
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 82
CYPERMETHRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CYPERMETHRIN
INTRODUCTION
1. SUMMARY
1.1. General
1.2. Environmental transport, distribution, and transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.6. Effects on experimental animals and in vitro test systems
1.7. Mechanism of toxicity
1.8. Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial production
3.2. Use patterns
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Transport from soil to water
4.1.2. Transport within water bodies
4.2. Abiotic degradation
4.2.1. Photodegradation
4.2.1.1 Basic studies
4.2.1.2 Photodegradation
4.3. Biological degradation in soil
4.3.1. Mechanism
4.3.2. Degradation pathways (separate isomers)
4.3.3. Rates of degradation
4.3.3.1 Laboratory studies (separate isomers)
4.3.3.2 Field studies
4.4. Degradation in water and sediments
4.4.1. Laboratory studies
4.4.2. Field studies
4.5. Bioaccumulation and biomagnification
4.5.1. n-Octanol water-partition coefficient
4.5.2. Bioaccumulation in fish
4.5.3. Bioaccumulation in aquatic invertebrates
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food
5.1.4.1 Residues in food commodities from
treated crops
5.1.4.2 Residues in food of animal origin
5.2. General population exposure
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption, excretion, and distribution
6.1.1. Oral
6.1.1.1 Rat
6.1.1.2 Mouse
6.1.1.3 Dog
6.1.1.4 Cow
6.1.1.5 Sheep
6.1.1.6 Chicken
6.1.1.7 Man
6.1.2. Dermal
6.1.2.1 Cow
6.1.2.2 Sheep
6.1.2.3 Man
6.2. Metabolic transformation
6.2.1. In vitro studies
6.2.2. In vivo studies
6.2.3. Metabolism of the glucoside conjugate of
3-phenoxybenzoic acid
6.3. Metabolism in plants
6.4. Metabolism in fish
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.2.1. Fish
7.2.1.1 Acute toxicity
7.2.1.2 Long-term toxicity
7.2.2. Invertebrates
7.2.2.1 Acute toxicity
7.2.2.2 Long-term toxicity
7.2.3. Field studies
7.2.3.1 Deliberate overspraying
7.2.3.2 Monitoring of drift from ground
and aerial applications
7.3. Terrestrial organisms
7.3.1. Laboratory studies
7.3.1.1 Acute toxicity
7.3.1.2 Short-term toxicity
7.3.2. Field studies
7.3.2.1 Applications for tsetse fly control in
Nigeria
7.3.2.2 Honey bees
7.3.2.3 Soil fauna
7.3.2.4 Foliar predators and parasites
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Oral
8.1.2. Dermal
8.1.3. Intraperitoneal
8.1.4. Inhalation
8.1.5. Skin and eye irritation
8.1.6. Sensitization
8.2. Short-term exposures
8.2.1. Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.2. Dermal
8.2.2.1 Rabbit
8.2.3. Intravenous
8.2.3.1 Rat
8.3. Long-term exposures
8.3.1. Rat
8.3.2. Mouse
8.3.3. Dog
8.4. Special studies
8.4.1. Synergism/potentiation studies
8.4.1.1 Organophosphate mixture
8.4.1.2 Organochlorine mixture
8.4.2. Neurotoxicity
8.4.2.1 Characterization of the neurotoxic
effects
8.4.2.2 Neuropathological studies
8.4.2.3 Biochemical and electro-physiological
studies
8.4.2.4 Appraisal
8.4.3. Immunosuppressive action
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. Reproduction
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Rat
8.5.2.2 Rabbit
8.6. Mutagenicity and related end-points
8.6.1. In vitro studies
8.6.1.1 Microorganisms
8.6.1.2 Mammalian cells
8.6.2. In vivo studies
8.6.2.1 Host-mediated assay
8.6.2.2 Dominant lethal assay
8.6.2.3 Bone marrow chromosome study
8.6.2.4 Micronucleus test
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1 Rat
8.7.1.2 Mouse
8.8. Mechanisms of toxicity - mode of action
9. EFFECTS ON MAN
9.1. General population exposure
9.1.1. Acute toxicity: poisoning incidents
9.1.2. Controlled human studies
9.1.3. Epidemiological studies
9.2. Occupational exposure
9.2.1. Acute toxicity: poisoning incidents
9.2.2. Effects of short- and long-term exposure
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation
10.2. Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
WHO TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR CYPERMETHRIN
Members
Dr L. Albert, Environmental Pollution Programme, National
Institute of Biological Resource Research, Veracruz, Mexico
Dr E. Budd, Office of Pesticide Programs, US Environmental
Protection Agency, Washington DC, USA
Mr T.P. Bwititi, Ministry of Health, Causeway, Harare, Zimbabwe
Dr S. Deema, Ministry of Agriculture and Cooperatives, Bangkok
Thailand
Dr I. Desi, Department of Hygiene & Epidemiology, Szeged
University Medical School, Szeged, Hungary
Dr A.K.H. El Sebae, Pesticides Division, Faculty of
Agriculture, Alexandria University, Alexandria, Egypt
Dr R. Goulding, Keats House, Guy's Hospital, London, United
Kingdom (Chairman)
Dr J. Jeyaratnam, National University of Singapore, Department
of Social Medicine & Public Health, Faculty of Medicine,
National University Hospital, Singapore (Vice-Chairman)
Dr Y. Osman, Occupational Health Department, Ministry of Health
Khartoum, Sudan
Dr A. Takanaka, Division of Pharmacology, National Institute
of Hygienic Sciences, Tokyo, Japan
Representatives of Other Organizations
Dr Nazim Punja, European Chemical Industry, Ecology &
Toxicology Centre, (ECETOC), Brussels, Belgium
Miss J. Shaw, International Group of National Associations
of Manufacturers of Agrochemical Products (GIFAP), Brussels,
Belgium
Secretariat
Dr M. Gilbert, United Nations Environment Programme,
International Register of Potentially Toxic Chemicals,
Geneva, Switzerland
Dr T. Ng, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
Dr G. Quélennec, Pesticides Development & Safe Use Unit,
World Health Organization, Geneva, Switzerland
Secretariat (contd.)
Dr G.J. van Esch, Bilthoven, The Netherlands (Temporary
Adviser) (Rapporteur)
Dr E.A.H. van Heemstra-Lequin, Laren, The Netherlands
(Temporary Adviser)
Dr K.W. Jager, International Programme on Chemical Safety,
Division of Environmental Health, World Health Organization
Geneva, Switzerland (Secretary)
Dr R.C. Tincknell, Beaconsfield, Buckinghamshire, United
Kingdom (Temporary Adviser) (Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 988400 -
985850).
* * *
NOTE:
The proprietary information contained in this document cannot
be used in place of the documentation required for registration
purposes, because the latter has to be closely linked to the
source, the manufacturing route, and the purity/impurities of the
substance to be registered. The data should be used in accordance
with paragraphs 82-84 and recommendations paragraph 90 of the 2nd
FAO Government Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR CYPERMETHRIN
A WHO Task Group on Environmental Health Criteria for
Cypermethrin met in Geneva from 1 to 5 December 1986.
Dr M. Mercier, Manager, IPCS, opened the meeting and welcomed the
participants on behalf of the heads of the three IPCS co-sponsoring
organizations (UNEP/ILO/WHO). The group reviewed and revised the
draft criteria document and made an evaluation of the risks for
human health and the environment from exposure to cypermethrin.
The first draft of the criteria document was prepared by
Dr G.J. van Esch of the Netherlands on the basis of two data
sources:
1. A draft document based on published literature prepared by
Dr J. Miyamoto and Dr M. Matsuo of Sumitomo Chemical Co., Ltd.
with the assistance of the staff of the National Institute of
Hygienic Sciences, Tokyo, Japan. Dr I. Yamamoto of the Tokyo
University of Agriculture and Dr M. Eto of Kyushu University,
Japan assisted in the finalization of this draft.
2. A review of all studies on Cypermethrin, including the
proprietary information, made available to the IPCS by Shell
International Chemical Company Limited, London, United Kingdom.
The second draft of the criteria document was prepared by
Dr van Esch, incorporating comments received following the
circulation of the first draft to the IPCS contact points for
Environmental Health Criteria documents.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
INTRODUCTION
SYNTHETIC PYRETHROIDS - A PROFILE
1. During investigations to modify the chemical structures of
natural pyrethrins, a certain number of synthetic pyrethroids
were produced with improved physical and chemical properties
and greater biological activity. Several of the earlier
synthetic pyrethroids were successfully commercialized,
mainly for the control of household insects. Other more
recent pyrethroids have been introduced as agricultural
insecticides because of their excellent activity against a
wide range of insect pests and their non-persistence in the
environment.
2. The pyrethroids constitute another group of insecticides in
addition to organochlorine, organophosphorus, carbamate, and
other compounds. Pyrethroids commercially available to date
include allethrin, resmethrin, d-phenothrin, and tetramethrin
(for insects of public health importance), and cypermethrin,
deltamethrin, fenvalerate, and permethrin (mainly for
agricultural insects). Other pyrethroids are also available
including furamethrin, kadethrin, and tellallethrin (usually
for household insects), fenpropathrin, tralomethrin,
cyhalothrin, lambda-cyhalothrin, tefluthrin, cufluthrin,
flucythrinate, fluvalinate, and biphenate (for agricultural
insects).
3. Toxicological evaluations of several synthetic pyrethroids
have been performed by the FAO/WHO Joint Meeting on Pesticide
Residues (JMPR). The acceptable daily intake (ADI) has been
estimated by the JMPR for cypermethrin, deltamethrin,
fenvalerate, permethrin, d-phenothrin, cyfluthrin,
cyhalothrin, and flucythrinate.
4. Chemically, synthetic pyrethroids are esters of specific
acids (e.g., chrysanthemic acid, halo-substituted
chrysanthemic acid, 2-(4-chlorophenyl)-3-methylbutyric
acid) and alcohols (e.g., allethrolone, 3-phenoxybenzyl
alcohol). For certain pyrethroids, the asymmetric centre(s)
exist in the acid and/or alcohol moiety, and the commercial
products sometimes consist of a mixture of both optical
(1R/1S or d/1) and geometric ( cis/trans) isomers. However,
most of the insecticidal activity of such products may reside
in only one or two isomers. Some of the products (e.g.,
d-phenothrin, deltamethrin) consist only of such active
isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the axons in
the peripheral and central nervous systems by interacting
with sodium channels in mammals and/or insects. A single
dose produces toxic signs in mammals, such as tremors,
hyperexcitability, salivation, choreoathetosis, and
paralysis. The signs disappear fairly rapidly, and the
animals recover, generally within a week. At near-lethal
dose levels, synthetic pyrethroids cause transient changes in
the nervous system, such as axonal swelling and/or breaks and
myelin degeneration in sciatic nerves. They are not
considered to cause delayed neurotoxicity of the kind induced
by some organophosphorus compounds. The mechanism of
toxicity of synthetic pyrethroids and their classification
into two types are discussed in the Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate,
flucythrinate, and cypermethrin) may cause a transient
itching and/or burning sensation in exposed human skin.
7. Synthetic pyrethroids are generally metabolized in mammals
through ester hydrolysis, oxidation, and conjugation, and
there is no tendency to accumulate in tissues. In the
environment, synthetic pyrethroids are fairly rapidly
degraded in soil and in plants. Ester hydrolysis and
oxidation at various sites on the molecule are the major
degradation processes. The pyrethroids are strongly adsorbed
on soil and sediments, and hardly eluted with water. There
is little tendency for bioaccumulation in organisms.
8. Because of low application rates and rapid degradation in the
environment, residues in food are generally low.
9. Synthetic pyrethroids have been shown to be toxic for fish,
aquatic arthropods, and honey-bees in laboratory tests. But,
in practical usage, no serious adverse effects have been
noticed because of the low rates of application and lack of
persistence in the environment. The toxicity of synthetic
pyrethroids in birds and domestic animals is low.
10. In addition to the evaluation documents of FAO/WHO, there are
several good reviews and books on the chemistry, metabolism,
mammalian toxicity, environmental effects, etc. of synthetic
pyrethroids, including those by Elliot (1977), Miyamoto
(1981), Miyamoto & Kearney (1983), and Leahey (1985).
1. SUMMARY
1.1. General
Cypermethrin was initially synthesized in 1974 and first
marketed in 1977 as a highly active synthetic pyrethroid
insecticide, effective against a wide range of pests in
agriculture, public health, and animal husbandry. In agriculture,
its main use is against foliage pests and certain surface soil
pests, such as cutworms, but because of its rapid breakdown in
soil, it is not recommended for use against soil-borne pests below
the surface.
In 1980, 92.5% of all the cypermethrin produced in the world
was used on cotton; in 1982, world production was 340 tonnes of the
active material. It is mainly used in the form of an emulsifiable
concentrate, but ultra low volume concentrates, wettable powders,
and combined formulations with other pesticides are also available.
Chemically, cypermethrin is the alpha-cyano-3-phenoxy-benzyl
ester of the dichloro analogue of chrysanthemic acid, 2,2-dimethyl-
3-(2,2-dichlorovinyl) cyclopropanecarboxylic acid. The molecule
embodies three chiral centres, two in the cyclopropane ring and one
on the alpha cyano carbon. These isomers are commonly grouped into
four cis- and four trans-isomers, the cis-group being the more
powerful insecticide. The ratio of cis- to trans-isomers varies
from 50:50 to 40:60. Cypermethrin is the racemic mixture of all
eight isomers and, in this appraisal, cypermethrin refers
exclusively to the racemic mixture (ratio 50:50) unless otherwise
stated.
Most technical grades of cypermethrin contain more than 90% of
the active material. The material varies in physical form from a
brown-yellow viscous liquid to a semi-solid.
Cypermethrin has a very low vapour pressure and solubility in
water, but it is highly soluble in a wide range of organic
solvents. Analytical methods are available for the determination
of cypermethrin in commercially available preparations. In
addition, methods for the determination of residues of cypermethrin
in foods and in the environment are well established. In most
substrates, the practical limit of determination is 0.01 mg/kg.
1.2. Environmental Transport, Distribution, and Transformation
Unlike the natural pyrethrins, cypermethrin is relatively
stable to sunlight and, though it is probable that photo-
degradation plays a significant role in the degradation of the
product on leaf surfaces and in surface waters, its effects in
soils are limited. The most important photodegradation products,
2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropane-carboxylic acid
(CPA), 3-phenoxybenzoic acid (PBA) and, to some extent, the amide
of the intact ester, do not differ greatly from those resulting
from biological degradation.
Degradation in the soil occurs primarily through cleavage of
the ester linkage to give CPA, PBA, and carbon dioxide. Some of
the carbon dioxide is formed through the cleavage of both the
cyclopropyl and phenyl rings under oxidative conditions. The half-
life of cypermethrin in a typical fertile soil is between 2 and 4
weeks.
Cypermethrin is adsorbed very strongly on soil particles,
especially in soils containing large amounts of clay or organic
matter. Movement in the soil is therefore extremely limited and
downward leaching of the parent molecule through the soil does not
occur to an appreciable extent under normal conditions of use. The
two principal degradation products show, on the scale of Helling,
"intermediate mobility".
Cypermethrin is also relatively immobile in surface waters and,
when applied to the surface of a body of water at rates typical of
those used in agriculture applications, it is largely confined to
the surface film and does not reach deeper levels or the sediment
in appreciable concentrations. Cypermethrin also degrades readily
in natural waters with a typical half-life of about 2 weeks. It is
probable that both photochemical and biological processes play a
part. It has been shown that spray drift reaching surface waters
adjacent to sprayed fields does not result in long-term residues in
such waters.
Accumulation studies have shown that cypermethrin is rapidly
taken up by fish (accumulation factor approximately 1000); the
half-life of residues in rainbow trout was 8 days. In view of the
low concentrations of cypermethrin that are likely to arise in
water bodies and their rapid decline, it has been concluded that,
under practical conditions, residues in fish will not reach
measurable levels.
The results of field studies have shown that, when applied at
recommended rates, the levels of cypermethrin and its degradation
products in soil and surface waters are very low. Thus, it is
unlikely that the recommended use of cypermethrin will have any
effects on the environment.
1.3. Environmental Levels and Human Exposure
Cypermethrin is used in a wide range of crops. In general, the
maximum residue limits are low, ranging from 0.05 to 2.0 mg/kg in
the different food commodities. The residues will be further
reduced during food processing. In food of animal origin, residues
may range between 0.01 and 0.2 mg/kg product. Residues in non-food
commodities are generally higher, ranging up to 20 mg/kg product.
Total dietary intake values for man are not available, but it
can be expected that the oral exposure of the general population is
low to negligible.
1.4. Kinetics and Metabolism
Absorption of cypermethrin from the gastrointestinal tract and
its elimination are quite rapid. The major metabolic reaction is
cleavage of the ester bond. Elimination of the cyclopropane moiety
in the rat, over a 7-day period, ranged from 40 to 60% in the urine
and from 30 to 50% in the faeces; elimination of the phenoxybenzyl
moiety was about 30% in the urine and 55 to 60% in the faeces.
Biliary excretion is a minor route of elimination for the
cyclopropane moiety and small amounts are exhaled as carbon
dioxide. In principle, these absorption and elimination rates and
metabolic pathways hold for all animal species studied, including
domestic animals. In cows fed 100 mg cypermethrin/day, the highest
level found in milk was 0.03 mg/litre; levels of up to 0.1 mg/kg
tissue were found in subcutaneous fat. Under practical conditions,
the oral intake of cypermethrin with feed will be much lower.
Cypermethrin used as a spray or dip to combat parasites, may give
rise to maximum residues of 0.05 mg/kg tissue and 0.01 mg/litre
milk.
Laying hens exposed orally to 10 mg cypermethrin/kg diet for 2
weeks, showed cypermethrin levels of up to 0.1 mg/kg in the fat,
and up to 0.09 mg/kg in the eggs (predominantly in the yolk).
Consistent with the lipophilic nature of cypermethrin, the
highest mean tissue concentrations are found in body fat, skin,
liver, kidneys, adrenals, and ovaries. Only negligible
concentrations are found in the brain. The half-life of cis-
cypermethrin in the fat of the rat ranges from 12 to 19 days and
that of the trans-isomer, from 3 to 4 days. In mice, these half-
lives are 13 days and 1 day, respectively.
Overall, the metabolic transformation has been similar in the
different animals studied, including man. Differences that occur
have been related to the rate of formation rather than to the
nature of the metabolites formed and to conjugation reactions.
Cypermethrin (both the cis- and trans-isomers) is metabolized via
the cleavage of the ester bond to phenoxybenzoic acid and
cyclopropane carbolic acid. The fact that thiocyanate has been
identified in in vivo studies, indicates that the cyanide moiety
is further metabolized. The 3-phenoxybenzoic acid is mainly
excreted as a conjugate. The type of conjugate differs in a number
of animal species. Phenoxybenzoic acid is further metabolized to a
hydroxy derivative and conjugated with glucuronic acid or sulfate.
The cyclopropyl moiety is mainly excreted as a glucuronide
conjugate, hydroxylation of the methyl group only occurring to a
limited extent.
Ester cleavage is much slower in certain fish species than in
other animal species, the main metabolic pathway being
hydroxylation of the phenoxybenzoic and the cyclopropyl moieties.
Ester cleavage also takes place in plants. The phenoxybenzyl
and cyclopropyl moieties are readily converted into glucoside
conjugates. In mammals, these conjugates are hydrolysed into the
original acids and metabolized.
1.5. Effects on Organisms in the Environment
High doses of cypermethrin may exert transient minor effects on
microflora activity in the soil. However, no influence on
ammonification and nitrification has been found.
Cypermethrin is very toxic for fish (in laboratory tests
96-h LC50s were generally within the range of 0.4-2.8 µg/litre),
and aquatic invertebrates (LC50s in the range of 0.01-> 5
µg/litre). The presence of suspended solids decreases the toxicity
by at least a factor of 2, because of adsorption of cypermethrin to
the solids.
Cypermethrin is not very toxic for birds. Signs of
cypermethrin intoxication were seen at dose levels of 3000
mg/kg body weight or more. Administration of 1000 mg
cypermethrin/kg body weight to laying hens over a 5-day period did
not cause signs of intoxication. However, cypermethrin was highly
toxic for honey bees in laboratory tests, the oral LD50 ranging
from 0.03 to 0.12 µg/bee. Under field conditions, the hazard is
considerably lower, because of the repellent effect of cypermethrin
on worker honey bees, which lasts for at least 6 h after spraying.
Earthworms are not sensitive to cypermethrin. No deaths
occurred in worms exposed to levels of 100 mg/kg soil for 14 days.
In studies involving deliberate overspraying of experimental
ponds under field conditions, peak concentrations of 2.6 µg
cypermethrin/litre were measured in the water. Fish were not
affected, but populations of crustaceae, mites, and surface-
breathing insects were severely reduced. Most of these populations
returned to normal levels after 15 weeks. Free-swimming dipterous
larvae and bottom-dwelling invertebrates, snails, flatworms, etc.,
were not affected. Under normal agricultural conditions (during
which drifts may reach adjacent ditches or streams), the only
effects seen in surface-breathing or -dwelling insects were
hyperactivity or immobilization.
The relative toxicity of cypermethrin for pests and their
parasites and predators is such that the balance between host/prey
and parasites/predator may not be adversely affected in the field.
However, care should be taken where predatory mites are important
in pest management.
1.6. Effects on Experimental Animals and In Vitro Test Systems
The acute oral toxicity of cypermethrin is moderate. While LD50
values differed considerably among animal species depending on the
vehicle used and the cis-/ trans-isomeric ratios, the toxic
responses in all species were found to be very similar. The acute
toxicity of the trans-isomer in the rat (LD50 > 2000 mg/kg body
weight) was lower than that of the cis-isomer (LD50, 160 - 300
mg/kg body weight). The onset of toxic signs of poisoning was
rapid and they disappeared within several days in survivors. The
toxic signs are characterized by salivation, tremors, increased
startle response, sinuous writhing of the whole body
(choreoathetosis), and clonic seizures. Myelin and axon
degeneration were noted in the sciatic nerve at near lethal dose
levels.
Cypermethrin was moderately to severely irritating, when
applied to the skin or the eye of the rabbit. The severity was
partly dependent on the vehicle used. In guinea-pigs, a mild skin
sensitizing potential was found using the maximization test.
No toxic effects were observed in rats, fed cypermethrin at 100
mg/kg diet for 3 months. Furthermore, prolonged feeding of
cypermethrin (2 years) to dogs at a level of 300 mg/kg feed did not
produce any toxicological effects. A level of 600 mg/kg diet
resulted in reduced body weight gain, but no gross pathological or
histopathological effects were seen.
Two long-term studies on rats and one on mice were carried out.
The dose levels in the rat studies ranged up to 1500 mg/kg
diet, equivalent to 75 mg/kg body weight. No effects were seen at
150 mg/kg diet. At the highest dose level, reduced body weight
gain, increased liver weights (accompanied by increased smooth
endoplasmatic reticulum), and some haematological and biochemical
changes were observed. No increase in tumour incidence was noted.
The same type of effects were seen in the mouse study at 1600 mg
cypermethrin/kg diet. No effects were seen in the 400 mg/kg diet
group.
The effect of cypermethrin on the immune system was studied in
rats. The results showed the possibility of immunesuppression by
pyrethroids. More attention should be paid to this aspect, but, at
present, no opinion can be given about its relevance in the
extrapolation of these data for man.
Repeated oral administration of cypermethrin to rats and other
animal species at levels sufficiently high to produce significant
mortality in one group of animals, produced biochemical changes in
the peripheral nerves, consistent with sparse axonal degeneration.
Histopathological changes (swelling and/or disintegration of axons
of the sciatic nerve) were observed. There was no cumulative
effect. The magnitude of the change was substantially less than
that encountered with established neurotoxic agents. The
neurotoxic effects seem to be reversible; presumably the clinical
signs are not related to the induction of neuro-pathological
lesions.
Further evidence to support the minor nature of the nerve
lesions has been afforded by electrophysiological studies on rats.
Measurements of the maximal motor conduction velocities of the
sciatic and tail nerves of rats were made before, and at intervals
of up to 5 weeks after, exposure to a single dose or repeated high
doses of cypermethrin. It was concluded from the results that,
even at near-lethal doses, cypermethrin did not cause any effects
on maximal motor conduction velocities and conduction velocities of
the slower motor fibres in rat peripheral nerves. No delayed
neurotoxicity was observed in domestic hens.
The ability of the major metabolite of cypermethrin,
3-phenoxybenzoic acid, to produce axonal changes has been
investigated and found to be negative.
In a multigeneration reproduction study on rats, dose levels up
to 500 mg/kg feed were tested. The parent animals at the highest
dose level showed decreased food intake and reduction in body
weight gain. No influence on reproductive performance or on
survival of the offspring was found. However, at the highest dose
level, reductions in litter size and total litter weights were
seen. The pooled body weights of weaning pups of the 500 mg/kg
group were decreased over 3 generations. No effect was found with
100 mg cypermethrin/kg diet.
Embryotoxic and teratogenic effects were not found in rats
administered dose levels of up to 70 mg/kg body weight and clear
teratogenic effects were not observed in rabbits given dose levels
of up to 30 mg/kg body weight during days 6 - 18 of gestation.
Cypermethrin did not show any mutagenic activity in bacteria or
in yeast, with or without metabolic activation, or in V79 Chinese
hamster cells. Furthermore, cypermethrin gave negative results in
an in vivo chromosomal aberration test with Chinese hamsters and
in dominant lethal studies on mice. In a host-mediated assay with
mice, no increase in the rate of mitotic gene conversion in
Saccharomyces cerevisae was found. In a chromosome study using the
bone marrow cells of Chinese hamsters, cypermethrin did not
increase the number of chromosome abnormalities. However, in a
micronucleus test with mouse bone marrow cells, an increase in the
frequency of polychromatic erythrocytes with micronuclei was found
after oral and dermal applications of cypermethrin. Intraperitoneal
application gave a negative result. A sister chromatid exchange
study using bone marrow cells of mice showed a dose-response
related increase in sister chromatid exchanges of dividing cells.
In long-term/carcinogenicity studies, oral administration of
cypermethrin to rats did not induce an increase in the incidence of
tumours. In a mouse study, dose levels of up to 1600 mg
cypermethrin/kg diet did not produce any increase in tumours of
types not commonly associated with the mouse strain employed. The
incidence of tumours was similar in all groups with the exception
of a slight increase in the incidence of benign alveolar lung
tumours in the females in the 1600 mg/kg diet group. However, the
increased incidence, when compared with concurrent and historical
control incidence, was not sufficient to warrant concern. There
was no suggestion of increased malignancy and no evidence of a
decrease in the latency of the tumours. Furthermore, there was no
evidence of a carcinogenic response in the male mice in this study
and, as the results of mutagenicity studies on cypermethrin have
been mainly negative, it is concluded that there is no evidence for
the carcinogenic potential of cypermethrin.
1.7. Mechanism of Toxicity
Extensive studies have been carried out to explain the
mechanism of toxicity of cypermethrin, especially with regard to
the effects on the nervous system. The results strongly suggest
that the primary target site of cypermethrin (and of pyrethroid
insecticides in general) in the vertebrate nervous system is the
sodium channel in the nerve membrane. The alpha-cyano pyrethroids,
such as cypermethrin, cause a long-lasting prolongation of the
normally transient increase in sodium permeability of the nerve
membrane during excitation, resulting in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. Since the
mechanisms responsible for nerve impulse generation and conduction
are basically the same throughout the entire nervous system,
pyrethroids may well act in a similar way in various parts of the
central nervous system. It is suggested that the facial skin
sensations that may be experienced by people handling cypermethrin
are brought about by repetitive firing of sensory nerve terminals
in the skin, and may be considered as an early warning signal that
exposure has occurred.
1.8. Effects on Man
No cases of accidental poisoning have been reported as a result
of occupational exposure.
Skin sensations, reported by a number of authors to have
occurred during field studies, generally lasted only a few hours
and did not persist for more than one day after exposure.
Neurological signs were not observed. General medical and
extensive clinical blood-chemistry studies, and
electrophysiological studies on selected motor and sensory nerves
in the legs and arms did not show any abnormalities.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
 IUPAC chemical name (RS)-alpha-cyano-3-phenoxybenzyl(1RS)-
cis-, trans-3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylate
CAS chemical name (RS)-cyano(3-phenoxyphenyl)methyl(1RS)-
cis- trans-3-(2,2-dichloroethenyl)-2.2-
dimethylcyclopropane carboxylate
CAS registry number 52315-07-8 (formerly 69865-47-0)
RTECS registry number GZ1250000
Common synonyms NRDC 149, WL43467, PP 383, CG-A 55186
Common trade names Ammo, Avicade, Barricade, CCN 52,
Cymbush, Folcord, Imperator, Kafil
Super, Polytrin, Ripcord, Stockade
The asymmetric carbons are marked with an arrow and give rise
to the 8 isomers shown in Fig. 1. Conventionally, the 4 isomers
where the dichlorovinyl group is trans in relation to the
phenoxybenzyl group are referred to as trans-isomers, and the
other 4 as cis-isomers.
Cypermethrin is the ISO name for the pure racemic compound.
The technical products commonly available contain more than 90%
cypermethrin and the ratio of cis- to trans-isomers varies from
50:50 to 40:60. The data presented in this document refer to
products within this range of composition, unless otherwise stated.
2.2. Physical and Chemical Properties
Some physical and chemical properties of cypermethrin are given
in Table 1.
Cypermethrin is highly stable to light and at temperatures
below 220 °C. It is more resistant to acidic than to alkaline
media, with an optimum stability at pH 4. Cypermethrin is
hydrolysed under alkaline conditions in the same way as simple
aliphatic esters: the rate-determining step is the nucleophilic
attack by a hydroxyl group (Camilleri, 1984). Dilute aqueous
solutions are subject to photolysis, which occurs at a moderate
rate (Martin & Worthing 1977; FAO/WHO, 1980b; Meister et al., 1983;
Worthing & Walker, 1983).
Table 1. Some physical and chemical properties of cypermethrina
--------------------------------------------------------------------
Physical state varies from a viscous yellow liquid
to a semi-solid crystalline mass at
ambient temperatures
Relative molecular mass 416.3
Melting point up to 80 °C depending on purity
and cis: trans ratio
Boiling point decomposes at 220 °C
Density (22 °C) 1.12 g/ml
Solubility in water (20 °C) 0.009 mg/litre
Solubility in organic solvents:
hexane 103 g/litre
xylene > 450 g/litre
also comparable solubility in
cyclohexanone, ethanol, acetone,
and chloroform
Vapour pressure (20 °C) 1.9 x 10-7 Pa (1.4 x 10-9 mmHg)
n-octanol/water partition 2 x 106 (log Pow 6.3)
coefficient
--------------------------------------------------------------------
a From: FAO/WHO (1980b); Grayson et al (1982); Working & Walker (1983).
2.3. Analytical Methods
The most widely adopted procedures for the determination of
cypermethrin residues in crops, soil, animal tissues and products,
and environmental samples are based on extraction of the residue
with organic solvent, clean-up of the extract, as necessary, by
means of solvent-solvent partition and adsorption column
chromatography, followed by determination of the residue using gas
chromatography with electron capture detector (GC/ECD). The
identity of residues can be confirmed by GC with mass selective
detection (GC-MSD) or by thin-layer chromatography (TLC) followed
by GC/ECD.
Methods using these procedures have been applied for the
determination of cypermethrin residues in the presence of other
synthetic pyrethroids or other classes of pesticides, including
organochlorine insecticides.
IUPAC chemical name (RS)-alpha-cyano-3-phenoxybenzyl(1RS)-
cis-, trans-3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylate
CAS chemical name (RS)-cyano(3-phenoxyphenyl)methyl(1RS)-
cis- trans-3-(2,2-dichloroethenyl)-2.2-
dimethylcyclopropane carboxylate
CAS registry number 52315-07-8 (formerly 69865-47-0)
RTECS registry number GZ1250000
Common synonyms NRDC 149, WL43467, PP 383, CG-A 55186
Common trade names Ammo, Avicade, Barricade, CCN 52,
Cymbush, Folcord, Imperator, Kafil
Super, Polytrin, Ripcord, Stockade
The asymmetric carbons are marked with an arrow and give rise
to the 8 isomers shown in Fig. 1. Conventionally, the 4 isomers
where the dichlorovinyl group is trans in relation to the
phenoxybenzyl group are referred to as trans-isomers, and the
other 4 as cis-isomers.
Cypermethrin is the ISO name for the pure racemic compound.
The technical products commonly available contain more than 90%
cypermethrin and the ratio of cis- to trans-isomers varies from
50:50 to 40:60. The data presented in this document refer to
products within this range of composition, unless otherwise stated.
2.2. Physical and Chemical Properties
Some physical and chemical properties of cypermethrin are given
in Table 1.
Cypermethrin is highly stable to light and at temperatures
below 220 °C. It is more resistant to acidic than to alkaline
media, with an optimum stability at pH 4. Cypermethrin is
hydrolysed under alkaline conditions in the same way as simple
aliphatic esters: the rate-determining step is the nucleophilic
attack by a hydroxyl group (Camilleri, 1984). Dilute aqueous
solutions are subject to photolysis, which occurs at a moderate
rate (Martin & Worthing 1977; FAO/WHO, 1980b; Meister et al., 1983;
Worthing & Walker, 1983).
Table 1. Some physical and chemical properties of cypermethrina
--------------------------------------------------------------------
Physical state varies from a viscous yellow liquid
to a semi-solid crystalline mass at
ambient temperatures
Relative molecular mass 416.3
Melting point up to 80 °C depending on purity
and cis: trans ratio
Boiling point decomposes at 220 °C
Density (22 °C) 1.12 g/ml
Solubility in water (20 °C) 0.009 mg/litre
Solubility in organic solvents:
hexane 103 g/litre
xylene > 450 g/litre
also comparable solubility in
cyclohexanone, ethanol, acetone,
and chloroform
Vapour pressure (20 °C) 1.9 x 10-7 Pa (1.4 x 10-9 mmHg)
n-octanol/water partition 2 x 106 (log Pow 6.3)
coefficient
--------------------------------------------------------------------
a From: FAO/WHO (1980b); Grayson et al (1982); Working & Walker (1983).
2.3. Analytical Methods
The most widely adopted procedures for the determination of
cypermethrin residues in crops, soil, animal tissues and products,
and environmental samples are based on extraction of the residue
with organic solvent, clean-up of the extract, as necessary, by
means of solvent-solvent partition and adsorption column
chromatography, followed by determination of the residue using gas
chromatography with electron capture detector (GC/ECD). The
identity of residues can be confirmed by GC with mass selective
detection (GC-MSD) or by thin-layer chromatography (TLC) followed
by GC/ECD.
Methods using these procedures have been applied for the
determination of cypermethrin residues in the presence of other
synthetic pyrethroids or other classes of pesticides, including
organochlorine insecticides.
 Alternative procedures, based on high-performance liquid
chromatography with UV detection (HPLC/UV) and TLC with a
colorimetric end point, have been described, but have not been
widely adopted, because of the simplicity and sensitivity of the
GC/ECD methods. This is also true for more elaborate procedures
based on hydrolysis and derivatization.
Procedures have also been developed for the determination of
the more important cypermethrin metabolites, 3-phenoxybenzoic acid
(PBA), the cyclopropane carboxylic acid (CPA), and the amide.
Following extraction and clean-up, these materials are determined
by HPLC/UV or by GC procedures, after derivatization in the case of
the two acids.
The Codex Committee on Pesticide Residues lists recommended
methods for the determination of cypermethrin residues (FAO/WHO
1986).
The methods for residue, environmental, and product analysis
for cypermethrin are summarized in Table 2.
Table 2. Published analytical methods for cypermethrin
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Residue
analysis
Apple n-hexane:acetone extraction silica gel/ electron capture 0.01 Baker &
Pear (1:1) solvent:H2O CH2Cl2 detection-gas 0.01 Bottomley
Cabbage chromatography 0.01 (1982)
Potato 0.01
Apple n-hexane:acetone extraction silica gel/ high-performance liquid 0.2 Baker &
Pear (1:1) solvent:H2O CH2Cl2 chromatography 0.2 Bottomley
Cabbage 0.2 (1982)
Potato 0.2
Onion CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
Carrot (2:1) ether: n- detection-gas al. (1982)
hexane chromatography
Celery CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Wheat n-hexane:acetone 2% NaCl:extrac- Florisil/ electron capture 0.02 Joia et
grain (1:1) tion solvent benzene detection-gas al. (1981,
flour chromatography 1985a)
bran
middling
Beef CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
muscle (85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Egg yolk CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
(85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Milk CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Cotton n-hexane Florisil/ n- electron capture Estesen et
foliage hexane:EtOAc detection-gas al. (1982)
(dislodgable chromatography
residue)
Environmental
analysis
Fish n-hexane:acetone alumina/ n- electron capture McLeese et
Shrimp (1:1) hexane:benzene detection-gas al. (1980)
chromatography
Water XAD-2 resin: extraction sol- electron capture McLeese et
Seawater acetone vent: n-hexane detection-gas al. (1980)
chromatography
Soil acetone sat. Na2SO4: electron capture Harris et
n-hexane detection-gas al. (1981)
chromatography
Soil CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
(2:1) ether: n-hexane detection-gas al. (1982)
chromatography
Product
analysis
Technical n-hexane flame ionization Chapman &
grade detection-gas Simmons
chromatography (1977)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Technical methylene flame ionization Bland
and chloride (con- detection-capillary gas (1985)
formulated taining as chromatography
material internal stan-
dard dicyclo-
hexylphthalate)
---------------------------------------------------------------------------------------------------------
a GLC = gas-liquid chromatography.
HPLC = high-performance liquid chromatography.
b LD = limit of determination. (The lower practical limit of determination for most of the analytical
methods based on GLC is usually 0.01 mg/kg. The actual level achievable, however, depends to some
extent on the substrate and to a great extent on the intensity of the clean-up steps in the
procedure).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial Production
Cypermethrin was synthesized by Elliott et al. in 1974. It was
prepared by the esterification of a chloro analogue of chrysanthemic
acid (1R, 1S or 1RS, 3R, 3S, or cis-, trans-)-2,2-dimethyl-3-(2,2-
dichlorovinyl)cyclopropanecarboxylic acid with (alphaR, alphaS, or
alphaRS)-alpha-cyano-3-phenoxybenzyl alcohol. Today there are many
other methods of preparation.
Cypermethrin has been marketed since 1977. Recent global
production figures are given in Table 3.
Table 3. Global production of cypermethrin
-------------------------------------------------
Year Production Reference
(tonnes)
-------------------------------------------------
1979 200 Wood, Mackenzie, & Co. (1980)
1980 380 Wood, Mackenzie, & Co. (1981)
1981 375 Wood, Mackenzie, & Co. (1982)
1982 340 Wood, Mackenzie, & Co. (1983)
-------------------------------------------------
3.2. Use Patterns
Cypermethrin is a highly active synthetic pyrethroid
insecticide, effective against a wide range of pests in many crops.
According to Battelle (1982), global consumption of cypermethrin
amounted to 159 tonnes in 1980. Fifty-eight tonnes were consumed
in Africa and 9 tonnes in western Europe. Global production in
1982 was 340 tonnes. Cypermethrin was mainly (92.5%) used on
cotton, the major consumer areas being Turkey (47 tonnes), Central
America (44 tonnes), and Egypt (25 tonnes) (Battelle, 1982). Other
agricultural uses included the treatment of hop, vegetables, and
maize. Cypermethrin is also used for the control of veterinary and
public health insects, such as flies, lice, and mites and, in the
United Kingdom, it is used as a wood preservative.
Cypermethrin is formulated as emulsifiable concentrates (100
and 250 g/litre), ultra-low-volume concentrate (10 - 50 g/litre),
wettable powder (125 g/kg), and animal dip concentrate (5 - 15%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSPORTATION
4.1. Transport and Distribution between Media
Because of its physical and chemical characteristics,
cypermethrin is comparatively immobile in the outdoor environment
and transport between media is restricted. It has a very low
vapour pressure and water solubility and is strongly adsorbed from
aqueous solutions by solid surfaces. This drastically restricts
its movement in air and water, and particularly in soils.
4.1.1. Transport from soil to water
Kaufman et al. (1981), working in the laboratory with radio-
labelled cypermethrin in soil columns, down which a volume of water
equivalent to their moisture equivalent was allowed to percolate,
reported virtually no movement of radioactivity below the top
2.5 cm. Using the procedure introduced by Helling & Turner (1968),
where the mobility was studied using thin layer chromatographic
(TLC) plates, very little movement of cypermethrin occurred in
soils. However, radio-labelled PBA leached down the soil columns
to a level of about 8 cm and CPA reached a maximum concentration at
this level. On the basis of the Helling nomenclature for soil
TLC, CPA and PBA were of "intermediate mobility" to "mobile".
While the mobilities of CPA and PBA were relatively little affected
by the organic matter content of the soil, pH appeared to be a most
important factor, mobility being greatest in soils of highest pH,
presumably because of increased dissociation.
Stevens & Hill (1980) studied the leaching of cypermethrin in
the laboratory in 4 different soil types, a clay loam, a loamy
sand, a coarse sand, and a fen peat. The compound was incubated
for three weeks with each soil under aerobic conditions. The soils
were then packed into glass columns and leached with 67.5 cm water
over a 10-week period. At the end of the period of incubation, a
substantial proportion of the cypermethrin had been lost as 14CO2
(up to a third in one case), and only minor amounts of degradation
products had been formed. It was found that, after the leaching
period, more than 99% of the 14C residue remained within the top
5 cm in all the soils. Radioactivity in the leachate was below the
limit of determination in all cases.
In laboratory studies using labelled cypermethrin and soil
columns, Jackson (1977) found little penetration of cypermethrin
below the top 2-cm layer, even after the percolation of 1.35 metres
of water.
In a further study on the percolation of distilled water
through sandy loam soils containing 14C-benzyl cypermethrin from
spent sheep-dip baths, Standen (1977) reported that up to 0.3% of
the applied radioactivity was leached out. However, most of the
radioactivity was associated with fine soil particles in the
leachate and could not be extracted with organic solvents. The
water contained small amounts of unchanged cypermethrin and PBA.
Most (89%) of the radioactivity was contained in the top 14 cm of
the columns, mainly as cypermethrin itself.
Sakata et al. (1986) studied the leaching with distilled water
of radio-labelled cypermethrin through columns of 4 different types
of soil in the laboratory. The water flow was at a rate of 3 ml/h
and was continued for 3 weeks at 25 °C, so that the total flow
through the column was equivalent to about 3 metres. Cypermethrin
was relatively resistent to leaching but radioactivity was found in
the leachate, especially in one sandy soil where, after 30 days of
incubation, about 30% of the cyclopropyl label first added was
collected in the leachate. The major products associated with
radioactivity in the leachates were either CPA or PBA, depending on
the position of the label. Unchanged cypermethrin was present only
in trace amounts in sand containing less than 0.1% organic matter.
4.1.2. Transport within water bodies
Cypermethrin moves slowly in water bodies. In the experimental
overspraying of ponds carried out by Crossland (1982) and described
in detail in section 4.4.2, it was calculated that 48 h after
treatment with 100 g cypermethrin/ha, only about 8 - 16% of the
amount applied could be found underneath the surface film of 0.05
mm depth. In all of Crossland's studies, the levels of
cypermethrin residues in the sediment at the bottom of the ponds
were below 7 µg/kg.
With spray levels applied according to normal agricultural
practice, Crossland et al. (1982) found that water bodies adjacent
to sprayed arable fields in the United Kingdom received only four-
five orders of magnitude less cypermethrin per m2 than the land
itself and that the initial concentration in the surface film of
water was between 6 and 20 µg/litre. Residues in the water below
the surface film did not reach more than 0.1 µg/litre and within
24 h the levels had nearly all fallen to below the limit of
determination of 0.01 µg/litre. A similar study in French
vineyards showed comparable results, though the initial
concentrations reached in the surface films were higher, probably
because conditions in the area were more favourable for spray drift
than those in the British study.
Shires & Bennett (1985) reported similar results concerning
water in drainage ditches adjacent to cereal fields in the United
Kingdom treated with an aerial spray application of 25 g
cypermethrin/ha.
From the available studies, it can be concluded that
contamination of water bodies by overspray is likely to be very
superficial and of comparatively short duration.
4.2. Abiotic Degradation
4.2.1. Photodegradation
4.2.1.1. Basic studies
According to Ruzo et al. (1977), cypermethrin is one of the
more light-stable pyrethroids. Thus, when exposed in the solid
phase to sunlight for 30 h, no loss of cypermethrin was detected.
When exposed in methanol solution to light of wavelength > 290 nm
for about 2 days, 55% of cypermethrin was recovered, but no data on
the photodecomposition products formed were reported. According to
Ruzo & Casida (1980), the reaction quantum yield at 300 nm in
methanol was low, at 0.022. Ruzo (1983) further demonstrated the
comparative resistance of cypermethrin to irradiation in his
studies on the involvement of oxygen in the photodegradation of
pyrethroids.
Cypermethrin is more susceptible to radiation of lower
wavelengths; Lauren & Henzel (1977) reported that under ultra-
violet radiation, 90% of cypermethrin on a glass petri dish was
decomposed after 3 days, but only 45% was decomposed after 3 days
when the cypermethrin was deposited on grass and placed under an
UV-lamp.
4.2.1.2. Photodegradation
(a) Water
Day & Leahey (1980) studied the effects of sunlight on dilute
aqueous solutions of cypermethrin. In their studies, 14C-labelled
cis- or trans-isomers were used with the label in either the
cyclopropyl or the benzyl ring. They were dissolved in sterile
aqueous acetonitrile at a concentration of 1 mg/litre, irradiated
in sunlight for 32 days and the irradiated solutions compared with
controls stored for the same length of time in the dark. The
degree of photodegradation was very limited. At the end of the
study, 89.4% of the cypermethrin remained in the case of the
irradiated benzyl label, compared with 97.4% in the dark control.
Corresponding figures for the cyclopropyl label were 92.3 and
96.8%. Six of the 8 photodegradation products separated by
chromatography were positively identified; cis- and trans-CPA,
phenoxybenzyl alcohol, aldehyde and acid, and alpha-cyano-3-
phenoxybenzyl alcohol.
The effects of natural sunlight on aqueous solutions of the
(1R, cis-, alpha RS) and (1R, trans-, alpha RS) isomers were
studied by Takahashi et al. (1985a,b). The products were labelled
with 14C in either the cyclopropyl ring, the benzyl ring, or the
cyano carbon. The aqueous solutions were made from distilled
water, 2% acetone, aqueous humic acid, sea water, or natural river
water (both of which had been filtered). The isomers were added to
the water in the form of a stabilized suspension using Tween 20 to
give 50 µg/litre test suspension. The rates of degradation of the
isomers were very rapid compared with those reported by other
authors, however, a large part of the changes involved
transformation to other isomers. The degradation was more rapid in
river or sea water (half-life of cis-isomer, 0.6 - 0.7 days) than
in distilled water or humic acid (half-life of cis-isomers, 2.3 -
2.6 days), but the most rapid change of all occurred in the
presence of acetone. Presumably the differences were due to the
well known effect of photosensitizaton by the acetone or organic
constituents of the natural waters. The main degradation products,
in addition to the different isomers, were CPA, PBA together with
smaller amounts of the corresponding aldehyde, and carbon dioxide,
especially in the case of the cyano label. There was evidence of
further degradation of CPA and PBA.
(b) Soil
Hall et al. (1981) studied the photodegradation of cypermethrin
on the soil surface. Labelled cypermethrin, as used by Day &
Leahey, was applied to very thin soil plates (0.5 mm) at a rate
equivalent to about 200 g/ha. The plates were exposed to sunlight
in the open air protected against rain by polythene sheeting, when
necessary; the sheeting was transparent to the UV component of
sunlight. The plates were extracted after an exposure period of 32
days and the extracts chromatographed. In the case of the
cyclopropyl label, 63% of the radioactivity initially applied to
the irradiated plate was recovered, compared with 103.5% from the
plate that had been kept in the dark. The half-life of the
cyclopropyl-labelled cypermethrin was reduced from > 32 days to 8
- 16 days by irradiation with natural light. The main degradation
products appear to have been the amide, together with cis- and
trans-CPA, and some unidentified (partly volatile) products. In
the case of the benzyl label, the degradation products identified
were mainly the amide analogue of cypermethrin and various
phenoxybenzyl derivatives, such as the alcohol, aldehyde, and acid.
In these studies, the amide was the most prominent, even in the
unirradiated sample, and in this respect, the results differ
somewhat from those obtained in other soil incubation studies,
where the main metabolite from benzyl-labelled cypermethrin was
PBA, with the amide occurring only as a very minor product.
Takahashi et al. (1985a,b), working with the same products as
they used for their study on water, applied the labelled products
at 1.1 µg/cm2 to half-millimetre layers of 3 different soils and
found very rapid degradation in the irradiated soils compared with
those kept in the dark. The half-lives ranged from between 0.6 and
1.9 days with sunlight and > 7 days in the dark. With regard to
degradation products, the results were rather similar to those
reported by Hall et al. (1981) in that the main degradation product
was the amide of the otherwise intact isomers. In addition, they
found smaller amounts of PBA, virtually no CPA, but occasionally
small amounts of alpha carbamoyl- and alpha carboxyphenoxybenzyl
alcohol. In one of the soils in which degradation was the
highest, nearly half of the radio-labelled carbon was unextractable
at the end of the exposure period. In contrast with the water
study, there was very little evidence of isomerization of the
parent isomers.
There is no obvious explanation for the different rates of
degradation under the influence of irradiation and it is difficult
to extrapolate the results of these studies to the practical
situation. It appears likely that photochemical reactions will
hasten the degradation of deposits of cypermethrin on exposed
surfaces and possible residues in water, but there is little
indication that they greatly change the degradation pathways.
4.3. Biological Degradation in Soil
Cypermethrin degrades relatively quickly in soils, primarily by
biological processes involving cleavage of the ester linkages, to
give the two main degradation products, CPA and PBA. These
products are themselves subsequently mineralized. There is also
evidence for the formation, as an intermediate, of the amide of the
intact molecule and occasionally the 4-hydroxy phenoxy analogue.
Neither of the latter products appears to persist in the soil
(Leahey 1979; Sakata et al., 1986).
4.3.1. Mechanism
Chapman et al. (1981), who studied the effects of sterilization
of soils on the rate of cypermethrin degradation in the laboratory,
demonstrated that degradation in the soil was essentially a
biological process. Cypermethrin was added at 1 mg/kg to the soils
(either untreated or sterilized) and the soils incubated for 16
weeks, by which time the sterilized soils were considered to have
become contaminated. The experiment was conducted under strictly
aerobic conditions. It was found that 84% of the added material
had degraded in natural organic soil compared with only 8% in the
sterilized organic soil. The corresponding values for the mineral
soil were 96% and 7%. The small amounts of degradation in the
sterilized soils presumably resulted from residual microbial
activity, especially in the later stages of the study.
4.3.2. Degradation pathways (separate isomers)
In order to study degradation pathways, Roberts & Standen
(1977, 1981) carried out a series of soil studies in the laboratory
using either the racemic cis- or trans-isomers of cypermethrin or
mixtures of the two. The compounds were 14C-radio-labelled in
either the benzyl or the cyclopropyl ring and were added to the
soil at a rate of 2.5 mg/kg moist soil. Incubation with 3
different soils was carried out under either aerobic or anaerobic
conditions, initially for 16 weeks and subsequently for a total of
52 weeks. Experiments were also conducted using biometer flasks,
in order to measure the output of radio-labelled carbon dioxide.
In the case of the cis-isomer, the main degradation products
extracted from the soils were PBA, cis-CPA with small amounts of
trans-CPA, and limited amounts of the 4-hydroxy derivative of
cypermethrin. Between 25% and 30% of the added radioactivity could
not be extracted with acetonitrile/water. A similar spectrum of
degradation products was extracted in the trans-isomer experiments,
except that the cis-isomer was absent. Some of the remaining
radioactivity was identified in a further degradation product of
CPA, the dicarboxylic acid.
A further study was undertaken by Roberts & Standen (1981) in
which ring-labelled cis- and trans-isomers of CPA were added to a
sandy loam soil at 2.5 - 13.5 mg/kg. In most cases, the soils were
contained in loosely stoppered vessels but, in one experiment, a
biometer flask fitted with a caustic potash trap was used, in order
to measure carbon dioxide (CO2) production. In spite of the
production of labelled CO2 in the initial study with cyclopropyl-
labelled cypermethrin, very little was produced in the latter study
and most of the radioactivity was shown to be still present in the
soil. At the end of the 8-week exposure period, it was found that
the greater part of the radioactivity in the soil was still
associated with unchanged CPA, 33 - 65% in the case of the trans-
acid and 78% in the case of the cis-acid. There was also evidence
that some of the trans-CPA was transformed to the cis-isomer, but
not vice-versa. This finding was analogous to that with the parent
compound where a certain amount of cis-CPA was produced from trans-
cypermethrin.
A similar series of studies was carried out by Sakata et al.
(1986) who incubated 2 Japanese soils with the 1R cis-RS alpha and
1R trans-RS alpha isomers of cypermethrin for up to 168 days at
25 °C. While ester cleavage was the principal pathway of
degradation, limited production of the amides of the intact esters
and production of the 4-hydroxy derivatives (on the phenoxy group)
were also reported. The latter were often present in greater
amounts than the PBA or CPA fragments. The authors also reported
the presence of small amounts of the desphenoxy derivative derived
from ether cleavage, not previously reported for cypermethrin.
However, the level of 14C associated with extractable breakdown
products was low (1 - 17% of the amount initially added, at 56
days) compared with that of bound radio carbon (14 - 58% at 56
days), the actual levels being very dependent on the type of soil
under study. Since the trans-isomers degraded more readily than
the cis-isomers and since the level of free degradation products
was considerably lower for the trans- than the cis-isomers, it is
possible that a substantial proportion of the bound radio carbon
had reverted to the general carbon pool of the soil organic matter.
A major proportion of the added label was recovered as carbon
dioxide (16 - 48% at 56 days) and the amount was highest when the
label was on the benzyl carbon, indicating, as Roberts & Standen
had found, that the PBA was mineralized more readily than the CPA.
Sakata et al. also found that, under comparable circumstances, the
cis-isomers produced carbon dioxide more slowly than the trans-
isomers.
The principal degradation products in soils, prior to breakage
of the benzyl and cyclopropane rings, are shown in Fig. 2.
4.3.3. Rates of degradation
4.3.3.1. Laboratory studies
(a) Separate isomers
In the laboratory studies carried out by Roberts & Standen
(1977, 1981), the half-lives of the cis-isomers were around 4
weeks, except in the inactive Los Palacios soils, where the figure
was nearer to 10 - 12 weeks. The trans-isomer generally exhibited
a much shorter half-life of less than 2 weeks and less than 4 weeks
on the less active soil. After a year, the amounts of unchanged
material left in the soils were very low and nearly always below
10% of the amount applied. But, even at the low levels remaining
after such a long interval, residues of the trans- were still
substantially less than those of the cis-product.
Alternative procedures, based on high-performance liquid
chromatography with UV detection (HPLC/UV) and TLC with a
colorimetric end point, have been described, but have not been
widely adopted, because of the simplicity and sensitivity of the
GC/ECD methods. This is also true for more elaborate procedures
based on hydrolysis and derivatization.
Procedures have also been developed for the determination of
the more important cypermethrin metabolites, 3-phenoxybenzoic acid
(PBA), the cyclopropane carboxylic acid (CPA), and the amide.
Following extraction and clean-up, these materials are determined
by HPLC/UV or by GC procedures, after derivatization in the case of
the two acids.
The Codex Committee on Pesticide Residues lists recommended
methods for the determination of cypermethrin residues (FAO/WHO
1986).
The methods for residue, environmental, and product analysis
for cypermethrin are summarized in Table 2.
Table 2. Published analytical methods for cypermethrin
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Residue
analysis
Apple n-hexane:acetone extraction silica gel/ electron capture 0.01 Baker &
Pear (1:1) solvent:H2O CH2Cl2 detection-gas 0.01 Bottomley
Cabbage chromatography 0.01 (1982)
Potato 0.01
Apple n-hexane:acetone extraction silica gel/ high-performance liquid 0.2 Baker &
Pear (1:1) solvent:H2O CH2Cl2 chromatography 0.2 Bottomley
Cabbage 0.2 (1982)
Potato 0.2
Onion CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
Carrot (2:1) ether: n- detection-gas al. (1982)
hexane chromatography
Celery CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Wheat n-hexane:acetone 2% NaCl:extrac- Florisil/ electron capture 0.02 Joia et
grain (1:1) tion solvent benzene detection-gas al. (1981,
flour chromatography 1985a)
bran
middling
Beef CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
muscle (85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Egg yolk CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
(85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Milk CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Cotton n-hexane Florisil/ n- electron capture Estesen et
foliage hexane:EtOAc detection-gas al. (1982)
(dislodgable chromatography
residue)
Environmental
analysis
Fish n-hexane:acetone alumina/ n- electron capture McLeese et
Shrimp (1:1) hexane:benzene detection-gas al. (1980)
chromatography
Water XAD-2 resin: extraction sol- electron capture McLeese et
Seawater acetone vent: n-hexane detection-gas al. (1980)
chromatography
Soil acetone sat. Na2SO4: electron capture Harris et
n-hexane detection-gas al. (1981)
chromatography
Soil CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
(2:1) ether: n-hexane detection-gas al. (1982)
chromatography
Product
analysis
Technical n-hexane flame ionization Chapman &
grade detection-gas Simmons
chromatography (1977)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Technical methylene flame ionization Bland
and chloride (con- detection-capillary gas (1985)
formulated taining as chromatography
material internal stan-
dard dicyclo-
hexylphthalate)
---------------------------------------------------------------------------------------------------------
a GLC = gas-liquid chromatography.
HPLC = high-performance liquid chromatography.
b LD = limit of determination. (The lower practical limit of determination for most of the analytical
methods based on GLC is usually 0.01 mg/kg. The actual level achievable, however, depends to some
extent on the substrate and to a great extent on the intensity of the clean-up steps in the
procedure).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial Production
Cypermethrin was synthesized by Elliott et al. in 1974. It was
prepared by the esterification of a chloro analogue of chrysanthemic
acid (1R, 1S or 1RS, 3R, 3S, or cis-, trans-)-2,2-dimethyl-3-(2,2-
dichlorovinyl)cyclopropanecarboxylic acid with (alphaR, alphaS, or
alphaRS)-alpha-cyano-3-phenoxybenzyl alcohol. Today there are many
other methods of preparation.
Cypermethrin has been marketed since 1977. Recent global
production figures are given in Table 3.
Table 3. Global production of cypermethrin
-------------------------------------------------
Year Production Reference
(tonnes)
-------------------------------------------------
1979 200 Wood, Mackenzie, & Co. (1980)
1980 380 Wood, Mackenzie, & Co. (1981)
1981 375 Wood, Mackenzie, & Co. (1982)
1982 340 Wood, Mackenzie, & Co. (1983)
-------------------------------------------------
3.2. Use Patterns
Cypermethrin is a highly active synthetic pyrethroid
insecticide, effective against a wide range of pests in many crops.
According to Battelle (1982), global consumption of cypermethrin
amounted to 159 tonnes in 1980. Fifty-eight tonnes were consumed
in Africa and 9 tonnes in western Europe. Global production in
1982 was 340 tonnes. Cypermethrin was mainly (92.5%) used on
cotton, the major consumer areas being Turkey (47 tonnes), Central
America (44 tonnes), and Egypt (25 tonnes) (Battelle, 1982). Other
agricultural uses included the treatment of hop, vegetables, and
maize. Cypermethrin is also used for the control of veterinary and
public health insects, such as flies, lice, and mites and, in the
United Kingdom, it is used as a wood preservative.
Cypermethrin is formulated as emulsifiable concentrates (100
and 250 g/litre), ultra-low-volume concentrate (10 - 50 g/litre),
wettable powder (125 g/kg), and animal dip concentrate (5 - 15%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSPORTATION
4.1. Transport and Distribution between Media
Because of its physical and chemical characteristics,
cypermethrin is comparatively immobile in the outdoor environment
and transport between media is restricted. It has a very low
vapour pressure and water solubility and is strongly adsorbed from
aqueous solutions by solid surfaces. This drastically restricts
its movement in air and water, and particularly in soils.
4.1.1. Transport from soil to water
Kaufman et al. (1981), working in the laboratory with radio-
labelled cypermethrin in soil columns, down which a volume of water
equivalent to their moisture equivalent was allowed to percolate,
reported virtually no movement of radioactivity below the top
2.5 cm. Using the procedure introduced by Helling & Turner (1968),
where the mobility was studied using thin layer chromatographic
(TLC) plates, very little movement of cypermethrin occurred in
soils. However, radio-labelled PBA leached down the soil columns
to a level of about 8 cm and CPA reached a maximum concentration at
this level. On the basis of the Helling nomenclature for soil
TLC, CPA and PBA were of "intermediate mobility" to "mobile".
