
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 192
Flame Retardants: A General Introduction
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Environmental Health Criteria 192
First draft prepared by Dr G.J. van Esch, Bilthoven, Netherlands
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1997
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Flame Retardants: A General Introduction
(Environmental health criteria ; 192)
1.Flame retardants - toxicity 2.Occupational exposure
3.Environmental exposure I.Series
ISBN 92 4 157192 6 (NLM Classification: WA 250)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS: A GENERAL
INTRODUCTION
PREAMBLE
GLOSSARY AND ABBREVIATIONS
PROLOGUE
1. INTRODUCTION
2. TYPES OF FLAME RETARDANTS
2.1. Inorganic flame retardants
2.1.1. Metal hydroxides
2.1.2. Antimony compounds
2.1.3. Boron compounds
2.1.4. Other metal compounds
2.1.5. Phosphorus compounds
2.1.6. Other inorganic flame retardants
2.2. Halogenated organic flame retardants
2.2.1. Brominated flame retardants
2.2.2. Chlorinated flame retardants
2.3. Organophosphoros flame retardants
2.3.1. Non-halogenated compounds
2.3.2. Halogenated phosphates
2.4. Nitrogen-based flame retardants
3. MECHANISM OF ACTION OF FLAME RETARDANTS
3.1. General aspects
3.1.1. Physical action
3.1.2. Chemical action
3.2. Condensed phase mechanisms
3.3. Gas-phase mechanisms
3.4. Co-additives for use with flame retardants
3.5. Smoke suppressants
3.5.1. Condensed phase
3.5.2. Gas phase
4. PERFORMANCE CRITERIA FOR AND CHOICE OF FLAME RETARDANTS
5. PRODUCTION AND USES OF FLAME RETARDANTS AND FLAME-RETARDED
POLYMERS
5.1. Production
5.2. Uses
5.2.1. Plastics
5.2.2. Textile/furnishing industry
6. FORMATION OF TOXIC PRODUCTS ON HEATING OR COMBUSTION OF
FLAME-RETARDED PRODUCTS
6.1. Toxic products in general
6.2. Formation of halogenated dibenzofurans and dibenzodioxins
6.3. Exposure to PBDD/PBDF from polymers containing halogenated
flame retardants
6.3.1. Exposure from contact or emission from products
containing halogenated flame retardants
6.3.2. Workplace exposure studies
6.3.3. Formation of PBDD/PBDF from combustion
6.3.3.1 Laboratory pyrolysis experiments
6.3.3.2 Fire tests and fire accidents
7. OVERVIEW OF EXPOSURE AND HAZARDS TO HUMANS AND THE ENVIRONMENT
7.1. Human exposure
7.1.1. General population
7.1.2. Occupational exposure
7.2. Exposure of the environment
7.3. Hazards to humans
7.4. Hazards to the environment
8. REGULATIONS WITH RESPECT TO FLAME RETARDANTS
9. CONCLUSIONS AND RECOMMENDATIONS FOR THE PROTECTION OF HUMAN
HEALTH AND THE ENVIRONMENT
10. FURTHER RESEARCH
REFERENCES
ANNEX I: Terminology
ANNEX II: Flame retardants in commercial use or
used formerly
ANNEX III: Fire tests
ANNEX IV: US Interagency Testing Commission
recommendations on brominated
flame retardants
CONCLUSIONS ET RECOMMANDATIONS
CONCLUSIONES Y RECOMMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22 -
9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number 5 U01 ES02617-
15 from the National Institute of Environmental Health Sciences,
National Institutes of Health, USA, and by financial support from the
European Commission.
* * *
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for this
publication.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
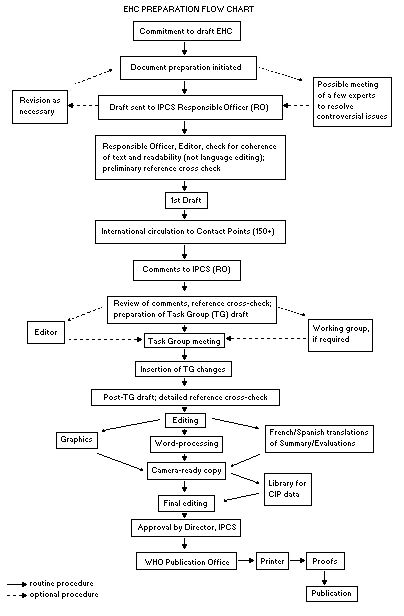 The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
A GENERAL INTRODUCTION
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico
Dr P. Arias, Brussels, Belgium
Dr S.A. Assimon, Contaminants Branch, US Food and Drug Administration,
Washington, DC, USA
Dr H. Hofer, Toxicology, Austrian Research Centre, Seibersdorf,
Austria
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Stockholm, Sweden ( Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health, Ahmedabad,
India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany ( Vice-Chairman)
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Sœderlund, Folkehelsa, National Institute of Public Health,
Oslo, Norway ( Rapporteur)
Dr J. Troitzsch, Fire and Environment Protection Service, Wiesbaden,
Germany
Observers
Dr M.L. Hardy, Albermarle Corporation, Baton Rouge, USA
Mr T.A. Jay, Applications Laboratory, Great Lakes Chemical (Europe)
N.V., Geel, Belgium
Dr M. Papez, European Flame Retardants Association, Brussels, Belgium
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization ( Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS: A GENERAL
INTRODUCTION
A WHO Task Group on Environmental Health Criteria for Flame
Retardants met at the World Health Organization, Geneva, from 4 to 8
December 1995. Dr K.W. Jager, IPCS, welcomed the participants on
behalf of Dr M. Mercier, Director of the IPCS, and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and prepared conclusions and
recommendations.
The first draft of the monograph was prepared by Dr G.J. van
Esch, Bilthoven, the Netherlands. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ABS acrylonitrile-butadiene-styrene
APP ammonium polyphosphate
ATH alumina trihydrate
DeBDE decabromodiphenyl ether
EPDM ethylene propylene rubber
EPS expandable polystyrene
FR flame retardant
HBCD hexabromocyclododecane
HIPS high impact polystyrene
HDPE high density polyethylene
LDPE low density polyethylene
PA polyamides
PBDE polybrominated diphenyl ether
PBB polybrominated biphenyl
PBDD polybrominated dibenzodioxin
PBDF polybrominated dibenzofuran
PBT polybutylene terephthalate
PCDD polychlorinated dibenzodioxin
PCDF polychlorinated dibenzofuran
PE polyethylene
PET polyethylene terephthalate
PP polypropylene
PVC polyvinyl chloride
TBBPA tetrabromobisphenol A
TCDD 2,3,7,8-tetrachlorinated dibenzo- p-dioxin
TCPP tris(1-chloro-2-propyl)phosphate
PROLOGUE
The intent of this Environmental Health Criteria (EHC) monograph
is to provide a general overview of the nature, mechanism of action,
use and production volume of compounds used to improve the flame
retardancy of polymeric materials and textiles. The monograph also
indicates some of the known health and environmental hazards for
certain of the flame retardants that have been assessed by the IPCS.
A large number of compounds have been identified as being used as
flame retardants. Detailed international assessments of the risks
posed to human health and the environment by a number of these
substances have been published previously as EHC monographs. In Table
1 are listed the monographs on flame retardants and compounds related
to flame retardants that have already been published by IPCS or are in
preparation.
This monograph is intended to provide a starting point for those
interested in obtaining general information on flame-retardant
chemicals. More detailed information on use patterns, sources of
exposure, and health and environmental risks posed by these substances
can be found in the appropriate EHC monograph.
Table 1. EHC monographs on chemicals associated with
flame-retardant use
Substance EHC monograph
Mirex (1,1a,2,2,3,3a,4,5,5,5a,5b,6- EHC 44 (1984)
dodecachloroocta-hydro-1,3,4-metheno-1H-
cyclobuta( cd)pentalene)
Polychlorinated dibenzo- p-dioxins and EHC 88 (1989)
dibenzofurans
Tricresyl phosphate EHC 110 (1990)
Triphenyl phosphate EHC 111 (1991)
Hexachlorocyclopentadiene EHC 120 (1991)
Polychlorinated biphenyls EHC 140 (1992)
Polybrominated biphenyls EHC 152 (1994)
Brominated diphenyl ethers EHC 162 (1994)
Tetrabromobisphenol A and some of its EHC 172 (1995)
derivatives
Tris(2,3-dibromopropyl) phosphate and EHC 173 (1995)
bis(2,3-dibromopropyl) phosphate
Chlorendic acid and anhydride EHC 185 (1996)
Chlorinated paraffins EHC 181 (1996)
Polybrominated dibenzo- p-dioxins and EHC in preparation
dibenzofurans
Vinylbromide EHC in prepraration
Tris(chloropropyl) phosphates EHC in prepraration
Tris(2-butoxyethyl) phosphate EHC in prepraration
Tris(2-chloroethyl) phosphate EHC in prepraration
Tris(2-ethylhexyl) phosphate EHC in prepraration
Tetrakis(hydroxymethyl) phosphonium salts EHC in prepraration
1. INTRODUCTION
In today's society, there is an unprecedented development in the
size and number of buildings, skyscrapers, warehouses and methods of
transport. Carpeting, furnishings, equipment, oil and gas for heating
all increase the fire load in a building. New technologies, new
processes and new applications introduce new fire hazards (e.g., new
ignition sources such as welding sparks and short circuits)
(Troitzsch, 1990). Modern fire-fighting techniques, equipment and
building design have reduced the destruction due to fires. However,
a high fuel load in either a residential or a commercial building can
offset even the best of building construction (Gann, 1993).
Each year, over 3 million fires leading to 29 000 injuries and
4500 deaths are reported in the USA. The direct property losses
exceed $8 billion and the total annual cost has been estimated at over
$100 billion. Personal losses occur mostly in residences where
furniture, wall coverings and clothes are frequently the fuel. Large
financial losses occur in commercial structures such as office
buildings and warehouses. Fires also occur in aeroplanes, buses and
trains (Gann, 1993).
To provide additional protection from fires and to increase
escape time when a fire occurs, methods to enhance the flame
retardance of consumer goods have been developed. Flame retardants
are chemicals added to polymeric materials, both natural and
synthetic, to enhance flame-retardance properties. Flame-retardant
chemicals are most often used to improve the fire performance of low-
to-moderate cost commodity polymers. These flame retardants may be
physically blended with or chemically bonded to the host polymer.
They generally either lower ignition susceptibility or lower flame
spread once ignition has occurred. Some polymers are inherently less
flammable due to more stable polymeric structures; these are usually
higher priced engineering plastics such as polyimides,
polybenzimidazoles and polyetherketones (Gann, 1993).
Flame-retardant systems for synthetic or organic polymers act in
five basic ways: (1) gas dilution; (2) thermal quenching;
(3) protective coating; (4) physical dilution; (5) chemical
interaction (Pettigrew, 1993); or through a combination of these
mechanisms.
1. Inert gas dilution involves using additives that produce large
volumes of non-combustible gases on decomposition. These gases
dilute the oxygen supply to the flame or dilute the fuel
concentration below the flammability limit. Metal hydroxides,
metal salts and some nitrogen compounds function in this way.
2. Thermal quenching is the result of endothermic decomposition of
the flame retardant. Metal hydroxides, metal salts and nitrogen
compounds act to decrease surface temperature and the rate of
burning.
3. Some flame retardants form a protective liquid or char barrier.
This limits the amount of polymer available to the flame front
and/or acts as an insulating layer to reduce the heat transfer
from the flame to the polymer. Phosphorus compounds and
intumescent systems based on melamine and other nitrogen
compounds are examples of this category.
4. Inert fillers (glass fibres and microspheres) and minerals (talc)
act as thermal sinks to increase the heat capacity of the polymer
or reduce its fuel content.
5. Halogens and some phosphorus flame retardants act by chemical
interaction. The flame retardant dissociates into radical
species that compete with chain-propagating steps in the
combustion process.
Chemicals that are used as flame retardants can be inorganic,
organic, mineral, halogen-containing or phosphorus-containing. The
term flame "retardant" represents a class of use and not a class of
chemical structure (Pettigrew, 1993).
Preventive flame protection, including the use of flame
retardants, has been practised since ancient times. Some examples of
early historical developments in flame retardants are shown in
Table 2.
Table 2. Early historical fire-retardant developmentsa
Development Date
Alum used to reduce the flammability of wood by the About 450 BC
Egyptians
The Romans used a mixture of alum and vinegar on About 200 BC
wood
Mixture of clay and gypsum used to reduce 1638
flammability of theatre curtains
Mixture of alum, ferrous sulfate and borax used 1735
on wood and textiles by Wyld in Britain
Alum used to reduce flammability of balloons 1783
Gay-Lussac reported a mixture of (NH4)3PO4, 1821
NH4Cl and borax to be effective on linen and hemp
Perkin described a flame-retardant treatment for 1912
cotton using a mixture of sodium stannate and
ammonium sulfate
a From: Hindersinn (1990)
The advent of synthetic polymers earlier this century was of
special significance, since the water-soluble inorganic salts used up
to that time were of little or no utility in these largely hydrophobic
materials. Modern developments were, therefore, concentrated on the
development of polymer-compatible flame retardants.
By the outbreak of the Second World War, flame-proof canvas
tentage for outdoor use by the military was produced with a treatment
of chlorinated paraffins and an insoluble metal oxide, mostly antimony
oxide as a glow inhibitor, together with a binder resin.
After the war, non-cellulosic thermoplastic polymers became more
and more important as the basic fibres used for flame-retardant
applications. A dramatic example of the superiority of the non-
cellulosic compounds is provided by the diminished use of cotton fibre
in children's sleepwear since the inception of new standards. In
1971, cotton supplied 78% of the fibres used to produce children's
sleepwear, whereas in 1973 it supplied less than 10% in the USA (US
EPA, 1976).
With the increasing use of thermoplastics and thermosets on a
large scale for applications in building, transportation, electrical
engineering and electronics, new flame-retardant systems were
developed. They mainly consist of inorganic and organic compounds
based on bromine, chlorine, phosphorus, nitrogen, boron, and metallic
oxides and hydroxides.
Today, these flame-retardant systems fulfill the multiple
flammability requirements developed for the above-mentioned
applications.
A glossary of terms concerning flammability and flame retardants
is given in Annex 1.
2. TYPES OF FLAME RETARDANTS
A distinction is made between reactive and additive flame
retardants. Reactive flame retardants are reactive components
chemically built into a polymer molecule. Additive flame retardants
are incorporated into the polymer either prior to, during or (most
frequently) following polymerization.
There are three main families of flame-retardant chemicals
(Troitzsch, 1990; Wolf & Kaul, 1992; Green, 1992; Touval, 1993;
Pettigrew, 1993; Weil, 1993).
1. The main inorganic flame retardants are aluminium trihydroxide,
magnesium hydroxide, ammonium polyphosphate and red phosphorus.
This group represents about 50% by volume of the worldwide flame
retardant production. Some of these chemicals are also used as
flame retardant synergists, of which antimony trioxide is the
most important (OECD, 1994).
2. Halogenated products are based primarily on chlorine and
bromine. This group represents about 25% by volume of the
worldwide production (OECD, 1994).
3. Organophosphorus products are primarily phosphate esters and
represent about 20% by volume of the worldwide production.
Products containing phosphorus, chlorine and/or bromine are also
important.
In addition, nitrogen-based flame retardants are used for a
limited number of polymers.
Annex II comprises lists of the different flame retardant
families commercially used at present (A) and those no more in
use (B).
2.1 Inorganic flame retardants
Metal hydroxides form the largest class of all flame retardants
used commercially today and are employed alone or in combination with
other flame retardants to achieve necessary improvements in flame
retardancy. Antimony compounds are used as synergistic co-additives
in combination with halogen compounds, facilitating the reduction in
total flame retardant levels needed to achieve a desired level of
flame retardancy. To a limited extent, compounds of other metals also
act as synergists with halogen compounds. They may be used alone but
are most commonly used with antimony trioxide to enhance other
characteristics, for example, smoke reduction or afterglow
suppression. Ionic compounds have a very long history as flame
retardants for wool- or cellulose-based products. Inorganic
phosphorus compounds are primarily used in polyamides and phenolic
resins, or as components in intumescent formulations.
2.1.1 Metal hydroxides
Metal hydroxides function in both the condensed and gas phases of
a fire by absorbing heat and decomposing to release their water of
hydration. This process cools both the polymer and the flame and
dilutes the flammable gas mixture. The very high concentrations (50
to 80%) required to impart flame retardancy often adversely affect the
mechanical properties of the polymer into which they are incorporated.
Aluminium hydroxide, also known as alumina trihydrate (ATH) is
the largest volume flame retardant in use today. It decomposes when
exposed to temperatures over 200°C, which limits the polymers in
which it can be incorporated. Magnesium hydroxide is stable to
temperatures above 300°C and can be processed into several polymers.
2.1.2 Antimony compounds
Antimony trioxide is not a flame retardant per se, but it is
used as a synergist. It is utilized in plastics, rubbers, textiles,
paper and paints, typically 2-10% by weight, with organochlorine and
organobromine compounds to diminish the flammability of a wide range
of plastics and textiles (IARC, 1989).
Antimony oxides and antimonates must be converted to volatile
species. This is usually accomplished by release of halogen acids at
fire temperatures. The halogen acids react with the antimony-
containing materials to form antimony trihalide and/or antimony halide
oxide. These materials act both in the substrate (condensed phase)
and in the flame to suppress flame propagation. In the condensed
phase, they promote char formation, which acts as a physical barrier
to flame and inhibits the volatilization of flammable materials. In
the flame, the antimony halides and halide oxides, generated in
sufficient volume, provide an inert gas blanket over the substrate,
thus excluding oxygen and preventing flame spread. These compounds
alter the chemical reactions occurring at fire temperatures in the
flame, thus reducing the ease with which oxygen can combine with the
volatile products. It is also suggested that antimony oxychloride or
trichloride reduces the rate at which the halogen leaves the flame
zone, thus increasing the probability of reaction with the reactive
species. Antimony trichloride probably evolves heavy vapours which
form a layer over the condensed phase, stop oxygen attack and thus
choke the flame. It is also assumed that the liquid and solid
antimony trichloride particles contained in the gas phase reduce the
energy content of the flames by wall or surface effects (Troitzsch,
1990).
Other antimony compounds include antimony pentoxide, available
primarily as a stable colloid or as a redispersible powder. It is
designed primarily for highly specialized applications, although
manufacturers suggest it has potential use in fibre and fabric
treatment.
Sodium antimonate (Na2OSb2O5Ê´H2O) is recommended for
formulations in which deep tone colours are required or where antimony
trioxide may promote unwanted chemical reactions.
2.1.3 Boron compounds
Within the class of boron compounds, by far the most widely used
is boric acid. Boric acid (H3BO3) and sodium borate (borax)
(Na2B4O7. 10H2O) are the two flame retardants with the longest
history, and are used primarily with cellulosic material, e.g., cotton
and paper. Both products are effective, but their use is limited to
products for which non-durable flame retardancy is acceptable since
both are very water-soluble.
Zinc borate, however, is water-insoluble and is mostly used in
plastics and rubber products. It is used either as a complete or
partial replacement for antimony oxide in PVC, nylon, polyolefin,
epoxy, EPDM, etc. In most systems, it displays synergism with
antimony oxide. Zinc borate can function as a flame retardant, smoke
suppressant and anti-arcing agent in condensed phase. Recently, zinc
borate has also been used in halogen-free, fire-retardant polymers.
2.1.4 Other metal compounds
Molybdenum compounds have been used as flame retardants in
cellulosic materials for many years and more recently with other
polymers, mainly as smoke suppressants (see section 3.4) (Troitzsch,
1990). They appear to function as condensed-phase flame retardants
(Avento & Touval, 1980). Titanium and zirconium compounds are used
for textiles, especially wool (Calamari & Harper, 1993).
Zinc compounds, such as zinc stannate and zinc hydroxy-stannate,
are also used as synergists and as partial replacements for antimony
trioxide.
2.1.5 Phosphorus compounds
Red phosphorus and ammonium polyphosphate (APP) are used in
various plastics.
Red phosphorus was first investigated in polyurethane foams and
found to be very effective as a flame retardant. It is now used
particularly for polyamides and phenolic applications. The flame-
retarding effect is due, in all probability, to the oxidation of
elemental phosphorus during the combustion process to phosphoric acid
or phosphorus pentoxide. The latter acts by the formation of a
carbonaceous layer in the condensed phase. The formation of fragments
that act by interrupting the radical chain mechanism is also likely.
Ammonium polyphosphate is mainly applied in intumescent coatings
and paints. Intumescent systems puff up to produce foams. Because of
this characteristic they are used to protect materials such as wood
and plastics that are combustible and those like steel that lose their
strength when exposed to high temperatures. Intumescent agents have
been available commercially for many years and are used mainly as
fire-protective coatings. They are now used as flame-retardant
systems for plastics by incorporating the intumescent components in
the polymer matrix, mainly polyolefins, particularly polypropylene
(Troitzsch, 1990).
2.1.6 Other inorganic flame retardants
Other inorganic flame retardants, including ammonium sulfamate
(NH4SONH2) and ammonium bromide (NH4Br), are used primarily with
cellulose-based products and in forest fire-fighting (Weil, 1993).
2.2 Halogenated organic flame retardants
Halogenated flame retardants can be divided into three classes:
aromatic, aliphatic and cycloaliphatic. Bromine and chlorine
compounds are the only halogen compounds having commercial
significance as flame-retardant chemicals. Fluorine compounds are
expensive and, except in special cases, are ineffective because the
C-F bond is too strong. Iodine compounds, although effective, are
expensive and too unstable to be useful (Cullis, 1987; Pettigrew,
1993). The brominated flame retardants are much more numerous than
the chlorinated types because of their higher efficacy (Cullis, 1987).
With repect to processability, halogenated flame retardants vary
in their thermal stability. In general, aromatic brominated flame
retardants are more thermally stable than chlorinated aliphatics,
which are more thermally stable than brominated aliphatics.
Brominated aromatic compounds can be used in thermoplastics at fairly
high temperatures without the use of stabilizers and at very high
temperatures with stabilizers. The thermal stability of the
chlorinated and brominated aliphatics is such that, with few
exceptions, they must be used with thermal stabilizers, such as a tin
compound.
Halogenated flame retardants are either added to or reacted with
the base polymer. Additive flame retardants are those that do not
react in the application designated. There are a few compounds that
can be used as an additive in one application and as a reactive in
another; tetrabromobisphenol A is the most notable example. Reactive
flame retardants become a part of the polymer either by becoming a
part of the backbone or by grafting onto the backbone. The choice of
a reactive flame retardant is more complex than the choice of an
additive type. The development of systems based on reactive flame
retardants is more expensive for the manufacturer, who in effect has
to develop novel co-polymers with the desired chemical, physical and
mechanical properties, as well as the appropriate degree of flame
retardance (Cullis, 1987; Pettigrew, 1993). Synergists such as
antimony oxides are frequently used with halogenated flame retardants.
2.2.1 Brominated flame retardants
Bromine-based flame retardants are highly brominated organic
compounds with a relative molecular mass ranging from 200 to that of
large molecule polymers. They usually contain 50 to 85% (by weight)
of bromine (Cullis, 1987).
The highest volume brominated flame retardant in use today is
tetrabromobisphenol A (TBBPA) (IPCS, 1995a) followed by
decabromodiphenyl ether (DeBDE) (IPCS, 1994b). Both of these flame
retardants are aromatic compounds. The primary use of TBBPA is as a
reactive intermediate in the production of flame-retarded epoxy resins
used in printed circuit boards (IPCS, 1995a). A secondary use for
TBBPA is as an additive flame retardant in ABS systems. DeBDE is the
second largest volume brominated flame retardant and is the largest
volume brominated flame retardant used solely as an additive. The
greatest use (by volume) of DeBDE is in high-impact polystyrene, which
is primarily used to produce television cabinets. Secondary uses
include ABS, engineering thermoplastics, polyolefins, thermosets, PVC
and elastomers. DeBDE is also widely used in textile applications as
the flame retardant in latex-based back coatings (Pettigrew, 1993).
Hexabromocyclododecane (HBCD), a major brominated cycloaliphatic
flame retardant, is primarily used in polystyrene foam. It is also
used to flame-retard textiles.
2.2.2 Chlorinated flame retardants
Chlorine-containing flame retardants belong to three chemical
groups: aliphatic, cycloaliphatic and aromatic compounds. Chlorinated
paraffins are by far the most widely used aliphatic chlorine-
containing flame retardants. They have applications in plastics,
fabrics, paints and coatings (IPCS, 1996b).
Bis(hexachlorocyclopentadieno)cyclo-octane is a flame retardant
having unusually good thermal stability for a chlorinated
cycloaliphatic. In fact, this compound is comparable in thermal
stability to brominated aromatics in some applications. It is used in
several polymers, especially polyamides and polyolefins for wire and
cable applications. Its principal drawback is the relatively high use
levels required, compared to some brominated flame retardants
(Pettigrew, 1993).
Aromatic chlorinated flame retardants are not used for flame-
retarding polymers.
2.3 Organophosphorus flame retardants
One of the principal classes of flame retardants used in plastics
and textiles is that of phosphorus, phosphorus-nitrogen and
phosphorus-halogen compounds. Phosphate esters, with or without
halogen, are the predominant phosphorus-based flame retardants in use.
For textiles, phosphorus-containing materials are by far the most
important class of compounds used to impart durable flame resistance
to cellulose. These textile flame retardant finishes usually also
contain nitrogen or halogen, or sometimes both (Weil, 1993; Calamari &
Harper, 1993).
2.3.1 Non-halogenated compounds
Although many phosphorus derivatives have flame-retardant
properties, the number of those with commercial importance is limited.
Some are additive and some reactive. The major groups of additive
organophosphorus compounds are phosphate esters, polyols, phosphonium
derivatives and phosphonates. The phosphate esters include trialkyl
derivatives such as triethyl or trioctyl phosphate, triaryl
derivatives such as triphenyl phosphate and aryl-alkyl derivatives
such as 2-ethylhexyl-diphenyl phosphate.
The flame retardancy of cellulosic products can be improved
through the application of phosphonium salts. The flame-retardant
treatments attained by phosphorylation of cellulose in the presence of
a nitrogen compound are also of importance (Calamari & Harper, 1993).
Plasticizers are mixed into polymers to increase flexibility and
workability. The esters formed by reaction of the three functional
groups of phosphoric acid with alcohols or phenols are excellent
plasticizers. The phosphoric acid esters are also remarkable flame
retardants, and for this reason are extensively used in plastics
(Liepins & Pearce, 1976).
Aryl phosphate plasticizers are used in PVC-based products. They
are also used as lubricants for industrial air compressors and gas
turbines. Miscellaneous uses of aryl phosphates are as pigment
dispersants and peroxide carriers, and as additives in adhesives,
lacquer coatings and wood preservatives (Boethling & Cooper, 1985).
2.3.2 Halogenated phosphates
In addition to the above types, flame retardants containing both
chlorine and phosphorus or bromine and phosphorus are used widely.
Halogenated phosphorus flame retardants combine the flame-retardant
properties of both the halogen and the phosphorus groups. In
addition, the halogens reduce the vapour pressure and water solubility
of the flame retardant, thereby contributing to the retention of the
flame retardant in the polymer.
One of the largest selling members of this group, tris(1-chloro-
2-propyl) phosphate (TCPP) is used in polyurethane foam. Tris(2-
chloroethyl) phosphate is used in the manufacture of polyester resins,
polyacrylates, polyurethanes and cellulose derivatives.
The most widely used bromine- and phosphorus-containing flame
retardant used to be tris(2,3-dibromopropyl)phosphate, but it was
withdrawn from use in many countries due to carcinogenic properties in
animals (Liepins & Pearce, 1976; Green, 1992).
2.4 Nitrogen-based flame retardants
Nitrogen-based compounds can be employed in flame-retardant
systems or form part of intumescent flame-retardant formulations.
Nitrogen-based flame retardants are used primarily in nitrogen-
containing polymers such as polyurethanes and polyamides. They are
also utilized in PVC and polyolefins and in the formulation of
intumescent paint systems (Grabner, 1993).
Melamine, melamine cyanurate, other melamine salts and guanidine
compounds are currently the most used group of nitrogen-containing
flame retardants. Melamine is used as a flame retardant additive for
polypropylene and polyethylene. Melamine cyanurate is employed
commercially as a flame retardant for polyamides and terephthalates
(PET/PBT) and is being developed for use in epoxy and polyurethane
resins. Melamine phosphate is also used as a flame retardant for
terephthalates (PET/PBT) and is currently being developed for use in
epoxy and polyurethane flame retardant formulations. Also in the
development stages for use as flame-retardant additives are melamine
salts and melamine formaldehyde for their application in thermoset
resins (Grabner, 1993).
3. MECHANISM OF ACTION OF FLAME RETARDANTS
3.1 General aspects
To understand flame retardants, it is necessary to understand
fire. Fire is a gas-phase reaction. Thus, in order for a substance to
burn, it must become a gas. In the case of a candle the wax melts and
migrates up the wick by capillary action. The wax is pyrolysed to
volatile hydrocarbon fragments on the wick's surface at 600-800°C.
There is no oxygen at the nucleus of the flame. Some of the
hydrocarbon fragments aromatize to soot particles and, in the
luminescent region of the flame, react with water and carbon dioxide
to form carbon monoxide. Most of the pyrolysis gases are carried to
the exterior of the flame and encounter oxygen diffusing inwards. They
react exothermically to produce heat, which melts and decomposes more
wax, maintaining the combustion reaction. If there is adequate oxygen,
the combustion products from the candle are carbon dioxide and water
(Anderson & Christy, 1992).
Natural and synthetic polymers can ignite on exposure to heat.
Ignition occurs either spontaneously or results from an external
source such as a spark or flame. If the heat evolved by the flame is
sufficient to keep the decomposition rate of the polymer above that
required to maintain the evolved combustibles within the flammability
limits, then a self-sustaining combustion cycle will be established
(Fig. 1).
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
A GENERAL INTRODUCTION
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico
Dr P. Arias, Brussels, Belgium
Dr S.A. Assimon, Contaminants Branch, US Food and Drug Administration,
Washington, DC, USA
Dr H. Hofer, Toxicology, Austrian Research Centre, Seibersdorf,
Austria
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Stockholm, Sweden ( Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health, Ahmedabad,
India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany ( Vice-Chairman)
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Sœderlund, Folkehelsa, National Institute of Public Health,
Oslo, Norway ( Rapporteur)
Dr J. Troitzsch, Fire and Environment Protection Service, Wiesbaden,
Germany
Observers
Dr M.L. Hardy, Albermarle Corporation, Baton Rouge, USA
Mr T.A. Jay, Applications Laboratory, Great Lakes Chemical (Europe)
N.V., Geel, Belgium
Dr M. Papez, European Flame Retardants Association, Brussels, Belgium
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization ( Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS: A GENERAL
INTRODUCTION
A WHO Task Group on Environmental Health Criteria for Flame
Retardants met at the World Health Organization, Geneva, from 4 to 8
December 1995. Dr K.W. Jager, IPCS, welcomed the participants on
behalf of Dr M. Mercier, Director of the IPCS, and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and prepared conclusions and
recommendations.
The first draft of the monograph was prepared by Dr G.J. van
Esch, Bilthoven, the Netherlands. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ABS acrylonitrile-butadiene-styrene
APP ammonium polyphosphate
ATH alumina trihydrate
DeBDE decabromodiphenyl ether
EPDM ethylene propylene rubber
EPS expandable polystyrene
FR flame retardant
HBCD hexabromocyclododecane
HIPS high impact polystyrene
HDPE high density polyethylene
LDPE low density polyethylene
PA polyamides
PBDE polybrominated diphenyl ether
PBB polybrominated biphenyl
PBDD polybrominated dibenzodioxin
PBDF polybrominated dibenzofuran
PBT polybutylene terephthalate
PCDD polychlorinated dibenzodioxin
PCDF polychlorinated dibenzofuran
PE polyethylene
PET polyethylene terephthalate
PP polypropylene
PVC polyvinyl chloride
TBBPA tetrabromobisphenol A
TCDD 2,3,7,8-tetrachlorinated dibenzo- p-dioxin
TCPP tris(1-chloro-2-propyl)phosphate
PROLOGUE
The intent of this Environmental Health Criteria (EHC) monograph
is to provide a general overview of the nature, mechanism of action,
use and production volume of compounds used to improve the flame
retardancy of polymeric materials and textiles. The monograph also
indicates some of the known health and environmental hazards for
certain of the flame retardants that have been assessed by the IPCS.
A large number of compounds have been identified as being used as
flame retardants. Detailed international assessments of the risks
posed to human health and the environment by a number of these
substances have been published previously as EHC monographs. In Table
1 are listed the monographs on flame retardants and compounds related
to flame retardants that have already been published by IPCS or are in
preparation.
This monograph is intended to provide a starting point for those
interested in obtaining general information on flame-retardant
chemicals. More detailed information on use patterns, sources of
exposure, and health and environmental risks posed by these substances
can be found in the appropriate EHC monograph.
Table 1. EHC monographs on chemicals associated with
flame-retardant use
Substance EHC monograph
Mirex (1,1a,2,2,3,3a,4,5,5,5a,5b,6- EHC 44 (1984)
dodecachloroocta-hydro-1,3,4-metheno-1H-
cyclobuta( cd)pentalene)
Polychlorinated dibenzo- p-dioxins and EHC 88 (1989)
dibenzofurans
Tricresyl phosphate EHC 110 (1990)
Triphenyl phosphate EHC 111 (1991)
Hexachlorocyclopentadiene EHC 120 (1991)
Polychlorinated biphenyls EHC 140 (1992)
Polybrominated biphenyls EHC 152 (1994)
Brominated diphenyl ethers EHC 162 (1994)
Tetrabromobisphenol A and some of its EHC 172 (1995)
derivatives
Tris(2,3-dibromopropyl) phosphate and EHC 173 (1995)
bis(2,3-dibromopropyl) phosphate
Chlorendic acid and anhydride EHC 185 (1996)
Chlorinated paraffins EHC 181 (1996)
Polybrominated dibenzo- p-dioxins and EHC in preparation
dibenzofurans
Vinylbromide EHC in prepraration
Tris(chloropropyl) phosphates EHC in prepraration
Tris(2-butoxyethyl) phosphate EHC in prepraration
Tris(2-chloroethyl) phosphate EHC in prepraration
Tris(2-ethylhexyl) phosphate EHC in prepraration
Tetrakis(hydroxymethyl) phosphonium salts EHC in prepraration
1. INTRODUCTION
In today's society, there is an unprecedented development in the
size and number of buildings, skyscrapers, warehouses and methods of
transport. Carpeting, furnishings, equipment, oil and gas for heating
all increase the fire load in a building. New technologies, new
processes and new applications introduce new fire hazards (e.g., new
ignition sources such as welding sparks and short circuits)
(Troitzsch, 1990). Modern fire-fighting techniques, equipment and
building design have reduced the destruction due to fires. However,
a high fuel load in either a residential or a commercial building can
offset even the best of building construction (Gann, 1993).
Each year, over 3 million fires leading to 29 000 injuries and
4500 deaths are reported in the USA. The direct property losses
exceed $8 billion and the total annual cost has been estimated at over
$100 billion. Personal losses occur mostly in residences where
furniture, wall coverings and clothes are frequently the fuel. Large
financial losses occur in commercial structures such as office
buildings and warehouses. Fires also occur in aeroplanes, buses and
trains (Gann, 1993).
To provide additional protection from fires and to increase
escape time when a fire occurs, methods to enhance the flame
retardance of consumer goods have been developed. Flame retardants
are chemicals added to polymeric materials, both natural and
synthetic, to enhance flame-retardance properties. Flame-retardant
chemicals are most often used to improve the fire performance of low-
to-moderate cost commodity polymers. These flame retardants may be
physically blended with or chemically bonded to the host polymer.
They generally either lower ignition susceptibility or lower flame
spread once ignition has occurred. Some polymers are inherently less
flammable due to more stable polymeric structures; these are usually
higher priced engineering plastics such as polyimides,
polybenzimidazoles and polyetherketones (Gann, 1993).
Flame-retardant systems for synthetic or organic polymers act in
five basic ways: (1) gas dilution; (2) thermal quenching;
(3) protective coating; (4) physical dilution; (5) chemical
interaction (Pettigrew, 1993); or through a combination of these
mechanisms.
1. Inert gas dilution involves using additives that produce large
volumes of non-combustible gases on decomposition. These gases
dilute the oxygen supply to the flame or dilute the fuel
concentration below the flammability limit. Metal hydroxides,
metal salts and some nitrogen compounds function in this way.
2. Thermal quenching is the result of endothermic decomposition of
the flame retardant. Metal hydroxides, metal salts and nitrogen
compounds act to decrease surface temperature and the rate of
burning.
3. Some flame retardants form a protective liquid or char barrier.
This limits the amount of polymer available to the flame front
and/or acts as an insulating layer to reduce the heat transfer
from the flame to the polymer. Phosphorus compounds and
intumescent systems based on melamine and other nitrogen
compounds are examples of this category.
4. Inert fillers (glass fibres and microspheres) and minerals (talc)
act as thermal sinks to increase the heat capacity of the polymer
or reduce its fuel content.
5. Halogens and some phosphorus flame retardants act by chemical
interaction. The flame retardant dissociates into radical
species that compete with chain-propagating steps in the
combustion process.
Chemicals that are used as flame retardants can be inorganic,
organic, mineral, halogen-containing or phosphorus-containing. The
term flame "retardant" represents a class of use and not a class of
chemical structure (Pettigrew, 1993).
Preventive flame protection, including the use of flame
retardants, has been practised since ancient times. Some examples of
early historical developments in flame retardants are shown in
Table 2.
Table 2. Early historical fire-retardant developmentsa
Development Date
Alum used to reduce the flammability of wood by the About 450 BC
Egyptians
The Romans used a mixture of alum and vinegar on About 200 BC
wood
Mixture of clay and gypsum used to reduce 1638
flammability of theatre curtains
Mixture of alum, ferrous sulfate and borax used 1735
on wood and textiles by Wyld in Britain
Alum used to reduce flammability of balloons 1783
Gay-Lussac reported a mixture of (NH4)3PO4, 1821
NH4Cl and borax to be effective on linen and hemp
Perkin described a flame-retardant treatment for 1912
cotton using a mixture of sodium stannate and
ammonium sulfate
a From: Hindersinn (1990)
The advent of synthetic polymers earlier this century was of
special significance, since the water-soluble inorganic salts used up
to that time were of little or no utility in these largely hydrophobic
materials. Modern developments were, therefore, concentrated on the
development of polymer-compatible flame retardants.
By the outbreak of the Second World War, flame-proof canvas
tentage for outdoor use by the military was produced with a treatment
of chlorinated paraffins and an insoluble metal oxide, mostly antimony
oxide as a glow inhibitor, together with a binder resin.
After the war, non-cellulosic thermoplastic polymers became more
and more important as the basic fibres used for flame-retardant
applications. A dramatic example of the superiority of the non-
cellulosic compounds is provided by the diminished use of cotton fibre
in children's sleepwear since the inception of new standards. In
1971, cotton supplied 78% of the fibres used to produce children's
sleepwear, whereas in 1973 it supplied less than 10% in the USA (US
EPA, 1976).
With the increasing use of thermoplastics and thermosets on a
large scale for applications in building, transportation, electrical
engineering and electronics, new flame-retardant systems were
developed. They mainly consist of inorganic and organic compounds
based on bromine, chlorine, phosphorus, nitrogen, boron, and metallic
oxides and hydroxides.
Today, these flame-retardant systems fulfill the multiple
flammability requirements developed for the above-mentioned
applications.
A glossary of terms concerning flammability and flame retardants
is given in Annex 1.
2. TYPES OF FLAME RETARDANTS
A distinction is made between reactive and additive flame
retardants. Reactive flame retardants are reactive components
chemically built into a polymer molecule. Additive flame retardants
are incorporated into the polymer either prior to, during or (most
frequently) following polymerization.
There are three main families of flame-retardant chemicals
(Troitzsch, 1990; Wolf & Kaul, 1992; Green, 1992; Touval, 1993;
Pettigrew, 1993; Weil, 1993).
1. The main inorganic flame retardants are aluminium trihydroxide,
magnesium hydroxide, ammonium polyphosphate and red phosphorus.
This group represents about 50% by volume of the worldwide flame
retardant production. Some of these chemicals are also used as
flame retardant synergists, of which antimony trioxide is the
most important (OECD, 1994).
2. Halogenated products are based primarily on chlorine and
bromine. This group represents about 25% by volume of the
worldwide production (OECD, 1994).
3. Organophosphorus products are primarily phosphate esters and
represent about 20% by volume of the worldwide production.
Products containing phosphorus, chlorine and/or bromine are also
important.
In addition, nitrogen-based flame retardants are used for a
limited number of polymers.
Annex II comprises lists of the different flame retardant
families commercially used at present (A) and those no more in
use (B).
2.1 Inorganic flame retardants
Metal hydroxides form the largest class of all flame retardants
used commercially today and are employed alone or in combination with
other flame retardants to achieve necessary improvements in flame
retardancy. Antimony compounds are used as synergistic co-additives
in combination with halogen compounds, facilitating the reduction in
total flame retardant levels needed to achieve a desired level of
flame retardancy. To a limited extent, compounds of other metals also
act as synergists with halogen compounds. They may be used alone but
are most commonly used with antimony trioxide to enhance other
characteristics, for example, smoke reduction or afterglow
suppression. Ionic compounds have a very long history as flame
retardants for wool- or cellulose-based products. Inorganic
phosphorus compounds are primarily used in polyamides and phenolic
resins, or as components in intumescent formulations.
2.1.1 Metal hydroxides
Metal hydroxides function in both the condensed and gas phases of
a fire by absorbing heat and decomposing to release their water of
hydration. This process cools both the polymer and the flame and
dilutes the flammable gas mixture. The very high concentrations (50
to 80%) required to impart flame retardancy often adversely affect the
mechanical properties of the polymer into which they are incorporated.
Aluminium hydroxide, also known as alumina trihydrate (ATH) is
the largest volume flame retardant in use today. It decomposes when
exposed to temperatures over 200°C, which limits the polymers in
which it can be incorporated. Magnesium hydroxide is stable to
temperatures above 300°C and can be processed into several polymers.
2.1.2 Antimony compounds
Antimony trioxide is not a flame retardant per se, but it is
used as a synergist. It is utilized in plastics, rubbers, textiles,
paper and paints, typically 2-10% by weight, with organochlorine and
organobromine compounds to diminish the flammability of a wide range
of plastics and textiles (IARC, 1989).
Antimony oxides and antimonates must be converted to volatile
species. This is usually accomplished by release of halogen acids at
fire temperatures. The halogen acids react with the antimony-
containing materials to form antimony trihalide and/or antimony halide
oxide. These materials act both in the substrate (condensed phase)
and in the flame to suppress flame propagation. In the condensed
phase, they promote char formation, which acts as a physical barrier
to flame and inhibits the volatilization of flammable materials. In
the flame, the antimony halides and halide oxides, generated in
sufficient volume, provide an inert gas blanket over the substrate,
thus excluding oxygen and preventing flame spread. These compounds
alter the chemical reactions occurring at fire temperatures in the
flame, thus reducing the ease with which oxygen can combine with the
volatile products. It is also suggested that antimony oxychloride or
trichloride reduces the rate at which the halogen leaves the flame
zone, thus increasing the probability of reaction with the reactive
species. Antimony trichloride probably evolves heavy vapours which
form a layer over the condensed phase, stop oxygen attack and thus
choke the flame. It is also assumed that the liquid and solid
antimony trichloride particles contained in the gas phase reduce the
energy content of the flames by wall or surface effects (Troitzsch,
1990).
Other antimony compounds include antimony pentoxide, available
primarily as a stable colloid or as a redispersible powder. It is
designed primarily for highly specialized applications, although
manufacturers suggest it has potential use in fibre and fabric
treatment.
Sodium antimonate (Na2OSb2O5Ê´H2O) is recommended for
formulations in which deep tone colours are required or where antimony
trioxide may promote unwanted chemical reactions.
2.1.3 Boron compounds
Within the class of boron compounds, by far the most widely used
is boric acid. Boric acid (H3BO3) and sodium borate (borax)
(Na2B4O7. 10H2O) are the two flame retardants with the longest
history, and are used primarily with cellulosic material, e.g., cotton
and paper. Both products are effective, but their use is limited to
products for which non-durable flame retardancy is acceptable since
both are very water-soluble.
Zinc borate, however, is water-insoluble and is mostly used in
plastics and rubber products. It is used either as a complete or
partial replacement for antimony oxide in PVC, nylon, polyolefin,
epoxy, EPDM, etc. In most systems, it displays synergism with
antimony oxide. Zinc borate can function as a flame retardant, smoke
suppressant and anti-arcing agent in condensed phase. Recently, zinc
borate has also been used in halogen-free, fire-retardant polymers.
2.1.4 Other metal compounds
Molybdenum compounds have been used as flame retardants in
cellulosic materials for many years and more recently with other
polymers, mainly as smoke suppressants (see section 3.4) (Troitzsch,
1990). They appear to function as condensed-phase flame retardants
(Avento & Touval, 1980). Titanium and zirconium compounds are used
for textiles, especially wool (Calamari & Harper, 1993).
Zinc compounds, such as zinc stannate and zinc hydroxy-stannate,
are also used as synergists and as partial replacements for antimony
trioxide.
2.1.5 Phosphorus compounds
Red phosphorus and ammonium polyphosphate (APP) are used in
various plastics.
Red phosphorus was first investigated in polyurethane foams and
found to be very effective as a flame retardant. It is now used
particularly for polyamides and phenolic applications. The flame-
retarding effect is due, in all probability, to the oxidation of
elemental phosphorus during the combustion process to phosphoric acid
or phosphorus pentoxide. The latter acts by the formation of a
carbonaceous layer in the condensed phase. The formation of fragments
that act by interrupting the radical chain mechanism is also likely.
Ammonium polyphosphate is mainly applied in intumescent coatings
and paints. Intumescent systems puff up to produce foams. Because of
this characteristic they are used to protect materials such as wood
and plastics that are combustible and those like steel that lose their
strength when exposed to high temperatures. Intumescent agents have
been available commercially for many years and are used mainly as
fire-protective coatings. They are now used as flame-retardant
systems for plastics by incorporating the intumescent components in
the polymer matrix, mainly polyolefins, particularly polypropylene
(Troitzsch, 1990).
2.1.6 Other inorganic flame retardants
Other inorganic flame retardants, including ammonium sulfamate
(NH4SONH2) and ammonium bromide (NH4Br), are used primarily with
cellulose-based products and in forest fire-fighting (Weil, 1993).
2.2 Halogenated organic flame retardants
Halogenated flame retardants can be divided into three classes:
aromatic, aliphatic and cycloaliphatic. Bromine and chlorine
compounds are the only halogen compounds having commercial
significance as flame-retardant chemicals. Fluorine compounds are
expensive and, except in special cases, are ineffective because the
C-F bond is too strong. Iodine compounds, although effective, are
expensive and too unstable to be useful (Cullis, 1987; Pettigrew,
1993). The brominated flame retardants are much more numerous than
the chlorinated types because of their higher efficacy (Cullis, 1987).
With repect to processability, halogenated flame retardants vary
in their thermal stability. In general, aromatic brominated flame
retardants are more thermally stable than chlorinated aliphatics,
which are more thermally stable than brominated aliphatics.
Brominated aromatic compounds can be used in thermoplastics at fairly
high temperatures without the use of stabilizers and at very high
temperatures with stabilizers. The thermal stability of the
chlorinated and brominated aliphatics is such that, with few
exceptions, they must be used with thermal stabilizers, such as a tin
compound.
Halogenated flame retardants are either added to or reacted with
the base polymer. Additive flame retardants are those that do not
react in the application designated. There are a few compounds that
can be used as an additive in one application and as a reactive in
another; tetrabromobisphenol A is the most notable example. Reactive
flame retardants become a part of the polymer either by becoming a
part of the backbone or by grafting onto the backbone. The choice of
a reactive flame retardant is more complex than the choice of an
additive type. The development of systems based on reactive flame
retardants is more expensive for the manufacturer, who in effect has
to develop novel co-polymers with the desired chemical, physical and
mechanical properties, as well as the appropriate degree of flame
retardance (Cullis, 1987; Pettigrew, 1993). Synergists such as
antimony oxides are frequently used with halogenated flame retardants.
2.2.1 Brominated flame retardants
Bromine-based flame retardants are highly brominated organic
compounds with a relative molecular mass ranging from 200 to that of
large molecule polymers. They usually contain 50 to 85% (by weight)
of bromine (Cullis, 1987).
The highest volume brominated flame retardant in use today is
tetrabromobisphenol A (TBBPA) (IPCS, 1995a) followed by
decabromodiphenyl ether (DeBDE) (IPCS, 1994b). Both of these flame
retardants are aromatic compounds. The primary use of TBBPA is as a
reactive intermediate in the production of flame-retarded epoxy resins
used in printed circuit boards (IPCS, 1995a). A secondary use for
TBBPA is as an additive flame retardant in ABS systems. DeBDE is the
second largest volume brominated flame retardant and is the largest
volume brominated flame retardant used solely as an additive. The
greatest use (by volume) of DeBDE is in high-impact polystyrene, which
is primarily used to produce television cabinets. Secondary uses
include ABS, engineering thermoplastics, polyolefins, thermosets, PVC
and elastomers. DeBDE is also widely used in textile applications as
the flame retardant in latex-based back coatings (Pettigrew, 1993).
Hexabromocyclododecane (HBCD), a major brominated cycloaliphatic
flame retardant, is primarily used in polystyrene foam. It is also
used to flame-retard textiles.
2.2.2 Chlorinated flame retardants
Chlorine-containing flame retardants belong to three chemical
groups: aliphatic, cycloaliphatic and aromatic compounds. Chlorinated
paraffins are by far the most widely used aliphatic chlorine-
containing flame retardants. They have applications in plastics,
fabrics, paints and coatings (IPCS, 1996b).
Bis(hexachlorocyclopentadieno)cyclo-octane is a flame retardant
having unusually good thermal stability for a chlorinated
cycloaliphatic. In fact, this compound is comparable in thermal
stability to brominated aromatics in some applications. It is used in
several polymers, especially polyamides and polyolefins for wire and
cable applications. Its principal drawback is the relatively high use
levels required, compared to some brominated flame retardants
(Pettigrew, 1993).
Aromatic chlorinated flame retardants are not used for flame-
retarding polymers.
2.3 Organophosphorus flame retardants
One of the principal classes of flame retardants used in plastics
and textiles is that of phosphorus, phosphorus-nitrogen and
phosphorus-halogen compounds. Phosphate esters, with or without
halogen, are the predominant phosphorus-based flame retardants in use.
For textiles, phosphorus-containing materials are by far the most
important class of compounds used to impart durable flame resistance
to cellulose. These textile flame retardant finishes usually also
contain nitrogen or halogen, or sometimes both (Weil, 1993; Calamari &
Harper, 1993).
2.3.1 Non-halogenated compounds
Although many phosphorus derivatives have flame-retardant
properties, the number of those with commercial importance is limited.
Some are additive and some reactive. The major groups of additive
organophosphorus compounds are phosphate esters, polyols, phosphonium
derivatives and phosphonates. The phosphate esters include trialkyl
derivatives such as triethyl or trioctyl phosphate, triaryl
derivatives such as triphenyl phosphate and aryl-alkyl derivatives
such as 2-ethylhexyl-diphenyl phosphate.
The flame retardancy of cellulosic products can be improved
through the application of phosphonium salts. The flame-retardant
treatments attained by phosphorylation of cellulose in the presence of
a nitrogen compound are also of importance (Calamari & Harper, 1993).
Plasticizers are mixed into polymers to increase flexibility and
workability. The esters formed by reaction of the three functional
groups of phosphoric acid with alcohols or phenols are excellent
plasticizers. The phosphoric acid esters are also remarkable flame
retardants, and for this reason are extensively used in plastics
(Liepins & Pearce, 1976).
Aryl phosphate plasticizers are used in PVC-based products. They
are also used as lubricants for industrial air compressors and gas
turbines. Miscellaneous uses of aryl phosphates are as pigment
dispersants and peroxide carriers, and as additives in adhesives,
lacquer coatings and wood preservatives (Boethling & Cooper, 1985).
2.3.2 Halogenated phosphates
In addition to the above types, flame retardants containing both
chlorine and phosphorus or bromine and phosphorus are used widely.
Halogenated phosphorus flame retardants combine the flame-retardant
properties of both the halogen and the phosphorus groups. In
addition, the halogens reduce the vapour pressure and water solubility
of the flame retardant, thereby contributing to the retention of the
flame retardant in the polymer.
One of the largest selling members of this group, tris(1-chloro-
2-propyl) phosphate (TCPP) is used in polyurethane foam. Tris(2-
chloroethyl) phosphate is used in the manufacture of polyester resins,
polyacrylates, polyurethanes and cellulose derivatives.
The most widely used bromine- and phosphorus-containing flame
retardant used to be tris(2,3-dibromopropyl)phosphate, but it was
withdrawn from use in many countries due to carcinogenic properties in
animals (Liepins & Pearce, 1976; Green, 1992).
2.4 Nitrogen-based flame retardants
Nitrogen-based compounds can be employed in flame-retardant
systems or form part of intumescent flame-retardant formulations.
Nitrogen-based flame retardants are used primarily in nitrogen-
containing polymers such as polyurethanes and polyamides. They are
also utilized in PVC and polyolefins and in the formulation of
intumescent paint systems (Grabner, 1993).
Melamine, melamine cyanurate, other melamine salts and guanidine
compounds are currently the most used group of nitrogen-containing
flame retardants. Melamine is used as a flame retardant additive for
polypropylene and polyethylene. Melamine cyanurate is employed
commercially as a flame retardant for polyamides and terephthalates
(PET/PBT) and is being developed for use in epoxy and polyurethane
resins. Melamine phosphate is also used as a flame retardant for
terephthalates (PET/PBT) and is currently being developed for use in
epoxy and polyurethane flame retardant formulations. Also in the
development stages for use as flame-retardant additives are melamine
salts and melamine formaldehyde for their application in thermoset
resins (Grabner, 1993).
3. MECHANISM OF ACTION OF FLAME RETARDANTS
3.1 General aspects
To understand flame retardants, it is necessary to understand
fire. Fire is a gas-phase reaction. Thus, in order for a substance to
burn, it must become a gas. In the case of a candle the wax melts and
migrates up the wick by capillary action. The wax is pyrolysed to
volatile hydrocarbon fragments on the wick's surface at 600-800°C.
There is no oxygen at the nucleus of the flame. Some of the
hydrocarbon fragments aromatize to soot particles and, in the
luminescent region of the flame, react with water and carbon dioxide
to form carbon monoxide. Most of the pyrolysis gases are carried to
the exterior of the flame and encounter oxygen diffusing inwards. They
react exothermically to produce heat, which melts and decomposes more
wax, maintaining the combustion reaction. If there is adequate oxygen,
the combustion products from the candle are carbon dioxide and water
(Anderson & Christy, 1992).
Natural and synthetic polymers can ignite on exposure to heat.
Ignition occurs either spontaneously or results from an external
source such as a spark or flame. If the heat evolved by the flame is
sufficient to keep the decomposition rate of the polymer above that
required to maintain the evolved combustibles within the flammability
limits, then a self-sustaining combustion cycle will be established
(Fig. 1).
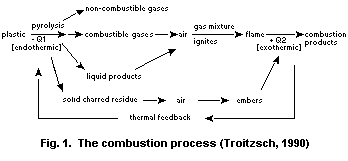 This self-sustaining combustion cycle occurs across both the gas
and condensed phases. Fire retardants act to break this cycle by
affecting chemical and/or physical processes occurring in one or both
of the phases. There are a number of ways in which the self-sustaining
combustion cycle can be interrupted. Whatever the method used, the
end effect is to reduce the rate of heat transfer to the polymer and
thus remove the fuel supply. Troitzsch (1990) described the general
physical and chemical mechanisms of flame-retardant action, in both
the gas and condensed phases and the behaviour of flame retardants.
Fundamentally, four processes are involved in polymer
flammability: preheating, decomposition, ignition and combustion/
propagation. Preheating involves heating of the material by means of
an external source, which raises the temperature of the material at a
rate dependent upon the thermal intensity of the ignition source, the
thermal conductivity of the material, the specific heat of the
material, and the latent heat of fusion and vaporization of the
material. When sufficiently heated, the material begins to degrade,
i.e., it loses its original properties as the weakest bonds begin to
break. Gaseous combustion products are formed, the rate being
dependent upon such factors as intensity of external heat, temperature
required for decomposition, and rate of decomposition. The
concentration of flammable gases increases until it reaches a level
that allows sustained oxidation in the presence of the ignition
source. The ignition characteristics of the gas and the availability
of oxygen are two important variables in any ignition process. After
ignition and removal of the ignition source, combustion becomes self-
propagating if sufficient heat is generated and is radiated back to
the material to continue the decomposition process. The combustion
process is governed by such variables as rate of heat generation, rate
of heat transfer to the surface, surface area, and rates of
decomposition. Flame retardancy, therefore, can be achieved by
eliminating (or improved by retarding) any one of these variables. A
flame retardant should inhibit or even suppress the combustion
process. Depending on their nature, flame retardants can act
chemically and/or physically in the solid, liquid or gas phase. They
interfere with combustion during a particular stage of this process,
i.e. during heating, decomposition, ignition or flame spread
(Troitzsch, 1990).
