 6
h/day; 10 days) and killed immediately after the last dose.
Biochemical analyses showed that glutathione S-alkyl transferase was
significantly increased in the lung (12%), liver (11.9%), and kidney
(6.9%). Non-protein sulfhydryl was significantly reduced by 11.4% in
the liver and 13.9% in the kidney. Glucose-6-phosphate dehydrogenase
was significantly increased in the kidney (8.5%), but not in the lung
or liver.
Studies on rats by Davenport et al. (1992) showed that
glutathione was depleted and regional brain
glutathione- S-transferase inhibited by methyl bromide inhalation
(584 mg methyl bromide/m3; 6 h/day; 5 days) [see sections 8.8.2 and
8.10].
In a preliminary report, Thomas & Morgan (1988) reported that
treatment of rats with buthionine sulfoximine (BSO) depleted
glutathione levels, prior to methyl bromide exposure, and increased
the toxicity of methyl bromide. This is in contrast to the findings of
Chellman et al. (1986) for methyl chloride in mice, who found that
glutathione depletion by BSO decreased methyl chloride toxicity in the
brain and kidney. However, the observation is consistent with those of
Tanaka et al. (1988), who showed that treatment of rats with
glutathione reduced the detrimental effects of methyl bromide on
sleep-wakefulness and its circadian rhythm and increased the LD50
value (section 8.8.2).
In whole body inhalation studies on rats exposed for 6 h/day, for
5 or 10 days, to 117 mg methyl bromide/m3 (30 ppm), glutathione
(GSH) S-transferase and glucose-6-phosphate dehydrogenase (G-6-PDH)
activities were increased in the lung. Decreases in GSH-reductase and
GSH- S-transferase activities were found in the liver (Jaskot et al.,
1988).
When human erythrocyte cytoplasm was incubated with methyl
bromide or methyl iodide in the presence of excess glutathione (GSH),
a spontaneous non-enzymatic conjugation was observed (Deutschmann et
al., 1989). This was verified in parallel experiments with boiled
cytoplasm and GSH added after boiling.
Enzymatic conjugation of methyl bromide with reduced glutathione
to produce S-methyl cysteine (the analysis) appears to be
isoenzyme-specific, since conjugation was observed in the erythrocyte
cytoplasm of a majority (13/20) of the population. The same
individuals also conjugated methyl chloride, a reaction that is
dependent on glutathione- S-transferase rho (GSTrho), a minor form of
the enzyme (Hallier et al., 1990b).
Later studies on the properties of the glutathione-
S-transferase (GST) responsible for methyl bromide conjugation with
GSH (measured by methyl bromide depletion) have separated the enzyme
GSTsigma from human erythrocytes; this new enzyme has not been found
in various non-human species (Schröder et al., 1992).
Measurement of methyl bromide disappearance in head-space vials
containing whole human blood cultures in an atmosphere of 19 460
mg/m3 (5000 ppm) at 37 °C indicated that the methyl bromide
concentration had fallen to zero within 1 h, in the presence of blood
from glutathione conjugators, whereas it had fallen to approximately
5836 mg/m3 (1500 ppm) at 1 h, in the presence of blood from
non-conjugators. Further reduction was slow, the methyl bromide
concentration being about 3890 mg/m3 (1000 ppm) after 6 h (Hallier
et al., 1993).
6.3.3.2 Insects
Glutathione is depleted and glutathione S-transferase can be
induced in the larvae of the khapra beetle (Trogoderma granarium) by
fumigation with methyl bromide at a lethal dose (Shivanandappa &
Rajendran, 1987).
Starratt & Bond (1981) demonstrated that, in the granary weevil
( Sitophilus granarius L.), the major pathway for the detoxication of
methyl bromide residues was by conjugation, primarily with
glutathione, and that increasing amounts of glutathione in the insects
resulted in increasing tolerance to methyl bromide exposure. Both
strains of the insect (methyl bromide sensitive and methyl bromide
resistant) metabolized methyl bromide primarily to substances that, on
thin layer chromatography, behaved consistently with their
identification as S-methyl glutathione.
6.4 Elimination and excretion in expired air, faeces, urine
The route of administration of methyl bromide affects the
pathways of excretion.
Miller & Haggard (1943) investigated the amount of methyl bromide
eliminated and the amount of bromide retained in the body after i.p.
administration of methyl bromide. Following a single i.p. injection of
60 mg methyl bromide/kg body weight, elimination continued for about
45 min and more than 90% was eliminated in the first 30 min. With
lethal i.p. doses of 120-180 mg methyl bromide/kg, 24- 45% of the
methyl bromide was eliminated; the amounts of fixed and nonvolatile
bromide being 94.9-126.6 mg/kg. In rats dosed orally (75-100 mg methyl
bromide/kg), similar bromide levels were found, but the methyl bromide
eliminated was given as only 2.4-4.6%.
These results have been confirmed by studies using radioactively
labelled methyl bromide (Medinsky et al., 1984). In rats dosed
intraperitoneally with [14C] methyl bromide, the major route of
elimination was the exhalation of 14CO2 (46%). In contrast,
urinary excretion of [14C] was the major route of elimination (43%
of the dose), when methyl bromide was given orally. Very little
appeared in the faeces (<3% of the dose), regardless of the route of
administration. In rats with bile duct cannulations, 46% of an oral
dose appeared in the bile over a 24-h period (Medinsky et al., 1984).
The authors suggested that reabsorption of biliary metabolites from
the gut played a significant role in the disposition of [14C] methyl
bromide.
In inhalation studies on rats exposed for 6 h to 337 nmol [14C]
methyl bromide/litre air, Bond et al. (1985) showed that excretion of
[14C] as 14CO2 was the major route of elimination, about 47%
(3900 nmol/rat) of the total [14C] methyl bromide absorbed being
excreted by this route. CO2 excretion exhibited a biphasic
elimination pattern with 85% of the 14CO2 being excreted with a
half-time of 3.9 h and 15% excreted with a half-time of 11.2 h.
Half-times for the elimination of 14C in urine and faeces were 9.6
and 16.1 h, respectively; 65 h after exposure, about 75% of the
initial radioactivity had been excreted with 25% remaining in the body
(Bond et al., 1985). Elimination half-times of [14C] from tissues
were 1.5-8 h. In all tissues examined, over 90% of the 14C in the
tissues were methyl bromide metabolites (Bond et al., 1985). The data
from this study indicate that, after inhalation, methyl bromide is
rapidly metabolized in tissues and readily eliminated.
Male CD rats were exposed (nose only) to [14C]-methyl bromide
at 214 mg/m3 (55 ppm) for 3 min. The liver, lung, and kidney were
the major organs of [14C] distribution, immediately after exposure.
Up to 32 h after exposure, the major routes of excretion were
pulmonary and renal with elimination of 43% and 21% of the total
inhaled label, respectively (Jaskot et al., 1988).
Studies into the time course of methyl bromide and bromide
elimination in rat tissue have been described by Honma et al. (1985).
Male Sprague-Dawley rats were exposed to 973 mg methyl bromide/m3
(250 ppm) for 8 h and methyl bromide and bromine concentrations
measured at successive time intervals (see also section 6.2). The
results are shown in Fig. 7 (bromine) and Fig. 8 (methyl bromide). The
methyl bromide levels in all tissues described reached a maximum in 1
h following exposure and remained at almost the same levels during
exposure. Methyl bromide levels decreased rapidly after exposure;
after 30 min only half of the methyl bromide concentrations in adipose
tissue and blood were still present (Fig. 7). The methyl bromide
levels in the brain and liver were very low, but the elimination of
methyl bromide from these organs was slower. Forty-eight hours after
exposure, methyl bromide could not be detected in any tissue examined
(Honma et al., 1985). In contrast to the methyl bromide study, peak
concentrations of bromine in blood, kidneys, and liver occurred 4-8 h
after bromide exposure, and the half-life in these tissues was about
5 days.
Honma et al. (1985) carried out a regression analysis of methyl
bromide and bromine concentrations in blood, kidney, liver, brain and
adipose in male rats after a 2-h exposure to 0, 973, 1946, 2918, or
3890 mg methyl bromide/m3 (0, 250, 500, 750, or 1000 ppm). Linear
relationships were obtained between exposure concentrations and the
tissue methyl bromide or bromine values.
Over 95% of the bromide ion in mice exposed to methyl bromide was
eliminated within 2.5 days, bringing the concentration close to the
control levels and the limit of detection (Alexeeff et al., 1985).
Lactating cows fed a methyl bromide fumigated grain ration (220
mg bromide/kg food) compared with unfumigated diets secreted increased
levels of bromide in the milk, i.e., 10-20 mg/litre instead of about
5 mg/litre (Lynn et al., 1963).
6.5 Retention and turnover
The biological half-life of bromide ions in human blood was found
to be about 12 days (Söremark, 1960).
The half-lives of bromide in the blood and brain of rats after
i.p. injection of methyl bromide were approximately 8.7 and 4.3 days,
respectively (Tanaka et al., 1988). In inhalation studies, a half-life
of bromide of about 5 days was reported in the blood, kidneys, and
liver (Honma et al., 1985). In contrast, methyl bromide concentrations
in the blood and adipose tissue were reduced by half after 30 min,
though elimination from the brain and liver was much slower (Honma et
al., 1985).
6.6 Reaction with body components
Honma et al. (1987) suggested that the main target of methyl
bromide in the body was the central nervous system. The nor-
epinephrine content of the hypothalamus and cortex with hippo-campus
was reduced on exposure to methyl bromide (Honma et al., 1982) and
changes in the amino acid content and metabolism in the brain were
noted (Honma et al., 1983). The results of further studies suggested
that alterations in catecholamine metabolism might be a factor in
methyl bromide-induced neurotoxicity (Honma et al., 1987) (see section
8.8). Kato et al. (1986) described histopathological changes in the
brain.
Eustis et al, (1986, 1988) found clear species- and sex- related
differences in the susceptibility of specific organs and tissues to
methyl bromide effects (section 8.8.1). In rats, neuronal necrosis
occurred primarily in the cerebral cortex, hippocampus, and thalamus
of the brain, whereas in mice, necrosis of the internal granular layer
of the cerebellar folia was more frequently observed. Nephrosis
occurred in all treated mice. Myocardial degeneration was observed in
male and female rats more frequently than male mice. Atrophy of the
adrenal cortex and testis and necrosis of the olfactory epithelium
were described. Similar findings were described by Hurtt et al.
(1987). Degeneration and regeneration of the olfactory epithelium were
also described by Hurtt et al. (1988) and Hastings et al. (1989);
Hastings (1990).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Soil microorganisms
Methyl bromide is used commercially to control soil- borne fungi
that cause:
- damping off: Rhizoctonia solani, Pythium spp., Sclerotium
bataticola (Macrophomina phaseolina), Phytophtora spp.,
and Thielaviopsis basicola.
- crown rot: Sclerotium rolfsii and Sclerotinia spp.
- root rot: Pythium spp., Stromatinia, Fusarium spp.,
Sclerotium bataticola, (Macrophomina phaseolina),
Rhizoctonia solani, Pyrenochaeta spp. (corky root, pink
root), Armillaria and Phytophthora spp.
- wilt: Fusarium spp., and Verticillium spp.
Generally, concentration-time products of methyl bromide required
to kill fungi are much higher than those needed to control insect and
nematode pests, susceptibility increasing with temperature. To control
fungi, methyl bromide is generally used at rates of 40-100 g/m2
(Davis et al., 1977).
Filip & Roth (1977) demonstrated the efficacy of methyl bromide
against Armillaria root rot in the stumps of ponderosa pine ( Pinus
ponderosa Laws). The survival of young pine trees in areas infected
with such fungi was increased after the elimination of the fungus,
using the fumigant.
Heungens & Roos (1982) recovered non-pathogenic fungi
(Penicillium and Mucor) following the application of methyl
bromide (300 g/m2) to pine litter, but no pathogenic fungi were
recovered.
After an initial reduction, the numbers of bacteria and fungi in
fumigated soil remained high and low, respectively, in comparison with
those in untreated soil (Sivasithamparam et al., 1987). In the
fumigated soil, Trichoderma species rapidly recolonized the soil,
becoming the dominant fungus within 15 days. In a study on the
microflora in the rhizosphere of wheat, the same authors found that,
though there was no difference in the total number of bacteria,
actinomycetes, and fungi, before and after fumigation with methyl
bromide, there were some fungal species differences with Fusarium
merismoides, T. koningii , and T. viride present in significantly
higher numbers, other fungi being less abundant.
Kelley & Rodriguez-Kabana (1979) reported that methyl bromide did
not cause any permanent changes in soil enzyme activities or adversely
affect the mycorrhizal root development of pine seedlings.
Methyl bromide is used for controlling fungal infections in the
poultry industry (Harry et al., 1972; Davis et al., 1977).
Methyl bromide is much less frequently used as a bactericide than
as an insecticide (Davis et al., 1977). It is used to control certain
soil-borne bacteria, e.g., bacterial canker (Corynebacterium
michiganese) , and bacterial wilt (Pseudomonas solanacerum)
(Bromine & Chemicals Ltd.,1990). A summary of the acute toxic effects
of methyl bromide on bacteria and viruses is given in Table 36.
Methyl bromide, used at a concentration-time product of 800
mg.h/litre at 25 °C, with a relative humidity of 70%, eliminated
salmonellae from artificially contaminated poultry foodstuffs (Tucker
et al., 1974).
Although there have been many studies on the effectiveness of
methyl bromide in reducing diseases produced by pathogenic
micro-organisms, there are fewer data on its effects on soil microbes.
Matta & Porta-Puglia (1968) tested methyl bromide on several
morphologically and functionally different groups of soil microbes. In
isolated soil samples, treated and maintained under constant
temperature (22-23 °C) and humidity (16-18%), microorganisms were
counted at 2, 21, 54, and 87 days following fumigation with 300 g
methyl bromide/m3. After 2 days, most bacteria were dead; after 87
days, there were very low counts of fungi, aerobic nitrogen-fixing,
nitrifying, and cellulolytic bacteria, whereas denitrifying,
proteolytic, amylolytic, and ammonifying bacteria showed a marked
resurgence in recolonization (Matta & Porta-Puglia, 1968). The
selective-action effects of methyl bromide fumigation on a given
microbe population in soil appear to be more significant than the
effects on microbe number.
Table 36. Effects of methyl bromide fumigation on bacteria and virusesa
Organism Dosage Conditions Reference
Vibrio cholera, Shigella 33 g/m3 10-h exposure LD100 Saiki (1952)
dysenteriae, Salmonella
typhi, Salmonella paratyphi A,
Salmonella paratyphi B
Corynebacterium 10 % bromomethane, 18-h exposure LD100 Richardson & Monro (1962)
sepedonicum 5 % ethylene oxide 85 % CO2
Arabis mosaic virus 0.32 kg/m3 controlled virus on strawberry Harrison et al. (1963)
plants
Tobacco mosaic virus, 640 g/m3 inactivated virus Inouye et al. (1967)
Cucumber green mottle virus 110 g/m3 inactivated at 27°C
320 g/m3 inactivated at 14-16°C
Escherichia coli 1257 1000 g/m3 40°C and 90 % relative humidity Prishchep &Nikiforova
provided control (1969)
Bacillus larvae, Bacillus 5000 g/m3 5-day exposure controlled Smirnov (1970)
paraalvei, Streptococcus bacteria in bee honeycombs
apis, Streptococcus pluton,
Pseudomonas apisepticus
Tobacco mosaic virus 200 g/m3 inactivated virus in 3 kg soil Doraiswamy et al. (1972)
in which tomatoes were grown
Salmonella typhimurium 800 mg-h/litre 25 °C and 70% relative humidity Tucker et al. (1974)
provided control
Xanthomonas begoniae 0.32 kg/m3 24-h exposure eliminated Strider (1975)
bacterial blight from Rieger
begonia
a Adapted from: Davis et al. (1977).
In a study on bacterial flora involved in the nitrogen cycle, hot
fumigation with methyl bromide at concentrations of 80 g/m3 was
carried out in greenhouses at 6 different sites (Turtura et al.,
1988). Seven months after treatment, the total aerobic mesophile
bacteria count, aerobic nitrogen-fixing, ammonifying,
ammonia-oxidizing, and nitrite-oxidizing bacteria, always showed
higher values in fumigated than in unfumigated control soils.
Recolonization was more marked in the upper 0-30 cm soil samples in
which the development of ammonifying and nitrifying bacteria was
highly significant.
Rovira & Ridge (1979) found no long-term effects on aerobic soil
bacteria or actinomycetes with application of 22 g methyl bromide/m2.
Yeates et al. (1991) described the recolonization of soils
sterilized in the laboratory and returned to their original pasture
and forest sites, under four different types of field conditions.
Sampling took place over 166 days (midsummer to midwinter) with two of
the sites having a moderate, and two a high, rainfall. Both microbial
biomass and dehydrogenase activity recovered rapidly but remained
consistently lower in the fumigated than in the untreated samples in
all four sites. Bacterial numbers also recovered rapidly. Fungal
hyphal lengths were 25% lower in the fumigated soil. Fumigation showed
no detectable effects on the subsequent rates of nitrogen
mineralization and little effect on nitrification rates. Protozoa were
almost completely eliminated by fumigation, numbers recovering most
rapidly in moist forest soil and slowly in dry pasture soil. Nematodes
were eliminated by fumigation; recolonization was first detected on
day 26. Numbers (10 and 62/g, respectively) and species (10 and 31,
respectively) remained much lower in fumigated, compared with
untreated, soil.
After sterilization of greenhouse soil with methyl bromide (75
g/m2), there were profound qualitative and quantitative disturbances
up to a soil depth of 30 cm (Bourbos & Skoudridakis, 1991); 7-9
species of soil mycoflora were isolated from the fumigated soil
compared with 107 from control soils. The 31-40 cm soil layer was not
affected by disinfestation. After two months, recolonization had taken
place of only 35-40% in species and 60-63% in density of the primary
microflora.
The use of methyl bromide as an effective fumigant for
greenhouses has been questioned (Bourbos & Skoudridakis, 1991).
Certain saprophytic fungi have developed a degree of tolerance.
Disinfested soil was quickly contaminated by certain pathogens
( Fusarium and Pythium spp.) and there was also a possibility of
reinfection from the lower layer of soil not reached by the fumigant.
Some fungi controlled by methyl bromide are listed in Table 37.
7.2 Aquatic organisms
7.2.1 Effect of methyl bromide
LC50 (96-h) values of 11 mg methyl bromide/litre and 12 mg
methyl bromide/litre for bluegill sunfish, Lepomis macrochirus
(freshwater) and tidewater silversides, Menidia beryllina
(saltwater), respectively, have been determined following exposure to
methyl bromide (Dawson et al., 1975/77).
The acute toxicity of methyl bromide for carp ( Cyprinus carpio
L.) was determined in studies with a 4-h exposure period (Segers et
al., 1984). The 4-h LC50 was calculated to be approximately 17
mg/litre. Damage to the gill epithelium was the most pronounced
morphological damage, which probably caused the death of the fish by
suffocation.
In a short-term study, triplicate groups of 10 fish ( P.
reticulata (guppy) and Oryzias latipes (medaka)) were exposed to
0.56, 1.0, or 1.8 mg methyl bromide/litre for 4 days. All methyl
bromide-exposed fish displayed abnormal behaviour (reduced activity).
Limited mortality was noted in P. reticulata exposed to 1.0 and 1.8
mg/litre and O. latipes exposed to 1.8 mg/litre, but there was no
increase in mortality in the next lower concentration groups (Wester
et al., 1988).
In a long-term study, P. reticulata and O. latipes were
exposed for 1 and 3 months to 0.032-3.2 mg methyl bromide/litre
(Wester et al., 1988). All guppies died in the 3.2 mg/litre group
within 3 days, and, in the 1.0 mg/litre group, within 3 weeks. The
NOLC (no observed lethal concentration) and NOEC (no observed effect
concentration: behaviour, appearance) values were 0.32 and 0.1
mg/litre, respectively. A significant decrease in weight was noted in
both sexes in the 0.32 mg/litre group.
All medaka embryos exposed to 1.8 or 3.2 mg/litre and most of the
1.0 mg/litre group died before hatching. The NOLC after 3 months was
0.32 mg/litre. The NOEC values, on the basis of behaviour and
appearance, were 0.56 mg/litre and 0.32 mg/litre after 1 and 3 months,
respectively (Wester et al., 1988).
A short-term study with (lethal) concentrations of 0.56, 1.0, or
1.8 mg methyl bromide/litre showed (using scanning electron
microscopy) major degenerative and regenerative changes in the
superficial epithelia, especially of the gills and oral mucosa,
caused, apparently, by the local irritating action of this compound.
Necrotic changes were also seen in the thymic cortex and the testis
(Wester et al., 1988). In a long-term study on guppies and medakas
exposed to the highest concentrations for 1 month and 3 months,
respectively, no significant organ or tissue changes could be detected
in routine histopathology.
Table 37. Some fungi controlled by methyl bromide
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Alternaria sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Armillaria melea 4-98 g/m2 citrus/ Munnecke et al. (1969)
grape Kissler et al. (1973)
Aspergillus sp. 1.6-5 g/m2 pecan/ Wells & Payne (1975)
honeycomb Smirnov (1970)
Byssochalamy fuloa 60-120 mg/kg starch Ito et al. (1972)
Cladosporium sp. 1.6-3.3 g/m2 pecan Wells & Payne (1975)
Eumargodes laivgi 24 g/m2 not stated Hitchcock (1968)
Fusarium sp. 45-100 g/m3 tomato Westeijn (1973)
Weihing et al. (1971)
Wells & Payne (1975)
Perrotta (1968)
Vanachter (1974)
Monochaeta sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Penicillium sp. 1.6-3.3 g/m3 pecan
Pestalotia sp. 1.6-3.3 g/m3 pecan
Phoma sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Phytophora parasitica 49 g/m2 citrus Grimm & Alexander (1971)
Phytophora capsici 40 g/m2 green peppers Alfaro Moreno & Vehg (1971)
Table 37 (continued)
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Plasmodiophora brassicae 50-150 g/m2 cabbage Winstead & Garriss (1960)
Plasmosisphora brassicae 48 g/m2 cabbage Wimalajewa (1975)
Pyrenochaeta lycopersici 125 g/m3 soil Vanachter (1974)
Rhizoctania solani 50-150 g/m2 soil Winstead & Garriss (1960)
Sclerotium rolfsii 50 g/m2 iris Kiewnick (1968)
Sclerotina sclerotiorum 50 g/m2 tobacco Hartill & Campbell (1973)
Thielaviopsis basicola 50 g/m3 tobacco Mounat & Hitier (1959)
Verticillium sp. 45-70 g/m2 tomato Perrotta (1968)
Table 38. Effects on aquatic organisms of short-term exposure to sodium bromidea
Results (g Br-/litre) at:
Test species Parameter 24 h 48 h 72 h 96 h
Scenedesmus EC50 (growth) 5.8 7.8 8.5 10
pannonicus NOEC (growth) 2.5 2.5 2.5 2.5
(alga)
Daphnia magna LC50 (mortality) 11 11 - -
(crustacea) EC50 (mort./abn. behaviour) 5.8 5.8 - -
NOLC (mortality) 7.8 7.8 - -
NOEC (mort./abn. behaviour) 4.3 4.3 - -
Poecilia LC50 (mortality) 16 16 16 16
reticulata EC50 (mort./abn. behaviour) 0.44 0.14 0.044 0.044
(fish) NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.078 0.025 0.025
Oryzias latipes LC50 (mortality) 26 25 24 24
(fish) EC50 (mort./abn. behaviour) 0.44 0.44 0.44 0.44
NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.25 0.25 0.25
a From: Canton et al. (1983).
b NOE(L)C = no observed (specified) effect concentration.
7.2.2 Effect of bromide ion on aquatic organisms
The main degradation product of methyl bromide is inorganic
bromide.
To evaluate the potential impact of pollution with the bromide
ion, Canton et al.(1983) investigated the short-term effects of sodium
bromide on various freshwater organisms, using algae (Scenedesmus
pannonicus) , crustaceans (Daphnia magna), and fish (P. reticulata
and O. latipes) (Table 38). Depending on the species tested, acute
toxic effects were seen at concentrations ranging from 44 to 5800 mg
Br-/litre and, in long-term tests, the NOEC varied from 7.8 to 250
mg Br-/litre. Bromide ion markedly impaired reproduction in both
crustaceans and fish.
Further tests were performed on P. reticulata (guppy) and O.
latipes (medaka) following sodium bromide exposure for 1 and 3
months at concentration ranges of 10-32 000 mg/litre (guppy) and
180-56 000 mg/litre (medaka). NOLC values for guppies were 10 000
mg/litre and 1000 mg/litre after 1 and 3 months, respectively. The
NOEC value was 32 mg/litre in both studies. Histopathological changes
were observed at concentrations of 100 mg/litre or more (Wester et
al., 1988). For medakas, the NOLC values were 5600 and 3200 mg/litre
after 3 weeks and 3 months, respectively, whereas the NOEC value
(behaviour) for both periods was 320 mg/litre (Wester et al., 1988).
The relative susceptibility to sodium bromide of 11 taxonomically
different freshwater species was determined in medium-term toxicity
tests by Slooff & Canton (1983). The data are summarized in Table 39.
In semi-static, long-term toxicity tests on Daphnia magna, EC50
and EC10 values of 27 and 18 mg/litre, respectively, were determined
(Van Leeuwen et al., 1986).
7.3 Terrestrial organisms
7.3.1 Protozoa
Long et al. (1972) studied the effect of methyl bromide on
protozoa (Eimeria tenella and E. acervulina) at a dosage of 5
g/m3 for 20 h at 25 °C; 100 % control (destruction of oocysts) was
achieved.
7.3.2 Plants
Methyl bromide is often applied, as a fumigant, directly to plant
seeds, plant cuttings, or harvested plant products to disinfect
before, and during, transportation or storage (Davis et al., 1977).
Additionally, methyl bromide is used as a soil fumigant to control
certain plant pathogens and weed seeds in areas to be planted.
Table 39. Summary of the results of medium-term toxicity tests using
sodium bromide on 11 different freshwater test speciesa
Test species Exposure Criteria NOL(E)C
time valuesb
(days) (mg/litre)
Pseudomonas 0.3 specific growth rate 3200
fluorescens
(bacterium)
Microcytis aeruginosa 4 specific growth rate 3200
(cyanobacterium)
Scenedesmus 4 growth (biomass) 3200
pannonicus
(alga)
Lemna minor (plant) 7 specific growth rate 3200
Daphnia magna 21 mortality 3200
(crustacea) reproduction 10
Culex pipiens 25 mortality 100
(insect) development 100
Hydra oligactis 21 specific growth rate 1000
(hydrozoan)
Lymnaea stagnalis 40 mortality 3200
(mollusc) reproduction 10
hatching 3200
Poecilia recticulata 28 mortality 100
(viviparous fish) mortality and behaviour 32
growth 320
Oryzias latipes 40 mortality 3200
(viviparous fish) mortality and behaviour 320
hatching growth 10 000
Xenopus laevis 100 mortality 32
(amphibian) development 320
development 320
a Adapted from: Slooff & Canton (1983).
b NOL(E)C = no observed (specified) effect concentration.
As chloropicrin is phytotoxic, methyl bromide formulations that
contain it as a warning agent are not used on nursery stock or other
living plants (Bond, 1984).
7.3.2.1 Seed fumigation
As shown in Table 40, fumigation with methyl bromide can result
in delay in the germination of seeds and some loss of total
germinative capacity, depending on the variety, moisture content, and
the extent of exposure to the gas (Davis et al., 1977). This was
confirmed by Sittisuang & Nakakita (1985) who compared the effects of
methyl bromide on the germination of rice seeds ( Oryza sativa L.,
Japicona type) and corn (maize) seeds ( Zea mays L.). No detrimental
effect of methyl bromide up to 4 mg/litre was observed in rice seeds
at a moisture content of 11%, but as the moisture content and
temperature increased, methyl bromide had an increasing effect on
germination. Maize seeds were much more tolerant to methyl bromide.
Exposure to 5 mg methyl bromide/litre, which caused heavy damage to
rice seeds in most cases, did not generally produce any harmful effect
on the germination of maize seeds, regardless of the moisture content
and temperature. At concentrations higher than 10 mg/litre, the
viability of maize seeds declined in a similar way to that of rice
seeds. Rice seeds were found to absorb more methyl bromide than corn
seeds. Seeds with a higher moisture content absorbed more methyl
bromide and seeds with the same moisture content absorbed more methyl
bromide at higher temperatures than at lower temperatures. The authors
suggested that changes in certain proteins and enzymes were major
factors in seed viability.
The effects of methyl bromide fumigation on the germination of
different cultivars of wheat seed have been investigated. The
germination of all cultivars was reduced following fumigation at a
dose of 16 mg/litre for 24 h (11% moisture, temperature not given).
The optimal conditions were a moisture content of 9% and a temperature
of 18 °C. With increasing moisture or temperature, the percentage
germination decreased. At higher levels of moisture, the concentration
of methyl bromide appeared to be a more important factor than exposure
time at a constant concentration x time product (CTP) of 768
mg.h/litre (Khanna & Yadav, 1987).
Hanson et al. (1987) found that fumigation with methyl bromide
not only caused a delay in germination and loss of germinative
capacity but also that certain varieties of seed barley were damaged,
exhibiting symptoms of albinism and stunted growth. The authors
suggested that great care should be taken in the selection of stored
barley intended for seed and, in particular, in the fumigation of
samples in standard reference collections. A CTP of 200 mg.h/litre is
used commercially, but higher concentrations may be attained if the
distribution during fumigation is poor.
Table 40. Effects on germination of seeds fumigated with methyl bromidea
Seed Fumigation conditions Germination results Reference
hemp 70-140 g/m3 5-23 % reduction Tkalich (1974)
onion 42 g/m3 for 24 h 95 % reduction in laboratory Powell (1975)
11.5% reduction in cool soil
peanuts 32 mg/litre (24 h, 27°C, 80% reduction of: Leesch et al. (1974)
paper container relative humidity), applied 21.7%
burlap bags under cover, aerated 72 h 11.4%
oat, wheat, rye, 0, 600, or 1200 g.h/m3 at 8, 11at 18% moisture content: Blackith & Lubatti (1965)
barley 14, or 18% moisture content - no germination after 6 years storage
at 8% moisture content:
- 90% germination after 6 years storageb
Picea abies, Picea seeds at various moisture germination normal after storage Jones (1968)
glauca, Pinus mugo content; 48 g/m3, 24°C, 2-5 h, only if seeds aerated 24 h before
mughus, Pinus then aerated 1-25 h and stored storage; all but P. sylvestris
sylvestris (seeds) in sealed containers at 7°C for required drying to 5% moisture content
1 year before storage
tobacco seed 16-32 g/m3 or germination satisfactory at <10 % seed Guthrie & Kincaid (1957)
32-48 g/m3 moisture content: germination dcreased at
seed moisture contents above 10%
barley, corn, grain 32 g/m3 (< 24 h, 26°C); unimpaired germination seed Whitney et al. (1958)
sorghum, oats, wheat moisture content less than 12%
a From: Davis et al. (1977).
b Except rye, germinated well only up to 3 years storage.
7.3.2.2 Fumigation of plants or plant products
Direct fumigation of plants or plant products is used to retard,
or prevent, pest infestations and to overcome quarantine barriers.
Post-harvest fumigation is discussed in section 5.1.4.2.
7.3.2.3 The effects on plants of soil fumigation
Methyl bromide can have adverse as well as positive effects on
plants.
The phytotoxic effects of methyl bromide as a soil sterilant can
be caused by:
(1) the action on plants of methyl bromide itself;
(2) the action of inorganic bromide formed by the breakdown of
methyl bromide in the soil;
(3) indirect action through effects of either methyl bromide or
inorganic bromide on soil microflora, soil structure, or
composition (Maw & Kempton, 1973).
Where the crops are affected by lack of mycorrhizae, the plants
are stunted. Experiments have proved that this problem can be
rectified by fertilizing with phosphoric acid in the irrigation water,
using a trickle system (Bromine & Chemicals Ltd., 1990).
The phytotoxicity of methyl bromide is thought to be due mainly
to the high level of bromide ion. Drosihn et al. (1968) showed that
the degree of susceptibility of carnations to methyl bromide
fumigation of the soil depends on the intensity of subsequent leaching
of the soil. Similar findings were described by Kempton & Maw (1974).
In contrast to this, tomato plants were relatively insensitive to
bromide; growing tomatoes tolerated up to 0.1 mg bromide/g soil
without signs of injury or growth retardation (Maw & Kempton, 1973).
These authors found lettuce to be particularly resistant to inorganic
bromide, with some varieties growing in the presence of as much as 5
mg Br-/g soil.
Reichmuth & Noack (1983) determined the threshold concentration
of methyl bromide in air that should not be exceeded in the vicinity
of fumigated buildings, in order to protect plants. The test plants
( Lactuca sativa capitata (lettuce) and Nasturtium officinale
(water cress)) were exposed to concentrations of between 4 and 1400
mg methyl bromide/m3 for 72 h. At 400 mg/m3, yellowing of lettuce
leaves became apparent, while no visible effects were observed on
water cress up to the highest concentration.
(a) Cultivated plants
Only a limited number of genera, species, or varieties of plants
are susceptible to methyl bromide. Of the 441 species of glasshouse
plants tested by Latta & Cowgill (1941), 414 (93.9%) were not affected
and only 27 sustained various levels of damage; of these, five species
were severely burned. For example, roses showed no pronounced toxic
effects, when planted in soils aerated for four days after methyl
bromide fumigation, however, carnations were extremely sensitive to
both residual methyl bromide gas and inorganic bromide in the soil
(Kempton & Maw, 1974). Other crops, such as cotton, celery, pepper,
and onion, do not reach adequate growth, when grown in fumigated soil
(Bromine & Chemicals Ltd., 1990). Plants, actively growing, are more
likely to sustain injury than dormant plants (Bond, 1984).
(b) Weeds
The phytotoxic effects of methyl bromide on weeds are important
in soil fumigation.
Table 11 shows that the recommended dose rates to eradicate weeds
is 35-50 g/m2, though purple nutsedge (nut grass), corms and seeds
of horseweed Erigeron (Conyza) , mallow (Malva) , and legumes are
not efficiently controlled at this dose (Bromine & Chemicals Ltd.,
1990).
Methyl bromide (40-80 g/m2) is mentioned as being the best soil
fumigant against yellow nutsedge ( Cyperus esculentus L.), but the
weed was not completely eradicated because dormant tubers below the
tillage depth survived (Rotteveel & Naber, 1987).
7.3.3 Soil invertebrates
Soil fumigation with methyl bromide (and chloropicrin) results
generally in toxic effects in both target and non-target organisms.
The concentrations used are sufficiently high to eradicate populations
of a wide variety of organisms. Fumigants, including methyl bromide
and chloropicrin, were all strongly nematocidal. Methyl bromide killed
virtually all soil arthropods, including mites; Collembola were almost
completely eradicated. Methyl bromide was very toxic for symphylids
and millipedes (details of dose not given) (Edwards & Thompson, 1973).
Methyl bromide (concentration not given) and chloropicrin were very
toxic for earthworms, even those that lived in deep burrows (Van Rhee,
1977). Chloropicrin was repellent to most arthropods in soil (Edwards
& Thompson, 1973). To control nematodes, methyl bromide is generally
used at rates of up to 80 g/m2 (Table 41).
7.3.4 Insects and arachnids
Methyl bromide is used as a fumigant to control insect pests.
Although it is not as toxic for insect species as some other
fumigants, such as HCN, acrylonitrile, and ethylene dibromide, its
ability to penetrate quickly and deeply into sorptive materials makes
it an effective and versatile fumigant (Davis et al., 1977; Sassaman
et al., 1986). The commercial dosage for methyl bromide as a storage
fumigant ranges from 16 to 100 g/m3 for up to 3 days (Tables 12 and
13).
The dosage required depends also on the temperature. The
threshold concentration levels identified at 15 and 25 °C differed by
a factor of two or three. These investigations by Bell (1988) were
carried out on the adult beetle. The dosage of methyl bromide required
to kill eggs and pupae is greater than that required to kill all
adults. Pupae and older larvae of Tribolium spp., for example,
required CTPs of up to 180 mg.h/litre for control (Hole, 1981). For
other species and exposure conditions see Table 42.
In an FAO study, it was found that there was a variation in
tolerance to methyl bromide in different strains of eight species of
stored product beetles collected from different parts of the world
(Hole, 1981). Although resistant strains have been identified in the
laboratory, there have been no reports of resistance to methyl bromide
in practice (Bell, 1988).
The effects of lethal concentrations of methyl bromide (48 g/m3
for 2 h) on embryos of the codling moth ( Cydia pomonella L.) were
assessed using light microscopy and transmission electron microscopy
(Cheetham, 1990). Cell division stopped within one hour in nearly all
embryos, a small number of terata being produced.
Methyl bromide is used to eradicate various wood and household
pests, particularly in the warmer climates of southern and western
USA. The primary targets are drywood termites (Kalotermitidiae).
Scheffrahn & Su (1992) have assessed the toxicity of methyl bromide at
27 °C against pseudergates, nymphs, or alates of several species.
Estimates of lethal accumulated doses for 50 and 99% mortality ranged,
respectively, from 11.4 and 16.5 for R. hesperus pseudergates to
45.9 and 75.0 mg h/litre for C. cavifrons pseudergates and nymphs.
Alates were more susceptible to methyl bromide than pseudergates or
nymphs. Boczek et al. (1975) reported three periods in the development
of Acarus siro that have increased sensitivity to methyl bromide:
(a) before the beginning of gastrulation movements in the germ band;
(b) during the formation of the nervous system; and (c) the period
preceding dorsal closure. For this species, Burkholder (1966) found
LD100 values ranging from 3.4 to 16.8 g/m3 at various exposure
times at 16 °C and 85% relative humidity whilst achieving the same CTP
(65-83 g.h/m3).
Table 41. Effects of methyl bromide on nematodes
Nematode Effective Exposure Reference
control Conditions
concentration
Anguina agrostis CTP 600-800 12% moisture Hague (1963)
mg.h/litre
Belonolaimus 98 g/m2 98% methyl bromide Darby et al. (1962)
longicaudans 2% chloropicrin: covered 48 h
Ditylenchus dipsaci CTP 850 10-14% moisture Hague & Clark (1959)
mg.h/litre
Dorylaimus sp. 2.3 g/m3 40 h Van Gundy et al. (1972)
Hemicyclophora parvana 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Heterodera rostochiensis Ct 500-1000 treatment with water before fumigation Hague (1959)
mg.h/litre enhanced penetration of methyl bromide
Heterodera rostochiensis 111 g/m2 covered 16 days: 98 % methyl bromide Whitehead et al.
and 2% chloropicrin (1972)
Heterodera schachtii 0.5-30 g/m3 1-21 days Abdalla & Lear (1975)
Hoplolaimus columbus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Hoplolaimus tylenchiformis 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Meloidogyne sp. 50 g/m2 manure applied prior to fumigation Scotto La Massese
decreased nematocidal effect & Mars (1975)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Meloidogyne incognita 2.3 g/m3 38 h Van Gundy et al. (1972)
Meloidogyne incognita 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Meloidogyne incognita 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne incognita 0.6-2.5 g/m3 1-21 days Abdalla & Lear (1975)
Meloidogyne incognita 17-22 g/m2 chisel applicator, covered or rolled Sher et al. (1958)
acrita
Meloidogyne javanica 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne javanica 22-34 g/m2 chisel application, covered Thomason (1959)
Meloidogyne javanica 56-112 g/m2 98% methyl bromide Milne (1962)
and 2% chloropicrin
Pratylenchus sp. 45-67 g/m2 covered Raski et al. (1975)
Pratylenchus sp. 4.9-9.7 g/m3 1-3 days Abdalla & Lear (1975)
Pratylenchus brachynrus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Pratylenchus brachyurus 25-51 g/m3 24 hours; 25°C Minton & Gillenwater
(1973)
Pratylenchus penetrans 50 g/m2 not stated Chen et al. (1962)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Pratylenchus thornei 49 g/m2 covered after application Van Gundy et al. (1974)
for unspecified time
Pratylenchus zeae 100 g/m2 covered for 48 h Oakes et al. (1956)
following fumigation
Trichoderus christiei 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin:
covered 48 h
Xiphinema americanum 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 2.3 g/m3 28 h Van Gundy et al. (1972)
Xiphinema index 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 0.2-2.0 g/m3 1-21 days Abdalla & Lear (1975)
Table 42. Some insects controlled by methyl bromidea
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Antagenus picus 32 Pence & Morganroth
(black carpet beetle) (1962)
Anthonomus grandis 16-80 Roth & Kennedy (1972)
(cotton boll weevil)
Anthrenus flavipes 32 Pence & Morgenroth
(furniture carpet beetle) (1962)
Anthrenus verbasci 32
(varied carpet beetle)
Araecerus fasciculatus 6.2 (eggs) Majumder et al. (1961)
3.4 (larvae)
7.4 (pupae)
4.5 (adults)
Blatta orientalis 64 Hickin (1961)
(cockroach)
Blatella germanica 64
(cockroach)
Bruchus rufimanus 28 Roth & Richardson
(broad bean weevil) (1974)
Cadra cautella 32 Leesch et al. (1974)
(almond moth)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Callosobruchus 0.85 (eggs) 1.25 (eggs) Adu & Muthu (1985)
chinensis (L) 2.2 (instar 2.72 (instar
(cowpea weevil) larvae) larvae)
0.89 (pupae) 3.98 (pupae)
1.17 (adult) 1.4 (adult)
Chilo agamemnon 20 (larvae) Isa et al. (1970)
(corn borer)
Corcyra cephalonica 1.66-1.78 (eggs) El-Buzz et al. (1974)
(rice moth) 1.10-1.68 (instar
larvae)
2.79 (pupae)
Curcilio caryae 32-112 Leesch & Gillenwater
(pecan weevil) (1976)
Ephestia kuehniella 2.02-2.46 Mostafa et al. (1972)
(mediterranean flour moth)
Gnorimoschema operculella 11.74 (larvae) Pradhan et al. (1960)
(potato tuber moth)
Gryllotalpa 70-100 g/m2 Dzidzariya (1972)
(mole cricket)
Hemp leaf roller 40-45 Tkalich (1972)
Laspeyresia pomonella 32 Morgan et al. (1974)
(codling moth) 32 Anthon et al. (1975)
Megastigmus acuelatus 50 Vodolagin (1971)
(dog rose weevil)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Musca domestica 64 Hickin (1961)
(housefly)
Onychuirus hortensis 89 g/m2 Edwards (1962)
(springtail)
Oryzaephilus mercator 200 Joshi (1974)
(merchant grain beetle) 32 Leesch et al. (1974)
Ostrinia nubilalis 20 (larvae) Isa et al. (1970)
(corn borer)
Periplaneta americana 64 Hickin (1961)
(cockroach)
Plodia interpunctella 5.5 (normal larvae) Sardesai (1972)
(indian meal moth) 10.2 (diapausing larvae)
32 Leesch et al. (1974)
Sitophilus oryzae 5.45-6.19
(rice weevil)
Sitotroga cerealella 1.85-2.21
(angoumois grain moth)
Tenebroides mauritanicus 16 23 Bond (1956)
(cadelle) 25.5-43.3 Monro et al. (1966)
Isoptera
(termites; 5 species) 64 Hickin (1961)
Tribolium castaneum 0.009 (adults)
(red flour beetle) 3.06-6.19 Mostafa et al. (1972)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Trilobium confusum 3.6 - 91 4.64-145.14 Kenaga (1961)
(confused flour beetle) 15b
21.5-23.7 Monro et al. (1966)
Trogoderma granaria 0.038 (larvae) Pradhan & Govindan
(grain beetle) (1954)
Trogoderma variable 32-40 (eggs) Vincent & Lindgren
(warehouse beetle) (1975)
16-56 (instar larvae)
32-72 (pupae)
24-36 (adults)
a A range of LD values reflects effects of time, temperature, or pressure.
b Preceded by gamma radiation (50 krad).
LD100 values have been determined for Rhipicephalus sanguineus
(brown dog tick) ranging from 6 to 96 g/m3 (3.5 h, 22 °C and 6 h, 11
°C, respectively) (Roth, 1973).
7.3.5 Gastropods
The effects of methyl bromide on various gastropods (slugs,
snails, limpets) have been studied. Roth & Kennedy (1973) found an
LD100 for Helidella candidula and H. conspurcata, exposed for 24
h at a dosage of 240 g/m3 . Similarly, for Cochicella barbara (72
h, 13 °C) and Theba pisana (10 h, 13 °C) a dosage of 128 g/m3 was
lethal (Richardson & Roth, 1965).
7.3.6 Birds
Rhode Island Red female hens were fed, from hatching, on diets
that had been fumigated with methyl bromide at the concentration
recommended for the elimination of salmonellae (800 mg.h/litre) or at
1´ times this value (Cooper et al., 1978). Body weight, egg weight,
and egg number were not significantly affected by treatments, but
sexual maturity may have been slightly delayed. The egg flavour was
adversely affected. The same group had previously shown that the taste
of meat from broiler chickens was similarly tainted (Griffiths et al.,
1978).
No adverse effects on either the fertility or hatchability of
hens' eggs, previously fumigated with methyl bromide at 32 g/m3 for
24 h, were observed (Devaney & Beerwinkle, 1982).
7.3.7 Other animals
Data are not available on the direct environmental exposure to
methyl bromide of other animals. Effects on test animals are given in
section 8.
Bromide intoxication was reported by Knight & Costner (1977)
after horses, goats, and cattle were accidentally fed oat hay that had
been cut from a field treated with methyl bromide the previous autumn.
The bromide content of the hay ranged from 6800 to 8400 mg/kg so that
the estimated mean daily intake was 9, 49, and 70 g of bromide ion in
goats, horses, and cattle, respectively. Signs of intoxication
reported included lethargy, weakness, and ataxia. Similar symptoms
were noticed between the 7th and 9th days in animals fed this hay on
an experimental basis. Signs of in-coordination (between 10th and 12th
days) were correlated with serum bromide concentrations of 30
mEq/litre (2.4 g/litre) or more (Knight & Reina-Guerra, 1977). Serum
bromide concentrations and the associated neurological signs subsided
markedly 14 days after feeding discontinued (Knight & Reina-Guerra,
1977). Methyl bromide is not approved for use prior to the planting of
forage crops.
7.4 Population and ecosystem effects
Application of methyl bromide as a soil fumigant resulted in the
almost complete eradication of populations of a wide variety of
microflora and fauna, as well as other soil organisms, thus altering,
at least temporarily, the trophic structure of the soil environment
(Sassaman et al., 1986).
Treatment with 100% methyl bromide and other methyl
bromide/chloropicrin formulations reduced populations of Fusarium,
Pythium, and Rhizooctonia species in soil. Nine weeks after
application, populations were still significantly lower. Seedlings
grown in treated plots had the least amount of damping off and root
rot (Enebak et al., 1988).
Methyl bromide dosed under plastic sheeting at a rate of 300
g/m3 (for 30 cm depth 100 g/m2) killed all insects, though small
numbers of soil nematodes and mites were collected during subsequent
sampling (Heungens & Roos, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
A summary of acute oral toxicity data is given in Table 43. Very
few studies have been carried out, mainly because methyl bromide is a
gas at temperatures above 4 °C. The minimum lethal oral dose of methyl
bromide for rabbits was found to be 60-65 mg/kg body weight (Dudley et
al., 1940; Dudley & Neal, 1942). Miller & Haggard (1943) found that
all rats given a single oral dose of 100 mg/kg body weight in olive
oil died in 5-7 h.
8.1.2 Inhalation
8.1.2.1 Guinea-pig and rabbit
Single exposure toxicity tests conducted on various mammalian
species have shown that methyl bromide is highly toxic. A summary of
the acute toxicity data is presented in Table 44.
Studies on guinea-pigs (Sayers et al., 1929) and rabbits (Irish
et al., 1940) were carried out. Rabbits were exposed to concentrations
of 420, 852, 1000, 2000, 10 000, 20 000, and 50 000 mg methyl
bromide/m3. Table 45 shows the exposure times giving 100% survival
and 100% mortality. Concentrations of methyl bromide above 10 000
mg/m3 sometimes caused the rabbits to close their eyes; otherwise
they appeared normal until they became too weak to hold up their heads
(Irish et al., 1940). Rabbits that survived 1000 mg methyl
bromide/m3 for 2 days after exposure usually became paralysed (Irish
et al., 1940).
Toxicity is a function of the concentration levels and the
exposure times (see also Table 45). The steep dose-mortality response
to methyl bromide found by many authors can be seen in Fig. 9.
8.1.2.2 Mouse
The results of single exposure inhalation studies on mice,
carried out by Alexeeff et al. (1985), Yamano (1991), and the Japanese
Ministry of Labour (1992), are given in Tables 46 and 47. The sharp
onset of lethal toxicity was shown in all cases. Alexeeff et al.
(1985) exposed male mice to methyl bromide (870-5930 mg/m3) for 1 h
and observed that clinical signs and mortality were dose related, with
the possibility of delayed effects in target organs, such as the
kidney.
Table 43. Acute and short-term oral (gavage) toxicity
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rabbit n.d. single 56-71 all rabbits given an oral dose of Dudley et al. (1940)
63.9 mg/kg died; one rabbit receiving Dudley & Neal (1942)
56.3 mg/kg died; all rabbits given
56.1 mg/kg or less survived;
destruction of superficial layers of
stomach and duodenum with accompanying
haemorrhage and hyperaemia; minimal lethal
dose: 60-65 mg/kg body weight
rat n.d. single 100 all died in 5-7 h Miller & Haggard (1943)
rat n.d; single 190-239 LD50, 214 mg/kg Danse et al. (1984)
rat n.d. 4 weeks; 50 epithelial hyperplasia, hyperkeratosis
7 days/week and ulceration of the forestomach
rat 10 (male) 13 weeks; 0 10 and 50 mg/kg: proliferative
(Wistar) 10 (female) 5 days/week 0.4 alterations of forestomach mucosa;
2 50 mg/kg: haematological changes
10 13/20 squamous cell carcinomas of
50 forestomach
rat 15 13-25 weeks; 0 treated group: week 13: forestomach Boorman et al.(1986)
12 weeks 50 acanthosis, fibrosis, pseudoepitheliomatous
recovery for hyperplasia; week 25: hyperplastic lesions
some groups of forestomach
recovery group: regression of stomach lesions,
but adhesions, fibrosis, and mild acanthosis
remained; evidence of malignancy in one rat
Table 43 (continued)
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rat n.d. 4,8,13, and 0 treated groups: forestomach ulceration Hubbs & Hartington (1986)
(n.d.) 17 weeks; 25 pseudoepitheliomatous hyperplasia
5 days/week 50
n.d. 13 weeks; 0 recovery period: marked but incomplete
5 days/week 25 regression of lesions;
recovery for 50 no evidence of malignancy
4-8 weeks
a n.d.= no details given.
Table 44. Single exposure inhalation studies of methyl bromide on mammalsa
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
mouse 94 950 25 100 % died within 6 h Bachem (1927)
mouse 700 n.d. 100 % survived Bachem (1927)
rat 20 000 6 100 % survived Irish et al. (1940)
24 100 % died
rat 43 000 3 survived Clarke et al. (1945)
rat 50 000 3 100 % survived Irish et al. (1940)
6 100 % died
rabbit 70 40 change in motor Balander & Polyak
reflex behaviour (1962)
rabbit 19 000 25 deep (fatigued) Beyne & Goett
breathing (1934)
rabbit 20 000 36 100 % survived Irish et al. (1940)
rabbit 20 000 84 100 % died
rabbit 25 000 30 died Beyne & Goett
(1934)
rabbit 31 600 5 died after 8-10 h Duvoir et al. (1937)
rabbit 36 000 25 died Beyne & Goett
(1934)
rabbit 50 000 12 100 % survived Irish et al. (1940)
30 100 % died
dog 10 000 5-6 h died Beyne & Goett
(1934)
dog 17 000 n.d. died Merzbach (1928)
dog 19 000 n.d. died Beyne & Goett
(1934)
dog 34 000 60 died Merzbach (1928)
Table 44 (continued)
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
dog 48 000 40 died Duvoir et al. (1937)
dog 50 000 45 died Merzbach (1928)
a Adapted from Henschler (1990).
b n.d. = no details given.
6.2.2 Human studies
Data on the concentrations of bromide in various human tissues after
methyl bromide poisoning are scarce. In an autopsy study of a methyl
bromide-exposed patient, Heimann (1944) reported the following bromide
values: lung (127 mg/kg), liver (187 mg/kg), brain (207 mg/kg), and,
in a composite sample of heart, kidney, and pancreas (107 mg/kg).
Traces of methyl alcohol and formaldehyde were also found in all the
tissues examined.
In four lethal cases of people exposed to methyl bromide,
Marraccini et al. (1983) found bromide ion concentrations in serum or
plasma ranging from 40 to 583 mg/litre. Methyl bromide was detected in
the brain of one patient (detection limit <1 mg/kg).
Values of 0.9 mg/kg in lymph nodes, 3.3 and 5.1 in ovaries and
testes, 7.5 in lung, and 8.2 mg/kg wet weight in kidney cortex have
been reported in autopsy samples (from accident victims) (Hamilton et
al., 1972/73). A study of the levels of bromide in adipose tissue from
human subjects in three countries showed the highest levels in the
United Kingdom, where 5.6% of the specimens contained levels ranging
from 4.0 to 4.5 mg/kg fat; the lowest levels were found in Germany
(0-0.9 mg/kg fat) whereas levels of 1-3.7 mg/kg were found in the
Netherlands samples (Crampton et al., 1971). Van Leeuwen & Sangster
(1987) stated that there was no evidence in humans of bromide
concentration in any particular organ that might indicate a specific
physiological function of this ion.
6.3 Metabolic transformation
The metabolism of methyl bromide has not been elucidated.
Bromide concentrations in blood (and target organs) were reported
to be increased in humans (Clarke et al., 1945; Rathus & Landy, 1961;
Hine, 1969) and in laboratory animals (Irish et al., 1940, 1941) after
exposure to methyl bromide. Miller & Haggard (1943) postulated that
methyl bromide is hydrolysed in the body with the formation of
inorganic bromide and methyl alcohol. In part, this hydrolysis may
occur intracellularly, resulting in a distribution of bromide that
differs from that for bromide given orally as sodium bromide. Sodium
bromide and methyl alcohol, given at the levels produced after methyl
bromide exposure, did not produce the same toxic and functional
response (Irish et al., 1940, 1941). This suggested that the toxicity
of methyl bromide was due to the reaction of the halide molecule with
the tissue and was not attributable to the hydrolytic products.
Hallier et al. (1990a) measured the cytosolic turnover rate of
methyl bromide in both liver and kidney from five different strains of
mice and rats, with in vitro incubation. The turnover rate in both
organs was consistently higher in tissues isolated from females. On
the basis of a similar effect with methyl chloride, this effect could
be attributed to a higher rate of glutathione conjugation in females.
The reaction products of methyl bromide in wheat were
characterized by Winteringham et al. (1955) using 14C- or
82Br-labelled methyl bromide. It was pointed out that, although most
attention is given to the bromide ion because it is the only part of
the residue that is easily determined, methyl methionine sulfonium
bromide, the methylated histidines, and possibly other residues of
methylation were also produced.
6.3.1 Binding to proteins and lipids
Methylation of cysteine- S and histidine- N residues of haemo-
globin in suspended mouse erythrocytes was found after in vitro
treatment with radiolabelled methyl bromide (Djalali-Behzad et al.,
1981). After inhalation of methyl bromide, alkylation of cysteine- S
residues was seen in mouse haemoglobin and liver proteins
(Djalali-Behzad et al., 1981).
Adducts result from reactions between toxic chemicals and amino
acids in haemoglobin or other proteins (or nucleosides in DNA - see
below). S-methyl-cysteine has been studied as a haemoglobin adduct
in mice (Iwasaki, 1988a,b) and rats (Xu et al., 1990) and as a serum
albumin adduct in human blood samples (Müller et al., 1991, 1992).
Both haemoglobin and serum albumin adducts have been studied in blood
samples of workers occupationally exposed to methyl bromide and these
have been proposed as suitable parameters for the biomonitoring of
exposure to the fumigant (Iwasaki et al., 1989; Müller et al., 1991,
1992) (see also section 9.4.4).
In the insect Triatoma infestans , Castro et al. (1976)
demonstrated that methyl bromide is irreversibly bound to lipids and
to proteins in both the nymph adult and eggs and that exposure to
methyl bromide significantly decreased the content of sulfhydryl
groups in nymph adult and egg proteins.
Studies on methylation by methyl bromide of wool, silk, collagen,
and gelatin (Blackburn & Phillips, 1944), wheat flour (Bridges, 1955;
Winteringham et al., 1955), and cocoa beans (Asante-Poku et al., 1974)
indicated that N-, O-, and S- methylation of proteins occurs
(Cova et al., 1986; Starratt & Bond, 1990b). The main site of
decomposition of methyl bromide in cocoa beans was shown to be in the
alcohol- insoluble proteins of the shell (Asante-Poku et al., 1974).
The methyl group of the fumigant became covalently bound to the
alpha-amino group of the various amino acids, the imidazole ring of
histidine, and the epsilon-amino group of lysine.
Winteringham et al. (1955) found that the gluten fraction of
whole-wheat flour exposed to [14C]methyl bromide was responsible for
80% of the decomposition of the absorbed fumigant with N-methyl,
dimethylsulfonium, and methoxyl and thiomethoxyl derivatives
accounting for 50, 30, and 20%, respectively, in this fraction.
Bridges (1955) reported that 1- N-methylhistidine,
3- N-methylhistidine and 1,3- N,N-dimethylhistidine accounted for
75% of the N-methyl derivatives, and that 10% was due to probably
epsilon- N-methyllysine.
Starratt & Bond (1990a,b) used [14C]methyl bromide to
distinguish naturally occurring residues from those formed during the
fumigation of a variety of commodities: maize, wheat, oatmeal,
peanuts, almonds, alfalfa, potatoes, oranges, and apples. In order to
get higher incorporation of the tracer, fumigation was carried out at
a level of 48 mg/litre, for 3 days. Methyl bromide was bound both
physically and chemically to the commodities. To measure the
physically-bound residue, a parallel test was run using unlabelled
fumigant. Methyl bromide levels were determined 1 h following
fumigation and then at 1, 2, 4, and 10 days. The levels in all the
commodities declined rapidly.
Extraction of the [14C]methyl bromide-fumigated commodities
with diethyl ether removed very little radioactivity, showing that
fats and other non-polar lipids were not methylated during treatment.
In maize, fractions corresponding to albumins, glutamines, zein, and
glutelin were all methylated.
Methylation of methionine is one of the main reactions forming
the relatively unstable methylmethionylsulfonium derivative.
Spontaneous decomposition yields dimethyl sulfide. Analysis of the
sites of methylation was uncertain as both acidic and basic hydrolysis
caused partial decomposition (Starratt & Bond, 1990a,b). The volatile
products included methanol, methyl mercaptan, and dimethyl sulfide.
Different commodities produced different residues (Starratt & Bond,
1990a,b). In potato and orange extracts, the main methylated
components were identified as S-methyl-glutathione,
gamma-glutamyl- S-methylcysteine and S-methyl cysteine. These
compounds were not found in maize. 1- N-methylhistidine and
3- N-methylhistidine were the major components from the fumigated
maize, almonds and other commodities. The highest level of histidine
methylation occurred in almonds, accounting for about 54% of the
chemically-bound residue.
6.3.2 Binding to DNA
Labelled 7-methylguanine was identified in DNA from liver and
spleen cells of mice exposed to [14C] methyl bromide (Djalali-Behzad
et al., 1981). In in vitro experiments with DNA solutions treated
with [14C] methyl bromide, predominantly [14C] 7-methylguanine was
identified (Starratt & Bond, 1988b).
Calf thymus DNA treated in the solid state with [14C] methyl
bromide, showed, on analysis, four major radiolabelled peaks with
retention times corresponding to 1-methyladenine, 7-methylguanine,
3-methyladenine, and 3- methylcytosine (Starratt & Bond, 1988b).
The DNA adducts, [14C]3-methyladenine, [14C]7-methylguanine,
and [14C]O6-methylguanine, have been found in the stomach and
forestomach of rats after both oral and inhalation exposure of
[14C]methyl bromide (Gansewendt et al., 1991).
In maize and wheat, 7-methylguanine and 1-methyladenine were
identified as major products after hydrolysis together with lesser
amounts of 3-methylcytosine and 3-methyladenine (Starratt & Bond,
1988a). Although the yields of DNA were low, Starratt & Bond (1990a)
also found evidence of radioactively-labelled 7-methylguanine and
1-methyladenine in [14C]methyl bromide-fumigated almonds and
potatoes.
6.3.3 The role of glutathione in methyl bromide metabolism
6.3.3.1 Mammals
Liver, kidney, lung, and brain from mice, exposed via inhalation
for 1 h to methyl bromide concentrations of from 870 to 5930 mg/m3,
were analysed for glutathione and bromide ion (Alexeeff et al., 1985).
The liver glutathione levels of the 4700 and 5930 mg/m3 exposure
groups were significantly lower than that of the controls. Bromide ion
levels were highest in the liver and kidney and lowest in the whole
blood. The lung and brain bromide levels were intermediate.
Methyl bromide has been shown in rats to increase the activity of
glutathione S-alkyl transferase and decrease the nonprotein
sulfhydryl content (Roycroft et al., 1981). A group of male
Sprague-Dawley rats were exposed to methyl bromide (117 mg/m3; 6
h/day; 10 days) and killed immediately after the last dose.
Biochemical analyses showed that glutathione S-alkyl transferase was
significantly increased in the lung (12%), liver (11.9%), and kidney
(6.9%). Non-protein sulfhydryl was significantly reduced by 11.4% in
the liver and 13.9% in the kidney. Glucose-6-phosphate dehydrogenase
was significantly increased in the kidney (8.5%), but not in the lung
or liver.
Studies on rats by Davenport et al. (1992) showed that
glutathione was depleted and regional brain
glutathione- S-transferase inhibited by methyl bromide inhalation
(584 mg methyl bromide/m3; 6 h/day; 5 days) [see sections 8.8.2 and
8.10].
In a preliminary report, Thomas & Morgan (1988) reported that
treatment of rats with buthionine sulfoximine (BSO) depleted
glutathione levels, prior to methyl bromide exposure, and increased
the toxicity of methyl bromide. This is in contrast to the findings of
Chellman et al. (1986) for methyl chloride in mice, who found that
glutathione depletion by BSO decreased methyl chloride toxicity in the
brain and kidney. However, the observation is consistent with those of
Tanaka et al. (1988), who showed that treatment of rats with
glutathione reduced the detrimental effects of methyl bromide on
sleep-wakefulness and its circadian rhythm and increased the LD50
value (section 8.8.2).
In whole body inhalation studies on rats exposed for 6 h/day, for
5 or 10 days, to 117 mg methyl bromide/m3 (30 ppm), glutathione
(GSH) S-transferase and glucose-6-phosphate dehydrogenase (G-6-PDH)
activities were increased in the lung. Decreases in GSH-reductase and
GSH- S-transferase activities were found in the liver (Jaskot et al.,
1988).
When human erythrocyte cytoplasm was incubated with methyl
bromide or methyl iodide in the presence of excess glutathione (GSH),
a spontaneous non-enzymatic conjugation was observed (Deutschmann et
al., 1989). This was verified in parallel experiments with boiled
cytoplasm and GSH added after boiling.
Enzymatic conjugation of methyl bromide with reduced glutathione
to produce S-methyl cysteine (the analysis) appears to be
isoenzyme-specific, since conjugation was observed in the erythrocyte
cytoplasm of a majority (13/20) of the population. The same
individuals also conjugated methyl chloride, a reaction that is
dependent on glutathione- S-transferase rho (GSTrho), a minor form of
the enzyme (Hallier et al., 1990b).
Later studies on the properties of the glutathione-
S-transferase (GST) responsible for methyl bromide conjugation with
GSH (measured by methyl bromide depletion) have separated the enzyme
GSTsigma from human erythrocytes; this new enzyme has not been found
in various non-human species (Schröder et al., 1992).
Measurement of methyl bromide disappearance in head-space vials
containing whole human blood cultures in an atmosphere of 19 460
mg/m3 (5000 ppm) at 37 °C indicated that the methyl bromide
concentration had fallen to zero within 1 h, in the presence of blood
from glutathione conjugators, whereas it had fallen to approximately
5836 mg/m3 (1500 ppm) at 1 h, in the presence of blood from
non-conjugators. Further reduction was slow, the methyl bromide
concentration being about 3890 mg/m3 (1000 ppm) after 6 h (Hallier
et al., 1993).