While the mobilities of CPA and PBA were relatively little affected
by the organic matter content of the soil, pH appeared to be a most
important factor, mobility being greatest in soils of highest pH,
presumably because of increased dissociation.
Stevens & Hill (1980) studied the leaching of cypermethrin in
the laboratory in 4 different soil types, a clay loam, a loamy
sand, a coarse sand, and a fen peat. The compound was incubated
for three weeks with each soil under aerobic conditions. The soils
were then packed into glass columns and leached with 67.5 cm water
over a 10-week period. At the end of the period of incubation, a
substantial proportion of the cypermethrin had been lost as 14CO2
(up to a third in one case), and only minor amounts of degradation
products had been formed. It was found that, after the leaching
period, more than 99% of the 14C residue remained within the top
5 cm in all the soils. Radioactivity in the leachate was below the
limit of determination in all cases.
In laboratory studies using labelled cypermethrin and soil
columns, Jackson (1977) found little penetration of cypermethrin
below the top 2-cm layer, even after the percolation of 1.35 metres
of water.
In a further study on the percolation of distilled water
through sandy loam soils containing 14C-benzyl cypermethrin from
spent sheep-dip baths, Standen (1977) reported that up to 0.3% of
the applied radioactivity was leached out. However, most of the
radioactivity was associated with fine soil particles in the
leachate and could not be extracted with organic solvents. The
water contained small amounts of unchanged cypermethrin and PBA.
Most (89%) of the radioactivity was contained in the top 14 cm of
the columns, mainly as cypermethrin itself.
Sakata et al. (1986) studied the leaching with distilled water
of radio-labelled cypermethrin through columns of 4 different types
of soil in the laboratory. The water flow was at a rate of 3 ml/h
and was continued for 3 weeks at 25 °C, so that the total flow
through the column was equivalent to about 3 metres. Cypermethrin
was relatively resistent to leaching but radioactivity was found in
the leachate, especially in one sandy soil where, after 30 days of
incubation, about 30% of the cyclopropyl label first added was
collected in the leachate. The major products associated with
radioactivity in the leachates were either CPA or PBA, depending on
the position of the label. Unchanged cypermethrin was present only
in trace amounts in sand containing less than 0.1% organic matter.
4.1.2. Transport within water bodies
Cypermethrin moves slowly in water bodies. In the experimental
overspraying of ponds carried out by Crossland (1982) and described
in detail in section 4.4.2, it was calculated that 48 h after
treatment with 100 g cypermethrin/ha, only about 8 - 16% of the
amount applied could be found underneath the surface film of 0.05
mm depth. In all of Crossland's studies, the levels of
cypermethrin residues in the sediment at the bottom of the ponds
were below 7 µg/kg.
With spray levels applied according to normal agricultural
practice, Crossland et al. (1982) found that water bodies adjacent
to sprayed arable fields in the United Kingdom received only four-
five orders of magnitude less cypermethrin per m2 than the land
itself and that the initial concentration in the surface film of
water was between 6 and 20 µg/litre. Residues in the water below
the surface film did not reach more than 0.1 µg/litre and within
24 h the levels had nearly all fallen to below the limit of
determination of 0.01 µg/litre. A similar study in French
vineyards showed comparable results, though the initial
concentrations reached in the surface films were higher, probably
because conditions in the area were more favourable for spray drift
than those in the British study.
Shires & Bennett (1985) reported similar results concerning
water in drainage ditches adjacent to cereal fields in the United
Kingdom treated with an aerial spray application of 25 g
cypermethrin/ha.
From the available studies, it can be concluded that
contamination of water bodies by overspray is likely to be very
superficial and of comparatively short duration.
4.2. Abiotic Degradation
4.2.1. Photodegradation
4.2.1.1. Basic studies
According to Ruzo et al. (1977), cypermethrin is one of the
more light-stable pyrethroids. Thus, when exposed in the solid
phase to sunlight for 30 h, no loss of cypermethrin was detected.
When exposed in methanol solution to light of wavelength > 290 nm
for about 2 days, 55% of cypermethrin was recovered, but no data on
the photodecomposition products formed were reported. According to
Ruzo & Casida (1980), the reaction quantum yield at 300 nm in
methanol was low, at 0.022. Ruzo (1983) further demonstrated the
comparative resistance of cypermethrin to irradiation in his
studies on the involvement of oxygen in the photodegradation of
pyrethroids.
Cypermethrin is more susceptible to radiation of lower
wavelengths; Lauren & Henzel (1977) reported that under ultra-
violet radiation, 90% of cypermethrin on a glass petri dish was
decomposed after 3 days, but only 45% was decomposed after 3 days
when the cypermethrin was deposited on grass and placed under an
UV-lamp.
4.2.1.2. Photodegradation
(a) Water
Day & Leahey (1980) studied the effects of sunlight on dilute
aqueous solutions of cypermethrin. In their studies, 14C-labelled
cis- or trans-isomers were used with the label in either the
cyclopropyl or the benzyl ring. They were dissolved in sterile
aqueous acetonitrile at a concentration of 1 mg/litre, irradiated
in sunlight for 32 days and the irradiated solutions compared with
controls stored for the same length of time in the dark. The
degree of photodegradation was very limited. At the end of the
study, 89.4% of the cypermethrin remained in the case of the
irradiated benzyl label, compared with 97.4% in the dark control.
Corresponding figures for the cyclopropyl label were 92.3 and
96.8%. Six of the 8 photodegradation products separated by
chromatography were positively identified; cis- and trans-CPA,
phenoxybenzyl alcohol, aldehyde and acid, and alpha-cyano-3-
phenoxybenzyl alcohol.
The effects of natural sunlight on aqueous solutions of the
(1R, cis-, alpha RS) and (1R, trans-, alpha RS) isomers were
studied by Takahashi et al. (1985a,b). The products were labelled
with 14C in either the cyclopropyl ring, the benzyl ring, or the
cyano carbon. The aqueous solutions were made from distilled
water, 2% acetone, aqueous humic acid, sea water, or natural river
water (both of which had been filtered). The isomers were added to
the water in the form of a stabilized suspension using Tween 20 to
give 50 µg/litre test suspension. The rates of degradation of the
isomers were very rapid compared with those reported by other
authors, however, a large part of the changes involved
transformation to other isomers. The degradation was more rapid in
river or sea water (half-life of cis-isomer, 0.6 - 0.7 days) than
in distilled water or humic acid (half-life of cis-isomers, 2.3 -
2.6 days), but the most rapid change of all occurred in the
presence of acetone. Presumably the differences were due to the
well known effect of photosensitizaton by the acetone or organic
constituents of the natural waters. The main degradation products,
in addition to the different isomers, were CPA, PBA together with
smaller amounts of the corresponding aldehyde, and carbon dioxide,
especially in the case of the cyano label. There was evidence of
further degradation of CPA and PBA.
(b) Soil
Hall et al. (1981) studied the photodegradation of cypermethrin
on the soil surface. Labelled cypermethrin, as used by Day &
Leahey, was applied to very thin soil plates (0.5 mm) at a rate
equivalent to about 200 g/ha. The plates were exposed to sunlight
in the open air protected against rain by polythene sheeting, when
necessary; the sheeting was transparent to the UV component of
sunlight. The plates were extracted after an exposure period of 32
days and the extracts chromatographed. In the case of the
cyclopropyl label, 63% of the radioactivity initially applied to
the irradiated plate was recovered, compared with 103.5% from the
plate that had been kept in the dark. The half-life of the
cyclopropyl-labelled cypermethrin was reduced from > 32 days to 8
- 16 days by irradiation with natural light. The main degradation
products appear to have been the amide, together with cis- and
trans-CPA, and some unidentified (partly volatile) products. In
the case of the benzyl label, the degradation products identified
were mainly the amide analogue of cypermethrin and various
phenoxybenzyl derivatives, such as the alcohol, aldehyde, and acid.
In these studies, the amide was the most prominent, even in the
unirradiated sample, and in this respect, the results differ
somewhat from those obtained in other soil incubation studies,
where the main metabolite from benzyl-labelled cypermethrin was
PBA, with the amide occurring only as a very minor product.
Takahashi et al. (1985a,b), working with the same products as
they used for their study on water, applied the labelled products
at 1.1 µg/cm2 to half-millimetre layers of 3 different soils and
found very rapid degradation in the irradiated soils compared with
those kept in the dark. The half-lives ranged from between 0.6 and
1.9 days with sunlight and > 7 days in the dark. With regard to
degradation products, the results were rather similar to those
reported by Hall et al. (1981) in that the main degradation product
was the amide of the otherwise intact isomers. In addition, they
found smaller amounts of PBA, virtually no CPA, but occasionally
small amounts of alpha carbamoyl- and alpha carboxyphenoxybenzyl
alcohol. In one of the soils in which degradation was the
highest, nearly half of the radio-labelled carbon was unextractable
at the end of the exposure period. In contrast with the water
study, there was very little evidence of isomerization of the
parent isomers.
There is no obvious explanation for the different rates of
degradation under the influence of irradiation and it is difficult
to extrapolate the results of these studies to the practical
situation. It appears likely that photochemical reactions will
hasten the degradation of deposits of cypermethrin on exposed
surfaces and possible residues in water, but there is little
indication that they greatly change the degradation pathways.
4.3. Biological Degradation in Soil
Cypermethrin degrades relatively quickly in soils, primarily by
biological processes involving cleavage of the ester linkages, to
give the two main degradation products, CPA and PBA. These
products are themselves subsequently mineralized. There is also
evidence for the formation, as an intermediate, of the amide of the
intact molecule and occasionally the 4-hydroxy phenoxy analogue.
Neither of the latter products appears to persist in the soil
(Leahey 1979; Sakata et al., 1986).
4.3.1. Mechanism
Chapman et al. (1981), who studied the effects of sterilization
of soils on the rate of cypermethrin degradation in the laboratory,
demonstrated that degradation in the soil was essentially a
biological process. Cypermethrin was added at 1 mg/kg to the soils
(either untreated or sterilized) and the soils incubated for 16
weeks, by which time the sterilized soils were considered to have
become contaminated. The experiment was conducted under strictly
aerobic conditions. It was found that 84% of the added material
had degraded in natural organic soil compared with only 8% in the
sterilized organic soil. The corresponding values for the mineral
soil were 96% and 7%. The small amounts of degradation in the
sterilized soils presumably resulted from residual microbial
activity, especially in the later stages of the study.
4.3.2. Degradation pathways (separate isomers)
In order to study degradation pathways, Roberts & Standen
(1977, 1981) carried out a series of soil studies in the laboratory
using either the racemic cis- or trans-isomers of cypermethrin or
mixtures of the two. The compounds were 14C-radio-labelled in
either the benzyl or the cyclopropyl ring and were added to the
soil at a rate of 2.5 mg/kg moist soil. Incubation with 3
different soils was carried out under either aerobic or anaerobic
conditions, initially for 16 weeks and subsequently for a total of
52 weeks. Experiments were also conducted using biometer flasks,
in order to measure the output of radio-labelled carbon dioxide.
In the case of the cis-isomer, the main degradation products
extracted from the soils were PBA, cis-CPA with small amounts of
trans-CPA, and limited amounts of the 4-hydroxy derivative of
cypermethrin. Between 25% and 30% of the added radioactivity could
not be extracted with acetonitrile/water. A similar spectrum of
degradation products was extracted in the trans-isomer experiments,
except that the cis-isomer was absent. Some of the remaining
radioactivity was identified in a further degradation product of
CPA, the dicarboxylic acid.
A further study was undertaken by Roberts & Standen (1981) in
which ring-labelled cis- and trans-isomers of CPA were added to a
sandy loam soil at 2.5 - 13.5 mg/kg. In most cases, the soils were
contained in loosely stoppered vessels but, in one experiment, a
biometer flask fitted with a caustic potash trap was used, in order
to measure carbon dioxide (CO2) production. In spite of the
production of labelled CO2 in the initial study with cyclopropyl-
labelled cypermethrin, very little was produced in the latter study
and most of the radioactivity was shown to be still present in the
soil. At the end of the 8-week exposure period, it was found that
the greater part of the radioactivity in the soil was still
associated with unchanged CPA, 33 - 65% in the case of the trans-
acid and 78% in the case of the cis-acid. There was also evidence
that some of the trans-CPA was transformed to the cis-isomer, but
not vice-versa. This finding was analogous to that with the parent
compound where a certain amount of cis-CPA was produced from trans-
cypermethrin.
A similar series of studies was carried out by Sakata et al.
(1986) who incubated 2 Japanese soils with the 1R cis-RS alpha and
1R trans-RS alpha isomers of cypermethrin for up to 168 days at
25 °C. While ester cleavage was the principal pathway of
degradation, limited production of the amides of the intact esters
and production of the 4-hydroxy derivatives (on the phenoxy group)
were also reported. The latter were often present in greater
amounts than the PBA or CPA fragments. The authors also reported
the presence of small amounts of the desphenoxy derivative derived
from ether cleavage, not previously reported for cypermethrin.
However, the level of 14C associated with extractable breakdown
products was low (1 - 17% of the amount initially added, at 56
days) compared with that of bound radio carbon (14 - 58% at 56
days), the actual levels being very dependent on the type of soil
under study. Since the trans-isomers degraded more readily than
the cis-isomers and since the level of free degradation products
was considerably lower for the trans- than the cis-isomers, it is
possible that a substantial proportion of the bound radio carbon
had reverted to the general carbon pool of the soil organic matter.
A major proportion of the added label was recovered as carbon
dioxide (16 - 48% at 56 days) and the amount was highest when the
label was on the benzyl carbon, indicating, as Roberts & Standen
had found, that the PBA was mineralized more readily than the CPA.
Sakata et al. also found that, under comparable circumstances, the
cis-isomers produced carbon dioxide more slowly than the trans-
isomers.
The principal degradation products in soils, prior to breakage
of the benzyl and cyclopropane rings, are shown in Fig. 2.
4.3.3. Rates of degradation
4.3.3.1. Laboratory studies
(a) Separate isomers
In the laboratory studies carried out by Roberts & Standen
(1977, 1981), the half-lives of the cis-isomers were around 4
weeks, except in the inactive Los Palacios soils, where the figure
was nearer to 10 - 12 weeks. The trans-isomer generally exhibited
a much shorter half-life of less than 2 weeks and less than 4 weeks
on the less active soil. After a year, the amounts of unchanged
material left in the soils were very low and nearly always below
10% of the amount applied. But, even at the low levels remaining
after such a long interval, residues of the trans- were still
substantially less than those of the cis-product.
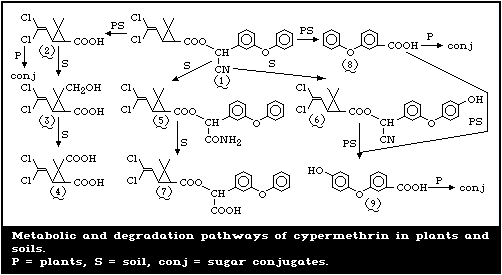 Sakata et al. (1986) in their incubation studies reported half-
lives of between 4.1 and 17.6 days for trans-cypermethrin and 12.5
and 56.4 days for the cis-isomer, under aerobic upland conditions.
Degradation was much slower in one of the soils than in the other,
as was also shown by Roberts & Standen (1977, 1981). Miyamoto &
Mikami (1983) reported data on the half-lives in soil incubation
tests for all 4 of the 1R isomers of cypermethrin. The alpha S
isomers of both cis- and trans-isomers degraded much more rapidly
than the alpha R isomers, sometimes nearly twice as fast. Again,
the cis-isomers were slower to degrade than the trans-isomers.
The greater readiness of the trans-isomers to degrade has been
observed extensively by other workers, i.e., Kaufman et al. (1978),
Chapman et al. (1981), Chapman & Harris, (1981), Harris et al.
(1981). The Japanese studies did not produce data for the 1S
isomers, but Chapman & Harris did not detect appreciable
differences between the rates of degradation of the 1R and 1S
isomers, either trans or cis. On the other hand, Harris et al.
(1981) reported a substantial decrease in the 1S/1R ratio for trans-
cypermethrin, as degradation in the soil proceeded suggesting
that, in these studies, the 1S trans-isomers degraded more quickly
than the 1R trans-isomers.
4.3.3.2. Field studies
(a) Cypermethrin, and separate isomers
Roberts & Standen (1981) showed that the rates of degradation
of cypermethrin observed in the laboratory and in the field did not
differ greatly. On the basis of their data, 2 - 4 weeks in the
growing season would appear to be a typical half-life for the
parent racemic cypermethrin, bearing in mind that the half-lives of
the cis-isomers were often approximately twice those of the trans-
isomers.
Shorter half-lives of less than 2 weeks on a mineral soil and
about 3 weeks on a peat soil were reported by Chapman & Harris
(1981). Harris et al. (1981) reported a half-life for cypermethrin
in Plainfield sand of about 2.5 weeks. The persistence of the
insecticidal activity of surface applications of cypermethrin, as
measured by toxicity for cutworms was studied by Cheng (1984).
Although these data cannot be expressed in terms of the half-life
of cypermethrin, it is interesting to note that initial
applications, giving 100% mortality, were only producing about 50%
mortality after 12 days.
However, Chapman & Harris (1981) warned that a simple half-life
expression was not necessarily a valid way of defining the rates of
degradation of cypermethrin, because these tend to decrease with
time. A possible explanation for this effect is that there is a
gradual increase in the proportion of cis-isomers in the residues.
Since these degrade more slowly, overall degradation rates are
bound to decrease with time. But the results of Harris et al.
(1981) cast doubt on whether this change in isomer ratio provides
the sole explanation. These authors reported that, in their
studies, the ratio of cis- to trans-isomers increased during the
early part of their studies, but decreased substantially
afterwards.
Chapman & Harris (1981) also reported that the degradation was
slowed down by high soil contents of organic matter or clay (c.f.,
the slow rates of degradation reported by Roberts & Standen (1977,
1981) on the very high clay soil, Los Palacios) and by anaerobic
conditions. Contrary to what might be expected in light of the
behaviour of other pesticides, they reported that cypermethrin
degraded more quickly on dry than on wet soils. They also
identified the level of cypermethrin in the soil as a very
important factor. Thus, degradation, expressed on a proportionate
basis, was 2 - 3 times slower with an initial concentration in the
soil of 10 mg/kg, than that with an initial concentration of
0.5 mg/kg. Kaufman et al. (1978) also reported faster degradation
with lower rates of application.
(b) Metabolites
In studies on the 2 metabolites (PBA and CPA), Roberts &
Standen (1977, 1981) reported that PBA was quicker to degrade than
CPA.
In the Leiston soil, only about 2% of applied radioactivity
was recovered as PBA after 16 weeks, though in the soil from Los
Palacios, the figure was just under 30% for the soil treated with
cis-cypermethrin and some 50% for the soil treated with trans-
cypermethrin. The higher figure for PBA derived from trans-
cypermethrin was, presumably, due to the more rapid rate of
degradation of this parent isomer.
The degradation of PBA is an oxidative process and, under
anaerobic conditions, its degradation was greatly retarded (Roberts
& Standen, 1977).
The data of Roberts & Standen (1977) on CPA showed that, in
Brenes soil treated with the parent cypermethrin cis-isomers,
radioactivity recovered as CPA reached a maximum (about 17% of the
total radioactivity initially added) at the 8th week. The maximum
level of CPA from the trans-cypermethrin was reached at about the
same time, but constituted nearly 50% of the radioactivity
originally applied. Moreover, by the 52nd week, whilst CPA from
the cis-product had practically disappeared, there was still a
residue of CPA from the trans-isomers, equivalent to some 10% of
the radioactivity originally applied.
The rate of decay of the unextractable radioactivity in soils
previously treated by Roberts & Standen (1977) with labelled
cypermethrin, as described above, was studied by incubating some of
the soils (Brenes & Leiston soils) for a further 26 weeks in
admixture with fresh soil. Substantial additional losses of radio
carbon were observed. At the end of this time, 25 - 45% of the
"bound" radioactivity initially present was lost. Perhaps
unexpectedly, the losses from cypermethrin labelled in the
cyclopropyl ring was almost double that from product labelled in
the benzyl ring. It is clear from these studies that the binding
of residues of breakdown products did not prevent their continued
degradation. Although some of the evidence of Roberts & Standen
relating to the rate of degradation of CPA itself appears to be
anomalous, it can be inferred that cypermethrin degrades rapidly in
the soil and that the subsequent degradation products are
mineralized, as shown by the liberation of labelled carbon dioxide
from cypermethrin labelled in either the cyclopropyl or benzyl
rings. As Miyamoto (1981) concluded, there appears to be little
likelihood of cypermethrin or its metabolites persisting for
lengthy periods in soils.
4.4. Degradation in Water and Sediments
4.4.1. Laboratory studies
(a) Cypermethrin and separate isomers
Camilleri (1984), using 10-5mol/litre solutions of the cis-2
isomer pair of enantiomers in dioxan-water, showed that, at
alkaline pH values, cypermethrin is readily degraded by ester
cleavage to give CPA and PBA. The alternative route of
degradation, hydrolysis of the cyano group to amide, required a
much higher energy of activation and could not be detected.
Takahashi et al. (1985a) demonstrated the effects of pH on the
hydrolysis of 1R cis- or 1R trans-cypermethrin in abiotic
buffered aqueous solutions. At acidic pH values, the half-life of
the isomers was one or more years, but it was appreciably shorter
at pH 7 and had fallen to a matter of minutes at pH 11 (all at
25 °C). In natural waters, sterilized by filtration and having a
pH of about 8, the half-life was about 3 weeks at 25 °C. The trans-
isomers were hydrolysed more readily than the cis-isomers.
The fate of cypermethrin under biotic conditions, simulating
those in rivers and ponds, was studied by Rapley et al. (1981)
using a radio-labelled product, with the label in either the
cyclopropyl or benzyl ring. Samples of water and sediments from 3
rivers and a pond were used in a laboratory experiment in which
mixtures of water and sediment were placed in pairs of glass
cylinders. The insecticide was added at a rate equivalent to 140
g/ha and the vessels incubated at 16 °C for up to 60 weeks,
periodic determinations being made of the level of cypermethrin
remaining and the amount of labelled CO2 evolved. One series of
vessels was aerated and the other left undisturbed. Degradation
was rapid in all cases, even in the non-aerated series. Some 50%
of cypermethrin was lost in less than 2 weeks and 90% within 2 - 9
weeks. After approximately one year, 40-70% of the 14C label from
the benzyl-labelled material was lost as 14CO2, but only 4% from
the cyclopropyl-labelled material, though this proportion rose to
10% after 63 weeks. In the case of the cyclopropyl-labelled
material, the main degradation product detected was CPA with a
small amount of dicarboxylic acid. Subsequent degradation of the
CPA was slow. When the label was in the benzyl ring, the main
product was PBA though, in the sediment, precursors (aldehyde and
to some extent the alcohol) were the most prominent, possibly
because aeration was defective.
Muir et al. (1985) studied the behaviour of cis- and trans-
cypermethrin isomers, labelled with 14C in the cyclopropyl ring, in
3 bottom sediments (sand, a river silty clay, and a pond bottom
clay). In each case, 0.064 or 0.64 mg of the trans-isomers/kg or
0.012, 0.017, or 0.17 mg of the cis-isomers/kg was added to the
sediment. Each sediment was covered with dechlorinated tap water
and allowed to equilibrate for 24 h. The system was sampled at 6
and 24 h and the level of radioactivity determined in the sediment,
pore water from the sediment (in a separate study), and in the
supernatant water. The radioactivity was much less strongly
absorbed on sediment treated with the trans-isomer than on that
treated with cis-isomer, indicating that a substantial proportion
of the radioactivity was associated with degradation products
rather than with the parent compound, because it is unlikely that
major differences in adsorption between the cis- and trans-isomers
of the parent molecule would have been noticed.
4.4.2. Field Studies
(a) Cypermethrin
Crossland (1982) studied the effects of deliberately
overspraying experimental ponds with cypermethrin at the rate of
100 g/ha. Water was sampled either from the surface (2.5 - 10 cm)
or from a depth of 50 cm. Approximately 4 h after treatment, the
concentration of cypermethrin in the surface was 0.1 mg/litre, but
it fell to about a tenth of this value in 24 h. By 13 days, the
surface concentration had fallen to 0.0007 mg/litre. Concentrations
at a depth of 50 cm rose to a plateau of 0.0023 - 0.0026 mg/litre,
4 h after treatment, and then started to fall. By 13 days after
treatment, the concentration had decreased to 0.0009 mg/litre.
Residues were also found in the sediment at the bottom of the pond;
these reached a concentration of 0.006 mg/kg by the thirteenth day.
In a second study with similar treatment, a procedure for
surface sampling was introduced that enabled water films of only
0.05 mm to be sampled. In this extremely thin surface film, the
initial concentration reached 24 mg/litre. There was a very rapid
fall to around 50 µg/litre after the first week, and by the third
week, none could be detected (the limit of determination was 1 - 2
µg/litre). In the subsurface water, where the limit of
determination was only 0.1 µg/litre, concentrations reached 1
µg/litre shortly after treatment but fell rapidly to about a fifth
of this value by the end of the first week. By the end of the
fourth week, the concentration was below the limit of
determination. Sporadic amounts were found in the sediments, but
most had disappeared by the end of the study (16 weeks).
The effects of overspraying ponds or streams adjacent to arable
fields in the United Kingdom and of treating vineyards in France
with cypermethrin were studied by Crossland et al. (1982). The
fields in the United Kingdom were treated with a tractor-drawn
sprayer at the rate of 70 g/ha and the French vineyards with
mistblowers at the rate of 30 - 45 g/ha. One objective of this
work was to determine the possible occurrence of the insecticide in
the water as a result of spray drift from the treated areas. In
the United Kingdom study, deposits on the soil where the spray had
been applied were in the range of 4 - 7 mg/m2, but those on the
surface of the water of the adjacent pond were 4 - 5 orders of
magnitude less. The concentration of cypermethrin in the surface
layer of water (0.06 mm) was between 6 and 20 µg/litre but, after
24 h, only one of the 14 surface samples showed any cypermethrin,
the concentration in this sample being 6 µg/litre. Residues in
the subsurface layers reached between 0.01 and 0.07 µg/litre after
5 h but then declined; after 24 h, levels in most samples were
below the limit of determination (0.01 µg/litre) with only the
occasional sample reaching 0.03 µg/litre.
In the French vineyards, deposits on the surface of the water
were considerably higher (0.04 - 0.5 mg/m2). Concentrations in the
surface water were initially between 0.14 and 1 mg/litre falling to
0.02 mg/litre within 3 h. Even in the subsurface samples,
concentrations of up to 2 µg/litre were occasionally reached, but
they fell rapidly and had generally decreased to 0.1 µg/litre or
less within a few hours.
Further experiments along similar lines were carried out by
Shires & Bennett (1985) who used a fixed wing aircraft to apply
cypermethrin at 25 g/ha to a large field of winter wheat that was
bordered on 3 sides by drainage ditches.
The deposit on the land was about 60% of the nominal rate of
application, while on the water it was only about a tenth of this
value (equivalent to 1.5 g/ha). Analysis of subsurface water
showed that any spray drift reaching the ditches resulted only in
very low levels ranging from below the level of determination of
0.01 µg/litre to a maximum of 0.03 µg/litre. By the fourth day,
none could be detected.
It appears unlikely that spray drift during properly conducted
spray operations will give rise to high concentrations of
cypermethrin in adjacent surface waters. It is also evident that
if cypermethrin residues do occur in natural waters, they are
relatively short lived.
4.5. Bioaccumulation and Biomagnification
4.5.1. n-Octanol/water partition coefficient
In common with those of other synthetic pyrethroids, the
n-octanol/water partition coefficient of cypermethrin is high; a
value of 2 x 106 (log Pow = 6.3) was obtained by extrapolation from
chromatographic data (Gray & Grayson, 1980). McLeese et al. (1980)
reported a calculated log n-octanol/water partition coefficient of
2.44.
4.5.2. Bioaccumulation in fish
The accumulation by fish of cypermethrin from water and its
subsequent elimination have been studied. In a preliminary study,
rainbow trout were exposed to 14C-benzyl-labelled cypermethrin in
water at 14 °C for a period of 22 days. The initial concentration
each day of 0.165 µg/litre decreased over the 24-h period to 0.064
µg/litre. Radioactivity in whole fish rose to a plateau equivalent
to 0.083 mg cypermethrin/kg wet weight after approximately 11 days.
During the steady state, at least 67% of the radioactivity was
unchanged cypermethrin, but unidentified materials were also
present. When the fish were transferred to clean water after 22
days, the concentration of radioactivity decreased to half the
plateau level in about 11 days. According to this study, allowing
for the cyclical nature of the exposure concentration, the best
estimate of the accumulation factor is approximately 1000 (Baldwin
& Lad, 1978b).
In a follow-up study, 2 groups of rainbow trout of different
sizes were exposed to steady, low concentrations of unlabelled
cypermethrin in a continuous-flow system. When exposed to a mean
concentration of 0.19 µg cypermethrin/litre, residues in small
trout (2-13 g) increased rapidly to approximately 0.15 mg/kg wet
weight over 10 days and 0.23 mg/kg wet weight in 10 - 18 days.
After 18 days, the fish were placed in clean water and depuration
followed. Using a one-compartment mathematical model, it was
calculated that the bioaccumulation factor at equilibrium was 1200.
The calculated depuration half-life was approximately 8 days. In
the larger trout (130 - 160 g), exposed to a mean concentration of
0.18 µg cypermethrin/litre in a continuous-flow system, the uptake
was slower, and residues in whole fish reached 0.12 mg/kg wet
weight after 24 days. Cypermethrin residues in fish were fairly
uniformly distributed (mean values 1 - 2 mg/kg tissue), when
expressed on a lipid-weight rather than a wet-weight basis, except
that the brain contained lower residues than the other tissues
(Bennett, 1981a).
Rainbow trout and common carp were exposed to cypermethrin
concentrations of 0.4 - 1.9 µg/litre in a continuous-flow study for
up to 21 days. It was found that residues in both species were
very similar on a wet- and on a lipid-weight basis, but that there
was only a small difference in residue burden between the fish that
died and those that survived. The mean residue concentrations
(mg/kg tissue) for trout and carp were respectively: 0.91 (died)
and 0.67 (survived), 0.68 (died) and 0.72 (survived), on a wet-
weight basis and 44 (died) and 29 (survived), and 43 (died) and 25
(survived) on a lipid-weight basis, (Bennett, 1981b).
McLeese et al. (1980) studied the concentration factors for
cypermethrin in salmon from various toxicity tests. The results
are given in Table 4.
Table 4. Concentration factors for
cypermethrin in salmona
-------------------------------------------
Concentration Exposure Concentration CFb
in water time fish
(µg/litre) (h) (mg/kg)
-------------------------------------------
12 12 0.04 3.5
7.8 21 0.02 3.6
3.0 62 0.02 6.7
1.4 96 0.01 7.1
-------------------------------------------
a From: McLeese et al. (1980).
b CF = Concentration in fish/concentration
in water.
The low CFs for cypermethrin may indicate rapid metabolism and
elimination of the compound by salmon.
Accumulation of cypermethrin by fish exposed under field
conditions was studied in rudd taken at various time intervals from
a pond treated with 100 g cypermethrin a.i./ha (Table 5).
These results show a rapid uptake of cypermethrin in the fish
followed by elimination from the fish as the compound is lost from
the water in the pond system. In such a dynamic situation, it is
not possible to give a definite accumulation factor (Crossland et
al., 1978).
Table 5. Residues of cypermethrin in rudd and water from a
pond treated at 100 g active ingredient/ha
---------------------------------------------------------------
Time after treatment Concentration of cypermethrin in:
subsurface water rudd (µg/kg wet weight)
(µg/litre) (individual values)
---------------------------------------------------------------
1 day 1.0 50 and 41
1 week 0.21 45 and 49
2 weeks 0.06 42 and 65
4 weeks 0.01 26 and 30
8 weeks 0.01 19 and 5
16 weeks - 5a
---------------------------------------------------------------
a Average of 8 fish.
In view of the very low concentrations of cypermethrin that are
likely to arise in water from normal agricultural use and the
rapidity with which concentrations decline, fish in the wild will
not contain measurable residues of cypermethrin, in spite of the
concentration factors reported.
4.5.3. Bioaccumulation in aquatic invertebrates
Muir et al. (1985) studied the accumulation of cypermethrin in
sediment-dwelling larvae of the midge Chironomus tentans. These
were allowed to establish themselves in the sediments or were kept
suspended in the water under the conditions of the study described
in section 4.4.1. Bioaccumulation factors were calculated for both
the water and sediment larvae; these varied from 43 to 245 for the
trans-compound and from 34 to 385 for the cis-, expressed as the
ratios of total radioactivity per gram of larvae to that per ml of
water.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air
No data are available.
5.1.2. Water
No data are available.
5.1.3. Soil
See section 4.3.
5.1.4. Food
Cypermethrin is used to control insect pests on a very wide
range of crops, and residues of the parent compound can sometimes
be found in agricultural commodities from treated crops. Foods of
animal origin can also contain limited residues arising either from
the use of the product for the control of ectoparasites or from the
occurrence of residues in the animal feed.
5.1.4.1. Residues in food commodities from treated crops
A large body of information on the levels of residues arising
in crop commodities where cypermethrin has been used according to
Good Agricultural Practice (GAP), was available to the Task Group.
The data had already been comprehensively reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues and summarized in their
published Monographs of the meetings in 1979, 1981, 1982, and 1984.
(FAO/WHO 1980b, 1982b, 1983b, and 1985c). As a consequence of
their reviews, the JMPR were able to propose a series of Maximum
Residue Limits (MRLs) for cypermethrin in a wide range of food
commodities (treated according to GAP) below which the actual
residue levels would be expected to fall. They range from 0.05 to
2 mg/kg. These MRLs are now at various steps in the Codex
procedure and many have already been fully adopted by the Codex
Alimentarious Commission, shown as step "CLX" in Table 6 (Codex
Alimentarius Commission, 1986).
Dried tea is an exception to this range of levels in food
commodities in that the level proposed is 20 mg/kg, but it was
shown that only 0.1% of the residues in dried tea enter the
infusion so that the brew, as drunk, will only contain negligible
amounts (FAO/WHO 1985b). Cereal straws also fall outside this
range in that the MRL is 5 mg/kg, but these are not foodstuffs.
Table 6. Codex limits for cypermethrin
residues in treated crops
--------------------------------------------
Crop MRL (mg/kg) Step
--------------------------------------------
Brassica leafy vegetables 1 CLX
Citrus 2 CLX
Lettuce 2 8
Oil seeds except peanuts 0.2 8
Peas 0.05 CLX
Root and tuber vegetables 0.05 CLX
Tomatoes 0.5 CLX
Wheat grain 0.2 8
--------------------------------------------
In addition to these data, limited information on residues have
been published by Lauren & Henzel (1977), Braun et al. (1982),
Frank et al. (1982), and Awasthi & Anand (1983).
Research has also been carried out on the fate of residues in
stored grain treated experimentally (Joia et al., 1985b; Noble &
Hamilton, 1985). Residues proved to be relatively persistent and a
knowledge of storage times and conditions would be required to
estimate the levels that would occur in the grain trade, should
this use of cypermethrin become accepted practice.
5.1.4.2. Residues in food of animal origin
Residues of cypermethrin can arise in foods of animal origin
(milk or milk products, eggs, meat or meat products), either from
topical application to livestock for the control of ecotoparasites
or from residues in livestock rations. In the USA, the actual
residues in meat and milk are expected to be less than the
tolerances of 0.05 mg/kg per litre product (US EPA, 1984). By
referring to available residue data, the JMPR was able to propose
MRLs for carcass meat and meat products, eggs, and milk.
Subsequently, the following Codex Limits (CLXs) were established
(Codex Alimentarius Commission-1986) (Table 7).
Table 7. Codex limits for cypermethrin residues
in foodstuffs
-------------------------------------------------
Commodity Maximum Residue Limit
mg/kg
-------------------------------------------------
Carcass meat (carcass fat) 0.2
Meat products 0.2
Eggs 0.05
Milk (whole milk) 0.01
-------------------------------------------------
5.2. General Population Exposure
Taking into consideration: (a) the levels of cypermethrin
residues that may occur in food commodities from crops or in foods
of animal origin, where cypermethrin has been used according to
GAP; (b) the contribution of the relevant commodities to the diet;
and (c) the losses that occur during the processing of these
commodities, it can confidently be inferred that the daily intake
of cypermethrin in the human diet will be well below the officially
adopted Acceptable Daily Intake. However, no total diet or market
basket studies are available.
5.3. Occupational Exposure
See section 9.2.2.
6. KINETICS AND METABOLISM
6.1. Absorption, Excretion, and Distribution
6.1.1. Oral
6.1.1.1. Rat
(a) Cypermethrin mixture
Three rats of each sex were given a single oral dose of 0.5 mg
(approximately 1.2 mg/kg body weight for males and 2.1 mg/kg body
weight for females) of a cis/trans mixture of 14C-cyclopropyl-
labelled cypermethrin. Three days after dosing, low concentrations
of radioactivity were found for both sexes in the kidneys, muscle,
brain, and blood. The level in the liver of male rats was 3 times
higher than that in the liver of female rats (0.37 and 0.12 mg/kg
tissue, respectively). The residues in the fat of the female rats
were 2 - 3 times higher than those in the male rats (0.72 and 0.31
mg/kg tissue respectively). Concentrations in muscle, brain, and
blood were < 0.05 mg/kg. The mean percentage recovery of the
administered dose was more than 100% (Crawford, 1977; Crawford et
al., 1981a).
Urinary excretion of the compound was rapid in both sexes;
approximately 50 - 65% of the dose being excreted in 48 h.
Elimination via the faeces was slower, the mean rate being
approximately 30% of the dose in 3 days. The amount of
radioactivity excreted via expired CO2, measured in a separate
study using one rat of each sex, was up to 0.1% of the dose in 15
days.
Studies with 14C-cyclopropyl-labelled cypermethrin indicated
that biliary excretion of the cyclopropyl moiety is a minor route
of elimination (up to 2% in 4 h) (Crawford et al., 1981a).
The metabolism of cypermethrin in maize oil was studied in male
and female Wistar rats following a single toxic oral dose of 200
mg/kg body weight of 2 radio-labelled forms (14C-benzyl and 14C-
cyclopropyl) of the insecticide. Minimal amounts of 14CO2 were
expired from both types of labelled cypermethrin: viz < 0.005 -
0.06% of dose. The elimination of radioactivity within 7 days was
29 - 33% (14C-benzyl label) and 41-56% (14C-cyclopropyl label) in
the urine and 55 - 59% and 34 - 46%, respectively, in the faeces.
The differences between the sexes were small (Rhodes et al., 1984).
The distribution and tissue retention of cypermethrin was
studied in 5 male and 5 female Wistar rats receiving daily oral
doses of 2 mg (14C-benzyl)-labelled cypermethrin/kg body weight for
28 days. Consistent with the lipophilic nature of cypermethrin,
the highest mean tissue concentration was found in the fat (4.1
mg/kg in males and 5.1 mg/kg in females). Concentrations in the
liver, kidneys, adrenals, gut, ovaries, and skin were of the order
of 0.4 - 0.9 mg/kg tissue. Small amounts of radioactivity (0.04 -
0.07 mg/kg) were detected in the muscle, spleen, and bone.
Negligible concentrations (< 0.01 mg/kg) were detected in the
brain (Rhodes et al., 1984). In a further study, the tissues
identified as containing the highest concentrations of 14C-benzyl-
labelled cypermethrin (fat, liver, kidneys, skin, and ovaries) as
well as whole blood and plasma were used to study the extent of
accumulation and rate of elimination of cypermethrin. A total of
60 female rats were dosed orally with 14C-benzyl-labelled
cypermethrin at 2 mg/kg body weight per day, for up to 70
consecutive days. Levels in all tissues reached a plateau after 56
days of dosing. The extent of accumulation, expressed as mg
equivalents of cypermethrin per kg tissue, was: fat, 3.91; liver,
0.97; kidneys, 0.69; ovaries, 0.03; skin, 1.89; whole blood, 0.35;
and plasma 0.64. Analysis of fat samples, 24 h after the final
dose, revealed that higher levels of the cis-isomer of cypermethrin
had been retained than of the trans-isomer. The rate of
elimination of radioactivity from fat was biphasic in nature, with
rapid elimination of trans-cypermethrin (half-life = 3.4 days) and
slower elimination of the less-readily hydrolysed cis-cypermethrin
(half-life = 18.9 days). Levels of 14C residues in the liver,
kidneys, and blood reached control background levels within 29, 8,
and 15 days, respectively, of the final dose. Apart from fat, the
only other tissue that contained radioactivity was the skin; the
rate of elimination of radioactivity from the skin was similar to
that for fat. Accumulation in the sciatic nerve was also studied
in rats dosed for 26 days. No appreciable bioaccumulation was
found to occur (Jones, 1981; Rhodes et al., 1984).
Three Wistar rats of each sex, given a single oral dose of
(14C-cyano)-cypermethrin (4.3 mg/kg body weight), eliminated 30 -
66% of the dose in the faeces over 3 days. Urinary excretion of
14CN-label was slow, accounting for 6 - 12% of the dose and
elimination of expired 14CO2 accounted for only 1.2 - 1.5% of the
dose. Tissue retention in major organs apart from fat, was higher
than that in similar studies involving 14C-benzyl or 14C-
cyclopropyl labelling, thus reflecting metabolism typical of the
14C-labelled cyanide moiety (Crawford et al., 1981a).
(b) Separate isomers
The fates of both cis- and trans-isomers have been studied
separately. Groups of 3 - 6 Wistar rats of each sex were given
single oral doses (approximately 2.5 mg/kg body weight) of either
the cis-isomer or the trans-isomer, both 14C-labelled in the
benzyl ring. Both isomers were rapidly eliminated. The greater
part of the administered dose was excreted in the urine; 40% and
60% for males and females, respectively, of the cis-isomer and 70%
and 80% of the trans-isomer within 48 h. Elimination of the cis-
isomer in the faeces amounted to 26% and 48% for male and females,
respectively; elimination of the trans-isomer was 24%. The
results for the cis-isomer show a clear sex difference in the route
of elimination. After 72 h, less than 5% of the administered dose
of either isomer remained in the animal tissues with the exception
of the intestines and skin. Fat and skin contained the highest
concentrations (Crawford, 1976a,b; Crawford et al., 1981a). It has
been demonstrated (Crawford & Hutson, 1977a, Crawford et al.,
1981a) that the residue derived from cis-cypermethrin is
eliminated more slowly from fat than from other tissues. In one
study, 8 female rats were given (14C-benzyl)- cis-cypermethrin at
2.5 mg/kg body weight orally, and elimination of radioactivity was
measured in fat samples from 8 up to 42 days after dosing. The
radioactivity was calculated to have a half-life of 11.7 (3.4 -
16.7) days. Ninety to 100% of the radioactivity still remaining in
the fat at 25 days was present as unchanged cypermethrin. The
residues in the liver and kidneys were much lower than those in the
fat but were eliminated at a similar rate (Crawford et al., 1981a).
6.1.1.2. Mouse
(a) Separate isomers
Elimination of radioactivity was measured in male Swiss-Webster
mice, dosed once orally with cis- or trans-cypermethrin, 14C-
labelled in either the benzyl (8 mg/kg body weight) or cyclopropyl
(7 mg/kg body weight) moiety. The 14C-benzyl-dosed mice eliminated
22% and 34% of the administered dose of cis-isomer in the urine
and faeces, respectively, in one day; values for the trans-isomer
were 41% and 16%, respectively. The 14C-cyclopropyl-dosed mice
eliminated 20% of the administered dose of cis-isomer in the urine
and 50% in the faeces in one day; the values for the trans-isomer
were 55% and 16%, respectively. Thus, radioactivity from the
trans-isomer was mainly eliminated in the urine and that from the
cis-isomer in the faeces. The 14C-benzyl-treated mice were killed
1, 3, or 8 days after dosing; the 14C-cyclopropyl-treated mice, 3
days after dosing. Residues of radioactivity from both labels, 3
days after dosing, were low in all tissues except for the fat. The
sequence of the residues in different organs was fat > liver ~
kidneys > blood ~ muscle > brain. Residues fell rapidly during
the 14C-benzyl study, with the exception of the residues derived
from the cis-isomer in fat, which did not decrease during the
study period (Hutson, 1978a; Hutson et al., 1981). However, in a
further study, radioactivity was measured in fat samples from 10
male mice taken up to 42 days after a single oral dose of
approximately 8.8 mg/kg body weight (14C-benzyl)- cis-cypermethrin.
The residue was eliminated exponentially with a half-life of 13.1
(3.6 - 18.4) days. At 8 and 22 days after dosing, approximately
90% of the radioactivity present in two pooled fat samples was
attributable to unchanged cis-cypermethrin (Crawford & Hutson,
1978; Crayford et al., 1980; Hutson et al., 1981).
6.1.1.3. Dog
(a) Cypermethrin mixture
Two male beagle dogs were given single oral doses of (14C-
cyclopropyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979a).
Elimination of labelled material was rapid in both dogs, though a
variable distribution between urine and faeces was observed between
the 2 dogs, i.e., 21 and 57% in urine and 78 and 48%, respectively,
in faeces. In a further study, one dog was dosed orally with
(14C-benzyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979b).
Over 4 days, 80% of the radioactivity was recovered in the faeces
and 11% in the urine. Analysis of tissues, 4 days after dosing,
revealed that the gall bladder (1.5 mg/kg tissue) and renal fat
(0.3 mg/kg tissue) contained the highest levels of radioactivity
expressed as cypermethrin. Negligible amounts were detected in the
brain (0.006 mg/kg tissue) and sciatic nerve (0.09 mg/kg tissue).
In the liver, adrenals, bone marrow, pituitary gland, and
mesenteric fat, levels of cypermethrin of 0.1 - 0.2 mg/kg tissue
were found.
(b) Separate isomers
Administration of (14C-benzyl)- cis-cypermethrin or
(14C-benzyl)- trans-cypermethrin separately to groups of 2 male
dogs as a single (2 mg/kg body weight) oral dose resulted in
83.4% of cis-isomer and 88% of trans-isomer being recovered in
the urine plus faeces over 6 - 7 days (Crawford, 1979b).
Quantitative differences existed between the amounts eliminated
via the 2 routes. As already mentioned, a variable distribution
was found. These data are consistent with the results of the study
involving 14C-cyclopropyl-labelled cypermethrin (Crawford, 1979a),
and the variation in amounts according to the route of elimination
probably reflects the inter-group differences in rates of
absorption of labelled material.
6.1.1.4. Cow
Three studies were carried out on lactating cows fed diets
containing 0.2, 5, or 10 mg 14C-benzyl and/or 14C-cyclo-propyl-
cypermethrin/kg feed, respectively, twice daily, for 7 or 21 days.
The estimated daily intake was 2, 50, or 100 mg cypermethrin/cow.
The radioactivity was rapidly eliminated following ingestion.
Equilibrium between ingestion and elimination was reached after
about 4 days. The amounts eliminated via the major routes were
similar for both labels, i.e., approximately 50% in the urine, and
approximately 40% in the faeces (mainly unchanged cypermethrin).
Polar and acidic components were found in the urine. Up to 0.2% of
the administered radioactivity was found in the milk, mainly in the
cream phase (about 88%). Feeding 0.2, 5, or 10 mg/kg feed, the
residues in the milk were 0.0006, 0.012, or 0.03 mg cypermethrin
/litre, respectively. Radioactivity (expressed as mg cypermethrin
/kg tissue) in the carcasses of the animals of the 3 groups at
slaughter was not detectable in muscle and brain (< 0.001- < 0.04
mg/kg). Levels in other tissues were: blood < 0.04 - 0.07 mg/kg,
liver 0.004 - 0.21 mg/kg, kidneys 0.003 - 0.11 mg/kg, and
subcutaneous and renal fat 0.01 - 0.1 mg/kg (Croucher et al.,
1985).
Swaine & Sapiets cf. FAO/WHO (1982b) dosed cows daily with 0.2,
5, or 50 mg cypermethrin (43% cis-isomers, 35% trans-isomers) per
kg feed for up to 29 days. Residues in milk and tissues were
comparable to those reported by Croucher et al. (1985).
6.1.1.5. Sheep
The elimination pattern in a single sheep, given one oral
dose of a mixture consisting of unlabelled cypermethrin with 14C-
benzyl- and 14C-cyclopropyl-labelled material (3.9 mg/kg body
weight) in a gelatin capsule, showed that 41% of the administered
dose was excreted in the urine and 20% was eliminated in faeces,
within 48 h. Tissue residues, 2 days after treatment, were muscle,
0.04 mg/kg; and liver, kidneys, and renal fat approximately 0.4
mg/kg tissue (Crawford & Hutson, 1977b).
6.1.1.6. Chicken
14C-phenoxy-labelled cypermethrin ( cis:trans, 55:45) was
administered orally to laying hens, daily for 14 days, at a rate
equivalent to 10 mg/kg diet (about 0.7 mg/kg body weight).
Radioactivity in the eggs reached a plateau, equivalent to about
0.05 mg cypermethrin/kg, after 8 days. Most of the radioactivity
was found in the yolk (up to 0.19 mg/kg) and about half of it was
identified as cypermethrin. The rest was closely associated with
neutral lipids and phosphatidyl cholines. Residues in the
carcasses, at slaughter, were low; values were between 0.01 and
0.02 mg/kg in muscle tissue, about 0.08 mg/kg in the subcutaneous
and peritoneal fat, and 0.37 mg/kg in the liver. The composition
of residues in the liver was not conclusively established. Apart
from small amounts of unchanged cypermethrin, the radioactivity was
also associated with highly polar material. However, it is evident
that the hen has a very effective mechanism for the metabolism of
cypermethrin (Hutson & Stoydin, 1987).
Comparable results were obtained from non-labelled studies with
laying hens in which dietary levels of up to 40 mg cypermethrin/kg
diet were fed for 28 days (Wallace et al., 1982).
6.1.1.7. Man
Male volunteers were each given a single oral dose of 0.25,
0.5, 1, or 1.5 mg cypermethrin in corn oil in a capsule. Urinary
excretion of cypermethrin metabolites was rapid. The subjects
excreted an average 78% of the dose of trans-isomer and 49% of the
cis-isomer within 24 h. These values did not differ from the
results in rats. The ester cleavage was a major route of
metabolism of cypermethrin in man. As reported in other animal
species, the trans-isomer was metabolized more readily than the
cis-isomer. Concentrations of both isomers excreted in the urine
between 2 and 5 days after dosing 0.5 or 1 mg cypermethrin were
below the limit of detection of 0.01 mg/litre (Eadsforth & Baldwin,
1983).
Groups of 2 male subjects were given cypermethrin in daily oral
doses of 0.25, 0.75, or 1.5 mg/man, by capsule, for 5 consecutive
days. During the dosing period and the following 5 days, 24-h
urine samples were collected daily and analysed for the
concentration of the cyclopropane carboxylic acid metabolite. The
results showed that the respective percent-ages of the cis- and
trans-isomers of cypermethrin, excreted in the 24-h period
following each of the oral doses, were similar to the percentage
excretion of these isomers measured in the single oral dose study.
Therefore, no accumulation in the body occurred (van Sittert et
al., 1985a).
6.1.2. Dermal
6.1.2.1. Cow
Two lactating cows were sprayed 3 times with 1.1 g
cypermethrin/animal, with 2-week intervals between treatments.
Milk samples were analysed during this period. Tissue samples
were analysed approximately three weeks after the final spraying.
The residues were: in whole milk, < 0.01 mg/litre; muscle, liver,
and kidneys, < 0.01 mg/kg tissue and in fat samples, 0.02 mg/kg
tissue or less (Baldwin et al., 1977).
Comparable results were obtained when 2 barns were sprayed with
either 0.05% or 0.1% of cypermethrin prepared from a 10% a.i.
formulation. Cows were present during spraying. Milk was
collected up to 4 weeks after spraying (0.05% application) or 4
days after spraying (0.1% application). Only the samples collected
4 days after the 0.05% treatment and 2 days after the 0.1%
treatment contained detectable residues (0.005 mg/kg milk). No
residues were found (< 0.002 mg/kg milk) in any of the other
samples (Baldwin & Lad, 1978a).
Cows were dipped twice in approximately 170 mg cypermethrin
/litre with a 10-week interval between treatments. The animals
were sacrificed 4 or 14 days after the second dipping. Residues in
muscle and liver did not exceed 0.01 mg/kg tissue. Fat samples
contained detectable residues. The highest was 0.13 mg/kg in renal
fat. The fat residue did not decline between 4 and 14 days after
treatment (Baldwin, 1977a).
Cattle sprayed once with 0.1 and 0.2% a.i. showed the same
level of residues (< 0.005 mg/kg tissue) in muscle, liver, and
kidneys, and a level of < 0.01 mg/kg in fat samples, 1, 3, 8, and
15 days after treatment. In cattle treated twice, fat samples
contained residues ranging from 0.01 to 0.05 mg/kg tissue (Bosio,
1979).
Many trials in which cows were sprayed with, or dipped in,
cypermethrin solutions were carried out in Australia. The milk
from cows sprayed with 0.1% cypermethrin did not contain any
detectable residues. The highest residue (0.03 mg/kg) in butterfat
was found one day after spraying. When the cows were dipped in a
dipwash containing 75 mg cypermethrin/litre, residues in the milk
determined 1, 3, and 7 days after dipping ranged from 0.01 to <
0.002 mg/litre. Omental fat contained the highest residue level
(0.02 mg/kg) 3 and 4 days after dipping. Liver, kidneys, and
muscle did not contain any detectable residues. A second dipping,
7 days after the first, did not cause any build-up of cypermethrin
in the tissues of the cattle (FAO/WHO, 1982b).
Detectable residues of cypermethrin of up to 0.01 mg/kg
butterfat were found in milk samples taken over 21 days from 5 of
10 cows wearing cypermethrin-integrated ear tags (Braun et al.,
1985).
Taylor et al. (1985) found cypermethrin in the hair of cattle,
in concentrations of up to 2.8 mg/kg, after application of
impregnated ear tags.
6.1.2.2. Sheep
Two sheep were each treated dermally with a mixture consisting
of unlabelled cypermethrin mixed with 14C-benzyl-and 14C-
cyclopropyl-labelled material at 22 mg/kg body weight. The
cypermethrin was slowly absorbed. Less than 0.5% of the dose was
excreted in the urine within 24 h and only 2% over a 6-day period.
Faecal elimination was also slow, 0.5% of the dose being eliminated
in 6 days. Approximately 30% of the dose was recovered from the
application area. Tissue residues, 6 days after treatment, were:
muscle, 0.04; renal fat, 0.3; and liver and kidneys 0.12 mg/kg
tissue (Crawford & Hutson, 1977b).
6.1.2.3. Man
A male subject was given a single dermal application of a ULV
formulation of cypermethrin (50 mg cypermethrin in hexylene
glycol/Shellsol AB) on the underside of the forearm. The majority
of this application (35 mg) was removed from the skin after 4 h.
Urine was monitored for residues of the acid metabolite [3-(2,2-
dichlorovinyl)-2,2-dimethylcyclo-propane-carboxylic acid] and its
glucuronide, for a 96-h period after dosing. The metabolites were
not detected over this period (Coveney & Eadsforth, 1982).
In a study by van Sittert et al. (1985b), 2 male volunteers
were given a single dermal application of a ULV formulation, 25 mg
cypermethrin in hexylene glycol/Shellsol A, on the underside of the
forearm. An average of 53% of the original amount of cypermethrin
applied was removed from the skin, 4 h after application.
Approximately 0.1% was excreted as the urinary metabolite,
cyclopropane carboxylic acid, during a 72-h period. Measurements
were made using gas liquid chromatography - mass spectrometry, a
method with a higher sensitivity and selectivity than gas liquid
chromatography - electron capture detection, which was used in the
previous study.
6.2. Metabolic Transformation
6.2.1. In vitro studies
In vitro studies on mouse liver homogenates have shown that
ester cleavage is more extensive for the trans-isomer than for the
cis-isomer. One mg of each of (1RS,trans)- and (1RS, cis)-
cypermethrin was incubated with 2.2 ml of approximately 10% mouse
microsome substrate at 37 °C for 30 min, under the following
conditions: (a) tetraethyl pyrophosphate (TEPP)-treated microsomes
(neither esterase nor oxidase activity); (b) normal microsomes
(esterase activity); (c) TEPP-treated microsomes plus NADPH
(oxidase activity); and (d) normal microsomes plus NADPH (esterase
plus oxidase activity). Each esterase preparation hydrolysed about
twice as much trans-cypermethrin as cis-cypermethrin. In contrast,
cis-cypermethrin was metabolized more rapidly in an oxidation
system than trans-cypermethrin. The major site of ring
hydroxylation was the 4' position and the secondary site was the 5
position. The trans-methyl group was an important site of
hydroxylation in the ester metabolites and cis-methyl oxidation was
predominant in the ester-cleaved acid metabolites. The
hydroxymethyl derivatives were further oxidized to the
corresponding aldehydes and carboxylic acids.
3-Phenoxybenzaldehyde-cyanohydrin was detected as a minor
metabolite. The preferred sites of hydroxylation were: with
trans-cypermethrin, cis-methyl > 4' position > trans-methyl > 5
position; with cis-cypermethrin, trans > cis > 4' position > 5
position (Shone & Casida, 1978; Shone et al., 1979). With cis-
cypermethrin, at least, cleavage of cypermethrin to cyanohydrin may
result from both hydrolytic and oxidative mechanisms, since large
amounts of the cleavage products were also evident in the oxidase
system, which lacks esterase activity (Shono & Casida, 1978; Shono
et al., 1979). However, at approximately 35-times higher substrate
levels, the hydrolysis rate of cypermethrin isomers was depressed
(Söderlund & Casida, 1977).
In studies on the metabolism of 14C-cypermethrin by rat liver
microsomes, the overall rates of metabolism of cis- and trans-
cypermethrin were similar, though their metabolic routes differed.
The cis-isomer was metabolized almost exclusively by an NADPH-
dependent oxidative pathway to 4'hydroxy- cis-cypermethrin with
subsequent oxidative ester cleavage. The predominant route for the
metabolism of the trans-isomer was hydrolysis to the trans-acid by
microsomal carboxylesterase (Crawford, 1979c). The in vitro
esteratic capacity was determined in rat, rabbit, and human liver
microsomes using p-nitrophenyl acetate and cypermethrin as
substrate. The relative ability to hydrolyse cypermethrin was
rabbit > man > rat. Rabbit and rat microsomes metabolized the
trans-isomer 6 times faster than the cis-isomer. Human
microsomes showed a similar capacity for metabolizing both cis-
and trans-isomers (Croucher et al., 1982a,b).
6.2.2. In vivo studies
The identification of the metabolites of cypermethrin has been
studied in mice (Hutson, 1978b, Casida et al., 1979; Hutson et al.,
1981), rats (Crawford & Hutson, 1977a; Casida et al., 1979; Hutson,
1979a,b; Crawford et al., 1981b; Rhodes et al., 1984), dogs
(Crawford, 1979d,e), and cows (Swaine & Sapiets, 1980a,b; Croucher
et al., 1985).
Overall, metabolism in these species is similar. Differences
that occur are related to the rate of metabolite formation rather
than to the nature of the metabolites formed. The only major
differences between species relate to conjugation reactions.
Cypermethrin (both isomers) is metabolized via cleavage of the
ester bond. The cyclopropane carboxylic acid moiety is mainly
excreted as the glucuronide conjugate; hydroxylation of the methyl
group occurs only to a limited extent (Crawford, 1979e; Rhodes et
al., 1984). The 3-phenoxy-benzyl product of the ester hydrolysis
is converted to PBA. The cyanide moiety is metabolized to
thiocyanate (Hutson, 1979b). The PBA moiety is mainly excreted as
a glutamic acid conjugate in the cow (Croucher et al., 1985), as a
taurine conjugate ( N-(3-phenoxy-benzoyl)taurine) in 2 strains of
mouse (Hutson & Casida, 1978; Hutson, 1978b, 1979a; Hutson et al.,
1981), and as a glycine conjugate in the rat and dog (Crawford &
Hutson, 1977a; Crawford, 1979d) and in the sheep, cat, and gerbil
(Huckle et al., 1981a). PBA is further metabolized (rat >
mouse > dog) via the 4'-hydroxylation to 3-(4'-hydroxyphenoxy)
benzoic acid and its sulfate conjugate (Crawford & Hutson, 1977a;
Hutson, 1978b; Crawford, 1979d). Glucuronic acid conjugates of PBA
and its 4'hydroxy derivative are the major urinary metabolites in
the marmoset, rabbit, guinea-pig, and hamster. The rat was unique
among the animal species tested in utilizing sulfuric acid for the
conjugation of the 4'-hydroxy derivative (Huckle et al., 1981a).