3.1.1 Physical action
There are several ways in which the combustion process can be
retarded by physical action (Troitzsch, 1990).
(a) By cooling. Endothermic processes triggered by additives cool
the substrate to a temperature below that required to sustain the
combustion process.
(b) By formation of a protective layer (coating). The condensed
combustible layer can be shielded from the gaseous phase with a solid
or gaseous protective layer. The condensed phase is thus cooled,
smaller quantities of pyrolysis gases are evolved, the oxygen
necessary for the combustion process is excluded and heat transfer is
impeded.
(c) By dilution. The incorporation of inert substances (e.g.,
fillers) and additives that evolve inert gases on decomposition
dilutes the fuel in the solid and gaseous phases so that the lower
ignition limit of the gas mixture is not exceeded.
3.1.2 Chemical action
The most significant chemical reactions interfering with the
combustion process take place in the solid and gas phases (Troitzsch,
1990).
(a) Reaction in the gas phase. The free radical mechanism of the
combustion process which takes place in the gas phase is interrupted
by the flame retardant. The exothermic processes are thus stopped,
the system cools down, and the supply of flammable gases is reduced
and eventually completely suppressed.
(b) Reaction in the solid phase. Here two types of reaction can
take place. Firstly, breakdown of the polymer can be accelerated by
the flame retardant, causing pronounced flow of the polymer and,
hence, its withdrawal from the sphere of influence of the flame, which
breaks away. Secondly, the flame retardant can cause a layer of
carbon to form on the polymer surface. This can occur, for example,
through the dehydrating action of the flame retardant generating
double bonds in the polymer. These form the carbonaceous layer by
cyclizing and cross-linking.
Flame retardancy is improved by flame retardants that cause the
formation of a surface film of low thermal conductivity and/or high
reflectivity, which reduces the rate of heating. It is also improved
by flame retardants that might serve as a heat sink by being
preferentially decomposed at low temperature. Finally, it is improved
by flame retardant coatings that, upon exposure to heat, intumesce
into a foamed surface layer with low thermal conductivity properties.
A flame retardant can promote transformation of a plastic into char
and thus limit production of combustible carbon-containing gases.
Simultaneously, the char will decrease thermal conductivity of the
surface. Flame retardants can also chemically alter the decomposition
products, resulting in a lower concentration of combustible gases.
Reduced fuel will result in less heat generation by the flame and may
lead to self-extinction.
Structural modification of the plastic, or use of an additive
flame retardant, might induce decomposition or melting upon exposure
to a heat source so that the material shrinks or drips away from the
heat source. It is also possible to significantly retard the
decomposition process through selection of chemically stable
structural components or structural modifications of a polymer.
In general, anything that will prevent the formation of a
combustible mixture of gases will prevent ignition. However, we may
also distinguish those cases in which the flame retardant or the
modified polymer unit, upon exposure to a heat source, will form gas
mixtures that will react chemically in the gas phase to inhibit
ignition. The goal of flame retardance in the combustion and
propagation stages is to decrease the rate of heat generated or
radiated back to the substrate. Any or all of the above-mentioned
mechanisms could function to prevent a self-sustaining flame (Pearce &
Liepins, 1975).
Flame retardancy occurs both as already stated in the vapour
phase (by interfering with oxidation through removal of free radicals)
and in the condensed phase (charring or altering thermal degradation
processes). Phosphorus acts primarily in the condensed phase by
promoting charring, presumably through the formation of phosphoric
acid and a decreased release of flammable volatiles. However, some
reports indicate that certain organic phosphorus compounds may also
work in the gas phase by scavenging free radicals. Antimony (which
functions only in the presence of a halogen) is believed to work
similarly to phosphorus in the condensed phase and combine with
halogens in the gas phase to scavenge free radicals (HÊ and OHÊ) that
are necessary for combustion. The role of nitrogen is not completely
understood. Nitrogen is known to impart flame retardancy in
combination with phosphorus and also by itself, as in polyamides and
aminoplasts. Bromine and chlorine act in the gas phase by reacting
with free radicals (Ulsamer et al., 1980).
The mechanism for imparting durable flame retardance to cellulose
is that of increasing the quantity of carbon, or char, formed instead
of volatile products of combustion, and flammable tars. Salts that
dissociate to form acids or bases upon heating are usually effective
flame retardants. Salts of strong acids and weak bases are the most
effective compounds. Ammonium and amine salts are generally
effective, as are Lewis acids and bases, either by themselves or when
formed in combustion.
3.2 Condensed phase mechanisms
The role of phosphorus compounds has been extensively studied. In
both cellulose and thermoplastics, phosphorus salts of volatile metals
and most organophosphorus compounds are known to be effective flame
retardants. The formation of char appears to be the key. For example,
although triphenyl phosphate, triphenyl phosphite and triphenyl
phosphine are all equivalent on a phosphorus basis, the more effective
flame retardant compounds act by forming phosphoric acid, which
changes the course of the decomposition of cellulose to form carbon
and water (US EPA, 1976).
The flame-retardant action of phosphorus compounds in cellulose
is believed to proceed by way of initial phosphorylation of the
cellulose, probably by initially formed phosphoric or polyphosphoric
acid. The phosphorylated cellulose then breaks down to water,
phosphoric acid and an unsaturated cellulose analogue, and eventually
to char by repetition of these steps. Certain nitrogen compounds such
as melamines, guanidines, ureas and other amides appear to catalyse
the steps forming cellulose phosphate and are found to enhance or
synergize the flame-retardant action of phosphorus on cellulose.
In polyethylene terephthalate and polymethyl methacrylate the
mechanism of action of phosphorus-based flame retardants has been
shown to involve both a similar decrease in the amount of combustible
volatiles and a similar increase in the amount of residues (aromatic
residues and char). The char formed also acts as a physical barrier to
heat and gases. In rigid polyurethane foams the action of phosphorus
flame retardants also appears to involve char enhancement. In flexible
foam the mechanism is less well understood (Weil,1993).
3.3 Gas-phase mechanisms
In addition to the condensed-phase mechanism, phosphorus flame
retardants can exert gas-phase flame-retardant action. It has been
demonstrated that trimethyl phosphate retards the velocity of a
methane-oxygen flame with about the same molar efficiency as antimony
trioxide (Weil, 1993). The mechanisms of action can differ depending
on the type of compound used as a flame retardant. The mechanism
affects the generation of products of combustion, some of which are
potentially corrosive and toxic.
One mechanism of improving the flame retardancy of thermoplastic
materials is to lower their melting point. This results in the
formation of free radical inhibitors in the flame front and causes the
material to recede from the flame without burning.
Free radical inhibition involves the reduction of gaseous fuels
generated by burning materials. Heating of combustible materials
results in the generation of hydrogen, oxygen, and hydroxide and
peroxide radicals that are subsequently oxidized with flame. Certain
flame retardants act to trap these radicals and thereby prevent their
oxidation. Bromine is more effective than chlorine. For example:
RBr + HÊ -> HBr + RÊ
If the resulting compound R is less readily oxidized than the radical
that is removed, the result is reduced flammability.
Measurements of the limiting oxygen index of polymers show that,
in contrast to the situation with chlorine, the effect of bromine does
depend on the gaseous oxidant involved. This suggests that bromine
compounds act to some extent by interfering with the flame reactions
and it is generally believed that this is probably their principal
mode of action, although they can also affect the condensed-phase
decomposition of the polymer.
Any gas-phase mechanism of flame retardancy by bromine compounds
must by definition involve the release of volatile bromine-containing
species, which then inhibit the flame reactions. In the case of
brominated flame retardants, it is generally assumed that hydrogen
bromide is liberated and reacts with the free radicals responsible for
the propagation of combustion, replacing them by the relatively
unreactive bromine atom.
HÊ + Hbr -> H2 + BrÊ
OHÊ + Hbr -> H2O + BrÊ
The mechanism operating in a particular polymer system will
depend on the mode and ease of breakdown of the brominated flame
retardant present. Some of these compounds are thermally stable and
volatilize when the associated polymer is heated to sufficiently high
temperatures. Others decompose to give substantial amounts of either
lower molecular weight organic bromine compounds or hydrogen bromide
(Cullis, 1987).
The presence of chemically bound bromine can also affect the
rates and modes of thermal decomposition of organic polymers in the
condensed phase. Brominated flame retardants vary considerably in
both their volatility and thermal stability. Although some very
stable compounds volatilize chemically unchanged, others break down
within the polymer or react directly with it in the condensed phase.
Hydrogen bromide is often a product and can significantly influence
the rate and course of polymer decomposition, although its effect is
small in comparison with those which it exerts on polymer combustion
as a whole. However, even thermally stable brominated flame
retardants can affect the decomposition of polymers in the condensed
phase, causing the original polymer breakdown stage to be replaced by
two separate stages, both of which involve polymer and additive. Thus,
it is clear that hydrogen bromide is not the only bromine-containing
compound which affects condensed-phase polymer decomposition and that
organic bromine compounds can also markedly change the rate and mode
of breakdown of organic polymers (Cullis, 1987).
A critical factor governing the effectiveness of brominated flame
retardants and indeed their mechanism of action is their thermal
stability relative to that of the polymers with which they are
associated. The most favourable situation for a flame retardant to be
effective will be one in which its decomposition temperature lies
50°C or so below that of the polymer. In general, decomposition at
this temperature with the liberation of substantial quantities of
hydrogen bromide or elemental bromine is likely to enhance flame-
retardant activity. Owing to the relatively low C-Br bond energy,
bromine compounds generally breakdown at quite low temperatures
(typically 200-300°C). Temperatures in this range overlap well with
the decomposition of many common polymers. This is probably a factor
determining the superior flame-retardant effectiveness of bromine
compounds compared with that of chlorine compounds (Cullis, 1987).
3.4 Co-additives for use with flame retardants
Brominated flame retardants are in some cases used on their own,
but their effectiveness is increased by a variety of co-additives, so
that in practice they are more often used in conjunction with other
compounds or with other elements incorporated into them. Thus, for
example, the addition of small quantities of organic peroxides to
polystyrene greatly reduces the amount of hexabromocyclododecane
needed to give a flame-retardant foam; other free radical initiators
behave in a similar fashion. These compounds appear to act by
promoting depolymerization of the hot polymer, giving a more fluid
melt. More heat is therefore required to keep the polymer alight,
because there is a greater tendency for the more molten material to
drip away from the neighbourhood of the flame (Cullis, 1987;
Troitzsch, 1990).
The flame-retardant properties of bromine compounds, like those
of chlorine compounds, will be considerably enhanced when they are
used in conjunction with other hetero-elements, notably phosphorus,
antimony and certain other metals.
The simultaneous presence of phosphorus in bromine-containing
polymer systems usually serves to improve their degree of flame
retardance, although, contrary to general opinion, bromine and
phosphorus generally exert effects that are largely additive rather
than synergistic.
Sometimes the two elements are present in the same molecule,
e.g., tris(2,3,-dibromopropyl)phosphate. In other systems, however,
it is more convenient to use mixtures of a bromine compound and a
phosphorus compound so that the ratio of the two elements can be
readily adjusted. It has already been pointed out that brominated
flame retardants on their own act predominantly in the gas phase. In
contrast, phosphorus compounds act mainly in the condensed phase,
especially with oxygen-containing polymers. It is therefore of
interest to discover whether, when both elements are present together,
each continues to act in the usual way or new mechanisms come into
operation. However, the evidence here is somewhat conflicting. Studies
of the effects of phosphate esters, with or without bromine present,
on the combustion of polyesters show that more char is formed when the
flame retardant contains bromine, and that most of this bromine
remains in the char. This suggests that the bromine-phosphorus
compound affects primarily the condensed-phase processes. However,
studies of the flammability of rigid polyurethane foams show that the
inhibiting effect of tris(2,3-dibromopropyl)- phosphate on combustion
depends on the nature of the gaseous oxidant, suggesting that the
flame retardant acts here, at least in part, by interfering with
reactions in the gas phase. With hydrocarbon polymers, such as
polyolefins and polystyrene, the major part of the phosphorus present
volatilizes and acts in the gas phase, being apparently converted to
simple species, such as phosphorus and phosphorus oxide free radicals.
These species can then interfere chemically with the reactions
responsible for flame propagation by catalysing the recombination of
the active free radicals involved. In such cases, any bromine present
simultaneously is presumably converted to species such as Brœ and HBr,
which function in the gas phase in the usual way (Cullis, 1987).
Antimony is a much more effective co-additive than phosphorus,
generally in the form of its oxide, Sb2O3. On its own this compound
has no flame-retardant activity and is therefore almost always used in
conjunction with a halogen compound. In general, bromine-antimony
mixtures are more effective than the corresponding chlorine-antimony
systems. The use of antimony trioxide greatly reduces the high levels
normally needed for effective flame retardance of bromine compounds on
their own. The principal mode of action is in the gas phase. If
bromine and antimony are present simultaneously in a burning organic
polymer, the major part of the antimony is volatilized, probably as
SbBr3 or SbOBr. These compounds then provide a ready source of
hydrogen bromide and they also produce in the middle of the combustion
zone a mist of fine particles of solid SbO, which can catalyse the
recombination of the free radicals responsible for flame propagation,
via the formation of transient species like SbOH. A number of other
metal oxides have been investigated as partial or total replacements
for antimony trioxide. Their use, however, has a number of
disadvantages. The most important point is that volatilization of the
bromine occurs at the right stage of the combustion cycle. With zinc
oxide, volatilization takes place too early and the bromine has
disappeared from the system before it can become effective (Cullis,
1987).
It can be concluded that in many, if not most, polymer systems in
which bromine and phosphorus are both present, the two elements tend
to act independently and therefore additively. The important mode of
action of metal oxides as co-additives for brominated flame retardants
is to catalyse the breakdown of the bromine compound and therefore the
release of volatile bromine compounds into the gas phase. However,
metal-bromine compounds may also be formed, and these may have more
specific modes of action in inhibiting polymer combustion (Cullis,
1987).
3.5 Smoke suppressants
Smoke production is determined by numerous parameters. No
comprehensive theory yet exists to describe the formation and
constitution of smoke.
Smoke suppressants rarely act by influencing just one of the
parameters determining smoke generation. Ferrocene, for example, is
effective in suppressing smoke by oxidizing soot in the gas phase as
well as by pronounced charring of the substrate in the condensed
phase. Intumescent systems also contribute to smoke suppression
through creation of a protective char. It is extremely difficult to
divide these multifunctional effects into primary and subsidiary
actions since they are so closely interwoven. At present no uniform
theory on the mode of action of smoke suppressants has been
established (Troitzsch, 1990).
3.5.1 Condensed phase
Smoke suppressants can act physically or chemically in the
condensed phase. Additives can act physically in a similar fashion to
flame retardants, i.e., by coating (glassy coatings, intumescent
foams) or dilution (addition of inert fillers), thus limiting the
formation of pyrolysis products and hence of smoke. Chalk (CaCO3),
frequently used as a filler, acts in some cases not only physically as
a dilutent but also chemically (in PVC, for example) by absorbing
hydrogen chloride or by effecting cross-linking so that the smoke
density is reduced in various ways. The processes contributing to
smoke suppression can be extremely complex.
Smoke can be suppressed by the formation of a charred layer on
the surface of the substrate, e.g., by the use of organic phosphates
in unsaturated polyester resins. In halogen-containing polymers, such
as PVC, iron compounds, e.g., iron (III) chloride, cause charring by
the formation of strong Lewis acids.
Certain compounds such as ferrocene cause condensed-phase
oxidation reactions that are visible as a glow. There is pronounced
evolution of CO and CO2, so that less aromatic precursors are given
off in the gas phase.
Compounds such as MoO3 can reduce the formation of benzene
during the thermal degradation of PVC, probably via chemisorption
reactions in the condensed phase. Relatively stable benzene-MoO3
complexes that suppress smoke development are formed (Troitzsch,
1990).
3.5.2 Gas phase
Smoke suppressants can also act chemically and physically in the
gas phase. The physical effect takes place mainly by shielding the
substrate with heavy gases against thermal attack. They also dilute
the smoke gases and reduce smoke density. In principle, two ways of
suppressing smoke chemically in the gas phase exist: the elimination
of either the soot precursors or the soot itself. Removal of soot
precursors occurs by oxidation of the aromatic species with the help
of transition metal complexes. Soot can also be destroyed oxidatively
by high-energy OH radicals formed by the catalytic action of metal
oxides or hydroxides. Smoke suppression can also be achieved by
eliminating the ionized nuclei necessary for forming soot with the aid
of metal oxides. Finally soot particles can be made to flocculate by
certain transition metal oxides (Troitzsch, 1990).
4. PERFORMANCE CRITERIA FOR AND CHOICE OF FLAME RETARDANTS
At present, the selection of a suitable flame retardant depends
on a variety of factors that severely limit the number of acceptable
materials.
Many countries require extensive information on human and
environmental health effects for new substances before they are
allowed to be put on the market. For existing chemicals such data are
not always available but several national and international programmes
are in the process of gathering this information.
For most chemicals, including flame retardants, the following
information regarding human and environmental health is essential to
understanding a chemical's potential hazards:
1. Data from adequate acute and repeated dose toxicity studies is
needed to understand potential health effects.
2. Data on biodegradability and bioaccumulation potential is needed
as a first step in understanding a chemical's environmental
behaviour and effects.
3. Information on the chemical's possible breakdown and/or
combustion products may also be needed.
4. Since flame retardants are often processed into polymers at
elevated temperatures, consideration of the stability of the
material at the temperature inherent to the polymer processing is
needed, as well as on whether or not the material volatilizes at
that temperature or during use.
5. Consideration should be given to the need for information on the
possible formation of toxic and/or persistent breakdown products
during accidental fires or incineration.
Successfully achieving the desired improvement in flame
retardancy is a necessary precursor to other performance
considerations. The basic flammability characteristics of the polymer
to be used play a major role in the flame-retardant selection process,
as some polymers burn much more readily than others.
Flame-retardant selection is also affected by the test method to
be used to assess flame retardancy. Some tests can be passed with
relatively low levels of many flame retardants, while high levels of
very powerful flame retardants are needed to pass other tests. It is
not possible to provide a comprehensive review in this monograph, but
a short introduction is given in Annex III.
There are many performance issues other than flame retardancy
that must be considered during the selection of a flame retardant for
any use. Just as in applications not needing improved flame
retardancy, a long list of processing and performance requirements
must be met before a material can be accepted for use. The
development of a polymer formulation that meets all of these
requirements involves finding the optimum combination of polymer(s),
flame retardant(s), synergist(s), stabilizer(s), processing aid(s),
and all other additives. This is complex and difficult work requiring
a great deal of time, effort and expense.
Flame retardants may adversely affect the processing
characteristics of polymers. Changes occurring in the viscosity of
liquid systems or in the flow of polymers that are melted during
processing can cause major problems. Significant alteration of the
rate of reaction of thermoset polymers or the speed and degree of
crystallization of thermoplastic polymers may result from the use of
some flame retardants. The temperatures routinely used to process
many polymers severely restrict the number of flame retardants
suitable for incorporation.
Since flame retardants are frequently used at high levels, they
often have a dramatic effect on the basic mechanical properties of
polymers in which they are used. Reduction of strength (tensile,
compression), rigidity, toughness and/or heat resistance are common
problems.
When flame retardants are added to polymers their appearance
(colour, gloss, transparency) and physical properties (density,
hardness, melting and glass transition temperatures, thermal
expansion) often change significantly. Electrical properties
(resistance, dielectric, tracking) are frequently altered, and aging
due to factors such as oxidation, UV radiation, high temperature may
be reduced.
The chemical properties of a flame retardant are often of great
importance in its selection. Resistance to exposure to water,
solvents, acids, bases, oils or other substances may be a requirement
for use. Issues related to solubility, hydrolysis resistance or
reactivity with other formulation components may prevent the use of an
otherwise desirable flame retardant.
The relationship between cost and performance is an essential
consideration in the selection of a flame retardant.
All of the above-mentioned issues also apply to textiles. In
addition, the durability (resistance to cleaning with water or by
other techniques) of the flame retardant system is critical (Jay,
1990).
5. PRODUCTION AND USES OF FLAME RETARDANTS AND FLAME-RETARDED
POLYMERS
5.1 Production
The worldwide demand for flame-retardant chemicals in 1992 was
estimated to be 600 000 tonnes (OECD, 1994). This includes over a
hundred different products, which can be classified according to base
chemical content as depicted in Table 3.
Table 3. Demand for flame retardants according to base chemical
content (from: OECD, 1994)
Base chemicals Demand
(tonnes)
Bromine 150 000
Chlorine 60 000
Phosphorus 100 000
Antimony 50 000
Nitrogen 30 000
Aluminium 170 000
Others 50 000
It is difficult to obtain an accurate picture of market volumes
of flame retardants as reports from different sources appear to
conflict.
Table 4 shows USA market volume trends between 1986 and 1991.
Table 5 presents the annual consumption of different flame
retardants in Japan over the period 1986 - 1994. A comparable table of
global use was not available. Table 5 indicates that the consumption
of brominated flame retardants and antimony oxide in Japan has more
than doubled over this period, compared to the moderate increase in
other flame retardants. The market for hydrated aluminium as a flame
retardant seems to have decreased in Japan, whereas Table 4 (Gann,
1993) shows that an increase occurred in the USA.
Chlorinated paraffins had an estimated world production of
300 000 tonnes/year in 1985 (IPCS, 1996b).
Table 4. Flame retardant market volume (from: Gann, 1993)
Group 1986 1991
(tonnes) (tonnes)
Phosphate esters 20 000 18 000
Halogenated phosphates 13 000 16 000
Chlorinated hydrocarbons 15 000 15 000
Brominated hydrocarbons 28 000 36 000
Brominated bisphenol A 16 000 18 000
Antimony trioxide 22 000 25 000
Borates 8 000 8 000
Aluminium trihydrate 140 000 170 000
Magnesium hydroxide
2 000 3 000
Total
264 000 301 000
5.2 Uses
The consumption of flame retardants in plastics and other
combustible materials is closely linked to regulations covering fire
precautions. The principle regulations relate to the building,
transportation, electrical engineering, furnishing and mining sectors
(Troitzsch, 1990).
A worldwide estimate of the consumption of flame retardants
according to materials is not available but the figures for Europe
listed in the Table 6 should reflect the market in general.
Table 5. Trends in the annual consumption of flame retardants in Japana
Type Compound Amount (tonnes)
1986 1990 1994
Brominated
Tetrabromobisphenol A (TBBPA) 12 000 23 000 24 000
Decabromobiphenyl ether 3 000 10 000 5 500
Octabromobiphenyl ether 600 1 100 500
Tetrabromobiphenyl ether 1 000 1 000 0
Hexabromocyclododecane 600 700 1 600
Bis(tetrabromophthalimido) ethane - 1 000 2 500
Tribromophenol 100 450 3 500
Bis(tribromophenoxy) ethane 400 400 900
TBBPA polycarbonate oligomer - - 2 500
Brominated polystyrene - - 1 300
TBBPA epoxy oligomer - 3 000 7 000
Others 2 400 - 2 150
Subtotal 20 000 40 650 51 450
Chlorinated
Chlorinated paraffins 4 000 4 500 4 300
Others 850 700 900
Subtotal 4 850 5 200 5 200
Phosphoric
Halogenated ester 3 000 3 000 3 100
Non-halogenated ester 4 000 4 400 4 400
Others 1 750 1 750 3 310
Subtotal 8 750 9 150 10 810
Table 5. (contd.)
Type Compound Amount (tonnes)
1986 1990 1994
Inorganic
Antimony oxide 8 300 16 000 17 000
Hydrated aluminium 48 000 37 000 42 000
Others 7 200 8 400 9 000
Subtotal 63 500 61 400 68 000
TOTAL 97 100 116 400 135 460
a Based on the investigation made by Kagaku Kogyo Nippon Co. Ltd. (Japan).
(Personal communication from Isao Watanabe, Osaka Prefectural Institute
of Public Health, Japan).
Table 6. Estimated consumption of flame retardants in western
Europe for 1985 and 1992 according to materials
(from: Sutker, 1988)
Product group Consumption (103 tonnes)
1985 1992
Polystyrene 4.0-4.5 4.5-5.0
ABS 1.0-1.5 1.2-1.8
Polyesters 7.5-8.0 8.5-9.0
Epoxy resins 3.5-4.0 4.0-4.5
Polyolefins 10.0-12.0 11.0-13.0
Polyvinyl chloride) 25.0-27.0 27.0-29.0
Polyurethanes 12.0-13.5 13.5-15.0
Engineering plastics 1.5-1.8 1.7-2.0
Paper and textiles 9.0-10.0 10.0-11.0
Rubber and elastomers 5.0-6.0 6.0-7.0
Other 11.5-11.7 12.6-12.7
Total 90.0-100.0 100.0-110.0
5.2.1 Plastics
The plastics industry is the largest consumer of flame
retardants, estimated at about 95% for the USA in 1991. About 10% of
all plastics contain flame retardants (Wolf & Kaul, 1992). The main
applications are in building materials and furnishings (structural
elements, roofing films, pipes, foamed plastics for insulation,
furniture and wall and floor coverings), transportation (equipment and
fittings for aircraft, ships, automobiles and railroad cars), and in
the electrical industry (cable housings and components for television
sets, office machines, household appliances and lamination of printed
circuits).
The growth in the flame retardant market reflects the enormous
expansion of the plastics industry in recent decades. Between 1988 and
1994, there was a worldwide increase of 20%. Although the USA,
western Europe and Japan are still the largest plastic producers (30,
24 and 12% of the market, respectively), other countries showed the
largest increases between 1988 and 1994, e.g., South Korea (170%);
China (60%); Taiwan (54%) (Anon, 1995).
Examples of flame retardants used in various plastics (Wolf &
Kaul, 1992) are as follows:
PVC: Chlorinated paraffins or phosphate esters, antimony trioxide,
aluminium hydroxide
Acrylonitrile-butadiene-styrene (ABS): Octabromodiphenyl ether,
antimony trioxide
Expandable polystyrene: Hexabromocyclododecane
High-Impact polystyrene (HIPS): Decabromodiphenyl ether or
tetrabromobisphenol A, antimony trioxide
Linear polyester: Brominated organics
Polypropylene: Tetrabromobisphenol A, bis(2,3-dibromopropyl ether),
antimony trioxide
Low-density polyethylene (LDPE) films: chlorinated paraffins,
antimony trioxide
High-density polyethylene (HDPE) and cross-linked polyethylene:
Brominated aromatics
Polyurethane foams: Organophosphates, brominated organic compounds,
alumina trihydrate
Polyamides: Brominated aromatic compounds, chlorinated
cycloaliphatic compounds, antimony trioxide, red phosphorus, melamine
Polycarbonates: Tetrabromobisphenol A, brominated organic oligomers,
sulfonate salts
Unsaturated polyesters: Chlorinated and brominated organic
compounds, antimony trioxide, alumina trihydrate
Epoxy resins: Tetrabromobisphenol A
5.2.2 Textile/furnishing industry
In contrast to the plastics industry, the textile industry is a
much smaller market for flame retardants. However, rather than
employing just one flame retardant, the use of a combination of
chemicals is usually necessary for textiles.
Phosphorus-containing materials are the most important class of
compounds to impart durable flame resistance to cellulose (Calamari &
Harper, 1993). Flame-retardant finishes containing phosphorus
compounds usually also contain nitrogen or bromine, or sometimes both.
Another system is based on halogens (usually bromine) in conjunction
with nitrogen or antimony.
Flame retardants used in furniture/textiles include the following
(Anon, 1992; Calamari & Harper, 1993; EFRA, 1995):
* organic phosphates such as tri-alkyl or tri-aryl phosphates, tri-
chloroalkyl phosphates, dialkyl phosphites,
tetrakis(hydroxymethyl)phosphonium chloride (THPC) and related
structures;
* halogenated compounds such as polybrominated diphenyl ethers
(found in over 50% of treated furniture) and chlorinated
paraffins (rainproof applications);
* inorganic compounds such as antimony trioxide, ammonium bromide,
boric acid and aluminium hydrate.
Details on the flame-retardant types were reported by Calamari &
Harper (1993) and can be found in Annex II.
6. FORMATION OF TOXIC PRODUCTS ON HEATING OR COMBUSTION OF
FLAME-RETARDED PRODUCTS
Natural or synthetic material that burns produces potentially
toxic products. There has been considerable debate on whether
addition of organic flame retardants results in the generation of a
smoke that is more toxic and may result in adverse health effects on
those exposed. There has been concern in particular about the
emission of polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) during manufacture, use and combustion of
brominated flame retardants.
6.1 Toxic products in general
Combustion of any organic chemical may generate carbon monoxide
(CO), which is a highly toxic non-irritating gas, and a variety of
other potentially toxic chemicals. Some of the major toxic products
that can be produced by pyrolysis of flame retardants are: CO, CO2,
HCl, POX, ammonia vapour, bromofurans, HBr, HCN, NOX and phosphoric
acid (Anon, 1992).
In general the acute toxicity of fire atmospheres is determined
mainly by the amount of CO, the source of which is the amount of
generally available flammable material. Most fire victims die in post
flash-over fires where the emission of CO is maximized and the
emission of HCN and other gases is less. The acute toxic potency of
smoke from most materials is lower than that of CO (Hirschler, 1995;
Nelson, 1995).
Flame retardants significantly decrease the burning rate of the
product, reducing heat yields and quantities of toxic gas. In most
cases, smoke was not significantly different in room fire tests
between flame-retarded and non-flame-retarded products (Babrauskas
et al. 1988).
Morikawa et al. (1995) reported toxicity studies on gases from
full-scale room fires involving fire retardant materials (a variety,
but not specified). HCN and CO were the two major toxicants. There was
no evidence that the smoke from flame-retarded materials was more
toxic to rabbits than the smoke from non-flame-retarded materials.
Regarding brominated flame retardants, Cullis (1987) stated that
unless suitable metal oxides or metal carbonates are also present,
virtually all the bromine is eventually converted to gaseous hydrogen
bromide (HBr). This is a corrosive and powerful sensory irritant. In a
fire situation however, it is always carbon monoxide (CO) or hydrogen
cyanide (HCN), rather than an irritant which causes rapid
incapacitation. Owing to its high reactivity, hydrogen bromide is
unlikely to reach dangerously high concentrations (Cullis, 1987).
6.2 Formation of halogenated dibenzofurans and dibenzodioxins
PBDFs and PBDDs can be formed from polybrominated diphenyl ethers
(PBDEs), polybrominated phenols, polybrominated biphenyls (PBBs) and
other brominated flame retardants under various laboratory conditions,
including heating. Because chlorinated derivatives are preferably
formed during pyrolysis, mixed halogen compounds will be predominantly
produced if a chlorine source is also available (Buser, 1987a,b).
As in the case of PCDD/PCDF, it is the 2,3,7,8-substituted
isomers that are toxic.
6.3 Exposure to PBDD/PBDF from polymers containing halogenated
flame retardants
6.3.1 Exposure due to contact or emission from products
containing halogenated flame retardants
Exposure of the general public to PBDD/PBDF impurities in flame-
retardant polymers is unlikely to be of significance. The possible
exposure to PBDD/PBDF from TV sets and computer monitors flame-
retarded with halogenated flame retardants has been discussed in
Environmental Health Criteria 162: Brominated diphenyl ethers and is
unlikely to be of significance (IPCS, 1994b).
6.3.2 Workplace exposure studies
Several studies have been performed to determine whether
PBDD/PBDF is present in the fumes emitted during thermal processes,
such as the extrusion of resins containing halogenated flame
retardants under normal processing conditions at temperatures in the
range of 200 to 250°C (IPCS 1994b, 1995a, in preparation).
Epidemiological studies of workers engaged in processing polymers
with PBDEs have been reported (IPCS, 1994b). Results of PBDD/PBDF
workplace monitoring during polymer processing have also been reported
(IPCS 1994b, 1995a). PBDD/PBDF personnel and room air levels during
processing of PBDEs were < 2 ng/m3 (TCDD equivalent) with the
exception of two samples at the extruder head (128 ng/m3, TCDD
equivalent) (IPCS, 1994b). Engineering controls were successful in
reducing these levels. Workplace control measures should also include
appropriate industrial hygiene measures and monitoring of exposure
(IPCS, 1994b, 1995a).
6.3.3 Formation of PBDD/PBDF from combustion
6.3.3.1 Laboratory pyrolysis experiments
In the late 1980s many pyrolysis experiments (at temperatures of
400-900°C) on brominated flame retardants and flame-retardant systems
were performed and the breakdown products measured. Flame retardants
or intermediates tested included PBBs, PBDEs, 2,4,6-tribromophenol,
pentabromophenol, tetrabromobisphenol A and tetrabromophthalic
anhydride (IPCS 1994b, 1995a, in preparation). Pyrolysis of the flame
retardants alone, as well as with polymer mixtures, was investigated.
As different laboratories carried out the experiments using a variety
of testing methods and conditions, a direct comparison of the many
experiments is not possible. Details of the pyrolysis experiments
involving PBDEs, tetrabromobisphenol A and derivatives, and PBBs are
given in the respective EHC monographs (IPCS 1994b, 1995a, in
preparation).
Although they indicate which flame retardants are likely to form
PBDF (and to a lesser extent PBDD) pyrolysis experiments are not
generally comparable to actual fire situations.
6.3.3.2 Fire tests and fire accidents
Fire tests on televisions have shown smoke and combustion
residues containing high levels of PBDD/PBDF. However, levels from
actual fire accidents involving televisions revealed much lower levels
than those produced under fire test conditions (IPCS, 1994b). Further
studies are discussed in the EHC monograph on PBDD/PBDFs (IPCS, in
preparation).
7. OVERVIEW OF EXPOSURE AND HAZARDS TO HUMANS AND THE ENVIRONMENT
Since flame retardants are a heterogeneous group of diverse
chemicals (see chapter 2 and Annex II), the information presented in
this section only provides a general overview of possible routes of
exposure to chemicals associated with flame-retardant use. This
section also provides a brief summary of the hazards to human health
and to the environment posed by chemicals connected with flame-
retardant use. For detailed information on the extent of exposure and
health and environmental effects of individual substances, the
appropriate specific EHC monographs should be consulted.
The toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles has discussed
in a report by the European Economic Community (Anon, 1992).
7.1 Human exposure
7.1.1 General population
Potential sources of exposure include consumer products,
manufacturing and disposal facilities, and environmental media.
Factors affecting exposure of the general population include the
physical and chemical properties of the product, the extent of
manufacturing and emission controls, the use made of the product
(surface coating, durability of fabric finishes, incorporation into a
polymer, etc.), the end use, and the method of disposal. Potential
routes of exposure for the general population include the dermal route
(contact with flame-retarded textiles), inhalation and ingestion.
7.1.2 Occupational exposure
Occupational exposure may occur during the manufacture,
transport, processing and disposal/recycling of flame retardants.
Routes of exposure could include inhalation, dermal contact and
ingestion. Factors affecting the extent of exposure include industrial
hygiene practices, engineering controls, manufacturing processes and
the type of product. As with any other industrial chemical, workplace
monitoring and good industrial practice can delineate the extent of
any exposure.
7.2 Exposure of the environment
Environmental exposure may occur as a result of the manufacture,
transport, use or waste disposal of flame retardants. Routes of
environmental exposure can include water, air and soil. Factors
affecting exposure include the physical and chemical properties of the
product, emission controls, disposal/recycling methods, volume and
biodegradability/persistence. Environmental monitoring can determine
the extent of environmental exposure.
On the basis of the estimated demand for flame retardants (see
Table 3), more than 1 million tonnes of flame-retardant polymers are
produced each year.
Most flame-retarded products eventually become waste. Municipal
waste is generally disposed of via incineration or landfill.
Incineration of flame-retarded products can produce various toxic
compounds, including halogenated dioxins and furans. The formation of
such compounds and their subsequent release to the environment is a
function of the operating conditions of the incineration plant and the
plant's emission controls.
There is a possibility of flame retardants leaching from products
disposed of in landfills. However, potential risks arising from
landfill processes are also dependent on local management of the whole
landfill. The significance of any release of flame retardants from
disposal sites has yet to be determined.
Some products containing flame retardants, including some
plastics, have been identified as suitable for recycling (Lorenz &
Bahadir, 1993; Meyer et al., 1993).
7.3 Hazards to humans
The hazards to humans associated with some flame retardants have
been outlined in the relevant EHC monographs. For example, the use of
tris(2,3-dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate
was banned in 1977 by the US Consumer Product Safety Commission and in
several other developed countries for use in children's clothing
because of concerns that the chemical might be a human carcinogen and
because of the possibility of significant human exposure through
contact with treated fabrics (IPCS, 1995b). Delayed neurotoxicity
due to tri- ortho-cresyl phosphate (TOCP), one of the tricresyl
phosphate isomers, has been observed in humans (IPCS, 1990). Some
polybrominated biphenyl (PBB) congeners have been shown to produce
chronic toxicity and cancer in experimental animals. However, no
definitive human health effects, correlatable with exposure, were
found in a population in Michigan, USA, accidentally exposed to PBBs
(IPCS, 1994a).
7.4 Hazards to the environment
EHC monographs outline the hazards to the environment associated
with some flame retardants (see Table 1). Some PBB congeners are
persistent and bioaccumulative and may pose a threat especially to
higher levels of the food chain (IPCS, 1994a). Hexachloro-
cyclopentadiene is highly toxic to aquatic organisms. However,
information obtained under environmentally realistic conditions is
limited. The potential hazard to the general environment is expected
to be low (IPCS, 1991b). Low concentrations of triphenyl phosphate
have been detected in environmental samples. Triphenyl phosphate is
rapidly degraded in the environment. However, sediment-dwelling
organisms near production plants may have been exposed to
concentrations high enough to exert toxic effects (IPCS, 1991a).
Tricresyl phosphate is also degraded rapidly in the environment, and
subsequent environmental concentrations are therefore low. The acute
toxicity of tricresyl phosphate to aquatic organisms is low (IPCS,
1990).
Persistence of pentabromodiphenyl ether (PeBDE) and lower
brominated diphenylethers in the environment suggest that commercial
PeBDE should not be used (IPCS, 1994b).
Some flame retardants have come under intense environmental
scrutiny. US EPA has called for additional testing (US EPA, 1992).
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas close to release sources there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate- and long-chain chlorinated paraffin products (IPCS,
1996b).
8. REGULATIONS WITH RESPECT TO FLAME RETARDANTS
Several national regulatory bodies have implemented regulations
on specific substances associated with flame-retardant applications.
In the USA the Interagency Testing Committee (ITC), under the
auspices of the Toxic Substances Control Act (TSCA), makes
recommendations concerning the need for additional testing on
chemicals in the TSCA inventory, including flame retardants. Based on
the information published since 1978, the ITC has made initial testing
recommendations upon 128 brominated flame retardants (US TSCA, 1992;
Walker, 1994; Annex IV).
In the European Community, the use of tris(2,3-dibromopropyl)
phosphate (EC Directive 76/769/EEC) and tris(1-aziridinyl)phosphine
oxide (EC Directive 83/264/EEC) in textiles has been banned. In 1977,
the US Consumer Product Safety Commission banned the use of tris(2,3-
dibromopropyl)phosphate in children's clothing (IPCS, 1995b).
The European Community has also banned the use of PBBs in
textiles (EC Directive 83/264/EEC). Several countries have either
taken or proposed regulatory actions on PBBs, as outlined in Table 7.
Controls on the emissions of dioxins and furans from municipal
solid waste incinerators have been implemented in the United Kingdom
under the Environmental Protection Act (1990). In Germany, a second
modification of the Chemicals Prohibition Ordinance, which was adopted
in 1994, imposes limits on 2,3,7,8-substituted chlorinated dioxins and
furans and, for the first time, on some 2,3,7,8-substituted brominated
dioxins and furans (OECD, 1994).
Table 7. Country-specific actions on PBBs either taken or proposeda
Country Actions
Austria Prohibits the manufacture, placing on the market,
import and use of PBBs and products containing
these substances.
Canada Prohibits the manufacture, use, processing, offer
for sale, selling or importation of PBBs for
commercial, manufacturing or processing purposes.
Denmark Implements EC Directive 89/677 banning the use of
PBBs in textiles.
Finland PBB may not be used in textile articles intended
to come into contact with the skin (in accordance
with EC Directive 83/264).
France Implements EC Directive concerning PBBs and their
use on textiles.
Netherlands Proposed resolution would prohibit the storage of
PBBs or products or preparations containing these
substances or making them available to third
parties. (Exports are excluded from the
resolution).
Norway Ban on PBBs in textiles intended to come into
contact with skin, implementation of EC Directives
76/769/EEC, 83/264 and 89/677.
Sweden Ban on PBBs in textiles intended to come into
contact with skin by implementation of EC
Directive 76/769.
Switzerland Prohibits manufacture, supply, import and use of
PBBs and products containing these substances.
Supply and import of capacitors and transformers
containing PBBs is forbidden.
USA No current production or use. Companies intending
to resume manufacture must notify US EPA 90 days
in advance for approval.
a Adapted from: OECD (1994)
9. CONCLUSIONS AND RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
9.1 Conclusions
Flame retardants are a diverse group of compounds used to improve
the flame retardancy of polymers and other materials. A large variety
of compounds, from inorganic to complex organic molecules, are used as
flame retardants, synergists and smoke suppressants. This overview is
focused on organic compounds, which typically contain halogen and/or
phosphorus.
It is difficult to find accurate figures for the global use of
flame retardants but estimates indicate that more than 600 000 tonnes
are produced annually. Available data indicate a substantial increase
of brominated organic product consumption during the last decade.
There are obvious benefits in using flame retardants, as many
human lifes and property are saved from fire. At present, knowledge
of long-term effects resulting from exposure to flame retardants and
their breakdown products is limited. Most people that die in fires
are killed by carbon monoxide.
The majority of the organic flame retardants are either
covalently bound into polymer molecules (reactive) or mixed into the
polymer (additive). They can act in several ways, either physically
(by cooling, by formation of a protective layer or by dilution of the
matrix) or chemically (by reactions in either the gas or the solid
phase).
A number of factors govern the selection of the type of flame
retardant to be used in a specific application. Some of these are the
flammability of the matrix, processing and performance requirements,
chemical properties and possible hazards to human and environmental
health.
Exposure of the general population to flame retardants can occur
via inhalation, dermal contact and ingestion. Potential sources of
exposure are consumer products, manufacturing/disposal facilities and
environmental media (including food intake). The same routes are
possible for occupational exposure, mainly during production,
processing, transportation and disposal/recycling of the flame
retardants or the treated products. Occupational exposure to the
breakdown products may also occur during fire fighting. As several of
the compounds used are lipophilic and persistent, they may
bioaccumulate. Some of the compounds have been shown to cause organ
damage, genotoxic effects and cancer.
There is also concern for occupational health and environmental
effects from combustion/pyrolysis products, especially the
polyhalogenated dibenzofurans and dibenzo- p-dioxins, from some
organic flame retardants. Other breakdown products also need to be
taken into account.
The properties of a number of flame retardants make them
persistent and/or bioaccumulative, and they may therefore pose hazards
to the environment. Some of the compounds that have been evaluated so
far (polybrominated biphenyls, polybrominated diphenyl ethers and
chlorinated paraffins) have been found to belong to this group. Some
of these have therefore been recommended to not be used.
Several countries have developed regulations affecting the
production, use and disposal of flame retardants. Some include
restrictions on the use of compounds because of potential toxic
effects in humans. Germany has developed rules for the maximum
content of some 2,3,7,8-substituted polychlorinated dibenzo- para-
dioxins and dibenzofurans in products.
The availability of relevant data on flame retardants in the open
literature is limited, especially for some existing chemicals
produced before regulations for commercialization were strengthened
in several countries.
IPCS has issued evaluations for some flame retardants and is
preparing evaluations for others.
9.2 Recommendations for the protection of human health and the
environment
a) Information on the content and nature of flame retardants,
including impurities in products, should be made available to
national authorities.
b) More complete information on the volume of flame retardants
production and consumption should be made available.
c) In view of the increased recycling of flame-retarded products,
consideration could be given to harmonized labelling by an
international forum.
d) Compounds that present a toxic risk to humans and/or the
environment should not be used as flame retardants.
e) Occupational exposure to flame retardants and their breakdown
products should be minimized using appropriate engineering and
good industrial hygiene practices. The exposure of people
working in these operations should be monitored.
f) There is a need for proper assessment of occupational health and
environmental effects from combustion or pyrolysis products of
flame retardants.
g) Emissions to the environment from manufacturing, processing,
transportation and disposal/recycling of products containing
persistent bioaccumulative compounds should be minimized using
best available techniques. The environment in the vicinity of
such operations should be monitored for the compounds used.
h) The use of flame retardants with properties that make them
persistent and bioaccumulative should be avoided.
i) The levels of the major persistent bioaccumulating flame
retardants should be monitored routinely in environmental
matrices (biota and sediments). Some compounds that are no
longer produced should likewise be monitored, in order to
indicate the long-term influence of such products.
10. FURTHER RESEARCH
a) Further studies should be undertaken to elucidate the fate of
flame retardants in disposal/recycling operations.
b) There is a need for further evaluations of flame retardants.
Useful criteria for setting priorities are volume of use,
intrinsic toxic effects on human health and the environment,
exposure assessments, and persistence and bioaccumulation/
biomagnification of flame retardants or their breakdown products.
REFERENCES
Anderson GA & Christy MR (1992) Standards, bans and flame retardants.
Presented at Structural Plastics "92", 5-8 April 1992. Dallas, Texas,
Society of the Plastics Industry, Inc., Structural Plastics Division.
Anon (1992) Toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles - Report to the
European Commission, DG-III, Brussels.
Anon (1995) [Plastic industry of the world.] Kunststoffe, 85(10):
1761-1772 (in German).
Arias P (1992) Brominated diphenyloxides as flame retardants. Bromine
based chemicals. Paris, Organisation for Economic Co-operation and
Development (Unpublished document).
Avento JM & Touval I (1980) Antimony and other inorganic compounds.
In: Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
John Wiley and Sons, vol 10, pp 486-487.
Babrauksas V, Harris RH Jr, Gann RG, Levin BC, Lee BT, Peacock RD,
Paabo M, Twilley W, Yoklavich MF, & Clark HM (1988) Fire hazard
comparison of fire-retarded and non-fire-retarded products.
Washington, DC, US Department of Commerce, National Bureau of
Standards (NBS Special Publication No. 749).
BFR/CEM Working Group (1989) [Polybrominated dibenzodioxins and
dibenzofurans (PBDDs/PBDFs) from flame retardants containing bromine:
Assessment of risk and proposed measures - Report by the Brominated
Flame Retardants/CEM Working Group to the Conference of Environmental
Ministers Bonn, Germany, September 1989.] (Report No. III/4299/89) (in
German).
Boethling RS & Cooper JC (1985) Environmental fate and effects of
triaryl and tri-alkyl/aryl phosphate esters. Residue Rev, 94: 49-99.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants. Chemosphere,
16(8-9): 1873-1876.
Calamari TA & Harper RJ (1993) Flame retardants for textiles. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 998-1022.
Cullis CF (1987) Bromine compounds as flame retardants. In:
Proceedings of the International Conference on Fire Safety, vol 12, pp
307-323.
Dynamac Corporation (1982) An overview of flame retardants: Volumes I
and II (Contract No. 68-01-6239). Rockville, Maryland, Dynamac
Corporation, Environmental Control Division (Prepared for the US
Environmental Protection Agency).
EFRA (1995) European flame retardants and their application. Brussels,
European Flame Retardants Association.
Flick EW (1986) Plastics additives: An industrial guide - Section IX:
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Gann RG (1993) Flame retardants: Overview. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 930-936.
Grabner RS (1993) N-containing flame retardants: An alternative.
Handout at OECD Workshop on Brominated Flame Retardants, Neuchâtel,
Switzerland, 22-25 February 1993.
Green JC (1992) A review of phosphorus-containing flame retardants. J
Fire Sci, 10: 470-487.
Hindersinn RR (1990) Historical aspects of polymer fire retardance.
In: Nelson GL ed. Fire and polymers hazards identification and
prevention. New York, American Chemical Society (ACS Symposium Series
No. 415).
Hirschler MM (1995) Smoke toxicity: How important is it for fire
safety? Handout at the 6th Annual BCC Conference on Flame Retardancy:
Recent advances in flame retardancy of polymeric materials, Stamford,
Connecticut, May 1995, 16pp.
Hutzinger O, Sundström G, & Safe S (1976) Environmental chemistry of
flame retardants: Part I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1975) Some aziridines, N-, S- & O-mustards and selenium.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
18).
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
IARC (1986a) Some chemicals used in plastics and elastomers. Lyon,
International Agency for Research on Cancer (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 39).
IARC (1986b) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
IARC (1989) Some organic solvents, resin monomers and related
compounds, pigments and occupational exposures in paint manufacture
and painting. Lyon, International Agency for Research on Cancer, pp
291-305 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Volume 47).
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 48).
IPCS (1984) Environmental health criteria 44: Mirex. Geneva, World
Health Organization, International Programme on Chemical Safety,
70 pp.
IPCS (1989) Environmental health criteria 88: Polychlorinated dibenzo-
p-dioxins and dibenzofurans. Geneva, World Health Organization,
International Programme on Chemical Safety, 409 pp.
IPCS (1990) Environmental health criteria 110: Tricresyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 122 pp.
IPCS (1991) Environmental health criteria 111: Triphenyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 80 pp.
IPCS (1991) Environmental health criteria 120: Hexachloro-
cyclopentadiene. Geneva, World Health Organization, International
Programme on Chemical Safety, 126 pp.
IPCS (1992) Environmental health criteria 140: Polychlorinated
biphenyls and terphenyls, 2nd ed. Geneva, World Health Organization,
International Programme on Chemical Safety, 692 pp.
IPCS (1994a) Environmental health criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, International Programme
on Chemical Safety, 577 pp.
IPCS (1994b) Environmental health criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, International Programme on
Chemical Safety, 347 pp.
IPCS (1995a) Environmental health criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization, International
Programme on Chemical Safety, 139 pp.
IPCS (1995b) Environmental health criteria 173: Tris(2,3-
dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate. Geneva,
World Health Organization, International Programme on Chemical Safety,
129 pp.
IPCS (1996a) Environmental health criteria 185: Chlorendic acid and
anhydride. Geneva, World Health Organization, International Programme
on Chemical Safety.
IPCS (1996b) Environmental health criteria 181: Chlorinated paraffins.
Geneva, World Health Organization, International Programme on Chemical
Safety.
IPCS (in preparation) Environmental health criteria: Polybrominated
dibenzo-p-dioxins and dibenzofurans. Geneva, World Health
Organization, International Programme on Chemical Safety.
ISO/IEC Guide (1990) Glossary of fire terms and definitions, 1st ed.
Geneva, International Organization for Standardization.
Japan Fire Retardant Association (1988) Voluntary rules of the flame
retardancy and the toxicological (safety) examination for flame
retardant products under the guide-line of the Fire Defence Agency,
Fire Retardant Products Approval Commission. Tokyo, Japan Fire
Retardant Association.
Jay TA (1990) Flame retardants. Presentation to the USSR All-Union
Congress on Flame Retardants at Saki, Crimea, USSR, 10 October 1990.
Kirk-Othmer (1993) Flame retardants. In: Kirk-Othmer encyclopedia of
chemical technology, 4th ed. New York, John Wiley & Sons, vol 10, pp
930-1022.
Kopp A (1990) [Discussions on bromine-containing flame-retardants.]
Berlin, Germany, Federal Ministry for Environment, Nature Conservation
and Nuclear Safety (Document sent to the European Commission,
Brussels) (in German)
Liepins R & Pearce EM (1976) Chemistry and toxicity of flame
retardants for plastics. Environ Health Perspect, 17: 55-63.
Lorenz W & Bahadir M (1993) Recycling of flame retardants containing
printed circuits: a study of the possible formation of polyhalogenated
dibenzodioxins/-furans. Chemosphere, 26(12): 2221-2229.
Meyer H, Neupert M, Pump W, & Willenberg B (1993) [Flame retardants
determine reusability.] Kunststoffe, 83(4): 253-257 (in German).
Morikawa T, Okada T, Kajiwara M, Sato Y, & Tsuda Y (1995) Toxicity of
gases from full-scale room fires involving fire retardant contents. J
Fire Sci, 13(1): 23-42.
Nelson GL (1995) Carbon monoxide and fire toxicity. Handout at the 6th
Annual BCC Conference on Flame Retardancy: "Recent Advances in Flame
Retardancy of Polymeric Materials, Stamford, Connecticut, May 1995 -
16pp.
OECD (1994) Selected brominated flame retardants. Paris, Organisation
for Economic Co-operation and Development, Environment Directorate,
152 pp (Risk Reduction Monograph No. 3).
Pearce EM & Liepins R (1975) Flame retardants. Environ Health
Perspect, 11: 59-69.
Pettigrew A (1993) Halogenated flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 954-976.
Soderlung E & Dybing E (1982) [Flame-retardant toxicology: A
literature survey.] Oslo, National Institute for Public Health
(Unpublished report ) (in Norwegian).
Sutker BJ (1988) Flame retardants. In: Ullmann's encyclopedia of
industrial chemistry, 5th ed. Weinheim, Germany, VCH Verlag, vol A11,
pp 123-140.
Teuerstein A (1990) Brominated FRS, production and uses (Summary).
Data from Eurobrom BV. In: Workshop on Brominated Aromatic Flame
Retardants. Proceedings of a Workshop held at Skoloster, Sweden, 24-26
October 1989. Solna, Swedish National Chemicals Inspectorate, pp
49-52.
Touval I (1993) Antimony and other inorganic flame retardants. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 936-954.
Troitzsch JH (1990) International plastics flammability handbook:
Principles, regulations, testing and approval, 2nd ed. Münich,
Germany, Hanser Publishers.
Ullmann (1988) Ullmann's encyclopedia of industrial chemistry: Volumes
A11 et A26, 5th ed. Weinheim, Germany, VCH Verlag.
Ulsamer AG, Osterberg RE, & McLauglin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US EPA (1976) A study of flame retardants for textiles. Washington,
DC, US Environmental Protection Agency (EPA-560/1-76-001; NTIS
PB-251-44).
US EPA (1989) Twenty-fifth report of the Interagency Testing Committee
to the administrator. Fed Reg, 54(237): 51114-51130.
US EPA (1992) Aryl phosphate base stocks: Proposed test rule and
reporting requirements. Fed Reg, 57(12): 2136-2158.
Walker JD (1994) Testing decisions of the TSCA Interagency Testing
Committee for Brominated Flame Retardants: A review of decisions and
health and safety data. Presented at the Meeting of the Fire Retardant
Chemicals Association, Williamsburg, Virginia, 9-12 October 1994.
Washington, DC, US Environmental Protection Agency.
Weil ED (1993) Phosphorus flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley and
Sons, vol 10, pp 976-998.
Wolf R & Kaul BL (1992) Plastics, additives. In: Ullmann's
encyclopedia of industrial chemistry, ed. Weinheim, Gemany, VCH
Verlag, vol A20, pp 459-507.
ANNEX I
TERMINOLOGY
There is no clear definition for many of the terms used to
describe fire, flammability and flame retardants. As a result, some
confusion has been created by the interchangeable use of terms such as
fire retardant, flame retardant, flame-proof and fire-proof. The
meaning of these and other terms is often clear only in the context.
Therefore, many efforts have been undertaken, at an international
level, to harmonize definitions and terms related to fire, including
fire protection and flame retardants. The definitions compiled in
Table 8 have been taken from the ISO/IEC Guide (1990) and other
sources (e.g., Troitzsch, 1990).
Other terms, which mostly concern mostly textile flame
retardancy, are defined below (US EPA, 1976, Kirk-Othmer, 1993):
Fireproof textile
This term applies only to those fabrics that undergo virtually no
change when exposed to a flame.
Flame-retardant textile
The term flame-retardant textile refers to any fabric that will
not support combustion after the source of ignition is removed. It is
synonymous with the term fire-resistant textile. The textile is
expected to char or melt. The term covers all treatments short of
fire-proofing.
Durability
Durability refers to the ability of a flame-retardant textile to
withstand washing/cleaning, chlorine bleaching, weathering and sun
exposure. A durable treatment is any chemical process which imparts
flame-retardant properties to textiles and textile products that will
last for at least 50 launderings and dry cleaning for the life of the
fabric. A semi-durable treatment will resist water but not withstand
dry cleaning or more than 10 to 15 launderings. A non-durable
treatment is readily removed by water or perspiration and requires
replacement after each exposure of the textile to water. The
definition of durability must be related to the conditions of use of
the textile and the product.
Table 8. Definitions of terms connected with fire
Term Definition
Afterflame Persistence of flaming of a material after the
ignition source has been removed
Afterglow Persistence of glowing of a material after
cessation of flaming or, if no flaming occurs,
after the ignition source has been removed
Burn To undergo combustion
Burning All the physical and/or chemical changes that
behaviour take place when a material or product is exposed
to a specified ignition source
Char Carbonaceous residue resulting from pyrolysis or
incomplete combustion
Combustible Capable of burning
Combustion Exothermic reaction of a substance with an
oxidizer, generally accompanied by flames and/or
glowing and/or emission of smoke
Fire a) A process of combustion characterized by the
emission of heat accompanied by smoke and/or
flame
b) Rapid combustion spreading uncontrolled in
time and space
Fire All the physical and/or chemical changes
behaviour that take place when a material, product and/or
structure is exposed to an uncontrolled fire
Fire The total gaseous, particulate or
effluent aerosol effluent from combustion or pyrolysis
Fire The ability of an element of building
resistance construction to fulfil for a stated period of
time the required load-bearing function,
integrity and/or thermal insulation specified
in the standard fire-resistance test
(see ISO 834)
Flame Zone of combustion in the gaseous phase from
which light is emitted
Table 8. (contd.)
