
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 81
VANADIUM
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1988
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154281 0
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1988
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR VANADIUM
1. SUMMARY AND CONCLUSIONS
1.1. Identity, physical and chemical properties, analytical methods
1.2. Sources in the environment, environmental transport and
distribution
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on experimental animals and in vitro test systems
1.6. Effects on man
1.6.1. Local effects and dose-response relationships
1.6.2. Systemic effects and dose-response relationships
1.7. Evaluation of health risks for man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
2.3.1. Atomic absorption analysis and emission spectrometry
2.3.2. Neutron activation analysis
2.3.3. Spark-source mass spectrometry
2.3.4. Spectrophotometric analysis
2.3.5. Electrochemical methods
2.3.6. Chromatography
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1. Natural occurrence
3.1.1. Rocks
3.1.2. Soils
3.1.3. Water
3.1.4. Air
3.1.5. Plants
3.1.6. Animals
3.2. Man-made sources
3.2.1. Production levels and processes
3.2.1.1 Extraction from ores
3.2.1.2 Extraction from fossil fuels
3.2.1.3 Extraction from slag
3.3. Consumption and use
3.3.1. Metallurgy
3.3.2. Other industries
3.4. Environmental pollution resulting from production, use, and
waste disposal
3.4.1. Metallurgy
3.4.2. Fossil fuel combustion
3.4.3. Agriculture
3.5. Transport and transformation
3.5.1. Geochemical processes
3.5.2. Biogeochemical processes
3.5.2.1 Transport in, and removal from, water
3.5.2.2 Occurrence in hydrocarbons
3.5.2.3 Biospheric redox processes
3.5.2.4 Transport in air
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1. General population exposure
4.1.1. Air
4.1.2. Water
4.1.3. Food
4.1.3.1 Individual foods
4.1.3.2 Complete diets
4.2. Occupational exposure
4.2.1. Metallurgy
4.2.2. Cleaning of oil-fired boilers
4.2.3. Occupational exposure limits
5. KINETICS AND METABOLISM
5.1. Physiological role
5.1.1. Microorganisms
5.1.2. Animals
5.2. Absorption
5.2.1. Absorption by inhalation
5.2.1.1 Human studies
5.2.1.2 Animal studies
5.2.2. Absorption from the gastrointestinal tract
5.2.2.1 Human studies
5.2.2.2 Animal studies
5.2.3. Absorption through the skin
5.3. Distribution and transformation
5.3.1. Human studies
5.3.2. Animal studies
5.4. Retention
5.4.1. Human studies
5.4.2. Animal studies
5.5. Elimination
5.5.1. Human studies
5.5.2. Animal studies
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1. Aquatic organisms
6.1.1. Microorganisms and higher plants
6.1.2. Invertebrates
6.1.3. Fish
6.2. Terrestrial organisms
6.2.1. Uptake of vanadium by plants
6.2.2. Effects on plants
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. General toxicity
7.2. Effects on metabolic processes
7.2.1. Mechanisms of action
7.3. Effects on the nervous system
7.4. Effects on the respiratory system
7.5. Effects on the cardiovascular system
7.6. Effects on the kidney
7.7. Effects on the immune system
7.8. Reproduction, embryotoxicity, and teratogenicity
7.8.1. Reproduction and embryotoxicity
7.8.2. Teratogenicity
7.9. Mutagenicity and related end-points
7.10. Carcinogenicity
8. EFFECTS ON MAN
8.1. Therapeutic exposure and controlled studies
8.1.1. Therapeutic exposure
8.1.2. Controlled studies
8.1.2.1 Effects on metabolism
8.1.2.2 Effects on the respiratory system
8.2. Clinical studies
8.2.1. Acute toxicity
8.2.2. Chronic toxicity
8.2.3. Diagnosis
8.2.4. Treatment of poisoning
8.3. General population exposure
8.3.1. Low vanadium intake
8.3.2. Epidemiological studies
8.4. Occupational exposure
8.4.1. Metallurgy
8.4.2. Cleaning and related operations on oil-fired boilers
8.4.3. Handling of pure vanadium pentoxide or vanadate dusts
8.4.4. Other industries
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1. Environmental levels and exposures
9.2. Physiological role
9.3. Effects and dose-response relationships
9.3.1. Local effects and dose-response relationships
9.3.2. Systemic effects and dose-response relationships
9.3.2.1 Metabolic effects
9.3.2.2 Effects on the nervous system
9.3.2.3 Effects on the liver
9.3.2.4 Effects on the kidney
9.3.2.5 Cardiovascular effects
9.3.2.6 Pulmonary effects
9.3.2.7 Effects on the immune system
9.3.3. Reproduction, embryotoxicity, and teratogenicity
9.3.4. Mutagenicity
9.3.5. Carcinogenicity
9.3.6. Risks from exposure of the general population
10. RECOMMENDATIONS
REFERENCES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR VANADIUM
Members
Professor A.D. Dashash, Department of Community Medicine,
Medical Faculty, University of Damascus, Damascus, Syria
(Chairman)
Dr R. Frentzel-Beyme, German Cancer Research Centre, Institute
of Epidemiology and Biometry, Heidelberg, Federal Republic
of Germany
Mrs Chantana Jutiteparak, Technical Division, Food and Drug
Administration, Ministry of Public Health, Bangkok,
Thailand
Professor G.N. Krasovsky, Department of Water, Hygiene and
Sanitary Waterbodies Protection, A.N. Sysin Research
Institute of General and Community Hygiene, Moscow, USSR
Dr G.D.E. Njagi, Genotoxicology Unit, Botany Department,
Kenyatta University, Nairobi, Kenya (Rapporteur)
Dr H. Nordman, Clinical Section, Institute of Occupational
Health, Helsinki, Finland
Professor A.V. Roshchin, Department of Occupational Hygiene,
Central Institute for Advanced Medical Training, Moscow,
USSR (Vice-Chairman)
Professor Sun Mian Ling, Department of Environmental Hygiene,
School of Public Health, West China University of Medical
Sciences, Chengdu, China
Representatives from Other Organizations
Dr Z.P. Grigorevskaya, Centre for International Projects, USSR
State Committee for Science and Technology, Moscow, USSR
Dr G.F. Shkolenok, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Observers
Professor V.Yu. Kogan, Institute for General Hygiene and Prof-
pathology, Yerevan, Armenia, USSR
Dr S.M. Sokolov, Minsk Medical Institute, Minsk, USSR
Secretariat
Dr E. Smith, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr M. Gounar, Centre for International Projects, USSR State
Committee for Science and Technology, Moscow, USSR
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to
the Manager of the International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland, in order that
they may be included in corrigenda, which will appear in
subsequent volumes.
* * *
A legal file can be obtained from the International Register
of Potentially Toxic Chemicals, Palais des Nations, 1211 Geneva
10, Switzerland (Telephone No. 988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR VANADIUM
A WHO Task Group on Environmental Health Criteria for
Vanadium met in Moscow, USSR from 30 March to 3 April 1987.
Dr M.I. Gounar opened the meeting and greeted the members on
behalf of the Centre for International Projects, Moscow, USSR.
Dr E. Smith addressed the meeting on behalf of the three co-
operating organizations of the IPCS (ILO/UNEP/WHO). The Task
Group reviewed and revised the draft criteria document and made
an evaluation of the health risks of exposure to vanadium.
The efforts of all those who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects.
Financial support was also generously provided by the Institute
of Occupational Health, Helsinki, Finland - an IPCS
Participating Institution.
1. SUMMARY AND CONCLUSIONS
1.1 Identity, Physical and Chemical Properties, Analytical Methods
Vanadium (V) is a greyish metal that occurs in the form of
two natural isotopes 50V and 51V. It forms oxidation states of
-1, 0, +2, +3, +4, and +5, the oxidation states +3, +4, and +5
being the most common. Oxidation state +4 is the most stable.
Vanadium pentoxide (V2O5) is the most common commercial form of
vanadium. It dissolves in water and acids and forms vanadates
with bases. Vanadium in the +3 oxidation state (e.g., V2O3) is
basic and dissolves in acid forming a green hexa-aquo ion.
Vanadium+3 salts are strong reducing agents. Organic compounds
of vanadium are generally unstable.
Analytical methods have improved during recent years, and
extremely small amounts of vanadium can be detected in various
media. Atomic absorption assays are suitable for the routine
determination of vanadium in different media. Some refractory
oxides do not dissociate in flame. Sensitivity can be improved
by the use of a high temperature oxyacetylene flame. Flameless
atomic absorption for the determination of vanadium in air has a
detection limit of 1 µg/litre. The same method can also be
used for the determination of vanadium in water and biological
samples, with a detection limit of 0.1 - 0.4 ng. Inductively
coupled plasma optical emission spectrometry has proved an
accurate and scientific development of atomic absorption.
Neutron activation analysis, which is both rapid and
accurate, has been successfully used for the determination of
vanadium in biological fluids, such as serum and blood, and in
air, water, and biological materials. The quantitative
separation of the components to be analysed is not necessary
with this method. The detection limit of neutron activation
assays is lower than that of atomic absorption methods, and
vanadium in air can be determined at a level of 10-12 g.
Spark-source mass spectrometry is suitable for the
simultaneous determination of several elements in air and
biological materials and has a detection limit of 10-11 -
10-12 g. Neutron activation and spark-source mass spectrometry
are sophisticated methods that, because of their high cost, are
not always feasible. Various electrochemical and spectrophoto-
metric assays are being widely used for the determination of
vanadium in a variety of media. These methods have the
advantage of being relatively cheap. Coulometric titration and
controlled potential coulometry are accurate methods for the
determination of vanadium in solutions. They are not as
sensitive as the more sophisticated methods.
Stripping voltammetry and other modern modifications of
polarography, as well as electrometrical methods based on
catalytic reactions, are considered highly sensitive for the
determination of vanadium in solutions and biological materials;
however, depending on the composition of the sample, they could
involve operations to separate out interfering elements.
1.2 Sources in the Environment, Environmental Transport and Distribution
Metallic vanadium does not occur in nature. Over 70
vanadium minerals are known, carnatite and vanadinite being the
most important from the point of view of mining. Production of
vanadium is linked with that of other metals such as iron,
uranium, titanium, and aluminium. As rich minerals rarely occur
in large deposits, ores with a low vanadium content, which exist
in large amounts, are important. Extraction of vanadium from
fossil fuels, including vanadium-rich oil and coal, tars,
bitumens, and asphaltites, is important in several countries.
During the first half of the 1980s, the global production of
vanadium (as vanadium pentoxide, V2O5) ranged from 34 to 45
million kg, China, Finland, South Africa, the USA, and the USSR
being the biggest producers.
Vanadium is mainly (75 - 85%) used in ferrous metallurgy as
an alloy additive in various types of steel. Its use in non-
ferrous metals is important for the atomic energy industry,
aircraft construction, and space technology. Vanadium is also
widely used as a catalyst in the chemical industry, where
vanadium pentoxide and metavanadates are especially important
for the production of sulfuric acid and plastics. Small
quantities of vanadium are used in a variety of other
applications.
From the point of view of environmental pollution, power-
and heat-producing plants using fossil fuels (petroleum, coal,
oil) cause the most widespread discharge of vanadium into the
environment. Burning of coal wastes or dumps of coal dust in
mining areas are other sources of vanadium discharge into the
atmosphere. In the distillation and purification of crude oil,
most of the vanadium remains in the residues. Burning of
distilled petroleum fuels contributes less vanadium to the
atmosphere.
Emissions of vanadium may be high in the vicinity of large
plants producing steel alloys. Vanadium is also released into
the air: during the re-smelting of scrap steel and the
transformation of titaniferrous and vanadic magnetite iron ores
into steel; from the roasting of vanadium slags; from vanadium
pentoxide smelting furnaces; and from electric furnaces in which
ferrovanadium is smelted.
Most of the vanadium that enters sea water is in suspension
or adsorbed on colloids. It does not react chemically with sea
water and passes mechanically through it. This is reflected in
its distribution on the sea bed in the form of silt. Only about
10% of the vanadium is present in a soluble form. The very low
concentrations of vanadium in sea water indicate that vanadium
is continuously removed from sea water, but the actual
mechanisms are largely unknown. Vanadium that accumulates in
ascidians, holothurians, and in marine algae will end up in the
silt.
1.3 Environmental Levels and Human Exposure
Concentrations of vanadium in ambient air vary considerably.
Elevated vanadium levels are believed to result from the burning
of fossil fuels with a high vanadium content. Thus, heating
requirements and seasonal differences in atmospheric inversions
are reflected by fluctuations in vanadium levels in air. Air
levels of vanadium can be reduced by using distilled instead of
residual fuel oil. In remote rural areas, levels are below
1 ng/m3, but burning of fossil fuels can exceptionally increase
local levels to about 75 ng/m3. Typical concentrations in urban
air vary over a wide range of about 0.25 - 300 ng/m3. Large
cities may have annual average air levels of the order of 20 -
100 ng/m3, with markedly higher concentrations during the winter
months compared with the summer months. In the vicinity of
metallurgical industries, concentrations of 1 µg/m3 are often
found. Assuming an average air concentration of about 50 ng/m3,
about 1 µg of vanadium may enter the respiratory tract daily.
Vanadium concentrations in drinking-water are generally less
than 10 µg/litre. A typical range is 1 - 30 µg/litre with an
average of about 5 µg/litre.
The main source of vanadium intake for the general
population is food. Reported vanadium concentrations in food
tended to be higher in early studies compared with more recent
measurements, which have shown concentrations in the range of
0.1 - 10 µg/kg wet weight, with typical concentrations of about
1 µg/kg. Recent estimates of daily intake suggest a range of
10 - 70 µg with the majority of estimates below 30 µg: higher
estimates of up to 2 mg suggested in earlier studies were most
likely due to analytical differences.
Exposure to high concentrations of vanadium in the air may
occur in working environments. In the production of vanadium
pentoxide, dust concentrations containing the pentoxide can
range from 0.1 to 30 mg/m3, and concentrations of about 0.5 -
5 mg/m3 are not uncommon in the production of vanadium metal and
vanadium catalysts. The highest vanadium concentrations in air
occur in boiler cleaning where dust concentrations of 50 -
100 mg/m3, but sometimes reaching 500 mg/m3, have been
encountered. Such dusts contain 5 - 17% of vanadium pentoxide
and 3 - 10% of lower oxides. These levels are not
representative of vanadium concentrations in the air in modern
plants, where levels are usually much lower.
1.4 Kinetics and Metabolism
The rate of pulmonary absorption of various vanadium
compounds has not been determined, but it has been estimated
that about 25% of soluble vanadium compounds may be absorbed.
The results of experimental animal studies have shown complete
clearance of the relatively soluble vanadium pentoxide from the
lung in 1 - 3 days following acute exposure. When 48VOCl3 was
instilled intratracheally in the rat lung, 50% was cleared
within the first day; 3% remained after 63 days.
Vanadium salts are poorly absorbed from the human gastro-
intestinal tract, only 0.1 - 1% of the very soluble oxytartaro-
vanadate being absorbed. A very low level of gastrointestinal
absorption has also been seen in animal studies, and, though it
has been shown that soluble vanadium compounds may be absorbed
through the skin of rabbits, the dermal absorption of vanadium
compounds is likely to be extremely small.
Absorbed vanadium is mainly transported in the plasma.
Vanadium concentrations in all tissues are generally low, but
are higher in the liver, kidney, and lung than in other tissues.
Levels in the liver may be in the range of 4.5 - 19 µg/kg wet
weight and those in kidney, 3 - 7 µg/kg. Higher levels may be
found in lung tissue with mean concentrations ranging from 10 to
130 µg/kg wet weight. Small amounts have been found in the
placenta, and vanadium passes through into the membranes rather
than the fetus. Vanadium is present in breast milk and saliva.
It also passes through the blood-brain barrier. Reported levels
in human blood differ widely, levels in whole blood and serum
lying within the range of 0.01 - 0.4 mg/litre. Most studies
have shown levels below 0.1 mg/litre.
Because of the low level of absorption in the gastro-
intestinal tract, ingested vanadium is mainly eliminated
unabsorbed with the faeces. The principal route of excretion of
absorbed vanadium is through the kidneys. Vanadium concentra-
tions in urine are of the order of 0.1 - 0.2 µg/litre. The
majority of studies on occupationally exposed populations have
shown a poor correlation between vanadium concentrations in air
and the amounts excreted in urine. However, in very highly
exposed workers, urine-vanadium levels increased 20 - 30 times
over a work-shift.
1.5 Effects on Experimental Animals and In Vitro Test Systems
Vanadium is an essential element for chicks and rats.
Vanadium deficiency in these species causes reduced growth,
impairment of reproduction, and disturbance of the lipid
metabolism. Vanadium has a diuretic and natriuretic action in
rats and inhibits the Na+-K+-ATPase (EC 3.6.1.3) in microsomal
fractions of kidney, brain, and heart of several species. The
observation of the inhibiting effects on Na+-K+-ATPase led to
the discovery that a variety of enzymes are vanadium sensitive.
For instance, ATP phosphohydrolase, ribonuclease, adenylate-
kinase, phosphofructokinase, and glucose-6-phosphatase are
inhibited by vanadium compounds.
In general, vanadium is better tolerated by small animals,
such as the rat and mouse, than by larger animals including
the rabbit and horse. The toxicity of vanadium is low when
administered orally, moderate when inhaled, and high when
injected. A 1-h LC50 of 70 mg/m3 has been reported for the
inhalation of vanadium pentoxide in the rat. The minimum
concentration of vanadium pentoxide that caused mild signs
of acute poisoning in the rat was 10 mg/m3 air. Exposure of
rabbits to vanadium pentoxide at 205 mg/m3 resulted in
conjunctivitis and tracheitis, pulmonary oedema,
bronchopneumonia, and death within 7 h. The exposure of rats
for 2 h every other day for 3 months to 3 - 5 mg/m3 caused
pathological changes only in the lungs. The endothelium was
swollen, there was capillary congestion, perivascular oedema,
and small haemorrhages indicating altered vascular permeability.
Similarly, respiratory symptoms, such as nasal discharge,
sneezing, dyspnoea, and asthmatic reactions, were seen in
rabbits exposed to vanadium trioxide aerosol at 40 - 75 mg/m3,
for 2 h/day over 9 - 12 months.
The effects of acute and long-term inhalation exposure on
the respiratory tract may partly be due to the effect of
vanadium on the macrophages. A 50% reduction in the viability
of cultured rabbit macrophages was seen after exposure to 13 µg
vanadium/ml (as vanadium pentoxide) for 20 h. Exposure for 2 h
(vanadium pentoxide) reduced the viability of murine pulmonary
alveolar macrophages at a dose of 7 µg vanadium/ml.
Vanadium pentoxide administered in the diet at 0.05 - 0.5 mg
vanadium/kg, per day, for 80 days, caused impairment of
conditioned reflexes in the rat. Daily parenteral injection of
sodium metavanadate (3.2 µg/kg body weight per day, for 10 - 15
days) increased the reactivity of cytochrome oxidase in guinea-
pig brain, whereas a dose of 128 µg/kg per day did not induce
any effects and 5.12 mg/kg body weight per day reduced the
activity. Cholinesterase activity in the rat brain was reduced
by the intraperitoneal administration of 1 - 10 mg vanadyl
sulfate/kg.
Fatty changes with partial cell necrosis of the liver
occurred in rats and rabbits exposed by inhalation to vanadium
pentoxide, trioxide, or trichloride (10 - 70 mg/kg, 2 h/day, for
9 - 12 months). Fatty changes in the liver of rats also
occurred after exposure to ammonium vanadate.
Vanadate has a diuretic and natriuretic effect on rat
kidneys, but not on those of the dog or cat. This effect is
thought to be due to the inhibition of Na+-K+-ATPase, which, in
turn, inhibits the tubular reabsorption. Fatty changes in the
myocardium of both the rat and rabbit were seen after long-term
inhalation of vanadium pentoxide, trioxide, or trichloride (10 -
70 mg/m3, 2 h/day, for 9 - 12 months). Perivascular swelling of
the myocardium was also seen.
Rats given metavanadate subcutaneously (0.85 mg/kg body
weight) showed shedding of spermatogenic epithelium.
Gonadotoxic effects were suggested by the absence of
fertilization of female rats by male rats that had been exposed
subcutaneously to vanadium at 0.85 mg/kg body weight. The same
dose administered to female rats on the fourth day of pregnancy
increased the mortality of embryos.
Parenteral administration of ammonium vanadate (intra-
peritoneal) to pregnant Syrian golden hamsters and of vanadium
pentoxide (subcutaneous and intravenous) to pregnant rats
resulted in increased numbers of fetal deaths and significantly
increased skeletal abnormalities. These studies indicate a
possible teratogenic effect of vanadium.
There are few data on the mutagenicity and carcinogenicity
of vanadium compounds and limited indications of the muta-
genicity of vanadium. In a rec assay with Bacillus subtilis,
testing DNA damaging capacity, three compounds (VOCl2, V2O5,
NH4VO3), gave mildly positive results, while results of tests in
Escherichia coli and Salmonella strains were mostly negative.
Bacterial assays have given conflicting results and no firm
conclusions can be drawn.
No information is available indicating a carcinogenic action
of vanadium.
1.6 Effects on Man
No data are available on the effects of vanadium deficiency
in man, and, though possible regulatory roles of vanadium have
been suggested, a daily dietary requirement of vanadium for man
has not been defined.
1.6.1 Local effects and dose-response relationships
There are comparatively few reports about the effects of
vanadium exposure on the skin. Eczematous dermatitis has been
reported in workers exposed to vanadium pentoxide, with dust
levels as low as 6.5 µg/m3.
Inhalation of vanadium pentoxide produces local irritation.
The exposure of 2 volunteers to 1 mg/m3 for 8 h resulted, 5 h
later, in coughing that lasted for 8 days. Inhalation of
0.2 mg/m3 by 5 volunteers resulted in similar symptoms, i.e.,
coughing that started a little later (20 h after exposure) and
lasted for 7 - 10 days. Similar irritation was noted in 2
volunteers exposed to 0.1 mg/m3 for 8 h. A dose-response
relationship was observed when 11 volunteers were exposed to
0.4 mg vanadium pentoxide/m3 condensation aerosol. Tickling and
itching with dryness of the mucous membranes of the mouth were
reported by 5 subjects at 0.16 mg/m3, whereas, at 0.08 mg/m3,
none of the subjects noted any effects.
Workers exposed to dust containing vanadium at 0.01 -
0.04 mg/m3, for about 10 months, showed irritant effects on the
mucous membranes of the upper respiratory tract. Cough,
increased production of sputum and irritation of the eyes, nose,
and throat occurred among workers exposed to a maximum of 0.9 -
5 mg vanadium/m3. At high exposures (dust concentrations
ranging between 5 and 150 mg/m3), workers developed atrophic
rhinitis, and chronic bronchitis. Blood-stained sputum,
haemoptysis, and bronchospasm were seen in a proportion of those
exposed. In workers exhibiting asthmatic reactions, when
exposed to vanadium pentoxide, there was no indication of
specific sensitization; the mechanism is thought to be a direct
chemical one.
Among the local effects caused by vanadium exposure, the
green tongue occurring in a proportion of the exposed is
considered a sign of exposure rather than a toxic effect.
1.6.2 Systemic effects and dose-response relationships
The effects of vanadium on dental caries is a debatable
issue. It has been claimed that, when added to the diet of
hamsters, vanadium had a favourable effect on dental caries. It
has also been shown, in one report, that the application of an
ammonium salt of vanadium reduced caries in children. However,
other studies between 1955 and 1968 failed to demonstrate such
beneficial effects of vanadium, and in one study, an increase in
caries was observed after administration of vanadium in the
drinking-water at 2 mg/litre.
The effects of vanadium on cholesterol levels have not been
fully elucidated. Studies in the 1950s and 1960s claimed a
temporary drop in cholesterol levels in patients fed ammonium
oxytartarovanadate and ammonium vanadyltartrate, for several
weeks, at 50 - 200 mg/day. Although some data on experimental
animals have indicated that vanadium reduces cholesterol levels,
this has not been convincingly shown in human beings.
The results of studies on the effects of vanadium pentoxide
on rats have shown a decrease in cysteine in hair and also a
reduction of co-enzyme A in the liver, which could explain the
mechanism behind the reduction of cysteine. Data on the effects
of vanadium on haematopoiesis are inconsistent, and it has not
been possible to assess the effects of low-level vanadium
exposure on iron metabolism. Vanadium has been shown to inhibit
the Na+-K+-ATPase (EC 3.6.1.3) in human red blood cells.
Systemic effects are rare in workers exposed to vanadium
compounds. Nonspecific signs and symptoms including headache,
weakness, nausea, vomiting, and ringing in the ears have been
reported, and there have been reports of dizziness or giddiness
and neuraesthenic and vegetative symptoms. A few early reports
mention tremor. It is not possible to derive dose-response
relationships for these effects on the nervous system. They are
likely to be associated only with fairly high exposure levels.
Systemic effects such as anaemia, leukopenia, and basophilic
granulation of leukocytes have been reported, but cannot be
expressed in relation to any particular exposure level.
Although fatty changes in the liver and kidney have been seen in
experimental animals, there are no data on human beings to
evaluate these effects.
In exposed workers, palpitations of the heart at rest and on
exercise have been reported. Transient coronary insufficiency
and a high incidence of extrasystoles have also been reported.
The association between these symptoms and vanadium is doubtful.
Low-level exposure of workers to vanadium pentoxide at 0.01 -
0.04 mg/m3 air for about 10 months, preceded by exposure to
0.2 - 0.5 mg/m3 for about 11 years, did not cause any
pathological effects on the blood picture, the cysteine level in
hair, or the respiratory function. Wheezing was more common in
exposed workers than in controls.
A few attempts to relate vanadium levels in ambient air to
adverse effects on the general population have been made.
Positive correlations between mortality from cardiovascular
disease, lung carcinoma, and bronchitis, and vanadium air
concentrations have been reported, but, so far, causal
associations have not been reported. Further studies on the
possible effects of vanadium exposure on the general population
are needed, with better control of confounding factors and
various intercorrelations than that in available studies.
1.7 Evaluation of Health Risks for Man
There is no convincing evidence that vanadium is an
essential element for man. Vanadium interferes with a multitude
of biochemical processes, and its physiological role should be
carefully assessed. Vanadium penetrates the blood-brain barrier
and is present in breast milk. Effects on the fetuses of rats
and hamsters when vanadium was administered to pregnant animals
indicate transfer across the placental barrier; however,
Vanadium appears to concentrate in the membranes rather than in
the fetus.
Current levels of vanadium in the ambient air have been
associated with mortality in the general population due to
various diseases of the heart and lung. All studies reporting
such relationships have had serious flaws, and no causal
relationships between vanadium and disease in the general
population have been established.
Practically all the information on adverse effects on human
beings has been derived from controlled, therapeutic, or
occupational exposure to concentrations that do not occur under
normal conditions. Exposed workers may suffer from irritation
of the eyes and the respiratory tract. There is a dose-response
relationship between the concentration of vanadium in air and
its irritant effects. With short-term inhalation exposure to
vanadium pentoxide at a concentration of about 0.1 mg/m3,
irritation is manifested as coughing with increased production
of mucous. Continuous exposure to even lower levels (0.01 -
0.04 mg/m3) may cause some irritation, but does not impair lung
function. A reversible decrease in forced vital capacity (FVC)
has been reported with exposure to a dust containing 15%
vanadium at a level of about 0.5 mg/m3. High exposure levels of
5 - 150 mg/m3 cause atrophic rhinitis and bronchitis with a risk
of bronchospastic effects. Eczematous dermatitis may occur with
low-level exposure to vanadium pentoxide (6.5 µg/m3).
Non-specific effects, such as headache, nausea, weakness,
ringing in the ears, and palpitation, have been reported in
exposed workers. These effects have not been related to any
specific exposure level, but, on such occasions, it has been in
the mg/m3 air range. Such symptoms may be taken as an
indication of the need for personal protection in work tasks
associated with the risk of heavy exposure to dusts containing
vanadium.
Several reported effects of vanadium need further research,
including the effects on cholesterol levels, iron metabolism,
and haematopoiesis. Available data do not imply any risk of
carcinogenic effects; however, the data cannot be considered
conclusive. There are only weak indications of possible
mutagenic effects of vanadium compounds. The results of studies
on point mutations in bacteria are conflicting, and there are
too few studies to draw definite conclusions with respect to
mutagenicity. The scanty evidence of spermato- and gonadotoxic
effects needs corroboration. The available data suggest that
vanadium may be embryotoxic and gonadotoxic. However, the
results indicating the induction of teratogenicity require
further confirmation.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Vanadium (V) is a greyish ductile metal with an atomic
number of 23, an atomic mass of 50.942, a melting point of 1890
± 10 °C, a boiling point of 3380 °C at 1 atm (1.013 x 105 Pa),
and a specific gravity of 6.11 at 18.7 °C (Weast, 1986-87).
Vanadium has two natural isotopes, 50V and 51V, and several
radioactive isotopes (46-49V, 52-54V) have been obtained
artificially (Clark, 1975; Weast, 1987).
2.2 Physical and Chemical Properties
Vanadium has a maximum oxidation state of +5. Compounds of
vanadium may contain vanadium in oxidation states of -1, 0, +2,
+3, +4, and +5. Vanadium is usually found bound to oxygen as a
negatively charged polymeric oxyanion that tends to complex to
polarizable ligands, such as phosphorus and sulfur (Buckingham,
1973; Cotton & Wilkinson 1980).
Vanadium's ability to be either an electronegative or an
electropositive metal results in a great variety of chemical
compounds (vanadium is second only to carbon in the number of
chemical compounds). Physical properties of some important
compounds are shown in Table 1 (Weast, 1987).
Vanadium usually occurs in the pentavalent state.
Pentavalent vanadium is stable in aqueous solutions over a wide
range of pH.
The formation of isopoly and heteropoly compounds is most
characteristic of pentavalent vanadium in aqueous solutions.
The tendency of vanadium compounds to form V-0-V-0 linkages is
due to the electronegativity of vanadium and to electronic
hybridization (Zolotavin, 1954).
Vanadium pentoxide (V205), the most common commercial form
of vanadium, dissolves in water (8 g/litre) to give a pale
yellow acidic solution containing vanadium species that are
moderately strong oxidizing agents (Cotton & Wilkinson, 1962).
Vanadium5+ is reduced to vanadium4+ by relatively mild
reducing agents. The 4+ state is the most stable oxidation
state for vanadium. Nearly all of the complexes of vanadium4+
are derived from the vanadyl ion (VO2+). Most of these complexes
are anionic and a few are non-electrolytes. Vanadium in this
oxidation state forms a large number of five or six coordinate
complexes, such as vanadyl acetylacetonate and vanadyl
porphyrins found in crude petroleum.
Vanadium3+ (e.g., V203) is completely basic and dissolves in
acid to give the green hexa-aquo ion (V(H2O)6)3+. Vanadium3+ is
a strong reducing agent that slowly attacks water with the
liberation of hydrogen and the production of vanadium4+. The
hexa-aquo ion of vanadium is easily oxidized to vanadium4+.
Table 1. Physical properties of some vanadium compounds
--------------------------------------------------------------------
Compound Melting Boiling Solubility in water (g/litre)
point (°C) point (°C) Cold Hot
--------------------------------------------------------------------
Vanadiuma 690 1750 0.7b no data
pentoxide
Vanadiuma 1970 no data slightly soluble
trioxide soluble
Sodiuma 630 no data 211 388
metavanadate
Vanadium -28 ± 2 148.5 decomposes no data
tetrachloridea
Vanadium no data 127 decomposes no data
oxychloridea
Ammonium 200c no data 5.2 69.5c
vanadatea
--------------------------------------------------------------------
a From: Weast (1987).
b From: Cotton & Wilkinson (1980).
c Decomposes.
2.3 Analytical Methods
A review of analytical methods used to determine vanadium in
different media suggests that atomic absorption and
spectrophotometric assays are the most suitable for routine
analysis. Neutron activation analysis has been widely and
successfully used for the determination of vanadium in serum and
blood.
2.3.1 Atomic absorption analysis and emission spectrometry
Atomic absorption techniques have been most widely used for
the determination of vanadium in various media. Vanadium forms
heat-stable refractory oxides that are not completely
dissociated in a flame. The use of a high temperature
oxyacetylene flame improves the sensitivity of the method
(L'vov, 1970). Other ways of improving sensitivity have been
suggested (Christian & Feldman, 1970; Omang, 1971; Kragten,
1981; Wood et al., 1982). High sensitivity has been achieved
using flameless electrothermal AAS assays with a graphite
furnace. A flameless atomic absorption method using a graphite
furnace was recommended by NIOSH (1977) for the determination of
vanadium in air. A detection limit of 1 ng/ml for a maximum
sample injection of 100 µlitre was given, corresponding to an
absolute sensitivity of 0.1 ng of vanadium. A detection limit of
0.4 ng was reported by Hwang et al. (1972) using a flameless
atomic absorption method that was applicable to air, water, and
biological samples.
A method for the determination of vanadium in work-place air
using direct current plasma atomic emission spectrometry (DCP-
AES) was reported by Pyy et al. (1983). A detection limit for
vanadium in air of 0.004 mg/m3 and a practical working range of
0.01 - 100 mg/litre were suggested. The precision was given as
1%. The results of this assay correlated with those obtained
with both flame (FAAS) and electrothermal atomization (EIA-AAS)
atomic absorption spectrometry.
Vanadium and 11 other trace elements in natural water were
determined using AAS and the stabilized temperature platform
furnace. A detection limit of 0.6 µg/litre with a precision of
10 - 15% was achieved (Manning & Slavin, 1983).
Electrothermal AAS methods have been used to determine
vanadium in urine. Buchet et al. (1982) detected concentrations
in the range of 1 - 500 µg/litre, giving a coefficient of
variation for triplicate samples of less than 8% for 10 µg
vanadium/litre. A practical detection limit for vanadium in
urine of 2 µg/litre was reported by Pyy et al. (1984) also
using an electrothermal AAS method with a graphite furnace.
Extraction of vanadium with ammonium 1-pyrrolidine-
carbodithioate into 4-methylopentan-2-one reduced the detection
limit to 0.5 µg/litre.
Atomic absorption is widely used for the determination of
vanadium in biological materials, such as tissues and serum. A
detection limit of 30 pg and a sensitivity of 65 pg have been
reported using a flameless apparatus and graphite tubes (Stroop
et al., 1982).
AAS methods can also be used in the determination of
vanadium in other media such as crude petroleum (Wood et al.,
1982) and sewage sludge (Kempton et al., 1982). Improved
techniques have been developed (Barbooti & Jasim 1982; Slavin et
al., 1983) including the use of simultaneous AAS and mass
spectrometry (Styris & Kaye, 1982).
In general, emission spectral analysis has been considered
less accurate than colorimetric methods (Bagget & Huyck, 1959;
Sandell, 1959). However, it is a universal selective method by
which small amounts of vanadium can be determined in the
presence of numerous other elements. The relative sensitivity
of spectral analysis is 10-3 - 10-5%. Sensitivity can be
increased by prior separation of the element to be determined.
Inductively coupled plasma optical emission spectrometry has
been used for the simultaneous determination of several elements
in aerosol samples collected with cascade impactors (Broekaert
et al., 1982) and also for the determination of vanadium in
urine (Barnes et al., 1983).
2.3.2 Neutron activation analysis
Neutron activation analysis is more rapid and accurate than
other methods. Using this method, it has been possible to
determine up to 70 elements in amounts of 10-12 g in air (Dams
et al., 1970; Gershkovich & Stykan, 1972). In carrying out
neutron activation analysis, a weighted sample or test solution
is irradiated with a thermal neutron flux in an atomic reactor,
for a certain length of time (Flaherty & Eldrige, 1970; Frolov,
1970). During irradiation, one or several isotopes of the
element being tested are formed. The activity of the isotopes
formed is determined from the gamma peak by means of a
scintillation gamma spectrometer. The sensitivity of the method
depends on many factors including: size of the particle flux,
duration of sample irradiation, efficiency of the counter, time
elapsed since the beginning of irradiation, background response
of the counter, etc.
The chemical form of vanadium in water can be determined
using a 48V tracer and neutron activation (Orvini et al.,
1979).
Neutron activation determination of vanadium in biological
material is complicated by the high concentration of sodium,
even when a Ge/Li detector is used. Because of the short life
of the isotope, the sodium must be eliminated before
irradiation, normally by absorption on antimony pentoxide
(Ralston & Sato, 1971). Neutron activation has been
successfully used to determine vanadium in serum (Byrne & Kosta,
1978; Sabbioni et al., 1979; Cornelis et al., 1980, 1981) and
body tissues (Yukawa et al., 1980).
2.3.3 Spark-source mass spectrometry
Spark-source mass spectrometry is an excellent analytical
tool (Johnson et al., 1974). The absolute sensitivity of the
method is 10-11 - 10-12 g, and the relative sensitivity is
10-7 g-atom. Up to 70 elements can be recorded simultaneously
on the photographic plate and only a few milligrams of sample
are needed (Chupahin et al., 1972). This method is used for the
multi-element analysis of air and biological materials.
Evans & Morrison (1968) described the problems that occur in
analysing ashed biological material for vanadium using spark-
source mass spectrometry. The ash must be completely free of
organic mixtures, since vanadium belongs to the class of
elements in which inorganic compounds are completely bound to
biological material. The concentration of vanadium found by
spark-source mass spectrometry was 10 times as high as that
found by the spectral method in the same samples.
Vanadium levels in urine and biological tissues were
determined by Pilz & Komischke (1972) using salicylhydroxamic
acid. The vanadium complex was extracted with N-pentanol, and
the vanadium was determined in the extract by spectrophotometry.
Beer's law was observed, with concentrations of vanadium ranging
from 1 to 2000 µg/25 ml extract. There was no interference by
cobalt, nickel, zinc, molybdenum, tungsten, iron, calcium, lead,
or chromium.
2.3.4 Spectrophotometric analysis
Organic reagents are often used to improve the specificity
of spectrophotometric analysis. Over 80 organic reagents have
been suggested for the direct quantitative determination of
vanadium (Mustafin et al., 1969; Muzquin et al., 1981). The
specificity of the organic reagents can be increased when
complexing agents are used to bind the interfering ions. In
most cases, particularly those based on complexing reactions,
specificity and sensitivity are enhanced by prior separation of
vanadium, mostly by extraction. Acyl derivatives of
hydroxylamine containing the OC-NOH group show high selectivity
for pentavalent vanadium, when the product of interaction in a
highly acid medium is extracted (Tandon & Bhattacharya, 1961;
Majumdar & Das, 1965).
Spectrophotometric analysis based on catalytic reactions,
e.g., on acceleration of the oxidation of aromatic amines and
aminophenols with chlorates, bromates, periodates, and
persulfates in the presence of pentavalent vanadium compounds,
is widely used to determine trace amounts of vanadium (Bakal &
Liseckaja, 1971; Zheljazkova et al., 1972). The sensitivity of
kinetic methods is theoretically unlimited and their use for
analysing biological materials is quite promising, because of
the considerably reduced amounts of material needed for analysis
(Jacimirskij, 1967).
Welch & Allaway (1972) proposed a method to determine
nanogram quantities of vanadium by means of an acid oxidation
reaction catalysed by vanadium pentoxide. Christian (1971)
determined vanadium in blood and urine using a method based on
the catalytic effect of vanadium on the oxidation reaction of
phenylehydrazine-N-sulfonic acid with potassium chlorate.
Vanadium concentrations of 0.056 ± 0.033 mg/litre in blood-
plasma, 0.061 ± 0.019 mg/litre in red blood cells, and
0.022 ± 0.015 mg/litre in urine were detected using this
method.
2.3.5 Electrochemical methods
Vanadium is commonly determined by electrochemical methods,
namely by volumetric titration with electrometric detection,
such as potentiometry (Cassani, 1968), amperometry (Singh &
Sharma, 1970), as well as by coulometric titrations (Kostromin
et al., 1970), polarography (Shevchenko & Gorodynskij, 1964;
Budnikov & Medjantseva, 1973), and coulometry (Rigdon & Harrar,
1969). Catalytic reactions with polarographic, potentiometric,
and amperometric detection (Weisz et al., 1974) are also used.
Stripping voltammetry (Van den Berg & Huang Fi Qiang, 1984)
and other modifications of polarography (Veys, 1983), as well as
electrometric methods based on catalytic reactions are highly
sensitive but, depending on the composition of the sample, they
can involve operations to separate out interfering elements in
the sample. The selectivity of controlled potential coulometry
is high, making the separate determination of vanadium compounds
of different valencies possible. The introduction of
differential techniques into both coulometric titration and
controlled potential coulometry results in very high accuracy
(Agasyan et al., 1975; Shkolenok et al., 1977).
2.3.6 Chromatography
The chromatographic method has found little practical
application in determining trace quantities of vanadium though
Bonig & Heigener (1971) used selective paper chromatography to
determine microgram quantities of vanadium.
3. SOURCES IN THE ENVIRONMENT, ENVIRONMENTAL TRANSPORT AND DISTRIBUTION
3.1 Natural Occurrence
3.1.1 Rocks
Vanadium is a typical rare element, present in the earth's
crust at concentrations of around 0.015 g/kg, which is roughly
in the same proportions as chromium, strontium, and zirconium.
It is considerably more widespread than copper, lead, zinc, and
other minor elements. Some 70 vanadium minerals are known, of
which 40 are vanadates. The main vanadium minerals are
vanadinite (19% vanadium pentoxide), descloizite (220 g/kg),
cuprodescloizite (170 - 220 g/kg), carnotite (200 g/kg),
roscoelite (210 - 290 g/kg), and patronite (170 - 290 g/kg).
Admixtures are found in the ore minerals titaniferromagnetite
(up to 88 g vanadium pentoxide/kg), magnesioferrite (160 g/kg),
magnetite (6 g/kg), rutile (1 g/kg), and ilimenite (4 g/kg).
Metallic vanadium does not occur in nature, and the richer
minerals rarely occur in large deposits. Vanadium compounds are
present in fossil fuels (oil, coal, shale), and some oilfields
have a high vanadium content (NAS, 1974).
Vanadiferrous phosphorites (1 - 10 g/kg), asphaltites (up to
500 g/kg in ash), and titaniferrous magnetite placers, mainly of
the sea-beach type (about 3 g/kg), are important sources of
vanadium. Oolite brown iron ore (ferrophosphorous ore), which
contains only small amounts of vanadium pentoxide (0.7 - 2 g/kg)
but occurs extensively, carbonaceous cherts (15 - 20 g/kg),
bauxites (0.2 - 0.4 g/kg), the ash of coal and combustible shale
(2 g/kg), and ferromanganese nodules in the ocean, may all
provide sources for vanadium extraction (Todria, 1963; Holodov,
1968, Schumann-Vogt, 1969; Borisenko, 1973; Rose, 1973; NAS,
1974). The most important deposits of vanadium ores are found
in Canada, Finland, Namibia, South Africa, Sweden, the USA, the
USSR, and Zambia (Borisenko, 1973).
3.1.2 Soils
The vanadium contents of soils are related to those of the
parent rocks from which they are formed and range from 3 to 310
mg/kg, the highest concentrations being found in shales and
clays (Waters, 1977). Vanadium is evenly distributed in the
soil horizons, but there is sometimes a higher level in the A
horizon, possibly connected with the vital activity of plants.
In the neighbourhood of vanadium-bearing rocks or of large
amounts of iron oxides, a moderate local increase in soil-
vanadium levels may be found. Vanadium is present in the soils
of France, Japan, Spain, the United Kingdom, and the USA at
levels that are partly determined by the distribution of iron in
these soils (Holodov, 1968); levels ranged from 1 to 680 g/kg
(Vinogradov 1957). The lowest concentration was reported from
Japan, and the highest from Spain. Vinogradov (1957) found
lower vanadium concentrations in USSR podzols than in tundra and
chernozem soils.
3.1.3 Water
The levels of vanadium in fresh water in different parts of
the world vary from undetectable to 0.220 mg/litre (Table 2).
The geographical differences in fresh water vanadium levels are
due to differences in rainwater runoff from natural sources or
in industrial effluent. Data on vanadium levels in waters
contaminated with industrial effluent are presented in section
3.4.1. Some data on vanadium in sea-water are presented in
Table 3.
3.1.4 Air
Natural sources of airborne vanadium are marine aerosols and
continental dust. The concentration of vanadium in the air at
the South Pole is very low (0.001 - 0.002 ng/m3) (Zoller et al.,
1974). Levels in ocean air in the middle latitudes are about
two orders of magnitude higher (Hoffman et al., 1972; Martens et
al., 1973).
Atmospheric concentrations of vanadium from continental dust
and sea spray can be predicted using various models (NAS, 1974).
Concentrations of 0.1 ng/m3 (range, 0.02 - 0.8 ng/m3) measured
over the eastern Pacific Ocean and 0.72 ng/m3 (range, 0.21 - 1.9
ng/m3) over rural northwestern Canada agree with these
predictions, and can be regarded as natural background levels.
Many rural areas in the USA display similar or only slightly
higher levels. However, in northeastern USA, rural air
concentrations are higher, ranging from 2 to 64 ng/m3, and are
attributed to the local burning of fuel oil with a very high
vanadium content (section 4.1.1).
Only small amounts of airborne vanadium are produced as a
result of volcanic action (Zoller et al., 1973).
3.1.5 Plants
Vanadium occurs in small amounts in all plants, usually at
concentrations of a few mg/kg dry weight. Within a given
species, variation is influenced by soil-vanadium levels, soil
acidity, and growing conditions, but the range of variation is
not large. The vanadium concentrations in roots are nearly the
same as the level in the soil in which they are grown. Vanadium
levels are lowest in the aerial portions of most plants and are
unrelated to soil levels. Bertrand (1950) found vanadium in
each of 62 plant species analysed; mean concentrations in higher
plants were 0.16 mg/kg fresh weight, 1 mg/kg dry weight, and
7 mg/kg ash. A mean level of 1.2 mg/kg was found in the leaves
of woody plants by Hanna & Grant (1962).
Vanadium accumulation occurs in the fly agaric mushroom
(Amanita muscaria) , which contains about 100 times as much as
other mushrooms or plants (Bertrand, 1950). Cowgill (1973)
determined vanadium concentrations in fresh-water plants in the
range of 0.4 - 80 mg/kg. The higher value of 80 mg/kg was found
in the pickerel weed (Pontedaris cordata) , which is a probable
accumulator. Mosses (Hypnum cupressiforme) also accumulate
vanadium; concentrations of about 10 mg/kg have been measured in
rural mosses, whereas concentrations may be as high as 50 -
250 mg/kg in mosses from city areas (Tyler, 1970; Ruhling,
1971).
Table 2. Vanadium levels in fresh water
-----------------------------------------------------------------------------
Source of water Vanadium level Reference
-----------------------------------------------------------------------------
Japan
Rivers of Japan 0.001 mg/litre Sugawara et al. (1956)
Waters of 5 Japanese 0.0001-0.087 mg/litre Sugawara et al. (1956)
lakes (average, 0.0007
mg/litre)
USA
Rivers of Colorado 0.2 - 49.2 µg/litre Linstedt & Kruger (1969)
Rivers of New Mexico up to 19 µg/litre NAS (1974)
Rivers of the USA 0.001 mg/litre Durum & Haffty (1963)
Rivers of the Colorado to 70 µg/litre Schroeder (197Oa)
plateau
Wyoming River 30 - 220 mg/litre Schroeder (197Oa)
USSR
30 large rivers traces to 0.43 mg/litre Konovalov et al. (1968)
average 0.037 mg/litre;
average in dissolved
form, 0.0012 mg/litre
Protva and Tarusa 0.007-0.0135 mg/litre Tjurjukanov (1963)
Rivers
Moscow River 0.0025-0.0074 mg/litre Tjurjukanov (1963)
Rivers of the Klinsk- 0.005-0.0074 mg/litre Tjurjukanov (1963)
Dmitrovsk ridge
Waters of the area west 0 - 34 µg/litre Petuhov et al. (1969)
of the Kama River in the
Tartar ASSR: rivers and
lakes
Uzbek SSR: surface 0.0003 - 0.003% Mirzaeva (1965)
waters
-----------------------------------------------------------------------------
Table 3. Vanadium levels in Sea-watera
------------------------------------------------------------
Water Vanadium level Reference
(mg/litre)
------------------------------------------------------------
Sea-water 0.0005 (average) Vinogradov (1944)
Sea-water 0.0003 Sverdrup et al.
(1950)
Near the Japanese coast 0.001 - 0.002 Sugawara et al.
(1956)
Sea-water 0.002 Goldberg (1961)
Western Pacific 0.003 Sugawara et al.
(1956)
Sea-water 0.002 - 0.029 NAS (1974)
------------------------------------------------------------
a Modified from: Holodov (1968).
3.1.6 Animals
Vanadium appears to be present in all animals, but tissue
levels in most vertebrates (especially land mammals) are so low
that detection is difficult. Higher concentrations have been
found in marine species, especially invertebrates (Bertrand,
1950). In land mammals, the highest levels occur in the liver
and skeletal tissues.
Estimates by Vinogradov (1959) and Schroeder (197Oa) of
vanadium concentrations in animals are shown in Table 4.
Limited data for several tissues of wild animals are shown in
Table 5, and some concentrations in domestic animal tissues,
measured by sensitive methods, are given in Table 11 (section
4.1.3.1). On the whole, these agree with Bertrand's
observations.
Using neutron activation analysis, Fukai & Meinke (1962)
reported that concentrations of vanadium in the soft tissues of
fish were 1000 times those in seaweeds and molluscs. The
highest concentrations of vanadium in marine organisms have been
found in certain ascidians (sea squirts) (e.g., Phallusia
mamillata , 1900 mg/kg), certain holothurians (sea cucumbers)
(e.g., Sticopus mobii , 1200 mg/kg), a mollusc (Pleurobranchus
plumula , 150 mg/kg), and marine algae.
Table 4. Vanadium levels in animalsa
----------------------------------------------
Animal Vanadium concentration
(mg/kg dry weight)
----------------------------------------------
Coelenterate 2.3
Annelid 1.2
Mollusc 0.7
Echinoderm 1.9
Crustacean 0.4
Insect 0.15
Fish 0.14
Mammal 0.4
----------------------------------------------
a From: Vinogradov (1959) and Schroeder (197Oa).
Table 5. Vanadium levels in tissues of wild animalsa
--------------------------------------------------
Tissueb Number of Vanadium concentration
samples (mg/kg wet weight)
Mean Range
--------------------------------------------------
Kidney 4 0.94 0 - 2.07
Liver 4 0.25 0 - 0.94
Heart 4 1.16 0 - 3.40
Spleen 1 1.16
--------------------------------------------------
a From: Schroeder (197Oa).
b Beaver, deer, woodchuck, rabbit, muskrat, and fox.
In certain ascidians, trivalent vanadium is present as a
chromoprotein called haemovanadin together with sulfuric acid in
green cells termed vanodocytes; in other forms, the free
haemovanadin is present in plasma (Hudson, 1964).
3.2 Man-Made Sources
3.2.1 Production levels and processes
The annual production of vanadium (as vanadium pentoxide)
ing 1980-84 was between 34 and 46 million kg (Table 6). The
estimated world capacity up to 1990 is shown in Table 7.
Table 6. Production of vanadium by major producersa,b,c
--------------------------------------------------------
Country 1981 1982 1983 1984
--------------------------------------------------------
Australia 0.1 0 0 0
China, Peoples Republic of 4.5 4.5 4.5 1.8
Finland 5.2 4.8 5.0 4.5
Japan 0.7 0.7 0.8 0.9
Norway 0.9 0.4 0 0
USA 13.9 10.1 3.6 5.9
South Africa 21.0 19.6 14.5 20.4
--------------------------------------------------------
a From: Wentzel (1985).
b Production in millions of kg V2O5 equivalent.
c Data on production in the USSR are lacking. It is
probably about 10 000-15 000 tonnes.
Table 7. World vanadium capacitya,b
------------------------------------------------------------------
Country 1981 1982 1983 1984 1985 1990
------------------------------------------------------------------
Australia/New Zealand 0.4 1.6 0 0 0 3.6
China, People's Republic of 5.4 5.4 5.4 5.4 5.4 5.4
Finland 5.2 5.2 5.2 5.2 3.1 0
Japan 1.3 1.3 1.3 1.3 1.3 1.3
Norway 0.9 0.9 0 0 0 0
South Africa 28.4 28.4 27.2 27.2 27.2 29.5
USA 13.9 16.4 14.3 14.3 9.5 15.4
Venezuela 0 0 0 0 0 2.7
------------------------------------------------------------------
a From: Wentzel (1985).
b Capacity in millions of kg V2O5 equivalent.
The major producers of vanadium are China, Finland, South
Africa, the USA, and the USSR.
European countries, together with Japan and the USA, use 85%
of the total output.
3.2.1.1 Extraction from ores
The production of vanadium is closely linked with that of
other metals (particularly iron, but also uranium, titanium, and
aluminium). It is sometimes extracted from ores directly as a
vanadium-rich alloy (e.g., ferrovanadium).
3.2.1.2 Extraction from fossil fuels
Petroleum is a source of vanadium. A number of oilfields
have a high vanadium content; the vanadium level in vanadium-
rich oil ash amounts to as much as 600 - 700 g/kg (Holodov,
1968; Borisenko, 1973; Aleshin et al., 1974; NAS, 1974). For
this reason, vanadium is extracted from petroleum ash in some of
countries (e.g., Canada, Italy, USA).
All coals contain vanadium, concentrations in various
coalfields ranging from extremely low to 10 g/kg (in coal)
(e.g., Argentina, USSR) (Holodov, 1968, 1973; Borisenko, 1973;
NAS, 1974). Coal ash constitutes a supplementary source of
vanadium (up to 300 g/kg).
Tar sands (Canada), bitumens, and asphaltites (Argentina,
Peru, USA, USSR) are potential sources of vanadium. For
instance, burning bitumen from the Sadkinskoe deposit (USSR)
yielded an ash containing 43 - 66% vanadium pentoxide (Holodov,
1968, 1973).
3.2.1.3 Extraction from slag
In some countries, vanadium is extracted from slag resulting
from the metallurgical production of catalysts (Pilz &
Komischke, 1972; Rose, 1973) or the processing of vanadium
catalysts. The levels of vanadium pentoxide in slags obtained
from Bessemer converters of pig iron made from Kachkanar ores
(USSR) were 135 - 140 g/kg (Pastuhov & Tretjakov, 1959). Slag
obtained at a factory in South Africa contained a vanadium
pentoxide concentration of about 250 g/kg (NAS, 1974).
3.3 Consumption and Use
3.3.1 Metallurgy
Vanadium has important industrial uses, mainly in ferrous
metallurgy, where 75 - 85% of all vanadium produced is used as
an alloy additive in making special steels. Pure vanadium is
very seldom used as it reacts easily with oxygen, nitrogen, and
carbon at a relatively low temperature (300 °C).
To produce various high-resistance carbon steels, vanadium
is combined with chromium, nickel, manganese, boron, tungsten,
and other elements. The amount of vanadium in the steel ranges
from 0.3 to 51 g/kg (Goldshtejn, 1967; Grin et al., 1971).
Vanadium may be a component of structural steels used in
building, transport, engineering, and boiler-making and in tool
steels. It is added to steel in the form of either
ferrovanadium (an iron/vanadium alloy containing 400 - 800 g
vanadium/kg) or vanadium carbide. Vanadium is also a major
alloying element in high-strength titanium alloys. The amounts
of vanadium used in recent years in the ferrous metal industries
of four major consumer countries are listed Table 8.
Table 8. Use of vanadium in ferrous metallurgy (tonnes)a
---------------------------------------------------------------------
1964 1965 1966 1967 1968 1969 1970 1971 1972
---------------------------------------------------------------------
Canada 115 113 - - - 187 231 - -
France 209 239 225 340 342 539 518 402 409
United 600 600 600 500 600 800 800 600 500
Kingdom
USA - 3709 4180 3425 3997 4333 3667 3346 -
---------------------------------------------------------------------
a From: US Bureau of Mines (1974).
3.3.2 Other industries
The consumption of vanadium by branches of industry other
than metallurgy has increased, as can be seen from the values
given for the USA in Table 9.
Table 9. Use of vanadium in non-ferrous
USA industries (tonnes)a
-----------------------------------------
1965 1966 1967 1968 1969 1970 1971
-----------------------------------------
562 703 1325 988 1250 991 810
-----------------------------------------
a From: US Bureau of Mines (1974).
Alloys of vanadium with non-ferrous metals (aluminium,
titanium, copper, etc.) are widely used in the atomic energy
industry, aircraft construction, and space technology. Vanadium
disilicide is used in the production of high-temperature
refractory products (Kubasky, 1957). With regard to the
production of chemicals, vanadium oxides and vanadates have
important applications as catalysts in: the synthesis of
sulfuric acid; the oxidation of organic compounds; petroleum
cracking; purifying exhaust gases; and oxidizing ethanol. These
vanadium compounds are also used in producing glass of different
types and colours, organic ion exchangers, luminescent
compounds, ethylene-propylene synthetic rubber, thermistors, and
switching elements. The pentoxide and various other salts of
vanadium are used in preparing glazes and enamels for porcelain
and pottery, in producing lacquers and paints, and as
developers, sensitizers, and colouring agents in photography and
cinematography. Vanadium is also used as a mordant in the
dyeing and printing of cotton, particularly for fixing aniline
black on silk. Europium-activated yttrium vanadate is used in
colour television tubes. Vanadium hydride can be used as a
neutron moderator in atomic reactors. Soluble salts of
arsenous-vanadous acid have been used as fungicides and
insecticides. Vanadium slags are used in casting shops as a
moulding material to improve the quality of the casting surface
and to facilitate cleaning.
In most of these applications, the quantities of vanadium
used are small. Some recycling takes place (e.g., with
catalysts).
3.4 Environmental Pollution Resulting from Production, Use, and Waste Disposal
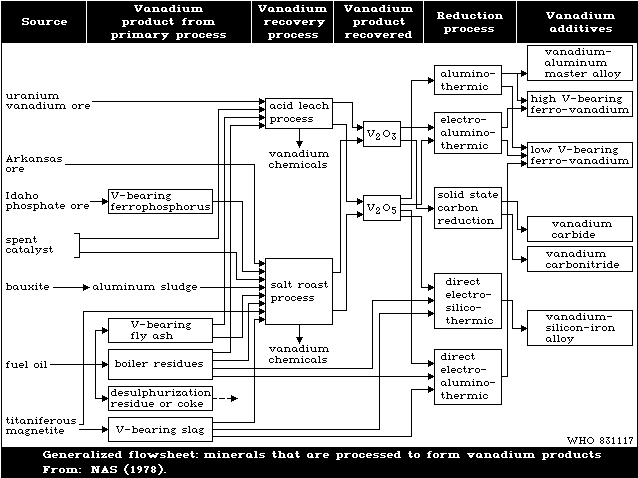
Fig. 1 shows the cycle of the various chemical processes
involved in the production and recovery of vanadium. Expansion
of the mining and processing of vanadiferrous materials, and of
the use of vanadium in metallurgical and other industries, and
the use of petroleum at power stations and in engineering, can
lead to increased pollution of the atmosphere and watercourses
with vanadium compounds. Pollution is mainly by the penta- and
trivalent oxides.
3.4.1 Metallurgy
The most important industry with respect to vanadium
pollution is the metallurgical industry, in which vanadium is
used to obtain steel alloys. Because of the relatively low
melting point of vanadium pentoxide (690 °C), its fumes may
enter the air, condense, and form an aerosol with particle
diameters of up to 2 µm (Roshchin, 1968). Obviously, these
processes may lead not only to contamination of the air in
industrial premises, but also to contamination of the outdoor
atmosphere (where an aerosol of vanadium pentoxide forms part of
the smoke emission). Vanadium pentoxide was found in 87% of all
air samples taken in the vicinity of large metallurgical plants,
in concentrations ranging from 0.98 to 1.49 µg/m3. Concentra-
tions in 11% of the samples exceeded 2 µg/m3 (Pazhynich,
1967).
The process of re-smelting steel scrap also leads to the
discharge of a vanadium-containing aerosol into the atmosphere.
In 1968, in the USA, 43.5 million tonnes of steel were produced
from the re-smelting of scrap in basic oxygen furnaces. The
emission factor was calculated to be 21 kg particulates per
tonne of steel produced and the degree of emission control 97%.
The aerosol discharged into the air during this process
contained a vanadium concentration of 0.02%. Based on these
figures, an estimated 6 tonnes of vanadium escaped into the
atmosphere (US EPA, 1977).
Ferrovanadium used for alloys in steelmaking is produced in
electric arc furnaces. The charge consists of scrap steel,
fused sodium metavanadate, and carbon with silicon, aluminium,
or a combination of the last two elements, as a reducing agent.
An estimated 131 tonnes of vanadium were discharged into the air
as a result of ferrovanadium production in the USA in 1968 (US
EPA, 1977).
Discharge into the atmosphere is greatest from furnaces
roasting vanadium slags, vanadium pentoxide smelting furnaces,
electric furnaces, crucibles in which ferrovanadium is melted,
and crushing equipment (US EPA, 1977).
Metallurgical slag may contain significant concentrations of
vanadium. When titaniferrous and vanadic magnetite iron ores
are converted into steel, the resulting slag contains vanadium
pentoxide concentrations of up to 250 g/kg (Dovgopol et al.,
1974; NAS, 1974). Vanadium is released into the atmosphere
during the loading, transporting, unloading, and crushing of
slag. The slag formed when iron is smelted contains
considerably less vanadium than converter slag. However, in
view of the considerable and increasing use of blast-furnace
slags as building-materials and in motorway construction, they
can be sources of environmental pollution. For example, in a
plant smelting vanadium-containing titaniferrous magnetite, the
losses of vanadium in slag represented 18% of the vanadium
content of the original raw material (Dovgopol et al., 1974).
The solid wastes formed as a result of roasting slag during
the production of technical vanadium pentoxide may be another
source of environmental pollution. In this process, an average
of 5.16 tonnes of solid waste containing 1.2% vanadium pentoxide
are formed for every tonne of vanadium pentoxide produced
(Kurmaev, 1974).
 The liquid waste and wash water from metallurgical plants
often contain large amounts of vanadium, up to several hundred
milligrams per litre, measured as vanadium pentoxide. Kurmaev
(1974) detected vanadium at a level of 702.8 mg/litre in
effluent from a vanadium pentoxide plant. In a new
ferrovanadium plant, purified waste water contained 340 mg
vanadium pentoxide/litre (Kurmaev, 1974). Unpurified waste-
water discharge from a vanadium pentoxide plant into an open
watercourse produced a vanadium level in the watercourse of
2 mg/litre (Seljankina, 1961). Linstedt & Kruger (1969) found
the highest river concentrations of vanadium near uranium-
vanadium plants and the lowest in water samples taken upstream
from the industrial areas (Table 2) (section 3.1.3). In
studies by Shilina & Malakhov (1974), water samples taken from
the Moscow River below the city contained 6 times as much
vanadium as samples taken above the city (0.06 and
0.01 mg/litre, respectively).
The pickling of steel casts or steel articles can include
vanadium in the pickling mixture. Waste hydrochloric, nitric,
hydrofluoric, and sulfuric acids from a steel smelting plant
contained 0.02% vanadium. The solid residue on the bottom of
the acid vats contained 2.4 g vanadium/kg (hydrochloric acid
vat) and 1.6 g vanadium/kg (nitric and hydrofluoric acid vats)
(Kurmaev, 1974); vanadium in waste acid that is not properly
treated may be a source of water pollution.
3.4.2 Fossil fuel combustion
Industrial plants producing power and heat and operating on
petroleum, coal, and heavy oils are the most widespread source
of vanadium discharge into the environment. In 1969, as a
result of the combustion of fossil fuels (coal and oil), about
20 000 tonnes of vanadium were discharged into the air in the
USA (NAS, 1974). The estimated levels of emission from burning
coal in the USA for 1969 are shown in Table 10.
In a study of the vanadium discharged from coal burning in
six electric power generating stations, the total amount
discharged into the air in 1968 was 3760 tonnes. Where there
were no ash-trapping devices, 65% of the ash entered the
atmosphere. The degree of atmospheric dispersion of ash
particles depended on the original coalfield, the size of coal
used, the type of furnace, the combustion conditions, and the
presence and type of ash-trapping devices (NAS, 1974).
In a study of the metal aerosol content of waste gas
emissions from an oil-fired electric power station, it was
reported that the concentration of vanadium pentoxide (and
oxides of aluminium, chromium, iron, and manganese) was not
affected by the type of boiler or mode of operation. Comparison
of the metal content in the fuel oil and waste gases showed that
90% was released into the atmosphere (Sokolov, 1986). In the
USSR, there is a trend towards a reduction in the use of heavy
fuel oils in electric power stations.
Other possible sources of vanadium discharge into the air
are the burning of coal tips or dumps of coal dust in mining
areas, but data are not available.
All crude petroleum oils contain vanadium at levels ranging
from 1 to 400 g/tonne, depending on the oilfield (Holodov, 1968,
1973; Shah et al., 1970; Christian & Robinson, 1971; Nelson,
1973; NAS, 1974). In the distillation of crude oil, almost all
the vanadium remains in the high relative molecular mass
hydrocarbon fractions. The vanadium contents of heavy fuel oils
range widely from 1 to 200 g/tonne and are almost a thousand
times greater than those of petroleum distillates. Assuming
that 10% of the vanadium is precipitated inside the plant (flue
and ash trap) while 90% is discharged into the air, it has been
calculated that atmospheric emissions in the USA as a result of
heavy fuel oil combustion were about 14 100 - 21 800 tonnes in
1970 (Holodov, 1968, 1973; NAS, 1974). Similar percentages were
found by Sokolov (1986).
Table 10. Estimated emissions of vanadium resulting from coal
burning in the USA, 1969a
-------------------------------------------------------------------
Type and use Coal Vanadium Vanadium Control Vanadium
of coal (1000 in coal in fly of discharged
tonnes) (tonnes) ash fly into the
(tonnes) ash (%) air
(tonnes)
-------------------------------------------------------------------
Bituminous coal
Electric power 308 642 9254 6015 85 902
utilities
Manufacturing 93 248 2797 1818 60 727
Retail 12 665 380 247 50 124
deliveries
Coking 92 901 2787 - 100 0
Subtotal 507 276 15 218 8080 1753
Anthracite coal 9275 1159 753 50 377
Total 2130
-------------------------------------------------------------------
a From: NAS (1974).
Distilled petroleum fuels produced in the USA (gasoline,
kerosene, diesel fuel, home-heating oils) contain 0.05 mg
vanadium/kg (NAS, 1974). The distillation process used leaves
nearly all of the vanadium originally present in the residual
fractions. Analyses of the spent gases from petrol engines,
sampled directly at the exhaust outlet, showed vanadium
concentrations of 0.1 - 0.2 mg/kg, and exhaust gases of diesel
engines contained 10 - 15 mg vanadium/kg. Six to 12 mg/kg of
vanadium was found in soot collected from the edges of flues in
a small oil-fired power station (Pilz & Komischke, 1972).
The amount of vanadium in natural gas is less than
0.5 g/tonne, and almost no vanadium is released into the
atmosphere on combustion (NAS, 1974).
3.4.3 Agriculture
Vanadium has been used as a trace fertilizer applied at the
rate of 0.75 - 1 mg/kg soil (Peterburgskij & Tormasova, 1969).
This practice must lead to an increased level of vanadium in the
soil, but further information on agricultural use is not
available.
3.5 Transport and Transformation
3.5.1 Geochemical processes
Vanadium is involved in various geochemical processes
occurring in the earth's crust. There is extremely wide
dispersion of vanadium during the formation of volcanic rocks
and sporadic accumulation with the formation of vanadium
minerals as a result of postmagmatic processes. Like all trace
elements that accumulate in soils, vanadium migrates within the
soil itself and within the system: rock-water-soil-vegetation-
animals-man.
During geochemical processes in the soil and in weathering
and podzolization, vanadium is shifted from the A horizon to the
B horizon (Kovda et al., 1959). However, vanadium shifting does
not occur during weathering processes that do not involve
movement of sesquioxides.
Vanadium concentrations in rocks are linked to the pH of the
rocks (Borisenko, 1973). Neutral and acid rocks contain lower
vanadium concentrations than basic rocks, and acid rocks contain
lower concentrations than neutral rocks. In magma of various
types (the main carrier of vanadium), about 92% of all vanadium
occurs in basic rocks (basalts, gabbro, amphibolites, and
eclogites), and about 8% occurs in acid and neutral rocks. Less
than 1% of the total amount of vanadium is found in ultrabasic
alkaline rocks.
The main carriers of vanadium in the sedimentation process
are ferric hydroxides and solid bitumens. The great affinity
between the crystallochemical properties of V3+ and Fe3+ is of
vital importance for the diffusion of vanadium. There is
roughly 400 - 500 times more iron than vanadium in the earth's
crust. Thus, iron is a "solvent" of trivalent vanadium, and is
responsible for its diffusion in magmatic rocks. The bulk of
the ferromagnesian rock-forming minerals (and also titaniferrous
magnetite and magnetite itself) trap vanadium during the
crystallization of rocks, and, during endogenous processes,
vanadium is very closely linked with trivalent iron. In the
formation of igneous rocks, vanadium is preferentially
concentrated in those with a high iron content.
3.5.2 Biogeochemical processes
The accumulation of vanadium in soils and in all other
materials depends directly on its concentration in the soil-
forming rocks, the atmosphere, and the oceans of the world.
Migration, diffusion, and concentration of vanadium in the
biosphere takes place as a result of its extraction by living
organisms from water, from food of both vegetable and animal
origin, and from different types of rock during their
decomposition and the formation of soils.
3.5.2.1 Transport in, and removal from, water
In assessing the relative importance of the two ways in
which vanadium is transported in water, Konovalov et al. (1968)
and Holodov (1968) concluded that 87% is carried away by the
rivers in suspended form, and 13% in solution. The average
level of dissolved vanadium in the rivers of Japan and the USA
(Sugawara et al., 1956; Durum & Haffty, 1963) were the same as
that recorded by Konovalov et al. (1968), i.e., 0.001 mg/litre.
The bulk of vanadium enters sea-water in suspended form or
sorbed on colloids. It accumulates in recent deposits, passes
through watercourses mechanically, and does not react chemically
with sea-water. This peculiarity of vanadium transport is
reflected in its distribution on the sea-bed in the form of
silt.
The fate of vanadium that is dissolved in water is more
complex. As very large amounts of dissolved vanadium have been
carried out into the oceans throughout all geological periods,
vanadium levels in sea-water of about 60 mg/litre might be
expected; in fact, levels do not exceed 0.003 mg/litre
(Goldschmidt, 1938; NAS, 1974), indicating that vanadium is
continuously removed from sea-water. Krauskopf (1963) concluded
that the vanadium content of sea-water is not dependent on
solubility and that natural reagents remove vanadium from the
water. There are two possible pathways, namely sorption and
biochemical processes. The migratory qualities of vanadium are
poor. The content of vanadium in the earth's crust is 0.015 g/kg
(Vinogradov, 1959), and a mean content in river water is
0.001 mg/litre. Thus, very little vanadium is transported via
water. The bulk of vanadium is precipitated on to the seabed
and becomes bound to silts (Petkevich et al. 1967; Strahov,
1968). Levels of vanadium dissolved in sea-water amount to
0.001 - 0.003 mg/litre. The vanadium comes from the 10%
dissolved in river water and is continuously precipitated from
the water by ferric hydroxides and organic matter (Krauskopf,
1963).
Biochemical reactions play an important role in the
extraction of vanadium from sea-water and conversion into a
sediment (Vinogradov, 1937; Holodov, 1973). This is confirmed
by the link between the concentrations of vanadium and organic
substances in sedimentary rocks and silt. However, in practice,
it is extremely difficult to determine the part of the vanadium
that has been assimilated by organisms and the part that has
been sorbed by the decomposing mass of organic matter or
introduced in dissolved form.
An important role in the biogenic migration of vanadium is
played by live marine organisms and plants. The ascidians and
holothurians are noteworthy vanadium accumulators (Bertrand,
1950) (section 3.1.6). Some marine algae are also capable of
accumulating vanadium (Krauskopf 1963). When they die, these
organisms promote accumulation of vanadium in the silt.
Thus, vanadium dissolved in sea-water is continuously
removed either by sorption or biochemical processes. In the
first case, the main precipitant is hydrated ferric trioxide; in
the second, vanadium is accumulated by marine animals, plankton,
and, less commonly, algal and plant organic material.
3.5.2.2 Occurrence in hydrocarbons
The accumulation of vanadium in organic concentrators is
linked with its occurrence in hydrocarbons (petroleum, asphalts,
peats, bitumens, and coal). Vanadium may enter petroleum
together with organic matter or accumulate in already-formed
petroleum from underground waters and petroliferous strata.
Apparently, both processes occur in nature. Secondary
transformation of petroleum into asphalt is accompanied by a
proportionate increase in the concentrations of the tar-asphalt
component and the vanadium linked to it (Vernadskij, 1940).
3.5.2.3 Biospheric redox processes
Vanadium takes part in redox processes not only of a
geochemical nature but also in plants and animals. Pejve &
Ajzupiet (1974), studying the intracellular distribution of
metals in plants, showed that a considerable number, including
vanadium, were linked to complex lipid substances of
comparatively high stability that persisted, even after the
cells had died. These lipids persisted in soils and silts and
served as a source of metal in sedimentary rocks. In various
types of plants, the proportional relationship between the level
of iron and those of manganese, copper, titanium, nickel,
chromium, cobalt, and vanadium decreased in the course of
evolution, and was least significant in the leaves of flowering
plants.
In a number of soil organisms, such as Microcococus lacto-
lyticus, Thiobacillus terrooxidans, and Ferrobacillus thio-
oxidans, there was a link between iron and vanadium in bio-
chemical processes (Zajic, 1969).
3.5.2.4 Transport in air
Information on the local movement and deposition of airborne
vanadium is presented in section 4.1.1. Strahov (1947) and
Ronov (1964) concluded that the gaseous envelope of the earth is
not of significant importance in the transport of vanadium, but
a study by Duce & Hoffman (1976) documented some transport of
man-made airborne vanadium over ocean areas. The authors
estimated that about 10% of this material was deposited in the
ocean. In a study of trace metals in European glaciers,
Jaworowski et al. (1973) showed the presence of small amounts of
vanadium, apparently deposited from the air, which had increased
in recent decades.
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 General Population Exposure
4.1.1 Air
Natural sources of vanadium, such as continental dust and
marine aerosols, cause only low natural background levels of
vanadium in air. In remote areas, such as the South Pole, the
concentrations range from 0.001 to 0.002 ng/m3 (Zoller et al.,
1974), and in the eastern Pacific Ocean, from 0.02 to 0.8 ng/m3
(Hoffman et al., 1969). In rural areas in Canada, the United
Kingdom, and the USA, concentrations have been reported to range
from 0.2 to about 75 ng/m3, with annual averages frequently
below 1 ng/m3 (Rahm, 1971; Cawse & Peirson, 1972; US EPA, 1977).
In general, air levels of vanadium are higher in urban areas
than rural areas. Annual averages may often be in the range of
20 - 100 ng/m3, though, exceptionally, higher averages exceeding
200 - 300 ng/m3 have been recorded in large cities, and the
maximum 24-h average may exceed 1000 ng/m3 (US EPA, 1977). In
all surveys, there have been conspicuous geographical and
seasonal variations. High concentrations of vanadium in air
have been attributed to the local burning of fuel oil with a
high vanadium content. The uptake of vanadium fall-out by
mosses (Hypnum capressiforme and Bryum argenteum) in the
Stockholm area indicates that heating oil is a major source of
vanadium (Rühling, 1971). In this regard, Faoro & McMullen
(1977) presented some interesting data (Fig. 2). The values
shown, especially for the period prior to 1971, are typical of
cold-climate cities where high-vanadium heavy fuel oils are
extensively used. The marked seasonal variations are due to
alterations in heating requirements and seasonal differences in
atmospheric inversions. The marked decline after 1970 is due to
the introduction of low-sulfur fuels; reduction of sulfur in oil
results in a proportional reduction in the vanadium content. A
similar effect can be produced by changing from heavy to
distilled fuel oil, as noted in Boston by Barry et al. (1975),
where levels of airborne vanadium for comparable months in 1966
and 1972 were 1.07 and 0.114 µg/m3, respectively.
These data illustrate the importance of fossil fuels as
sources of vanadium in urban air. The patterns observed in
other areas are similar but less extreme in concentration and
fluctuation. Because of the variations in vanadium
concentrations in oil and coal, community levels will depend
mainly on the actual vanadium concentrations in the fuels used
and on meteorological factors.
Pollution of the air by industrial facilities may be less
than that by power stations and heating equipment. At a steel
plant in the USA in 1967, concentrations of vanadium ranged from
0.04 to 0.107 µg/m3 and averaged 0.072 µg/m3, corresponding to
the mean concentration over 13 Pennsylvania towns (also
0.072 µg/m3) (NAS, 1974). The following levels of vanadium
pentoxide were found by Pazhynich (1967) in the USSR in areas of
extensive metallurgical activity not connected with vanadium
production: in the years 1964-65: 1.49 µg/m3 at 150 m from the
source of discharge; 0.47 µg/m3 at 500 m; 1.35 µg/m3 at
1000 m; and 0.98 µg/m3 at 1500 m. Near a plant producing
technical vanadium pentoxide, Kurmaev (1974) detected the
following 24-h mean levels of vanadium pentoxide: 0.004 -
0.012 mg/m3 at 500 m from the source; 0.001 - 0.006 mg/m3 at
1000 m; and 0.001 - 0.004 mg/m3 at 2000 m. Seventy to 72% of
the particles were less than 2 µg in diameter.
The liquid waste and wash water from metallurgical plants
often contain large amounts of vanadium, up to several hundred
milligrams per litre, measured as vanadium pentoxide. Kurmaev
(1974) detected vanadium at a level of 702.8 mg/litre in
effluent from a vanadium pentoxide plant. In a new
ferrovanadium plant, purified waste water contained 340 mg
vanadium pentoxide/litre (Kurmaev, 1974). Unpurified waste-
water discharge from a vanadium pentoxide plant into an open
watercourse produced a vanadium level in the watercourse of
2 mg/litre (Seljankina, 1961). Linstedt & Kruger (1969) found
the highest river concentrations of vanadium near uranium-
vanadium plants and the lowest in water samples taken upstream
from the industrial areas (Table 2) (section 3.1.3). In
studies by Shilina & Malakhov (1974), water samples taken from
the Moscow River below the city contained 6 times as much
vanadium as samples taken above the city (0.06 and
0.01 mg/litre, respectively).
The pickling of steel casts or steel articles can include
vanadium in the pickling mixture. Waste hydrochloric, nitric,
hydrofluoric, and sulfuric acids from a steel smelting plant
contained 0.02% vanadium. The solid residue on the bottom of
the acid vats contained 2.4 g vanadium/kg (hydrochloric acid
vat) and 1.6 g vanadium/kg (nitric and hydrofluoric acid vats)
(Kurmaev, 1974); vanadium in waste acid that is not properly
treated may be a source of water pollution.
3.4.2 Fossil fuel combustion
Industrial plants producing power and heat and operating on
petroleum, coal, and heavy oils are the most widespread source
of vanadium discharge into the environment. In 1969, as a
result of the combustion of fossil fuels (coal and oil), about
20 000 tonnes of vanadium were discharged into the air in the
USA (NAS, 1974). The estimated levels of emission from burning
coal in the USA for 1969 are shown in Table 10.
In a study of the vanadium discharged from coal burning in
six electric power generating stations, the total amount
discharged into the air in 1968 was 3760 tonnes. Where there
were no ash-trapping devices, 65% of the ash entered the
atmosphere. The degree of atmospheric dispersion of ash
particles depended on the original coalfield, the size of coal
used, the type of furnace, the combustion conditions, and the
presence and type of ash-trapping devices (NAS, 1974).
In a study of the metal aerosol content of waste gas
emissions from an oil-fired electric power station, it was
reported that the concentration of vanadium pentoxide (and
oxides of aluminium, chromium, iron, and manganese) was not
affected by the type of boiler or mode of operation. Comparison
of the metal content in the fuel oil and waste gases showed that
90% was released into the atmosphere (Sokolov, 1986). In the
USSR, there is a trend towards a reduction in the use of heavy
fuel oils in electric power stations.
Other possible sources of vanadium discharge into the air
are the burning of coal tips or dumps of coal dust in mining
areas, but data are not available.
All crude petroleum oils contain vanadium at levels ranging
from 1 to 400 g/tonne, depending on the oilfield (Holodov, 1968,
1973; Shah et al., 1970; Christian & Robinson, 1971; Nelson,
1973; NAS, 1974). In the distillation of crude oil, almost all
the vanadium remains in the high relative molecular mass
hydrocarbon fractions. The vanadium contents of heavy fuel oils
range widely from 1 to 200 g/tonne and are almost a thousand
times greater than those of petroleum distillates. Assuming
that 10% of the vanadium is precipitated inside the plant (flue
and ash trap) while 90% is discharged into the air, it has been
calculated that atmospheric emissions in the USA as a result of
heavy fuel oil combustion were about 14 100 - 21 800 tonnes in
1970 (Holodov, 1968, 1973; NAS, 1974). Similar percentages were
found by Sokolov (1986).
Table 10. Estimated emissions of vanadium resulting from coal
burning in the USA, 1969a
-------------------------------------------------------------------
Type and use Coal Vanadium Vanadium Control Vanadium
of coal (1000 in coal in fly of discharged
tonnes) (tonnes) ash fly into the
(tonnes) ash (%) air
(tonnes)
-------------------------------------------------------------------
Bituminous coal
Electric power 308 642 9254 6015 85 902
utilities
Manufacturing 93 248 2797 1818 60 727
Retail 12 665 380 247 50 124
deliveries
Coking 92 901 2787 - 100 0
Subtotal 507 276 15 218 8080 1753
Anthracite coal 9275 1159 753 50 377
Total 2130
-------------------------------------------------------------------
a From: NAS (1974).
Distilled petroleum fuels produced in the USA (gasoline,
kerosene, diesel fuel, home-heating oils) contain 0.05 mg
vanadium/kg (NAS, 1974). The distillation process used leaves
nearly all of the vanadium originally present in the residual
fractions. Analyses of the spent gases from petrol engines,
sampled directly at the exhaust outlet, showed vanadium
concentrations of 0.1 - 0.2 mg/kg, and exhaust gases of diesel
engines contained 10 - 15 mg vanadium/kg. Six to 12 mg/kg of
vanadium was found in soot collected from the edges of flues in
a small oil-fired power station (Pilz & Komischke, 1972).
The amount of vanadium in natural gas is less than
0.5 g/tonne, and almost no vanadium is released into the
atmosphere on combustion (NAS, 1974).
3.4.3 Agriculture
Vanadium has been used as a trace fertilizer applied at the
rate of 0.75 - 1 mg/kg soil (Peterburgskij & Tormasova, 1969).
This practice must lead to an increased level of vanadium in the
soil, but further information on agricultural use is not
available.
3.5 Transport and Transformation
3.5.1 Geochemical processes
Vanadium is involved in various geochemical processes
occurring in the earth's crust. There is extremely wide
dispersion of vanadium during the formation of volcanic rocks
and sporadic accumulation with the formation of vanadium
minerals as a result of postmagmatic processes. Like all trace
elements that accumulate in soils, vanadium migrates within the
soil itself and within the system: rock-water-soil-vegetation-
animals-man.
During geochemical processes in the soil and in weathering
and podzolization, vanadium is shifted from the A horizon to the
B horizon (Kovda et al., 1959). However, vanadium shifting does
not occur during weathering processes that do not involve
movement of sesquioxides.
Vanadium concentrations in rocks are linked to the pH of the
rocks (Borisenko, 1973). Neutral and acid rocks contain lower
vanadium concentrations than basic rocks, and acid rocks contain
lower concentrations than neutral rocks. In magma of various
types (the main carrier of vanadium), about 92% of all vanadium
occurs in basic rocks (basalts, gabbro, amphibolites, and
eclogites), and about 8% occurs in acid and neutral rocks. Less
than 1% of the total amount of vanadium is found in ultrabasic
alkaline rocks.
The main carriers of vanadium in the sedimentation process
are ferric hydroxides and solid bitumens. The great affinity
between the crystallochemical properties of V3+ and Fe3+ is of
vital importance for the diffusion of vanadium. There is
roughly 400 - 500 times more iron than vanadium in the earth's
crust. Thus, iron is a "solvent" of trivalent vanadium, and is
responsible for its diffusion in magmatic rocks. The bulk of
the ferromagnesian rock-forming minerals (and also titaniferrous
magnetite and magnetite itself) trap vanadium during the
crystallization of rocks, and, during endogenous processes,
vanadium is very closely linked with trivalent iron. In the
formation of igneous rocks, vanadium is preferentially
concentrated in those with a high iron content.
3.5.2 Biogeochemical processes
The accumulation of vanadium in soils and in all other
materials depends directly on its concentration in the soil-
forming rocks, the atmosphere, and the oceans of the world.
Migration, diffusion, and concentration of vanadium in the
biosphere takes place as a result of its extraction by living
organisms from water, from food of both vegetable and animal
origin, and from different types of rock during their
decomposition and the formation of soils.
3.5.2.1 Transport in, and removal from, water
In assessing the relative importance of the two ways in
which vanadium is transported in water, Konovalov et al. (1968)
and Holodov (1968) concluded that 87% is carried away by the
rivers in suspended form, and 13% in solution. The average
level of dissolved vanadium in the rivers of Japan and the USA
(Sugawara et al., 1956; Durum & Haffty, 1963) were the same as
that recorded by Konovalov et al. (1968), i.e., 0.001 mg/litre.
The bulk of vanadium enters sea-water in suspended form or
sorbed on colloids. It accumulates in recent deposits, passes
through watercourses mechanically, and does not react chemically
with sea-water. This peculiarity of vanadium transport is
reflected in its distribution on the sea-bed in the form of
silt.
The fate of vanadium that is dissolved in water is more
complex. As very large amounts of dissolved vanadium have been
carried out into the oceans throughout all geological periods,
vanadium levels in sea-water of about 60 mg/litre might be
expected; in fact, levels do not exceed 0.003 mg/litre
(Goldschmidt, 1938; NAS, 1974), indicating that vanadium is
continuously removed from sea-water. Krauskopf (1963) concluded
that the vanadium content of sea-water is not dependent on
solubility and that natural reagents remove vanadium from the
water. There are two possible pathways, namely sorption and
biochemical processes. The migratory qualities of vanadium are
poor. The content of vanadium in the earth's crust is 0.015 g/kg
(Vinogradov, 1959), and a mean content in river water is
0.001 mg/litre. Thus, very little vanadium is transported via
water. The bulk of vanadium is precipitated on to the seabed
and becomes bound to silts (Petkevich et al. 1967; Strahov,
1968). Levels of vanadium dissolved in sea-water amount to
0.001 - 0.003 mg/litre. The vanadium comes from the 10%
dissolved in river water and is continuously precipitated from
the water by ferric hydroxides and organic matter (Krauskopf,
1963).
Biochemical reactions play an important role in the
extraction of vanadium from sea-water and conversion into a
sediment (Vinogradov, 1937; Holodov, 1973). This is confirmed
by the link between the concentrations of vanadium and organic
substances in sedimentary rocks and silt. However, in practice,
it is extremely difficult to determine the part of the vanadium
that has been assimilated by organisms and the part that has
been sorbed by the decomposing mass of organic matter or
introduced in dissolved form.
An important role in the biogenic migration of vanadium is
played by live marine organisms and plants. The ascidians and
holothurians are noteworthy vanadium accumulators (Bertrand,
1950) (section 3.1.6). Some marine algae are also capable of
accumulating vanadium (Krauskopf 1963). When they die, these
organisms promote accumulation of vanadium in the silt.
Thus, vanadium dissolved in sea-water is continuously
removed either by sorption or biochemical processes. In the
first case, the main precipitant is hydrated ferric trioxide; in
the second, vanadium is accumulated by marine animals, plankton,
and, less commonly, algal and plant organic material.
3.5.2.2 Occurrence in hydrocarbons
The accumulation of vanadium in organic concentrators is
linked with its occurrence in hydrocarbons (petroleum, asphalts,
peats, bitumens, and coal). Vanadium may enter petroleum
together with organic matter or accumulate in already-formed
petroleum from underground waters and petroliferous strata.
Apparently, both processes occur in nature. Secondary
transformation of petroleum into asphalt is accompanied by a
proportionate increase in the concentrations of the tar-asphalt
component and the vanadium linked to it (Vernadskij, 1940).
3.5.2.3 Biospheric redox processes
Vanadium takes part in redox processes not only of a
geochemical nature but also in plants and animals. Pejve &
Ajzupiet (1974), studying the intracellular distribution of
metals in plants, showed that a considerable number, including
vanadium, were linked to complex lipid substances of
comparatively high stability that persisted, even after the
cells had died. These lipids persisted in soils and silts and
served as a source of metal in sedimentary rocks. In various
types of plants, the proportional relationship between the level
of iron and those of manganese, copper, titanium, nickel,
chromium, cobalt, and vanadium decreased in the course of
evolution, and was least significant in the leaves of flowering
plants.
In a number of soil organisms, such as Microcococus lacto-
lyticus, Thiobacillus terrooxidans, and Ferrobacillus thio-
oxidans, there was a link between iron and vanadium in bio-
chemical processes (Zajic, 1969).
3.5.2.4 Transport in air
Information on the local movement and deposition of airborne
vanadium is presented in section 4.1.1. Strahov (1947) and
Ronov (1964) concluded that the gaseous envelope of the earth is
not of significant importance in the transport of vanadium, but
a study by Duce & Hoffman (1976) documented some transport of
man-made airborne vanadium over ocean areas. The authors
estimated that about 10% of this material was deposited in the
ocean. In a study of trace metals in European glaciers,
Jaworowski et al. (1973) showed the presence of small amounts of
vanadium, apparently deposited from the air, which had increased
in recent decades.
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 General Population Exposure
4.1.1 Air
Natural sources of vanadium, such as continental dust and
marine aerosols, cause only low natural background levels of
vanadium in air. In remote areas, such as the South Pole, the
concentrations range from 0.001 to 0.002 ng/m3 (Zoller et al.,
1974), and in the eastern Pacific Ocean, from 0.02 to 0.8 ng/m3
(Hoffman et al., 1969). In rural areas in Canada, the United
Kingdom, and the USA, concentrations have been reported to range
from 0.2 to about 75 ng/m3, with annual averages frequently
below 1 ng/m3 (Rahm, 1971; Cawse & Peirson, 1972; US EPA, 1977).
In general, air levels of vanadium are higher in urban areas
than rural areas. Annual averages may often be in the range of
20 - 100 ng/m3, though, exceptionally, higher averages exceeding
200 - 300 ng/m3 have been recorded in large cities, and the
maximum 24-h average may exceed 1000 ng/m3 (US EPA, 1977). In
all surveys, there have been conspicuous geographical and
seasonal variations. High concentrations of vanadium in air
have been attributed to the local burning of fuel oil with a
high vanadium content. The uptake of vanadium fall-out by
mosses (Hypnum capressiforme and Bryum argenteum) in the
Stockholm area indicates that heating oil is a major source of
vanadium (Rühling, 1971). In this regard, Faoro & McMullen
(1977) presented some interesting data (Fig. 2). The values
shown, especially for the period prior to 1971, are typical of
cold-climate cities where high-vanadium heavy fuel oils are
extensively used. The marked seasonal variations are due to
alterations in heating requirements and seasonal differences in
atmospheric inversions. The marked decline after 1970 is due to
the introduction of low-sulfur fuels; reduction of sulfur in oil
results in a proportional reduction in the vanadium content. A
similar effect can be produced by changing from heavy to
distilled fuel oil, as noted in Boston by Barry et al. (1975),
where levels of airborne vanadium for comparable months in 1966
and 1972 were 1.07 and 0.114 µg/m3, respectively.
These data illustrate the importance of fossil fuels as
sources of vanadium in urban air. The patterns observed in
other areas are similar but less extreme in concentration and
fluctuation. Because of the variations in vanadium
concentrations in oil and coal, community levels will depend
mainly on the actual vanadium concentrations in the fuels used
and on meteorological factors.
Pollution of the air by industrial facilities may be less
than that by power stations and heating equipment. At a steel
plant in the USA in 1967, concentrations of vanadium ranged from
0.04 to 0.107 µg/m3 and averaged 0.072 µg/m3, corresponding to
the mean concentration over 13 Pennsylvania towns (also
0.072 µg/m3) (NAS, 1974). The following levels of vanadium
pentoxide were found by Pazhynich (1967) in the USSR in areas of
extensive metallurgical activity not connected with vanadium
production: in the years 1964-65: 1.49 µg/m3 at 150 m from the
source of discharge; 0.47 µg/m3 at 500 m; 1.35 µg/m3 at
1000 m; and 0.98 µg/m3 at 1500 m. Near a plant producing
technical vanadium pentoxide, Kurmaev (1974) detected the
following 24-h mean levels of vanadium pentoxide: 0.004 -
0.012 mg/m3 at 500 m from the source; 0.001 - 0.006 mg/m3 at
1000 m; and 0.001 - 0.004 mg/m3 at 2000 m. Seventy to 72% of
the particles were less than 2 µg in diameter.
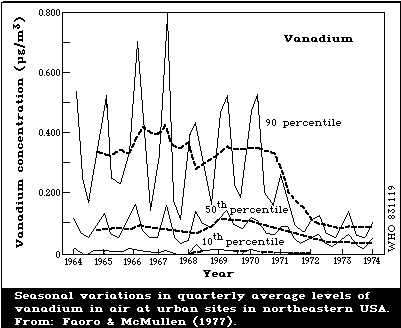 Boyarkina et al. (1978) studied vanadium precipitation in
and around an industrial city, in winter, by measuring
concentrations in snow. In the central area, a concentration of
24 µg/litre was found, decreasing to 3.2 µg/litre at a
distance of 40 - 50 km.
4.1.2 Water
Natural background levels of vanadium in water have been
discussed in section 3.1.3, and levels in industrial effluent,
in section 3.4.1. Durfor & Becker (1963) included vanadium in
their analyses for trace elements in drinking-water supplies in
large cities in the USA. Of the samples analysed, 91% showed
less than 10 µg vanadium/litre; the maximum concentration was
70 µg/litre, and the average was about 4.3 µg/litre. Many of
the samples were negative. Twenty-six percent of 3676 tap water
samples from 34 areas in the USA contained vanadium at
concentrations ranging from 1.3 to 33 µg/litre with a mean of
4.85 µg/litre (Greathouse & Craun, 1979).
Hoffmann et al. (1972) published data from a regional well-
water survey in Poland. The average vanadium concentrations
were 0.06 - 6 µg/litre, with a maximum single value of
15 µg/litre. Bottled waters from mineral springs frequently
contained higher levels; Schlettwein-Gzell & Mommsen-Straub
(1973) reported a range of 4 - 290 µg/litre in bottled waters
from Switzerland.
Highly-mineralized waters in Argentina contained 0.3 -
10 µg vanadium/litre (Trelles et al., 1970). These concentra-
tions often occur in conjunction with high concentrations of
arsenic and/or fluorides.
4.1.3 Food
4.1.3.1 Individual foods
Information on the vanadium contents of human food is
sparse. Data from two studies (Myron et al., 1977; Byrne &
Kosta, 1978) are combined in Table 11 with those from an earlier
study using a similar method (Söremark, 1967). The results of
these studies agree reasonably well, but differ in some respects
from the results of earlier work, especially that of Schroeder
et al. (1963). The principal difference is that Schroeder noted
high vanadium concentrations in fats and oils, whereas low
concentrations were found in the more recent studies. Such a
difference may be accounted for by the use of different
analytical methods. Similar discrepancies have been encountered
in the study of other trace elements, i.e., lower concentrations
have been found using more recently developed methods.
The data presented in Table 11 show low levels of vanadium
in most elements of the human diet. There are also some
interesting differences among specific foods. Grains contain
higher levels of vanadium than fruits and vegetables. Levels in
oils and fats and beef and pork are low, but those in the liver
and kidneys of cows and pigs are higher. Higher levels are
found in both the flesh and internal organs of the chicken, and
levels in fish flesh are also high. Vanadium levels in milk and
eggs are low, and those in beer and wine are high. Myron et al.
(1977) pointed out that processing appears to raise levels in
food (e.g., white versus brown rice; cereal, flour, bread, and
gluten versus grain; peanut butter; bologna and bacon versus
pork). No explanation was given for the very high levels in
dill and parsley.
Table 11. Vanadium concentrations in foods (µg/kg)a
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Grains
Wheat 3.6
Flour 15, 40
Bread 11.20 10, 13
Gluten 33
Oats 3
Oatmeal 6
Corn 0.7
Cornmeal 2
Brown rice 1
White rice 21 12, 30
Barley 14 1.6
Cereal 93
Fruits
Apple 1.1 4 0.3
Pear 0 0.2
Banana 3 0.2
Orange 1
Cherry 0.4
Apricot 0.2
Peach 0.2
Strawberry 31.41 (dry)
Blueberry 1.6
Vegetables
Potato 0.8 1 1.2, 1.9
Radish 52 5 0.6
Carrot 0 1 2.3, 2.4
Beet 0
Garlic 0.6
Onion 0.6
Leek 0.3
Navy bean 14
Pea 0 7 0.4
Tomato 0.03 2 0.3
Cucumber 2.1
Squash 4
Brussels sprout 0.5
Cauliflower 0.08 1 0.9
Cabbage 2 0.3
Lettuce 21 4 1.0, 2.7
Table 11 (contd).
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Spinach 35
Parsley 790 1800 (dry)
Mushroom 50 - 2 000 (dry)
Dill 140 431
Meats
Beef 0 1 0.4 - 1.3
Beef liver 2.4, 10 6 7.3
Pork 0 1 0.6, 0.9
Pork liver 8.4
Pork kidney 8.5
Bacon 5
Bolgna 8
Chicken, white 22 1.7
Chicken, dark 12
Chicken liver 37, 38
Chicken kidney 18
Cod 28 7.2
Mackerel 2.6 3.5
Tuna 11 10, 3
Lobster 43 5
Scallop 22
Oils and fats
Margarine 4
Soybean oil 1
Corn oil 1 3
Pumpkin seed oil 0.2
Lard 2 0.2
Nuts
Hazel nut 3.7
Peanut butter 44
Dairy products
Milk 0 - 0.1 3 0.2, 0.2
Powdered milk 0 - 0.2 25
Chocolate milk 21
Butter 1
Egg white 0.3 - 1.8
Egg yolk 2.0 - 3.6
Beverages
Coffee 1.6
Tea 1.3 0.3
Cola 0.7 1.5
Beer 11 8.4
Wine 3.5 - 3.2
---------------------------------------------------------
a Study 1: Söremark (1967) (neutron activation analysis).
Study 2: Myron et al. (1977) (atomic absorption spectroscopy).
Study 3: Byrne & Kosta (1978) (neutron activation analysis).
4.1.3.2 Complete diets
Byrne & Kosta (1978) estimated the daily intake of vanadium
to be "a few tens of micrograms," but added that it may vary
considerably. Assuming a very low rate of intestinal absorption
of vanadium in man (section 5.1.2), Byrne & Kosta calculated
intakes for 3 adults in whom they measured dietary
concentrations. The calculated intakes were 36, 66, and
11 µg/day, respectively.
Analyses of 9 selected hospital diets (Myron et al., 1978)
are given in Table 12. Each meal was prepared and analysed
separately by atomic absorption spectroscopy. The results were
similar to concentrations found in individual foodstuffs in
other studies given above. In a later study, Byrne & Kosta
(1979) reported on the determination of vanadium in total diet
samples obtained during a nutrition survey in 5 Italian towns
(Table 13). The concentrations agree with those reported by
Myron et al. (1978).
Table 12. Daily vanadium intake in dieta
------------------------------------------------------
Diet type (µg/day) (µg/g) (µg/1000
cal)
------------------------------------------------------
General-1 13.6 0.019 4.7
Cholesterol reducing-1 25.6 0.034 8.8
Cholesterol reducing-2 16.8 0.022 5.8
Cholesterol raising 30.1 0.046 10.5
General-2 28.0 0.040 9.8
Low calorie 12.4 0.029 10.6
Low salt 15.5 0.028 9.1
Puree 26.0 0.050 14.1
Soft 15.8 0.024 6.4
------------------------------------------------------
a Adapted from: Myron et al. (1978).
4.2 Occupational Exposure
In terms of occupational exposure, the most important
vanadium compounds are vanadium pentoxide, vanadium trioxide,
ferrovanadium, vanadium carbide, and vanadium salts, such as
sodium and ammonium vanadate. The oxides and salts are commonly
used in industry in powder form, giving rise to the possibility
of dust and aerosol formation, when the substances are crushed
or ground. Many metallurgical processes involve the production
of vapour containing vanadium pentoxide, which condenses to form
respirable aerosols. Boiler-cleaning operations generate dusts
containing the pentoxide and trioxide compounds. Combustion of
residual fuels with a high vanadium content is likely to produce
aerosols of the pentoxide as well as oxide complexes of vanadium
with other metals.
Table 13. Vanadium contents of Italian freeze-dried
total diet samplesa
--------------------------------------------------------
Town Dry Vanadium Daily
weight concentrationb vanadium
(g) (ng/g) intake (µg)
--------------------------------------------------------
Aosta 310 32.1 0.9 (2) 10.0
L'Aquila 173 46.0 3.4 (2) 8.0
Montfalcone 278 39.7 4.1 (2) 11.0
Mt. Amiata 377 29.7 0.8 (2) 11.2
Rome 285 42.3 4.6 (4) 12.0
Mean values 38.0 6.9 10.4 1.5
--------------------------------------------------------
a From: Byrne & Kosta (1979).
b Average and standard deviation; number of aliquots
in parentheses.
4.2.1 Metallurgy
The processing of metals containing vanadium includes
chemical treatment and high-temperature operations. However,
only moderate concentrations of vanadium were found in the
breathing zone of workers engaged in operations that would be
expected to produce the greatest fume exposure. During the
addition of vanadium to furnaces, concentrations ranged from
0.006 to 0.08 mg/m3 and, during tapping, from 0.004 to
0.02 mg/m3. Concentrations found in oxyacetylene cutting ranged
from 0.008 to 0.015 mg/m3 and, in arc-welding, from 0.002 to
0.006 mg/m3 (NAS, 1974).
Vanadium levels in metallurgical plants have been studied in
detail (Roshchin, 1968). Vanadium slag contains about 11 - 13%,
mainly in the form of trioxides of vanadium (measured as
vanadium pentoxide). Slags are used for the production of
vanadium pentoxide and ferrovanadium, and the process is
accompanied by extensive formation of iron oxide aerosol. Air
concentrations of the dust (mainly vanadium trioxide) found in
the main working positions (converter operator, mixer, and crane
driver) ranged from 20 to 55 mg/m3. Measured as vanadium
pentoxide, the contents of the swirling dust did not exceed
0.17 mg/m3. About 75% of the dust particles had a diameter of
less than 2 µm and 20% had a diameter of between 2 µm and
4 µm.
Breaking, loading and unloading, crushing and grinding, and
magnetic separation of vanadium slag causes thick dust
formation, with concentrations ranging from 30 to 120 mg/m3.
The slag contains 111 - 129 g vanadium pentoxide/kg. A diameter
of less than 2 µm was recorded for 70 - 72% of the particles;
86 - 96% had a diameter of less than 5 µm. When the slag is
roasted, free vanadium pentoxide is discharged into the work-
place air; atmospheric concentrations in the vicinity of
furnaces ranged from 0.04 to 1.56 mg/m3. During leaching and
precipitation, concentrations of vanadium in the air may be
high, sometimes exceeding 0.5 mg/m3.
The smelting and granulation of technical vanadium pentoxide
are accompanied by the formation of an aerosol. This aerosol
escapes when the product is poured for granulation. During the
loading of smelting furnaces, concentrations of vanadium
pentoxide ranged from 0.15 to 0.80 mg/m3. During smelting and
granulation, concentrations ranged from 0.7 to 11.7 mg/m3. In
other parts of the work-place, concentrations may range from
0.03 to 0.2 mg/m3.
In aluminium production, when bauxite is being converted
into alumina, the aluminate solutions accumulate vanadium salts,
which crystallize and precipitate out. Precipitated sodium
polyvanadate is smelted to form vanadium pentoxide, which is
cooled and settles in the form of thin plates. Vanadium
pentoxide dust (concentrations of up to 2.3 mg/m3) is given off
only in the terminal phase during tapping of the liquid product,
packing, and loading.
During the drying, sieving, and calcination of ammonium
vanadate and during the crushing, unloading, and packaging of
pure vanadium pentoxide, dusts are formed. When vanadium
pentoxide is sieved after calcination, the concentration in air
may range from 2.2 to 26 mg/m3. In plants with less
mechanization, incomplete sealing of equipment, and inefficient
local exhaust ventilation, concentrations of dust during these
operations ranged from 4.9 to 48.9 mg/m3.
In the production of ferrovanadium, there is a continuous
source of discharge of vanadium pentoxide and lower oxides
during the smelting process. Data on vanadium in air at various
sites are shown in Table 14.
Using spectrophotometric techniques, Roshchin (1968),
Katayeva & Sapunov (1974) and Kazimov (1977) found high
concentrations of vanadium during smelting and granulation
(range, 0.16 - 1.89 mg/m3; mean, 0.59 mg/m3; 104 samples),
production of ferrovanadium (range, 0.58 - 4.81 mg/m3; mean,
1.7 mg/m3; 110 samples), and roasting of the charge (range,
0.44 - 3.64 mg/m3; mean, 1.52 mg/m3; 112 samples).
Table 14. Vanadium levels in the air of a ferrovanadium planta
--------------------------------------------------------------------
Work-place/operations Vanadium pentoxide Lower oxides of
(mg/m3) vanadium (mg/m3)
--------------------------------------------------------------------
Work area of smelters and 0.1 - 2.6 0.05 - 1.2
helpers
Unloading of vanadium pent- 2 - 124.6
oxide from the bin and
charging of electric furnace
Crane driver's cabin during 0.07 - 9.43 0.03 - 0.1
smelting
Cutting up ferrovanadium 0.97 - 12.6
Maintenance of the furnace 7.5 - 30
--------------------------------------------------------------------
a From: Roshchin (1968).
Using high-volume sampling and atomic absorption analysis,
Usutani et al. (1979) measured vanadium pentoxide
concentrations in the air at several places in a vanadium
refinery. The highest concentrations (higher than 1 mg/m3) were
detected in samples collected during the removal of the vanadium
pentoxide flake. High-volume samples from other locations as
well as low-volume samples obtained over 6.5-h work shifts
showed lower concentrations (0.002 - 0.735 mg/m3).
When ductile vanadium is produced by the aluminothermic
process (based on the reduction of pure vanadium pentoxide with
aluminium powder), the violent exothermic reaction leads to the
release of a condensation aerosol of vanadium pentoxide. During
preparation of the charge mixture, work-place concentrations of
vanadium pentoxide ranged from 19 to 25.1 mg/m3. When the
burden was placed in crucibles inside the smelting chambers,
concentrations ranged from 64 to 240 mg/m3. During smelting,
concentrations at the operators' workplaces ranged from 0.17 to
0.6 mg/m3. Twenty to 30 min after smelting, levels declined to
0 -0.3 mg/m3. Ninety-eight percent of the condensation aerosol
particles produced had a diameter of less than 5 µm, and 82%
had a diameter of less than 2 µm (Roshchin, 1968).
In the production of vanadium by the vacuum carbon thermic
method, most of the pollution occurs during operations with
vanadium trioxide (Roshchin, 1968). Mixing of vanadium trioxide
in a closed mixer led to air concentrations in the workplace of
from 0.019 to 0.58 mg/m3. Unloading of the charge resulted in
high concentrations of 14.7 - 29.4 mg/m3. In the packing
department, when the charge was sifted in a fume cupboard,
concentrations in the breathing zone ranged from 0.58 to
4.7 mg/m3. When the charge was being weighed out and packed in
a fume cupboard, concentrations ranged from 3.38 to 6.76 mg/m3.
Levels of vanadium-containing dust and vanadium pentoxide in
the air during catalyst production are shown in Table 15.
Table 15. Air contamination in vanadium catalyst productiona
-----------------------------------------------------------------------------
Operation Dust (mg/m3) Vanadium pentoxide (mg/m3)
Minimum Maximum Most Minimum Maximum Most
frequent frequent
-----------------------------------------------------------------------------
Grinding and un- 5 45 7 - 9 1 7 1.5 - 3
loading vanadium
pentoxide
Loading ground 12 53 14 - 17 3.2 7.5 4 - 4.2
pentoxide into
the bin
Sifting and pack- 5 17.5 5 - 7 0.1 1 0.4 - 0.5
ing granules of
bulk contact
substance
-----------------------------------------------------------------------------
a From: Roshchin (1968).
4.2.2 Cleaning of oil-fired boilers
Significant occupational exposure to vanadium occurs during
the cleaning of boilers in oil-fired heating and power plants
and ships (Symanski, 1939; Roshchin, 1968; Kuzelova et al.,
1977; Levy et al., 1984). Fuel oil combustion results in the
formation of vanadium-containing dust, and large amounts of dust
result from operations connected with removing ash encrustations
in boiler cleaning and in cleaning the blades of gas turbines.
Most of these operations are carried out by hand, and the dust
in the air inside the boilers may range from 20 to 400 mg/m3,
the most common range being 50 - 100 mg/m3, with the dust
containing 5 - 17% vanadium pentoxide and from 3 to 10% of the
lower vanadium oxides (Roshchin, 1968). Kuzelova et al. (1977)
reported dust concentrations of 136 - 36 036 ml/m3 in the air
with vanadium concentrations ranging between 1.7 and 18.4
mg/m3.
Williams (1952) published air sampling data on boiler-
cleaning operations in the British power industry. He found
concentrations of soot dust at different points ranging from 239
to 659 mg/m3. The vanadium concentrations in the dust of the
superheater chamber was 40.2 mg/m3, while, in the combustion
chamber, the concentration was 58.6 mg/m3. Most (93.6%) of the
dust particles had a diameter of between 0.15 and 1 µm.
4.2.3 Occupational exposure limits
Some national occupational exposure limits for vanadium in
work-place air are shown in Table 16.
Table 16. Examples of occupational exposure limits for vanadium in various countries
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Australia Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Belgium Recommendation Time-weighted average (TWA) (fume) 0.05 ILO (1980)
Czechoslovakia Regulatory requirement Maximum allowable concentration (MAC) Hygienic Regula-
- Time-weighted average (TWA) 0.1 tions of the
- Ceiling value (fume) 0.3 Ministry of Health
- Ceiling value (dust) 1.5 of CSR (1985) (58)
(Regulation No. 7;
section 24/ZB)
Finland Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA) (fume)a 0.05
German Democratic Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1980)
Republic Short-term exposure limit (STEL)a 0.1
(fume)
Germany, Federal Recommendation 8-h time-weighted average (TWA)a 0.5 Federal Republic
Republic of of Germany Comm-
Short-term exposure limit (STEL)a 2.5 ision for Maximum
(30 min, 2x/shift) Work-Place Con-
centrations
8-h time-weighted average (TWA)a 0.1 (1985) (xxi, 16)
(fume)
Recommendation Short-term exposure limit (STEL)a 0.5
(30 min, 2x/shift) (fume)
Italy Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1985)
Hungary Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1984)
Netherlands Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Romania Regulatory requirement Ceiling value (fume)a 0.1 ILO (1980)
Sweden Regulatory requirement Ceiling valuea 0 ILO (1980)
Switzerland Regulatory requirement Time-weighted average (TWA)a 0.09 Permitted Values
in the Work-Place,
Berne (1984)
USA Recommendation Time-weighted average (TWA)a 0.05 ACGIH (1986)
(dust and fume)
Time-weighted average (TWA)a 0.5 OSHA (1977) (29
(dust) CFR.1910.1000)
Ceiling value (15 m)a 0.05 NIOSH (1977)
(dust and fume)
Time-weighted average (TWA)a 0.1 OSHA (1977) (29
(fume) CFR.1910.1000)
USSR Regulatory requirement Time-weighted average (TWA)a 0.1 ILO (1980)
Yugoslavia Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA)a 0.1
---------------------------------------------------------------------------------------------------------
a Measured as V2O5.
5. KINETICS AND METABOLISM
5.1 Physiological Role
5.1.1 Microorganisms
Vanadium is essential for the mould Aspergillus niger
(Bertrand, 1942) and the green alga Scenedesmus obliguus (Arnon
& Wessel, 1953; Arnon, 1958). It may play a role in photo-
synthesis in the latter. A need for vanadium has been shown by
the yeast Candida slooffii at high temperatures (Roitman et al.,
1969). The growth effects of vanadium in Azotobacter and other
bacteria have been related to the ability of vanadium, in lieu
of molybdenum, to catalyse nitrogen fixation reactions (Horner
et al., 1942; Takahashi & Nason, 1957).
5.1.2 Animals
Vanadium deficiency has been reported in chicks (Hopkins &
Mohr 1971a; Nielsen & Ollerich, 1973) and rats (Schwarz & Milne,
1971; Strasia, 1971), and vanadium is considered essential for
these animals (Underwood, 1977; Vouk, 1979; Nechay, 1984).
Hopkins & Mohr (1974) observed reduced feather growth in
chickens. They also reported impaired reproduction, due to
decreased fertility, and increased perinatal mortality in rats
fed a low vanadium diet (10 µg/kg) over 4 generations. A
positive growth response in rats was observed by Schwarz & Milne
(1971) when vanadium salts, at levels of between 50 and
500 µg/kg were added to a semi-purified diet (vanadium content
unspecified) fed for 26 - 28 days.
In male Leghorn chicks fed vanadium in the diet at levels of
12.5 mg/kg and 25 mg/kg, body-weight gain was normal at 1, 2, 3,
and 4 weeks of age (Kubena et al., 1986).
Slower growth, higher haematocrits, and higher levels of
plasma- and bone-iron were reported by Strasia (1971) in rats
fed diets containing less than 100 µg vanadium/kg compared with
controls receiving diets containing 500 µg vanadium/kg, but
these results were not confirmed in another study by Williams
(1973). Hopkins & Mohr (1971a,b, 1974) reported that a diet
containing less than 10 µg vanadium/kg decreased plasma-
cholesterol levels in chicks at 28 days of age, increased
plasma-cholesterol levels at 49 days of age, and increased
plasma-triglyceride levels at 28 days of age. Nielsen &
Ollerich (1973) found that administration of a diet containing
30 - 35 µg vanadium/kg to chicks decreased growth, increased
haematocrits and plasma-cholesterol levels, and impaired bone
development.
Nielsen (1980) carried out further studies on chicks and
rats, given different types of low-vanadium experimental diets,
to examine the effects of vanadium deficiency. In rats,
vanadium deficiency adversely affected prenatal survival,
growth, physical appearance, haematocrit, plasma-cholesterol
levels, and hepatic lipid and phospholipid levels. In chicks,
low vanadium intake produced adverse effects on growth,
feathering, haematocrit, bone development, plasma-cholesterol
levels, and hepatic lipid, phospholipid, and cholesterol levels.
However, consistent deficiency effects were not observed in
chicks or rats in any of the studies. Nielsen (1980) suggested
that the inconsistency in vanadium deficiency effects might be
due to the fact that different experimental diets had different
effects on the metabolism of vanadium.
Vanadate appears to have an insulin-like action (Heyliger et
al., 1985). In female Wistar rats made diabetic with
streptozotocin (single iv injection of 55 mg/kg) and given
sodium orthovanadate in the drinking-water at a concentration of
0.6 - 0.8 mg/ml (corresponding to calculated daily intakes of
between 75 ± 3 and 100 ± 3 mg/kg body weight per day,
respectively) for 4 weeks, the serum-insulin level was low, but
there was no increase in blood-glucose levels compared with
controls. In diabetic rats not treated with vanadate, serum-
insulin levels were also low, but blood-glucose levels were
increased 3-fold. The cardiac performance of vanadate-treated
animals did not differ significantly from that of non-diabetic
controls. It was concluded that vanadate controlled blood-
glucose levels and prevented the decline in cardiac performance
due to diabetes.
The results of in vitro studies suggest that vanadium may
play a specific physiological role as a regulator of the sodium
pump (Macara, 1980). Vanadate has been shown to inhibit Na+K+-
ATPase (EC 3.6.1.3) in intact human red blood cells (Rifkin,
1965; Cantley et al., 1977, 1978a,b). It also has potent
diuretic and natriuretic effects in rats (Balfour et al., 1978;
Westenfelder et al. 1981). It was a powerful inhibitor of
Na+K+-ATPase in microsomal fractions of the kidney, brain, and
heart in several species, including human beings (kidney).
Mg2+-ATPase was up to 10 000 times more resistant to vanadium
inhibition than Na+K+-ATPase (Nechay & Saunders, 1978).
Similarly, in vivo studies on laying chickens fed calcium
orthovanadate for 15 months, at levels of 0.25, 50, or 100 mg/kg
diet, showed clear inhibition of Na+K+-ATPase activity in the
kidney (Phillips et al., 1982). The inhibition of Na+K+-ATPase
by vanadate can be reversed by catecholamines, though these do
not have any effects on the Na+K+-ATPase activity in the absence
of vanadate (Hudgins & Bond, 1979). The inhibition is also
partially prevented by the reducing agents ascorbic acid and
glutathione (Grantham & Glynn, 1979).
The mechanism by which cells reduce cytoplasmic vanadium5+
to vanadium4+ was investigated using human red cells (Macara et
al., 1980). The authors concluded that vanadate is reduced by
cytoplasmic glutathione and that this explains the resistance of
the Na++K+-ATPase to vanadium in intact cells.
Since vanadate (V5+) is a potent inhibitor of Na+K+-ATPase,
many physiological and biochemical processes are vanadium
sensitive (Grantham, 1980; Nechay, 1984). For example, vanadium
compounds inhibit ATP phosphohydrolases, ribonuclease, adenylate
kinase, phosphofructokinase, squalene synthetase, glycer-
aldehyde-3-phosphate dehydrogenase (Macara, 1980), glucose-6-
phosphatase (Singh et al., 1981), and phosphotyrosyl-protein-
phosphatase (Swarup et al., 1982). The membrane neutral (K+)-
p-nitrophenylphosphatase in the membrane fraction of skeletal
muscle was inhibited equally by vanadium4+ and vanadium5+ at 4 x
10-8 mol/litre (Vyskocil et al., 1981). Extracellular
application of both forms of vanadium failed to inhibit the
electrogenic (Na+ - K+) pump in intact mouse diaphragm fibres
(Vyskocil et al., 1981). In the concentration range from 10-5 to
10-3 mol/litre, vanadium4+ even potentiated the hyper-
polarization of the muscle fibres from -74 to -82 mV (Zemkova et
al., 1982), probably by increasing the intracellular potassium
level. These findings have led to the hypothesis that vanadate
could control sodium pump activity in vivo , perhaps via a
vanadium5+ : vanadium4+ equilibrium connecting pump activity to
the cellular redox state. However, vanadate in the red cell is
reduced to vanadium4+, which then binds to haemoglobin (Cantley
& Aisen, 1979), a reaction that seems to be essentially
quantitatively driven by glutathione (Macara et al., 1980), NADH
(Vyskocil et al., 1980), or by other mild reducing agents, such
as ascorbate or norepinephrine (Svoboda et al., 1984). The role
of the vanadium5+ : vanadium4+ redox equilibrium in the
regulation of cation flow across cell membranes has yet to be
unequivocally demonstrated.
Vanadium appears to be essential for chicks and rats, but it
does not appear to be essential in other species, as defined by
Mertz (1970), i.e., an element is essential if its deficiency
reproducibly results in an impairment of a function from optimal
to suboptimal. However, more research is needed before definite
conclusions can be drawn regarding the role of vanadium as a
nutritionally essential trace element for animals.
5.2 Absorption
The absorption and distribution of vanadium compounds depend
on the route of entry and the solubility of the compounds in
body fluids. The solubility of vanadium compounds in biological
media varies (Reznik, 1954). The following compounds are listed
in decreasing order of solubility: (a) in gastric juices,
vanadyl sulfate, sodium vanadate, ammonium vanadate, vanadium
pentoxide; (b) in blood-serum and in 0.22% sodium carbonate
solution, sodium vanadate, ammonium vanadate, vanadium
pentoxide, and vanadyl sulfate. The higher the solubility in
water and biological media, the more toxic the compound,
presumably because of better absorption (Roshchin, 1968).
5.2.1 Absorption by inhalation
5.2.1.1 Human studies
There is little information on the deposition of vanadium
compounds in the respiratory tract following inhalation.
However, the greatest deposition would be expected in the
submicrometre particle size fraction and particle size
distribution studies (Lee et al., 1972) have shown that most
vanadium-bearing particulate matter is very small and well
within the respirable range for human beings.
Soluble vanadium compounds inhaled and deposited in the
lung, are readily absorbed but the rates of absorption have not
been quantified and estimates have not been made of the amounts
of inhaled vanadium that are transported back to the pharynx by
mucociliary clearance, swallowed, and are then available for
absorption via the gastrointestinal tract. It has been
estimated that about 25% of soluble vanadium compounds may be
absorbed via the respiratory tract (ICRP, 1960). Absorption
from the respiratory tract was demonstrated in workers exposed
to vanadium dust, who showed increased concentrations of
vanadium in the urine (Lewis, 1959b; Gylseth et al., 1979;
Maroni et al., 1983).
5.2.1.2 Animal studies
Following acute exposure, there is complete clearance of the
relatively soluble vanadium pentoxide from the lung within 1 -
3 days (Sjöberg, 1950; Levina, 1972). Stokinger et al (1953)
demonstrated that vanadium is present for more than 40 days
following cessation of long-term exposure.
Intratracheal administration of 48V-vanadium nitrate (0.4
and 20 mg/kg body weight) to albino rats showed that the 48V
absorption rate was maximum after 5 min and could be detected in
internal organs after 30 min. Blood levels were initially high,
but fell to trace levels after 2 days. 48V was not detectable
after 4 days, but reappeared at 8 days and accumulated in all
internal organs, the greatest quantity accumulating in the bone
(Ordzhonikidze, 1977). In female Fischer rats exposed by intra-
tracheal instillation to 40 µg vanadium pentoxide in 0.9%
saline solution, the time for removal of 50% of the initial
burden was 18 min, but traces remained for a considerable time.
At 14 days, the vanadium was distributed principally in the
carcass (40%) and skeleton (12%) (Rhoads & Sanders, 1985). In
a study of the kinetics of vanadium following single or multiple
intratracheal administration, the blood concentration was high
initially and vanadium accumulated in the liver and kidney
reaching the highest level after 24 h (Roshchin & Ordzhonikidze,
1986).
Vanadium trioxide was cleared from the lung more rapidly
than pentoxide or ammonium vanadate following intratracheal
instillation in rats (Levina, 1972).
5.2.2 Absorption from the gastrointestinal tract
5.2.2.1 Human studies
In general, vanadium salts are poorly absorbed from the
human gastrointestinal tract. In a study by Curran et al.
(1959), from 0.1 to 1% of 100 mg vanadium (as highly soluble
diammonium oxytartratovanadate) was absorbed from the
gastrointestinal tract and 60% of this was excreted via the
kidneys within 24 h. The remainder was retained in the liver
and bone, until the oral administration ceased, when it was
mobilized rapidly from the liver and slowly from the bone.
Sodium metavanadate (12.5 mg/day for 12 days) was recovered
largely unabsorbed in the faeces (87.6%) and the remainder in
urine (12.4%) (Proescher et al., 1917). The International
Commission on Radiological Protection (ICRP, 1960) estimate for
the gastrointestinal absorption of soluble vanadium compounds
was 2%. A low degree of absorption was also found by Roshchin
et al. (1980).
5.2.2.2 Animal studies
Mountain (1959)a reported an unpublished study in which
vanadyl sulfate was fed to adult male rats in daily doses
ranging from 650 to 1250 µg (160 - 310 µg of vanadium). The
mean absorption was about 0.5%, but urinary values varied
considerably. The duration of the study was not given.
5.2.3 Absorption through the skin
Dermal absorption and skin irritation were reported in a
study in which a nearly saturated solution (20%) of sodium
metavanadate was applied to the skin of the rabbit (Stokinger,
1967).
However, according to US EPA (1977), the skin appears to be
a minor route of vanadium uptake for human beings. In an in
vitro study using 48V radiotracer, there was no penetration of
human skin samples (Roshchin, 1980).
5.3 Distribution and Transformation
5.3.1 Human studies
Vanadium levels in man, reported in earlier studies, were
considerably higher than those reported more recently. The
difference is illustrated by the whole-body content of 17 -
43 mg vanadium for a 70-kg man calculated by Schroeder (1963)
compared with estimates of 100 µg derived by Byrne & Kosta
(1978). The influence of different sampling procedures and
analysis (colorimetric determination, neutron activation) should
be clarified before concluding that such effects are real
(Lagerkvist et al., 1986) or that they reflect decreasing
environmental exposure.
__________________
a Mountain, J.T. (1959) Unpublished results, Toxicologic
Services, Occupational Health Field Headquarters,
Cincinnati, Ohio.
Absorbed vanadium is transported mainly in the plasma
(Schroeder et al. 1963; Ordhzonikidze et al., 1977; Roshchin et
al., 1980). Some mean values obtained using different methods
of analysis are given in Table 17. There is a wide divergence
in the results, the ratio between the highest and the lowest
mean values being about 104. In general, levels tend to decline
chronologically as analytical techniques become more sensitive.
There is also a difference in results obtained by neutron
activation analysis according to whether separation was carried
out before, or after, irradiation.
In an extensive investigation on human tissues, the
inadequate sensitivity of the analytical method meant that
quantitative information could only be obtained for the lung
and intestine (Tipton & Cook, 1963). Using neutron activation
analysis, Byrne & Kosta (1978) obtained information on other
organs. Table 18 includes autopsy tissue values and some
results from two other investigations using neutron activation
analysis. It is apparent that vanadium concentrations are low
in all tissues, though the liver, kidney, and lung often show
higher levels than other tissues. In another investigation
on a selection of organs, using spark-source mass spectrometry,
the vanadium levels detected were: brain, 30 µg vanadium/kg
wet weight (average from 10 specimens); liver, 40 µg/kg
(average from 11 specimens); lung, 100 µg/kg (average from 11
specimens); lymph node, 400 µg/kg (average from 6 specimens);
and testis, 20 µg/kg (average from 5 specimens) (Hamilton et
al., 1972/73). The values reported are in reasonable agreement
with those found in other animals. However, there is
considerable disagreement between investigators and between
analytical methods, which has not been resolved, since
interlaboratory and intermethod investigations have not been
carried out.
In the general population, which is mainly exposed to low
levels of vanadium in food with poor absorption from the
intestine, vanadium is usually undetectable in the urine, even
using very sensitive methods (Byrne & Kosta, 1978). Examination
of urine samples from 50 normal individuals using atomic
absorption spectrometry showed that vanadium was present in only
13; 11 samples showed a level of 0.1 µg/litre and two,
0.2 µg/litre (Ueno & Ishizaki, 1980). In industry, where
exposure is mainly through air and absorption from the lung is
high, vanadium concentrations in the urine cover a considerable
range (section 5.4.1).
Table 17. Vanadium levels in human blooda,b
---------------------------------------------------------------------------------------------------------
Analytical method Whole blood Serum/plasma Reference
(mg/litre) (mg/litre)
---------------------------------------------------------------------------------------------------------
X-ray emission 0.01 (43) Gofman (1962)
Spectrography 0.0078 (24) Lifschitz (1962)
Colorimetry 0.23 (calculated) 0.42 (13) Schroeder et al. (1963)
Neutron activation analy- 0.016 Bowen (1963)
sis (preseparated)
Neutron activation analysis 0.67 ± 0.32 ng/ml Simonoff et al. (1986)
Spectrography 0.126 (47) Butt et al. (1964)
Neutron activation analy- 0.0046 (36) Heydorn & Lukens (1966)
sis (preseparated)
Catalytic 0.01 (82) Allaway (1968)
0.02 - 0.01 (7)
Spectrophotometric 0.057 (10) Christian (1971)
Neutron activation analy- 0.0046 Kirzhner et al. (1974)
sis (preseparated)
Neutron activation analy- 0.022 (5) Buono et al. (1977)
sis (preseparated)
Neutron activation analy- 0.0005 (5) Byrne & Kosta (1978)
sis (preseparated)
Neutron activation analy-
sis (preseparated) 0.0066c (5) Sabbioni et al. (1979)
Neutron activation analysis 0.000047 (9 males) Cornelis et al. (1979)
0.000024 (8 females) Cornelis et al. (1979)
Neutron activation analy-
sis 0.000033 (17 females) Cornelis et al. (1980)
--------------------------------------------------------------------------------------------------------
a Adapted from: Byrne & Kosta (1978) and Versieck & Cornelis (1980).
b Number of subjects in parentheses.
c Recalculated values assuming an haematocrit of 0.45.
Table 18. Vanadium levels in human organs (µg/kg wet weight)a
-----------------------------------------------------------------------------
Tissue Study
-------------------------------------------------------------------
Byrne & Kosta (1978)b,c Damsgaard (1972)c,d Lievens (1977)b
-----------------------------------------------------------------------------
Kidney 3.3 3.2 2.6 nd 7
Liver 7.5 4.5 5 19 7 - 19 (5)
Brain 0.7 0.75
Thyroid 3.2 3.0
Heart 1.1
Cardiac 0.45 0.3
fat
Subcut- 0.63 0.80
aneous
fat
Muscle 0.45 0.62 0-59 7 nd
Spleen 3 4
Pancreas nd 14
Lung 19 - 40 (7) nd 13
median 30
-----------------------------------------------------------------------------
a From: Byrne & Kosta (1978).
b Parentheses enclose number of subjects.
c Brackets embrace specimens from one autopsy.
d nd = not detected.
Studies on vanadium levels in human bone have produced
widely varying results. Byrne & Kosta (1978) reported the
following data from human bones (lg/kg wet weight): skull,
2.5, 3, 4.5, 8.3; sternum, 3.1; rib, 0.8, 2.1; tooth enamel
(fragmented), 2, 3.4, 4, 5.1; and tooth enamel (drill powdered),
18. The high concentration in tooth enamel may be due to diet;
concentrations of vanadium tend to be higher in processed than
in unprocessed foods (Myron et al., 1977), but vanadium is
commonly present in steel alloys, especially tool steels, and
vanadium contamination may have occurred from the drill. Using
atomic absorption spectroscopy, Sumino et al. (1975) found a
range of 100 - 200 µg/kg (wet weight) in 6 specimens of rib.
Using emission spectral analysis, Shevchenko (1965) detected a
mean vanadium level of 150 ± 2 µg/kg (0.015 mg%) (dry weight)
in 14 samples of healthy bone tissue; bone tumours contained
higher levels.
Metal levels have been extensively studied in hair, because
of its potential value for exposure and body burden studies.
Gordus et al. (1974) and Gibson & DeWolfe (1979), using the same
method as Byrne & Kosta (1978), found levels of 20 - 40 µg/kg
in 42 subjects and 20 - 41 µg/kg in 370 subjects, respectively.
These results compare favourably with those of Byrne & Kosta,
i.e., 12 - 87 µg/kg in 12 subjects. Ueno & Ishizaki (1980)
used atomic absorption spectrometry to determine vanadium in
hair specimens from 130 men and 132 women. They found mean
concentrations of 53.6 µg/kg (range, 5 - 155 µg/kg) and
44.2 µg/kg (range, 1.8 - 118.8 µg/kg), respectively. However,
Creason et al. (1975), using emission spectroscopy in extensive
surveys in the USA, found levels that were about 10 times
higher.
There is considerable information on vanadium levels in lung
tissue. In studies by Schroeder et al. (1963), vanadium was
found in 97 out of 173 lungs examined in groups from 7 USA
cities, group mean concentrations ranging from 10 to 130 µg/kg
wet weight. Byrne & Kosta (1978) found levels of from 19 to
140 µg/kg (median, 30 µg/kg) in 7 cases; Hamilton et al.
(1972/73) detected a mean level of 100 µg/kg in 11 cases; and
Sumino et al. (1975) determined a range of 100 - 300 µg/kg for
13 observations. Statistical analyses of lung tissue trace
metal data were performed by Tipton & Shafer (1964) using data
taken from Tipton & Cook (1963). One approach was examination
of the relationship between lung metal concentrations and age.
The levels of a number of metals, including vanadium, increased
in the lung with age, indicating accumulation of insoluble
compounds, and Schroeder (1970b) calculated an annual increment
rate of 1.3 µg for lung-vanadium. However, earlier analysis of
the same data (Schroeder et al., 1963) did not show an age-
related increase in lung-vanadium. The later analysis by Tipton
& Shafer (1964) was performed on a subset of the original data
from Tipton & Cook (1963), which may account for some of the
discrepancy. The Tipton & Shafer (1964) data did not show a
graded increase in lung-vanadium with age, and there was a high
mean level only in the oldest age group. This could have been
produced by a single very high value in this age group. The
concept of vanadium accumulation in the human lung with age
remains doubtful, and the results of animal studies do not
indicate significant accumulation in the lung (sections 5.1.1.2
and 5.3.2).
5.3.2 Animal studies
Observations on pregnant rats, that received vanadium on the
21st - 22nd day of pregnancy, revealed accumulation in the
placenta but not "in perceivable quantities" in the organs of
the fetus. Vanadium was reported to be secreted in the milk
(Roshchin et al., 1980).
The identification of cellular components that react with
vanadium has been investigated in vivo and in vitro using 48V
radiotracer (Marafante & Sabbioni, 1983). Vanadium has a high
affinity for nuclear and mitochondrial components. When rats
were treated every day with 10 µg vanadium/rat for up to 8
days, a dose-related increase in vanadium incorporation in the
subcellular fractions was observed. There is evidence that non-
haem Fe-containing proteins, such as transferrin and ferritin,
have a high affinity for vanadium, while Fe haemoproteins are
not able to incorporate the metal.
In rat serum, both Vanadium4+ and Vanadium5+ form metal-
protein complexes with transferrins. Specific intracellular
vanadyl-ferritin complexes are formed in rat liver, spleen, and
kidney (Chasteen et al., 1986).
Ermolaev (1969) studied the distribution of vanadium in the
organs and tissues of 3 groups of rabbits, i.e., animals fed
under ordinary conditions, animals fed a diet supplemented with
vanadium (0.5% solution of vanadyl sulfate) at a dose of
0.05 mg/kg body weight, and animals fed the vanadium-
supplemented diet and subcutaneously injected with vanadium
also at 0.05 mg/kg body weight. The results of this
investigation are given in Table 19. The method of
administration had little effect on the resultant blood, brain,
and stomach levels, but there were distinct differences in the
case of the other organs and tissues.
The distribution and kinetics of vanadium, administered ip
as 80 mg vanadocene dichloride/kg to strain A mice, were
determined in blood, kidney, liver, small intestine, and brain.
The vanadium concentration decreased rapidly and exponentially
in the blood (half-time = 118 ± 43 min) and small intestine
(half-time a = 18 ± 0.14 min; half-time b = 341 ± 45 min).
Vanadium accumulated in kidney (maximum concentration,
1.12 ± 0.06 mmol at 12 h) and liver (maximum concentration,
0.56 ± 0.06 mmol at 8 h) and was then excreted (estimated half-
time, 7.9 ± 0.7 h for kidney; 12.1 ± 0.1 h for liver). Vanadium
was not detected in the brain (Toney et al., 1985).
Table 19. Vanadium levels in rabbit organs and tissues following
oral and subcutaneous administration (mg %)a
-----------------------------------------------------------------------------
Organ/ Control group First group Second group
tissue (0.05 mg/kg (0.05 mg/kg with
with food) food and injected
subcutaneously)
-----------------------------------------------------------------------------
liver 66 88 632
muscle tissue 27 41 30
blood 29 82 90
spleen 68 116 180
kidneys 71 90 464
lungs 62 82 168
brain 20 26 24
heart muscle 52 68 71
intestine 20 22 32
stomach 30 70 74
-----------------------------------------------------------------------------
a From: Ermolaev (1969).
The influence of the oxidation state of intravenously
injected compounds of 48V on uptake and distribution to selected
organs and subcellular elements of the rat liver was studied by
Hopkins & Tilton (1966). They did not observe any significant
differences in the rate or amount of uptake of nanogram
quantities of vanadium in three different oxidation states
(VOC13, VOC12, and VC13) and concluded that either the oxidation
state was not critical to transport or that the vanadium was
converted to a common oxidation state in vivo. Similar results
with respect to oxidation state and uptake and also the
distribution of vanadium in the rat were reported by Sabbioni et
al. (1978) and Conklin et al. (1982). In contrast, Parker &
Sharma (1978) found that levels of vanadium in the tissues of
male Wistar rats given sodium orthovanadate in the drinking-
water at 50 mg/litre for 3 months were higher than those in the
tissues of animals given vanadyl sulfate at the same
concentration. Roshchin et al. (1964) found evidence of the
partial conversion of vanadium trioxide to the pentavalent form
in blood-serum and in weakly acidic and basic solutions in
vitro. In a later study, Johnson et al. (1974) reported
the in vivo conversion of vanadium pentoxide to the tetravalent
state.
Information on vanadium distribution in rats after intra-
peritoneal, intratracheal, oral, and subcutaneous administration
of radioactive (48V) vanadium nitrate in single doses of
20 mg/kg body weight (LD50) and 0.4 mg/kg body weight (non-toxic
dose) is given in Table 20 (Roshchin et al., 1980). The study,
which involved 1060 albino rats, showed that whatever the method
of administration, vanadium was present in blood in significant
quantities only during the first 24 h, and that, after 2 days,
only traces of vanadium were detectable. No vanadium was found
in the blood after 4 - 8 days. However, in the groups
administered the radioactive compound intratracheally or
subcutaneously, low amounts of vanadium were detected between 8
and 16 days after administration as a result of reabsorption
from the organs. The highest level occurred 5 min after intra-
tracheal administration, 30 min after intraperitoneal admini-
stration, and 15 min - 1 h after subcutaneous or intragastric
administration. Vanadium was detected in all tissues and
organs. In 2 days, vanadium had accumulated in the bone, kidney,
liver, and lung, which were also the primary targets in rats
after intratracheal administration of vanadium pentoxide
(48V2O5) or chloride (48VO2C1) (Conklin et al., 1982) and after
oral administration of vanadyl sulfate or sodium orthovanadate
in the drinking-water (50 mg/litre) for three months (Parker &
Sharma, 1978).
In another study, 48VOCl3, which is fairly soluble, was
administered intratracheally to juvenile male Wistar rats at a
dose of 12.6 µCi in 1 ml buffered solution. Within 15 min, the
vanadium isotope was found in all major organs, except the
brain. The largest fractions were found in the blood, heart,
spleen, liver, and kidneys. Peak uptake occurred between 4 and
24 h after administration and activity was maintained throughout
a 9-week period (Oberg et al., 1978).
5.4 Retention
5.4.1 Human studies
Vanadium levels in human tissues (Table 18, section 5.3.1)
are low, the highest concentrations tending to occur in the
liver, kidney, and lung.
Storage of available vanadium in man occurs mainly in fat
and serum lipids (Schroeder et al., 1963).
Shevchenko (1965) used emission spectral analysis to
determine the vanadium contents of bone tumours and of the
cortical layer of bone adjacent to the tumours. He found that
vanadium accumulated in tumours. Healthy bone tissue contained
a mean vanadium concentration of 0.15 ± 0.002 mg/kg dry weight,
osteoblastomas contained 6.38 ± 1.17 mg/kg, and osteosarcomas,
4.16 ± 0.77 mg/kg. The cortical layer adjacent to
osteoblastomas contained 2.01 ± 0.42 mg/kg, and that adjacent to
osteosarcomas contained 1.77 ± 0.48 mg/kg. In comparison to
normal bone, bone cysts, osteochondromas, and exostoses also
showed higher vanadium levels, but to a lesser extent than bone
tumours.
The significant accumulation of vanadium in the tissue of
benign and malignant tumours and, to a lesser extent, in the
neighbouring tissue suggests that quantitative changes in the
amounts observed may indicate disturbances in its metabolism.
This may be in connection with the role of vanadium in the
phospholipid and cholesterol metabolism, which may become
indirect indicators of the intensity of the phospholipid
turnover in tumour tissue.
5.4.2 Animal studies
Large amounts of vanadium were reported in the crude fat
from beef, pork, and lamb (Schroeder et al., 1963), but the
results of subsequent studies do not support this finding, which
is probably erroneous because of the analytical methods used
(NRC, 1980).
In rats, after oral administration of 20 mg vanadium
nitrate/kg body weight, levels of vanadium in the blood were
detectable only during the first 24 h, and only traces remained
after 48 h; after 4 days, no vanadium was found in blood. For
48 h after the administration of vanadium, concentrations in the
organs increased by 0.4% of the administered dose in the liver,
by 0.69% in the kidneys, and by 0.16% in the spleen. The most
significant increase in vanadium (1.01% of the dose
administered) was found in the bones. After 16 days, the level
in the bone tissue had increased by 1.72% of the dose
administered, while in other organs it had significantly
decreased (Roshchin et al., 1980).
Studies on rats showed that liver, kidney, spleen, and
testes continued to accumulate intravenously injected vanadium-
48 for up to 4 h and that most of the radioactivity was retained
for up to 96 h (Hopkins & Tilton, 1966) . After 96 h, 14 - 84%
of the 10-min uptake was retained in other organs, and 46% and
9% of the vanadium-48 had been eliminated in the urine and
faeces, respectively. Levels of vanadium-48 in the
mitochondrial and nuclear fractions of homogenized liver
increased from 14 to 40% of the total during the first 96 h,
while the level of radioactivity in the microsomal fraction
remained relatively constant.
When strain A mice were given 80 mg vanadocene dichloride/kg
ip, vanadium accumulated in the liver and kidney. Maximum
concentrations of 1.12 ± 0.06 mmol in the kidney and 0.56 ±
0.06 mmol in the liver were reached after 12 h and 8 h,
respectively (Toney et al., 1985).
Table 20. Concentrations of 48vanadium in the organs and tissues of rats after intraperitoneal,
subcutaneous, intratracheal, and intragastric administration of a radioactive vanadium compound
(expressed as % radioactivity equivalent to 10 uCi per animal)a
---------------------------------------------------------------------------------------------------------
Organ/tissue Intraperitoneal administration Intratracheal administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 2.20 ± 0.55 0.43 ± 0.02 0.22 ± 0 0.43 ± 0.19 0.36 ± 0.19 1.25 ± 0.38
Kidney 7.20 ± 0.85 1.60 ± 0.02 0.66 ± 0 1.55 ± 0.67 1.56 ± 0.19 4.03 ± 0.74
Spleen 2.16 ± 0.23 0.17 ± 0.01 0.09 ± 0 0.15 ± 0.06 0.18 ± 0.02 1.92 ± 0.09
Lung 2.53 ± 0.73 0.11 ± 0 0 9.09 ± 5.91 0.20 ± 0.39 4.64 ± 2.11
Stomach 2.93 ± 0.73 0.08 ± 0 0 2.56 ± 0.94 0.13 ± 0.05 0
Small intestine 2.53 ± 0.31 0.08 ± 0 0 0.71 ± 0.22 0.21 ± 0.15 0
Large intestine 3.33 ± 0.88 0.13 ± 0.03 0 0.19 ± 0.08 0.17 ± 0.05 0.47 ± 0
Muscle 0.40 ± 0.05 0.03 ± 0 0 0.28 ± 0.20 0.03 ± 0 0
Bone 2.13 ± 0.12 3.45 ± 0.48 1.72 ± 0.31 0.79 ± 0.31 2.19 ± 0.31 9.70 ± 1.93
Testicle 1.30 ± 0.15 0.09 ± 0 0.06 ± 0 0.09 ± 0 0.08 ± 0 0.54 ± 0.08
Thyroid gland 0.60 ± 0 0.23 ± 0.05 0 1.21 ± 0.69 0.54 ± 0.40 0
Adrenals 2.53 ± 1.01 0.11 ± 0.02 0 0.67 ± 0 0.06 ± 0.02 0
Pancreas 7.88 ± 0.26 0.31 ± 0.06 0 0.14 ± 0.04 0.08 ± 0.02 0
Brain 0.16 ± 0.04 0.01 ± 0 0 0.02 ± 0 0 0
Heart 1.50 ± 0.34 0.07 ± 0 0 0.04 ± 0 0.08 ± 0 0.41 ± 0.15
---------------------------------------------------------------------------------------------------------
Table 20 (contd.)
---------------------------------------------------------------------------------------------------------
Organ/tissue Intragastric administration Subcutaneous administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 0.08 ± 0.03 0.33 ± 0.11 0.27 ± 0 0.05 ± 0.01 1.00 ± 0.15 1.67 ± 0.42
Kidney 0.17 ± 0.13 0.69 ± 0.20 0.67 ± 0 0.13 ± 0.03 2.30 ± 0 4.47 ± 1.48
Spleen 0.04 ± 0 0.16 ± 0.05 0.08 ± 0 0.03 ± 0 0.31 ± 0.03 1.38 ± 0.75
Lung 0.06 ± 0.02 0.09 ± 0.01 0.06 ± 0 0.10 ± 0.01 0.19 ± 0.01 0.89 ± 0.18
Stomach 8.08 ± 1.49 1.55 ± 0.05 0.05 ± 0 0.29 ± 0.01 0.12 ± 0 0.39 ± 0.06
Small intestine 4.65 ± 9.95 0.22 ± 0.09 0.06 ± 0 0.77 ± 0.66 0.11 ± 0.01 0
Large intestine 0.12 ± 0 0.14 ± 0 0.01 ± 0 0.46 ± 0.21 0.14 ± 0 0
Muscle 0.01 ± 0 0.02 ± 0 0.04 ± 0 0.40 ± 0.30 0.03 ± 0 0.46 ± 0.15
Bone 0.05 ± 0.02 1.01 ± 0.31 1.72 ± 0 0.43 ± 0.30 3.23 ± 0.41 1.86 ± 0.52
Testicle 0.26 ± 0.20 0.04 ± 0 0.06 ± 0 0.01 ± 0 0.12 ± 0.01 0.64 ± 0.07
Thyroid gland 0.08 ± 0 0.32 ± 0.24 0 0.15 ± 0.05 0 0
Adrenals - 0.09 ± 0.03 0 0.10 ± 0 0.16 ± 0.04 0.39 ± 0
Pancreas 0.32 ± 0.29 0.04 ± 0.04 0.08 ± 0 0.03 ± 0 0.09 ± 0.01 0.83 ± 0.04
Brain 0.02 ± 0 0.01 ± 0 0.02 ± 0 0.03 ± 0 0.02 ± 0 0
Heart 0.06 ± 0 0.05 ± 0 0 0.06 ± 0 0.12 ± 0 0.81 ± 0.08
---------------------------------------------------------------------------------------------------------
a From: Roshchin et al. (1980).
In a study on 2 young rats (strain unspecified), the highest
uptake of vanadium-48 from V205 occurred in rapidly mineralizing
areas of dentine and bone (Söremark et al., 1962). In mice,
high uptake occurred in the mammary glands, liver, renal cortex,
and lung (Söremark & Ullberg, 1962).
In studies by Belehova (1966, 1969), vanadium levels were
lower in carious than in normal canine teeth. In other studies,
intramuscular injection of vanadium in dogs at a dose of 2 mg/kg
body weight resulted in vanadium levels in the hard tissues of
the teeth that were 1.3 times higher than those in the controls.
On the 7th day, the concentration in the enamel had almost
returned to its initial level of 8.8 mg/kg; the level in the
dentine was still 32% higher.
Vanadium was reported to decrease the incidence of dental
caries, when added to the diet of hamsters (Geyer, 1953).
However, Hein & Wisotzky (1955) reported significant increases
in dental caries in hamsters given drinking-water containing
vanadium pentoxide equivalent to 10 mg vanadium/litre over an
80-day period. Muhler (1957) studied the effect of vanadium
pentoxide on caries in Sprague-Dawley rats. Groups of 50 rats
received vanadium (as vanadium pentoxide) at 10 mg/litre,
20 mg/litre, or 40 mg/litre in their drinking-water. One
control group received drinking-water containing ammonium
fluorosilicate (20 mg/litre), the other received untreated
water. Vanadium did not produce any reduction in the incidence
of dental caries, in fact there was a slight, but not
significant increase in dental caries in the groups receiving
10 mg/litre and 20 mg/litre for 140 days. All the rats showed
signs of vanadium toxicity and all animals in the highest dose
group (40 mg/litre) died within 65 days.
In an in vitro study on the effects of vanadate on bone
formation in 21-day-old fetal rat calvariae, sodium vanadate at
concentrations of 0.1 - 10 mmol stimulated the incorporation of
3H-thymidine into DNA and increased the bone DNA content and the
mitotic index. Sodium vanadate at a concentration of 100 mmol
produced a marked and irreversible inhibition of DNA labelling
and protein synthesis. Concentrations of 1 mmol (24 h) and
10 mmol (96 h) inhibited alkaline phosphatase activity. Sodium
vanadate also stimulated collagen and non-collagen protein at
low concentrations, but again had an irreversible inhibitory
effect at a high concentration of 100 mmol (Canalis, 1985).
5.5 Elimination
5.5.1 Human studies
Owing to low gastrointestinal absorption, ingested vanadium
is predominantly eliminated unabsorbed in the faeces. The
principle route of excretion of absorbed vanadium is through the
kidneys.
The relationship between urinary excretion and the extent of
exposure has been studied. As part of a clinical study on the
effects on cholesterol level, Dimond et al. (1963) gave ammonium
vanadyl tartrate to patients (5 female, 1 male) and did not find
any correlation between urinary excretion and oral dose.
Variable absorption was suggested as the reason for wide
variation in urinary excretion. In a 50-week study on 2
volunteers, Tipton et al. (1969) reported a urine/diet ratio of
0.13. This is in agreement with the figure of 12.4% excretion
in urine in a man given sodium metavanadate orally (12.5 mg
daily for 12 days) (Proescher et al., 1917).
Studies on occupationally exposed populations have shown a
poor correlation between vanadium concentrations in air and
amounts excreted in urine (Williams, 1952; Lewis, 1959b;
Jaraczewska & Jakubowski, 1964; Watanabe et al., 1966; Troppens,
1969; Köhler, 1972). Differences in laboratories and methods
may contribute to the wide range of urinary concentrations
reported.
In a study on power-station workers exposed to vanadium
during maintenance work on an oil-fired boiler, it was shown
that urinary excretion increased in those most heavily exposed,
in spite of the use of protective masks. For instance, urinary
levels of vanadium in 6 welders and 4 cleaners increased over a
work shift from 2.7 to 43.8 mg vanadium/kg creatinine and from
1.65 to 52.8 mg/kg, respectively. The vanadium concentration
in air during boiler cleaning was estimated to vary between 0.1
and 5 mg/m3 (Maroni et al., 1983). These results resemble those
reported by Thürauf et al. (1979) on 54 workers exposed to
vanadium in a metallurgical plant. Exposed workers had a urinary
vanadium concentration of 33.9 mg/kg creatinine, whereas the
level in unexposed workers was 0.6 mg/kg creatinine.
Roshchin et al. (1980) used polarography in a study of the
urine samples of 100 workers who were exposed daily to vanadium
pentoxide, and found vanadium in 50% of subjects. The mean
concentration of vanadium in the urine was 0.18 ± 0.03 mg/litre.
In workers with a duration of exposure in the range of 1 - 2
years, the mean concentration was 0.14 ± 0.08 mg/litre; after 2
years, the mean concentration was 0.20 ± 0.009 mg/litre. In
workers employed for 2 - 6 months, the mean concentration of
vanadium in the urine was 0.16 ± 0.12 mg/litre. There appeared
to be a correlation between the urinary levels and the
concentration of vanadium in the air. For example, in workers
exposed to mean vanadium pentoxide atmospheric concentrations of
0.28 ± 0.06 mg/m3, the concentration in the urine was 0.20 ±
0.05 mg/litre; at an atmospheric concentration of 0.17 ±
0.01 mg/m3, the urinary concentration was 0.19 ± 0.007
mg/litre.
The relationships between personal exposure and blood and
urine levels of vanadium were examined in 16 workers in a
ferrovanadium plant in Norway (Gylseth et al., 1979).
Individual levels did not show any clear relationship between
exposure and response, but, when the data were divided into
high- and low-exposure groups, significant differences were
found for both blood and urine levels in relation to exposure
levels. There was also a reasonably good correlation between
blood and urine levels. However, the authors concluded that
"the differences are small and the method difficult and
expensive, so for a routine control other criteria should be
sought".
Kiviluoto et al. (1979a,c) studied serum- and urinary-
vanadium levels in relation to exposure levels. Grouped and
individual data were examined at intervals during vacation
periods and compared with those for an unexposed group. The
levels of exposure were low (0.01 - 0.05 mg/m3), and no
correlations between exposure levels and serum or urine
concentrations were found. However, there was a conspicuous
decline in the urine levels at the beginning of the vacation
period, but they did not decline down to the level of the
unexposed group. It was considered that this provided some
measure of the extent of exposure.
5.5.2 Animal studies
Talvitie & Wagner (1954) administered sodium metavanadate
monohydrate in saline to albino Webster rats and albino rabbits
by intraperitoneal and intravenous injection, respectively. In
one part of the study, rats were given a single ip dose
equivalent to 0.5 mg vanadium/kg body weight and, in another,
twice daily ip injections, each equivalent to 0.25 mg
vanadium/kg body weight, for 5 days. Rabbits were injected
intravenously twice daily for a total of 7 or 9 days, with doses
equivalent to 0.25 mg vanadium/kg body weight, except for 1
rabbit that received doses of 0.4 mg/kg body weight for the
second 5-day period. No histological changes were noted. The
ratio of vanadium eliminated in the urine and faeces was 5:1.
Following oral administration of vanadium sulfate and
vanadium pentoxide to guinea-pigs (equivalent to 2 mg vanadium/
kg body weight), Reznik (1954) found vanadium in the urine and
faeces for 7 - 10 days, though elimination in the urine ceased
before that in faeces. The author concluded that the presence
of vanadium in the faeces, several days after administration,
was due to its return to the intestine after internal resorption
and excretion. Biliary excretion of less than 2% of the
intravenously injected dose (between 0.9 and 30 µg penta-
vanadate/kg body weight) was demonstrated in rats during the
first 6 h after administration. By comparison, 20% was excreted
with the urine during the same period of time (Sabbioni et al.,
1981).
Roshchin (1968) administered a dose of 3 mg vanadium
trichloride to 180-g albino rats. Most of the vanadium was
excreted via the kidneys. Of the dose administered, 18% was
found in the urine after 24 h and 25%, after 48 h. The amount
of vanadium excreted in the urine fell and, after 6 days, was
low. Elimination of vanadium via the intestine occurred on a
much smaller scale and remained relatively stable; 30.9% of the
administered dose was excreted over a 6-day period. Thus, the
largest quantity of vanadium was eliminated during the first 2
days, and the rest was eliminated gradually. The ratio of
amounts eliminated in the urine and faeces was 5:1,
corroborating the findings by Talvitie & Wagner (1954).
In a study on mice, rats, and dogs, Mitchell & Floyd (1954)
showed that ascorbic acid increased vanadium elimination in the
urine during the first few days and later in the faeces.
CaNa3DTPA (salicylic salt of diethylenetriamine-pentaacetic
acid) increased vanadium excretion in the urine and reduced
elimination via faeces. Following the combined administration
of both preparations, elimination in the faeces increased.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Microorganisms and higher plants
Trace quantities (1 - 10 µg/litre) of vanadium stimulated
the growth of some algae, including Scenedesmus or Chlorella
(Arnon & Wessel, 1953; Hopkins et al., 1977; Patrick, 1978).
Toxicity studies on phytoplankton, mainly using pentavalent
vanadium, have revealed differences in susceptibility between
various species. A concentration of 0.02 mg/litre, as ammonium
vanadate, interfered with the cell division of the fresh-water
algae Chlorella pyrenoidosa, whereas 0.25 mg/litre was lethal
(Meish & Benzschawel, 1978). The 15-day LC50 for an estuarine
and salt-water green alga (Dunaliella marina) was given as
0.5 mg/litre of sodium metavanadate and that of a salt-water
pennote diatom (Asterionella japonica) as 2 mg/litre (Minamand &
Unsal, 1978).
Vanadium does not appear to be essential for higher plants.