The major route of excretion for cypermethrin metabolites is via
the urine; unchanged cypermethrin accounted for the majority of
radioactivity found in faeces in radiolabel studies. The amount of
cyclopropyl-radioactivity eliminated in the bile (1%) suggests that
the biliary-intestinal-faecal route is of minor importance for this
moiety (Crawford & Hutson, 1977a; Crawford, 1979e; Rhodes et al.,
1984). Biliary excretion of PBA occurred as glucuronide and that
of 4'-OH-PBA as ether and ester glucuronic acid conjugates. Very
little was eliminated in the faeces, indicating that the biliary
glucuronides decompose and/or are enzymatically cleaved in the
gastro-intestinal tract to the respective benzoic acids. The
latter are subsequently reabsorbed and undergo further metabolism,
principally to the sulfate ester, which is excreted in the urine
(Huckle et al., 1981b). As already noted, the major urinary
metabolite of cypermethrin in cows is N-(3-phenoxy-benzoyl)glutamic
acid. This metabolite is also found in the organs and tissues with
only a small quantity of unchanged cypermethrin. The residues in
body fat consist mainly of cypermethrin. An unidentified polar
metabolite, present in the liver and kidneys, is suspected of being
a conjugate of 3-(4'-hydroxyphenoxy)benzoic acid. The small
portion of radioactivity appearing in milk was associated with
lipid components and consisted mainly of unchanged cypermethrin
(Croucher et al., 1985). The metabolic pathway of cypermethrin is
shown in Fig. 3.
As in other mammals, ester cleavage and elimination of the
cyclopropyl acid moieties in the free and glucuronidated form is a
major route of metabolism of cypermethrin in man (Eadsforth &
Baldwin, 1983).
6.2.3. Metabolism of the glucoside conjugate of 3-phenoxy-benzoic
acid
Studies have been carried out on rats on the metabolism of the
glucoside conjugate of 3-phenoxybenzoic acid, which occurs
occasionally as a metabolite in plants (Crayford, 1978). The
results indicated that the rat hydrolyses the glucoside and then
metabolizes the 3-phenoxybenzoic acid in virtually the same way as
it would metabolize PBA liberated during the metabolism of
cypermethrin.
Sakata et al. (1986) in their incubation studies reported half-
lives of between 4.1 and 17.6 days for trans-cypermethrin and 12.5
and 56.4 days for the cis-isomer, under aerobic upland conditions.
Degradation was much slower in one of the soils than in the other,
as was also shown by Roberts & Standen (1977, 1981). Miyamoto &
Mikami (1983) reported data on the half-lives in soil incubation
tests for all 4 of the 1R isomers of cypermethrin. The alpha S
isomers of both cis- and trans-isomers degraded much more rapidly
than the alpha R isomers, sometimes nearly twice as fast. Again,
the cis-isomers were slower to degrade than the trans-isomers.
The greater readiness of the trans-isomers to degrade has been
observed extensively by other workers, i.e., Kaufman et al. (1978),
Chapman et al. (1981), Chapman & Harris, (1981), Harris et al.
(1981). The Japanese studies did not produce data for the 1S
isomers, but Chapman & Harris did not detect appreciable
differences between the rates of degradation of the 1R and 1S
isomers, either trans or cis. On the other hand, Harris et al.
(1981) reported a substantial decrease in the 1S/1R ratio for trans-
cypermethrin, as degradation in the soil proceeded suggesting
that, in these studies, the 1S trans-isomers degraded more quickly
than the 1R trans-isomers.
4.3.3.2. Field studies
(a) Cypermethrin, and separate isomers
Roberts & Standen (1981) showed that the rates of degradation
of cypermethrin observed in the laboratory and in the field did not
differ greatly. On the basis of their data, 2 - 4 weeks in the
growing season would appear to be a typical half-life for the
parent racemic cypermethrin, bearing in mind that the half-lives of
the cis-isomers were often approximately twice those of the trans-
isomers.
Shorter half-lives of less than 2 weeks on a mineral soil and
about 3 weeks on a peat soil were reported by Chapman & Harris
(1981). Harris et al. (1981) reported a half-life for cypermethrin
in Plainfield sand of about 2.5 weeks. The persistence of the
insecticidal activity of surface applications of cypermethrin, as
measured by toxicity for cutworms was studied by Cheng (1984).
Although these data cannot be expressed in terms of the half-life
of cypermethrin, it is interesting to note that initial
applications, giving 100% mortality, were only producing about 50%
mortality after 12 days.
However, Chapman & Harris (1981) warned that a simple half-life
expression was not necessarily a valid way of defining the rates of
degradation of cypermethrin, because these tend to decrease with
time. A possible explanation for this effect is that there is a
gradual increase in the proportion of cis-isomers in the residues.
Since these degrade more slowly, overall degradation rates are
bound to decrease with time. But the results of Harris et al.
(1981) cast doubt on whether this change in isomer ratio provides
the sole explanation. These authors reported that, in their
studies, the ratio of cis- to trans-isomers increased during the
early part of their studies, but decreased substantially
afterwards.
Chapman & Harris (1981) also reported that the degradation was
slowed down by high soil contents of organic matter or clay (c.f.,
the slow rates of degradation reported by Roberts & Standen (1977,
1981) on the very high clay soil, Los Palacios) and by anaerobic
conditions. Contrary to what might be expected in light of the
behaviour of other pesticides, they reported that cypermethrin
degraded more quickly on dry than on wet soils. They also
identified the level of cypermethrin in the soil as a very
important factor. Thus, degradation, expressed on a proportionate
basis, was 2 - 3 times slower with an initial concentration in the
soil of 10 mg/kg, than that with an initial concentration of
0.5 mg/kg. Kaufman et al. (1978) also reported faster degradation
with lower rates of application.
(b) Metabolites
In studies on the 2 metabolites (PBA and CPA), Roberts &
Standen (1977, 1981) reported that PBA was quicker to degrade than
CPA.
In the Leiston soil, only about 2% of applied radioactivity
was recovered as PBA after 16 weeks, though in the soil from Los
Palacios, the figure was just under 30% for the soil treated with
cis-cypermethrin and some 50% for the soil treated with trans-
cypermethrin. The higher figure for PBA derived from trans-
cypermethrin was, presumably, due to the more rapid rate of
degradation of this parent isomer.
The degradation of PBA is an oxidative process and, under
anaerobic conditions, its degradation was greatly retarded (Roberts
& Standen, 1977).
The data of Roberts & Standen (1977) on CPA showed that, in
Brenes soil treated with the parent cypermethrin cis-isomers,
radioactivity recovered as CPA reached a maximum (about 17% of the
total radioactivity initially added) at the 8th week. The maximum
level of CPA from the trans-cypermethrin was reached at about the
same time, but constituted nearly 50% of the radioactivity
originally applied. Moreover, by the 52nd week, whilst CPA from
the cis-product had practically disappeared, there was still a
residue of CPA from the trans-isomers, equivalent to some 10% of
the radioactivity originally applied.
The rate of decay of the unextractable radioactivity in soils
previously treated by Roberts & Standen (1977) with labelled
cypermethrin, as described above, was studied by incubating some of
the soils (Brenes & Leiston soils) for a further 26 weeks in
admixture with fresh soil. Substantial additional losses of radio
carbon were observed. At the end of this time, 25 - 45% of the
"bound" radioactivity initially present was lost. Perhaps
unexpectedly, the losses from cypermethrin labelled in the
cyclopropyl ring was almost double that from product labelled in
the benzyl ring. It is clear from these studies that the binding
of residues of breakdown products did not prevent their continued
degradation. Although some of the evidence of Roberts & Standen
relating to the rate of degradation of CPA itself appears to be
anomalous, it can be inferred that cypermethrin degrades rapidly in
the soil and that the subsequent degradation products are
mineralized, as shown by the liberation of labelled carbon dioxide
from cypermethrin labelled in either the cyclopropyl or benzyl
rings. As Miyamoto (1981) concluded, there appears to be little
likelihood of cypermethrin or its metabolites persisting for
lengthy periods in soils.
4.4. Degradation in Water and Sediments
4.4.1. Laboratory studies
(a) Cypermethrin and separate isomers
Camilleri (1984), using 10-5mol/litre solutions of the cis-2
isomer pair of enantiomers in dioxan-water, showed that, at
alkaline pH values, cypermethrin is readily degraded by ester
cleavage to give CPA and PBA. The alternative route of
degradation, hydrolysis of the cyano group to amide, required a
much higher energy of activation and could not be detected.
Takahashi et al. (1985a) demonstrated the effects of pH on the
hydrolysis of 1R cis- or 1R trans-cypermethrin in abiotic
buffered aqueous solutions. At acidic pH values, the half-life of
the isomers was one or more years, but it was appreciably shorter
at pH 7 and had fallen to a matter of minutes at pH 11 (all at
25 °C). In natural waters, sterilized by filtration and having a
pH of about 8, the half-life was about 3 weeks at 25 °C. The trans-
isomers were hydrolysed more readily than the cis-isomers.
The fate of cypermethrin under biotic conditions, simulating
those in rivers and ponds, was studied by Rapley et al. (1981)
using a radio-labelled product, with the label in either the
cyclopropyl or benzyl ring. Samples of water and sediments from 3
rivers and a pond were used in a laboratory experiment in which
mixtures of water and sediment were placed in pairs of glass
cylinders. The insecticide was added at a rate equivalent to 140
g/ha and the vessels incubated at 16 °C for up to 60 weeks,
periodic determinations being made of the level of cypermethrin
remaining and the amount of labelled CO2 evolved. One series of
vessels was aerated and the other left undisturbed. Degradation
was rapid in all cases, even in the non-aerated series. Some 50%
of cypermethrin was lost in less than 2 weeks and 90% within 2 - 9
weeks. After approximately one year, 40-70% of the 14C label from
the benzyl-labelled material was lost as 14CO2, but only 4% from
the cyclopropyl-labelled material, though this proportion rose to
10% after 63 weeks. In the case of the cyclopropyl-labelled
material, the main degradation product detected was CPA with a
small amount of dicarboxylic acid. Subsequent degradation of the
CPA was slow. When the label was in the benzyl ring, the main
product was PBA though, in the sediment, precursors (aldehyde and
to some extent the alcohol) were the most prominent, possibly
because aeration was defective.
Muir et al. (1985) studied the behaviour of cis- and trans-
cypermethrin isomers, labelled with 14C in the cyclopropyl ring, in
3 bottom sediments (sand, a river silty clay, and a pond bottom
clay). In each case, 0.064 or 0.64 mg of the trans-isomers/kg or
0.012, 0.017, or 0.17 mg of the cis-isomers/kg was added to the
sediment. Each sediment was covered with dechlorinated tap water
and allowed to equilibrate for 24 h. The system was sampled at 6
and 24 h and the level of radioactivity determined in the sediment,
pore water from the sediment (in a separate study), and in the
supernatant water. The radioactivity was much less strongly
absorbed on sediment treated with the trans-isomer than on that
treated with cis-isomer, indicating that a substantial proportion
of the radioactivity was associated with degradation products
rather than with the parent compound, because it is unlikely that
major differences in adsorption between the cis- and trans-isomers
of the parent molecule would have been noticed.
4.4.2. Field Studies
(a) Cypermethrin
Crossland (1982) studied the effects of deliberately
overspraying experimental ponds with cypermethrin at the rate of
100 g/ha. Water was sampled either from the surface (2.5 - 10 cm)
or from a depth of 50 cm. Approximately 4 h after treatment, the
concentration of cypermethrin in the surface was 0.1 mg/litre, but
it fell to about a tenth of this value in 24 h. By 13 days, the
surface concentration had fallen to 0.0007 mg/litre. Concentrations
at a depth of 50 cm rose to a plateau of 0.0023 - 0.0026 mg/litre,
4 h after treatment, and then started to fall. By 13 days after
treatment, the concentration had decreased to 0.0009 mg/litre.
Residues were also found in the sediment at the bottom of the pond;
these reached a concentration of 0.006 mg/kg by the thirteenth day.
In a second study with similar treatment, a procedure for
surface sampling was introduced that enabled water films of only
0.05 mm to be sampled. In this extremely thin surface film, the
initial concentration reached 24 mg/litre. There was a very rapid
fall to around 50 µg/litre after the first week, and by the third
week, none could be detected (the limit of determination was 1 - 2
µg/litre). In the subsurface water, where the limit of
determination was only 0.1 µg/litre, concentrations reached 1
µg/litre shortly after treatment but fell rapidly to about a fifth
of this value by the end of the first week. By the end of the
fourth week, the concentration was below the limit of
determination. Sporadic amounts were found in the sediments, but
most had disappeared by the end of the study (16 weeks).
The effects of overspraying ponds or streams adjacent to arable
fields in the United Kingdom and of treating vineyards in France
with cypermethrin were studied by Crossland et al. (1982). The
fields in the United Kingdom were treated with a tractor-drawn
sprayer at the rate of 70 g/ha and the French vineyards with
mistblowers at the rate of 30 - 45 g/ha. One objective of this
work was to determine the possible occurrence of the insecticide in
the water as a result of spray drift from the treated areas. In
the United Kingdom study, deposits on the soil where the spray had
been applied were in the range of 4 - 7 mg/m2, but those on the
surface of the water of the adjacent pond were 4 - 5 orders of
magnitude less. The concentration of cypermethrin in the surface
layer of water (0.06 mm) was between 6 and 20 µg/litre but, after
24 h, only one of the 14 surface samples showed any cypermethrin,
the concentration in this sample being 6 µg/litre. Residues in
the subsurface layers reached between 0.01 and 0.07 µg/litre after
5 h but then declined; after 24 h, levels in most samples were
below the limit of determination (0.01 µg/litre) with only the
occasional sample reaching 0.03 µg/litre.
In the French vineyards, deposits on the surface of the water
were considerably higher (0.04 - 0.5 mg/m2). Concentrations in the
surface water were initially between 0.14 and 1 mg/litre falling to
0.02 mg/litre within 3 h. Even in the subsurface samples,
concentrations of up to 2 µg/litre were occasionally reached, but
they fell rapidly and had generally decreased to 0.1 µg/litre or
less within a few hours.
Further experiments along similar lines were carried out by
Shires & Bennett (1985) who used a fixed wing aircraft to apply
cypermethrin at 25 g/ha to a large field of winter wheat that was
bordered on 3 sides by drainage ditches.
The deposit on the land was about 60% of the nominal rate of
application, while on the water it was only about a tenth of this
value (equivalent to 1.5 g/ha). Analysis of subsurface water
showed that any spray drift reaching the ditches resulted only in
very low levels ranging from below the level of determination of
0.01 µg/litre to a maximum of 0.03 µg/litre. By the fourth day,
none could be detected.
It appears unlikely that spray drift during properly conducted
spray operations will give rise to high concentrations of
cypermethrin in adjacent surface waters. It is also evident that
if cypermethrin residues do occur in natural waters, they are
relatively short lived.
4.5. Bioaccumulation and Biomagnification
4.5.1. n-Octanol/water partition coefficient
In common with those of other synthetic pyrethroids, the
n-octanol/water partition coefficient of cypermethrin is high; a
value of 2 x 106 (log Pow = 6.3) was obtained by extrapolation from
chromatographic data (Gray & Grayson, 1980). McLeese et al. (1980)
reported a calculated log n-octanol/water partition coefficient of
2.44.
4.5.2. Bioaccumulation in fish
The accumulation by fish of cypermethrin from water and its
subsequent elimination have been studied. In a preliminary study,
rainbow trout were exposed to 14C-benzyl-labelled cypermethrin in
water at 14 °C for a period of 22 days. The initial concentration
each day of 0.165 µg/litre decreased over the 24-h period to 0.064
µg/litre. Radioactivity in whole fish rose to a plateau equivalent
to 0.083 mg cypermethrin/kg wet weight after approximately 11 days.
During the steady state, at least 67% of the radioactivity was
unchanged cypermethrin, but unidentified materials were also
present. When the fish were transferred to clean water after 22
days, the concentration of radioactivity decreased to half the
plateau level in about 11 days. According to this study, allowing
for the cyclical nature of the exposure concentration, the best
estimate of the accumulation factor is approximately 1000 (Baldwin
& Lad, 1978b).
In a follow-up study, 2 groups of rainbow trout of different
sizes were exposed to steady, low concentrations of unlabelled
cypermethrin in a continuous-flow system. When exposed to a mean
concentration of 0.19 µg cypermethrin/litre, residues in small
trout (2-13 g) increased rapidly to approximately 0.15 mg/kg wet
weight over 10 days and 0.23 mg/kg wet weight in 10 - 18 days.
After 18 days, the fish were placed in clean water and depuration
followed. Using a one-compartment mathematical model, it was
calculated that the bioaccumulation factor at equilibrium was 1200.
The calculated depuration half-life was approximately 8 days. In
the larger trout (130 - 160 g), exposed to a mean concentration of
0.18 µg cypermethrin/litre in a continuous-flow system, the uptake
was slower, and residues in whole fish reached 0.12 mg/kg wet
weight after 24 days. Cypermethrin residues in fish were fairly
uniformly distributed (mean values 1 - 2 mg/kg tissue), when
expressed on a lipid-weight rather than a wet-weight basis, except
that the brain contained lower residues than the other tissues
(Bennett, 1981a).
Rainbow trout and common carp were exposed to cypermethrin
concentrations of 0.4 - 1.9 µg/litre in a continuous-flow study for
up to 21 days. It was found that residues in both species were
very similar on a wet- and on a lipid-weight basis, but that there
was only a small difference in residue burden between the fish that
died and those that survived. The mean residue concentrations
(mg/kg tissue) for trout and carp were respectively: 0.91 (died)
and 0.67 (survived), 0.68 (died) and 0.72 (survived), on a wet-
weight basis and 44 (died) and 29 (survived), and 43 (died) and 25
(survived) on a lipid-weight basis, (Bennett, 1981b).
McLeese et al. (1980) studied the concentration factors for
cypermethrin in salmon from various toxicity tests. The results
are given in Table 4.
Table 4. Concentration factors for
cypermethrin in salmona
-------------------------------------------
Concentration Exposure Concentration CFb
in water time fish
(µg/litre) (h) (mg/kg)
-------------------------------------------
12 12 0.04 3.5
7.8 21 0.02 3.6
3.0 62 0.02 6.7
1.4 96 0.01 7.1
-------------------------------------------
a From: McLeese et al. (1980).
b CF = Concentration in fish/concentration
in water.
The low CFs for cypermethrin may indicate rapid metabolism and
elimination of the compound by salmon.
Accumulation of cypermethrin by fish exposed under field
conditions was studied in rudd taken at various time intervals from
a pond treated with 100 g cypermethrin a.i./ha (Table 5).
These results show a rapid uptake of cypermethrin in the fish
followed by elimination from the fish as the compound is lost from
the water in the pond system. In such a dynamic situation, it is
not possible to give a definite accumulation factor (Crossland et
al., 1978).
Table 5. Residues of cypermethrin in rudd and water from a
pond treated at 100 g active ingredient/ha
---------------------------------------------------------------
Time after treatment Concentration of cypermethrin in:
subsurface water rudd (µg/kg wet weight)
(µg/litre) (individual values)
---------------------------------------------------------------
1 day 1.0 50 and 41
1 week 0.21 45 and 49
2 weeks 0.06 42 and 65
4 weeks 0.01 26 and 30
8 weeks 0.01 19 and 5
16 weeks - 5a
---------------------------------------------------------------
a Average of 8 fish.
In view of the very low concentrations of cypermethrin that are
likely to arise in water from normal agricultural use and the
rapidity with which concentrations decline, fish in the wild will
not contain measurable residues of cypermethrin, in spite of the
concentration factors reported.
4.5.3. Bioaccumulation in aquatic invertebrates
Muir et al. (1985) studied the accumulation of cypermethrin in
sediment-dwelling larvae of the midge Chironomus tentans. These
were allowed to establish themselves in the sediments or were kept
suspended in the water under the conditions of the study described
in section 4.4.1. Bioaccumulation factors were calculated for both
the water and sediment larvae; these varied from 43 to 245 for the
trans-compound and from 34 to 385 for the cis-, expressed as the
ratios of total radioactivity per gram of larvae to that per ml of
water.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air
No data are available.
5.1.2. Water
No data are available.
5.1.3. Soil
See section 4.3.
5.1.4. Food
Cypermethrin is used to control insect pests on a very wide
range of crops, and residues of the parent compound can sometimes
be found in agricultural commodities from treated crops. Foods of
animal origin can also contain limited residues arising either from
the use of the product for the control of ectoparasites or from the
occurrence of residues in the animal feed.
5.1.4.1. Residues in food commodities from treated crops
A large body of information on the levels of residues arising
in crop commodities where cypermethrin has been used according to
Good Agricultural Practice (GAP), was available to the Task Group.
The data had already been comprehensively reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues and summarized in their
published Monographs of the meetings in 1979, 1981, 1982, and 1984.
(FAO/WHO 1980b, 1982b, 1983b, and 1985c). As a consequence of
their reviews, the JMPR were able to propose a series of Maximum
Residue Limits (MRLs) for cypermethrin in a wide range of food
commodities (treated according to GAP) below which the actual
residue levels would be expected to fall. They range from 0.05 to
2 mg/kg. These MRLs are now at various steps in the Codex
procedure and many have already been fully adopted by the Codex
Alimentarious Commission, shown as step "CLX" in Table 6 (Codex
Alimentarius Commission, 1986).
Dried tea is an exception to this range of levels in food
commodities in that the level proposed is 20 mg/kg, but it was
shown that only 0.1% of the residues in dried tea enter the
infusion so that the brew, as drunk, will only contain negligible
amounts (FAO/WHO 1985b). Cereal straws also fall outside this
range in that the MRL is 5 mg/kg, but these are not foodstuffs.
Table 6. Codex limits for cypermethrin
residues in treated crops
--------------------------------------------
Crop MRL (mg/kg) Step
--------------------------------------------
Brassica leafy vegetables 1 CLX
Citrus 2 CLX
Lettuce 2 8
Oil seeds except peanuts 0.2 8
Peas 0.05 CLX
Root and tuber vegetables 0.05 CLX
Tomatoes 0.5 CLX
Wheat grain 0.2 8
--------------------------------------------
In addition to these data, limited information on residues have
been published by Lauren & Henzel (1977), Braun et al. (1982),
Frank et al. (1982), and Awasthi & Anand (1983).
Research has also been carried out on the fate of residues in
stored grain treated experimentally (Joia et al., 1985b; Noble &
Hamilton, 1985). Residues proved to be relatively persistent and a
knowledge of storage times and conditions would be required to
estimate the levels that would occur in the grain trade, should
this use of cypermethrin become accepted practice.
5.1.4.2. Residues in food of animal origin
Residues of cypermethrin can arise in foods of animal origin
(milk or milk products, eggs, meat or meat products), either from
topical application to livestock for the control of ecotoparasites
or from residues in livestock rations. In the USA, the actual
residues in meat and milk are expected to be less than the
tolerances of 0.05 mg/kg per litre product (US EPA, 1984). By
referring to available residue data, the JMPR was able to propose
MRLs for carcass meat and meat products, eggs, and milk.
Subsequently, the following Codex Limits (CLXs) were established
(Codex Alimentarius Commission-1986) (Table 7).
Table 7. Codex limits for cypermethrin residues
in foodstuffs
-------------------------------------------------
Commodity Maximum Residue Limit
mg/kg
-------------------------------------------------
Carcass meat (carcass fat) 0.2
Meat products 0.2
Eggs 0.05
Milk (whole milk) 0.01
-------------------------------------------------
5.2. General Population Exposure
Taking into consideration: (a) the levels of cypermethrin
residues that may occur in food commodities from crops or in foods
of animal origin, where cypermethrin has been used according to
GAP; (b) the contribution of the relevant commodities to the diet;
and (c) the losses that occur during the processing of these
commodities, it can confidently be inferred that the daily intake
of cypermethrin in the human diet will be well below the officially
adopted Acceptable Daily Intake. However, no total diet or market
basket studies are available.
5.3. Occupational Exposure
See section 9.2.2.
6. KINETICS AND METABOLISM
6.1. Absorption, Excretion, and Distribution
6.1.1. Oral
6.1.1.1. Rat
(a) Cypermethrin mixture
Three rats of each sex were given a single oral dose of 0.5 mg
(approximately 1.2 mg/kg body weight for males and 2.1 mg/kg body
weight for females) of a cis/trans mixture of 14C-cyclopropyl-
labelled cypermethrin. Three days after dosing, low concentrations
of radioactivity were found for both sexes in the kidneys, muscle,
brain, and blood. The level in the liver of male rats was 3 times
higher than that in the liver of female rats (0.37 and 0.12 mg/kg
tissue, respectively). The residues in the fat of the female rats
were 2 - 3 times higher than those in the male rats (0.72 and 0.31
mg/kg tissue respectively). Concentrations in muscle, brain, and
blood were < 0.05 mg/kg. The mean percentage recovery of the
administered dose was more than 100% (Crawford, 1977; Crawford et
al., 1981a).
Urinary excretion of the compound was rapid in both sexes;
approximately 50 - 65% of the dose being excreted in 48 h.
Elimination via the faeces was slower, the mean rate being
approximately 30% of the dose in 3 days. The amount of
radioactivity excreted via expired CO2, measured in a separate
study using one rat of each sex, was up to 0.1% of the dose in 15
days.
Studies with 14C-cyclopropyl-labelled cypermethrin indicated
that biliary excretion of the cyclopropyl moiety is a minor route
of elimination (up to 2% in 4 h) (Crawford et al., 1981a).
The metabolism of cypermethrin in maize oil was studied in male
and female Wistar rats following a single toxic oral dose of 200
mg/kg body weight of 2 radio-labelled forms (14C-benzyl and 14C-
cyclopropyl) of the insecticide. Minimal amounts of 14CO2 were
expired from both types of labelled cypermethrin: viz < 0.005 -
0.06% of dose. The elimination of radioactivity within 7 days was
29 - 33% (14C-benzyl label) and 41-56% (14C-cyclopropyl label) in
the urine and 55 - 59% and 34 - 46%, respectively, in the faeces.
The differences between the sexes were small (Rhodes et al., 1984).
The distribution and tissue retention of cypermethrin was
studied in 5 male and 5 female Wistar rats receiving daily oral
doses of 2 mg (14C-benzyl)-labelled cypermethrin/kg body weight for
28 days. Consistent with the lipophilic nature of cypermethrin,
the highest mean tissue concentration was found in the fat (4.1
mg/kg in males and 5.1 mg/kg in females). Concentrations in the
liver, kidneys, adrenals, gut, ovaries, and skin were of the order
of 0.4 - 0.9 mg/kg tissue. Small amounts of radioactivity (0.04 -
0.07 mg/kg) were detected in the muscle, spleen, and bone.
Negligible concentrations (< 0.01 mg/kg) were detected in the
brain (Rhodes et al., 1984). In a further study, the tissues
identified as containing the highest concentrations of 14C-benzyl-
labelled cypermethrin (fat, liver, kidneys, skin, and ovaries) as
well as whole blood and plasma were used to study the extent of
accumulation and rate of elimination of cypermethrin. A total of
60 female rats were dosed orally with 14C-benzyl-labelled
cypermethrin at 2 mg/kg body weight per day, for up to 70
consecutive days. Levels in all tissues reached a plateau after 56
days of dosing. The extent of accumulation, expressed as mg
equivalents of cypermethrin per kg tissue, was: fat, 3.91; liver,
0.97; kidneys, 0.69; ovaries, 0.03; skin, 1.89; whole blood, 0.35;
and plasma 0.64. Analysis of fat samples, 24 h after the final
dose, revealed that higher levels of the cis-isomer of cypermethrin
had been retained than of the trans-isomer. The rate of
elimination of radioactivity from fat was biphasic in nature, with
rapid elimination of trans-cypermethrin (half-life = 3.4 days) and
slower elimination of the less-readily hydrolysed cis-cypermethrin
(half-life = 18.9 days). Levels of 14C residues in the liver,
kidneys, and blood reached control background levels within 29, 8,
and 15 days, respectively, of the final dose. Apart from fat, the
only other tissue that contained radioactivity was the skin; the
rate of elimination of radioactivity from the skin was similar to
that for fat. Accumulation in the sciatic nerve was also studied
in rats dosed for 26 days. No appreciable bioaccumulation was
found to occur (Jones, 1981; Rhodes et al., 1984).
Three Wistar rats of each sex, given a single oral dose of
(14C-cyano)-cypermethrin (4.3 mg/kg body weight), eliminated 30 -
66% of the dose in the faeces over 3 days. Urinary excretion of
14CN-label was slow, accounting for 6 - 12% of the dose and
elimination of expired 14CO2 accounted for only 1.2 - 1.5% of the
dose. Tissue retention in major organs apart from fat, was higher
than that in similar studies involving 14C-benzyl or 14C-
cyclopropyl labelling, thus reflecting metabolism typical of the
14C-labelled cyanide moiety (Crawford et al., 1981a).
(b) Separate isomers
The fates of both cis- and trans-isomers have been studied
separately. Groups of 3 - 6 Wistar rats of each sex were given
single oral doses (approximately 2.5 mg/kg body weight) of either
the cis-isomer or the trans-isomer, both 14C-labelled in the
benzyl ring. Both isomers were rapidly eliminated. The greater
part of the administered dose was excreted in the urine; 40% and
60% for males and females, respectively, of the cis-isomer and 70%
and 80% of the trans-isomer within 48 h. Elimination of the cis-
isomer in the faeces amounted to 26% and 48% for male and females,
respectively; elimination of the trans-isomer was 24%. The
results for the cis-isomer show a clear sex difference in the route
of elimination. After 72 h, less than 5% of the administered dose
of either isomer remained in the animal tissues with the exception
of the intestines and skin. Fat and skin contained the highest
concentrations (Crawford, 1976a,b; Crawford et al., 1981a). It has
been demonstrated (Crawford & Hutson, 1977a, Crawford et al.,
1981a) that the residue derived from cis-cypermethrin is
eliminated more slowly from fat than from other tissues. In one
study, 8 female rats were given (14C-benzyl)- cis-cypermethrin at
2.5 mg/kg body weight orally, and elimination of radioactivity was
measured in fat samples from 8 up to 42 days after dosing. The
radioactivity was calculated to have a half-life of 11.7 (3.4 -
16.7) days. Ninety to 100% of the radioactivity still remaining in
the fat at 25 days was present as unchanged cypermethrin. The
residues in the liver and kidneys were much lower than those in the
fat but were eliminated at a similar rate (Crawford et al., 1981a).
6.1.1.2. Mouse
(a) Separate isomers
Elimination of radioactivity was measured in male Swiss-Webster
mice, dosed once orally with cis- or trans-cypermethrin, 14C-
labelled in either the benzyl (8 mg/kg body weight) or cyclopropyl
(7 mg/kg body weight) moiety. The 14C-benzyl-dosed mice eliminated
22% and 34% of the administered dose of cis-isomer in the urine
and faeces, respectively, in one day; values for the trans-isomer
were 41% and 16%, respectively. The 14C-cyclopropyl-dosed mice
eliminated 20% of the administered dose of cis-isomer in the urine
and 50% in the faeces in one day; the values for the trans-isomer
were 55% and 16%, respectively. Thus, radioactivity from the
trans-isomer was mainly eliminated in the urine and that from the
cis-isomer in the faeces. The 14C-benzyl-treated mice were killed
1, 3, or 8 days after dosing; the 14C-cyclopropyl-treated mice, 3
days after dosing. Residues of radioactivity from both labels, 3
days after dosing, were low in all tissues except for the fat. The
sequence of the residues in different organs was fat > liver ~
kidneys > blood ~ muscle > brain. Residues fell rapidly during
the 14C-benzyl study, with the exception of the residues derived
from the cis-isomer in fat, which did not decrease during the
study period (Hutson, 1978a; Hutson et al., 1981). However, in a
further study, radioactivity was measured in fat samples from 10
male mice taken up to 42 days after a single oral dose of
approximately 8.8 mg/kg body weight (14C-benzyl)- cis-cypermethrin.
The residue was eliminated exponentially with a half-life of 13.1
(3.6 - 18.4) days. At 8 and 22 days after dosing, approximately
90% of the radioactivity present in two pooled fat samples was
attributable to unchanged cis-cypermethrin (Crawford & Hutson,
1978; Crayford et al., 1980; Hutson et al., 1981).
6.1.1.3. Dog
(a) Cypermethrin mixture
Two male beagle dogs were given single oral doses of (14C-
cyclopropyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979a).
Elimination of labelled material was rapid in both dogs, though a
variable distribution between urine and faeces was observed between
the 2 dogs, i.e., 21 and 57% in urine and 78 and 48%, respectively,
in faeces. In a further study, one dog was dosed orally with
(14C-benzyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979b).
Over 4 days, 80% of the radioactivity was recovered in the faeces
and 11% in the urine. Analysis of tissues, 4 days after dosing,
revealed that the gall bladder (1.5 mg/kg tissue) and renal fat
(0.3 mg/kg tissue) contained the highest levels of radioactivity
expressed as cypermethrin. Negligible amounts were detected in the
brain (0.006 mg/kg tissue) and sciatic nerve (0.09 mg/kg tissue).
In the liver, adrenals, bone marrow, pituitary gland, and
mesenteric fat, levels of cypermethrin of 0.1 - 0.2 mg/kg tissue
were found.
(b) Separate isomers
Administration of (14C-benzyl)- cis-cypermethrin or
(14C-benzyl)- trans-cypermethrin separately to groups of 2 male
dogs as a single (2 mg/kg body weight) oral dose resulted in
83.4% of cis-isomer and 88% of trans-isomer being recovered in
the urine plus faeces over 6 - 7 days (Crawford, 1979b).
Quantitative differences existed between the amounts eliminated
via the 2 routes. As already mentioned, a variable distribution
was found. These data are consistent with the results of the study
involving 14C-cyclopropyl-labelled cypermethrin (Crawford, 1979a),
and the variation in amounts according to the route of elimination
probably reflects the inter-group differences in rates of
absorption of labelled material.
6.1.1.4. Cow
Three studies were carried out on lactating cows fed diets
containing 0.2, 5, or 10 mg 14C-benzyl and/or 14C-cyclo-propyl-
cypermethrin/kg feed, respectively, twice daily, for 7 or 21 days.
The estimated daily intake was 2, 50, or 100 mg cypermethrin/cow.
The radioactivity was rapidly eliminated following ingestion.
Equilibrium between ingestion and elimination was reached after
about 4 days. The amounts eliminated via the major routes were
similar for both labels, i.e., approximately 50% in the urine, and
approximately 40% in the faeces (mainly unchanged cypermethrin).
Polar and acidic components were found in the urine. Up to 0.2% of
the administered radioactivity was found in the milk, mainly in the
cream phase (about 88%). Feeding 0.2, 5, or 10 mg/kg feed, the
residues in the milk were 0.0006, 0.012, or 0.03 mg cypermethrin
/litre, respectively. Radioactivity (expressed as mg cypermethrin
/kg tissue) in the carcasses of the animals of the 3 groups at
slaughter was not detectable in muscle and brain (< 0.001- < 0.04
mg/kg). Levels in other tissues were: blood < 0.04 - 0.07 mg/kg,
liver 0.004 - 0.21 mg/kg, kidneys 0.003 - 0.11 mg/kg, and
subcutaneous and renal fat 0.01 - 0.1 mg/kg (Croucher et al.,
1985).
Swaine & Sapiets cf. FAO/WHO (1982b) dosed cows daily with 0.2,
5, or 50 mg cypermethrin (43% cis-isomers, 35% trans-isomers) per
kg feed for up to 29 days. Residues in milk and tissues were
comparable to those reported by Croucher et al. (1985).
6.1.1.5. Sheep
The elimination pattern in a single sheep, given one oral
dose of a mixture consisting of unlabelled cypermethrin with 14C-
benzyl- and 14C-cyclopropyl-labelled material (3.9 mg/kg body
weight) in a gelatin capsule, showed that 41% of the administered
dose was excreted in the urine and 20% was eliminated in faeces,
within 48 h. Tissue residues, 2 days after treatment, were muscle,
0.04 mg/kg; and liver, kidneys, and renal fat approximately 0.4
mg/kg tissue (Crawford & Hutson, 1977b).
6.1.1.6. Chicken
14C-phenoxy-labelled cypermethrin ( cis:trans, 55:45) was
administered orally to laying hens, daily for 14 days, at a rate
equivalent to 10 mg/kg diet (about 0.7 mg/kg body weight).
Radioactivity in the eggs reached a plateau, equivalent to about
0.05 mg cypermethrin/kg, after 8 days. Most of the radioactivity
was found in the yolk (up to 0.19 mg/kg) and about half of it was
identified as cypermethrin. The rest was closely associated with
neutral lipids and phosphatidyl cholines. Residues in the
carcasses, at slaughter, were low; values were between 0.01 and
0.02 mg/kg in muscle tissue, about 0.08 mg/kg in the subcutaneous
and peritoneal fat, and 0.37 mg/kg in the liver. The composition
of residues in the liver was not conclusively established. Apart
from small amounts of unchanged cypermethrin, the radioactivity was
also associated with highly polar material. However, it is evident
that the hen has a very effective mechanism for the metabolism of
cypermethrin (Hutson & Stoydin, 1987).
Comparable results were obtained from non-labelled studies with
laying hens in which dietary levels of up to 40 mg cypermethrin/kg
diet were fed for 28 days (Wallace et al., 1982).
6.1.1.7. Man
Male volunteers were each given a single oral dose of 0.25,
0.5, 1, or 1.5 mg cypermethrin in corn oil in a capsule. Urinary
excretion of cypermethrin metabolites was rapid. The subjects
excreted an average 78% of the dose of trans-isomer and 49% of the
cis-isomer within 24 h. These values did not differ from the
results in rats. The ester cleavage was a major route of
metabolism of cypermethrin in man. As reported in other animal
species, the trans-isomer was metabolized more readily than the
cis-isomer. Concentrations of both isomers excreted in the urine
between 2 and 5 days after dosing 0.5 or 1 mg cypermethrin were
below the limit of detection of 0.01 mg/litre (Eadsforth & Baldwin,
1983).
Groups of 2 male subjects were given cypermethrin in daily oral
doses of 0.25, 0.75, or 1.5 mg/man, by capsule, for 5 consecutive
days. During the dosing period and the following 5 days, 24-h
urine samples were collected daily and analysed for the
concentration of the cyclopropane carboxylic acid metabolite. The
results showed that the respective percent-ages of the cis- and
trans-isomers of cypermethrin, excreted in the 24-h period
following each of the oral doses, were similar to the percentage
excretion of these isomers measured in the single oral dose study.
Therefore, no accumulation in the body occurred (van Sittert et
al., 1985a).
6.1.2. Dermal
6.1.2.1. Cow
Two lactating cows were sprayed 3 times with 1.1 g
cypermethrin/animal, with 2-week intervals between treatments.
Milk samples were analysed during this period. Tissue samples
were analysed approximately three weeks after the final spraying.
The residues were: in whole milk, < 0.01 mg/litre; muscle, liver,
and kidneys, < 0.01 mg/kg tissue and in fat samples, 0.02 mg/kg
tissue or less (Baldwin et al., 1977).
Comparable results were obtained when 2 barns were sprayed with
either 0.05% or 0.1% of cypermethrin prepared from a 10% a.i.
formulation. Cows were present during spraying. Milk was
collected up to 4 weeks after spraying (0.05% application) or 4
days after spraying (0.1% application). Only the samples collected
4 days after the 0.05% treatment and 2 days after the 0.1%
treatment contained detectable residues (0.005 mg/kg milk). No
residues were found (< 0.002 mg/kg milk) in any of the other
samples (Baldwin & Lad, 1978a).
Cows were dipped twice in approximately 170 mg cypermethrin
/litre with a 10-week interval between treatments. The animals
were sacrificed 4 or 14 days after the second dipping. Residues in
muscle and liver did not exceed 0.01 mg/kg tissue. Fat samples
contained detectable residues. The highest was 0.13 mg/kg in renal
fat. The fat residue did not decline between 4 and 14 days after
treatment (Baldwin, 1977a).
Cattle sprayed once with 0.1 and 0.2% a.i. showed the same
level of residues (< 0.005 mg/kg tissue) in muscle, liver, and
kidneys, and a level of < 0.01 mg/kg in fat samples, 1, 3, 8, and
15 days after treatment. In cattle treated twice, fat samples
contained residues ranging from 0.01 to 0.05 mg/kg tissue (Bosio,
1979).
Many trials in which cows were sprayed with, or dipped in,
cypermethrin solutions were carried out in Australia. The milk
from cows sprayed with 0.1% cypermethrin did not contain any
detectable residues. The highest residue (0.03 mg/kg) in butterfat
was found one day after spraying. When the cows were dipped in a
dipwash containing 75 mg cypermethrin/litre, residues in the milk
determined 1, 3, and 7 days after dipping ranged from 0.01 to <
0.002 mg/litre. Omental fat contained the highest residue level
(0.02 mg/kg) 3 and 4 days after dipping. Liver, kidneys, and
muscle did not contain any detectable residues. A second dipping,
7 days after the first, did not cause any build-up of cypermethrin
in the tissues of the cattle (FAO/WHO, 1982b).
Detectable residues of cypermethrin of up to 0.01 mg/kg
butterfat were found in milk samples taken over 21 days from 5 of
10 cows wearing cypermethrin-integrated ear tags (Braun et al.,
1985).
Taylor et al. (1985) found cypermethrin in the hair of cattle,
in concentrations of up to 2.8 mg/kg, after application of
impregnated ear tags.
6.1.2.2. Sheep
Two sheep were each treated dermally with a mixture consisting
of unlabelled cypermethrin mixed with 14C-benzyl-and 14C-
cyclopropyl-labelled material at 22 mg/kg body weight. The
cypermethrin was slowly absorbed. Less than 0.5% of the dose was
excreted in the urine within 24 h and only 2% over a 6-day period.
Faecal elimination was also slow, 0.5% of the dose being eliminated
in 6 days. Approximately 30% of the dose was recovered from the
application area. Tissue residues, 6 days after treatment, were:
muscle, 0.04; renal fat, 0.3; and liver and kidneys 0.12 mg/kg
tissue (Crawford & Hutson, 1977b).
6.1.2.3. Man
A male subject was given a single dermal application of a ULV
formulation of cypermethrin (50 mg cypermethrin in hexylene
glycol/Shellsol AB) on the underside of the forearm. The majority
of this application (35 mg) was removed from the skin after 4 h.
Urine was monitored for residues of the acid metabolite [3-(2,2-
dichlorovinyl)-2,2-dimethylcyclo-propane-carboxylic acid] and its
glucuronide, for a 96-h period after dosing. The metabolites were
not detected over this period (Coveney & Eadsforth, 1982).
In a study by van Sittert et al. (1985b), 2 male volunteers
were given a single dermal application of a ULV formulation, 25 mg
cypermethrin in hexylene glycol/Shellsol A, on the underside of the
forearm. An average of 53% of the original amount of cypermethrin
applied was removed from the skin, 4 h after application.
Approximately 0.1% was excreted as the urinary metabolite,
cyclopropane carboxylic acid, during a 72-h period. Measurements
were made using gas liquid chromatography - mass spectrometry, a
method with a higher sensitivity and selectivity than gas liquid
chromatography - electron capture detection, which was used in the
previous study.
6.2. Metabolic Transformation
6.2.1. In vitro studies
In vitro studies on mouse liver homogenates have shown that
ester cleavage is more extensive for the trans-isomer than for the
cis-isomer. One mg of each of (1RS,trans)- and (1RS, cis)-
cypermethrin was incubated with 2.2 ml of approximately 10% mouse
microsome substrate at 37 °C for 30 min, under the following
conditions: (a) tetraethyl pyrophosphate (TEPP)-treated microsomes
(neither esterase nor oxidase activity); (b) normal microsomes
(esterase activity); (c) TEPP-treated microsomes plus NADPH
(oxidase activity); and (d) normal microsomes plus NADPH (esterase
plus oxidase activity). Each esterase preparation hydrolysed about
twice as much trans-cypermethrin as cis-cypermethrin. In contrast,
cis-cypermethrin was metabolized more rapidly in an oxidation
system than trans-cypermethrin. The major site of ring
hydroxylation was the 4' position and the secondary site was the 5
position. The trans-methyl group was an important site of
hydroxylation in the ester metabolites and cis-methyl oxidation was
predominant in the ester-cleaved acid metabolites. The
hydroxymethyl derivatives were further oxidized to the
corresponding aldehydes and carboxylic acids.
3-Phenoxybenzaldehyde-cyanohydrin was detected as a minor
metabolite. The preferred sites of hydroxylation were: with
trans-cypermethrin, cis-methyl > 4' position > trans-methyl > 5
position; with cis-cypermethrin, trans > cis > 4' position > 5
position (Shone & Casida, 1978; Shone et al., 1979). With cis-
cypermethrin, at least, cleavage of cypermethrin to cyanohydrin may
result from both hydrolytic and oxidative mechanisms, since large
amounts of the cleavage products were also evident in the oxidase
system, which lacks esterase activity (Shono & Casida, 1978; Shono
et al., 1979). However, at approximately 35-times higher substrate
levels, the hydrolysis rate of cypermethrin isomers was depressed
(Söderlund & Casida, 1977).
In studies on the metabolism of 14C-cypermethrin by rat liver
microsomes, the overall rates of metabolism of cis- and trans-
cypermethrin were similar, though their metabolic routes differed.
The cis-isomer was metabolized almost exclusively by an NADPH-
dependent oxidative pathway to 4'hydroxy- cis-cypermethrin with
subsequent oxidative ester cleavage. The predominant route for the
metabolism of the trans-isomer was hydrolysis to the trans-acid by
microsomal carboxylesterase (Crawford, 1979c). The in vitro
esteratic capacity was determined in rat, rabbit, and human liver
microsomes using p-nitrophenyl acetate and cypermethrin as
substrate. The relative ability to hydrolyse cypermethrin was
rabbit > man > rat. Rabbit and rat microsomes metabolized the
trans-isomer 6 times faster than the cis-isomer. Human
microsomes showed a similar capacity for metabolizing both cis-
and trans-isomers (Croucher et al., 1982a,b).
6.2.2. In vivo studies
The identification of the metabolites of cypermethrin has been
studied in mice (Hutson, 1978b, Casida et al., 1979; Hutson et al.,
1981), rats (Crawford & Hutson, 1977a; Casida et al., 1979; Hutson,
1979a,b; Crawford et al., 1981b; Rhodes et al., 1984), dogs
(Crawford, 1979d,e), and cows (Swaine & Sapiets, 1980a,b; Croucher
et al., 1985).
Overall, metabolism in these species is similar. Differences
that occur are related to the rate of metabolite formation rather
than to the nature of the metabolites formed. The only major
differences between species relate to conjugation reactions.
Cypermethrin (both isomers) is metabolized via cleavage of the
ester bond. The cyclopropane carboxylic acid moiety is mainly
excreted as the glucuronide conjugate; hydroxylation of the methyl
group occurs only to a limited extent (Crawford, 1979e; Rhodes et
al., 1984). The 3-phenoxy-benzyl product of the ester hydrolysis
is converted to PBA. The cyanide moiety is metabolized to
thiocyanate (Hutson, 1979b). The PBA moiety is mainly excreted as
a glutamic acid conjugate in the cow (Croucher et al., 1985), as a
taurine conjugate ( N-(3-phenoxy-benzoyl)taurine) in 2 strains of
mouse (Hutson & Casida, 1978; Hutson, 1978b, 1979a; Hutson et al.,
1981), and as a glycine conjugate in the rat and dog (Crawford &
Hutson, 1977a; Crawford, 1979d) and in the sheep, cat, and gerbil
(Huckle et al., 1981a). PBA is further metabolized (rat >
mouse > dog) via the 4'-hydroxylation to 3-(4'-hydroxyphenoxy)
benzoic acid and its sulfate conjugate (Crawford & Hutson, 1977a;
Hutson, 1978b; Crawford, 1979d). Glucuronic acid conjugates of PBA
and its 4'hydroxy derivative are the major urinary metabolites in
the marmoset, rabbit, guinea-pig, and hamster. The rat was unique
among the animal species tested in utilizing sulfuric acid for the
conjugation of the 4'-hydroxy derivative (Huckle et al., 1981a).
The major route of excretion for cypermethrin metabolites is via
the urine; unchanged cypermethrin accounted for the majority of
radioactivity found in faeces in radiolabel studies. The amount of
cyclopropyl-radioactivity eliminated in the bile (1%) suggests that
the biliary-intestinal-faecal route is of minor importance for this
moiety (Crawford & Hutson, 1977a; Crawford, 1979e; Rhodes et al.,
1984). Biliary excretion of PBA occurred as glucuronide and that
of 4'-OH-PBA as ether and ester glucuronic acid conjugates. Very
little was eliminated in the faeces, indicating that the biliary
glucuronides decompose and/or are enzymatically cleaved in the
gastro-intestinal tract to the respective benzoic acids. The
latter are subsequently reabsorbed and undergo further metabolism,
principally to the sulfate ester, which is excreted in the urine
(Huckle et al., 1981b). As already noted, the major urinary
metabolite of cypermethrin in cows is N-(3-phenoxy-benzoyl)glutamic
acid. This metabolite is also found in the organs and tissues with
only a small quantity of unchanged cypermethrin. The residues in
body fat consist mainly of cypermethrin. An unidentified polar
metabolite, present in the liver and kidneys, is suspected of being
a conjugate of 3-(4'-hydroxyphenoxy)benzoic acid. The small
portion of radioactivity appearing in milk was associated with
lipid components and consisted mainly of unchanged cypermethrin
(Croucher et al., 1985). The metabolic pathway of cypermethrin is
shown in Fig. 3.
As in other mammals, ester cleavage and elimination of the
cyclopropyl acid moieties in the free and glucuronidated form is a
major route of metabolism of cypermethrin in man (Eadsforth &
Baldwin, 1983).
6.2.3. Metabolism of the glucoside conjugate of 3-phenoxy-benzoic
acid
Studies have been carried out on rats on the metabolism of the
glucoside conjugate of 3-phenoxybenzoic acid, which occurs
occasionally as a metabolite in plants (Crayford, 1978). The
results indicated that the rat hydrolyses the glucoside and then
metabolizes the 3-phenoxybenzoic acid in virtually the same way as
it would metabolize PBA liberated during the metabolism of
cypermethrin.
 The same conclusion was also reached by Mikami et al. (1985).
During this study involving the metabolism of the glucoside
conjugate of PBA, it was noticed that the skin and carcasses
contained high residues (4 - 7% of the administered dose) of
radioactivity. To characterize the metabolites of PBA in the skin
and carcasses, rats were given (14C-benzyl) 3-PBA in a single oral
dose (0.8 mg/kg body weight) or a higher dose (totalling
approximately 750 mg/kg body weight) for 7 consecutive days. Two
components were identified in the skin: unchanged PBA and a
mixture of 3-phenoxybenzoyl-dipalmitins. The components were
present in the skin of the high-dose animals in the approximate
ratio of 3:7 and in the carcasses at 9:1 (Crayford & Hutson, 1979,
1980).
6.3. Metabolism in Plants
Lettuce plants were sprayed outdoors with 14C cypermethrin
labelled in the cyclopropyl ring (Wright et al., 1980; Roberts,
1981). The plants were sprayed twice, at a rate equivalent to 0.3
kg/ha, and harvested for analysis 21 days after the last treatment.
Most of the residue was in the form of unchanged cypermethrin (33%
of the total label present) and polar materials (54%), which were
shown to be mainly conjugates of trans-CPA. One of these
conjugates was identified as the beta-D-glucopyranose ester.
Evidence for this was obtained from studies on abscised cotton
leaves. The acid, trans-CPA was shown to be readily converted
into a mixture of the beta-D-glucopyranose ester, an acidic
derivative of this, and disaccharide derivatives, including the
glucosyl arabinose ester and the glucosylxylose ester.
Small apple trees were grown in cages outdoors (Roberts, 1981)
and the cis- or trans-isomers, labelled in either the cyclopropyl
or the benzyl rings, were applied to either the leaves or fruit.
In the leaves, it was shown that the main component of the residue
was cypermethrin itself with smaller amounts of sugar conjugates
that gave rise on hydrolysis to the 3-phenoxybenzyl alcohol,
3-phenoxybenzyl aldehyde, or PBA. A small amount of 4-hydroxy
cypermethrin was also detected. There was also evidence that some
30% of the cis-cypermethrin was converted to the trans-isomer,
though the conversion of trans- to cis- was not observed. Less
extensive metabolism occurred in the apple fruit. More than 98% of
the total label recovered was associated with the peel. Of this,
up to 77% was cypermethrin. Small amounts of the other free
compounds, together with polar compounds, were detected (Roberts,
1981).
Furuzawa et al. (1986) treated young cabbage plants in the
greenhouse with known amounts of either cis- or trans-
cypermethrin labelled in either the cyclopropyl or benzyl rings.
After 42 days, 4 - 6% of the applied dose was found on the surface
of the leaves, 57 - 63% in the acetone extract of the whole leaves,
and 13 - 26% was present as a bound residue. The observed half-
lives were 4 - 5 days for the trans- and 7 - 8 days for the cis-
cypermethrin. Isomerization was carefully followed in these
studies. First, there was practically no change in the ratio of
alpha-R to alpha-S isomers. On the other hand, both of the 1R
isomers ( cis and trans) were converted, in part, to the
corresponding 1S isomers, though the transformation appeared
greater where cis- had been the starting material, compared with
trans-cypermethrin. However, the position was complicated by the
simultaneous conversion of trans to cis-isomers. Apparently, these
changes were much less in the case of the extracts from the whole
leaf and the authors considered that this isomerization, as well as
the 1R-1S epimerization, were probably photochemical. These
findings on the conversion of cis to trans are consistent with the
formation of trans-cypermethrin from cis- reported by Cole et al.
(1982) in bean and cotton leaves in the greenhouse. These authors
also considered this to be a photochemical reaction. Roberts
(1981) reported the conversion of cis- to trans-cypermethrin on
apple leaves, but not of trans to cis.
The results of studies by Furuzawa et al. (1986) showed that,
in the intact ester, there was some hydration of the cyano group to
the amide, and subsequently to the corresponding acid, as well as
hydroxylation at the 4-benzyl or one of the gem methyl groups in
the cyclopropyl moiety. However, by far the most important
metabolites were glycoside conjugates of 4'-OH-PBA and CPA (with a
limited degree of isomerization from cis to trans and vice versa).
Hydroxylated CPA, either free or conjugated, was a relatively minor
metabolite.
More et al. (1978) studied the uptake of 14C radio-labelled PBA
in the abscised leaves of a range of different plants, which were
dipped in aqueous solutions. The PBA was labelled in the benzyl
ring. Mainly cotton plants and vines were studied, but broad
beans, tomatoes, lettuce, peas, and soybeans were also included.
In the case of cotton, some of the leaves were exposed to 13C-
labelled CO2 and allowed to take up the labelled PBA after a 2-h
period of photosynthesis; the 13C label was used to assist
identification by mass spectroscopy. A large part of the PBA in
the cotton leaves was conjugated within a few hours; it was shown
that most of the conjugated material was the glucose ester. It was
also shown, in the 13CO2 study, that at least a part of the glucose
moiety had been derived from recent photosynthesis. On more
prolonged exposure, other conjugates were formed. In the case of
vine leaves, these proved to be a series of disaccharides that
contained pentose sugars as well as glucose. The evidence
suggested that these had been formed by the addition of the pentose
sugar to the glucose conjugate rather than by the PBA becoming
conjugated with the preformed disaccharide. The conjugates in the
other plant species were not always positively identified, but the
authors commented that the interspecies differences were likely to
be mainly of degree rather than of any fundamental differences in
process.
Mikami et al. (1984) studied the metabolism of PBA in abscised
leaves of cabbage, cotton, cucumber, kidney bean, and tomato
plants. PBA was rapidly converted into more polar products by
esterification with glucose, malonylglucose, gentiobiose,
cellobiose, glycosylxylose, and tri-glucose. The main metabolites
were malonyl glucoside in cabbage, kidney bean, and cucumber, and
the glucosylxylose ester in cotton. The gentiobiose and tri-glucose
esters were predominant in tomato. The metabolism of cypermethrin
in plants is summarized in Fig. 4.
6.4. Metabolism in Fish
The metabolism of cis-cypermethrin (14C-labelled in either the
acid or the alcohol moiety) in trout was studied by Edwards &
Millburn (1985a). In contrast with that in other animals (frogs,
mice, and quail), ester cleavage of cypermethrin was very slow,
and the main metabolic pathway was hydroxylation to give the 4-
hydroxyphenoxy derivative, which was excreted in the bile as the
glucuronide. Edwards & Millburn (1985b) identified the glucuronide
of CPA and the ether glucuronide and sulfate of 4-hydroxy PBA, but
only as minor metabolites.
The same conclusion was also reached by Mikami et al. (1985).
During this study involving the metabolism of the glucoside
conjugate of PBA, it was noticed that the skin and carcasses
contained high residues (4 - 7% of the administered dose) of
radioactivity. To characterize the metabolites of PBA in the skin
and carcasses, rats were given (14C-benzyl) 3-PBA in a single oral
dose (0.8 mg/kg body weight) or a higher dose (totalling
approximately 750 mg/kg body weight) for 7 consecutive days. Two
components were identified in the skin: unchanged PBA and a
mixture of 3-phenoxybenzoyl-dipalmitins. The components were
present in the skin of the high-dose animals in the approximate
ratio of 3:7 and in the carcasses at 9:1 (Crayford & Hutson, 1979,
1980).
6.3. Metabolism in Plants
Lettuce plants were sprayed outdoors with 14C cypermethrin
labelled in the cyclopropyl ring (Wright et al., 1980; Roberts,
1981). The plants were sprayed twice, at a rate equivalent to 0.3
kg/ha, and harvested for analysis 21 days after the last treatment.
Most of the residue was in the form of unchanged cypermethrin (33%
of the total label present) and polar materials (54%), which were
shown to be mainly conjugates of trans-CPA. One of these
conjugates was identified as the beta-D-glucopyranose ester.
Evidence for this was obtained from studies on abscised cotton
leaves. The acid, trans-CPA was shown to be readily converted
into a mixture of the beta-D-glucopyranose ester, an acidic
derivative of this, and disaccharide derivatives, including the
glucosyl arabinose ester and the glucosylxylose ester.
Small apple trees were grown in cages outdoors (Roberts, 1981)
and the cis- or trans-isomers, labelled in either the cyclopropyl
or the benzyl rings, were applied to either the leaves or fruit.
In the leaves, it was shown that the main component of the residue
was cypermethrin itself with smaller amounts of sugar conjugates
that gave rise on hydrolysis to the 3-phenoxybenzyl alcohol,
3-phenoxybenzyl aldehyde, or PBA. A small amount of 4-hydroxy
cypermethrin was also detected. There was also evidence that some
30% of the cis-cypermethrin was converted to the trans-isomer,
though the conversion of trans- to cis- was not observed. Less
extensive metabolism occurred in the apple fruit. More than 98% of
the total label recovered was associated with the peel. Of this,
up to 77% was cypermethrin. Small amounts of the other free
compounds, together with polar compounds, were detected (Roberts,
1981).
Furuzawa et al. (1986) treated young cabbage plants in the
greenhouse with known amounts of either cis- or trans-
cypermethrin labelled in either the cyclopropyl or benzyl rings.
After 42 days, 4 - 6% of the applied dose was found on the surface
of the leaves, 57 - 63% in the acetone extract of the whole leaves,
and 13 - 26% was present as a bound residue. The observed half-
lives were 4 - 5 days for the trans- and 7 - 8 days for the cis-
cypermethrin. Isomerization was carefully followed in these
studies. First, there was practically no change in the ratio of
alpha-R to alpha-S isomers. On the other hand, both of the 1R
isomers ( cis and trans) were converted, in part, to the
corresponding 1S isomers, though the transformation appeared
greater where cis- had been the starting material, compared with
trans-cypermethrin. However, the position was complicated by the
simultaneous conversion of trans to cis-isomers. Apparently, these
changes were much less in the case of the extracts from the whole
leaf and the authors considered that this isomerization, as well as
the 1R-1S epimerization, were probably photochemical. These
findings on the conversion of cis to trans are consistent with the
formation of trans-cypermethrin from cis- reported by Cole et al.
(1982) in bean and cotton leaves in the greenhouse. These authors
also considered this to be a photochemical reaction. Roberts
(1981) reported the conversion of cis- to trans-cypermethrin on
apple leaves, but not of trans to cis.
The results of studies by Furuzawa et al. (1986) showed that,
in the intact ester, there was some hydration of the cyano group to
the amide, and subsequently to the corresponding acid, as well as
hydroxylation at the 4-benzyl or one of the gem methyl groups in
the cyclopropyl moiety. However, by far the most important
metabolites were glycoside conjugates of 4'-OH-PBA and CPA (with a
limited degree of isomerization from cis to trans and vice versa).
Hydroxylated CPA, either free or conjugated, was a relatively minor
metabolite.
More et al. (1978) studied the uptake of 14C radio-labelled PBA
in the abscised leaves of a range of different plants, which were
dipped in aqueous solutions. The PBA was labelled in the benzyl
ring. Mainly cotton plants and vines were studied, but broad
beans, tomatoes, lettuce, peas, and soybeans were also included.