Term Definition
Flame The property of a material either
retardance inherent or by virtue of a substance added or a
treatment applied to suppress, significantly
reduce or delay the propagation of flame
Flame A substance added or a treatment applied
retardant to a material in order to suppress, significantly
reduce or delay the combustion of the material
Flame spread Propagation of a flame front
Flame spread Distance travelled by a flame front
rate during its propagation per unit time under
specified test conditions
Flammability Ability of a material or product to burn with a
flame under specified test conditions
Flammable Capable of burning with a flame under specified
test conditions
Flash over The rapid transition to a state of total surface
involvement in a fire of combustible materials
within an enclosure
Fully The state of total involvement of
developed fire combustible materials in a fire
Glowing Combustion of a material in the solid
combustion phase without flame but with emission of light
from the combustion zone
Heat release The calorific energy released per unit
rate time by a material during combustion under
specified test conditions
Ignition Minimum temperature of a material at
temperature which sustained combustion can be initiated under
specified test conditions
Melting Phenomena accompanying the softening of
behaviour a material under the influence of heat (including
shrinking, dripping, burning of molten material,
etc.)
Table 8. (contd.)
Term Definition
Pyrolysis Irreversible chemical decomposition of a material
due to an increase in temperature without
oxidation
Reaction The response of a material under
to fire specified test conditions in contributing by its
own decomposition to a fire to which it is exposed
Smoke A visible suspension of solid and/or liquid
particles in gases resulting from combustion or
pyrolysis
Smoke The reduction in luminous intensity due
obscuration to passage through smoke
Smouldering The slow combustion of a material without light
being visible and generally evidenced by an
increase in temperature and/or by smoke
Soot Finely divided particles, mainly carbon, produced
and/or deposited during the incomplete combustion
of organic materials
ANNEX II
Flame retardants in commercial use or used formerly
Introduction
Tables 9 and 10 have been compiled on the basis of all the
information on flame retardants available to the IPCS and from the
following sources:
Arias (1992) BFR/CEM Working Group (1989)
Boethling & Cooper (1985) Dynamac Corporation (1982)
EFRA (1995) Flick (1986)
Hutzinger et al. (1976) IARC (1975, 1978, 1979, 1986a,b,
1987, 1989, 1990)
IRPTC (1987) Japan Fire Retardant Association
(1988)
Kirk-Othmer (1993) Kopp (1990)
Liepins & Pearce (1976) Pearce & Liepins (1975)
Sœderlund & Dybing (1982) Teuerstein (1990)
Troitzsch (1990) Ulsamer et al. (1980)
Ullmann (1988) US EPA (1989)
US TSCA (1992)
More than 175 flame retardants, or groups of them are tabulated
in these two tables. On just 17 of them the database was adequate for
preparing a hazard and risk evaluation for man and the environment,
and Environmental Health Criteria (EHC) monographs have been, or are
being prepared, on these. For the others the hazard to man and the
environment has not been evaluated internationally. Most of these
substances also have other major uses. In addition, some chemicals
used as intermediates in the production of flame retardants have beeen
listed. It is likely that these lists are not exhaustive, and that
new chemical structures are being developed as flame retardants.
Table 9 lists flame retardants in commercial use today and some
intermediates, while Table 10 lists flame retardants that have been
used in the past.
The tables list the chemical name, the chemical structure, the
CAS registry number and the uses as flame retardants. The "Use" column
also contains information on the global production volume of those
compounds that are currently being produced commercially (estimated
for the IPCS Task Group by P. Arias, 1995). The international
evaluation status of the substances is indicated in the column
"Remarks".
The following abbreviations are used in this table:
EHC An Environmental Health Criteria monograph on the chemical
has been published. If no EHC number is given, the document
is still in preparation.
IARC The International Agency for Research on Cancer of WHO in
Lyon has evaluated the carcinogenicity.
H High production volume (> 5000 tons per year)
M Moderate production volume (1000-5000 tons per year)
L Low production volume (<1000 tons per year or in
the developmental stage)
Table 9. Flame retardants being used commercially today
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Inorganic flame retardants
Potassium fluorotitanate K2TiF6 16919-27-0 Wool
Potassium fluorozirconate K2ZrF6 16923-95-8 Wool
Aluminium hydroxide Al(OH)3 21645-51-2 Rubber compounds, PVC, polyolefins, thermosets H
Antimony pentoxide Sb2O5 1314-60-9 Additive type flame-retardant synergist M
Antimony trioxide Sb2O3 1309-64-4 Additive type flame-retardant synergist H IARC
(1989)
Zinc oxide ZnO 1314-13-2 Additive type flame-retardant synergist
polyamides, rubber M
Boric acid H3BO3 11113-50-1 Wool, cellulosic, textiles H
Sodium borate (borax) Na2B4O7.10H2O 1303-96-4 Flame retardant and synergist H
Zinc borate 3ZnO.2B2O3. 1332-07-6 Synergist and smoke supressant M
Ammonium sulfamate NH4SO3NH2 7773-06-0 Cellulosic and textiles
Ammonium orthophosphate (NH4)3PO4 10124-31-9 Cellulosic and textiles
Ammonium carbamate phosphate Textiles
Di-ammonium phosphate (NH4)2HP04 7783-28-0 Textiles
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ammonium polyphosphate 68333-79-9 Cellulosics and mastics, paints, polyolefins H
Huntite- Mg3Ca(CO3)4 - 19569-21-2 Thermoplastics, coatings H
hydromagnesite Mg5(CO3)4Ê(OH)2Ê4H2O 12411-64-2 Smoke supressant M
12125-28-9
Ammonium octamolybdate (NH4)4Mo8O26
Magnesium hydroxide Mg(OH)2 1309-42-8 Thermoplastics, thermosets, rubbers H
Ammonium bromide NH4 Br 12124-97-9 Cellulosics H
Barium metaborate BaB2O4ÊxH2O 14701-59-2 Flame retardant additive, synergist M
Molybdenum trioxide MoO3 1313-27-5 Smoke suppressant L
Ammonium sulfate (NH4)2 SO4 7783-20-2 Cellulosic textiles
Ammonium chloride NH4Cl 12125-02-9 Cellulosics
Zinc hydroxystannate ZnSn(OH)6 12027-96-2 Smoke suppressant and
flame-retardant synergist L
Red phosphorus P 7723-14-0 Polyamides, phenolics, engineering
thermoplastics M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Sodium tungstate Na2WO4.2H2O 13472-45-2 Textiles
Sodium antimonate NaSbO3 15432-85-6 Flame-retardant additive, synergist
Brominated flame retardants
Decabromobiphenyl 13654-09-6 ABS, polystyrene M EHC 152
IARC
(1986b)
This self-sustaining combustion cycle occurs across both the gas
and condensed phases. Fire retardants act to break this cycle by
affecting chemical and/or physical processes occurring in one or both
of the phases. There are a number of ways in which the self-sustaining
combustion cycle can be interrupted. Whatever the method used, the
end effect is to reduce the rate of heat transfer to the polymer and
thus remove the fuel supply. Troitzsch (1990) described the general
physical and chemical mechanisms of flame-retardant action, in both
the gas and condensed phases and the behaviour of flame retardants.
Fundamentally, four processes are involved in polymer
flammability: preheating, decomposition, ignition and combustion/
propagation. Preheating involves heating of the material by means of
an external source, which raises the temperature of the material at a
rate dependent upon the thermal intensity of the ignition source, the
thermal conductivity of the material, the specific heat of the
material, and the latent heat of fusion and vaporization of the
material. When sufficiently heated, the material begins to degrade,
i.e., it loses its original properties as the weakest bonds begin to
break. Gaseous combustion products are formed, the rate being
dependent upon such factors as intensity of external heat, temperature
required for decomposition, and rate of decomposition. The
concentration of flammable gases increases until it reaches a level
that allows sustained oxidation in the presence of the ignition
source. The ignition characteristics of the gas and the availability
of oxygen are two important variables in any ignition process. After
ignition and removal of the ignition source, combustion becomes self-
propagating if sufficient heat is generated and is radiated back to
the material to continue the decomposition process. The combustion
process is governed by such variables as rate of heat generation, rate
of heat transfer to the surface, surface area, and rates of
decomposition. Flame retardancy, therefore, can be achieved by
eliminating (or improved by retarding) any one of these variables. A
flame retardant should inhibit or even suppress the combustion
process. Depending on their nature, flame retardants can act
chemically and/or physically in the solid, liquid or gas phase. They
interfere with combustion during a particular stage of this process,
i.e. during heating, decomposition, ignition or flame spread
(Troitzsch, 1990).
3.1.1 Physical action
There are several ways in which the combustion process can be
retarded by physical action (Troitzsch, 1990).
(a) By cooling. Endothermic processes triggered by additives cool
the substrate to a temperature below that required to sustain the
combustion process.
(b) By formation of a protective layer (coating). The condensed
combustible layer can be shielded from the gaseous phase with a solid
or gaseous protective layer. The condensed phase is thus cooled,
smaller quantities of pyrolysis gases are evolved, the oxygen
necessary for the combustion process is excluded and heat transfer is
impeded.
(c) By dilution. The incorporation of inert substances (e.g.,
fillers) and additives that evolve inert gases on decomposition
dilutes the fuel in the solid and gaseous phases so that the lower
ignition limit of the gas mixture is not exceeded.
3.1.2 Chemical action
The most significant chemical reactions interfering with the
combustion process take place in the solid and gas phases (Troitzsch,
1990).
(a) Reaction in the gas phase. The free radical mechanism of the
combustion process which takes place in the gas phase is interrupted
by the flame retardant. The exothermic processes are thus stopped,
the system cools down, and the supply of flammable gases is reduced
and eventually completely suppressed.
(b) Reaction in the solid phase. Here two types of reaction can
take place. Firstly, breakdown of the polymer can be accelerated by
the flame retardant, causing pronounced flow of the polymer and,
hence, its withdrawal from the sphere of influence of the flame, which
breaks away. Secondly, the flame retardant can cause a layer of
carbon to form on the polymer surface. This can occur, for example,
through the dehydrating action of the flame retardant generating
double bonds in the polymer. These form the carbonaceous layer by
cyclizing and cross-linking.
Flame retardancy is improved by flame retardants that cause the
formation of a surface film of low thermal conductivity and/or high
reflectivity, which reduces the rate of heating. It is also improved
by flame retardants that might serve as a heat sink by being
preferentially decomposed at low temperature. Finally, it is improved
by flame retardant coatings that, upon exposure to heat, intumesce
into a foamed surface layer with low thermal conductivity properties.
A flame retardant can promote transformation of a plastic into char
and thus limit production of combustible carbon-containing gases.
Simultaneously, the char will decrease thermal conductivity of the
surface. Flame retardants can also chemically alter the decomposition
products, resulting in a lower concentration of combustible gases.
Reduced fuel will result in less heat generation by the flame and may
lead to self-extinction.
Structural modification of the plastic, or use of an additive
flame retardant, might induce decomposition or melting upon exposure
to a heat source so that the material shrinks or drips away from the
heat source. It is also possible to significantly retard the
decomposition process through selection of chemically stable
structural components or structural modifications of a polymer.
In general, anything that will prevent the formation of a
combustible mixture of gases will prevent ignition. However, we may
also distinguish those cases in which the flame retardant or the
modified polymer unit, upon exposure to a heat source, will form gas
mixtures that will react chemically in the gas phase to inhibit
ignition. The goal of flame retardance in the combustion and
propagation stages is to decrease the rate of heat generated or
radiated back to the substrate. Any or all of the above-mentioned
mechanisms could function to prevent a self-sustaining flame (Pearce &
Liepins, 1975).
Flame retardancy occurs both as already stated in the vapour
phase (by interfering with oxidation through removal of free radicals)
and in the condensed phase (charring or altering thermal degradation
processes). Phosphorus acts primarily in the condensed phase by
promoting charring, presumably through the formation of phosphoric
acid and a decreased release of flammable volatiles. However, some
reports indicate that certain organic phosphorus compounds may also
work in the gas phase by scavenging free radicals. Antimony (which
functions only in the presence of a halogen) is believed to work
similarly to phosphorus in the condensed phase and combine with
halogens in the gas phase to scavenge free radicals (HÊ and OHÊ) that
are necessary for combustion. The role of nitrogen is not completely
understood. Nitrogen is known to impart flame retardancy in
combination with phosphorus and also by itself, as in polyamides and
aminoplasts. Bromine and chlorine act in the gas phase by reacting
with free radicals (Ulsamer et al., 1980).
The mechanism for imparting durable flame retardance to cellulose
is that of increasing the quantity of carbon, or char, formed instead
of volatile products of combustion, and flammable tars. Salts that
dissociate to form acids or bases upon heating are usually effective
flame retardants. Salts of strong acids and weak bases are the most
effective compounds. Ammonium and amine salts are generally
effective, as are Lewis acids and bases, either by themselves or when
formed in combustion.
3.2 Condensed phase mechanisms
The role of phosphorus compounds has been extensively studied. In
both cellulose and thermoplastics, phosphorus salts of volatile metals
and most organophosphorus compounds are known to be effective flame
retardants. The formation of char appears to be the key. For example,
although triphenyl phosphate, triphenyl phosphite and triphenyl
phosphine are all equivalent on a phosphorus basis, the more effective
flame retardant compounds act by forming phosphoric acid, which
changes the course of the decomposition of cellulose to form carbon
and water (US EPA, 1976).
The flame-retardant action of phosphorus compounds in cellulose
is believed to proceed by way of initial phosphorylation of the
cellulose, probably by initially formed phosphoric or polyphosphoric
acid. The phosphorylated cellulose then breaks down to water,
phosphoric acid and an unsaturated cellulose analogue, and eventually
to char by repetition of these steps. Certain nitrogen compounds such
as melamines, guanidines, ureas and other amides appear to catalyse
the steps forming cellulose phosphate and are found to enhance or
synergize the flame-retardant action of phosphorus on cellulose.
In polyethylene terephthalate and polymethyl methacrylate the
mechanism of action of phosphorus-based flame retardants has been
shown to involve both a similar decrease in the amount of combustible
volatiles and a similar increase in the amount of residues (aromatic
residues and char). The char formed also acts as a physical barrier to
heat and gases. In rigid polyurethane foams the action of phosphorus
flame retardants also appears to involve char enhancement. In flexible
foam the mechanism is less well understood (Weil,1993).
3.3 Gas-phase mechanisms
In addition to the condensed-phase mechanism, phosphorus flame
retardants can exert gas-phase flame-retardant action. It has been
demonstrated that trimethyl phosphate retards the velocity of a
methane-oxygen flame with about the same molar efficiency as antimony
trioxide (Weil, 1993). The mechanisms of action can differ depending
on the type of compound used as a flame retardant. The mechanism
affects the generation of products of combustion, some of which are
potentially corrosive and toxic.
One mechanism of improving the flame retardancy of thermoplastic
materials is to lower their melting point. This results in the
formation of free radical inhibitors in the flame front and causes the
material to recede from the flame without burning.
Free radical inhibition involves the reduction of gaseous fuels
generated by burning materials. Heating of combustible materials
results in the generation of hydrogen, oxygen, and hydroxide and
peroxide radicals that are subsequently oxidized with flame. Certain
flame retardants act to trap these radicals and thereby prevent their
oxidation. Bromine is more effective than chlorine. For example:
RBr + HÊ -> HBr + RÊ
If the resulting compound R is less readily oxidized than the radical
that is removed, the result is reduced flammability.
Measurements of the limiting oxygen index of polymers show that,
in contrast to the situation with chlorine, the effect of bromine does
depend on the gaseous oxidant involved. This suggests that bromine
compounds act to some extent by interfering with the flame reactions
and it is generally believed that this is probably their principal
mode of action, although they can also affect the condensed-phase
decomposition of the polymer.
Any gas-phase mechanism of flame retardancy by bromine compounds
must by definition involve the release of volatile bromine-containing
species, which then inhibit the flame reactions. In the case of
brominated flame retardants, it is generally assumed that hydrogen
bromide is liberated and reacts with the free radicals responsible for
the propagation of combustion, replacing them by the relatively
unreactive bromine atom.
HÊ + Hbr -> H2 + BrÊ
OHÊ + Hbr -> H2O + BrÊ
The mechanism operating in a particular polymer system will
depend on the mode and ease of breakdown of the brominated flame
retardant present. Some of these compounds are thermally stable and
volatilize when the associated polymer is heated to sufficiently high
temperatures. Others decompose to give substantial amounts of either
lower molecular weight organic bromine compounds or hydrogen bromide
(Cullis, 1987).
The presence of chemically bound bromine can also affect the
rates and modes of thermal decomposition of organic polymers in the
condensed phase. Brominated flame retardants vary considerably in
both their volatility and thermal stability. Although some very
stable compounds volatilize chemically unchanged, others break down
within the polymer or react directly with it in the condensed phase.
Hydrogen bromide is often a product and can significantly influence
the rate and course of polymer decomposition, although its effect is
small in comparison with those which it exerts on polymer combustion
as a whole. However, even thermally stable brominated flame
retardants can affect the decomposition of polymers in the condensed
phase, causing the original polymer breakdown stage to be replaced by
two separate stages, both of which involve polymer and additive. Thus,
it is clear that hydrogen bromide is not the only bromine-containing
compound which affects condensed-phase polymer decomposition and that
organic bromine compounds can also markedly change the rate and mode
of breakdown of organic polymers (Cullis, 1987).
A critical factor governing the effectiveness of brominated flame
retardants and indeed their mechanism of action is their thermal
stability relative to that of the polymers with which they are
associated. The most favourable situation for a flame retardant to be
effective will be one in which its decomposition temperature lies
50°C or so below that of the polymer. In general, decomposition at
this temperature with the liberation of substantial quantities of
hydrogen bromide or elemental bromine is likely to enhance flame-
retardant activity. Owing to the relatively low C-Br bond energy,
bromine compounds generally breakdown at quite low temperatures
(typically 200-300°C). Temperatures in this range overlap well with
the decomposition of many common polymers. This is probably a factor
determining the superior flame-retardant effectiveness of bromine
compounds compared with that of chlorine compounds (Cullis, 1987).
3.4 Co-additives for use with flame retardants
Brominated flame retardants are in some cases used on their own,
but their effectiveness is increased by a variety of co-additives, so
that in practice they are more often used in conjunction with other
compounds or with other elements incorporated into them. Thus, for
example, the addition of small quantities of organic peroxides to
polystyrene greatly reduces the amount of hexabromocyclododecane
needed to give a flame-retardant foam; other free radical initiators
behave in a similar fashion. These compounds appear to act by
promoting depolymerization of the hot polymer, giving a more fluid
melt. More heat is therefore required to keep the polymer alight,
because there is a greater tendency for the more molten material to
drip away from the neighbourhood of the flame (Cullis, 1987;
Troitzsch, 1990).
The flame-retardant properties of bromine compounds, like those
of chlorine compounds, will be considerably enhanced when they are
used in conjunction with other hetero-elements, notably phosphorus,
antimony and certain other metals.
The simultaneous presence of phosphorus in bromine-containing
polymer systems usually serves to improve their degree of flame
retardance, although, contrary to general opinion, bromine and
phosphorus generally exert effects that are largely additive rather
than synergistic.
Sometimes the two elements are present in the same molecule,
e.g., tris(2,3,-dibromopropyl)phosphate. In other systems, however,
it is more convenient to use mixtures of a bromine compound and a
phosphorus compound so that the ratio of the two elements can be
readily adjusted. It has already been pointed out that brominated
flame retardants on their own act predominantly in the gas phase. In
contrast, phosphorus compounds act mainly in the condensed phase,
especially with oxygen-containing polymers. It is therefore of
interest to discover whether, when both elements are present together,
each continues to act in the usual way or new mechanisms come into
operation. However, the evidence here is somewhat conflicting. Studies
of the effects of phosphate esters, with or without bromine present,
on the combustion of polyesters show that more char is formed when the
flame retardant contains bromine, and that most of this bromine
remains in the char. This suggests that the bromine-phosphorus
compound affects primarily the condensed-phase processes. However,
studies of the flammability of rigid polyurethane foams show that the
inhibiting effect of tris(2,3-dibromopropyl)- phosphate on combustion
depends on the nature of the gaseous oxidant, suggesting that the
flame retardant acts here, at least in part, by interfering with
reactions in the gas phase. With hydrocarbon polymers, such as
polyolefins and polystyrene, the major part of the phosphorus present
volatilizes and acts in the gas phase, being apparently converted to
simple species, such as phosphorus and phosphorus oxide free radicals.
These species can then interfere chemically with the reactions
responsible for flame propagation by catalysing the recombination of
the active free radicals involved. In such cases, any bromine present
simultaneously is presumably converted to species such as Brœ and HBr,
which function in the gas phase in the usual way (Cullis, 1987).
Antimony is a much more effective co-additive than phosphorus,
generally in the form of its oxide, Sb2O3. On its own this compound
has no flame-retardant activity and is therefore almost always used in
conjunction with a halogen compound. In general, bromine-antimony
mixtures are more effective than the corresponding chlorine-antimony
systems. The use of antimony trioxide greatly reduces the high levels
normally needed for effective flame retardance of bromine compounds on
their own. The principal mode of action is in the gas phase. If
bromine and antimony are present simultaneously in a burning organic
polymer, the major part of the antimony is volatilized, probably as
SbBr3 or SbOBr. These compounds then provide a ready source of
hydrogen bromide and they also produce in the middle of the combustion
zone a mist of fine particles of solid SbO, which can catalyse the
recombination of the free radicals responsible for flame propagation,
via the formation of transient species like SbOH. A number of other
metal oxides have been investigated as partial or total replacements
for antimony trioxide. Their use, however, has a number of
disadvantages. The most important point is that volatilization of the
bromine occurs at the right stage of the combustion cycle. With zinc
oxide, volatilization takes place too early and the bromine has
disappeared from the system before it can become effective (Cullis,
1987).
It can be concluded that in many, if not most, polymer systems in
which bromine and phosphorus are both present, the two elements tend
to act independently and therefore additively. The important mode of
action of metal oxides as co-additives for brominated flame retardants
is to catalyse the breakdown of the bromine compound and therefore the
release of volatile bromine compounds into the gas phase. However,
metal-bromine compounds may also be formed, and these may have more
specific modes of action in inhibiting polymer combustion (Cullis,
1987).
3.5 Smoke suppressants
Smoke production is determined by numerous parameters. No
comprehensive theory yet exists to describe the formation and
constitution of smoke.
Smoke suppressants rarely act by influencing just one of the
parameters determining smoke generation. Ferrocene, for example, is
effective in suppressing smoke by oxidizing soot in the gas phase as
well as by pronounced charring of the substrate in the condensed
phase. Intumescent systems also contribute to smoke suppression
through creation of a protective char. It is extremely difficult to
divide these multifunctional effects into primary and subsidiary
actions since they are so closely interwoven. At present no uniform
theory on the mode of action of smoke suppressants has been
established (Troitzsch, 1990).
3.5.1 Condensed phase
Smoke suppressants can act physically or chemically in the
condensed phase. Additives can act physically in a similar fashion to
flame retardants, i.e., by coating (glassy coatings, intumescent
foams) or dilution (addition of inert fillers), thus limiting the
formation of pyrolysis products and hence of smoke. Chalk (CaCO3),
frequently used as a filler, acts in some cases not only physically as
a dilutent but also chemically (in PVC, for example) by absorbing
hydrogen chloride or by effecting cross-linking so that the smoke
density is reduced in various ways. The processes contributing to
smoke suppression can be extremely complex.
Smoke can be suppressed by the formation of a charred layer on
the surface of the substrate, e.g., by the use of organic phosphates
in unsaturated polyester resins. In halogen-containing polymers, such
as PVC, iron compounds, e.g., iron (III) chloride, cause charring by
the formation of strong Lewis acids.
Certain compounds such as ferrocene cause condensed-phase
oxidation reactions that are visible as a glow. There is pronounced
evolution of CO and CO2, so that less aromatic precursors are given
off in the gas phase.
Compounds such as MoO3 can reduce the formation of benzene
during the thermal degradation of PVC, probably via chemisorption
reactions in the condensed phase. Relatively stable benzene-MoO3
complexes that suppress smoke development are formed (Troitzsch,
1990).
3.5.2 Gas phase
Smoke suppressants can also act chemically and physically in the
gas phase. The physical effect takes place mainly by shielding the
substrate with heavy gases against thermal attack. They also dilute
the smoke gases and reduce smoke density. In principle, two ways of
suppressing smoke chemically in the gas phase exist: the elimination
of either the soot precursors or the soot itself. Removal of soot
precursors occurs by oxidation of the aromatic species with the help
of transition metal complexes. Soot can also be destroyed oxidatively
by high-energy OH radicals formed by the catalytic action of metal
oxides or hydroxides. Smoke suppression can also be achieved by
eliminating the ionized nuclei necessary for forming soot with the aid
of metal oxides. Finally soot particles can be made to flocculate by
certain transition metal oxides (Troitzsch, 1990).
4. PERFORMANCE CRITERIA FOR AND CHOICE OF FLAME RETARDANTS
At present, the selection of a suitable flame retardant depends
on a variety of factors that severely limit the number of acceptable
materials.
Many countries require extensive information on human and
environmental health effects for new substances before they are
allowed to be put on the market. For existing chemicals such data are
not always available but several national and international programmes
are in the process of gathering this information.
For most chemicals, including flame retardants, the following
information regarding human and environmental health is essential to
understanding a chemical's potential hazards:
1. Data from adequate acute and repeated dose toxicity studies is
needed to understand potential health effects.
2. Data on biodegradability and bioaccumulation potential is needed
as a first step in understanding a chemical's environmental
behaviour and effects.
3. Information on the chemical's possible breakdown and/or
combustion products may also be needed.
4. Since flame retardants are often processed into polymers at
elevated temperatures, consideration of the stability of the
material at the temperature inherent to the polymer processing is
needed, as well as on whether or not the material volatilizes at
that temperature or during use.
5. Consideration should be given to the need for information on the
possible formation of toxic and/or persistent breakdown products
during accidental fires or incineration.
Successfully achieving the desired improvement in flame
retardancy is a necessary precursor to other performance
considerations. The basic flammability characteristics of the polymer
to be used play a major role in the flame-retardant selection process,
as some polymers burn much more readily than others.
Flame-retardant selection is also affected by the test method to
be used to assess flame retardancy. Some tests can be passed with
relatively low levels of many flame retardants, while high levels of
very powerful flame retardants are needed to pass other tests. It is
not possible to provide a comprehensive review in this monograph, but
a short introduction is given in Annex III.
There are many performance issues other than flame retardancy
that must be considered during the selection of a flame retardant for
any use. Just as in applications not needing improved flame
retardancy, a long list of processing and performance requirements
must be met before a material can be accepted for use. The
development of a polymer formulation that meets all of these
requirements involves finding the optimum combination of polymer(s),
flame retardant(s), synergist(s), stabilizer(s), processing aid(s),
and all other additives. This is complex and difficult work requiring
a great deal of time, effort and expense.
Flame retardants may adversely affect the processing
characteristics of polymers. Changes occurring in the viscosity of
liquid systems or in the flow of polymers that are melted during
processing can cause major problems. Significant alteration of the
rate of reaction of thermoset polymers or the speed and degree of
crystallization of thermoplastic polymers may result from the use of
some flame retardants. The temperatures routinely used to process
many polymers severely restrict the number of flame retardants
suitable for incorporation.
Since flame retardants are frequently used at high levels, they
often have a dramatic effect on the basic mechanical properties of
polymers in which they are used. Reduction of strength (tensile,
compression), rigidity, toughness and/or heat resistance are common
problems.
When flame retardants are added to polymers their appearance
(colour, gloss, transparency) and physical properties (density,
hardness, melting and glass transition temperatures, thermal
expansion) often change significantly. Electrical properties
(resistance, dielectric, tracking) are frequently altered, and aging
due to factors such as oxidation, UV radiation, high temperature may
be reduced.
The chemical properties of a flame retardant are often of great
importance in its selection. Resistance to exposure to water,
solvents, acids, bases, oils or other substances may be a requirement
for use. Issues related to solubility, hydrolysis resistance or
reactivity with other formulation components may prevent the use of an
otherwise desirable flame retardant.
The relationship between cost and performance is an essential
consideration in the selection of a flame retardant.
All of the above-mentioned issues also apply to textiles. In
addition, the durability (resistance to cleaning with water or by
other techniques) of the flame retardant system is critical (Jay,
1990).
5. PRODUCTION AND USES OF FLAME RETARDANTS AND FLAME-RETARDED
POLYMERS
5.1 Production
The worldwide demand for flame-retardant chemicals in 1992 was
estimated to be 600 000 tonnes (OECD, 1994). This includes over a
hundred different products, which can be classified according to base
chemical content as depicted in Table 3.
Table 3. Demand for flame retardants according to base chemical
content (from: OECD, 1994)
Base chemicals Demand
(tonnes)
Bromine 150 000
Chlorine 60 000
Phosphorus 100 000
Antimony 50 000
Nitrogen 30 000
Aluminium 170 000
Others 50 000
It is difficult to obtain an accurate picture of market volumes
of flame retardants as reports from different sources appear to
conflict.
Table 4 shows USA market volume trends between 1986 and 1991.
Table 5 presents the annual consumption of different flame
retardants in Japan over the period 1986 - 1994. A comparable table of
global use was not available. Table 5 indicates that the consumption
of brominated flame retardants and antimony oxide in Japan has more
than doubled over this period, compared to the moderate increase in
other flame retardants. The market for hydrated aluminium as a flame
retardant seems to have decreased in Japan, whereas Table 4 (Gann,
1993) shows that an increase occurred in the USA.
Chlorinated paraffins had an estimated world production of
300 000 tonnes/year in 1985 (IPCS, 1996b).
Table 4. Flame retardant market volume (from: Gann, 1993)
Group 1986 1991
(tonnes) (tonnes)
Phosphate esters 20 000 18 000
Halogenated phosphates 13 000 16 000
Chlorinated hydrocarbons 15 000 15 000
Brominated hydrocarbons 28 000 36 000
Brominated bisphenol A 16 000 18 000
Antimony trioxide 22 000 25 000
Borates 8 000 8 000
Aluminium trihydrate 140 000 170 000
Magnesium hydroxide
2 000 3 000
Total
264 000 301 000
5.2 Uses
The consumption of flame retardants in plastics and other
combustible materials is closely linked to regulations covering fire
precautions. The principle regulations relate to the building,
transportation, electrical engineering, furnishing and mining sectors
(Troitzsch, 1990).
A worldwide estimate of the consumption of flame retardants
according to materials is not available but the figures for Europe
listed in the Table 6 should reflect the market in general.
Table 5. Trends in the annual consumption of flame retardants in Japana
Type Compound Amount (tonnes)
1986 1990 1994
Brominated
Tetrabromobisphenol A (TBBPA) 12 000 23 000 24 000
Decabromobiphenyl ether 3 000 10 000 5 500
Octabromobiphenyl ether 600 1 100 500
Tetrabromobiphenyl ether 1 000 1 000 0
Hexabromocyclododecane 600 700 1 600
Bis(tetrabromophthalimido) ethane - 1 000 2 500
Tribromophenol 100 450 3 500
Bis(tribromophenoxy) ethane 400 400 900
TBBPA polycarbonate oligomer - - 2 500
Brominated polystyrene - - 1 300
TBBPA epoxy oligomer - 3 000 7 000
Others 2 400 - 2 150
Subtotal 20 000 40 650 51 450
Chlorinated
Chlorinated paraffins 4 000 4 500 4 300
Others 850 700 900
Subtotal 4 850 5 200 5 200
Phosphoric
Halogenated ester 3 000 3 000 3 100
Non-halogenated ester 4 000 4 400 4 400
Others 1 750 1 750 3 310
Subtotal 8 750 9 150 10 810
Table 5. (contd.)
Type Compound Amount (tonnes)
1986 1990 1994
Inorganic
Antimony oxide 8 300 16 000 17 000
Hydrated aluminium 48 000 37 000 42 000
Others 7 200 8 400 9 000
Subtotal 63 500 61 400 68 000
TOTAL 97 100 116 400 135 460
a Based on the investigation made by Kagaku Kogyo Nippon Co. Ltd. (Japan).
(Personal communication from Isao Watanabe, Osaka Prefectural Institute
of Public Health, Japan).
Table 6. Estimated consumption of flame retardants in western
Europe for 1985 and 1992 according to materials
(from: Sutker, 1988)
Product group Consumption (103 tonnes)
1985 1992
Polystyrene 4.0-4.5 4.5-5.0
ABS 1.0-1.5 1.2-1.8
Polyesters 7.5-8.0 8.5-9.0
Epoxy resins 3.5-4.0 4.0-4.5
Polyolefins 10.0-12.0 11.0-13.0
Polyvinyl chloride) 25.0-27.0 27.0-29.0
Polyurethanes 12.0-13.5 13.5-15.0
Engineering plastics 1.5-1.8 1.7-2.0
Paper and textiles 9.0-10.0 10.0-11.0
Rubber and elastomers 5.0-6.0 6.0-7.0
Other 11.5-11.7 12.6-12.7
Total 90.0-100.0 100.0-110.0
5.2.1 Plastics
The plastics industry is the largest consumer of flame
retardants, estimated at about 95% for the USA in 1991. About 10% of
all plastics contain flame retardants (Wolf & Kaul, 1992). The main
applications are in building materials and furnishings (structural
elements, roofing films, pipes, foamed plastics for insulation,
furniture and wall and floor coverings), transportation (equipment and
fittings for aircraft, ships, automobiles and railroad cars), and in
the electrical industry (cable housings and components for television
sets, office machines, household appliances and lamination of printed
circuits).
The growth in the flame retardant market reflects the enormous
expansion of the plastics industry in recent decades. Between 1988 and
1994, there was a worldwide increase of 20%. Although the USA,
western Europe and Japan are still the largest plastic producers (30,
24 and 12% of the market, respectively), other countries showed the
largest increases between 1988 and 1994, e.g., South Korea (170%);
China (60%); Taiwan (54%) (Anon, 1995).
Examples of flame retardants used in various plastics (Wolf &
Kaul, 1992) are as follows:
PVC: Chlorinated paraffins or phosphate esters, antimony trioxide,
aluminium hydroxide
Acrylonitrile-butadiene-styrene (ABS): Octabromodiphenyl ether,
antimony trioxide
Expandable polystyrene: Hexabromocyclododecane
High-Impact polystyrene (HIPS): Decabromodiphenyl ether or
tetrabromobisphenol A, antimony trioxide
Linear polyester: Brominated organics
Polypropylene: Tetrabromobisphenol A, bis(2,3-dibromopropyl ether),
antimony trioxide
Low-density polyethylene (LDPE) films: chlorinated paraffins,
antimony trioxide
High-density polyethylene (HDPE) and cross-linked polyethylene:
Brominated aromatics
Polyurethane foams: Organophosphates, brominated organic compounds,
alumina trihydrate
Polyamides: Brominated aromatic compounds, chlorinated
cycloaliphatic compounds, antimony trioxide, red phosphorus, melamine
Polycarbonates: Tetrabromobisphenol A, brominated organic oligomers,
sulfonate salts
Unsaturated polyesters: Chlorinated and brominated organic
compounds, antimony trioxide, alumina trihydrate
Epoxy resins: Tetrabromobisphenol A
5.2.2 Textile/furnishing industry
In contrast to the plastics industry, the textile industry is a
much smaller market for flame retardants. However, rather than
employing just one flame retardant, the use of a combination of
chemicals is usually necessary for textiles.
Phosphorus-containing materials are the most important class of
compounds to impart durable flame resistance to cellulose (Calamari &
Harper, 1993). Flame-retardant finishes containing phosphorus
compounds usually also contain nitrogen or bromine, or sometimes both.
Another system is based on halogens (usually bromine) in conjunction
with nitrogen or antimony.
Flame retardants used in furniture/textiles include the following
(Anon, 1992; Calamari & Harper, 1993; EFRA, 1995):
* organic phosphates such as tri-alkyl or tri-aryl phosphates, tri-
chloroalkyl phosphates, dialkyl phosphites,
tetrakis(hydroxymethyl)phosphonium chloride (THPC) and related
structures;
* halogenated compounds such as polybrominated diphenyl ethers
(found in over 50% of treated furniture) and chlorinated
paraffins (rainproof applications);
* inorganic compounds such as antimony trioxide, ammonium bromide,
boric acid and aluminium hydrate.
Details on the flame-retardant types were reported by Calamari &
Harper (1993) and can be found in Annex II.
6. FORMATION OF TOXIC PRODUCTS ON HEATING OR COMBUSTION OF
FLAME-RETARDED PRODUCTS
Natural or synthetic material that burns produces potentially
toxic products. There has been considerable debate on whether
addition of organic flame retardants results in the generation of a
smoke that is more toxic and may result in adverse health effects on
those exposed. There has been concern in particular about the
emission of polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) during manufacture, use and combustion of
brominated flame retardants.
6.1 Toxic products in general
Combustion of any organic chemical may generate carbon monoxide
(CO), which is a highly toxic non-irritating gas, and a variety of
other potentially toxic chemicals. Some of the major toxic products
that can be produced by pyrolysis of flame retardants are: CO, CO2,
HCl, POX, ammonia vapour, bromofurans, HBr, HCN, NOX and phosphoric
acid (Anon, 1992).
In general the acute toxicity of fire atmospheres is determined
mainly by the amount of CO, the source of which is the amount of
generally available flammable material. Most fire victims die in post
flash-over fires where the emission of CO is maximized and the
emission of HCN and other gases is less. The acute toxic potency of
smoke from most materials is lower than that of CO (Hirschler, 1995;
Nelson, 1995).
Flame retardants significantly decrease the burning rate of the
product, reducing heat yields and quantities of toxic gas. In most
cases, smoke was not significantly different in room fire tests
between flame-retarded and non-flame-retarded products (Babrauskas
et al. 1988).
Morikawa et al. (1995) reported toxicity studies on gases from
full-scale room fires involving fire retardant materials (a variety,
but not specified). HCN and CO were the two major toxicants. There was
no evidence that the smoke from flame-retarded materials was more
toxic to rabbits than the smoke from non-flame-retarded materials.
Regarding brominated flame retardants, Cullis (1987) stated that
unless suitable metal oxides or metal carbonates are also present,
virtually all the bromine is eventually converted to gaseous hydrogen
bromide (HBr). This is a corrosive and powerful sensory irritant. In a
fire situation however, it is always carbon monoxide (CO) or hydrogen
cyanide (HCN), rather than an irritant which causes rapid
incapacitation. Owing to its high reactivity, hydrogen bromide is
unlikely to reach dangerously high concentrations (Cullis, 1987).
6.2 Formation of halogenated dibenzofurans and dibenzodioxins
PBDFs and PBDDs can be formed from polybrominated diphenyl ethers
(PBDEs), polybrominated phenols, polybrominated biphenyls (PBBs) and
other brominated flame retardants under various laboratory conditions,
including heating. Because chlorinated derivatives are preferably
formed during pyrolysis, mixed halogen compounds will be predominantly
produced if a chlorine source is also available (Buser, 1987a,b).
As in the case of PCDD/PCDF, it is the 2,3,7,8-substituted
isomers that are toxic.
6.3 Exposure to PBDD/PBDF from polymers containing halogenated
flame retardants
6.3.1 Exposure due to contact or emission from products
containing halogenated flame retardants
Exposure of the general public to PBDD/PBDF impurities in flame-
retardant polymers is unlikely to be of significance. The possible
exposure to PBDD/PBDF from TV sets and computer monitors flame-
retarded with halogenated flame retardants has been discussed in
Environmental Health Criteria 162: Brominated diphenyl ethers and is
unlikely to be of significance (IPCS, 1994b).
6.3.2 Workplace exposure studies
Several studies have been performed to determine whether
PBDD/PBDF is present in the fumes emitted during thermal processes,
such as the extrusion of resins containing halogenated flame
retardants under normal processing conditions at temperatures in the
range of 200 to 250°C (IPCS 1994b, 1995a, in preparation).
Epidemiological studies of workers engaged in processing polymers
with PBDEs have been reported (IPCS, 1994b). Results of PBDD/PBDF
workplace monitoring during polymer processing have also been reported
(IPCS 1994b, 1995a). PBDD/PBDF personnel and room air levels during
processing of PBDEs were < 2 ng/m3 (TCDD equivalent) with the
exception of two samples at the extruder head (128 ng/m3, TCDD
equivalent) (IPCS, 1994b). Engineering controls were successful in
reducing these levels. Workplace control measures should also include
appropriate industrial hygiene measures and monitoring of exposure
(IPCS, 1994b, 1995a).
6.3.3 Formation of PBDD/PBDF from combustion
6.3.3.1 Laboratory pyrolysis experiments
In the late 1980s many pyrolysis experiments (at temperatures of
400-900°C) on brominated flame retardants and flame-retardant systems
were performed and the breakdown products measured. Flame retardants
or intermediates tested included PBBs, PBDEs, 2,4,6-tribromophenol,
pentabromophenol, tetrabromobisphenol A and tetrabromophthalic
anhydride (IPCS 1994b, 1995a, in preparation). Pyrolysis of the flame
retardants alone, as well as with polymer mixtures, was investigated.
As different laboratories carried out the experiments using a variety
of testing methods and conditions, a direct comparison of the many
experiments is not possible. Details of the pyrolysis experiments
involving PBDEs, tetrabromobisphenol A and derivatives, and PBBs are
given in the respective EHC monographs (IPCS 1994b, 1995a, in
preparation).
Although they indicate which flame retardants are likely to form
PBDF (and to a lesser extent PBDD) pyrolysis experiments are not
generally comparable to actual fire situations.
6.3.3.2 Fire tests and fire accidents
Fire tests on televisions have shown smoke and combustion
residues containing high levels of PBDD/PBDF. However, levels from
actual fire accidents involving televisions revealed much lower levels
than those produced under fire test conditions (IPCS, 1994b). Further
studies are discussed in the EHC monograph on PBDD/PBDFs (IPCS, in
preparation).
7. OVERVIEW OF EXPOSURE AND HAZARDS TO HUMANS AND THE ENVIRONMENT
Since flame retardants are a heterogeneous group of diverse
chemicals (see chapter 2 and Annex II), the information presented in
this section only provides a general overview of possible routes of
exposure to chemicals associated with flame-retardant use. This
section also provides a brief summary of the hazards to human health
and to the environment posed by chemicals connected with flame-
retardant use. For detailed information on the extent of exposure and
health and environmental effects of individual substances, the
appropriate specific EHC monographs should be consulted.
The toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles has discussed
in a report by the European Economic Community (Anon, 1992).
7.1 Human exposure
7.1.1 General population
Potential sources of exposure include consumer products,
manufacturing and disposal facilities, and environmental media.
Factors affecting exposure of the general population include the
physical and chemical properties of the product, the extent of
manufacturing and emission controls, the use made of the product
(surface coating, durability of fabric finishes, incorporation into a
polymer, etc.), the end use, and the method of disposal. Potential
routes of exposure for the general population include the dermal route
(contact with flame-retarded textiles), inhalation and ingestion.
7.1.2 Occupational exposure
Occupational exposure may occur during the manufacture,
transport, processing and disposal/recycling of flame retardants.
Routes of exposure could include inhalation, dermal contact and
ingestion. Factors affecting the extent of exposure include industrial
hygiene practices, engineering controls, manufacturing processes and
the type of product. As with any other industrial chemical, workplace
monitoring and good industrial practice can delineate the extent of
any exposure.
7.2 Exposure of the environment
Environmental exposure may occur as a result of the manufacture,
transport, use or waste disposal of flame retardants. Routes of
environmental exposure can include water, air and soil. Factors
affecting exposure include the physical and chemical properties of the
product, emission controls, disposal/recycling methods, volume and
biodegradability/persistence. Environmental monitoring can determine
the extent of environmental exposure.
On the basis of the estimated demand for flame retardants (see
Table 3), more than 1 million tonnes of flame-retardant polymers are
produced each year.
Most flame-retarded products eventually become waste. Municipal
waste is generally disposed of via incineration or landfill.
Incineration of flame-retarded products can produce various toxic
compounds, including halogenated dioxins and furans. The formation of
such compounds and their subsequent release to the environment is a
function of the operating conditions of the incineration plant and the
plant's emission controls.
There is a possibility of flame retardants leaching from products
disposed of in landfills. However, potential risks arising from
landfill processes are also dependent on local management of the whole
landfill. The significance of any release of flame retardants from
disposal sites has yet to be determined.
Some products containing flame retardants, including some
plastics, have been identified as suitable for recycling (Lorenz &
Bahadir, 1993; Meyer et al., 1993).
7.3 Hazards to humans
The hazards to humans associated with some flame retardants have
been outlined in the relevant EHC monographs. For example, the use of
tris(2,3-dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate
was banned in 1977 by the US Consumer Product Safety Commission and in
several other developed countries for use in children's clothing
because of concerns that the chemical might be a human carcinogen and
because of the possibility of significant human exposure through
contact with treated fabrics (IPCS, 1995b). Delayed neurotoxicity
due to tri- ortho-cresyl phosphate (TOCP), one of the tricresyl
phosphate isomers, has been observed in humans (IPCS, 1990). Some
polybrominated biphenyl (PBB) congeners have been shown to produce
chronic toxicity and cancer in experimental animals. However, no
definitive human health effects, correlatable with exposure, were
found in a population in Michigan, USA, accidentally exposed to PBBs
(IPCS, 1994a).
7.4 Hazards to the environment
EHC monographs outline the hazards to the environment associated
with some flame retardants (see Table 1). Some PBB congeners are
persistent and bioaccumulative and may pose a threat especially to
higher levels of the food chain (IPCS, 1994a). Hexachloro-
cyclopentadiene is highly toxic to aquatic organisms. However,
information obtained under environmentally realistic conditions is
limited. The potential hazard to the general environment is expected
to be low (IPCS, 1991b). Low concentrations of triphenyl phosphate
have been detected in environmental samples. Triphenyl phosphate is
rapidly degraded in the environment. However, sediment-dwelling
organisms near production plants may have been exposed to
concentrations high enough to exert toxic effects (IPCS, 1991a).
Tricresyl phosphate is also degraded rapidly in the environment, and
subsequent environmental concentrations are therefore low. The acute
toxicity of tricresyl phosphate to aquatic organisms is low (IPCS,
1990).
Persistence of pentabromodiphenyl ether (PeBDE) and lower
brominated diphenylethers in the environment suggest that commercial
PeBDE should not be used (IPCS, 1994b).
Some flame retardants have come under intense environmental
scrutiny. US EPA has called for additional testing (US EPA, 1992).
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas close to release sources there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate- and long-chain chlorinated paraffin products (IPCS,
1996b).
8. REGULATIONS WITH RESPECT TO FLAME RETARDANTS
Several national regulatory bodies have implemented regulations
on specific substances associated with flame-retardant applications.
In the USA the Interagency Testing Committee (ITC), under the
auspices of the Toxic Substances Control Act (TSCA), makes
recommendations concerning the need for additional testing on
chemicals in the TSCA inventory, including flame retardants. Based on
the information published since 1978, the ITC has made initial testing
recommendations upon 128 brominated flame retardants (US TSCA, 1992;
Walker, 1994; Annex IV).
In the European Community, the use of tris(2,3-dibromopropyl)
phosphate (EC Directive 76/769/EEC) and tris(1-aziridinyl)phosphine
oxide (EC Directive 83/264/EEC) in textiles has been banned. In 1977,
the US Consumer Product Safety Commission banned the use of tris(2,3-
dibromopropyl)phosphate in children's clothing (IPCS, 1995b).
The European Community has also banned the use of PBBs in
textiles (EC Directive 83/264/EEC). Several countries have either
taken or proposed regulatory actions on PBBs, as outlined in Table 7.
Controls on the emissions of dioxins and furans from municipal
solid waste incinerators have been implemented in the United Kingdom
under the Environmental Protection Act (1990). In Germany, a second
modification of the Chemicals Prohibition Ordinance, which was adopted
in 1994, imposes limits on 2,3,7,8-substituted chlorinated dioxins and
furans and, for the first time, on some 2,3,7,8-substituted brominated
dioxins and furans (OECD, 1994).
Table 7. Country-specific actions on PBBs either taken or proposeda
Country Actions
Austria Prohibits the manufacture, placing on the market,
import and use of PBBs and products containing
these substances.
Canada Prohibits the manufacture, use, processing, offer
for sale, selling or importation of PBBs for
commercial, manufacturing or processing purposes.
Denmark Implements EC Directive 89/677 banning the use of
PBBs in textiles.
Finland PBB may not be used in textile articles intended
to come into contact with the skin (in accordance
with EC Directive 83/264).
France Implements EC Directive concerning PBBs and their
use on textiles.
Netherlands Proposed resolution would prohibit the storage of
PBBs or products or preparations containing these
substances or making them available to third
parties. (Exports are excluded from the
resolution).
Norway Ban on PBBs in textiles intended to come into
contact with skin, implementation of EC Directives
76/769/EEC, 83/264 and 89/677.
Sweden Ban on PBBs in textiles intended to come into
contact with skin by implementation of EC
Directive 76/769.
Switzerland Prohibits manufacture, supply, import and use of
PBBs and products containing these substances.
Supply and import of capacitors and transformers
containing PBBs is forbidden.
USA No current production or use. Companies intending
to resume manufacture must notify US EPA 90 days
in advance for approval.
a Adapted from: OECD (1994)
9. CONCLUSIONS AND RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
9.1 Conclusions
Flame retardants are a diverse group of compounds used to improve
the flame retardancy of polymers and other materials. A large variety
of compounds, from inorganic to complex organic molecules, are used as
flame retardants, synergists and smoke suppressants. This overview is
focused on organic compounds, which typically contain halogen and/or
phosphorus.
It is difficult to find accurate figures for the global use of
flame retardants but estimates indicate that more than 600 000 tonnes
are produced annually. Available data indicate a substantial increase
of brominated organic product consumption during the last decade.
There are obvious benefits in using flame retardants, as many
human lifes and property are saved from fire. At present, knowledge
of long-term effects resulting from exposure to flame retardants and
their breakdown products is limited. Most people that die in fires
are killed by carbon monoxide.
The majority of the organic flame retardants are either
covalently bound into polymer molecules (reactive) or mixed into the
polymer (additive). They can act in several ways, either physically
(by cooling, by formation of a protective layer or by dilution of the
matrix) or chemically (by reactions in either the gas or the solid
phase).
A number of factors govern the selection of the type of flame
retardant to be used in a specific application. Some of these are the
flammability of the matrix, processing and performance requirements,
chemical properties and possible hazards to human and environmental
health.
Exposure of the general population to flame retardants can occur
via inhalation, dermal contact and ingestion. Potential sources of
exposure are consumer products, manufacturing/disposal facilities and
environmental media (including food intake). The same routes are
possible for occupational exposure, mainly during production,
processing, transportation and disposal/recycling of the flame
retardants or the treated products. Occupational exposure to the
breakdown products may also occur during fire fighting. As several of
the compounds used are lipophilic and persistent, they may
bioaccumulate. Some of the compounds have been shown to cause organ
damage, genotoxic effects and cancer.
There is also concern for occupational health and environmental
effects from combustion/pyrolysis products, especially the
polyhalogenated dibenzofurans and dibenzo- p-dioxins, from some
organic flame retardants. Other breakdown products also need to be
taken into account.
The properties of a number of flame retardants make them
persistent and/or bioaccumulative, and they may therefore pose hazards
to the environment. Some of the compounds that have been evaluated so
far (polybrominated biphenyls, polybrominated diphenyl ethers and
chlorinated paraffins) have been found to belong to this group. Some
of these have therefore been recommended to not be used.
Several countries have developed regulations affecting the
production, use and disposal of flame retardants. Some include
restrictions on the use of compounds because of potential toxic
effects in humans. Germany has developed rules for the maximum
content of some 2,3,7,8-substituted polychlorinated dibenzo- para-
dioxins and dibenzofurans in products.
The availability of relevant data on flame retardants in the open
literature is limited, especially for some existing chemicals
produced before regulations for commercialization were strengthened
in several countries.
IPCS has issued evaluations for some flame retardants and is
preparing evaluations for others.
9.2 Recommendations for the protection of human health and the
environment
a) Information on the content and nature of flame retardants,
including impurities in products, should be made available to
national authorities.
b) More complete information on the volume of flame retardants
production and consumption should be made available.
c) In view of the increased recycling of flame-retarded products,
consideration could be given to harmonized labelling by an
international forum.
d) Compounds that present a toxic risk to humans and/or the
environment should not be used as flame retardants.
e) Occupational exposure to flame retardants and their breakdown
products should be minimized using appropriate engineering and
good industrial hygiene practices. The exposure of people
working in these operations should be monitored.
f) There is a need for proper assessment of occupational health and
environmental effects from combustion or pyrolysis products of
flame retardants.
g) Emissions to the environment from manufacturing, processing,
transportation and disposal/recycling of products containing
persistent bioaccumulative compounds should be minimized using
best available techniques. The environment in the vicinity of
such operations should be monitored for the compounds used.
h) The use of flame retardants with properties that make them
persistent and bioaccumulative should be avoided.
i) The levels of the major persistent bioaccumulating flame
retardants should be monitored routinely in environmental
matrices (biota and sediments). Some compounds that are no
longer produced should likewise be monitored, in order to
indicate the long-term influence of such products.
10. FURTHER RESEARCH
a) Further studies should be undertaken to elucidate the fate of
flame retardants in disposal/recycling operations.
b) There is a need for further evaluations of flame retardants.
Useful criteria for setting priorities are volume of use,
intrinsic toxic effects on human health and the environment,
exposure assessments, and persistence and bioaccumulation/
biomagnification of flame retardants or their breakdown products.
REFERENCES
Anderson GA & Christy MR (1992) Standards, bans and flame retardants.
Presented at Structural Plastics "92", 5-8 April 1992. Dallas, Texas,
Society of the Plastics Industry, Inc., Structural Plastics Division.
Anon (1992) Toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles - Report to the
European Commission, DG-III, Brussels.
Anon (1995) [Plastic industry of the world.] Kunststoffe, 85(10):
1761-1772 (in German).
Arias P (1992) Brominated diphenyloxides as flame retardants. Bromine
based chemicals. Paris, Organisation for Economic Co-operation and
Development (Unpublished document).
Avento JM & Touval I (1980) Antimony and other inorganic compounds.
In: Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
John Wiley and Sons, vol 10, pp 486-487.
Babrauksas V, Harris RH Jr, Gann RG, Levin BC, Lee BT, Peacock RD,
Paabo M, Twilley W, Yoklavich MF, & Clark HM (1988) Fire hazard
comparison of fire-retarded and non-fire-retarded products.
Washington, DC, US Department of Commerce, National Bureau of
Standards (NBS Special Publication No. 749).
BFR/CEM Working Group (1989) [Polybrominated dibenzodioxins and
dibenzofurans (PBDDs/PBDFs) from flame retardants containing bromine:
Assessment of risk and proposed measures - Report by the Brominated
Flame Retardants/CEM Working Group to the Conference of Environmental
Ministers Bonn, Germany, September 1989.] (Report No. III/4299/89) (in
German).
Boethling RS & Cooper JC (1985) Environmental fate and effects of
triaryl and tri-alkyl/aryl phosphate esters. Residue Rev, 94: 49-99.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants. Chemosphere,
16(8-9): 1873-1876.
Calamari TA & Harper RJ (1993) Flame retardants for textiles. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 998-1022.
Cullis CF (1987) Bromine compounds as flame retardants. In:
Proceedings of the International Conference on Fire Safety, vol 12, pp
307-323.
Dynamac Corporation (1982) An overview of flame retardants: Volumes I
and II (Contract No. 68-01-6239). Rockville, Maryland, Dynamac
Corporation, Environmental Control Division (Prepared for the US
Environmental Protection Agency).
EFRA (1995) European flame retardants and their application. Brussels,
European Flame Retardants Association.
Flick EW (1986) Plastics additives: An industrial guide - Section IX:
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Gann RG (1993) Flame retardants: Overview. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 930-936.
Grabner RS (1993) N-containing flame retardants: An alternative.
Handout at OECD Workshop on Brominated Flame Retardants, Neuchâtel,
Switzerland, 22-25 February 1993.
Green JC (1992) A review of phosphorus-containing flame retardants. J
Fire Sci, 10: 470-487.
Hindersinn RR (1990) Historical aspects of polymer fire retardance.
In: Nelson GL ed. Fire and polymers hazards identification and
prevention. New York, American Chemical Society (ACS Symposium Series
No. 415).
Hirschler MM (1995) Smoke toxicity: How important is it for fire
safety? Handout at the 6th Annual BCC Conference on Flame Retardancy:
Recent advances in flame retardancy of polymeric materials, Stamford,
Connecticut, May 1995, 16pp.
Hutzinger O, Sundström G, & Safe S (1976) Environmental chemistry of
flame retardants: Part I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1975) Some aziridines, N-, S- & O-mustards and selenium.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
18).
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
IARC (1986a) Some chemicals used in plastics and elastomers. Lyon,
International Agency for Research on Cancer (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 39).
IARC (1986b) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
IARC (1989) Some organic solvents, resin monomers and related
compounds, pigments and occupational exposures in paint manufacture
and painting. Lyon, International Agency for Research on Cancer, pp
291-305 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Volume 47).