6.1.2 Invertebrates
In a study using sea-water with a background level of 0.0017
mg vanadium/litre, the 9-day LC50s for vanadium (as sodium
metavanadate) for the worm (Nereis diversicolor), mussel
(Mytilus galloprovincialis), and crab (Carcinas maenas) were
10, 35, and 65 mg vanadium/litre, respectively (Miramand &
Unsal, 1978). Some marine invertebrates, such as the tunicates,
accumulate vanadium to levels that may be 10-5 to 10-6 times the
sea-water concentrations (Table 3.). Vanadium levels in such
species may exceed 3000 mg/kg dry weight (Biggs & Swinehart,
1976; Carlson, 1977). It was stated that the uptake of vanadium
by the mussel (Mytilus edulis) from food (plankton) was of the
same magnitude as that from water (Unsal, 1978). It appears
that benthic aquatic organisms tolerate higher concentrations of
vanadium than fish (section 6.1.3).
6.1.3 Fish
There are some data on the acute toxicity of vanadium for
fish (Van Zinderen Bakker & Jaworski, 1980). The 4- to 6-day
LC50s for fresh-water species are in the range 0.5 -10 mg/litre.
Factors influencing toxicity include water hardness, and the
LC50 values are higher in hard water. Giles et al. (1979)
studied the influence of pH on the toxicity of vanadium
pentoxide for rainbow trout fingerlings. The 96-h LC50 ranged
from 6.43 to 21.75 mg/litre. There was some indication of
vanadium pentoxide being most toxic at a pH of 7. Also, Sprague
et al. (1978) tested zebrafish (Brachydanio rerio) with vanadium
pentoxide and found that a pH of 7.5 provided the most toxic
conditions. At this pH, death occurred between 23.5 and 45 h at
a concentration of 22 mg/litre, whereas at pH 8.2, the time
decreased to 32 h and, at pH 8.8 - 9, to 37 - 39 h.
Studying the chronic effects of vanadium pentoxide on
flagfish, Sprague et al. (1978) and Holdway & Sprague (1979)
concluded that the sublethal threshold concentration would be
about 0.08 mg vanadium/litre.
The rainbow trout (Salmo gairdneri) is the most commonly
used fish for toxicity studies. Sprague et al. (1978) reported
7-day LC50 values in one series of studies ranging from 2.4 to
5.6 mg/litre. Increasing the exposure time resulted in
progressively lower LC50 values, the lowest being 1.99 mg/litre
for an 11-day exposure period. Similar results were reported by
Giles et al. (1979) using experimental conditions of pH 8,
15 °C, and hardness 90 mg CaCO3/litre. The LC50 values
decreased from 4.34 mg CaCO3/litre for 5 days exposure to 1.95
mg CaCO3/litre for 14 days. Neither of these groups was able to
define a minimum lethal level for rainbow trout. Studies by
Stendahl & Sprague (1982) indicated that small rainbow trout
were more resistant than larger fish to vanadium pentoxide. In
general, rainbow trout eggs were 10 - 15 times more resistant to
pentavalent vanadium than fingerlings, suggesting the
possibility of a protective function by the chorion (Giles et
al., 1979). It should be noted that the available studies on
vanadium toxicity have been performed on fresh-water species
only. The effects of salinity remain to be studied.
6.2 Terrestrial Organisms
6.2.1 Uptake of vanadium by plants
In general, the highest concentrations of vanadium in plants
growing in natural soils occur in the roots and decrease towards
the aerial portions of the plant. The concentration of vanadium
in soil is, by and large, 10 times the concentration in the
plant (Cannon, 1963). Absorption appears to be passive (Welch,
1973).
When grown in culture solution, several plant species absorb
vanadium, which is also translocated to the aerial parts and
seeds (Hopkins et al., 1977).
6.2.2 Effects on plants
Vanadium has not been demonstrated to be essential for
higher land plants (Hopkins et al., 1977). However, traces of
vanadate (0.02 mmol vanadium as VO2+ or VO3-) were shown to
promote chloroplast development and oxygen production in higher
plants. Vanadium also had a function as a redox catalyst in the
electron flow from photosystem II to photosystem I (Meisch &
Becker, 1981).
Small amounts of aqueous vanadium (10 - 20 mg/litre) have
detrimental effects on most plants (Cannon, 1963). The growth
of flax, peas, soybeans, and cabbage was reduced in nutrient
solutions containing 0.5 mg vanadium/litre (given as VOCl2 or
VCl3) (Warington, 1955; Hara et al., 1976). Vanadium can
induce iron deficiency chlorosis (Cannon, 1963) or affect the
trace element nutrition by reducing the levels of, e.g.,
manganese, copper, calcium, and phosphorus (Warington, 1955;
Wallace et al., 1977). Similarly, 5 mg vanadium/litre (as VO3-)
in irrigation water reduced the growth of sugar beets by 30 -
50% and caused iron deficiency chlorosis (Hewitt, 1953).
In soils, the toxicity of vanadium may range between 10 and
1258 mg/kg, depending on plant species and type of soil (Hopkins
et al., 1977). Ten mg/kg added to sandy soil as Ca(VO3)2
decreased the growth of sour orange, whereas 150 mg/kg was
lethal (Vanselow, 1950).
Fertilizers may have a high vanadium content. For instance,
rock phosphate, super phosphate, and basic slag may contain
several thousand mg vanadium/kg. These may cause unacceptable
levels of accumulation of vanadium in soil (Mitchell, 1964;
Hopkins et al., 1977). Urban sewage sludges usually contain
less than 35 mg/kg of vanadium (Bradford et al., 1975; Oliver &
Cosgrove, 1975). On the other hand, vanadium in sewage sludge
may be up to 6 times more easily available in sludge than in
soil (Bernow & Webber, 1972 ).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 General Toxicity
The toxicity of vanadium for experimental animals varies
with the species and route of administration. Smaller animals,
including the rat and mouse, tolerate the metal well; the rabbit
and horse are more sensitive (Hudson, 1964). In general,
toxicity is low when the metal is given by the oral route,
moderate by the respiratory route, and high by injection.
Lethal doses for various vanadium salts injected intravenously
in rabbits and subcutaneously in guinea-pigs, rats, and mice are
shown in Table 21.
Table 21. Lethal doses of vanadium (mg V2O5/kg body weight) in
experimental animalsa
-----------------------------------------------------------------------------
Compound Rabbitb Guinea-pig Rat Mouse
-----------------------------------------------------------------------------
colloidal vanadium 1 - 2 20 - 28 - 87.5 - 117.5
pentoxide
ammonium metavanadate 1.5 - 2 1 - 2 20 - 30 25 - 30
sodium orthovanadate 2 - 3 1 - 2 50 - 60 50 - 100
sodium pyrovanadate 3 - 4 1 - 2 40 - 50 50 - 100
sodium tetravanadate 6 - 8 18 - 20 30 - 40 25 - 50
sodium hexavanadate 30 - 40 40 - 50 40 - 50 100 - 150
vanadyl sulfate 18 - 20 34 - 45 58 - 190 125 - 150
sodium vanadate - 30 - 40 10 - 20 100 - 150
-----------------------------------------------------------------------------
a From: Hudson (1964).
b Rabbits were injected intravenously; other animals subcutaneously.
The toxicity of vanadium also varies considerably with the
nature of the compound, vanadium being toxic both as a cation
and as an anion. As a general rule, toxicity increases as
valency increases, vanadium5+ being the most toxic. Among the
oxides of vanadium, vanadium pentoxide is more soluble and more
toxic than the trioxide or dioxide.
Roshchin (1967b, 1968) described the results of acute
inhalation studies on albino rats exposed to vanadium pentoxide
in the form of a condensation aerosol (fume) at 10 - 70 mg/m3
or in the form of a dispersion aerosol (dust) at 80 - 700 mg/m3,
ammonium vanadate (presumably as a dispersion aerosol) at
1000 mg/m3, and ferrovanadium, as a dispersion aerosol at 1000 -
10 000 mg/m3. The minimum concentration of vanadium pentoxide
(condensation aerosol) that gave rise to mild signs of acute
poisoning was 10 mg/m3 air. The absolute lethal concentration
for the condensation aerosol was 70 mg/m3. Dispersion aerosols
(containing large particles) were only one-fifth as toxic as
condensation aerosols. Inhalation of dispersion aerosols of
ferrovanadium did not produce any acute toxic effects, probably
because the particles were too large. However, acute toxic
effects were observed following intratracheal instillation of
ferrovanadium. These may have been related to the biological
solubility and the extent of absorption.
The effects of vanadium on experimental animals have been
investigated in a number of studies using various compounds,
species, and test protocols. Some representative acute and long-
term studies are summarized in Tables 22 and 23. Subsequent
discussions on the effects of vanadium on specific biological
systems are frequently based on the results of these studies.
7.2 Effects on Metabolic Processes
Rabbits exposed to a dispersion aerosol of vanadium trioxide
(40 - 75 mg/m3, 2 h/day for up to 12 months) exhibited a
progressive weight loss amounting to an average of 4.6% at the
termination of the study. Control animals gained weight by an
average of 12.3%. The number of white blood cells declined by
the end of the test from between 7000 and 8000 down to 5000/mm3;
no change was noted in controls. Haemoglobin levels in the test
animals decreased from 75 to 68% of normal levelsa. Serum-
ascorbate levels in the blood progressively decreased to about
20% of controls between 7 and 8 months. Protein sulfhydryl
levels in the serum of exposed animals decreased by 30% compared
with those of the controls. Respiration in the liver and brain
tissues of test animals was reduced by one-half by the end of
the study compared with controls, but the respiratory quotient
was unchanged. Blood-cholinesterase levels in exposed rabbits
increased by an average of 25% after the fifth month (Roshchin
et al., 1964; Roshchin, 1968).
In these studies, the weight loss together with the
depression in levels of white cells, haemoglobin, and protein
sulfhydryl groups in the blood and the decreased liver tissue
respiration were taken as indicators of the "general toxic
effect" of vanadium. Increased cholinesterase activity was held
to be indicative of sensitization.
_________________
a Normal haemoglobin level in the rabbit is 80 - 150 g/litre
and normal rabbit haematocrit, 30 - 50%.
Table 22. Acute studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Dose Concentration Reference
administration index or dose
---------------------------------------------------------------------------------------------------------
vanadium pentoxide mouse intragastric LD50 23.4 mg/kg body weight Roshchin (1967a)
rat inhalation LC50 70 mg/m3 Roshchin (1967a)
rat inhalation minimum 10 mg/m3 Roshchin (1967a)
effective
rat inhalation LC50 70 mg/m3 Sjöberg (1950)
cat inhalation LC50 500 mg/m3 Faulkner Hudson
(1964)
rabbit inhalation LC100 205 mg/m3 Sjöberg (1950)
rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
ammonium vanadate mouse intragastric LD50 10 mg/kg body weight Roshchin (1968)
rat intragastric effective 20 mg/kg body weight Kaku et al. (1971)
rat subcutaneous effective 5-30 mg/kg body weight Kaku et al. (1971)
vanadium mouse intragastric LD50 24 mg/kg body weight Roshchin (1968)
trichloride
vanadium di-iodide mouse intragastric LD50 68 mg/kg body weight Roshchin (1968)
vanadium dibromide mouse intragastric LD50 88 mg/kg body weight Roshchin (1968)
vanadium trioxide mouse intragastric LD50 130 mg/kg body weight Reznik (1954)
ammonium rat intragastric LD50 10 mg/kg body weight Gulko (1956)
metavanadate
vanadyl sulfate rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
rabbit subcutaneous LC50 59.1 mg/kg body weight Korkhov (1965)
rabbit subcutaneous maximum 25 mg/kg body weight Korkhov (1965)
tolerated
guinea-pig subcutaneous LD100 800 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous LD50 560 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous maximum 300 mg/kg body weight Kopylova (1971)
tolerated
water-soluble mouse intragastric LD50 5 mg/kg body weight Seljankina (1961)
vanadium compounds mouse intragastric no effect 0.005 mg/kg body weight Seljankina (1961)
(or 0.1 mg/litre
in water)
---------------------------------------------------------------------------------------------------------
Table 23. Long-term studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Concentration Duration of Reference
administration exposure
---------------------------------------------------------------------------------------------------------
vanadium pentoxide rabbit inhalation 20 - 40 mg/m3 several months Sjöberg (1950)
rabbit inhalation 25 mg/m3 10 months Gulko (1956)
guinea- inhalation 25 mg/m3 10 months Gulko (1956)
pig
rabbit inhalation 8 - 18 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat inhalation 10 - 30 mg/m3 several months Roshchin (1967b)
rat inhalation 3 - 5 mg/m3 several months Roshchin (1967b)
rat inhalation 0.027 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.002 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.006 mg/m3 40 days Pazhynich (1966)
ammonium rat subcutaneous 1 mg/kg 30 days Kaku et al. (1971)
metavanadate
sodium guinea- subcutaneous 3.2 - 128 ug/kg days Kulieva (1974)
metavanadate pig
guinea- subcutaneous 5.12 mg/kg days Kulieva (1974)
pig
vanadium trioxide rabbit inhalation 40 - 75 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
vanadium rabbit oral 5 mg/kg 2 - 3 months Roshchin (1968)
trichloride
vanadium carbide rabbit inhalation 40 - 80 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
ferrovanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
metallic vanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
---------------------------------------------------------------------------------------------------------
Chronic poisoning, following the inhalation of trivalent
vanadium (V203) or the oral administration of vanadium tri-
chloride (VCl3), resulted in blood changes by the end of the
second and third months (Table 24). These changes were
characterized by decreased albumin and increased globulins
(mainly y-globulins) with a halving of the albumin-globulin
ratio, and by an increase in serum concentrations of the amino
acids cystine, arginine, and histidine. There was also a 10%
increase in nucleic acid levels in the blood and a
"considerable" increase in the blood-chloride concentration.
The effects of vanadium on the metabolism of proteins and
nucleic acids were considered to be responsible for the
immunological and allergic reactions that may occur in vanadium
poisoning (Roshchin, 1967a).
Metabolic changes were observed in a study by Pazhynich
(1966) in which albino rats were exposed by inhalation for 70
days to condensation aerosols of vanadium pentoxide at levels of
0.027 ± 0.002 mg/m3 and 0.002 ± 0.00013 mg/m3. Statistically
significant changes were observed at the higher level of
exposure, but not at the lower level. The observations included
decreases in: whole blood-cholinesterase levels, total serum-
protein levels, serum-globulin levels, and the oxyhaemoglobin
content of venous blood. Elevated serum-globulin levels,
increased numbers of blood leukocytes showing yellow, orange,
and red nuclear fluorescence with acridine orange, and increased
oxygen consumption as indicated by isolated liver preparations
were also observed in the high-level exposure group. The
pattern of leukocyte nuclear fluorescence returned to normal 20
days after cessation of exposure. In a second study, albino
rats were continuously exposed to vanadium pentoxide at 0.006 ±
0.00056 mg/m3 for 40 days. No changes in blood-leukocyte
nuclear fluorescence were observed. During the sixth week of
exposure, animals received water but no food. After 3.5 days of
this treatment, the number of leukocytes displaying altered
nuclear fluorescence increased by a factor of 4.83.
In an in vitro study, ammonium metavanadate was found to
inhibit microsomal ketamine N -demethylation, lipid peroxida-
tion, and hydrogen peroxide formation in rat liver. The
inhibiting doses of NH4VO3 ranged from 10-5 to 10-3 mol/litre.
Cytochrome c reductase was also inhibited, whereas cytochrome
oxidase activity was stimulated (Beyhl, 1983).
Parenteral injection of guinea-pigs with sodium metavanadate
in daily doses of 3.2 µg/kg, 128 µg/kg, or 5.12 mg/kg body
weight resulted in dose-dependent increased succinate dehydro-
genase activity in the liver and kidneys and cytochrome oxidase
activity in the liver (Kulieva, 1974).
Table 24. Respiratory effects of vanadium pentoxide on experimental animalsa
---------------------------------------------------------------------------------------------------------
Reference Species Form Concentration Exposure Pathological findings
(mg/m3)
---------------------------------------------------------------------------------------------------------
Sjöberg (1950) rabbit dust 205 7 h conjunctivitis, tracheitis,
pulmonary oedema, broncho-
pneumonia, enteritis, fatty
liver, death
Sjöberg (1950) rabbit dust 20 - 40 1 h/day chronic rhinitis, trache-
several months itis, emphysema, atelect-
asis, bronchopneumonia,
pyelonephritis
Gulko (1956) rabbit dust 10 - 30 continuous, bronchitis, pneumonia,
acute weight loss, bloody diarr-
hoea
Roshchin (1963) rat dust, 80 - 700 continuous, haemorrhagic inflammation in
fume 10 - 70 acute lungs, haemorrhage in inter-
nal organs, paralysis, res-
piratory failure, death
Roshchin (1963) rat dust, 10 - 30 2 h/day, haemorrhagic inflammation in
fume 3 - 5 several months lungs purulent bronchitis,
pneumonia
Pazhynich (1966) rat fume 0.027 continuous haemorrhagic inflammation in
70 days lungs, vascular congestion,
haemorrhage in liver, kid-
neys, and heart
---------------------------------------------------------------------------------------------------------
a From: Waters (1977).
In acute studies on rats (Donryu strain) weighing 200 g,
Kaku et al. (1971) administered vanadium (as ammonium vanadate)
by gavage at a dose of 20 mg/kg body weight or injected it
subcutaneously (doses of between 5 and 30 mg/kg body weight).
There were dose-dependent reversible increases in the trigly-
ceride concentrations in the liver and blood-serum, a decrease
in the serum-cholesterol level, and increases in glutamate-
oxalo-acetate transaminase and glutamate-pyruvate transaminase
activity. After subcutaneous injection, there was a fall in
cholesterol levels. The lowest values were reached 24 h after
the injection; values then returned to normal. Dose-dependent
increases were also observed in the concentrations of triglyc-
erides in the liver and serum. Levels peaked 48 h after the
injection and then gradually declined. When ammonium vandate
solution equivalent to 1 mg vanadium/kg body weight was
administered daily by subcutaneous injection for 30 days more
marked changes in serum cholestrol were observed.
Korkhov (1965) injected 0.3 mg vanadyl sulfate/kg body
weight subcutaneously in rabbits with experimental athero-
sclerosis and observed a lowering of the hypercholesterolaemia
and inhibition of the rise in lecithin. Combined administration
of vanadyl sulfate and phenylethylacetic acid lowered the aortic
cholesterol level 3.5 times compared with controls. Korkhov
(1965) also showed that cholesterol biosynthesis in liver tissue
culture was inhibited by the addition of 10-4 vanadyl sulfate.
When 30 rabbits with experimental atherosclerosis were
administered a mixture of trace elements (copper, cobalt,
manganese, and zinc) in combination with cholesterol, Babenko &
Vandzhura (1969) detected increases in the blood lipids and
decreases in vanadium concentrations, compared with healthy
control animals. The same results were noted following combined
administration of copper and cobalt plus cholesterol at
0.2 g/kg. After administration of manganese, the decrease in
the vanadium concentration was delayed; after administration of
zinc, vanadium remained at the same level as in healthy animals.
Vanadium concentrations in body tissues decreased as cholesterol
levels increased, but manganese and zinc, when given together
with cholesterol, helped maintain vanadium concentrations in the
tissues of the body, the highest vanadium concentrations being
found in the liver, aorta, and muscles.
In the studies by Novakova et al. (1981), daily oral
administration to rabbits for 4 months of vanadium pentoxide and
cholesterol (1st group: vanadium at 0.5 mg/kg body weight and
cholesterol at 0.3 mg/kg body weight; 2nd group: vanadium at
1.5 mg/kg body weight and cholesterol at 0.5 mg/kg body weight)
resulted in high levels of cholesterol in the blood and
extensive atherosclerosis of the aorta. In the animals
administered either cholesterol (0.5 mg/kg body weight) or
vanadium (0.5 mg/kg body weight), hypercholesterolaemia was also
observed, but the increase was less pronounced. Increases in
levels of lipids, lipoproteins, and triglycerides were also
observed. After one month of administration, cholesterol levels
in treated animals were more than ten times those in the
controls. At the end of the study (after the 4th month), the
cholesterol levels in the treated animals were considerably
higher than those in the controls.
The effects of vanadium on iron metabolism have not been
fully elucidated. In some studies, a stimulative effect on
haemoglobin and erythrocyte levels has been claimed. The
results of studies by Myers & Beard (1931) suggested that
vanadium chloride given at 0.6 mg/kg diet to rats, previously
rendered anaemic, had a favourable effect on the haemoglobin
level, and Kopylova (1971) obtained increases in the erythrocyte
count and haemoglobin level in rabbits by subcutaneous admini-
stration of vanadyl sulfate (1 mg/kg body weight, daily, for 2
months). Trummert & Boehm (1957) observed an increase in the
erythrocyte count following intravenous injection of vanadium
gluconate (0.3 - 1.5 mg/kg body weight, daily, for 40 days), but
the haemoglobin level was not significantly affected.
7.2.1 Mechanisms of action
Many of the metabolic effects observed can be explained by
the biochemical effects of vanadium exposure in vivo and in
vitro.
Roschin (1967a) presented the hypothesis that the mechanism
of the initial step in the non-specific haematopoietic effect of
vanadium and the subsequent anaemia was the inhibition of the
redox system of hydrogen carriers. In response to the resulting
hypoxia, there is increased haematopoiesis. Possibly vanadium
interferes with tissue respiration at the stage of dehydrogena-
tion effected by nicotinamide adenine dinucleotide (NAD). By
inhibiting this coenzyme, vanadium interferes with the
incorporation of iron in the porphyrin complexes and haemoglobin
synthesis. The anti-vitamin C effect of vanadium and the
consequent vitamin C deficiency also inhibits the utilization of
iron for haemoglobin synthesis. Iron accumulates in the
reticuloendothelial tissue.
It is known that vanadium also inhibits the activity of
monoamine oxidase, which catalyses the conversion of serotonin
to 5-hydroxyindoleacetic acid. In a study on rabbits exposed
to vanadium pentoxide dust for 3 months, urine levels of 5-
hydroxyindoleacetic acid fell to 33% below control values
(Roschin, 1967a). It was suggested by the author that
inhibition of monoamine oxidase might result in accumulation of
serotonin in the central nervous system, leading to functional
disturbances, such as bronchospasm, diarrhoea, and urinary
incontinence. Elevated serotonin levels could also be
responsible for the dystrophic and necrotic process in the
kidneys and the high permeability of the blood vessels. A
decrease in sulfhydryl groups in blood-proteins and a reduction
in the cystine content of keratinized tissues might be due to an
interaction between vanadium and an unspecified enzyme.
The catabolism of cystine and cysteine was increased by
exposure to vanadium (Bergel et al., 1958). In vitro, pyridoxal
5-phosphate induced the catabolism of sulfhydryl amino acids in
the presence of VO2+ (Anbar & Inbar, 1962). The authors
suggested that the activation of pyridoxal phosphate by vanadyl
ions was specific to elimination and strongly suggested a
decrease in -SH groups in the organism. A reduction in cystine
levels was observed in keratinized tissues (hair of rats fed
vanadium compounds and the fingernails of vanadium workers)
by Mountain et al. (1953, 1955). It was suggested that this
effect was the result of decreased synthesis of cysteine and
cystine and that metabolic processes depending on either of
these amino acids may be depressed in the presence of vanadium.
According to Mahler & Cordes (1966), coenzyme A plays a
central role in many biosynthetic and oxidative pathways. In
the biosynthesis of coenzyme A, cysteine reacts with 4-
phosphopantothenic acid in the presence of adenosine triphos-
phate (ATP) to form the intermediate 4'-phosphopantothenyl
cystine, and Mascitelli-Coriandoli & Citterio (1959a,b) showed
that treatment with sodium metavanadate reduced the content of
coenzyme A in rat liver.
As coenzyme A is involved in biochemical pathways starting
with acetate, these processes can also be affected by vanadium;
Curran (1954) showed that the synthesis of cholesterol from 14C-
acetate in rat liver was reduced in the presence of vanadium.
Later studies indicated that one site of the inhibitory action
of vanadium in the synthesis of cholesterol was at the level of
the enzyme squalene synthetase, which catalyses the conversion
of farnesyl pyrophosphate to squalene (Azarnoff & Curran, 1957;
Azarnoff et al., 1961). In a study by Curran & Costello (1956),
aortic cholesterol was mobilized more rapidly in vanadium-
treated atherosclerotic rabbits than in controls. Cholesterol
levels appear to be reduced by vanadium in young animals,
including human beings, but not in older ones. It has been
suggested, but not demonstrated, that a regulatory enzyme in the
synthesis of cholesterol (acetoacetyl coenzyme A deacylase) is
activated by vanadium in the former but inhibited in the latter.
Vanadium might reduce the synthesis of triglycerides and
phospholipids, since acetyl coenzyme A is a precursor of fatty
acids. However, the results of studies by Curran and colleagues
showed that, while the levels of triglycerides decreased in the
livers of rats given vanadium (Curran, 1954), serum-
triglycerides levels in human beings increased following the
ingestion of vanadium (Curran et al., 1959). Snyder & Cornatzer
(1958) reported that the incorporation of labelled phosphate
into rat liver phospholipids decreased following injection of
vanadyl sulfate. This could have been due to the inhibition of
phospho-lipid biosynthesis or to increased oxidative
degradation, as originally suggested by Bernheim & Bernheim
(1938, 1939). Using an isotope method with radioactive
phosphorus as the indicator, Prokopenko (1961) observed a
disturbance in the intensity of phosphorus exchange between
organic acid-soluble compounds and inorganic phosphates in
albino rats and mice with acute ammonium vanadate poisoning. No
changes were found in the intensity of total metabolism and in
liver tissue phosphate levels. After administration for 6
months in doses of 1 mg/kg body weight, phosphorylation
processes in the organs and tissues were disturbed and the
concentration of inorganic phosphorus in the blood and urine was
increased.
Coenzyme A is also involved in the synthesis of coenzyme Q
(ubiquinone) in the mitochondrial electron transport chain.
Ubiquinone synthesis in isolated rat mitochondria was reduced by
vanadium, but this effect was partially reversed when cysteine
was given with vanadium. Addition of ATP and coenzyme A
prevented the inhibition of ubiquinone synthesis (Aiyar &
Sreenivasan, 1961).
It has been suggested that mitochondrial oxidative
phosphorylation in liver homogenates in vitro is uncoupled by
vanadium with a resulting depletion in ATP energy stores (Wright
et al., 1960). In young chicks, the addition of ammonium
metavanadate to the diet equivalent to 25 mg vanadium/kg body
weight uncoupled oxidative phosphorylation in the liver
mitochondria (Hathcock et al., 1966). These authors suggested
that vanadate might replace the phosphate ion in ATP synthesis,
and a high-energy vanadyl intermediate (vanadium X) or ADP-
vanadium be formed and hydrolysed. The results of studies by
DeMaster & Mitchell (1973) supported the theory of the mechanism
involving the uncoupling of glyceraldehyde-3-phosphate
dehydrogenase by vanadium. It was also shown that the
vanadium5+ oxyanion inhibited oxidative phosphorylation in
intact rat liver mitochondria, but did not act as an uncoupler.
Vanadium salts inhibit the activity of succinic dehydro-
genase, a key enzyme in the citric acid cycle and the electron
transport system that is activated by sulfhydryl groups, and
this would also reduce ATP synthesis (Aiyar & Sreenivasan,
1961). The inhibiting effects of vanadium on succinic
dehydrogenase could involve a reduction in available -SH
groups.
The oxidation of tryptamine by monoamine oxidase from
guinea-pig liver and kidney was accelerated by 125% in the
presence of vanadium (Perry et al., 1955, 1969); however, the
results of studies by Lewis (1959c) on dogs injected with sodium
metavanadate indicated that vanadium inhibited monoamine
oxidase, because the urinary output of 5-hydroxyindoleacetic
acid was reduced. The decreased output of 5-hydroxyindoleacetic
acid, suggests the possibility of accumulation of serotonin,
which was also reported by Roshchin (1967a).
When rats were administered daily injections of sodium
metavanadate (1.25 - 2.5 mg/kg body weight), weight loss was
correlated with accumulation of the metal in the liver (Johnson
et al., 1974). The activities of the hepatic enzymes, xanthine
oxidase and sulfite oxidase, which have molybdenum groups, and
total liver concentrations of molybdenum were not affected by
vanadium. It was concluded that vanadium toxicity in rats was
not related to molybdenum utilization. Though vanadium was
administered in the pentavalent state, the rat livers had an
electron paramagnetic resonance (EPR) spectrum characteristic of
vanadium4+. It was suggested that vanadium is in a protein-
bound form in the livers of rats. Vanadium4+ was also found in
the kidneys and, to a limited extent, in the lungs of rats
injected with sodium metavanadate, but not in the hearts; it was
considered that the ability of liver and kidney to reduce the
vanadate by one electron might be related to a specific
detoxification mechanism present in these organs. Johnson et
al. (1974) and Minden & Rothe (1966) studied the effects of
pentavalent vanadium salts on various enzyme systems in rat
liver homogenates, rabbit blood-serum, and suspensions of
erythrocytes and concluded that there was nothing to suggest
that vanadium salts reacted directly with coenzymes (NAD and
NADP, pyroxidal sulfate) or -SH groups.
The results of in vitro studies, conducted by Tolman et al.
(1979), indicated that vanadium stimulated glucose oxidation and
transport in adipocytes and glycogen synthesis in the liver and
diaphragm, and inhibited hepatic gluconeogenesis and intestinal
glucose transport. There is no evidence in vivo showing a role
of vanadium in the regulation of glucose metabolism.
The intimate mechanism of interaction between vanadium and
enzymes is not yet known. Experimentally observed disturbances
of the cardiovascular system, changes in the concentration of
-SH groups and cystine, disturbances in the metabolism of
sulfur-containing, glycogen-forming, and keto-forming amino
acids, and of the functioning of the liver and kidneys, of RNA
and DNA synthesis, and of cholesterol metabolism, indicate that
vanadium possesses a broad spectrum of action in the body and
that its toxic action is not analogous to that of any other
metal (Roshchin, 1968).
7.3 Effects on the Nervous System
The Na+K+-ATPase in rat brain is inhibited by vanadium
pentoxide, though not as strongly as that in kidney and heart
(Nechay & Saunders, 1978). Svoboda et al. (1984) showed that
the brain microsomal N+K+-ATPase in rat is equally inhibited by
vanadate (VO3-) or the vanadyl ion (VO+2).
In a short-term exposure study, rats received single intra-
peritoneal injections of sodium metavanadate equal to 20% of the
LD50 (1 mg vanadium/kg body weight). The level of noradrenaline
decreased and those of dopamine and 5-hydroxy-tryptamine
increased. A long-term study involved the oral administration
of sodium metavanadate (3 mg vanadium/kg body weight). The
findings were similar to, but more pronounced than, those in the
short-term study (Witkowska & Brzezinski, 1979).
When adult male CD1 mice were treated with vanadate in the
drinking-water for 30 days, there was a dose-related decrease in
norepinephrine levels in the hypothalamus. Dopamine levels also
decreased significantly, but 5-hydroxytryptamine levels were not
affected. Levels of dopamine in the corpus striatum were
unchanged and there were only marginal effects on the amines in
the other brain regions. It is suggested that vanadium has a
selective effect on adrenergic pathways (Sharma et al., 1986).
Several inhibitors of Na+K+-ATPase, such as ouabain, have
been reported to block DNA and protein synthesis in cell
cultures (Kaplan, 1978). It has also been shown that vanadate
inhibits protein synthesis in neuroblastoma cells and in the
brain homogenates of rats fed sodium monovanadate (125 mg/litre)
ad libitum for 30 or 60 days (Montero et al., 1981).
Neurophysiological effects have been reported following
acute exposure (oral and sc injection) of dogs and rabbits to
vanadium oxides and salts (V2O3, V2O5, VCl3, NH4VO3) (Roshchin,
1967a). These include disturbances of the central nervous
system (impaired conditioned reflexes and neuromuscular
excitability).
In studies by Seljankina (1961), solutions of vanadium
pentoxide or ammonium vanadate were administered orally to rats
or mice in doses of 0.005 - 1 mg vanadium/kg body weight per day
for periods ranging from 21 days at the higher levels to 6
months at the lower levels. A dose of 0.05 mg vanadium/kg body
weight was found to be the threshold dose for functional
disturbances in conditioned reflex activity in both mice and
rats; a dose of 0.005 mg vanadium/kg body weight did not produce
any adverse effects.
In a study by Pazhynich (1966), albino rats underwent con-
tinuous inhalation exposure to condensation aerosols of vanadium
pentoxide at levels of 0.002 mg/m3 and 0.027 mg/m3. Animals in
both treated and control groups showed normal weight gain.
After 30 days, the motor chronaxy of the extensor muscles of the
tibia in the group exposed to 0.027 mg/m3 decreased by an average
of 0.8 µs ( P < 0.01), and the chronaxy of the corresponding
flexor muscles increased by an average of 4 µs ( P < 0.001).
Thus, the chronaxy ratio of antagonistic muscles fell from 1.5
at the beginning of the studies to 1.0 on day 20 ( P < 0.02),
and 0.5 on day 30 ( P < 0.01). The decrease continued until a
level of about 0.25 was reached. About 18 days after cessation
of exposure on the 70th day, the chronaxy ratio returned to
normal (1.5). Changes in motor chronaxy were not observed in
rats exposed at 0.002 mg/m3 or in the controls.
In a second study, albino rats were exposed continuously to
vanadium pentoxide at 0.006 ± 0.00056 mg/m3 for 40 days. No
changes were observed in the chronaxy of antagonistic muscles of
the tibia in exposed animals, compared with the controls, during
the first month of exposure. However, after 30 days, there was
a statistically significant decrease in chronaxy ratio. When
animals were given only water and no food for 3.5 days, during
the sixth week of exposure, chronaxy ratios decreased to 0.92,
compared with 1.5 in the controls.
7.4 Effects on the Respiratory System
Studies on respiratory exposure to vanadium pentoxide are
summarized in Table 23 (section 7.1).
Sjöberg (1950) exposed rabbits to vanadium pentoxide dust
particles, nearly all of which were smaller than 10 µm in
diameter at concentrations of 77, 109, 205, or 525 mg/m3 for
periods of 7 h, 4 h, 7 h, or 1 h, respectively. Death occurred
only in the 205 mg V205/m3 (7 h) group (equivalent to 115 mg
vanadium/m3). There was marked tracheitis accompanied by
pulmonary oedema and bronchopneumonia. Conjunctivitis,
enteritis, and fatty infiltration of the liver were also
observed.
In further studies by the same author, rabbits were exposed
to 20 - 40 mg V205/m3 (equivalent to 11 - 22 mg vanadium/m3)
intermittently for 1 h each day for several months. At autopsy,
pathological changes observed included chronic rhinitis and
tracheitis, emphysema, patches of lung atelectasis, broncho-
pneumonia and, in some cases, pyelonephritis. Vanadium was
detected in ashed lung, liver, and kidney, but not in the
intestines. Changes of a fibrotic nature and specific chronic
lesions were not observed in the lungs, and there was no visible
accumulation of particles. These findings, plus the fact that
vanadium was present in the liver and kidney, were considered
evidence of rapid clearance and/or absorption from the lung.
Continuous inhalation exposure of rabbits to 10 - 30 mg
V205/m3 (5.6 - 16.8 mg vanadium/m3) caused bronchitis,
pneumonia, loss of weight, and bloody diarrhoea (Gulko, 1956).
In studies by Roshchin (1967b, 1968) described in section
7.2, acute inhalation toxicity in albino rats was characterized
by irritation of the respiratory mucosa and nasal discharge that
sometimes contained blood. Animals breathed with difficulty and
there were crepitations. The animals behaved passively,
refusing to eat, and lost weight. In cases of severe poisoning,
diarrhoea, paralysis of the hind limbs, and respiratory failure
were followed by death. Pulmonary abscesses were frequently
seen in animals that recovered. Animals that died or were
killed at various times after exposure, showed severe
congestion, particularly in the capillaries, and small
haemorrhages were observed in all internal organs. Signs of
increased intracranial pressure, and fatty degeneration of the
liver and kidneys were also seen. In the lungs, there was
capillary congestion together with tiny haemorrhages,
perivascular and focal oedema, bronchitis, and focal
interstitial pneumonia. The bronchitis and bronchopneumonia
were often purulent, and the small bronchi were constricted.
There was a relationship between the severity of the
pathological changes and the vanadium concentration in the air.
In cases of slight toxicity, pathological changes were mainly
confined to the lungs.
Pathological changes were also seen in the lungs when rats
were exposed intermittently to a condensation aerosol of
vanadium pentoxide at 3 - 5 mg/m3 for 2 h, every other day, for
3 months, or to a dispersion aerosol of V205 at 10 - 30 mg/m3
for 4 months. Blood vessels were engorged and the endothelium
was swollen; capillary congestion, perivascular oedema,
lymphostasis, and small haemorrhages indicated altered vascular
permeability and disturbances in the circulation of the
pulmonary blood and lymph. Occasionally, foci of oedema and
desquamative bronchitis were observed and small bronchi were
often constricted. Lymphocytes and histiocytes had infiltrated
interstitial tissue. Connective tissue proliferation was
sometimes seen in the zone of lymphocytic infiltration.
Purulent bronchitis or pneumonia occurred in some animals, and
occasionally lung abscesses developed.
Roshchin (1967a) observed similar effects with vanadium
trioxide and vanadium trichloride. As vanadium trichloride was
more soluble, more marked histopathological effects were seen in
internal organs. Pentavalent compounds of vanadium were 3 - 5
times more toxic (in terms of median lethal concentration) than
trivalent compounds. Although dispersion aerosols of vanadium
metal, vanadium carbide, and ferrovanadium were not highly
toxic, long-term exposure at high concentrations resulted in
many of the signs and symptoms produced by vanadium pentoxide.
In studies by Pazhynich (1966) (section 7.2), histopatho-
logical changes observed in rats following high-level inhalation
exposure included marked lung congestion, focal lung haemor-
rhages, and extensive bronchitis.
Effects on the lung were observed in albino rats exposed by
inhalation for 2 weeks to uncoated bismuth orthovanadate dust
(0.11 mg/litre, 1.2 mg/litre), silica-coated bismuth ortho-
vanadate (0.15 mg/litre, 1.3 mg/litre), or silica-coated
titanium dioxide (1.19 mg/litre). In rats sacrificed at the end
of the 2-week exposure, there was a dose-related macrophage
(dust cell) response to both forms of bismuth orthovanadate.
Three months after exposure, the bismuth orthovanadate rats had
alveolar proteinosis, foamy macrophages with cholesterol clefts,
and hyperplastic type II pneumocytes. Six months after exposure,
these changes were more marked with cholesterol granulomas and
degeneration of foamy macrophages. In rats sacrificed after 1
year, the pulmonary lesions were reduced, but alveolar protein-
osis and cholesterol granulomas persisted. Initially, similar
changes were observed in rats exposed to the silica-coated
titanium dioxide, but recovery was obvious at 6 months and, at 1
year, the lungs were almost normal with only a few remaining
macrophage (dust cell) aggregates (Lee & Gillies, 1986).
The respiratory and related histopathological effects of
vanadium exposure in experimental animals were marked irritation
of the respiratory mucosa; vascular injury resulting in
capillary stasis, perivascular oedema, and small haemorrhages;
and an asthmatic-type bronchitis and expiratory difficulty on
acute exposure (Roschin, 1967b).
In adult male cynomolgus monkeys exposed by inhalation to
vanadium pentoxide dust concentrations of 0.5 mg/m3 or 5 mg/m3
at weekly intervals, significant central and peripheral airflow
restriction was measured one day after each exposure. There
were also significant increases in respiratory cell counts
obtained by bronchoalveolar lavage. The increased cell count
was due to a marked increase in the number (absolute and
relative percentage) of polymorphonuclear leukocytes,
indicating pulmonary inflammatory changes (Knecht et al.,
1985).
In the respiratory tract of experimental animals, the main
differences between the acute and chronic effects of vanadium
are the development, after prolonged exposure, of chronic
inflammation in the bronchi and a greater tendency to septic
bronchopneumonia. Atelectasis, interstitial infiltration and
proliferation, and emphysema also occur.
Macrophages are engaged in a variety of pulmonary defence
mechanisms. Theoretically, effects of vanadium on these cells
may explain some of the observations on vanadium toxicity on the
respiratory system. In in vitro studies, Waters et al. (1974)
demonstrated a 50% reduction in the viability of cultured rabbit
macrophages after exposure to 13 mg vanadium/litre (as vanadium
pentoxide) for 20 h. Short-term exposure (2 h) to vanadium
pentoxide at a dose of 7 mg vanadium/litre also reduced the
viability of mouse pulmonary alveolar macrophages to 87%. In
another study, the phagocytic index was reduced to 71% (Fisher
et al., 1978). The incubation of bovine alveolar macrophages
with ammonium metavanadate (NH4VO3) at 0.5 or 1 mg
vanadium/litre, for 4 h, reduced viability to 95 and 85%,
respectively. After 8 h incubation, viability was reduced by 24
and 38%, respectively, and, after 16 h, no viable cells
remained. Low levels of vanadium (0.01, 0.1 mg vanadium/litre)
stimulated the phagocytic activity of macrophages, whereas a
striking decrease in phagocytic activity was noted with 0.5 and
1 mg/litre at 8 h, though, initially, there was stimulation of
activity. Doses of 0.01 and 0.1 mg/litre did not affect
viability (Wei & Misra, 1982).
7.5 Effects on the Cardiovascular System
Severe exposure of animals to vanadium oxides and salts
produced cardiovascular changes (occurrence of arrythmias and
extrasystole, prolongation of the Q-RST interval, and decrease
in the height of the P and T waves of the EKG) (Roshchin,
1967a).
Intense vasoconstriction has been reported in the spleen,
kidney, and intestines following intravenous injections of sub-
lethal doses of sodium ortho- and metavanadate (Hudson, 1964).
Intravenous injection of sodium metavanadate at 2.5 mg/kg in
dogs provoked an increased amplitude of T-waves in the electro-
cardiogram followed by depression of S-T segments (Lewis,
1959c). Perivascular swelling, as well as fatty changes in the
myocardium, were observed by Roshchin (1968) following long-term
inhalation exposure of rats and rabbits to vanadium pentoxide,
trioxide, and chloride (10 - 70 mg/m3, 2 h/day, 9 - 12 months).
Vanadium sulfate (500 mg/kg diet, 6 weeks) mobilized excess
arterial cholesterol in rabbits previously maintained on a
cholesterol-rich diet (Curran & Costello, 1956).
Feeding rats sodium orthovanadate at 100 or 200 mg/kg body
weight (added to normal rat chow) for up to 56 weeks resulted in
a gradual increase in systolic blood pressure. The effect was
unrelated to water intake, urine output, or urinary-sodium
excretion. The increased pressure was sustained in a dose-
related manner and was positively correlated with plasma levels
of vanadium that ranged from 0.04 to 0.27 mg/litre (Steffen et
al., 1981). Similar increases in mean arterial blood pressure
have been reported in both conscious (Day et al., 1980) and
anaesthetized rats (Hatfield & Churchill, 1981).
7.6 Effects on the Kidney
In earlier studies, glomerular hyperaemia and necrosis of
convoluted tubules were reported to be related to acute vanadium
exposure (Hudson, 1964). Nephrotoxicity, manifested as
albuminuria, was reported after intravenous injection of sodium
metavanadate at 2.5 - 5 mg/kg body weight in male dogs by Lewis
(1959c). Inhalation of 10 - 70 mg vanadium chloride/m3, for 2 h
daily, for 9 - 12 months, was followed by fatty changes in the
kidney of the rat and rabbit (Roshchin, 1968). In an inhalation
study on rats exposed continuously to vanadium pentoxide
condensation aerosols at concentrations of 0.002 and 0.027 mg/m3
for 70 days, Pazhynich (1966) reported granular degeneration of
the epithelial cells of the convoluted tubules, with areas of
necrosis. Acute tubular necrosis followed subcutaneous injection
of NH4VO3-solutions in 0.1 mol/litre tris-HCl-NaCl buffers in
albino mice (20 mg vanadium/kg body weight). The mortality rate
was higher at a pH of 7.8 (68%) than at pH 6.1 (20%) (Wei et
al., 1982).
There are distinct species differences with regard to the
renal effects of vanadate (Grantham, 1980; Phillips et al. 1983;
Nechay, 1984). Vanadate has both diuretic and natriuretic
effects on the rat kidney (Balfour et al., 1978; Day et al.
1980; Kumar & Corder, 1980; Hatfield & Churchill, 1981; Roman et
al., 1981), but not on that of the dog (Inciarte et al., 1980;
Lopez Novoa et al., 1982a,b) or the cat (Larsen et al., 1979).
In the rat, the tubular effect is independent of changes in
glomercular filtration rate, whereas the tubular effect in the
cat is either absent or masked by pronounced renal vasoconstric-
tion and anuria (Larsen et al., 1979; Larsen & Thomsen, 1980).
Vanadate has also been reported to increase the urinary
excretion of calcium, phosphate, bicarbonate, and chloride in
the rat kidney (Kumar & Corder, 1980).
7.7 Effects on the Immune System
Exposure of mice to vanadium in drinking-water resulted in a
dose-related, but not statistically-significant, decrease in
antibody-forming cells in the spleens of mice challenged with
sheep erythrocytes; serum immunoglobulins were not affected.
Splenic lymphocytes obtained at 1, 4, 8, and 13 weeks from male
Swiss-Webster mice treated with 1, 10, or 50 mg vanadium/litre
drinking-water showed increased DNA synthesis in vitro (Sharma
et al., 1981). In female B6C3F1 mice given ammonium
metavanadate ip at doses of 2.5, 5, or 10 mg/kg body weight,
every 3 days, for 3, 6, or 9 weeks, there was a dose-related
increase in resistance to Escherichia coli endotoxin lethality
up to 6 weeks and a dose-related decrease in resistance
to Listeria lethality. Peritoneal macrophage activity also
decreased in a dose-related manner, but without any effects on
viability. The rosetting capability of splenic lymphocytes was
increased. There was enlargement of the liver and spleen with
enhanced formation of splenic mega-karyocytes and red blood cell
percursors. The authors concluded that vanadium may affect the
normal functioning of the immune system (Cohen et al., 1986).
7.8 Reproduction, Embryotoxicity, and Teratogenicity
7.8.1 Reproduction and embryotoxicity
Roshchin at al. (1980) studied the gonado- and embryotoxic
effects of 0.85 mg metavanadate/kg body weight (1/20 LD50),
administered subcutaneously to albino rats. Vanadium
administered to pregnant rats on days 21 - 22 of pregnancy
accumulated in the placenta but was not reported to penetrate
the placental barrier and reach the fetus. During the period of
lactation, vanadium was found in the mammary glands (0.14% of
administered dose/g tissue) and excreted with the milk. In new-
born rats, the uptake of vanadium was in the range of 0.018 -
0.032% of the original administered dose to the lactating dams
per g neonate. Impairment of spermatogenesis was manifested as
a 10 - 33% decrease in the mobility of spermatozoa, a decrease
in osmotic resistance of 7.9 - 11.4% and a 31% rise in the
number of dead spermatozoa. There were morphological changes in
spermatozoa and desquamation of the spermatogenic epithelium in
the seminal tubuli. The impairment of spermatogenesis affected
the reproduction of animals, resulting in pre-implantation
deaths of embryos. Gonadotoxic effects were suggested by the
absence of fertilization of female rats by male rats exposed
daily to vanadium at 0.85 mg/kg body weight. In other studies
(Hackett & Kelman, 1983) in pregnant rats vanadium tended to
localize initially in the placenta and then to preferentially
concentrate in the membranes rather than in the fetus.
The administration of similar doses of vanadium to female
rats on the 4th day of pregnancy increased the mortality of
embryos as a result of pre-implantation deaths; the number of
fetuses in each female rat was only half that in the untreated
animals. These effects were observed in the absence of general
toxicity in the experimental animals.
In vitro, orthovanadate (0.2 - 2 mmol) inhibited
luteinizing hormone-induced cyclic adenosine monophosphate
(cAMP) in isolated corpora lutea from pseudopregnant rats.
When added simultaneously with luteinizing hormone, inhibition
occurred within 25 min, but not when the corpora lutea had been
pretreated with luteinizing hormone for 60 min. A decrease was
also observed when corpora lutea were exposed to vanadate in the
presence of 3-isobutyl-1-methylxanthene (0.5 mmol), a phospho-
diesterase inhibitor. Cyclic adenosine monophosphate was also
inhibited by vanadate in copora lutea incubated in a calcium-
depleted medium. Vanadyl sulfate (0.4, 2 mmol) was as effective
as vanadate in inhibiting luteinizing hormone-induced cyclic
adenosine monophosphate accumulation (Lahav et al., 1986).
7.8.2 Teratogenicity
Carlton et al. (1982) injected 80 mature Syrian golden
hamsters ip with ammonium vanadate at 0, 0.47, 1.88, or
3.75 mg/kg body weight. Injections were carried out on days 5 -
10 of gestation. A significant increase in skeletal anomalies
was observed in all groups exposed to vanadate compared with
control animals. A significant increase in deaths was registered
in the 1.88 mg/kg group. There was no dose-response relationship
as regards anomalies.
Pregnant NMRI albino mice were injected intravenously with
1 mmol vanadium pentoxide dissolved in distilled water on day 3
or day 8 of pregnancy (day 1 = finding of vaginal plug). Control
groups were given 0.1 ml physiological saline on the same days.
Mice were killed on day 17, the uterine horns examined for
resorbed embryos, and the fetuses were removed for detailed
examination. Vanadium pentoxide did not produce any effects in
implantation, and fetuses from the day 3-treated and control
groups did not show any differences in litter size, fetal
weight, or external and internal morphology. The fetuses from
the day 8-treated group showed a statistically significant high
frequency (71%) of delayed ossification (supraoccipital bone,
sternum, metatarsals, and caudal vertebrae), and broken spinal
cord occurred in a few fetuses (Wide, 1984).
Vanadium pentoxide was administered subcutaneously to
pregnant rats (strain unspecified) at doses of 0.5, 1, or 4
mg/kg body weight for 10 days, from day 7 to day 16 of
gestation. The incidences of resorbed and dead fetuses in the 1
and 4 mg/kg groups were 17% and 27.2%, respectively. These were
significantly higher than those found in the controls (3.5%). In
the 4 mg/kg group, 52.38% of fetuses showed wavy ribs. In a
separate study, pregnant rats were given an aqueous solution of
V2O5 by ip injection at concentrations of 0.3, 1, or 3 mg/kg
body weight. This induced a higher level of resorbed and dead
fetuses than oral administration. Both ip injection of 0.3
mg/kg and oral administration of 9 mg/kg induced an array of
skeletal anomalies, namely wavy ribs, supernumerary ribs, and
fused sternebrae and vertebrae (Sun, ed., 1987). Though
tentative, these results suggest that vanadium may have the
potential to induce teratogenicity in a mammalian system.
7.9 Mutagenicity and Related End-Points
There are few studies on the mutagenicity and carcino-
genicity of vanadium compounds. Mutagens in the air can be
divided into two groups: a non-polar extract rich in polycyclic
aromatic hydrocarbons (PAHs) and other promutagens, and a polar
extract containing direct acting mutagens, i.e., not requiring
microsomal activation (Madsen et al., 1982). The non-polar
fraction was strongly influenced by automobile exhaust products,
whereas the polar was more attributable to secondary emissions
transformed by atmospheric reactions, and to primary emissions
from stationary sources. The role of PAHs in the overall
mutagenicity was estimated to be modest; thus, the importance of
other substances increases. Vanadium was found at all sample
sites and is a well-known constituent of, for instance, coal fly
ash (section 3.4.2).
Vanadium5+ has been shown both to inhibit or enhance DNA
synthesis in vitro , depending on the concentration in the media
(Hori & Oka, 1980; Carpenter, 1981; Jackson & Linskens, 1982;
Smith, 1983).
In a DNA synthesis inhibition assay in male mice, vanadium
pentoxide suspended in 3% starch solution was administered
orally at doses of 14.6, 29.2, or 58.4 mg/kg body weight. The
animals were killed 24 h after dosing; 3 h before sacrifice,
1 µCi 3H-thymidine/g body weight was administered ip.
Incorporation of 3H-thymidine in the testes, spleen, liver, and
blood was measured in a liquid scintillation spectrometer.
There were no significant differences between the experimental
groups and the solvent control group (Sun, ed., 1987).
In a study using FADU (Fluorescence Analysis of DNA
Unwinding), vanadyl chloride at a concentration of 5 x 10-5 mol
failed to induce DNA damage (strand breaks) in human peripheral
white blood cells (McLean et al., 1982).
Kanematsu et al. (1980) carried out rec assays on 127 metal
compounds with Bacillus subtilis to test their DNA-damaging
capacity. Mild positive results were noted for three vanadium
compounds (VOCl2, V2O5, NH4VO3).
A lack of induction of spot mutations in Escherichia coli
and in Salmonella typhimurium was demonstrated by Kanematsu &
Kada (1978) and Kada et al. (1980). Similar results were
obtained by Si Rongshan et al. (1982) with E. coli . However,
ammonium metavanadate was found to be mutagenic in S.
typhimurium TA1535 in a modified plate incorporation assay and
in the fluctuation test with TA100 (Arlauskas et al., 1985). In
a recent study, Sun, ed. (1987) demonstrated the induction of
reverse mutations by vanadium pentoxide with E. coli WP2,
WP2uvrA, and Cm-981, but no frameshift mutations with strains
ND-160 and MR102. Vanadium pentoxide showed negative results
with S. typhimurium strains TA1535, TA1537, TA98, and TA100.
Thus, the results of mutagenicity studies of vanadium with
bacterial assays are conflicting, and no firm conclusions can be
drawn.
In a micronucleus test, vanadium pentoxide was administered
to two strains of mice (615 and Kunming albino) by ip injection
at doses of 6.4, 2.13, or 0.17 mg/kg body weight for 5 consecu-
tive days; cyclophosphamide was used as a positive control (Sun,
ed., 1987). Significant levels of induced micronuclei in both
strains were observed. Both subcutaneous injection of vanadium
pentoxide solution (0.25, 1, or 4 mg/kg) and inhalation of
vanadium pentoxide dust (0.5, 2, or 8 mg/m3) also induced micro-
nuclei in mice strain 615. However, negative results were
obtained following oral administration of a 3% starch suspension
of vanadium pentoxide at doses of 1.44, 2.83, 5.65, or
11.3 mg/kg body weight, daily, for 6 weeks, to Kunming albino
mice (Sun, ed., 1987).
In an in vitro study on human peripheral lymphocyte cultures
with vanadium pentoxide concentrations of 0.047, 0.47, or
4.7 moles (mitomycin c was used as positive control), no
increases in the frequency of sister chromatid exchange were
observed (Sun, ed., 1987).
In a dominant-lethal mutation assay, vanadium pentoxide
(0.2, 1, or 4 mg/kg body weight) was administered daily by
subcutaneous injection to 5 groups of male mice, aged 5 - 6
weeks at the start of the study, for 3 months, following which
they were mated with females. Ethylmethanesulfonate and
distilled water were used as positive and negative controls,
respectively. The females were killed 17 days after conception
and the numbers of fetuses and resorptions recorded. The
results were considered negative for the induction of dominant-
lethal mutations (Sun, ed., 1987).
7.10 Carcinogenicity
In a study on 13 metallic compounds, intraperitoneal
injections of vanadium3+ 2.4-pentanedione at doses of 24, 60, or
120 mg/kg body weight did not significantly increase the
incidence of lung adenomas in mice (Stoner et al., 1976).
In life-span studies, the incidence of tumours in mice
given vanadyl ions (as the sulfate) at 5 µg/litre drinking-
water was similar to that in control animals (Kanisawa &
Schroeder, 1967; Schroeder et al., 1970; Schroeder & Mitchener,
1975).
In rats, the induction of mammary carcinogenesis by 1-
methyl-1-nitrosurea was blocked by feeding a purified diet
supplemented with 25 mg vanadyl4+ sulfate/kg during the post-
initiation stages of the neoplastic process. Both cancer
incidence and the average number of cancers per rat were reduced
by the vanadium4+ diet without inhibiting the overall growth of
the animals (Thompson et al., 1984). It has also been shown
that metallocene dichlorides, (C2H5)2MCl2 (where M = titanium,
vanadium, molybdenum, or niobium), exhibit cancerostatic
activity against the Erlich ascites tumour system in mice, and
that treatment with such substances cured the tumour (Köpf-Maier
et al., 1980). Vanadocene dichloride was reported to have a
chemotherapeutic activity similar to that of cis-dichlordiamine
platinum (II) when used against liver tumours in mice (Köpf-
Maier & Köpf, 1979). The mechanism of the preventive effect of
vanadium is not clear. Modulation of one or more aspects of the
DNA metabolism could account for these results.
The effects of ammonium vanadate on the development of large
bowel neoplasms in mice treated with 1,2-dimethylhydrazine (DMH)
were studied by Kingsnorth et al. (1986). Mice were treated
with DMH (20 mg/kg body weight per week) for 20 weeks. Ammonium
vanadate was given in the drinking-water (10 or 20 mg/litre) to
groups of mice during the study. At 30 weeks, the colons of
DMH-treated mice (not receiving ammonium vanadate) showed
increases in RNA content (+14%) and DNA content (+18%) and
deeper crypts (+33%). In the mice treated with DMH and receiving
ammonium vanadate at 10 or 20 mg/litre, the RNA content was
decreased by 11%. Although thymidine incorporation was
increased, ammonium vanadate did not have any effects on the
incidence or type of tumour induced by DMH (Kingsnorth et al.,
1986).
In a long-term study in which the carcinogenic activity of
various materials was studied using intrabronchial pellet
implantation in the lower left bronchus of rats, vanadium solids
produced chronic inflammatory changes in 44/100 rats, bronchial
inflammation in 50/100, squamous metaplasia in 10/100, and one
bronchial carcinoma in a male rat after 645 days. These results
were not significant for carcinogenicity (Levy et al., 1986).
8. EFFECTS ON MAN
8.1 Therapeutic Exposure and Controlled Studies
8.1.1 Therapeutic exposure
In the past, vanadium compounds were prescribed as thera-
peutic agents for anaemia, chlorosis, tuberculosis, and
diabetes. They were also used as an antiseptic, a spiro-
chetocide, and a tonic. For example, sodium metavanadate was
given therapeutically by mouth in doses of 1 - 8 mg, and sodium
tartrate was injected intramuscularly at levels as high as
150 mg. Due to poor absorption from the gastrointestinal tract,
the metal is not very toxic for human beings when ingested, but,
if introduced directly into the circulation in a soluble form,
Hudson (1964) estimated that the lethal dose for a 70-kg person
would be only 30 mg V205 (0.42 mg V205/kg body weight).
8.1.2 Controlled studies
8.1.2.1 Effects on metabolism
Vanadium has been administered under controlled conditions
to study its effects on blood-cholesterol levels. Curran et al.
(1959) conducted a clinical study in which 5 healthy adult male
volunteers were fed soluble diammonium oxytartarovanadate at
150 - 200 mg/day (21 - 30 mg vanadium/day) for 6 weeks. At the
end of the period, plasma-cholesterol was significantly
reduced.
Lewis (1959a) compared age-matched groups of 32 vanadium
workers with 45 controls, all over 45 years of age. Vanadium
workers exposed for at least 6 months excreted greater amounts
of vanadium and exhibited slightly lower serum-cholesterol
levels than the controls. Mean cholesterol values for the 2
reference groups (representing 2 geographical areas) were 2309
and 2267 mg/litre. Mean levels for vanadium workers from
corresponding areas were lower at 2049 and 2067 mg/litre (P <
0.05), respectively.
A clinical study by Somerville & Davies (1962) on 12
patients (9 of whom were hypercholesterolaemic) given diammonium
vanadotartrate orally for 6 months (25 mg 3 times daily for 2
weeks, increased to 125 mg daily in 10 patients) did not show
any significant changes in serum-cholesterol levels over 5.5
months. The mean pretreatment control level of serum-cholesterol
was 4110 mg/litre, and the mean age was 49.2 years. The study
is not comparable with that of Curran et al. (1959), as the
patients were hypercholesterolaemic and older. Dimond et al.
(1963) observed temporary drops (not statistically significant)
in cholesterol levels in 2 out of 6 patients given ammonium
vanadyl tartrate for several weeks at levels of between 50 and
100 mg/day. No statistically significant changes were observed
in blood-lipids, phospholipids, triglycerides, 17-ketosteroids,
or 17-hydroxycorticosteroids. Two patients complained of fatigue
and lethargy while they were taking vanadium. All complained of
cramps and loosened stools, and all developed green tongue.
Schroeder et al. (1963) reporting findings similar to those of
Dimond et al. (1963) considered that the slight effects of
vanadium on serum-cholesterol were pharmacological rather than
caused by correction of a physiological deficiency. They
further pointed out that dietary regimens based on the consump-
tion of unsaturated fats, which reduce plasma-cholesterol in
human beings, are associated with the intake of 1 - 4 mg
vanadium/day and that the feeding of vanadium-poor saturated
fats raises cholesterol.
Studies have been undertaken on the effects of vanadium on
human dental caries. Belehova (1969) studied 583 school
children ranging in age from 7 to 11 years. The subjects were
divided into 4 groups. Children in Group I received fluoride
twice a year, those in Group II received a local application of
a 50% paste of an ammonium salt of vanadium and glycerol, Group
III received both fluoride and vanadium, and Group IV acted as
the control. The incidence of caries was 11% in Group III,
15.4% in Group II, 29.7% in Group I, and 43% in the control
group. Belehova concluded that the lower incidence of caries in
subjects receiving vanadium suggested a possible prophylactic
action. However, other studies (Hein & Wisotzky, 1955; Muhler,
1957; Hadjimarkos, 1966, 1968; McLundie et al., 1968) failed to
demonstrate a clearly beneficial effect with regard to dental
caries in human beings.
Data on the effects of vanadium on the haematopoiesis are
inconsistent. Lewis (1959a) did not observe any effects of
exposure to vanadium on haematocrit levels in 32 vanadium
workers compared with 45 controls matched for age. A beneficial
effect of low-level vanadium administration on nutritional
anaemia has been suggested (Beard et al., 1931; Myers & Beard
1931; Hadjimarkos, 1966; Kopylova, 1971). However, the effects
of vanadium on iron metabolism have not been fully assessed
(Vouk, 1979).
The administration of w -methylpantothenic acid, an
antimetabolite of pantothenic acid, to human beings resulted in
a syndrome consisting of postural hypotension, dizziness,
tachycardia, fatigue, drowsiness, epigastric distress, anorexia,
numbness and tingling of the hands and feet, and hyperactive
deep reflexes. It is not known whether these symptoms are the
result of an induced deficiency of pantothenic acid or are toxic
effects of the anti-metabolite. However, the symptoms resemble
those resulting from exposure to high concentrations of
vanadium. The common denominator in both cases may be a
reduction in hepatic coenzyme A levels (Waters, 1977).
8.1.2.2 Effects on the respiratory system
Zenz & Berg (1967) studied the effects of vanadium
inhalation in 9 healthy volunteers aged 27 - 44 years, for whom
baseline lung function data were available. Two volunteers,
exposed to vanadium pentoxide dust at 1 mg/m3 for 8 h,
developed sporadic coughing after 5 h and a frequent cough after
nearly 7 h. Coughing lasted 8 days, but lung sounds remained
clear and there were no other signs of irritation. Lung
function tests, complete blood counts, urinalyses, and nasal
smears were normal up to 3 weeks. Three weeks later, the same 2
volunteers were accidently exposed for 5 min to a "heavy cloud"
of vanadium pentoxide dust. A productive cough developed within
16 h, and, within 24 h, rales and expiratory rhonchi developed
throughout the lung, but pulmonary function remained normal.
Isoproterenol (1:2000) relieved the symptoms for about 1 h, but
coughing then resumed and continued for 7 days. There were no
other symptoms. Eosinophils were not present in the nasal
mucous.
Exposure of 5 volunteers to a lower concentration
(0.2 mg/m3, 98% of particle size 5 µm) had similar effects,
though the symptoms took longer to develop, i.e., after 20 h.
Coughing, without other systemic effects, persisted for 7 - 10
days. Pulmonary function tests and differential white blood
counts remained normal. The vanadium concentration in the urine
was highest (0.13 mg/litre) on the third day, with none
detectable after 7 days. The maximal faecal-vanadium level was
3 mg/kg, with none detectable after 14 days. Exposure to a
concentration of 0.1 mg/m3 for 8 h did not produce any coughing
in 2 subjects not previously exposed. However, an increase in
the production of mucous, 24 h later, indicated some respiratory
irritation. Then there was slight coughing, which became more
severe after 48 h, subsided after 72 h, and disappeared after
96 h. Pulmonary function tests and differential white blood
counts remained normal.
Pazhynich (1967) studied the irritant effects of vanadium
pentoxide condensation aerosol on 11 volunteers. At a
concentration of 0.4 mg/m3, all reported a tickling or itching
sensation and a feeling of dryness in the region of the root of
the tongue, the posterior wall of the pharynx, and the fauces,
as well as a slight prickling sensation in the nose and
posterior pharyngeal wall. These symptoms were easily tolerated.
A concentration of 0.16 mg/m3 caused mild signs of irritation in
only 5 volunteers, and a concentration of 0.08 mg/m3 was not
noticed by any volunteer. It was concluded that the mean
perceptible concentration for human beings is 0.27 mg/m3 and
that 0.16 mg/m3 is imperceptible.
8.2 Clinical Studies
The clinical picture of poisoning shows the broad spectrum
of toxic effects of vanadium. The lesions observed affect the
respiratory system, circulatory system, central nervous system,
digestive organs, kidneys, and skin. Poisoning can be divided
into acute and chronic forms.
8.2.1 Acute toxicity
Acute toxicity is characterized by a latent period, which
depends on the concentration of vanadium, the individual
sensitivity of the subject, and the properties of the specific
vanadium compound. The more soluble salts of vanadium pentoxide
have a more rapid action than the vanadium oxides. Chemically-
pure vanadium pentoxide acts more rapidly than the technical
grade. A condensation aerosol of vanadium pentoxide is more
toxic than a disintegration aerosol (Roshchin, 1964). Vanadium
chloride is toxic more rapidly than other compounds.
Roshchin (1968) subdivided acute vanadium effects into
"mild", "moderate", and "severe" forms. The clinical features
of mild toxicity include rhinitis with a profuse and often
bloody discharge, sneezing, and an itching and burning sensation
in the throat. The rhinitis may be followed by the development
of a dry cough with expectoration of small amounts of viscid
sputum, general weakness, and exhaustion. A sub-normal
temperature may be present; in other cases, the temperature may
be high or normal. The patient is afebrile in the absence of
pneumonic disease (Sjöberg, 1950). Conjunctivitis is frequently
observed. The symptoms and course of mild toxicity resemble an
upper respiratory tract infection. Other symptoms include
diarrhoea due to intensified intestinal peristalsis. The
symptoms disappear from 2 - 5 days after cessation of contact
with the dust.
In moderate toxicity, in addition to conjunctivitis and
irritation of the upper respiratory tract, there is bronchitis
with expiratory dyspnoea and bronchospasm. There are frequent
disturbances in the activity of the gastrointestinal tract,
including vomiting and diarrhoea. Taken together with the
bronchospasm, this points to a response of the smooth muscle to
vanadium exposure. Some affected persons have cutaneous
manifestations of toxicity in the form of rashes and eczema with
itching papules and dry patches (Browne, 1955; Zenz et al.,
1962).
Bronchitis and bronchopneumonia are features of severe toxic
effects. Other symptoms may also be more prominent, such as
headache, vomiting, diarrhoea, palpitations, sweating, and
general weakness. Disorders of the nervous system include
severe neurotic states and tremor of the fingers and hands
(Wyers, 1946; Sjöberg, 1955). Functional disturbances of the
respiratory system can be expected, and X-ray examination will
reveal intensification of the lung pattern.
Kidney damage, highlighted by grave dystrophic changes in
the epithelium of the convoluted tubules and disturbed tubular
secretion, occurs immediately after the start of exposure to low
vanadium doses in both acute and chronic intoxication. Once
triggered off, the changes are irreversible, even if exposure is
discontinued. Therefore, the kidneys are a critical organ for
vanadium poisoning (Korkhov, 1965).
8.2.2 Chronic toxicity
Chronic vanadium intoxication produces profound changes in
the respiratory organs, because of the irritant action of
vanadium and the biochemical and functional disturbances
connected with its general resorptive action. Chronic
respiratory illness takes the form of diffuse pneumosclerosis,
chronic bronchitis, chronic rhinitis, and pharyngitis (Roshchin,
1968). However, Parkes (1982) claimed that the available
evidence (Sjöberg, 1950; Williams, 1952; Zenz & Berg, 1967) did
not support the contention that prolonged exposure to vanadium
compounds leads to chronic bronchitis, with or without
emphysema. Although wheezing is more common among vanadium
pentoxide workers than among unexposed workers, lung function
tests and chest radiography have not revealed persistent lung
damage (Kiviluoto, 1980). The cardiovascular system (Wyers,
1946; Sjöberg, 1950; Izycki et al., 1971) is commonly affected
in chronic respiratory disorders by a diagnosable accentuated
second cardiac sound on the pulmonary artery and an attenuated
first sound on the apex cordis. Most of these workers exhibit
heavy sinus arrhythmia and a shift of the EGG-axis to the right.
After extensive exposure, workers may develop bradycardia and a
change of the P wave in the second and third standard leads;
coronary spasm is also usually recognizable in such workers. A
statistically significant increase in the incidence of enlarged
liver and a decrease in functional tests together with
bilirubinaemia and a direct reaction to bilirubin have been seen
in the blood of exposed workers (Roshchin, 1968). Biochemical
alterations have also been found, such as reduction in albumins,
and expansion of the globulin fractions at the expense of gamma-
globulins, even though the total protein content remained
normal. Furthermore, a marked reduction in sulfhydryl groups in
blood-serum and in vitamin C levels in the blood, and a less
marked drop in cholesterol levels have been observed. Systemic
effects, such as a tendency towards anaemia and leukopaenia, and
basophilic granulation of leukocytes have been reported
(Watanabe et al., 1966).
Vanadium levels in whole blood and serum have been studied
to investigate the possible role of vanadium in depressive
states. In a study involving neutron activation analysis of
vanadium levels in the whole blood, serum, and hair of patients
suffering from mania or depression, and of patients who had
recovered from these conditions, as well as normal controls,
manic patients were reported to have normal levels in whole
blood and serum, but significantly raised levels in hair.
Depressed patients had raised levels in whole blood and serum.
In both conditions, raised levels fell with recovery. The
levels of vanadium in serum were correlated with those in whole
blood but not with hair levels (Naylor et al., 1984). In another
study, serum-vanadium levels, measured by neutron activation
analysis, were reported to be 3.10 ± 1.38 mg/litre in patients
suffering from depressive illness and 0.67 ± 0.32 µg/litre in
normal subjects (Simonoff et al., 1986). However, in a series
of 25 depressive, 13 recovered depressive patients, and 24
controls, the whole-blood concentrations of vanadium were
similar to normal levels, and vanadium levels did not change in
depressive patients after recovery (Ali et al., 1985).
8.2.3 Diagnosis
Information on likely exposure, the clinical picture, and
certain biochemical indications of probable exposure can aid
diagnosis, but no specific test can be recommended. Determina-
tion of the vanadium contents of the blood and especially of the
urine provides documentation of exposure, though the correlation
between vanadium levels in the urine or serum and air is poor
(Kiviluoto et al., 1979a,c). In view of the work of Schroeder
et al. (1963), it would seem desirable to measure the vanadium
contents of the serum separately from that of the cellular
elements, since the concentration of vanadium in the latter may
be more indicative of exposure levels. As reported by Watanabe
et al. (1966), a decreased urinary output of ascorbic acid may
be one characteristic of vanadium exposure, but differences from
controls do not appear sufficient to make the test clinically
useful.
Green colouration of the tongue is also an indication of
vanadium exposure (Wyers 1946; Williams, 1952; Lewis 1959b). The
green hexaquo ion (V(H2O))63+ is probably responsible for the
green-coloured tongue. However, several other bright green
complexes of vanadium+4 are known and may also account for the
sign (Cotton & Wilkinson, 1962; Durrant & Durrant, 1970). The
"green tongue" may be absent, even in prolonged exposure
(Sjöberg, 1950).
During continuous exposure, measurement of the cystine
content of fingernails was reported to be a sensitive indicator
of exposure. This parameter was negatively correlated with
vanadium exposure in workers. A decrease in cystine levels in
fingernails was demonstrated when urinary-vanadium levels were
only 0.02 - 0.03 mg/litre (Mountain et al., 1955). A similar
reduction in the cystine content of rat hair was noted when
vanadium in the diet ranged from 25 -1000 mg/kg (Mountain et
al., 1953). Some evidence suggesting that vanadium may directly
inhibit the synthesis of cystine or cysteine has also been
reported (Mountain et al., 1953, 1955).
In a recent study on workers exposed to low levels of
vanadium pentoxide (0.1 - 0.6 mg/m3) for about 14 years,
Kiviluoto et al. (198O) could not corroborate the observations
by Mountain and co-workers as no differences were found in
fingernail cystine contents between the 22 exposed workers and
22 unexposed controls. A small reduction in the cystine content
of fingernails (89 mg cystine/kg fingernail for exposed and
99 mg/kg for controls) was found by Thürauf et al. (1979) in 54
exposed workers with an increased urine-vanadium concentration
of 37.8 µg/litre (controls 0.8 µg/litre).
8.2.4 Treatment of poisoning
There are few published data on the treatment of human
poisoning by vanadium. BAL has been used successfully in two
cases of overexposure (Sjöberg, 1955). Experimentally, ascorbic
acid in doses of 125 mg/kg body weight given 20 min prior to an
LD70 dose of NaVO3H2O had a strong protective effect in mice
(Mitchell & Floyd 1956). CaNa2-EDTA was also antidotal in dogs,
when given intraperitoneally in doses of 100 mg/kg body weight
after the first sign of poisoning became evident and again 2 and
4 h later. Jones & Basinger (1983) tested various chelating
agents and their protective effects in mice and found that
efficient antidotes for both vanadate (VO33- and vanadyl (VO2+)
were ascorbic acid, deferoxamine D-penicillamine, sodium,
calcium, Na3CaDTPA, Na2CaEDTa, and glutathione. Ascorbic acid
appeared best suited for human use as an antidote.
Intraperitoneal doses of NaVO3 (0.3 - 1.2 mmol/kg body
weight) were injected in mice followed by chelating and reducing
agents at one-quarter of their respective LD50s. Significant
increases in the survival rate, 14 days after the treatment,
were noted with ascorbic acid, deferoxamine, and tiron (4,5-
dihydroxy-1,3-benzene-disulfonic acid). Other chelators tested
included EOTA, DTPA (Na3Ca-diethylene triaminepenta-acetate) and
L-cysteine. Ascorbic acid was the most effective substance in
preventing vanadium intoxication (Domingo et al., 1986). In
another report, sodium salicylate and D-L-penicillamine were
found useless as antidotes for acute toxicity caused by NaVO3.
8.3 General Population Exposure
8.3.1 Low vanadium intake
Because conditions required to achieve reproducible vanadium
deficiency in animals have not been defined precisely, it is
difficult to predict the consequences of a low vanadium intake
on human health.
Statistical studies have shown negative correlations between
environmental levels of vanadium and certain other trace
elements and the incidence of cardiovascular disease. Consump-
tion of hard water containing vanadium was associated with a
lower incidence of cardiovascular disease (Strain, 1961)a.
Schroeder (1966) reported a significant negative correlation
between the vanadium content of municipal waters and death rates
due to arteriosclerotic heart disease. In a study by Voors
(1971) on the correlation between 7 metals (calcium, chromium,
lithium, zinc, manganese, nickel, vanadium) and arteriosclerotic
heart disease, a low vanadium intake was associated signifi-
cantly with a higher incidence of arteriosclerotic heart disease
in non-white populations, but no direct correlation was
demonstrated for white populations.
____________________
a Strain, W.H. (1961) Effects of some minor elements in
animals and people. Paper presented at the meeting of the
American Association for the Advancement of Science, Denver,
29 December 1961 (unpublished).
In a joint WHO and IAEA study on the role of trace elements
in the etiology of cardiovascular diseases in 20 countries, a
significant role was shown for environmental lack of vanadium as
well as chromium, zinc, manganese, calcium, and magnesium
(Masironi, 1969). Conversely, Hickey et al. (1967) noted a
positive correlation between airborne vanadium levels and the
incidence of cardiovascular disease (section 8.3.2).
The evidence implicating vanadium as an essential trace
element for human beings is not satisfactory. Although certain
statistical studies have indicated that low vanadium intake may
be associated with human cardiovascular disease, these
relationships do not furnish any direct proof for a nutritional
role of vanadium in human health. However, they do suggest leads
for further laboratory and epidemiological investigations.
8.3.2 Epidemiological studies
Descriptive epidemiological work has been published using
a correlational approach, which has well-known limitations,
though it imitates a population-based cohort study.
Despite their limitations, such studies can give indications for
more intensive and detailed controlled studies into suspected
health hazards, comparing incidences of diseases in defined
exposure groups (such as production workers) with those obtained
from reference populations.
Stocks (1960) reported the results of a study in which
airborne concentrations of 13 trace elements were correlated
with mortality from lung cancer, pneumonia, and bronchitis in 23
localities in the United Kingdom. At concentrations ranging
from 1.1 to 42 µg/1000 m3, vanadium showed a weak association
with mortality from lung cancer (taking into consideration
population density, sex, and age), with a correlation
coefficient of 0.347. Airborne vanadium levels were also
correlated with mortality from pneumonia in males, with a
correlation coefficient for mortality from pneumonia of 0.443.
For mortality involving bronchitis, vanadium gave a correlation
coefficient of 0.563. Vanadium also showed an association with
mortality from cancers other than lung cancer in males, but not
in females. However, in this study, as is usual in studies of
this kind, it is not certain that cases of interest (lung
cancer, pneumonia) had been exposed at all. There are also the
uncertainties of mortality data and failure to consider
confounding factors.
In another study, Hickey et al. (1967) considered 10 metals
in the air, including vanadium, in 25 communities in the USA.
Various techniques, including canonical analysis, were used to
correlate airborne metal concentrations with mortality indices
for 1962 and 1963 involving 8 disease categories. The mean
atmospheric concentrations for vanadium at the various locations
ranged from 0.001 to 0.672 µg/m3. The incidence of several
diseases, including "diseases of the heart", nephritis, and
"arteriosclerotic heart", could be correlated reasonably well
with air levels of vanadium and other metals. A high inter-
correlation between vanadium and nickel was unexplained. This
study was of a very preliminary nature, with no adjustments for
the basic pertinent variables normally employed. Other studies
have demonstrated significant negative correlations between the
incidence of cardiovascular disease and environmental levels of
vanadium (section 8.3.1).
An additional multivariate analysis of air-vanadium levels
in relation to selected white male mortality levels was included
in an unpublished US Environmental Protection Agency staff study
by Pinkerton et al. (1972)a. Several categories of cardio-
vascular disease were used, and also influenza-pneumonia.
Vanadium was not correlated with the latter, but was correlated
with the cardiovascular categories. However, adjustment for
population density produced a considerable reduction in some of
these relationships. It was concluded that the observed
statistical associations of air-manganese and air-vanadium
levels were not causal associations, and represented either a
reflection of other more directly associated causes or
statistical artifacts.
Barannik et al. (1969) studied the role of certain trace
elements and the natural radioactivity of food products in the
etiology of endemic goitre in the USSR. More chromium and
vanadium and less lead were found in most of the vegetable
products from a region where goitre was endemic compared with
those from a goitre-free region. The differences in the mean
concentrations of these trace elements were statistically
significant.
The differences between these general population-based
observations and the occupational studies on health effects in
vanadium workers (section 8.3) are connected to different
approaches. The correlational epidemiological studies, based
exclusively on long-term effects and causes of death, are
considered at the expense of lack of individual exposure data,
while the medical studies, cross-sectional in nature, cannot
consider the selection effects and lack long-term information
(such as cause of death). Their strength, however, lies in the
fact that they permit analysis according to different levels of
exposure, though further occupational and population studies on
chronic illness in unambiguous relationship to vanadium exposure
are needed to verify previous work and determine if there is
evidence of a dose-response relationship. In such studies,
morbidity as well as mortality should be considered.
______________
a Pinkerton, C., Hammer, D.I., McClain, K., Williams, M.E.,
Bridbord, K., & Riggins, W.B. (1972) Relationship of
manganese and vanadium in the ambient air to heart disease
and influenza-pneumonia mortality rates, Research Triangle
Park, North Carolina, US Environmental Protection Agency
(unpublished data).
8.4 Occupational Exposure
Occupational poisoning occurs mainly during the industrial
production and use of vanadium and in boiler cleaning
operations. Under these conditions, vanadium may enter the
human body through the respiratory tract; an unknown quantity
will be transported to the alimentary tract when swallowed.
Vanadium can also enter through the skin (Roshchin, 1968).
Both acute and chronic poisoning can occur. Vanadium-
containing industrial aerosols differ in chemical and structural
composition and thus evoke different responses in the human
body.
In sections 8.3.1 - 8.3.4, a survey is made of the available
clinical and epidemiological data on the health effects of
vanadium in workers occupationally exposed to vanadium
compounds. Most of the reported clinical symptoms reflect
irritant effects of vanadium on the respiratory tract and eyes.
8.4.1 Metallurgy
Dutton (1911) first described the effects of industrial
exposure to vanadium-bearing ores. He reported a dry, paroxysmal
cough with haemoptysis and irritation of the eyes, nose, and
throat. Temporary increases in haemoglobin levels and red blood
cells were followed by reductions in both and the onset of
anaemia. Vanadium was recovered in all bodily secretions.
Postmortem examination revealed highly congested lungs with
destruction of the alveolar epithelium and congested kidneys
with evidence of haemorrhagic nephritis. Unfortunately, the
workers frequently suffered from pulmonary tuberculosis, which
undoubtedly produced many symptoms that were aggravated by
vanadium exposure, and no details regarding the number of
workers examined or the incidence of the signs and symptoms were
provided.
A later study by Symanski (1939) on relatively healthy metal
workers exposed to vanadium pentoxide dust for periods ranging
from a few months up to several years reported severe conjunct-
ivitis, rhinitis, pharyngitis, chronic productive cough, and
tightness of the chest; severe chronic bronchitis and bronchi-
ectasis sometimes occurred with longer exposure. There was no
evidence of a generalized systemic action of vanadium.
Rundberg (1939) observed bronchitis with purulent sputum,
general weakness, and skin irritation of the face and hands in
20 men handling vanadium pentoxide in a metallurgical works.
Productive cough, bronchitis, and shortness of breath were
reported by Balestra & Molfino (1942) in 25 workers exposed to
vanadium pentoxide dust from petroleum ash. Other substances
were involved, and chest X-rays showed definite lung markings
suggesting pneumonoconiosis. Bronchiectasis was suspected in 2
cases.
Studies were reported by Wyers (1946, 1948) on 50 - 90
workers exposed to vanadium pentoxide as an oil combustion
residue and to slag from the production of ferrovanadium.
Findings included bronchospasm, often with elevated blood
pressure and accentuated pulmonary second sound, a paroxsymal
cough, dyspnoea, skin pallor, tremor of fingers, palpitation,
chest pains, and reticulation of the lungs. Wyers emphasized
the irritant effects of vanadium pentoxide on the respiratory
tract, but also found evidence of systemic toxicity.
An extensive report including data on the dust contents of
the air in a metallurgical plant producing vanadium pentoxide
was published by Sjöberg (1950). The dust particles were
relatively large in size (39% less than 12 µm, 22% less than
8 µm). It was estimated that a concentration of 6.5 µg
V205/m3 represented the worst exposure conditions. Thirty-six
men between 20 and 50 years of age had been employed in the
plant since 1946: 22 had a dry cough; wheezing sounds could be
detected in 31; and 27 were short of breath. One man developed
acute pneumonitis, and 4 others developed bronchopneumonia.
There was no evidence of systemic toxicity.
A dry eczematous dermatitis developed in 9 men in Sjöberg's
(1950) study, but only 1 man showed a positive patch test.
Sjöberg (1951) and Sjöberg & Rigner (1956) believed that allergy
might play a role in the development of eczema and pneumonitis
following vanadium exposure. Zenz et al. (1962) also considered
this an explanation for the more severe symptoms found on re-
exposure in their study. In a follow-up to the 1950 study,
Sjöberg & Rigner (1956) reported that the 16 men most severely
affected still complained of shortness of breath, cough,
fatigue, and wheezing. Bronchitis was present in 2 men.
However, spirometric measurements, cardiac function tests,
electrocardiograms, haematological tests, and urinanalyses were
essentially normal.
Lewis (1959b) studied 24 male workers in an environment in
which the maximum exposure was only 0.925 mg vanadium (as
V205)/m3 of air. In most cases, the exposure was to 0.3 mg
vanadium/m3. More than 92% of the dust particles were smaller
than 0.5 µg in every process area sampled. Symptoms of cough
with sputum production, eye, nose, and throat irritation, and
wheezing were related to physical findings of wheezes, rales, or
rhonchi, injected pharynx, and green tongue. All of these
symptoms and physical findings were statistically significant in
comparison to those in 45 referents (Tables 25 and 26).
A report by Rajner (1960) on 30 vanadium workers in a
metallurgical plant described particularly severe signs and
symptoms, but did not give any estimates of exposure except in
conjunction with urinary-vanadium levels. In acutely poisoned
workers, vanadium values were about 4000 µg/litre urine. The
average value among permanent employees was 45 µg/litre;
vanadium pentoxide smelter workers had maximum values of about
400 µg/litre. When a new production process was introduced,
symptoms of acute vanadium poisoning occurred in 3 workers after
16 h of work including severe respiratory difficulties, head-
ache, dejection, and loss of appetite. Acute inflammatory
changes of the upper respiratory tract with copious mucous
production, oedema of the vocal cords, and profuse epistaxes
were reported. All workers who had been exposed for a long time
(up to 22 years in 27 subjects, mostly in ferrovanadium and
vanadium pentoxide smelting operations) complained of coughing
and eye, nose, and throat irritation, breathing difficulties
during physical exertion ("more than two-thirds of the
workers"), and headache (12 cases). Clinical findings included
intense hyperaemia of the mucosa of the nasal septum in 20
workers; perforation of the nasal septum was seen in 4 workers
exposed for an average of 18 years. Intense hyperaemia of the
mucosa of the throat and larynx with dilated fine capillaries
was found in 50% of the workers. Bronchoscopy indicated the
presence of chronic bronchitis, and bronchial smears revealed
sloughed epithelium.
Matantseva (1960) studied 77 workers in contact with
vanadium pentoxide in the form of dust and fume in concentra-
tions exceeding the MAC value (dust = 0.5 mg/m3; fumes =
0.1 mg/m3) for periods ranging from 1 to 12 years. Nearly all
the subjects had various complaints relating to the upper
respiratory tract including unpleasant sensations in the nose, a
liquid mucous discharge from the nose, obstructed nasal
breathing, a sensation of burning and dryness in the naso-
pharynx, scratching, dryness, and tickling in the throat,
hoarseness of the voice, and cough. Physical examination showed
rhinitis, which was of a simple catarrhal form in workers
exposed for less than 3 years, a hypertrophic and subatrophic
form if the exposure was for more than 3 years, and an atrophic
form if the exposure was for between 7 and 12 years. Examina-
tion of the lungs revealed acute and chronic lesions in the form
of bronchitis, peribronchitis, and pneumosclerosis. Hyper-
ventilation and an elevated basal metabolic rate were noted.
Table 25. Symptoms in 24 vanadium workers and 45 unexposed referentsa
-----------------------------------------------------------------------------
Symptom Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Cough 33.3 83.4 13.71b
Sputum 13.3 41.5 5.55c
Exertional dyspnoea 24.4 12.5 0.592
Eyes, nose, throat 6.6 62.5 23.17b
irritation
Headache 20 12.5 0.124
Palpitations 11.1 20.8 0.538
Epistaxis 0 4.2 0.148
Wheezing 0 16.6 5.20c
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
c Significant at P = 0.02.
Table 26. Physical findings in 24 vanadium workers and 45 unexposed
referentsa
-----------------------------------------------------------------------------
Physical finding Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Tremors of hands 4.5 4.2 0.0320
Hypertension 13.3 16.6 0.0002
Wheezes, rales, 0 20.8 6.93b
or rhonchi
Hepatomegaly 8.9 12.5 0.003
Eye irritation 2.2 16.6 2.94
Injected pharynx 4.4 41.5 12.62b
Green tongue 0 37.5 14.53b
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
Roshchin (1963b) published an account of the effects of
vanadium-containing Bessemer slag dust on 45 workers. Dust
concentrations in the air during various phases of this opera-
tion ranged from 5 to 150 mg/m3, with the highest concentrations
occurring during loading/unloading of broken slag as the
trivalent oxide, mostly within spinellide. Repeated examinations
of the 45 workers showed the slag dust to have an effect on the
respiratory mucosa. Subatrophic rhinitis, bronchitis, and
pneumosclerosis were seen in subjects with long occupational
exposure (11 workers). Chronic bronchitis was found in every
worker employed for 5 years or more. Clinical and X-ray
examination of all 45 subjects showed radiological changes in 24
employed for 10 years or more; in 11 subjects, pneumoconiosis of
stage I-II was diagnosed. X-ray examination showed diffuse
sclerotic changes over the whole extent of the lung fields
(except for the supraclavicular zones), small focal opacities,
intensified and enlarged shadows at the root of the lungs, and
marked signs of bullous emphysema. Predominant involvement of
the lower regions of the lungs (characteristic of silicosis) was
not present. This pneumosclerosis was accompanied by changes in
the cardiovascular and nervous systems, biochemical disturbances
(hyper-vitaminosis with dysproteinaemia and an increase in the
serum concentration of sulfhydryl groups), a tendency to anaemia
and leukopaenia, and changes in the liver.
In another study, Roshchin (1964) described chronic effects
of vanadium in 193 workers who had been exposed to aerosols of
free vanadium pentoxide: 127 worked in vanadium metallurgy and
66 were boiler cleaners (section 8.3.2). The length of
occupational contact with vanadium was over 10 years for 60%,
from 5 to 10 years for 30%, and under 5 years for the remaining
10%. Practically all complained of irritation of the nasal and
pharyngeal mucosa including itching, a profusely running nose
(especially during work), and unpleasant sensations in the
throat and nose. Epistaxis was frequent in 20%. Physical
examination revealed a high incidence of changes in the nasal
mucosa: dryness (40%), erosion (23%), scars (8%), perforation
(4%), hyperaemia (10%), and hypertrophy (7%). Also noted were
dryness of the pharynx (5%), hyperaemia of the pharynx (5%),
hyperaemia of the larynx (4%), and tonsillitis (5%). The most
common pathological changes in the upper respiratory tract were
subatrophic rhinitis (40%) and destructive changes in nasal
mucosa (35%), while hypertrophic rhinitis was less frequently
seen (7%). The overwhelming majority had a dry cough; cough
with viscid sputum was less common. Workers with longer
occupational exposure complained of shortness of breath, which
appeared sometimes after 5 but mostly after 10 years of work in
the industry. Nearly all complained of aching or shooting pains
in the chest and of lassitude and weakness. The main respiratory
diseases diagnosed were chronic bronchitis (40%) and diffuse
pneumosclerosis (13%). Haematological tests showed the total
serum-protein concentration to be normal, y-globulins to be
raised (19.4% compared with 12.2% in controls), and the albumin-
globulin ratio to be 1:1 - 1:2 (1:9 in controls). Determination
of total, residual, and protein sulfhydryl groups in the blood-
serum revealed a marked decrease of 7 - 13% compared with the
controls. Regular observations over a period of 14 years showed
that the chronic bronchitis tended to get worse, with develop-
ment of bronchospasm. After a long period of time, some subjects
developed pneumosclerosis; in others, the disease progressed
slowly from chronic bronchitis to diffuse pneumosclerosis and
pulmonary emphysema.
Eisler et al. (1968) studied 48 metallurgical workers
occupationally exposed to vanadium for between 17.6 ± 9 years.
Definite clinical evidence of chronic bronchitis was present in
90% of the subjects, and 50% had severe obstructive bronchitis.
In control groups, which included basic-slag crushers and
furnace operators (99 and 50, respectively), chronic bronchitis
was observed in 33% and 26%, respectively.
A study on 13 workers engaged in the extraction and refining
of vanadium pentoxide from soot generated by the combustion of
heavy fuel oil was reported by Nishiyama et al. (1977).
Concentrations of vanadium in the air at various locations in
the work environment were all less than 0.5 mg/m3 (mean, 1.2 -
12 µg/m3). There was a significant incidence of injection of
the pharynx (58.3%) compared with controls. Elevated levels of
vanadium in the urine and hair were detected both in currently-
exposed as well as in previously-exposed subjects. Apart from a
slight depression in serum-cholesterol levels, haematological
results were normal.
Roshchin (1968) analysed the incidence of influenza and
upper respiratory catarrh as a cause of lost working time in
workers in vanadium metallurgical plants compared with ferrous
metallurgical workers in adjacent plants. The results are given
in Table 27. The morbidity was consistently higher among
workers producing vanadium than among workers in other
departments in all years.
Table 27. Morbidity from influenza and upper respiratory catarrha
-----------------------------------------------------------------------------
Department Cases Days off work
(per 100 workers) (per 100 workers)
1958 1959 1960 1962 1958 1959 1960 1962
-----------------------------------------------------------------------------
Vanadium plant 40.6 68.4 58.8 59.8 180.6 376.7 271.9 336.5
Open hearth furnace 22.2 53.2 45.6 62.1 76.9 331.1 176 213.3
Blast furnace 21.9 46.7 37.8 47.6 36.3 262.8 166.6 215.5
Engineering shop 19.6 39.1 33.9 44.9 86.8 224.8 154.3 223.6
-----------------------------------------------------------------------------
a From: Roshchin (1968).
Asthma was reported in 4 workers exposed to vanadium
pentoxide dust in a newly established vanadium pentoxide
refinery (Musk & Tees, 1982). One of the workers had positive
skin tests to environmental allergens; the others were non-
atopic. Three were smokers; one was an ex-smoker. One of the
subjects experienced irritation of the upper respiratory tract
after a single exposure; dyspnoea and wheezing developed 2 weeks
later. All workers had similar irritant symptoms and green
tongue. Two showed bronchial hyperreactivity when challenged
with histamine; these were the workers with the most recent
exposure. In one worker, the asthmatic symptoms continued for 8
weeks after cessation of exposure. There was no indication of
an immunological aetiology, and the authors concluded that the
effect was likely to be a direct chemical one.
Kiviluoto et al. (1979a,b, 1980, 1981a,b) and Kiviluoto
(1980) reported the results of a cross-sectional study on 63
males exposed to vanadium-containing dust in a vanadium factory;
a reference group matched for age and smoking was selected from
a magnetite ore mine. The workers had been exposed to vanadium
dust for an average of 11 years at concentrations ranging from
0.1 to 3.9 mg/m3 (estimated average exposure levels of 0.2 -
0.5 mg/m3); the respirable fraction (< 5 µm) was 20%. Nasal
biopsies and lung function tests were taken at the end of the
summer holidays (duration, 2 - 4 weeks). Nasal smears and
biopsies were repeated in 31 workers, 7 - 11 months later, after
hygienic improvements had reduced the exposure levels to 0.01 -
0.04 mg/m3. Microscopic examination of nasal smears revealed a
significant increase in neutrophils and biopsies of nasal mucosa
showed significantly elevated numbers of plasma and round cells
in the exposed workers. There was no further increase in the
cell findings after 10 months of exposure to 0.01 - 0.04 mg/m3
vanadium dust; eosinophils did not show any differences between
the exposed and the referents. The authors attributed these
findings to "an irritating effect of vanadium dust on the mucous
membranes of the upper respiratory tract". Biopsies from
workers with the longest exposures (170 - 241 months) showed "a
zone-like sub-epithelial infiltration of mononuclear cells and
frequent papillarity in the mucous membrane surface with its
hyperaemic capillaries". The similarity between this pattern
and that seen in vanadium-exposed rabbits (Sjöberg, 1950) was
noted (Kiviluoto et al., 1979b). A random sample of 12 nasal
biopsies was further investigated for the amount and classes of
immunoglobulins (IgE, IgG, IgM, and IgD). IgG subclasses were
not studied. There were no differences between the 12 workers
and their referents, which was construed as a further indication
of non-specific inflammation (Kiviluoto et al., 1981b).
Pulmonary condition was assessed by means of questionnaires,
X-ray, and pulmonary function testing. There was only one
significant difference between the workers exposed for an
average of 11 years to 0.1 - 3.9 mg/m3 (estimated average, 0.2 -
0.5 mg/m3) and at the time of investigation to 0.01 -
0.04 mg/m3, and their matched referents; complaints of wheeze
were more common in the exposed worker group (Kiviluoto, 1980).
The importance of this finding remained doubtful. It may
reflect the respiratory findings mentioned above, since upper
respiratory irritation may be accompanied by transient reflex
bronchospasm. A series of laboratory tests were designed to
evaluate electrolyte, protein fractions, carbohydrate, and
lipids, liver, renal, muscle, pancreatic, and bone marrow
functions, and immunological status. There were no decreases in
serum-cholesterol or triglycerides, and no clinical differences
between worker and control groups (Kiviluoto et al., 1981a).
The effects of vanadium compounds on health is not confined
to the development of local respiratory or other lesions.
Various studies, most of them rather old, on patterns of lost
working time due to morbidity have shown that the incidence of
disease among workers in plants producing vanadium compounds is
considerably higher than among other workers (Symanski, 1939;
Syers, 1946; Sjöberg, 1950, 1956; Reznik, 1954; Reinl, 1958;
Matantseva, 1961; Watanabe et al., 1966; Roshchin, 1968, 1969;
Athanassiadis, 1969; Schumann-Vogt, 1969; Chiriatti, 1971). The
most significant differences are found in the incidences of
influenza, upper respiratory catarrh, and inflammation of the
lungs. The difference in the incidence of bronchitis is
particularly marked.
8.4.2 Cleaning and related operations on oil-fired boilers
Bronchitis and conjunctivitis resulting from exposure to
soot (containing 6 - 11% vanadium) during the cleaning of the
stacks of oil-fired boilers were first recognized by Frost
(1951). Frost did not report any other effects, but, in a
subsequent report of a boiler-cleaning operation by Williams
(1952), sneezing, nasal discharge, lachrymation, sore throat,
and substernal pain occurred within 0.5 - 12 h of exposure.
Within 6 - 24 h, secondary symptoms developed; these consisted
of dry cough, wheezing, laboured breathing, lassitude, and
depression. In some cases, the cough became paroxysmal and
productive. Symptoms lessened only after removal from the
working environment for 3 days. Air sampling showed most of the
dust particles to be smaller than 1 µg. The vanadium concen-
tration ranged from 17.2 mg/m3 in a superheater chamber to
58.6 mg/m3 in a combustion chamber. Roshchin (1962) observed 8
cases of acute vanadium poisoning in workers who cleaned boiler
flues at power stations burning high-sulfur oil. Analysis of
soot deposits showed that the soot in the region of greatest
dust formation (the pipes of the steam superheater and water
economizer) contained from 24 to 40% vanadium pentoxide. The
workers carried out cleaning operations without respirators or
with respirators that did not provide the necessary protection.
After cleaning the boilers, the workers developed acute vanadium
poisoning: itching in the throat, sneezing, cough with difficult
expectoration, and smarting eyes. On the following days, the
symptoms became more severe. Tightness in the chest, sweating,
general weakness, conjunctivitis, and noticeable loss of weight
developed. On examination one week later, hyperaemia and oedema
of the fauces and posterior pharyngeal wall were observed.
Harsh breathing sounds and dry crepitations were heard in the
lungs. X-ray examination showed intensified lung markings in
the middle zones of the right and left lungs and thickening of
the fissure on the right. One month later, only one worker
still had cough, weakness, perspiration, loss of energy, and
dyspnoea. The other workers recovered quickly, with complete
disappearance of cough and shortness of breath.
In another study on workers engaged in boiler-cleaning
operations (Troppens, 1969), the symptoms were described as
similar to mild coryza or influenza with bronchitis. Following
recovery, workers were tired, debilitated, irritable, without
any appetite, and complained of watery eyes. The first symptoms
were swelling of face and eyes as early as 20 min after entering
the boiler area. Removal from exposure for 2 - 3 weeks resulted
in the disappearance of symptoms. Skin blemishes described as
allergic dermatoses were attributed to absorption of vanadium
through sensitive skin. Troppens claimed that there was an
increased susceptibility of the vanadium worker to asthmatic
bronchitis and emphysema.
An investigation is reported on 53 workers performing
emergency repair work on oil-fired power station boilers (Izycki
et al., 1971). They were exposed to vanadium pentoxide in
average concentrations of from 1.2 to 11 mg/m3 and also to
manganese, calcium, and nickel oxides, and sulfur compounds.
Characteristic features of both acute and chronic vanadium
poisoning included upper respiratory catarrh in 45%, increased
lung markings in 24.5%, and bradycardia in 22% of cases.
Persistent chronic changes in the respiratory tract (rhinitis,
pharyngeal catarrh, laryngitis, and changes in the paranasal
sinuses) were present in 45%.
Milby (1974) reported 21 cases of vanadium poisoning in
boilermakers installing new catalytic-converter tubes. This
work involved marble-sized pellets of vanadium containing 11.7%
V2O5. The dust formed during the shaking of these pellets had a
particle size of 1.1 - 1.5 µm. After working for 72 h, the
workers began to complain of nasal, eye, and bronchial
irritation. By the 4th day, most felt very ill, with signs of
irritation of the upper respiratory tract and eyes and pains in
the chest.
In a study by Garlej (1974) 50 workers engaged in the
cleaning of oil-fired boilers were compared with a control group
of 60 other workers. Boiler deposits contained 44 -65% V2O5;
the maximum exposure was estimated to be 10 mg/m3. Although no
clinical evidence of vanadium poisoning was seen, a number of
exposure-dependent positive biochemical reactions were found in
the boiler-cleaning group. Urinary excretion of delta-amino-
levulinic acid (ALA), porphobilinogen (PBG), and porphyrin
increased beyond the physiological limit, and the positive Nadi
reaction (with associated green fluorescence) occurred. The
increased excretion of cytochrome (as indicated by the Nadi
reaction) suggested oxidation through V2O5 of the thiol group
-SH cysteine in the protein carrier, resulting in decreased
binding of cytochrome in the mitochondria.
A study on 17 men who were engaged in cleaning boilers at an
electric generating station was reported by Lees (1980). In
addition to clinical findings, which were similar to those
described above, urine-vanadium levels were determined, and
pulmonary function measurements were made for a week following
exposure. Sixteen of the men wore protective clothing, and
respirators that were found to have about 9% leakage. One
workman volunteered to wear only a simple oro-nasal dust mask
for 1 h of exposure. The dust exposure level was estimated to
be 26 mg/m3; respirable dust (under 10 µm) was measured at
523 µg/m3 with a vanadium content of 15.3%. All of the men
developed reduced pulmonary function that had not fully returned
to normal in one week, but did so after one month. Reduced
function outlasted the clinical symptoms by several days. Fig. 3
shows the contrast in pulmonary reaction between the more
heavily exposed individual and one of the other workmen. The
urine-vanadium level of the volunteer was 280 µg/litre, whereas
those of the remainder of the workers were below 40 µg/litre.
Boyarkina et al. (1978) studied vanadium precipitation in
and around an industrial city, in winter, by measuring
concentrations in snow. In the central area, a concentration of
24 µg/litre was found, decreasing to 3.2 µg/litre at a
distance of 40 - 50 km.
4.1.2 Water
Natural background levels of vanadium in water have been
discussed in section 3.1.3, and levels in industrial effluent,
in section 3.4.1. Durfor & Becker (1963) included vanadium in
their analyses for trace elements in drinking-water supplies in
large cities in the USA. Of the samples analysed, 91% showed
less than 10 µg vanadium/litre; the maximum concentration was
70 µg/litre, and the average was about 4.3 µg/litre. Many of
the samples were negative. Twenty-six percent of 3676 tap water
samples from 34 areas in the USA contained vanadium at
concentrations ranging from 1.3 to 33 µg/litre with a mean of
4.85 µg/litre (Greathouse & Craun, 1979).
Hoffmann et al. (1972) published data from a regional well-
water survey in Poland. The average vanadium concentrations
were 0.06 - 6 µg/litre, with a maximum single value of
15 µg/litre. Bottled waters from mineral springs frequently
contained higher levels; Schlettwein-Gzell & Mommsen-Straub
(1973) reported a range of 4 - 290 µg/litre in bottled waters
from Switzerland.
Highly-mineralized waters in Argentina contained 0.3 -
10 µg vanadium/litre (Trelles et al., 1970). These concentra-
tions often occur in conjunction with high concentrations of
arsenic and/or fluorides.
4.1.3 Food
4.1.3.1 Individual foods
Information on the vanadium contents of human food is
sparse. Data from two studies (Myron et al., 1977; Byrne &
Kosta, 1978) are combined in Table 11 with those from an earlier
study using a similar method (Söremark, 1967). The results of
these studies agree reasonably well, but differ in some respects
from the results of earlier work, especially that of Schroeder
et al. (1963). The principal difference is that Schroeder noted
high vanadium concentrations in fats and oils, whereas low
concentrations were found in the more recent studies. Such a
difference may be accounted for by the use of different
analytical methods. Similar discrepancies have been encountered
in the study of other trace elements, i.e., lower concentrations
have been found using more recently developed methods.
The data presented in Table 11 show low levels of vanadium
in most elements of the human diet. There are also some
interesting differences among specific foods. Grains contain
higher levels of vanadium than fruits and vegetables. Levels in
oils and fats and beef and pork are low, but those in the liver
and kidneys of cows and pigs are higher. Higher levels are
found in both the flesh and internal organs of the chicken, and
levels in fish flesh are also high. Vanadium levels in milk and
eggs are low, and those in beer and wine are high. Myron et al.
(1977) pointed out that processing appears to raise levels in
food (e.g., white versus brown rice; cereal, flour, bread, and
gluten versus grain; peanut butter; bologna and bacon versus
pork). No explanation was given for the very high levels in
dill and parsley.
Table 11. Vanadium concentrations in foods (µg/kg)a
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Grains
Wheat 3.6
Flour 15, 40
Bread 11.20 10, 13
Gluten 33
Oats 3
Oatmeal 6
Corn 0.7
Cornmeal 2
Brown rice 1
White rice 21 12, 30
Barley 14 1.6
Cereal 93
Fruits
Apple 1.1 4 0.3
Pear 0 0.2
Banana 3 0.2
Orange 1
Cherry 0.4
Apricot 0.2
Peach 0.2
Strawberry 31.41 (dry)
Blueberry 1.6
Vegetables
Potato 0.8 1 1.2, 1.9
Radish 52 5 0.6
Carrot 0 1 2.3, 2.4
Beet 0
Garlic 0.6
Onion 0.6
Leek 0.3
Navy bean 14
Pea 0 7 0.4
Tomato 0.03 2 0.3
Cucumber 2.1
Squash 4
Brussels sprout 0.5
Cauliflower 0.08 1 0.9
Cabbage 2 0.3
Lettuce 21 4 1.0, 2.7
Table 11 (contd).
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Spinach 35
Parsley 790 1800 (dry)
Mushroom 50 - 2 000 (dry)
Dill 140 431
Meats
Beef 0 1 0.4 - 1.3
Beef liver 2.4, 10 6 7.3
Pork 0 1 0.6, 0.9
Pork liver 8.4
Pork kidney 8.5
Bacon 5
Bolgna 8
Chicken, white 22 1.7
Chicken, dark 12
Chicken liver 37, 38
Chicken kidney 18
Cod 28 7.2
Mackerel 2.6 3.5
Tuna 11 10, 3
Lobster 43 5
Scallop 22
Oils and fats
Margarine 4
Soybean oil 1
Corn oil 1 3
Pumpkin seed oil 0.2
Lard 2 0.2
Nuts
Hazel nut 3.7
Peanut butter 44
Dairy products
Milk 0 - 0.1 3 0.2, 0.2
Powdered milk 0 - 0.2 25
Chocolate milk 21
Butter 1
Egg white 0.3 - 1.8
Egg yolk 2.0 - 3.6
Beverages
Coffee 1.6
Tea 1.3 0.3
Cola 0.7 1.5
Beer 11 8.4
Wine 3.5 - 3.2
---------------------------------------------------------
a Study 1: Söremark (1967) (neutron activation analysis).
Study 2: Myron et al. (1977) (atomic absorption spectroscopy).
Study 3: Byrne & Kosta (1978) (neutron activation analysis).
4.1.3.2 Complete diets
Byrne & Kosta (1978) estimated the daily intake of vanadium
to be "a few tens of micrograms," but added that it may vary
considerably. Assuming a very low rate of intestinal absorption
of vanadium in man (section 5.1.2), Byrne & Kosta calculated
intakes for 3 adults in whom they measured dietary
concentrations. The calculated intakes were 36, 66, and
11 µg/day, respectively.
Analyses of 9 selected hospital diets (Myron et al., 1978)
are given in Table 12. Each meal was prepared and analysed
separately by atomic absorption spectroscopy. The results were
similar to concentrations found in individual foodstuffs in
other studies given above. In a later study, Byrne & Kosta
(1979) reported on the determination of vanadium in total diet
samples obtained during a nutrition survey in 5 Italian towns
(Table 13). The concentrations agree with those reported by
Myron et al. (1978).
Table 12. Daily vanadium intake in dieta
------------------------------------------------------
Diet type (µg/day) (µg/g) (µg/1000
cal)
------------------------------------------------------
General-1 13.6 0.019 4.7
Cholesterol reducing-1 25.6 0.034 8.8
Cholesterol reducing-2 16.8 0.022 5.8
Cholesterol raising 30.1 0.046 10.5
General-2 28.0 0.040 9.8
Low calorie 12.4 0.029 10.6
Low salt 15.5 0.028 9.1
Puree 26.0 0.050 14.1
Soft 15.8 0.024 6.4
------------------------------------------------------
a Adapted from: Myron et al. (1978).
4.2 Occupational Exposure
In terms of occupational exposure, the most important
vanadium compounds are vanadium pentoxide, vanadium trioxide,
ferrovanadium, vanadium carbide, and vanadium salts, such as
sodium and ammonium vanadate. The oxides and salts are commonly
used in industry in powder form, giving rise to the possibility
of dust and aerosol formation, when the substances are crushed
or ground. Many metallurgical processes involve the production
of vapour containing vanadium pentoxide, which condenses to form
respirable aerosols. Boiler-cleaning operations generate dusts
containing the pentoxide and trioxide compounds. Combustion of
residual fuels with a high vanadium content is likely to produce
aerosols of the pentoxide as well as oxide complexes of vanadium
with other metals.
Table 13. Vanadium contents of Italian freeze-dried
total diet samplesa
--------------------------------------------------------
Town Dry Vanadium Daily
weight concentrationb vanadium
(g) (ng/g) intake (µg)
--------------------------------------------------------
Aosta 310 32.1 0.9 (2) 10.0
L'Aquila 173 46.0 3.4 (2) 8.0
Montfalcone 278 39.7 4.1 (2) 11.0
Mt. Amiata 377 29.7 0.8 (2) 11.2
Rome 285 42.3 4.6 (4) 12.0
Mean values 38.0 6.9 10.4 1.5
--------------------------------------------------------
a From: Byrne & Kosta (1979).
b Average and standard deviation; number of aliquots
in parentheses.
4.2.1 Metallurgy
The processing of metals containing vanadium includes
chemical treatment and high-temperature operations. However,
only moderate concentrations of vanadium were found in the
breathing zone of workers engaged in operations that would be
expected to produce the greatest fume exposure. During the
addition of vanadium to furnaces, concentrations ranged from
0.006 to 0.08 mg/m3 and, during tapping, from 0.004 to
0.02 mg/m3. Concentrations found in oxyacetylene cutting ranged
from 0.008 to 0.015 mg/m3 and, in arc-welding, from 0.002 to
0.006 mg/m3 (NAS, 1974).
Vanadium levels in metallurgical plants have been studied in
detail (Roshchin, 1968). Vanadium slag contains about 11 - 13%,
mainly in the form of trioxides of vanadium (measured as
vanadium pentoxide). Slags are used for the production of
vanadium pentoxide and ferrovanadium, and the process is
accompanied by extensive formation of iron oxide aerosol. Air
concentrations of the dust (mainly vanadium trioxide) found in
the main working positions (converter operator, mixer, and crane
driver) ranged from 20 to 55 mg/m3. Measured as vanadium
pentoxide, the contents of the swirling dust did not exceed
0.17 mg/m3. About 75% of the dust particles had a diameter of
less than 2 µm and 20% had a diameter of between 2 µm and
4 µm.
Breaking, loading and unloading, crushing and grinding, and
magnetic separation of vanadium slag causes thick dust
formation, with concentrations ranging from 30 to 120 mg/m3.
The slag contains 111 - 129 g vanadium pentoxide/kg. A diameter
of less than 2 µm was recorded for 70 - 72% of the particles;
86 - 96% had a diameter of less than 5 µm. When the slag is
roasted, free vanadium pentoxide is discharged into the work-
place air; atmospheric concentrations in the vicinity of
furnaces ranged from 0.04 to 1.56 mg/m3. During leaching and
precipitation, concentrations of vanadium in the air may be
high, sometimes exceeding 0.5 mg/m3.
The smelting and granulation of technical vanadium pentoxide
are accompanied by the formation of an aerosol. This aerosol
escapes when the product is poured for granulation. During the
loading of smelting furnaces, concentrations of vanadium
pentoxide ranged from 0.15 to 0.80 mg/m3. During smelting and
granulation, concentrations ranged from 0.7 to 11.7 mg/m3. In
other parts of the work-place, concentrations may range from
0.03 to 0.2 mg/m3.
In aluminium production, when bauxite is being converted
into alumina, the aluminate solutions accumulate vanadium salts,
which crystallize and precipitate out. Precipitated sodium
polyvanadate is smelted to form vanadium pentoxide, which is
cooled and settles in the form of thin plates. Vanadium
pentoxide dust (concentrations of up to 2.3 mg/m3) is given off
only in the terminal phase during tapping of the liquid product,
packing, and loading.
During the drying, sieving, and calcination of ammonium
vanadate and during the crushing, unloading, and packaging of
pure vanadium pentoxide, dusts are formed. When vanadium
pentoxide is sieved after calcination, the concentration in air
may range from 2.2 to 26 mg/m3. In plants with less
mechanization, incomplete sealing of equipment, and inefficient
local exhaust ventilation, concentrations of dust during these
operations ranged from 4.9 to 48.9 mg/m3.
In the production of ferrovanadium, there is a continuous
source of discharge of vanadium pentoxide and lower oxides
during the smelting process. Data on vanadium in air at various
sites are shown in Table 14.
Using spectrophotometric techniques, Roshchin (1968),
Katayeva & Sapunov (1974) and Kazimov (1977) found high
concentrations of vanadium during smelting and granulation
(range, 0.16 - 1.89 mg/m3; mean, 0.59 mg/m3; 104 samples),
production of ferrovanadium (range, 0.58 - 4.81 mg/m3; mean,
1.7 mg/m3; 110 samples), and roasting of the charge (range,
0.44 - 3.64 mg/m3; mean, 1.52 mg/m3; 112 samples).
Table 14. Vanadium levels in the air of a ferrovanadium planta
--------------------------------------------------------------------
Work-place/operations Vanadium pentoxide Lower oxides of
(mg/m3) vanadium (mg/m3)
--------------------------------------------------------------------
Work area of smelters and 0.1 - 2.6 0.05 - 1.2
helpers
Unloading of vanadium pent- 2 - 124.6
oxide from the bin and
charging of electric furnace
Crane driver's cabin during 0.07 - 9.43 0.03 - 0.1
smelting
Cutting up ferrovanadium 0.97 - 12.6
Maintenance of the furnace 7.5 - 30
--------------------------------------------------------------------
a From: Roshchin (1968).
Using high-volume sampling and atomic absorption analysis,
Usutani et al. (1979) measured vanadium pentoxide
concentrations in the air at several places in a vanadium
refinery. The highest concentrations (higher than 1 mg/m3) were
detected in samples collected during the removal of the vanadium
pentoxide flake. High-volume samples from other locations as
well as low-volume samples obtained over 6.5-h work shifts
showed lower concentrations (0.002 - 0.735 mg/m3).
When ductile vanadium is produced by the aluminothermic
process (based on the reduction of pure vanadium pentoxide with
aluminium powder), the violent exothermic reaction leads to the
release of a condensation aerosol of vanadium pentoxide. During
preparation of the charge mixture, work-place concentrations of
vanadium pentoxide ranged from 19 to 25.1 mg/m3. When the
burden was placed in crucibles inside the smelting chambers,
concentrations ranged from 64 to 240 mg/m3. During smelting,
concentrations at the operators' workplaces ranged from 0.17 to
0.6 mg/m3. Twenty to 30 min after smelting, levels declined to
0 -0.3 mg/m3. Ninety-eight percent of the condensation aerosol
particles produced had a diameter of less than 5 µm, and 82%
had a diameter of less than 2 µm (Roshchin, 1968).
In the production of vanadium by the vacuum carbon thermic
method, most of the pollution occurs during operations with
vanadium trioxide (Roshchin, 1968). Mixing of vanadium trioxide
in a closed mixer led to air concentrations in the workplace of
from 0.019 to 0.58 mg/m3. Unloading of the charge resulted in
high concentrations of 14.7 - 29.4 mg/m3. In the packing
department, when the charge was sifted in a fume cupboard,
concentrations in the breathing zone ranged from 0.58 to
4.7 mg/m3. When the charge was being weighed out and packed in
a fume cupboard, concentrations ranged from 3.38 to 6.76 mg/m3.
Levels of vanadium-containing dust and vanadium pentoxide in
the air during catalyst production are shown in Table 15.
Table 15. Air contamination in vanadium catalyst productiona
-----------------------------------------------------------------------------
Operation Dust (mg/m3) Vanadium pentoxide (mg/m3)
Minimum Maximum Most Minimum Maximum Most
frequent frequent
-----------------------------------------------------------------------------
Grinding and un- 5 45 7 - 9 1 7 1.5 - 3
loading vanadium
pentoxide
Loading ground 12 53 14 - 17 3.2 7.5 4 - 4.2
pentoxide into
the bin
Sifting and pack- 5 17.5 5 - 7 0.1 1 0.4 - 0.5
ing granules of
bulk contact
substance
-----------------------------------------------------------------------------
a From: Roshchin (1968).
4.2.2 Cleaning of oil-fired boilers
Significant occupational exposure to vanadium occurs during
the cleaning of boilers in oil-fired heating and power plants
and ships (Symanski, 1939; Roshchin, 1968; Kuzelova et al.,
1977; Levy et al., 1984). Fuel oil combustion results in the
formation of vanadium-containing dust, and large amounts of dust
result from operations connected with removing ash encrustations
in boiler cleaning and in cleaning the blades of gas turbines.
Most of these operations are carried out by hand, and the dust
in the air inside the boilers may range from 20 to 400 mg/m3,
the most common range being 50 - 100 mg/m3, with the dust
containing 5 - 17% vanadium pentoxide and from 3 to 10% of the
lower vanadium oxides (Roshchin, 1968). Kuzelova et al. (1977)
reported dust concentrations of 136 - 36 036 ml/m3 in the air
with vanadium concentrations ranging between 1.7 and 18.4
mg/m3.
Williams (1952) published air sampling data on boiler-
cleaning operations in the British power industry. He found
concentrations of soot dust at different points ranging from 239
to 659 mg/m3. The vanadium concentrations in the dust of the
superheater chamber was 40.2 mg/m3, while, in the combustion
chamber, the concentration was 58.6 mg/m3. Most (93.6%) of the
dust particles had a diameter of between 0.15 and 1 µm.
4.2.3 Occupational exposure limits
Some national occupational exposure limits for vanadium in
work-place air are shown in Table 16.
Table 16. Examples of occupational exposure limits for vanadium in various countries
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Australia Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Belgium Recommendation Time-weighted average (TWA) (fume) 0.05 ILO (1980)
Czechoslovakia Regulatory requirement Maximum allowable concentration (MAC) Hygienic Regula-
- Time-weighted average (TWA) 0.1 tions of the
- Ceiling value (fume) 0.3 Ministry of Health
- Ceiling value (dust) 1.5 of CSR (1985) (58)
(Regulation No. 7;
section 24/ZB)
Finland Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA) (fume)a 0.05
German Democratic Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1980)
Republic Short-term exposure limit (STEL)a 0.1
(fume)
Germany, Federal Recommendation 8-h time-weighted average (TWA)a 0.5 Federal Republic
Republic of of Germany Comm-
Short-term exposure limit (STEL)a 2.5 ision for Maximum
(30 min, 2x/shift) Work-Place Con-
centrations
8-h time-weighted average (TWA)a 0.1 (1985) (xxi, 16)
(fume)
Recommendation Short-term exposure limit (STEL)a 0.5
(30 min, 2x/shift) (fume)
Italy Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1985)
Hungary Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1984)
Netherlands Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Romania Regulatory requirement Ceiling value (fume)a 0.1 ILO (1980)
Sweden Regulatory requirement Ceiling valuea 0 ILO (1980)
Switzerland Regulatory requirement Time-weighted average (TWA)a 0.09 Permitted Values
in the Work-Place,
Berne (1984)
USA Recommendation Time-weighted average (TWA)a 0.05 ACGIH (1986)
(dust and fume)
Time-weighted average (TWA)a 0.5 OSHA (1977) (29
(dust) CFR.1910.1000)
Ceiling value (15 m)a 0.05 NIOSH (1977)
(dust and fume)
Time-weighted average (TWA)a 0.1 OSHA (1977) (29
(fume) CFR.1910.1000)
USSR Regulatory requirement Time-weighted average (TWA)a 0.1 ILO (1980)
Yugoslavia Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA)a 0.1
---------------------------------------------------------------------------------------------------------
a Measured as V2O5.
5. KINETICS AND METABOLISM
5.1 Physiological Role
5.1.1 Microorganisms
Vanadium is essential for the mould Aspergillus niger
(Bertrand, 1942) and the green alga Scenedesmus obliguus (Arnon
& Wessel, 1953; Arnon, 1958). It may play a role in photo-
synthesis in the latter. A need for vanadium has been shown by
the yeast Candida slooffii at high temperatures (Roitman et al.,
1969). The growth effects of vanadium in Azotobacter and other
bacteria have been related to the ability of vanadium, in lieu
of molybdenum, to catalyse nitrogen fixation reactions (Horner
et al., 1942; Takahashi & Nason, 1957).
5.1.2 Animals
Vanadium deficiency has been reported in chicks (Hopkins &
Mohr 1971a; Nielsen & Ollerich, 1973) and rats (Schwarz & Milne,
1971; Strasia, 1971), and vanadium is considered essential for
these animals (Underwood, 1977; Vouk, 1979; Nechay, 1984).
Hopkins & Mohr (1974) observed reduced feather growth in
chickens. They also reported impaired reproduction, due to
decreased fertility, and increased perinatal mortality in rats
fed a low vanadium diet (10 µg/kg) over 4 generations. A
positive growth response in rats was observed by Schwarz & Milne
(1971) when vanadium salts, at levels of between 50 and
500 µg/kg were added to a semi-purified diet (vanadium content
unspecified) fed for 26 - 28 days.
In male Leghorn chicks fed vanadium in the diet at levels of
12.5 mg/kg and 25 mg/kg, body-weight gain was normal at 1, 2, 3,
and 4 weeks of age (Kubena et al., 1986).
Slower growth, higher haematocrits, and higher levels of
plasma- and bone-iron were reported by Strasia (1971) in rats
fed diets containing less than 100 µg vanadium/kg compared with
controls receiving diets containing 500 µg vanadium/kg, but
these results were not confirmed in another study by Williams
(1973). Hopkins & Mohr (1971a,b, 1974) reported that a diet
containing less than 10 µg vanadium/kg decreased plasma-
cholesterol levels in chicks at 28 days of age, increased
plasma-cholesterol levels at 49 days of age, and increased
plasma-triglyceride levels at 28 days of age. Nielsen &
Ollerich (1973) found that administration of a diet containing
30 - 35 µg vanadium/kg to chicks decreased growth, increased
haematocrits and plasma-cholesterol levels, and impaired bone
development.
Nielsen (1980) carried out further studies on chicks and
rats, given different types of low-vanadium experimental diets,
to examine the effects of vanadium deficiency. In rats,
vanadium deficiency adversely affected prenatal survival,
growth, physical appearance, haematocrit, plasma-cholesterol
levels, and hepatic lipid and phospholipid levels. In chicks,
low vanadium intake produced adverse effects on growth,
feathering, haematocrit, bone development, plasma-cholesterol
levels, and hepatic lipid, phospholipid, and cholesterol levels.
However, consistent deficiency effects were not observed in
chicks or rats in any of the studies. Nielsen (1980) suggested
that the inconsistency in vanadium deficiency effects might be
due to the fact that different experimental diets had different
effects on the metabolism of vanadium.
Vanadate appears to have an insulin-like action (Heyliger et
al., 1985). In female Wistar rats made diabetic with
streptozotocin (single iv injection of 55 mg/kg) and given
sodium orthovanadate in the drinking-water at a concentration of
0.6 - 0.8 mg/ml (corresponding to calculated daily intakes of
between 75 ± 3 and 100 ± 3 mg/kg body weight per day,
respectively) for 4 weeks, the serum-insulin level was low, but
there was no increase in blood-glucose levels compared with
controls. In diabetic rats not treated with vanadate, serum-
insulin levels were also low, but blood-glucose levels were
increased 3-fold. The cardiac performance of vanadate-treated
animals did not differ significantly from that of non-diabetic
controls. It was concluded that vanadate controlled blood-
glucose levels and prevented the decline in cardiac performance
due to diabetes.
The results of in vitro studies suggest that vanadium may
play a specific physiological role as a regulator of the sodium
pump (Macara, 1980). Vanadate has been shown to inhibit Na+K+-
ATPase (EC 3.6.1.3) in intact human red blood cells (Rifkin,
1965; Cantley et al., 1977, 1978a,b). It also has potent
diuretic and natriuretic effects in rats (Balfour et al., 1978;
Westenfelder et al. 1981). It was a powerful inhibitor of
Na+K+-ATPase in microsomal fractions of the kidney, brain, and
heart in several species, including human beings (kidney).
Mg2+-ATPase was up to 10 000 times more resistant to vanadium
inhibition than Na+K+-ATPase (Nechay & Saunders, 1978).
Similarly, in vivo studies on laying chickens fed calcium
orthovanadate for 15 months, at levels of 0.25, 50, or 100 mg/kg
diet, showed clear inhibition of Na+K+-ATPase activity in the
kidney (Phillips et al., 1982). The inhibition of Na+K+-ATPase
by vanadate can be reversed by catecholamines, though these do
not have any effects on the Na+K+-ATPase activity in the absence
of vanadate (Hudgins & Bond, 1979). The inhibition is also
partially prevented by the reducing agents ascorbic acid and
glutathione (Grantham & Glynn, 1979).
The mechanism by which cells reduce cytoplasmic vanadium5+
to vanadium4+ was investigated using human red cells (Macara et
al., 1980). The authors concluded that vanadate is reduced by
cytoplasmic glutathione and that this explains the resistance of
the Na++K+-ATPase to vanadium in intact cells.
Since vanadate (V5+) is a potent inhibitor of Na+K+-ATPase,
many physiological and biochemical processes are vanadium
sensitive (Grantham, 1980; Nechay, 1984). For example, vanadium
compounds inhibit ATP phosphohydrolases, ribonuclease, adenylate
kinase, phosphofructokinase, squalene synthetase, glycer-
aldehyde-3-phosphate dehydrogenase (Macara, 1980), glucose-6-
phosphatase (Singh et al., 1981), and phosphotyrosyl-protein-
phosphatase (Swarup et al., 1982). The membrane neutral (K+)-
p-nitrophenylphosphatase in the membrane fraction of skeletal
muscle was inhibited equally by vanadium4+ and vanadium5+ at 4 x
10-8 mol/litre (Vyskocil et al., 1981). Extracellular
application of both forms of vanadium failed to inhibit the
electrogenic (Na+ - K+) pump in intact mouse diaphragm fibres
(Vyskocil et al., 1981). In the concentration range from 10-5 to
10-3 mol/litre, vanadium4+ even potentiated the hyper-
polarization of the muscle fibres from -74 to -82 mV (Zemkova et
al., 1982), probably by increasing the intracellular potassium
level. These findings have led to the hypothesis that vanadate
could control sodium pump activity in vivo , perhaps via a
vanadium5+ : vanadium4+ equilibrium connecting pump activity to
the cellular redox state. However, vanadate in the red cell is
reduced to vanadium4+, which then binds to haemoglobin (Cantley
& Aisen, 1979), a reaction that seems to be essentially
quantitatively driven by glutathione (Macara et al., 1980), NADH
(Vyskocil et al., 1980), or by other mild reducing agents, such
as ascorbate or norepinephrine (Svoboda et al., 1984). The role
of the vanadium5+ : vanadium4+ redox equilibrium in the
regulation of cation flow across cell membranes has yet to be
unequivocally demonstrated.
Vanadium appears to be essential for chicks and rats, but it
does not appear to be essential in other species, as defined by
Mertz (1970), i.e., an element is essential if its deficiency
reproducibly results in an impairment of a function from optimal
to suboptimal. However, more research is needed before definite
conclusions can be drawn regarding the role of vanadium as a
nutritionally essential trace element for animals.
5.2 Absorption
The absorption and distribution of vanadium compounds depend
on the route of entry and the solubility of the compounds in
body fluids. The solubility of vanadium compounds in biological
media varies (Reznik, 1954). The following compounds are listed
in decreasing order of solubility: (a) in gastric juices,
vanadyl sulfate, sodium vanadate, ammonium vanadate, vanadium
pentoxide; (b) in blood-serum and in 0.22% sodium carbonate
solution, sodium vanadate, ammonium vanadate, vanadium
pentoxide, and vanadyl sulfate. The higher the solubility in
water and biological media, the more toxic the compound,
presumably because of better absorption (Roshchin, 1968).
5.2.1 Absorption by inhalation
5.2.1.1 Human studies
There is little information on the deposition of vanadium
compounds in the respiratory tract following inhalation.
However, the greatest deposition would be expected in the
submicrometre particle size fraction and particle size
distribution studies (Lee et al., 1972) have shown that most
vanadium-bearing particulate matter is very small and well
within the respirable range for human beings.
Soluble vanadium compounds inhaled and deposited in the
lung, are readily absorbed but the rates of absorption have not
been quantified and estimates have not been made of the amounts
of inhaled vanadium that are transported back to the pharynx by
mucociliary clearance, swallowed, and are then available for
absorption via the gastrointestinal tract. It has been
estimated that about 25% of soluble vanadium compounds may be
absorbed via the respiratory tract (ICRP, 1960). Absorption
from the respiratory tract was demonstrated in workers exposed
to vanadium dust, who showed increased concentrations of
vanadium in the urine (Lewis, 1959b; Gylseth et al., 1979;
Maroni et al., 1983).
5.2.1.2 Animal studies
Following acute exposure, there is complete clearance of the
relatively soluble vanadium pentoxide from the lung within 1 -
3 days (Sjöberg, 1950; Levina, 1972). Stokinger et al (1953)
demonstrated that vanadium is present for more than 40 days
following cessation of long-term exposure.
Intratracheal administration of 48V-vanadium nitrate (0.4
and 20 mg/kg body weight) to albino rats showed that the 48V
absorption rate was maximum after 5 min and could be detected in
internal organs after 30 min. Blood levels were initially high,
but fell to trace levels after 2 days. 48V was not detectable
after 4 days, but reappeared at 8 days and accumulated in all
internal organs, the greatest quantity accumulating in the bone
(Ordzhonikidze, 1977). In female Fischer rats exposed by intra-
tracheal instillation to 40 µg vanadium pentoxide in 0.9%
saline solution, the time for removal of 50% of the initial
burden was 18 min, but traces remained for a considerable time.
At 14 days, the vanadium was distributed principally in the
carcass (40%) and skeleton (12%) (Rhoads & Sanders, 1985). In
a study of the kinetics of vanadium following single or multiple
intratracheal administration, the blood concentration was high
initially and vanadium accumulated in the liver and kidney
reaching the highest level after 24 h (Roshchin & Ordzhonikidze,
1986).
Vanadium trioxide was cleared from the lung more rapidly
than pentoxide or ammonium vanadate following intratracheal
instillation in rats (Levina, 1972).
5.2.2 Absorption from the gastrointestinal tract
5.2.2.1 Human studies
In general, vanadium salts are poorly absorbed from the
human gastrointestinal tract. In a study by Curran et al.
(1959), from 0.1 to 1% of 100 mg vanadium (as highly soluble
diammonium oxytartratovanadate) was absorbed from the
gastrointestinal tract and 60% of this was excreted via the
kidneys within 24 h. The remainder was retained in the liver
and bone, until the oral administration ceased, when it was
mobilized rapidly from the liver and slowly from the bone.
Sodium metavanadate (12.5 mg/day for 12 days) was recovered
largely unabsorbed in the faeces (87.6%) and the remainder in
urine (12.4%) (Proescher et al., 1917). The International
Commission on Radiological Protection (ICRP, 1960) estimate for
the gastrointestinal absorption of soluble vanadium compounds
was 2%. A low degree of absorption was also found by Roshchin
et al. (1980).
5.2.2.2 Animal studies
Mountain (1959)a reported an unpublished study in which
vanadyl sulfate was fed to adult male rats in daily doses
ranging from 650 to 1250 µg (160 - 310 µg of vanadium). The
mean absorption was about 0.5%, but urinary values varied
considerably. The duration of the study was not given.
5.2.3 Absorption through the skin
Dermal absorption and skin irritation were reported in a
study in which a nearly saturated solution (20%) of sodium
metavanadate was applied to the skin of the rabbit (Stokinger,
1967).
However, according to US EPA (1977), the skin appears to be
a minor route of vanadium uptake for human beings. In an in
vitro study using 48V radiotracer, there was no penetration of
human skin samples (Roshchin, 1980).
5.3 Distribution and Transformation
5.3.1 Human studies
Vanadium levels in man, reported in earlier studies, were
considerably higher than those reported more recently. The
difference is illustrated by the whole-body content of 17 -
43 mg vanadium for a 70-kg man calculated by Schroeder (1963)
compared with estimates of 100 µg derived by Byrne & Kosta
(1978). The influence of different sampling procedures and
analysis (colorimetric determination, neutron activation) should
be clarified before concluding that such effects are real
(Lagerkvist et al., 1986) or that they reflect decreasing
environmental exposure.
__________________
a Mountain, J.T. (1959) Unpublished results, Toxicologic
Services, Occupational Health Field Headquarters,
Cincinnati, Ohio.
Absorbed vanadium is transported mainly in the plasma
(Schroeder et al. 1963; Ordhzonikidze et al., 1977; Roshchin et
al., 1980). Some mean values obtained using different methods
of analysis are given in Table 17. There is a wide divergence
in the results, the ratio between the highest and the lowest
mean values being about 104. In general, levels tend to decline
chronologically as analytical techniques become more sensitive.
There is also a difference in results obtained by neutron
activation analysis according to whether separation was carried
out before, or after, irradiation.
In an extensive investigation on human tissues, the
inadequate sensitivity of the analytical method meant that
quantitative information could only be obtained for the lung
and intestine (Tipton & Cook, 1963). Using neutron activation
analysis, Byrne & Kosta (1978) obtained information on other
organs. Table 18 includes autopsy tissue values and some
results from two other investigations using neutron activation
analysis. It is apparent that vanadium concentrations are low
in all tissues, though the liver, kidney, and lung often show
higher levels than other tissues. In another investigation
on a selection of organs, using spark-source mass spectrometry,
the vanadium levels detected were: brain, 30 µg vanadium/kg
wet weight (average from 10 specimens); liver, 40 µg/kg
(average from 11 specimens); lung, 100 µg/kg (average from 11
specimens); lymph node, 400 µg/kg (average from 6 specimens);
and testis, 20 µg/kg (average from 5 specimens) (Hamilton et
al., 1972/73). The values reported are in reasonable agreement
with those found in other animals. However, there is
considerable disagreement between investigators and between
analytical methods, which has not been resolved, since
interlaboratory and intermethod investigations have not been
carried out.
In the general population, which is mainly exposed to low
levels of vanadium in food with poor absorption from the
intestine, vanadium is usually undetectable in the urine, even
using very sensitive methods (Byrne & Kosta, 1978). Examination
of urine samples from 50 normal individuals using atomic
absorption spectrometry showed that vanadium was present in only
13; 11 samples showed a level of 0.1 µg/litre and two,
0.2 µg/litre (Ueno & Ishizaki, 1980). In industry, where
exposure is mainly through air and absorption from the lung is
high, vanadium concentrations in the urine cover a considerable
range (section 5.4.1).
Table 17. Vanadium levels in human blooda,b
---------------------------------------------------------------------------------------------------------
Analytical method Whole blood Serum/plasma Reference
(mg/litre) (mg/litre)
---------------------------------------------------------------------------------------------------------
X-ray emission 0.01 (43) Gofman (1962)
Spectrography 0.0078 (24) Lifschitz (1962)
Colorimetry 0.23 (calculated) 0.42 (13) Schroeder et al. (1963)
Neutron activation analy- 0.016 Bowen (1963)
sis (preseparated)
Neutron activation analysis 0.67 ± 0.32 ng/ml Simonoff et al. (1986)
Spectrography 0.126 (47) Butt et al. (1964)
Neutron activation analy- 0.0046 (36) Heydorn & Lukens (1966)
sis (preseparated)
Catalytic 0.01 (82) Allaway (1968)
0.02 - 0.01 (7)
Spectrophotometric 0.057 (10) Christian (1971)
Neutron activation analy- 0.0046 Kirzhner et al. (1974)
sis (preseparated)
Neutron activation analy- 0.022 (5) Buono et al. (1977)
sis (preseparated)
Neutron activation analy- 0.0005 (5) Byrne & Kosta (1978)
sis (preseparated)
Neutron activation analy-
sis (preseparated) 0.0066c (5) Sabbioni et al. (1979)
Neutron activation analysis 0.000047 (9 males) Cornelis et al. (1979)
0.000024 (8 females) Cornelis et al. (1979)
Neutron activation analy-
sis 0.000033 (17 females) Cornelis et al. (1980)
--------------------------------------------------------------------------------------------------------
a Adapted from: Byrne & Kosta (1978) and Versieck & Cornelis (1980).
b Number of subjects in parentheses.
c Recalculated values assuming an haematocrit of 0.45.
Table 18. Vanadium levels in human organs (µg/kg wet weight)a
-----------------------------------------------------------------------------
Tissue Study
-------------------------------------------------------------------
Byrne & Kosta (1978)b,c Damsgaard (1972)c,d Lievens (1977)b
-----------------------------------------------------------------------------
Kidney 3.3 3.2 2.6 nd 7
Liver 7.5 4.5 5 19 7 - 19 (5)
Brain 0.7 0.75
Thyroid 3.2 3.0
Heart 1.1
Cardiac 0.45 0.3
fat
Subcut- 0.63 0.80
aneous
fat
Muscle 0.45 0.62 0-59 7 nd
Spleen 3 4
Pancreas nd 14
Lung 19 - 40 (7) nd 13
median 30
-----------------------------------------------------------------------------
a From: Byrne & Kosta (1978).
b Parentheses enclose number of subjects.
c Brackets embrace specimens from one autopsy.
d nd = not detected.
Studies on vanadium levels in human bone have produced
widely varying results. Byrne & Kosta (1978) reported the
following data from human bones (lg/kg wet weight): skull,
2.5, 3, 4.5, 8.3; sternum, 3.1; rib, 0.8, 2.1; tooth enamel
(fragmented), 2, 3.4, 4, 5.1; and tooth enamel (drill powdered),
18. The high concentration in tooth enamel may be due to diet;
concentrations of vanadium tend to be higher in processed than
in unprocessed foods (Myron et al., 1977), but vanadium is
commonly present in steel alloys, especially tool steels, and
vanadium contamination may have occurred from the drill. Using
atomic absorption spectroscopy, Sumino et al. (1975) found a
range of 100 - 200 µg/kg (wet weight) in 6 specimens of rib.
Using emission spectral analysis, Shevchenko (1965) detected a
mean vanadium level of 150 ± 2 µg/kg (0.015 mg%) (dry weight)
in 14 samples of healthy bone tissue; bone tumours contained
higher levels.
Metal levels have been extensively studied in hair, because
of its potential value for exposure and body burden studies.
Gordus et al. (1974) and Gibson & DeWolfe (1979), using the same
method as Byrne & Kosta (1978), found levels of 20 - 40 µg/kg
in 42 subjects and 20 - 41 µg/kg in 370 subjects, respectively.
These results compare favourably with those of Byrne & Kosta,
i.e., 12 - 87 µg/kg in 12 subjects. Ueno & Ishizaki (1980)
used atomic absorption spectrometry to determine vanadium in
hair specimens from 130 men and 132 women. They found mean
concentrations of 53.6 µg/kg (range, 5 - 155 µg/kg) and
44.2 µg/kg (range, 1.8 - 118.8 µg/kg), respectively. However,
Creason et al. (1975), using emission spectroscopy in extensive
surveys in the USA, found levels that were about 10 times
higher.
There is considerable information on vanadium levels in lung
tissue. In studies by Schroeder et al. (1963), vanadium was
found in 97 out of 173 lungs examined in groups from 7 USA
cities, group mean concentrations ranging from 10 to 130 µg/kg
wet weight. Byrne & Kosta (1978) found levels of from 19 to
140 µg/kg (median, 30 µg/kg) in 7 cases; Hamilton et al.
(1972/73) detected a mean level of 100 µg/kg in 11 cases; and
Sumino et al. (1975) determined a range of 100 - 300 µg/kg for
13 observations. Statistical analyses of lung tissue trace
metal data were performed by Tipton & Shafer (1964) using data
taken from Tipton & Cook (1963). One approach was examination
of the relationship between lung metal concentrations and age.
The levels of a number of metals, including vanadium, increased
in the lung with age, indicating accumulation of insoluble
compounds, and Schroeder (1970b) calculated an annual increment
rate of 1.3 µg for lung-vanadium. However, earlier analysis of
the same data (Schroeder et al., 1963) did not show an age-
related increase in lung-vanadium. The later analysis by Tipton
& Shafer (1964) was performed on a subset of the original data
from Tipton & Cook (1963), which may account for some of the
discrepancy. The Tipton & Shafer (1964) data did not show a
graded increase in lung-vanadium with age, and there was a high
mean level only in the oldest age group. This could have been
produced by a single very high value in this age group. The
concept of vanadium accumulation in the human lung with age
remains doubtful, and the results of animal studies do not
indicate significant accumulation in the lung (sections 5.1.1.2
and 5.3.2).
5.3.2 Animal studies
Observations on pregnant rats, that received vanadium on the
21st - 22nd day of pregnancy, revealed accumulation in the
placenta but not "in perceivable quantities" in the organs of
the fetus. Vanadium was reported to be secreted in the milk
(Roshchin et al., 1980).
The identification of cellular components that react with
vanadium has been investigated in vivo and in vitro using 48V
radiotracer (Marafante & Sabbioni, 1983). Vanadium has a high
affinity for nuclear and mitochondrial components. When rats
were treated every day with 10 µg vanadium/rat for up to 8
days, a dose-related increase in vanadium incorporation in the
subcellular fractions was observed. There is evidence that non-
haem Fe-containing proteins, such as transferrin and ferritin,
have a high affinity for vanadium, while Fe haemoproteins are
not able to incorporate the metal.
In rat serum, both Vanadium4+ and Vanadium5+ form metal-
protein complexes with transferrins. Specific intracellular
vanadyl-ferritin complexes are formed in rat liver, spleen, and
kidney (Chasteen et al., 1986).
Ermolaev (1969) studied the distribution of vanadium in the
organs and tissues of 3 groups of rabbits, i.e., animals fed
under ordinary conditions, animals fed a diet supplemented with
vanadium (0.5% solution of vanadyl sulfate) at a dose of
0.05 mg/kg body weight, and animals fed the vanadium-
supplemented diet and subcutaneously injected with vanadium
also at 0.05 mg/kg body weight. The results of this
investigation are given in Table 19. The method of
administration had little effect on the resultant blood, brain,
and stomach levels, but there were distinct differences in the
case of the other organs and tissues.
The distribution and kinetics of vanadium, administered ip
as 80 mg vanadocene dichloride/kg to strain A mice, were
determined in blood, kidney, liver, small intestine, and brain.
The vanadium concentration decreased rapidly and exponentially
in the blood (half-time = 118 ± 43 min) and small intestine
(half-time a = 18 ± 0.14 min; half-time b = 341 ± 45 min).
Vanadium accumulated in kidney (maximum concentration,
1.12 ± 0.06 mmol at 12 h) and liver (maximum concentration,
0.56 ± 0.06 mmol at 8 h) and was then excreted (estimated half-
time, 7.9 ± 0.7 h for kidney; 12.1 ± 0.1 h for liver). Vanadium
was not detected in the brain (Toney et al., 1985).
Table 19. Vanadium levels in rabbit organs and tissues following
oral and subcutaneous administration (mg %)a
-----------------------------------------------------------------------------
Organ/ Control group First group Second group
tissue (0.05 mg/kg (0.05 mg/kg with
with food) food and injected
subcutaneously)
-----------------------------------------------------------------------------
liver 66 88 632
muscle tissue 27 41 30
blood 29 82 90
spleen 68 116 180
kidneys 71 90 464
lungs 62 82 168
brain 20 26 24
heart muscle 52 68 71
intestine 20 22 32
stomach 30 70 74
-----------------------------------------------------------------------------
a From: Ermolaev (1969).
The influence of the oxidation state of intravenously
injected compounds of 48V on uptake and distribution to selected
organs and subcellular elements of the rat liver was studied by
Hopkins & Tilton (1966). They did not observe any significant
differences in the rate or amount of uptake of nanogram
quantities of vanadium in three different oxidation states
(VOC13, VOC12, and VC13) and concluded that either the oxidation
state was not critical to transport or that the vanadium was
converted to a common oxidation state in vivo. Similar results
with respect to oxidation state and uptake and also the
distribution of vanadium in the rat were reported by Sabbioni et
al. (1978) and Conklin et al. (1982). In contrast, Parker &
Sharma (1978) found that levels of vanadium in the tissues of
male Wistar rats given sodium orthovanadate in the drinking-
water at 50 mg/litre for 3 months were higher than those in the
tissues of animals given vanadyl sulfate at the same
concentration. Roshchin et al. (1964) found evidence of the
partial conversion of vanadium trioxide to the pentavalent form
in blood-serum and in weakly acidic and basic solutions in
vitro. In a later study, Johnson et al. (1974) reported
the in vivo conversion of vanadium pentoxide to the tetravalent
state.
Information on vanadium distribution in rats after intra-
peritoneal, intratracheal, oral, and subcutaneous administration
of radioactive (48V) vanadium nitrate in single doses of
20 mg/kg body weight (LD50) and 0.4 mg/kg body weight (non-toxic
dose) is given in Table 20 (Roshchin et al., 1980). The study,
which involved 1060 albino rats, showed that whatever the method
of administration, vanadium was present in blood in significant
quantities only during the first 24 h, and that, after 2 days,
only traces of vanadium were detectable. No vanadium was found
in the blood after 4 - 8 days. However, in the groups
administered the radioactive compound intratracheally or
subcutaneously, low amounts of vanadium were detected between 8
and 16 days after administration as a result of reabsorption
from the organs. The highest level occurred 5 min after intra-
tracheal administration, 30 min after intraperitoneal admini-
stration, and 15 min - 1 h after subcutaneous or intragastric
administration. Vanadium was detected in all tissues and
organs. In 2 days, vanadium had accumulated in the bone, kidney,
liver, and lung, which were also the primary targets in rats
after intratracheal administration of vanadium pentoxide
(48V2O5) or chloride (48VO2C1) (Conklin et al., 1982) and after
oral administration of vanadyl sulfate or sodium orthovanadate
in the drinking-water (50 mg/litre) for three months (Parker &
Sharma, 1978).
In another study, 48VOCl3, which is fairly soluble, was
administered intratracheally to juvenile male Wistar rats at a
dose of 12.6 µCi in 1 ml buffered solution. Within 15 min, the
vanadium isotope was found in all major organs, except the
brain. The largest fractions were found in the blood, heart,
spleen, liver, and kidneys. Peak uptake occurred between 4 and
24 h after administration and activity was maintained throughout
a 9-week period (Oberg et al., 1978).
5.4 Retention
5.4.1 Human studies
Vanadium levels in human tissues (Table 18, section 5.3.1)
are low, the highest concentrations tending to occur in the
liver, kidney, and lung.
Storage of available vanadium in man occurs mainly in fat
and serum lipids (Schroeder et al., 1963).
Shevchenko (1965) used emission spectral analysis to
determine the vanadium contents of bone tumours and of the
cortical layer of bone adjacent to the tumours. He found that
vanadium accumulated in tumours. Healthy bone tissue contained
a mean vanadium concentration of 0.15 ± 0.002 mg/kg dry weight,
osteoblastomas contained 6.38 ± 1.17 mg/kg, and osteosarcomas,
4.16 ± 0.77 mg/kg. The cortical layer adjacent to
osteoblastomas contained 2.01 ± 0.42 mg/kg, and that adjacent to
osteosarcomas contained 1.77 ± 0.48 mg/kg. In comparison to
normal bone, bone cysts, osteochondromas, and exostoses also
showed higher vanadium levels, but to a lesser extent than bone
tumours.
The significant accumulation of vanadium in the tissue of
benign and malignant tumours and, to a lesser extent, in the
neighbouring tissue suggests that quantitative changes in the
amounts observed may indicate disturbances in its metabolism.
This may be in connection with the role of vanadium in the
phospholipid and cholesterol metabolism, which may become
indirect indicators of the intensity of the phospholipid
turnover in tumour tissue.
5.4.2 Animal studies
Large amounts of vanadium were reported in the crude fat
from beef, pork, and lamb (Schroeder et al., 1963), but the
results of subsequent studies do not support this finding, which
is probably erroneous because of the analytical methods used
(NRC, 1980).
In rats, after oral administration of 20 mg vanadium
nitrate/kg body weight, levels of vanadium in the blood were
detectable only during the first 24 h, and only traces remained
after 48 h; after 4 days, no vanadium was found in blood. For
48 h after the administration of vanadium, concentrations in the
organs increased by 0.4% of the administered dose in the liver,
by 0.69% in the kidneys, and by 0.16% in the spleen. The most
significant increase in vanadium (1.01% of the dose
administered) was found in the bones. After 16 days, the level
in the bone tissue had increased by 1.72% of the dose
administered, while in other organs it had significantly
decreased (Roshchin et al., 1980).
Studies on rats showed that liver, kidney, spleen, and
testes continued to accumulate intravenously injected vanadium-
48 for up to 4 h and that most of the radioactivity was retained
for up to 96 h (Hopkins & Tilton, 1966) . After 96 h, 14 - 84%
of the 10-min uptake was retained in other organs, and 46% and
9% of the vanadium-48 had been eliminated in the urine and
faeces, respectively. Levels of vanadium-48 in the
mitochondrial and nuclear fractions of homogenized liver
increased from 14 to 40% of the total during the first 96 h,
while the level of radioactivity in the microsomal fraction
remained relatively constant.
When strain A mice were given 80 mg vanadocene dichloride/kg
ip, vanadium accumulated in the liver and kidney. Maximum
concentrations of 1.12 ± 0.06 mmol in the kidney and 0.56 ±
0.06 mmol in the liver were reached after 12 h and 8 h,
respectively (Toney et al., 1985).
Table 20. Concentrations of 48vanadium in the organs and tissues of rats after intraperitoneal,
subcutaneous, intratracheal, and intragastric administration of a radioactive vanadium compound
(expressed as % radioactivity equivalent to 10 uCi per animal)a
---------------------------------------------------------------------------------------------------------
Organ/tissue Intraperitoneal administration Intratracheal administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 2.20 ± 0.55 0.43 ± 0.02 0.22 ± 0 0.43 ± 0.19 0.36 ± 0.19 1.25 ± 0.38
Kidney 7.20 ± 0.85 1.60 ± 0.02 0.66 ± 0 1.55 ± 0.67 1.56 ± 0.19 4.03 ± 0.74
Spleen 2.16 ± 0.23 0.17 ± 0.01 0.09 ± 0 0.15 ± 0.06 0.18 ± 0.02 1.92 ± 0.09
Lung 2.53 ± 0.73 0.11 ± 0 0 9.09 ± 5.91 0.20 ± 0.39 4.64 ± 2.11
Stomach 2.93 ± 0.73 0.08 ± 0 0 2.56 ± 0.94 0.13 ± 0.05 0
Small intestine 2.53 ± 0.31 0.08 ± 0 0 0.71 ± 0.22 0.21 ± 0.15 0
Large intestine 3.33 ± 0.88 0.13 ± 0.03 0 0.19 ± 0.08 0.17 ± 0.05 0.47 ± 0
Muscle 0.40 ± 0.05 0.03 ± 0 0 0.28 ± 0.20 0.03 ± 0 0
Bone 2.13 ± 0.12 3.45 ± 0.48 1.72 ± 0.31 0.79 ± 0.31 2.19 ± 0.31 9.70 ± 1.93
Testicle 1.30 ± 0.15 0.09 ± 0 0.06 ± 0 0.09 ± 0 0.08 ± 0 0.54 ± 0.08
Thyroid gland 0.60 ± 0 0.23 ± 0.05 0 1.21 ± 0.69 0.54 ± 0.40 0
Adrenals 2.53 ± 1.01 0.11 ± 0.02 0 0.67 ± 0 0.06 ± 0.02 0
Pancreas 7.88 ± 0.26 0.31 ± 0.06 0 0.14 ± 0.04 0.08 ± 0.02 0
Brain 0.16 ± 0.04 0.01 ± 0 0 0.02 ± 0 0 0
Heart 1.50 ± 0.34 0.07 ± 0 0 0.04 ± 0 0.08 ± 0 0.41 ± 0.15
---------------------------------------------------------------------------------------------------------
Table 20 (contd.)
---------------------------------------------------------------------------------------------------------
Organ/tissue Intragastric administration Subcutaneous administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 0.08 ± 0.03 0.33 ± 0.11 0.27 ± 0 0.05 ± 0.01 1.00 ± 0.15 1.67 ± 0.42
Kidney 0.17 ± 0.13 0.69 ± 0.20 0.67 ± 0 0.13 ± 0.03 2.30 ± 0 4.47 ± 1.48
Spleen 0.04 ± 0 0.16 ± 0.05 0.08 ± 0 0.03 ± 0 0.31 ± 0.03 1.38 ± 0.75
Lung 0.06 ± 0.02 0.09 ± 0.01 0.06 ± 0 0.10 ± 0.01 0.19 ± 0.01 0.89 ± 0.18
Stomach 8.08 ± 1.49 1.55 ± 0.05 0.05 ± 0 0.29 ± 0.01 0.12 ± 0 0.39 ± 0.06
Small intestine 4.65 ± 9.95 0.22 ± 0.09 0.06 ± 0 0.77 ± 0.66 0.11 ± 0.01 0
Large intestine 0.12 ± 0 0.14 ± 0 0.01 ± 0 0.46 ± 0.21 0.14 ± 0 0
Muscle 0.01 ± 0 0.02 ± 0 0.04 ± 0 0.40 ± 0.30 0.03 ± 0 0.46 ± 0.15
Bone 0.05 ± 0.02 1.01 ± 0.31 1.72 ± 0 0.43 ± 0.30 3.23 ± 0.41 1.86 ± 0.52
Testicle 0.26 ± 0.20 0.04 ± 0 0.06 ± 0 0.01 ± 0 0.12 ± 0.01 0.64 ± 0.07
Thyroid gland 0.08 ± 0 0.32 ± 0.24 0 0.15 ± 0.05 0 0
Adrenals - 0.09 ± 0.03 0 0.10 ± 0 0.16 ± 0.04 0.39 ± 0
Pancreas 0.32 ± 0.29 0.04 ± 0.04 0.08 ± 0 0.03 ± 0 0.09 ± 0.01 0.83 ± 0.04
Brain 0.02 ± 0 0.01 ± 0 0.02 ± 0 0.03 ± 0 0.02 ± 0 0
Heart 0.06 ± 0 0.05 ± 0 0 0.06 ± 0 0.12 ± 0 0.81 ± 0.08
---------------------------------------------------------------------------------------------------------
a From: Roshchin et al. (1980).