In the case of cotton, some of the leaves were exposed to 13C-
labelled CO2 and allowed to take up the labelled PBA after a 2-h
period of photosynthesis; the 13C label was used to assist
identification by mass spectroscopy. A large part of the PBA in
the cotton leaves was conjugated within a few hours; it was shown
that most of the conjugated material was the glucose ester. It was
also shown, in the 13CO2 study, that at least a part of the glucose
moiety had been derived from recent photosynthesis. On more
prolonged exposure, other conjugates were formed. In the case of
vine leaves, these proved to be a series of disaccharides that
contained pentose sugars as well as glucose. The evidence
suggested that these had been formed by the addition of the pentose
sugar to the glucose conjugate rather than by the PBA becoming
conjugated with the preformed disaccharide. The conjugates in the
other plant species were not always positively identified, but the
authors commented that the interspecies differences were likely to
be mainly of degree rather than of any fundamental differences in
process.
Mikami et al. (1984) studied the metabolism of PBA in abscised
leaves of cabbage, cotton, cucumber, kidney bean, and tomato
plants. PBA was rapidly converted into more polar products by
esterification with glucose, malonylglucose, gentiobiose,
cellobiose, glycosylxylose, and tri-glucose. The main metabolites
were malonyl glucoside in cabbage, kidney bean, and cucumber, and
the glucosylxylose ester in cotton. The gentiobiose and tri-glucose
esters were predominant in tomato. The metabolism of cypermethrin
in plants is summarized in Fig. 4.
6.4. Metabolism in Fish
The metabolism of cis-cypermethrin (14C-labelled in either the
acid or the alcohol moiety) in trout was studied by Edwards &
Millburn (1985a). In contrast with that in other animals (frogs,
mice, and quail), ester cleavage of cypermethrin was very slow,
and the main metabolic pathway was hydroxylation to give the 4-
hydroxyphenoxy derivative, which was excreted in the bile as the
glucuronide. Edwards & Millburn (1985b) identified the glucuronide
of CPA and the ether glucuronide and sulfate of 4-hydroxy PBA, but
only as minor metabolites.
 Trout liver microsomes metabolize both cis- and trans-isomers
of cypermethrin to the 4'-hydroxy derivatives of the intact esters
and their corresponding ether glucuronic acid conjugates at
comparable rates. There are indications, derived from studies with
other pyrethroids, that the liver microsomes of the carp are more
able to metabolize pyrethroids than those of the salmonids (Edwards
& Millburn, 1985b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Leahey (1985) and Smith & Stratton (1986) give extensive
reviews on this matter.
7.1. Microorganisms
Effects of cypermethrin on microbial activity in the soil have
been investigated under laboratory conditions. Cypermethrin was
applied to a sandy loam soil at a rate of 2.5 or 250 mg/kg soil.
No effect was found on the rate of carbon dioxide evolution (Cook,
1978a) or oxygen uptake (Loveridge & Cook, 1978a) at the lower
rate. With 250 mg/kg, slight, but significant, inhibition of the
CO2 evolution and a decrease in oxygen uptake were noted after long
incubation periods. Nitrogen fixation was assessed by the
acetylene reduction method (Loveridge & Cook, 1978b). No
significant effects were found. Ammonification and nitrification
were studied in soils, either alone, or amended with urea or
ammonium chloride. Again, no significant effects were found, even
at the high rate of application (Cook, 1978b). Finally, glucose
utilization was studied in the short term (2 days) and after pre-
incubation with cypermethrin for up to 38 weeks. No significant
effects were noted, even at the high rate (Bromley & Cook, 1979).
A second series of experiments, in which cypermethrin was
applied to a sandy loam at 0.5 or 5 mg/kg soil has been reported.
Antimicrobial activity was observed in the early stages of
incubation with fungal population numbers returning to normal
within 2 - 4 weeks, and with a subsequent stimulation of microbial
growth. There was no inhibition of acetylene reduction activity at
either dosage. Nitrification and microbial respiration were
increased at both dosages, suggesting microbial degradation of
cypermethrin. Dehydrogenase activity was not affected, while
urease activity was stimulated. This study indicates that
cypermethrin may exert transient effects on the populations and
activities of microflora, but they are short-lived and minor in
nature (Tu, 1980).
The toxicity of cypermethrin for 4 pathogens of soybean and for
the nodulation organism Rhizobium has been investigated. In a
paper disc inhibition test, cypermethrin proved non-toxic for
Rhizobium, the EC50 exceeding 10 000 mg/litre. The toxicity for
the pathogens was higher, EC50 values ranging from 390 to 1700
mg/litre (Tu, 1982).
7.2. Aquatic Organisms
7.2.1. Fish
7.2.1.1. Acute toxicity
In common with other pyrethroids, cypermethrin is very toxic
for fish in clean water under laboratory conditions. The available
data are summarized in Table 8. The data demonstrate a similar
high acute toxicity for both cold- and warm-water species of fish.
Certain of these data have been reviewed by Stephenson (1982e).
There is no evidence of a significant effect of temperature on
toxicity, though a negative temperature coefficient has been
claimed for certain pyrethroids (Kumaraguru & Beamish, 1981).
Furthermore, the toxicity of pyrethroids for fish was not
influenced by the hardness or pH of the water (Mauck et al., 1976).
To determine the acute toxicity of cypermethrin for Tilapia
nilotica, an EC formulation of cypermethrin (25 g/litre) was
sprayed on the surface of static water held in stainless steel
tanks in a glasshouse. The concentration of cypermethrin in the
water was determined over a 96-h period. Depending on the
application rate, the concentration of cypermethrin in the water
rose rapidly during the first 24 h after application and decreased
slowly over the following 3 days. The tanks each contained 5 fish
and water to a depth of 30 cm. Water temperatures were 23 - 26 °C.
No fish deaths occurred when the peak concentration of cypermethrin
was less than 1.5 µg/litre, while all fish died when the
concentration exceeded approximately 5 µg/litre (Stephenson,
1981a).
To study the influence of suspended solids on water
concentrations and the toxicity for fish of cypermethrin, Rainbow
trout were exposed to nominal concentrations of 2 or 5 µg
cypermethrin/litre, which was added to microfiltered mains water or
to pond water containing 14.5 mg suspended solids/litre. The
actual concentrations were measured for water samples taken 30 min
after initial mixing. Two samples of pond water were taken. One
was analysed as such while the second was centrifuged (60 min at
3000 rev/min) to precipitate the suspended solids; the clear
supernatant liquid was then analysed. The results are summarized
in Table 9.
The data from this study show that suspended solids absorb 40%
of the cypermethrin initially present. Fish exposed to an actual
concentration of 4.9 µg cypermethrin/litre in mains water died
within 24 h, those in pond water containing an actual concentration
of 2.5 - 4 µg/litre survived (Reiff, 1978a).
The high degree of adsorption of cypermethrin on soils was
demonstrated by Riley & Hill (1983) in their studies on the
toxicity of cypermethrin for Daphnia and other aquatic organisms.
The addition of soil to the system reduced the toxicity of the
aqueous solution by 200 - 350 times.
The stainless steel tank study with Tilapia nilotica was
repeated with the water initially containing 100 mg suspended
solids/litre. In this study too, the presence of suspended solids
reduced the toxic effects of cypermethrin (about 2-fold)
(Stephenson, 1982a).
Table 8. Acute toxicity of cypermethrin for fish
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Brown trout 5 - 8 technical, dispersed via 15 2 - 2.8 Reiff (1976)
(Salmo trutta) DMSO
5 - 8 technical, absorbed on 15 1.2a,b Reiff (1976)
pumice
Rainbow trout 1 - 11 technical, absorbed on 10 - 15 0.5a,b,c Reiff (1978b)
(Salmo gairdneri) pumice
1 - 2 technical, via acetone 10 0.5 Stephenson
(1982b)
3.3 technical, via acetone 15 2.8 Stephenson
(1982b)
3 emulsifiable concentrate 10 11d,g Coats &
(40% ai) O'Donnell-Jeffery
(1979)
3 technical, dispersed via 10 55d Coats &
acetone O'Donnell-Jeffery
(1979)
Common carp 4 - 10 technical, absorbed on 10 0.9a,b,c Reiff (1978b)
(Cyprinus carpio) pumice
4 - 10 technical, absorbed on 20 - 25 1.1a,b,c Reiff (1978b)
pumice
8 - 10 technical, via acetone 10 0.9 Stephenson
(1982c)
2.1 emulsifiable concentrate 22 - 26 3.4f Stephenson
(5% a.i.) (1982c)
Stephenson et
al. (1984)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Rudd (Scardinius 9 - 10 technical, absorbed on 15 0.4a,b,c Reiff (1978b)
erythrophthalmus) pumice
Atlantic salmon 5.3 technical, dispersed via 10 2 - 2.4b McLeese et al.
( Salmo salar) ethanol (1980)
9.4 1-R- cis- 10 0.74 Zitko et al.
(1979)
Tilapia nilotica 1 - 3 technical, absorbed on 25 2.0a,b,c Stephenson
pumice (1981b)
Stephenson et
al. (1984)
Fathead minnow 0.74 technical, absorbed on 23 - 25 1.2a,b Stephenson
(Pimephales (juvenile) pumice (1982d)
promelas)
Mugil cephalus 0.1 30% EC ? 24g Tag El-Din et al.
30 days (1981)
Gambusia affinis 4 weeks 30% EC 25 6.6g El-Sebae et al.
(1983)
---------------------------------------------------------------------------------------------------------
a Flow-through system. Otherwise, static test.
b Measured concentration. Otherwise, nominal.
c The data quoted have been recalculated from the primary data given in the original reports following
current statistical methods.
d 24-h LC50.
e Lethal threshold value (geometric mean of lowest concentration with and highest concentration without
mortality).
f 48-h LC50.
g Formulation.
Remark: In most tests, the pH of the water was 7.5 - 8.5; the hardness was 260 mg/litre as CaCO3 (except
for a few cases).
Table 9. Influence of suspended solids on the toxicity of cypermethrin
for Rainbow trout
-------------------------------------------------------------------------
Source Suspended Initial cypermethrin Response of Rainbow
solids concentration (µg/litre) trout
(mg/litre) actual
nominal total in water
-------------------------------------------------------------------------
Microfiltered 0 2 1.7 1.7 1/6 fish dead at 48 h
mains water 5 4.9 4.9 6/6 fish dead at 24 h
Pond water 14.5 2 1.2 0.65 no symptoms or deaths
5 4.0 2.5 at 7 days
-------------------------------------------------------------------------
7.2.1.2. Long-term toxicity
The effects of cypermethrin on the most sensitive stage in the
life cycle of the Fathead minnow (Pimephales promelas) were
investigated using a flow-through system. Total hardness, pH,
concentration of dissolved oxygen, and temperature were controlled.
Within 24 h of fertilization, eggs were exposed to nominal
concentrations of 0, 0.03, 0.1, 0.3, or 1.0 µg/litre (mean exposure
concentration 0, 0.03, 0.12, 0.17, and 0.79 µg cypermethrin/litre),
for a total of 34 days. Hatching occurred between the 3rd and 6th
day, while egg hatch was not affected at the highest concentration.
No fry survived day 34. Survival was reduced at concentrations of
0.3 and 0.1 µg/litre but not at 0.03 µg/litre. On the basis of the
most sensitive parameter, i.e., survival of young fry, the no-
observed-adverse-effect level for cypermethrin lay between 0.03 and
0.12 µg/litre (Stephenson, 1982d).
7.2.2. Invertebrates
7.2.2.1. Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cypermethrin. The data in Table 10 are from static, clean water
test systems and some of these data have been reviewed by
Stephenson (1982e). It can be seen that snails do not show any
effects at 5 µg/litre (close to the water solubility of
cypermethrin), while some insects show behavioural changes, but no
mortality at this level. Crustacea, particularly marine decapod
Crustacea, are highly susceptible to cypermethrin, mortality
occurring at levels below 0.05 µg/litre. Water mites are also very
susceptible (Zitko et al., 1979).
In addition to these static water tests, continuous-flow tests
in clean water have been undertaken with 2 sensitive species
(Stephenson, 1980b) (Table 11).
It can be concluded that effects can be expected when
concentrations of cypermethrin of the order of 0.01 µg/litre are
maintained in the water phase for more than 96 h.
It has been shown that, at 100 µg/litre, cypermethrin does not
affect the growth of the single-celled green alga Selenastrum
capricornutum, over a period of 2 - 4 days (Stephenson, 1982b).
7.2.2.2. Long-term toxicity
The effects of cypermethrin on the survival, growth, and
reproduction of Daphnia magna were investigated, over 21 days, in
a static water test with daily renewal of test solutions. The
nominal concentrations tested were 0, 0.003, 0.01, 0.03, 0.1, and
0.3 µg/litre. Cypermethrin affected all 3 parameters at a nominal
concentration of 0.3 µg/litre, but no effects were noted at 0.1
µg/litre. Chemical analysis suggested that the Daphnia were
exposed to about 50% of the nominal concentration, two-thirds in
solution, the remainder adsorbed on suspended solids. These
results show that the no-observed-adverse-effect level of
cypermethrin throughout the life cycle for Daphnia is of the order
of 0.05 µg/litre (Garforth, 1982).
7.2.3. Field studies
7.2.3.1. Deliberate overspraying
This subject has received wide attention, and various aspects
have been reviewed in a number of publications (Crossland &
Stephenson, 1979; Crossland, 1982; Crossland et al., 1982;
Crossland & Elgar, 1983; Shires, 1983a; Stephenson, 1983).
The effects of field applications of cypermethrin on fish were
studied in a preliminary study by Crossland & Bennett (1976). A
small pond was deliberately oversprayed with 100 g cypermethrin/ha
as an EC formulation. The peak concentration of cypermethrin in
the water was 2.6 µg/litre. Wild populations of fish and amphibia
were not affected by this treatment. Large numbers of young fish
seen during the 2 weeks following treatment did not appear to be
affected.
In a second study at the same rate of application, the peak
concentration of cypermethrin was 1.4 µg/litre. There was no
observed mortality among 12 small rudd that had been placed in the
pond 12 days before treatment, and no effects of treatment were
noted in wild populations of rudd or newts (Crossland et al.,
1978).
Table 10. Acute toxicity of cypermethrin for aquatic invertebrates in static tests
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Fresh-water
Crustacea
Water flea up to 24 h 22 acetone 4.2 Reiff (1977)
(Daphnia magna) old 18 water 2 2 Stephenson (1980a)b
20 acetone 1.2 - Stephenson (1982b)
20 acetone 0.3c - Stephenson (1982b)
Water hog louse 3 - 8 mm 15 water 0.02 0.2 Stephenson (1980a)
(Asellus spp.)
Freshwater shrimp 3 - 8 mm 15 id 0.04 0.1 Stephenson (1980a)
(Gammarus pulex)
Insecta
Mayfly larvae 15 id 0.07 0.6 Stephenson (1980a)
(Cloeon dipterum)
Whirligig beetle adult 15 id 0.07 > 5 Stephenson (1980a)
(Gyrinus natator)
Bloodworm larvae 15 id 0.2 > 5 Stephenson (1980a)
(Chironomus thummi)
Mosquito larvae 18 id 0.03 1 Stephenson (1980a)
(Aedes aegypti)
Midge (Chaoborus larvae 15 id 0.03 0.2 Stephenson (1980a)
crystallinus)
Water boatman adult 15 water 0.7 > 5 Stephenson (1980a)
(Corixa punctata)
Water boatman adult 15 id 0.3 > 5 Stephenson (1980a)
(Notonecta spp.)
---------------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Arachnida
Water mite adult 15 0.02 0.05 Stephenson (1980a)
(Piona carnea)
Mollusca
Snail (Lymnaea < 8 mm 15 id > 5 > 5 Stephenson (1980a)
peregra)
Marine
Crustacea
Lobster (Homarus 450 g 10 ethanol - 0.04g McLeese et al.
americanus) (1980)
450 g 10 id - 0.003d,f,g Zitko et al. (1979)
Shrimp (Crangon 1.3 g 10 id - 0.01d,e McLeese et al.
septemspinosa) (1980)
---------------------------------------------------------------------------------------------------------
a EC50 values are based on effects, usually immobilization, other than death.
b Stephenson (1980a) used cypermethrin (85% a.i.) dissolved in acetone and absorbed on pumice.
c 48-h EC50.
d 96-h LC50.
e Dispersed via ethanol.
f 1-R- cis-.
g Salinity, 30%.
Remark: In most tests, the pH of the water was 7.5 - 8.5; and the hardness, 260 mg/litre as CaCO3.
Table 11. Acute toxicity of cypermethrin for aquatic invertebrates in
continuous-flow tests
------------------------------------------------------------------------
Species Stage Temperature EC50 LC50
size (°C) (µg/litre) (µg/litre)
24-h 96-h 24-h 96-h
------------------------------------------------------------------------
Freshwater shrimp 3 - 8 mm 15 0.005 0.004 0.030 0.009
(Gammarus pulex)
Mayfly larvae 15 0.008 0.004 0.070a 0.020
(Cloeon dipterum) 0.5 - 1 cm
------------------------------------------------------------------------
a 48-h LC50.
Observations on invertebrates were included in these two pond
studies. In the first study, which lasted 2 weeks, populations of
Crustacea, mites, and insects were severely reduced. Surface
breathing insects were affected most rapidly, within hours of
treatment. Free-swimming dipterous larvae were not noticeably
affected for 24 h, while zooplankton were killed between 1 and 2
days after treatment. Bottom dwelling invertebrates, including
chironomid larvae, snails, leaches, and flatworms, did not appear
to be affected, though the numbers in the last 2 groups were low in
pre-treatment samples.
The second study was continued for 15 weeks after treatment.
Initial results were similar to those reported above. Macro-
invertebrates were markedly reduced in numbers, 2 weeks after
treatment. However, both numbers and diversity returned to normal
levels after 15 weeks. Snails and flat-worms (again numbers low in
this group in pretreatment counts) were unaffected, but no
arthropods were present in the samples taken at 2 weeks.
Recolonization by flying insects (beetles and chironomids)
commenced 4 weeks after treatment. The Crustacean Asellus had not
reappeared by the end of the study.
No daphnids or copepods were found in the zooplankton samples,
1 week after treatment, and they only reappeared in the 8-week
post-treatment sample. Populations returned to normal levels in
10 - 12 weeks. Some 2 weeks after treatment, an increase in
filamentous algae was noted, and this persisted until the end of
the study. It was inferred that this was a secondary effect
following from the elimination of known feeders on algae, for
instance, the mayfly, Cloeon dipterum, and the daphnid,
Simocephalus sp. (Crossland & Bennett, 1976; Crossland et al.,
1978).
7.2.3.2. Monitoring of drift from ground and aerial applications
Two field studies were undertaken to assess the fate and
effects in adjacent waters of spray drift from ground-based
agricultural applications of cypermethrin.
(a) Mistblower applications on vines (France)
Three sites were chosen, each with vines planted up to the
banks of adjacent streams. One application of cypermethrin was
monitored at each site and a second application at one of the
sites. A diluted EC formulation of cypermethrin was applied at
30 g a.i./ha using a backpack, swinging-head mistblower, and at
45 g a.i./ha using a tractor-mounted, fixed-head mistblower.
The deposition on the ground and an adjacent stream of 0.37 -
4.5 g/ha led to peak concentrations in the subsurface water ranging
from 0.17 to 1.7 µg/litre during the first hour after spraying; the
concentrations decreased to less than 0.1 µg/litre within a few
hours. No effects on fish, tadpoles, or frogs were observed. Some
aquatic invertebrates in adjacent streams were seen to be
hyperactive or immobilized during the first 2 h after application.
This was most obvious with mayfly larvae, water boatmen, water
beetles, pond skaters, and syrphid larvae. During the first 3 h
following application, there was an increase in the number of
invertebrates caught in drift nets. However, there were no
significant population effects in zooplankton or macro-
invertebrates (Bennett et al., 1980).
(b) Boom-and-nozzle applications to row crops (United Kingdom)
Two sites were chosen each with row crops (potatoes or
sugarbeet or both) planted up to within 1 - 3 m of the edge of
ponds. Two applications of cypermethrin were monitored at each of
3 ponds. In each case, a diluted EC formulation of cypermethrin
was applied at 70 g a.i./ha using tractor-mounted, boom-and-nozzle
equipment. Deposition on the ground and over the pond was
estimated. The deposition led to peak concentrations in subsurface
water in the range of 0.01 - 0.05 µg/litre. In one case, the
concentration in subsurface waters after 24 h was 0.02 - 0.03
µg/litre. In all other cases, this value was at, or below, 0.01
µg/litre, the limit of detection. No effects on fish were
observed. No residues of cypermethrin were found in fish (limit of
detection, 5 µg/kg wet weight). The only effects on invertebrates
in adjacent waters were noted in the corner of 1 pond. Some pond
skaters, one water-boatman, and 2 syrphid larvae were found
immobilized. They had recovered by the next day. There was no
evidence from zooplankton or sweep-net samples of any effects on
invertebrate populations (Shires et al., 1980).
(c) Aerial application to winter wheat (United Kingdom)
A large field of winter wheat was chosen, which was surrounded
on 3 sides by drainage ditches. An EC formulation of cypermethrin
was applied at 25 g a.i./ha by fixed-wing aircraft. Up to 6% of
the nominal dose was deposited on the water surfaces, resulting in
maximum levels in subsurface waters of 0.03 µg/litre, which
declined rapidly after spraying. No effects were observed on caged
or wild fish, and no significant residues of cypermethrin were
found in the fish. A few air-breathing water boatmen and water
mites, which are very susceptible, showed minor short-term
reductions in abundance (Shires & Bennett, 1982, 1985).
(d) Application for rice insect control (Korea, Spain)
An EC formulation of cypermethrin was applied to rice paddies
in Korea, in which carp had been caged, by hand-spraying at 15 or
40 g a.i./ha. Mortality at the higher rate was significantly
higher than that in the control, i.e., 15 and 7%, respectively
(Stephenson, 1982c).
Another field study was carried out in Spain. Cypermethrin was
applied by air to paddy rice and also to caged fish (Cyprinus
carpio) within the area. The application rate was 15 - 40 g
a.i./ha. The limited toxic effects of cypermethrin on fish under
field conditions were confirmed in this study. Aerial overspraying
of the rice paddies at 25 g a.i./ha did not produce any mortality
(Stephenson et al., 1984).
(e) Applications for tsetse fly control (Nigeria)
Field studies have been reported on the assessment of the
environmental impact of the application of cypermethrin for tsetse
fly control in Nigeria. Searches for affected fish were made in
areas that had been selectively sprayed from the ground with an EC
formulation of cypermethrin at 2 - 3 g a.i./litre, or sprayed from
the air with an oil-based solution of cypermethrin at 100 g a.i./ha
in 1977 and at 60 and 150 g a.i./ha in 1978. The ground spray
application was successful in controlling the tsetse fly, but only
the highest rate applied from the air approached a satisfactory
level of tsetse control. None of these searches revealed any dead
fish in the rivers within the areas sprayed with cypermethrin.
Pre- and post-treatment net samples of aquatic invertebrates were
taken in an area sprayed at 100/150 g a.i./ha. Acute mortality
occurred in terrestrial and aquatic arthropods, such as the water
beetle and Crustaceans. Shrimps and mayfly larvae disappeared from
river benthos after spraying, but reappeared one year later (Smies
et al., 1980).
7.3. Terrestrial Organisms
7.3.1. Laboratory studies
7.3.1.1. Acute toxicity
(a) Birds
The acute oral toxicity of cypermethrin for birds is summarized
in Table 12.
In the first study (Coombs et al., 1976), 4 birds of each
species were observed for a 3-week period after dosing; no deaths
or toxic signs were noted at the highest dosages applied. In the
second study (Rose, 1981), clinical signs of cypermethrin
intoxication, including ataxia and lethargy, were noted at dosages
of 3000 mg/kg body weight and above. One male duckling, out of 28
of each sex, died at the top dosage of 10 000 mg/kg body weight.
7.3.1.2. Short-term toxicity
(a) Birds
Six laying hens were given 5 successive daily oral doses of
1000 mg cypermethrin/kg body weight in DMSO followed after 3 weeks
by a second 5-day dosing regime and were observed for a further 3
weeks. Control hens did not receive any treatment. No signs of
intoxication or histological changes in nervous tissue were noted
at any time (Owen & Butterworth, 1977).
Table 12. Acute oral toxicity of cypermethrin for birds
--------------------------------------------------------------------------
Species Age Vehicle Observation LD50 Reference
period (mg/kg
body
weight)
--------------------------------------------------------------------------
Domestic fowl adult 40% DMSO 21 days > 2000 Coombs et al.
(Gallus (1976)
domesticus)
French partridge adult 40% DMSO 21 days > 3000 Coombs et al.
(Allectoris (1976)
rufa)
Mallard duck 2 - 6 40% emul- 14 days > 10 000 Rose (1981)
(Anas months sifiable
platyrhynchos) concentrate
--------------------------------------------------------------------------
Cypermethrin was administered for 5 consecutive days to Mallard
ducks at dosages of 5000 or 10 000 mg/kg diet. No deaths or
clinical signs of intoxication were noted during the feeding period
or over a subsequent 40-day holding period. Food intake and body
weights were slightly depressed during the feeding period, though
most ducks had regained or exceeded their initial body weights by
the end of the holding period (Rose, 1981).
Riviere et al. (1983) studied the influence of cypermethrin on
the microsomal drug metabolizing enzymes in Japanese quail
(Coturnix coturnix). Cypermethrin had no or only a very weak
inducing effect.
(b) Honey bees
Cypermethrin was highly toxic for worker honey bees (Apis
mellifera) in laboratory tests (24-h oral LD50 0.035 µg a.i. per
bee) (Table 13). Cypermethrin applied on the dorsal side of the
thorax was more toxic for honey-bees at lower breeding
temperatures, i.e., at 12 - 20 °C, the toxic level was 0.01 - 0.02
µg/bee and at 32 °C, 0.02 - 0.03 µg/bee. In addition, the
sensitivity of the bees to cypermethrin increased with age (Delabie
et al., 1985).
An aqueous dispersion of cypermethrin was sprayed directly on
worker honey bees. Only 5% of the bees sprayed with a 0.01
g/litre solution were killed. All bees sprayed with 0.1 and 1
g/litre died (Harris & Turnbull, 1978).
First indications that cypermethrin might not be hazardous for
bees under field conditions were obtained in cage tests. Worker
honey bees were exposed for 2 h to residues produced by treating
flowering Solidago (Golden rod) to run-off with a spray containing
0.5 g cypermethrin/litre and aging the residue for 1 h. Some
knock-down of the bees (up to 9%) was noted during the first 8 h
following initial exposure. However, most bees recovered, and
mortality after 1 day and 10 days did not differ from that in
untreated control bees. Higher mortality had been noted in an
earlier study using the same technique but with a higher dosage
rate of 1.5 g cypermethrin/litre (Gerig, 1979, 1981).
Table 13. Toxicity of cypermethrin for worker honey bees
-------------------------------------------------------------------------
Formulation 24-h LD50 (mg/bee) Reference
Topical application Oral administration
-------------------------------------------------------------------------
Technical 0.02 0.035 Badmin & Twydell
(1976)
Technical 0.053 0.12 Knight (1982)
Technical 0.056 - Smart & Stevenson
(1982), Westlake
et al. (1985)
Emulsifiable - 0.031 Badmin & Twydell
concentrate (1976)
(40%)
-------------------------------------------------------------------------
(c) Predatory and parasitic species
Cypermethrin has also been evaluated against a number of
predatory or parasitic insects and mites. Laboratory data are
available for a number of predators (Coleoptera) and parasites
(Hymenoptera) of insect pests.
The available data indicate that cypermethrin can show useful
selectivity between insect predators and parasites and the relevant
pest species (Mulla et al., 1978; du Toit, 1978; Waddill, 1978;
Abdel-Aal et al., 1979; Coats et al., 1979; Holden, 1979; Leake et
al., 1979; McDonald, 1979; European Patent Office, 1980; Harris &
Turnbull, 1980; Hagley et al., 1981; Jordan & Chang, 1981; Saad et
al., 1981; Ascher et al., 1982; Baicu, 1982; Bayoumi, 1982; Chang &
Jordan, 1982; Osman et al., 1982; Rajakulendran & Plapp, 1982;
Surulivelu & Menon, 1982; Brempong-Yeboah et al., 1983; Chang &
Jordan, 1983; El-Guindy et al., 1983; El-Minshawy et al., 1983;
El-Sebae et al., 1983; Ho et al., 1983; Ishaaya et al., 1983;
Watters et al., 1983; Abbassy et al., 1984; Bariola & Lingren,
1984; Brempong-Yeboah et al., 1984a,b,c; Cheng & Hanlon, 1984; Dai
& Sun, 1984; El-Sayed & Knowles, 1984; Ewen et al., 1984; Fabellar
& Heinrichs, 1984; Hopkins et al., 1984; Liu et al., 1984; Mani &
Krishnamoorthy, 1984; Meisner et al., 1984; Le Patourel & Singh,
1984; Riskallah, 1984; Scott & Georghiou, 1984; Wilde et al., 1984;
Zohdy et al., 1984; Ahmed et al., 1985; Abou-Awad & El-Banhawy,
1985; Bostanian & Belanger, 1985; Bostanian et al., 1985; Corbitt
et al., 1985; Edwards et al., 1985; El-Sebae et al., 1985; Harris
et al., 1985; Knapp et al., 1985; Knowles & El-Sayed, 1985; McKee &
Knowles, 1985; Pree & Hagley, 1985; Suhas & Devaiah, 1985; Tewari &
Krishnamoorthy, 1985; Vekaria & Vyas, 1985; Barlow et al., 1986).
(d) Earthworms
The toxicity of cypermethrin for the earthworm (Eisenia
foetida) has been assessed in an artificial soil test system. Worms
were exposed to dosages of 0, 0.1, 1.0, 10, or 100 mg/kg soil for
14 days. No mortality was found (Inglesfield & Sherwood, 1983;
Inglesfield, 1984).
The LC50 for cypermethrin in Eisenia foetida was found to be
26.1 µg/cm2 (16.3 - 44.4 µg/cm2) (Roberts & Dorough, 1984).
(e) Higher plants
In studies on effects of cypermethrin on seed germination in
the soybean, seedling emergence and survival were not reduced by
levels of up to 5000 mg/litre (Tu, 1982). Cypermethrin has been
demonstrated not to be phytotoxic for crops when applied at
recommended dosages (Hargreaves & Cooper, 1979).
7.3.2. Field Studies
7.3.2.1. Applications for tsetse fly control in Nigeria
Field trials in Nigeria of cypermethrin against Tsetse flies
( Glossina palpalis (RD) and Glossina tachnoides Westw.) were
carried out, over a 2-year period by Spielberger et al. (1979).
Ground applications of cypermethrin (0.3%) were made on fly-resting
sites in vegetation using pressurized knapsack sprayers.
Populations of both species were eradicated after a single
application. Levels of over 150 g/ha were needed for complete
eradication by residual spraying from a helicopter.
The effects on the general insect population of aerial
applications of cypermethrin for tsetse fly control were studied by
Smies et al. (1980). Within a few hours of application, dead and
dying insects were found, exemplifying the rapid knockdown effect
of cypermethrin. Insect fallout, as assessed by funnel traps, was
markedly increased for 1 day by 100 g cypermethrin a.i./ha, and for
3 days by 150 g a.i./ha. General insect activity as assessed by
Malaise trap catches was not affected by 150 g cypermethrin a.i./ha
(Smies et al., 1980).
7.3.2.2. Honey bees
This subject has been reviewed by Shires & Debray (1982) and
Shires (1983b).
The hazard of practical field applications of cypermethrin for
worker honey bees was assessed in 2 field trials. In both trials,
cypermethrin was applied to flowering oilseed rape, by helicopter,
at a time when the crops were being actively worked by bees from
nearby colonies, thus representing a worst case exposure of the
bees. The rate of application in both trials was 25 g a.i./ha. Of
the 8 hives, placed adjacent to the treated crops, 6 had been
fitted with dead bee traps and 2 with pollen traps. Observations
were made on bee mortality, pollen collection, foraging activity,
hive populations, and brood areas. In addition, in the second
trial, cypermethrin residues were determined in the bees, pollen,
wax, honey, and in the leaves and flowers of the treated crop
(Pearson & Shires, 1981; Shires, 1982b).
In the first trial, only a small increase in bee mortality was
noted, following the cypermethrin treatment (Table 14).
Cypermethrin appeared to be repellent to honey bees foraging the
crop, but the duration of this effect could not be established
because of poor weather during the first few days following
treatment. Cypermethrin did not have any effects on hive
populations of adult bees or brood areas.
In the second trial, a slight increase in bee mortality was
found at the time of treatment (Table 14). Cypermethrin had a
repellent effect on honey bees for up to 24 h after application.
Following this period, foraging activity and pollen collection
returned to normal. Cypermethrin residues in dead bees, collected
on the day of spraying and on the following morning, were higher
than would be expected to be lethal. However, residue levels in
dead bees collected after this period were considerably lower than
those required to produce toxic effects, confirming that they
represented the natural mortality level. Very low levels of
cypermethrin were found in live bees and levels in honey and wax
were close to the limit of detection. Concentrations of
cypermethrin in flowers and pollen declined rapidly after
treatment. Again, cypermethrin did not have any effects on hive
populations of adult bees or of brood areas (Shires, 1982b).
Table 14. Mean number of dead bees per hive during large-scale
field trials with cypermethrina
-----------------------------------------------------------------
Treatment Time from treatment
compound -2 to -1 -1 to 0 0 to +4 +1 to +2 +3 to +4
(dosage) days days h days days
-----------------------------------------------------------------
Winter-sown rape; 65 6 260 40 2
cypermethrin
(25 g a.i./ha)
water control 75 17 0 26 15
Spring-sown rape 9 36 310 2 13
cypermethrin
(25 g a.i./ha)
-----------------------------------------------------------------
a From: Shires (1983b).
In glasshouse studies, in which insecticide treatment was
carried out during foraging by spraying the rape with 0.12 ml of
Cymbush in 100 ml water (equivalent to 50 g a.i./ha), high
mortality was observed 2 days after treatment, but no residual
mortality appeared during the following 2-month period. In this
study, oilseed rape flowers were not attractive to bees for at
least 2 days following treatment.
A field test was carried out on a 38-ha field of oilseed rape.
The insecticide was sprayed on a central area of 13 ha during the
morning, at a rate of 50 g a.i./ha. The weather was sunny and the
temperature about 18 °C. A high level of mortality occurred only
on the 3 days following treatment. The results also showed that the
bees avoided visiting the flowers as soon as the treatment was
made, especially during the first 2 days. From the third day after
the treatment, the repellent effect decreased, and the visits to
the rape flowers increased reaching normal on the fifth day. It
was suggested by the authors that the repellent effect appeared to
be due to the formulation ingredients.
No residues of cypermetherin were found in hive products
(pollen, wax, or honey) (Delabie et al., 1985).
In a study by Gerig (1981), cypermethrin was repellent to
worker honey bees for a short period after spraying. Flowering
Phaselia plants, within a flight tent, were treated with a spray
containing 0.5 g cypermethrin/litre. Honey bee visits to the
treated flowers were very few over the first 0.5 h following
treatment and remained at a reduced level during the remaining
5.5 h of observation on the day of treatment. Flower visits
returned to normal levels on the following day (Gerig, 1981).
7.3.2.3. Soil fauna
This topic has been reviewed by Shires (1980).
In an initial study on the effects of cypermethrin on the soil
surface fauna and earthworms, cypermethrin was applied at 100 g
a.i./ha to small plots of spring wheat. This treatment did not
have any effect on earthworm populations or on leaf litter
breakdown, which is an indirect indicator of earthworm activity.
The general population of soil surface predators, mainly beetles
and spiders, was reduced to about 50% of the control value soon
after treatment, but increased to a higher level than that in the
controls after 5 weeks. A subsequent secondary decrease in
predator populations was probably related to the efficiency of
control of cereal aphids by cypermethrin (Shires et al., 1979). In
a second study, cypermethrin was applied at 25 g a.i./ha to newly-
emerged winter barley to control the aphid vectors of barley yellow
dwarf virus. Populations of non-target arthropods were assessed
using pitfall and water traps. Populations of soil Collembola
were only slightly affected by cypermethrin. Populations of
predators (beetles, spiders, and centipedes) were already declining
because of the season, but there were significantly fewer predators
in the cypermethrin plots compared with the controls at the end of
the trial. Cypermethrin treatment did not affect the numbers of
invertebrates collected from the water traps (Sherwood & Shires,
1981).
Two studies have been reported on the summer application of
cypermethrin for aphid control in winter wheat. In France, ground
applications of cypermethrin at 50 and 75 g a.i./ha were made in
June and effects on the fauna in the air, on the crop, and on the
soil surface were studied. Cypermethrin proved effective against
the phytophagous (pest) species. Effects on non-target organisms
were only minor, with the exception of spiders. In the second
study in the United Kingdom, cypermethrin was applied in June using
a fixed-wing aircraft at 25 g a.i./ha. Again, cypermethrin proved
effective against the pest species, while, generally, effects on
non-target organisms were minor and short-lived (Shires, 1982a,c).
Similar results have been obtained in studies on the aerial
application of cypermethrin at 25 g a.i./ha on oilseed rape and at
50 and 75 g a.i./ha on maize, in France (Inglesfield, 1982;
Garforth, 1983).
7.3.2.4. Foliar predators and parasites
The relative toxicity of cypermethrin for pests and their
parasites and predators is such that the balance between host/prey
and parasites/predators may not be adversely affected in the field.
However, care should be taken where predatory mites are important
in pest management. The high toxicity of cypermethrin for
predatory mites has been confirmed under field conditions (Wong &
Chapman, 1979; Aliniazee & Cranham, 1980; Shires & Tipton, 1982).
The effects of cypermethrin treatments, included in a spray
programme for cotton insect control, on white fly parasitism have
been studied in the Sudan. The treatment regime that gave the
consistently highest level of control of parasitism included
cypermethrin at 40 g a.i./ha in 4 early season sprays (Shires &
Tipton, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Leahey (1985) and Smith & Stratton (1986) have reviewed the
available literature on this subject.
8.1. Single Exposures
8.1.1. Oral
The acute oral toxicity of cypermethrin (mixture of cis-/ trans-
isomers) in experimental animals is of a moderate order (Tables 15,
16). Signs of intoxication, indicative of an action on the central
nervous system consist of sedation, ataxia, splayed gait, tip-toe
walk, with occasional tremors and convulsions. These signs of
toxicity appear within a few hours following dosing, and survivors
show clinical recovery within 3 days (Coombs et al., 1976).
Table 16 shows the effects of the ratio of cis-: trans-isomers
on the acute oral toxicity (FAO/WHO, 1980b).
Factors known to influence the oral LD50 value of cypermethrin
include concentration, vehicle, temperature, age of the animals,
and the animal strain used (Coombs et al., 1976; Dewar & Owen,
1979). The acute toxicity of cypermethrin was approximately 3
times greater in 3-week-old, than in 12-week-old rats (Rose &
Dewar, 1978). The cis-: trans-ratio plays a role in determining
acute toxicity, the cis-isomers being more toxic than the trans-
isomers. The oral LD50 of the cis-isomers in rats (Carworth Farm
E strain) was 229 mg/kg body weight while, with the trans-isomers,
no deaths were found at 2000 mg/kg body weight (Brown, 1979a,b).
8.1.2. Dermal
The acute dermal toxicity of technical cypermethrin is of a low
order. There were no deaths in CFE rats at any dose level tested
up to 1600 mg/kg body weight using 20% w/v xylene as the vehicle
(Coombs et al., 1976). The dermal LD50 for the cis-isomer of
cypermethrin, applied to the skin of rats as a 10% solution in
DMSO, was 219 mg/kg body weight (Brown, 1979a).
The acute dermal toxicity of cypermethrin for the rabbit
is > 2460 mg/kg body weight (US EPA 1984).
8.1.3. Intraperitoneal
The intraperitoneal (ip) LD50 (20% w/v solution in corn oil)
for technical cypermethrin in CFI mice is 485 mg/kg body weight
(Coombs et al., 1976). In rats, the LD50 is between 198 and 315
mg/kg body weight (administered as 5% solution in DMSO) (Price,
1981a).
Table 15. Oral LD50 values for technical cypermethrin
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
-----------------------------------------------------------------------
rat 5% in corn oil 251 (203 - 295) Coombs et al.
(1976)
rat 5% in dimethylsulf- 303 (277 - 329) Coombs et al.
oxide (1976)
rat 5% in dimethylsulf- 570 (485 - 823) Price (1981a)
oxide
rat 40% in dimethylsulf- approximately 4000 Rose (1982)
oxide
rat 5% in glycerol 200 - 400 (male); Coombs et al.
formal approximately 200 (1976)
(female)
rat 10% in aqueous 400 - 800 (male); Coombs et al.
suspension approximately 400 (1976)
(female)
rat 50% in aqueous 3423 (2815 - 4328)a Rose (1982)
suspension
mouse 5% in corn oil 82 (68 - 116) Rose (1982)
mouse 5% in dimethylsulf- 138 (105 - 199) Coombs et al.
oxide (1976)
mouse 50% in aqueous 657 (439 - 1003) Rose (1982)
suspension
Syrian 10% in corn oil approximately 400 Coombs et al.
hamster (male and female) (1976)
Chinese 5% in corn oil 203 (144 - 255) Coombs et al.
hamster (1976)
guinea- 20% in corn oil approximately 500 Coombs et al.
pig (male); > 1000 (1976)
(female)
bovine 15% formulation in 142 - 284 (male) Cassidy (1979)
calves Shellsol A
piglets 15% formulation in > 600 (male) Cassidy (1979)
Shellsol A
-----------------------------------------------------------------------
Table 15. (contd.)
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
----------------------------------------------------------------------
lamb 15% formulation in 283 - 567 (male) Cassidy (1979)
Shellsol A
domestic 40% in dimethylsulf- > 2000 Coombs et al.
fowl oxide (1976)
partridge 40% in dimethylsulf- > 3000 Coombs et al.
oxide (1976)
-----------------------------------------------------------------------
a 40:60 cis/trans.
Table 16. Effects of cis-: trans-ratio on the acute toxicity of
cypermethrina
------------------------------------------------------------------
Species Sex Vehicle cis-: trans-ratio LD50 (mg/kg)
------------------------------------------------------------------
rat male, dimethylsulfoxide cis-only 160 - 300
female
rat male, dimethylsulfoxide trans-only > 2000
female
rat female corn oil 90:10 367
rat female corn oil 40:60 891
------------------------------------------------------------------
a From: FAO/WHO (1980b).
8.1.4. Inhalation
Groups of CD rats (4 of each sex) were exposed for 4 h to an
aqueous spray of cypermethrin as a 400 g/litre emulsifiable
concentrate. The liquid phase contained 3 g cypermethrin/litre and
the atmospheric concentration of droplets in the vicinity of the
rats was calculated to be 0.7 mg/litre. The median droplet size
was 130 µm with a geometric standard deviation of 1.6 µm,
comparable with the droplet spectrum of a field-spraying device.
(It should be noted that this droplet size is nearly not
inhalable). The rats became thoroughly soaked during the exposure,
thereby indicating a significant dermal exposure with probable oral
exposure during post-exposure grooming. Immediately after the
exposure, the female rats showed the abnormal gait and urinary
incontinence typical of pyrethroid intoxication. Recovery took
place within 3 days. No deaths or other adverse signs occurred
during the exposure period or the subsequent 14-day observation
period (Blair & Roderick, 1976).
In another study on possible effects on the peripheral nerves,
rats (8 of each sex) were exposed for 4 h to an aqueous spray of
cypermethrin as a 400 g/litre emulsifiable concentrate under
conditions similar to those in the previous study. Half of the
rats were necropsied immediately after exposure and the others
after 66 h. Examination of the sciatic nerves from all of the rats
did not reveal any abnormalities, even though signs of intoxication
had been observed (Blair et al., 1976).
8.1.5. Skin and eye irritation
Technical cypermethrin was moderately irritant to occluded
rabbit skin (both intact and abraded) and to the eyes of New
Zealand white rabbits. In both cases, recovery was complete in a
few days. The severity of the irritation depended on the
formulation and the solvent used (Hine & Zuidema, 1970; Coombs et
al., 1976).
The skin on the backs of guinea-pigs was treated with
cypermethrin. The animals responded by licking, rubbing,
scratching, or biting the sides tested. This reaction may be
associated with skin sensory effects characterized by transient
itching and tingling sensations (Cagen et al., 1984).
8.1.6. Sensitization
A skin sensitization test on guinea-pigs (maximization
procedure of Magnusson & Kligman) indicated that technical
cypermethrin may have a mild skin sensitizing potential (Coombs et
al., 1976; US EPA, 1984).
8.2. Short-Term Exposures
8.2.1. Oral
8.2.1.1. Rat
Groups of 6 male and 6 female Charles River rats were fed diets
containing 0, 25, 100, 250, 750, or 1500 mg cypermethrin/kg feed
for 5 weeks. In the 1500 mg/kg group, reduced body weight gain and
food intake, piloerection, nervousness, incoordinated movement,
increased liver weight, and increases in blood urea and haemoglobin
concentrations were observed, but there were no pathological
changes. No changes were detected in the groups receiving 750
mg/kg or less (Coombs et al., 1976).
Groups of Charles River rats, 12 of each sex (24 of each sex as
controls), were fed dietary concentrations of 0, 25, 100, 400, or
1600 mg cypermethrin/kg feed for 3 months. Signs of intoxication,
such as hypersensitivity and abnormal gait, were observed in the
1600 mg/kg group, during the first 5 weeks, and one animal died.
The survivors showed clinical recovery in the second half of the
study. However, body weight gain was reduced, liver and kidney
weights increased, and there were increases in plasma-urea
concentrations and plasma-alkaline phosphatase activity, and
decreases in haemoglobin concentrations and the red blood cell
count in females, and in the kaolin cephalin clothing time in males
and packed cell volume were observed. Two out of 4 male rats,
killed prematurely, showed axonal breaks and vacuolization of
myelin in the sciatic nerve. None of the survivors showed nerve
lesions, and no other pathological effects were found. In the
400 mg/kg group, males showed increased kidney weights but no
histopathological changes. No effects were found in the 100 mg/kg
group (Hend & Butterworth, 1976).
Male and female rats (20 in each group) were fed cypermethrin
( cis-: trans- = 44:56) with a purity of 92% in the diet at levels
of 0, 75, 150, or 1500 mg/kg diet, for 90 days. Haematology and
the results of urinalysis were normal. Both males and females
receiving 1500 mg/kg diet showed reduced body weight and reduced
food consumption during the first month of the study. Increased
liver microsomal oxidase activity was noted in both males and
females in the 1500 mg/kg group and in the males in the 150 mg/kg
group. These changes were substantially reversed within the 4-week
recovery period. Gross and microscopic (including electron
microscopic) examination of the tissues and organs did not reveal
any signficant differences between the treated groups and the
controls. Examination of the sciatic nerve of animals in the
control and the 1500 mg/kg groups did not reveal any changes that
could be directly attributed to cypermethrin (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats per group and 14 of
each sex as controls) were fed trans-isomer at concentrations of
0, 30, 100, 1000, or 3000 mg/kg diet, for 5 weeks. No gross or
microscopic findings were observed and mortality did not occur.
Changes in several haematological parameters, alkaline phosphatase
activity, and liver, spleen, and kidney weight were observed at the
2 highest dose levels. Special histopathological studies of the
sciatic nerve did not show any damage (Hend & Butterworth, 1977a).
Groups of rats (6 male and 6 female rats per group and 10 of
each sex used as controls) were fed concentrations of the cis-
isomer at 0, 30, 100, 300, 750, or 1500 mg/kg diet for 5 weeks
(protocol similar to that described above). Mortality was observed
at the 1500 mg/kg level as well as neurotoxic signs of poisoning.
Growth was reduced at the 750 mg/kg level. Significant reductions
in food intake were also noted at doses of 300 mg/kg or more,
during the initial phase of the study. Relative kidney weights
showed a statistically-significant increase at doses of 300 mg/kg
or more and an increase in liver weight was observed at 750 mg/kg.
Histopathological examinations revealed substantial degeneration
in both the liver and sciatic nerve at 1500 mg/kg. No lesions were
observed in the brain or spinal cord (Hend & Butterworth, 1977b).
8.2.1.2. Dog
Beagle hounds (4 of each sex per dose level) were fed diets
containing 0, 5, 50, 500, or 1500 mg cypermethrin/kg diet for 13
weeks. At 1500 mg/kg, severe signs of intoxication consisting of
diminished food intake, weight loss, diarrhoea, anorexia, licking
and chewing of the paws, whole body tremors, a stiff exaggerated
gait, ataxia, incoordination, and hyperaesthesia were observed.
Half of the dogs in this group were necropsied because of severe
clinical signs of intoxication. However, no changes in
haematology, organ weight, or histopathology were observed and
despite the severe signs of intoxication, no lesions of the sciatic
nerves were observed in the dogs. No effects were seen at 500
mg/kg feed (Buckwell & Butterworth, 1977).
8.2.2. Dermal
8.2.2.1. Rabbit
Groups of 10 male and 10 female New Zealand white rabbits
received occluded dermal applications (abraded and non-abraded
skin) of 2, 20, or 200 mg cypermethrin/kg body weight in
polyethylene glycol (PEG 300) for 6 h per day, 5 days per week,
for 3 weeks. A control group was similarly treated with the
vehicle only (PEG 300). Slight-to-moderate skin irritation was
observed in rabbits receiving 2 and 20 mg/kg body weight; those
receiving 200 mg/kg body weight showed slight-to-severe irritation.
In the rabbits treated at 200 mg/kg, reductions were observed in
food intake, body weight gain, and weight of gonads. No other
changes were noticed. No such effects were found at 20 mg/kg body
weight (Henderson & Parkinson, 1981).
8.2.3. Intraveneous
8.2.3.1. Rat
Lock & Berry (1981) found biochemical changes in the rat
cerebellum after a single iv administration of 25 mg
cypermethrin/kg body weight. Signs of toxicity included:
salivation, clonic convulsions, and sinuous writhing movements.
The concentrations of cerebellar amino acids and cyclic nucleotides
were determined at these stages of toxicity. Treatment with
cypermethrin resulted in an increase in cerebellar cyclic GMP at
the earliest stage, without changing cyclic AMP. Levels of blood-
and cerebellar-glucose, lactate, and ammonia were also increased in
most of the stages of toxicity. No changes were seen in
concentrations of glutamate, glutamine, gamma-aminobutyrate,
creatine, phosphate, or ATP in the cerebellum. The increase in
cerebellar cyclic GMP did not appear to be related to the
convulsive state of the animal or to the muscarinic cholinergic
stimulation.
8.3. Long-Term Exposures
8.3.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) were fed dietary concentrations of 0, 1, 10, 100, or
1000 mg cypermethrin/kg diet for 2 years. Additional groups (6 or
12 rats of each sex per dose level) were fed the diets for only 6,
12, or 18 months.
Throughout the 2-year study, the control and cypermethrin-
treated animals were similar in terms of behaviour; no compound-
related clinical signs were observed, except for a significantly-
reduced growth rate in both males and females fed 1000 mg/kg diet.
Survival was not affected by cypermethrin. No clinical-chemical,
haematological, or histopathological changes were observed. The
sciatic nerves from a number of animals of all groups, sacrificed
after 6, 12, 18, or 24 months, showed a small number of nerve
fibres exhibiting Wallerian degeneration. The incidence increased
with age, but there was no difference in severity between the
control and treated groups. It is concluded that doses of 100 mg
cypermethrin/kg diet or less did not produce significant
toxicological effects in rats over a 2-year period (McAusland et
al., 1978).
Assays of liver microsomal enzyme activity in 6 rats of each
sex fed 0 or 1000 mg cypermethrin/kg feed for 2 years showed
cypermethrin to be a weak inducer of the microsomal enzyme hepatic
p-nitroanisole- O-dimethylase (PNOD), used as an index of
monooxygenase activity (Potter & McAusland, 1980).
Five groups of Wistar rats, each composed of 52 males and 52
females, were given diets containing 0 (2 groups), 20, 150, or 1500
mg/kg diet (equivalent to 0, 1, 7.5, and 75 mg/kg body weight) for
2 years. Satellite groups of 12 male and 12 female rats were
sacrificed at 52 weeks. For the first 6 weeks of the study, the
rats in the 1500 mg/kg group received 1000 mg/kg. The purity of
the cypermethrin was between 88% and 93% ( cis-: trans-ratio;
55:45). After 104 weeks at the highest dose level, body weight
loss, increased liver weight, increased smooth endoplasmatic
reticulum in hepatocytes, and some haematological and other slight
clinical changes were observed. No other changes or increase in
the incidence of tumours were found. The dose of 150 mg/kg diet
was considered to be the no-observed-adverse-effect level in this
study (only a summary is given) (US-EPA, 1984).
8.3.2. Mouse
The effects of cypermethrin were investigated in groups of 70
male and 70 female Swiss mice fed diets containing 0 (2 groups),
100, 400, or 1600 mg/kg feed (equivalent to 0, 15, 60, or 240 mg/kg
body weight) for up to 101 weeks. The purity of the cypermethrin
was between 91.5 and 94.2%; cis-: trans-ratio; 53:47 or 54:46.
Ten males and 10 females per group were killed at 52 weeks for
interim haematological evaluation. Appearance, behaviour, and
survival were similar in all groups. Body weight gain was reduced
in mice fed 1600 mg cypermethrin/kg diet compared with the combined
control groups. Food intake of both males and females was not
significantly changed. Several haematological changes, consistent
with a mild anaemia, were found in the 1600 mg/kg group of animals
at the interim kill (decreased haemoglobin, haematocrit, and red
blood cell counts in males; decreased mean cell volume and mean
cell haemoglobin concentration in females) but were not found at
termination. Thrombocytosis and increased liver weight were also
observed in this group, at both the interim and terminal kills.
There were no accompanying histopathological changes (for
carcinogenic potential, see section 8.7). No effects were observed
at dose levels of 400 mg/kg diet or less (Lindsay et al., 1982).
8.3.3. Dog
Cypermethrin (dissolved in corn oil) was administered to 4
groups of 8 beagle dogs of each sex at dose levels of 0, 1, 5, or
15 mg/kg body weight per day, by capsule, for a period of 52 weeks.
The purity of the cypermethrin was 90.6% ( cis-: trans-ratio;
54:46). The dogs in the highest dose group exhibited loss of
appetite, tremors, gait changes, incoordination, disorientation,
and hypersensitivity. No other abnormalities were found in the
composition of the blood or urine or in organ weights. Dogs
receiving 5 mg/kg or more showed liquid stools by this mode of
administration. However, this effect was not found when
cypermethrin was fed in the diet for 2 years. No effects were seen
at 1 mg/kg (but only a summary is given) (US-EPA, 1984).
Groups of 4 male and 4 female Beagle hounds were fed diets
containing 0, 3, 30, or 300 mg cypermethrin/kg feed for 2 years.
An additional group received 1000 mg/kg feed but, because of severe
intoxication signs, the dose was decreased to 750 mg/kg. The signs
of intoxication consisted of licking and chewing of the paws, a
stiff high-stepping gait, whole body tremors, head shaking,
incoordination, ataxia, and convulsions. After 3 weeks of feeding
at 750 mg/kg, the animals received the control diet, until signs of
intoxication could no longer be seen. The dogs were then fed a
diet containing 600 mg cypermethrin/kg from week 8 until
termination at 2 years. No signs of intoxication were observed in
dogs fed diets containing 0, 3, 30, or 300 mg/kg during the course
of the study. There was reduced body weight gain in male dogs in
the 600 mg/kg group. Clinical chemistry and haematological
investigations, performed at 6-week intervals for 2 years, did not
reveal any consistent differences between treated groups and
controls. The minor changes in absolute organ weight observed in
the brain and thyroid in the 300 mg/kg group were not present when
the differences were corrected for terminal body weight.
Furthermore, a dose relationship was not apparent, and there were
no accompanying histopathological changes. Opthalmological
observations performed during the course of the study did not
reveal any ocular differences between treated groups and control.
No abnormalities were found in the sciatic nerves, brain, or spinal
cord in any of the treated groups. The feeding of diets containing
up to 600 mg cypermethrin/kg diet to dogs for 2 years did not
reveal any treatment-related gross or histopathological effects,
although the dogs receiving diets containing 1000 mg/kg decreased
to 600 mg cypermethrin/kg showed reduced body weight gain.
Cypermethrin did not produce any toxicological effects in dogs fed
dietary concentrations of 300 mg/kg feed or less, over 2 years
(Buckwell, 1981).
8.4. Special Studies
8.4.1. Synergism/potentiation studies
8.4.1.1. Organophosphate mixture
In a study on rats, designed to determine whether an
organophosphate insecticide, monocrotophos, potentiated the
neurotoxic effect of cypermethrin, 5 groups of 7 or 8 male and 7 or
8 female rats were given 7 consecutive oral doses of monocrotophos
(100 g/litre), cypermethrin (25g/litre), or a mixture of
monocrotophos and cypermethrin (100 g/litre and 25 g/litre,
respectively). Signs of intoxication and neurotoxic effects
(biochemical changes consistent with very sparse axonal
degeneration in sciatic/tibial nerves) were found to be wholly
attributable to the monocrotophos component of the mixture, and
there was no evidence of potentiation of neurotoxic effects (Rose &
Dewar, 1979a).
8.4.1.2. Organochlorine mixture
The oral and dermal toxicities of cypermethrin and endosulfan
for rats were determined both individually and combined. No
evidence for enhancement of toxicity was found for either
cypermethrin or endosulfan when administered as a 1:1 mixture in
corn oil (Price, 1981b).
8.4.2. Neurotoxicity
8.4.2.1. Characterization of the neurotoxic effects
During preliminary short-term studies on cypermethrin, 2
observations were made:
(a) high doses of cypermethrin given orally to rats
caused an unusual gait in intoxicated animals; and
(b) at lethal or near-lethal dermal or oral doses,
histopathological changes (swelling and/or
disintegration of axons of the sciatic nerve of
rats) were observed; such observations have been
reported to occur with other synthetic pyrethroids
(Okuno et al., 1976a) and natural pyrethrins (Okuno
et al., 1976b).
As a consequence of these findings, an extensive programme of
work has been undertaken to characterize the neurotoxic effects of
cypermethrin.
8.4.2.2. Neuropathological studies
(a) Rat
Single oral doses of cypermethrin in corn oil were given to
groups of 6 - 12 rats of each sex at 100, 200, or 400 mg/kg body
weight. All rats showed signs of intoxication, which varied in
severity according to the dose level. At 400 mg/kg, severe
clinical signs were seen within 2 days of dosing. Most of the
animals showed swelling of the myelin sheaths and breaks of some of
the axons of the sciatic nerves. At 200 mg/kg, 8 rats of each sex
died or were killed within 48 h of dosing, and the remaining 4 of
each sex survived the 9 days of the study. Nearly half of the
animals showed lesions of the sciatic nerve. At 100 mg/kg, all
rats survived 9 days of the trial. Only one female out of 12
showed minimal lesions of the sciatic nerve. Neuropathological
changes were found in a number of animals dosed with 200 mg
cypermethrin/kg body weight or more. Dose-related increases in
signs of intoxication and neuropathy were observed; however,
neuropathy was not detected in all the animals showing signs of
intoxication (Carter & Butterworth, 1976).
Groups of rats (10 males per group) were fed dietary levels of
cypermethrin ( cis-: trans- = 45:55) at 0, 1250, 2500, or 5000
mg/kg diet for 14 days. Mortality was observed at the 2 higher
doses and growth inhibition was seen in all treated groups.
Clinical signs of neurotoxicity were characterized by an impaired
ability to walk, splayed hind limbs, ataxia, and paralysis. Other
clinical signs were hypersensitivity, gross disorientation, and
convulsions. The neurotoxic signs of poisoning observed at 1250
mg/kg diet were reversible. Remission of ataxia at 2500 mg/kg diet
was also noted. Ultrastructural changes in the sciatic nerve were
observed in a small number of animals at the 2 highest dose levels.
Some evidence of axonal damage in the myelinated nerves was
observed, mainly in these groups (Glaister et al., 1977a).
In another study, rats were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg diet for 2 years (section 8.3).
At the 12-month interim kill, part of the sciatic nerve was removed
from 6 males and 6 females in both the control and 1000 mg/kg
group. The dissected nerves were divided and teased into a fan
shape, allowing each fibre to be examined. No significant
difference was found in the incidence of abnormal nerve fibres in
the control and the 1000 mg/kg group (Trigg et al., 1977; McAusland
et al., 1978).
(b) Hamster
Groups of 6 male and 6 female Syrian hamsters were given single
oral doses of cypermethrin in corn oil at 0, 794, 1000, and 1260
mg/kg body weight. Signs of intoxication (generally tiptoe
walking, tremors, irregular movements) occurred in all groups
receiving cypermethrin. Animals died or were killed at times
varying from a few hours to 9 days after dosing. Of the 31 animals
of the different test groups that were examined, 3 had nerve
lesions, axonal swelling, and axonal breaks in the sciatic and
posterior tibial nerves (many animals died within a few hours)
(Butterworth & Clark, 1977).