6.3.3.2 Insects
Glutathione is depleted and glutathione S-transferase can be
induced in the larvae of the khapra beetle (Trogoderma granarium) by
fumigation with methyl bromide at a lethal dose (Shivanandappa &
Rajendran, 1987).
Starratt & Bond (1981) demonstrated that, in the granary weevil
( Sitophilus granarius L.), the major pathway for the detoxication of
methyl bromide residues was by conjugation, primarily with
glutathione, and that increasing amounts of glutathione in the insects
resulted in increasing tolerance to methyl bromide exposure. Both
strains of the insect (methyl bromide sensitive and methyl bromide
resistant) metabolized methyl bromide primarily to substances that, on
thin layer chromatography, behaved consistently with their
identification as S-methyl glutathione.
6.4 Elimination and excretion in expired air, faeces, urine
The route of administration of methyl bromide affects the
pathways of excretion.
Miller & Haggard (1943) investigated the amount of methyl bromide
eliminated and the amount of bromide retained in the body after i.p.
administration of methyl bromide. Following a single i.p. injection of
60 mg methyl bromide/kg body weight, elimination continued for about
45 min and more than 90% was eliminated in the first 30 min. With
lethal i.p. doses of 120-180 mg methyl bromide/kg, 24- 45% of the
methyl bromide was eliminated; the amounts of fixed and nonvolatile
bromide being 94.9-126.6 mg/kg. In rats dosed orally (75-100 mg methyl
bromide/kg), similar bromide levels were found, but the methyl bromide
eliminated was given as only 2.4-4.6%.
These results have been confirmed by studies using radioactively
labelled methyl bromide (Medinsky et al., 1984). In rats dosed
intraperitoneally with [14C] methyl bromide, the major route of
elimination was the exhalation of 14CO2 (46%). In contrast,
urinary excretion of [14C] was the major route of elimination (43%
of the dose), when methyl bromide was given orally. Very little
appeared in the faeces (<3% of the dose), regardless of the route of
administration. In rats with bile duct cannulations, 46% of an oral
dose appeared in the bile over a 24-h period (Medinsky et al., 1984).
The authors suggested that reabsorption of biliary metabolites from
the gut played a significant role in the disposition of [14C] methyl
bromide.
In inhalation studies on rats exposed for 6 h to 337 nmol [14C]
methyl bromide/litre air, Bond et al. (1985) showed that excretion of
[14C] as 14CO2 was the major route of elimination, about 47%
(3900 nmol/rat) of the total [14C] methyl bromide absorbed being
excreted by this route. CO2 excretion exhibited a biphasic
elimination pattern with 85% of the 14CO2 being excreted with a
half-time of 3.9 h and 15% excreted with a half-time of 11.2 h.
Half-times for the elimination of 14C in urine and faeces were 9.6
and 16.1 h, respectively; 65 h after exposure, about 75% of the
initial radioactivity had been excreted with 25% remaining in the body
(Bond et al., 1985). Elimination half-times of [14C] from tissues
were 1.5-8 h. In all tissues examined, over 90% of the 14C in the
tissues were methyl bromide metabolites (Bond et al., 1985). The data
from this study indicate that, after inhalation, methyl bromide is
rapidly metabolized in tissues and readily eliminated.
Male CD rats were exposed (nose only) to [14C]-methyl bromide
at 214 mg/m3 (55 ppm) for 3 min. The liver, lung, and kidney were
the major organs of [14C] distribution, immediately after exposure.
Up to 32 h after exposure, the major routes of excretion were
pulmonary and renal with elimination of 43% and 21% of the total
inhaled label, respectively (Jaskot et al., 1988).
Studies into the time course of methyl bromide and bromide
elimination in rat tissue have been described by Honma et al. (1985).
Male Sprague-Dawley rats were exposed to 973 mg methyl bromide/m3
(250 ppm) for 8 h and methyl bromide and bromine concentrations
measured at successive time intervals (see also section 6.2). The
results are shown in Fig. 7 (bromine) and Fig. 8 (methyl bromide). The
methyl bromide levels in all tissues described reached a maximum in 1
h following exposure and remained at almost the same levels during
exposure. Methyl bromide levels decreased rapidly after exposure;
after 30 min only half of the methyl bromide concentrations in adipose
tissue and blood were still present (Fig. 7). The methyl bromide
levels in the brain and liver were very low, but the elimination of
methyl bromide from these organs was slower. Forty-eight hours after
exposure, methyl bromide could not be detected in any tissue examined
(Honma et al., 1985). In contrast to the methyl bromide study, peak
concentrations of bromine in blood, kidneys, and liver occurred 4-8 h
after bromide exposure, and the half-life in these tissues was about
5 days.
Honma et al. (1985) carried out a regression analysis of methyl
bromide and bromine concentrations in blood, kidney, liver, brain and
adipose in male rats after a 2-h exposure to 0, 973, 1946, 2918, or
3890 mg methyl bromide/m3 (0, 250, 500, 750, or 1000 ppm). Linear
relationships were obtained between exposure concentrations and the
tissue methyl bromide or bromine values.
Over 95% of the bromide ion in mice exposed to methyl bromide was
eliminated within 2.5 days, bringing the concentration close to the
control levels and the limit of detection (Alexeeff et al., 1985).
Lactating cows fed a methyl bromide fumigated grain ration (220
mg bromide/kg food) compared with unfumigated diets secreted increased
levels of bromide in the milk, i.e., 10-20 mg/litre instead of about
5 mg/litre (Lynn et al., 1963).
6.5 Retention and turnover
The biological half-life of bromide ions in human blood was found
to be about 12 days (Söremark, 1960).
The half-lives of bromide in the blood and brain of rats after
i.p. injection of methyl bromide were approximately 8.7 and 4.3 days,
respectively (Tanaka et al., 1988). In inhalation studies, a half-life
of bromide of about 5 days was reported in the blood, kidneys, and
liver (Honma et al., 1985). In contrast, methyl bromide concentrations
in the blood and adipose tissue were reduced by half after 30 min,
though elimination from the brain and liver was much slower (Honma et
al., 1985).
6.6 Reaction with body components
Honma et al. (1987) suggested that the main target of methyl
bromide in the body was the central nervous system. The nor-
epinephrine content of the hypothalamus and cortex with hippo-campus
was reduced on exposure to methyl bromide (Honma et al., 1982) and
changes in the amino acid content and metabolism in the brain were
noted (Honma et al., 1983). The results of further studies suggested
that alterations in catecholamine metabolism might be a factor in
methyl bromide-induced neurotoxicity (Honma et al., 1987) (see section
8.8). Kato et al. (1986) described histopathological changes in the
brain.
Eustis et al, (1986, 1988) found clear species- and sex- related
differences in the susceptibility of specific organs and tissues to
methyl bromide effects (section 8.8.1). In rats, neuronal necrosis
occurred primarily in the cerebral cortex, hippocampus, and thalamus
of the brain, whereas in mice, necrosis of the internal granular layer
of the cerebellar folia was more frequently observed. Nephrosis
occurred in all treated mice. Myocardial degeneration was observed in
male and female rats more frequently than male mice. Atrophy of the
adrenal cortex and testis and necrosis of the olfactory epithelium
were described. Similar findings were described by Hurtt et al.
(1987). Degeneration and regeneration of the olfactory epithelium were
also described by Hurtt et al. (1988) and Hastings et al. (1989);
Hastings (1990).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Soil microorganisms
Methyl bromide is used commercially to control soil- borne fungi
that cause:
- damping off: Rhizoctonia solani, Pythium spp., Sclerotium
bataticola (Macrophomina phaseolina), Phytophtora spp.,
and Thielaviopsis basicola.
- crown rot: Sclerotium rolfsii and Sclerotinia spp.
- root rot: Pythium spp., Stromatinia, Fusarium spp.,
Sclerotium bataticola, (Macrophomina phaseolina),
Rhizoctonia solani, Pyrenochaeta spp. (corky root, pink
root), Armillaria and Phytophthora spp.
- wilt: Fusarium spp., and Verticillium spp.
Generally, concentration-time products of methyl bromide required
to kill fungi are much higher than those needed to control insect and
nematode pests, susceptibility increasing with temperature. To control
fungi, methyl bromide is generally used at rates of 40-100 g/m2
(Davis et al., 1977).
Filip & Roth (1977) demonstrated the efficacy of methyl bromide
against Armillaria root rot in the stumps of ponderosa pine ( Pinus
ponderosa Laws). The survival of young pine trees in areas infected
with such fungi was increased after the elimination of the fungus,
using the fumigant.
Heungens & Roos (1982) recovered non-pathogenic fungi
(Penicillium and Mucor) following the application of methyl
bromide (300 g/m2) to pine litter, but no pathogenic fungi were
recovered.
After an initial reduction, the numbers of bacteria and fungi in
fumigated soil remained high and low, respectively, in comparison with
those in untreated soil (Sivasithamparam et al., 1987). In the
fumigated soil, Trichoderma species rapidly recolonized the soil,
becoming the dominant fungus within 15 days. In a study on the
microflora in the rhizosphere of wheat, the same authors found that,
though there was no difference in the total number of bacteria,
actinomycetes, and fungi, before and after fumigation with methyl
bromide, there were some fungal species differences with Fusarium
merismoides, T. koningii , and T. viride present in significantly
higher numbers, other fungi being less abundant.
Kelley & Rodriguez-Kabana (1979) reported that methyl bromide did
not cause any permanent changes in soil enzyme activities or adversely
affect the mycorrhizal root development of pine seedlings.
Methyl bromide is used for controlling fungal infections in the
poultry industry (Harry et al., 1972; Davis et al., 1977).
Methyl bromide is much less frequently used as a bactericide than
as an insecticide (Davis et al., 1977). It is used to control certain
soil-borne bacteria, e.g., bacterial canker (Corynebacterium
michiganese) , and bacterial wilt (Pseudomonas solanacerum)
(Bromine & Chemicals Ltd.,1990). A summary of the acute toxic effects
of methyl bromide on bacteria and viruses is given in Table 36.
Methyl bromide, used at a concentration-time product of 800
mg.h/litre at 25 °C, with a relative humidity of 70%, eliminated
salmonellae from artificially contaminated poultry foodstuffs (Tucker
et al., 1974).
Although there have been many studies on the effectiveness of
methyl bromide in reducing diseases produced by pathogenic
micro-organisms, there are fewer data on its effects on soil microbes.
Matta & Porta-Puglia (1968) tested methyl bromide on several
morphologically and functionally different groups of soil microbes. In
isolated soil samples, treated and maintained under constant
temperature (22-23 °C) and humidity (16-18%), microorganisms were
counted at 2, 21, 54, and 87 days following fumigation with 300 g
methyl bromide/m3. After 2 days, most bacteria were dead; after 87
days, there were very low counts of fungi, aerobic nitrogen-fixing,
nitrifying, and cellulolytic bacteria, whereas denitrifying,
proteolytic, amylolytic, and ammonifying bacteria showed a marked
resurgence in recolonization (Matta & Porta-Puglia, 1968). The
selective-action effects of methyl bromide fumigation on a given
microbe population in soil appear to be more significant than the
effects on microbe number.
Table 36. Effects of methyl bromide fumigation on bacteria and virusesa
Organism Dosage Conditions Reference
Vibrio cholera, Shigella 33 g/m3 10-h exposure LD100 Saiki (1952)
dysenteriae, Salmonella
typhi, Salmonella paratyphi A,
Salmonella paratyphi B
Corynebacterium 10 % bromomethane, 18-h exposure LD100 Richardson & Monro (1962)
sepedonicum 5 % ethylene oxide 85 % CO2
Arabis mosaic virus 0.32 kg/m3 controlled virus on strawberry Harrison et al. (1963)
plants
Tobacco mosaic virus, 640 g/m3 inactivated virus Inouye et al. (1967)
Cucumber green mottle virus 110 g/m3 inactivated at 27°C
320 g/m3 inactivated at 14-16°C
Escherichia coli 1257 1000 g/m3 40°C and 90 % relative humidity Prishchep &Nikiforova
provided control (1969)
Bacillus larvae, Bacillus 5000 g/m3 5-day exposure controlled Smirnov (1970)
paraalvei, Streptococcus bacteria in bee honeycombs
apis, Streptococcus pluton,
Pseudomonas apisepticus
Tobacco mosaic virus 200 g/m3 inactivated virus in 3 kg soil Doraiswamy et al. (1972)
in which tomatoes were grown
Salmonella typhimurium 800 mg-h/litre 25 °C and 70% relative humidity Tucker et al. (1974)
provided control
Xanthomonas begoniae 0.32 kg/m3 24-h exposure eliminated Strider (1975)
bacterial blight from Rieger
begonia
a Adapted from: Davis et al. (1977).
In a study on bacterial flora involved in the nitrogen cycle, hot
fumigation with methyl bromide at concentrations of 80 g/m3 was
carried out in greenhouses at 6 different sites (Turtura et al.,
1988). Seven months after treatment, the total aerobic mesophile
bacteria count, aerobic nitrogen-fixing, ammonifying,
ammonia-oxidizing, and nitrite-oxidizing bacteria, always showed
higher values in fumigated than in unfumigated control soils.
Recolonization was more marked in the upper 0-30 cm soil samples in
which the development of ammonifying and nitrifying bacteria was
highly significant.
Rovira & Ridge (1979) found no long-term effects on aerobic soil
bacteria or actinomycetes with application of 22 g methyl bromide/m2.
Yeates et al. (1991) described the recolonization of soils
sterilized in the laboratory and returned to their original pasture
and forest sites, under four different types of field conditions.
Sampling took place over 166 days (midsummer to midwinter) with two of
the sites having a moderate, and two a high, rainfall. Both microbial
biomass and dehydrogenase activity recovered rapidly but remained
consistently lower in the fumigated than in the untreated samples in
all four sites. Bacterial numbers also recovered rapidly. Fungal
hyphal lengths were 25% lower in the fumigated soil. Fumigation showed
no detectable effects on the subsequent rates of nitrogen
mineralization and little effect on nitrification rates. Protozoa were
almost completely eliminated by fumigation, numbers recovering most
rapidly in moist forest soil and slowly in dry pasture soil. Nematodes
were eliminated by fumigation; recolonization was first detected on
day 26. Numbers (10 and 62/g, respectively) and species (10 and 31,
respectively) remained much lower in fumigated, compared with
untreated, soil.
After sterilization of greenhouse soil with methyl bromide (75
g/m2), there were profound qualitative and quantitative disturbances
up to a soil depth of 30 cm (Bourbos & Skoudridakis, 1991); 7-9
species of soil mycoflora were isolated from the fumigated soil
compared with 107 from control soils. The 31-40 cm soil layer was not
affected by disinfestation. After two months, recolonization had taken
place of only 35-40% in species and 60-63% in density of the primary
microflora.
The use of methyl bromide as an effective fumigant for
greenhouses has been questioned (Bourbos & Skoudridakis, 1991).
Certain saprophytic fungi have developed a degree of tolerance.
Disinfested soil was quickly contaminated by certain pathogens
( Fusarium and Pythium spp.) and there was also a possibility of
reinfection from the lower layer of soil not reached by the fumigant.
Some fungi controlled by methyl bromide are listed in Table 37.
7.2 Aquatic organisms
7.2.1 Effect of methyl bromide
LC50 (96-h) values of 11 mg methyl bromide/litre and 12 mg
methyl bromide/litre for bluegill sunfish, Lepomis macrochirus
(freshwater) and tidewater silversides, Menidia beryllina
(saltwater), respectively, have been determined following exposure to
methyl bromide (Dawson et al., 1975/77).
The acute toxicity of methyl bromide for carp ( Cyprinus carpio
L.) was determined in studies with a 4-h exposure period (Segers et
al., 1984). The 4-h LC50 was calculated to be approximately 17
mg/litre. Damage to the gill epithelium was the most pronounced
morphological damage, which probably caused the death of the fish by
suffocation.
In a short-term study, triplicate groups of 10 fish ( P.
reticulata (guppy) and Oryzias latipes (medaka)) were exposed to
0.56, 1.0, or 1.8 mg methyl bromide/litre for 4 days. All methyl
bromide-exposed fish displayed abnormal behaviour (reduced activity).
Limited mortality was noted in P. reticulata exposed to 1.0 and 1.8
mg/litre and O. latipes exposed to 1.8 mg/litre, but there was no
increase in mortality in the next lower concentration groups (Wester
et al., 1988).
In a long-term study, P. reticulata and O. latipes were
exposed for 1 and 3 months to 0.032-3.2 mg methyl bromide/litre
(Wester et al., 1988). All guppies died in the 3.2 mg/litre group
within 3 days, and, in the 1.0 mg/litre group, within 3 weeks. The
NOLC (no observed lethal concentration) and NOEC (no observed effect
concentration: behaviour, appearance) values were 0.32 and 0.1
mg/litre, respectively. A significant decrease in weight was noted in
both sexes in the 0.32 mg/litre group.
All medaka embryos exposed to 1.8 or 3.2 mg/litre and most of the
1.0 mg/litre group died before hatching. The NOLC after 3 months was
0.32 mg/litre. The NOEC values, on the basis of behaviour and
appearance, were 0.56 mg/litre and 0.32 mg/litre after 1 and 3 months,
respectively (Wester et al., 1988).
A short-term study with (lethal) concentrations of 0.56, 1.0, or
1.8 mg methyl bromide/litre showed (using scanning electron
microscopy) major degenerative and regenerative changes in the
superficial epithelia, especially of the gills and oral mucosa,
caused, apparently, by the local irritating action of this compound.
Necrotic changes were also seen in the thymic cortex and the testis
(Wester et al., 1988). In a long-term study on guppies and medakas
exposed to the highest concentrations for 1 month and 3 months,
respectively, no significant organ or tissue changes could be detected
in routine histopathology.
Table 37. Some fungi controlled by methyl bromide
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Alternaria sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Armillaria melea 4-98 g/m2 citrus/ Munnecke et al. (1969)
grape Kissler et al. (1973)
Aspergillus sp. 1.6-5 g/m2 pecan/ Wells & Payne (1975)
honeycomb Smirnov (1970)
Byssochalamy fuloa 60-120 mg/kg starch Ito et al. (1972)
Cladosporium sp. 1.6-3.3 g/m2 pecan Wells & Payne (1975)
Eumargodes laivgi 24 g/m2 not stated Hitchcock (1968)
Fusarium sp. 45-100 g/m3 tomato Westeijn (1973)
Weihing et al. (1971)
Wells & Payne (1975)
Perrotta (1968)
Vanachter (1974)
Monochaeta sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Penicillium sp. 1.6-3.3 g/m3 pecan
Pestalotia sp. 1.6-3.3 g/m3 pecan
Phoma sp. 1.6-3.3 g/m3 pecan Wells & Payne (1975)
Phytophora parasitica 49 g/m2 citrus Grimm & Alexander (1971)
Phytophora capsici 40 g/m2 green peppers Alfaro Moreno & Vehg (1971)
Table 37 (continued)
Fungus Dose, Crop/ Reference
Surface application/m2, commodity
commodity fumigation/m3
Plasmodiophora brassicae 50-150 g/m2 cabbage Winstead & Garriss (1960)
Plasmosisphora brassicae 48 g/m2 cabbage Wimalajewa (1975)
Pyrenochaeta lycopersici 125 g/m3 soil Vanachter (1974)
Rhizoctania solani 50-150 g/m2 soil Winstead & Garriss (1960)
Sclerotium rolfsii 50 g/m2 iris Kiewnick (1968)
Sclerotina sclerotiorum 50 g/m2 tobacco Hartill & Campbell (1973)
Thielaviopsis basicola 50 g/m3 tobacco Mounat & Hitier (1959)
Verticillium sp. 45-70 g/m2 tomato Perrotta (1968)
Table 38. Effects on aquatic organisms of short-term exposure to sodium bromidea
Results (g Br-/litre) at:
Test species Parameter 24 h 48 h 72 h 96 h
Scenedesmus EC50 (growth) 5.8 7.8 8.5 10
pannonicus NOEC (growth) 2.5 2.5 2.5 2.5
(alga)
Daphnia magna LC50 (mortality) 11 11 - -
(crustacea) EC50 (mort./abn. behaviour) 5.8 5.8 - -
NOLC (mortality) 7.8 7.8 - -
NOEC (mort./abn. behaviour) 4.3 4.3 - -
Poecilia LC50 (mortality) 16 16 16 16
reticulata EC50 (mort./abn. behaviour) 0.44 0.14 0.044 0.044
(fish) NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.078 0.025 0.025
Oryzias latipes LC50 (mortality) 26 25 24 24
(fish) EC50 (mort./abn. behaviour) 0.44 0.44 0.44 0.44
NOLC (mortality) 7.8 7.8 7.8 7.8
NOEC (mort./abn. behaviour) 0.25 0.25 0.25 0.25
a From: Canton et al. (1983).
b NOE(L)C = no observed (specified) effect concentration.
7.2.2 Effect of bromide ion on aquatic organisms
The main degradation product of methyl bromide is inorganic
bromide.
To evaluate the potential impact of pollution with the bromide
ion, Canton et al.(1983) investigated the short-term effects of sodium
bromide on various freshwater organisms, using algae (Scenedesmus
pannonicus) , crustaceans (Daphnia magna), and fish (P. reticulata
and O. latipes) (Table 38). Depending on the species tested, acute
toxic effects were seen at concentrations ranging from 44 to 5800 mg
Br-/litre and, in long-term tests, the NOEC varied from 7.8 to 250
mg Br-/litre. Bromide ion markedly impaired reproduction in both
crustaceans and fish.
Further tests were performed on P. reticulata (guppy) and O.
latipes (medaka) following sodium bromide exposure for 1 and 3
months at concentration ranges of 10-32 000 mg/litre (guppy) and
180-56 000 mg/litre (medaka). NOLC values for guppies were 10 000
mg/litre and 1000 mg/litre after 1 and 3 months, respectively. The
NOEC value was 32 mg/litre in both studies. Histopathological changes
were observed at concentrations of 100 mg/litre or more (Wester et
al., 1988). For medakas, the NOLC values were 5600 and 3200 mg/litre
after 3 weeks and 3 months, respectively, whereas the NOEC value
(behaviour) for both periods was 320 mg/litre (Wester et al., 1988).
The relative susceptibility to sodium bromide of 11 taxonomically
different freshwater species was determined in medium-term toxicity
tests by Slooff & Canton (1983). The data are summarized in Table 39.
In semi-static, long-term toxicity tests on Daphnia magna, EC50
and EC10 values of 27 and 18 mg/litre, respectively, were determined
(Van Leeuwen et al., 1986).
7.3 Terrestrial organisms
7.3.1 Protozoa
Long et al. (1972) studied the effect of methyl bromide on
protozoa (Eimeria tenella and E. acervulina) at a dosage of 5
g/m3 for 20 h at 25 °C; 100 % control (destruction of oocysts) was
achieved.
7.3.2 Plants
Methyl bromide is often applied, as a fumigant, directly to plant
seeds, plant cuttings, or harvested plant products to disinfect
before, and during, transportation or storage (Davis et al., 1977).
Additionally, methyl bromide is used as a soil fumigant to control
certain plant pathogens and weed seeds in areas to be planted.
Table 39. Summary of the results of medium-term toxicity tests using
sodium bromide on 11 different freshwater test speciesa
Test species Exposure Criteria NOL(E)C
time valuesb
(days) (mg/litre)
Pseudomonas 0.3 specific growth rate 3200
fluorescens
(bacterium)
Microcytis aeruginosa 4 specific growth rate 3200
(cyanobacterium)
Scenedesmus 4 growth (biomass) 3200
pannonicus
(alga)
Lemna minor (plant) 7 specific growth rate 3200
Daphnia magna 21 mortality 3200
(crustacea) reproduction 10
Culex pipiens 25 mortality 100
(insect) development 100
Hydra oligactis 21 specific growth rate 1000
(hydrozoan)
Lymnaea stagnalis 40 mortality 3200
(mollusc) reproduction 10
hatching 3200
Poecilia recticulata 28 mortality 100
(viviparous fish) mortality and behaviour 32
growth 320
Oryzias latipes 40 mortality 3200
(viviparous fish) mortality and behaviour 320
hatching growth 10 000
Xenopus laevis 100 mortality 32
(amphibian) development 320
development 320
a Adapted from: Slooff & Canton (1983).
b NOL(E)C = no observed (specified) effect concentration.
As chloropicrin is phytotoxic, methyl bromide formulations that
contain it as a warning agent are not used on nursery stock or other
living plants (Bond, 1984).
7.3.2.1 Seed fumigation
As shown in Table 40, fumigation with methyl bromide can result
in delay in the germination of seeds and some loss of total
germinative capacity, depending on the variety, moisture content, and
the extent of exposure to the gas (Davis et al., 1977). This was
confirmed by Sittisuang & Nakakita (1985) who compared the effects of
methyl bromide on the germination of rice seeds ( Oryza sativa L.,
Japicona type) and corn (maize) seeds ( Zea mays L.). No detrimental
effect of methyl bromide up to 4 mg/litre was observed in rice seeds
at a moisture content of 11%, but as the moisture content and
temperature increased, methyl bromide had an increasing effect on
germination. Maize seeds were much more tolerant to methyl bromide.
Exposure to 5 mg methyl bromide/litre, which caused heavy damage to
rice seeds in most cases, did not generally produce any harmful effect
on the germination of maize seeds, regardless of the moisture content
and temperature. At concentrations higher than 10 mg/litre, the
viability of maize seeds declined in a similar way to that of rice
seeds. Rice seeds were found to absorb more methyl bromide than corn
seeds. Seeds with a higher moisture content absorbed more methyl
bromide and seeds with the same moisture content absorbed more methyl
bromide at higher temperatures than at lower temperatures. The authors
suggested that changes in certain proteins and enzymes were major
factors in seed viability.
The effects of methyl bromide fumigation on the germination of
different cultivars of wheat seed have been investigated. The
germination of all cultivars was reduced following fumigation at a
dose of 16 mg/litre for 24 h (11% moisture, temperature not given).
The optimal conditions were a moisture content of 9% and a temperature
of 18 °C. With increasing moisture or temperature, the percentage
germination decreased. At higher levels of moisture, the concentration
of methyl bromide appeared to be a more important factor than exposure
time at a constant concentration x time product (CTP) of 768
mg.h/litre (Khanna & Yadav, 1987).
Hanson et al. (1987) found that fumigation with methyl bromide
not only caused a delay in germination and loss of germinative
capacity but also that certain varieties of seed barley were damaged,
exhibiting symptoms of albinism and stunted growth. The authors
suggested that great care should be taken in the selection of stored
barley intended for seed and, in particular, in the fumigation of
samples in standard reference collections. A CTP of 200 mg.h/litre is
used commercially, but higher concentrations may be attained if the
distribution during fumigation is poor.
Table 40. Effects on germination of seeds fumigated with methyl bromidea
Seed Fumigation conditions Germination results Reference
hemp 70-140 g/m3 5-23 % reduction Tkalich (1974)
onion 42 g/m3 for 24 h 95 % reduction in laboratory Powell (1975)
11.5% reduction in cool soil
peanuts 32 mg/litre (24 h, 27°C, 80% reduction of: Leesch et al. (1974)
paper container relative humidity), applied 21.7%
burlap bags under cover, aerated 72 h 11.4%
oat, wheat, rye, 0, 600, or 1200 g.h/m3 at 8, 11at 18% moisture content: Blackith & Lubatti (1965)
barley 14, or 18% moisture content - no germination after 6 years storage
at 8% moisture content:
- 90% germination after 6 years storageb
Picea abies, Picea seeds at various moisture germination normal after storage Jones (1968)
glauca, Pinus mugo content; 48 g/m3, 24°C, 2-5 h, only if seeds aerated 24 h before
mughus, Pinus then aerated 1-25 h and stored storage; all but P. sylvestris
sylvestris (seeds) in sealed containers at 7°C for required drying to 5% moisture content
1 year before storage
tobacco seed 16-32 g/m3 or germination satisfactory at <10 % seed Guthrie & Kincaid (1957)
32-48 g/m3 moisture content: germination dcreased at
seed moisture contents above 10%
barley, corn, grain 32 g/m3 (< 24 h, 26°C); unimpaired germination seed Whitney et al. (1958)
sorghum, oats, wheat moisture content less than 12%
a From: Davis et al. (1977).
b Except rye, germinated well only up to 3 years storage.
7.3.2.2 Fumigation of plants or plant products
Direct fumigation of plants or plant products is used to retard,
or prevent, pest infestations and to overcome quarantine barriers.
Post-harvest fumigation is discussed in section 5.1.4.2.
7.3.2.3 The effects on plants of soil fumigation
Methyl bromide can have adverse as well as positive effects on
plants.
The phytotoxic effects of methyl bromide as a soil sterilant can
be caused by:
(1) the action on plants of methyl bromide itself;
(2) the action of inorganic bromide formed by the breakdown of
methyl bromide in the soil;
(3) indirect action through effects of either methyl bromide or
inorganic bromide on soil microflora, soil structure, or
composition (Maw & Kempton, 1973).
Where the crops are affected by lack of mycorrhizae, the plants
are stunted. Experiments have proved that this problem can be
rectified by fertilizing with phosphoric acid in the irrigation water,
using a trickle system (Bromine & Chemicals Ltd., 1990).
The phytotoxicity of methyl bromide is thought to be due mainly
to the high level of bromide ion. Drosihn et al. (1968) showed that
the degree of susceptibility of carnations to methyl bromide
fumigation of the soil depends on the intensity of subsequent leaching
of the soil. Similar findings were described by Kempton & Maw (1974).
In contrast to this, tomato plants were relatively insensitive to
bromide; growing tomatoes tolerated up to 0.1 mg bromide/g soil
without signs of injury or growth retardation (Maw & Kempton, 1973).
These authors found lettuce to be particularly resistant to inorganic
bromide, with some varieties growing in the presence of as much as 5
mg Br-/g soil.
Reichmuth & Noack (1983) determined the threshold concentration
of methyl bromide in air that should not be exceeded in the vicinity
of fumigated buildings, in order to protect plants. The test plants
( Lactuca sativa capitata (lettuce) and Nasturtium officinale
(water cress)) were exposed to concentrations of between 4 and 1400
mg methyl bromide/m3 for 72 h. At 400 mg/m3, yellowing of lettuce
leaves became apparent, while no visible effects were observed on
water cress up to the highest concentration.
(a) Cultivated plants
Only a limited number of genera, species, or varieties of plants
are susceptible to methyl bromide. Of the 441 species of glasshouse
plants tested by Latta & Cowgill (1941), 414 (93.9%) were not affected
and only 27 sustained various levels of damage; of these, five species
were severely burned. For example, roses showed no pronounced toxic
effects, when planted in soils aerated for four days after methyl
bromide fumigation, however, carnations were extremely sensitive to
both residual methyl bromide gas and inorganic bromide in the soil
(Kempton & Maw, 1974). Other crops, such as cotton, celery, pepper,
and onion, do not reach adequate growth, when grown in fumigated soil
(Bromine & Chemicals Ltd., 1990). Plants, actively growing, are more
likely to sustain injury than dormant plants (Bond, 1984).
(b) Weeds
The phytotoxic effects of methyl bromide on weeds are important
in soil fumigation.
Table 11 shows that the recommended dose rates to eradicate weeds
is 35-50 g/m2, though purple nutsedge (nut grass), corms and seeds
of horseweed Erigeron (Conyza) , mallow (Malva) , and legumes are
not efficiently controlled at this dose (Bromine & Chemicals Ltd.,
1990).
Methyl bromide (40-80 g/m2) is mentioned as being the best soil
fumigant against yellow nutsedge ( Cyperus esculentus L.), but the
weed was not completely eradicated because dormant tubers below the
tillage depth survived (Rotteveel & Naber, 1987).
7.3.3 Soil invertebrates
Soil fumigation with methyl bromide (and chloropicrin) results
generally in toxic effects in both target and non-target organisms.
The concentrations used are sufficiently high to eradicate populations
of a wide variety of organisms. Fumigants, including methyl bromide
and chloropicrin, were all strongly nematocidal. Methyl bromide killed
virtually all soil arthropods, including mites; Collembola were almost
completely eradicated. Methyl bromide was very toxic for symphylids
and millipedes (details of dose not given) (Edwards & Thompson, 1973).
Methyl bromide (concentration not given) and chloropicrin were very
toxic for earthworms, even those that lived in deep burrows (Van Rhee,
1977). Chloropicrin was repellent to most arthropods in soil (Edwards
& Thompson, 1973). To control nematodes, methyl bromide is generally
used at rates of up to 80 g/m2 (Table 41).
7.3.4 Insects and arachnids
Methyl bromide is used as a fumigant to control insect pests.
Although it is not as toxic for insect species as some other
fumigants, such as HCN, acrylonitrile, and ethylene dibromide, its
ability to penetrate quickly and deeply into sorptive materials makes
it an effective and versatile fumigant (Davis et al., 1977; Sassaman
et al., 1986). The commercial dosage for methyl bromide as a storage
fumigant ranges from 16 to 100 g/m3 for up to 3 days (Tables 12 and
13).
The dosage required depends also on the temperature. The
threshold concentration levels identified at 15 and 25 °C differed by
a factor of two or three. These investigations by Bell (1988) were
carried out on the adult beetle. The dosage of methyl bromide required
to kill eggs and pupae is greater than that required to kill all
adults. Pupae and older larvae of Tribolium spp., for example,
required CTPs of up to 180 mg.h/litre for control (Hole, 1981). For
other species and exposure conditions see Table 42.
In an FAO study, it was found that there was a variation in
tolerance to methyl bromide in different strains of eight species of
stored product beetles collected from different parts of the world
(Hole, 1981). Although resistant strains have been identified in the
laboratory, there have been no reports of resistance to methyl bromide
in practice (Bell, 1988).
The effects of lethal concentrations of methyl bromide (48 g/m3
for 2 h) on embryos of the codling moth ( Cydia pomonella L.) were
assessed using light microscopy and transmission electron microscopy
(Cheetham, 1990). Cell division stopped within one hour in nearly all
embryos, a small number of terata being produced.
Methyl bromide is used to eradicate various wood and household
pests, particularly in the warmer climates of southern and western
USA. The primary targets are drywood termites (Kalotermitidiae).
Scheffrahn & Su (1992) have assessed the toxicity of methyl bromide at
27 °C against pseudergates, nymphs, or alates of several species.
Estimates of lethal accumulated doses for 50 and 99% mortality ranged,
respectively, from 11.4 and 16.5 for R. hesperus pseudergates to
45.9 and 75.0 mg h/litre for C. cavifrons pseudergates and nymphs.
Alates were more susceptible to methyl bromide than pseudergates or
nymphs. Boczek et al. (1975) reported three periods in the development
of Acarus siro that have increased sensitivity to methyl bromide:
(a) before the beginning of gastrulation movements in the germ band;
(b) during the formation of the nervous system; and (c) the period
preceding dorsal closure. For this species, Burkholder (1966) found
LD100 values ranging from 3.4 to 16.8 g/m3 at various exposure
times at 16 °C and 85% relative humidity whilst achieving the same CTP
(65-83 g.h/m3).
Table 41. Effects of methyl bromide on nematodes
Nematode Effective Exposure Reference
control Conditions
concentration
Anguina agrostis CTP 600-800 12% moisture Hague (1963)
mg.h/litre
Belonolaimus 98 g/m2 98% methyl bromide Darby et al. (1962)
longicaudans 2% chloropicrin: covered 48 h
Ditylenchus dipsaci CTP 850 10-14% moisture Hague & Clark (1959)
mg.h/litre
Dorylaimus sp. 2.3 g/m3 40 h Van Gundy et al. (1972)
Hemicyclophora parvana 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Heterodera rostochiensis Ct 500-1000 treatment with water before fumigation Hague (1959)
mg.h/litre enhanced penetration of methyl bromide
Heterodera rostochiensis 111 g/m2 covered 16 days: 98 % methyl bromide Whitehead et al.
and 2% chloropicrin (1972)
Heterodera schachtii 0.5-30 g/m3 1-21 days Abdalla & Lear (1975)
Hoplolaimus columbus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Hoplolaimus tylenchiformis 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin: covered 48 h
Meloidogyne sp. 50 g/m2 manure applied prior to fumigation Scotto La Massese
decreased nematocidal effect & Mars (1975)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Meloidogyne incognita 2.3 g/m3 38 h Van Gundy et al. (1972)
Meloidogyne incognita 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Meloidogyne incognita 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne incognita 0.6-2.5 g/m3 1-21 days Abdalla & Lear (1975)
Meloidogyne incognita 17-22 g/m2 chisel applicator, covered or rolled Sher et al. (1958)
acrita
Meloidogyne javanica 45-67 g/m2 covered Raski et al. (1975)
Meloidogyne javanica 22-34 g/m2 chisel application, covered Thomason (1959)
Meloidogyne javanica 56-112 g/m2 98% methyl bromide Milne (1962)
and 2% chloropicrin
Pratylenchus sp. 45-67 g/m2 covered Raski et al. (1975)
Pratylenchus sp. 4.9-9.7 g/m3 1-3 days Abdalla & Lear (1975)
Pratylenchus brachynrus 23 g/m2 potted seedlings, covered, Bird et al. (1974)
aerated for 1 h after 24 h
Pratylenchus brachyurus 25-51 g/m3 24 hours; 25°C Minton & Gillenwater
(1973)
Pratylenchus penetrans 50 g/m2 not stated Chen et al. (1962)
Table 41 (continued)
Nematode Effective Exposure Reference
control Conditions
concentration
Pratylenchus thornei 49 g/m2 covered after application Van Gundy et al. (1974)
for unspecified time
Pratylenchus zeae 100 g/m2 covered for 48 h Oakes et al. (1956)
following fumigation
Trichoderus christiei 98 g/m2 98% methyl bromide Darby et al. (1962)
2% chloropicrin:
covered 48 h
Xiphinema americanum 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 2.3 g/m3 28 h Van Gundy et al. (1972)
Xiphinema index 45-67 g/m2 covered Raski et al. (1975)
Xiphinema index 0.2-2.0 g/m3 1-21 days Abdalla & Lear (1975)
Table 42. Some insects controlled by methyl bromidea
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Antagenus picus 32 Pence & Morganroth
(black carpet beetle) (1962)
Anthonomus grandis 16-80 Roth & Kennedy (1972)
(cotton boll weevil)
Anthrenus flavipes 32 Pence & Morgenroth
(furniture carpet beetle) (1962)
Anthrenus verbasci 32
(varied carpet beetle)
Araecerus fasciculatus 6.2 (eggs) Majumder et al. (1961)
3.4 (larvae)
7.4 (pupae)
4.5 (adults)
Blatta orientalis 64 Hickin (1961)
(cockroach)
Blatella germanica 64
(cockroach)
Bruchus rufimanus 28 Roth & Richardson
(broad bean weevil) (1974)
Cadra cautella 32 Leesch et al. (1974)
(almond moth)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Callosobruchus 0.85 (eggs) 1.25 (eggs) Adu & Muthu (1985)
chinensis (L) 2.2 (instar 2.72 (instar
(cowpea weevil) larvae) larvae)
0.89 (pupae) 3.98 (pupae)
1.17 (adult) 1.4 (adult)
Chilo agamemnon 20 (larvae) Isa et al. (1970)
(corn borer)
Corcyra cephalonica 1.66-1.78 (eggs) El-Buzz et al. (1974)
(rice moth) 1.10-1.68 (instar
larvae)
2.79 (pupae)
Curcilio caryae 32-112 Leesch & Gillenwater
(pecan weevil) (1976)
Ephestia kuehniella 2.02-2.46 Mostafa et al. (1972)
(mediterranean flour moth)
Gnorimoschema operculella 11.74 (larvae) Pradhan et al. (1960)
(potato tuber moth)
Gryllotalpa 70-100 g/m2 Dzidzariya (1972)
(mole cricket)
Hemp leaf roller 40-45 Tkalich (1972)
Laspeyresia pomonella 32 Morgan et al. (1974)
(codling moth) 32 Anthon et al. (1975)
Megastigmus acuelatus 50 Vodolagin (1971)
(dog rose weevil)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Musca domestica 64 Hickin (1961)
(housefly)
Onychuirus hortensis 89 g/m2 Edwards (1962)
(springtail)
Oryzaephilus mercator 200 Joshi (1974)
(merchant grain beetle) 32 Leesch et al. (1974)
Ostrinia nubilalis 20 (larvae) Isa et al. (1970)
(corn borer)
Periplaneta americana 64 Hickin (1961)
(cockroach)
Plodia interpunctella 5.5 (normal larvae) Sardesai (1972)
(indian meal moth) 10.2 (diapausing larvae)
32 Leesch et al. (1974)
Sitophilus oryzae 5.45-6.19
(rice weevil)
Sitotroga cerealella 1.85-2.21
(angoumois grain moth)
Tenebroides mauritanicus 16 23 Bond (1956)
(cadelle) 25.5-43.3 Monro et al. (1966)
Isoptera
(termites; 5 species) 64 Hickin (1961)
Tribolium castaneum 0.009 (adults)
(red flour beetle) 3.06-6.19 Mostafa et al. (1972)
Table 42 (continued)
Insect LD50 (g/m3) LD95 (g/m3) LD100 (g/m3) Reference
Trilobium confusum 3.6 - 91 4.64-145.14 Kenaga (1961)
(confused flour beetle) 15b
21.5-23.7 Monro et al. (1966)
Trogoderma granaria 0.038 (larvae) Pradhan & Govindan
(grain beetle) (1954)
Trogoderma variable 32-40 (eggs) Vincent & Lindgren
(warehouse beetle) (1975)
16-56 (instar larvae)
32-72 (pupae)
24-36 (adults)
a A range of LD values reflects effects of time, temperature, or pressure.
b Preceded by gamma radiation (50 krad).
LD100 values have been determined for Rhipicephalus sanguineus
(brown dog tick) ranging from 6 to 96 g/m3 (3.5 h, 22 °C and 6 h, 11
°C, respectively) (Roth, 1973).
7.3.5 Gastropods
The effects of methyl bromide on various gastropods (slugs,
snails, limpets) have been studied. Roth & Kennedy (1973) found an
LD100 for Helidella candidula and H. conspurcata, exposed for 24
h at a dosage of 240 g/m3 . Similarly, for Cochicella barbara (72
h, 13 °C) and Theba pisana (10 h, 13 °C) a dosage of 128 g/m3 was
lethal (Richardson & Roth, 1965).
7.3.6 Birds
Rhode Island Red female hens were fed, from hatching, on diets
that had been fumigated with methyl bromide at the concentration
recommended for the elimination of salmonellae (800 mg.h/litre) or at
1´ times this value (Cooper et al., 1978). Body weight, egg weight,
and egg number were not significantly affected by treatments, but
sexual maturity may have been slightly delayed. The egg flavour was
adversely affected. The same group had previously shown that the taste
of meat from broiler chickens was similarly tainted (Griffiths et al.,
1978).
No adverse effects on either the fertility or hatchability of
hens' eggs, previously fumigated with methyl bromide at 32 g/m3 for
24 h, were observed (Devaney & Beerwinkle, 1982).
7.3.7 Other animals
Data are not available on the direct environmental exposure to
methyl bromide of other animals. Effects on test animals are given in
section 8.
Bromide intoxication was reported by Knight & Costner (1977)
after horses, goats, and cattle were accidentally fed oat hay that had
been cut from a field treated with methyl bromide the previous autumn.
The bromide content of the hay ranged from 6800 to 8400 mg/kg so that
the estimated mean daily intake was 9, 49, and 70 g of bromide ion in
goats, horses, and cattle, respectively. Signs of intoxication
reported included lethargy, weakness, and ataxia. Similar symptoms
were noticed between the 7th and 9th days in animals fed this hay on
an experimental basis. Signs of in-coordination (between 10th and 12th
days) were correlated with serum bromide concentrations of 30
mEq/litre (2.4 g/litre) or more (Knight & Reina-Guerra, 1977). Serum
bromide concentrations and the associated neurological signs subsided
markedly 14 days after feeding discontinued (Knight & Reina-Guerra,
1977). Methyl bromide is not approved for use prior to the planting of
forage crops.
7.4 Population and ecosystem effects
Application of methyl bromide as a soil fumigant resulted in the
almost complete eradication of populations of a wide variety of
microflora and fauna, as well as other soil organisms, thus altering,
at least temporarily, the trophic structure of the soil environment
(Sassaman et al., 1986).
Treatment with 100% methyl bromide and other methyl
bromide/chloropicrin formulations reduced populations of Fusarium,
Pythium, and Rhizooctonia species in soil. Nine weeks after
application, populations were still significantly lower. Seedlings
grown in treated plots had the least amount of damping off and root
rot (Enebak et al., 1988).
Methyl bromide dosed under plastic sheeting at a rate of 300
g/m3 (for 30 cm depth 100 g/m2) killed all insects, though small
numbers of soil nematodes and mites were collected during subsequent
sampling (Heungens & Roos, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
A summary of acute oral toxicity data is given in Table 43. Very
few studies have been carried out, mainly because methyl bromide is a
gas at temperatures above 4 °C. The minimum lethal oral dose of methyl
bromide for rabbits was found to be 60-65 mg/kg body weight (Dudley et
al., 1940; Dudley & Neal, 1942). Miller & Haggard (1943) found that
all rats given a single oral dose of 100 mg/kg body weight in olive
oil died in 5-7 h.
8.1.2 Inhalation
8.1.2.1 Guinea-pig and rabbit
Single exposure toxicity tests conducted on various mammalian
species have shown that methyl bromide is highly toxic. A summary of
the acute toxicity data is presented in Table 44.
Studies on guinea-pigs (Sayers et al., 1929) and rabbits (Irish
et al., 1940) were carried out. Rabbits were exposed to concentrations
of 420, 852, 1000, 2000, 10 000, 20 000, and 50 000 mg methyl
bromide/m3. Table 45 shows the exposure times giving 100% survival
and 100% mortality. Concentrations of methyl bromide above 10 000
mg/m3 sometimes caused the rabbits to close their eyes; otherwise
they appeared normal until they became too weak to hold up their heads
(Irish et al., 1940). Rabbits that survived 1000 mg methyl
bromide/m3 for 2 days after exposure usually became paralysed (Irish
et al., 1940).
Toxicity is a function of the concentration levels and the
exposure times (see also Table 45). The steep dose-mortality response
to methyl bromide found by many authors can be seen in Fig. 9.
8.1.2.2 Mouse
The results of single exposure inhalation studies on mice,
carried out by Alexeeff et al. (1985), Yamano (1991), and the Japanese
Ministry of Labour (1992), are given in Tables 46 and 47. The sharp
onset of lethal toxicity was shown in all cases. Alexeeff et al.
(1985) exposed male mice to methyl bromide (870-5930 mg/m3) for 1 h
and observed that clinical signs and mortality were dose related, with
the possibility of delayed effects in target organs, such as the
kidney.
Table 43. Acute and short-term oral (gavage) toxicity
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rabbit n.d. single 56-71 all rabbits given an oral dose of Dudley et al. (1940)
63.9 mg/kg died; one rabbit receiving Dudley & Neal (1942)
56.3 mg/kg died; all rabbits given
56.1 mg/kg or less survived;
destruction of superficial layers of
stomach and duodenum with accompanying
haemorrhage and hyperaemia; minimal lethal
dose: 60-65 mg/kg body weight
rat n.d. single 100 all died in 5-7 h Miller & Haggard (1943)
rat n.d; single 190-239 LD50, 214 mg/kg Danse et al. (1984)
rat n.d. 4 weeks; 50 epithelial hyperplasia, hyperkeratosis
7 days/week and ulceration of the forestomach
rat 10 (male) 13 weeks; 0 10 and 50 mg/kg: proliferative
(Wistar) 10 (female) 5 days/week 0.4 alterations of forestomach mucosa;
2 50 mg/kg: haematological changes
10 13/20 squamous cell carcinomas of
50 forestomach
rat 15 13-25 weeks; 0 treated group: week 13: forestomach Boorman et al.(1986)
12 weeks 50 acanthosis, fibrosis, pseudoepitheliomatous
recovery for hyperplasia; week 25: hyperplastic lesions
some groups of forestomach
recovery group: regression of stomach lesions,
but adhesions, fibrosis, and mild acanthosis
remained; evidence of malignancy in one rat
Table 43 (continued)
Species/ Number of Exposure Dose Effect Reference
strain animals/ time (mg/kg
groupa body weight)
rat n.d. 4,8,13, and 0 treated groups: forestomach ulceration Hubbs & Hartington (1986)
(n.d.) 17 weeks; 25 pseudoepitheliomatous hyperplasia
5 days/week 50
n.d. 13 weeks; 0 recovery period: marked but incomplete
5 days/week 25 regression of lesions;
recovery for 50 no evidence of malignancy
4-8 weeks
a n.d.= no details given.
Table 44. Single exposure inhalation studies of methyl bromide on mammalsa
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
mouse 94 950 25 100 % died within 6 h Bachem (1927)
mouse 700 n.d. 100 % survived Bachem (1927)
rat 20 000 6 100 % survived Irish et al. (1940)
24 100 % died
rat 43 000 3 survived Clarke et al. (1945)
rat 50 000 3 100 % survived Irish et al. (1940)
6 100 % died
rabbit 70 40 change in motor Balander & Polyak
reflex behaviour (1962)
rabbit 19 000 25 deep (fatigued) Beyne & Goett
breathing (1934)
rabbit 20 000 36 100 % survived Irish et al. (1940)
rabbit 20 000 84 100 % died
rabbit 25 000 30 died Beyne & Goett
(1934)
rabbit 31 600 5 died after 8-10 h Duvoir et al. (1937)
rabbit 36 000 25 died Beyne & Goett
(1934)
rabbit 50 000 12 100 % survived Irish et al. (1940)
30 100 % died
dog 10 000 5-6 h died Beyne & Goett
(1934)
dog 17 000 n.d. died Merzbach (1928)
dog 19 000 n.d. died Beyne & Goett
(1934)
dog 34 000 60 died Merzbach (1928)
Table 44 (continued)
Species Concentration Length of Effect References
(mg/m3) exposure
(min)b
dog 48 000 40 died Duvoir et al. (1937)
dog 50 000 45 died Merzbach (1928)
a Adapted from Henschler (1990).
b n.d. = no details given.
 Table 45. Acute inhalation toxicity of methyl bromide for rats and rabbitsa
Concentration Exposure time in hours
(mg/m3) Rats Rabbits
100 % fatality 100 % survival 100 % fatality 100 % survival
50 000 0.1 0.03 0.5 0.2
20 000 0.4 0.1 1.4 0.6
10 000 0.7 0.4 2.2 1.0
2 000 6 2 11 6
1 000 22 8 24 15
852 26 12 32 20
420 -b 22 -b -b
a From: Irish et al. (1940).
b No data.
Table 46. LC50 values for methyl bromide
Species Concentration Exposure Reference
(mg/m3) time
mouse 6 600 30 min Bakhishev (1973)
mouse 4 680 1 h Alexeeff et al. (1985)
mouse 1 540 2 h Balander & Polyak (1962)
mouse 1 575 4 h Yamano (1991)
rat 11 000 30 min Bakhishev (1973)
rat 7 300 1 h Zwart (1988); Zwart et al.
(1992)
rat 3 034 4 h Kato et al.(1986)
rat 1 175 8 h Honma et al. (1985)
Further details of target organ studies and biochemical findings
from the series of mouse studies (Alexeeff et al., 1985) are given in
sections 8.8 and 6.3, respectively.
An LC50 of 1575 mg methyl bromide/m3 (405 ppm ± 20) was
determined after a 4 h exposure (Yamano, 1991). In a further study,
the author exposed mice to 1945 mg methyl bromide/m3 (500 ppm).
After 2 h of exposure, there were no deaths, but, after a further 30
min, there was 85% mortality. Mice treated prior to exposure with
glutathione (500 mg/kg i.p.) showed only 5.3% mortality after this
time.
Table 47. Some single exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 6 (male) (nose only) 0 Alexeeff et al.
(Swiss-Webster) 1 h, surviving 870 (+); no toxic response (1985)
mice sacrificed 1720 (+); no toxic response
one week 2200 (+); significantly decreased lung and
later 2720 liver weights
3500 (+); additionally enlarged, pale kidneys
and kidney lesions
3820 (-); additionally abnormal clinical
signs, weight loss and mortality;
cerebral haemorrhage
4700 (-); additionally liver lesion, liver
congestion and haemorrhage
5770 (-); additionally decreased motor
coordination; cerebral congestion;
colonic haemorrhage; congested
kidneys
5930 (-); all effects mentioned above
(1 h-LC50 of 4680 mg/m3 determined)
a (+) = Able to recall a single task passive avoidance test.
b (-) = Not able to recall a single task passive avoidance test.
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 10 (male) 4 h 389 0% mortality Japanese
(Crj: BDF1) 10 (female) 584 0% mortality Ministry of
873 0% mortality Labour (1992)
1315 0% mortality
Pathology: respiratory metaplasia of the
olfactory epithelium of the nasal cavity
(female)
1970 80% mortality (male) and 100% mortality
(female)
Clinical signs: decrease in locomotor
movement, tremor, convulsion, diarrhoea,
bradypnoea, dyspnoea (dead)
Pathology:
Dead: congestion of the lung, necrosis
and degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis of the
thymus and lymph node, necrosis of the
olfactory epithelium of the nasal cavity
Survived: tubular necrosis and regeneration
of the kidney, necrosis and respiratory
metaplasia of the olfactory epithelium of the
nasal cavity
2950 100% mortality
clinical sign and pathology: same as
1970 mg/m3 dead animals
mouse 1 h 45 min 1945 0% mortality Yamano (1991)
2 h 0% mortality
2 h 10 min 11% mortality
2 h 20 min 15% mortality
2 h 30 min 85% mortality
3 h 90% mortality
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 10(male) 4 h 584 0% mortality Japanese
(F344/DuCrj) 10(female) 875 0% mortality Ministry of
Pathology: disarrangement and Labour (1992)
respiratory metaplasia of the olfactory
epithelium of the nasal cavity
1315 0% mortality; Pathology: same as 875 mg/m3
1970 0% mortality; Pathology: same as 875 mg/m3
2956 100% mortality
Clinical signs: closed eyelid, decrease in
locomotor movement, ataxic gait, serous
discharge of nose, lacrimation, diarrhoea,
irregular breathing and bradypnoea
Pathology: congestion of the lung,
degeneration of the liver, tubular necrosis
of the kidney, haemorrhage of heart,
haemorrhage or necrosis of the adrenal glands,
necrosis of the olfactory epithelium of the
nasal cavity, congestion of the thymus
4435 100% mortality
Clinical signs and pathology: same as 2956 mg/m3
rat 2 (male) 5.2-86 min 7500- 1-h LC50 was 7300 mg/m3. Zwart (1988)
(SPF-Wistar) 57 000 Rangec: 3.5-min LC50 = 75 700 mg/m3 Zwart et al.
480-min LC50 = 1300 mg/m3 (1992)
rat 5 (male) 8 h 1042- LC50 = 1175 mg/m3 Honma et al.
(Sprague-Dawley) 2085 (1985)
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 8 (male) 8 h 245- locomotor activity decreased at
(Sprague-Dawley) 972 731 mg/m3; rectal temperature fell
2°C at 486 mg/m3; decrease in feed
consumption and body weight gain at
486 mg/m3; all animals lost righting
reflex at 245 mg/m3
rat 8 (male) 6 h 778 extensive destruction of the Hurtt et
(F-344) olfactory epithelium al. (1988)
c Total of 23 combinations of time and dosage; the animals were observed for up to 2 weeks.
Groups (10 male+10 female) of BDF1 mice were exposed to methyl
bromide (99.9% pure) concentrations of 389, 584, 873, 1315, 1970, or
2950 mg/m3 (100, 150, 225, 338, 506, or 760 ppm) for 4 h (Japanese
Ministry of Labour, 1992). Mice exposed to concentrations of 1970 and
2950 mg/m3 showed decreased locomotor activity, tremor, convulsions,
diarrhoea, dyspnoea, and bradypnoea. In the 2950 mg/m3 group, all
the mice died; at 1970 mg/m3, 2 males survived. Mice exposed to
concentrations of between 389 and 1315 mg/m3 did not exhibit any
abnormal clinical signs.
Pathology in a female mouse exposed to 1315 mg/m3 showed
metaplasia of the olfactory epithelium. In the 2 male mice surviving
exposure to 1970 mg/m3, there was renal tubular necrosis and
regeneration, and necrosis and metaplasia of the olfactory epithelium.
In the other mice exposed to 1970 and 2950 mg/m3, there was
pulmonary congestion, hepatic degeneration and necrosis, renal tubular
necrosis, karyorrhexis of the thymus and lymph nodes, and necrosis of
the olfactory epithelium.
8.1.2.3 Rat
Irish et al. (1940) exposed rats to concentrations of 420, 852,
1000, 2000, 10 000, 20 000, or 50 000 mg methyl bromide/m3. Table 45
shows the exposure time in hours resulting in 100 % survival and 100
% fatality. Rats exposed to concentrations below 10 000 mg/m3 showed
roughening of the fur, hunching of the back, drowsiness, heavy
breathing, and sometimes lacrimation. At higher concentrations, the
first signs were nose irritation and lacrimation followed by the
reactions already mentioned. Those exposed for 20 h to 1000 mg methyl
bromide/m3 often became hyperactive until exhausted.
As well as neurological manifestations of toxicity in rats,
methyl bromide at concentrations of 1000-20 000 mg/m3 caused
irritation of the lungs, producing acute congestion and oedema (Irish
et al., 1940).
Groups (10 male + 10 female) of F344 rats were exposed to methyl
bromide (99.9% pure) concentrations of 584, 875, 1315, 1970, 2956, or
4435 mg/m3 (150, 225, 338, 506, 760, or 1140 ppm) for 4 h in a
chamber (Japanese Ministry of Labour, 1992). At concentrations of 1315
mg/m3 and above, there was decreased locomotor activity, ataxia,
nasal discharge, lacrimation, diarrhoea, and irregular breathing and
bradypnoea. In the 2956 and 4435 mg/m3 exposure groups, all the rats
died. Pathology of these groups showed pulmonary congestion, hepatic
degeneration, renal necrosis, myocardial haemorrhages, haemorrhage and
necrosis of the adrenal glands, and congestion of the thymus. In rats
exposed to 875, 1315, or 1970 mg/m3, there was metaplasia of the
olfactory epithelium and, in those exposed to the two highest doses,
also necrosis of the olfactory epithelium.
A single 6-h exposure of rats to 780 mg methyl bromide/m3
caused extensive destruction of the olfactory mucosal epithelium
(Hurtt et al., 1988).
Kato et al. (1986) determined a 4-h LC50 for methyl bromide in
male Sprague-Dawley rats (Fig. 9). Groups of 5 rats were exposed for
4 h to methyl bromide at concentrations of 1952, 2420, 3108, or 3485
mg/m3 (502, 622, 799, or 896 ppm), and approximate values of 100%
survival and 100% lethal concentration were determined (2529 and 3501
mg/m3, respectively). In a further test, 10 rats each were exposed
to 2727, 2984, 3143, 3178, or 3236 mg/m3 (701, 767, 808, 817, or 832
ppm). An LC50 value of 3034 mg/m3 (780 ppm) was calculated from
mortality at one week after exposure (Kato et al., 1986).
The dependence of methyl bromide toxicity on time and
concentration was demonstrated in studies performed by Zwart (1988)
and Zwart et al. (1992). Male SPF-Wistar rats were exposed to a total
of 23 combinations (2 rats each) of time and concentration and LC50
values were determined at seven time points ranging from 3.5 to 480
min. LC50s ranged from 75 700 mg/m3 at 3.5 min to 1300 mg/m3 at
480 min. The 1-h LC50 was 7300 mg/m3. Most animals showed some
incoordination, decreased response to stimuli, and had lame limbs,
directly after exposure. All mortalities occurred during the first
week and, on examination, red discoloured lungs and red/black spots in
the thymus were found in most dead rats. After two weeks, the
surviving animals were sacrificed. Some of these rats showed clear or
light red stained fluid in the lungs (Zwart, 1988).
Honma et al. (1985) carried out various investigations into the
effects of a single, 8-h exposure to methyl bromide on male
Sprague-Dawley rats. An acute toxicity study was carried out with five
groups of five animals exposed to 1042, 1303, 1564, 1824, or 2085
mg/m3 (268, 335, 402, 469, or 536 ppm), respectively. An 8-h LC50
of 1175 mg/m3 (302 ppm) with 95% confidence limits of 1040-1323
mg/m3 (267-340 ppm) was determined.
Body temperature was measured in four groups of five rats each
exposed to 245, 486, or 972 mg methyl bromide/m3 (63, 125, or 250
ppm). Exposure to 245 mg/m3 did not effect rectal temperature, while
8-h exposure at 486 or 972 mg/m3 decreased body temperature by about
2°C; however, this normalized within one day (Honma et al., 1985).
The effects of methyl bromide on body weight gain were
investigated. Food deprivation (feeding only twice a day) was started
at least 2 weeks before exposure. Rats were exposed to 245, 486, or
972 mg methyl bromide/m3 (63, 125, or 250 ppm) for 8 h and feed was
provided immediately afterwards. Decrease in food consumption and
depression in body weight gain were observed in groups exposed to 486
and 972 mg methyl bromide/m3, but not in groups exposed to 245 mg
methyl bromide/m3. The control group gained 15 g/day whereas with
exposure to 972 mg methyl bromide/m3, weight gain was almost fully
suppressed and was still partially depressed (+10 g) the following day
(Honma et al., 1985).
8.1.3 Dermal
Toxicity studies concerning the dermal route of exposure in
animals have not been reported.
8.1.4 Subcutaneous administration
For a single subcutaneous administration in Sprague-Dawley male
rats (9 rats/group) an LD50 for methyl bromide was found to be 135
mg/kg body weight (range 75- 250 mg/kg body weight) (Tanaka et al.,
1988).
8.2 Short-term exposure
8.2.1 Oral
A summary of studies concerned with oral exposure to methyl
bromide by gavage is given in Table 43.
A group of 12 rabbits was fed a mixed diet that had been
fumigated with methyl bromide for 24 h. The rabbits were fed
immediately after the fumigation was completed and the content of
methyl bromide in the feed was 3865 mg/kg. The first animal died 3
days after feeding was begun and the last in 13 days. All were
paralysed prior to death and all showed pulmonary damage. No changes
in the gastrointestinal tract were found (Dudley et al., 1940).
Studies on rats (8-week preliminary test, 16-week, and 20-week
test) and rabbits (52 weeks) were carried out by Dudley & Neal (1942).
Results from the rat study showed that, when high (5290-6200 mg Br/kg
food) amounts of organic and inorganic bromides were present in food
after fumigation with methyl bromide, mortality increased, body weight
gain and activity were reduced, and general health and reproductivity
were adversely affected. When feed containing 240-300 mg Br/kg,
following fumigation with 58 g methyl bromide/m3 for 24 h, was fed,
or when fumigated fruits and vegetables were fed, few or no
deleterious effects were noted (Dudley & Neal, 1942). Activity,
general condition, body weight gain, and reproductivity were normal.
Dudley & Neal (1942) carried out similar studies on rabbits. All 12
rabbits died within 2 weeks of being fed a diet containing about 3000
mg Br/kg. However, the 12 rabbits fed a diet of 60-100 mg Br/kg for 52
weeks showed few or no deleterious effects.
No apparent effects on appearance and general behaviour were
observed in Wistar rats (male and female) given doses, by gavage, of
up to 50 mg methyl bromide/kg body weight in a 90-day study (Danse et
al., 1984). Body weight gain in the male rats was significantly less
than that of controls, though this was not the case for females. There
were slight haematological changes and, in the two higher dosage
groups, several animals showed proliferative alterations of the
forestomach mucosa, characterized by hyperkeratosis and papilloma
(section 8.7).
A study by Boorman et al. (1986), based on a study design by
Danse et al. (1984), included dose groups with a recovery period, in
order to study the progression or regression of lesions. Details are
given in section 8.7 and Table 43. Boorman et al. (1986) found
forestomach lesions similar to those described by Danse et al. (1984),
but these lesions regressed in the 60-day recovery period.
Similar findings were reported by Hubbs & Harrington (1986). They
administered methyl bromide in peanut oil to rats at doses of 0, 25,
or 50 mg/kg body weight per day for up to 120 days. In a regression
study, some of the rats were treated for 90 days and then allowed to
recover for 30-60 days (Table 43 and section 8.7).