In a study on 2 young rats (strain unspecified), the highest
uptake of vanadium-48 from V205 occurred in rapidly mineralizing
areas of dentine and bone (Söremark et al., 1962). In mice,
high uptake occurred in the mammary glands, liver, renal cortex,
and lung (Söremark & Ullberg, 1962).
In studies by Belehova (1966, 1969), vanadium levels were
lower in carious than in normal canine teeth. In other studies,
intramuscular injection of vanadium in dogs at a dose of 2 mg/kg
body weight resulted in vanadium levels in the hard tissues of
the teeth that were 1.3 times higher than those in the controls.
On the 7th day, the concentration in the enamel had almost
returned to its initial level of 8.8 mg/kg; the level in the
dentine was still 32% higher.
Vanadium was reported to decrease the incidence of dental
caries, when added to the diet of hamsters (Geyer, 1953).
However, Hein & Wisotzky (1955) reported significant increases
in dental caries in hamsters given drinking-water containing
vanadium pentoxide equivalent to 10 mg vanadium/litre over an
80-day period. Muhler (1957) studied the effect of vanadium
pentoxide on caries in Sprague-Dawley rats. Groups of 50 rats
received vanadium (as vanadium pentoxide) at 10 mg/litre,
20 mg/litre, or 40 mg/litre in their drinking-water. One
control group received drinking-water containing ammonium
fluorosilicate (20 mg/litre), the other received untreated
water. Vanadium did not produce any reduction in the incidence
of dental caries, in fact there was a slight, but not
significant increase in dental caries in the groups receiving
10 mg/litre and 20 mg/litre for 140 days. All the rats showed
signs of vanadium toxicity and all animals in the highest dose
group (40 mg/litre) died within 65 days.
In an in vitro study on the effects of vanadate on bone
formation in 21-day-old fetal rat calvariae, sodium vanadate at
concentrations of 0.1 - 10 mmol stimulated the incorporation of
3H-thymidine into DNA and increased the bone DNA content and the
mitotic index. Sodium vanadate at a concentration of 100 mmol
produced a marked and irreversible inhibition of DNA labelling
and protein synthesis. Concentrations of 1 mmol (24 h) and
10 mmol (96 h) inhibited alkaline phosphatase activity. Sodium
vanadate also stimulated collagen and non-collagen protein at
low concentrations, but again had an irreversible inhibitory
effect at a high concentration of 100 mmol (Canalis, 1985).
5.5 Elimination
5.5.1 Human studies
Owing to low gastrointestinal absorption, ingested vanadium
is predominantly eliminated unabsorbed in the faeces. The
principle route of excretion of absorbed vanadium is through the
kidneys.
The relationship between urinary excretion and the extent of
exposure has been studied. As part of a clinical study on the
effects on cholesterol level, Dimond et al. (1963) gave ammonium
vanadyl tartrate to patients (5 female, 1 male) and did not find
any correlation between urinary excretion and oral dose.
Variable absorption was suggested as the reason for wide
variation in urinary excretion. In a 50-week study on 2
volunteers, Tipton et al. (1969) reported a urine/diet ratio of
0.13. This is in agreement with the figure of 12.4% excretion
in urine in a man given sodium metavanadate orally (12.5 mg
daily for 12 days) (Proescher et al., 1917).
Studies on occupationally exposed populations have shown a
poor correlation between vanadium concentrations in air and
amounts excreted in urine (Williams, 1952; Lewis, 1959b;
Jaraczewska & Jakubowski, 1964; Watanabe et al., 1966; Troppens,
1969; Köhler, 1972). Differences in laboratories and methods
may contribute to the wide range of urinary concentrations
reported.
In a study on power-station workers exposed to vanadium
during maintenance work on an oil-fired boiler, it was shown
that urinary excretion increased in those most heavily exposed,
in spite of the use of protective masks. For instance, urinary
levels of vanadium in 6 welders and 4 cleaners increased over a
work shift from 2.7 to 43.8 mg vanadium/kg creatinine and from
1.65 to 52.8 mg/kg, respectively. The vanadium concentration
in air during boiler cleaning was estimated to vary between 0.1
and 5 mg/m3 (Maroni et al., 1983). These results resemble those
reported by Thürauf et al. (1979) on 54 workers exposed to
vanadium in a metallurgical plant. Exposed workers had a urinary
vanadium concentration of 33.9 mg/kg creatinine, whereas the
level in unexposed workers was 0.6 mg/kg creatinine.
Roshchin et al. (1980) used polarography in a study of the
urine samples of 100 workers who were exposed daily to vanadium
pentoxide, and found vanadium in 50% of subjects. The mean
concentration of vanadium in the urine was 0.18 ± 0.03 mg/litre.
In workers with a duration of exposure in the range of 1 - 2
years, the mean concentration was 0.14 ± 0.08 mg/litre; after 2
years, the mean concentration was 0.20 ± 0.009 mg/litre. In
workers employed for 2 - 6 months, the mean concentration of
vanadium in the urine was 0.16 ± 0.12 mg/litre. There appeared
to be a correlation between the urinary levels and the
concentration of vanadium in the air. For example, in workers
exposed to mean vanadium pentoxide atmospheric concentrations of
0.28 ± 0.06 mg/m3, the concentration in the urine was 0.20 ±
0.05 mg/litre; at an atmospheric concentration of 0.17 ±
0.01 mg/m3, the urinary concentration was 0.19 ± 0.007
mg/litre.
The relationships between personal exposure and blood and
urine levels of vanadium were examined in 16 workers in a
ferrovanadium plant in Norway (Gylseth et al., 1979).
Individual levels did not show any clear relationship between
exposure and response, but, when the data were divided into
high- and low-exposure groups, significant differences were
found for both blood and urine levels in relation to exposure
levels. There was also a reasonably good correlation between
blood and urine levels. However, the authors concluded that
"the differences are small and the method difficult and
expensive, so for a routine control other criteria should be
sought".
Kiviluoto et al. (1979a,c) studied serum- and urinary-
vanadium levels in relation to exposure levels. Grouped and
individual data were examined at intervals during vacation
periods and compared with those for an unexposed group. The
levels of exposure were low (0.01 - 0.05 mg/m3), and no
correlations between exposure levels and serum or urine
concentrations were found. However, there was a conspicuous
decline in the urine levels at the beginning of the vacation
period, but they did not decline down to the level of the
unexposed group. It was considered that this provided some
measure of the extent of exposure.
5.5.2 Animal studies
Talvitie & Wagner (1954) administered sodium metavanadate
monohydrate in saline to albino Webster rats and albino rabbits
by intraperitoneal and intravenous injection, respectively. In
one part of the study, rats were given a single ip dose
equivalent to 0.5 mg vanadium/kg body weight and, in another,
twice daily ip injections, each equivalent to 0.25 mg
vanadium/kg body weight, for 5 days. Rabbits were injected
intravenously twice daily for a total of 7 or 9 days, with doses
equivalent to 0.25 mg vanadium/kg body weight, except for 1
rabbit that received doses of 0.4 mg/kg body weight for the
second 5-day period. No histological changes were noted. The
ratio of vanadium eliminated in the urine and faeces was 5:1.
Following oral administration of vanadium sulfate and
vanadium pentoxide to guinea-pigs (equivalent to 2 mg vanadium/
kg body weight), Reznik (1954) found vanadium in the urine and
faeces for 7 - 10 days, though elimination in the urine ceased
before that in faeces. The author concluded that the presence
of vanadium in the faeces, several days after administration,
was due to its return to the intestine after internal resorption
and excretion. Biliary excretion of less than 2% of the
intravenously injected dose (between 0.9 and 30 µg penta-
vanadate/kg body weight) was demonstrated in rats during the
first 6 h after administration. By comparison, 20% was excreted
with the urine during the same period of time (Sabbioni et al.,
1981).
Roshchin (1968) administered a dose of 3 mg vanadium
trichloride to 180-g albino rats. Most of the vanadium was
excreted via the kidneys. Of the dose administered, 18% was
found in the urine after 24 h and 25%, after 48 h. The amount
of vanadium excreted in the urine fell and, after 6 days, was
low. Elimination of vanadium via the intestine occurred on a
much smaller scale and remained relatively stable; 30.9% of the
administered dose was excreted over a 6-day period. Thus, the
largest quantity of vanadium was eliminated during the first 2
days, and the rest was eliminated gradually. The ratio of
amounts eliminated in the urine and faeces was 5:1,
corroborating the findings by Talvitie & Wagner (1954).
In a study on mice, rats, and dogs, Mitchell & Floyd (1954)
showed that ascorbic acid increased vanadium elimination in the
urine during the first few days and later in the faeces.
CaNa3DTPA (salicylic salt of diethylenetriamine-pentaacetic
acid) increased vanadium excretion in the urine and reduced
elimination via faeces. Following the combined administration
of both preparations, elimination in the faeces increased.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Microorganisms and higher plants
Trace quantities (1 - 10 µg/litre) of vanadium stimulated
the growth of some algae, including Scenedesmus or Chlorella
(Arnon & Wessel, 1953; Hopkins et al., 1977; Patrick, 1978).
Toxicity studies on phytoplankton, mainly using pentavalent
vanadium, have revealed differences in susceptibility between
various species. A concentration of 0.02 mg/litre, as ammonium
vanadate, interfered with the cell division of the fresh-water
algae Chlorella pyrenoidosa, whereas 0.25 mg/litre was lethal
(Meish & Benzschawel, 1978). The 15-day LC50 for an estuarine
and salt-water green alga (Dunaliella marina) was given as
0.5 mg/litre of sodium metavanadate and that of a salt-water
pennote diatom (Asterionella japonica) as 2 mg/litre (Minamand &
Unsal, 1978).
Vanadium does not appear to be essential for higher plants.
6.1.2 Invertebrates
In a study using sea-water with a background level of 0.0017
mg vanadium/litre, the 9-day LC50s for vanadium (as sodium
metavanadate) for the worm (Nereis diversicolor), mussel
(Mytilus galloprovincialis), and crab (Carcinas maenas) were
10, 35, and 65 mg vanadium/litre, respectively (Miramand &
Unsal, 1978). Some marine invertebrates, such as the tunicates,
accumulate vanadium to levels that may be 10-5 to 10-6 times the
sea-water concentrations (Table 3.). Vanadium levels in such
species may exceed 3000 mg/kg dry weight (Biggs & Swinehart,
1976; Carlson, 1977). It was stated that the uptake of vanadium
by the mussel (Mytilus edulis) from food (plankton) was of the
same magnitude as that from water (Unsal, 1978). It appears
that benthic aquatic organisms tolerate higher concentrations of
vanadium than fish (section 6.1.3).
6.1.3 Fish
There are some data on the acute toxicity of vanadium for
fish (Van Zinderen Bakker & Jaworski, 1980). The 4- to 6-day
LC50s for fresh-water species are in the range 0.5 -10 mg/litre.
Factors influencing toxicity include water hardness, and the
LC50 values are higher in hard water. Giles et al. (1979)
studied the influence of pH on the toxicity of vanadium
pentoxide for rainbow trout fingerlings. The 96-h LC50 ranged
from 6.43 to 21.75 mg/litre. There was some indication of
vanadium pentoxide being most toxic at a pH of 7. Also, Sprague
et al. (1978) tested zebrafish (Brachydanio rerio) with vanadium
pentoxide and found that a pH of 7.5 provided the most toxic
conditions. At this pH, death occurred between 23.5 and 45 h at
a concentration of 22 mg/litre, whereas at pH 8.2, the time
decreased to 32 h and, at pH 8.8 - 9, to 37 - 39 h.
Studying the chronic effects of vanadium pentoxide on
flagfish, Sprague et al. (1978) and Holdway & Sprague (1979)
concluded that the sublethal threshold concentration would be
about 0.08 mg vanadium/litre.
The rainbow trout (Salmo gairdneri) is the most commonly
used fish for toxicity studies. Sprague et al. (1978) reported
7-day LC50 values in one series of studies ranging from 2.4 to
5.6 mg/litre. Increasing the exposure time resulted in
progressively lower LC50 values, the lowest being 1.99 mg/litre
for an 11-day exposure period. Similar results were reported by
Giles et al. (1979) using experimental conditions of pH 8,
15 °C, and hardness 90 mg CaCO3/litre. The LC50 values
decreased from 4.34 mg CaCO3/litre for 5 days exposure to 1.95
mg CaCO3/litre for 14 days. Neither of these groups was able to
define a minimum lethal level for rainbow trout. Studies by
Stendahl & Sprague (1982) indicated that small rainbow trout
were more resistant than larger fish to vanadium pentoxide. In
general, rainbow trout eggs were 10 - 15 times more resistant to
pentavalent vanadium than fingerlings, suggesting the
possibility of a protective function by the chorion (Giles et
al., 1979). It should be noted that the available studies on
vanadium toxicity have been performed on fresh-water species
only. The effects of salinity remain to be studied.
6.2 Terrestrial Organisms
6.2.1 Uptake of vanadium by plants
In general, the highest concentrations of vanadium in plants
growing in natural soils occur in the roots and decrease towards
the aerial portions of the plant. The concentration of vanadium
in soil is, by and large, 10 times the concentration in the
plant (Cannon, 1963). Absorption appears to be passive (Welch,
1973).
When grown in culture solution, several plant species absorb
vanadium, which is also translocated to the aerial parts and
seeds (Hopkins et al., 1977).
6.2.2 Effects on plants
Vanadium has not been demonstrated to be essential for
higher land plants (Hopkins et al., 1977). However, traces of
vanadate (0.02 mmol vanadium as VO2+ or VO3-) were shown to
promote chloroplast development and oxygen production in higher
plants. Vanadium also had a function as a redox catalyst in the
electron flow from photosystem II to photosystem I (Meisch &
Becker, 1981).
Small amounts of aqueous vanadium (10 - 20 mg/litre) have
detrimental effects on most plants (Cannon, 1963). The growth
of flax, peas, soybeans, and cabbage was reduced in nutrient
solutions containing 0.5 mg vanadium/litre (given as VOCl2 or
VCl3) (Warington, 1955; Hara et al., 1976). Vanadium can
induce iron deficiency chlorosis (Cannon, 1963) or affect the
trace element nutrition by reducing the levels of, e.g.,
manganese, copper, calcium, and phosphorus (Warington, 1955;
Wallace et al., 1977). Similarly, 5 mg vanadium/litre (as VO3-)
in irrigation water reduced the growth of sugar beets by 30 -
50% and caused iron deficiency chlorosis (Hewitt, 1953).
In soils, the toxicity of vanadium may range between 10 and
1258 mg/kg, depending on plant species and type of soil (Hopkins
et al., 1977). Ten mg/kg added to sandy soil as Ca(VO3)2
decreased the growth of sour orange, whereas 150 mg/kg was
lethal (Vanselow, 1950).
Fertilizers may have a high vanadium content. For instance,
rock phosphate, super phosphate, and basic slag may contain
several thousand mg vanadium/kg. These may cause unacceptable
levels of accumulation of vanadium in soil (Mitchell, 1964;
Hopkins et al., 1977). Urban sewage sludges usually contain
less than 35 mg/kg of vanadium (Bradford et al., 1975; Oliver &
Cosgrove, 1975). On the other hand, vanadium in sewage sludge
may be up to 6 times more easily available in sludge than in
soil (Bernow & Webber, 1972 ).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 General Toxicity
The toxicity of vanadium for experimental animals varies
with the species and route of administration. Smaller animals,
including the rat and mouse, tolerate the metal well; the rabbit
and horse are more sensitive (Hudson, 1964). In general,
toxicity is low when the metal is given by the oral route,
moderate by the respiratory route, and high by injection.
Lethal doses for various vanadium salts injected intravenously
in rabbits and subcutaneously in guinea-pigs, rats, and mice are
shown in Table 21.
Table 21. Lethal doses of vanadium (mg V2O5/kg body weight) in
experimental animalsa
-----------------------------------------------------------------------------
Compound Rabbitb Guinea-pig Rat Mouse
-----------------------------------------------------------------------------
colloidal vanadium 1 - 2 20 - 28 - 87.5 - 117.5
pentoxide
ammonium metavanadate 1.5 - 2 1 - 2 20 - 30 25 - 30
sodium orthovanadate 2 - 3 1 - 2 50 - 60 50 - 100
sodium pyrovanadate 3 - 4 1 - 2 40 - 50 50 - 100
sodium tetravanadate 6 - 8 18 - 20 30 - 40 25 - 50
sodium hexavanadate 30 - 40 40 - 50 40 - 50 100 - 150
vanadyl sulfate 18 - 20 34 - 45 58 - 190 125 - 150
sodium vanadate - 30 - 40 10 - 20 100 - 150
-----------------------------------------------------------------------------
a From: Hudson (1964).
b Rabbits were injected intravenously; other animals subcutaneously.
The toxicity of vanadium also varies considerably with the
nature of the compound, vanadium being toxic both as a cation
and as an anion. As a general rule, toxicity increases as
valency increases, vanadium5+ being the most toxic. Among the
oxides of vanadium, vanadium pentoxide is more soluble and more
toxic than the trioxide or dioxide.
Roshchin (1967b, 1968) described the results of acute
inhalation studies on albino rats exposed to vanadium pentoxide
in the form of a condensation aerosol (fume) at 10 - 70 mg/m3
or in the form of a dispersion aerosol (dust) at 80 - 700 mg/m3,
ammonium vanadate (presumably as a dispersion aerosol) at
1000 mg/m3, and ferrovanadium, as a dispersion aerosol at 1000 -
10 000 mg/m3. The minimum concentration of vanadium pentoxide
(condensation aerosol) that gave rise to mild signs of acute
poisoning was 10 mg/m3 air. The absolute lethal concentration
for the condensation aerosol was 70 mg/m3. Dispersion aerosols
(containing large particles) were only one-fifth as toxic as
condensation aerosols. Inhalation of dispersion aerosols of
ferrovanadium did not produce any acute toxic effects, probably
because the particles were too large. However, acute toxic
effects were observed following intratracheal instillation of
ferrovanadium. These may have been related to the biological
solubility and the extent of absorption.
The effects of vanadium on experimental animals have been
investigated in a number of studies using various compounds,
species, and test protocols. Some representative acute and long-
term studies are summarized in Tables 22 and 23. Subsequent
discussions on the effects of vanadium on specific biological
systems are frequently based on the results of these studies.
7.2 Effects on Metabolic Processes
Rabbits exposed to a dispersion aerosol of vanadium trioxide
(40 - 75 mg/m3, 2 h/day for up to 12 months) exhibited a
progressive weight loss amounting to an average of 4.6% at the
termination of the study. Control animals gained weight by an
average of 12.3%. The number of white blood cells declined by
the end of the test from between 7000 and 8000 down to 5000/mm3;
no change was noted in controls. Haemoglobin levels in the test
animals decreased from 75 to 68% of normal levelsa. Serum-
ascorbate levels in the blood progressively decreased to about
20% of controls between 7 and 8 months. Protein sulfhydryl
levels in the serum of exposed animals decreased by 30% compared
with those of the controls. Respiration in the liver and brain
tissues of test animals was reduced by one-half by the end of
the study compared with controls, but the respiratory quotient
was unchanged. Blood-cholinesterase levels in exposed rabbits
increased by an average of 25% after the fifth month (Roshchin
et al., 1964; Roshchin, 1968).
In these studies, the weight loss together with the
depression in levels of white cells, haemoglobin, and protein
sulfhydryl groups in the blood and the decreased liver tissue
respiration were taken as indicators of the "general toxic
effect" of vanadium. Increased cholinesterase activity was held
to be indicative of sensitization.
_________________
a Normal haemoglobin level in the rabbit is 80 - 150 g/litre
and normal rabbit haematocrit, 30 - 50%.
Table 22. Acute studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Dose Concentration Reference
administration index or dose
---------------------------------------------------------------------------------------------------------
vanadium pentoxide mouse intragastric LD50 23.4 mg/kg body weight Roshchin (1967a)
rat inhalation LC50 70 mg/m3 Roshchin (1967a)
rat inhalation minimum 10 mg/m3 Roshchin (1967a)
effective
rat inhalation LC50 70 mg/m3 Sjöberg (1950)
cat inhalation LC50 500 mg/m3 Faulkner Hudson
(1964)
rabbit inhalation LC100 205 mg/m3 Sjöberg (1950)
rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
ammonium vanadate mouse intragastric LD50 10 mg/kg body weight Roshchin (1968)
rat intragastric effective 20 mg/kg body weight Kaku et al. (1971)
rat subcutaneous effective 5-30 mg/kg body weight Kaku et al. (1971)
vanadium mouse intragastric LD50 24 mg/kg body weight Roshchin (1968)
trichloride
vanadium di-iodide mouse intragastric LD50 68 mg/kg body weight Roshchin (1968)
vanadium dibromide mouse intragastric LD50 88 mg/kg body weight Roshchin (1968)
vanadium trioxide mouse intragastric LD50 130 mg/kg body weight Reznik (1954)
ammonium rat intragastric LD50 10 mg/kg body weight Gulko (1956)
metavanadate
vanadyl sulfate rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
rabbit subcutaneous LC50 59.1 mg/kg body weight Korkhov (1965)
rabbit subcutaneous maximum 25 mg/kg body weight Korkhov (1965)
tolerated
guinea-pig subcutaneous LD100 800 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous LD50 560 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous maximum 300 mg/kg body weight Kopylova (1971)
tolerated
water-soluble mouse intragastric LD50 5 mg/kg body weight Seljankina (1961)
vanadium compounds mouse intragastric no effect 0.005 mg/kg body weight Seljankina (1961)
(or 0.1 mg/litre
in water)
---------------------------------------------------------------------------------------------------------
Table 23. Long-term studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Concentration Duration of Reference
administration exposure
---------------------------------------------------------------------------------------------------------
vanadium pentoxide rabbit inhalation 20 - 40 mg/m3 several months Sjöberg (1950)
rabbit inhalation 25 mg/m3 10 months Gulko (1956)
guinea- inhalation 25 mg/m3 10 months Gulko (1956)
pig
rabbit inhalation 8 - 18 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat inhalation 10 - 30 mg/m3 several months Roshchin (1967b)
rat inhalation 3 - 5 mg/m3 several months Roshchin (1967b)
rat inhalation 0.027 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.002 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.006 mg/m3 40 days Pazhynich (1966)
ammonium rat subcutaneous 1 mg/kg 30 days Kaku et al. (1971)
metavanadate
sodium guinea- subcutaneous 3.2 - 128 ug/kg days Kulieva (1974)
metavanadate pig
guinea- subcutaneous 5.12 mg/kg days Kulieva (1974)
pig
vanadium trioxide rabbit inhalation 40 - 75 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
vanadium rabbit oral 5 mg/kg 2 - 3 months Roshchin (1968)
trichloride
vanadium carbide rabbit inhalation 40 - 80 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
ferrovanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
metallic vanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
---------------------------------------------------------------------------------------------------------
Chronic poisoning, following the inhalation of trivalent
vanadium (V203) or the oral administration of vanadium tri-
chloride (VCl3), resulted in blood changes by the end of the
second and third months (Table 24). These changes were
characterized by decreased albumin and increased globulins
(mainly y-globulins) with a halving of the albumin-globulin
ratio, and by an increase in serum concentrations of the amino
acids cystine, arginine, and histidine. There was also a 10%
increase in nucleic acid levels in the blood and a
"considerable" increase in the blood-chloride concentration.
The effects of vanadium on the metabolism of proteins and
nucleic acids were considered to be responsible for the
immunological and allergic reactions that may occur in vanadium
poisoning (Roshchin, 1967a).
Metabolic changes were observed in a study by Pazhynich
(1966) in which albino rats were exposed by inhalation for 70
days to condensation aerosols of vanadium pentoxide at levels of
0.027 ± 0.002 mg/m3 and 0.002 ± 0.00013 mg/m3. Statistically
significant changes were observed at the higher level of
exposure, but not at the lower level. The observations included
decreases in: whole blood-cholinesterase levels, total serum-
protein levels, serum-globulin levels, and the oxyhaemoglobin
content of venous blood. Elevated serum-globulin levels,
increased numbers of blood leukocytes showing yellow, orange,
and red nuclear fluorescence with acridine orange, and increased
oxygen consumption as indicated by isolated liver preparations
were also observed in the high-level exposure group. The
pattern of leukocyte nuclear fluorescence returned to normal 20
days after cessation of exposure. In a second study, albino
rats were continuously exposed to vanadium pentoxide at 0.006 ±
0.00056 mg/m3 for 40 days. No changes in blood-leukocyte
nuclear fluorescence were observed. During the sixth week of
exposure, animals received water but no food. After 3.5 days of
this treatment, the number of leukocytes displaying altered
nuclear fluorescence increased by a factor of 4.83.
In an in vitro study, ammonium metavanadate was found to
inhibit microsomal ketamine N -demethylation, lipid peroxida-
tion, and hydrogen peroxide formation in rat liver. The
inhibiting doses of NH4VO3 ranged from 10-5 to 10-3 mol/litre.
Cytochrome c reductase was also inhibited, whereas cytochrome
oxidase activity was stimulated (Beyhl, 1983).
Parenteral injection of guinea-pigs with sodium metavanadate
in daily doses of 3.2 µg/kg, 128 µg/kg, or 5.12 mg/kg body
weight resulted in dose-dependent increased succinate dehydro-
genase activity in the liver and kidneys and cytochrome oxidase
activity in the liver (Kulieva, 1974).
Table 24. Respiratory effects of vanadium pentoxide on experimental animalsa
---------------------------------------------------------------------------------------------------------
Reference Species Form Concentration Exposure Pathological findings
(mg/m3)
---------------------------------------------------------------------------------------------------------
Sjöberg (1950) rabbit dust 205 7 h conjunctivitis, tracheitis,
pulmonary oedema, broncho-
pneumonia, enteritis, fatty
liver, death
Sjöberg (1950) rabbit dust 20 - 40 1 h/day chronic rhinitis, trache-
several months itis, emphysema, atelect-
asis, bronchopneumonia,
pyelonephritis
Gulko (1956) rabbit dust 10 - 30 continuous, bronchitis, pneumonia,
acute weight loss, bloody diarr-
hoea
Roshchin (1963) rat dust, 80 - 700 continuous, haemorrhagic inflammation in
fume 10 - 70 acute lungs, haemorrhage in inter-
nal organs, paralysis, res-
piratory failure, death
Roshchin (1963) rat dust, 10 - 30 2 h/day, haemorrhagic inflammation in
fume 3 - 5 several months lungs purulent bronchitis,
pneumonia
Pazhynich (1966) rat fume 0.027 continuous haemorrhagic inflammation in
70 days lungs, vascular congestion,
haemorrhage in liver, kid-
neys, and heart
---------------------------------------------------------------------------------------------------------
a From: Waters (1977).
In acute studies on rats (Donryu strain) weighing 200 g,
Kaku et al. (1971) administered vanadium (as ammonium vanadate)
by gavage at a dose of 20 mg/kg body weight or injected it
subcutaneously (doses of between 5 and 30 mg/kg body weight).
There were dose-dependent reversible increases in the trigly-
ceride concentrations in the liver and blood-serum, a decrease
in the serum-cholesterol level, and increases in glutamate-
oxalo-acetate transaminase and glutamate-pyruvate transaminase
activity. After subcutaneous injection, there was a fall in
cholesterol levels. The lowest values were reached 24 h after
the injection; values then returned to normal. Dose-dependent
increases were also observed in the concentrations of triglyc-
erides in the liver and serum. Levels peaked 48 h after the
injection and then gradually declined. When ammonium vandate
solution equivalent to 1 mg vanadium/kg body weight was
administered daily by subcutaneous injection for 30 days more
marked changes in serum cholestrol were observed.
Korkhov (1965) injected 0.3 mg vanadyl sulfate/kg body
weight subcutaneously in rabbits with experimental athero-
sclerosis and observed a lowering of the hypercholesterolaemia
and inhibition of the rise in lecithin. Combined administration
of vanadyl sulfate and phenylethylacetic acid lowered the aortic
cholesterol level 3.5 times compared with controls. Korkhov
(1965) also showed that cholesterol biosynthesis in liver tissue
culture was inhibited by the addition of 10-4 vanadyl sulfate.
When 30 rabbits with experimental atherosclerosis were
administered a mixture of trace elements (copper, cobalt,
manganese, and zinc) in combination with cholesterol, Babenko &
Vandzhura (1969) detected increases in the blood lipids and
decreases in vanadium concentrations, compared with healthy
control animals. The same results were noted following combined
administration of copper and cobalt plus cholesterol at
0.2 g/kg. After administration of manganese, the decrease in
the vanadium concentration was delayed; after administration of
zinc, vanadium remained at the same level as in healthy animals.
Vanadium concentrations in body tissues decreased as cholesterol
levels increased, but manganese and zinc, when given together
with cholesterol, helped maintain vanadium concentrations in the
tissues of the body, the highest vanadium concentrations being
found in the liver, aorta, and muscles.
In the studies by Novakova et al. (1981), daily oral
administration to rabbits for 4 months of vanadium pentoxide and
cholesterol (1st group: vanadium at 0.5 mg/kg body weight and
cholesterol at 0.3 mg/kg body weight; 2nd group: vanadium at
1.5 mg/kg body weight and cholesterol at 0.5 mg/kg body weight)
resulted in high levels of cholesterol in the blood and
extensive atherosclerosis of the aorta. In the animals
administered either cholesterol (0.5 mg/kg body weight) or
vanadium (0.5 mg/kg body weight), hypercholesterolaemia was also
observed, but the increase was less pronounced. Increases in
levels of lipids, lipoproteins, and triglycerides were also
observed. After one month of administration, cholesterol levels
in treated animals were more than ten times those in the
controls. At the end of the study (after the 4th month), the
cholesterol levels in the treated animals were considerably
higher than those in the controls.
The effects of vanadium on iron metabolism have not been
fully elucidated. In some studies, a stimulative effect on
haemoglobin and erythrocyte levels has been claimed. The
results of studies by Myers & Beard (1931) suggested that
vanadium chloride given at 0.6 mg/kg diet to rats, previously
rendered anaemic, had a favourable effect on the haemoglobin
level, and Kopylova (1971) obtained increases in the erythrocyte
count and haemoglobin level in rabbits by subcutaneous admini-
stration of vanadyl sulfate (1 mg/kg body weight, daily, for 2
months). Trummert & Boehm (1957) observed an increase in the
erythrocyte count following intravenous injection of vanadium
gluconate (0.3 - 1.5 mg/kg body weight, daily, for 40 days), but
the haemoglobin level was not significantly affected.
7.2.1 Mechanisms of action
Many of the metabolic effects observed can be explained by
the biochemical effects of vanadium exposure in vivo and in
vitro.
Roschin (1967a) presented the hypothesis that the mechanism
of the initial step in the non-specific haematopoietic effect of
vanadium and the subsequent anaemia was the inhibition of the
redox system of hydrogen carriers. In response to the resulting
hypoxia, there is increased haematopoiesis. Possibly vanadium
interferes with tissue respiration at the stage of dehydrogena-
tion effected by nicotinamide adenine dinucleotide (NAD). By
inhibiting this coenzyme, vanadium interferes with the
incorporation of iron in the porphyrin complexes and haemoglobin
synthesis. The anti-vitamin C effect of vanadium and the
consequent vitamin C deficiency also inhibits the utilization of
iron for haemoglobin synthesis. Iron accumulates in the
reticuloendothelial tissue.
It is known that vanadium also inhibits the activity of
monoamine oxidase, which catalyses the conversion of serotonin
to 5-hydroxyindoleacetic acid. In a study on rabbits exposed
to vanadium pentoxide dust for 3 months, urine levels of 5-
hydroxyindoleacetic acid fell to 33% below control values
(Roschin, 1967a). It was suggested by the author that
inhibition of monoamine oxidase might result in accumulation of
serotonin in the central nervous system, leading to functional
disturbances, such as bronchospasm, diarrhoea, and urinary
incontinence. Elevated serotonin levels could also be
responsible for the dystrophic and necrotic process in the
kidneys and the high permeability of the blood vessels. A
decrease in sulfhydryl groups in blood-proteins and a reduction
in the cystine content of keratinized tissues might be due to an
interaction between vanadium and an unspecified enzyme.
The catabolism of cystine and cysteine was increased by
exposure to vanadium (Bergel et al., 1958). In vitro, pyridoxal
5-phosphate induced the catabolism of sulfhydryl amino acids in
the presence of VO2+ (Anbar & Inbar, 1962). The authors
suggested that the activation of pyridoxal phosphate by vanadyl
ions was specific to elimination and strongly suggested a
decrease in -SH groups in the organism. A reduction in cystine
levels was observed in keratinized tissues (hair of rats fed
vanadium compounds and the fingernails of vanadium workers)
by Mountain et al. (1953, 1955). It was suggested that this
effect was the result of decreased synthesis of cysteine and
cystine and that metabolic processes depending on either of
these amino acids may be depressed in the presence of vanadium.
According to Mahler & Cordes (1966), coenzyme A plays a
central role in many biosynthetic and oxidative pathways. In
the biosynthesis of coenzyme A, cysteine reacts with 4-
phosphopantothenic acid in the presence of adenosine triphos-
phate (ATP) to form the intermediate 4'-phosphopantothenyl
cystine, and Mascitelli-Coriandoli & Citterio (1959a,b) showed
that treatment with sodium metavanadate reduced the content of
coenzyme A in rat liver.
As coenzyme A is involved in biochemical pathways starting
with acetate, these processes can also be affected by vanadium;
Curran (1954) showed that the synthesis of cholesterol from 14C-
acetate in rat liver was reduced in the presence of vanadium.
Later studies indicated that one site of the inhibitory action
of vanadium in the synthesis of cholesterol was at the level of
the enzyme squalene synthetase, which catalyses the conversion
of farnesyl pyrophosphate to squalene (Azarnoff & Curran, 1957;
Azarnoff et al., 1961). In a study by Curran & Costello (1956),
aortic cholesterol was mobilized more rapidly in vanadium-
treated atherosclerotic rabbits than in controls. Cholesterol
levels appear to be reduced by vanadium in young animals,
including human beings, but not in older ones. It has been
suggested, but not demonstrated, that a regulatory enzyme in the
synthesis of cholesterol (acetoacetyl coenzyme A deacylase) is
activated by vanadium in the former but inhibited in the latter.
Vanadium might reduce the synthesis of triglycerides and
phospholipids, since acetyl coenzyme A is a precursor of fatty
acids. However, the results of studies by Curran and colleagues
showed that, while the levels of triglycerides decreased in the
livers of rats given vanadium (Curran, 1954), serum-
triglycerides levels in human beings increased following the
ingestion of vanadium (Curran et al., 1959). Snyder & Cornatzer
(1958) reported that the incorporation of labelled phosphate
into rat liver phospholipids decreased following injection of
vanadyl sulfate. This could have been due to the inhibition of
phospho-lipid biosynthesis or to increased oxidative
degradation, as originally suggested by Bernheim & Bernheim
(1938, 1939). Using an isotope method with radioactive
phosphorus as the indicator, Prokopenko (1961) observed a
disturbance in the intensity of phosphorus exchange between
organic acid-soluble compounds and inorganic phosphates in
albino rats and mice with acute ammonium vanadate poisoning. No
changes were found in the intensity of total metabolism and in
liver tissue phosphate levels. After administration for 6
months in doses of 1 mg/kg body weight, phosphorylation
processes in the organs and tissues were disturbed and the
concentration of inorganic phosphorus in the blood and urine was
increased.
Coenzyme A is also involved in the synthesis of coenzyme Q
(ubiquinone) in the mitochondrial electron transport chain.
Ubiquinone synthesis in isolated rat mitochondria was reduced by
vanadium, but this effect was partially reversed when cysteine
was given with vanadium. Addition of ATP and coenzyme A
prevented the inhibition of ubiquinone synthesis (Aiyar &
Sreenivasan, 1961).
It has been suggested that mitochondrial oxidative
phosphorylation in liver homogenates in vitro is uncoupled by
vanadium with a resulting depletion in ATP energy stores (Wright
et al., 1960). In young chicks, the addition of ammonium
metavanadate to the diet equivalent to 25 mg vanadium/kg body
weight uncoupled oxidative phosphorylation in the liver
mitochondria (Hathcock et al., 1966). These authors suggested
that vanadate might replace the phosphate ion in ATP synthesis,
and a high-energy vanadyl intermediate (vanadium X) or ADP-
vanadium be formed and hydrolysed. The results of studies by
DeMaster & Mitchell (1973) supported the theory of the mechanism
involving the uncoupling of glyceraldehyde-3-phosphate
dehydrogenase by vanadium. It was also shown that the
vanadium5+ oxyanion inhibited oxidative phosphorylation in
intact rat liver mitochondria, but did not act as an uncoupler.
Vanadium salts inhibit the activity of succinic dehydro-
genase, a key enzyme in the citric acid cycle and the electron
transport system that is activated by sulfhydryl groups, and
this would also reduce ATP synthesis (Aiyar & Sreenivasan,
1961). The inhibiting effects of vanadium on succinic
dehydrogenase could involve a reduction in available -SH
groups.
The oxidation of tryptamine by monoamine oxidase from
guinea-pig liver and kidney was accelerated by 125% in the
presence of vanadium (Perry et al., 1955, 1969); however, the
results of studies by Lewis (1959c) on dogs injected with sodium
metavanadate indicated that vanadium inhibited monoamine
oxidase, because the urinary output of 5-hydroxyindoleacetic
acid was reduced. The decreased output of 5-hydroxyindoleacetic
acid, suggests the possibility of accumulation of serotonin,
which was also reported by Roshchin (1967a).
When rats were administered daily injections of sodium
metavanadate (1.25 - 2.5 mg/kg body weight), weight loss was
correlated with accumulation of the metal in the liver (Johnson
et al., 1974). The activities of the hepatic enzymes, xanthine
oxidase and sulfite oxidase, which have molybdenum groups, and
total liver concentrations of molybdenum were not affected by
vanadium. It was concluded that vanadium toxicity in rats was
not related to molybdenum utilization. Though vanadium was
administered in the pentavalent state, the rat livers had an
electron paramagnetic resonance (EPR) spectrum characteristic of
vanadium4+. It was suggested that vanadium is in a protein-
bound form in the livers of rats. Vanadium4+ was also found in
the kidneys and, to a limited extent, in the lungs of rats
injected with sodium metavanadate, but not in the hearts; it was
considered that the ability of liver and kidney to reduce the
vanadate by one electron might be related to a specific
detoxification mechanism present in these organs. Johnson et
al. (1974) and Minden & Rothe (1966) studied the effects of
pentavalent vanadium salts on various enzyme systems in rat
liver homogenates, rabbit blood-serum, and suspensions of
erythrocytes and concluded that there was nothing to suggest
that vanadium salts reacted directly with coenzymes (NAD and
NADP, pyroxidal sulfate) or -SH groups.
The results of in vitro studies, conducted by Tolman et al.
(1979), indicated that vanadium stimulated glucose oxidation and
transport in adipocytes and glycogen synthesis in the liver and
diaphragm, and inhibited hepatic gluconeogenesis and intestinal
glucose transport. There is no evidence in vivo showing a role
of vanadium in the regulation of glucose metabolism.
The intimate mechanism of interaction between vanadium and
enzymes is not yet known. Experimentally observed disturbances
of the cardiovascular system, changes in the concentration of
-SH groups and cystine, disturbances in the metabolism of
sulfur-containing, glycogen-forming, and keto-forming amino
acids, and of the functioning of the liver and kidneys, of RNA
and DNA synthesis, and of cholesterol metabolism, indicate that
vanadium possesses a broad spectrum of action in the body and
that its toxic action is not analogous to that of any other
metal (Roshchin, 1968).
7.3 Effects on the Nervous System
The Na+K+-ATPase in rat brain is inhibited by vanadium
pentoxide, though not as strongly as that in kidney and heart
(Nechay & Saunders, 1978). Svoboda et al. (1984) showed that
the brain microsomal N+K+-ATPase in rat is equally inhibited by
vanadate (VO3-) or the vanadyl ion (VO+2).
In a short-term exposure study, rats received single intra-
peritoneal injections of sodium metavanadate equal to 20% of the
LD50 (1 mg vanadium/kg body weight). The level of noradrenaline
decreased and those of dopamine and 5-hydroxy-tryptamine
increased. A long-term study involved the oral administration
of sodium metavanadate (3 mg vanadium/kg body weight). The
findings were similar to, but more pronounced than, those in the
short-term study (Witkowska & Brzezinski, 1979).
When adult male CD1 mice were treated with vanadate in the
drinking-water for 30 days, there was a dose-related decrease in
norepinephrine levels in the hypothalamus. Dopamine levels also
decreased significantly, but 5-hydroxytryptamine levels were not
affected. Levels of dopamine in the corpus striatum were
unchanged and there were only marginal effects on the amines in
the other brain regions. It is suggested that vanadium has a
selective effect on adrenergic pathways (Sharma et al., 1986).
Several inhibitors of Na+K+-ATPase, such as ouabain, have
been reported to block DNA and protein synthesis in cell
cultures (Kaplan, 1978). It has also been shown that vanadate
inhibits protein synthesis in neuroblastoma cells and in the
brain homogenates of rats fed sodium monovanadate (125 mg/litre)
ad libitum for 30 or 60 days (Montero et al., 1981).
Neurophysiological effects have been reported following
acute exposure (oral and sc injection) of dogs and rabbits to
vanadium oxides and salts (V2O3, V2O5, VCl3, NH4VO3) (Roshchin,
1967a). These include disturbances of the central nervous
system (impaired conditioned reflexes and neuromuscular
excitability).
In studies by Seljankina (1961), solutions of vanadium
pentoxide or ammonium vanadate were administered orally to rats
or mice in doses of 0.005 - 1 mg vanadium/kg body weight per day
for periods ranging from 21 days at the higher levels to 6
months at the lower levels. A dose of 0.05 mg vanadium/kg body
weight was found to be the threshold dose for functional
disturbances in conditioned reflex activity in both mice and
rats; a dose of 0.005 mg vanadium/kg body weight did not produce
any adverse effects.
In a study by Pazhynich (1966), albino rats underwent con-
tinuous inhalation exposure to condensation aerosols of vanadium
pentoxide at levels of 0.002 mg/m3 and 0.027 mg/m3. Animals in
both treated and control groups showed normal weight gain.
After 30 days, the motor chronaxy of the extensor muscles of the
tibia in the group exposed to 0.027 mg/m3 decreased by an average
of 0.8 µs ( P < 0.01), and the chronaxy of the corresponding
flexor muscles increased by an average of 4 µs ( P < 0.001).
Thus, the chronaxy ratio of antagonistic muscles fell from 1.5
at the beginning of the studies to 1.0 on day 20 ( P < 0.02),
and 0.5 on day 30 ( P < 0.01). The decrease continued until a
level of about 0.25 was reached. About 18 days after cessation
of exposure on the 70th day, the chronaxy ratio returned to
normal (1.5). Changes in motor chronaxy were not observed in
rats exposed at 0.002 mg/m3 or in the controls.
In a second study, albino rats were exposed continuously to
vanadium pentoxide at 0.006 ± 0.00056 mg/m3 for 40 days. No
changes were observed in the chronaxy of antagonistic muscles of
the tibia in exposed animals, compared with the controls, during
the first month of exposure. However, after 30 days, there was
a statistically significant decrease in chronaxy ratio. When
animals were given only water and no food for 3.5 days, during
the sixth week of exposure, chronaxy ratios decreased to 0.92,
compared with 1.5 in the controls.
7.4 Effects on the Respiratory System
Studies on respiratory exposure to vanadium pentoxide are
summarized in Table 23 (section 7.1).
Sjöberg (1950) exposed rabbits to vanadium pentoxide dust
particles, nearly all of which were smaller than 10 µm in
diameter at concentrations of 77, 109, 205, or 525 mg/m3 for
periods of 7 h, 4 h, 7 h, or 1 h, respectively. Death occurred
only in the 205 mg V205/m3 (7 h) group (equivalent to 115 mg
vanadium/m3). There was marked tracheitis accompanied by
pulmonary oedema and bronchopneumonia. Conjunctivitis,
enteritis, and fatty infiltration of the liver were also
observed.
In further studies by the same author, rabbits were exposed
to 20 - 40 mg V205/m3 (equivalent to 11 - 22 mg vanadium/m3)
intermittently for 1 h each day for several months. At autopsy,
pathological changes observed included chronic rhinitis and
tracheitis, emphysema, patches of lung atelectasis, broncho-
pneumonia and, in some cases, pyelonephritis. Vanadium was
detected in ashed lung, liver, and kidney, but not in the
intestines. Changes of a fibrotic nature and specific chronic
lesions were not observed in the lungs, and there was no visible
accumulation of particles. These findings, plus the fact that
vanadium was present in the liver and kidney, were considered
evidence of rapid clearance and/or absorption from the lung.
Continuous inhalation exposure of rabbits to 10 - 30 mg
V205/m3 (5.6 - 16.8 mg vanadium/m3) caused bronchitis,
pneumonia, loss of weight, and bloody diarrhoea (Gulko, 1956).
In studies by Roshchin (1967b, 1968) described in section
7.2, acute inhalation toxicity in albino rats was characterized
by irritation of the respiratory mucosa and nasal discharge that
sometimes contained blood. Animals breathed with difficulty and
there were crepitations. The animals behaved passively,
refusing to eat, and lost weight. In cases of severe poisoning,
diarrhoea, paralysis of the hind limbs, and respiratory failure
were followed by death. Pulmonary abscesses were frequently
seen in animals that recovered. Animals that died or were
killed at various times after exposure, showed severe
congestion, particularly in the capillaries, and small
haemorrhages were observed in all internal organs. Signs of
increased intracranial pressure, and fatty degeneration of the
liver and kidneys were also seen. In the lungs, there was
capillary congestion together with tiny haemorrhages,
perivascular and focal oedema, bronchitis, and focal
interstitial pneumonia. The bronchitis and bronchopneumonia
were often purulent, and the small bronchi were constricted.
There was a relationship between the severity of the
pathological changes and the vanadium concentration in the air.
In cases of slight toxicity, pathological changes were mainly
confined to the lungs.
Pathological changes were also seen in the lungs when rats
were exposed intermittently to a condensation aerosol of
vanadium pentoxide at 3 - 5 mg/m3 for 2 h, every other day, for
3 months, or to a dispersion aerosol of V205 at 10 - 30 mg/m3
for 4 months. Blood vessels were engorged and the endothelium
was swollen; capillary congestion, perivascular oedema,
lymphostasis, and small haemorrhages indicated altered vascular
permeability and disturbances in the circulation of the
pulmonary blood and lymph. Occasionally, foci of oedema and
desquamative bronchitis were observed and small bronchi were
often constricted. Lymphocytes and histiocytes had infiltrated
interstitial tissue. Connective tissue proliferation was
sometimes seen in the zone of lymphocytic infiltration.
Purulent bronchitis or pneumonia occurred in some animals, and
occasionally lung abscesses developed.
Roshchin (1967a) observed similar effects with vanadium
trioxide and vanadium trichloride. As vanadium trichloride was
more soluble, more marked histopathological effects were seen in
internal organs. Pentavalent compounds of vanadium were 3 - 5
times more toxic (in terms of median lethal concentration) than
trivalent compounds. Although dispersion aerosols of vanadium
metal, vanadium carbide, and ferrovanadium were not highly
toxic, long-term exposure at high concentrations resulted in
many of the signs and symptoms produced by vanadium pentoxide.
In studies by Pazhynich (1966) (section 7.2), histopatho-
logical changes observed in rats following high-level inhalation
exposure included marked lung congestion, focal lung haemor-
rhages, and extensive bronchitis.
Effects on the lung were observed in albino rats exposed by
inhalation for 2 weeks to uncoated bismuth orthovanadate dust
(0.11 mg/litre, 1.2 mg/litre), silica-coated bismuth ortho-
vanadate (0.15 mg/litre, 1.3 mg/litre), or silica-coated
titanium dioxide (1.19 mg/litre). In rats sacrificed at the end
of the 2-week exposure, there was a dose-related macrophage
(dust cell) response to both forms of bismuth orthovanadate.
Three months after exposure, the bismuth orthovanadate rats had
alveolar proteinosis, foamy macrophages with cholesterol clefts,
and hyperplastic type II pneumocytes. Six months after exposure,
these changes were more marked with cholesterol granulomas and
degeneration of foamy macrophages. In rats sacrificed after 1
year, the pulmonary lesions were reduced, but alveolar protein-
osis and cholesterol granulomas persisted. Initially, similar
changes were observed in rats exposed to the silica-coated
titanium dioxide, but recovery was obvious at 6 months and, at 1
year, the lungs were almost normal with only a few remaining
macrophage (dust cell) aggregates (Lee & Gillies, 1986).
The respiratory and related histopathological effects of
vanadium exposure in experimental animals were marked irritation
of the respiratory mucosa; vascular injury resulting in
capillary stasis, perivascular oedema, and small haemorrhages;
and an asthmatic-type bronchitis and expiratory difficulty on
acute exposure (Roschin, 1967b).
In adult male cynomolgus monkeys exposed by inhalation to
vanadium pentoxide dust concentrations of 0.5 mg/m3 or 5 mg/m3
at weekly intervals, significant central and peripheral airflow
restriction was measured one day after each exposure. There
were also significant increases in respiratory cell counts
obtained by bronchoalveolar lavage. The increased cell count
was due to a marked increase in the number (absolute and
relative percentage) of polymorphonuclear leukocytes,
indicating pulmonary inflammatory changes (Knecht et al.,
1985).
In the respiratory tract of experimental animals, the main
differences between the acute and chronic effects of vanadium
are the development, after prolonged exposure, of chronic
inflammation in the bronchi and a greater tendency to septic
bronchopneumonia. Atelectasis, interstitial infiltration and
proliferation, and emphysema also occur.
Macrophages are engaged in a variety of pulmonary defence
mechanisms. Theoretically, effects of vanadium on these cells
may explain some of the observations on vanadium toxicity on the
respiratory system. In in vitro studies, Waters et al. (1974)
demonstrated a 50% reduction in the viability of cultured rabbit
macrophages after exposure to 13 mg vanadium/litre (as vanadium
pentoxide) for 20 h. Short-term exposure (2 h) to vanadium
pentoxide at a dose of 7 mg vanadium/litre also reduced the
viability of mouse pulmonary alveolar macrophages to 87%. In
another study, the phagocytic index was reduced to 71% (Fisher
et al., 1978). The incubation of bovine alveolar macrophages
with ammonium metavanadate (NH4VO3) at 0.5 or 1 mg
vanadium/litre, for 4 h, reduced viability to 95 and 85%,
respectively. After 8 h incubation, viability was reduced by 24
and 38%, respectively, and, after 16 h, no viable cells
remained. Low levels of vanadium (0.01, 0.1 mg vanadium/litre)
stimulated the phagocytic activity of macrophages, whereas a
striking decrease in phagocytic activity was noted with 0.5 and
1 mg/litre at 8 h, though, initially, there was stimulation of
activity. Doses of 0.01 and 0.1 mg/litre did not affect
viability (Wei & Misra, 1982).
7.5 Effects on the Cardiovascular System
Severe exposure of animals to vanadium oxides and salts
produced cardiovascular changes (occurrence of arrythmias and
extrasystole, prolongation of the Q-RST interval, and decrease
in the height of the P and T waves of the EKG) (Roshchin,
1967a).
Intense vasoconstriction has been reported in the spleen,
kidney, and intestines following intravenous injections of sub-
lethal doses of sodium ortho- and metavanadate (Hudson, 1964).
Intravenous injection of sodium metavanadate at 2.5 mg/kg in
dogs provoked an increased amplitude of T-waves in the electro-
cardiogram followed by depression of S-T segments (Lewis,
1959c). Perivascular swelling, as well as fatty changes in the
myocardium, were observed by Roshchin (1968) following long-term
inhalation exposure of rats and rabbits to vanadium pentoxide,
trioxide, and chloride (10 - 70 mg/m3, 2 h/day, 9 - 12 months).
Vanadium sulfate (500 mg/kg diet, 6 weeks) mobilized excess
arterial cholesterol in rabbits previously maintained on a
cholesterol-rich diet (Curran & Costello, 1956).
Feeding rats sodium orthovanadate at 100 or 200 mg/kg body
weight (added to normal rat chow) for up to 56 weeks resulted in
a gradual increase in systolic blood pressure. The effect was
unrelated to water intake, urine output, or urinary-sodium
excretion. The increased pressure was sustained in a dose-
related manner and was positively correlated with plasma levels
of vanadium that ranged from 0.04 to 0.27 mg/litre (Steffen et
al., 1981). Similar increases in mean arterial blood pressure
have been reported in both conscious (Day et al., 1980) and
anaesthetized rats (Hatfield & Churchill, 1981).
7.6 Effects on the Kidney
In earlier studies, glomerular hyperaemia and necrosis of
convoluted tubules were reported to be related to acute vanadium
exposure (Hudson, 1964). Nephrotoxicity, manifested as
albuminuria, was reported after intravenous injection of sodium
metavanadate at 2.5 - 5 mg/kg body weight in male dogs by Lewis
(1959c). Inhalation of 10 - 70 mg vanadium chloride/m3, for 2 h
daily, for 9 - 12 months, was followed by fatty changes in the
kidney of the rat and rabbit (Roshchin, 1968). In an inhalation
study on rats exposed continuously to vanadium pentoxide
condensation aerosols at concentrations of 0.002 and 0.027 mg/m3
for 70 days, Pazhynich (1966) reported granular degeneration of
the epithelial cells of the convoluted tubules, with areas of
necrosis. Acute tubular necrosis followed subcutaneous injection
of NH4VO3-solutions in 0.1 mol/litre tris-HCl-NaCl buffers in
albino mice (20 mg vanadium/kg body weight). The mortality rate
was higher at a pH of 7.8 (68%) than at pH 6.1 (20%) (Wei et
al., 1982).
There are distinct species differences with regard to the
renal effects of vanadate (Grantham, 1980; Phillips et al. 1983;
Nechay, 1984). Vanadate has both diuretic and natriuretic
effects on the rat kidney (Balfour et al., 1978; Day et al.
1980; Kumar & Corder, 1980; Hatfield & Churchill, 1981; Roman et
al., 1981), but not on that of the dog (Inciarte et al., 1980;
Lopez Novoa et al., 1982a,b) or the cat (Larsen et al., 1979).
In the rat, the tubular effect is independent of changes in
glomercular filtration rate, whereas the tubular effect in the
cat is either absent or masked by pronounced renal vasoconstric-
tion and anuria (Larsen et al., 1979; Larsen & Thomsen, 1980).
Vanadate has also been reported to increase the urinary
excretion of calcium, phosphate, bicarbonate, and chloride in
the rat kidney (Kumar & Corder, 1980).
7.7 Effects on the Immune System
Exposure of mice to vanadium in drinking-water resulted in a
dose-related, but not statistically-significant, decrease in
antibody-forming cells in the spleens of mice challenged with
sheep erythrocytes; serum immunoglobulins were not affected.
Splenic lymphocytes obtained at 1, 4, 8, and 13 weeks from male
Swiss-Webster mice treated with 1, 10, or 50 mg vanadium/litre
drinking-water showed increased DNA synthesis in vitro (Sharma
et al., 1981). In female B6C3F1 mice given ammonium
metavanadate ip at doses of 2.5, 5, or 10 mg/kg body weight,
every 3 days, for 3, 6, or 9 weeks, there was a dose-related
increase in resistance to Escherichia coli endotoxin lethality
up to 6 weeks and a dose-related decrease in resistance
to Listeria lethality. Peritoneal macrophage activity also
decreased in a dose-related manner, but without any effects on
viability. The rosetting capability of splenic lymphocytes was
increased. There was enlargement of the liver and spleen with
enhanced formation of splenic mega-karyocytes and red blood cell
percursors. The authors concluded that vanadium may affect the
normal functioning of the immune system (Cohen et al., 1986).
7.8 Reproduction, Embryotoxicity, and Teratogenicity
7.8.1 Reproduction and embryotoxicity
Roshchin at al. (1980) studied the gonado- and embryotoxic
effects of 0.85 mg metavanadate/kg body weight (1/20 LD50),
administered subcutaneously to albino rats. Vanadium
administered to pregnant rats on days 21 - 22 of pregnancy
accumulated in the placenta but was not reported to penetrate
the placental barrier and reach the fetus. During the period of
lactation, vanadium was found in the mammary glands (0.14% of
administered dose/g tissue) and excreted with the milk. In new-
born rats, the uptake of vanadium was in the range of 0.018 -
0.032% of the original administered dose to the lactating dams
per g neonate. Impairment of spermatogenesis was manifested as
a 10 - 33% decrease in the mobility of spermatozoa, a decrease
in osmotic resistance of 7.9 - 11.4% and a 31% rise in the
number of dead spermatozoa. There were morphological changes in
spermatozoa and desquamation of the spermatogenic epithelium in
the seminal tubuli. The impairment of spermatogenesis affected
the reproduction of animals, resulting in pre-implantation
deaths of embryos. Gonadotoxic effects were suggested by the
absence of fertilization of female rats by male rats exposed
daily to vanadium at 0.85 mg/kg body weight. In other studies
(Hackett & Kelman, 1983) in pregnant rats vanadium tended to
localize initially in the placenta and then to preferentially
concentrate in the membranes rather than in the fetus.
The administration of similar doses of vanadium to female
rats on the 4th day of pregnancy increased the mortality of
embryos as a result of pre-implantation deaths; the number of
fetuses in each female rat was only half that in the untreated
animals. These effects were observed in the absence of general
toxicity in the experimental animals.
In vitro, orthovanadate (0.2 - 2 mmol) inhibited
luteinizing hormone-induced cyclic adenosine monophosphate
(cAMP) in isolated corpora lutea from pseudopregnant rats.
When added simultaneously with luteinizing hormone, inhibition
occurred within 25 min, but not when the corpora lutea had been
pretreated with luteinizing hormone for 60 min. A decrease was
also observed when corpora lutea were exposed to vanadate in the
presence of 3-isobutyl-1-methylxanthene (0.5 mmol), a phospho-
diesterase inhibitor. Cyclic adenosine monophosphate was also
inhibited by vanadate in copora lutea incubated in a calcium-
depleted medium. Vanadyl sulfate (0.4, 2 mmol) was as effective
as vanadate in inhibiting luteinizing hormone-induced cyclic
adenosine monophosphate accumulation (Lahav et al., 1986).
7.8.2 Teratogenicity
Carlton et al. (1982) injected 80 mature Syrian golden
hamsters ip with ammonium vanadate at 0, 0.47, 1.88, or
3.75 mg/kg body weight. Injections were carried out on days 5 -
10 of gestation. A significant increase in skeletal anomalies
was observed in all groups exposed to vanadate compared with
control animals. A significant increase in deaths was registered
in the 1.88 mg/kg group. There was no dose-response relationship
as regards anomalies.
Pregnant NMRI albino mice were injected intravenously with
1 mmol vanadium pentoxide dissolved in distilled water on day 3
or day 8 of pregnancy (day 1 = finding of vaginal plug). Control
groups were given 0.1 ml physiological saline on the same days.
Mice were killed on day 17, the uterine horns examined for
resorbed embryos, and the fetuses were removed for detailed
examination. Vanadium pentoxide did not produce any effects in
implantation, and fetuses from the day 3-treated and control
groups did not show any differences in litter size, fetal
weight, or external and internal morphology. The fetuses from
the day 8-treated group showed a statistically significant high
frequency (71%) of delayed ossification (supraoccipital bone,
sternum, metatarsals, and caudal vertebrae), and broken spinal
cord occurred in a few fetuses (Wide, 1984).
Vanadium pentoxide was administered subcutaneously to
pregnant rats (strain unspecified) at doses of 0.5, 1, or 4
mg/kg body weight for 10 days, from day 7 to day 16 of
gestation. The incidences of resorbed and dead fetuses in the 1
and 4 mg/kg groups were 17% and 27.2%, respectively. These were
significantly higher than those found in the controls (3.5%). In
the 4 mg/kg group, 52.38% of fetuses showed wavy ribs. In a
separate study, pregnant rats were given an aqueous solution of
V2O5 by ip injection at concentrations of 0.3, 1, or 3 mg/kg
body weight. This induced a higher level of resorbed and dead
fetuses than oral administration. Both ip injection of 0.3
mg/kg and oral administration of 9 mg/kg induced an array of
skeletal anomalies, namely wavy ribs, supernumerary ribs, and
fused sternebrae and vertebrae (Sun, ed., 1987). Though
tentative, these results suggest that vanadium may have the
potential to induce teratogenicity in a mammalian system.
7.9 Mutagenicity and Related End-Points
There are few studies on the mutagenicity and carcino-
genicity of vanadium compounds. Mutagens in the air can be
divided into two groups: a non-polar extract rich in polycyclic
aromatic hydrocarbons (PAHs) and other promutagens, and a polar
extract containing direct acting mutagens, i.e., not requiring
microsomal activation (Madsen et al., 1982). The non-polar
fraction was strongly influenced by automobile exhaust products,
whereas the polar was more attributable to secondary emissions
transformed by atmospheric reactions, and to primary emissions
from stationary sources. The role of PAHs in the overall
mutagenicity was estimated to be modest; thus, the importance of
other substances increases. Vanadium was found at all sample
sites and is a well-known constituent of, for instance, coal fly
ash (section 3.4.2).
Vanadium5+ has been shown both to inhibit or enhance DNA
synthesis in vitro , depending on the concentration in the media
(Hori & Oka, 1980; Carpenter, 1981; Jackson & Linskens, 1982;
Smith, 1983).
In a DNA synthesis inhibition assay in male mice, vanadium
pentoxide suspended in 3% starch solution was administered
orally at doses of 14.6, 29.2, or 58.4 mg/kg body weight. The
animals were killed 24 h after dosing; 3 h before sacrifice,
1 µCi 3H-thymidine/g body weight was administered ip.
Incorporation of 3H-thymidine in the testes, spleen, liver, and
blood was measured in a liquid scintillation spectrometer.
There were no significant differences between the experimental
groups and the solvent control group (Sun, ed., 1987).
In a study using FADU (Fluorescence Analysis of DNA
Unwinding), vanadyl chloride at a concentration of 5 x 10-5 mol
failed to induce DNA damage (strand breaks) in human peripheral
white blood cells (McLean et al., 1982).
Kanematsu et al. (1980) carried out rec assays on 127 metal
compounds with Bacillus subtilis to test their DNA-damaging
capacity. Mild positive results were noted for three vanadium
compounds (VOCl2, V2O5, NH4VO3).
A lack of induction of spot mutations in Escherichia coli
and in Salmonella typhimurium was demonstrated by Kanematsu &
Kada (1978) and Kada et al. (1980). Similar results were
obtained by Si Rongshan et al. (1982) with E. coli . However,
ammonium metavanadate was found to be mutagenic in S.
typhimurium TA1535 in a modified plate incorporation assay and
in the fluctuation test with TA100 (Arlauskas et al., 1985). In
a recent study, Sun, ed. (1987) demonstrated the induction of
reverse mutations by vanadium pentoxide with E. coli WP2,
WP2uvrA, and Cm-981, but no frameshift mutations with strains
ND-160 and MR102. Vanadium pentoxide showed negative results
with S. typhimurium strains TA1535, TA1537, TA98, and TA100.
Thus, the results of mutagenicity studies of vanadium with
bacterial assays are conflicting, and no firm conclusions can be
drawn.
In a micronucleus test, vanadium pentoxide was administered
to two strains of mice (615 and Kunming albino) by ip injection
at doses of 6.4, 2.13, or 0.17 mg/kg body weight for 5 consecu-
tive days; cyclophosphamide was used as a positive control (Sun,
ed., 1987). Significant levels of induced micronuclei in both
strains were observed. Both subcutaneous injection of vanadium
pentoxide solution (0.25, 1, or 4 mg/kg) and inhalation of
vanadium pentoxide dust (0.5, 2, or 8 mg/m3) also induced micro-
nuclei in mice strain 615. However, negative results were
obtained following oral administration of a 3% starch suspension
of vanadium pentoxide at doses of 1.44, 2.83, 5.65, or
11.3 mg/kg body weight, daily, for 6 weeks, to Kunming albino
mice (Sun, ed., 1987).
In an in vitro study on human peripheral lymphocyte cultures
with vanadium pentoxide concentrations of 0.047, 0.47, or
4.7 moles (mitomycin c was used as positive control), no
increases in the frequency of sister chromatid exchange were
observed (Sun, ed., 1987).
In a dominant-lethal mutation assay, vanadium pentoxide
(0.2, 1, or 4 mg/kg body weight) was administered daily by
subcutaneous injection to 5 groups of male mice, aged 5 - 6
weeks at the start of the study, for 3 months, following which
they were mated with females. Ethylmethanesulfonate and
distilled water were used as positive and negative controls,
respectively. The females were killed 17 days after conception
and the numbers of fetuses and resorptions recorded. The
results were considered negative for the induction of dominant-
lethal mutations (Sun, ed., 1987).
7.10 Carcinogenicity
In a study on 13 metallic compounds, intraperitoneal
injections of vanadium3+ 2.4-pentanedione at doses of 24, 60, or
120 mg/kg body weight did not significantly increase the
incidence of lung adenomas in mice (Stoner et al., 1976).
In life-span studies, the incidence of tumours in mice
given vanadyl ions (as the sulfate) at 5 µg/litre drinking-
water was similar to that in control animals (Kanisawa &
Schroeder, 1967; Schroeder et al., 1970; Schroeder & Mitchener,
1975).
In rats, the induction of mammary carcinogenesis by 1-
methyl-1-nitrosurea was blocked by feeding a purified diet
supplemented with 25 mg vanadyl4+ sulfate/kg during the post-
initiation stages of the neoplastic process. Both cancer
incidence and the average number of cancers per rat were reduced
by the vanadium4+ diet without inhibiting the overall growth of
the animals (Thompson et al., 1984). It has also been shown
that metallocene dichlorides, (C2H5)2MCl2 (where M = titanium,
vanadium, molybdenum, or niobium), exhibit cancerostatic
activity against the Erlich ascites tumour system in mice, and
that treatment with such substances cured the tumour (Köpf-Maier
et al., 1980). Vanadocene dichloride was reported to have a
chemotherapeutic activity similar to that of cis-dichlordiamine
platinum (II) when used against liver tumours in mice (Köpf-
Maier & Köpf, 1979). The mechanism of the preventive effect of
vanadium is not clear. Modulation of one or more aspects of the
DNA metabolism could account for these results.
The effects of ammonium vanadate on the development of large
bowel neoplasms in mice treated with 1,2-dimethylhydrazine (DMH)
were studied by Kingsnorth et al. (1986). Mice were treated
with DMH (20 mg/kg body weight per week) for 20 weeks. Ammonium
vanadate was given in the drinking-water (10 or 20 mg/litre) to
groups of mice during the study. At 30 weeks, the colons of
DMH-treated mice (not receiving ammonium vanadate) showed
increases in RNA content (+14%) and DNA content (+18%) and
deeper crypts (+33%). In the mice treated with DMH and receiving
ammonium vanadate at 10 or 20 mg/litre, the RNA content was
decreased by 11%. Although thymidine incorporation was
increased, ammonium vanadate did not have any effects on the
incidence or type of tumour induced by DMH (Kingsnorth et al.,
1986).
In a long-term study in which the carcinogenic activity of
various materials was studied using intrabronchial pellet
implantation in the lower left bronchus of rats, vanadium solids
produced chronic inflammatory changes in 44/100 rats, bronchial
inflammation in 50/100, squamous metaplasia in 10/100, and one
bronchial carcinoma in a male rat after 645 days. These results
were not significant for carcinogenicity (Levy et al., 1986).
8. EFFECTS ON MAN
8.1 Therapeutic Exposure and Controlled Studies
8.1.1 Therapeutic exposure
In the past, vanadium compounds were prescribed as thera-
peutic agents for anaemia, chlorosis, tuberculosis, and
diabetes. They were also used as an antiseptic, a spiro-
chetocide, and a tonic. For example, sodium metavanadate was
given therapeutically by mouth in doses of 1 - 8 mg, and sodium
tartrate was injected intramuscularly at levels as high as
150 mg. Due to poor absorption from the gastrointestinal tract,
the metal is not very toxic for human beings when ingested, but,
if introduced directly into the circulation in a soluble form,
Hudson (1964) estimated that the lethal dose for a 70-kg person
would be only 30 mg V205 (0.42 mg V205/kg body weight).
8.1.2 Controlled studies
8.1.2.1 Effects on metabolism
Vanadium has been administered under controlled conditions
to study its effects on blood-cholesterol levels. Curran et al.
(1959) conducted a clinical study in which 5 healthy adult male
volunteers were fed soluble diammonium oxytartarovanadate at
150 - 200 mg/day (21 - 30 mg vanadium/day) for 6 weeks. At the
end of the period, plasma-cholesterol was significantly
reduced.
Lewis (1959a) compared age-matched groups of 32 vanadium
workers with 45 controls, all over 45 years of age. Vanadium
workers exposed for at least 6 months excreted greater amounts
of vanadium and exhibited slightly lower serum-cholesterol
levels than the controls. Mean cholesterol values for the 2
reference groups (representing 2 geographical areas) were 2309
and 2267 mg/litre. Mean levels for vanadium workers from
corresponding areas were lower at 2049 and 2067 mg/litre (P <
0.05), respectively.
A clinical study by Somerville & Davies (1962) on 12
patients (9 of whom were hypercholesterolaemic) given diammonium
vanadotartrate orally for 6 months (25 mg 3 times daily for 2
weeks, increased to 125 mg daily in 10 patients) did not show
any significant changes in serum-cholesterol levels over 5.5
months. The mean pretreatment control level of serum-cholesterol
was 4110 mg/litre, and the mean age was 49.2 years. The study
is not comparable with that of Curran et al. (1959), as the
patients were hypercholesterolaemic and older. Dimond et al.
(1963) observed temporary drops (not statistically significant)
in cholesterol levels in 2 out of 6 patients given ammonium
vanadyl tartrate for several weeks at levels of between 50 and
100 mg/day. No statistically significant changes were observed
in blood-lipids, phospholipids, triglycerides, 17-ketosteroids,
or 17-hydroxycorticosteroids. Two patients complained of fatigue
and lethargy while they were taking vanadium. All complained of
cramps and loosened stools, and all developed green tongue.
Schroeder et al. (1963) reporting findings similar to those of
Dimond et al. (1963) considered that the slight effects of
vanadium on serum-cholesterol were pharmacological rather than
caused by correction of a physiological deficiency. They
further pointed out that dietary regimens based on the consump-
tion of unsaturated fats, which reduce plasma-cholesterol in
human beings, are associated with the intake of 1 - 4 mg
vanadium/day and that the feeding of vanadium-poor saturated
fats raises cholesterol.
Studies have been undertaken on the effects of vanadium on
human dental caries. Belehova (1969) studied 583 school
children ranging in age from 7 to 11 years. The subjects were
divided into 4 groups. Children in Group I received fluoride
twice a year, those in Group II received a local application of
a 50% paste of an ammonium salt of vanadium and glycerol, Group
III received both fluoride and vanadium, and Group IV acted as
the control. The incidence of caries was 11% in Group III,
15.4% in Group II, 29.7% in Group I, and 43% in the control
group. Belehova concluded that the lower incidence of caries in
subjects receiving vanadium suggested a possible prophylactic
action. However, other studies (Hein & Wisotzky, 1955; Muhler,
1957; Hadjimarkos, 1966, 1968; McLundie et al., 1968) failed to
demonstrate a clearly beneficial effect with regard to dental
caries in human beings.
Data on the effects of vanadium on the haematopoiesis are
inconsistent. Lewis (1959a) did not observe any effects of
exposure to vanadium on haematocrit levels in 32 vanadium
workers compared with 45 controls matched for age. A beneficial
effect of low-level vanadium administration on nutritional
anaemia has been suggested (Beard et al., 1931; Myers & Beard
1931; Hadjimarkos, 1966; Kopylova, 1971). However, the effects
of vanadium on iron metabolism have not been fully assessed
(Vouk, 1979).
The administration of w -methylpantothenic acid, an
antimetabolite of pantothenic acid, to human beings resulted in
a syndrome consisting of postural hypotension, dizziness,
tachycardia, fatigue, drowsiness, epigastric distress, anorexia,
numbness and tingling of the hands and feet, and hyperactive
deep reflexes. It is not known whether these symptoms are the
result of an induced deficiency of pantothenic acid or are toxic
effects of the anti-metabolite. However, the symptoms resemble
those resulting from exposure to high concentrations of
vanadium. The common denominator in both cases may be a
reduction in hepatic coenzyme A levels (Waters, 1977).
8.1.2.2 Effects on the respiratory system
Zenz & Berg (1967) studied the effects of vanadium
inhalation in 9 healthy volunteers aged 27 - 44 years, for whom
baseline lung function data were available. Two volunteers,
exposed to vanadium pentoxide dust at 1 mg/m3 for 8 h,
developed sporadic coughing after 5 h and a frequent cough after
nearly 7 h. Coughing lasted 8 days, but lung sounds remained
clear and there were no other signs of irritation. Lung
function tests, complete blood counts, urinalyses, and nasal
smears were normal up to 3 weeks. Three weeks later, the same 2
volunteers were accidently exposed for 5 min to a "heavy cloud"
of vanadium pentoxide dust. A productive cough developed within
16 h, and, within 24 h, rales and expiratory rhonchi developed
throughout the lung, but pulmonary function remained normal.
Isoproterenol (1:2000) relieved the symptoms for about 1 h, but
coughing then resumed and continued for 7 days. There were no
other symptoms. Eosinophils were not present in the nasal
mucous.
Exposure of 5 volunteers to a lower concentration
(0.2 mg/m3, 98% of particle size 5 µm) had similar effects,
though the symptoms took longer to develop, i.e., after 20 h.
Coughing, without other systemic effects, persisted for 7 - 10
days. Pulmonary function tests and differential white blood
counts remained normal. The vanadium concentration in the urine
was highest (0.13 mg/litre) on the third day, with none
detectable after 7 days. The maximal faecal-vanadium level was
3 mg/kg, with none detectable after 14 days. Exposure to a
concentration of 0.1 mg/m3 for 8 h did not produce any coughing
in 2 subjects not previously exposed. However, an increase in
the production of mucous, 24 h later, indicated some respiratory
irritation. Then there was slight coughing, which became more
severe after 48 h, subsided after 72 h, and disappeared after
96 h. Pulmonary function tests and differential white blood
counts remained normal.
Pazhynich (1967) studied the irritant effects of vanadium
pentoxide condensation aerosol on 11 volunteers. At a
concentration of 0.4 mg/m3, all reported a tickling or itching
sensation and a feeling of dryness in the region of the root of
the tongue, the posterior wall of the pharynx, and the fauces,
as well as a slight prickling sensation in the nose and
posterior pharyngeal wall. These symptoms were easily tolerated.
A concentration of 0.16 mg/m3 caused mild signs of irritation in
only 5 volunteers, and a concentration of 0.08 mg/m3 was not
noticed by any volunteer. It was concluded that the mean
perceptible concentration for human beings is 0.27 mg/m3 and
that 0.16 mg/m3 is imperceptible.
8.2 Clinical Studies
The clinical picture of poisoning shows the broad spectrum
of toxic effects of vanadium. The lesions observed affect the
respiratory system, circulatory system, central nervous system,
digestive organs, kidneys, and skin. Poisoning can be divided
into acute and chronic forms.
8.2.1 Acute toxicity
Acute toxicity is characterized by a latent period, which
depends on the concentration of vanadium, the individual
sensitivity of the subject, and the properties of the specific
vanadium compound. The more soluble salts of vanadium pentoxide
have a more rapid action than the vanadium oxides. Chemically-
pure vanadium pentoxide acts more rapidly than the technical
grade. A condensation aerosol of vanadium pentoxide is more
toxic than a disintegration aerosol (Roshchin, 1964). Vanadium
chloride is toxic more rapidly than other compounds.
Roshchin (1968) subdivided acute vanadium effects into
"mild", "moderate", and "severe" forms. The clinical features
of mild toxicity include rhinitis with a profuse and often
bloody discharge, sneezing, and an itching and burning sensation
in the throat. The rhinitis may be followed by the development
of a dry cough with expectoration of small amounts of viscid
sputum, general weakness, and exhaustion. A sub-normal
temperature may be present; in other cases, the temperature may
be high or normal. The patient is afebrile in the absence of
pneumonic disease (Sjöberg, 1950). Conjunctivitis is frequently
observed. The symptoms and course of mild toxicity resemble an
upper respiratory tract infection. Other symptoms include
diarrhoea due to intensified intestinal peristalsis. The
symptoms disappear from 2 - 5 days after cessation of contact
with the dust.
In moderate toxicity, in addition to conjunctivitis and
irritation of the upper respiratory tract, there is bronchitis
with expiratory dyspnoea and bronchospasm. There are frequent
disturbances in the activity of the gastrointestinal tract,
including vomiting and diarrhoea. Taken together with the
bronchospasm, this points to a response of the smooth muscle to
vanadium exposure. Some affected persons have cutaneous
manifestations of toxicity in the form of rashes and eczema with
itching papules and dry patches (Browne, 1955; Zenz et al.,
1962).
Bronchitis and bronchopneumonia are features of severe toxic
effects. Other symptoms may also be more prominent, such as
headache, vomiting, diarrhoea, palpitations, sweating, and
general weakness. Disorders of the nervous system include
severe neurotic states and tremor of the fingers and hands
(Wyers, 1946; Sjöberg, 1955). Functional disturbances of the
respiratory system can be expected, and X-ray examination will
reveal intensification of the lung pattern.
Kidney damage, highlighted by grave dystrophic changes in
the epithelium of the convoluted tubules and disturbed tubular
secretion, occurs immediately after the start of exposure to low
vanadium doses in both acute and chronic intoxication. Once
triggered off, the changes are irreversible, even if exposure is
discontinued. Therefore, the kidneys are a critical organ for
vanadium poisoning (Korkhov, 1965).
8.2.2 Chronic toxicity
Chronic vanadium intoxication produces profound changes in
the respiratory organs, because of the irritant action of
vanadium and the biochemical and functional disturbances
connected with its general resorptive action. Chronic
respiratory illness takes the form of diffuse pneumosclerosis,
chronic bronchitis, chronic rhinitis, and pharyngitis (Roshchin,
1968). However, Parkes (1982) claimed that the available
evidence (Sjöberg, 1950; Williams, 1952; Zenz & Berg, 1967) did
not support the contention that prolonged exposure to vanadium
compounds leads to chronic bronchitis, with or without
emphysema. Although wheezing is more common among vanadium
pentoxide workers than among unexposed workers, lung function
tests and chest radiography have not revealed persistent lung
damage (Kiviluoto, 1980). The cardiovascular system (Wyers,
1946; Sjöberg, 1950; Izycki et al., 1971) is commonly affected
in chronic respiratory disorders by a diagnosable accentuated
second cardiac sound on the pulmonary artery and an attenuated
first sound on the apex cordis. Most of these workers exhibit
heavy sinus arrhythmia and a shift of the EGG-axis to the right.
After extensive exposure, workers may develop bradycardia and a
change of the P wave in the second and third standard leads;
coronary spasm is also usually recognizable in such workers. A
statistically significant increase in the incidence of enlarged
liver and a decrease in functional tests together with
bilirubinaemia and a direct reaction to bilirubin have been seen
in the blood of exposed workers (Roshchin, 1968). Biochemical
alterations have also been found, such as reduction in albumins,
and expansion of the globulin fractions at the expense of gamma-
globulins, even though the total protein content remained
normal. Furthermore, a marked reduction in sulfhydryl groups in
blood-serum and in vitamin C levels in the blood, and a less
marked drop in cholesterol levels have been observed. Systemic
effects, such as a tendency towards anaemia and leukopaenia, and
basophilic granulation of leukocytes have been reported
(Watanabe et al., 1966).
Vanadium levels in whole blood and serum have been studied
to investigate the possible role of vanadium in depressive
states. In a study involving neutron activation analysis of
vanadium levels in the whole blood, serum, and hair of patients
suffering from mania or depression, and of patients who had
recovered from these conditions, as well as normal controls,
manic patients were reported to have normal levels in whole
blood and serum, but significantly raised levels in hair.
Depressed patients had raised levels in whole blood and serum.
In both conditions, raised levels fell with recovery. The
levels of vanadium in serum were correlated with those in whole
blood but not with hair levels (Naylor et al., 1984). In another
study, serum-vanadium levels, measured by neutron activation
analysis, were reported to be 3.10 ± 1.38 mg/litre in patients
suffering from depressive illness and 0.67 ± 0.32 µg/litre in
normal subjects (Simonoff et al., 1986). However, in a series
of 25 depressive, 13 recovered depressive patients, and 24
controls, the whole-blood concentrations of vanadium were
similar to normal levels, and vanadium levels did not change in
depressive patients after recovery (Ali et al., 1985).
8.2.3 Diagnosis
Information on likely exposure, the clinical picture, and
certain biochemical indications of probable exposure can aid
diagnosis, but no specific test can be recommended. Determina-
tion of the vanadium contents of the blood and especially of the
urine provides documentation of exposure, though the correlation
between vanadium levels in the urine or serum and air is poor
(Kiviluoto et al., 1979a,c). In view of the work of Schroeder
et al. (1963), it would seem desirable to measure the vanadium
contents of the serum separately from that of the cellular
elements, since the concentration of vanadium in the latter may
be more indicative of exposure levels. As reported by Watanabe
et al. (1966), a decreased urinary output of ascorbic acid may
be one characteristic of vanadium exposure, but differences from
controls do not appear sufficient to make the test clinically
useful.
Green colouration of the tongue is also an indication of
vanadium exposure (Wyers 1946; Williams, 1952; Lewis 1959b). The
green hexaquo ion (V(H2O))63+ is probably responsible for the
green-coloured tongue. However, several other bright green
complexes of vanadium+4 are known and may also account for the
sign (Cotton & Wilkinson, 1962; Durrant & Durrant, 1970). The
"green tongue" may be absent, even in prolonged exposure
(Sjöberg, 1950).
During continuous exposure, measurement of the cystine
content of fingernails was reported to be a sensitive indicator
of exposure. This parameter was negatively correlated with
vanadium exposure in workers. A decrease in cystine levels in
fingernails was demonstrated when urinary-vanadium levels were
only 0.02 - 0.03 mg/litre (Mountain et al., 1955). A similar
reduction in the cystine content of rat hair was noted when
vanadium in the diet ranged from 25 -1000 mg/kg (Mountain et
al., 1953). Some evidence suggesting that vanadium may directly
inhibit the synthesis of cystine or cysteine has also been
reported (Mountain et al., 1953, 1955).
In a recent study on workers exposed to low levels of
vanadium pentoxide (0.1 - 0.6 mg/m3) for about 14 years,
Kiviluoto et al. (198O) could not corroborate the observations
by Mountain and co-workers as no differences were found in
fingernail cystine contents between the 22 exposed workers and
22 unexposed controls. A small reduction in the cystine content
of fingernails (89 mg cystine/kg fingernail for exposed and
99 mg/kg for controls) was found by Thürauf et al. (1979) in 54
exposed workers with an increased urine-vanadium concentration
of 37.8 µg/litre (controls 0.8 µg/litre).
8.2.4 Treatment of poisoning
There are few published data on the treatment of human
poisoning by vanadium. BAL has been used successfully in two
cases of overexposure (Sjöberg, 1955). Experimentally, ascorbic
acid in doses of 125 mg/kg body weight given 20 min prior to an
LD70 dose of NaVO3H2O had a strong protective effect in mice
(Mitchell & Floyd 1956). CaNa2-EDTA was also antidotal in dogs,
when given intraperitoneally in doses of 100 mg/kg body weight
after the first sign of poisoning became evident and again 2 and
4 h later. Jones & Basinger (1983) tested various chelating
agents and their protective effects in mice and found that
efficient antidotes for both vanadate (VO33- and vanadyl (VO2+)
were ascorbic acid, deferoxamine D-penicillamine, sodium,
calcium, Na3CaDTPA, Na2CaEDTa, and glutathione. Ascorbic acid
appeared best suited for human use as an antidote.
Intraperitoneal doses of NaVO3 (0.3 - 1.2 mmol/kg body
weight) were injected in mice followed by chelating and reducing
agents at one-quarter of their respective LD50s. Significant
increases in the survival rate, 14 days after the treatment,
were noted with ascorbic acid, deferoxamine, and tiron (4,5-
dihydroxy-1,3-benzene-disulfonic acid). Other chelators tested
included EOTA, DTPA (Na3Ca-diethylene triaminepenta-acetate) and
L-cysteine. Ascorbic acid was the most effective substance in
preventing vanadium intoxication (Domingo et al., 1986). In
another report, sodium salicylate and D-L-penicillamine were
found useless as antidotes for acute toxicity caused by NaVO3.
8.3 General Population Exposure
8.3.1 Low vanadium intake
Because conditions required to achieve reproducible vanadium
deficiency in animals have not been defined precisely, it is
difficult to predict the consequences of a low vanadium intake
on human health.
Statistical studies have shown negative correlations between
environmental levels of vanadium and certain other trace
elements and the incidence of cardiovascular disease. Consump-
tion of hard water containing vanadium was associated with a
lower incidence of cardiovascular disease (Strain, 1961)a.
Schroeder (1966) reported a significant negative correlation
between the vanadium content of municipal waters and death rates
due to arteriosclerotic heart disease. In a study by Voors
(1971) on the correlation between 7 metals (calcium, chromium,
lithium, zinc, manganese, nickel, vanadium) and arteriosclerotic
heart disease, a low vanadium intake was associated signifi-
cantly with a higher incidence of arteriosclerotic heart disease
in non-white populations, but no direct correlation was
demonstrated for white populations.
____________________
a Strain, W.H. (1961) Effects of some minor elements in
animals and people. Paper presented at the meeting of the
American Association for the Advancement of Science, Denver,
29 December 1961 (unpublished).
In a joint WHO and IAEA study on the role of trace elements
in the etiology of cardiovascular diseases in 20 countries, a
significant role was shown for environmental lack of vanadium as
well as chromium, zinc, manganese, calcium, and magnesium
(Masironi, 1969). Conversely, Hickey et al. (1967) noted a
positive correlation between airborne vanadium levels and the
incidence of cardiovascular disease (section 8.3.2).
The evidence implicating vanadium as an essential trace
element for human beings is not satisfactory. Although certain
statistical studies have indicated that low vanadium intake may
be associated with human cardiovascular disease, these
relationships do not furnish any direct proof for a nutritional
role of vanadium in human health. However, they do suggest leads
for further laboratory and epidemiological investigations.
8.3.2 Epidemiological studies
Descriptive epidemiological work has been published using
a correlational approach, which has well-known limitations,
though it imitates a population-based cohort study.
Despite their limitations, such studies can give indications for
more intensive and detailed controlled studies into suspected
health hazards, comparing incidences of diseases in defined
exposure groups (such as production workers) with those obtained
from reference populations.
Stocks (1960) reported the results of a study in which
airborne concentrations of 13 trace elements were correlated
with mortality from lung cancer, pneumonia, and bronchitis in 23
localities in the United Kingdom. At concentrations ranging
from 1.1 to 42 µg/1000 m3, vanadium showed a weak association
with mortality from lung cancer (taking into consideration
population density, sex, and age), with a correlation
coefficient of 0.347. Airborne vanadium levels were also
correlated with mortality from pneumonia in males, with a
correlation coefficient for mortality from pneumonia of 0.443.
For mortality involving bronchitis, vanadium gave a correlation
coefficient of 0.563. Vanadium also showed an association with
mortality from cancers other than lung cancer in males, but not
in females. However, in this study, as is usual in studies of
this kind, it is not certain that cases of interest (lung
cancer, pneumonia) had been exposed at all. There are also the
uncertainties of mortality data and failure to consider
confounding factors.
In another study, Hickey et al. (1967) considered 10 metals
in the air, including vanadium, in 25 communities in the USA.
Various techniques, including canonical analysis, were used to
correlate airborne metal concentrations with mortality indices
for 1962 and 1963 involving 8 disease categories. The mean
atmospheric concentrations for vanadium at the various locations
ranged from 0.001 to 0.672 µg/m3. The incidence of several
diseases, including "diseases of the heart", nephritis, and
"arteriosclerotic heart", could be correlated reasonably well
with air levels of vanadium and other metals. A high inter-
correlation between vanadium and nickel was unexplained. This
study was of a very preliminary nature, with no adjustments for
the basic pertinent variables normally employed. Other studies
have demonstrated significant negative correlations between the
incidence of cardiovascular disease and environmental levels of
vanadium (section 8.3.1).
An additional multivariate analysis of air-vanadium levels
in relation to selected white male mortality levels was included
in an unpublished US Environmental Protection Agency staff study
by Pinkerton et al. (1972)a. Several categories of cardio-
vascular disease were used, and also influenza-pneumonia.
Vanadium was not correlated with the latter, but was correlated
with the cardiovascular categories. However, adjustment for
population density produced a considerable reduction in some of
these relationships. It was concluded that the observed
statistical associations of air-manganese and air-vanadium
levels were not causal associations, and represented either a
reflection of other more directly associated causes or
statistical artifacts.
Barannik et al. (1969) studied the role of certain trace
elements and the natural radioactivity of food products in the
etiology of endemic goitre in the USSR. More chromium and
vanadium and less lead were found in most of the vegetable
products from a region where goitre was endemic compared with
those from a goitre-free region. The differences in the mean
concentrations of these trace elements were statistically
significant.
The differences between these general population-based
observations and the occupational studies on health effects in
vanadium workers (section 8.3) are connected to different
approaches. The correlational epidemiological studies, based
exclusively on long-term effects and causes of death, are
considered at the expense of lack of individual exposure data,
while the medical studies, cross-sectional in nature, cannot
consider the selection effects and lack long-term information
(such as cause of death). Their strength, however, lies in the
fact that they permit analysis according to different levels of
exposure, though further occupational and population studies on
chronic illness in unambiguous relationship to vanadium exposure
are needed to verify previous work and determine if there is
evidence of a dose-response relationship. In such studies,
morbidity as well as mortality should be considered.
______________
a Pinkerton, C., Hammer, D.I., McClain, K., Williams, M.E.,
Bridbord, K., & Riggins, W.B. (1972) Relationship of
manganese and vanadium in the ambient air to heart disease
and influenza-pneumonia mortality rates, Research Triangle
Park, North Carolina, US Environmental Protection Agency
(unpublished data).
8.4 Occupational Exposure
Occupational poisoning occurs mainly during the industrial
production and use of vanadium and in boiler cleaning
operations. Under these conditions, vanadium may enter the
human body through the respiratory tract; an unknown quantity
will be transported to the alimentary tract when swallowed.
Vanadium can also enter through the skin (Roshchin, 1968).
Both acute and chronic poisoning can occur. Vanadium-
containing industrial aerosols differ in chemical and structural
composition and thus evoke different responses in the human
body.
In sections 8.3.1 - 8.3.4, a survey is made of the available
clinical and epidemiological data on the health effects of
vanadium in workers occupationally exposed to vanadium
compounds. Most of the reported clinical symptoms reflect
irritant effects of vanadium on the respiratory tract and eyes.
8.4.1 Metallurgy
Dutton (1911) first described the effects of industrial
exposure to vanadium-bearing ores. He reported a dry, paroxysmal
cough with haemoptysis and irritation of the eyes, nose, and
throat. Temporary increases in haemoglobin levels and red blood
cells were followed by reductions in both and the onset of
anaemia. Vanadium was recovered in all bodily secretions.
Postmortem examination revealed highly congested lungs with
destruction of the alveolar epithelium and congested kidneys
with evidence of haemorrhagic nephritis. Unfortunately, the
workers frequently suffered from pulmonary tuberculosis, which
undoubtedly produced many symptoms that were aggravated by
vanadium exposure, and no details regarding the number of
workers examined or the incidence of the signs and symptoms were
provided.
A later study by Symanski (1939) on relatively healthy metal
workers exposed to vanadium pentoxide dust for periods ranging
from a few months up to several years reported severe conjunct-
ivitis, rhinitis, pharyngitis, chronic productive cough, and
tightness of the chest; severe chronic bronchitis and bronchi-
ectasis sometimes occurred with longer exposure. There was no
evidence of a generalized systemic action of vanadium.
Rundberg (1939) observed bronchitis with purulent sputum,
general weakness, and skin irritation of the face and hands in
20 men handling vanadium pentoxide in a metallurgical works.
Productive cough, bronchitis, and shortness of breath were
reported by Balestra & Molfino (1942) in 25 workers exposed to
vanadium pentoxide dust from petroleum ash. Other substances
were involved, and chest X-rays showed definite lung markings
suggesting pneumonoconiosis. Bronchiectasis was suspected in 2
cases.
Studies were reported by Wyers (1946, 1948) on 50 - 90
workers exposed to vanadium pentoxide as an oil combustion
residue and to slag from the production of ferrovanadium.
Findings included bronchospasm, often with elevated blood
pressure and accentuated pulmonary second sound, a paroxsymal
cough, dyspnoea, skin pallor, tremor of fingers, palpitation,
chest pains, and reticulation of the lungs. Wyers emphasized
the irritant effects of vanadium pentoxide on the respiratory
tract, but also found evidence of systemic toxicity.
An extensive report including data on the dust contents of
the air in a metallurgical plant producing vanadium pentoxide
was published by Sjöberg (1950). The dust particles were
relatively large in size (39% less than 12 µm, 22% less than
8 µm). It was estimated that a concentration of 6.5 µg
V205/m3 represented the worst exposure conditions. Thirty-six
men between 20 and 50 years of age had been employed in the
plant since 1946: 22 had a dry cough; wheezing sounds could be
detected in 31; and 27 were short of breath. One man developed
acute pneumonitis, and 4 others developed bronchopneumonia.
There was no evidence of systemic toxicity.
A dry eczematous dermatitis developed in 9 men in Sjöberg's
(1950) study, but only 1 man showed a positive patch test.
Sjöberg (1951) and Sjöberg & Rigner (1956) believed that allergy
might play a role in the development of eczema and pneumonitis
following vanadium exposure. Zenz et al. (1962) also considered
this an explanation for the more severe symptoms found on re-
exposure in their study. In a follow-up to the 1950 study,
Sjöberg & Rigner (1956) reported that the 16 men most severely
affected still complained of shortness of breath, cough,
fatigue, and wheezing. Bronchitis was present in 2 men.
However, spirometric measurements, cardiac function tests,
electrocardiograms, haematological tests, and urinanalyses were
essentially normal.
Lewis (1959b) studied 24 male workers in an environment in
which the maximum exposure was only 0.925 mg vanadium (as
V205)/m3 of air. In most cases, the exposure was to 0.3 mg
vanadium/m3. More than 92% of the dust particles were smaller
than 0.5 µg in every process area sampled. Symptoms of cough
with sputum production, eye, nose, and throat irritation, and
wheezing were related to physical findings of wheezes, rales, or
rhonchi, injected pharynx, and green tongue. All of these
symptoms and physical findings were statistically significant in
comparison to those in 45 referents (Tables 25 and 26).
A report by Rajner (1960) on 30 vanadium workers in a
metallurgical plant described particularly severe signs and
symptoms, but did not give any estimates of exposure except in
conjunction with urinary-vanadium levels. In acutely poisoned
workers, vanadium values were about 4000 µg/litre urine. The
average value among permanent employees was 45 µg/litre;
vanadium pentoxide smelter workers had maximum values of about
400 µg/litre. When a new production process was introduced,
symptoms of acute vanadium poisoning occurred in 3 workers after
16 h of work including severe respiratory difficulties, head-
ache, dejection, and loss of appetite. Acute inflammatory
changes of the upper respiratory tract with copious mucous
production, oedema of the vocal cords, and profuse epistaxes
were reported. All workers who had been exposed for a long time
(up to 22 years in 27 subjects, mostly in ferrovanadium and
vanadium pentoxide smelting operations) complained of coughing
and eye, nose, and throat irritation, breathing difficulties
during physical exertion ("more than two-thirds of the
workers"), and headache (12 cases). Clinical findings included
intense hyperaemia of the mucosa of the nasal septum in 20
workers; perforation of the nasal septum was seen in 4 workers
exposed for an average of 18 years. Intense hyperaemia of the
mucosa of the throat and larynx with dilated fine capillaries
was found in 50% of the workers. Bronchoscopy indicated the
presence of chronic bronchitis, and bronchial smears revealed
sloughed epithelium.
Matantseva (1960) studied 77 workers in contact with
vanadium pentoxide in the form of dust and fume in concentra-
tions exceeding the MAC value (dust = 0.5 mg/m3; fumes =
0.1 mg/m3) for periods ranging from 1 to 12 years. Nearly all
the subjects had various complaints relating to the upper
respiratory tract including unpleasant sensations in the nose, a
liquid mucous discharge from the nose, obstructed nasal
breathing, a sensation of burning and dryness in the naso-
pharynx, scratching, dryness, and tickling in the throat,
hoarseness of the voice, and cough. Physical examination showed
rhinitis, which was of a simple catarrhal form in workers
exposed for less than 3 years, a hypertrophic and subatrophic
form if the exposure was for more than 3 years, and an atrophic
form if the exposure was for between 7 and 12 years. Examina-
tion of the lungs revealed acute and chronic lesions in the form
of bronchitis, peribronchitis, and pneumosclerosis. Hyper-
ventilation and an elevated basal metabolic rate were noted.
Table 25. Symptoms in 24 vanadium workers and 45 unexposed referentsa
-----------------------------------------------------------------------------
Symptom Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Cough 33.3 83.4 13.71b
Sputum 13.3 41.5 5.55c
Exertional dyspnoea 24.4 12.5 0.592
Eyes, nose, throat 6.6 62.5 23.17b
irritation
Headache 20 12.5 0.124
Palpitations 11.1 20.8 0.538
Epistaxis 0 4.2 0.148
Wheezing 0 16.6 5.20c
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
c Significant at P = 0.02.
Table 26. Physical findings in 24 vanadium workers and 45 unexposed
referentsa
-----------------------------------------------------------------------------
Physical finding Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Tremors of hands 4.5 4.2 0.0320
Hypertension 13.3 16.6 0.0002
Wheezes, rales, 0 20.8 6.93b
or rhonchi
Hepatomegaly 8.9 12.5 0.003
Eye irritation 2.2 16.6 2.94
Injected pharynx 4.4 41.5 12.62b
Green tongue 0 37.5 14.53b
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
Roshchin (1963b) published an account of the effects of
vanadium-containing Bessemer slag dust on 45 workers. Dust
concentrations in the air during various phases of this opera-
tion ranged from 5 to 150 mg/m3, with the highest concentrations
occurring during loading/unloading of broken slag as the
trivalent oxide, mostly within spinellide. Repeated examinations
of the 45 workers showed the slag dust to have an effect on the
respiratory mucosa. Subatrophic rhinitis, bronchitis, and
pneumosclerosis were seen in subjects with long occupational
exposure (11 workers). Chronic bronchitis was found in every
worker employed for 5 years or more. Clinical and X-ray
examination of all 45 subjects showed radiological changes in 24
employed for 10 years or more; in 11 subjects, pneumoconiosis of
stage I-II was diagnosed. X-ray examination showed diffuse
sclerotic changes over the whole extent of the lung fields
(except for the supraclavicular zones), small focal opacities,
intensified and enlarged shadows at the root of the lungs, and
marked signs of bullous emphysema. Predominant involvement of
the lower regions of the lungs (characteristic of silicosis) was
not present. This pneumosclerosis was accompanied by changes in
the cardiovascular and nervous systems, biochemical disturbances
(hyper-vitaminosis with dysproteinaemia and an increase in the
serum concentration of sulfhydryl groups), a tendency to anaemia
and leukopaenia, and changes in the liver.
In another study, Roshchin (1964) described chronic effects
of vanadium in 193 workers who had been exposed to aerosols of
free vanadium pentoxide: 127 worked in vanadium metallurgy and
66 were boiler cleaners (section 8.3.2). The length of
occupational contact with vanadium was over 10 years for 60%,
from 5 to 10 years for 30%, and under 5 years for the remaining
10%. Practically all complained of irritation of the nasal and
pharyngeal mucosa including itching, a profusely running nose
(especially during work), and unpleasant sensations in the
throat and nose. Epistaxis was frequent in 20%. Physical
examination revealed a high incidence of changes in the nasal
mucosa: dryness (40%), erosion (23%), scars (8%), perforation
(4%), hyperaemia (10%), and hypertrophy (7%). Also noted were
dryness of the pharynx (5%), hyperaemia of the pharynx (5%),
hyperaemia of the larynx (4%), and tonsillitis (5%). The most
common pathological changes in the upper respiratory tract were
subatrophic rhinitis (40%) and destructive changes in nasal
mucosa (35%), while hypertrophic rhinitis was less frequently
seen (7%). The overwhelming majority had a dry cough; cough
with viscid sputum was less common. Workers with longer
occupational exposure complained of shortness of breath, which
appeared sometimes after 5 but mostly after 10 years of work in
the industry. Nearly all complained of aching or shooting pains
in the chest and of lassitude and weakness. The main respiratory
diseases diagnosed were chronic bronchitis (40%) and diffuse
pneumosclerosis (13%). Haematological tests showed the total
serum-protein concentration to be normal, y-globulins to be
raised (19.4% compared with 12.2% in controls), and the albumin-
globulin ratio to be 1:1 - 1:2 (1:9 in controls). Determination
of total, residual, and protein sulfhydryl groups in the blood-
serum revealed a marked decrease of 7 - 13% compared with the
controls. Regular observations over a period of 14 years showed
that the chronic bronchitis tended to get worse, with develop-
ment of bronchospasm. After a long period of time, some subjects
developed pneumosclerosis; in others, the disease progressed
slowly from chronic bronchitis to diffuse pneumosclerosis and
pulmonary emphysema.
Eisler et al. (1968) studied 48 metallurgical workers
occupationally exposed to vanadium for between 17.6 ± 9 years.
Definite clinical evidence of chronic bronchitis was present in
90% of the subjects, and 50% had severe obstructive bronchitis.
In control groups, which included basic-slag crushers and
furnace operators (99 and 50, respectively), chronic bronchitis
was observed in 33% and 26%, respectively.
A study on 13 workers engaged in the extraction and refining
of vanadium pentoxide from soot generated by the combustion of
heavy fuel oil was reported by Nishiyama et al. (1977).
Concentrations of vanadium in the air at various locations in
the work environment were all less than 0.5 mg/m3 (mean, 1.2 -
12 µg/m3). There was a significant incidence of injection of
the pharynx (58.3%) compared with controls. Elevated levels of
vanadium in the urine and hair were detected both in currently-
exposed as well as in previously-exposed subjects. Apart from a
slight depression in serum-cholesterol levels, haematological
results were normal.
Roshchin (1968) analysed the incidence of influenza and
upper respiratory catarrh as a cause of lost working time in
workers in vanadium metallurgical plants compared with ferrous
metallurgical workers in adjacent plants. The results are given
in Table 27. The morbidity was consistently higher among
workers producing vanadium than among workers in other
departments in all years.
Table 27. Morbidity from influenza and upper respiratory catarrha
-----------------------------------------------------------------------------
Department Cases Days off work
(per 100 workers) (per 100 workers)
1958 1959 1960 1962 1958 1959 1960 1962
-----------------------------------------------------------------------------
Vanadium plant 40.6 68.4 58.8 59.8 180.6 376.7 271.9 336.5
Open hearth furnace 22.2 53.2 45.6 62.1 76.9 331.1 176 213.3
Blast furnace 21.9 46.7 37.8 47.6 36.3 262.8 166.6 215.5
Engineering shop 19.6 39.1 33.9 44.9 86.8 224.8 154.3 223.6
-----------------------------------------------------------------------------
a From: Roshchin (1968).
Asthma was reported in 4 workers exposed to vanadium
pentoxide dust in a newly established vanadium pentoxide
refinery (Musk & Tees, 1982). One of the workers had positive
skin tests to environmental allergens; the others were non-
atopic. Three were smokers; one was an ex-smoker. One of the
subjects experienced irritation of the upper respiratory tract
after a single exposure; dyspnoea and wheezing developed 2 weeks
later. All workers had similar irritant symptoms and green
tongue. Two showed bronchial hyperreactivity when challenged
with histamine; these were the workers with the most recent
exposure. In one worker, the asthmatic symptoms continued for 8
weeks after cessation of exposure. There was no indication of
an immunological aetiology, and the authors concluded that the
effect was likely to be a direct chemical one.
Kiviluoto et al. (1979a,b, 1980, 1981a,b) and Kiviluoto
(1980) reported the results of a cross-sectional study on 63
males exposed to vanadium-containing dust in a vanadium factory;
a reference group matched for age and smoking was selected from
a magnetite ore mine. The workers had been exposed to vanadium
dust for an average of 11 years at concentrations ranging from
0.1 to 3.9 mg/m3 (estimated average exposure levels of 0.2 -
0.5 mg/m3); the respirable fraction (< 5 µm) was 20%. Nasal
biopsies and lung function tests were taken at the end of the
summer holidays (duration, 2 - 4 weeks). Nasal smears and
biopsies were repeated in 31 workers, 7 - 11 months later, after
hygienic improvements had reduced the exposure levels to 0.01 -
0.04 mg/m3. Microscopic examination of nasal smears revealed a
significant increase in neutrophils and biopsies of nasal mucosa
showed significantly elevated numbers of plasma and round cells
in the exposed workers. There was no further increase in the
cell findings after 10 months of exposure to 0.01 - 0.04 mg/m3
vanadium dust; eosinophils did not show any differences between
the exposed and the referents. The authors attributed these
findings to "an irritating effect of vanadium dust on the mucous
membranes of the upper respiratory tract". Biopsies from
workers with the longest exposures (170 - 241 months) showed "a
zone-like sub-epithelial infiltration of mononuclear cells and
frequent papillarity in the mucous membrane surface with its
hyperaemic capillaries". The similarity between this pattern
and that seen in vanadium-exposed rabbits (Sjöberg, 1950) was
noted (Kiviluoto et al., 1979b). A random sample of 12 nasal
biopsies was further investigated for the amount and classes of
immunoglobulins (IgE, IgG, IgM, and IgD). IgG subclasses were
not studied. There were no differences between the 12 workers
and their referents, which was construed as a further indication
of non-specific inflammation (Kiviluoto et al., 1981b).
Pulmonary condition was assessed by means of questionnaires,
X-ray, and pulmonary function testing. There was only one
significant difference between the workers exposed for an
average of 11 years to 0.1 - 3.9 mg/m3 (estimated average, 0.2 -
0.5 mg/m3) and at the time of investigation to 0.01 -
0.04 mg/m3, and their matched referents; complaints of wheeze
were more common in the exposed worker group (Kiviluoto, 1980).
The importance of this finding remained doubtful. It may
reflect the respiratory findings mentioned above, since upper
respiratory irritation may be accompanied by transient reflex
bronchospasm. A series of laboratory tests were designed to
evaluate electrolyte, protein fractions, carbohydrate, and
lipids, liver, renal, muscle, pancreatic, and bone marrow
functions, and immunological status. There were no decreases in
serum-cholesterol or triglycerides, and no clinical differences
between worker and control groups (Kiviluoto et al., 1981a).
The effects of vanadium compounds on health is not confined
to the development of local respiratory or other lesions.
Various studies, most of them rather old, on patterns of lost
working time due to morbidity have shown that the incidence of
disease among workers in plants producing vanadium compounds is
considerably higher than among other workers (Symanski, 1939;
Syers, 1946; Sjöberg, 1950, 1956; Reznik, 1954; Reinl, 1958;
Matantseva, 1961; Watanabe et al., 1966; Roshchin, 1968, 1969;
Athanassiadis, 1969; Schumann-Vogt, 1969; Chiriatti, 1971). The
most significant differences are found in the incidences of
influenza, upper respiratory catarrh, and inflammation of the
lungs. The difference in the incidence of bronchitis is
particularly marked.
8.4.2 Cleaning and related operations on oil-fired boilers
Bronchitis and conjunctivitis resulting from exposure to
soot (containing 6 - 11% vanadium) during the cleaning of the
stacks of oil-fired boilers were first recognized by Frost
(1951). Frost did not report any other effects, but, in a
subsequent report of a boiler-cleaning operation by Williams
(1952), sneezing, nasal discharge, lachrymation, sore throat,
and substernal pain occurred within 0.5 - 12 h of exposure.
Within 6 - 24 h, secondary symptoms developed; these consisted
of dry cough, wheezing, laboured breathing, lassitude, and
depression. In some cases, the cough became paroxysmal and
productive. Symptoms lessened only after removal from the
working environment for 3 days. Air sampling showed most of the
dust particles to be smaller than 1 µg. The vanadium concen-
tration ranged from 17.2 mg/m3 in a superheater chamber to
58.6 mg/m3 in a combustion chamber. Roshchin (1962) observed 8
cases of acute vanadium poisoning in workers who cleaned boiler
flues at power stations burning high-sulfur oil. Analysis of
soot deposits showed that the soot in the region of greatest
dust formation (the pipes of the steam superheater and water
economizer) contained from 24 to 40% vanadium pentoxide. The
workers carried out cleaning operations without respirators or
with respirators that did not provide the necessary protection.
After cleaning the boilers, the workers developed acute vanadium
poisoning: itching in the throat, sneezing, cough with difficult
expectoration, and smarting eyes. On the following days, the
symptoms became more severe. Tightness in the chest, sweating,
general weakness, conjunctivitis, and noticeable loss of weight
developed. On examination one week later, hyperaemia and oedema
of the fauces and posterior pharyngeal wall were observed.
Harsh breathing sounds and dry crepitations were heard in the
lungs. X-ray examination showed intensified lung markings in
the middle zones of the right and left lungs and thickening of
the fissure on the right. One month later, only one worker
still had cough, weakness, perspiration, loss of energy, and
dyspnoea. The other workers recovered quickly, with complete
disappearance of cough and shortness of breath.
In another study on workers engaged in boiler-cleaning
operations (Troppens, 1969), the symptoms were described as
similar to mild coryza or influenza with bronchitis. Following
recovery, workers were tired, debilitated, irritable, without
any appetite, and complained of watery eyes. The first symptoms
were swelling of face and eyes as early as 20 min after entering
the boiler area. Removal from exposure for 2 - 3 weeks resulted
in the disappearance of symptoms. Skin blemishes described as
allergic dermatoses were attributed to absorption of vanadium
through sensitive skin. Troppens claimed that there was an
increased susceptibility of the vanadium worker to asthmatic
bronchitis and emphysema.
An investigation is reported on 53 workers performing
emergency repair work on oil-fired power station boilers (Izycki
et al., 1971). They were exposed to vanadium pentoxide in
average concentrations of from 1.2 to 11 mg/m3 and also to
manganese, calcium, and nickel oxides, and sulfur compounds.
Characteristic features of both acute and chronic vanadium
poisoning included upper respiratory catarrh in 45%, increased
lung markings in 24.5%, and bradycardia in 22% of cases.
Persistent chronic changes in the respiratory tract (rhinitis,
pharyngeal catarrh, laryngitis, and changes in the paranasal
sinuses) were present in 45%.
Milby (1974) reported 21 cases of vanadium poisoning in
boilermakers installing new catalytic-converter tubes. This
work involved marble-sized pellets of vanadium containing 11.7%
V2O5. The dust formed during the shaking of these pellets had a
particle size of 1.1 - 1.5 µm. After working for 72 h, the
workers began to complain of nasal, eye, and bronchial
irritation. By the 4th day, most felt very ill, with signs of
irritation of the upper respiratory tract and eyes and pains in
the chest.
In a study by Garlej (1974) 50 workers engaged in the
cleaning of oil-fired boilers were compared with a control group
of 60 other workers. Boiler deposits contained 44 -65% V2O5;
the maximum exposure was estimated to be 10 mg/m3. Although no
clinical evidence of vanadium poisoning was seen, a number of
exposure-dependent positive biochemical reactions were found in
the boiler-cleaning group. Urinary excretion of delta-amino-
levulinic acid (ALA), porphobilinogen (PBG), and porphyrin
increased beyond the physiological limit, and the positive Nadi
reaction (with associated green fluorescence) occurred. The
increased excretion of cytochrome (as indicated by the Nadi
reaction) suggested oxidation through V2O5 of the thiol group
-SH cysteine in the protein carrier, resulting in decreased
binding of cytochrome in the mitochondria.
A study on 17 men who were engaged in cleaning boilers at an
electric generating station was reported by Lees (1980). In
addition to clinical findings, which were similar to those
described above, urine-vanadium levels were determined, and
pulmonary function measurements were made for a week following
exposure. Sixteen of the men wore protective clothing, and
respirators that were found to have about 9% leakage. One
workman volunteered to wear only a simple oro-nasal dust mask
for 1 h of exposure. The dust exposure level was estimated to
be 26 mg/m3; respirable dust (under 10 µm) was measured at
523 µg/m3 with a vanadium content of 15.3%. All of the men
developed reduced pulmonary function that had not fully returned
to normal in one week, but did so after one month. Reduced
function outlasted the clinical symptoms by several days. Fig. 3
shows the contrast in pulmonary reaction between the more
heavily exposed individual and one of the other workmen. The
urine-vanadium level of the volunteer was 280 µg/litre, whereas
those of the remainder of the workers were below 40 µg/litre.
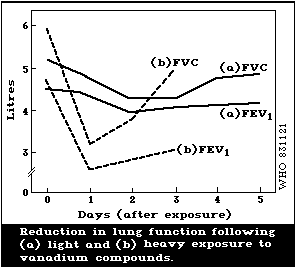 Other observations of boiler-cleaning operations have been
made by Fallentin & Frost (1954), Sjöberg (1955), Thomas &
Stiebris (1956), Hickling (1958), and Kuzelova et al. (1975).
In terms of respiratory symptoms relating to boiler-cleaning, it
should be noted that sulfates and sulfuric acid may be present
in boiler soot and may be partly responsible for irritative
effects. Hudson (1964) suggested that the quick onset of
symptoms (lachrymation with nose and throat irritation) with
rapid recovery following removal from exposure is character-
istic of exposure to acid sulfates. Response to vanadium expo-
sure is characterized by some delay in the onset of irritative
symptoms (a few hours to several days) and persistence of
symptoms following removal from exposure (Hudson, 1964).
A recent report by Levy et al. (1984) concerned a
comparatively high incidence of severe respiratory tract
irritation in boilermakers (74/100), many of them welders in
areas without adequate ventilation, exposed to vanadium
pentoxide fumes in a power plant where conversion from oil- to
coal-burning occurred. The severe illness of 70 men caused an
average of 5 days of absence, some objective tests (e.g., FVC)
being markedly affected. The vanadium pentoxide content was
above the permissible exposure limit in 8 samples, and this
resulted in litigation for inadequate protection of the
workers.
Kuzelova et al. (1977) drew attention to the occupational
risk of chimney sweeps cleaning large-capacity heating
facilities in large housing settlements. This coincided with a
report of a detailed cross-sectional examination of 121 chimney
sweeps by Holzhauer & Schaller (1977) in the Federal Republic of
Germany with an average exposure duration of 19 years ( ± 5
years). Vanadium exposure was determined by personal samples,
and measurements between 0.73 and 13.7 mg vanadium pentoxide/day
were determined compared with 4 µg in the normal (average)
population. Urinary excretion was determined to be between 0.15
and 13 µg/litre, which was significantly higher than the values
in 31 referents. The main complaints of the chimney sweeps were
wheezing, rhinitis, conjunctival irritation, cough, sputum
dyspnoea, and hoarseness; there were no skin symptoms. A
prospective follow-up of the cohort was emphasized, but the
results are not yet available.
8.4.3 Handling of pure vanadium pentoxide or vanadate dusts
Health effects due to occupational handling of pure vanadium
pentoxide or vanadate dusts have been reported. Tara et al.
(1953) described the effects of vanadium exposure in 4 dock
workers who unloaded and bagged spilled calcium vanadate. The
symptoms (bronchitic wheezing sounds, dyspnoea, productive
cough, haemoptysis in one case, and headache) necessitated
interruption of the work after 1´ days. Zenz et al. (1962)
described an acute illness that occurred in 18 workers pellet-
izing pure vanadium pentoxide; it was characterized by a rapidly
developing mild conjunctivitis, severe pharyngeal irritation, a
non-productive persistent cough, diffuse rales, and broncho-
spasm. With severe exposure, 4 men complained of itching skin
and a sensation of heat in the face and forearms. The symptoms
became more severe after each exposure, suggesting a sensitivity
reaction, but their duration was not prolonged by repeated
exposures.
8.4.4 Other industries
Browne (1955) studied vanadium poisoning in 12 patients
exposed to exhaust fumes from gas turbines using heavy fuel oil.
Evidence of poisoning appeared between the first and 14th day of
exposure and consisted of conjunctivitis, rhinitis, cough,
crepitations, and dyspnoea. Bleeding appeared before the
rhinorrhoea.
Other occupations in which respiratory effects of vanadium
exposure have been reported include operations connected with
the gasification of fuel oil (Fear & Tyrer, 1958) and the
manufacture of phosphor for television picture tubes (Tebrock &
Machle, 1968). In the latter study, elevated blood pressure was
noted in men exposed to vanadium pentoxide.
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1 Environmental Levels and Exposures
While vanadium concentrations in the air of remote rural
areas are less than 1 ng/m3, other rural areas show concentra-
tions in excess of 50 ng/m3. This is generally considered to
reflect the local burning of fuel oil with a high vanadium
content. Typical concentrations in urban air may range from
below 1 ng/m3 to over 300 ng/m3, with annual averages for large
cities of about 20 - 100 ng/m3. At an annual average of
50 ng/m3 and a respiration rate of 20 m3, the total amount of
vanadium reaching the respiratory tract would be only 1 µg.
Assuming a rate of absorption of about 25%, the direct daily
contribution of vanadium from air would be about 250 ng.
Drinking-water supplies without excessive vanadium pollution
contain from less than 1 µg/litre to occasional maximum
concentrations of 15 - 30 µg/litre. Two comprehensive surveys
have shown average concentrations of 4.3 and 4.85 µg/litre,
respectively. At a daily intake of 2 litres of water, the
average daily intake of vanadium with water would be about
10 µg, ranging from about 1 µg to 30 - 60 µg. Although
levels in ordinary water supplies would vary considerably,
intake should rarely exceed 100 µg/day. Intake with bottled
waters from mineral springs may exceed these values.
As a rule, the concentration of vanadium in food is low.
High levels reported in early studies have been attributed to
analytical differences. Recent studies on complete diets
suggest a daily intake of vanadium of about 10 - 70 µg, with
the majority of estimates remaining below 30 µg. Assuming an
absorption rate from the gastrointestinal tract of 1 - 2%, the
contribution from food and water is unlikely to exceed 4 -
5 µg/day.
Vanadium concentrations in air in the vicinity of metal-
lurgical industries are often about 1 µg/m3. In the production
of vanadium metal or compounds, concentrations may reach a few
mg/m3. In boiler-cleaning operations, dust concentrations in
air are frequently around 50 - 100 mg/m3, and concentrations as
high as 500 mg/m3 have been reported; the vanadium content of
the dust is about 5 - 17% as vanadium pentoxide and 3 - 10 % as
lower vanadium oxides. The need for personal protection devices
in such operations is obvious.
9.2 Physiological Role
While present knowledge indicates that vanadium is an
essential element for chicks and rats, conclusive evidence that
vanadium is essential for other species, including man, is
lacking. A variety of physiological and biochemical processes
have been found to be vanadium sensitive. However, so far,
there is no evidence of adverse effects arising from vanadium
deficiency in man, and the daily requirement of vanadium in the
diet is not known.
9.3 Effects and Dose-Response Relationships
The toxicity of vanadium varies in experimental animals with
both the species and route of administration. Small animals,
such as the rat and mouse, tolerate the metal better than the
rabbit and horse. The toxicity of vanadium is low when given
orally, moderate when inhaled, and high when injected. As a
rule, the toxicity of vanadium increases as the valency
increases, pentavalent vanadium being the most toxic.
9.3.1 Local effects and dose-response relationships
Exposure of 2 volunteers to vanadium pentoxide dust at
1 mg/m3 for 8 h resulted in irritation of the respiratory tract
with cough starting 5 h later. The cough lasted for 8 days.
Exposure of 5 volunteers to a concentration of 0.2 mg/m3 caused
the same symptoms, i.e., coughing, but with an onset at 20 h.
The cough lasted for 7 - 10 days. Respiratory irritation was
noted in 2 subjects exposed to 0.1 mg/m3 for 8 h. The irritant
effect was manifested as an increase in mucous production, 24 h
after exposure, and total recovery within 4 days. Tickling and
itching, together with dryness of the mucous membranes of the
mouth, was reported by 11 volunteers exposed to 0.4 mg/m3 of
vanadium pentoxide condensation aerosol; 0.16 mg/m3 caused
irritation in only 5 subjects, and 0.08 mg/m3 did not induce
symptoms in any of the subjects.
Exposure to high concentrations of vanadium is possible in
the industrial production and use of vanadium, especially in the
cleaning of oil-fired boilers. Frequently reported irritant
symptoms include sneezing, nasal discharge, irritation of the
eyes with lachrymation, sore throat, dry or productive cough,
and chest pain. Normally, such symptoms disappear in a few days
when exposure has ceased. Cough, increased sputum, and
particularly irritation of the eyes, nose, and throat occurred
among 24 male workers exposed to a maximum of 0.9 - 5 mg
vanadium/m3 (measured as vanadium pentoxide V2O5). Most workers
had been exposed to 0.3 mg/m3 (section 8.4.1). A cross-
sectional study of 63 male workers exposed for an average of 11
years to vanadium-containing dust at 0.2 - 0.5 mg vanadium/m3
(range, 0.1 - 3.9 mg/m3) showed chronic irritant effects in the
mucous membranes of the nose. The nasal changes persisted
unchanged during subsequent exposure to much lower levels
(0.01 - 0.04 mg/m3).
Heavily exposed workers (dust concentrations of 5 -
150 mg/m3) developed atrophic rhinitis and chronic bronchitis.
Bronchospasm is also a feature in heavily exposed workers.
The effects on 63 workers of long-term exposure to vanadium
at 0.2 - 0.5 mg/m3 were studied using matched referents, a
questionnaire on respiratory symptoms, chest radiography, and
lung function testing (section 8.4). There was no change in
ventilatory function compared with the matched reference group;
only complaints of wheezing were significantly more common among
exposed workers than among referents. However, in another study
the forced vital capacity (FVC) was reversibly reduced in 17
boiler cleaners who had been exposed to a time-weighted average
respirable dust of 523 µg/3 containing 15% of vanadium (section
8.3.1).
Vanadium poses weak sensitizing properties when skin and
mucous membranes of the upper respiratory tract are exposed to
high concentrations, manifested by the development of allergic
dermatitis and rhinitis in workers in contact with vanadium.
The allergic nature of these manifestations is proved by
positive reactions of epicutaneous tests with a 2% solution of
sodium vanadate. There is information on the sensitizing effect
of vanadium in tests on animals.
In one study (section 8.4.1), 9 workers out of 36 developed
a dry eczematous dermatitis. These workers had been exposed to
vanadium pentoxide at about 6.5 µg/m3.
Green tongue is seen in a proportion of workers exposed to
vanadium-containing dust, and is an indication of exposure.
9.3.2 Systemic effects and dose-response relationships
9.3.2.1 Metabolic effects
The effect of vanadium on dental caries remains a debatable
issue (section 5.4.2). The application of a 50% paste of an
ammonium salt of vanadium and glycerol was reported to reduce
caries in children aged 7 - 11 years. Other studies between
1955 and 1968 have failed to demonstrate a clearly beneficial
effect (section 8.1.2.1).
Soluble diammonium oxytartarovanadate (150 - 200 mg/day for
6 weeks) was administered to 5 healthy adult male volunteers.
There was a significant reduction of plasma-cholesterol levels
at the end of the period. A temporary drop in the cholesterol
level was also observed in 2 out of 6 patients given ammonium
vanadyl tartrate for 7 weeks at 50 or 100 mg/day. The results
were not convincing and the temporary drops in cholesterol
levels were not statistically significant. No significant
changes in serum-cholesterol levels were noted in 12 patients (9
of whom were hypercholesterolaemic) given diammonium vanado-
tartrate orally for 6 months, (25 mg three times daily for 2
weeks, increased to 125 mg daily in 10 patients). Although some
studies on rats and rabbits have indicated decreasing
cholesterol levels following administration of vanadium and have
corroborated the reduced levels of cholesterol observed by other
authors, this effect of vanadium has not been convincingly
demonstrated in human beings so far (section 8.1.2.1).
Vanadium pentoxide in the diet (25 - 1000 mg vanadium/kg)
resulted in lower levels of cystine in the hair of rats compared
with those in controls, indicating the inhibition of cystine
synthesis (section 7.2.1). Rats administered sodium vanadate
intraperitoneally (5 - 10 mg/kg body weight as a single
injection or a dietary concentration of 500 mg/kg) showed
reduction of co-enzyme A in the liver; this has been construed
as an explanation of the reduction of cystine (section 7.2.1).
When workers were exposed to vanadium-containing dust (0.2 -
0.5 mg vanadium/m3, at the time of the study), there was no
correlation between exposure level and cystine levels in
fingernails, and no decrease in levels of serum-cholesterol or
triglycerides (section 8.4.1).
The data on the effects of vanadium on haematopoiesis are
inconsistent. A favourable effect of vanadium chloride (0.6 mg
vanadium/kg diet) on haemoglobin levels in rats, previously made
anaemic, had already been suggested in 1931 (section 7.2). A
small increase in erythrocytes and haemoglobin levels was
observed in rabbits given vanadyl sulfate subcutaneously, at
1 mg/kg body weight daily, for 2 months). When 32 vanadium
workers who had been exposed for more than 6 months were
compared with 45 referents, matched for age, no differences were
seen in haematocrit levels (8.2.1.1). It is not possible to
assess the effects of low-level vanadium exposure on iron
metabolism.
9.3.2.2 Effects on the nervous system
Systemic effects are rare in workers exposed to vanadium
compounds. Non-specific signs and symptoms including headache,
weakness, nausea, vomiting, and tinnitus have been reported.
Such signs and symptoms have mostly occurred in workers exposed
to extremely high dust concentrations, when cleaning oil-fired
boilers, but it has not been possible to derive dose-response
relationships for them.
Elevated vanadium levels in whole blood and serum have been
reported in patients suffering from depressive illness. In one
report, the vanadium levels fell to normal with recovery of the
patients. The role of vanadium in depressive states is not
known (section 8.2.2).
In mice and rats, repeated oral administration of vanadium
pentoxide or ammonium vanadate at doses of 0.05 - 0.5 mg
vanadium/kg body weight, daily, for 6 months and 21 days,
respectively, resulted in impaired conditioned reflexes. Daily
oral doses of sodium metavanadate (3.2 µg/kg body weight per
day for 10 - 15 days) caused increases in the activity of
cytochrome oxidase in the brain of guinea-pigs; a dose of
128 µg/kg per day did not have any effect, whereas 5.12 mg/kg
per day reduced the activity (section 7.2). Total
cholinesterase activity in the brain of rats was significantly
reduced by the intraperitoneal administration of 1 mg vanadyl
sulfate/kg body weight (section 7.3).
9.3.2.3 Effects on the liver
There are insufficient human data to make an assessment of
the effects of vanadium on the liver. Rats and rabbits exposed
through inhalation to vanadium pentoxide, trioxide, or tri-
chloride (10 - 70 mg/kg, 2 h/day, for 9 - 12 months) showed
fatty changes with partial cell necrosis in the liver. A clear
reduction in the liver tissue respiration and a decrease in the
albumin/globulin ratio in the serum were also observed. Subcu-
taneous injection of ammonium vanadate (1 mg vanadium/kg body
weight per day, for 30 days) caused similar fatty changes in the
liver of rats. Intraperitoneal injections of sodium meta-
vanadate (1.25 - 2.5 mg vanadium/kg body weight) in rats caused
loss of weight. The toxic effects observed were correlated with
the concentration of vanadium in the liver (section 7.2).
9.3.2.4 Effects on the kidney
Data on the effects of vanadium on the human kidney are
lacking. Intravenous injection of sodium metavanadate (2.5 -
5 mg/kg body weight) in male dogs resulted in albuminuria. In
mice, acute tubular necrosis followed subcutaneous injection of
ammonium vanadate at a dose equivalent to 20 mg vanadium/kg body
weight. Rats and rabbits inhaling vanadium chloride (70 mg/m3
2 h/day, for 9 - 12 months) caused fatty changes in the kidney.
Vanadate has diuretic and natriuretic effects on the kidney in
the rat but not in the dog or cat. Vanadate has also been
reported to increase the urinary excretion of calcium,
phosphate, bicarbonate, and chloride by the rat kidney. These
diuretic and natriuretic effects are thought to be due to the
inhibition of Na+-K+-ATPase causing inhibition of the tubular
reabsorption.
9.3.2.5 Cardiovascular effects
Palpitation of the heart at rest and on exercise has been
reported in workers occupationally exposed to vanadium.
Transient coronary insufficiency, a high incidence of extra-
systoles and bradycardia were reported (section 8.3). Exposure
of workers to low levels of vanadium pentoxide (0.2 - 0.5 mg/m3)
did not cause any pathological changes in the blood picture
(section 8.4.1).
Electrocardiographic changes (ST-segment depression,
increased T-wave amplitudes) were seen after intravenous
injection of sodium metavanadate in dogs (2.5 - 5 mg/kg body
weight). Long-term inhalation exposure of rats and rabbits to
vanadium pentoxide, trioxide, or chloride (10 - 70 mg/m3,
2 h/day, for 9 - 12 months) caused fatty changes in the myo-
cardium as well as perivascular swelling.
9.3.2.6 Pulmonary effects
Asthmatic reactions in conjunction with non-specific
bronchial hyperreactivity have occasionally been reported in
refinery workers exposed to vanadium pentoxide dust. There has
not been any evidence of an immunological mechanism behind such
cases. A dose-dependent decline in forced expiratory volume in
one second (FEV1) and forced vital capacity (FVC) has been
demonstrated in boiler cleaners. The functional increase did
not return to normal during the first week following exposure,
but fully recovered within one month. The mechanism leading to
the obstructive pulmonary impairment has not been clarified.
9.3.2.7 Effects on the immune system
In mice, vanadium and ammonium vanadate affect the normal
function of the immune system. Vanadium had slight depressant
effects on antibody-forming cells and increased DNA synthesis in
splenic leukocytes. Ammonium vanadate increased resistance
to E. coli endotoxin, but decreased resistance to Listeria
lethality. In the spleen, it increased the rosetting capability
of leukocytes, the formation of megakaryocytes, and red blood
cell precursors (section 7.7).
9.3.3 Reproduction, embryotoxicity, and teratogenicity
Human data on the effects of vanadium on reproduction and
embryotoxicity are lacking. Vanadium administered to pregnant
rats by subcutaneous administration of metavanadate (0.85 mg/kg,
equal to 1/20 LD50) accumulated in the placenta. However, the
extent to which it reaches the fetus has not been clearly
established. During the lactation period, vanadium was found in
the mammary glands and was excreted with milk. Morphological
changes in spermatozoa as well as desquamation of spermatogenic
epithelium in the seminal tubuli were observed. Gonadotoxic
effects were suggested by the absence of fertilization of female
rats by male rats that had been exposed to 0.85 mg vanadium/kg
body weight. The same doses of vanadium given to female rats on
the fourth day of pregnancy significantly decreased the number
of fetuses (section 7.8.1).
Weanling pigs receiving vanadate (200 mg vanadium/kg body
weight) showed a suppressed growth rate and increased mortality.
Vanadium was not markedly toxic when fed to growing lambs
(200 mg/kg, 84 days) (section 7.8.1).
Tentative results suggest that vanadium is teratogenic for
rats and hamsters causing skeletal anomalies and death of the
fetuses. Dose-response relationships have not been demonstrated
(section 7.8.2). There are no human data concerning possible
teratogenic effects of vanadium.
9.3.4 Mutagenicity
The data on the mutagenic potential of vanadium in bacterial
systems are inconclusive. There are positive and negative
results with E. coli and Salmonella tests (section 7.9). Data
suggest the induction of micronuclei, but not sister chromatid
exchange or dominant-lethal mutations. Chromosome effects in
vivo and in vitro have not been studied.
9.3.5 Carcinogenicity
Life-time studies on mice given 5 µg vanadyl ions/ml as the
sulfate in drinking-water did not increase the incidence of
spontaneous tumours and intraperitoneal injections of
vanadium(III)2.4pentanedione (24, 60, or 120 mg/kg body weight)
did not increase the incidence of lung adenomas in mice. The
results of a long-term study on rats with intrabronchiolar
implants of vanadium solids were negative. The few studies
available do not provide any indications of carcinogenic effects
of vanadium (section 7.10).
9.3.6 Risks from exposure of the general population
There are only a few studies on the possible effects of
vanadium in ambient air on the general population (section
8.3.2). In one study, air concentrations of vanadium together
with 12 other trace elements were found to be correlated with
mortality from pneumonia and lung cancer (coefficients of
correlation of 0.443 and 0.347, respectively) and also with
mortality from bronchitis (coefficient of correlation of 0.563).
In another study, a correlation between the levels of vanadium,
cadmium, zinc, tin, and nickel and the incidence of several
diseases including "diseases of the heart", "nephritis", and
"arteriosclerotic heart" was claimed, but the results of these
studies do not establish any causal relationships.
10. RECOMMENDATIONS
There is a conspicuous lack of data on several aspects of
the health effects of vanadium compounds. The overwhelming bulk
of recent research focuses on the effects of vanadium on
biochemical systems, especially specific effects on enzymes, and
there are major gaps in knowledge with respect to analytical,
metabolic, and exposure data.
There are indications of a weak mutagenic effect of
vanadium, but the data are partly conflicting and
uncorroborated. Further confirmative mutagenicity studies,
including studies on chromosomal effects, should be given high
priority. Data on the carcinogenicity of vanadium in various
species are practically non-existent. Such studies are urgent
and should be conducted as long-term exposure studies.
Vanadium induces toxic effects on the fetus. However,
whether these are direct effects or indirect effects resulting
from effects of vanadium on the mother is not known. Studies to
assess the nature of the teratogenic effects and the mechanism
behind them should be encouraged.
Effects resulting from high occupational exposure to
vanadium dusts have been reasonably well described. Such
exposure levels may cause a variety of clinical manifestations.
However, they should be remedied by hygienic and technical
improvements. Dose-effect and dose-response relationships have
not been well defined at low exposure levels in the range of
approximately 0.01 - 0.5 mg/m3. It is considered important to
develop specific indicators for the detection of early adverse
effects of vanadium on man.
Epidemiological studies on occupational cohorts, paying
particular attention to exposure levels and using proper
referent groups, should be encouraged. The literature is
inconsistent regarding the documentation of sensitizing
properties of vanadium compounds. This is an important aspect
with regard to worker protection.
An area of great importance is the exposure of the general
population. There are considerable geographical variations in
vanadium concentrations in air and water. Epidemiological
studies on populations living in areas with high vanadium
exposure should be carried out, relating possible adverse
effects to exposure levels. Such studies should take into
account possible interactions with other pollutants.
REFERENCES
ACGIH (1986) Threshold limit values for chemical substances
and physical agents in the work environment , Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists.
AGASYAN, P.K., LAKEEVA, T.N., NIKOLAEVA, E.R., PARTASHNIKOVA,
M.Z., SHKOLENOK, G.F., & KHREPINYUK, T.E. (1975) [Determina-
tion of the bulk substances in preparations of potassium
dichromate and ammonium vanadate by means of differential
coulometry.] Zavod. Lab ., 41(4): 385-387 (in Russian).
AIYAR, A.S. & SREENIVASAN, A. (1961) Effect of vanadium
administration on coenzyme Q metabolism in rats. Proc. Soc.
Exp. Biol. Med ., 107: 914-916.
ALESHIN, G.N., SMOLJANINOV, S.I., MESHEYAKOV, R.P., & GLOUCHOV,
G.G. (1974) [The chemistry and technology of vanadium
compounds.] In: Ivakin, A.A. & Voronova, E.M., ed. [ Proceedings
of the International Conference on the Chemistry and Technology
of Vanadium Compounds ,] Perm, Scientific Institute of Black
Metals (in Russian).
ALI, S.A., PEET, M., & WARD, N.I. (1985) Blood levels of
vanadium, caesium, and other elements in depressive patients. J.
affective Disord ., 9(2): 187-191.
ALLAWAY, W.H. (l968) Selenium, molybdenum, and vanadium in
human blood. Arch. environ. Health , 16: 342-348.
ANBAR, M. & INBAR, M. (1962) Effect of pyrodoxial 5-phosphate
in the presence of vanadyl ions of the de-iodination of
thyroxine. Nature (Lond.) , 196: 1213.
ARNON, D.I. (1958) The role of micronutrients in plant
nutrition with special reference to photosynthesis and nitrogen
assimilation. In: Lamb, C.A., Bentley, 0.G., & Beattie, J.M.,
ed. Trace elements , New York, Academic Press, pp. 1-32.
ARNON, D.I. & WESSEL, G. (1953) Vanadium as an essential
element for green plants. Nature (Lond.) , 172: 1039-1040.
ATHANASSIADIS, Y.C. (1969) Preliminary air pollution survey
of vanadium and its compounds: A literature review , Raleigh,
North Carolina, National Air Pollution Control Administration
(Publication No. APTD 69-48).
AZARNOFF, D.L. & CURRAN, G.L. (1957) Site of vanadium
inhibition of cholesterol biosynthesis (letter to the editor).
J. Am. Chem. Soc ., 79: 2968.
AZARNOFF, D.L., BROCK, F.E., & CURRAN, G.L. (1961) A specific
site of vanadium inhibition of cholesterol biosynthesis.
Biochem. Biophys. Acta , 51: 379-398.
BABENKO, G.A. & VANDZHURA, I.N. (1969) [Vanadium metabolism
after supplementary administration of certain microelements in
experimental atherosclerosis.] Bull. exp. Biol. Med ., 67(6):
72-73 (in Russian).
BAGGETT, W.L. & HUYCK, H.P. (1959) Spectrochemical determina-
tion of vanadium in alkali brines. Anal. Chem ., 31(8): 1320-
1322.
BAKAL, G.F. & LISECKAJA, G.S. (1971) [Determination of
vanadium in mercury using the reaction of oxidation of
pyrocatechin by ammonium persulfate.] Ukr. khim Zh ., 37(6):
594-597 (in Russian).
BALESTRA, G. & MOLFINO, F. (1942) [Lung impairments due to
dusts in operations to extract vanadium from petroleum ash.]
Rass. Med. Ind. Ig. Lav ., 13: 5-12 (in Italian).
BALFOUR, W.E., GRANTHAM, J.J., & GLYNN, I.M. (1978) Vanadate -
stimulated natriuresis. Nature (Lond.) , 275: 768.
BARANNIK, P.I., MIKHALYUK, I.A., & MOTUZKOV, I.N. (1969) Data
on the role of chromium, lead, vanadium and the natural radio-
activity of foodstuffs in the etiology of endemic goiter. Hyg.
Sanit ., 35(2): 289-291.
BARBOOTI, M.M. & JASIM, F. (1982) Electrothermal atomic-
absorption determination of vanadium. Talanta , 29: 107-111.
BARNES, R.M., FODOR, P., INAGAKI, K., & FODOR, M. (1983)
Determination of trace elements in urine using inductively
coupled plasma spectroscopy with a poly(dithiocarbamate)
chelating resin. Spectrochim. Acta , 38B: 245-257.
BARRY, E.F., REI, M.T., REYNOLDS, H.H., & O'BRIEN, J. (1975)
Determination of nickel and vanadium in the atmosphere of
eastern Massachusetts. Environ. Lett ., 8(4): 381-385.
BEARD, H.H., BAKER, R.W., & MYERS, V.C. (1931) Studies in the
nutritional anemia of the rat. J. biol. Chem ., 94: 123.
BELEHOVA, V.A. (1966) [Spectral analysis of vanadium content
in intact teeth and in teeth damaged by caries.] Stomatologija ,
4: 85-86 (in Russian).
BELEHOVA, V.A. (1969) [Vanadization of teeth as a means of
preventing dental caries.] Nauchn. Tr. Irkutsk. Med. Inst ., 95:
20-33 (in Russian).
BERGEL, F., BRAY, R.C., & HARRAP, Y.R. (1958) A model system
for cysteine desulphydrase action: pyridoxal phosphate-
vanadium. Nature (Lond.) , 181: 1654-1655.
BERNHEIM, F. & BERNHEIM, M.L.C. (1938) Action of vanadium on
tissue oxidation. Science, 88: 481.
BERNHEIM, F. & BERNHEIM, M.L.C. (1939) The action of vanadium
on the oxidation of phospholipids by certain tissues. J. biol.
Chem ., 127: 353.
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric ., 23: 93-100.
BERTRAND, D. (1942) Le vanadium comme élément oligosynergique
pour l'Aspergillus niger. Ann. Inst. Pasteur , 68: 226-244.
BERTRAND, D. (1950) Survey of contemporary knowledge of
biochemistry. II. The biogeochemistry of vanadium. Bull. Am.
Mus. Natl Hist ., 94(7): 407-455.
BEYHL, F.E. (1983) Action of ammonium metavanadate on hepatic
enzymes in vitro . Arch. Toxicol ., Suppl. 6: 250-253.
BIGGS, W.R. & SWINEHART, J.H. (1976) Vanadium in selected
biological systems. In: Sigel, H., ed. Metal ions in biological
systems , New York, Marcel Dekker, Vol. 6 , pp. 141-196.
BONIG, G. & HEIGENER, H. (l971) [Micro-determination of
vanadium in biological substances by selective paper
chromatography.] Landwirtsch. Forsch ., 25: 139-143 (in German).
BORISENKO, L.F. (1973) [ Vanadium ,] Moscow, Nedra Publishing
House (in Russian).
BOWEN, H.J.M. (1963) The elementary composition of mammalian
blood , Berkshire, Wantage Research Laboratory, Isotope Research
Division (AERE-R 4196).
BOYARKINA, A.P., VASILEV, N.V., KRIVYAKORA, E.N., NIKOLAEV,
A.V., & TIUKIUPO, E.B. (1978) [On the accumulation of nickel
and vanadium in the vicinity of industrial centres.] Gig. i
Sanit ., 43(2): 92-95 (in Russian).
BRODERICK, G.N. (1977) Vanadium - 1977 , Washington DC, US
Department of the Interior, Bureau of Mines (Mineral Commodity
Profiles, MCP-8).
BROEKAERT, J.A.C., WOPENKA, B., & PUXBAUM, H. (1982) Inductively
coupled plasma optical emission spectrometry for the analysis of
aerosol samples collected by cascade impactors. Anal. Chem ., 54:
2174-2179.
BROWNE, R.C. (1955) Vanadium poisoning from gas turbines. Br.
J. ind. Med ., 12: 57-59.
BROWNE, R. & STEEL, J. (1963) The control of the vanadium
hazard in catalytic oil-gas plants. Ann. occup. Hyg ., 6: 75-79.
BROWNING, E. (1969) Vanadium. In: Toxicity of industrial
metals , 2nd ed., London, Butterworths, pp. 340-347.
BUCHET, J.P., KNEPPER, E., & LAUWERYS, R. (1982) Determination
of vanadium in urine by electrothermal atomic absorption
spectrometry. Anal. Chim. Acta , 136: 243-248.
BUCKINGHAM, D.A. (1973) Structure and stereochemistry of
coordination compounds. Part 1. Coordination chemistry. In:
Eichhorn, G.L., ed. Inorganic biochemistry , Amsterdam, New York,
Oxford, Elsevier Science Publishers, Vol. 1 , p. 18.
BUDNIKOV, G.K. & MEDJANCEVA, E.P. (1973) [Determination of
vanadium and nickel in bitumen by oscillographic polarography
with linear change of potential.] Zh. anal. Khim ., 28(2): 301-
305 (in Russian).
BUONO, J.A., BUONO, J.C., & FASCHING, J.L. (1977) Simultaneous
determination of Al, V. Mn and Cu from neutronactivated saline
matrices by precipitation with poly-5-vinyl-8-hydroxyquinoline.
J. radioanal. Chem ., 36: 353.
BUTT, E.M., NUSBAUM, R.E., GILMOUR, T.C., DIDIO, S.L., & Sister
MARIANO (1964) Trace metal levels in human serum and blood.
Arch. environ. Health , 8: 60.
BYRNE, A.R. & KOSTA, L. (1978) Vanadium in foods and in human
body fluids and tissues. Sci. total Environ ., 10: 17-30.
BYRNE, A.R. & KOSTA, L. (1979) On the vanadium and tin
contents of diet and human blood. Sci. total Environ ., 11: 87-
90.
CANALIS, E. (1985) Effect of sodium vanadate on deoxyribo-
nucleic acid and protein synthesis in cultured rat calvariae.
Endocrinology , 116(3): 855-862.
CANNON, H.L. (1963) The biogeochemistry of vanadium. Soil
Sci., 96(3): 196-204.
CANTLEY, L.C., Jr & AISEN, P. (1979) The fate of cytoplasmic
vanadium. Implications on (Na, K)-ATPase inhibition. J. biol.
Chem ., 254: 1781-1784.
CANTLEY, L.C., Jr, JOSEPHSON, L., WARNER, R., YANAGISAWA, M.,
LECHENE, C., & GUIDOTTI, G. (1977) Vanadate is a potent
(Na++,K)-ATPase inhibitor found in ATP derived from muscle. J.
biol. Chem ., 252(21): 7421-7423.
CANTLEY, L.C., Jr, CANTLEY, L.G., & JOSEPHSON, L. (1978a) A
characterization of vanadate interactions with the (Na,K)-
ATPase. J. biol. Chem ., 253: 7361-7368.
CANTLEY, L.C., Jr, RESH, M.D., & GUIDOTTI, G. (1978b) Vanadate
inhibits red cell, (Na+, K+) ATPase from the cytoplasmic side.
Nature (Lond.) , 272: 552-554.
CARLSON, R.M.K. (1977) The structure of the native vanadium
chromagen in tunicate blood cells , Palo Alto, California,
Stanford University, 252 pp (Ph.D. Thesis) (University Micro-
films No. 78-2142).
CARLTON, B.D., BENEKE, M.B., & FISHER, G.L. (1982) Assessment
of the teratogenicity of ammonium vanadate using Syrian golden
hamsters. Environ. Res ., 29(2): 256-262.
CARPENTER, G. (1981) Vanadate, epidermal growth factor and the
stimulation of DNA synthesis. Biochem. biophys. Res. Commun.,
102: 1115-1121.
CASSANI, F. (1969) [Potentiometric determination of titanium
and vanadium.] Chim. Ind. (Milan) , 51: 1248-1251 (in Italian).
CAWSE, P.A. & PEIRSON, D.H., ed. (1972) Analytical study of
trace elements in the atmospheric environment , Harwell, Atomic
Energy Research Establishment (No. AERE-R 7134).
CHASTEEN, N.D., LORD, E.M., THOMPSON, H.J., & GRADY, J.K.
(1986) Vandium complexes of transferrin and ferritin in the
rat. Biochim. Biophys. Acta , 884(1): 84-92.
CHIRIATTI, G.N. (1971) [Prevention of occupational vanadium
poisoning.] Folia med ., 54: 57-76 (in Italian).
CHRISTIAN, G.D. (1971) Catalytic determination of vanadium in
blood and urine. Anal. Lett ., 4(4): 187-196.
CHRISTIAN, G.D. & FELDMAN, F.I. (1970) Atomic absorption
spectroscopy. In: Applications in agriculture, biology, and
medicine , New York, John Wiley and Sons, pp. 276-281.
CHRISTIAN, H.C.M. & ROBINSON, J.W. (1971) The determination of
vanadium and nickel in mineral oils by flameless graphite tube
atomization. Anal. Chim . Acta, 56: 470-473.
CHUPAHIN, S.M., KRIUCHKOV, O.M., & RAMENDI, G.I. (1972)
[ Analytical capabilities of spark-source mass spectrometry ,]
Moscow, Atom Publishers (in Russian).
CLARK, R.J.H. (1975) The chemistry of vanadium, niobium, and
tantalum , Oxford, Pergamon Press, pp. 491-535.
COHEN, M.D., WEI, C.I., TAN, H., & KAO, K.J. (1986) Effect of
ammonium metavanadate on the murine immune response. J. Toxicol.
environ. Health , 19(2): 279-298.
CONKLIN, A.W., SKINNER, C.S., FELTEN, T.L., & SANDERS, C.L.
(1982) Clearance and distribution of intratracheally instilled
48vanadium compounds in the rat. Toxicol. Lett ., 11: 199-203.
CORNELIS, R., MEES, L., HOSTE, J., RYCKEBUSCH, J., VERSIECK, J.,
& BARBIER, F. (1979) Neutron activation analysis of vanadium
in human liver and serum. In: Proceedings of an International
Symposium on Nuclear Activation Techniques in the Life Sciences,
Vienna, 22-26 May, 1978 , Vienna, International Atomic Energy
Agency, pp. 165-177.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1980) Determination of vanadium in human serum by neutron
activation analysis. J. radioanal. Chem ., 55(1): 35-43.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1981) The ultratrace element vanadium in human serum. Biol.
Trace Elem. Res ., 3: 257-263.
COTTON, F.A. & WILKINSON, G. (1962) Advanced inorganic
chemistry , New York, Interscience.
COTTON, F.A. & WILKINSON, G. (1980) Advanced inorganic
chemistry , 4th ed., New York, John Wiley and Sons, pp. 708-719.
COWGILL, U.M. (1973) The determination of all detectable
elements in the aquatic plants of Linsley Pond and Cedar Lake
(North Branford, Connecticut) by X-ray emission and optical
emission spectroscopy. Appl. Spectrosci ., 27: 5-9.
CREASON, J.P., HINNERS, T.A., BUMGARNER, J.E., & PINKERTON, C.
(l975) Trace elements in hair, as related to exposure in
Metropolitan New York. Clin. Chem ., 21: 603-612.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
CURRAN, G.L. & BURCH, R.E. (1967) Biological and health
effects of vanadium. In: Proceedings of the First Annual
Conference on Trace Substances in Environmental Health,
Columbia, 10-11 July, 1967 , Columbia, University of Missouri,
pp. 96-104.
CURRAN, G.L. & COSTELLO, R.L. (1956) Reduction of excess
cholesterol in the rabbit aorta by inhibition of endogenous
cholesterol synthesis. J. exp. Med ., 103: 49-56.
CURRAN, G.L., AZARNOFF, D.L., & BOLINGER, R.E. (1959) Effect
of cholesterol synthesis inhibition in normocholesteremic young
men. J. clin. Invest ., 38: 1251-1261.
DAMS, R., ROBBINS, J.A., RAHN, K.A., & WINCHESTER, J.W. (1970)
Nondestructive neutron activation analyses of air pollution
particulates. Anal. Chem ., 42: 861-867.
DAY, H., MIDDENDORF, D., LUKERT, C., HEINZ, A., & GRANTHAM, J.J.
(1980) The renal response to intravenous vanadate in rats. J.
lab. clin. Med., 96: 382-395.
DEMASTER, E.G. & MITCHELL, R.A. (1973) A comparison of
arsenate and vanadate as inhibitors or uncouplers of
mitochondria and glycolytic energy metabolism. Biochemistry,
12(19): 3616-3621.
DHEW (1968) Air quality data from the National Air
Surveillance Networks and contributing state and local networks ,
1966 ed., Durham, North Carolina, US Department of Health,
Education, and Welfare, National Air Pollution Control
Administration.
DIMOND, E.G., CARAVACA, J., & BENCHIMOL, A. (1963) Vanadium:
Excretion, toxicity, lipid effect in man. Am. J. clin. Nutr .,
12: 49-53.
DOMINGO, J.L., LLOBET, J.M., & CORBELLA, J. (1985) Protection
of mice against the lethal effects of sodium metavanadate: a
quantitative comparison of a number of chelating agents.
Toxicol. Lett ., 26(2-3): 95-99.
DOMINGO, J.L., LLOBET, J.M., TOMAS, J.M., & CORBELLA, J. (1986)
Influence of chelating agents on the toxicity, distribution, and
excretion of vanadium in mice. J. appl. Toxicol ., 6(5): 337-
341.
DOVGOPOL, V.I., BENOUNI, A.CH., MEDVEDEV, A.A., SHAPOVALOV,
J.P., & MEDVEDEVA, L.A. (1974) [The chemistry and engineering
technology of vanadium compounds.] In: Ivakin, A.A. & Voronova,
E.M., ed. [ Proceedings of the International Conference on the
Chemistry and Technology of Vanadium Compounds ,] Perm,
Scientific Institute of Black Metals, pp. 477-480 (in Russian).
DUCE, R.A. & HOFFMAN, G.L. (1976) Atmospheric vanadium
transport to the ocean. Atmos. Environ ., 10: 989-996.
DURFOR, C.N. & BECKER, E. (1963) Public water supplies of
the 100 largest cities in the United States, 1962 , Washington
DC, US Geological Survey (Water-Supply Paper No. 1812).
DURRANT, P.J. & DURRANT, B. (1970) Introduction to advanced
inorganic chemistry , 2nd ed., New York, John Wiley and Sons.
DURUM, W.H. & HAFFTY, J. (1963) Implications of the minor
element content of some major streams of the world. Geochim.
Cosmochim. Acta , 23: 1-11.
DUTTON, W.F. (1911) Vanadiumism. J. Am. Med. Assoc ., 56(22):
1648.
EISLER, L., SIMECEK, R., & USTUPSKY, J. (1968) [Effect of work
in vanadium workshop on respiratory ways.] Prac. Lek., 20(2/3):
52-57 (in Czech).
ELINDER, C.G. (1984) Metabolism and toxicity of metals ,
Stockholm, Department of Environmental Hygiene, Karolinska
Institute and the National Institute of Environmental Medicine,
pp. 265-274 (Life Sciences Research Report No. 28).
ELVES, M.V., WILSON, J.N., SCALES, J.T., & KEMP, H.B.S. (1975)
Incidence of metal sensitivity in patients with total joint
replacements. Br. med. J ., 4: 376-378.
ERMOLAEV, G.F. (1969) [Effect of vanadium on certain
biochemical processes in rabbit organs.] In: [ Trace elements in
medicine ,] Moscow, Ivano-Frankovsk, pp. 190-192 (in Russian).
EVANS, C.A. & MORRISON, G.H. (1968) Trace element survey
analysis of biological materials by spark source mass
spectrometry. Anal. Chem ., 40: 869-875.
FALLENTIN, B. & FROST, J. (1954) [Health hazards from cleaning
oil-fired boilers caused by vanadium and sulfuric acid in the
soot.] Nord. Hyg. Tidskr ., 3: 58-65 (in Danish).
FAORO, R.B. & MCMULLEN, T.B. (1977) National trends in trace
metals in ambient air 1965-1974 , Research Triangle Park, North
Carolina, US Environmental Protection Agency (EPA-450/1-77-
003).
FAULKNER HUDSON, T.G. (1964) Vanadium: toxicology and
biological significance , Amsterdam, New York, Oxford, Elsevier
Science Publishers, 135 pp.
FEAR, E.C. & TYRER, F.H. (1958) A study of vanadium poisoning
in gas workers. Trans. Assoc. Ind. Med. Off ., 8: 153-155.
FENNELLY, P.F. (1976) The origin and influence of airborne
particulates. Am. Sci ., 64(1): 46-56.
FISHER, G.L., MCNEILL, K.L., WHALEY, C.B., & FONG, J. (1978)
Attachment and phagocytosis studies with murine pulmonary
alveolar macrophages. J. Reticuloendothel. Soc ., 24: 243-252.
FISHER, G.L., MCNEILL, K.L., & DEMOCKO, C.J. (1986) Trace
element interactions affecting pulmonary macrophage cyto-
toxicity. Environ. Res ., 39(1): 164-171.
FLAHERTY, J.P. & ELDRIDGE, H.B. (1970) Neutron activation
analysis of residual oil for vanadium. Appl. Spectrosci ., 24(5):
534-538.
FROLOV, V.V. (1970) [Determination of chlorine and vanadium in
graphite by the activation method.] Zavod. Lab ., 36(7): 807-809
(in Russian).
FROST, J (1951) [Vanadium poisoning from cleaning the smoke
stacks of boilers burning fuel oil.] Ugeskr. Laeger , 113: 1309-
1312 (in Danish).
FUKAI, R. & MEINKE, W.W. (1962) Activation analyses of
vanadium, arsenic, molybdenum, tungsten, rhenium, and gold in
marine organisms. Limnol. Oceanogr ., 7: 186.