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 48).
IPCS (1984) Environmental health criteria 44: Mirex. Geneva, World
Health Organization, International Programme on Chemical Safety,
70 pp.
IPCS (1989) Environmental health criteria 88: Polychlorinated dibenzo-
p-dioxins and dibenzofurans. Geneva, World Health Organization,
International Programme on Chemical Safety, 409 pp.
IPCS (1990) Environmental health criteria 110: Tricresyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 122 pp.
IPCS (1991) Environmental health criteria 111: Triphenyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 80 pp.
IPCS (1991) Environmental health criteria 120: Hexachloro-
cyclopentadiene. Geneva, World Health Organization, International
Programme on Chemical Safety, 126 pp.
IPCS (1992) Environmental health criteria 140: Polychlorinated
biphenyls and terphenyls, 2nd ed. Geneva, World Health Organization,
International Programme on Chemical Safety, 692 pp.
IPCS (1994a) Environmental health criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, International Programme
on Chemical Safety, 577 pp.
IPCS (1994b) Environmental health criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, International Programme on
Chemical Safety, 347 pp.
IPCS (1995a) Environmental health criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization, International
Programme on Chemical Safety, 139 pp.
IPCS (1995b) Environmental health criteria 173: Tris(2,3-
dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate. Geneva,
World Health Organization, International Programme on Chemical Safety,
129 pp.
IPCS (1996a) Environmental health criteria 185: Chlorendic acid and
anhydride. Geneva, World Health Organization, International Programme
on Chemical Safety.
IPCS (1996b) Environmental health criteria 181: Chlorinated paraffins.
Geneva, World Health Organization, International Programme on Chemical
Safety.
IPCS (in preparation) Environmental health criteria: Polybrominated
dibenzo-p-dioxins and dibenzofurans. Geneva, World Health
Organization, International Programme on Chemical Safety.
ISO/IEC Guide (1990) Glossary of fire terms and definitions, 1st ed.
Geneva, International Organization for Standardization.
Japan Fire Retardant Association (1988) Voluntary rules of the flame
retardancy and the toxicological (safety) examination for flame
retardant products under the guide-line of the Fire Defence Agency,
Fire Retardant Products Approval Commission. Tokyo, Japan Fire
Retardant Association.
Jay TA (1990) Flame retardants. Presentation to the USSR All-Union
Congress on Flame Retardants at Saki, Crimea, USSR, 10 October 1990.
Kirk-Othmer (1993) Flame retardants. In: Kirk-Othmer encyclopedia of
chemical technology, 4th ed. New York, John Wiley & Sons, vol 10, pp
930-1022.
Kopp A (1990) [Discussions on bromine-containing flame-retardants.]
Berlin, Germany, Federal Ministry for Environment, Nature Conservation
and Nuclear Safety (Document sent to the European Commission,
Brussels) (in German)
Liepins R & Pearce EM (1976) Chemistry and toxicity of flame
retardants for plastics. Environ Health Perspect, 17: 55-63.
Lorenz W & Bahadir M (1993) Recycling of flame retardants containing
printed circuits: a study of the possible formation of polyhalogenated
dibenzodioxins/-furans. Chemosphere, 26(12): 2221-2229.
Meyer H, Neupert M, Pump W, & Willenberg B (1993) [Flame retardants
determine reusability.] Kunststoffe, 83(4): 253-257 (in German).
Morikawa T, Okada T, Kajiwara M, Sato Y, & Tsuda Y (1995) Toxicity of
gases from full-scale room fires involving fire retardant contents. J
Fire Sci, 13(1): 23-42.
Nelson GL (1995) Carbon monoxide and fire toxicity. Handout at the 6th
Annual BCC Conference on Flame Retardancy: "Recent Advances in Flame
Retardancy of Polymeric Materials, Stamford, Connecticut, May 1995 -
16pp.
OECD (1994) Selected brominated flame retardants. Paris, Organisation
for Economic Co-operation and Development, Environment Directorate,
152 pp (Risk Reduction Monograph No. 3).
Pearce EM & Liepins R (1975) Flame retardants. Environ Health
Perspect, 11: 59-69.
Pettigrew A (1993) Halogenated flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 954-976.
Soderlung E & Dybing E (1982) [Flame-retardant toxicology: A
literature survey.] Oslo, National Institute for Public Health
(Unpublished report ) (in Norwegian).
Sutker BJ (1988) Flame retardants. In: Ullmann's encyclopedia of
industrial chemistry, 5th ed. Weinheim, Germany, VCH Verlag, vol A11,
pp 123-140.
Teuerstein A (1990) Brominated FRS, production and uses (Summary).
Data from Eurobrom BV. In: Workshop on Brominated Aromatic Flame
Retardants. Proceedings of a Workshop held at Skoloster, Sweden, 24-26
October 1989. Solna, Swedish National Chemicals Inspectorate, pp
49-52.
Touval I (1993) Antimony and other inorganic flame retardants. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 936-954.
Troitzsch JH (1990) International plastics flammability handbook:
Principles, regulations, testing and approval, 2nd ed. Münich,
Germany, Hanser Publishers.
Ullmann (1988) Ullmann's encyclopedia of industrial chemistry: Volumes
A11 et A26, 5th ed. Weinheim, Germany, VCH Verlag.
Ulsamer AG, Osterberg RE, & McLauglin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US EPA (1976) A study of flame retardants for textiles. Washington,
DC, US Environmental Protection Agency (EPA-560/1-76-001; NTIS
PB-251-44).
US EPA (1989) Twenty-fifth report of the Interagency Testing Committee
to the administrator. Fed Reg, 54(237): 51114-51130.
US EPA (1992) Aryl phosphate base stocks: Proposed test rule and
reporting requirements. Fed Reg, 57(12): 2136-2158.
Walker JD (1994) Testing decisions of the TSCA Interagency Testing
Committee for Brominated Flame Retardants: A review of decisions and
health and safety data. Presented at the Meeting of the Fire Retardant
Chemicals Association, Williamsburg, Virginia, 9-12 October 1994.
Washington, DC, US Environmental Protection Agency.
Weil ED (1993) Phosphorus flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley and
Sons, vol 10, pp 976-998.
Wolf R & Kaul BL (1992) Plastics, additives. In: Ullmann's
encyclopedia of industrial chemistry, ed. Weinheim, Gemany, VCH
Verlag, vol A20, pp 459-507.
ANNEX I
TERMINOLOGY
There is no clear definition for many of the terms used to
describe fire, flammability and flame retardants. As a result, some
confusion has been created by the interchangeable use of terms such as
fire retardant, flame retardant, flame-proof and fire-proof. The
meaning of these and other terms is often clear only in the context.
Therefore, many efforts have been undertaken, at an international
level, to harmonize definitions and terms related to fire, including
fire protection and flame retardants. The definitions compiled in
Table 8 have been taken from the ISO/IEC Guide (1990) and other
sources (e.g., Troitzsch, 1990).
Other terms, which mostly concern mostly textile flame
retardancy, are defined below (US EPA, 1976, Kirk-Othmer, 1993):
Fireproof textile
This term applies only to those fabrics that undergo virtually no
change when exposed to a flame.
Flame-retardant textile
The term flame-retardant textile refers to any fabric that will
not support combustion after the source of ignition is removed. It is
synonymous with the term fire-resistant textile. The textile is
expected to char or melt. The term covers all treatments short of
fire-proofing.
Durability
Durability refers to the ability of a flame-retardant textile to
withstand washing/cleaning, chlorine bleaching, weathering and sun
exposure. A durable treatment is any chemical process which imparts
flame-retardant properties to textiles and textile products that will
last for at least 50 launderings and dry cleaning for the life of the
fabric. A semi-durable treatment will resist water but not withstand
dry cleaning or more than 10 to 15 launderings. A non-durable
treatment is readily removed by water or perspiration and requires
replacement after each exposure of the textile to water. The
definition of durability must be related to the conditions of use of
the textile and the product.
Table 8. Definitions of terms connected with fire
Term Definition
Afterflame Persistence of flaming of a material after the
ignition source has been removed
Afterglow Persistence of glowing of a material after
cessation of flaming or, if no flaming occurs,
after the ignition source has been removed
Burn To undergo combustion
Burning All the physical and/or chemical changes that
behaviour take place when a material or product is exposed
to a specified ignition source
Char Carbonaceous residue resulting from pyrolysis or
incomplete combustion
Combustible Capable of burning
Combustion Exothermic reaction of a substance with an
oxidizer, generally accompanied by flames and/or
glowing and/or emission of smoke
Fire a) A process of combustion characterized by the
emission of heat accompanied by smoke and/or
flame
b) Rapid combustion spreading uncontrolled in
time and space
Fire All the physical and/or chemical changes
behaviour that take place when a material, product and/or
structure is exposed to an uncontrolled fire
Fire The total gaseous, particulate or
effluent aerosol effluent from combustion or pyrolysis
Fire The ability of an element of building
resistance construction to fulfil for a stated period of
time the required load-bearing function,
integrity and/or thermal insulation specified
in the standard fire-resistance test
(see ISO 834)
Flame Zone of combustion in the gaseous phase from
which light is emitted
Table 8. (contd.)
Term Definition
Flame The property of a material either
retardance inherent or by virtue of a substance added or a
treatment applied to suppress, significantly
reduce or delay the propagation of flame
Flame A substance added or a treatment applied
retardant to a material in order to suppress, significantly
reduce or delay the combustion of the material
Flame spread Propagation of a flame front
Flame spread Distance travelled by a flame front
rate during its propagation per unit time under
specified test conditions
Flammability Ability of a material or product to burn with a
flame under specified test conditions
Flammable Capable of burning with a flame under specified
test conditions
Flash over The rapid transition to a state of total surface
involvement in a fire of combustible materials
within an enclosure
Fully The state of total involvement of
developed fire combustible materials in a fire
Glowing Combustion of a material in the solid
combustion phase without flame but with emission of light
from the combustion zone
Heat release The calorific energy released per unit
rate time by a material during combustion under
specified test conditions
Ignition Minimum temperature of a material at
temperature which sustained combustion can be initiated under
specified test conditions
Melting Phenomena accompanying the softening of
behaviour a material under the influence of heat (including
shrinking, dripping, burning of molten material,
etc.)
Table 8. (contd.)
Term Definition
Pyrolysis Irreversible chemical decomposition of a material
due to an increase in temperature without
oxidation
Reaction The response of a material under
to fire specified test conditions in contributing by its
own decomposition to a fire to which it is exposed
Smoke A visible suspension of solid and/or liquid
particles in gases resulting from combustion or
pyrolysis
Smoke The reduction in luminous intensity due
obscuration to passage through smoke
Smouldering The slow combustion of a material without light
being visible and generally evidenced by an
increase in temperature and/or by smoke
Soot Finely divided particles, mainly carbon, produced
and/or deposited during the incomplete combustion
of organic materials
ANNEX II
Flame retardants in commercial use or used formerly
Introduction
Tables 9 and 10 have been compiled on the basis of all the
information on flame retardants available to the IPCS and from the
following sources:
Arias (1992) BFR/CEM Working Group (1989)
Boethling & Cooper (1985) Dynamac Corporation (1982)
EFRA (1995) Flick (1986)
Hutzinger et al. (1976) IARC (1975, 1978, 1979, 1986a,b,
1987, 1989, 1990)
IRPTC (1987) Japan Fire Retardant Association
(1988)
Kirk-Othmer (1993) Kopp (1990)
Liepins & Pearce (1976) Pearce & Liepins (1975)
Sœderlund & Dybing (1982) Teuerstein (1990)
Troitzsch (1990) Ulsamer et al. (1980)
Ullmann (1988) US EPA (1989)
US TSCA (1992)
More than 175 flame retardants, or groups of them are tabulated
in these two tables. On just 17 of them the database was adequate for
preparing a hazard and risk evaluation for man and the environment,
and Environmental Health Criteria (EHC) monographs have been, or are
being prepared, on these. For the others the hazard to man and the
environment has not been evaluated internationally. Most of these
substances also have other major uses. In addition, some chemicals
used as intermediates in the production of flame retardants have beeen
listed. It is likely that these lists are not exhaustive, and that
new chemical structures are being developed as flame retardants.
Table 9 lists flame retardants in commercial use today and some
intermediates, while Table 10 lists flame retardants that have been
used in the past.
The tables list the chemical name, the chemical structure, the
CAS registry number and the uses as flame retardants. The "Use" column
also contains information on the global production volume of those
compounds that are currently being produced commercially (estimated
for the IPCS Task Group by P. Arias, 1995). The international
evaluation status of the substances is indicated in the column
"Remarks".
The following abbreviations are used in this table:
EHC An Environmental Health Criteria monograph on the chemical
has been published. If no EHC number is given, the document
is still in preparation.
IARC The International Agency for Research on Cancer of WHO in
Lyon has evaluated the carcinogenicity.
H High production volume (> 5000 tons per year)
M Moderate production volume (1000-5000 tons per year)
L Low production volume (<1000 tons per year or in
the developmental stage)
Table 9. Flame retardants being used commercially today
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Inorganic flame retardants
Potassium fluorotitanate K2TiF6 16919-27-0 Wool
Potassium fluorozirconate K2ZrF6 16923-95-8 Wool
Aluminium hydroxide Al(OH)3 21645-51-2 Rubber compounds, PVC, polyolefins, thermosets H
Antimony pentoxide Sb2O5 1314-60-9 Additive type flame-retardant synergist M
Antimony trioxide Sb2O3 1309-64-4 Additive type flame-retardant synergist H IARC
(1989)
Zinc oxide ZnO 1314-13-2 Additive type flame-retardant synergist
polyamides, rubber M
Boric acid H3BO3 11113-50-1 Wool, cellulosic, textiles H
Sodium borate (borax) Na2B4O7.10H2O 1303-96-4 Flame retardant and synergist H
Zinc borate 3ZnO.2B2O3. 1332-07-6 Synergist and smoke supressant M
Ammonium sulfamate NH4SO3NH2 7773-06-0 Cellulosic and textiles
Ammonium orthophosphate (NH4)3PO4 10124-31-9 Cellulosic and textiles
Ammonium carbamate phosphate Textiles
Di-ammonium phosphate (NH4)2HP04 7783-28-0 Textiles
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ammonium polyphosphate 68333-79-9 Cellulosics and mastics, paints, polyolefins H
Huntite- Mg3Ca(CO3)4 - 19569-21-2 Thermoplastics, coatings H
hydromagnesite Mg5(CO3)4Ê(OH)2Ê4H2O 12411-64-2 Smoke supressant M
12125-28-9
Ammonium octamolybdate (NH4)4Mo8O26
Magnesium hydroxide Mg(OH)2 1309-42-8 Thermoplastics, thermosets, rubbers H
Ammonium bromide NH4 Br 12124-97-9 Cellulosics H
Barium metaborate BaB2O4ÊxH2O 14701-59-2 Flame retardant additive, synergist M
Molybdenum trioxide MoO3 1313-27-5 Smoke suppressant L
Ammonium sulfate (NH4)2 SO4 7783-20-2 Cellulosic textiles
Ammonium chloride NH4Cl 12125-02-9 Cellulosics
Zinc hydroxystannate ZnSn(OH)6 12027-96-2 Smoke suppressant and
flame-retardant synergist L
Red phosphorus P 7723-14-0 Polyamides, phenolics, engineering
thermoplastics M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Sodium tungstate Na2WO4.2H2O 13472-45-2 Textiles
Sodium antimonate NaSbO3 15432-85-6 Flame-retardant additive, synergist
Brominated flame retardants
Decabromobiphenyl 13654-09-6 ABS, polystyrene M EHC 152
IARC
(1986b)
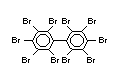 Decabromodiphenyl ethane 61262-53-1 Additive flame retardant for thermoplastics
such as high impact polystyrene, ABS
polypropylene, polyamide and polyester/
cotton M
Decabromodiphenyl ethane 61262-53-1 Additive flame retardant for thermoplastics
such as high impact polystyrene, ABS
polypropylene, polyamide and polyester/
cotton M
 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Decabromodiphenyl ether 1163-19-5 Polystyrene, polyesters, polyamides, textiles EHC 162
H IARC
(1990)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Decabromodiphenyl ether 1163-19-5 Polystyrene, polyesters, polyamides, textiles EHC 162
H IARC
(1990)
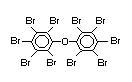 Octabromodiphenyl ether 32536-52-0 ABS H EHC
162
Octabromodiphenyl ether 32536-52-0 ABS H EHC
162
 Pentabromodiphenyl ether 32534-81-9 Textiles, polyurethanes H EHC
162
Pentabromodiphenyl ether 32534-81-9 Textiles, polyurethanes H EHC
162
 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobis phenol A 79-94-7 Intermediates for epoxy resins, polyester EHC
(30496-13-0) resins, polycarbonate resins, unsaturated 172
polyesters. ABS, phenolic resins H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobis phenol A 79-94-7 Intermediates for epoxy resins, polyester EHC
(30496-13-0) resins, polycarbonate resins, unsaturated 172
polyesters. ABS, phenolic resins H
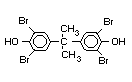 Tetrabromobisphenol 21850-44-2 Polyolefin resins M EHC
A-bis-(2,3-dibromopropylether) 172
Tetrabromobisphenol 21850-44-2 Polyolefin resins M EHC
A-bis-(2,3-dibromopropylether) 172
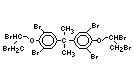 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol 4162-45-2 Unsaturated and linear polyesters; intermediates; EHC
A-bis-(2-hydroxyethylether) epoxy thermoset resins: polyurethanes. 172
Reactive flame retardant M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol 4162-45-2 Unsaturated and linear polyesters; intermediates; EHC
A-bis-(2-hydroxyethylether) epoxy thermoset resins: polyurethanes. 172
Reactive flame retardant M
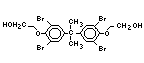 Tetrabromobisphenol 25327-89-3 EPS, foamed polystyrene M EHC
A-bis-(allylether) 172
Tetrabromobisphenol 25327-89-3 EPS, foamed polystyrene M EHC
A-bis-(allylether) 172
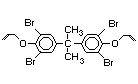 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol 37853-61-5 Expandable polystyrene L EHC
A-dimethylether 172
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol 37853-61-5 Expandable polystyrene L EHC
A-dimethylether 172
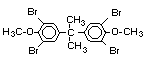 Tetrabromobisphenol 32844-27-2 Reactive and active flame retardants; EHC
A diglycidyl-ether epoxy 71342-77-3 polyethylenes, polypropylenes, polystyrenes, 172
oligomer carbonate oligomer ABS, polyamides, linear polyester,
polycarbonate, epoxide resins, unsaturated
polyester, phenolic resins H
Tetrabromobisphenol S 39635-79-5 Intermediate for flame-retardant production L
Tetrabromobisphenol 32844-27-2 Reactive and active flame retardants; EHC
A diglycidyl-ether epoxy 71342-77-3 polyethylenes, polypropylenes, polystyrenes, 172
oligomer carbonate oligomer ABS, polyamides, linear polyester,
polycarbonate, epoxide resins, unsaturated
polyester, phenolic resins H
Tetrabromobisphenol S 39635-79-5 Intermediate for flame-retardant production L
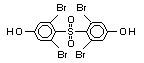 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ethylene-bistetrabromophthalimide Polyethylene, polypropylene M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ethylene-bistetrabromophthalimide Polyethylene, polypropylene M
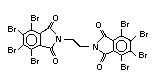 Dibromoneopentylglycol CH2OH 3296-90-0 Unsaturated polyesters; rigid polyurethane
(1,3-propanediol, BrH2C--|--CH2Br foams; intermediates; elastomers H
2,2-bis(bromomethyl)) CH2OH
Tribromoneopentylalcohol CH2Br 36483-57-5 Substantially used as reactive flame retardant
BrH2C--|--CH2OH Rigid and flexible polyurethane foam;
CH2Br intermediates for flame retardants M
Vinylbromide H2C=CHBr 593-60-2 Monomeric reactive flame retardant. EHC
Modacrylic fibers M IARC
(1986a)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tribromophenyl allylether 3278-89-5 (EPS) Expandable polystyrene L
Dibromoneopentylglycol CH2OH 3296-90-0 Unsaturated polyesters; rigid polyurethane
(1,3-propanediol, BrH2C--|--CH2Br foams; intermediates; elastomers H
2,2-bis(bromomethyl)) CH2OH
Tribromoneopentylalcohol CH2Br 36483-57-5 Substantially used as reactive flame retardant
BrH2C--|--CH2OH Rigid and flexible polyurethane foam;
CH2Br intermediates for flame retardants M
Vinylbromide H2C=CHBr 593-60-2 Monomeric reactive flame retardant. EHC
Modacrylic fibers M IARC
(1986a)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tribromophenyl allylether 3278-89-5 (EPS) Expandable polystyrene L
 (Poly)pentabromobenzyl acrylate 59447-55-1 Polyamide; PBT; PET; ABS; polypropylene;
(polymer) Polystyrene and others - polyamides,
59447-57-3 polyesters, polycarbonates M
(Poly)pentabromobenzyl acrylate 59447-55-1 Polyamide; PBT; PET; ABS; polypropylene;
(polymer) Polystyrene and others - polyamides,
59447-57-3 polyesters, polycarbonates M
 Pentabromotoluene 87-83-2 Unsaturated polyesters; polyethylene;
polypropylenes; polystyrene; SBR-latex,
textiles, rubbers. ABS M
Pentabromotoluene 87-83-2 Unsaturated polyesters; polyethylene;
polypropylenes; polystyrene; SBR-latex,
textiles, rubbers. ABS M
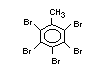 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,3-Dibromo-2-butene-1,4-diol 3234-02-4 Intermediate for the production of flame
retardants M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,3-Dibromo-2-butene-1,4-diol 3234-02-4 Intermediate for the production of flame
retardants M
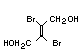 (Poly)bromophenols: 615-58-7 Epoxy resins; phenolic resins; intermediates
2,4-Dibromophenol 118-79-6 polyester resins; polyolefins H
2,4,6-Tribromophenol 608-71-9
Pentabromophenol
(Poly)bromophenols: 615-58-7 Epoxy resins; phenolic resins; intermediates
2,4-Dibromophenol 118-79-6 polyester resins; polyolefins H
2,4,6-Tribromophenol 608-71-9
Pentabromophenol
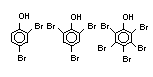 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Bis(2,4,6- 37853-59-1 Additive flame retardant for thermoplastics
tribromophenoxy)ethane ABS polymer systems. High impact polystyrene L
1,1-(1,2-ethanediylbis(oxy),
bis 2,4,6-tribromo-benzene
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Bis(2,4,6- 37853-59-1 Additive flame retardant for thermoplastics
tribromophenoxy)ethane ABS polymer systems. High impact polystyrene L
1,1-(1,2-ethanediylbis(oxy),
bis 2,4,6-tribromo-benzene
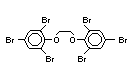 Tetrabromophthalic acid Na salt 25357-79-3 Additive flame retardant. Unsaturated
polyesters and rigid polyurethane foams.
Reactive intermediates for polyols: esters;
imides; paper; textiles; epoxides L
Tetrabromophthalic acid Na salt 25357-79-3 Additive flame retardant. Unsaturated
polyesters and rigid polyurethane foams.
Reactive intermediates for polyols: esters;
imides; paper; textiles; epoxides L
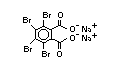 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromophthalic acid diol 20566-35-2 Wool, leather and polyurethane foams M
[2-Hydroxypropyl-oxy-2-
(2-hydroxyethyl)-
ethyltetrabromophthalate]
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromophthalic acid diol 20566-35-2 Wool, leather and polyurethane foams M
[2-Hydroxypropyl-oxy-2-
(2-hydroxyethyl)-
ethyltetrabromophthalate]
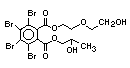 Tetrabromophthalic anhydride 632-79-1 Reactive flame retardant. Unsaturated poly
esters and rigid polyurethane foams. Reactive
intermediates for polyols; esters; imides;
paper; textiles; epoxides H
Tetrabromophthalic anhydride 632-79-1 Reactive flame retardant. Unsaturated poly
esters and rigid polyurethane foams. Reactive
intermediates for polyols; esters; imides;
paper; textiles; epoxides H
 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
N,N'-Ethylene-bis-(tetrabromophthalimide) 32588-76-4 High impact polystyrene; polyethylene;
polypropylene; thermoplastic polyesters;
polyamide; EPDM; rubbers; polycarbonate;
ethylene co-polymers; ionomer resins;
textiles M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
N,N'-Ethylene-bis-(tetrabromophthalimide) 32588-76-4 High impact polystyrene; polyethylene;
polypropylene; thermoplastic polyesters;
polyamide; EPDM; rubbers; polycarbonate;
ethylene co-polymers; ionomer resins;
textiles M
 1,3-Butadiene homopolymer brominated 68441-46-3 Elastomers L
Bis(tribromophenoxy)ethane Polystyrene, polycarbonate, coatings M
1,3-Butadiene homopolymer brominated 68441-46-3 Elastomers L
Bis(tribromophenoxy)ethane Polystyrene, polycarbonate, coatings M
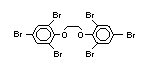 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetradecabromodi 58965-66-5 Engineering thermoplastics M
phenoxybenzene
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetradecabromodi 58965-66-5 Engineering thermoplastics M
phenoxybenzene
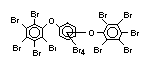 Poly(2,6-dibromophenylene oxide) 69882-11-7 For crystalline polymer polyamide, thermoplastic
polyester resins, polystyrenes, polyamides,
polycarbonate, ABS M
Poly(2,6-dibromophenylene oxide) 69882-11-7 For crystalline polymer polyamide, thermoplastic
polyester resins, polystyrenes, polyamides,
polycarbonate, ABS M
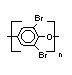 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Poly-tribromostyrene 57137-10-7 Polyethylene, linear polyester, epoxide resins,
Brominated polystyrene unsaturated polyester resin, polyamides, ABS M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Poly-tribromostyrene 57137-10-7 Polyethylene, linear polyester, epoxide resins,
Brominated polystyrene unsaturated polyester resin, polyamides, ABS M
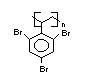 Polydibromostyrene 31780-26-4 Styrenic polymers, engineering plastics M
Polydibromostyrene 31780-26-4 Styrenic polymers, engineering plastics M
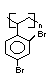 Hexabromocyclododecane 25637-99-4 Expandable polystyrene; latex; textiles; adhesives;
(1,2,5,6,9,10-HBCD) also coatings; foamed and high-impact polysytrene;
3194-55-6 unsaturated polyesters H
Hexabromocyclododecane 25637-99-4 Expandable polystyrene; latex; textiles; adhesives;
(1,2,5,6,9,10-HBCD) also coatings; foamed and high-impact polysytrene;
3194-55-6 unsaturated polyesters H
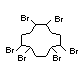 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Dibromo-4(1,2 dibromomethyl) 3322-93-8 Expandable polystyrene L
cyclohexane
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Dibromo-4(1,2 dibromomethyl) 3322-93-8 Expandable polystyrene L
cyclohexane
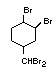 Ethylene-bis(5,6-dibromonorbornane-2, 41291-34-3 Polypropylene M
3-dicarboximide 52907-07-0
Ethylene-bis(5,6-dibromonorbornane-2, 41291-34-3 Polypropylene M
3-dicarboximide 52907-07-0
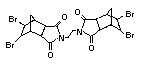 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibromostyrene grafted PP 171091-06-8 Polyolefins
1,3,5-tris(2,3-dibromo-propoxy)- 52434-59-0 Polypropylene L
2,4,6-triazine
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibromostyrene grafted PP 171091-06-8 Polyolefins
1,3,5-tris(2,3-dibromo-propoxy)- 52434-59-0 Polypropylene L
2,4,6-triazine
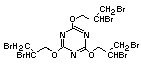 Diester of tetrabromophthalic acid 20566-35-2 PVC, rubber, thermoplastics, PUR, coatings H
Diester of tetrabromophthalic acid 20566-35-2 PVC, rubber, thermoplastics, PUR, coatings H
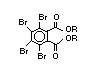 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated flame retardants
Chlorinated paraffins CxH(2x+2-y) Cly 63449-39-8 High and low density polyethylene; high EHC 181
(at least 20 impact polystyrene; PVC, unsaturated polyester IARC
other CAS resins; polypropylene, rubber, textiles H (1990)
numbers)
Chlorendic acid 115-28-6 Reactive flame retardant for polyester resins, EHC
alkyl paints M 185
IARC
(1990)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated flame retardants
Chlorinated paraffins CxH(2x+2-y) Cly 63449-39-8 High and low density polyethylene; high EHC 181
(at least 20 impact polystyrene; PVC, unsaturated polyester IARC
other CAS resins; polypropylene, rubber, textiles H (1990)
numbers)
Chlorendic acid 115-28-6 Reactive flame retardant for polyester resins, EHC
alkyl paints M 185
IARC
(1990)
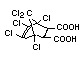 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorendic anhydride 115-17-5 Reactive flame retardant, used as flame EHC
retardant for unsaturated polyester, 185
epoxides, alkyl paints, epoxy hardener M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorendic anhydride 115-17-5 Reactive flame retardant, used as flame EHC
retardant for unsaturated polyester, 185
epoxides, alkyl paints, epoxy hardener M
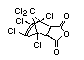 Dodecachlorodimethano- 13560-89-9 Polyamides, polystyrene M
dibenzocyclo-octane
Dodecachlorodimethano- 13560-89-9 Polyamides, polystyrene M
dibenzocyclo-octane
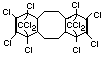 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopentadiene 77-47-4 Intermediate for production of flame EHC
retardants H 120
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopentadiene 77-47-4 Intermediate for production of flame EHC
retardants H 120
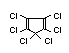 Tetrachlorophthalic anhydride - TCPA 117-08-8 Unsaturated polyester resins. Alkyds. M
Tetrachlorophthalic anhydride - TCPA 117-08-8 Unsaturated polyester resins. Alkyds. M
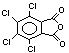 Bromo-chlorinated paraffins CxH(2x+2-y-z) BryClz 61090-89-9 Textile fabrics, PVC, Polyurethane L
68527-01-5
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,2',6,6-Tetrachlorobisphenol A 79-95-8 Expoxy intermediate L
Bromo-chlorinated paraffins CxH(2x+2-y-z) BryClz 61090-89-9 Textile fabrics, PVC, Polyurethane L
68527-01-5
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,2',6,6-Tetrachlorobisphenol A 79-95-8 Expoxy intermediate L
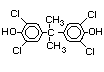 Tetrachlorophthalic anhydride 117-08-8 Intermediate, unsaturated polyesters, alkyds M
Tetrachlorophthalic anhydride 117-08-8 Intermediate, unsaturated polyesters, alkyds M
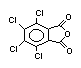 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Organophosphorus flame retardants
Dimethylphosphono- 20120-33-6 Cotton; cotton/polyester; rayon H
N-hydroxymethyl-3-propionamide
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Organophosphorus flame retardants
Dimethylphosphono- 20120-33-6 Cotton; cotton/polyester; rayon H
N-hydroxymethyl-3-propionamide
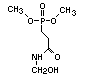 Tris(2-butoxyethyl) phosphate 78-51-3 Additive flame retardant and EHC
plasticizer in plastics and synthetic
rubbers M
Tris(2-butoxyethyl) phosphate 78-51-3 Additive flame retardant and EHC
plasticizer in plastics and synthetic
rubbers M
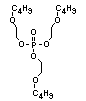 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isopropylphenyl diphenyl phosphate 68937-41-7 Plasticizer; hydraulic fluid; lubricant
and in engineering thermoplastics H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isopropylphenyl diphenyl phosphate 68937-41-7 Plasticizer; hydraulic fluid; lubricant
and in engineering thermoplastics H
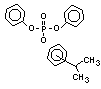 Tricresyl phosphate 1330-78-5 Solvent; additive for pressure lubricants EHC
and hydraulic systems, cutting oils, 110
transmission fluids, PVC H
Tricresyl phosphate 1330-78-5 Solvent; additive for pressure lubricants EHC
and hydraulic systems, cutting oils, 110
transmission fluids, PVC H
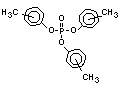 Triphenylphosphate 115-86-6 PVC, phenolics resins; phenylene-oxide-based EHC
resins H 111
Triphenylphosphate 115-86-6 PVC, phenolics resins; phenylene-oxide-based EHC
resins H 111
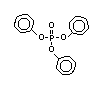 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl-methyl- O 756-79-6 Unsaturated polyesters; paints and coatings:
phosphonate (DMMP) H3C " CH3 urethane rigid foam M
\ /
O-P--O
|
CH3
Resorcinol 57583-54-7 Engeneering thermoplastics
dipheny-lphosphate H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl-methyl- O 756-79-6 Unsaturated polyesters; paints and coatings:
phosphonate (DMMP) H3C " CH3 urethane rigid foam M
\ /
O-P--O
|
CH3
Resorcinol 57583-54-7 Engeneering thermoplastics
dipheny-lphosphate H
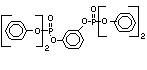 Diethyl-ethyl- H5C2 O C2H5 78-38-6 Unsaturated polyesters; paints and coatings:
phosphonate \ " / urethane rigid foam L
(DEEP) O-P--O
|
C2H5
Cyclic phosphonate ester 61840-22-0 Polyester fibres; rigid urethane foams L
(including:
42595-45-9
41203-81-0
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isodecyldiphenyl phosphate 29761-21-5 PVC
H
Diethyl-ethyl- H5C2 O C2H5 78-38-6 Unsaturated polyesters; paints and coatings:
phosphonate \ " / urethane rigid foam L
(DEEP) O-P--O
|
C2H5
Cyclic phosphonate ester 61840-22-0 Polyester fibres; rigid urethane foams L
(including:
42595-45-9
41203-81-0
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isodecyldiphenyl phosphate 29761-21-5 PVC
H
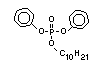 O,O-Diethyl-N,N- 2781-11-5 Polyurethane foam textiles L
bis(2-hydroxyethyl)
aminomethyl
phosphonate
O,O-Diethyl-N,N- 2781-11-5 Polyurethane foam textiles L
bis(2-hydroxyethyl)
aminomethyl
phosphonate
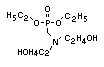 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl- Cellulosic fabrics
3-(hydroxymethylamino)-3- H
oxopropyl phosphonate
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl- Cellulosic fabrics
3-(hydroxymethylamino)-3- H
oxopropyl phosphonate
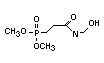 Dimethyl phosphonate H3C O CH3 868-85-9 Flame retardant to cotton textile IARC
\ " / and polyamide paints (1990)
O-P--O
|
H
Cresyl diphenyl phosphate 26444-49-5 PVC, hydraulic fluid, lubricant, food
packaging, ABS pc-blends, engineering
thermoplastics, rubber phenolics, paints
H
Dimethyl phosphonate H3C O CH3 868-85-9 Flame retardant to cotton textile IARC
\ " / and polyamide paints (1990)
O-P--O
|
H
Cresyl diphenyl phosphate 26444-49-5 PVC, hydraulic fluid, lubricant, food
packaging, ABS pc-blends, engineering
thermoplastics, rubber phenolics, paints
H
 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Octyl diphenyl phosphate 115-88-8 PVC, rubber, paints, coatings
H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Octyl diphenyl phosphate 115-88-8 PVC, rubber, paints, coatings
H
 Tris(2-ethyl hexyl) phosphate 78-42-2 PVC, solvents, rubber; paints, polyurethane EHC
M
Tris(2-ethyl hexyl) phosphate 78-42-2 PVC, solvents, rubber; paints, polyurethane EHC
M
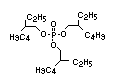 Trioctyl phosphate H17C8 O C8H17 1806-54-8 PVC, solvent paints, rubber, polyurethane
\ " /
O-P--O
|
O
\
C8H17
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Triethyl phosphate H5C2 O C2H5 78-40-0 PVC, polyester resins, polyurethane
\ " / M
O-P--O
|
O
\
C2H5
2-Ethylhexyldiphenyl phosphate 1241-94-7 Plasticizer in food packaging, hydraulic
fluid, PVC H
Trioctyl phosphate H17C8 O C8H17 1806-54-8 PVC, solvent paints, rubber, polyurethane
\ " /
O-P--O
|
O
\
C8H17
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Triethyl phosphate H5C2 O C2H5 78-40-0 PVC, polyester resins, polyurethane
\ " / M
O-P--O
|
O
\
C2H5
2-Ethylhexyldiphenyl phosphate 1241-94-7 Plasticizer in food packaging, hydraulic
fluid, PVC H
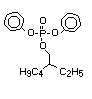 CH2OH
Tetrakis (hydroxymethyl)- | Reactive flame retardant for cotton; EHC
phosphonium HOH2C-P+--CH2OH X- rayon and other cellulosic materials as IARC
salts (THP salts): | well as polyester fabrics (1990)
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Acetate 7580-37-2 Chloride H
Acetate-phosphate (3:1) 55818-96-7 Sulfate H
Acetate phosphate (1:1) 62588-94-7 Others L
Bromide 5940-69-2
6-Carboxycellulose salt 73082-49-2
Cellulose carboxymethyl ether 73083-23-5
Chloride 124-64-1
Ethanedioate 52221-67-7
Formate 25151-36-4
Hydroxybutanedioate 39734-92-4
2-Hydroxypropionate 39686-78-7
Iodide 69248-12-0
1-Naphthalenesulfonate 79481-21-3
2-Naphthalenesulfonate 79481-22-4
Oxalate (1:1) 53211-22-6
Oxalate (2:1) 52221-67-7
Phosphate 22031-17-0
Sulfate 55566-30-8
Tetraphenylborate- 15652-65-0
tetraacetate
p-Toluenesulfonate 75019-90-8
n addition the following
complex condensates
are used:
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
- Tetrakis
(hydroxymethyl)
phosphonium
sulfate-urea
precondensate
- Tetrakis(hydroxymethyl)-
phosphonium chloride-urea-
melamine condensate
- Tetrakis(hydroxymethyl)-
phosphonium sulfate-urea-
N-hydroxymethyl-dimethyl-
phosphonopropionamide
precondensate
Phosphonic acid 4351-70-6 Polyurethane foam
derivative M
Bis(5,5-dimethyl-2- 4090-51-1 Rayon
thiono-1,3,2-
dioxaphosphorinamyl)
oxide
O
Tris(hydroxymethyl) " High impact polystyrene
phosphine oxide HOH2C-P--CH2OH
"
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trixylenyl phosphate 25155-23-1 PVC, hydraulic fluids
M
CH2OH
Tetrakis (hydroxymethyl)- | Reactive flame retardant for cotton; EHC
phosphonium HOH2C-P+--CH2OH X- rayon and other cellulosic materials as IARC
salts (THP salts): | well as polyester fabrics (1990)
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Acetate 7580-37-2 Chloride H
Acetate-phosphate (3:1) 55818-96-7 Sulfate H
Acetate phosphate (1:1) 62588-94-7 Others L
Bromide 5940-69-2
6-Carboxycellulose salt 73082-49-2
Cellulose carboxymethyl ether 73083-23-5
Chloride 124-64-1
Ethanedioate 52221-67-7
Formate 25151-36-4
Hydroxybutanedioate 39734-92-4
2-Hydroxypropionate 39686-78-7
Iodide 69248-12-0
1-Naphthalenesulfonate 79481-21-3
2-Naphthalenesulfonate 79481-22-4
Oxalate (1:1) 53211-22-6
Oxalate (2:1) 52221-67-7
Phosphate 22031-17-0
Sulfate 55566-30-8
Tetraphenylborate- 15652-65-0
tetraacetate
p-Toluenesulfonate 75019-90-8
n addition the following
complex condensates
are used:
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
- Tetrakis
(hydroxymethyl)
phosphonium
sulfate-urea
precondensate
- Tetrakis(hydroxymethyl)-
phosphonium chloride-urea-
melamine condensate
- Tetrakis(hydroxymethyl)-
phosphonium sulfate-urea-
N-hydroxymethyl-dimethyl-
phosphonopropionamide
precondensate
Phosphonic acid 4351-70-6 Polyurethane foam
derivative M
Bis(5,5-dimethyl-2- 4090-51-1 Rayon
thiono-1,3,2-
dioxaphosphorinamyl)
oxide
O
Tris(hydroxymethyl) " High impact polystyrene
phosphine oxide HOH2C-P--CH2OH
"
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trixylenyl phosphate 25155-23-1 PVC, hydraulic fluids
M
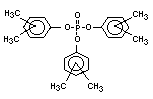 Tris(isopropy-lphenyl) 68937-41-7 PVC, engineering
phosphate thermoplastics H
Tris(isopropy-lphenyl) 68937-41-7 PVC, engineering
phosphate thermoplastics H
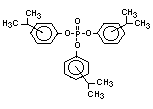 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Halogenated organophosphorus flame retardants
Tris(1,3-dichloro- 13674-87-8 Additive flame retardant in polyurethane EHC
2-propyl) phosphate and styrene-butadiene rubber; synthetic
fibres H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Halogenated organophosphorus flame retardants
Tris(1,3-dichloro- 13674-87-8 Additive flame retardant in polyurethane EHC
2-propyl) phosphate and styrene-butadiene rubber; synthetic
fibres H
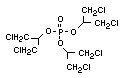 Tris(2-chloroethyl) 115-96-8 Polyester resins, polyurethanes, cellulose EHC
phosphate derivatives, PVC H IARC
(1990)
Tris(2-chloroethyl) 115-96-8 Polyester resins, polyurethanes, cellulose EHC
phosphate derivatives, PVC H IARC
(1990)
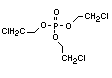 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2-chloroethyl) 28205-79-0 Polyurethane EHC
phosphate polymer M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2-chloroethyl) 28205-79-0 Polyurethane EHC
phosphate polymer M
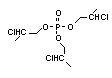 Tris(2-chloro-1-propyl) 6145-73-9 Polyurethane foam EHC
phosphate H
Tris(2-chloro-1-propyl) 6145-73-9 Polyurethane foam EHC
phosphate H
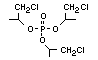 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-chloro-2-propyl) 13674-84-5 Polyurethane foam, polyesters foams EHC
phosphate H
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-chloro-2-propyl) 13674-84-5 Polyurethane foam, polyesters foams EHC
phosphate H
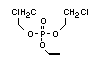 Bis(2-chloroethyl) vinyl 115-98-0 Cotton; rayon, polyolefins;
phosphate intermediate
L
Mixture of monomeric 64176-42-7 Textiles
chloroethyl
phosphonates and
high-boiling
phosphonates
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4-Dibromophenyl 49690-63-3 Engineering thermoplastics
phosphate L
Bis(2-chloroethyl) vinyl 115-98-0 Cotton; rayon, polyolefins;
phosphate intermediate
L
Mixture of monomeric 64176-42-7 Textiles
chloroethyl
phosphonates and
high-boiling
phosphonates
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4-Dibromophenyl 49690-63-3 Engineering thermoplastics
phosphate L
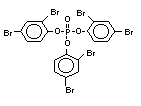 Tris(tribromoneopentyl) Thermoplastics
phosphate L
Tris(tribromoneopentyl) Thermoplastics
phosphate L
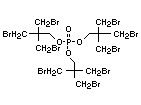 Chlorinated brominated 35-37% Br, 8-9% 125997-20-8 Polyurethane foams,
phosphate ester Cl, 6-8% P thermosets, coating
(Firemaster 836) and M
(HP-36)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bromine-, chlorine and Polyurethane foams
phosphorus-containing H
polyol
Nitrogen-based and miscellaneous flame retardants
Melamine Polyurethane foams
H
Chlorinated brominated 35-37% Br, 8-9% 125997-20-8 Polyurethane foams,
phosphate ester Cl, 6-8% P thermosets, coating
(Firemaster 836) and M
(HP-36)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bromine-, chlorine and Polyurethane foams
phosphorus-containing H
polyol
Nitrogen-based and miscellaneous flame retardants
Melamine Polyurethane foams
H
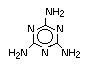 Melamine phosphate Polypropylene
L
Melamine cyanurate 37640-57-6 Polyamides, polyurethanes,
polyolefines, polyester,
epoxy resins
M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ferrocene 102-54-5 Additive smoke
suppressant
L
Melamine phosphate Polypropylene
L
Melamine cyanurate 37640-57-6 Polyamides, polyurethanes,
polyolefines, polyester,
epoxy resins
M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ferrocene 102-54-5 Additive smoke
suppressant
L
 Table 10. Flame retardants that have been used commercially in the past
Chemical name Chemical structure CAS registry Use as flame retardant Remarks
number
Inorganic flame retardants
Sodium stannate Na2SnO3 12058-66-1 Textiles
Sodium aluminate NaAlO2 1302-42-7 Textiles
Sodium silicate Na2SiO3Ê9H2O 1344-09-8 Textiles
Sodium bisulfate NaHSO4Ê2O 7631-90-5 Textiles
Ammonium borate NH4BO3 12007-58-8 Textiles
Ammonium iodide NH4I 12027-06-4 Textiles
Zinc chloride ZnCl2 7646-85-7 Non-durable finish, textiles
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Calcium chloride CaCl2Ê6H2O 10043-52-4 Non-durable finish, textiles
Magnesium chloride MgCl2 7786-30-3 Non-durable finish, textiles
Brominated flame retardants
Dibromopropylacrylate 19660-16-3 Acrylic fibres
Table 10. Flame retardants that have been used commercially in the past
Chemical name Chemical structure CAS registry Use as flame retardant Remarks
number
Inorganic flame retardants
Sodium stannate Na2SnO3 12058-66-1 Textiles
Sodium aluminate NaAlO2 1302-42-7 Textiles
Sodium silicate Na2SiO3Ê9H2O 1344-09-8 Textiles
Sodium bisulfate NaHSO4Ê2O 7631-90-5 Textiles
Ammonium borate NH4BO3 12007-58-8 Textiles
Ammonium iodide NH4I 12027-06-4 Textiles
Zinc chloride ZnCl2 7646-85-7 Non-durable finish, textiles
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Calcium chloride CaCl2Ê6H2O 10043-52-4 Non-durable finish, textiles
Magnesium chloride MgCl2 7786-30-3 Non-durable finish, textiles
Brominated flame retardants
Dibromopropylacrylate 19660-16-3 Acrylic fibres
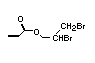 Tetrabromodipenta-erythritol 109678-33-3 Polyester Polyurethane
Tetrabromodipenta-erythritol 109678-33-3 Polyester Polyurethane
 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Pentabromoethylbenzene 85-22-3 Textiles; adhesives; polyurethane foam.
Thermoset polyester resins, coatings.
Additive for unsaturated polyesters
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Pentabromoethylbenzene 85-22-3 Textiles; adhesives; polyurethane foam.
Thermoset polyester resins, coatings.
Additive for unsaturated polyesters
 Tetrabromoxylene 23488-38-2 Additive for styrene thermoplastics
and polyolefines and textiles
Tetrabromoxylene 23488-38-2 Additive for styrene thermoplastics
and polyolefines and textiles
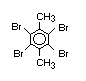 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromophenoxy- 35109-60-5 Extrusion grade of PP
2,3-dibromopropane
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromophenoxy- 35109-60-5 Extrusion grade of PP
2,3-dibromopropane
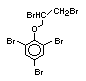 Hexabromobenzene 87-82-1 Paper, electric goods, polyamides, PES
fibres, PP and PBT
Hexabromobenzene 87-82-1 Paper, electric goods, polyamides, PES
fibres, PP and PBT
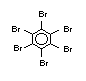 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexabromobiphenyl 59536-65-1 Thermoplastic polymers EHC 152
67774-32-7 IARC
(1986b)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexabromobiphenyl 59536-65-1 Thermoplastic polymers EHC 152
67774-32-7 IARC
(1986b)
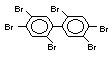 Octabromobiphenyl 61288-13-9 Thermoplastic polymers EHC 152
Octabromobiphenyl 61288-13-9 Thermoplastic polymers EHC 152
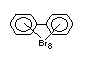 Hexabromodiphenyl ether 61262-53-1 Variety of resins (high thermal stability). EHC
36483-60-0 Polystyrene, ABS polycarbonate, unsaturated 162
polyester
Hexabromodiphenyl ether 61262-53-1 Variety of resins (high thermal stability). EHC
36483-60-0 Polystyrene, ABS polycarbonate, unsaturated 162
polyester
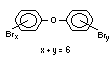 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol A-bis- 66710-97-2 High thermal stability
(2-ethylether acrylate)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol A-bis- 66710-97-2 High thermal stability
(2-ethylether acrylate)
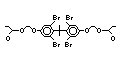 Pentabromochlorocyclo-hexane 87-84-3 Polystyrene foam and polypropylene
Pentabromochlorocyclo-hexane 87-84-3 Polystyrene foam and polypropylene
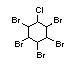 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,3-dibromopropyl) phosphate 126-72-7 Polyesters, urea and melamine resins, textiles EHC 173
IARC
(1979,
1987)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,3-dibromopropyl) phosphate 126-72-7 Polyesters, urea and melamine resins, textiles EHC 173
IARC
(1979,
1987)
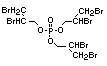 Bis(2,3-dibromopropyl) phosphate 66519-18-4 K salt EHC 173
and salts 64864-08-0 Na salt
36711-31-6 Mg salt
5412-25-9 H (base)
34432-82-1 Ammonium salt
Bis(2,3-dibromopropyl) phosphate 66519-18-4 K salt EHC 173
and salts 64864-08-0 Na salt
36711-31-6 Mg salt
5412-25-9 H (base)
34432-82-1 Ammonium salt
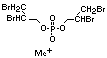 Tetrabromo-2,3-dimethylbutane 00-00-0 EPS
Tetrabromo-2,3-dimethylbutane 00-00-0 EPS
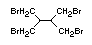 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromoaniline 147-82-0 Reactive flame retardant
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromoaniline 147-82-0 Reactive flame retardant
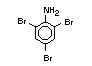 1-Pentabromophenoxy-2-propene 3555-11-1 Synergist
1-Pentabromophenoxy-2-propene 3555-11-1 Synergist
 2,4-dibromophenylglycidyl ether 20217-01-0 Reactive flame retardant
2,4-dibromophenylglycidyl ether 20217-01-0 Reactive flame retardant
 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trichloromethyl ABS, polystyrene, polyester
tetrabromobenzene
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trichloromethyl ABS, polystyrene, polyester
tetrabromobenzene
 Pentabromophenyl
benzoate ABS, polyester, polystyrene
Pentabromophenyl
benzoate ABS, polyester, polystyrene
 1,4-Bis(bromomethyl)-tetrabromo Polyolefines
benzene
1,4-Bis(bromomethyl)-tetrabromo Polyolefines
benzene
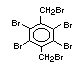 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bis-(2,3-dibromo-1-propyl) 7415-86-3 Polyesters, alkyl
phthalate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bis-(2,3-dibromo-1-propyl) 7415-86-3 Polyesters, alkyl
phthalate
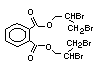 Hexabromocyclohexane 1837-91-8 Styrene foams
Hexabromocyclohexane 1837-91-8 Styrene foams
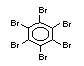 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
5,6-dibromohexahydro-2-phenyl- 40703-79-5 Styrenic polymers
4,7-methano-1H-isoindole-
1,3(2H)-dione
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
5,6-dibromohexahydro-2-phenyl- 40703-79-5 Styrenic polymers
4,7-methano-1H-isoindole-
1,3(2H)-dione
 Chlorinated flame retardants
Dimethyl chlorendate Reactive flame
retardant for polyester resins,
alkyl paints
Chlorinated flame retardants
Dimethyl chlorendate Reactive flame
retardant for polyester resins,
alkyl paints
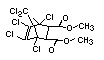 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibutyl chlorendate 1770-80-5 Reactive flame
retardant for polyester
resins, alkyl paints
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibutyl chlorendate 1770-80-5 Reactive flame
retardant for polyester
resins, alkyl paints
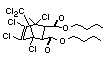 1,2,3,4,6,7,8,9.10,10,11, 31107-44-5 Cross-linked polyethylene, polyolefins,
11-Dodecachloro-1,4,4a,5a,6, polystyrene
9,9a,9b-octahydro-1,4:6,9-
dimethanodibenzofuran
1,2,3,4,6,7,8,9.10,10,11, 31107-44-5 Cross-linked polyethylene, polyolefins,
11-Dodecachloro-1,4,4a,5a,6, polystyrene
9,9a,9b-octahydro-1,4:6,9-
dimethanodibenzofuran
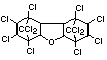 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,1a,2,2,3,3a,4,5,5,5a,5b, 2385-85-5 Plastics, rubber, paints, paper, and electrical EHC
6-Dodecachloro- goods No. 44
octahydro- (Mirex)
1,3,4-metheno-1H- IARC
cyclobuta(cd)pentalene (1979)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,1a,2,2,3,3a,4,5,5,5a,5b, 2385-85-5 Plastics, rubber, paints, paper, and electrical EHC
6-Dodecachloro- goods No. 44
octahydro- (Mirex)
1,3,4-metheno-1H- IARC
cyclobuta(cd)pentalene (1979)
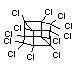 Polychlorinated biphenyls 1336-36-3 Fire-resistant liquid in closed-system EHC
hydraulic fluids polystyrene, polyolefines No. 140
IARC
(1978,
1987)
Polychlorinated biphenyls 1336-36-3 Fire-resistant liquid in closed-system EHC
hydraulic fluids polystyrene, polyolefines No. 140
IARC
(1978,
1987)
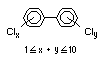 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopenta- 51936-55-1 Styrenic polymers
dienyl-dibromocyclooctane
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopenta- 51936-55-1 Styrenic polymers
dienyl-dibromocyclooctane
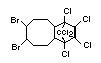 Dibromochlordene 18300-04-4 Styrenic polymers
[4,7-Methano-1H-indene,
1,2-dibromo-4,5,6,7,8,8-
hexachloro-2,3,3a,4,7,7a-
hexahydro]
Dibromochlordene 18300-04-4 Styrenic polymers
[4,7-Methano-1H-indene,
1,2-dibromo-4,5,6,7,8,8-
hexachloro-2,3,3a,4,7,7a-
hexahydro]
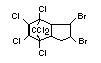 Organophosphorus flame retardants
Trimethylphosphoramide Cotton, rayon
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-aziridinyl)phosphine 545-55-1 Cotton fabrics, polyester fibres IARC (1975,
oxide 1987)
Organophosphorus flame retardants
Trimethylphosphoramide Cotton, rayon
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-aziridinyl)phosphine 545-55-1 Cotton fabrics, polyester fibres IARC (1975,
oxide 1987)
 Cyanamide-phosphoric acid H2NCN + H3PO4f Finishes
Halogenated organophosphorus flame retardants
Ethylene bis[tris 10310-38-0 Additive for thermoplastics, textiles
(2-yanoethyl)phosphonium]
bromide
Cyanamide-phosphoric acid H2NCN + H3PO4f Finishes
Halogenated organophosphorus flame retardants
Ethylene bis[tris 10310-38-0 Additive for thermoplastics, textiles
(2-yanoethyl)phosphonium]
bromide
 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrakis(2-chloroethyl) 33125-86-9 Plastics
ethylene diphosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrakis(2-chloroethyl) 33125-86-9 Plastics
ethylene diphosphate
 Tris(2,3-dichloro-1-propyl) 78-43-3 Additive flame retardant in plastics, plasticizer EHC
phosphate
Tris(2,3-dichloro-1-propyl) 78-43-3 Additive flame retardant in plastics, plasticizer EHC
phosphate
 Condensate of bis Cellulosic textiles, cotton and rayon
(betachloroethyl)
phosphonate and alkyl
phosphonate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,4,6-tribromophenyl) Engineering thermoplastics
phosphate
Condensate of bis Cellulosic textiles, cotton and rayon
(betachloroethyl)
phosphonate and alkyl
phosphonate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,4,6-tribromophenyl) Engineering thermoplastics
phosphate
 Bis(1,3-dichloro-2-propyl)- 61090-89-9 Polyolefins
(3-chloro-2,2-
dibromomethylpropyl) phosphate
Bis(1,3-dichloro-2-propyl)- 61090-89-9 Polyolefins
(3-chloro-2,2-
dibromomethylpropyl) phosphate
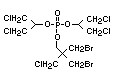 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated phosphonic acid Polyurethane foam
ester condensate with tris-
dibromo-propyl-iso-cyanurate
Tris(dichloropropyl) phosphite 6749-73-1 Textiles
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated phosphonic acid Polyurethane foam
ester condensate with tris-
dibromo-propyl-iso-cyanurate
Tris(dichloropropyl) phosphite 6749-73-1 Textiles
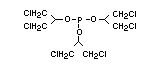 Bis[bis(2-chloroethoxy)-phosphinyl] Textiles
isopropylchloro-ethyl phosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris-(2-chloroethyl)- 140-08-9 Textiles, hydraulic fluids
phosphite
Bis[bis(2-chloroethoxy)-phosphinyl] Textiles
isopropylchloro-ethyl phosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris-(2-chloroethyl)- 140-08-9 Textiles, hydraulic fluids
phosphite
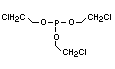 Ethylene-bis[bis 33125-86-9 Polyurethane foams
(2-chloroethyl)phosphate]
Ethylene-bis[bis 33125-86-9 Polyurethane foams
(2-chloroethyl)phosphate]
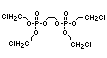 Nitrogen-based flame retardants
Pyrophosphate dimelamine salts 70776-17-9 Polyurethane, polyester
ANNEX III
Fire tests
Fire tests are usually carried out to comply with specific
regulations or voluntary agreements.