Three groups of four beagle dogs (3 male, 1 female) were fed
methyl bromide-fumigated food for 6-8 weeks in doses equivalent to an
average daily ingestion of 35, 75, or 150 mg/kg body weight of bromide
ion, respectively (Rosenblum et al., 1960). A further group of 4 dogs
received 128 mg sodium bromide/kg per day (equivalent to 100 mg
bromide ion/kg per day). A control group of 6 dogs (3 male and 3
female) received only dog chow. After one year of observation and
monthly blood and urine tests, the remaining dogs were killed, the
organs weighed, and histological studies carried out. No evidence of
toxicity that could be attributed to bromide was observed in animals
that received 35 or 75 mg bromide/kg per day. Dogs in the group
receiving 150 mg/kg per day became lethargic and had occasional
episodes of salivation and diarrhoea. No significant effects on blood
chemistry, haematology, or urinary values were reported, nor were
treatment-related deaths or histological lesions noted.
8.2.2 Inhalation studies
8.2.2.1 Guinea-pig, rabbit, monkey
A summary of short-term exposure studies is given in Table 48.
Irish et al. (1940) carried out extensive studies into the
long-term exposure of animals to methyl bromide. A total number of 135
rats, 98 guinea-pigs, 104 rabbits, and 13 monkeys were exposed to 65,
130, 250, 420, or 850 mg methyl bromide/m3, 7-8 h/day, 5 days/week
for 6 months, or, until the majority had either died or shown a severe
reaction (Table 49).
8.2.2.2 Mouse
In the short-term studies on male and female B6C3F1 mice, exposed
6 h/day for 10 days over 14 days (778 mg methyl bromide/m3),
described by NTP (1992), five mice/dose group per sex were evaluated
for haematology, serum pseudocholinesterase activity, and pathology.
Necropsied animals from the two highest dosage groups were examined
histopathologically. The results are summarized in Table 48.
Eustis et al. (1988) carried out a special target organ study on
B6C3F1 mice (and F344/N rats). Male and female B6C3F1 mice were
exposed to either 622 mg methyl bromide/m3 (160 ppm) or air for 6
h/day, 5 days/week. The animals were scheduled for sacrifice after 3,
10, or 30 exposures. When 50% mortality was observed in any group, the
surviving animals in that group were sacrificed. Mice were evaluated
for body weight, mortality, organ weights, haematology, and
histopathology. In addition to these end-points, urine chemistry and
plasma enzymes were assessed in the rats. Significantly different
mortality rates were observed between the two species, with the mice
demonstrating a higher sensitivity to 622 mg methyl bromide/m3 than
rats. Body weight differences were exposure-related (Eustis et al.,
1988). The results are summarized in Table 48. Mortality exceeded 50%
after 8 and 6 exposures in male and female mice, respectively. The
remaining male mice were killed after 10 exposures and the females
after 8 exposures. There were significant reductions in body weight
and corresponding reductions in organ weights in both sexes, whereas
there were sex differences in the haematological parameters. The
responses to exposure were minimal in males, but marked in females, in
which there were large and significant reductions in RBC, haemoglobin,
haematocrit values, and mean corpuscular haemoglobin concentrations,
and increases in WBC and mean corpuscular volume (Eustis et al.,
1988). Histopathological changes in target organs are described in
section 8.8.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). Survival was reduced at all
exposure concentrations and none of the mice exposed to 1315 or 1712
mg methyl bromide/m3 survived. At all exposure concentrations, mice
exhibited decreased locomotor activity, piloerection, lacrimation,
ataxia, and tremor.
Table 48. Short-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 10 (male) 6 h/day; 5 days/week; 477 78 mg/m3 group: 9 male, 6 female died; NTP (1992)
(B6C3F1) 10 (female) 2 weeks 97 All groups: no body weight changes; bloody
195 urine; trembling, jumpiness, paralysis in
389 all groups, but most pronounced in highest
778 dosage groups; haematology parameters/
pseudo cholinesterase activity - no
consistent dose-related effects
1 female mouse showed minimal
hyperaemia of lungs, liver, kidneys
Mouse 20 (male) 6 h/day; 5 days/week; 622 50% mortality (male) after 8 exp. days, Eustis et
(B6C3F1) 20 (female) 10 exp. days 50% mortality (female) after 6 exp. days; al. (1988)
for males and exposure-related lesions were seen in
8 exp. days the brain, heart, kidneys, thymus, and
for females spleen of both sexes; in the testes,
nose, and lungs of males, and in the
adrenal glands of females
Mouse 15 (male) 6 h/day; 5 days/week; 0 Eustis et al. (1988)
(B6C3F1) 15 (female) 6 weeks 622 lethargy; curling and crossing
(continued) of hind-limbs, forelimb twitching
and tremors; decrease in body weight
gain after 5 days; decrease in organ
weight (lung, heart, thymus, brain,
liver) neuronal necrosis; nephrosis;
atrophy of inner zone of adrenal
cortex; testicular degeneration;
decrease in RBC, increase in WBC
(females only)
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 18-30 (males) 5 days/week; 6 h/day; 0 All dose groups: no significant organ NTP (1992)
(B6C3F1) 18-30 (female) 13 weeks 39 weight effects;
78
156 decrease in Hb and MCV, increase in RBC (males);
311 decrease in Hb and MCV, increase in RBC (males);
467 17 % mortality in males; decrease in body weight,
additionally severe curling and crossing
of hindlimbs and twitching of forelimbs
(male > female)
Mouse 10 (male) 6 h/day; 5 days/week 0 Japanese
(Crj:BDF1) 10 (female) 13 weeks 29 no toxic effects; Ministry of
58 no toxic effects; Labour
117 no toxic effects; (1992)
234 depression of body weight gain;
Haematology: increase in MCV in females;
Urinalysis: increased protein in females
Mouse 10 (male) 6 h/day; 5 days/week 599 10% mortality (male) and 0% (female) Japanese
(Crj:BDF1) 10 (female) 2 weeks depression of body weight gain; Ministry of
(continued) Clinical signs: Labour (1992)
Dead mice: decrease in locomotor activity,
piloerection, lacrimation, bradypnoea,
opacity of eye, diarrhoea
Surviving mice: decrease in locomotor activity,
bradypnoea, ataxic gait, sub-normal
temperature, tremor, lacrimation, soiled,
pallor, hunched posture, piloerection
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology:
Dead mice: tubular necrosis of the kidney,
ulcer of the stomach, testicular
atrophy, atrophy of the spleen, atrophy
and karyorrhexis of the lymph node,
myocardial necrosis
Surviving mice: degeneration of the granular
layer of the cerebellum, tubular necrosis and
regeneration of the kidney, necrosis and
respiratory metaplasia of the olfactory
epithelium
Mouse 778 50% mortality of males and 80% mortality of Japanese Ministry
(Crj:BDF1) females, depression of body weight gain; of Labour (1992)
(continued) Clinical signs: same as 599 mg/m3 group
Pathology: Dead mice: degeneration of the
granular layer of the cerebellum, tubular
necrosis and regeneration of the kidney,
extramedullary haematopoiesis and atrophy
of the spleen, karyorrhexis of the thymus,
myocardial necrosis, necrosis of the
olfactory epithelium
1011 90% mortality (male and female)
depression of body weight gain;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
1315 100% mortality (male and female);
Clinical signs: same as mice dying
in 599 mg/m3 group
Pathology: congestion of the lung,
degeneration of the liver, hyaline droplet
and tubular necrosis of the kidney,
karyorrhexis of the thymus and spleen,
myocardial necrosis, necrosis of the olfactory
epithelium.
Mouse 1712 100% mortality (male and female); Japanese Ministry
(Crj:BDF1) Clinical signs: same as mice dying of Labour
(continued) in the 599 mg/m3 group (1992)
Pathology: congestion of the lung,
degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis
of the thymus and spleen.
467 mortality, 10% in males and 90% in females;
depression of body weight gain;
Clinical signs: ataxic gait;
Haematology: increased MCV in males;
Urinalysis: same as 234 mg/m3 group;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, congestion of the lung, karyorrhexis
and atrophy of the thymus, tubular necrosis
of the kidney, necrosis of the heart,
vacuolic change of the adrenal glands
Rat 6 (male) 6 h/day; 5 days 0 NTP (1992)
(SPF Wistar) (week 1) 150 brain weight depression 4-5%
6 h/day; 3 days 375 (dose related) at 750 mg/m3;
(week 2) 750 additionally, marked growth retardation;
tremors, motor incoordination; liver
weights decreased by 26%; no distinct
microscopic changes in eight organs (but
lungs of three high-dose rats were
hyperaemic)
Rat 6 (male) 6 h/day; 5 days/week 0 no toxic effects; NTP (1992)
(SPF Wistar) 6 (female) (week 1,2,3) 70 no toxic effects (marginal no-effect level); (Dutch
6 h/day; 7 days/week 200 decrease in feed consumption, decrease Study)
(week 4) in body weight gain;
disturbed gait and tremors;
600 histopathological changes in heart
and lungs, 8 rats died before end
of study
Rat 10 (male) 6 h/day; 5 day/week; 0 no deaths; no clinical findings; no Wilmer et
(Wistar) 10 (female) 13 weeks 4 change in body weight al. (1983)
25
166 minimal changes in liver
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 10 (male) 6 h/day; 5 days/week; 599 depression of body weight gain Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
Pathology: disarrangement and
respiratory metaplasia of the
olfactory epithelium
778 depression of body weight gain;
Pathology: disarrangement and respiratory
metaplasia of the olfactory epithelium
of the nasal cavity, cellular
vacuolization in the adrenal glands
1011 depression of body weight gain;
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
Rat 10 (male) 6 h/day; 5 days/week; 1315 70% mortality in males and 10% mortality Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
(continued) Dead mice: depression of body weight gain; (continued)
Clinical signs: decrease in locomotor
movement, soiled, piloerection, lacrimation,
serous or bloody nose discharge, diarrhoea,
pallor, irregular breathing;
Dead and surviving mice: decrease in
locomotor movement, hunched posture, soiled,
piloerection, haemorrhagic discharge of nose,
lacrimation
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: interstitial pneumonia,
karryorrhexis of of the thymus,
myocardial damage, cellular vacuolization
of the adrenal glands, disarrangement and
respiratory metaplasia of the olfactory
epithelium
1712 100% mortality;
Clinical signs: same as 338 ppm group dead
rats;
Pathology: congestion and haemorrhage of
the lung, congestion, necrosis, and fatty
changes in the liver, tubular necrosis of
the kidney, myocardial necrosis, haemorrhage,
necrosis and cellular vacuolization of the
adrenal glands, necrosis and
respiratory metaplasia of the olfactory
epithelium, congestion of the thymus,
inflammation of the bone marrow
Rat 10-12 (male) 4 h/day; 6 weeks 584 decrease in body weight, Kato et
(Sprague- no clinical changes; al. (1986)
Dawley) 778 decrease in body weight,
no clinical changes;
1167 3/12: paralysis of hindlimbs;
1556 5/10: ataxia after 2 weeks, paralysis
after 3 weeks, 1/10: died after 4 weeks,
3/10: died after 5 weeks;
Haematology: no change in RBC, Hb, Hct, WBC
1167 mg/m3 group: increase in serum enzyme
activities;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Organ weights: decrease in all groups, but
no clear dose dependency;
Residual bromide: increase in all groups
584 mg/m3: spleen>kidney>liver;
higher dosage groups: kidney>spleen>liver;
Histopathological changes: in brain, heart
and testes
Rat 5 (male) 3 weeks 4 biochemical changes Sato et
(Sprague- 20 al. (1985)
Dawley) 39
Rat 10 (male) 6 h/day; 5 days 350 no observable effects; Hurtt et
(F-344) 680 dose-dependent vacuolar degeneration al. (1987)
of zona fasciculata (adrenal gland),
cerebellar granular cell degeneration
and olfactory sensory cell degeneration;
973 as above, plus: diarrhoea, haemoglobinuria,
1264 some gait disturbances and convulsions;
hepatocellular degeneration (at 1264
mg/m3 only) - cerebral cortical degeneation
ation and minor alterations in testicular
histology
Rat total 84 6 h/day; 5 days 778 increase in mean body weight; Hurtt et al. (1988)
(F-344) (male) degeneration and regeneration
of olfactory epithelium
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 40 (male); 6 h/day; 5 days 778 decrease in plasma testosterone Hurtt & Working
(F-344) 10 (male) concentration and nonprotein sulfhydryl (1988)
killed on contents of liver and testis
each of day
1,3,5 and 8
35 (male); 6 h/day; 5 days
5 killed on
each of day 6,
10, 17, 24, 38,
52, 73
Rat 5 (male) 6 h/day (3 days) 622 50 % mortality in males after 14 exp. Eustis et
(F-344) 5 (female) days; remaining males sacrificed; al. (1988)
females killed after 6 weeks;clear
5 + 10 (male) 6 h/day, 5 days/week sex-related differences in susceptibility
5 (female) (2 weeks) of specific organs to CH3Br:brain, kidney,
nasal cavity, heart, adrenal, liver, and
10 (female) 6 h/day, 5 days/week testis; neuronal necrosis in cerebral cortex,
(6 weeks) hippocampus and thalamus of brain; necrosis of
olfactory epithelium; myocardial degeneration;
testicular degeneration
Rat 18 6 h/day; 5 day/week; 0 All dose groups: no deaths or clinical Haber et al.
(F-344/N) 18 (female) 13 weeks 117 signs; no consistent organ weight effects; (1985) [abstract]
234 * body weight (females) NTP (1990)
467 * body weight (both sexes);
minor neurobehavioural changes; (females)
* Hct, * Hb, * RBC;
olfactory epithelial dysplasia and cysts
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 36 (male) 6 h/day; 5 and 117 GSH and G-6-PDH activities: increase in Jaskot et
(CD) 10 days lung and decrease in liver; al. (1988)
serum: decrease in cholinesterase, BUN,
uric acid, cholesterol,
increase in leucine amino-peptidase
Rat total 30 4 h/day; 4 days/week, 778 damage of olfactory epithelium; Hastings
(Long-Evans) (15 control) 2 weeks repair by day 4; impaired nasal (1990)
function recovered after 4 days
Rat 10 (male) 6 h/day; 5 day/week; 0 Japanese Ministry
10 (female) 13 weeks 29 no toxic effects of Labour
73 Blood biochemistry: * K (male), ** total (1992)
cholesterol (female)
183 Blood biochemistry: * in potassium (male)
** in total cholesterol, GOT, GPT (female)
455 * of body weight gain
Haematology: ** Hct, ** MCV, ** platelet
(male) ** MCV (female)
Blood biochemistry: * K (male), ** total
** total cholesterol, GOT, GPT * glucose,
creatinine (female)
Rat 1140 100% mortality; Japanese
(continued) Clinical signs: * locomotor activity, Ministry of Labour
hunchback position, piloerection, (1992) (continued)
soiled, ataxic gait, tremor, convulsion,
diarrhoea, loose stool or cyanosis,
haematuria, serous or haemorrhagic
discharge of nose, haemorrhagic discharge of
eye, lacrimation, irregular breathing
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, karyorrhexis, haemorrhage and atrophy
of the thymus, tubular necrosis of the kidney,
atrophy of the testis, foamy cell accumulation
and interstitial pneumonia, myocardial damage,
cellular vacuolization of the adrenal glands,
pigmentation of the Harderian glands,
necrosis, disarrangement and respiratory
metaplasia of the olfactory epithelium
GSH = glutathione S-transferase; G-6-PDH = glucose-6-phosphate dehydrogenase; BUN = blood urea nitrogen;
RBC = red blood cell count; exp. = exposure; p.c. = post copulation.
* = decrease; ** = increase
Histopathology showed degenerative cerebellar changes, renal
tubular necrosis and regeneration, and metaplasia and necrosis of
olfactory epithelium in all exposed groups. In male mice exposed to
599 mg/m3, there were also stomach ulceration, testicular atrophy,
atrophy of the spleen, and atrophy and karyorrhexis in the lymph
nodes. At concentrations above 778 mg/m3, there were, also karyor-
rhexis of the thymus, and myocardial necrosis. Hepatic degeneration
and pulmonary congestion were found at 1315 and 1712 mg methyl
bromide/m3. For further details see Table 48.
B6C3F1 mice were exposed to methyl bromide concentrations of 0,
39, 78, 156, 312, or 468 mg/m3 for 6 h per day on five days a week
for 13 weeks (NTP, 1992). There were reductions in survival and body
weight gain among male mice exposed to 468 mg/m3. No reduction was
observed among males in the other groups or in female mice in any
group. Clinical findings in the high-dose group were severe curling
and crossing of the hindlimbs and twitching of the forelimbs. These
signs were more severe among male than among female mice. Mild
behavioural test response deviations reached a maximum after about 6
weeks with no further increase in severity in the later 7 weeks of the
study. Plasma cholinesterase activity was unaffected by treatment. No
exposure-related histopathological changes were described.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 58, 117, 234, or 467 mg/m3 (0,
7.5, 15, 30, 60, or 120 ppm) for 13 weeks, 6 h/day, and 5 days/week
(Japanese Ministry of Labour, 1992). At 467 mg/m3, there was a
mortality rate of 10% (males) and 90% (females). Ataxia was noted in
mice exposed to 467 mg/m3. There were no abnormal clinical signs at
concentrations of between 29 and 234 mg/m3. Slight increases in mean
corpuscular volume (MCV) in female mice exposed to 234 mg/m3 and in
male mice exposed to 467 mg/m3 were not accompanied by other
haematological effects. Blood biochemistry showed no abnormalities. No
treatment-related, histopathological effects were observed in the 29
and 234 mg/m3 groups. In the mice that did not survive exposure to
467 mg/m3, there were cerebellar degeneration and brain necrosis,
pulmonary congestion, thymic atrophy, myocardial damage, renal tubular
necrosis, and vacuolization of the adrenal glands (Table 49).
Table 49. Long-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
mouse 86 (male) 5 days/week; 0 All dose groups: no biologically significant NTP (1992)
(B6C3F1) 86 (female) 6 h/day;2 year 39 haematology values; no carcinogenic
[planned; (interim 128 effects; increased incidence of
because of high sacrifice at 6 389 nonneoplastic lesions in brain, bone,
mortality, and 15 months) heart, and nose
regime altered] 389 mg/m3 dosage group: high mortality
(40/86 males and 9/86 females) after 20
weeks; decrease in body weight and
in thymus weight;
tremors, abnormal posture and limb paralysis;
significant behavioural differences at 3
months (males), less so in females
mouse 50 (male) 6 h/day; 5 days/ 0 Japanese
(Crj: 50 (female) week; 16 no toxic effects; Ministry of
BDF1) 2 years 62 no toxic effects; Labour (1992)
250 no mortality change;
depression of body weight gain
Blood biochemistry: increase in CPK, inorganic
phosphorus (male), chloride; decrease
in albumin (female);
Pathology: atrophy of the granular layer of
the cerebellum no increase in neoplastic change
was considered
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 90 (male) 6 h/day; 5 days/ 0 All dose groups: degenerative and Dreef-van der
(Wistar) 80 (female) week; 29 months 12 hyperplastic lesions in nasal mucosa; no Meulen et al.
117 tumours induced by methyl bromide; (1989)
350 350 mg/m3 group: * body weight gain; Reuzel et al.
* absolute brain weight; hyperkeratosis in (1991)
oesophagus and forestomach; ** incidence
of haemothorax, myocardial degeneration;
thrombi in the heart; and increased
mortality
rat 50 (male) 6 h/day; 5 days/ 16 Pathology: ** incidence and severity of Japanese
(F344/ 50 (female) week; 2 years inflammation of the nasal cavity (male); Ministry of
DuCrj) 78 Urinalysis: * in protein (male); Labour (1992)
Pathology: same as 16 mg/m3
389 no mortality change, * body weight gain;
Haematology: ** RBC, Hb, Hct (male)
** Hb, Hct (female);
Blood biochemistry: * LDH, CPK, Na+, K+,
Cl- (male), * Glu, CPK, Ca2+, ** LAP (female);
rat Urinalysis: * protein
(F344/DuCrj) Pathology: ** incidence of necrosis and
(continued) respiratory metaplasia of the olfactory
epithelium; ** incidence and severity of
inflammation of the nasal cavity (male);
marginal ** necrosis of the olfactory
epithelium and inflammation of the nasal
cavity (female);
no increase in neoplasms observed
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 8 6 h/day; 5 days/ 0 no effect on nerve conduction velocity, Anger et al.
(Sprague- 32 (n.g.)b week; 36 weeks 214 open field activity, or coordination (1981)
Dawley) over 12 months
a RBC = red blood count; Hb = haemoglobin; Hct = haematocrit; WBC = white blood count; MCV = mean cell volume.
b n.g.= sex not given; * = decrease; ** = increase.
8.2.2.3 Rat
F344-rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). All rats in the 1712 mg/m3
group and 7 males and one female in the 1315 mg/m3 group died before
the end of the study. Piloerection and haemorrhagic nasal discharge
were reported at exposure concentrations of 1011, 1315, and 1712
mg/m3. In addition, decreased locomotor activity, a hunched position
and lacrimation were observed in rats that survived exposure to 1315
mg/m3. Diarrhoea and irregular breathing were noted in the rats that
died following exposure to 1315 and 1712 mg/m3.
Pathology showed metaplasia of the olfactory epithelium at methyl
bromide concentrations of 599 and 778 mg/m3, plus necrosis at
concentrations between 1011 and 1712 mg/m3. Vacuolization of the
adrenal glands was found at concentrations of 778, 1011, and 1315
mg/m3 with necrosis at 1712 mg/m3. Myocardial damage was reported
at concentrations between 1011 and 1712 mg/m3. In the rats exposed
to 1315 and 1712 mg/m3, there was also pulmonary congestion and
haemorrhage, renal tubular necrosis and congestion of the thymus; bone
marrow inflammation was reported at 1712 mg/m3 (Table 48).
Two short-term range finding studies were conducted in SPF Wistar
rats (NTP, 1992). In the first study, groups of six male rats were
exposed to up to 750 mg methyl bromide/m3 for 6 h/day for 2 weeks
(see Table 48). In the second range-finding study, groups of six male
and six female rats were exposed to up to 600 mg methyl bromide/m3
for 4 weeks. Five male and three female rats in the high-dose group
died before the end of the study. The most important histopathological
changes occurred in the heart and lungs of animals in the high-dose
group. Diffuse fatty vacuolization and diffuse myocardial fibre
degeneration appeared. The lungs showed hyperaemic and dilated
alveoli; in some animals, interstitial pneumonia was noted (NTP,
1992).
Male Sprague-Dawley rats were exposed to up to 1556 mg methyl
bromide/m3 for 4 h/day for 6 weeks (Kato et al., 1986). Changes in
body weight, general condition, haematology parameters, organ weight,
tissue bromide ion concentration, and the histopathology of several
organs were determined. Suppression of body weight gain, abnormal
clinical signs, and severe weakness were observed at 1556 mg methyl
bromide/m3. Bromide ion accumulation was seen, especially in the
kidney and spleen, without significant dose-related change. Pronounced
histopathological changes were noted in the brain (section 8.8) and
multiple small necrotic foci in the heart.
Other short-term, inhalation exposure studies concentrated on one
aspect/target organ of methyl bromide exposure.
Sato et al. (1985) described biochemical findings in rats exposed
to 4, 20, or 39 mg methyl bromide/m3, continuously, for 3 weeks.
After sacrifice, the organs were weighed and biochemical examinations
were performed on the blood and a homogenate of heart, liver, and
lungs. The results showed no differences between the 4 mg/m3 and the
control group. In the 20 mg/m3 group, several changes were observed;
serum creatine phosphokinase (CPK), phospholipids (PL), and blood
glucose levels, and thymus weight decreased, while blood haemoglobin
(Hb), reduced glutathione (GSH), serum total protein, and lung
gamma-glutamate-pyruvate transaminase (GTP) levels increased after
exposure. In the 39 mg/m3 group, increases were observed in serum
glutamate oxaloacetate transaminase (GOT), lactate dehydrogenase
(LDH), alpha-hydroxy-butyrate dehydrogenase (alpha-HBDH), total
protein, blood Hb, GSH, lung acid phosphatase (AcP), gamma-GTP, LDH
total, liver PL, and triglycerides (TriG), while decreases were noted
in serum cholinesterase (ChE), CPK, PL, TriG, lung alkaline
phosphatase (AlP), free-cholesterol (f-Chol), lactate, blood glucose,
heart lactate, glucose, lung AlP, liver free fatty acids (FFA), and
glycogen. Pulmonary haemorrhage was observed in almost all animals in
this group.
The effects of short-term inhalation exposure to 1264 mg methyl
bromide/m3 (6 h/day for 5 days) including those on the target organ
histopathology of male F-344 rats were studied by Hurtt et al. (1987).
Clinical changes (noted only in the 973 and 1264 mg/m3 groups) were
diarrhoea, haemoglobinuria, and, in a few cases, gait disturbances and
convulsions. A dose-dependent vacuolar degeneration of the zona
fasciculata of the adrenal glands, cerebellar granule cell
degeneration, and olfactory sensory cell degeneration were seen in the
680, 973, and 1264 mg/m3 groups. Cerebral cortical degeneration and
minor alterations in testicular histology were seen only in the 1264
mg/m3 group whereas hepatocellular degeneration was also found in
the 973 mg/m3 group. No changes were found in the kidneys or
epididymides.
In a further study, the degeneration and regeneration of the
olfactory epithelium were examined following exposure of a total of 84
rats to 778 mg methyl bromide/m3 for 6 h/day for 5 days (Hurtt et
al., 1988). Groups of five rats were sacrificed after days 1, 3, and
5 and after exposure weeks 1, 2, 3, 5, and 10. Cell replication rate
and histopathology were used to assess the kinetics of repair. In
addition, olfactory function was assessed using a buried food test.
Extensive damage to the olfactory epithelium was evident in animals
killed directly after 6 h of exposure. The specific site of damage
appeared to be in the olfactory sustentacular cells and mature sensory
cells, the basal cells generally being unaffected. By day 3, despite
continuous exposure, there was replacement of the olfactory epithelium
by a squamous cell layer that increased in thickness and basophilic
staining over the next 2 days. One week after exposure, the epithelial
region was covered by a layer of polyhedral, basophilic cells and from
2 to 10 weeks exposure, the epithelium exhibited progressive
reorganization to restore the original olfactory epithelium pattern;
75-80% of the olfactory epithelium appeared morphologically normal by
week 10.
In the same study, the ability of the rats to locate feed was not
affected by exposure to 350 mg methyl bromide/m3, but treatment with
778 mg methyl bromide/m3 rendered all animals temporarily incapable
of locating buried pellets, though they demonstrated searching
activity. Four to six days after treatment the animals recovered
sufficient olfactory function to find food pellets.
Similar results were reported by Hastings et al. (1989) and
Hastings (1990). Studies on Long-Evans rats showed that, after 4 h
exposure to methyl bromide (778 mg/m3), recovery of buried food was
greatly impaired but, even with continuous exposure, recovery occurred
until, by day 4 of exposure, olfactory function was essentially
normal. Extensive damage to the olfactory epithelium was evident after
day 1 of exposure; repair of the epithelium was in progress by day 4.
The specific site of damage appeared to be in the olfactory
sustentacular cell population while the respiratory epithelium was
largely spared (Hastings et al.,1989; Hastings, 1990). Bolon et al.
(1990) suggested that prior exposure to methyl bromide as well as
caging conditions (e.g., inhalation of ammonia from soiled bedding)
could influence the olfactory epithelial response.
Evans & Hastings (1992) reported that methyl bromide induced an
olfactory function deficit in rats. The olfactory threshold to ethyl
acetate was measured in six rats using a conditioned suppression
behavioural protocol. Three out of the six rats showed an increase in
absolute threshold or threshold response variability after a single,
6-h exposure to 778 mg methyl bromide/m3.
F-344 rats (20 male + 20 female) were exposed (6 h/day; 5
days/week) to 0 or 622 mg methyl bromide/m3 (0 or 160 ppm) for 3,
10, or 30 days (Eustis et al., 1988). Toxicological end-points
assessed included clinical observations, mortality, body and organ
weights, haematology, clinical chemistry, urinalysis, gross pathology,
and histopathology. There were no apparent treatment- related changes
in any of the clinical chemistry and urinalysis analytes measured.
Treatment-related effects in rats included neuronal necrosis in the
brain, myocardial degeneration, olfactory epithelial degeneration and
atrophy, and testicular degeneration and atrophy.
A study by Eustis et al. (1988) confirmed the very steep
concentration-response curve for methyl bromide in rats (and mice).
The authors also found clear species and sex differences in
sensitivity to methyl bromide toxicity. When rats were compared with
mice, the order of susceptibility was female mice>male mice>male
rats> female rats. Species and sex-related differences in the
histopathology of certain organs were also observed.
Kato et al. (1986) exposed Sprague-Dawley rats for 11 weeks, 4
h/day, to 584 mg methyl bromide/m3. No abnormal clinical signs were
reported. Small necrotic foci and fibrosis were observed in the heart
muscle.
F-344 rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 73, 183, 455, or 1140 mg/m3 (0,
7.5, 18.8, 46.9, 117, or 293 ppm) for 13 weeks for 6 h/day, 5
days/week (Japanese Ministry of Labour, 1992). At 1140 mg/m3, there
was 100% mortality. At this concentration of methyl bromide, rats
exhibited decreased locomotor activity, piloerection, ataxia, tremor,
cyanosis, haematuria, and nasal and ocular discharge. No clinical
signs were reported at lower doses.
Haematological studies showed increased MCV in male and female
rats exposed to 455 mg/m3; there was also an increased number of
platelets in the male rats. Serum potassium was decreased in male rats
exposed to concentrations of 73 mg/m3 or more. Glutamic oxalacetic
transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were
significantly increased in female rats exposed to concentrations of
183, 455, and 1140 mg/m3. Serum glucose and creatinine levels were
significantly decreased in female rats at 455 mg/m3.
There were no abnormal histopathological findings in rats exposed
to methyl bromide concentrations of between 29 and 455 mg/m3. Rats
exposed to 1140 mg/m3 exhibited cerebellar degeneration and brain
necrosis, thymic haemorrhages and atrophy, renal tubular necrosis,
pneumonitis, myocardial damage, adrenal vacuolization, Harderian gland
pigmentation and metaplasia, and necrosis of the olfactory epithelium
(Table 49).
Thirteen-week inhalation toxicity studies were conducted in which
groups of ten male and ten female Wistar rats were exposed to methyl
bromide at target concentrations of up to 166 mg/m3 for 6 h/day, 5
days/week (Wilmer et al., 1983). No deaths occurred, and no abnormal
clinical findings were observed. Body weight gain was not affected in
any of the exposed groups. Leukocyte counts were 22% higher in high-
dose males than in male control animals. Plasma alkaline phosphatase
activity was lower in both high-dose males (32%) and females (53%)
than in controls, and the plasma albumin concentration was 10% higher
in high-dose females than in controls. The absolute and relative liver
weights were up to 16% lower for high-dose males and females compared
with controls. The only exposure-related histopathological change,
which occurred in the liver of high-dose male and female rats, was
characterized by small hepatocytes with homogeneous eosinophilic
cytoplasm. This alteration varied in extent from slight to severe and
was seen in 6/10 males and 7/10 females. The no-adverse-effect level
for these 13-week inhalation toxicity studies was considered to be 25
mg/m3.
Groups of 18 rats of each sex were exposed to up to 467 mg/m3
for 6 h/day and 5 days/week over 13 weeks (NTP, 1992). All rats
survived to the end of the studies, few or no clinical effects of
exposure to methyl bromide could be seen. Significant decreases in
body weight gain were noted in both sexes at 467 mg/m3 and in
females at 234 mg/m3. No consistent organ weight effects were
observed. Minor neurobehavioural changes were noted in both sexes in
the highest dosage group. Females, but not males, of this group had
significantly lower haematocrit, haemoglobin levels, and erythrocyte
counts compared with those of controls. Olfactory epithelial dysplasia
and cysts, characterized by irregularity in mucosal thickness and
focal cavitated spaces, respectively, were seen in both sexes at 467
mg/m3.
8.2.3 Dermal
There are no reports of studies on short-term dermal exposure.
8.3 Skin and eye irritation
Irish et al. (1940) noticed lacrimation in rats after inhalation
of methyl bromide levels above 10 000 mg/m3. Irritation of the eye
membranes in mice at concentrations of 3200 mg methyl bromide/m3 was
described by Balander & Polyak (1962). There are no reports on skin
effects in animals.
8.4 Long-term exposure
8.4.1 Oral
8.4.1.1 Rat
Rats were fed for 7-8 months on wheat grain or peanuts fumigated
with methyl bromide having residual bromide levels of 20 and 22-46
mg/kg, respectively. There were no effects on weight gain of the
animals, haemoglobin content, or red or white blood cell numbers.
However, a decrease in iodine and calcium levels in the blood and
abnormal changes in the thyroid and parathyroid glands were reported
(Vitte et al., 1970).
A two-year, oral, long-term toxicity and carcinogenicity study
was carried out on 60 male and 60 female F-344 rats per group fed
diets fumigated with methyl bromide (Mitsumori et al., 1990). Diets
containing 80, 200, or 500 mg total bromide/kg food (methyl bromide
concentration <20 mg/kg food) were fed, the controls receiving
commercial basal diet (containing 30 mg bromide/kg) and a diet
containing 500 mg potassium bromide/kg. No effects were observed on
the behaviour of the rats in any of the groups fed the fumigated or
KBr- containing diets. In rats fed the diets fumigated with methyl
bromide, there were no marked toxic changes, except for a slight
depression in body weight from week 60 onwards in males in the 500
mg/kg group. Tumour incidence was unaffected. Rats given a diet
containing KBr did not show any treatment-related changes. The
no-effect level was 200 mg/kg (equivalent to 6.77 mg total bromine/kg
body weight per day) in males. No effects were observed in females at
the doses studied.
8.4.2 Inhalation studies
Long-term exposure inhalation studies are summarized in Table 49.
8.4.2.1 Mouse
In a carcinogenicity study, 86 B6C3F1 mice/sex were exposed to
39, 128, or 389 mg methyl bromide/m3 for 6 h/day, 5 days/week, for
2 years with scheduled interim sacrifices at 6 months and 15 months
(NTP, 1992). Because of the high mortality early in this study in the
389 mg/m3 group (27/86 males, 7/86 females), the exposure to methyl
bromide at this level was stopped at 20 weeks and the surviving mice
were exposed to air for the rest of the study.
Terminal survival rates (Kaplan-Meier determinations) in the
control, 39 mg/m3, 128 mg/m3, and 389 mg/m3 groups were: males
82%, 74%, 80%, and 23%; and females, 71%, 82%, 90%, and 65%,
respectively. Significantly lower mean body weights of the highest
dosage group compared with controls appeared by week 11 and persisted
after termination of dosing at week 20 until the end of the studies.
The only biologically significant change appeared to be reduced
absolute and relative thymus weights in both sexes. Neurological signs
were also observed, mostly in the highest dosage group, including
tremors, abnormal posture (lateral curvature of the spine), and limb
paralysis. These symptoms generally persisted (NTP, 1992).
Exposure-related histological lesions occurred primarily in the 389
mg/m3 exposure group and included degeneration in the cerebellum,
cerebrum and heart, chronic cardiomyopathy, sternal dysplasia, and
either necrosis or metaplasia of the olfactory epithelium. There were
no neoplasms attributed to exposure to methyl bromide.
Quantitative neurobehavioural testing showed significant
differences in the behaviour of the high-dose males at 3 months. The
animals were less active and demonstrated a reduction in startle
response latency. Because of the high early mortality in high-dose
males, only males in the controls and two lower dosage groups were
tested after 3 months. After 6 months of exposure, the 389 mg/m3
females had significantly lower activity scores than females in other
groups, but their higher startle responses had disappeared. Although
at 9 months of exposure no behavioural differences were apparent,
lower activity and heightened startle response were again noted at the
2-year testing time (NTP, 1992).
Toxicity and carcinogenicity studies were conducted by inhalation
exposure of groups of 50 male and 50 female Crj:BDF1 mice (6 h/day, 5
days/week) to methyl bromide (99.9% pure) for 104 weeks (Japanese
Ministry of Labour, 1992). Methyl bromide concentrations of 0, 16, 62,
and 250 mg/m3 were used.
Body weight gains in male and female mice exposed to 250 mg/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of atrophy (slight) of the granular layer of
the cerebellum were observed in male and female mice exposed to 250
mg/m3. There were no treatment-related neoplasms in male or female
mice.
8.4.2.2 Rat
Toxicity and carcinogenicity studies were also conducted by
inhalation exposure to methyl bromide (99.9% pure) of groups of 50
male and 50 female F344/DuCrj rats (6 h/day, 5 days/week) for 104
weeks (Japanese Ministry of Labour, 1992). Methyl bromide
concentrations of 0, 16, 78, and 389 mg/m3 were used. Body weight
gains in males and female rats exposed to 389 mg methyl bromide/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of necrosis and respiratory metaplasia of
the olfactory epithelium of the nasal cavity were observed in male
rats exposed to 389 mg methyl bromide/m3, and increased incidence
and severity of inflammation of the nasal cavity were observed in male
rats exposed at all concentrations used. Necrosis of the olfactory
epithelium and inflammation of the nasal cavity were marginally
increased in female rats exposed to 389 mg methyl bromide/m3. There
were no exposure-related increased incidences of neoplasms in male and
female rats.
In a long-term inhalation study, a total of 360 male and 360
female rats (Wistar) were exposed to concentrations of up to 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 29 months (Dreef-van
der Meulen et al., 1989; Reuzel et al., 1991). Non-neoplastic and
neoplastic lesions were scored in all control and high-level animals
of the main group, and the nose examined histopathologically at all
exposure levels. Mortality was higher in males and females at the 350
mg/m3 level than in controls from the end of the second year
onwards. Body weights in the 350 mg/m3 group (both sexes) were
slightly lower than controls from week 4 onwards. No essential
differences between groups were observed in haematology, clinical and
blood chemistry, and urine analysis at three months and one year. The
absolute brain weight was decreased in females at 350 mg/m3. A
higher incidence of haemothorax was seen in males and females at 350
mg/m3 than in controls. In the heart there was an increased
incidence of thrombi and myocardial degeneration in rats exposed to
350 mg/m3. There was hyperkeratosis of the oesophagus and
forestomach. The incidences of neoplastic lesions did not differ
significantly among the groups.
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction and embryotoxicity
McGregor (1981) conducted a sperm abnormality assay exposing
groups of 10 male B6C3F1 mice to air containing 0, 78, or 272 mg
methyl bromide/m3 (0, 20, or 70 ppm) for 7 h/day, for 5 days. No
sperm abnormalities were found at these concentrations.
Atrophy of seminal epithelium, incomplete spermatogenesis, and
giant cells in the seminal tubules, were detected unilaterally in one
rat (group of 10) after inhalation of 778 mg methyl bromide/m3 (200
ppm) (4 h/day; 5 days/week for 6 weeks) and in six rats (group of 10)
after inhalation of 1167 mg methyl bromide/m3 (same length of
exposure). In the tubules of the epididymis adjacent to the atrophied
testis, necrotic spermatocytes had accumulated in seminal fluid, but
spermatozoa were not seen (Kato et al., 1986).
Spermatogenesis and sperm quality were evaluated in the rat
(F-344/N) following exposure to 778 mg methyl bromide/m3, 6 h/day
for 5 days (Hurtt & Working, 1988). Although methyl bromide caused a
transient decrease in plasma testosterone and testicular nonprotein
sulfhydryl concentrations during acute exposure, no other reproductive
indices, including testis weight, daily sperm production, cauda
epididymal sperm count, sperm morphology, percentage motile sperm,
linear sperm velocity, and epididymal and testicular histology, were
affected by methyl bromide exposure.
Testicular degeneration and atrophy occurred in several rats and
mice following repeated (6 h/day, 5 days/week for up to 6 weeks)
inhalation exposure to 622 mg methyl bromide/m3 (Eustis et al.,
1988). In rats, degeneration included separation and sloughing of
spermatocytes and late stage spermatids and/or formation of
intratubular multinucleate giant cells. Atrophy was characterized by
variable loss of all components of the spermatogenic epithelium. In
exposed mice, degeneration of testes occurred frequently, but it was
not always severe.
Thirteen-week inhalation studies on sperm morphology and vaginal
cytology examinations (SMVCEs) in B6C3F1 mice and F-344 rats were
carried out by Morrissey et al. (1988). Results from mouse studies
(inhalation of 39, 156, or 467 mg methyl bromide/m3) showed a
decrease in terminal body weight, and a relative increase in the
weight of epididymis and testis. A decrease in sperm density and an
increase in the percentage of abnormal sperm were also noted. In rats
that inhaled 117, 233, or 467 mg methyl bromide/m3, a decrease in
terminal body weight as well as a decrease in the weight of the cauda
epididymis, and a relative increase in the weight of the testis were
noted. A decrease in sperm motility was also observed. In females,
exposure to methyl bromide did not affect the length of the estrous
cycle (Morrissey et al., 1988).
Female rats exposed to 272 mg methyl bromide/m3 (70 ppm) for up
to 40 days survived and reproduced without impairment (Hardin et al.,
1981; Sikov et al., 1981).
In a dominant lethal assay carried out in rats (McGregor, 1981),
groups of 10 male, adult CD rats were exposed to 0, 78, or 272 mg
methyl bromide/m3 for 7 h/day on five consecutive days, then
serially mated at weekly intervals for 10 weeks with untreated virgin
females in the ratio one male: two females. When the females were
sacrificed, their ovaries were examined for corpora lutea graviditatis
and the uteri were opened and examined for live implantations, late
deaths, and early deaths. The frequency of pregnancy was determined by
(a) females with corpora lutea graviditatis and (b) females with
implantations. With either method, methyl bromide did not cause a
significant decrease in the frequency of pregnancy, and the number of
corpora lutea per pregnancy and the frequency of early deaths were
unaffected.
The effects of inhalation exposure to 0, 12, 117, or 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week, for around 8 months, on
the growth, reproduction, and offspring in two consecutive generations
of CD Sprague-Dawley rats were investigated (American Biogenics
Corporation, 1986). Body weight depressions were noted in the males in
the highest concentration group at 5 of the 10 pre-mating collection
intervals and at final sacrifice. The pre-mating and total weight
gains were also less than those of the untreated control males. In the
F1 generation, parental body weight was comparable in exposed and
control animals. In the F2a litter, a slight body weight depression
was noted in gestating and lactating dams in the highest concentration
group compared with controls. Otherwise, maternal body weights were
comparable with those of the controls.
There was a marginal reduction in female fertility index in the
two top dose groups of the F2a litter. The mean numbers of pups
delivered viable were comparable with controls. Survival of the pups
was reduced in the 350 mg/m3 dose group in late lactation in the F1a
generation. Body weights of pups were reduced in both the 350 and 117
mg methyl bromide/m3 dose groups of the F1a, F2a and F2b
generations, though no consistent changes were observed in the F1b
generation.
No anomalies were noted in the treated progeny that were
attributed to methyl bromide exposure.
Gross pathological examination of parent animals from both
generations and randomly selected F1b and F2b progeny did not reveal
any treatment-related lesions. A statistically significant decrease in
the mean brain weight in the F0 males and an increased liver to body
weight ratio in both sexes were found in the upper concentration
group. In the F1 generation, both male and female brain weights were
decreased at 350 mg methyl bromide/m3.
Statistical analyses of the F1b progeny final body weight and
organ weight data revealed no significant differences. Statistical
reductions were found in the final body weight data obtained for the
F2b males (350 mg methyl bromide/m3) and F2b females (117 and 350 mg
methyl bromide/m3) compared with untreated control progeny. Analysis
of F2b progeny organ weight data revealed significant decreases,
compared with controls, for the female brain, heart, kidney, and liver
weights (350 mg methyl bromide/m3) and female liver weight (117 mg
methyl bromide/m3). In these two upper concentration groups, the
females brain to body weight ratio was increased and the liver to
brain ratio, decreased. Microscopic examination of the reproductive
organs and abnormal tissues did not reveal any treatment-related
lesions.
8.5.2 Teratogenicity
Female Wistar rats were exposed for 7 h/day, 5 days/week for 3
weeks to methyl bromide concentrations of 78 or 272 mg/m3. After
this time, they were mated. There was a total of 7 different exposure
groups (Table 48). Female rats whose vaginal smears showed evidence of
sperm were exposed for 7 h daily from day 1 (=day of finding sperm in
vaginal lavage) to 19 of gestation. The day before term (gestation day
21), they were sacrificed and necropsied. The results showed no
effects of the exposure on the female rats, nor were embryotoxic or
other teratogenic effects found (Sikov et al., 1981).
In a further series of studies by Sikov et al. (1981), groups of
24 female New Zealand white rabbits were exposed for 7 h daily to 78
or 272 mg methyl bromide/m3 (20 or 70 ppm) from the day of
artificial insemination. The 78 mg/m3 group was exposed up to day 24
of gestation; ecause of toxic effects, exposure had to be stopped on
day 15 for the 272 mg/m3 group, in which all but one pregnant rabbit
died. The remaining rabbits from the control and 78 mg/m3 exposure
group were sacrificed on the 30th day of gestation. The low exposure
group showed no fetotoxic or eratogenic effects. An evaluation of the
fetotoxicity or teratogenic results from the high dose group was not
possible because of the high mortality rate in the pregnant rabbits
(Hardin et al., 1981; Sikov et al., 1981).
In a preliminary teratology study (Breslin et al., 1990),
pregnant New Zealand White rabbits were exposed via inhalation for 6
h/day to 0, 39, 117, 195, 272, or 545 mg methyl bromide/m3 (0, 10,
30, 50, 70, or 140 ppm). Toxicity was observed in rabbits exposed to
545 mg methyl bromide/m3 and these were sacrificed before the end of
the study. No apparent embryotoxic effects were observed at any
exposure level (Breslin et al., 1990).
A subsequent study by the same investigators was conducted in two
parts. In Part I, groups of 26 inseminated New Zealand White rabbits
were exposed via inhalation to 0, 78, 156, or 311 mg methyl
bromide/m3 (0, 20, 40, or 80 ppm) on days 7-19 of gestation. In Part
II, groups of 17 inseminated rabbits were exposed for 6 h/day to 0 or
311 mg methyl bromide/m3 (0 or 80 ppm) on days 7-19 of gestation. An
additional group of 16 females, inseminated by a single male, acted as
controls. On day 28 of gestation, all surviving animals were
necropsied. Maternal liver, kidney, lung, brain weights, gravid
uterine weights, and the number of corpora lutea, implantations,
resorptions, and live/dead fetuses were noted. The fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
alterations.
Rabbits exposed to 311 mg methyl bromide/m3 showed moderate to
severe maternal toxicity. Decreased body weight and/or body weight
gain were noted. Clinical alterations included lethargy, right-sided
head tilt, ataxia, and lateral recumbency. The last three signs were
associated with significant histopathological lesions of the brain
(multifocal areas of inflammation of the meninges and/or bilaterally
symmetrical necrosis or spongiosis in the midbrain) in the
above-mentioned probe study with 545 mg methyl bromide/m3. At an
exposure level of 311 mg methyl bromide/m3, developmental effects
were observed consisting of decreased fetal weights, an increase in
the incidence of fused sternebrae, and an increase in the incidence of
malformations [18/23] (mostly missing gallbladder or missing caudal
lobe of the lung).
No adverse maternal, embryonal, or fetal effects were observed in
rabbits exposed to 78 or 156 mg methyl bromide/m3 (Breslin et al.,
1990).
Methyl bromide at 0, 0.5, 5, 25, or 50 mg/kg body weight was
administered in peanut oil, by gavage, to pregnant rats on days 5-20
of gestation (Peters et al., 1982). Signs of maternal toxicity were
evident in the two highest dose groups. Total resorption of embryos
was observed in the highest dose group and was considered to be the
result of the poor health of the pregnant rats and not a primary toxic
effect. In the control and 25 mg/kg groups, no effects on the skeleton
or internal organs were reported. In this study, methyl bromide was
not considered teratogenic and adverse effects on prenatal development
only occurred when maternal toxicity was present.
8.6 Mutagenicity and related end-points
Table 50 summarizes the results of tests for the genotoxic
activity of methyl bromide.
8.6.1 DNA damage
DNA adducts have been demonstrated in F344 rats, following oral
or inhalation administration 14C-methyl bromide. The adducts were
isolated from liver, lung, stomach, and forestomach and identified as
3-methylguanine, 7-methylguanine, and 0 6-methylguanine. Following
exposure by either route, the levels of adducts were higher in female
than in male rats and the guanine adducts were particularly prominent
in the stomach and forestomach (Gansewendt et al., 1991).
Alkylation of guanine-N-7 in the DNA of liver and spleen was
observed after treatment of male CBA mice with 14C-labelled methyl
bromide (4.9-5.0 mCi/mmol) by inhalation (340 mCi/kg body weight for
4 h) or i.p. injection (4.4 µmol/kg body weight) (Djalali-Behzad et
al., 1981). They noted that the extent of DNA alkylation in the liver
and spleen in vivo was 200 and 20 times lower, respectively, than
expected from the extent of the alkylation of haemoglobin and from the
relative reactivities of DNA and haemoglobin towards methyl bromide
in vitro.
Table 50. Mutagenicity tests with methyl bromide
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Reverse mutation Salmonella 0.02-0.2% +/- positive, Simmon et al.
typhimurium (in desiccators) TA 100 (1977)
TA 100, TA 98,
TA 1535, TA 1537,2 h
TA 1538
Reverse mutation Salmonella 500-5000 mg/m3, +/- positive, Moriya et al.
typhimurium 2 days TA 100, (1983)
TA 100, 1535
TA 98, 1537, 1538 TA 1535
Escherichia coli positive
WP 2 hcr
Reverse mutation Salmonella 500-50 000 mg/m3 +/- positive at Kramers et al.
typhimurium TA 100 (plate), 5 days 1900 mg/m3 (1985a)
TA 98 negative
SOS-umu (modified Salmonella 1.5 litre/min for positive Ong et al. (1987)
Ames test) typhimurium TA 1535/ 30 min
pSK 1002
Forward mutations Klebsiella pneumoniae 950-19 000 mg/m3, positive at Kramers et al.
streptomycin ur- pro- 20 h 4750 mg/m3 (1985a)
resistance
Forward mutation Escherichia coli 0.5-6 mmol/litre, 1 mutation/108 Djalali-Behzad et
Sd 4 1 h surviving al. (1981)
bacteria/mmol*h
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Forward mutation L5178Y mouse 0.03-30 mg/litre, positive Kramers et al.
24 h (1985a)
Sex-linked Drosophila 78 or 272 mg/m3, negative McGregor (1981)
recessive lethal melanogaster 5 h
Sex-linked Drosophila 750 mg/m3 (6 h) negative Kramers et al.
recessive melanogaster 375 mg/m3 (5 x 6 h) positive (1985a,b)
200 mg/m3 (15 x 6 h) positive
Sister chromatid Phytohaemagglutinin- 4.3% increased SCE Tucker et al.
exchanges (SCE) stimulated frequency from (1985)
human whole blood 10-16.84/cell
after 100-second
exposures
SCE cultured human 10-4-10-6 +/- positive Garry et al.
lymphocytes mol/litre (1990)
SCE bone marrow cells 47-778 mg/m3 dose response NTP (1992)
of exposed B6C3F1 6 h/day; 5 days/ observed at 14 days
mice week; 14 days and only; higher in
12 weeks females than males
same dose range negative
and exposure
Unscheduled DNA human diploid up to 70% +/- negative McGregor (1981)
synthesis fibroblasts 3 h
(human embryonic
intestinal cells)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Unscheduled DNA SPF male Wistar 10-30 mg/litre negative Kramers et al.
synthesis rats, primary liver (1985a)
Cell Syrian hamster 3890-31 120 mg/m3 negative Hatch et al.
transformation embryo cells 2-20 h in sealed (1983)
chambers
Chromosomal male and female CD 78 or 272 mg/m3 negative McGregor (1981)
aberrations (ex Sprague-Dawley) single 7 h or
rat bone marrow 7 h/day; 5 days
cells
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
BDF1 mice 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
(778 mg/m3) and
6-fold in females
(600 mg/m3); in
peripheral blood
increased 32-fold
in males (778 mg/m3)
and 3-fold in females
(600 mg/m3)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
F344 rats 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
and 3-fold in females
at 1314 mg/m3
Micronucleus in peripheral 47-778 mg/m3 elevated (only at NTP (1992)
erythrocytes of 6 h/day; 5 days/ 14 days) responses
B6C3F1 mice week; 14 days over entire dose
range with greatest
response at 389 and
778 mg/m3 in
females; males less
responsive
same dose range and negative
exposure routine;
13 weeks
Dominant lethal rats, male CD 78 or 272 mg/m3 negative McGregor
(Sprague-Dawley) 7 h/day; 5 days (1981)
Starratt & Bond (1988a) found that methylation of DNA in maize
and wheat took place during fumigation using 14C- labelled methyl
bromide (48 mg/litre for 72 h). They identified 7-methylguanine and
1-methyladenine as major products and lesser amounts of
3-methyl-cytosine and 3- methyladenine and 7-methyladenine; 0.5-1% of
the guanine residues in the DNA were methylated. Methylation of solid
samples of calf thymus DNA and salmon testes DNA gave similar results,
except that the quantity of 1-methyladenine exceeded that of
7-methylguanine (Starratt & Bond, 1988b).
In contrast, a different pattern of methylated bases was found
when solutions of DNA were treated with 14C-labelled methyl bromide.
Here, predominantly 7-methylguanine with a small amount of
3-methyladenine and only traces of 1-methyladenine and
3-methyl-cytosine were identified (Starratt & Bond, 1988b). The
authors noted that these results agreed better with the in vivo
studies of Djalali-Behzad et al. (1981).
An in vitro assay for unscheduled DNA synthesis (UDS) was
carried out in human diploid fibroblasts with exposures of 3 h and
concentrations up to 70% in air. No increase in UDS was found
(McGregor, 1981).
As measured by autoradiography, methyl bromide (tested at 10-30
mg/litre) did not induce unscheduled DNA synthesis in primary cultures
of rat hepatocytes treated in air-tight bottles (Kramers et al.,
1985a).
8.6.2 Mutation
Methyl bromide has been tested in various in vitro and in vivo
test systems (Table 50).
Methyl bromide was mutagenic to Salmonella typhimurium TA 100
when tested at concentrations of 0.02-0.2%, in desiccators, in the
absence of an exogenous metabolizing system (Simmon et al., 1977).
Positive results were also obtained in strain TA 100 in a liquid
assay (tested at 10-1000 mg/litre) and a plate assay (tested in closed
containers at concentrations of 500-50 000 mg/m3). Methyl bromide
was mutagenic at concentrations of 1900 mg/m3 and higher (plate
tests), and concentrations of 285 mg/litre (medium) and higher
(suspension test). The activity in the plate assay was unaffected by
the presence of liver homogenates from Aroclor(R)-induced rats
(Kramers et al., 1985a).
Methyl bromide (tested at 0.5-5 g/m3 in a closed container) was
mutagenic to S. typhimurium TA 1535 and TA 100 and to E. coli WP2
her, in the absence of an exogenous metabolic system (Moriya et al.,
1983).
An aqueous solution of methyl bromide (tested at 0.5-6 µmol/
litre) induced mutations to streptomycin independence in E. coli
Sd-4 (Djalali-Behzad et al., 1981).
Methyl bromide showed no mutagenic activity in a modified Ames
test using the impingement (in situ) test system, but, with the SOS
umu-test, this compound induced a significant SOS response, even
with only 30 min impingement (Ong et al., 1987). The SOS function
induced by genotoxic agents was detected by a colorimetric measurement
of beta-galactosidase activity encoded by the 1acZ gene, which is
regulated by the Umu operon.
Mutations to streptomycin resistance were induced in the
fluctuation test with Klebsiella pneumoniae at concentrations of
4750 mg methyl bromide/m3 and higher (tested at 950- 19 000 mg/m3)
(Kramers et al., 1985a).
In barley, a few mutations were induced after treatment of
kernels with 1.4 mmol methyl bromide/litre for 24 h, in closed vessels
(Ehrenberg et al., 1974).
A sex-related recessive lethal assay was carried out on
Drosophila melanogaster (McGregor, 1981). Male strain Oregon K
Drosophila were exposed to 78 or 272 mg methyl bromide/m3 for 5 h
and were mated on days 1,3, or 8 following exposure. The F1 progeny
from these matings were then mated brother to sister, 1-4 days after
emergence from pupae and the F2 generation was ex-amined for the
absence of wild-type males. At 78 mg methyl bromide/m3, the
frequencies of lethal mutations in the F2 generations from one of the
stocks were elevated, but this was not thought by the authors to be
compound-related as these were higher than those at the higher
concentration range.
In a sex-linked recessive lethal test on Drosophila
melanogaster , flies of the Berlin K strain were exposed to methyl
bromide at concentrations of 70-750 mg/m3 for increasing periods;
mutation frequencies were significantly increased at the highest
nontoxic concentrations. At a concentration of 600 mg/m3, all flies
died within a short time during the fourth day of exposure (Kramers et
al., 1985a). Prolongation of the exposure time permitted lower
concentrations to be detected as mutagenic; 487 mg/m3 for 5 x 6 h
and 200 mg/m3 for 15 x 6 h were effective exposures whereas
treatment with up to 750 mg/m3 for 6 h was not sufficient to produce
significantly increased mutation frequencies. The mutagenic effect of
methyl bromide was most pronounced in postmeiotic germ cell stages
(Kramers et al., 1985b).
Treatment of L5178Y mouse lymphoma cells with 0.03-30 mg methyl
bromide/litre, in air-tight bottles, resulted in a dose-related
increase in 6-thioguanine- and bromodeoxyuridine-resistant mutants
(Kramers et al., 1985a).
8.6.3 Chromosomal effects
8.6.3.1 In vitro studies
Exposure of human lymphocyte cultures to 4.3% methyl bromide for 100
seconds increased the frequency of sister chromatid exchanges (SCEs)
from 10.0 to 16.8 per cell (Tucker et al., 1985, 1986).
Human G0 lymphocytes were treated with methyl bromide (0-24
µg/ml) for 30 min with, and without, addition of rat liver homogenate.
After culture, the prepared slides were studied and dose-related
sister chromatid exchanges (SCEs) and chromosome aberrations (CAs)
were found. Methyl bromide significantly induced chromosome
aberrations in the presence of S-9 (Garry et al., 1990).
Inhalation of methyl bromide gas induced mitotic recombination in
somatic cells (somatic wing-spot assay) of Drosophila melanogaster
(Katz, 1985, 1987). Third instar larvae trans-dihybrid for two
recessive wing-hair mutations were exposed via inhalation to methyl
bromide (0-20 000 mg/m3) for 1 h. Wings of surviving adults were
scored for the presence of clones of cells possessing malformed wing-
hairs. Small and large, single (indicating a variety of genetic
alterations) as well as twin spots (from mitotic recom bination) were
found.
8.6.3.2 In vivo studies
The results of in vivo mammalian tests for chromosomal
aberrations in rat bone marrow were negative (McGregor, 1981).
Micronuclei formation was studied on F-344 rats and BDF1 mice
exposed to 0, 600, 778, 1011, 1314, or 1712 mg methyl bromide/m3 (0,
154, 200, 260, 338, or 440 ppm) for 6 h/day and 5 days/week for 14
days (Ikawa et al., 1986). In the surviving mice, poly-chromatic
erythrocytes with micronuclei in the bone marrow increased 10-fold in
males (778 mg/m3) and 6-fold in females (600 mg/m3) and those in
peripheral blood increased 32-fold in males (778 mg/m3) and 3-fold
in females (600 mg/m3). In rats, poly-chromatic erythrocytes
containing micronuclei in the bone marrow increased 10-fold in males
and 3-fold in females (both 1314 mg/m3).
Increases in SCEs and micronuclei were observed in the bone
marrow cells of male and female B6C3F1 mice exposed via inhalation to
a concentration of 778 mg methyl bromide/m3 (200 ppm) for 14 days (6
h/day, 5 days/week), the increases were more pronounced in female
mice. In contrast, no significant increases in either SCEs or
micronuclei were observed in male or female mice exposed via
inhalation to a concentration of 467 mg methyl bromide/m3 (120 ppm)
for 13 weeks (NTP, 1992).
8.6.4 Cell transformation
Transformation in Syrian hamster embryo cells by SA/adenovirus
was not enhanced by exposure to 4000-16 000 mg methyl bromide/m3
(1000-4000 ppm) for 2 or 20 h, in sealed chambers (Hatch et al.,
1983).
8.7 Carcinogenicity and related end-points
8.7.1 Gavage studies
Danse et al. (1984) administered methyl bromide, by gavage, to
groups (10 male + 10 female) of weanling Wistar rats for 90 days at
doses of 0.4, 2, 10, or 50 mg/kg body weight. At the highest dose
level of 50 mg/kg, squamous cell carcinomas of the forestomach
developed in 13 out of 20 animals. A marked diffuse hyperplasia of the
epithelium of the forestomach was seen in all animals in this group.
However, from subsequent examination of the slides from this study it
was concluded that the forestomach lesions reported at 50 mg
represented inflammation and hyperplasia rather than malignant lesions
(Pesticide & Toxic Chemical News, 1984).
Boorman et al. (1986) administered methyl bromide in peanut oil
(50 mg/kg body weight), by gavage, to groups of 15 male Wistar rats
for 13 weeks (five times a week) with a 12 week recovery period; other
groups were exposed for 17, 21, or 25 weeks to methyl bromide;
necropsies were performed at 13, 17, 21, and 25 weeks. At week 13,
inflammation, acanthosis, fibrosis, and pseudo- epitheliomatous
hyperplasia in the forestomach were observed microscopically in dosed
animals. At week 25, all rats had hyperplastic lesions of the
forestomach that were more severe than those at 13 weeks. Evidence of
malignancy, seen in one rat, was considered to be a very early
carcinoma. In the stop treatment group that had received methyl
bromide for 13 weeks, there was regression of the stomach lesions, but
at the 12-week final sacrifice, adhesions, fibrosis, and mild
acanthosis remained (Table 43).
Hubbs & Harrington (1986) reported similar results. Gross and
microscopic alterations were most pronounced in the non-glandular part
of the stomach of the treated rats. Histological change in the
squamous epithelial portion of the stomach included ulceration and
pseudoepitheliomatous hyperplasia characterized by hyperkeratosis,
acanthosis, and epithelial peg formation. Fibrosis, foreign material
(hair) and subacute to chronic inflammation were found in the
muscularis mucosa, submucosa, and tunica muscularis of some rats. A
30-60 day recovery period was accompanied by marked, but incomplete,
regression of lesions. No evidence of malignancy was seen in the
stomachs of treated rats (Hubbs & Harrington, 1986).
A two-year, oral, carcinogenicity study was carried out on 60
male and 60 female F-344 rats fed on diets fumigated with methyl
bromide (Mitsumori et al., 1990). Diets contained 80, 200, or 500 mg
total bromide/kg, respectively, (methyl bromide concentration being
<20 mg/kg), the controls were fed commercial basal diet (containing
30 mg bromide/kg) and a diet containing 500 mg potassium bromide
(KBr)/kg. No carcinogenic effects were observed (see also section
8.4.1.2).
8.7.2 Inhalation studies
In lifetime, inhalation, carcinogenicity studies (Table 49),
groups of 90 male and 80 female Wistar rats were exposed to methyl
bromide concentrations of up to 350 mg/m3 for 6 h/day, 5 days/ week,
for up to 130 weeks (Dreef-van der Meulen et al., 1989; Reuzel et al.,
1991). Methyl bromide was a mild nasal irritant at all exposure
concentrations. There was no increased incidence of neoplasms.
In a 2-year inhalation study (NTP, 1992) on male and female
B6C3F1 mice exposed to 39, 128, or 389 mg methyl bromide/m3 (Table
49), there was no evidence of carcinogenic activity in either male or
female mice.
8.8 Special studies
8.8.1 Target organ effects
8.8.1.1 Inhalation studies
Studies on laboratory animals have shown that, following
inhalation exposure, the primary target organs are the brain, kidney,
nasal cavity, heart, adrenal gland, lung, liver, and testis (Irish et
al., 1940; Hurtt et al., 1987; Eustis et al., 1988) and lung (Bond et
al., 1985; Kato et al., 1986; Jaskot et al., 1988) (Table 48).
Extensive target organ studies have been carried out by Eustis et
al. (1988) on B6C3F1 mice and F-344 rats after inhalation of 622 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 6 weeks. The
histopathological changes observed were as follows:
Brain: neuronal necrosis in the internal granular layer of the
cerebellar folia (mice); neurosis and loss of neurons in the cerebral
cortex, hippocampus, and thalamus (rats).