GARLEJ, T. (1974) [The influence of vanadium-bearing dust on
the workers' health in the Plock thermal-electric power station:
New exposure tests.] Pol. Tyg. Lek ., 29(49): 2143-2144 (in
Polish).
GERSHKOVICH, E.E. & STYKAN, T.P. (1972) [A catalytic method of
measuring hydrogen sulfide levels in air.] Gig. Tr. prof.
Zabol ., 2: 60-61 (in Russian).
GEYER, C.F. (1953) Vanadium, a caries-inhibiting trace element
in the Syrian hamster. J. dent. Res ., 35: 590-595.
GIBSON, R.S. & DEWOLFE, M.S. (1979) Copper, zinc, manganese,
vanadium, and iodine concentrations in the hair of Canadian low
birth wight neonates. Am. J. clin. Nutr ., 32: 1728-1733.
GILES, M.A., KLAVERKAMP, J.F., & LAWRENCE, S.G. (1979) The
acute toxicity of saline groundwater and of vanadium to fish and
aquatic invertebrates , Edmonton, Environment Alberta, 172 pp
(Prepared for the Alberta Oil Sands Environmental Research
Program) (Project No. AF 3.2.1).
GOFMAN J.W. (1962) Chemical elements of the blood of man in
health. Adv. biol. med. Phys ., 8: 1.
GOLDBERG, E.D. (1961) Marine geochemistry. In: Eyring, H.,
Christensen, C.J., & Johnston, H.S., ed. Annual review of
physical chemistry , Vol. 12, Palo Alto, California, Annual
Reviews, Inc., pp. 29-48.
GOLDSCHMIDT, V.M. (1938) Collected articles on the
geochemistry of rare elements. In: [ Collection of papers on the
geochemistry of rare metals ,] Moscow, Amalgamated State
Technical and Scientific Publishing Houses (in Russian).
GOLDSHTEJN, M.I. (1967) [ Vanadium-containing steels ,] Moscow,
Chermetinformacija (in Russian).
GORDUS, A.A., MAHER, C.C., III, & BIRD, G.C. (1974) Human hair
as an indicator of trace metal environmental exposure. In:
Proceedings of the First Annual NSF Trace Contaminants
Conference , Springfield, Virginia, National Technical
Information Service, pp. 463-487 (Conf. 730802).
GOYER, R.A. & MEHLMANN, M.A., ed. (1977) Toxicology of trace
elements. In: Advances in modern toxicology, Washington DC,
Hemisphere, Vol. 2 , 303 pp.
GRANTHAM, J.J. (1980) The renal sodium pump and vanadate. Am.
J. Physiol ., 239: F97-F106.
GRANTHAM, J.J. & GLYNN, I.M. (1979) Renal Na, K-ATPase:
determinants of inhibition by vanadium. Am. J. Physiol ., 236(6):
F530-F535.
GRIN, A.V., BLUM, A.A., SELETKOV, A.L., FREIDENZON, YU.E., &
NAIMUCHINA, L.F. (1971) [Low-alloyed welded steels containing
vanadium for construction.] In: [ Problems of Kachkanar ,]
Sverdlovsk, Central Bureau of Technical Information, pp. 186-196
(in Russian).
GULKO, A.G. (1956) [On the characteristics of vanadium as an
industrial poison.] Gig. i Sanit ., 21(11): 24-28 (in Russian).
GYLSETH, B., LEIRA, H.L., STEINNES, E., & THOMASSEN, Y. (1979)
Vanadium in the blood and urine of workers in a feraalloy plant.
Scand. J. Work environ. Health , 5: 188-194.
HACKETT, P.L. & KELMAN, B.J. (1983) Availability of toxic trace
metals to the conceptus. Sci. total environ .,28: 433-442.
HADJIMARKOS, D.M. (1966) Vanadium and dental caries. Nature
(Lond.) , 209: 1137.
HADJIMARKOS, D.M. (1968) Effect of trace elements on dental
caries. Adv. oral Biol ., 3: 282-285.
HAMILTON, E.I., MINSKI, M.J., & CLEARY, J.J. (1972-73) The
concentration and distribution of some stable elements in
healthy human tissues from the United Kingdom. Sci. total
Environ ., 1: 341-374.
HAMMOND, P.B. & FOULKES, E.C. (1986) Metal ion toxicity in man
and animals. In: Metal ions in biological systems , Cincinatti,
Ohio, Kettering Laboratory, Vol. 20 , pp. 157-200.
HANNA, W.J. & GRANT, C.L. (1962) Spectrochemical analyses of
certain trees and ornamentals for 23 elements. Bull. Torrey Bot.
Club , 89: 293-302.
HARA, T., SONODA, Y., & IWAI, I. (1976) Growth response of
cabbage plants to transition elements under water culture
conditions. I. Titanium, vanadium, chromium, manganese, and
iron. Soil Sci. plant Nutr ., 22(3): 307-315.
HARRIS, W.R., FRIEDMAN, S.B., & SILBERMAN, D. (1984) Behavior
of vanadate and vanadyl ion in canine blood. J. inorg. Biochem .,
20(2): 157-169.
HATFIELD, M. & CHURCHILL, P. (1981) Renal vascular and tubular
effects of vanadate in the anaesthetized rat. J. Pharmacol. exp.
Ther ., 217: 406-410.
HATHCOCK, J.N., HILL, C.H., & TOVE, S.B. (1966) Uncoupling of
oxidative phosphorylation by vanadate. Can. J. Biochem ., 44:
983-988.
HEIN, J.W. & WISOTZKY, J. (1955) The effect of a 10 ppm
vanadium drinking solution on dental caries in male and female
Syrian hamsters. J. dent. Res ., 34: 756.
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand culture.
J. exp. Bot ., 4(10): 59-64.
HEYDORN, K. & LUKENS, H.R. (1966) Pre-irradiation separation
for the determination of vanadium in blood serum by reactor
neutron activation analysis , Roskilde, Danish Atomic Energy
Commission, Research Establishment Risö (Risö Report No. 138).
HEYLIGER, C.E., TAHILIANI, A.G., & MCNEILL, J.H. (1985) Effect
of vanadate on elevated blood-glucose and depressed cardiac
performance of diabetic rats. Science , 227(4693): 1474-1477.
HICKEY, R.J., SCHOFF, E.0., & CLELLAND, R.C. (1967)
Relationship between air pollution and certain chronic disease
death rates. Arch. environ. Health , 15: 728-738.
HICKLING, S. (1958) Vanadium poisoning. N.Z. med. J ., 57: 607-
608.
HOFFMAN, B., KOMENDA, W., SADOWSKA, A.M., & ROZANSKI, A. (1972)
[The occurence of vanadium in the drinking-water in the region
of Bialystock Province.] Rocz. Panstw. Zakl. Hig ., 23(2): 155-
159 (in Polish).
HOFFMAN, G.L., DUCE, R.A., & ZOLLER, W.H. (1969) Vanadium,
copper, and aluminum in the lower atmosphere between California
and Hawaii. Environ. Sci. Technol ., 3: 1207-1210.
HOFFMAN, G.L., DUCE, R.A., & HOFFMAN, E.J. (1972) Trace metals
in the Hawaiian marine atmosphere. J. geophys. Res ., 77: 5322-
5329.
HOLDWAY, D.A. & SPRAGUE, J.B. (1979) Chronic toxicity of
vanadium to flagfish. Water Res ., 13: 905-910.
HOLODOV, V.N. (1968) [ Vanadium ,] Moscow, Nauka Publishing
House (in Russian).
HOLODOV, V.N. (1973) [ Sedimentary ore formation and
metallogeny on vanadium ,] Moscow, Nauka Publishing House (in
Russian).
HOLZHAUER, K.P. & SCHALLER, K.-H. (1977) [ Occupational
medicine studies in chimney sweeps. Hazards at the workplace and
occupation-linked health damage ,] Stuttgart, G. Thieme (in
German).
HOPKINS, L.L., Jr & MOHR, H.E. (197la) The biological
essentiality of vanadium. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition , New York, Marcel Dekker,
pp. 195-213.
HOPKINS, L.L., Jr & MOHR, H.E. (197lb) Effect of vanadium
deficiency on plasma cholesterol of chicks. Fed. Proc ., 30:
462.
HOPKINS, L.L., Jr & MOHR, H.E. (1974) Vanadium as an essential
nutrient. Fed. Proc ., 33(6): 1173-1175.
HOPKINS, L.L., Jr & TILTON, B.E. (1966) Metabolism of trace
amounts of vanadium 48 in rat organs and liver subcellular
particles. Am. J. Physiol ., 211: 169-172.
HOPKINS, L.L., CANNON, H.L., MUSCH, A.T., WELCH, R.M., &
NIELSEN, F.H. (1977) Vanadium. Geochem. Environ ., 2: 93-107.
HORI, C. & OKA, T. (1980) Vanadate enhances the stimulatory
action of insulin on DNA synthesis in cultured mouse mammary
glands. Biochim. Biophys. Acta , 610: 235-240.
HORNER, C.K., BURCK, D., ALLISON, F., & SHERMAN, M.S. (1942)
Nitrogen fixation by azotobacter as influenced by molybdenum and
vanadium. J. agric. Res ., 65: 173-193.
HUDGINS, P.M. & BOND, G.H. (1979) Reversal of vanadate
inhibition of Na++K-ATPase by catecholamines. Res. Commun. chem.
Pathol. Pharmacol ., 23(2): 313-326.
HUDSON, T.G.F. (1964) Vanadium: toxicology and biological
significance , Amsterdam, New York, Oxford, Elsevier Science
Publishers.
HWANG, I.Y., ULLICCI, P.A., & SMITH, S.B. (1972) A simple
flameless atomizer. Am. Lab ., 3: 41-43.
ICRP (1960) Report of Committee II on Permissible Dose for
Internal Radiation (1959). Recommendations of the International
Commission on Radiological Protection , Oxford, Pergamon Press
(ICRP Publication No. 2).
ILO (1980) Occupational Exposure Limits for Airborne Toxic
Substances; Occupational Safety and Health Series, No. 37, 2nd
revised ed ., Geneva, International Labour Office.
INCIARTE, D.J., STEFFEN, R.P., DOBBINS, D.E., SWINDALL, B.T.,
JOHNSTON, J., & HADDY, F.J. (1980) Cardiovascular effects of
vanadate in the dog. Am. J. Physiol ., 239: 47-56.
IZYCKI, J., SULKOWSKI, W., ADAMIAK-ZIEMBA, J., & STROSZEJN, G.
(1971) [Evaluation of professional exposure to vanadium
compounds and other environmental factors of workers employed in
cleaning of oil-fired boilers with special regard to the state
of respiratory tract.] Med. Pr ., 22(4): 421-431 (in Polish).
JACIMIRSKIJ, K.B. (1967) [ Kinetic methods of analysis ,]
Moscow, Himija (in Russian).
JACKSON, J.F. & LINSKENS, H.F. (1982) Metal ion induced
unscheduled DNA synthesis in Petunia pollen. Mol. gen. Genet .,
187: 112.
JANDHYALA, B.S. & HOM, G.J. (1983) Physiological and pharmaco-
logical properties of vanadium. Life Sci ., 33: 1325-1340.
JARACZEWSKA, W. & JAKUBOWSKI, M. (1964) [The attempt of
evaluation of vanadium exposure in the chemical industry.] Med.
Pr ., 15(6): 375-383 (in Polish).
JAWOROWSKI, Z., BILKIEWICZ, J., DIBOSZ, E., GRYBOWSKA, D.,
KOWNACKA, L., & WRONSKI, Z. (1973) Radiation hazards to
population from conventional and nuclear power production. In:
Symposium on environmental surveillance around nuclear
installations, Warsaw, 5-9 November, 1973, Vienna, International
Atomic Energy Agency (IAEA-SM-180/20).
JOHNSON, J.L., COHEN, H.L., & RAJAGOPALAN, K.V. (1974) Studies
of vanadium toxicity in the rat; lack of correlation with
molybdenum utilization. Biochem. biophys. Res. Commun ., 56(4):
940-946.
JONES, M.M. & BASINGER, M.A. (1983) Chelate antidotes for
sodium vanadate and vanadyl sulfate intoxication in mice. J.
Toxicol. environ. Health , 12(4-6): 749-756.
KADA, T., HIRANO, K., & SHIRASU, Y. (1980) Screening of
environmental chemical mutagens by the rec-assay system with
Bacillus subtilis. In: de Serres, F.J. & Hollaender, A., ed.
Chemical mutagens: principles and methods for their detection ,
New York, Plenum Press, pp. 149-173.
KAKU, S., HOSHIDE, M., MATSUO, S., NUNOTANI, H., ISHIDA, N.,
KURATA, S., MATSUMOTO, H., YAMAGUCHI, S., SANO, T., KONO, Y.,
KOGA, S., & KOGA, M. (1971) [Experimental studies on vanadium
poisoning. I. Vanadium-induced hepatic injuries in rats.] Jap.
J. ind. Health , 13: 263-267 (in Japanese).
KANEMATSU, K. & KADA, T. (1978) Mutagenicity of metal
compounds. Mutat. Res ., 53(2): 207-208.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec-assay and
mutagenicity studies on metal compounds. Mutat. Res ., 77: 109-
116.
KANISAWA, M. & SCHROEDER, H.A. (1967) Life-term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumours in mice. Cancer Res ., 27: 1192-1195.
KATAYEVA, I.G. & SAPUNOV, S.F. (1974) [Sanitary-hygienic
characteristics of vanadium ferroalloy production at the
Chusovoy Metallurgical Plant.] Gig. Tr. prof. Zabol ., 18(1):
37-39 (in Russian).
KAZIMOV, M.A. (1977) [Occupational hygiene with new methods of
the ferrovanadium production.] Gig. Tr. prof. Zabol ., 6: 8-12
(in Russian).
KEJT, M. & DEGENS, E. (1961) [Geometrical indicators.] In:
[ Geometrical research at low temperatures and pressures ,]
Moscow, Foreign Language Publishers, pp. 56-84 (in Russian).
KEMPTON, S., STERRITT, R.M., & LESTER, J.N. (1982) Atomic-
absorption spectrophotometric determination of antimony,
arsenic, bismuth, tellurium, thallium and vanadium in sewage
sludge. Talanta , 29: 675-681.
KINGSNORTH, A.N., LAMURAGLIA, G.M., ROSS, J.S., & MALT, R.A.
(1986) Vanadate supplements and 1,2-dimethylhydrazine induced
colon cancer in mice: increased thymidine incorporation without
enhanced carcinogenesis. Br. J. Cancer, 53(5): 683-686.
KIRZHNER, M.A., KRYZHENKOVA, N.A., & KIST, A.A. (1974) [Scope
for the preliminary concentration in the use of neutron
activation in matrix analysis for aluminium and vanadium.] At.
Energ ., 37: 498 (in Russian).
KIVILUOTO, M. (1980) Observations on the lungs of vanadium
workers. Br. J. ind. Med ., 37: 363-366.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1979a) Serum and
urinary vanadium pentoxide. Int. Arch. occup. Health , 48: 251-
256.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M. (1979b)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory. Scand. J. Work environ. Health , 5: 50-58.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M., (1979c)
Serum and urinary vanadium of vanadium exposed workers. Scand.
J. Work environ. Health , 5: 362-367.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health,
46: 179-182.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1981a) Clinical
laboratory results of vanadium-exposed workers. Arch. environ.
Health , 36(3): 109-113.
KIVILUOTO, M., RASANEN, O., RINNE, A., & RISSANEN, M. (1981b)
Intracellular immunoglobulins in plasma cells of nasal biopsies
taken from vanadium-exposed workers. Anat. Anz. Jena , 149: 446-
450.
KNECHT, E.A., MOORMAN, W.J., CLARK, J.C., LYNCH, D.W., & LEWIS,
T.R. (1985) Pulmonary effects of acute vanadium pentoxide
inhalation in monkeys. Am. Rev. respir. Dis ., 132(6): 1181-
1185.
KOHLER, R. (1972) [Rebuttal: problems of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 27: 815-816 (in German).
KOLPAKOV, L.E., DOBOSH, V.G., GREKOV, S.D., JUFEREV, A.JU.,
LLOKAZOV, E.D., AZFALOVA, R.U., & SANDLER, V.I. (1971)
[Improvement of technology of utilisation of vanadium contained
in waste water.] In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 229-231 (in
Russian).
KONOVALOV, G.S., IVANOVA, A.A., & KOLESNIKOV, T.CH. (1968)
[Scattered rare elements dissolved in water contained in
colloidal substances of the major USSR rivers.] In: [ A
collection of papers on the geochemistry of sedimentary rocks
and ores. Documentation of the Seventh All-Union Conference on
Lithology of the Academy of Sciences of the USSR], Moscow,
Nauka Publishing House, pp. 72-87 (in Russian).
KÖPF-MAIER, P. & KÖPF, H. (1979) [Vanadocene dichloride:
another anti-tumour agent from the metallocene series.] Z.
Naturforsch ., 34B: 805-807 (in German).
KÖPF-MAIER, P., HESSE, B., & KÖPF, H. (1980) Tumour inhibition
by metallocenes: effect of titanocene and hafnocene dichlorides
on Ehrlich ascites tumour in mice. J. Cancer Res. clin. Oncol .,
96(1): 43-51.
KOPYLOVA, L.M. (1971) [A study of some blood indicators
following prolonged administration of vanadium sulfate.] Tr.
Voronezh. Med. Inst ., 85: 62-65 (in Russian).
KORKHOV, V.V. (1965) [Vanadium in prophylaxis and treatment of
experimental atheroslerosis.] Farmakol. i Toksikol ., 28(1): 83-
87 (in Russian).
KOSTROMIN, A.I., AKHMETOV, A.A., & ORLOVA, L.N. (1970)
[Coulometric determination of manganese (II), cesium (III), and
vanadium (IV).] Zh. anal. Khim ., 25: 195-196 (in Russian).
KOVDA, V.A., JAKUSHEVSKAYA, I.V., & TURUKANOV, A.N. (1959)
[ Trace elements in the soils of the Soviet Union ,] Moscow,
Moscow University Publishing House (in Russian).
KRAGTEN, J. (1981) Improved determination of vanadium by flame
AAS by preventing polynuclear hydroxide complex formation. At.
Spectrosci ., 2(4): 135-136.
KRAUSKOPF, F.K. (1963) Factors governing the concentration of
thirteen rare metals in seawater. Collected articles on the
geochemistry of rock formation. In: [ The geochemistry of
lithogenesis ,] Moscow, Foreign Languages Publishing House, pp.
294-338 (in Russian).
KUBASKY, A. (1957) Waste from a vanadium work as moulding
material. Slevarenstvi , 5(1): 4-7.
KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D., & FLETCHER, O.J.
(1986) Influence of ochratoxin A and vanadium on various
parameters in growing chicks. Poult. Sci ., 65(9): 1671-1678.
KULIEVA, T.K. (1974) [Effect of vanadium on the activity of
some tissue-respiration enzymes.] Zdravookhr. Turkm ., 3: 7-17
(in Russian).
KUMAR, A. & CORDER, C.N. (1980) Diuretic and vasoconstrictor
effects of sodium orthovanadate on the isolated perfused rat
kidney. J. Pharmacol. exp. Ther ., 213: 85-90.
KURMAEV, R.H. (1974) [Solid, liquid and aerosol waste in the
production of technical vanadium pentoxide.] In: Ivakin, A.A. &
Voronova, E.M., ed. [ Proceedings of the International
Conference on the Chemistry and Technology of Vanadium
Compounds ,] Perm, Scientific Institute of Black Metals, pp. 43-
46 (in Russian).
KUZELOVA, M., SIRL, J., & POPLER, A. (1975) [Occupational
poisonings by vanadium compounds in workers at mazut boiler
houses.] Prac. Lek ., 27(5): 170-173 (in Czech).
KUZELOVA, M., HAVEL, V., POPLER, A., & STEPANEK, O. (1977)
[The problems of occupational medicine in the work of chimney
sweeps.] Prac. Lek ., 29: 225-228 (in Czech).
LAGERKVIST, B., NORDBERG, G.F., & VOUK, V. (1986) Vanadium.
In: Friberg, G.F., Nordberg, G.F., & Vouk, V.B., ed. Handbook on
the toxicology of metals. II. Specific metals , Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 638-663.
LAHAV, M., RENNERT, H., & BARZILAI, D. (1986) Inhibition by
vanadate of cyclic AMP production in rat corpora lutea incubated
in vitro . Life Sci ., 39(26): 2557-2564.
LANDER, D.W., STEINER, R.L., ANDERSON, D.H., & DEHM, R.L.
(1971) Spectrographic determination of elments in airborne
dirt. Appl. Spectrosci ., 25: 270-275.
LARSEN, J.A. & THOMSEN, O. (1980) Vanadate-induced oliguria
and vasoconstriction in the cat. Acta physiol. Scand ., 110:
367-374.
LARSEN, J.A., THOMSEN, O., & HANSEN, O. (1979) Vanadate-
induced oliguria in the anaesthetized cat. Acta physiol. Scand .,
106: 495-496.
LEE, K.P. & GILLIES, P.J. (1986) Pulmonary response and
intrapulmonary lipids in rats exposed to bismuth orthovanadate
dust by inhalation. Environ. Res ., 40(1): 115-135.
LEE, R.E., GORANSON, S.S., ENROINE, R.E., & MORGAN, G.B. (1972)
National air surveillance Cascade Impactor Network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol ., 6: 1025-1030.
LEE, K., NALEWAJKO, C., & JACK, T.R. (1978) Effects of
vanadium on freshwater algae. In: Abstracts of the 5th Annual
Aquatic Toxicity Workshop, Hamilton, Ontario, 7-9 November ,
Burlington, Ontario, Canada Centre for Inland Waters.
LEES, R.E.M. (1980) Changes in lung function after exposure to
vanadium compounds in fuel oil ash. Br. J. ind. Med ., 37: 253-
256.
LEVlNA, E.N. (1972) [Relationships between solubility of metal
compounds and their toxicity, distribution, and elimination from
the body.] Gig. Tr. prof. Zabol ., 16: 40-43 (in Russian).
LEVY, B.S., HOFFMAN, L., & GOTTSEGEN, S. (1984) Boilermakers'
bronchitis: respiratory tract irritation associated with
vanadium pentoxide exposure during oil-to-coal conversion of a
power plant. J. occup. Med ., 26(8): 567-570.
LEVY, L.S., MARTIN, P.A., & BIDSTRUP, P.L. (1986) Investigation
of the potential carcinogenicity of a range of chromium
containing materials on rat lung. Br. J. ind. Med ., 43(4): 243-
256.
LEWIS, C.E. (1959a) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 19: 419-424.
LEWIS, C.E. (1959b) The biological effects of vanadium. II.
The signs and symptoms of occupational vanadium exposure. Am.
Med. Assoc. Arch. Ind. Health , 19: 497-503.
LEWIS, C.E. (1959c) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 20(5): 455-466.
LIFSCHITZ, V.M. (1962) [Spectral determination of trace
elements in human blood (group determination of Ni, Ag, Zn, Cu,
Cd, V, Pb, Mn, Fe, and Co).] Tr. Voronezh. Med. Inst ., 49: 110
(in Russian).
LINSTEDT, K.D. & KRUGER, P. (1969) Vanadium concentrations in
Colorado River Basin waters. J. Am. Water Works Assoc ., 61(2):
85-88.
LINTER, C.M. (1985) Neuropsychiatric aspects of trace
elements. Br. J. hosp. Med ., 34(6): 361-365.
LOPEZ-NOVOA, J.M., GARCIA, J.C., CRUZ-SOTO, M.A., BENABE, J.E.,
& MARTINEZ-MALDONADO, M. (1982a) Effect of sodium ortho-
vanadate on renal renin secretion in vivo. J. Pharmacol. exp.
Ther ., 222: 447-451.
LOPEZ-NOVOA, J.M., MAYOL, V., & MARTINEZ-MALDONADO, M. (1982b)
Renal actions of orthovanadate in the dog. Proc. Soc. Exp. Biol.
Med ., 170: 418-426.
L'VOV, B.V. (1970) Atomic absorption spectrochemical analysis,
2nd ed., London, Adam Hilger.
MACARA, I.G. (1980) Vanadium - an element in search of a role.
Trends biochem. Sci ., April: 92-94.
MACARA, I.G., KUSTIN, K., & CANTLEY, L.C., Jr (1980) Gluta-
thione reduces cytoplasmic vanadate mechanism and physiological
implications. Biochim. Biophys. Acta , 629: 95-106.
MCLEAN, J.R., MCWILLIAMS, R.S., KAPLAN, J.G., & BIRNBOIM, H.C.
(1982) Rapid detection of DNA strand breaks in human peripheral
blood cells and animal organs following treatment with physical
and chemical agents. In: Bora, K.C., Douglas, G.R., & Nestmann,
E.R., ed. Chemical mutagenesis, human population monitoring, and
genetic risk assessment , Amsterdam, Oxford, New York, Elsevier
Science Publishers, p. 137 (Progress in Mutation Research,
Vol. 3).
MCLUNDIE, A.C., SHEPHERD, J.B., & MOBBS, D.R.A. (1968) Studies
on the effects of various ions on enamel solubility. Arch. oral
Biol ., 13: 1321-1330.
MADSEN, E.S., NIELSEN, P.A., & PEDERSEN, J.C. (1982) The
distribution and origin of mutagens in airborne particulates,
detected by the Salmonella /microsome assay in relation to
levels of lead, vanadium, and PAH. Sci. total Environ ., 24(1):
13-25.
MAHLER, H.R. & CORDES, E.H. (1966) Biological chemistry , New
York, Harper & Row.
MAJUMDAR, A.K. & DAS, G. (1965) Spectrophotometric determin-
ation of vanadium with N -benzoyl- N -p-chlorophenylhydroxyl-
amine. J. Indian Chem. Soc ., 42(3): 189.
MANNING, D.C. & SLAVIN, W. (1983) The determination of trace
elements in natural waters using the stabilized temperature
platform furnace. Appl. Spectrosci ., 37(1): 1-10.
MARAFANTE, E. & SABBIONI, E. (1983) Metabolic studies on
vanadium: in vivo and in vitro association with cellular
components. In: Proceedings of an International Conference on
Heavy Metals in the Environment, London, 18-21 September,
1979 , Edinburgh, CEC Consultants, pp. 171-174.
MARONI, M., COLOMBI, A., BURATTI, M., CALTZAFERRI, G., FOA, V.,
SABBIONI, E., & PIETRA, R. (1983) Urinary elimination of
vanadium in boiler cleaners. In: Proceedings of an International
Conference on Heavy Metals in the Environment, London, 18-21
September, 1979 , Edinburgh, CEC Consultants, pp. 66-69.
MARTENS, C.S., WESOLOWSKI, J.J., KAIFER, R., & JOHN, W. (1973)
Sources of vanadium in Puerto Rican and San Francisco bay area
aerosols. Environ. Sci. Technol ., 7: 817-819.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959a) Effect of
vanadium upon liver coenzyme A in rats. Nature (Lond.) , 183:
1527-1528.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959b) Intracellular
thioctic acid and coenzyme A following vanadium treatment.
Nature (Lond.) , 184: 1641.
MASIRONl, R. (1969) Trace elements and cardiovascular
diseases. Bull. World Health Organ ., 40: 305-312.
MATANTSEVA, E.I. (1960) [The state of the respiratory organs
in workers coming into contact with vanadium pentoxide.] Gig.
Tr. prof. Zabol ., 7: 41-44 (in Russian).
MEISCH, H.U. & BECKER, L.J. (1981) Vanadium in photosynthesis
of Chlorellafusca and higher plants. Biochim. Biophys. Acta ,
636: 119-125.
MEISCH, H.U. & BENZSCHAWEL, H. (1978) Role of vanadium in
green plants. III. Influence on cell division of chlorella.
Arch. Microbiol ., 116(1): 91-95.
MERTZ, W. (1970) Some aspects of nutritional trace element
research. Fed. Proc ., 29: 1482-1488.
MINDEN, H. & ROTHE, R. (1966) [Biochemical mechanism of action
of vanadium.] Z. gesamte Hyg ., 12(5): 315-321 (in German).
MIRAMAND, P. & UNSAL, M. (1978) Acute toxicity of vanadium to
some marine benthic and phytoplanktonic species. Chemosphere ,
10: 827-832.
MIRZAEVA, K.H. (1965) [Trace elements in natural waters in
some districts of Uzbekistan.] In: [ Trace elements in agri-
culture ,] Tashkent, Science Publishers of Uzbekistan, pp. 122-
126 (in Russian).
MISIEWICZ, A. (1980) [Effect of air containing gasoline,
tungsten, titanium, cobalt, and vanadium on the phagocytic
activity of leucocytes.] Pol. Tyg. Lek ., 35(50): 1965-1967 (in
Polish).
MISIEWICZ, A. (1983) [Effect of air containing benzine,
wolfram, titanium, cobalt, and vanadium on peripheral blood.]
Med. Pr ., 34(3): 251-257 (in Polish).
MITCHELL, R.L. (1964) Trace elements in soil. In: Bear, F.E.,
ed. Chemistry of the soil , New York, Reinhold Publishing,
pp. 320-368.
MITCHELL, W.G. & FLOYD, E.P. (1954) Ascorbic acid and
ethylenediaminetetracetate (EDTA) as antidotes in experimental
vanadium poisoning. Proc. Soc. Exp. Biol. Med ., 85: 206-208.
MONTERO, M.R., GUERRI, C., RIBELLES, M., & GRISOLIA, S. (1981)
Inhibition of protein synthesis in cell cultures by vanadate and
in brain homogenates of rats fed vanadate. Physiol. Chem.
Physics , 13: 281-287.
MORGAN, W.K.C. & SEATON, A. (1984) Occupational lung diseases ,
2nd ed., Philadelphia, W.B. Saunders & Co.
MOUNTAIN, J.T. (1963) Detecting hypersusceptibility to toxic
substances. Arch. environ. Health , 6: 357-365.
MOUNTAIN, J.T., DELKER, L.L., & STOKINGER, H.E. (1953) Studies
in vanadium toxicology. I. Reduction in the cystine content of
rat hair. Am. Med. Assoc. Arch. Ind. Hyg. Occup. Med ., 8: 406.
MOUNTAIN, J.T., STOCKELL, F.R., Jr, & STOKINGER, H.E. (1955)
Studies in vanadium toxicology. Am. Med. Assoc. Arch. Ind.
Health , 12: 494.
MUHLER, J.C. (1957) The effect of vanadium pentoxide,
fluorides, and tin compounds on the dental caries experience in
rats. J. dent. Res ., 36: 787-794.
MUSK, A.W. & TEES, J.G. (1982) Asthma caused by occupational
exposure to vanadium compounds. Med. J. Aust ., 1: 183-184.
MUSTAFIN, I.S., NECHAENKO, T.M., & FRUMINA, N.S. (1969) [ The
range of reagents available for vanadium ,] Moscow, Moscow State
University (in Russian).
MUZQUIN, V.N., KHAVZINA, L.B., ZOLOTAVIN, V.L., & BERRUKOV,
I.YA. (1981) [ Analytical chemistry of vanadium ,] Moscow,
Nauka Publishing House, 216 pp (in Russian).
MYERS, V.C. & BEARD, H.H. (1931) Studies in the nutritional
anaemia of the rat. II. Influence of iron plus supplements of
other inorganic elements upon blood regeneration. J. biol.
Chem ., 94: 89.
MYRON, D.R., GIVAND, S.H., & NIELSEN, F.H. (1977) Vanadium
content of selected foods as determined by flameless atomic
absorption spectroscopy. J. agric. food Chem ., 25(2): 297-299.
MYRON, D.R., ZIMMERMAN, T.J., SHULER, T.R., KLEVAY, L.M., LEE,
D.E., & NIELSEN, F.H. (1978) Intake of nickel and vanadium by
humans. A survey of selected diets. Am. J. clin. Nutr ., 31(3):
527-531.
NAS (1974) Vanadium , Washington DC, National Academy of
Sciences.
NAS (1978) Vanadium supply and demand outlook , Washington DC,
National Academy of Sciences, National Materials Advisory Board
(Publication NMAB-346).
NASON, A. (1958) The metabolic role of vanadium and molybdenum
in plants and animals. In: Lamb, C.A., Bentley, 0.G., & Beattie,
J.M., ed. Trace elements , New York, Academic Press, pp. 269-
296.
NAYLOR, G.J. (1984) Vanadium and manic depressive psychosis.
Nutr. Health , 3(1-2): 79-85.
NAYLOR, G.J., SMITH, A.H., BRYCE-SMITH, D., & WARD, N.I. (1984)
Tissue vanadium levels in manic-depressive psychosis. Psychol.
Med ., 14(4): 767-772.
NECHAY, R. (1984) Mechanisms of action of vanadate. Ann. Rev.
Pharmacol. Toxicol ., 24: 501-524.
NECHAY, R. & SAUNDERS, J.P. (1978) Inhibition by vanadium of
sodium and potassium dependent adenosinetriphosphatase derived
from animal and human tissues. J. environ. Pathol. Toxicol ., 2:
247-262.
NELSON, W.L. (1973) Direct desulfurization handles low-metals
topped crudes. Oil Gas J ., 19 November: 54-56.
NIELSEN, F.H. & OLLERICH, D.A. (1973) Studies on a vanadium
deficiency in chicks. Fed. Proc ., 32: 929.
NIOSH (1977) Occupational exposure to vanadium , Washington DC,
National Institute for Occupational Safety and Health (Document
No. 77-222), 142 pp.
NISHIYAMA, K., ONISHI, K., MIYAI, T., SUGIURA, S., SAITO, K., &
TOJYO, Y. (1977) [A survey of workers on vanadium pentoxide.]
Shikoku igaku zasshi , 31(6): 389-393 (in Japanese).
NOVAKOVA, S., NIKOLCHEV, G., ANGELIEVA, R., DINOEVA, S., &
MAUTNER, G. (1981) [Effect of vanadium on experimental
atherosclerosis.] Gig. i Sanit ., 11: 58-59 (in Russian).
NRC (1980) Drinking-water and health , Washington DC, National
Academy Press, Vol. 3, pp. 350-354 (Safe Drinking-Water
Committee).
OBERG, S.G., PARKER, R.D.R., & SHARMA, R.P. (1978)
Distribution and elimination of an intratracheally administered
vanadium compound in the rat. Toxicology , 11: 315-323.
OLEFFE, J. & WILMET, J. (1980) Generalized dermatitis from an
osteosynthesis screw. Contact Dermat ., 6: 365.
OLIVER, B.G. & COSGROVE, E.G. (1975) Metal concentrations in
the sewage, effluents and sludges of some Southern Ontario
wastewater treatment plants. Environ. Lett ., 9(1): 75-90.
OMANG, S.H. (1971) The determination of vanadium and nickel in
mineral oils by flameless graphite tube atomization. Anal. Chim.
Acta , 56: 470-473.
ORDHZONIKIDZE, E.K., ROSCHIN, A.V., SHALGANOVA, I.V., BOGOMAZOV,
M.YA., & KAZIMOV, M.A. (1977) [On the distribution and
elimination of vanadium from the organisms.] Gig. Tr. prof.
Zabol ., 6: 29-34 (in Russian).
ORVINI, E., LODOLA, L., SABBIONI, E., PIETRA, R., & GOETZ, L.
(1979) Determination of the chemical forms of dissolved
vanadium in freshwater as determined by 48V radiotracer experi-
ments and neutron activation analysis. Sci. total Environ ., 13:
195-207.
PARKER, R.D.R. & SHARMA, R.P. (1978) Accumulation and
depletion of vanadium in selected tissues of rats treated with
vanadyl sulfate and sodium orthovanadate. J. environ. Pathol.
Toxicol ., 2: 235-245.
PARKES, W.R. (1982) Occupational lung disorders , 2nd ed.,
London, Butterworths & Co.
PASTUHOV, A.I. & TRETJAKOV, M.A. (1959) Metallurgical
processing of cast iron from titanomagnetites from the Kackanar
district into steel. In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 95-104 (in
Russian).
PATRICK, R. (1978) Effects of trace metals in the aquatic
ecosystem. Am. Sci ., 66: 185-191.
PAZHYNICH, V.M. (1966) Maximum permissible concentration of
vanadium pentoxide in the atmosphere. Hyg. Sanit ., 31: 6-11.
PAZHYNICH, V.M. (1967) [Experimental basis for the determina-
tion of maximum allowable concentration of vanadium pentoxide in
atmospheric air.] In: Rjazanov, V.A., ed. [ The biological
effect and hygienic importance of atmospheric pollutants ,]
Moscow, Medicina Publishing House, pp. 201-217 (in Russian).
PAZHYNICH, V.M. (1980) [Effect of aerosols of rare metals on
the functional state of the body of workers in conditions of
powder wire production.] Vrach. Delo , 3: 102-104 (in Russian).
PEJVE, J.V. & AJZUPIET, I.P. (1974) [A short review of the
results of research on the problem "Trace elements in plant
cultivation and livestock rearing in 1972".] In: [ Trace
elements in the USSR. XV. Methodological material ,] Riga,
Knowledge Publishers (in Russian).
PERRY, H.M., TEITLEBAUM, S., & SCHWARTZ, P.L. (1955) Effects
of antihypertensive agents on amino acid decarboxylation and
amine oxidation. Fed. Proc ., 14: 113-114.
PERRY, H.M., Jr, SCHWARTZ, P.L., & SAHAGIAN, B.M. (1969)
Effects of transition metals and of metal-binding antihyper-
tensive agents on tryptamine oxidase sand dopa decarboxylase.
Proc. Soc. Exp. Biol. Med ., 130: 273-277.
PETERBURGSKIJ, A.V. & TORMASOVA, E.E. (1969) [The effect of
vanadium on certain physiologycal processes in the pea.] Dok.
Timiryazevsk. Akad ., 149: (in Russian).
PETKEVICH, A.N., VILLER, G.E., & VOROTNIKOV, B.A. (1967)
[Some trace elements in natural waters of Kama's district.] In:
[ Trace elements in the biosphere and their application in
agriculture and medicine in Siberia and the Far East ,] Ulan-
Ude, Ulan-Ude Book Publishers, pp. 223-227 (in Russian).
PETUHOV, N.I., STANKEVICH, E.F., & VALACHANOVICH, V.I. (1969)
Some microelements in natural waters near the source of the
Kama. Tr. Kazanskogo Med. Inst ., 29: 100-103.
PHILLIPS, T.D., NECHAY, B.R., NELDON, S.L., KUBENA, L.F.,
HEIDELBAUGH, N.D., SHEPHERD, E.C., STEIN, A.F., & HAYES, A.W.
(1982) Vanadium-induced inhibition of renal Na+ K+-
adenosinetriphosphatase in the chicken after chronic dietary
exposure. J. Toxicol. environ. Health , 9: 651-661.
PHILLIPS, T.D., NECHAY, B.R., & HEIDELBAUGH, N.D. (1983)
Vanadium: chemistry and the kidney. Fed. Proc ., 42(13): 2969-
2973.
PIELSTICKER, F. (1954) [Health effects due to vanadium
compounds. Symptoms and prognosis.] Arch. Gewerbepathol.
Gewerbehyg ., 13: 73-96 (in German).
PILZ, W. & KOMISCHKE, S. (1972) [Determination of vanadium in
biological material and air. II. The decomposition of biological
material: air analyses.] Int. J. environ. anal. Chem ., 1(4):
275-282 (in German).
POERSEL, B. (1977) [ The biological and toxicological
significance of vanadium ,] Dusseldorf, Med. Diss., 86 pp (in
German).
PROESCHER, F., SEIL, H.H., & STILLIANS, A.W. (1917) A
contribution to the action of vanadium with particular reference
to syphilis. Am. J. Syph ., 1: 347-405.
PROKOPENKO, T.A. (1961) [Effect of industrial poisons,
fluorine, vanadium, manganese, and some drugs tested in
treatment on the tissue metabolism of compounds containing
phosphorus.] In: [ Proceedings of the 9th All-Union Pharmaco-
logical Conference, Sverdlovsk ,] Sverdlovsk, Central Bureau of
Technical Information, pp. 210-211 (in Russian).
PYY, L., KIVILUOTO, M., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health ,
46(2): 179-182.
PYY, L., LAJUNEN, L.H.J., & HAKALA, E. (1983) Determination of
vanadium in work-place air by DCP emission spectrometry. Am.
Ind. Hyg. Assoc. J ., 44(8): 609-614.
PYY, L., HAKALA, E., & LAJUNEN, L.H.J. (1984) Screening for
vanadium in urine- and blood-serum by electrothermal atomic
absorption spectrometry and dc plasma atomic emission
spectrometry. Anal. Chim. Acta , 158: 297-303.
RAESAENEN, O., KIVILUOTO, M., RINNE, A., & RISSANEN, M. (1979)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory: a macroscopic and microscopic study. Scand.
J. Work environ. Health , 5(1): 50-58.
RAJNER, V. (1960) [Effect of vanadium in the respiratory
tract.] Cesk. Otolaryngol ., 9: 202-204 (in Czech).
RALSTON, H.R. & SATO, E.S. (1971) Sodium removal as an aid to
neutron activation analysis. Anal. Chem ., 43: 129-131.
REINL, W. (1958) [Illnesses caused by basic slag, vanadium
slag, and vanadium compounds.] Staub , 18(5): 143-148 (in
German).
REZNIK, J.B. (1954) [The importance of vanadium compounds from
the point of view of industrial toxicology.] Vrach. Delo , 7:
627-632 (in Russian).
RHAN, K.A. (1971) Sources of trace elements in aerosols: an
approach to clean air , Ann Arbor, Michigan, University of
Michigan, Department of Meteorology, 309 pp (US Atomic Energy
Commission Contract No. COO-1705-9).
RHOADS, K. & SANDERS, C.L. (1985) Lung clearance, trans-
location, and acute toxicity of arsenic, beryllium, cadmium,
cobalt, lead, selenium, vanadium, and ytterbium oxides following
deposition in rat lung. Environ. Res ., 36(2): 359-378.
RIFKIN, R.J. (1965) In vitro inhibition of Na+ -K+ and Mg2+
ATPases by mono-, di-, and trivalent cations. Proc. Soc. Exp.
Biol. Med ., 120: 802-804.
RIGDON, L.P. & HARRAR, J.E. (1969) Determination of vanadium
by controlled-potential coulometry. Anal. Chem ., 41: 1673-1675.
ROITMAN, I., TRAVASSAS, L.R., AZEVEDO, H.P., & CURRY, A. (1969)
Choline, trace elements, and amino acids as factors for growth
of an enteric yeast, Candida slooffii. Sabouraudia J. Int. Soc.
Hum. Anim. Mycol ., 7: 15-19.
ROMAN, R.J., BONVENTRE, J.V., SILVA, P., & LECHENE, C. (1981)
Sodium orthovanadate diuresis in rats. J. Pharmacol. exp. Ther .,
218: 168-174.
RONOV, A.B. (1964) [General tendencies in the evolution of the
composition of the Earth's crust, the oceans and the
atmosphere.] Geohimija , 8: 715-743 (in Russian).
ROSE, E.R. (1973) Geology of vanadium and vanadiferous
occurrences in Canada , Ottawa, Geological Survey of Canada,
Department of Energy, Mines and Resources, pp. 7-101 (Economic
Geology Report No. 27).
ROSHCHIN, A.V. (1962) [The hazards of occupational vanadium
intoxication in boiler cleaning operations at electric power
stations operating on fuel oil, and prevention problems.] Gig.
Tr. prof. Zabol ., 6: 17-22 (in Russian).
ROSHCHIN, A.V. (1963a) [Biological effects of rare, dispersed,
and other metals and their compounds used in industry:
vanadium.] In: [ Toxicology of the less common metals ,] Moscow,
Meditsina, pp. 83-95 (in Russian).
ROSHCHIN, A.V. (1963b) [Hygienic evaluation of dust from
vanadium-containing slag.] Gig. i Sanit ., 12: 23-29 (in
Russian).
ROSHCHIN, A.V. (1964) [Vanadium metallurgy in the light of
industrial hygiene and questions concerned with the prevention
of occupational diseases and intoxication.] Gig. Tr. prof.
Zabol ., 8: 3-10 (in Russian).
ROSHCHIN, A.V. (1967a) Toxicology of vanadium compounds used
in modern industry. Hyg. Sanit ., 32: 345-352.
ROSHCHIN, A.V. (1967b) Vanadium. In: Izraelson, Z.I., ed.
Toxicology of the rare metals , Jerusalem, Israel Program for
Scientific Translations Ltd., pp. 52-59 (AEC-tr-6710).
ROSHCHIN, A.V. (1968) [ Vanadium and its compounds ,] Moscow,
Medicina Publishing House (in Russian).
ROSHCHIN, A.V. & ORDZHONIKIDZE, E.K. (1986) [Metal toxico-
kinetics and its significance for prevention of occupational
poisoning.] Gig. Tr. prof. Zabol ., 3: 1-6 (in Russian).
ROSHCHIN, A.V., ILNITSKAYA, A.V., LUTSENKO, L.A., & ZHIDKOVA,
L.A. (1964) [Research into effects of vanadium trioxide dust
on organisms.] Gig. Tr. prof. Zabol ., 8: 25-30 (in Russian).
ROSHCHIN, A.V., ORDZHONIKIDZE, E.K., & SHALGANOVA, I.V. (1980)
Vanadium - toxicity, metabolism, carrier state. J. Hyg.
Epidemiol. Microbiol. Immunol ., 24(4): 377-383.
RUHLING, A. (1971) [Pollution with heavy metals in the greater
Stockholm area.] In: [ Report No. 12 on ecological heavy metal
research ,] Lund, University of Lund, Department of Plant
Ecology (in Swedish).
RUNDBERG, G. (1939) Modern problems of occupational diseases
in Sweden. Nord. Med ., 2: 1845-1856.
SABBIONI, E., MARAFANTE, E., AMANTINI, L., & UBERTALLI, L.
(1978) Similarity in metabolic patterns of different chemical
species of vanadium in the rat. Bioinorg. Chem ., 8: 503-515.
SABBIONI, E., MARAFANTE, E., PIETRA, R., GOETZ, L., GIRARDI, F.,
& ORVINI, E. (1979) The association of vanadium with the iron
transport system in human blood as determined by gel filtration
and neutron activation analysis. In: Proceedings of a Symposium
on Nuclear Activation Techniques in the Life Sciences, Vienna,
22-26 May, 1978 , Vienna, International Atomic Energy Agency, pp.
179-192.
SABBIONI, E., MARAFANTE, E., RADE, J., GREGOTTI, C., DI NUCCI,
A., & MANZO, L. (1981) Biliary excretion of vanadium in rats.
Toxicol. Eur. Res ., 3(2): 93-98.
SANDELL, E., ed. (1959) Vanadium. In: Colorimetric determina-
tion of traces of metals , 3rd ed., New York, Interscience
Publishers, Vol. 3, pp. 923-940 (Chemical Analysis Series).
SCHLETTWEIN-GZELL, D. & MOMMSEN-STRAUB, S. (1973) [Trace
elements in foodstuffs. XI. Vanadium.] Int. Z. Vitam.-
Ernährungsforsch ., 43(2): 242-250 (in German).
SCHROEDER, H.A. (1966) Municipal drinking-water and cardio-
vascular death rates. J. Am. Med. Assoc ., 195: 81-85.
SCHROEDER, H.A. (197Oa) Vanadium , Washington DC, American
Petroleum Institute (Air Quality Monograph No. 70-13).
SCHROEDER, H.A. (197Ob) A sensible look at air pollution by
metals. Arch. environ. Health , 21: 798-806.
SCHROEDER, H.A. & MITCHENER, M. (1975) Life-term effects of
mercury, methylmercury, and nine other trace metals on mice. J.
Nutr ., 105(4): 452-458.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1963) Abnormal
trace metals in man: vanadium. J. chron. Dis ., 16: 1047-1071.
SCHROEDER, H.A., MITCHENER, M., & NASON, A.P. (1970) Zirconium,
niobium, antimony, vanadium, and lead in rats: life-term
studies. J. Nutr ., 100(1): 59-68.
SCHUMANN-VOGT, B. (1969) [Health hazards in industry caused by
vanadium.] Zentralbl. Arbeitsmed. Arbeitsschutz , 19(2): 33-39
(in German).
SCHWARZ, K. & MILNE, D.B. (1971) Growth effects of vanadium in
the rat. Science , 174: 426-428.
SELJANKINA, K.P. (1961) [Data for determining the maximum
permissible content of vanadium in water basins.] Gig. i Sanit .,
26(10): 6-12 (in Russian).
SHAH, K.R., FILBY, R.H., & HALLER, W.A. (1970) Determination
of trace elements in petroleum by neutron activation analysis.
1. Determination of Na, S, Li, La, V, Mn, Cu, Ga, and Br. J.
radioanal. Chem ., 6: 185-192.
SHARMA, R.P., BOURCIER, D.R., BRINKERHOFF, C.R., & CHRISTENSEN,
S.A. (1981) Effects of vanadium on immunologic functions. Am.
J. ind. Med ., 2(2): 91-99.
SHARMA, R.P., COULOMBE, R.A., Jr, & SRISUCHART, B. (1986)
Effects of dietary vanadium exposure on levels of regional brain
neurotransmitters and their metabolites. Biochem. Pharmacol .,
35(3): 461-465.
SHEVCHENKO, I.T. & GORODYNSKYJ, V.I. (1964) [ Polarography in
medicine and biology ,] Kiev, State Medical Publishers of the
Ukraine (in Russian).
SHEVCHENKO, S.D. (1965) [Content of vanadium in bone tumours.]
Ortop. Travmatol. Protez ., 8: 83 (in Russian).
SHILINA, A.I. & MALAKHOV, S.G. (1974) [Trace elements in
natural waters and the atmosphere.] In: [ A collection of papers
on experimental meteorology ,] Moscow, Gidrometeozdat (in
Russian).
SHKOLENOK, G.F., NIKOLAEVA, E.R., & AGASYAN, P.K. (1977)
[Differential controlled-potential coulometry. Determination of
vanadium(V) and vanadium(IV).] Zavod. Lab ., 43(9): 1048-1050 (in
Russian).
SIMONOFF, M., CONRI, C., & SIMONOFF, G. (1986) Vanadium in
depressive states. Acta pharmacol. toxicol . (Copenhagen) ,
59(Suppl. 7): 463-466.
SINGH, D. & SHARMA, S. (1970) Amperometric permanganometric
estimations at low concentrations in stirred solutions. Indian
J. Chem ., 8(2): 192.
SINGH, J., NORDLIE, R.C., & JORGENSON, R.A. (1981) Vanadate:
a potent inhibitor of multifunctional glucose-6-phosphatase.
Biochim. Biophys . Acta , 678: 477-482.
SJÖBERG, S.-G. (1950) Vanadium pentoxide dust: A clinical and
experimental investigation on its effect after inhalation. Acta
med. Scand ., 138(Suppl. 238): 1-188.
SJÖBERG, S.-G. (1951) Possible importance of allergy in
diseases caused by inhalation of inorganic dust. Acta allergol .,
4(4): 357-361.
SJÖBERG, S.-G. (1955) Vanadium bronchitis from cleaning oil-
fired boilers. Arch. ind. Health , 11(6): 505-512.
SJÖBERG, S.-G. (1956) Vanadium dust, chronic bronchitis and
possible risk of emphysema. A follow-up investigation of workers
at a vanadium factory. Acta med. Scand ., 44(5): 381-386.
SJÖBERG, S.-G. & RIGNER, K.-G. (1956) [Skin, eye, and
respiratory tract symptoms associated with the cleaning of oil-
fired boilers.] Nord. Hyg. Tidskr ., 37: 217-228 (in Swedish).
SLAVIN, W., CARNRICK, G.R., MANNING, D.C., & PRUSZKOWSKA, E.
(1983) Recent experiences with the stabilized temperature
platform furnace and zeeman background correction. At.
Spectrosci ., 4(3): 69-86.
SMITH, J.B. (1983) Vanadium ions stimulate DNA synthesis in
Swiss mouse 3T3 and 3T6 cells. Proc. Natl Acad. Sci. (USA) ,
80(20): 6162-6166.
SNYDER, F. & CORNATZER, W.E. (1958) Vanadium inhibition of
phospholipid sulphydryl activity in rat liver. Nature (Lond.) ,
182: 462.
SOKOLOV, S.M. (1986) Metholodological aspects of assessing
atmospheric contamination with metal aerosols in the vicinity of
thermal power complexes. J. Hyg. Epidemiol. Microbiol.
Immunol ., 30(3): 249-254.
SOMERVILLE, J. & DAVIES, B. (1962) Effect of vanadium on serum
cholesterol. Am. Heart J ., 64: 54-56.
SÖREMARK, R. (1967) Vanadium in some biological specimens. J.
Nutr ., 92: 183-190.
SÖREMARK, R. & ULLBERG, S. (1962) Distribution and kinetics of
48V2O5 in mice. In: Friend, N., ed. Proceedings of a Symposium
on the Use of Radioisotopes in Animal Biology and the Medical
Sciences, Mexico City, 21 November-1 December, 1961 , New York,
Academic Press, Vol. 2, pp. 103-114.
SÖREMARK, R., ULLBERG, S., & APPELGREN, L.E. (1962) Auto-
radiographic localization of vanadium pentoxide (V24805) in
developing teeth and bones of rats. Acta odontol. Scand ., 20:
225-232.
SPRAGUE, J.B., HOLDWAY, D.A., & STENDAHL, D.H. (1978) Acute
and chronic toxicity of vanadium to fish , Edmonton, Environment
Alberta, 92 pp (Prepared for the Alberta Oil Sands Research
Program (Project No. AF 3.5.1).
STEFFEN, R.P., PAMNANI, M.B., CLOUGH, D.L., HUOT, S.J., MULDOON,
S.M., & HADDY, F.J. (1981) Effect of prolonged dietary
administration of vanadate on blood pressure in the rat.
Hypertension , 3: I/173-I/178.
STENDAHL, D.H. & SPRAGUE, J.B. (1982) Effects of water
hardness and pH on vanadium lethality to rainbow trout. Water
Res ., 16: 1479-1488.
STOCKS, P. (1960) On the relations between atmospheric
pollution in urban and rural localitites and mortality from
cancer, bronchitis and pneumonia, with particular reference to
3:4 benzopyrene, beryllium, molybdenum, vanadium, and arsenic.
Br. J. Cancer , 14: 397-418.
STOKINGER, H.E., WAGNER, W.D., MOUNTAIN, J.T., STOCKELL, F.R.,
DOBROGORSKI, O.J., & KEENAN, R.G. (1967) In Vanadium V. In:
Clayton, G.D. & Clayton, F.E., eds. Patty's Industrial hygiene
and toxicology , 3rd revised ed. (1981), New York, Interscience,
Vol. 2A, pp. 2013-2033.
STONER, G.D., SHIMKIN, M.B., TROXELL, M.C., THOMPSON, T.L., &
TERRY, L.S. (1976) Test for carcinogenicity of metallic
compounds by the pulmonary tumor response in strain a mice.
Cancer Res ., 36: 1744-1747.
STRAHOV, N.F. (1947) [Iron-ore facies and their analogues in
the history of the earth.] Tr. Inst. Geol. AN SSSR, geol. Ser .,
73: 22 (in Russian).
STRAHOV, N.M. (1968) [On the theory of geochemical process in
humid zones.] In: [ A collection of papers on the geochemistry
of sedimentary rocks and ores ,] Moscow, Na++uka Publishing House,
pp. 102-134 (Documentation of the Seventh All-Union Conference
on Lithology of the Academy of Sciences of the USSR) (in
Russian).
STRASIA, C.A. (1971) Vanadium: essentiality and toxicity in
the laboratory rat , Ann Arbor, Michigan Purdue University (Ph.D.
Thesis).
STROOP, S.D., HELINEK, G., & GREENE, H.L. (1982) More
sensitive flameless atomic absorption analysis of vanadium in
tissue and serum. Clin. Chem ., 28(1): 79-82.
STYRIS, D.L. & KAYE, J.H. (1982) Mechanisms of vaporization of
vanadium pentaoxide from vitreous carbon and tantalum furnaces
by combined atomic absorption/mass spectrometry. Anal. Chem .,
54: 864-869.
SUGAWARA, K., NAITO, H., & YAMADA, S. (1956) Geochemistry of
vanadium in natural waters. J. earth Sci. Nagoya Univ ., 4(1):
44-61.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975)
Heavy metals in normal Japanese tissues. Arch. environ. Health ,
30: 487-494.
SUN, MIANLING, ed. (1987) Toxicity of vanadium and its
environmental health standard , Chengdu, West China University of
Medical Sciences, 20 pp.
SVERDRUP, H.U., JOHNSON, M.W., & FLEMING, R.H. (1950) The
oceans, their physics, chemistry and general biology , Englewood
Cliffs, New Jersey, Prentice-Hall Inc., pp. 175-177.
SVOBODA, P., TEISINGER, J., PILAR, J., & VYSKOCHIL, F. (1984)
Vanadyl (VO2+) and vanadate (VO3-) ions inhibit the brain
microsomal Na, K-AtPase with similar affinities. Protection by
transferrin and noradrenaline. Biochem. Pharmacol ., 33: 2485-
2491.
SWARUP, G., COHEN, S., & GARBERS, D.L. (1982) Inhibition of
membrane phosphotyrosyl-protein phosphatase activity by
vanadate. Biochem. biophys. Res. Commun ., 107: 1104-1109.
SYMANSKI, J. (1939) [Occupational vanadium poisoning, its
occurrence and symptomatology.] Arch. Gewerbepathol.
Gewerbehyg ., 9: 295-313 (in German).
TAKAHASHI, H. & NASON, A. (1957) Tungstate as a competitive
inhibitor of molybdate in nitrate assimilation and N2 fixation
by azotobacter. Biochim. Biophys. Acta , 23: 433-435.
TALVITIE, N.A. & WAGNER, W.D. (1954) Studies in vanadium
toxicology. II. Distribution and excretion of vanadium in
animals. Arch. ind. Hyg ., 9: 414-422.
TANDON, A.G. & BHATTACHARYA, S.C. (1961) Use of N-2-thio-
phenecarbonyl- N-p-tolylhydroxylamine and N-2-thiophene-
carbonyl- N-phenylhydroxylamine as reagents for vanadium. Anal.
Chem ., 33(9): 1267.
TARA, S., CAVIGNEAUX, A., & DEPLACE, Y. (1953) Intoxication
par le vanadate de calcium. Arch. Mal. prof. Méd. Trav. Séc.
soc ., 14: 378-380.
TEBROCK, H.E. & MACHLE, W. (1968) Exposure to europium-
activated yttrium orthovanadate: a cathodoluminescent phosphor.
J. occup. Med ., 10(12): 692-696.
THOMAS, D.L.G. & STIEBRIS, K. (1956) Vanadium poisoning in
industry. Med. J. Aust ., 1: 607-609.
THOMPSON, H.J., CHASTEEN, N.D., & MEEKER, L.D. (1984) Dietary
vanadyl (IV) sulfate inhibits chemically-induced mammary
carcinogenesis. Carcinogenicity , 5: 849-851.
THURAUF, J., SYGA, G., & SCHALLER, K.H. (1979) Field tests
carried out to determine the occupational exposure to vanadium.
Zbl. Bakteriol. Hyg., I. Abt. Orig. B , 168: 273-290.
TIPTON, I.H. & COOK, M.J. (1963) Trace elements in human
tissue. II. Adult subjects from the United States. Health Phys .,
9: 103-145.
TIPTON, I.H. & SHAFER, J.J. (1964) Statistical analysis of
lung trace element levels. Arch. environ. Health , 8: 58-67.
TIPTON, I.H., STEWART, P.L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys .,
16: 455-462.
TJURJUKANOV, A.N. (1963) Vanadium. In: Trace elements in
agriculture , Kiev, Gosselhozizdat, pp. 472-475.
TODRIA, F.F. (1963) [Identification of vanadium in ashes of
Tkibul coals.] In: [ Abstracts of scientific papers of Tbilisi
Medical Institute ,] Tbilisi, Tbilisi State Medical Institute,
p. 144 (in Russian).
TOLMAN, E.L., BARRIS, E., BURNS, M., PANSINI, A., & PARTRIDGE,
R. (1979) Effects of vanadium on glucose metabolism in vitro .
Life Sci ., 25: 1159-1164.
TONEY, J.H., MURTHY, M.S., & MARKS, T.J. (1985) Biodistribu-
tion and pharmacokinetics of vanadium following intraperitoneal
administration of vanadocene dichloride to mice. Chem.-biol.
Interact ., 56(1): 45-54.
TRELLES, R.A., LARGHI, L.A., & PAEZ, L.J.P. (1970) [The health
problem of human drinking-water with high contents of arsenic,
vanadium, and fluorine.] Saneamiento , 34(217): 31-80 (in
Spanish).
TROPPENS, H. (1969) [The uncertainties of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 24: 1089-1092 (in
German).
TRUMMERT, W. & BOEHM, G. (1957) [The trace element vanadium
and its haemopoietic action.] Blut , 3: 210-216 (in German).
TYLER, G. (1970) [Pollution with heavy metals in the
Norrkkoping area. II. Molybdenum and vanadium.] In: [ Report No.
12 on ecological heavy metal research ,] Lund, University of
Lund, Department of Plant Ecology (in Swedish).
UENO, S. & ISHIZAKI, M. (1980) Concentrations of vanadium in
human urine and hair. Jpn. J. ind. Health , 22: 318-379.
UNDERWOOD, E.J. (1977) Vanadium. In: Trace elements in human
and animal nutrition , 4th ed., New York, Academic Press,
pp. 388-397.
UNSAL, M. (1978) Study of the transfer pathways and the
accumulation phenomena of vanadium in mollusca Mytilus edulis.
Rev. int. oceanogr. Med ., 51/52: 71-81.
US BUREAU OF MINES (1974) Metals Information Bulletin. Washing-
ton DC, US Department of the Interior, Bureau of Mines, Branch
of Ferrous Metals.
US EPA (1977) Scientific and technical assessment report on
vanadium , Washington DC, US Environmental Protection Agency
(STAR series, EPA-600/6-77-002).
USMONOV, S.M. (1973) [Vanadium content in constituents of
bone, urine, and faeces in sufferers of acute and chronic
dysentery.] In: [ Epidemiological, microbiological, and clinical
problems in the infectious pathology of Uzbekistan ,] Tashkent,
Institute of Hygiene, Sanitation, and Occupational Diseases,
pp. 125-128 (in Russian).
USUTANI, S., NISHIAMA, K., SATO, I., MATSURA, K., SAWADA, Y.,
HOSOKAWA, Y., & IZUMI, S. (1979) [An investigation of the
environment in a certain vanadium refinery.] Jpn. J. ind.
Health , 21: 11-20 (in Japanese).
VAN DEN BERG, C.M.G. & HUANG ZI QIANG (1984) Direct electro-
chemical determination of dissolved vanadium in seawater by
cathodic stripping voltammetry with the hanging mercury drop
electrode. Anal. Chem ., 56(13): 2383-2386.
VANSELOW, A.P. (1950) Unpublished data cited by Pratt, P.F.
In: Diagnostic criteria for plants and soils, University of
California, Division of Agricultural Sciences , University of
California, pp. 480-482.
VAN ZINDEREN BAKKER, E.M. & JAWORSKI, J.F. (1980) Effects of
vanadium in the Canadian environment , Ottawa, National Research
Council of Canada (Publication No. 18132).
VERSIECK, J. & CORNELIS, R. (1980) Normal levels of trace
elements in human blood-plasma or -serum. Anal. Chim. Acta , 116:
217-254.
VEYS, C. (1983) Possibilités offertes par les méthodes
modernes de polarographie au dosage à éléments tracés. Ind.
Sci ., 59(3): 1-6.
VIGLIANI, E.C. (1969) WHO Technical Report Series No. 415:
Permissible levels of occupational exposure to airborne toxic
substances (Sixth Report of the Joint ILO/WHO Committee on
Occupational Health) , Geneva, World Health Organization, pp. 5-
16.
VlNOGRADOV, A.P. (1937) [The chemical elements entering into
the composition of marine organisms. Part II.] Tr. biogeokhim.
Lab ., 4: 217 (in Russian).
VINOGRADOV A.P. (1944) [The geochemistry of trace elements in
seawater.] Uspehi Himii , 13(1): 123-129 (in Russian).
VINOGRADOV, A.P. (1957) [ Geochemistry of rare and dispersed
chemical elements in soils ,] 2nd ed (revised), Moscow, USSR
Academy of Sciences, pp. 122-129, 216-217 (in Russian).
VINOGRADOV, A.P. (1959) [ The geochemistry of rare and
dispersed elements in soil ,] 2nd ed., New York, Consultants
Bureau Inc., 209 pp (in Russian).
VINTINNER, F.J., VALLENAS, R., CARLIN, C.E., WEISS, R., MACHER,
C., & OCHOA, R. (1955) Study of the health of workers employed
in mining and processing of vanadium ore. Arch. ind. Health ,
12: 635.
VOORS, A.W. (1971) Minerals in the municipal water and
atherosclerotic heart death. Am. J. Epidemiol ., 93: 259-266.
VOUK, V. (1979) Vanadium. In: Friberg, L., Nordberg, G.F., &
Vouk, V., ed. Handbook on the toxicology of metals , Amsterdam,
New York, Oxford, Elsevier Science Publishers, pp. 659-674.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1980) A specific
enzyme is not necessary for vanadate-induced oxidation of NADH.
Nature (Lond.) , 286(5772): 516-517.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1981) The disparity
between effects of vanadate (V) and vanadyl (IV) ions on (Na+ -
K+) AtPase and K+-phosphatase in skeletal muscle. Biochem.
biophys. Res. Commun ., 100: 982-987.
WALDRON, H.A. (1979) Metal poisoning: the three common causes.
Mod. Med ., 24(9): 45-47.
WALLACE, A., ALEXANDER, G.V., & CHADHURY, F.M. (1977) Phyto-
toxicity of cobalt, vanadium, titanium, silver, and chromium.
Commun. soil. Sci. plant Anal ., 8(9): 751-756.
WARINGTON, K. (1955) The influence of iron supply on toxic
effects of manganese, molybdenum and vanadium in soybeans, peas,
and flax. Ann. appl. Biol ., 41: 1-22.
WATANABE, H., MURAYAMA, H., & YAMAOKA, S. (1966) [Some
clinical findings in vanadium workers.] Jpn. J. ind. Health ,
8(7): 23-27 (in Japanese).
WATERS, M.D. (1977) Toxicology of vanadium. Adv. mod.
Toxicol ., 2: 147-189.
WATERS, M.D., GARDNER, D.E., & COFFIN. D.L. (1974) Cytotoxic
effects of vanadium on rabbit alvolar macrophages in vitro .
Toxicol. appl. Pharmacol ., 28: 253-263.
WATERS, M.D., GARDNER, D.E., ARANYI, C., & COFFIN, D.L. (1975)
Metal toxicity for rabbit alveolar macrophages in vitro.
Environ. Res ., 9: 32-47.
WEAST, R.C. (1987) CRC handbook of chemistry and physics , 67th
ed., Boca Raton, Florida, CRC Press.
WEI, C.I. & MISRA, H.P. (1982) Cytotoxicity of ammonium meta-
vanadate to cultured bovine alveolar macrophage. J. Toxicol.
environ. Health , 9(5-6): 995-1006.
WEI, C.I., AL BAYATI, M.A., CULBERTSON, M.R., ROSENBLATT, L.S.,
& HANSEN, L.D. (1982) Acute toxicity of ammonium metavanadate
in mice. J. Toxicol. environ. Health , 10(4/5): 673-687.
WEISZ, H., ROTHMAIER, K., & LUDWIG, H. (1974) Kinetic
determination of thorium, vanadium, and iodide with the use of a
potentiometer. Anal. Chim. Acta , 73(1): 224-228.
WELCH, R.M. (1973) Vanadium uptake by plants. Absorption
kinetics and the effects of pH, metabolic inhibitors, and other
anions and cations. Plant Physiol ., 51: 828-832.
WELCH, R.M. & ALLAWAY, W.H. (1972) Vanadium determination in
biological materials at nanogram levels by a catalytic method.
Anal. Chem ., 44(9): 1644-1647.
WENTZEL, C.T. (1985) Vanadium: a moderate recovery in 1984.
Eng. mining J ., March: 87-88.
WESTENFELDER, C., HAMBURGER, R.K., & GARCIA, M.E. (1981)
Effect of vanadate on renal tubular function in rats. Am. J.
Physiol ., 240: F522-F529.
WIDE, M. (1984) Effect of short-term exposure to five
industrial metals on the embryonic and fetal development of the
mouse. Environ. Res ., 33: 47-53.
WILLIAMS, D.L. (1973) Biological value of vanadium for rats,
chickens, and sheep , Ann Arbor, Michigan, Purdue University
(Ph.D. Thesis).
WILLIAMS, N. (1952) Vanadium poisoning from cleaning oil-fired
boilers. Br. J. ind. Med ., 9: 50-55.
WITKOWSKA, D. & BRZEZINSKI, J. (1979) Alteration of brain
noradrenaline, dopamine, and 5-hydroxytryptamine levels during
vanadium poisoning. Pol. J. Pharmacol. Pharm ., 31: 393-398.
WOOD, B.J., GALOBARDES, J.F., & ROGERS, L.B. (1982) Effect of
the organic ligand on the response for vanadium in flame atomic
emission spectrometry. Anal. Chim. Acta , 135: 145-152.
WRIGHT, L.D., LI, L.F., & TRAGER, R. (1960) The site of
vanadyl inhibition of chloesterol biosynthesis in liver
homogenates. Biochem. biophys. Res. Commun ., 3: 264-267.
WYERS, H. (1946) Some toxic effects of vanadium pentoxide.
Br. J. ind. Med ., 3: 177-182.
WYERS, H. (1948) Some recent observations on hazards in the
chemical industry. In: Proceedings of the 9th International
Congress of Industrial Medicine, London, 13-17 September ,
Bristol, John Wright, pp. 900-903.
YUKAWA, M., SUZUKI-YASUMOTO, M., AMANO, K., & TERAI, M. (1980)
Distribution of trace elements in the human body determined by
neutron activation analysis. Arch. environ. Health , 35(1): 36-
44.
ZAJIC, J.E. (1969) Microbial biochemistry. 12. Vanadium
biogeochemistry , New York, Academic Press, pp. 126-202.
ZEMKOVA, H., TEISINGER, J., & VYSKOCIL, F. (1982) The
comparison of vanadyl (IV) and insulin-induced hyperpolarization
of the mammalian muscle cell. Biochim. Biophys. Acta , 720: 405-
410.
ZENZ, C. & BERG, B.A. (1967) Human responses to controlled
vanadium pentoxide exposure. Arch. environ. Health , 14: 709-
712.
ZENZ, C., BARTLETT, J.P., & THIEDE, W.H. (1962) Acute vanadium
pentoxide intoxication. Arch. environ. Health , 5(6): 542-546.
ZHELJAZKOVA, B.G., CVETANOVA, A.L., & JACIMIRSKIJ, K.B. (1972)
[A catalytic method for determining nanogram quantities of
vanadium.] Zh. anal. Khim ., 27(4): 795-798 (in Russian).
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentration and sources of trace metals at the South Pole.
Science , 183: 198-200.
ZOLLER, W.H., GORDON, G.E., GLADNEY, E.S., & JONES, A.G. (1973)
The sources and distribution of vanadium in the atmosphere. In:
Kothy, E.L., ed. Trace elements in the environment , Washington
DC, American Chemical Society, pp. 31-47 (Advances in Chemistry
Series 123).
ZOLOTAVIN, V.L. (1954) [Achieving a pure solution of chlor-
vanadyl.] Zh. obshch. Khim ., 24: 433 (in Russian).
Other observations of boiler-cleaning operations have been
made by Fallentin & Frost (1954), Sjöberg (1955), Thomas &
Stiebris (1956), Hickling (1958), and Kuzelova et al. (1975).
In terms of respiratory symptoms relating to boiler-cleaning, it
should be noted that sulfates and sulfuric acid may be present
in boiler soot and may be partly responsible for irritative
effects. Hudson (1964) suggested that the quick onset of
symptoms (lachrymation with nose and throat irritation) with
rapid recovery following removal from exposure is character-
istic of exposure to acid sulfates. Response to vanadium expo-
sure is characterized by some delay in the onset of irritative
symptoms (a few hours to several days) and persistence of
symptoms following removal from exposure (Hudson, 1964).
A recent report by Levy et al. (1984) concerned a
comparatively high incidence of severe respiratory tract
irritation in boilermakers (74/100), many of them welders in
areas without adequate ventilation, exposed to vanadium
pentoxide fumes in a power plant where conversion from oil- to
coal-burning occurred. The severe illness of 70 men caused an
average of 5 days of absence, some objective tests (e.g., FVC)
being markedly affected. The vanadium pentoxide content was
above the permissible exposure limit in 8 samples, and this
resulted in litigation for inadequate protection of the
workers.
Kuzelova et al. (1977) drew attention to the occupational
risk of chimney sweeps cleaning large-capacity heating
facilities in large housing settlements. This coincided with a
report of a detailed cross-sectional examination of 121 chimney
sweeps by Holzhauer & Schaller (1977) in the Federal Republic of
Germany with an average exposure duration of 19 years ( ± 5
years). Vanadium exposure was determined by personal samples,
and measurements between 0.73 and 13.7 mg vanadium pentoxide/day
were determined compared with 4 µg in the normal (average)
population. Urinary excretion was determined to be between 0.15
and 13 µg/litre, which was significantly higher than the values
in 31 referents. The main complaints of the chimney sweeps were
wheezing, rhinitis, conjunctival irritation, cough, sputum
dyspnoea, and hoarseness; there were no skin symptoms. A
prospective follow-up of the cohort was emphasized, but the
results are not yet available.
8.4.3 Handling of pure vanadium pentoxide or vanadate dusts
Health effects due to occupational handling of pure vanadium
pentoxide or vanadate dusts have been reported. Tara et al.
(1953) described the effects of vanadium exposure in 4 dock
workers who unloaded and bagged spilled calcium vanadate. The
symptoms (bronchitic wheezing sounds, dyspnoea, productive
cough, haemoptysis in one case, and headache) necessitated
interruption of the work after 1´ days. Zenz et al. (1962)
described an acute illness that occurred in 18 workers pellet-
izing pure vanadium pentoxide; it was characterized by a rapidly
developing mild conjunctivitis, severe pharyngeal irritation, a
non-productive persistent cough, diffuse rales, and broncho-
spasm. With severe exposure, 4 men complained of itching skin
and a sensation of heat in the face and forearms. The symptoms
became more severe after each exposure, suggesting a sensitivity
reaction, but their duration was not prolonged by repeated
exposures.
8.4.4 Other industries
Browne (1955) studied vanadium poisoning in 12 patients
exposed to exhaust fumes from gas turbines using heavy fuel oil.
Evidence of poisoning appeared between the first and 14th day of
exposure and consisted of conjunctivitis, rhinitis, cough,
crepitations, and dyspnoea. Bleeding appeared before the
rhinorrhoea.
Other occupations in which respiratory effects of vanadium
exposure have been reported include operations connected with
the gasification of fuel oil (Fear & Tyrer, 1958) and the
manufacture of phosphor for television picture tubes (Tebrock &
Machle, 1968). In the latter study, elevated blood pressure was
noted in men exposed to vanadium pentoxide.
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1 Environmental Levels and Exposures
While vanadium concentrations in the air of remote rural
areas are less than 1 ng/m3, other rural areas show concentra-
tions in excess of 50 ng/m3. This is generally considered to
reflect the local burning of fuel oil with a high vanadium
content. Typical concentrations in urban air may range from
below 1 ng/m3 to over 300 ng/m3, with annual averages for large
cities of about 20 - 100 ng/m3. At an annual average of
50 ng/m3 and a respiration rate of 20 m3, the total amount of
vanadium reaching the respiratory tract would be only 1 µg.
Assuming a rate of absorption of about 25%, the direct daily
contribution of vanadium from air would be about 250 ng.
Drinking-water supplies without excessive vanadium pollution
contain from less than 1 µg/litre to occasional maximum
concentrations of 15 - 30 µg/litre. Two comprehensive surveys
have shown average concentrations of 4.3 and 4.85 µg/litre,
respectively. At a daily intake of 2 litres of water, the
average daily intake of vanadium with water would be about
10 µg, ranging from about 1 µg to 30 - 60 µg. Although
levels in ordinary water supplies would vary considerably,
intake should rarely exceed 100 µg/day. Intake with bottled
waters from mineral springs may exceed these values.
As a rule, the concentration of vanadium in food is low.
High levels reported in early studies have been attributed to
analytical differences. Recent studies on complete diets
suggest a daily intake of vanadium of about 10 - 70 µg, with
the majority of estimates remaining below 30 µg. Assuming an
absorption rate from the gastrointestinal tract of 1 - 2%, the
contribution from food and water is unlikely to exceed 4 -
5 µg/day.
Vanadium concentrations in air in the vicinity of metal-
lurgical industries are often about 1 µg/m3. In the production
of vanadium metal or compounds, concentrations may reach a few
mg/m3. In boiler-cleaning operations, dust concentrations in
air are frequently around 50 - 100 mg/m3, and concentrations as
high as 500 mg/m3 have been reported; the vanadium content of
the dust is about 5 - 17% as vanadium pentoxide and 3 - 10 % as
lower vanadium oxides. The need for personal protection devices
in such operations is obvious.
9.2 Physiological Role
While present knowledge indicates that vanadium is an
essential element for chicks and rats, conclusive evidence that
vanadium is essential for other species, including man, is
lacking. A variety of physiological and biochemical processes
have been found to be vanadium sensitive. However, so far,
there is no evidence of adverse effects arising from vanadium
deficiency in man, and the daily requirement of vanadium in the
diet is not known.
9.3 Effects and Dose-Response Relationships
The toxicity of vanadium varies in experimental animals with
both the species and route of administration. Small animals,
such as the rat and mouse, tolerate the metal better than the
rabbit and horse. The toxicity of vanadium is low when given
orally, moderate when inhaled, and high when injected. As a
rule, the toxicity of vanadium increases as the valency
increases, pentavalent vanadium being the most toxic.
9.3.1 Local effects and dose-response relationships
Exposure of 2 volunteers to vanadium pentoxide dust at
1 mg/m3 for 8 h resulted in irritation of the respiratory tract
with cough starting 5 h later. The cough lasted for 8 days.
Exposure of 5 volunteers to a concentration of 0.2 mg/m3 caused
the same symptoms, i.e., coughing, but with an onset at 20 h.
The cough lasted for 7 - 10 days. Respiratory irritation was
noted in 2 subjects exposed to 0.1 mg/m3 for 8 h. The irritant
effect was manifested as an increase in mucous production, 24 h
after exposure, and total recovery within 4 days. Tickling and
itching, together with dryness of the mucous membranes of the
mouth, was reported by 11 volunteers exposed to 0.4 mg/m3 of
vanadium pentoxide condensation aerosol; 0.16 mg/m3 caused
irritation in only 5 subjects, and 0.08 mg/m3 did not induce
symptoms in any of the subjects.
Exposure to high concentrations of vanadium is possible in
the industrial production and use of vanadium, especially in the
cleaning of oil-fired boilers. Frequently reported irritant
symptoms include sneezing, nasal discharge, irritation of the
eyes with lachrymation, sore throat, dry or productive cough,
and chest pain. Normally, such symptoms disappear in a few days
when exposure has ceased. Cough, increased sputum, and
particularly irritation of the eyes, nose, and throat occurred
among 24 male workers exposed to a maximum of 0.9 - 5 mg
vanadium/m3 (measured as vanadium pentoxide V2O5). Most workers
had been exposed to 0.3 mg/m3 (section 8.4.1). A cross-
sectional study of 63 male workers exposed for an average of 11
years to vanadium-containing dust at 0.2 - 0.5 mg vanadium/m3
(range, 0.1 - 3.9 mg/m3) showed chronic irritant effects in the
mucous membranes of the nose. The nasal changes persisted
unchanged during subsequent exposure to much lower levels
(0.01 - 0.04 mg/m3).
Heavily exposed workers (dust concentrations of 5 -
150 mg/m3) developed atrophic rhinitis and chronic bronchitis.
Bronchospasm is also a feature in heavily exposed workers.
The effects on 63 workers of long-term exposure to vanadium
at 0.2 - 0.5 mg/m3 were studied using matched referents, a
questionnaire on respiratory symptoms, chest radiography, and
lung function testing (section 8.4). There was no change in
ventilatory function compared with the matched reference group;
only complaints of wheezing were significantly more common among
exposed workers than among referents. However, in another study
the forced vital capacity (FVC) was reversibly reduced in 17
boiler cleaners who had been exposed to a time-weighted average
respirable dust of 523 µg/3 containing 15% of vanadium (section
8.3.1).
Vanadium poses weak sensitizing properties when skin and
mucous membranes of the upper respiratory tract are exposed to
high concentrations, manifested by the development of allergic
dermatitis and rhinitis in workers in contact with vanadium.
The allergic nature of these manifestations is proved by
positive reactions of epicutaneous tests with a 2% solution of
sodium vanadate. There is information on the sensitizing effect
of vanadium in tests on animals.
In one study (section 8.4.1), 9 workers out of 36 developed
a dry eczematous dermatitis. These workers had been exposed to
vanadium pentoxide at about 6.5 µg/m3.
Green tongue is seen in a proportion of workers exposed to
vanadium-containing dust, and is an indication of exposure.
9.3.2 Systemic effects and dose-response relationships
9.3.2.1 Metabolic effects
The effect of vanadium on dental caries remains a debatable
issue (section 5.4.2). The application of a 50% paste of an
ammonium salt of vanadium and glycerol was reported to reduce
caries in children aged 7 - 11 years. Other studies between
1955 and 1968 have failed to demonstrate a clearly beneficial
effect (section 8.1.2.1).
Soluble diammonium oxytartarovanadate (150 - 200 mg/day for
6 weeks) was administered to 5 healthy adult male volunteers.
There was a significant reduction of plasma-cholesterol levels
at the end of the period. A temporary drop in the cholesterol
level was also observed in 2 out of 6 patients given ammonium
vanadyl tartrate for 7 weeks at 50 or 100 mg/day. The results
were not convincing and the temporary drops in cholesterol
levels were not statistically significant. No significant
changes in serum-cholesterol levels were noted in 12 patients (9
of whom were hypercholesterolaemic) given diammonium vanado-
tartrate orally for 6 months, (25 mg three times daily for 2
weeks, increased to 125 mg daily in 10 patients). Although some
studies on rats and rabbits have indicated decreasing
cholesterol levels following administration of vanadium and have
corroborated the reduced levels of cholesterol observed by other
authors, this effect of vanadium has not been convincingly
demonstrated in human beings so far (section 8.1.2.1).
Vanadium pentoxide in the diet (25 - 1000 mg vanadium/kg)
resulted in lower levels of cystine in the hair of rats compared
with those in controls, indicating the inhibition of cystine
synthesis (section 7.2.1). Rats administered sodium vanadate
intraperitoneally (5 - 10 mg/kg body weight as a single
injection or a dietary concentration of 500 mg/kg) showed
reduction of co-enzyme A in the liver; this has been construed
as an explanation of the reduction of cystine (section 7.2.1).
When workers were exposed to vanadium-containing dust (0.2 -
0.5 mg vanadium/m3, at the time of the study), there was no
correlation between exposure level and cystine levels in
fingernails, and no decrease in levels of serum-cholesterol or
triglycerides (section 8.4.1).
The data on the effects of vanadium on haematopoiesis are
inconsistent. A favourable effect of vanadium chloride (0.6 mg
vanadium/kg diet) on haemoglobin levels in rats, previously made
anaemic, had already been suggested in 1931 (section 7.2). A
small increase in erythrocytes and haemoglobin levels was
observed in rabbits given vanadyl sulfate subcutaneously, at
1 mg/kg body weight daily, for 2 months). When 32 vanadium
workers who had been exposed for more than 6 months were
compared with 45 referents, matched for age, no differences were
seen in haematocrit levels (8.2.1.1). It is not possible to
assess the effects of low-level vanadium exposure on iron
metabolism.
9.3.2.2 Effects on the nervous system
Systemic effects are rare in workers exposed to vanadium
compounds. Non-specific signs and symptoms including headache,
weakness, nausea, vomiting, and tinnitus have been reported.
Such signs and symptoms have mostly occurred in workers exposed
to extremely high dust concentrations, when cleaning oil-fired
boilers, but it has not been possible to derive dose-response
relationships for them.
Elevated vanadium levels in whole blood and serum have been
reported in patients suffering from depressive illness. In one
report, the vanadium levels fell to normal with recovery of the
patients. The role of vanadium in depressive states is not
known (section 8.2.2).
In mice and rats, repeated oral administration of vanadium
pentoxide or ammonium vanadate at doses of 0.05 - 0.5 mg
vanadium/kg body weight, daily, for 6 months and 21 days,
respectively, resulted in impaired conditioned reflexes. Daily
oral doses of sodium metavanadate (3.2 µg/kg body weight per
day for 10 - 15 days) caused increases in the activity of
cytochrome oxidase in the brain of guinea-pigs; a dose of
128 µg/kg per day did not have any effect, whereas 5.12 mg/kg
per day reduced the activity (section 7.2). Total
cholinesterase activity in the brain of rats was significantly
reduced by the intraperitoneal administration of 1 mg vanadyl
sulfate/kg body weight (section 7.3).
9.3.2.3 Effects on the liver
There are insufficient human data to make an assessment of
the effects of vanadium on the liver. Rats and rabbits exposed
through inhalation to vanadium pentoxide, trioxide, or tri-
chloride (10 - 70 mg/kg, 2 h/day, for 9 - 12 months) showed
fatty changes with partial cell necrosis in the liver. A clear
reduction in the liver tissue respiration and a decrease in the
albumin/globulin ratio in the serum were also observed. Subcu-
taneous injection of ammonium vanadate (1 mg vanadium/kg body
weight per day, for 30 days) caused similar fatty changes in the
liver of rats. Intraperitoneal injections of sodium meta-
vanadate (1.25 - 2.5 mg vanadium/kg body weight) in rats caused
loss of weight. The toxic effects observed were correlated with
the concentration of vanadium in the liver (section 7.2).
9.3.2.4 Effects on the kidney
Data on the effects of vanadium on the human kidney are
lacking. Intravenous injection of sodium metavanadate (2.5 -
5 mg/kg body weight) in male dogs resulted in albuminuria. In
mice, acute tubular necrosis followed subcutaneous injection of
ammonium vanadate at a dose equivalent to 20 mg vanadium/kg body
weight. Rats and rabbits inhaling vanadium chloride (70 mg/m3
2 h/day, for 9 - 12 months) caused fatty changes in the kidney.
Vanadate has diuretic and natriuretic effects on the kidney in
the rat but not in the dog or cat. Vanadate has also been
reported to increase the urinary excretion of calcium,
phosphate, bicarbonate, and chloride by the rat kidney. These
diuretic and natriuretic effects are thought to be due to the
inhibition of Na+-K+-ATPase causing inhibition of the tubular
reabsorption.
9.3.2.5 Cardiovascular effects
Palpitation of the heart at rest and on exercise has been
reported in workers occupationally exposed to vanadium.
Transient coronary insufficiency, a high incidence of extra-
systoles and bradycardia were reported (section 8.3). Exposure
of workers to low levels of vanadium pentoxide (0.2 - 0.5 mg/m3)
did not cause any pathological changes in the blood picture
(section 8.4.1).
Electrocardiographic changes (ST-segment depression,
increased T-wave amplitudes) were seen after intravenous
injection of sodium metavanadate in dogs (2.5 - 5 mg/kg body
weight). Long-term inhalation exposure of rats and rabbits to
vanadium pentoxide, trioxide, or chloride (10 - 70 mg/m3,
2 h/day, for 9 - 12 months) caused fatty changes in the myo-
cardium as well as perivascular swelling.
9.3.2.6 Pulmonary effects
Asthmatic reactions in conjunction with non-specific
bronchial hyperreactivity have occasionally been reported in
refinery workers exposed to vanadium pentoxide dust. There has
not been any evidence of an immunological mechanism behind such
cases. A dose-dependent decline in forced expiratory volume in
one second (FEV1) and forced vital capacity (FVC) has been
demonstrated in boiler cleaners. The functional increase did
not return to normal during the first week following exposure,
but fully recovered within one month. The mechanism leading to
the obstructive pulmonary impairment has not been clarified.
9.3.2.7 Effects on the immune system
In mice, vanadium and ammonium vanadate affect the normal
function of the immune system. Vanadium had slight depressant
effects on antibody-forming cells and increased DNA synthesis in
splenic leukocytes. Ammonium vanadate increased resistance
to E. coli endotoxin, but decreased resistance to Listeria
lethality. In the spleen, it increased the rosetting capability
of leukocytes, the formation of megakaryocytes, and red blood
cell precursors (section 7.7).
9.3.3 Reproduction, embryotoxicity, and teratogenicity
Human data on the effects of vanadium on reproduction and
embryotoxicity are lacking. Vanadium administered to pregnant
rats by subcutaneous administration of metavanadate (0.85 mg/kg,
equal to 1/20 LD50) accumulated in the placenta. However, the
extent to which it reaches the fetus has not been clearly
established. During the lactation period, vanadium was found in
the mammary glands and was excreted with milk. Morphological
changes in spermatozoa as well as desquamation of spermatogenic
epithelium in the seminal tubuli were observed. Gonadotoxic
effects were suggested by the absence of fertilization of female
rats by male rats that had been exposed to 0.85 mg vanadium/kg
body weight. The same doses of vanadium given to female rats on
the fourth day of pregnancy significantly decreased the number
of fetuses (section 7.8.1).
Weanling pigs receiving vanadate (200 mg vanadium/kg body
weight) showed a suppressed growth rate and increased mortality.
Vanadium was not markedly toxic when fed to growing lambs
(200 mg/kg, 84 days) (section 7.8.1).
Tentative results suggest that vanadium is teratogenic for
rats and hamsters causing skeletal anomalies and death of the
fetuses. Dose-response relationships have not been demonstrated
(section 7.8.2). There are no human data concerning possible
teratogenic effects of vanadium.
9.3.4 Mutagenicity
The data on the mutagenic potential of vanadium in bacterial
systems are inconclusive. There are positive and negative
results with E. coli and Salmonella tests (section 7.9). Data
suggest the induction of micronuclei, but not sister chromatid
exchange or dominant-lethal mutations. Chromosome effects in
vivo and in vitro have not been studied.
9.3.5 Carcinogenicity
Life-time studies on mice given 5 µg vanadyl ions/ml as the
sulfate in drinking-water did not increase the incidence of
spontaneous tumours and intraperitoneal injections of
vanadium(III)2.4pentanedione (24, 60, or 120 mg/kg body weight)
did not increase the incidence of lung adenomas in mice. The
results of a long-term study on rats with intrabronchiolar
implants of vanadium solids were negative. The few studies
available do not provide any indications of carcinogenic effects
of vanadium (section 7.10).
9.3.6 Risks from exposure of the general population
There are only a few studies on the possible effects of
vanadium in ambient air on the general population (section
8.3.2). In one study, air concentrations of vanadium together
with 12 other trace elements were found to be correlated with
mortality from pneumonia and lung cancer (coefficients of
correlation of 0.443 and 0.347, respectively) and also with
mortality from bronchitis (coefficient of correlation of 0.563).
In another study, a correlation between the levels of vanadium,
cadmium, zinc, tin, and nickel and the incidence of several
diseases including "diseases of the heart", "nephritis", and
"arteriosclerotic heart" was claimed, but the results of these
studies do not establish any causal relationships.
10. RECOMMENDATIONS
There is a conspicuous lack of data on several aspects of
the health effects of vanadium compounds. The overwhelming bulk
of recent research focuses on the effects of vanadium on
biochemical systems, especially specific effects on enzymes, and
there are major gaps in knowledge with respect to analytical,
metabolic, and exposure data.
There are indications of a weak mutagenic effect of
vanadium, but the data are partly conflicting and
uncorroborated. Further confirmative mutagenicity studies,
including studies on chromosomal effects, should be given high
priority. Data on the carcinogenicity of vanadium in various
species are practically non-existent. Such studies are urgent
and should be conducted as long-term exposure studies.
Vanadium induces toxic effects on the fetus. However,
whether these are direct effects or indirect effects resulting
from effects of vanadium on the mother is not known. Studies to
assess the nature of the teratogenic effects and the mechanism
behind them should be encouraged.
Effects resulting from high occupational exposure to
vanadium dusts have been reasonably well described. Such
exposure levels may cause a variety of clinical manifestations.
However, they should be remedied by hygienic and technical
improvements. Dose-effect and dose-response relationships have
not been well defined at low exposure levels in the range of
approximately 0.01 - 0.5 mg/m3. It is considered important to
develop specific indicators for the detection of early adverse
effects of vanadium on man.
Epidemiological studies on occupational cohorts, paying
particular attention to exposure levels and using proper
referent groups, should be encouraged. The literature is
inconsistent regarding the documentation of sensitizing
properties of vanadium compounds. This is an important aspect
with regard to worker protection.
An area of great importance is the exposure of the general
population. There are considerable geographical variations in
vanadium concentrations in air and water. Epidemiological
studies on populations living in areas with high vanadium
exposure should be carried out, relating possible adverse
effects to exposure levels. Such studies should take into
account possible interactions with other pollutants.
REFERENCES
ACGIH (1986) Threshold limit values for chemical substances
and physical agents in the work environment , Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists.
AGASYAN, P.K., LAKEEVA, T.N., NIKOLAEVA, E.R., PARTASHNIKOVA,
M.Z., SHKOLENOK, G.F., & KHREPINYUK, T.E. (1975) [Determina-
tion of the bulk substances in preparations of potassium
dichromate and ammonium vanadate by means of differential
coulometry.] Zavod. Lab ., 41(4): 385-387 (in Russian).
AIYAR, A.S. & SREENIVASAN, A. (1961) Effect of vanadium
administration on coenzyme Q metabolism in rats. Proc. Soc.
Exp. Biol. Med ., 107: 914-916.
ALESHIN, G.N., SMOLJANINOV, S.I., MESHEYAKOV, R.P., & GLOUCHOV,
G.G. (1974) [The chemistry and technology of vanadium
compounds.] In: Ivakin, A.A. & Voronova, E.M., ed. [ Proceedings
of the International Conference on the Chemistry and Technology
of Vanadium Compounds ,] Perm, Scientific Institute of Black
Metals (in Russian).
ALI, S.A., PEET, M., & WARD, N.I. (1985) Blood levels of
vanadium, caesium, and other elements in depressive patients. J.
affective Disord ., 9(2): 187-191.
ALLAWAY, W.H. (l968) Selenium, molybdenum, and vanadium in
human blood. Arch. environ. Health , 16: 342-348.
ANBAR, M. & INBAR, M. (1962) Effect of pyrodoxial 5-phosphate
in the presence of vanadyl ions of the de-iodination of
thyroxine. Nature (Lond.) , 196: 1213.
ARNON, D.I. (1958) The role of micronutrients in plant
nutrition with special reference to photosynthesis and nitrogen
assimilation. In: Lamb, C.A., Bentley, 0.G., & Beattie, J.M.,
ed. Trace elements , New York, Academic Press, pp. 1-32.
ARNON, D.I. & WESSEL, G. (1953) Vanadium as an essential
element for green plants. Nature (Lond.) , 172: 1039-1040.
ATHANASSIADIS, Y.C. (1969) Preliminary air pollution survey
of vanadium and its compounds: A literature review , Raleigh,
North Carolina, National Air Pollution Control Administration
(Publication No. APTD 69-48).
AZARNOFF, D.L. & CURRAN, G.L. (1957) Site of vanadium
inhibition of cholesterol biosynthesis (letter to the editor).
J. Am. Chem. Soc ., 79: 2968.
AZARNOFF, D.L., BROCK, F.E., & CURRAN, G.L. (1961) A specific
site of vanadium inhibition of cholesterol biosynthesis.
Biochem. Biophys. Acta , 51: 379-398.
BABENKO, G.A. & VANDZHURA, I.N. (1969) [Vanadium metabolism
after supplementary administration of certain microelements in
experimental atherosclerosis.] Bull. exp. Biol. Med ., 67(6):
72-73 (in Russian).
BAGGETT, W.L. & HUYCK, H.P. (1959) Spectrochemical determina-
tion of vanadium in alkali brines. Anal. Chem ., 31(8): 1320-
1322.
BAKAL, G.F. & LISECKAJA, G.S. (1971) [Determination of
vanadium in mercury using the reaction of oxidation of
pyrocatechin by ammonium persulfate.] Ukr. khim Zh ., 37(6):
594-597 (in Russian).
BALESTRA, G. & MOLFINO, F. (1942) [Lung impairments due to
dusts in operations to extract vanadium from petroleum ash.]
Rass. Med. Ind. Ig. Lav ., 13: 5-12 (in Italian).
BALFOUR, W.E., GRANTHAM, J.J., & GLYNN, I.M. (1978) Vanadate -
stimulated natriuresis. Nature (Lond.) , 275: 768.
BARANNIK, P.I., MIKHALYUK, I.A., & MOTUZKOV, I.N. (1969) Data
on the role of chromium, lead, vanadium and the natural radio-
activity of foodstuffs in the etiology of endemic goiter. Hyg.
Sanit ., 35(2): 289-291.
BARBOOTI, M.M. & JASIM, F. (1982) Electrothermal atomic-
absorption determination of vanadium. Talanta , 29: 107-111.
BARNES, R.M., FODOR, P., INAGAKI, K., & FODOR, M. (1983)
Determination of trace elements in urine using inductively
coupled plasma spectroscopy with a poly(dithiocarbamate)
chelating resin. Spectrochim. Acta , 38B: 245-257.
BARRY, E.F., REI, M.T., REYNOLDS, H.H., & O'BRIEN, J. (1975)
Determination of nickel and vanadium in the atmosphere of
eastern Massachusetts. Environ. Lett ., 8(4): 381-385.
BEARD, H.H., BAKER, R.W., & MYERS, V.C. (1931) Studies in the
nutritional anemia of the rat. J. biol. Chem ., 94: 123.
BELEHOVA, V.A. (1966) [Spectral analysis of vanadium content
in intact teeth and in teeth damaged by caries.] Stomatologija ,
4: 85-86 (in Russian).
BELEHOVA, V.A. (1969) [Vanadization of teeth as a means of
preventing dental caries.] Nauchn. Tr. Irkutsk. Med. Inst ., 95:
20-33 (in Russian).
BERGEL, F., BRAY, R.C., & HARRAP, Y.R. (1958) A model system
for cysteine desulphydrase action: pyridoxal phosphate-
vanadium. Nature (Lond.) , 181: 1654-1655.
BERNHEIM, F. & BERNHEIM, M.L.C. (1938) Action of vanadium on
tissue oxidation. Science, 88: 481.
BERNHEIM, F. & BERNHEIM, M.L.C. (1939) The action of vanadium
on the oxidation of phospholipids by certain tissues. J. biol.
Chem ., 127: 353.
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric ., 23: 93-100.
BERTRAND, D. (1942) Le vanadium comme élément oligosynergique
pour l'Aspergillus niger. Ann. Inst. Pasteur , 68: 226-244.
BERTRAND, D. (1950) Survey of contemporary knowledge of
biochemistry. II. The biogeochemistry of vanadium. Bull. Am.
Mus. Natl Hist ., 94(7): 407-455.
BEYHL, F.E. (1983) Action of ammonium metavanadate on hepatic
enzymes in vitro . Arch. Toxicol ., Suppl. 6: 250-253.
BIGGS, W.R. & SWINEHART, J.H. (1976) Vanadium in selected
biological systems. In: Sigel, H., ed. Metal ions in biological
systems , New York, Marcel Dekker, Vol. 6 , pp. 141-196.
BONIG, G. & HEIGENER, H. (l971) [Micro-determination of
vanadium in biological substances by selective paper
chromatography.] Landwirtsch. Forsch ., 25: 139-143 (in German).
BORISENKO, L.F. (1973) [ Vanadium ,] Moscow, Nedra Publishing
House (in Russian).
BOWEN, H.J.M. (1963) The elementary composition of mammalian
blood , Berkshire, Wantage Research Laboratory, Isotope Research
Division (AERE-R 4196).
BOYARKINA, A.P., VASILEV, N.V., KRIVYAKORA, E.N., NIKOLAEV,
A.V., & TIUKIUPO, E.B. (1978) [On the accumulation of nickel
and vanadium in the vicinity of industrial centres.] Gig. i
Sanit ., 43(2): 92-95 (in Russian).
BRODERICK, G.N. (1977) Vanadium - 1977 , Washington DC, US
Department of the Interior, Bureau of Mines (Mineral Commodity
Profiles, MCP-8).
BROEKAERT, J.A.C., WOPENKA, B., & PUXBAUM, H. (1982) Inductively
coupled plasma optical emission spectrometry for the analysis of
aerosol samples collected by cascade impactors. Anal. Chem ., 54:
2174-2179.
BROWNE, R.C. (1955) Vanadium poisoning from gas turbines. Br.
J. ind. Med ., 12: 57-59.
BROWNE, R. & STEEL, J. (1963) The control of the vanadium
hazard in catalytic oil-gas plants. Ann. occup. Hyg ., 6: 75-79.
BROWNING, E. (1969) Vanadium. In: Toxicity of industrial
metals , 2nd ed., London, Butterworths, pp. 340-347.
BUCHET, J.P., KNEPPER, E., & LAUWERYS, R. (1982) Determination
of vanadium in urine by electrothermal atomic absorption
spectrometry. Anal. Chim. Acta , 136: 243-248.
BUCKINGHAM, D.A. (1973) Structure and stereochemistry of
coordination compounds. Part 1. Coordination chemistry. In:
Eichhorn, G.L., ed. Inorganic biochemistry , Amsterdam, New York,
Oxford, Elsevier Science Publishers, Vol. 1 , p. 18.
BUDNIKOV, G.K. & MEDJANCEVA, E.P. (1973) [Determination of
vanadium and nickel in bitumen by oscillographic polarography
with linear change of potential.] Zh. anal. Khim ., 28(2): 301-
305 (in Russian).
BUONO, J.A., BUONO, J.C., & FASCHING, J.L. (1977) Simultaneous
determination of Al, V. Mn and Cu from neutronactivated saline
matrices by precipitation with poly-5-vinyl-8-hydroxyquinoline.
J. radioanal. Chem ., 36: 353.
BUTT, E.M., NUSBAUM, R.E., GILMOUR, T.C., DIDIO, S.L., & Sister
MARIANO (1964) Trace metal levels in human serum and blood.
Arch. environ. Health , 8: 60.
BYRNE, A.R. & KOSTA, L. (1978) Vanadium in foods and in human
body fluids and tissues. Sci. total Environ ., 10: 17-30.
BYRNE, A.R. & KOSTA, L. (1979) On the vanadium and tin
contents of diet and human blood. Sci. total Environ ., 11: 87-
90.
CANALIS, E. (1985) Effect of sodium vanadate on deoxyribo-
nucleic acid and protein synthesis in cultured rat calvariae.
Endocrinology , 116(3): 855-862.
CANNON, H.L. (1963) The biogeochemistry of vanadium. Soil
Sci., 96(3): 196-204.
CANTLEY, L.C., Jr & AISEN, P. (1979) The fate of cytoplasmic
vanadium. Implications on (Na, K)-ATPase inhibition. J. biol.
Chem ., 254: 1781-1784.
CANTLEY, L.C., Jr, JOSEPHSON, L., WARNER, R., YANAGISAWA, M.,
LECHENE, C., & GUIDOTTI, G. (1977) Vanadate is a potent
(Na++,K)-ATPase inhibitor found in ATP derived from muscle. J.
biol. Chem ., 252(21): 7421-7423.
CANTLEY, L.C., Jr, CANTLEY, L.G., & JOSEPHSON, L. (1978a) A
characterization of vanadate interactions with the (Na,K)-
ATPase. J. biol. Chem ., 253: 7361-7368.
CANTLEY, L.C., Jr, RESH, M.D., & GUIDOTTI, G. (1978b) Vanadate
inhibits red cell, (Na+, K+) ATPase from the cytoplasmic side.
Nature (Lond.) , 272: 552-554.
CARLSON, R.M.K. (1977) The structure of the native vanadium
chromagen in tunicate blood cells , Palo Alto, California,
Stanford University, 252 pp (Ph.D. Thesis) (University Micro-
films No. 78-2142).
CARLTON, B.D., BENEKE, M.B., & FISHER, G.L. (1982) Assessment
of the teratogenicity of ammonium vanadate using Syrian golden
hamsters. Environ. Res ., 29(2): 256-262.
CARPENTER, G. (1981) Vanadate, epidermal growth factor and the
stimulation of DNA synthesis. Biochem. biophys. Res. Commun.,
102: 1115-1121.
CASSANI, F. (1969) [Potentiometric determination of titanium
and vanadium.] Chim. Ind. (Milan) , 51: 1248-1251 (in Italian).
CAWSE, P.A. & PEIRSON, D.H., ed. (1972) Analytical study of
trace elements in the atmospheric environment , Harwell, Atomic
Energy Research Establishment (No. AERE-R 7134).
CHASTEEN, N.D., LORD, E.M., THOMPSON, H.J., & GRADY, J.K.
(1986) Vandium complexes of transferrin and ferritin in the
rat. Biochim. Biophys. Acta , 884(1): 84-92.
CHIRIATTI, G.N. (1971) [Prevention of occupational vanadium
poisoning.] Folia med ., 54: 57-76 (in Italian).
CHRISTIAN, G.D. (1971) Catalytic determination of vanadium in
blood and urine. Anal. Lett ., 4(4): 187-196.
CHRISTIAN, G.D. & FELDMAN, F.I. (1970) Atomic absorption
spectroscopy. In: Applications in agriculture, biology, and
medicine , New York, John Wiley and Sons, pp. 276-281.
CHRISTIAN, H.C.M. & ROBINSON, J.W. (1971) The determination of
vanadium and nickel in mineral oils by flameless graphite tube
atomization. Anal. Chim . Acta, 56: 470-473.
CHUPAHIN, S.M., KRIUCHKOV, O.M., & RAMENDI, G.I. (1972)
[ Analytical capabilities of spark-source mass spectrometry ,]
Moscow, Atom Publishers (in Russian).
CLARK, R.J.H. (1975) The chemistry of vanadium, niobium, and
tantalum , Oxford, Pergamon Press, pp. 491-535.
COHEN, M.D., WEI, C.I., TAN, H., & KAO, K.J. (1986) Effect of
ammonium metavanadate on the murine immune response. J. Toxicol.
environ. Health , 19(2): 279-298.
CONKLIN, A.W., SKINNER, C.S., FELTEN, T.L., & SANDERS, C.L.
(1982) Clearance and distribution of intratracheally instilled
48vanadium compounds in the rat. Toxicol. Lett ., 11: 199-203.
CORNELIS, R., MEES, L., HOSTE, J., RYCKEBUSCH, J., VERSIECK, J.,
& BARBIER, F. (1979) Neutron activation analysis of vanadium
in human liver and serum. In: Proceedings of an International
Symposium on Nuclear Activation Techniques in the Life Sciences,
Vienna, 22-26 May, 1978 , Vienna, International Atomic Energy
Agency, pp. 165-177.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1980) Determination of vanadium in human serum by neutron
activation analysis. J. radioanal. Chem ., 55(1): 35-43.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1981) The ultratrace element vanadium in human serum. Biol.
Trace Elem. Res ., 3: 257-263.
COTTON, F.A. & WILKINSON, G. (1962) Advanced inorganic
chemistry , New York, Interscience.
COTTON, F.A. & WILKINSON, G. (1980) Advanced inorganic
chemistry , 4th ed., New York, John Wiley and Sons, pp. 708-719.
COWGILL, U.M. (1973) The determination of all detectable
elements in the aquatic plants of Linsley Pond and Cedar Lake
(North Branford, Connecticut) by X-ray emission and optical
emission spectroscopy. Appl. Spectrosci ., 27: 5-9.
CREASON, J.P., HINNERS, T.A., BUMGARNER, J.E., & PINKERTON, C.
(l975) Trace elements in hair, as related to exposure in
Metropolitan New York. Clin. Chem ., 21: 603-612.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
CURRAN, G.L. & BURCH, R.E. (1967) Biological and health
effects of vanadium. In: Proceedings of the First Annual
Conference on Trace Substances in Environmental Health,
Columbia, 10-11 July, 1967 , Columbia, University of Missouri,
pp. 96-104.
CURRAN, G.L. & COSTELLO, R.L. (1956) Reduction of excess
cholesterol in the rabbit aorta by inhibition of endogenous
cholesterol synthesis. J. exp. Med ., 103: 49-56.
CURRAN, G.L., AZARNOFF, D.L., & BOLINGER, R.E. (1959) Effect
of cholesterol synthesis inhibition in normocholesteremic young
men. J. clin. Invest ., 38: 1251-1261.
DAMS, R., ROBBINS, J.A., RAHN, K.A., & WINCHESTER, J.W. (1970)
Nondestructive neutron activation analyses of air pollution
particulates. Anal. Chem ., 42: 861-867.
DAY, H., MIDDENDORF, D., LUKERT, C., HEINZ, A., & GRANTHAM, J.J.
(1980) The renal response to intravenous vanadate in rats. J.
lab. clin. Med., 96: 382-395.
DEMASTER, E.G. & MITCHELL, R.A. (1973) A comparison of
arsenate and vanadate as inhibitors or uncouplers of
mitochondria and glycolytic energy metabolism. Biochemistry,
12(19): 3616-3621.
DHEW (1968) Air quality data from the National Air
Surveillance Networks and contributing state and local networks ,
1966 ed., Durham, North Carolina, US Department of Health,
Education, and Welfare, National Air Pollution Control
Administration.
DIMOND, E.G., CARAVACA, J., & BENCHIMOL, A. (1963) Vanadium:
Excretion, toxicity, lipid effect in man. Am. J. clin. Nutr .,
12: 49-53.
DOMINGO, J.L., LLOBET, J.M., & CORBELLA, J. (1985) Protection
of mice against the lethal effects of sodium metavanadate: a
quantitative comparison of a number of chelating agents.
Toxicol. Lett ., 26(2-3): 95-99.
DOMINGO, J.L., LLOBET, J.M., TOMAS, J.M., & CORBELLA, J. (1986)
Influence of chelating agents on the toxicity, distribution, and
excretion of vanadium in mice. J. appl. Toxicol ., 6(5): 337-
341.
DOVGOPOL, V.I., BENOUNI, A.CH., MEDVEDEV, A.A., SHAPOVALOV,
J.P., & MEDVEDEVA, L.A. (1974) [The chemistry and engineering
technology of vanadium compounds.] In: Ivakin, A.A. & Voronova,
E.M., ed. [ Proceedings of the International Conference on the
Chemistry and Technology of Vanadium Compounds ,] Perm,
Scientific Institute of Black Metals, pp. 477-480 (in Russian).
DUCE, R.A. & HOFFMAN, G.L. (1976) Atmospheric vanadium
transport to the ocean. Atmos. Environ ., 10: 989-996.
DURFOR, C.N. & BECKER, E. (1963) Public water supplies of
the 100 largest cities in the United States, 1962 , Washington
DC, US Geological Survey (Water-Supply Paper No. 1812).
DURRANT, P.J. & DURRANT, B. (1970) Introduction to advanced
inorganic chemistry , 2nd ed., New York, John Wiley and Sons.
DURUM, W.H. & HAFFTY, J. (1963) Implications of the minor
element content of some major streams of the world. Geochim.
Cosmochim. Acta , 23: 1-11.
DUTTON, W.F. (1911) Vanadiumism. J. Am. Med. Assoc ., 56(22):
1648.
EISLER, L., SIMECEK, R., & USTUPSKY, J. (1968) [Effect of work
in vanadium workshop on respiratory ways.] Prac. Lek., 20(2/3):
52-57 (in Czech).
ELINDER, C.G. (1984) Metabolism and toxicity of metals ,
Stockholm, Department of Environmental Hygiene, Karolinska
Institute and the National Institute of Environmental Medicine,
pp. 265-274 (Life Sciences Research Report No. 28).
ELVES, M.V., WILSON, J.N., SCALES, J.T., & KEMP, H.B.S. (1975)
Incidence of metal sensitivity in patients with total joint
replacements. Br. med. J ., 4: 376-378.
ERMOLAEV, G.F. (1969) [Effect of vanadium on certain
biochemical processes in rabbit organs.] In: [ Trace elements in
medicine ,] Moscow, Ivano-Frankovsk, pp. 190-192 (in Russian).
EVANS, C.A. & MORRISON, G.H. (1968) Trace element survey
analysis of biological materials by spark source mass
spectrometry. Anal. Chem ., 40: 869-875.
FALLENTIN, B. & FROST, J. (1954) [Health hazards from cleaning
oil-fired boilers caused by vanadium and sulfuric acid in the
soot.] Nord. Hyg. Tidskr ., 3: 58-65 (in Danish).
FAORO, R.B. & MCMULLEN, T.B. (1977) National trends in trace
metals in ambient air 1965-1974 , Research Triangle Park, North
Carolina, US Environmental Protection Agency (EPA-450/1-77-
003).
FAULKNER HUDSON, T.G. (1964) Vanadium: toxicology and
biological significance , Amsterdam, New York, Oxford, Elsevier
Science Publishers, 135 pp.
FEAR, E.C. & TYRER, F.H. (1958) A study of vanadium poisoning
in gas workers. Trans. Assoc. Ind. Med. Off ., 8: 153-155.
FENNELLY, P.F. (1976) The origin and influence of airborne
particulates. Am. Sci ., 64(1): 46-56.
FISHER, G.L., MCNEILL, K.L., WHALEY, C.B., & FONG, J. (1978)
Attachment and phagocytosis studies with murine pulmonary
alveolar macrophages. J. Reticuloendothel. Soc ., 24: 243-252.
FISHER, G.L., MCNEILL, K.L., & DEMOCKO, C.J. (1986) Trace
element interactions affecting pulmonary macrophage cyto-
toxicity. Environ. Res ., 39(1): 164-171.
FLAHERTY, J.P. & ELDRIDGE, H.B. (1970) Neutron activation
analysis of residual oil for vanadium. Appl. Spectrosci ., 24(5):
534-538.
FROLOV, V.V. (1970) [Determination of chlorine and vanadium in
graphite by the activation method.] Zavod. Lab ., 36(7): 807-809
(in Russian).
FROST, J (1951) [Vanadium poisoning from cleaning the smoke
stacks of boilers burning fuel oil.] Ugeskr. Laeger , 113: 1309-
1312 (in Danish).
FUKAI, R. & MEINKE, W.W. (1962) Activation analyses of
vanadium, arsenic, molybdenum, tungsten, rhenium, and gold in
marine organisms. Limnol. Oceanogr ., 7: 186.
GARLEJ, T. (1974) [The influence of vanadium-bearing dust on
the workers' health in the Plock thermal-electric power station:
New exposure tests.] Pol. Tyg. Lek ., 29(49): 2143-2144 (in
Polish).
GERSHKOVICH, E.E. & STYKAN, T.P. (1972) [A catalytic method of
measuring hydrogen sulfide levels in air.] Gig. Tr. prof.
Zabol ., 2: 60-61 (in Russian).
GEYER, C.F. (1953) Vanadium, a caries-inhibiting trace element
in the Syrian hamster. J. dent. Res ., 35: 590-595.
GIBSON, R.S. & DEWOLFE, M.S. (1979) Copper, zinc, manganese,
vanadium, and iodine concentrations in the hair of Canadian low
birth wight neonates. Am. J. clin. Nutr ., 32: 1728-1733.
GILES, M.A., KLAVERKAMP, J.F., & LAWRENCE, S.G. (1979) The
acute toxicity of saline groundwater and of vanadium to fish and
aquatic invertebrates , Edmonton, Environment Alberta, 172 pp
(Prepared for the Alberta Oil Sands Environmental Research
Program) (Project No. AF 3.2.1).
GOFMAN J.W. (1962) Chemical elements of the blood of man in
health. Adv. biol. med. Phys ., 8: 1.
GOLDBERG, E.D. (1961) Marine geochemistry. In: Eyring, H.,
Christensen, C.J., & Johnston, H.S., ed. Annual review of
physical chemistry , Vol. 12, Palo Alto, California, Annual
Reviews, Inc., pp. 29-48.
GOLDSCHMIDT, V.M. (1938) Collected articles on the
geochemistry of rare elements. In: [ Collection of papers on the
geochemistry of rare metals ,] Moscow, Amalgamated State
Technical and Scientific Publishing Houses (in Russian).
GOLDSHTEJN, M.I. (1967) [ Vanadium-containing steels ,] Moscow,
Chermetinformacija (in Russian).
GORDUS, A.A., MAHER, C.C., III, & BIRD, G.C. (1974) Human hair
as an indicator of trace metal environmental exposure. In:
Proceedings of the First Annual NSF Trace Contaminants
Conference , Springfield, Virginia, National Technical
Information Service, pp. 463-487 (Conf. 730802).
GOYER, R.A. & MEHLMANN, M.A., ed. (1977) Toxicology of trace
elements. In: Advances in modern toxicology, Washington DC,
Hemisphere, Vol. 2 , 303 pp.
GRANTHAM, J.J. (1980) The renal sodium pump and vanadate. Am.
J. Physiol ., 239: F97-F106.
GRANTHAM, J.J. & GLYNN, I.M. (1979) Renal Na, K-ATPase:
determinants of inhibition by vanadium. Am. J. Physiol ., 236(6):
F530-F535.
GRIN, A.V., BLUM, A.A., SELETKOV, A.L., FREIDENZON, YU.E., &
NAIMUCHINA, L.F. (1971) [Low-alloyed welded steels containing
vanadium for construction.] In: [ Problems of Kachkanar ,]
Sverdlovsk, Central Bureau of Technical Information, pp. 186-196
(in Russian).
GULKO, A.G. (1956) [On the characteristics of vanadium as an
industrial poison.] Gig. i Sanit ., 21(11): 24-28 (in Russian).
GYLSETH, B., LEIRA, H.L., STEINNES, E., & THOMASSEN, Y. (1979)
Vanadium in the blood and urine of workers in a feraalloy plant.
Scand. J. Work environ. Health , 5: 188-194.
HACKETT, P.L. & KELMAN, B.J. (1983) Availability of toxic trace
metals to the conceptus. Sci. total environ .,28: 433-442.
HADJIMARKOS, D.M. (1966) Vanadium and dental caries. Nature
(Lond.) , 209: 1137.
HADJIMARKOS, D.M. (1968) Effect of trace elements on dental
caries. Adv. oral Biol ., 3: 282-285.
HAMILTON, E.I., MINSKI, M.J., & CLEARY, J.J. (1972-73) The
concentration and distribution of some stable elements in
healthy human tissues from the United Kingdom. Sci. total
Environ ., 1: 341-374.
HAMMOND, P.B. & FOULKES, E.C. (1986) Metal ion toxicity in man
and animals. In: Metal ions in biological systems , Cincinatti,
Ohio, Kettering Laboratory, Vol. 20 , pp. 157-200.
HANNA, W.J. & GRANT, C.L. (1962) Spectrochemical analyses of
certain trees and ornamentals for 23 elements. Bull. Torrey Bot.
Club , 89: 293-302.
HARA, T., SONODA, Y., & IWAI, I. (1976) Growth response of
cabbage plants to transition elements under water culture
conditions. I. Titanium, vanadium, chromium, manganese, and
iron. Soil Sci. plant Nutr ., 22(3): 307-315.
HARRIS, W.R., FRIEDMAN, S.B., & SILBERMAN, D. (1984) Behavior
of vanadate and vanadyl ion in canine blood. J. inorg. Biochem .,
20(2): 157-169.
HATFIELD, M. & CHURCHILL, P. (1981) Renal vascular and tubular
effects of vanadate in the anaesthetized rat. J. Pharmacol. exp.
Ther ., 217: 406-410.
HATHCOCK, J.N., HILL, C.H., & TOVE, S.B. (1966) Uncoupling of
oxidative phosphorylation by vanadate. Can. J. Biochem ., 44:
983-988.
HEIN, J.W. & WISOTZKY, J. (1955) The effect of a 10 ppm
vanadium drinking solution on dental caries in male and female
Syrian hamsters. J. dent. Res ., 34: 756.
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand culture.
J. exp. Bot ., 4(10): 59-64.
HEYDORN, K. & LUKENS, H.R. (1966) Pre-irradiation separation
for the determination of vanadium in blood serum by reactor
neutron activation analysis , Roskilde, Danish Atomic Energy
Commission, Research Establishment Risö (Risö Report No. 138).
HEYLIGER, C.E., TAHILIANI, A.G., & MCNEILL, J.H. (1985) Effect
of vanadate on elevated blood-glucose and depressed cardiac
performance of diabetic rats. Science , 227(4693): 1474-1477.
HICKEY, R.J., SCHOFF, E.0., & CLELLAND, R.C. (1967)
Relationship between air pollution and certain chronic disease
death rates. Arch. environ. Health , 15: 728-738.
HICKLING, S. (1958) Vanadium poisoning. N.Z. med. J ., 57: 607-
608.
HOFFMAN, B., KOMENDA, W., SADOWSKA, A.M., & ROZANSKI, A. (1972)
[The occurence of vanadium in the drinking-water in the region
of Bialystock Province.] Rocz. Panstw. Zakl. Hig ., 23(2): 155-
159 (in Polish).
HOFFMAN, G.L., DUCE, R.A., & ZOLLER, W.H. (1969) Vanadium,
copper, and aluminum in the lower atmosphere between California
and Hawaii. Environ. Sci. Technol ., 3: 1207-1210.
HOFFMAN, G.L., DUCE, R.A., & HOFFMAN, E.J. (1972) Trace metals
in the Hawaiian marine atmosphere. J. geophys. Res ., 77: 5322-
5329.
HOLDWAY, D.A. & SPRAGUE, J.B. (1979) Chronic toxicity of
vanadium to flagfish. Water Res ., 13: 905-910.
HOLODOV, V.N. (1968) [ Vanadium ,] Moscow, Nauka Publishing
House (in Russian).
HOLODOV, V.N. (1973) [ Sedimentary ore formation and
metallogeny on vanadium ,] Moscow, Nauka Publishing House (in
Russian).
HOLZHAUER, K.P. & SCHALLER, K.-H. (1977) [ Occupational
medicine studies in chimney sweeps. Hazards at the workplace and
occupation-linked health damage ,] Stuttgart, G. Thieme (in
German).
HOPKINS, L.L., Jr & MOHR, H.E. (197la) The biological
essentiality of vanadium. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition , New York, Marcel Dekker,
pp. 195-213.
HOPKINS, L.L., Jr & MOHR, H.E. (197lb) Effect of vanadium
deficiency on plasma cholesterol of chicks. Fed. Proc ., 30:
462.
HOPKINS, L.L., Jr & MOHR, H.E. (1974) Vanadium as an essential
nutrient. Fed. Proc ., 33(6): 1173-1175.
HOPKINS, L.L., Jr & TILTON, B.E. (1966) Metabolism of trace
amounts of vanadium 48 in rat organs and liver subcellular
particles. Am. J. Physiol ., 211: 169-172.
HOPKINS, L.L., CANNON, H.L., MUSCH, A.T., WELCH, R.M., &
NIELSEN, F.H. (1977) Vanadium. Geochem. Environ ., 2: 93-107.
HORI, C. & OKA, T. (1980) Vanadate enhances the stimulatory
action of insulin on DNA synthesis in cultured mouse mammary
glands. Biochim. Biophys. Acta , 610: 235-240.
HORNER, C.K., BURCK, D., ALLISON, F., & SHERMAN, M.S. (1942)
Nitrogen fixation by azotobacter as influenced by molybdenum and
vanadium. J. agric. Res ., 65: 173-193.
HUDGINS, P.M. & BOND, G.H. (1979) Reversal of vanadate
inhibition of Na++K-ATPase by catecholamines. Res. Commun. chem.
Pathol. Pharmacol ., 23(2): 313-326.
HUDSON, T.G.F. (1964) Vanadium: toxicology and biological
significance , Amsterdam, New York, Oxford, Elsevier Science
Publishers.
HWANG, I.Y., ULLICCI, P.A., & SMITH, S.B. (1972) A simple
flameless atomizer. Am. Lab ., 3: 41-43.
ICRP (1960) Report of Committee II on Permissible Dose for
Internal Radiation (1959). Recommendations of the International
Commission on Radiological Protection , Oxford, Pergamon Press
(ICRP Publication No. 2).
ILO (1980) Occupational Exposure Limits for Airborne Toxic
Substances; Occupational Safety and Health Series, No. 37, 2nd
revised ed ., Geneva, International Labour Office.
INCIARTE, D.J., STEFFEN, R.P., DOBBINS, D.E., SWINDALL, B.T.,
JOHNSTON, J., & HADDY, F.J. (1980) Cardiovascular effects of
vanadate in the dog. Am. J. Physiol ., 239: 47-56.
IZYCKI, J., SULKOWSKI, W., ADAMIAK-ZIEMBA, J., & STROSZEJN, G.
(1971) [Evaluation of professional exposure to vanadium
compounds and other environmental factors of workers employed in
cleaning of oil-fired boilers with special regard to the state
of respiratory tract.] Med. Pr ., 22(4): 421-431 (in Polish).
JACIMIRSKIJ, K.B. (1967) [ Kinetic methods of analysis ,]
Moscow, Himija (in Russian).
JACKSON, J.F. & LINSKENS, H.F. (1982) Metal ion induced
unscheduled DNA synthesis in Petunia pollen. Mol. gen. Genet .,
187: 112.
JANDHYALA, B.S. & HOM, G.J. (1983) Physiological and pharmaco-
logical properties of vanadium. Life Sci ., 33: 1325-1340.
JARACZEWSKA, W. & JAKUBOWSKI, M. (1964) [The attempt of
evaluation of vanadium exposure in the chemical industry.] Med.
Pr ., 15(6): 375-383 (in Polish).
JAWOROWSKI, Z., BILKIEWICZ, J., DIBOSZ, E., GRYBOWSKA, D.,
KOWNACKA, L., & WRONSKI, Z. (1973) Radiation hazards to
population from conventional and nuclear power production. In:
Symposium on environmental surveillance around nuclear
installations, Warsaw, 5-9 November, 1973, Vienna, International
Atomic Energy Agency (IAEA-SM-180/20).
JOHNSON, J.L., COHEN, H.L., & RAJAGOPALAN, K.V. (1974) Studies
of vanadium toxicity in the rat; lack of correlation with
molybdenum utilization. Biochem. biophys. Res. Commun ., 56(4):
940-946.
JONES, M.M. & BASINGER, M.A. (1983) Chelate antidotes for
sodium vanadate and vanadyl sulfate intoxication in mice. J.
Toxicol. environ. Health , 12(4-6): 749-756.
KADA, T., HIRANO, K., & SHIRASU, Y. (1980) Screening of
environmental chemical mutagens by the rec-assay system with
Bacillus subtilis. In: de Serres, F.J. & Hollaender, A., ed.
Chemical mutagens: principles and methods for their detection ,
New York, Plenum Press, pp. 149-173.
KAKU, S., HOSHIDE, M., MATSUO, S., NUNOTANI, H., ISHIDA, N.,
KURATA, S., MATSUMOTO, H., YAMAGUCHI, S., SANO, T., KONO, Y.,
KOGA, S., & KOGA, M. (1971) [Experimental studies on vanadium
poisoning. I. Vanadium-induced hepatic injuries in rats.] Jap.
J. ind. Health , 13: 263-267 (in Japanese).
KANEMATSU, K. & KADA, T. (1978) Mutagenicity of metal
compounds. Mutat. Res ., 53(2): 207-208.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec-assay and
mutagenicity studies on metal compounds. Mutat. Res ., 77: 109-
116.
KANISAWA, M. & SCHROEDER, H.A. (1967) Life-term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumours in mice. Cancer Res ., 27: 1192-1195.
KATAYEVA, I.G. & SAPUNOV, S.F. (1974) [Sanitary-hygienic
characteristics of vanadium ferroalloy production at the
Chusovoy Metallurgical Plant.] Gig. Tr. prof. Zabol ., 18(1):
37-39 (in Russian).
KAZIMOV, M.A. (1977) [Occupational hygiene with new methods of
the ferrovanadium production.] Gig. Tr. prof. Zabol ., 6: 8-12
(in Russian).
KEJT, M. & DEGENS, E. (1961) [Geometrical indicators.] In:
[ Geometrical research at low temperatures and pressures ,]
Moscow, Foreign Language Publishers, pp. 56-84 (in Russian).
KEMPTON, S., STERRITT, R.M., & LESTER, J.N. (1982) Atomic-
absorption spectrophotometric determination of antimony,
arsenic, bismuth, tellurium, thallium and vanadium in sewage
sludge. Talanta , 29: 675-681.
KINGSNORTH, A.N., LAMURAGLIA, G.M., ROSS, J.S., & MALT, R.A.
(1986) Vanadate supplements and 1,2-dimethylhydrazine induced
colon cancer in mice: increased thymidine incorporation without
enhanced carcinogenesis. Br. J. Cancer, 53(5): 683-686.
KIRZHNER, M.A., KRYZHENKOVA, N.A., & KIST, A.A. (1974) [Scope
for the preliminary concentration in the use of neutron
activation in matrix analysis for aluminium and vanadium.] At.
Energ ., 37: 498 (in Russian).
KIVILUOTO, M. (1980) Observations on the lungs of vanadium
workers. Br. J. ind. Med ., 37: 363-366.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1979a) Serum and
urinary vanadium pentoxide. Int. Arch. occup. Health , 48: 251-
256.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M. (1979b)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory. Scand. J. Work environ. Health , 5: 50-58.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M., (1979c)
Serum and urinary vanadium of vanadium exposed workers. Scand.
J. Work environ. Health , 5: 362-367.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health,
46: 179-182.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1981a) Clinical
laboratory results of vanadium-exposed workers. Arch. environ.
Health , 36(3): 109-113.
KIVILUOTO, M., RASANEN, O., RINNE, A., & RISSANEN, M. (1981b)
Intracellular immunoglobulins in plasma cells of nasal biopsies
taken from vanadium-exposed workers. Anat. Anz. Jena , 149: 446-
450.
KNECHT, E.A., MOORMAN, W.J., CLARK, J.C., LYNCH, D.W., & LEWIS,
T.R. (1985) Pulmonary effects of acute vanadium pentoxide
inhalation in monkeys. Am. Rev. respir. Dis ., 132(6): 1181-
1185.
KOHLER, R. (1972) [Rebuttal: problems of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 27: 815-816 (in German).
KOLPAKOV, L.E., DOBOSH, V.G., GREKOV, S.D., JUFEREV, A.JU.,
LLOKAZOV, E.D., AZFALOVA, R.U., & SANDLER, V.I. (1971)
[Improvement of technology of utilisation of vanadium contained
in waste water.] In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 229-231 (in
Russian).
KONOVALOV, G.S., IVANOVA, A.A., & KOLESNIKOV, T.CH. (1968)
[Scattered rare elements dissolved in water contained in
colloidal substances of the major USSR rivers.] In: [ A
collection of papers on the geochemistry of sedimentary rocks
and ores. Documentation of the Seventh All-Union Conference on
Lithology of the Academy of Sciences of the USSR], Moscow,
Nauka Publishing House, pp. 72-87 (in Russian).
KÖPF-MAIER, P. & KÖPF, H. (1979) [Vanadocene dichloride:
another anti-tumour agent from the metallocene series.] Z.
Naturforsch ., 34B: 805-807 (in German).
KÖPF-MAIER, P., HESSE, B., & KÖPF, H. (1980) Tumour inhibition
by metallocenes: effect of titanocene and hafnocene dichlorides
on Ehrlich ascites tumour in mice. J. Cancer Res. clin. Oncol .,
96(1): 43-51.
KOPYLOVA, L.M. (1971) [A study of some blood indicators
following prolonged administration of vanadium sulfate.] Tr.
Voronezh. Med. Inst ., 85: 62-65 (in Russian).
KORKHOV, V.V. (1965) [Vanadium in prophylaxis and treatment of
experimental atheroslerosis.] Farmakol. i Toksikol ., 28(1): 83-
87 (in Russian).
KOSTROMIN, A.I., AKHMETOV, A.A., & ORLOVA, L.N. (1970)
[Coulometric determination of manganese (II), cesium (III), and
vanadium (IV).] Zh. anal. Khim ., 25: 195-196 (in Russian).
KOVDA, V.A., JAKUSHEVSKAYA, I.V., & TURUKANOV, A.N. (1959)
[ Trace elements in the soils of the Soviet Union ,] Moscow,
Moscow University Publishing House (in Russian).
KRAGTEN, J. (1981) Improved determination of vanadium by flame
AAS by preventing polynuclear hydroxide complex formation. At.
Spectrosci ., 2(4): 135-136.
KRAUSKOPF, F.K. (1963) Factors governing the concentration of
thirteen rare metals in seawater. Collected articles on the
geochemistry of rock formation. In: [ The geochemistry of
lithogenesis ,] Moscow, Foreign Languages Publishing House, pp.
294-338 (in Russian).
KUBASKY, A. (1957) Waste from a vanadium work as moulding
material. Slevarenstvi , 5(1): 4-7.
KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D., & FLETCHER, O.J.
(1986) Influence of ochratoxin A and vanadium on various
parameters in growing chicks. Poult. Sci ., 65(9): 1671-1678.
KULIEVA, T.K. (1974) [Effect of vanadium on the activity of
some tissue-respiration enzymes.] Zdravookhr. Turkm ., 3: 7-17
(in Russian).
KUMAR, A. & CORDER, C.N. (1980) Diuretic and vasoconstrictor
effects of sodium orthovanadate on the isolated perfused rat
kidney. J. Pharmacol. exp. Ther ., 213: 85-90.
KURMAEV, R.H. (1974) [Solid, liquid and aerosol waste in the
production of technical vanadium pentoxide.] In: Ivakin, A.A. &
Voronova, E.M., ed. [ Proceedings of the International
Conference on the Chemistry and Technology of Vanadium
Compounds ,] Perm, Scientific Institute of Black Metals, pp. 43-
46 (in Russian).
KUZELOVA, M., SIRL, J., & POPLER, A. (1975) [Occupational
poisonings by vanadium compounds in workers at mazut boiler
houses.] Prac. Lek ., 27(5): 170-173 (in Czech).
KUZELOVA, M., HAVEL, V., POPLER, A., & STEPANEK, O. (1977)
[The problems of occupational medicine in the work of chimney
sweeps.] Prac. Lek ., 29: 225-228 (in Czech).
LAGERKVIST, B., NORDBERG, G.F., & VOUK, V. (1986) Vanadium.
In: Friberg, G.F., Nordberg, G.F., & Vouk, V.B., ed. Handbook on
the toxicology of metals. II. Specific metals , Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 638-663.
LAHAV, M., RENNERT, H., & BARZILAI, D. (1986) Inhibition by
vanadate of cyclic AMP production in rat corpora lutea incubated
in vitro . Life Sci ., 39(26): 2557-2564.
LANDER, D.W., STEINER, R.L., ANDERSON, D.H., & DEHM, R.L.
(1971) Spectrographic determination of elments in airborne
dirt. Appl. Spectrosci ., 25: 270-275.
LARSEN, J.A. & THOMSEN, O. (1980) Vanadate-induced oliguria
and vasoconstriction in the cat. Acta physiol. Scand ., 110:
367-374.
LARSEN, J.A., THOMSEN, O., & HANSEN, O. (1979) Vanadate-
induced oliguria in the anaesthetized cat. Acta physiol. Scand .,
106: 495-496.
LEE, K.P. & GILLIES, P.J. (1986) Pulmonary response and
intrapulmonary lipids in rats exposed to bismuth orthovanadate
dust by inhalation. Environ. Res ., 40(1): 115-135.
LEE, R.E., GORANSON, S.S., ENROINE, R.E., & MORGAN, G.B. (1972)
National air surveillance Cascade Impactor Network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol ., 6: 1025-1030.
LEE, K., NALEWAJKO, C., & JACK, T.R. (1978) Effects of
vanadium on freshwater algae. In: Abstracts of the 5th Annual
Aquatic Toxicity Workshop, Hamilton, Ontario, 7-9 November ,
Burlington, Ontario, Canada Centre for Inland Waters.
LEES, R.E.M. (1980) Changes in lung function after exposure to
vanadium compounds in fuel oil ash. Br. J. ind. Med ., 37: 253-
256.
LEVlNA, E.N. (1972) [Relationships between solubility of metal
compounds and their toxicity, distribution, and elimination from
the body.] Gig. Tr. prof. Zabol ., 16: 40-43 (in Russian).
LEVY, B.S., HOFFMAN, L., & GOTTSEGEN, S. (1984) Boilermakers'
bronchitis: respiratory tract irritation associated with
vanadium pentoxide exposure during oil-to-coal conversion of a
power plant. J. occup. Med ., 26(8): 567-570.
LEVY, L.S., MARTIN, P.A., & BIDSTRUP, P.L. (1986) Investigation
of the potential carcinogenicity of a range of chromium
containing materials on rat lung. Br. J. ind. Med ., 43(4): 243-
256.
LEWIS, C.E. (1959a) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 19: 419-424.
LEWIS, C.E. (1959b) The biological effects of vanadium. II.
The signs and symptoms of occupational vanadium exposure. Am.
Med. Assoc. Arch. Ind. Health , 19: 497-503.
LEWIS, C.E. (1959c) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 20(5): 455-466.
LIFSCHITZ, V.M. (1962) [Spectral determination of trace
elements in human blood (group determination of Ni, Ag, Zn, Cu,
Cd, V, Pb, Mn, Fe, and Co).] Tr. Voronezh. Med. Inst ., 49: 110
(in Russian).
LINSTEDT, K.D. & KRUGER, P. (1969) Vanadium concentrations in
Colorado River Basin waters. J. Am. Water Works Assoc ., 61(2):
85-88.
LINTER, C.M. (1985) Neuropsychiatric aspects of trace
elements. Br. J. hosp. Med ., 34(6): 361-365.
LOPEZ-NOVOA, J.M., GARCIA, J.C., CRUZ-SOTO, M.A., BENABE, J.E.,
& MARTINEZ-MALDONADO, M. (1982a) Effect of sodium ortho-
vanadate on renal renin secretion in vivo. J. Pharmacol. exp.
Ther ., 222: 447-451.
LOPEZ-NOVOA, J.M., MAYOL, V., & MARTINEZ-MALDONADO, M. (1982b)
Renal actions of orthovanadate in the dog. Proc. Soc. Exp. Biol.
Med ., 170: 418-426.
L'VOV, B.V. (1970) Atomic absorption spectrochemical analysis,
2nd ed., London, Adam Hilger.
MACARA, I.G. (1980) Vanadium - an element in search of a role.
Trends biochem. Sci ., April: 92-94.
MACARA, I.G., KUSTIN, K., & CANTLEY, L.C., Jr (1980) Gluta-
thione reduces cytoplasmic vanadate mechanism and physiological
implications. Biochim. Biophys. Acta , 629: 95-106.
MCLEAN, J.R., MCWILLIAMS, R.S., KAPLAN, J.G., & BIRNBOIM, H.C.
(1982) Rapid detection of DNA strand breaks in human peripheral
blood cells and animal organs following treatment with physical
and chemical agents. In: Bora, K.C., Douglas, G.R., & Nestmann,
E.R., ed. Chemical mutagenesis, human population monitoring, and
genetic risk assessment , Amsterdam, Oxford, New York, Elsevier
Science Publishers, p. 137 (Progress in Mutation Research,
Vol. 3).
MCLUNDIE, A.C., SHEPHERD, J.B., & MOBBS, D.R.A. (1968) Studies
on the effects of various ions on enamel solubility. Arch. oral
Biol ., 13: 1321-1330.
MADSEN, E.S., NIELSEN, P.A., & PEDERSEN, J.C. (1982) The
distribution and origin of mutagens in airborne particulates,
detected by the Salmonella /microsome assay in relation to
levels of lead, vanadium, and PAH. Sci. total Environ ., 24(1):
13-25.
MAHLER, H.R. & CORDES, E.H. (1966) Biological chemistry , New
York, Harper & Row.
MAJUMDAR, A.K. & DAS, G. (1965) Spectrophotometric determin-
ation of vanadium with N -benzoyl- N -p-chlorophenylhydroxyl-
amine. J. Indian Chem. Soc ., 42(3): 189.
MANNING, D.C. & SLAVIN, W. (1983) The determination of trace
elements in natural waters using the stabilized temperature
platform furnace. Appl. Spectrosci ., 37(1): 1-10.
MARAFANTE, E. & SABBIONI, E. (1983) Metabolic studies on
vanadium: in vivo and in vitro association with cellular
components. In: Proceedings of an International Conference on
Heavy Metals in the Environment, London, 18-21 September,
1979 , Edinburgh, CEC Consultants, pp. 171-174.
MARONI, M., COLOMBI, A., BURATTI, M., CALTZAFERRI, G., FOA, V.,
SABBIONI, E., & PIETRA, R. (1983) Urinary elimination of
vanadium in boiler cleaners. In: Proceedings of an International
Conference on Heavy Metals in the Environment, London, 18-21
September, 1979 , Edinburgh, CEC Consultants, pp. 66-69.
MARTENS, C.S., WESOLOWSKI, J.J., KAIFER, R., & JOHN, W. (1973)
Sources of vanadium in Puerto Rican and San Francisco bay area
aerosols. Environ. Sci. Technol ., 7: 817-819.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959a) Effect of
vanadium upon liver coenzyme A in rats. Nature (Lond.) , 183:
1527-1528.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959b) Intracellular
thioctic acid and coenzyme A following vanadium treatment.
Nature (Lond.) , 184: 1641.
MASIRONl, R. (1969) Trace elements and cardiovascular
diseases. Bull. World Health Organ ., 40: 305-312.
MATANTSEVA, E.I. (1960) [The state of the respiratory organs
in workers coming into contact with vanadium pentoxide.] Gig.
Tr. prof. Zabol ., 7: 41-44 (in Russian).
MEISCH, H.U. & BECKER, L.J. (1981) Vanadium in photosynthesis
of Chlorellafusca and higher plants. Biochim. Biophys. Acta ,
636: 119-125.
MEISCH, H.U. & BENZSCHAWEL, H. (1978) Role of vanadium in
green plants. III. Influence on cell division of chlorella.
Arch. Microbiol ., 116(1): 91-95.
MERTZ, W. (1970) Some aspects of nutritional trace element
research. Fed. Proc ., 29: 1482-1488.
MINDEN, H. & ROTHE, R. (1966) [Biochemical mechanism of action
of vanadium.] Z. gesamte Hyg ., 12(5): 315-321 (in German).
MIRAMAND, P. & UNSAL, M. (1978) Acute toxicity of vanadium to
some marine benthic and phytoplanktonic species. Chemosphere ,
10: 827-832.
MIRZAEVA, K.H. (1965) [Trace elements in natural waters in
some districts of Uzbekistan.] In: [ Trace elements in agri-
culture ,] Tashkent, Science Publishers of Uzbekistan, pp. 122-
126 (in Russian).
MISIEWICZ, A. (1980) [Effect of air containing gasoline,
tungsten, titanium, cobalt, and vanadium on the phagocytic
activity of leucocytes.] Pol. Tyg. Lek ., 35(50): 1965-1967 (in
Polish).
MISIEWICZ, A. (1983) [Effect of air containing benzine,
wolfram, titanium, cobalt, and vanadium on peripheral blood.]
Med. Pr ., 34(3): 251-257 (in Polish).
MITCHELL, R.L. (1964) Trace elements in soil. In: Bear, F.E.,
ed. Chemistry of the soil , New York, Reinhold Publishing,
pp. 320-368.
MITCHELL, W.G. & FLOYD, E.P. (1954) Ascorbic acid and
ethylenediaminetetracetate (EDTA) as antidotes in experimental
vanadium poisoning. Proc. Soc. Exp. Biol. Med ., 85: 206-208.
MONTERO, M.R., GUERRI, C., RIBELLES, M., & GRISOLIA, S. (1981)
Inhibition of protein synthesis in cell cultures by vanadate and
in brain homogenates of rats fed vanadate. Physiol. Chem.
Physics , 13: 281-287.
MORGAN, W.K.C. & SEATON, A. (1984) Occupational lung diseases ,
2nd ed., Philadelphia, W.B. Saunders & Co.
MOUNTAIN, J.T. (1963) Detecting hypersusceptibility to toxic
substances. Arch. environ. Health , 6: 357-365.
MOUNTAIN, J.T., DELKER, L.L., & STOKINGER, H.E. (1953) Studies
in vanadium toxicology. I. Reduction in the cystine content of
rat hair. Am. Med. Assoc. Arch. Ind. Hyg. Occup. Med ., 8: 406.
MOUNTAIN, J.T., STOCKELL, F.R., Jr, & STOKINGER, H.E. (1955)
Studies in vanadium toxicology. Am. Med. Assoc. Arch. Ind.
Health , 12: 494.
MUHLER, J.C. (1957) The effect of vanadium pentoxide,
fluorides, and tin compounds on the dental caries experience in
rats. J. dent. Res ., 36: 787-794.
MUSK, A.W. & TEES, J.G. (1982) Asthma caused by occupational
exposure to vanadium compounds. Med. J. Aust ., 1: 183-184.
MUSTAFIN, I.S., NECHAENKO, T.M., & FRUMINA, N.S. (1969) [ The
range of reagents available for vanadium ,] Moscow, Moscow State
University (in Russian).
MUZQUIN, V.N., KHAVZINA, L.B., ZOLOTAVIN, V.L., & BERRUKOV,
I.YA. (1981) [ Analytical chemistry of vanadium ,] Moscow,
Nauka Publishing House, 216 pp (in Russian).
MYERS, V.C. & BEARD, H.H. (1931) Studies in the nutritional
anaemia of the rat. II. Influence of iron plus supplements of
other inorganic elements upon blood regeneration. J. biol.
Chem ., 94: 89.
MYRON, D.R., GIVAND, S.H., & NIELSEN, F.H. (1977) Vanadium
content of selected foods as determined by flameless atomic
absorption spectroscopy. J. agric. food Chem ., 25(2): 297-299.
MYRON, D.R., ZIMMERMAN, T.J., SHULER, T.R., KLEVAY, L.M., LEE,
D.E., & NIELSEN, F.H. (1978) Intake of nickel and vanadium by
humans. A survey of selected diets. Am. J. clin. Nutr ., 31(3):
527-531.
NAS (1974) Vanadium , Washington DC, National Academy of
Sciences.
NAS (1978) Vanadium supply and demand outlook , Washington DC,
National Academy of Sciences, National Materials Advisory Board
(Publication NMAB-346).
NASON, A. (1958) The metabolic role of vanadium and molybdenum
in plants and animals. In: Lamb, C.A., Bentley, 0.G., & Beattie,
J.M., ed. Trace elements , New York, Academic Press, pp. 269-
296.
NAYLOR, G.J. (1984) Vanadium and manic depressive psychosis.
Nutr. Health , 3(1-2): 79-85.
NAYLOR, G.J., SMITH, A.H., BRYCE-SMITH, D., & WARD, N.I. (1984)
Tissue vanadium levels in manic-depressive psychosis. Psychol.
Med ., 14(4): 767-772.
NECHAY, R. (1984) Mechanisms of action of vanadate. Ann. Rev.
Pharmacol. Toxicol ., 24: 501-524.
NECHAY, R. & SAUNDERS, J.P. (1978) Inhibition by vanadium of
sodium and potassium dependent adenosinetriphosphatase derived
from animal and human tissues. J. environ. Pathol. Toxicol ., 2:
247-262.
NELSON, W.L. (1973) Direct desulfurization handles low-metals
topped crudes. Oil Gas J ., 19 November: 54-56.
NIELSEN, F.H. & OLLERICH, D.A. (1973) Studies on a vanadium
deficiency in chicks. Fed. Proc ., 32: 929.
NIOSH (1977) Occupational exposure to vanadium , Washington DC,
National Institute for Occupational Safety and Health (Document
No. 77-222), 142 pp.
NISHIYAMA, K., ONISHI, K., MIYAI, T., SUGIURA, S., SAITO, K., &
TOJYO, Y. (1977) [A survey of workers on vanadium pentoxide.]
Shikoku igaku zasshi , 31(6): 389-393 (in Japanese).
NOVAKOVA, S., NIKOLCHEV, G., ANGELIEVA, R., DINOEVA, S., &
MAUTNER, G. (1981) [Effect of vanadium on experimental
atherosclerosis.] Gig. i Sanit ., 11: 58-59 (in Russian).
NRC (1980) Drinking-water and health , Washington DC, National
Academy Press, Vol. 3, pp. 350-354 (Safe Drinking-Water
Committee).
OBERG, S.G., PARKER, R.D.R., & SHARMA, R.P. (1978)
Distribution and elimination of an intratracheally administered
vanadium compound in the rat. Toxicology , 11: 315-323.
OLEFFE, J. & WILMET, J. (1980) Generalized dermatitis from an
osteosynthesis screw. Contact Dermat ., 6: 365.
OLIVER, B.G. & COSGROVE, E.G. (1975) Metal concentrations in
the sewage, effluents and sludges of some Southern Ontario
wastewater treatment plants. Environ. Lett ., 9(1): 75-90.
OMANG, S.H. (1971) The determination of vanadium and nickel in
mineral oils by flameless graphite tube atomization. Anal. Chim.
Acta , 56: 470-473.
ORDHZONIKIDZE, E.K., ROSCHIN, A.V., SHALGANOVA, I.V., BOGOMAZOV,
M.YA., & KAZIMOV, M.A. (1977) [On the distribution and
elimination of vanadium from the organisms.] Gig. Tr. prof.
Zabol ., 6: 29-34 (in Russian).
ORVINI, E., LODOLA, L., SABBIONI, E., PIETRA, R., & GOETZ, L.
(1979) Determination of the chemical forms of dissolved
vanadium in freshwater as determined by 48V radiotracer experi-
ments and neutron activation analysis. Sci. total Environ ., 13:
195-207.
PARKER, R.D.R. & SHARMA, R.P. (1978) Accumulation and
depletion of vanadium in selected tissues of rats treated with
vanadyl sulfate and sodium orthovanadate. J. environ. Pathol.
Toxicol ., 2: 235-245.
PARKES, W.R. (1982) Occupational lung disorders , 2nd ed.,
London, Butterworths & Co.
PASTUHOV, A.I. & TRETJAKOV, M.A. (1959) Metallurgical
processing of cast iron from titanomagnetites from the Kackanar
district into steel. In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 95-104 (in
Russian).
PATRICK, R. (1978) Effects of trace metals in the aquatic
ecosystem. Am. Sci ., 66: 185-191.
PAZHYNICH, V.M. (1966) Maximum permissible concentration of
vanadium pentoxide in the atmosphere. Hyg. Sanit ., 31: 6-11.
PAZHYNICH, V.M. (1967) [Experimental basis for the determina-
tion of maximum allowable concentration of vanadium pentoxide in
atmospheric air.] In: Rjazanov, V.A., ed. [ The biological
effect and hygienic importance of atmospheric pollutants ,]
Moscow, Medicina Publishing House, pp. 201-217 (in Russian).
PAZHYNICH, V.M. (1980) [Effect of aerosols of rare metals on
the functional state of the body of workers in conditions of
powder wire production.] Vrach. Delo , 3: 102-104 (in Russian).
PEJVE, J.V. & AJZUPIET, I.P. (1974) [A short review of the
results of research on the problem "Trace elements in plant
cultivation and livestock rearing in 1972".] In: [ Trace
elements in the USSR. XV. Methodological material ,] Riga,
Knowledge Publishers (in Russian).
PERRY, H.M., TEITLEBAUM, S., & SCHWARTZ, P.L. (1955) Effects
of antihypertensive agents on amino acid decarboxylation and
amine oxidation. Fed. Proc ., 14: 113-114.
PERRY, H.M., Jr, SCHWARTZ, P.L., & SAHAGIAN, B.M. (1969)
Effects of transition metals and of metal-binding antihyper-
tensive agents on tryptamine oxidase sand dopa decarboxylase.
Proc. Soc. Exp. Biol. Med ., 130: 273-277.
PETERBURGSKIJ, A.V. & TORMASOVA, E.E. (1969) [The effect of
vanadium on certain physiologycal processes in the pea.] Dok.
Timiryazevsk. Akad ., 149: (in Russian).
PETKEVICH, A.N., VILLER, G.E., & VOROTNIKOV, B.A. (1967)
[Some trace elements in natural waters of Kama's district.] In:
[ Trace elements in the biosphere and their application in
agriculture and medicine in Siberia and the Far East ,] Ulan-
Ude, Ulan-Ude Book Publishers, pp. 223-227 (in Russian).
PETUHOV, N.I., STANKEVICH, E.F., & VALACHANOVICH, V.I. (1969)
Some microelements in natural waters near the source of the
Kama. Tr. Kazanskogo Med. Inst ., 29: 100-103.
PHILLIPS, T.D., NECHAY, B.R., NELDON, S.L., KUBENA, L.F.,
HEIDELBAUGH, N.D., SHEPHERD, E.C., STEIN, A.F., & HAYES, A.W.
(1982) Vanadium-induced inhibition of renal Na+ K+-
adenosinetriphosphatase in the chicken after chronic dietary
exposure. J. Toxicol. environ. Health , 9: 651-661.
PHILLIPS, T.D., NECHAY, B.R., & HEIDELBAUGH, N.D. (1983)
Vanadium: chemistry and the kidney. Fed. Proc ., 42(13): 2969-
2973.
PIELSTICKER, F. (1954) [Health effects due to vanadium
compounds. Symptoms and prognosis.] Arch. Gewerbepathol.
Gewerbehyg ., 13: 73-96 (in German).
PILZ, W. & KOMISCHKE, S. (1972) [Determination of vanadium in
biological material and air. II. The decomposition of biological
material: air analyses.] Int. J. environ. anal. Chem ., 1(4):
275-282 (in German).
POERSEL, B. (1977) [ The biological and toxicological
significance of vanadium ,] Dusseldorf, Med. Diss., 86 pp (in
German).
PROESCHER, F., SEIL, H.H., & STILLIANS, A.W. (1917) A
contribution to the action of vanadium with particular reference
to syphilis. Am. J. Syph ., 1: 347-405.
PROKOPENKO, T.A. (1961) [Effect of industrial poisons,
fluorine, vanadium, manganese, and some drugs tested in
treatment on the tissue metabolism of compounds containing
phosphorus.] In: [ Proceedings of the 9th All-Union Pharmaco-
logical Conference, Sverdlovsk ,] Sverdlovsk, Central Bureau of
Technical Information, pp. 210-211 (in Russian).
PYY, L., KIVILUOTO, M., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health ,
46(2): 179-182.
PYY, L., LAJUNEN, L.H.J., & HAKALA, E. (1983) Determination of
vanadium in work-place air by DCP emission spectrometry. Am.
Ind. Hyg. Assoc. J ., 44(8): 609-614.
PYY, L., HAKALA, E., & LAJUNEN, L.H.J. (1984) Screening for
vanadium in urine- and blood-serum by electrothermal atomic
absorption spectrometry and dc plasma atomic emission
spectrometry. Anal. Chim. Acta , 158: 297-303.
RAESAENEN, O., KIVILUOTO, M., RINNE, A., & RISSANEN, M. (1979)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory: a macroscopic and microscopic study. Scand.
J. Work environ. Health , 5(1): 50-58.
RAJNER, V. (1960) [Effect of vanadium in the respiratory
tract.] Cesk. Otolaryngol ., 9: 202-204 (in Czech).
RALSTON, H.R. & SATO, E.S. (1971) Sodium removal as an aid to
neutron activation analysis. Anal. Chem ., 43: 129-131.
REINL, W. (1958) [Illnesses caused by basic slag, vanadium
slag, and vanadium compounds.] Staub , 18(5): 143-148 (in
German).
REZNIK, J.B. (1954) [The importance of vanadium compounds from
the point of view of industrial toxicology.] Vrach. Delo , 7:
627-632 (in Russian).
RHAN, K.A. (1971) Sources of trace elements in aerosols: an
approach to clean air , Ann Arbor, Michigan, University of
Michigan, Department of Meteorology, 309 pp (US Atomic Energy
Commission Contract No. COO-1705-9).
RHOADS, K. & SANDERS, C.L. (1985) Lung clearance, trans-
location, and acute toxicity of arsenic, beryllium, cadmium,
cobalt, lead, selenium, vanadium, and ytterbium oxides following
deposition in rat lung. Environ. Res ., 36(2): 359-378.
RIFKIN, R.J. (1965) In vitro inhibition of Na+ -K+ and Mg2+
ATPases by mono-, di-, and trivalent cations. Proc. Soc. Exp.
Biol. Med ., 120: 802-804.