They can be classified into three groups (Troitzsch, 1990; Arias,
1992; OECD, 1994):
(a) Tests reflecting single events in a fire. For historical
reasons, many different national fire tests now exist,
particularly for building products. These may not necessarily
correlate well. Within the European Union, efforts are being
made to harmonize testing.
(b) Tests addressing flammability. These tests are mainly used
with respect to transportation and electrical/electronic
products. They are mostly used internationally.
(c) Screening tests. These are used to screen materials during
product development or for quality control.
ANNEX IV
US Interagency Testing Commission recommendations on
brominated flame retardants
Chemical
Designateda
1,2-Epoxy-3-bromopropane
2,4,6-Tribromoaniline
1,2-Dibromo-4-(1,2-dibromomethyl) cyclohexane
Pentabromoethylbenzene
Tetrabromobisphenol A
Decabromodiphenyl ether
Hexabromocyclododecane
Pentabromodiphenyl ether
Octabromodiphenyl ether
1,2-Bis(2,4,6-tribromophenoxy)ethane
Recommendedb
2,4,6-Tribromophenol
Tetrabromophthalic anhydride
Dibromoneopentyl glycol
Ethylene bis(tetrabromophthalimide)
Ethylene bis(tetrabromonorbornane-2,3-dicarboximide)
Tribrominated polystyrene
Ethylene bis(pentabromophenoxide)
Bromochloromethane
3,4',5-Tribromosalicylanilide
2,3,4,5,6-Pentabromotoluene
1,2,3,4,5-Pentabromo-6-chlorocyclohexane
2,3-Dibromopropanol
Vinyl bromide
2,4-Dibromophenol
Ethoxylated tetrabromobisphenol A
Tetrabromobisphenol A, bis(allyl ether)
Annex IV (contd).
Chemical
Recommended (contd).
Tetrabromodichlorocyclohexane
Tribromotrichlorocyclohexane
Tribromoneopentyl alcohol
Tetrabromobisphenol A diacrylate
Alkanes, C10-16, bromochloro
2,4-(or 2,6)-Dibromophenol, homopolymer
Benzene, ethenyl-, homopolymer, brominated
DeferredC
Polybromobiphenyl
Tetrabromo-o-chlorotoluene
Bromophenol (Br1, Br2, Br5)
Bis(dibromopropyl) carbamate
Bis(2,3-dibromopropyl) phosphite
Bis(dibromopropyl) phosphite
Tetrabromochlorotoluene
Brominated terphenyls
Dibromopropyl carbamate
Allyl bis(2,3-dibromopropyl) phosphite
Bis(dibromopropyl) phosphoryl chloride
2,3-Dibromo-1-propanol phosphate
Pentabromophenol
3,3-Bis(bromomethyl)oxetane
(bromomethyl)oxirane
pentabromophenyl allyl ether
2,6-Dibromo-4-[1-(3-bromo-4-hydroxyphenyl)-1-methylethyl]phenol
bis(2,3-dibromopropyl) phthalate
1,2-Ethanediylbis[tris(2-cyanoethyl)phosphonium] dibromide
(bromoethyl)oxirane
Decabromobiphenyl
Tetrabromophthalic acid, aluminium salt
Fumaric acid, bis(pentabromophenyl) ester
1,2-Dibromo-4,5,6,7,8,8-hexachloro-2,3,3a,4,7,7a-hexahydro-4,7
-methano-1H-indene
tetrabromophthalic acid, dipotassium salt
2-(2,4,6-Tribromophenoxy)ethanol
Bis(2,4,6-tribromophenyl) fumarate
Annex IV (contd).
Chemical
Deferred (contd)
Tribromophenol
Dibromoethane
Poly(2,6-dibromophenylene oxide)
Tribromophenyl allyl ether
Nonabromobiphenyl
Octabromobiphenyl
Bromophenol
3,3',5,5'-Tetrabromobisphenol A diacetate
3,3',5,5'-Tetrabromobisphenol S
Tris(dibromophenyl) phosphate
Dibromopropyl carbamate
2,3,4,6-Tetrabromo-5-methylphenol
2-(Pentabromophenoxy)ethanol
Dibromopropyl carbamate
3,9-Bis[3-bromo-2,2-bis(bromomethyl)propoxy]-
2,2-Bis(bromomethyl)-3-chloropropyl phosphoric acid
Tribromophenyl allyl ether
Decabrominated diphenoxyethane
2,4,6-Tribromophenol carbonate
pentabromophenol, aluminium salt
3,4,5,6-Tetrabromo-1,2-benzenedicarboxylic acid, magnesium salt
2-Butenedioic acid (z), bis(pentabromophenyl) ester
Pentabromo[2-(tetrabromophenoxy)ethoxy]benzene
Pentabromo[2-(tetrabromochlorophenoxy)ethoxy]benzene
1,3,5-Tribromo-2-(2-bromoethoxy)benzene
Brominated and chlorinated benzene
Bis[3-bromo-2-(bromomethyl)-2-(hydroxymethyl) propyl]hexanoate
Adapted from Walker (1994) and from a Memorandum dated 22 October 1992
entitled Actions on Brominated Flame Retardants; Toxic Substances
Control Act, Interagency Testing Committee (ITC), US Environmental
Protection Agency, Washington DC
a ITC designated chemical to US EPA for a decision about testing.
b ITC required additional information for further recommendation.
c Consideration for testing by ITC.
9. CONCLUSIONS ET RECOMMANDATIONS POUR LA PROTECTION DE LA SANTE
HUMAINE ET DE L'ENVIRONNEMENT
9.1 Conclusions
Les retardateurs de flamme sont un groupe de composés très divers
qu'on utilise pour retarder l'inflammation des polymères et autres
matériaux. On utilise une grande variété de composés, des substances
minérales aux molécules organiques complexes, comme retardateurs de
flamme, synergisants et inhibiteurs de fumée. Les considérations
générales qui suivent portent essentiellement sur des composés
organiques caractérisés par la présence d'halogènes ou de phosphore.
Il est difficile de se faire une idée exacte de l'utilisation des
retardateurs de flammes au niveau mondial, mais selon les estimations,
ce sont plus de 600 000 tonnes qui sont produites chaque année. Les
données dont on dispose indiquent qu'au cours de la dernière décennie,
les dérivés organiques bromés ont vu leur production s'accroître de
façon substantielle.
L'utilisation de retardateurs de flamme présente des avantages
évidents puisqu'ils permettent de sauver nombre de vies humaines et de
biens matériels. A l'heure actuelle, on ne connaît pas très bien les
effets à long terme pouvant résulter d'une exposition à ces composés
et à leurs produits de décomposition. La plupart des personnes qui
décèdent lors d'incendies sont victimes de l'oxyde de carbone.
La majorité des retardateurs de flamme organiques sont fixés soit
par une liaison covalente aux molécules de polymères (réactifs) , soit
incorporés aux polymères (additifs). Ils peuvent agir de plusieurs
manières, soit physiquement (par refroidissement, par formation d'une
couche protectrice ou par dilution de la matrice) ou chimiquement (par
des réactions dans la phase gazeuse ou solide).
Un certain nombre de facteurs président au choix de tel ou tel
type de retardateurs de flamme à utiliser pour une application donnée.
Il peut s'agir notamment de l'inflammabilité de la matrice, de
certaines exigences de fabrication ou de comportement, des propriétés
chimiques et des risques éventuels pour la santé de l'homme et pour
l'environnement.
L'exposition de la population générale à ces composés se produit
par la voie respiratoire, le contact cutané ou l'ingestion. Cette
exposition peut avoir lieu lors de l'utilisation des produits de
consommation, en cas de présence sur les lieux de fabrication ou
d'élimination ou encore par l'intermédiaire des différents
compartiments du milieu (y compris par l'absorption de nourriture).
Ces mêmes voies d'exposition se retrouvent en cas d'exposition
professionnelle, principalement lors de la production, de la
transformation, du transport, de l'élimination ou du recyclage des
retardateurs de flamme ou des produits traités avec ces composés. Il
peut également y avoir exposition professionnelle aux produits de
décomposition lors de la lutte contre les incendies. Comme plusieurs
de ces composés sont lipophiles et persistants, ils peuvent subir une
bioaccumulation. On a montré que certains d'entre eux pouvaient
entraîner des lésions au niveau de certains organes, des effets
génotoxiques et des cancers.
On se préoccupe également de l'exposition professionnelle aux
produits de combustion et de pyrolyse, en particulier les
dibenzofuranes polyhalogénés et les dibenzo-p-dioxines contenus dans
certains retardateurs de flamme organiques, ainsi d'ailleurs que des
effets que ces produits peuvent exercer sur l'environnement. Il existe
également d'autres produits de décomposition dont il faut tenir
compte.
Les retardateurs de flamme ont des propriétés qui les rendent
persistants ou enclins à la bioaccumulation , donc dangereux pour
l'environnement. Certains des composés qui ont été évalués jusqu'ici
(polybromobiphényles, éthers diphényliques polybromés et paraffines
chlorées) se sont révélés appartenir à ce groupe. L'usage de certains
de ces produits est donc déconseillé.
Plusieurs pays ont promulgué une réglementation relative à la
production, à l'utilisation et à l'élimination des retardateurs de
flamme. Certaines de ces réglementations prévoient des restrictions à
l'utilisation de ces composés en raison de leurs effets toxiques
potentiels pour l'homme. En Allemagne, la réglementation fixe la
teneur maximale en dibenzo-para-dioxines et en dibenzofuranes
polychlorés substitués en position 2,3,7,8.
Il y a peu de données intéressantes sur les retardateurs de
flamme dans les publications non soumises à restriction, en
particulier en ce qui concerne un certain nombre de produits chimiques
produits avant que la réglementation concernant leur commercialisation
n'ait été renforcée dans un certain nombre de pays.
Le PISC a déjà évalué un certain nombre de retardateurs de flamme
et les évaluations concernant d'autres produits de ce type seront
publiées ultérieurement.
9.2 Recommandations pour la protection de la santé humaine et de
l'environnement
a) Les autorités nationales doivent avoir communication de données
sur la teneur et la nature des retardateurs de flamme, et
notamment sur les impuretés qu'ils peuvent contenir.
b) On doit avoir accès à une information plus complète sur l'ampleur
de la production et de la consommation des retardateurs de
flamme.
c) Etant donné que les produits contenant des retardateurs de flamme
sont de plus en plus souvent recyclés, il faudrait faire
harmoniser l'étiquetage par une commission internationale.
d) Les composés qui présentent un risque toxique pour l'homme ou
pour l'environnement ne doivent pas être utilisés comme
retardateurs de flamme.
e) Il convient de réduire au minimum l'exposition professionnelle
aux retardateurs de flamme et à leurs produits de décomposition
en ayant recours à des techniques appropriées et en respectant
les règles de l'hygiène industrielle. Il convient en outre de
surveiller l'exposition des personnes qui sont exposées
professionnellement.
f) Il y a nécessité d'une bonne évaluation des effets que les
produits de combustion et de pyrolyse des retardateurs de flamme
peuvent exercer sur l'environnement ou sur les personnes exposées
de par leur profession.
g) Les émissions dans l'environnement qui résultent de la
fabrication, de la transformation, du transport , de
l'élimination ou du recyclage de produits contenant des composés
persistants ayant tendance à s'accumuler dans les tissus
biologiques doivent être réduites au minimum par le recours aux
meilleures techniques disponibles. Il convient de surveiller la
présence des composés utilisés dans l'environnement immédiat des
sites où il est procédé à ces opérations.
h) Il convient d'éviter d'utiliser des retardateurs de flamme dont
les propriétés sont telles qu'ils persistent dans l'environnement
et s'accumulent dans les tissus biologiques.
i) Il faut surveiller systématiquement la concentration, dans
certaines matrices environnementales (biotes et sédiments), des
principaux retardateurs de flamme persistants et susceptibles de
bioaccumulation. La même surveillance doit s'exercer sur certains
composés qui ne sont plus produits afin de voir quelle peut être
l'influence à long terme de ces substances.
9. CONCLUSIONES Y RECOMENDACIONES PARA La PROTECCIœN DE LA SALUD
HUMANA Y EL MEDIO AMBIENTE
9.1 Conclusiones
Los pirorretardadores son un grupo variado de compuestos
utilizados para mejorar la pirorretardancia de polímeros y otro
material. Hay una amplia variedad de compuestos, desde inorgánicos
hasta complejas moléculas orgánicas, que se utilizan como
pirorretardadores, sinergistas y supresores del humo. El presente
resumen se refiere a los compuestos orgánicos, que normalmente
contienen un halógeno y/o fósforo.
Es difícil encontrar cifras precisas sobre el uso de los
pirorretardadores a nivel mundial, pero se estima que se producen más
de 600 000 toneladas anuales. Los datos disponibles indican un
aumento sustancial del consumo de productos orgánicos bromados durante
el último decenio.
La utilización de los pirorretardadores tiene beneficios
evidentes, ya que éstos permiten salvar muchas vidas humanas y bienes
materiales del fuego. En la actualidad disponemos de conocimientos
limitados sobre los efectos a largo plazo de la exposición a los
pirorretardadores y productos de la descomposición de éstos. La mayor
parte de las muertes ocurridas en los incendios están causadas por el
monóxido de carbono.
La mayor parte de los pirorretardadores orgánicos se hallan
combinados mediante enlace covalente en moléculas de polímeros (por
reacción) o mezclados en el polímero (por adición). Pueden actuar de
varias maneras, ya sea físicamente (por enfriamiento, por formación de
una capa protectora o por dilución de la matriz) o químicamente (por
reacciones en el gas o en la fase sólida).
La selección del tipo de pirorretardador que se ha de utilizar en
una aplicación específica se basa en varios factores. Algunos de
ellos son la inflamabilidad de la matriz, requisitos de elaboración y
rendimiento, propiedades químicas y riesgos posibles para la salud
humana y el medio ambiente.
La población en general puede verse expuesta a los
pirorretardadores por inhalación, contacto dérmico o ingestión. Las
fuentes potenciales de exposición son productos de consumo,
instalaciones de fabricación/eliminación y diversos medios ambientales
(inclusive los alimentos que se ingieren). La exposición ocupacional
puede tener las mismas vías, principalmente durante la producción, la
elaboración, el transporte y la eliminación/reciclado de los
pirorretardadores o los productos tratados con ellos. También puede
haber exposición ocupacional a los productos de descomposición durante
la extinción de incendios. Varios de los compuestos utilizados son
lipofílicos y persistentes, por lo que son bioacumulables. Se ha
observado que algunos de los compuestos ocasionan lesiones orgánicas,
efectos genotóxicos y cáncer.
Los productos de la combustión/pirólisis, especialmente
dibenzofuranos polihalogenados y dibenzo- p-dioxinas, de algunos
pirorretardadores orgánicos son motivo de preocupación en lo
concerniente a la salud ocupacional y los efectos ambientales.
También es preciso tener en cuenta otros productos de la
descomposición.
Cierto número de pirorretardadores tienen propiedades que los
hacen persistentes y/o bioacumulativos, por lo que pueden constituir
un riesgo para el medio ambiente. Algunos de los compuestos que se
han evaluado hasta hoy (bifenilos polibromados, difenil éteres
polibromados y parafinas cloradas) pertenecen a este grupo. Por lo
tanto, se ha recomendado la no utilización de algunos de ellos.
Varios países han establecido reglamentaciones que afecta a la
producción, la utilización y la eliminación de los pirorretardadores.
Algunas comprenden restricciones sobre la utilización de compuestos en
razón de sus efectos tóxicos potenciales en el ser humano. Alemania
ha establecido normas aplicables al contenido máximo de algunos
dibenzofuranos y dibenzo- para-dioxinas 2,3,7,8-policloradas y en los
productos.
En la bibliografía abierta son limitados los datos pertinentes
que pueden encontrarse sobre los pirorretardadores, especialmente
sobre algunas sustancias químicas existentes producidas antes de que
en varios países se reforzara la reglamentación aplicable a la
comercialización.
El IPCS ha emitido evaluaciones sobre algunos pirorretardadores y
está preparando evaluaciones de otros.
9.2 Recomendaciones para la protección de la salud humana y del medio
ambiente
a) Debe ponerse a disposición de las autoridades nacionales la
información sobre el contenido y la naturaleza de los
pirorretardadores, inclusive sobre las impurezas presentes en los
productos.
b) Debe ponerse a disposición información más completa sobre el
volumen de la producción y el consumo de pirorretardadores.
c) En vista del creciente reciclado de productos pirorretardados,
debe considerarse la posibilidad de que un foro internacional
armonice el etiquetado.
d) Los compuestos que conlleven un riesgo de toxicidad para el ser
humano y/o el medio ambiente no deben utilizarse como
pirorretardadores.
e) La exposición ocupacional a los pirorretardadores y los productos
de la descomposición de éstos debe reducirse al mínimo mediante
una ingeniería apropiada y buenas prácticas de higiene
industrial. Debe vigilarse la exposición de las personas que
trabajan en esas actividades.
f) Es necesario que se haga una evaluación apropiada de los efectos
de los productos de la combustión o la pirólisis de los
pirorretardadores en la salud ocupacional y el medio ambiente.
g) Las emisiones en el medio ambiente resultantes de la fabricación,
la elaboración, el transporte y la eliminación/ reciclado de los
productos que contengan compuestos bioacumulativos persistentes
en el medio ambiente debe reducirse al mínimo mediante la
utilización de las mejores técnicas disponibles. El medio
ambiente próximo a los lugares donde se realizan dichas
operaciones debe vigilarse para detectar la presencia de los
compuestos utilizados.
h) Debe evitarse la utilización de pirorretardadores con propiedades
que los hagan persistentes y bioacumulativos.
i) Deben vigilarse sistemáticamente en las matrices ambientales
(biota y sedimentos) los niveles de los principales
pirorretardadores bioacumulativos persistentes. Algunos
compuestos que han dejado de producirse deben someterse
igualmente a vigilancia para determinar la influencia a largo
plazo de dichos productos.
Nitrogen-based flame retardants
Pyrophosphate dimelamine salts 70776-17-9 Polyurethane, polyester
ANNEX III
Fire tests
Fire tests are usually carried out to comply with specific
regulations or voluntary agreements.
They can be classified into three groups (Troitzsch, 1990; Arias,
1992; OECD, 1994):
(a) Tests reflecting single events in a fire. For historical
reasons, many different national fire tests now exist,
particularly for building products. These may not necessarily
correlate well. Within the European Union, efforts are being
made to harmonize testing.
(b) Tests addressing flammability. These tests are mainly used
with respect to transportation and electrical/electronic
products. They are mostly used internationally.
(c) Screening tests. These are used to screen materials during
product development or for quality control.
ANNEX IV
US Interagency Testing Commission recommendations on
brominated flame retardants
Chemical
Designateda
1,2-Epoxy-3-bromopropane
2,4,6-Tribromoaniline
1,2-Dibromo-4-(1,2-dibromomethyl) cyclohexane
Pentabromoethylbenzene
Tetrabromobisphenol A
Decabromodiphenyl ether
Hexabromocyclododecane
Pentabromodiphenyl ether
Octabromodiphenyl ether
1,2-Bis(2,4,6-tribromophenoxy)ethane
Recommendedb
2,4,6-Tribromophenol
Tetrabromophthalic anhydride
Dibromoneopentyl glycol
Ethylene bis(tetrabromophthalimide)
Ethylene bis(tetrabromonorbornane-2,3-dicarboximide)
Tribrominated polystyrene
Ethylene bis(pentabromophenoxide)
Bromochloromethane
3,4',5-Tribromosalicylanilide
2,3,4,5,6-Pentabromotoluene
1,2,3,4,5-Pentabromo-6-chlorocyclohexane
2,3-Dibromopropanol
Vinyl bromide
2,4-Dibromophenol
Ethoxylated tetrabromobisphenol A
Tetrabromobisphenol A, bis(allyl ether)
Annex IV (contd).
Chemical
Recommended (contd).
Tetrabromodichlorocyclohexane
Tribromotrichlorocyclohexane
Tribromoneopentyl alcohol
Tetrabromobisphenol A diacrylate
Alkanes, C10-16, bromochloro
2,4-(or 2,6)-Dibromophenol, homopolymer
Benzene, ethenyl-, homopolymer, brominated
DeferredC
Polybromobiphenyl
Tetrabromo-o-chlorotoluene
Bromophenol (Br1, Br2, Br5)
Bis(dibromopropyl) carbamate
Bis(2,3-dibromopropyl) phosphite
Bis(dibromopropyl) phosphite
Tetrabromochlorotoluene
Brominated terphenyls
Dibromopropyl carbamate
Allyl bis(2,3-dibromopropyl) phosphite
Bis(dibromopropyl) phosphoryl chloride
2,3-Dibromo-1-propanol phosphate
Pentabromophenol
3,3-Bis(bromomethyl)oxetane
(bromomethyl)oxirane
pentabromophenyl allyl ether
2,6-Dibromo-4-[1-(3-bromo-4-hydroxyphenyl)-1-methylethyl]phenol
bis(2,3-dibromopropyl) phthalate
1,2-Ethanediylbis[tris(2-cyanoethyl)phosphonium] dibromide
(bromoethyl)oxirane
Decabromobiphenyl
Tetrabromophthalic acid, aluminium salt
Fumaric acid, bis(pentabromophenyl) ester
1,2-Dibromo-4,5,6,7,8,8-hexachloro-2,3,3a,4,7,7a-hexahydro-4,7
-methano-1H-indene
tetrabromophthalic acid, dipotassium salt
2-(2,4,6-Tribromophenoxy)ethanol
Bis(2,4,6-tribromophenyl) fumarate
Annex IV (contd).
Chemical
Deferred (contd)
Tribromophenol
Dibromoethane
Poly(2,6-dibromophenylene oxide)
Tribromophenyl allyl ether
Nonabromobiphenyl
Octabromobiphenyl
Bromophenol
3,3',5,5'-Tetrabromobisphenol A diacetate
3,3',5,5'-Tetrabromobisphenol S
Tris(dibromophenyl) phosphate
Dibromopropyl carbamate
2,3,4,6-Tetrabromo-5-methylphenol
2-(Pentabromophenoxy)ethanol
Dibromopropyl carbamate
3,9-Bis[3-bromo-2,2-bis(bromomethyl)propoxy]-
2,2-Bis(bromomethyl)-3-chloropropyl phosphoric acid
Tribromophenyl allyl ether
Decabrominated diphenoxyethane
2,4,6-Tribromophenol carbonate
pentabromophenol, aluminium salt
3,4,5,6-Tetrabromo-1,2-benzenedicarboxylic acid, magnesium salt
2-Butenedioic acid (z), bis(pentabromophenyl) ester
Pentabromo[2-(tetrabromophenoxy)ethoxy]benzene
Pentabromo[2-(tetrabromochlorophenoxy)ethoxy]benzene
1,3,5-Tribromo-2-(2-bromoethoxy)benzene
Brominated and chlorinated benzene
Bis[3-bromo-2-(bromomethyl)-2-(hydroxymethyl) propyl]hexanoate
Adapted from Walker (1994) and from a Memorandum dated 22 October 1992
entitled Actions on Brominated Flame Retardants; Toxic Substances
Control Act, Interagency Testing Committee (ITC), US Environmental
Protection Agency, Washington DC
a ITC designated chemical to US EPA for a decision about testing.
b ITC required additional information for further recommendation.
c Consideration for testing by ITC.
9. CONCLUSIONS ET RECOMMANDATIONS POUR LA PROTECTION DE LA SANTE
HUMAINE ET DE L'ENVIRONNEMENT
9.1 Conclusions
Les retardateurs de flamme sont un groupe de composés très divers
qu'on utilise pour retarder l'inflammation des polymères et autres
matériaux. On utilise une grande variété de composés, des substances
minérales aux molécules organiques complexes, comme retardateurs de
flamme, synergisants et inhibiteurs de fumée. Les considérations
générales qui suivent portent essentiellement sur des composés
organiques caractérisés par la présence d'halogènes ou de phosphore.
Il est difficile de se faire une idée exacte de l'utilisation des
retardateurs de flammes au niveau mondial, mais selon les estimations,
ce sont plus de 600 000 tonnes qui sont produites chaque année. Les
données dont on dispose indiquent qu'au cours de la dernière décennie,
les dérivés organiques bromés ont vu leur production s'accroître de
façon substantielle.
L'utilisation de retardateurs de flamme présente des avantages
évidents puisqu'ils permettent de sauver nombre de vies humaines et de
biens matériels. A l'heure actuelle, on ne connaît pas très bien les
effets à long terme pouvant résulter d'une exposition à ces composés
et à leurs produits de décomposition. La plupart des personnes qui
décèdent lors d'incendies sont victimes de l'oxyde de carbone.
La majorité des retardateurs de flamme organiques sont fixés soit
par une liaison covalente aux molécules de polymères (réactifs) , soit
incorporés aux polymères (additifs). Ils peuvent agir de plusieurs
manières, soit physiquement (par refroidissement, par formation d'une
couche protectrice ou par dilution de la matrice) ou chimiquement (par
des réactions dans la phase gazeuse ou solide).
Un certain nombre de facteurs président au choix de tel ou tel
type de retardateurs de flamme à utiliser pour une application donnée.
Il peut s'agir notamment de l'inflammabilité de la matrice, de
certaines exigences de fabrication ou de comportement, des propriétés
chimiques et des risques éventuels pour la santé de l'homme et pour
l'environnement.
L'exposition de la population générale à ces composés se produit
par la voie respiratoire, le contact cutané ou l'ingestion. Cette
exposition peut avoir lieu lors de l'utilisation des produits de
consommation, en cas de présence sur les lieux de fabrication ou
d'élimination ou encore par l'intermédiaire des différents
compartiments du milieu (y compris par l'absorption de nourriture).
Ces mêmes voies d'exposition se retrouvent en cas d'exposition
professionnelle, principalement lors de la production, de la
transformation, du transport, de l'élimination ou du recyclage des
retardateurs de flamme ou des produits traités avec ces composés. Il
peut également y avoir exposition professionnelle aux produits de
décomposition lors de la lutte contre les incendies. Comme plusieurs
de ces composés sont lipophiles et persistants, ils peuvent subir une
bioaccumulation. On a montré que certains d'entre eux pouvaient
entraîner des lésions au niveau de certains organes, des effets
génotoxiques et des cancers.
On se préoccupe également de l'exposition professionnelle aux
produits de combustion et de pyrolyse, en particulier les
dibenzofuranes polyhalogénés et les dibenzo-p-dioxines contenus dans
certains retardateurs de flamme organiques, ainsi d'ailleurs que des
effets que ces produits peuvent exercer sur l'environnement. Il existe
également d'autres produits de décomposition dont il faut tenir
compte.
Les retardateurs de flamme ont des propriétés qui les rendent
persistants ou enclins à la bioaccumulation , donc dangereux pour
l'environnement. Certains des composés qui ont été évalués jusqu'ici
(polybromobiphényles, éthers diphényliques polybromés et paraffines
chlorées) se sont révélés appartenir à ce groupe. L'usage de certains
de ces produits est donc déconseillé.
Plusieurs pays ont promulgué une réglementation relative à la
production, à l'utilisation et à l'élimination des retardateurs de
flamme. Certaines de ces réglementations prévoient des restrictions à
l'utilisation de ces composés en raison de leurs effets toxiques
potentiels pour l'homme. En Allemagne, la réglementation fixe la
teneur maximale en dibenzo-para-dioxines et en dibenzofuranes
polychlorés substitués en position 2,3,7,8.
Il y a peu de données intéressantes sur les retardateurs de
flamme dans les publications non soumises à restriction, en
particulier en ce qui concerne un certain nombre de produits chimiques
produits avant que la réglementation concernant leur commercialisation
n'ait été renforcée dans un certain nombre de pays.
Le PISC a déjà évalué un certain nombre de retardateurs de flamme
et les évaluations concernant d'autres produits de ce type seront
publiées ultérieurement.
9.2 Recommandations pour la protection de la santé humaine et de
l'environnement
a) Les autorités nationales doivent avoir communication de données
sur la teneur et la nature des retardateurs de flamme, et
notamment sur les impuretés qu'ils peuvent contenir.
b) On doit avoir accès à une information plus complète sur l'ampleur
de la production et de la consommation des retardateurs de
flamme.
c) Etant donné que les produits contenant des retardateurs de flamme
sont de plus en plus souvent recyclés, il faudrait faire
harmoniser l'étiquetage par une commission internationale.
d) Les composés qui présentent un risque toxique pour l'homme ou
pour l'environnement ne doivent pas être utilisés comme
retardateurs de flamme.
e) Il convient de réduire au minimum l'exposition professionnelle
aux retardateurs de flamme et à leurs produits de décomposition
en ayant recours à des techniques appropriées et en respectant
les règles de l'hygiène industrielle. Il convient en outre de
surveiller l'exposition des personnes qui sont exposées
professionnellement.
f) Il y a nécessité d'une bonne évaluation des effets que les
produits de combustion et de pyrolyse des retardateurs de flamme
peuvent exercer sur l'environnement ou sur les personnes exposées
de par leur profession.
g) Les émissions dans l'environnement qui résultent de la
fabrication, de la transformation, du transport , de
l'élimination ou du recyclage de produits contenant des composés
persistants ayant tendance à s'accumuler dans les tissus
biologiques doivent être réduites au minimum par le recours aux
meilleures techniques disponibles. Il convient de surveiller la
présence des composés utilisés dans l'environnement immédiat des
sites où il est procédé à ces opérations.
h) Il convient d'éviter d'utiliser des retardateurs de flamme dont
les propriétés sont telles qu'ils persistent dans l'environnement
et s'accumulent dans les tissus biologiques.
i) Il faut surveiller systématiquement la concentration, dans
certaines matrices environnementales (biotes et sédiments), des
principaux retardateurs de flamme persistants et susceptibles de
bioaccumulation. La même surveillance doit s'exercer sur certains
composés qui ne sont plus produits afin de voir quelle peut être
l'influence à long terme de ces substances.
9. CONCLUSIONES Y RECOMENDACIONES PARA La PROTECCIœN DE LA SALUD
HUMANA Y EL MEDIO AMBIENTE
9.1 Conclusiones
Los pirorretardadores son un grupo variado de compuestos
utilizados para mejorar la pirorretardancia de polímeros y otro
material. Hay una amplia variedad de compuestos, desde inorgánicos
hasta complejas moléculas orgánicas, que se utilizan como
pirorretardadores, sinergistas y supresores del humo. El presente
resumen se refiere a los compuestos orgánicos, que normalmente
contienen un halógeno y/o fósforo.
Es difícil encontrar cifras precisas sobre el uso de los
pirorretardadores a nivel mundial, pero se estima que se producen más
de 600 000 toneladas anuales. Los datos disponibles indican un
aumento sustancial del consumo de productos orgánicos bromados durante
el último decenio.
La utilización de los pirorretardadores tiene beneficios
evidentes, ya que éstos permiten salvar muchas vidas humanas y bienes
materiales del fuego. En la actualidad disponemos de conocimientos
limitados sobre los efectos a largo plazo de la exposición a los
pirorretardadores y productos de la descomposición de éstos. La mayor
parte de las muertes ocurridas en los incendios están causadas por el
monóxido de carbono.
La mayor parte de los pirorretardadores orgánicos se hallan
combinados mediante enlace covalente en moléculas de polímeros (por
reacción) o mezclados en el polímero (por adición). Pueden actuar de
varias maneras, ya sea físicamente (por enfriamiento, por formación de
una capa protectora o por dilución de la matriz) o químicamente (por
reacciones en el gas o en la fase sólida).
La selección del tipo de pirorretardador que se ha de utilizar en
una aplicación específica se basa en varios factores. Algunos de
ellos son la inflamabilidad de la matriz, requisitos de elaboración y
rendimiento, propiedades químicas y riesgos posibles para la salud
humana y el medio ambiente.
La población en general puede verse expuesta a los
pirorretardadores por inhalación, contacto dérmico o ingestión. Las
fuentes potenciales de exposición son productos de consumo,
instalaciones de fabricación/eliminación y diversos medios ambientales
(inclusive los alimentos que se ingieren). La exposición ocupacional
puede tener las mismas vías, principalmente durante la producción, la
elaboración, el transporte y la eliminación/reciclado de los
pirorretardadores o los productos tratados con ellos. También puede
haber exposición ocupacional a los productos de descomposición durante
la extinción de incendios. Varios de los compuestos utilizados son
lipofílicos y persistentes, por lo que son bioacumulables. Se ha
observado que algunos de los compuestos ocasionan lesiones orgánicas,
efectos genotóxicos y cáncer.
Los productos de la combustión/pirólisis, especialmente
dibenzofuranos polihalogenados y dibenzo- p-dioxinas, de algunos
pirorretardadores orgánicos son motivo de preocupación en lo
concerniente a la salud ocupacional y los efectos ambientales.
También es preciso tener en cuenta otros productos de la
descomposición.
Cierto número de pirorretardadores tienen propiedades que los
hacen persistentes y/o bioacumulativos, por lo que pueden constituir
un riesgo para el medio ambiente. Algunos de los compuestos que se
han evaluado hasta hoy (bifenilos polibromados, difenil éteres
polibromados y parafinas cloradas) pertenecen a este grupo. Por lo
tanto, se ha recomendado la no utilización de algunos de ellos.
Varios países han establecido reglamentaciones que afecta a la
producción, la utilización y la eliminación de los pirorretardadores.
Algunas comprenden restricciones sobre la utilización de compuestos en
razón de sus efectos tóxicos potenciales en el ser humano. Alemania
ha establecido normas aplicables al contenido máximo de algunos
dibenzofuranos y dibenzo- para-dioxinas 2,3,7,8-policloradas y en los
productos.
En la bibliografía abierta son limitados los datos pertinentes
que pueden encontrarse sobre los pirorretardadores, especialmente
sobre algunas sustancias químicas existentes producidas antes de que
en varios países se reforzara la reglamentación aplicable a la
comercialización.
El IPCS ha emitido evaluaciones sobre algunos pirorretardadores y
está preparando evaluaciones de otros.
9.2 Recomendaciones para la protección de la salud humana y del medio
ambiente
a) Debe ponerse a disposición de las autoridades nacionales la
información sobre el contenido y la naturaleza de los
pirorretardadores, inclusive sobre las impurezas presentes en los
productos.
b) Debe ponerse a disposición información más completa sobre el
volumen de la producción y el consumo de pirorretardadores.
c) En vista del creciente reciclado de productos pirorretardados,
debe considerarse la posibilidad de que un foro internacional
armonice el etiquetado.
d) Los compuestos que conlleven un riesgo de toxicidad para el ser
humano y/o el medio ambiente no deben utilizarse como
pirorretardadores.
e) La exposición ocupacional a los pirorretardadores y los productos
de la descomposición de éstos debe reducirse al mínimo mediante
una ingeniería apropiada y buenas prácticas de higiene
industrial. Debe vigilarse la exposición de las personas que
trabajan en esas actividades.
f) Es necesario que se haga una evaluación apropiada de los efectos
de los productos de la combustión o la pirólisis de los
pirorretardadores en la salud ocupacional y el medio ambiente.
g) Las emisiones en el medio ambiente resultantes de la fabricación,
la elaboración, el transporte y la eliminación/ reciclado de los
productos que contengan compuestos bioacumulativos persistentes
en el medio ambiente debe reducirse al mínimo mediante la
utilización de las mejores técnicas disponibles. El medio
ambiente próximo a los lugares donde se realizan dichas
operaciones debe vigilarse para detectar la presencia de los
compuestos utilizados.
h) Debe evitarse la utilización de pirorretardadores con propiedades
que los hagan persistentes y bioacumulativos.
i) Deben vigilarse sistemáticamente en las matrices ambientales
(biota y sedimentos) los niveles de los principales
pirorretardadores bioacumulativos persistentes. Algunos
compuestos que han dejado de producirse deben someterse
igualmente a vigilancia para determinar la influencia a largo
plazo de dichos productos.
See Also:
Toxicological Abbreviations
Flame retardants (EHC 209, 1998)
Flame retardants (EHC 218, 2000)
 The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
A GENERAL INTRODUCTION
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico
Dr P. Arias, Brussels, Belgium
Dr S.A. Assimon, Contaminants Branch, US Food and Drug Administration,
Washington, DC, USA
Dr H. Hofer, Toxicology, Austrian Research Centre, Seibersdorf,
Austria
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Stockholm, Sweden ( Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health, Ahmedabad,
India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany ( Vice-Chairman)
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Sœderlund, Folkehelsa, National Institute of Public Health,
Oslo, Norway ( Rapporteur)
Dr J. Troitzsch, Fire and Environment Protection Service, Wiesbaden,
Germany
Observers
Dr M.L. Hardy, Albermarle Corporation, Baton Rouge, USA
Mr T.A. Jay, Applications Laboratory, Great Lakes Chemical (Europe)
N.V., Geel, Belgium
Dr M. Papez, European Flame Retardants Association, Brussels, Belgium
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization ( Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS: A GENERAL
INTRODUCTION
A WHO Task Group on Environmental Health Criteria for Flame
Retardants met at the World Health Organization, Geneva, from 4 to 8
December 1995. Dr K.W. Jager, IPCS, welcomed the participants on
behalf of Dr M. Mercier, Director of the IPCS, and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and prepared conclusions and
recommendations.
The first draft of the monograph was prepared by Dr G.J. van
Esch, Bilthoven, the Netherlands. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ABS acrylonitrile-butadiene-styrene
APP ammonium polyphosphate
ATH alumina trihydrate
DeBDE decabromodiphenyl ether
EPDM ethylene propylene rubber
EPS expandable polystyrene
FR flame retardant
HBCD hexabromocyclododecane
HIPS high impact polystyrene
HDPE high density polyethylene
LDPE low density polyethylene
PA polyamides
PBDE polybrominated diphenyl ether
PBB polybrominated biphenyl
PBDD polybrominated dibenzodioxin
PBDF polybrominated dibenzofuran
PBT polybutylene terephthalate
PCDD polychlorinated dibenzodioxin
PCDF polychlorinated dibenzofuran
PE polyethylene
PET polyethylene terephthalate
PP polypropylene
PVC polyvinyl chloride
TBBPA tetrabromobisphenol A
TCDD 2,3,7,8-tetrachlorinated dibenzo- p-dioxin
TCPP tris(1-chloro-2-propyl)phosphate
PROLOGUE
The intent of this Environmental Health Criteria (EHC) monograph
is to provide a general overview of the nature, mechanism of action,
use and production volume of compounds used to improve the flame
retardancy of polymeric materials and textiles. The monograph also
indicates some of the known health and environmental hazards for
certain of the flame retardants that have been assessed by the IPCS.
A large number of compounds have been identified as being used as
flame retardants. Detailed international assessments of the risks
posed to human health and the environment by a number of these
substances have been published previously as EHC monographs. In Table
1 are listed the monographs on flame retardants and compounds related
to flame retardants that have already been published by IPCS or are in
preparation.
This monograph is intended to provide a starting point for those
interested in obtaining general information on flame-retardant
chemicals. More detailed information on use patterns, sources of
exposure, and health and environmental risks posed by these substances
can be found in the appropriate EHC monograph.
Table 1. EHC monographs on chemicals associated with
flame-retardant use
Substance EHC monograph
Mirex (1,1a,2,2,3,3a,4,5,5,5a,5b,6- EHC 44 (1984)
dodecachloroocta-hydro-1,3,4-metheno-1H-
cyclobuta( cd)pentalene)
Polychlorinated dibenzo- p-dioxins and EHC 88 (1989)
dibenzofurans
Tricresyl phosphate EHC 110 (1990)
Triphenyl phosphate EHC 111 (1991)
Hexachlorocyclopentadiene EHC 120 (1991)
Polychlorinated biphenyls EHC 140 (1992)
Polybrominated biphenyls EHC 152 (1994)
Brominated diphenyl ethers EHC 162 (1994)
Tetrabromobisphenol A and some of its EHC 172 (1995)
derivatives
Tris(2,3-dibromopropyl) phosphate and EHC 173 (1995)
bis(2,3-dibromopropyl) phosphate
Chlorendic acid and anhydride EHC 185 (1996)
Chlorinated paraffins EHC 181 (1996)
Polybrominated dibenzo- p-dioxins and EHC in preparation
dibenzofurans
Vinylbromide EHC in prepraration
Tris(chloropropyl) phosphates EHC in prepraration
Tris(2-butoxyethyl) phosphate EHC in prepraration
Tris(2-chloroethyl) phosphate EHC in prepraration
Tris(2-ethylhexyl) phosphate EHC in prepraration
Tetrakis(hydroxymethyl) phosphonium salts EHC in prepraration
1. INTRODUCTION
In today's society, there is an unprecedented development in the
size and number of buildings, skyscrapers, warehouses and methods of
transport. Carpeting, furnishings, equipment, oil and gas for heating
all increase the fire load in a building. New technologies, new
processes and new applications introduce new fire hazards (e.g., new
ignition sources such as welding sparks and short circuits)
(Troitzsch, 1990). Modern fire-fighting techniques, equipment and
building design have reduced the destruction due to fires. However,
a high fuel load in either a residential or a commercial building can
offset even the best of building construction (Gann, 1993).
Each year, over 3 million fires leading to 29 000 injuries and
4500 deaths are reported in the USA. The direct property losses
exceed $8 billion and the total annual cost has been estimated at over
$100 billion. Personal losses occur mostly in residences where
furniture, wall coverings and clothes are frequently the fuel. Large
financial losses occur in commercial structures such as office
buildings and warehouses. Fires also occur in aeroplanes, buses and
trains (Gann, 1993).
To provide additional protection from fires and to increase
escape time when a fire occurs, methods to enhance the flame
retardance of consumer goods have been developed. Flame retardants
are chemicals added to polymeric materials, both natural and
synthetic, to enhance flame-retardance properties. Flame-retardant
chemicals are most often used to improve the fire performance of low-
to-moderate cost commodity polymers. These flame retardants may be
physically blended with or chemically bonded to the host polymer.
They generally either lower ignition susceptibility or lower flame
spread once ignition has occurred. Some polymers are inherently less
flammable due to more stable polymeric structures; these are usually
higher priced engineering plastics such as polyimides,
polybenzimidazoles and polyetherketones (Gann, 1993).
Flame-retardant systems for synthetic or organic polymers act in
five basic ways: (1) gas dilution; (2) thermal quenching;
(3) protective coating; (4) physical dilution; (5) chemical
interaction (Pettigrew, 1993); or through a combination of these
mechanisms.
1. Inert gas dilution involves using additives that produce large
volumes of non-combustible gases on decomposition. These gases
dilute the oxygen supply to the flame or dilute the fuel
concentration below the flammability limit. Metal hydroxides,
metal salts and some nitrogen compounds function in this way.
2. Thermal quenching is the result of endothermic decomposition of
the flame retardant. Metal hydroxides, metal salts and nitrogen
compounds act to decrease surface temperature and the rate of
burning.
3. Some flame retardants form a protective liquid or char barrier.
This limits the amount of polymer available to the flame front
and/or acts as an insulating layer to reduce the heat transfer
from the flame to the polymer. Phosphorus compounds and
intumescent systems based on melamine and other nitrogen
compounds are examples of this category.
4. Inert fillers (glass fibres and microspheres) and minerals (talc)
act as thermal sinks to increase the heat capacity of the polymer
or reduce its fuel content.
5. Halogens and some phosphorus flame retardants act by chemical
interaction. The flame retardant dissociates into radical
species that compete with chain-propagating steps in the
combustion process.
Chemicals that are used as flame retardants can be inorganic,
organic, mineral, halogen-containing or phosphorus-containing. The
term flame "retardant" represents a class of use and not a class of
chemical structure (Pettigrew, 1993).
Preventive flame protection, including the use of flame
retardants, has been practised since ancient times. Some examples of
early historical developments in flame retardants are shown in
Table 2.
Table 2. Early historical fire-retardant developmentsa
Development Date
Alum used to reduce the flammability of wood by the About 450 BC
Egyptians
The Romans used a mixture of alum and vinegar on About 200 BC
wood
Mixture of clay and gypsum used to reduce 1638
flammability of theatre curtains
Mixture of alum, ferrous sulfate and borax used 1735
on wood and textiles by Wyld in Britain
Alum used to reduce flammability of balloons 1783
Gay-Lussac reported a mixture of (NH4)3PO4, 1821
NH4Cl and borax to be effective on linen and hemp
Perkin described a flame-retardant treatment for 1912
cotton using a mixture of sodium stannate and
ammonium sulfate
a From: Hindersinn (1990)
The advent of synthetic polymers earlier this century was of
special significance, since the water-soluble inorganic salts used up
to that time were of little or no utility in these largely hydrophobic
materials. Modern developments were, therefore, concentrated on the
development of polymer-compatible flame retardants.
By the outbreak of the Second World War, flame-proof canvas
tentage for outdoor use by the military was produced with a treatment
of chlorinated paraffins and an insoluble metal oxide, mostly antimony
oxide as a glow inhibitor, together with a binder resin.
After the war, non-cellulosic thermoplastic polymers became more
and more important as the basic fibres used for flame-retardant
applications. A dramatic example of the superiority of the non-
cellulosic compounds is provided by the diminished use of cotton fibre
in children's sleepwear since the inception of new standards. In
1971, cotton supplied 78% of the fibres used to produce children's
sleepwear, whereas in 1973 it supplied less than 10% in the USA (US
EPA, 1976).
With the increasing use of thermoplastics and thermosets on a
large scale for applications in building, transportation, electrical
engineering and electronics, new flame-retardant systems were
developed. They mainly consist of inorganic and organic compounds
based on bromine, chlorine, phosphorus, nitrogen, boron, and metallic
oxides and hydroxides.
Today, these flame-retardant systems fulfill the multiple
flammability requirements developed for the above-mentioned
applications.
A glossary of terms concerning flammability and flame retardants
is given in Annex 1.
2. TYPES OF FLAME RETARDANTS
A distinction is made between reactive and additive flame
retardants. Reactive flame retardants are reactive components
chemically built into a polymer molecule. Additive flame retardants
are incorporated into the polymer either prior to, during or (most
frequently) following polymerization.
There are three main families of flame-retardant chemicals
(Troitzsch, 1990; Wolf & Kaul, 1992; Green, 1992; Touval, 1993;
Pettigrew, 1993; Weil, 1993).
1. The main inorganic flame retardants are aluminium trihydroxide,
magnesium hydroxide, ammonium polyphosphate and red phosphorus.
This group represents about 50% by volume of the worldwide flame
retardant production. Some of these chemicals are also used as
flame retardant synergists, of which antimony trioxide is the
most important (OECD, 1994).
2. Halogenated products are based primarily on chlorine and
bromine. This group represents about 25% by volume of the
worldwide production (OECD, 1994).
3. Organophosphorus products are primarily phosphate esters and
represent about 20% by volume of the worldwide production.
Products containing phosphorus, chlorine and/or bromine are also
important.
In addition, nitrogen-based flame retardants are used for a
limited number of polymers.
Annex II comprises lists of the different flame retardant
families commercially used at present (A) and those no more in
use (B).
2.1 Inorganic flame retardants
Metal hydroxides form the largest class of all flame retardants
used commercially today and are employed alone or in combination with
other flame retardants to achieve necessary improvements in flame
retardancy. Antimony compounds are used as synergistic co-additives
in combination with halogen compounds, facilitating the reduction in
total flame retardant levels needed to achieve a desired level of
flame retardancy. To a limited extent, compounds of other metals also
act as synergists with halogen compounds. They may be used alone but
are most commonly used with antimony trioxide to enhance other
characteristics, for example, smoke reduction or afterglow
suppression. Ionic compounds have a very long history as flame
retardants for wool- or cellulose-based products. Inorganic
phosphorus compounds are primarily used in polyamides and phenolic
resins, or as components in intumescent formulations.
2.1.1 Metal hydroxides
Metal hydroxides function in both the condensed and gas phases of
a fire by absorbing heat and decomposing to release their water of
hydration. This process cools both the polymer and the flame and
dilutes the flammable gas mixture. The very high concentrations (50
to 80%) required to impart flame retardancy often adversely affect the
mechanical properties of the polymer into which they are incorporated.
Aluminium hydroxide, also known as alumina trihydrate (ATH) is
the largest volume flame retardant in use today. It decomposes when
exposed to temperatures over 200°C, which limits the polymers in
which it can be incorporated. Magnesium hydroxide is stable to
temperatures above 300°C and can be processed into several polymers.
2.1.2 Antimony compounds
Antimony trioxide is not a flame retardant per se, but it is
used as a synergist. It is utilized in plastics, rubbers, textiles,
paper and paints, typically 2-10% by weight, with organochlorine and
organobromine compounds to diminish the flammability of a wide range
of plastics and textiles (IARC, 1989).
Antimony oxides and antimonates must be converted to volatile
species. This is usually accomplished by release of halogen acids at
fire temperatures. The halogen acids react with the antimony-
containing materials to form antimony trihalide and/or antimony halide
oxide. These materials act both in the substrate (condensed phase)
and in the flame to suppress flame propagation. In the condensed
phase, they promote char formation, which acts as a physical barrier
to flame and inhibits the volatilization of flammable materials. In
the flame, the antimony halides and halide oxides, generated in
sufficient volume, provide an inert gas blanket over the substrate,
thus excluding oxygen and preventing flame spread. These compounds
alter the chemical reactions occurring at fire temperatures in the
flame, thus reducing the ease with which oxygen can combine with the
volatile products. It is also suggested that antimony oxychloride or
trichloride reduces the rate at which the halogen leaves the flame
zone, thus increasing the probability of reaction with the reactive
species. Antimony trichloride probably evolves heavy vapours which
form a layer over the condensed phase, stop oxygen attack and thus
choke the flame. It is also assumed that the liquid and solid
antimony trichloride particles contained in the gas phase reduce the
energy content of the flames by wall or surface effects (Troitzsch,
1990).
Other antimony compounds include antimony pentoxide, available
primarily as a stable colloid or as a redispersible powder. It is
designed primarily for highly specialized applications, although
manufacturers suggest it has potential use in fibre and fabric
treatment.
Sodium antimonate (Na2OSb2O5Ê´H2O) is recommended for
formulations in which deep tone colours are required or where antimony
trioxide may promote unwanted chemical reactions.
2.1.3 Boron compounds
Within the class of boron compounds, by far the most widely used
is boric acid. Boric acid (H3BO3) and sodium borate (borax)
(Na2B4O7. 10H2O) are the two flame retardants with the longest
history, and are used primarily with cellulosic material, e.g., cotton
and paper. Both products are effective, but their use is limited to
products for which non-durable flame retardancy is acceptable since
both are very water-soluble.
Zinc borate, however, is water-insoluble and is mostly used in
plastics and rubber products. It is used either as a complete or
partial replacement for antimony oxide in PVC, nylon, polyolefin,
epoxy, EPDM, etc. In most systems, it displays synergism with
antimony oxide. Zinc borate can function as a flame retardant, smoke
suppressant and anti-arcing agent in condensed phase. Recently, zinc
borate has also been used in halogen-free, fire-retardant polymers.
2.1.4 Other metal compounds
Molybdenum compounds have been used as flame retardants in
cellulosic materials for many years and more recently with other
polymers, mainly as smoke suppressants (see section 3.4) (Troitzsch,
1990). They appear to function as condensed-phase flame retardants
(Avento & Touval, 1980). Titanium and zirconium compounds are used
for textiles, especially wool (Calamari & Harper, 1993).
Zinc compounds, such as zinc stannate and zinc hydroxy-stannate,
are also used as synergists and as partial replacements for antimony
trioxide.
2.1.5 Phosphorus compounds
Red phosphorus and ammonium polyphosphate (APP) are used in
various plastics.
Red phosphorus was first investigated in polyurethane foams and
found to be very effective as a flame retardant. It is now used
particularly for polyamides and phenolic applications. The flame-
retarding effect is due, in all probability, to the oxidation of
elemental phosphorus during the combustion process to phosphoric acid
or phosphorus pentoxide. The latter acts by the formation of a
carbonaceous layer in the condensed phase. The formation of fragments
that act by interrupting the radical chain mechanism is also likely.
Ammonium polyphosphate is mainly applied in intumescent coatings
and paints. Intumescent systems puff up to produce foams. Because of
this characteristic they are used to protect materials such as wood
and plastics that are combustible and those like steel that lose their
strength when exposed to high temperatures. Intumescent agents have
been available commercially for many years and are used mainly as
fire-protective coatings. They are now used as flame-retardant
systems for plastics by incorporating the intumescent components in
the polymer matrix, mainly polyolefins, particularly polypropylene
(Troitzsch, 1990).
2.1.6 Other inorganic flame retardants
Other inorganic flame retardants, including ammonium sulfamate
(NH4SONH2) and ammonium bromide (NH4Br), are used primarily with
cellulose-based products and in forest fire-fighting (Weil, 1993).
2.2 Halogenated organic flame retardants
Halogenated flame retardants can be divided into three classes:
aromatic, aliphatic and cycloaliphatic. Bromine and chlorine
compounds are the only halogen compounds having commercial
significance as flame-retardant chemicals. Fluorine compounds are
expensive and, except in special cases, are ineffective because the
C-F bond is too strong. Iodine compounds, although effective, are
expensive and too unstable to be useful (Cullis, 1987; Pettigrew,
1993). The brominated flame retardants are much more numerous than
the chlorinated types because of their higher efficacy (Cullis, 1987).
With repect to processability, halogenated flame retardants vary
in their thermal stability. In general, aromatic brominated flame
retardants are more thermally stable than chlorinated aliphatics,
which are more thermally stable than brominated aliphatics.
Brominated aromatic compounds can be used in thermoplastics at fairly
high temperatures without the use of stabilizers and at very high
temperatures with stabilizers. The thermal stability of the
chlorinated and brominated aliphatics is such that, with few
exceptions, they must be used with thermal stabilizers, such as a tin
compound.
Halogenated flame retardants are either added to or reacted with
the base polymer. Additive flame retardants are those that do not
react in the application designated. There are a few compounds that
can be used as an additive in one application and as a reactive in
another; tetrabromobisphenol A is the most notable example. Reactive
flame retardants become a part of the polymer either by becoming a
part of the backbone or by grafting onto the backbone. The choice of
a reactive flame retardant is more complex than the choice of an
additive type. The development of systems based on reactive flame
retardants is more expensive for the manufacturer, who in effect has
to develop novel co-polymers with the desired chemical, physical and
mechanical properties, as well as the appropriate degree of flame
retardance (Cullis, 1987; Pettigrew, 1993). Synergists such as
antimony oxides are frequently used with halogenated flame retardants.
2.2.1 Brominated flame retardants
Bromine-based flame retardants are highly brominated organic
compounds with a relative molecular mass ranging from 200 to that of
large molecule polymers. They usually contain 50 to 85% (by weight)
of bromine (Cullis, 1987).
The highest volume brominated flame retardant in use today is
tetrabromobisphenol A (TBBPA) (IPCS, 1995a) followed by
decabromodiphenyl ether (DeBDE) (IPCS, 1994b). Both of these flame
retardants are aromatic compounds. The primary use of TBBPA is as a
reactive intermediate in the production of flame-retarded epoxy resins
used in printed circuit boards (IPCS, 1995a). A secondary use for
TBBPA is as an additive flame retardant in ABS systems. DeBDE is the
second largest volume brominated flame retardant and is the largest
volume brominated flame retardant used solely as an additive. The
greatest use (by volume) of DeBDE is in high-impact polystyrene, which
is primarily used to produce television cabinets. Secondary uses
include ABS, engineering thermoplastics, polyolefins, thermosets, PVC
and elastomers. DeBDE is also widely used in textile applications as
the flame retardant in latex-based back coatings (Pettigrew, 1993).
Hexabromocyclododecane (HBCD), a major brominated cycloaliphatic
flame retardant, is primarily used in polystyrene foam. It is also
used to flame-retard textiles.
2.2.2 Chlorinated flame retardants
Chlorine-containing flame retardants belong to three chemical
groups: aliphatic, cycloaliphatic and aromatic compounds. Chlorinated
paraffins are by far the most widely used aliphatic chlorine-
containing flame retardants. They have applications in plastics,
fabrics, paints and coatings (IPCS, 1996b).
Bis(hexachlorocyclopentadieno)cyclo-octane is a flame retardant
having unusually good thermal stability for a chlorinated
cycloaliphatic. In fact, this compound is comparable in thermal
stability to brominated aromatics in some applications. It is used in
several polymers, especially polyamides and polyolefins for wire and
cable applications. Its principal drawback is the relatively high use
levels required, compared to some brominated flame retardants
(Pettigrew, 1993).
Aromatic chlorinated flame retardants are not used for flame-
retarding polymers.
2.3 Organophosphorus flame retardants
One of the principal classes of flame retardants used in plastics
and textiles is that of phosphorus, phosphorus-nitrogen and
phosphorus-halogen compounds. Phosphate esters, with or without
halogen, are the predominant phosphorus-based flame retardants in use.
For textiles, phosphorus-containing materials are by far the most
important class of compounds used to impart durable flame resistance
to cellulose. These textile flame retardant finishes usually also
contain nitrogen or halogen, or sometimes both (Weil, 1993; Calamari &
Harper, 1993).
2.3.1 Non-halogenated compounds
Although many phosphorus derivatives have flame-retardant
properties, the number of those with commercial importance is limited.