(c) Chicken
In a delayed neurotoxicity study on 6 adult domestic hens,
cypermethrin in DMSO did not cause histological lesions in the
nervous system (brain, spinal cord, and sciatic nerves) or signs of
intoxication at an oral dose of 1000 mg/kg body weight per day for
5 days, compared with a positive control group. No delayed
neurotoxicity was observed (Owen & Butterworth, 1977).
8.4.2.3. Biochemical and electrophysiological studies
Biochemical and electrophysiological studies have been carried
out because the sparse axonal degeneration described above is
difficult to demonstrate by conventional histopathological
techniques. Furthermore, evidence was required to quantify the
neurological dysfunction attributable to cypermethrin. The
rationale for measuring the activities of beta-glucuronidase and
beta-galactosidase in conjunction with an assessment of "mean slip
angle" and "mean landing foot spread" as markers for axonal
degeneration has been described by Dewar (1977a,b).
(a) Rat
Using biochemical markers, it has been shown that, at high oral
doses of cypermethrin, sparse axonal degeneration occurs in the
sciatic/posterior tibial nerves (Dewar, 1977b; Rose & Dewar, 1983),
and the trigeminal nerve and trigeminal ganglion of Wistar rats
(Dewar & Moffett, 1978a).
Three studies involving repeated oral administration of
cypermethrin to rats at 150 mg/kg body weight for 5 or 7 days and
0, 25, 50, 100, or 200 mg/kg body weight for 5 or 7 days have been
carried out. Mortality occurred from 100 mg/kg body weight
onwards. A dose-related transient functional impairment, assessed
by means of the inclined plane test, was found in the first week.
Significant increases in beta-glucuronidase or beta-galactosidase
activity of the sciatic, tibial, or trigeminal nerves only occurred
with 5 or 7 doses of 150 or 200 mg/kg body weight. The sensitivity
of these nerves is comparable. Complete functional recovery
occurred within 26 days. Increased activity of the enzymes in the
distal portion of nerves was found but, even in the most severely
intoxicated animals, the magnitude of the increases was less than
that induced by the known neurotoxic agent methylmercury chloride
(Dewar, 1977b; Dewar & Moffett, 1978a; Rose & Dewar, 1983).
Biochemical changes, consistent with primary axonal
degeneration, were detected in rats aged 3, 6, or 12 weeks.
Cypermethrin was more acutely toxic for 3-week-old rats than for
12-week-old rats and the dose required to cause a sparse axonal
degeneration, as demonstrated by biochemical changes, was
proportionally smaller for the younger rats (Rose & Dewar, 1978).
The time-course for development and recovery from axonopathy
was investigated in groups of rats (5 of each sex) at periods of
2 - 12 weeks after the start of dosing. The daily doses were 150
mg/kg body weight for the first 11 doses and 100 mg/kg body weight
for the subsequent 9 doses, over a period of 4 weeks. More than
half of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication, characterized by abnormal
gait, ataxia, salivation, lethargy, chromodacryorrhoea,
piloerection, and hypersensitivity to external stimuli. Maximum
enzyme activities in the sciatic posterior tibial nerves were found
after 5 weeks and had returned to control values by 12 weeks. In a
second phase of the study, 10 male and 10 female rats were given
20 oral doses of cypermethrin at 37.5, 75, or 150 mg/kg body weight
per day, (concurrent control group dosed with DMSO), over a 4-week
period. The animals given 75 and 150 mg/kg showed clinical signs
of intoxication. Mortality also occurred at the highest dose
level. The behaviour and appearance of animals in the 37.5 mg/kg
body weight per day group were similar to those of the controls.
Five weeks after the initial dose, biochemical changes, which were
indicative of a mild axonal degeneration, were observed in animals
in the highest dose group. No consistent or biologically
significant neurobiochemical changes were found in the 37.5 or 75
mg/kg body weight groups. The results of this study demonstrated
that 20 oral doses of cypermethrin at 75 mg/kg body weight per day
administered over a 4-week period, produced mild clinical signs of
intoxication; however, no biochemical changes which were indicative
of peripheral neuropathy were seen (Rose, 1983).
Further evidence to support the minor nature of the nerve
lesions has been afforded by electrophysiological studies on rats.
In these studies, measurements of the maximal motor conduction
velocities and conduction velocity of slower fibres in the sciatic
and tail nerves of Wistar rats were made before, and at intervals
of up to 5 weeks after, exposure to single (200 mg/kg body weight)
or repeated doses (7 doses of 150 mg/kg body weight) of
cypermethrin. Even at near-lethal doses, cypermethrin did not
cause any effects on these parameters (Dewar & Deacon, 1977).
The ability of the major metabolite of cypermethrin, PBA, to
produce axonal changes has been investigated. Four groups of 8
male and 8 female rats given 7 consecutive daily oral doses of 0,
25, 77, or 375 mg/kg body weight did not show any biochemical
changes indicative of peripheral nerve damage. The sparse axonal
degeneration observed with high doses of cypermethrin is,
therefore, not caused by PBA (Rose & Dewar, 1979b).
(b) Hamster
In 3 studies, Chinese hamsters were given oral doses of
5 - 30 mg cypermethrin/kg body weight per day, for 5 consecutive
days. Clinical signs of intoxication were observed and tests
made to assess whether the compound produced degenerative changes
in peripheral nerves and sensory ganglia. Beta-glucuronidase,
and beta-galactosidase activities were measured in the
sciatic/posterior tibial nerves and trigeminal ganglion. A
positive control group received 7.5 mg methylmercury chloride/kg
body weight per day, for 5 consecutive days.
The most striking feature in the hamsters treated with doses
exceeding 5 times 20 mg/kg body weight was a marked loss of fur in
the facial area, neck, and back, due to repeated scratching. The
results of the enzyme determinations suggest that the Chinese
hamster like the rat developed sparse axonal degeneration following
oral doses in excess of 5 times 10 mg/kg body weight (Dewar &
Moffet, 1978b)
(c) Rabbit
Rabbits were administered capsules with 0 (corn oil), 75,
150, or 300 mg cypermethrin (93.5%)/kg body weight in corn oil,
5 times a week, for 6 weeks. At the end of the 6th week,
electroencephalographic records were taken. The waves of the
complex EEG activity as well as the performance of the 6
constituting bands of different frequency were computer analysed.
No significant alterations were found (Desi et al., 1986a).
8.4.2.4. Appraisal
Repeated oral administration of cypermethrin to rats, at doses
sufficiently high to produce significant mortality in a group of
animals, produced biochemical changes in peripheral nerves that
were consistent with sparse axonal degeneration. The magnitude of
the biochemical changes was similar to that of changes produced
with either a single (Dewar, 1977b) or repeated doses of shorter
duration (Dewar & Moffet, 1978a), thus demonstrating a lack of
cumulative effect. The magnitude of change was substantially less
than that encountered with established neurotoxic agents and the
site of maximal enzyme change (distal portion of the nerve)
suggests that the degeneration is more likely to be of a Wallerian
type rather than segmental demyelination. The short time required
to produce ataxia and/or abnormal gait rules out a causal
relationship between ataxia and axonopathy. The clinical signs are
consistent with a pharmacologically-mediated effect whereas the
axonopathy is a minor reversible lesion that occurs several days
after exposure and has also been detected in animals that do not
show clinical signs.
Histopathological lesions of some fibres of the sciatic nerve
have only been demonstrated in rats or hamsters receiving extremely
high, lethal or near lethal, oral doses of cypermethrin (Carter &
Butterworth, 1976; Butterworth & Clark, 1977). Compound related
histopathological changes have not been observed in rats exposed to
cypermethrin through inhalation (Blair et al., 1976) or in rats fed
diets containing 1000 mg cypermethrin/kg over a 1 - 2 year period
(Trigg et al., 1977; McAusland et al., 1978). Furthermore, dogs
fed 1500 or 600 mg cypermethrin/kg diet for 3 or 24 months,
respectively, did not have any compound-related lesions of the
sciatic nerve (Buckwell & Butterworth, 1977; Buckwell, 1981).
The results of biochemical studies, used as sensitive
indicators of change, confirmed that minor changes that occur after
massive exposure to cypermethrin are reversible and are not
necessarily associated with clinical signs (Dewar, 1977b; Dewar &
Moffet, 1978a; Rose, 1983). There was no direct correlation
between the time course of the neuromuscular dysfunction and the
neurobiochemical changes (Rose & Dewar, 1983). The metabolite of
cypermethrin, PBA, did not produce any biochemical changes in
peripheral nerves (Rose & Dewar, 1979b).
8.4.3. Immunosuppressive action
Stelzer & Gordon (1984) studied the in vitro effects of
pyrethroids on the mitogenic responsiveness of murine splenic
lymphocytes to concornavalin A and lipopolysaccharide. The results
of these studies showed that at concentrations of the order of 1 -
10 µmol/litre, inhibition of murine splenic lymphocytes to both B
and T cell mitogens was found. These results support the
possibility of immune suppression by pyrethroid (cypermethrin)
exposure.
Desi et al., (1985, 1986a) studied the short-term effects of
6.25, 12.5 or 25 mg cypermethrin (93.5%)/kg body weight in rats, on
the immune system by measuring the autologous rosette formation of
T-lymphocytes and determining the ovalbumin titre. These studies
were performed over a period of 6 or 12 weeks.
The humoral immune response after vaccination with Salmonella
typhimurium and the cell-mediated immune response were determined
in rabbits. The antibody titre was measured by tube-agglutination
and complement-binding tests and the tuberculin skin test was used
for the immune response test. The dose levels in this study were
75, 150, or 300 mg/kg body weight, for 7 weeks. A significant
dose-dependent decrease was observed in both the anti-ovalbumin
titre of the blood-serum and in the autologous rosette formation of
the T-lymphocytes. In rabbits, the 2 highest dose levels induced a
significant dose-dependent decrease in serum antibody titres and
the tuberculin skin test showed the same dose-dependent tendency.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
Cypermethrin was fed to Wistar rats (30 male and 30 female per
group) at dietary concentrations of 0, 10, 100, or 500 mg/kg for 5
weeks, after which the males and females (10 weeks of age) from
each treatment group were mated. Two successive litters were
produced from each pair. The first of these litters was discarded,
and randomly-selected male and female pups of the second litters
were mated to produce the next generation. The study was continued
until 2 litters from each of 3 successive generations had been
bred. The parent animals in the 500 mg/kg group consumed less food
than the controls and this was accompanied by a reduction in body
weight. Otherwise, the parent animals of control and treatment
groups behaved similarly. Cypermethrin did not cause any adverse
effects on the reproductive performance of the rats or on the
survival of the offspring. No consistent changes were observed in
mean litter weight between birth and weaning in any treatment
group, with the exception of a reduction in total litter weights in
the 500 mg/kg F1a litters on days 4, 14, and 21. There was also a
statistically-significant decrease compared with controls in total
litter weights and size in the F1b litters at 500 mg
cypermethrin/kg diet. In the 500 mg/kg Fo group, one animal showed
a squamous cell carcinoma of the skin. However, this was
considered not to be related to the compound, because no increase
in tumour incidence was found in the long-term studies on rats and
mice. No changes were observed in rats administered 100 mg/kg diet
(Hend et al., 1978).
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1. Rat
Groups of pregnant female Sprague Dawley CD rats (25 animals
per group) were administered cypermethrin orally as a 1% solution
in corn oil at doses of 0, 17.5, 35, or 70 mg/kg body weight per
day, from days 6 to 15 (inclusive) of gestation. Cypermethrin at
17.5 mg/kg body weight per day did not affect maternal performance
or fetal survival and development. At the higher doses of 35 and
70 mg/kg body weight, respectively, slight and significant
retardation of maternal body weight gain was recorded. In
addition, at 70 mg/kg per day, slight to severe neurological
disturbances were observed in nearly half of the females including:
slight splaying of the hind legs while walking ranging to severe
splaying of all limbs, involuntary movements of the jaws,
convulsive spasms, and hypersensitivity to noise. Despite this
maternal toxicity, there were no indications of any embryotoxic or
teratogenic effects of cypermethrin (Tesh et al., 1978).
8.5.2.2. Rabbit
Groups of pregnant female Banded Dutch rabbits (30 controls and
20 for each dose group) were dosed orally with 0, 3, 10, or 30 mg
cypermethrin/kg body weight per day in corn oil (by gelatin
capsule) during days 6 - 18 (inclusive) of gestation. No influence
was found on growth, pre-implantation losses, resorptions, fetal
deaths, or numbers and sizes of fetuses. The incidence of fetal
visceral and/or skeletal abnormalities was comparable to that in
the vehicle control group, except for a slight increase in the mean
percentages of fetuses showing visceral and/or skeletal
abnormalities in the group receiving 30 mg/kg body weight per day.
No teratogenic effects were found in this study (Dix, 1978).
8.6. Mutagenicity and Related End-Points
8.6.1. In vitro studies
8.6.1.1. Microorganisms
Using bacterial assays, no increases in the reversion rates of
Escherichia coli WP2, E. coli WP2 uvrA, Salmonella typhimurium
TA 1535, TA 1537, TA 1538, TA 98, and TA 100 were detected with
cypermethrin (concentrations of up to 2 mg per plate) in the
presence or absence of a rat liver microsomal activation system.
Exposing Saccharomyces cerevisiae JD1 in liquid culture to
cypermethrin at concentrations of up to 5 mg/ml, both in the
presence and absence of a rat liver microsomal activation system,
did not result in any increase in the rate of mitotic gene
conversion (Brooks, 1980).
In a study in which cypermethrin was tested for mutagenicity in
Salmonella typhimurium TA 100 or TA 98, in the presence or absence
of a rat liver activation system, using the plate incorporation
assay, concentrations of up to 1 mg/plate gave negative results.
The same was true with the fluctuation test, with concentrations of
up to 10 µg/ml (Pluymen et al., 1984).
8.6.1.2. Mammalian cells
Cypermethrin was tested for mutagenicity in V79 Chinese hamster
cells. No cytotoxicity was observed when the assay was carried out
in the presence of rat hepatocytes. Cypermethrin was found not to
be mutagenic for either genetic locus (OUA' and TG') in V79 cells,
when tested in concentrations up to 20 µg/ml, in the presence or
absence of rat hepatocytes (Pluymen et al., 1984).
8.6.2. In vivo studies
8.6.2.1. Host-mediated assay
There was no increase in the rate of mitotic gene
conversion in Saccharomyces cerevisiae JD1 harvested from male
mice following a single oral dose of cypermethrin at 25 or 50 mg/kg
body weight for 2 days (Brooks, 1980).
8.6.2.2. Dominant lethal assay
No evidence of dominant lethality was found when male CD1 mice
(3 groups of 12 animals and a control group with 36 animals) were
given single oral doses of 6.25, 12.5, or 25 mg cypermethrin/kg
body weight. Two other groups were given 5 consecutive daily oral
doses of 2.5 or 5 mg/kg body weight (controls received the vehicle
DMSO). A significant reduction in fetal implants during the second
week of mating was noted and a marginal increase in early fetal
deaths was observed at 5 mg/kg per day. This effect was not
confirmed in a second study using oral doses of 2.5, 5, 7.5, or
10 mg/kg body weight for 5 consecutive days, when no effects were
found on reproductive capability or the histopathology of the
testes and epididymis. The marginal differences found in the
5 mg/kg per day group in the second study were considered not to
be related to the compound.
In summary, cypermethrin did not show detectable dominant
lethality after administration of doses of up to 10 mg/kg body
weight per day, for 5 days, or as a single oral dose of 25 mg/kg
body weight (Dean et al., 1977).
8.6.2.3. Bone marrow chromosome study
Chinese hamsters (12 of each sex per group) were orally dosed
with 20 or 40 mg cypermethrin/kg body weight, in DMSO, for 2
successive days. The incidence of chromosome abnormalities in
bone marrow cells, 8 and 24 h after dosing, did not differ from
that in the DMSO control animals. The positive control group
(100 mg/kg cyclophosphamide) showed many chromosomal aberrations
(Dean, 1977).
The effects of cypermethrin on sister chromatid exchange were
studied in the bone marrow cells of 3-month-old mice. Cypermethrin
was injected subcutaneously at 0.75, 1.5, or 3 mg/kg body weight
or 2.5, 5.0, or 10 mg Ripcord/kg body weight. Both the technical
and the formulated products showed a dose-related increase in
sister chromatid exchanges in the dividing cells at all dose
levels. The highest doses of both cypermethrin and Ripcord
completely inhibited mitotic division (Seehy et al., 1983).
8.6.2.4. Micronucleus test
The induction of micronuclei was studied in mouse bone marrow.
Cypermethrin was administered by 3 routes: intraperitoneal, oral,
and dermal. Cypermethrin showed mutagenic potential after oral
administration of 900 mg/kg diet for 7 and 14 consecutive days. No
effects were seen after ip administration of a single injection of
60 or 180 mg/kg body weight or double and triple injections of 60
mg/kg body weight.
Up to 4 dermal treatments with 360 mg cypermethrin/kg body
weight resulted in a significant increase in the frequency of
polychromatic erythrocytes with micronuclei (Amer & Aboul-Ela,
1985).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) (section 8.3) were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg for up to 2 years in a combined
long-term/carcinogenicity study. No evidence for carcinogenicity
was found in this study (McAusland et al., 1978) (section 8.3.1).
No increase in tumour incidence was seen when Wistar rats were
given diets containing 0, 20, 150, or 1500 mg cypermethrin/kg diet
(equivalent to 0, 1, 7.5, or 75 mg/kg body weight) for 2 years.
The purity of the cypermethrin ( cis-: trans- ratio; 55:45) was
between 88% and 93% (section 8.3.1) (US EPA, 1984).
8.7.1.2. Mouse
Swiss mice (70 male and 70 female) were fed diets containing 0
(2 groups), 100, 400, or 1600 mg cypermethrin/kg feed for up to 101
weeks. Effects consisted of increased liver weights at 400 and
1600 mg/kg diet and decreased body weight, thrombocytosis, and mild
anaemia at 1600 mg/kg diet (section 8.3.2).
There were no compound-related changes in non-neoplastic
histopathology or increases in tumours of types that are not
commonly associated with the mouse strain used. The incidence of
tumours was similar in all groups with the exception of a slightly
increased incidence of benign alveolar lung tumours in the females
in the 1600 mg/kg group. However, the magnitude of this increased
incidence was insufficient when compared with concurrent and
historical control incidence, to warrant concern. There was no
evidence for a decreased latency of benign alveolar lung tumours in
the female mice receiving the highest dose, and this tumour type
was not accompanied by any increase in malignancy. There was also
no evidence of a carcinogenic response in the male mice in this
study. Furthermore, benign alveolar lung tumours are know to occur
in both sexes at a high and variable incidence in this strain of
mouse. Therefore, it is considered that the occurrence of benign
alveolar lung tumours in the female mice receiving the highest dose
was not related to treatment with cypermethrin. Feeding
cypermethrin at levels of up to 1600 mg/kg diet to mice for a life-
time did not produce any evidence of carcinogenicity (Lindsay et
al., 1982).
8.8. Mechanisms of Toxicity - Mode of Action
In the classification in the Appendix, cypermethrin is
classified among the Type II compounds, which cause toxic signs of
choreoathetosis with salivation (CS-syndrome) in the rat
(Verschoyle & Aldridge, 1980) and bursts of spikes in the cercal
motor nerve of the cockroach (Gammon et al., 1981). Pyrethroids
having alpha-cyano-3-phenoxy-benzyl alcohol, such as cypermethrin,
do not induce repetitive firing in the cercal sensory nerves of the
cockroach in vivo or in vitro, but cause different signs including
a pronounced convulsive phase (Gammon et al., 1981).
The intravenous toxicity of 1RS- cis-, and 1RS- trans-
cypermethrin for the rat has been examined. Both compounds cause
the CS-syndrome, which is characterized by initial pawing and
burrowing behaviour, followed within 2 - 5 min by profuse
salivation, coarse whole-body tremor, increased startle response
and abnormal locomotion involving the hind limbs. The coarse
tremor progresses into sinuous writhing (choreoathetosis) of the
whole body, which gradually becomes more violent and is enhanced by
sensory stimuli. Clonic seizures are occasionally observed as a
terminal event. No increase in core temperature occurs and, in
fact, it may fall. In the electroencephalogram (EEG) during the
CS-syndrome, poisoned rats showed generalized spike and wave
discharges, prior to choreoathetosis (Verschoyle & Aldridge, 1980).
The primary site of action resulting in the CS-syndrome has not yet
been decided.
It has been suggested (Van den Bercken, personal communication,
1981) that the facial skin sensations experienced by people who
handle cypermethrin or other pyrethroids (section 9.2.2) are
brought about by repetitive firing of sensory nerve terminals in
the skin. In the opinion of the author, this is a strictly local
effect that may occur as soon as the pyrethroid concentration on or
in the skin reaches a certain level and is not a sign of general
intoxication. It is considered by the author that the occurrence
of these skin sensations are a warning signal indicating that
exposure has occurred. No undue hazard is likely, provided the
pyrethroid does not reach the blood in any significant
concentration. In this connection, it was stressed that, although
cypermethrin is among the least toxic insecticides when
administered orally, it may become highly toxic, whenever it
reaches the nervous system in sufficient concentrations.
The question remains whether there is a causal relation between
the intense repetitive activity induced by alpha-cyano pyrethroids
(of which cypermethrin is an example) and the nerve damage that
occurs in experimental animals after prolonged exposure to nearly
lethal concentrations of these compounds. Because of the large
number of different chemicals, most of which do not cause
repetitive activity in the nervous system but are known to cause
serious nerve damage, such a correlation is hardly expected.
Furthermore, the extensive literature on DDT (which causes the same
type of repetitive activity in sense organs and in sensory nerve
terminals as the non-cyano pyrethroids) does not contain any
indication that this insecticide may cause axonal degeneration.
However, in theory, there is a possibility that repeated occurrence
of intense repetitive firing will eventually lead to dysfunction of
sensory nerve terminals and sense organs, and, finally, to
degeneration of sensory nerve fibres (Van den Bercken, personal
communication, 1981; Van den Bercken & Vijverberg, 1980a).
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity: poisoning incidents
One report of accidental intoxication has been described. A
family who ate food cooked in 10% cypermethrin developed nausea,
prolonged vomiting with colicky pain, tenesmus, and diarrhoea
within minutes of ingestion. One male adult had convulsions,
passed into coma, and died due to respiratory paralysis. The
symptoms were less severe in other members of the family (Poulos et
al., 1982). There is some doubt whether this was a cypermethrin
intoxication (Suthers, personal communication).
9.1.2. Controlled human studies
Flannigan & Tucker (1985) studied the differences in the extent
of paraesthesia induced by a number of pyrethroids. Field strength
formulated cypermethrin (0.13 mg/cm2) was applied (0.05 ml) to a
4 cm2 area of the earlobe of volunteers on 5 occasions. Distilled
water was applied to the opposite earlobe. Participant evaluation
after each application continued for 48 h and involved description
of the cutaneous sensations. Each participant was treated after
each application with one of the other pyrethroids. Cypermethrin
(as the other pyrethroids) induced sensation. The paraesthesia
developed with a latency period of approximately 30 min, peaked by
8 h and deteriorated as early as 24 h. Dl-alpha tocopheryl acetate
markedly inhibited the occurrence of the paraesthesia.
9.1.3. Epidemiological studies
No information is available.
9.2. Occupational Exposure
9.2.1. Acute toxicity: poisoning incidents
No information is available.
9.2.2. Effects of short- and long-term exposure
Laboratory workers and field operators handling natural and
synthetic pyrethroids, including cypermethrin, have noticed a
transient sensation (described as "tingling" or "burning"
sensations) of the skin in the periorbital area of the face and of
other sites after direct skin exposure. The skin sensations have
been interpreted (section 8.8) as being caused by spontaneous
repetitive firing of local sensory nerve fibres or nerve endings,
with thresholds that have been transiently lowered by the compound
(Wouters & Van den Bercken, 1978). There is a delay of about
30 min before onset of these symptoms following pyrethroid
exposure; the sensation generally lasts only a few hours and does
not persist for more than one day after exposure (Van den Bercken &
Vijverberg, 1980a, Le Quesne et al., 1980).
In a clinical and electrophysiological assessment of these skin
sensations (paraesthesia), 23 laboratory workers, who had been
exposed to synthetic pyrethroids during the preceding months, were
examined. Nineteen of the 23 workers had experienced at least one
or more episodes of facial sensations. Neurological signs were not
observed and electrophysiological studies of selected motor and
sensory nerves in the legs and arms of the 23 subjects showed no
abnormalities (Le Quesne et al., 1980).
Field studies have been performed in the Côte d'Ivoire in which
cypermethrin was sprayed on cotton using a hand-held, ultra-low-
volume (ULV) commercial spraying machine. This method of
application represented a situation of high potential exposure.
The first study, in which 17 workers were involved, concerned a
single exposure to cypermethrin (Prinsen & Van Sittert, 1978),
while a second study was conducted in which 7 workers took part in
7 consecutive spray sessions (Prinsen & Van Sittert, 1979). Skin
exposure to cypermethrin was assessed by quantitative measurement
of the compound deposited on aluminium foil pads on the body of the
sprayer during the spraying operation. The rate of dermal exposure
of the operators during spraying ranged from 1.5 to 46.1 mg/h.
Total absorption of cypermethrin in the body was monitored by
urinary analysis for 2,2-dichlorovinyl-3,3-dimethyl-cyclopropane-
1-carboxylic acid, a metabolite of cypermethrin. In most of the
samples, the excretion was below the limit of detection
(0.05 mg/litre), indicating a low level of absorption. It was
estimated that approximately 3% of the total dermal dose was
absorbed. General medical, extensive clinical blood chemistry, and
electrophysiological examinations were carried out of selected
motor and sensory peripheral nerves in the legs and arms, of the
trigeminal nerve, and of the facial nerve. No effects were found.
In another field study in India, 18 workers, including spraymen
(spraying an emulsifiable concentrate formulation by mist-blower or
knap-sack), mixers, and loaders, handled cypermethrin daily for 5
consecutive days. Medical examination, with special attention to
the sensory function of the peripheral nervous system, was carried
out before, during, and after spraying (Suthers & Marlow, 1981).
No compound-related adverse clinical effects of peripheral
neuropathy were noticed in any of the workers. The urinary
excretion of the cypermethrin metabolite (methyl ester of the
cyclopropane carboxylic acid moiety) increased from day 1 to 5 of
the study, but decreased 24 h after spraying had ceased. On the
fifth day, concentrations of up to 0.18 mg (average 0.1 mg) were
found in the 24-h urine of workers using the mist-blower.
Concentrations were lower in workers using the knap-sack sprayer.
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
A field study was carried out on Indian workers in the Satara
district in India prior to, during, and after spraying cotton with
the synthetic pyrethroid formulations, permethrin (Ambush) and
cypermethrin (Cymbush). The formulations were applied using a
mist-blower or knap-sack spray. Exposure of 7 spraymen and 2
loaders/mixers was monitored by measuring the 24-h urinary
excretion of 3-(4'-hydroxyphenoxy)-benzoic acid, and medical
assessments were carried out by 4 medical doctors. The sensory
function of the peripheral nervous system was also assessed. The
formulations were applied at the recommended rates; Ambush, 150 g
a.i./ha and Cymbush, 70 g a.i./ha. Average exposure was
approximately 8 h per day for 5 days. No compound-related adverse
clinical effects were noticed. The average urinary excretion of
3-(4'-hydroxy-phenoxy)-benzoic acid increased from day 1 to day 5,
but then decreased 24 h after spraying had ceased (Hart et al.,
1982).
In another study, two agricultural pilots and five
mixer/loaders were monitored for dermal exposure during aerial
"ultra-low-volume" (ULV) applications of cypermethrin in vegetable
oil to cotton. This study was conducted in Greenwood, Mississippi.
The mixer/loaders wore protective equipment. The actual dermal
exposure for pilots was 0.67 mg/8 h and for mixer/loaders 2.43
mg/8h. The exposure of pilots was predominantly of the hands,
whereas that of mixer/loaders was more uniform (hands were
protected). The urinary excretion of cypermethrin metabolites was
very low; between 4 and 22 µg cypermethrin equivalents/day. This
study demonstrates that exposure of pilots and mixer/loaders during
the aerial ULV applications of cypermethrin is minimal and that
skin absorption is very slight (Chester et al., 1986).
Desi et al. (1986b) carried out a biological monitoring and
health surveillance study on 11 workers spraying organophosphate
carbamate and pyrethroid pesticides in greenhouses during the whole
year in comparison with 10 control persons. During the work,
protective clothing and masks were worn before and after a regular
spraying period with pyrethroids (including cypermethrin).
Extensive medical examinations, such as urinalysis, haematology,
immunoglobulin levels, whole blood cholinesterase activity, serum-
gamma-glutamyltransferase activity, chromosome analysis and
electro-cardiography were performed over a period of 3 months. The
amount of cypermethrin in the blood was just at the limit of
detection. No health injuries or other significant changes in the
parameters studied were found.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereo-isomers, is a highly active insecticide effective
against a wide range of pests in many food and non-food
commodities.
It is stable to light and heat, it has a low vapour pressure
and is more stable in acidic than in alkaline media. Sensitive
analytical methods for the determination of residues in food and
the environment are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly
rapid and residues do not accumulate in the environment.
Photodegradation is unlikely to play an important role. The main
route of degradation is cleavage of the ester linkage to give 2
main degradation products containing the cyclopropane, and the
phenoxybenzyl moiety. The half-lives in the soil are determined by
many factors, but are in the range of 2 - 4 weeks. Cypermethrin is
strongly adsorbed by soil and downward leaching is negligible.
Because of its rather fast breakdown forming less toxic products,
and the low dose rates used in good agricultural practice, it is
unlikely that cypermethrin will attain significant levels in the
environment.
Bioaccummulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the
levels of cypermethrin residues in food commodities are generally
low. Total diet studies are not available, but from the available
residue information, it can be inferred that the oral intake by man
is well below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are
generally rather resistant to cypermethrin, while fish and other
aquatic invertebrates are very sensitive. Because of its strong
adsorption on soil, only low levels of cypermethrin may leak into
surface water. These may have transient effects, mainly on surface
breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to
be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect for bees.
Absorption and elimination of cypermethrin has been rapid in
the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation
and conjugation of the cyclopropane- and phenoxybenzyl moiety. The
highest levels are found in body fat, which is consistent with the
lipophilic nature of cypermethrin. The half-life in the fat of the
rat is about 12 - 19 days for the cis-isomer and 3 - 4 days for the
trans-isomer. Breakdown products in plants are bound as
glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg
body weight. Short-term and long-term toxicity studies on rats,
mice, and dogs have shown effects on growth, on the liver and
kidneys, and the nervous system, and on haematology. A no-
observed-adverse-effect level of 7.5 mg/kg body weight has been
adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at
70 mg/kg body weight or rabbits at 30 mg/kg body weight. It was
also shown not to have any effects on reproductive performance
during a 3-generation reproduction study in rats administered
100 mg/kg diet. In a variety of mutagenicity studies, cypermethrin
was shown to be mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational
studies available, it seems that the skin sensation seen in workers
handling cypermethrin, generally lasts only a few hours and does
not persist for more than one day after exposure. Other
neurological signs were not observed. These skin sensations can be
considered to be an early warning that exposure has occurred and
that work practice should be reviewed. Cypermethrin may cause eye
irritation and may be a sensitizer for certain persons.
10.2. Conclusions
It can be concluded that:
General population: When applied according to good
agricultural practice, exposure of the general population to
cypermethrin is negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices,
hygiene measures, and safety precautions, the use of cypermethrin
is unlikely to present a hazard to those occupationally exposed to
it. The occurrence of "facial sensations" is an indication of
exposure. Under these circumstances work practice should be
reviewed.
Environment: With recommended application rates it is
unlikely that cypermethrin or its degradation products will attain
levels of environmental significance. Notwithstanding its high
toxicity for fish and honey bees, this is only likely to cause a
problem in the case of spillage and overspraying.
11. RECOMMENDATIONS
- Cypermethrin should be included among the residues looked for
in surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human
beings should be conducted, in order to better understand this
phenomenon.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cypermethrin was discussed at meetings of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) during the period 1979-84
(FAO/WHO, 1980a,b; 1982a,b; 1983a,b; 1984; 1985a,b,c; Vettorazzi &
Van den Hurk, 1984). In 1981, the JMPR established an Acceptable
Daily Intake (ADI) for cypermethrin of 0 - 0.05 mg/kg body weight.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, classified cypermethrin as "irritant to
eyes and sensitizer of skin" in the list of "technical products
unlikely to present an acute hazard in normal use" (Plestina, 1984;
WHO, 1979; WHO, 1985). The same division published a data sheet on
cypermethrin, No. 84.58 (WHO/FAO, 1975-85).
REFERENCES
ABBASSY, M.A., ASHRY, M., ADAM, F., KHALIL, F., & ABOU-SHLOU,
M.A. (1984) Toxicological and histopathological studies on
the cotton bollworm. ( Pectinophera gossypiella Saund). Meded.
Fac. Landbouwwet. Rijksuniv. Gent, 49(3a): 691-698.
ABDEL-AAL, Y.A.I., EL-SAYED, A.M.K., NEGM, A.A., HUSSEIN,
M.H., & EL-SEBAE, A.H. (1979) The relative toxicity of
certain insecticides to Spodoptera littoralis (Boisd) and
Coccinella undecimpunctata L. Int. Pest Control, 21(4): 79-80,
82.
ABOU-AWAD, B.A. & EL-BANHAWY, E.M. (1985) Comparison between
the toxicity of synthetic pyrethroids and other compounds to
the predacious mite Amblyseius gossipi (Mesostigmata:
Phytoseiidae). Exp. appl. Acarol., 1: 185-191.
AHMED, Y.M., MOSTAFA, A.M.A., & ELEWA, M.A. (1985) Toxicity
of certain dyes as insecticides and their joint action with
some pyrethroids. J. environ. Sci. Health, B20(6): 689-699.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four
synthetic pyrethroids on a predatory mite, Typhlodromus pyri,
and its prey, Panonychus ulmi, on apples in Southeast England.
Environ. Entomol., 9: 436-439.
AMER, S.M. & ABOUL-ELA, E.I. (1985) Cytogenetic effects of
pesticides. III. Induction of micronuclei in mouse bone marrow
by the insecticides cypermethrin and rotenone. Mutat. Res.,
155(3): 135-142.
ASCHER, K.R.S., ELIYAHU, M., NEMNY, N.E., & ISHAAYA, I.
(1982) The toxicity of some novel pesticides - synthetic
pyrethroids and benzoylphenylurea chitin synthesis inhibitors
- for eggs of Spodoptera littoralis (Boisd). Z. angew.
Entomol., 94: 504-509.
AWASTHI, M.D. & ANAND, L. (1983) Dissipation and persistence
of synthetic pyrethroids on fruits of okra. J. entomol. Res.,
7(1): 55-59.
BADMIN, J.S. & TWYDELL, R.S. (1976) Evaluation of the
insecticide WL 43467 against the honey bee Apis mellifera,
Sittingbourne, Shell Research (WKSR.0021.76).
BAICU, T. (1982) Toxicity of some pesticides to Trichoderma
viride pers., Crop Prot., 1(3): 349-358.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues
of synthetic pyrethroids in fruit and vegetables by gas-liquid
and high-performance liquid chromatography. Analyst, 107:
206-212.
BALDWIN, M.K. (1977a) Residues of the pyrethroid insecticide
WL43467 in tissues of cattle following a dip-bath application,
Sittingbourne, Shell Research (TLGR.0.115.77).
BALDWIN, M.K. & LAD, D.D. (1978a) Residue data for milk
following application of WL 43467 in a barn, Sittingbourne,
Shell Research (TLGR.0042.78).
BALDWIN, M.K. & LAD, D.D. (1978b) The accumulation and
elimination of WL 43467 by the Rainbow trout (Salmo
gairdneri), Sittingbourne, Shell Research (TLGR.0041.78).
BALDWIN, M.K., BUCKWELL, A.C., & LAD, D.D. (1977) Residue
data following the application of WL 43467 for nuisance fly
control on cattle, Sittingbourne, Shell Research (TGLR.0.112.
77).
BARIOLA, L.A. & LINGREN, P.D. (1984) Comparative toxicities
of selected insecticides against pink bollworm (Lepidoptera:
Gelechiidae) moths. J. econ. Entomol., 77: 207-210.
BARLOW, F., HADAWAY, A.B., FLOWER, L.S., & TURNER, C.R.
(1986) Residual contact toxicity of some insecticides to tse
tse flies in laboratory tests, London, Centre for Overseas
Pest Research, pp. 12.
BATTELLE (1982) Pesticide programme of research and market
planning. I. Insecticides, Geneva, Institute Battelle (October
1982).
BAYOUMI, O.C. (1982) The susceptibility of Myzus persicae
Sulz. to some insecticides in artificial diets. Parasitica,
38(4): 193-199.
BENNETT, D. (1981a) The accumulation, distribution, and
elimination of RIPCORD by Rainbow trout using a continuous-
flow procedure, Sittingbourne, Shell Research (SBGR.81.026 and
Addendum).
BENNETT, D. (1981b) Residues of RIPCORD in Rainbow trout
(Salmo gairdneri) and common carp (Cyprinus carpio) exposed to
lethal concentrations in water, Sittingbourne, Shell Research
(SBGR.81.075).
BENNETT, D., CROSSLAND, N.O., & SHIRES, S.W. (1980) Spray
drift from RIPCORD applications to vineyards in France: fate
and effects in adjacent streams, Sittingbourne, Shell Research
(TLGR.80.095).
BLAIR, D. & RODERICK, H.R. (1976) Toxicity studies on the
pyrethroid insecticide WL 43467, emulsifiable concentrate FX
3315. Acute inhalation exposure of rats to an aqueous spray,
Sittingbourne, Shell Research (TLGR.0001.76).
BLAIR, D., BUTTERWORTH, S.T.G., & RODERICK, H.R. (1976)
Toxicity studies on the pyrethroid insecticide WL 43467,
emulsifiable concentrate FX 3315. Histopathology of rats
exposed to an aqueous spray, Sittingbourne, Shell Research
(TLGR.0102.76).
BLAND, P.D. (1985) Capillary gas chromatographic
determination of cypermethrin in formulations: collaborative
study. J. Assoc. Off. Anal. Chem., 68(3): 592-595.
BOSIO, P.G. (1979) Residues of Barricade (cypermethrin) in
cattle from Australia, Berre, France, Shell Chimie (BEGR.79.
117) (Unpublished Shell data).
BOSTANIAN, N.J. & BELANGER, A. (1985) The toxicity of three
pyrethroids to Amblyseius fallacis (Garman) Acari.
Phytoseiidae and their residues on apple foliage. Agric.
Ecosyst. Environ., 14(3/4): 243-250.
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC
multi-residue method to determination of synthetic pyrethroid
residues in celery and animal products. J. Assoc. Off. Anal.
Chem., 65(3): 685-689.
BRAUN, H.E., FRANK, R., & MILLER, L.A. (1985) Residues of
cypermethrin in milk from cows wearing impregnated ear tags.
Bull. environ. Contam. Toxicol., 35: 61-64.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1983)
Injection toxicity of some pyrethroids in the armyworm.
J. Pestic. Sci., 8: 95-98.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984a)
Topical and injection toxicities of some pyrethroids in the
tobacco cutworm, Spodoptera litteralis Fabricius. J. Pestic.
Sci., 9: 481-487.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984b)
Topical and injection toxicities of some pyrethroids to the
german cockroach, Blattella germanica (Dictyoptera:
Blattellidae). Appl. Entomol. Zool., 19(3): 348-355.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984c) The
selective toxicity of some synthetic pyrethroids in the
armyworm. Pseudaletia separata (Walker). III Cuticle
permeabilities of some pyrethroids. Appl. Entomol. Zool.,
19(1): 87-94.
BROMLEY, S. & COOK, K.A. (1979) Determination of the effects
of WL 43467 on microbial activity in soil. V. Effects on
glucose utilization, Sittingbourne, Shell Research
(BLGR.79.099).
BROOKS, T.M. (1980) Toxicity studies with agricultural
chemicals: mutagenicity studies with RIPCORD in microorganisms
in vitro and in the host-mediated assay, Sittingbourne, Shell
Research (TLGR.80.059).
BROWN, V.K. (1979a) Toxicology of WL 43467 isomers: acute
toxicity of WL 43481 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.117).
BROWN, V.K. (1979b) Toxicology of WL 43467 isomers: acute
toxicity of WL 42641 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.116).
BUCKWELL, A.C. (1981) A 2-year feeding study in dogs on WL
43467, Sittingbourne, Shell Research (SBGR.81.126).
BUCKWELL, A.C. & BUTTERWORTH, S.T.G. (1977) Toxicity studies
on the pyrethroid insecticide WL 43467: a 13-week feeding
study in dogs, Sittingbourne, Shell Research (TLGR.0127.77).
BUTTERWORTH, S.T.G. & CLARK, D.G. (1977) Toxicity studies on
the insecticide WL 43467: acute oral toxicity and neuro-
pathological effects in Syrian hamsters, Sittingbourne, Shell
Research (TLGR.0094.76).
CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN
GELDER, G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin
sensory stimulation characterized by a new behavioral
paradigm. Toxicol. appl. Pharmacol., 76: 270-279.
CAMILLERI, P. (1984) Alkaline hydrolysis of some pyrethroid
insecticides. J. agric. food Chem., 32: 1122-1124.
CARTER, B.I. & BUTTERWORTH, S.T.G. (1976) Toxicity of
insecticides: the acute oral toxicity and neuropathological
effects of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0055.76).
CASIDA, J.E., GAUGHAN, L.C., & RUZO, L.O. (1979) Comparative
metabolism of pyrethroids derived from 3-phenoxybenzyl and
alpha-cyano-3-phenoxybenzyl alcohols. Adv. Pestic. Sci., 2:
182-189.
CASSIDY, S.L. (1979) Acute oral toxicity of the spray
formulation EF 5288 to calves, piglets, and lambs,
Sittingbourne, Shell Research (TLGR.0001.79).
CHANG, C.K. & JORDAN, T.W. (1982) Penetration and metabolism
of topically applied permethrin and cypermethrin in
pyrethroid-tolerant Wiscana cervinata larvae. Pestic. Biochem.
Physiol., 17: 196-204.
CHANG, C.K. & JORDAN, T.W. (1983) Insecticide handling
mechanisms in some New Zealand pasture pests. N.Z. J. Sci.,
26: 509-516.
CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four
pyrethroid insecticides in a mineral and an organic soil.
J. environ. Sci. Health, B16(5): 605-615.
CHAPMAN, R.A. & SIMMONS, H.S. (1977) Gas-liquid chromato-
graphy of picogram quantities of pyrethroid insecticides.
J. Assoc. Off. Anal. Chem., 60: 977-978.
CHAPMAN, R.A., TU, C.M., HARRIS, C.R., & COLE, C. (1981)
Persistence of five pyrethroid insecticides in sterile and
natural, mineral and organic soil. Bull. environ. Contam.
Toxicol., 26: 513-519.
CHENG, H.H. (1984) Residual toxicity of six pyrethroid and
two organophosphorus insecticides on the soil surface against
dark-sided cutworm (Lepidoptera: Noctuidae) on tobacco in
Ontario. Can. Entomol., 116: 11-17.
CHENG, H.H. & HANLON, J.J. (1984) Residual toxicity of six
insecticides and a herbicide applied sequentially or in tank
mix combinations on tobacco seedlings against dark-sided
cutworm (Lepidoptera: Noctuidae). Tob. Int., 186(22): 39-42.
CHESTER, G., HATFIELD, L.D., HART, T.B., LEPPERT, B.C.,
SWAINE, H., & TUMMON, O.J. (1986) Worker exposure to, and
absorption of cypermethrin during aerial application of an
"ultra low volume" formulation to cotton, Fernhurst, Imperial
Chemical Industries, Plant Protection Division (Unpublished
report).
COATS, S.A., COATS, J.R., & ELLIS, C.R. (1979) Selective
toxicity of three synthetic pyrethroids to eight coccinellids,
a eulophid parasitoid and two pest chrysomelids. Environ.
Entomol., 8(4): 720-722.
CODEX ALIMENTARIUS COMMISSION (1986) Guide to Codex
recommendations concerning pesticide residues. Part 2: Maximum
limits for pesticide residues, third preliminary issue, Rome,
Food and Agriculture Organization of the United Nations
(CAC/PR 2-1986).
COLE, L.M., CASIDA, J.E., & RUZO, L.O. (1982) Comparative
degradation of the pyrethroids tralomethrin, tralocythrin,
deltamethrin and cypermethrin on cotton and bean foliage.
J. agric. food Chem., 30: 916-920.
COOK, K.A. (1978a) Determination of the effects of WL 43467
on microbial activity in soil. I. Effects on carbon dioxide
evolution, Sittingbourne, Shell Research (BLGR.0003.78).
COOK, K.A. (1978b) Determination of the effects of WL 43467
on microbial activity in soil. IV. Effects on nitrification,
Sittingbourne, Shell Research (BLGR.0015.78).
COOMBS, A.D., CARTER, B.I., HEND, R.W., BUTTERWORTH, S.G., &
BUCKWELL, A.C. (1976) Toxicity studies on the insecticide WL
43467: Summary of results of preliminary experiments,
Sittingbourne, Shell Research (TLGR.0104.76).
CORBITT, T.S., WRIGHT, D.J., & GREEN, A.St.J. (1985) The
toxicity of abamectin (MK. 936) on cabbage to first and third
larval instars of Spodoptera littoralis (Boisd.). Meded. Fac.
Landbourwwet. Rijksuniv. Gent, 50(2b): 639-642.
COVENEY, P.C. & EADSFORTH, C.V. (1982) The metabolism of
cypermethrin in man (3). Urinary excretion following a single
dermal dose of cypermethrin, Sittingbourne, Shell Research
(SBGR.82.290).
CRAWFORD, M.J. (1976a) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of (14 C-benzyl)WL
43481 (cis-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0046.76).
CRAWFORD, M.J. (1976b) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of 14 C-WL 42641
(trans-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0077.76).
CRAWFORD, M.J. (1977) The metabolism of WL 43467 in mammals.
The fate of a single oral dose of (14 C-cyclopropyl)WL 43467 in
the rat, Sittingbourne, Shell Research (TLGR.0004.77).
CRAWFORD, M.J. (1979a) The metabolism of cypermethrin (WL
43467) in mammals. The fate of a single oral dose of
(14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne, Shell
Research (TLGR.79.029).
CRAWFORD, M.J. (1979b) The metabolism of cypermethrin (WL
43467) in mammals. The fate of single oral doses of cis- and
trans-(14 C-benzyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.0011.79).
CRAWFORD, M.J. (1979c) The metabolism of 14 C-cypermethrin by
rat liver microsomes, Sittingbourne, Shell Research
(TLGR.79.057).
CRAWFORD, M.J. (1979d) The metabolic fate of the cis- and
trans-isomers of WL 43467 (cypermethrin) and of 3-phenoxy-
benzoic acid in the dog, Sittingbourne, Shell Research
(TLGR.79.012).
CRAWFORD, M.J. (1979e) The metabolism of cypermethrin (WL
43467) in mammals. Metabolites derived from a single oral dose
of (14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.79.096).
CRAWFORD, M.J. & HUTSON, D.H. (1977a) The metabolic fate of
the cis- and trans-isomers of WL 43467 (cypermethrin).
Metabolism and elimination of 14 C-aryl-labelled cis- and
trans-isomers in rats, Sittingbourne, Shell Research
(TLGR.0131.77).
CRAWFORD, M.J. & HUTSON, D.H. (1977b) The elimination and
retention of WL 43467 when administered dermally or orally to
sheep, Sittingbourne, Shell Research (TLGR.0098.77).
CRAWFORD, M.J. & HUTSON, D.H. (1978) The elimination of
residues from the fat of mice following the oral
administration of (14 C-benzyl)WL 43481 (cis-WL 43467),
Sittingbourne, Shell Research (TLGR.0080.78).
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981a)
Metabolism of cis- and trans-cypermethrin in rats. Balance and
tissue retention study. J. agric. food Chem., 29: 130-135.
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981b) The
metabolism of the pyrethroid insecticide cypermethrin in rats:
excreted metabolites. Pestic. Sci., 12: 399-411.
CRAYFORD, J.V. (1978) A study of the metabolism of
3-phenoxybenzoic acid and its glucoside conjugate in rats,
Sittingbourne, Shell Research (TLGR.0186.78).
CRAYFORD, J.V. & HUTSON, D.H. (1979) The identification of
metabolites in the tissues of rats treated orally with
3- phenoxybenzoic acid, Sittingbourne, Shell Research (TLGR.0043.
79).
CRAYFORD, J.V. & HUTSON, D.H. (1980) Xenobiotic triglyceride
formation. Xenobiotica, 10(5): 349-354.
CRAYFORD, J.V., HUTSON, D.H., & THORPE, E. (1980) The
elimination of residues from the fat of mice following the
oral administration of 14 C-benzyl WL 43481 (cis-WL 43467),
Tunstall, Shell Toxicology Laboratory (TLGR.0080.78,
additional report).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin.
II. Fate and biological effects in pond experiments. Aquat.
Toxicol., 2: 205-222.
CROSSLAND, N.O. & BENNETT, D. (1976) A field trial to assess
the dispersion and toxicity of an EC formulation of the
insecticide WL 43467 in a pond system, Sittingbourne, Shell
Research (TLGR.0101.76).
CROSSLAND, N.O. & ELGAR, K.E. (1983) Fate and biological
effects of insecticides in ponds. In: Miyamoto, J. et al., ed.
IUPAC. Pesticide chemistry: human welfare and the environment,
Oxford, Pergamon Press, Vol. 3, pp. 551-556.
CROSSLAND, N.O. & STEPHENSON, R.R. (1979) The role of pond
studies in assessing the hazard of toxic chemicals to
freshwater ecosystems. In: Proceedings of the British Crop
Protection Conference, 1979: Pests and Diseases, Croydon,
British Crop Protection Council, Vol. 2, pp. 453-459.
CROSSLAND, N.O., BENNETT, D., KANE, D.F., & STEPHENSON, R.R.
(1978) The dispersion and toxic effects of the insecticide WL
43467 in a pond, Sittingbourne, Shell Research (TLGR.0076.78).
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic
toxicology of cypermethrin. III. Fate and biological effects
of spray drift deposits in freshwater adjacent to agricultural
land. Aquat. Toxicol., 2: 253-270.
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982a) Hepatic
esterases: characterization and quantitation in vitro of rat,
rabbit, and human liver esterases. Part I, Sittingbourne,
Shell Research (SBGR.82.204).
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982b) In vitro
metabolism of the pyrethroid insecticide cypermethrin by liver
esterases. In: Proceedings of the 5th International Congress
on Pesticide Chemicals, Vol. 4.
CROUCHER, A., HUTSON, D.H., & STOYDIN, G. (1985) Excretion
and residues of the pyrethroid insecticide cypermethrin in
lactating cows. Pestic. Sci., 16: 287-301.
DAI, S.M. & SUN, C.N. (1984) Pyrethroid resistance and
synergism in Nilaparvata lugens Stal (Homoptera: Delphacidae)
in Taiwan. J. econ. Entomol., 77: 891-897.
DAY, S.R. & LEAHEY, J.P. (1980) 14 C-cypermethrin: aqueous
photodegradation in sunlight, Fernhurst, Imperial Chemical
Industries (Unpublished ICI Report No. RJ0154B).
DEAN, B.J. (1977) Toxicity studies with SL 43467: chromosome
studies on bone marrow cells of Chinese hamsters after two
daily oral doses of WL 43467, Sittingbourne, Shell Research
(TLGR.0136.77).
DEAN, B.J., PAUW, C.L, VAN DER, & BUTTERWORTH, S.T.B. (1977)
Toxicity studies with WL 43467: dominant lethal assay in male
mice after single oral doses of WL 43467, Sittingbourne, Shell
Research (TLGR.0042.77).
DELABIE, J., BOS, C., FONTA, C., & MASSON, C. (1985) Toxic
and repellent effects of cypermethrin on the honey bee:
laboratory, glasshouse and field experiments. Pestic. Sci.,
16: 409-415.
DESI, I., VARGA, L., DOBRONYI, I., & SZKLENARIK, G. (1985)
Immunotoxicological investigation of the effects of a
pesticide: cypermethrin. Arch. Toxicol., Suppl. 8: 305-309.
DESI, I., DOBRONYI, I., & VARGA, L. (1986a) Immuno-, neuro-
and general toxicologic animal studies on a synthetic
pyrethroid: cypermethrin. Ecotoxicol. environ. Saf., 12:
220-232.
DESI, I., PALOTAS, M., VETRO, G., CSOLLE, I., NEHEZ, M.,
ZIMANYI, M., FERKE, A., HUSZTA, E., & NAGYMAJTENYI, L.
(1986b) Biological monitoring and health surveillance of a
group of greenhouse pesticide sprayers. Toxicol. Lett., 33:
91-105.
DEWAR, A.J. (1977a) The use of lysosomal enzyme measurements
as an indicator of chemically-induced peripheral neuropathy,
Sittingbourne, Shell Research (TLGR.0074.77).
DEWAR, A.J. (1977b) Toxicity studies on the insecticide WL
43467: biochemical and functional studies on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research (TLGR.0082.
77).
DEWAR, A.J. & DEACON, P.A. (1977) Toxicity studies on the
insecticide WL 43467: electrophysiological studies on the
neurotoxicity of WL 43467 to rats. I. The effect on motor
conduction velocity in the sciatic and tail nerves,
Sittingbourne, Shell Research (TLGR.0133.77).
DEWAR, A.J. & MOFFETT, B.J. (1978a) Toxicity studies on the
insecticide WL 43467: biochemical studies on the effect of WL
43467 on the rat trigeminal nerve and ganglion, Sittingbourne,
Shell Research (TLGR.0162.77).
DEWAR, A.J. & MOFFETT, B.J. (1978b) Toxicity studies on the
insecticide WL 43467: biochemical and functional studies on
the neurotoxicity of WL 43467 to Chinese hamsters,
Sittingbourne, Shell Research (TLGR.0038.78).
DEWAR, A.J. & OWEN, D.E. (1979) Toxicology of pyrethroids:
the acute oral toxicity to rats of a sample of ICI
cypermethrin, Sittingbourne, Shell Research (TLGR.79.019).
DIX, K.M. (1978) Toxicity of WL 43467: teratological studies
in rabbits given WL 43467 orally, Sittingbourne, Shell
Research (TLGR.0010.78).
DU TOIT, G.D.G. (1978) Evaluation by topical application of
selected insecticides against adult grass grub. In: Proceeding
of the 31st New Zealand Weed and Pest Control Conference, pp.
160-163.
EADSFORTH, C.V. & BALDWIN, M.K. (1983) Human dose-excretion
studies with the pyrethroid insecticide cypermethrin.
Xenobiotica, 13: 67-72.
EDWARDS, P.J., WILKINSON, W., & COULSON, M. (1985)
Laboratory toxicity test for carabid beetles. Proceedings of
the British Crop Protection Conference, 1984: Pests and
Diseases, Croydon, British Crop Protection Council, Vol. 1,
pp. 359-362.
EDWARDS, R. & MILLBURN, P. (1985a) Toxicity and metabolism
of cypermethrin in fish compared with other vertebrates.
Pestic. Sci., 16: 201-202.
EDWARDS, R. & MILLBURN, P. (1985b) The metabolism and
toxicity of insecticides in fish. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry and toxicology,
New York, John Wiley and Sons, Vol. 5, pp. 249-274.
ELLIOTT, M., FARNHAN, A.W., JONES, N.F., NEEDHAM, P.H., &
PULMAN, D.A. (1974) Synthetic insecticide with a new order
of activity. Nature (Lond.), 248: 710-711.
EL-MINSHAWY, A., MACKLAD, F., RAGAB, F., & DONIA, A. (1983)
Selective toxicity of certain pyrethroids to the cotton
leafworm Spodoptera littoralis (Boisd.) and to one of its
major parasites Microplitis rufiventris (Kok). Proceedings of
the International Conference on Environmental Hazards of
Agrochemicals, Alexandria, Egypt, 8-12 November, 1983, London,
Paris, New York, Harwood Academic Publishers, Vol. 1, pp. 551-
561.
EL-SAYED, G.N. & KNOWLES, C.O. (1984) Formamidine synergism
of pyrethroid toxicity to two-spotted spider mites (Acari,
Tetranychidae). J. econ. Entomol., 77: 23-30.
EL-SEBAE, A.H., EL-BAKARY, A.S., LE PATOUREL, J., KADOUS, E.,
& MACKLAD, M.F. (1983) Effect of photoperiodism on fish
susceptibility to insecticides. In: Zewail, A.H., ed.
Proceedings of the International Conference on Photochemistry
and Photobiology, Alexandria, Egypt, 8-12 November, 1983,
London, Paris, New York, Harwood Academic Publishers, pp. 960-
966.
EL-SEBAE, A.H., ENAN, E.E., DAOUD, A.S., & ZEID, M.I. (1985)
Selective toxicity of synthetic pyrethroids and some
synergists to mice and cotton leafworm in relation to some
biochemical enzyme activities. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 50(3a): 939-950.
ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1982) Dislodgable
insecticide residues on cotton foliage: carbaryl, cyper-
methrin, and methamidophos. Bull. environ. Contam. Toxicol.,
28: 490-493.
EUROPEAN PATENT OFFICE (1980) European patent application
(Publication No. 0015598(A2)).
EVANS, M.H. (1976) End-plate potentials in frog muscle
exposed to a synthetic pyrethroid. Pestic. Biochem. Physiol.,
6: 547-550.
EWEN, A.B., MUKERJI, M.K., & HINKS, C.F. (1984) Effect of
temperature on the toxicity of cypermethrin to nymphs of the
migratory grasshopper. Melanoplus sanguinipes (Orthoptera:
Acridoidea). Can. Entomol., 116(9): 1153-1156.
FABELLAR, L.T. & HEINRICHS, E.A. (1984) Toxicity of
insecticides to predators of rice brown planthoppers,
Nilaparvata lugens (Stal) (Homoptera: Delphacidae) Environ.
Entomol., 13: 832-837.
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20;
Suppl).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 56).
FAO/WHO (1985a) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985c) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Protection and Protection Paper No. 67).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
FRANK, R., BRAUN, H.E., RITCEY, G., MCEWEN, F.L., & SIRONS,
G.J. (1982) Pesticide residues in onions and carrots grown
on organic soils, Ontario, 1975-80. J. econ. Entomol., 75:
560-565.
FURUZAWA, K., MIKAMI, N., YAMADA, H., & MIYAMOTO, J. (1986)
Metabolism of the pyrethroid insecticide cypermethrin in
cabbages. J. Pestic. Sci., 11: 253-260.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes
of pyrethroid action in the cockroach. Pestic. Biochem.
Physiol., 15: 181-191.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most
potent class antagonize GABA action at the crayfish
neuromuscular junction. Neurosci. Letters, 40: 163-168.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982)
Pyrethroid toxicology: Protective effects of Diazepam and
phenobarbital in the mouse and the cockroach. Toxicol. applied
Pharmacol., 66:290-296.
GARFORTH, B.M. (1982) WL 85871 and cypermethrin: chronic
toxicity to Daphnia magna, Sittingbourne, Shell Research
(SBGR.82.119).
GARFORTH, B.M. (1983) The effect of RIPCORD on the non-
target fauna of maize, Sittingbourne, Shell Research (SBGR.83.
045).
GERIG, L. (1979) [The toxicity of synthetic pyrethrines to
foraging bees.] Schwei z. Bienen-Ztg, 101: 228-236 (in German).
GERIG, L. (1981) [The toxicity of synthetic pyrethrines to
foraging bees. Part 2.] Schweiz. Bienen-Ztg, 104: 155-174 (in
German).
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977a) Effects of
high dietary levels on clinical behaviour and structure of
sciatic nerves in the rat, Fernhurst, Imperial Chemical
Industries, Central Toxicology Laboratory, pp. 383
(Unpublished ICI report).
GLAISTER, J.R., GARE, C.W., MARSAT, G.J., PHILLIPS, C., &
PRATT, I. (1977b) 90-day feeding study in rats, Fernhurst,
Imperial Chemical Industries, Central Toxicology Laboratory,
pp. 383 (Unpublished ICI report).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4(6): 793-799.
GRAY, K. & GRAYSON, B.T. (1980) Determination of the
n-octanol/water partition coefficients of BIRLANE AND GARDONA
and a literature search for the values of other group
compounds, Sittingbourne, Shell Research (BLGR.80.103).
GRAYSON, B.T., LANGNER, E., & WELLS, D. (1982) Comparison of
two gas saturation methods for the determination of the vapour
pressure of cypermethrin. Pestic. Sci., 13: 552-556.
HAGLEY, E.A.C., PREE, D.J., & SIMPSON, C.M. (1981) Toxicity
of insecticides to parasites of the spotted tentiform
leafminer (Lepidoptera: Gracillariidae). Can. Entomol., 113:
899-906.