RIGDON, L.P. & HARRAR, J.E. (1969) Determination of vanadium
by controlled-potential coulometry. Anal. Chem ., 41: 1673-1675.
ROITMAN, I., TRAVASSAS, L.R., AZEVEDO, H.P., & CURRY, A. (1969)
Choline, trace elements, and amino acids as factors for growth
of an enteric yeast, Candida slooffii. Sabouraudia J. Int. Soc.
Hum. Anim. Mycol ., 7: 15-19.
ROMAN, R.J., BONVENTRE, J.V., SILVA, P., & LECHENE, C. (1981)
Sodium orthovanadate diuresis in rats. J. Pharmacol. exp. Ther .,
218: 168-174.
RONOV, A.B. (1964) [General tendencies in the evolution of the
composition of the Earth's crust, the oceans and the
atmosphere.] Geohimija , 8: 715-743 (in Russian).
ROSE, E.R. (1973) Geology of vanadium and vanadiferous
occurrences in Canada , Ottawa, Geological Survey of Canada,
Department of Energy, Mines and Resources, pp. 7-101 (Economic
Geology Report No. 27).
ROSHCHIN, A.V. (1962) [The hazards of occupational vanadium
intoxication in boiler cleaning operations at electric power
stations operating on fuel oil, and prevention problems.] Gig.
Tr. prof. Zabol ., 6: 17-22 (in Russian).
ROSHCHIN, A.V. (1963a) [Biological effects of rare, dispersed,
and other metals and their compounds used in industry:
vanadium.] In: [ Toxicology of the less common metals ,] Moscow,
Meditsina, pp. 83-95 (in Russian).
ROSHCHIN, A.V. (1963b) [Hygienic evaluation of dust from
vanadium-containing slag.] Gig. i Sanit ., 12: 23-29 (in
Russian).
ROSHCHIN, A.V. (1964) [Vanadium metallurgy in the light of
industrial hygiene and questions concerned with the prevention
of occupational diseases and intoxication.] Gig. Tr. prof.
Zabol ., 8: 3-10 (in Russian).
ROSHCHIN, A.V. (1967a) Toxicology of vanadium compounds used
in modern industry. Hyg. Sanit ., 32: 345-352.
ROSHCHIN, A.V. (1967b) Vanadium. In: Izraelson, Z.I., ed.
Toxicology of the rare metals , Jerusalem, Israel Program for
Scientific Translations Ltd., pp. 52-59 (AEC-tr-6710).
ROSHCHIN, A.V. (1968) [ Vanadium and its compounds ,] Moscow,
Medicina Publishing House (in Russian).
ROSHCHIN, A.V. & ORDZHONIKIDZE, E.K. (1986) [Metal toxico-
kinetics and its significance for prevention of occupational
poisoning.] Gig. Tr. prof. Zabol ., 3: 1-6 (in Russian).
ROSHCHIN, A.V., ILNITSKAYA, A.V., LUTSENKO, L.A., & ZHIDKOVA,
L.A. (1964) [Research into effects of vanadium trioxide dust
on organisms.] Gig. Tr. prof. Zabol ., 8: 25-30 (in Russian).
ROSHCHIN, A.V., ORDZHONIKIDZE, E.K., & SHALGANOVA, I.V. (1980)
Vanadium - toxicity, metabolism, carrier state. J. Hyg.
Epidemiol. Microbiol. Immunol ., 24(4): 377-383.
RUHLING, A. (1971) [Pollution with heavy metals in the greater
Stockholm area.] In: [ Report No. 12 on ecological heavy metal
research ,] Lund, University of Lund, Department of Plant
Ecology (in Swedish).
RUNDBERG, G. (1939) Modern problems of occupational diseases
in Sweden. Nord. Med ., 2: 1845-1856.
SABBIONI, E., MARAFANTE, E., AMANTINI, L., & UBERTALLI, L.
(1978) Similarity in metabolic patterns of different chemical
species of vanadium in the rat. Bioinorg. Chem ., 8: 503-515.
SABBIONI, E., MARAFANTE, E., PIETRA, R., GOETZ, L., GIRARDI, F.,
& ORVINI, E. (1979) The association of vanadium with the iron
transport system in human blood as determined by gel filtration
and neutron activation analysis. In: Proceedings of a Symposium
on Nuclear Activation Techniques in the Life Sciences, Vienna,
22-26 May, 1978 , Vienna, International Atomic Energy Agency, pp.
179-192.
SABBIONI, E., MARAFANTE, E., RADE, J., GREGOTTI, C., DI NUCCI,
A., & MANZO, L. (1981) Biliary excretion of vanadium in rats.
Toxicol. Eur. Res ., 3(2): 93-98.
SANDELL, E., ed. (1959) Vanadium. In: Colorimetric determina-
tion of traces of metals , 3rd ed., New York, Interscience
Publishers, Vol. 3, pp. 923-940 (Chemical Analysis Series).
SCHLETTWEIN-GZELL, D. & MOMMSEN-STRAUB, S. (1973) [Trace
elements in foodstuffs. XI. Vanadium.] Int. Z. Vitam.-
Ernährungsforsch ., 43(2): 242-250 (in German).
SCHROEDER, H.A. (1966) Municipal drinking-water and cardio-
vascular death rates. J. Am. Med. Assoc ., 195: 81-85.
SCHROEDER, H.A. (197Oa) Vanadium , Washington DC, American
Petroleum Institute (Air Quality Monograph No. 70-13).
SCHROEDER, H.A. (197Ob) A sensible look at air pollution by
metals. Arch. environ. Health , 21: 798-806.
SCHROEDER, H.A. & MITCHENER, M. (1975) Life-term effects of
mercury, methylmercury, and nine other trace metals on mice. J.
Nutr ., 105(4): 452-458.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1963) Abnormal
trace metals in man: vanadium. J. chron. Dis ., 16: 1047-1071.
SCHROEDER, H.A., MITCHENER, M., & NASON, A.P. (1970) Zirconium,
niobium, antimony, vanadium, and lead in rats: life-term
studies. J. Nutr ., 100(1): 59-68.
SCHUMANN-VOGT, B. (1969) [Health hazards in industry caused by
vanadium.] Zentralbl. Arbeitsmed. Arbeitsschutz , 19(2): 33-39
(in German).
SCHWARZ, K. & MILNE, D.B. (1971) Growth effects of vanadium in
the rat. Science , 174: 426-428.
SELJANKINA, K.P. (1961) [Data for determining the maximum
permissible content of vanadium in water basins.] Gig. i Sanit .,
26(10): 6-12 (in Russian).
SHAH, K.R., FILBY, R.H., & HALLER, W.A. (1970) Determination
of trace elements in petroleum by neutron activation analysis.
1. Determination of Na, S, Li, La, V, Mn, Cu, Ga, and Br. J.
radioanal. Chem ., 6: 185-192.
SHARMA, R.P., BOURCIER, D.R., BRINKERHOFF, C.R., & CHRISTENSEN,
S.A. (1981) Effects of vanadium on immunologic functions. Am.
J. ind. Med ., 2(2): 91-99.
SHARMA, R.P., COULOMBE, R.A., Jr, & SRISUCHART, B. (1986)
Effects of dietary vanadium exposure on levels of regional brain
neurotransmitters and their metabolites. Biochem. Pharmacol .,
35(3): 461-465.
SHEVCHENKO, I.T. & GORODYNSKYJ, V.I. (1964) [ Polarography in
medicine and biology ,] Kiev, State Medical Publishers of the
Ukraine (in Russian).
SHEVCHENKO, S.D. (1965) [Content of vanadium in bone tumours.]
Ortop. Travmatol. Protez ., 8: 83 (in Russian).
SHILINA, A.I. & MALAKHOV, S.G. (1974) [Trace elements in
natural waters and the atmosphere.] In: [ A collection of papers
on experimental meteorology ,] Moscow, Gidrometeozdat (in
Russian).
SHKOLENOK, G.F., NIKOLAEVA, E.R., & AGASYAN, P.K. (1977)
[Differential controlled-potential coulometry. Determination of
vanadium(V) and vanadium(IV).] Zavod. Lab ., 43(9): 1048-1050 (in
Russian).
SIMONOFF, M., CONRI, C., & SIMONOFF, G. (1986) Vanadium in
depressive states. Acta pharmacol. toxicol . (Copenhagen) ,
59(Suppl. 7): 463-466.
SINGH, D. & SHARMA, S. (1970) Amperometric permanganometric
estimations at low concentrations in stirred solutions. Indian
J. Chem ., 8(2): 192.
SINGH, J., NORDLIE, R.C., & JORGENSON, R.A. (1981) Vanadate:
a potent inhibitor of multifunctional glucose-6-phosphatase.
Biochim. Biophys . Acta , 678: 477-482.
SJÖBERG, S.-G. (1950) Vanadium pentoxide dust: A clinical and
experimental investigation on its effect after inhalation. Acta
med. Scand ., 138(Suppl. 238): 1-188.
SJÖBERG, S.-G. (1951) Possible importance of allergy in
diseases caused by inhalation of inorganic dust. Acta allergol .,
4(4): 357-361.
SJÖBERG, S.-G. (1955) Vanadium bronchitis from cleaning oil-
fired boilers. Arch. ind. Health , 11(6): 505-512.
SJÖBERG, S.-G. (1956) Vanadium dust, chronic bronchitis and
possible risk of emphysema. A follow-up investigation of workers
at a vanadium factory. Acta med. Scand ., 44(5): 381-386.
SJÖBERG, S.-G. & RIGNER, K.-G. (1956) [Skin, eye, and
respiratory tract symptoms associated with the cleaning of oil-
fired boilers.] Nord. Hyg. Tidskr ., 37: 217-228 (in Swedish).
SLAVIN, W., CARNRICK, G.R., MANNING, D.C., & PRUSZKOWSKA, E.
(1983) Recent experiences with the stabilized temperature
platform furnace and zeeman background correction. At.
Spectrosci ., 4(3): 69-86.
SMITH, J.B. (1983) Vanadium ions stimulate DNA synthesis in
Swiss mouse 3T3 and 3T6 cells. Proc. Natl Acad. Sci. (USA) ,
80(20): 6162-6166.
SNYDER, F. & CORNATZER, W.E. (1958) Vanadium inhibition of
phospholipid sulphydryl activity in rat liver. Nature (Lond.) ,
182: 462.
SOKOLOV, S.M. (1986) Metholodological aspects of assessing
atmospheric contamination with metal aerosols in the vicinity of
thermal power complexes. J. Hyg. Epidemiol. Microbiol.
Immunol ., 30(3): 249-254.
SOMERVILLE, J. & DAVIES, B. (1962) Effect of vanadium on serum
cholesterol. Am. Heart J ., 64: 54-56.
SÖREMARK, R. (1967) Vanadium in some biological specimens. J.
Nutr ., 92: 183-190.
SÖREMARK, R. & ULLBERG, S. (1962) Distribution and kinetics of
48V2O5 in mice. In: Friend, N., ed. Proceedings of a Symposium
on the Use of Radioisotopes in Animal Biology and the Medical
Sciences, Mexico City, 21 November-1 December, 1961 , New York,
Academic Press, Vol. 2, pp. 103-114.
SÖREMARK, R., ULLBERG, S., & APPELGREN, L.E. (1962) Auto-
radiographic localization of vanadium pentoxide (V24805) in
developing teeth and bones of rats. Acta odontol. Scand ., 20:
225-232.
SPRAGUE, J.B., HOLDWAY, D.A., & STENDAHL, D.H. (1978) Acute
and chronic toxicity of vanadium to fish , Edmonton, Environment
Alberta, 92 pp (Prepared for the Alberta Oil Sands Research
Program (Project No. AF 3.5.1).
STEFFEN, R.P., PAMNANI, M.B., CLOUGH, D.L., HUOT, S.J., MULDOON,
S.M., & HADDY, F.J. (1981) Effect of prolonged dietary
administration of vanadate on blood pressure in the rat.
Hypertension , 3: I/173-I/178.
STENDAHL, D.H. & SPRAGUE, J.B. (1982) Effects of water
hardness and pH on vanadium lethality to rainbow trout. Water
Res ., 16: 1479-1488.
STOCKS, P. (1960) On the relations between atmospheric
pollution in urban and rural localitites and mortality from
cancer, bronchitis and pneumonia, with particular reference to
3:4 benzopyrene, beryllium, molybdenum, vanadium, and arsenic.
Br. J. Cancer , 14: 397-418.
STOKINGER, H.E., WAGNER, W.D., MOUNTAIN, J.T., STOCKELL, F.R.,
DOBROGORSKI, O.J., & KEENAN, R.G. (1967) In Vanadium V. In:
Clayton, G.D. & Clayton, F.E., eds. Patty's Industrial hygiene
and toxicology , 3rd revised ed. (1981), New York, Interscience,
Vol. 2A, pp. 2013-2033.
STONER, G.D., SHIMKIN, M.B., TROXELL, M.C., THOMPSON, T.L., &
TERRY, L.S. (1976) Test for carcinogenicity of metallic
compounds by the pulmonary tumor response in strain a mice.
Cancer Res ., 36: 1744-1747.
STRAHOV, N.F. (1947) [Iron-ore facies and their analogues in
the history of the earth.] Tr. Inst. Geol. AN SSSR, geol. Ser .,
73: 22 (in Russian).
STRAHOV, N.M. (1968) [On the theory of geochemical process in
humid zones.] In: [ A collection of papers on the geochemistry
of sedimentary rocks and ores ,] Moscow, Na++uka Publishing House,
pp. 102-134 (Documentation of the Seventh All-Union Conference
on Lithology of the Academy of Sciences of the USSR) (in
Russian).
STRASIA, C.A. (1971) Vanadium: essentiality and toxicity in
the laboratory rat , Ann Arbor, Michigan Purdue University (Ph.D.
Thesis).
STROOP, S.D., HELINEK, G., & GREENE, H.L. (1982) More
sensitive flameless atomic absorption analysis of vanadium in
tissue and serum. Clin. Chem ., 28(1): 79-82.
STYRIS, D.L. & KAYE, J.H. (1982) Mechanisms of vaporization of
vanadium pentaoxide from vitreous carbon and tantalum furnaces
by combined atomic absorption/mass spectrometry. Anal. Chem .,
54: 864-869.
SUGAWARA, K., NAITO, H., & YAMADA, S. (1956) Geochemistry of
vanadium in natural waters. J. earth Sci. Nagoya Univ ., 4(1):
44-61.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975)
Heavy metals in normal Japanese tissues. Arch. environ. Health ,
30: 487-494.
SUN, MIANLING, ed. (1987) Toxicity of vanadium and its
environmental health standard , Chengdu, West China University of
Medical Sciences, 20 pp.
SVERDRUP, H.U., JOHNSON, M.W., & FLEMING, R.H. (1950) The
oceans, their physics, chemistry and general biology , Englewood
Cliffs, New Jersey, Prentice-Hall Inc., pp. 175-177.
SVOBODA, P., TEISINGER, J., PILAR, J., & VYSKOCHIL, F. (1984)
Vanadyl (VO2+) and vanadate (VO3-) ions inhibit the brain
microsomal Na, K-AtPase with similar affinities. Protection by
transferrin and noradrenaline. Biochem. Pharmacol ., 33: 2485-
2491.
SWARUP, G., COHEN, S., & GARBERS, D.L. (1982) Inhibition of
membrane phosphotyrosyl-protein phosphatase activity by
vanadate. Biochem. biophys. Res. Commun ., 107: 1104-1109.
SYMANSKI, J. (1939) [Occupational vanadium poisoning, its
occurrence and symptomatology.] Arch. Gewerbepathol.
Gewerbehyg ., 9: 295-313 (in German).
TAKAHASHI, H. & NASON, A. (1957) Tungstate as a competitive
inhibitor of molybdate in nitrate assimilation and N2 fixation
by azotobacter. Biochim. Biophys. Acta , 23: 433-435.
TALVITIE, N.A. & WAGNER, W.D. (1954) Studies in vanadium
toxicology. II. Distribution and excretion of vanadium in
animals. Arch. ind. Hyg ., 9: 414-422.
TANDON, A.G. & BHATTACHARYA, S.C. (1961) Use of N-2-thio-
phenecarbonyl- N-p-tolylhydroxylamine and N-2-thiophene-
carbonyl- N-phenylhydroxylamine as reagents for vanadium. Anal.
Chem ., 33(9): 1267.
TARA, S., CAVIGNEAUX, A., & DEPLACE, Y. (1953) Intoxication
par le vanadate de calcium. Arch. Mal. prof. Méd. Trav. Séc.
soc ., 14: 378-380.
TEBROCK, H.E. & MACHLE, W. (1968) Exposure to europium-
activated yttrium orthovanadate: a cathodoluminescent phosphor.
J. occup. Med ., 10(12): 692-696.
THOMAS, D.L.G. & STIEBRIS, K. (1956) Vanadium poisoning in
industry. Med. J. Aust ., 1: 607-609.
THOMPSON, H.J., CHASTEEN, N.D., & MEEKER, L.D. (1984) Dietary
vanadyl (IV) sulfate inhibits chemically-induced mammary
carcinogenesis. Carcinogenicity , 5: 849-851.
THURAUF, J., SYGA, G., & SCHALLER, K.H. (1979) Field tests
carried out to determine the occupational exposure to vanadium.
Zbl. Bakteriol. Hyg., I. Abt. Orig. B , 168: 273-290.
TIPTON, I.H. & COOK, M.J. (1963) Trace elements in human
tissue. II. Adult subjects from the United States. Health Phys .,
9: 103-145.
TIPTON, I.H. & SHAFER, J.J. (1964) Statistical analysis of
lung trace element levels. Arch. environ. Health , 8: 58-67.
TIPTON, I.H., STEWART, P.L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys .,
16: 455-462.
TJURJUKANOV, A.N. (1963) Vanadium. In: Trace elements in
agriculture , Kiev, Gosselhozizdat, pp. 472-475.
TODRIA, F.F. (1963) [Identification of vanadium in ashes of
Tkibul coals.] In: [ Abstracts of scientific papers of Tbilisi
Medical Institute ,] Tbilisi, Tbilisi State Medical Institute,
p. 144 (in Russian).
TOLMAN, E.L., BARRIS, E., BURNS, M., PANSINI, A., & PARTRIDGE,
R. (1979) Effects of vanadium on glucose metabolism in vitro .
Life Sci ., 25: 1159-1164.
TONEY, J.H., MURTHY, M.S., & MARKS, T.J. (1985) Biodistribu-
tion and pharmacokinetics of vanadium following intraperitoneal
administration of vanadocene dichloride to mice. Chem.-biol.
Interact ., 56(1): 45-54.
TRELLES, R.A., LARGHI, L.A., & PAEZ, L.J.P. (1970) [The health
problem of human drinking-water with high contents of arsenic,
vanadium, and fluorine.] Saneamiento , 34(217): 31-80 (in
Spanish).
TROPPENS, H. (1969) [The uncertainties of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 24: 1089-1092 (in
German).
TRUMMERT, W. & BOEHM, G. (1957) [The trace element vanadium
and its haemopoietic action.] Blut , 3: 210-216 (in German).
TYLER, G. (1970) [Pollution with heavy metals in the
Norrkkoping area. II. Molybdenum and vanadium.] In: [ Report No.
12 on ecological heavy metal research ,] Lund, University of
Lund, Department of Plant Ecology (in Swedish).
UENO, S. & ISHIZAKI, M. (1980) Concentrations of vanadium in
human urine and hair. Jpn. J. ind. Health , 22: 318-379.
UNDERWOOD, E.J. (1977) Vanadium. In: Trace elements in human
and animal nutrition , 4th ed., New York, Academic Press,
pp. 388-397.
UNSAL, M. (1978) Study of the transfer pathways and the
accumulation phenomena of vanadium in mollusca Mytilus edulis.
Rev. int. oceanogr. Med ., 51/52: 71-81.
US BUREAU OF MINES (1974) Metals Information Bulletin. Washing-
ton DC, US Department of the Interior, Bureau of Mines, Branch
of Ferrous Metals.
US EPA (1977) Scientific and technical assessment report on
vanadium , Washington DC, US Environmental Protection Agency
(STAR series, EPA-600/6-77-002).
USMONOV, S.M. (1973) [Vanadium content in constituents of
bone, urine, and faeces in sufferers of acute and chronic
dysentery.] In: [ Epidemiological, microbiological, and clinical
problems in the infectious pathology of Uzbekistan ,] Tashkent,
Institute of Hygiene, Sanitation, and Occupational Diseases,
pp. 125-128 (in Russian).
USUTANI, S., NISHIAMA, K., SATO, I., MATSURA, K., SAWADA, Y.,
HOSOKAWA, Y., & IZUMI, S. (1979) [An investigation of the
environment in a certain vanadium refinery.] Jpn. J. ind.
Health , 21: 11-20 (in Japanese).
VAN DEN BERG, C.M.G. & HUANG ZI QIANG (1984) Direct electro-
chemical determination of dissolved vanadium in seawater by
cathodic stripping voltammetry with the hanging mercury drop
electrode. Anal. Chem ., 56(13): 2383-2386.
VANSELOW, A.P. (1950) Unpublished data cited by Pratt, P.F.
In: Diagnostic criteria for plants and soils, University of
California, Division of Agricultural Sciences , University of
California, pp. 480-482.
VAN ZINDEREN BAKKER, E.M. & JAWORSKI, J.F. (1980) Effects of
vanadium in the Canadian environment , Ottawa, National Research
Council of Canada (Publication No. 18132).
VERSIECK, J. & CORNELIS, R. (1980) Normal levels of trace
elements in human blood-plasma or -serum. Anal. Chim. Acta , 116:
217-254.
VEYS, C. (1983) Possibilités offertes par les méthodes
modernes de polarographie au dosage à éléments tracés. Ind.
Sci ., 59(3): 1-6.
VIGLIANI, E.C. (1969) WHO Technical Report Series No. 415:
Permissible levels of occupational exposure to airborne toxic
substances (Sixth Report of the Joint ILO/WHO Committee on
Occupational Health) , Geneva, World Health Organization, pp. 5-
16.
VlNOGRADOV, A.P. (1937) [The chemical elements entering into
the composition of marine organisms. Part II.] Tr. biogeokhim.
Lab ., 4: 217 (in Russian).
VINOGRADOV A.P. (1944) [The geochemistry of trace elements in
seawater.] Uspehi Himii , 13(1): 123-129 (in Russian).
VINOGRADOV, A.P. (1957) [ Geochemistry of rare and dispersed
chemical elements in soils ,] 2nd ed (revised), Moscow, USSR
Academy of Sciences, pp. 122-129, 216-217 (in Russian).
VINOGRADOV, A.P. (1959) [ The geochemistry of rare and
dispersed elements in soil ,] 2nd ed., New York, Consultants
Bureau Inc., 209 pp (in Russian).
VINTINNER, F.J., VALLENAS, R., CARLIN, C.E., WEISS, R., MACHER,
C., & OCHOA, R. (1955) Study of the health of workers employed
in mining and processing of vanadium ore. Arch. ind. Health ,
12: 635.
VOORS, A.W. (1971) Minerals in the municipal water and
atherosclerotic heart death. Am. J. Epidemiol ., 93: 259-266.
VOUK, V. (1979) Vanadium. In: Friberg, L., Nordberg, G.F., &
Vouk, V., ed. Handbook on the toxicology of metals , Amsterdam,
New York, Oxford, Elsevier Science Publishers, pp. 659-674.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1980) A specific
enzyme is not necessary for vanadate-induced oxidation of NADH.
Nature (Lond.) , 286(5772): 516-517.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1981) The disparity
between effects of vanadate (V) and vanadyl (IV) ions on (Na+ -
K+) AtPase and K+-phosphatase in skeletal muscle. Biochem.
biophys. Res. Commun ., 100: 982-987.
WALDRON, H.A. (1979) Metal poisoning: the three common causes.
Mod. Med ., 24(9): 45-47.
WALLACE, A., ALEXANDER, G.V., & CHADHURY, F.M. (1977) Phyto-
toxicity of cobalt, vanadium, titanium, silver, and chromium.
Commun. soil. Sci. plant Anal ., 8(9): 751-756.
WARINGTON, K. (1955) The influence of iron supply on toxic
effects of manganese, molybdenum and vanadium in soybeans, peas,
and flax. Ann. appl. Biol ., 41: 1-22.
WATANABE, H., MURAYAMA, H., & YAMAOKA, S. (1966) [Some
clinical findings in vanadium workers.] Jpn. J. ind. Health ,
8(7): 23-27 (in Japanese).
WATERS, M.D. (1977) Toxicology of vanadium. Adv. mod.
Toxicol ., 2: 147-189.
WATERS, M.D., GARDNER, D.E., & COFFIN. D.L. (1974) Cytotoxic
effects of vanadium on rabbit alvolar macrophages in vitro .
Toxicol. appl. Pharmacol ., 28: 253-263.
WATERS, M.D., GARDNER, D.E., ARANYI, C., & COFFIN, D.L. (1975)
Metal toxicity for rabbit alveolar macrophages in vitro.
Environ. Res ., 9: 32-47.
WEAST, R.C. (1987) CRC handbook of chemistry and physics , 67th
ed., Boca Raton, Florida, CRC Press.
WEI, C.I. & MISRA, H.P. (1982) Cytotoxicity of ammonium meta-
vanadate to cultured bovine alveolar macrophage. J. Toxicol.
environ. Health , 9(5-6): 995-1006.
WEI, C.I., AL BAYATI, M.A., CULBERTSON, M.R., ROSENBLATT, L.S.,
& HANSEN, L.D. (1982) Acute toxicity of ammonium metavanadate
in mice. J. Toxicol. environ. Health , 10(4/5): 673-687.
WEISZ, H., ROTHMAIER, K., & LUDWIG, H. (1974) Kinetic
determination of thorium, vanadium, and iodide with the use of a
potentiometer. Anal. Chim. Acta , 73(1): 224-228.
WELCH, R.M. (1973) Vanadium uptake by plants. Absorption
kinetics and the effects of pH, metabolic inhibitors, and other
anions and cations. Plant Physiol ., 51: 828-832.
WELCH, R.M. & ALLAWAY, W.H. (1972) Vanadium determination in
biological materials at nanogram levels by a catalytic method.
Anal. Chem ., 44(9): 1644-1647.
WENTZEL, C.T. (1985) Vanadium: a moderate recovery in 1984.
Eng. mining J ., March: 87-88.
WESTENFELDER, C., HAMBURGER, R.K., & GARCIA, M.E. (1981)
Effect of vanadate on renal tubular function in rats. Am. J.
Physiol ., 240: F522-F529.
WIDE, M. (1984) Effect of short-term exposure to five
industrial metals on the embryonic and fetal development of the
mouse. Environ. Res ., 33: 47-53.
WILLIAMS, D.L. (1973) Biological value of vanadium for rats,
chickens, and sheep , Ann Arbor, Michigan, Purdue University
(Ph.D. Thesis).
WILLIAMS, N. (1952) Vanadium poisoning from cleaning oil-fired
boilers. Br. J. ind. Med ., 9: 50-55.
WITKOWSKA, D. & BRZEZINSKI, J. (1979) Alteration of brain
noradrenaline, dopamine, and 5-hydroxytryptamine levels during
vanadium poisoning. Pol. J. Pharmacol. Pharm ., 31: 393-398.
WOOD, B.J., GALOBARDES, J.F., & ROGERS, L.B. (1982) Effect of
the organic ligand on the response for vanadium in flame atomic
emission spectrometry. Anal. Chim. Acta , 135: 145-152.
WRIGHT, L.D., LI, L.F., & TRAGER, R. (1960) The site of
vanadyl inhibition of chloesterol biosynthesis in liver
homogenates. Biochem. biophys. Res. Commun ., 3: 264-267.
WYERS, H. (1946) Some toxic effects of vanadium pentoxide.
Br. J. ind. Med ., 3: 177-182.
WYERS, H. (1948) Some recent observations on hazards in the
chemical industry. In: Proceedings of the 9th International
Congress of Industrial Medicine, London, 13-17 September ,
Bristol, John Wright, pp. 900-903.
YUKAWA, M., SUZUKI-YASUMOTO, M., AMANO, K., & TERAI, M. (1980)
Distribution of trace elements in the human body determined by
neutron activation analysis. Arch. environ. Health , 35(1): 36-
44.
ZAJIC, J.E. (1969) Microbial biochemistry. 12. Vanadium
biogeochemistry , New York, Academic Press, pp. 126-202.
ZEMKOVA, H., TEISINGER, J., & VYSKOCIL, F. (1982) The
comparison of vanadyl (IV) and insulin-induced hyperpolarization
of the mammalian muscle cell. Biochim. Biophys. Acta , 720: 405-
410.
ZENZ, C. & BERG, B.A. (1967) Human responses to controlled
vanadium pentoxide exposure. Arch. environ. Health , 14: 709-
712.
ZENZ, C., BARTLETT, J.P., & THIEDE, W.H. (1962) Acute vanadium
pentoxide intoxication. Arch. environ. Health , 5(6): 542-546.
ZHELJAZKOVA, B.G., CVETANOVA, A.L., & JACIMIRSKIJ, K.B. (1972)
[A catalytic method for determining nanogram quantities of
vanadium.] Zh. anal. Khim ., 27(4): 795-798 (in Russian).
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentration and sources of trace metals at the South Pole.
Science , 183: 198-200.
ZOLLER, W.H., GORDON, G.E., GLADNEY, E.S., & JONES, A.G. (1973)
The sources and distribution of vanadium in the atmosphere. In:
Kothy, E.L., ed. Trace elements in the environment , Washington
DC, American Chemical Society, pp. 31-47 (Advances in Chemistry
Series 123).
ZOLOTAVIN, V.L. (1954) [Achieving a pure solution of chlor-
vanadyl.] Zh. obshch. Khim ., 24: 433 (in Russian).
See Also:
Toxicological Abbreviations
 The liquid waste and wash water from metallurgical plants
often contain large amounts of vanadium, up to several hundred
milligrams per litre, measured as vanadium pentoxide. Kurmaev
(1974) detected vanadium at a level of 702.8 mg/litre in
effluent from a vanadium pentoxide plant. In a new
ferrovanadium plant, purified waste water contained 340 mg
vanadium pentoxide/litre (Kurmaev, 1974). Unpurified waste-
water discharge from a vanadium pentoxide plant into an open
watercourse produced a vanadium level in the watercourse of
2 mg/litre (Seljankina, 1961). Linstedt & Kruger (1969) found
the highest river concentrations of vanadium near uranium-
vanadium plants and the lowest in water samples taken upstream
from the industrial areas (Table 2) (section 3.1.3). In
studies by Shilina & Malakhov (1974), water samples taken from
the Moscow River below the city contained 6 times as much
vanadium as samples taken above the city (0.06 and
0.01 mg/litre, respectively).
The pickling of steel casts or steel articles can include
vanadium in the pickling mixture. Waste hydrochloric, nitric,
hydrofluoric, and sulfuric acids from a steel smelting plant
contained 0.02% vanadium. The solid residue on the bottom of
the acid vats contained 2.4 g vanadium/kg (hydrochloric acid
vat) and 1.6 g vanadium/kg (nitric and hydrofluoric acid vats)
(Kurmaev, 1974); vanadium in waste acid that is not properly
treated may be a source of water pollution.
3.4.2 Fossil fuel combustion
Industrial plants producing power and heat and operating on
petroleum, coal, and heavy oils are the most widespread source
of vanadium discharge into the environment. In 1969, as a
result of the combustion of fossil fuels (coal and oil), about
20 000 tonnes of vanadium were discharged into the air in the
USA (NAS, 1974). The estimated levels of emission from burning
coal in the USA for 1969 are shown in Table 10.
In a study of the vanadium discharged from coal burning in
six electric power generating stations, the total amount
discharged into the air in 1968 was 3760 tonnes. Where there
were no ash-trapping devices, 65% of the ash entered the
atmosphere. The degree of atmospheric dispersion of ash
particles depended on the original coalfield, the size of coal
used, the type of furnace, the combustion conditions, and the
presence and type of ash-trapping devices (NAS, 1974).
In a study of the metal aerosol content of waste gas
emissions from an oil-fired electric power station, it was
reported that the concentration of vanadium pentoxide (and
oxides of aluminium, chromium, iron, and manganese) was not
affected by the type of boiler or mode of operation. Comparison
of the metal content in the fuel oil and waste gases showed that
90% was released into the atmosphere (Sokolov, 1986). In the
USSR, there is a trend towards a reduction in the use of heavy
fuel oils in electric power stations.
Other possible sources of vanadium discharge into the air
are the burning of coal tips or dumps of coal dust in mining
areas, but data are not available.
All crude petroleum oils contain vanadium at levels ranging
from 1 to 400 g/tonne, depending on the oilfield (Holodov, 1968,
1973; Shah et al., 1970; Christian & Robinson, 1971; Nelson,
1973; NAS, 1974). In the distillation of crude oil, almost all
the vanadium remains in the high relative molecular mass
hydrocarbon fractions. The vanadium contents of heavy fuel oils
range widely from 1 to 200 g/tonne and are almost a thousand
times greater than those of petroleum distillates. Assuming
that 10% of the vanadium is precipitated inside the plant (flue
and ash trap) while 90% is discharged into the air, it has been
calculated that atmospheric emissions in the USA as a result of
heavy fuel oil combustion were about 14 100 - 21 800 tonnes in
1970 (Holodov, 1968, 1973; NAS, 1974). Similar percentages were
found by Sokolov (1986).
Table 10. Estimated emissions of vanadium resulting from coal
burning in the USA, 1969a
-------------------------------------------------------------------
Type and use Coal Vanadium Vanadium Control Vanadium
of coal (1000 in coal in fly of discharged
tonnes) (tonnes) ash fly into the
(tonnes) ash (%) air
(tonnes)
-------------------------------------------------------------------
Bituminous coal
Electric power 308 642 9254 6015 85 902
utilities
Manufacturing 93 248 2797 1818 60 727
Retail 12 665 380 247 50 124
deliveries
Coking 92 901 2787 - 100 0
Subtotal 507 276 15 218 8080 1753
Anthracite coal 9275 1159 753 50 377
Total 2130
-------------------------------------------------------------------
a From: NAS (1974).
Distilled petroleum fuels produced in the USA (gasoline,
kerosene, diesel fuel, home-heating oils) contain 0.05 mg
vanadium/kg (NAS, 1974). The distillation process used leaves
nearly all of the vanadium originally present in the residual
fractions. Analyses of the spent gases from petrol engines,
sampled directly at the exhaust outlet, showed vanadium
concentrations of 0.1 - 0.2 mg/kg, and exhaust gases of diesel
engines contained 10 - 15 mg vanadium/kg. Six to 12 mg/kg of
vanadium was found in soot collected from the edges of flues in
a small oil-fired power station (Pilz & Komischke, 1972).
The amount of vanadium in natural gas is less than
0.5 g/tonne, and almost no vanadium is released into the
atmosphere on combustion (NAS, 1974).
3.4.3 Agriculture
Vanadium has been used as a trace fertilizer applied at the
rate of 0.75 - 1 mg/kg soil (Peterburgskij & Tormasova, 1969).
This practice must lead to an increased level of vanadium in the
soil, but further information on agricultural use is not
available.
3.5 Transport and Transformation
3.5.1 Geochemical processes
Vanadium is involved in various geochemical processes
occurring in the earth's crust. There is extremely wide
dispersion of vanadium during the formation of volcanic rocks
and sporadic accumulation with the formation of vanadium
minerals as a result of postmagmatic processes. Like all trace
elements that accumulate in soils, vanadium migrates within the
soil itself and within the system: rock-water-soil-vegetation-
animals-man.
During geochemical processes in the soil and in weathering
and podzolization, vanadium is shifted from the A horizon to the
B horizon (Kovda et al., 1959). However, vanadium shifting does
not occur during weathering processes that do not involve
movement of sesquioxides.
Vanadium concentrations in rocks are linked to the pH of the
rocks (Borisenko, 1973). Neutral and acid rocks contain lower
vanadium concentrations than basic rocks, and acid rocks contain
lower concentrations than neutral rocks. In magma of various
types (the main carrier of vanadium), about 92% of all vanadium
occurs in basic rocks (basalts, gabbro, amphibolites, and
eclogites), and about 8% occurs in acid and neutral rocks. Less
than 1% of the total amount of vanadium is found in ultrabasic
alkaline rocks.
The main carriers of vanadium in the sedimentation process
are ferric hydroxides and solid bitumens. The great affinity
between the crystallochemical properties of V3+ and Fe3+ is of
vital importance for the diffusion of vanadium. There is
roughly 400 - 500 times more iron than vanadium in the earth's
crust. Thus, iron is a "solvent" of trivalent vanadium, and is
responsible for its diffusion in magmatic rocks. The bulk of
the ferromagnesian rock-forming minerals (and also titaniferrous
magnetite and magnetite itself) trap vanadium during the
crystallization of rocks, and, during endogenous processes,
vanadium is very closely linked with trivalent iron. In the
formation of igneous rocks, vanadium is preferentially
concentrated in those with a high iron content.
3.5.2 Biogeochemical processes
The accumulation of vanadium in soils and in all other
materials depends directly on its concentration in the soil-
forming rocks, the atmosphere, and the oceans of the world.
Migration, diffusion, and concentration of vanadium in the
biosphere takes place as a result of its extraction by living
organisms from water, from food of both vegetable and animal
origin, and from different types of rock during their
decomposition and the formation of soils.
3.5.2.1 Transport in, and removal from, water
In assessing the relative importance of the two ways in
which vanadium is transported in water, Konovalov et al. (1968)
and Holodov (1968) concluded that 87% is carried away by the
rivers in suspended form, and 13% in solution. The average
level of dissolved vanadium in the rivers of Japan and the USA
(Sugawara et al., 1956; Durum & Haffty, 1963) were the same as
that recorded by Konovalov et al. (1968), i.e., 0.001 mg/litre.
The bulk of vanadium enters sea-water in suspended form or
sorbed on colloids. It accumulates in recent deposits, passes
through watercourses mechanically, and does not react chemically
with sea-water. This peculiarity of vanadium transport is
reflected in its distribution on the sea-bed in the form of
silt.
The fate of vanadium that is dissolved in water is more
complex. As very large amounts of dissolved vanadium have been
carried out into the oceans throughout all geological periods,
vanadium levels in sea-water of about 60 mg/litre might be
expected; in fact, levels do not exceed 0.003 mg/litre
(Goldschmidt, 1938; NAS, 1974), indicating that vanadium is
continuously removed from sea-water. Krauskopf (1963) concluded
that the vanadium content of sea-water is not dependent on
solubility and that natural reagents remove vanadium from the
water. There are two possible pathways, namely sorption and
biochemical processes. The migratory qualities of vanadium are
poor. The content of vanadium in the earth's crust is 0.015 g/kg
(Vinogradov, 1959), and a mean content in river water is
0.001 mg/litre. Thus, very little vanadium is transported via
water. The bulk of vanadium is precipitated on to the seabed
and becomes bound to silts (Petkevich et al. 1967; Strahov,
1968). Levels of vanadium dissolved in sea-water amount to
0.001 - 0.003 mg/litre. The vanadium comes from the 10%
dissolved in river water and is continuously precipitated from
the water by ferric hydroxides and organic matter (Krauskopf,
1963).
Biochemical reactions play an important role in the
extraction of vanadium from sea-water and conversion into a
sediment (Vinogradov, 1937; Holodov, 1973). This is confirmed
by the link between the concentrations of vanadium and organic
substances in sedimentary rocks and silt. However, in practice,
it is extremely difficult to determine the part of the vanadium
that has been assimilated by organisms and the part that has
been sorbed by the decomposing mass of organic matter or
introduced in dissolved form.
An important role in the biogenic migration of vanadium is
played by live marine organisms and plants. The ascidians and
holothurians are noteworthy vanadium accumulators (Bertrand,
1950) (section 3.1.6). Some marine algae are also capable of
accumulating vanadium (Krauskopf 1963). When they die, these
organisms promote accumulation of vanadium in the silt.
Thus, vanadium dissolved in sea-water is continuously
removed either by sorption or biochemical processes. In the
first case, the main precipitant is hydrated ferric trioxide; in
the second, vanadium is accumulated by marine animals, plankton,
and, less commonly, algal and plant organic material.
3.5.2.2 Occurrence in hydrocarbons
The accumulation of vanadium in organic concentrators is
linked with its occurrence in hydrocarbons (petroleum, asphalts,
peats, bitumens, and coal). Vanadium may enter petroleum
together with organic matter or accumulate in already-formed
petroleum from underground waters and petroliferous strata.
Apparently, both processes occur in nature. Secondary
transformation of petroleum into asphalt is accompanied by a
proportionate increase in the concentrations of the tar-asphalt
component and the vanadium linked to it (Vernadskij, 1940).
3.5.2.3 Biospheric redox processes
Vanadium takes part in redox processes not only of a
geochemical nature but also in plants and animals. Pejve &
Ajzupiet (1974), studying the intracellular distribution of
metals in plants, showed that a considerable number, including
vanadium, were linked to complex lipid substances of
comparatively high stability that persisted, even after the
cells had died. These lipids persisted in soils and silts and
served as a source of metal in sedimentary rocks. In various
types of plants, the proportional relationship between the level
of iron and those of manganese, copper, titanium, nickel,
chromium, cobalt, and vanadium decreased in the course of
evolution, and was least significant in the leaves of flowering
plants.
In a number of soil organisms, such as Microcococus lacto-
lyticus, Thiobacillus terrooxidans, and Ferrobacillus thio-
oxidans, there was a link between iron and vanadium in bio-
chemical processes (Zajic, 1969).
3.5.2.4 Transport in air
Information on the local movement and deposition of airborne
vanadium is presented in section 4.1.1. Strahov (1947) and
Ronov (1964) concluded that the gaseous envelope of the earth is
not of significant importance in the transport of vanadium, but
a study by Duce & Hoffman (1976) documented some transport of
man-made airborne vanadium over ocean areas. The authors
estimated that about 10% of this material was deposited in the
ocean. In a study of trace metals in European glaciers,
Jaworowski et al. (1973) showed the presence of small amounts of
vanadium, apparently deposited from the air, which had increased
in recent decades.
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 General Population Exposure
4.1.1 Air
Natural sources of vanadium, such as continental dust and
marine aerosols, cause only low natural background levels of
vanadium in air. In remote areas, such as the South Pole, the
concentrations range from 0.001 to 0.002 ng/m3 (Zoller et al.,
1974), and in the eastern Pacific Ocean, from 0.02 to 0.8 ng/m3
(Hoffman et al., 1969). In rural areas in Canada, the United
Kingdom, and the USA, concentrations have been reported to range
from 0.2 to about 75 ng/m3, with annual averages frequently
below 1 ng/m3 (Rahm, 1971; Cawse & Peirson, 1972; US EPA, 1977).
In general, air levels of vanadium are higher in urban areas
than rural areas. Annual averages may often be in the range of
20 - 100 ng/m3, though, exceptionally, higher averages exceeding
200 - 300 ng/m3 have been recorded in large cities, and the
maximum 24-h average may exceed 1000 ng/m3 (US EPA, 1977). In
all surveys, there have been conspicuous geographical and
seasonal variations. High concentrations of vanadium in air
have been attributed to the local burning of fuel oil with a
high vanadium content. The uptake of vanadium fall-out by
mosses (Hypnum capressiforme and Bryum argenteum) in the
Stockholm area indicates that heating oil is a major source of
vanadium (Rühling, 1971). In this regard, Faoro & McMullen
(1977) presented some interesting data (Fig. 2). The values
shown, especially for the period prior to 1971, are typical of
cold-climate cities where high-vanadium heavy fuel oils are
extensively used. The marked seasonal variations are due to
alterations in heating requirements and seasonal differences in
atmospheric inversions. The marked decline after 1970 is due to
the introduction of low-sulfur fuels; reduction of sulfur in oil
results in a proportional reduction in the vanadium content. A
similar effect can be produced by changing from heavy to
distilled fuel oil, as noted in Boston by Barry et al. (1975),
where levels of airborne vanadium for comparable months in 1966
and 1972 were 1.07 and 0.114 µg/m3, respectively.
These data illustrate the importance of fossil fuels as
sources of vanadium in urban air. The patterns observed in
other areas are similar but less extreme in concentration and
fluctuation. Because of the variations in vanadium
concentrations in oil and coal, community levels will depend
mainly on the actual vanadium concentrations in the fuels used
and on meteorological factors.
Pollution of the air by industrial facilities may be less
than that by power stations and heating equipment. At a steel
plant in the USA in 1967, concentrations of vanadium ranged from
0.04 to 0.107 µg/m3 and averaged 0.072 µg/m3, corresponding to
the mean concentration over 13 Pennsylvania towns (also
0.072 µg/m3) (NAS, 1974). The following levels of vanadium
pentoxide were found by Pazhynich (1967) in the USSR in areas of
extensive metallurgical activity not connected with vanadium
production: in the years 1964-65: 1.49 µg/m3 at 150 m from the
source of discharge; 0.47 µg/m3 at 500 m; 1.35 µg/m3 at
1000 m; and 0.98 µg/m3 at 1500 m. Near a plant producing
technical vanadium pentoxide, Kurmaev (1974) detected the
following 24-h mean levels of vanadium pentoxide: 0.004 -
0.012 mg/m3 at 500 m from the source; 0.001 - 0.006 mg/m3 at
1000 m; and 0.001 - 0.004 mg/m3 at 2000 m. Seventy to 72% of
the particles were less than 2 µg in diameter.
The liquid waste and wash water from metallurgical plants
often contain large amounts of vanadium, up to several hundred
milligrams per litre, measured as vanadium pentoxide. Kurmaev
(1974) detected vanadium at a level of 702.8 mg/litre in
effluent from a vanadium pentoxide plant. In a new
ferrovanadium plant, purified waste water contained 340 mg
vanadium pentoxide/litre (Kurmaev, 1974). Unpurified waste-
water discharge from a vanadium pentoxide plant into an open
watercourse produced a vanadium level in the watercourse of
2 mg/litre (Seljankina, 1961). Linstedt & Kruger (1969) found
the highest river concentrations of vanadium near uranium-
vanadium plants and the lowest in water samples taken upstream
from the industrial areas (Table 2) (section 3.1.3). In
studies by Shilina & Malakhov (1974), water samples taken from
the Moscow River below the city contained 6 times as much
vanadium as samples taken above the city (0.06 and
0.01 mg/litre, respectively).
The pickling of steel casts or steel articles can include
vanadium in the pickling mixture. Waste hydrochloric, nitric,
hydrofluoric, and sulfuric acids from a steel smelting plant
contained 0.02% vanadium. The solid residue on the bottom of
the acid vats contained 2.4 g vanadium/kg (hydrochloric acid
vat) and 1.6 g vanadium/kg (nitric and hydrofluoric acid vats)
(Kurmaev, 1974); vanadium in waste acid that is not properly
treated may be a source of water pollution.
3.4.2 Fossil fuel combustion
Industrial plants producing power and heat and operating on
petroleum, coal, and heavy oils are the most widespread source
of vanadium discharge into the environment. In 1969, as a
result of the combustion of fossil fuels (coal and oil), about
20 000 tonnes of vanadium were discharged into the air in the
USA (NAS, 1974). The estimated levels of emission from burning
coal in the USA for 1969 are shown in Table 10.
In a study of the vanadium discharged from coal burning in
six electric power generating stations, the total amount
discharged into the air in 1968 was 3760 tonnes. Where there
were no ash-trapping devices, 65% of the ash entered the
atmosphere. The degree of atmospheric dispersion of ash
particles depended on the original coalfield, the size of coal
used, the type of furnace, the combustion conditions, and the
presence and type of ash-trapping devices (NAS, 1974).
In a study of the metal aerosol content of waste gas
emissions from an oil-fired electric power station, it was
reported that the concentration of vanadium pentoxide (and
oxides of aluminium, chromium, iron, and manganese) was not
affected by the type of boiler or mode of operation. Comparison
of the metal content in the fuel oil and waste gases showed that
90% was released into the atmosphere (Sokolov, 1986). In the
USSR, there is a trend towards a reduction in the use of heavy
fuel oils in electric power stations.
Other possible sources of vanadium discharge into the air
are the burning of coal tips or dumps of coal dust in mining
areas, but data are not available.
All crude petroleum oils contain vanadium at levels ranging
from 1 to 400 g/tonne, depending on the oilfield (Holodov, 1968,
1973; Shah et al., 1970; Christian & Robinson, 1971; Nelson,
1973; NAS, 1974). In the distillation of crude oil, almost all
the vanadium remains in the high relative molecular mass
hydrocarbon fractions. The vanadium contents of heavy fuel oils
range widely from 1 to 200 g/tonne and are almost a thousand
times greater than those of petroleum distillates. Assuming
that 10% of the vanadium is precipitated inside the plant (flue
and ash trap) while 90% is discharged into the air, it has been
calculated that atmospheric emissions in the USA as a result of
heavy fuel oil combustion were about 14 100 - 21 800 tonnes in
1970 (Holodov, 1968, 1973; NAS, 1974). Similar percentages were
found by Sokolov (1986).
Table 10. Estimated emissions of vanadium resulting from coal
burning in the USA, 1969a
-------------------------------------------------------------------
Type and use Coal Vanadium Vanadium Control Vanadium
of coal (1000 in coal in fly of discharged
tonnes) (tonnes) ash fly into the
(tonnes) ash (%) air
(tonnes)
-------------------------------------------------------------------
Bituminous coal
Electric power 308 642 9254 6015 85 902
utilities
Manufacturing 93 248 2797 1818 60 727
Retail 12 665 380 247 50 124
deliveries
Coking 92 901 2787 - 100 0
Subtotal 507 276 15 218 8080 1753
Anthracite coal 9275 1159 753 50 377
Total 2130
-------------------------------------------------------------------
a From: NAS (1974).
Distilled petroleum fuels produced in the USA (gasoline,
kerosene, diesel fuel, home-heating oils) contain 0.05 mg
vanadium/kg (NAS, 1974). The distillation process used leaves
nearly all of the vanadium originally present in the residual
fractions. Analyses of the spent gases from petrol engines,
sampled directly at the exhaust outlet, showed vanadium
concentrations of 0.1 - 0.2 mg/kg, and exhaust gases of diesel
engines contained 10 - 15 mg vanadium/kg. Six to 12 mg/kg of
vanadium was found in soot collected from the edges of flues in
a small oil-fired power station (Pilz & Komischke, 1972).
The amount of vanadium in natural gas is less than
0.5 g/tonne, and almost no vanadium is released into the
atmosphere on combustion (NAS, 1974).
3.4.3 Agriculture
Vanadium has been used as a trace fertilizer applied at the
rate of 0.75 - 1 mg/kg soil (Peterburgskij & Tormasova, 1969).
This practice must lead to an increased level of vanadium in the
soil, but further information on agricultural use is not
available.
3.5 Transport and Transformation
3.5.1 Geochemical processes
Vanadium is involved in various geochemical processes
occurring in the earth's crust. There is extremely wide
dispersion of vanadium during the formation of volcanic rocks
and sporadic accumulation with the formation of vanadium
minerals as a result of postmagmatic processes. Like all trace
elements that accumulate in soils, vanadium migrates within the
soil itself and within the system: rock-water-soil-vegetation-
animals-man.
During geochemical processes in the soil and in weathering
and podzolization, vanadium is shifted from the A horizon to the
B horizon (Kovda et al., 1959). However, vanadium shifting does
not occur during weathering processes that do not involve
movement of sesquioxides.
Vanadium concentrations in rocks are linked to the pH of the
rocks (Borisenko, 1973). Neutral and acid rocks contain lower
vanadium concentrations than basic rocks, and acid rocks contain
lower concentrations than neutral rocks. In magma of various
types (the main carrier of vanadium), about 92% of all vanadium
occurs in basic rocks (basalts, gabbro, amphibolites, and
eclogites), and about 8% occurs in acid and neutral rocks. Less
than 1% of the total amount of vanadium is found in ultrabasic
alkaline rocks.
The main carriers of vanadium in the sedimentation process
are ferric hydroxides and solid bitumens. The great affinity
between the crystallochemical properties of V3+ and Fe3+ is of
vital importance for the diffusion of vanadium. There is
roughly 400 - 500 times more iron than vanadium in the earth's
crust. Thus, iron is a "solvent" of trivalent vanadium, and is
responsible for its diffusion in magmatic rocks. The bulk of
the ferromagnesian rock-forming minerals (and also titaniferrous
magnetite and magnetite itself) trap vanadium during the
crystallization of rocks, and, during endogenous processes,
vanadium is very closely linked with trivalent iron. In the
formation of igneous rocks, vanadium is preferentially
concentrated in those with a high iron content.
3.5.2 Biogeochemical processes
The accumulation of vanadium in soils and in all other
materials depends directly on its concentration in the soil-
forming rocks, the atmosphere, and the oceans of the world.
Migration, diffusion, and concentration of vanadium in the
biosphere takes place as a result of its extraction by living
organisms from water, from food of both vegetable and animal
origin, and from different types of rock during their
decomposition and the formation of soils.
3.5.2.1 Transport in, and removal from, water
In assessing the relative importance of the two ways in
which vanadium is transported in water, Konovalov et al. (1968)
and Holodov (1968) concluded that 87% is carried away by the
rivers in suspended form, and 13% in solution. The average
level of dissolved vanadium in the rivers of Japan and the USA
(Sugawara et al., 1956; Durum & Haffty, 1963) were the same as
that recorded by Konovalov et al. (1968), i.e., 0.001 mg/litre.
The bulk of vanadium enters sea-water in suspended form or
sorbed on colloids. It accumulates in recent deposits, passes
through watercourses mechanically, and does not react chemically
with sea-water. This peculiarity of vanadium transport is
reflected in its distribution on the sea-bed in the form of
silt.
The fate of vanadium that is dissolved in water is more
complex. As very large amounts of dissolved vanadium have been
carried out into the oceans throughout all geological periods,
vanadium levels in sea-water of about 60 mg/litre might be
expected; in fact, levels do not exceed 0.003 mg/litre
(Goldschmidt, 1938; NAS, 1974), indicating that vanadium is
continuously removed from sea-water. Krauskopf (1963) concluded
that the vanadium content of sea-water is not dependent on
solubility and that natural reagents remove vanadium from the
water. There are two possible pathways, namely sorption and
biochemical processes. The migratory qualities of vanadium are
poor. The content of vanadium in the earth's crust is 0.015 g/kg
(Vinogradov, 1959), and a mean content in river water is
0.001 mg/litre. Thus, very little vanadium is transported via
water. The bulk of vanadium is precipitated on to the seabed
and becomes bound to silts (Petkevich et al. 1967; Strahov,
1968). Levels of vanadium dissolved in sea-water amount to
0.001 - 0.003 mg/litre. The vanadium comes from the 10%
dissolved in river water and is continuously precipitated from
the water by ferric hydroxides and organic matter (Krauskopf,
1963).
Biochemical reactions play an important role in the
extraction of vanadium from sea-water and conversion into a
sediment (Vinogradov, 1937; Holodov, 1973). This is confirmed
by the link between the concentrations of vanadium and organic
substances in sedimentary rocks and silt. However, in practice,
it is extremely difficult to determine the part of the vanadium
that has been assimilated by organisms and the part that has
been sorbed by the decomposing mass of organic matter or
introduced in dissolved form.
An important role in the biogenic migration of vanadium is
played by live marine organisms and plants. The ascidians and
holothurians are noteworthy vanadium accumulators (Bertrand,
1950) (section 3.1.6). Some marine algae are also capable of
accumulating vanadium (Krauskopf 1963). When they die, these
organisms promote accumulation of vanadium in the silt.
Thus, vanadium dissolved in sea-water is continuously
removed either by sorption or biochemical processes. In the
first case, the main precipitant is hydrated ferric trioxide; in
the second, vanadium is accumulated by marine animals, plankton,
and, less commonly, algal and plant organic material.
3.5.2.2 Occurrence in hydrocarbons
The accumulation of vanadium in organic concentrators is
linked with its occurrence in hydrocarbons (petroleum, asphalts,
peats, bitumens, and coal). Vanadium may enter petroleum
together with organic matter or accumulate in already-formed
petroleum from underground waters and petroliferous strata.
Apparently, both processes occur in nature. Secondary
transformation of petroleum into asphalt is accompanied by a
proportionate increase in the concentrations of the tar-asphalt
component and the vanadium linked to it (Vernadskij, 1940).
3.5.2.3 Biospheric redox processes
Vanadium takes part in redox processes not only of a
geochemical nature but also in plants and animals. Pejve &
Ajzupiet (1974), studying the intracellular distribution of
metals in plants, showed that a considerable number, including
vanadium, were linked to complex lipid substances of
comparatively high stability that persisted, even after the
cells had died. These lipids persisted in soils and silts and
served as a source of metal in sedimentary rocks. In various
types of plants, the proportional relationship between the level
of iron and those of manganese, copper, titanium, nickel,
chromium, cobalt, and vanadium decreased in the course of
evolution, and was least significant in the leaves of flowering
plants.
In a number of soil organisms, such as Microcococus lacto-
lyticus, Thiobacillus terrooxidans, and Ferrobacillus thio-
oxidans, there was a link between iron and vanadium in bio-
chemical processes (Zajic, 1969).
3.5.2.4 Transport in air
Information on the local movement and deposition of airborne
vanadium is presented in section 4.1.1. Strahov (1947) and
Ronov (1964) concluded that the gaseous envelope of the earth is
not of significant importance in the transport of vanadium, but
a study by Duce & Hoffman (1976) documented some transport of
man-made airborne vanadium over ocean areas. The authors
estimated that about 10% of this material was deposited in the
ocean. In a study of trace metals in European glaciers,
Jaworowski et al. (1973) showed the presence of small amounts of
vanadium, apparently deposited from the air, which had increased
in recent decades.
4. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
4.1 General Population Exposure
4.1.1 Air
Natural sources of vanadium, such as continental dust and
marine aerosols, cause only low natural background levels of
vanadium in air. In remote areas, such as the South Pole, the
concentrations range from 0.001 to 0.002 ng/m3 (Zoller et al.,
1974), and in the eastern Pacific Ocean, from 0.02 to 0.8 ng/m3
(Hoffman et al., 1969). In rural areas in Canada, the United
Kingdom, and the USA, concentrations have been reported to range
from 0.2 to about 75 ng/m3, with annual averages frequently
below 1 ng/m3 (Rahm, 1971; Cawse & Peirson, 1972; US EPA, 1977).
In general, air levels of vanadium are higher in urban areas
than rural areas. Annual averages may often be in the range of
20 - 100 ng/m3, though, exceptionally, higher averages exceeding
200 - 300 ng/m3 have been recorded in large cities, and the
maximum 24-h average may exceed 1000 ng/m3 (US EPA, 1977). In
all surveys, there have been conspicuous geographical and
seasonal variations. High concentrations of vanadium in air
have been attributed to the local burning of fuel oil with a
high vanadium content. The uptake of vanadium fall-out by
mosses (Hypnum capressiforme and Bryum argenteum) in the
Stockholm area indicates that heating oil is a major source of
vanadium (Rühling, 1971). In this regard, Faoro & McMullen
(1977) presented some interesting data (Fig. 2). The values
shown, especially for the period prior to 1971, are typical of
cold-climate cities where high-vanadium heavy fuel oils are
extensively used. The marked seasonal variations are due to
alterations in heating requirements and seasonal differences in
atmospheric inversions. The marked decline after 1970 is due to
the introduction of low-sulfur fuels; reduction of sulfur in oil
results in a proportional reduction in the vanadium content. A
similar effect can be produced by changing from heavy to
distilled fuel oil, as noted in Boston by Barry et al. (1975),
where levels of airborne vanadium for comparable months in 1966
and 1972 were 1.07 and 0.114 µg/m3, respectively.
These data illustrate the importance of fossil fuels as
sources of vanadium in urban air. The patterns observed in
other areas are similar but less extreme in concentration and
fluctuation. Because of the variations in vanadium
concentrations in oil and coal, community levels will depend
mainly on the actual vanadium concentrations in the fuels used
and on meteorological factors.
Pollution of the air by industrial facilities may be less
than that by power stations and heating equipment. At a steel
plant in the USA in 1967, concentrations of vanadium ranged from
0.04 to 0.107 µg/m3 and averaged 0.072 µg/m3, corresponding to
the mean concentration over 13 Pennsylvania towns (also
0.072 µg/m3) (NAS, 1974). The following levels of vanadium
pentoxide were found by Pazhynich (1967) in the USSR in areas of
extensive metallurgical activity not connected with vanadium
production: in the years 1964-65: 1.49 µg/m3 at 150 m from the
source of discharge; 0.47 µg/m3 at 500 m; 1.35 µg/m3 at
1000 m; and 0.98 µg/m3 at 1500 m. Near a plant producing
technical vanadium pentoxide, Kurmaev (1974) detected the
following 24-h mean levels of vanadium pentoxide: 0.004 -
0.012 mg/m3 at 500 m from the source; 0.001 - 0.006 mg/m3 at
1000 m; and 0.001 - 0.004 mg/m3 at 2000 m. Seventy to 72% of
the particles were less than 2 µg in diameter.
 Boyarkina et al. (1978) studied vanadium precipitation in
and around an industrial city, in winter, by measuring
concentrations in snow. In the central area, a concentration of
24 µg/litre was found, decreasing to 3.2 µg/litre at a
distance of 40 - 50 km.
4.1.2 Water
Natural background levels of vanadium in water have been
discussed in section 3.1.3, and levels in industrial effluent,
in section 3.4.1. Durfor & Becker (1963) included vanadium in
their analyses for trace elements in drinking-water supplies in
large cities in the USA. Of the samples analysed, 91% showed
less than 10 µg vanadium/litre; the maximum concentration was
70 µg/litre, and the average was about 4.3 µg/litre. Many of
the samples were negative. Twenty-six percent of 3676 tap water
samples from 34 areas in the USA contained vanadium at
concentrations ranging from 1.3 to 33 µg/litre with a mean of
4.85 µg/litre (Greathouse & Craun, 1979).
Hoffmann et al. (1972) published data from a regional well-
water survey in Poland. The average vanadium concentrations
were 0.06 - 6 µg/litre, with a maximum single value of
15 µg/litre. Bottled waters from mineral springs frequently
contained higher levels; Schlettwein-Gzell & Mommsen-Straub
(1973) reported a range of 4 - 290 µg/litre in bottled waters
from Switzerland.
Highly-mineralized waters in Argentina contained 0.3 -
10 µg vanadium/litre (Trelles et al., 1970). These concentra-
tions often occur in conjunction with high concentrations of
arsenic and/or fluorides.
4.1.3 Food
4.1.3.1 Individual foods
Information on the vanadium contents of human food is
sparse. Data from two studies (Myron et al., 1977; Byrne &
Kosta, 1978) are combined in Table 11 with those from an earlier
study using a similar method (Söremark, 1967). The results of
these studies agree reasonably well, but differ in some respects
from the results of earlier work, especially that of Schroeder
et al. (1963). The principal difference is that Schroeder noted
high vanadium concentrations in fats and oils, whereas low
concentrations were found in the more recent studies. Such a
difference may be accounted for by the use of different
analytical methods. Similar discrepancies have been encountered
in the study of other trace elements, i.e., lower concentrations
have been found using more recently developed methods.
The data presented in Table 11 show low levels of vanadium
in most elements of the human diet. There are also some
interesting differences among specific foods. Grains contain
higher levels of vanadium than fruits and vegetables. Levels in
oils and fats and beef and pork are low, but those in the liver
and kidneys of cows and pigs are higher. Higher levels are
found in both the flesh and internal organs of the chicken, and
levels in fish flesh are also high. Vanadium levels in milk and
eggs are low, and those in beer and wine are high. Myron et al.
(1977) pointed out that processing appears to raise levels in
food (e.g., white versus brown rice; cereal, flour, bread, and
gluten versus grain; peanut butter; bologna and bacon versus
pork). No explanation was given for the very high levels in
dill and parsley.
Table 11. Vanadium concentrations in foods (µg/kg)a
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Grains
Wheat 3.6
Flour 15, 40
Bread 11.20 10, 13
Gluten 33
Oats 3
Oatmeal 6
Corn 0.7
Cornmeal 2
Brown rice 1
White rice 21 12, 30
Barley 14 1.6
Cereal 93
Fruits
Apple 1.1 4 0.3
Pear 0 0.2
Banana 3 0.2
Orange 1
Cherry 0.4
Apricot 0.2
Peach 0.2
Strawberry 31.41 (dry)
Blueberry 1.6
Vegetables
Potato 0.8 1 1.2, 1.9
Radish 52 5 0.6
Carrot 0 1 2.3, 2.4
Beet 0
Garlic 0.6
Onion 0.6
Leek 0.3
Navy bean 14
Pea 0 7 0.4
Tomato 0.03 2 0.3
Cucumber 2.1
Squash 4
Brussels sprout 0.5
Cauliflower 0.08 1 0.9
Cabbage 2 0.3
Lettuce 21 4 1.0, 2.7
Table 11 (contd).
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Spinach 35
Parsley 790 1800 (dry)
Mushroom 50 - 2 000 (dry)
Dill 140 431
Meats
Beef 0 1 0.4 - 1.3
Beef liver 2.4, 10 6 7.3
Pork 0 1 0.6, 0.9
Pork liver 8.4
Pork kidney 8.5
Bacon 5
Bolgna 8
Chicken, white 22 1.7
Chicken, dark 12
Chicken liver 37, 38
Chicken kidney 18
Cod 28 7.2
Mackerel 2.6 3.5
Tuna 11 10, 3
Lobster 43 5
Scallop 22
Oils and fats
Margarine 4
Soybean oil 1
Corn oil 1 3
Pumpkin seed oil 0.2
Lard 2 0.2
Nuts
Hazel nut 3.7
Peanut butter 44
Dairy products
Milk 0 - 0.1 3 0.2, 0.2
Powdered milk 0 - 0.2 25
Chocolate milk 21
Butter 1
Egg white 0.3 - 1.8
Egg yolk 2.0 - 3.6
Beverages
Coffee 1.6
Tea 1.3 0.3
Cola 0.7 1.5
Beer 11 8.4
Wine 3.5 - 3.2
---------------------------------------------------------
a Study 1: Söremark (1967) (neutron activation analysis).
Study 2: Myron et al. (1977) (atomic absorption spectroscopy).
Study 3: Byrne & Kosta (1978) (neutron activation analysis).
4.1.3.2 Complete diets
Byrne & Kosta (1978) estimated the daily intake of vanadium
to be "a few tens of micrograms," but added that it may vary
considerably. Assuming a very low rate of intestinal absorption
of vanadium in man (section 5.1.2), Byrne & Kosta calculated
intakes for 3 adults in whom they measured dietary
concentrations. The calculated intakes were 36, 66, and
11 µg/day, respectively.
Analyses of 9 selected hospital diets (Myron et al., 1978)
are given in Table 12. Each meal was prepared and analysed
separately by atomic absorption spectroscopy. The results were
similar to concentrations found in individual foodstuffs in
other studies given above. In a later study, Byrne & Kosta
(1979) reported on the determination of vanadium in total diet
samples obtained during a nutrition survey in 5 Italian towns
(Table 13). The concentrations agree with those reported by
Myron et al. (1978).
Table 12. Daily vanadium intake in dieta
------------------------------------------------------
Diet type (µg/day) (µg/g) (µg/1000
cal)
------------------------------------------------------
General-1 13.6 0.019 4.7
Cholesterol reducing-1 25.6 0.034 8.8
Cholesterol reducing-2 16.8 0.022 5.8
Cholesterol raising 30.1 0.046 10.5
General-2 28.0 0.040 9.8
Low calorie 12.4 0.029 10.6
Low salt 15.5 0.028 9.1
Puree 26.0 0.050 14.1
Soft 15.8 0.024 6.4
------------------------------------------------------
a Adapted from: Myron et al. (1978).
4.2 Occupational Exposure
In terms of occupational exposure, the most important
vanadium compounds are vanadium pentoxide, vanadium trioxide,
ferrovanadium, vanadium carbide, and vanadium salts, such as
sodium and ammonium vanadate. The oxides and salts are commonly
used in industry in powder form, giving rise to the possibility
of dust and aerosol formation, when the substances are crushed
or ground. Many metallurgical processes involve the production
of vapour containing vanadium pentoxide, which condenses to form
respirable aerosols. Boiler-cleaning operations generate dusts
containing the pentoxide and trioxide compounds. Combustion of
residual fuels with a high vanadium content is likely to produce
aerosols of the pentoxide as well as oxide complexes of vanadium
with other metals.
Table 13. Vanadium contents of Italian freeze-dried
total diet samplesa
--------------------------------------------------------
Town Dry Vanadium Daily
weight concentrationb vanadium
(g) (ng/g) intake (µg)
--------------------------------------------------------
Aosta 310 32.1 0.9 (2) 10.0
L'Aquila 173 46.0 3.4 (2) 8.0
Montfalcone 278 39.7 4.1 (2) 11.0
Mt. Amiata 377 29.7 0.8 (2) 11.2
Rome 285 42.3 4.6 (4) 12.0
Mean values 38.0 6.9 10.4 1.5
--------------------------------------------------------
a From: Byrne & Kosta (1979).
b Average and standard deviation; number of aliquots
in parentheses.
4.2.1 Metallurgy
The processing of metals containing vanadium includes
chemical treatment and high-temperature operations. However,
only moderate concentrations of vanadium were found in the
breathing zone of workers engaged in operations that would be
expected to produce the greatest fume exposure. During the
addition of vanadium to furnaces, concentrations ranged from
0.006 to 0.08 mg/m3 and, during tapping, from 0.004 to
0.02 mg/m3. Concentrations found in oxyacetylene cutting ranged
from 0.008 to 0.015 mg/m3 and, in arc-welding, from 0.002 to
0.006 mg/m3 (NAS, 1974).
Vanadium levels in metallurgical plants have been studied in
detail (Roshchin, 1968). Vanadium slag contains about 11 - 13%,
mainly in the form of trioxides of vanadium (measured as
vanadium pentoxide). Slags are used for the production of
vanadium pentoxide and ferrovanadium, and the process is
accompanied by extensive formation of iron oxide aerosol. Air
concentrations of the dust (mainly vanadium trioxide) found in
the main working positions (converter operator, mixer, and crane
driver) ranged from 20 to 55 mg/m3. Measured as vanadium
pentoxide, the contents of the swirling dust did not exceed
0.17 mg/m3. About 75% of the dust particles had a diameter of
less than 2 µm and 20% had a diameter of between 2 µm and
4 µm.
Breaking, loading and unloading, crushing and grinding, and
magnetic separation of vanadium slag causes thick dust
formation, with concentrations ranging from 30 to 120 mg/m3.
The slag contains 111 - 129 g vanadium pentoxide/kg. A diameter
of less than 2 µm was recorded for 70 - 72% of the particles;
86 - 96% had a diameter of less than 5 µm. When the slag is
roasted, free vanadium pentoxide is discharged into the work-
place air; atmospheric concentrations in the vicinity of
furnaces ranged from 0.04 to 1.56 mg/m3. During leaching and
precipitation, concentrations of vanadium in the air may be
high, sometimes exceeding 0.5 mg/m3.
The smelting and granulation of technical vanadium pentoxide
are accompanied by the formation of an aerosol. This aerosol
escapes when the product is poured for granulation. During the
loading of smelting furnaces, concentrations of vanadium
pentoxide ranged from 0.15 to 0.80 mg/m3. During smelting and
granulation, concentrations ranged from 0.7 to 11.7 mg/m3. In
other parts of the work-place, concentrations may range from
0.03 to 0.2 mg/m3.
In aluminium production, when bauxite is being converted
into alumina, the aluminate solutions accumulate vanadium salts,
which crystallize and precipitate out. Precipitated sodium
polyvanadate is smelted to form vanadium pentoxide, which is
cooled and settles in the form of thin plates. Vanadium
pentoxide dust (concentrations of up to 2.3 mg/m3) is given off
only in the terminal phase during tapping of the liquid product,
packing, and loading.
During the drying, sieving, and calcination of ammonium
vanadate and during the crushing, unloading, and packaging of
pure vanadium pentoxide, dusts are formed. When vanadium
pentoxide is sieved after calcination, the concentration in air
may range from 2.2 to 26 mg/m3. In plants with less
mechanization, incomplete sealing of equipment, and inefficient
local exhaust ventilation, concentrations of dust during these
operations ranged from 4.9 to 48.9 mg/m3.
In the production of ferrovanadium, there is a continuous
source of discharge of vanadium pentoxide and lower oxides
during the smelting process. Data on vanadium in air at various
sites are shown in Table 14.
Using spectrophotometric techniques, Roshchin (1968),
Katayeva & Sapunov (1974) and Kazimov (1977) found high
concentrations of vanadium during smelting and granulation
(range, 0.16 - 1.89 mg/m3; mean, 0.59 mg/m3; 104 samples),
production of ferrovanadium (range, 0.58 - 4.81 mg/m3; mean,
1.7 mg/m3; 110 samples), and roasting of the charge (range,
0.44 - 3.64 mg/m3; mean, 1.52 mg/m3; 112 samples).
Table 14. Vanadium levels in the air of a ferrovanadium planta
--------------------------------------------------------------------
Work-place/operations Vanadium pentoxide Lower oxides of
(mg/m3) vanadium (mg/m3)
--------------------------------------------------------------------
Work area of smelters and 0.1 - 2.6 0.05 - 1.2
helpers
Unloading of vanadium pent- 2 - 124.6
oxide from the bin and
charging of electric furnace
Crane driver's cabin during 0.07 - 9.43 0.03 - 0.1
smelting
Cutting up ferrovanadium 0.97 - 12.6
Maintenance of the furnace 7.5 - 30
--------------------------------------------------------------------
a From: Roshchin (1968).
Using high-volume sampling and atomic absorption analysis,
Usutani et al. (1979) measured vanadium pentoxide
concentrations in the air at several places in a vanadium
refinery. The highest concentrations (higher than 1 mg/m3) were
detected in samples collected during the removal of the vanadium
pentoxide flake. High-volume samples from other locations as
well as low-volume samples obtained over 6.5-h work shifts
showed lower concentrations (0.002 - 0.735 mg/m3).
When ductile vanadium is produced by the aluminothermic
process (based on the reduction of pure vanadium pentoxide with
aluminium powder), the violent exothermic reaction leads to the
release of a condensation aerosol of vanadium pentoxide. During
preparation of the charge mixture, work-place concentrations of
vanadium pentoxide ranged from 19 to 25.1 mg/m3. When the
burden was placed in crucibles inside the smelting chambers,
concentrations ranged from 64 to 240 mg/m3. During smelting,
concentrations at the operators' workplaces ranged from 0.17 to
0.6 mg/m3. Twenty to 30 min after smelting, levels declined to
0 -0.3 mg/m3. Ninety-eight percent of the condensation aerosol
particles produced had a diameter of less than 5 µm, and 82%
had a diameter of less than 2 µm (Roshchin, 1968).
In the production of vanadium by the vacuum carbon thermic
method, most of the pollution occurs during operations with
vanadium trioxide (Roshchin, 1968). Mixing of vanadium trioxide
in a closed mixer led to air concentrations in the workplace of
from 0.019 to 0.58 mg/m3. Unloading of the charge resulted in
high concentrations of 14.7 - 29.4 mg/m3. In the packing
department, when the charge was sifted in a fume cupboard,
concentrations in the breathing zone ranged from 0.58 to
4.7 mg/m3. When the charge was being weighed out and packed in
a fume cupboard, concentrations ranged from 3.38 to 6.76 mg/m3.
Levels of vanadium-containing dust and vanadium pentoxide in
the air during catalyst production are shown in Table 15.
Table 15. Air contamination in vanadium catalyst productiona
-----------------------------------------------------------------------------
Operation Dust (mg/m3) Vanadium pentoxide (mg/m3)
Minimum Maximum Most Minimum Maximum Most
frequent frequent
-----------------------------------------------------------------------------
Grinding and un- 5 45 7 - 9 1 7 1.5 - 3
loading vanadium
pentoxide
Loading ground 12 53 14 - 17 3.2 7.5 4 - 4.2
pentoxide into
the bin
Sifting and pack- 5 17.5 5 - 7 0.1 1 0.4 - 0.5
ing granules of
bulk contact
substance
-----------------------------------------------------------------------------
a From: Roshchin (1968).
4.2.2 Cleaning of oil-fired boilers
Significant occupational exposure to vanadium occurs during
the cleaning of boilers in oil-fired heating and power plants
and ships (Symanski, 1939; Roshchin, 1968; Kuzelova et al.,
1977; Levy et al., 1984). Fuel oil combustion results in the
formation of vanadium-containing dust, and large amounts of dust
result from operations connected with removing ash encrustations
in boiler cleaning and in cleaning the blades of gas turbines.
Most of these operations are carried out by hand, and the dust
in the air inside the boilers may range from 20 to 400 mg/m3,
the most common range being 50 - 100 mg/m3, with the dust
containing 5 - 17% vanadium pentoxide and from 3 to 10% of the
lower vanadium oxides (Roshchin, 1968). Kuzelova et al. (1977)
reported dust concentrations of 136 - 36 036 ml/m3 in the air
with vanadium concentrations ranging between 1.7 and 18.4
mg/m3.
Williams (1952) published air sampling data on boiler-
cleaning operations in the British power industry. He found
concentrations of soot dust at different points ranging from 239
to 659 mg/m3. The vanadium concentrations in the dust of the
superheater chamber was 40.2 mg/m3, while, in the combustion
chamber, the concentration was 58.6 mg/m3. Most (93.6%) of the
dust particles had a diameter of between 0.15 and 1 µm.
4.2.3 Occupational exposure limits
Some national occupational exposure limits for vanadium in
work-place air are shown in Table 16.
Table 16. Examples of occupational exposure limits for vanadium in various countries
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Australia Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Belgium Recommendation Time-weighted average (TWA) (fume) 0.05 ILO (1980)
Czechoslovakia Regulatory requirement Maximum allowable concentration (MAC) Hygienic Regula-
- Time-weighted average (TWA) 0.1 tions of the
- Ceiling value (fume) 0.3 Ministry of Health
- Ceiling value (dust) 1.5 of CSR (1985) (58)
(Regulation No. 7;
section 24/ZB)
Finland Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA) (fume)a 0.05
German Democratic Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1980)
Republic Short-term exposure limit (STEL)a 0.1
(fume)
Germany, Federal Recommendation 8-h time-weighted average (TWA)a 0.5 Federal Republic
Republic of of Germany Comm-
Short-term exposure limit (STEL)a 2.5 ision for Maximum
(30 min, 2x/shift) Work-Place Con-
centrations
8-h time-weighted average (TWA)a 0.1 (1985) (xxi, 16)
(fume)
Recommendation Short-term exposure limit (STEL)a 0.5
(30 min, 2x/shift) (fume)
Italy Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1985)
Hungary Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1984)
Netherlands Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Romania Regulatory requirement Ceiling value (fume)a 0.1 ILO (1980)
Sweden Regulatory requirement Ceiling valuea 0 ILO (1980)
Switzerland Regulatory requirement Time-weighted average (TWA)a 0.09 Permitted Values
in the Work-Place,
Berne (1984)
USA Recommendation Time-weighted average (TWA)a 0.05 ACGIH (1986)
(dust and fume)
Time-weighted average (TWA)a 0.5 OSHA (1977) (29
(dust) CFR.1910.1000)
Ceiling value (15 m)a 0.05 NIOSH (1977)
(dust and fume)
Time-weighted average (TWA)a 0.1 OSHA (1977) (29
(fume) CFR.1910.1000)
USSR Regulatory requirement Time-weighted average (TWA)a 0.1 ILO (1980)
Yugoslavia Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA)a 0.1
---------------------------------------------------------------------------------------------------------
a Measured as V2O5.
5. KINETICS AND METABOLISM
5.1 Physiological Role
5.1.1 Microorganisms
Vanadium is essential for the mould Aspergillus niger
(Bertrand, 1942) and the green alga Scenedesmus obliguus (Arnon
& Wessel, 1953; Arnon, 1958). It may play a role in photo-
synthesis in the latter. A need for vanadium has been shown by
the yeast Candida slooffii at high temperatures (Roitman et al.,
1969). The growth effects of vanadium in Azotobacter and other
bacteria have been related to the ability of vanadium, in lieu
of molybdenum, to catalyse nitrogen fixation reactions (Horner
et al., 1942; Takahashi & Nason, 1957).
5.1.2 Animals
Vanadium deficiency has been reported in chicks (Hopkins &
Mohr 1971a; Nielsen & Ollerich, 1973) and rats (Schwarz & Milne,
1971; Strasia, 1971), and vanadium is considered essential for
these animals (Underwood, 1977; Vouk, 1979; Nechay, 1984).
Hopkins & Mohr (1974) observed reduced feather growth in
chickens. They also reported impaired reproduction, due to
decreased fertility, and increased perinatal mortality in rats
fed a low vanadium diet (10 µg/kg) over 4 generations. A
positive growth response in rats was observed by Schwarz & Milne
(1971) when vanadium salts, at levels of between 50 and
500 µg/kg were added to a semi-purified diet (vanadium content
unspecified) fed for 26 - 28 days.
In male Leghorn chicks fed vanadium in the diet at levels of
12.5 mg/kg and 25 mg/kg, body-weight gain was normal at 1, 2, 3,
and 4 weeks of age (Kubena et al., 1986).
Slower growth, higher haematocrits, and higher levels of
plasma- and bone-iron were reported by Strasia (1971) in rats
fed diets containing less than 100 µg vanadium/kg compared with
controls receiving diets containing 500 µg vanadium/kg, but
these results were not confirmed in another study by Williams
(1973). Hopkins & Mohr (1971a,b, 1974) reported that a diet
containing less than 10 µg vanadium/kg decreased plasma-
cholesterol levels in chicks at 28 days of age, increased
plasma-cholesterol levels at 49 days of age, and increased
plasma-triglyceride levels at 28 days of age. Nielsen &
Ollerich (1973) found that administration of a diet containing
30 - 35 µg vanadium/kg to chicks decreased growth, increased
haematocrits and plasma-cholesterol levels, and impaired bone
development.
Nielsen (1980) carried out further studies on chicks and
rats, given different types of low-vanadium experimental diets,
to examine the effects of vanadium deficiency. In rats,
vanadium deficiency adversely affected prenatal survival,
growth, physical appearance, haematocrit, plasma-cholesterol
levels, and hepatic lipid and phospholipid levels. In chicks,
low vanadium intake produced adverse effects on growth,
feathering, haematocrit, bone development, plasma-cholesterol
levels, and hepatic lipid, phospholipid, and cholesterol levels.
However, consistent deficiency effects were not observed in
chicks or rats in any of the studies. Nielsen (1980) suggested
that the inconsistency in vanadium deficiency effects might be
due to the fact that different experimental diets had different
effects on the metabolism of vanadium.
Vanadate appears to have an insulin-like action (Heyliger et
al., 1985). In female Wistar rats made diabetic with
streptozotocin (single iv injection of 55 mg/kg) and given
sodium orthovanadate in the drinking-water at a concentration of
0.6 - 0.8 mg/ml (corresponding to calculated daily intakes of
between 75 ± 3 and 100 ± 3 mg/kg body weight per day,
respectively) for 4 weeks, the serum-insulin level was low, but
there was no increase in blood-glucose levels compared with
controls. In diabetic rats not treated with vanadate, serum-
insulin levels were also low, but blood-glucose levels were
increased 3-fold. The cardiac performance of vanadate-treated
animals did not differ significantly from that of non-diabetic
controls. It was concluded that vanadate controlled blood-
glucose levels and prevented the decline in cardiac performance
due to diabetes.
The results of in vitro studies suggest that vanadium may
play a specific physiological role as a regulator of the sodium
pump (Macara, 1980). Vanadate has been shown to inhibit Na+K+-
ATPase (EC 3.6.1.3) in intact human red blood cells (Rifkin,
1965; Cantley et al., 1977, 1978a,b). It also has potent
diuretic and natriuretic effects in rats (Balfour et al., 1978;
Westenfelder et al. 1981). It was a powerful inhibitor of
Na+K+-ATPase in microsomal fractions of the kidney, brain, and
heart in several species, including human beings (kidney).
Mg2+-ATPase was up to 10 000 times more resistant to vanadium
inhibition than Na+K+-ATPase (Nechay & Saunders, 1978).
Similarly, in vivo studies on laying chickens fed calcium
orthovanadate for 15 months, at levels of 0.25, 50, or 100 mg/kg
diet, showed clear inhibition of Na+K+-ATPase activity in the
kidney (Phillips et al., 1982). The inhibition of Na+K+-ATPase
by vanadate can be reversed by catecholamines, though these do
not have any effects on the Na+K+-ATPase activity in the absence
of vanadate (Hudgins & Bond, 1979). The inhibition is also
partially prevented by the reducing agents ascorbic acid and
glutathione (Grantham & Glynn, 1979).
The mechanism by which cells reduce cytoplasmic vanadium5+
to vanadium4+ was investigated using human red cells (Macara et
al., 1980). The authors concluded that vanadate is reduced by
cytoplasmic glutathione and that this explains the resistance of
the Na++K+-ATPase to vanadium in intact cells.
Since vanadate (V5+) is a potent inhibitor of Na+K+-ATPase,
many physiological and biochemical processes are vanadium
sensitive (Grantham, 1980; Nechay, 1984). For example, vanadium
compounds inhibit ATP phosphohydrolases, ribonuclease, adenylate
kinase, phosphofructokinase, squalene synthetase, glycer-
aldehyde-3-phosphate dehydrogenase (Macara, 1980), glucose-6-
phosphatase (Singh et al., 1981), and phosphotyrosyl-protein-
phosphatase (Swarup et al., 1982). The membrane neutral (K+)-
p-nitrophenylphosphatase in the membrane fraction of skeletal
muscle was inhibited equally by vanadium4+ and vanadium5+ at 4 x
10-8 mol/litre (Vyskocil et al., 1981). Extracellular
application of both forms of vanadium failed to inhibit the
electrogenic (Na+ - K+) pump in intact mouse diaphragm fibres
(Vyskocil et al., 1981). In the concentration range from 10-5 to
10-3 mol/litre, vanadium4+ even potentiated the hyper-
polarization of the muscle fibres from -74 to -82 mV (Zemkova et
al., 1982), probably by increasing the intracellular potassium
level. These findings have led to the hypothesis that vanadate
could control sodium pump activity in vivo , perhaps via a
vanadium5+ : vanadium4+ equilibrium connecting pump activity to
the cellular redox state. However, vanadate in the red cell is
reduced to vanadium4+, which then binds to haemoglobin (Cantley
& Aisen, 1979), a reaction that seems to be essentially
quantitatively driven by glutathione (Macara et al., 1980), NADH
(Vyskocil et al., 1980), or by other mild reducing agents, such
as ascorbate or norepinephrine (Svoboda et al., 1984). The role
of the vanadium5+ : vanadium4+ redox equilibrium in the
regulation of cation flow across cell membranes has yet to be
unequivocally demonstrated.
Vanadium appears to be essential for chicks and rats, but it
does not appear to be essential in other species, as defined by
Mertz (1970), i.e., an element is essential if its deficiency
reproducibly results in an impairment of a function from optimal
to suboptimal. However, more research is needed before definite
conclusions can be drawn regarding the role of vanadium as a
nutritionally essential trace element for animals.
5.2 Absorption
The absorption and distribution of vanadium compounds depend
on the route of entry and the solubility of the compounds in
body fluids. The solubility of vanadium compounds in biological
media varies (Reznik, 1954). The following compounds are listed
in decreasing order of solubility: (a) in gastric juices,
vanadyl sulfate, sodium vanadate, ammonium vanadate, vanadium
pentoxide; (b) in blood-serum and in 0.22% sodium carbonate
solution, sodium vanadate, ammonium vanadate, vanadium
pentoxide, and vanadyl sulfate. The higher the solubility in
water and biological media, the more toxic the compound,
presumably because of better absorption (Roshchin, 1968).
5.2.1 Absorption by inhalation
5.2.1.1 Human studies
There is little information on the deposition of vanadium
compounds in the respiratory tract following inhalation.
However, the greatest deposition would be expected in the
submicrometre particle size fraction and particle size
distribution studies (Lee et al., 1972) have shown that most
vanadium-bearing particulate matter is very small and well
within the respirable range for human beings.
Soluble vanadium compounds inhaled and deposited in the
lung, are readily absorbed but the rates of absorption have not
been quantified and estimates have not been made of the amounts
of inhaled vanadium that are transported back to the pharynx by
mucociliary clearance, swallowed, and are then available for
absorption via the gastrointestinal tract. It has been
estimated that about 25% of soluble vanadium compounds may be
absorbed via the respiratory tract (ICRP, 1960). Absorption
from the respiratory tract was demonstrated in workers exposed
to vanadium dust, who showed increased concentrations of
vanadium in the urine (Lewis, 1959b; Gylseth et al., 1979;
Maroni et al., 1983).
5.2.1.2 Animal studies
Following acute exposure, there is complete clearance of the
relatively soluble vanadium pentoxide from the lung within 1 -
3 days (Sjöberg, 1950; Levina, 1972). Stokinger et al (1953)
demonstrated that vanadium is present for more than 40 days
following cessation of long-term exposure.
Intratracheal administration of 48V-vanadium nitrate (0.4
and 20 mg/kg body weight) to albino rats showed that the 48V
absorption rate was maximum after 5 min and could be detected in
internal organs after 30 min. Blood levels were initially high,
but fell to trace levels after 2 days. 48V was not detectable
after 4 days, but reappeared at 8 days and accumulated in all
internal organs, the greatest quantity accumulating in the bone
(Ordzhonikidze, 1977). In female Fischer rats exposed by intra-
tracheal instillation to 40 µg vanadium pentoxide in 0.9%
saline solution, the time for removal of 50% of the initial
burden was 18 min, but traces remained for a considerable time.
At 14 days, the vanadium was distributed principally in the
carcass (40%) and skeleton (12%) (Rhoads & Sanders, 1985). In
a study of the kinetics of vanadium following single or multiple
intratracheal administration, the blood concentration was high
initially and vanadium accumulated in the liver and kidney
reaching the highest level after 24 h (Roshchin & Ordzhonikidze,
1986).
Vanadium trioxide was cleared from the lung more rapidly
than pentoxide or ammonium vanadate following intratracheal
instillation in rats (Levina, 1972).
5.2.2 Absorption from the gastrointestinal tract
5.2.2.1 Human studies
In general, vanadium salts are poorly absorbed from the
human gastrointestinal tract. In a study by Curran et al.
(1959), from 0.1 to 1% of 100 mg vanadium (as highly soluble
diammonium oxytartratovanadate) was absorbed from the
gastrointestinal tract and 60% of this was excreted via the
kidneys within 24 h. The remainder was retained in the liver
and bone, until the oral administration ceased, when it was
mobilized rapidly from the liver and slowly from the bone.
Sodium metavanadate (12.5 mg/day for 12 days) was recovered
largely unabsorbed in the faeces (87.6%) and the remainder in
urine (12.4%) (Proescher et al., 1917). The International
Commission on Radiological Protection (ICRP, 1960) estimate for
the gastrointestinal absorption of soluble vanadium compounds
was 2%. A low degree of absorption was also found by Roshchin
et al. (1980).
5.2.2.2 Animal studies
Mountain (1959)a reported an unpublished study in which
vanadyl sulfate was fed to adult male rats in daily doses
ranging from 650 to 1250 µg (160 - 310 µg of vanadium). The
mean absorption was about 0.5%, but urinary values varied
considerably. The duration of the study was not given.
5.2.3 Absorption through the skin
Dermal absorption and skin irritation were reported in a
study in which a nearly saturated solution (20%) of sodium
metavanadate was applied to the skin of the rabbit (Stokinger,
1967).
However, according to US EPA (1977), the skin appears to be
a minor route of vanadium uptake for human beings. In an in
vitro study using 48V radiotracer, there was no penetration of
human skin samples (Roshchin, 1980).
5.3 Distribution and Transformation
5.3.1 Human studies
Vanadium levels in man, reported in earlier studies, were
considerably higher than those reported more recently. The
difference is illustrated by the whole-body content of 17 -
43 mg vanadium for a 70-kg man calculated by Schroeder (1963)
compared with estimates of 100 µg derived by Byrne & Kosta
(1978). The influence of different sampling procedures and
analysis (colorimetric determination, neutron activation) should
be clarified before concluding that such effects are real
(Lagerkvist et al., 1986) or that they reflect decreasing
environmental exposure.
__________________
a Mountain, J.T. (1959) Unpublished results, Toxicologic
Services, Occupational Health Field Headquarters,
Cincinnati, Ohio.
Absorbed vanadium is transported mainly in the plasma
(Schroeder et al. 1963; Ordhzonikidze et al., 1977; Roshchin et
al., 1980). Some mean values obtained using different methods
of analysis are given in Table 17. There is a wide divergence
in the results, the ratio between the highest and the lowest
mean values being about 104. In general, levels tend to decline
chronologically as analytical techniques become more sensitive.
There is also a difference in results obtained by neutron
activation analysis according to whether separation was carried
out before, or after, irradiation.
In an extensive investigation on human tissues, the
inadequate sensitivity of the analytical method meant that
quantitative information could only be obtained for the lung
and intestine (Tipton & Cook, 1963). Using neutron activation
analysis, Byrne & Kosta (1978) obtained information on other
organs. Table 18 includes autopsy tissue values and some
results from two other investigations using neutron activation
analysis. It is apparent that vanadium concentrations are low
in all tissues, though the liver, kidney, and lung often show
higher levels than other tissues. In another investigation
on a selection of organs, using spark-source mass spectrometry,
the vanadium levels detected were: brain, 30 µg vanadium/kg
wet weight (average from 10 specimens); liver, 40 µg/kg
(average from 11 specimens); lung, 100 µg/kg (average from 11
specimens); lymph node, 400 µg/kg (average from 6 specimens);
and testis, 20 µg/kg (average from 5 specimens) (Hamilton et
al., 1972/73). The values reported are in reasonable agreement
with those found in other animals. However, there is
considerable disagreement between investigators and between
analytical methods, which has not been resolved, since
interlaboratory and intermethod investigations have not been
carried out.
In the general population, which is mainly exposed to low
levels of vanadium in food with poor absorption from the
intestine, vanadium is usually undetectable in the urine, even
using very sensitive methods (Byrne & Kosta, 1978). Examination
of urine samples from 50 normal individuals using atomic
absorption spectrometry showed that vanadium was present in only
13; 11 samples showed a level of 0.1 µg/litre and two,
0.2 µg/litre (Ueno & Ishizaki, 1980). In industry, where
exposure is mainly through air and absorption from the lung is
high, vanadium concentrations in the urine cover a considerable
range (section 5.4.1).
Table 17. Vanadium levels in human blooda,b
---------------------------------------------------------------------------------------------------------
Analytical method Whole blood Serum/plasma Reference
(mg/litre) (mg/litre)
---------------------------------------------------------------------------------------------------------
X-ray emission 0.01 (43) Gofman (1962)
Spectrography 0.0078 (24) Lifschitz (1962)
Colorimetry 0.23 (calculated) 0.42 (13) Schroeder et al. (1963)
Neutron activation analy- 0.016 Bowen (1963)
sis (preseparated)
Neutron activation analysis 0.67 ± 0.32 ng/ml Simonoff et al. (1986)
Spectrography 0.126 (47) Butt et al. (1964)
Neutron activation analy- 0.0046 (36) Heydorn & Lukens (1966)
sis (preseparated)
Catalytic 0.01 (82) Allaway (1968)
0.02 - 0.01 (7)
Spectrophotometric 0.057 (10) Christian (1971)
Neutron activation analy- 0.0046 Kirzhner et al. (1974)
sis (preseparated)
Neutron activation analy- 0.022 (5) Buono et al. (1977)
sis (preseparated)
Neutron activation analy- 0.0005 (5) Byrne & Kosta (1978)
sis (preseparated)
Neutron activation analy-
sis (preseparated) 0.0066c (5) Sabbioni et al. (1979)
Neutron activation analysis 0.000047 (9 males) Cornelis et al. (1979)
0.000024 (8 females) Cornelis et al. (1979)
Neutron activation analy-
sis 0.000033 (17 females) Cornelis et al. (1980)
--------------------------------------------------------------------------------------------------------
a Adapted from: Byrne & Kosta (1978) and Versieck & Cornelis (1980).
b Number of subjects in parentheses.
c Recalculated values assuming an haematocrit of 0.45.
Table 18. Vanadium levels in human organs (µg/kg wet weight)a
-----------------------------------------------------------------------------
Tissue Study
-------------------------------------------------------------------
Byrne & Kosta (1978)b,c Damsgaard (1972)c,d Lievens (1977)b
-----------------------------------------------------------------------------
Kidney 3.3 3.2 2.6 nd 7
Liver 7.5 4.5 5 19 7 - 19 (5)
Brain 0.7 0.75
Thyroid 3.2 3.0
Heart 1.1
Cardiac 0.45 0.3
fat
Subcut- 0.63 0.80
aneous
fat
Muscle 0.45 0.62 0-59 7 nd
Spleen 3 4
Pancreas nd 14
Lung 19 - 40 (7) nd 13
median 30
-----------------------------------------------------------------------------
a From: Byrne & Kosta (1978).
b Parentheses enclose number of subjects.
c Brackets embrace specimens from one autopsy.
d nd = not detected.
Studies on vanadium levels in human bone have produced
widely varying results. Byrne & Kosta (1978) reported the
following data from human bones (lg/kg wet weight): skull,
2.5, 3, 4.5, 8.3; sternum, 3.1; rib, 0.8, 2.1; tooth enamel
(fragmented), 2, 3.4, 4, 5.1; and tooth enamel (drill powdered),
18. The high concentration in tooth enamel may be due to diet;
concentrations of vanadium tend to be higher in processed than
in unprocessed foods (Myron et al., 1977), but vanadium is
commonly present in steel alloys, especially tool steels, and
vanadium contamination may have occurred from the drill. Using
atomic absorption spectroscopy, Sumino et al. (1975) found a
range of 100 - 200 µg/kg (wet weight) in 6 specimens of rib.
Using emission spectral analysis, Shevchenko (1965) detected a
mean vanadium level of 150 ± 2 µg/kg (0.015 mg%) (dry weight)
in 14 samples of healthy bone tissue; bone tumours contained
higher levels.
Metal levels have been extensively studied in hair, because
of its potential value for exposure and body burden studies.
Gordus et al. (1974) and Gibson & DeWolfe (1979), using the same
method as Byrne & Kosta (1978), found levels of 20 - 40 µg/kg
in 42 subjects and 20 - 41 µg/kg in 370 subjects, respectively.
These results compare favourably with those of Byrne & Kosta,
i.e., 12 - 87 µg/kg in 12 subjects. Ueno & Ishizaki (1980)
used atomic absorption spectrometry to determine vanadium in
hair specimens from 130 men and 132 women. They found mean
concentrations of 53.6 µg/kg (range, 5 - 155 µg/kg) and
44.2 µg/kg (range, 1.8 - 118.8 µg/kg), respectively. However,
Creason et al. (1975), using emission spectroscopy in extensive
surveys in the USA, found levels that were about 10 times
higher.
There is considerable information on vanadium levels in lung
tissue. In studies by Schroeder et al. (1963), vanadium was
found in 97 out of 173 lungs examined in groups from 7 USA
cities, group mean concentrations ranging from 10 to 130 µg/kg
wet weight. Byrne & Kosta (1978) found levels of from 19 to
140 µg/kg (median, 30 µg/kg) in 7 cases; Hamilton et al.
(1972/73) detected a mean level of 100 µg/kg in 11 cases; and
Sumino et al. (1975) determined a range of 100 - 300 µg/kg for
13 observations. Statistical analyses of lung tissue trace
metal data were performed by Tipton & Shafer (1964) using data
taken from Tipton & Cook (1963). One approach was examination
of the relationship between lung metal concentrations and age.
The levels of a number of metals, including vanadium, increased
in the lung with age, indicating accumulation of insoluble
compounds, and Schroeder (1970b) calculated an annual increment
rate of 1.3 µg for lung-vanadium. However, earlier analysis of
the same data (Schroeder et al., 1963) did not show an age-
related increase in lung-vanadium. The later analysis by Tipton
& Shafer (1964) was performed on a subset of the original data
from Tipton & Cook (1963), which may account for some of the
discrepancy. The Tipton & Shafer (1964) data did not show a
graded increase in lung-vanadium with age, and there was a high
mean level only in the oldest age group. This could have been
produced by a single very high value in this age group. The
concept of vanadium accumulation in the human lung with age
remains doubtful, and the results of animal studies do not
indicate significant accumulation in the lung (sections 5.1.1.2
and 5.3.2).
5.3.2 Animal studies
Observations on pregnant rats, that received vanadium on the
21st - 22nd day of pregnancy, revealed accumulation in the
placenta but not "in perceivable quantities" in the organs of
the fetus. Vanadium was reported to be secreted in the milk
(Roshchin et al., 1980).
The identification of cellular components that react with
vanadium has been investigated in vivo and in vitro using 48V
radiotracer (Marafante & Sabbioni, 1983). Vanadium has a high
affinity for nuclear and mitochondrial components. When rats
were treated every day with 10 µg vanadium/rat for up to 8
days, a dose-related increase in vanadium incorporation in the
subcellular fractions was observed. There is evidence that non-
haem Fe-containing proteins, such as transferrin and ferritin,
have a high affinity for vanadium, while Fe haemoproteins are
not able to incorporate the metal.
In rat serum, both Vanadium4+ and Vanadium5+ form metal-
protein complexes with transferrins. Specific intracellular
vanadyl-ferritin complexes are formed in rat liver, spleen, and
kidney (Chasteen et al., 1986).
Ermolaev (1969) studied the distribution of vanadium in the
organs and tissues of 3 groups of rabbits, i.e., animals fed
under ordinary conditions, animals fed a diet supplemented with
vanadium (0.5% solution of vanadyl sulfate) at a dose of
0.05 mg/kg body weight, and animals fed the vanadium-
supplemented diet and subcutaneously injected with vanadium
also at 0.05 mg/kg body weight. The results of this
investigation are given in Table 19. The method of
administration had little effect on the resultant blood, brain,
and stomach levels, but there were distinct differences in the
case of the other organs and tissues.
The distribution and kinetics of vanadium, administered ip
as 80 mg vanadocene dichloride/kg to strain A mice, were
determined in blood, kidney, liver, small intestine, and brain.
The vanadium concentration decreased rapidly and exponentially
in the blood (half-time = 118 ± 43 min) and small intestine
(half-time a = 18 ± 0.14 min; half-time b = 341 ± 45 min).
Vanadium accumulated in kidney (maximum concentration,
1.12 ± 0.06 mmol at 12 h) and liver (maximum concentration,
0.56 ± 0.06 mmol at 8 h) and was then excreted (estimated half-
time, 7.9 ± 0.7 h for kidney; 12.1 ± 0.1 h for liver). Vanadium
was not detected in the brain (Toney et al., 1985).
Table 19. Vanadium levels in rabbit organs and tissues following
oral and subcutaneous administration (mg %)a
-----------------------------------------------------------------------------
Organ/ Control group First group Second group
tissue (0.05 mg/kg (0.05 mg/kg with
with food) food and injected
subcutaneously)
-----------------------------------------------------------------------------
liver 66 88 632
muscle tissue 27 41 30
blood 29 82 90
spleen 68 116 180
kidneys 71 90 464
lungs 62 82 168
brain 20 26 24
heart muscle 52 68 71
intestine 20 22 32
stomach 30 70 74
-----------------------------------------------------------------------------
a From: Ermolaev (1969).
The influence of the oxidation state of intravenously
injected compounds of 48V on uptake and distribution to selected
organs and subcellular elements of the rat liver was studied by
Hopkins & Tilton (1966). They did not observe any significant
differences in the rate or amount of uptake of nanogram
quantities of vanadium in three different oxidation states
(VOC13, VOC12, and VC13) and concluded that either the oxidation
state was not critical to transport or that the vanadium was
converted to a common oxidation state in vivo. Similar results
with respect to oxidation state and uptake and also the
distribution of vanadium in the rat were reported by Sabbioni et
al. (1978) and Conklin et al. (1982). In contrast, Parker &
Sharma (1978) found that levels of vanadium in the tissues of
male Wistar rats given sodium orthovanadate in the drinking-
water at 50 mg/litre for 3 months were higher than those in the
tissues of animals given vanadyl sulfate at the same
concentration. Roshchin et al. (1964) found evidence of the
partial conversion of vanadium trioxide to the pentavalent form
in blood-serum and in weakly acidic and basic solutions in
vitro. In a later study, Johnson et al. (1974) reported
the in vivo conversion of vanadium pentoxide to the tetravalent
state.
Information on vanadium distribution in rats after intra-
peritoneal, intratracheal, oral, and subcutaneous administration
of radioactive (48V) vanadium nitrate in single doses of
20 mg/kg body weight (LD50) and 0.4 mg/kg body weight (non-toxic
dose) is given in Table 20 (Roshchin et al., 1980). The study,
which involved 1060 albino rats, showed that whatever the method
of administration, vanadium was present in blood in significant
quantities only during the first 24 h, and that, after 2 days,
only traces of vanadium were detectable. No vanadium was found
in the blood after 4 - 8 days. However, in the groups
administered the radioactive compound intratracheally or
subcutaneously, low amounts of vanadium were detected between 8
and 16 days after administration as a result of reabsorption
from the organs. The highest level occurred 5 min after intra-
tracheal administration, 30 min after intraperitoneal admini-
stration, and 15 min - 1 h after subcutaneous or intragastric
administration. Vanadium was detected in all tissues and
organs. In 2 days, vanadium had accumulated in the bone, kidney,
liver, and lung, which were also the primary targets in rats
after intratracheal administration of vanadium pentoxide
(48V2O5) or chloride (48VO2C1) (Conklin et al., 1982) and after
oral administration of vanadyl sulfate or sodium orthovanadate
in the drinking-water (50 mg/litre) for three months (Parker &
Sharma, 1978).
In another study, 48VOCl3, which is fairly soluble, was
administered intratracheally to juvenile male Wistar rats at a
dose of 12.6 µCi in 1 ml buffered solution. Within 15 min, the
vanadium isotope was found in all major organs, except the
brain. The largest fractions were found in the blood, heart,
spleen, liver, and kidneys. Peak uptake occurred between 4 and
24 h after administration and activity was maintained throughout
a 9-week period (Oberg et al., 1978).
5.4 Retention
5.4.1 Human studies
Vanadium levels in human tissues (Table 18, section 5.3.1)
are low, the highest concentrations tending to occur in the
liver, kidney, and lung.
Storage of available vanadium in man occurs mainly in fat
and serum lipids (Schroeder et al., 1963).
Shevchenko (1965) used emission spectral analysis to
determine the vanadium contents of bone tumours and of the
cortical layer of bone adjacent to the tumours. He found that
vanadium accumulated in tumours. Healthy bone tissue contained
a mean vanadium concentration of 0.15 ± 0.002 mg/kg dry weight,
osteoblastomas contained 6.38 ± 1.17 mg/kg, and osteosarcomas,
4.16 ± 0.77 mg/kg. The cortical layer adjacent to
osteoblastomas contained 2.01 ± 0.42 mg/kg, and that adjacent to
osteosarcomas contained 1.77 ± 0.48 mg/kg. In comparison to
normal bone, bone cysts, osteochondromas, and exostoses also
showed higher vanadium levels, but to a lesser extent than bone
tumours.
The significant accumulation of vanadium in the tissue of
benign and malignant tumours and, to a lesser extent, in the
neighbouring tissue suggests that quantitative changes in the
amounts observed may indicate disturbances in its metabolism.
This may be in connection with the role of vanadium in the
phospholipid and cholesterol metabolism, which may become
indirect indicators of the intensity of the phospholipid
turnover in tumour tissue.
5.4.2 Animal studies
Large amounts of vanadium were reported in the crude fat
from beef, pork, and lamb (Schroeder et al., 1963), but the
results of subsequent studies do not support this finding, which
is probably erroneous because of the analytical methods used
(NRC, 1980).
In rats, after oral administration of 20 mg vanadium
nitrate/kg body weight, levels of vanadium in the blood were
detectable only during the first 24 h, and only traces remained
after 48 h; after 4 days, no vanadium was found in blood. For
48 h after the administration of vanadium, concentrations in the
organs increased by 0.4% of the administered dose in the liver,
by 0.69% in the kidneys, and by 0.16% in the spleen. The most
significant increase in vanadium (1.01% of the dose
administered) was found in the bones. After 16 days, the level
in the bone tissue had increased by 1.72% of the dose
administered, while in other organs it had significantly
decreased (Roshchin et al., 1980).
Studies on rats showed that liver, kidney, spleen, and
testes continued to accumulate intravenously injected vanadium-
48 for up to 4 h and that most of the radioactivity was retained
for up to 96 h (Hopkins & Tilton, 1966) . After 96 h, 14 - 84%
of the 10-min uptake was retained in other organs, and 46% and
9% of the vanadium-48 had been eliminated in the urine and
faeces, respectively. Levels of vanadium-48 in the
mitochondrial and nuclear fractions of homogenized liver
increased from 14 to 40% of the total during the first 96 h,
while the level of radioactivity in the microsomal fraction
remained relatively constant.
When strain A mice were given 80 mg vanadocene dichloride/kg
ip, vanadium accumulated in the liver and kidney. Maximum
concentrations of 1.12 ± 0.06 mmol in the kidney and 0.56 ±
0.06 mmol in the liver were reached after 12 h and 8 h,
respectively (Toney et al., 1985).
Table 20. Concentrations of 48vanadium in the organs and tissues of rats after intraperitoneal,
subcutaneous, intratracheal, and intragastric administration of a radioactive vanadium compound
(expressed as % radioactivity equivalent to 10 uCi per animal)a
---------------------------------------------------------------------------------------------------------
Organ/tissue Intraperitoneal administration Intratracheal administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 2.20 ± 0.55 0.43 ± 0.02 0.22 ± 0 0.43 ± 0.19 0.36 ± 0.19 1.25 ± 0.38
Kidney 7.20 ± 0.85 1.60 ± 0.02 0.66 ± 0 1.55 ± 0.67 1.56 ± 0.19 4.03 ± 0.74
Spleen 2.16 ± 0.23 0.17 ± 0.01 0.09 ± 0 0.15 ± 0.06 0.18 ± 0.02 1.92 ± 0.09
Lung 2.53 ± 0.73 0.11 ± 0 0 9.09 ± 5.91 0.20 ± 0.39 4.64 ± 2.11
Stomach 2.93 ± 0.73 0.08 ± 0 0 2.56 ± 0.94 0.13 ± 0.05 0
Small intestine 2.53 ± 0.31 0.08 ± 0 0 0.71 ± 0.22 0.21 ± 0.15 0
Large intestine 3.33 ± 0.88 0.13 ± 0.03 0 0.19 ± 0.08 0.17 ± 0.05 0.47 ± 0
Muscle 0.40 ± 0.05 0.03 ± 0 0 0.28 ± 0.20 0.03 ± 0 0
Bone 2.13 ± 0.12 3.45 ± 0.48 1.72 ± 0.31 0.79 ± 0.31 2.19 ± 0.31 9.70 ± 1.93
Testicle 1.30 ± 0.15 0.09 ± 0 0.06 ± 0 0.09 ± 0 0.08 ± 0 0.54 ± 0.08
Thyroid gland 0.60 ± 0 0.23 ± 0.05 0 1.21 ± 0.69 0.54 ± 0.40 0
Adrenals 2.53 ± 1.01 0.11 ± 0.02 0 0.67 ± 0 0.06 ± 0.02 0
Pancreas 7.88 ± 0.26 0.31 ± 0.06 0 0.14 ± 0.04 0.08 ± 0.02 0
Brain 0.16 ± 0.04 0.01 ± 0 0 0.02 ± 0 0 0
Heart 1.50 ± 0.34 0.07 ± 0 0 0.04 ± 0 0.08 ± 0 0.41 ± 0.15
---------------------------------------------------------------------------------------------------------
Table 20 (contd.)
---------------------------------------------------------------------------------------------------------
Organ/tissue Intragastric administration Subcutaneous administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 0.08 ± 0.03 0.33 ± 0.11 0.27 ± 0 0.05 ± 0.01 1.00 ± 0.15 1.67 ± 0.42
Kidney 0.17 ± 0.13 0.69 ± 0.20 0.67 ± 0 0.13 ± 0.03 2.30 ± 0 4.47 ± 1.48
Spleen 0.04 ± 0 0.16 ± 0.05 0.08 ± 0 0.03 ± 0 0.31 ± 0.03 1.38 ± 0.75
Lung 0.06 ± 0.02 0.09 ± 0.01 0.06 ± 0 0.10 ± 0.01 0.19 ± 0.01 0.89 ± 0.18
Stomach 8.08 ± 1.49 1.55 ± 0.05 0.05 ± 0 0.29 ± 0.01 0.12 ± 0 0.39 ± 0.06
Small intestine 4.65 ± 9.95 0.22 ± 0.09 0.06 ± 0 0.77 ± 0.66 0.11 ± 0.01 0
Large intestine 0.12 ± 0 0.14 ± 0 0.01 ± 0 0.46 ± 0.21 0.14 ± 0 0
Muscle 0.01 ± 0 0.02 ± 0 0.04 ± 0 0.40 ± 0.30 0.03 ± 0 0.46 ± 0.15
Bone 0.05 ± 0.02 1.01 ± 0.31 1.72 ± 0 0.43 ± 0.30 3.23 ± 0.41 1.86 ± 0.52
Testicle 0.26 ± 0.20 0.04 ± 0 0.06 ± 0 0.01 ± 0 0.12 ± 0.01 0.64 ± 0.07
Thyroid gland 0.08 ± 0 0.32 ± 0.24 0 0.15 ± 0.05 0 0
Adrenals - 0.09 ± 0.03 0 0.10 ± 0 0.16 ± 0.04 0.39 ± 0
Pancreas 0.32 ± 0.29 0.04 ± 0.04 0.08 ± 0 0.03 ± 0 0.09 ± 0.01 0.83 ± 0.04
Brain 0.02 ± 0 0.01 ± 0 0.02 ± 0 0.03 ± 0 0.02 ± 0 0
Heart 0.06 ± 0 0.05 ± 0 0 0.06 ± 0 0.12 ± 0 0.81 ± 0.08
---------------------------------------------------------------------------------------------------------
a From: Roshchin et al. (1980).
In a study on 2 young rats (strain unspecified), the highest
uptake of vanadium-48 from V205 occurred in rapidly mineralizing
areas of dentine and bone (Söremark et al., 1962). In mice,
high uptake occurred in the mammary glands, liver, renal cortex,
and lung (Söremark & Ullberg, 1962).
In studies by Belehova (1966, 1969), vanadium levels were
lower in carious than in normal canine teeth. In other studies,
intramuscular injection of vanadium in dogs at a dose of 2 mg/kg
body weight resulted in vanadium levels in the hard tissues of
the teeth that were 1.3 times higher than those in the controls.
On the 7th day, the concentration in the enamel had almost
returned to its initial level of 8.8 mg/kg; the level in the
dentine was still 32% higher.
Vanadium was reported to decrease the incidence of dental
caries, when added to the diet of hamsters (Geyer, 1953).
However, Hein & Wisotzky (1955) reported significant increases
in dental caries in hamsters given drinking-water containing
vanadium pentoxide equivalent to 10 mg vanadium/litre over an
80-day period. Muhler (1957) studied the effect of vanadium
pentoxide on caries in Sprague-Dawley rats. Groups of 50 rats
received vanadium (as vanadium pentoxide) at 10 mg/litre,
20 mg/litre, or 40 mg/litre in their drinking-water. One
control group received drinking-water containing ammonium
fluorosilicate (20 mg/litre), the other received untreated
water. Vanadium did not produce any reduction in the incidence
of dental caries, in fact there was a slight, but not
significant increase in dental caries in the groups receiving
10 mg/litre and 20 mg/litre for 140 days. All the rats showed
signs of vanadium toxicity and all animals in the highest dose
group (40 mg/litre) died within 65 days.
In an in vitro study on the effects of vanadate on bone
formation in 21-day-old fetal rat calvariae, sodium vanadate at
concentrations of 0.1 - 10 mmol stimulated the incorporation of
3H-thymidine into DNA and increased the bone DNA content and the
mitotic index. Sodium vanadate at a concentration of 100 mmol
produced a marked and irreversible inhibition of DNA labelling
and protein synthesis. Concentrations of 1 mmol (24 h) and
10 mmol (96 h) inhibited alkaline phosphatase activity. Sodium
vanadate also stimulated collagen and non-collagen protein at
low concentrations, but again had an irreversible inhibitory
effect at a high concentration of 100 mmol (Canalis, 1985).
5.5 Elimination
5.5.1 Human studies
Owing to low gastrointestinal absorption, ingested vanadium
is predominantly eliminated unabsorbed in the faeces. The
principle route of excretion of absorbed vanadium is through the
kidneys.
The relationship between urinary excretion and the extent of
exposure has been studied. As part of a clinical study on the
effects on cholesterol level, Dimond et al. (1963) gave ammonium
vanadyl tartrate to patients (5 female, 1 male) and did not find
any correlation between urinary excretion and oral dose.
Variable absorption was suggested as the reason for wide
variation in urinary excretion. In a 50-week study on 2
volunteers, Tipton et al. (1969) reported a urine/diet ratio of
0.13. This is in agreement with the figure of 12.4% excretion
in urine in a man given sodium metavanadate orally (12.5 mg
daily for 12 days) (Proescher et al., 1917).
Studies on occupationally exposed populations have shown a
poor correlation between vanadium concentrations in air and
amounts excreted in urine (Williams, 1952; Lewis, 1959b;
Jaraczewska & Jakubowski, 1964; Watanabe et al., 1966; Troppens,
1969; Köhler, 1972). Differences in laboratories and methods
may contribute to the wide range of urinary concentrations
reported.
In a study on power-station workers exposed to vanadium
during maintenance work on an oil-fired boiler, it was shown
that urinary excretion increased in those most heavily exposed,
in spite of the use of protective masks. For instance, urinary
levels of vanadium in 6 welders and 4 cleaners increased over a
work shift from 2.7 to 43.8 mg vanadium/kg creatinine and from
1.65 to 52.8 mg/kg, respectively. The vanadium concentration
in air during boiler cleaning was estimated to vary between 0.1
and 5 mg/m3 (Maroni et al., 1983). These results resemble those
reported by Thürauf et al. (1979) on 54 workers exposed to
vanadium in a metallurgical plant. Exposed workers had a urinary
vanadium concentration of 33.9 mg/kg creatinine, whereas the
level in unexposed workers was 0.6 mg/kg creatinine.
Roshchin et al. (1980) used polarography in a study of the
urine samples of 100 workers who were exposed daily to vanadium
pentoxide, and found vanadium in 50% of subjects. The mean
concentration of vanadium in the urine was 0.18 ± 0.03 mg/litre.
In workers with a duration of exposure in the range of 1 - 2
years, the mean concentration was 0.14 ± 0.08 mg/litre; after 2
years, the mean concentration was 0.20 ± 0.009 mg/litre. In
workers employed for 2 - 6 months, the mean concentration of
vanadium in the urine was 0.16 ± 0.12 mg/litre. There appeared
to be a correlation between the urinary levels and the
concentration of vanadium in the air. For example, in workers
exposed to mean vanadium pentoxide atmospheric concentrations of
0.28 ± 0.06 mg/m3, the concentration in the urine was 0.20 ±
0.05 mg/litre; at an atmospheric concentration of 0.17 ±
0.01 mg/m3, the urinary concentration was 0.19 ± 0.007
mg/litre.
The relationships between personal exposure and blood and
urine levels of vanadium were examined in 16 workers in a
ferrovanadium plant in Norway (Gylseth et al., 1979).
Individual levels did not show any clear relationship between
exposure and response, but, when the data were divided into
high- and low-exposure groups, significant differences were
found for both blood and urine levels in relation to exposure
levels. There was also a reasonably good correlation between
blood and urine levels. However, the authors concluded that
"the differences are small and the method difficult and
expensive, so for a routine control other criteria should be
sought".
Kiviluoto et al. (1979a,c) studied serum- and urinary-
vanadium levels in relation to exposure levels. Grouped and
individual data were examined at intervals during vacation
periods and compared with those for an unexposed group. The
levels of exposure were low (0.01 - 0.05 mg/m3), and no
correlations between exposure levels and serum or urine
concentrations were found. However, there was a conspicuous
decline in the urine levels at the beginning of the vacation
period, but they did not decline down to the level of the
unexposed group. It was considered that this provided some
measure of the extent of exposure.
5.5.2 Animal studies
Talvitie & Wagner (1954) administered sodium metavanadate
monohydrate in saline to albino Webster rats and albino rabbits
by intraperitoneal and intravenous injection, respectively. In
one part of the study, rats were given a single ip dose
equivalent to 0.5 mg vanadium/kg body weight and, in another,
twice daily ip injections, each equivalent to 0.25 mg
vanadium/kg body weight, for 5 days. Rabbits were injected
intravenously twice daily for a total of 7 or 9 days, with doses
equivalent to 0.25 mg vanadium/kg body weight, except for 1
rabbit that received doses of 0.4 mg/kg body weight for the
second 5-day period. No histological changes were noted. The
ratio of vanadium eliminated in the urine and faeces was 5:1.
Following oral administration of vanadium sulfate and
vanadium pentoxide to guinea-pigs (equivalent to 2 mg vanadium/
kg body weight), Reznik (1954) found vanadium in the urine and
faeces for 7 - 10 days, though elimination in the urine ceased
before that in faeces. The author concluded that the presence
of vanadium in the faeces, several days after administration,
was due to its return to the intestine after internal resorption
and excretion. Biliary excretion of less than 2% of the
intravenously injected dose (between 0.9 and 30 µg penta-
vanadate/kg body weight) was demonstrated in rats during the
first 6 h after administration. By comparison, 20% was excreted
with the urine during the same period of time (Sabbioni et al.,
1981).
Roshchin (1968) administered a dose of 3 mg vanadium
trichloride to 180-g albino rats. Most of the vanadium was
excreted via the kidneys. Of the dose administered, 18% was
found in the urine after 24 h and 25%, after 48 h. The amount
of vanadium excreted in the urine fell and, after 6 days, was
low. Elimination of vanadium via the intestine occurred on a
much smaller scale and remained relatively stable; 30.9% of the
administered dose was excreted over a 6-day period. Thus, the
largest quantity of vanadium was eliminated during the first 2
days, and the rest was eliminated gradually. The ratio of
amounts eliminated in the urine and faeces was 5:1,
corroborating the findings by Talvitie & Wagner (1954).
In a study on mice, rats, and dogs, Mitchell & Floyd (1954)
showed that ascorbic acid increased vanadium elimination in the
urine during the first few days and later in the faeces.
CaNa3DTPA (salicylic salt of diethylenetriamine-pentaacetic
acid) increased vanadium excretion in the urine and reduced
elimination via faeces. Following the combined administration
of both preparations, elimination in the faeces increased.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Microorganisms and higher plants
Trace quantities (1 - 10 µg/litre) of vanadium stimulated
the growth of some algae, including Scenedesmus or Chlorella
(Arnon & Wessel, 1953; Hopkins et al., 1977; Patrick, 1978).
Toxicity studies on phytoplankton, mainly using pentavalent
vanadium, have revealed differences in susceptibility between
various species. A concentration of 0.02 mg/litre, as ammonium
vanadate, interfered with the cell division of the fresh-water
algae Chlorella pyrenoidosa, whereas 0.25 mg/litre was lethal
(Meish & Benzschawel, 1978). The 15-day LC50 for an estuarine
and salt-water green alga (Dunaliella marina) was given as
0.5 mg/litre of sodium metavanadate and that of a salt-water
pennote diatom (Asterionella japonica) as 2 mg/litre (Minamand &
Unsal, 1978).
Vanadium does not appear to be essential for higher plants.
6.1.2 Invertebrates
In a study using sea-water with a background level of 0.0017
mg vanadium/litre, the 9-day LC50s for vanadium (as sodium
metavanadate) for the worm (Nereis diversicolor), mussel
(Mytilus galloprovincialis), and crab (Carcinas maenas) were
10, 35, and 65 mg vanadium/litre, respectively (Miramand &
Unsal, 1978). Some marine invertebrates, such as the tunicates,
accumulate vanadium to levels that may be 10-5 to 10-6 times the
sea-water concentrations (Table 3.). Vanadium levels in such
species may exceed 3000 mg/kg dry weight (Biggs & Swinehart,
1976; Carlson, 1977). It was stated that the uptake of vanadium
by the mussel (Mytilus edulis) from food (plankton) was of the
same magnitude as that from water (Unsal, 1978). It appears
that benthic aquatic organisms tolerate higher concentrations of
vanadium than fish (section 6.1.3).
6.1.3 Fish
There are some data on the acute toxicity of vanadium for
fish (Van Zinderen Bakker & Jaworski, 1980). The 4- to 6-day
LC50s for fresh-water species are in the range 0.5 -10 mg/litre.
Factors influencing toxicity include water hardness, and the
LC50 values are higher in hard water. Giles et al. (1979)
studied the influence of pH on the toxicity of vanadium
pentoxide for rainbow trout fingerlings. The 96-h LC50 ranged
from 6.43 to 21.75 mg/litre. There was some indication of
vanadium pentoxide being most toxic at a pH of 7. Also, Sprague
et al. (1978) tested zebrafish (Brachydanio rerio) with vanadium
pentoxide and found that a pH of 7.5 provided the most toxic
conditions. At this pH, death occurred between 23.5 and 45 h at
a concentration of 22 mg/litre, whereas at pH 8.2, the time
decreased to 32 h and, at pH 8.8 - 9, to 37 - 39 h.
Studying the chronic effects of vanadium pentoxide on
flagfish, Sprague et al. (1978) and Holdway & Sprague (1979)
concluded that the sublethal threshold concentration would be
about 0.08 mg vanadium/litre.
The rainbow trout (Salmo gairdneri) is the most commonly
used fish for toxicity studies. Sprague et al. (1978) reported
7-day LC50 values in one series of studies ranging from 2.4 to
5.6 mg/litre. Increasing the exposure time resulted in
progressively lower LC50 values, the lowest being 1.99 mg/litre
for an 11-day exposure period. Similar results were reported by
Giles et al. (1979) using experimental conditions of pH 8,
15 °C, and hardness 90 mg CaCO3/litre. The LC50 values
decreased from 4.34 mg CaCO3/litre for 5 days exposure to 1.95
mg CaCO3/litre for 14 days. Neither of these groups was able to
define a minimum lethal level for rainbow trout. Studies by
Stendahl & Sprague (1982) indicated that small rainbow trout
were more resistant than larger fish to vanadium pentoxide. In
general, rainbow trout eggs were 10 - 15 times more resistant to
pentavalent vanadium than fingerlings, suggesting the
possibility of a protective function by the chorion (Giles et
al., 1979). It should be noted that the available studies on
vanadium toxicity have been performed on fresh-water species
only. The effects of salinity remain to be studied.
6.2 Terrestrial Organisms
6.2.1 Uptake of vanadium by plants
In general, the highest concentrations of vanadium in plants
growing in natural soils occur in the roots and decrease towards
the aerial portions of the plant. The concentration of vanadium
in soil is, by and large, 10 times the concentration in the
plant (Cannon, 1963). Absorption appears to be passive (Welch,
1973).
When grown in culture solution, several plant species absorb
vanadium, which is also translocated to the aerial parts and
seeds (Hopkins et al., 1977).
6.2.2 Effects on plants
Vanadium has not been demonstrated to be essential for
higher land plants (Hopkins et al., 1977). However, traces of
vanadate (0.02 mmol vanadium as VO2+ or VO3-) were shown to
promote chloroplast development and oxygen production in higher
plants. Vanadium also had a function as a redox catalyst in the
electron flow from photosystem II to photosystem I (Meisch &
Becker, 1981).
Small amounts of aqueous vanadium (10 - 20 mg/litre) have
detrimental effects on most plants (Cannon, 1963). The growth
of flax, peas, soybeans, and cabbage was reduced in nutrient
solutions containing 0.5 mg vanadium/litre (given as VOCl2 or
VCl3) (Warington, 1955; Hara et al., 1976). Vanadium can
induce iron deficiency chlorosis (Cannon, 1963) or affect the
trace element nutrition by reducing the levels of, e.g.,
manganese, copper, calcium, and phosphorus (Warington, 1955;
Wallace et al., 1977). Similarly, 5 mg vanadium/litre (as VO3-)
in irrigation water reduced the growth of sugar beets by 30 -
50% and caused iron deficiency chlorosis (Hewitt, 1953).
In soils, the toxicity of vanadium may range between 10 and
1258 mg/kg, depending on plant species and type of soil (Hopkins
et al., 1977). Ten mg/kg added to sandy soil as Ca(VO3)2
decreased the growth of sour orange, whereas 150 mg/kg was
lethal (Vanselow, 1950).
Fertilizers may have a high vanadium content. For instance,
rock phosphate, super phosphate, and basic slag may contain
several thousand mg vanadium/kg. These may cause unacceptable
levels of accumulation of vanadium in soil (Mitchell, 1964;
Hopkins et al., 1977). Urban sewage sludges usually contain
less than 35 mg/kg of vanadium (Bradford et al., 1975; Oliver &
Cosgrove, 1975). On the other hand, vanadium in sewage sludge
may be up to 6 times more easily available in sludge than in
soil (Bernow & Webber, 1972 ).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 General Toxicity
The toxicity of vanadium for experimental animals varies
with the species and route of administration. Smaller animals,
including the rat and mouse, tolerate the metal well; the rabbit
and horse are more sensitive (Hudson, 1964). In general,
toxicity is low when the metal is given by the oral route,
moderate by the respiratory route, and high by injection.
Lethal doses for various vanadium salts injected intravenously
in rabbits and subcutaneously in guinea-pigs, rats, and mice are
shown in Table 21.
Table 21. Lethal doses of vanadium (mg V2O5/kg body weight) in
experimental animalsa
-----------------------------------------------------------------------------
Compound Rabbitb Guinea-pig Rat Mouse
-----------------------------------------------------------------------------
colloidal vanadium 1 - 2 20 - 28 - 87.5 - 117.5
pentoxide
ammonium metavanadate 1.5 - 2 1 - 2 20 - 30 25 - 30
sodium orthovanadate 2 - 3 1 - 2 50 - 60 50 - 100
sodium pyrovanadate 3 - 4 1 - 2 40 - 50 50 - 100
sodium tetravanadate 6 - 8 18 - 20 30 - 40 25 - 50
sodium hexavanadate 30 - 40 40 - 50 40 - 50 100 - 150
vanadyl sulfate 18 - 20 34 - 45 58 - 190 125 - 150
sodium vanadate - 30 - 40 10 - 20 100 - 150
-----------------------------------------------------------------------------
a From: Hudson (1964).
b Rabbits were injected intravenously; other animals subcutaneously.
The toxicity of vanadium also varies considerably with the
nature of the compound, vanadium being toxic both as a cation
and as an anion. As a general rule, toxicity increases as
valency increases, vanadium5+ being the most toxic. Among the
oxides of vanadium, vanadium pentoxide is more soluble and more
toxic than the trioxide or dioxide.
Roshchin (1967b, 1968) described the results of acute
inhalation studies on albino rats exposed to vanadium pentoxide
in the form of a condensation aerosol (fume) at 10 - 70 mg/m3
or in the form of a dispersion aerosol (dust) at 80 - 700 mg/m3,
ammonium vanadate (presumably as a dispersion aerosol) at
1000 mg/m3, and ferrovanadium, as a dispersion aerosol at 1000 -
10 000 mg/m3. The minimum concentration of vanadium pentoxide
(condensation aerosol) that gave rise to mild signs of acute
poisoning was 10 mg/m3 air. The absolute lethal concentration
for the condensation aerosol was 70 mg/m3. Dispersion aerosols
(containing large particles) were only one-fifth as toxic as
condensation aerosols. Inhalation of dispersion aerosols of
ferrovanadium did not produce any acute toxic effects, probably
because the particles were too large. However, acute toxic
effects were observed following intratracheal instillation of
ferrovanadium. These may have been related to the biological
solubility and the extent of absorption.
The effects of vanadium on experimental animals have been
investigated in a number of studies using various compounds,
species, and test protocols. Some representative acute and long-
term studies are summarized in Tables 22 and 23. Subsequent
discussions on the effects of vanadium on specific biological
systems are frequently based on the results of these studies.
7.2 Effects on Metabolic Processes
Rabbits exposed to a dispersion aerosol of vanadium trioxide
(40 - 75 mg/m3, 2 h/day for up to 12 months) exhibited a
progressive weight loss amounting to an average of 4.6% at the
termination of the study. Control animals gained weight by an
average of 12.3%. The number of white blood cells declined by
the end of the test from between 7000 and 8000 down to 5000/mm3;
no change was noted in controls. Haemoglobin levels in the test
animals decreased from 75 to 68% of normal levelsa. Serum-
ascorbate levels in the blood progressively decreased to about
20% of controls between 7 and 8 months. Protein sulfhydryl
levels in the serum of exposed animals decreased by 30% compared
with those of the controls. Respiration in the liver and brain
tissues of test animals was reduced by one-half by the end of
the study compared with controls, but the respiratory quotient
was unchanged. Blood-cholinesterase levels in exposed rabbits
increased by an average of 25% after the fifth month (Roshchin
et al., 1964; Roshchin, 1968).
In these studies, the weight loss together with the
depression in levels of white cells, haemoglobin, and protein
sulfhydryl groups in the blood and the decreased liver tissue
respiration were taken as indicators of the "general toxic
effect" of vanadium. Increased cholinesterase activity was held
to be indicative of sensitization.
_________________
a Normal haemoglobin level in the rabbit is 80 - 150 g/litre
and normal rabbit haematocrit, 30 - 50%.
Table 22. Acute studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Dose Concentration Reference
administration index or dose
---------------------------------------------------------------------------------------------------------
vanadium pentoxide mouse intragastric LD50 23.4 mg/kg body weight Roshchin (1967a)
rat inhalation LC50 70 mg/m3 Roshchin (1967a)
rat inhalation minimum 10 mg/m3 Roshchin (1967a)
effective
rat inhalation LC50 70 mg/m3 Sjöberg (1950)
cat inhalation LC50 500 mg/m3 Faulkner Hudson
(1964)
rabbit inhalation LC100 205 mg/m3 Sjöberg (1950)
rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
ammonium vanadate mouse intragastric LD50 10 mg/kg body weight Roshchin (1968)
rat intragastric effective 20 mg/kg body weight Kaku et al. (1971)
rat subcutaneous effective 5-30 mg/kg body weight Kaku et al. (1971)
vanadium mouse intragastric LD50 24 mg/kg body weight Roshchin (1968)
trichloride
vanadium di-iodide mouse intragastric LD50 68 mg/kg body weight Roshchin (1968)
vanadium dibromide mouse intragastric LD50 88 mg/kg body weight Roshchin (1968)
vanadium trioxide mouse intragastric LD50 130 mg/kg body weight Reznik (1954)
ammonium rat intragastric LD50 10 mg/kg body weight Gulko (1956)
metavanadate
vanadyl sulfate rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
rabbit subcutaneous LC50 59.1 mg/kg body weight Korkhov (1965)
rabbit subcutaneous maximum 25 mg/kg body weight Korkhov (1965)
tolerated
guinea-pig subcutaneous LD100 800 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous LD50 560 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous maximum 300 mg/kg body weight Kopylova (1971)
tolerated
water-soluble mouse intragastric LD50 5 mg/kg body weight Seljankina (1961)
vanadium compounds mouse intragastric no effect 0.005 mg/kg body weight Seljankina (1961)
(or 0.1 mg/litre
in water)
---------------------------------------------------------------------------------------------------------
Table 23. Long-term studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Concentration Duration of Reference
administration exposure
---------------------------------------------------------------------------------------------------------
vanadium pentoxide rabbit inhalation 20 - 40 mg/m3 several months Sjöberg (1950)
rabbit inhalation 25 mg/m3 10 months Gulko (1956)
guinea- inhalation 25 mg/m3 10 months Gulko (1956)
pig
rabbit inhalation 8 - 18 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat inhalation 10 - 30 mg/m3 several months Roshchin (1967b)
rat inhalation 3 - 5 mg/m3 several months Roshchin (1967b)
rat inhalation 0.027 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.002 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.006 mg/m3 40 days Pazhynich (1966)
ammonium rat subcutaneous 1 mg/kg 30 days Kaku et al. (1971)
metavanadate
sodium guinea- subcutaneous 3.2 - 128 ug/kg days Kulieva (1974)
metavanadate pig
guinea- subcutaneous 5.12 mg/kg days Kulieva (1974)
pig
vanadium trioxide rabbit inhalation 40 - 75 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
vanadium rabbit oral 5 mg/kg 2 - 3 months Roshchin (1968)
trichloride
vanadium carbide rabbit inhalation 40 - 80 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
ferrovanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
metallic vanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
---------------------------------------------------------------------------------------------------------
Chronic poisoning, following the inhalation of trivalent
vanadium (V203) or the oral administration of vanadium tri-
chloride (VCl3), resulted in blood changes by the end of the
second and third months (Table 24). These changes were
characterized by decreased albumin and increased globulins
(mainly y-globulins) with a halving of the albumin-globulin
ratio, and by an increase in serum concentrations of the amino
acids cystine, arginine, and histidine. There was also a 10%
increase in nucleic acid levels in the blood and a
"considerable" increase in the blood-chloride concentration.
The effects of vanadium on the metabolism of proteins and
nucleic acids were considered to be responsible for the
immunological and allergic reactions that may occur in vanadium
poisoning (Roshchin, 1967a).
Metabolic changes were observed in a study by Pazhynich
(1966) in which albino rats were exposed by inhalation for 70
days to condensation aerosols of vanadium pentoxide at levels of
0.027 ± 0.002 mg/m3 and 0.002 ± 0.00013 mg/m3. Statistically
significant changes were observed at the higher level of
exposure, but not at the lower level. The observations included
decreases in: whole blood-cholinesterase levels, total serum-
protein levels, serum-globulin levels, and the oxyhaemoglobin
content of venous blood. Elevated serum-globulin levels,
increased numbers of blood leukocytes showing yellow, orange,
and red nuclear fluorescence with acridine orange, and increased
oxygen consumption as indicated by isolated liver preparations
were also observed in the high-level exposure group. The
pattern of leukocyte nuclear fluorescence returned to normal 20
days after cessation of exposure. In a second study, albino
rats were continuously exposed to vanadium pentoxide at 0.006 ±
0.00056 mg/m3 for 40 days. No changes in blood-leukocyte
nuclear fluorescence were observed. During the sixth week of
exposure, animals received water but no food. After 3.5 days of
this treatment, the number of leukocytes displaying altered
nuclear fluorescence increased by a factor of 4.83.
In an in vitro study, ammonium metavanadate was found to
inhibit microsomal ketamine N -demethylation, lipid peroxida-
tion, and hydrogen peroxide formation in rat liver. The
inhibiting doses of NH4VO3 ranged from 10-5 to 10-3 mol/litre.
Cytochrome c reductase was also inhibited, whereas cytochrome
oxidase activity was stimulated (Beyhl, 1983).
Parenteral injection of guinea-pigs with sodium metavanadate
in daily doses of 3.2 µg/kg, 128 µg/kg, or 5.12 mg/kg body
weight resulted in dose-dependent increased succinate dehydro-
genase activity in the liver and kidneys and cytochrome oxidase
activity in the liver (Kulieva, 1974).
Table 24. Respiratory effects of vanadium pentoxide on experimental animalsa
---------------------------------------------------------------------------------------------------------
Reference Species Form Concentration Exposure Pathological findings
(mg/m3)
---------------------------------------------------------------------------------------------------------
Sjöberg (1950) rabbit dust 205 7 h conjunctivitis, tracheitis,
pulmonary oedema, broncho-
pneumonia, enteritis, fatty
liver, death
Sjöberg (1950) rabbit dust 20 - 40 1 h/day chronic rhinitis, trache-
several months itis, emphysema, atelect-
asis, bronchopneumonia,
pyelonephritis
Gulko (1956) rabbit dust 10 - 30 continuous, bronchitis, pneumonia,
acute weight loss, bloody diarr-
hoea
Roshchin (1963) rat dust, 80 - 700 continuous, haemorrhagic inflammation in
fume 10 - 70 acute lungs, haemorrhage in inter-
nal organs, paralysis, res-
piratory failure, death
Roshchin (1963) rat dust, 10 - 30 2 h/day, haemorrhagic inflammation in
fume 3 - 5 several months lungs purulent bronchitis,
pneumonia
Pazhynich (1966) rat fume 0.027 continuous haemorrhagic inflammation in
70 days lungs, vascular congestion,
haemorrhage in liver, kid-
neys, and heart
---------------------------------------------------------------------------------------------------------
a From: Waters (1977).
In acute studies on rats (Donryu strain) weighing 200 g,
Kaku et al. (1971) administered vanadium (as ammonium vanadate)
by gavage at a dose of 20 mg/kg body weight or injected it
subcutaneously (doses of between 5 and 30 mg/kg body weight).
There were dose-dependent reversible increases in the trigly-
ceride concentrations in the liver and blood-serum, a decrease
in the serum-cholesterol level, and increases in glutamate-
oxalo-acetate transaminase and glutamate-pyruvate transaminase
activity. After subcutaneous injection, there was a fall in
cholesterol levels. The lowest values were reached 24 h after
the injection; values then returned to normal. Dose-dependent
increases were also observed in the concentrations of triglyc-
erides in the liver and serum. Levels peaked 48 h after the
injection and then gradually declined. When ammonium vandate
solution equivalent to 1 mg vanadium/kg body weight was
administered daily by subcutaneous injection for 30 days more
marked changes in serum cholestrol were observed.
Korkhov (1965) injected 0.3 mg vanadyl sulfate/kg body
weight subcutaneously in rabbits with experimental athero-
sclerosis and observed a lowering of the hypercholesterolaemia
and inhibition of the rise in lecithin. Combined administration
of vanadyl sulfate and phenylethylacetic acid lowered the aortic
cholesterol level 3.5 times compared with controls. Korkhov
(1965) also showed that cholesterol biosynthesis in liver tissue
culture was inhibited by the addition of 10-4 vanadyl sulfate.
When 30 rabbits with experimental atherosclerosis were
administered a mixture of trace elements (copper, cobalt,
manganese, and zinc) in combination with cholesterol, Babenko &
Vandzhura (1969) detected increases in the blood lipids and
decreases in vanadium concentrations, compared with healthy
control animals. The same results were noted following combined
administration of copper and cobalt plus cholesterol at
0.2 g/kg. After administration of manganese, the decrease in
the vanadium concentration was delayed; after administration of
zinc, vanadium remained at the same level as in healthy animals.
Vanadium concentrations in body tissues decreased as cholesterol
levels increased, but manganese and zinc, when given together
with cholesterol, helped maintain vanadium concentrations in the
tissues of the body, the highest vanadium concentrations being
found in the liver, aorta, and muscles.
In the studies by Novakova et al. (1981), daily oral
administration to rabbits for 4 months of vanadium pentoxide and
cholesterol (1st group: vanadium at 0.5 mg/kg body weight and
cholesterol at 0.3 mg/kg body weight; 2nd group: vanadium at
1.5 mg/kg body weight and cholesterol at 0.5 mg/kg body weight)
resulted in high levels of cholesterol in the blood and
extensive atherosclerosis of the aorta. In the animals
administered either cholesterol (0.5 mg/kg body weight) or
vanadium (0.5 mg/kg body weight), hypercholesterolaemia was also
observed, but the increase was less pronounced. Increases in
levels of lipids, lipoproteins, and triglycerides were also
observed. After one month of administration, cholesterol levels
in treated animals were more than ten times those in the
controls. At the end of the study (after the 4th month), the
cholesterol levels in the treated animals were considerably
higher than those in the controls.
The effects of vanadium on iron metabolism have not been
fully elucidated. In some studies, a stimulative effect on
haemoglobin and erythrocyte levels has been claimed. The
results of studies by Myers & Beard (1931) suggested that
vanadium chloride given at 0.6 mg/kg diet to rats, previously
rendered anaemic, had a favourable effect on the haemoglobin
level, and Kopylova (1971) obtained increases in the erythrocyte
count and haemoglobin level in rabbits by subcutaneous admini-
stration of vanadyl sulfate (1 mg/kg body weight, daily, for 2
months). Trummert & Boehm (1957) observed an increase in the
erythrocyte count following intravenous injection of vanadium
gluconate (0.3 - 1.5 mg/kg body weight, daily, for 40 days), but
the haemoglobin level was not significantly affected.
7.2.1 Mechanisms of action
Many of the metabolic effects observed can be explained by
the biochemical effects of vanadium exposure in vivo and in
vitro.
Roschin (1967a) presented the hypothesis that the mechanism
of the initial step in the non-specific haematopoietic effect of
vanadium and the subsequent anaemia was the inhibition of the
redox system of hydrogen carriers. In response to the resulting
hypoxia, there is increased haematopoiesis. Possibly vanadium
interferes with tissue respiration at the stage of dehydrogena-
tion effected by nicotinamide adenine dinucleotide (NAD). By
inhibiting this coenzyme, vanadium interferes with the
incorporation of iron in the porphyrin complexes and haemoglobin
synthesis. The anti-vitamin C effect of vanadium and the
consequent vitamin C deficiency also inhibits the utilization of
iron for haemoglobin synthesis. Iron accumulates in the
reticuloendothelial tissue.
It is known that vanadium also inhibits the activity of
monoamine oxidase, which catalyses the conversion of serotonin
to 5-hydroxyindoleacetic acid. In a study on rabbits exposed
to vanadium pentoxide dust for 3 months, urine levels of 5-
hydroxyindoleacetic acid fell to 33% below control values
(Roschin, 1967a). It was suggested by the author that
inhibition of monoamine oxidase might result in accumulation of
serotonin in the central nervous system, leading to functional
disturbances, such as bronchospasm, diarrhoea, and urinary
incontinence. Elevated serotonin levels could also be
responsible for the dystrophic and necrotic process in the
kidneys and the high permeability of the blood vessels. A
decrease in sulfhydryl groups in blood-proteins and a reduction
in the cystine content of keratinized tissues might be due to an
interaction between vanadium and an unspecified enzyme.
The catabolism of cystine and cysteine was increased by
exposure to vanadium (Bergel et al., 1958). In vitro, pyridoxal
5-phosphate induced the catabolism of sulfhydryl amino acids in
the presence of VO2+ (Anbar & Inbar, 1962). The authors
suggested that the activation of pyridoxal phosphate by vanadyl
ions was specific to elimination and strongly suggested a
decrease in -SH groups in the organism. A reduction in cystine
levels was observed in keratinized tissues (hair of rats fed
vanadium compounds and the fingernails of vanadium workers)
by Mountain et al. (1953, 1955). It was suggested that this
effect was the result of decreased synthesis of cysteine and
cystine and that metabolic processes depending on either of
these amino acids may be depressed in the presence of vanadium.
According to Mahler & Cordes (1966), coenzyme A plays a
central role in many biosynthetic and oxidative pathways. In
the biosynthesis of coenzyme A, cysteine reacts with 4-
phosphopantothenic acid in the presence of adenosine triphos-
phate (ATP) to form the intermediate 4'-phosphopantothenyl
cystine, and Mascitelli-Coriandoli & Citterio (1959a,b) showed
that treatment with sodium metavanadate reduced the content of
coenzyme A in rat liver.
As coenzyme A is involved in biochemical pathways starting
with acetate, these processes can also be affected by vanadium;
Curran (1954) showed that the synthesis of cholesterol from 14C-
acetate in rat liver was reduced in the presence of vanadium.
Later studies indicated that one site of the inhibitory action
of vanadium in the synthesis of cholesterol was at the level of
the enzyme squalene synthetase, which catalyses the conversion
of farnesyl pyrophosphate to squalene (Azarnoff & Curran, 1957;
Azarnoff et al., 1961). In a study by Curran & Costello (1956),
aortic cholesterol was mobilized more rapidly in vanadium-
treated atherosclerotic rabbits than in controls. Cholesterol
levels appear to be reduced by vanadium in young animals,
including human beings, but not in older ones. It has been
suggested, but not demonstrated, that a regulatory enzyme in the
synthesis of cholesterol (acetoacetyl coenzyme A deacylase) is
activated by vanadium in the former but inhibited in the latter.
Vanadium might reduce the synthesis of triglycerides and
phospholipids, since acetyl coenzyme A is a precursor of fatty
acids. However, the results of studies by Curran and colleagues
showed that, while the levels of triglycerides decreased in the
livers of rats given vanadium (Curran, 1954), serum-
triglycerides levels in human beings increased following the
ingestion of vanadium (Curran et al., 1959). Snyder & Cornatzer
(1958) reported that the incorporation of labelled phosphate
into rat liver phospholipids decreased following injection of
vanadyl sulfate. This could have been due to the inhibition of
phospho-lipid biosynthesis or to increased oxidative
degradation, as originally suggested by Bernheim & Bernheim
(1938, 1939). Using an isotope method with radioactive
phosphorus as the indicator, Prokopenko (1961) observed a
disturbance in the intensity of phosphorus exchange between
organic acid-soluble compounds and inorganic phosphates in
albino rats and mice with acute ammonium vanadate poisoning. No
changes were found in the intensity of total metabolism and in
liver tissue phosphate levels. After administration for 6
months in doses of 1 mg/kg body weight, phosphorylation
processes in the organs and tissues were disturbed and the
concentration of inorganic phosphorus in the blood and urine was
increased.
Coenzyme A is also involved in the synthesis of coenzyme Q
(ubiquinone) in the mitochondrial electron transport chain.
Ubiquinone synthesis in isolated rat mitochondria was reduced by
vanadium, but this effect was partially reversed when cysteine
was given with vanadium. Addition of ATP and coenzyme A
prevented the inhibition of ubiquinone synthesis (Aiyar &
Sreenivasan, 1961).
It has been suggested that mitochondrial oxidative
phosphorylation in liver homogenates in vitro is uncoupled by
vanadium with a resulting depletion in ATP energy stores (Wright
et al., 1960). In young chicks, the addition of ammonium
metavanadate to the diet equivalent to 25 mg vanadium/kg body
weight uncoupled oxidative phosphorylation in the liver
mitochondria (Hathcock et al., 1966). These authors suggested
that vanadate might replace the phosphate ion in ATP synthesis,
and a high-energy vanadyl intermediate (vanadium X) or ADP-
vanadium be formed and hydrolysed. The results of studies by
DeMaster & Mitchell (1973) supported the theory of the mechanism
involving the uncoupling of glyceraldehyde-3-phosphate
dehydrogenase by vanadium. It was also shown that the
vanadium5+ oxyanion inhibited oxidative phosphorylation in
intact rat liver mitochondria, but did not act as an uncoupler.
Vanadium salts inhibit the activity of succinic dehydro-
genase, a key enzyme in the citric acid cycle and the electron
transport system that is activated by sulfhydryl groups, and
this would also reduce ATP synthesis (Aiyar & Sreenivasan,
1961). The inhibiting effects of vanadium on succinic
dehydrogenase could involve a reduction in available -SH
groups.
The oxidation of tryptamine by monoamine oxidase from
guinea-pig liver and kidney was accelerated by 125% in the
presence of vanadium (Perry et al., 1955, 1969); however, the
results of studies by Lewis (1959c) on dogs injected with sodium
metavanadate indicated that vanadium inhibited monoamine
oxidase, because the urinary output of 5-hydroxyindoleacetic
acid was reduced. The decreased output of 5-hydroxyindoleacetic
acid, suggests the possibility of accumulation of serotonin,
which was also reported by Roshchin (1967a).
When rats were administered daily injections of sodium
metavanadate (1.25 - 2.5 mg/kg body weight), weight loss was
correlated with accumulation of the metal in the liver (Johnson
et al., 1974). The activities of the hepatic enzymes, xanthine
oxidase and sulfite oxidase, which have molybdenum groups, and
total liver concentrations of molybdenum were not affected by
vanadium. It was concluded that vanadium toxicity in rats was
not related to molybdenum utilization. Though vanadium was
administered in the pentavalent state, the rat livers had an
electron paramagnetic resonance (EPR) spectrum characteristic of
vanadium4+. It was suggested that vanadium is in a protein-
bound form in the livers of rats. Vanadium4+ was also found in
the kidneys and, to a limited extent, in the lungs of rats
injected with sodium metavanadate, but not in the hearts; it was
considered that the ability of liver and kidney to reduce the
vanadate by one electron might be related to a specific
detoxification mechanism present in these organs. Johnson et
al. (1974) and Minden & Rothe (1966) studied the effects of
pentavalent vanadium salts on various enzyme systems in rat
liver homogenates, rabbit blood-serum, and suspensions of
erythrocytes and concluded that there was nothing to suggest
that vanadium salts reacted directly with coenzymes (NAD and
NADP, pyroxidal sulfate) or -SH groups.
The results of in vitro studies, conducted by Tolman et al.
(1979), indicated that vanadium stimulated glucose oxidation and
transport in adipocytes and glycogen synthesis in the liver and
diaphragm, and inhibited hepatic gluconeogenesis and intestinal
glucose transport. There is no evidence in vivo showing a role
of vanadium in the regulation of glucose metabolism.
The intimate mechanism of interaction between vanadium and
enzymes is not yet known. Experimentally observed disturbances
of the cardiovascular system, changes in the concentration of
-SH groups and cystine, disturbances in the metabolism of
sulfur-containing, glycogen-forming, and keto-forming amino
acids, and of the functioning of the liver and kidneys, of RNA
and DNA synthesis, and of cholesterol metabolism, indicate that
vanadium possesses a broad spectrum of action in the body and
that its toxic action is not analogous to that of any other
metal (Roshchin, 1968).
7.3 Effects on the Nervous System
The Na+K+-ATPase in rat brain is inhibited by vanadium
pentoxide, though not as strongly as that in kidney and heart
(Nechay & Saunders, 1978). Svoboda et al. (1984) showed that
the brain microsomal N+K+-ATPase in rat is equally inhibited by
vanadate (VO3-) or the vanadyl ion (VO+2).
In a short-term exposure study, rats received single intra-
peritoneal injections of sodium metavanadate equal to 20% of the
LD50 (1 mg vanadium/kg body weight). The level of noradrenaline
decreased and those of dopamine and 5-hydroxy-tryptamine
increased. A long-term study involved the oral administration
of sodium metavanadate (3 mg vanadium/kg body weight). The
findings were similar to, but more pronounced than, those in the
short-term study (Witkowska & Brzezinski, 1979).
When adult male CD1 mice were treated with vanadate in the
drinking-water for 30 days, there was a dose-related decrease in
norepinephrine levels in the hypothalamus. Dopamine levels also
decreased significantly, but 5-hydroxytryptamine levels were not
affected. Levels of dopamine in the corpus striatum were
unchanged and there were only marginal effects on the amines in
the other brain regions. It is suggested that vanadium has a
selective effect on adrenergic pathways (Sharma et al., 1986).
Several inhibitors of Na+K+-ATPase, such as ouabain, have
been reported to block DNA and protein synthesis in cell
cultures (Kaplan, 1978). It has also been shown that vanadate
inhibits protein synthesis in neuroblastoma cells and in the
brain homogenates of rats fed sodium monovanadate (125 mg/litre)
ad libitum for 30 or 60 days (Montero et al., 1981).
Neurophysiological effects have been reported following
acute exposure (oral and sc injection) of dogs and rabbits to
vanadium oxides and salts (V2O3, V2O5, VCl3, NH4VO3) (Roshchin,
1967a). These include disturbances of the central nervous
system (impaired conditioned reflexes and neuromuscular
excitability).
In studies by Seljankina (1961), solutions of vanadium
pentoxide or ammonium vanadate were administered orally to rats
or mice in doses of 0.005 - 1 mg vanadium/kg body weight per day
for periods ranging from 21 days at the higher levels to 6
months at the lower levels. A dose of 0.05 mg vanadium/kg body
weight was found to be the threshold dose for functional
disturbances in conditioned reflex activity in both mice and
rats; a dose of 0.005 mg vanadium/kg body weight did not produce
any adverse effects.
In a study by Pazhynich (1966), albino rats underwent con-
tinuous inhalation exposure to condensation aerosols of vanadium
pentoxide at levels of 0.002 mg/m3 and 0.027 mg/m3. Animals in
both treated and control groups showed normal weight gain.
After 30 days, the motor chronaxy of the extensor muscles of the
tibia in the group exposed to 0.027 mg/m3 decreased by an average
of 0.8 µs ( P < 0.01), and the chronaxy of the corresponding
flexor muscles increased by an average of 4 µs ( P < 0.001).