Kidney: nephrosis, which occurred in all treated mice, was
charac-terized by degeneration, necrosis, and sloughing of the
epithelium of convoluted tubules in the renal cortex. In rats, there
was dilatation of tubules with atrophy of the epithelium, hyaline and
granular casts in the tubules, and increased cytoplasmic basophilia,
indicative of epithelial regeneration. There was minimal nephrosis in
one female rat.
Testes: degeneration of the testes occurred frequently in exposed
mice and mild bilateral atrophy was present in two. Degeneration and
atrophy of seminiferous tubules of the testes was noted in several
exposed rats.
Nasal cavity: exposed male mice had various levels of degeneration
and atrophy of the nasal olfactory epithelium. Minimal degeneration of
the olfactory epithelium was seen in only one female mouse. In male
and female rats killed after three exposures to methyl bromide, there
was moderate to marked degeneration of the olfactory epithelium of the
ethmoturbinates and posterior dorsal nasal septum. There was no
inflammatory response. In rats, killed or dying after 10 or more
exposures, actual degeneration of the olfactory epithelium was minimal
or mild, but there was focal or multifocal loss of olfactory sensory
cells.
Heart: degeneration of the myocardium primarily occurred in treated
males with minimal myocardial degeneration in two female mice.
Degeneration of the myocardium occurred more frequently and with
greater severity in treated than in control male and female rats.
There were increased numbers of mononuclear and fusiform nuclei that
may represent interstitial cells, the foci showing a relative increase
in fine reticular fibres, and scattered clear vacuoles.
Adrenal gland: exposed female mice showed diminished cellularity of
the x-zone of the adrenal cortex and the affected cells contained less
cytoplasm and smaller, more hyperchromatic nuclei than normal cells.
Minimal to mild cytoplasmic vacuolation occurred in the adrenal cortex
of exposed rats.
Liver: individual cell necrosis was noted in the liver of several
treated rats and was generally more severe in affected males than
females. In three males and one female, an inflammatory reaction
occurred. There were no data for mice.
Thymus and spleen: thymic atrophy and lymphoid depletion of the
spleen was noted in mice and rats of both sexes.
8.8.2 Neurotoxicity
Irish et al. (1940) examined the effects of a wide range of
inhalation exposure conditions in rats, rabbits, guinea- pigs, and
monkeys, using visual inspection to assess behavioural deficits. They
observed that nearly 60% (34 out of 58) of the rabbits surviving
exposure to 128 mg/m3 (33 ppm) developed hindlimb paralysis during
the 6-month exposure period. This was the lowest concentration at
which definite neurobehavioural effects could be detected in the
rabbit, the other species being unaffected at this concentration.
Anger et al. (1981) studied the neurobehavioural effects of long-
and short-term methyl bromide inhalation exposure on Sprague-Dawley
rats and New Zealand White rabbits using more sensitive tests than
those available in 1940 when the study of Irish et al., was performed.
Exposure to 252 mg/m3 (65 ppm) for weeks caused sig-nificantly
reduced eye blink responses and nerve conduction velocity in rabbits,
but not in rats. No effect on nerve conduction velocity, open-field
activity, or coordination in rats could be seen after extended
exposure at 214 mg/m3 (55 ppm) for 36 weeks. In further studies
(Russo et al., 1984), rabbits did not show any neurobehavioural
effects after inhalation exposure to 105 mg methyl bromide/m3 (27
ppm) for 7.5 h per day, 4 days per week over 8 months. A separate
group of rabbits exposed to 252 mg/m3 exhibited severe neuromuscular
effects followed by partial recovery, 6-8 weeks after exposure ceased.
Behavioural effects in rats following repeated exposure to methyl
bromide at concentrations of 778 or 1167 mg/m3 (200 or 300 ppm) for
3 weeks were investigated by Ikeda et al. (1980). Relatively prolonged
(12 days) motor incoordination (rotarod) was observed.
Conditioned taste aversion induced by inhalation exposure to
methyl bromide in rats has been reported (Miyagawa, 1982). Rats kept
under a water restriction schedule for 7 days, were permitted access
to 0.3% (w/v) sodium saccharin, and were exposed to 0, 97, 195, or 389
mg methyl bromide/m3 (0, 25, 50, or 100 ppm) for 4 h. Three days
after exposure, saccharin preference tests were carried out, revealing
dose-dependent saccharin aversion in the exposure group (Miyagawa,
1982).
Male Swiss-Webster mice exposed to methyl bromide concentrations
up to 3500 mg/m3 showed no effects on single-task passive avoidance
but had significantly lower rotarod performance (Alexeeff et al.,
1985).
Effects of methyl bromide on locomotor activity were observed in
five groups of three rats after exposure to 245, 486, 731, or 972
mg/m3 (63, 125, 188, or 250 ppm) for 8 h. The locomotor activity was
measured in an activity cage before, and immediately after, the
exposure, shown as an average count per hour in 12-h blocks (day -2
(before exposure) and day 1) or 4-h blocks (day 0). In the 245 mg/m3
group, the activity was the same as that in the control group. The
activity was decreased by 731 mg/m3 and in-hibited strongly by 972
mg methyl bromide/m3 for up to 6 h after exposure (Honma et al.,
1985).
The effects of methyl bromide exposure on the thiopental-induced
reduction of righting reflex of rats was investigated. Three groups of
eight rats were exposed to 0, 245, or 486 mg/m3 (63 or 125 ppm) and
then were injected i.p. with 60 mg thiopental/kg to induce a loss of
righting reflex. The time of loss of righting reflex was measured and
group means were calculated. In the control group, two out of eight
rats lost the righting reflex, while in the 245 and 486 mg methyl
bromide/m3-exposure groups, the loss of righting reflex was observed
in all rats and was considerably more prolonged (Honma et al., 1985).
Kato et al. (1986) described neurological signs in rats, such as
paralysis and ataxia, after exposure to 1167 or 1556 mg methyl
bromide/m3, for 4 h/day over 6 weeks (section 8.4).
The acute effects of methyl bromide on electroencephalographic
activity and on sleep-wakefulness and circadian rhythms have been
investigated in rats (Tanaka et al., 1988). Slowing of the EEG
frequency in the wakefulness (W) stage and spike-wave activity
appeared at a single subcutaneous dose of 135 mg methyl bromide/kg
body weight. These abnormal EEG activities did not occur at lower dose
levels. Administration of methyl bromide at doses of 45, 15, or 5
mg/kg produced dose-related changes in amounts of W, non-REM sleep,
and REM sleep and in their circadian rhythms. Pretreatment with
glutathione effectively lessened the detrimental effects of methyl
bromide on sleep-wakefulness and its circadian rhythms and increased
the LD50 (Tanaka et al., 1988).
The effects were studied on regional brain glutathione- S-
transferase (GST) activity and the concentrations of glutathione
(GSH), monoamines, and amino acids in F-344 rats exposed to methyl
bromide (Davenport et al., 1992). Exposure to 584 mg/m3 was for 6
h/day for five days. While no histological evidence of brain lesions
was noted, there was GSH depletion and GST inhibition in the frontal
cortex, caudate nucleus, hippocampus (examined for GSH only), brain
stem, and cerebellum. No significant changes were observed in the
concentrations of monoamines (only measured in males), but there were
increases in the concentrations of aspartic acid and glycine in the
frontal cortex and of aspartic acid only in the cerebellum (only
measured in males).
Honma et al. (1982) exposed groups of 5-6 SD rats to methyl
bromide concentrations of 39-467 mg/m3 for 24 h or to 4-40 mg/m3
for 3 weeks. After sacrifice (microwave), the brain was dissected and
analysed for monoamines. A reduction in the norepinephrine (NE)
contents of the hypothalamus and cortex + hippocampus was found at
concentrations of 390 mg/m3 and above (24-h study) or 39 mg/m3
(3-week study), whereas levels of dopamine (DA), sero-tonin (5HT), and
acetylcholine (ACh), were only slightly affected by exposure. In more
detailed studies, rats were exposed to 120, 240, 486, or 973 mg/m3
for 8 h (Honma, 1987; Honma et al., 1987). Decreases in DA and NE and
increases in the metabolites homovanillic acid (HVA) and
3-methoxy-4-hydroxyphenylglycol (MHPG) were observed in various
regions of the CNS in rats exposed to 319 mg/m3. The maximal effects
were obtained 0 or 2 h after exposure with the largest changes in DA
(-24%) and NE (-34%) seen in the striatum. HVA and MHPG levels were
increased primarily in the frontal cortex (81%) and striatum (38%).
The tyrosine hydroxylase (TH) activity in the striatum, hypothalamus,
frontal cortex, midbrain, and medulla oblongata was measured in brain
homogenates from rats exposed to 62-973 mg/m3 (16-250 ppm) for 8 h
(Honma et al., 1991). The rats were sacrificed serially (decapitation)
between 0 and 16 h after exposure. TH was also measured in vivo
following administration of decarboxylase inhibitor. Exposure to
methyl bromide dose-dependently inhibited both TH activity in vitro
and in vivo . TH activity in the hypothalamus was most sensitive to
methyl bromide compared with the other brain areas. The authors
suggested that methyl bromide reduces catecholaminergic neuronal
activity in the brain via inhibition of TH activity.
The direct effects of methyl bromide on the rat brain have been
investigated using a two-probe (right striatum and left ventricle)
microdialysis method (Honma, 1992). Methyl bromide (1 µg or 0.5
µg/µlitre) dissolved in 10% ethanol/90% artificial cerebrospinal fluid
was perfused through the ventricle probe at a rate of 2.5 µlitre/min
and changes in 3,4-dihydroxyphenylacetic acid (DOPAC), HVA, and
5-hydroxyindoleacetic acid, a serotonin metabolite, (5HIAA) content in
the striatum perfusate were measured. DOPAC and HVA increased
following the initiation of ventricle perfusion but the 5HIAA content
of the striatum perfusate was reduced. The increases in DOPAC and HVA
persisted after perfusion and the decreased 5HIAA level returned to
normal. A concentration of 0.1 µg methyl bromide/µlitre had no effects
on DOPAC and HVA levels, though the 5HIAA level was reduced. These
findings support the findings of an increase in DOPAC and HVA in the
brain homogenate of rats exposed to methyl bromide (Honma, 1987; Honma
et al., 1987) indicating that dopamine metabolites in the
extracellular, and, probably, intracellular, regions of dopamine nerve
terminals were increased by methyl bromide itself. The extracellular
level of the 5HIAA in microdialized perfusate was reduced by
intraventricularly administered methyl bromide (Honma, 1992).
Systematic neuropathological studies of their central and
peripheral nervous systems were carried out on male Wistar rats
exposed to methyl bromide (1128 or 1945 mg/m3) for 6 h/day, 3
days/week for 3-8 weeks (Furuta et al. 1993). Among the rats exposed
to 1945 mg methyl bromide/m3 for 10-18 days, the following
neuropathological changes were noted: axonal degeneration of
myelinated fibres at the cervical level of the fasciculus gracilis and
the necrosis and atrophy of neurons in the caudate-putamen, thalamus,
and cingulate cortex. Rats exposed to a concentration of 1128 mg
methyl bromide/m3 for 8 weeks did not show any abnormalities.
8.8.3 Immunotoxicity
No data on the immunotoxicity of methyl bromide are available.
8.9 Factors modifying toxicity; toxicity of metabolites
Cysteine has been shown to reduce the toxicity of methyl bromide,
when administered to rats, mice, and rabbits, orally or s.c., 30 min
before, or s.c. within 5 min following, acute poisoning (Mizyukova &
Bakhishev, 1971). Cysteine treatment prevented the death, paralysis,
paresis, and spasms that developed on the 3rd-4th days after methyl
bromide inhalation in other animals.
8.10 Mechanisms of toxicity - mode of action
The mode of action of methyl bromide is still not understood.
Proposed mechanisms of toxicity include the direct cytotoxic effect of
the intact methyl bromide molecule or toxicity due to one of its
metabolites. The bromide ion concentrations are insufficient to
explain methyl bromide toxicity, but it may be related to its
alkylating ability.
Honma et al. (1985) concluded that the CNS toxicity seems to be
due to the methyl bromide molecule itself or the methyl moiety
incorporated into the tissue and does not appear to be attributable to
inorganic bromide or methyl alcohol (Honma et al., 1985). In humans
showing medium or severe toxic symptoms of the CNS, methanol
concentrations in the blood ranged between 200 and 3000 mg/litre
(Swartz et al., 1981).
The direct cytotoxic effect of methyl bromide on HeLa cells in
vitro has been shown, whereas bromide appeared not to cause cell
damage (Nishimura et al., 1980).
Methyl bromide reacted in vitro with a number of SH enzymes and
caused progressive and irreversible inhibition (Lewis, 1948). DNA and
protein alkylation by methyl bromide have previously been discussed
(section 6.3).
The role of glutathione (GSH) in reducing toxicity is also not
clear. Studies on the possible role of glutathione are given in
section 6.3. One hypothesis for the toxicity of methyl bromide
proposes the formation of a reactive species through a methyl
bromide-glutathione conjugation process, but it is more probable that
glutathione acts as a detoxifying agent.
9. EFFECTS ON HUMANS
Humans can be exposed to methyl bromide via inhalation, by skin
contact with the compound, or through residues in food that has been
fumigated with the gas to control pests. Exposure is also possible
through drinking- water from wells contaminated with leaching water.
In this section, only direct exposure to gaseous or liquid methyl
bromide is considered.
Since the first case reported by Schuler in 1899, there have been
hundreds of cases of methyl bromide poisoning involving fatalities,
systemic poisoning, skin and eye injuries, and damage to the central
nervous system (von Oettingen, 1946, 1964; Hine, 1969; Torkelson &
Rowe, 1981; Weller, 1982; Alexeeff & Kilgore, 1983).
Table 51 shows acute cases up to 1983 reported throughout the
world. Up to 1955, most cases were from chemical manufacture or fire
extinguisher incidents. Since this date, cases of poisoning from its
use as a fumigant have predominated.
9.1 Clinical findings
It is important to note that the manifestations of methyl bromide
poisoning may be delayed. The latent period may vary from 2 to 48 h
(Holling & Clarke, 1944).
The acute and long-term effects of methyl bromide can be divided
broadly into two categories: neurological and non-neurological.
The principal non-neurological symptoms reported after acute
inhalation of methyl bromide are associated with the respiratory
system. Chest pain or difficulty in breathing have been reported,
which is consistent with pathological findings at autopsy including
pulmonary oedema, bronchopneumonia, congestion, and haemorrhage
(Holling & Clarke, 1944; Hine, 1969).
In fatal poisoning, the early symptoms and signs are headache,
visual disturbances, nausea and vomiting, smarting of the eyes,
itching of the skin, listlessness, vertigo, and tremor. Progression is
usually rapid, with the development of convulsions, often with a
Jacksonian-type of progress, fever, tachypnoea associated with signs
of severe pulmonary oedema, cyanosis, pallor, and death. Several
neuropsychiatric signs and symptoms, such as mental confusion, mania,
muscular twitches, and slurring of speech, may precede death (Wyers,
1945; Sax et al., 1984; Gosselin et al., 1984).
Table 51. Methyl bromide poisoning incidents up to 1955 and between 1955 and 1983a
Resulting from: Fatalities Systemic Poisoning Skin Injuries Eye Injuries Other or unspecified injuries
up to 1955- up to 1955- up to 1955- up to 1955- up to 1955-
1955 1983 1955 1983 1955 1983 1955 1983 1955 1983
Chemical manufacture, 16 1 108 45 26 4 0b 0b
filling, storage,
transportation, or
disposal
Use or leaking fire 24 11 38 28 10 0 0b 0b
extinguishers
Uses as a fumigant 44 16 3 298 0 145 0b 57 0b 78
a Adapted from: Alexeeff & Kilgore (1983). Data up to 1955 were taken from von Oettingen (1955).
b No cases known.
The clinical picture in non-fatal poisoning is extremely
variable. Fatigue, blurred or double vision, nausea, and vomiting are
frequent; incoordination, tremors, convulsions, exaggeration of the
patellar reflexes, and a positive Babinski's sign may develop. Nearly
every type of nervous disturbance has been reported. The pulmonary
symptoms are comparatively slight (Sax et al., 1984). Low-level,
short-term exposures to the vapour have produced a syndrome of
polyneuropathy without overt central manifestations, characterized by
persistent numbness in the hands and legs, impaired superficial
sensation, muscle weakness, unsteadiness of gait, and absent or
hypoactive distal tendon reflexes (Kantarjian & Shaheen, 1963). Victor
& Adams (1980) stated that the signs and symptoms of chronic bromide
poisoning intoxication consisted of dizziness, drowsiness,
irritability, emotional lability, impairment of thought or memory,
and, in severe cases, delirium and mania or stupor or coma.
Locally, methyl bromide is an intense vesicant on human skin. The
blisters produced by methyl bromide are enormous, but rarely deep
enough to destroy the entire skin layer, even though it may produce
severe burns (Watrous, 1942; Butler et al., 1945; Wyers, 1945).
Both central and peripheral neurological deficits may persist;
organic brain syndrome has occurred (Greenberg, 1971; Goulon et al.,
1975). Profound psychological depression can occur during prolonged
convalescence (Hine, 1969). Late sequelae include bronchopneumonia
after severe pulmonary lesions, renal failure, and severe weakness
with, or without, evidence of paralysis. Renal failure due to tubular
necrosis may be a late sequela, but this is uncommon and usually of
mild proportions (Benatt & Courtney, 1948; Prain & Smith, 1952). There
have been occasional reports of jaundice, elevations of liver enzyme
activity in serum, and abnormal liver function tests suggestive of
mild hepatotoxicity after exposure to methyl bromide (Verberk et al.,
1979). In a 6- year-old boy, signs of liver involvement in conjunction
with encephalopathy after exposure to methyl bromide vapour were at
first mistaken for Reye's syndrome (Shield et al., 1977).
Recovery is frequently prolonged and there may be permanent
injury, commonly characterized by sensory disturbances, weakness,
disturbances of gait, irritability, and blurred vision.
The effects of methyl bromide on the nervous system, based on
case histories, showed that dysfunction affected the peripheral and
optic nerves, cerebellar connections, and certain spinal cord tracts
(Carter, 1945). Brain stem damage was described in a man who died
after 30 days of unconsciousness following methyl bromide exposure
(Cavanagh, 1992; Squier et al., 1992). Serum bromide levels, 2 days
after admission, were 160 mg/litre (normal 3-4 mg/litre). At
post-mortem examination, the brain showed mild generalized swelling,
normal ventricles, and well- defined symmetrical lesions, including
loss of neurones, in the mammilary bodies and inferior colliculi. The
cerebellar dentate nuclei had occasional foci of neuronal loss; other
brain stem regions were normal. The spinal cord was normal, but dorsal
root ganglia had scattered nodules of neuronal loss. Nerve roots and
thoracic peripheral nerve showed sparse chronic inflammatory exudate
and patchy axonal and myelin loss. The thyroid gland was densely
infiltrated with lymphocytes, plasma cells were rare, and there was no
significant fibrosis. The lungs were slightly oedematous and
congested. In another case of methyl bromide poisoning, a woman
remained in a coma for 4 years and 8 months before death. At
post-mortem, there was necrosis of both colliculi, with gliosis in the
upper brainstem, reticular formation, and moderate changes in the
dentate and pontine nuclei (Goulon et al., 1975).
Various visual disturbances have been reported following acute
methyl bromide poisoning. These include blurring of vision, diplopia,
lacrimation from eye irritation, accommodative disturbance, and
central scotomata for shades of green (Grant, 1974). Ocular findings
in a case of chronic methyl bromide poisoning included persistent
bilateral decrease in vision, temporal optic nerve head pallor, marked
attenuation of visual evoked response amplitude with normal latencies,
normal electro-retinogram but abnormal electro-oculogram, and
deuteranomalous (green) defect (Chavez et al., 1985).
Electrophysiological studies have been performed on patients as
part of a clinical investigation of the neurological effects of methyl
bromide poisoning (Goulon et al., 1975; Verberk et al., 1979; Audry et
al., 1985; Lopez et al., 1986a, b; Uncini et al., 1990; Mazzini et
al., 1992; Hustinx et al., 1993). Abnormal findings included
epileptiform patterns in electroencephalograms, enlarged and
asymmetric somatosensory evoked potentials, and evidence of axonal
neuropathy in peripheral nerves.
9.1.1 Bromide levels in body tissues and fluids
In cases of methyl bromide poisoning, methyl bromide itself has
been detected in human tissue on only one occcasion. This may be due
to the absence of methyl bromide or, more probably, to its very short
half-life in tissues, as well as difficulties in analysis. There is an
inconsistency in the data as to whether there is a correlation between
bromide levels and the symptoms of methyl bromide poisoning. Some
authors have suggested a direct correlation between blood bromide
levels and the degree of intoxication (Rathus & Landy, 1961; Hine,
1969): 400 mg/litre (ppm) - severe disability and death in some cases;
250 mg/litre (ppm) - convulsive seizure and sometimes death; 176
mg/litre (ppm) - slight residual ataxia; 135 mg/litre (ppm) - moderate
disability; 100 mg/litre (ppm) or less -recovery, but symptoms of
poisoning have been reported at bromide levels as low as 28 mg/litre
blood and severe symptoms at blood bromide levels of 120 mg/litre
(Rathus & Landy, 1961). Brenner (1978) and Bowers & Onoreski (1990)
considered that the toxic threshold level for bromide in serum in
humans was 500 mg/kg, though effects were observed in patients with
lower levels.
However, Weller (1982) disputed a correlation between the bromide
levels in serum and the severity of poisoning. In workers in a methyl
bromide manufacturing plant, no definite association was found between
symptoms and urine bromine concentrations (Kishi et al., 1991). A
direct association between serum bromine concentrations and the
severity of neurological symptoms was not found in a poisoning
incident involving greenhouse workers (Hustinx et al., 1993).
9.1.2 Dermal exposure
Dermal exposure can result from direct contact with liquid methyl
bromide, e.g., from accidental splashing, or through contact with
contaminated boots, clothing, bandages, or gloves (Alexeeff & Kilgore,
1983). These articles are often made of rubber, which can absorb
methyl bromide (Watrous, 1942). Direct eye injury or irritation can
also occur. Dermal exposure to gaseous methyl bromide can also cause
poisoning.
When liquid methyl bromide is spilled on the skin, it evaporates
rapidly producing a cool, but not a cold, or burning sensation
(Watrous, 1942). Repeated application of the liquid caused a burning
or tingling sensation in the skin, which, in more severe cases, led to
a sense of numbness, followed later by aching. In the early stages,
the skin appeared red and slightly swollen, with blisters and
second-degree burns appearing after 2-12 h in severe cases. In less
severe cases, an itching dermatitis may develop after a latent period
of about seven days (Watrous, 1942).
Cases of skin burns have been reported during fumigation (Bruhin,
1942) and from methyl bromide fire extinguishers (Butler et al.,
1945). Bruhin (1942) reported three cases of dermal exposure during
bulk fumigation of a mill. Methyl bromide poisoning occurred due to
percutaneous absorption; the men were using breathing apparatus. All
three men were exposed to the same conditions, they suffered first or
second degree skin burns and one died as a consequence of the
exposure.
Skin lesions occurred in 6 patients who were exposed to methyl
bromide during the fumigation of a castle (Zwaveling et al., 1987;
Hezemans-Boer et al., 1988). They had adequate airway protection, so
poisoning was by dermal exposure and not by inhalation. Exposure to
high concentrations of methyl bromide (about 40 g/m3) for 40 min led
to redness and blistering of the skin. Standard protective clothing
(overalls - 35% cotton/65% polyester), worn over normal clothing, PVC
gloves, and working shoes did not prevent this. Redness, oedema, and
blistering of the skin were limited to areas where perspiration is
relatively high, i.e., armpits, groin, genitals, and the skin under
the waistbelt. The authors suggested that methyl bromide absorption
might be increased in this partly lipophilic (sebaceous glands),
partly hydrophilic (sweat glands) environment, perhaps leading to
increased absorption through the skin as well. The skin in these parts
is also thinner, favouring absorption. Plasma bromide levels were
highest about 13 h after exposure (mean 9.0± 1.4 mg/litre) and fell in
subsequent hours (mean 6.8±2.3 mg/litre; 25 h after exposure),
suggesting absorption of methyl bromide through the skin. Since the
published half-life of bromide is about 100 h, the falls in plasma
levels must be due almost exclusively to the distribution of bromide
in tissues. No systemic effects were noted.
9.1.3 Inhalation
Inhalation is the primary route of exposure. Poisoning has
occurred following acute, short-, and long-term exposures. Acute cases
have occurred involving spilling or leaks while handling methyl
bromide or where the persons were unaware of its presence (Alexeeff &
Kilgore, 1983).
In spite of stronger regulations for the safe and licensed use of
methyl bromide, cases of poisoning still occur. Cases have been
reported in California (Edmiston & Maddy, 1987; Maddy et al., 1990)
and incidents of poisoning have been reported with occupational
exposure (Cantineau et al., 1988; Herzstein & Cullen, 1990), in
members of the general public (Goldman et al., 1987; Polkowski et al.,
1990), and in burglars entering fumigated premises (Marraccini et al.,
1983; O'Neal, 1987).
9.2 General population exposure
9.2.1 Poisoning incidents
Earlier poisoning incidents involving the general public were
mainly from the methyl bromide in fire extinguishers. More poisoning
incidents have involved unauthorized entry into fumigated buildings or
persons living near fumigated buildings, greenhouses, or fields being
fumigated with methyl bromide.
9.2.1.1 Poisoning associated with fire extinguishers
Incidents involving fire extinguishers filled with methyl bromide
often involved exposure to the gas by inhalation and severe burns when
the liquid was inadvertently squirted on the feet or clothing. Butler
et al. (1945) described two cases where drivers suffered burns after
extinguishing a fire in an armoured car. Both recovered after 2 and 8
weeks, respectively. Twenty-two cases of methyl bromide poisoning in
incidents involving fire extinguishers in ships, including 6
fatalities, were reported between 1939 and 1945 (Holling & Clarke,
1944; Clarke et al., 1945). In another incident, 8 boys, 6 of whom
died, were exposed accidentally to methyl bromide from a fire
extinguisher (Prain & Harvey Smith, 1952). Longley & Jones (1965)
described an accident during methyl bromide filling where there was
massive skin contamination. Although the victim removed the
contaminated clothing and washed himself thoroughly, symptoms and
signs of poisoning, commencing with severe nausea appeared within 2 h,
and CNS effects 5 h after the exposure. The CNS effects persisted and
6 months later there was still some disability. There were no dermal
effects apart from oedema and itching of the eyelids with subsequent
peeling of the skin and no evidence of pulmonary or renal involvement.
Goulon et al. (1975) reported a detailed study of three cases
where one patient died after 5 years in a stuporous state with
myoclonus. Two of these were females who had slept in bedrooms
containing fire extinguishers filled with methyl bromide; one of these
patients died, still in coma, 4 years and 8 months later. The other
case was a male lorry driver, who was found in a coma after spending
a night in his lorry cab, which contained a leaking fire extinguisher;
4 years later he showed only partial recovery with persisting lack of
coordination, ataxia, and dysarthria. Behrens & Dukes (1986) described
the case of a scrap-dealer who was poisoned by methyl bromide from
fire extinguishers in obsolete aircraft engines.
A case of poisoning and the resulting death of a man (and his
dog) from methyl bromide leaking from an old corroded fire
extinguisher has been reported (Cavanagh, 1992; Squier et al., 1992).
Since methyl bromide is no longer in general use for fire
extinguishers, the occurrence of such cases is now extremely rare.
9.2.1.2 Poisoning associated with bulk or house fumigation
In May 1978, severe methyl bromide poisoning occurred in 4 people
living above a warehouse in Japan (Ishizu et al., 1988). The
warehouse, containing herbs, was accidentally fumigated with a greater
quantity of methyl bromide than normal giving an estimated
concentration of 38 900-58 350 mg/m3 (10 000-15 000 ppm). Three days
later, one member of the family, a girl aged 12 years, developed
severe convulsions and then 2 others had severe convulsions and the
fourth had marked mental confusion. The serum or plasma bromide ion
levels ranged from 280 to 600 mg/litre. Clinical laboratory tests
showed that GOT exceeded the normal level in 3 out of 4 cases.
Moreover, the LDH activity was above the normal range in three cases
and CPK activity was increased in all the cases (Ishizu et al., 1988).
In California, the most frequent cause of death from
non-occupational exposure, in recent years, has been unauthorized
entry into structures under fumigation with methyl bromide. Most often
the entry was by burglars, transients, or intoxicated persons who
broke into buildings that were covered with gas resistant sheeting,
locked, and posted with warning signs. During 1982-87 there were 13
such fatalities (Maddy et al., 1990).
In Florida, where homes are fumigated to destroy termites by
placing a huge coloured tent over the house, the methyl bromide being
released by trained technicians, 27 non-fatal cases and four
fatalities following unauthorized entry were reported between 1957 and
1982 (Marraccini et al., 1983). The intervals between exposure and
death ranged between 2.5 and 36 h. Three of these deaths together with
three additional hospitalizations followed burglaries of tented houses
during a nine-month period.
O'Neal (1987) reported that, at one hospital in Florida, 15-25
patients a year, including fatalities, were treated for methyl bromide
poisoning. Proper diagnosis and therapy were hampered by the latency
period (4-48 h before onset of symptoms) and the fact that the toxic
state also mimics other diseases.
Even after apartments have been cleared for habitation, deaths
due to methyl bromide poisoning have been reported. The fumigant can
be absorbed by furniture, water beds, and walls, and can sequester in
enclosed spaces, with subsequent desorption (Dempsey et al., 1992).
Prockop & Smith (1986) reported a case in which a house fumigator
wearing a faulty protective face mask was exposed briefly on one day.
This was followed by malaise, although he continued to work, and then
again, ten days later, when tremor and increasing malaise resulted in
admission to hospital. On the next day, he was comatose with
generalized major motor convulsions, and myoclonic limb movements
between convulsions. On admission, the serum bromide level was 350
mg/litre (falling to 30 mg/litre 29 days later). Five years later, the
patient still exhibited dysarthria, impaired arm coordination, and
disturbance of gait.
A fatal case of methyl bromide poisoning was reported after
fumigation of a restaurant. Although the methyl bromide levels were
checked, for some reason the apparatus showed a reading of "no
detectable levels" and, without putting the ventilation system on, the
premises were declared safe. A restaurant employee, who then entered
the building, was found dead 2.5 h later; the post-mortem inorganic
bromide level in serum was 48 mg/litre. Four other workers who were in
the building for 45 min had blood bromide concentrations ranging from
40 to 51 mg/litre. Another worker who was in the restaurant for 1 h 15
min, had a blood bromide concentration of 101 mg /litre. These 5
workers had immediate symptoms of malaise and fatigue, but no chronic
symptoms (Fuortes, 1992).
A 13-year-old girl accidentally exposed to excessive methyl
bromide concentrations after a warehouse fumigation had early symptoms
of headache, dizziness, and nausea, followed a few hours later by
unconsciousness, from which she recovered three days later. At that
time, myoclonic jerks developed, the intensity of which progressed and
was unresponsive to treatment over a two-month period. In a two-year
follow-up, treatment with clonazepam and phenobarbitol decreased the
intensity of myoclonus and she was able to return to school.
Electrophysiological findings suggested that the methyl bromide
exposure had induced a type of cortical reflex myoclonus (Uncini et
al., 1990).
9.2.1.3 Poisoning associated with soil fumigation
Goldman et al. (1987) reported four episodes of community
exposure to methyl bromide and chloropicrin soil fumigation in
California in 1973, 1980, and two in 1984. A total of over sixty cases
were involved, and, in all episodes, either evacuation of homes or
cessation of fumigation was the outcome. In one episode, a strawberry
field was fumigated preplanting with a methyl bromide/ chloropicrin
formulation (dosage and % composition not given). Local weather
conditions included a temperature of 30 °C with an inversion; 32
adults and 4 children were treated with incident-related symptoms,
such as eye irritation, sore throat, headache, shortness of breath,
and cough. One child was hallucinating. Fumigant-related symptoms
dropped off sharply with distance, 30% within 1 km compared with 4.5%
more than 2 km from the field. It should be noted that these symptoms
could be attributed to either methyl bromide or chloropicrin.
A nurseryman was poisoned after glasshouses near his house had
been fumigated with methyl bromide (Bishop, 1992). The symptoms were
delayed. By day 3 after the fumigation, he had convulsions and became
comatose. There was a long recovery period and persistence of
neurological signs and symptoms.
9.2.1.4 Miscellaneous incidents
Inhalation of methyl bromide from leaking canisters caused acute
methyl bromide poisoning. Two grandparents and a child showed various
levels of poisoning. The man showed predominantly mental disturbances,
with only mild neurological signs and no convulsions; initial euphoria
and lack of concern progressed to a florid psychosis. His mental state
was normal 6 months later. The woman was severely affected and
remained in status epilepticus for 7 days. The boy had an upper
respiratory illness 24 h before methyl bromide poisoning, which might
have increased his susceptibility to the toxic agent. He developed an
acute encephalopathy with fluctuating conscious state, hypotonia,
araflexia, and extensor plantar responses. Before methyl bromide was
identified as the cause of the illness, Reye's syndrome had been
diagnosed (Shield et al., 1977).
A tractor trailer-truck overturned and a cylinder (max. capacity
680 kg) was punctured. Although people were warned of the risk, ten
were admitted to hospital. The symptoms included nausea, vomiting,
breathing difficulty, headache, dizziness, burning throat, coughing,
and chest tightness. The primary route of exposure was inhalation for
seven patients, dermal for two, and both dermal and inhalation for
one. All patients recovered without neurological sequelae (Polkowski
et al., 1990).
Leakage from a compressed gas cylinder caused unconsciousness and
status epilepticus in a man (Mazzini et al., 1992). After awaking, he
suffered from severe myoclonic jerks and major epileptic convulsions.
Six months later, he still had severe action myoclonus of the limbs.
A standardized neuropsychological evaluation revealed severe cognitive
impairment.
9.3 Controlled human studies
Raabe (1988) carried out an inhalation study using [14C]-
labelled methyl bromide up to 0.1 mg/m3 to determine the percentage
of methyl bromide absorbed by the human body from air containing
ambient concentrations of the gas. The uptake was 55.4% nasal and
52.1% oral (section 6.1.1.2 and Fig. 6).
9.4 Occupational exposure
Up to 1955, the majority of methyl bromide poisoning incidents
resulted from chemical manufacture and filling operations, followed by
fire extinguishers and fumigation (von Oettingen, 1955). Since 1955,
fumigation has become the major source of fatalities (Alexeeff &
Kilgore, 1983).
9.4.1 Occupational exposure during manufacture
Incidents of methyl bromide poisoning in industry have been
described by Watrous (1942) and Wyers (1945). The main incidents were
dermatological cases occurring principally among those who filled the
small cylinders for distribution from the larger ones. Other sources
of danger were defects in the plant, such as ill-fitting joints and
bursting of cylinders by an undue rise in temperature. Carelessness
with handling was also a cause.
Kishi et al. (1991) carried out a questionnaire survey to
determine the symptoms of workers exposed to methyl bromide in the
manufacturing process. Seventy five male workers (exposure length:
1-25 years) were compared with a reference group of railway workers.
The questionnaire covered acute symptoms during workshift (16
questions), and general symptoms (61 questions). Acute and general
symptoms were statistically higher in frequency among the exposed
workers (sign test of pairwise comparison). Methyl bromide
concentrations in the air in the workers' breathing zone were measured
every 6 months, and were normally less than 4 mg/m3, but, during
some accidental events, they exceeded 20 mg/m3. The mean bromide ion
concentration in the urine of men working in the manufacture of methyl
bromide was 18.9 mg/litre± 10.4 (range 3.2-54.0 mg/litre), with no
correlation with the symptoms reported.
Previous studies had shown that urinary bromide concentrations
were an index of relatively acute exposure, correlating with the air
concentration of the working site. The concentration in the work-place
air sometimes accidently exceeded 58 mg/m3. There were some cases of
sub-acute poisoning with lethargy, ataxia, and retrobulbar optic
neuritis. In one incident, the mean urine concentration of 20 workers
exposed to methyl bromide was 277 mg/litre. On that occasion, 14 of
the 21 exposed workers had abnormally accelerated tendon reflexes, 8
had paraesthesia, and 4 showed disturbance of the convergence reflex
of the eyes (Kishi et al., 1991).
9.4.2 Occupational exposure due to methyl bromide fumigation
In many countries, the use of methyl bromide is restricted to
trained and licensed personnel.
Occupational exposure to methyl bromide can occur during the
manufacturing, filling, and packaging processes, as well as during its
use as a fumigant. As described in section 3.2.2, methyl bromide is
used mainly for soil fumigation as well as for the fumigation of
buildings and commodities, to prevent pest infestation. The various
occupations involved are given in section 5.3.
Methyl bromide was used as a fumigant in the USA by an estimated
105 000 workers between 1972 and 1974 (NIOSH, 1984) and 75 000 workers
in 1980 (Anger et al., 1981).
9.4.2.1 Incidents involving bulk fumigation
A poisoning incident in a mill where three fumigators were
poisoned through dermal exposure to gaseous methyl bromide has been
described in section 9.1.2 (Bruhin, 1942).
Johnstone (1945) and Ingram (1951) reported incidents in
data-processing factories where over 200 employees were taken ill,
with over 50 cases of frank methyl bromide poisoning, including two
workers suspected of insanity. All 34 packers, described by Johnstone
(1945), had visual disturbances and there was a high incidence of
speech difficulties, mental confusion, hallucinations, and
paraesthesia. Four workers claimed permanent and six temporary
disabilities. The main factor in these poisonings was that the workers
ignored the necessary precautionary measures (Johnstone, 1945).
Kantarjian & Shaheen (1963) described 8 cases of polyneuropathy in
date factory workers who suffered repeated exposure to the vapour due
to faulty working techniques over a period of three months. Within 6
months all had recovered. Only 8 out of 14 employees developed
symptoms, suggesting a variation in susceptibility in different
individuals.
Seven workers employed in the bulk fumigation of houses were
poisoned by methyl bromide (Rathus & Landy (1961). The cause was the
insufficient absorption properties of the canisters in their
respirators. Three of the men were burned on the area of the face
covered by the respirators.
Drawneek et al. (1964) described a case of irreversible brain
damage in a methyl bromide fumigator. Seven other workers, without any
recognisable illness, were found to have serum bromide levels of over
50 mg/litre, at which level mild euphoria may develop and lead to
carelessness in the handling of methyl bromide, and, therefore, to
possible acute exposure.
Hine (1969) reported two acute and eight chronic cases of
fumigation-related poisoning in California from 1957 to 1966. Four of
the patients died. The most common symptoms were malaise, weakness,
and dyspnoea. The deaths were preceded by convulsions and coma. All
cases could be traced to a failure in preventative procedures.
A few hours after exposure, during the fumigation of cocoa beans,
a dock worker developed status epilepticus, coma, and pulmonary oedema
(Greenberg, 1971).
Ten cases of methyl bromide poisoning occurred in the hold of a
ship whilst a rice cargo was being fumigated in port (Brodniewicz,
1967). The accident was the result of several circumstances: sealing
of the crew's quarters was forgotten, some of the crew remained on the
ship during, and after, fumigation, and the port fumigating team
lacked experience and proper qualifications. One victim died following
convulsions, acute pulmonary oedema, and heart failure. In the other
cases, the main complaints were dizziness and nausea.
In a fatal case of methyl bromide poisoning of a ship's boy who
slept in the crew's quarters, the levels of methyl bromide measured
after fumigation had not exceeded 40 mg/m3, but the boy was
poison-ed by a high concentration of the gas leaking from the
fumigated commodity, because of large temperature differences (Weller,
1982).
An employee habitually not wearing a mask in a fumigating plant
spraying fruits and vegetables was initially treated for psychosis, as
the early symptoms of methyl bromide poisoning are similar (Zatuchni
& Hong, 1981).
Cases of dermal exposure occurring during the fumigation of a
castle have been reported (Zwaveling et al., 1987; Hezemans-Boer et
al., 1988). Details are given in section 9.1.2.
Systemic and neuro-ophthalmic manifestations of methyl bromide
poisoning including increased serum bromide level (66 mg/litre),
paraesthesia and burning dysesthesia on hands and feet, and visual
impairment, were described in an assistant fumigator who had been
exposed acutely, twice, during the year previous to examination
(Chavez et al., 1985).
9.4.2.2 Incidents involving soil fumigation
(a) Field workers
Table 52 shows occupational poisoning incidents, reported in
California, due to methyl bromide exposure in the years 1950, 1986,
and 1987, and analysed according to work activity (Edmiston & Maddy,
1987; Maddy et al., 1990).
Seven deaths through occupational exposure were reported in
California between 1951 and 1965. The distribution of cases was: 1952
(1), 1956 (2), 1958 (1), 1959 (2), and 1965 (1) (Maddy et al., 1990).
Methyl bromide poisoning of four field workers occurred during
the removal of soil fumigation sheets under cool weather conditions
(Herzstein & Cullen, 1990). Ten days after injection of methyl bromide
into the soil, the polyethylene sheets covering the soil were removed.
The 4 field workers developed fatigue and light-headedness and 3 had
progressive respiratory, gastrointestinal, and neurological symptoms.
The acute systemic symptoms improved over several days, but
neuropsychiatric symptoms that developed later persisted for several
weeks. The 2% chloropicrin was not enough to warn the workers of the
presence of methyl bromide.
(b) Greenhouse workers
Methyl bromide has been used extensively in the USA since the
1950s, and, in Western Europe, since the 1960s, in the fumigation of
soils in greenhouses. This can be hazardous to the health of the
workers because of the enclosed area in which methyl bromide is
applied (Roosels et al., 1981). Poisoning incidents have occurred in
operators and other people entering fumigated areas. An analysis of
eight severe cases was made by Van den Oever et al. (1978). The signs
and symptoms followed the usual pattern. Nearly all incidents were
associated with professional fumigation workers using the injection
method.
In a case of acute occupational methyl bromide intoxication, a
27-year-old man, using methyl bromide in a greenhouse, suffered
continuous epileptic convulsions for 3 weeks, followed by repeated
generalized convulsions. Myoclony of the face and the fingers
accompanied by a complete motor deficiency persisted; there was also
bilateral deafness (Cantineau et al., 1988).
In an accident involving nine greenhouse workers (2 women, 7 men;
aged 21-40 years), exposed to an inadvertent spread of methyl bromide
during fumigation, two patients needed intensive care for several
weeks because of severe reactive myoclonus and tonic-clonic
generalized convulsions (Hustinx et al., 1993).
Table 52. Summary of occupational cases of methyl bromide poisoning in California
as reported by physicians, listed according to work activity and type of illness/injury a
Year Work activity Systemic Eye Skin Eye/ Total
skin cases
1950 all occupational 3 0 0 0 3
exposures
1986 chamber fumigator 2 0 1 0 3
1987 chamber fumigator 2 0 0 0 2
1986 field fumigator 0 1 5 0 6
1987 field fumigator 1 1 4 0 6
1987b field fumigator 6 2 1 0 9
1986 "tarp" cover fumigator 4 0 1 1 6
1987 "tarp" cover fumigator 1 0 1 0 2
1987b "tarp" cover fumigator 1 0 0 0 1
1986 coincidental exposure 0 1 0 0 1
1987 coincidental exposure 4 0 0 0 4
1987b coincidental exposure 2 0 0 0 2
1987 emergency response 2 1 0 0 3
1987b personnel 1 0 0 1 1
a 1950 and 1987 figures taken from Maddy et al. (1990);
1986 figures taken from Edmiston & Maddy (1987)
b A methyl bromide/chloropicrin formulation was used.
9.4.3 Studies measuring the levels of bromide ion in biological
fluids and tissues
There have been a number of studies to investigate whether there
is a correlation between methyl bromide exposure levels and bromide
ion concentrations, but the results are conflicting.
9.4.3.1 Manufacturing
Ohmori & Hirata (1982), using radioactivation analysis, reported
mean bromine concentrations in serum (66 µg/g) and hair (11 µg/g) from
14 methyl bromide workers, and mean bromine concentrations in serum
(40 µg/g) and hair (4 µg/g) in controls. A worker suspected of methyl
bromide poisoning had a level of 412 µg/g serum, 13 days after
exposure.
Average bromide ion concentrations detected in 36 urine samples
of workers exposed to methyl bromide were 13.3±7.7 mg/litre compared
with 7.1±2.1 mg/litre in a non-exposed group (Koga et al., 1991). The
atmospheric methyl bromide concentrations of exposed workers were
monitored with passive samplers during their work shifts (8 h). No
significant correlation was found between these methyl bromide
readings and the bromide ion concentrations in urine.
9.4.3.2 Fumigation
Routine monitoring of bromide levels in the blood of a group of
fumigators, undertaking both bulk and soil fumigation, was carried out
in England between 1971 and 1979 (Cornwell, 1979). It was found that
the plasma bromide levels between January and the end of the year were
correlated with the number of gas applications, irrespective of the
type of fumigation work undertaken (Fig. 10).
The plasma bromide level was thought to rise as a result of repeated
exposure to low concentrations of methyl bromide with small amounts
accumulating in the body faster than the rate of elimination. During
the Christmas period, when all the men took a break at the same time,
the plasma bromide levels were substantially reduced. Results from
October 1974 to January 1978 indicated that average plasma levels
increased from 15 mg/litre in January to 25 mg/litre in October. Some
fumigators carried out disproportionally more treatments of stacks,
containers, and lighters, and reached plasma bromide levels of 30-60
mg/litre. A relationship between plasma bromide levels and the number
of fumigation applications was found (Fig. 11).
Table 45. Acute inhalation toxicity of methyl bromide for rats and rabbitsa
Concentration Exposure time in hours
(mg/m3) Rats Rabbits
100 % fatality 100 % survival 100 % fatality 100 % survival
50 000 0.1 0.03 0.5 0.2
20 000 0.4 0.1 1.4 0.6
10 000 0.7 0.4 2.2 1.0
2 000 6 2 11 6
1 000 22 8 24 15
852 26 12 32 20
420 -b 22 -b -b
a From: Irish et al. (1940).
b No data.
Table 46. LC50 values for methyl bromide
Species Concentration Exposure Reference
(mg/m3) time
mouse 6 600 30 min Bakhishev (1973)
mouse 4 680 1 h Alexeeff et al. (1985)
mouse 1 540 2 h Balander & Polyak (1962)
mouse 1 575 4 h Yamano (1991)
rat 11 000 30 min Bakhishev (1973)
rat 7 300 1 h Zwart (1988); Zwart et al.
(1992)
rat 3 034 4 h Kato et al.(1986)
rat 1 175 8 h Honma et al. (1985)
Further details of target organ studies and biochemical findings
from the series of mouse studies (Alexeeff et al., 1985) are given in
sections 8.8 and 6.3, respectively.
An LC50 of 1575 mg methyl bromide/m3 (405 ppm ± 20) was
determined after a 4 h exposure (Yamano, 1991). In a further study,
the author exposed mice to 1945 mg methyl bromide/m3 (500 ppm).
After 2 h of exposure, there were no deaths, but, after a further 30
min, there was 85% mortality. Mice treated prior to exposure with
glutathione (500 mg/kg i.p.) showed only 5.3% mortality after this
time.
Table 47. Some single exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 6 (male) (nose only) 0 Alexeeff et al.
(Swiss-Webster) 1 h, surviving 870 (+); no toxic response (1985)
mice sacrificed 1720 (+); no toxic response
one week 2200 (+); significantly decreased lung and
later 2720 liver weights
3500 (+); additionally enlarged, pale kidneys
and kidney lesions
3820 (-); additionally abnormal clinical
signs, weight loss and mortality;
cerebral haemorrhage
4700 (-); additionally liver lesion, liver
congestion and haemorrhage
5770 (-); additionally decreased motor
coordination; cerebral congestion;
colonic haemorrhage; congested
kidneys
5930 (-); all effects mentioned above
(1 h-LC50 of 4680 mg/m3 determined)
a (+) = Able to recall a single task passive avoidance test.
b (-) = Not able to recall a single task passive avoidance test.
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
mouse 10 (male) 4 h 389 0% mortality Japanese
(Crj: BDF1) 10 (female) 584 0% mortality Ministry of
873 0% mortality Labour (1992)
1315 0% mortality
Pathology: respiratory metaplasia of the
olfactory epithelium of the nasal cavity
(female)
1970 80% mortality (male) and 100% mortality
(female)
Clinical signs: decrease in locomotor
movement, tremor, convulsion, diarrhoea,
bradypnoea, dyspnoea (dead)
Pathology:
Dead: congestion of the lung, necrosis
and degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis of the
thymus and lymph node, necrosis of the
olfactory epithelium of the nasal cavity
Survived: tubular necrosis and regeneration
of the kidney, necrosis and respiratory
metaplasia of the olfactory epithelium of the
nasal cavity
2950 100% mortality
clinical sign and pathology: same as
1970 mg/m3 dead animals
mouse 1 h 45 min 1945 0% mortality Yamano (1991)
2 h 0% mortality
2 h 10 min 11% mortality
2 h 20 min 15% mortality
2 h 30 min 85% mortality
3 h 90% mortality
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 10(male) 4 h 584 0% mortality Japanese
(F344/DuCrj) 10(female) 875 0% mortality Ministry of
Pathology: disarrangement and Labour (1992)
respiratory metaplasia of the olfactory
epithelium of the nasal cavity
1315 0% mortality; Pathology: same as 875 mg/m3
1970 0% mortality; Pathology: same as 875 mg/m3
2956 100% mortality
Clinical signs: closed eyelid, decrease in
locomotor movement, ataxic gait, serous
discharge of nose, lacrimation, diarrhoea,
irregular breathing and bradypnoea
Pathology: congestion of the lung,
degeneration of the liver, tubular necrosis
of the kidney, haemorrhage of heart,
haemorrhage or necrosis of the adrenal glands,
necrosis of the olfactory epithelium of the
nasal cavity, congestion of the thymus
4435 100% mortality
Clinical signs and pathology: same as 2956 mg/m3
rat 2 (male) 5.2-86 min 7500- 1-h LC50 was 7300 mg/m3. Zwart (1988)
(SPF-Wistar) 57 000 Rangec: 3.5-min LC50 = 75 700 mg/m3 Zwart et al.
480-min LC50 = 1300 mg/m3 (1992)
rat 5 (male) 8 h 1042- LC50 = 1175 mg/m3 Honma et al.
(Sprague-Dawley) 2085 (1985)
Table 47 (continued)
Species/ No. of Exposure time Concentration Observed effectsa Reference
strain animals/ (mg/m3)
exposure group
rat 8 (male) 8 h 245- locomotor activity decreased at
(Sprague-Dawley) 972 731 mg/m3; rectal temperature fell
2°C at 486 mg/m3; decrease in feed
consumption and body weight gain at
486 mg/m3; all animals lost righting
reflex at 245 mg/m3
rat 8 (male) 6 h 778 extensive destruction of the Hurtt et
(F-344) olfactory epithelium al. (1988)
c Total of 23 combinations of time and dosage; the animals were observed for up to 2 weeks.
Groups (10 male+10 female) of BDF1 mice were exposed to methyl
bromide (99.9% pure) concentrations of 389, 584, 873, 1315, 1970, or
2950 mg/m3 (100, 150, 225, 338, 506, or 760 ppm) for 4 h (Japanese
Ministry of Labour, 1992). Mice exposed to concentrations of 1970 and
2950 mg/m3 showed decreased locomotor activity, tremor, convulsions,
diarrhoea, dyspnoea, and bradypnoea. In the 2950 mg/m3 group, all
the mice died; at 1970 mg/m3, 2 males survived. Mice exposed to
concentrations of between 389 and 1315 mg/m3 did not exhibit any
abnormal clinical signs.
Pathology in a female mouse exposed to 1315 mg/m3 showed
metaplasia of the olfactory epithelium. In the 2 male mice surviving
exposure to 1970 mg/m3, there was renal tubular necrosis and
regeneration, and necrosis and metaplasia of the olfactory epithelium.
In the other mice exposed to 1970 and 2950 mg/m3, there was
pulmonary congestion, hepatic degeneration and necrosis, renal tubular
necrosis, karyorrhexis of the thymus and lymph nodes, and necrosis of
the olfactory epithelium.
8.1.2.3 Rat
Irish et al. (1940) exposed rats to concentrations of 420, 852,
1000, 2000, 10 000, 20 000, or 50 000 mg methyl bromide/m3. Table 45
shows the exposure time in hours resulting in 100 % survival and 100
% fatality. Rats exposed to concentrations below 10 000 mg/m3 showed
roughening of the fur, hunching of the back, drowsiness, heavy
breathing, and sometimes lacrimation. At higher concentrations, the
first signs were nose irritation and lacrimation followed by the
reactions already mentioned. Those exposed for 20 h to 1000 mg methyl
bromide/m3 often became hyperactive until exhausted.
As well as neurological manifestations of toxicity in rats,
methyl bromide at concentrations of 1000-20 000 mg/m3 caused
irritation of the lungs, producing acute congestion and oedema (Irish
et al., 1940).
Groups (10 male + 10 female) of F344 rats were exposed to methyl
bromide (99.9% pure) concentrations of 584, 875, 1315, 1970, 2956, or
4435 mg/m3 (150, 225, 338, 506, 760, or 1140 ppm) for 4 h in a
chamber (Japanese Ministry of Labour, 1992). At concentrations of 1315
mg/m3 and above, there was decreased locomotor activity, ataxia,
nasal discharge, lacrimation, diarrhoea, and irregular breathing and
bradypnoea. In the 2956 and 4435 mg/m3 exposure groups, all the rats
died. Pathology of these groups showed pulmonary congestion, hepatic
degeneration, renal necrosis, myocardial haemorrhages, haemorrhage and
necrosis of the adrenal glands, and congestion of the thymus. In rats
exposed to 875, 1315, or 1970 mg/m3, there was metaplasia of the
olfactory epithelium and, in those exposed to the two highest doses,
also necrosis of the olfactory epithelium.
A single 6-h exposure of rats to 780 mg methyl bromide/m3
caused extensive destruction of the olfactory mucosal epithelium
(Hurtt et al., 1988).
Kato et al. (1986) determined a 4-h LC50 for methyl bromide in
male Sprague-Dawley rats (Fig. 9). Groups of 5 rats were exposed for
4 h to methyl bromide at concentrations of 1952, 2420, 3108, or 3485
mg/m3 (502, 622, 799, or 896 ppm), and approximate values of 100%
survival and 100% lethal concentration were determined (2529 and 3501
mg/m3, respectively). In a further test, 10 rats each were exposed
to 2727, 2984, 3143, 3178, or 3236 mg/m3 (701, 767, 808, 817, or 832
ppm). An LC50 value of 3034 mg/m3 (780 ppm) was calculated from
mortality at one week after exposure (Kato et al., 1986).
The dependence of methyl bromide toxicity on time and
concentration was demonstrated in studies performed by Zwart (1988)
and Zwart et al. (1992). Male SPF-Wistar rats were exposed to a total
of 23 combinations (2 rats each) of time and concentration and LC50
values were determined at seven time points ranging from 3.5 to 480
min. LC50s ranged from 75 700 mg/m3 at 3.5 min to 1300 mg/m3 at
480 min. The 1-h LC50 was 7300 mg/m3. Most animals showed some
incoordination, decreased response to stimuli, and had lame limbs,
directly after exposure. All mortalities occurred during the first
week and, on examination, red discoloured lungs and red/black spots in
the thymus were found in most dead rats. After two weeks, the
surviving animals were sacrificed. Some of these rats showed clear or
light red stained fluid in the lungs (Zwart, 1988).
Honma et al. (1985) carried out various investigations into the
effects of a single, 8-h exposure to methyl bromide on male
Sprague-Dawley rats. An acute toxicity study was carried out with five
groups of five animals exposed to 1042, 1303, 1564, 1824, or 2085
mg/m3 (268, 335, 402, 469, or 536 ppm), respectively. An 8-h LC50
of 1175 mg/m3 (302 ppm) with 95% confidence limits of 1040-1323
mg/m3 (267-340 ppm) was determined.
Body temperature was measured in four groups of five rats each
exposed to 245, 486, or 972 mg methyl bromide/m3 (63, 125, or 250
ppm). Exposure to 245 mg/m3 did not effect rectal temperature, while
8-h exposure at 486 or 972 mg/m3 decreased body temperature by about
2°C; however, this normalized within one day (Honma et al., 1985).
The effects of methyl bromide on body weight gain were
investigated. Food deprivation (feeding only twice a day) was started
at least 2 weeks before exposure. Rats were exposed to 245, 486, or
972 mg methyl bromide/m3 (63, 125, or 250 ppm) for 8 h and feed was
provided immediately afterwards. Decrease in food consumption and
depression in body weight gain were observed in groups exposed to 486
and 972 mg methyl bromide/m3, but not in groups exposed to 245 mg
methyl bromide/m3. The control group gained 15 g/day whereas with
exposure to 972 mg methyl bromide/m3, weight gain was almost fully
suppressed and was still partially depressed (+10 g) the following day
(Honma et al., 1985).
8.1.3 Dermal
Toxicity studies concerning the dermal route of exposure in
animals have not been reported.
8.1.4 Subcutaneous administration
For a single subcutaneous administration in Sprague-Dawley male
rats (9 rats/group) an LD50 for methyl bromide was found to be 135
mg/kg body weight (range 75- 250 mg/kg body weight) (Tanaka et al.,
1988).
8.2 Short-term exposure
8.2.1 Oral
A summary of studies concerned with oral exposure to methyl
bromide by gavage is given in Table 43.
A group of 12 rabbits was fed a mixed diet that had been
fumigated with methyl bromide for 24 h. The rabbits were fed
immediately after the fumigation was completed and the content of
methyl bromide in the feed was 3865 mg/kg. The first animal died 3
days after feeding was begun and the last in 13 days. All were
paralysed prior to death and all showed pulmonary damage. No changes
in the gastrointestinal tract were found (Dudley et al., 1940).
Studies on rats (8-week preliminary test, 16-week, and 20-week
test) and rabbits (52 weeks) were carried out by Dudley & Neal (1942).
Results from the rat study showed that, when high (5290-6200 mg Br/kg
food) amounts of organic and inorganic bromides were present in food
after fumigation with methyl bromide, mortality increased, body weight
gain and activity were reduced, and general health and reproductivity
were adversely affected. When feed containing 240-300 mg Br/kg,
following fumigation with 58 g methyl bromide/m3 for 24 h, was fed,
or when fumigated fruits and vegetables were fed, few or no
deleterious effects were noted (Dudley & Neal, 1942). Activity,
general condition, body weight gain, and reproductivity were normal.
Dudley & Neal (1942) carried out similar studies on rabbits. All 12
rabbits died within 2 weeks of being fed a diet containing about 3000
mg Br/kg. However, the 12 rabbits fed a diet of 60-100 mg Br/kg for 52
weeks showed few or no deleterious effects.
No apparent effects on appearance and general behaviour were
observed in Wistar rats (male and female) given doses, by gavage, of
up to 50 mg methyl bromide/kg body weight in a 90-day study (Danse et
al., 1984). Body weight gain in the male rats was significantly less
than that of controls, though this was not the case for females. There
were slight haematological changes and, in the two higher dosage
groups, several animals showed proliferative alterations of the
forestomach mucosa, characterized by hyperkeratosis and papilloma
(section 8.7).
A study by Boorman et al. (1986), based on a study design by
Danse et al. (1984), included dose groups with a recovery period, in
order to study the progression or regression of lesions. Details are
given in section 8.7 and Table 43. Boorman et al. (1986) found
forestomach lesions similar to those described by Danse et al. (1984),
but these lesions regressed in the 60-day recovery period.
Similar findings were reported by Hubbs & Harrington (1986). They
administered methyl bromide in peanut oil to rats at doses of 0, 25,
or 50 mg/kg body weight per day for up to 120 days. In a regression
study, some of the rats were treated for 90 days and then allowed to
recover for 30-60 days (Table 43 and section 8.7).
Three groups of four beagle dogs (3 male, 1 female) were fed
methyl bromide-fumigated food for 6-8 weeks in doses equivalent to an
average daily ingestion of 35, 75, or 150 mg/kg body weight of bromide
ion, respectively (Rosenblum et al., 1960). A further group of 4 dogs
received 128 mg sodium bromide/kg per day (equivalent to 100 mg
bromide ion/kg per day). A control group of 6 dogs (3 male and 3
female) received only dog chow. After one year of observation and
monthly blood and urine tests, the remaining dogs were killed, the
organs weighed, and histological studies carried out. No evidence of
toxicity that could be attributed to bromide was observed in animals
that received 35 or 75 mg bromide/kg per day. Dogs in the group
receiving 150 mg/kg per day became lethargic and had occasional
episodes of salivation and diarrhoea. No significant effects on blood
chemistry, haematology, or urinary values were reported, nor were
treatment-related deaths or histological lesions noted.
8.2.2 Inhalation studies
8.2.2.1 Guinea-pig, rabbit, monkey
A summary of short-term exposure studies is given in Table 48.
Irish et al. (1940) carried out extensive studies into the
long-term exposure of animals to methyl bromide. A total number of 135
rats, 98 guinea-pigs, 104 rabbits, and 13 monkeys were exposed to 65,
130, 250, 420, or 850 mg methyl bromide/m3, 7-8 h/day, 5 days/week
for 6 months, or, until the majority had either died or shown a severe
reaction (Table 49).
8.2.2.2 Mouse
In the short-term studies on male and female B6C3F1 mice, exposed
6 h/day for 10 days over 14 days (778 mg methyl bromide/m3),
described by NTP (1992), five mice/dose group per sex were evaluated
for haematology, serum pseudocholinesterase activity, and pathology.
Necropsied animals from the two highest dosage groups were examined
histopathologically. The results are summarized in Table 48.
Eustis et al. (1988) carried out a special target organ study on
B6C3F1 mice (and F344/N rats). Male and female B6C3F1 mice were
exposed to either 622 mg methyl bromide/m3 (160 ppm) or air for 6
h/day, 5 days/week. The animals were scheduled for sacrifice after 3,
10, or 30 exposures. When 50% mortality was observed in any group, the
surviving animals in that group were sacrificed. Mice were evaluated
for body weight, mortality, organ weights, haematology, and
histopathology. In addition to these end-points, urine chemistry and
plasma enzymes were assessed in the rats. Significantly different
mortality rates were observed between the two species, with the mice
demonstrating a higher sensitivity to 622 mg methyl bromide/m3 than
rats. Body weight differences were exposure-related (Eustis et al.,
1988). The results are summarized in Table 48. Mortality exceeded 50%
after 8 and 6 exposures in male and female mice, respectively. The
remaining male mice were killed after 10 exposures and the females
after 8 exposures. There were significant reductions in body weight
and corresponding reductions in organ weights in both sexes, whereas
there were sex differences in the haematological parameters. The
responses to exposure were minimal in males, but marked in females, in
which there were large and significant reductions in RBC, haemoglobin,
haematocrit values, and mean corpuscular haemoglobin concentrations,
and increases in WBC and mean corpuscular volume (Eustis et al.,
1988). Histopathological changes in target organs are described in
section 8.8.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). Survival was reduced at all
exposure concentrations and none of the mice exposed to 1315 or 1712
mg methyl bromide/m3 survived. At all exposure concentrations, mice
exhibited decreased locomotor activity, piloerection, lacrimation,
ataxia, and tremor.