HALL, J.S., LEAHEY, J.P., & CURL, E.A. (1981) Cypermethrin:
photodegradation on a soil surface, Fernhurst, Imperial
Chemical Industries (Unpublished ICI Report No. RJ0192B).
HARGREAVES, J.R. & COOPER, L.P. (1979) Phytotoxicity tests
with pyrethroid insecticides on glasshouse grown tomato
seedlings. Queensland J. Agric. anim. Sci., 36(2): 151-154.
HARRIS, C.R. & TURNBULL, S.A. (1978) Laboratory studies on
the contact toxicity and activity in soil of four pyrethroid
insecticides. Can. Entomol., 110: 285-288.
HARRIS, C.R. & TURNBULL, S.A. (1980) Toxicity of some
insecticides to insecticide-susceptible strains of the onion,
cabbage and seedcorn maggots (Diptera: Anthomyiidae) and the
dark-sided cutworm (Lepidoptera: Nocturidae). Can. Entomol.,
112(10): 1029-1032.
HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory
studies on the persistence and behaviour in soil of 4
pyrethroid insecticides. Can. Entomol., 113: 685-694.
HARRIS, C.R., TURNBULL, S.A., & MCLEOD, D.G.R. (1985)
Contact toxicity of twenty-one insecticides to adults of the
carrot rust fly (Diptera: Psilidae). Can. Entomol., 117:
1025-1027.
HART, T.B., SPINKS, C.A., MARLOW, R.G., & SUTHERS, J.R.
(1982) A study of the exposure and health of Indian workers
spraying, Ambush and Cymbush on cotton using high volume
hand-held spray applicators, Fernhurst, Imperial Chemical
Industries, Plant Protection Division (Report TMF.1841-B).
HELLING, C.S. & TURNER, B.C. (1968) Pesticide mobility:
determination in soil by thin-layer chromatography. Science,
162: 562-563.
HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies on
the insecticide WL 43467: a three-month feeding study in rats,
Sittingbourne, Shell Research (TLGR.0027.76) (Unpublished
report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977a) Toxicity studies on
the insecticide WL 42641: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977b) Toxicity studies on
the insecticide WL 43481: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W., HENDY, R., & FLEMING, D.J. (1978) Toxicity
studies on the insecticide WL 43467: a three-generation
reproduction study in rats, Sittingbourne, Shell Research
(TLGR.0188.78).
HENDERSON, C. & PARKINSON, G.R. (1981) Cypermethrin
technical: subacute dermal toxicity study in rabbits, Alderley
Park, Imperial Chemical Industries (Report No. CTL/P/588).
HINE, C.H. & ZUIDEMA, H.H. (1970) The toxicological
properties of hydrocarbon solvents. Ind. Med., 39(5): 39-44.
HO, S.H., LEE, B.H., & SEE, D. (1983) Toxicity of
deltamethrin and cypermethrin to the larvae of the diamond-
back moth, Plutella xylostella L. Toxicol. Lett., 19: 127-131.
HOLDEN, J.S. (1979) Absorption and metabolism of permethrin
and cypermethrin in the cockroach and the cotton leaf-worm
larvae. Pestic. Sci., 10: 295-307.
HOPKINS, A.R., MOORE, R.F., & JAMES, W. (1984) Contact and
residual toxicities of pyrethroids and organophosphorus
compounds to the Boll weevil (Coleoptera: Curculionidae) J.
Georgia Entomol. Soc., 19(1): 27-34.
HUCKLE, K.R., HUTSON, D.H., & MILLBURN, P. (1981a) Species
differences in the metabolism of 3-phenoxybenzoic acid. Drug
Metab. Disp., 9: 352-359.
HUCKLE, K.R., CHIPMAN, J.K., HUTSON, D.H., & MILLBURN, P.
(1981b) Metabolism of 3-phenoxybenzoic acid and the
enterohepatorenal disposition of its metabolites in the rat.
Drug Metab. Disp., 9(4): 360-368.
HUTSON, D.H. (1978a) The elimination of radioactivity by
mice following oral dosing with 14 C-cis- and 14 C-trans-WL
43467 (cypermethrin), Sittingbourne, Shell Research
(TLGR.0079.78).
HUTSON, D.H. (1978b) The metabolites of cis- and trans-
cypermethrin (WL 43467) in mice, Sittingbourne, Shell Research
(TLGR.102.78).
HUTSON, D.H. (1979a) The metabolic fate of synthetic
pyrethroid insecticide in mammals. Prog. Drug Metab., 3:
215-252.
HUTSON, D.H. (1979b) The metabolism of WL 43467 in mammals.
Metabolites derived from (14 C-cyano)cypermethrin (WL 43467) in
rats, Sittingbourne, Shell Research (TLGR.79.183).
HUTSON, D.H. & CASIDA, J.E. (1978) Taurine conjugation in
metabolism of 3-phenoxybenzoic acid and the pyrethroid
insecticide cypermethrin in mouse. Xenobiotica, 8(9): 565-571.
HUTSON, D.H. & STOYDIN, G. (1987) Excretion and residues of
the pyrethroid insecticide cypermethrin in laying hens.
Pestic. Sci., 18: 157-168.
HUTSON, D.H., GAUGHAN, L.C., & CASIDA, J.E. (1981)
Metabolism of the cis- and trans-isomers of cypermethrin in
mice. Pestic. Sci., 12: 385-398.
INGLESFIELD, C. (1982) The effects of an aerial application
of RIPCORD on the non-target fauna of oil seed rape in France,
Sittingbourne, Shell Research (SBGR.82.364).
INGLESFIELD, C. (1984) Toxicity of the pyrethroid
insecticides cypermethrin and WL 85871 to the earthworm
Eisenia foetida Savigny. Bull. environ. Contam. Toxicol.,
33(5): 568-570.
INGLESFIELD, C. & SHERWOOD, C.M. (1983) Toxicity of
cypermethrin to the earthworm, Eisenia foetida L (Oligochaeta:
Lumbriculidae) in laboratory tests, Sittingbourne, Shell
Research (SBGR.83.070).
ISHAAYA, I., ASCHER, K.R.S., & CASIDA, J.E. (1983)
Pyrethroid synergism by esterase inhibition in Spodoptera
littoralis (Boisd.) larvae, Crop Prot., 2(3): 335-343.
JACKSON, C. (1977) The leaching of WL 43467 through
laboratory soil columns, Sittingbourne, Shell Research
(Unpublished Report BLGR.0150.77).
JOIA, B.S., LOSCHIAVO, S.R., & WEBSTER, G.R.B. (1981) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat. In: Proceedings of the 16th Annual Workshop
on Pesticide Residue Analysis, Western Canada, pp. 14-16.
JOIA, B.S., SARMA, L.P., & WEBSTER, G.R.B. (1985a) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat and milled fractions. J. environ. anal.
Chem., 21: 179-184.
JOIA, B.S., WEBSTER, G.R.B., & LOSCHIAVO, S.R. (1985b)
Cypermethrin and fenvalerate residues in stored wheat and
milled fractions. J. agric. food Chem., 33: 618-622.
JONES, B.J. (1981) Cypermethrin: bioaccumulation study in
the rat, Alderley Park, Imperial Chemical Industries (Report
No. CTL/P/599).
JORDAN, T.W. & CHANG, C.K. (1981) Pyrethroid resistance
mechanisms in Porina caterpillars. In: Proceedings of the 34th
New Zealand Weed and Pest Control Conference, pp. 167-169.
KAUFMAN, D.D., JORDAN, E.G., & KAYSER, A.J. (1978)
Degradation of cypermethrin and permethrin in soil. In:
Proceedings of the 175th Meeting on Pesticides of the American
Chemical Society, Washington, DC, American Chemical Society
(Abstract Paper No. 47).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J.
(1981) Movement of cypermethrin, decamethrin, permethrin, and
their degradation products in soil. J. agric. food Chem., 29:
239-245.
KNAPP, F.W., HERALD, F., & SCHWINGHAMMER, K.A. (1985)
Comparative toxicity of selected insecticides to laboratory-
reared and field-collected face flies (Diptera: Muscidae) J.
econ. Entomol., 78: 860-862.
KNIGHT, R.J. (1982) The toxicity of the pyrethroid WL 85871
against honey bee Apis mellifera, Sittingbourne, Shell
Research (SBGR.82.023).
KNOWLES, C.O. & EL-SAYED, G.N. (1985) Formanilide
enhancement of acaricide toxicity to Tetranychus urticae Koch
(Acari: Tetranychidae), J. econ. Entomol., 78: 308-310.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in
relation to body weight and temperature. Water Res., 15:
503-505.
LAUREN, D.R. & HENZEL, R.F. (1977) Residual lives of two
synthetic prethroid insecticides applied to pasture. In:
Proceedings of the 30th New Zealand Weed and Pest Control
Conference, pp. 207-210.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology:
mouse intracerebral structure-toxicity relationship. Pestic.
Biochem. Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action
of pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Inter-
actions of pyrethroid insecticides with chloride ionophore-
associated binding sites. Neurotoxicol., 6: 87-98.
LEAHEY, J.P. (1979) The metabolism and environmental
degradation of the pyrethroid insecticides. Outlook Agric.,
10(3): 135-142.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London,
Philadelphia, Taylor and Francis.
LEAKE, L.D., LAUCKNER, S.M., & FORD, M.G. (1979)
Relationship between neurophysiological effects of selected
pyrethroids and toxicity to the leech Haemopsis sanguisuga and
the locust Schistocerca gregaria. Neurobiol. Pest. Action.,
London, pp. 423-430.
LE PATOUREL, G.N.J. & SINGH, J. (1984) Toxicity of amorphous
silicas and silica-pyrethroid mixtures to Tribolium castaneum
(Herbst) (Coleoptera: Tenebrionidae). J. stored Prod. Res.,
20(4): 183-190.
LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to
synthetic pyrethroids: a clinical and electrophysiological
assessment. Neurotoxicology, 2: 1-11.
LINDSAY, S., BANHAM, P.B., CHART, I.S., CHALMERS, D.T.,
GODLEY, M.J., & TAYLOR, K. (1982) Cypermethrin: life-time
feeding study in mice, Alderley Park, Imperial Chemical
Industries (Report No. CTL/P/687).
LIU, M.Y., CHEN, J.S., & SUN, C.N. (1984) Synergism of
pyrethroids by several compounds in larvae of the Diamondback
moth (Lepidoptera: Plutellidae) J. econ. Entomol., 77: 851-856.
LOCK, E.A. & BERRY, P.N. (1981) Biochemical changes in the
rat cerebellum following cypermethrin administration. Toxicol.
appl. Pharmacol., 59(3): 508-514.
LOVERIDGE, D. & COOK, K.A. (1978a) Determination of the
effects of WL 43467 on microbial activity in soil. II. Effects
on oxygen uptake, Sittingbourne, Shell Research (BLGR.0004.78).
LOVERIDGE, D. & COOK, K.A. (1978b) Determination of the
effects of WL 43467 on microbial activity in soil. III.
Effects on nitrogen fixation, Sittingbourne, Shell Research
(BLGR.0007.78).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve
membrane effects of pyrethroids and DDT analogs. Pestic.
Biochem. Physiol., 20: 203-216.
MCAUSLAND, H.E., BUTTERWORTH, S.T.G., & HUNT, P.F. (1978)
Toxicity studies on the insecticide WL 43467: a two-year
feeding study in rats, Sittingbourne, Shell Research
(TLGR.0189.78).
MCDONALD, S. (1979) Evaluation of insecticides for control
of the army cutworm. J. econ. Entomol., 72(2): 277-280.
MCKEE, M.J. & KNOWLES, C.O. (1985) Pharmacokinetics of
pyrethroids in two-spotted spider mites. Pestic. Biochem.
Physiol., 24: 326-335.
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality
of permethrin, cypermethrin, and fenvalerate to salmon,
lobster, and shrimp. Bull. environ. Contam. Toxicol., 25:
950-955.
MANI, M. & KRISHNAMOORTHY, A. (1984) Toxicity of some
insecticides to Apanteles plutellae, a parasite of the
diamondback moth. Trop. Pestic. Manage., 30(2): 130-132.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th
ed., Croydon, British Crop Protection Council.
MAUCK, W.L., OLSEN, L.E., & MARKING, L.L. (1976) Toxicity of
natural pyrethrins and five pyrethroids to fish. Arch.
environ. Contam. Toxicol., 4: 18-29.
MEISNER, J., ASCHER, K.R.S., & EIZICK, C. (1984) Effect of
the commercial phagostimulants coax and gustol on the toxicity
of cypermethrin and deltamethrin against Spodoptera littoralis
(Lepidoptera: Noctuidae). J. econ. Entomol., 77: 1123-1126.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK,
J. (1983) Farm chemical handbook. C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, pp. C67.
MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J.
(1984) New conjugated metabolites of 3-phenoxybenzoic acid in
plants. Pestic. Sci., 15: 531-542.
MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO,
J. (1985) Metabolism in rats of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid glucoside conjugates formed in plants.
Pestic. Sci., 16: 33-45.
MIYAMOTO, J. (1981) The chemistry, metabolism, and residue
analysis of synthetic pyrethroids. Pure appl. Chem., 53:
1967-2022.
MIYAMOTO, J. & MIKAMI, N. (1983) Degradation of pyrethroid
insecticides in the field. In: IUPAC Pesticide chemistry,
human welfare and the environment, Oxford, Pergamon Press, pp.
193-200.
MORE, J.E., ROBERTS, T.R., & WRIGHT, A.N. (1978) Studies of
the metabolism of 3-phenoxybenzoic acid in plants. Pestic.
Biochem. Physiol., 9: 268-280.
MUIR, D.C.G., RAWN, G.P., TOWNSEND, B.E., LOCKHART, W.L., &
GREENHALGH, R. (1985) Bioconcentration of cypermethrin,
deltamethrin, fenvalerate, and permethrin by Chironomus
tentans larvae in sediment and water. Environ. Toxicol. Chem.,
4(1): 51-61.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some non-target insects. Mosq. News.,
38(1): 90-96.
NOBLE, R.M. & HAMILTON, D.J. (1985) Stability of
cypermethrin and cyfluthrin on wheat in storage. Pestic. Sci.,
16: 179-185.
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976a) Neurotoxic
effects of S 5602 and NRDC 149 by dermal application in rats,
Osaka, Sumitomo Chemical Company (AT-60-0047).
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976b) Neurotoxic
effects of natural pyrethrins and resmethrin by oral application
in rats, Osaka, Sumitomo Chemical Company (AT-60-0052).
OSMAN, A.A., ZOHDY, G.I., & MOMEN, F.M. (1982) Effect of
some pesticides on the food requirements of the predatory
mite, Amblyseius gossipiu-El-Badry. In: Griffiths, D.A. &
Bowman, C.E., ed. Acarology VI, Chichester, Ellis Horwood Ltd,
Vol. 2, pp. 669-672.
OWEN, D.E. & BUTTERWORTH, S.T.G. (1977) Toxicity of
pyrethroid insecticides: investigation of the neurotoxic
potential of WL 43467 to adult domestic hens, Sittingbourne,
Shell Research (TLGR.0134.77).
PEARSON, N. & SHIRES, S.W. (1981) A field study in France of
the effects on honey bees of an aerial application of RIPCORD
in winter-sown oil seed rape, Sittingbourne, Shell Research
(SBGR.81.304).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PLUYMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity
of synthetic pyrethroids in Salmonella typhimurium strains and
in V79 Chinese hamster cells. Mutat. Res., 137: 7-15.
POTTER, D. & MCAUSLAND, H.E. (1980) Toxicity studies on the
insecticide WL 43467: a study of liver microsomal enzyme
activity in rats fed WL 43467 for 2 years, Sittingbourne,
Shell Research (TLGR.80.057).
POULOS, L., ATHANASELIS, S., & COUTSELINIS, A. (1982) Acute
intoxication with cypermethrin (NRDC 149). J. Toxicol. clin.
Toxicol., 19(5): 519-520.
PREE, D.J. & HAGLEY, E.A.C. (1985) Toxicity of pesticides to
Chrysopa oculata Say (Neuroptera : Chrysopidae). J. econ.
Entomol., 78(1): 129-132.
PRICE, J.B. (1981a) Toxicology of pyrethroids: the acute
oral and intraperitoneal toxicity of cypermethrin,
Sittingbourne, Shell Research (SBGR.81.069).
PRICE, J.B. (1981b) Toxicology of pyrethroids: the acute
oral and percutaneous toxicities of RIPCORD and endosulfan,
individually or combined in a 1:1 mixture, Sittingbourne,
Shell Research (SBGR.81.121).
PRINSEN, G.H. & SITTERT, N.J., VAN (1978) Exposure and
medical monitoring study of the pyrethroid WL 43467 after
single application on cotton in Ivory Coast, The Hague, Shell
Internationale Research Mij (TOX 78-004).
PRINSEN, G.H. & SITTERT, N.J., VAN (1979) Exposure and
medical monitoring study of the pyrethroid RIPCORD (WL 43467)
after one season of spraying on cotton in Ivory Coast, The
Hague, Shell Internationale Research Mij (TOX 79-001).
RAJAKULENDRAN, S.V. & PLAPP, F.W., Jr (1982) Comparative
toxicities of five synthetic pyrethroids to the tobacco
budworm (Lepidoptera: Noctuidae), an Ichneumonid parasite,
Campoletis sonorensis and a predator, Chrysopa carnea. J.
econ. Entomol., 75: 769-772.
RAPLEY, J.H., ARNOLD, D.J., & VINCENT, J. (1981)
Cypermethrin: degradation in river and pond waters and
sediments, Fernhurst, Imperial Chemical Industries, Plant
Protection Division (Report No. RJ0175B) (Unpublished ICI
data).
REIFF, B. (1976) The acute toxicity of the pyrethroid
insecticide WL43467 to Brown trout (S. trutta), Sittingbourne,
Shell Research (TLBR.0096.76).
REIFF, B. (1977) The acute toxicity of the pyrethroid WL
43467 to Daphnia magna, Sittingbourne, Shell Research (TLGR.
0155.77).
REIFF, B. (1978a) The effect of suspended solids on the
toxicity of WL 43467 to Rainbow trout (Salmo gairdneri),
Sittingbourne, Shell Research (TLGR.0007.78).
REIFF, B. (1978b) The acute toxicity of the pyrethroid
insecticide WL 43467 to Rainbow trout (Salmo gairdneri),
Common carp (Cyprinus carpio), and Rudd (Scardinius
erythrophthalmus), Sittingbourne, Shell Research (TLGR.0067.
78).
RHODES, C., JONES, B.K., CROUCHER, A., HUTSON, D.H., LOGAN,
C.J., HOPKINS, R., HALL, B.E., & VICKERS, J.A. (1984) The
bioaccumulation and biotransformation of cis, trans-
cypermethrin in the rat. Pestic. Sci., 25: 471-480.
RILEY, D. & HILL, I.R. (1983) Adsorption reduces activity of
pesticides in soil and water. In: Proceedings of the 10th
International Congress on Plant Protection, Brighton, 20-25
November, 1983, Vol. 2, pp. 728 (4B-R18).
RISKALLAH, M.R. (1984) Influence of posttreatment
temperature on the toxicity of pyrethroid insecticides to
susceptible and resistant larvae of the Egyptian cotton
leafworm, Spodoptera littoralis (Boisd.) Experientia (Basel),
40(2): 188-190.
RIVIERE, J.L., BACH, J., & GROLLEAU, G. (1983) Effect of
pyrethroid insecticides and N-(3,5-dichlorophenyl)
dicarboximide fungicides on microsomal drug metabolizing
enzymes in the Japanese quail (Coturnix coturnix). Bull.
environ. Contam. Toxicol., 31: 479-485.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3(1): 67-68.
ROBERTS, T.R. (1981) The metabolism of the synthetic
pyrethroids in plants and soils. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry, New York, John
Wiley and Sons, Vol. 1, pp. 115-146.
ROBERTS, T.R. & STANDEN, M.E. (1977) Degradation of the
pyrethroid cypermethrin NRDC 149(±)-alpha-cyano-3-phenoxy-
benzyl(±)- cis,trans-3-(2,2-dichlorovinyl-2,2-dimethylcyclo-
propanecarboxylate and the respective cis-(NRDC 160) and the
trans-(NRDC 159) isomers in soils. Pestic. Sci., 8: 305-319.
ROBERTS, T.R. & STANDEN, M.E. (1981) Further studies of the
degradation of the pyrethroid insecticide cypermethrin in
soils. Pestic. Sci., 12: 285-296.
ROSE, B. (1981) A dietary toxicity study of WL 43467 in
Mallard ducks, Sittingbourne, Shell Research (TLGR.80.033).
ROSE, G.P. (1982) Toxicology of pyrethroids: the acute oral
and percutaneous toxicity of WL 85871 (cis-2-RIPCORD)
comparison with RIPCORD, Sittingbourne, Shell Research
(SBGR.82.130).
ROSE, G.P. (1983) Neurotoxicity of WL 85871 comparison with
WL 43467: the effect of twenty oral doses of WL 85871 or WL
43467 over a period of 4 weeks on the rat sciatic/posterior
tibial nerve, trigeminal nerve, and trigeminal ganglion,
Sittingbourne, Shell Research (SBGR.83.185).
ROSE, G.P. & DEWAR, A.J. (1978) Toxicity studies on the
insecticide WL 43467: the effect of age on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0039.78).
ROSE, G.P. & DEWAR, A.J. (1979a) Toxicity studies on the
RIPCORD/AZODRIN formulation EF 5254: biochemical and
functional studies on the neurotoxicity of the formulation EF
5254 in the rat, Sittingbourne, Shell Research (TLGR.79.027).
ROSE, G.P. & DEWAR, A.J. (1979b) A neurotoxicity study on
the pyrethroid metabolite 3-phenoxybenzoic acid (3-PBA),
Sittingbourne, Shell Research (TLGR.79.076).
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four
synthetic pyrethroids fails to show any correlation between
neuromuscular dysfunction and neurobiochemical abnormalities
in rats. Arch. Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of
pyrethroids on a nerve - muscle preparation of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 25: 176-187.
RUZO, L.O. (1983) Involvement of oxygen in the
photoreactions of cypermethrin and other halogenated
pyrethroids. J. agric. food Chem., 31: 1113-1115.
RUZO, L.O. & CASIDA, J.E. (1980) Pyrethroid photochemistry:
mechanistic aspects in reactions of the (dihalogenovinyl)-
cyclopropanecarboxylate substituent. J.C.S. Perkin Trans. I,
728-732.
RUZO, L.O., HOLMSTEAD, R.L., & CASIDA, J.E. (1977)
Pyrethroid photochemistry: decamethrin. J. agric. food Chem.,
25(6): 1385-1389.
SAAD, A.S.A., ELEWA, M.A., ALY, N.M., AUDA, M., & EL-SEBAE,
A.H. (1981) Toxicological studies on the Egyptian cotton
leafworm Spodoptera littoralis. I. Potentiation and antagonism
of synthetic pyrethroid, organophosphorus and urea derivative
insecticides. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(2):
559-571.
SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1986)
Degradation and leaching behaviour of the pyrethroid
insecticide cypermethrin in soils. J. Pestic. Sci., 11: 71-79.
SCOTT, J.G. & GEORGHIOU, G.P. (1984) Influence of
temperature on knockdown, toxicity and resistance to
pyrethroids in the housefly, Musca domestica. Pestic. Biochem.
Physiol., 21: 53-62.
SEEHY, M.A., SHALABI, H.G., SHAKER, N., & BADR, E. (1983) In
vivo induction of sister chromatid exchanges in mice by
cypermethrin. Proceedings of the International Conference on
Environmental Hazards of Agrochemicals, 1982, Vol. 1, 659-673.
SHERWOOD, C.M. & SHIRES, S.W. (1981) The effect of RIPCORD
on the invertebrate fauna of winter barley in France,
Sittingbourne, Shell Research (SBGR.81.070).
SHIRES, S.W. (1980) Soil surface predators in arable land:
the effects of farming practices. Span, 23(2): 62-64.
SHIRES, S.W. (1982a) The effect of RIPCORD on the
invertebrate fauna of winter wheat in France, Sittingbourne,
Shell Research (SBGR.81.303).
SHIRES, S.W. (1982b) A field study in France of the effects
on honey bees of an aerial application of RIPCORD in spring-
grown oil seed rape, Sittingbourne, Shell Research
(SBGR.82.066).
SHIRES, S.W. (1982c) A study of the effects of an aerial
application of RIPCORD on the invertebrate fauna of winter
wheat, Sittingbourne, Shell Research (SBGR.82.304).
SHIRES, S.W. (1983a) The use of small enclosures to assess
the toxic effects of cypermethrin in fish under field
conditions. Pestic. Sci., 14: 475-480.
SHIRES, S.W. (1983b) Pesticides and honey bees. Case studies
with RIPCORD and FASTAC. Span, 26(3): 118-120.
SHIRES, S.W. & BENNETT, D. (1982) Spray drift from aerial
application of RIPCORD to winter wheat in Kent, UK: fate and
effects in adjacent drainage ditches, Sittingbourne, Shell
Research (SBGR.82.274).
SHIRES, S.W. & BENNETT, D. (1985) Contamination and effects
in freshwater ditches, resulting from an aerial application of
cypermethrin. Ecotoxicol. environ. Saf., 9: 145-158.
SHIRES, S.W. & DEBRAY, P. (1982) Pyrethroids and the bee
problem. Shell Agric., May: 1-3.
SHIRES, S.W. & TIPTON, J.D. (1982) A study of the effects of
BIRLANE and RIPCORD on the hymenopterous parasites of white
flies on cotton in the Sudan, Sittingbourne, Shell Research
(SBGR.82.342).
SHIRES, S.W., BENNETT, D., & KANE, D.F. (1979) The effects
of WL 43467 on soil surface fauna, earthworms, and litter
composition, Sittingbourne, Shell Research (TLGR.79.074).
SHIRES, S.W., BENNETT, D., & CROSSLAND, N.O. (1980) Spray
drift from RIPCORD applications to arable crops in Suffolk,
UK: fate and effects in adjacent farm ponds, Sittingbourne,
Shell Research (TLGR.80.150).
SHONO, T. & CASIDA, J.E. (1978) Species-specificity in
enzymatic oxidation of pyrethroid insecticides:
3-phenoxybenzyl and alpha-cyano-3-phenoxybenzyl 3-(2,2-dihalo-
vinyl)-2,2-dimethylcyclopropanecarboxylates. J. Pestic. Sci.,
3: 165-168.
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of
trans- and cis-permethrin and trans- and cis-cypermethrin, and
decamethrin by microsomal enzymes. J. agric. food Chem.,
27(2): 316-325.
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation
of toxicity of pyrethroid insecticides to honey bees:
relevance to hazard in the field. Bee World, 63(4): 150-152.
SMIES, M., EVERS, R.H.J., PEIJNENBURG, F.H.M., & KOEMAN, J.H.
(1980) Environmental aspects of field trials with pyrethroids
to eradicate tsetse fly in Nigeria. Ecotoxicol. environ. Saf.,
4: 114-128.
SMITH, T.M. & STRATTON, G.W. (1986) Effects of synthetic
pyrethroid insecticides on non-target organisms. Residue Rev.,
97: 93-120.
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid
structure on rates of hydrolysis and oxidation by mouse liver
microsomal enzymes. Pestic. Biochem. Physiol., 7: 391-401.
SPIELBERGER, U., NA'ISA, B.K., KOCH, K., MANNO, A., SKIDMORE,
P.R., & COUHS, H.H. (1979) Field trials with the synthetic
pyrethroids permethrin, cypermethrin, and decamethrin against
Glossina (Diptera: gloninidae) in Nigeria. Bull. entomol.
Res., 69: 667-689.
STANDEN, M.E. (1977) The leaching and degradation of the
insecticide WL43467 when applied in sheep-dip solution to
soil, Sittingbourne, Shell Research (BLGR.0141.77)
(Unpublished report).
STELZER, K.J. & GORDON, M.A. (1984) Effects of pyrethroids
on lymphocyte mitogenic responsiveness. Res. Commun. chem.
Pathol. Pharmacol., 46(1): 137-150.
STEPHENSON, R.R. (1980a) The acute toxicity of WL 43467 to
some freshwater invertebrates in static water tests,
Sittingbourne, Shell Research (TLGR.80.040).
STEPHENSON, R.R. (1980b) The acute toxicity of cypermethrin
(WL 43467) to the freshwater shrimp (Gammarus pulex) and
larvae of the mayfly (Cloeon dipterum) in continuous-flow
tests, Sittingbourne, Shell Research (TLGR.80.079).
STEPHENSON, R.R. (1981a) RIPCORD: the acute toxicity of an
EC formulation to Tilapia nilotica in the laboratory,
Sittingbourne, Shell Research (SBGR.81.028).
STEPHENSON, R.R. (1981b) Cypermethrin: acute toxicity to
Tilapia nilotica in a continuous-flow test, Sittingbourne,
Shell Research (SBGR.81.080).
STEPHENSON, R.R. (1982a) RIPCORD: a laboratory study of the
acute toxicity of an EC formulation to Tilapia nilotica in the
presence of suspended solids, Sittingbourne, Shell Research
(SBGR.81.235).
STEPHENSON, R.R. (1982b) WL 85871 and cypermethrin: a
comparison of their acute toxicity to Salmo gairdneri, Daphnia
magna, and Selenastrum capricornutum, Sittingbourne, Shell
Research (SBGR.81.277).
STEPHENSON, R.R. (1982c) RIPCORD, BIRLANE, and FURADAN:
acute toxicity to common carp (Cyprinus carpio L.) in the
laboratory and in rice paddies, Sittingbourne, Shell Research
(SBGR.82.030).
STEPHENSON, R.R. (1982d) WL 85871 and cypermethrin: a
comparative study of their toxicity to the Fathead minnow
Pimephales promelas (Rafinesque), Sittingbourne, Shell
Research (SBGR.82.298).
STEPHENSON, R.R. (1982e) Aquatic toxicology of cypermethrin.
I. Acute toxicity to some freshwater fish and invertebrates in
laboratory tests. Aquat. Toxicol., 2: 175-185.
STEPHENSON, R.R. (1983) Pesticides and freshwater animals. A
case study with RIPCORD. Span, 26(3): 121-122.
STEPHENSON, R.R., CHOI, S.Y., & OLMOS-JEREZ, A. (1984)
Determining the toxicity and hazard to fish of a rice
insecticide. Crop Prot., 3(2): 151-165.
STEVENS, J.E.B. & HILL, I.R. (1980) Mobility of cypermethrin
and its degradation products in soil columns, Fernhurst,
Imperial Chemical Industries (Report No. RJ/0166-B)
(Unpublished ICI data).
SUHAS, Y. & DEVAIAH, M.C. (1985) Studies on the effect of
insecticides sprayed mulberry leaves to silkworm, Bombyx mori
L. Pesticides, 19(10): 53-54, 57.
SUNDARARAJAN, R. & CHAWLA, R.P. (1983) Simple, sensitive
technique for detection and separation of halogenated
synthetic pyrethroids by thin layer chromatography. J. Assoc.
Off. Anal. Chem., 66(4): 1009-1012.
SURULIVELU, T. & MENON, M.V. (1982) Contact toxicity of
synthetic pyrethroids, organophosphorus and carbamate
insecticides to adults of the parasite Chelonus blackburni
Cameron. J. Agric. Sci. Camb., 98: 331-334.
SUTHERS, J.R. & MARLOW, R.G. (1981) A study of the exposure
and health of Indian workers spraying RIPCORD on cotton over
five consecutive days using mistblower and knapsack
applicators, The Hague, Shell Internationale Research Mij (TOX
81-003).
SWAINE, H. & SAPIETS, A. (1980a) Residue transfer study with
dairy cows fed on a diet containing the insecticide.
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0186-B).
SWAINE, H. & SAPIETS, A. (1980b) Residue levels of the major
metabolites of the insecticide in the milk and tissues of
dairy cows fed on a diet containing cypermethrin at 50 mg/kg,
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0198-B).
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., & ASKAR,
A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46(1): 387-391.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985a) Hydrolysis of the pyrethroid insecticide cypermethrin
in aqueous media. J. Pestic. Sci., 10: 643-648.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985b) Photodegradation of the pyrethroid insecticide
cypermethrin in water and on soil surface. J. Pestic. Sci.,
10: 629-642.
TAYLOR, S.M., ELLIOTT, C.T., & BLANCHFLOWER, J. (1985)
Cypermethrin concentrations in hair of cattle after
application of impregnated ear tags. Vet. Rec., 116(23): 620.
TESH, J.M., TESH, S.A., & DAVIES, W. (1978) WL 43467:
effects upon the progress and outcome of pregnancy in rat,
Stock, Life Science Research (LSR Report No. 78/SHL2/364).
TEWARI, G.C. & KRISHNAMOORTHY, P.N. (1985) Selective
toxicity of some synthetic pyrethroids and conventional
insecticides to aphid predator, Menochilus sexmaculatus
Fabricius. Indian J. agric. Sci., 55(1): 40-43.
TRIGG, C.E., BUTTERWORTH, S., & HUNT, P.F. (1977)
Neurotoxicity of pyrethroids: a study of teased nerves from
rats fed WL 43467 for 12 months, Sittingbourne, Shell Research
(TLGR.0137.77).
TU, C.M. (1980) Influence of five pyrethroids insecticides
on microbial populations and activities in soil. Microbiol.
Ecol., 5: 321-327.
TU, C.M. (1982) Effects of some pesticides on Rhizobium
japonicum and on the seed germination and pathogens of
soybean. Chemosphere, 11(10): 1027-1033.
US EPA (1984) Cypermethrin: tolerances for residues in or on
raw agricultural commodities: final rule. Part III. Fed. Reg.,
49(117): 24865-24872.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the
peripheral nervous system of the frog. Pestic. Sci., 8:
692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Effects of
insecticides on the sensory system of Xenopus. Insect neurobiology
and pesticide action, London, Society of Chemical
Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M.
(1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effects of insecticides on the sensory nervous system.
In: Narashashi, T., ed. Neurotoxicology of insecticides and
pheromones, New York, London, Plenum Publishing Corporation,
pp. 183-210.
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985a)
Human oral dose-excretion study with RIPCORD, The Hague, Shell
Internationale Petroleum Maatschappy (HSE.85.008).
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985b)
Human dermal dose-excretion study with RIPCORD, The Hague,
Shell Internationale Petroleum Maatschappy (HSE.85.009).
VEKARIA, M.V. & VYAS, H.N. (1985) Studies on ovicidal
toxicity of certain insecticides against the eggs of Heliothis
armigera Hubner. Pesticides, 19(10): 43-44.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationships of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index. JMPR 1961-84, Geneva, World Health
Organization.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in
frog myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system.
Neuropathol. appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides
on the lateral-line sense organ and on peripheral nerves of
the clawed frog, Xenopus laevis. Pestic. Biochem. Physiol.,
18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on
sodium channel gating in myelinated nerves. Nature (Lond.),
295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature- and
structure-dependent interaction of pyrethroids with the sodium
channels in frog node of Ranvier. Biochim. biophys. Acta, 728:
73-82.
WADDILL, V.H. (1978) Contact toxicity of four synthetic
pyrethroids and methomyl to some adult insect parasites.
Florida Entomol., 61(1): 27-30.
WALLACE, B.G., ROBERTS, T.R., & MCKERRELL, E.H. (1982)
Cypermethrin. A residue transfer study with laying hens,
Sittingbourne, Shell Research (SBTR.82.059).
WATTERS, F.L., WHITE, N.D.G., & COTE, D. (1983) Effect of
temperature on toxicity and persistence of three pyrethroid
insecticides applied to fir plywood for the control of the red
flour beetle (Coleoptera: Tenebrionidae). J. econ. Entomol.,
76: 11-16.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23 .
WHO (1985) WHO Technical Report Series, No. 720 (Safe use of
pesticides. Ninth Report of the WHO Expert Committee on Vector
Biology and Control), pp. 14-19 .
WHO/FAO (1984) Cypermethrin, Geneva, World Health
Organization (Data Sheets on Pesticides, No. 84.58).
WILDE, G., KADOUM, A., & MIZE, T. (1984) Absence of
synergism with insecticides combinations used on chinch bugs
(Heteroptera: Lygaeridae). J. econ. Entomol., 77: 1297-1298.
WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic
pyrethoid insecticides to predaceous phytoseiid. Aust. J.
agric. Res., 30: 497-501.
WOOD, MACKENZIE, & CO. (1980) Pyrethroids, Agrochem. Monit.,
9: 3-14.
WOOD, MACKENZIE, & CO. (1981) Pyrethroids. Agrochem. Monit.,
15: 3-27.
WOOD, MACKENZIE, & CO. (1982) Pyrethroids. Agrochem. Monit.,
21: 3-17.
WOOD, MACKENZIE, & CO. (1983) Pyrethroids. Agrochem. Monit.,
27: 3-12.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual,
7th ed., Croydon, British Crop Protection Council, pp. 150-151.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of
pyrethroids. Gen. Pharmacol., 9: 387-398.
WRIGHT, A.N., ROBERTS, T.R., DUTTON, A.J., & DOIG, M.V.
(1980) The metabolism of cypermethrin in plants: the
conjugation of the cyclopropyl moiety. Pestic. Biochem.
Physiol., 13: 71-80.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G.
(1979) Toxicity of permethrin, decamethrin, and related
pyrethroids to salmon and lobster. Bull. environ. Contam.
Toxicol., 21: 338-343.
ZOHDY, G.I., OSMAN, A.A., & MOMEN, F.M. (1984) Toxicity of
some pyrethroid compounds to the predatory mite, Amblyseius
gossipi El-Badry. In: Griffiths, D.A. & Bowman, C.E., ed.
Acarology VI, Chichester, Ellis Horwood Ltd., Vol. 2, pp.
659-662.
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana esculenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3 - 10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittantly, cause large
depolarizing afterpotentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids
(allethrin, cismethrin, bioresmethrin, and 1R, cis-phenothrin)
caused moderate presynaptic repetitive activity, resulting in the
occurrence of multiple end-plate potentials (Ruigt & Van den
Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses.
The duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependent depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butyl-bicyclophosphoro-thionate [35S]-TBPS. Studies with 37
pyrethroids revealed an absolute correlation, without any false
positive or negative, between mouse intracerebral toxicity and in
vitro inhibition: all toxic cyano compounds including
deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin, and
[2S, alphaS]-fenvalerate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin
and [2S,alpha S]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA
receptorionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive muscle
action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids,
is not necessarily due to an exclusive central site of action. It
may be related to the long-lasting repetitive activity in sense
organs and possibly in other parts of the nervous system, which, in
a more advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established
that sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
Trout liver microsomes metabolize both cis- and trans-isomers
of cypermethrin to the 4'-hydroxy derivatives of the intact esters
and their corresponding ether glucuronic acid conjugates at
comparable rates. There are indications, derived from studies with
other pyrethroids, that the liver microsomes of the carp are more
able to metabolize pyrethroids than those of the salmonids (Edwards
& Millburn, 1985b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Leahey (1985) and Smith & Stratton (1986) give extensive
reviews on this matter.
7.1. Microorganisms
Effects of cypermethrin on microbial activity in the soil have
been investigated under laboratory conditions. Cypermethrin was
applied to a sandy loam soil at a rate of 2.5 or 250 mg/kg soil.
No effect was found on the rate of carbon dioxide evolution (Cook,
1978a) or oxygen uptake (Loveridge & Cook, 1978a) at the lower
rate. With 250 mg/kg, slight, but significant, inhibition of the
CO2 evolution and a decrease in oxygen uptake were noted after long
incubation periods. Nitrogen fixation was assessed by the
acetylene reduction method (Loveridge & Cook, 1978b). No
significant effects were found. Ammonification and nitrification
were studied in soils, either alone, or amended with urea or
ammonium chloride. Again, no significant effects were found, even
at the high rate of application (Cook, 1978b). Finally, glucose
utilization was studied in the short term (2 days) and after pre-
incubation with cypermethrin for up to 38 weeks. No significant
effects were noted, even at the high rate (Bromley & Cook, 1979).
A second series of experiments, in which cypermethrin was
applied to a sandy loam at 0.5 or 5 mg/kg soil has been reported.
Antimicrobial activity was observed in the early stages of
incubation with fungal population numbers returning to normal
within 2 - 4 weeks, and with a subsequent stimulation of microbial
growth. There was no inhibition of acetylene reduction activity at
either dosage. Nitrification and microbial respiration were
increased at both dosages, suggesting microbial degradation of
cypermethrin. Dehydrogenase activity was not affected, while
urease activity was stimulated. This study indicates that
cypermethrin may exert transient effects on the populations and
activities of microflora, but they are short-lived and minor in
nature (Tu, 1980).
The toxicity of cypermethrin for 4 pathogens of soybean and for
the nodulation organism Rhizobium has been investigated. In a
paper disc inhibition test, cypermethrin proved non-toxic for
Rhizobium, the EC50 exceeding 10 000 mg/litre. The toxicity for
the pathogens was higher, EC50 values ranging from 390 to 1700
mg/litre (Tu, 1982).
7.2. Aquatic Organisms
7.2.1. Fish
7.2.1.1. Acute toxicity
In common with other pyrethroids, cypermethrin is very toxic
for fish in clean water under laboratory conditions. The available
data are summarized in Table 8. The data demonstrate a similar
high acute toxicity for both cold- and warm-water species of fish.
Certain of these data have been reviewed by Stephenson (1982e).
There is no evidence of a significant effect of temperature on
toxicity, though a negative temperature coefficient has been
claimed for certain pyrethroids (Kumaraguru & Beamish, 1981).
Furthermore, the toxicity of pyrethroids for fish was not
influenced by the hardness or pH of the water (Mauck et al., 1976).
To determine the acute toxicity of cypermethrin for Tilapia
nilotica, an EC formulation of cypermethrin (25 g/litre) was
sprayed on the surface of static water held in stainless steel
tanks in a glasshouse. The concentration of cypermethrin in the
water was determined over a 96-h period. Depending on the
application rate, the concentration of cypermethrin in the water
rose rapidly during the first 24 h after application and decreased
slowly over the following 3 days. The tanks each contained 5 fish
and water to a depth of 30 cm. Water temperatures were 23 - 26 °C.
No fish deaths occurred when the peak concentration of cypermethrin
was less than 1.5 µg/litre, while all fish died when the
concentration exceeded approximately 5 µg/litre (Stephenson,
1981a).
To study the influence of suspended solids on water
concentrations and the toxicity for fish of cypermethrin, Rainbow
trout were exposed to nominal concentrations of 2 or 5 µg
cypermethrin/litre, which was added to microfiltered mains water or
to pond water containing 14.5 mg suspended solids/litre. The
actual concentrations were measured for water samples taken 30 min
after initial mixing. Two samples of pond water were taken. One
was analysed as such while the second was centrifuged (60 min at
3000 rev/min) to precipitate the suspended solids; the clear
supernatant liquid was then analysed. The results are summarized
in Table 9.
The data from this study show that suspended solids absorb 40%
of the cypermethrin initially present. Fish exposed to an actual
concentration of 4.9 µg cypermethrin/litre in mains water died
within 24 h, those in pond water containing an actual concentration
of 2.5 - 4 µg/litre survived (Reiff, 1978a).
The high degree of adsorption of cypermethrin on soils was
demonstrated by Riley & Hill (1983) in their studies on the
toxicity of cypermethrin for Daphnia and other aquatic organisms.
The addition of soil to the system reduced the toxicity of the
aqueous solution by 200 - 350 times.
The stainless steel tank study with Tilapia nilotica was
repeated with the water initially containing 100 mg suspended
solids/litre. In this study too, the presence of suspended solids
reduced the toxic effects of cypermethrin (about 2-fold)
(Stephenson, 1982a).
Table 8. Acute toxicity of cypermethrin for fish
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Brown trout 5 - 8 technical, dispersed via 15 2 - 2.8 Reiff (1976)
(Salmo trutta) DMSO
5 - 8 technical, absorbed on 15 1.2a,b Reiff (1976)
pumice
Rainbow trout 1 - 11 technical, absorbed on 10 - 15 0.5a,b,c Reiff (1978b)
(Salmo gairdneri) pumice
1 - 2 technical, via acetone 10 0.5 Stephenson
(1982b)
3.3 technical, via acetone 15 2.8 Stephenson
(1982b)
3 emulsifiable concentrate 10 11d,g Coats &
(40% ai) O'Donnell-Jeffery
(1979)
3 technical, dispersed via 10 55d Coats &
acetone O'Donnell-Jeffery
(1979)
Common carp 4 - 10 technical, absorbed on 10 0.9a,b,c Reiff (1978b)
(Cyprinus carpio) pumice
4 - 10 technical, absorbed on 20 - 25 1.1a,b,c Reiff (1978b)
pumice
8 - 10 technical, via acetone 10 0.9 Stephenson
(1982c)
2.1 emulsifiable concentrate 22 - 26 3.4f Stephenson
(5% a.i.) (1982c)
Stephenson et
al. (1984)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Rudd (Scardinius 9 - 10 technical, absorbed on 15 0.4a,b,c Reiff (1978b)
erythrophthalmus) pumice
Atlantic salmon 5.3 technical, dispersed via 10 2 - 2.4b McLeese et al.
( Salmo salar) ethanol (1980)
9.4 1-R- cis- 10 0.74 Zitko et al.
(1979)
Tilapia nilotica 1 - 3 technical, absorbed on 25 2.0a,b,c Stephenson
pumice (1981b)
Stephenson et
al. (1984)
Fathead minnow 0.74 technical, absorbed on 23 - 25 1.2a,b Stephenson
(Pimephales (juvenile) pumice (1982d)
promelas)
Mugil cephalus 0.1 30% EC ? 24g Tag El-Din et al.
30 days (1981)
Gambusia affinis 4 weeks 30% EC 25 6.6g El-Sebae et al.
(1983)
---------------------------------------------------------------------------------------------------------
a Flow-through system. Otherwise, static test.
b Measured concentration. Otherwise, nominal.
c The data quoted have been recalculated from the primary data given in the original reports following
current statistical methods.
d 24-h LC50.
e Lethal threshold value (geometric mean of lowest concentration with and highest concentration without
mortality).
f 48-h LC50.
g Formulation.
Remark: In most tests, the pH of the water was 7.5 - 8.5; the hardness was 260 mg/litre as CaCO3 (except
for a few cases).
Table 9. Influence of suspended solids on the toxicity of cypermethrin
for Rainbow trout
-------------------------------------------------------------------------
Source Suspended Initial cypermethrin Response of Rainbow
solids concentration (µg/litre) trout
(mg/litre) actual
nominal total in water
-------------------------------------------------------------------------
Microfiltered 0 2 1.7 1.7 1/6 fish dead at 48 h
mains water 5 4.9 4.9 6/6 fish dead at 24 h
Pond water 14.5 2 1.2 0.65 no symptoms or deaths
5 4.0 2.5 at 7 days
-------------------------------------------------------------------------
7.2.1.2. Long-term toxicity
The effects of cypermethrin on the most sensitive stage in the
life cycle of the Fathead minnow (Pimephales promelas) were
investigated using a flow-through system. Total hardness, pH,
concentration of dissolved oxygen, and temperature were controlled.
Within 24 h of fertilization, eggs were exposed to nominal
concentrations of 0, 0.03, 0.1, 0.3, or 1.0 µg/litre (mean exposure
concentration 0, 0.03, 0.12, 0.17, and 0.79 µg cypermethrin/litre),
for a total of 34 days. Hatching occurred between the 3rd and 6th
day, while egg hatch was not affected at the highest concentration.
No fry survived day 34. Survival was reduced at concentrations of
0.3 and 0.1 µg/litre but not at 0.03 µg/litre. On the basis of the
most sensitive parameter, i.e., survival of young fry, the no-
observed-adverse-effect level for cypermethrin lay between 0.03 and
0.12 µg/litre (Stephenson, 1982d).
7.2.2. Invertebrates
7.2.2.1. Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cypermethrin. The data in Table 10 are from static, clean water
test systems and some of these data have been reviewed by
Stephenson (1982e). It can be seen that snails do not show any
effects at 5 µg/litre (close to the water solubility of
cypermethrin), while some insects show behavioural changes, but no
mortality at this level. Crustacea, particularly marine decapod
Crustacea, are highly susceptible to cypermethrin, mortality
occurring at levels below 0.05 µg/litre. Water mites are also very
susceptible (Zitko et al., 1979).
In addition to these static water tests, continuous-flow tests
in clean water have been undertaken with 2 sensitive species
(Stephenson, 1980b) (Table 11).
It can be concluded that effects can be expected when
concentrations of cypermethrin of the order of 0.01 µg/litre are
maintained in the water phase for more than 96 h.
It has been shown that, at 100 µg/litre, cypermethrin does not
affect the growth of the single-celled green alga Selenastrum
capricornutum, over a period of 2 - 4 days (Stephenson, 1982b).
7.2.2.2. Long-term toxicity
The effects of cypermethrin on the survival, growth, and
reproduction of Daphnia magna were investigated, over 21 days, in
a static water test with daily renewal of test solutions. The
nominal concentrations tested were 0, 0.003, 0.01, 0.03, 0.1, and
0.3 µg/litre. Cypermethrin affected all 3 parameters at a nominal
concentration of 0.3 µg/litre, but no effects were noted at 0.1
µg/litre. Chemical analysis suggested that the Daphnia were
exposed to about 50% of the nominal concentration, two-thirds in
solution, the remainder adsorbed on suspended solids. These
results show that the no-observed-adverse-effect level of
cypermethrin throughout the life cycle for Daphnia is of the order
of 0.05 µg/litre (Garforth, 1982).
7.2.3. Field studies
7.2.3.1. Deliberate overspraying
This subject has received wide attention, and various aspects
have been reviewed in a number of publications (Crossland &
Stephenson, 1979; Crossland, 1982; Crossland et al., 1982;
Crossland & Elgar, 1983; Shires, 1983a; Stephenson, 1983).
The effects of field applications of cypermethrin on fish were
studied in a preliminary study by Crossland & Bennett (1976). A
small pond was deliberately oversprayed with 100 g cypermethrin/ha
as an EC formulation. The peak concentration of cypermethrin in
the water was 2.6 µg/litre. Wild populations of fish and amphibia
were not affected by this treatment. Large numbers of young fish
seen during the 2 weeks following treatment did not appear to be
affected.
In a second study at the same rate of application, the peak
concentration of cypermethrin was 1.4 µg/litre. There was no
observed mortality among 12 small rudd that had been placed in the
pond 12 days before treatment, and no effects of treatment were
noted in wild populations of rudd or newts (Crossland et al.,
1978).
Table 10. Acute toxicity of cypermethrin for aquatic invertebrates in static tests
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Fresh-water
Crustacea
Water flea up to 24 h 22 acetone 4.2 Reiff (1977)
(Daphnia magna) old 18 water 2 2 Stephenson (1980a)b
20 acetone 1.2 - Stephenson (1982b)
20 acetone 0.3c - Stephenson (1982b)
Water hog louse 3 - 8 mm 15 water 0.02 0.2 Stephenson (1980a)
(Asellus spp.)
Freshwater shrimp 3 - 8 mm 15 id 0.04 0.1 Stephenson (1980a)
(Gammarus pulex)
Insecta
Mayfly larvae 15 id 0.07 0.6 Stephenson (1980a)
(Cloeon dipterum)
Whirligig beetle adult 15 id 0.07 > 5 Stephenson (1980a)
(Gyrinus natator)
Bloodworm larvae 15 id 0.2 > 5 Stephenson (1980a)
(Chironomus thummi)
Mosquito larvae 18 id 0.03 1 Stephenson (1980a)
(Aedes aegypti)
Midge (Chaoborus larvae 15 id 0.03 0.2 Stephenson (1980a)
crystallinus)
Water boatman adult 15 water 0.7 > 5 Stephenson (1980a)
(Corixa punctata)
Water boatman adult 15 id 0.3 > 5 Stephenson (1980a)
(Notonecta spp.)
---------------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Arachnida
Water mite adult 15 0.02 0.05 Stephenson (1980a)
(Piona carnea)
Mollusca
Snail (Lymnaea < 8 mm 15 id > 5 > 5 Stephenson (1980a)
peregra)
Marine
Crustacea
Lobster (Homarus 450 g 10 ethanol - 0.04g McLeese et al.
americanus) (1980)
450 g 10 id - 0.003d,f,g Zitko et al. (1979)
Shrimp (Crangon 1.3 g 10 id - 0.01d,e McLeese et al.
septemspinosa) (1980)
---------------------------------------------------------------------------------------------------------
a EC50 values are based on effects, usually immobilization, other than death.
b Stephenson (1980a) used cypermethrin (85% a.i.) dissolved in acetone and absorbed on pumice.
c 48-h EC50.
d 96-h LC50.
e Dispersed via ethanol.
f 1-R- cis-.
g Salinity, 30%.
Remark: In most tests, the pH of the water was 7.5 - 8.5; and the hardness, 260 mg/litre as CaCO3.
Table 11. Acute toxicity of cypermethrin for aquatic invertebrates in
continuous-flow tests
------------------------------------------------------------------------
Species Stage Temperature EC50 LC50
size (°C) (µg/litre) (µg/litre)
24-h 96-h 24-h 96-h
------------------------------------------------------------------------
Freshwater shrimp 3 - 8 mm 15 0.005 0.004 0.030 0.009
(Gammarus pulex)
Mayfly larvae 15 0.008 0.004 0.070a 0.020
(Cloeon dipterum) 0.5 - 1 cm
------------------------------------------------------------------------
a 48-h LC50.
Observations on invertebrates were included in these two pond
studies. In the first study, which lasted 2 weeks, populations of
Crustacea, mites, and insects were severely reduced. Surface
breathing insects were affected most rapidly, within hours of
treatment. Free-swimming dipterous larvae were not noticeably
affected for 24 h, while zooplankton were killed between 1 and 2
days after treatment. Bottom dwelling invertebrates, including
chironomid larvae, snails, leaches, and flatworms, did not appear
to be affected, though the numbers in the last 2 groups were low in
pre-treatment samples.
The second study was continued for 15 weeks after treatment.
Initial results were similar to those reported above. Macro-
invertebrates were markedly reduced in numbers, 2 weeks after
treatment. However, both numbers and diversity returned to normal
levels after 15 weeks. Snails and flat-worms (again numbers low in
this group in pretreatment counts) were unaffected, but no
arthropods were present in the samples taken at 2 weeks.
Recolonization by flying insects (beetles and chironomids)
commenced 4 weeks after treatment. The Crustacean Asellus had not
reappeared by the end of the study.
No daphnids or copepods were found in the zooplankton samples,
1 week after treatment, and they only reappeared in the 8-week
post-treatment sample. Populations returned to normal levels in
10 - 12 weeks. Some 2 weeks after treatment, an increase in
filamentous algae was noted, and this persisted until the end of
the study. It was inferred that this was a secondary effect
following from the elimination of known feeders on algae, for
instance, the mayfly, Cloeon dipterum, and the daphnid,
Simocephalus sp. (Crossland & Bennett, 1976; Crossland et al.,
1978).
7.2.3.2. Monitoring of drift from ground and aerial applications
Two field studies were undertaken to assess the fate and
effects in adjacent waters of spray drift from ground-based
agricultural applications of cypermethrin.
(a) Mistblower applications on vines (France)
Three sites were chosen, each with vines planted up to the
banks of adjacent streams. One application of cypermethrin was
monitored at each site and a second application at one of the
sites. A diluted EC formulation of cypermethrin was applied at
30 g a.i./ha using a backpack, swinging-head mistblower, and at
45 g a.i./ha using a tractor-mounted, fixed-head mistblower.
The deposition on the ground and an adjacent stream of 0.37 -
4.5 g/ha led to peak concentrations in the subsurface water ranging
from 0.17 to 1.7 µg/litre during the first hour after spraying; the
concentrations decreased to less than 0.1 µg/litre within a few
hours. No effects on fish, tadpoles, or frogs were observed. Some
aquatic invertebrates in adjacent streams were seen to be
hyperactive or immobilized during the first 2 h after application.
This was most obvious with mayfly larvae, water boatmen, water
beetles, pond skaters, and syrphid larvae. During the first 3 h
following application, there was an increase in the number of
invertebrates caught in drift nets. However, there were no
significant population effects in zooplankton or macro-
invertebrates (Bennett et al., 1980).
(b) Boom-and-nozzle applications to row crops (United Kingdom)
Two sites were chosen each with row crops (potatoes or
sugarbeet or both) planted up to within 1 - 3 m of the edge of
ponds. Two applications of cypermethrin were monitored at each of
3 ponds. In each case, a diluted EC formulation of cypermethrin
was applied at 70 g a.i./ha using tractor-mounted, boom-and-nozzle
equipment. Deposition on the ground and over the pond was
estimated. The deposition led to peak concentrations in subsurface
water in the range of 0.01 - 0.05 µg/litre. In one case, the
concentration in subsurface waters after 24 h was 0.02 - 0.03
µg/litre. In all other cases, this value was at, or below, 0.01
µg/litre, the limit of detection. No effects on fish were
observed. No residues of cypermethrin were found in fish (limit of
detection, 5 µg/kg wet weight). The only effects on invertebrates
in adjacent waters were noted in the corner of 1 pond. Some pond
skaters, one water-boatman, and 2 syrphid larvae were found
immobilized. They had recovered by the next day. There was no
evidence from zooplankton or sweep-net samples of any effects on
invertebrate populations (Shires et al., 1980).
(c) Aerial application to winter wheat (United Kingdom)
A large field of winter wheat was chosen, which was surrounded
on 3 sides by drainage ditches. An EC formulation of cypermethrin
was applied at 25 g a.i./ha by fixed-wing aircraft. Up to 6% of
the nominal dose was deposited on the water surfaces, resulting in
maximum levels in subsurface waters of 0.03 µg/litre, which
declined rapidly after spraying. No effects were observed on caged
or wild fish, and no significant residues of cypermethrin were
found in the fish. A few air-breathing water boatmen and water
mites, which are very susceptible, showed minor short-term
reductions in abundance (Shires & Bennett, 1982, 1985).
(d) Application for rice insect control (Korea, Spain)
An EC formulation of cypermethrin was applied to rice paddies
in Korea, in which carp had been caged, by hand-spraying at 15 or
40 g a.i./ha. Mortality at the higher rate was significantly
higher than that in the control, i.e., 15 and 7%, respectively
(Stephenson, 1982c).
Another field study was carried out in Spain. Cypermethrin was
applied by air to paddy rice and also to caged fish (Cyprinus
carpio) within the area. The application rate was 15 - 40 g
a.i./ha. The limited toxic effects of cypermethrin on fish under
field conditions were confirmed in this study. Aerial overspraying
of the rice paddies at 25 g a.i./ha did not produce any mortality
(Stephenson et al., 1984).
(e) Applications for tsetse fly control (Nigeria)
Field studies have been reported on the assessment of the
environmental impact of the application of cypermethrin for tsetse
fly control in Nigeria. Searches for affected fish were made in
areas that had been selectively sprayed from the ground with an EC
formulation of cypermethrin at 2 - 3 g a.i./litre, or sprayed from
the air with an oil-based solution of cypermethrin at 100 g a.i./ha
in 1977 and at 60 and 150 g a.i./ha in 1978. The ground spray
application was successful in controlling the tsetse fly, but only
the highest rate applied from the air approached a satisfactory
level of tsetse control. None of these searches revealed any dead
fish in the rivers within the areas sprayed with cypermethrin.
Pre- and post-treatment net samples of aquatic invertebrates were
taken in an area sprayed at 100/150 g a.i./ha. Acute mortality
occurred in terrestrial and aquatic arthropods, such as the water
beetle and Crustaceans. Shrimps and mayfly larvae disappeared from
river benthos after spraying, but reappeared one year later (Smies
et al., 1980).
7.3. Terrestrial Organisms
7.3.1. Laboratory studies
7.3.1.1. Acute toxicity
(a) Birds
The acute oral toxicity of cypermethrin for birds is summarized
in Table 12.
In the first study (Coombs et al., 1976), 4 birds of each
species were observed for a 3-week period after dosing; no deaths
or toxic signs were noted at the highest dosages applied. In the
second study (Rose, 1981), clinical signs of cypermethrin
intoxication, including ataxia and lethargy, were noted at dosages
of 3000 mg/kg body weight and above. One male duckling, out of 28
of each sex, died at the top dosage of 10 000 mg/kg body weight.
7.3.1.2. Short-term toxicity
(a) Birds
Six laying hens were given 5 successive daily oral doses of
1000 mg cypermethrin/kg body weight in DMSO followed after 3 weeks
by a second 5-day dosing regime and were observed for a further 3
weeks. Control hens did not receive any treatment. No signs of
intoxication or histological changes in nervous tissue were noted
at any time (Owen & Butterworth, 1977).
Table 12. Acute oral toxicity of cypermethrin for birds
--------------------------------------------------------------------------
Species Age Vehicle Observation LD50 Reference
period (mg/kg
body
weight)
--------------------------------------------------------------------------
Domestic fowl adult 40% DMSO 21 days > 2000 Coombs et al.