Thus, the chronaxy ratio of antagonistic muscles fell from 1.5
at the beginning of the studies to 1.0 on day 20 ( P < 0.02),
and 0.5 on day 30 ( P < 0.01). The decrease continued until a
level of about 0.25 was reached. About 18 days after cessation
of exposure on the 70th day, the chronaxy ratio returned to
normal (1.5). Changes in motor chronaxy were not observed in
rats exposed at 0.002 mg/m3 or in the controls.
In a second study, albino rats were exposed continuously to
vanadium pentoxide at 0.006 ± 0.00056 mg/m3 for 40 days. No
changes were observed in the chronaxy of antagonistic muscles of
the tibia in exposed animals, compared with the controls, during
the first month of exposure. However, after 30 days, there was
a statistically significant decrease in chronaxy ratio. When
animals were given only water and no food for 3.5 days, during
the sixth week of exposure, chronaxy ratios decreased to 0.92,
compared with 1.5 in the controls.
7.4 Effects on the Respiratory System
Studies on respiratory exposure to vanadium pentoxide are
summarized in Table 23 (section 7.1).
Sjöberg (1950) exposed rabbits to vanadium pentoxide dust
particles, nearly all of which were smaller than 10 µm in
diameter at concentrations of 77, 109, 205, or 525 mg/m3 for
periods of 7 h, 4 h, 7 h, or 1 h, respectively. Death occurred
only in the 205 mg V205/m3 (7 h) group (equivalent to 115 mg
vanadium/m3). There was marked tracheitis accompanied by
pulmonary oedema and bronchopneumonia. Conjunctivitis,
enteritis, and fatty infiltration of the liver were also
observed.
In further studies by the same author, rabbits were exposed
to 20 - 40 mg V205/m3 (equivalent to 11 - 22 mg vanadium/m3)
intermittently for 1 h each day for several months. At autopsy,
pathological changes observed included chronic rhinitis and
tracheitis, emphysema, patches of lung atelectasis, broncho-
pneumonia and, in some cases, pyelonephritis. Vanadium was
detected in ashed lung, liver, and kidney, but not in the
intestines. Changes of a fibrotic nature and specific chronic
lesions were not observed in the lungs, and there was no visible
accumulation of particles. These findings, plus the fact that
vanadium was present in the liver and kidney, were considered
evidence of rapid clearance and/or absorption from the lung.
Continuous inhalation exposure of rabbits to 10 - 30 mg
V205/m3 (5.6 - 16.8 mg vanadium/m3) caused bronchitis,
pneumonia, loss of weight, and bloody diarrhoea (Gulko, 1956).
In studies by Roshchin (1967b, 1968) described in section
7.2, acute inhalation toxicity in albino rats was characterized
by irritation of the respiratory mucosa and nasal discharge that
sometimes contained blood. Animals breathed with difficulty and
there were crepitations. The animals behaved passively,
refusing to eat, and lost weight. In cases of severe poisoning,
diarrhoea, paralysis of the hind limbs, and respiratory failure
were followed by death. Pulmonary abscesses were frequently
seen in animals that recovered. Animals that died or were
killed at various times after exposure, showed severe
congestion, particularly in the capillaries, and small
haemorrhages were observed in all internal organs. Signs of
increased intracranial pressure, and fatty degeneration of the
liver and kidneys were also seen. In the lungs, there was
capillary congestion together with tiny haemorrhages,
perivascular and focal oedema, bronchitis, and focal
interstitial pneumonia. The bronchitis and bronchopneumonia
were often purulent, and the small bronchi were constricted.
There was a relationship between the severity of the
pathological changes and the vanadium concentration in the air.
In cases of slight toxicity, pathological changes were mainly
confined to the lungs.
Pathological changes were also seen in the lungs when rats
were exposed intermittently to a condensation aerosol of
vanadium pentoxide at 3 - 5 mg/m3 for 2 h, every other day, for
3 months, or to a dispersion aerosol of V205 at 10 - 30 mg/m3
for 4 months. Blood vessels were engorged and the endothelium
was swollen; capillary congestion, perivascular oedema,
lymphostasis, and small haemorrhages indicated altered vascular
permeability and disturbances in the circulation of the
pulmonary blood and lymph. Occasionally, foci of oedema and
desquamative bronchitis were observed and small bronchi were
often constricted. Lymphocytes and histiocytes had infiltrated
interstitial tissue. Connective tissue proliferation was
sometimes seen in the zone of lymphocytic infiltration.
Purulent bronchitis or pneumonia occurred in some animals, and
occasionally lung abscesses developed.
Roshchin (1967a) observed similar effects with vanadium
trioxide and vanadium trichloride. As vanadium trichloride was
more soluble, more marked histopathological effects were seen in
internal organs. Pentavalent compounds of vanadium were 3 - 5
times more toxic (in terms of median lethal concentration) than
trivalent compounds. Although dispersion aerosols of vanadium
metal, vanadium carbide, and ferrovanadium were not highly
toxic, long-term exposure at high concentrations resulted in
many of the signs and symptoms produced by vanadium pentoxide.
In studies by Pazhynich (1966) (section 7.2), histopatho-
logical changes observed in rats following high-level inhalation
exposure included marked lung congestion, focal lung haemor-
rhages, and extensive bronchitis.
Effects on the lung were observed in albino rats exposed by
inhalation for 2 weeks to uncoated bismuth orthovanadate dust
(0.11 mg/litre, 1.2 mg/litre), silica-coated bismuth ortho-
vanadate (0.15 mg/litre, 1.3 mg/litre), or silica-coated
titanium dioxide (1.19 mg/litre). In rats sacrificed at the end
of the 2-week exposure, there was a dose-related macrophage
(dust cell) response to both forms of bismuth orthovanadate.
Three months after exposure, the bismuth orthovanadate rats had
alveolar proteinosis, foamy macrophages with cholesterol clefts,
and hyperplastic type II pneumocytes. Six months after exposure,
these changes were more marked with cholesterol granulomas and
degeneration of foamy macrophages. In rats sacrificed after 1
year, the pulmonary lesions were reduced, but alveolar protein-
osis and cholesterol granulomas persisted. Initially, similar
changes were observed in rats exposed to the silica-coated
titanium dioxide, but recovery was obvious at 6 months and, at 1
year, the lungs were almost normal with only a few remaining
macrophage (dust cell) aggregates (Lee & Gillies, 1986).
The respiratory and related histopathological effects of
vanadium exposure in experimental animals were marked irritation
of the respiratory mucosa; vascular injury resulting in
capillary stasis, perivascular oedema, and small haemorrhages;
and an asthmatic-type bronchitis and expiratory difficulty on
acute exposure (Roschin, 1967b).
In adult male cynomolgus monkeys exposed by inhalation to
vanadium pentoxide dust concentrations of 0.5 mg/m3 or 5 mg/m3
at weekly intervals, significant central and peripheral airflow
restriction was measured one day after each exposure. There
were also significant increases in respiratory cell counts
obtained by bronchoalveolar lavage. The increased cell count
was due to a marked increase in the number (absolute and
relative percentage) of polymorphonuclear leukocytes,
indicating pulmonary inflammatory changes (Knecht et al.,
1985).
In the respiratory tract of experimental animals, the main
differences between the acute and chronic effects of vanadium
are the development, after prolonged exposure, of chronic
inflammation in the bronchi and a greater tendency to septic
bronchopneumonia. Atelectasis, interstitial infiltration and
proliferation, and emphysema also occur.
Macrophages are engaged in a variety of pulmonary defence
mechanisms. Theoretically, effects of vanadium on these cells
may explain some of the observations on vanadium toxicity on the
respiratory system. In in vitro studies, Waters et al. (1974)
demonstrated a 50% reduction in the viability of cultured rabbit
macrophages after exposure to 13 mg vanadium/litre (as vanadium
pentoxide) for 20 h. Short-term exposure (2 h) to vanadium
pentoxide at a dose of 7 mg vanadium/litre also reduced the
viability of mouse pulmonary alveolar macrophages to 87%. In
another study, the phagocytic index was reduced to 71% (Fisher
et al., 1978). The incubation of bovine alveolar macrophages
with ammonium metavanadate (NH4VO3) at 0.5 or 1 mg
vanadium/litre, for 4 h, reduced viability to 95 and 85%,
respectively. After 8 h incubation, viability was reduced by 24
and 38%, respectively, and, after 16 h, no viable cells
remained. Low levels of vanadium (0.01, 0.1 mg vanadium/litre)
stimulated the phagocytic activity of macrophages, whereas a
striking decrease in phagocytic activity was noted with 0.5 and
1 mg/litre at 8 h, though, initially, there was stimulation of
activity. Doses of 0.01 and 0.1 mg/litre did not affect
viability (Wei & Misra, 1982).
7.5 Effects on the Cardiovascular System
Severe exposure of animals to vanadium oxides and salts
produced cardiovascular changes (occurrence of arrythmias and
extrasystole, prolongation of the Q-RST interval, and decrease
in the height of the P and T waves of the EKG) (Roshchin,
1967a).
Intense vasoconstriction has been reported in the spleen,
kidney, and intestines following intravenous injections of sub-
lethal doses of sodium ortho- and metavanadate (Hudson, 1964).
Intravenous injection of sodium metavanadate at 2.5 mg/kg in
dogs provoked an increased amplitude of T-waves in the electro-
cardiogram followed by depression of S-T segments (Lewis,
1959c). Perivascular swelling, as well as fatty changes in the
myocardium, were observed by Roshchin (1968) following long-term
inhalation exposure of rats and rabbits to vanadium pentoxide,
trioxide, and chloride (10 - 70 mg/m3, 2 h/day, 9 - 12 months).
Vanadium sulfate (500 mg/kg diet, 6 weeks) mobilized excess
arterial cholesterol in rabbits previously maintained on a
cholesterol-rich diet (Curran & Costello, 1956).
Feeding rats sodium orthovanadate at 100 or 200 mg/kg body
weight (added to normal rat chow) for up to 56 weeks resulted in
a gradual increase in systolic blood pressure. The effect was
unrelated to water intake, urine output, or urinary-sodium
excretion. The increased pressure was sustained in a dose-
related manner and was positively correlated with plasma levels
of vanadium that ranged from 0.04 to 0.27 mg/litre (Steffen et
al., 1981). Similar increases in mean arterial blood pressure
have been reported in both conscious (Day et al., 1980) and
anaesthetized rats (Hatfield & Churchill, 1981).
7.6 Effects on the Kidney
In earlier studies, glomerular hyperaemia and necrosis of
convoluted tubules were reported to be related to acute vanadium
exposure (Hudson, 1964). Nephrotoxicity, manifested as
albuminuria, was reported after intravenous injection of sodium
metavanadate at 2.5 - 5 mg/kg body weight in male dogs by Lewis
(1959c). Inhalation of 10 - 70 mg vanadium chloride/m3, for 2 h
daily, for 9 - 12 months, was followed by fatty changes in the
kidney of the rat and rabbit (Roshchin, 1968). In an inhalation
study on rats exposed continuously to vanadium pentoxide
condensation aerosols at concentrations of 0.002 and 0.027 mg/m3
for 70 days, Pazhynich (1966) reported granular degeneration of
the epithelial cells of the convoluted tubules, with areas of
necrosis. Acute tubular necrosis followed subcutaneous injection
of NH4VO3-solutions in 0.1 mol/litre tris-HCl-NaCl buffers in
albino mice (20 mg vanadium/kg body weight). The mortality rate
was higher at a pH of 7.8 (68%) than at pH 6.1 (20%) (Wei et
al., 1982).
There are distinct species differences with regard to the
renal effects of vanadate (Grantham, 1980; Phillips et al. 1983;
Nechay, 1984). Vanadate has both diuretic and natriuretic
effects on the rat kidney (Balfour et al., 1978; Day et al.
1980; Kumar & Corder, 1980; Hatfield & Churchill, 1981; Roman et
al., 1981), but not on that of the dog (Inciarte et al., 1980;
Lopez Novoa et al., 1982a,b) or the cat (Larsen et al., 1979).
In the rat, the tubular effect is independent of changes in
glomercular filtration rate, whereas the tubular effect in the
cat is either absent or masked by pronounced renal vasoconstric-
tion and anuria (Larsen et al., 1979; Larsen & Thomsen, 1980).
Vanadate has also been reported to increase the urinary
excretion of calcium, phosphate, bicarbonate, and chloride in
the rat kidney (Kumar & Corder, 1980).
7.7 Effects on the Immune System
Exposure of mice to vanadium in drinking-water resulted in a
dose-related, but not statistically-significant, decrease in
antibody-forming cells in the spleens of mice challenged with
sheep erythrocytes; serum immunoglobulins were not affected.
Splenic lymphocytes obtained at 1, 4, 8, and 13 weeks from male
Swiss-Webster mice treated with 1, 10, or 50 mg vanadium/litre
drinking-water showed increased DNA synthesis in vitro (Sharma
et al., 1981). In female B6C3F1 mice given ammonium
metavanadate ip at doses of 2.5, 5, or 10 mg/kg body weight,
every 3 days, for 3, 6, or 9 weeks, there was a dose-related
increase in resistance to Escherichia coli endotoxin lethality
up to 6 weeks and a dose-related decrease in resistance
to Listeria lethality. Peritoneal macrophage activity also
decreased in a dose-related manner, but without any effects on
viability. The rosetting capability of splenic lymphocytes was
increased. There was enlargement of the liver and spleen with
enhanced formation of splenic mega-karyocytes and red blood cell
percursors. The authors concluded that vanadium may affect the
normal functioning of the immune system (Cohen et al., 1986).
7.8 Reproduction, Embryotoxicity, and Teratogenicity
7.8.1 Reproduction and embryotoxicity
Roshchin at al. (1980) studied the gonado- and embryotoxic
effects of 0.85 mg metavanadate/kg body weight (1/20 LD50),
administered subcutaneously to albino rats. Vanadium
administered to pregnant rats on days 21 - 22 of pregnancy
accumulated in the placenta but was not reported to penetrate
the placental barrier and reach the fetus. During the period of
lactation, vanadium was found in the mammary glands (0.14% of
administered dose/g tissue) and excreted with the milk. In new-
born rats, the uptake of vanadium was in the range of 0.018 -
0.032% of the original administered dose to the lactating dams
per g neonate. Impairment of spermatogenesis was manifested as
a 10 - 33% decrease in the mobility of spermatozoa, a decrease
in osmotic resistance of 7.9 - 11.4% and a 31% rise in the
number of dead spermatozoa. There were morphological changes in
spermatozoa and desquamation of the spermatogenic epithelium in
the seminal tubuli. The impairment of spermatogenesis affected
the reproduction of animals, resulting in pre-implantation
deaths of embryos. Gonadotoxic effects were suggested by the
absence of fertilization of female rats by male rats exposed
daily to vanadium at 0.85 mg/kg body weight. In other studies
(Hackett & Kelman, 1983) in pregnant rats vanadium tended to
localize initially in the placenta and then to preferentially
concentrate in the membranes rather than in the fetus.
The administration of similar doses of vanadium to female
rats on the 4th day of pregnancy increased the mortality of
embryos as a result of pre-implantation deaths; the number of
fetuses in each female rat was only half that in the untreated
animals. These effects were observed in the absence of general
toxicity in the experimental animals.
In vitro, orthovanadate (0.2 - 2 mmol) inhibited
luteinizing hormone-induced cyclic adenosine monophosphate
(cAMP) in isolated corpora lutea from pseudopregnant rats.
When added simultaneously with luteinizing hormone, inhibition
occurred within 25 min, but not when the corpora lutea had been
pretreated with luteinizing hormone for 60 min. A decrease was
also observed when corpora lutea were exposed to vanadate in the
presence of 3-isobutyl-1-methylxanthene (0.5 mmol), a phospho-
diesterase inhibitor. Cyclic adenosine monophosphate was also
inhibited by vanadate in copora lutea incubated in a calcium-
depleted medium. Vanadyl sulfate (0.4, 2 mmol) was as effective
as vanadate in inhibiting luteinizing hormone-induced cyclic
adenosine monophosphate accumulation (Lahav et al., 1986).
7.8.2 Teratogenicity
Carlton et al. (1982) injected 80 mature Syrian golden
hamsters ip with ammonium vanadate at 0, 0.47, 1.88, or
3.75 mg/kg body weight. Injections were carried out on days 5 -
10 of gestation. A significant increase in skeletal anomalies
was observed in all groups exposed to vanadate compared with
control animals. A significant increase in deaths was registered
in the 1.88 mg/kg group. There was no dose-response relationship
as regards anomalies.
Pregnant NMRI albino mice were injected intravenously with
1 mmol vanadium pentoxide dissolved in distilled water on day 3
or day 8 of pregnancy (day 1 = finding of vaginal plug). Control
groups were given 0.1 ml physiological saline on the same days.
Mice were killed on day 17, the uterine horns examined for
resorbed embryos, and the fetuses were removed for detailed
examination. Vanadium pentoxide did not produce any effects in
implantation, and fetuses from the day 3-treated and control
groups did not show any differences in litter size, fetal
weight, or external and internal morphology. The fetuses from
the day 8-treated group showed a statistically significant high
frequency (71%) of delayed ossification (supraoccipital bone,
sternum, metatarsals, and caudal vertebrae), and broken spinal
cord occurred in a few fetuses (Wide, 1984).
Vanadium pentoxide was administered subcutaneously to
pregnant rats (strain unspecified) at doses of 0.5, 1, or 4
mg/kg body weight for 10 days, from day 7 to day 16 of
gestation. The incidences of resorbed and dead fetuses in the 1
and 4 mg/kg groups were 17% and 27.2%, respectively. These were
significantly higher than those found in the controls (3.5%). In
the 4 mg/kg group, 52.38% of fetuses showed wavy ribs. In a
separate study, pregnant rats were given an aqueous solution of
V2O5 by ip injection at concentrations of 0.3, 1, or 3 mg/kg
body weight. This induced a higher level of resorbed and dead
fetuses than oral administration. Both ip injection of 0.3
mg/kg and oral administration of 9 mg/kg induced an array of
skeletal anomalies, namely wavy ribs, supernumerary ribs, and
fused sternebrae and vertebrae (Sun, ed., 1987). Though
tentative, these results suggest that vanadium may have the
potential to induce teratogenicity in a mammalian system.
7.9 Mutagenicity and Related End-Points
There are few studies on the mutagenicity and carcino-
genicity of vanadium compounds. Mutagens in the air can be
divided into two groups: a non-polar extract rich in polycyclic
aromatic hydrocarbons (PAHs) and other promutagens, and a polar
extract containing direct acting mutagens, i.e., not requiring
microsomal activation (Madsen et al., 1982). The non-polar
fraction was strongly influenced by automobile exhaust products,
whereas the polar was more attributable to secondary emissions
transformed by atmospheric reactions, and to primary emissions
from stationary sources. The role of PAHs in the overall
mutagenicity was estimated to be modest; thus, the importance of
other substances increases. Vanadium was found at all sample
sites and is a well-known constituent of, for instance, coal fly
ash (section 3.4.2).
Vanadium5+ has been shown both to inhibit or enhance DNA
synthesis in vitro , depending on the concentration in the media
(Hori & Oka, 1980; Carpenter, 1981; Jackson & Linskens, 1982;
Smith, 1983).
In a DNA synthesis inhibition assay in male mice, vanadium
pentoxide suspended in 3% starch solution was administered
orally at doses of 14.6, 29.2, or 58.4 mg/kg body weight. The
animals were killed 24 h after dosing; 3 h before sacrifice,
1 µCi 3H-thymidine/g body weight was administered ip.
Incorporation of 3H-thymidine in the testes, spleen, liver, and
blood was measured in a liquid scintillation spectrometer.
There were no significant differences between the experimental
groups and the solvent control group (Sun, ed., 1987).
In a study using FADU (Fluorescence Analysis of DNA
Unwinding), vanadyl chloride at a concentration of 5 x 10-5 mol
failed to induce DNA damage (strand breaks) in human peripheral
white blood cells (McLean et al., 1982).
Kanematsu et al. (1980) carried out rec assays on 127 metal
compounds with Bacillus subtilis to test their DNA-damaging
capacity. Mild positive results were noted for three vanadium
compounds (VOCl2, V2O5, NH4VO3).
A lack of induction of spot mutations in Escherichia coli
and in Salmonella typhimurium was demonstrated by Kanematsu &
Kada (1978) and Kada et al. (1980). Similar results were
obtained by Si Rongshan et al. (1982) with E. coli . However,
ammonium metavanadate was found to be mutagenic in S.
typhimurium TA1535 in a modified plate incorporation assay and
in the fluctuation test with TA100 (Arlauskas et al., 1985). In
a recent study, Sun, ed. (1987) demonstrated the induction of
reverse mutations by vanadium pentoxide with E. coli WP2,
WP2uvrA, and Cm-981, but no frameshift mutations with strains
ND-160 and MR102. Vanadium pentoxide showed negative results
with S. typhimurium strains TA1535, TA1537, TA98, and TA100.
Thus, the results of mutagenicity studies of vanadium with
bacterial assays are conflicting, and no firm conclusions can be
drawn.
In a micronucleus test, vanadium pentoxide was administered
to two strains of mice (615 and Kunming albino) by ip injection
at doses of 6.4, 2.13, or 0.17 mg/kg body weight for 5 consecu-
tive days; cyclophosphamide was used as a positive control (Sun,
ed., 1987). Significant levels of induced micronuclei in both
strains were observed. Both subcutaneous injection of vanadium
pentoxide solution (0.25, 1, or 4 mg/kg) and inhalation of
vanadium pentoxide dust (0.5, 2, or 8 mg/m3) also induced micro-
nuclei in mice strain 615. However, negative results were
obtained following oral administration of a 3% starch suspension
of vanadium pentoxide at doses of 1.44, 2.83, 5.65, or
11.3 mg/kg body weight, daily, for 6 weeks, to Kunming albino
mice (Sun, ed., 1987).
In an in vitro study on human peripheral lymphocyte cultures
with vanadium pentoxide concentrations of 0.047, 0.47, or
4.7 moles (mitomycin c was used as positive control), no
increases in the frequency of sister chromatid exchange were
observed (Sun, ed., 1987).
In a dominant-lethal mutation assay, vanadium pentoxide
(0.2, 1, or 4 mg/kg body weight) was administered daily by
subcutaneous injection to 5 groups of male mice, aged 5 - 6
weeks at the start of the study, for 3 months, following which
they were mated with females. Ethylmethanesulfonate and
distilled water were used as positive and negative controls,
respectively. The females were killed 17 days after conception
and the numbers of fetuses and resorptions recorded. The
results were considered negative for the induction of dominant-
lethal mutations (Sun, ed., 1987).
7.10 Carcinogenicity
In a study on 13 metallic compounds, intraperitoneal
injections of vanadium3+ 2.4-pentanedione at doses of 24, 60, or
120 mg/kg body weight did not significantly increase the
incidence of lung adenomas in mice (Stoner et al., 1976).
In life-span studies, the incidence of tumours in mice
given vanadyl ions (as the sulfate) at 5 µg/litre drinking-
water was similar to that in control animals (Kanisawa &
Schroeder, 1967; Schroeder et al., 1970; Schroeder & Mitchener,
1975).
In rats, the induction of mammary carcinogenesis by 1-
methyl-1-nitrosurea was blocked by feeding a purified diet
supplemented with 25 mg vanadyl4+ sulfate/kg during the post-
initiation stages of the neoplastic process. Both cancer
incidence and the average number of cancers per rat were reduced
by the vanadium4+ diet without inhibiting the overall growth of
the animals (Thompson et al., 1984). It has also been shown
that metallocene dichlorides, (C2H5)2MCl2 (where M = titanium,
vanadium, molybdenum, or niobium), exhibit cancerostatic
activity against the Erlich ascites tumour system in mice, and
that treatment with such substances cured the tumour (Köpf-Maier
et al., 1980). Vanadocene dichloride was reported to have a
chemotherapeutic activity similar to that of cis-dichlordiamine
platinum (II) when used against liver tumours in mice (Köpf-
Maier & Köpf, 1979). The mechanism of the preventive effect of
vanadium is not clear. Modulation of one or more aspects of the
DNA metabolism could account for these results.
The effects of ammonium vanadate on the development of large
bowel neoplasms in mice treated with 1,2-dimethylhydrazine (DMH)
were studied by Kingsnorth et al. (1986). Mice were treated
with DMH (20 mg/kg body weight per week) for 20 weeks. Ammonium
vanadate was given in the drinking-water (10 or 20 mg/litre) to
groups of mice during the study. At 30 weeks, the colons of
DMH-treated mice (not receiving ammonium vanadate) showed
increases in RNA content (+14%) and DNA content (+18%) and
deeper crypts (+33%). In the mice treated with DMH and receiving
ammonium vanadate at 10 or 20 mg/litre, the RNA content was
decreased by 11%. Although thymidine incorporation was
increased, ammonium vanadate did not have any effects on the
incidence or type of tumour induced by DMH (Kingsnorth et al.,
1986).
In a long-term study in which the carcinogenic activity of
various materials was studied using intrabronchial pellet
implantation in the lower left bronchus of rats, vanadium solids
produced chronic inflammatory changes in 44/100 rats, bronchial
inflammation in 50/100, squamous metaplasia in 10/100, and one
bronchial carcinoma in a male rat after 645 days. These results
were not significant for carcinogenicity (Levy et al., 1986).
8. EFFECTS ON MAN
8.1 Therapeutic Exposure and Controlled Studies
8.1.1 Therapeutic exposure
In the past, vanadium compounds were prescribed as thera-
peutic agents for anaemia, chlorosis, tuberculosis, and
diabetes. They were also used as an antiseptic, a spiro-
chetocide, and a tonic. For example, sodium metavanadate was
given therapeutically by mouth in doses of 1 - 8 mg, and sodium
tartrate was injected intramuscularly at levels as high as
150 mg. Due to poor absorption from the gastrointestinal tract,
the metal is not very toxic for human beings when ingested, but,
if introduced directly into the circulation in a soluble form,
Hudson (1964) estimated that the lethal dose for a 70-kg person
would be only 30 mg V205 (0.42 mg V205/kg body weight).
8.1.2 Controlled studies
8.1.2.1 Effects on metabolism
Vanadium has been administered under controlled conditions
to study its effects on blood-cholesterol levels. Curran et al.
(1959) conducted a clinical study in which 5 healthy adult male
volunteers were fed soluble diammonium oxytartarovanadate at
150 - 200 mg/day (21 - 30 mg vanadium/day) for 6 weeks. At the
end of the period, plasma-cholesterol was significantly
reduced.
Lewis (1959a) compared age-matched groups of 32 vanadium
workers with 45 controls, all over 45 years of age. Vanadium
workers exposed for at least 6 months excreted greater amounts
of vanadium and exhibited slightly lower serum-cholesterol
levels than the controls. Mean cholesterol values for the 2
reference groups (representing 2 geographical areas) were 2309
and 2267 mg/litre. Mean levels for vanadium workers from
corresponding areas were lower at 2049 and 2067 mg/litre (P <
0.05), respectively.
A clinical study by Somerville & Davies (1962) on 12
patients (9 of whom were hypercholesterolaemic) given diammonium
vanadotartrate orally for 6 months (25 mg 3 times daily for 2
weeks, increased to 125 mg daily in 10 patients) did not show
any significant changes in serum-cholesterol levels over 5.5
months. The mean pretreatment control level of serum-cholesterol
was 4110 mg/litre, and the mean age was 49.2 years. The study
is not comparable with that of Curran et al. (1959), as the
patients were hypercholesterolaemic and older. Dimond et al.
(1963) observed temporary drops (not statistically significant)
in cholesterol levels in 2 out of 6 patients given ammonium
vanadyl tartrate for several weeks at levels of between 50 and
100 mg/day. No statistically significant changes were observed
in blood-lipids, phospholipids, triglycerides, 17-ketosteroids,
or 17-hydroxycorticosteroids. Two patients complained of fatigue
and lethargy while they were taking vanadium. All complained of
cramps and loosened stools, and all developed green tongue.
Schroeder et al. (1963) reporting findings similar to those of
Dimond et al. (1963) considered that the slight effects of
vanadium on serum-cholesterol were pharmacological rather than
caused by correction of a physiological deficiency. They
further pointed out that dietary regimens based on the consump-
tion of unsaturated fats, which reduce plasma-cholesterol in
human beings, are associated with the intake of 1 - 4 mg
vanadium/day and that the feeding of vanadium-poor saturated
fats raises cholesterol.
Studies have been undertaken on the effects of vanadium on
human dental caries. Belehova (1969) studied 583 school
children ranging in age from 7 to 11 years. The subjects were
divided into 4 groups. Children in Group I received fluoride
twice a year, those in Group II received a local application of
a 50% paste of an ammonium salt of vanadium and glycerol, Group
III received both fluoride and vanadium, and Group IV acted as
the control. The incidence of caries was 11% in Group III,
15.4% in Group II, 29.7% in Group I, and 43% in the control
group. Belehova concluded that the lower incidence of caries in
subjects receiving vanadium suggested a possible prophylactic
action. However, other studies (Hein & Wisotzky, 1955; Muhler,
1957; Hadjimarkos, 1966, 1968; McLundie et al., 1968) failed to
demonstrate a clearly beneficial effect with regard to dental
caries in human beings.
Data on the effects of vanadium on the haematopoiesis are
inconsistent. Lewis (1959a) did not observe any effects of
exposure to vanadium on haematocrit levels in 32 vanadium
workers compared with 45 controls matched for age. A beneficial
effect of low-level vanadium administration on nutritional
anaemia has been suggested (Beard et al., 1931; Myers & Beard
1931; Hadjimarkos, 1966; Kopylova, 1971). However, the effects
of vanadium on iron metabolism have not been fully assessed
(Vouk, 1979).
The administration of w -methylpantothenic acid, an
antimetabolite of pantothenic acid, to human beings resulted in
a syndrome consisting of postural hypotension, dizziness,
tachycardia, fatigue, drowsiness, epigastric distress, anorexia,
numbness and tingling of the hands and feet, and hyperactive
deep reflexes. It is not known whether these symptoms are the
result of an induced deficiency of pantothenic acid or are toxic
effects of the anti-metabolite. However, the symptoms resemble
those resulting from exposure to high concentrations of
vanadium. The common denominator in both cases may be a
reduction in hepatic coenzyme A levels (Waters, 1977).
8.1.2.2 Effects on the respiratory system
Zenz & Berg (1967) studied the effects of vanadium
inhalation in 9 healthy volunteers aged 27 - 44 years, for whom
baseline lung function data were available. Two volunteers,
exposed to vanadium pentoxide dust at 1 mg/m3 for 8 h,
developed sporadic coughing after 5 h and a frequent cough after
nearly 7 h. Coughing lasted 8 days, but lung sounds remained
clear and there were no other signs of irritation. Lung
function tests, complete blood counts, urinalyses, and nasal
smears were normal up to 3 weeks. Three weeks later, the same 2
volunteers were accidently exposed for 5 min to a "heavy cloud"
of vanadium pentoxide dust. A productive cough developed within
16 h, and, within 24 h, rales and expiratory rhonchi developed
throughout the lung, but pulmonary function remained normal.
Isoproterenol (1:2000) relieved the symptoms for about 1 h, but
coughing then resumed and continued for 7 days. There were no
other symptoms. Eosinophils were not present in the nasal
mucous.
Exposure of 5 volunteers to a lower concentration
(0.2 mg/m3, 98% of particle size 5 µm) had similar effects,
though the symptoms took longer to develop, i.e., after 20 h.
Coughing, without other systemic effects, persisted for 7 - 10
days. Pulmonary function tests and differential white blood
counts remained normal. The vanadium concentration in the urine
was highest (0.13 mg/litre) on the third day, with none
detectable after 7 days. The maximal faecal-vanadium level was
3 mg/kg, with none detectable after 14 days. Exposure to a
concentration of 0.1 mg/m3 for 8 h did not produce any coughing
in 2 subjects not previously exposed. However, an increase in
the production of mucous, 24 h later, indicated some respiratory
irritation. Then there was slight coughing, which became more
severe after 48 h, subsided after 72 h, and disappeared after
96 h. Pulmonary function tests and differential white blood
counts remained normal.
Pazhynich (1967) studied the irritant effects of vanadium
pentoxide condensation aerosol on 11 volunteers. At a
concentration of 0.4 mg/m3, all reported a tickling or itching
sensation and a feeling of dryness in the region of the root of
the tongue, the posterior wall of the pharynx, and the fauces,
as well as a slight prickling sensation in the nose and
posterior pharyngeal wall. These symptoms were easily tolerated.
A concentration of 0.16 mg/m3 caused mild signs of irritation in
only 5 volunteers, and a concentration of 0.08 mg/m3 was not
noticed by any volunteer. It was concluded that the mean
perceptible concentration for human beings is 0.27 mg/m3 and
that 0.16 mg/m3 is imperceptible.
8.2 Clinical Studies
The clinical picture of poisoning shows the broad spectrum
of toxic effects of vanadium. The lesions observed affect the
respiratory system, circulatory system, central nervous system,
digestive organs, kidneys, and skin. Poisoning can be divided
into acute and chronic forms.
8.2.1 Acute toxicity
Acute toxicity is characterized by a latent period, which
depends on the concentration of vanadium, the individual
sensitivity of the subject, and the properties of the specific
vanadium compound. The more soluble salts of vanadium pentoxide
have a more rapid action than the vanadium oxides. Chemically-
pure vanadium pentoxide acts more rapidly than the technical
grade. A condensation aerosol of vanadium pentoxide is more
toxic than a disintegration aerosol (Roshchin, 1964). Vanadium
chloride is toxic more rapidly than other compounds.
Roshchin (1968) subdivided acute vanadium effects into
"mild", "moderate", and "severe" forms. The clinical features
of mild toxicity include rhinitis with a profuse and often
bloody discharge, sneezing, and an itching and burning sensation
in the throat. The rhinitis may be followed by the development
of a dry cough with expectoration of small amounts of viscid
sputum, general weakness, and exhaustion. A sub-normal
temperature may be present; in other cases, the temperature may
be high or normal. The patient is afebrile in the absence of
pneumonic disease (Sjöberg, 1950). Conjunctivitis is frequently
observed. The symptoms and course of mild toxicity resemble an
upper respiratory tract infection. Other symptoms include
diarrhoea due to intensified intestinal peristalsis. The
symptoms disappear from 2 - 5 days after cessation of contact
with the dust.
In moderate toxicity, in addition to conjunctivitis and
irritation of the upper respiratory tract, there is bronchitis
with expiratory dyspnoea and bronchospasm. There are frequent
disturbances in the activity of the gastrointestinal tract,
including vomiting and diarrhoea. Taken together with the
bronchospasm, this points to a response of the smooth muscle to
vanadium exposure. Some affected persons have cutaneous
manifestations of toxicity in the form of rashes and eczema with
itching papules and dry patches (Browne, 1955; Zenz et al.,
1962).
Bronchitis and bronchopneumonia are features of severe toxic
effects. Other symptoms may also be more prominent, such as
headache, vomiting, diarrhoea, palpitations, sweating, and
general weakness. Disorders of the nervous system include
severe neurotic states and tremor of the fingers and hands
(Wyers, 1946; Sjöberg, 1955). Functional disturbances of the
respiratory system can be expected, and X-ray examination will
reveal intensification of the lung pattern.
Kidney damage, highlighted by grave dystrophic changes in
the epithelium of the convoluted tubules and disturbed tubular
secretion, occurs immediately after the start of exposure to low
vanadium doses in both acute and chronic intoxication. Once
triggered off, the changes are irreversible, even if exposure is
discontinued. Therefore, the kidneys are a critical organ for
vanadium poisoning (Korkhov, 1965).
8.2.2 Chronic toxicity
Chronic vanadium intoxication produces profound changes in
the respiratory organs, because of the irritant action of
vanadium and the biochemical and functional disturbances
connected with its general resorptive action. Chronic
respiratory illness takes the form of diffuse pneumosclerosis,
chronic bronchitis, chronic rhinitis, and pharyngitis (Roshchin,
1968). However, Parkes (1982) claimed that the available
evidence (Sjöberg, 1950; Williams, 1952; Zenz & Berg, 1967) did
not support the contention that prolonged exposure to vanadium
compounds leads to chronic bronchitis, with or without
emphysema. Although wheezing is more common among vanadium
pentoxide workers than among unexposed workers, lung function
tests and chest radiography have not revealed persistent lung
damage (Kiviluoto, 1980). The cardiovascular system (Wyers,
1946; Sjöberg, 1950; Izycki et al., 1971) is commonly affected
in chronic respiratory disorders by a diagnosable accentuated
second cardiac sound on the pulmonary artery and an attenuated
first sound on the apex cordis. Most of these workers exhibit
heavy sinus arrhythmia and a shift of the EGG-axis to the right.
After extensive exposure, workers may develop bradycardia and a
change of the P wave in the second and third standard leads;
coronary spasm is also usually recognizable in such workers. A
statistically significant increase in the incidence of enlarged
liver and a decrease in functional tests together with
bilirubinaemia and a direct reaction to bilirubin have been seen
in the blood of exposed workers (Roshchin, 1968). Biochemical
alterations have also been found, such as reduction in albumins,
and expansion of the globulin fractions at the expense of gamma-
globulins, even though the total protein content remained
normal. Furthermore, a marked reduction in sulfhydryl groups in
blood-serum and in vitamin C levels in the blood, and a less
marked drop in cholesterol levels have been observed. Systemic
effects, such as a tendency towards anaemia and leukopaenia, and
basophilic granulation of leukocytes have been reported
(Watanabe et al., 1966).
Vanadium levels in whole blood and serum have been studied
to investigate the possible role of vanadium in depressive
states. In a study involving neutron activation analysis of
vanadium levels in the whole blood, serum, and hair of patients
suffering from mania or depression, and of patients who had
recovered from these conditions, as well as normal controls,
manic patients were reported to have normal levels in whole
blood and serum, but significantly raised levels in hair.
Depressed patients had raised levels in whole blood and serum.
In both conditions, raised levels fell with recovery. The
levels of vanadium in serum were correlated with those in whole
blood but not with hair levels (Naylor et al., 1984). In another
study, serum-vanadium levels, measured by neutron activation
analysis, were reported to be 3.10 ± 1.38 mg/litre in patients
suffering from depressive illness and 0.67 ± 0.32 µg/litre in
normal subjects (Simonoff et al., 1986). However, in a series
of 25 depressive, 13 recovered depressive patients, and 24
controls, the whole-blood concentrations of vanadium were
similar to normal levels, and vanadium levels did not change in
depressive patients after recovery (Ali et al., 1985).
8.2.3 Diagnosis
Information on likely exposure, the clinical picture, and
certain biochemical indications of probable exposure can aid
diagnosis, but no specific test can be recommended. Determina-
tion of the vanadium contents of the blood and especially of the
urine provides documentation of exposure, though the correlation
between vanadium levels in the urine or serum and air is poor
(Kiviluoto et al., 1979a,c). In view of the work of Schroeder
et al. (1963), it would seem desirable to measure the vanadium
contents of the serum separately from that of the cellular
elements, since the concentration of vanadium in the latter may
be more indicative of exposure levels. As reported by Watanabe
et al. (1966), a decreased urinary output of ascorbic acid may
be one characteristic of vanadium exposure, but differences from
controls do not appear sufficient to make the test clinically
useful.
Green colouration of the tongue is also an indication of
vanadium exposure (Wyers 1946; Williams, 1952; Lewis 1959b). The
green hexaquo ion (V(H2O))63+ is probably responsible for the
green-coloured tongue. However, several other bright green
complexes of vanadium+4 are known and may also account for the
sign (Cotton & Wilkinson, 1962; Durrant & Durrant, 1970). The
"green tongue" may be absent, even in prolonged exposure
(Sjöberg, 1950).
During continuous exposure, measurement of the cystine
content of fingernails was reported to be a sensitive indicator
of exposure. This parameter was negatively correlated with
vanadium exposure in workers. A decrease in cystine levels in
fingernails was demonstrated when urinary-vanadium levels were
only 0.02 - 0.03 mg/litre (Mountain et al., 1955). A similar
reduction in the cystine content of rat hair was noted when
vanadium in the diet ranged from 25 -1000 mg/kg (Mountain et
al., 1953). Some evidence suggesting that vanadium may directly
inhibit the synthesis of cystine or cysteine has also been
reported (Mountain et al., 1953, 1955).
In a recent study on workers exposed to low levels of
vanadium pentoxide (0.1 - 0.6 mg/m3) for about 14 years,
Kiviluoto et al. (198O) could not corroborate the observations
by Mountain and co-workers as no differences were found in
fingernail cystine contents between the 22 exposed workers and
22 unexposed controls. A small reduction in the cystine content
of fingernails (89 mg cystine/kg fingernail for exposed and
99 mg/kg for controls) was found by Thürauf et al. (1979) in 54
exposed workers with an increased urine-vanadium concentration
of 37.8 µg/litre (controls 0.8 µg/litre).
8.2.4 Treatment of poisoning
There are few published data on the treatment of human
poisoning by vanadium. BAL has been used successfully in two
cases of overexposure (Sjöberg, 1955). Experimentally, ascorbic
acid in doses of 125 mg/kg body weight given 20 min prior to an
LD70 dose of NaVO3H2O had a strong protective effect in mice
(Mitchell & Floyd 1956). CaNa2-EDTA was also antidotal in dogs,
when given intraperitoneally in doses of 100 mg/kg body weight
after the first sign of poisoning became evident and again 2 and
4 h later. Jones & Basinger (1983) tested various chelating
agents and their protective effects in mice and found that
efficient antidotes for both vanadate (VO33- and vanadyl (VO2+)
were ascorbic acid, deferoxamine D-penicillamine, sodium,
calcium, Na3CaDTPA, Na2CaEDTa, and glutathione. Ascorbic acid
appeared best suited for human use as an antidote.
Intraperitoneal doses of NaVO3 (0.3 - 1.2 mmol/kg body
weight) were injected in mice followed by chelating and reducing
agents at one-quarter of their respective LD50s. Significant
increases in the survival rate, 14 days after the treatment,
were noted with ascorbic acid, deferoxamine, and tiron (4,5-
dihydroxy-1,3-benzene-disulfonic acid). Other chelators tested
included EOTA, DTPA (Na3Ca-diethylene triaminepenta-acetate) and
L-cysteine. Ascorbic acid was the most effective substance in
preventing vanadium intoxication (Domingo et al., 1986). In
another report, sodium salicylate and D-L-penicillamine were
found useless as antidotes for acute toxicity caused by NaVO3.
8.3 General Population Exposure
8.3.1 Low vanadium intake
Because conditions required to achieve reproducible vanadium
deficiency in animals have not been defined precisely, it is
difficult to predict the consequences of a low vanadium intake
on human health.
Statistical studies have shown negative correlations between
environmental levels of vanadium and certain other trace
elements and the incidence of cardiovascular disease. Consump-
tion of hard water containing vanadium was associated with a
lower incidence of cardiovascular disease (Strain, 1961)a.
Schroeder (1966) reported a significant negative correlation
between the vanadium content of municipal waters and death rates
due to arteriosclerotic heart disease. In a study by Voors
(1971) on the correlation between 7 metals (calcium, chromium,
lithium, zinc, manganese, nickel, vanadium) and arteriosclerotic
heart disease, a low vanadium intake was associated signifi-
cantly with a higher incidence of arteriosclerotic heart disease
in non-white populations, but no direct correlation was
demonstrated for white populations.
____________________
a Strain, W.H. (1961) Effects of some minor elements in
animals and people. Paper presented at the meeting of the
American Association for the Advancement of Science, Denver,
29 December 1961 (unpublished).
In a joint WHO and IAEA study on the role of trace elements
in the etiology of cardiovascular diseases in 20 countries, a
significant role was shown for environmental lack of vanadium as
well as chromium, zinc, manganese, calcium, and magnesium
(Masironi, 1969). Conversely, Hickey et al. (1967) noted a
positive correlation between airborne vanadium levels and the
incidence of cardiovascular disease (section 8.3.2).
The evidence implicating vanadium as an essential trace
element for human beings is not satisfactory. Although certain
statistical studies have indicated that low vanadium intake may
be associated with human cardiovascular disease, these
relationships do not furnish any direct proof for a nutritional
role of vanadium in human health. However, they do suggest leads
for further laboratory and epidemiological investigations.
8.3.2 Epidemiological studies
Descriptive epidemiological work has been published using
a correlational approach, which has well-known limitations,
though it imitates a population-based cohort study.
Despite their limitations, such studies can give indications for
more intensive and detailed controlled studies into suspected
health hazards, comparing incidences of diseases in defined
exposure groups (such as production workers) with those obtained
from reference populations.
Stocks (1960) reported the results of a study in which
airborne concentrations of 13 trace elements were correlated
with mortality from lung cancer, pneumonia, and bronchitis in 23
localities in the United Kingdom. At concentrations ranging
from 1.1 to 42 µg/1000 m3, vanadium showed a weak association
with mortality from lung cancer (taking into consideration
population density, sex, and age), with a correlation
coefficient of 0.347. Airborne vanadium levels were also
correlated with mortality from pneumonia in males, with a
correlation coefficient for mortality from pneumonia of 0.443.
For mortality involving bronchitis, vanadium gave a correlation
coefficient of 0.563. Vanadium also showed an association with
mortality from cancers other than lung cancer in males, but not
in females. However, in this study, as is usual in studies of
this kind, it is not certain that cases of interest (lung
cancer, pneumonia) had been exposed at all. There are also the
uncertainties of mortality data and failure to consider
confounding factors.
In another study, Hickey et al. (1967) considered 10 metals
in the air, including vanadium, in 25 communities in the USA.
Various techniques, including canonical analysis, were used to
correlate airborne metal concentrations with mortality indices
for 1962 and 1963 involving 8 disease categories. The mean
atmospheric concentrations for vanadium at the various locations
ranged from 0.001 to 0.672 µg/m3. The incidence of several
diseases, including "diseases of the heart", nephritis, and
"arteriosclerotic heart", could be correlated reasonably well
with air levels of vanadium and other metals. A high inter-
correlation between vanadium and nickel was unexplained. This
study was of a very preliminary nature, with no adjustments for
the basic pertinent variables normally employed. Other studies
have demonstrated significant negative correlations between the
incidence of cardiovascular disease and environmental levels of
vanadium (section 8.3.1).
An additional multivariate analysis of air-vanadium levels
in relation to selected white male mortality levels was included
in an unpublished US Environmental Protection Agency staff study
by Pinkerton et al. (1972)a. Several categories of cardio-
vascular disease were used, and also influenza-pneumonia.
Vanadium was not correlated with the latter, but was correlated
with the cardiovascular categories. However, adjustment for
population density produced a considerable reduction in some of
these relationships. It was concluded that the observed
statistical associations of air-manganese and air-vanadium
levels were not causal associations, and represented either a
reflection of other more directly associated causes or
statistical artifacts.
Barannik et al. (1969) studied the role of certain trace
elements and the natural radioactivity of food products in the
etiology of endemic goitre in the USSR. More chromium and
vanadium and less lead were found in most of the vegetable
products from a region where goitre was endemic compared with
those from a goitre-free region. The differences in the mean
concentrations of these trace elements were statistically
significant.
The differences between these general population-based
observations and the occupational studies on health effects in
vanadium workers (section 8.3) are connected to different
approaches. The correlational epidemiological studies, based
exclusively on long-term effects and causes of death, are
considered at the expense of lack of individual exposure data,
while the medical studies, cross-sectional in nature, cannot
consider the selection effects and lack long-term information
(such as cause of death). Their strength, however, lies in the
fact that they permit analysis according to different levels of
exposure, though further occupational and population studies on
chronic illness in unambiguous relationship to vanadium exposure
are needed to verify previous work and determine if there is
evidence of a dose-response relationship. In such studies,
morbidity as well as mortality should be considered.
______________
a Pinkerton, C., Hammer, D.I., McClain, K., Williams, M.E.,
Bridbord, K., & Riggins, W.B. (1972) Relationship of
manganese and vanadium in the ambient air to heart disease
and influenza-pneumonia mortality rates, Research Triangle
Park, North Carolina, US Environmental Protection Agency
(unpublished data).
8.4 Occupational Exposure
Occupational poisoning occurs mainly during the industrial
production and use of vanadium and in boiler cleaning
operations. Under these conditions, vanadium may enter the
human body through the respiratory tract; an unknown quantity
will be transported to the alimentary tract when swallowed.
Vanadium can also enter through the skin (Roshchin, 1968).
Both acute and chronic poisoning can occur. Vanadium-
containing industrial aerosols differ in chemical and structural
composition and thus evoke different responses in the human
body.
In sections 8.3.1 - 8.3.4, a survey is made of the available
clinical and epidemiological data on the health effects of
vanadium in workers occupationally exposed to vanadium
compounds. Most of the reported clinical symptoms reflect
irritant effects of vanadium on the respiratory tract and eyes.
8.4.1 Metallurgy
Dutton (1911) first described the effects of industrial
exposure to vanadium-bearing ores. He reported a dry, paroxysmal
cough with haemoptysis and irritation of the eyes, nose, and
throat. Temporary increases in haemoglobin levels and red blood
cells were followed by reductions in both and the onset of
anaemia. Vanadium was recovered in all bodily secretions.
Postmortem examination revealed highly congested lungs with
destruction of the alveolar epithelium and congested kidneys
with evidence of haemorrhagic nephritis. Unfortunately, the
workers frequently suffered from pulmonary tuberculosis, which
undoubtedly produced many symptoms that were aggravated by
vanadium exposure, and no details regarding the number of
workers examined or the incidence of the signs and symptoms were
provided.
A later study by Symanski (1939) on relatively healthy metal
workers exposed to vanadium pentoxide dust for periods ranging
from a few months up to several years reported severe conjunct-
ivitis, rhinitis, pharyngitis, chronic productive cough, and
tightness of the chest; severe chronic bronchitis and bronchi-
ectasis sometimes occurred with longer exposure. There was no
evidence of a generalized systemic action of vanadium.
Rundberg (1939) observed bronchitis with purulent sputum,
general weakness, and skin irritation of the face and hands in
20 men handling vanadium pentoxide in a metallurgical works.
Productive cough, bronchitis, and shortness of breath were
reported by Balestra & Molfino (1942) in 25 workers exposed to
vanadium pentoxide dust from petroleum ash. Other substances
were involved, and chest X-rays showed definite lung markings
suggesting pneumonoconiosis. Bronchiectasis was suspected in 2
cases.
Studies were reported by Wyers (1946, 1948) on 50 - 90
workers exposed to vanadium pentoxide as an oil combustion
residue and to slag from the production of ferrovanadium.
Findings included bronchospasm, often with elevated blood
pressure and accentuated pulmonary second sound, a paroxsymal
cough, dyspnoea, skin pallor, tremor of fingers, palpitation,
chest pains, and reticulation of the lungs. Wyers emphasized
the irritant effects of vanadium pentoxide on the respiratory
tract, but also found evidence of systemic toxicity.
An extensive report including data on the dust contents of
the air in a metallurgical plant producing vanadium pentoxide
was published by Sjöberg (1950). The dust particles were
relatively large in size (39% less than 12 µm, 22% less than
8 µm). It was estimated that a concentration of 6.5 µg
V205/m3 represented the worst exposure conditions. Thirty-six
men between 20 and 50 years of age had been employed in the
plant since 1946: 22 had a dry cough; wheezing sounds could be
detected in 31; and 27 were short of breath. One man developed
acute pneumonitis, and 4 others developed bronchopneumonia.
There was no evidence of systemic toxicity.
A dry eczematous dermatitis developed in 9 men in Sjöberg's
(1950) study, but only 1 man showed a positive patch test.
Sjöberg (1951) and Sjöberg & Rigner (1956) believed that allergy
might play a role in the development of eczema and pneumonitis
following vanadium exposure. Zenz et al. (1962) also considered
this an explanation for the more severe symptoms found on re-
exposure in their study. In a follow-up to the 1950 study,
Sjöberg & Rigner (1956) reported that the 16 men most severely
affected still complained of shortness of breath, cough,
fatigue, and wheezing. Bronchitis was present in 2 men.
However, spirometric measurements, cardiac function tests,
electrocardiograms, haematological tests, and urinanalyses were
essentially normal.
Lewis (1959b) studied 24 male workers in an environment in
which the maximum exposure was only 0.925 mg vanadium (as
V205)/m3 of air. In most cases, the exposure was to 0.3 mg
vanadium/m3. More than 92% of the dust particles were smaller
than 0.5 µg in every process area sampled. Symptoms of cough
with sputum production, eye, nose, and throat irritation, and
wheezing were related to physical findings of wheezes, rales, or
rhonchi, injected pharynx, and green tongue. All of these
symptoms and physical findings were statistically significant in
comparison to those in 45 referents (Tables 25 and 26).
A report by Rajner (1960) on 30 vanadium workers in a
metallurgical plant described particularly severe signs and
symptoms, but did not give any estimates of exposure except in
conjunction with urinary-vanadium levels. In acutely poisoned
workers, vanadium values were about 4000 µg/litre urine. The
average value among permanent employees was 45 µg/litre;
vanadium pentoxide smelter workers had maximum values of about
400 µg/litre. When a new production process was introduced,
symptoms of acute vanadium poisoning occurred in 3 workers after
16 h of work including severe respiratory difficulties, head-
ache, dejection, and loss of appetite. Acute inflammatory
changes of the upper respiratory tract with copious mucous
production, oedema of the vocal cords, and profuse epistaxes
were reported. All workers who had been exposed for a long time
(up to 22 years in 27 subjects, mostly in ferrovanadium and
vanadium pentoxide smelting operations) complained of coughing
and eye, nose, and throat irritation, breathing difficulties
during physical exertion ("more than two-thirds of the
workers"), and headache (12 cases). Clinical findings included
intense hyperaemia of the mucosa of the nasal septum in 20
workers; perforation of the nasal septum was seen in 4 workers
exposed for an average of 18 years. Intense hyperaemia of the
mucosa of the throat and larynx with dilated fine capillaries
was found in 50% of the workers. Bronchoscopy indicated the
presence of chronic bronchitis, and bronchial smears revealed
sloughed epithelium.
Matantseva (1960) studied 77 workers in contact with
vanadium pentoxide in the form of dust and fume in concentra-
tions exceeding the MAC value (dust = 0.5 mg/m3; fumes =
0.1 mg/m3) for periods ranging from 1 to 12 years. Nearly all
the subjects had various complaints relating to the upper
respiratory tract including unpleasant sensations in the nose, a
liquid mucous discharge from the nose, obstructed nasal
breathing, a sensation of burning and dryness in the naso-
pharynx, scratching, dryness, and tickling in the throat,
hoarseness of the voice, and cough. Physical examination showed
rhinitis, which was of a simple catarrhal form in workers
exposed for less than 3 years, a hypertrophic and subatrophic
form if the exposure was for more than 3 years, and an atrophic
form if the exposure was for between 7 and 12 years. Examina-
tion of the lungs revealed acute and chronic lesions in the form
of bronchitis, peribronchitis, and pneumosclerosis. Hyper-
ventilation and an elevated basal metabolic rate were noted.
Table 25. Symptoms in 24 vanadium workers and 45 unexposed referentsa
-----------------------------------------------------------------------------
Symptom Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Cough 33.3 83.4 13.71b
Sputum 13.3 41.5 5.55c
Exertional dyspnoea 24.4 12.5 0.592
Eyes, nose, throat 6.6 62.5 23.17b
irritation
Headache 20 12.5 0.124
Palpitations 11.1 20.8 0.538
Epistaxis 0 4.2 0.148
Wheezing 0 16.6 5.20c
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
c Significant at P = 0.02.
Table 26. Physical findings in 24 vanadium workers and 45 unexposed
referentsa
-----------------------------------------------------------------------------
Physical finding Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Tremors of hands 4.5 4.2 0.0320
Hypertension 13.3 16.6 0.0002
Wheezes, rales, 0 20.8 6.93b
or rhonchi
Hepatomegaly 8.9 12.5 0.003
Eye irritation 2.2 16.6 2.94
Injected pharynx 4.4 41.5 12.62b
Green tongue 0 37.5 14.53b
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
Roshchin (1963b) published an account of the effects of
vanadium-containing Bessemer slag dust on 45 workers. Dust
concentrations in the air during various phases of this opera-
tion ranged from 5 to 150 mg/m3, with the highest concentrations
occurring during loading/unloading of broken slag as the
trivalent oxide, mostly within spinellide. Repeated examinations
of the 45 workers showed the slag dust to have an effect on the
respiratory mucosa. Subatrophic rhinitis, bronchitis, and
pneumosclerosis were seen in subjects with long occupational
exposure (11 workers). Chronic bronchitis was found in every
worker employed for 5 years or more. Clinical and X-ray
examination of all 45 subjects showed radiological changes in 24
employed for 10 years or more; in 11 subjects, pneumoconiosis of
stage I-II was diagnosed. X-ray examination showed diffuse
sclerotic changes over the whole extent of the lung fields
(except for the supraclavicular zones), small focal opacities,
intensified and enlarged shadows at the root of the lungs, and
marked signs of bullous emphysema. Predominant involvement of
the lower regions of the lungs (characteristic of silicosis) was
not present. This pneumosclerosis was accompanied by changes in
the cardiovascular and nervous systems, biochemical disturbances
(hyper-vitaminosis with dysproteinaemia and an increase in the
serum concentration of sulfhydryl groups), a tendency to anaemia
and leukopaenia, and changes in the liver.
In another study, Roshchin (1964) described chronic effects
of vanadium in 193 workers who had been exposed to aerosols of
free vanadium pentoxide: 127 worked in vanadium metallurgy and
66 were boiler cleaners (section 8.3.2). The length of
occupational contact with vanadium was over 10 years for 60%,
from 5 to 10 years for 30%, and under 5 years for the remaining
10%. Practically all complained of irritation of the nasal and
pharyngeal mucosa including itching, a profusely running nose
(especially during work), and unpleasant sensations in the
throat and nose. Epistaxis was frequent in 20%. Physical
examination revealed a high incidence of changes in the nasal
mucosa: dryness (40%), erosion (23%), scars (8%), perforation
(4%), hyperaemia (10%), and hypertrophy (7%). Also noted were
dryness of the pharynx (5%), hyperaemia of the pharynx (5%),
hyperaemia of the larynx (4%), and tonsillitis (5%). The most
common pathological changes in the upper respiratory tract were
subatrophic rhinitis (40%) and destructive changes in nasal
mucosa (35%), while hypertrophic rhinitis was less frequently
seen (7%). The overwhelming majority had a dry cough; cough
with viscid sputum was less common. Workers with longer
occupational exposure complained of shortness of breath, which
appeared sometimes after 5 but mostly after 10 years of work in
the industry. Nearly all complained of aching or shooting pains
in the chest and of lassitude and weakness. The main respiratory
diseases diagnosed were chronic bronchitis (40%) and diffuse
pneumosclerosis (13%). Haematological tests showed the total
serum-protein concentration to be normal, y-globulins to be
raised (19.4% compared with 12.2% in controls), and the albumin-
globulin ratio to be 1:1 - 1:2 (1:9 in controls). Determination
of total, residual, and protein sulfhydryl groups in the blood-
serum revealed a marked decrease of 7 - 13% compared with the
controls. Regular observations over a period of 14 years showed
that the chronic bronchitis tended to get worse, with develop-
ment of bronchospasm. After a long period of time, some subjects
developed pneumosclerosis; in others, the disease progressed
slowly from chronic bronchitis to diffuse pneumosclerosis and
pulmonary emphysema.
Eisler et al. (1968) studied 48 metallurgical workers
occupationally exposed to vanadium for between 17.6 ± 9 years.
Definite clinical evidence of chronic bronchitis was present in
90% of the subjects, and 50% had severe obstructive bronchitis.
In control groups, which included basic-slag crushers and
furnace operators (99 and 50, respectively), chronic bronchitis
was observed in 33% and 26%, respectively.
A study on 13 workers engaged in the extraction and refining
of vanadium pentoxide from soot generated by the combustion of
heavy fuel oil was reported by Nishiyama et al. (1977).
Concentrations of vanadium in the air at various locations in
the work environment were all less than 0.5 mg/m3 (mean, 1.2 -
12 µg/m3). There was a significant incidence of injection of
the pharynx (58.3%) compared with controls. Elevated levels of
vanadium in the urine and hair were detected both in currently-
exposed as well as in previously-exposed subjects. Apart from a
slight depression in serum-cholesterol levels, haematological
results were normal.
Roshchin (1968) analysed the incidence of influenza and
upper respiratory catarrh as a cause of lost working time in
workers in vanadium metallurgical plants compared with ferrous
metallurgical workers in adjacent plants. The results are given
in Table 27. The morbidity was consistently higher among
workers producing vanadium than among workers in other
departments in all years.
Table 27. Morbidity from influenza and upper respiratory catarrha
-----------------------------------------------------------------------------
Department Cases Days off work
(per 100 workers) (per 100 workers)
1958 1959 1960 1962 1958 1959 1960 1962
-----------------------------------------------------------------------------
Vanadium plant 40.6 68.4 58.8 59.8 180.6 376.7 271.9 336.5
Open hearth furnace 22.2 53.2 45.6 62.1 76.9 331.1 176 213.3
Blast furnace 21.9 46.7 37.8 47.6 36.3 262.8 166.6 215.5
Engineering shop 19.6 39.1 33.9 44.9 86.8 224.8 154.3 223.6
-----------------------------------------------------------------------------
a From: Roshchin (1968).
Asthma was reported in 4 workers exposed to vanadium
pentoxide dust in a newly established vanadium pentoxide
refinery (Musk & Tees, 1982). One of the workers had positive
skin tests to environmental allergens; the others were non-
atopic. Three were smokers; one was an ex-smoker. One of the
subjects experienced irritation of the upper respiratory tract
after a single exposure; dyspnoea and wheezing developed 2 weeks
later. All workers had similar irritant symptoms and green
tongue. Two showed bronchial hyperreactivity when challenged
with histamine; these were the workers with the most recent
exposure. In one worker, the asthmatic symptoms continued for 8
weeks after cessation of exposure. There was no indication of
an immunological aetiology, and the authors concluded that the
effect was likely to be a direct chemical one.
Kiviluoto et al. (1979a,b, 1980, 1981a,b) and Kiviluoto
(1980) reported the results of a cross-sectional study on 63
males exposed to vanadium-containing dust in a vanadium factory;
a reference group matched for age and smoking was selected from
a magnetite ore mine. The workers had been exposed to vanadium
dust for an average of 11 years at concentrations ranging from
0.1 to 3.9 mg/m3 (estimated average exposure levels of 0.2 -
0.5 mg/m3); the respirable fraction (< 5 µm) was 20%. Nasal
biopsies and lung function tests were taken at the end of the
summer holidays (duration, 2 - 4 weeks). Nasal smears and
biopsies were repeated in 31 workers, 7 - 11 months later, after
hygienic improvements had reduced the exposure levels to 0.01 -
0.04 mg/m3. Microscopic examination of nasal smears revealed a
significant increase in neutrophils and biopsies of nasal mucosa
showed significantly elevated numbers of plasma and round cells
in the exposed workers. There was no further increase in the
cell findings after 10 months of exposure to 0.01 - 0.04 mg/m3
vanadium dust; eosinophils did not show any differences between
the exposed and the referents. The authors attributed these
findings to "an irritating effect of vanadium dust on the mucous
membranes of the upper respiratory tract". Biopsies from
workers with the longest exposures (170 - 241 months) showed "a
zone-like sub-epithelial infiltration of mononuclear cells and
frequent papillarity in the mucous membrane surface with its
hyperaemic capillaries". The similarity between this pattern
and that seen in vanadium-exposed rabbits (Sjöberg, 1950) was
noted (Kiviluoto et al., 1979b). A random sample of 12 nasal
biopsies was further investigated for the amount and classes of
immunoglobulins (IgE, IgG, IgM, and IgD). IgG subclasses were
not studied. There were no differences between the 12 workers
and their referents, which was construed as a further indication
of non-specific inflammation (Kiviluoto et al., 1981b).
Pulmonary condition was assessed by means of questionnaires,
X-ray, and pulmonary function testing. There was only one
significant difference between the workers exposed for an
average of 11 years to 0.1 - 3.9 mg/m3 (estimated average, 0.2 -
0.5 mg/m3) and at the time of investigation to 0.01 -
0.04 mg/m3, and their matched referents; complaints of wheeze
were more common in the exposed worker group (Kiviluoto, 1980).
The importance of this finding remained doubtful. It may
reflect the respiratory findings mentioned above, since upper
respiratory irritation may be accompanied by transient reflex
bronchospasm. A series of laboratory tests were designed to
evaluate electrolyte, protein fractions, carbohydrate, and
lipids, liver, renal, muscle, pancreatic, and bone marrow
functions, and immunological status. There were no decreases in
serum-cholesterol or triglycerides, and no clinical differences
between worker and control groups (Kiviluoto et al., 1981a).
The effects of vanadium compounds on health is not confined
to the development of local respiratory or other lesions.
Various studies, most of them rather old, on patterns of lost
working time due to morbidity have shown that the incidence of
disease among workers in plants producing vanadium compounds is
considerably higher than among other workers (Symanski, 1939;
Syers, 1946; Sjöberg, 1950, 1956; Reznik, 1954; Reinl, 1958;
Matantseva, 1961; Watanabe et al., 1966; Roshchin, 1968, 1969;
Athanassiadis, 1969; Schumann-Vogt, 1969; Chiriatti, 1971). The
most significant differences are found in the incidences of
influenza, upper respiratory catarrh, and inflammation of the
lungs. The difference in the incidence of bronchitis is
particularly marked.
8.4.2 Cleaning and related operations on oil-fired boilers
Bronchitis and conjunctivitis resulting from exposure to
soot (containing 6 - 11% vanadium) during the cleaning of the
stacks of oil-fired boilers were first recognized by Frost
(1951). Frost did not report any other effects, but, in a
subsequent report of a boiler-cleaning operation by Williams
(1952), sneezing, nasal discharge, lachrymation, sore throat,
and substernal pain occurred within 0.5 - 12 h of exposure.
Within 6 - 24 h, secondary symptoms developed; these consisted
of dry cough, wheezing, laboured breathing, lassitude, and
depression. In some cases, the cough became paroxysmal and
productive. Symptoms lessened only after removal from the
working environment for 3 days. Air sampling showed most of the
dust particles to be smaller than 1 µg. The vanadium concen-
tration ranged from 17.2 mg/m3 in a superheater chamber to
58.6 mg/m3 in a combustion chamber. Roshchin (1962) observed 8
cases of acute vanadium poisoning in workers who cleaned boiler
flues at power stations burning high-sulfur oil. Analysis of
soot deposits showed that the soot in the region of greatest
dust formation (the pipes of the steam superheater and water
economizer) contained from 24 to 40% vanadium pentoxide. The
workers carried out cleaning operations without respirators or
with respirators that did not provide the necessary protection.
After cleaning the boilers, the workers developed acute vanadium
poisoning: itching in the throat, sneezing, cough with difficult
expectoration, and smarting eyes. On the following days, the
symptoms became more severe. Tightness in the chest, sweating,
general weakness, conjunctivitis, and noticeable loss of weight
developed. On examination one week later, hyperaemia and oedema
of the fauces and posterior pharyngeal wall were observed.
Harsh breathing sounds and dry crepitations were heard in the
lungs. X-ray examination showed intensified lung markings in
the middle zones of the right and left lungs and thickening of
the fissure on the right. One month later, only one worker
still had cough, weakness, perspiration, loss of energy, and
dyspnoea. The other workers recovered quickly, with complete
disappearance of cough and shortness of breath.
In another study on workers engaged in boiler-cleaning
operations (Troppens, 1969), the symptoms were described as
similar to mild coryza or influenza with bronchitis. Following
recovery, workers were tired, debilitated, irritable, without
any appetite, and complained of watery eyes. The first symptoms
were swelling of face and eyes as early as 20 min after entering
the boiler area. Removal from exposure for 2 - 3 weeks resulted
in the disappearance of symptoms. Skin blemishes described as
allergic dermatoses were attributed to absorption of vanadium
through sensitive skin. Troppens claimed that there was an
increased susceptibility of the vanadium worker to asthmatic
bronchitis and emphysema.
An investigation is reported on 53 workers performing
emergency repair work on oil-fired power station boilers (Izycki
et al., 1971). They were exposed to vanadium pentoxide in
average concentrations of from 1.2 to 11 mg/m3 and also to
manganese, calcium, and nickel oxides, and sulfur compounds.
Characteristic features of both acute and chronic vanadium
poisoning included upper respiratory catarrh in 45%, increased
lung markings in 24.5%, and bradycardia in 22% of cases.
Persistent chronic changes in the respiratory tract (rhinitis,
pharyngeal catarrh, laryngitis, and changes in the paranasal
sinuses) were present in 45%.
Milby (1974) reported 21 cases of vanadium poisoning in
boilermakers installing new catalytic-converter tubes. This
work involved marble-sized pellets of vanadium containing 11.7%
V2O5. The dust formed during the shaking of these pellets had a
particle size of 1.1 - 1.5 µm. After working for 72 h, the
workers began to complain of nasal, eye, and bronchial
irritation. By the 4th day, most felt very ill, with signs of
irritation of the upper respiratory tract and eyes and pains in
the chest.
In a study by Garlej (1974) 50 workers engaged in the
cleaning of oil-fired boilers were compared with a control group
of 60 other workers. Boiler deposits contained 44 -65% V2O5;
the maximum exposure was estimated to be 10 mg/m3. Although no
clinical evidence of vanadium poisoning was seen, a number of
exposure-dependent positive biochemical reactions were found in
the boiler-cleaning group. Urinary excretion of delta-amino-
levulinic acid (ALA), porphobilinogen (PBG), and porphyrin
increased beyond the physiological limit, and the positive Nadi
reaction (with associated green fluorescence) occurred. The
increased excretion of cytochrome (as indicated by the Nadi
reaction) suggested oxidation through V2O5 of the thiol group
-SH cysteine in the protein carrier, resulting in decreased
binding of cytochrome in the mitochondria.
A study on 17 men who were engaged in cleaning boilers at an
electric generating station was reported by Lees (1980). In
addition to clinical findings, which were similar to those
described above, urine-vanadium levels were determined, and
pulmonary function measurements were made for a week following
exposure. Sixteen of the men wore protective clothing, and
respirators that were found to have about 9% leakage. One
workman volunteered to wear only a simple oro-nasal dust mask
for 1 h of exposure. The dust exposure level was estimated to
be 26 mg/m3; respirable dust (under 10 µm) was measured at
523 µg/m3 with a vanadium content of 15.3%. All of the men
developed reduced pulmonary function that had not fully returned
to normal in one week, but did so after one month. Reduced
function outlasted the clinical symptoms by several days. Fig. 3
shows the contrast in pulmonary reaction between the more
heavily exposed individual and one of the other workmen. The
urine-vanadium level of the volunteer was 280 µg/litre, whereas
those of the remainder of the workers were below 40 µg/litre.
Boyarkina et al. (1978) studied vanadium precipitation in
and around an industrial city, in winter, by measuring
concentrations in snow. In the central area, a concentration of
24 µg/litre was found, decreasing to 3.2 µg/litre at a
distance of 40 - 50 km.
4.1.2 Water
Natural background levels of vanadium in water have been
discussed in section 3.1.3, and levels in industrial effluent,
in section 3.4.1. Durfor & Becker (1963) included vanadium in
their analyses for trace elements in drinking-water supplies in
large cities in the USA. Of the samples analysed, 91% showed
less than 10 µg vanadium/litre; the maximum concentration was
70 µg/litre, and the average was about 4.3 µg/litre. Many of
the samples were negative. Twenty-six percent of 3676 tap water
samples from 34 areas in the USA contained vanadium at
concentrations ranging from 1.3 to 33 µg/litre with a mean of
4.85 µg/litre (Greathouse & Craun, 1979).
Hoffmann et al. (1972) published data from a regional well-
water survey in Poland. The average vanadium concentrations
were 0.06 - 6 µg/litre, with a maximum single value of
15 µg/litre. Bottled waters from mineral springs frequently
contained higher levels; Schlettwein-Gzell & Mommsen-Straub
(1973) reported a range of 4 - 290 µg/litre in bottled waters
from Switzerland.
Highly-mineralized waters in Argentina contained 0.3 -
10 µg vanadium/litre (Trelles et al., 1970). These concentra-
tions often occur in conjunction with high concentrations of
arsenic and/or fluorides.
4.1.3 Food
4.1.3.1 Individual foods
Information on the vanadium contents of human food is
sparse. Data from two studies (Myron et al., 1977; Byrne &
Kosta, 1978) are combined in Table 11 with those from an earlier
study using a similar method (Söremark, 1967). The results of
these studies agree reasonably well, but differ in some respects
from the results of earlier work, especially that of Schroeder
et al. (1963). The principal difference is that Schroeder noted
high vanadium concentrations in fats and oils, whereas low
concentrations were found in the more recent studies. Such a
difference may be accounted for by the use of different
analytical methods. Similar discrepancies have been encountered
in the study of other trace elements, i.e., lower concentrations
have been found using more recently developed methods.
The data presented in Table 11 show low levels of vanadium
in most elements of the human diet. There are also some
interesting differences among specific foods. Grains contain
higher levels of vanadium than fruits and vegetables. Levels in
oils and fats and beef and pork are low, but those in the liver
and kidneys of cows and pigs are higher. Higher levels are
found in both the flesh and internal organs of the chicken, and
levels in fish flesh are also high. Vanadium levels in milk and
eggs are low, and those in beer and wine are high. Myron et al.
(1977) pointed out that processing appears to raise levels in
food (e.g., white versus brown rice; cereal, flour, bread, and
gluten versus grain; peanut butter; bologna and bacon versus
pork). No explanation was given for the very high levels in
dill and parsley.
Table 11. Vanadium concentrations in foods (µg/kg)a
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Grains
Wheat 3.6
Flour 15, 40
Bread 11.20 10, 13
Gluten 33
Oats 3
Oatmeal 6
Corn 0.7
Cornmeal 2
Brown rice 1
White rice 21 12, 30
Barley 14 1.6
Cereal 93
Fruits
Apple 1.1 4 0.3
Pear 0 0.2
Banana 3 0.2
Orange 1
Cherry 0.4
Apricot 0.2
Peach 0.2
Strawberry 31.41 (dry)
Blueberry 1.6
Vegetables
Potato 0.8 1 1.2, 1.9
Radish 52 5 0.6
Carrot 0 1 2.3, 2.4
Beet 0
Garlic 0.6
Onion 0.6
Leek 0.3
Navy bean 14
Pea 0 7 0.4
Tomato 0.03 2 0.3
Cucumber 2.1
Squash 4
Brussels sprout 0.5
Cauliflower 0.08 1 0.9
Cabbage 2 0.3
Lettuce 21 4 1.0, 2.7
Table 11 (contd).
---------------------------------------------------------
Study 1 Study 2 Study 3
---------------------------------------------------------
Spinach 35
Parsley 790 1800 (dry)
Mushroom 50 - 2 000 (dry)
Dill 140 431
Meats
Beef 0 1 0.4 - 1.3
Beef liver 2.4, 10 6 7.3
Pork 0 1 0.6, 0.9
Pork liver 8.4
Pork kidney 8.5
Bacon 5
Bolgna 8
Chicken, white 22 1.7
Chicken, dark 12
Chicken liver 37, 38
Chicken kidney 18
Cod 28 7.2
Mackerel 2.6 3.5
Tuna 11 10, 3
Lobster 43 5
Scallop 22
Oils and fats
Margarine 4
Soybean oil 1
Corn oil 1 3
Pumpkin seed oil 0.2
Lard 2 0.2
Nuts
Hazel nut 3.7
Peanut butter 44
Dairy products
Milk 0 - 0.1 3 0.2, 0.2
Powdered milk 0 - 0.2 25
Chocolate milk 21
Butter 1
Egg white 0.3 - 1.8
Egg yolk 2.0 - 3.6
Beverages
Coffee 1.6
Tea 1.3 0.3
Cola 0.7 1.5
Beer 11 8.4
Wine 3.5 - 3.2
---------------------------------------------------------
a Study 1: Söremark (1967) (neutron activation analysis).
Study 2: Myron et al. (1977) (atomic absorption spectroscopy).
Study 3: Byrne & Kosta (1978) (neutron activation analysis).
4.1.3.2 Complete diets
Byrne & Kosta (1978) estimated the daily intake of vanadium
to be "a few tens of micrograms," but added that it may vary
considerably. Assuming a very low rate of intestinal absorption
of vanadium in man (section 5.1.2), Byrne & Kosta calculated
intakes for 3 adults in whom they measured dietary
concentrations. The calculated intakes were 36, 66, and
11 µg/day, respectively.
Analyses of 9 selected hospital diets (Myron et al., 1978)
are given in Table 12. Each meal was prepared and analysed
separately by atomic absorption spectroscopy. The results were
similar to concentrations found in individual foodstuffs in
other studies given above. In a later study, Byrne & Kosta
(1979) reported on the determination of vanadium in total diet
samples obtained during a nutrition survey in 5 Italian towns
(Table 13). The concentrations agree with those reported by
Myron et al. (1978).
Table 12. Daily vanadium intake in dieta
------------------------------------------------------
Diet type (µg/day) (µg/g) (µg/1000
cal)
------------------------------------------------------
General-1 13.6 0.019 4.7
Cholesterol reducing-1 25.6 0.034 8.8
Cholesterol reducing-2 16.8 0.022 5.8
Cholesterol raising 30.1 0.046 10.5
General-2 28.0 0.040 9.8
Low calorie 12.4 0.029 10.6
Low salt 15.5 0.028 9.1
Puree 26.0 0.050 14.1
Soft 15.8 0.024 6.4
------------------------------------------------------
a Adapted from: Myron et al. (1978).
4.2 Occupational Exposure
In terms of occupational exposure, the most important
vanadium compounds are vanadium pentoxide, vanadium trioxide,
ferrovanadium, vanadium carbide, and vanadium salts, such as
sodium and ammonium vanadate. The oxides and salts are commonly
used in industry in powder form, giving rise to the possibility
of dust and aerosol formation, when the substances are crushed
or ground. Many metallurgical processes involve the production
of vapour containing vanadium pentoxide, which condenses to form
respirable aerosols. Boiler-cleaning operations generate dusts
containing the pentoxide and trioxide compounds. Combustion of
residual fuels with a high vanadium content is likely to produce
aerosols of the pentoxide as well as oxide complexes of vanadium
with other metals.
Table 13. Vanadium contents of Italian freeze-dried
total diet samplesa
--------------------------------------------------------
Town Dry Vanadium Daily
weight concentrationb vanadium
(g) (ng/g) intake (µg)
--------------------------------------------------------
Aosta 310 32.1 0.9 (2) 10.0
L'Aquila 173 46.0 3.4 (2) 8.0
Montfalcone 278 39.7 4.1 (2) 11.0
Mt. Amiata 377 29.7 0.8 (2) 11.2
Rome 285 42.3 4.6 (4) 12.0
Mean values 38.0 6.9 10.4 1.5
--------------------------------------------------------
a From: Byrne & Kosta (1979).
b Average and standard deviation; number of aliquots
in parentheses.
4.2.1 Metallurgy
The processing of metals containing vanadium includes
chemical treatment and high-temperature operations. However,
only moderate concentrations of vanadium were found in the
breathing zone of workers engaged in operations that would be
expected to produce the greatest fume exposure. During the
addition of vanadium to furnaces, concentrations ranged from
0.006 to 0.08 mg/m3 and, during tapping, from 0.004 to
0.02 mg/m3. Concentrations found in oxyacetylene cutting ranged
from 0.008 to 0.015 mg/m3 and, in arc-welding, from 0.002 to
0.006 mg/m3 (NAS, 1974).
Vanadium levels in metallurgical plants have been studied in
detail (Roshchin, 1968). Vanadium slag contains about 11 - 13%,
mainly in the form of trioxides of vanadium (measured as
vanadium pentoxide). Slags are used for the production of
vanadium pentoxide and ferrovanadium, and the process is
accompanied by extensive formation of iron oxide aerosol. Air
concentrations of the dust (mainly vanadium trioxide) found in
the main working positions (converter operator, mixer, and crane
driver) ranged from 20 to 55 mg/m3. Measured as vanadium
pentoxide, the contents of the swirling dust did not exceed
0.17 mg/m3. About 75% of the dust particles had a diameter of
less than 2 µm and 20% had a diameter of between 2 µm and
4 µm.
Breaking, loading and unloading, crushing and grinding, and
magnetic separation of vanadium slag causes thick dust
formation, with concentrations ranging from 30 to 120 mg/m3.
The slag contains 111 - 129 g vanadium pentoxide/kg. A diameter
of less than 2 µm was recorded for 70 - 72% of the particles;
86 - 96% had a diameter of less than 5 µm. When the slag is
roasted, free vanadium pentoxide is discharged into the work-
place air; atmospheric concentrations in the vicinity of
furnaces ranged from 0.04 to 1.56 mg/m3. During leaching and
precipitation, concentrations of vanadium in the air may be
high, sometimes exceeding 0.5 mg/m3.
The smelting and granulation of technical vanadium pentoxide
are accompanied by the formation of an aerosol. This aerosol
escapes when the product is poured for granulation. During the
loading of smelting furnaces, concentrations of vanadium
pentoxide ranged from 0.15 to 0.80 mg/m3. During smelting and
granulation, concentrations ranged from 0.7 to 11.7 mg/m3. In
other parts of the work-place, concentrations may range from
0.03 to 0.2 mg/m3.
In aluminium production, when bauxite is being converted
into alumina, the aluminate solutions accumulate vanadium salts,
which crystallize and precipitate out. Precipitated sodium
polyvanadate is smelted to form vanadium pentoxide, which is
cooled and settles in the form of thin plates. Vanadium
pentoxide dust (concentrations of up to 2.3 mg/m3) is given off
only in the terminal phase during tapping of the liquid product,
packing, and loading.
During the drying, sieving, and calcination of ammonium
vanadate and during the crushing, unloading, and packaging of
pure vanadium pentoxide, dusts are formed. When vanadium
pentoxide is sieved after calcination, the concentration in air
may range from 2.2 to 26 mg/m3. In plants with less
mechanization, incomplete sealing of equipment, and inefficient
local exhaust ventilation, concentrations of dust during these
operations ranged from 4.9 to 48.9 mg/m3.
In the production of ferrovanadium, there is a continuous
source of discharge of vanadium pentoxide and lower oxides
during the smelting process. Data on vanadium in air at various
sites are shown in Table 14.
Using spectrophotometric techniques, Roshchin (1968),
Katayeva & Sapunov (1974) and Kazimov (1977) found high
concentrations of vanadium during smelting and granulation
(range, 0.16 - 1.89 mg/m3; mean, 0.59 mg/m3; 104 samples),
production of ferrovanadium (range, 0.58 - 4.81 mg/m3; mean,
1.7 mg/m3; 110 samples), and roasting of the charge (range,
0.44 - 3.64 mg/m3; mean, 1.52 mg/m3; 112 samples).
Table 14. Vanadium levels in the air of a ferrovanadium planta
--------------------------------------------------------------------
Work-place/operations Vanadium pentoxide Lower oxides of
(mg/m3) vanadium (mg/m3)
--------------------------------------------------------------------
Work area of smelters and 0.1 - 2.6 0.05 - 1.2
helpers
Unloading of vanadium pent- 2 - 124.6
oxide from the bin and
charging of electric furnace
Crane driver's cabin during 0.07 - 9.43 0.03 - 0.1
smelting
Cutting up ferrovanadium 0.97 - 12.6
Maintenance of the furnace 7.5 - 30
--------------------------------------------------------------------
a From: Roshchin (1968).
Using high-volume sampling and atomic absorption analysis,
Usutani et al. (1979) measured vanadium pentoxide
concentrations in the air at several places in a vanadium
refinery. The highest concentrations (higher than 1 mg/m3) were
detected in samples collected during the removal of the vanadium
pentoxide flake. High-volume samples from other locations as
well as low-volume samples obtained over 6.5-h work shifts
showed lower concentrations (0.002 - 0.735 mg/m3).
When ductile vanadium is produced by the aluminothermic
process (based on the reduction of pure vanadium pentoxide with
aluminium powder), the violent exothermic reaction leads to the
release of a condensation aerosol of vanadium pentoxide. During
preparation of the charge mixture, work-place concentrations of
vanadium pentoxide ranged from 19 to 25.1 mg/m3. When the
burden was placed in crucibles inside the smelting chambers,
concentrations ranged from 64 to 240 mg/m3. During smelting,
concentrations at the operators' workplaces ranged from 0.17 to
0.6 mg/m3. Twenty to 30 min after smelting, levels declined to
0 -0.3 mg/m3. Ninety-eight percent of the condensation aerosol
particles produced had a diameter of less than 5 µm, and 82%
had a diameter of less than 2 µm (Roshchin, 1968).
In the production of vanadium by the vacuum carbon thermic
method, most of the pollution occurs during operations with
vanadium trioxide (Roshchin, 1968). Mixing of vanadium trioxide
in a closed mixer led to air concentrations in the workplace of
from 0.019 to 0.58 mg/m3. Unloading of the charge resulted in
high concentrations of 14.7 - 29.4 mg/m3. In the packing
department, when the charge was sifted in a fume cupboard,
concentrations in the breathing zone ranged from 0.58 to
4.7 mg/m3. When the charge was being weighed out and packed in
a fume cupboard, concentrations ranged from 3.38 to 6.76 mg/m3.
Levels of vanadium-containing dust and vanadium pentoxide in
the air during catalyst production are shown in Table 15.
Table 15. Air contamination in vanadium catalyst productiona
-----------------------------------------------------------------------------
Operation Dust (mg/m3) Vanadium pentoxide (mg/m3)
Minimum Maximum Most Minimum Maximum Most
frequent frequent
-----------------------------------------------------------------------------
Grinding and un- 5 45 7 - 9 1 7 1.5 - 3
loading vanadium
pentoxide
Loading ground 12 53 14 - 17 3.2 7.5 4 - 4.2
pentoxide into
the bin
Sifting and pack- 5 17.5 5 - 7 0.1 1 0.4 - 0.5
ing granules of
bulk contact
substance
-----------------------------------------------------------------------------
a From: Roshchin (1968).
4.2.2 Cleaning of oil-fired boilers
Significant occupational exposure to vanadium occurs during
the cleaning of boilers in oil-fired heating and power plants
and ships (Symanski, 1939; Roshchin, 1968; Kuzelova et al.,
1977; Levy et al., 1984). Fuel oil combustion results in the
formation of vanadium-containing dust, and large amounts of dust
result from operations connected with removing ash encrustations
in boiler cleaning and in cleaning the blades of gas turbines.
Most of these operations are carried out by hand, and the dust
in the air inside the boilers may range from 20 to 400 mg/m3,
the most common range being 50 - 100 mg/m3, with the dust
containing 5 - 17% vanadium pentoxide and from 3 to 10% of the
lower vanadium oxides (Roshchin, 1968). Kuzelova et al. (1977)
reported dust concentrations of 136 - 36 036 ml/m3 in the air
with vanadium concentrations ranging between 1.7 and 18.4
mg/m3.
Williams (1952) published air sampling data on boiler-
cleaning operations in the British power industry. He found
concentrations of soot dust at different points ranging from 239
to 659 mg/m3. The vanadium concentrations in the dust of the
superheater chamber was 40.2 mg/m3, while, in the combustion
chamber, the concentration was 58.6 mg/m3. Most (93.6%) of the
dust particles had a diameter of between 0.15 and 1 µm.
4.2.3 Occupational exposure limits
Some national occupational exposure limits for vanadium in
work-place air are shown in Table 16.
Table 16. Examples of occupational exposure limits for vanadium in various countries
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Australia Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Belgium Recommendation Time-weighted average (TWA) (fume) 0.05 ILO (1980)
Czechoslovakia Regulatory requirement Maximum allowable concentration (MAC) Hygienic Regula-
- Time-weighted average (TWA) 0.1 tions of the
- Ceiling value (fume) 0.3 Ministry of Health
- Ceiling value (dust) 1.5 of CSR (1985) (58)
(Regulation No. 7;
section 24/ZB)
Finland Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA) (fume)a 0.05
German Democratic Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1980)
Republic Short-term exposure limit (STEL)a 0.1
(fume)
Germany, Federal Recommendation 8-h time-weighted average (TWA)a 0.5 Federal Republic
Republic of of Germany Comm-
Short-term exposure limit (STEL)a 2.5 ision for Maximum
(30 min, 2x/shift) Work-Place Con-
centrations
8-h time-weighted average (TWA)a 0.1 (1985) (xxi, 16)
(fume)
Recommendation Short-term exposure limit (STEL)a 0.5
(30 min, 2x/shift) (fume)
Italy Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1985)
Hungary Regulatory requirement Time-weighted average (TWA) (fume)a 0.1 ILO (1984)
Netherlands Recommendation Time-weighted average (TWA) (fume)a 0.05 ILO (1980)
Table 16. (contd.)
---------------------------------------------------------------------------------------------------------
Country Legal status Exposure limit description Value Source
(mg/m3)
---------------------------------------------------------------------------------------------------------
Romania Regulatory requirement Ceiling value (fume)a 0.1 ILO (1980)
Sweden Regulatory requirement Ceiling valuea 0 ILO (1980)
Switzerland Regulatory requirement Time-weighted average (TWA)a 0.09 Permitted Values
in the Work-Place,
Berne (1984)
USA Recommendation Time-weighted average (TWA)a 0.05 ACGIH (1986)
(dust and fume)
Time-weighted average (TWA)a 0.5 OSHA (1977) (29
(dust) CFR.1910.1000)
Ceiling value (15 m)a 0.05 NIOSH (1977)
(dust and fume)
Time-weighted average (TWA)a 0.1 OSHA (1977) (29
(fume) CFR.1910.1000)
USSR Regulatory requirement Time-weighted average (TWA)a 0.1 ILO (1980)
Yugoslavia Regulatory requirement Maximum allowable concentration (MAC)a ILO (1980)
- Time-weighted average (TWA)a 0.1
---------------------------------------------------------------------------------------------------------
a Measured as V2O5.
5. KINETICS AND METABOLISM
5.1 Physiological Role
5.1.1 Microorganisms
Vanadium is essential for the mould Aspergillus niger
(Bertrand, 1942) and the green alga Scenedesmus obliguus (Arnon
& Wessel, 1953; Arnon, 1958). It may play a role in photo-
synthesis in the latter. A need for vanadium has been shown by
the yeast Candida slooffii at high temperatures (Roitman et al.,
1969). The growth effects of vanadium in Azotobacter and other
bacteria have been related to the ability of vanadium, in lieu
of molybdenum, to catalyse nitrogen fixation reactions (Horner
et al., 1942; Takahashi & Nason, 1957).
5.1.2 Animals
Vanadium deficiency has been reported in chicks (Hopkins &
Mohr 1971a; Nielsen & Ollerich, 1973) and rats (Schwarz & Milne,
1971; Strasia, 1971), and vanadium is considered essential for
these animals (Underwood, 1977; Vouk, 1979; Nechay, 1984).
Hopkins & Mohr (1974) observed reduced feather growth in
chickens. They also reported impaired reproduction, due to
decreased fertility, and increased perinatal mortality in rats
fed a low vanadium diet (10 µg/kg) over 4 generations. A
positive growth response in rats was observed by Schwarz & Milne
(1971) when vanadium salts, at levels of between 50 and
500 µg/kg were added to a semi-purified diet (vanadium content
unspecified) fed for 26 - 28 days.
In male Leghorn chicks fed vanadium in the diet at levels of
12.5 mg/kg and 25 mg/kg, body-weight gain was normal at 1, 2, 3,
and 4 weeks of age (Kubena et al., 1986).
Slower growth, higher haematocrits, and higher levels of
plasma- and bone-iron were reported by Strasia (1971) in rats
fed diets containing less than 100 µg vanadium/kg compared with
controls receiving diets containing 500 µg vanadium/kg, but
these results were not confirmed in another study by Williams
(1973). Hopkins & Mohr (1971a,b, 1974) reported that a diet
containing less than 10 µg vanadium/kg decreased plasma-
cholesterol levels in chicks at 28 days of age, increased
plasma-cholesterol levels at 49 days of age, and increased
plasma-triglyceride levels at 28 days of age. Nielsen &
Ollerich (1973) found that administration of a diet containing
30 - 35 µg vanadium/kg to chicks decreased growth, increased
haematocrits and plasma-cholesterol levels, and impaired bone
development.
Nielsen (1980) carried out further studies on chicks and
rats, given different types of low-vanadium experimental diets,
to examine the effects of vanadium deficiency. In rats,
vanadium deficiency adversely affected prenatal survival,
growth, physical appearance, haematocrit, plasma-cholesterol
levels, and hepatic lipid and phospholipid levels. In chicks,
low vanadium intake produced adverse effects on growth,
feathering, haematocrit, bone development, plasma-cholesterol
levels, and hepatic lipid, phospholipid, and cholesterol levels.
However, consistent deficiency effects were not observed in
chicks or rats in any of the studies. Nielsen (1980) suggested
that the inconsistency in vanadium deficiency effects might be
due to the fact that different experimental diets had different
effects on the metabolism of vanadium.
Vanadate appears to have an insulin-like action (Heyliger et
al., 1985). In female Wistar rats made diabetic with
streptozotocin (single iv injection of 55 mg/kg) and given
sodium orthovanadate in the drinking-water at a concentration of
0.6 - 0.8 mg/ml (corresponding to calculated daily intakes of
between 75 ± 3 and 100 ± 3 mg/kg body weight per day,
respectively) for 4 weeks, the serum-insulin level was low, but
there was no increase in blood-glucose levels compared with
controls. In diabetic rats not treated with vanadate, serum-
insulin levels were also low, but blood-glucose levels were
increased 3-fold. The cardiac performance of vanadate-treated
animals did not differ significantly from that of non-diabetic
controls. It was concluded that vanadate controlled blood-
glucose levels and prevented the decline in cardiac performance
due to diabetes.
The results of in vitro studies suggest that vanadium may
play a specific physiological role as a regulator of the sodium
pump (Macara, 1980). Vanadate has been shown to inhibit Na+K+-
ATPase (EC 3.6.1.3) in intact human red blood cells (Rifkin,
1965; Cantley et al., 1977, 1978a,b). It also has potent
diuretic and natriuretic effects in rats (Balfour et al., 1978;
Westenfelder et al. 1981). It was a powerful inhibitor of
Na+K+-ATPase in microsomal fractions of the kidney, brain, and
heart in several species, including human beings (kidney).
Mg2+-ATPase was up to 10 000 times more resistant to vanadium
inhibition than Na+K+-ATPase (Nechay & Saunders, 1978).
Similarly, in vivo studies on laying chickens fed calcium
orthovanadate for 15 months, at levels of 0.25, 50, or 100 mg/kg
diet, showed clear inhibition of Na+K+-ATPase activity in the
kidney (Phillips et al., 1982). The inhibition of Na+K+-ATPase
by vanadate can be reversed by catecholamines, though these do
not have any effects on the Na+K+-ATPase activity in the absence
of vanadate (Hudgins & Bond, 1979). The inhibition is also
partially prevented by the reducing agents ascorbic acid and
glutathione (Grantham & Glynn, 1979).
The mechanism by which cells reduce cytoplasmic vanadium5+
to vanadium4+ was investigated using human red cells (Macara et
al., 1980). The authors concluded that vanadate is reduced by
cytoplasmic glutathione and that this explains the resistance of
the Na++K+-ATPase to vanadium in intact cells.
Since vanadate (V5+) is a potent inhibitor of Na+K+-ATPase,
many physiological and biochemical processes are vanadium
sensitive (Grantham, 1980; Nechay, 1984). For example, vanadium
compounds inhibit ATP phosphohydrolases, ribonuclease, adenylate
kinase, phosphofructokinase, squalene synthetase, glycer-
aldehyde-3-phosphate dehydrogenase (Macara, 1980), glucose-6-
phosphatase (Singh et al., 1981), and phosphotyrosyl-protein-
phosphatase (Swarup et al., 1982). The membrane neutral (K+)-
p-nitrophenylphosphatase in the membrane fraction of skeletal
muscle was inhibited equally by vanadium4+ and vanadium5+ at 4 x
10-8 mol/litre (Vyskocil et al., 1981). Extracellular
application of both forms of vanadium failed to inhibit the
electrogenic (Na+ - K+) pump in intact mouse diaphragm fibres
(Vyskocil et al., 1981). In the concentration range from 10-5 to
10-3 mol/litre, vanadium4+ even potentiated the hyper-
polarization of the muscle fibres from -74 to -82 mV (Zemkova et
al., 1982), probably by increasing the intracellular potassium
level. These findings have led to the hypothesis that vanadate
could control sodium pump activity in vivo , perhaps via a
vanadium5+ : vanadium4+ equilibrium connecting pump activity to
the cellular redox state. However, vanadate in the red cell is
reduced to vanadium4+, which then binds to haemoglobin (Cantley
& Aisen, 1979), a reaction that seems to be essentially
quantitatively driven by glutathione (Macara et al., 1980), NADH
(Vyskocil et al., 1980), or by other mild reducing agents, such
as ascorbate or norepinephrine (Svoboda et al., 1984). The role
of the vanadium5+ : vanadium4+ redox equilibrium in the
regulation of cation flow across cell membranes has yet to be
unequivocally demonstrated.
Vanadium appears to be essential for chicks and rats, but it
does not appear to be essential in other species, as defined by
Mertz (1970), i.e., an element is essential if its deficiency
reproducibly results in an impairment of a function from optimal
to suboptimal. However, more research is needed before definite
conclusions can be drawn regarding the role of vanadium as a
nutritionally essential trace element for animals.
5.2 Absorption
The absorption and distribution of vanadium compounds depend
on the route of entry and the solubility of the compounds in
body fluids. The solubility of vanadium compounds in biological
media varies (Reznik, 1954). The following compounds are listed
in decreasing order of solubility: (a) in gastric juices,
vanadyl sulfate, sodium vanadate, ammonium vanadate, vanadium
pentoxide; (b) in blood-serum and in 0.22% sodium carbonate
solution, sodium vanadate, ammonium vanadate, vanadium
pentoxide, and vanadyl sulfate. The higher the solubility in
water and biological media, the more toxic the compound,
presumably because of better absorption (Roshchin, 1968).
5.2.1 Absorption by inhalation
5.2.1.1 Human studies
There is little information on the deposition of vanadium
compounds in the respiratory tract following inhalation.
However, the greatest deposition would be expected in the
submicrometre particle size fraction and particle size
distribution studies (Lee et al., 1972) have shown that most
vanadium-bearing particulate matter is very small and well
within the respirable range for human beings.
Soluble vanadium compounds inhaled and deposited in the
lung, are readily absorbed but the rates of absorption have not
been quantified and estimates have not been made of the amounts
of inhaled vanadium that are transported back to the pharynx by
mucociliary clearance, swallowed, and are then available for
absorption via the gastrointestinal tract. It has been
estimated that about 25% of soluble vanadium compounds may be
absorbed via the respiratory tract (ICRP, 1960). Absorption
from the respiratory tract was demonstrated in workers exposed
to vanadium dust, who showed increased concentrations of
vanadium in the urine (Lewis, 1959b; Gylseth et al., 1979;
Maroni et al., 1983).
5.2.1.2 Animal studies
Following acute exposure, there is complete clearance of the
relatively soluble vanadium pentoxide from the lung within 1 -
3 days (Sjöberg, 1950; Levina, 1972). Stokinger et al (1953)
demonstrated that vanadium is present for more than 40 days
following cessation of long-term exposure.
Intratracheal administration of 48V-vanadium nitrate (0.4
and 20 mg/kg body weight) to albino rats showed that the 48V
absorption rate was maximum after 5 min and could be detected in
internal organs after 30 min. Blood levels were initially high,
but fell to trace levels after 2 days. 48V was not detectable
after 4 days, but reappeared at 8 days and accumulated in all
internal organs, the greatest quantity accumulating in the bone
(Ordzhonikidze, 1977). In female Fischer rats exposed by intra-
tracheal instillation to 40 µg vanadium pentoxide in 0.9%
saline solution, the time for removal of 50% of the initial
burden was 18 min, but traces remained for a considerable time.
At 14 days, the vanadium was distributed principally in the
carcass (40%) and skeleton (12%) (Rhoads & Sanders, 1985). In
a study of the kinetics of vanadium following single or multiple
intratracheal administration, the blood concentration was high
initially and vanadium accumulated in the liver and kidney
reaching the highest level after 24 h (Roshchin & Ordzhonikidze,
1986).
Vanadium trioxide was cleared from the lung more rapidly
than pentoxide or ammonium vanadate following intratracheal
instillation in rats (Levina, 1972).
5.2.2 Absorption from the gastrointestinal tract
5.2.2.1 Human studies
In general, vanadium salts are poorly absorbed from the
human gastrointestinal tract. In a study by Curran et al.
(1959), from 0.1 to 1% of 100 mg vanadium (as highly soluble
diammonium oxytartratovanadate) was absorbed from the
gastrointestinal tract and 60% of this was excreted via the
kidneys within 24 h. The remainder was retained in the liver
and bone, until the oral administration ceased, when it was
mobilized rapidly from the liver and slowly from the bone.
Sodium metavanadate (12.5 mg/day for 12 days) was recovered
largely unabsorbed in the faeces (87.6%) and the remainder in
urine (12.4%) (Proescher et al., 1917). The International
Commission on Radiological Protection (ICRP, 1960) estimate for
the gastrointestinal absorption of soluble vanadium compounds
was 2%. A low degree of absorption was also found by Roshchin
et al. (1980).
5.2.2.2 Animal studies
Mountain (1959)a reported an unpublished study in which
vanadyl sulfate was fed to adult male rats in daily doses
ranging from 650 to 1250 µg (160 - 310 µg of vanadium). The
mean absorption was about 0.5%, but urinary values varied
considerably. The duration of the study was not given.
5.2.3 Absorption through the skin
Dermal absorption and skin irritation were reported in a
study in which a nearly saturated solution (20%) of sodium
metavanadate was applied to the skin of the rabbit (Stokinger,
1967).
However, according to US EPA (1977), the skin appears to be
a minor route of vanadium uptake for human beings. In an in
vitro study using 48V radiotracer, there was no penetration of
human skin samples (Roshchin, 1980).
5.3 Distribution and Transformation
5.3.1 Human studies
Vanadium levels in man, reported in earlier studies, were
considerably higher than those reported more recently. The
difference is illustrated by the whole-body content of 17 -
43 mg vanadium for a 70-kg man calculated by Schroeder (1963)
compared with estimates of 100 µg derived by Byrne & Kosta
(1978). The influence of different sampling procedures and
analysis (colorimetric determination, neutron activation) should
be clarified before concluding that such effects are real
(Lagerkvist et al., 1986) or that they reflect decreasing
environmental exposure.
__________________
a Mountain, J.T. (1959) Unpublished results, Toxicologic
Services, Occupational Health Field Headquarters,
Cincinnati, Ohio.
Absorbed vanadium is transported mainly in the plasma
(Schroeder et al. 1963; Ordhzonikidze et al., 1977; Roshchin et
al., 1980). Some mean values obtained using different methods
of analysis are given in Table 17. There is a wide divergence
in the results, the ratio between the highest and the lowest
mean values being about 104. In general, levels tend to decline
chronologically as analytical techniques become more sensitive.
There is also a difference in results obtained by neutron
activation analysis according to whether separation was carried
out before, or after, irradiation.
In an extensive investigation on human tissues, the
inadequate sensitivity of the analytical method meant that
quantitative information could only be obtained for the lung
and intestine (Tipton & Cook, 1963). Using neutron activation
analysis, Byrne & Kosta (1978) obtained information on other
organs. Table 18 includes autopsy tissue values and some
results from two other investigations using neutron activation
analysis. It is apparent that vanadium concentrations are low
in all tissues, though the liver, kidney, and lung often show
higher levels than other tissues. In another investigation
on a selection of organs, using spark-source mass spectrometry,
the vanadium levels detected were: brain, 30 µg vanadium/kg
wet weight (average from 10 specimens); liver, 40 µg/kg
(average from 11 specimens); lung, 100 µg/kg (average from 11
specimens); lymph node, 400 µg/kg (average from 6 specimens);
and testis, 20 µg/kg (average from 5 specimens) (Hamilton et
al., 1972/73). The values reported are in reasonable agreement
with those found in other animals. However, there is
considerable disagreement between investigators and between
analytical methods, which has not been resolved, since
interlaboratory and intermethod investigations have not been
carried out.
In the general population, which is mainly exposed to low
levels of vanadium in food with poor absorption from the
intestine, vanadium is usually undetectable in the urine, even
using very sensitive methods (Byrne & Kosta, 1978). Examination
of urine samples from 50 normal individuals using atomic
absorption spectrometry showed that vanadium was present in only
13; 11 samples showed a level of 0.1 µg/litre and two,
0.2 µg/litre (Ueno & Ishizaki, 1980). In industry, where
exposure is mainly through air and absorption from the lung is
high, vanadium concentrations in the urine cover a considerable
range (section 5.4.1).
Table 17. Vanadium levels in human blooda,b
---------------------------------------------------------------------------------------------------------
Analytical method Whole blood Serum/plasma Reference
(mg/litre) (mg/litre)
---------------------------------------------------------------------------------------------------------
X-ray emission 0.01 (43) Gofman (1962)
Spectrography 0.0078 (24) Lifschitz (1962)
Colorimetry 0.23 (calculated) 0.42 (13) Schroeder et al. (1963)
Neutron activation analy- 0.016 Bowen (1963)
sis (preseparated)
Neutron activation analysis 0.67 ± 0.32 ng/ml Simonoff et al. (1986)
Spectrography 0.126 (47) Butt et al. (1964)
Neutron activation analy- 0.0046 (36) Heydorn & Lukens (1966)
sis (preseparated)
Catalytic 0.01 (82) Allaway (1968)
0.02 - 0.01 (7)
Spectrophotometric 0.057 (10) Christian (1971)
Neutron activation analy- 0.0046 Kirzhner et al. (1974)
sis (preseparated)
Neutron activation analy- 0.022 (5) Buono et al. (1977)
sis (preseparated)
Neutron activation analy- 0.0005 (5) Byrne & Kosta (1978)
sis (preseparated)
Neutron activation analy-
sis (preseparated) 0.0066c (5) Sabbioni et al. (1979)
Neutron activation analysis 0.000047 (9 males) Cornelis et al. (1979)
0.000024 (8 females) Cornelis et al. (1979)
Neutron activation analy-
sis 0.000033 (17 females) Cornelis et al. (1980)
--------------------------------------------------------------------------------------------------------
a Adapted from: Byrne & Kosta (1978) and Versieck & Cornelis (1980).
b Number of subjects in parentheses.
c Recalculated values assuming an haematocrit of 0.45.
Table 18. Vanadium levels in human organs (µg/kg wet weight)a
-----------------------------------------------------------------------------
Tissue Study
-------------------------------------------------------------------
Byrne & Kosta (1978)b,c Damsgaard (1972)c,d Lievens (1977)b
-----------------------------------------------------------------------------
Kidney 3.3 3.2 2.6 nd 7
Liver 7.5 4.5 5 19 7 - 19 (5)
Brain 0.7 0.75
Thyroid 3.2 3.0
Heart 1.1
Cardiac 0.45 0.3
fat
Subcut- 0.63 0.80
aneous
fat
Muscle 0.45 0.62 0-59 7 nd
Spleen 3 4
Pancreas nd 14
Lung 19 - 40 (7) nd 13
median 30
-----------------------------------------------------------------------------
a From: Byrne & Kosta (1978).
b Parentheses enclose number of subjects.
c Brackets embrace specimens from one autopsy.
d nd = not detected.
Studies on vanadium levels in human bone have produced
widely varying results. Byrne & Kosta (1978) reported the
following data from human bones (lg/kg wet weight): skull,
2.5, 3, 4.5, 8.3; sternum, 3.1; rib, 0.8, 2.1; tooth enamel
(fragmented), 2, 3.4, 4, 5.1; and tooth enamel (drill powdered),
18. The high concentration in tooth enamel may be due to diet;
concentrations of vanadium tend to be higher in processed than
in unprocessed foods (Myron et al., 1977), but vanadium is
commonly present in steel alloys, especially tool steels, and
vanadium contamination may have occurred from the drill. Using
atomic absorption spectroscopy, Sumino et al. (1975) found a
range of 100 - 200 µg/kg (wet weight) in 6 specimens of rib.
Using emission spectral analysis, Shevchenko (1965) detected a
mean vanadium level of 150 ± 2 µg/kg (0.015 mg%) (dry weight)
in 14 samples of healthy bone tissue; bone tumours contained
higher levels.
Metal levels have been extensively studied in hair, because
of its potential value for exposure and body burden studies.
Gordus et al. (1974) and Gibson & DeWolfe (1979), using the same
method as Byrne & Kosta (1978), found levels of 20 - 40 µg/kg
in 42 subjects and 20 - 41 µg/kg in 370 subjects, respectively.
These results compare favourably with those of Byrne & Kosta,
i.e., 12 - 87 µg/kg in 12 subjects. Ueno & Ishizaki (1980)
used atomic absorption spectrometry to determine vanadium in
hair specimens from 130 men and 132 women. They found mean
concentrations of 53.6 µg/kg (range, 5 - 155 µg/kg) and
44.2 µg/kg (range, 1.8 - 118.8 µg/kg), respectively. However,
Creason et al. (1975), using emission spectroscopy in extensive
surveys in the USA, found levels that were about 10 times
higher.
There is considerable information on vanadium levels in lung
tissue. In studies by Schroeder et al. (1963), vanadium was
found in 97 out of 173 lungs examined in groups from 7 USA
cities, group mean concentrations ranging from 10 to 130 µg/kg
wet weight. Byrne & Kosta (1978) found levels of from 19 to
140 µg/kg (median, 30 µg/kg) in 7 cases; Hamilton et al.
(1972/73) detected a mean level of 100 µg/kg in 11 cases; and
Sumino et al. (1975) determined a range of 100 - 300 µg/kg for
13 observations. Statistical analyses of lung tissue trace
metal data were performed by Tipton & Shafer (1964) using data
taken from Tipton & Cook (1963). One approach was examination
of the relationship between lung metal concentrations and age.
The levels of a number of metals, including vanadium, increased
in the lung with age, indicating accumulation of insoluble
compounds, and Schroeder (1970b) calculated an annual increment
rate of 1.3 µg for lung-vanadium. However, earlier analysis of
the same data (Schroeder et al., 1963) did not show an age-
related increase in lung-vanadium. The later analysis by Tipton
& Shafer (1964) was performed on a subset of the original data
from Tipton & Cook (1963), which may account for some of the
discrepancy. The Tipton & Shafer (1964) data did not show a
graded increase in lung-vanadium with age, and there was a high
mean level only in the oldest age group. This could have been
produced by a single very high value in this age group. The
concept of vanadium accumulation in the human lung with age
remains doubtful, and the results of animal studies do not
indicate significant accumulation in the lung (sections 5.1.1.2
and 5.3.2).
5.3.2 Animal studies
Observations on pregnant rats, that received vanadium on the
21st - 22nd day of pregnancy, revealed accumulation in the
placenta but not "in perceivable quantities" in the organs of
the fetus. Vanadium was reported to be secreted in the milk
(Roshchin et al., 1980).
The identification of cellular components that react with
vanadium has been investigated in vivo and in vitro using 48V
radiotracer (Marafante & Sabbioni, 1983). Vanadium has a high
affinity for nuclear and mitochondrial components. When rats
were treated every day with 10 µg vanadium/rat for up to 8
days, a dose-related increase in vanadium incorporation in the
subcellular fractions was observed. There is evidence that non-
haem Fe-containing proteins, such as transferrin and ferritin,
have a high affinity for vanadium, while Fe haemoproteins are
not able to incorporate the metal.
In rat serum, both Vanadium4+ and Vanadium5+ form metal-
protein complexes with transferrins. Specific intracellular
vanadyl-ferritin complexes are formed in rat liver, spleen, and
kidney (Chasteen et al., 1986).
Ermolaev (1969) studied the distribution of vanadium in the
organs and tissues of 3 groups of rabbits, i.e., animals fed
under ordinary conditions, animals fed a diet supplemented with
vanadium (0.5% solution of vanadyl sulfate) at a dose of
0.05 mg/kg body weight, and animals fed the vanadium-
supplemented diet and subcutaneously injected with vanadium
also at 0.05 mg/kg body weight. The results of this
investigation are given in Table 19. The method of
administration had little effect on the resultant blood, brain,
and stomach levels, but there were distinct differences in the
case of the other organs and tissues.
The distribution and kinetics of vanadium, administered ip
as 80 mg vanadocene dichloride/kg to strain A mice, were
determined in blood, kidney, liver, small intestine, and brain.
The vanadium concentration decreased rapidly and exponentially
in the blood (half-time = 118 ± 43 min) and small intestine
(half-time a = 18 ± 0.14 min; half-time b = 341 ± 45 min).
Vanadium accumulated in kidney (maximum concentration,
1.12 ± 0.06 mmol at 12 h) and liver (maximum concentration,
0.56 ± 0.06 mmol at 8 h) and was then excreted (estimated half-
time, 7.9 ± 0.7 h for kidney; 12.1 ± 0.1 h for liver). Vanadium
was not detected in the brain (Toney et al., 1985).
Table 19. Vanadium levels in rabbit organs and tissues following
oral and subcutaneous administration (mg %)a
-----------------------------------------------------------------------------
Organ/ Control group First group Second group
tissue (0.05 mg/kg (0.05 mg/kg with
with food) food and injected
subcutaneously)
-----------------------------------------------------------------------------
liver 66 88 632
muscle tissue 27 41 30
blood 29 82 90
spleen 68 116 180
kidneys 71 90 464
lungs 62 82 168
brain 20 26 24
heart muscle 52 68 71
intestine 20 22 32
stomach 30 70 74
-----------------------------------------------------------------------------
a From: Ermolaev (1969).
The influence of the oxidation state of intravenously
injected compounds of 48V on uptake and distribution to selected
organs and subcellular elements of the rat liver was studied by
Hopkins & Tilton (1966). They did not observe any significant
differences in the rate or amount of uptake of nanogram
quantities of vanadium in three different oxidation states
(VOC13, VOC12, and VC13) and concluded that either the oxidation
state was not critical to transport or that the vanadium was
converted to a common oxidation state in vivo. Similar results
with respect to oxidation state and uptake and also the
distribution of vanadium in the rat were reported by Sabbioni et
al. (1978) and Conklin et al. (1982). In contrast, Parker &
Sharma (1978) found that levels of vanadium in the tissues of
male Wistar rats given sodium orthovanadate in the drinking-
water at 50 mg/litre for 3 months were higher than those in the
tissues of animals given vanadyl sulfate at the same
concentration. Roshchin et al. (1964) found evidence of the
partial conversion of vanadium trioxide to the pentavalent form
in blood-serum and in weakly acidic and basic solutions in
vitro. In a later study, Johnson et al. (1974) reported
the in vivo conversion of vanadium pentoxide to the tetravalent
state.
Information on vanadium distribution in rats after intra-
peritoneal, intratracheal, oral, and subcutaneous administration
of radioactive (48V) vanadium nitrate in single doses of
20 mg/kg body weight (LD50) and 0.4 mg/kg body weight (non-toxic
dose) is given in Table 20 (Roshchin et al., 1980). The study,
which involved 1060 albino rats, showed that whatever the method
of administration, vanadium was present in blood in significant
quantities only during the first 24 h, and that, after 2 days,
only traces of vanadium were detectable. No vanadium was found
in the blood after 4 - 8 days. However, in the groups
administered the radioactive compound intratracheally or
subcutaneously, low amounts of vanadium were detected between 8
and 16 days after administration as a result of reabsorption
from the organs. The highest level occurred 5 min after intra-
tracheal administration, 30 min after intraperitoneal admini-
stration, and 15 min - 1 h after subcutaneous or intragastric
administration. Vanadium was detected in all tissues and
organs. In 2 days, vanadium had accumulated in the bone, kidney,
liver, and lung, which were also the primary targets in rats
after intratracheal administration of vanadium pentoxide
(48V2O5) or chloride (48VO2C1) (Conklin et al., 1982) and after
oral administration of vanadyl sulfate or sodium orthovanadate
in the drinking-water (50 mg/litre) for three months (Parker &
Sharma, 1978).
In another study, 48VOCl3, which is fairly soluble, was
administered intratracheally to juvenile male Wistar rats at a
dose of 12.6 µCi in 1 ml buffered solution. Within 15 min, the
vanadium isotope was found in all major organs, except the
brain. The largest fractions were found in the blood, heart,
spleen, liver, and kidneys. Peak uptake occurred between 4 and
24 h after administration and activity was maintained throughout
a 9-week period (Oberg et al., 1978).
5.4 Retention
5.4.1 Human studies
Vanadium levels in human tissues (Table 18, section 5.3.1)
are low, the highest concentrations tending to occur in the
liver, kidney, and lung.
Storage of available vanadium in man occurs mainly in fat
and serum lipids (Schroeder et al., 1963).
Shevchenko (1965) used emission spectral analysis to
determine the vanadium contents of bone tumours and of the
cortical layer of bone adjacent to the tumours. He found that
vanadium accumulated in tumours. Healthy bone tissue contained
a mean vanadium concentration of 0.15 ± 0.002 mg/kg dry weight,
osteoblastomas contained 6.38 ± 1.17 mg/kg, and osteosarcomas,
4.16 ± 0.77 mg/kg. The cortical layer adjacent to
osteoblastomas contained 2.01 ± 0.42 mg/kg, and that adjacent to
osteosarcomas contained 1.77 ± 0.48 mg/kg. In comparison to
normal bone, bone cysts, osteochondromas, and exostoses also
showed higher vanadium levels, but to a lesser extent than bone
tumours.
The significant accumulation of vanadium in the tissue of
benign and malignant tumours and, to a lesser extent, in the
neighbouring tissue suggests that quantitative changes in the
amounts observed may indicate disturbances in its metabolism.
This may be in connection with the role of vanadium in the
phospholipid and cholesterol metabolism, which may become
indirect indicators of the intensity of the phospholipid
turnover in tumour tissue.
5.4.2 Animal studies
Large amounts of vanadium were reported in the crude fat
from beef, pork, and lamb (Schroeder et al., 1963), but the
results of subsequent studies do not support this finding, which
is probably erroneous because of the analytical methods used
(NRC, 1980).
In rats, after oral administration of 20 mg vanadium
nitrate/kg body weight, levels of vanadium in the blood were
detectable only during the first 24 h, and only traces remained
after 48 h; after 4 days, no vanadium was found in blood. For
48 h after the administration of vanadium, concentrations in the
organs increased by 0.4% of the administered dose in the liver,
by 0.69% in the kidneys, and by 0.16% in the spleen. The most
significant increase in vanadium (1.01% of the dose
administered) was found in the bones. After 16 days, the level
in the bone tissue had increased by 1.72% of the dose
administered, while in other organs it had significantly
decreased (Roshchin et al., 1980).
Studies on rats showed that liver, kidney, spleen, and
testes continued to accumulate intravenously injected vanadium-
48 for up to 4 h and that most of the radioactivity was retained
for up to 96 h (Hopkins & Tilton, 1966) . After 96 h, 14 - 84%
of the 10-min uptake was retained in other organs, and 46% and
9% of the vanadium-48 had been eliminated in the urine and
faeces, respectively. Levels of vanadium-48 in the
mitochondrial and nuclear fractions of homogenized liver
increased from 14 to 40% of the total during the first 96 h,
while the level of radioactivity in the microsomal fraction
remained relatively constant.
When strain A mice were given 80 mg vanadocene dichloride/kg
ip, vanadium accumulated in the liver and kidney. Maximum
concentrations of 1.12 ± 0.06 mmol in the kidney and 0.56 ±
0.06 mmol in the liver were reached after 12 h and 8 h,
respectively (Toney et al., 1985).
Table 20. Concentrations of 48vanadium in the organs and tissues of rats after intraperitoneal,
subcutaneous, intratracheal, and intragastric administration of a radioactive vanadium compound
(expressed as % radioactivity equivalent to 10 uCi per animal)a
---------------------------------------------------------------------------------------------------------
Organ/tissue Intraperitoneal administration Intratracheal administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 2.20 ± 0.55 0.43 ± 0.02 0.22 ± 0 0.43 ± 0.19 0.36 ± 0.19 1.25 ± 0.38
Kidney 7.20 ± 0.85 1.60 ± 0.02 0.66 ± 0 1.55 ± 0.67 1.56 ± 0.19 4.03 ± 0.74
Spleen 2.16 ± 0.23 0.17 ± 0.01 0.09 ± 0 0.15 ± 0.06 0.18 ± 0.02 1.92 ± 0.09
Lung 2.53 ± 0.73 0.11 ± 0 0 9.09 ± 5.91 0.20 ± 0.39 4.64 ± 2.11
Stomach 2.93 ± 0.73 0.08 ± 0 0 2.56 ± 0.94 0.13 ± 0.05 0
Small intestine 2.53 ± 0.31 0.08 ± 0 0 0.71 ± 0.22 0.21 ± 0.15 0
Large intestine 3.33 ± 0.88 0.13 ± 0.03 0 0.19 ± 0.08 0.17 ± 0.05 0.47 ± 0
Muscle 0.40 ± 0.05 0.03 ± 0 0 0.28 ± 0.20 0.03 ± 0 0
Bone 2.13 ± 0.12 3.45 ± 0.48 1.72 ± 0.31 0.79 ± 0.31 2.19 ± 0.31 9.70 ± 1.93
Testicle 1.30 ± 0.15 0.09 ± 0 0.06 ± 0 0.09 ± 0 0.08 ± 0 0.54 ± 0.08
Thyroid gland 0.60 ± 0 0.23 ± 0.05 0 1.21 ± 0.69 0.54 ± 0.40 0
Adrenals 2.53 ± 1.01 0.11 ± 0.02 0 0.67 ± 0 0.06 ± 0.02 0
Pancreas 7.88 ± 0.26 0.31 ± 0.06 0 0.14 ± 0.04 0.08 ± 0.02 0
Brain 0.16 ± 0.04 0.01 ± 0 0 0.02 ± 0 0 0
Heart 1.50 ± 0.34 0.07 ± 0 0 0.04 ± 0 0.08 ± 0 0.41 ± 0.15
---------------------------------------------------------------------------------------------------------
Table 20 (contd.)
---------------------------------------------------------------------------------------------------------
Organ/tissue Intragastric administration Subcutaneous administration
Time after administration
30 min 2 days 16 days 30 min 2 days 16 days
---------------------------------------------------------------------------------------------------------
Liver 0.08 ± 0.03 0.33 ± 0.11 0.27 ± 0 0.05 ± 0.01 1.00 ± 0.15 1.67 ± 0.42
Kidney 0.17 ± 0.13 0.69 ± 0.20 0.67 ± 0 0.13 ± 0.03 2.30 ± 0 4.47 ± 1.48
Spleen 0.04 ± 0 0.16 ± 0.05 0.08 ± 0 0.03 ± 0 0.31 ± 0.03 1.38 ± 0.75
Lung 0.06 ± 0.02 0.09 ± 0.01 0.06 ± 0 0.10 ± 0.01 0.19 ± 0.01 0.89 ± 0.18
Stomach 8.08 ± 1.49 1.55 ± 0.05 0.05 ± 0 0.29 ± 0.01 0.12 ± 0 0.39 ± 0.06
Small intestine 4.65 ± 9.95 0.22 ± 0.09 0.06 ± 0 0.77 ± 0.66 0.11 ± 0.01 0
Large intestine 0.12 ± 0 0.14 ± 0 0.01 ± 0 0.46 ± 0.21 0.14 ± 0 0
Muscle 0.01 ± 0 0.02 ± 0 0.04 ± 0 0.40 ± 0.30 0.03 ± 0 0.46 ± 0.15
Bone 0.05 ± 0.02 1.01 ± 0.31 1.72 ± 0 0.43 ± 0.30 3.23 ± 0.41 1.86 ± 0.52
Testicle 0.26 ± 0.20 0.04 ± 0 0.06 ± 0 0.01 ± 0 0.12 ± 0.01 0.64 ± 0.07
Thyroid gland 0.08 ± 0 0.32 ± 0.24 0 0.15 ± 0.05 0 0
Adrenals - 0.09 ± 0.03 0 0.10 ± 0 0.16 ± 0.04 0.39 ± 0
Pancreas 0.32 ± 0.29 0.04 ± 0.04 0.08 ± 0 0.03 ± 0 0.09 ± 0.01 0.83 ± 0.04
Brain 0.02 ± 0 0.01 ± 0 0.02 ± 0 0.03 ± 0 0.02 ± 0 0
Heart 0.06 ± 0 0.05 ± 0 0 0.06 ± 0 0.12 ± 0 0.81 ± 0.08
---------------------------------------------------------------------------------------------------------
a From: Roshchin et al. (1980).
In a study on 2 young rats (strain unspecified), the highest
uptake of vanadium-48 from V205 occurred in rapidly mineralizing
areas of dentine and bone (Söremark et al., 1962). In mice,
high uptake occurred in the mammary glands, liver, renal cortex,
and lung (Söremark & Ullberg, 1962).
In studies by Belehova (1966, 1969), vanadium levels were
lower in carious than in normal canine teeth. In other studies,
intramuscular injection of vanadium in dogs at a dose of 2 mg/kg
body weight resulted in vanadium levels in the hard tissues of
the teeth that were 1.3 times higher than those in the controls.
On the 7th day, the concentration in the enamel had almost
returned to its initial level of 8.8 mg/kg; the level in the
dentine was still 32% higher.
Vanadium was reported to decrease the incidence of dental
caries, when added to the diet of hamsters (Geyer, 1953).
However, Hein & Wisotzky (1955) reported significant increases
in dental caries in hamsters given drinking-water containing
vanadium pentoxide equivalent to 10 mg vanadium/litre over an
80-day period. Muhler (1957) studied the effect of vanadium
pentoxide on caries in Sprague-Dawley rats. Groups of 50 rats
received vanadium (as vanadium pentoxide) at 10 mg/litre,
20 mg/litre, or 40 mg/litre in their drinking-water. One
control group received drinking-water containing ammonium
fluorosilicate (20 mg/litre), the other received untreated
water. Vanadium did not produce any reduction in the incidence
of dental caries, in fact there was a slight, but not
significant increase in dental caries in the groups receiving
10 mg/litre and 20 mg/litre for 140 days. All the rats showed
signs of vanadium toxicity and all animals in the highest dose
group (40 mg/litre) died within 65 days.
In an in vitro study on the effects of vanadate on bone
formation in 21-day-old fetal rat calvariae, sodium vanadate at
concentrations of 0.1 - 10 mmol stimulated the incorporation of
3H-thymidine into DNA and increased the bone DNA content and the
mitotic index. Sodium vanadate at a concentration of 100 mmol
produced a marked and irreversible inhibition of DNA labelling
and protein synthesis. Concentrations of 1 mmol (24 h) and
10 mmol (96 h) inhibited alkaline phosphatase activity. Sodium
vanadate also stimulated collagen and non-collagen protein at
low concentrations, but again had an irreversible inhibitory
effect at a high concentration of 100 mmol (Canalis, 1985).
5.5 Elimination
5.5.1 Human studies
Owing to low gastrointestinal absorption, ingested vanadium
is predominantly eliminated unabsorbed in the faeces. The
principle route of excretion of absorbed vanadium is through the
kidneys.
The relationship between urinary excretion and the extent of
exposure has been studied. As part of a clinical study on the
effects on cholesterol level, Dimond et al. (1963) gave ammonium
vanadyl tartrate to patients (5 female, 1 male) and did not find
any correlation between urinary excretion and oral dose.
Variable absorption was suggested as the reason for wide
variation in urinary excretion. In a 50-week study on 2
volunteers, Tipton et al. (1969) reported a urine/diet ratio of
0.13. This is in agreement with the figure of 12.4% excretion
in urine in a man given sodium metavanadate orally (12.5 mg
daily for 12 days) (Proescher et al., 1917).
Studies on occupationally exposed populations have shown a
poor correlation between vanadium concentrations in air and
amounts excreted in urine (Williams, 1952; Lewis, 1959b;
Jaraczewska & Jakubowski, 1964; Watanabe et al., 1966; Troppens,
1969; Köhler, 1972). Differences in laboratories and methods
may contribute to the wide range of urinary concentrations
reported.
In a study on power-station workers exposed to vanadium
during maintenance work on an oil-fired boiler, it was shown
that urinary excretion increased in those most heavily exposed,
in spite of the use of protective masks. For instance, urinary
levels of vanadium in 6 welders and 4 cleaners increased over a
work shift from 2.7 to 43.8 mg vanadium/kg creatinine and from
1.65 to 52.8 mg/kg, respectively. The vanadium concentration
in air during boiler cleaning was estimated to vary between 0.1
and 5 mg/m3 (Maroni et al., 1983). These results resemble those
reported by Thürauf et al. (1979) on 54 workers exposed to
vanadium in a metallurgical plant. Exposed workers had a urinary
vanadium concentration of 33.9 mg/kg creatinine, whereas the
level in unexposed workers was 0.6 mg/kg creatinine.
Roshchin et al. (1980) used polarography in a study of the
urine samples of 100 workers who were exposed daily to vanadium
pentoxide, and found vanadium in 50% of subjects. The mean
concentration of vanadium in the urine was 0.18 ± 0.03 mg/litre.
In workers with a duration of exposure in the range of 1 - 2
years, the mean concentration was 0.14 ± 0.08 mg/litre; after 2
years, the mean concentration was 0.20 ± 0.009 mg/litre. In
workers employed for 2 - 6 months, the mean concentration of
vanadium in the urine was 0.16 ± 0.12 mg/litre. There appeared
to be a correlation between the urinary levels and the
concentration of vanadium in the air. For example, in workers
exposed to mean vanadium pentoxide atmospheric concentrations of
0.28 ± 0.06 mg/m3, the concentration in the urine was 0.20 ±
0.05 mg/litre; at an atmospheric concentration of 0.17 ±
0.01 mg/m3, the urinary concentration was 0.19 ± 0.007
mg/litre.
The relationships between personal exposure and blood and
urine levels of vanadium were examined in 16 workers in a
ferrovanadium plant in Norway (Gylseth et al., 1979).
Individual levels did not show any clear relationship between
exposure and response, but, when the data were divided into
high- and low-exposure groups, significant differences were
found for both blood and urine levels in relation to exposure
levels. There was also a reasonably good correlation between
blood and urine levels. However, the authors concluded that
"the differences are small and the method difficult and
expensive, so for a routine control other criteria should be
sought".
Kiviluoto et al. (1979a,c) studied serum- and urinary-
vanadium levels in relation to exposure levels. Grouped and
individual data were examined at intervals during vacation
periods and compared with those for an unexposed group. The
levels of exposure were low (0.01 - 0.05 mg/m3), and no
correlations between exposure levels and serum or urine
concentrations were found. However, there was a conspicuous
decline in the urine levels at the beginning of the vacation
period, but they did not decline down to the level of the
unexposed group. It was considered that this provided some
measure of the extent of exposure.
5.5.2 Animal studies
Talvitie & Wagner (1954) administered sodium metavanadate
monohydrate in saline to albino Webster rats and albino rabbits
by intraperitoneal and intravenous injection, respectively. In
one part of the study, rats were given a single ip dose
equivalent to 0.5 mg vanadium/kg body weight and, in another,
twice daily ip injections, each equivalent to 0.25 mg
vanadium/kg body weight, for 5 days. Rabbits were injected
intravenously twice daily for a total of 7 or 9 days, with doses
equivalent to 0.25 mg vanadium/kg body weight, except for 1
rabbit that received doses of 0.4 mg/kg body weight for the
second 5-day period. No histological changes were noted. The
ratio of vanadium eliminated in the urine and faeces was 5:1.
Following oral administration of vanadium sulfate and
vanadium pentoxide to guinea-pigs (equivalent to 2 mg vanadium/
kg body weight), Reznik (1954) found vanadium in the urine and
faeces for 7 - 10 days, though elimination in the urine ceased
before that in faeces. The author concluded that the presence
of vanadium in the faeces, several days after administration,
was due to its return to the intestine after internal resorption
and excretion. Biliary excretion of less than 2% of the
intravenously injected dose (between 0.9 and 30 µg penta-
vanadate/kg body weight) was demonstrated in rats during the
first 6 h after administration. By comparison, 20% was excreted
with the urine during the same period of time (Sabbioni et al.,
1981).
Roshchin (1968) administered a dose of 3 mg vanadium
trichloride to 180-g albino rats. Most of the vanadium was
excreted via the kidneys. Of the dose administered, 18% was
found in the urine after 24 h and 25%, after 48 h. The amount
of vanadium excreted in the urine fell and, after 6 days, was
low. Elimination of vanadium via the intestine occurred on a
much smaller scale and remained relatively stable; 30.9% of the
administered dose was excreted over a 6-day period. Thus, the
largest quantity of vanadium was eliminated during the first 2
days, and the rest was eliminated gradually. The ratio of
amounts eliminated in the urine and faeces was 5:1,
corroborating the findings by Talvitie & Wagner (1954).
In a study on mice, rats, and dogs, Mitchell & Floyd (1954)
showed that ascorbic acid increased vanadium elimination in the
urine during the first few days and later in the faeces.
CaNa3DTPA (salicylic salt of diethylenetriamine-pentaacetic
acid) increased vanadium excretion in the urine and reduced
elimination via faeces. Following the combined administration
of both preparations, elimination in the faeces increased.
6. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
6.1 Aquatic Organisms
6.1.1 Microorganisms and higher plants
Trace quantities (1 - 10 µg/litre) of vanadium stimulated
the growth of some algae, including Scenedesmus or Chlorella
(Arnon & Wessel, 1953; Hopkins et al., 1977; Patrick, 1978).
Toxicity studies on phytoplankton, mainly using pentavalent
vanadium, have revealed differences in susceptibility between
various species. A concentration of 0.02 mg/litre, as ammonium
vanadate, interfered with the cell division of the fresh-water
algae Chlorella pyrenoidosa, whereas 0.25 mg/litre was lethal
(Meish & Benzschawel, 1978). The 15-day LC50 for an estuarine
and salt-water green alga (Dunaliella marina) was given as
0.5 mg/litre of sodium metavanadate and that of a salt-water
pennote diatom (Asterionella japonica) as 2 mg/litre (Minamand &
Unsal, 1978).
Vanadium does not appear to be essential for higher plants.
6.1.2 Invertebrates
In a study using sea-water with a background level of 0.0017
mg vanadium/litre, the 9-day LC50s for vanadium (as sodium
metavanadate) for the worm (Nereis diversicolor), mussel
(Mytilus galloprovincialis), and crab (Carcinas maenas) were
10, 35, and 65 mg vanadium/litre, respectively (Miramand &
Unsal, 1978). Some marine invertebrates, such as the tunicates,
accumulate vanadium to levels that may be 10-5 to 10-6 times the
sea-water concentrations (Table 3.). Vanadium levels in such
species may exceed 3000 mg/kg dry weight (Biggs & Swinehart,
1976; Carlson, 1977). It was stated that the uptake of vanadium
by the mussel (Mytilus edulis) from food (plankton) was of the
same magnitude as that from water (Unsal, 1978). It appears
that benthic aquatic organisms tolerate higher concentrations of
vanadium than fish (section 6.1.3).
6.1.3 Fish
There are some data on the acute toxicity of vanadium for
fish (Van Zinderen Bakker & Jaworski, 1980). The 4- to 6-day
LC50s for fresh-water species are in the range 0.5 -10 mg/litre.
Factors influencing toxicity include water hardness, and the
LC50 values are higher in hard water. Giles et al. (1979)
studied the influence of pH on the toxicity of vanadium
pentoxide for rainbow trout fingerlings. The 96-h LC50 ranged
from 6.43 to 21.75 mg/litre. There was some indication of
vanadium pentoxide being most toxic at a pH of 7. Also, Sprague
et al. (1978) tested zebrafish (Brachydanio rerio) with vanadium
pentoxide and found that a pH of 7.5 provided the most toxic
conditions. At this pH, death occurred between 23.5 and 45 h at
a concentration of 22 mg/litre, whereas at pH 8.2, the time
decreased to 32 h and, at pH 8.8 - 9, to 37 - 39 h.
Studying the chronic effects of vanadium pentoxide on
flagfish, Sprague et al. (1978) and Holdway & Sprague (1979)
concluded that the sublethal threshold concentration would be
about 0.08 mg vanadium/litre.
The rainbow trout (Salmo gairdneri) is the most commonly
used fish for toxicity studies. Sprague et al. (1978) reported
7-day LC50 values in one series of studies ranging from 2.4 to
5.6 mg/litre. Increasing the exposure time resulted in
progressively lower LC50 values, the lowest being 1.99 mg/litre
for an 11-day exposure period. Similar results were reported by
Giles et al. (1979) using experimental conditions of pH 8,
15 °C, and hardness 90 mg CaCO3/litre. The LC50 values
decreased from 4.34 mg CaCO3/litre for 5 days exposure to 1.95
mg CaCO3/litre for 14 days. Neither of these groups was able to
define a minimum lethal level for rainbow trout. Studies by
Stendahl & Sprague (1982) indicated that small rainbow trout
were more resistant than larger fish to vanadium pentoxide. In
general, rainbow trout eggs were 10 - 15 times more resistant to
pentavalent vanadium than fingerlings, suggesting the
possibility of a protective function by the chorion (Giles et
al., 1979). It should be noted that the available studies on
vanadium toxicity have been performed on fresh-water species
only. The effects of salinity remain to be studied.
6.2 Terrestrial Organisms
6.2.1 Uptake of vanadium by plants
In general, the highest concentrations of vanadium in plants
growing in natural soils occur in the roots and decrease towards
the aerial portions of the plant. The concentration of vanadium
in soil is, by and large, 10 times the concentration in the
plant (Cannon, 1963). Absorption appears to be passive (Welch,
1973).
When grown in culture solution, several plant species absorb
vanadium, which is also translocated to the aerial parts and
seeds (Hopkins et al., 1977).
6.2.2 Effects on plants
Vanadium has not been demonstrated to be essential for
higher land plants (Hopkins et al., 1977). However, traces of
vanadate (0.02 mmol vanadium as VO2+ or VO3-) were shown to
promote chloroplast development and oxygen production in higher
plants. Vanadium also had a function as a redox catalyst in the
electron flow from photosystem II to photosystem I (Meisch &
Becker, 1981).
Small amounts of aqueous vanadium (10 - 20 mg/litre) have
detrimental effects on most plants (Cannon, 1963). The growth
of flax, peas, soybeans, and cabbage was reduced in nutrient
solutions containing 0.5 mg vanadium/litre (given as VOCl2 or
VCl3) (Warington, 1955; Hara et al., 1976). Vanadium can
induce iron deficiency chlorosis (Cannon, 1963) or affect the
trace element nutrition by reducing the levels of, e.g.,
manganese, copper, calcium, and phosphorus (Warington, 1955;
Wallace et al., 1977). Similarly, 5 mg vanadium/litre (as VO3-)
in irrigation water reduced the growth of sugar beets by 30 -
50% and caused iron deficiency chlorosis (Hewitt, 1953).
In soils, the toxicity of vanadium may range between 10 and
1258 mg/kg, depending on plant species and type of soil (Hopkins
et al., 1977). Ten mg/kg added to sandy soil as Ca(VO3)2
decreased the growth of sour orange, whereas 150 mg/kg was
lethal (Vanselow, 1950).
Fertilizers may have a high vanadium content. For instance,
rock phosphate, super phosphate, and basic slag may contain
several thousand mg vanadium/kg. These may cause unacceptable
levels of accumulation of vanadium in soil (Mitchell, 1964;
Hopkins et al., 1977). Urban sewage sludges usually contain
less than 35 mg/kg of vanadium (Bradford et al., 1975; Oliver &
Cosgrove, 1975). On the other hand, vanadium in sewage sludge
may be up to 6 times more easily available in sludge than in
soil (Bernow & Webber, 1972 ).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1 General Toxicity
The toxicity of vanadium for experimental animals varies
with the species and route of administration. Smaller animals,
including the rat and mouse, tolerate the metal well; the rabbit
and horse are more sensitive (Hudson, 1964). In general,
toxicity is low when the metal is given by the oral route,
moderate by the respiratory route, and high by injection.
Lethal doses for various vanadium salts injected intravenously
in rabbits and subcutaneously in guinea-pigs, rats, and mice are
shown in Table 21.
Table 21. Lethal doses of vanadium (mg V2O5/kg body weight) in
experimental animalsa
-----------------------------------------------------------------------------
Compound Rabbitb Guinea-pig Rat Mouse
-----------------------------------------------------------------------------
colloidal vanadium 1 - 2 20 - 28 - 87.5 - 117.5
pentoxide
ammonium metavanadate 1.5 - 2 1 - 2 20 - 30 25 - 30
sodium orthovanadate 2 - 3 1 - 2 50 - 60 50 - 100
sodium pyrovanadate 3 - 4 1 - 2 40 - 50 50 - 100
sodium tetravanadate 6 - 8 18 - 20 30 - 40 25 - 50
sodium hexavanadate 30 - 40 40 - 50 40 - 50 100 - 150
vanadyl sulfate 18 - 20 34 - 45 58 - 190 125 - 150
sodium vanadate - 30 - 40 10 - 20 100 - 150
-----------------------------------------------------------------------------
a From: Hudson (1964).
b Rabbits were injected intravenously; other animals subcutaneously.
The toxicity of vanadium also varies considerably with the
nature of the compound, vanadium being toxic both as a cation
and as an anion. As a general rule, toxicity increases as
valency increases, vanadium5+ being the most toxic. Among the
oxides of vanadium, vanadium pentoxide is more soluble and more
toxic than the trioxide or dioxide.
Roshchin (1967b, 1968) described the results of acute
inhalation studies on albino rats exposed to vanadium pentoxide
in the form of a condensation aerosol (fume) at 10 - 70 mg/m3
or in the form of a dispersion aerosol (dust) at 80 - 700 mg/m3,
ammonium vanadate (presumably as a dispersion aerosol) at
1000 mg/m3, and ferrovanadium, as a dispersion aerosol at 1000 -
10 000 mg/m3. The minimum concentration of vanadium pentoxide
(condensation aerosol) that gave rise to mild signs of acute
poisoning was 10 mg/m3 air. The absolute lethal concentration
for the condensation aerosol was 70 mg/m3. Dispersion aerosols
(containing large particles) were only one-fifth as toxic as
condensation aerosols. Inhalation of dispersion aerosols of
ferrovanadium did not produce any acute toxic effects, probably
because the particles were too large. However, acute toxic
effects were observed following intratracheal instillation of
ferrovanadium. These may have been related to the biological
solubility and the extent of absorption.
The effects of vanadium on experimental animals have been
investigated in a number of studies using various compounds,
species, and test protocols. Some representative acute and long-
term studies are summarized in Tables 22 and 23. Subsequent
discussions on the effects of vanadium on specific biological
systems are frequently based on the results of these studies.
7.2 Effects on Metabolic Processes
Rabbits exposed to a dispersion aerosol of vanadium trioxide
(40 - 75 mg/m3, 2 h/day for up to 12 months) exhibited a
progressive weight loss amounting to an average of 4.6% at the
termination of the study. Control animals gained weight by an
average of 12.3%. The number of white blood cells declined by
the end of the test from between 7000 and 8000 down to 5000/mm3;
no change was noted in controls. Haemoglobin levels in the test
animals decreased from 75 to 68% of normal levelsa. Serum-
ascorbate levels in the blood progressively decreased to about
20% of controls between 7 and 8 months. Protein sulfhydryl
levels in the serum of exposed animals decreased by 30% compared
with those of the controls. Respiration in the liver and brain
tissues of test animals was reduced by one-half by the end of
the study compared with controls, but the respiratory quotient
was unchanged. Blood-cholinesterase levels in exposed rabbits
increased by an average of 25% after the fifth month (Roshchin
et al., 1964; Roshchin, 1968).
In these studies, the weight loss together with the
depression in levels of white cells, haemoglobin, and protein
sulfhydryl groups in the blood and the decreased liver tissue
respiration were taken as indicators of the "general toxic
effect" of vanadium. Increased cholinesterase activity was held
to be indicative of sensitization.
_________________
a Normal haemoglobin level in the rabbit is 80 - 150 g/litre
and normal rabbit haematocrit, 30 - 50%.
Table 22. Acute studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Dose Concentration Reference
administration index or dose
---------------------------------------------------------------------------------------------------------
vanadium pentoxide mouse intragastric LD50 23.4 mg/kg body weight Roshchin (1967a)
rat inhalation LC50 70 mg/m3 Roshchin (1967a)
rat inhalation minimum 10 mg/m3 Roshchin (1967a)
effective
rat inhalation LC50 70 mg/m3 Sjöberg (1950)
cat inhalation LC50 500 mg/m3 Faulkner Hudson
(1964)
rabbit inhalation LC100 205 mg/m3 Sjöberg (1950)
rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
ammonium vanadate mouse intragastric LD50 10 mg/kg body weight Roshchin (1968)
rat intragastric effective 20 mg/kg body weight Kaku et al. (1971)
rat subcutaneous effective 5-30 mg/kg body weight Kaku et al. (1971)
vanadium mouse intragastric LD50 24 mg/kg body weight Roshchin (1968)
trichloride
vanadium di-iodide mouse intragastric LD50 68 mg/kg body weight Roshchin (1968)
vanadium dibromide mouse intragastric LD50 88 mg/kg body weight Roshchin (1968)
vanadium trioxide mouse intragastric LD50 130 mg/kg body weight Reznik (1954)
ammonium rat intragastric LD50 10 mg/kg body weight Gulko (1956)
metavanadate
vanadyl sulfate rat intragastric LD100 10 mg/kg body weight Lewis (1959b)
rabbit subcutaneous LC50 59.1 mg/kg body weight Korkhov (1965)
rabbit subcutaneous maximum 25 mg/kg body weight Korkhov (1965)
tolerated
guinea-pig subcutaneous LD100 800 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous LD50 560 mg/kg body weight Kopylova (1971)
guinea-pig subcutaneous maximum 300 mg/kg body weight Kopylova (1971)
tolerated
water-soluble mouse intragastric LD50 5 mg/kg body weight Seljankina (1961)
vanadium compounds mouse intragastric no effect 0.005 mg/kg body weight Seljankina (1961)
(or 0.1 mg/litre
in water)
---------------------------------------------------------------------------------------------------------
Table 23. Long-term studies on experimental animals
---------------------------------------------------------------------------------------------------------
Compound Species Route of Concentration Duration of Reference
administration exposure
---------------------------------------------------------------------------------------------------------
vanadium pentoxide rabbit inhalation 20 - 40 mg/m3 several months Sjöberg (1950)
rabbit inhalation 25 mg/m3 10 months Gulko (1956)
guinea- inhalation 25 mg/m3 10 months Gulko (1956)
pig
rabbit inhalation 8 - 18 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat inhalation 10 - 30 mg/m3 several months Roshchin (1967b)
rat inhalation 3 - 5 mg/m3 several months Roshchin (1967b)
rat inhalation 0.027 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.002 mg/m3 70 days Pazhynich (1966)
rat inhalation 0.006 mg/m3 40 days Pazhynich (1966)
ammonium rat subcutaneous 1 mg/kg 30 days Kaku et al. (1971)
metavanadate
sodium guinea- subcutaneous 3.2 - 128 ug/kg days Kulieva (1974)
metavanadate pig
guinea- subcutaneous 5.12 mg/kg days Kulieva (1974)
pig
vanadium trioxide rabbit inhalation 40 - 75 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
vanadium rabbit oral 5 mg/kg 2 - 3 months Roshchin (1968)
trichloride
vanadium carbide rabbit inhalation 40 - 80 mg/m3 9 - 12 months Roshchin (1968)
rat oral 5 - 30 mg/kg 6 months Roshchin (1968)
rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
ferrovanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
metallic vanadium rat intratracheal 25 mg per rat 9 - 12 months Roshchin (1968)
---------------------------------------------------------------------------------------------------------
Chronic poisoning, following the inhalation of trivalent
vanadium (V203) or the oral administration of vanadium tri-
chloride (VCl3), resulted in blood changes by the end of the
second and third months (Table 24). These changes were
characterized by decreased albumin and increased globulins
(mainly y-globulins) with a halving of the albumin-globulin
ratio, and by an increase in serum concentrations of the amino
acids cystine, arginine, and histidine. There was also a 10%
increase in nucleic acid levels in the blood and a
"considerable" increase in the blood-chloride concentration.
The effects of vanadium on the metabolism of proteins and
nucleic acids were considered to be responsible for the
immunological and allergic reactions that may occur in vanadium
poisoning (Roshchin, 1967a).
Metabolic changes were observed in a study by Pazhynich
(1966) in which albino rats were exposed by inhalation for 70
days to condensation aerosols of vanadium pentoxide at levels of
0.027 ± 0.002 mg/m3 and 0.002 ± 0.00013 mg/m3. Statistically
significant changes were observed at the higher level of
exposure, but not at the lower level. The observations included
decreases in: whole blood-cholinesterase levels, total serum-
protein levels, serum-globulin levels, and the oxyhaemoglobin
content of venous blood. Elevated serum-globulin levels,
increased numbers of blood leukocytes showing yellow, orange,
and red nuclear fluorescence with acridine orange, and increased
oxygen consumption as indicated by isolated liver preparations
were also observed in the high-level exposure group. The
pattern of leukocyte nuclear fluorescence returned to normal 20
days after cessation of exposure. In a second study, albino
rats were continuously exposed to vanadium pentoxide at 0.006 ±
0.00056 mg/m3 for 40 days. No changes in blood-leukocyte
nuclear fluorescence were observed. During the sixth week of
exposure, animals received water but no food. After 3.5 days of
this treatment, the number of leukocytes displaying altered
nuclear fluorescence increased by a factor of 4.83.
In an in vitro study, ammonium metavanadate was found to
inhibit microsomal ketamine N -demethylation, lipid peroxida-
tion, and hydrogen peroxide formation in rat liver. The
inhibiting doses of NH4VO3 ranged from 10-5 to 10-3 mol/litre.
Cytochrome c reductase was also inhibited, whereas cytochrome
oxidase activity was stimulated (Beyhl, 1983).
Parenteral injection of guinea-pigs with sodium metavanadate
in daily doses of 3.2 µg/kg, 128 µg/kg, or 5.12 mg/kg body
weight resulted in dose-dependent increased succinate dehydro-
genase activity in the liver and kidneys and cytochrome oxidase
activity in the liver (Kulieva, 1974).
Table 24. Respiratory effects of vanadium pentoxide on experimental animalsa
---------------------------------------------------------------------------------------------------------
Reference Species Form Concentration Exposure Pathological findings
(mg/m3)
---------------------------------------------------------------------------------------------------------
Sjöberg (1950) rabbit dust 205 7 h conjunctivitis, tracheitis,
pulmonary oedema, broncho-
pneumonia, enteritis, fatty
liver, death
Sjöberg (1950) rabbit dust 20 - 40 1 h/day chronic rhinitis, trache-
several months itis, emphysema, atelect-
asis, bronchopneumonia,
pyelonephritis
Gulko (1956) rabbit dust 10 - 30 continuous, bronchitis, pneumonia,
acute weight loss, bloody diarr-
hoea
Roshchin (1963) rat dust, 80 - 700 continuous, haemorrhagic inflammation in
fume 10 - 70 acute lungs, haemorrhage in inter-
nal organs, paralysis, res-
piratory failure, death
Roshchin (1963) rat dust, 10 - 30 2 h/day, haemorrhagic inflammation in
fume 3 - 5 several months lungs purulent bronchitis,
pneumonia
Pazhynich (1966) rat fume 0.027 continuous haemorrhagic inflammation in
70 days lungs, vascular congestion,
haemorrhage in liver, kid-
neys, and heart
---------------------------------------------------------------------------------------------------------
a From: Waters (1977).
In acute studies on rats (Donryu strain) weighing 200 g,
Kaku et al. (1971) administered vanadium (as ammonium vanadate)
by gavage at a dose of 20 mg/kg body weight or injected it
subcutaneously (doses of between 5 and 30 mg/kg body weight).
There were dose-dependent reversible increases in the trigly-
ceride concentrations in the liver and blood-serum, a decrease
in the serum-cholesterol level, and increases in glutamate-
oxalo-acetate transaminase and glutamate-pyruvate transaminase
activity. After subcutaneous injection, there was a fall in
cholesterol levels. The lowest values were reached 24 h after
the injection; values then returned to normal. Dose-dependent
increases were also observed in the concentrations of triglyc-
erides in the liver and serum. Levels peaked 48 h after the
injection and then gradually declined. When ammonium vandate
solution equivalent to 1 mg vanadium/kg body weight was
administered daily by subcutaneous injection for 30 days more
marked changes in serum cholestrol were observed.
Korkhov (1965) injected 0.3 mg vanadyl sulfate/kg body
weight subcutaneously in rabbits with experimental athero-
sclerosis and observed a lowering of the hypercholesterolaemia
and inhibition of the rise in lecithin. Combined administration
of vanadyl sulfate and phenylethylacetic acid lowered the aortic
cholesterol level 3.5 times compared with controls. Korkhov
(1965) also showed that cholesterol biosynthesis in liver tissue
culture was inhibited by the addition of 10-4 vanadyl sulfate.
When 30 rabbits with experimental atherosclerosis were
administered a mixture of trace elements (copper, cobalt,
manganese, and zinc) in combination with cholesterol, Babenko &
Vandzhura (1969) detected increases in the blood lipids and
decreases in vanadium concentrations, compared with healthy
control animals. The same results were noted following combined
administration of copper and cobalt plus cholesterol at
0.2 g/kg. After administration of manganese, the decrease in
the vanadium concentration was delayed; after administration of
zinc, vanadium remained at the same level as in healthy animals.
Vanadium concentrations in body tissues decreased as cholesterol
levels increased, but manganese and zinc, when given together
with cholesterol, helped maintain vanadium concentrations in the
tissues of the body, the highest vanadium concentrations being
found in the liver, aorta, and muscles.
In the studies by Novakova et al. (1981), daily oral
administration to rabbits for 4 months of vanadium pentoxide and
cholesterol (1st group: vanadium at 0.5 mg/kg body weight and
cholesterol at 0.3 mg/kg body weight; 2nd group: vanadium at
1.5 mg/kg body weight and cholesterol at 0.5 mg/kg body weight)
resulted in high levels of cholesterol in the blood and
extensive atherosclerosis of the aorta. In the animals
administered either cholesterol (0.5 mg/kg body weight) or
vanadium (0.5 mg/kg body weight), hypercholesterolaemia was also
observed, but the increase was less pronounced. Increases in
levels of lipids, lipoproteins, and triglycerides were also
observed. After one month of administration, cholesterol levels
in treated animals were more than ten times those in the
controls. At the end of the study (after the 4th month), the
cholesterol levels in the treated animals were considerably
higher than those in the controls.
The effects of vanadium on iron metabolism have not been
fully elucidated. In some studies, a stimulative effect on
haemoglobin and erythrocyte levels has been claimed. The
results of studies by Myers & Beard (1931) suggested that
vanadium chloride given at 0.6 mg/kg diet to rats, previously
rendered anaemic, had a favourable effect on the haemoglobin
level, and Kopylova (1971) obtained increases in the erythrocyte
count and haemoglobin level in rabbits by subcutaneous admini-
stration of vanadyl sulfate (1 mg/kg body weight, daily, for 2
months). Trummert & Boehm (1957) observed an increase in the
erythrocyte count following intravenous injection of vanadium
gluconate (0.3 - 1.5 mg/kg body weight, daily, for 40 days), but
the haemoglobin level was not significantly affected.
7.2.1 Mechanisms of action
Many of the metabolic effects observed can be explained by
the biochemical effects of vanadium exposure in vivo and in
vitro.
Roschin (1967a) presented the hypothesis that the mechanism
of the initial step in the non-specific haematopoietic effect of
vanadium and the subsequent anaemia was the inhibition of the
redox system of hydrogen carriers. In response to the resulting
hypoxia, there is increased haematopoiesis. Possibly vanadium
interferes with tissue respiration at the stage of dehydrogena-
tion effected by nicotinamide adenine dinucleotide (NAD). By
inhibiting this coenzyme, vanadium interferes with the
incorporation of iron in the porphyrin complexes and haemoglobin
synthesis. The anti-vitamin C effect of vanadium and the
consequent vitamin C deficiency also inhibits the utilization of
iron for haemoglobin synthesis. Iron accumulates in the
reticuloendothelial tissue.