Table 48. Short-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 10 (male) 6 h/day; 5 days/week; 477 78 mg/m3 group: 9 male, 6 female died; NTP (1992)
(B6C3F1) 10 (female) 2 weeks 97 All groups: no body weight changes; bloody
195 urine; trembling, jumpiness, paralysis in
389 all groups, but most pronounced in highest
778 dosage groups; haematology parameters/
pseudo cholinesterase activity - no
consistent dose-related effects
1 female mouse showed minimal
hyperaemia of lungs, liver, kidneys
Mouse 20 (male) 6 h/day; 5 days/week; 622 50% mortality (male) after 8 exp. days, Eustis et
(B6C3F1) 20 (female) 10 exp. days 50% mortality (female) after 6 exp. days; al. (1988)
for males and exposure-related lesions were seen in
8 exp. days the brain, heart, kidneys, thymus, and
for females spleen of both sexes; in the testes,
nose, and lungs of males, and in the
adrenal glands of females
Mouse 15 (male) 6 h/day; 5 days/week; 0 Eustis et al. (1988)
(B6C3F1) 15 (female) 6 weeks 622 lethargy; curling and crossing
(continued) of hind-limbs, forelimb twitching
and tremors; decrease in body weight
gain after 5 days; decrease in organ
weight (lung, heart, thymus, brain,
liver) neuronal necrosis; nephrosis;
atrophy of inner zone of adrenal
cortex; testicular degeneration;
decrease in RBC, increase in WBC
(females only)
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Mouse 18-30 (males) 5 days/week; 6 h/day; 0 All dose groups: no significant organ NTP (1992)
(B6C3F1) 18-30 (female) 13 weeks 39 weight effects;
78
156 decrease in Hb and MCV, increase in RBC (males);
311 decrease in Hb and MCV, increase in RBC (males);
467 17 % mortality in males; decrease in body weight,
additionally severe curling and crossing
of hindlimbs and twitching of forelimbs
(male > female)
Mouse 10 (male) 6 h/day; 5 days/week 0 Japanese
(Crj:BDF1) 10 (female) 13 weeks 29 no toxic effects; Ministry of
58 no toxic effects; Labour
117 no toxic effects; (1992)
234 depression of body weight gain;
Haematology: increase in MCV in females;
Urinalysis: increased protein in females
Mouse 10 (male) 6 h/day; 5 days/week 599 10% mortality (male) and 0% (female) Japanese
(Crj:BDF1) 10 (female) 2 weeks depression of body weight gain; Ministry of
(continued) Clinical signs: Labour (1992)
Dead mice: decrease in locomotor activity,
piloerection, lacrimation, bradypnoea,
opacity of eye, diarrhoea
Surviving mice: decrease in locomotor activity,
bradypnoea, ataxic gait, sub-normal
temperature, tremor, lacrimation, soiled,
pallor, hunched posture, piloerection
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology:
Dead mice: tubular necrosis of the kidney,
ulcer of the stomach, testicular
atrophy, atrophy of the spleen, atrophy
and karyorrhexis of the lymph node,
myocardial necrosis
Surviving mice: degeneration of the granular
layer of the cerebellum, tubular necrosis and
regeneration of the kidney, necrosis and
respiratory metaplasia of the olfactory
epithelium
Mouse 778 50% mortality of males and 80% mortality of Japanese Ministry
(Crj:BDF1) females, depression of body weight gain; of Labour (1992)
(continued) Clinical signs: same as 599 mg/m3 group
Pathology: Dead mice: degeneration of the
granular layer of the cerebellum, tubular
necrosis and regeneration of the kidney,
extramedullary haematopoiesis and atrophy
of the spleen, karyorrhexis of the thymus,
myocardial necrosis, necrosis of the
olfactory epithelium
1011 90% mortality (male and female)
depression of body weight gain;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
1315 100% mortality (male and female);
Clinical signs: same as mice dying
in 599 mg/m3 group
Pathology: congestion of the lung,
degeneration of the liver, hyaline droplet
and tubular necrosis of the kidney,
karyorrhexis of the thymus and spleen,
myocardial necrosis, necrosis of the olfactory
epithelium.
Mouse 1712 100% mortality (male and female); Japanese Ministry
(Crj:BDF1) Clinical signs: same as mice dying of Labour
(continued) in the 599 mg/m3 group (1992)
Pathology: congestion of the lung,
degeneration of the liver, tubular
necrosis of the kidney, karyorrhexis
of the thymus and spleen.
467 mortality, 10% in males and 90% in females;
depression of body weight gain;
Clinical signs: ataxic gait;
Haematology: increased MCV in males;
Urinalysis: same as 234 mg/m3 group;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, congestion of the lung, karyorrhexis
and atrophy of the thymus, tubular necrosis
of the kidney, necrosis of the heart,
vacuolic change of the adrenal glands
Rat 6 (male) 6 h/day; 5 days 0 NTP (1992)
(SPF Wistar) (week 1) 150 brain weight depression 4-5%
6 h/day; 3 days 375 (dose related) at 750 mg/m3;
(week 2) 750 additionally, marked growth retardation;
tremors, motor incoordination; liver
weights decreased by 26%; no distinct
microscopic changes in eight organs (but
lungs of three high-dose rats were
hyperaemic)
Rat 6 (male) 6 h/day; 5 days/week 0 no toxic effects; NTP (1992)
(SPF Wistar) 6 (female) (week 1,2,3) 70 no toxic effects (marginal no-effect level); (Dutch
6 h/day; 7 days/week 200 decrease in feed consumption, decrease Study)
(week 4) in body weight gain;
disturbed gait and tremors;
600 histopathological changes in heart
and lungs, 8 rats died before end
of study
Rat 10 (male) 6 h/day; 5 day/week; 0 no deaths; no clinical findings; no Wilmer et
(Wistar) 10 (female) 13 weeks 4 change in body weight al. (1983)
25
166 minimal changes in liver
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 10 (male) 6 h/day; 5 days/week; 599 depression of body weight gain Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
Pathology: disarrangement and
respiratory metaplasia of the
olfactory epithelium
778 depression of body weight gain;
Pathology: disarrangement and respiratory
metaplasia of the olfactory epithelium
of the nasal cavity, cellular
vacuolization in the adrenal glands
1011 depression of body weight gain;
Clinical signs: piloerection, soiled,
bloody nose discharge;
Pathology: disarrangement, necrosis and
respiratory metaplasia of the olfactory
epithelium of the nasal cavity, vacuolic
change of the adrenal glands, myocardial
damage
Rat 10 (male) 6 h/day; 5 days/week; 1315 70% mortality in males and 10% mortality Japanese Ministry
(F344/DuCrj) 10 (female) 2 weeks in females; of Labour (1992)
(continued) Dead mice: depression of body weight gain; (continued)
Clinical signs: decrease in locomotor
movement, soiled, piloerection, lacrimation,
serous or bloody nose discharge, diarrhoea,
pallor, irregular breathing;
Dead and surviving mice: decrease in
locomotor movement, hunched posture, soiled,
piloerection, haemorrhagic discharge of nose,
lacrimation
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Pathology: interstitial pneumonia,
karryorrhexis of of the thymus,
myocardial damage, cellular vacuolization
of the adrenal glands, disarrangement and
respiratory metaplasia of the olfactory
epithelium
1712 100% mortality;
Clinical signs: same as 338 ppm group dead
rats;
Pathology: congestion and haemorrhage of
the lung, congestion, necrosis, and fatty
changes in the liver, tubular necrosis of
the kidney, myocardial necrosis, haemorrhage,
necrosis and cellular vacuolization of the
adrenal glands, necrosis and
respiratory metaplasia of the olfactory
epithelium, congestion of the thymus,
inflammation of the bone marrow
Rat 10-12 (male) 4 h/day; 6 weeks 584 decrease in body weight, Kato et
(Sprague- no clinical changes; al. (1986)
Dawley) 778 decrease in body weight,
no clinical changes;
1167 3/12: paralysis of hindlimbs;
1556 5/10: ataxia after 2 weeks, paralysis
after 3 weeks, 1/10: died after 4 weeks,
3/10: died after 5 weeks;
Haematology: no change in RBC, Hb, Hct, WBC
1167 mg/m3 group: increase in serum enzyme
activities;
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Organ weights: decrease in all groups, but
no clear dose dependency;
Residual bromide: increase in all groups
584 mg/m3: spleen>kidney>liver;
higher dosage groups: kidney>spleen>liver;
Histopathological changes: in brain, heart
and testes
Rat 5 (male) 3 weeks 4 biochemical changes Sato et
(Sprague- 20 al. (1985)
Dawley) 39
Rat 10 (male) 6 h/day; 5 days 350 no observable effects; Hurtt et
(F-344) 680 dose-dependent vacuolar degeneration al. (1987)
of zona fasciculata (adrenal gland),
cerebellar granular cell degeneration
and olfactory sensory cell degeneration;
973 as above, plus: diarrhoea, haemoglobinuria,
1264 some gait disturbances and convulsions;
hepatocellular degeneration (at 1264
mg/m3 only) - cerebral cortical degeneation
ation and minor alterations in testicular
histology
Rat total 84 6 h/day; 5 days 778 increase in mean body weight; Hurtt et al. (1988)
(F-344) (male) degeneration and regeneration
of olfactory epithelium
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 40 (male); 6 h/day; 5 days 778 decrease in plasma testosterone Hurtt & Working
(F-344) 10 (male) concentration and nonprotein sulfhydryl (1988)
killed on contents of liver and testis
each of day
1,3,5 and 8
35 (male); 6 h/day; 5 days
5 killed on
each of day 6,
10, 17, 24, 38,
52, 73
Rat 5 (male) 6 h/day (3 days) 622 50 % mortality in males after 14 exp. Eustis et
(F-344) 5 (female) days; remaining males sacrificed; al. (1988)
females killed after 6 weeks;clear
5 + 10 (male) 6 h/day, 5 days/week sex-related differences in susceptibility
5 (female) (2 weeks) of specific organs to CH3Br:brain, kidney,
nasal cavity, heart, adrenal, liver, and
10 (female) 6 h/day, 5 days/week testis; neuronal necrosis in cerebral cortex,
(6 weeks) hippocampus and thalamus of brain; necrosis of
olfactory epithelium; myocardial degeneration;
testicular degeneration
Rat 18 6 h/day; 5 day/week; 0 All dose groups: no deaths or clinical Haber et al.
(F-344/N) 18 (female) 13 weeks 117 signs; no consistent organ weight effects; (1985) [abstract]
234 * body weight (females) NTP (1990)
467 * body weight (both sexes);
minor neurobehavioural changes; (females)
* Hct, * Hb, * RBC;
olfactory epithelial dysplasia and cysts
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
Rat 36 (male) 6 h/day; 5 and 117 GSH and G-6-PDH activities: increase in Jaskot et
(CD) 10 days lung and decrease in liver; al. (1988)
serum: decrease in cholinesterase, BUN,
uric acid, cholesterol,
increase in leucine amino-peptidase
Rat total 30 4 h/day; 4 days/week, 778 damage of olfactory epithelium; Hastings
(Long-Evans) (15 control) 2 weeks repair by day 4; impaired nasal (1990)
function recovered after 4 days
Rat 10 (male) 6 h/day; 5 day/week; 0 Japanese Ministry
10 (female) 13 weeks 29 no toxic effects of Labour
73 Blood biochemistry: * K (male), ** total (1992)
cholesterol (female)
183 Blood biochemistry: * in potassium (male)
** in total cholesterol, GOT, GPT (female)
455 * of body weight gain
Haematology: ** Hct, ** MCV, ** platelet
(male) ** MCV (female)
Blood biochemistry: * K (male), ** total
** total cholesterol, GOT, GPT * glucose,
creatinine (female)
Rat 1140 100% mortality; Japanese
(continued) Clinical signs: * locomotor activity, Ministry of Labour
hunchback position, piloerection, (1992) (continued)
soiled, ataxic gait, tremor, convulsion,
diarrhoea, loose stool or cyanosis,
haematuria, serous or haemorrhagic
discharge of nose, haemorrhagic discharge of
eye, lacrimation, irregular breathing
Table 48 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals/ (mg/m3)
exposure group
pathology: degeneration of the granular
layer of the cerebellum, necrosis of the
brain, karyorrhexis, haemorrhage and atrophy
of the thymus, tubular necrosis of the kidney,
atrophy of the testis, foamy cell accumulation
and interstitial pneumonia, myocardial damage,
cellular vacuolization of the adrenal glands,
pigmentation of the Harderian glands,
necrosis, disarrangement and respiratory
metaplasia of the olfactory epithelium
GSH = glutathione S-transferase; G-6-PDH = glucose-6-phosphate dehydrogenase; BUN = blood urea nitrogen;
RBC = red blood cell count; exp. = exposure; p.c. = post copulation.
* = decrease; ** = increase
Histopathology showed degenerative cerebellar changes, renal
tubular necrosis and regeneration, and metaplasia and necrosis of
olfactory epithelium in all exposed groups. In male mice exposed to
599 mg/m3, there were also stomach ulceration, testicular atrophy,
atrophy of the spleen, and atrophy and karyorrhexis in the lymph
nodes. At concentrations above 778 mg/m3, there were, also karyor-
rhexis of the thymus, and myocardial necrosis. Hepatic degeneration
and pulmonary congestion were found at 1315 and 1712 mg methyl
bromide/m3. For further details see Table 48.
B6C3F1 mice were exposed to methyl bromide concentrations of 0,
39, 78, 156, 312, or 468 mg/m3 for 6 h per day on five days a week
for 13 weeks (NTP, 1992). There were reductions in survival and body
weight gain among male mice exposed to 468 mg/m3. No reduction was
observed among males in the other groups or in female mice in any
group. Clinical findings in the high-dose group were severe curling
and crossing of the hindlimbs and twitching of the forelimbs. These
signs were more severe among male than among female mice. Mild
behavioural test response deviations reached a maximum after about 6
weeks with no further increase in severity in the later 7 weeks of the
study. Plasma cholinesterase activity was unaffected by treatment. No
exposure-related histopathological changes were described.
BDF1 mice (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 58, 117, 234, or 467 mg/m3 (0,
7.5, 15, 30, 60, or 120 ppm) for 13 weeks, 6 h/day, and 5 days/week
(Japanese Ministry of Labour, 1992). At 467 mg/m3, there was a
mortality rate of 10% (males) and 90% (females). Ataxia was noted in
mice exposed to 467 mg/m3. There were no abnormal clinical signs at
concentrations of between 29 and 234 mg/m3. Slight increases in mean
corpuscular volume (MCV) in female mice exposed to 234 mg/m3 and in
male mice exposed to 467 mg/m3 were not accompanied by other
haematological effects. Blood biochemistry showed no abnormalities. No
treatment-related, histopathological effects were observed in the 29
and 234 mg/m3 groups. In the mice that did not survive exposure to
467 mg/m3, there were cerebellar degeneration and brain necrosis,
pulmonary congestion, thymic atrophy, myocardial damage, renal tubular
necrosis, and vacuolization of the adrenal glands (Table 49).
Table 49. Long-term exposure inhalation studies
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
mouse 86 (male) 5 days/week; 0 All dose groups: no biologically significant NTP (1992)
(B6C3F1) 86 (female) 6 h/day;2 year 39 haematology values; no carcinogenic
[planned; (interim 128 effects; increased incidence of
because of high sacrifice at 6 389 nonneoplastic lesions in brain, bone,
mortality, and 15 months) heart, and nose
regime altered] 389 mg/m3 dosage group: high mortality
(40/86 males and 9/86 females) after 20
weeks; decrease in body weight and
in thymus weight;
tremors, abnormal posture and limb paralysis;
significant behavioural differences at 3
months (males), less so in females
mouse 50 (male) 6 h/day; 5 days/ 0 Japanese
(Crj: 50 (female) week; 16 no toxic effects; Ministry of
BDF1) 2 years 62 no toxic effects; Labour (1992)
250 no mortality change;
depression of body weight gain
Blood biochemistry: increase in CPK, inorganic
phosphorus (male), chloride; decrease
in albumin (female);
Pathology: atrophy of the granular layer of
the cerebellum no increase in neoplastic change
was considered
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 90 (male) 6 h/day; 5 days/ 0 All dose groups: degenerative and Dreef-van der
(Wistar) 80 (female) week; 29 months 12 hyperplastic lesions in nasal mucosa; no Meulen et al.
117 tumours induced by methyl bromide; (1989)
350 350 mg/m3 group: * body weight gain; Reuzel et al.
* absolute brain weight; hyperkeratosis in (1991)
oesophagus and forestomach; ** incidence
of haemothorax, myocardial degeneration;
thrombi in the heart; and increased
mortality
rat 50 (male) 6 h/day; 5 days/ 16 Pathology: ** incidence and severity of Japanese
(F344/ 50 (female) week; 2 years inflammation of the nasal cavity (male); Ministry of
DuCrj) 78 Urinalysis: * in protein (male); Labour (1992)
Pathology: same as 16 mg/m3
389 no mortality change, * body weight gain;
Haematology: ** RBC, Hb, Hct (male)
** Hb, Hct (female);
Blood biochemistry: * LDH, CPK, Na+, K+,
Cl- (male), * Glu, CPK, Ca2+, ** LAP (female);
rat Urinalysis: * protein
(F344/DuCrj) Pathology: ** incidence of necrosis and
(continued) respiratory metaplasia of the olfactory
epithelium; ** incidence and severity of
inflammation of the nasal cavity (male);
marginal ** necrosis of the olfactory
epithelium and inflammation of the nasal
cavity (female);
no increase in neoplasms observed
Table 49 (continued)
Species/ No. of Exposure time Concentration Observed effects Reference
strain animals per (mg/m3)
exposure group
rat 8 6 h/day; 5 days/ 0 no effect on nerve conduction velocity, Anger et al.
(Sprague- 32 (n.g.)b week; 36 weeks 214 open field activity, or coordination (1981)
Dawley) over 12 months
a RBC = red blood count; Hb = haemoglobin; Hct = haematocrit; WBC = white blood count; MCV = mean cell volume.
b n.g.= sex not given; * = decrease; ** = increase.
8.2.2.3 Rat
F344-rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 599, 778, 1011, 1315, or 1712 mg/m3
(154, 200, 260, 338, or 440 ppm) 6 h/day, 5 days/week, for 2 weeks
(Japanese Ministry of Labour, 1992). All rats in the 1712 mg/m3
group and 7 males and one female in the 1315 mg/m3 group died before
the end of the study. Piloerection and haemorrhagic nasal discharge
were reported at exposure concentrations of 1011, 1315, and 1712
mg/m3. In addition, decreased locomotor activity, a hunched position
and lacrimation were observed in rats that survived exposure to 1315
mg/m3. Diarrhoea and irregular breathing were noted in the rats that
died following exposure to 1315 and 1712 mg/m3.
Pathology showed metaplasia of the olfactory epithelium at methyl
bromide concentrations of 599 and 778 mg/m3, plus necrosis at
concentrations between 1011 and 1712 mg/m3. Vacuolization of the
adrenal glands was found at concentrations of 778, 1011, and 1315
mg/m3 with necrosis at 1712 mg/m3. Myocardial damage was reported
at concentrations between 1011 and 1712 mg/m3. In the rats exposed
to 1315 and 1712 mg/m3, there was also pulmonary congestion and
haemorrhage, renal tubular necrosis and congestion of the thymus; bone
marrow inflammation was reported at 1712 mg/m3 (Table 48).
Two short-term range finding studies were conducted in SPF Wistar
rats (NTP, 1992). In the first study, groups of six male rats were
exposed to up to 750 mg methyl bromide/m3 for 6 h/day for 2 weeks
(see Table 48). In the second range-finding study, groups of six male
and six female rats were exposed to up to 600 mg methyl bromide/m3
for 4 weeks. Five male and three female rats in the high-dose group
died before the end of the study. The most important histopathological
changes occurred in the heart and lungs of animals in the high-dose
group. Diffuse fatty vacuolization and diffuse myocardial fibre
degeneration appeared. The lungs showed hyperaemic and dilated
alveoli; in some animals, interstitial pneumonia was noted (NTP,
1992).
Male Sprague-Dawley rats were exposed to up to 1556 mg methyl
bromide/m3 for 4 h/day for 6 weeks (Kato et al., 1986). Changes in
body weight, general condition, haematology parameters, organ weight,
tissue bromide ion concentration, and the histopathology of several
organs were determined. Suppression of body weight gain, abnormal
clinical signs, and severe weakness were observed at 1556 mg methyl
bromide/m3. Bromide ion accumulation was seen, especially in the
kidney and spleen, without significant dose-related change. Pronounced
histopathological changes were noted in the brain (section 8.8) and
multiple small necrotic foci in the heart.
Other short-term, inhalation exposure studies concentrated on one
aspect/target organ of methyl bromide exposure.
Sato et al. (1985) described biochemical findings in rats exposed
to 4, 20, or 39 mg methyl bromide/m3, continuously, for 3 weeks.
After sacrifice, the organs were weighed and biochemical examinations
were performed on the blood and a homogenate of heart, liver, and
lungs. The results showed no differences between the 4 mg/m3 and the
control group. In the 20 mg/m3 group, several changes were observed;
serum creatine phosphokinase (CPK), phospholipids (PL), and blood
glucose levels, and thymus weight decreased, while blood haemoglobin
(Hb), reduced glutathione (GSH), serum total protein, and lung
gamma-glutamate-pyruvate transaminase (GTP) levels increased after
exposure. In the 39 mg/m3 group, increases were observed in serum
glutamate oxaloacetate transaminase (GOT), lactate dehydrogenase
(LDH), alpha-hydroxy-butyrate dehydrogenase (alpha-HBDH), total
protein, blood Hb, GSH, lung acid phosphatase (AcP), gamma-GTP, LDH
total, liver PL, and triglycerides (TriG), while decreases were noted
in serum cholinesterase (ChE), CPK, PL, TriG, lung alkaline
phosphatase (AlP), free-cholesterol (f-Chol), lactate, blood glucose,
heart lactate, glucose, lung AlP, liver free fatty acids (FFA), and
glycogen. Pulmonary haemorrhage was observed in almost all animals in
this group.
The effects of short-term inhalation exposure to 1264 mg methyl
bromide/m3 (6 h/day for 5 days) including those on the target organ
histopathology of male F-344 rats were studied by Hurtt et al. (1987).
Clinical changes (noted only in the 973 and 1264 mg/m3 groups) were
diarrhoea, haemoglobinuria, and, in a few cases, gait disturbances and
convulsions. A dose-dependent vacuolar degeneration of the zona
fasciculata of the adrenal glands, cerebellar granule cell
degeneration, and olfactory sensory cell degeneration were seen in the
680, 973, and 1264 mg/m3 groups. Cerebral cortical degeneration and
minor alterations in testicular histology were seen only in the 1264
mg/m3 group whereas hepatocellular degeneration was also found in
the 973 mg/m3 group. No changes were found in the kidneys or
epididymides.
In a further study, the degeneration and regeneration of the
olfactory epithelium were examined following exposure of a total of 84
rats to 778 mg methyl bromide/m3 for 6 h/day for 5 days (Hurtt et
al., 1988). Groups of five rats were sacrificed after days 1, 3, and
5 and after exposure weeks 1, 2, 3, 5, and 10. Cell replication rate
and histopathology were used to assess the kinetics of repair. In
addition, olfactory function was assessed using a buried food test.
Extensive damage to the olfactory epithelium was evident in animals
killed directly after 6 h of exposure. The specific site of damage
appeared to be in the olfactory sustentacular cells and mature sensory
cells, the basal cells generally being unaffected. By day 3, despite
continuous exposure, there was replacement of the olfactory epithelium
by a squamous cell layer that increased in thickness and basophilic
staining over the next 2 days. One week after exposure, the epithelial
region was covered by a layer of polyhedral, basophilic cells and from
2 to 10 weeks exposure, the epithelium exhibited progressive
reorganization to restore the original olfactory epithelium pattern;
75-80% of the olfactory epithelium appeared morphologically normal by
week 10.
In the same study, the ability of the rats to locate feed was not
affected by exposure to 350 mg methyl bromide/m3, but treatment with
778 mg methyl bromide/m3 rendered all animals temporarily incapable
of locating buried pellets, though they demonstrated searching
activity. Four to six days after treatment the animals recovered
sufficient olfactory function to find food pellets.
Similar results were reported by Hastings et al. (1989) and
Hastings (1990). Studies on Long-Evans rats showed that, after 4 h
exposure to methyl bromide (778 mg/m3), recovery of buried food was
greatly impaired but, even with continuous exposure, recovery occurred
until, by day 4 of exposure, olfactory function was essentially
normal. Extensive damage to the olfactory epithelium was evident after
day 1 of exposure; repair of the epithelium was in progress by day 4.
The specific site of damage appeared to be in the olfactory
sustentacular cell population while the respiratory epithelium was
largely spared (Hastings et al.,1989; Hastings, 1990). Bolon et al.
(1990) suggested that prior exposure to methyl bromide as well as
caging conditions (e.g., inhalation of ammonia from soiled bedding)
could influence the olfactory epithelial response.
Evans & Hastings (1992) reported that methyl bromide induced an
olfactory function deficit in rats. The olfactory threshold to ethyl
acetate was measured in six rats using a conditioned suppression
behavioural protocol. Three out of the six rats showed an increase in
absolute threshold or threshold response variability after a single,
6-h exposure to 778 mg methyl bromide/m3.
F-344 rats (20 male + 20 female) were exposed (6 h/day; 5
days/week) to 0 or 622 mg methyl bromide/m3 (0 or 160 ppm) for 3,
10, or 30 days (Eustis et al., 1988). Toxicological end-points
assessed included clinical observations, mortality, body and organ
weights, haematology, clinical chemistry, urinalysis, gross pathology,
and histopathology. There were no apparent treatment- related changes
in any of the clinical chemistry and urinalysis analytes measured.
Treatment-related effects in rats included neuronal necrosis in the
brain, myocardial degeneration, olfactory epithelial degeneration and
atrophy, and testicular degeneration and atrophy.
A study by Eustis et al. (1988) confirmed the very steep
concentration-response curve for methyl bromide in rats (and mice).
The authors also found clear species and sex differences in
sensitivity to methyl bromide toxicity. When rats were compared with
mice, the order of susceptibility was female mice>male mice>male
rats> female rats. Species and sex-related differences in the
histopathology of certain organs were also observed.
Kato et al. (1986) exposed Sprague-Dawley rats for 11 weeks, 4
h/day, to 584 mg methyl bromide/m3. No abnormal clinical signs were
reported. Small necrotic foci and fibrosis were observed in the heart
muscle.
F-344 rats (groups 10 males/10 females) were exposed to methyl
bromide at concentrations of 0, 29, 73, 183, 455, or 1140 mg/m3 (0,
7.5, 18.8, 46.9, 117, or 293 ppm) for 13 weeks for 6 h/day, 5
days/week (Japanese Ministry of Labour, 1992). At 1140 mg/m3, there
was 100% mortality. At this concentration of methyl bromide, rats
exhibited decreased locomotor activity, piloerection, ataxia, tremor,
cyanosis, haematuria, and nasal and ocular discharge. No clinical
signs were reported at lower doses.
Haematological studies showed increased MCV in male and female
rats exposed to 455 mg/m3; there was also an increased number of
platelets in the male rats. Serum potassium was decreased in male rats
exposed to concentrations of 73 mg/m3 or more. Glutamic oxalacetic
transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels were
significantly increased in female rats exposed to concentrations of
183, 455, and 1140 mg/m3. Serum glucose and creatinine levels were
significantly decreased in female rats at 455 mg/m3.
There were no abnormal histopathological findings in rats exposed
to methyl bromide concentrations of between 29 and 455 mg/m3. Rats
exposed to 1140 mg/m3 exhibited cerebellar degeneration and brain
necrosis, thymic haemorrhages and atrophy, renal tubular necrosis,
pneumonitis, myocardial damage, adrenal vacuolization, Harderian gland
pigmentation and metaplasia, and necrosis of the olfactory epithelium
(Table 49).
Thirteen-week inhalation toxicity studies were conducted in which
groups of ten male and ten female Wistar rats were exposed to methyl
bromide at target concentrations of up to 166 mg/m3 for 6 h/day, 5
days/week (Wilmer et al., 1983). No deaths occurred, and no abnormal
clinical findings were observed. Body weight gain was not affected in
any of the exposed groups. Leukocyte counts were 22% higher in high-
dose males than in male control animals. Plasma alkaline phosphatase
activity was lower in both high-dose males (32%) and females (53%)
than in controls, and the plasma albumin concentration was 10% higher
in high-dose females than in controls. The absolute and relative liver
weights were up to 16% lower for high-dose males and females compared
with controls. The only exposure-related histopathological change,
which occurred in the liver of high-dose male and female rats, was
characterized by small hepatocytes with homogeneous eosinophilic
cytoplasm. This alteration varied in extent from slight to severe and
was seen in 6/10 males and 7/10 females. The no-adverse-effect level
for these 13-week inhalation toxicity studies was considered to be 25
mg/m3.
Groups of 18 rats of each sex were exposed to up to 467 mg/m3
for 6 h/day and 5 days/week over 13 weeks (NTP, 1992). All rats
survived to the end of the studies, few or no clinical effects of
exposure to methyl bromide could be seen. Significant decreases in
body weight gain were noted in both sexes at 467 mg/m3 and in
females at 234 mg/m3. No consistent organ weight effects were
observed. Minor neurobehavioural changes were noted in both sexes in
the highest dosage group. Females, but not males, of this group had
significantly lower haematocrit, haemoglobin levels, and erythrocyte
counts compared with those of controls. Olfactory epithelial dysplasia
and cysts, characterized by irregularity in mucosal thickness and
focal cavitated spaces, respectively, were seen in both sexes at 467
mg/m3.
8.2.3 Dermal
There are no reports of studies on short-term dermal exposure.
8.3 Skin and eye irritation
Irish et al. (1940) noticed lacrimation in rats after inhalation
of methyl bromide levels above 10 000 mg/m3. Irritation of the eye
membranes in mice at concentrations of 3200 mg methyl bromide/m3 was
described by Balander & Polyak (1962). There are no reports on skin
effects in animals.
8.4 Long-term exposure
8.4.1 Oral
8.4.1.1 Rat
Rats were fed for 7-8 months on wheat grain or peanuts fumigated
with methyl bromide having residual bromide levels of 20 and 22-46
mg/kg, respectively. There were no effects on weight gain of the
animals, haemoglobin content, or red or white blood cell numbers.
However, a decrease in iodine and calcium levels in the blood and
abnormal changes in the thyroid and parathyroid glands were reported
(Vitte et al., 1970).
A two-year, oral, long-term toxicity and carcinogenicity study
was carried out on 60 male and 60 female F-344 rats per group fed
diets fumigated with methyl bromide (Mitsumori et al., 1990). Diets
containing 80, 200, or 500 mg total bromide/kg food (methyl bromide
concentration <20 mg/kg food) were fed, the controls receiving
commercial basal diet (containing 30 mg bromide/kg) and a diet
containing 500 mg potassium bromide/kg. No effects were observed on
the behaviour of the rats in any of the groups fed the fumigated or
KBr- containing diets. In rats fed the diets fumigated with methyl
bromide, there were no marked toxic changes, except for a slight
depression in body weight from week 60 onwards in males in the 500
mg/kg group. Tumour incidence was unaffected. Rats given a diet
containing KBr did not show any treatment-related changes. The
no-effect level was 200 mg/kg (equivalent to 6.77 mg total bromine/kg
body weight per day) in males. No effects were observed in females at
the doses studied.
8.4.2 Inhalation studies
Long-term exposure inhalation studies are summarized in Table 49.
8.4.2.1 Mouse
In a carcinogenicity study, 86 B6C3F1 mice/sex were exposed to
39, 128, or 389 mg methyl bromide/m3 for 6 h/day, 5 days/week, for
2 years with scheduled interim sacrifices at 6 months and 15 months
(NTP, 1992). Because of the high mortality early in this study in the
389 mg/m3 group (27/86 males, 7/86 females), the exposure to methyl
bromide at this level was stopped at 20 weeks and the surviving mice
were exposed to air for the rest of the study.
Terminal survival rates (Kaplan-Meier determinations) in the
control, 39 mg/m3, 128 mg/m3, and 389 mg/m3 groups were: males
82%, 74%, 80%, and 23%; and females, 71%, 82%, 90%, and 65%,
respectively. Significantly lower mean body weights of the highest
dosage group compared with controls appeared by week 11 and persisted
after termination of dosing at week 20 until the end of the studies.
The only biologically significant change appeared to be reduced
absolute and relative thymus weights in both sexes. Neurological signs
were also observed, mostly in the highest dosage group, including
tremors, abnormal posture (lateral curvature of the spine), and limb
paralysis. These symptoms generally persisted (NTP, 1992).
Exposure-related histological lesions occurred primarily in the 389
mg/m3 exposure group and included degeneration in the cerebellum,
cerebrum and heart, chronic cardiomyopathy, sternal dysplasia, and
either necrosis or metaplasia of the olfactory epithelium. There were
no neoplasms attributed to exposure to methyl bromide.
Quantitative neurobehavioural testing showed significant
differences in the behaviour of the high-dose males at 3 months. The
animals were less active and demonstrated a reduction in startle
response latency. Because of the high early mortality in high-dose
males, only males in the controls and two lower dosage groups were
tested after 3 months. After 6 months of exposure, the 389 mg/m3
females had significantly lower activity scores than females in other
groups, but their higher startle responses had disappeared. Although
at 9 months of exposure no behavioural differences were apparent,
lower activity and heightened startle response were again noted at the
2-year testing time (NTP, 1992).
Toxicity and carcinogenicity studies were conducted by inhalation
exposure of groups of 50 male and 50 female Crj:BDF1 mice (6 h/day, 5
days/week) to methyl bromide (99.9% pure) for 104 weeks (Japanese
Ministry of Labour, 1992). Methyl bromide concentrations of 0, 16, 62,
and 250 mg/m3 were used.
Body weight gains in male and female mice exposed to 250 mg/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of atrophy (slight) of the granular layer of
the cerebellum were observed in male and female mice exposed to 250
mg/m3. There were no treatment-related neoplasms in male or female
mice.
8.4.2.2 Rat
Toxicity and carcinogenicity studies were also conducted by
inhalation exposure to methyl bromide (99.9% pure) of groups of 50
male and 50 female F344/DuCrj rats (6 h/day, 5 days/week) for 104
weeks (Japanese Ministry of Labour, 1992). Methyl bromide
concentrations of 0, 16, 78, and 389 mg/m3 were used. Body weight
gains in males and female rats exposed to 389 mg methyl bromide/m3
were lower than those in chamber controls. No significant differences
in survival were observed between exposed and control groups of either
sex. Increased incidences of necrosis and respiratory metaplasia of
the olfactory epithelium of the nasal cavity were observed in male
rats exposed to 389 mg methyl bromide/m3, and increased incidence
and severity of inflammation of the nasal cavity were observed in male
rats exposed at all concentrations used. Necrosis of the olfactory
epithelium and inflammation of the nasal cavity were marginally
increased in female rats exposed to 389 mg methyl bromide/m3. There
were no exposure-related increased incidences of neoplasms in male and
female rats.
In a long-term inhalation study, a total of 360 male and 360
female rats (Wistar) were exposed to concentrations of up to 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 29 months (Dreef-van
der Meulen et al., 1989; Reuzel et al., 1991). Non-neoplastic and
neoplastic lesions were scored in all control and high-level animals
of the main group, and the nose examined histopathologically at all
exposure levels. Mortality was higher in males and females at the 350
mg/m3 level than in controls from the end of the second year
onwards. Body weights in the 350 mg/m3 group (both sexes) were
slightly lower than controls from week 4 onwards. No essential
differences between groups were observed in haematology, clinical and
blood chemistry, and urine analysis at three months and one year. The
absolute brain weight was decreased in females at 350 mg/m3. A
higher incidence of haemothorax was seen in males and females at 350
mg/m3 than in controls. In the heart there was an increased
incidence of thrombi and myocardial degeneration in rats exposed to
350 mg/m3. There was hyperkeratosis of the oesophagus and
forestomach. The incidences of neoplastic lesions did not differ
significantly among the groups.
8.5 Reproduction, embryotoxicity, and teratogenicity
8.5.1 Reproduction and embryotoxicity
McGregor (1981) conducted a sperm abnormality assay exposing
groups of 10 male B6C3F1 mice to air containing 0, 78, or 272 mg
methyl bromide/m3 (0, 20, or 70 ppm) for 7 h/day, for 5 days. No
sperm abnormalities were found at these concentrations.
Atrophy of seminal epithelium, incomplete spermatogenesis, and
giant cells in the seminal tubules, were detected unilaterally in one
rat (group of 10) after inhalation of 778 mg methyl bromide/m3 (200
ppm) (4 h/day; 5 days/week for 6 weeks) and in six rats (group of 10)
after inhalation of 1167 mg methyl bromide/m3 (same length of
exposure). In the tubules of the epididymis adjacent to the atrophied
testis, necrotic spermatocytes had accumulated in seminal fluid, but
spermatozoa were not seen (Kato et al., 1986).
Spermatogenesis and sperm quality were evaluated in the rat
(F-344/N) following exposure to 778 mg methyl bromide/m3, 6 h/day
for 5 days (Hurtt & Working, 1988). Although methyl bromide caused a
transient decrease in plasma testosterone and testicular nonprotein
sulfhydryl concentrations during acute exposure, no other reproductive
indices, including testis weight, daily sperm production, cauda
epididymal sperm count, sperm morphology, percentage motile sperm,
linear sperm velocity, and epididymal and testicular histology, were
affected by methyl bromide exposure.
Testicular degeneration and atrophy occurred in several rats and
mice following repeated (6 h/day, 5 days/week for up to 6 weeks)
inhalation exposure to 622 mg methyl bromide/m3 (Eustis et al.,
1988). In rats, degeneration included separation and sloughing of
spermatocytes and late stage spermatids and/or formation of
intratubular multinucleate giant cells. Atrophy was characterized by
variable loss of all components of the spermatogenic epithelium. In
exposed mice, degeneration of testes occurred frequently, but it was
not always severe.
Thirteen-week inhalation studies on sperm morphology and vaginal
cytology examinations (SMVCEs) in B6C3F1 mice and F-344 rats were
carried out by Morrissey et al. (1988). Results from mouse studies
(inhalation of 39, 156, or 467 mg methyl bromide/m3) showed a
decrease in terminal body weight, and a relative increase in the
weight of epididymis and testis. A decrease in sperm density and an
increase in the percentage of abnormal sperm were also noted. In rats
that inhaled 117, 233, or 467 mg methyl bromide/m3, a decrease in
terminal body weight as well as a decrease in the weight of the cauda
epididymis, and a relative increase in the weight of the testis were
noted. A decrease in sperm motility was also observed. In females,
exposure to methyl bromide did not affect the length of the estrous
cycle (Morrissey et al., 1988).
Female rats exposed to 272 mg methyl bromide/m3 (70 ppm) for up
to 40 days survived and reproduced without impairment (Hardin et al.,
1981; Sikov et al., 1981).
In a dominant lethal assay carried out in rats (McGregor, 1981),
groups of 10 male, adult CD rats were exposed to 0, 78, or 272 mg
methyl bromide/m3 for 7 h/day on five consecutive days, then
serially mated at weekly intervals for 10 weeks with untreated virgin
females in the ratio one male: two females. When the females were
sacrificed, their ovaries were examined for corpora lutea graviditatis
and the uteri were opened and examined for live implantations, late
deaths, and early deaths. The frequency of pregnancy was determined by
(a) females with corpora lutea graviditatis and (b) females with
implantations. With either method, methyl bromide did not cause a
significant decrease in the frequency of pregnancy, and the number of
corpora lutea per pregnancy and the frequency of early deaths were
unaffected.
The effects of inhalation exposure to 0, 12, 117, or 350 mg
methyl bromide/m3 for 6 h/day, 5 days/week, for around 8 months, on
the growth, reproduction, and offspring in two consecutive generations
of CD Sprague-Dawley rats were investigated (American Biogenics
Corporation, 1986). Body weight depressions were noted in the males in
the highest concentration group at 5 of the 10 pre-mating collection
intervals and at final sacrifice. The pre-mating and total weight
gains were also less than those of the untreated control males. In the
F1 generation, parental body weight was comparable in exposed and
control animals. In the F2a litter, a slight body weight depression
was noted in gestating and lactating dams in the highest concentration
group compared with controls. Otherwise, maternal body weights were
comparable with those of the controls.
There was a marginal reduction in female fertility index in the
two top dose groups of the F2a litter. The mean numbers of pups
delivered viable were comparable with controls. Survival of the pups
was reduced in the 350 mg/m3 dose group in late lactation in the F1a
generation. Body weights of pups were reduced in both the 350 and 117
mg methyl bromide/m3 dose groups of the F1a, F2a and F2b
generations, though no consistent changes were observed in the F1b
generation.
No anomalies were noted in the treated progeny that were
attributed to methyl bromide exposure.
Gross pathological examination of parent animals from both
generations and randomly selected F1b and F2b progeny did not reveal
any treatment-related lesions. A statistically significant decrease in
the mean brain weight in the F0 males and an increased liver to body
weight ratio in both sexes were found in the upper concentration
group. In the F1 generation, both male and female brain weights were
decreased at 350 mg methyl bromide/m3.
Statistical analyses of the F1b progeny final body weight and
organ weight data revealed no significant differences. Statistical
reductions were found in the final body weight data obtained for the
F2b males (350 mg methyl bromide/m3) and F2b females (117 and 350 mg
methyl bromide/m3) compared with untreated control progeny. Analysis
of F2b progeny organ weight data revealed significant decreases,
compared with controls, for the female brain, heart, kidney, and liver
weights (350 mg methyl bromide/m3) and female liver weight (117 mg
methyl bromide/m3). In these two upper concentration groups, the
females brain to body weight ratio was increased and the liver to
brain ratio, decreased. Microscopic examination of the reproductive
organs and abnormal tissues did not reveal any treatment-related
lesions.
8.5.2 Teratogenicity
Female Wistar rats were exposed for 7 h/day, 5 days/week for 3
weeks to methyl bromide concentrations of 78 or 272 mg/m3. After
this time, they were mated. There was a total of 7 different exposure
groups (Table 48). Female rats whose vaginal smears showed evidence of
sperm were exposed for 7 h daily from day 1 (=day of finding sperm in
vaginal lavage) to 19 of gestation. The day before term (gestation day
21), they were sacrificed and necropsied. The results showed no
effects of the exposure on the female rats, nor were embryotoxic or
other teratogenic effects found (Sikov et al., 1981).
In a further series of studies by Sikov et al. (1981), groups of
24 female New Zealand white rabbits were exposed for 7 h daily to 78
or 272 mg methyl bromide/m3 (20 or 70 ppm) from the day of
artificial insemination. The 78 mg/m3 group was exposed up to day 24
of gestation; ecause of toxic effects, exposure had to be stopped on
day 15 for the 272 mg/m3 group, in which all but one pregnant rabbit
died. The remaining rabbits from the control and 78 mg/m3 exposure
group were sacrificed on the 30th day of gestation. The low exposure
group showed no fetotoxic or eratogenic effects. An evaluation of the
fetotoxicity or teratogenic results from the high dose group was not
possible because of the high mortality rate in the pregnant rabbits
(Hardin et al., 1981; Sikov et al., 1981).
In a preliminary teratology study (Breslin et al., 1990),
pregnant New Zealand White rabbits were exposed via inhalation for 6
h/day to 0, 39, 117, 195, 272, or 545 mg methyl bromide/m3 (0, 10,
30, 50, 70, or 140 ppm). Toxicity was observed in rabbits exposed to
545 mg methyl bromide/m3 and these were sacrificed before the end of
the study. No apparent embryotoxic effects were observed at any
exposure level (Breslin et al., 1990).
A subsequent study by the same investigators was conducted in two
parts. In Part I, groups of 26 inseminated New Zealand White rabbits
were exposed via inhalation to 0, 78, 156, or 311 mg methyl
bromide/m3 (0, 20, 40, or 80 ppm) on days 7-19 of gestation. In Part
II, groups of 17 inseminated rabbits were exposed for 6 h/day to 0 or
311 mg methyl bromide/m3 (0 or 80 ppm) on days 7-19 of gestation. An
additional group of 16 females, inseminated by a single male, acted as
controls. On day 28 of gestation, all surviving animals were
necropsied. Maternal liver, kidney, lung, brain weights, gravid
uterine weights, and the number of corpora lutea, implantations,
resorptions, and live/dead fetuses were noted. The fetuses were
weighed, sexed, and examined for external, visceral, and skeletal
alterations.
Rabbits exposed to 311 mg methyl bromide/m3 showed moderate to
severe maternal toxicity. Decreased body weight and/or body weight
gain were noted. Clinical alterations included lethargy, right-sided
head tilt, ataxia, and lateral recumbency. The last three signs were
associated with significant histopathological lesions of the brain
(multifocal areas of inflammation of the meninges and/or bilaterally
symmetrical necrosis or spongiosis in the midbrain) in the
above-mentioned probe study with 545 mg methyl bromide/m3. At an
exposure level of 311 mg methyl bromide/m3, developmental effects
were observed consisting of decreased fetal weights, an increase in
the incidence of fused sternebrae, and an increase in the incidence of
malformations [18/23] (mostly missing gallbladder or missing caudal
lobe of the lung).
No adverse maternal, embryonal, or fetal effects were observed in
rabbits exposed to 78 or 156 mg methyl bromide/m3 (Breslin et al.,
1990).
Methyl bromide at 0, 0.5, 5, 25, or 50 mg/kg body weight was
administered in peanut oil, by gavage, to pregnant rats on days 5-20
of gestation (Peters et al., 1982). Signs of maternal toxicity were
evident in the two highest dose groups. Total resorption of embryos
was observed in the highest dose group and was considered to be the
result of the poor health of the pregnant rats and not a primary toxic
effect. In the control and 25 mg/kg groups, no effects on the skeleton
or internal organs were reported. In this study, methyl bromide was
not considered teratogenic and adverse effects on prenatal development
only occurred when maternal toxicity was present.
8.6 Mutagenicity and related end-points
Table 50 summarizes the results of tests for the genotoxic
activity of methyl bromide.
8.6.1 DNA damage
DNA adducts have been demonstrated in F344 rats, following oral
or inhalation administration 14C-methyl bromide. The adducts were
isolated from liver, lung, stomach, and forestomach and identified as
3-methylguanine, 7-methylguanine, and 0 6-methylguanine. Following
exposure by either route, the levels of adducts were higher in female
than in male rats and the guanine adducts were particularly prominent
in the stomach and forestomach (Gansewendt et al., 1991).
Alkylation of guanine-N-7 in the DNA of liver and spleen was
observed after treatment of male CBA mice with 14C-labelled methyl
bromide (4.9-5.0 mCi/mmol) by inhalation (340 mCi/kg body weight for
4 h) or i.p. injection (4.4 µmol/kg body weight) (Djalali-Behzad et
al., 1981). They noted that the extent of DNA alkylation in the liver
and spleen in vivo was 200 and 20 times lower, respectively, than
expected from the extent of the alkylation of haemoglobin and from the
relative reactivities of DNA and haemoglobin towards methyl bromide
in vitro.
Table 50. Mutagenicity tests with methyl bromide
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Reverse mutation Salmonella 0.02-0.2% +/- positive, Simmon et al.
typhimurium (in desiccators) TA 100 (1977)
TA 100, TA 98,
TA 1535, TA 1537,2 h
TA 1538
Reverse mutation Salmonella 500-5000 mg/m3, +/- positive, Moriya et al.
typhimurium 2 days TA 100, (1983)
TA 100, 1535
TA 98, 1537, 1538 TA 1535
Escherichia coli positive
WP 2 hcr
Reverse mutation Salmonella 500-50 000 mg/m3 +/- positive at Kramers et al.
typhimurium TA 100 (plate), 5 days 1900 mg/m3 (1985a)
TA 98 negative
SOS-umu (modified Salmonella 1.5 litre/min for positive Ong et al. (1987)
Ames test) typhimurium TA 1535/ 30 min
pSK 1002
Forward mutations Klebsiella pneumoniae 950-19 000 mg/m3, positive at Kramers et al.
streptomycin ur- pro- 20 h 4750 mg/m3 (1985a)
resistance
Forward mutation Escherichia coli 0.5-6 mmol/litre, 1 mutation/108 Djalali-Behzad et
Sd 4 1 h surviving al. (1981)
bacteria/mmol*h
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Forward mutation L5178Y mouse 0.03-30 mg/litre, positive Kramers et al.
24 h (1985a)
Sex-linked Drosophila 78 or 272 mg/m3, negative McGregor (1981)
recessive lethal melanogaster 5 h
Sex-linked Drosophila 750 mg/m3 (6 h) negative Kramers et al.
recessive melanogaster 375 mg/m3 (5 x 6 h) positive (1985a,b)
200 mg/m3 (15 x 6 h) positive
Sister chromatid Phytohaemagglutinin- 4.3% increased SCE Tucker et al.
exchanges (SCE) stimulated frequency from (1985)
human whole blood 10-16.84/cell
after 100-second
exposures
SCE cultured human 10-4-10-6 +/- positive Garry et al.
lymphocytes mol/litre (1990)
SCE bone marrow cells 47-778 mg/m3 dose response NTP (1992)
of exposed B6C3F1 6 h/day; 5 days/ observed at 14 days
mice week; 14 days and only; higher in
12 weeks females than males
same dose range negative
and exposure
Unscheduled DNA human diploid up to 70% +/- negative McGregor (1981)
synthesis fibroblasts 3 h
(human embryonic
intestinal cells)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Unscheduled DNA SPF male Wistar 10-30 mg/litre negative Kramers et al.
synthesis rats, primary liver (1985a)
Cell Syrian hamster 3890-31 120 mg/m3 negative Hatch et al.
transformation embryo cells 2-20 h in sealed (1983)
chambers
Chromosomal male and female CD 78 or 272 mg/m3 negative McGregor (1981)
aberrations (ex Sprague-Dawley) single 7 h or
rat bone marrow 7 h/day; 5 days
cells
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
BDF1 mice 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
(778 mg/m3) and
6-fold in females
(600 mg/m3); in
peripheral blood
increased 32-fold
in males (778 mg/m3)
and 3-fold in females
(600 mg/m3)
Table 50 (continued)
Test Test system Dose level, Metabolic Response Reference
concentration activation
presence/
absence
Micronucleus male and female 600-1712 mg/m3 polychromatic Ikawa et al.
F344 rats 6 h/day; 5 days/ erythrocytes with (1986)
week; 14 days micronuclei in the
bone marrow increased
10-fold in males
and 3-fold in females
at 1314 mg/m3
Micronucleus in peripheral 47-778 mg/m3 elevated (only at NTP (1992)
erythrocytes of 6 h/day; 5 days/ 14 days) responses
B6C3F1 mice week; 14 days over entire dose
range with greatest
response at 389 and
778 mg/m3 in
females; males less
responsive
same dose range and negative
exposure routine;
13 weeks
Dominant lethal rats, male CD 78 or 272 mg/m3 negative McGregor
(Sprague-Dawley) 7 h/day; 5 days (1981)
Starratt & Bond (1988a) found that methylation of DNA in maize
and wheat took place during fumigation using 14C- labelled methyl
bromide (48 mg/litre for 72 h). They identified 7-methylguanine and
1-methyladenine as major products and lesser amounts of
3-methyl-cytosine and 3- methyladenine and 7-methyladenine; 0.5-1% of
the guanine residues in the DNA were methylated. Methylation of solid
samples of calf thymus DNA and salmon testes DNA gave similar results,
except that the quantity of 1-methyladenine exceeded that of
7-methylguanine (Starratt & Bond, 1988b).
In contrast, a different pattern of methylated bases was found
when solutions of DNA were treated with 14C-labelled methyl bromide.
Here, predominantly 7-methylguanine with a small amount of
3-methyladenine and only traces of 1-methyladenine and
3-methyl-cytosine were identified (Starratt & Bond, 1988b). The
authors noted that these results agreed better with the in vivo
studies of Djalali-Behzad et al. (1981).
An in vitro assay for unscheduled DNA synthesis (UDS) was
carried out in human diploid fibroblasts with exposures of 3 h and
concentrations up to 70% in air. No increase in UDS was found
(McGregor, 1981).
As measured by autoradiography, methyl bromide (tested at 10-30
mg/litre) did not induce unscheduled DNA synthesis in primary cultures
of rat hepatocytes treated in air-tight bottles (Kramers et al.,
1985a).
8.6.2 Mutation
Methyl bromide has been tested in various in vitro and in vivo
test systems (Table 50).
Methyl bromide was mutagenic to Salmonella typhimurium TA 100
when tested at concentrations of 0.02-0.2%, in desiccators, in the
absence of an exogenous metabolizing system (Simmon et al., 1977).
Positive results were also obtained in strain TA 100 in a liquid
assay (tested at 10-1000 mg/litre) and a plate assay (tested in closed
containers at concentrations of 500-50 000 mg/m3). Methyl bromide
was mutagenic at concentrations of 1900 mg/m3 and higher (plate
tests), and concentrations of 285 mg/litre (medium) and higher
(suspension test). The activity in the plate assay was unaffected by
the presence of liver homogenates from Aroclor(R)-induced rats
(Kramers et al., 1985a).
Methyl bromide (tested at 0.5-5 g/m3 in a closed container) was
mutagenic to S. typhimurium TA 1535 and TA 100 and to E. coli WP2
her, in the absence of an exogenous metabolic system (Moriya et al.,
1983).
An aqueous solution of methyl bromide (tested at 0.5-6 µmol/
litre) induced mutations to streptomycin independence in E. coli
Sd-4 (Djalali-Behzad et al., 1981).
Methyl bromide showed no mutagenic activity in a modified Ames
test using the impingement (in situ) test system, but, with the SOS
umu-test, this compound induced a significant SOS response, even
with only 30 min impingement (Ong et al., 1987). The SOS function
induced by genotoxic agents was detected by a colorimetric measurement
of beta-galactosidase activity encoded by the 1acZ gene, which is
regulated by the Umu operon.
Mutations to streptomycin resistance were induced in the
fluctuation test with Klebsiella pneumoniae at concentrations of
4750 mg methyl bromide/m3 and higher (tested at 950- 19 000 mg/m3)
(Kramers et al., 1985a).
In barley, a few mutations were induced after treatment of
kernels with 1.4 mmol methyl bromide/litre for 24 h, in closed vessels
(Ehrenberg et al., 1974).
A sex-related recessive lethal assay was carried out on
Drosophila melanogaster (McGregor, 1981). Male strain Oregon K
Drosophila were exposed to 78 or 272 mg methyl bromide/m3 for 5 h
and were mated on days 1,3, or 8 following exposure. The F1 progeny
from these matings were then mated brother to sister, 1-4 days after
emergence from pupae and the F2 generation was ex-amined for the
absence of wild-type males. At 78 mg methyl bromide/m3, the
frequencies of lethal mutations in the F2 generations from one of the
stocks were elevated, but this was not thought by the authors to be
compound-related as these were higher than those at the higher
concentration range.
In a sex-linked recessive lethal test on Drosophila
melanogaster , flies of the Berlin K strain were exposed to methyl
bromide at concentrations of 70-750 mg/m3 for increasing periods;
mutation frequencies were significantly increased at the highest
nontoxic concentrations. At a concentration of 600 mg/m3, all flies
died within a short time during the fourth day of exposure (Kramers et
al., 1985a). Prolongation of the exposure time permitted lower
concentrations to be detected as mutagenic; 487 mg/m3 for 5 x 6 h
and 200 mg/m3 for 15 x 6 h were effective exposures whereas
treatment with up to 750 mg/m3 for 6 h was not sufficient to produce
significantly increased mutation frequencies. The mutagenic effect of
methyl bromide was most pronounced in postmeiotic germ cell stages
(Kramers et al., 1985b).
Treatment of L5178Y mouse lymphoma cells with 0.03-30 mg methyl
bromide/litre, in air-tight bottles, resulted in a dose-related
increase in 6-thioguanine- and bromodeoxyuridine-resistant mutants
(Kramers et al., 1985a).
8.6.3 Chromosomal effects
8.6.3.1 In vitro studies
Exposure of human lymphocyte cultures to 4.3% methyl bromide for 100
seconds increased the frequency of sister chromatid exchanges (SCEs)
from 10.0 to 16.8 per cell (Tucker et al., 1985, 1986).
Human G0 lymphocytes were treated with methyl bromide (0-24
µg/ml) for 30 min with, and without, addition of rat liver homogenate.
After culture, the prepared slides were studied and dose-related
sister chromatid exchanges (SCEs) and chromosome aberrations (CAs)
were found. Methyl bromide significantly induced chromosome
aberrations in the presence of S-9 (Garry et al., 1990).
Inhalation of methyl bromide gas induced mitotic recombination in
somatic cells (somatic wing-spot assay) of Drosophila melanogaster
(Katz, 1985, 1987). Third instar larvae trans-dihybrid for two
recessive wing-hair mutations were exposed via inhalation to methyl
bromide (0-20 000 mg/m3) for 1 h. Wings of surviving adults were
scored for the presence of clones of cells possessing malformed wing-
hairs. Small and large, single (indicating a variety of genetic
alterations) as well as twin spots (from mitotic recom bination) were
found.
8.6.3.2 In vivo studies
The results of in vivo mammalian tests for chromosomal
aberrations in rat bone marrow were negative (McGregor, 1981).
Micronuclei formation was studied on F-344 rats and BDF1 mice
exposed to 0, 600, 778, 1011, 1314, or 1712 mg methyl bromide/m3 (0,
154, 200, 260, 338, or 440 ppm) for 6 h/day and 5 days/week for 14
days (Ikawa et al., 1986). In the surviving mice, poly-chromatic
erythrocytes with micronuclei in the bone marrow increased 10-fold in
males (778 mg/m3) and 6-fold in females (600 mg/m3) and those in
peripheral blood increased 32-fold in males (778 mg/m3) and 3-fold
in females (600 mg/m3). In rats, poly-chromatic erythrocytes
containing micronuclei in the bone marrow increased 10-fold in males
and 3-fold in females (both 1314 mg/m3).
Increases in SCEs and micronuclei were observed in the bone
marrow cells of male and female B6C3F1 mice exposed via inhalation to
a concentration of 778 mg methyl bromide/m3 (200 ppm) for 14 days (6
h/day, 5 days/week), the increases were more pronounced in female
mice. In contrast, no significant increases in either SCEs or
micronuclei were observed in male or female mice exposed via
inhalation to a concentration of 467 mg methyl bromide/m3 (120 ppm)
for 13 weeks (NTP, 1992).
8.6.4 Cell transformation
Transformation in Syrian hamster embryo cells by SA/adenovirus
was not enhanced by exposure to 4000-16 000 mg methyl bromide/m3
(1000-4000 ppm) for 2 or 20 h, in sealed chambers (Hatch et al.,
1983).
8.7 Carcinogenicity and related end-points
8.7.1 Gavage studies
Danse et al. (1984) administered methyl bromide, by gavage, to
groups (10 male + 10 female) of weanling Wistar rats for 90 days at
doses of 0.4, 2, 10, or 50 mg/kg body weight. At the highest dose
level of 50 mg/kg, squamous cell carcinomas of the forestomach
developed in 13 out of 20 animals. A marked diffuse hyperplasia of the
epithelium of the forestomach was seen in all animals in this group.
However, from subsequent examination of the slides from this study it
was concluded that the forestomach lesions reported at 50 mg
represented inflammation and hyperplasia rather than malignant lesions
(Pesticide & Toxic Chemical News, 1984).
Boorman et al. (1986) administered methyl bromide in peanut oil
(50 mg/kg body weight), by gavage, to groups of 15 male Wistar rats
for 13 weeks (five times a week) with a 12 week recovery period; other
groups were exposed for 17, 21, or 25 weeks to methyl bromide;
necropsies were performed at 13, 17, 21, and 25 weeks. At week 13,
inflammation, acanthosis, fibrosis, and pseudo- epitheliomatous
hyperplasia in the forestomach were observed microscopically in dosed
animals. At week 25, all rats had hyperplastic lesions of the
forestomach that were more severe than those at 13 weeks. Evidence of
malignancy, seen in one rat, was considered to be a very early
carcinoma. In the stop treatment group that had received methyl
bromide for 13 weeks, there was regression of the stomach lesions, but
at the 12-week final sacrifice, adhesions, fibrosis, and mild
acanthosis remained (Table 43).
Hubbs & Harrington (1986) reported similar results. Gross and
microscopic alterations were most pronounced in the non-glandular part
of the stomach of the treated rats. Histological change in the
squamous epithelial portion of the stomach included ulceration and
pseudoepitheliomatous hyperplasia characterized by hyperkeratosis,
acanthosis, and epithelial peg formation. Fibrosis, foreign material
(hair) and subacute to chronic inflammation were found in the
muscularis mucosa, submucosa, and tunica muscularis of some rats. A
30-60 day recovery period was accompanied by marked, but incomplete,
regression of lesions. No evidence of malignancy was seen in the
stomachs of treated rats (Hubbs & Harrington, 1986).
A two-year, oral, carcinogenicity study was carried out on 60
male and 60 female F-344 rats fed on diets fumigated with methyl
bromide (Mitsumori et al., 1990). Diets contained 80, 200, or 500 mg
total bromide/kg, respectively, (methyl bromide concentration being
<20 mg/kg), the controls were fed commercial basal diet (containing
30 mg bromide/kg) and a diet containing 500 mg potassium bromide
(KBr)/kg. No carcinogenic effects were observed (see also section
8.4.1.2).
8.7.2 Inhalation studies
In lifetime, inhalation, carcinogenicity studies (Table 49),
groups of 90 male and 80 female Wistar rats were exposed to methyl
bromide concentrations of up to 350 mg/m3 for 6 h/day, 5 days/ week,
for up to 130 weeks (Dreef-van der Meulen et al., 1989; Reuzel et al.,
1991). Methyl bromide was a mild nasal irritant at all exposure
concentrations. There was no increased incidence of neoplasms.
In a 2-year inhalation study (NTP, 1992) on male and female
B6C3F1 mice exposed to 39, 128, or 389 mg methyl bromide/m3 (Table
49), there was no evidence of carcinogenic activity in either male or
female mice.
8.8 Special studies
8.8.1 Target organ effects
8.8.1.1 Inhalation studies
Studies on laboratory animals have shown that, following
inhalation exposure, the primary target organs are the brain, kidney,
nasal cavity, heart, adrenal gland, lung, liver, and testis (Irish et
al., 1940; Hurtt et al., 1987; Eustis et al., 1988) and lung (Bond et
al., 1985; Kato et al., 1986; Jaskot et al., 1988) (Table 48).
Extensive target organ studies have been carried out by Eustis et
al. (1988) on B6C3F1 mice and F-344 rats after inhalation of 622 mg
methyl bromide/m3 for 6 h/day, 5 days/week for 6 weeks. The
histopathological changes observed were as follows:
Brain: neuronal necrosis in the internal granular layer of the
cerebellar folia (mice); neurosis and loss of neurons in the cerebral
cortex, hippocampus, and thalamus (rats).
Kidney: nephrosis, which occurred in all treated mice, was
charac-terized by degeneration, necrosis, and sloughing of the
epithelium of convoluted tubules in the renal cortex. In rats, there
was dilatation of tubules with atrophy of the epithelium, hyaline and
granular casts in the tubules, and increased cytoplasmic basophilia,
indicative of epithelial regeneration. There was minimal nephrosis in
one female rat.
Testes: degeneration of the testes occurred frequently in exposed
mice and mild bilateral atrophy was present in two. Degeneration and
atrophy of seminiferous tubules of the testes was noted in several
exposed rats.
Nasal cavity: exposed male mice had various levels of degeneration
and atrophy of the nasal olfactory epithelium. Minimal degeneration of
the olfactory epithelium was seen in only one female mouse. In male
and female rats killed after three exposures to methyl bromide, there
was moderate to marked degeneration of the olfactory epithelium of the
ethmoturbinates and posterior dorsal nasal septum. There was no
inflammatory response. In rats, killed or dying after 10 or more
exposures, actual degeneration of the olfactory epithelium was minimal
or mild, but there was focal or multifocal loss of olfactory sensory
cells.
Heart: degeneration of the myocardium primarily occurred in treated
males with minimal myocardial degeneration in two female mice.
Degeneration of the myocardium occurred more frequently and with
greater severity in treated than in control male and female rats.
There were increased numbers of mononuclear and fusiform nuclei that
may represent interstitial cells, the foci showing a relative increase
in fine reticular fibres, and scattered clear vacuoles.
Adrenal gland: exposed female mice showed diminished cellularity of
the x-zone of the adrenal cortex and the affected cells contained less
cytoplasm and smaller, more hyperchromatic nuclei than normal cells.
Minimal to mild cytoplasmic vacuolation occurred in the adrenal cortex
of exposed rats.
Liver: individual cell necrosis was noted in the liver of several
treated rats and was generally more severe in affected males than
females. In three males and one female, an inflammatory reaction
occurred. There were no data for mice.
Thymus and spleen: thymic atrophy and lymphoid depletion of the
spleen was noted in mice and rats of both sexes.
8.8.2 Neurotoxicity
Irish et al. (1940) examined the effects of a wide range of
inhalation exposure conditions in rats, rabbits, guinea- pigs, and
monkeys, using visual inspection to assess behavioural deficits. They
observed that nearly 60% (34 out of 58) of the rabbits surviving
exposure to 128 mg/m3 (33 ppm) developed hindlimb paralysis during
the 6-month exposure period. This was the lowest concentration at
which definite neurobehavioural effects could be detected in the
rabbit, the other species being unaffected at this concentration.
Anger et al. (1981) studied the neurobehavioural effects of long-
and short-term methyl bromide inhalation exposure on Sprague-Dawley
rats and New Zealand White rabbits using more sensitive tests than
those available in 1940 when the study of Irish et al., was performed.