Some are additive and some reactive. The major groups of additive
organophosphorus compounds are phosphate esters, polyols, phosphonium
derivatives and phosphonates. The phosphate esters include trialkyl
derivatives such as triethyl or trioctyl phosphate, triaryl
derivatives such as triphenyl phosphate and aryl-alkyl derivatives
such as 2-ethylhexyl-diphenyl phosphate.
The flame retardancy of cellulosic products can be improved
through the application of phosphonium salts. The flame-retardant
treatments attained by phosphorylation of cellulose in the presence of
a nitrogen compound are also of importance (Calamari & Harper, 1993).
Plasticizers are mixed into polymers to increase flexibility and
workability. The esters formed by reaction of the three functional
groups of phosphoric acid with alcohols or phenols are excellent
plasticizers. The phosphoric acid esters are also remarkable flame
retardants, and for this reason are extensively used in plastics
(Liepins & Pearce, 1976).
Aryl phosphate plasticizers are used in PVC-based products. They
are also used as lubricants for industrial air compressors and gas
turbines. Miscellaneous uses of aryl phosphates are as pigment
dispersants and peroxide carriers, and as additives in adhesives,
lacquer coatings and wood preservatives (Boethling & Cooper, 1985).
2.3.2 Halogenated phosphates
In addition to the above types, flame retardants containing both
chlorine and phosphorus or bromine and phosphorus are used widely.
Halogenated phosphorus flame retardants combine the flame-retardant
properties of both the halogen and the phosphorus groups. In
addition, the halogens reduce the vapour pressure and water solubility
of the flame retardant, thereby contributing to the retention of the
flame retardant in the polymer.
One of the largest selling members of this group, tris(1-chloro-
2-propyl) phosphate (TCPP) is used in polyurethane foam. Tris(2-
chloroethyl) phosphate is used in the manufacture of polyester resins,
polyacrylates, polyurethanes and cellulose derivatives.
The most widely used bromine- and phosphorus-containing flame
retardant used to be tris(2,3-dibromopropyl)phosphate, but it was
withdrawn from use in many countries due to carcinogenic properties in
animals (Liepins & Pearce, 1976; Green, 1992).
2.4 Nitrogen-based flame retardants
Nitrogen-based compounds can be employed in flame-retardant
systems or form part of intumescent flame-retardant formulations.
Nitrogen-based flame retardants are used primarily in nitrogen-
containing polymers such as polyurethanes and polyamides. They are
also utilized in PVC and polyolefins and in the formulation of
intumescent paint systems (Grabner, 1993).
Melamine, melamine cyanurate, other melamine salts and guanidine
compounds are currently the most used group of nitrogen-containing
flame retardants. Melamine is used as a flame retardant additive for
polypropylene and polyethylene. Melamine cyanurate is employed
commercially as a flame retardant for polyamides and terephthalates
(PET/PBT) and is being developed for use in epoxy and polyurethane
resins. Melamine phosphate is also used as a flame retardant for
terephthalates (PET/PBT) and is currently being developed for use in
epoxy and polyurethane flame retardant formulations. Also in the
development stages for use as flame-retardant additives are melamine
salts and melamine formaldehyde for their application in thermoset
resins (Grabner, 1993).
3. MECHANISM OF ACTION OF FLAME RETARDANTS
3.1 General aspects
To understand flame retardants, it is necessary to understand
fire. Fire is a gas-phase reaction. Thus, in order for a substance to
burn, it must become a gas. In the case of a candle the wax melts and
migrates up the wick by capillary action. The wax is pyrolysed to
volatile hydrocarbon fragments on the wick's surface at 600-800°C.
There is no oxygen at the nucleus of the flame. Some of the
hydrocarbon fragments aromatize to soot particles and, in the
luminescent region of the flame, react with water and carbon dioxide
to form carbon monoxide. Most of the pyrolysis gases are carried to
the exterior of the flame and encounter oxygen diffusing inwards. They
react exothermically to produce heat, which melts and decomposes more
wax, maintaining the combustion reaction. If there is adequate oxygen,
the combustion products from the candle are carbon dioxide and water
(Anderson & Christy, 1992).
Natural and synthetic polymers can ignite on exposure to heat.
Ignition occurs either spontaneously or results from an external
source such as a spark or flame. If the heat evolved by the flame is
sufficient to keep the decomposition rate of the polymer above that
required to maintain the evolved combustibles within the flammability
limits, then a self-sustaining combustion cycle will be established
(Fig. 1).
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
A GENERAL INTRODUCTION
Members
Dr L.A. Albert, Xalapa, Veracruz, Mexico
Dr P. Arias, Brussels, Belgium
Dr S.A. Assimon, Contaminants Branch, US Food and Drug Administration,
Washington, DC, USA
Dr H. Hofer, Toxicology, Austrian Research Centre, Seibersdorf,
Austria
Dr B. Jansson, Institute of Applied Environmental Research, Stockholm
University, Stockholm, Sweden ( Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health, Ahmedabad,
India
Dr J. Kielhorn, Fraunhofer Institute of Toxicology and Aerosol
Research, Hanover, Germany ( Vice-Chairman)
Dr R.G. Liteplo, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada
Mr H. Malcolm, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, United Kingdom
Dr E. Sœderlund, Folkehelsa, National Institute of Public Health,
Oslo, Norway ( Rapporteur)
Dr J. Troitzsch, Fire and Environment Protection Service, Wiesbaden,
Germany
Observers
Dr M.L. Hardy, Albermarle Corporation, Baton Rouge, USA
Mr T.A. Jay, Applications Laboratory, Great Lakes Chemical (Europe)
N.V., Geel, Belgium
Dr M. Papez, European Flame Retardants Association, Brussels, Belgium
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization ( Secretary)
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS: A GENERAL
INTRODUCTION
A WHO Task Group on Environmental Health Criteria for Flame
Retardants met at the World Health Organization, Geneva, from 4 to 8
December 1995. Dr K.W. Jager, IPCS, welcomed the participants on
behalf of Dr M. Mercier, Director of the IPCS, and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft monograph and prepared conclusions and
recommendations.
The first draft of the monograph was prepared by Dr G.J. van
Esch, Bilthoven, the Netherlands. The second draft, incorporating
comments received following circulation of the first draft to the IPCS
contact points for Environmental Health Criteria monographs, was
prepared by the IPCS Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both of the IPCS Central Unit,
were responsible for the scientific content of the monograph and the
technical editing, respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ABS acrylonitrile-butadiene-styrene
APP ammonium polyphosphate
ATH alumina trihydrate
DeBDE decabromodiphenyl ether
EPDM ethylene propylene rubber
EPS expandable polystyrene
FR flame retardant
HBCD hexabromocyclododecane
HIPS high impact polystyrene
HDPE high density polyethylene
LDPE low density polyethylene
PA polyamides
PBDE polybrominated diphenyl ether
PBB polybrominated biphenyl
PBDD polybrominated dibenzodioxin
PBDF polybrominated dibenzofuran
PBT polybutylene terephthalate
PCDD polychlorinated dibenzodioxin
PCDF polychlorinated dibenzofuran
PE polyethylene
PET polyethylene terephthalate
PP polypropylene
PVC polyvinyl chloride
TBBPA tetrabromobisphenol A
TCDD 2,3,7,8-tetrachlorinated dibenzo- p-dioxin
TCPP tris(1-chloro-2-propyl)phosphate
PROLOGUE
The intent of this Environmental Health Criteria (EHC) monograph
is to provide a general overview of the nature, mechanism of action,
use and production volume of compounds used to improve the flame
retardancy of polymeric materials and textiles. The monograph also
indicates some of the known health and environmental hazards for
certain of the flame retardants that have been assessed by the IPCS.
A large number of compounds have been identified as being used as
flame retardants. Detailed international assessments of the risks
posed to human health and the environment by a number of these
substances have been published previously as EHC monographs. In Table
1 are listed the monographs on flame retardants and compounds related
to flame retardants that have already been published by IPCS or are in
preparation.
This monograph is intended to provide a starting point for those
interested in obtaining general information on flame-retardant
chemicals. More detailed information on use patterns, sources of
exposure, and health and environmental risks posed by these substances
can be found in the appropriate EHC monograph.
Table 1. EHC monographs on chemicals associated with
flame-retardant use
Substance EHC monograph
Mirex (1,1a,2,2,3,3a,4,5,5,5a,5b,6- EHC 44 (1984)
dodecachloroocta-hydro-1,3,4-metheno-1H-
cyclobuta( cd)pentalene)
Polychlorinated dibenzo- p-dioxins and EHC 88 (1989)
dibenzofurans
Tricresyl phosphate EHC 110 (1990)
Triphenyl phosphate EHC 111 (1991)
Hexachlorocyclopentadiene EHC 120 (1991)
Polychlorinated biphenyls EHC 140 (1992)
Polybrominated biphenyls EHC 152 (1994)
Brominated diphenyl ethers EHC 162 (1994)
Tetrabromobisphenol A and some of its EHC 172 (1995)
derivatives
Tris(2,3-dibromopropyl) phosphate and EHC 173 (1995)
bis(2,3-dibromopropyl) phosphate
Chlorendic acid and anhydride EHC 185 (1996)
Chlorinated paraffins EHC 181 (1996)
Polybrominated dibenzo- p-dioxins and EHC in preparation
dibenzofurans
Vinylbromide EHC in prepraration
Tris(chloropropyl) phosphates EHC in prepraration
Tris(2-butoxyethyl) phosphate EHC in prepraration
Tris(2-chloroethyl) phosphate EHC in prepraration
Tris(2-ethylhexyl) phosphate EHC in prepraration
Tetrakis(hydroxymethyl) phosphonium salts EHC in prepraration
1. INTRODUCTION
In today's society, there is an unprecedented development in the
size and number of buildings, skyscrapers, warehouses and methods of
transport. Carpeting, furnishings, equipment, oil and gas for heating
all increase the fire load in a building. New technologies, new
processes and new applications introduce new fire hazards (e.g., new
ignition sources such as welding sparks and short circuits)
(Troitzsch, 1990). Modern fire-fighting techniques, equipment and
building design have reduced the destruction due to fires. However,
a high fuel load in either a residential or a commercial building can
offset even the best of building construction (Gann, 1993).
Each year, over 3 million fires leading to 29 000 injuries and
4500 deaths are reported in the USA. The direct property losses
exceed $8 billion and the total annual cost has been estimated at over
$100 billion. Personal losses occur mostly in residences where
furniture, wall coverings and clothes are frequently the fuel. Large
financial losses occur in commercial structures such as office
buildings and warehouses. Fires also occur in aeroplanes, buses and
trains (Gann, 1993).
To provide additional protection from fires and to increase
escape time when a fire occurs, methods to enhance the flame
retardance of consumer goods have been developed. Flame retardants
are chemicals added to polymeric materials, both natural and
synthetic, to enhance flame-retardance properties. Flame-retardant
chemicals are most often used to improve the fire performance of low-
to-moderate cost commodity polymers. These flame retardants may be
physically blended with or chemically bonded to the host polymer.
They generally either lower ignition susceptibility or lower flame
spread once ignition has occurred. Some polymers are inherently less
flammable due to more stable polymeric structures; these are usually
higher priced engineering plastics such as polyimides,
polybenzimidazoles and polyetherketones (Gann, 1993).
Flame-retardant systems for synthetic or organic polymers act in
five basic ways: (1) gas dilution; (2) thermal quenching;
(3) protective coating; (4) physical dilution; (5) chemical
interaction (Pettigrew, 1993); or through a combination of these
mechanisms.
1. Inert gas dilution involves using additives that produce large
volumes of non-combustible gases on decomposition. These gases
dilute the oxygen supply to the flame or dilute the fuel
concentration below the flammability limit. Metal hydroxides,
metal salts and some nitrogen compounds function in this way.
2. Thermal quenching is the result of endothermic decomposition of
the flame retardant. Metal hydroxides, metal salts and nitrogen
compounds act to decrease surface temperature and the rate of
burning.
3. Some flame retardants form a protective liquid or char barrier.
This limits the amount of polymer available to the flame front
and/or acts as an insulating layer to reduce the heat transfer
from the flame to the polymer. Phosphorus compounds and
intumescent systems based on melamine and other nitrogen
compounds are examples of this category.
4. Inert fillers (glass fibres and microspheres) and minerals (talc)
act as thermal sinks to increase the heat capacity of the polymer
or reduce its fuel content.
5. Halogens and some phosphorus flame retardants act by chemical
interaction. The flame retardant dissociates into radical
species that compete with chain-propagating steps in the
combustion process.
Chemicals that are used as flame retardants can be inorganic,
organic, mineral, halogen-containing or phosphorus-containing. The
term flame "retardant" represents a class of use and not a class of
chemical structure (Pettigrew, 1993).
Preventive flame protection, including the use of flame
retardants, has been practised since ancient times. Some examples of
early historical developments in flame retardants are shown in
Table 2.
Table 2. Early historical fire-retardant developmentsa
Development Date
Alum used to reduce the flammability of wood by the About 450 BC
Egyptians
The Romans used a mixture of alum and vinegar on About 200 BC
wood
Mixture of clay and gypsum used to reduce 1638
flammability of theatre curtains
Mixture of alum, ferrous sulfate and borax used 1735
on wood and textiles by Wyld in Britain
Alum used to reduce flammability of balloons 1783
Gay-Lussac reported a mixture of (NH4)3PO4, 1821
NH4Cl and borax to be effective on linen and hemp
Perkin described a flame-retardant treatment for 1912
cotton using a mixture of sodium stannate and
ammonium sulfate
a From: Hindersinn (1990)
The advent of synthetic polymers earlier this century was of
special significance, since the water-soluble inorganic salts used up
to that time were of little or no utility in these largely hydrophobic
materials. Modern developments were, therefore, concentrated on the
development of polymer-compatible flame retardants.
By the outbreak of the Second World War, flame-proof canvas
tentage for outdoor use by the military was produced with a treatment
of chlorinated paraffins and an insoluble metal oxide, mostly antimony
oxide as a glow inhibitor, together with a binder resin.
After the war, non-cellulosic thermoplastic polymers became more
and more important as the basic fibres used for flame-retardant
applications. A dramatic example of the superiority of the non-
cellulosic compounds is provided by the diminished use of cotton fibre
in children's sleepwear since the inception of new standards. In
1971, cotton supplied 78% of the fibres used to produce children's
sleepwear, whereas in 1973 it supplied less than 10% in the USA (US
EPA, 1976).
With the increasing use of thermoplastics and thermosets on a
large scale for applications in building, transportation, electrical
engineering and electronics, new flame-retardant systems were
developed. They mainly consist of inorganic and organic compounds
based on bromine, chlorine, phosphorus, nitrogen, boron, and metallic
oxides and hydroxides.
Today, these flame-retardant systems fulfill the multiple
flammability requirements developed for the above-mentioned
applications.
A glossary of terms concerning flammability and flame retardants
is given in Annex 1.
2. TYPES OF FLAME RETARDANTS
A distinction is made between reactive and additive flame
retardants. Reactive flame retardants are reactive components
chemically built into a polymer molecule. Additive flame retardants
are incorporated into the polymer either prior to, during or (most
frequently) following polymerization.
There are three main families of flame-retardant chemicals
(Troitzsch, 1990; Wolf & Kaul, 1992; Green, 1992; Touval, 1993;
Pettigrew, 1993; Weil, 1993).
1. The main inorganic flame retardants are aluminium trihydroxide,
magnesium hydroxide, ammonium polyphosphate and red phosphorus.
This group represents about 50% by volume of the worldwide flame
retardant production. Some of these chemicals are also used as
flame retardant synergists, of which antimony trioxide is the
most important (OECD, 1994).
2. Halogenated products are based primarily on chlorine and
bromine. This group represents about 25% by volume of the
worldwide production (OECD, 1994).
3. Organophosphorus products are primarily phosphate esters and
represent about 20% by volume of the worldwide production.
Products containing phosphorus, chlorine and/or bromine are also
important.
In addition, nitrogen-based flame retardants are used for a
limited number of polymers.
Annex II comprises lists of the different flame retardant
families commercially used at present (A) and those no more in
use (B).
2.1 Inorganic flame retardants
Metal hydroxides form the largest class of all flame retardants
used commercially today and are employed alone or in combination with
other flame retardants to achieve necessary improvements in flame
retardancy. Antimony compounds are used as synergistic co-additives
in combination with halogen compounds, facilitating the reduction in
total flame retardant levels needed to achieve a desired level of
flame retardancy. To a limited extent, compounds of other metals also
act as synergists with halogen compounds. They may be used alone but
are most commonly used with antimony trioxide to enhance other
characteristics, for example, smoke reduction or afterglow
suppression. Ionic compounds have a very long history as flame
retardants for wool- or cellulose-based products. Inorganic
phosphorus compounds are primarily used in polyamides and phenolic
resins, or as components in intumescent formulations.
2.1.1 Metal hydroxides
Metal hydroxides function in both the condensed and gas phases of
a fire by absorbing heat and decomposing to release their water of
hydration. This process cools both the polymer and the flame and
dilutes the flammable gas mixture. The very high concentrations (50
to 80%) required to impart flame retardancy often adversely affect the
mechanical properties of the polymer into which they are incorporated.
Aluminium hydroxide, also known as alumina trihydrate (ATH) is
the largest volume flame retardant in use today. It decomposes when
exposed to temperatures over 200°C, which limits the polymers in
which it can be incorporated. Magnesium hydroxide is stable to
temperatures above 300°C and can be processed into several polymers.
2.1.2 Antimony compounds
Antimony trioxide is not a flame retardant per se, but it is
used as a synergist. It is utilized in plastics, rubbers, textiles,
paper and paints, typically 2-10% by weight, with organochlorine and
organobromine compounds to diminish the flammability of a wide range
of plastics and textiles (IARC, 1989).
Antimony oxides and antimonates must be converted to volatile
species. This is usually accomplished by release of halogen acids at
fire temperatures. The halogen acids react with the antimony-
containing materials to form antimony trihalide and/or antimony halide
oxide. These materials act both in the substrate (condensed phase)
and in the flame to suppress flame propagation. In the condensed
phase, they promote char formation, which acts as a physical barrier
to flame and inhibits the volatilization of flammable materials. In
the flame, the antimony halides and halide oxides, generated in
sufficient volume, provide an inert gas blanket over the substrate,
thus excluding oxygen and preventing flame spread. These compounds
alter the chemical reactions occurring at fire temperatures in the
flame, thus reducing the ease with which oxygen can combine with the
volatile products. It is also suggested that antimony oxychloride or
trichloride reduces the rate at which the halogen leaves the flame
zone, thus increasing the probability of reaction with the reactive
species. Antimony trichloride probably evolves heavy vapours which
form a layer over the condensed phase, stop oxygen attack and thus
choke the flame. It is also assumed that the liquid and solid
antimony trichloride particles contained in the gas phase reduce the
energy content of the flames by wall or surface effects (Troitzsch,
1990).
Other antimony compounds include antimony pentoxide, available
primarily as a stable colloid or as a redispersible powder. It is
designed primarily for highly specialized applications, although
manufacturers suggest it has potential use in fibre and fabric
treatment.
Sodium antimonate (Na2OSb2O5Ê´H2O) is recommended for
formulations in which deep tone colours are required or where antimony
trioxide may promote unwanted chemical reactions.
2.1.3 Boron compounds
Within the class of boron compounds, by far the most widely used
is boric acid. Boric acid (H3BO3) and sodium borate (borax)
(Na2B4O7. 10H2O) are the two flame retardants with the longest
history, and are used primarily with cellulosic material, e.g., cotton
and paper. Both products are effective, but their use is limited to
products for which non-durable flame retardancy is acceptable since
both are very water-soluble.
Zinc borate, however, is water-insoluble and is mostly used in
plastics and rubber products. It is used either as a complete or
partial replacement for antimony oxide in PVC, nylon, polyolefin,
epoxy, EPDM, etc. In most systems, it displays synergism with
antimony oxide. Zinc borate can function as a flame retardant, smoke
suppressant and anti-arcing agent in condensed phase. Recently, zinc
borate has also been used in halogen-free, fire-retardant polymers.
2.1.4 Other metal compounds
Molybdenum compounds have been used as flame retardants in
cellulosic materials for many years and more recently with other
polymers, mainly as smoke suppressants (see section 3.4) (Troitzsch,
1990). They appear to function as condensed-phase flame retardants
(Avento & Touval, 1980). Titanium and zirconium compounds are used
for textiles, especially wool (Calamari & Harper, 1993).
Zinc compounds, such as zinc stannate and zinc hydroxy-stannate,
are also used as synergists and as partial replacements for antimony
trioxide.
2.1.5 Phosphorus compounds
Red phosphorus and ammonium polyphosphate (APP) are used in
various plastics.
Red phosphorus was first investigated in polyurethane foams and
found to be very effective as a flame retardant. It is now used
particularly for polyamides and phenolic applications. The flame-
retarding effect is due, in all probability, to the oxidation of
elemental phosphorus during the combustion process to phosphoric acid
or phosphorus pentoxide. The latter acts by the formation of a
carbonaceous layer in the condensed phase. The formation of fragments
that act by interrupting the radical chain mechanism is also likely.
Ammonium polyphosphate is mainly applied in intumescent coatings
and paints. Intumescent systems puff up to produce foams. Because of
this characteristic they are used to protect materials such as wood
and plastics that are combustible and those like steel that lose their
strength when exposed to high temperatures. Intumescent agents have
been available commercially for many years and are used mainly as
fire-protective coatings. They are now used as flame-retardant
systems for plastics by incorporating the intumescent components in
the polymer matrix, mainly polyolefins, particularly polypropylene
(Troitzsch, 1990).
2.1.6 Other inorganic flame retardants
Other inorganic flame retardants, including ammonium sulfamate
(NH4SONH2) and ammonium bromide (NH4Br), are used primarily with
cellulose-based products and in forest fire-fighting (Weil, 1993).
2.2 Halogenated organic flame retardants
Halogenated flame retardants can be divided into three classes:
aromatic, aliphatic and cycloaliphatic. Bromine and chlorine
compounds are the only halogen compounds having commercial
significance as flame-retardant chemicals. Fluorine compounds are
expensive and, except in special cases, are ineffective because the
C-F bond is too strong. Iodine compounds, although effective, are
expensive and too unstable to be useful (Cullis, 1987; Pettigrew,
1993). The brominated flame retardants are much more numerous than
the chlorinated types because of their higher efficacy (Cullis, 1987).
With repect to processability, halogenated flame retardants vary
in their thermal stability. In general, aromatic brominated flame
retardants are more thermally stable than chlorinated aliphatics,
which are more thermally stable than brominated aliphatics.
Brominated aromatic compounds can be used in thermoplastics at fairly
high temperatures without the use of stabilizers and at very high
temperatures with stabilizers. The thermal stability of the
chlorinated and brominated aliphatics is such that, with few
exceptions, they must be used with thermal stabilizers, such as a tin
compound.
Halogenated flame retardants are either added to or reacted with
the base polymer. Additive flame retardants are those that do not
react in the application designated. There are a few compounds that
can be used as an additive in one application and as a reactive in
another; tetrabromobisphenol A is the most notable example. Reactive
flame retardants become a part of the polymer either by becoming a
part of the backbone or by grafting onto the backbone. The choice of
a reactive flame retardant is more complex than the choice of an
additive type. The development of systems based on reactive flame
retardants is more expensive for the manufacturer, who in effect has
to develop novel co-polymers with the desired chemical, physical and
mechanical properties, as well as the appropriate degree of flame
retardance (Cullis, 1987; Pettigrew, 1993). Synergists such as
antimony oxides are frequently used with halogenated flame retardants.
2.2.1 Brominated flame retardants
Bromine-based flame retardants are highly brominated organic
compounds with a relative molecular mass ranging from 200 to that of
large molecule polymers. They usually contain 50 to 85% (by weight)
of bromine (Cullis, 1987).
The highest volume brominated flame retardant in use today is
tetrabromobisphenol A (TBBPA) (IPCS, 1995a) followed by
decabromodiphenyl ether (DeBDE) (IPCS, 1994b). Both of these flame
retardants are aromatic compounds. The primary use of TBBPA is as a
reactive intermediate in the production of flame-retarded epoxy resins
used in printed circuit boards (IPCS, 1995a). A secondary use for
TBBPA is as an additive flame retardant in ABS systems. DeBDE is the
second largest volume brominated flame retardant and is the largest
volume brominated flame retardant used solely as an additive. The
greatest use (by volume) of DeBDE is in high-impact polystyrene, which
is primarily used to produce television cabinets. Secondary uses
include ABS, engineering thermoplastics, polyolefins, thermosets, PVC
and elastomers. DeBDE is also widely used in textile applications as
the flame retardant in latex-based back coatings (Pettigrew, 1993).
Hexabromocyclododecane (HBCD), a major brominated cycloaliphatic
flame retardant, is primarily used in polystyrene foam. It is also
used to flame-retard textiles.
2.2.2 Chlorinated flame retardants
Chlorine-containing flame retardants belong to three chemical
groups: aliphatic, cycloaliphatic and aromatic compounds. Chlorinated
paraffins are by far the most widely used aliphatic chlorine-
containing flame retardants. They have applications in plastics,
fabrics, paints and coatings (IPCS, 1996b).
Bis(hexachlorocyclopentadieno)cyclo-octane is a flame retardant
having unusually good thermal stability for a chlorinated
cycloaliphatic. In fact, this compound is comparable in thermal
stability to brominated aromatics in some applications. It is used in
several polymers, especially polyamides and polyolefins for wire and
cable applications. Its principal drawback is the relatively high use
levels required, compared to some brominated flame retardants
(Pettigrew, 1993).
Aromatic chlorinated flame retardants are not used for flame-
retarding polymers.
2.3 Organophosphorus flame retardants
One of the principal classes of flame retardants used in plastics
and textiles is that of phosphorus, phosphorus-nitrogen and
phosphorus-halogen compounds. Phosphate esters, with or without
halogen, are the predominant phosphorus-based flame retardants in use.
For textiles, phosphorus-containing materials are by far the most
important class of compounds used to impart durable flame resistance
to cellulose. These textile flame retardant finishes usually also
contain nitrogen or halogen, or sometimes both (Weil, 1993; Calamari &
Harper, 1993).
2.3.1 Non-halogenated compounds
Although many phosphorus derivatives have flame-retardant
properties, the number of those with commercial importance is limited.
Some are additive and some reactive. The major groups of additive
organophosphorus compounds are phosphate esters, polyols, phosphonium
derivatives and phosphonates. The phosphate esters include trialkyl
derivatives such as triethyl or trioctyl phosphate, triaryl
derivatives such as triphenyl phosphate and aryl-alkyl derivatives
such as 2-ethylhexyl-diphenyl phosphate.
The flame retardancy of cellulosic products can be improved
through the application of phosphonium salts. The flame-retardant
treatments attained by phosphorylation of cellulose in the presence of
a nitrogen compound are also of importance (Calamari & Harper, 1993).
Plasticizers are mixed into polymers to increase flexibility and
workability. The esters formed by reaction of the three functional
groups of phosphoric acid with alcohols or phenols are excellent
plasticizers. The phosphoric acid esters are also remarkable flame
retardants, and for this reason are extensively used in plastics
(Liepins & Pearce, 1976).
Aryl phosphate plasticizers are used in PVC-based products. They
are also used as lubricants for industrial air compressors and gas
turbines. Miscellaneous uses of aryl phosphates are as pigment
dispersants and peroxide carriers, and as additives in adhesives,
lacquer coatings and wood preservatives (Boethling & Cooper, 1985).
2.3.2 Halogenated phosphates
In addition to the above types, flame retardants containing both
chlorine and phosphorus or bromine and phosphorus are used widely.
Halogenated phosphorus flame retardants combine the flame-retardant
properties of both the halogen and the phosphorus groups. In
addition, the halogens reduce the vapour pressure and water solubility
of the flame retardant, thereby contributing to the retention of the
flame retardant in the polymer.
One of the largest selling members of this group, tris(1-chloro-
2-propyl) phosphate (TCPP) is used in polyurethane foam. Tris(2-
chloroethyl) phosphate is used in the manufacture of polyester resins,
polyacrylates, polyurethanes and cellulose derivatives.
The most widely used bromine- and phosphorus-containing flame
retardant used to be tris(2,3-dibromopropyl)phosphate, but it was
withdrawn from use in many countries due to carcinogenic properties in
animals (Liepins & Pearce, 1976; Green, 1992).
2.4 Nitrogen-based flame retardants
Nitrogen-based compounds can be employed in flame-retardant
systems or form part of intumescent flame-retardant formulations.
Nitrogen-based flame retardants are used primarily in nitrogen-
containing polymers such as polyurethanes and polyamides. They are
also utilized in PVC and polyolefins and in the formulation of
intumescent paint systems (Grabner, 1993).
Melamine, melamine cyanurate, other melamine salts and guanidine
compounds are currently the most used group of nitrogen-containing
flame retardants. Melamine is used as a flame retardant additive for
polypropylene and polyethylene. Melamine cyanurate is employed
commercially as a flame retardant for polyamides and terephthalates
(PET/PBT) and is being developed for use in epoxy and polyurethane
resins. Melamine phosphate is also used as a flame retardant for
terephthalates (PET/PBT) and is currently being developed for use in
epoxy and polyurethane flame retardant formulations. Also in the
development stages for use as flame-retardant additives are melamine
salts and melamine formaldehyde for their application in thermoset
resins (Grabner, 1993).
3. MECHANISM OF ACTION OF FLAME RETARDANTS
3.1 General aspects
To understand flame retardants, it is necessary to understand
fire. Fire is a gas-phase reaction. Thus, in order for a substance to
burn, it must become a gas. In the case of a candle the wax melts and
migrates up the wick by capillary action. The wax is pyrolysed to
volatile hydrocarbon fragments on the wick's surface at 600-800°C.
There is no oxygen at the nucleus of the flame. Some of the
hydrocarbon fragments aromatize to soot particles and, in the
luminescent region of the flame, react with water and carbon dioxide
to form carbon monoxide. Most of the pyrolysis gases are carried to
the exterior of the flame and encounter oxygen diffusing inwards. They
react exothermically to produce heat, which melts and decomposes more
wax, maintaining the combustion reaction. If there is adequate oxygen,
the combustion products from the candle are carbon dioxide and water
(Anderson & Christy, 1992).
Natural and synthetic polymers can ignite on exposure to heat.
Ignition occurs either spontaneously or results from an external
source such as a spark or flame. If the heat evolved by the flame is
sufficient to keep the decomposition rate of the polymer above that
required to maintain the evolved combustibles within the flammability
limits, then a self-sustaining combustion cycle will be established
(Fig. 1).
 This self-sustaining combustion cycle occurs across both the gas
and condensed phases. Fire retardants act to break this cycle by
affecting chemical and/or physical processes occurring in one or both
of the phases. There are a number of ways in which the self-sustaining
combustion cycle can be interrupted. Whatever the method used, the
end effect is to reduce the rate of heat transfer to the polymer and
thus remove the fuel supply. Troitzsch (1990) described the general
physical and chemical mechanisms of flame-retardant action, in both
the gas and condensed phases and the behaviour of flame retardants.
Fundamentally, four processes are involved in polymer
flammability: preheating, decomposition, ignition and combustion/
propagation. Preheating involves heating of the material by means of
an external source, which raises the temperature of the material at a
rate dependent upon the thermal intensity of the ignition source, the
thermal conductivity of the material, the specific heat of the
material, and the latent heat of fusion and vaporization of the
material. When sufficiently heated, the material begins to degrade,
i.e., it loses its original properties as the weakest bonds begin to
break. Gaseous combustion products are formed, the rate being
dependent upon such factors as intensity of external heat, temperature
required for decomposition, and rate of decomposition. The
concentration of flammable gases increases until it reaches a level
that allows sustained oxidation in the presence of the ignition
source. The ignition characteristics of the gas and the availability
of oxygen are two important variables in any ignition process. After
ignition and removal of the ignition source, combustion becomes self-
propagating if sufficient heat is generated and is radiated back to
the material to continue the decomposition process. The combustion
process is governed by such variables as rate of heat generation, rate
of heat transfer to the surface, surface area, and rates of
decomposition. Flame retardancy, therefore, can be achieved by
eliminating (or improved by retarding) any one of these variables. A
flame retardant should inhibit or even suppress the combustion
process. Depending on their nature, flame retardants can act
chemically and/or physically in the solid, liquid or gas phase. They
interfere with combustion during a particular stage of this process,
i.e. during heating, decomposition, ignition or flame spread
(Troitzsch, 1990).
3.1.1 Physical action
There are several ways in which the combustion process can be
retarded by physical action (Troitzsch, 1990).
(a) By cooling. Endothermic processes triggered by additives cool
the substrate to a temperature below that required to sustain the
combustion process.
(b) By formation of a protective layer (coating). The condensed
combustible layer can be shielded from the gaseous phase with a solid
or gaseous protective layer. The condensed phase is thus cooled,
smaller quantities of pyrolysis gases are evolved, the oxygen
necessary for the combustion process is excluded and heat transfer is
impeded.
(c) By dilution. The incorporation of inert substances (e.g.,
fillers) and additives that evolve inert gases on decomposition
dilutes the fuel in the solid and gaseous phases so that the lower
ignition limit of the gas mixture is not exceeded.
3.1.2 Chemical action
The most significant chemical reactions interfering with the
combustion process take place in the solid and gas phases (Troitzsch,
1990).
(a) Reaction in the gas phase. The free radical mechanism of the
combustion process which takes place in the gas phase is interrupted
by the flame retardant. The exothermic processes are thus stopped,
the system cools down, and the supply of flammable gases is reduced
and eventually completely suppressed.
(b) Reaction in the solid phase. Here two types of reaction can
take place. Firstly, breakdown of the polymer can be accelerated by
the flame retardant, causing pronounced flow of the polymer and,
hence, its withdrawal from the sphere of influence of the flame, which
breaks away. Secondly, the flame retardant can cause a layer of
carbon to form on the polymer surface. This can occur, for example,
through the dehydrating action of the flame retardant generating
double bonds in the polymer. These form the carbonaceous layer by
cyclizing and cross-linking.
Flame retardancy is improved by flame retardants that cause the
formation of a surface film of low thermal conductivity and/or high
reflectivity, which reduces the rate of heating. It is also improved
by flame retardants that might serve as a heat sink by being
preferentially decomposed at low temperature. Finally, it is improved
by flame retardant coatings that, upon exposure to heat, intumesce
into a foamed surface layer with low thermal conductivity properties.
A flame retardant can promote transformation of a plastic into char
and thus limit production of combustible carbon-containing gases.
Simultaneously, the char will decrease thermal conductivity of the
surface. Flame retardants can also chemically alter the decomposition
products, resulting in a lower concentration of combustible gases.
Reduced fuel will result in less heat generation by the flame and may
lead to self-extinction.
Structural modification of the plastic, or use of an additive
flame retardant, might induce decomposition or melting upon exposure
to a heat source so that the material shrinks or drips away from the
heat source. It is also possible to significantly retard the
decomposition process through selection of chemically stable
structural components or structural modifications of a polymer.
In general, anything that will prevent the formation of a
combustible mixture of gases will prevent ignition. However, we may
also distinguish those cases in which the flame retardant or the
modified polymer unit, upon exposure to a heat source, will form gas
mixtures that will react chemically in the gas phase to inhibit
ignition. The goal of flame retardance in the combustion and
propagation stages is to decrease the rate of heat generated or
radiated back to the substrate. Any or all of the above-mentioned
mechanisms could function to prevent a self-sustaining flame (Pearce &
Liepins, 1975).
Flame retardancy occurs both as already stated in the vapour
phase (by interfering with oxidation through removal of free radicals)
and in the condensed phase (charring or altering thermal degradation
processes). Phosphorus acts primarily in the condensed phase by
promoting charring, presumably through the formation of phosphoric
acid and a decreased release of flammable volatiles. However, some
reports indicate that certain organic phosphorus compounds may also
work in the gas phase by scavenging free radicals. Antimony (which
functions only in the presence of a halogen) is believed to work
similarly to phosphorus in the condensed phase and combine with
halogens in the gas phase to scavenge free radicals (HÊ and OHÊ) that
are necessary for combustion. The role of nitrogen is not completely
understood. Nitrogen is known to impart flame retardancy in
combination with phosphorus and also by itself, as in polyamides and
aminoplasts. Bromine and chlorine act in the gas phase by reacting
with free radicals (Ulsamer et al., 1980).
The mechanism for imparting durable flame retardance to cellulose
is that of increasing the quantity of carbon, or char, formed instead
of volatile products of combustion, and flammable tars. Salts that
dissociate to form acids or bases upon heating are usually effective
flame retardants. Salts of strong acids and weak bases are the most
effective compounds. Ammonium and amine salts are generally
effective, as are Lewis acids and bases, either by themselves or when
formed in combustion.
3.2 Condensed phase mechanisms
The role of phosphorus compounds has been extensively studied. In
both cellulose and thermoplastics, phosphorus salts of volatile metals
and most organophosphorus compounds are known to be effective flame
retardants. The formation of char appears to be the key. For example,
although triphenyl phosphate, triphenyl phosphite and triphenyl
phosphine are all equivalent on a phosphorus basis, the more effective
flame retardant compounds act by forming phosphoric acid, which
changes the course of the decomposition of cellulose to form carbon
and water (US EPA, 1976).
The flame-retardant action of phosphorus compounds in cellulose
is believed to proceed by way of initial phosphorylation of the
cellulose, probably by initially formed phosphoric or polyphosphoric
acid. The phosphorylated cellulose then breaks down to water,
phosphoric acid and an unsaturated cellulose analogue, and eventually
to char by repetition of these steps. Certain nitrogen compounds such
as melamines, guanidines, ureas and other amides appear to catalyse
the steps forming cellulose phosphate and are found to enhance or
synergize the flame-retardant action of phosphorus on cellulose.
In polyethylene terephthalate and polymethyl methacrylate the
mechanism of action of phosphorus-based flame retardants has been
shown to involve both a similar decrease in the amount of combustible
volatiles and a similar increase in the amount of residues (aromatic
residues and char). The char formed also acts as a physical barrier to
heat and gases. In rigid polyurethane foams the action of phosphorus
flame retardants also appears to involve char enhancement. In flexible
foam the mechanism is less well understood (Weil,1993).
3.3 Gas-phase mechanisms
In addition to the condensed-phase mechanism, phosphorus flame
retardants can exert gas-phase flame-retardant action. It has been
demonstrated that trimethyl phosphate retards the velocity of a
methane-oxygen flame with about the same molar efficiency as antimony
trioxide (Weil, 1993). The mechanisms of action can differ depending
on the type of compound used as a flame retardant. The mechanism
affects the generation of products of combustion, some of which are
potentially corrosive and toxic.
One mechanism of improving the flame retardancy of thermoplastic
materials is to lower their melting point. This results in the
formation of free radical inhibitors in the flame front and causes the
material to recede from the flame without burning.
Free radical inhibition involves the reduction of gaseous fuels
generated by burning materials. Heating of combustible materials
results in the generation of hydrogen, oxygen, and hydroxide and
peroxide radicals that are subsequently oxidized with flame. Certain
flame retardants act to trap these radicals and thereby prevent their
oxidation. Bromine is more effective than chlorine. For example:
RBr + HÊ -> HBr + RÊ
If the resulting compound R is less readily oxidized than the radical
that is removed, the result is reduced flammability.
Measurements of the limiting oxygen index of polymers show that,
in contrast to the situation with chlorine, the effect of bromine does
depend on the gaseous oxidant involved. This suggests that bromine
compounds act to some extent by interfering with the flame reactions
and it is generally believed that this is probably their principal
mode of action, although they can also affect the condensed-phase
decomposition of the polymer.
Any gas-phase mechanism of flame retardancy by bromine compounds
must by definition involve the release of volatile bromine-containing
species, which then inhibit the flame reactions. In the case of
brominated flame retardants, it is generally assumed that hydrogen
bromide is liberated and reacts with the free radicals responsible for
the propagation of combustion, replacing them by the relatively
unreactive bromine atom.
HÊ + Hbr -> H2 + BrÊ
OHÊ + Hbr -> H2O + BrÊ
The mechanism operating in a particular polymer system will
depend on the mode and ease of breakdown of the brominated flame
retardant present. Some of these compounds are thermally stable and
volatilize when the associated polymer is heated to sufficiently high
temperatures. Others decompose to give substantial amounts of either
lower molecular weight organic bromine compounds or hydrogen bromide
(Cullis, 1987).
The presence of chemically bound bromine can also affect the
rates and modes of thermal decomposition of organic polymers in the
condensed phase. Brominated flame retardants vary considerably in
both their volatility and thermal stability. Although some very
stable compounds volatilize chemically unchanged, others break down
within the polymer or react directly with it in the condensed phase.
Hydrogen bromide is often a product and can significantly influence
the rate and course of polymer decomposition, although its effect is
small in comparison with those which it exerts on polymer combustion
as a whole. However, even thermally stable brominated flame
retardants can affect the decomposition of polymers in the condensed
phase, causing the original polymer breakdown stage to be replaced by
two separate stages, both of which involve polymer and additive. Thus,
it is clear that hydrogen bromide is not the only bromine-containing
compound which affects condensed-phase polymer decomposition and that
organic bromine compounds can also markedly change the rate and mode
of breakdown of organic polymers (Cullis, 1987).
A critical factor governing the effectiveness of brominated flame
retardants and indeed their mechanism of action is their thermal
stability relative to that of the polymers with which they are
associated. The most favourable situation for a flame retardant to be
effective will be one in which its decomposition temperature lies
50°C or so below that of the polymer. In general, decomposition at
this temperature with the liberation of substantial quantities of
hydrogen bromide or elemental bromine is likely to enhance flame-
retardant activity. Owing to the relatively low C-Br bond energy,
bromine compounds generally breakdown at quite low temperatures
(typically 200-300°C). Temperatures in this range overlap well with
the decomposition of many common polymers. This is probably a factor
determining the superior flame-retardant effectiveness of bromine
compounds compared with that of chlorine compounds (Cullis, 1987).
3.4 Co-additives for use with flame retardants
Brominated flame retardants are in some cases used on their own,
but their effectiveness is increased by a variety of co-additives, so
that in practice they are more often used in conjunction with other
compounds or with other elements incorporated into them. Thus, for
example, the addition of small quantities of organic peroxides to
polystyrene greatly reduces the amount of hexabromocyclododecane
needed to give a flame-retardant foam; other free radical initiators
behave in a similar fashion. These compounds appear to act by
promoting depolymerization of the hot polymer, giving a more fluid
melt. More heat is therefore required to keep the polymer alight,
because there is a greater tendency for the more molten material to
drip away from the neighbourhood of the flame (Cullis, 1987;
Troitzsch, 1990).
The flame-retardant properties of bromine compounds, like those
of chlorine compounds, will be considerably enhanced when they are
used in conjunction with other hetero-elements, notably phosphorus,
antimony and certain other metals.
The simultaneous presence of phosphorus in bromine-containing
polymer systems usually serves to improve their degree of flame
retardance, although, contrary to general opinion, bromine and
phosphorus generally exert effects that are largely additive rather
than synergistic.
Sometimes the two elements are present in the same molecule,
e.g., tris(2,3,-dibromopropyl)phosphate. In other systems, however,
it is more convenient to use mixtures of a bromine compound and a
phosphorus compound so that the ratio of the two elements can be
readily adjusted. It has already been pointed out that brominated
flame retardants on their own act predominantly in the gas phase. In
contrast, phosphorus compounds act mainly in the condensed phase,
especially with oxygen-containing polymers. It is therefore of
interest to discover whether, when both elements are present together,
each continues to act in the usual way or new mechanisms come into
operation. However, the evidence here is somewhat conflicting. Studies
of the effects of phosphate esters, with or without bromine present,
on the combustion of polyesters show that more char is formed when the
flame retardant contains bromine, and that most of this bromine
remains in the char. This suggests that the bromine-phosphorus
compound affects primarily the condensed-phase processes. However,
studies of the flammability of rigid polyurethane foams show that the
inhibiting effect of tris(2,3-dibromopropyl)- phosphate on combustion
depends on the nature of the gaseous oxidant, suggesting that the
flame retardant acts here, at least in part, by interfering with
reactions in the gas phase. With hydrocarbon polymers, such as
polyolefins and polystyrene, the major part of the phosphorus present
volatilizes and acts in the gas phase, being apparently converted to
simple species, such as phosphorus and phosphorus oxide free radicals.
These species can then interfere chemically with the reactions
responsible for flame propagation by catalysing the recombination of
the active free radicals involved. In such cases, any bromine present
simultaneously is presumably converted to species such as Brœ and HBr,
which function in the gas phase in the usual way (Cullis, 1987).
Antimony is a much more effective co-additive than phosphorus,
generally in the form of its oxide, Sb2O3. On its own this compound
has no flame-retardant activity and is therefore almost always used in
conjunction with a halogen compound. In general, bromine-antimony
mixtures are more effective than the corresponding chlorine-antimony
systems. The use of antimony trioxide greatly reduces the high levels
normally needed for effective flame retardance of bromine compounds on
their own. The principal mode of action is in the gas phase. If
bromine and antimony are present simultaneously in a burning organic
polymer, the major part of the antimony is volatilized, probably as
SbBr3 or SbOBr. These compounds then provide a ready source of
hydrogen bromide and they also produce in the middle of the combustion
zone a mist of fine particles of solid SbO, which can catalyse the
recombination of the free radicals responsible for flame propagation,
via the formation of transient species like SbOH. A number of other
metal oxides have been investigated as partial or total replacements
for antimony trioxide. Their use, however, has a number of
disadvantages. The most important point is that volatilization of the
bromine occurs at the right stage of the combustion cycle. With zinc
oxide, volatilization takes place too early and the bromine has
disappeared from the system before it can become effective (Cullis,
1987).
It can be concluded that in many, if not most, polymer systems in
which bromine and phosphorus are both present, the two elements tend
to act independently and therefore additively. The important mode of
action of metal oxides as co-additives for brominated flame retardants
is to catalyse the breakdown of the bromine compound and therefore the
release of volatile bromine compounds into the gas phase. However,
metal-bromine compounds may also be formed, and these may have more
specific modes of action in inhibiting polymer combustion (Cullis,
1987).
3.5 Smoke suppressants
Smoke production is determined by numerous parameters. No
comprehensive theory yet exists to describe the formation and
constitution of smoke.
Smoke suppressants rarely act by influencing just one of the
parameters determining smoke generation. Ferrocene, for example, is
effective in suppressing smoke by oxidizing soot in the gas phase as
well as by pronounced charring of the substrate in the condensed
phase. Intumescent systems also contribute to smoke suppression
through creation of a protective char. It is extremely difficult to
divide these multifunctional effects into primary and subsidiary
actions since they are so closely interwoven. At present no uniform
theory on the mode of action of smoke suppressants has been
established (Troitzsch, 1990).
3.5.1 Condensed phase
Smoke suppressants can act physically or chemically in the
condensed phase. Additives can act physically in a similar fashion to
flame retardants, i.e., by coating (glassy coatings, intumescent
foams) or dilution (addition of inert fillers), thus limiting the
formation of pyrolysis products and hence of smoke. Chalk (CaCO3),
frequently used as a filler, acts in some cases not only physically as
a dilutent but also chemically (in PVC, for example) by absorbing
hydrogen chloride or by effecting cross-linking so that the smoke
density is reduced in various ways. The processes contributing to
smoke suppression can be extremely complex.
Smoke can be suppressed by the formation of a charred layer on
the surface of the substrate, e.g., by the use of organic phosphates
in unsaturated polyester resins. In halogen-containing polymers, such
as PVC, iron compounds, e.g., iron (III) chloride, cause charring by
the formation of strong Lewis acids.
Certain compounds such as ferrocene cause condensed-phase
oxidation reactions that are visible as a glow. There is pronounced
evolution of CO and CO2, so that less aromatic precursors are given
off in the gas phase.
Compounds such as MoO3 can reduce the formation of benzene
during the thermal degradation of PVC, probably via chemisorption
reactions in the condensed phase. Relatively stable benzene-MoO3
complexes that suppress smoke development are formed (Troitzsch,
1990).
3.5.2 Gas phase
Smoke suppressants can also act chemically and physically in the
gas phase. The physical effect takes place mainly by shielding the
substrate with heavy gases against thermal attack. They also dilute
the smoke gases and reduce smoke density. In principle, two ways of
suppressing smoke chemically in the gas phase exist: the elimination
of either the soot precursors or the soot itself. Removal of soot
precursors occurs by oxidation of the aromatic species with the help
of transition metal complexes. Soot can also be destroyed oxidatively
by high-energy OH radicals formed by the catalytic action of metal
oxides or hydroxides. Smoke suppression can also be achieved by
eliminating the ionized nuclei necessary for forming soot with the aid
of metal oxides. Finally soot particles can be made to flocculate by
certain transition metal oxides (Troitzsch, 1990).
4. PERFORMANCE CRITERIA FOR AND CHOICE OF FLAME RETARDANTS
At present, the selection of a suitable flame retardant depends
on a variety of factors that severely limit the number of acceptable
materials.
Many countries require extensive information on human and
environmental health effects for new substances before they are
allowed to be put on the market. For existing chemicals such data are
not always available but several national and international programmes
are in the process of gathering this information.
For most chemicals, including flame retardants, the following
information regarding human and environmental health is essential to
understanding a chemical's potential hazards:
1. Data from adequate acute and repeated dose toxicity studies is
needed to understand potential health effects.
2. Data on biodegradability and bioaccumulation potential is needed
as a first step in understanding a chemical's environmental
behaviour and effects.
3. Information on the chemical's possible breakdown and/or
combustion products may also be needed.
4. Since flame retardants are often processed into polymers at
elevated temperatures, consideration of the stability of the
material at the temperature inherent to the polymer processing is
needed, as well as on whether or not the material volatilizes at
that temperature or during use.
5. Consideration should be given to the need for information on the
possible formation of toxic and/or persistent breakdown products
during accidental fires or incineration.
Successfully achieving the desired improvement in flame
retardancy is a necessary precursor to other performance
considerations. The basic flammability characteristics of the polymer
to be used play a major role in the flame-retardant selection process,
as some polymers burn much more readily than others.
Flame-retardant selection is also affected by the test method to
be used to assess flame retardancy. Some tests can be passed with
relatively low levels of many flame retardants, while high levels of
very powerful flame retardants are needed to pass other tests. It is
not possible to provide a comprehensive review in this monograph, but
a short introduction is given in Annex III.
There are many performance issues other than flame retardancy
that must be considered during the selection of a flame retardant for
any use. Just as in applications not needing improved flame
retardancy, a long list of processing and performance requirements
must be met before a material can be accepted for use. The
development of a polymer formulation that meets all of these
requirements involves finding the optimum combination of polymer(s),
flame retardant(s), synergist(s), stabilizer(s), processing aid(s),
and all other additives. This is complex and difficult work requiring
a great deal of time, effort and expense.
Flame retardants may adversely affect the processing
characteristics of polymers. Changes occurring in the viscosity of
liquid systems or in the flow of polymers that are melted during
processing can cause major problems. Significant alteration of the
rate of reaction of thermoset polymers or the speed and degree of
crystallization of thermoplastic polymers may result from the use of
some flame retardants. The temperatures routinely used to process
many polymers severely restrict the number of flame retardants
suitable for incorporation.
Since flame retardants are frequently used at high levels, they
often have a dramatic effect on the basic mechanical properties of
polymers in which they are used. Reduction of strength (tensile,
compression), rigidity, toughness and/or heat resistance are common
problems.
When flame retardants are added to polymers their appearance
(colour, gloss, transparency) and physical properties (density,
hardness, melting and glass transition temperatures, thermal
expansion) often change significantly. Electrical properties
(resistance, dielectric, tracking) are frequently altered, and aging
due to factors such as oxidation, UV radiation, high temperature may
be reduced.
The chemical properties of a flame retardant are often of great
importance in its selection. Resistance to exposure to water,
solvents, acids, bases, oils or other substances may be a requirement
for use. Issues related to solubility, hydrolysis resistance or
reactivity with other formulation components may prevent the use of an
otherwise desirable flame retardant.
The relationship between cost and performance is an essential
consideration in the selection of a flame retardant.
All of the above-mentioned issues also apply to textiles. In
addition, the durability (resistance to cleaning with water or by
other techniques) of the flame retardant system is critical (Jay,
1990).
5. PRODUCTION AND USES OF FLAME RETARDANTS AND FLAME-RETARDED
POLYMERS
5.1 Production
The worldwide demand for flame-retardant chemicals in 1992 was
estimated to be 600 000 tonnes (OECD, 1994). This includes over a
hundred different products, which can be classified according to base
chemical content as depicted in Table 3.
Table 3. Demand for flame retardants according to base chemical
content (from: OECD, 1994)
Base chemicals Demand
(tonnes)
Bromine 150 000
Chlorine 60 000
Phosphorus 100 000
Antimony 50 000
Nitrogen 30 000
Aluminium 170 000
Others 50 000
It is difficult to obtain an accurate picture of market volumes
of flame retardants as reports from different sources appear to
conflict.
Table 4 shows USA market volume trends between 1986 and 1991.
Table 5 presents the annual consumption of different flame
retardants in Japan over the period 1986 - 1994. A comparable table of
global use was not available. Table 5 indicates that the consumption
of brominated flame retardants and antimony oxide in Japan has more
than doubled over this period, compared to the moderate increase in
other flame retardants. The market for hydrated aluminium as a flame
retardant seems to have decreased in Japan, whereas Table 4 (Gann,
1993) shows that an increase occurred in the USA.
Chlorinated paraffins had an estimated world production of
300 000 tonnes/year in 1985 (IPCS, 1996b).
Table 4. Flame retardant market volume (from: Gann, 1993)
Group 1986 1991
(tonnes) (tonnes)
Phosphate esters 20 000 18 000
Halogenated phosphates 13 000 16 000
Chlorinated hydrocarbons 15 000 15 000
Brominated hydrocarbons 28 000 36 000
Brominated bisphenol A 16 000 18 000
Antimony trioxide 22 000 25 000
Borates 8 000 8 000
Aluminium trihydrate 140 000 170 000
Magnesium hydroxide
2 000 3 000
Total
264 000 301 000
5.2 Uses
The consumption of flame retardants in plastics and other
combustible materials is closely linked to regulations covering fire
precautions. The principle regulations relate to the building,
transportation, electrical engineering, furnishing and mining sectors
(Troitzsch, 1990).
A worldwide estimate of the consumption of flame retardants
according to materials is not available but the figures for Europe
listed in the Table 6 should reflect the market in general.
Table 5. Trends in the annual consumption of flame retardants in Japana
Type Compound Amount (tonnes)
1986 1990 1994
Brominated
Tetrabromobisphenol A (TBBPA) 12 000 23 000 24 000
Decabromobiphenyl ether 3 000 10 000 5 500
Octabromobiphenyl ether 600 1 100 500
Tetrabromobiphenyl ether 1 000 1 000 0
Hexabromocyclododecane 600 700 1 600
Bis(tetrabromophthalimido) ethane - 1 000 2 500
Tribromophenol 100 450 3 500
Bis(tribromophenoxy) ethane 400 400 900
TBBPA polycarbonate oligomer - - 2 500
Brominated polystyrene - - 1 300
TBBPA epoxy oligomer - 3 000 7 000
Others 2 400 - 2 150
Subtotal 20 000 40 650 51 450
Chlorinated
Chlorinated paraffins 4 000 4 500 4 300
Others 850 700 900
Subtotal 4 850 5 200 5 200
Phosphoric
Halogenated ester 3 000 3 000 3 100
Non-halogenated ester 4 000 4 400 4 400
Others 1 750 1 750 3 310
Subtotal 8 750 9 150 10 810
Table 5. (contd.)
Type Compound Amount (tonnes)
1986 1990 1994
Inorganic
Antimony oxide 8 300 16 000 17 000
Hydrated aluminium 48 000 37 000 42 000
Others 7 200 8 400 9 000
Subtotal 63 500 61 400 68 000
TOTAL 97 100 116 400 135 460
a Based on the investigation made by Kagaku Kogyo Nippon Co. Ltd. (Japan).
(Personal communication from Isao Watanabe, Osaka Prefectural Institute
of Public Health, Japan).
Table 6. Estimated consumption of flame retardants in western
Europe for 1985 and 1992 according to materials
(from: Sutker, 1988)
Product group Consumption (103 tonnes)
1985 1992
Polystyrene 4.0-4.5 4.5-5.0
ABS 1.0-1.5 1.2-1.8
Polyesters 7.5-8.0 8.5-9.0
Epoxy resins 3.5-4.0 4.0-4.5
Polyolefins 10.0-12.0 11.0-13.0
Polyvinyl chloride) 25.0-27.0 27.0-29.0
Polyurethanes 12.0-13.5 13.5-15.0
Engineering plastics 1.5-1.8 1.7-2.0
Paper and textiles 9.0-10.0 10.0-11.0
Rubber and elastomers 5.0-6.0 6.0-7.0
Other 11.5-11.7 12.6-12.7
Total 90.0-100.0 100.0-110.0
5.2.1 Plastics
The plastics industry is the largest consumer of flame
retardants, estimated at about 95% for the USA in 1991. About 10% of
all plastics contain flame retardants (Wolf & Kaul, 1992). The main
applications are in building materials and furnishings (structural
elements, roofing films, pipes, foamed plastics for insulation,
furniture and wall and floor coverings), transportation (equipment and
fittings for aircraft, ships, automobiles and railroad cars), and in
the electrical industry (cable housings and components for television
sets, office machines, household appliances and lamination of printed
circuits).