It is known that vanadium also inhibits the activity of
monoamine oxidase, which catalyses the conversion of serotonin
to 5-hydroxyindoleacetic acid. In a study on rabbits exposed
to vanadium pentoxide dust for 3 months, urine levels of 5-
hydroxyindoleacetic acid fell to 33% below control values
(Roschin, 1967a). It was suggested by the author that
inhibition of monoamine oxidase might result in accumulation of
serotonin in the central nervous system, leading to functional
disturbances, such as bronchospasm, diarrhoea, and urinary
incontinence. Elevated serotonin levels could also be
responsible for the dystrophic and necrotic process in the
kidneys and the high permeability of the blood vessels. A
decrease in sulfhydryl groups in blood-proteins and a reduction
in the cystine content of keratinized tissues might be due to an
interaction between vanadium and an unspecified enzyme.
The catabolism of cystine and cysteine was increased by
exposure to vanadium (Bergel et al., 1958). In vitro, pyridoxal
5-phosphate induced the catabolism of sulfhydryl amino acids in
the presence of VO2+ (Anbar & Inbar, 1962). The authors
suggested that the activation of pyridoxal phosphate by vanadyl
ions was specific to elimination and strongly suggested a
decrease in -SH groups in the organism. A reduction in cystine
levels was observed in keratinized tissues (hair of rats fed
vanadium compounds and the fingernails of vanadium workers)
by Mountain et al. (1953, 1955). It was suggested that this
effect was the result of decreased synthesis of cysteine and
cystine and that metabolic processes depending on either of
these amino acids may be depressed in the presence of vanadium.
According to Mahler & Cordes (1966), coenzyme A plays a
central role in many biosynthetic and oxidative pathways. In
the biosynthesis of coenzyme A, cysteine reacts with 4-
phosphopantothenic acid in the presence of adenosine triphos-
phate (ATP) to form the intermediate 4'-phosphopantothenyl
cystine, and Mascitelli-Coriandoli & Citterio (1959a,b) showed
that treatment with sodium metavanadate reduced the content of
coenzyme A in rat liver.
As coenzyme A is involved in biochemical pathways starting
with acetate, these processes can also be affected by vanadium;
Curran (1954) showed that the synthesis of cholesterol from 14C-
acetate in rat liver was reduced in the presence of vanadium.
Later studies indicated that one site of the inhibitory action
of vanadium in the synthesis of cholesterol was at the level of
the enzyme squalene synthetase, which catalyses the conversion
of farnesyl pyrophosphate to squalene (Azarnoff & Curran, 1957;
Azarnoff et al., 1961). In a study by Curran & Costello (1956),
aortic cholesterol was mobilized more rapidly in vanadium-
treated atherosclerotic rabbits than in controls. Cholesterol
levels appear to be reduced by vanadium in young animals,
including human beings, but not in older ones. It has been
suggested, but not demonstrated, that a regulatory enzyme in the
synthesis of cholesterol (acetoacetyl coenzyme A deacylase) is
activated by vanadium in the former but inhibited in the latter.
Vanadium might reduce the synthesis of triglycerides and
phospholipids, since acetyl coenzyme A is a precursor of fatty
acids. However, the results of studies by Curran and colleagues
showed that, while the levels of triglycerides decreased in the
livers of rats given vanadium (Curran, 1954), serum-
triglycerides levels in human beings increased following the
ingestion of vanadium (Curran et al., 1959). Snyder & Cornatzer
(1958) reported that the incorporation of labelled phosphate
into rat liver phospholipids decreased following injection of
vanadyl sulfate. This could have been due to the inhibition of
phospho-lipid biosynthesis or to increased oxidative
degradation, as originally suggested by Bernheim & Bernheim
(1938, 1939). Using an isotope method with radioactive
phosphorus as the indicator, Prokopenko (1961) observed a
disturbance in the intensity of phosphorus exchange between
organic acid-soluble compounds and inorganic phosphates in
albino rats and mice with acute ammonium vanadate poisoning. No
changes were found in the intensity of total metabolism and in
liver tissue phosphate levels. After administration for 6
months in doses of 1 mg/kg body weight, phosphorylation
processes in the organs and tissues were disturbed and the
concentration of inorganic phosphorus in the blood and urine was
increased.
Coenzyme A is also involved in the synthesis of coenzyme Q
(ubiquinone) in the mitochondrial electron transport chain.
Ubiquinone synthesis in isolated rat mitochondria was reduced by
vanadium, but this effect was partially reversed when cysteine
was given with vanadium. Addition of ATP and coenzyme A
prevented the inhibition of ubiquinone synthesis (Aiyar &
Sreenivasan, 1961).
It has been suggested that mitochondrial oxidative
phosphorylation in liver homogenates in vitro is uncoupled by
vanadium with a resulting depletion in ATP energy stores (Wright
et al., 1960). In young chicks, the addition of ammonium
metavanadate to the diet equivalent to 25 mg vanadium/kg body
weight uncoupled oxidative phosphorylation in the liver
mitochondria (Hathcock et al., 1966). These authors suggested
that vanadate might replace the phosphate ion in ATP synthesis,
and a high-energy vanadyl intermediate (vanadium X) or ADP-
vanadium be formed and hydrolysed. The results of studies by
DeMaster & Mitchell (1973) supported the theory of the mechanism
involving the uncoupling of glyceraldehyde-3-phosphate
dehydrogenase by vanadium. It was also shown that the
vanadium5+ oxyanion inhibited oxidative phosphorylation in
intact rat liver mitochondria, but did not act as an uncoupler.
Vanadium salts inhibit the activity of succinic dehydro-
genase, a key enzyme in the citric acid cycle and the electron
transport system that is activated by sulfhydryl groups, and
this would also reduce ATP synthesis (Aiyar & Sreenivasan,
1961). The inhibiting effects of vanadium on succinic
dehydrogenase could involve a reduction in available -SH
groups.
The oxidation of tryptamine by monoamine oxidase from
guinea-pig liver and kidney was accelerated by 125% in the
presence of vanadium (Perry et al., 1955, 1969); however, the
results of studies by Lewis (1959c) on dogs injected with sodium
metavanadate indicated that vanadium inhibited monoamine
oxidase, because the urinary output of 5-hydroxyindoleacetic
acid was reduced. The decreased output of 5-hydroxyindoleacetic
acid, suggests the possibility of accumulation of serotonin,
which was also reported by Roshchin (1967a).
When rats were administered daily injections of sodium
metavanadate (1.25 - 2.5 mg/kg body weight), weight loss was
correlated with accumulation of the metal in the liver (Johnson
et al., 1974). The activities of the hepatic enzymes, xanthine
oxidase and sulfite oxidase, which have molybdenum groups, and
total liver concentrations of molybdenum were not affected by
vanadium. It was concluded that vanadium toxicity in rats was
not related to molybdenum utilization. Though vanadium was
administered in the pentavalent state, the rat livers had an
electron paramagnetic resonance (EPR) spectrum characteristic of
vanadium4+. It was suggested that vanadium is in a protein-
bound form in the livers of rats. Vanadium4+ was also found in
the kidneys and, to a limited extent, in the lungs of rats
injected with sodium metavanadate, but not in the hearts; it was
considered that the ability of liver and kidney to reduce the
vanadate by one electron might be related to a specific
detoxification mechanism present in these organs. Johnson et
al. (1974) and Minden & Rothe (1966) studied the effects of
pentavalent vanadium salts on various enzyme systems in rat
liver homogenates, rabbit blood-serum, and suspensions of
erythrocytes and concluded that there was nothing to suggest
that vanadium salts reacted directly with coenzymes (NAD and
NADP, pyroxidal sulfate) or -SH groups.
The results of in vitro studies, conducted by Tolman et al.
(1979), indicated that vanadium stimulated glucose oxidation and
transport in adipocytes and glycogen synthesis in the liver and
diaphragm, and inhibited hepatic gluconeogenesis and intestinal
glucose transport. There is no evidence in vivo showing a role
of vanadium in the regulation of glucose metabolism.
The intimate mechanism of interaction between vanadium and
enzymes is not yet known. Experimentally observed disturbances
of the cardiovascular system, changes in the concentration of
-SH groups and cystine, disturbances in the metabolism of
sulfur-containing, glycogen-forming, and keto-forming amino
acids, and of the functioning of the liver and kidneys, of RNA
and DNA synthesis, and of cholesterol metabolism, indicate that
vanadium possesses a broad spectrum of action in the body and
that its toxic action is not analogous to that of any other
metal (Roshchin, 1968).
7.3 Effects on the Nervous System
The Na+K+-ATPase in rat brain is inhibited by vanadium
pentoxide, though not as strongly as that in kidney and heart
(Nechay & Saunders, 1978). Svoboda et al. (1984) showed that
the brain microsomal N+K+-ATPase in rat is equally inhibited by
vanadate (VO3-) or the vanadyl ion (VO+2).
In a short-term exposure study, rats received single intra-
peritoneal injections of sodium metavanadate equal to 20% of the
LD50 (1 mg vanadium/kg body weight). The level of noradrenaline
decreased and those of dopamine and 5-hydroxy-tryptamine
increased. A long-term study involved the oral administration
of sodium metavanadate (3 mg vanadium/kg body weight). The
findings were similar to, but more pronounced than, those in the
short-term study (Witkowska & Brzezinski, 1979).
When adult male CD1 mice were treated with vanadate in the
drinking-water for 30 days, there was a dose-related decrease in
norepinephrine levels in the hypothalamus. Dopamine levels also
decreased significantly, but 5-hydroxytryptamine levels were not
affected. Levels of dopamine in the corpus striatum were
unchanged and there were only marginal effects on the amines in
the other brain regions. It is suggested that vanadium has a
selective effect on adrenergic pathways (Sharma et al., 1986).
Several inhibitors of Na+K+-ATPase, such as ouabain, have
been reported to block DNA and protein synthesis in cell
cultures (Kaplan, 1978). It has also been shown that vanadate
inhibits protein synthesis in neuroblastoma cells and in the
brain homogenates of rats fed sodium monovanadate (125 mg/litre)
ad libitum for 30 or 60 days (Montero et al., 1981).
Neurophysiological effects have been reported following
acute exposure (oral and sc injection) of dogs and rabbits to
vanadium oxides and salts (V2O3, V2O5, VCl3, NH4VO3) (Roshchin,
1967a). These include disturbances of the central nervous
system (impaired conditioned reflexes and neuromuscular
excitability).
In studies by Seljankina (1961), solutions of vanadium
pentoxide or ammonium vanadate were administered orally to rats
or mice in doses of 0.005 - 1 mg vanadium/kg body weight per day
for periods ranging from 21 days at the higher levels to 6
months at the lower levels. A dose of 0.05 mg vanadium/kg body
weight was found to be the threshold dose for functional
disturbances in conditioned reflex activity in both mice and
rats; a dose of 0.005 mg vanadium/kg body weight did not produce
any adverse effects.
In a study by Pazhynich (1966), albino rats underwent con-
tinuous inhalation exposure to condensation aerosols of vanadium
pentoxide at levels of 0.002 mg/m3 and 0.027 mg/m3. Animals in
both treated and control groups showed normal weight gain.
After 30 days, the motor chronaxy of the extensor muscles of the
tibia in the group exposed to 0.027 mg/m3 decreased by an average
of 0.8 µs ( P < 0.01), and the chronaxy of the corresponding
flexor muscles increased by an average of 4 µs ( P < 0.001).
Thus, the chronaxy ratio of antagonistic muscles fell from 1.5
at the beginning of the studies to 1.0 on day 20 ( P < 0.02),
and 0.5 on day 30 ( P < 0.01). The decrease continued until a
level of about 0.25 was reached. About 18 days after cessation
of exposure on the 70th day, the chronaxy ratio returned to
normal (1.5). Changes in motor chronaxy were not observed in
rats exposed at 0.002 mg/m3 or in the controls.
In a second study, albino rats were exposed continuously to
vanadium pentoxide at 0.006 ± 0.00056 mg/m3 for 40 days. No
changes were observed in the chronaxy of antagonistic muscles of
the tibia in exposed animals, compared with the controls, during
the first month of exposure. However, after 30 days, there was
a statistically significant decrease in chronaxy ratio. When
animals were given only water and no food for 3.5 days, during
the sixth week of exposure, chronaxy ratios decreased to 0.92,
compared with 1.5 in the controls.
7.4 Effects on the Respiratory System
Studies on respiratory exposure to vanadium pentoxide are
summarized in Table 23 (section 7.1).
Sjöberg (1950) exposed rabbits to vanadium pentoxide dust
particles, nearly all of which were smaller than 10 µm in
diameter at concentrations of 77, 109, 205, or 525 mg/m3 for
periods of 7 h, 4 h, 7 h, or 1 h, respectively. Death occurred
only in the 205 mg V205/m3 (7 h) group (equivalent to 115 mg
vanadium/m3). There was marked tracheitis accompanied by
pulmonary oedema and bronchopneumonia. Conjunctivitis,
enteritis, and fatty infiltration of the liver were also
observed.
In further studies by the same author, rabbits were exposed
to 20 - 40 mg V205/m3 (equivalent to 11 - 22 mg vanadium/m3)
intermittently for 1 h each day for several months. At autopsy,
pathological changes observed included chronic rhinitis and
tracheitis, emphysema, patches of lung atelectasis, broncho-
pneumonia and, in some cases, pyelonephritis. Vanadium was
detected in ashed lung, liver, and kidney, but not in the
intestines. Changes of a fibrotic nature and specific chronic
lesions were not observed in the lungs, and there was no visible
accumulation of particles. These findings, plus the fact that
vanadium was present in the liver and kidney, were considered
evidence of rapid clearance and/or absorption from the lung.
Continuous inhalation exposure of rabbits to 10 - 30 mg
V205/m3 (5.6 - 16.8 mg vanadium/m3) caused bronchitis,
pneumonia, loss of weight, and bloody diarrhoea (Gulko, 1956).
In studies by Roshchin (1967b, 1968) described in section
7.2, acute inhalation toxicity in albino rats was characterized
by irritation of the respiratory mucosa and nasal discharge that
sometimes contained blood. Animals breathed with difficulty and
there were crepitations. The animals behaved passively,
refusing to eat, and lost weight. In cases of severe poisoning,
diarrhoea, paralysis of the hind limbs, and respiratory failure
were followed by death. Pulmonary abscesses were frequently
seen in animals that recovered. Animals that died or were
killed at various times after exposure, showed severe
congestion, particularly in the capillaries, and small
haemorrhages were observed in all internal organs. Signs of
increased intracranial pressure, and fatty degeneration of the
liver and kidneys were also seen. In the lungs, there was
capillary congestion together with tiny haemorrhages,
perivascular and focal oedema, bronchitis, and focal
interstitial pneumonia. The bronchitis and bronchopneumonia
were often purulent, and the small bronchi were constricted.
There was a relationship between the severity of the
pathological changes and the vanadium concentration in the air.
In cases of slight toxicity, pathological changes were mainly
confined to the lungs.
Pathological changes were also seen in the lungs when rats
were exposed intermittently to a condensation aerosol of
vanadium pentoxide at 3 - 5 mg/m3 for 2 h, every other day, for
3 months, or to a dispersion aerosol of V205 at 10 - 30 mg/m3
for 4 months. Blood vessels were engorged and the endothelium
was swollen; capillary congestion, perivascular oedema,
lymphostasis, and small haemorrhages indicated altered vascular
permeability and disturbances in the circulation of the
pulmonary blood and lymph. Occasionally, foci of oedema and
desquamative bronchitis were observed and small bronchi were
often constricted. Lymphocytes and histiocytes had infiltrated
interstitial tissue. Connective tissue proliferation was
sometimes seen in the zone of lymphocytic infiltration.
Purulent bronchitis or pneumonia occurred in some animals, and
occasionally lung abscesses developed.
Roshchin (1967a) observed similar effects with vanadium
trioxide and vanadium trichloride. As vanadium trichloride was
more soluble, more marked histopathological effects were seen in
internal organs. Pentavalent compounds of vanadium were 3 - 5
times more toxic (in terms of median lethal concentration) than
trivalent compounds. Although dispersion aerosols of vanadium
metal, vanadium carbide, and ferrovanadium were not highly
toxic, long-term exposure at high concentrations resulted in
many of the signs and symptoms produced by vanadium pentoxide.
In studies by Pazhynich (1966) (section 7.2), histopatho-
logical changes observed in rats following high-level inhalation
exposure included marked lung congestion, focal lung haemor-
rhages, and extensive bronchitis.
Effects on the lung were observed in albino rats exposed by
inhalation for 2 weeks to uncoated bismuth orthovanadate dust
(0.11 mg/litre, 1.2 mg/litre), silica-coated bismuth ortho-
vanadate (0.15 mg/litre, 1.3 mg/litre), or silica-coated
titanium dioxide (1.19 mg/litre). In rats sacrificed at the end
of the 2-week exposure, there was a dose-related macrophage
(dust cell) response to both forms of bismuth orthovanadate.
Three months after exposure, the bismuth orthovanadate rats had
alveolar proteinosis, foamy macrophages with cholesterol clefts,
and hyperplastic type II pneumocytes. Six months after exposure,
these changes were more marked with cholesterol granulomas and
degeneration of foamy macrophages. In rats sacrificed after 1
year, the pulmonary lesions were reduced, but alveolar protein-
osis and cholesterol granulomas persisted. Initially, similar
changes were observed in rats exposed to the silica-coated
titanium dioxide, but recovery was obvious at 6 months and, at 1
year, the lungs were almost normal with only a few remaining
macrophage (dust cell) aggregates (Lee & Gillies, 1986).
The respiratory and related histopathological effects of
vanadium exposure in experimental animals were marked irritation
of the respiratory mucosa; vascular injury resulting in
capillary stasis, perivascular oedema, and small haemorrhages;
and an asthmatic-type bronchitis and expiratory difficulty on
acute exposure (Roschin, 1967b).
In adult male cynomolgus monkeys exposed by inhalation to
vanadium pentoxide dust concentrations of 0.5 mg/m3 or 5 mg/m3
at weekly intervals, significant central and peripheral airflow
restriction was measured one day after each exposure. There
were also significant increases in respiratory cell counts
obtained by bronchoalveolar lavage. The increased cell count
was due to a marked increase in the number (absolute and
relative percentage) of polymorphonuclear leukocytes,
indicating pulmonary inflammatory changes (Knecht et al.,
1985).
In the respiratory tract of experimental animals, the main
differences between the acute and chronic effects of vanadium
are the development, after prolonged exposure, of chronic
inflammation in the bronchi and a greater tendency to septic
bronchopneumonia. Atelectasis, interstitial infiltration and
proliferation, and emphysema also occur.
Macrophages are engaged in a variety of pulmonary defence
mechanisms. Theoretically, effects of vanadium on these cells
may explain some of the observations on vanadium toxicity on the
respiratory system. In in vitro studies, Waters et al. (1974)
demonstrated a 50% reduction in the viability of cultured rabbit
macrophages after exposure to 13 mg vanadium/litre (as vanadium
pentoxide) for 20 h. Short-term exposure (2 h) to vanadium
pentoxide at a dose of 7 mg vanadium/litre also reduced the
viability of mouse pulmonary alveolar macrophages to 87%. In
another study, the phagocytic index was reduced to 71% (Fisher
et al., 1978). The incubation of bovine alveolar macrophages
with ammonium metavanadate (NH4VO3) at 0.5 or 1 mg
vanadium/litre, for 4 h, reduced viability to 95 and 85%,
respectively. After 8 h incubation, viability was reduced by 24
and 38%, respectively, and, after 16 h, no viable cells
remained. Low levels of vanadium (0.01, 0.1 mg vanadium/litre)
stimulated the phagocytic activity of macrophages, whereas a
striking decrease in phagocytic activity was noted with 0.5 and
1 mg/litre at 8 h, though, initially, there was stimulation of
activity. Doses of 0.01 and 0.1 mg/litre did not affect
viability (Wei & Misra, 1982).
7.5 Effects on the Cardiovascular System
Severe exposure of animals to vanadium oxides and salts
produced cardiovascular changes (occurrence of arrythmias and
extrasystole, prolongation of the Q-RST interval, and decrease
in the height of the P and T waves of the EKG) (Roshchin,
1967a).
Intense vasoconstriction has been reported in the spleen,
kidney, and intestines following intravenous injections of sub-
lethal doses of sodium ortho- and metavanadate (Hudson, 1964).
Intravenous injection of sodium metavanadate at 2.5 mg/kg in
dogs provoked an increased amplitude of T-waves in the electro-
cardiogram followed by depression of S-T segments (Lewis,
1959c). Perivascular swelling, as well as fatty changes in the
myocardium, were observed by Roshchin (1968) following long-term
inhalation exposure of rats and rabbits to vanadium pentoxide,
trioxide, and chloride (10 - 70 mg/m3, 2 h/day, 9 - 12 months).
Vanadium sulfate (500 mg/kg diet, 6 weeks) mobilized excess
arterial cholesterol in rabbits previously maintained on a
cholesterol-rich diet (Curran & Costello, 1956).
Feeding rats sodium orthovanadate at 100 or 200 mg/kg body
weight (added to normal rat chow) for up to 56 weeks resulted in
a gradual increase in systolic blood pressure. The effect was
unrelated to water intake, urine output, or urinary-sodium
excretion. The increased pressure was sustained in a dose-
related manner and was positively correlated with plasma levels
of vanadium that ranged from 0.04 to 0.27 mg/litre (Steffen et
al., 1981). Similar increases in mean arterial blood pressure
have been reported in both conscious (Day et al., 1980) and
anaesthetized rats (Hatfield & Churchill, 1981).
7.6 Effects on the Kidney
In earlier studies, glomerular hyperaemia and necrosis of
convoluted tubules were reported to be related to acute vanadium
exposure (Hudson, 1964). Nephrotoxicity, manifested as
albuminuria, was reported after intravenous injection of sodium
metavanadate at 2.5 - 5 mg/kg body weight in male dogs by Lewis
(1959c). Inhalation of 10 - 70 mg vanadium chloride/m3, for 2 h
daily, for 9 - 12 months, was followed by fatty changes in the
kidney of the rat and rabbit (Roshchin, 1968). In an inhalation
study on rats exposed continuously to vanadium pentoxide
condensation aerosols at concentrations of 0.002 and 0.027 mg/m3
for 70 days, Pazhynich (1966) reported granular degeneration of
the epithelial cells of the convoluted tubules, with areas of
necrosis. Acute tubular necrosis followed subcutaneous injection
of NH4VO3-solutions in 0.1 mol/litre tris-HCl-NaCl buffers in
albino mice (20 mg vanadium/kg body weight). The mortality rate
was higher at a pH of 7.8 (68%) than at pH 6.1 (20%) (Wei et
al., 1982).
There are distinct species differences with regard to the
renal effects of vanadate (Grantham, 1980; Phillips et al. 1983;
Nechay, 1984). Vanadate has both diuretic and natriuretic
effects on the rat kidney (Balfour et al., 1978; Day et al.
1980; Kumar & Corder, 1980; Hatfield & Churchill, 1981; Roman et
al., 1981), but not on that of the dog (Inciarte et al., 1980;
Lopez Novoa et al., 1982a,b) or the cat (Larsen et al., 1979).
In the rat, the tubular effect is independent of changes in
glomercular filtration rate, whereas the tubular effect in the
cat is either absent or masked by pronounced renal vasoconstric-
tion and anuria (Larsen et al., 1979; Larsen & Thomsen, 1980).
Vanadate has also been reported to increase the urinary
excretion of calcium, phosphate, bicarbonate, and chloride in
the rat kidney (Kumar & Corder, 1980).
7.7 Effects on the Immune System
Exposure of mice to vanadium in drinking-water resulted in a
dose-related, but not statistically-significant, decrease in
antibody-forming cells in the spleens of mice challenged with
sheep erythrocytes; serum immunoglobulins were not affected.
Splenic lymphocytes obtained at 1, 4, 8, and 13 weeks from male
Swiss-Webster mice treated with 1, 10, or 50 mg vanadium/litre
drinking-water showed increased DNA synthesis in vitro (Sharma
et al., 1981). In female B6C3F1 mice given ammonium
metavanadate ip at doses of 2.5, 5, or 10 mg/kg body weight,
every 3 days, for 3, 6, or 9 weeks, there was a dose-related
increase in resistance to Escherichia coli endotoxin lethality
up to 6 weeks and a dose-related decrease in resistance
to Listeria lethality. Peritoneal macrophage activity also
decreased in a dose-related manner, but without any effects on
viability. The rosetting capability of splenic lymphocytes was
increased. There was enlargement of the liver and spleen with
enhanced formation of splenic mega-karyocytes and red blood cell
percursors. The authors concluded that vanadium may affect the
normal functioning of the immune system (Cohen et al., 1986).
7.8 Reproduction, Embryotoxicity, and Teratogenicity
7.8.1 Reproduction and embryotoxicity
Roshchin at al. (1980) studied the gonado- and embryotoxic
effects of 0.85 mg metavanadate/kg body weight (1/20 LD50),
administered subcutaneously to albino rats. Vanadium
administered to pregnant rats on days 21 - 22 of pregnancy
accumulated in the placenta but was not reported to penetrate
the placental barrier and reach the fetus. During the period of
lactation, vanadium was found in the mammary glands (0.14% of
administered dose/g tissue) and excreted with the milk. In new-
born rats, the uptake of vanadium was in the range of 0.018 -
0.032% of the original administered dose to the lactating dams
per g neonate. Impairment of spermatogenesis was manifested as
a 10 - 33% decrease in the mobility of spermatozoa, a decrease
in osmotic resistance of 7.9 - 11.4% and a 31% rise in the
number of dead spermatozoa. There were morphological changes in
spermatozoa and desquamation of the spermatogenic epithelium in
the seminal tubuli. The impairment of spermatogenesis affected
the reproduction of animals, resulting in pre-implantation
deaths of embryos. Gonadotoxic effects were suggested by the
absence of fertilization of female rats by male rats exposed
daily to vanadium at 0.85 mg/kg body weight. In other studies
(Hackett & Kelman, 1983) in pregnant rats vanadium tended to
localize initially in the placenta and then to preferentially
concentrate in the membranes rather than in the fetus.
The administration of similar doses of vanadium to female
rats on the 4th day of pregnancy increased the mortality of
embryos as a result of pre-implantation deaths; the number of
fetuses in each female rat was only half that in the untreated
animals. These effects were observed in the absence of general
toxicity in the experimental animals.
In vitro, orthovanadate (0.2 - 2 mmol) inhibited
luteinizing hormone-induced cyclic adenosine monophosphate
(cAMP) in isolated corpora lutea from pseudopregnant rats.
When added simultaneously with luteinizing hormone, inhibition
occurred within 25 min, but not when the corpora lutea had been
pretreated with luteinizing hormone for 60 min. A decrease was
also observed when corpora lutea were exposed to vanadate in the
presence of 3-isobutyl-1-methylxanthene (0.5 mmol), a phospho-
diesterase inhibitor. Cyclic adenosine monophosphate was also
inhibited by vanadate in copora lutea incubated in a calcium-
depleted medium. Vanadyl sulfate (0.4, 2 mmol) was as effective
as vanadate in inhibiting luteinizing hormone-induced cyclic
adenosine monophosphate accumulation (Lahav et al., 1986).
7.8.2 Teratogenicity
Carlton et al. (1982) injected 80 mature Syrian golden
hamsters ip with ammonium vanadate at 0, 0.47, 1.88, or
3.75 mg/kg body weight. Injections were carried out on days 5 -
10 of gestation. A significant increase in skeletal anomalies
was observed in all groups exposed to vanadate compared with
control animals. A significant increase in deaths was registered
in the 1.88 mg/kg group. There was no dose-response relationship
as regards anomalies.
Pregnant NMRI albino mice were injected intravenously with
1 mmol vanadium pentoxide dissolved in distilled water on day 3
or day 8 of pregnancy (day 1 = finding of vaginal plug). Control
groups were given 0.1 ml physiological saline on the same days.
Mice were killed on day 17, the uterine horns examined for
resorbed embryos, and the fetuses were removed for detailed
examination. Vanadium pentoxide did not produce any effects in
implantation, and fetuses from the day 3-treated and control
groups did not show any differences in litter size, fetal
weight, or external and internal morphology. The fetuses from
the day 8-treated group showed a statistically significant high
frequency (71%) of delayed ossification (supraoccipital bone,
sternum, metatarsals, and caudal vertebrae), and broken spinal
cord occurred in a few fetuses (Wide, 1984).
Vanadium pentoxide was administered subcutaneously to
pregnant rats (strain unspecified) at doses of 0.5, 1, or 4
mg/kg body weight for 10 days, from day 7 to day 16 of
gestation. The incidences of resorbed and dead fetuses in the 1
and 4 mg/kg groups were 17% and 27.2%, respectively. These were
significantly higher than those found in the controls (3.5%). In
the 4 mg/kg group, 52.38% of fetuses showed wavy ribs. In a
separate study, pregnant rats were given an aqueous solution of
V2O5 by ip injection at concentrations of 0.3, 1, or 3 mg/kg
body weight. This induced a higher level of resorbed and dead
fetuses than oral administration. Both ip injection of 0.3
mg/kg and oral administration of 9 mg/kg induced an array of
skeletal anomalies, namely wavy ribs, supernumerary ribs, and
fused sternebrae and vertebrae (Sun, ed., 1987). Though
tentative, these results suggest that vanadium may have the
potential to induce teratogenicity in a mammalian system.
7.9 Mutagenicity and Related End-Points
There are few studies on the mutagenicity and carcino-
genicity of vanadium compounds. Mutagens in the air can be
divided into two groups: a non-polar extract rich in polycyclic
aromatic hydrocarbons (PAHs) and other promutagens, and a polar
extract containing direct acting mutagens, i.e., not requiring
microsomal activation (Madsen et al., 1982). The non-polar
fraction was strongly influenced by automobile exhaust products,
whereas the polar was more attributable to secondary emissions
transformed by atmospheric reactions, and to primary emissions
from stationary sources. The role of PAHs in the overall
mutagenicity was estimated to be modest; thus, the importance of
other substances increases. Vanadium was found at all sample
sites and is a well-known constituent of, for instance, coal fly
ash (section 3.4.2).
Vanadium5+ has been shown both to inhibit or enhance DNA
synthesis in vitro , depending on the concentration in the media
(Hori & Oka, 1980; Carpenter, 1981; Jackson & Linskens, 1982;
Smith, 1983).
In a DNA synthesis inhibition assay in male mice, vanadium
pentoxide suspended in 3% starch solution was administered
orally at doses of 14.6, 29.2, or 58.4 mg/kg body weight. The
animals were killed 24 h after dosing; 3 h before sacrifice,
1 µCi 3H-thymidine/g body weight was administered ip.
Incorporation of 3H-thymidine in the testes, spleen, liver, and
blood was measured in a liquid scintillation spectrometer.
There were no significant differences between the experimental
groups and the solvent control group (Sun, ed., 1987).
In a study using FADU (Fluorescence Analysis of DNA
Unwinding), vanadyl chloride at a concentration of 5 x 10-5 mol
failed to induce DNA damage (strand breaks) in human peripheral
white blood cells (McLean et al., 1982).
Kanematsu et al. (1980) carried out rec assays on 127 metal
compounds with Bacillus subtilis to test their DNA-damaging
capacity. Mild positive results were noted for three vanadium
compounds (VOCl2, V2O5, NH4VO3).
A lack of induction of spot mutations in Escherichia coli
and in Salmonella typhimurium was demonstrated by Kanematsu &
Kada (1978) and Kada et al. (1980). Similar results were
obtained by Si Rongshan et al. (1982) with E. coli . However,
ammonium metavanadate was found to be mutagenic in S.
typhimurium TA1535 in a modified plate incorporation assay and
in the fluctuation test with TA100 (Arlauskas et al., 1985). In
a recent study, Sun, ed. (1987) demonstrated the induction of
reverse mutations by vanadium pentoxide with E. coli WP2,
WP2uvrA, and Cm-981, but no frameshift mutations with strains
ND-160 and MR102. Vanadium pentoxide showed negative results
with S. typhimurium strains TA1535, TA1537, TA98, and TA100.
Thus, the results of mutagenicity studies of vanadium with
bacterial assays are conflicting, and no firm conclusions can be
drawn.
In a micronucleus test, vanadium pentoxide was administered
to two strains of mice (615 and Kunming albino) by ip injection
at doses of 6.4, 2.13, or 0.17 mg/kg body weight for 5 consecu-
tive days; cyclophosphamide was used as a positive control (Sun,
ed., 1987). Significant levels of induced micronuclei in both
strains were observed. Both subcutaneous injection of vanadium
pentoxide solution (0.25, 1, or 4 mg/kg) and inhalation of
vanadium pentoxide dust (0.5, 2, or 8 mg/m3) also induced micro-
nuclei in mice strain 615. However, negative results were
obtained following oral administration of a 3% starch suspension
of vanadium pentoxide at doses of 1.44, 2.83, 5.65, or
11.3 mg/kg body weight, daily, for 6 weeks, to Kunming albino
mice (Sun, ed., 1987).
In an in vitro study on human peripheral lymphocyte cultures
with vanadium pentoxide concentrations of 0.047, 0.47, or
4.7 moles (mitomycin c was used as positive control), no
increases in the frequency of sister chromatid exchange were
observed (Sun, ed., 1987).
In a dominant-lethal mutation assay, vanadium pentoxide
(0.2, 1, or 4 mg/kg body weight) was administered daily by
subcutaneous injection to 5 groups of male mice, aged 5 - 6
weeks at the start of the study, for 3 months, following which
they were mated with females. Ethylmethanesulfonate and
distilled water were used as positive and negative controls,
respectively. The females were killed 17 days after conception
and the numbers of fetuses and resorptions recorded. The
results were considered negative for the induction of dominant-
lethal mutations (Sun, ed., 1987).
7.10 Carcinogenicity
In a study on 13 metallic compounds, intraperitoneal
injections of vanadium3+ 2.4-pentanedione at doses of 24, 60, or
120 mg/kg body weight did not significantly increase the
incidence of lung adenomas in mice (Stoner et al., 1976).
In life-span studies, the incidence of tumours in mice
given vanadyl ions (as the sulfate) at 5 µg/litre drinking-
water was similar to that in control animals (Kanisawa &
Schroeder, 1967; Schroeder et al., 1970; Schroeder & Mitchener,
1975).
In rats, the induction of mammary carcinogenesis by 1-
methyl-1-nitrosurea was blocked by feeding a purified diet
supplemented with 25 mg vanadyl4+ sulfate/kg during the post-
initiation stages of the neoplastic process. Both cancer
incidence and the average number of cancers per rat were reduced
by the vanadium4+ diet without inhibiting the overall growth of
the animals (Thompson et al., 1984). It has also been shown
that metallocene dichlorides, (C2H5)2MCl2 (where M = titanium,
vanadium, molybdenum, or niobium), exhibit cancerostatic
activity against the Erlich ascites tumour system in mice, and
that treatment with such substances cured the tumour (Köpf-Maier
et al., 1980). Vanadocene dichloride was reported to have a
chemotherapeutic activity similar to that of cis-dichlordiamine
platinum (II) when used against liver tumours in mice (Köpf-
Maier & Köpf, 1979). The mechanism of the preventive effect of
vanadium is not clear. Modulation of one or more aspects of the
DNA metabolism could account for these results.
The effects of ammonium vanadate on the development of large
bowel neoplasms in mice treated with 1,2-dimethylhydrazine (DMH)
were studied by Kingsnorth et al. (1986). Mice were treated
with DMH (20 mg/kg body weight per week) for 20 weeks. Ammonium
vanadate was given in the drinking-water (10 or 20 mg/litre) to
groups of mice during the study. At 30 weeks, the colons of
DMH-treated mice (not receiving ammonium vanadate) showed
increases in RNA content (+14%) and DNA content (+18%) and
deeper crypts (+33%). In the mice treated with DMH and receiving
ammonium vanadate at 10 or 20 mg/litre, the RNA content was
decreased by 11%. Although thymidine incorporation was
increased, ammonium vanadate did not have any effects on the
incidence or type of tumour induced by DMH (Kingsnorth et al.,
1986).
In a long-term study in which the carcinogenic activity of
various materials was studied using intrabronchial pellet
implantation in the lower left bronchus of rats, vanadium solids
produced chronic inflammatory changes in 44/100 rats, bronchial
inflammation in 50/100, squamous metaplasia in 10/100, and one
bronchial carcinoma in a male rat after 645 days. These results
were not significant for carcinogenicity (Levy et al., 1986).
8. EFFECTS ON MAN
8.1 Therapeutic Exposure and Controlled Studies
8.1.1 Therapeutic exposure
In the past, vanadium compounds were prescribed as thera-
peutic agents for anaemia, chlorosis, tuberculosis, and
diabetes. They were also used as an antiseptic, a spiro-
chetocide, and a tonic. For example, sodium metavanadate was
given therapeutically by mouth in doses of 1 - 8 mg, and sodium
tartrate was injected intramuscularly at levels as high as
150 mg. Due to poor absorption from the gastrointestinal tract,
the metal is not very toxic for human beings when ingested, but,
if introduced directly into the circulation in a soluble form,
Hudson (1964) estimated that the lethal dose for a 70-kg person
would be only 30 mg V205 (0.42 mg V205/kg body weight).
8.1.2 Controlled studies
8.1.2.1 Effects on metabolism
Vanadium has been administered under controlled conditions
to study its effects on blood-cholesterol levels. Curran et al.
(1959) conducted a clinical study in which 5 healthy adult male
volunteers were fed soluble diammonium oxytartarovanadate at
150 - 200 mg/day (21 - 30 mg vanadium/day) for 6 weeks. At the
end of the period, plasma-cholesterol was significantly
reduced.
Lewis (1959a) compared age-matched groups of 32 vanadium
workers with 45 controls, all over 45 years of age. Vanadium
workers exposed for at least 6 months excreted greater amounts
of vanadium and exhibited slightly lower serum-cholesterol
levels than the controls. Mean cholesterol values for the 2
reference groups (representing 2 geographical areas) were 2309
and 2267 mg/litre. Mean levels for vanadium workers from
corresponding areas were lower at 2049 and 2067 mg/litre (P <
0.05), respectively.
A clinical study by Somerville & Davies (1962) on 12
patients (9 of whom were hypercholesterolaemic) given diammonium
vanadotartrate orally for 6 months (25 mg 3 times daily for 2
weeks, increased to 125 mg daily in 10 patients) did not show
any significant changes in serum-cholesterol levels over 5.5
months. The mean pretreatment control level of serum-cholesterol
was 4110 mg/litre, and the mean age was 49.2 years. The study
is not comparable with that of Curran et al. (1959), as the
patients were hypercholesterolaemic and older. Dimond et al.
(1963) observed temporary drops (not statistically significant)
in cholesterol levels in 2 out of 6 patients given ammonium
vanadyl tartrate for several weeks at levels of between 50 and
100 mg/day. No statistically significant changes were observed
in blood-lipids, phospholipids, triglycerides, 17-ketosteroids,
or 17-hydroxycorticosteroids. Two patients complained of fatigue
and lethargy while they were taking vanadium. All complained of
cramps and loosened stools, and all developed green tongue.
Schroeder et al. (1963) reporting findings similar to those of
Dimond et al. (1963) considered that the slight effects of
vanadium on serum-cholesterol were pharmacological rather than
caused by correction of a physiological deficiency. They
further pointed out that dietary regimens based on the consump-
tion of unsaturated fats, which reduce plasma-cholesterol in
human beings, are associated with the intake of 1 - 4 mg
vanadium/day and that the feeding of vanadium-poor saturated
fats raises cholesterol.
Studies have been undertaken on the effects of vanadium on
human dental caries. Belehova (1969) studied 583 school
children ranging in age from 7 to 11 years. The subjects were
divided into 4 groups. Children in Group I received fluoride
twice a year, those in Group II received a local application of
a 50% paste of an ammonium salt of vanadium and glycerol, Group
III received both fluoride and vanadium, and Group IV acted as
the control. The incidence of caries was 11% in Group III,
15.4% in Group II, 29.7% in Group I, and 43% in the control
group. Belehova concluded that the lower incidence of caries in
subjects receiving vanadium suggested a possible prophylactic
action. However, other studies (Hein & Wisotzky, 1955; Muhler,
1957; Hadjimarkos, 1966, 1968; McLundie et al., 1968) failed to
demonstrate a clearly beneficial effect with regard to dental
caries in human beings.
Data on the effects of vanadium on the haematopoiesis are
inconsistent. Lewis (1959a) did not observe any effects of
exposure to vanadium on haematocrit levels in 32 vanadium
workers compared with 45 controls matched for age. A beneficial
effect of low-level vanadium administration on nutritional
anaemia has been suggested (Beard et al., 1931; Myers & Beard
1931; Hadjimarkos, 1966; Kopylova, 1971). However, the effects
of vanadium on iron metabolism have not been fully assessed
(Vouk, 1979).
The administration of w -methylpantothenic acid, an
antimetabolite of pantothenic acid, to human beings resulted in
a syndrome consisting of postural hypotension, dizziness,
tachycardia, fatigue, drowsiness, epigastric distress, anorexia,
numbness and tingling of the hands and feet, and hyperactive
deep reflexes. It is not known whether these symptoms are the
result of an induced deficiency of pantothenic acid or are toxic
effects of the anti-metabolite. However, the symptoms resemble
those resulting from exposure to high concentrations of
vanadium. The common denominator in both cases may be a
reduction in hepatic coenzyme A levels (Waters, 1977).
8.1.2.2 Effects on the respiratory system
Zenz & Berg (1967) studied the effects of vanadium
inhalation in 9 healthy volunteers aged 27 - 44 years, for whom
baseline lung function data were available. Two volunteers,
exposed to vanadium pentoxide dust at 1 mg/m3 for 8 h,
developed sporadic coughing after 5 h and a frequent cough after
nearly 7 h. Coughing lasted 8 days, but lung sounds remained
clear and there were no other signs of irritation. Lung
function tests, complete blood counts, urinalyses, and nasal
smears were normal up to 3 weeks. Three weeks later, the same 2
volunteers were accidently exposed for 5 min to a "heavy cloud"
of vanadium pentoxide dust. A productive cough developed within
16 h, and, within 24 h, rales and expiratory rhonchi developed
throughout the lung, but pulmonary function remained normal.
Isoproterenol (1:2000) relieved the symptoms for about 1 h, but
coughing then resumed and continued for 7 days. There were no
other symptoms. Eosinophils were not present in the nasal
mucous.
Exposure of 5 volunteers to a lower concentration
(0.2 mg/m3, 98% of particle size 5 µm) had similar effects,
though the symptoms took longer to develop, i.e., after 20 h.
Coughing, without other systemic effects, persisted for 7 - 10
days. Pulmonary function tests and differential white blood
counts remained normal. The vanadium concentration in the urine
was highest (0.13 mg/litre) on the third day, with none
detectable after 7 days. The maximal faecal-vanadium level was
3 mg/kg, with none detectable after 14 days. Exposure to a
concentration of 0.1 mg/m3 for 8 h did not produce any coughing
in 2 subjects not previously exposed. However, an increase in
the production of mucous, 24 h later, indicated some respiratory
irritation. Then there was slight coughing, which became more
severe after 48 h, subsided after 72 h, and disappeared after
96 h. Pulmonary function tests and differential white blood
counts remained normal.
Pazhynich (1967) studied the irritant effects of vanadium
pentoxide condensation aerosol on 11 volunteers. At a
concentration of 0.4 mg/m3, all reported a tickling or itching
sensation and a feeling of dryness in the region of the root of
the tongue, the posterior wall of the pharynx, and the fauces,
as well as a slight prickling sensation in the nose and
posterior pharyngeal wall. These symptoms were easily tolerated.
A concentration of 0.16 mg/m3 caused mild signs of irritation in
only 5 volunteers, and a concentration of 0.08 mg/m3 was not
noticed by any volunteer. It was concluded that the mean
perceptible concentration for human beings is 0.27 mg/m3 and
that 0.16 mg/m3 is imperceptible.
8.2 Clinical Studies
The clinical picture of poisoning shows the broad spectrum
of toxic effects of vanadium. The lesions observed affect the
respiratory system, circulatory system, central nervous system,
digestive organs, kidneys, and skin. Poisoning can be divided
into acute and chronic forms.
8.2.1 Acute toxicity
Acute toxicity is characterized by a latent period, which
depends on the concentration of vanadium, the individual
sensitivity of the subject, and the properties of the specific
vanadium compound. The more soluble salts of vanadium pentoxide
have a more rapid action than the vanadium oxides. Chemically-
pure vanadium pentoxide acts more rapidly than the technical
grade. A condensation aerosol of vanadium pentoxide is more
toxic than a disintegration aerosol (Roshchin, 1964). Vanadium
chloride is toxic more rapidly than other compounds.
Roshchin (1968) subdivided acute vanadium effects into
"mild", "moderate", and "severe" forms. The clinical features
of mild toxicity include rhinitis with a profuse and often
bloody discharge, sneezing, and an itching and burning sensation
in the throat. The rhinitis may be followed by the development
of a dry cough with expectoration of small amounts of viscid
sputum, general weakness, and exhaustion. A sub-normal
temperature may be present; in other cases, the temperature may
be high or normal. The patient is afebrile in the absence of
pneumonic disease (Sjöberg, 1950). Conjunctivitis is frequently
observed. The symptoms and course of mild toxicity resemble an
upper respiratory tract infection. Other symptoms include
diarrhoea due to intensified intestinal peristalsis. The
symptoms disappear from 2 - 5 days after cessation of contact
with the dust.
In moderate toxicity, in addition to conjunctivitis and
irritation of the upper respiratory tract, there is bronchitis
with expiratory dyspnoea and bronchospasm. There are frequent
disturbances in the activity of the gastrointestinal tract,
including vomiting and diarrhoea. Taken together with the
bronchospasm, this points to a response of the smooth muscle to
vanadium exposure. Some affected persons have cutaneous
manifestations of toxicity in the form of rashes and eczema with
itching papules and dry patches (Browne, 1955; Zenz et al.,
1962).
Bronchitis and bronchopneumonia are features of severe toxic
effects. Other symptoms may also be more prominent, such as
headache, vomiting, diarrhoea, palpitations, sweating, and
general weakness. Disorders of the nervous system include
severe neurotic states and tremor of the fingers and hands
(Wyers, 1946; Sjöberg, 1955). Functional disturbances of the
respiratory system can be expected, and X-ray examination will
reveal intensification of the lung pattern.
Kidney damage, highlighted by grave dystrophic changes in
the epithelium of the convoluted tubules and disturbed tubular
secretion, occurs immediately after the start of exposure to low
vanadium doses in both acute and chronic intoxication. Once
triggered off, the changes are irreversible, even if exposure is
discontinued. Therefore, the kidneys are a critical organ for
vanadium poisoning (Korkhov, 1965).
8.2.2 Chronic toxicity
Chronic vanadium intoxication produces profound changes in
the respiratory organs, because of the irritant action of
vanadium and the biochemical and functional disturbances
connected with its general resorptive action. Chronic
respiratory illness takes the form of diffuse pneumosclerosis,
chronic bronchitis, chronic rhinitis, and pharyngitis (Roshchin,
1968). However, Parkes (1982) claimed that the available
evidence (Sjöberg, 1950; Williams, 1952; Zenz & Berg, 1967) did
not support the contention that prolonged exposure to vanadium
compounds leads to chronic bronchitis, with or without
emphysema. Although wheezing is more common among vanadium
pentoxide workers than among unexposed workers, lung function
tests and chest radiography have not revealed persistent lung
damage (Kiviluoto, 1980). The cardiovascular system (Wyers,
1946; Sjöberg, 1950; Izycki et al., 1971) is commonly affected
in chronic respiratory disorders by a diagnosable accentuated
second cardiac sound on the pulmonary artery and an attenuated
first sound on the apex cordis. Most of these workers exhibit
heavy sinus arrhythmia and a shift of the EGG-axis to the right.
After extensive exposure, workers may develop bradycardia and a
change of the P wave in the second and third standard leads;
coronary spasm is also usually recognizable in such workers. A
statistically significant increase in the incidence of enlarged
liver and a decrease in functional tests together with
bilirubinaemia and a direct reaction to bilirubin have been seen
in the blood of exposed workers (Roshchin, 1968). Biochemical
alterations have also been found, such as reduction in albumins,
and expansion of the globulin fractions at the expense of gamma-
globulins, even though the total protein content remained
normal. Furthermore, a marked reduction in sulfhydryl groups in
blood-serum and in vitamin C levels in the blood, and a less
marked drop in cholesterol levels have been observed. Systemic
effects, such as a tendency towards anaemia and leukopaenia, and
basophilic granulation of leukocytes have been reported
(Watanabe et al., 1966).
Vanadium levels in whole blood and serum have been studied
to investigate the possible role of vanadium in depressive
states. In a study involving neutron activation analysis of
vanadium levels in the whole blood, serum, and hair of patients
suffering from mania or depression, and of patients who had
recovered from these conditions, as well as normal controls,
manic patients were reported to have normal levels in whole
blood and serum, but significantly raised levels in hair.
Depressed patients had raised levels in whole blood and serum.
In both conditions, raised levels fell with recovery. The
levels of vanadium in serum were correlated with those in whole
blood but not with hair levels (Naylor et al., 1984). In another
study, serum-vanadium levels, measured by neutron activation
analysis, were reported to be 3.10 ± 1.38 mg/litre in patients
suffering from depressive illness and 0.67 ± 0.32 µg/litre in
normal subjects (Simonoff et al., 1986). However, in a series
of 25 depressive, 13 recovered depressive patients, and 24
controls, the whole-blood concentrations of vanadium were
similar to normal levels, and vanadium levels did not change in
depressive patients after recovery (Ali et al., 1985).
8.2.3 Diagnosis
Information on likely exposure, the clinical picture, and
certain biochemical indications of probable exposure can aid
diagnosis, but no specific test can be recommended. Determina-
tion of the vanadium contents of the blood and especially of the
urine provides documentation of exposure, though the correlation
between vanadium levels in the urine or serum and air is poor
(Kiviluoto et al., 1979a,c). In view of the work of Schroeder
et al. (1963), it would seem desirable to measure the vanadium
contents of the serum separately from that of the cellular
elements, since the concentration of vanadium in the latter may
be more indicative of exposure levels. As reported by Watanabe
et al. (1966), a decreased urinary output of ascorbic acid may
be one characteristic of vanadium exposure, but differences from
controls do not appear sufficient to make the test clinically
useful.
Green colouration of the tongue is also an indication of
vanadium exposure (Wyers 1946; Williams, 1952; Lewis 1959b). The
green hexaquo ion (V(H2O))63+ is probably responsible for the
green-coloured tongue. However, several other bright green
complexes of vanadium+4 are known and may also account for the
sign (Cotton & Wilkinson, 1962; Durrant & Durrant, 1970). The
"green tongue" may be absent, even in prolonged exposure
(Sjöberg, 1950).
During continuous exposure, measurement of the cystine
content of fingernails was reported to be a sensitive indicator
of exposure. This parameter was negatively correlated with
vanadium exposure in workers. A decrease in cystine levels in
fingernails was demonstrated when urinary-vanadium levels were
only 0.02 - 0.03 mg/litre (Mountain et al., 1955). A similar
reduction in the cystine content of rat hair was noted when
vanadium in the diet ranged from 25 -1000 mg/kg (Mountain et
al., 1953). Some evidence suggesting that vanadium may directly
inhibit the synthesis of cystine or cysteine has also been
reported (Mountain et al., 1953, 1955).
In a recent study on workers exposed to low levels of
vanadium pentoxide (0.1 - 0.6 mg/m3) for about 14 years,
Kiviluoto et al. (198O) could not corroborate the observations
by Mountain and co-workers as no differences were found in
fingernail cystine contents between the 22 exposed workers and
22 unexposed controls. A small reduction in the cystine content
of fingernails (89 mg cystine/kg fingernail for exposed and
99 mg/kg for controls) was found by Thürauf et al. (1979) in 54
exposed workers with an increased urine-vanadium concentration
of 37.8 µg/litre (controls 0.8 µg/litre).
8.2.4 Treatment of poisoning
There are few published data on the treatment of human
poisoning by vanadium. BAL has been used successfully in two
cases of overexposure (Sjöberg, 1955). Experimentally, ascorbic
acid in doses of 125 mg/kg body weight given 20 min prior to an
LD70 dose of NaVO3H2O had a strong protective effect in mice
(Mitchell & Floyd 1956). CaNa2-EDTA was also antidotal in dogs,
when given intraperitoneally in doses of 100 mg/kg body weight
after the first sign of poisoning became evident and again 2 and
4 h later. Jones & Basinger (1983) tested various chelating
agents and their protective effects in mice and found that
efficient antidotes for both vanadate (VO33- and vanadyl (VO2+)
were ascorbic acid, deferoxamine D-penicillamine, sodium,
calcium, Na3CaDTPA, Na2CaEDTa, and glutathione. Ascorbic acid
appeared best suited for human use as an antidote.
Intraperitoneal doses of NaVO3 (0.3 - 1.2 mmol/kg body
weight) were injected in mice followed by chelating and reducing
agents at one-quarter of their respective LD50s. Significant
increases in the survival rate, 14 days after the treatment,
were noted with ascorbic acid, deferoxamine, and tiron (4,5-
dihydroxy-1,3-benzene-disulfonic acid). Other chelators tested
included EOTA, DTPA (Na3Ca-diethylene triaminepenta-acetate) and
L-cysteine. Ascorbic acid was the most effective substance in
preventing vanadium intoxication (Domingo et al., 1986). In
another report, sodium salicylate and D-L-penicillamine were
found useless as antidotes for acute toxicity caused by NaVO3.
8.3 General Population Exposure
8.3.1 Low vanadium intake
Because conditions required to achieve reproducible vanadium
deficiency in animals have not been defined precisely, it is
difficult to predict the consequences of a low vanadium intake
on human health.
Statistical studies have shown negative correlations between
environmental levels of vanadium and certain other trace
elements and the incidence of cardiovascular disease. Consump-
tion of hard water containing vanadium was associated with a
lower incidence of cardiovascular disease (Strain, 1961)a.
Schroeder (1966) reported a significant negative correlation
between the vanadium content of municipal waters and death rates
due to arteriosclerotic heart disease. In a study by Voors
(1971) on the correlation between 7 metals (calcium, chromium,
lithium, zinc, manganese, nickel, vanadium) and arteriosclerotic
heart disease, a low vanadium intake was associated signifi-
cantly with a higher incidence of arteriosclerotic heart disease
in non-white populations, but no direct correlation was
demonstrated for white populations.
____________________
a Strain, W.H. (1961) Effects of some minor elements in
animals and people. Paper presented at the meeting of the
American Association for the Advancement of Science, Denver,
29 December 1961 (unpublished).
In a joint WHO and IAEA study on the role of trace elements
in the etiology of cardiovascular diseases in 20 countries, a
significant role was shown for environmental lack of vanadium as
well as chromium, zinc, manganese, calcium, and magnesium
(Masironi, 1969). Conversely, Hickey et al. (1967) noted a
positive correlation between airborne vanadium levels and the
incidence of cardiovascular disease (section 8.3.2).
The evidence implicating vanadium as an essential trace
element for human beings is not satisfactory. Although certain
statistical studies have indicated that low vanadium intake may
be associated with human cardiovascular disease, these
relationships do not furnish any direct proof for a nutritional
role of vanadium in human health. However, they do suggest leads
for further laboratory and epidemiological investigations.
8.3.2 Epidemiological studies
Descriptive epidemiological work has been published using
a correlational approach, which has well-known limitations,
though it imitates a population-based cohort study.
Despite their limitations, such studies can give indications for
more intensive and detailed controlled studies into suspected
health hazards, comparing incidences of diseases in defined
exposure groups (such as production workers) with those obtained
from reference populations.
Stocks (1960) reported the results of a study in which
airborne concentrations of 13 trace elements were correlated
with mortality from lung cancer, pneumonia, and bronchitis in 23
localities in the United Kingdom. At concentrations ranging
from 1.1 to 42 µg/1000 m3, vanadium showed a weak association
with mortality from lung cancer (taking into consideration
population density, sex, and age), with a correlation
coefficient of 0.347. Airborne vanadium levels were also
correlated with mortality from pneumonia in males, with a
correlation coefficient for mortality from pneumonia of 0.443.
For mortality involving bronchitis, vanadium gave a correlation
coefficient of 0.563. Vanadium also showed an association with
mortality from cancers other than lung cancer in males, but not
in females. However, in this study, as is usual in studies of
this kind, it is not certain that cases of interest (lung
cancer, pneumonia) had been exposed at all. There are also the
uncertainties of mortality data and failure to consider
confounding factors.
In another study, Hickey et al. (1967) considered 10 metals
in the air, including vanadium, in 25 communities in the USA.
Various techniques, including canonical analysis, were used to
correlate airborne metal concentrations with mortality indices
for 1962 and 1963 involving 8 disease categories. The mean
atmospheric concentrations for vanadium at the various locations
ranged from 0.001 to 0.672 µg/m3. The incidence of several
diseases, including "diseases of the heart", nephritis, and
"arteriosclerotic heart", could be correlated reasonably well
with air levels of vanadium and other metals. A high inter-
correlation between vanadium and nickel was unexplained. This
study was of a very preliminary nature, with no adjustments for
the basic pertinent variables normally employed. Other studies
have demonstrated significant negative correlations between the
incidence of cardiovascular disease and environmental levels of
vanadium (section 8.3.1).
An additional multivariate analysis of air-vanadium levels
in relation to selected white male mortality levels was included
in an unpublished US Environmental Protection Agency staff study
by Pinkerton et al. (1972)a. Several categories of cardio-
vascular disease were used, and also influenza-pneumonia.
Vanadium was not correlated with the latter, but was correlated
with the cardiovascular categories. However, adjustment for
population density produced a considerable reduction in some of
these relationships. It was concluded that the observed
statistical associations of air-manganese and air-vanadium
levels were not causal associations, and represented either a
reflection of other more directly associated causes or
statistical artifacts.
Barannik et al. (1969) studied the role of certain trace
elements and the natural radioactivity of food products in the
etiology of endemic goitre in the USSR. More chromium and
vanadium and less lead were found in most of the vegetable
products from a region where goitre was endemic compared with
those from a goitre-free region. The differences in the mean
concentrations of these trace elements were statistically
significant.
The differences between these general population-based
observations and the occupational studies on health effects in
vanadium workers (section 8.3) are connected to different
approaches. The correlational epidemiological studies, based
exclusively on long-term effects and causes of death, are
considered at the expense of lack of individual exposure data,
while the medical studies, cross-sectional in nature, cannot
consider the selection effects and lack long-term information
(such as cause of death). Their strength, however, lies in the
fact that they permit analysis according to different levels of
exposure, though further occupational and population studies on
chronic illness in unambiguous relationship to vanadium exposure
are needed to verify previous work and determine if there is
evidence of a dose-response relationship. In such studies,
morbidity as well as mortality should be considered.
______________
a Pinkerton, C., Hammer, D.I., McClain, K., Williams, M.E.,
Bridbord, K., & Riggins, W.B. (1972) Relationship of
manganese and vanadium in the ambient air to heart disease
and influenza-pneumonia mortality rates, Research Triangle
Park, North Carolina, US Environmental Protection Agency
(unpublished data).
8.4 Occupational Exposure
Occupational poisoning occurs mainly during the industrial
production and use of vanadium and in boiler cleaning
operations. Under these conditions, vanadium may enter the
human body through the respiratory tract; an unknown quantity
will be transported to the alimentary tract when swallowed.
Vanadium can also enter through the skin (Roshchin, 1968).
Both acute and chronic poisoning can occur. Vanadium-
containing industrial aerosols differ in chemical and structural
composition and thus evoke different responses in the human
body.
In sections 8.3.1 - 8.3.4, a survey is made of the available
clinical and epidemiological data on the health effects of
vanadium in workers occupationally exposed to vanadium
compounds. Most of the reported clinical symptoms reflect
irritant effects of vanadium on the respiratory tract and eyes.
8.4.1 Metallurgy
Dutton (1911) first described the effects of industrial
exposure to vanadium-bearing ores. He reported a dry, paroxysmal
cough with haemoptysis and irritation of the eyes, nose, and
throat. Temporary increases in haemoglobin levels and red blood
cells were followed by reductions in both and the onset of
anaemia. Vanadium was recovered in all bodily secretions.
Postmortem examination revealed highly congested lungs with
destruction of the alveolar epithelium and congested kidneys
with evidence of haemorrhagic nephritis. Unfortunately, the
workers frequently suffered from pulmonary tuberculosis, which
undoubtedly produced many symptoms that were aggravated by
vanadium exposure, and no details regarding the number of
workers examined or the incidence of the signs and symptoms were
provided.
A later study by Symanski (1939) on relatively healthy metal
workers exposed to vanadium pentoxide dust for periods ranging
from a few months up to several years reported severe conjunct-
ivitis, rhinitis, pharyngitis, chronic productive cough, and
tightness of the chest; severe chronic bronchitis and bronchi-
ectasis sometimes occurred with longer exposure. There was no
evidence of a generalized systemic action of vanadium.
Rundberg (1939) observed bronchitis with purulent sputum,
general weakness, and skin irritation of the face and hands in
20 men handling vanadium pentoxide in a metallurgical works.
Productive cough, bronchitis, and shortness of breath were
reported by Balestra & Molfino (1942) in 25 workers exposed to
vanadium pentoxide dust from petroleum ash. Other substances
were involved, and chest X-rays showed definite lung markings
suggesting pneumonoconiosis. Bronchiectasis was suspected in 2
cases.
Studies were reported by Wyers (1946, 1948) on 50 - 90
workers exposed to vanadium pentoxide as an oil combustion
residue and to slag from the production of ferrovanadium.
Findings included bronchospasm, often with elevated blood
pressure and accentuated pulmonary second sound, a paroxsymal
cough, dyspnoea, skin pallor, tremor of fingers, palpitation,
chest pains, and reticulation of the lungs. Wyers emphasized
the irritant effects of vanadium pentoxide on the respiratory
tract, but also found evidence of systemic toxicity.
An extensive report including data on the dust contents of
the air in a metallurgical plant producing vanadium pentoxide
was published by Sjöberg (1950). The dust particles were
relatively large in size (39% less than 12 µm, 22% less than
8 µm). It was estimated that a concentration of 6.5 µg
V205/m3 represented the worst exposure conditions. Thirty-six
men between 20 and 50 years of age had been employed in the
plant since 1946: 22 had a dry cough; wheezing sounds could be
detected in 31; and 27 were short of breath. One man developed
acute pneumonitis, and 4 others developed bronchopneumonia.
There was no evidence of systemic toxicity.
A dry eczematous dermatitis developed in 9 men in Sjöberg's
(1950) study, but only 1 man showed a positive patch test.
Sjöberg (1951) and Sjöberg & Rigner (1956) believed that allergy
might play a role in the development of eczema and pneumonitis
following vanadium exposure. Zenz et al. (1962) also considered
this an explanation for the more severe symptoms found on re-
exposure in their study. In a follow-up to the 1950 study,
Sjöberg & Rigner (1956) reported that the 16 men most severely
affected still complained of shortness of breath, cough,
fatigue, and wheezing. Bronchitis was present in 2 men.
However, spirometric measurements, cardiac function tests,
electrocardiograms, haematological tests, and urinanalyses were
essentially normal.
Lewis (1959b) studied 24 male workers in an environment in
which the maximum exposure was only 0.925 mg vanadium (as
V205)/m3 of air. In most cases, the exposure was to 0.3 mg
vanadium/m3. More than 92% of the dust particles were smaller
than 0.5 µg in every process area sampled. Symptoms of cough
with sputum production, eye, nose, and throat irritation, and
wheezing were related to physical findings of wheezes, rales, or
rhonchi, injected pharynx, and green tongue. All of these
symptoms and physical findings were statistically significant in
comparison to those in 45 referents (Tables 25 and 26).
A report by Rajner (1960) on 30 vanadium workers in a
metallurgical plant described particularly severe signs and
symptoms, but did not give any estimates of exposure except in
conjunction with urinary-vanadium levels. In acutely poisoned
workers, vanadium values were about 4000 µg/litre urine. The
average value among permanent employees was 45 µg/litre;
vanadium pentoxide smelter workers had maximum values of about
400 µg/litre. When a new production process was introduced,
symptoms of acute vanadium poisoning occurred in 3 workers after
16 h of work including severe respiratory difficulties, head-
ache, dejection, and loss of appetite. Acute inflammatory
changes of the upper respiratory tract with copious mucous
production, oedema of the vocal cords, and profuse epistaxes
were reported. All workers who had been exposed for a long time
(up to 22 years in 27 subjects, mostly in ferrovanadium and
vanadium pentoxide smelting operations) complained of coughing
and eye, nose, and throat irritation, breathing difficulties
during physical exertion ("more than two-thirds of the
workers"), and headache (12 cases). Clinical findings included
intense hyperaemia of the mucosa of the nasal septum in 20
workers; perforation of the nasal septum was seen in 4 workers
exposed for an average of 18 years. Intense hyperaemia of the
mucosa of the throat and larynx with dilated fine capillaries
was found in 50% of the workers. Bronchoscopy indicated the
presence of chronic bronchitis, and bronchial smears revealed
sloughed epithelium.
Matantseva (1960) studied 77 workers in contact with
vanadium pentoxide in the form of dust and fume in concentra-
tions exceeding the MAC value (dust = 0.5 mg/m3; fumes =
0.1 mg/m3) for periods ranging from 1 to 12 years. Nearly all
the subjects had various complaints relating to the upper
respiratory tract including unpleasant sensations in the nose, a
liquid mucous discharge from the nose, obstructed nasal
breathing, a sensation of burning and dryness in the naso-
pharynx, scratching, dryness, and tickling in the throat,
hoarseness of the voice, and cough. Physical examination showed
rhinitis, which was of a simple catarrhal form in workers
exposed for less than 3 years, a hypertrophic and subatrophic
form if the exposure was for more than 3 years, and an atrophic
form if the exposure was for between 7 and 12 years. Examina-
tion of the lungs revealed acute and chronic lesions in the form
of bronchitis, peribronchitis, and pneumosclerosis. Hyper-
ventilation and an elevated basal metabolic rate were noted.
Table 25. Symptoms in 24 vanadium workers and 45 unexposed referentsa
-----------------------------------------------------------------------------
Symptom Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Cough 33.3 83.4 13.71b
Sputum 13.3 41.5 5.55c
Exertional dyspnoea 24.4 12.5 0.592
Eyes, nose, throat 6.6 62.5 23.17b
irritation
Headache 20 12.5 0.124
Palpitations 11.1 20.8 0.538
Epistaxis 0 4.2 0.148
Wheezing 0 16.6 5.20c
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
c Significant at P = 0.02.
Table 26. Physical findings in 24 vanadium workers and 45 unexposed
referentsa
-----------------------------------------------------------------------------
Physical finding Incidence (%) X2 value
Referents Exposed
-----------------------------------------------------------------------------
Tremors of hands 4.5 4.2 0.0320
Hypertension 13.3 16.6 0.0002
Wheezes, rales, 0 20.8 6.93b
or rhonchi
Hepatomegaly 8.9 12.5 0.003
Eye irritation 2.2 16.6 2.94
Injected pharynx 4.4 41.5 12.62b
Green tongue 0 37.5 14.53b
-----------------------------------------------------------------------------
a From: Lewis (1959b).
b Significant beyond P = 0.01.
Roshchin (1963b) published an account of the effects of
vanadium-containing Bessemer slag dust on 45 workers. Dust
concentrations in the air during various phases of this opera-
tion ranged from 5 to 150 mg/m3, with the highest concentrations
occurring during loading/unloading of broken slag as the
trivalent oxide, mostly within spinellide. Repeated examinations
of the 45 workers showed the slag dust to have an effect on the
respiratory mucosa. Subatrophic rhinitis, bronchitis, and
pneumosclerosis were seen in subjects with long occupational
exposure (11 workers). Chronic bronchitis was found in every
worker employed for 5 years or more. Clinical and X-ray
examination of all 45 subjects showed radiological changes in 24
employed for 10 years or more; in 11 subjects, pneumoconiosis of
stage I-II was diagnosed. X-ray examination showed diffuse
sclerotic changes over the whole extent of the lung fields
(except for the supraclavicular zones), small focal opacities,
intensified and enlarged shadows at the root of the lungs, and
marked signs of bullous emphysema. Predominant involvement of
the lower regions of the lungs (characteristic of silicosis) was
not present. This pneumosclerosis was accompanied by changes in
the cardiovascular and nervous systems, biochemical disturbances
(hyper-vitaminosis with dysproteinaemia and an increase in the
serum concentration of sulfhydryl groups), a tendency to anaemia
and leukopaenia, and changes in the liver.
In another study, Roshchin (1964) described chronic effects
of vanadium in 193 workers who had been exposed to aerosols of
free vanadium pentoxide: 127 worked in vanadium metallurgy and
66 were boiler cleaners (section 8.3.2). The length of
occupational contact with vanadium was over 10 years for 60%,
from 5 to 10 years for 30%, and under 5 years for the remaining
10%. Practically all complained of irritation of the nasal and
pharyngeal mucosa including itching, a profusely running nose
(especially during work), and unpleasant sensations in the
throat and nose. Epistaxis was frequent in 20%. Physical
examination revealed a high incidence of changes in the nasal
mucosa: dryness (40%), erosion (23%), scars (8%), perforation
(4%), hyperaemia (10%), and hypertrophy (7%). Also noted were
dryness of the pharynx (5%), hyperaemia of the pharynx (5%),
hyperaemia of the larynx (4%), and tonsillitis (5%). The most
common pathological changes in the upper respiratory tract were
subatrophic rhinitis (40%) and destructive changes in nasal
mucosa (35%), while hypertrophic rhinitis was less frequently
seen (7%). The overwhelming majority had a dry cough; cough
with viscid sputum was less common. Workers with longer
occupational exposure complained of shortness of breath, which
appeared sometimes after 5 but mostly after 10 years of work in
the industry. Nearly all complained of aching or shooting pains
in the chest and of lassitude and weakness. The main respiratory
diseases diagnosed were chronic bronchitis (40%) and diffuse
pneumosclerosis (13%). Haematological tests showed the total
serum-protein concentration to be normal, y-globulins to be
raised (19.4% compared with 12.2% in controls), and the albumin-
globulin ratio to be 1:1 - 1:2 (1:9 in controls). Determination
of total, residual, and protein sulfhydryl groups in the blood-
serum revealed a marked decrease of 7 - 13% compared with the
controls. Regular observations over a period of 14 years showed
that the chronic bronchitis tended to get worse, with develop-
ment of bronchospasm. After a long period of time, some subjects
developed pneumosclerosis; in others, the disease progressed
slowly from chronic bronchitis to diffuse pneumosclerosis and
pulmonary emphysema.
Eisler et al. (1968) studied 48 metallurgical workers
occupationally exposed to vanadium for between 17.6 ± 9 years.
Definite clinical evidence of chronic bronchitis was present in
90% of the subjects, and 50% had severe obstructive bronchitis.
In control groups, which included basic-slag crushers and
furnace operators (99 and 50, respectively), chronic bronchitis
was observed in 33% and 26%, respectively.
A study on 13 workers engaged in the extraction and refining
of vanadium pentoxide from soot generated by the combustion of
heavy fuel oil was reported by Nishiyama et al. (1977).
Concentrations of vanadium in the air at various locations in
the work environment were all less than 0.5 mg/m3 (mean, 1.2 -
12 µg/m3). There was a significant incidence of injection of
the pharynx (58.3%) compared with controls. Elevated levels of
vanadium in the urine and hair were detected both in currently-
exposed as well as in previously-exposed subjects. Apart from a
slight depression in serum-cholesterol levels, haematological
results were normal.
Roshchin (1968) analysed the incidence of influenza and
upper respiratory catarrh as a cause of lost working time in
workers in vanadium metallurgical plants compared with ferrous
metallurgical workers in adjacent plants. The results are given
in Table 27. The morbidity was consistently higher among
workers producing vanadium than among workers in other
departments in all years.
Table 27. Morbidity from influenza and upper respiratory catarrha
-----------------------------------------------------------------------------
Department Cases Days off work
(per 100 workers) (per 100 workers)
1958 1959 1960 1962 1958 1959 1960 1962
-----------------------------------------------------------------------------
Vanadium plant 40.6 68.4 58.8 59.8 180.6 376.7 271.9 336.5
Open hearth furnace 22.2 53.2 45.6 62.1 76.9 331.1 176 213.3
Blast furnace 21.9 46.7 37.8 47.6 36.3 262.8 166.6 215.5
Engineering shop 19.6 39.1 33.9 44.9 86.8 224.8 154.3 223.6
-----------------------------------------------------------------------------
a From: Roshchin (1968).
Asthma was reported in 4 workers exposed to vanadium
pentoxide dust in a newly established vanadium pentoxide
refinery (Musk & Tees, 1982). One of the workers had positive
skin tests to environmental allergens; the others were non-
atopic. Three were smokers; one was an ex-smoker. One of the
subjects experienced irritation of the upper respiratory tract
after a single exposure; dyspnoea and wheezing developed 2 weeks
later. All workers had similar irritant symptoms and green
tongue. Two showed bronchial hyperreactivity when challenged
with histamine; these were the workers with the most recent
exposure. In one worker, the asthmatic symptoms continued for 8
weeks after cessation of exposure. There was no indication of
an immunological aetiology, and the authors concluded that the
effect was likely to be a direct chemical one.
Kiviluoto et al. (1979a,b, 1980, 1981a,b) and Kiviluoto
(1980) reported the results of a cross-sectional study on 63
males exposed to vanadium-containing dust in a vanadium factory;
a reference group matched for age and smoking was selected from
a magnetite ore mine. The workers had been exposed to vanadium
dust for an average of 11 years at concentrations ranging from
0.1 to 3.9 mg/m3 (estimated average exposure levels of 0.2 -
0.5 mg/m3); the respirable fraction (< 5 µm) was 20%. Nasal
biopsies and lung function tests were taken at the end of the
summer holidays (duration, 2 - 4 weeks). Nasal smears and
biopsies were repeated in 31 workers, 7 - 11 months later, after
hygienic improvements had reduced the exposure levels to 0.01 -
0.04 mg/m3. Microscopic examination of nasal smears revealed a
significant increase in neutrophils and biopsies of nasal mucosa
showed significantly elevated numbers of plasma and round cells
in the exposed workers. There was no further increase in the
cell findings after 10 months of exposure to 0.01 - 0.04 mg/m3
vanadium dust; eosinophils did not show any differences between
the exposed and the referents. The authors attributed these
findings to "an irritating effect of vanadium dust on the mucous
membranes of the upper respiratory tract". Biopsies from
workers with the longest exposures (170 - 241 months) showed "a
zone-like sub-epithelial infiltration of mononuclear cells and
frequent papillarity in the mucous membrane surface with its
hyperaemic capillaries". The similarity between this pattern
and that seen in vanadium-exposed rabbits (Sjöberg, 1950) was
noted (Kiviluoto et al., 1979b). A random sample of 12 nasal
biopsies was further investigated for the amount and classes of
immunoglobulins (IgE, IgG, IgM, and IgD). IgG subclasses were
not studied. There were no differences between the 12 workers
and their referents, which was construed as a further indication
of non-specific inflammation (Kiviluoto et al., 1981b).
Pulmonary condition was assessed by means of questionnaires,
X-ray, and pulmonary function testing. There was only one
significant difference between the workers exposed for an
average of 11 years to 0.1 - 3.9 mg/m3 (estimated average, 0.2 -
0.5 mg/m3) and at the time of investigation to 0.01 -
0.04 mg/m3, and their matched referents; complaints of wheeze
were more common in the exposed worker group (Kiviluoto, 1980).
The importance of this finding remained doubtful. It may
reflect the respiratory findings mentioned above, since upper
respiratory irritation may be accompanied by transient reflex
bronchospasm. A series of laboratory tests were designed to
evaluate electrolyte, protein fractions, carbohydrate, and
lipids, liver, renal, muscle, pancreatic, and bone marrow
functions, and immunological status. There were no decreases in
serum-cholesterol or triglycerides, and no clinical differences
between worker and control groups (Kiviluoto et al., 1981a).
The effects of vanadium compounds on health is not confined
to the development of local respiratory or other lesions.
Various studies, most of them rather old, on patterns of lost
working time due to morbidity have shown that the incidence of
disease among workers in plants producing vanadium compounds is
considerably higher than among other workers (Symanski, 1939;
Syers, 1946; Sjöberg, 1950, 1956; Reznik, 1954; Reinl, 1958;
Matantseva, 1961; Watanabe et al., 1966; Roshchin, 1968, 1969;
Athanassiadis, 1969; Schumann-Vogt, 1969; Chiriatti, 1971). The
most significant differences are found in the incidences of
influenza, upper respiratory catarrh, and inflammation of the
lungs. The difference in the incidence of bronchitis is
particularly marked.
8.4.2 Cleaning and related operations on oil-fired boilers
Bronchitis and conjunctivitis resulting from exposure to
soot (containing 6 - 11% vanadium) during the cleaning of the
stacks of oil-fired boilers were first recognized by Frost
(1951). Frost did not report any other effects, but, in a
subsequent report of a boiler-cleaning operation by Williams
(1952), sneezing, nasal discharge, lachrymation, sore throat,
and substernal pain occurred within 0.5 - 12 h of exposure.
Within 6 - 24 h, secondary symptoms developed; these consisted
of dry cough, wheezing, laboured breathing, lassitude, and
depression. In some cases, the cough became paroxysmal and
productive. Symptoms lessened only after removal from the
working environment for 3 days. Air sampling showed most of the
dust particles to be smaller than 1 µg. The vanadium concen-
tration ranged from 17.2 mg/m3 in a superheater chamber to
58.6 mg/m3 in a combustion chamber. Roshchin (1962) observed 8
cases of acute vanadium poisoning in workers who cleaned boiler
flues at power stations burning high-sulfur oil. Analysis of
soot deposits showed that the soot in the region of greatest
dust formation (the pipes of the steam superheater and water
economizer) contained from 24 to 40% vanadium pentoxide. The
workers carried out cleaning operations without respirators or
with respirators that did not provide the necessary protection.
After cleaning the boilers, the workers developed acute vanadium
poisoning: itching in the throat, sneezing, cough with difficult
expectoration, and smarting eyes. On the following days, the
symptoms became more severe. Tightness in the chest, sweating,
general weakness, conjunctivitis, and noticeable loss of weight
developed. On examination one week later, hyperaemia and oedema
of the fauces and posterior pharyngeal wall were observed.
Harsh breathing sounds and dry crepitations were heard in the
lungs. X-ray examination showed intensified lung markings in
the middle zones of the right and left lungs and thickening of
the fissure on the right. One month later, only one worker
still had cough, weakness, perspiration, loss of energy, and
dyspnoea. The other workers recovered quickly, with complete
disappearance of cough and shortness of breath.
In another study on workers engaged in boiler-cleaning
operations (Troppens, 1969), the symptoms were described as
similar to mild coryza or influenza with bronchitis. Following
recovery, workers were tired, debilitated, irritable, without
any appetite, and complained of watery eyes. The first symptoms
were swelling of face and eyes as early as 20 min after entering
the boiler area. Removal from exposure for 2 - 3 weeks resulted
in the disappearance of symptoms. Skin blemishes described as
allergic dermatoses were attributed to absorption of vanadium
through sensitive skin. Troppens claimed that there was an
increased susceptibility of the vanadium worker to asthmatic
bronchitis and emphysema.
An investigation is reported on 53 workers performing
emergency repair work on oil-fired power station boilers (Izycki
et al., 1971). They were exposed to vanadium pentoxide in
average concentrations of from 1.2 to 11 mg/m3 and also to
manganese, calcium, and nickel oxides, and sulfur compounds.
Characteristic features of both acute and chronic vanadium
poisoning included upper respiratory catarrh in 45%, increased
lung markings in 24.5%, and bradycardia in 22% of cases.
Persistent chronic changes in the respiratory tract (rhinitis,
pharyngeal catarrh, laryngitis, and changes in the paranasal
sinuses) were present in 45%.
Milby (1974) reported 21 cases of vanadium poisoning in
boilermakers installing new catalytic-converter tubes. This
work involved marble-sized pellets of vanadium containing 11.7%
V2O5. The dust formed during the shaking of these pellets had a
particle size of 1.1 - 1.5 µm. After working for 72 h, the
workers began to complain of nasal, eye, and bronchial
irritation. By the 4th day, most felt very ill, with signs of
irritation of the upper respiratory tract and eyes and pains in
the chest.
In a study by Garlej (1974) 50 workers engaged in the
cleaning of oil-fired boilers were compared with a control group
of 60 other workers. Boiler deposits contained 44 -65% V2O5;
the maximum exposure was estimated to be 10 mg/m3. Although no
clinical evidence of vanadium poisoning was seen, a number of
exposure-dependent positive biochemical reactions were found in
the boiler-cleaning group. Urinary excretion of delta-amino-
levulinic acid (ALA), porphobilinogen (PBG), and porphyrin
increased beyond the physiological limit, and the positive Nadi
reaction (with associated green fluorescence) occurred. The
increased excretion of cytochrome (as indicated by the Nadi
reaction) suggested oxidation through V2O5 of the thiol group
-SH cysteine in the protein carrier, resulting in decreased
binding of cytochrome in the mitochondria.
A study on 17 men who were engaged in cleaning boilers at an
electric generating station was reported by Lees (1980). In
addition to clinical findings, which were similar to those
described above, urine-vanadium levels were determined, and
pulmonary function measurements were made for a week following
exposure. Sixteen of the men wore protective clothing, and
respirators that were found to have about 9% leakage. One
workman volunteered to wear only a simple oro-nasal dust mask
for 1 h of exposure. The dust exposure level was estimated to
be 26 mg/m3; respirable dust (under 10 µm) was measured at
523 µg/m3 with a vanadium content of 15.3%. All of the men
developed reduced pulmonary function that had not fully returned
to normal in one week, but did so after one month. Reduced
function outlasted the clinical symptoms by several days. Fig. 3
shows the contrast in pulmonary reaction between the more
heavily exposed individual and one of the other workmen. The
urine-vanadium level of the volunteer was 280 µg/litre, whereas
those of the remainder of the workers were below 40 µg/litre.
 Other observations of boiler-cleaning operations have been
made by Fallentin & Frost (1954), Sjöberg (1955), Thomas &
Stiebris (1956), Hickling (1958), and Kuzelova et al. (1975).
In terms of respiratory symptoms relating to boiler-cleaning, it
should be noted that sulfates and sulfuric acid may be present
in boiler soot and may be partly responsible for irritative
effects. Hudson (1964) suggested that the quick onset of
symptoms (lachrymation with nose and throat irritation) with
rapid recovery following removal from exposure is character-
istic of exposure to acid sulfates. Response to vanadium expo-
sure is characterized by some delay in the onset of irritative
symptoms (a few hours to several days) and persistence of
symptoms following removal from exposure (Hudson, 1964).
A recent report by Levy et al. (1984) concerned a
comparatively high incidence of severe respiratory tract
irritation in boilermakers (74/100), many of them welders in
areas without adequate ventilation, exposed to vanadium
pentoxide fumes in a power plant where conversion from oil- to
coal-burning occurred. The severe illness of 70 men caused an
average of 5 days of absence, some objective tests (e.g., FVC)
being markedly affected. The vanadium pentoxide content was
above the permissible exposure limit in 8 samples, and this
resulted in litigation for inadequate protection of the
workers.
Kuzelova et al. (1977) drew attention to the occupational
risk of chimney sweeps cleaning large-capacity heating
facilities in large housing settlements. This coincided with a
report of a detailed cross-sectional examination of 121 chimney
sweeps by Holzhauer & Schaller (1977) in the Federal Republic of
Germany with an average exposure duration of 19 years ( ± 5
years). Vanadium exposure was determined by personal samples,
and measurements between 0.73 and 13.7 mg vanadium pentoxide/day
were determined compared with 4 µg in the normal (average)
population. Urinary excretion was determined to be between 0.15
and 13 µg/litre, which was significantly higher than the values
in 31 referents. The main complaints of the chimney sweeps were
wheezing, rhinitis, conjunctival irritation, cough, sputum
dyspnoea, and hoarseness; there were no skin symptoms. A
prospective follow-up of the cohort was emphasized, but the
results are not yet available.
8.4.3 Handling of pure vanadium pentoxide or vanadate dusts
Health effects due to occupational handling of pure vanadium
pentoxide or vanadate dusts have been reported. Tara et al.
(1953) described the effects of vanadium exposure in 4 dock
workers who unloaded and bagged spilled calcium vanadate. The
symptoms (bronchitic wheezing sounds, dyspnoea, productive
cough, haemoptysis in one case, and headache) necessitated
interruption of the work after 1´ days. Zenz et al. (1962)
described an acute illness that occurred in 18 workers pellet-
izing pure vanadium pentoxide; it was characterized by a rapidly
developing mild conjunctivitis, severe pharyngeal irritation, a
non-productive persistent cough, diffuse rales, and broncho-
spasm. With severe exposure, 4 men complained of itching skin
and a sensation of heat in the face and forearms. The symptoms
became more severe after each exposure, suggesting a sensitivity
reaction, but their duration was not prolonged by repeated
exposures.
8.4.4 Other industries
Browne (1955) studied vanadium poisoning in 12 patients
exposed to exhaust fumes from gas turbines using heavy fuel oil.
Evidence of poisoning appeared between the first and 14th day of
exposure and consisted of conjunctivitis, rhinitis, cough,
crepitations, and dyspnoea. Bleeding appeared before the
rhinorrhoea.
Other occupations in which respiratory effects of vanadium
exposure have been reported include operations connected with
the gasification of fuel oil (Fear & Tyrer, 1958) and the
manufacture of phosphor for television picture tubes (Tebrock &
Machle, 1968). In the latter study, elevated blood pressure was
noted in men exposed to vanadium pentoxide.
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1 Environmental Levels and Exposures
While vanadium concentrations in the air of remote rural
areas are less than 1 ng/m3, other rural areas show concentra-
tions in excess of 50 ng/m3. This is generally considered to
reflect the local burning of fuel oil with a high vanadium
content. Typical concentrations in urban air may range from
below 1 ng/m3 to over 300 ng/m3, with annual averages for large
cities of about 20 - 100 ng/m3. At an annual average of
50 ng/m3 and a respiration rate of 20 m3, the total amount of
vanadium reaching the respiratory tract would be only 1 µg.
Assuming a rate of absorption of about 25%, the direct daily
contribution of vanadium from air would be about 250 ng.
Drinking-water supplies without excessive vanadium pollution
contain from less than 1 µg/litre to occasional maximum
concentrations of 15 - 30 µg/litre. Two comprehensive surveys
have shown average concentrations of 4.3 and 4.85 µg/litre,
respectively. At a daily intake of 2 litres of water, the
average daily intake of vanadium with water would be about
10 µg, ranging from about 1 µg to 30 - 60 µg. Although
levels in ordinary water supplies would vary considerably,
intake should rarely exceed 100 µg/day. Intake with bottled
waters from mineral springs may exceed these values.
As a rule, the concentration of vanadium in food is low.
High levels reported in early studies have been attributed to
analytical differences. Recent studies on complete diets
suggest a daily intake of vanadium of about 10 - 70 µg, with
the majority of estimates remaining below 30 µg. Assuming an
absorption rate from the gastrointestinal tract of 1 - 2%, the
contribution from food and water is unlikely to exceed 4 -
5 µg/day.
Vanadium concentrations in air in the vicinity of metal-
lurgical industries are often about 1 µg/m3. In the production
of vanadium metal or compounds, concentrations may reach a few
mg/m3. In boiler-cleaning operations, dust concentrations in
air are frequently around 50 - 100 mg/m3, and concentrations as
high as 500 mg/m3 have been reported; the vanadium content of
the dust is about 5 - 17% as vanadium pentoxide and 3 - 10 % as
lower vanadium oxides. The need for personal protection devices
in such operations is obvious.
9.2 Physiological Role
While present knowledge indicates that vanadium is an
essential element for chicks and rats, conclusive evidence that
vanadium is essential for other species, including man, is
lacking. A variety of physiological and biochemical processes
have been found to be vanadium sensitive. However, so far,
there is no evidence of adverse effects arising from vanadium
deficiency in man, and the daily requirement of vanadium in the
diet is not known.
9.3 Effects and Dose-Response Relationships
The toxicity of vanadium varies in experimental animals with
both the species and route of administration. Small animals,
such as the rat and mouse, tolerate the metal better than the
rabbit and horse. The toxicity of vanadium is low when given
orally, moderate when inhaled, and high when injected. As a
rule, the toxicity of vanadium increases as the valency
increases, pentavalent vanadium being the most toxic.
9.3.1 Local effects and dose-response relationships
Exposure of 2 volunteers to vanadium pentoxide dust at
1 mg/m3 for 8 h resulted in irritation of the respiratory tract
with cough starting 5 h later. The cough lasted for 8 days.
Exposure of 5 volunteers to a concentration of 0.2 mg/m3 caused
the same symptoms, i.e., coughing, but with an onset at 20 h.
The cough lasted for 7 - 10 days. Respiratory irritation was
noted in 2 subjects exposed to 0.1 mg/m3 for 8 h. The irritant
effect was manifested as an increase in mucous production, 24 h
after exposure, and total recovery within 4 days. Tickling and
itching, together with dryness of the mucous membranes of the
mouth, was reported by 11 volunteers exposed to 0.4 mg/m3 of
vanadium pentoxide condensation aerosol; 0.16 mg/m3 caused
irritation in only 5 subjects, and 0.08 mg/m3 did not induce
symptoms in any of the subjects.
Exposure to high concentrations of vanadium is possible in
the industrial production and use of vanadium, especially in the
cleaning of oil-fired boilers. Frequently reported irritant
symptoms include sneezing, nasal discharge, irritation of the
eyes with lachrymation, sore throat, dry or productive cough,
and chest pain. Normally, such symptoms disappear in a few days
when exposure has ceased. Cough, increased sputum, and
particularly irritation of the eyes, nose, and throat occurred
among 24 male workers exposed to a maximum of 0.9 - 5 mg
vanadium/m3 (measured as vanadium pentoxide V2O5). Most workers
had been exposed to 0.3 mg/m3 (section 8.4.1). A cross-
sectional study of 63 male workers exposed for an average of 11
years to vanadium-containing dust at 0.2 - 0.5 mg vanadium/m3
(range, 0.1 - 3.9 mg/m3) showed chronic irritant effects in the
mucous membranes of the nose. The nasal changes persisted
unchanged during subsequent exposure to much lower levels
(0.01 - 0.04 mg/m3).
Heavily exposed workers (dust concentrations of 5 -
150 mg/m3) developed atrophic rhinitis and chronic bronchitis.
Bronchospasm is also a feature in heavily exposed workers.
The effects on 63 workers of long-term exposure to vanadium
at 0.2 - 0.5 mg/m3 were studied using matched referents, a
questionnaire on respiratory symptoms, chest radiography, and
lung function testing (section 8.4). There was no change in
ventilatory function compared with the matched reference group;
only complaints of wheezing were significantly more common among
exposed workers than among referents. However, in another study
the forced vital capacity (FVC) was reversibly reduced in 17
boiler cleaners who had been exposed to a time-weighted average
respirable dust of 523 µg/3 containing 15% of vanadium (section
8.3.1).
Vanadium poses weak sensitizing properties when skin and
mucous membranes of the upper respiratory tract are exposed to
high concentrations, manifested by the development of allergic
dermatitis and rhinitis in workers in contact with vanadium.
The allergic nature of these manifestations is proved by
positive reactions of epicutaneous tests with a 2% solution of
sodium vanadate. There is information on the sensitizing effect
of vanadium in tests on animals.
In one study (section 8.4.1), 9 workers out of 36 developed
a dry eczematous dermatitis. These workers had been exposed to
vanadium pentoxide at about 6.5 µg/m3.
Green tongue is seen in a proportion of workers exposed to
vanadium-containing dust, and is an indication of exposure.
9.3.2 Systemic effects and dose-response relationships
9.3.2.1 Metabolic effects
The effect of vanadium on dental caries remains a debatable
issue (section 5.4.2). The application of a 50% paste of an
ammonium salt of vanadium and glycerol was reported to reduce
caries in children aged 7 - 11 years. Other studies between
1955 and 1968 have failed to demonstrate a clearly beneficial
effect (section 8.1.2.1).
Soluble diammonium oxytartarovanadate (150 - 200 mg/day for
6 weeks) was administered to 5 healthy adult male volunteers.
There was a significant reduction of plasma-cholesterol levels
at the end of the period. A temporary drop in the cholesterol
level was also observed in 2 out of 6 patients given ammonium
vanadyl tartrate for 7 weeks at 50 or 100 mg/day. The results
were not convincing and the temporary drops in cholesterol
levels were not statistically significant. No significant
changes in serum-cholesterol levels were noted in 12 patients (9
of whom were hypercholesterolaemic) given diammonium vanado-
tartrate orally for 6 months, (25 mg three times daily for 2
weeks, increased to 125 mg daily in 10 patients). Although some
studies on rats and rabbits have indicated decreasing
cholesterol levels following administration of vanadium and have
corroborated the reduced levels of cholesterol observed by other
authors, this effect of vanadium has not been convincingly
demonstrated in human beings so far (section 8.1.2.1).
Vanadium pentoxide in the diet (25 - 1000 mg vanadium/kg)
resulted in lower levels of cystine in the hair of rats compared
with those in controls, indicating the inhibition of cystine
synthesis (section 7.2.1). Rats administered sodium vanadate
intraperitoneally (5 - 10 mg/kg body weight as a single
injection or a dietary concentration of 500 mg/kg) showed
reduction of co-enzyme A in the liver; this has been construed
as an explanation of the reduction of cystine (section 7.2.1).
When workers were exposed to vanadium-containing dust (0.2 -
0.5 mg vanadium/m3, at the time of the study), there was no
correlation between exposure level and cystine levels in
fingernails, and no decrease in levels of serum-cholesterol or
triglycerides (section 8.4.1).
The data on the effects of vanadium on haematopoiesis are
inconsistent. A favourable effect of vanadium chloride (0.6 mg
vanadium/kg diet) on haemoglobin levels in rats, previously made
anaemic, had already been suggested in 1931 (section 7.2). A
small increase in erythrocytes and haemoglobin levels was
observed in rabbits given vanadyl sulfate subcutaneously, at
1 mg/kg body weight daily, for 2 months). When 32 vanadium
workers who had been exposed for more than 6 months were
compared with 45 referents, matched for age, no differences were
seen in haematocrit levels (8.2.1.1). It is not possible to
assess the effects of low-level vanadium exposure on iron
metabolism.
9.3.2.2 Effects on the nervous system
Systemic effects are rare in workers exposed to vanadium
compounds. Non-specific signs and symptoms including headache,
weakness, nausea, vomiting, and tinnitus have been reported.
Such signs and symptoms have mostly occurred in workers exposed
to extremely high dust concentrations, when cleaning oil-fired
boilers, but it has not been possible to derive dose-response
relationships for them.
Elevated vanadium levels in whole blood and serum have been
reported in patients suffering from depressive illness. In one
report, the vanadium levels fell to normal with recovery of the
patients. The role of vanadium in depressive states is not
known (section 8.2.2).
In mice and rats, repeated oral administration of vanadium
pentoxide or ammonium vanadate at doses of 0.05 - 0.5 mg
vanadium/kg body weight, daily, for 6 months and 21 days,
respectively, resulted in impaired conditioned reflexes. Daily
oral doses of sodium metavanadate (3.2 µg/kg body weight per
day for 10 - 15 days) caused increases in the activity of
cytochrome oxidase in the brain of guinea-pigs; a dose of
128 µg/kg per day did not have any effect, whereas 5.12 mg/kg
per day reduced the activity (section 7.2). Total
cholinesterase activity in the brain of rats was significantly
reduced by the intraperitoneal administration of 1 mg vanadyl
sulfate/kg body weight (section 7.3).
9.3.2.3 Effects on the liver
There are insufficient human data to make an assessment of
the effects of vanadium on the liver. Rats and rabbits exposed
through inhalation to vanadium pentoxide, trioxide, or tri-
chloride (10 - 70 mg/kg, 2 h/day, for 9 - 12 months) showed
fatty changes with partial cell necrosis in the liver. A clear
reduction in the liver tissue respiration and a decrease in the
albumin/globulin ratio in the serum were also observed. Subcu-
taneous injection of ammonium vanadate (1 mg vanadium/kg body
weight per day, for 30 days) caused similar fatty changes in the
liver of rats. Intraperitoneal injections of sodium meta-
vanadate (1.25 - 2.5 mg vanadium/kg body weight) in rats caused
loss of weight. The toxic effects observed were correlated with
the concentration of vanadium in the liver (section 7.2).
9.3.2.4 Effects on the kidney
Data on the effects of vanadium on the human kidney are
lacking. Intravenous injection of sodium metavanadate (2.5 -
5 mg/kg body weight) in male dogs resulted in albuminuria. In
mice, acute tubular necrosis followed subcutaneous injection of
ammonium vanadate at a dose equivalent to 20 mg vanadium/kg body
weight. Rats and rabbits inhaling vanadium chloride (70 mg/m3
2 h/day, for 9 - 12 months) caused fatty changes in the kidney.
Vanadate has diuretic and natriuretic effects on the kidney in
the rat but not in the dog or cat. Vanadate has also been
reported to increase the urinary excretion of calcium,
phosphate, bicarbonate, and chloride by the rat kidney. These
diuretic and natriuretic effects are thought to be due to the
inhibition of Na+-K+-ATPase causing inhibition of the tubular
reabsorption.
9.3.2.5 Cardiovascular effects
Palpitation of the heart at rest and on exercise has been
reported in workers occupationally exposed to vanadium.
Transient coronary insufficiency, a high incidence of extra-
systoles and bradycardia were reported (section 8.3). Exposure
of workers to low levels of vanadium pentoxide (0.2 - 0.5 mg/m3)
did not cause any pathological changes in the blood picture
(section 8.4.1).
Electrocardiographic changes (ST-segment depression,
increased T-wave amplitudes) were seen after intravenous
injection of sodium metavanadate in dogs (2.5 - 5 mg/kg body
weight). Long-term inhalation exposure of rats and rabbits to
vanadium pentoxide, trioxide, or chloride (10 - 70 mg/m3,
2 h/day, for 9 - 12 months) caused fatty changes in the myo-
cardium as well as perivascular swelling.
9.3.2.6 Pulmonary effects
Asthmatic reactions in conjunction with non-specific
bronchial hyperreactivity have occasionally been reported in
refinery workers exposed to vanadium pentoxide dust. There has
not been any evidence of an immunological mechanism behind such
cases. A dose-dependent decline in forced expiratory volume in
one second (FEV1) and forced vital capacity (FVC) has been
demonstrated in boiler cleaners. The functional increase did
not return to normal during the first week following exposure,
but fully recovered within one month. The mechanism leading to
the obstructive pulmonary impairment has not been clarified.
9.3.2.7 Effects on the immune system
In mice, vanadium and ammonium vanadate affect the normal
function of the immune system. Vanadium had slight depressant
effects on antibody-forming cells and increased DNA synthesis in
splenic leukocytes. Ammonium vanadate increased resistance
to E. coli endotoxin, but decreased resistance to Listeria
lethality. In the spleen, it increased the rosetting capability
of leukocytes, the formation of megakaryocytes, and red blood
cell precursors (section 7.7).
9.3.3 Reproduction, embryotoxicity, and teratogenicity
Human data on the effects of vanadium on reproduction and
embryotoxicity are lacking. Vanadium administered to pregnant
rats by subcutaneous administration of metavanadate (0.85 mg/kg,
equal to 1/20 LD50) accumulated in the placenta. However, the
extent to which it reaches the fetus has not been clearly
established. During the lactation period, vanadium was found in
the mammary glands and was excreted with milk. Morphological
changes in spermatozoa as well as desquamation of spermatogenic
epithelium in the seminal tubuli were observed. Gonadotoxic
effects were suggested by the absence of fertilization of female
rats by male rats that had been exposed to 0.85 mg vanadium/kg
body weight. The same doses of vanadium given to female rats on
the fourth day of pregnancy significantly decreased the number
of fetuses (section 7.8.1).
Weanling pigs receiving vanadate (200 mg vanadium/kg body
weight) showed a suppressed growth rate and increased mortality.
Vanadium was not markedly toxic when fed to growing lambs
(200 mg/kg, 84 days) (section 7.8.1).
Tentative results suggest that vanadium is teratogenic for
rats and hamsters causing skeletal anomalies and death of the
fetuses. Dose-response relationships have not been demonstrated
(section 7.8.2). There are no human data concerning possible
teratogenic effects of vanadium.
9.3.4 Mutagenicity
The data on the mutagenic potential of vanadium in bacterial
systems are inconclusive. There are positive and negative
results with E. coli and Salmonella tests (section 7.9). Data
suggest the induction of micronuclei, but not sister chromatid
exchange or dominant-lethal mutations. Chromosome effects in
vivo and in vitro have not been studied.
9.3.5 Carcinogenicity
Life-time studies on mice given 5 µg vanadyl ions/ml as the
sulfate in drinking-water did not increase the incidence of
spontaneous tumours and intraperitoneal injections of
vanadium(III)2.4pentanedione (24, 60, or 120 mg/kg body weight)
did not increase the incidence of lung adenomas in mice. The
results of a long-term study on rats with intrabronchiolar
implants of vanadium solids were negative. The few studies
available do not provide any indications of carcinogenic effects
of vanadium (section 7.10).
9.3.6 Risks from exposure of the general population
There are only a few studies on the possible effects of
vanadium in ambient air on the general population (section
8.3.2). In one study, air concentrations of vanadium together
with 12 other trace elements were found to be correlated with
mortality from pneumonia and lung cancer (coefficients of
correlation of 0.443 and 0.347, respectively) and also with
mortality from bronchitis (coefficient of correlation of 0.563).
In another study, a correlation between the levels of vanadium,
cadmium, zinc, tin, and nickel and the incidence of several
diseases including "diseases of the heart", "nephritis", and
"arteriosclerotic heart" was claimed, but the results of these
studies do not establish any causal relationships.
10. RECOMMENDATIONS
There is a conspicuous lack of data on several aspects of
the health effects of vanadium compounds. The overwhelming bulk
of recent research focuses on the effects of vanadium on
biochemical systems, especially specific effects on enzymes, and
there are major gaps in knowledge with respect to analytical,
metabolic, and exposure data.
There are indications of a weak mutagenic effect of
vanadium, but the data are partly conflicting and
uncorroborated. Further confirmative mutagenicity studies,
including studies on chromosomal effects, should be given high
priority. Data on the carcinogenicity of vanadium in various
species are practically non-existent. Such studies are urgent
and should be conducted as long-term exposure studies.
Vanadium induces toxic effects on the fetus. However,
whether these are direct effects or indirect effects resulting
from effects of vanadium on the mother is not known. Studies to
assess the nature of the teratogenic effects and the mechanism
behind them should be encouraged.
Effects resulting from high occupational exposure to
vanadium dusts have been reasonably well described. Such
exposure levels may cause a variety of clinical manifestations.
However, they should be remedied by hygienic and technical
improvements. Dose-effect and dose-response relationships have
not been well defined at low exposure levels in the range of
approximately 0.01 - 0.5 mg/m3. It is considered important to
develop specific indicators for the detection of early adverse
effects of vanadium on man.
Epidemiological studies on occupational cohorts, paying
particular attention to exposure levels and using proper
referent groups, should be encouraged. The literature is
inconsistent regarding the documentation of sensitizing
properties of vanadium compounds. This is an important aspect
with regard to worker protection.
An area of great importance is the exposure of the general
population. There are considerable geographical variations in
vanadium concentrations in air and water. Epidemiological
studies on populations living in areas with high vanadium
exposure should be carried out, relating possible adverse
effects to exposure levels. Such studies should take into
account possible interactions with other pollutants.
REFERENCES
ACGIH (1986) Threshold limit values for chemical substances
and physical agents in the work environment , Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists.
AGASYAN, P.K., LAKEEVA, T.N., NIKOLAEVA, E.R., PARTASHNIKOVA,
M.Z., SHKOLENOK, G.F., & KHREPINYUK, T.E. (1975) [Determina-
tion of the bulk substances in preparations of potassium
dichromate and ammonium vanadate by means of differential
coulometry.] Zavod. Lab ., 41(4): 385-387 (in Russian).
AIYAR, A.S. & SREENIVASAN, A. (1961) Effect of vanadium
administration on coenzyme Q metabolism in rats. Proc. Soc.
Exp. Biol. Med ., 107: 914-916.
ALESHIN, G.N., SMOLJANINOV, S.I., MESHEYAKOV, R.P., & GLOUCHOV,
G.G. (1974) [The chemistry and technology of vanadium
compounds.] In: Ivakin, A.A. & Voronova, E.M., ed. [ Proceedings
of the International Conference on the Chemistry and Technology
of Vanadium Compounds ,] Perm, Scientific Institute of Black
Metals (in Russian).
ALI, S.A., PEET, M., & WARD, N.I. (1985) Blood levels of
vanadium, caesium, and other elements in depressive patients. J.
affective Disord ., 9(2): 187-191.
ALLAWAY, W.H. (l968) Selenium, molybdenum, and vanadium in
human blood. Arch. environ. Health , 16: 342-348.
ANBAR, M. & INBAR, M. (1962) Effect of pyrodoxial 5-phosphate
in the presence of vanadyl ions of the de-iodination of
thyroxine. Nature (Lond.) , 196: 1213.
ARNON, D.I. (1958) The role of micronutrients in plant
nutrition with special reference to photosynthesis and nitrogen
assimilation. In: Lamb, C.A., Bentley, 0.G., & Beattie, J.M.,
ed. Trace elements , New York, Academic Press, pp. 1-32.
ARNON, D.I. & WESSEL, G. (1953) Vanadium as an essential
element for green plants. Nature (Lond.) , 172: 1039-1040.
ATHANASSIADIS, Y.C. (1969) Preliminary air pollution survey
of vanadium and its compounds: A literature review , Raleigh,
North Carolina, National Air Pollution Control Administration
(Publication No. APTD 69-48).
AZARNOFF, D.L. & CURRAN, G.L. (1957) Site of vanadium
inhibition of cholesterol biosynthesis (letter to the editor).
J. Am. Chem. Soc ., 79: 2968.
AZARNOFF, D.L., BROCK, F.E., & CURRAN, G.L. (1961) A specific
site of vanadium inhibition of cholesterol biosynthesis.
Biochem. Biophys. Acta , 51: 379-398.
BABENKO, G.A. & VANDZHURA, I.N. (1969) [Vanadium metabolism
after supplementary administration of certain microelements in
experimental atherosclerosis.] Bull. exp. Biol. Med ., 67(6):
72-73 (in Russian).
BAGGETT, W.L. & HUYCK, H.P. (1959) Spectrochemical determina-
tion of vanadium in alkali brines. Anal. Chem ., 31(8): 1320-
1322.
BAKAL, G.F. & LISECKAJA, G.S. (1971) [Determination of
vanadium in mercury using the reaction of oxidation of
pyrocatechin by ammonium persulfate.] Ukr. khim Zh ., 37(6):
594-597 (in Russian).
BALESTRA, G. & MOLFINO, F. (1942) [Lung impairments due to
dusts in operations to extract vanadium from petroleum ash.]
Rass. Med. Ind. Ig. Lav ., 13: 5-12 (in Italian).
BALFOUR, W.E., GRANTHAM, J.J., & GLYNN, I.M. (1978) Vanadate -
stimulated natriuresis. Nature (Lond.) , 275: 768.
BARANNIK, P.I., MIKHALYUK, I.A., & MOTUZKOV, I.N. (1969) Data
on the role of chromium, lead, vanadium and the natural radio-
activity of foodstuffs in the etiology of endemic goiter. Hyg.
Sanit ., 35(2): 289-291.
BARBOOTI, M.M. & JASIM, F. (1982) Electrothermal atomic-
absorption determination of vanadium. Talanta , 29: 107-111.
BARNES, R.M., FODOR, P., INAGAKI, K., & FODOR, M. (1983)
Determination of trace elements in urine using inductively
coupled plasma spectroscopy with a poly(dithiocarbamate)
chelating resin. Spectrochim. Acta , 38B: 245-257.
BARRY, E.F., REI, M.T., REYNOLDS, H.H., & O'BRIEN, J. (1975)
Determination of nickel and vanadium in the atmosphere of
eastern Massachusetts. Environ. Lett ., 8(4): 381-385.
BEARD, H.H., BAKER, R.W., & MYERS, V.C. (1931) Studies in the
nutritional anemia of the rat. J. biol. Chem ., 94: 123.
BELEHOVA, V.A. (1966) [Spectral analysis of vanadium content
in intact teeth and in teeth damaged by caries.] Stomatologija ,
4: 85-86 (in Russian).
BELEHOVA, V.A. (1969) [Vanadization of teeth as a means of
preventing dental caries.] Nauchn. Tr. Irkutsk. Med. Inst ., 95:
20-33 (in Russian).
BERGEL, F., BRAY, R.C., & HARRAP, Y.R. (1958) A model system
for cysteine desulphydrase action: pyridoxal phosphate-
vanadium. Nature (Lond.) , 181: 1654-1655.
BERNHEIM, F. & BERNHEIM, M.L.C. (1938) Action of vanadium on
tissue oxidation. Science, 88: 481.
BERNHEIM, F. & BERNHEIM, M.L.C. (1939) The action of vanadium
on the oxidation of phospholipids by certain tissues. J. biol.
Chem ., 127: 353.
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric ., 23: 93-100.
BERTRAND, D. (1942) Le vanadium comme élément oligosynergique
pour l'Aspergillus niger. Ann. Inst. Pasteur , 68: 226-244.
BERTRAND, D. (1950) Survey of contemporary knowledge of
biochemistry. II. The biogeochemistry of vanadium. Bull. Am.
Mus. Natl Hist ., 94(7): 407-455.
BEYHL, F.E. (1983) Action of ammonium metavanadate on hepatic
enzymes in vitro . Arch. Toxicol ., Suppl. 6: 250-253.
BIGGS, W.R. & SWINEHART, J.H. (1976) Vanadium in selected
biological systems. In: Sigel, H., ed. Metal ions in biological
systems , New York, Marcel Dekker, Vol. 6 , pp. 141-196.
BONIG, G. & HEIGENER, H. (l971) [Micro-determination of
vanadium in biological substances by selective paper
chromatography.] Landwirtsch. Forsch ., 25: 139-143 (in German).
BORISENKO, L.F. (1973) [ Vanadium ,] Moscow, Nedra Publishing
House (in Russian).
BOWEN, H.J.M. (1963) The elementary composition of mammalian
blood , Berkshire, Wantage Research Laboratory, Isotope Research
Division (AERE-R 4196).
BOYARKINA, A.P., VASILEV, N.V., KRIVYAKORA, E.N., NIKOLAEV,
A.V., & TIUKIUPO, E.B. (1978) [On the accumulation of nickel
and vanadium in the vicinity of industrial centres.] Gig. i
Sanit ., 43(2): 92-95 (in Russian).
BRODERICK, G.N. (1977) Vanadium - 1977 , Washington DC, US
Department of the Interior, Bureau of Mines (Mineral Commodity
Profiles, MCP-8).
BROEKAERT, J.A.C., WOPENKA, B., & PUXBAUM, H. (1982) Inductively
coupled plasma optical emission spectrometry for the analysis of
aerosol samples collected by cascade impactors. Anal. Chem ., 54:
2174-2179.
BROWNE, R.C. (1955) Vanadium poisoning from gas turbines. Br.
J. ind. Med ., 12: 57-59.
BROWNE, R. & STEEL, J. (1963) The control of the vanadium
hazard in catalytic oil-gas plants. Ann. occup. Hyg ., 6: 75-79.
BROWNING, E. (1969) Vanadium. In: Toxicity of industrial
metals , 2nd ed., London, Butterworths, pp. 340-347.
BUCHET, J.P., KNEPPER, E., & LAUWERYS, R. (1982) Determination
of vanadium in urine by electrothermal atomic absorption
spectrometry. Anal. Chim. Acta , 136: 243-248.
BUCKINGHAM, D.A. (1973) Structure and stereochemistry of
coordination compounds. Part 1. Coordination chemistry. In:
Eichhorn, G.L., ed. Inorganic biochemistry , Amsterdam, New York,
Oxford, Elsevier Science Publishers, Vol. 1 , p. 18.
BUDNIKOV, G.K. & MEDJANCEVA, E.P. (1973) [Determination of
vanadium and nickel in bitumen by oscillographic polarography
with linear change of potential.] Zh. anal. Khim ., 28(2): 301-
305 (in Russian).
BUONO, J.A., BUONO, J.C., & FASCHING, J.L. (1977) Simultaneous
determination of Al, V. Mn and Cu from neutronactivated saline
matrices by precipitation with poly-5-vinyl-8-hydroxyquinoline.
J. radioanal. Chem ., 36: 353.
BUTT, E.M., NUSBAUM, R.E., GILMOUR, T.C., DIDIO, S.L., & Sister
MARIANO (1964) Trace metal levels in human serum and blood.
Arch. environ. Health , 8: 60.
BYRNE, A.R. & KOSTA, L. (1978) Vanadium in foods and in human
body fluids and tissues. Sci. total Environ ., 10: 17-30.
BYRNE, A.R. & KOSTA, L. (1979) On the vanadium and tin
contents of diet and human blood. Sci. total Environ ., 11: 87-
90.
CANALIS, E. (1985) Effect of sodium vanadate on deoxyribo-
nucleic acid and protein synthesis in cultured rat calvariae.
Endocrinology , 116(3): 855-862.
CANNON, H.L. (1963) The biogeochemistry of vanadium. Soil
Sci., 96(3): 196-204.
CANTLEY, L.C., Jr & AISEN, P. (1979) The fate of cytoplasmic
vanadium. Implications on (Na, K)-ATPase inhibition. J. biol.
Chem ., 254: 1781-1784.
CANTLEY, L.C., Jr, JOSEPHSON, L., WARNER, R., YANAGISAWA, M.,
LECHENE, C., & GUIDOTTI, G. (1977) Vanadate is a potent
(Na++,K)-ATPase inhibitor found in ATP derived from muscle. J.
biol. Chem ., 252(21): 7421-7423.
CANTLEY, L.C., Jr, CANTLEY, L.G., & JOSEPHSON, L. (1978a) A
characterization of vanadate interactions with the (Na,K)-
ATPase. J. biol. Chem ., 253: 7361-7368.
CANTLEY, L.C., Jr, RESH, M.D., & GUIDOTTI, G. (1978b) Vanadate
inhibits red cell, (Na+, K+) ATPase from the cytoplasmic side.
Nature (Lond.) , 272: 552-554.
CARLSON, R.M.K. (1977) The structure of the native vanadium
chromagen in tunicate blood cells , Palo Alto, California,
Stanford University, 252 pp (Ph.D. Thesis) (University Micro-
films No. 78-2142).
CARLTON, B.D., BENEKE, M.B., & FISHER, G.L. (1982) Assessment
of the teratogenicity of ammonium vanadate using Syrian golden
hamsters. Environ. Res ., 29(2): 256-262.
CARPENTER, G. (1981) Vanadate, epidermal growth factor and the
stimulation of DNA synthesis. Biochem. biophys. Res. Commun.,
102: 1115-1121.
CASSANI, F. (1969) [Potentiometric determination of titanium
and vanadium.] Chim. Ind. (Milan) , 51: 1248-1251 (in Italian).
CAWSE, P.A. & PEIRSON, D.H., ed. (1972) Analytical study of
trace elements in the atmospheric environment , Harwell, Atomic
Energy Research Establishment (No. AERE-R 7134).
CHASTEEN, N.D., LORD, E.M., THOMPSON, H.J., & GRADY, J.K.
(1986) Vandium complexes of transferrin and ferritin in the
rat. Biochim. Biophys. Acta , 884(1): 84-92.
CHIRIATTI, G.N. (1971) [Prevention of occupational vanadium
poisoning.] Folia med ., 54: 57-76 (in Italian).
CHRISTIAN, G.D. (1971) Catalytic determination of vanadium in
blood and urine. Anal. Lett ., 4(4): 187-196.
CHRISTIAN, G.D. & FELDMAN, F.I. (1970) Atomic absorption
spectroscopy. In: Applications in agriculture, biology, and
medicine , New York, John Wiley and Sons, pp. 276-281.
CHRISTIAN, H.C.M. & ROBINSON, J.W. (1971) The determination of
vanadium and nickel in mineral oils by flameless graphite tube
atomization. Anal. Chim . Acta, 56: 470-473.
CHUPAHIN, S.M., KRIUCHKOV, O.M., & RAMENDI, G.I. (1972)
[ Analytical capabilities of spark-source mass spectrometry ,]
Moscow, Atom Publishers (in Russian).
CLARK, R.J.H. (1975) The chemistry of vanadium, niobium, and
tantalum , Oxford, Pergamon Press, pp. 491-535.
COHEN, M.D., WEI, C.I., TAN, H., & KAO, K.J. (1986) Effect of
ammonium metavanadate on the murine immune response. J. Toxicol.
environ. Health , 19(2): 279-298.
CONKLIN, A.W., SKINNER, C.S., FELTEN, T.L., & SANDERS, C.L.
(1982) Clearance and distribution of intratracheally instilled
48vanadium compounds in the rat. Toxicol. Lett ., 11: 199-203.
CORNELIS, R., MEES, L., HOSTE, J., RYCKEBUSCH, J., VERSIECK, J.,
& BARBIER, F. (1979) Neutron activation analysis of vanadium
in human liver and serum. In: Proceedings of an International
Symposium on Nuclear Activation Techniques in the Life Sciences,
Vienna, 22-26 May, 1978 , Vienna, International Atomic Energy
Agency, pp. 165-177.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1980) Determination of vanadium in human serum by neutron
activation analysis. J. radioanal. Chem ., 55(1): 35-43.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1981) The ultratrace element vanadium in human serum. Biol.
Trace Elem. Res ., 3: 257-263.
COTTON, F.A. & WILKINSON, G. (1962) Advanced inorganic
chemistry , New York, Interscience.
COTTON, F.A. & WILKINSON, G. (1980) Advanced inorganic
chemistry , 4th ed., New York, John Wiley and Sons, pp. 708-719.
COWGILL, U.M. (1973) The determination of all detectable
elements in the aquatic plants of Linsley Pond and Cedar Lake
(North Branford, Connecticut) by X-ray emission and optical
emission spectroscopy. Appl. Spectrosci ., 27: 5-9.
CREASON, J.P., HINNERS, T.A., BUMGARNER, J.E., & PINKERTON, C.
(l975) Trace elements in hair, as related to exposure in
Metropolitan New York. Clin. Chem ., 21: 603-612.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
CURRAN, G.L. & BURCH, R.E. (1967) Biological and health
effects of vanadium. In: Proceedings of the First Annual
Conference on Trace Substances in Environmental Health,
Columbia, 10-11 July, 1967 , Columbia, University of Missouri,
pp. 96-104.
CURRAN, G.L. & COSTELLO, R.L. (1956) Reduction of excess
cholesterol in the rabbit aorta by inhibition of endogenous
cholesterol synthesis. J. exp. Med ., 103: 49-56.
CURRAN, G.L., AZARNOFF, D.L., & BOLINGER, R.E. (1959) Effect
of cholesterol synthesis inhibition in normocholesteremic young
men. J. clin. Invest ., 38: 1251-1261.
DAMS, R., ROBBINS, J.A., RAHN, K.A., & WINCHESTER, J.W. (1970)
Nondestructive neutron activation analyses of air pollution
particulates. Anal. Chem ., 42: 861-867.
DAY, H., MIDDENDORF, D., LUKERT, C., HEINZ, A., & GRANTHAM, J.J.
(1980) The renal response to intravenous vanadate in rats. J.
lab. clin. Med., 96: 382-395.
DEMASTER, E.G. & MITCHELL, R.A. (1973) A comparison of
arsenate and vanadate as inhibitors or uncouplers of
mitochondria and glycolytic energy metabolism. Biochemistry,
12(19): 3616-3621.
DHEW (1968) Air quality data from the National Air
Surveillance Networks and contributing state and local networks ,
1966 ed., Durham, North Carolina, US Department of Health,
Education, and Welfare, National Air Pollution Control
Administration.
DIMOND, E.G., CARAVACA, J., & BENCHIMOL, A. (1963) Vanadium:
Excretion, toxicity, lipid effect in man. Am. J. clin. Nutr .,
12: 49-53.
DOMINGO, J.L., LLOBET, J.M., & CORBELLA, J. (1985) Protection
of mice against the lethal effects of sodium metavanadate: a
quantitative comparison of a number of chelating agents.
Toxicol. Lett ., 26(2-3): 95-99.
DOMINGO, J.L., LLOBET, J.M., TOMAS, J.M., & CORBELLA, J. (1986)
Influence of chelating agents on the toxicity, distribution, and
excretion of vanadium in mice. J. appl. Toxicol ., 6(5): 337-
341.
DOVGOPOL, V.I., BENOUNI, A.CH., MEDVEDEV, A.A., SHAPOVALOV,
J.P., & MEDVEDEVA, L.A. (1974) [The chemistry and engineering
technology of vanadium compounds.] In: Ivakin, A.A. & Voronova,
E.M., ed. [ Proceedings of the International Conference on the
Chemistry and Technology of Vanadium Compounds ,] Perm,
Scientific Institute of Black Metals, pp. 477-480 (in Russian).
DUCE, R.A. & HOFFMAN, G.L. (1976) Atmospheric vanadium
transport to the ocean. Atmos. Environ ., 10: 989-996.
DURFOR, C.N. & BECKER, E. (1963) Public water supplies of
the 100 largest cities in the United States, 1962 , Washington
DC, US Geological Survey (Water-Supply Paper No. 1812).
DURRANT, P.J. & DURRANT, B. (1970) Introduction to advanced
inorganic chemistry , 2nd ed., New York, John Wiley and Sons.
DURUM, W.H. & HAFFTY, J. (1963) Implications of the minor
element content of some major streams of the world. Geochim.
Cosmochim. Acta , 23: 1-11.
DUTTON, W.F. (1911) Vanadiumism. J. Am. Med. Assoc ., 56(22):
1648.
EISLER, L., SIMECEK, R., & USTUPSKY, J. (1968) [Effect of work
in vanadium workshop on respiratory ways.] Prac. Lek., 20(2/3):
52-57 (in Czech).
ELINDER, C.G. (1984) Metabolism and toxicity of metals ,
Stockholm, Department of Environmental Hygiene, Karolinska
Institute and the National Institute of Environmental Medicine,
pp. 265-274 (Life Sciences Research Report No. 28).
ELVES, M.V., WILSON, J.N., SCALES, J.T., & KEMP, H.B.S. (1975)
Incidence of metal sensitivity in patients with total joint
replacements. Br. med. J ., 4: 376-378.
ERMOLAEV, G.F. (1969) [Effect of vanadium on certain
biochemical processes in rabbit organs.] In: [ Trace elements in
medicine ,] Moscow, Ivano-Frankovsk, pp. 190-192 (in Russian).
EVANS, C.A. & MORRISON, G.H. (1968) Trace element survey
analysis of biological materials by spark source mass
spectrometry. Anal. Chem ., 40: 869-875.
FALLENTIN, B. & FROST, J. (1954) [Health hazards from cleaning
oil-fired boilers caused by vanadium and sulfuric acid in the
soot.] Nord. Hyg. Tidskr ., 3: 58-65 (in Danish).
FAORO, R.B. & MCMULLEN, T.B. (1977) National trends in trace
metals in ambient air 1965-1974 , Research Triangle Park, North
Carolina, US Environmental Protection Agency (EPA-450/1-77-
003).
FAULKNER HUDSON, T.G. (1964) Vanadium: toxicology and
biological significance , Amsterdam, New York, Oxford, Elsevier
Science Publishers, 135 pp.
FEAR, E.C. & TYRER, F.H. (1958) A study of vanadium poisoning
in gas workers. Trans. Assoc. Ind. Med. Off ., 8: 153-155.
FENNELLY, P.F. (1976) The origin and influence of airborne
particulates. Am. Sci ., 64(1): 46-56.
FISHER, G.L., MCNEILL, K.L., WHALEY, C.B., & FONG, J. (1978)
Attachment and phagocytosis studies with murine pulmonary
alveolar macrophages. J. Reticuloendothel. Soc ., 24: 243-252.
FISHER, G.L., MCNEILL, K.L., & DEMOCKO, C.J. (1986) Trace
element interactions affecting pulmonary macrophage cyto-
toxicity. Environ. Res ., 39(1): 164-171.
FLAHERTY, J.P. & ELDRIDGE, H.B. (1970) Neutron activation
analysis of residual oil for vanadium. Appl. Spectrosci ., 24(5):
534-538.
FROLOV, V.V. (1970) [Determination of chlorine and vanadium in
graphite by the activation method.] Zavod. Lab ., 36(7): 807-809
(in Russian).
FROST, J (1951) [Vanadium poisoning from cleaning the smoke
stacks of boilers burning fuel oil.] Ugeskr. Laeger , 113: 1309-
1312 (in Danish).
FUKAI, R. & MEINKE, W.W. (1962) Activation analyses of
vanadium, arsenic, molybdenum, tungsten, rhenium, and gold in
marine organisms. Limnol. Oceanogr ., 7: 186.
GARLEJ, T. (1974) [The influence of vanadium-bearing dust on
the workers' health in the Plock thermal-electric power station:
New exposure tests.] Pol. Tyg. Lek ., 29(49): 2143-2144 (in
Polish).
GERSHKOVICH, E.E. & STYKAN, T.P. (1972) [A catalytic method of
measuring hydrogen sulfide levels in air.] Gig. Tr. prof.
Zabol ., 2: 60-61 (in Russian).
GEYER, C.F. (1953) Vanadium, a caries-inhibiting trace element
in the Syrian hamster. J. dent. Res ., 35: 590-595.
GIBSON, R.S. & DEWOLFE, M.S. (1979) Copper, zinc, manganese,
vanadium, and iodine concentrations in the hair of Canadian low
birth wight neonates. Am. J. clin. Nutr ., 32: 1728-1733.
GILES, M.A., KLAVERKAMP, J.F., & LAWRENCE, S.G. (1979) The
acute toxicity of saline groundwater and of vanadium to fish and
aquatic invertebrates , Edmonton, Environment Alberta, 172 pp
(Prepared for the Alberta Oil Sands Environmental Research
Program) (Project No. AF 3.2.1).
GOFMAN J.W. (1962) Chemical elements of the blood of man in
health. Adv. biol. med. Phys ., 8: 1.
GOLDBERG, E.D. (1961) Marine geochemistry. In: Eyring, H.,
Christensen, C.J., & Johnston, H.S., ed. Annual review of
physical chemistry , Vol. 12, Palo Alto, California, Annual
Reviews, Inc., pp. 29-48.
GOLDSCHMIDT, V.M. (1938) Collected articles on the
geochemistry of rare elements. In: [ Collection of papers on the
geochemistry of rare metals ,] Moscow, Amalgamated State
Technical and Scientific Publishing Houses (in Russian).
GOLDSHTEJN, M.I. (1967) [ Vanadium-containing steels ,] Moscow,
Chermetinformacija (in Russian).
GORDUS, A.A., MAHER, C.C., III, & BIRD, G.C. (1974) Human hair
as an indicator of trace metal environmental exposure. In:
Proceedings of the First Annual NSF Trace Contaminants
Conference , Springfield, Virginia, National Technical
Information Service, pp. 463-487 (Conf. 730802).
GOYER, R.A. & MEHLMANN, M.A., ed. (1977) Toxicology of trace
elements. In: Advances in modern toxicology, Washington DC,
Hemisphere, Vol. 2 , 303 pp.
GRANTHAM, J.J. (1980) The renal sodium pump and vanadate. Am.
J. Physiol ., 239: F97-F106.
GRANTHAM, J.J. & GLYNN, I.M. (1979) Renal Na, K-ATPase:
determinants of inhibition by vanadium. Am. J. Physiol ., 236(6):
F530-F535.
GRIN, A.V., BLUM, A.A., SELETKOV, A.L., FREIDENZON, YU.E., &
NAIMUCHINA, L.F. (1971) [Low-alloyed welded steels containing
vanadium for construction.] In: [ Problems of Kachkanar ,]
Sverdlovsk, Central Bureau of Technical Information, pp. 186-196
(in Russian).
GULKO, A.G. (1956) [On the characteristics of vanadium as an
industrial poison.] Gig. i Sanit ., 21(11): 24-28 (in Russian).
GYLSETH, B., LEIRA, H.L., STEINNES, E., & THOMASSEN, Y. (1979)
Vanadium in the blood and urine of workers in a feraalloy plant.
Scand. J. Work environ. Health , 5: 188-194.
HACKETT, P.L. & KELMAN, B.J. (1983) Availability of toxic trace
metals to the conceptus. Sci. total environ .,28: 433-442.
HADJIMARKOS, D.M. (1966) Vanadium and dental caries. Nature
(Lond.) , 209: 1137.
HADJIMARKOS, D.M. (1968) Effect of trace elements on dental
caries. Adv. oral Biol ., 3: 282-285.
HAMILTON, E.I., MINSKI, M.J., & CLEARY, J.J. (1972-73) The
concentration and distribution of some stable elements in
healthy human tissues from the United Kingdom. Sci. total
Environ ., 1: 341-374.
HAMMOND, P.B. & FOULKES, E.C. (1986) Metal ion toxicity in man
and animals. In: Metal ions in biological systems , Cincinatti,
Ohio, Kettering Laboratory, Vol. 20 , pp. 157-200.
HANNA, W.J. & GRANT, C.L. (1962) Spectrochemical analyses of
certain trees and ornamentals for 23 elements. Bull. Torrey Bot.
Club , 89: 293-302.
HARA, T., SONODA, Y., & IWAI, I. (1976) Growth response of
cabbage plants to transition elements under water culture
conditions. I. Titanium, vanadium, chromium, manganese, and
iron. Soil Sci. plant Nutr ., 22(3): 307-315.
HARRIS, W.R., FRIEDMAN, S.B., & SILBERMAN, D. (1984) Behavior
of vanadate and vanadyl ion in canine blood. J. inorg. Biochem .,
20(2): 157-169.
HATFIELD, M. & CHURCHILL, P. (1981) Renal vascular and tubular
effects of vanadate in the anaesthetized rat. J. Pharmacol. exp.
Ther ., 217: 406-410.
HATHCOCK, J.N., HILL, C.H., & TOVE, S.B. (1966) Uncoupling of
oxidative phosphorylation by vanadate. Can. J. Biochem ., 44:
983-988.
HEIN, J.W. & WISOTZKY, J. (1955) The effect of a 10 ppm
vanadium drinking solution on dental caries in male and female
Syrian hamsters. J. dent. Res ., 34: 756.
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand culture.
J. exp. Bot ., 4(10): 59-64.
HEYDORN, K. & LUKENS, H.R. (1966) Pre-irradiation separation
for the determination of vanadium in blood serum by reactor
neutron activation analysis , Roskilde, Danish Atomic Energy
Commission, Research Establishment Risö (Risö Report No. 138).
HEYLIGER, C.E., TAHILIANI, A.G., & MCNEILL, J.H. (1985) Effect
of vanadate on elevated blood-glucose and depressed cardiac
performance of diabetic rats. Science , 227(4693): 1474-1477.
HICKEY, R.J., SCHOFF, E.0., & CLELLAND, R.C. (1967)
Relationship between air pollution and certain chronic disease
death rates. Arch. environ. Health , 15: 728-738.
HICKLING, S. (1958) Vanadium poisoning. N.Z. med. J ., 57: 607-
608.
HOFFMAN, B., KOMENDA, W., SADOWSKA, A.M., & ROZANSKI, A. (1972)
[The occurence of vanadium in the drinking-water in the region
of Bialystock Province.] Rocz. Panstw. Zakl. Hig ., 23(2): 155-
159 (in Polish).
HOFFMAN, G.L., DUCE, R.A., & ZOLLER, W.H. (1969) Vanadium,
copper, and aluminum in the lower atmosphere between California
and Hawaii. Environ. Sci. Technol ., 3: 1207-1210.
HOFFMAN, G.L., DUCE, R.A., & HOFFMAN, E.J. (1972) Trace metals
in the Hawaiian marine atmosphere. J. geophys. Res ., 77: 5322-
5329.
HOLDWAY, D.A. & SPRAGUE, J.B. (1979) Chronic toxicity of
vanadium to flagfish. Water Res ., 13: 905-910.
HOLODOV, V.N. (1968) [ Vanadium ,] Moscow, Nauka Publishing
House (in Russian).
HOLODOV, V.N. (1973) [ Sedimentary ore formation and
metallogeny on vanadium ,] Moscow, Nauka Publishing House (in
Russian).
HOLZHAUER, K.P. & SCHALLER, K.-H. (1977) [ Occupational
medicine studies in chimney sweeps. Hazards at the workplace and
occupation-linked health damage ,] Stuttgart, G. Thieme (in
German).
HOPKINS, L.L., Jr & MOHR, H.E. (197la) The biological
essentiality of vanadium. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition , New York, Marcel Dekker,
pp. 195-213.
HOPKINS, L.L., Jr & MOHR, H.E. (197lb) Effect of vanadium
deficiency on plasma cholesterol of chicks. Fed. Proc ., 30:
462.
HOPKINS, L.L., Jr & MOHR, H.E. (1974) Vanadium as an essential
nutrient. Fed. Proc ., 33(6): 1173-1175.
HOPKINS, L.L., Jr & TILTON, B.E. (1966) Metabolism of trace
amounts of vanadium 48 in rat organs and liver subcellular
particles. Am. J. Physiol ., 211: 169-172.
HOPKINS, L.L., CANNON, H.L., MUSCH, A.T., WELCH, R.M., &
NIELSEN, F.H. (1977) Vanadium. Geochem. Environ ., 2: 93-107.
HORI, C. & OKA, T. (1980) Vanadate enhances the stimulatory
action of insulin on DNA synthesis in cultured mouse mammary
glands. Biochim. Biophys. Acta , 610: 235-240.
HORNER, C.K., BURCK, D., ALLISON, F., & SHERMAN, M.S. (1942)
Nitrogen fixation by azotobacter as influenced by molybdenum and
vanadium. J. agric. Res ., 65: 173-193.
HUDGINS, P.M. & BOND, G.H. (1979) Reversal of vanadate
inhibition of Na++K-ATPase by catecholamines. Res. Commun. chem.
Pathol. Pharmacol ., 23(2): 313-326.
HUDSON, T.G.F. (1964) Vanadium: toxicology and biological
significance , Amsterdam, New York, Oxford, Elsevier Science
Publishers.
HWANG, I.Y., ULLICCI, P.A., & SMITH, S.B. (1972) A simple
flameless atomizer. Am. Lab ., 3: 41-43.
ICRP (1960) Report of Committee II on Permissible Dose for
Internal Radiation (1959). Recommendations of the International
Commission on Radiological Protection , Oxford, Pergamon Press
(ICRP Publication No. 2).
ILO (1980) Occupational Exposure Limits for Airborne Toxic
Substances; Occupational Safety and Health Series, No. 37, 2nd
revised ed ., Geneva, International Labour Office.
INCIARTE, D.J., STEFFEN, R.P., DOBBINS, D.E., SWINDALL, B.T.,
JOHNSTON, J., & HADDY, F.J. (1980) Cardiovascular effects of
vanadate in the dog. Am. J. Physiol ., 239: 47-56.
IZYCKI, J., SULKOWSKI, W., ADAMIAK-ZIEMBA, J., & STROSZEJN, G.
(1971) [Evaluation of professional exposure to vanadium
compounds and other environmental factors of workers employed in
cleaning of oil-fired boilers with special regard to the state
of respiratory tract.] Med. Pr ., 22(4): 421-431 (in Polish).
JACIMIRSKIJ, K.B. (1967) [ Kinetic methods of analysis ,]
Moscow, Himija (in Russian).
JACKSON, J.F. & LINSKENS, H.F. (1982) Metal ion induced
unscheduled DNA synthesis in Petunia pollen. Mol. gen. Genet .,
187: 112.
JANDHYALA, B.S. & HOM, G.J. (1983) Physiological and pharmaco-
logical properties of vanadium. Life Sci ., 33: 1325-1340.
JARACZEWSKA, W. & JAKUBOWSKI, M. (1964) [The attempt of
evaluation of vanadium exposure in the chemical industry.] Med.
Pr ., 15(6): 375-383 (in Polish).
JAWOROWSKI, Z., BILKIEWICZ, J., DIBOSZ, E., GRYBOWSKA, D.,
KOWNACKA, L., & WRONSKI, Z. (1973) Radiation hazards to
population from conventional and nuclear power production. In:
Symposium on environmental surveillance around nuclear
installations, Warsaw, 5-9 November, 1973, Vienna, International
Atomic Energy Agency (IAEA-SM-180/20).
JOHNSON, J.L., COHEN, H.L., & RAJAGOPALAN, K.V. (1974) Studies
of vanadium toxicity in the rat; lack of correlation with
molybdenum utilization. Biochem. biophys. Res. Commun ., 56(4):
940-946.
JONES, M.M. & BASINGER, M.A. (1983) Chelate antidotes for
sodium vanadate and vanadyl sulfate intoxication in mice. J.
Toxicol. environ. Health , 12(4-6): 749-756.
KADA, T., HIRANO, K., & SHIRASU, Y. (1980) Screening of
environmental chemical mutagens by the rec-assay system with
Bacillus subtilis. In: de Serres, F.J. & Hollaender, A., ed.
Chemical mutagens: principles and methods for their detection ,
New York, Plenum Press, pp. 149-173.
KAKU, S., HOSHIDE, M., MATSUO, S., NUNOTANI, H., ISHIDA, N.,
KURATA, S., MATSUMOTO, H., YAMAGUCHI, S., SANO, T., KONO, Y.,
KOGA, S., & KOGA, M. (1971) [Experimental studies on vanadium
poisoning. I. Vanadium-induced hepatic injuries in rats.] Jap.
J. ind. Health , 13: 263-267 (in Japanese).
KANEMATSU, K. & KADA, T. (1978) Mutagenicity of metal
compounds. Mutat. Res ., 53(2): 207-208.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec-assay and
mutagenicity studies on metal compounds. Mutat. Res ., 77: 109-
116.
KANISAWA, M. & SCHROEDER, H.A. (1967) Life-term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumours in mice. Cancer Res ., 27: 1192-1195.
KATAYEVA, I.G. & SAPUNOV, S.F. (1974) [Sanitary-hygienic
characteristics of vanadium ferroalloy production at the
Chusovoy Metallurgical Plant.] Gig. Tr. prof. Zabol ., 18(1):
37-39 (in Russian).
KAZIMOV, M.A. (1977) [Occupational hygiene with new methods of
the ferrovanadium production.] Gig. Tr. prof. Zabol ., 6: 8-12
(in Russian).
KEJT, M. & DEGENS, E. (1961) [Geometrical indicators.] In:
[ Geometrical research at low temperatures and pressures ,]
Moscow, Foreign Language Publishers, pp. 56-84 (in Russian).
KEMPTON, S., STERRITT, R.M., & LESTER, J.N. (1982) Atomic-
absorption spectrophotometric determination of antimony,
arsenic, bismuth, tellurium, thallium and vanadium in sewage
sludge. Talanta , 29: 675-681.
KINGSNORTH, A.N., LAMURAGLIA, G.M., ROSS, J.S., & MALT, R.A.
(1986) Vanadate supplements and 1,2-dimethylhydrazine induced
colon cancer in mice: increased thymidine incorporation without
enhanced carcinogenesis. Br. J. Cancer, 53(5): 683-686.
KIRZHNER, M.A., KRYZHENKOVA, N.A., & KIST, A.A. (1974) [Scope
for the preliminary concentration in the use of neutron
activation in matrix analysis for aluminium and vanadium.] At.
Energ ., 37: 498 (in Russian).
KIVILUOTO, M. (1980) Observations on the lungs of vanadium
workers. Br. J. ind. Med ., 37: 363-366.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1979a) Serum and
urinary vanadium pentoxide. Int. Arch. occup. Health , 48: 251-
256.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M. (1979b)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory. Scand. J. Work environ. Health , 5: 50-58.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M., (1979c)
Serum and urinary vanadium of vanadium exposed workers. Scand.
J. Work environ. Health , 5: 362-367.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health,
46: 179-182.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1981a) Clinical
laboratory results of vanadium-exposed workers. Arch. environ.
Health , 36(3): 109-113.
KIVILUOTO, M., RASANEN, O., RINNE, A., & RISSANEN, M. (1981b)
Intracellular immunoglobulins in plasma cells of nasal biopsies
taken from vanadium-exposed workers. Anat. Anz. Jena , 149: 446-
450.
KNECHT, E.A., MOORMAN, W.J., CLARK, J.C., LYNCH, D.W., & LEWIS,
T.R. (1985) Pulmonary effects of acute vanadium pentoxide
inhalation in monkeys. Am. Rev. respir. Dis ., 132(6): 1181-
1185.
KOHLER, R. (1972) [Rebuttal: problems of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 27: 815-816 (in German).
KOLPAKOV, L.E., DOBOSH, V.G., GREKOV, S.D., JUFEREV, A.JU.,
LLOKAZOV, E.D., AZFALOVA, R.U., & SANDLER, V.I. (1971)
[Improvement of technology of utilisation of vanadium contained
in waste water.] In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 229-231 (in
Russian).
KONOVALOV, G.S., IVANOVA, A.A., & KOLESNIKOV, T.CH. (1968)
[Scattered rare elements dissolved in water contained in
colloidal substances of the major USSR rivers.] In: [ A
collection of papers on the geochemistry of sedimentary rocks
and ores. Documentation of the Seventh All-Union Conference on
Lithology of the Academy of Sciences of the USSR], Moscow,
Nauka Publishing House, pp. 72-87 (in Russian).
KÖPF-MAIER, P. & KÖPF, H. (1979) [Vanadocene dichloride:
another anti-tumour agent from the metallocene series.] Z.
Naturforsch ., 34B: 805-807 (in German).
KÖPF-MAIER, P., HESSE, B., & KÖPF, H. (1980) Tumour inhibition
by metallocenes: effect of titanocene and hafnocene dichlorides
on Ehrlich ascites tumour in mice. J. Cancer Res. clin. Oncol .,
96(1): 43-51.
KOPYLOVA, L.M. (1971) [A study of some blood indicators
following prolonged administration of vanadium sulfate.] Tr.
Voronezh. Med. Inst ., 85: 62-65 (in Russian).
KORKHOV, V.V. (1965) [Vanadium in prophylaxis and treatment of
experimental atheroslerosis.] Farmakol. i Toksikol ., 28(1): 83-
87 (in Russian).
KOSTROMIN, A.I., AKHMETOV, A.A., & ORLOVA, L.N. (1970)
[Coulometric determination of manganese (II), cesium (III), and
vanadium (IV).] Zh. anal. Khim ., 25: 195-196 (in Russian).
KOVDA, V.A., JAKUSHEVSKAYA, I.V., & TURUKANOV, A.N. (1959)
[ Trace elements in the soils of the Soviet Union ,] Moscow,
Moscow University Publishing House (in Russian).
KRAGTEN, J. (1981) Improved determination of vanadium by flame
AAS by preventing polynuclear hydroxide complex formation. At.
Spectrosci ., 2(4): 135-136.
KRAUSKOPF, F.K. (1963) Factors governing the concentration of
thirteen rare metals in seawater. Collected articles on the
geochemistry of rock formation. In: [ The geochemistry of
lithogenesis ,] Moscow, Foreign Languages Publishing House, pp.
294-338 (in Russian).
KUBASKY, A. (1957) Waste from a vanadium work as moulding
material. Slevarenstvi , 5(1): 4-7.
KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D., & FLETCHER, O.J.
(1986) Influence of ochratoxin A and vanadium on various
parameters in growing chicks. Poult. Sci ., 65(9): 1671-1678.
KULIEVA, T.K. (1974) [Effect of vanadium on the activity of
some tissue-respiration enzymes.] Zdravookhr. Turkm ., 3: 7-17
(in Russian).
KUMAR, A. & CORDER, C.N. (1980) Diuretic and vasoconstrictor
effects of sodium orthovanadate on the isolated perfused rat
kidney. J. Pharmacol. exp. Ther ., 213: 85-90.
KURMAEV, R.H. (1974) [Solid, liquid and aerosol waste in the
production of technical vanadium pentoxide.] In: Ivakin, A.A. &
Voronova, E.M., ed. [ Proceedings of the International
Conference on the Chemistry and Technology of Vanadium
Compounds ,] Perm, Scientific Institute of Black Metals, pp. 43-
46 (in Russian).
KUZELOVA, M., SIRL, J., & POPLER, A. (1975) [Occupational
poisonings by vanadium compounds in workers at mazut boiler
houses.] Prac. Lek ., 27(5): 170-173 (in Czech).
KUZELOVA, M., HAVEL, V., POPLER, A., & STEPANEK, O. (1977)
[The problems of occupational medicine in the work of chimney
sweeps.] Prac. Lek ., 29: 225-228 (in Czech).
LAGERKVIST, B., NORDBERG, G.F., & VOUK, V. (1986) Vanadium.
In: Friberg, G.F., Nordberg, G.F., & Vouk, V.B., ed. Handbook on
the toxicology of metals. II. Specific metals , Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 638-663.
LAHAV, M., RENNERT, H., & BARZILAI, D. (1986) Inhibition by
vanadate of cyclic AMP production in rat corpora lutea incubated
in vitro . Life Sci ., 39(26): 2557-2564.
LANDER, D.W., STEINER, R.L., ANDERSON, D.H., & DEHM, R.L.
(1971) Spectrographic determination of elments in airborne
dirt. Appl. Spectrosci ., 25: 270-275.
LARSEN, J.A. & THOMSEN, O. (1980) Vanadate-induced oliguria
and vasoconstriction in the cat. Acta physiol. Scand ., 110:
367-374.
LARSEN, J.A., THOMSEN, O., & HANSEN, O. (1979) Vanadate-
induced oliguria in the anaesthetized cat. Acta physiol. Scand .,
106: 495-496.
LEE, K.P. & GILLIES, P.J. (1986) Pulmonary response and
intrapulmonary lipids in rats exposed to bismuth orthovanadate
dust by inhalation. Environ. Res ., 40(1): 115-135.
LEE, R.E., GORANSON, S.S., ENROINE, R.E., & MORGAN, G.B. (1972)
National air surveillance Cascade Impactor Network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol ., 6: 1025-1030.
LEE, K., NALEWAJKO, C., & JACK, T.R. (1978) Effects of
vanadium on freshwater algae. In: Abstracts of the 5th Annual
Aquatic Toxicity Workshop, Hamilton, Ontario, 7-9 November ,
Burlington, Ontario, Canada Centre for Inland Waters.
LEES, R.E.M. (1980) Changes in lung function after exposure to
vanadium compounds in fuel oil ash. Br. J. ind. Med ., 37: 253-
256.
LEVlNA, E.N. (1972) [Relationships between solubility of metal
compounds and their toxicity, distribution, and elimination from
the body.] Gig. Tr. prof. Zabol ., 16: 40-43 (in Russian).
LEVY, B.S., HOFFMAN, L., & GOTTSEGEN, S. (1984) Boilermakers'
bronchitis: respiratory tract irritation associated with
vanadium pentoxide exposure during oil-to-coal conversion of a
power plant. J. occup. Med ., 26(8): 567-570.
LEVY, L.S., MARTIN, P.A., & BIDSTRUP, P.L. (1986) Investigation
of the potential carcinogenicity of a range of chromium
containing materials on rat lung. Br. J. ind. Med ., 43(4): 243-
256.
LEWIS, C.E. (1959a) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 19: 419-424.
LEWIS, C.E. (1959b) The biological effects of vanadium. II.
The signs and symptoms of occupational vanadium exposure. Am.
Med. Assoc. Arch. Ind. Health , 19: 497-503.
LEWIS, C.E. (1959c) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 20(5): 455-466.
LIFSCHITZ, V.M. (1962) [Spectral determination of trace
elements in human blood (group determination of Ni, Ag, Zn, Cu,
Cd, V, Pb, Mn, Fe, and Co).] Tr. Voronezh. Med. Inst ., 49: 110
(in Russian).
LINSTEDT, K.D. & KRUGER, P. (1969) Vanadium concentrations in
Colorado River Basin waters. J. Am. Water Works Assoc ., 61(2):
85-88.
LINTER, C.M. (1985) Neuropsychiatric aspects of trace
elements. Br. J. hosp. Med ., 34(6): 361-365.
LOPEZ-NOVOA, J.M., GARCIA, J.C., CRUZ-SOTO, M.A., BENABE, J.E.,
& MARTINEZ-MALDONADO, M. (1982a) Effect of sodium ortho-
vanadate on renal renin secretion in vivo. J. Pharmacol. exp.
Ther ., 222: 447-451.
LOPEZ-NOVOA, J.M., MAYOL, V., & MARTINEZ-MALDONADO, M. (1982b)
Renal actions of orthovanadate in the dog. Proc. Soc. Exp. Biol.
Med ., 170: 418-426.
L'VOV, B.V. (1970) Atomic absorption spectrochemical analysis,
2nd ed., London, Adam Hilger.
MACARA, I.G. (1980) Vanadium - an element in search of a role.
Trends biochem. Sci ., April: 92-94.
MACARA, I.G., KUSTIN, K., & CANTLEY, L.C., Jr (1980) Gluta-
thione reduces cytoplasmic vanadate mechanism and physiological
implications. Biochim. Biophys. Acta , 629: 95-106.
MCLEAN, J.R., MCWILLIAMS, R.S., KAPLAN, J.G., & BIRNBOIM, H.C.
(1982) Rapid detection of DNA strand breaks in human peripheral
blood cells and animal organs following treatment with physical
and chemical agents. In: Bora, K.C., Douglas, G.R., & Nestmann,
E.R., ed. Chemical mutagenesis, human population monitoring, and
genetic risk assessment , Amsterdam, Oxford, New York, Elsevier
Science Publishers, p. 137 (Progress in Mutation Research,
Vol. 3).
MCLUNDIE, A.C., SHEPHERD, J.B., & MOBBS, D.R.A. (1968) Studies
on the effects of various ions on enamel solubility. Arch. oral
Biol ., 13: 1321-1330.
MADSEN, E.S., NIELSEN, P.A., & PEDERSEN, J.C. (1982) The
distribution and origin of mutagens in airborne particulates,
detected by the Salmonella /microsome assay in relation to
levels of lead, vanadium, and PAH. Sci. total Environ ., 24(1):
13-25.
MAHLER, H.R. & CORDES, E.H. (1966) Biological chemistry , New
York, Harper & Row.
MAJUMDAR, A.K. & DAS, G. (1965) Spectrophotometric determin-
ation of vanadium with N -benzoyl- N -p-chlorophenylhydroxyl-
amine. J. Indian Chem. Soc ., 42(3): 189.
MANNING, D.C. & SLAVIN, W. (1983) The determination of trace
elements in natural waters using the stabilized temperature
platform furnace. Appl. Spectrosci ., 37(1): 1-10.
MARAFANTE, E. & SABBIONI, E. (1983) Metabolic studies on
vanadium: in vivo and in vitro association with cellular
components. In: Proceedings of an International Conference on
Heavy Metals in the Environment, London, 18-21 September,
1979 , Edinburgh, CEC Consultants, pp. 171-174.
MARONI, M., COLOMBI, A., BURATTI, M., CALTZAFERRI, G., FOA, V.,
SABBIONI, E., & PIETRA, R. (1983) Urinary elimination of
vanadium in boiler cleaners. In: Proceedings of an International
Conference on Heavy Metals in the Environment, London, 18-21
September, 1979 , Edinburgh, CEC Consultants, pp. 66-69.
MARTENS, C.S., WESOLOWSKI, J.J., KAIFER, R., & JOHN, W. (1973)
Sources of vanadium in Puerto Rican and San Francisco bay area
aerosols. Environ. Sci. Technol ., 7: 817-819.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959a) Effect of
vanadium upon liver coenzyme A in rats. Nature (Lond.) , 183:
1527-1528.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959b) Intracellular
thioctic acid and coenzyme A following vanadium treatment.
Nature (Lond.) , 184: 1641.
MASIRONl, R. (1969) Trace elements and cardiovascular
diseases. Bull. World Health Organ ., 40: 305-312.
MATANTSEVA, E.I. (1960) [The state of the respiratory organs
in workers coming into contact with vanadium pentoxide.] Gig.
Tr. prof. Zabol ., 7: 41-44 (in Russian).
MEISCH, H.U. & BECKER, L.J. (1981) Vanadium in photosynthesis
of Chlorellafusca and higher plants. Biochim. Biophys. Acta ,
636: 119-125.
MEISCH, H.U. & BENZSCHAWEL, H. (1978) Role of vanadium in
green plants. III. Influence on cell division of chlorella.
Arch. Microbiol ., 116(1): 91-95.
MERTZ, W. (1970) Some aspects of nutritional trace element
research. Fed. Proc ., 29: 1482-1488.
MINDEN, H. & ROTHE, R. (1966) [Biochemical mechanism of action
of vanadium.] Z. gesamte Hyg ., 12(5): 315-321 (in German).
MIRAMAND, P. & UNSAL, M. (1978) Acute toxicity of vanadium to
some marine benthic and phytoplanktonic species. Chemosphere ,
10: 827-832.
MIRZAEVA, K.H. (1965) [Trace elements in natural waters in
some districts of Uzbekistan.] In: [ Trace elements in agri-
culture ,] Tashkent, Science Publishers of Uzbekistan, pp. 122-
126 (in Russian).
MISIEWICZ, A. (1980) [Effect of air containing gasoline,
tungsten, titanium, cobalt, and vanadium on the phagocytic
activity of leucocytes.] Pol. Tyg. Lek ., 35(50): 1965-1967 (in
Polish).
MISIEWICZ, A. (1983) [Effect of air containing benzine,
wolfram, titanium, cobalt, and vanadium on peripheral blood.]
Med. Pr ., 34(3): 251-257 (in Polish).
MITCHELL, R.L. (1964) Trace elements in soil. In: Bear, F.E.,
ed. Chemistry of the soil , New York, Reinhold Publishing,
pp. 320-368.
MITCHELL, W.G. & FLOYD, E.P. (1954) Ascorbic acid and
ethylenediaminetetracetate (EDTA) as antidotes in experimental
vanadium poisoning. Proc. Soc. Exp. Biol. Med ., 85: 206-208.
MONTERO, M.R., GUERRI, C., RIBELLES, M., & GRISOLIA, S. (1981)
Inhibition of protein synthesis in cell cultures by vanadate and
in brain homogenates of rats fed vanadate. Physiol. Chem.
Physics , 13: 281-287.
MORGAN, W.K.C. & SEATON, A. (1984) Occupational lung diseases ,
2nd ed., Philadelphia, W.B. Saunders & Co.
MOUNTAIN, J.T. (1963) Detecting hypersusceptibility to toxic
substances. Arch. environ. Health , 6: 357-365.
MOUNTAIN, J.T., DELKER, L.L., & STOKINGER, H.E. (1953) Studies
in vanadium toxicology. I. Reduction in the cystine content of
rat hair. Am. Med. Assoc. Arch. Ind. Hyg. Occup. Med ., 8: 406.
MOUNTAIN, J.T., STOCKELL, F.R., Jr, & STOKINGER, H.E. (1955)
Studies in vanadium toxicology. Am. Med. Assoc. Arch. Ind.
Health , 12: 494.
MUHLER, J.C. (1957) The effect of vanadium pentoxide,
fluorides, and tin compounds on the dental caries experience in
rats. J. dent. Res ., 36: 787-794.
MUSK, A.W. & TEES, J.G. (1982) Asthma caused by occupational
exposure to vanadium compounds. Med. J. Aust ., 1: 183-184.
MUSTAFIN, I.S., NECHAENKO, T.M., & FRUMINA, N.S. (1969) [ The
range of reagents available for vanadium ,] Moscow, Moscow State
University (in Russian).
MUZQUIN, V.N., KHAVZINA, L.B., ZOLOTAVIN, V.L., & BERRUKOV,
I.YA. (1981) [ Analytical chemistry of vanadium ,] Moscow,
Nauka Publishing House, 216 pp (in Russian).
MYERS, V.C. & BEARD, H.H. (1931) Studies in the nutritional
anaemia of the rat. II. Influence of iron plus supplements of
other inorganic elements upon blood regeneration. J. biol.
Chem ., 94: 89.
MYRON, D.R., GIVAND, S.H., & NIELSEN, F.H. (1977) Vanadium
content of selected foods as determined by flameless atomic
absorption spectroscopy. J. agric. food Chem ., 25(2): 297-299.
MYRON, D.R., ZIMMERMAN, T.J., SHULER, T.R., KLEVAY, L.M., LEE,
D.E., & NIELSEN, F.H. (1978) Intake of nickel and vanadium by
humans. A survey of selected diets. Am. J. clin. Nutr ., 31(3):
527-531.
NAS (1974) Vanadium , Washington DC, National Academy of
Sciences.