(Gallus (1976)
domesticus)
French partridge adult 40% DMSO 21 days > 3000 Coombs et al.
(Allectoris (1976)
rufa)
Mallard duck 2 - 6 40% emul- 14 days > 10 000 Rose (1981)
(Anas months sifiable
platyrhynchos) concentrate
--------------------------------------------------------------------------
Cypermethrin was administered for 5 consecutive days to Mallard
ducks at dosages of 5000 or 10 000 mg/kg diet. No deaths or
clinical signs of intoxication were noted during the feeding period
or over a subsequent 40-day holding period. Food intake and body
weights were slightly depressed during the feeding period, though
most ducks had regained or exceeded their initial body weights by
the end of the holding period (Rose, 1981).
Riviere et al. (1983) studied the influence of cypermethrin on
the microsomal drug metabolizing enzymes in Japanese quail
(Coturnix coturnix). Cypermethrin had no or only a very weak
inducing effect.
(b) Honey bees
Cypermethrin was highly toxic for worker honey bees (Apis
mellifera) in laboratory tests (24-h oral LD50 0.035 µg a.i. per
bee) (Table 13). Cypermethrin applied on the dorsal side of the
thorax was more toxic for honey-bees at lower breeding
temperatures, i.e., at 12 - 20 °C, the toxic level was 0.01 - 0.02
µg/bee and at 32 °C, 0.02 - 0.03 µg/bee. In addition, the
sensitivity of the bees to cypermethrin increased with age (Delabie
et al., 1985).
An aqueous dispersion of cypermethrin was sprayed directly on
worker honey bees. Only 5% of the bees sprayed with a 0.01
g/litre solution were killed. All bees sprayed with 0.1 and 1
g/litre died (Harris & Turnbull, 1978).
First indications that cypermethrin might not be hazardous for
bees under field conditions were obtained in cage tests. Worker
honey bees were exposed for 2 h to residues produced by treating
flowering Solidago (Golden rod) to run-off with a spray containing
0.5 g cypermethrin/litre and aging the residue for 1 h. Some
knock-down of the bees (up to 9%) was noted during the first 8 h
following initial exposure. However, most bees recovered, and
mortality after 1 day and 10 days did not differ from that in
untreated control bees. Higher mortality had been noted in an
earlier study using the same technique but with a higher dosage
rate of 1.5 g cypermethrin/litre (Gerig, 1979, 1981).
Table 13. Toxicity of cypermethrin for worker honey bees
-------------------------------------------------------------------------
Formulation 24-h LD50 (mg/bee) Reference
Topical application Oral administration
-------------------------------------------------------------------------
Technical 0.02 0.035 Badmin & Twydell
(1976)
Technical 0.053 0.12 Knight (1982)
Technical 0.056 - Smart & Stevenson
(1982), Westlake
et al. (1985)
Emulsifiable - 0.031 Badmin & Twydell
concentrate (1976)
(40%)
-------------------------------------------------------------------------
(c) Predatory and parasitic species
Cypermethrin has also been evaluated against a number of
predatory or parasitic insects and mites. Laboratory data are
available for a number of predators (Coleoptera) and parasites
(Hymenoptera) of insect pests.
The available data indicate that cypermethrin can show useful
selectivity between insect predators and parasites and the relevant
pest species (Mulla et al., 1978; du Toit, 1978; Waddill, 1978;
Abdel-Aal et al., 1979; Coats et al., 1979; Holden, 1979; Leake et
al., 1979; McDonald, 1979; European Patent Office, 1980; Harris &
Turnbull, 1980; Hagley et al., 1981; Jordan & Chang, 1981; Saad et
al., 1981; Ascher et al., 1982; Baicu, 1982; Bayoumi, 1982; Chang &
Jordan, 1982; Osman et al., 1982; Rajakulendran & Plapp, 1982;
Surulivelu & Menon, 1982; Brempong-Yeboah et al., 1983; Chang &
Jordan, 1983; El-Guindy et al., 1983; El-Minshawy et al., 1983;
El-Sebae et al., 1983; Ho et al., 1983; Ishaaya et al., 1983;
Watters et al., 1983; Abbassy et al., 1984; Bariola & Lingren,
1984; Brempong-Yeboah et al., 1984a,b,c; Cheng & Hanlon, 1984; Dai
& Sun, 1984; El-Sayed & Knowles, 1984; Ewen et al., 1984; Fabellar
& Heinrichs, 1984; Hopkins et al., 1984; Liu et al., 1984; Mani &
Krishnamoorthy, 1984; Meisner et al., 1984; Le Patourel & Singh,
1984; Riskallah, 1984; Scott & Georghiou, 1984; Wilde et al., 1984;
Zohdy et al., 1984; Ahmed et al., 1985; Abou-Awad & El-Banhawy,
1985; Bostanian & Belanger, 1985; Bostanian et al., 1985; Corbitt
et al., 1985; Edwards et al., 1985; El-Sebae et al., 1985; Harris
et al., 1985; Knapp et al., 1985; Knowles & El-Sayed, 1985; McKee &
Knowles, 1985; Pree & Hagley, 1985; Suhas & Devaiah, 1985; Tewari &
Krishnamoorthy, 1985; Vekaria & Vyas, 1985; Barlow et al., 1986).
(d) Earthworms
The toxicity of cypermethrin for the earthworm (Eisenia
foetida) has been assessed in an artificial soil test system. Worms
were exposed to dosages of 0, 0.1, 1.0, 10, or 100 mg/kg soil for
14 days. No mortality was found (Inglesfield & Sherwood, 1983;
Inglesfield, 1984).
The LC50 for cypermethrin in Eisenia foetida was found to be
26.1 µg/cm2 (16.3 - 44.4 µg/cm2) (Roberts & Dorough, 1984).
(e) Higher plants
In studies on effects of cypermethrin on seed germination in
the soybean, seedling emergence and survival were not reduced by
levels of up to 5000 mg/litre (Tu, 1982). Cypermethrin has been
demonstrated not to be phytotoxic for crops when applied at
recommended dosages (Hargreaves & Cooper, 1979).
7.3.2. Field Studies
7.3.2.1. Applications for tsetse fly control in Nigeria
Field trials in Nigeria of cypermethrin against Tsetse flies
( Glossina palpalis (RD) and Glossina tachnoides Westw.) were
carried out, over a 2-year period by Spielberger et al. (1979).
Ground applications of cypermethrin (0.3%) were made on fly-resting
sites in vegetation using pressurized knapsack sprayers.
Populations of both species were eradicated after a single
application. Levels of over 150 g/ha were needed for complete
eradication by residual spraying from a helicopter.
The effects on the general insect population of aerial
applications of cypermethrin for tsetse fly control were studied by
Smies et al. (1980). Within a few hours of application, dead and
dying insects were found, exemplifying the rapid knockdown effect
of cypermethrin. Insect fallout, as assessed by funnel traps, was
markedly increased for 1 day by 100 g cypermethrin a.i./ha, and for
3 days by 150 g a.i./ha. General insect activity as assessed by
Malaise trap catches was not affected by 150 g cypermethrin a.i./ha
(Smies et al., 1980).
7.3.2.2. Honey bees
This subject has been reviewed by Shires & Debray (1982) and
Shires (1983b).
The hazard of practical field applications of cypermethrin for
worker honey bees was assessed in 2 field trials. In both trials,
cypermethrin was applied to flowering oilseed rape, by helicopter,
at a time when the crops were being actively worked by bees from
nearby colonies, thus representing a worst case exposure of the
bees. The rate of application in both trials was 25 g a.i./ha. Of
the 8 hives, placed adjacent to the treated crops, 6 had been
fitted with dead bee traps and 2 with pollen traps. Observations
were made on bee mortality, pollen collection, foraging activity,
hive populations, and brood areas. In addition, in the second
trial, cypermethrin residues were determined in the bees, pollen,
wax, honey, and in the leaves and flowers of the treated crop
(Pearson & Shires, 1981; Shires, 1982b).
In the first trial, only a small increase in bee mortality was
noted, following the cypermethrin treatment (Table 14).
Cypermethrin appeared to be repellent to honey bees foraging the
crop, but the duration of this effect could not be established
because of poor weather during the first few days following
treatment. Cypermethrin did not have any effects on hive
populations of adult bees or brood areas.
In the second trial, a slight increase in bee mortality was
found at the time of treatment (Table 14). Cypermethrin had a
repellent effect on honey bees for up to 24 h after application.
Following this period, foraging activity and pollen collection
returned to normal. Cypermethrin residues in dead bees, collected
on the day of spraying and on the following morning, were higher
than would be expected to be lethal. However, residue levels in
dead bees collected after this period were considerably lower than
those required to produce toxic effects, confirming that they
represented the natural mortality level. Very low levels of
cypermethrin were found in live bees and levels in honey and wax
were close to the limit of detection. Concentrations of
cypermethrin in flowers and pollen declined rapidly after
treatment. Again, cypermethrin did not have any effects on hive
populations of adult bees or of brood areas (Shires, 1982b).
Table 14. Mean number of dead bees per hive during large-scale
field trials with cypermethrina
-----------------------------------------------------------------
Treatment Time from treatment
compound -2 to -1 -1 to 0 0 to +4 +1 to +2 +3 to +4
(dosage) days days h days days
-----------------------------------------------------------------
Winter-sown rape; 65 6 260 40 2
cypermethrin
(25 g a.i./ha)
water control 75 17 0 26 15
Spring-sown rape 9 36 310 2 13
cypermethrin
(25 g a.i./ha)
-----------------------------------------------------------------
a From: Shires (1983b).
In glasshouse studies, in which insecticide treatment was
carried out during foraging by spraying the rape with 0.12 ml of
Cymbush in 100 ml water (equivalent to 50 g a.i./ha), high
mortality was observed 2 days after treatment, but no residual
mortality appeared during the following 2-month period. In this
study, oilseed rape flowers were not attractive to bees for at
least 2 days following treatment.
A field test was carried out on a 38-ha field of oilseed rape.
The insecticide was sprayed on a central area of 13 ha during the
morning, at a rate of 50 g a.i./ha. The weather was sunny and the
temperature about 18 °C. A high level of mortality occurred only
on the 3 days following treatment. The results also showed that the
bees avoided visiting the flowers as soon as the treatment was
made, especially during the first 2 days. From the third day after
the treatment, the repellent effect decreased, and the visits to
the rape flowers increased reaching normal on the fifth day. It
was suggested by the authors that the repellent effect appeared to
be due to the formulation ingredients.
No residues of cypermetherin were found in hive products
(pollen, wax, or honey) (Delabie et al., 1985).
In a study by Gerig (1981), cypermethrin was repellent to
worker honey bees for a short period after spraying. Flowering
Phaselia plants, within a flight tent, were treated with a spray
containing 0.5 g cypermethrin/litre. Honey bee visits to the
treated flowers were very few over the first 0.5 h following
treatment and remained at a reduced level during the remaining
5.5 h of observation on the day of treatment. Flower visits
returned to normal levels on the following day (Gerig, 1981).
7.3.2.3. Soil fauna
This topic has been reviewed by Shires (1980).
In an initial study on the effects of cypermethrin on the soil
surface fauna and earthworms, cypermethrin was applied at 100 g
a.i./ha to small plots of spring wheat. This treatment did not
have any effect on earthworm populations or on leaf litter
breakdown, which is an indirect indicator of earthworm activity.
The general population of soil surface predators, mainly beetles
and spiders, was reduced to about 50% of the control value soon
after treatment, but increased to a higher level than that in the
controls after 5 weeks. A subsequent secondary decrease in
predator populations was probably related to the efficiency of
control of cereal aphids by cypermethrin (Shires et al., 1979). In
a second study, cypermethrin was applied at 25 g a.i./ha to newly-
emerged winter barley to control the aphid vectors of barley yellow
dwarf virus. Populations of non-target arthropods were assessed
using pitfall and water traps. Populations of soil Collembola
were only slightly affected by cypermethrin. Populations of
predators (beetles, spiders, and centipedes) were already declining
because of the season, but there were significantly fewer predators
in the cypermethrin plots compared with the controls at the end of
the trial. Cypermethrin treatment did not affect the numbers of
invertebrates collected from the water traps (Sherwood & Shires,
1981).
Two studies have been reported on the summer application of
cypermethrin for aphid control in winter wheat. In France, ground
applications of cypermethrin at 50 and 75 g a.i./ha were made in
June and effects on the fauna in the air, on the crop, and on the
soil surface were studied. Cypermethrin proved effective against
the phytophagous (pest) species. Effects on non-target organisms
were only minor, with the exception of spiders. In the second
study in the United Kingdom, cypermethrin was applied in June using
a fixed-wing aircraft at 25 g a.i./ha. Again, cypermethrin proved
effective against the pest species, while, generally, effects on
non-target organisms were minor and short-lived (Shires, 1982a,c).
Similar results have been obtained in studies on the aerial
application of cypermethrin at 25 g a.i./ha on oilseed rape and at
50 and 75 g a.i./ha on maize, in France (Inglesfield, 1982;
Garforth, 1983).
7.3.2.4. Foliar predators and parasites
The relative toxicity of cypermethrin for pests and their
parasites and predators is such that the balance between host/prey
and parasites/predators may not be adversely affected in the field.
However, care should be taken where predatory mites are important
in pest management. The high toxicity of cypermethrin for
predatory mites has been confirmed under field conditions (Wong &
Chapman, 1979; Aliniazee & Cranham, 1980; Shires & Tipton, 1982).
The effects of cypermethrin treatments, included in a spray
programme for cotton insect control, on white fly parasitism have
been studied in the Sudan. The treatment regime that gave the
consistently highest level of control of parasitism included
cypermethrin at 40 g a.i./ha in 4 early season sprays (Shires &
Tipton, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Leahey (1985) and Smith & Stratton (1986) have reviewed the
available literature on this subject.
8.1. Single Exposures
8.1.1. Oral
The acute oral toxicity of cypermethrin (mixture of cis-/ trans-
isomers) in experimental animals is of a moderate order (Tables 15,
16). Signs of intoxication, indicative of an action on the central
nervous system consist of sedation, ataxia, splayed gait, tip-toe
walk, with occasional tremors and convulsions. These signs of
toxicity appear within a few hours following dosing, and survivors
show clinical recovery within 3 days (Coombs et al., 1976).
Table 16 shows the effects of the ratio of cis-: trans-isomers
on the acute oral toxicity (FAO/WHO, 1980b).
Factors known to influence the oral LD50 value of cypermethrin
include concentration, vehicle, temperature, age of the animals,
and the animal strain used (Coombs et al., 1976; Dewar & Owen,
1979). The acute toxicity of cypermethrin was approximately 3
times greater in 3-week-old, than in 12-week-old rats (Rose &
Dewar, 1978). The cis-: trans-ratio plays a role in determining
acute toxicity, the cis-isomers being more toxic than the trans-
isomers. The oral LD50 of the cis-isomers in rats (Carworth Farm
E strain) was 229 mg/kg body weight while, with the trans-isomers,
no deaths were found at 2000 mg/kg body weight (Brown, 1979a,b).
8.1.2. Dermal
The acute dermal toxicity of technical cypermethrin is of a low
order. There were no deaths in CFE rats at any dose level tested
up to 1600 mg/kg body weight using 20% w/v xylene as the vehicle
(Coombs et al., 1976). The dermal LD50 for the cis-isomer of
cypermethrin, applied to the skin of rats as a 10% solution in
DMSO, was 219 mg/kg body weight (Brown, 1979a).
The acute dermal toxicity of cypermethrin for the rabbit
is > 2460 mg/kg body weight (US EPA 1984).
8.1.3. Intraperitoneal
The intraperitoneal (ip) LD50 (20% w/v solution in corn oil)
for technical cypermethrin in CFI mice is 485 mg/kg body weight
(Coombs et al., 1976). In rats, the LD50 is between 198 and 315
mg/kg body weight (administered as 5% solution in DMSO) (Price,
1981a).
Table 15. Oral LD50 values for technical cypermethrin
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
-----------------------------------------------------------------------
rat 5% in corn oil 251 (203 - 295) Coombs et al.
(1976)
rat 5% in dimethylsulf- 303 (277 - 329) Coombs et al.
oxide (1976)
rat 5% in dimethylsulf- 570 (485 - 823) Price (1981a)
oxide
rat 40% in dimethylsulf- approximately 4000 Rose (1982)
oxide
rat 5% in glycerol 200 - 400 (male); Coombs et al.
formal approximately 200 (1976)
(female)
rat 10% in aqueous 400 - 800 (male); Coombs et al.
suspension approximately 400 (1976)
(female)
rat 50% in aqueous 3423 (2815 - 4328)a Rose (1982)
suspension
mouse 5% in corn oil 82 (68 - 116) Rose (1982)
mouse 5% in dimethylsulf- 138 (105 - 199) Coombs et al.
oxide (1976)
mouse 50% in aqueous 657 (439 - 1003) Rose (1982)
suspension
Syrian 10% in corn oil approximately 400 Coombs et al.
hamster (male and female) (1976)
Chinese 5% in corn oil 203 (144 - 255) Coombs et al.
hamster (1976)
guinea- 20% in corn oil approximately 500 Coombs et al.
pig (male); > 1000 (1976)
(female)
bovine 15% formulation in 142 - 284 (male) Cassidy (1979)
calves Shellsol A
piglets 15% formulation in > 600 (male) Cassidy (1979)
Shellsol A
-----------------------------------------------------------------------
Table 15. (contd.)
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
----------------------------------------------------------------------
lamb 15% formulation in 283 - 567 (male) Cassidy (1979)
Shellsol A
domestic 40% in dimethylsulf- > 2000 Coombs et al.
fowl oxide (1976)
partridge 40% in dimethylsulf- > 3000 Coombs et al.
oxide (1976)
-----------------------------------------------------------------------
a 40:60 cis/trans.
Table 16. Effects of cis-: trans-ratio on the acute toxicity of
cypermethrina
------------------------------------------------------------------
Species Sex Vehicle cis-: trans-ratio LD50 (mg/kg)
------------------------------------------------------------------
rat male, dimethylsulfoxide cis-only 160 - 300
female
rat male, dimethylsulfoxide trans-only > 2000
female
rat female corn oil 90:10 367
rat female corn oil 40:60 891
------------------------------------------------------------------
a From: FAO/WHO (1980b).
8.1.4. Inhalation
Groups of CD rats (4 of each sex) were exposed for 4 h to an
aqueous spray of cypermethrin as a 400 g/litre emulsifiable
concentrate. The liquid phase contained 3 g cypermethrin/litre and
the atmospheric concentration of droplets in the vicinity of the
rats was calculated to be 0.7 mg/litre. The median droplet size
was 130 µm with a geometric standard deviation of 1.6 µm,
comparable with the droplet spectrum of a field-spraying device.
(It should be noted that this droplet size is nearly not
inhalable). The rats became thoroughly soaked during the exposure,
thereby indicating a significant dermal exposure with probable oral
exposure during post-exposure grooming. Immediately after the
exposure, the female rats showed the abnormal gait and urinary
incontinence typical of pyrethroid intoxication. Recovery took
place within 3 days. No deaths or other adverse signs occurred
during the exposure period or the subsequent 14-day observation
period (Blair & Roderick, 1976).
In another study on possible effects on the peripheral nerves,
rats (8 of each sex) were exposed for 4 h to an aqueous spray of
cypermethrin as a 400 g/litre emulsifiable concentrate under
conditions similar to those in the previous study. Half of the
rats were necropsied immediately after exposure and the others
after 66 h. Examination of the sciatic nerves from all of the rats
did not reveal any abnormalities, even though signs of intoxication
had been observed (Blair et al., 1976).
8.1.5. Skin and eye irritation
Technical cypermethrin was moderately irritant to occluded
rabbit skin (both intact and abraded) and to the eyes of New
Zealand white rabbits. In both cases, recovery was complete in a
few days. The severity of the irritation depended on the
formulation and the solvent used (Hine & Zuidema, 1970; Coombs et
al., 1976).
The skin on the backs of guinea-pigs was treated with
cypermethrin. The animals responded by licking, rubbing,
scratching, or biting the sides tested. This reaction may be
associated with skin sensory effects characterized by transient
itching and tingling sensations (Cagen et al., 1984).
8.1.6. Sensitization
A skin sensitization test on guinea-pigs (maximization
procedure of Magnusson & Kligman) indicated that technical
cypermethrin may have a mild skin sensitizing potential (Coombs et
al., 1976; US EPA, 1984).
8.2. Short-Term Exposures
8.2.1. Oral
8.2.1.1. Rat
Groups of 6 male and 6 female Charles River rats were fed diets
containing 0, 25, 100, 250, 750, or 1500 mg cypermethrin/kg feed
for 5 weeks. In the 1500 mg/kg group, reduced body weight gain and
food intake, piloerection, nervousness, incoordinated movement,
increased liver weight, and increases in blood urea and haemoglobin
concentrations were observed, but there were no pathological
changes. No changes were detected in the groups receiving 750
mg/kg or less (Coombs et al., 1976).
Groups of Charles River rats, 12 of each sex (24 of each sex as
controls), were fed dietary concentrations of 0, 25, 100, 400, or
1600 mg cypermethrin/kg feed for 3 months. Signs of intoxication,
such as hypersensitivity and abnormal gait, were observed in the
1600 mg/kg group, during the first 5 weeks, and one animal died.
The survivors showed clinical recovery in the second half of the
study. However, body weight gain was reduced, liver and kidney
weights increased, and there were increases in plasma-urea
concentrations and plasma-alkaline phosphatase activity, and
decreases in haemoglobin concentrations and the red blood cell
count in females, and in the kaolin cephalin clothing time in males
and packed cell volume were observed. Two out of 4 male rats,
killed prematurely, showed axonal breaks and vacuolization of
myelin in the sciatic nerve. None of the survivors showed nerve
lesions, and no other pathological effects were found. In the
400 mg/kg group, males showed increased kidney weights but no
histopathological changes. No effects were found in the 100 mg/kg
group (Hend & Butterworth, 1976).
Male and female rats (20 in each group) were fed cypermethrin
( cis-: trans- = 44:56) with a purity of 92% in the diet at levels
of 0, 75, 150, or 1500 mg/kg diet, for 90 days. Haematology and
the results of urinalysis were normal. Both males and females
receiving 1500 mg/kg diet showed reduced body weight and reduced
food consumption during the first month of the study. Increased
liver microsomal oxidase activity was noted in both males and
females in the 1500 mg/kg group and in the males in the 150 mg/kg
group. These changes were substantially reversed within the 4-week
recovery period. Gross and microscopic (including electron
microscopic) examination of the tissues and organs did not reveal
any signficant differences between the treated groups and the
controls. Examination of the sciatic nerve of animals in the
control and the 1500 mg/kg groups did not reveal any changes that
could be directly attributed to cypermethrin (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats per group and 14 of
each sex as controls) were fed trans-isomer at concentrations of
0, 30, 100, 1000, or 3000 mg/kg diet, for 5 weeks. No gross or
microscopic findings were observed and mortality did not occur.
Changes in several haematological parameters, alkaline phosphatase
activity, and liver, spleen, and kidney weight were observed at the
2 highest dose levels. Special histopathological studies of the
sciatic nerve did not show any damage (Hend & Butterworth, 1977a).
Groups of rats (6 male and 6 female rats per group and 10 of
each sex used as controls) were fed concentrations of the cis-
isomer at 0, 30, 100, 300, 750, or 1500 mg/kg diet for 5 weeks
(protocol similar to that described above). Mortality was observed
at the 1500 mg/kg level as well as neurotoxic signs of poisoning.
Growth was reduced at the 750 mg/kg level. Significant reductions
in food intake were also noted at doses of 300 mg/kg or more,
during the initial phase of the study. Relative kidney weights
showed a statistically-significant increase at doses of 300 mg/kg
or more and an increase in liver weight was observed at 750 mg/kg.
Histopathological examinations revealed substantial degeneration
in both the liver and sciatic nerve at 1500 mg/kg. No lesions were
observed in the brain or spinal cord (Hend & Butterworth, 1977b).
8.2.1.2. Dog
Beagle hounds (4 of each sex per dose level) were fed diets
containing 0, 5, 50, 500, or 1500 mg cypermethrin/kg diet for 13
weeks. At 1500 mg/kg, severe signs of intoxication consisting of
diminished food intake, weight loss, diarrhoea, anorexia, licking
and chewing of the paws, whole body tremors, a stiff exaggerated
gait, ataxia, incoordination, and hyperaesthesia were observed.
Half of the dogs in this group were necropsied because of severe
clinical signs of intoxication. However, no changes in
haematology, organ weight, or histopathology were observed and
despite the severe signs of intoxication, no lesions of the sciatic
nerves were observed in the dogs. No effects were seen at 500
mg/kg feed (Buckwell & Butterworth, 1977).
8.2.2. Dermal
8.2.2.1. Rabbit
Groups of 10 male and 10 female New Zealand white rabbits
received occluded dermal applications (abraded and non-abraded
skin) of 2, 20, or 200 mg cypermethrin/kg body weight in
polyethylene glycol (PEG 300) for 6 h per day, 5 days per week,
for 3 weeks. A control group was similarly treated with the
vehicle only (PEG 300). Slight-to-moderate skin irritation was
observed in rabbits receiving 2 and 20 mg/kg body weight; those
receiving 200 mg/kg body weight showed slight-to-severe irritation.
In the rabbits treated at 200 mg/kg, reductions were observed in
food intake, body weight gain, and weight of gonads. No other
changes were noticed. No such effects were found at 20 mg/kg body
weight (Henderson & Parkinson, 1981).
8.2.3. Intraveneous
8.2.3.1. Rat
Lock & Berry (1981) found biochemical changes in the rat
cerebellum after a single iv administration of 25 mg
cypermethrin/kg body weight. Signs of toxicity included:
salivation, clonic convulsions, and sinuous writhing movements.
The concentrations of cerebellar amino acids and cyclic nucleotides
were determined at these stages of toxicity. Treatment with
cypermethrin resulted in an increase in cerebellar cyclic GMP at
the earliest stage, without changing cyclic AMP. Levels of blood-
and cerebellar-glucose, lactate, and ammonia were also increased in
most of the stages of toxicity. No changes were seen in
concentrations of glutamate, glutamine, gamma-aminobutyrate,
creatine, phosphate, or ATP in the cerebellum. The increase in
cerebellar cyclic GMP did not appear to be related to the
convulsive state of the animal or to the muscarinic cholinergic
stimulation.
8.3. Long-Term Exposures
8.3.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) were fed dietary concentrations of 0, 1, 10, 100, or
1000 mg cypermethrin/kg diet for 2 years. Additional groups (6 or
12 rats of each sex per dose level) were fed the diets for only 6,
12, or 18 months.
Throughout the 2-year study, the control and cypermethrin-
treated animals were similar in terms of behaviour; no compound-
related clinical signs were observed, except for a significantly-
reduced growth rate in both males and females fed 1000 mg/kg diet.
Survival was not affected by cypermethrin. No clinical-chemical,
haematological, or histopathological changes were observed. The
sciatic nerves from a number of animals of all groups, sacrificed
after 6, 12, 18, or 24 months, showed a small number of nerve
fibres exhibiting Wallerian degeneration. The incidence increased
with age, but there was no difference in severity between the
control and treated groups. It is concluded that doses of 100 mg
cypermethrin/kg diet or less did not produce significant
toxicological effects in rats over a 2-year period (McAusland et
al., 1978).
Assays of liver microsomal enzyme activity in 6 rats of each
sex fed 0 or 1000 mg cypermethrin/kg feed for 2 years showed
cypermethrin to be a weak inducer of the microsomal enzyme hepatic
p-nitroanisole- O-dimethylase (PNOD), used as an index of
monooxygenase activity (Potter & McAusland, 1980).
Five groups of Wistar rats, each composed of 52 males and 52
females, were given diets containing 0 (2 groups), 20, 150, or 1500
mg/kg diet (equivalent to 0, 1, 7.5, and 75 mg/kg body weight) for
2 years. Satellite groups of 12 male and 12 female rats were
sacrificed at 52 weeks. For the first 6 weeks of the study, the
rats in the 1500 mg/kg group received 1000 mg/kg. The purity of
the cypermethrin was between 88% and 93% ( cis-: trans-ratio;
55:45). After 104 weeks at the highest dose level, body weight
loss, increased liver weight, increased smooth endoplasmatic
reticulum in hepatocytes, and some haematological and other slight
clinical changes were observed. No other changes or increase in
the incidence of tumours were found. The dose of 150 mg/kg diet
was considered to be the no-observed-adverse-effect level in this
study (only a summary is given) (US-EPA, 1984).
8.3.2. Mouse
The effects of cypermethrin were investigated in groups of 70
male and 70 female Swiss mice fed diets containing 0 (2 groups),
100, 400, or 1600 mg/kg feed (equivalent to 0, 15, 60, or 240 mg/kg
body weight) for up to 101 weeks. The purity of the cypermethrin
was between 91.5 and 94.2%; cis-: trans-ratio; 53:47 or 54:46.
Ten males and 10 females per group were killed at 52 weeks for
interim haematological evaluation. Appearance, behaviour, and
survival were similar in all groups. Body weight gain was reduced
in mice fed 1600 mg cypermethrin/kg diet compared with the combined
control groups. Food intake of both males and females was not
significantly changed. Several haematological changes, consistent
with a mild anaemia, were found in the 1600 mg/kg group of animals
at the interim kill (decreased haemoglobin, haematocrit, and red
blood cell counts in males; decreased mean cell volume and mean
cell haemoglobin concentration in females) but were not found at
termination. Thrombocytosis and increased liver weight were also
observed in this group, at both the interim and terminal kills.
There were no accompanying histopathological changes (for
carcinogenic potential, see section 8.7). No effects were observed
at dose levels of 400 mg/kg diet or less (Lindsay et al., 1982).
8.3.3. Dog
Cypermethrin (dissolved in corn oil) was administered to 4
groups of 8 beagle dogs of each sex at dose levels of 0, 1, 5, or
15 mg/kg body weight per day, by capsule, for a period of 52 weeks.
The purity of the cypermethrin was 90.6% ( cis-: trans-ratio;
54:46). The dogs in the highest dose group exhibited loss of
appetite, tremors, gait changes, incoordination, disorientation,
and hypersensitivity. No other abnormalities were found in the
composition of the blood or urine or in organ weights. Dogs
receiving 5 mg/kg or more showed liquid stools by this mode of
administration. However, this effect was not found when
cypermethrin was fed in the diet for 2 years. No effects were seen
at 1 mg/kg (but only a summary is given) (US-EPA, 1984).
Groups of 4 male and 4 female Beagle hounds were fed diets
containing 0, 3, 30, or 300 mg cypermethrin/kg feed for 2 years.
An additional group received 1000 mg/kg feed but, because of severe
intoxication signs, the dose was decreased to 750 mg/kg. The signs
of intoxication consisted of licking and chewing of the paws, a
stiff high-stepping gait, whole body tremors, head shaking,
incoordination, ataxia, and convulsions. After 3 weeks of feeding
at 750 mg/kg, the animals received the control diet, until signs of
intoxication could no longer be seen. The dogs were then fed a
diet containing 600 mg cypermethrin/kg from week 8 until
termination at 2 years. No signs of intoxication were observed in
dogs fed diets containing 0, 3, 30, or 300 mg/kg during the course
of the study. There was reduced body weight gain in male dogs in
the 600 mg/kg group. Clinical chemistry and haematological
investigations, performed at 6-week intervals for 2 years, did not
reveal any consistent differences between treated groups and
controls. The minor changes in absolute organ weight observed in
the brain and thyroid in the 300 mg/kg group were not present when
the differences were corrected for terminal body weight.
Furthermore, a dose relationship was not apparent, and there were
no accompanying histopathological changes. Opthalmological
observations performed during the course of the study did not
reveal any ocular differences between treated groups and control.
No abnormalities were found in the sciatic nerves, brain, or spinal
cord in any of the treated groups. The feeding of diets containing
up to 600 mg cypermethrin/kg diet to dogs for 2 years did not
reveal any treatment-related gross or histopathological effects,
although the dogs receiving diets containing 1000 mg/kg decreased
to 600 mg cypermethrin/kg showed reduced body weight gain.
Cypermethrin did not produce any toxicological effects in dogs fed
dietary concentrations of 300 mg/kg feed or less, over 2 years
(Buckwell, 1981).
8.4. Special Studies
8.4.1. Synergism/potentiation studies
8.4.1.1. Organophosphate mixture
In a study on rats, designed to determine whether an
organophosphate insecticide, monocrotophos, potentiated the
neurotoxic effect of cypermethrin, 5 groups of 7 or 8 male and 7 or
8 female rats were given 7 consecutive oral doses of monocrotophos
(100 g/litre), cypermethrin (25g/litre), or a mixture of
monocrotophos and cypermethrin (100 g/litre and 25 g/litre,
respectively). Signs of intoxication and neurotoxic effects
(biochemical changes consistent with very sparse axonal
degeneration in sciatic/tibial nerves) were found to be wholly
attributable to the monocrotophos component of the mixture, and
there was no evidence of potentiation of neurotoxic effects (Rose &
Dewar, 1979a).
8.4.1.2. Organochlorine mixture
The oral and dermal toxicities of cypermethrin and endosulfan
for rats were determined both individually and combined. No
evidence for enhancement of toxicity was found for either
cypermethrin or endosulfan when administered as a 1:1 mixture in
corn oil (Price, 1981b).
8.4.2. Neurotoxicity
8.4.2.1. Characterization of the neurotoxic effects
During preliminary short-term studies on cypermethrin, 2
observations were made:
(a) high doses of cypermethrin given orally to rats
caused an unusual gait in intoxicated animals; and
(b) at lethal or near-lethal dermal or oral doses,
histopathological changes (swelling and/or
disintegration of axons of the sciatic nerve of
rats) were observed; such observations have been
reported to occur with other synthetic pyrethroids
(Okuno et al., 1976a) and natural pyrethrins (Okuno
et al., 1976b).
As a consequence of these findings, an extensive programme of
work has been undertaken to characterize the neurotoxic effects of
cypermethrin.
8.4.2.2. Neuropathological studies
(a) Rat
Single oral doses of cypermethrin in corn oil were given to
groups of 6 - 12 rats of each sex at 100, 200, or 400 mg/kg body
weight. All rats showed signs of intoxication, which varied in
severity according to the dose level. At 400 mg/kg, severe
clinical signs were seen within 2 days of dosing. Most of the
animals showed swelling of the myelin sheaths and breaks of some of
the axons of the sciatic nerves. At 200 mg/kg, 8 rats of each sex
died or were killed within 48 h of dosing, and the remaining 4 of
each sex survived the 9 days of the study. Nearly half of the
animals showed lesions of the sciatic nerve. At 100 mg/kg, all
rats survived 9 days of the trial. Only one female out of 12
showed minimal lesions of the sciatic nerve. Neuropathological
changes were found in a number of animals dosed with 200 mg
cypermethrin/kg body weight or more. Dose-related increases in
signs of intoxication and neuropathy were observed; however,
neuropathy was not detected in all the animals showing signs of
intoxication (Carter & Butterworth, 1976).
Groups of rats (10 males per group) were fed dietary levels of
cypermethrin ( cis-: trans- = 45:55) at 0, 1250, 2500, or 5000
mg/kg diet for 14 days. Mortality was observed at the 2 higher
doses and growth inhibition was seen in all treated groups.
Clinical signs of neurotoxicity were characterized by an impaired
ability to walk, splayed hind limbs, ataxia, and paralysis. Other
clinical signs were hypersensitivity, gross disorientation, and
convulsions. The neurotoxic signs of poisoning observed at 1250
mg/kg diet were reversible. Remission of ataxia at 2500 mg/kg diet
was also noted. Ultrastructural changes in the sciatic nerve were
observed in a small number of animals at the 2 highest dose levels.
Some evidence of axonal damage in the myelinated nerves was
observed, mainly in these groups (Glaister et al., 1977a).
In another study, rats were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg diet for 2 years (section 8.3).
At the 12-month interim kill, part of the sciatic nerve was removed
from 6 males and 6 females in both the control and 1000 mg/kg
group. The dissected nerves were divided and teased into a fan
shape, allowing each fibre to be examined. No significant
difference was found in the incidence of abnormal nerve fibres in
the control and the 1000 mg/kg group (Trigg et al., 1977; McAusland
et al., 1978).
(b) Hamster
Groups of 6 male and 6 female Syrian hamsters were given single
oral doses of cypermethrin in corn oil at 0, 794, 1000, and 1260
mg/kg body weight. Signs of intoxication (generally tiptoe
walking, tremors, irregular movements) occurred in all groups
receiving cypermethrin. Animals died or were killed at times
varying from a few hours to 9 days after dosing. Of the 31 animals
of the different test groups that were examined, 3 had nerve
lesions, axonal swelling, and axonal breaks in the sciatic and
posterior tibial nerves (many animals died within a few hours)
(Butterworth & Clark, 1977).
(c) Chicken
In a delayed neurotoxicity study on 6 adult domestic hens,
cypermethrin in DMSO did not cause histological lesions in the
nervous system (brain, spinal cord, and sciatic nerves) or signs of
intoxication at an oral dose of 1000 mg/kg body weight per day for
5 days, compared with a positive control group. No delayed
neurotoxicity was observed (Owen & Butterworth, 1977).
8.4.2.3. Biochemical and electrophysiological studies
Biochemical and electrophysiological studies have been carried
out because the sparse axonal degeneration described above is
difficult to demonstrate by conventional histopathological
techniques. Furthermore, evidence was required to quantify the
neurological dysfunction attributable to cypermethrin. The
rationale for measuring the activities of beta-glucuronidase and
beta-galactosidase in conjunction with an assessment of "mean slip
angle" and "mean landing foot spread" as markers for axonal
degeneration has been described by Dewar (1977a,b).
(a) Rat
Using biochemical markers, it has been shown that, at high oral
doses of cypermethrin, sparse axonal degeneration occurs in the
sciatic/posterior tibial nerves (Dewar, 1977b; Rose & Dewar, 1983),
and the trigeminal nerve and trigeminal ganglion of Wistar rats
(Dewar & Moffett, 1978a).
Three studies involving repeated oral administration of
cypermethrin to rats at 150 mg/kg body weight for 5 or 7 days and
0, 25, 50, 100, or 200 mg/kg body weight for 5 or 7 days have been
carried out. Mortality occurred from 100 mg/kg body weight
onwards. A dose-related transient functional impairment, assessed
by means of the inclined plane test, was found in the first week.
Significant increases in beta-glucuronidase or beta-galactosidase
activity of the sciatic, tibial, or trigeminal nerves only occurred
with 5 or 7 doses of 150 or 200 mg/kg body weight. The sensitivity
of these nerves is comparable. Complete functional recovery
occurred within 26 days. Increased activity of the enzymes in the
distal portion of nerves was found but, even in the most severely
intoxicated animals, the magnitude of the increases was less than
that induced by the known neurotoxic agent methylmercury chloride
(Dewar, 1977b; Dewar & Moffett, 1978a; Rose & Dewar, 1983).
Biochemical changes, consistent with primary axonal
degeneration, were detected in rats aged 3, 6, or 12 weeks.
Cypermethrin was more acutely toxic for 3-week-old rats than for
12-week-old rats and the dose required to cause a sparse axonal
degeneration, as demonstrated by biochemical changes, was
proportionally smaller for the younger rats (Rose & Dewar, 1978).
The time-course for development and recovery from axonopathy
was investigated in groups of rats (5 of each sex) at periods of
2 - 12 weeks after the start of dosing. The daily doses were 150
mg/kg body weight for the first 11 doses and 100 mg/kg body weight
for the subsequent 9 doses, over a period of 4 weeks. More than
half of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication, characterized by abnormal
gait, ataxia, salivation, lethargy, chromodacryorrhoea,
piloerection, and hypersensitivity to external stimuli. Maximum
enzyme activities in the sciatic posterior tibial nerves were found
after 5 weeks and had returned to control values by 12 weeks. In a
second phase of the study, 10 male and 10 female rats were given
20 oral doses of cypermethrin at 37.5, 75, or 150 mg/kg body weight
per day, (concurrent control group dosed with DMSO), over a 4-week
period. The animals given 75 and 150 mg/kg showed clinical signs
of intoxication. Mortality also occurred at the highest dose
level. The behaviour and appearance of animals in the 37.5 mg/kg
body weight per day group were similar to those of the controls.
Five weeks after the initial dose, biochemical changes, which were
indicative of a mild axonal degeneration, were observed in animals
in the highest dose group. No consistent or biologically
significant neurobiochemical changes were found in the 37.5 or 75
mg/kg body weight groups. The results of this study demonstrated
that 20 oral doses of cypermethrin at 75 mg/kg body weight per day
administered over a 4-week period, produced mild clinical signs of
intoxication; however, no biochemical changes which were indicative
of peripheral neuropathy were seen (Rose, 1983).
Further evidence to support the minor nature of the nerve
lesions has been afforded by electrophysiological studies on rats.
In these studies, measurements of the maximal motor conduction
velocities and conduction velocity of slower fibres in the sciatic
and tail nerves of Wistar rats were made before, and at intervals
of up to 5 weeks after, exposure to single (200 mg/kg body weight)
or repeated doses (7 doses of 150 mg/kg body weight) of
cypermethrin. Even at near-lethal doses, cypermethrin did not
cause any effects on these parameters (Dewar & Deacon, 1977).
The ability of the major metabolite of cypermethrin, PBA, to
produce axonal changes has been investigated. Four groups of 8
male and 8 female rats given 7 consecutive daily oral doses of 0,
25, 77, or 375 mg/kg body weight did not show any biochemical
changes indicative of peripheral nerve damage. The sparse axonal
degeneration observed with high doses of cypermethrin is,
therefore, not caused by PBA (Rose & Dewar, 1979b).
(b) Hamster
In 3 studies, Chinese hamsters were given oral doses of
5 - 30 mg cypermethrin/kg body weight per day, for 5 consecutive
days. Clinical signs of intoxication were observed and tests
made to assess whether the compound produced degenerative changes
in peripheral nerves and sensory ganglia. Beta-glucuronidase,
and beta-galactosidase activities were measured in the
sciatic/posterior tibial nerves and trigeminal ganglion. A
positive control group received 7.5 mg methylmercury chloride/kg
body weight per day, for 5 consecutive days.
The most striking feature in the hamsters treated with doses
exceeding 5 times 20 mg/kg body weight was a marked loss of fur in
the facial area, neck, and back, due to repeated scratching. The
results of the enzyme determinations suggest that the Chinese
hamster like the rat developed sparse axonal degeneration following
oral doses in excess of 5 times 10 mg/kg body weight (Dewar &
Moffet, 1978b)
(c) Rabbit
Rabbits were administered capsules with 0 (corn oil), 75,
150, or 300 mg cypermethrin (93.5%)/kg body weight in corn oil,
5 times a week, for 6 weeks. At the end of the 6th week,
electroencephalographic records were taken. The waves of the
complex EEG activity as well as the performance of the 6
constituting bands of different frequency were computer analysed.
No significant alterations were found (Desi et al., 1986a).
8.4.2.4. Appraisal
Repeated oral administration of cypermethrin to rats, at doses
sufficiently high to produce significant mortality in a group of
animals, produced biochemical changes in peripheral nerves that
were consistent with sparse axonal degeneration. The magnitude of
the biochemical changes was similar to that of changes produced
with either a single (Dewar, 1977b) or repeated doses of shorter
duration (Dewar & Moffet, 1978a), thus demonstrating a lack of
cumulative effect. The magnitude of change was substantially less
than that encountered with established neurotoxic agents and the
site of maximal enzyme change (distal portion of the nerve)
suggests that the degeneration is more likely to be of a Wallerian
type rather than segmental demyelination. The short time required
to produce ataxia and/or abnormal gait rules out a causal
relationship between ataxia and axonopathy. The clinical signs are
consistent with a pharmacologically-mediated effect whereas the
axonopathy is a minor reversible lesion that occurs several days
after exposure and has also been detected in animals that do not
show clinical signs.
Histopathological lesions of some fibres of the sciatic nerve
have only been demonstrated in rats or hamsters receiving extremely
high, lethal or near lethal, oral doses of cypermethrin (Carter &
Butterworth, 1976; Butterworth & Clark, 1977). Compound related
histopathological changes have not been observed in rats exposed to
cypermethrin through inhalation (Blair et al., 1976) or in rats fed
diets containing 1000 mg cypermethrin/kg over a 1 - 2 year period
(Trigg et al., 1977; McAusland et al., 1978). Furthermore, dogs
fed 1500 or 600 mg cypermethrin/kg diet for 3 or 24 months,
respectively, did not have any compound-related lesions of the
sciatic nerve (Buckwell & Butterworth, 1977; Buckwell, 1981).
The results of biochemical studies, used as sensitive
indicators of change, confirmed that minor changes that occur after
massive exposure to cypermethrin are reversible and are not
necessarily associated with clinical signs (Dewar, 1977b; Dewar &
Moffet, 1978a; Rose, 1983). There was no direct correlation
between the time course of the neuromuscular dysfunction and the
neurobiochemical changes (Rose & Dewar, 1983). The metabolite of
cypermethrin, PBA, did not produce any biochemical changes in
peripheral nerves (Rose & Dewar, 1979b).
8.4.3. Immunosuppressive action
Stelzer & Gordon (1984) studied the in vitro effects of
pyrethroids on the mitogenic responsiveness of murine splenic
lymphocytes to concornavalin A and lipopolysaccharide. The results
of these studies showed that at concentrations of the order of 1 -
10 µmol/litre, inhibition of murine splenic lymphocytes to both B
and T cell mitogens was found. These results support the
possibility of immune suppression by pyrethroid (cypermethrin)
exposure.
Desi et al., (1985, 1986a) studied the short-term effects of
6.25, 12.5 or 25 mg cypermethrin (93.5%)/kg body weight in rats, on
the immune system by measuring the autologous rosette formation of
T-lymphocytes and determining the ovalbumin titre. These studies
were performed over a period of 6 or 12 weeks.
The humoral immune response after vaccination with Salmonella
typhimurium and the cell-mediated immune response were determined
in rabbits. The antibody titre was measured by tube-agglutination
and complement-binding tests and the tuberculin skin test was used
for the immune response test. The dose levels in this study were
75, 150, or 300 mg/kg body weight, for 7 weeks. A significant
dose-dependent decrease was observed in both the anti-ovalbumin
titre of the blood-serum and in the autologous rosette formation of
the T-lymphocytes. In rabbits, the 2 highest dose levels induced a
significant dose-dependent decrease in serum antibody titres and
the tuberculin skin test showed the same dose-dependent tendency.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
Cypermethrin was fed to Wistar rats (30 male and 30 female per
group) at dietary concentrations of 0, 10, 100, or 500 mg/kg for 5
weeks, after which the males and females (10 weeks of age) from
each treatment group were mated. Two successive litters were
produced from each pair. The first of these litters was discarded,
and randomly-selected male and female pups of the second litters
were mated to produce the next generation. The study was continued
until 2 litters from each of 3 successive generations had been
bred. The parent animals in the 500 mg/kg group consumed less food
than the controls and this was accompanied by a reduction in body
weight. Otherwise, the parent animals of control and treatment
groups behaved similarly. Cypermethrin did not cause any adverse
effects on the reproductive performance of the rats or on the
survival of the offspring. No consistent changes were observed in
mean litter weight between birth and weaning in any treatment
group, with the exception of a reduction in total litter weights in
the 500 mg/kg F1a litters on days 4, 14, and 21. There was also a
statistically-significant decrease compared with controls in total
litter weights and size in the F1b litters at 500 mg
cypermethrin/kg diet. In the 500 mg/kg Fo group, one animal showed
a squamous cell carcinoma of the skin. However, this was
considered not to be related to the compound, because no increase
in tumour incidence was found in the long-term studies on rats and
mice. No changes were observed in rats administered 100 mg/kg diet
(Hend et al., 1978).
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1. Rat
Groups of pregnant female Sprague Dawley CD rats (25 animals
per group) were administered cypermethrin orally as a 1% solution
in corn oil at doses of 0, 17.5, 35, or 70 mg/kg body weight per
day, from days 6 to 15 (inclusive) of gestation. Cypermethrin at
17.5 mg/kg body weight per day did not affect maternal performance
or fetal survival and development. At the higher doses of 35 and
70 mg/kg body weight, respectively, slight and significant
retardation of maternal body weight gain was recorded. In
addition, at 70 mg/kg per day, slight to severe neurological
disturbances were observed in nearly half of the females including:
slight splaying of the hind legs while walking ranging to severe
splaying of all limbs, involuntary movements of the jaws,
convulsive spasms, and hypersensitivity to noise. Despite this
maternal toxicity, there were no indications of any embryotoxic or
teratogenic effects of cypermethrin (Tesh et al., 1978).
8.5.2.2. Rabbit
Groups of pregnant female Banded Dutch rabbits (30 controls and
20 for each dose group) were dosed orally with 0, 3, 10, or 30 mg
cypermethrin/kg body weight per day in corn oil (by gelatin
capsule) during days 6 - 18 (inclusive) of gestation. No influence
was found on growth, pre-implantation losses, resorptions, fetal
deaths, or numbers and sizes of fetuses. The incidence of fetal
visceral and/or skeletal abnormalities was comparable to that in
the vehicle control group, except for a slight increase in the mean
percentages of fetuses showing visceral and/or skeletal
abnormalities in the group receiving 30 mg/kg body weight per day.
No teratogenic effects were found in this study (Dix, 1978).
8.6. Mutagenicity and Related End-Points
8.6.1. In vitro studies
8.6.1.1. Microorganisms
Using bacterial assays, no increases in the reversion rates of
Escherichia coli WP2, E. coli WP2 uvrA, Salmonella typhimurium
TA 1535, TA 1537, TA 1538, TA 98, and TA 100 were detected with
cypermethrin (concentrations of up to 2 mg per plate) in the
presence or absence of a rat liver microsomal activation system.
Exposing Saccharomyces cerevisiae JD1 in liquid culture to
cypermethrin at concentrations of up to 5 mg/ml, both in the
presence and absence of a rat liver microsomal activation system,
did not result in any increase in the rate of mitotic gene
conversion (Brooks, 1980).
In a study in which cypermethrin was tested for mutagenicity in
Salmonella typhimurium TA 100 or TA 98, in the presence or absence
of a rat liver activation system, using the plate incorporation
assay, concentrations of up to 1 mg/plate gave negative results.
The same was true with the fluctuation test, with concentrations of
up to 10 µg/ml (Pluymen et al., 1984).
8.6.1.2. Mammalian cells
Cypermethrin was tested for mutagenicity in V79 Chinese hamster
cells. No cytotoxicity was observed when the assay was carried out
in the presence of rat hepatocytes. Cypermethrin was found not to
be mutagenic for either genetic locus (OUA' and TG') in V79 cells,
when tested in concentrations up to 20 µg/ml, in the presence or
absence of rat hepatocytes (Pluymen et al., 1984).
8.6.2. In vivo studies
8.6.2.1. Host-mediated assay
There was no increase in the rate of mitotic gene
conversion in Saccharomyces cerevisiae JD1 harvested from male
mice following a single oral dose of cypermethrin at 25 or 50 mg/kg
body weight for 2 days (Brooks, 1980).
8.6.2.2. Dominant lethal assay
No evidence of dominant lethality was found when male CD1 mice
(3 groups of 12 animals and a control group with 36 animals) were
given single oral doses of 6.25, 12.5, or 25 mg cypermethrin/kg
body weight. Two other groups were given 5 consecutive daily oral
doses of 2.5 or 5 mg/kg body weight (controls received the vehicle
DMSO). A significant reduction in fetal implants during the second
week of mating was noted and a marginal increase in early fetal
deaths was observed at 5 mg/kg per day. This effect was not
confirmed in a second study using oral doses of 2.5, 5, 7.5, or
10 mg/kg body weight for 5 consecutive days, when no effects were
found on reproductive capability or the histopathology of the
testes and epididymis. The marginal differences found in the
5 mg/kg per day group in the second study were considered not to
be related to the compound.
In summary, cypermethrin did not show detectable dominant
lethality after administration of doses of up to 10 mg/kg body
weight per day, for 5 days, or as a single oral dose of 25 mg/kg
body weight (Dean et al., 1977).
8.6.2.3. Bone marrow chromosome study
Chinese hamsters (12 of each sex per group) were orally dosed
with 20 or 40 mg cypermethrin/kg body weight, in DMSO, for 2
successive days. The incidence of chromosome abnormalities in
bone marrow cells, 8 and 24 h after dosing, did not differ from
that in the DMSO control animals. The positive control group
(100 mg/kg cyclophosphamide) showed many chromosomal aberrations
(Dean, 1977).
The effects of cypermethrin on sister chromatid exchange were
studied in the bone marrow cells of 3-month-old mice. Cypermethrin
was injected subcutaneously at 0.75, 1.5, or 3 mg/kg body weight
or 2.5, 5.0, or 10 mg Ripcord/kg body weight. Both the technical
and the formulated products showed a dose-related increase in
sister chromatid exchanges in the dividing cells at all dose
levels. The highest doses of both cypermethrin and Ripcord
completely inhibited mitotic division (Seehy et al., 1983).
8.6.2.4. Micronucleus test
The induction of micronuclei was studied in mouse bone marrow.
Cypermethrin was administered by 3 routes: intraperitoneal, oral,
and dermal. Cypermethrin showed mutagenic potential after oral
administration of 900 mg/kg diet for 7 and 14 consecutive days. No
effects were seen after ip administration of a single injection of
60 or 180 mg/kg body weight or double and triple injections of 60
mg/kg body weight.
Up to 4 dermal treatments with 360 mg cypermethrin/kg body
weight resulted in a significant increase in the frequency of
polychromatic erythrocytes with micronuclei (Amer & Aboul-Ela,
1985).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) (section 8.3) were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg for up to 2 years in a combined
long-term/carcinogenicity study. No evidence for carcinogenicity
was found in this study (McAusland et al., 1978) (section 8.3.1).
No increase in tumour incidence was seen when Wistar rats were
given diets containing 0, 20, 150, or 1500 mg cypermethrin/kg diet
(equivalent to 0, 1, 7.5, or 75 mg/kg body weight) for 2 years.
The purity of the cypermethrin ( cis-: trans- ratio; 55:45) was
between 88% and 93% (section 8.3.1) (US EPA, 1984).
8.7.1.2. Mouse
Swiss mice (70 male and 70 female) were fed diets containing 0
(2 groups), 100, 400, or 1600 mg cypermethrin/kg feed for up to 101
weeks. Effects consisted of increased liver weights at 400 and
1600 mg/kg diet and decreased body weight, thrombocytosis, and mild
anaemia at 1600 mg/kg diet (section 8.3.2).
There were no compound-related changes in non-neoplastic
histopathology or increases in tumours of types that are not
commonly associated with the mouse strain used. The incidence of
tumours was similar in all groups with the exception of a slightly
increased incidence of benign alveolar lung tumours in the females
in the 1600 mg/kg group. However, the magnitude of this increased
incidence was insufficient when compared with concurrent and
historical control incidence, to warrant concern. There was no
evidence for a decreased latency of benign alveolar lung tumours in
the female mice receiving the highest dose, and this tumour type
was not accompanied by any increase in malignancy. There was also
no evidence of a carcinogenic response in the male mice in this
study. Furthermore, benign alveolar lung tumours are know to occur
in both sexes at a high and variable incidence in this strain of
mouse. Therefore, it is considered that the occurrence of benign
alveolar lung tumours in the female mice receiving the highest dose
was not related to treatment with cypermethrin. Feeding
cypermethrin at levels of up to 1600 mg/kg diet to mice for a life-
time did not produce any evidence of carcinogenicity (Lindsay et
al., 1982).
8.8. Mechanisms of Toxicity - Mode of Action
In the classification in the Appendix, cypermethrin is
classified among the Type II compounds, which cause toxic signs of
choreoathetosis with salivation (CS-syndrome) in the rat
(Verschoyle & Aldridge, 1980) and bursts of spikes in the cercal
motor nerve of the cockroach (Gammon et al., 1981). Pyrethroids
having alpha-cyano-3-phenoxy-benzyl alcohol, such as cypermethrin,
do not induce repetitive firing in the cercal sensory nerves of the
cockroach in vivo or in vitro, but cause different signs including
a pronounced convulsive phase (Gammon et al., 1981).
The intravenous toxicity of 1RS- cis-, and 1RS- trans-
cypermethrin for the rat has been examined. Both compounds cause
the CS-syndrome, which is characterized by initial pawing and
burrowing behaviour, followed within 2 - 5 min by profuse
salivation, coarse whole-body tremor, increased startle response
and abnormal locomotion involving the hind limbs. The coarse
tremor progresses into sinuous writhing (choreoathetosis) of the
whole body, which gradually becomes more violent and is enhanced by
sensory stimuli. Clonic seizures are occasionally observed as a
terminal event. No increase in core temperature occurs and, in
fact, it may fall. In the electroencephalogram (EEG) during the
CS-syndrome, poisoned rats showed generalized spike and wave
discharges, prior to choreoathetosis (Verschoyle & Aldridge, 1980).
The primary site of action resulting in the CS-syndrome has not yet
been decided.
It has been suggested (Van den Bercken, personal communication,
1981) that the facial skin sensations experienced by people who
handle cypermethrin or other pyrethroids (section 9.2.2) are
brought about by repetitive firing of sensory nerve terminals in
the skin. In the opinion of the author, this is a strictly local
effect that may occur as soon as the pyrethroid concentration on or
in the skin reaches a certain level and is not a sign of general
intoxication. It is considered by the author that the occurrence
of these skin sensations are a warning signal indicating that
exposure has occurred. No undue hazard is likely, provided the
pyrethroid does not reach the blood in any significant
concentration. In this connection, it was stressed that, although
cypermethrin is among the least toxic insecticides when
administered orally, it may become highly toxic, whenever it
reaches the nervous system in sufficient concentrations.
The question remains whether there is a causal relation between
the intense repetitive activity induced by alpha-cyano pyrethroids
(of which cypermethrin is an example) and the nerve damage that
occurs in experimental animals after prolonged exposure to nearly
lethal concentrations of these compounds. Because of the large
number of different chemicals, most of which do not cause
repetitive activity in the nervous system but are known to cause
serious nerve damage, such a correlation is hardly expected.
Furthermore, the extensive literature on DDT (which causes the same
type of repetitive activity in sense organs and in sensory nerve
terminals as the non-cyano pyrethroids) does not contain any
indication that this insecticide may cause axonal degeneration.
However, in theory, there is a possibility that repeated occurrence
of intense repetitive firing will eventually lead to dysfunction of
sensory nerve terminals and sense organs, and, finally, to
degeneration of sensory nerve fibres (Van den Bercken, personal
communication, 1981; Van den Bercken & Vijverberg, 1980a).
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity: poisoning incidents
One report of accidental intoxication has been described. A
family who ate food cooked in 10% cypermethrin developed nausea,
prolonged vomiting with colicky pain, tenesmus, and diarrhoea
within minutes of ingestion. One male adult had convulsions,
passed into coma, and died due to respiratory paralysis. The
symptoms were less severe in other members of the family (Poulos et
al., 1982). There is some doubt whether this was a cypermethrin
intoxication (Suthers, personal communication).
9.1.2. Controlled human studies
Flannigan & Tucker (1985) studied the differences in the extent
of paraesthesia induced by a number of pyrethroids. Field strength
formulated cypermethrin (0.13 mg/cm2) was applied (0.05 ml) to a
4 cm2 area of the earlobe of volunteers on 5 occasions. Distilled
water was applied to the opposite earlobe. Participant evaluation
after each application continued for 48 h and involved description
of the cutaneous sensations. Each participant was treated after
each application with one of the other pyrethroids. Cypermethrin
(as the other pyrethroids) induced sensation. The paraesthesia
developed with a latency period of approximately 30 min, peaked by
8 h and deteriorated as early as 24 h. Dl-alpha tocopheryl acetate
markedly inhibited the occurrence of the paraesthesia.
9.1.3. Epidemiological studies
No information is available.
9.2. Occupational Exposure
9.2.1. Acute toxicity: poisoning incidents
No information is available.
9.2.2. Effects of short- and long-term exposure
Laboratory workers and field operators handling natural and
synthetic pyrethroids, including cypermethrin, have noticed a
transient sensation (described as "tingling" or "burning"
sensations) of the skin in the periorbital area of the face and of
other sites after direct skin exposure. The skin sensations have
been interpreted (section 8.8) as being caused by spontaneous
repetitive firing of local sensory nerve fibres or nerve endings,
with thresholds that have been transiently lowered by the compound
(Wouters & Van den Bercken, 1978). There is a delay of about
30 min before onset of these symptoms following pyrethroid
exposure; the sensation generally lasts only a few hours and does
not persist for more than one day after exposure (Van den Bercken &
Vijverberg, 1980a, Le Quesne et al., 1980).
In a clinical and electrophysiological assessment of these skin
sensations (paraesthesia), 23 laboratory workers, who had been
exposed to synthetic pyrethroids during the preceding months, were
examined. Nineteen of the 23 workers had experienced at least one
or more episodes of facial sensations. Neurological signs were not
observed and electrophysiological studies of selected motor and
sensory nerves in the legs and arms of the 23 subjects showed no
abnormalities (Le Quesne et al., 1980).