The growth in the flame retardant market reflects the enormous
expansion of the plastics industry in recent decades. Between 1988 and
1994, there was a worldwide increase of 20%. Although the USA,
western Europe and Japan are still the largest plastic producers (30,
24 and 12% of the market, respectively), other countries showed the
largest increases between 1988 and 1994, e.g., South Korea (170%);
China (60%); Taiwan (54%) (Anon, 1995).
Examples of flame retardants used in various plastics (Wolf &
Kaul, 1992) are as follows:
PVC: Chlorinated paraffins or phosphate esters, antimony trioxide,
aluminium hydroxide
Acrylonitrile-butadiene-styrene (ABS): Octabromodiphenyl ether,
antimony trioxide
Expandable polystyrene: Hexabromocyclododecane
High-Impact polystyrene (HIPS): Decabromodiphenyl ether or
tetrabromobisphenol A, antimony trioxide
Linear polyester: Brominated organics
Polypropylene: Tetrabromobisphenol A, bis(2,3-dibromopropyl ether),
antimony trioxide
Low-density polyethylene (LDPE) films: chlorinated paraffins,
antimony trioxide
High-density polyethylene (HDPE) and cross-linked polyethylene:
Brominated aromatics
Polyurethane foams: Organophosphates, brominated organic compounds,
alumina trihydrate
Polyamides: Brominated aromatic compounds, chlorinated
cycloaliphatic compounds, antimony trioxide, red phosphorus, melamine
Polycarbonates: Tetrabromobisphenol A, brominated organic oligomers,
sulfonate salts
Unsaturated polyesters: Chlorinated and brominated organic
compounds, antimony trioxide, alumina trihydrate
Epoxy resins: Tetrabromobisphenol A
5.2.2 Textile/furnishing industry
In contrast to the plastics industry, the textile industry is a
much smaller market for flame retardants. However, rather than
employing just one flame retardant, the use of a combination of
chemicals is usually necessary for textiles.
Phosphorus-containing materials are the most important class of
compounds to impart durable flame resistance to cellulose (Calamari &
Harper, 1993). Flame-retardant finishes containing phosphorus
compounds usually also contain nitrogen or bromine, or sometimes both.
Another system is based on halogens (usually bromine) in conjunction
with nitrogen or antimony.
Flame retardants used in furniture/textiles include the following
(Anon, 1992; Calamari & Harper, 1993; EFRA, 1995):
* organic phosphates such as tri-alkyl or tri-aryl phosphates, tri-
chloroalkyl phosphates, dialkyl phosphites,
tetrakis(hydroxymethyl)phosphonium chloride (THPC) and related
structures;
* halogenated compounds such as polybrominated diphenyl ethers
(found in over 50% of treated furniture) and chlorinated
paraffins (rainproof applications);
* inorganic compounds such as antimony trioxide, ammonium bromide,
boric acid and aluminium hydrate.
Details on the flame-retardant types were reported by Calamari &
Harper (1993) and can be found in Annex II.
6. FORMATION OF TOXIC PRODUCTS ON HEATING OR COMBUSTION OF
FLAME-RETARDED PRODUCTS
Natural or synthetic material that burns produces potentially
toxic products. There has been considerable debate on whether
addition of organic flame retardants results in the generation of a
smoke that is more toxic and may result in adverse health effects on
those exposed. There has been concern in particular about the
emission of polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) during manufacture, use and combustion of
brominated flame retardants.
6.1 Toxic products in general
Combustion of any organic chemical may generate carbon monoxide
(CO), which is a highly toxic non-irritating gas, and a variety of
other potentially toxic chemicals. Some of the major toxic products
that can be produced by pyrolysis of flame retardants are: CO, CO2,
HCl, POX, ammonia vapour, bromofurans, HBr, HCN, NOX and phosphoric
acid (Anon, 1992).
In general the acute toxicity of fire atmospheres is determined
mainly by the amount of CO, the source of which is the amount of
generally available flammable material. Most fire victims die in post
flash-over fires where the emission of CO is maximized and the
emission of HCN and other gases is less. The acute toxic potency of
smoke from most materials is lower than that of CO (Hirschler, 1995;
Nelson, 1995).
Flame retardants significantly decrease the burning rate of the
product, reducing heat yields and quantities of toxic gas. In most
cases, smoke was not significantly different in room fire tests
between flame-retarded and non-flame-retarded products (Babrauskas
et al. 1988).
Morikawa et al. (1995) reported toxicity studies on gases from
full-scale room fires involving fire retardant materials (a variety,
but not specified). HCN and CO were the two major toxicants. There was
no evidence that the smoke from flame-retarded materials was more
toxic to rabbits than the smoke from non-flame-retarded materials.
Regarding brominated flame retardants, Cullis (1987) stated that
unless suitable metal oxides or metal carbonates are also present,
virtually all the bromine is eventually converted to gaseous hydrogen
bromide (HBr). This is a corrosive and powerful sensory irritant. In a
fire situation however, it is always carbon monoxide (CO) or hydrogen
cyanide (HCN), rather than an irritant which causes rapid
incapacitation. Owing to its high reactivity, hydrogen bromide is
unlikely to reach dangerously high concentrations (Cullis, 1987).
6.2 Formation of halogenated dibenzofurans and dibenzodioxins
PBDFs and PBDDs can be formed from polybrominated diphenyl ethers
(PBDEs), polybrominated phenols, polybrominated biphenyls (PBBs) and
other brominated flame retardants under various laboratory conditions,
including heating. Because chlorinated derivatives are preferably
formed during pyrolysis, mixed halogen compounds will be predominantly
produced if a chlorine source is also available (Buser, 1987a,b).
As in the case of PCDD/PCDF, it is the 2,3,7,8-substituted
isomers that are toxic.
6.3 Exposure to PBDD/PBDF from polymers containing halogenated
flame retardants
6.3.1 Exposure due to contact or emission from products
containing halogenated flame retardants
Exposure of the general public to PBDD/PBDF impurities in flame-
retardant polymers is unlikely to be of significance. The possible
exposure to PBDD/PBDF from TV sets and computer monitors flame-
retarded with halogenated flame retardants has been discussed in
Environmental Health Criteria 162: Brominated diphenyl ethers and is
unlikely to be of significance (IPCS, 1994b).
6.3.2 Workplace exposure studies
Several studies have been performed to determine whether
PBDD/PBDF is present in the fumes emitted during thermal processes,
such as the extrusion of resins containing halogenated flame
retardants under normal processing conditions at temperatures in the
range of 200 to 250°C (IPCS 1994b, 1995a, in preparation).
Epidemiological studies of workers engaged in processing polymers
with PBDEs have been reported (IPCS, 1994b). Results of PBDD/PBDF
workplace monitoring during polymer processing have also been reported
(IPCS 1994b, 1995a). PBDD/PBDF personnel and room air levels during
processing of PBDEs were < 2 ng/m3 (TCDD equivalent) with the
exception of two samples at the extruder head (128 ng/m3, TCDD
equivalent) (IPCS, 1994b). Engineering controls were successful in
reducing these levels. Workplace control measures should also include
appropriate industrial hygiene measures and monitoring of exposure
(IPCS, 1994b, 1995a).
6.3.3 Formation of PBDD/PBDF from combustion
6.3.3.1 Laboratory pyrolysis experiments
In the late 1980s many pyrolysis experiments (at temperatures of
400-900°C) on brominated flame retardants and flame-retardant systems
were performed and the breakdown products measured. Flame retardants
or intermediates tested included PBBs, PBDEs, 2,4,6-tribromophenol,
pentabromophenol, tetrabromobisphenol A and tetrabromophthalic
anhydride (IPCS 1994b, 1995a, in preparation). Pyrolysis of the flame
retardants alone, as well as with polymer mixtures, was investigated.
As different laboratories carried out the experiments using a variety
of testing methods and conditions, a direct comparison of the many
experiments is not possible. Details of the pyrolysis experiments
involving PBDEs, tetrabromobisphenol A and derivatives, and PBBs are
given in the respective EHC monographs (IPCS 1994b, 1995a, in
preparation).
Although they indicate which flame retardants are likely to form
PBDF (and to a lesser extent PBDD) pyrolysis experiments are not
generally comparable to actual fire situations.
6.3.3.2 Fire tests and fire accidents
Fire tests on televisions have shown smoke and combustion
residues containing high levels of PBDD/PBDF. However, levels from
actual fire accidents involving televisions revealed much lower levels
than those produced under fire test conditions (IPCS, 1994b). Further
studies are discussed in the EHC monograph on PBDD/PBDFs (IPCS, in
preparation).
7. OVERVIEW OF EXPOSURE AND HAZARDS TO HUMANS AND THE ENVIRONMENT
Since flame retardants are a heterogeneous group of diverse
chemicals (see chapter 2 and Annex II), the information presented in
this section only provides a general overview of possible routes of
exposure to chemicals associated with flame-retardant use. This
section also provides a brief summary of the hazards to human health
and to the environment posed by chemicals connected with flame-
retardant use. For detailed information on the extent of exposure and
health and environmental effects of individual substances, the
appropriate specific EHC monographs should be consulted.
The toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles has discussed
in a report by the European Economic Community (Anon, 1992).
7.1 Human exposure
7.1.1 General population
Potential sources of exposure include consumer products,
manufacturing and disposal facilities, and environmental media.
Factors affecting exposure of the general population include the
physical and chemical properties of the product, the extent of
manufacturing and emission controls, the use made of the product
(surface coating, durability of fabric finishes, incorporation into a
polymer, etc.), the end use, and the method of disposal. Potential
routes of exposure for the general population include the dermal route
(contact with flame-retarded textiles), inhalation and ingestion.
7.1.2 Occupational exposure
Occupational exposure may occur during the manufacture,
transport, processing and disposal/recycling of flame retardants.
Routes of exposure could include inhalation, dermal contact and
ingestion. Factors affecting the extent of exposure include industrial
hygiene practices, engineering controls, manufacturing processes and
the type of product. As with any other industrial chemical, workplace
monitoring and good industrial practice can delineate the extent of
any exposure.
7.2 Exposure of the environment
Environmental exposure may occur as a result of the manufacture,
transport, use or waste disposal of flame retardants. Routes of
environmental exposure can include water, air and soil. Factors
affecting exposure include the physical and chemical properties of the
product, emission controls, disposal/recycling methods, volume and
biodegradability/persistence. Environmental monitoring can determine
the extent of environmental exposure.
On the basis of the estimated demand for flame retardants (see
Table 3), more than 1 million tonnes of flame-retardant polymers are
produced each year.
Most flame-retarded products eventually become waste. Municipal
waste is generally disposed of via incineration or landfill.
Incineration of flame-retarded products can produce various toxic
compounds, including halogenated dioxins and furans. The formation of
such compounds and their subsequent release to the environment is a
function of the operating conditions of the incineration plant and the
plant's emission controls.
There is a possibility of flame retardants leaching from products
disposed of in landfills. However, potential risks arising from
landfill processes are also dependent on local management of the whole
landfill. The significance of any release of flame retardants from
disposal sites has yet to be determined.
Some products containing flame retardants, including some
plastics, have been identified as suitable for recycling (Lorenz &
Bahadir, 1993; Meyer et al., 1993).
7.3 Hazards to humans
The hazards to humans associated with some flame retardants have
been outlined in the relevant EHC monographs. For example, the use of
tris(2,3-dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate
was banned in 1977 by the US Consumer Product Safety Commission and in
several other developed countries for use in children's clothing
because of concerns that the chemical might be a human carcinogen and
because of the possibility of significant human exposure through
contact with treated fabrics (IPCS, 1995b). Delayed neurotoxicity
due to tri- ortho-cresyl phosphate (TOCP), one of the tricresyl
phosphate isomers, has been observed in humans (IPCS, 1990). Some
polybrominated biphenyl (PBB) congeners have been shown to produce
chronic toxicity and cancer in experimental animals. However, no
definitive human health effects, correlatable with exposure, were
found in a population in Michigan, USA, accidentally exposed to PBBs
(IPCS, 1994a).
7.4 Hazards to the environment
EHC monographs outline the hazards to the environment associated
with some flame retardants (see Table 1). Some PBB congeners are
persistent and bioaccumulative and may pose a threat especially to
higher levels of the food chain (IPCS, 1994a). Hexachloro-
cyclopentadiene is highly toxic to aquatic organisms. However,
information obtained under environmentally realistic conditions is
limited. The potential hazard to the general environment is expected
to be low (IPCS, 1991b). Low concentrations of triphenyl phosphate
have been detected in environmental samples. Triphenyl phosphate is
rapidly degraded in the environment. However, sediment-dwelling
organisms near production plants may have been exposed to
concentrations high enough to exert toxic effects (IPCS, 1991a).
Tricresyl phosphate is also degraded rapidly in the environment, and
subsequent environmental concentrations are therefore low. The acute
toxicity of tricresyl phosphate to aquatic organisms is low (IPCS,
1990).
Persistence of pentabromodiphenyl ether (PeBDE) and lower
brominated diphenylethers in the environment suggest that commercial
PeBDE should not be used (IPCS, 1994b).
Some flame retardants have come under intense environmental
scrutiny. US EPA has called for additional testing (US EPA, 1992).
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas close to release sources there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate- and long-chain chlorinated paraffin products (IPCS,
1996b).
8. REGULATIONS WITH RESPECT TO FLAME RETARDANTS
Several national regulatory bodies have implemented regulations
on specific substances associated with flame-retardant applications.
In the USA the Interagency Testing Committee (ITC), under the
auspices of the Toxic Substances Control Act (TSCA), makes
recommendations concerning the need for additional testing on
chemicals in the TSCA inventory, including flame retardants. Based on
the information published since 1978, the ITC has made initial testing
recommendations upon 128 brominated flame retardants (US TSCA, 1992;
Walker, 1994; Annex IV).
In the European Community, the use of tris(2,3-dibromopropyl)
phosphate (EC Directive 76/769/EEC) and tris(1-aziridinyl)phosphine
oxide (EC Directive 83/264/EEC) in textiles has been banned. In 1977,
the US Consumer Product Safety Commission banned the use of tris(2,3-
dibromopropyl)phosphate in children's clothing (IPCS, 1995b).
The European Community has also banned the use of PBBs in
textiles (EC Directive 83/264/EEC). Several countries have either
taken or proposed regulatory actions on PBBs, as outlined in Table 7.
Controls on the emissions of dioxins and furans from municipal
solid waste incinerators have been implemented in the United Kingdom
under the Environmental Protection Act (1990). In Germany, a second
modification of the Chemicals Prohibition Ordinance, which was adopted
in 1994, imposes limits on 2,3,7,8-substituted chlorinated dioxins and
furans and, for the first time, on some 2,3,7,8-substituted brominated
dioxins and furans (OECD, 1994).
Table 7. Country-specific actions on PBBs either taken or proposeda
Country Actions
Austria Prohibits the manufacture, placing on the market,
import and use of PBBs and products containing
these substances.
Canada Prohibits the manufacture, use, processing, offer
for sale, selling or importation of PBBs for
commercial, manufacturing or processing purposes.
Denmark Implements EC Directive 89/677 banning the use of
PBBs in textiles.
Finland PBB may not be used in textile articles intended
to come into contact with the skin (in accordance
with EC Directive 83/264).
France Implements EC Directive concerning PBBs and their
use on textiles.
Netherlands Proposed resolution would prohibit the storage of
PBBs or products or preparations containing these
substances or making them available to third
parties. (Exports are excluded from the
resolution).
Norway Ban on PBBs in textiles intended to come into
contact with skin, implementation of EC Directives
76/769/EEC, 83/264 and 89/677.
Sweden Ban on PBBs in textiles intended to come into
contact with skin by implementation of EC
Directive 76/769.
Switzerland Prohibits manufacture, supply, import and use of
PBBs and products containing these substances.
Supply and import of capacitors and transformers
containing PBBs is forbidden.
USA No current production or use. Companies intending
to resume manufacture must notify US EPA 90 days
in advance for approval.
a Adapted from: OECD (1994)
9. CONCLUSIONS AND RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
9.1 Conclusions
Flame retardants are a diverse group of compounds used to improve
the flame retardancy of polymers and other materials. A large variety
of compounds, from inorganic to complex organic molecules, are used as
flame retardants, synergists and smoke suppressants. This overview is
focused on organic compounds, which typically contain halogen and/or
phosphorus.
It is difficult to find accurate figures for the global use of
flame retardants but estimates indicate that more than 600 000 tonnes
are produced annually. Available data indicate a substantial increase
of brominated organic product consumption during the last decade.
There are obvious benefits in using flame retardants, as many
human lifes and property are saved from fire. At present, knowledge
of long-term effects resulting from exposure to flame retardants and
their breakdown products is limited. Most people that die in fires
are killed by carbon monoxide.
The majority of the organic flame retardants are either
covalently bound into polymer molecules (reactive) or mixed into the
polymer (additive). They can act in several ways, either physically
(by cooling, by formation of a protective layer or by dilution of the
matrix) or chemically (by reactions in either the gas or the solid
phase).
A number of factors govern the selection of the type of flame
retardant to be used in a specific application. Some of these are the
flammability of the matrix, processing and performance requirements,
chemical properties and possible hazards to human and environmental
health.
Exposure of the general population to flame retardants can occur
via inhalation, dermal contact and ingestion. Potential sources of
exposure are consumer products, manufacturing/disposal facilities and
environmental media (including food intake). The same routes are
possible for occupational exposure, mainly during production,
processing, transportation and disposal/recycling of the flame
retardants or the treated products. Occupational exposure to the
breakdown products may also occur during fire fighting. As several of
the compounds used are lipophilic and persistent, they may
bioaccumulate. Some of the compounds have been shown to cause organ
damage, genotoxic effects and cancer.
There is also concern for occupational health and environmental
effects from combustion/pyrolysis products, especially the
polyhalogenated dibenzofurans and dibenzo- p-dioxins, from some
organic flame retardants. Other breakdown products also need to be
taken into account.
The properties of a number of flame retardants make them
persistent and/or bioaccumulative, and they may therefore pose hazards
to the environment. Some of the compounds that have been evaluated so
far (polybrominated biphenyls, polybrominated diphenyl ethers and
chlorinated paraffins) have been found to belong to this group. Some
of these have therefore been recommended to not be used.
Several countries have developed regulations affecting the
production, use and disposal of flame retardants. Some include
restrictions on the use of compounds because of potential toxic
effects in humans. Germany has developed rules for the maximum
content of some 2,3,7,8-substituted polychlorinated dibenzo- para-
dioxins and dibenzofurans in products.
The availability of relevant data on flame retardants in the open
literature is limited, especially for some existing chemicals
produced before regulations for commercialization were strengthened
in several countries.
IPCS has issued evaluations for some flame retardants and is
preparing evaluations for others.
9.2 Recommendations for the protection of human health and the
environment
a) Information on the content and nature of flame retardants,
including impurities in products, should be made available to
national authorities.
b) More complete information on the volume of flame retardants
production and consumption should be made available.
c) In view of the increased recycling of flame-retarded products,
consideration could be given to harmonized labelling by an
international forum.
d) Compounds that present a toxic risk to humans and/or the
environment should not be used as flame retardants.
e) Occupational exposure to flame retardants and their breakdown
products should be minimized using appropriate engineering and
good industrial hygiene practices. The exposure of people
working in these operations should be monitored.
f) There is a need for proper assessment of occupational health and
environmental effects from combustion or pyrolysis products of
flame retardants.
g) Emissions to the environment from manufacturing, processing,
transportation and disposal/recycling of products containing
persistent bioaccumulative compounds should be minimized using
best available techniques. The environment in the vicinity of
such operations should be monitored for the compounds used.
h) The use of flame retardants with properties that make them
persistent and bioaccumulative should be avoided.
i) The levels of the major persistent bioaccumulating flame
retardants should be monitored routinely in environmental
matrices (biota and sediments). Some compounds that are no
longer produced should likewise be monitored, in order to
indicate the long-term influence of such products.
10. FURTHER RESEARCH
a) Further studies should be undertaken to elucidate the fate of
flame retardants in disposal/recycling operations.
b) There is a need for further evaluations of flame retardants.
Useful criteria for setting priorities are volume of use,
intrinsic toxic effects on human health and the environment,
exposure assessments, and persistence and bioaccumulation/
biomagnification of flame retardants or their breakdown products.
REFERENCES
Anderson GA & Christy MR (1992) Standards, bans and flame retardants.
Presented at Structural Plastics "92", 5-8 April 1992. Dallas, Texas,
Society of the Plastics Industry, Inc., Structural Plastics Division.
Anon (1992) Toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles - Report to the
European Commission, DG-III, Brussels.
Anon (1995) [Plastic industry of the world.] Kunststoffe, 85(10):
1761-1772 (in German).
Arias P (1992) Brominated diphenyloxides as flame retardants. Bromine
based chemicals. Paris, Organisation for Economic Co-operation and
Development (Unpublished document).
Avento JM & Touval I (1980) Antimony and other inorganic compounds.
In: Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
John Wiley and Sons, vol 10, pp 486-487.
Babrauksas V, Harris RH Jr, Gann RG, Levin BC, Lee BT, Peacock RD,
Paabo M, Twilley W, Yoklavich MF, & Clark HM (1988) Fire hazard
comparison of fire-retarded and non-fire-retarded products.
Washington, DC, US Department of Commerce, National Bureau of
Standards (NBS Special Publication No. 749).
BFR/CEM Working Group (1989) [Polybrominated dibenzodioxins and
dibenzofurans (PBDDs/PBDFs) from flame retardants containing bromine:
Assessment of risk and proposed measures - Report by the Brominated
Flame Retardants/CEM Working Group to the Conference of Environmental
Ministers Bonn, Germany, September 1989.] (Report No. III/4299/89) (in
German).
Boethling RS & Cooper JC (1985) Environmental fate and effects of
triaryl and tri-alkyl/aryl phosphate esters. Residue Rev, 94: 49-99.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants. Chemosphere,
16(8-9): 1873-1876.
Calamari TA & Harper RJ (1993) Flame retardants for textiles. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 998-1022.
Cullis CF (1987) Bromine compounds as flame retardants. In:
Proceedings of the International Conference on Fire Safety, vol 12, pp
307-323.
Dynamac Corporation (1982) An overview of flame retardants: Volumes I
and II (Contract No. 68-01-6239). Rockville, Maryland, Dynamac
Corporation, Environmental Control Division (Prepared for the US
Environmental Protection Agency).
EFRA (1995) European flame retardants and their application. Brussels,
European Flame Retardants Association.
Flick EW (1986) Plastics additives: An industrial guide - Section IX:
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Gann RG (1993) Flame retardants: Overview. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 930-936.
Grabner RS (1993) N-containing flame retardants: An alternative.
Handout at OECD Workshop on Brominated Flame Retardants, Neuchâtel,
Switzerland, 22-25 February 1993.
Green JC (1992) A review of phosphorus-containing flame retardants. J
Fire Sci, 10: 470-487.
Hindersinn RR (1990) Historical aspects of polymer fire retardance.
In: Nelson GL ed. Fire and polymers hazards identification and
prevention. New York, American Chemical Society (ACS Symposium Series
No. 415).
Hirschler MM (1995) Smoke toxicity: How important is it for fire
safety? Handout at the 6th Annual BCC Conference on Flame Retardancy:
Recent advances in flame retardancy of polymeric materials, Stamford,
Connecticut, May 1995, 16pp.
Hutzinger O, Sundström G, & Safe S (1976) Environmental chemistry of
flame retardants: Part I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1975) Some aziridines, N-, S- & O-mustards and selenium.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
18).
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
IARC (1986a) Some chemicals used in plastics and elastomers. Lyon,
International Agency for Research on Cancer (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 39).
IARC (1986b) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
IARC (1989) Some organic solvents, resin monomers and related
compounds, pigments and occupational exposures in paint manufacture
and painting. Lyon, International Agency for Research on Cancer, pp
291-305 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Volume 47).
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 48).
IPCS (1984) Environmental health criteria 44: Mirex. Geneva, World
Health Organization, International Programme on Chemical Safety,
70 pp.
IPCS (1989) Environmental health criteria 88: Polychlorinated dibenzo-
p-dioxins and dibenzofurans. Geneva, World Health Organization,
International Programme on Chemical Safety, 409 pp.
IPCS (1990) Environmental health criteria 110: Tricresyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 122 pp.
IPCS (1991) Environmental health criteria 111: Triphenyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 80 pp.
IPCS (1991) Environmental health criteria 120: Hexachloro-
cyclopentadiene. Geneva, World Health Organization, International
Programme on Chemical Safety, 126 pp.
IPCS (1992) Environmental health criteria 140: Polychlorinated
biphenyls and terphenyls, 2nd ed. Geneva, World Health Organization,
International Programme on Chemical Safety, 692 pp.
IPCS (1994a) Environmental health criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, International Programme
on Chemical Safety, 577 pp.
IPCS (1994b) Environmental health criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, International Programme on
Chemical Safety, 347 pp.
IPCS (1995a) Environmental health criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization, International
Programme on Chemical Safety, 139 pp.
IPCS (1995b) Environmental health criteria 173: Tris(2,3-
dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate. Geneva,
World Health Organization, International Programme on Chemical Safety,
129 pp.
IPCS (1996a) Environmental health criteria 185: Chlorendic acid and
anhydride. Geneva, World Health Organization, International Programme
on Chemical Safety.
IPCS (1996b) Environmental health criteria 181: Chlorinated paraffins.
Geneva, World Health Organization, International Programme on Chemical
Safety.
IPCS (in preparation) Environmental health criteria: Polybrominated
dibenzo-p-dioxins and dibenzofurans. Geneva, World Health
Organization, International Programme on Chemical Safety.
ISO/IEC Guide (1990) Glossary of fire terms and definitions, 1st ed.
Geneva, International Organization for Standardization.
Japan Fire Retardant Association (1988) Voluntary rules of the flame
retardancy and the toxicological (safety) examination for flame
retardant products under the guide-line of the Fire Defence Agency,
Fire Retardant Products Approval Commission. Tokyo, Japan Fire
Retardant Association.
Jay TA (1990) Flame retardants. Presentation to the USSR All-Union
Congress on Flame Retardants at Saki, Crimea, USSR, 10 October 1990.
Kirk-Othmer (1993) Flame retardants. In: Kirk-Othmer encyclopedia of
chemical technology, 4th ed. New York, John Wiley & Sons, vol 10, pp
930-1022.
Kopp A (1990) [Discussions on bromine-containing flame-retardants.]
Berlin, Germany, Federal Ministry for Environment, Nature Conservation
and Nuclear Safety (Document sent to the European Commission,
Brussels) (in German)
Liepins R & Pearce EM (1976) Chemistry and toxicity of flame
retardants for plastics. Environ Health Perspect, 17: 55-63.
Lorenz W & Bahadir M (1993) Recycling of flame retardants containing
printed circuits: a study of the possible formation of polyhalogenated
dibenzodioxins/-furans. Chemosphere, 26(12): 2221-2229.
Meyer H, Neupert M, Pump W, & Willenberg B (1993) [Flame retardants
determine reusability.] Kunststoffe, 83(4): 253-257 (in German).
Morikawa T, Okada T, Kajiwara M, Sato Y, & Tsuda Y (1995) Toxicity of
gases from full-scale room fires involving fire retardant contents. J
Fire Sci, 13(1): 23-42.
Nelson GL (1995) Carbon monoxide and fire toxicity. Handout at the 6th
Annual BCC Conference on Flame Retardancy: "Recent Advances in Flame
Retardancy of Polymeric Materials, Stamford, Connecticut, May 1995 -
16pp.
OECD (1994) Selected brominated flame retardants. Paris, Organisation
for Economic Co-operation and Development, Environment Directorate,
152 pp (Risk Reduction Monograph No. 3).
Pearce EM & Liepins R (1975) Flame retardants. Environ Health
Perspect, 11: 59-69.
Pettigrew A (1993) Halogenated flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 954-976.
Soderlung E & Dybing E (1982) [Flame-retardant toxicology: A
literature survey.] Oslo, National Institute for Public Health
(Unpublished report ) (in Norwegian).
Sutker BJ (1988) Flame retardants. In: Ullmann's encyclopedia of
industrial chemistry, 5th ed. Weinheim, Germany, VCH Verlag, vol A11,
pp 123-140.
Teuerstein A (1990) Brominated FRS, production and uses (Summary).
Data from Eurobrom BV. In: Workshop on Brominated Aromatic Flame
Retardants. Proceedings of a Workshop held at Skoloster, Sweden, 24-26
October 1989. Solna, Swedish National Chemicals Inspectorate, pp
49-52.
Touval I (1993) Antimony and other inorganic flame retardants. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 936-954.
Troitzsch JH (1990) International plastics flammability handbook:
Principles, regulations, testing and approval, 2nd ed. Münich,
Germany, Hanser Publishers.
Ullmann (1988) Ullmann's encyclopedia of industrial chemistry: Volumes
A11 et A26, 5th ed. Weinheim, Germany, VCH Verlag.
Ulsamer AG, Osterberg RE, & McLauglin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US EPA (1976) A study of flame retardants for textiles. Washington,
DC, US Environmental Protection Agency (EPA-560/1-76-001; NTIS
PB-251-44).
US EPA (1989) Twenty-fifth report of the Interagency Testing Committee
to the administrator. Fed Reg, 54(237): 51114-51130.
US EPA (1992) Aryl phosphate base stocks: Proposed test rule and
reporting requirements. Fed Reg, 57(12): 2136-2158.
Walker JD (1994) Testing decisions of the TSCA Interagency Testing
Committee for Brominated Flame Retardants: A review of decisions and
health and safety data. Presented at the Meeting of the Fire Retardant
Chemicals Association, Williamsburg, Virginia, 9-12 October 1994.
Washington, DC, US Environmental Protection Agency.
Weil ED (1993) Phosphorus flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley and
Sons, vol 10, pp 976-998.
Wolf R & Kaul BL (1992) Plastics, additives. In: Ullmann's
encyclopedia of industrial chemistry, ed. Weinheim, Gemany, VCH
Verlag, vol A20, pp 459-507.
ANNEX I
TERMINOLOGY
There is no clear definition for many of the terms used to
describe fire, flammability and flame retardants. As a result, some
confusion has been created by the interchangeable use of terms such as
fire retardant, flame retardant, flame-proof and fire-proof. The
meaning of these and other terms is often clear only in the context.
Therefore, many efforts have been undertaken, at an international
level, to harmonize definitions and terms related to fire, including
fire protection and flame retardants. The definitions compiled in
Table 8 have been taken from the ISO/IEC Guide (1990) and other
sources (e.g., Troitzsch, 1990).
Other terms, which mostly concern mostly textile flame
retardancy, are defined below (US EPA, 1976, Kirk-Othmer, 1993):
Fireproof textile
This term applies only to those fabrics that undergo virtually no
change when exposed to a flame.
Flame-retardant textile
The term flame-retardant textile refers to any fabric that will
not support combustion after the source of ignition is removed. It is
synonymous with the term fire-resistant textile. The textile is
expected to char or melt. The term covers all treatments short of
fire-proofing.
Durability
Durability refers to the ability of a flame-retardant textile to
withstand washing/cleaning, chlorine bleaching, weathering and sun
exposure. A durable treatment is any chemical process which imparts
flame-retardant properties to textiles and textile products that will
last for at least 50 launderings and dry cleaning for the life of the
fabric. A semi-durable treatment will resist water but not withstand
dry cleaning or more than 10 to 15 launderings. A non-durable
treatment is readily removed by water or perspiration and requires
replacement after each exposure of the textile to water. The
definition of durability must be related to the conditions of use of
the textile and the product.
Table 8. Definitions of terms connected with fire
Term Definition
Afterflame Persistence of flaming of a material after the
ignition source has been removed
Afterglow Persistence of glowing of a material after
cessation of flaming or, if no flaming occurs,
after the ignition source has been removed
Burn To undergo combustion
Burning All the physical and/or chemical changes that
behaviour take place when a material or product is exposed
to a specified ignition source
Char Carbonaceous residue resulting from pyrolysis or
incomplete combustion
Combustible Capable of burning
Combustion Exothermic reaction of a substance with an
oxidizer, generally accompanied by flames and/or
glowing and/or emission of smoke
Fire a) A process of combustion characterized by the
emission of heat accompanied by smoke and/or
flame
b) Rapid combustion spreading uncontrolled in
time and space
Fire All the physical and/or chemical changes
behaviour that take place when a material, product and/or
structure is exposed to an uncontrolled fire
Fire The total gaseous, particulate or
effluent aerosol effluent from combustion or pyrolysis
Fire The ability of an element of building
resistance construction to fulfil for a stated period of
time the required load-bearing function,
integrity and/or thermal insulation specified
in the standard fire-resistance test
(see ISO 834)
Flame Zone of combustion in the gaseous phase from
which light is emitted
Table 8. (contd.)
Term Definition
Flame The property of a material either
retardance inherent or by virtue of a substance added or a
treatment applied to suppress, significantly
reduce or delay the propagation of flame
Flame A substance added or a treatment applied
retardant to a material in order to suppress, significantly
reduce or delay the combustion of the material
Flame spread Propagation of a flame front
Flame spread Distance travelled by a flame front
rate during its propagation per unit time under
specified test conditions
Flammability Ability of a material or product to burn with a
flame under specified test conditions
Flammable Capable of burning with a flame under specified
test conditions
Flash over The rapid transition to a state of total surface
involvement in a fire of combustible materials
within an enclosure
Fully The state of total involvement of
developed fire combustible materials in a fire
Glowing Combustion of a material in the solid
combustion phase without flame but with emission of light
from the combustion zone
Heat release The calorific energy released per unit
rate time by a material during combustion under
specified test conditions
Ignition Minimum temperature of a material at
temperature which sustained combustion can be initiated under
specified test conditions
Melting Phenomena accompanying the softening of
behaviour a material under the influence of heat (including
shrinking, dripping, burning of molten material,
etc.)
Table 8. (contd.)
Term Definition
Pyrolysis Irreversible chemical decomposition of a material
due to an increase in temperature without
oxidation
Reaction The response of a material under
to fire specified test conditions in contributing by its
own decomposition to a fire to which it is exposed
Smoke A visible suspension of solid and/or liquid
particles in gases resulting from combustion or
pyrolysis
Smoke The reduction in luminous intensity due
obscuration to passage through smoke
Smouldering The slow combustion of a material without light
being visible and generally evidenced by an
increase in temperature and/or by smoke
Soot Finely divided particles, mainly carbon, produced
and/or deposited during the incomplete combustion
of organic materials
ANNEX II
Flame retardants in commercial use or used formerly
Introduction
Tables 9 and 10 have been compiled on the basis of all the
information on flame retardants available to the IPCS and from the
following sources:
Arias (1992) BFR/CEM Working Group (1989)
Boethling & Cooper (1985) Dynamac Corporation (1982)
EFRA (1995) Flick (1986)
Hutzinger et al. (1976) IARC (1975, 1978, 1979, 1986a,b,
1987, 1989, 1990)
IRPTC (1987) Japan Fire Retardant Association
(1988)
Kirk-Othmer (1993) Kopp (1990)
Liepins & Pearce (1976) Pearce & Liepins (1975)
Sœderlund & Dybing (1982) Teuerstein (1990)
Troitzsch (1990) Ulsamer et al. (1980)
Ullmann (1988) US EPA (1989)
US TSCA (1992)
More than 175 flame retardants, or groups of them are tabulated
in these two tables. On just 17 of them the database was adequate for
preparing a hazard and risk evaluation for man and the environment,
and Environmental Health Criteria (EHC) monographs have been, or are
being prepared, on these. For the others the hazard to man and the
environment has not been evaluated internationally. Most of these
substances also have other major uses. In addition, some chemicals
used as intermediates in the production of flame retardants have beeen
listed. It is likely that these lists are not exhaustive, and that
new chemical structures are being developed as flame retardants.
Table 9 lists flame retardants in commercial use today and some
intermediates, while Table 10 lists flame retardants that have been
used in the past.
The tables list the chemical name, the chemical structure, the
CAS registry number and the uses as flame retardants. The "Use" column
also contains information on the global production volume of those
compounds that are currently being produced commercially (estimated
for the IPCS Task Group by P. Arias, 1995). The international
evaluation status of the substances is indicated in the column
"Remarks".
The following abbreviations are used in this table:
EHC An Environmental Health Criteria monograph on the chemical
has been published. If no EHC number is given, the document
is still in preparation.
IARC The International Agency for Research on Cancer of WHO in
Lyon has evaluated the carcinogenicity.
H High production volume (> 5000 tons per year)
M Moderate production volume (1000-5000 tons per year)
L Low production volume (<1000 tons per year or in
the developmental stage)
Table 9. Flame retardants being used commercially today
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Inorganic flame retardants
Potassium fluorotitanate K2TiF6
This self-sustaining combustion cycle occurs across both the gas
and condensed phases. Fire retardants act to break this cycle by
affecting chemical and/or physical processes occurring in one or both
of the phases. There are a number of ways in which the self-sustaining
combustion cycle can be interrupted. Whatever the method used, the
end effect is to reduce the rate of heat transfer to the polymer and
thus remove the fuel supply. Troitzsch (1990) described the general
physical and chemical mechanisms of flame-retardant action, in both
the gas and condensed phases and the behaviour of flame retardants.
Fundamentally, four processes are involved in polymer
flammability: preheating, decomposition, ignition and combustion/
propagation. Preheating involves heating of the material by means of
an external source, which raises the temperature of the material at a
rate dependent upon the thermal intensity of the ignition source, the
thermal conductivity of the material, the specific heat of the
material, and the latent heat of fusion and vaporization of the
material. When sufficiently heated, the material begins to degrade,
i.e., it loses its original properties as the weakest bonds begin to
break. Gaseous combustion products are formed, the rate being
dependent upon such factors as intensity of external heat, temperature
required for decomposition, and rate of decomposition. The
concentration of flammable gases increases until it reaches a level
that allows sustained oxidation in the presence of the ignition
source. The ignition characteristics of the gas and the availability
of oxygen are two important variables in any ignition process. After
ignition and removal of the ignition source, combustion becomes self-
propagating if sufficient heat is generated and is radiated back to
the material to continue the decomposition process. The combustion
process is governed by such variables as rate of heat generation, rate
of heat transfer to the surface, surface area, and rates of
decomposition. Flame retardancy, therefore, can be achieved by
eliminating (or improved by retarding) any one of these variables. A
flame retardant should inhibit or even suppress the combustion
process. Depending on their nature, flame retardants can act
chemically and/or physically in the solid, liquid or gas phase. They
interfere with combustion during a particular stage of this process,
i.e. during heating, decomposition, ignition or flame spread
(Troitzsch, 1990).
3.1.1 Physical action
There are several ways in which the combustion process can be
retarded by physical action (Troitzsch, 1990).
(a) By cooling. Endothermic processes triggered by additives cool
the substrate to a temperature below that required to sustain the
combustion process.
(b) By formation of a protective layer (coating). The condensed
combustible layer can be shielded from the gaseous phase with a solid
or gaseous protective layer. The condensed phase is thus cooled,
smaller quantities of pyrolysis gases are evolved, the oxygen
necessary for the combustion process is excluded and heat transfer is
impeded.
(c) By dilution. The incorporation of inert substances (e.g.,
fillers) and additives that evolve inert gases on decomposition
dilutes the fuel in the solid and gaseous phases so that the lower
ignition limit of the gas mixture is not exceeded.
3.1.2 Chemical action
The most significant chemical reactions interfering with the
combustion process take place in the solid and gas phases (Troitzsch,
1990).
(a) Reaction in the gas phase. The free radical mechanism of the
combustion process which takes place in the gas phase is interrupted
by the flame retardant. The exothermic processes are thus stopped,
the system cools down, and the supply of flammable gases is reduced
and eventually completely suppressed.
(b) Reaction in the solid phase. Here two types of reaction can
take place. Firstly, breakdown of the polymer can be accelerated by
the flame retardant, causing pronounced flow of the polymer and,
hence, its withdrawal from the sphere of influence of the flame, which
breaks away. Secondly, the flame retardant can cause a layer of
carbon to form on the polymer surface. This can occur, for example,
through the dehydrating action of the flame retardant generating
double bonds in the polymer. These form the carbonaceous layer by
cyclizing and cross-linking.
Flame retardancy is improved by flame retardants that cause the
formation of a surface film of low thermal conductivity and/or high
reflectivity, which reduces the rate of heating. It is also improved
by flame retardants that might serve as a heat sink by being
preferentially decomposed at low temperature. Finally, it is improved
by flame retardant coatings that, upon exposure to heat, intumesce
into a foamed surface layer with low thermal conductivity properties.
A flame retardant can promote transformation of a plastic into char
and thus limit production of combustible carbon-containing gases.
Simultaneously, the char will decrease thermal conductivity of the
surface. Flame retardants can also chemically alter the decomposition
products, resulting in a lower concentration of combustible gases.
Reduced fuel will result in less heat generation by the flame and may
lead to self-extinction.
Structural modification of the plastic, or use of an additive
flame retardant, might induce decomposition or melting upon exposure
to a heat source so that the material shrinks or drips away from the
heat source. It is also possible to significantly retard the
decomposition process through selection of chemically stable
structural components or structural modifications of a polymer.
In general, anything that will prevent the formation of a
combustible mixture of gases will prevent ignition. However, we may
also distinguish those cases in which the flame retardant or the
modified polymer unit, upon exposure to a heat source, will form gas
mixtures that will react chemically in the gas phase to inhibit
ignition. The goal of flame retardance in the combustion and
propagation stages is to decrease the rate of heat generated or
radiated back to the substrate. Any or all of the above-mentioned
mechanisms could function to prevent a self-sustaining flame (Pearce &
Liepins, 1975).
Flame retardancy occurs both as already stated in the vapour
phase (by interfering with oxidation through removal of free radicals)
and in the condensed phase (charring or altering thermal degradation
processes). Phosphorus acts primarily in the condensed phase by
promoting charring, presumably through the formation of phosphoric
acid and a decreased release of flammable volatiles. However, some
reports indicate that certain organic phosphorus compounds may also
work in the gas phase by scavenging free radicals. Antimony (which
functions only in the presence of a halogen) is believed to work
similarly to phosphorus in the condensed phase and combine with
halogens in the gas phase to scavenge free radicals (HÊ and OHÊ) that
are necessary for combustion. The role of nitrogen is not completely
understood. Nitrogen is known to impart flame retardancy in
combination with phosphorus and also by itself, as in polyamides and
aminoplasts. Bromine and chlorine act in the gas phase by reacting
with free radicals (Ulsamer et al., 1980).
The mechanism for imparting durable flame retardance to cellulose
is that of increasing the quantity of carbon, or char, formed instead
of volatile products of combustion, and flammable tars. Salts that
dissociate to form acids or bases upon heating are usually effective
flame retardants. Salts of strong acids and weak bases are the most
effective compounds. Ammonium and amine salts are generally
effective, as are Lewis acids and bases, either by themselves or when
formed in combustion.
3.2 Condensed phase mechanisms
The role of phosphorus compounds has been extensively studied. In
both cellulose and thermoplastics, phosphorus salts of volatile metals
and most organophosphorus compounds are known to be effective flame
retardants. The formation of char appears to be the key. For example,
although triphenyl phosphate, triphenyl phosphite and triphenyl
phosphine are all equivalent on a phosphorus basis, the more effective
flame retardant compounds act by forming phosphoric acid, which
changes the course of the decomposition of cellulose to form carbon
and water (US EPA, 1976).
The flame-retardant action of phosphorus compounds in cellulose
is believed to proceed by way of initial phosphorylation of the
cellulose, probably by initially formed phosphoric or polyphosphoric
acid. The phosphorylated cellulose then breaks down to water,
phosphoric acid and an unsaturated cellulose analogue, and eventually
to char by repetition of these steps. Certain nitrogen compounds such
as melamines, guanidines, ureas and other amides appear to catalyse
the steps forming cellulose phosphate and are found to enhance or
synergize the flame-retardant action of phosphorus on cellulose.
In polyethylene terephthalate and polymethyl methacrylate the
mechanism of action of phosphorus-based flame retardants has been
shown to involve both a similar decrease in the amount of combustible
volatiles and a similar increase in the amount of residues (aromatic
residues and char). The char formed also acts as a physical barrier to
heat and gases. In rigid polyurethane foams the action of phosphorus
flame retardants also appears to involve char enhancement. In flexible
foam the mechanism is less well understood (Weil,1993).
3.3 Gas-phase mechanisms
In addition to the condensed-phase mechanism, phosphorus flame
retardants can exert gas-phase flame-retardant action. It has been
demonstrated that trimethyl phosphate retards the velocity of a
methane-oxygen flame with about the same molar efficiency as antimony
trioxide (Weil, 1993). The mechanisms of action can differ depending
on the type of compound used as a flame retardant. The mechanism
affects the generation of products of combustion, some of which are
potentially corrosive and toxic.
One mechanism of improving the flame retardancy of thermoplastic
materials is to lower their melting point. This results in the
formation of free radical inhibitors in the flame front and causes the
material to recede from the flame without burning.
Free radical inhibition involves the reduction of gaseous fuels
generated by burning materials. Heating of combustible materials
results in the generation of hydrogen, oxygen, and hydroxide and
peroxide radicals that are subsequently oxidized with flame. Certain
flame retardants act to trap these radicals and thereby prevent their
oxidation. Bromine is more effective than chlorine. For example:
RBr + HÊ -> HBr + RÊ
If the resulting compound R is less readily oxidized than the radical
that is removed, the result is reduced flammability.
Measurements of the limiting oxygen index of polymers show that,
in contrast to the situation with chlorine, the effect of bromine does
depend on the gaseous oxidant involved. This suggests that bromine
compounds act to some extent by interfering with the flame reactions
and it is generally believed that this is probably their principal
mode of action, although they can also affect the condensed-phase
decomposition of the polymer.
Any gas-phase mechanism of flame retardancy by bromine compounds
must by definition involve the release of volatile bromine-containing
species, which then inhibit the flame reactions. In the case of
brominated flame retardants, it is generally assumed that hydrogen
bromide is liberated and reacts with the free radicals responsible for
the propagation of combustion, replacing them by the relatively
unreactive bromine atom.
HÊ + Hbr -> H2 + BrÊ
OHÊ + Hbr -> H2O + BrÊ
The mechanism operating in a particular polymer system will
depend on the mode and ease of breakdown of the brominated flame
retardant present. Some of these compounds are thermally stable and
volatilize when the associated polymer is heated to sufficiently high
temperatures. Others decompose to give substantial amounts of either
lower molecular weight organic bromine compounds or hydrogen bromide
(Cullis, 1987).
The presence of chemically bound bromine can also affect the
rates and modes of thermal decomposition of organic polymers in the
condensed phase. Brominated flame retardants vary considerably in
both their volatility and thermal stability. Although some very
stable compounds volatilize chemically unchanged, others break down
within the polymer or react directly with it in the condensed phase.
Hydrogen bromide is often a product and can significantly influence
the rate and course of polymer decomposition, although its effect is
small in comparison with those which it exerts on polymer combustion
as a whole. However, even thermally stable brominated flame
retardants can affect the decomposition of polymers in the condensed
phase, causing the original polymer breakdown stage to be replaced by
two separate stages, both of which involve polymer and additive. Thus,
it is clear that hydrogen bromide is not the only bromine-containing
compound which affects condensed-phase polymer decomposition and that
organic bromine compounds can also markedly change the rate and mode
of breakdown of organic polymers (Cullis, 1987).
A critical factor governing the effectiveness of brominated flame
retardants and indeed their mechanism of action is their thermal
stability relative to that of the polymers with which they are
associated. The most favourable situation for a flame retardant to be
effective will be one in which its decomposition temperature lies
50°C or so below that of the polymer. In general, decomposition at
this temperature with the liberation of substantial quantities of
hydrogen bromide or elemental bromine is likely to enhance flame-
retardant activity. Owing to the relatively low C-Br bond energy,
bromine compounds generally breakdown at quite low temperatures
(typically 200-300°C). Temperatures in this range overlap well with
the decomposition of many common polymers. This is probably a factor
determining the superior flame-retardant effectiveness of bromine
compounds compared with that of chlorine compounds (Cullis, 1987).
3.4 Co-additives for use with flame retardants
Brominated flame retardants are in some cases used on their own,
but their effectiveness is increased by a variety of co-additives, so
that in practice they are more often used in conjunction with other
compounds or with other elements incorporated into them. Thus, for
example, the addition of small quantities of organic peroxides to
polystyrene greatly reduces the amount of hexabromocyclododecane
needed to give a flame-retardant foam; other free radical initiators
behave in a similar fashion. These compounds appear to act by
promoting depolymerization of the hot polymer, giving a more fluid
melt. More heat is therefore required to keep the polymer alight,
because there is a greater tendency for the more molten material to
drip away from the neighbourhood of the flame (Cullis, 1987;
Troitzsch, 1990).
The flame-retardant properties of bromine compounds, like those
of chlorine compounds, will be considerably enhanced when they are
used in conjunction with other hetero-elements, notably phosphorus,
antimony and certain other metals.
The simultaneous presence of phosphorus in bromine-containing
polymer systems usually serves to improve their degree of flame
retardance, although, contrary to general opinion, bromine and
phosphorus generally exert effects that are largely additive rather
than synergistic.
Sometimes the two elements are present in the same molecule,
e.g., tris(2,3,-dibromopropyl)phosphate. In other systems, however,
it is more convenient to use mixtures of a bromine compound and a
phosphorus compound so that the ratio of the two elements can be
readily adjusted. It has already been pointed out that brominated
flame retardants on their own act predominantly in the gas phase. In
contrast, phosphorus compounds act mainly in the condensed phase,
especially with oxygen-containing polymers. It is therefore of
interest to discover whether, when both elements are present together,
each continues to act in the usual way or new mechanisms come into
operation. However, the evidence here is somewhat conflicting. Studies
of the effects of phosphate esters, with or without bromine present,
on the combustion of polyesters show that more char is formed when the
flame retardant contains bromine, and that most of this bromine
remains in the char. This suggests that the bromine-phosphorus
compound affects primarily the condensed-phase processes. However,
studies of the flammability of rigid polyurethane foams show that the
inhibiting effect of tris(2,3-dibromopropyl)- phosphate on combustion
depends on the nature of the gaseous oxidant, suggesting that the
flame retardant acts here, at least in part, by interfering with
reactions in the gas phase. With hydrocarbon polymers, such as
polyolefins and polystyrene, the major part of the phosphorus present
volatilizes and acts in the gas phase, being apparently converted to
simple species, such as phosphorus and phosphorus oxide free radicals.
These species can then interfere chemically with the reactions
responsible for flame propagation by catalysing the recombination of
the active free radicals involved. In such cases, any bromine present
simultaneously is presumably converted to species such as Brœ and HBr,
which function in the gas phase in the usual way (Cullis, 1987).
Antimony is a much more effective co-additive than phosphorus,
generally in the form of its oxide, Sb2O3. On its own this compound
has no flame-retardant activity and is therefore almost always used in
conjunction with a halogen compound. In general, bromine-antimony
mixtures are more effective than the corresponding chlorine-antimony
systems. The use of antimony trioxide greatly reduces the high levels
normally needed for effective flame retardance of bromine compounds on
their own. The principal mode of action is in the gas phase. If
bromine and antimony are present simultaneously in a burning organic
polymer, the major part of the antimony is volatilized, probably as
SbBr3 or SbOBr. These compounds then provide a ready source of
hydrogen bromide and they also produce in the middle of the combustion
zone a mist of fine particles of solid SbO, which can catalyse the
recombination of the free radicals responsible for flame propagation,
via the formation of transient species like SbOH. A number of other
metal oxides have been investigated as partial or total replacements
for antimony trioxide. Their use, however, has a number of
disadvantages. The most important point is that volatilization of the
bromine occurs at the right stage of the combustion cycle. With zinc
oxide, volatilization takes place too early and the bromine has
disappeared from the system before it can become effective (Cullis,
1987).
It can be concluded that in many, if not most, polymer systems in
which bromine and phosphorus are both present, the two elements tend
to act independently and therefore additively. The important mode of
action of metal oxides as co-additives for brominated flame retardants
is to catalyse the breakdown of the bromine compound and therefore the
release of volatile bromine compounds into the gas phase. However,
metal-bromine compounds may also be formed, and these may have more
specific modes of action in inhibiting polymer combustion (Cullis,
1987).
3.5 Smoke suppressants
Smoke production is determined by numerous parameters. No
comprehensive theory yet exists to describe the formation and
constitution of smoke.
Smoke suppressants rarely act by influencing just one of the
parameters determining smoke generation. Ferrocene, for example, is
effective in suppressing smoke by oxidizing soot in the gas phase as
well as by pronounced charring of the substrate in the condensed
phase. Intumescent systems also contribute to smoke suppression
through creation of a protective char. It is extremely difficult to
divide these multifunctional effects into primary and subsidiary
actions since they are so closely interwoven. At present no uniform
theory on the mode of action of smoke suppressants has been
established (Troitzsch, 1990).
3.5.1 Condensed phase
Smoke suppressants can act physically or chemically in the
condensed phase. Additives can act physically in a similar fashion to
flame retardants, i.e., by coating (glassy coatings, intumescent
foams) or dilution (addition of inert fillers), thus limiting the
formation of pyrolysis products and hence of smoke. Chalk (CaCO3),
frequently used as a filler, acts in some cases not only physically as
a dilutent but also chemically (in PVC, for example) by absorbing
hydrogen chloride or by effecting cross-linking so that the smoke
density is reduced in various ways. The processes contributing to
smoke suppression can be extremely complex.
Smoke can be suppressed by the formation of a charred layer on
the surface of the substrate, e.g., by the use of organic phosphates
in unsaturated polyester resins. In halogen-containing polymers, such
as PVC, iron compounds, e.g., iron (III) chloride, cause charring by
the formation of strong Lewis acids.
Certain compounds such as ferrocene cause condensed-phase
oxidation reactions that are visible as a glow. There is pronounced
evolution of CO and CO2, so that less aromatic precursors are given
off in the gas phase.
Compounds such as MoO3 can reduce the formation of benzene
during the thermal degradation of PVC, probably via chemisorption
reactions in the condensed phase. Relatively stable benzene-MoO3
complexes that suppress smoke development are formed (Troitzsch,
1990).
3.5.2 Gas phase
Smoke suppressants can also act chemically and physically in the
gas phase. The physical effect takes place mainly by shielding the
substrate with heavy gases against thermal attack. They also dilute
the smoke gases and reduce smoke density. In principle, two ways of
suppressing smoke chemically in the gas phase exist: the elimination
of either the soot precursors or the soot itself. Removal of soot
precursors occurs by oxidation of the aromatic species with the help
of transition metal complexes. Soot can also be destroyed oxidatively
by high-energy OH radicals formed by the catalytic action of metal
oxides or hydroxides. Smoke suppression can also be achieved by
eliminating the ionized nuclei necessary for forming soot with the aid
of metal oxides. Finally soot particles can be made to flocculate by
certain transition metal oxides (Troitzsch, 1990).
4. PERFORMANCE CRITERIA FOR AND CHOICE OF FLAME RETARDANTS
At present, the selection of a suitable flame retardant depends
on a variety of factors that severely limit the number of acceptable
materials.
Many countries require extensive information on human and
environmental health effects for new substances before they are
allowed to be put on the market. For existing chemicals such data are
not always available but several national and international programmes
are in the process of gathering this information.
For most chemicals, including flame retardants, the following
information regarding human and environmental health is essential to
understanding a chemical's potential hazards:
1. Data from adequate acute and repeated dose toxicity studies is
needed to understand potential health effects.
2. Data on biodegradability and bioaccumulation potential is needed
as a first step in understanding a chemical's environmental
behaviour and effects.
3. Information on the chemical's possible breakdown and/or
combustion products may also be needed.
4. Since flame retardants are often processed into polymers at
elevated temperatures, consideration of the stability of the
material at the temperature inherent to the polymer processing is
needed, as well as on whether or not the material volatilizes at
that temperature or during use.
5. Consideration should be given to the need for information on the
possible formation of toxic and/or persistent breakdown products
during accidental fires or incineration.
Successfully achieving the desired improvement in flame
retardancy is a necessary precursor to other performance
considerations. The basic flammability characteristics of the polymer
to be used play a major role in the flame-retardant selection process,
as some polymers burn much more readily than others.
Flame-retardant selection is also affected by the test method to
be used to assess flame retardancy. Some tests can be passed with
relatively low levels of many flame retardants, while high levels of
very powerful flame retardants are needed to pass other tests. It is
not possible to provide a comprehensive review in this monograph, but
a short introduction is given in Annex III.
There are many performance issues other than flame retardancy
that must be considered during the selection of a flame retardant for
any use. Just as in applications not needing improved flame
retardancy, a long list of processing and performance requirements
must be met before a material can be accepted for use. The
development of a polymer formulation that meets all of these
requirements involves finding the optimum combination of polymer(s),
flame retardant(s), synergist(s), stabilizer(s), processing aid(s),
and all other additives. This is complex and difficult work requiring
a great deal of time, effort and expense.
Flame retardants may adversely affect the processing
characteristics of polymers. Changes occurring in the viscosity of
liquid systems or in the flow of polymers that are melted during
processing can cause major problems. Significant alteration of the
rate of reaction of thermoset polymers or the speed and degree of
crystallization of thermoplastic polymers may result from the use of
some flame retardants. The temperatures routinely used to process
many polymers severely restrict the number of flame retardants
suitable for incorporation.
Since flame retardants are frequently used at high levels, they
often have a dramatic effect on the basic mechanical properties of
polymers in which they are used. Reduction of strength (tensile,
compression), rigidity, toughness and/or heat resistance are common
problems.