NAS (1978) Vanadium supply and demand outlook , Washington DC,
National Academy of Sciences, National Materials Advisory Board
(Publication NMAB-346).
NASON, A. (1958) The metabolic role of vanadium and molybdenum
in plants and animals. In: Lamb, C.A., Bentley, 0.G., & Beattie,
J.M., ed. Trace elements , New York, Academic Press, pp. 269-
296.
NAYLOR, G.J. (1984) Vanadium and manic depressive psychosis.
Nutr. Health , 3(1-2): 79-85.
NAYLOR, G.J., SMITH, A.H., BRYCE-SMITH, D., & WARD, N.I. (1984)
Tissue vanadium levels in manic-depressive psychosis. Psychol.
Med ., 14(4): 767-772.
NECHAY, R. (1984) Mechanisms of action of vanadate. Ann. Rev.
Pharmacol. Toxicol ., 24: 501-524.
NECHAY, R. & SAUNDERS, J.P. (1978) Inhibition by vanadium of
sodium and potassium dependent adenosinetriphosphatase derived
from animal and human tissues. J. environ. Pathol. Toxicol ., 2:
247-262.
NELSON, W.L. (1973) Direct desulfurization handles low-metals
topped crudes. Oil Gas J ., 19 November: 54-56.
NIELSEN, F.H. & OLLERICH, D.A. (1973) Studies on a vanadium
deficiency in chicks. Fed. Proc ., 32: 929.
NIOSH (1977) Occupational exposure to vanadium , Washington DC,
National Institute for Occupational Safety and Health (Document
No. 77-222), 142 pp.
NISHIYAMA, K., ONISHI, K., MIYAI, T., SUGIURA, S., SAITO, K., &
TOJYO, Y. (1977) [A survey of workers on vanadium pentoxide.]
Shikoku igaku zasshi , 31(6): 389-393 (in Japanese).
NOVAKOVA, S., NIKOLCHEV, G., ANGELIEVA, R., DINOEVA, S., &
MAUTNER, G. (1981) [Effect of vanadium on experimental
atherosclerosis.] Gig. i Sanit ., 11: 58-59 (in Russian).
NRC (1980) Drinking-water and health , Washington DC, National
Academy Press, Vol. 3, pp. 350-354 (Safe Drinking-Water
Committee).
OBERG, S.G., PARKER, R.D.R., & SHARMA, R.P. (1978)
Distribution and elimination of an intratracheally administered
vanadium compound in the rat. Toxicology , 11: 315-323.
OLEFFE, J. & WILMET, J. (1980) Generalized dermatitis from an
osteosynthesis screw. Contact Dermat ., 6: 365.
OLIVER, B.G. & COSGROVE, E.G. (1975) Metal concentrations in
the sewage, effluents and sludges of some Southern Ontario
wastewater treatment plants. Environ. Lett ., 9(1): 75-90.
OMANG, S.H. (1971) The determination of vanadium and nickel in
mineral oils by flameless graphite tube atomization. Anal. Chim.
Acta , 56: 470-473.
ORDHZONIKIDZE, E.K., ROSCHIN, A.V., SHALGANOVA, I.V., BOGOMAZOV,
M.YA., & KAZIMOV, M.A. (1977) [On the distribution and
elimination of vanadium from the organisms.] Gig. Tr. prof.
Zabol ., 6: 29-34 (in Russian).
ORVINI, E., LODOLA, L., SABBIONI, E., PIETRA, R., & GOETZ, L.
(1979) Determination of the chemical forms of dissolved
vanadium in freshwater as determined by 48V radiotracer experi-
ments and neutron activation analysis. Sci. total Environ ., 13:
195-207.
PARKER, R.D.R. & SHARMA, R.P. (1978) Accumulation and
depletion of vanadium in selected tissues of rats treated with
vanadyl sulfate and sodium orthovanadate. J. environ. Pathol.
Toxicol ., 2: 235-245.
PARKES, W.R. (1982) Occupational lung disorders , 2nd ed.,
London, Butterworths & Co.
PASTUHOV, A.I. & TRETJAKOV, M.A. (1959) Metallurgical
processing of cast iron from titanomagnetites from the Kackanar
district into steel. In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 95-104 (in
Russian).
PATRICK, R. (1978) Effects of trace metals in the aquatic
ecosystem. Am. Sci ., 66: 185-191.
PAZHYNICH, V.M. (1966) Maximum permissible concentration of
vanadium pentoxide in the atmosphere. Hyg. Sanit ., 31: 6-11.
PAZHYNICH, V.M. (1967) [Experimental basis for the determina-
tion of maximum allowable concentration of vanadium pentoxide in
atmospheric air.] In: Rjazanov, V.A., ed. [ The biological
effect and hygienic importance of atmospheric pollutants ,]
Moscow, Medicina Publishing House, pp. 201-217 (in Russian).
PAZHYNICH, V.M. (1980) [Effect of aerosols of rare metals on
the functional state of the body of workers in conditions of
powder wire production.] Vrach. Delo , 3: 102-104 (in Russian).
PEJVE, J.V. & AJZUPIET, I.P. (1974) [A short review of the
results of research on the problem "Trace elements in plant
cultivation and livestock rearing in 1972".] In: [ Trace
elements in the USSR. XV. Methodological material ,] Riga,
Knowledge Publishers (in Russian).
PERRY, H.M., TEITLEBAUM, S., & SCHWARTZ, P.L. (1955) Effects
of antihypertensive agents on amino acid decarboxylation and
amine oxidation. Fed. Proc ., 14: 113-114.
PERRY, H.M., Jr, SCHWARTZ, P.L., & SAHAGIAN, B.M. (1969)
Effects of transition metals and of metal-binding antihyper-
tensive agents on tryptamine oxidase sand dopa decarboxylase.
Proc. Soc. Exp. Biol. Med ., 130: 273-277.
PETERBURGSKIJ, A.V. & TORMASOVA, E.E. (1969) [The effect of
vanadium on certain physiologycal processes in the pea.] Dok.
Timiryazevsk. Akad ., 149: (in Russian).
PETKEVICH, A.N., VILLER, G.E., & VOROTNIKOV, B.A. (1967)
[Some trace elements in natural waters of Kama's district.] In:
[ Trace elements in the biosphere and their application in
agriculture and medicine in Siberia and the Far East ,] Ulan-
Ude, Ulan-Ude Book Publishers, pp. 223-227 (in Russian).
PETUHOV, N.I., STANKEVICH, E.F., & VALACHANOVICH, V.I. (1969)
Some microelements in natural waters near the source of the
Kama. Tr. Kazanskogo Med. Inst ., 29: 100-103.
PHILLIPS, T.D., NECHAY, B.R., NELDON, S.L., KUBENA, L.F.,
HEIDELBAUGH, N.D., SHEPHERD, E.C., STEIN, A.F., & HAYES, A.W.
(1982) Vanadium-induced inhibition of renal Na+ K+-
adenosinetriphosphatase in the chicken after chronic dietary
exposure. J. Toxicol. environ. Health , 9: 651-661.
PHILLIPS, T.D., NECHAY, B.R., & HEIDELBAUGH, N.D. (1983)
Vanadium: chemistry and the kidney. Fed. Proc ., 42(13): 2969-
2973.
PIELSTICKER, F. (1954) [Health effects due to vanadium
compounds. Symptoms and prognosis.] Arch. Gewerbepathol.
Gewerbehyg ., 13: 73-96 (in German).
PILZ, W. & KOMISCHKE, S. (1972) [Determination of vanadium in
biological material and air. II. The decomposition of biological
material: air analyses.] Int. J. environ. anal. Chem ., 1(4):
275-282 (in German).
POERSEL, B. (1977) [ The biological and toxicological
significance of vanadium ,] Dusseldorf, Med. Diss., 86 pp (in
German).
PROESCHER, F., SEIL, H.H., & STILLIANS, A.W. (1917) A
contribution to the action of vanadium with particular reference
to syphilis. Am. J. Syph ., 1: 347-405.
PROKOPENKO, T.A. (1961) [Effect of industrial poisons,
fluorine, vanadium, manganese, and some drugs tested in
treatment on the tissue metabolism of compounds containing
phosphorus.] In: [ Proceedings of the 9th All-Union Pharmaco-
logical Conference, Sverdlovsk ,] Sverdlovsk, Central Bureau of
Technical Information, pp. 210-211 (in Russian).
PYY, L., KIVILUOTO, M., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health ,
46(2): 179-182.
PYY, L., LAJUNEN, L.H.J., & HAKALA, E. (1983) Determination of
vanadium in work-place air by DCP emission spectrometry. Am.
Ind. Hyg. Assoc. J ., 44(8): 609-614.
PYY, L., HAKALA, E., & LAJUNEN, L.H.J. (1984) Screening for
vanadium in urine- and blood-serum by electrothermal atomic
absorption spectrometry and dc plasma atomic emission
spectrometry. Anal. Chim. Acta , 158: 297-303.
RAESAENEN, O., KIVILUOTO, M., RINNE, A., & RISSANEN, M. (1979)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory: a macroscopic and microscopic study. Scand.
J. Work environ. Health , 5(1): 50-58.
RAJNER, V. (1960) [Effect of vanadium in the respiratory
tract.] Cesk. Otolaryngol ., 9: 202-204 (in Czech).
RALSTON, H.R. & SATO, E.S. (1971) Sodium removal as an aid to
neutron activation analysis. Anal. Chem ., 43: 129-131.
REINL, W. (1958) [Illnesses caused by basic slag, vanadium
slag, and vanadium compounds.] Staub , 18(5): 143-148 (in
German).
REZNIK, J.B. (1954) [The importance of vanadium compounds from
the point of view of industrial toxicology.] Vrach. Delo , 7:
627-632 (in Russian).
RHAN, K.A. (1971) Sources of trace elements in aerosols: an
approach to clean air , Ann Arbor, Michigan, University of
Michigan, Department of Meteorology, 309 pp (US Atomic Energy
Commission Contract No. COO-1705-9).
RHOADS, K. & SANDERS, C.L. (1985) Lung clearance, trans-
location, and acute toxicity of arsenic, beryllium, cadmium,
cobalt, lead, selenium, vanadium, and ytterbium oxides following
deposition in rat lung. Environ. Res ., 36(2): 359-378.
RIFKIN, R.J. (1965) In vitro inhibition of Na+ -K+ and Mg2+
ATPases by mono-, di-, and trivalent cations. Proc. Soc. Exp.
Biol. Med ., 120: 802-804.
RIGDON, L.P. & HARRAR, J.E. (1969) Determination of vanadium
by controlled-potential coulometry. Anal. Chem ., 41: 1673-1675.
ROITMAN, I., TRAVASSAS, L.R., AZEVEDO, H.P., & CURRY, A. (1969)
Choline, trace elements, and amino acids as factors for growth
of an enteric yeast, Candida slooffii. Sabouraudia J. Int. Soc.
Hum. Anim. Mycol ., 7: 15-19.
ROMAN, R.J., BONVENTRE, J.V., SILVA, P., & LECHENE, C. (1981)
Sodium orthovanadate diuresis in rats. J. Pharmacol. exp. Ther .,
218: 168-174.
RONOV, A.B. (1964) [General tendencies in the evolution of the
composition of the Earth's crust, the oceans and the
atmosphere.] Geohimija , 8: 715-743 (in Russian).
ROSE, E.R. (1973) Geology of vanadium and vanadiferous
occurrences in Canada , Ottawa, Geological Survey of Canada,
Department of Energy, Mines and Resources, pp. 7-101 (Economic
Geology Report No. 27).
ROSHCHIN, A.V. (1962) [The hazards of occupational vanadium
intoxication in boiler cleaning operations at electric power
stations operating on fuel oil, and prevention problems.] Gig.
Tr. prof. Zabol ., 6: 17-22 (in Russian).
ROSHCHIN, A.V. (1963a) [Biological effects of rare, dispersed,
and other metals and their compounds used in industry:
vanadium.] In: [ Toxicology of the less common metals ,] Moscow,
Meditsina, pp. 83-95 (in Russian).
ROSHCHIN, A.V. (1963b) [Hygienic evaluation of dust from
vanadium-containing slag.] Gig. i Sanit ., 12: 23-29 (in
Russian).
ROSHCHIN, A.V. (1964) [Vanadium metallurgy in the light of
industrial hygiene and questions concerned with the prevention
of occupational diseases and intoxication.] Gig. Tr. prof.
Zabol ., 8: 3-10 (in Russian).
ROSHCHIN, A.V. (1967a) Toxicology of vanadium compounds used
in modern industry. Hyg. Sanit ., 32: 345-352.
ROSHCHIN, A.V. (1967b) Vanadium. In: Izraelson, Z.I., ed.
Toxicology of the rare metals , Jerusalem, Israel Program for
Scientific Translations Ltd., pp. 52-59 (AEC-tr-6710).
ROSHCHIN, A.V. (1968) [ Vanadium and its compounds ,] Moscow,
Medicina Publishing House (in Russian).
ROSHCHIN, A.V. & ORDZHONIKIDZE, E.K. (1986) [Metal toxico-
kinetics and its significance for prevention of occupational
poisoning.] Gig. Tr. prof. Zabol ., 3: 1-6 (in Russian).
ROSHCHIN, A.V., ILNITSKAYA, A.V., LUTSENKO, L.A., & ZHIDKOVA,
L.A. (1964) [Research into effects of vanadium trioxide dust
on organisms.] Gig. Tr. prof. Zabol ., 8: 25-30 (in Russian).
ROSHCHIN, A.V., ORDZHONIKIDZE, E.K., & SHALGANOVA, I.V. (1980)
Vanadium - toxicity, metabolism, carrier state. J. Hyg.
Epidemiol. Microbiol. Immunol ., 24(4): 377-383.
RUHLING, A. (1971) [Pollution with heavy metals in the greater
Stockholm area.] In: [ Report No. 12 on ecological heavy metal
research ,] Lund, University of Lund, Department of Plant
Ecology (in Swedish).
RUNDBERG, G. (1939) Modern problems of occupational diseases
in Sweden. Nord. Med ., 2: 1845-1856.
SABBIONI, E., MARAFANTE, E., AMANTINI, L., & UBERTALLI, L.
(1978) Similarity in metabolic patterns of different chemical
species of vanadium in the rat. Bioinorg. Chem ., 8: 503-515.
SABBIONI, E., MARAFANTE, E., PIETRA, R., GOETZ, L., GIRARDI, F.,
& ORVINI, E. (1979) The association of vanadium with the iron
transport system in human blood as determined by gel filtration
and neutron activation analysis. In: Proceedings of a Symposium
on Nuclear Activation Techniques in the Life Sciences, Vienna,
22-26 May, 1978 , Vienna, International Atomic Energy Agency, pp.
179-192.
SABBIONI, E., MARAFANTE, E., RADE, J., GREGOTTI, C., DI NUCCI,
A., & MANZO, L. (1981) Biliary excretion of vanadium in rats.
Toxicol. Eur. Res ., 3(2): 93-98.
SANDELL, E., ed. (1959) Vanadium. In: Colorimetric determina-
tion of traces of metals , 3rd ed., New York, Interscience
Publishers, Vol. 3, pp. 923-940 (Chemical Analysis Series).
SCHLETTWEIN-GZELL, D. & MOMMSEN-STRAUB, S. (1973) [Trace
elements in foodstuffs. XI. Vanadium.] Int. Z. Vitam.-
Ernährungsforsch ., 43(2): 242-250 (in German).
SCHROEDER, H.A. (1966) Municipal drinking-water and cardio-
vascular death rates. J. Am. Med. Assoc ., 195: 81-85.
SCHROEDER, H.A. (197Oa) Vanadium , Washington DC, American
Petroleum Institute (Air Quality Monograph No. 70-13).
SCHROEDER, H.A. (197Ob) A sensible look at air pollution by
metals. Arch. environ. Health , 21: 798-806.
SCHROEDER, H.A. & MITCHENER, M. (1975) Life-term effects of
mercury, methylmercury, and nine other trace metals on mice. J.
Nutr ., 105(4): 452-458.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1963) Abnormal
trace metals in man: vanadium. J. chron. Dis ., 16: 1047-1071.
SCHROEDER, H.A., MITCHENER, M., & NASON, A.P. (1970) Zirconium,
niobium, antimony, vanadium, and lead in rats: life-term
studies. J. Nutr ., 100(1): 59-68.
SCHUMANN-VOGT, B. (1969) [Health hazards in industry caused by
vanadium.] Zentralbl. Arbeitsmed. Arbeitsschutz , 19(2): 33-39
(in German).
SCHWARZ, K. & MILNE, D.B. (1971) Growth effects of vanadium in
the rat. Science , 174: 426-428.
SELJANKINA, K.P. (1961) [Data for determining the maximum
permissible content of vanadium in water basins.] Gig. i Sanit .,
26(10): 6-12 (in Russian).
SHAH, K.R., FILBY, R.H., & HALLER, W.A. (1970) Determination
of trace elements in petroleum by neutron activation analysis.
1. Determination of Na, S, Li, La, V, Mn, Cu, Ga, and Br. J.
radioanal. Chem ., 6: 185-192.
SHARMA, R.P., BOURCIER, D.R., BRINKERHOFF, C.R., & CHRISTENSEN,
S.A. (1981) Effects of vanadium on immunologic functions. Am.
J. ind. Med ., 2(2): 91-99.
SHARMA, R.P., COULOMBE, R.A., Jr, & SRISUCHART, B. (1986)
Effects of dietary vanadium exposure on levels of regional brain
neurotransmitters and their metabolites. Biochem. Pharmacol .,
35(3): 461-465.
SHEVCHENKO, I.T. & GORODYNSKYJ, V.I. (1964) [ Polarography in
medicine and biology ,] Kiev, State Medical Publishers of the
Ukraine (in Russian).
SHEVCHENKO, S.D. (1965) [Content of vanadium in bone tumours.]
Ortop. Travmatol. Protez ., 8: 83 (in Russian).
SHILINA, A.I. & MALAKHOV, S.G. (1974) [Trace elements in
natural waters and the atmosphere.] In: [ A collection of papers
on experimental meteorology ,] Moscow, Gidrometeozdat (in
Russian).
SHKOLENOK, G.F., NIKOLAEVA, E.R., & AGASYAN, P.K. (1977)
[Differential controlled-potential coulometry. Determination of
vanadium(V) and vanadium(IV).] Zavod. Lab ., 43(9): 1048-1050 (in
Russian).
SIMONOFF, M., CONRI, C., & SIMONOFF, G. (1986) Vanadium in
depressive states. Acta pharmacol. toxicol . (Copenhagen) ,
59(Suppl. 7): 463-466.
SINGH, D. & SHARMA, S. (1970) Amperometric permanganometric
estimations at low concentrations in stirred solutions. Indian
J. Chem ., 8(2): 192.
SINGH, J., NORDLIE, R.C., & JORGENSON, R.A. (1981) Vanadate:
a potent inhibitor of multifunctional glucose-6-phosphatase.
Biochim. Biophys . Acta , 678: 477-482.
SJÖBERG, S.-G. (1950) Vanadium pentoxide dust: A clinical and
experimental investigation on its effect after inhalation. Acta
med. Scand ., 138(Suppl. 238): 1-188.
SJÖBERG, S.-G. (1951) Possible importance of allergy in
diseases caused by inhalation of inorganic dust. Acta allergol .,
4(4): 357-361.
SJÖBERG, S.-G. (1955) Vanadium bronchitis from cleaning oil-
fired boilers. Arch. ind. Health , 11(6): 505-512.
SJÖBERG, S.-G. (1956) Vanadium dust, chronic bronchitis and
possible risk of emphysema. A follow-up investigation of workers
at a vanadium factory. Acta med. Scand ., 44(5): 381-386.
SJÖBERG, S.-G. & RIGNER, K.-G. (1956) [Skin, eye, and
respiratory tract symptoms associated with the cleaning of oil-
fired boilers.] Nord. Hyg. Tidskr ., 37: 217-228 (in Swedish).
SLAVIN, W., CARNRICK, G.R., MANNING, D.C., & PRUSZKOWSKA, E.
(1983) Recent experiences with the stabilized temperature
platform furnace and zeeman background correction. At.
Spectrosci ., 4(3): 69-86.
SMITH, J.B. (1983) Vanadium ions stimulate DNA synthesis in
Swiss mouse 3T3 and 3T6 cells. Proc. Natl Acad. Sci. (USA) ,
80(20): 6162-6166.
SNYDER, F. & CORNATZER, W.E. (1958) Vanadium inhibition of
phospholipid sulphydryl activity in rat liver. Nature (Lond.) ,
182: 462.
SOKOLOV, S.M. (1986) Metholodological aspects of assessing
atmospheric contamination with metal aerosols in the vicinity of
thermal power complexes. J. Hyg. Epidemiol. Microbiol.
Immunol ., 30(3): 249-254.
SOMERVILLE, J. & DAVIES, B. (1962) Effect of vanadium on serum
cholesterol. Am. Heart J ., 64: 54-56.
SÖREMARK, R. (1967) Vanadium in some biological specimens. J.
Nutr ., 92: 183-190.
SÖREMARK, R. & ULLBERG, S. (1962) Distribution and kinetics of
48V2O5 in mice. In: Friend, N., ed. Proceedings of a Symposium
on the Use of Radioisotopes in Animal Biology and the Medical
Sciences, Mexico City, 21 November-1 December, 1961 , New York,
Academic Press, Vol. 2, pp. 103-114.
SÖREMARK, R., ULLBERG, S., & APPELGREN, L.E. (1962) Auto-
radiographic localization of vanadium pentoxide (V24805) in
developing teeth and bones of rats. Acta odontol. Scand ., 20:
225-232.
SPRAGUE, J.B., HOLDWAY, D.A., & STENDAHL, D.H. (1978) Acute
and chronic toxicity of vanadium to fish , Edmonton, Environment
Alberta, 92 pp (Prepared for the Alberta Oil Sands Research
Program (Project No. AF 3.5.1).
STEFFEN, R.P., PAMNANI, M.B., CLOUGH, D.L., HUOT, S.J., MULDOON,
S.M., & HADDY, F.J. (1981) Effect of prolonged dietary
administration of vanadate on blood pressure in the rat.
Hypertension , 3: I/173-I/178.
STENDAHL, D.H. & SPRAGUE, J.B. (1982) Effects of water
hardness and pH on vanadium lethality to rainbow trout. Water
Res ., 16: 1479-1488.
STOCKS, P. (1960) On the relations between atmospheric
pollution in urban and rural localitites and mortality from
cancer, bronchitis and pneumonia, with particular reference to
3:4 benzopyrene, beryllium, molybdenum, vanadium, and arsenic.
Br. J. Cancer , 14: 397-418.
STOKINGER, H.E., WAGNER, W.D., MOUNTAIN, J.T., STOCKELL, F.R.,
DOBROGORSKI, O.J., & KEENAN, R.G. (1967) In Vanadium V. In:
Clayton, G.D. & Clayton, F.E., eds. Patty's Industrial hygiene
and toxicology , 3rd revised ed. (1981), New York, Interscience,
Vol. 2A, pp. 2013-2033.
STONER, G.D., SHIMKIN, M.B., TROXELL, M.C., THOMPSON, T.L., &
TERRY, L.S. (1976) Test for carcinogenicity of metallic
compounds by the pulmonary tumor response in strain a mice.
Cancer Res ., 36: 1744-1747.
STRAHOV, N.F. (1947) [Iron-ore facies and their analogues in
the history of the earth.] Tr. Inst. Geol. AN SSSR, geol. Ser .,
73: 22 (in Russian).
STRAHOV, N.M. (1968) [On the theory of geochemical process in
humid zones.] In: [ A collection of papers on the geochemistry
of sedimentary rocks and ores ,] Moscow, Na++uka Publishing House,
pp. 102-134 (Documentation of the Seventh All-Union Conference
on Lithology of the Academy of Sciences of the USSR) (in
Russian).
STRASIA, C.A. (1971) Vanadium: essentiality and toxicity in
the laboratory rat , Ann Arbor, Michigan Purdue University (Ph.D.
Thesis).
STROOP, S.D., HELINEK, G., & GREENE, H.L. (1982) More
sensitive flameless atomic absorption analysis of vanadium in
tissue and serum. Clin. Chem ., 28(1): 79-82.
STYRIS, D.L. & KAYE, J.H. (1982) Mechanisms of vaporization of
vanadium pentaoxide from vitreous carbon and tantalum furnaces
by combined atomic absorption/mass spectrometry. Anal. Chem .,
54: 864-869.
SUGAWARA, K., NAITO, H., & YAMADA, S. (1956) Geochemistry of
vanadium in natural waters. J. earth Sci. Nagoya Univ ., 4(1):
44-61.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975)
Heavy metals in normal Japanese tissues. Arch. environ. Health ,
30: 487-494.
SUN, MIANLING, ed. (1987) Toxicity of vanadium and its
environmental health standard , Chengdu, West China University of
Medical Sciences, 20 pp.
SVERDRUP, H.U., JOHNSON, M.W., & FLEMING, R.H. (1950) The
oceans, their physics, chemistry and general biology , Englewood
Cliffs, New Jersey, Prentice-Hall Inc., pp. 175-177.
SVOBODA, P., TEISINGER, J., PILAR, J., & VYSKOCHIL, F. (1984)
Vanadyl (VO2+) and vanadate (VO3-) ions inhibit the brain
microsomal Na, K-AtPase with similar affinities. Protection by
transferrin and noradrenaline. Biochem. Pharmacol ., 33: 2485-
2491.
SWARUP, G., COHEN, S., & GARBERS, D.L. (1982) Inhibition of
membrane phosphotyrosyl-protein phosphatase activity by
vanadate. Biochem. biophys. Res. Commun ., 107: 1104-1109.
SYMANSKI, J. (1939) [Occupational vanadium poisoning, its
occurrence and symptomatology.] Arch. Gewerbepathol.
Gewerbehyg ., 9: 295-313 (in German).
TAKAHASHI, H. & NASON, A. (1957) Tungstate as a competitive
inhibitor of molybdate in nitrate assimilation and N2 fixation
by azotobacter. Biochim. Biophys. Acta , 23: 433-435.
TALVITIE, N.A. & WAGNER, W.D. (1954) Studies in vanadium
toxicology. II. Distribution and excretion of vanadium in
animals. Arch. ind. Hyg ., 9: 414-422.
TANDON, A.G. & BHATTACHARYA, S.C. (1961) Use of N-2-thio-
phenecarbonyl- N-p-tolylhydroxylamine and N-2-thiophene-
carbonyl- N-phenylhydroxylamine as reagents for vanadium. Anal.
Chem ., 33(9): 1267.
TARA, S., CAVIGNEAUX, A., & DEPLACE, Y. (1953) Intoxication
par le vanadate de calcium. Arch. Mal. prof. Méd. Trav. Séc.
soc ., 14: 378-380.
TEBROCK, H.E. & MACHLE, W. (1968) Exposure to europium-
activated yttrium orthovanadate: a cathodoluminescent phosphor.
J. occup. Med ., 10(12): 692-696.
THOMAS, D.L.G. & STIEBRIS, K. (1956) Vanadium poisoning in
industry. Med. J. Aust ., 1: 607-609.
THOMPSON, H.J., CHASTEEN, N.D., & MEEKER, L.D. (1984) Dietary
vanadyl (IV) sulfate inhibits chemically-induced mammary
carcinogenesis. Carcinogenicity , 5: 849-851.
THURAUF, J., SYGA, G., & SCHALLER, K.H. (1979) Field tests
carried out to determine the occupational exposure to vanadium.
Zbl. Bakteriol. Hyg., I. Abt. Orig. B , 168: 273-290.
TIPTON, I.H. & COOK, M.J. (1963) Trace elements in human
tissue. II. Adult subjects from the United States. Health Phys .,
9: 103-145.
TIPTON, I.H. & SHAFER, J.J. (1964) Statistical analysis of
lung trace element levels. Arch. environ. Health , 8: 58-67.
TIPTON, I.H., STEWART, P.L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys .,
16: 455-462.
TJURJUKANOV, A.N. (1963) Vanadium. In: Trace elements in
agriculture , Kiev, Gosselhozizdat, pp. 472-475.
TODRIA, F.F. (1963) [Identification of vanadium in ashes of
Tkibul coals.] In: [ Abstracts of scientific papers of Tbilisi
Medical Institute ,] Tbilisi, Tbilisi State Medical Institute,
p. 144 (in Russian).
TOLMAN, E.L., BARRIS, E., BURNS, M., PANSINI, A., & PARTRIDGE,
R. (1979) Effects of vanadium on glucose metabolism in vitro .
Life Sci ., 25: 1159-1164.
TONEY, J.H., MURTHY, M.S., & MARKS, T.J. (1985) Biodistribu-
tion and pharmacokinetics of vanadium following intraperitoneal
administration of vanadocene dichloride to mice. Chem.-biol.
Interact ., 56(1): 45-54.
TRELLES, R.A., LARGHI, L.A., & PAEZ, L.J.P. (1970) [The health
problem of human drinking-water with high contents of arsenic,
vanadium, and fluorine.] Saneamiento , 34(217): 31-80 (in
Spanish).
TROPPENS, H. (1969) [The uncertainties of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 24: 1089-1092 (in
German).
TRUMMERT, W. & BOEHM, G. (1957) [The trace element vanadium
and its haemopoietic action.] Blut , 3: 210-216 (in German).
TYLER, G. (1970) [Pollution with heavy metals in the
Norrkkoping area. II. Molybdenum and vanadium.] In: [ Report No.
12 on ecological heavy metal research ,] Lund, University of
Lund, Department of Plant Ecology (in Swedish).
UENO, S. & ISHIZAKI, M. (1980) Concentrations of vanadium in
human urine and hair. Jpn. J. ind. Health , 22: 318-379.
UNDERWOOD, E.J. (1977) Vanadium. In: Trace elements in human
and animal nutrition , 4th ed., New York, Academic Press,
pp. 388-397.
UNSAL, M. (1978) Study of the transfer pathways and the
accumulation phenomena of vanadium in mollusca Mytilus edulis.
Rev. int. oceanogr. Med ., 51/52: 71-81.
US BUREAU OF MINES (1974) Metals Information Bulletin. Washing-
ton DC, US Department of the Interior, Bureau of Mines, Branch
of Ferrous Metals.
US EPA (1977) Scientific and technical assessment report on
vanadium , Washington DC, US Environmental Protection Agency
(STAR series, EPA-600/6-77-002).
USMONOV, S.M. (1973) [Vanadium content in constituents of
bone, urine, and faeces in sufferers of acute and chronic
dysentery.] In: [ Epidemiological, microbiological, and clinical
problems in the infectious pathology of Uzbekistan ,] Tashkent,
Institute of Hygiene, Sanitation, and Occupational Diseases,
pp. 125-128 (in Russian).
USUTANI, S., NISHIAMA, K., SATO, I., MATSURA, K., SAWADA, Y.,
HOSOKAWA, Y., & IZUMI, S. (1979) [An investigation of the
environment in a certain vanadium refinery.] Jpn. J. ind.
Health , 21: 11-20 (in Japanese).
VAN DEN BERG, C.M.G. & HUANG ZI QIANG (1984) Direct electro-
chemical determination of dissolved vanadium in seawater by
cathodic stripping voltammetry with the hanging mercury drop
electrode. Anal. Chem ., 56(13): 2383-2386.
VANSELOW, A.P. (1950) Unpublished data cited by Pratt, P.F.
In: Diagnostic criteria for plants and soils, University of
California, Division of Agricultural Sciences , University of
California, pp. 480-482.
VAN ZINDEREN BAKKER, E.M. & JAWORSKI, J.F. (1980) Effects of
vanadium in the Canadian environment , Ottawa, National Research
Council of Canada (Publication No. 18132).
VERSIECK, J. & CORNELIS, R. (1980) Normal levels of trace
elements in human blood-plasma or -serum. Anal. Chim. Acta , 116:
217-254.
VEYS, C. (1983) Possibilités offertes par les méthodes
modernes de polarographie au dosage à éléments tracés. Ind.
Sci ., 59(3): 1-6.
VIGLIANI, E.C. (1969) WHO Technical Report Series No. 415:
Permissible levels of occupational exposure to airborne toxic
substances (Sixth Report of the Joint ILO/WHO Committee on
Occupational Health) , Geneva, World Health Organization, pp. 5-
16.
VlNOGRADOV, A.P. (1937) [The chemical elements entering into
the composition of marine organisms. Part II.] Tr. biogeokhim.
Lab ., 4: 217 (in Russian).
VINOGRADOV A.P. (1944) [The geochemistry of trace elements in
seawater.] Uspehi Himii , 13(1): 123-129 (in Russian).
VINOGRADOV, A.P. (1957) [ Geochemistry of rare and dispersed
chemical elements in soils ,] 2nd ed (revised), Moscow, USSR
Academy of Sciences, pp. 122-129, 216-217 (in Russian).
VINOGRADOV, A.P. (1959) [ The geochemistry of rare and
dispersed elements in soil ,] 2nd ed., New York, Consultants
Bureau Inc., 209 pp (in Russian).
VINTINNER, F.J., VALLENAS, R., CARLIN, C.E., WEISS, R., MACHER,
C., & OCHOA, R. (1955) Study of the health of workers employed
in mining and processing of vanadium ore. Arch. ind. Health ,
12: 635.
VOORS, A.W. (1971) Minerals in the municipal water and
atherosclerotic heart death. Am. J. Epidemiol ., 93: 259-266.
VOUK, V. (1979) Vanadium. In: Friberg, L., Nordberg, G.F., &
Vouk, V., ed. Handbook on the toxicology of metals , Amsterdam,
New York, Oxford, Elsevier Science Publishers, pp. 659-674.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1980) A specific
enzyme is not necessary for vanadate-induced oxidation of NADH.
Nature (Lond.) , 286(5772): 516-517.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1981) The disparity
between effects of vanadate (V) and vanadyl (IV) ions on (Na+ -
K+) AtPase and K+-phosphatase in skeletal muscle. Biochem.
biophys. Res. Commun ., 100: 982-987.
WALDRON, H.A. (1979) Metal poisoning: the three common causes.
Mod. Med ., 24(9): 45-47.
WALLACE, A., ALEXANDER, G.V., & CHADHURY, F.M. (1977) Phyto-
toxicity of cobalt, vanadium, titanium, silver, and chromium.
Commun. soil. Sci. plant Anal ., 8(9): 751-756.
WARINGTON, K. (1955) The influence of iron supply on toxic
effects of manganese, molybdenum and vanadium in soybeans, peas,
and flax. Ann. appl. Biol ., 41: 1-22.
WATANABE, H., MURAYAMA, H., & YAMAOKA, S. (1966) [Some
clinical findings in vanadium workers.] Jpn. J. ind. Health ,
8(7): 23-27 (in Japanese).
WATERS, M.D. (1977) Toxicology of vanadium. Adv. mod.
Toxicol ., 2: 147-189.
WATERS, M.D., GARDNER, D.E., & COFFIN. D.L. (1974) Cytotoxic
effects of vanadium on rabbit alvolar macrophages in vitro .
Toxicol. appl. Pharmacol ., 28: 253-263.
WATERS, M.D., GARDNER, D.E., ARANYI, C., & COFFIN, D.L. (1975)
Metal toxicity for rabbit alveolar macrophages in vitro.
Environ. Res ., 9: 32-47.
WEAST, R.C. (1987) CRC handbook of chemistry and physics , 67th
ed., Boca Raton, Florida, CRC Press.
WEI, C.I. & MISRA, H.P. (1982) Cytotoxicity of ammonium meta-
vanadate to cultured bovine alveolar macrophage. J. Toxicol.
environ. Health , 9(5-6): 995-1006.
WEI, C.I., AL BAYATI, M.A., CULBERTSON, M.R., ROSENBLATT, L.S.,
& HANSEN, L.D. (1982) Acute toxicity of ammonium metavanadate
in mice. J. Toxicol. environ. Health , 10(4/5): 673-687.
WEISZ, H., ROTHMAIER, K., & LUDWIG, H. (1974) Kinetic
determination of thorium, vanadium, and iodide with the use of a
potentiometer. Anal. Chim. Acta , 73(1): 224-228.
WELCH, R.M. (1973) Vanadium uptake by plants. Absorption
kinetics and the effects of pH, metabolic inhibitors, and other
anions and cations. Plant Physiol ., 51: 828-832.
WELCH, R.M. & ALLAWAY, W.H. (1972) Vanadium determination in
biological materials at nanogram levels by a catalytic method.
Anal. Chem ., 44(9): 1644-1647.
WENTZEL, C.T. (1985) Vanadium: a moderate recovery in 1984.
Eng. mining J ., March: 87-88.
WESTENFELDER, C., HAMBURGER, R.K., & GARCIA, M.E. (1981)
Effect of vanadate on renal tubular function in rats. Am. J.
Physiol ., 240: F522-F529.
WIDE, M. (1984) Effect of short-term exposure to five
industrial metals on the embryonic and fetal development of the
mouse. Environ. Res ., 33: 47-53.
WILLIAMS, D.L. (1973) Biological value of vanadium for rats,
chickens, and sheep , Ann Arbor, Michigan, Purdue University
(Ph.D. Thesis).
WILLIAMS, N. (1952) Vanadium poisoning from cleaning oil-fired
boilers. Br. J. ind. Med ., 9: 50-55.
WITKOWSKA, D. & BRZEZINSKI, J. (1979) Alteration of brain
noradrenaline, dopamine, and 5-hydroxytryptamine levels during
vanadium poisoning. Pol. J. Pharmacol. Pharm ., 31: 393-398.
WOOD, B.J., GALOBARDES, J.F., & ROGERS, L.B. (1982) Effect of
the organic ligand on the response for vanadium in flame atomic
emission spectrometry. Anal. Chim. Acta , 135: 145-152.
WRIGHT, L.D., LI, L.F., & TRAGER, R. (1960) The site of
vanadyl inhibition of chloesterol biosynthesis in liver
homogenates. Biochem. biophys. Res. Commun ., 3: 264-267.
WYERS, H. (1946) Some toxic effects of vanadium pentoxide.
Br. J. ind. Med ., 3: 177-182.
WYERS, H. (1948) Some recent observations on hazards in the
chemical industry. In: Proceedings of the 9th International
Congress of Industrial Medicine, London, 13-17 September ,
Bristol, John Wright, pp. 900-903.
YUKAWA, M., SUZUKI-YASUMOTO, M., AMANO, K., & TERAI, M. (1980)
Distribution of trace elements in the human body determined by
neutron activation analysis. Arch. environ. Health , 35(1): 36-
44.
ZAJIC, J.E. (1969) Microbial biochemistry. 12. Vanadium
biogeochemistry , New York, Academic Press, pp. 126-202.
ZEMKOVA, H., TEISINGER, J., & VYSKOCIL, F. (1982) The
comparison of vanadyl (IV) and insulin-induced hyperpolarization
of the mammalian muscle cell. Biochim. Biophys. Acta , 720: 405-
410.
ZENZ, C. & BERG, B.A. (1967) Human responses to controlled
vanadium pentoxide exposure. Arch. environ. Health , 14: 709-
712.
ZENZ, C., BARTLETT, J.P., & THIEDE, W.H. (1962) Acute vanadium
pentoxide intoxication. Arch. environ. Health , 5(6): 542-546.
ZHELJAZKOVA, B.G., CVETANOVA, A.L., & JACIMIRSKIJ, K.B. (1972)
[A catalytic method for determining nanogram quantities of
vanadium.] Zh. anal. Khim ., 27(4): 795-798 (in Russian).
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentration and sources of trace metals at the South Pole.
Science , 183: 198-200.
ZOLLER, W.H., GORDON, G.E., GLADNEY, E.S., & JONES, A.G. (1973)
The sources and distribution of vanadium in the atmosphere. In:
Kothy, E.L., ed. Trace elements in the environment , Washington
DC, American Chemical Society, pp. 31-47 (Advances in Chemistry
Series 123).
ZOLOTAVIN, V.L. (1954) [Achieving a pure solution of chlor-
vanadyl.] Zh. obshch. Khim ., 24: 433 (in Russian).
Other observations of boiler-cleaning operations have been
made by Fallentin & Frost (1954), Sjöberg (1955), Thomas &
Stiebris (1956), Hickling (1958), and Kuzelova et al. (1975).
In terms of respiratory symptoms relating to boiler-cleaning, it
should be noted that sulfates and sulfuric acid may be present
in boiler soot and may be partly responsible for irritative
effects. Hudson (1964) suggested that the quick onset of
symptoms (lachrymation with nose and throat irritation) with
rapid recovery following removal from exposure is character-
istic of exposure to acid sulfates. Response to vanadium expo-
sure is characterized by some delay in the onset of irritative
symptoms (a few hours to several days) and persistence of
symptoms following removal from exposure (Hudson, 1964).
A recent report by Levy et al. (1984) concerned a
comparatively high incidence of severe respiratory tract
irritation in boilermakers (74/100), many of them welders in
areas without adequate ventilation, exposed to vanadium
pentoxide fumes in a power plant where conversion from oil- to
coal-burning occurred. The severe illness of 70 men caused an
average of 5 days of absence, some objective tests (e.g., FVC)
being markedly affected. The vanadium pentoxide content was
above the permissible exposure limit in 8 samples, and this
resulted in litigation for inadequate protection of the
workers.
Kuzelova et al. (1977) drew attention to the occupational
risk of chimney sweeps cleaning large-capacity heating
facilities in large housing settlements. This coincided with a
report of a detailed cross-sectional examination of 121 chimney
sweeps by Holzhauer & Schaller (1977) in the Federal Republic of
Germany with an average exposure duration of 19 years ( ± 5
years). Vanadium exposure was determined by personal samples,
and measurements between 0.73 and 13.7 mg vanadium pentoxide/day
were determined compared with 4 µg in the normal (average)
population. Urinary excretion was determined to be between 0.15
and 13 µg/litre, which was significantly higher than the values
in 31 referents. The main complaints of the chimney sweeps were
wheezing, rhinitis, conjunctival irritation, cough, sputum
dyspnoea, and hoarseness; there were no skin symptoms. A
prospective follow-up of the cohort was emphasized, but the
results are not yet available.
8.4.3 Handling of pure vanadium pentoxide or vanadate dusts
Health effects due to occupational handling of pure vanadium
pentoxide or vanadate dusts have been reported. Tara et al.
(1953) described the effects of vanadium exposure in 4 dock
workers who unloaded and bagged spilled calcium vanadate. The
symptoms (bronchitic wheezing sounds, dyspnoea, productive
cough, haemoptysis in one case, and headache) necessitated
interruption of the work after 1´ days. Zenz et al. (1962)
described an acute illness that occurred in 18 workers pellet-
izing pure vanadium pentoxide; it was characterized by a rapidly
developing mild conjunctivitis, severe pharyngeal irritation, a
non-productive persistent cough, diffuse rales, and broncho-
spasm. With severe exposure, 4 men complained of itching skin
and a sensation of heat in the face and forearms. The symptoms
became more severe after each exposure, suggesting a sensitivity
reaction, but their duration was not prolonged by repeated
exposures.
8.4.4 Other industries
Browne (1955) studied vanadium poisoning in 12 patients
exposed to exhaust fumes from gas turbines using heavy fuel oil.
Evidence of poisoning appeared between the first and 14th day of
exposure and consisted of conjunctivitis, rhinitis, cough,
crepitations, and dyspnoea. Bleeding appeared before the
rhinorrhoea.
Other occupations in which respiratory effects of vanadium
exposure have been reported include operations connected with
the gasification of fuel oil (Fear & Tyrer, 1958) and the
manufacture of phosphor for television picture tubes (Tebrock &
Machle, 1968). In the latter study, elevated blood pressure was
noted in men exposed to vanadium pentoxide.
9. EVALUATION OF HEALTH RISKS FOR MAN
9.1 Environmental Levels and Exposures
While vanadium concentrations in the air of remote rural
areas are less than 1 ng/m3, other rural areas show concentra-
tions in excess of 50 ng/m3. This is generally considered to
reflect the local burning of fuel oil with a high vanadium
content. Typical concentrations in urban air may range from
below 1 ng/m3 to over 300 ng/m3, with annual averages for large
cities of about 20 - 100 ng/m3. At an annual average of
50 ng/m3 and a respiration rate of 20 m3, the total amount of
vanadium reaching the respiratory tract would be only 1 µg.
Assuming a rate of absorption of about 25%, the direct daily
contribution of vanadium from air would be about 250 ng.
Drinking-water supplies without excessive vanadium pollution
contain from less than 1 µg/litre to occasional maximum
concentrations of 15 - 30 µg/litre. Two comprehensive surveys
have shown average concentrations of 4.3 and 4.85 µg/litre,
respectively. At a daily intake of 2 litres of water, the
average daily intake of vanadium with water would be about
10 µg, ranging from about 1 µg to 30 - 60 µg. Although
levels in ordinary water supplies would vary considerably,
intake should rarely exceed 100 µg/day. Intake with bottled
waters from mineral springs may exceed these values.
As a rule, the concentration of vanadium in food is low.
High levels reported in early studies have been attributed to
analytical differences. Recent studies on complete diets
suggest a daily intake of vanadium of about 10 - 70 µg, with
the majority of estimates remaining below 30 µg. Assuming an
absorption rate from the gastrointestinal tract of 1 - 2%, the
contribution from food and water is unlikely to exceed 4 -
5 µg/day.
Vanadium concentrations in air in the vicinity of metal-
lurgical industries are often about 1 µg/m3. In the production
of vanadium metal or compounds, concentrations may reach a few
mg/m3. In boiler-cleaning operations, dust concentrations in
air are frequently around 50 - 100 mg/m3, and concentrations as
high as 500 mg/m3 have been reported; the vanadium content of
the dust is about 5 - 17% as vanadium pentoxide and 3 - 10 % as
lower vanadium oxides. The need for personal protection devices
in such operations is obvious.
9.2 Physiological Role
While present knowledge indicates that vanadium is an
essential element for chicks and rats, conclusive evidence that
vanadium is essential for other species, including man, is
lacking. A variety of physiological and biochemical processes
have been found to be vanadium sensitive. However, so far,
there is no evidence of adverse effects arising from vanadium
deficiency in man, and the daily requirement of vanadium in the
diet is not known.
9.3 Effects and Dose-Response Relationships
The toxicity of vanadium varies in experimental animals with
both the species and route of administration. Small animals,
such as the rat and mouse, tolerate the metal better than the
rabbit and horse. The toxicity of vanadium is low when given
orally, moderate when inhaled, and high when injected. As a
rule, the toxicity of vanadium increases as the valency
increases, pentavalent vanadium being the most toxic.
9.3.1 Local effects and dose-response relationships
Exposure of 2 volunteers to vanadium pentoxide dust at
1 mg/m3 for 8 h resulted in irritation of the respiratory tract
with cough starting 5 h later. The cough lasted for 8 days.
Exposure of 5 volunteers to a concentration of 0.2 mg/m3 caused
the same symptoms, i.e., coughing, but with an onset at 20 h.
The cough lasted for 7 - 10 days. Respiratory irritation was
noted in 2 subjects exposed to 0.1 mg/m3 for 8 h. The irritant
effect was manifested as an increase in mucous production, 24 h
after exposure, and total recovery within 4 days. Tickling and
itching, together with dryness of the mucous membranes of the
mouth, was reported by 11 volunteers exposed to 0.4 mg/m3 of
vanadium pentoxide condensation aerosol; 0.16 mg/m3 caused
irritation in only 5 subjects, and 0.08 mg/m3 did not induce
symptoms in any of the subjects.
Exposure to high concentrations of vanadium is possible in
the industrial production and use of vanadium, especially in the
cleaning of oil-fired boilers. Frequently reported irritant
symptoms include sneezing, nasal discharge, irritation of the
eyes with lachrymation, sore throat, dry or productive cough,
and chest pain. Normally, such symptoms disappear in a few days
when exposure has ceased. Cough, increased sputum, and
particularly irritation of the eyes, nose, and throat occurred
among 24 male workers exposed to a maximum of 0.9 - 5 mg
vanadium/m3 (measured as vanadium pentoxide V2O5). Most workers
had been exposed to 0.3 mg/m3 (section 8.4.1). A cross-
sectional study of 63 male workers exposed for an average of 11
years to vanadium-containing dust at 0.2 - 0.5 mg vanadium/m3
(range, 0.1 - 3.9 mg/m3) showed chronic irritant effects in the
mucous membranes of the nose. The nasal changes persisted
unchanged during subsequent exposure to much lower levels
(0.01 - 0.04 mg/m3).
Heavily exposed workers (dust concentrations of 5 -
150 mg/m3) developed atrophic rhinitis and chronic bronchitis.
Bronchospasm is also a feature in heavily exposed workers.
The effects on 63 workers of long-term exposure to vanadium
at 0.2 - 0.5 mg/m3 were studied using matched referents, a
questionnaire on respiratory symptoms, chest radiography, and
lung function testing (section 8.4). There was no change in
ventilatory function compared with the matched reference group;
only complaints of wheezing were significantly more common among
exposed workers than among referents. However, in another study
the forced vital capacity (FVC) was reversibly reduced in 17
boiler cleaners who had been exposed to a time-weighted average
respirable dust of 523 µg/3 containing 15% of vanadium (section
8.3.1).
Vanadium poses weak sensitizing properties when skin and
mucous membranes of the upper respiratory tract are exposed to
high concentrations, manifested by the development of allergic
dermatitis and rhinitis in workers in contact with vanadium.
The allergic nature of these manifestations is proved by
positive reactions of epicutaneous tests with a 2% solution of
sodium vanadate. There is information on the sensitizing effect
of vanadium in tests on animals.
In one study (section 8.4.1), 9 workers out of 36 developed
a dry eczematous dermatitis. These workers had been exposed to
vanadium pentoxide at about 6.5 µg/m3.
Green tongue is seen in a proportion of workers exposed to
vanadium-containing dust, and is an indication of exposure.
9.3.2 Systemic effects and dose-response relationships
9.3.2.1 Metabolic effects
The effect of vanadium on dental caries remains a debatable
issue (section 5.4.2). The application of a 50% paste of an
ammonium salt of vanadium and glycerol was reported to reduce
caries in children aged 7 - 11 years. Other studies between
1955 and 1968 have failed to demonstrate a clearly beneficial
effect (section 8.1.2.1).
Soluble diammonium oxytartarovanadate (150 - 200 mg/day for
6 weeks) was administered to 5 healthy adult male volunteers.
There was a significant reduction of plasma-cholesterol levels
at the end of the period. A temporary drop in the cholesterol
level was also observed in 2 out of 6 patients given ammonium
vanadyl tartrate for 7 weeks at 50 or 100 mg/day. The results
were not convincing and the temporary drops in cholesterol
levels were not statistically significant. No significant
changes in serum-cholesterol levels were noted in 12 patients (9
of whom were hypercholesterolaemic) given diammonium vanado-
tartrate orally for 6 months, (25 mg three times daily for 2
weeks, increased to 125 mg daily in 10 patients). Although some
studies on rats and rabbits have indicated decreasing
cholesterol levels following administration of vanadium and have
corroborated the reduced levels of cholesterol observed by other
authors, this effect of vanadium has not been convincingly
demonstrated in human beings so far (section 8.1.2.1).
Vanadium pentoxide in the diet (25 - 1000 mg vanadium/kg)
resulted in lower levels of cystine in the hair of rats compared
with those in controls, indicating the inhibition of cystine
synthesis (section 7.2.1). Rats administered sodium vanadate
intraperitoneally (5 - 10 mg/kg body weight as a single
injection or a dietary concentration of 500 mg/kg) showed
reduction of co-enzyme A in the liver; this has been construed
as an explanation of the reduction of cystine (section 7.2.1).
When workers were exposed to vanadium-containing dust (0.2 -
0.5 mg vanadium/m3, at the time of the study), there was no
correlation between exposure level and cystine levels in
fingernails, and no decrease in levels of serum-cholesterol or
triglycerides (section 8.4.1).
The data on the effects of vanadium on haematopoiesis are
inconsistent. A favourable effect of vanadium chloride (0.6 mg
vanadium/kg diet) on haemoglobin levels in rats, previously made
anaemic, had already been suggested in 1931 (section 7.2). A
small increase in erythrocytes and haemoglobin levels was
observed in rabbits given vanadyl sulfate subcutaneously, at
1 mg/kg body weight daily, for 2 months). When 32 vanadium
workers who had been exposed for more than 6 months were
compared with 45 referents, matched for age, no differences were
seen in haematocrit levels (8.2.1.1). It is not possible to
assess the effects of low-level vanadium exposure on iron
metabolism.
9.3.2.2 Effects on the nervous system
Systemic effects are rare in workers exposed to vanadium
compounds. Non-specific signs and symptoms including headache,
weakness, nausea, vomiting, and tinnitus have been reported.
Such signs and symptoms have mostly occurred in workers exposed
to extremely high dust concentrations, when cleaning oil-fired
boilers, but it has not been possible to derive dose-response
relationships for them.
Elevated vanadium levels in whole blood and serum have been
reported in patients suffering from depressive illness. In one
report, the vanadium levels fell to normal with recovery of the
patients. The role of vanadium in depressive states is not
known (section 8.2.2).
In mice and rats, repeated oral administration of vanadium
pentoxide or ammonium vanadate at doses of 0.05 - 0.5 mg
vanadium/kg body weight, daily, for 6 months and 21 days,
respectively, resulted in impaired conditioned reflexes. Daily
oral doses of sodium metavanadate (3.2 µg/kg body weight per
day for 10 - 15 days) caused increases in the activity of
cytochrome oxidase in the brain of guinea-pigs; a dose of
128 µg/kg per day did not have any effect, whereas 5.12 mg/kg
per day reduced the activity (section 7.2). Total
cholinesterase activity in the brain of rats was significantly
reduced by the intraperitoneal administration of 1 mg vanadyl
sulfate/kg body weight (section 7.3).
9.3.2.3 Effects on the liver
There are insufficient human data to make an assessment of
the effects of vanadium on the liver. Rats and rabbits exposed
through inhalation to vanadium pentoxide, trioxide, or tri-
chloride (10 - 70 mg/kg, 2 h/day, for 9 - 12 months) showed
fatty changes with partial cell necrosis in the liver. A clear
reduction in the liver tissue respiration and a decrease in the
albumin/globulin ratio in the serum were also observed. Subcu-
taneous injection of ammonium vanadate (1 mg vanadium/kg body
weight per day, for 30 days) caused similar fatty changes in the
liver of rats. Intraperitoneal injections of sodium meta-
vanadate (1.25 - 2.5 mg vanadium/kg body weight) in rats caused
loss of weight. The toxic effects observed were correlated with
the concentration of vanadium in the liver (section 7.2).
9.3.2.4 Effects on the kidney
Data on the effects of vanadium on the human kidney are
lacking. Intravenous injection of sodium metavanadate (2.5 -
5 mg/kg body weight) in male dogs resulted in albuminuria. In
mice, acute tubular necrosis followed subcutaneous injection of
ammonium vanadate at a dose equivalent to 20 mg vanadium/kg body
weight. Rats and rabbits inhaling vanadium chloride (70 mg/m3
2 h/day, for 9 - 12 months) caused fatty changes in the kidney.
Vanadate has diuretic and natriuretic effects on the kidney in
the rat but not in the dog or cat. Vanadate has also been
reported to increase the urinary excretion of calcium,
phosphate, bicarbonate, and chloride by the rat kidney. These
diuretic and natriuretic effects are thought to be due to the
inhibition of Na+-K+-ATPase causing inhibition of the tubular
reabsorption.
9.3.2.5 Cardiovascular effects
Palpitation of the heart at rest and on exercise has been
reported in workers occupationally exposed to vanadium.
Transient coronary insufficiency, a high incidence of extra-
systoles and bradycardia were reported (section 8.3). Exposure
of workers to low levels of vanadium pentoxide (0.2 - 0.5 mg/m3)
did not cause any pathological changes in the blood picture
(section 8.4.1).
Electrocardiographic changes (ST-segment depression,
increased T-wave amplitudes) were seen after intravenous
injection of sodium metavanadate in dogs (2.5 - 5 mg/kg body
weight). Long-term inhalation exposure of rats and rabbits to
vanadium pentoxide, trioxide, or chloride (10 - 70 mg/m3,
2 h/day, for 9 - 12 months) caused fatty changes in the myo-
cardium as well as perivascular swelling.
9.3.2.6 Pulmonary effects
Asthmatic reactions in conjunction with non-specific
bronchial hyperreactivity have occasionally been reported in
refinery workers exposed to vanadium pentoxide dust. There has
not been any evidence of an immunological mechanism behind such
cases. A dose-dependent decline in forced expiratory volume in
one second (FEV1) and forced vital capacity (FVC) has been
demonstrated in boiler cleaners. The functional increase did
not return to normal during the first week following exposure,
but fully recovered within one month. The mechanism leading to
the obstructive pulmonary impairment has not been clarified.
9.3.2.7 Effects on the immune system
In mice, vanadium and ammonium vanadate affect the normal
function of the immune system. Vanadium had slight depressant
effects on antibody-forming cells and increased DNA synthesis in
splenic leukocytes. Ammonium vanadate increased resistance
to E. coli endotoxin, but decreased resistance to Listeria
lethality. In the spleen, it increased the rosetting capability
of leukocytes, the formation of megakaryocytes, and red blood
cell precursors (section 7.7).
9.3.3 Reproduction, embryotoxicity, and teratogenicity
Human data on the effects of vanadium on reproduction and
embryotoxicity are lacking. Vanadium administered to pregnant
rats by subcutaneous administration of metavanadate (0.85 mg/kg,
equal to 1/20 LD50) accumulated in the placenta. However, the
extent to which it reaches the fetus has not been clearly
established. During the lactation period, vanadium was found in
the mammary glands and was excreted with milk. Morphological
changes in spermatozoa as well as desquamation of spermatogenic
epithelium in the seminal tubuli were observed. Gonadotoxic
effects were suggested by the absence of fertilization of female
rats by male rats that had been exposed to 0.85 mg vanadium/kg
body weight. The same doses of vanadium given to female rats on
the fourth day of pregnancy significantly decreased the number
of fetuses (section 7.8.1).
Weanling pigs receiving vanadate (200 mg vanadium/kg body
weight) showed a suppressed growth rate and increased mortality.
Vanadium was not markedly toxic when fed to growing lambs
(200 mg/kg, 84 days) (section 7.8.1).
Tentative results suggest that vanadium is teratogenic for
rats and hamsters causing skeletal anomalies and death of the
fetuses. Dose-response relationships have not been demonstrated
(section 7.8.2). There are no human data concerning possible
teratogenic effects of vanadium.
9.3.4 Mutagenicity
The data on the mutagenic potential of vanadium in bacterial
systems are inconclusive. There are positive and negative
results with E. coli and Salmonella tests (section 7.9). Data
suggest the induction of micronuclei, but not sister chromatid
exchange or dominant-lethal mutations. Chromosome effects in
vivo and in vitro have not been studied.
9.3.5 Carcinogenicity
Life-time studies on mice given 5 µg vanadyl ions/ml as the
sulfate in drinking-water did not increase the incidence of
spontaneous tumours and intraperitoneal injections of
vanadium(III)2.4pentanedione (24, 60, or 120 mg/kg body weight)
did not increase the incidence of lung adenomas in mice. The
results of a long-term study on rats with intrabronchiolar
implants of vanadium solids were negative. The few studies
available do not provide any indications of carcinogenic effects
of vanadium (section 7.10).
9.3.6 Risks from exposure of the general population
There are only a few studies on the possible effects of
vanadium in ambient air on the general population (section
8.3.2). In one study, air concentrations of vanadium together
with 12 other trace elements were found to be correlated with
mortality from pneumonia and lung cancer (coefficients of
correlation of 0.443 and 0.347, respectively) and also with
mortality from bronchitis (coefficient of correlation of 0.563).
In another study, a correlation between the levels of vanadium,
cadmium, zinc, tin, and nickel and the incidence of several
diseases including "diseases of the heart", "nephritis", and
"arteriosclerotic heart" was claimed, but the results of these
studies do not establish any causal relationships.
10. RECOMMENDATIONS
There is a conspicuous lack of data on several aspects of
the health effects of vanadium compounds. The overwhelming bulk
of recent research focuses on the effects of vanadium on
biochemical systems, especially specific effects on enzymes, and
there are major gaps in knowledge with respect to analytical,
metabolic, and exposure data.
There are indications of a weak mutagenic effect of
vanadium, but the data are partly conflicting and
uncorroborated. Further confirmative mutagenicity studies,
including studies on chromosomal effects, should be given high
priority. Data on the carcinogenicity of vanadium in various
species are practically non-existent. Such studies are urgent
and should be conducted as long-term exposure studies.
Vanadium induces toxic effects on the fetus. However,
whether these are direct effects or indirect effects resulting
from effects of vanadium on the mother is not known. Studies to
assess the nature of the teratogenic effects and the mechanism
behind them should be encouraged.
Effects resulting from high occupational exposure to
vanadium dusts have been reasonably well described. Such
exposure levels may cause a variety of clinical manifestations.
However, they should be remedied by hygienic and technical
improvements. Dose-effect and dose-response relationships have
not been well defined at low exposure levels in the range of
approximately 0.01 - 0.5 mg/m3. It is considered important to
develop specific indicators for the detection of early adverse
effects of vanadium on man.
Epidemiological studies on occupational cohorts, paying
particular attention to exposure levels and using proper
referent groups, should be encouraged. The literature is
inconsistent regarding the documentation of sensitizing
properties of vanadium compounds. This is an important aspect
with regard to worker protection.
An area of great importance is the exposure of the general
population. There are considerable geographical variations in
vanadium concentrations in air and water. Epidemiological
studies on populations living in areas with high vanadium
exposure should be carried out, relating possible adverse
effects to exposure levels. Such studies should take into
account possible interactions with other pollutants.
REFERENCES
ACGIH (1986) Threshold limit values for chemical substances
and physical agents in the work environment , Cincinnati, Ohio,
American Conference of Governmental Industrial Hygienists.
AGASYAN, P.K., LAKEEVA, T.N., NIKOLAEVA, E.R., PARTASHNIKOVA,
M.Z., SHKOLENOK, G.F., & KHREPINYUK, T.E. (1975) [Determina-
tion of the bulk substances in preparations of potassium
dichromate and ammonium vanadate by means of differential
coulometry.] Zavod. Lab ., 41(4): 385-387 (in Russian).
AIYAR, A.S. & SREENIVASAN, A. (1961) Effect of vanadium
administration on coenzyme Q metabolism in rats. Proc. Soc.
Exp. Biol. Med ., 107: 914-916.
ALESHIN, G.N., SMOLJANINOV, S.I., MESHEYAKOV, R.P., & GLOUCHOV,
G.G. (1974) [The chemistry and technology of vanadium
compounds.] In: Ivakin, A.A. & Voronova, E.M., ed. [ Proceedings
of the International Conference on the Chemistry and Technology
of Vanadium Compounds ,] Perm, Scientific Institute of Black
Metals (in Russian).
ALI, S.A., PEET, M., & WARD, N.I. (1985) Blood levels of
vanadium, caesium, and other elements in depressive patients. J.
affective Disord ., 9(2): 187-191.
ALLAWAY, W.H. (l968) Selenium, molybdenum, and vanadium in
human blood. Arch. environ. Health , 16: 342-348.
ANBAR, M. & INBAR, M. (1962) Effect of pyrodoxial 5-phosphate
in the presence of vanadyl ions of the de-iodination of
thyroxine. Nature (Lond.) , 196: 1213.
ARNON, D.I. (1958) The role of micronutrients in plant
nutrition with special reference to photosynthesis and nitrogen
assimilation. In: Lamb, C.A., Bentley, 0.G., & Beattie, J.M.,
ed. Trace elements , New York, Academic Press, pp. 1-32.
ARNON, D.I. & WESSEL, G. (1953) Vanadium as an essential
element for green plants. Nature (Lond.) , 172: 1039-1040.
ATHANASSIADIS, Y.C. (1969) Preliminary air pollution survey
of vanadium and its compounds: A literature review , Raleigh,
North Carolina, National Air Pollution Control Administration
(Publication No. APTD 69-48).
AZARNOFF, D.L. & CURRAN, G.L. (1957) Site of vanadium
inhibition of cholesterol biosynthesis (letter to the editor).
J. Am. Chem. Soc ., 79: 2968.
AZARNOFF, D.L., BROCK, F.E., & CURRAN, G.L. (1961) A specific
site of vanadium inhibition of cholesterol biosynthesis.
Biochem. Biophys. Acta , 51: 379-398.
BABENKO, G.A. & VANDZHURA, I.N. (1969) [Vanadium metabolism
after supplementary administration of certain microelements in
experimental atherosclerosis.] Bull. exp. Biol. Med ., 67(6):
72-73 (in Russian).
BAGGETT, W.L. & HUYCK, H.P. (1959) Spectrochemical determina-
tion of vanadium in alkali brines. Anal. Chem ., 31(8): 1320-
1322.
BAKAL, G.F. & LISECKAJA, G.S. (1971) [Determination of
vanadium in mercury using the reaction of oxidation of
pyrocatechin by ammonium persulfate.] Ukr. khim Zh ., 37(6):
594-597 (in Russian).
BALESTRA, G. & MOLFINO, F. (1942) [Lung impairments due to
dusts in operations to extract vanadium from petroleum ash.]
Rass. Med. Ind. Ig. Lav ., 13: 5-12 (in Italian).
BALFOUR, W.E., GRANTHAM, J.J., & GLYNN, I.M. (1978) Vanadate -
stimulated natriuresis. Nature (Lond.) , 275: 768.
BARANNIK, P.I., MIKHALYUK, I.A., & MOTUZKOV, I.N. (1969) Data
on the role of chromium, lead, vanadium and the natural radio-
activity of foodstuffs in the etiology of endemic goiter. Hyg.
Sanit ., 35(2): 289-291.
BARBOOTI, M.M. & JASIM, F. (1982) Electrothermal atomic-
absorption determination of vanadium. Talanta , 29: 107-111.
BARNES, R.M., FODOR, P., INAGAKI, K., & FODOR, M. (1983)
Determination of trace elements in urine using inductively
coupled plasma spectroscopy with a poly(dithiocarbamate)
chelating resin. Spectrochim. Acta , 38B: 245-257.
BARRY, E.F., REI, M.T., REYNOLDS, H.H., & O'BRIEN, J. (1975)
Determination of nickel and vanadium in the atmosphere of
eastern Massachusetts. Environ. Lett ., 8(4): 381-385.
BEARD, H.H., BAKER, R.W., & MYERS, V.C. (1931) Studies in the
nutritional anemia of the rat. J. biol. Chem ., 94: 123.
BELEHOVA, V.A. (1966) [Spectral analysis of vanadium content
in intact teeth and in teeth damaged by caries.] Stomatologija ,
4: 85-86 (in Russian).
BELEHOVA, V.A. (1969) [Vanadization of teeth as a means of
preventing dental caries.] Nauchn. Tr. Irkutsk. Med. Inst ., 95:
20-33 (in Russian).
BERGEL, F., BRAY, R.C., & HARRAP, Y.R. (1958) A model system
for cysteine desulphydrase action: pyridoxal phosphate-
vanadium. Nature (Lond.) , 181: 1654-1655.
BERNHEIM, F. & BERNHEIM, M.L.C. (1938) Action of vanadium on
tissue oxidation. Science, 88: 481.
BERNHEIM, F. & BERNHEIM, M.L.C. (1939) The action of vanadium
on the oxidation of phospholipids by certain tissues. J. biol.
Chem ., 127: 353.
BERROW, M.L. & WEBBER, J. (1972) Trace elements in sewage
sludges. J. Sci. Food Agric ., 23: 93-100.
BERTRAND, D. (1942) Le vanadium comme élément oligosynergique
pour l'Aspergillus niger. Ann. Inst. Pasteur , 68: 226-244.
BERTRAND, D. (1950) Survey of contemporary knowledge of
biochemistry. II. The biogeochemistry of vanadium. Bull. Am.
Mus. Natl Hist ., 94(7): 407-455.
BEYHL, F.E. (1983) Action of ammonium metavanadate on hepatic
enzymes in vitro . Arch. Toxicol ., Suppl. 6: 250-253.
BIGGS, W.R. & SWINEHART, J.H. (1976) Vanadium in selected
biological systems. In: Sigel, H., ed. Metal ions in biological
systems , New York, Marcel Dekker, Vol. 6 , pp. 141-196.
BONIG, G. & HEIGENER, H. (l971) [Micro-determination of
vanadium in biological substances by selective paper
chromatography.] Landwirtsch. Forsch ., 25: 139-143 (in German).
BORISENKO, L.F. (1973) [ Vanadium ,] Moscow, Nedra Publishing
House (in Russian).
BOWEN, H.J.M. (1963) The elementary composition of mammalian
blood , Berkshire, Wantage Research Laboratory, Isotope Research
Division (AERE-R 4196).
BOYARKINA, A.P., VASILEV, N.V., KRIVYAKORA, E.N., NIKOLAEV,
A.V., & TIUKIUPO, E.B. (1978) [On the accumulation of nickel
and vanadium in the vicinity of industrial centres.] Gig. i
Sanit ., 43(2): 92-95 (in Russian).
BRODERICK, G.N. (1977) Vanadium - 1977 , Washington DC, US
Department of the Interior, Bureau of Mines (Mineral Commodity
Profiles, MCP-8).
BROEKAERT, J.A.C., WOPENKA, B., & PUXBAUM, H. (1982) Inductively
coupled plasma optical emission spectrometry for the analysis of
aerosol samples collected by cascade impactors. Anal. Chem ., 54:
2174-2179.
BROWNE, R.C. (1955) Vanadium poisoning from gas turbines. Br.
J. ind. Med ., 12: 57-59.
BROWNE, R. & STEEL, J. (1963) The control of the vanadium
hazard in catalytic oil-gas plants. Ann. occup. Hyg ., 6: 75-79.
BROWNING, E. (1969) Vanadium. In: Toxicity of industrial
metals , 2nd ed., London, Butterworths, pp. 340-347.
BUCHET, J.P., KNEPPER, E., & LAUWERYS, R. (1982) Determination
of vanadium in urine by electrothermal atomic absorption
spectrometry. Anal. Chim. Acta , 136: 243-248.
BUCKINGHAM, D.A. (1973) Structure and stereochemistry of
coordination compounds. Part 1. Coordination chemistry. In:
Eichhorn, G.L., ed. Inorganic biochemistry , Amsterdam, New York,
Oxford, Elsevier Science Publishers, Vol. 1 , p. 18.
BUDNIKOV, G.K. & MEDJANCEVA, E.P. (1973) [Determination of
vanadium and nickel in bitumen by oscillographic polarography
with linear change of potential.] Zh. anal. Khim ., 28(2): 301-
305 (in Russian).
BUONO, J.A., BUONO, J.C., & FASCHING, J.L. (1977) Simultaneous
determination of Al, V. Mn and Cu from neutronactivated saline
matrices by precipitation with poly-5-vinyl-8-hydroxyquinoline.
J. radioanal. Chem ., 36: 353.
BUTT, E.M., NUSBAUM, R.E., GILMOUR, T.C., DIDIO, S.L., & Sister
MARIANO (1964) Trace metal levels in human serum and blood.
Arch. environ. Health , 8: 60.
BYRNE, A.R. & KOSTA, L. (1978) Vanadium in foods and in human
body fluids and tissues. Sci. total Environ ., 10: 17-30.
BYRNE, A.R. & KOSTA, L. (1979) On the vanadium and tin
contents of diet and human blood. Sci. total Environ ., 11: 87-
90.
CANALIS, E. (1985) Effect of sodium vanadate on deoxyribo-
nucleic acid and protein synthesis in cultured rat calvariae.
Endocrinology , 116(3): 855-862.
CANNON, H.L. (1963) The biogeochemistry of vanadium. Soil
Sci., 96(3): 196-204.
CANTLEY, L.C., Jr & AISEN, P. (1979) The fate of cytoplasmic
vanadium. Implications on (Na, K)-ATPase inhibition. J. biol.
Chem ., 254: 1781-1784.
CANTLEY, L.C., Jr, JOSEPHSON, L., WARNER, R., YANAGISAWA, M.,
LECHENE, C., & GUIDOTTI, G. (1977) Vanadate is a potent
(Na++,K)-ATPase inhibitor found in ATP derived from muscle. J.
biol. Chem ., 252(21): 7421-7423.
CANTLEY, L.C., Jr, CANTLEY, L.G., & JOSEPHSON, L. (1978a) A
characterization of vanadate interactions with the (Na,K)-
ATPase. J. biol. Chem ., 253: 7361-7368.
CANTLEY, L.C., Jr, RESH, M.D., & GUIDOTTI, G. (1978b) Vanadate
inhibits red cell, (Na+, K+) ATPase from the cytoplasmic side.
Nature (Lond.) , 272: 552-554.
CARLSON, R.M.K. (1977) The structure of the native vanadium
chromagen in tunicate blood cells , Palo Alto, California,
Stanford University, 252 pp (Ph.D. Thesis) (University Micro-
films No. 78-2142).
CARLTON, B.D., BENEKE, M.B., & FISHER, G.L. (1982) Assessment
of the teratogenicity of ammonium vanadate using Syrian golden
hamsters. Environ. Res ., 29(2): 256-262.
CARPENTER, G. (1981) Vanadate, epidermal growth factor and the
stimulation of DNA synthesis. Biochem. biophys. Res. Commun.,
102: 1115-1121.
CASSANI, F. (1969) [Potentiometric determination of titanium
and vanadium.] Chim. Ind. (Milan) , 51: 1248-1251 (in Italian).
CAWSE, P.A. & PEIRSON, D.H., ed. (1972) Analytical study of
trace elements in the atmospheric environment , Harwell, Atomic
Energy Research Establishment (No. AERE-R 7134).
CHASTEEN, N.D., LORD, E.M., THOMPSON, H.J., & GRADY, J.K.
(1986) Vandium complexes of transferrin and ferritin in the
rat. Biochim. Biophys. Acta , 884(1): 84-92.
CHIRIATTI, G.N. (1971) [Prevention of occupational vanadium
poisoning.] Folia med ., 54: 57-76 (in Italian).
CHRISTIAN, G.D. (1971) Catalytic determination of vanadium in
blood and urine. Anal. Lett ., 4(4): 187-196.
CHRISTIAN, G.D. & FELDMAN, F.I. (1970) Atomic absorption
spectroscopy. In: Applications in agriculture, biology, and
medicine , New York, John Wiley and Sons, pp. 276-281.
CHRISTIAN, H.C.M. & ROBINSON, J.W. (1971) The determination of
vanadium and nickel in mineral oils by flameless graphite tube
atomization. Anal. Chim . Acta, 56: 470-473.
CHUPAHIN, S.M., KRIUCHKOV, O.M., & RAMENDI, G.I. (1972)
[ Analytical capabilities of spark-source mass spectrometry ,]
Moscow, Atom Publishers (in Russian).
CLARK, R.J.H. (1975) The chemistry of vanadium, niobium, and
tantalum , Oxford, Pergamon Press, pp. 491-535.
COHEN, M.D., WEI, C.I., TAN, H., & KAO, K.J. (1986) Effect of
ammonium metavanadate on the murine immune response. J. Toxicol.
environ. Health , 19(2): 279-298.
CONKLIN, A.W., SKINNER, C.S., FELTEN, T.L., & SANDERS, C.L.
(1982) Clearance and distribution of intratracheally instilled
48vanadium compounds in the rat. Toxicol. Lett ., 11: 199-203.
CORNELIS, R., MEES, L., HOSTE, J., RYCKEBUSCH, J., VERSIECK, J.,
& BARBIER, F. (1979) Neutron activation analysis of vanadium
in human liver and serum. In: Proceedings of an International
Symposium on Nuclear Activation Techniques in the Life Sciences,
Vienna, 22-26 May, 1978 , Vienna, International Atomic Energy
Agency, pp. 165-177.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1980) Determination of vanadium in human serum by neutron
activation analysis. J. radioanal. Chem ., 55(1): 35-43.
CORNELIS, R., VERSIECK, J., MEES, L., HOSTE, J., & BARBIER, F.
(1981) The ultratrace element vanadium in human serum. Biol.
Trace Elem. Res ., 3: 257-263.
COTTON, F.A. & WILKINSON, G. (1962) Advanced inorganic
chemistry , New York, Interscience.
COTTON, F.A. & WILKINSON, G. (1980) Advanced inorganic
chemistry , 4th ed., New York, John Wiley and Sons, pp. 708-719.
COWGILL, U.M. (1973) The determination of all detectable
elements in the aquatic plants of Linsley Pond and Cedar Lake
(North Branford, Connecticut) by X-ray emission and optical
emission spectroscopy. Appl. Spectrosci ., 27: 5-9.
CREASON, J.P., HINNERS, T.A., BUMGARNER, J.E., & PINKERTON, C.
(l975) Trace elements in hair, as related to exposure in
Metropolitan New York. Clin. Chem ., 21: 603-612.
CURRAN, G.L. (1954) Effect of certain transition group
elements on hepatic synthesis of cholesterol in the rat. J.
biol. Chem., 210: 765-770.
CURRAN, G.L. & BURCH, R.E. (1967) Biological and health
effects of vanadium. In: Proceedings of the First Annual
Conference on Trace Substances in Environmental Health,
Columbia, 10-11 July, 1967 , Columbia, University of Missouri,
pp. 96-104.
CURRAN, G.L. & COSTELLO, R.L. (1956) Reduction of excess
cholesterol in the rabbit aorta by inhibition of endogenous
cholesterol synthesis. J. exp. Med ., 103: 49-56.
CURRAN, G.L., AZARNOFF, D.L., & BOLINGER, R.E. (1959) Effect
of cholesterol synthesis inhibition in normocholesteremic young
men. J. clin. Invest ., 38: 1251-1261.
DAMS, R., ROBBINS, J.A., RAHN, K.A., & WINCHESTER, J.W. (1970)
Nondestructive neutron activation analyses of air pollution
particulates. Anal. Chem ., 42: 861-867.
DAY, H., MIDDENDORF, D., LUKERT, C., HEINZ, A., & GRANTHAM, J.J.
(1980) The renal response to intravenous vanadate in rats. J.
lab. clin. Med., 96: 382-395.
DEMASTER, E.G. & MITCHELL, R.A. (1973) A comparison of
arsenate and vanadate as inhibitors or uncouplers of
mitochondria and glycolytic energy metabolism. Biochemistry,
12(19): 3616-3621.
DHEW (1968) Air quality data from the National Air
Surveillance Networks and contributing state and local networks ,
1966 ed., Durham, North Carolina, US Department of Health,
Education, and Welfare, National Air Pollution Control
Administration.
DIMOND, E.G., CARAVACA, J., & BENCHIMOL, A. (1963) Vanadium:
Excretion, toxicity, lipid effect in man. Am. J. clin. Nutr .,
12: 49-53.
DOMINGO, J.L., LLOBET, J.M., & CORBELLA, J. (1985) Protection
of mice against the lethal effects of sodium metavanadate: a
quantitative comparison of a number of chelating agents.
Toxicol. Lett ., 26(2-3): 95-99.
DOMINGO, J.L., LLOBET, J.M., TOMAS, J.M., & CORBELLA, J. (1986)
Influence of chelating agents on the toxicity, distribution, and
excretion of vanadium in mice. J. appl. Toxicol ., 6(5): 337-
341.
DOVGOPOL, V.I., BENOUNI, A.CH., MEDVEDEV, A.A., SHAPOVALOV,
J.P., & MEDVEDEVA, L.A. (1974) [The chemistry and engineering
technology of vanadium compounds.] In: Ivakin, A.A. & Voronova,
E.M., ed. [ Proceedings of the International Conference on the
Chemistry and Technology of Vanadium Compounds ,] Perm,
Scientific Institute of Black Metals, pp. 477-480 (in Russian).
DUCE, R.A. & HOFFMAN, G.L. (1976) Atmospheric vanadium
transport to the ocean. Atmos. Environ ., 10: 989-996.
DURFOR, C.N. & BECKER, E. (1963) Public water supplies of
the 100 largest cities in the United States, 1962 , Washington
DC, US Geological Survey (Water-Supply Paper No. 1812).
DURRANT, P.J. & DURRANT, B. (1970) Introduction to advanced
inorganic chemistry , 2nd ed., New York, John Wiley and Sons.
DURUM, W.H. & HAFFTY, J. (1963) Implications of the minor
element content of some major streams of the world. Geochim.
Cosmochim. Acta , 23: 1-11.
DUTTON, W.F. (1911) Vanadiumism. J. Am. Med. Assoc ., 56(22):
1648.
EISLER, L., SIMECEK, R., & USTUPSKY, J. (1968) [Effect of work
in vanadium workshop on respiratory ways.] Prac. Lek., 20(2/3):
52-57 (in Czech).
ELINDER, C.G. (1984) Metabolism and toxicity of metals ,
Stockholm, Department of Environmental Hygiene, Karolinska
Institute and the National Institute of Environmental Medicine,
pp. 265-274 (Life Sciences Research Report No. 28).
ELVES, M.V., WILSON, J.N., SCALES, J.T., & KEMP, H.B.S. (1975)
Incidence of metal sensitivity in patients with total joint
replacements. Br. med. J ., 4: 376-378.
ERMOLAEV, G.F. (1969) [Effect of vanadium on certain
biochemical processes in rabbit organs.] In: [ Trace elements in
medicine ,] Moscow, Ivano-Frankovsk, pp. 190-192 (in Russian).
EVANS, C.A. & MORRISON, G.H. (1968) Trace element survey
analysis of biological materials by spark source mass
spectrometry. Anal. Chem ., 40: 869-875.
FALLENTIN, B. & FROST, J. (1954) [Health hazards from cleaning
oil-fired boilers caused by vanadium and sulfuric acid in the
soot.] Nord. Hyg. Tidskr ., 3: 58-65 (in Danish).
FAORO, R.B. & MCMULLEN, T.B. (1977) National trends in trace
metals in ambient air 1965-1974 , Research Triangle Park, North
Carolina, US Environmental Protection Agency (EPA-450/1-77-
003).
FAULKNER HUDSON, T.G. (1964) Vanadium: toxicology and
biological significance , Amsterdam, New York, Oxford, Elsevier
Science Publishers, 135 pp.
FEAR, E.C. & TYRER, F.H. (1958) A study of vanadium poisoning
in gas workers. Trans. Assoc. Ind. Med. Off ., 8: 153-155.
FENNELLY, P.F. (1976) The origin and influence of airborne
particulates. Am. Sci ., 64(1): 46-56.
FISHER, G.L., MCNEILL, K.L., WHALEY, C.B., & FONG, J. (1978)
Attachment and phagocytosis studies with murine pulmonary
alveolar macrophages. J. Reticuloendothel. Soc ., 24: 243-252.
FISHER, G.L., MCNEILL, K.L., & DEMOCKO, C.J. (1986) Trace
element interactions affecting pulmonary macrophage cyto-
toxicity. Environ. Res ., 39(1): 164-171.
FLAHERTY, J.P. & ELDRIDGE, H.B. (1970) Neutron activation
analysis of residual oil for vanadium. Appl. Spectrosci ., 24(5):
534-538.
FROLOV, V.V. (1970) [Determination of chlorine and vanadium in
graphite by the activation method.] Zavod. Lab ., 36(7): 807-809
(in Russian).
FROST, J (1951) [Vanadium poisoning from cleaning the smoke
stacks of boilers burning fuel oil.] Ugeskr. Laeger , 113: 1309-
1312 (in Danish).
FUKAI, R. & MEINKE, W.W. (1962) Activation analyses of
vanadium, arsenic, molybdenum, tungsten, rhenium, and gold in
marine organisms. Limnol. Oceanogr ., 7: 186.
GARLEJ, T. (1974) [The influence of vanadium-bearing dust on
the workers' health in the Plock thermal-electric power station:
New exposure tests.] Pol. Tyg. Lek ., 29(49): 2143-2144 (in
Polish).
GERSHKOVICH, E.E. & STYKAN, T.P. (1972) [A catalytic method of
measuring hydrogen sulfide levels in air.] Gig. Tr. prof.
Zabol ., 2: 60-61 (in Russian).
GEYER, C.F. (1953) Vanadium, a caries-inhibiting trace element
in the Syrian hamster. J. dent. Res ., 35: 590-595.
GIBSON, R.S. & DEWOLFE, M.S. (1979) Copper, zinc, manganese,
vanadium, and iodine concentrations in the hair of Canadian low
birth wight neonates. Am. J. clin. Nutr ., 32: 1728-1733.
GILES, M.A., KLAVERKAMP, J.F., & LAWRENCE, S.G. (1979) The
acute toxicity of saline groundwater and of vanadium to fish and
aquatic invertebrates , Edmonton, Environment Alberta, 172 pp
(Prepared for the Alberta Oil Sands Environmental Research
Program) (Project No. AF 3.2.1).
GOFMAN J.W. (1962) Chemical elements of the blood of man in
health. Adv. biol. med. Phys ., 8: 1.
GOLDBERG, E.D. (1961) Marine geochemistry. In: Eyring, H.,
Christensen, C.J., & Johnston, H.S., ed. Annual review of
physical chemistry , Vol. 12, Palo Alto, California, Annual
Reviews, Inc., pp. 29-48.
GOLDSCHMIDT, V.M. (1938) Collected articles on the
geochemistry of rare elements. In: [ Collection of papers on the
geochemistry of rare metals ,] Moscow, Amalgamated State
Technical and Scientific Publishing Houses (in Russian).
GOLDSHTEJN, M.I. (1967) [ Vanadium-containing steels ,] Moscow,
Chermetinformacija (in Russian).
GORDUS, A.A., MAHER, C.C., III, & BIRD, G.C. (1974) Human hair
as an indicator of trace metal environmental exposure. In:
Proceedings of the First Annual NSF Trace Contaminants
Conference , Springfield, Virginia, National Technical
Information Service, pp. 463-487 (Conf. 730802).
GOYER, R.A. & MEHLMANN, M.A., ed. (1977) Toxicology of trace
elements. In: Advances in modern toxicology, Washington DC,
Hemisphere, Vol. 2 , 303 pp.
GRANTHAM, J.J. (1980) The renal sodium pump and vanadate. Am.
J. Physiol ., 239: F97-F106.
GRANTHAM, J.J. & GLYNN, I.M. (1979) Renal Na, K-ATPase:
determinants of inhibition by vanadium. Am. J. Physiol ., 236(6):
F530-F535.
GRIN, A.V., BLUM, A.A., SELETKOV, A.L., FREIDENZON, YU.E., &
NAIMUCHINA, L.F. (1971) [Low-alloyed welded steels containing
vanadium for construction.] In: [ Problems of Kachkanar ,]
Sverdlovsk, Central Bureau of Technical Information, pp. 186-196
(in Russian).
GULKO, A.G. (1956) [On the characteristics of vanadium as an
industrial poison.] Gig. i Sanit ., 21(11): 24-28 (in Russian).
GYLSETH, B., LEIRA, H.L., STEINNES, E., & THOMASSEN, Y. (1979)
Vanadium in the blood and urine of workers in a feraalloy plant.
Scand. J. Work environ. Health , 5: 188-194.
HACKETT, P.L. & KELMAN, B.J. (1983) Availability of toxic trace
metals to the conceptus. Sci. total environ .,28: 433-442.
HADJIMARKOS, D.M. (1966) Vanadium and dental caries. Nature
(Lond.) , 209: 1137.
HADJIMARKOS, D.M. (1968) Effect of trace elements on dental
caries. Adv. oral Biol ., 3: 282-285.
HAMILTON, E.I., MINSKI, M.J., & CLEARY, J.J. (1972-73) The
concentration and distribution of some stable elements in
healthy human tissues from the United Kingdom. Sci. total
Environ ., 1: 341-374.
HAMMOND, P.B. & FOULKES, E.C. (1986) Metal ion toxicity in man
and animals. In: Metal ions in biological systems , Cincinatti,
Ohio, Kettering Laboratory, Vol. 20 , pp. 157-200.
HANNA, W.J. & GRANT, C.L. (1962) Spectrochemical analyses of
certain trees and ornamentals for 23 elements. Bull. Torrey Bot.
Club , 89: 293-302.
HARA, T., SONODA, Y., & IWAI, I. (1976) Growth response of
cabbage plants to transition elements under water culture
conditions. I. Titanium, vanadium, chromium, manganese, and
iron. Soil Sci. plant Nutr ., 22(3): 307-315.
HARRIS, W.R., FRIEDMAN, S.B., & SILBERMAN, D. (1984) Behavior
of vanadate and vanadyl ion in canine blood. J. inorg. Biochem .,
20(2): 157-169.
HATFIELD, M. & CHURCHILL, P. (1981) Renal vascular and tubular
effects of vanadate in the anaesthetized rat. J. Pharmacol. exp.
Ther ., 217: 406-410.
HATHCOCK, J.N., HILL, C.H., & TOVE, S.B. (1966) Uncoupling of
oxidative phosphorylation by vanadate. Can. J. Biochem ., 44:
983-988.
HEIN, J.W. & WISOTZKY, J. (1955) The effect of a 10 ppm
vanadium drinking solution on dental caries in male and female
Syrian hamsters. J. dent. Res ., 34: 756.
HEWITT, E.J. (1953) Metal interrelationships in plant
nutrition. I. Effects of some metal toxicities on sugar beet,
tomato, oat, potato, and marrowstem kale grown in sand culture.
J. exp. Bot ., 4(10): 59-64.
HEYDORN, K. & LUKENS, H.R. (1966) Pre-irradiation separation
for the determination of vanadium in blood serum by reactor
neutron activation analysis , Roskilde, Danish Atomic Energy
Commission, Research Establishment Risö (Risö Report No. 138).
HEYLIGER, C.E., TAHILIANI, A.G., & MCNEILL, J.H. (1985) Effect
of vanadate on elevated blood-glucose and depressed cardiac
performance of diabetic rats. Science , 227(4693): 1474-1477.
HICKEY, R.J., SCHOFF, E.0., & CLELLAND, R.C. (1967)
Relationship between air pollution and certain chronic disease
death rates. Arch. environ. Health , 15: 728-738.
HICKLING, S. (1958) Vanadium poisoning. N.Z. med. J ., 57: 607-
608.
HOFFMAN, B., KOMENDA, W., SADOWSKA, A.M., & ROZANSKI, A. (1972)
[The occurence of vanadium in the drinking-water in the region
of Bialystock Province.] Rocz. Panstw. Zakl. Hig ., 23(2): 155-
159 (in Polish).
HOFFMAN, G.L., DUCE, R.A., & ZOLLER, W.H. (1969) Vanadium,
copper, and aluminum in the lower atmosphere between California
and Hawaii. Environ. Sci. Technol ., 3: 1207-1210.
HOFFMAN, G.L., DUCE, R.A., & HOFFMAN, E.J. (1972) Trace metals
in the Hawaiian marine atmosphere. J. geophys. Res ., 77: 5322-
5329.
HOLDWAY, D.A. & SPRAGUE, J.B. (1979) Chronic toxicity of
vanadium to flagfish. Water Res ., 13: 905-910.
HOLODOV, V.N. (1968) [ Vanadium ,] Moscow, Nauka Publishing
House (in Russian).
HOLODOV, V.N. (1973) [ Sedimentary ore formation and
metallogeny on vanadium ,] Moscow, Nauka Publishing House (in
Russian).
HOLZHAUER, K.P. & SCHALLER, K.-H. (1977) [ Occupational
medicine studies in chimney sweeps. Hazards at the workplace and
occupation-linked health damage ,] Stuttgart, G. Thieme (in
German).
HOPKINS, L.L., Jr & MOHR, H.E. (197la) The biological
essentiality of vanadium. In: Mertz, W. & Cornatzer, W.E., ed.
Newer trace elements in nutrition , New York, Marcel Dekker,
pp. 195-213.
HOPKINS, L.L., Jr & MOHR, H.E. (197lb) Effect of vanadium
deficiency on plasma cholesterol of chicks. Fed. Proc ., 30:
462.
HOPKINS, L.L., Jr & MOHR, H.E. (1974) Vanadium as an essential
nutrient. Fed. Proc ., 33(6): 1173-1175.
HOPKINS, L.L., Jr & TILTON, B.E. (1966) Metabolism of trace
amounts of vanadium 48 in rat organs and liver subcellular
particles. Am. J. Physiol ., 211: 169-172.
HOPKINS, L.L., CANNON, H.L., MUSCH, A.T., WELCH, R.M., &
NIELSEN, F.H. (1977) Vanadium. Geochem. Environ ., 2: 93-107.
HORI, C. & OKA, T. (1980) Vanadate enhances the stimulatory
action of insulin on DNA synthesis in cultured mouse mammary
glands. Biochim. Biophys. Acta , 610: 235-240.
HORNER, C.K., BURCK, D., ALLISON, F., & SHERMAN, M.S. (1942)
Nitrogen fixation by azotobacter as influenced by molybdenum and
vanadium. J. agric. Res ., 65: 173-193.
HUDGINS, P.M. & BOND, G.H. (1979) Reversal of vanadate
inhibition of Na++K-ATPase by catecholamines. Res. Commun. chem.
Pathol. Pharmacol ., 23(2): 313-326.
HUDSON, T.G.F. (1964) Vanadium: toxicology and biological
significance , Amsterdam, New York, Oxford, Elsevier Science
Publishers.
HWANG, I.Y., ULLICCI, P.A., & SMITH, S.B. (1972) A simple
flameless atomizer. Am. Lab ., 3: 41-43.
ICRP (1960) Report of Committee II on Permissible Dose for
Internal Radiation (1959). Recommendations of the International
Commission on Radiological Protection , Oxford, Pergamon Press
(ICRP Publication No. 2).
ILO (1980) Occupational Exposure Limits for Airborne Toxic
Substances; Occupational Safety and Health Series, No. 37, 2nd
revised ed ., Geneva, International Labour Office.
INCIARTE, D.J., STEFFEN, R.P., DOBBINS, D.E., SWINDALL, B.T.,
JOHNSTON, J., & HADDY, F.J. (1980) Cardiovascular effects of
vanadate in the dog. Am. J. Physiol ., 239: 47-56.
IZYCKI, J., SULKOWSKI, W., ADAMIAK-ZIEMBA, J., & STROSZEJN, G.
(1971) [Evaluation of professional exposure to vanadium
compounds and other environmental factors of workers employed in
cleaning of oil-fired boilers with special regard to the state
of respiratory tract.] Med. Pr ., 22(4): 421-431 (in Polish).
JACIMIRSKIJ, K.B. (1967) [ Kinetic methods of analysis ,]
Moscow, Himija (in Russian).
JACKSON, J.F. & LINSKENS, H.F. (1982) Metal ion induced
unscheduled DNA synthesis in Petunia pollen. Mol. gen. Genet .,
187: 112.
JANDHYALA, B.S. & HOM, G.J. (1983) Physiological and pharmaco-
logical properties of vanadium. Life Sci ., 33: 1325-1340.
JARACZEWSKA, W. & JAKUBOWSKI, M. (1964) [The attempt of
evaluation of vanadium exposure in the chemical industry.] Med.
Pr ., 15(6): 375-383 (in Polish).
JAWOROWSKI, Z., BILKIEWICZ, J., DIBOSZ, E., GRYBOWSKA, D.,
KOWNACKA, L., & WRONSKI, Z. (1973) Radiation hazards to
population from conventional and nuclear power production. In:
Symposium on environmental surveillance around nuclear
installations, Warsaw, 5-9 November, 1973, Vienna, International
Atomic Energy Agency (IAEA-SM-180/20).
JOHNSON, J.L., COHEN, H.L., & RAJAGOPALAN, K.V. (1974) Studies
of vanadium toxicity in the rat; lack of correlation with
molybdenum utilization. Biochem. biophys. Res. Commun ., 56(4):
940-946.
JONES, M.M. & BASINGER, M.A. (1983) Chelate antidotes for
sodium vanadate and vanadyl sulfate intoxication in mice. J.
Toxicol. environ. Health , 12(4-6): 749-756.
KADA, T., HIRANO, K., & SHIRASU, Y. (1980) Screening of
environmental chemical mutagens by the rec-assay system with
Bacillus subtilis. In: de Serres, F.J. & Hollaender, A., ed.
Chemical mutagens: principles and methods for their detection ,
New York, Plenum Press, pp. 149-173.
KAKU, S., HOSHIDE, M., MATSUO, S., NUNOTANI, H., ISHIDA, N.,
KURATA, S., MATSUMOTO, H., YAMAGUCHI, S., SANO, T., KONO, Y.,
KOGA, S., & KOGA, M. (1971) [Experimental studies on vanadium
poisoning. I. Vanadium-induced hepatic injuries in rats.] Jap.
J. ind. Health , 13: 263-267 (in Japanese).
KANEMATSU, K. & KADA, T. (1978) Mutagenicity of metal
compounds. Mutat. Res ., 53(2): 207-208.
KANEMATSU, N., HARA, M., & KADA, T. (1980) Rec-assay and
mutagenicity studies on metal compounds. Mutat. Res ., 77: 109-
116.
KANISAWA, M. & SCHROEDER, H.A. (1967) Life-term studies on the
effects of arsenic, germanium, tin, and vanadium on spontaneous
tumours in mice. Cancer Res ., 27: 1192-1195.
KATAYEVA, I.G. & SAPUNOV, S.F. (1974) [Sanitary-hygienic
characteristics of vanadium ferroalloy production at the
Chusovoy Metallurgical Plant.] Gig. Tr. prof. Zabol ., 18(1):
37-39 (in Russian).
KAZIMOV, M.A. (1977) [Occupational hygiene with new methods of
the ferrovanadium production.] Gig. Tr. prof. Zabol ., 6: 8-12
(in Russian).
KEJT, M. & DEGENS, E. (1961) [Geometrical indicators.] In:
[ Geometrical research at low temperatures and pressures ,]
Moscow, Foreign Language Publishers, pp. 56-84 (in Russian).
KEMPTON, S., STERRITT, R.M., & LESTER, J.N. (1982) Atomic-
absorption spectrophotometric determination of antimony,
arsenic, bismuth, tellurium, thallium and vanadium in sewage
sludge. Talanta , 29: 675-681.
KINGSNORTH, A.N., LAMURAGLIA, G.M., ROSS, J.S., & MALT, R.A.
(1986) Vanadate supplements and 1,2-dimethylhydrazine induced
colon cancer in mice: increased thymidine incorporation without
enhanced carcinogenesis. Br. J. Cancer, 53(5): 683-686.
KIRZHNER, M.A., KRYZHENKOVA, N.A., & KIST, A.A. (1974) [Scope
for the preliminary concentration in the use of neutron
activation in matrix analysis for aluminium and vanadium.] At.
Energ ., 37: 498 (in Russian).
KIVILUOTO, M. (1980) Observations on the lungs of vanadium
workers. Br. J. ind. Med ., 37: 363-366.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1979a) Serum and
urinary vanadium pentoxide. Int. Arch. occup. Health , 48: 251-
256.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M. (1979b)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory. Scand. J. Work environ. Health , 5: 50-58.
KIVILUOTO, M., RASANEN, 0., RINNE, A., & RISSANEN, M., (1979c)
Serum and urinary vanadium of vanadium exposed workers. Scand.
J. Work environ. Health , 5: 362-367.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health,
46: 179-182.
KIVILUOTO, M., PYY, L., & PAKARINEN, A. (1981a) Clinical
laboratory results of vanadium-exposed workers. Arch. environ.
Health , 36(3): 109-113.
KIVILUOTO, M., RASANEN, O., RINNE, A., & RISSANEN, M. (1981b)
Intracellular immunoglobulins in plasma cells of nasal biopsies
taken from vanadium-exposed workers. Anat. Anz. Jena , 149: 446-
450.
KNECHT, E.A., MOORMAN, W.J., CLARK, J.C., LYNCH, D.W., & LEWIS,
T.R. (1985) Pulmonary effects of acute vanadium pentoxide
inhalation in monkeys. Am. Rev. respir. Dis ., 132(6): 1181-
1185.
KOHLER, R. (1972) [Rebuttal: problems of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 27: 815-816 (in German).
KOLPAKOV, L.E., DOBOSH, V.G., GREKOV, S.D., JUFEREV, A.JU.,
LLOKAZOV, E.D., AZFALOVA, R.U., & SANDLER, V.I. (1971)
[Improvement of technology of utilisation of vanadium contained
in waste water.] In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 229-231 (in
Russian).
KONOVALOV, G.S., IVANOVA, A.A., & KOLESNIKOV, T.CH. (1968)
[Scattered rare elements dissolved in water contained in
colloidal substances of the major USSR rivers.] In: [ A
collection of papers on the geochemistry of sedimentary rocks
and ores. Documentation of the Seventh All-Union Conference on
Lithology of the Academy of Sciences of the USSR], Moscow,
Nauka Publishing House, pp. 72-87 (in Russian).
KÖPF-MAIER, P. & KÖPF, H. (1979) [Vanadocene dichloride:
another anti-tumour agent from the metallocene series.] Z.
Naturforsch ., 34B: 805-807 (in German).
KÖPF-MAIER, P., HESSE, B., & KÖPF, H. (1980) Tumour inhibition
by metallocenes: effect of titanocene and hafnocene dichlorides
on Ehrlich ascites tumour in mice. J. Cancer Res. clin. Oncol .,
96(1): 43-51.
KOPYLOVA, L.M. (1971) [A study of some blood indicators
following prolonged administration of vanadium sulfate.] Tr.
Voronezh. Med. Inst ., 85: 62-65 (in Russian).
KORKHOV, V.V. (1965) [Vanadium in prophylaxis and treatment of
experimental atheroslerosis.] Farmakol. i Toksikol ., 28(1): 83-
87 (in Russian).
KOSTROMIN, A.I., AKHMETOV, A.A., & ORLOVA, L.N. (1970)
[Coulometric determination of manganese (II), cesium (III), and
vanadium (IV).] Zh. anal. Khim ., 25: 195-196 (in Russian).
KOVDA, V.A., JAKUSHEVSKAYA, I.V., & TURUKANOV, A.N. (1959)
[ Trace elements in the soils of the Soviet Union ,] Moscow,
Moscow University Publishing House (in Russian).
KRAGTEN, J. (1981) Improved determination of vanadium by flame
AAS by preventing polynuclear hydroxide complex formation. At.
Spectrosci ., 2(4): 135-136.
KRAUSKOPF, F.K. (1963) Factors governing the concentration of
thirteen rare metals in seawater. Collected articles on the
geochemistry of rock formation. In: [ The geochemistry of
lithogenesis ,] Moscow, Foreign Languages Publishing House, pp.
294-338 (in Russian).
KUBASKY, A. (1957) Waste from a vanadium work as moulding
material. Slevarenstvi , 5(1): 4-7.
KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D., & FLETCHER, O.J.
(1986) Influence of ochratoxin A and vanadium on various
parameters in growing chicks. Poult. Sci ., 65(9): 1671-1678.
KULIEVA, T.K. (1974) [Effect of vanadium on the activity of
some tissue-respiration enzymes.] Zdravookhr. Turkm ., 3: 7-17
(in Russian).
KUMAR, A. & CORDER, C.N. (1980) Diuretic and vasoconstrictor
effects of sodium orthovanadate on the isolated perfused rat
kidney. J. Pharmacol. exp. Ther ., 213: 85-90.
KURMAEV, R.H. (1974) [Solid, liquid and aerosol waste in the
production of technical vanadium pentoxide.] In: Ivakin, A.A. &
Voronova, E.M., ed. [ Proceedings of the International
Conference on the Chemistry and Technology of Vanadium
Compounds ,] Perm, Scientific Institute of Black Metals, pp. 43-
46 (in Russian).
KUZELOVA, M., SIRL, J., & POPLER, A. (1975) [Occupational
poisonings by vanadium compounds in workers at mazut boiler
houses.] Prac. Lek ., 27(5): 170-173 (in Czech).
KUZELOVA, M., HAVEL, V., POPLER, A., & STEPANEK, O. (1977)
[The problems of occupational medicine in the work of chimney
sweeps.] Prac. Lek ., 29: 225-228 (in Czech).
LAGERKVIST, B., NORDBERG, G.F., & VOUK, V. (1986) Vanadium.
In: Friberg, G.F., Nordberg, G.F., & Vouk, V.B., ed. Handbook on
the toxicology of metals. II. Specific metals , Amsterdam, New
York, Oxford, Elsevier Science Publishers, pp. 638-663.
LAHAV, M., RENNERT, H., & BARZILAI, D. (1986) Inhibition by
vanadate of cyclic AMP production in rat corpora lutea incubated
in vitro . Life Sci ., 39(26): 2557-2564.
LANDER, D.W., STEINER, R.L., ANDERSON, D.H., & DEHM, R.L.
(1971) Spectrographic determination of elments in airborne
dirt. Appl. Spectrosci ., 25: 270-275.
LARSEN, J.A. & THOMSEN, O. (1980) Vanadate-induced oliguria
and vasoconstriction in the cat. Acta physiol. Scand ., 110:
367-374.
LARSEN, J.A., THOMSEN, O., & HANSEN, O. (1979) Vanadate-
induced oliguria in the anaesthetized cat. Acta physiol. Scand .,
106: 495-496.
LEE, K.P. & GILLIES, P.J. (1986) Pulmonary response and
intrapulmonary lipids in rats exposed to bismuth orthovanadate
dust by inhalation. Environ. Res ., 40(1): 115-135.
LEE, R.E., GORANSON, S.S., ENROINE, R.E., & MORGAN, G.B. (1972)
National air surveillance Cascade Impactor Network. II. Size
distribution measurements of trace metal components. Environ.
Sci. Technol ., 6: 1025-1030.
LEE, K., NALEWAJKO, C., & JACK, T.R. (1978) Effects of
vanadium on freshwater algae. In: Abstracts of the 5th Annual
Aquatic Toxicity Workshop, Hamilton, Ontario, 7-9 November ,
Burlington, Ontario, Canada Centre for Inland Waters.
LEES, R.E.M. (1980) Changes in lung function after exposure to
vanadium compounds in fuel oil ash. Br. J. ind. Med ., 37: 253-
256.
LEVlNA, E.N. (1972) [Relationships between solubility of metal
compounds and their toxicity, distribution, and elimination from
the body.] Gig. Tr. prof. Zabol ., 16: 40-43 (in Russian).
LEVY, B.S., HOFFMAN, L., & GOTTSEGEN, S. (1984) Boilermakers'
bronchitis: respiratory tract irritation associated with
vanadium pentoxide exposure during oil-to-coal conversion of a
power plant. J. occup. Med ., 26(8): 567-570.
LEVY, L.S., MARTIN, P.A., & BIDSTRUP, P.L. (1986) Investigation
of the potential carcinogenicity of a range of chromium
containing materials on rat lung. Br. J. ind. Med ., 43(4): 243-
256.
LEWIS, C.E. (1959a) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 19: 419-424.
LEWIS, C.E. (1959b) The biological effects of vanadium. II.
The signs and symptoms of occupational vanadium exposure. Am.
Med. Assoc. Arch. Ind. Health , 19: 497-503.
LEWIS, C.E. (1959c) The biological actions of vanadium. Am.
Med. Assoc. Arch. Ind. Health , 20(5): 455-466.
LIFSCHITZ, V.M. (1962) [Spectral determination of trace
elements in human blood (group determination of Ni, Ag, Zn, Cu,
Cd, V, Pb, Mn, Fe, and Co).] Tr. Voronezh. Med. Inst ., 49: 110
(in Russian).
LINSTEDT, K.D. & KRUGER, P. (1969) Vanadium concentrations in
Colorado River Basin waters. J. Am. Water Works Assoc ., 61(2):
85-88.
LINTER, C.M. (1985) Neuropsychiatric aspects of trace
elements. Br. J. hosp. Med ., 34(6): 361-365.
LOPEZ-NOVOA, J.M., GARCIA, J.C., CRUZ-SOTO, M.A., BENABE, J.E.,
& MARTINEZ-MALDONADO, M. (1982a) Effect of sodium ortho-
vanadate on renal renin secretion in vivo. J. Pharmacol. exp.
Ther ., 222: 447-451.
LOPEZ-NOVOA, J.M., MAYOL, V., & MARTINEZ-MALDONADO, M. (1982b)
Renal actions of orthovanadate in the dog. Proc. Soc. Exp. Biol.
Med ., 170: 418-426.
L'VOV, B.V. (1970) Atomic absorption spectrochemical analysis,
2nd ed., London, Adam Hilger.
MACARA, I.G. (1980) Vanadium - an element in search of a role.
Trends biochem. Sci ., April: 92-94.
MACARA, I.G., KUSTIN, K., & CANTLEY, L.C., Jr (1980) Gluta-
thione reduces cytoplasmic vanadate mechanism and physiological
implications. Biochim. Biophys. Acta , 629: 95-106.
MCLEAN, J.R., MCWILLIAMS, R.S., KAPLAN, J.G., & BIRNBOIM, H.C.
(1982) Rapid detection of DNA strand breaks in human peripheral
blood cells and animal organs following treatment with physical
and chemical agents. In: Bora, K.C., Douglas, G.R., & Nestmann,
E.R., ed. Chemical mutagenesis, human population monitoring, and
genetic risk assessment , Amsterdam, Oxford, New York, Elsevier
Science Publishers, p. 137 (Progress in Mutation Research,
Vol. 3).
MCLUNDIE, A.C., SHEPHERD, J.B., & MOBBS, D.R.A. (1968) Studies
on the effects of various ions on enamel solubility. Arch. oral
Biol ., 13: 1321-1330.
MADSEN, E.S., NIELSEN, P.A., & PEDERSEN, J.C. (1982) The
distribution and origin of mutagens in airborne particulates,
detected by the Salmonella /microsome assay in relation to
levels of lead, vanadium, and PAH. Sci. total Environ ., 24(1):
13-25.
MAHLER, H.R. & CORDES, E.H. (1966) Biological chemistry , New
York, Harper & Row.
MAJUMDAR, A.K. & DAS, G. (1965) Spectrophotometric determin-
ation of vanadium with N -benzoyl- N -p-chlorophenylhydroxyl-
amine. J. Indian Chem. Soc ., 42(3): 189.
MANNING, D.C. & SLAVIN, W. (1983) The determination of trace
elements in natural waters using the stabilized temperature
platform furnace. Appl. Spectrosci ., 37(1): 1-10.
MARAFANTE, E. & SABBIONI, E. (1983) Metabolic studies on
vanadium: in vivo and in vitro association with cellular
components. In: Proceedings of an International Conference on
Heavy Metals in the Environment, London, 18-21 September,
1979 , Edinburgh, CEC Consultants, pp. 171-174.
MARONI, M., COLOMBI, A., BURATTI, M., CALTZAFERRI, G., FOA, V.,
SABBIONI, E., & PIETRA, R. (1983) Urinary elimination of
vanadium in boiler cleaners. In: Proceedings of an International
Conference on Heavy Metals in the Environment, London, 18-21
September, 1979 , Edinburgh, CEC Consultants, pp. 66-69.
MARTENS, C.S., WESOLOWSKI, J.J., KAIFER, R., & JOHN, W. (1973)
Sources of vanadium in Puerto Rican and San Francisco bay area
aerosols. Environ. Sci. Technol ., 7: 817-819.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959a) Effect of
vanadium upon liver coenzyme A in rats. Nature (Lond.) , 183:
1527-1528.
MASCITELLI-CORIANDOLI, E. & CITTERIO, C. (1959b) Intracellular
thioctic acid and coenzyme A following vanadium treatment.
Nature (Lond.) , 184: 1641.
MASIRONl, R. (1969) Trace elements and cardiovascular
diseases. Bull. World Health Organ ., 40: 305-312.
MATANTSEVA, E.I. (1960) [The state of the respiratory organs
in workers coming into contact with vanadium pentoxide.] Gig.
Tr. prof. Zabol ., 7: 41-44 (in Russian).
MEISCH, H.U. & BECKER, L.J. (1981) Vanadium in photosynthesis
of Chlorellafusca and higher plants. Biochim. Biophys. Acta ,
636: 119-125.
MEISCH, H.U. & BENZSCHAWEL, H. (1978) Role of vanadium in
green plants. III. Influence on cell division of chlorella.
Arch. Microbiol ., 116(1): 91-95.
MERTZ, W. (1970) Some aspects of nutritional trace element
research. Fed. Proc ., 29: 1482-1488.
MINDEN, H. & ROTHE, R. (1966) [Biochemical mechanism of action
of vanadium.] Z. gesamte Hyg ., 12(5): 315-321 (in German).
MIRAMAND, P. & UNSAL, M. (1978) Acute toxicity of vanadium to
some marine benthic and phytoplanktonic species. Chemosphere ,
10: 827-832.
MIRZAEVA, K.H. (1965) [Trace elements in natural waters in
some districts of Uzbekistan.] In: [ Trace elements in agri-
culture ,] Tashkent, Science Publishers of Uzbekistan, pp. 122-
126 (in Russian).
MISIEWICZ, A. (1980) [Effect of air containing gasoline,
tungsten, titanium, cobalt, and vanadium on the phagocytic
activity of leucocytes.] Pol. Tyg. Lek ., 35(50): 1965-1967 (in
Polish).
MISIEWICZ, A. (1983) [Effect of air containing benzine,
wolfram, titanium, cobalt, and vanadium on peripheral blood.]
Med. Pr ., 34(3): 251-257 (in Polish).
MITCHELL, R.L. (1964) Trace elements in soil. In: Bear, F.E.,
ed. Chemistry of the soil , New York, Reinhold Publishing,
pp. 320-368.
MITCHELL, W.G. & FLOYD, E.P. (1954) Ascorbic acid and
ethylenediaminetetracetate (EDTA) as antidotes in experimental
vanadium poisoning. Proc. Soc. Exp. Biol. Med ., 85: 206-208.
MONTERO, M.R., GUERRI, C., RIBELLES, M., & GRISOLIA, S. (1981)
Inhibition of protein synthesis in cell cultures by vanadate and
in brain homogenates of rats fed vanadate. Physiol. Chem.
Physics , 13: 281-287.
MORGAN, W.K.C. & SEATON, A. (1984) Occupational lung diseases ,
2nd ed., Philadelphia, W.B. Saunders & Co.
MOUNTAIN, J.T. (1963) Detecting hypersusceptibility to toxic
substances. Arch. environ. Health , 6: 357-365.
MOUNTAIN, J.T., DELKER, L.L., & STOKINGER, H.E. (1953) Studies
in vanadium toxicology. I. Reduction in the cystine content of
rat hair. Am. Med. Assoc. Arch. Ind. Hyg. Occup. Med ., 8: 406.
MOUNTAIN, J.T., STOCKELL, F.R., Jr, & STOKINGER, H.E. (1955)
Studies in vanadium toxicology. Am. Med. Assoc. Arch. Ind.
Health , 12: 494.
MUHLER, J.C. (1957) The effect of vanadium pentoxide,
fluorides, and tin compounds on the dental caries experience in
rats. J. dent. Res ., 36: 787-794.
MUSK, A.W. & TEES, J.G. (1982) Asthma caused by occupational
exposure to vanadium compounds. Med. J. Aust ., 1: 183-184.
MUSTAFIN, I.S., NECHAENKO, T.M., & FRUMINA, N.S. (1969) [ The
range of reagents available for vanadium ,] Moscow, Moscow State
University (in Russian).
MUZQUIN, V.N., KHAVZINA, L.B., ZOLOTAVIN, V.L., & BERRUKOV,
I.YA. (1981) [ Analytical chemistry of vanadium ,] Moscow,
Nauka Publishing House, 216 pp (in Russian).
MYERS, V.C. & BEARD, H.H. (1931) Studies in the nutritional
anaemia of the rat. II. Influence of iron plus supplements of
other inorganic elements upon blood regeneration. J. biol.
Chem ., 94: 89.
MYRON, D.R., GIVAND, S.H., & NIELSEN, F.H. (1977) Vanadium
content of selected foods as determined by flameless atomic
absorption spectroscopy. J. agric. food Chem ., 25(2): 297-299.
MYRON, D.R., ZIMMERMAN, T.J., SHULER, T.R., KLEVAY, L.M., LEE,
D.E., & NIELSEN, F.H. (1978) Intake of nickel and vanadium by
humans. A survey of selected diets. Am. J. clin. Nutr ., 31(3):
527-531.
NAS (1974) Vanadium , Washington DC, National Academy of
Sciences.
NAS (1978) Vanadium supply and demand outlook , Washington DC,
National Academy of Sciences, National Materials Advisory Board
(Publication NMAB-346).
NASON, A. (1958) The metabolic role of vanadium and molybdenum
in plants and animals. In: Lamb, C.A., Bentley, 0.G., & Beattie,
J.M., ed. Trace elements , New York, Academic Press, pp. 269-
296.
NAYLOR, G.J. (1984) Vanadium and manic depressive psychosis.
Nutr. Health , 3(1-2): 79-85.
NAYLOR, G.J., SMITH, A.H., BRYCE-SMITH, D., & WARD, N.I. (1984)
Tissue vanadium levels in manic-depressive psychosis. Psychol.
Med ., 14(4): 767-772.
NECHAY, R. (1984) Mechanisms of action of vanadate. Ann. Rev.
Pharmacol. Toxicol ., 24: 501-524.
NECHAY, R. & SAUNDERS, J.P. (1978) Inhibition by vanadium of
sodium and potassium dependent adenosinetriphosphatase derived
from animal and human tissues. J. environ. Pathol. Toxicol ., 2:
247-262.
NELSON, W.L. (1973) Direct desulfurization handles low-metals
topped crudes. Oil Gas J ., 19 November: 54-56.
NIELSEN, F.H. & OLLERICH, D.A. (1973) Studies on a vanadium
deficiency in chicks. Fed. Proc ., 32: 929.
NIOSH (1977) Occupational exposure to vanadium , Washington DC,
National Institute for Occupational Safety and Health (Document
No. 77-222), 142 pp.
NISHIYAMA, K., ONISHI, K., MIYAI, T., SUGIURA, S., SAITO, K., &
TOJYO, Y. (1977) [A survey of workers on vanadium pentoxide.]
Shikoku igaku zasshi , 31(6): 389-393 (in Japanese).
NOVAKOVA, S., NIKOLCHEV, G., ANGELIEVA, R., DINOEVA, S., &
MAUTNER, G. (1981) [Effect of vanadium on experimental
atherosclerosis.] Gig. i Sanit ., 11: 58-59 (in Russian).
NRC (1980) Drinking-water and health , Washington DC, National
Academy Press, Vol. 3, pp. 350-354 (Safe Drinking-Water
Committee).
OBERG, S.G., PARKER, R.D.R., & SHARMA, R.P. (1978)
Distribution and elimination of an intratracheally administered
vanadium compound in the rat. Toxicology , 11: 315-323.
OLEFFE, J. & WILMET, J. (1980) Generalized dermatitis from an
osteosynthesis screw. Contact Dermat ., 6: 365.
OLIVER, B.G. & COSGROVE, E.G. (1975) Metal concentrations in
the sewage, effluents and sludges of some Southern Ontario
wastewater treatment plants. Environ. Lett ., 9(1): 75-90.
OMANG, S.H. (1971) The determination of vanadium and nickel in
mineral oils by flameless graphite tube atomization. Anal. Chim.
Acta , 56: 470-473.
ORDHZONIKIDZE, E.K., ROSCHIN, A.V., SHALGANOVA, I.V., BOGOMAZOV,
M.YA., & KAZIMOV, M.A. (1977) [On the distribution and
elimination of vanadium from the organisms.] Gig. Tr. prof.
Zabol ., 6: 29-34 (in Russian).
ORVINI, E., LODOLA, L., SABBIONI, E., PIETRA, R., & GOETZ, L.
(1979) Determination of the chemical forms of dissolved
vanadium in freshwater as determined by 48V radiotracer experi-
ments and neutron activation analysis. Sci. total Environ ., 13:
195-207.
PARKER, R.D.R. & SHARMA, R.P. (1978) Accumulation and
depletion of vanadium in selected tissues of rats treated with
vanadyl sulfate and sodium orthovanadate. J. environ. Pathol.
Toxicol ., 2: 235-245.
PARKES, W.R. (1982) Occupational lung disorders , 2nd ed.,
London, Butterworths & Co.
PASTUHOV, A.I. & TRETJAKOV, M.A. (1959) Metallurgical
processing of cast iron from titanomagnetites from the Kackanar
district into steel. In: [ Problems of Kachkanar ,] Sverdlovsk,
Central Bureau of Technical Information, pp. 95-104 (in
Russian).
PATRICK, R. (1978) Effects of trace metals in the aquatic
ecosystem. Am. Sci ., 66: 185-191.
PAZHYNICH, V.M. (1966) Maximum permissible concentration of
vanadium pentoxide in the atmosphere. Hyg. Sanit ., 31: 6-11.
PAZHYNICH, V.M. (1967) [Experimental basis for the determina-
tion of maximum allowable concentration of vanadium pentoxide in
atmospheric air.] In: Rjazanov, V.A., ed. [ The biological
effect and hygienic importance of atmospheric pollutants ,]
Moscow, Medicina Publishing House, pp. 201-217 (in Russian).
PAZHYNICH, V.M. (1980) [Effect of aerosols of rare metals on
the functional state of the body of workers in conditions of
powder wire production.] Vrach. Delo , 3: 102-104 (in Russian).
PEJVE, J.V. & AJZUPIET, I.P. (1974) [A short review of the
results of research on the problem "Trace elements in plant
cultivation and livestock rearing in 1972".] In: [ Trace
elements in the USSR. XV. Methodological material ,] Riga,
Knowledge Publishers (in Russian).
PERRY, H.M., TEITLEBAUM, S., & SCHWARTZ, P.L. (1955) Effects
of antihypertensive agents on amino acid decarboxylation and
amine oxidation. Fed. Proc ., 14: 113-114.
PERRY, H.M., Jr, SCHWARTZ, P.L., & SAHAGIAN, B.M. (1969)
Effects of transition metals and of metal-binding antihyper-
tensive agents on tryptamine oxidase sand dopa decarboxylase.
Proc. Soc. Exp. Biol. Med ., 130: 273-277.
PETERBURGSKIJ, A.V. & TORMASOVA, E.E. (1969) [The effect of
vanadium on certain physiologycal processes in the pea.] Dok.
Timiryazevsk. Akad ., 149: (in Russian).
PETKEVICH, A.N., VILLER, G.E., & VOROTNIKOV, B.A. (1967)
[Some trace elements in natural waters of Kama's district.] In:
[ Trace elements in the biosphere and their application in
agriculture and medicine in Siberia and the Far East ,] Ulan-
Ude, Ulan-Ude Book Publishers, pp. 223-227 (in Russian).
PETUHOV, N.I., STANKEVICH, E.F., & VALACHANOVICH, V.I. (1969)
Some microelements in natural waters near the source of the
Kama. Tr. Kazanskogo Med. Inst ., 29: 100-103.
PHILLIPS, T.D., NECHAY, B.R., NELDON, S.L., KUBENA, L.F.,
HEIDELBAUGH, N.D., SHEPHERD, E.C., STEIN, A.F., & HAYES, A.W.
(1982) Vanadium-induced inhibition of renal Na+ K+-
adenosinetriphosphatase in the chicken after chronic dietary
exposure. J. Toxicol. environ. Health , 9: 651-661.
PHILLIPS, T.D., NECHAY, B.R., & HEIDELBAUGH, N.D. (1983)
Vanadium: chemistry and the kidney. Fed. Proc ., 42(13): 2969-
2973.
PIELSTICKER, F. (1954) [Health effects due to vanadium
compounds. Symptoms and prognosis.] Arch. Gewerbepathol.
Gewerbehyg ., 13: 73-96 (in German).
PILZ, W. & KOMISCHKE, S. (1972) [Determination of vanadium in
biological material and air. II. The decomposition of biological
material: air analyses.] Int. J. environ. anal. Chem ., 1(4):
275-282 (in German).
POERSEL, B. (1977) [ The biological and toxicological
significance of vanadium ,] Dusseldorf, Med. Diss., 86 pp (in
German).
PROESCHER, F., SEIL, H.H., & STILLIANS, A.W. (1917) A
contribution to the action of vanadium with particular reference
to syphilis. Am. J. Syph ., 1: 347-405.
PROKOPENKO, T.A. (1961) [Effect of industrial poisons,
fluorine, vanadium, manganese, and some drugs tested in
treatment on the tissue metabolism of compounds containing
phosphorus.] In: [ Proceedings of the 9th All-Union Pharmaco-
logical Conference, Sverdlovsk ,] Sverdlovsk, Central Bureau of
Technical Information, pp. 210-211 (in Russian).
PYY, L., KIVILUOTO, M., & PAKARINEN, A. (1980) Fingernail
cystine of vanadium workers. Int. Arch. occup. environ. Health ,
46(2): 179-182.
PYY, L., LAJUNEN, L.H.J., & HAKALA, E. (1983) Determination of
vanadium in work-place air by DCP emission spectrometry. Am.
Ind. Hyg. Assoc. J ., 44(8): 609-614.
PYY, L., HAKALA, E., & LAJUNEN, L.H.J. (1984) Screening for
vanadium in urine- and blood-serum by electrothermal atomic
absorption spectrometry and dc plasma atomic emission
spectrometry. Anal. Chim. Acta , 158: 297-303.
RAESAENEN, O., KIVILUOTO, M., RINNE, A., & RISSANEN, M. (1979)
Effects of vanadium on the upper respiratory tract of workers in
a vanadium factory: a macroscopic and microscopic study. Scand.
J. Work environ. Health , 5(1): 50-58.
RAJNER, V. (1960) [Effect of vanadium in the respiratory
tract.] Cesk. Otolaryngol ., 9: 202-204 (in Czech).
RALSTON, H.R. & SATO, E.S. (1971) Sodium removal as an aid to
neutron activation analysis. Anal. Chem ., 43: 129-131.
REINL, W. (1958) [Illnesses caused by basic slag, vanadium
slag, and vanadium compounds.] Staub , 18(5): 143-148 (in
German).
REZNIK, J.B. (1954) [The importance of vanadium compounds from
the point of view of industrial toxicology.] Vrach. Delo , 7:
627-632 (in Russian).
RHAN, K.A. (1971) Sources of trace elements in aerosols: an
approach to clean air , Ann Arbor, Michigan, University of
Michigan, Department of Meteorology, 309 pp (US Atomic Energy
Commission Contract No. COO-1705-9).
RHOADS, K. & SANDERS, C.L. (1985) Lung clearance, trans-
location, and acute toxicity of arsenic, beryllium, cadmium,
cobalt, lead, selenium, vanadium, and ytterbium oxides following
deposition in rat lung. Environ. Res ., 36(2): 359-378.
RIFKIN, R.J. (1965) In vitro inhibition of Na+ -K+ and Mg2+
ATPases by mono-, di-, and trivalent cations. Proc. Soc. Exp.
Biol. Med ., 120: 802-804.
RIGDON, L.P. & HARRAR, J.E. (1969) Determination of vanadium
by controlled-potential coulometry. Anal. Chem ., 41: 1673-1675.
ROITMAN, I., TRAVASSAS, L.R., AZEVEDO, H.P., & CURRY, A. (1969)
Choline, trace elements, and amino acids as factors for growth
of an enteric yeast, Candida slooffii. Sabouraudia J. Int. Soc.
Hum. Anim. Mycol ., 7: 15-19.
ROMAN, R.J., BONVENTRE, J.V., SILVA, P., & LECHENE, C. (1981)
Sodium orthovanadate diuresis in rats. J. Pharmacol. exp. Ther .,
218: 168-174.
RONOV, A.B. (1964) [General tendencies in the evolution of the
composition of the Earth's crust, the oceans and the
atmosphere.] Geohimija , 8: 715-743 (in Russian).
ROSE, E.R. (1973) Geology of vanadium and vanadiferous
occurrences in Canada , Ottawa, Geological Survey of Canada,
Department of Energy, Mines and Resources, pp. 7-101 (Economic
Geology Report No. 27).
ROSHCHIN, A.V. (1962) [The hazards of occupational vanadium
intoxication in boiler cleaning operations at electric power
stations operating on fuel oil, and prevention problems.] Gig.
Tr. prof. Zabol ., 6: 17-22 (in Russian).
ROSHCHIN, A.V. (1963a) [Biological effects of rare, dispersed,
and other metals and their compounds used in industry:
vanadium.] In: [ Toxicology of the less common metals ,] Moscow,
Meditsina, pp. 83-95 (in Russian).
ROSHCHIN, A.V. (1963b) [Hygienic evaluation of dust from
vanadium-containing slag.] Gig. i Sanit ., 12: 23-29 (in
Russian).
ROSHCHIN, A.V. (1964) [Vanadium metallurgy in the light of
industrial hygiene and questions concerned with the prevention
of occupational diseases and intoxication.] Gig. Tr. prof.
Zabol ., 8: 3-10 (in Russian).
ROSHCHIN, A.V. (1967a) Toxicology of vanadium compounds used
in modern industry. Hyg. Sanit ., 32: 345-352.
ROSHCHIN, A.V. (1967b) Vanadium. In: Izraelson, Z.I., ed.
Toxicology of the rare metals , Jerusalem, Israel Program for
Scientific Translations Ltd., pp. 52-59 (AEC-tr-6710).
ROSHCHIN, A.V. (1968) [ Vanadium and its compounds ,] Moscow,
Medicina Publishing House (in Russian).
ROSHCHIN, A.V. & ORDZHONIKIDZE, E.K. (1986) [Metal toxico-
kinetics and its significance for prevention of occupational
poisoning.] Gig. Tr. prof. Zabol ., 3: 1-6 (in Russian).
ROSHCHIN, A.V., ILNITSKAYA, A.V., LUTSENKO, L.A., & ZHIDKOVA,
L.A. (1964) [Research into effects of vanadium trioxide dust
on organisms.] Gig. Tr. prof. Zabol ., 8: 25-30 (in Russian).
ROSHCHIN, A.V., ORDZHONIKIDZE, E.K., & SHALGANOVA, I.V. (1980)
Vanadium - toxicity, metabolism, carrier state. J. Hyg.
Epidemiol. Microbiol. Immunol ., 24(4): 377-383.
RUHLING, A. (1971) [Pollution with heavy metals in the greater
Stockholm area.] In: [ Report No. 12 on ecological heavy metal
research ,] Lund, University of Lund, Department of Plant
Ecology (in Swedish).
RUNDBERG, G. (1939) Modern problems of occupational diseases
in Sweden. Nord. Med ., 2: 1845-1856.
SABBIONI, E., MARAFANTE, E., AMANTINI, L., & UBERTALLI, L.
(1978) Similarity in metabolic patterns of different chemical
species of vanadium in the rat. Bioinorg. Chem ., 8: 503-515.
SABBIONI, E., MARAFANTE, E., PIETRA, R., GOETZ, L., GIRARDI, F.,
& ORVINI, E. (1979) The association of vanadium with the iron
transport system in human blood as determined by gel filtration
and neutron activation analysis. In: Proceedings of a Symposium
on Nuclear Activation Techniques in the Life Sciences, Vienna,
22-26 May, 1978 , Vienna, International Atomic Energy Agency, pp.
179-192.
SABBIONI, E., MARAFANTE, E., RADE, J., GREGOTTI, C., DI NUCCI,
A., & MANZO, L. (1981) Biliary excretion of vanadium in rats.
Toxicol. Eur. Res ., 3(2): 93-98.
SANDELL, E., ed. (1959) Vanadium. In: Colorimetric determina-
tion of traces of metals , 3rd ed., New York, Interscience
Publishers, Vol. 3, pp. 923-940 (Chemical Analysis Series).
SCHLETTWEIN-GZELL, D. & MOMMSEN-STRAUB, S. (1973) [Trace
elements in foodstuffs. XI. Vanadium.] Int. Z. Vitam.-
Ernährungsforsch ., 43(2): 242-250 (in German).
SCHROEDER, H.A. (1966) Municipal drinking-water and cardio-
vascular death rates. J. Am. Med. Assoc ., 195: 81-85.
SCHROEDER, H.A. (197Oa) Vanadium , Washington DC, American
Petroleum Institute (Air Quality Monograph No. 70-13).
SCHROEDER, H.A. (197Ob) A sensible look at air pollution by
metals. Arch. environ. Health , 21: 798-806.
SCHROEDER, H.A. & MITCHENER, M. (1975) Life-term effects of
mercury, methylmercury, and nine other trace metals on mice. J.
Nutr ., 105(4): 452-458.
SCHROEDER, H.A., BALASSA, J.J., & TIPTON, I.H. (1963) Abnormal
trace metals in man: vanadium. J. chron. Dis ., 16: 1047-1071.
SCHROEDER, H.A., MITCHENER, M., & NASON, A.P. (1970) Zirconium,
niobium, antimony, vanadium, and lead in rats: life-term
studies. J. Nutr ., 100(1): 59-68.
SCHUMANN-VOGT, B. (1969) [Health hazards in industry caused by
vanadium.] Zentralbl. Arbeitsmed. Arbeitsschutz , 19(2): 33-39
(in German).
SCHWARZ, K. & MILNE, D.B. (1971) Growth effects of vanadium in
the rat. Science , 174: 426-428.
SELJANKINA, K.P. (1961) [Data for determining the maximum
permissible content of vanadium in water basins.] Gig. i Sanit .,
26(10): 6-12 (in Russian).
SHAH, K.R., FILBY, R.H., & HALLER, W.A. (1970) Determination
of trace elements in petroleum by neutron activation analysis.
1. Determination of Na, S, Li, La, V, Mn, Cu, Ga, and Br. J.
radioanal. Chem ., 6: 185-192.
SHARMA, R.P., BOURCIER, D.R., BRINKERHOFF, C.R., & CHRISTENSEN,
S.A. (1981) Effects of vanadium on immunologic functions. Am.
J. ind. Med ., 2(2): 91-99.
SHARMA, R.P., COULOMBE, R.A., Jr, & SRISUCHART, B. (1986)
Effects of dietary vanadium exposure on levels of regional brain
neurotransmitters and their metabolites. Biochem. Pharmacol .,
35(3): 461-465.
SHEVCHENKO, I.T. & GORODYNSKYJ, V.I. (1964) [ Polarography in
medicine and biology ,] Kiev, State Medical Publishers of the
Ukraine (in Russian).
SHEVCHENKO, S.D. (1965) [Content of vanadium in bone tumours.]
Ortop. Travmatol. Protez ., 8: 83 (in Russian).
SHILINA, A.I. & MALAKHOV, S.G. (1974) [Trace elements in
natural waters and the atmosphere.] In: [ A collection of papers
on experimental meteorology ,] Moscow, Gidrometeozdat (in
Russian).
SHKOLENOK, G.F., NIKOLAEVA, E.R., & AGASYAN, P.K. (1977)
[Differential controlled-potential coulometry. Determination of
vanadium(V) and vanadium(IV).] Zavod. Lab ., 43(9): 1048-1050 (in
Russian).
SIMONOFF, M., CONRI, C., & SIMONOFF, G. (1986) Vanadium in
depressive states. Acta pharmacol. toxicol . (Copenhagen) ,
59(Suppl. 7): 463-466.
SINGH, D. & SHARMA, S. (1970) Amperometric permanganometric
estimations at low concentrations in stirred solutions. Indian
J. Chem ., 8(2): 192.
SINGH, J., NORDLIE, R.C., & JORGENSON, R.A. (1981) Vanadate:
a potent inhibitor of multifunctional glucose-6-phosphatase.
Biochim. Biophys . Acta , 678: 477-482.
SJÖBERG, S.-G. (1950) Vanadium pentoxide dust: A clinical and
experimental investigation on its effect after inhalation. Acta
med. Scand ., 138(Suppl. 238): 1-188.
SJÖBERG, S.-G. (1951) Possible importance of allergy in
diseases caused by inhalation of inorganic dust. Acta allergol .,
4(4): 357-361.
SJÖBERG, S.-G. (1955) Vanadium bronchitis from cleaning oil-
fired boilers. Arch. ind. Health , 11(6): 505-512.
SJÖBERG, S.-G. (1956) Vanadium dust, chronic bronchitis and
possible risk of emphysema. A follow-up investigation of workers
at a vanadium factory. Acta med. Scand ., 44(5): 381-386.
SJÖBERG, S.-G. & RIGNER, K.-G. (1956) [Skin, eye, and
respiratory tract symptoms associated with the cleaning of oil-
fired boilers.] Nord. Hyg. Tidskr ., 37: 217-228 (in Swedish).
SLAVIN, W., CARNRICK, G.R., MANNING, D.C., & PRUSZKOWSKA, E.
(1983) Recent experiences with the stabilized temperature
platform furnace and zeeman background correction. At.
Spectrosci ., 4(3): 69-86.
SMITH, J.B. (1983) Vanadium ions stimulate DNA synthesis in
Swiss mouse 3T3 and 3T6 cells. Proc. Natl Acad. Sci. (USA) ,
80(20): 6162-6166.
SNYDER, F. & CORNATZER, W.E. (1958) Vanadium inhibition of
phospholipid sulphydryl activity in rat liver. Nature (Lond.) ,
182: 462.
SOKOLOV, S.M. (1986) Metholodological aspects of assessing
atmospheric contamination with metal aerosols in the vicinity of
thermal power complexes. J. Hyg. Epidemiol. Microbiol.
Immunol ., 30(3): 249-254.
SOMERVILLE, J. & DAVIES, B. (1962) Effect of vanadium on serum
cholesterol. Am. Heart J ., 64: 54-56.
SÖREMARK, R. (1967) Vanadium in some biological specimens. J.
Nutr ., 92: 183-190.
SÖREMARK, R. & ULLBERG, S. (1962) Distribution and kinetics of
48V2O5 in mice. In: Friend, N., ed. Proceedings of a Symposium
on the Use of Radioisotopes in Animal Biology and the Medical
Sciences, Mexico City, 21 November-1 December, 1961 , New York,
Academic Press, Vol. 2, pp. 103-114.
SÖREMARK, R., ULLBERG, S., & APPELGREN, L.E. (1962) Auto-
radiographic localization of vanadium pentoxide (V24805) in
developing teeth and bones of rats. Acta odontol. Scand ., 20:
225-232.
SPRAGUE, J.B., HOLDWAY, D.A., & STENDAHL, D.H. (1978) Acute
and chronic toxicity of vanadium to fish , Edmonton, Environment
Alberta, 92 pp (Prepared for the Alberta Oil Sands Research
Program (Project No. AF 3.5.1).
STEFFEN, R.P., PAMNANI, M.B., CLOUGH, D.L., HUOT, S.J., MULDOON,
S.M., & HADDY, F.J. (1981) Effect of prolonged dietary
administration of vanadate on blood pressure in the rat.
Hypertension , 3: I/173-I/178.
STENDAHL, D.H. & SPRAGUE, J.B. (1982) Effects of water
hardness and pH on vanadium lethality to rainbow trout. Water
Res ., 16: 1479-1488.
STOCKS, P. (1960) On the relations between atmospheric
pollution in urban and rural localitites and mortality from
cancer, bronchitis and pneumonia, with particular reference to
3:4 benzopyrene, beryllium, molybdenum, vanadium, and arsenic.
Br. J. Cancer , 14: 397-418.
STOKINGER, H.E., WAGNER, W.D., MOUNTAIN, J.T., STOCKELL, F.R.,
DOBROGORSKI, O.J., & KEENAN, R.G. (1967) In Vanadium V. In:
Clayton, G.D. & Clayton, F.E., eds. Patty's Industrial hygiene
and toxicology , 3rd revised ed. (1981), New York, Interscience,
Vol. 2A, pp. 2013-2033.
STONER, G.D., SHIMKIN, M.B., TROXELL, M.C., THOMPSON, T.L., &
TERRY, L.S. (1976) Test for carcinogenicity of metallic
compounds by the pulmonary tumor response in strain a mice.
Cancer Res ., 36: 1744-1747.
STRAHOV, N.F. (1947) [Iron-ore facies and their analogues in
the history of the earth.] Tr. Inst. Geol. AN SSSR, geol. Ser .,
73: 22 (in Russian).
STRAHOV, N.M. (1968) [On the theory of geochemical process in
humid zones.] In: [ A collection of papers on the geochemistry
of sedimentary rocks and ores ,] Moscow, Na++uka Publishing House,
pp. 102-134 (Documentation of the Seventh All-Union Conference
on Lithology of the Academy of Sciences of the USSR) (in
Russian).
STRASIA, C.A. (1971) Vanadium: essentiality and toxicity in
the laboratory rat , Ann Arbor, Michigan Purdue University (Ph.D.
Thesis).
STROOP, S.D., HELINEK, G., & GREENE, H.L. (1982) More
sensitive flameless atomic absorption analysis of vanadium in
tissue and serum. Clin. Chem ., 28(1): 79-82.
STYRIS, D.L. & KAYE, J.H. (1982) Mechanisms of vaporization of
vanadium pentaoxide from vitreous carbon and tantalum furnaces
by combined atomic absorption/mass spectrometry. Anal. Chem .,
54: 864-869.
SUGAWARA, K., NAITO, H., & YAMADA, S. (1956) Geochemistry of
vanadium in natural waters. J. earth Sci. Nagoya Univ ., 4(1):
44-61.
SUMINO, K., HAYAKAWA, K., SHIBATA, T., & KITAMURA, S. (1975)
Heavy metals in normal Japanese tissues. Arch. environ. Health ,
30: 487-494.
SUN, MIANLING, ed. (1987) Toxicity of vanadium and its
environmental health standard , Chengdu, West China University of
Medical Sciences, 20 pp.
SVERDRUP, H.U., JOHNSON, M.W., & FLEMING, R.H. (1950) The
oceans, their physics, chemistry and general biology , Englewood
Cliffs, New Jersey, Prentice-Hall Inc., pp. 175-177.
SVOBODA, P., TEISINGER, J., PILAR, J., & VYSKOCHIL, F. (1984)
Vanadyl (VO2+) and vanadate (VO3-) ions inhibit the brain
microsomal Na, K-AtPase with similar affinities. Protection by
transferrin and noradrenaline. Biochem. Pharmacol ., 33: 2485-
2491.
SWARUP, G., COHEN, S., & GARBERS, D.L. (1982) Inhibition of
membrane phosphotyrosyl-protein phosphatase activity by
vanadate. Biochem. biophys. Res. Commun ., 107: 1104-1109.
SYMANSKI, J. (1939) [Occupational vanadium poisoning, its
occurrence and symptomatology.] Arch. Gewerbepathol.
Gewerbehyg ., 9: 295-313 (in German).
TAKAHASHI, H. & NASON, A. (1957) Tungstate as a competitive
inhibitor of molybdate in nitrate assimilation and N2 fixation
by azotobacter. Biochim. Biophys. Acta , 23: 433-435.
TALVITIE, N.A. & WAGNER, W.D. (1954) Studies in vanadium
toxicology. II. Distribution and excretion of vanadium in
animals. Arch. ind. Hyg ., 9: 414-422.
TANDON, A.G. & BHATTACHARYA, S.C. (1961) Use of N-2-thio-
phenecarbonyl- N-p-tolylhydroxylamine and N-2-thiophene-
carbonyl- N-phenylhydroxylamine as reagents for vanadium. Anal.
Chem ., 33(9): 1267.
TARA, S., CAVIGNEAUX, A., & DEPLACE, Y. (1953) Intoxication
par le vanadate de calcium. Arch. Mal. prof. Méd. Trav. Séc.
soc ., 14: 378-380.
TEBROCK, H.E. & MACHLE, W. (1968) Exposure to europium-
activated yttrium orthovanadate: a cathodoluminescent phosphor.
J. occup. Med ., 10(12): 692-696.
THOMAS, D.L.G. & STIEBRIS, K. (1956) Vanadium poisoning in
industry. Med. J. Aust ., 1: 607-609.
THOMPSON, H.J., CHASTEEN, N.D., & MEEKER, L.D. (1984) Dietary
vanadyl (IV) sulfate inhibits chemically-induced mammary
carcinogenesis. Carcinogenicity , 5: 849-851.
THURAUF, J., SYGA, G., & SCHALLER, K.H. (1979) Field tests
carried out to determine the occupational exposure to vanadium.
Zbl. Bakteriol. Hyg., I. Abt. Orig. B , 168: 273-290.
TIPTON, I.H. & COOK, M.J. (1963) Trace elements in human
tissue. II. Adult subjects from the United States. Health Phys .,
9: 103-145.
TIPTON, I.H. & SHAFER, J.J. (1964) Statistical analysis of
lung trace element levels. Arch. environ. Health , 8: 58-67.
TIPTON, I.H., STEWART, P.L., & DICKSON, J. (1969) Patterns of
elemental excretion in long-term balance studies. Health Phys .,
16: 455-462.
TJURJUKANOV, A.N. (1963) Vanadium. In: Trace elements in
agriculture , Kiev, Gosselhozizdat, pp. 472-475.
TODRIA, F.F. (1963) [Identification of vanadium in ashes of
Tkibul coals.] In: [ Abstracts of scientific papers of Tbilisi
Medical Institute ,] Tbilisi, Tbilisi State Medical Institute,
p. 144 (in Russian).
TOLMAN, E.L., BARRIS, E., BURNS, M., PANSINI, A., & PARTRIDGE,
R. (1979) Effects of vanadium on glucose metabolism in vitro .
Life Sci ., 25: 1159-1164.
TONEY, J.H., MURTHY, M.S., & MARKS, T.J. (1985) Biodistribu-
tion and pharmacokinetics of vanadium following intraperitoneal
administration of vanadocene dichloride to mice. Chem.-biol.
Interact ., 56(1): 45-54.
TRELLES, R.A., LARGHI, L.A., & PAEZ, L.J.P. (1970) [The health
problem of human drinking-water with high contents of arsenic,
vanadium, and fluorine.] Saneamiento , 34(217): 31-80 (in
Spanish).
TROPPENS, H. (1969) [The uncertainties of vanadium pentoxide
intoxication.] Dtsch. Gesundheitswes ., 24: 1089-1092 (in
German).
TRUMMERT, W. & BOEHM, G. (1957) [The trace element vanadium
and its haemopoietic action.] Blut , 3: 210-216 (in German).
TYLER, G. (1970) [Pollution with heavy metals in the
Norrkkoping area. II. Molybdenum and vanadium.] In: [ Report No.
12 on ecological heavy metal research ,] Lund, University of
Lund, Department of Plant Ecology (in Swedish).
UENO, S. & ISHIZAKI, M. (1980) Concentrations of vanadium in
human urine and hair. Jpn. J. ind. Health , 22: 318-379.
UNDERWOOD, E.J. (1977) Vanadium. In: Trace elements in human
and animal nutrition , 4th ed., New York, Academic Press,
pp. 388-397.
UNSAL, M. (1978) Study of the transfer pathways and the
accumulation phenomena of vanadium in mollusca Mytilus edulis.
Rev. int. oceanogr. Med ., 51/52: 71-81.
US BUREAU OF MINES (1974) Metals Information Bulletin. Washing-
ton DC, US Department of the Interior, Bureau of Mines, Branch
of Ferrous Metals.
US EPA (1977) Scientific and technical assessment report on
vanadium , Washington DC, US Environmental Protection Agency
(STAR series, EPA-600/6-77-002).
USMONOV, S.M. (1973) [Vanadium content in constituents of
bone, urine, and faeces in sufferers of acute and chronic
dysentery.] In: [ Epidemiological, microbiological, and clinical
problems in the infectious pathology of Uzbekistan ,] Tashkent,
Institute of Hygiene, Sanitation, and Occupational Diseases,
pp. 125-128 (in Russian).
USUTANI, S., NISHIAMA, K., SATO, I., MATSURA, K., SAWADA, Y.,
HOSOKAWA, Y., & IZUMI, S. (1979) [An investigation of the
environment in a certain vanadium refinery.] Jpn. J. ind.
Health , 21: 11-20 (in Japanese).
VAN DEN BERG, C.M.G. & HUANG ZI QIANG (1984) Direct electro-
chemical determination of dissolved vanadium in seawater by
cathodic stripping voltammetry with the hanging mercury drop
electrode. Anal. Chem ., 56(13): 2383-2386.
VANSELOW, A.P. (1950) Unpublished data cited by Pratt, P.F.
In: Diagnostic criteria for plants and soils, University of
California, Division of Agricultural Sciences , University of
California, pp. 480-482.
VAN ZINDEREN BAKKER, E.M. & JAWORSKI, J.F. (1980) Effects of
vanadium in the Canadian environment , Ottawa, National Research
Council of Canada (Publication No. 18132).
VERSIECK, J. & CORNELIS, R. (1980) Normal levels of trace
elements in human blood-plasma or -serum. Anal. Chim. Acta , 116:
217-254.
VEYS, C. (1983) Possibilités offertes par les méthodes
modernes de polarographie au dosage à éléments tracés. Ind.
Sci ., 59(3): 1-6.
VIGLIANI, E.C. (1969) WHO Technical Report Series No. 415:
Permissible levels of occupational exposure to airborne toxic
substances (Sixth Report of the Joint ILO/WHO Committee on
Occupational Health) , Geneva, World Health Organization, pp. 5-
16.
VlNOGRADOV, A.P. (1937) [The chemical elements entering into
the composition of marine organisms. Part II.] Tr. biogeokhim.
Lab ., 4: 217 (in Russian).
VINOGRADOV A.P. (1944) [The geochemistry of trace elements in
seawater.] Uspehi Himii , 13(1): 123-129 (in Russian).
VINOGRADOV, A.P. (1957) [ Geochemistry of rare and dispersed
chemical elements in soils ,] 2nd ed (revised), Moscow, USSR
Academy of Sciences, pp. 122-129, 216-217 (in Russian).
VINOGRADOV, A.P. (1959) [ The geochemistry of rare and
dispersed elements in soil ,] 2nd ed., New York, Consultants
Bureau Inc., 209 pp (in Russian).
VINTINNER, F.J., VALLENAS, R., CARLIN, C.E., WEISS, R., MACHER,
C., & OCHOA, R. (1955) Study of the health of workers employed
in mining and processing of vanadium ore. Arch. ind. Health ,
12: 635.
VOORS, A.W. (1971) Minerals in the municipal water and
atherosclerotic heart death. Am. J. Epidemiol ., 93: 259-266.
VOUK, V. (1979) Vanadium. In: Friberg, L., Nordberg, G.F., &
Vouk, V., ed. Handbook on the toxicology of metals , Amsterdam,
New York, Oxford, Elsevier Science Publishers, pp. 659-674.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1980) A specific
enzyme is not necessary for vanadate-induced oxidation of NADH.
Nature (Lond.) , 286(5772): 516-517.
VYSKOCIL, F., TEISINGER, J., & DLOUHA, H. (1981) The disparity
between effects of vanadate (V) and vanadyl (IV) ions on (Na+ -
K+) AtPase and K+-phosphatase in skeletal muscle. Biochem.
biophys. Res. Commun ., 100: 982-987.
WALDRON, H.A. (1979) Metal poisoning: the three common causes.
Mod. Med ., 24(9): 45-47.
WALLACE, A., ALEXANDER, G.V., & CHADHURY, F.M. (1977) Phyto-
toxicity of cobalt, vanadium, titanium, silver, and chromium.
Commun. soil. Sci. plant Anal ., 8(9): 751-756.
WARINGTON, K. (1955) The influence of iron supply on toxic
effects of manganese, molybdenum and vanadium in soybeans, peas,
and flax. Ann. appl. Biol ., 41: 1-22.
WATANABE, H., MURAYAMA, H., & YAMAOKA, S. (1966) [Some
clinical findings in vanadium workers.] Jpn. J. ind. Health ,
8(7): 23-27 (in Japanese).
WATERS, M.D. (1977) Toxicology of vanadium. Adv. mod.
Toxicol ., 2: 147-189.
WATERS, M.D., GARDNER, D.E., & COFFIN. D.L. (1974) Cytotoxic
effects of vanadium on rabbit alvolar macrophages in vitro .
Toxicol. appl. Pharmacol ., 28: 253-263.
WATERS, M.D., GARDNER, D.E., ARANYI, C., & COFFIN, D.L. (1975)
Metal toxicity for rabbit alveolar macrophages in vitro.
Environ. Res ., 9: 32-47.
WEAST, R.C. (1987) CRC handbook of chemistry and physics , 67th
ed., Boca Raton, Florida, CRC Press.
WEI, C.I. & MISRA, H.P. (1982) Cytotoxicity of ammonium meta-
vanadate to cultured bovine alveolar macrophage. J. Toxicol.
environ. Health , 9(5-6): 995-1006.
WEI, C.I., AL BAYATI, M.A., CULBERTSON, M.R., ROSENBLATT, L.S.,
& HANSEN, L.D. (1982) Acute toxicity of ammonium metavanadate
in mice. J. Toxicol. environ. Health , 10(4/5): 673-687.
WEISZ, H., ROTHMAIER, K., & LUDWIG, H. (1974) Kinetic
determination of thorium, vanadium, and iodide with the use of a
potentiometer. Anal. Chim. Acta , 73(1): 224-228.
WELCH, R.M. (1973) Vanadium uptake by plants. Absorption
kinetics and the effects of pH, metabolic inhibitors, and other
anions and cations. Plant Physiol ., 51: 828-832.
WELCH, R.M. & ALLAWAY, W.H. (1972) Vanadium determination in
biological materials at nanogram levels by a catalytic method.
Anal. Chem ., 44(9): 1644-1647.
WENTZEL, C.T. (1985) Vanadium: a moderate recovery in 1984.
Eng. mining J ., March: 87-88.
WESTENFELDER, C., HAMBURGER, R.K., & GARCIA, M.E. (1981)
Effect of vanadate on renal tubular function in rats. Am. J.
Physiol ., 240: F522-F529.
WIDE, M. (1984) Effect of short-term exposure to five
industrial metals on the embryonic and fetal development of the
mouse. Environ. Res ., 33: 47-53.
WILLIAMS, D.L. (1973) Biological value of vanadium for rats,
chickens, and sheep , Ann Arbor, Michigan, Purdue University
(Ph.D. Thesis).
WILLIAMS, N. (1952) Vanadium poisoning from cleaning oil-fired
boilers. Br. J. ind. Med ., 9: 50-55.
WITKOWSKA, D. & BRZEZINSKI, J. (1979) Alteration of brain
noradrenaline, dopamine, and 5-hydroxytryptamine levels during
vanadium poisoning. Pol. J. Pharmacol. Pharm ., 31: 393-398.
WOOD, B.J., GALOBARDES, J.F., & ROGERS, L.B. (1982) Effect of
the organic ligand on the response for vanadium in flame atomic
emission spectrometry. Anal. Chim. Acta , 135: 145-152.
WRIGHT, L.D., LI, L.F., & TRAGER, R. (1960) The site of
vanadyl inhibition of chloesterol biosynthesis in liver
homogenates. Biochem. biophys. Res. Commun ., 3: 264-267.
WYERS, H. (1946) Some toxic effects of vanadium pentoxide.
Br. J. ind. Med ., 3: 177-182.
WYERS, H. (1948) Some recent observations on hazards in the
chemical industry. In: Proceedings of the 9th International
Congress of Industrial Medicine, London, 13-17 September ,
Bristol, John Wright, pp. 900-903.
YUKAWA, M., SUZUKI-YASUMOTO, M., AMANO, K., & TERAI, M. (1980)
Distribution of trace elements in the human body determined by
neutron activation analysis. Arch. environ. Health , 35(1): 36-
44.
ZAJIC, J.E. (1969) Microbial biochemistry. 12. Vanadium
biogeochemistry , New York, Academic Press, pp. 126-202.
ZEMKOVA, H., TEISINGER, J., & VYSKOCIL, F. (1982) The
comparison of vanadyl (IV) and insulin-induced hyperpolarization
of the mammalian muscle cell. Biochim. Biophys. Acta , 720: 405-
410.
ZENZ, C. & BERG, B.A. (1967) Human responses to controlled
vanadium pentoxide exposure. Arch. environ. Health , 14: 709-
712.
ZENZ, C., BARTLETT, J.P., & THIEDE, W.H. (1962) Acute vanadium
pentoxide intoxication. Arch. environ. Health , 5(6): 542-546.
ZHELJAZKOVA, B.G., CVETANOVA, A.L., & JACIMIRSKIJ, K.B. (1972)
[A catalytic method for determining nanogram quantities of
vanadium.] Zh. anal. Khim ., 27(4): 795-798 (in Russian).
ZOLLER, W.H., GLADNEY, E.S., & DUCE, R.A. (1974) Atmospheric
concentration and sources of trace metals at the South Pole.
Science , 183: 198-200.
ZOLLER, W.H., GORDON, G.E., GLADNEY, E.S., & JONES, A.G. (1973)
The sources and distribution of vanadium in the atmosphere. In:
Kothy, E.L., ed. Trace elements in the environment , Washington
DC, American Chemical Society, pp. 31-47 (Advances in Chemistry
Series 123).
ZOLOTAVIN, V.L. (1954) [Achieving a pure solution of chlor-
vanadyl.] Zh. obshch. Khim ., 24: 433 (in Russian).