Exposure to 252 mg/m3 (65 ppm) for weeks caused sig-nificantly
reduced eye blink responses and nerve conduction velocity in rabbits,
but not in rats. No effect on nerve conduction velocity, open-field
activity, or coordination in rats could be seen after extended
exposure at 214 mg/m3 (55 ppm) for 36 weeks. In further studies
(Russo et al., 1984), rabbits did not show any neurobehavioural
effects after inhalation exposure to 105 mg methyl bromide/m3 (27
ppm) for 7.5 h per day, 4 days per week over 8 months. A separate
group of rabbits exposed to 252 mg/m3 exhibited severe neuromuscular
effects followed by partial recovery, 6-8 weeks after exposure ceased.
Behavioural effects in rats following repeated exposure to methyl
bromide at concentrations of 778 or 1167 mg/m3 (200 or 300 ppm) for
3 weeks were investigated by Ikeda et al. (1980). Relatively prolonged
(12 days) motor incoordination (rotarod) was observed.
Conditioned taste aversion induced by inhalation exposure to
methyl bromide in rats has been reported (Miyagawa, 1982). Rats kept
under a water restriction schedule for 7 days, were permitted access
to 0.3% (w/v) sodium saccharin, and were exposed to 0, 97, 195, or 389
mg methyl bromide/m3 (0, 25, 50, or 100 ppm) for 4 h. Three days
after exposure, saccharin preference tests were carried out, revealing
dose-dependent saccharin aversion in the exposure group (Miyagawa,
1982).
Male Swiss-Webster mice exposed to methyl bromide concentrations
up to 3500 mg/m3 showed no effects on single-task passive avoidance
but had significantly lower rotarod performance (Alexeeff et al.,
1985).
Effects of methyl bromide on locomotor activity were observed in
five groups of three rats after exposure to 245, 486, 731, or 972
mg/m3 (63, 125, 188, or 250 ppm) for 8 h. The locomotor activity was
measured in an activity cage before, and immediately after, the
exposure, shown as an average count per hour in 12-h blocks (day -2
(before exposure) and day 1) or 4-h blocks (day 0). In the 245 mg/m3
group, the activity was the same as that in the control group. The
activity was decreased by 731 mg/m3 and in-hibited strongly by 972
mg methyl bromide/m3 for up to 6 h after exposure (Honma et al.,
1985).
The effects of methyl bromide exposure on the thiopental-induced
reduction of righting reflex of rats was investigated. Three groups of
eight rats were exposed to 0, 245, or 486 mg/m3 (63 or 125 ppm) and
then were injected i.p. with 60 mg thiopental/kg to induce a loss of
righting reflex. The time of loss of righting reflex was measured and
group means were calculated. In the control group, two out of eight
rats lost the righting reflex, while in the 245 and 486 mg methyl
bromide/m3-exposure groups, the loss of righting reflex was observed
in all rats and was considerably more prolonged (Honma et al., 1985).
Kato et al. (1986) described neurological signs in rats, such as
paralysis and ataxia, after exposure to 1167 or 1556 mg methyl
bromide/m3, for 4 h/day over 6 weeks (section 8.4).
The acute effects of methyl bromide on electroencephalographic
activity and on sleep-wakefulness and circadian rhythms have been
investigated in rats (Tanaka et al., 1988). Slowing of the EEG
frequency in the wakefulness (W) stage and spike-wave activity
appeared at a single subcutaneous dose of 135 mg methyl bromide/kg
body weight. These abnormal EEG activities did not occur at lower dose
levels. Administration of methyl bromide at doses of 45, 15, or 5
mg/kg produced dose-related changes in amounts of W, non-REM sleep,
and REM sleep and in their circadian rhythms. Pretreatment with
glutathione effectively lessened the detrimental effects of methyl
bromide on sleep-wakefulness and its circadian rhythms and increased
the LD50 (Tanaka et al., 1988).
The effects were studied on regional brain glutathione- S-
transferase (GST) activity and the concentrations of glutathione
(GSH), monoamines, and amino acids in F-344 rats exposed to methyl
bromide (Davenport et al., 1992). Exposure to 584 mg/m3 was for 6
h/day for five days. While no histological evidence of brain lesions
was noted, there was GSH depletion and GST inhibition in the frontal
cortex, caudate nucleus, hippocampus (examined for GSH only), brain
stem, and cerebellum. No significant changes were observed in the
concentrations of monoamines (only measured in males), but there were
increases in the concentrations of aspartic acid and glycine in the
frontal cortex and of aspartic acid only in the cerebellum (only
measured in males).
Honma et al. (1982) exposed groups of 5-6 SD rats to methyl
bromide concentrations of 39-467 mg/m3 for 24 h or to 4-40 mg/m3
for 3 weeks. After sacrifice (microwave), the brain was dissected and
analysed for monoamines. A reduction in the norepinephrine (NE)
contents of the hypothalamus and cortex + hippocampus was found at
concentrations of 390 mg/m3 and above (24-h study) or 39 mg/m3
(3-week study), whereas levels of dopamine (DA), sero-tonin (5HT), and
acetylcholine (ACh), were only slightly affected by exposure. In more
detailed studies, rats were exposed to 120, 240, 486, or 973 mg/m3
for 8 h (Honma, 1987; Honma et al., 1987). Decreases in DA and NE and
increases in the metabolites homovanillic acid (HVA) and
3-methoxy-4-hydroxyphenylglycol (MHPG) were observed in various
regions of the CNS in rats exposed to 319 mg/m3. The maximal effects
were obtained 0 or 2 h after exposure with the largest changes in DA
(-24%) and NE (-34%) seen in the striatum. HVA and MHPG levels were
increased primarily in the frontal cortex (81%) and striatum (38%).
The tyrosine hydroxylase (TH) activity in the striatum, hypothalamus,
frontal cortex, midbrain, and medulla oblongata was measured in brain
homogenates from rats exposed to 62-973 mg/m3 (16-250 ppm) for 8 h
(Honma et al., 1991). The rats were sacrificed serially (decapitation)
between 0 and 16 h after exposure. TH was also measured in vivo
following administration of decarboxylase inhibitor. Exposure to
methyl bromide dose-dependently inhibited both TH activity in vitro
and in vivo . TH activity in the hypothalamus was most sensitive to
methyl bromide compared with the other brain areas. The authors
suggested that methyl bromide reduces catecholaminergic neuronal
activity in the brain via inhibition of TH activity.
The direct effects of methyl bromide on the rat brain have been
investigated using a two-probe (right striatum and left ventricle)
microdialysis method (Honma, 1992). Methyl bromide (1 µg or 0.5
µg/µlitre) dissolved in 10% ethanol/90% artificial cerebrospinal fluid
was perfused through the ventricle probe at a rate of 2.5 µlitre/min
and changes in 3,4-dihydroxyphenylacetic acid (DOPAC), HVA, and
5-hydroxyindoleacetic acid, a serotonin metabolite, (5HIAA) content in
the striatum perfusate were measured. DOPAC and HVA increased
following the initiation of ventricle perfusion but the 5HIAA content
of the striatum perfusate was reduced. The increases in DOPAC and HVA
persisted after perfusion and the decreased 5HIAA level returned to
normal. A concentration of 0.1 µg methyl bromide/µlitre had no effects
on DOPAC and HVA levels, though the 5HIAA level was reduced. These
findings support the findings of an increase in DOPAC and HVA in the
brain homogenate of rats exposed to methyl bromide (Honma, 1987; Honma
et al., 1987) indicating that dopamine metabolites in the
extracellular, and, probably, intracellular, regions of dopamine nerve
terminals were increased by methyl bromide itself. The extracellular
level of the 5HIAA in microdialized perfusate was reduced by
intraventricularly administered methyl bromide (Honma, 1992).
Systematic neuropathological studies of their central and
peripheral nervous systems were carried out on male Wistar rats
exposed to methyl bromide (1128 or 1945 mg/m3) for 6 h/day, 3
days/week for 3-8 weeks (Furuta et al. 1993). Among the rats exposed
to 1945 mg methyl bromide/m3 for 10-18 days, the following
neuropathological changes were noted: axonal degeneration of
myelinated fibres at the cervical level of the fasciculus gracilis and
the necrosis and atrophy of neurons in the caudate-putamen, thalamus,
and cingulate cortex. Rats exposed to a concentration of 1128 mg
methyl bromide/m3 for 8 weeks did not show any abnormalities.
8.8.3 Immunotoxicity
No data on the immunotoxicity of methyl bromide are available.
8.9 Factors modifying toxicity; toxicity of metabolites
Cysteine has been shown to reduce the toxicity of methyl bromide,
when administered to rats, mice, and rabbits, orally or s.c., 30 min
before, or s.c. within 5 min following, acute poisoning (Mizyukova &
Bakhishev, 1971). Cysteine treatment prevented the death, paralysis,
paresis, and spasms that developed on the 3rd-4th days after methyl
bromide inhalation in other animals.
8.10 Mechanisms of toxicity - mode of action
The mode of action of methyl bromide is still not understood.
Proposed mechanisms of toxicity include the direct cytotoxic effect of
the intact methyl bromide molecule or toxicity due to one of its
metabolites. The bromide ion concentrations are insufficient to
explain methyl bromide toxicity, but it may be related to its
alkylating ability.
Honma et al. (1985) concluded that the CNS toxicity seems to be
due to the methyl bromide molecule itself or the methyl moiety
incorporated into the tissue and does not appear to be attributable to
inorganic bromide or methyl alcohol (Honma et al., 1985). In humans
showing medium or severe toxic symptoms of the CNS, methanol
concentrations in the blood ranged between 200 and 3000 mg/litre
(Swartz et al., 1981).
The direct cytotoxic effect of methyl bromide on HeLa cells in
vitro has been shown, whereas bromide appeared not to cause cell
damage (Nishimura et al., 1980).
Methyl bromide reacted in vitro with a number of SH enzymes and
caused progressive and irreversible inhibition (Lewis, 1948). DNA and
protein alkylation by methyl bromide have previously been discussed
(section 6.3).
The role of glutathione (GSH) in reducing toxicity is also not
clear. Studies on the possible role of glutathione are given in
section 6.3. One hypothesis for the toxicity of methyl bromide
proposes the formation of a reactive species through a methyl
bromide-glutathione conjugation process, but it is more probable that
glutathione acts as a detoxifying agent.
9. EFFECTS ON HUMANS
Humans can be exposed to methyl bromide via inhalation, by skin
contact with the compound, or through residues in food that has been
fumigated with the gas to control pests. Exposure is also possible
through drinking- water from wells contaminated with leaching water.
In this section, only direct exposure to gaseous or liquid methyl
bromide is considered.
Since the first case reported by Schuler in 1899, there have been
hundreds of cases of methyl bromide poisoning involving fatalities,
systemic poisoning, skin and eye injuries, and damage to the central
nervous system (von Oettingen, 1946, 1964; Hine, 1969; Torkelson &
Rowe, 1981; Weller, 1982; Alexeeff & Kilgore, 1983).
Table 51 shows acute cases up to 1983 reported throughout the
world. Up to 1955, most cases were from chemical manufacture or fire
extinguisher incidents. Since this date, cases of poisoning from its
use as a fumigant have predominated.
9.1 Clinical findings
It is important to note that the manifestations of methyl bromide
poisoning may be delayed. The latent period may vary from 2 to 48 h
(Holling & Clarke, 1944).
The acute and long-term effects of methyl bromide can be divided
broadly into two categories: neurological and non-neurological.
The principal non-neurological symptoms reported after acute
inhalation of methyl bromide are associated with the respiratory
system. Chest pain or difficulty in breathing have been reported,
which is consistent with pathological findings at autopsy including
pulmonary oedema, bronchopneumonia, congestion, and haemorrhage
(Holling & Clarke, 1944; Hine, 1969).
In fatal poisoning, the early symptoms and signs are headache,
visual disturbances, nausea and vomiting, smarting of the eyes,
itching of the skin, listlessness, vertigo, and tremor. Progression is
usually rapid, with the development of convulsions, often with a
Jacksonian-type of progress, fever, tachypnoea associated with signs
of severe pulmonary oedema, cyanosis, pallor, and death. Several
neuropsychiatric signs and symptoms, such as mental confusion, mania,
muscular twitches, and slurring of speech, may precede death (Wyers,
1945; Sax et al., 1984; Gosselin et al., 1984).
Table 51. Methyl bromide poisoning incidents up to 1955 and between 1955 and 1983a
Resulting from: Fatalities Systemic Poisoning Skin Injuries Eye Injuries Other or unspecified injuries
up to 1955- up to 1955- up to 1955- up to 1955- up to 1955-
1955 1983 1955 1983 1955 1983 1955 1983 1955 1983
Chemical manufacture, 16 1 108 45 26 4 0b 0b
filling, storage,
transportation, or
disposal
Use or leaking fire 24 11 38 28 10 0 0b 0b
extinguishers
Uses as a fumigant 44 16 3 298 0 145 0b 57 0b 78
a Adapted from: Alexeeff & Kilgore (1983). Data up to 1955 were taken from von Oettingen (1955).
b No cases known.
The clinical picture in non-fatal poisoning is extremely
variable. Fatigue, blurred or double vision, nausea, and vomiting are
frequent; incoordination, tremors, convulsions, exaggeration of the
patellar reflexes, and a positive Babinski's sign may develop. Nearly
every type of nervous disturbance has been reported. The pulmonary
symptoms are comparatively slight (Sax et al., 1984). Low-level,
short-term exposures to the vapour have produced a syndrome of
polyneuropathy without overt central manifestations, characterized by
persistent numbness in the hands and legs, impaired superficial
sensation, muscle weakness, unsteadiness of gait, and absent or
hypoactive distal tendon reflexes (Kantarjian & Shaheen, 1963). Victor
& Adams (1980) stated that the signs and symptoms of chronic bromide
poisoning intoxication consisted of dizziness, drowsiness,
irritability, emotional lability, impairment of thought or memory,
and, in severe cases, delirium and mania or stupor or coma.
Locally, methyl bromide is an intense vesicant on human skin. The
blisters produced by methyl bromide are enormous, but rarely deep
enough to destroy the entire skin layer, even though it may produce
severe burns (Watrous, 1942; Butler et al., 1945; Wyers, 1945).
Both central and peripheral neurological deficits may persist;
organic brain syndrome has occurred (Greenberg, 1971; Goulon et al.,
1975). Profound psychological depression can occur during prolonged
convalescence (Hine, 1969). Late sequelae include bronchopneumonia
after severe pulmonary lesions, renal failure, and severe weakness
with, or without, evidence of paralysis. Renal failure due to tubular
necrosis may be a late sequela, but this is uncommon and usually of
mild proportions (Benatt & Courtney, 1948; Prain & Smith, 1952). There
have been occasional reports of jaundice, elevations of liver enzyme
activity in serum, and abnormal liver function tests suggestive of
mild hepatotoxicity after exposure to methyl bromide (Verberk et al.,
1979). In a 6- year-old boy, signs of liver involvement in conjunction
with encephalopathy after exposure to methyl bromide vapour were at
first mistaken for Reye's syndrome (Shield et al., 1977).
Recovery is frequently prolonged and there may be permanent
injury, commonly characterized by sensory disturbances, weakness,
disturbances of gait, irritability, and blurred vision.
The effects of methyl bromide on the nervous system, based on
case histories, showed that dysfunction affected the peripheral and
optic nerves, cerebellar connections, and certain spinal cord tracts
(Carter, 1945). Brain stem damage was described in a man who died
after 30 days of unconsciousness following methyl bromide exposure
(Cavanagh, 1992; Squier et al., 1992). Serum bromide levels, 2 days
after admission, were 160 mg/litre (normal 3-4 mg/litre). At
post-mortem examination, the brain showed mild generalized swelling,
normal ventricles, and well- defined symmetrical lesions, including
loss of neurones, in the mammilary bodies and inferior colliculi. The
cerebellar dentate nuclei had occasional foci of neuronal loss; other
brain stem regions were normal. The spinal cord was normal, but dorsal
root ganglia had scattered nodules of neuronal loss. Nerve roots and
thoracic peripheral nerve showed sparse chronic inflammatory exudate
and patchy axonal and myelin loss. The thyroid gland was densely
infiltrated with lymphocytes, plasma cells were rare, and there was no
significant fibrosis. The lungs were slightly oedematous and
congested. In another case of methyl bromide poisoning, a woman
remained in a coma for 4 years and 8 months before death. At
post-mortem, there was necrosis of both colliculi, with gliosis in the
upper brainstem, reticular formation, and moderate changes in the
dentate and pontine nuclei (Goulon et al., 1975).
Various visual disturbances have been reported following acute
methyl bromide poisoning. These include blurring of vision, diplopia,
lacrimation from eye irritation, accommodative disturbance, and
central scotomata for shades of green (Grant, 1974). Ocular findings
in a case of chronic methyl bromide poisoning included persistent
bilateral decrease in vision, temporal optic nerve head pallor, marked
attenuation of visual evoked response amplitude with normal latencies,
normal electro-retinogram but abnormal electro-oculogram, and
deuteranomalous (green) defect (Chavez et al., 1985).
Electrophysiological studies have been performed on patients as
part of a clinical investigation of the neurological effects of methyl
bromide poisoning (Goulon et al., 1975; Verberk et al., 1979; Audry et
al., 1985; Lopez et al., 1986a, b; Uncini et al., 1990; Mazzini et
al., 1992; Hustinx et al., 1993). Abnormal findings included
epileptiform patterns in electroencephalograms, enlarged and
asymmetric somatosensory evoked potentials, and evidence of axonal
neuropathy in peripheral nerves.
9.1.1 Bromide levels in body tissues and fluids
In cases of methyl bromide poisoning, methyl bromide itself has
been detected in human tissue on only one occcasion. This may be due
to the absence of methyl bromide or, more probably, to its very short
half-life in tissues, as well as difficulties in analysis. There is an
inconsistency in the data as to whether there is a correlation between
bromide levels and the symptoms of methyl bromide poisoning. Some
authors have suggested a direct correlation between blood bromide
levels and the degree of intoxication (Rathus & Landy, 1961; Hine,
1969): 400 mg/litre (ppm) - severe disability and death in some cases;
250 mg/litre (ppm) - convulsive seizure and sometimes death; 176
mg/litre (ppm) - slight residual ataxia; 135 mg/litre (ppm) - moderate
disability; 100 mg/litre (ppm) or less -recovery, but symptoms of
poisoning have been reported at bromide levels as low as 28 mg/litre
blood and severe symptoms at blood bromide levels of 120 mg/litre
(Rathus & Landy, 1961). Brenner (1978) and Bowers & Onoreski (1990)
considered that the toxic threshold level for bromide in serum in
humans was 500 mg/kg, though effects were observed in patients with
lower levels.
However, Weller (1982) disputed a correlation between the bromide
levels in serum and the severity of poisoning. In workers in a methyl
bromide manufacturing plant, no definite association was found between
symptoms and urine bromine concentrations (Kishi et al., 1991). A
direct association between serum bromine concentrations and the
severity of neurological symptoms was not found in a poisoning
incident involving greenhouse workers (Hustinx et al., 1993).
9.1.2 Dermal exposure
Dermal exposure can result from direct contact with liquid methyl
bromide, e.g., from accidental splashing, or through contact with
contaminated boots, clothing, bandages, or gloves (Alexeeff & Kilgore,
1983). These articles are often made of rubber, which can absorb
methyl bromide (Watrous, 1942). Direct eye injury or irritation can
also occur. Dermal exposure to gaseous methyl bromide can also cause
poisoning.
When liquid methyl bromide is spilled on the skin, it evaporates
rapidly producing a cool, but not a cold, or burning sensation
(Watrous, 1942). Repeated application of the liquid caused a burning
or tingling sensation in the skin, which, in more severe cases, led to
a sense of numbness, followed later by aching. In the early stages,
the skin appeared red and slightly swollen, with blisters and
second-degree burns appearing after 2-12 h in severe cases. In less
severe cases, an itching dermatitis may develop after a latent period
of about seven days (Watrous, 1942).
Cases of skin burns have been reported during fumigation (Bruhin,
1942) and from methyl bromide fire extinguishers (Butler et al.,
1945). Bruhin (1942) reported three cases of dermal exposure during
bulk fumigation of a mill. Methyl bromide poisoning occurred due to
percutaneous absorption; the men were using breathing apparatus. All
three men were exposed to the same conditions, they suffered first or
second degree skin burns and one died as a consequence of the
exposure.
Skin lesions occurred in 6 patients who were exposed to methyl
bromide during the fumigation of a castle (Zwaveling et al., 1987;
Hezemans-Boer et al., 1988). They had adequate airway protection, so
poisoning was by dermal exposure and not by inhalation. Exposure to
high concentrations of methyl bromide (about 40 g/m3) for 40 min led
to redness and blistering of the skin. Standard protective clothing
(overalls - 35% cotton/65% polyester), worn over normal clothing, PVC
gloves, and working shoes did not prevent this. Redness, oedema, and
blistering of the skin were limited to areas where perspiration is
relatively high, i.e., armpits, groin, genitals, and the skin under
the waistbelt. The authors suggested that methyl bromide absorption
might be increased in this partly lipophilic (sebaceous glands),
partly hydrophilic (sweat glands) environment, perhaps leading to
increased absorption through the skin as well. The skin in these parts
is also thinner, favouring absorption. Plasma bromide levels were
highest about 13 h after exposure (mean 9.0± 1.4 mg/litre) and fell in
subsequent hours (mean 6.8±2.3 mg/litre; 25 h after exposure),
suggesting absorption of methyl bromide through the skin. Since the
published half-life of bromide is about 100 h, the falls in plasma
levels must be due almost exclusively to the distribution of bromide
in tissues. No systemic effects were noted.
9.1.3 Inhalation
Inhalation is the primary route of exposure. Poisoning has
occurred following acute, short-, and long-term exposures. Acute cases
have occurred involving spilling or leaks while handling methyl
bromide or where the persons were unaware of its presence (Alexeeff &
Kilgore, 1983).
In spite of stronger regulations for the safe and licensed use of
methyl bromide, cases of poisoning still occur. Cases have been
reported in California (Edmiston & Maddy, 1987; Maddy et al., 1990)
and incidents of poisoning have been reported with occupational
exposure (Cantineau et al., 1988; Herzstein & Cullen, 1990), in
members of the general public (Goldman et al., 1987; Polkowski et al.,
1990), and in burglars entering fumigated premises (Marraccini et al.,
1983; O'Neal, 1987).
9.2 General population exposure
9.2.1 Poisoning incidents
Earlier poisoning incidents involving the general public were
mainly from the methyl bromide in fire extinguishers. More poisoning
incidents have involved unauthorized entry into fumigated buildings or
persons living near fumigated buildings, greenhouses, or fields being
fumigated with methyl bromide.
9.2.1.1 Poisoning associated with fire extinguishers
Incidents involving fire extinguishers filled with methyl bromide
often involved exposure to the gas by inhalation and severe burns when
the liquid was inadvertently squirted on the feet or clothing. Butler
et al. (1945) described two cases where drivers suffered burns after
extinguishing a fire in an armoured car. Both recovered after 2 and 8
weeks, respectively. Twenty-two cases of methyl bromide poisoning in
incidents involving fire extinguishers in ships, including 6
fatalities, were reported between 1939 and 1945 (Holling & Clarke,
1944; Clarke et al., 1945). In another incident, 8 boys, 6 of whom
died, were exposed accidentally to methyl bromide from a fire
extinguisher (Prain & Harvey Smith, 1952). Longley & Jones (1965)
described an accident during methyl bromide filling where there was
massive skin contamination. Although the victim removed the
contaminated clothing and washed himself thoroughly, symptoms and
signs of poisoning, commencing with severe nausea appeared within 2 h,
and CNS effects 5 h after the exposure. The CNS effects persisted and
6 months later there was still some disability. There were no dermal
effects apart from oedema and itching of the eyelids with subsequent
peeling of the skin and no evidence of pulmonary or renal involvement.
Goulon et al. (1975) reported a detailed study of three cases
where one patient died after 5 years in a stuporous state with
myoclonus. Two of these were females who had slept in bedrooms
containing fire extinguishers filled with methyl bromide; one of these
patients died, still in coma, 4 years and 8 months later. The other
case was a male lorry driver, who was found in a coma after spending
a night in his lorry cab, which contained a leaking fire extinguisher;
4 years later he showed only partial recovery with persisting lack of
coordination, ataxia, and dysarthria. Behrens & Dukes (1986) described
the case of a scrap-dealer who was poisoned by methyl bromide from
fire extinguishers in obsolete aircraft engines.
A case of poisoning and the resulting death of a man (and his
dog) from methyl bromide leaking from an old corroded fire
extinguisher has been reported (Cavanagh, 1992; Squier et al., 1992).
Since methyl bromide is no longer in general use for fire
extinguishers, the occurrence of such cases is now extremely rare.
9.2.1.2 Poisoning associated with bulk or house fumigation
In May 1978, severe methyl bromide poisoning occurred in 4 people
living above a warehouse in Japan (Ishizu et al., 1988). The
warehouse, containing herbs, was accidentally fumigated with a greater
quantity of methyl bromide than normal giving an estimated
concentration of 38 900-58 350 mg/m3 (10 000-15 000 ppm). Three days
later, one member of the family, a girl aged 12 years, developed
severe convulsions and then 2 others had severe convulsions and the
fourth had marked mental confusion. The serum or plasma bromide ion
levels ranged from 280 to 600 mg/litre. Clinical laboratory tests
showed that GOT exceeded the normal level in 3 out of 4 cases.
Moreover, the LDH activity was above the normal range in three cases
and CPK activity was increased in all the cases (Ishizu et al., 1988).
In California, the most frequent cause of death from
non-occupational exposure, in recent years, has been unauthorized
entry into structures under fumigation with methyl bromide. Most often
the entry was by burglars, transients, or intoxicated persons who
broke into buildings that were covered with gas resistant sheeting,
locked, and posted with warning signs. During 1982-87 there were 13
such fatalities (Maddy et al., 1990).
In Florida, where homes are fumigated to destroy termites by
placing a huge coloured tent over the house, the methyl bromide being
released by trained technicians, 27 non-fatal cases and four
fatalities following unauthorized entry were reported between 1957 and
1982 (Marraccini et al., 1983). The intervals between exposure and
death ranged between 2.5 and 36 h. Three of these deaths together with
three additional hospitalizations followed burglaries of tented houses
during a nine-month period.
O'Neal (1987) reported that, at one hospital in Florida, 15-25
patients a year, including fatalities, were treated for methyl bromide
poisoning. Proper diagnosis and therapy were hampered by the latency
period (4-48 h before onset of symptoms) and the fact that the toxic
state also mimics other diseases.
Even after apartments have been cleared for habitation, deaths
due to methyl bromide poisoning have been reported. The fumigant can
be absorbed by furniture, water beds, and walls, and can sequester in
enclosed spaces, with subsequent desorption (Dempsey et al., 1992).
Prockop & Smith (1986) reported a case in which a house fumigator
wearing a faulty protective face mask was exposed briefly on one day.
This was followed by malaise, although he continued to work, and then
again, ten days later, when tremor and increasing malaise resulted in
admission to hospital. On the next day, he was comatose with
generalized major motor convulsions, and myoclonic limb movements
between convulsions. On admission, the serum bromide level was 350
mg/litre (falling to 30 mg/litre 29 days later). Five years later, the
patient still exhibited dysarthria, impaired arm coordination, and
disturbance of gait.
A fatal case of methyl bromide poisoning was reported after
fumigation of a restaurant. Although the methyl bromide levels were
checked, for some reason the apparatus showed a reading of "no
detectable levels" and, without putting the ventilation system on, the
premises were declared safe. A restaurant employee, who then entered
the building, was found dead 2.5 h later; the post-mortem inorganic
bromide level in serum was 48 mg/litre. Four other workers who were in
the building for 45 min had blood bromide concentrations ranging from
40 to 51 mg/litre. Another worker who was in the restaurant for 1 h 15
min, had a blood bromide concentration of 101 mg /litre. These 5
workers had immediate symptoms of malaise and fatigue, but no chronic
symptoms (Fuortes, 1992).
A 13-year-old girl accidentally exposed to excessive methyl
bromide concentrations after a warehouse fumigation had early symptoms
of headache, dizziness, and nausea, followed a few hours later by
unconsciousness, from which she recovered three days later. At that
time, myoclonic jerks developed, the intensity of which progressed and
was unresponsive to treatment over a two-month period. In a two-year
follow-up, treatment with clonazepam and phenobarbitol decreased the
intensity of myoclonus and she was able to return to school.
Electrophysiological findings suggested that the methyl bromide
exposure had induced a type of cortical reflex myoclonus (Uncini et
al., 1990).
9.2.1.3 Poisoning associated with soil fumigation
Goldman et al. (1987) reported four episodes of community
exposure to methyl bromide and chloropicrin soil fumigation in
California in 1973, 1980, and two in 1984. A total of over sixty cases
were involved, and, in all episodes, either evacuation of homes or
cessation of fumigation was the outcome. In one episode, a strawberry
field was fumigated preplanting with a methyl bromide/ chloropicrin
formulation (dosage and % composition not given). Local weather
conditions included a temperature of 30 °C with an inversion; 32
adults and 4 children were treated with incident-related symptoms,
such as eye irritation, sore throat, headache, shortness of breath,
and cough. One child was hallucinating. Fumigant-related symptoms
dropped off sharply with distance, 30% within 1 km compared with 4.5%
more than 2 km from the field. It should be noted that these symptoms
could be attributed to either methyl bromide or chloropicrin.
A nurseryman was poisoned after glasshouses near his house had
been fumigated with methyl bromide (Bishop, 1992). The symptoms were
delayed. By day 3 after the fumigation, he had convulsions and became
comatose. There was a long recovery period and persistence of
neurological signs and symptoms.
9.2.1.4 Miscellaneous incidents
Inhalation of methyl bromide from leaking canisters caused acute
methyl bromide poisoning. Two grandparents and a child showed various
levels of poisoning. The man showed predominantly mental disturbances,
with only mild neurological signs and no convulsions; initial euphoria
and lack of concern progressed to a florid psychosis. His mental state
was normal 6 months later. The woman was severely affected and
remained in status epilepticus for 7 days. The boy had an upper
respiratory illness 24 h before methyl bromide poisoning, which might
have increased his susceptibility to the toxic agent. He developed an
acute encephalopathy with fluctuating conscious state, hypotonia,
araflexia, and extensor plantar responses. Before methyl bromide was
identified as the cause of the illness, Reye's syndrome had been
diagnosed (Shield et al., 1977).
A tractor trailer-truck overturned and a cylinder (max. capacity
680 kg) was punctured. Although people were warned of the risk, ten
were admitted to hospital. The symptoms included nausea, vomiting,
breathing difficulty, headache, dizziness, burning throat, coughing,
and chest tightness. The primary route of exposure was inhalation for
seven patients, dermal for two, and both dermal and inhalation for
one. All patients recovered without neurological sequelae (Polkowski
et al., 1990).
Leakage from a compressed gas cylinder caused unconsciousness and
status epilepticus in a man (Mazzini et al., 1992). After awaking, he
suffered from severe myoclonic jerks and major epileptic convulsions.
Six months later, he still had severe action myoclonus of the limbs.
A standardized neuropsychological evaluation revealed severe cognitive
impairment.
9.3 Controlled human studies
Raabe (1988) carried out an inhalation study using [14C]-
labelled methyl bromide up to 0.1 mg/m3 to determine the percentage
of methyl bromide absorbed by the human body from air containing
ambient concentrations of the gas. The uptake was 55.4% nasal and
52.1% oral (section 6.1.1.2 and Fig. 6).
9.4 Occupational exposure
Up to 1955, the majority of methyl bromide poisoning incidents
resulted from chemical manufacture and filling operations, followed by
fire extinguishers and fumigation (von Oettingen, 1955). Since 1955,
fumigation has become the major source of fatalities (Alexeeff &
Kilgore, 1983).
9.4.1 Occupational exposure during manufacture
Incidents of methyl bromide poisoning in industry have been
described by Watrous (1942) and Wyers (1945). The main incidents were
dermatological cases occurring principally among those who filled the
small cylinders for distribution from the larger ones. Other sources
of danger were defects in the plant, such as ill-fitting joints and
bursting of cylinders by an undue rise in temperature. Carelessness
with handling was also a cause.
Kishi et al. (1991) carried out a questionnaire survey to
determine the symptoms of workers exposed to methyl bromide in the
manufacturing process. Seventy five male workers (exposure length:
1-25 years) were compared with a reference group of railway workers.
The questionnaire covered acute symptoms during workshift (16
questions), and general symptoms (61 questions). Acute and general
symptoms were statistically higher in frequency among the exposed
workers (sign test of pairwise comparison). Methyl bromide
concentrations in the air in the workers' breathing zone were measured
every 6 months, and were normally less than 4 mg/m3, but, during
some accidental events, they exceeded 20 mg/m3. The mean bromide ion
concentration in the urine of men working in the manufacture of methyl
bromide was 18.9 mg/litre± 10.4 (range 3.2-54.0 mg/litre), with no
correlation with the symptoms reported.
Previous studies had shown that urinary bromide concentrations
were an index of relatively acute exposure, correlating with the air
concentration of the working site. The concentration in the work-place
air sometimes accidently exceeded 58 mg/m3. There were some cases of
sub-acute poisoning with lethargy, ataxia, and retrobulbar optic
neuritis. In one incident, the mean urine concentration of 20 workers
exposed to methyl bromide was 277 mg/litre. On that occasion, 14 of
the 21 exposed workers had abnormally accelerated tendon reflexes, 8
had paraesthesia, and 4 showed disturbance of the convergence reflex
of the eyes (Kishi et al., 1991).
9.4.2 Occupational exposure due to methyl bromide fumigation
In many countries, the use of methyl bromide is restricted to
trained and licensed personnel.
Occupational exposure to methyl bromide can occur during the
manufacturing, filling, and packaging processes, as well as during its
use as a fumigant. As described in section 3.2.2, methyl bromide is
used mainly for soil fumigation as well as for the fumigation of
buildings and commodities, to prevent pest infestation. The various
occupations involved are given in section 5.3.
Methyl bromide was used as a fumigant in the USA by an estimated
105 000 workers between 1972 and 1974 (NIOSH, 1984) and 75 000 workers
in 1980 (Anger et al., 1981).
9.4.2.1 Incidents involving bulk fumigation
A poisoning incident in a mill where three fumigators were
poisoned through dermal exposure to gaseous methyl bromide has been
described in section 9.1.2 (Bruhin, 1942).
Johnstone (1945) and Ingram (1951) reported incidents in
data-processing factories where over 200 employees were taken ill,
with over 50 cases of frank methyl bromide poisoning, including two
workers suspected of insanity. All 34 packers, described by Johnstone
(1945), had visual disturbances and there was a high incidence of
speech difficulties, mental confusion, hallucinations, and
paraesthesia. Four workers claimed permanent and six temporary
disabilities. The main factor in these poisonings was that the workers
ignored the necessary precautionary measures (Johnstone, 1945).
Kantarjian & Shaheen (1963) described 8 cases of polyneuropathy in
date factory workers who suffered repeated exposure to the vapour due
to faulty working techniques over a period of three months. Within 6
months all had recovered. Only 8 out of 14 employees developed
symptoms, suggesting a variation in susceptibility in different
individuals.
Seven workers employed in the bulk fumigation of houses were
poisoned by methyl bromide (Rathus & Landy (1961). The cause was the
insufficient absorption properties of the canisters in their
respirators. Three of the men were burned on the area of the face
covered by the respirators.
Drawneek et al. (1964) described a case of irreversible brain
damage in a methyl bromide fumigator. Seven other workers, without any
recognisable illness, were found to have serum bromide levels of over
50 mg/litre, at which level mild euphoria may develop and lead to
carelessness in the handling of methyl bromide, and, therefore, to
possible acute exposure.
Hine (1969) reported two acute and eight chronic cases of
fumigation-related poisoning in California from 1957 to 1966. Four of
the patients died. The most common symptoms were malaise, weakness,
and dyspnoea. The deaths were preceded by convulsions and coma. All
cases could be traced to a failure in preventative procedures.
A few hours after exposure, during the fumigation of cocoa beans,
a dock worker developed status epilepticus, coma, and pulmonary oedema
(Greenberg, 1971).
Ten cases of methyl bromide poisoning occurred in the hold of a
ship whilst a rice cargo was being fumigated in port (Brodniewicz,
1967). The accident was the result of several circumstances: sealing
of the crew's quarters was forgotten, some of the crew remained on the
ship during, and after, fumigation, and the port fumigating team
lacked experience and proper qualifications. One victim died following
convulsions, acute pulmonary oedema, and heart failure. In the other
cases, the main complaints were dizziness and nausea.
In a fatal case of methyl bromide poisoning of a ship's boy who
slept in the crew's quarters, the levels of methyl bromide measured
after fumigation had not exceeded 40 mg/m3, but the boy was
poison-ed by a high concentration of the gas leaking from the
fumigated commodity, because of large temperature differences (Weller,
1982).
An employee habitually not wearing a mask in a fumigating plant
spraying fruits and vegetables was initially treated for psychosis, as
the early symptoms of methyl bromide poisoning are similar (Zatuchni
& Hong, 1981).
Cases of dermal exposure occurring during the fumigation of a
castle have been reported (Zwaveling et al., 1987; Hezemans-Boer et
al., 1988). Details are given in section 9.1.2.
Systemic and neuro-ophthalmic manifestations of methyl bromide
poisoning including increased serum bromide level (66 mg/litre),
paraesthesia and burning dysesthesia on hands and feet, and visual
impairment, were described in an assistant fumigator who had been
exposed acutely, twice, during the year previous to examination
(Chavez et al., 1985).
9.4.2.2 Incidents involving soil fumigation
(a) Field workers
Table 52 shows occupational poisoning incidents, reported in
California, due to methyl bromide exposure in the years 1950, 1986,
and 1987, and analysed according to work activity (Edmiston & Maddy,
1987; Maddy et al., 1990).
Seven deaths through occupational exposure were reported in
California between 1951 and 1965. The distribution of cases was: 1952
(1), 1956 (2), 1958 (1), 1959 (2), and 1965 (1) (Maddy et al., 1990).
Methyl bromide poisoning of four field workers occurred during
the removal of soil fumigation sheets under cool weather conditions
(Herzstein & Cullen, 1990). Ten days after injection of methyl bromide
into the soil, the polyethylene sheets covering the soil were removed.
The 4 field workers developed fatigue and light-headedness and 3 had
progressive respiratory, gastrointestinal, and neurological symptoms.
The acute systemic symptoms improved over several days, but
neuropsychiatric symptoms that developed later persisted for several
weeks. The 2% chloropicrin was not enough to warn the workers of the
presence of methyl bromide.
(b) Greenhouse workers
Methyl bromide has been used extensively in the USA since the
1950s, and, in Western Europe, since the 1960s, in the fumigation of
soils in greenhouses. This can be hazardous to the health of the
workers because of the enclosed area in which methyl bromide is
applied (Roosels et al., 1981). Poisoning incidents have occurred in
operators and other people entering fumigated areas. An analysis of
eight severe cases was made by Van den Oever et al. (1978). The signs
and symptoms followed the usual pattern. Nearly all incidents were
associated with professional fumigation workers using the injection
method.
In a case of acute occupational methyl bromide intoxication, a
27-year-old man, using methyl bromide in a greenhouse, suffered
continuous epileptic convulsions for 3 weeks, followed by repeated
generalized convulsions. Myoclony of the face and the fingers
accompanied by a complete motor deficiency persisted; there was also
bilateral deafness (Cantineau et al., 1988).
In an accident involving nine greenhouse workers (2 women, 7 men;
aged 21-40 years), exposed to an inadvertent spread of methyl bromide
during fumigation, two patients needed intensive care for several
weeks because of severe reactive myoclonus and tonic-clonic
generalized convulsions (Hustinx et al., 1993).
Table 52. Summary of occupational cases of methyl bromide poisoning in California
as reported by physicians, listed according to work activity and type of illness/injury a
Year Work activity Systemic Eye Skin Eye/ Total
skin cases
1950 all occupational 3 0 0 0 3
exposures
1986 chamber fumigator 2 0 1 0 3
1987 chamber fumigator 2 0 0 0 2
1986 field fumigator 0 1 5 0 6
1987 field fumigator 1 1 4 0 6
1987b field fumigator 6 2 1 0 9
1986 "tarp" cover fumigator 4 0 1 1 6
1987 "tarp" cover fumigator 1 0 1 0 2
1987b "tarp" cover fumigator 1 0 0 0 1
1986 coincidental exposure 0 1 0 0 1
1987 coincidental exposure 4 0 0 0 4
1987b coincidental exposure 2 0 0 0 2
1987 emergency response 2 1 0 0 3
1987b personnel 1 0 0 1 1
a 1950 and 1987 figures taken from Maddy et al. (1990);
1986 figures taken from Edmiston & Maddy (1987)
b A methyl bromide/chloropicrin formulation was used.
9.4.3 Studies measuring the levels of bromide ion in biological
fluids and tissues
There have been a number of studies to investigate whether there
is a correlation between methyl bromide exposure levels and bromide
ion concentrations, but the results are conflicting.
9.4.3.1 Manufacturing
Ohmori & Hirata (1982), using radioactivation analysis, reported
mean bromine concentrations in serum (66 µg/g) and hair (11 µg/g) from
14 methyl bromide workers, and mean bromine concentrations in serum
(40 µg/g) and hair (4 µg/g) in controls. A worker suspected of methyl
bromide poisoning had a level of 412 µg/g serum, 13 days after
exposure.
Average bromide ion concentrations detected in 36 urine samples
of workers exposed to methyl bromide were 13.3±7.7 mg/litre compared
with 7.1±2.1 mg/litre in a non-exposed group (Koga et al., 1991). The
atmospheric methyl bromide concentrations of exposed workers were
monitored with passive samplers during their work shifts (8 h). No
significant correlation was found between these methyl bromide
readings and the bromide ion concentrations in urine.
9.4.3.2 Fumigation
Routine monitoring of bromide levels in the blood of a group of
fumigators, undertaking both bulk and soil fumigation, was carried out
in England between 1971 and 1979 (Cornwell, 1979). It was found that
the plasma bromide levels between January and the end of the year were
correlated with the number of gas applications, irrespective of the
type of fumigation work undertaken (Fig. 10).
The plasma bromide level was thought to rise as a result of repeated
exposure to low concentrations of methyl bromide with small amounts
accumulating in the body faster than the rate of elimination. During
the Christmas period, when all the men took a break at the same time,
the plasma bromide levels were substantially reduced. Results from
October 1974 to January 1978 indicated that average plasma levels
increased from 15 mg/litre in January to 25 mg/litre in October. Some
fumigators carried out disproportionally more treatments of stacks,
containers, and lighters, and reached plasma bromide levels of 30-60
mg/litre. A relationship between plasma bromide levels and the number
of fumigation applications was found (Fig. 11).
 Blood bromide levels, EEGs, and transaminases were measured in 33
methyl bromide workers engaged in soil disinfection inside
greenhouses. Blood bromide varied between 4 and 23 mg/litre (Verberk
et al., 1979).
In a study of the bromide ion concentration in the plasma of 39
methyl bromide workers, a mean level of 6.9 mg/litre was measured
compared with controls (100 workers) who had a mean of 3.7 mg/ litre
(Yamano et al., 1987).
Blood bromide levels, EEGs, and transaminases were measured in 33
methyl bromide workers engaged in soil disinfection inside
greenhouses. Blood bromide varied between 4 and 23 mg/litre (Verberk
et al., 1979).
In a study of the bromide ion concentration in the plasma of 39
methyl bromide workers, a mean level of 6.9 mg/litre was measured
compared with controls (100 workers) who had a mean of 3.7 mg/ litre
(Yamano et al., 1987).
 Tanaka et al. (1991) measured ambient methyl bromide
concen-trations and urinary bromine concentrations at various plant
quarantine fumigation sites in Japan. The geometric mean for exposed
workers was 9.0±1.85 mg/litre while the arithmetic mean for a matched
control population was 6.3±2.5 mg/litre. The authors found a
statistically significant positive correlation between the ambient
methyl bromide concentrations collected with a personal sampling
device and the urinary concentrations.
9.4.4 Haemoglobin adducts as a biological index to methyl bromide
exposure
Iwasaki et al. (1989) determined the haemoglobin adduct
(haemoglobin MeCys) in methyl bromide manufacturing workers, and
examined its effectiveness as a biological index of exposure to methyl
bromide. Methyl bromide reacts with cysteine to form
S-methylcysteine (MeCys) in haemoglobin (Djalali-Behzad et al.,
1981). It was suggested that determination of MeCys in haemoglobin
could detect previous methyl bromide exposure levels so low that they
were not detected by a special medical check-up (Iwasaki et al.,
1989). Haemoglobin adducts have a life span of about 2 months, so
workers only intermittently exposed to methyl bromide would also be
detected in such a survey. Previous studies by the same authors showed
individual differences in mice in the levels of MeCys within each dose
and time series group (Iwasaki, 1988a,b). These individual differences
may be caused by the differential susceptibility of haemoglobin in red
blood cells or individual differences in methyl bromide metabolism.
These differences were found to be minor in the human study (Iwasaki
et al., 1989).
Results from animal studies (Xu et al., 1990) supported the
suggestion of Iwasaki et al. (1989) that the Hb adduct of methyl
bromide might be a useful parameter in the biological exposure
monitoring of methyl bromide workers.
9.4.5 Neurobehavioural and other studies
Anger et al. (1986) carried out a neurobehavioural study on soil
fumigators who were exposed to 9 mg methyl bromide/m3 over an 8-h
day and structural fumigators who were exposed to 0-9 mg/m3 for 1.5
h per day. The fumigators, who reported a significantly higher
prevalence of 18 symptoms, consistent with methyl bromide toxicity,
than the controls, did not perform so well on 23 out of 27 behavioural
tests, and were significantly lower on one test of finger sensitivity
and one of cognitive performance.
EEGs and transaminases were measured in 33 methyl bromide workers
engaged in soil disinfection inside greenhouses in the Netherlands.
Symptoms of methyl bromide intoxication were determined by means of a
specially designed questionnaire. A general neurological examination
was performed. No differences compared with controls were found, with
the exception of slight electro-encephalographic changes in 10 workers
involving diffuse increase of beta and theta activity. Workers with
abnormal EEGs had higher blood bromide levels (geometric mean 10.9
mg/litre compared with a mean of 8.2 mg/litre in the other workers;
the difference was statistically significant ( P <0.05) (Verberk et
al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human exposure
Human exposure to methyl bromide occurs predominantly as an
occupational hazard, particularly during soil or bulk fumigation, but
also during manufacture. Individuals in the vicinity of fumigated
fields or buildings may also be exposed. Methyl bromide has widespread
use for fumigating post-harvest foods, such as cereals, spices,
dried-fruits, and nuts, as well as fresh fruits and vegetables. The
determination of levels of methyl bromide and inorganic bromide in the
fumigated food is important for the assessment of possible risks for
human and animal health. Methyl bromide levels in food commodities
usually decrease rapidly after aeration, but may be detected for some
weeks after treatment. Fumigation with methyl bromide results in
increased levels of inorganic bromide in food commodities and,
equally, in produce grown on fumigated soils. Fumigant dosage
standards should be adhered to, so that the bromide levels do not
exceed the recommended limits.
The major health concern is from acute exposure.
Delayed onset of symptoms may occur. Fatal poisoning has resulted
from exposures to relatively high concentrations (from 33 000 mg/m3
or 8600 ppm onwards) of methyl bromide vapours. Non-fatal poisoning
has resulted from exposure to concentrations as low as 390-1950
mg/m3 (100- 500 ppm). Organs affected by exposure include the
nervous system, lung, nasal mucosa, kidney, eye, and skin. There are
no epidemiological data on reproductive toxicity and carcinogenicity
in humans. There are no data on any human health effects of methyl
bromide residues in food or drinking-water.
10.1.1 Relevant animal studies
Methyl bromide is very toxic for all animal species by all routes
of administration studied. Deaths from exposure follow a steep
dose-response curve. LC50 (1-h) values for mice and rats are 4680
and 7300 mg/m3, respectively.
Deaths and neurotoxicity occur within hours or days after a
single inhalation exposure at high concentrations. Studies on mice
show that more prolonged exposure at low concentrations (6 h/day; 389
mg/m3) may also produce neurotoxicity or deaths, appearing with a
delayed onset of several months.
Absorption and distribution to various tissues is rapid, as is
elimination. The metabolic pathways and mode of action are unknown.
The principal toxic effects associated with lethality occur in
the brain and kidney. Histopathology of the brain shows necrosis of
granular cells of the cerebellum in mice and rats and neuronal
necrosis in the cerebral cortex, hippocampus, and thalamus, in rats.
The lowest doses at which these lesions were observed were 250
mg/m3, 6 h/day, for 2 years, in mice, and, 622 mg/m3, 6 h/day, for
5 weeks, in rats. In the kidney, necrosis of the convoluted tubule
epithelium occurs at doses of 599 mg/m3, 6 h/day, for 2 weeks, in
mice, and 1712 mg/m3, 6 h/day, for 2 weeks, in rats. Other effects
are observed in the heart (degeneration of focal necrosis), nose
(necrosis of olfactory epithelium), testis (degeneration of
seminiferous tubules), and other organs. In non-lethal exposure, nasal
irritation and neuro-toxicity (including histopathological lesions)
are the major toxic effects.
No teratogenic effects have been observed in rats or rabbits.
Embryotoxicity occurred in rats and rabbits only at doses that were
also maternally toxic. In a rat multigeneration study, there were
reductions in the fertility index in the second generation, when the
animals were exposed to 117 or 350 mg/m3 (30 or 90 ppm) for 6 h/
day, but effects were not observed following exposure to 12 mg/m3 (3
ppm) for 6 h/day.
Methyl bromide was mutagenic in several in vivo and in vitro
assays.
Long-term inhalation studies on rats and mice did not reveal any
evidence of carcinogenicity. Lesions originally interpreted as
carcinomas of the forestomach in rats, following gavage
administration, were shown in a subsequent study to regress after
termination of treatment, and were considered not relevant for human
risk assessment.
10.2 Environment
Methyl bromide is predominantly a naturally occurring compound.
Oceans are believed to be a major natural source of methyl bromide.
Another source(s) may exist in the tropics, which is yet to be
explained. Anthropogenic sources from fumigation and, to a much lesser
extent, motor vehicles (from the combustion of organic bromine
additives in leaded petrol) add to these. Present data indicate that
the globally averaged atmospheric abundance of methyl bromide is
between 9 and 13 pptv, equivalent to a total atmospheric loading of
150-220 thousand tonnes. If the atmospheric lifetime is two years
(assuming only atmospheric removal processes are significant),
anthropogenic sources of methyl bromide represent about 25% (±10%) of
the total emissions. The world production of methyl bromide in 1990
was 69 000 tonnes, having increased at a rate of 6% per year from 1984
to 1990. About 50% of the methyl bromide produced is released into the
atmosphere during, or after, use. Although methyl bromide reacts with
the hydroxyl radical in the troposphere, some methyl bromide is
transferred by upward diffusion to the stratosphere, where it
photolyses. Active bromine species react with ozone in the lower
stratosphere and are partly responsible for the depletion of the ozone
layer. It is estimated that anthropogenic releases of methyl bromide
cause about 3% of the present total stratospheric ozone loss.
Methyl bromide is used for soil fumigation (about 77%), for
quarantine, commodity fumigation (12%), for structural fumigation
(5%), and for chemical intermediates (6%).
In soil, methyl bromide is degraded by hydrolysis and microbial
activity. The remainder (about 50%) eventually dissipates into the
atmosphere. The degradation product, principally as inorganic bromide,
remains as a residue in soil.
Methyl bromide has herbicidal properties. In the vicinity of
greenhouses and fumigated structures, phytotoxic effects may occur.
Visible damage to the leaves of lettuce (a sensitive test plant) was
noticed at 400 mg/m3.
Soil fumigation using methyl bromide (with 2% chloropicrin)
affects both target and non-target organisms: various soil microflora
and fauna are adversely affected, at least temporarily, by fumigation.
High mortality of non-target insects has been noticed from fumigation
under plastic sheeting. Methyl bromide could be detected in different
soil types up to 3 weeks after the treatments, the highest levels
being found in the upper layers (0-40 cm) of the soil.
Methyl bromide is highly toxic for aquatic organisms, though it
is generally of no risk to the aquatic environment. The lowest EC50
or LC50 values reported are 2.8 mg/litre for algae, 1.7 mg/litre for
daphnids, and 0.3 mg/litre for fish. NOEC levels, derived from
long-term studies, as low as 0.06 mg/litre have been reported for
daphnids and fish. Toxic concentrations are not expected to be reached
under normal circumstances, because most of the methyl bromide applied
on soil is degraded or lost, due to evaporation, before it reaches
surface water via run-off. Only in very special situations (leaching
of greenhouse soils to reduce inorganic bromide residues), can levels
of methyl bromide in the mg/litre range occur in water. Concentrations
of up to 9.3 mg methyl bromide/litre were measured in drainage water.
Similarly, relatively high levels of bromide (up to 72 mg/litre)
that could adversely affect aquatic organisms were measured in
drainage water from greenhouses. Long-term exposure to bromide ion
resulted in an EC50 value of 27 mg bromide/litre for daphnia and the
lowest NOEC for different fish species was 25 mg bromide/litre.
11. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
11.1 Human health protection
In most countries, methyl bromide is strictly regulated for
application as a fumigant for soil, commodities, and structural
purposes. Adherence to good practices and guidelines should ensure no
adverse effects on persons exposed occupationally. Considerable care
should be taken by manufacturers in the production process of methyl
bromide and by suppliers in its transfer and use.
Methyl bromide does not pose a significant risk for the general
population. It is, however, a very toxic substance and precautions
have to be taken: exposure of the general public should be avoided
through adequate precautions in fumigated buildings, greenhouses, and
stores used for commodity fumigation. Fumigated premises should be
clearly marked, in order to prevent accidental exposure of
individuals.
11.2 Environmental protection
Emission of methyl bromide from anthropogenic sources should be
reduced as far as possible. It is, therefore, desirable to reduce
emissions from soil, commodity, and structural fumigation.
Improvements should be sought in these areas:
(1) improved injection methods;
(2) better barrier films;
(3) better measurement of the efficacy of methyl bromide, with
the goal of lower dosage rates where possible.
For commodity and, possibly, for structural fumigation,
techniques should be developed to:
(1) seal fumigation chambers more tightly;
(2) capture and recycle fumigation gas.
11.3 Recommendations for further research
There is a need for:
- study of the metabolism and toxic mechanisms of methyl
bromide;
- a postnatal behavioural study;
- a human epidemiological study including exposure
assessment;
- development of a treatment protocol for cases of human
poisoning.
Effects of methyl bromide on the depletion of the ozone layer are
not yet entirely understood, indicating a need for:
- studies quantifying the distribution sources of methyl
bromide and other organic bromine compounds.
There is also a need for:
- a study of the impact of sea spray on the formation of
organic bromine compounds;
- a study on the degradation products of methyl bromide in the
troposphere;
- further study of the rate constant for the reaction with
OH-;
- studies on soil fumigation with the aim of reducing
emissions while keeping efficacy.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12.1 FAO/WHO
The toxicology of methyl bromide was evaluated by the FAO/WHO
Joint Meeting on Pesticide Residues in 1965 and 1966 (FAO/ WHO, 1965
a,b; WHO, 1967, a,b). In 1965, no acceptable daily intake (ADI) was
allocated, but, in 1966, an ADI of 1 mg/kg body weight, as bromide
ion, was established. In 1988, the JMPR evaluated the toxicology of
the bromide ion (FAO/WHO 1988a,b) and concluded that the level causing
no toxicological effect was:
Rat: 240 ppm, equivalent to 12 mg bromide/kg body weight
per day
Human: 9 mg bromide/kg body weight per day
The acceptable daily intake (ADI) of 1 mg/kg body weight was
confirmed.
12.2 IARC
The carcinogenic risks for humans were evaluated by an
International Agency for Research on Cancer ad hoc expert group in
1986. The evaluation was updated in 1987 and it was concluded that
there was inadequate evidence for carcinogenicity to humans and
limited evidence for carcinogenicity to animals; the overall
evaluation of carcinogenicity to humans was "not classifiable" [group
3] (IARC, 1986, 1987).
12.3 UNEP
In 1992, the United Nations Environment Programme evaluated methyl
bromide on behalf of the Contracting Parties to the Montreal Protocol
(UNEP, 1992). The executive summary of the evaluation reads:
"The report covers the current understanding of the impact
of methyl bromide on the ozone layer and on the uses of, and
alternatives to, methyl bromide."
It was requested by the United Nations Environment Programme on behalf
of the Parties to the Montreal Protocol. The report includes
information presented and discussed at the International Methyl
Bromide Science Workshop held in Washington, DC, on 2-3 June 1992, and
at the International Workshop on Alternatives and Substitutes to
Methyl Bromide held in Washington, DC, on 16-18 June 1992.
The current state of scientific knowledge concerning bromine
compounds in the atmosphere is considerably less developed than the
corresponding understanding of chlorine compounds. In addition, the
evaluation of the alternatives and substitutes to methyl bromide as a
fumigant is in an earlier stage than that for chlorofluorocarbons
(CFCs), methylchloroform, carbon tetrachloride, and halons.
Methyl bromide is used as a fumigant for soils, commodities, and
structures. It is currently vital for the economic viability of
certain agricultural production (especially strawberries, tomatoes,
peppers, tobacco, eggplants, nursery stock, vines, and turf) and for
the quarantine treatment of certain products in international trade.
Many developing countries are particularly dependent on the export of
products currently fumigated using methyl bromide, before shipment and
at ports of entry.
Total annual production and sales of methyl bromide for
fumigation have increased from about 42 000 tonnes to about 63 000
tonnes between 1984 and 1990. Combining the approximate methyl bromide
use-pattern data with the currently estimated fraction that escapes to
the atmosphere from each use (soils: 80% of use, 50 % emitted;
commodities: 15 % of use, <80 % emitted; and structural: 5 % of use,
80% emitted) indicates that about half of the methyl bromide used as
a fumigant is emitted into the atmosphere. Based on current
understanding, this implies an anthropogenic emission from fumigation
applications of about 30 thousand tonnes in 1990, which represents
25±10 % of the total (natural and anthropogenic) emissions.
Ozone destruction by bromine is more efficient on a per molecule
basis than destruction by chlorine by a factor of 30-60. Therefore, 1
part per trillion by volume (pptv) of bromine is equivalent to
0.03-0.06 parts per billion by volume (ppbv) of stratospheric
chlorine.
The current best estimate of the steady-state value of the Ozone
Depletion Potential (ODP) for methyl bromide is 0.7. Because of the
short atmospheric lifetime of methyl bromide, its relative impact on
ozone is expected to be much greater over the next decade (when
chlorine abundances and ozone losses are predicted to reach their
maximum) than is indicated by its steady-state ODP.
There are significant uncertainties in the atmospheric budget and
ODP of methyl bromide, especially the quantification of possible
oceanic and terrestrial surface removal processes and the rate of
formation of unreactive bromine in the stratosphere.
Model calculations suggest that anthropogenic emissions of methyl
bromide used for fumigation applications could have accounted for
about one-twentieth to one-tenth of the current observed global ozone
loss of 4-6% and could grow to about one-sixth of the predicted ozone
loss by the year 2000, if methyl bromide emissions continue to
increase at the present rate of about 5-6% per year.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide from fumigation applications is about 3
pptv, which is equivalent to 0.09-0.18 ppbv of stratospheric chlorine.
An advance of the CFC and carbon tetrachloride phaseout schedule by
three years would reduce the peak chlorine loading by 0.18 ppbv.
Therefore, elimination of methyl bromide used as a fumigant could
provide an ozone-layer protection equivalent to that of an advance of
the CFC and carbon tetrachloride phaseout schedule by about 1.5-3
years.
There is no single alternative to methyl bromide in the broad
spectrum of applications for which it is currently used. There are,
however, many alternative chemicals and procedures for specific
applications. The introduction of some chemical alternatives may
require government approval, which could be a lengthy process.
Rough estimates indicate that a significant fraction (from as low
as 30% to as high as 90%), albeit uncertain, of methyl bromide used
for soil fumigation could be replaced by chemical substitutes during
the 1990s; that a substantial proportion of emissions from fumigation
chambers could be captured and recycled or destroyed; that a small
fraction (1-2%) of methyl bromide emissions could be eliminated by
better procedures during tank filling; and that significant reductions
in emissions could be made using other alternatives or techniques,
alone, or in combination. However, there are some applications for
which there are limited or no alternatives, including some
agricultural situations that have developed a dependence on methyl
bromide, quarantine treatments, and some structural fumigation uses.
REFERENCES
Abdalla N & Lear B (1975) Lethal dosages of methyl bromide for four
plant-parasitic nematodes and the effect of soil temperature on its
nematicidal activity. Plant Dis Rep, 59: 224-228.
Adu OO & Muthu M (1985) The relative toxicity of seven fumigants to
life cycle stages of Callosobruchus chinensis (L). Insect Sci Appl,
6: 75-78.
Alexeeff GV & Kilgore WW (1983) Methyl bromide. In: Gunther FA &
Gunther JD ed. Residue reviews. Residues of pesticides and other
contaminants in the total environment, Vol. 88. Berlin, Heidelberg,
New York, Springer-Verlag, pp 102-153.
Alexeeff GV, Kilgore WW, Munoz P, & Watt D (1985) Determination of
acute toxic effects in mice following exposure to methyl bromide. J
Toxicol Environ Health, 15: 109-123.
Alfaro Moreno A & Vegh I (1971) [Green pepper diseases produced by
Phytophthora capsici.] An Inst Nac Invest Agr Ser Prot Veg, 1: 9-42
(in Spanish).
American Biogenics Corporation (1986) Two-generation reproduction
study in albino rats with methyl bromide - results of both generations
(Study No. 4500-1525) (Unpublished final report).
Andersen ME, Gargas ML, Jones RA, & Jenkins LJ (1980) Determination of
the kinetic constants for metabolism of inhaled toxicants in vivo
using gas uptake measurements. Toxicol Appl Pharmacol, 54: 100-116.
Anger WK, Setzer JV, Russo JM, Brightwell WS, Wait RG, & Johnson BL
(1981) Neurobehavioral effects of methyl bromide inhalation exposures.
Scand J Work Environ Health, 7: 40-47.
Anger WK, Moody L, Burg J, Brightwell WS, Taylor BJ, Russo JM,
Dickerson N, Setzer JV, Johnson BL, & Hicks K (1986) Neurobehavioral
evaluation of soil and structural fumigators using methyl bromide and
sulfuryl fluoride. Neurotoxicology, 7: 137-156.
Angerer J (1977) [A method for determination of bromide in urine with
help of a ionsensitive electrode.] J Clin Chem Clin Biochem, 15:
201-204 (in German).
Angerer J (1980) [Bromide. Determination in urine.] In: Henschler D
ed. [Analytical methods for the investigation of work materials
detrimental to health. Analysis in biological materials.] Weinheim,
Verlag Chemie, vol 2/2, pp D1-D6, 1-9 (in German).
Angerer J (1982) [Methyl bromide. Air analysis]. In: Henschler D ed.
[Analytical methods for the investigation of work materials
detrimental to health. Air analysis.] Weinheim, Verlag Chemie, vol
1/2, D1-D2, 1-8 (in German).
Anthon EW, Moffit HR, Couey HM, & Smith LO (1975) Control of codling
moth in harvested sweet cherries with methyl bromide and effects upon
quality and taste of treated fruit. J Econ Entomol, 68: 524-526.
Arvieu JC (1983) Some physico-chemical aspects of methyl bromide
behaviour in soil. Acta Hortic, 152: 267-275.
Arvieu JC & Cuany A (1985) Effets de la matière organique sur la
bioactivité et la dégradation du bromure de méthyle dans le sol. EPPO
Bull, 15: 87-96.
Asante-Poku S, Aston WP, & Schmidt DE Jr (1974) Site of decomposition
of methyl bromide in cocoa beans. J Sci Food Agric, 25: 285-291.
Atochem (1987) Fumyl-o-gas. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 4 pp.
Atochem (1988) BM 98 SOBROM. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 2 pp.
Audry D, Soichot P, Giard MH, & Mauguiere F (1985) Une cause rare de
myoclonies avec PES "Géants": l'intoxication par le bromure de
méthyle. A propos d'une observation à prédominance unilaterale. Rev
EEG Neurophysiol, 15: 45-52.
Austin RK & Phillips DJ (1985) Use of a standard-addition
bromide-selective electrode technique to determine bromide and trace
its migration in peaches. J Agric Food Chem, 33: 1165-1168.
Bachem C (1927) [Contribution to the toxicology of the alkyl
halogens.] Arch Exp Pathol Pharmakol, 122: 69-76 (in German).