When flame retardants are added to polymers their appearance
(colour, gloss, transparency) and physical properties (density,
hardness, melting and glass transition temperatures, thermal
expansion) often change significantly. Electrical properties
(resistance, dielectric, tracking) are frequently altered, and aging
due to factors such as oxidation, UV radiation, high temperature may
be reduced.
The chemical properties of a flame retardant are often of great
importance in its selection. Resistance to exposure to water,
solvents, acids, bases, oils or other substances may be a requirement
for use. Issues related to solubility, hydrolysis resistance or
reactivity with other formulation components may prevent the use of an
otherwise desirable flame retardant.
The relationship between cost and performance is an essential
consideration in the selection of a flame retardant.
All of the above-mentioned issues also apply to textiles. In
addition, the durability (resistance to cleaning with water or by
other techniques) of the flame retardant system is critical (Jay,
1990).
5. PRODUCTION AND USES OF FLAME RETARDANTS AND FLAME-RETARDED
POLYMERS
5.1 Production
The worldwide demand for flame-retardant chemicals in 1992 was
estimated to be 600 000 tonnes (OECD, 1994). This includes over a
hundred different products, which can be classified according to base
chemical content as depicted in Table 3.
Table 3. Demand for flame retardants according to base chemical
content (from: OECD, 1994)
Base chemicals Demand
(tonnes)
Bromine 150 000
Chlorine 60 000
Phosphorus 100 000
Antimony 50 000
Nitrogen 30 000
Aluminium 170 000
Others 50 000
It is difficult to obtain an accurate picture of market volumes
of flame retardants as reports from different sources appear to
conflict.
Table 4 shows USA market volume trends between 1986 and 1991.
Table 5 presents the annual consumption of different flame
retardants in Japan over the period 1986 - 1994. A comparable table of
global use was not available. Table 5 indicates that the consumption
of brominated flame retardants and antimony oxide in Japan has more
than doubled over this period, compared to the moderate increase in
other flame retardants. The market for hydrated aluminium as a flame
retardant seems to have decreased in Japan, whereas Table 4 (Gann,
1993) shows that an increase occurred in the USA.
Chlorinated paraffins had an estimated world production of
300 000 tonnes/year in 1985 (IPCS, 1996b).
Table 4. Flame retardant market volume (from: Gann, 1993)
Group 1986 1991
(tonnes) (tonnes)
Phosphate esters 20 000 18 000
Halogenated phosphates 13 000 16 000
Chlorinated hydrocarbons 15 000 15 000
Brominated hydrocarbons 28 000 36 000
Brominated bisphenol A 16 000 18 000
Antimony trioxide 22 000 25 000
Borates 8 000 8 000
Aluminium trihydrate 140 000 170 000
Magnesium hydroxide
2 000 3 000
Total
264 000 301 000
5.2 Uses
The consumption of flame retardants in plastics and other
combustible materials is closely linked to regulations covering fire
precautions. The principle regulations relate to the building,
transportation, electrical engineering, furnishing and mining sectors
(Troitzsch, 1990).
A worldwide estimate of the consumption of flame retardants
according to materials is not available but the figures for Europe
listed in the Table 6 should reflect the market in general.
Table 5. Trends in the annual consumption of flame retardants in Japana
Type Compound Amount (tonnes)
1986 1990 1994
Brominated
Tetrabromobisphenol A (TBBPA) 12 000 23 000 24 000
Decabromobiphenyl ether 3 000 10 000 5 500
Octabromobiphenyl ether 600 1 100 500
Tetrabromobiphenyl ether 1 000 1 000 0
Hexabromocyclododecane 600 700 1 600
Bis(tetrabromophthalimido) ethane - 1 000 2 500
Tribromophenol 100 450 3 500
Bis(tribromophenoxy) ethane 400 400 900
TBBPA polycarbonate oligomer - - 2 500
Brominated polystyrene - - 1 300
TBBPA epoxy oligomer - 3 000 7 000
Others 2 400 - 2 150
Subtotal 20 000 40 650 51 450
Chlorinated
Chlorinated paraffins 4 000 4 500 4 300
Others 850 700 900
Subtotal 4 850 5 200 5 200
Phosphoric
Halogenated ester 3 000 3 000 3 100
Non-halogenated ester 4 000 4 400 4 400
Others 1 750 1 750 3 310
Subtotal 8 750 9 150 10 810
Table 5. (contd.)
Type Compound Amount (tonnes)
1986 1990 1994
Inorganic
Antimony oxide 8 300 16 000 17 000
Hydrated aluminium 48 000 37 000 42 000
Others 7 200 8 400 9 000
Subtotal 63 500 61 400 68 000
TOTAL 97 100 116 400 135 460
a Based on the investigation made by Kagaku Kogyo Nippon Co. Ltd. (Japan).
(Personal communication from Isao Watanabe, Osaka Prefectural Institute
of Public Health, Japan).
Table 6. Estimated consumption of flame retardants in western
Europe for 1985 and 1992 according to materials
(from: Sutker, 1988)
Product group Consumption (103 tonnes)
1985 1992
Polystyrene 4.0-4.5 4.5-5.0
ABS 1.0-1.5 1.2-1.8
Polyesters 7.5-8.0 8.5-9.0
Epoxy resins 3.5-4.0 4.0-4.5
Polyolefins 10.0-12.0 11.0-13.0
Polyvinyl chloride) 25.0-27.0 27.0-29.0
Polyurethanes 12.0-13.5 13.5-15.0
Engineering plastics 1.5-1.8 1.7-2.0
Paper and textiles 9.0-10.0 10.0-11.0
Rubber and elastomers 5.0-6.0 6.0-7.0
Other 11.5-11.7 12.6-12.7
Total 90.0-100.0 100.0-110.0
5.2.1 Plastics
The plastics industry is the largest consumer of flame
retardants, estimated at about 95% for the USA in 1991. About 10% of
all plastics contain flame retardants (Wolf & Kaul, 1992). The main
applications are in building materials and furnishings (structural
elements, roofing films, pipes, foamed plastics for insulation,
furniture and wall and floor coverings), transportation (equipment and
fittings for aircraft, ships, automobiles and railroad cars), and in
the electrical industry (cable housings and components for television
sets, office machines, household appliances and lamination of printed
circuits).
The growth in the flame retardant market reflects the enormous
expansion of the plastics industry in recent decades. Between 1988 and
1994, there was a worldwide increase of 20%. Although the USA,
western Europe and Japan are still the largest plastic producers (30,
24 and 12% of the market, respectively), other countries showed the
largest increases between 1988 and 1994, e.g., South Korea (170%);
China (60%); Taiwan (54%) (Anon, 1995).
Examples of flame retardants used in various plastics (Wolf &
Kaul, 1992) are as follows:
PVC: Chlorinated paraffins or phosphate esters, antimony trioxide,
aluminium hydroxide
Acrylonitrile-butadiene-styrene (ABS): Octabromodiphenyl ether,
antimony trioxide
Expandable polystyrene: Hexabromocyclododecane
High-Impact polystyrene (HIPS): Decabromodiphenyl ether or
tetrabromobisphenol A, antimony trioxide
Linear polyester: Brominated organics
Polypropylene: Tetrabromobisphenol A, bis(2,3-dibromopropyl ether),
antimony trioxide
Low-density polyethylene (LDPE) films: chlorinated paraffins,
antimony trioxide
High-density polyethylene (HDPE) and cross-linked polyethylene:
Brominated aromatics
Polyurethane foams: Organophosphates, brominated organic compounds,
alumina trihydrate
Polyamides: Brominated aromatic compounds, chlorinated
cycloaliphatic compounds, antimony trioxide, red phosphorus, melamine
Polycarbonates: Tetrabromobisphenol A, brominated organic oligomers,
sulfonate salts
Unsaturated polyesters: Chlorinated and brominated organic
compounds, antimony trioxide, alumina trihydrate
Epoxy resins: Tetrabromobisphenol A
5.2.2 Textile/furnishing industry
In contrast to the plastics industry, the textile industry is a
much smaller market for flame retardants. However, rather than
employing just one flame retardant, the use of a combination of
chemicals is usually necessary for textiles.
Phosphorus-containing materials are the most important class of
compounds to impart durable flame resistance to cellulose (Calamari &
Harper, 1993). Flame-retardant finishes containing phosphorus
compounds usually also contain nitrogen or bromine, or sometimes both.
Another system is based on halogens (usually bromine) in conjunction
with nitrogen or antimony.
Flame retardants used in furniture/textiles include the following
(Anon, 1992; Calamari & Harper, 1993; EFRA, 1995):
* organic phosphates such as tri-alkyl or tri-aryl phosphates, tri-
chloroalkyl phosphates, dialkyl phosphites,
tetrakis(hydroxymethyl)phosphonium chloride (THPC) and related
structures;
* halogenated compounds such as polybrominated diphenyl ethers
(found in over 50% of treated furniture) and chlorinated
paraffins (rainproof applications);
* inorganic compounds such as antimony trioxide, ammonium bromide,
boric acid and aluminium hydrate.
Details on the flame-retardant types were reported by Calamari &
Harper (1993) and can be found in Annex II.
6. FORMATION OF TOXIC PRODUCTS ON HEATING OR COMBUSTION OF
FLAME-RETARDED PRODUCTS
Natural or synthetic material that burns produces potentially
toxic products. There has been considerable debate on whether
addition of organic flame retardants results in the generation of a
smoke that is more toxic and may result in adverse health effects on
those exposed. There has been concern in particular about the
emission of polybrominated dibenzofurans (PBDF) and polybrominated
dibenzodioxins (PBDD) during manufacture, use and combustion of
brominated flame retardants.
6.1 Toxic products in general
Combustion of any organic chemical may generate carbon monoxide
(CO), which is a highly toxic non-irritating gas, and a variety of
other potentially toxic chemicals. Some of the major toxic products
that can be produced by pyrolysis of flame retardants are: CO, CO2,
HCl, POX, ammonia vapour, bromofurans, HBr, HCN, NOX and phosphoric
acid (Anon, 1992).
In general the acute toxicity of fire atmospheres is determined
mainly by the amount of CO, the source of which is the amount of
generally available flammable material. Most fire victims die in post
flash-over fires where the emission of CO is maximized and the
emission of HCN and other gases is less. The acute toxic potency of
smoke from most materials is lower than that of CO (Hirschler, 1995;
Nelson, 1995).
Flame retardants significantly decrease the burning rate of the
product, reducing heat yields and quantities of toxic gas. In most
cases, smoke was not significantly different in room fire tests
between flame-retarded and non-flame-retarded products (Babrauskas
et al. 1988).
Morikawa et al. (1995) reported toxicity studies on gases from
full-scale room fires involving fire retardant materials (a variety,
but not specified). HCN and CO were the two major toxicants. There was
no evidence that the smoke from flame-retarded materials was more
toxic to rabbits than the smoke from non-flame-retarded materials.
Regarding brominated flame retardants, Cullis (1987) stated that
unless suitable metal oxides or metal carbonates are also present,
virtually all the bromine is eventually converted to gaseous hydrogen
bromide (HBr). This is a corrosive and powerful sensory irritant. In a
fire situation however, it is always carbon monoxide (CO) or hydrogen
cyanide (HCN), rather than an irritant which causes rapid
incapacitation. Owing to its high reactivity, hydrogen bromide is
unlikely to reach dangerously high concentrations (Cullis, 1987).
6.2 Formation of halogenated dibenzofurans and dibenzodioxins
PBDFs and PBDDs can be formed from polybrominated diphenyl ethers
(PBDEs), polybrominated phenols, polybrominated biphenyls (PBBs) and
other brominated flame retardants under various laboratory conditions,
including heating. Because chlorinated derivatives are preferably
formed during pyrolysis, mixed halogen compounds will be predominantly
produced if a chlorine source is also available (Buser, 1987a,b).
As in the case of PCDD/PCDF, it is the 2,3,7,8-substituted
isomers that are toxic.
6.3 Exposure to PBDD/PBDF from polymers containing halogenated
flame retardants
6.3.1 Exposure due to contact or emission from products
containing halogenated flame retardants
Exposure of the general public to PBDD/PBDF impurities in flame-
retardant polymers is unlikely to be of significance. The possible
exposure to PBDD/PBDF from TV sets and computer monitors flame-
retarded with halogenated flame retardants has been discussed in
Environmental Health Criteria 162: Brominated diphenyl ethers and is
unlikely to be of significance (IPCS, 1994b).
6.3.2 Workplace exposure studies
Several studies have been performed to determine whether
PBDD/PBDF is present in the fumes emitted during thermal processes,
such as the extrusion of resins containing halogenated flame
retardants under normal processing conditions at temperatures in the
range of 200 to 250°C (IPCS 1994b, 1995a, in preparation).
Epidemiological studies of workers engaged in processing polymers
with PBDEs have been reported (IPCS, 1994b). Results of PBDD/PBDF
workplace monitoring during polymer processing have also been reported
(IPCS 1994b, 1995a). PBDD/PBDF personnel and room air levels during
processing of PBDEs were < 2 ng/m3 (TCDD equivalent) with the
exception of two samples at the extruder head (128 ng/m3, TCDD
equivalent) (IPCS, 1994b). Engineering controls were successful in
reducing these levels. Workplace control measures should also include
appropriate industrial hygiene measures and monitoring of exposure
(IPCS, 1994b, 1995a).
6.3.3 Formation of PBDD/PBDF from combustion
6.3.3.1 Laboratory pyrolysis experiments
In the late 1980s many pyrolysis experiments (at temperatures of
400-900°C) on brominated flame retardants and flame-retardant systems
were performed and the breakdown products measured. Flame retardants
or intermediates tested included PBBs, PBDEs, 2,4,6-tribromophenol,
pentabromophenol, tetrabromobisphenol A and tetrabromophthalic
anhydride (IPCS 1994b, 1995a, in preparation). Pyrolysis of the flame
retardants alone, as well as with polymer mixtures, was investigated.
As different laboratories carried out the experiments using a variety
of testing methods and conditions, a direct comparison of the many
experiments is not possible. Details of the pyrolysis experiments
involving PBDEs, tetrabromobisphenol A and derivatives, and PBBs are
given in the respective EHC monographs (IPCS 1994b, 1995a, in
preparation).
Although they indicate which flame retardants are likely to form
PBDF (and to a lesser extent PBDD) pyrolysis experiments are not
generally comparable to actual fire situations.
6.3.3.2 Fire tests and fire accidents
Fire tests on televisions have shown smoke and combustion
residues containing high levels of PBDD/PBDF. However, levels from
actual fire accidents involving televisions revealed much lower levels
than those produced under fire test conditions (IPCS, 1994b). Further
studies are discussed in the EHC monograph on PBDD/PBDFs (IPCS, in
preparation).
7. OVERVIEW OF EXPOSURE AND HAZARDS TO HUMANS AND THE ENVIRONMENT
Since flame retardants are a heterogeneous group of diverse
chemicals (see chapter 2 and Annex II), the information presented in
this section only provides a general overview of possible routes of
exposure to chemicals associated with flame-retardant use. This
section also provides a brief summary of the hazards to human health
and to the environment posed by chemicals connected with flame-
retardant use. For detailed information on the extent of exposure and
health and environmental effects of individual substances, the
appropriate specific EHC monographs should be consulted.
The toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles has discussed
in a report by the European Economic Community (Anon, 1992).
7.1 Human exposure
7.1.1 General population
Potential sources of exposure include consumer products,
manufacturing and disposal facilities, and environmental media.
Factors affecting exposure of the general population include the
physical and chemical properties of the product, the extent of
manufacturing and emission controls, the use made of the product
(surface coating, durability of fabric finishes, incorporation into a
polymer, etc.), the end use, and the method of disposal. Potential
routes of exposure for the general population include the dermal route
(contact with flame-retarded textiles), inhalation and ingestion.
7.1.2 Occupational exposure
Occupational exposure may occur during the manufacture,
transport, processing and disposal/recycling of flame retardants.
Routes of exposure could include inhalation, dermal contact and
ingestion. Factors affecting the extent of exposure include industrial
hygiene practices, engineering controls, manufacturing processes and
the type of product. As with any other industrial chemical, workplace
monitoring and good industrial practice can delineate the extent of
any exposure.
7.2 Exposure of the environment
Environmental exposure may occur as a result of the manufacture,
transport, use or waste disposal of flame retardants. Routes of
environmental exposure can include water, air and soil. Factors
affecting exposure include the physical and chemical properties of the
product, emission controls, disposal/recycling methods, volume and
biodegradability/persistence. Environmental monitoring can determine
the extent of environmental exposure.
On the basis of the estimated demand for flame retardants (see
Table 3), more than 1 million tonnes of flame-retardant polymers are
produced each year.
Most flame-retarded products eventually become waste. Municipal
waste is generally disposed of via incineration or landfill.
Incineration of flame-retarded products can produce various toxic
compounds, including halogenated dioxins and furans. The formation of
such compounds and their subsequent release to the environment is a
function of the operating conditions of the incineration plant and the
plant's emission controls.
There is a possibility of flame retardants leaching from products
disposed of in landfills. However, potential risks arising from
landfill processes are also dependent on local management of the whole
landfill. The significance of any release of flame retardants from
disposal sites has yet to be determined.
Some products containing flame retardants, including some
plastics, have been identified as suitable for recycling (Lorenz &
Bahadir, 1993; Meyer et al., 1993).
7.3 Hazards to humans
The hazards to humans associated with some flame retardants have
been outlined in the relevant EHC monographs. For example, the use of
tris(2,3-dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate
was banned in 1977 by the US Consumer Product Safety Commission and in
several other developed countries for use in children's clothing
because of concerns that the chemical might be a human carcinogen and
because of the possibility of significant human exposure through
contact with treated fabrics (IPCS, 1995b). Delayed neurotoxicity
due to tri- ortho-cresyl phosphate (TOCP), one of the tricresyl
phosphate isomers, has been observed in humans (IPCS, 1990). Some
polybrominated biphenyl (PBB) congeners have been shown to produce
chronic toxicity and cancer in experimental animals. However, no
definitive human health effects, correlatable with exposure, were
found in a population in Michigan, USA, accidentally exposed to PBBs
(IPCS, 1994a).
7.4 Hazards to the environment
EHC monographs outline the hazards to the environment associated
with some flame retardants (see Table 1). Some PBB congeners are
persistent and bioaccumulative and may pose a threat especially to
higher levels of the food chain (IPCS, 1994a). Hexachloro-
cyclopentadiene is highly toxic to aquatic organisms. However,
information obtained under environmentally realistic conditions is
limited. The potential hazard to the general environment is expected
to be low (IPCS, 1991b). Low concentrations of triphenyl phosphate
have been detected in environmental samples. Triphenyl phosphate is
rapidly degraded in the environment. However, sediment-dwelling
organisms near production plants may have been exposed to
concentrations high enough to exert toxic effects (IPCS, 1991a).
Tricresyl phosphate is also degraded rapidly in the environment, and
subsequent environmental concentrations are therefore low. The acute
toxicity of tricresyl phosphate to aquatic organisms is low (IPCS,
1990).
Persistence of pentabromodiphenyl ether (PeBDE) and lower
brominated diphenylethers in the environment suggest that commercial
PeBDE should not be used (IPCS, 1994b).
Some flame retardants have come under intense environmental
scrutiny. US EPA has called for additional testing (US EPA, 1992).
The data on environmental levels of short-chain chlorinated
paraffins indicate that in areas close to release sources there is a
risk to both freshwater and estuarine organisms. Recent data indicate
that there is also a potential risk to aquatic invertebrates from
intermediate- and long-chain chlorinated paraffin products (IPCS,
1996b).
8. REGULATIONS WITH RESPECT TO FLAME RETARDANTS
Several national regulatory bodies have implemented regulations
on specific substances associated with flame-retardant applications.
In the USA the Interagency Testing Committee (ITC), under the
auspices of the Toxic Substances Control Act (TSCA), makes
recommendations concerning the need for additional testing on
chemicals in the TSCA inventory, including flame retardants. Based on
the information published since 1978, the ITC has made initial testing
recommendations upon 128 brominated flame retardants (US TSCA, 1992;
Walker, 1994; Annex IV).
In the European Community, the use of tris(2,3-dibromopropyl)
phosphate (EC Directive 76/769/EEC) and tris(1-aziridinyl)phosphine
oxide (EC Directive 83/264/EEC) in textiles has been banned. In 1977,
the US Consumer Product Safety Commission banned the use of tris(2,3-
dibromopropyl)phosphate in children's clothing (IPCS, 1995b).
The European Community has also banned the use of PBBs in
textiles (EC Directive 83/264/EEC). Several countries have either
taken or proposed regulatory actions on PBBs, as outlined in Table 7.
Controls on the emissions of dioxins and furans from municipal
solid waste incinerators have been implemented in the United Kingdom
under the Environmental Protection Act (1990). In Germany, a second
modification of the Chemicals Prohibition Ordinance, which was adopted
in 1994, imposes limits on 2,3,7,8-substituted chlorinated dioxins and
furans and, for the first time, on some 2,3,7,8-substituted brominated
dioxins and furans (OECD, 1994).
Table 7. Country-specific actions on PBBs either taken or proposeda
Country Actions
Austria Prohibits the manufacture, placing on the market,
import and use of PBBs and products containing
these substances.
Canada Prohibits the manufacture, use, processing, offer
for sale, selling or importation of PBBs for
commercial, manufacturing or processing purposes.
Denmark Implements EC Directive 89/677 banning the use of
PBBs in textiles.
Finland PBB may not be used in textile articles intended
to come into contact with the skin (in accordance
with EC Directive 83/264).
France Implements EC Directive concerning PBBs and their
use on textiles.
Netherlands Proposed resolution would prohibit the storage of
PBBs or products or preparations containing these
substances or making them available to third
parties. (Exports are excluded from the
resolution).
Norway Ban on PBBs in textiles intended to come into
contact with skin, implementation of EC Directives
76/769/EEC, 83/264 and 89/677.
Sweden Ban on PBBs in textiles intended to come into
contact with skin by implementation of EC
Directive 76/769.
Switzerland Prohibits manufacture, supply, import and use of
PBBs and products containing these substances.
Supply and import of capacitors and transformers
containing PBBs is forbidden.
USA No current production or use. Companies intending
to resume manufacture must notify US EPA 90 days
in advance for approval.
a Adapted from: OECD (1994)
9. CONCLUSIONS AND RECOMMENDATIONS FOR THE PROTECTION OF HUMAN HEALTH
AND THE ENVIRONMENT
9.1 Conclusions
Flame retardants are a diverse group of compounds used to improve
the flame retardancy of polymers and other materials. A large variety
of compounds, from inorganic to complex organic molecules, are used as
flame retardants, synergists and smoke suppressants. This overview is
focused on organic compounds, which typically contain halogen and/or
phosphorus.
It is difficult to find accurate figures for the global use of
flame retardants but estimates indicate that more than 600 000 tonnes
are produced annually. Available data indicate a substantial increase
of brominated organic product consumption during the last decade.
There are obvious benefits in using flame retardants, as many
human lifes and property are saved from fire. At present, knowledge
of long-term effects resulting from exposure to flame retardants and
their breakdown products is limited. Most people that die in fires
are killed by carbon monoxide.
The majority of the organic flame retardants are either
covalently bound into polymer molecules (reactive) or mixed into the
polymer (additive). They can act in several ways, either physically
(by cooling, by formation of a protective layer or by dilution of the
matrix) or chemically (by reactions in either the gas or the solid
phase).
A number of factors govern the selection of the type of flame
retardant to be used in a specific application. Some of these are the
flammability of the matrix, processing and performance requirements,
chemical properties and possible hazards to human and environmental
health.
Exposure of the general population to flame retardants can occur
via inhalation, dermal contact and ingestion. Potential sources of
exposure are consumer products, manufacturing/disposal facilities and
environmental media (including food intake). The same routes are
possible for occupational exposure, mainly during production,
processing, transportation and disposal/recycling of the flame
retardants or the treated products. Occupational exposure to the
breakdown products may also occur during fire fighting. As several of
the compounds used are lipophilic and persistent, they may
bioaccumulate. Some of the compounds have been shown to cause organ
damage, genotoxic effects and cancer.
There is also concern for occupational health and environmental
effects from combustion/pyrolysis products, especially the
polyhalogenated dibenzofurans and dibenzo- p-dioxins, from some
organic flame retardants. Other breakdown products also need to be
taken into account.
The properties of a number of flame retardants make them
persistent and/or bioaccumulative, and they may therefore pose hazards
to the environment. Some of the compounds that have been evaluated so
far (polybrominated biphenyls, polybrominated diphenyl ethers and
chlorinated paraffins) have been found to belong to this group. Some
of these have therefore been recommended to not be used.
Several countries have developed regulations affecting the
production, use and disposal of flame retardants. Some include
restrictions on the use of compounds because of potential toxic
effects in humans. Germany has developed rules for the maximum
content of some 2,3,7,8-substituted polychlorinated dibenzo- para-
dioxins and dibenzofurans in products.
The availability of relevant data on flame retardants in the open
literature is limited, especially for some existing chemicals
produced before regulations for commercialization were strengthened
in several countries.
IPCS has issued evaluations for some flame retardants and is
preparing evaluations for others.
9.2 Recommendations for the protection of human health and the
environment
a) Information on the content and nature of flame retardants,
including impurities in products, should be made available to
national authorities.
b) More complete information on the volume of flame retardants
production and consumption should be made available.
c) In view of the increased recycling of flame-retarded products,
consideration could be given to harmonized labelling by an
international forum.
d) Compounds that present a toxic risk to humans and/or the
environment should not be used as flame retardants.
e) Occupational exposure to flame retardants and their breakdown
products should be minimized using appropriate engineering and
good industrial hygiene practices. The exposure of people
working in these operations should be monitored.
f) There is a need for proper assessment of occupational health and
environmental effects from combustion or pyrolysis products of
flame retardants.
g) Emissions to the environment from manufacturing, processing,
transportation and disposal/recycling of products containing
persistent bioaccumulative compounds should be minimized using
best available techniques. The environment in the vicinity of
such operations should be monitored for the compounds used.
h) The use of flame retardants with properties that make them
persistent and bioaccumulative should be avoided.
i) The levels of the major persistent bioaccumulating flame
retardants should be monitored routinely in environmental
matrices (biota and sediments). Some compounds that are no
longer produced should likewise be monitored, in order to
indicate the long-term influence of such products.
10. FURTHER RESEARCH
a) Further studies should be undertaken to elucidate the fate of
flame retardants in disposal/recycling operations.
b) There is a need for further evaluations of flame retardants.
Useful criteria for setting priorities are volume of use,
intrinsic toxic effects on human health and the environment,
exposure assessments, and persistence and bioaccumulation/
biomagnification of flame retardants or their breakdown products.
REFERENCES
Anderson GA & Christy MR (1992) Standards, bans and flame retardants.
Presented at Structural Plastics "92", 5-8 April 1992. Dallas, Texas,
Society of the Plastics Industry, Inc., Structural Plastics Division.
Anon (1992) Toxicity and ecotoxicity of flame retardants used in the
industry of upholstered furniture and related articles - Report to the
European Commission, DG-III, Brussels.
Anon (1995) [Plastic industry of the world.] Kunststoffe, 85(10):
1761-1772 (in German).
Arias P (1992) Brominated diphenyloxides as flame retardants. Bromine
based chemicals. Paris, Organisation for Economic Co-operation and
Development (Unpublished document).
Avento JM & Touval I (1980) Antimony and other inorganic compounds.
In: Kirk-Othmer encyclopedia of chemical technology, 3rd ed. New York,
John Wiley and Sons, vol 10, pp 486-487.
Babrauksas V, Harris RH Jr, Gann RG, Levin BC, Lee BT, Peacock RD,
Paabo M, Twilley W, Yoklavich MF, & Clark HM (1988) Fire hazard
comparison of fire-retarded and non-fire-retarded products.
Washington, DC, US Department of Commerce, National Bureau of
Standards (NBS Special Publication No. 749).
BFR/CEM Working Group (1989) [Polybrominated dibenzodioxins and
dibenzofurans (PBDDs/PBDFs) from flame retardants containing bromine:
Assessment of risk and proposed measures - Report by the Brominated
Flame Retardants/CEM Working Group to the Conference of Environmental
Ministers Bonn, Germany, September 1989.] (Report No. III/4299/89) (in
German).
Boethling RS & Cooper JC (1985) Environmental fate and effects of
triaryl and tri-alkyl/aryl phosphate esters. Residue Rev, 94: 49-99.
Buser HR (1987) Brominated and brominated/chlorinated dibenzodioxins
and dibenzofurans: potential environmental contaminants. Chemosphere,
16(8-9): 1873-1876.
Calamari TA & Harper RJ (1993) Flame retardants for textiles. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 998-1022.
Cullis CF (1987) Bromine compounds as flame retardants. In:
Proceedings of the International Conference on Fire Safety, vol 12, pp
307-323.
Dynamac Corporation (1982) An overview of flame retardants: Volumes I
and II (Contract No. 68-01-6239). Rockville, Maryland, Dynamac
Corporation, Environmental Control Division (Prepared for the US
Environmental Protection Agency).
EFRA (1995) European flame retardants and their application. Brussels,
European Flame Retardants Association.
Flick EW (1986) Plastics additives: An industrial guide - Section IX:
Fire and flame retardants. Park Ridge, New Jersey, Noyes Publications,
pp 213-248.
Gann RG (1993) Flame retardants: Overview. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 930-936.
Grabner RS (1993) N-containing flame retardants: An alternative.
Handout at OECD Workshop on Brominated Flame Retardants, Neuchâtel,
Switzerland, 22-25 February 1993.
Green JC (1992) A review of phosphorus-containing flame retardants. J
Fire Sci, 10: 470-487.
Hindersinn RR (1990) Historical aspects of polymer fire retardance.
In: Nelson GL ed. Fire and polymers hazards identification and
prevention. New York, American Chemical Society (ACS Symposium Series
No. 415).
Hirschler MM (1995) Smoke toxicity: How important is it for fire
safety? Handout at the 6th Annual BCC Conference on Flame Retardancy:
Recent advances in flame retardancy of polymeric materials, Stamford,
Connecticut, May 1995, 16pp.
Hutzinger O, Sundström G, & Safe S (1976) Environmental chemistry of
flame retardants: Part I. Introduction and principles. Chemosphere,
1: 3-10.
IARC (1975) Some aziridines, N-, S- & O-mustards and selenium.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of Carcinogenic Risk of Chemicals to Man, Volume 9).
IARC (1978) Polychlorinated biphenyls and polybrominated biphenyls.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume
18).
IARC (1979) Some halogenated hydrocarbons. Lyon, International Agency
for Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Volume 20).
IARC (1986a) Some chemicals used in plastics and elastomers. Lyon,
International Agency for Research on Cancer (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 39).
IARC (1986b) Some halogenated hydrocarbons and pesticide exposures.
Lyon, International Agency for Research on Cancer (IARC Monographs on
the Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Volume 41).
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Supplement 7).
IARC (1989) Some organic solvents, resin monomers and related
compounds, pigments and occupational exposures in paint manufacture
and painting. Lyon, International Agency for Research on Cancer, pp
291-305 (IARC Monographs on the Evaluation of Carcinogenic Risks to
Humans, Volume 47).
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic
Risks to Humans, Volume 48).
IPCS (1984) Environmental health criteria 44: Mirex. Geneva, World
Health Organization, International Programme on Chemical Safety,
70 pp.
IPCS (1989) Environmental health criteria 88: Polychlorinated dibenzo-
p-dioxins and dibenzofurans. Geneva, World Health Organization,
International Programme on Chemical Safety, 409 pp.
IPCS (1990) Environmental health criteria 110: Tricresyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 122 pp.
IPCS (1991) Environmental health criteria 111: Triphenyl phosphate.
Geneva, World Health Organization, International Programme on Chemical
Safety, 80 pp.
IPCS (1991) Environmental health criteria 120: Hexachloro-
cyclopentadiene. Geneva, World Health Organization, International
Programme on Chemical Safety, 126 pp.
IPCS (1992) Environmental health criteria 140: Polychlorinated
biphenyls and terphenyls, 2nd ed. Geneva, World Health Organization,
International Programme on Chemical Safety, 692 pp.
IPCS (1994a) Environmental health criteria 152: Polybrominated
biphenyls. Geneva, World Health Organization, International Programme
on Chemical Safety, 577 pp.
IPCS (1994b) Environmental health criteria 162: Brominated diphenyl
ethers. Geneva, World Health Organization, International Programme on
Chemical Safety, 347 pp.
IPCS (1995a) Environmental health criteria 172: Tetrabromobisphenol-A
and derivatives. Geneva, World Health Organization, International
Programme on Chemical Safety, 139 pp.
IPCS (1995b) Environmental health criteria 173: Tris(2,3-
dibromopropyl) phosphate and bis(2,3-dibromopropyl) phosphate. Geneva,
World Health Organization, International Programme on Chemical Safety,
129 pp.
IPCS (1996a) Environmental health criteria 185: Chlorendic acid and
anhydride. Geneva, World Health Organization, International Programme
on Chemical Safety.
IPCS (1996b) Environmental health criteria 181: Chlorinated paraffins.
Geneva, World Health Organization, International Programme on Chemical
Safety.
IPCS (in preparation) Environmental health criteria: Polybrominated
dibenzo-p-dioxins and dibenzofurans. Geneva, World Health
Organization, International Programme on Chemical Safety.
ISO/IEC Guide (1990) Glossary of fire terms and definitions, 1st ed.
Geneva, International Organization for Standardization.
Japan Fire Retardant Association (1988) Voluntary rules of the flame
retardancy and the toxicological (safety) examination for flame
retardant products under the guide-line of the Fire Defence Agency,
Fire Retardant Products Approval Commission. Tokyo, Japan Fire
Retardant Association.
Jay TA (1990) Flame retardants. Presentation to the USSR All-Union
Congress on Flame Retardants at Saki, Crimea, USSR, 10 October 1990.
Kirk-Othmer (1993) Flame retardants. In: Kirk-Othmer encyclopedia of
chemical technology, 4th ed. New York, John Wiley & Sons, vol 10, pp
930-1022.
Kopp A (1990) [Discussions on bromine-containing flame-retardants.]
Berlin, Germany, Federal Ministry for Environment, Nature Conservation
and Nuclear Safety (Document sent to the European Commission,
Brussels) (in German)
Liepins R & Pearce EM (1976) Chemistry and toxicity of flame
retardants for plastics. Environ Health Perspect, 17: 55-63.
Lorenz W & Bahadir M (1993) Recycling of flame retardants containing
printed circuits: a study of the possible formation of polyhalogenated
dibenzodioxins/-furans. Chemosphere, 26(12): 2221-2229.
Meyer H, Neupert M, Pump W, & Willenberg B (1993) [Flame retardants
determine reusability.] Kunststoffe, 83(4): 253-257 (in German).
Morikawa T, Okada T, Kajiwara M, Sato Y, & Tsuda Y (1995) Toxicity of
gases from full-scale room fires involving fire retardant contents. J
Fire Sci, 13(1): 23-42.
Nelson GL (1995) Carbon monoxide and fire toxicity. Handout at the 6th
Annual BCC Conference on Flame Retardancy: "Recent Advances in Flame
Retardancy of Polymeric Materials, Stamford, Connecticut, May 1995 -
16pp.
OECD (1994) Selected brominated flame retardants. Paris, Organisation
for Economic Co-operation and Development, Environment Directorate,
152 pp (Risk Reduction Monograph No. 3).
Pearce EM & Liepins R (1975) Flame retardants. Environ Health
Perspect, 11: 59-69.
Pettigrew A (1993) Halogenated flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley &
Sons, vol 10, pp 954-976.
Soderlung E & Dybing E (1982) [Flame-retardant toxicology: A
literature survey.] Oslo, National Institute for Public Health
(Unpublished report ) (in Norwegian).
Sutker BJ (1988) Flame retardants. In: Ullmann's encyclopedia of
industrial chemistry, 5th ed. Weinheim, Germany, VCH Verlag, vol A11,
pp 123-140.
Teuerstein A (1990) Brominated FRS, production and uses (Summary).
Data from Eurobrom BV. In: Workshop on Brominated Aromatic Flame
Retardants. Proceedings of a Workshop held at Skoloster, Sweden, 24-26
October 1989. Solna, Swedish National Chemicals Inspectorate, pp
49-52.
Touval I (1993) Antimony and other inorganic flame retardants. In:
Kirk-Othmer encyclopedia of chemical technology, 4th ed. New York,
John Wiley & Sons, vol 10, pp 936-954.
Troitzsch JH (1990) International plastics flammability handbook:
Principles, regulations, testing and approval, 2nd ed. Münich,
Germany, Hanser Publishers.
Ullmann (1988) Ullmann's encyclopedia of industrial chemistry: Volumes
A11 et A26, 5th ed. Weinheim, Germany, VCH Verlag.
Ulsamer AG, Osterberg RE, & McLauglin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US EPA (1976) A study of flame retardants for textiles. Washington,
DC, US Environmental Protection Agency (EPA-560/1-76-001; NTIS
PB-251-44).
US EPA (1989) Twenty-fifth report of the Interagency Testing Committee
to the administrator. Fed Reg, 54(237): 51114-51130.
US EPA (1992) Aryl phosphate base stocks: Proposed test rule and
reporting requirements. Fed Reg, 57(12): 2136-2158.
Walker JD (1994) Testing decisions of the TSCA Interagency Testing
Committee for Brominated Flame Retardants: A review of decisions and
health and safety data. Presented at the Meeting of the Fire Retardant
Chemicals Association, Williamsburg, Virginia, 9-12 October 1994.
Washington, DC, US Environmental Protection Agency.
Weil ED (1993) Phosphorus flame retardants. In: Kirk-Othmer
encyclopedia of chemical technology, 4th ed. New York, John Wiley and
Sons, vol 10, pp 976-998.
Wolf R & Kaul BL (1992) Plastics, additives. In: Ullmann's
encyclopedia of industrial chemistry, ed. Weinheim, Gemany, VCH
Verlag, vol A20, pp 459-507.
ANNEX I
TERMINOLOGY
There is no clear definition for many of the terms used to
describe fire, flammability and flame retardants. As a result, some
confusion has been created by the interchangeable use of terms such as
fire retardant, flame retardant, flame-proof and fire-proof. The
meaning of these and other terms is often clear only in the context.
Therefore, many efforts have been undertaken, at an international
level, to harmonize definitions and terms related to fire, including
fire protection and flame retardants. The definitions compiled in
Table 8 have been taken from the ISO/IEC Guide (1990) and other
sources (e.g., Troitzsch, 1990).
Other terms, which mostly concern mostly textile flame
retardancy, are defined below (US EPA, 1976, Kirk-Othmer, 1993):
Fireproof textile
This term applies only to those fabrics that undergo virtually no
change when exposed to a flame.
Flame-retardant textile
The term flame-retardant textile refers to any fabric that will
not support combustion after the source of ignition is removed. It is
synonymous with the term fire-resistant textile. The textile is
expected to char or melt. The term covers all treatments short of
fire-proofing.
Durability
Durability refers to the ability of a flame-retardant textile to
withstand washing/cleaning, chlorine bleaching, weathering and sun
exposure. A durable treatment is any chemical process which imparts
flame-retardant properties to textiles and textile products that will
last for at least 50 launderings and dry cleaning for the life of the
fabric. A semi-durable treatment will resist water but not withstand
dry cleaning or more than 10 to 15 launderings. A non-durable
treatment is readily removed by water or perspiration and requires
replacement after each exposure of the textile to water. The
definition of durability must be related to the conditions of use of
the textile and the product.
Table 8. Definitions of terms connected with fire
Term Definition
Afterflame Persistence of flaming of a material after the
ignition source has been removed
Afterglow Persistence of glowing of a material after
cessation of flaming or, if no flaming occurs,
after the ignition source has been removed
Burn To undergo combustion
Burning All the physical and/or chemical changes that
behaviour take place when a material or product is exposed
to a specified ignition source
Char Carbonaceous residue resulting from pyrolysis or
incomplete combustion
Combustible Capable of burning
Combustion Exothermic reaction of a substance with an
oxidizer, generally accompanied by flames and/or
glowing and/or emission of smoke
Fire a) A process of combustion characterized by the
emission of heat accompanied by smoke and/or
flame
b) Rapid combustion spreading uncontrolled in
time and space
Fire All the physical and/or chemical changes
behaviour that take place when a material, product and/or
structure is exposed to an uncontrolled fire
Fire The total gaseous, particulate or
effluent aerosol effluent from combustion or pyrolysis
Fire The ability of an element of building
resistance construction to fulfil for a stated period of
time the required load-bearing function,
integrity and/or thermal insulation specified
in the standard fire-resistance test
(see ISO 834)
Flame Zone of combustion in the gaseous phase from
which light is emitted
Table 8. (contd.)
Term Definition
Flame The property of a material either
retardance inherent or by virtue of a substance added or a
treatment applied to suppress, significantly
reduce or delay the propagation of flame
Flame A substance added or a treatment applied
retardant to a material in order to suppress, significantly
reduce or delay the combustion of the material
Flame spread Propagation of a flame front
Flame spread Distance travelled by a flame front
rate during its propagation per unit time under
specified test conditions
Flammability Ability of a material or product to burn with a
flame under specified test conditions
Flammable Capable of burning with a flame under specified
test conditions
Flash over The rapid transition to a state of total surface
involvement in a fire of combustible materials
within an enclosure
Fully The state of total involvement of
developed fire combustible materials in a fire
Glowing Combustion of a material in the solid
combustion phase without flame but with emission of light
from the combustion zone
Heat release The calorific energy released per unit
rate time by a material during combustion under
specified test conditions
Ignition Minimum temperature of a material at
temperature which sustained combustion can be initiated under
specified test conditions
Melting Phenomena accompanying the softening of
behaviour a material under the influence of heat (including
shrinking, dripping, burning of molten material,
etc.)
Table 8. (contd.)
Term Definition
Pyrolysis Irreversible chemical decomposition of a material
due to an increase in temperature without
oxidation
Reaction The response of a material under
to fire specified test conditions in contributing by its
own decomposition to a fire to which it is exposed
Smoke A visible suspension of solid and/or liquid
particles in gases resulting from combustion or
pyrolysis
Smoke The reduction in luminous intensity due
obscuration to passage through smoke
Smouldering The slow combustion of a material without light
being visible and generally evidenced by an
increase in temperature and/or by smoke
Soot Finely divided particles, mainly carbon, produced
and/or deposited during the incomplete combustion
of organic materials
ANNEX II
Flame retardants in commercial use or used formerly
Introduction
Tables 9 and 10 have been compiled on the basis of all the
information on flame retardants available to the IPCS and from the
following sources:
Arias (1992) BFR/CEM Working Group (1989)
Boethling & Cooper (1985) Dynamac Corporation (1982)
EFRA (1995) Flick (1986)
Hutzinger et al. (1976) IARC (1975, 1978, 1979, 1986a,b,
1987, 1989, 1990)
IRPTC (1987) Japan Fire Retardant Association
(1988)
Kirk-Othmer (1993) Kopp (1990)
Liepins & Pearce (1976) Pearce & Liepins (1975)
Sœderlund & Dybing (1982) Teuerstein (1990)
Troitzsch (1990) Ulsamer et al. (1980)
Ullmann (1988) US EPA (1989)
US TSCA (1992)
More than 175 flame retardants, or groups of them are tabulated
in these two tables. On just 17 of them the database was adequate for
preparing a hazard and risk evaluation for man and the environment,
and Environmental Health Criteria (EHC) monographs have been, or are
being prepared, on these. For the others the hazard to man and the
environment has not been evaluated internationally. Most of these
substances also have other major uses. In addition, some chemicals
used as intermediates in the production of flame retardants have beeen
listed. It is likely that these lists are not exhaustive, and that
new chemical structures are being developed as flame retardants.
Table 9 lists flame retardants in commercial use today and some
intermediates, while Table 10 lists flame retardants that have been
used in the past.
The tables list the chemical name, the chemical structure, the
CAS registry number and the uses as flame retardants. The "Use" column
also contains information on the global production volume of those
compounds that are currently being produced commercially (estimated
for the IPCS Task Group by P. Arias, 1995). The international
evaluation status of the substances is indicated in the column
"Remarks".
The following abbreviations are used in this table:
EHC An Environmental Health Criteria monograph on the chemical
has been published. If no EHC number is given, the document
is still in preparation.
IARC The International Agency for Research on Cancer of WHO in
Lyon has evaluated the carcinogenicity.
H High production volume (> 5000 tons per year)
M Moderate production volume (1000-5000 tons per year)
L Low production volume (<1000 tons per year or in
the developmental stage)
Table 9. Flame retardants being used commercially today
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Inorganic flame retardants
Potassium fluorotitanate K2TiF6  Decabromodiphenyl ethane
Decabromodiphenyl ethane  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Decabromodiphenyl ether
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Decabromodiphenyl ether  Octabromodiphenyl ether
Octabromodiphenyl ether 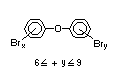 Pentabromodiphenyl ether
Pentabromodiphenyl ether 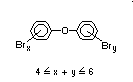 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobis phenol A
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobis phenol A  Tetrabromobisphenol
Tetrabromobisphenol 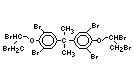 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol  Tetrabromobisphenol
Tetrabromobisphenol  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol  Tetrabromobisphenol
Tetrabromobisphenol  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ethylene-bistetrabromophthalimide Polyethylene, polypropylene M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Ethylene-bistetrabromophthalimide Polyethylene, polypropylene M
 Dibromoneopentylglycol CH2OH
Dibromoneopentylglycol CH2OH  (Poly)pentabromobenzyl acrylate
(Poly)pentabromobenzyl acrylate  Pentabromotoluene
Pentabromotoluene  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,3-Dibromo-2-butene-1,4-diol
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,3-Dibromo-2-butene-1,4-diol  (Poly)bromophenols:
(Poly)bromophenols: 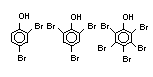 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Bis(2,4,6-
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Bis(2,4,6-  Tetrabromophthalic acid Na salt
Tetrabromophthalic acid Na salt 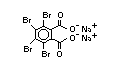 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromophthalic acid diol
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromophthalic acid diol  Tetrabromophthalic anhydride
Tetrabromophthalic anhydride  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
N,N'-Ethylene-bis-(tetrabromophthalimide)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
N,N'-Ethylene-bis-(tetrabromophthalimide)  1,3-Butadiene homopolymer brominated
1,3-Butadiene homopolymer brominated  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetradecabromodi
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetradecabromodi  Poly(2,6-dibromophenylene oxide)
Poly(2,6-dibromophenylene oxide)  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Poly-tribromostyrene
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Poly-tribromostyrene  Polydibromostyrene
Polydibromostyrene 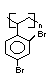 Hexabromocyclododecane
Hexabromocyclododecane  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Dibromo-4(1,2 dibromomethyl)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,2-Dibromo-4(1,2 dibromomethyl)  Ethylene-bis(5,6-dibromonorbornane-2,
Ethylene-bis(5,6-dibromonorbornane-2,  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibromostyrene grafted PP
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibromostyrene grafted PP 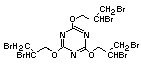 Diester of tetrabromophthalic acid
Diester of tetrabromophthalic acid  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated flame retardants
Chlorinated paraffins CxH(2x+2-y) Cly
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated flame retardants
Chlorinated paraffins CxH(2x+2-y) Cly  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorendic anhydride 115-17-5 Reactive flame retardant, used as flame EHC
retardant for unsaturated polyester, 185
epoxides, alkyl paints, epoxy hardener M
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorendic anhydride 115-17-5 Reactive flame retardant, used as flame EHC
retardant for unsaturated polyester, 185
epoxides, alkyl paints, epoxy hardener M
 Dodecachlorodimethano-
Dodecachlorodimethano-  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopentadiene
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopentadiene  Tetrachlorophthalic anhydride - TCPA
Tetrachlorophthalic anhydride - TCPA  Bromo-chlorinated paraffins CxH(2x+2-y-z) BryClz
Bromo-chlorinated paraffins CxH(2x+2-y-z) BryClz  Tetrachlorophthalic anhydride
Tetrachlorophthalic anhydride  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Organophosphorus flame retardants
Dimethylphosphono-
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Organophosphorus flame retardants
Dimethylphosphono-  Tris(2-butoxyethyl) phosphate
Tris(2-butoxyethyl) phosphate 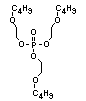 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isopropylphenyl diphenyl phosphate
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Isopropylphenyl diphenyl phosphate 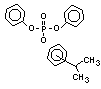 Tricresyl phosphate
Tricresyl phosphate 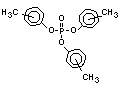 Triphenylphosphate
Triphenylphosphate 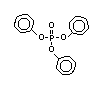 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl-methyl- O
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl-methyl- O 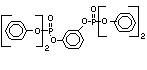 Diethyl-ethyl- H5C2 O C2H5
Diethyl-ethyl- H5C2 O C2H5 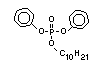 O,O-Diethyl-N,N-
O,O-Diethyl-N,N- 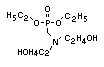 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl- Cellulosic fabrics
3-(hydroxymethylamino)-3- H
oxopropyl phosphonate
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dimethyl- Cellulosic fabrics
3-(hydroxymethylamino)-3- H
oxopropyl phosphonate
 Dimethyl phosphonate H3C O CH3
Dimethyl phosphonate H3C O CH3 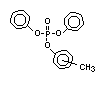 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Octyl diphenyl phosphate
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Octyl diphenyl phosphate 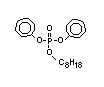 Tris(2-ethyl hexyl) phosphate
Tris(2-ethyl hexyl) phosphate 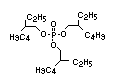 Trioctyl phosphate H17C8 O C8H17
Trioctyl phosphate H17C8 O C8H17 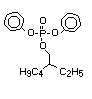 CH2OH
Tetrakis (hydroxymethyl)- | Reactive flame retardant for cotton; EHC
phosphonium HOH2C-P+--CH2OH X- rayon and other cellulosic materials as IARC
salts (THP salts): | well as polyester fabrics (1990)
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Acetate
CH2OH
Tetrakis (hydroxymethyl)- | Reactive flame retardant for cotton; EHC
phosphonium HOH2C-P+--CH2OH X- rayon and other cellulosic materials as IARC
salts (THP salts): | well as polyester fabrics (1990)
CH2OH
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Acetate 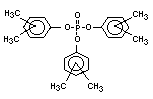 Tris(isopropy-lphenyl)
Tris(isopropy-lphenyl)  Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Halogenated organophosphorus flame retardants
Tris(1,3-dichloro-
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Halogenated organophosphorus flame retardants
Tris(1,3-dichloro- 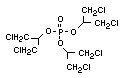 Tris(2-chloroethyl)
Tris(2-chloroethyl) 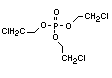 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2-chloroethyl)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2-chloroethyl) 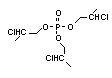 Tris(2-chloro-1-propyl)
Tris(2-chloro-1-propyl) 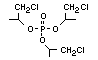 Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-chloro-2-propyl)
Table 9. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-chloro-2-propyl) 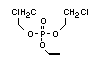 Bis(2-chloroethyl) vinyl
Bis(2-chloroethyl) vinyl  Tris(tribromoneopentyl) Thermoplastics
phosphate L
Tris(tribromoneopentyl) Thermoplastics
phosphate L
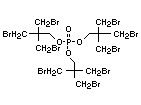 Chlorinated brominated 35-37% Br, 8-9%
Chlorinated brominated 35-37% Br, 8-9% 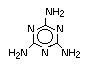 Melamine phosphate Polypropylene
L
Melamine cyanurate
Melamine phosphate Polypropylene
L
Melamine cyanurate  Table 10. Flame retardants that have been used commercially in the past
Chemical name Chemical structure CAS registry Use as flame retardant Remarks
number
Inorganic flame retardants
Sodium stannate Na2SnO3
Table 10. Flame retardants that have been used commercially in the past
Chemical name Chemical structure CAS registry Use as flame retardant Remarks
number
Inorganic flame retardants
Sodium stannate Na2SnO3 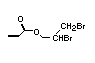 Tetrabromodipenta-erythritol
Tetrabromodipenta-erythritol 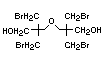 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Pentabromoethylbenzene
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Pentabromoethylbenzene 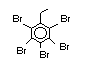 Tetrabromoxylene
Tetrabromoxylene  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromophenoxy-
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromophenoxy- 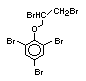 Hexabromobenzene
Hexabromobenzene  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexabromobiphenyl
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexabromobiphenyl  Octabromobiphenyl
Octabromobiphenyl 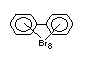 Hexabromodiphenyl ether
Hexabromodiphenyl ether 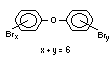 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol A-bis-
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrabromobisphenol A-bis- 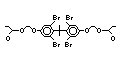 Pentabromochlorocyclo-hexane
Pentabromochlorocyclo-hexane  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,3-dibromopropyl) phosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,3-dibromopropyl) phosphate  Bis(2,3-dibromopropyl) phosphate
Bis(2,3-dibromopropyl) phosphate  Tetrabromo-2,3-dimethylbutane 00-00-0 EPS
Tetrabromo-2,3-dimethylbutane 00-00-0 EPS
 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromoaniline
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
2,4,6-Tribromoaniline 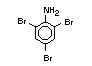 1-Pentabromophenoxy-2-propene
1-Pentabromophenoxy-2-propene  2,4-dibromophenylglycidyl ether
2,4-dibromophenylglycidyl ether  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trichloromethyl ABS, polystyrene, polyester
tetrabromobenzene
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Trichloromethyl ABS, polystyrene, polyester
tetrabromobenzene
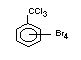 Pentabromophenyl
benzoate ABS, polyester, polystyrene
Pentabromophenyl
benzoate ABS, polyester, polystyrene
 1,4-Bis(bromomethyl)-tetrabromo Polyolefines
benzene
1,4-Bis(bromomethyl)-tetrabromo Polyolefines
benzene
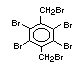 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bis-(2,3-dibromo-1-propyl)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Bis-(2,3-dibromo-1-propyl) 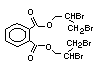 Hexabromocyclohexane
Hexabromocyclohexane  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
5,6-dibromohexahydro-2-phenyl-
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
5,6-dibromohexahydro-2-phenyl-  Chlorinated flame retardants
Dimethyl chlorendate Reactive flame
retardant for polyester resins,
alkyl paints
Chlorinated flame retardants
Dimethyl chlorendate Reactive flame
retardant for polyester resins,
alkyl paints
 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibutyl chlorendate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Dibutyl chlorendate  1,2,3,4,6,7,8,9.10,10,11,
1,2,3,4,6,7,8,9.10,10,11,  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,1a,2,2,3,3a,4,5,5,5a,5b,
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
1,1a,2,2,3,3a,4,5,5,5a,5b,  Polychlorinated biphenyls
Polychlorinated biphenyls 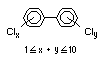 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopenta-
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Hexachlorocyclopenta-  Dibromochlordene
Dibromochlordene  Organophosphorus flame retardants
Trimethylphosphoramide Cotton, rayon
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-aziridinyl)phosphine
Organophosphorus flame retardants
Trimethylphosphoramide Cotton, rayon
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(1-aziridinyl)phosphine 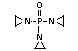 Cyanamide-phosphoric acid H2NCN + H3PO4f Finishes
Halogenated organophosphorus flame retardants
Ethylene bis[tris
Cyanamide-phosphoric acid H2NCN + H3PO4f Finishes
Halogenated organophosphorus flame retardants
Ethylene bis[tris  Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrakis(2-chloroethyl)
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tetrakis(2-chloroethyl) 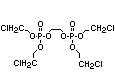 Tris(2,3-dichloro-1-propyl)
Tris(2,3-dichloro-1-propyl) 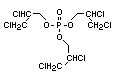 Condensate of bis Cellulosic textiles, cotton and rayon
(betachloroethyl)
phosphonate and alkyl
phosphonate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,4,6-tribromophenyl) Engineering thermoplastics
phosphate
Condensate of bis Cellulosic textiles, cotton and rayon
(betachloroethyl)
phosphonate and alkyl
phosphonate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris(2,4,6-tribromophenyl) Engineering thermoplastics
phosphate
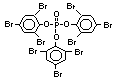 Bis(1,3-dichloro-2-propyl)-
Bis(1,3-dichloro-2-propyl)- 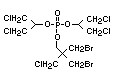 Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated phosphonic acid Polyurethane foam
ester condensate with tris-
dibromo-propyl-iso-cyanurate
Tris(dichloropropyl) phosphite
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Chlorinated phosphonic acid Polyurethane foam
ester condensate with tris-
dibromo-propyl-iso-cyanurate
Tris(dichloropropyl) phosphite 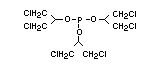 Bis[bis(2-chloroethoxy)-phosphinyl] Textiles
isopropylchloro-ethyl phosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris-(2-chloroethyl)-
Bis[bis(2-chloroethoxy)-phosphinyl] Textiles
isopropylchloro-ethyl phosphate
Table 10. (contd.)
Chemical name Chemical structure CAS registry Use as flame Remarks
number retardant
Tris-(2-chloroethyl)- 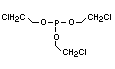 Ethylene-bis[bis
Ethylene-bis[bis 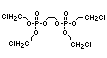 Nitrogen-based flame retardants
Pyrophosphate dimelamine salts
Nitrogen-based flame retardants
Pyrophosphate dimelamine salts 