Bakhishev GN (1973) [Relative toxicity of aliphatic halohydrocarbons
to rats.] Farmakol Toksikol, 8: 140-142 (in Russian).
Balander PA & Polyak MG (1962) [Toxicological characteristics of
methyl bromide.] Gig i Toksikol, 60: 412-419 (in Russian).
Basile M & Lamberti F (1981) Bromide residues in edible organs of
plants grown in soil treated with methyl bromide. Meded Fac
Landbouwwet Rijksuniv Gent, 46: 337-341.
Basile M, Lamberti F, & Basile AC (1987) [Inorganic bromide
concentrations in soil after fumigation with methyl bromide.] Essenze
Deriv Agrum, 57: 116-120 (in Italian).
Baumann H & Heumann KG (1987) Analysis of organobromine compounds and
hydrogen bromide in motor car exhaust gases with a GC/microwave plasma
system. Fresenius Z Anal Chem, 327: 186-192.
BBA (Biologische Bundesanstalt für Land- und Forstwirtschaft) (1989)
[Plant protective agent register, 1989/90, Part 5.] 37th ed.
Braunschweig, Federal Institute for Agriculture and Forestry, 66 pp
(in German).
Behrens RH & Dukes DCD (1986) Fatal methyl bromide poisoning. Br J Ind
Med, 43: 561-562.
Bell CH (1988) Minimum concentration levels of methyl bromide required
for full efficacy against seven species of stored-product beetle at
two temperatures. Pestic Sci, 24: 97-109.
Benatt AJ & Courtney TRB (1948) Uraemia in methyl bromide poisoning:
A case report. Br J Ind Med, 5: 21-25.
Berg WW, Heidt LE, Pollock W, Sperry PD, Cicerone RJ, & Gladney ES
(1984) Brominated organic species in the arctic atmosphere. Geophys
Res Lett, 11: 429-432.
Beyne M & Goett C (1934) Toxicité de certains appareils extincteurs
d'incendie et précautions qu'ils comportent dans leur emploi. Arch Méd
Pharm Nav, 124: 409.
Bird GW, Rich JR, & Glover SU (1974) Increased endomycorrhizae of
cotton roots in soil treated with nematicides. Phytopathology, 64:
48-51.
Bishop CM (1992) A case of methyl bromide poisoning. J Occup Med, 42:
107-109.
Blackburn S & Philips H (1944) Experiments on the methylation and
acetylation of wool, silk fibroin, collagen and gelatin. Biochem J,
38: 171-178.
Blackith RE & Lubatti OF (1965) Fumigation of agricultural products.
XX. Prolonged storage of cereals fumigated with methyl bromide. J Sci
Food Agric, 16: 455-457.
Boczek J, Klag J, & Komorowska B (1975) Effect of methyl bromide on
the embryonic development of Acarus siro (Acarina, Acaridae). J
Stored Prod Res, 11: 41-46.
Bolon B, Bonnefoi M, & Morgan KT (1990) Effect of olfactory epithelial
regeneration and cage environment on the sensitivity of the rat
olfactory epithelium to methyl bromide. Chem Senses, 15: 554-555.
Bond EJ (1956) The effect of methyl bromide on the respiration of the
cadelle Tenebroides mauritanicus L. (Coleoptera: Ostomidae). Can J
Zool, 34: 405-415.
Bond EJ (1984) Manual of fumigation for insect control. 6. Chemicals
used as fumigants. Rome, Food and Agriculture Organization of the
United Nations, pp 71-96 (Plant Production and Protection Paper 54).
Bond EJ & Dumas T (1987) Concentrations of methyl bromide inside flour
mills and in the atmosphere around the mills during and after
fumigation. Proc Entomol Soc Ont, 118: 1-6.
Bond JA, Dutcher JS, Medinsky MA, Henderson RF, & Birnbaum LS (1985)
Disposition of [14C]methyl bromide in rats after inhalation. Toxicol
Appl Pharmacol, 78: 259-267.
Boorman GA, Hong HL, Jamieson CW, Yoshitomi K, & Maronpot RR (1986)
Regression of methyl bromide induced forestomach lesions in the rat.
Toxicol Appl Pharmacol, 86: 131-139.
Bourbos VA & Skoudridakis MT (1991) Effect of methyl bromide and
chloropicrin on the soil mycoflora in greenhouse tomato. Plant Pathol
Lab, 56: 569-575.
Bowers GN Jr & Onoreski M (1990) Hyperchloremia and the incidence of
bromism in 1990. Clin Chem, 36: 1399-1403.
Brenner I (1978) Bromism: alive in well. Am J Psychiatry, 7: 857-858.
Breslin WJ, Zablotny CL, Bradley GF, & Lomax LG (1990) Methyl bromide
inhalation teratology study in New Zealand white rabbits. Midland,
Michigan, The Dow Chemical Company (Unpublished final report).
Bridges RG (1955) The fate of labelled insecticide residues in food
products. III. N-methylation as a result of fumigating wheat with
methyl bromide. J Sci Food Agric, 6: 261-268.
Brodniewicz A (1967) Poisoning of seamen with methyl bromide due to
fumigation of a Polish cargo ship in Haiphong (Vietnam). Arh Hig Rada,
18: 241-246 (in Polish with English translation).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: an assessment of available data (Contract-No. 68-02-3452).
Menlo Park, California, SRI International (EPA/DF-83/005a).
Bromine and Chemicals Ltd (1990) Methylbromide soil fumigation - use
and application instructions. London, Bromine and Chemicals Ltd, pp
1-36.
Brown G & Jenkinson DS (1971) Bromine in wheat grown on soil fumigated
with methyl bromide. Soil Sci Plant Anal, 2: 45-54.
Brown D & Rolston DE (1980) Transport and transformation of methyl
bromide in soils. Soil Sci, 130: 68-75.
Brown AL, Burau RG, Meyer RD, Raski DJ, Wilhelm S, & Quick J (1979)
Plant uptake of bromide following soil fumigation with methyl bromide.
Calif Agric, 33: 11-13.
Bruhin J (1942) [Percutaneous poisoning with methyl bromide during
pest control.] University of Zurich, Medical Faculty, 31 pp (M.D.
Thesis) (in German).
BUA (1987) [BUA Chemical Report 14: Methyl bromide.] Weinheim,
Advisory Committee for Environmental Priority Pollutants (BUA) of the
German Chemists Association, 64 pp (in German).
Burkholder WE (1966) Toxicity of methyl bromide to Acarus siro , a
cheese-infesting mite. J Econ Ent, 59: 110-112.
Butler ECB, Perry KMA, & Williams JRF (1945) Methyl bromide burns. Br
J Ind Med, 2: 30-31.
CAC (1986) Guide to Codex Alimentarius recommendations on pesticide
residues. Part 3. Guideline levels for pesticide residues. Rome, Food
and Agriculture Organization (CAC/PR 3-1986).
Cantineau A, Thomas R, Breurec JY, Bousser J, Baert A, Bonny JS,
Bouget J, Dubois C, Venisse T, & Curtes JP (1988) Acute methylbromide
poisoning. J Toxicol Clin Exp, 8: 83-87.
Canton JH, Wester PW, & Matthijssen-Spiekman, EAM (1983) Study on the
toxicity of sodium bromide to different freshwater organisms. Food
Chem Toxicol, 21: 369-378.
Carter B (1945) Methyl bromide poisoning. Effects on the nervous
system. Br Med J, 1: 43-45.
Castro CE & Belser NO (1981) Photohydrolysis of methyl bromide and
chloropicrin. J Agric Food Chem, 29: 1005-1008.
Castro JA, Zerba EN, De Licastro SA, Picollo MI, Wood EJ, Ruveda MA,
De Moutier Aldao EM, & Libertella R (1976) Toxicity of methyl bromide
and other gaseous insecticides to Triatoma infestans. Acta Physiol
Lat Am, 26: 106-114.
Cavanagh JB (1992) Methyl bromide intoxication and acute energy
deprivation syndromes. Neuropathol Appl Neurobiol, 18: 573-578.
CDFA (1988) Sampling for pesticide residues in California well water:
1988 update; well inventory data base. Sacramento, California,
Department of Food and Agriculture, Division of Pest Management,
Environmental Protection and Worker Safety, Environmental Monitoring
and Pest Management Branch, 66 pp (Report EH 88-10).
CEC (1985) Report of the Scientific Committee for Pesticides on the
use of methyl bromide as a fumigant for plant culture media.
Luxembourg, Commission of the European Communities, pp 15-33 (EUR
10211).
Chavez CT, Hepler RS, & Straatsma BR (1985) Methyl bromide optic
atrophy. Am J Ophthalmol, 99: 715-719.
Cheetham T (1990) Pathological alterations in embryos of the codling
moth (Lepidoptera: Tortricidae) induced by methyl bromide. Ann.
Entomol. Soc. Am., 83: 59-67.
Chellman GJ, White RD, Norton RM, & Bus JS (1986) Inhibition of the
acute toxicity of methyl chloride in male B6C3F1 mice by glutathione
depletion. Toxicol Appl Pharmacol, 86: 93-104.
Chen T, Kilpatrick RA, & Rich AE (1962) Stylet-bearing nematodes
associated with white clovers in New Hampshire. Plant Dis Rep, 46:
346-347.
Chisholm RD & Koblitsky L (1943) Sorption of methyl bromide by soil in
a fumigation chamber. J Econ Entomol, 36: 549-551.
Cicerone RJ, Heidt LE, & Pollock WH (1988) Measurements of atmospheric
methyl bromide and bromoform. J Geophys Res, 93: 3745-3749.
Cirilli L & Borgioli A (1986) Methyl bromide in surface drinking
waters. Gas-chromatographic determination by the headspace technique.
Water Res, 20: 273-275.
Clarke CA, Roworth CG, & Holling HE (1945) Methyl bromide poisoning.
Br J Ind Med, 2: 17-23.
Cochran JW (1988) A rapid, sensitive method for the analysis of
halogenated gases in water. J High Resol Chromatogr Chromatogr Commun,
11: 663-665.
Cooper DM, Griffiths NM, Hobson-Frohock A, Land DG, & Rowell JG (1978)
Fumigation of poultry food with methyl bromide: effects on egg
flavour, number and weight. Br Poult Sci, 19: 537-542.
Cornwell PB (1979) Health monitoring experience of fumigators using
methylbromide. Proceedings of the Fifth British Pest Control
Conference, Stratford-on-Avon, England, 26-29 September 1979. London,
British Pest Control Association (Sixth Session; Paper No. 17).
Cova D, Molinari GP, & Rossini L (1986) Residues after fumigation with
methyl bromide: bromide ion and methyl bromide in middlings and final
cereal foodstuffs. Food Addit Contam, 3: 235-240.
Crampton RF, Elias PS, & Gangolli SD (1971) The bromide content of
human tissue. Br J Nutr, 25: 317-322.
Daelemans A (1978) Uptake of methyl bromide by plantss and uptake of
bromide from decontaminated soils. Leuwen, Belgium, Catholic
University (Dissertation) (in Flemish).
Daft J (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71: 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1992) Headspace method for rapid determination of methyl
bromide in assorted nut samples. J Assoc Off Anal Chem, 75: 701-706.
Dagani MJ, Barda HJ, & Sanders DC (1985) Bromine compounds. Weinheim,
Verlag Chemie, pp 405-420.
Danse LHJC, Van elsen FL, & Van der Heijden CA (1984) Methylbromide:
carcinogenic effects in the rat forestomach. Toxicol Appl Pharmacol,
72: 262-271.
Darby JF, Dieter CE, & Rau GJ (1962) Evaluation of treatments for
control of soil-borne pests in celery seedbeds. Plant Dis Rep, 46:
441-443.
Davenport CJ, Ali SF, Miller FJ, Lipe GW, Morgan KT, & Bonnefoi MS
(1992) Effect of methyl bromide on regional brain glutathione,
glutathione- S-transferases, monoamines, and amino acids in F344
rats. Toxicol Appl Pharmacol, 112: 120-127.
Davis LN, Strange JR, Hoecker JE, Howard PH, & Santodonato J (1977)
Investigation of selected potential environmental contaminants:
monohalomethanes. Washington, DC, US Environmental Protection Agency
(EPA-560/2-77-007).
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1: 303-318.
De Heer H, Tuinstra LGMPH, Hamaker PH, & Van der burg AMM (1983) Use
of gas-tight plastic films during fumigation of glasshouse soils with
methylbromide. I. Significance of permeation and leakage for the
emission into the outside air. Acta Hortic, 152: 109-126.
Dempsey DA, Becker CE & Mihm FC (1992) Death after entering an
apartment building fumigated with methyl bromide and cleared for
habitation. Vet Hum Toxicol, 34: 356.
Deutschmann S, Reichel C, & Kettschau F (1989) Metabolism of methyl
bromide and methyl iodide in erythrocytes compared with methyl
chloride. Naunyn-Schmiedeberg's Arch Pharmacol, 339: R12.
Deutschmann S, Peter H, Reichel C, Bolt M, & Hallier E (1989)
[Kinetics of metabolism of methyl bromide and methyl iodide in
erythrocytes of humans and related species.] Verh Dtsch Gesundh
Arbeitsmed, 29: 517-519 (in German).
Devaney JA & Beerwinkle KR (1982) Control of the northern fowl mite on
inanimate objects by fumigation: field studies. Poult Sci, 62: 43-46.
Devries JW, Borge JM, Schroeder JP, Bowers RH, Larson PA, & Burns NM
(1985) Headspace gas chromatographic method for determination of
methyl bromide in food ingredients. J Assoc Off Anal Chem, 68:
1112-1116.
Djalali-Behzad G, Hussain S, Ostermann-Golker S, & Segerbaeck D (1981)
Estimation of genetic risks of alkylating agents. VI. Exposure of mice
and bacteria to methyl bromide. Mutat Res, 84: 1-9.
Doraiswamy S, Van Winckel A, & Van Assche C (1972) Effect in vitro of
soil fumigants on soil microbial population in relation to degradation
of tobacco mosaic virus. Meded Fac Landbouwwet Rijksuniv Gent, 37:
492-499.
Drawneek W, O'Brien MJ, Goldsmith HJ, & Bourdillon RE (1964)
Industrial methyl bromide poisoning in fumigators. Lancet, October 17:
855-856.
Dreef-Van der Meulen HC, Reuzel PGJ, Kuper CF, Hollanders VMH, & Feron
VJ (1989) Chronic inhalation toxicity of methyl bromide in rats. Third
International Symposium on Soil Disinfestation, Leuwen, Belgium,
September 26-30, 1988. Acta Hortic, 255: 313-315.
Driscoll JN, Duffy M, Pappas S, & Webb M (1987) Analysis for purgeable
organics in water by capillary GC/PID-ELCD. J chromatogr sci, 25:
369-375.
Drosihn UG, Stephan BR, & Hoffmann GM (1968) [Investigations into soil
contamination with methyl bromide.] Z Pflanzenkr Pflanzenschutz, 75:
272-287 (in German).
Dudley HC & Neal PA (1942) Methyl bromide as a fumigant for foods.
Food Res, 7: 412-429.
Dudley HC, Miller JW, Neal PA, & Sayers RR (1940) Studies on
foodstuffs fumigated with methyl bromide. Public Health Rep, 55:
2251-2282.
Duffy M, Driscoll JN, Pappas S, & Sanford W (1988) Analysis of ppb
levels of organics in water by means of purge-and-trap, capillary gas
chromatography and selective detectors. J Chromatogr, 441: 73-80.
Dumas T (1982) Trapping low levels of methyl bromide in air or as
residues at ambient and lower temperatures for gas chromatography. J
Assoc Off Anal Chem, 65: 913-915.
Dumas T & Bond EJ (1985) Analysis of methyl bromide at ultra low
concentration levels. J Agric Food Chem, 33: 276-278.
Duvoir M, Fabre R, & Layani F (1937). L'intoxication par le bromure de
méthyle. Bull Soc Méd Paris, 53: 1540.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards CA (1962) Springtail damage to bean seedlings. Plant Pathol,
11: 67-68.
Edwards CA & Thompson AR (1973) Pesticides and the soil fauna. In:
Gunther FA & Gunter JD, ed. Residue reviews. Residues of pesticides
and other contaminants in the total environment. Berlin, Heidelberg,
New York, Springer-Verlag, vol 45, pp 1-79.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
beta-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
El Buzz HK, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1974) Effect of
diet on the susceptibility of the different developmental stages of
Corcyra cephalonica staint. to carbon bisulphide and methyl bromide.
Agric Res Rev, 52: 21-29.
Eller PM (1985) NIOSH manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 2520/1-2520/-4 (NIOSH Publication No. 84-100).
EMBA (1988) Handling and transportation of methyl bromide. Code of
practice. Brussels, European Methyl Bromide Association, pp 1-8.
Enebak SA, Palmer MA, & Blanchette RA (1988) Effect of soil-borne
pathogen control methods on the production of white pine nursery
seedlings. Phytopathology, 78: 1532-1533.
Ethyl Corporation (1990a) Methyl bromide applications manual. Baton
Rouge, Louisiana, Ethyl Corporation, 3 pp.
Eustis SL, Haber SB, Drew RT, & Yang RSH (1988) Toxicology and
pathology of methyl bromide in F344 rats and B6C3F1 mice following
repeated inhalation exposure. Fundam Appl Toxicol, 11: 594-610.
Evans JE & Hastings L (1992) The effect of methyl bromide on measures
of olfactory function. Toxicologist, 12: 270.
Fabian P (1984) Atmosphere and environment. Berlin, Heidelberg, New
York, Springer-Verlag, p 41.
Fabian P, Borchers R, Penkett SA & Prosser NJD (1981) Halocarbons in
the stratosphere. Nature (Lond), 294: 733-735.
Fairall RJ & Scudamore KA (1980) Determination of residual methyl
bromide in fumigated commodities using derivative gas-liquid
chromatography. Analyst, 105: 251-256.
Fallico R & Ferrante M (1991) Exposure to inorganic bromides from
greenhouse crops where methyl bromide was applied for soil fumigation.
Zent.bl Hyg Umweltmed, 191: 555-562.
FAO (1980) Pesticide residues in food. Methyl bromide. Rome, Food and
Agriculture Organization of the United Nations, pp 89-97 (FAO Plant
Production and Protection Paper 20 Sup).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Group on Pesticide
Residues. Rome, Food and Agriculture Organization of the United
Nations; Geneva, World Health Organization (FAO Meeting Report No.
PL/1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1965b) Evaluation of hazards to consumers resulting from the
use of fumigants in the protection of food. Rome, Food and Agriculture
Organization of the United Nations; Geneva, World Health Organization
(FAO Meeting Report No. PL/1965/10/2; WHO/Food Add./28.65).
FAO/WHO (1988a) Pesticide residues in food - 1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environmental and the WHO Expert Group on Pesticide Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 92).
FAO/WHO (1988b) Pesticide residues in food - 1988 evaluations. Part II
-Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 93/2).
Filip GM & Roth LF (1977) Stump injections with soil fumigant to
eradicate Armillariella mellea from young-growth ponderosa pine
killed by root rot. Can J For Res, 7: 226-231.
Flick EW (1985) Industrial solvents handbook, 3rd ed. Park Ridge, New
Jersey, Noyes Data Corporation, 4 pp.
Foxboro (1989) Ambient air instrumentation sheet. East Bridgewater,
Maryland, The Foxboro Company.
Fransi A, Pons R, Sala A, Vallejo VR, & Bertran C (1987) Wheat and
soil bromide dynamics after fumigation with methyl bromide in a
mediterranean climate. Plant Soil, 98: 417-424.
Fuortes LJ (1992) A case of fatal methyl bromide poisoning. Vet Hum
Toxicol, 34: 240-241.
Furuta A, Hyakudo T, Ohnishi A, Hori H & Tanaka E (1993) Neurotoxicity
of methyl bromide - neuropathologic evaluation, preliminary study.
Sangyo Ika Daigaku Zasshi, 15: 21-27.
Gansewendt B, Foest U, Xu D, Hallier E, Bolt HM, & Peter H (1991)
Formation of DNA adducts in F-344 rats after oral administration or
inhalation of [14C]methyl bromide. Food Chem Toxicol, 29: 557-563.
Gargas ML & Andersen ME (1982) Metabolism of inhaled brominated
hydrocarbons: validation of gas uptake results by determination of a
stable metabolite. Toxicol Appl Pharmacol, 66: 55-68.
Garman JR, Freund T, & Lawless EW (1987) Testing for groundwater
contamination at hazardous waste sites. J Chromatogr Sci, 25: 328-337.
Garry VF, Nelson RL, Griffith J, & Harkins M (1990) Preparation for
human study of pesticide applicators: sister chromatid exchanges and
chromosome aberrations in cultured human lymphocytes exposed to
selected fumigants. Teratog Carcinog Mutagen, 10: 21-29.
Gentile IA, Ferraris L, Crespi S, & Belligno A (1989) The degradation
of methyl bromide in some natural fresh waters. Influence of
temperature, pH and light. Pestic Sci, 25: 261-272.
Gentile IA, Ferraris L, Sanguinetti M, Tiprigan M, & Fisichella G
(1992) Methyl bromide in natural fresh waters: hydrolysis and
volatilisation. Pestic Sci, 34: 297-301.
Gillotay D, Simon PC & Dierickx L (1989) Temperature dependence of
ultraviolet absorption cross-sections of brominated methanes an
ethanes. In: Bojkov RD & Fabian P, ed. Ozone in the atmosphere.
Proceedings of the Quadrennial Ozone Symposium 1988 and Tropospheric
Ozone Workshop, Göttingen, Germany, 4-13 August 1988. Hampton,
Virginia, Deepak Publishing, pp 706-709.
Goldman LR, Mengle D, Epstein DM, Feson D, Kelly K, & Jackson RJ
(1987) Acute symptoms in persons residing near a field treated with
the soil fumigants methyl bromide and chloropicrin. West J Med, 147:
95-98.
Gosselin RE, Smith RP, Hodge HC, & Braddock JE (1984) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams &
Wilkins Company, pp III/280-III/284.
Goulon M, Nouailhat F, Escourolle R, Zarranz-Imirizaldu JJ, Grosbuis
S, & Levy-Alcover MA (1975) Intoxication par le bromure de méthyle.
Trois observations, dont une mortelle. Etude neuro-pathologique d'un
cas de stupeur avec myoclonies, suivi pendant cinq ans. Rev. Neurol.,
131: 445-468.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Charles C. Thomas, pp 680-685.
Green M, Seiber JN, & Biermann HW (1991) in situ measurement of methyl
bromide in indoor air using long-path ftir spectroscopy. In: Schiff
HI, ed. Measurement of atmospheric gases. Proceedings spie - the
international society for optical engineering. Los angeles,
California, 2-3 January 1991 (Volume 1433). Washington, DC, The
International Society for Optical Engineering, pp 270-274.
Greenberg JO (1971) The neurological effects of methyl bromide
poisoning. Ind Med, 40: 27-29.
Greve PA & Grevenstuk WBF (1976) Optimization studies on the
determination of bromide residues in lettuce. Meded Fac Landbouwwet
Rijksuniv Gent, 41: 1371-1381.
Greve PA & Grevenstuk WBF (1979) Gas-liquid chromatographic
determination of bromide ion in lettuce: interlaboratory studies. J
Assoc Off Anal Chem, 62: 1155-1159.
Greve PA & Hogendoorn EA (1979) Determination of fumigant residues in
grain. Meded Fac Landbouwwet Rijksuniv Gent, 44: 877-884.
Griffiths NM, Hobson-Frohock A, Land DG, Levett JM, Cooper DM, &
Rowell JG (1978) Fumigation of poultry food with methyl bromide:
effects on flavour and acceptability of broiler meat. Br Poult Sci,
19: 529-535.
Grimm GR & Alexander AR (1971) Fumigation of phytophthora in sandy
soil by surface application of methyl bromide and methyl
bromide-chloropicrin. Plant Dis Rep, 55: 929-931.
Gryder-Boutet DE & Kennish JM (1988) Using headspace sampling with
capillary column GC-MS to analyze trace volatile organics in water and
wastewater. Am Water Works Assoc J, 80: 52-55.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Guillemin MP, Hillier RS, & Bernhard CA (1990) Occupational and
environmental hygiene assessment of fumigations with methyl bromide.
Ann Occup Hyg, 34: 591-607.
Guns MF (1989) Monitoring of the groundwater bromine content and plant
residues in methyl bromide fumigated glasshouses. Third International
Symposium on Soil Disinfestation, Leuven, Belgium, September 26-30,
1988. Acta Hortic, 255: 337-346.
Haber SB, Drew RT, Eustis S, & Yang RSH (1985) Methyl bromide
toxicity: a target organ? Toxicologist, 5: 130 (Abstract No. 518).
Haesanen E, Manninen PKG, Himberg K, & Väätäinen V (1990) Chlorine and
bromine contents in tobacco and tobacco smoke. J Radioanal Nucl Chem,
144: 367-372.
Hague NGM (1963) Fumigation of agricultural products. XVIII. Effect of
methyl bromide on the bent grass nematode, Anguina agrostis,
steinbuch 1799 , filipjev 1936, and on the germination of bent
grass, Agrostis tenuis. J Sci Food Agric, 14: 577-579.
Hague NGM & Clark WC (1959) Fumigation with methyl bromide and
chloropicrin to control seed-borne infestations of the stem eelworm.
Meded Landbouwhogesch Opzoekingasstn Gent, 24: 628-636.
Hallier E, Jaeger R, Deutschmann S, Bolt HM, & Peter H (1990a)
Glutathione conjugation and cytochrome p-450 metabolism of methyl
chloride in vitro. Toxic in vitro , 4: 513-517.
Hallier E, Deutschmann S, Reichel C, Bolt HM, & Peter H (1990b) A
comparative investigation of the metabolism of methyl bromide and
methyl iodide in human erythrocytes. Int Arch Occup Environ Health,
62: 221-225.
Hallier E, Langhof T, Dannappel D, Leutbecher M, Schröder K, Goergens
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methyl bromide, ethylene oxide and dichloromethane in human blood:
influence on the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol, 67: 173-178.
Hamilton I, Minski MJ, Milton EI, & Cleary JJ, (1972/73) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study. Sci
Total Environ, 1: 341-374.
Hansch C & Leo A (1979) Substituent constants for correlation in
chemistry and biology. New York, Chichester, Brisbane, Toronto, John
Wiley & Sons, p 173.
Hanson PR, Wainman HE, & Chakrabarti B (1987) The effects of methyl
bromide on the seed of some varieties of barley. Seed Sci Technol, 15:
155-162.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Belilies RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7: 66-75.
Harper DB (1985) Halomethane from halide ion - a highly efficient
fungal conversion of environmental significance. Nature (Lond), 315:
55-57.
Harrison BO, Peachey JE, & Winslow RO (1963) The use of nematicides to
control the spread of arabis mosaic virus by Xiphinema
diversicaudatum (Micol.). Ann Appl Biol, 52: 243-255.
Harry EG, Brown WB, & Goodship G (1972) The disinfecting activity of
methyl bromide on various microbes and infected material under
controlled conditions. J Appl Bacteriol, 35: 485-491.
Harsch DE & Rasmussen RA (1977) Identification of methyl bromide in
urban air. Anal Lett, 10: 1041-1047.
Hartill WFT & Campbell JM (1973) Control of sclerotinia in tobacco
seedbeds. Plant Dis Rep, 57: 932-934.
Hastings L (1990) Sensory neurotoxicology: use of the olfactory system
in the assessment of toxicity. Neurotoxicol Teratol, 12: 455-459.
Hastings L, Miller M, Minnema D, & Evans J (1989) Effects of methyl
bromide on rat olfactory system. Chem Senses, 14: 708.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res, 43:
1945-1950.
Heimann H (1944) Poisoning as result of industrial exposure to methyl
bromide. NY State Ind Bull, 23: 103-105.
Henschler D (1990) Chemicals harmful to health: toxicological-medical
establishment of MAK-values, 16th ed. Weinheim, VCH
Verlagsgesellschaft mbH.
Heungens A & Roos A (1982) L'influence du dazomet et du bromure de
méthyle sur la faune et la flore de la litière de pin. Rev Agric,
35(2): 2005-2050.
Heurtebise M & Ross WJ (1971) Application of 80m Br to the
semi-automated analysis of bromine in biological fluids. J Radioanal
Chem, 8: 5-12.
Heuser SG & Scudamore KA (1968) Fumigant residues in wheat and flour:
solvent extraction and gas-chromatographic determination of free
methyl bromide and ethylene oxide. Analyst, 93: 252-258.
Heuser SG & Scudamore KA (1970) Selective determination of ionised
bromide and organic bromides in foodstuffs by gas-liquid
chromatography with special reference to fumigant residues. Pestic
Sci, 1: 244-249.
Hezemans-Boer M, Toonstra J, Meulenbelt J, Zwaveling JH, Sangster B,
& Van Vloten WA (1988) Skin lesions due to exposure to methyl bromide.
Arch Dermatol, 124: 917-921.
Hicken NE (1961) Termite fumigation with methyl bromide. Chem Prod,
24: 205-207.
Hine CH (1969) Methyl bromide poisoning - a review of ten cases. J
Occup Med, 11: 1-10.
Hitchcock BE (1968) Progress of earth pearl studies in Queensland.
Proc Int Soc Sugar-Cane Technol, 13: 1382-1388.
Ho JSY (1989) A sequential analysis for volatile organics in water by
purge-and-trap capillary column gas chromatography with
photoionization and electrolytic conductivity detectors in series. J
Chromatogr Sci, 27: 91-98.
Hoffman GM & Malkomes HP (1974) Bromide residues in vegetable crops
after soil fumigation with methyl bromide. Agric Environ, 1: 321-328.
Hole BD (1981) Variation in tolerance of seven species of stored
product coleoptera to methyl bromide and phosphine in strains from
twenty-nine countries. Bull Entomol Res, 71: 299-306.
Holling HE & Clarke CA (1944) Methyl bromide intoxication. J R Navy
Med Serv, 30: 218-224.
Hommel G (1984) [Handbook of dangerous goods (Explanatory leaflet
127).] Berlin, Heidelberg, New York, Springer-Verlag (in German).
Honma T (1987) Alteration of catecholamine metabolism in rat brain
produced by inhalation exposure to methyl bromide. Jpn J Ind Health,
29: 218-219.
Honma T (1992) Brain microdialysis study of the effects of hazardous
chemicals on the central nervous system. 1. Changes in monoamine
metabolites induced by cerebral methyl bromide administration measured
by two-probe microdialysis (TPMD) method. Ind Health, 30: 47-60.
Honma T, Sudo A, Miyagawa M, & Sato M (1982) Significant changes in
monoamines in rat brain induced by exposure to methyl bromide.
Neurobehav Toxicol Teratol, 4: 521-524.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Changes in
free amino acid contents of rat brain induced by exposure to methyl
bromide. Toxicol Lett, 15: 317-321.
Honma T, Miyagawa M, Sato M, & Hasegawa H (1985) Neurotoxicity and
metabolism of methyl bromide in rats. Toxicol Appl Pharmacol, 81:
183-191.
Honma T, Muneyuki M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain. Neurotoxicol
Teratol, 9: 369-375.
Honma T, Miyagawa M, & Sato M (1991) Inhibition of tyrosine
hydroxylase activity by methyl bromide exposure. Neurotoxicol Teratol,
13: 1-4.
Hubbs AF & Harrington DD (1986) Further evaluation of the potential
gastric carcinogenic effects of subchronic methyl bromide
administration. In: Proceedings of the 36th Meeting of the American
College of Veterinary Pathology and the Annual Meeting of the American
Society for Veterinary and Clinical Pathology, Denver, Colorado,
December 1985. Denver, Colorado, American Society for Veterinary and
Clinical Pathology, p 92.
Hurtt ME & Working PK (1988) Evaluation of spermatogenesis and sperm
quality in the rat following acute inhalation exposure to methyl
bromide. Fundam Appl Toxicol, 10: 490-498.
Hurtt ME, Morgan KT, & Working PK (1987) Histopathology of acute toxic
responses in selected tissues from rats exposed by inhalation to
methyl bromide. Fundam Appl Toxicol, 9: 352-365.
Hurtt ME, Thomas DA, Working PK, Monticello TM, & Morgan KT (1988)
Degeneration and regeneration of the olfactory epithelium following
inhalation exposure to methyl bromide: pathology, cell kinetics, and
olfactory function. Toxicol Appl Pharmacol, 94: 311-328.
Hustinx WNM, Van de Laar RTH, Van Huffelen AC, Vrey JC, Meulenbelt J,
& Savelkoul TJF (1993) Systemic effects of inhalation methyl bromide
poisoning: a study of nine cases occupationally exposed due to
inadvertent spread during fumigation. Br J Ind Med, 50: 155-159.
Hyman MR & Wood PM (1984) Bromocarbon oxidations by Nitrosomonas
europaea. In: Crawford RL & Hanson RS ed. Microbial growth on C1
compounds. Washington, DC, American Society for Microbiology, pp
49-52.
IARC (1986) Methyl bromide. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 187-212 (IARC Monograph on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1-42. Lyon, International Agency for Research
on Cancer, p 245 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Ikawa N, Araki A, Nozaki K, & Matsushima T (1986) Micronucleus test of
methyl bromide by the inhalation method. Mutat Res, 164: 269
(Abstract).
Ikeda T, Kishi R, Yamamura K, Miyake H, & Sato M (1980) Behavioural
effects in rats following repeated exposure to methyl bromide. Toxicol
Lett, 6: 317-321.
Ingram FR (1951) Methyl bromide fumigation control in the date-packing
industry. Arch Ind Hyg Occup Med, 4: 193-198.
Inouye T, Inouye N, Asatani M, & Mitsuhata K (1967) Cucumber green
mottle mosaic virus in Japan. III. Inactivation of cucumber green
mottle mosaic virus in infected plant tissue buried in soil treated
with methyl bromide. Nogaku Kenkyu, 51: 199-207.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1940) The response
attending exposure of laboratory animals to vapors of methyl bromide.
J Ind Hyg Toxicol, 22: 218-230.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1941) Chemical changes of
methyl bromide in the animal body in relation to its physiological
effects. J Ind Hyg Toxicol, 23: 408-411.
Isa AL, Fam EZ, Kamel AH, & Awadallah WH (1970) On the effect of
certain fumigants on the overwintering corn borers larvae. Agric Res
Rev, 48: 43-47.
Ishizu S, Kato N, Morinobu S, Nagao N, Yamano Y, & Ito I (1988) [Cases
of severe methyl bromide poisoning residing above a warehouse.] Jpn J
Ind Health, 30: 54-60 (in Japanese with English abstract).
Ito KA, Seeger ML, & Lee WH (1972) Destruction of Byssochlamys fulva
asci by low concentrations of gaseous methyl bromide and by aqueous
solutions of chlorine, an iodophor, and peracetic acid. J Appl
Bacteriol, 35: 479-483.
Iwasaki K (1988a) Determination of S-methylcysteine in mouse
hemoglobin following exposure to methyl bromide. Ind Health, 26:
187-190.
Iwasaki K (1988b) Individual differences in the formation of
hemoglobin adducts following exposure to methyl bromide. Ind Health,
26: 257-262.
Iwasaki K, Ito I, & Kagawa J (1989) Biological exposure monitoring of
methyl bromide workers by determination of hemoglobin adducts. Ind
Health, 27: 181-183.
Japanese Ministry of Labour (1992) Toxicology and carcinogenesis
studies of methyl bromide in F344 rats and BDF mice (inhalation
studies). Tokyo, Industrial Safety and Health Association, Japanese
Bioassay Laboratory, 197 pp (Unpublished report).
Jaskot RH, Grose EC, Most BM, Menache MG, Williams TB, &
Roycroft JH (1988) The distribution and toxicological effects of
inhaled methyl bromide in the rat. J Am Coll Toxicol, 7: 631-642.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 3: 777-782.
JEA (Japanese Environmental Agency) (1981) Chemicals in the
environment. Tokyo, Japanese Environmental Agency, Office of Health
Studies, Environmental Health Department, pp 92-93.
Johnstone RT (1945) Methyl bromide intoxication of a large group of
workers. Ind Med, 14: 495-497.
Jones L (1968) effect of methyl bromide treatments on several species
of conifer seed. J For, 8: 858-860.
Joshi GP (1974) Toxicity of certain chemicals on Oryzaephilus
mercator F. (Coleoptra: Cucujidae). Appl Entomol Zool, 9: 280-281.
Kallio H & Shibamoto T (1988) Direct capillary trapping and gas
chromatographic analysis of bromomethane and other highly volatile air
pollutants. J Chromatogr, 454: 392-397.
Kantarjian AD & Shaheen AS (1963) Methyl bromide poisoning with
nervous system manifestations resembling polyneuropathy. Neurology,
13: 1054-1058.
Kato N, Morinobu S, & Ishizu S (1986) Subacute inhalation experiment
for methyl bromide in rats. Ind Health, 24: 87-103.
Katz AJ (1985) Genotoxicity of methyl bromide in somatic cells of
Drosophila larvae. Environ Mutagen, 7: 13 (Abstract).
Katz AJ (1987) Inhalation of methyl bromide gas induces mitotic
recombination in somatic cells of Drosophila melanogaster. Mutat Res,
192: 131-135.
Kelley WD & Rodriguez-Kabana R (1979) Effects of sodium azide and
methyl bromide on soil bacterial populations, enzymic activities and
other biological variables. Pestic Sci, 10: 207-215.
Kempton RJ & Maw GA (1974) Soil fumigation with methyl bromide: the
phytotoxicity of inorganic bromide to carnation plants. Ann Appl Biol,
76: 217-229.
Kenaga EE (1961) Time, temperature and dosage relationships of several
insecticidal fumigants. J Econ Entomol, 54: 537-542.
Khalil MAK & Rasmussen RA (1985) The trend of bromodifluoromethane
(CBrCIF2) and the concentration of other bromine containing gases at
the South Pole. Antarct J US, 21: 206-207.
Khalil MAK, Rasmussen RA, & Gunawardena R (1993) Atmospheric methyl
bromide: Trends and global mass balance. J Geophys Res, 98: 2887-2896.
Khanna SC & Yadav TD (1987) Effect of methyl bromide fumigation on the
germinability of wheat seed. Seed Res, 15: 183-186.
Kiewnick L (1968) Occurrence and control of sclerotium rolfsii.
Meded Fac Landbouwwet Rijksuniv Gent, 33: 987-995.
King JR & Benschoter CA (1991) Comparative methyl bromide residues in
Florida citrus: A basis for proposing quarantine treatments against
caribbean fruit fly. J Agric Food Chem, 39: 1307-1309.
King JR, Benschoter CA, & Burditt AK, Jr (1981) Residues of methyl
bromide in fumigated grapefruit determined by a rapid, headspace
assay. J Agric Food Chem, 29: 1003-1005.
Kishi R, Ishizu I, Ito I, Katoh N, Miyake H, & Harabuch I (1988)
Health research of methyl bromide manufacturing workers. Part 1.
Symptoms of long-term exposure. Occupational health in the chemical
industry. XXII ICOH (International Commission on Occupational Health)
Congress, Sydney, New South Wales, Australia, 27 September-2 October
1987. Copenhagen, World Health Organization Regional Office for
Europe, pp 120-134.
Kishi R, Itoh I, Ishizu S, Harabuchi I, & Miyake H (1991) Symptoms
among workers with long-term exposure to methyl bromide. An
epidemiological study. Jpn J Ind Health, 33: 241-250.
Kisser W (1967) [The detection and quantitative determination of
brominated urea derivatives in toxicology.] Arch Toxicol, 22: 404-409
(in German).
Kissler JJ, Lider JV, Raabe RD, Raski DJ, Schmitt RV, & Hurlimann JH
(1973) Soil fumigation for control of nematodes and oak root fungus in
vineyard replants. Plant Dis Rep, 57: 115-119.
Klein L (1989) Methyl bromide strip fumigation - problems encountered
and ways to combat them. Third International Symposium on Soil
Disinfestation, Leuven, Belgium, September 26-30, 1988. Acta Hortic,
255: 213-225.
Knight HD & Costner GC (1977) Bromide intoxication of horses, goats,
and cattle. J Am Vet Med Assoc, 171: 446-448.
Knight HD & Reina-Guerra M (1977) Intoxication of cattle with sodium
bromide-contaminated feed. Am J Vet Res, 38: 407-409.
Koga M, Hara K, Hori H, Kodama Y, & Okubo T (1991) [Determination of
bromide ion concentration in urine using a head-space gas
chromatography and an ion chromatography. Biological monitoring for
methyl bromide exposure.] J Univ Occup Environ Health, 13: 19-24 (in
Japanese with English summary).
Kolbezen MJ & Abu-El-Haj FJ (1972) Fumigation with methyl bromide. II.
Equipment and methods for sampling and analysing deep field soil
atmospheres. Pestic Sci, 3: 73-80.
Kramers PGN, Bissumbhar B, & Mout HCA (1985b) Studies with gaseous
mutagens in Drosophila melanogaster. In: Waters MD, Sandhu SS, Lewtas
J, Claxton L, Straus G, & Nesnow S, ed. Short-term bioassays in the
analysis of complex environmental mixtures IV., New York, London,
Plenum Press, pp 65-73.
Kramers PGN, Voogd CE, Knaap AGAC, & Van der Heijden CA (1985a)
Mutagenicity of methyl bromide in a series of short-term tests. Mutat
Res, 155: 41-47.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Latta R & Cowgill H (1941) Methyl bromide fumigation of greenhouse
plants at U.S. Plant Introduction Garden, Glenn Dale, Md. (Abstract
S.8189, Bureau of Entomology and Plant Quarantine). Washington, DC, US
Department of Agriculture (E-526).
Laug EP (1941) Bromide residues in foodstuffs. Volatile and
nonvolatile residues following experimental exposure to methyl
bromide. Ind Eng Chem, 33: 803-805.
Leesch JG, Gillenwater HB, & Woodward JO (1974) Methyl bromide
fumigation of shelled peanuts in bulk containers. J Econ Entomol, 67:
769-771.
Lefevre C, Ferrari P, Günier JP, & Muller J (1989) Sampling and
analysis of airborne methylbromide. Chromatographia, 27: 37-43.
Leichnitz K (1985) [Dräger-tubes methyl bromide 3/a. Detector-tubes
pocket book 6.] Lübeck, Dragerwerk (in German).
Lepschy J, Stark H, & Sub A (1979) [Behaviour of methyl bromide
(Terabol) in soil.] Gartenbauwissenschaften, 44: 84-89 (in German).
Lewis SE (1948) Inhibition of SH proteins by methyl bromide. Nature
(Lond), 161: 692-693.
Linenberg A, Davis R, & Robinson D (1991) Hazardous waste site
measurements of low levels of chlorinated hydrocarbons using a
portable gas chromatography. Hazard Mater Control, 4: 42-46.
Long PL, Brown WB, & Goodship G (1972) Effect of methyl bromide on
coccidial oocysts determined under controlled conditions. Vet Rec, 90:
562-566.
Longley EO & Jones AT (1965) Methyl bromide poisoning in man. Ind Med
Surg, 34: 499-502.
Lopez VC, Fernandez JAM, Yanez LP, & Vazquez MS (1986a) [Methyl
bromide poisoning: I. Some historical, clinical, legal and
neuropsychiatric aspects.] Arch Neurobiol, 49: 279-290 (in Spanish).
Lopez CV, Fernandez MJA, Yanez PL, & Vazquez SM (1986b) [Methyl
bromide poisoning. II. Contribution of 2 cases.] Arch Neurobiol, 49:
311-328 (in Spanish).
Lopez-Avila V, Wood R, Flanagan M, & Scott R (1987) Determination of
volatile priority pollutants in water by purge and trap and capillary
column gas chromatography/mass spectrometry. J Chromatogr Sci, 25:
286-291.
Love JL, Winchester RV, & Keating DL (1979) Fumigant and contact
insecticide residues on dried fruits, cereals, nuts, and spices
imported into New Zealand. N Z J Sci, 22: 95-98.
Lovelock JE (1975) Natural halocarbons in the air and in the sea.
Nature (Lond), 256: 193-194.
Lynn GE, Shrader SA, Hammer OH, & Lassiter CA (1963) Occurrence of
bromides in the milk of cows fed sodium bromide and grain fumigated
with methyl bromide. J Agric Food Chem, 11: 87-91.
Mabey W & Mill T (1978) Critical review of organic compounds in water
under environmental conditions. J Phys Chem Ref Data, 7: 383-415.
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds, Report No. 32 - Individual compound report: methyl bromide.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, 190 pp (PB83-130211).
Mackay D & Shiu WK (1981) A critical review of Henry's Law Constants
for chemicals of environmental interest. J Phys Chem Ref Data, 10:
1175-1199.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. In: Gunter FA
ed. Reviews of environmental contamination and toxicology. Berlin,
Heidelberg, New York, Springer-Verlag, vol 14, pp 57-123.
MAFF (1990) Report of the working party on pesticide residues:
1988-89. Supplement to issue No. 8 (1990) of the pesticides register.
London, Ministry of Agriculture, Fisheries and Food, Health and Safety
Executive, 11 pp.
MAFF (1989) Report of the working party on pesticide residues:
1985-88. The twenty-fifth report of the steering group on food
surveillance. London, Ministry of Agriculture, Fisheries and Food, 3
pp (Food Surveillance Paper No. 25).
Malkomes H-P (1970) [Investigations into the degradation of methyl
bromide during substrate fumigation and its influence on the following
crops.] Hanover, Technical University, pp 18-35 (Dissertation) (in
German).
Marraccini JV, Thomas GE, Ongley JP, Pfaffenberger D, Davis JH, &
Bednarczyk LR (1983) Death and injury caused by methyl bromide, an
insecticide fumigant. J Forensic Sci Soc, 28: 601-607.
Matheson gas data book (1980) The Matheson gas data book. East
Rutherford, New Jersey, Matheson Gas products (A Division of Will
Ross), vol 6, pp 361-363.
Matta A & Porta-Puglia A (1968) [Sensitivity to methyl bromide and
vapam and secondary growth in fumigated soil of distinct functional
groups of microorganisms.] Ann Fac Agrar, 4: 261-274 (in Italian).
Maw GA & Kempton RJ (1973) Methyl bromide as a soil fumigant. Soils
Fertil, 36: 41-47.
Mazzini L, Galante M, Rezzonico M, & Kokodoko A (1992) Methylbromide
intoxication: A case report. Schweiz Arch Neurol Psychiatr, 143:
75-80.
MBSW (1992) Proceedings of the Methyl Bromide Science Workshop,
Washington, DC, 2-3 June 1992. Washington, DC, Atmospheric
Environmental Research, Inc., pp 90-97.
Medinsky MA, Bond JA, Dutcher JS, & Birnbaum LS (1984) Disposition of
[14C]methyl bromide in Fischer-344 rats after oral or
intraperitoneal administration. Toxicology, 32: 187-196.
Medinsky MA, Dutcher JS, Bond JA, Henderson RF, Mauderly JL, Snipes
MB, Mewhinney JA, Cheng YS, & Birnbaum LS (1985) Uptake and excretion
of [14C]methyl bromide as influenced by exposure concentration.
Toxicol Appl Pharmacol, 78: 215-225.
Meister RT (1985) In: Meister RT ed. Farm chemicals handbook 85.
Pesticide dictionary -Section C. Willoughby, Ohio, Meister Publishing
Co., 154 pp.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992a) Atmospheric lifetimes and ozone
depletition potentials of methyl bromide (CH3Br) and dibromomethane
(CH2Br2). Geophys Res Lett, 19: 2059-2062.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992b) Correction to "atmospheric lifetimes and
ozone depletion potentials of methylbromide (CH3Br) and
dibromomethane (CH2Br2)". Geophys Res Lett, 19: 2279-2280.
Merzbach L (1928) [The pharmacology of methyl bromide and some related
compounds.] Z. Ges. Exp. Med., 63: 383-392 (in German).
Mignard E & Benet JC (1989) Diffusion of methyl bromide in soil. J
Soil Sci, 40: 151-165.
Miller DP & Haggard HW (1943) Intracellular penetration of bromide as
a feature in the toxicity of alkyl bromides. J Ind Hyg Toxicol, 25:
423-433.
Minton NA & Gillenwater HB (1973) Methyl bromide fumigation of
Pratylenchus brachyurus in peatnut shells. J Nematol, 5: 147-149.
Mitsumori K, Maita K, Kosaka T, Miyaoka T, & Shirasu Y (1990) Two-year
oral chronic toxicity and carcinogenicity study in rats of diets
fumigated with methyl bromide. Food Chem Toxicol, 28: 109-119.
Miyagawa M (1982) Conditioned taste aversion induced by inhalation
exposure to methyl bromide in rats. Toxicol Lett, 10: 411-416.
Mizyukova IG & Bakhishev CN (1971) [Specific treatment of acute
poisoning with methyl bromide.] Vrach Delo, 7: 128-131 (in Russian
with English abstract).
Moelwyn-Hughes EA (1938) The hydrolysis of the methyl halides. Proc R
Soc, A164: 295-306.
Moje W (1960) The chemistry and nematocidal activity of organic
halides. In: Metcalf RL, ed. Volume III. Advances in pest control
research. New York, Intersience Publishers, pp 181-217.
Molina LT, Molina MJ, & Rowland FS (1982) Ultraviolet absorption cross
sections of several brominated methanes and ethanes of atmospheric
interest. J Phys Chem, 86: 2672-2676.
Monro HAU, Dumas T, & Ruckland CT (1966) The influence of vapour
pressure of different fumigants on the mortality of two stored-product
insects in vacuum fumigation. J Stored Prod Res, 1: 207-222.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrissey RE, Schwetz BA, Lamb JCIV, Ross MD, Teague JL, & Morris RW
(1988) Evaluation of rodent sperm, vaginal cytology, and reproductive
organ weight data from National Toxicology Program 13-week studies.
Fundam Appl Toxicol, 11: 343-358.
Mostafa SAS, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1972) Toxicity
of carbon bisulphide and methyl bromide to the eggs of four stored
product insects. J Stored Prod Res, 8: 193-198.
Mounat A & Hitier H (1959) Chemical disinfection of the soil of
tobacco seed beds. Ann Inst Exp Tabac Bergerac, 3: 287-298.
Mueller A, Jorritsma U, Hindermeier U, & Gansewendt B (1991) Human
serum albumin adducts as a parameter for biomonitoring of exposure to
methyl bromide. Naunyn-Schmiedeberg's Arch Pharmacol, 344: R121.
Mueller A, Jorritsma U, & Gansewendt B (1992) Different binding of
methyl bromide to blood macromolecules due to conjugator status.
Naunyn-Schmiedeberg's Arch Pharmacol, 345: R 42.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol, 17:
405-429.
Munnecke DE, Kolbezen MJ, & Stolzy LH (1969) Factors affecting field
fumigation of citrus soils for control of Armillaria mellea.
Proceedings of the First International Citrus Symposium. Lancaster,
Pennsylvania, Society of the Plastics Industry, vol 3, pp 1273-1277.
Munnecke DE, Kolbezen MJ, & Wilbur WD (1978) Types and thickness of
plastic films in relation to methyl bromide fumigation. Proceedings of
the Agricultural Plastic Congress, San Diego, pp 482-487.
National Academy of Science (1978) Chloroform, carbon tetrachloride,
and other halomethanes: an environmental assessment. Washington, DC,
National Academy of Sciences, 294 pp.
NASA (1992) Chemical kinetics and photochemical data for use in
stratospheric modeling. Evaluation No. 10: NASA Panel for Data
Evaluation. Pasadena, California Institute of Toxicology, Jet
Propulsion Laboratory, National Aeronautics and Space Administration.
NFPA (1984) Fire protection guide on hazardous materials, 9th ed.
Quincy, Maryland, National Fire Protection Agency, 5 pp (NFPA No.
SPP-1E).
NIOSH (1984) Monohalomethanes: methyl chloride CH3Cl, methyl bromide
CH3Br, methyl iodide CH3I. Curr Intell Bull, 42: 1-22.
Nishimura M, Umeda M, Ishizu S, & Sato M (1980) Effect of methyl
bromide on cultured mammalian cells. J Toxicol Sci, 5: 321-330.
NTP (1992) Toxicology and carcinogenesis studies of methyl bromide
(CAS No.
Tanaka et al. (1991) measured ambient methyl bromide
concen-trations and urinary bromine concentrations at various plant
quarantine fumigation sites in Japan. The geometric mean for exposed
workers was 9.0±1.85 mg/litre while the arithmetic mean for a matched
control population was 6.3±2.5 mg/litre. The authors found a
statistically significant positive correlation between the ambient
methyl bromide concentrations collected with a personal sampling
device and the urinary concentrations.
9.4.4 Haemoglobin adducts as a biological index to methyl bromide
exposure
Iwasaki et al. (1989) determined the haemoglobin adduct
(haemoglobin MeCys) in methyl bromide manufacturing workers, and
examined its effectiveness as a biological index of exposure to methyl
bromide. Methyl bromide reacts with cysteine to form
S-methylcysteine (MeCys) in haemoglobin (Djalali-Behzad et al.,
1981). It was suggested that determination of MeCys in haemoglobin
could detect previous methyl bromide exposure levels so low that they
were not detected by a special medical check-up (Iwasaki et al.,
1989). Haemoglobin adducts have a life span of about 2 months, so
workers only intermittently exposed to methyl bromide would also be
detected in such a survey. Previous studies by the same authors showed
individual differences in mice in the levels of MeCys within each dose
and time series group (Iwasaki, 1988a,b). These individual differences
may be caused by the differential susceptibility of haemoglobin in red
blood cells or individual differences in methyl bromide metabolism.
These differences were found to be minor in the human study (Iwasaki
et al., 1989).
Results from animal studies (Xu et al., 1990) supported the
suggestion of Iwasaki et al. (1989) that the Hb adduct of methyl
bromide might be a useful parameter in the biological exposure
monitoring of methyl bromide workers.
9.4.5 Neurobehavioural and other studies
Anger et al. (1986) carried out a neurobehavioural study on soil
fumigators who were exposed to 9 mg methyl bromide/m3 over an 8-h
day and structural fumigators who were exposed to 0-9 mg/m3 for 1.5
h per day. The fumigators, who reported a significantly higher
prevalence of 18 symptoms, consistent with methyl bromide toxicity,
than the controls, did not perform so well on 23 out of 27 behavioural
tests, and were significantly lower on one test of finger sensitivity
and one of cognitive performance.
EEGs and transaminases were measured in 33 methyl bromide workers
engaged in soil disinfection inside greenhouses in the Netherlands.
Symptoms of methyl bromide intoxication were determined by means of a
specially designed questionnaire. A general neurological examination
was performed. No differences compared with controls were found, with
the exception of slight electro-encephalographic changes in 10 workers
involving diffuse increase of beta and theta activity. Workers with
abnormal EEGs had higher blood bromide levels (geometric mean 10.9
mg/litre compared with a mean of 8.2 mg/litre in the other workers;
the difference was statistically significant ( P <0.05) (Verberk et
al., 1979).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1 Human exposure
Human exposure to methyl bromide occurs predominantly as an
occupational hazard, particularly during soil or bulk fumigation, but
also during manufacture. Individuals in the vicinity of fumigated
fields or buildings may also be exposed. Methyl bromide has widespread
use for fumigating post-harvest foods, such as cereals, spices,
dried-fruits, and nuts, as well as fresh fruits and vegetables. The
determination of levels of methyl bromide and inorganic bromide in the
fumigated food is important for the assessment of possible risks for
human and animal health. Methyl bromide levels in food commodities
usually decrease rapidly after aeration, but may be detected for some
weeks after treatment. Fumigation with methyl bromide results in
increased levels of inorganic bromide in food commodities and,
equally, in produce grown on fumigated soils. Fumigant dosage
standards should be adhered to, so that the bromide levels do not
exceed the recommended limits.
The major health concern is from acute exposure.
Delayed onset of symptoms may occur. Fatal poisoning has resulted
from exposures to relatively high concentrations (from 33 000 mg/m3
or 8600 ppm onwards) of methyl bromide vapours. Non-fatal poisoning
has resulted from exposure to concentrations as low as 390-1950
mg/m3 (100- 500 ppm). Organs affected by exposure include the
nervous system, lung, nasal mucosa, kidney, eye, and skin. There are
no epidemiological data on reproductive toxicity and carcinogenicity
in humans. There are no data on any human health effects of methyl
bromide residues in food or drinking-water.
10.1.1 Relevant animal studies
Methyl bromide is very toxic for all animal species by all routes
of administration studied. Deaths from exposure follow a steep
dose-response curve. LC50 (1-h) values for mice and rats are 4680
and 7300 mg/m3, respectively.
Deaths and neurotoxicity occur within hours or days after a
single inhalation exposure at high concentrations. Studies on mice
show that more prolonged exposure at low concentrations (6 h/day; 389
mg/m3) may also produce neurotoxicity or deaths, appearing with a
delayed onset of several months.
Absorption and distribution to various tissues is rapid, as is
elimination. The metabolic pathways and mode of action are unknown.
The principal toxic effects associated with lethality occur in
the brain and kidney. Histopathology of the brain shows necrosis of
granular cells of the cerebellum in mice and rats and neuronal
necrosis in the cerebral cortex, hippocampus, and thalamus, in rats.
The lowest doses at which these lesions were observed were 250
mg/m3, 6 h/day, for 2 years, in mice, and, 622 mg/m3, 6 h/day, for
5 weeks, in rats. In the kidney, necrosis of the convoluted tubule
epithelium occurs at doses of 599 mg/m3, 6 h/day, for 2 weeks, in
mice, and 1712 mg/m3, 6 h/day, for 2 weeks, in rats. Other effects
are observed in the heart (degeneration of focal necrosis), nose
(necrosis of olfactory epithelium), testis (degeneration of
seminiferous tubules), and other organs. In non-lethal exposure, nasal
irritation and neuro-toxicity (including histopathological lesions)
are the major toxic effects.
No teratogenic effects have been observed in rats or rabbits.
Embryotoxicity occurred in rats and rabbits only at doses that were
also maternally toxic. In a rat multigeneration study, there were
reductions in the fertility index in the second generation, when the
animals were exposed to 117 or 350 mg/m3 (30 or 90 ppm) for 6 h/
day, but effects were not observed following exposure to 12 mg/m3 (3
ppm) for 6 h/day.
Methyl bromide was mutagenic in several in vivo and in vitro
assays.
Long-term inhalation studies on rats and mice did not reveal any
evidence of carcinogenicity. Lesions originally interpreted as
carcinomas of the forestomach in rats, following gavage
administration, were shown in a subsequent study to regress after
termination of treatment, and were considered not relevant for human
risk assessment.
10.2 Environment
Methyl bromide is predominantly a naturally occurring compound.
Oceans are believed to be a major natural source of methyl bromide.
Another source(s) may exist in the tropics, which is yet to be
explained. Anthropogenic sources from fumigation and, to a much lesser
extent, motor vehicles (from the combustion of organic bromine
additives in leaded petrol) add to these. Present data indicate that
the globally averaged atmospheric abundance of methyl bromide is
between 9 and 13 pptv, equivalent to a total atmospheric loading of
150-220 thousand tonnes. If the atmospheric lifetime is two years
(assuming only atmospheric removal processes are significant),
anthropogenic sources of methyl bromide represent about 25% (±10%) of
the total emissions. The world production of methyl bromide in 1990
was 69 000 tonnes, having increased at a rate of 6% per year from 1984
to 1990. About 50% of the methyl bromide produced is released into the
atmosphere during, or after, use. Although methyl bromide reacts with
the hydroxyl radical in the troposphere, some methyl bromide is
transferred by upward diffusion to the stratosphere, where it
photolyses. Active bromine species react with ozone in the lower
stratosphere and are partly responsible for the depletion of the ozone
layer. It is estimated that anthropogenic releases of methyl bromide
cause about 3% of the present total stratospheric ozone loss.
Methyl bromide is used for soil fumigation (about 77%), for
quarantine, commodity fumigation (12%), for structural fumigation
(5%), and for chemical intermediates (6%).
In soil, methyl bromide is degraded by hydrolysis and microbial
activity. The remainder (about 50%) eventually dissipates into the
atmosphere. The degradation product, principally as inorganic bromide,
remains as a residue in soil.
Methyl bromide has herbicidal properties. In the vicinity of
greenhouses and fumigated structures, phytotoxic effects may occur.
Visible damage to the leaves of lettuce (a sensitive test plant) was
noticed at 400 mg/m3.
Soil fumigation using methyl bromide (with 2% chloropicrin)
affects both target and non-target organisms: various soil microflora
and fauna are adversely affected, at least temporarily, by fumigation.
High mortality of non-target insects has been noticed from fumigation
under plastic sheeting. Methyl bromide could be detected in different
soil types up to 3 weeks after the treatments, the highest levels
being found in the upper layers (0-40 cm) of the soil.
Methyl bromide is highly toxic for aquatic organisms, though it
is generally of no risk to the aquatic environment. The lowest EC50
or LC50 values reported are 2.8 mg/litre for algae, 1.7 mg/litre for
daphnids, and 0.3 mg/litre for fish. NOEC levels, derived from
long-term studies, as low as 0.06 mg/litre have been reported for
daphnids and fish. Toxic concentrations are not expected to be reached
under normal circumstances, because most of the methyl bromide applied
on soil is degraded or lost, due to evaporation, before it reaches
surface water via run-off. Only in very special situations (leaching
of greenhouse soils to reduce inorganic bromide residues), can levels
of methyl bromide in the mg/litre range occur in water. Concentrations
of up to 9.3 mg methyl bromide/litre were measured in drainage water.
Similarly, relatively high levels of bromide (up to 72 mg/litre)
that could adversely affect aquatic organisms were measured in
drainage water from greenhouses. Long-term exposure to bromide ion
resulted in an EC50 value of 27 mg bromide/litre for daphnia and the
lowest NOEC for different fish species was 25 mg bromide/litre.
11. RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH AND THE
ENVIRONMENT
11.1 Human health protection
In most countries, methyl bromide is strictly regulated for
application as a fumigant for soil, commodities, and structural
purposes. Adherence to good practices and guidelines should ensure no
adverse effects on persons exposed occupationally. Considerable care
should be taken by manufacturers in the production process of methyl
bromide and by suppliers in its transfer and use.
Methyl bromide does not pose a significant risk for the general
population. It is, however, a very toxic substance and precautions
have to be taken: exposure of the general public should be avoided
through adequate precautions in fumigated buildings, greenhouses, and
stores used for commodity fumigation. Fumigated premises should be
clearly marked, in order to prevent accidental exposure of
individuals.
11.2 Environmental protection
Emission of methyl bromide from anthropogenic sources should be
reduced as far as possible. It is, therefore, desirable to reduce
emissions from soil, commodity, and structural fumigation.
Improvements should be sought in these areas:
(1) improved injection methods;
(2) better barrier films;
(3) better measurement of the efficacy of methyl bromide, with
the goal of lower dosage rates where possible.
For commodity and, possibly, for structural fumigation,
techniques should be developed to:
(1) seal fumigation chambers more tightly;
(2) capture and recycle fumigation gas.
11.3 Recommendations for further research
There is a need for:
- study of the metabolism and toxic mechanisms of methyl
bromide;
- a postnatal behavioural study;
- a human epidemiological study including exposure
assessment;
- development of a treatment protocol for cases of human
poisoning.
Effects of methyl bromide on the depletion of the ozone layer are
not yet entirely understood, indicating a need for:
- studies quantifying the distribution sources of methyl
bromide and other organic bromine compounds.
There is also a need for:
- a study of the impact of sea spray on the formation of
organic bromine compounds;
- a study on the degradation products of methyl bromide in the
troposphere;
- further study of the rate constant for the reaction with
OH-;
- studies on soil fumigation with the aim of reducing
emissions while keeping efficacy.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
12.1 FAO/WHO
The toxicology of methyl bromide was evaluated by the FAO/WHO
Joint Meeting on Pesticide Residues in 1965 and 1966 (FAO/ WHO, 1965
a,b; WHO, 1967, a,b). In 1965, no acceptable daily intake (ADI) was
allocated, but, in 1966, an ADI of 1 mg/kg body weight, as bromide
ion, was established. In 1988, the JMPR evaluated the toxicology of
the bromide ion (FAO/WHO 1988a,b) and concluded that the level causing
no toxicological effect was:
Rat: 240 ppm, equivalent to 12 mg bromide/kg body weight
per day
Human: 9 mg bromide/kg body weight per day
The acceptable daily intake (ADI) of 1 mg/kg body weight was
confirmed.
12.2 IARC
The carcinogenic risks for humans were evaluated by an
International Agency for Research on Cancer ad hoc expert group in
1986. The evaluation was updated in 1987 and it was concluded that
there was inadequate evidence for carcinogenicity to humans and
limited evidence for carcinogenicity to animals; the overall
evaluation of carcinogenicity to humans was "not classifiable" [group
3] (IARC, 1986, 1987).
12.3 UNEP
In 1992, the United Nations Environment Programme evaluated methyl
bromide on behalf of the Contracting Parties to the Montreal Protocol
(UNEP, 1992). The executive summary of the evaluation reads:
"The report covers the current understanding of the impact
of methyl bromide on the ozone layer and on the uses of, and
alternatives to, methyl bromide."
It was requested by the United Nations Environment Programme on behalf
of the Parties to the Montreal Protocol. The report includes
information presented and discussed at the International Methyl
Bromide Science Workshop held in Washington, DC, on 2-3 June 1992, and
at the International Workshop on Alternatives and Substitutes to
Methyl Bromide held in Washington, DC, on 16-18 June 1992.
The current state of scientific knowledge concerning bromine
compounds in the atmosphere is considerably less developed than the
corresponding understanding of chlorine compounds. In addition, the
evaluation of the alternatives and substitutes to methyl bromide as a
fumigant is in an earlier stage than that for chlorofluorocarbons
(CFCs), methylchloroform, carbon tetrachloride, and halons.
Methyl bromide is used as a fumigant for soils, commodities, and
structures. It is currently vital for the economic viability of
certain agricultural production (especially strawberries, tomatoes,
peppers, tobacco, eggplants, nursery stock, vines, and turf) and for
the quarantine treatment of certain products in international trade.
Many developing countries are particularly dependent on the export of
products currently fumigated using methyl bromide, before shipment and
at ports of entry.
Total annual production and sales of methyl bromide for
fumigation have increased from about 42 000 tonnes to about 63 000
tonnes between 1984 and 1990. Combining the approximate methyl bromide
use-pattern data with the currently estimated fraction that escapes to
the atmosphere from each use (soils: 80% of use, 50 % emitted;
commodities: 15 % of use, <80 % emitted; and structural: 5 % of use,
80% emitted) indicates that about half of the methyl bromide used as
a fumigant is emitted into the atmosphere. Based on current
understanding, this implies an anthropogenic emission from fumigation
applications of about 30 thousand tonnes in 1990, which represents
25±10 % of the total (natural and anthropogenic) emissions.
Ozone destruction by bromine is more efficient on a per molecule
basis than destruction by chlorine by a factor of 30-60. Therefore, 1
part per trillion by volume (pptv) of bromine is equivalent to
0.03-0.06 parts per billion by volume (ppbv) of stratospheric
chlorine.
The current best estimate of the steady-state value of the Ozone
Depletion Potential (ODP) for methyl bromide is 0.7. Because of the
short atmospheric lifetime of methyl bromide, its relative impact on
ozone is expected to be much greater over the next decade (when
chlorine abundances and ozone losses are predicted to reach their
maximum) than is indicated by its steady-state ODP.
There are significant uncertainties in the atmospheric budget and
ODP of methyl bromide, especially the quantification of possible
oceanic and terrestrial surface removal processes and the rate of
formation of unreactive bromine in the stratosphere.
Model calculations suggest that anthropogenic emissions of methyl
bromide used for fumigation applications could have accounted for
about one-twentieth to one-tenth of the current observed global ozone
loss of 4-6% and could grow to about one-sixth of the predicted ozone
loss by the year 2000, if methyl bromide emissions continue to
increase at the present rate of about 5-6% per year.
The anthropogenic contribution to the current atmospheric
abundance of methyl bromide from fumigation applications is about 3
pptv, which is equivalent to 0.09-0.18 ppbv of stratospheric chlorine.
An advance of the CFC and carbon tetrachloride phaseout schedule by
three years would reduce the peak chlorine loading by 0.18 ppbv.
Therefore, elimination of methyl bromide used as a fumigant could
provide an ozone-layer protection equivalent to that of an advance of
the CFC and carbon tetrachloride phaseout schedule by about 1.5-3
years.
There is no single alternative to methyl bromide in the broad
spectrum of applications for which it is currently used. There are,
however, many alternative chemicals and procedures for specific
applications. The introduction of some chemical alternatives may
require government approval, which could be a lengthy process.
Rough estimates indicate that a significant fraction (from as low
as 30% to as high as 90%), albeit uncertain, of methyl bromide used
for soil fumigation could be replaced by chemical substitutes during
the 1990s; that a substantial proportion of emissions from fumigation
chambers could be captured and recycled or destroyed; that a small
fraction (1-2%) of methyl bromide emissions could be eliminated by
better procedures during tank filling; and that significant reductions
in emissions could be made using other alternatives or techniques,
alone, or in combination. However, there are some applications for
which there are limited or no alternatives, including some
agricultural situations that have developed a dependence on methyl
bromide, quarantine treatments, and some structural fumigation uses.
REFERENCES
Abdalla N & Lear B (1975) Lethal dosages of methyl bromide for four
plant-parasitic nematodes and the effect of soil temperature on its
nematicidal activity. Plant Dis Rep, 59: 224-228.
Adu OO & Muthu M (1985) The relative toxicity of seven fumigants to
life cycle stages of Callosobruchus chinensis (L). Insect Sci Appl,
6: 75-78.
Alexeeff GV & Kilgore WW (1983) Methyl bromide. In: Gunther FA &
Gunther JD ed. Residue reviews. Residues of pesticides and other
contaminants in the total environment, Vol. 88. Berlin, Heidelberg,
New York, Springer-Verlag, pp 102-153.
Alexeeff GV, Kilgore WW, Munoz P, & Watt D (1985) Determination of
acute toxic effects in mice following exposure to methyl bromide. J
Toxicol Environ Health, 15: 109-123.
Alfaro Moreno A & Vegh I (1971) [Green pepper diseases produced by
Phytophthora capsici.] An Inst Nac Invest Agr Ser Prot Veg, 1: 9-42
(in Spanish).
American Biogenics Corporation (1986) Two-generation reproduction
study in albino rats with methyl bromide - results of both generations
(Study No. 4500-1525) (Unpublished final report).
Andersen ME, Gargas ML, Jones RA, & Jenkins LJ (1980) Determination of
the kinetic constants for metabolism of inhaled toxicants in vivo
using gas uptake measurements. Toxicol Appl Pharmacol, 54: 100-116.
Anger WK, Setzer JV, Russo JM, Brightwell WS, Wait RG, & Johnson BL
(1981) Neurobehavioral effects of methyl bromide inhalation exposures.
Scand J Work Environ Health, 7: 40-47.
Anger WK, Moody L, Burg J, Brightwell WS, Taylor BJ, Russo JM,
Dickerson N, Setzer JV, Johnson BL, & Hicks K (1986) Neurobehavioral
evaluation of soil and structural fumigators using methyl bromide and
sulfuryl fluoride. Neurotoxicology, 7: 137-156.
Angerer J (1977) [A method for determination of bromide in urine with
help of a ionsensitive electrode.] J Clin Chem Clin Biochem, 15:
201-204 (in German).
Angerer J (1980) [Bromide. Determination in urine.] In: Henschler D
ed. [Analytical methods for the investigation of work materials
detrimental to health. Analysis in biological materials.] Weinheim,
Verlag Chemie, vol 2/2, pp D1-D6, 1-9 (in German).
Angerer J (1982) [Methyl bromide. Air analysis]. In: Henschler D ed.
[Analytical methods for the investigation of work materials
detrimental to health. Air analysis.] Weinheim, Verlag Chemie, vol
1/2, D1-D2, 1-8 (in German).
Anthon EW, Moffit HR, Couey HM, & Smith LO (1975) Control of codling
moth in harvested sweet cherries with methyl bromide and effects upon
quality and taste of treated fruit. J Econ Entomol, 68: 524-526.
Arvieu JC (1983) Some physico-chemical aspects of methyl bromide
behaviour in soil. Acta Hortic, 152: 267-275.
Arvieu JC & Cuany A (1985) Effets de la matière organique sur la
bioactivité et la dégradation du bromure de méthyle dans le sol. EPPO
Bull, 15: 87-96.
Asante-Poku S, Aston WP, & Schmidt DE Jr (1974) Site of decomposition
of methyl bromide in cocoa beans. J Sci Food Agric, 25: 285-291.
Atochem (1987) Fumyl-o-gas. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 4 pp.
Atochem (1988) BM 98 SOBROM. Fiche de données de sécurité. Paris,
Groupe Elf Aquitaine, 2 pp.
Audry D, Soichot P, Giard MH, & Mauguiere F (1985) Une cause rare de
myoclonies avec PES "Géants": l'intoxication par le bromure de
méthyle. A propos d'une observation à prédominance unilaterale. Rev
EEG Neurophysiol, 15: 45-52.
Austin RK & Phillips DJ (1985) Use of a standard-addition
bromide-selective electrode technique to determine bromide and trace
its migration in peaches. J Agric Food Chem, 33: 1165-1168.
Bachem C (1927) [Contribution to the toxicology of the alkyl
halogens.] Arch Exp Pathol Pharmakol, 122: 69-76 (in German).
Bakhishev GN (1973) [Relative toxicity of aliphatic halohydrocarbons
to rats.] Farmakol Toksikol, 8: 140-142 (in Russian).
Balander PA & Polyak MG (1962) [Toxicological characteristics of
methyl bromide.] Gig i Toksikol, 60: 412-419 (in Russian).
Basile M & Lamberti F (1981) Bromide residues in edible organs of
plants grown in soil treated with methyl bromide. Meded Fac
Landbouwwet Rijksuniv Gent, 46: 337-341.
Basile M, Lamberti F, & Basile AC (1987) [Inorganic bromide
concentrations in soil after fumigation with methyl bromide.] Essenze
Deriv Agrum, 57: 116-120 (in Italian).
Baumann H & Heumann KG (1987) Analysis of organobromine compounds and
hydrogen bromide in motor car exhaust gases with a GC/microwave plasma
system. Fresenius Z Anal Chem, 327: 186-192.
BBA (Biologische Bundesanstalt für Land- und Forstwirtschaft) (1989)
[Plant protective agent register, 1989/90, Part 5.] 37th ed.
Braunschweig, Federal Institute for Agriculture and Forestry, 66 pp
(in German).
Behrens RH & Dukes DCD (1986) Fatal methyl bromide poisoning. Br J Ind
Med, 43: 561-562.
Bell CH (1988) Minimum concentration levels of methyl bromide required
for full efficacy against seven species of stored-product beetle at
two temperatures. Pestic Sci, 24: 97-109.
Benatt AJ & Courtney TRB (1948) Uraemia in methyl bromide poisoning:
A case report. Br J Ind Med, 5: 21-25.
Berg WW, Heidt LE, Pollock W, Sperry PD, Cicerone RJ, & Gladney ES
(1984) Brominated organic species in the arctic atmosphere. Geophys
Res Lett, 11: 429-432.
Beyne M & Goett C (1934) Toxicité de certains appareils extincteurs
d'incendie et précautions qu'ils comportent dans leur emploi. Arch Méd
Pharm Nav, 124: 409.
Bird GW, Rich JR, & Glover SU (1974) Increased endomycorrhizae of
cotton roots in soil treated with nematicides. Phytopathology, 64:
48-51.
Bishop CM (1992) A case of methyl bromide poisoning. J Occup Med, 42:
107-109.
Blackburn S & Philips H (1944) Experiments on the methylation and
acetylation of wool, silk fibroin, collagen and gelatin. Biochem J,
38: 171-178.
Blackith RE & Lubatti OF (1965) Fumigation of agricultural products.
XX. Prolonged storage of cereals fumigated with methyl bromide. J Sci
Food Agric, 16: 455-457.
Boczek J, Klag J, & Komorowska B (1975) Effect of methyl bromide on
the embryonic development of Acarus siro (Acarina, Acaridae). J
Stored Prod Res, 11: 41-46.
Bolon B, Bonnefoi M, & Morgan KT (1990) Effect of olfactory epithelial
regeneration and cage environment on the sensitivity of the rat
olfactory epithelium to methyl bromide. Chem Senses, 15: 554-555.
Bond EJ (1956) The effect of methyl bromide on the respiration of the
cadelle Tenebroides mauritanicus L. (Coleoptera: Ostomidae). Can J
Zool, 34: 405-415.
Bond EJ (1984) Manual of fumigation for insect control. 6. Chemicals
used as fumigants. Rome, Food and Agriculture Organization of the
United Nations, pp 71-96 (Plant Production and Protection Paper 54).
Bond EJ & Dumas T (1987) Concentrations of methyl bromide inside flour
mills and in the atmosphere around the mills during and after
fumigation. Proc Entomol Soc Ont, 118: 1-6.
Bond JA, Dutcher JS, Medinsky MA, Henderson RF, & Birnbaum LS (1985)
Disposition of [14C]methyl bromide in rats after inhalation. Toxicol
Appl Pharmacol, 78: 259-267.
Boorman GA, Hong HL, Jamieson CW, Yoshitomi K, & Maronpot RR (1986)
Regression of methyl bromide induced forestomach lesions in the rat.
Toxicol Appl Pharmacol, 86: 131-139.
Bourbos VA & Skoudridakis MT (1991) Effect of methyl bromide and
chloropicrin on the soil mycoflora in greenhouse tomato. Plant Pathol
Lab, 56: 569-575.
Bowers GN Jr & Onoreski M (1990) Hyperchloremia and the incidence of
bromism in 1990. Clin Chem, 36: 1399-1403.
Brenner I (1978) Bromism: alive in well. Am J Psychiatry, 7: 857-858.
Breslin WJ, Zablotny CL, Bradley GF, & Lomax LG (1990) Methyl bromide
inhalation teratology study in New Zealand white rabbits. Midland,
Michigan, The Dow Chemical Company (Unpublished final report).
Bridges RG (1955) The fate of labelled insecticide residues in food
products. III. N-methylation as a result of fumigating wheat with
methyl bromide. J Sci Food Agric, 6: 261-268.
Brodniewicz A (1967) Poisoning of seamen with methyl bromide due to
fumigation of a Polish cargo ship in Haiphong (Vietnam). Arh Hig Rada,
18: 241-246 (in Polish with English translation).
Brodzinsky R & Singh HB (1983) Volatile organic chemicals in the
atmosphere: an assessment of available data (Contract-No. 68-02-3452).
Menlo Park, California, SRI International (EPA/DF-83/005a).
Bromine and Chemicals Ltd (1990) Methylbromide soil fumigation - use
and application instructions. London, Bromine and Chemicals Ltd, pp
1-36.
Brown G & Jenkinson DS (1971) Bromine in wheat grown on soil fumigated
with methyl bromide. Soil Sci Plant Anal, 2: 45-54.
Brown D & Rolston DE (1980) Transport and transformation of methyl
bromide in soils. Soil Sci, 130: 68-75.
Brown AL, Burau RG, Meyer RD, Raski DJ, Wilhelm S, & Quick J (1979)
Plant uptake of bromide following soil fumigation with methyl bromide.
Calif Agric, 33: 11-13.
Bruhin J (1942) [Percutaneous poisoning with methyl bromide during
pest control.] University of Zurich, Medical Faculty, 31 pp (M.D.
Thesis) (in German).
BUA (1987) [BUA Chemical Report 14: Methyl bromide.] Weinheim,
Advisory Committee for Environmental Priority Pollutants (BUA) of the
German Chemists Association, 64 pp (in German).
Burkholder WE (1966) Toxicity of methyl bromide to Acarus siro , a
cheese-infesting mite. J Econ Ent, 59: 110-112.
Butler ECB, Perry KMA, & Williams JRF (1945) Methyl bromide burns. Br
J Ind Med, 2: 30-31.
CAC (1986) Guide to Codex Alimentarius recommendations on pesticide
residues. Part 3. Guideline levels for pesticide residues. Rome, Food
and Agriculture Organization (CAC/PR 3-1986).
Cantineau A, Thomas R, Breurec JY, Bousser J, Baert A, Bonny JS,
Bouget J, Dubois C, Venisse T, & Curtes JP (1988) Acute methylbromide
poisoning. J Toxicol Clin Exp, 8: 83-87.
Canton JH, Wester PW, & Matthijssen-Spiekman, EAM (1983) Study on the
toxicity of sodium bromide to different freshwater organisms. Food
Chem Toxicol, 21: 369-378.
Carter B (1945) Methyl bromide poisoning. Effects on the nervous
system. Br Med J, 1: 43-45.
Castro CE & Belser NO (1981) Photohydrolysis of methyl bromide and
chloropicrin. J Agric Food Chem, 29: 1005-1008.
Castro JA, Zerba EN, De Licastro SA, Picollo MI, Wood EJ, Ruveda MA,
De Moutier Aldao EM, & Libertella R (1976) Toxicity of methyl bromide
and other gaseous insecticides to Triatoma infestans. Acta Physiol
Lat Am, 26: 106-114.
Cavanagh JB (1992) Methyl bromide intoxication and acute energy
deprivation syndromes. Neuropathol Appl Neurobiol, 18: 573-578.
CDFA (1988) Sampling for pesticide residues in California well water:
1988 update; well inventory data base. Sacramento, California,
Department of Food and Agriculture, Division of Pest Management,
Environmental Protection and Worker Safety, Environmental Monitoring
and Pest Management Branch, 66 pp (Report EH 88-10).
CEC (1985) Report of the Scientific Committee for Pesticides on the
use of methyl bromide as a fumigant for plant culture media.
Luxembourg, Commission of the European Communities, pp 15-33 (EUR
10211).
Chavez CT, Hepler RS, & Straatsma BR (1985) Methyl bromide optic
atrophy. Am J Ophthalmol, 99: 715-719.
Cheetham T (1990) Pathological alterations in embryos of the codling
moth (Lepidoptera: Tortricidae) induced by methyl bromide. Ann.
Entomol. Soc. Am., 83: 59-67.
Chellman GJ, White RD, Norton RM, & Bus JS (1986) Inhibition of the
acute toxicity of methyl chloride in male B6C3F1 mice by glutathione
depletion. Toxicol Appl Pharmacol, 86: 93-104.
Chen T, Kilpatrick RA, & Rich AE (1962) Stylet-bearing nematodes
associated with white clovers in New Hampshire. Plant Dis Rep, 46:
346-347.
Chisholm RD & Koblitsky L (1943) Sorption of methyl bromide by soil in
a fumigation chamber. J Econ Entomol, 36: 549-551.
Cicerone RJ, Heidt LE, & Pollock WH (1988) Measurements of atmospheric
methyl bromide and bromoform. J Geophys Res, 93: 3745-3749.
Cirilli L & Borgioli A (1986) Methyl bromide in surface drinking
waters. Gas-chromatographic determination by the headspace technique.
Water Res, 20: 273-275.
Clarke CA, Roworth CG, & Holling HE (1945) Methyl bromide poisoning.
Br J Ind Med, 2: 17-23.
Cochran JW (1988) A rapid, sensitive method for the analysis of
halogenated gases in water. J High Resol Chromatogr Chromatogr Commun,
11: 663-665.
Cooper DM, Griffiths NM, Hobson-Frohock A, Land DG, & Rowell JG (1978)
Fumigation of poultry food with methyl bromide: effects on egg
flavour, number and weight. Br Poult Sci, 19: 537-542.
Cornwell PB (1979) Health monitoring experience of fumigators using
methylbromide. Proceedings of the Fifth British Pest Control
Conference, Stratford-on-Avon, England, 26-29 September 1979. London,
British Pest Control Association (Sixth Session; Paper No. 17).
Cova D, Molinari GP, & Rossini L (1986) Residues after fumigation with
methyl bromide: bromide ion and methyl bromide in middlings and final
cereal foodstuffs. Food Addit Contam, 3: 235-240.
Crampton RF, Elias PS, & Gangolli SD (1971) The bromide content of
human tissue. Br J Nutr, 25: 317-322.
Daelemans A (1978) Uptake of methyl bromide by plantss and uptake of
bromide from decontaminated soils. Leuwen, Belgium, Catholic
University (Dissertation) (in Flemish).
Daft J (1987) Determining multifumigants in whole grains and legumes,
milled and low-fat grain products, spices, citrus fruit, and
beverages. J Assoc Off Anal Chem, 70: 734-739.
Daft JL (1988) Rapid determination of fumigant and industrial chemical
residues in food. J Assoc Off Anal Chem, 71: 748-760.
Daft JL (1989) Determination of fumigants and related chemicals in
fatty and nonfatty foods. J Agric Food Chem, 37: 560-564.
Daft JL (1992) Headspace method for rapid determination of methyl
bromide in assorted nut samples. J Assoc Off Anal Chem, 75: 701-706.
Dagani MJ, Barda HJ, & Sanders DC (1985) Bromine compounds. Weinheim,
Verlag Chemie, pp 405-420.
Danse LHJC, Van elsen FL, & Van der Heijden CA (1984) Methylbromide:
carcinogenic effects in the rat forestomach. Toxicol Appl Pharmacol,
72: 262-271.
Darby JF, Dieter CE, & Rau GJ (1962) Evaluation of treatments for
control of soil-borne pests in celery seedbeds. Plant Dis Rep, 46:
441-443.
Davenport CJ, Ali SF, Miller FJ, Lipe GW, Morgan KT, & Bonnefoi MS
(1992) Effect of methyl bromide on regional brain glutathione,
glutathione- S-transferases, monoamines, and amino acids in F344
rats. Toxicol Appl Pharmacol, 112: 120-127.
Davis LN, Strange JR, Hoecker JE, Howard PH, & Santodonato J (1977)
Investigation of selected potential environmental contaminants:
monohalomethanes. Washington, DC, US Environmental Protection Agency
(EPA-560/2-77-007).
Dawson GW, Jennings AL, Drozdowski D, & Rider E (1975/77) The acute
toxicity of 47 industrial chemicals to fresh and saltwater fishes. J
Hazard Mater, 1: 303-318.
De Heer H, Tuinstra LGMPH, Hamaker PH, & Van der burg AMM (1983) Use
of gas-tight plastic films during fumigation of glasshouse soils with
methylbromide. I. Significance of permeation and leakage for the
emission into the outside air. Acta Hortic, 152: 109-126.
Dempsey DA, Becker CE & Mihm FC (1992) Death after entering an
apartment building fumigated with methyl bromide and cleared for
habitation. Vet Hum Toxicol, 34: 356.
Deutschmann S, Reichel C, & Kettschau F (1989) Metabolism of methyl
bromide and methyl iodide in erythrocytes compared with methyl
chloride. Naunyn-Schmiedeberg's Arch Pharmacol, 339: R12.
Deutschmann S, Peter H, Reichel C, Bolt M, & Hallier E (1989)
[Kinetics of metabolism of methyl bromide and methyl iodide in
erythrocytes of humans and related species.] Verh Dtsch Gesundh
Arbeitsmed, 29: 517-519 (in German).
Devaney JA & Beerwinkle KR (1982) Control of the northern fowl mite on
inanimate objects by fumigation: field studies. Poult Sci, 62: 43-46.
Devries JW, Borge JM, Schroeder JP, Bowers RH, Larson PA, & Burns NM
(1985) Headspace gas chromatographic method for determination of
methyl bromide in food ingredients. J Assoc Off Anal Chem, 68:
1112-1116.
Djalali-Behzad G, Hussain S, Ostermann-Golker S, & Segerbaeck D (1981)
Estimation of genetic risks of alkylating agents. VI. Exposure of mice
and bacteria to methyl bromide. Mutat Res, 84: 1-9.
Doraiswamy S, Van Winckel A, & Van Assche C (1972) Effect in vitro of
soil fumigants on soil microbial population in relation to degradation
of tobacco mosaic virus. Meded Fac Landbouwwet Rijksuniv Gent, 37:
492-499.
Drawneek W, O'Brien MJ, Goldsmith HJ, & Bourdillon RE (1964)
Industrial methyl bromide poisoning in fumigators. Lancet, October 17:
855-856.
Dreef-Van der Meulen HC, Reuzel PGJ, Kuper CF, Hollanders VMH, & Feron
VJ (1989) Chronic inhalation toxicity of methyl bromide in rats. Third
International Symposium on Soil Disinfestation, Leuwen, Belgium,
September 26-30, 1988. Acta Hortic, 255: 313-315.
Driscoll JN, Duffy M, Pappas S, & Webb M (1987) Analysis for purgeable
organics in water by capillary GC/PID-ELCD. J chromatogr sci, 25:
369-375.
Drosihn UG, Stephan BR, & Hoffmann GM (1968) [Investigations into soil
contamination with methyl bromide.] Z Pflanzenkr Pflanzenschutz, 75:
272-287 (in German).
Dudley HC & Neal PA (1942) Methyl bromide as a fumigant for foods.
Food Res, 7: 412-429.
Dudley HC, Miller JW, Neal PA, & Sayers RR (1940) Studies on
foodstuffs fumigated with methyl bromide. Public Health Rep, 55:
2251-2282.
Duffy M, Driscoll JN, Pappas S, & Sanford W (1988) Analysis of ppb
levels of organics in water by means of purge-and-trap, capillary gas
chromatography and selective detectors. J Chromatogr, 441: 73-80.
Dumas T (1982) Trapping low levels of methyl bromide in air or as
residues at ambient and lower temperatures for gas chromatography. J
Assoc Off Anal Chem, 65: 913-915.
Dumas T & Bond EJ (1985) Analysis of methyl bromide at ultra low
concentration levels. J Agric Food Chem, 33: 276-278.
Duvoir M, Fabre R, & Layani F (1937). L'intoxication par le bromure de
méthyle. Bull Soc Méd Paris, 53: 1540.
Easley DM, Kleopfer RD, & Carasea AM (1981) Gas chromatographic-mass
spectrometric determination of volatile organic compounds in fish. J
Assoc Off Anal Chem, 64: 653-656.
Edmiston S & Maddy KT (1987) Summary of illnesses and injuries
reported in California by physicians in 1986 as potentially related to
pesticides. Vet Hum Toxicol, 29: 391-397.
Edwards CA (1962) Springtail damage to bean seedlings. Plant Pathol,
11: 67-68.
Edwards CA & Thompson AR (1973) Pesticides and the soil fauna. In:
Gunther FA & Gunter JD, ed. Residue reviews. Residues of pesticides
and other contaminants in the total environment. Berlin, Heidelberg,
New York, Springer-Verlag, vol 45, pp 1-79.
Ehrenberg L, Osterman-Golkar S, Singh D, & Lundqvist U (1974) On the
reaction kinetics and mutagenic activity of methylating and
beta-halogenoethylating gasoline additives. Radiat Bot, 15: 185-194.
El Buzz HK, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1974) Effect of
diet on the susceptibility of the different developmental stages of
Corcyra cephalonica staint. to carbon bisulphide and methyl bromide.
Agric Res Rev, 52: 21-29.
Eller PM (1985) NIOSH manual of analytical methods, 3rd ed.
Cincinnati, Ohio, National Institute for Occupational Safety and
Health, pp 2520/1-2520/-4 (NIOSH Publication No. 84-100).
EMBA (1988) Handling and transportation of methyl bromide. Code of
practice. Brussels, European Methyl Bromide Association, pp 1-8.
Enebak SA, Palmer MA, & Blanchette RA (1988) Effect of soil-borne
pathogen control methods on the production of white pine nursery
seedlings. Phytopathology, 78: 1532-1533.
Ethyl Corporation (1990a) Methyl bromide applications manual. Baton
Rouge, Louisiana, Ethyl Corporation, 3 pp.
Eustis SL, Haber SB, Drew RT, & Yang RSH (1988) Toxicology and
pathology of methyl bromide in F344 rats and B6C3F1 mice following
repeated inhalation exposure. Fundam Appl Toxicol, 11: 594-610.
Evans JE & Hastings L (1992) The effect of methyl bromide on measures
of olfactory function. Toxicologist, 12: 270.
Fabian P (1984) Atmosphere and environment. Berlin, Heidelberg, New
York, Springer-Verlag, p 41.
Fabian P, Borchers R, Penkett SA & Prosser NJD (1981) Halocarbons in
the stratosphere. Nature (Lond), 294: 733-735.
Fairall RJ & Scudamore KA (1980) Determination of residual methyl
bromide in fumigated commodities using derivative gas-liquid
chromatography. Analyst, 105: 251-256.
Fallico R & Ferrante M (1991) Exposure to inorganic bromides from
greenhouse crops where methyl bromide was applied for soil fumigation.
Zent.bl Hyg Umweltmed, 191: 555-562.
FAO (1980) Pesticide residues in food. Methyl bromide. Rome, Food and
Agriculture Organization of the United Nations, pp 89-97 (FAO Plant
Production and Protection Paper 20 Sup).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Group on Pesticide
Residues. Rome, Food and Agriculture Organization of the United
Nations; Geneva, World Health Organization (FAO Meeting Report No.
PL/1965/10/1; WHO/Food Add./27.65).
FAO/WHO (1965b) Evaluation of hazards to consumers resulting from the
use of fumigants in the protection of food. Rome, Food and Agriculture
Organization of the United Nations; Geneva, World Health Organization
(FAO Meeting Report No. PL/1965/10/2; WHO/Food Add./28.65).
FAO/WHO (1988a) Pesticide residues in food - 1988. Report of the Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environmental and the WHO Expert Group on Pesticide Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 92).
FAO/WHO (1988b) Pesticide residues in food - 1988 evaluations. Part II
-Toxicology. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 93/2).
Filip GM & Roth LF (1977) Stump injections with soil fumigant to
eradicate Armillariella mellea from young-growth ponderosa pine
killed by root rot. Can J For Res, 7: 226-231.
Flick EW (1985) Industrial solvents handbook, 3rd ed. Park Ridge, New
Jersey, Noyes Data Corporation, 4 pp.
Foxboro (1989) Ambient air instrumentation sheet. East Bridgewater,
Maryland, The Foxboro Company.
Fransi A, Pons R, Sala A, Vallejo VR, & Bertran C (1987) Wheat and
soil bromide dynamics after fumigation with methyl bromide in a
mediterranean climate. Plant Soil, 98: 417-424.
Fuortes LJ (1992) A case of fatal methyl bromide poisoning. Vet Hum
Toxicol, 34: 240-241.
Furuta A, Hyakudo T, Ohnishi A, Hori H & Tanaka E (1993) Neurotoxicity
of methyl bromide - neuropathologic evaluation, preliminary study.
Sangyo Ika Daigaku Zasshi, 15: 21-27.
Gansewendt B, Foest U, Xu D, Hallier E, Bolt HM, & Peter H (1991)
Formation of DNA adducts in F-344 rats after oral administration or
inhalation of [14C]methyl bromide. Food Chem Toxicol, 29: 557-563.
Gargas ML & Andersen ME (1982) Metabolism of inhaled brominated
hydrocarbons: validation of gas uptake results by determination of a
stable metabolite. Toxicol Appl Pharmacol, 66: 55-68.
Garman JR, Freund T, & Lawless EW (1987) Testing for groundwater
contamination at hazardous waste sites. J Chromatogr Sci, 25: 328-337.
Garry VF, Nelson RL, Griffith J, & Harkins M (1990) Preparation for
human study of pesticide applicators: sister chromatid exchanges and
chromosome aberrations in cultured human lymphocytes exposed to
selected fumigants. Teratog Carcinog Mutagen, 10: 21-29.
Gentile IA, Ferraris L, Crespi S, & Belligno A (1989) The degradation
of methyl bromide in some natural fresh waters. Influence of
temperature, pH and light. Pestic Sci, 25: 261-272.
Gentile IA, Ferraris L, Sanguinetti M, Tiprigan M, & Fisichella G
(1992) Methyl bromide in natural fresh waters: hydrolysis and
volatilisation. Pestic Sci, 34: 297-301.
Gillotay D, Simon PC & Dierickx L (1989) Temperature dependence of
ultraviolet absorption cross-sections of brominated methanes an
ethanes. In: Bojkov RD & Fabian P, ed. Ozone in the atmosphere.
Proceedings of the Quadrennial Ozone Symposium 1988 and Tropospheric
Ozone Workshop, Göttingen, Germany, 4-13 August 1988. Hampton,
Virginia, Deepak Publishing, pp 706-709.
Goldman LR, Mengle D, Epstein DM, Feson D, Kelly K, & Jackson RJ
(1987) Acute symptoms in persons residing near a field treated with
the soil fumigants methyl bromide and chloropicrin. West J Med, 147:
95-98.
Gosselin RE, Smith RP, Hodge HC, & Braddock JE (1984) Clinical
toxicology of commercial products. Baltimore, Maryland, Williams &
Wilkins Company, pp III/280-III/284.
Goulon M, Nouailhat F, Escourolle R, Zarranz-Imirizaldu JJ, Grosbuis
S, & Levy-Alcover MA (1975) Intoxication par le bromure de méthyle.
Trois observations, dont une mortelle. Etude neuro-pathologique d'un
cas de stupeur avec myoclonies, suivi pendant cinq ans. Rev. Neurol.,
131: 445-468.
Grant WM (1974) Toxicology of the eye, 2nd ed. Springfield, Virginia,
Charles C. Thomas, pp 680-685.
Green M, Seiber JN, & Biermann HW (1991) in situ measurement of methyl
bromide in indoor air using long-path ftir spectroscopy. In: Schiff
HI, ed. Measurement of atmospheric gases. Proceedings spie - the
international society for optical engineering. Los angeles,
California, 2-3 January 1991 (Volume 1433). Washington, DC, The
International Society for Optical Engineering, pp 270-274.
Greenberg JO (1971) The neurological effects of methyl bromide
poisoning. Ind Med, 40: 27-29.
Greve PA & Grevenstuk WBF (1976) Optimization studies on the
determination of bromide residues in lettuce. Meded Fac Landbouwwet
Rijksuniv Gent, 41: 1371-1381.
Greve PA & Grevenstuk WBF (1979) Gas-liquid chromatographic
determination of bromide ion in lettuce: interlaboratory studies. J
Assoc Off Anal Chem, 62: 1155-1159.
Greve PA & Hogendoorn EA (1979) Determination of fumigant residues in
grain. Meded Fac Landbouwwet Rijksuniv Gent, 44: 877-884.
Griffiths NM, Hobson-Frohock A, Land DG, Levett JM, Cooper DM, &
Rowell JG (1978) Fumigation of poultry food with methyl bromide:
effects on flavour and acceptability of broiler meat. Br Poult Sci,
19: 529-535.
Grimm GR & Alexander AR (1971) Fumigation of phytophthora in sandy
soil by surface application of methyl bromide and methyl
bromide-chloropicrin. Plant Dis Rep, 55: 929-931.
Gryder-Boutet DE & Kennish JM (1988) Using headspace sampling with
capillary column GC-MS to analyze trace volatile organics in water and
wastewater. Am Water Works Assoc J, 80: 52-55.
Guicherit R & Schulting FL (1985) The occurrence of organic chemicals
in the atmosphere of the Netherlands. Sci Total Environ, 43: 193-219.
Guillemin MP, Hillier RS, & Bernhard CA (1990) Occupational and
environmental hygiene assessment of fumigations with methyl bromide.
Ann Occup Hyg, 34: 591-607.
Guns MF (1989) Monitoring of the groundwater bromine content and plant
residues in methyl bromide fumigated glasshouses. Third International
Symposium on Soil Disinfestation, Leuven, Belgium, September 26-30,
1988. Acta Hortic, 255: 337-346.
Haber SB, Drew RT, Eustis S, & Yang RSH (1985) Methyl bromide
toxicity: a target organ? Toxicologist, 5: 130 (Abstract No. 518).
Haesanen E, Manninen PKG, Himberg K, & Väätäinen V (1990) Chlorine and
bromine contents in tobacco and tobacco smoke. J Radioanal Nucl Chem,
144: 367-372.
Hague NGM (1963) Fumigation of agricultural products. XVIII. Effect of
methyl bromide on the bent grass nematode, Anguina agrostis,
steinbuch 1799 , filipjev 1936, and on the germination of bent
grass, Agrostis tenuis. J Sci Food Agric, 14: 577-579.
Hague NGM & Clark WC (1959) Fumigation with methyl bromide and
chloropicrin to control seed-borne infestations of the stem eelworm.
Meded Landbouwhogesch Opzoekingasstn Gent, 24: 628-636.
Hallier E, Jaeger R, Deutschmann S, Bolt HM, & Peter H (1990a)
Glutathione conjugation and cytochrome p-450 metabolism of methyl
chloride in vitro. Toxic in vitro , 4: 513-517.
Hallier E, Deutschmann S, Reichel C, Bolt HM, & Peter H (1990b) A
comparative investigation of the metabolism of methyl bromide and
methyl iodide in human erythrocytes. Int Arch Occup Environ Health,
62: 221-225.
Hallier E, Langhof T, Dannappel D, Leutbecher M, Schröder K, Goergens
HW, Müller A, & Bolt HM (1993) Polymorphism of glutathione conjugation
of methyl bromide, ethylene oxide and dichloromethane in human blood:
influence on the induction of sister chromatid exchanges (SCE) in
lymphocytes. Arch Toxicol, 67: 173-178.
Hamilton I, Minski MJ, Milton EI, & Cleary JJ, (1972/73) The
concentration and distribution of some stable elements in healthy
human tissues from the United Kingdom. An environmental study. Sci
Total Environ, 1: 341-374.
Hansch C & Leo A (1979) Substituent constants for correlation in
chemistry and biology. New York, Chichester, Brisbane, Toronto, John
Wiley & Sons, p 173.
Hanson PR, Wainman HE, & Chakrabarti B (1987) The effects of methyl
bromide on the seed of some varieties of barley. Seed Sci Technol, 15:
155-162.
Hardin BD, Bond GP, Sikov MR, Andrew FD, Belilies RP, & Niemeier RW
(1981) Testing of selected workplace chemicals for teratogenic
potential. Scand J Work Environ Health, 7: 66-75.
Harper DB (1985) Halomethane from halide ion - a highly efficient
fungal conversion of environmental significance. Nature (Lond), 315:
55-57.
Harrison BO, Peachey JE, & Winslow RO (1963) The use of nematicides to
control the spread of arabis mosaic virus by Xiphinema
diversicaudatum (Micol.). Ann Appl Biol, 52: 243-255.
Harry EG, Brown WB, & Goodship G (1972) The disinfecting activity of
methyl bromide on various microbes and infected material under
controlled conditions. J Appl Bacteriol, 35: 485-491.
Harsch DE & Rasmussen RA (1977) Identification of methyl bromide in
urban air. Anal Lett, 10: 1041-1047.
Hartill WFT & Campbell JM (1973) Control of sclerotinia in tobacco
seedbeds. Plant Dis Rep, 57: 932-934.
Hastings L (1990) Sensory neurotoxicology: use of the olfactory system
in the assessment of toxicity. Neurotoxicol Teratol, 12: 455-459.
Hastings L, Miller M, Minnema D, & Evans J (1989) Effects of methyl
bromide on rat olfactory system. Chem Senses, 14: 708.
Hatch GG, Mamay PD, Ayer ML, Casto BC, & Nesnow S (1983) Chemical
enhancement of viral transformation in Syrian hamster embryo cells by
gaseous and volatile chlorinated methanes and ethanes. Cancer Res, 43:
1945-1950.
Heimann H (1944) Poisoning as result of industrial exposure to methyl
bromide. NY State Ind Bull, 23: 103-105.
Henschler D (1990) Chemicals harmful to health: toxicological-medical
establishment of MAK-values, 16th ed. Weinheim, VCH
Verlagsgesellschaft mbH.
Heungens A & Roos A (1982) L'influence du dazomet et du bromure de
méthyle sur la faune et la flore de la litière de pin. Rev Agric,
35(2): 2005-2050.
Heurtebise M & Ross WJ (1971) Application of 80m Br to the
semi-automated analysis of bromine in biological fluids. J Radioanal
Chem, 8: 5-12.
Heuser SG & Scudamore KA (1968) Fumigant residues in wheat and flour:
solvent extraction and gas-chromatographic determination of free
methyl bromide and ethylene oxide. Analyst, 93: 252-258.
Heuser SG & Scudamore KA (1970) Selective determination of ionised
bromide and organic bromides in foodstuffs by gas-liquid
chromatography with special reference to fumigant residues. Pestic
Sci, 1: 244-249.
Hezemans-Boer M, Toonstra J, Meulenbelt J, Zwaveling JH, Sangster B,
& Van Vloten WA (1988) Skin lesions due to exposure to methyl bromide.
Arch Dermatol, 124: 917-921.
Hicken NE (1961) Termite fumigation with methyl bromide. Chem Prod,
24: 205-207.
Hine CH (1969) Methyl bromide poisoning - a review of ten cases. J
Occup Med, 11: 1-10.
Hitchcock BE (1968) Progress of earth pearl studies in Queensland.
Proc Int Soc Sugar-Cane Technol, 13: 1382-1388.
Ho JSY (1989) A sequential analysis for volatile organics in water by
purge-and-trap capillary column gas chromatography with
photoionization and electrolytic conductivity detectors in series. J
Chromatogr Sci, 27: 91-98.
Hoffman GM & Malkomes HP (1974) Bromide residues in vegetable crops
after soil fumigation with methyl bromide. Agric Environ, 1: 321-328.
Hole BD (1981) Variation in tolerance of seven species of stored
product coleoptera to methyl bromide and phosphine in strains from
twenty-nine countries. Bull Entomol Res, 71: 299-306.
Holling HE & Clarke CA (1944) Methyl bromide intoxication. J R Navy
Med Serv, 30: 218-224.
Hommel G (1984) [Handbook of dangerous goods (Explanatory leaflet
127).] Berlin, Heidelberg, New York, Springer-Verlag (in German).
Honma T (1987) Alteration of catecholamine metabolism in rat brain
produced by inhalation exposure to methyl bromide. Jpn J Ind Health,
29: 218-219.
Honma T (1992) Brain microdialysis study of the effects of hazardous
chemicals on the central nervous system. 1. Changes in monoamine
metabolites induced by cerebral methyl bromide administration measured
by two-probe microdialysis (TPMD) method. Ind Health, 30: 47-60.
Honma T, Sudo A, Miyagawa M, & Sato M (1982) Significant changes in
monoamines in rat brain induced by exposure to methyl bromide.
Neurobehav Toxicol Teratol, 4: 521-524.
Honma T, Sudo A, Miyagawa M, Sato M, & Hasegawa H (1983) Changes in
free amino acid contents of rat brain induced by exposure to methyl
bromide. Toxicol Lett, 15: 317-321.
Honma T, Miyagawa M, Sato M, & Hasegawa H (1985) Neurotoxicity and
metabolism of methyl bromide in rats. Toxicol Appl Pharmacol, 81:
183-191.
Honma T, Muneyuki M, & Sato M (1987) Methyl bromide alters
catecholamine and metabolite concentrations in rat brain. Neurotoxicol
Teratol, 9: 369-375.
Honma T, Miyagawa M, & Sato M (1991) Inhibition of tyrosine
hydroxylase activity by methyl bromide exposure. Neurotoxicol Teratol,
13: 1-4.
Hubbs AF & Harrington DD (1986) Further evaluation of the potential
gastric carcinogenic effects of subchronic methyl bromide
administration. In: Proceedings of the 36th Meeting of the American
College of Veterinary Pathology and the Annual Meeting of the American
Society for Veterinary and Clinical Pathology, Denver, Colorado,
December 1985. Denver, Colorado, American Society for Veterinary and
Clinical Pathology, p 92.
Hurtt ME & Working PK (1988) Evaluation of spermatogenesis and sperm
quality in the rat following acute inhalation exposure to methyl
bromide. Fundam Appl Toxicol, 10: 490-498.
Hurtt ME, Morgan KT, & Working PK (1987) Histopathology of acute toxic
responses in selected tissues from rats exposed by inhalation to
methyl bromide. Fundam Appl Toxicol, 9: 352-365.
Hurtt ME, Thomas DA, Working PK, Monticello TM, & Morgan KT (1988)
Degeneration and regeneration of the olfactory epithelium following
inhalation exposure to methyl bromide: pathology, cell kinetics, and
olfactory function. Toxicol Appl Pharmacol, 94: 311-328.
Hustinx WNM, Van de Laar RTH, Van Huffelen AC, Vrey JC, Meulenbelt J,
& Savelkoul TJF (1993) Systemic effects of inhalation methyl bromide
poisoning: a study of nine cases occupationally exposed due to
inadvertent spread during fumigation. Br J Ind Med, 50: 155-159.
Hyman MR & Wood PM (1984) Bromocarbon oxidations by Nitrosomonas
europaea. In: Crawford RL & Hanson RS ed. Microbial growth on C1
compounds. Washington, DC, American Society for Microbiology, pp
49-52.
IARC (1986) Methyl bromide. In: Some halogenated hydrocarbons and
pesticide exposures. Lyon, International Agency for Research on
Cancer, pp 187-212 (IARC Monograph on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1-42. Lyon, International Agency for Research
on Cancer, p 245 (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Ikawa N, Araki A, Nozaki K, & Matsushima T (1986) Micronucleus test of
methyl bromide by the inhalation method. Mutat Res, 164: 269
(Abstract).
Ikeda T, Kishi R, Yamamura K, Miyake H, & Sato M (1980) Behavioural
effects in rats following repeated exposure to methyl bromide. Toxicol
Lett, 6: 317-321.
Ingram FR (1951) Methyl bromide fumigation control in the date-packing
industry. Arch Ind Hyg Occup Med, 4: 193-198.
Inouye T, Inouye N, Asatani M, & Mitsuhata K (1967) Cucumber green
mottle mosaic virus in Japan. III. Inactivation of cucumber green
mottle mosaic virus in infected plant tissue buried in soil treated
with methyl bromide. Nogaku Kenkyu, 51: 199-207.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1940) The response
attending exposure of laboratory animals to vapors of methyl bromide.
J Ind Hyg Toxicol, 22: 218-230.
Irish DD, Adams EM, Spencer HC, & Rowe VK (1941) Chemical changes of
methyl bromide in the animal body in relation to its physiological
effects. J Ind Hyg Toxicol, 23: 408-411.
Isa AL, Fam EZ, Kamel AH, & Awadallah WH (1970) On the effect of
certain fumigants on the overwintering corn borers larvae. Agric Res
Rev, 48: 43-47.
Ishizu S, Kato N, Morinobu S, Nagao N, Yamano Y, & Ito I (1988) [Cases
of severe methyl bromide poisoning residing above a warehouse.] Jpn J
Ind Health, 30: 54-60 (in Japanese with English abstract).
Ito KA, Seeger ML, & Lee WH (1972) Destruction of Byssochlamys fulva
asci by low concentrations of gaseous methyl bromide and by aqueous
solutions of chlorine, an iodophor, and peracetic acid. J Appl
Bacteriol, 35: 479-483.
Iwasaki K (1988a) Determination of S-methylcysteine in mouse
hemoglobin following exposure to methyl bromide. Ind Health, 26:
187-190.
Iwasaki K (1988b) Individual differences in the formation of
hemoglobin adducts following exposure to methyl bromide. Ind Health,
26: 257-262.
Iwasaki K, Ito I, & Kagawa J (1989) Biological exposure monitoring of
methyl bromide workers by determination of hemoglobin adducts. Ind
Health, 27: 181-183.
Japanese Ministry of Labour (1992) Toxicology and carcinogenesis
studies of methyl bromide in F344 rats and BDF mice (inhalation
studies). Tokyo, Industrial Safety and Health Association, Japanese
Bioassay Laboratory, 197 pp (Unpublished report).
Jaskot RH, Grose EC, Most BM, Menache MG, Williams TB, &
Roycroft JH (1988) The distribution and toxicological effects of
inhaled methyl bromide in the rat. J Am Coll Toxicol, 7: 631-642.
Jayanty RKM (1989) Evaluation of sampling and analytical methods for
monitoring toxic organics in air. Atmos Environ, 3: 777-782.
JEA (Japanese Environmental Agency) (1981) Chemicals in the
environment. Tokyo, Japanese Environmental Agency, Office of Health
Studies, Environmental Health Department, pp 92-93.
Johnstone RT (1945) Methyl bromide intoxication of a large group of
workers. Ind Med, 14: 495-497.
Jones L (1968) effect of methyl bromide treatments on several species
of conifer seed. J For, 8: 858-860.
Joshi GP (1974) Toxicity of certain chemicals on Oryzaephilus
mercator F. (Coleoptra: Cucujidae). Appl Entomol Zool, 9: 280-281.
Kallio H & Shibamoto T (1988) Direct capillary trapping and gas
chromatographic analysis of bromomethane and other highly volatile air
pollutants. J Chromatogr, 454: 392-397.
Kantarjian AD & Shaheen AS (1963) Methyl bromide poisoning with
nervous system manifestations resembling polyneuropathy. Neurology,
13: 1054-1058.
Kato N, Morinobu S, & Ishizu S (1986) Subacute inhalation experiment
for methyl bromide in rats. Ind Health, 24: 87-103.
Katz AJ (1985) Genotoxicity of methyl bromide in somatic cells of
Drosophila larvae. Environ Mutagen, 7: 13 (Abstract).
Katz AJ (1987) Inhalation of methyl bromide gas induces mitotic
recombination in somatic cells of Drosophila melanogaster. Mutat Res,
192: 131-135.
Kelley WD & Rodriguez-Kabana R (1979) Effects of sodium azide and
methyl bromide on soil bacterial populations, enzymic activities and
other biological variables. Pestic Sci, 10: 207-215.
Kempton RJ & Maw GA (1974) Soil fumigation with methyl bromide: the
phytotoxicity of inorganic bromide to carnation plants. Ann Appl Biol,
76: 217-229.
Kenaga EE (1961) Time, temperature and dosage relationships of several
insecticidal fumigants. J Econ Entomol, 54: 537-542.
Khalil MAK & Rasmussen RA (1985) The trend of bromodifluoromethane
(CBrCIF2) and the concentration of other bromine containing gases at
the South Pole. Antarct J US, 21: 206-207.
Khalil MAK, Rasmussen RA, & Gunawardena R (1993) Atmospheric methyl
bromide: Trends and global mass balance. J Geophys Res, 98: 2887-2896.
Khanna SC & Yadav TD (1987) Effect of methyl bromide fumigation on the
germinability of wheat seed. Seed Res, 15: 183-186.
Kiewnick L (1968) Occurrence and control of sclerotium rolfsii.
Meded Fac Landbouwwet Rijksuniv Gent, 33: 987-995.
King JR & Benschoter CA (1991) Comparative methyl bromide residues in
Florida citrus: A basis for proposing quarantine treatments against
caribbean fruit fly. J Agric Food Chem, 39: 1307-1309.
King JR, Benschoter CA, & Burditt AK, Jr (1981) Residues of methyl
bromide in fumigated grapefruit determined by a rapid, headspace
assay. J Agric Food Chem, 29: 1003-1005.
Kishi R, Ishizu I, Ito I, Katoh N, Miyake H, & Harabuch I (1988)
Health research of methyl bromide manufacturing workers. Part 1.
Symptoms of long-term exposure. Occupational health in the chemical
industry. XXII ICOH (International Commission on Occupational Health)
Congress, Sydney, New South Wales, Australia, 27 September-2 October
1987. Copenhagen, World Health Organization Regional Office for
Europe, pp 120-134.
Kishi R, Itoh I, Ishizu S, Harabuchi I, & Miyake H (1991) Symptoms
among workers with long-term exposure to methyl bromide. An
epidemiological study. Jpn J Ind Health, 33: 241-250.
Kisser W (1967) [The detection and quantitative determination of
brominated urea derivatives in toxicology.] Arch Toxicol, 22: 404-409
(in German).
Kissler JJ, Lider JV, Raabe RD, Raski DJ, Schmitt RV, & Hurlimann JH
(1973) Soil fumigation for control of nematodes and oak root fungus in
vineyard replants. Plant Dis Rep, 57: 115-119.
Klein L (1989) Methyl bromide strip fumigation - problems encountered
and ways to combat them. Third International Symposium on Soil
Disinfestation, Leuven, Belgium, September 26-30, 1988. Acta Hortic,
255: 213-225.
Knight HD & Costner GC (1977) Bromide intoxication of horses, goats,
and cattle. J Am Vet Med Assoc, 171: 446-448.
Knight HD & Reina-Guerra M (1977) Intoxication of cattle with sodium
bromide-contaminated feed. Am J Vet Res, 38: 407-409.
Koga M, Hara K, Hori H, Kodama Y, & Okubo T (1991) [Determination of
bromide ion concentration in urine using a head-space gas
chromatography and an ion chromatography. Biological monitoring for
methyl bromide exposure.] J Univ Occup Environ Health, 13: 19-24 (in
Japanese with English summary).
Kolbezen MJ & Abu-El-Haj FJ (1972) Fumigation with methyl bromide. II.
Equipment and methods for sampling and analysing deep field soil
atmospheres. Pestic Sci, 3: 73-80.
Kramers PGN, Bissumbhar B, & Mout HCA (1985b) Studies with gaseous
mutagens in Drosophila melanogaster. In: Waters MD, Sandhu SS, Lewtas
J, Claxton L, Straus G, & Nesnow S, ed. Short-term bioassays in the
analysis of complex environmental mixtures IV., New York, London,
Plenum Press, pp 65-73.
Kramers PGN, Voogd CE, Knaap AGAC, & Van der Heijden CA (1985a)
Mutagenicity of methyl bromide in a series of short-term tests. Mutat
Res, 155: 41-47.
Krost KJ, Pellizzari ED, Walburn SG, & Hubbard SA (1982) Collection
and analysis of hazardous organic emissions. Anal Chem, 54: 810-817.
Latta R & Cowgill H (1941) Methyl bromide fumigation of greenhouse
plants at U.S. Plant Introduction Garden, Glenn Dale, Md. (Abstract
S.8189, Bureau of Entomology and Plant Quarantine). Washington, DC, US
Department of Agriculture (E-526).
Laug EP (1941) Bromide residues in foodstuffs. Volatile and
nonvolatile residues following experimental exposure to methyl
bromide. Ind Eng Chem, 33: 803-805.
Leesch JG, Gillenwater HB, & Woodward JO (1974) Methyl bromide
fumigation of shelled peanuts in bulk containers. J Econ Entomol, 67:
769-771.
Lefevre C, Ferrari P, Günier JP, & Muller J (1989) Sampling and
analysis of airborne methylbromide. Chromatographia, 27: 37-43.
Leichnitz K (1985) [Dräger-tubes methyl bromide 3/a. Detector-tubes
pocket book 6.] Lübeck, Dragerwerk (in German).
Lepschy J, Stark H, & Sub A (1979) [Behaviour of methyl bromide
(Terabol) in soil.] Gartenbauwissenschaften, 44: 84-89 (in German).
Lewis SE (1948) Inhibition of SH proteins by methyl bromide. Nature
(Lond), 161: 692-693.
Linenberg A, Davis R, & Robinson D (1991) Hazardous waste site
measurements of low levels of chlorinated hydrocarbons using a
portable gas chromatography. Hazard Mater Control, 4: 42-46.
Long PL, Brown WB, & Goodship G (1972) Effect of methyl bromide on
coccidial oocysts determined under controlled conditions. Vet Rec, 90:
562-566.
Longley EO & Jones AT (1965) Methyl bromide poisoning in man. Ind Med
Surg, 34: 499-502.
Lopez VC, Fernandez JAM, Yanez LP, & Vazquez MS (1986a) [Methyl
bromide poisoning: I. Some historical, clinical, legal and
neuropsychiatric aspects.] Arch Neurobiol, 49: 279-290 (in Spanish).
Lopez CV, Fernandez MJA, Yanez PL, & Vazquez SM (1986b) [Methyl
bromide poisoning. II. Contribution of 2 cases.] Arch Neurobiol, 49:
311-328 (in Spanish).
Lopez-Avila V, Wood R, Flanagan M, & Scott R (1987) Determination of
volatile priority pollutants in water by purge and trap and capillary
column gas chromatography/mass spectrometry. J Chromatogr Sci, 25:
286-291.
Love JL, Winchester RV, & Keating DL (1979) Fumigant and contact
insecticide residues on dried fruits, cereals, nuts, and spices
imported into New Zealand. N Z J Sci, 22: 95-98.
Lovelock JE (1975) Natural halocarbons in the air and in the sea.
Nature (Lond), 256: 193-194.
Lynn GE, Shrader SA, Hammer OH, & Lassiter CA (1963) Occurrence of
bromides in the milk of cows fed sodium bromide and grain fumigated
with methyl bromide. J Agric Food Chem, 11: 87-91.
Mabey W & Mill T (1978) Critical review of organic compounds in water
under environmental conditions. J Phys Chem Ref Data, 7: 383-415.
McGregor DB (1981) Tier II mutagenic screening of 13 NIOSH priority
compounds, Report No. 32 - Individual compound report: methyl bromide.
Cincinnati, Ohio, National Institute of Occupational Safety and
Health, 190 pp (PB83-130211).
Mackay D & Shiu WK (1981) A critical review of Henry's Law Constants
for chemicals of environmental interest. J Phys Chem Ref Data, 10:
1175-1199.
Maddy KT, Edmiston S, & Richmond D (1990) Illness, injuries, and
deaths from pesticide exposures in California 1949-1988. In: Gunter FA
ed. Reviews of environmental contamination and toxicology. Berlin,
Heidelberg, New York, Springer-Verlag, vol 14, pp 57-123.
MAFF (1990) Report of the working party on pesticide residues:
1988-89. Supplement to issue No. 8 (1990) of the pesticides register.
London, Ministry of Agriculture, Fisheries and Food, Health and Safety
Executive, 11 pp.
MAFF (1989) Report of the working party on pesticide residues:
1985-88. The twenty-fifth report of the steering group on food
surveillance. London, Ministry of Agriculture, Fisheries and Food, 3
pp (Food Surveillance Paper No. 25).
Malkomes H-P (1970) [Investigations into the degradation of methyl
bromide during substrate fumigation and its influence on the following
crops.] Hanover, Technical University, pp 18-35 (Dissertation) (in
German).
Marraccini JV, Thomas GE, Ongley JP, Pfaffenberger D, Davis JH, &
Bednarczyk LR (1983) Death and injury caused by methyl bromide, an
insecticide fumigant. J Forensic Sci Soc, 28: 601-607.
Matheson gas data book (1980) The Matheson gas data book. East
Rutherford, New Jersey, Matheson Gas products (A Division of Will
Ross), vol 6, pp 361-363.
Matta A & Porta-Puglia A (1968) [Sensitivity to methyl bromide and
vapam and secondary growth in fumigated soil of distinct functional
groups of microorganisms.] Ann Fac Agrar, 4: 261-274 (in Italian).
Maw GA & Kempton RJ (1973) Methyl bromide as a soil fumigant. Soils
Fertil, 36: 41-47.
Mazzini L, Galante M, Rezzonico M, & Kokodoko A (1992) Methylbromide
intoxication: A case report. Schweiz Arch Neurol Psychiatr, 143:
75-80.
MBSW (1992) Proceedings of the Methyl Bromide Science Workshop,
Washington, DC, 2-3 June 1992. Washington, DC, Atmospheric
Environmental Research, Inc., pp 90-97.
Medinsky MA, Bond JA, Dutcher JS, & Birnbaum LS (1984) Disposition of
[14C]methyl bromide in Fischer-344 rats after oral or
intraperitoneal administration. Toxicology, 32: 187-196.
Medinsky MA, Dutcher JS, Bond JA, Henderson RF, Mauderly JL, Snipes
MB, Mewhinney JA, Cheng YS, & Birnbaum LS (1985) Uptake and excretion
of [14C]methyl bromide as influenced by exposure concentration.
Toxicol Appl Pharmacol, 78: 215-225.
Meister RT (1985) In: Meister RT ed. Farm chemicals handbook 85.
Pesticide dictionary -Section C. Willoughby, Ohio, Meister Publishing
Co., 154 pp.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992a) Atmospheric lifetimes and ozone
depletition potentials of methyl bromide (CH3Br) and dibromomethane
(CH2Br2). Geophys Res Lett, 19: 2059-2062.
Mellouki A, Talukdar RK, Schmoltner A-M, Gierczak T, Mills MJ, Solomon
S, & Ravishankara AR (1992b) Correction to "atmospheric lifetimes and
ozone depletion potentials of methylbromide (CH3Br) and
dibromomethane (CH2Br2)". Geophys Res Lett, 19: 2279-2280.
Merzbach L (1928) [The pharmacology of methyl bromide and some related
compounds.] Z. Ges. Exp. Med., 63: 383-392 (in German).
Mignard E & Benet JC (1989) Diffusion of methyl bromide in soil. J
Soil Sci, 40: 151-165.
Miller DP & Haggard HW (1943) Intracellular penetration of bromide as
a feature in the toxicity of alkyl bromides. J Ind Hyg Toxicol, 25:
423-433.
Minton NA & Gillenwater HB (1973) Methyl bromide fumigation of
Pratylenchus brachyurus in peatnut shells. J Nematol, 5: 147-149.
Mitsumori K, Maita K, Kosaka T, Miyaoka T, & Shirasu Y (1990) Two-year
oral chronic toxicity and carcinogenicity study in rats of diets
fumigated with methyl bromide. Food Chem Toxicol, 28: 109-119.
Miyagawa M (1982) Conditioned taste aversion induced by inhalation
exposure to methyl bromide in rats. Toxicol Lett, 10: 411-416.
Mizyukova IG & Bakhishev CN (1971) [Specific treatment of acute
poisoning with methyl bromide.] Vrach Delo, 7: 128-131 (in Russian
with English abstract).
Moelwyn-Hughes EA (1938) The hydrolysis of the methyl halides. Proc R
Soc, A164: 295-306.
Moje W (1960) The chemistry and nematocidal activity of organic
halides. In: Metcalf RL, ed. Volume III. Advances in pest control
research. New York, Intersience Publishers, pp 181-217.
Molina LT, Molina MJ, & Rowland FS (1982) Ultraviolet absorption cross
sections of several brominated methanes and ethanes of atmospheric
interest. J Phys Chem, 86: 2672-2676.
Monro HAU, Dumas T, & Ruckland CT (1966) The influence of vapour
pressure of different fumigants on the mortality of two stored-product
insects in vacuum fumigation. J Stored Prod Res, 1: 207-222.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Morrissey RE, Schwetz BA, Lamb JCIV, Ross MD, Teague JL, & Morris RW
(1988) Evaluation of rodent sperm, vaginal cytology, and reproductive
organ weight data from National Toxicology Program 13-week studies.
Fundam Appl Toxicol, 11: 343-358.
Mostafa SAS, Kamel AH, El-Nahal AKM, & El-Borollosy FM (1972) Toxicity
of carbon bisulphide and methyl bromide to the eggs of four stored
product insects. J Stored Prod Res, 8: 193-198.
Mounat A & Hitier H (1959) Chemical disinfection of the soil of
tobacco seed beds. Ann Inst Exp Tabac Bergerac, 3: 287-298.
Mueller A, Jorritsma U, Hindermeier U, & Gansewendt B (1991) Human
serum albumin adducts as a parameter for biomonitoring of exposure to
methyl bromide. Naunyn-Schmiedeberg's Arch Pharmacol, 344: R121.
Mueller A, Jorritsma U, & Gansewendt B (1992) Different binding of
methyl bromide to blood macromolecules due to conjugator status.
Naunyn-Schmiedeberg's Arch Pharmacol, 345: R 42.
Munnecke DE & Van Gundy SD (1979) Movement of fumigants in soil,
dosage responses, and differential effects. Annu Rev Phytopathol, 17:
405-429.
Munnecke DE, Kolbezen MJ, & Stolzy LH (1969) Factors affecting field
fumigation of citrus soils for control of Armillaria mellea.
Proceedings of the First International Citrus Symposium. Lancaster,
Pennsylvania, Society of the Plastics Industry, vol 3, pp 1273-1277.
Munnecke DE, Kolbezen MJ, & Wilbur WD (1978) Types and thickness of
plastic films in relation to methyl bromide fumigation. Proceedings of
the Agricultural Plastic Congress, San Diego, pp 482-487.
National Academy of Science (1978) Chloroform, carbon tetrachloride,
and other halomethanes: an environmental assessment. Washington, DC,
National Academy of Sciences, 294 pp.
NASA (1992) Chemical kinetics and photochemical data for use in
stratospheric modeling. Evaluation No. 10: NASA Panel for Data
Evaluation. Pasadena, California Institute of Toxicology, Jet
Propulsion Laboratory, National Aeronautics and Space Administration.
NFPA (1984) Fire protection guide on hazardous materials, 9th ed.
Quincy, Maryland, National Fire Protection Agency, 5 pp (NFPA No.
SPP-1E).
NIOSH (1984) Monohalomethanes: methyl chloride CH3Cl, methyl bromide
CH3Br, methyl iodide CH3I. Curr Intell Bull, 42: 1-22.
Nishimura M, Umeda M, Ishizu S, & Sato M (1980) Effect of methyl
bromide on cultured mammalian cells. J Toxicol Sci, 5: 321-330.
NTP (1992) Toxicology and carcinogenesis studies of methyl bromide
(CAS No. 