Field studies have been performed in the Côte d'Ivoire in which
cypermethrin was sprayed on cotton using a hand-held, ultra-low-
volume (ULV) commercial spraying machine. This method of
application represented a situation of high potential exposure.
The first study, in which 17 workers were involved, concerned a
single exposure to cypermethrin (Prinsen & Van Sittert, 1978),
while a second study was conducted in which 7 workers took part in
7 consecutive spray sessions (Prinsen & Van Sittert, 1979). Skin
exposure to cypermethrin was assessed by quantitative measurement
of the compound deposited on aluminium foil pads on the body of the
sprayer during the spraying operation. The rate of dermal exposure
of the operators during spraying ranged from 1.5 to 46.1 mg/h.
Total absorption of cypermethrin in the body was monitored by
urinary analysis for 2,2-dichlorovinyl-3,3-dimethyl-cyclopropane-
1-carboxylic acid, a metabolite of cypermethrin. In most of the
samples, the excretion was below the limit of detection
(0.05 mg/litre), indicating a low level of absorption. It was
estimated that approximately 3% of the total dermal dose was
absorbed. General medical, extensive clinical blood chemistry, and
electrophysiological examinations were carried out of selected
motor and sensory peripheral nerves in the legs and arms, of the
trigeminal nerve, and of the facial nerve. No effects were found.
In another field study in India, 18 workers, including spraymen
(spraying an emulsifiable concentrate formulation by mist-blower or
knap-sack), mixers, and loaders, handled cypermethrin daily for 5
consecutive days. Medical examination, with special attention to
the sensory function of the peripheral nervous system, was carried
out before, during, and after spraying (Suthers & Marlow, 1981).
No compound-related adverse clinical effects of peripheral
neuropathy were noticed in any of the workers. The urinary
excretion of the cypermethrin metabolite (methyl ester of the
cyclopropane carboxylic acid moiety) increased from day 1 to 5 of
the study, but decreased 24 h after spraying had ceased. On the
fifth day, concentrations of up to 0.18 mg (average 0.1 mg) were
found in the 24-h urine of workers using the mist-blower.
Concentrations were lower in workers using the knap-sack sprayer.
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
A field study was carried out on Indian workers in the Satara
district in India prior to, during, and after spraying cotton with
the synthetic pyrethroid formulations, permethrin (Ambush) and
cypermethrin (Cymbush). The formulations were applied using a
mist-blower or knap-sack spray. Exposure of 7 spraymen and 2
loaders/mixers was monitored by measuring the 24-h urinary
excretion of 3-(4'-hydroxyphenoxy)-benzoic acid, and medical
assessments were carried out by 4 medical doctors. The sensory
function of the peripheral nervous system was also assessed. The
formulations were applied at the recommended rates; Ambush, 150 g
a.i./ha and Cymbush, 70 g a.i./ha. Average exposure was
approximately 8 h per day for 5 days. No compound-related adverse
clinical effects were noticed. The average urinary excretion of
3-(4'-hydroxy-phenoxy)-benzoic acid increased from day 1 to day 5,
but then decreased 24 h after spraying had ceased (Hart et al.,
1982).
In another study, two agricultural pilots and five
mixer/loaders were monitored for dermal exposure during aerial
"ultra-low-volume" (ULV) applications of cypermethrin in vegetable
oil to cotton. This study was conducted in Greenwood, Mississippi.
The mixer/loaders wore protective equipment. The actual dermal
exposure for pilots was 0.67 mg/8 h and for mixer/loaders 2.43
mg/8h. The exposure of pilots was predominantly of the hands,
whereas that of mixer/loaders was more uniform (hands were
protected). The urinary excretion of cypermethrin metabolites was
very low; between 4 and 22 µg cypermethrin equivalents/day. This
study demonstrates that exposure of pilots and mixer/loaders during
the aerial ULV applications of cypermethrin is minimal and that
skin absorption is very slight (Chester et al., 1986).
Desi et al. (1986b) carried out a biological monitoring and
health surveillance study on 11 workers spraying organophosphate
carbamate and pyrethroid pesticides in greenhouses during the whole
year in comparison with 10 control persons. During the work,
protective clothing and masks were worn before and after a regular
spraying period with pyrethroids (including cypermethrin).
Extensive medical examinations, such as urinalysis, haematology,
immunoglobulin levels, whole blood cholinesterase activity, serum-
gamma-glutamyltransferase activity, chromosome analysis and
electro-cardiography were performed over a period of 3 months. The
amount of cypermethrin in the blood was just at the limit of
detection. No health injuries or other significant changes in the
parameters studied were found.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereo-isomers, is a highly active insecticide effective
against a wide range of pests in many food and non-food
commodities.
It is stable to light and heat, it has a low vapour pressure
and is more stable in acidic than in alkaline media. Sensitive
analytical methods for the determination of residues in food and
the environment are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly
rapid and residues do not accumulate in the environment.
Photodegradation is unlikely to play an important role. The main
route of degradation is cleavage of the ester linkage to give 2
main degradation products containing the cyclopropane, and the
phenoxybenzyl moiety. The half-lives in the soil are determined by
many factors, but are in the range of 2 - 4 weeks. Cypermethrin is
strongly adsorbed by soil and downward leaching is negligible.
Because of its rather fast breakdown forming less toxic products,
and the low dose rates used in good agricultural practice, it is
unlikely that cypermethrin will attain significant levels in the
environment.
Bioaccummulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the
levels of cypermethrin residues in food commodities are generally
low. Total diet studies are not available, but from the available
residue information, it can be inferred that the oral intake by man
is well below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are
generally rather resistant to cypermethrin, while fish and other
aquatic invertebrates are very sensitive. Because of its strong
adsorption on soil, only low levels of cypermethrin may leak into
surface water. These may have transient effects, mainly on surface
breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to
be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect for bees.
Absorption and elimination of cypermethrin has been rapid in
the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation
and conjugation of the cyclopropane- and phenoxybenzyl moiety. The
highest levels are found in body fat, which is consistent with the
lipophilic nature of cypermethrin. The half-life in the fat of the
rat is about 12 - 19 days for the cis-isomer and 3 - 4 days for the
trans-isomer. Breakdown products in plants are bound as
glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg
body weight. Short-term and long-term toxicity studies on rats,
mice, and dogs have shown effects on growth, on the liver and
kidneys, and the nervous system, and on haematology. A no-
observed-adverse-effect level of 7.5 mg/kg body weight has been
adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at
70 mg/kg body weight or rabbits at 30 mg/kg body weight. It was
also shown not to have any effects on reproductive performance
during a 3-generation reproduction study in rats administered
100 mg/kg diet. In a variety of mutagenicity studies, cypermethrin
was shown to be mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational
studies available, it seems that the skin sensation seen in workers
handling cypermethrin, generally lasts only a few hours and does
not persist for more than one day after exposure. Other
neurological signs were not observed. These skin sensations can be
considered to be an early warning that exposure has occurred and
that work practice should be reviewed. Cypermethrin may cause eye
irritation and may be a sensitizer for certain persons.
10.2. Conclusions
It can be concluded that:
General population: When applied according to good
agricultural practice, exposure of the general population to
cypermethrin is negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices,
hygiene measures, and safety precautions, the use of cypermethrin
is unlikely to present a hazard to those occupationally exposed to
it. The occurrence of "facial sensations" is an indication of
exposure. Under these circumstances work practice should be
reviewed.
Environment: With recommended application rates it is
unlikely that cypermethrin or its degradation products will attain
levels of environmental significance. Notwithstanding its high
toxicity for fish and honey bees, this is only likely to cause a
problem in the case of spillage and overspraying.
11. RECOMMENDATIONS
- Cypermethrin should be included among the residues looked for
in surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human
beings should be conducted, in order to better understand this
phenomenon.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cypermethrin was discussed at meetings of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) during the period 1979-84
(FAO/WHO, 1980a,b; 1982a,b; 1983a,b; 1984; 1985a,b,c; Vettorazzi &
Van den Hurk, 1984). In 1981, the JMPR established an Acceptable
Daily Intake (ADI) for cypermethrin of 0 - 0.05 mg/kg body weight.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, classified cypermethrin as "irritant to
eyes and sensitizer of skin" in the list of "technical products
unlikely to present an acute hazard in normal use" (Plestina, 1984;
WHO, 1979; WHO, 1985). The same division published a data sheet on
cypermethrin, No. 84.58 (WHO/FAO, 1975-85).
REFERENCES
ABBASSY, M.A., ASHRY, M., ADAM, F., KHALIL, F., & ABOU-SHLOU,
M.A. (1984) Toxicological and histopathological studies on
the cotton bollworm. ( Pectinophera gossypiella Saund). Meded.
Fac. Landbouwwet. Rijksuniv. Gent, 49(3a): 691-698.
ABDEL-AAL, Y.A.I., EL-SAYED, A.M.K., NEGM, A.A., HUSSEIN,
M.H., & EL-SEBAE, A.H. (1979) The relative toxicity of
certain insecticides to Spodoptera littoralis (Boisd) and
Coccinella undecimpunctata L. Int. Pest Control, 21(4): 79-80,
82.
ABOU-AWAD, B.A. & EL-BANHAWY, E.M. (1985) Comparison between
the toxicity of synthetic pyrethroids and other compounds to
the predacious mite Amblyseius gossipi (Mesostigmata:
Phytoseiidae). Exp. appl. Acarol., 1: 185-191.
AHMED, Y.M., MOSTAFA, A.M.A., & ELEWA, M.A. (1985) Toxicity
of certain dyes as insecticides and their joint action with
some pyrethroids. J. environ. Sci. Health, B20(6): 689-699.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four
synthetic pyrethroids on a predatory mite, Typhlodromus pyri,
and its prey, Panonychus ulmi, on apples in Southeast England.
Environ. Entomol., 9: 436-439.
AMER, S.M. & ABOUL-ELA, E.I. (1985) Cytogenetic effects of
pesticides. III. Induction of micronuclei in mouse bone marrow
by the insecticides cypermethrin and rotenone. Mutat. Res.,
155(3): 135-142.
ASCHER, K.R.S., ELIYAHU, M., NEMNY, N.E., & ISHAAYA, I.
(1982) The toxicity of some novel pesticides - synthetic
pyrethroids and benzoylphenylurea chitin synthesis inhibitors
- for eggs of Spodoptera littoralis (Boisd). Z. angew.
Entomol., 94: 504-509.
AWASTHI, M.D. & ANAND, L. (1983) Dissipation and persistence
of synthetic pyrethroids on fruits of okra. J. entomol. Res.,
7(1): 55-59.
BADMIN, J.S. & TWYDELL, R.S. (1976) Evaluation of the
insecticide WL 43467 against the honey bee Apis mellifera,
Sittingbourne, Shell Research (WKSR.0021.76).
BAICU, T. (1982) Toxicity of some pesticides to Trichoderma
viride pers., Crop Prot., 1(3): 349-358.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues
of synthetic pyrethroids in fruit and vegetables by gas-liquid
and high-performance liquid chromatography. Analyst, 107:
206-212.
BALDWIN, M.K. (1977a) Residues of the pyrethroid insecticide
WL43467 in tissues of cattle following a dip-bath application,
Sittingbourne, Shell Research (TLGR.0.115.77).
BALDWIN, M.K. & LAD, D.D. (1978a) Residue data for milk
following application of WL 43467 in a barn, Sittingbourne,
Shell Research (TLGR.0042.78).
BALDWIN, M.K. & LAD, D.D. (1978b) The accumulation and
elimination of WL 43467 by the Rainbow trout (Salmo
gairdneri), Sittingbourne, Shell Research (TLGR.0041.78).
BALDWIN, M.K., BUCKWELL, A.C., & LAD, D.D. (1977) Residue
data following the application of WL 43467 for nuisance fly
control on cattle, Sittingbourne, Shell Research (TGLR.0.112.
77).
BARIOLA, L.A. & LINGREN, P.D. (1984) Comparative toxicities
of selected insecticides against pink bollworm (Lepidoptera:
Gelechiidae) moths. J. econ. Entomol., 77: 207-210.
BARLOW, F., HADAWAY, A.B., FLOWER, L.S., & TURNER, C.R.
(1986) Residual contact toxicity of some insecticides to tse
tse flies in laboratory tests, London, Centre for Overseas
Pest Research, pp. 12.
BATTELLE (1982) Pesticide programme of research and market
planning. I. Insecticides, Geneva, Institute Battelle (October
1982).
BAYOUMI, O.C. (1982) The susceptibility of Myzus persicae
Sulz. to some insecticides in artificial diets. Parasitica,
38(4): 193-199.
BENNETT, D. (1981a) The accumulation, distribution, and
elimination of RIPCORD by Rainbow trout using a continuous-
flow procedure, Sittingbourne, Shell Research (SBGR.81.026 and
Addendum).
BENNETT, D. (1981b) Residues of RIPCORD in Rainbow trout
(Salmo gairdneri) and common carp (Cyprinus carpio) exposed to
lethal concentrations in water, Sittingbourne, Shell Research
(SBGR.81.075).
BENNETT, D., CROSSLAND, N.O., & SHIRES, S.W. (1980) Spray
drift from RIPCORD applications to vineyards in France: fate
and effects in adjacent streams, Sittingbourne, Shell Research
(TLGR.80.095).
BLAIR, D. & RODERICK, H.R. (1976) Toxicity studies on the
pyrethroid insecticide WL 43467, emulsifiable concentrate FX
3315. Acute inhalation exposure of rats to an aqueous spray,
Sittingbourne, Shell Research (TLGR.0001.76).
BLAIR, D., BUTTERWORTH, S.T.G., & RODERICK, H.R. (1976)
Toxicity studies on the pyrethroid insecticide WL 43467,
emulsifiable concentrate FX 3315. Histopathology of rats
exposed to an aqueous spray, Sittingbourne, Shell Research
(TLGR.0102.76).
BLAND, P.D. (1985) Capillary gas chromatographic
determination of cypermethrin in formulations: collaborative
study. J. Assoc. Off. Anal. Chem., 68(3): 592-595.
BOSIO, P.G. (1979) Residues of Barricade (cypermethrin) in
cattle from Australia, Berre, France, Shell Chimie (BEGR.79.
117) (Unpublished Shell data).
BOSTANIAN, N.J. & BELANGER, A. (1985) The toxicity of three
pyrethroids to Amblyseius fallacis (Garman) Acari.
Phytoseiidae and their residues on apple foliage. Agric.
Ecosyst. Environ., 14(3/4): 243-250.
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC
multi-residue method to determination of synthetic pyrethroid
residues in celery and animal products. J. Assoc. Off. Anal.
Chem., 65(3): 685-689.
BRAUN, H.E., FRANK, R., & MILLER, L.A. (1985) Residues of
cypermethrin in milk from cows wearing impregnated ear tags.
Bull. environ. Contam. Toxicol., 35: 61-64.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1983)
Injection toxicity of some pyrethroids in the armyworm.
J. Pestic. Sci., 8: 95-98.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984a)
Topical and injection toxicities of some pyrethroids in the
tobacco cutworm, Spodoptera litteralis Fabricius. J. Pestic.
Sci., 9: 481-487.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984b)
Topical and injection toxicities of some pyrethroids to the
german cockroach, Blattella germanica (Dictyoptera:
Blattellidae). Appl. Entomol. Zool., 19(3): 348-355.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984c) The
selective toxicity of some synthetic pyrethroids in the
armyworm. Pseudaletia separata (Walker). III Cuticle
permeabilities of some pyrethroids. Appl. Entomol. Zool.,
19(1): 87-94.
BROMLEY, S. & COOK, K.A. (1979) Determination of the effects
of WL 43467 on microbial activity in soil. V. Effects on
glucose utilization, Sittingbourne, Shell Research
(BLGR.79.099).
BROOKS, T.M. (1980) Toxicity studies with agricultural
chemicals: mutagenicity studies with RIPCORD in microorganisms
in vitro and in the host-mediated assay, Sittingbourne, Shell
Research (TLGR.80.059).
BROWN, V.K. (1979a) Toxicology of WL 43467 isomers: acute
toxicity of WL 43481 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.117).
BROWN, V.K. (1979b) Toxicology of WL 43467 isomers: acute
toxicity of WL 42641 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.116).
BUCKWELL, A.C. (1981) A 2-year feeding study in dogs on WL
43467, Sittingbourne, Shell Research (SBGR.81.126).
BUCKWELL, A.C. & BUTTERWORTH, S.T.G. (1977) Toxicity studies
on the pyrethroid insecticide WL 43467: a 13-week feeding
study in dogs, Sittingbourne, Shell Research (TLGR.0127.77).
BUTTERWORTH, S.T.G. & CLARK, D.G. (1977) Toxicity studies on
the insecticide WL 43467: acute oral toxicity and neuro-
pathological effects in Syrian hamsters, Sittingbourne, Shell
Research (TLGR.0094.76).
CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN
GELDER, G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin
sensory stimulation characterized by a new behavioral
paradigm. Toxicol. appl. Pharmacol., 76: 270-279.
CAMILLERI, P. (1984) Alkaline hydrolysis of some pyrethroid
insecticides. J. agric. food Chem., 32: 1122-1124.
CARTER, B.I. & BUTTERWORTH, S.T.G. (1976) Toxicity of
insecticides: the acute oral toxicity and neuropathological
effects of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0055.76).
CASIDA, J.E., GAUGHAN, L.C., & RUZO, L.O. (1979) Comparative
metabolism of pyrethroids derived from 3-phenoxybenzyl and
alpha-cyano-3-phenoxybenzyl alcohols. Adv. Pestic. Sci., 2:
182-189.
CASSIDY, S.L. (1979) Acute oral toxicity of the spray
formulation EF 5288 to calves, piglets, and lambs,
Sittingbourne, Shell Research (TLGR.0001.79).
CHANG, C.K. & JORDAN, T.W. (1982) Penetration and metabolism
of topically applied permethrin and cypermethrin in
pyrethroid-tolerant Wiscana cervinata larvae. Pestic. Biochem.
Physiol., 17: 196-204.
CHANG, C.K. & JORDAN, T.W. (1983) Insecticide handling
mechanisms in some New Zealand pasture pests. N.Z. J. Sci.,
26: 509-516.
CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four
pyrethroid insecticides in a mineral and an organic soil.
J. environ. Sci. Health, B16(5): 605-615.
CHAPMAN, R.A. & SIMMONS, H.S. (1977) Gas-liquid chromato-
graphy of picogram quantities of pyrethroid insecticides.
J. Assoc. Off. Anal. Chem., 60: 977-978.
CHAPMAN, R.A., TU, C.M., HARRIS, C.R., & COLE, C. (1981)
Persistence of five pyrethroid insecticides in sterile and
natural, mineral and organic soil. Bull. environ. Contam.
Toxicol., 26: 513-519.
CHENG, H.H. (1984) Residual toxicity of six pyrethroid and
two organophosphorus insecticides on the soil surface against
dark-sided cutworm (Lepidoptera: Noctuidae) on tobacco in
Ontario. Can. Entomol., 116: 11-17.
CHENG, H.H. & HANLON, J.J. (1984) Residual toxicity of six
insecticides and a herbicide applied sequentially or in tank
mix combinations on tobacco seedlings against dark-sided
cutworm (Lepidoptera: Noctuidae). Tob. Int., 186(22): 39-42.
CHESTER, G., HATFIELD, L.D., HART, T.B., LEPPERT, B.C.,
SWAINE, H., & TUMMON, O.J. (1986) Worker exposure to, and
absorption of cypermethrin during aerial application of an
"ultra low volume" formulation to cotton, Fernhurst, Imperial
Chemical Industries, Plant Protection Division (Unpublished
report).
COATS, S.A., COATS, J.R., & ELLIS, C.R. (1979) Selective
toxicity of three synthetic pyrethroids to eight coccinellids,
a eulophid parasitoid and two pest chrysomelids. Environ.
Entomol., 8(4): 720-722.
CODEX ALIMENTARIUS COMMISSION (1986) Guide to Codex
recommendations concerning pesticide residues. Part 2: Maximum
limits for pesticide residues, third preliminary issue, Rome,
Food and Agriculture Organization of the United Nations
(CAC/PR 2-1986).
COLE, L.M., CASIDA, J.E., & RUZO, L.O. (1982) Comparative
degradation of the pyrethroids tralomethrin, tralocythrin,
deltamethrin and cypermethrin on cotton and bean foliage.
J. agric. food Chem., 30: 916-920.
COOK, K.A. (1978a) Determination of the effects of WL 43467
on microbial activity in soil. I. Effects on carbon dioxide
evolution, Sittingbourne, Shell Research (BLGR.0003.78).
COOK, K.A. (1978b) Determination of the effects of WL 43467
on microbial activity in soil. IV. Effects on nitrification,
Sittingbourne, Shell Research (BLGR.0015.78).
COOMBS, A.D., CARTER, B.I., HEND, R.W., BUTTERWORTH, S.G., &
BUCKWELL, A.C. (1976) Toxicity studies on the insecticide WL
43467: Summary of results of preliminary experiments,
Sittingbourne, Shell Research (TLGR.0104.76).
CORBITT, T.S., WRIGHT, D.J., & GREEN, A.St.J. (1985) The
toxicity of abamectin (MK. 936) on cabbage to first and third
larval instars of Spodoptera littoralis (Boisd.). Meded. Fac.
Landbourwwet. Rijksuniv. Gent, 50(2b): 639-642.
COVENEY, P.C. & EADSFORTH, C.V. (1982) The metabolism of
cypermethrin in man (3). Urinary excretion following a single
dermal dose of cypermethrin, Sittingbourne, Shell Research
(SBGR.82.290).
CRAWFORD, M.J. (1976a) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of (14 C-benzyl)WL
43481 (cis-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0046.76).
CRAWFORD, M.J. (1976b) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of 14 C-WL 42641
(trans-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0077.76).
CRAWFORD, M.J. (1977) The metabolism of WL 43467 in mammals.
The fate of a single oral dose of (14 C-cyclopropyl)WL 43467 in
the rat, Sittingbourne, Shell Research (TLGR.0004.77).
CRAWFORD, M.J. (1979a) The metabolism of cypermethrin (WL
43467) in mammals. The fate of a single oral dose of
(14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne, Shell
Research (TLGR.79.029).
CRAWFORD, M.J. (1979b) The metabolism of cypermethrin (WL
43467) in mammals. The fate of single oral doses of cis- and
trans-(14 C-benzyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.0011.79).
CRAWFORD, M.J. (1979c) The metabolism of 14 C-cypermethrin by
rat liver microsomes, Sittingbourne, Shell Research
(TLGR.79.057).
CRAWFORD, M.J. (1979d) The metabolic fate of the cis- and
trans-isomers of WL 43467 (cypermethrin) and of 3-phenoxy-
benzoic acid in the dog, Sittingbourne, Shell Research
(TLGR.79.012).
CRAWFORD, M.J. (1979e) The metabolism of cypermethrin (WL
43467) in mammals. Metabolites derived from a single oral dose
of (14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.79.096).
CRAWFORD, M.J. & HUTSON, D.H. (1977a) The metabolic fate of
the cis- and trans-isomers of WL 43467 (cypermethrin).
Metabolism and elimination of 14 C-aryl-labelled cis- and
trans-isomers in rats, Sittingbourne, Shell Research
(TLGR.0131.77).
CRAWFORD, M.J. & HUTSON, D.H. (1977b) The elimination and
retention of WL 43467 when administered dermally or orally to
sheep, Sittingbourne, Shell Research (TLGR.0098.77).
CRAWFORD, M.J. & HUTSON, D.H. (1978) The elimination of
residues from the fat of mice following the oral
administration of (14 C-benzyl)WL 43481 (cis-WL 43467),
Sittingbourne, Shell Research (TLGR.0080.78).
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981a)
Metabolism of cis- and trans-cypermethrin in rats. Balance and
tissue retention study. J. agric. food Chem., 29: 130-135.
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981b) The
metabolism of the pyrethroid insecticide cypermethrin in rats:
excreted metabolites. Pestic. Sci., 12: 399-411.
CRAYFORD, J.V. (1978) A study of the metabolism of
3-phenoxybenzoic acid and its glucoside conjugate in rats,
Sittingbourne, Shell Research (TLGR.0186.78).
CRAYFORD, J.V. & HUTSON, D.H. (1979) The identification of
metabolites in the tissues of rats treated orally with
3- phenoxybenzoic acid, Sittingbourne, Shell Research (TLGR.0043.
79).
CRAYFORD, J.V. & HUTSON, D.H. (1980) Xenobiotic triglyceride
formation. Xenobiotica, 10(5): 349-354.
CRAYFORD, J.V., HUTSON, D.H., & THORPE, E. (1980) The
elimination of residues from the fat of mice following the
oral administration of 14 C-benzyl WL 43481 (cis-WL 43467),
Tunstall, Shell Toxicology Laboratory (TLGR.0080.78,
additional report).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin.
II. Fate and biological effects in pond experiments. Aquat.
Toxicol., 2: 205-222.
CROSSLAND, N.O. & BENNETT, D. (1976) A field trial to assess
the dispersion and toxicity of an EC formulation of the
insecticide WL 43467 in a pond system, Sittingbourne, Shell
Research (TLGR.0101.76).
CROSSLAND, N.O. & ELGAR, K.E. (1983) Fate and biological
effects of insecticides in ponds. In: Miyamoto, J. et al., ed.
IUPAC. Pesticide chemistry: human welfare and the environment,
Oxford, Pergamon Press, Vol. 3, pp. 551-556.
CROSSLAND, N.O. & STEPHENSON, R.R. (1979) The role of pond
studies in assessing the hazard of toxic chemicals to
freshwater ecosystems. In: Proceedings of the British Crop
Protection Conference, 1979: Pests and Diseases, Croydon,
British Crop Protection Council, Vol. 2, pp. 453-459.
CROSSLAND, N.O., BENNETT, D., KANE, D.F., & STEPHENSON, R.R.
(1978) The dispersion and toxic effects of the insecticide WL
43467 in a pond, Sittingbourne, Shell Research (TLGR.0076.78).
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic
toxicology of cypermethrin. III. Fate and biological effects
of spray drift deposits in freshwater adjacent to agricultural
land. Aquat. Toxicol., 2: 253-270.
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982a) Hepatic
esterases: characterization and quantitation in vitro of rat,
rabbit, and human liver esterases. Part I, Sittingbourne,
Shell Research (SBGR.82.204).
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982b) In vitro
metabolism of the pyrethroid insecticide cypermethrin by liver
esterases. In: Proceedings of the 5th International Congress
on Pesticide Chemicals, Vol. 4.
CROUCHER, A., HUTSON, D.H., & STOYDIN, G. (1985) Excretion
and residues of the pyrethroid insecticide cypermethrin in
lactating cows. Pestic. Sci., 16: 287-301.
DAI, S.M. & SUN, C.N. (1984) Pyrethroid resistance and
synergism in Nilaparvata lugens Stal (Homoptera: Delphacidae)
in Taiwan. J. econ. Entomol., 77: 891-897.
DAY, S.R. & LEAHEY, J.P. (1980) 14 C-cypermethrin: aqueous
photodegradation in sunlight, Fernhurst, Imperial Chemical
Industries (Unpublished ICI Report No. RJ0154B).
DEAN, B.J. (1977) Toxicity studies with SL 43467: chromosome
studies on bone marrow cells of Chinese hamsters after two
daily oral doses of WL 43467, Sittingbourne, Shell Research
(TLGR.0136.77).
DEAN, B.J., PAUW, C.L, VAN DER, & BUTTERWORTH, S.T.B. (1977)
Toxicity studies with WL 43467: dominant lethal assay in male
mice after single oral doses of WL 43467, Sittingbourne, Shell
Research (TLGR.0042.77).
DELABIE, J., BOS, C., FONTA, C., & MASSON, C. (1985) Toxic
and repellent effects of cypermethrin on the honey bee:
laboratory, glasshouse and field experiments. Pestic. Sci.,
16: 409-415.
DESI, I., VARGA, L., DOBRONYI, I., & SZKLENARIK, G. (1985)
Immunotoxicological investigation of the effects of a
pesticide: cypermethrin. Arch. Toxicol., Suppl. 8: 305-309.
DESI, I., DOBRONYI, I., & VARGA, L. (1986a) Immuno-, neuro-
and general toxicologic animal studies on a synthetic
pyrethroid: cypermethrin. Ecotoxicol. environ. Saf., 12:
220-232.
DESI, I., PALOTAS, M., VETRO, G., CSOLLE, I., NEHEZ, M.,
ZIMANYI, M., FERKE, A., HUSZTA, E., & NAGYMAJTENYI, L.
(1986b) Biological monitoring and health surveillance of a
group of greenhouse pesticide sprayers. Toxicol. Lett., 33:
91-105.
DEWAR, A.J. (1977a) The use of lysosomal enzyme measurements
as an indicator of chemically-induced peripheral neuropathy,
Sittingbourne, Shell Research (TLGR.0074.77).
DEWAR, A.J. (1977b) Toxicity studies on the insecticide WL
43467: biochemical and functional studies on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research (TLGR.0082.
77).
DEWAR, A.J. & DEACON, P.A. (1977) Toxicity studies on the
insecticide WL 43467: electrophysiological studies on the
neurotoxicity of WL 43467 to rats. I. The effect on motor
conduction velocity in the sciatic and tail nerves,
Sittingbourne, Shell Research (TLGR.0133.77).
DEWAR, A.J. & MOFFETT, B.J. (1978a) Toxicity studies on the
insecticide WL 43467: biochemical studies on the effect of WL
43467 on the rat trigeminal nerve and ganglion, Sittingbourne,
Shell Research (TLGR.0162.77).
DEWAR, A.J. & MOFFETT, B.J. (1978b) Toxicity studies on the
insecticide WL 43467: biochemical and functional studies on
the neurotoxicity of WL 43467 to Chinese hamsters,
Sittingbourne, Shell Research (TLGR.0038.78).
DEWAR, A.J. & OWEN, D.E. (1979) Toxicology of pyrethroids:
the acute oral toxicity to rats of a sample of ICI
cypermethrin, Sittingbourne, Shell Research (TLGR.79.019).
DIX, K.M. (1978) Toxicity of WL 43467: teratological studies
in rabbits given WL 43467 orally, Sittingbourne, Shell
Research (TLGR.0010.78).
DU TOIT, G.D.G. (1978) Evaluation by topical application of
selected insecticides against adult grass grub. In: Proceeding
of the 31st New Zealand Weed and Pest Control Conference, pp.
160-163.
EADSFORTH, C.V. & BALDWIN, M.K. (1983) Human dose-excretion
studies with the pyrethroid insecticide cypermethrin.
Xenobiotica, 13: 67-72.
EDWARDS, P.J., WILKINSON, W., & COULSON, M. (1985)
Laboratory toxicity test for carabid beetles. Proceedings of
the British Crop Protection Conference, 1984: Pests and
Diseases, Croydon, British Crop Protection Council, Vol. 1,
pp. 359-362.
EDWARDS, R. & MILLBURN, P. (1985a) Toxicity and metabolism
of cypermethrin in fish compared with other vertebrates.
Pestic. Sci., 16: 201-202.
EDWARDS, R. & MILLBURN, P. (1985b) The metabolism and
toxicity of insecticides in fish. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry and toxicology,
New York, John Wiley and Sons, Vol. 5, pp. 249-274.
ELLIOTT, M., FARNHAN, A.W., JONES, N.F., NEEDHAM, P.H., &
PULMAN, D.A. (1974) Synthetic insecticide with a new order
of activity. Nature (Lond.), 248: 710-711.
EL-MINSHAWY, A., MACKLAD, F., RAGAB, F., & DONIA, A. (1983)
Selective toxicity of certain pyrethroids to the cotton
leafworm Spodoptera littoralis (Boisd.) and to one of its
major parasites Microplitis rufiventris (Kok). Proceedings of
the International Conference on Environmental Hazards of
Agrochemicals, Alexandria, Egypt, 8-12 November, 1983, London,
Paris, New York, Harwood Academic Publishers, Vol. 1, pp. 551-
561.
EL-SAYED, G.N. & KNOWLES, C.O. (1984) Formamidine synergism
of pyrethroid toxicity to two-spotted spider mites (Acari,
Tetranychidae). J. econ. Entomol., 77: 23-30.
EL-SEBAE, A.H., EL-BAKARY, A.S., LE PATOUREL, J., KADOUS, E.,
& MACKLAD, M.F. (1983) Effect of photoperiodism on fish
susceptibility to insecticides. In: Zewail, A.H., ed.
Proceedings of the International Conference on Photochemistry
and Photobiology, Alexandria, Egypt, 8-12 November, 1983,
London, Paris, New York, Harwood Academic Publishers, pp. 960-
966.
EL-SEBAE, A.H., ENAN, E.E., DAOUD, A.S., & ZEID, M.I. (1985)
Selective toxicity of synthetic pyrethroids and some
synergists to mice and cotton leafworm in relation to some
biochemical enzyme activities. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 50(3a): 939-950.
ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1982) Dislodgable
insecticide residues on cotton foliage: carbaryl, cyper-
methrin, and methamidophos. Bull. environ. Contam. Toxicol.,
28: 490-493.
EUROPEAN PATENT OFFICE (1980) European patent application
(Publication No. 0015598(A2)).
EVANS, M.H. (1976) End-plate potentials in frog muscle
exposed to a synthetic pyrethroid. Pestic. Biochem. Physiol.,
6: 547-550.
EWEN, A.B., MUKERJI, M.K., & HINKS, C.F. (1984) Effect of
temperature on the toxicity of cypermethrin to nymphs of the
migratory grasshopper. Melanoplus sanguinipes (Orthoptera:
Acridoidea). Can. Entomol., 116(9): 1153-1156.
FABELLAR, L.T. & HEINRICHS, E.A. (1984) Toxicity of
insecticides to predators of rice brown planthoppers,
Nilaparvata lugens (Stal) (Homoptera: Delphacidae) Environ.
Entomol., 13: 832-837.
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20;
Suppl).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 56).
FAO/WHO (1985a) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985c) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Protection and Protection Paper No. 67).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
FRANK, R., BRAUN, H.E., RITCEY, G., MCEWEN, F.L., & SIRONS,
G.J. (1982) Pesticide residues in onions and carrots grown
on organic soils, Ontario, 1975-80. J. econ. Entomol., 75:
560-565.
FURUZAWA, K., MIKAMI, N., YAMADA, H., & MIYAMOTO, J. (1986)
Metabolism of the pyrethroid insecticide cypermethrin in
cabbages. J. Pestic. Sci., 11: 253-260.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes
of pyrethroid action in the cockroach. Pestic. Biochem.
Physiol., 15: 181-191.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most
potent class antagonize GABA action at the crayfish
neuromuscular junction. Neurosci. Letters, 40: 163-168.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982)
Pyrethroid toxicology: Protective effects of Diazepam and
phenobarbital in the mouse and the cockroach. Toxicol. applied
Pharmacol., 66:290-296.
GARFORTH, B.M. (1982) WL 85871 and cypermethrin: chronic
toxicity to Daphnia magna, Sittingbourne, Shell Research
(SBGR.82.119).
GARFORTH, B.M. (1983) The effect of RIPCORD on the non-
target fauna of maize, Sittingbourne, Shell Research (SBGR.83.
045).
GERIG, L. (1979) [The toxicity of synthetic pyrethrines to
foraging bees.] Schwei z. Bienen-Ztg, 101: 228-236 (in German).
GERIG, L. (1981) [The toxicity of synthetic pyrethrines to
foraging bees. Part 2.] Schweiz. Bienen-Ztg, 104: 155-174 (in
German).
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977a) Effects of
high dietary levels on clinical behaviour and structure of
sciatic nerves in the rat, Fernhurst, Imperial Chemical
Industries, Central Toxicology Laboratory, pp. 383
(Unpublished ICI report).
GLAISTER, J.R., GARE, C.W., MARSAT, G.J., PHILLIPS, C., &
PRATT, I. (1977b) 90-day feeding study in rats, Fernhurst,
Imperial Chemical Industries, Central Toxicology Laboratory,
pp. 383 (Unpublished ICI report).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4(6): 793-799.
GRAY, K. & GRAYSON, B.T. (1980) Determination of the
n-octanol/water partition coefficients of BIRLANE AND GARDONA
and a literature search for the values of other group
compounds, Sittingbourne, Shell Research (BLGR.80.103).
GRAYSON, B.T., LANGNER, E., & WELLS, D. (1982) Comparison of
two gas saturation methods for the determination of the vapour
pressure of cypermethrin. Pestic. Sci., 13: 552-556.
HAGLEY, E.A.C., PREE, D.J., & SIMPSON, C.M. (1981) Toxicity
of insecticides to parasites of the spotted tentiform
leafminer (Lepidoptera: Gracillariidae). Can. Entomol., 113:
899-906.
HALL, J.S., LEAHEY, J.P., & CURL, E.A. (1981) Cypermethrin:
photodegradation on a soil surface, Fernhurst, Imperial
Chemical Industries (Unpublished ICI Report No. RJ0192B).
HARGREAVES, J.R. & COOPER, L.P. (1979) Phytotoxicity tests
with pyrethroid insecticides on glasshouse grown tomato
seedlings. Queensland J. Agric. anim. Sci., 36(2): 151-154.
HARRIS, C.R. & TURNBULL, S.A. (1978) Laboratory studies on
the contact toxicity and activity in soil of four pyrethroid
insecticides. Can. Entomol., 110: 285-288.
HARRIS, C.R. & TURNBULL, S.A. (1980) Toxicity of some
insecticides to insecticide-susceptible strains of the onion,
cabbage and seedcorn maggots (Diptera: Anthomyiidae) and the
dark-sided cutworm (Lepidoptera: Nocturidae). Can. Entomol.,
112(10): 1029-1032.
HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory
studies on the persistence and behaviour in soil of 4
pyrethroid insecticides. Can. Entomol., 113: 685-694.
HARRIS, C.R., TURNBULL, S.A., & MCLEOD, D.G.R. (1985)
Contact toxicity of twenty-one insecticides to adults of the
carrot rust fly (Diptera: Psilidae). Can. Entomol., 117:
1025-1027.
HART, T.B., SPINKS, C.A., MARLOW, R.G., & SUTHERS, J.R.
(1982) A study of the exposure and health of Indian workers
spraying, Ambush and Cymbush on cotton using high volume
hand-held spray applicators, Fernhurst, Imperial Chemical
Industries, Plant Protection Division (Report TMF.1841-B).
HELLING, C.S. & TURNER, B.C. (1968) Pesticide mobility:
determination in soil by thin-layer chromatography. Science,
162: 562-563.
HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies on
the insecticide WL 43467: a three-month feeding study in rats,
Sittingbourne, Shell Research (TLGR.0027.76) (Unpublished
report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977a) Toxicity studies on
the insecticide WL 42641: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977b) Toxicity studies on
the insecticide WL 43481: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W., HENDY, R., & FLEMING, D.J. (1978) Toxicity
studies on the insecticide WL 43467: a three-generation
reproduction study in rats, Sittingbourne, Shell Research
(TLGR.0188.78).
HENDERSON, C. & PARKINSON, G.R. (1981) Cypermethrin
technical: subacute dermal toxicity study in rabbits, Alderley
Park, Imperial Chemical Industries (Report No. CTL/P/588).
HINE, C.H. & ZUIDEMA, H.H. (1970) The toxicological
properties of hydrocarbon solvents. Ind. Med., 39(5): 39-44.
HO, S.H., LEE, B.H., & SEE, D. (1983) Toxicity of
deltamethrin and cypermethrin to the larvae of the diamond-
back moth, Plutella xylostella L. Toxicol. Lett., 19: 127-131.
HOLDEN, J.S. (1979) Absorption and metabolism of permethrin
and cypermethrin in the cockroach and the cotton leaf-worm
larvae. Pestic. Sci., 10: 295-307.
HOPKINS, A.R., MOORE, R.F., & JAMES, W. (1984) Contact and
residual toxicities of pyrethroids and organophosphorus
compounds to the Boll weevil (Coleoptera: Curculionidae) J.
Georgia Entomol. Soc., 19(1): 27-34.
HUCKLE, K.R., HUTSON, D.H., & MILLBURN, P. (1981a) Species
differences in the metabolism of 3-phenoxybenzoic acid. Drug
Metab. Disp., 9: 352-359.
HUCKLE, K.R., CHIPMAN, J.K., HUTSON, D.H., & MILLBURN, P.
(1981b) Metabolism of 3-phenoxybenzoic acid and the
enterohepatorenal disposition of its metabolites in the rat.
Drug Metab. Disp., 9(4): 360-368.
HUTSON, D.H. (1978a) The elimination of radioactivity by
mice following oral dosing with 14 C-cis- and 14 C-trans-WL
43467 (cypermethrin), Sittingbourne, Shell Research
(TLGR.0079.78).
HUTSON, D.H. (1978b) The metabolites of cis- and trans-
cypermethrin (WL 43467) in mice, Sittingbourne, Shell Research
(TLGR.102.78).
HUTSON, D.H. (1979a) The metabolic fate of synthetic
pyrethroid insecticide in mammals. Prog. Drug Metab., 3:
215-252.
HUTSON, D.H. (1979b) The metabolism of WL 43467 in mammals.
Metabolites derived from (14 C-cyano)cypermethrin (WL 43467) in
rats, Sittingbourne, Shell Research (TLGR.79.183).
HUTSON, D.H. & CASIDA, J.E. (1978) Taurine conjugation in
metabolism of 3-phenoxybenzoic acid and the pyrethroid
insecticide cypermethrin in mouse. Xenobiotica, 8(9): 565-571.
HUTSON, D.H. & STOYDIN, G. (1987) Excretion and residues of
the pyrethroid insecticide cypermethrin in laying hens.
Pestic. Sci., 18: 157-168.
HUTSON, D.H., GAUGHAN, L.C., & CASIDA, J.E. (1981)
Metabolism of the cis- and trans-isomers of cypermethrin in
mice. Pestic. Sci., 12: 385-398.
INGLESFIELD, C. (1982) The effects of an aerial application
of RIPCORD on the non-target fauna of oil seed rape in France,
Sittingbourne, Shell Research (SBGR.82.364).
INGLESFIELD, C. (1984) Toxicity of the pyrethroid
insecticides cypermethrin and WL 85871 to the earthworm
Eisenia foetida Savigny. Bull. environ. Contam. Toxicol.,
33(5): 568-570.
INGLESFIELD, C. & SHERWOOD, C.M. (1983) Toxicity of
cypermethrin to the earthworm, Eisenia foetida L (Oligochaeta:
Lumbriculidae) in laboratory tests, Sittingbourne, Shell
Research (SBGR.83.070).
ISHAAYA, I., ASCHER, K.R.S., & CASIDA, J.E. (1983)
Pyrethroid synergism by esterase inhibition in Spodoptera
littoralis (Boisd.) larvae, Crop Prot., 2(3): 335-343.
JACKSON, C. (1977) The leaching of WL 43467 through
laboratory soil columns, Sittingbourne, Shell Research
(Unpublished Report BLGR.0150.77).
JOIA, B.S., LOSCHIAVO, S.R., & WEBSTER, G.R.B. (1981) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat. In: Proceedings of the 16th Annual Workshop
on Pesticide Residue Analysis, Western Canada, pp. 14-16.
JOIA, B.S., SARMA, L.P., & WEBSTER, G.R.B. (1985a) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat and milled fractions. J. environ. anal.
Chem., 21: 179-184.
JOIA, B.S., WEBSTER, G.R.B., & LOSCHIAVO, S.R. (1985b)
Cypermethrin and fenvalerate residues in stored wheat and
milled fractions. J. agric. food Chem., 33: 618-622.
JONES, B.J. (1981) Cypermethrin: bioaccumulation study in
the rat, Alderley Park, Imperial Chemical Industries (Report
No. CTL/P/599).
JORDAN, T.W. & CHANG, C.K. (1981) Pyrethroid resistance
mechanisms in Porina caterpillars. In: Proceedings of the 34th
New Zealand Weed and Pest Control Conference, pp. 167-169.
KAUFMAN, D.D., JORDAN, E.G., & KAYSER, A.J. (1978)
Degradation of cypermethrin and permethrin in soil. In:
Proceedings of the 175th Meeting on Pesticides of the American
Chemical Society, Washington, DC, American Chemical Society
(Abstract Paper No. 47).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J.
(1981) Movement of cypermethrin, decamethrin, permethrin, and
their degradation products in soil. J. agric. food Chem., 29:
239-245.
KNAPP, F.W., HERALD, F., & SCHWINGHAMMER, K.A. (1985)
Comparative toxicity of selected insecticides to laboratory-
reared and field-collected face flies (Diptera: Muscidae) J.
econ. Entomol., 78: 860-862.
KNIGHT, R.J. (1982) The toxicity of the pyrethroid WL 85871
against honey bee Apis mellifera, Sittingbourne, Shell
Research (SBGR.82.023).
KNOWLES, C.O. & EL-SAYED, G.N. (1985) Formanilide
enhancement of acaricide toxicity to Tetranychus urticae Koch
(Acari: Tetranychidae), J. econ. Entomol., 78: 308-310.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in
relation to body weight and temperature. Water Res., 15:
503-505.
LAUREN, D.R. & HENZEL, R.F. (1977) Residual lives of two
synthetic prethroid insecticides applied to pasture. In:
Proceedings of the 30th New Zealand Weed and Pest Control
Conference, pp. 207-210.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology:
mouse intracerebral structure-toxicity relationship. Pestic.
Biochem. Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action
of pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Inter-
actions of pyrethroid insecticides with chloride ionophore-
associated binding sites. Neurotoxicol., 6: 87-98.
LEAHEY, J.P. (1979) The metabolism and environmental
degradation of the pyrethroid insecticides. Outlook Agric.,
10(3): 135-142.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London,
Philadelphia, Taylor and Francis.
LEAKE, L.D., LAUCKNER, S.M., & FORD, M.G. (1979)
Relationship between neurophysiological effects of selected
pyrethroids and toxicity to the leech Haemopsis sanguisuga and
the locust Schistocerca gregaria. Neurobiol. Pest. Action.,
London, pp. 423-430.
LE PATOUREL, G.N.J. & SINGH, J. (1984) Toxicity of amorphous
silicas and silica-pyrethroid mixtures to Tribolium castaneum
(Herbst) (Coleoptera: Tenebrionidae). J. stored Prod. Res.,
20(4): 183-190.
LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to
synthetic pyrethroids: a clinical and electrophysiological
assessment. Neurotoxicology, 2: 1-11.
LINDSAY, S., BANHAM, P.B., CHART, I.S., CHALMERS, D.T.,
GODLEY, M.J., & TAYLOR, K. (1982) Cypermethrin: life-time
feeding study in mice, Alderley Park, Imperial Chemical
Industries (Report No. CTL/P/687).
LIU, M.Y., CHEN, J.S., & SUN, C.N. (1984) Synergism of
pyrethroids by several compounds in larvae of the Diamondback
moth (Lepidoptera: Plutellidae) J. econ. Entomol., 77: 851-856.
LOCK, E.A. & BERRY, P.N. (1981) Biochemical changes in the
rat cerebellum following cypermethrin administration. Toxicol.
appl. Pharmacol., 59(3): 508-514.
LOVERIDGE, D. & COOK, K.A. (1978a) Determination of the
effects of WL 43467 on microbial activity in soil. II. Effects
on oxygen uptake, Sittingbourne, Shell Research (BLGR.0004.78).
LOVERIDGE, D. & COOK, K.A. (1978b) Determination of the
effects of WL 43467 on microbial activity in soil. III.
Effects on nitrogen fixation, Sittingbourne, Shell Research
(BLGR.0007.78).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve
membrane effects of pyrethroids and DDT analogs. Pestic.
Biochem. Physiol., 20: 203-216.
MCAUSLAND, H.E., BUTTERWORTH, S.T.G., & HUNT, P.F. (1978)
Toxicity studies on the insecticide WL 43467: a two-year
feeding study in rats, Sittingbourne, Shell Research
(TLGR.0189.78).
MCDONALD, S. (1979) Evaluation of insecticides for control
of the army cutworm. J. econ. Entomol., 72(2): 277-280.
MCKEE, M.J. & KNOWLES, C.O. (1985) Pharmacokinetics of
pyrethroids in two-spotted spider mites. Pestic. Biochem.
Physiol., 24: 326-335.
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality
of permethrin, cypermethrin, and fenvalerate to salmon,
lobster, and shrimp. Bull. environ. Contam. Toxicol., 25:
950-955.
MANI, M. & KRISHNAMOORTHY, A. (1984) Toxicity of some
insecticides to Apanteles plutellae, a parasite of the
diamondback moth. Trop. Pestic. Manage., 30(2): 130-132.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th
ed., Croydon, British Crop Protection Council.
MAUCK, W.L., OLSEN, L.E., & MARKING, L.L. (1976) Toxicity of
natural pyrethrins and five pyrethroids to fish. Arch.
environ. Contam. Toxicol., 4: 18-29.
MEISNER, J., ASCHER, K.R.S., & EIZICK, C. (1984) Effect of
the commercial phagostimulants coax and gustol on the toxicity
of cypermethrin and deltamethrin against Spodoptera littoralis
(Lepidoptera: Noctuidae). J. econ. Entomol., 77: 1123-1126.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK,
J. (1983) Farm chemical handbook. C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, pp. C67.
MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J.
(1984) New conjugated metabolites of 3-phenoxybenzoic acid in
plants. Pestic. Sci., 15: 531-542.
MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO,
J. (1985) Metabolism in rats of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid glucoside conjugates formed in plants.
Pestic. Sci., 16: 33-45.
MIYAMOTO, J. (1981) The chemistry, metabolism, and residue
analysis of synthetic pyrethroids. Pure appl. Chem., 53:
1967-2022.
MIYAMOTO, J. & MIKAMI, N. (1983) Degradation of pyrethroid
insecticides in the field. In: IUPAC Pesticide chemistry,
human welfare and the environment, Oxford, Pergamon Press, pp.
193-200.
MORE, J.E., ROBERTS, T.R., & WRIGHT, A.N. (1978) Studies of
the metabolism of 3-phenoxybenzoic acid in plants. Pestic.
Biochem. Physiol., 9: 268-280.
MUIR, D.C.G., RAWN, G.P., TOWNSEND, B.E., LOCKHART, W.L., &
GREENHALGH, R. (1985) Bioconcentration of cypermethrin,
deltamethrin, fenvalerate, and permethrin by Chironomus
tentans larvae in sediment and water. Environ. Toxicol. Chem.,
4(1): 51-61.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some non-target insects. Mosq. News.,
38(1): 90-96.
NOBLE, R.M. & HAMILTON, D.J. (1985) Stability of
cypermethrin and cyfluthrin on wheat in storage. Pestic. Sci.,
16: 179-185.
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976a) Neurotoxic
effects of S 5602 and NRDC 149 by dermal application in rats,
Osaka, Sumitomo Chemical Company (AT-60-0047).
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976b) Neurotoxic
effects of natural pyrethrins and resmethrin by oral application
in rats, Osaka, Sumitomo Chemical Company (AT-60-0052).
OSMAN, A.A., ZOHDY, G.I., & MOMEN, F.M. (1982) Effect of
some pesticides on the food requirements of the predatory
mite, Amblyseius gossipiu-El-Badry. In: Griffiths, D.A. &
Bowman, C.E., ed. Acarology VI, Chichester, Ellis Horwood Ltd,
Vol. 2, pp. 669-672.
OWEN, D.E. & BUTTERWORTH, S.T.G. (1977) Toxicity of
pyrethroid insecticides: investigation of the neurotoxic
potential of WL 43467 to adult domestic hens, Sittingbourne,
Shell Research (TLGR.0134.77).
PEARSON, N. & SHIRES, S.W. (1981) A field study in France of
the effects on honey bees of an aerial application of RIPCORD
in winter-sown oil seed rape, Sittingbourne, Shell Research
(SBGR.81.304).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PLUYMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity
of synthetic pyrethroids in Salmonella typhimurium strains and
in V79 Chinese hamster cells. Mutat. Res., 137: 7-15.
POTTER, D. & MCAUSLAND, H.E. (1980) Toxicity studies on the
insecticide WL 43467: a study of liver microsomal enzyme
activity in rats fed WL 43467 for 2 years, Sittingbourne,
Shell Research (TLGR.80.057).
POULOS, L., ATHANASELIS, S., & COUTSELINIS, A. (1982) Acute
intoxication with cypermethrin (NRDC 149). J. Toxicol. clin.
Toxicol., 19(5): 519-520.
PREE, D.J. & HAGLEY, E.A.C. (1985) Toxicity of pesticides to
Chrysopa oculata Say (Neuroptera : Chrysopidae). J. econ.
Entomol., 78(1): 129-132.
PRICE, J.B. (1981a) Toxicology of pyrethroids: the acute
oral and intraperitoneal toxicity of cypermethrin,
Sittingbourne, Shell Research (SBGR.81.069).
PRICE, J.B. (1981b) Toxicology of pyrethroids: the acute
oral and percutaneous toxicities of RIPCORD and endosulfan,
individually or combined in a 1:1 mixture, Sittingbourne,
Shell Research (SBGR.81.121).
PRINSEN, G.H. & SITTERT, N.J., VAN (1978) Exposure and
medical monitoring study of the pyrethroid WL 43467 after
single application on cotton in Ivory Coast, The Hague, Shell
Internationale Research Mij (TOX 78-004).
PRINSEN, G.H. & SITTERT, N.J., VAN (1979) Exposure and
medical monitoring study of the pyrethroid RIPCORD (WL 43467)
after one season of spraying on cotton in Ivory Coast, The
Hague, Shell Internationale Research Mij (TOX 79-001).
RAJAKULENDRAN, S.V. & PLAPP, F.W., Jr (1982) Comparative
toxicities of five synthetic pyrethroids to the tobacco
budworm (Lepidoptera: Noctuidae), an Ichneumonid parasite,
Campoletis sonorensis and a predator, Chrysopa carnea. J.
econ. Entomol., 75: 769-772.
RAPLEY, J.H., ARNOLD, D.J., & VINCENT, J. (1981)
Cypermethrin: degradation in river and pond waters and
sediments, Fernhurst, Imperial Chemical Industries, Plant
Protection Division (Report No. RJ0175B) (Unpublished ICI
data).
REIFF, B. (1976) The acute toxicity of the pyrethroid
insecticide WL43467 to Brown trout (S. trutta), Sittingbourne,
Shell Research (TLBR.0096.76).
REIFF, B. (1977) The acute toxicity of the pyrethroid WL
43467 to Daphnia magna, Sittingbourne, Shell Research (TLGR.
0155.77).
REIFF, B. (1978a) The effect of suspended solids on the
toxicity of WL 43467 to Rainbow trout (Salmo gairdneri),
Sittingbourne, Shell Research (TLGR.0007.78).
REIFF, B. (1978b) The acute toxicity of the pyrethroid
insecticide WL 43467 to Rainbow trout (Salmo gairdneri),
Common carp (Cyprinus carpio), and Rudd (Scardinius
erythrophthalmus), Sittingbourne, Shell Research (TLGR.0067.
78).
RHODES, C., JONES, B.K., CROUCHER, A., HUTSON, D.H., LOGAN,
C.J., HOPKINS, R., HALL, B.E., & VICKERS, J.A. (1984) The
bioaccumulation and biotransformation of cis, trans-
cypermethrin in the rat. Pestic. Sci., 25: 471-480.
RILEY, D. & HILL, I.R. (1983) Adsorption reduces activity of
pesticides in soil and water. In: Proceedings of the 10th
International Congress on Plant Protection, Brighton, 20-25
November, 1983, Vol. 2, pp. 728 (4B-R18).
RISKALLAH, M.R. (1984) Influence of posttreatment
temperature on the toxicity of pyrethroid insecticides to
susceptible and resistant larvae of the Egyptian cotton
leafworm, Spodoptera littoralis (Boisd.) Experientia (Basel),
40(2): 188-190.
RIVIERE, J.L., BACH, J., & GROLLEAU, G. (1983) Effect of
pyrethroid insecticides and N-(3,5-dichlorophenyl)
dicarboximide fungicides on microsomal drug metabolizing
enzymes in the Japanese quail (Coturnix coturnix). Bull.
environ. Contam. Toxicol., 31: 479-485.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3(1): 67-68.
ROBERTS, T.R. (1981) The metabolism of the synthetic
pyrethroids in plants and soils. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry, New York, John
Wiley and Sons, Vol. 1, pp. 115-146.
ROBERTS, T.R. & STANDEN, M.E. (1977) Degradation of the
pyrethroid cypermethrin NRDC 149(±)-alpha-cyano-3-phenoxy-
benzyl(±)- cis,trans-3-(2,2-dichlorovinyl-2,2-dimethylcyclo-
propanecarboxylate and the respective cis-(NRDC 160) and the
trans-(NRDC 159) isomers in soils. Pestic. Sci., 8: 305-319.
ROBERTS, T.R. & STANDEN, M.E. (1981) Further studies of the
degradation of the pyrethroid insecticide cypermethrin in
soils. Pestic. Sci., 12: 285-296.
ROSE, B. (1981) A dietary toxicity study of WL 43467 in
Mallard ducks, Sittingbourne, Shell Research (TLGR.80.033).
ROSE, G.P. (1982) Toxicology of pyrethroids: the acute oral
and percutaneous toxicity of WL 85871 (cis-2-RIPCORD)
comparison with RIPCORD, Sittingbourne, Shell Research
(SBGR.82.130).
ROSE, G.P. (1983) Neurotoxicity of WL 85871 comparison with
WL 43467: the effect of twenty oral doses of WL 85871 or WL
43467 over a period of 4 weeks on the rat sciatic/posterior
tibial nerve, trigeminal nerve, and trigeminal ganglion,
Sittingbourne, Shell Research (SBGR.83.185).
ROSE, G.P. & DEWAR, A.J. (1978) Toxicity studies on the
insecticide WL 43467: the effect of age on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0039.78).
ROSE, G.P. & DEWAR, A.J. (1979a) Toxicity studies on the
RIPCORD/AZODRIN formulation EF 5254: biochemical and
functional studies on the neurotoxicity of the formulation EF
5254 in the rat, Sittingbourne, Shell Research (TLGR.79.027).
ROSE, G.P. & DEWAR, A.J. (1979b) A neurotoxicity study on
the pyrethroid metabolite 3-phenoxybenzoic acid (3-PBA),
Sittingbourne, Shell Research (TLGR.79.076).
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four
synthetic pyrethroids fails to show any correlation between
neuromuscular dysfunction and neurobiochemical abnormalities
in rats. Arch. Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of
pyrethroids on a nerve - muscle preparation of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 25: 176-187.
RUZO, L.O. (1983) Involvement of oxygen in the
photoreactions of cypermethrin and other halogenated
pyrethroids. J. agric. food Chem., 31: 1113-1115.
RUZO, L.O. & CASIDA, J.E. (1980) Pyrethroid photochemistry:
mechanistic aspects in reactions of the (dihalogenovinyl)-
cyclopropanecarboxylate substituent. J.C.S. Perkin Trans. I,
728-732.
RUZO, L.O., HOLMSTEAD, R.L., & CASIDA, J.E. (1977)
Pyrethroid photochemistry: decamethrin. J. agric. food Chem.,
25(6): 1385-1389.
SAAD, A.S.A., ELEWA, M.A., ALY, N.M., AUDA, M., & EL-SEBAE,
A.H. (1981) Toxicological studies on the Egyptian cotton
leafworm Spodoptera littoralis. I. Potentiation and antagonism
of synthetic pyrethroid, organophosphorus and urea derivative
insecticides. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(2):
559-571.
SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1986)
Degradation and leaching behaviour of the pyrethroid
insecticide cypermethrin in soils. J. Pestic. Sci., 11: 71-79.
SCOTT, J.G. & GEORGHIOU, G.P. (1984) Influence of
temperature on knockdown, toxicity and resistance to
pyrethroids in the housefly, Musca domestica. Pestic. Biochem.
Physiol., 21: 53-62.
SEEHY, M.A., SHALABI, H.G., SHAKER, N., & BADR, E. (1983) In
vivo induction of sister chromatid exchanges in mice by
cypermethrin. Proceedings of the International Conference on
Environmental Hazards of Agrochemicals, 1982, Vol. 1, 659-673.
SHERWOOD, C.M. & SHIRES, S.W. (1981) The effect of RIPCORD
on the invertebrate fauna of winter barley in France,
Sittingbourne, Shell Research (SBGR.81.070).
SHIRES, S.W. (1980) Soil surface predators in arable land:
the effects of farming practices. Span, 23(2): 62-64.
SHIRES, S.W. (1982a) The effect of RIPCORD on the
invertebrate fauna of winter wheat in France, Sittingbourne,
Shell Research (SBGR.81.303).
SHIRES, S.W. (1982b) A field study in France of the effects
on honey bees of an aerial application of RIPCORD in spring-
grown oil seed rape, Sittingbourne, Shell Research
(SBGR.82.066).
SHIRES, S.W. (1982c) A study of the effects of an aerial
application of RIPCORD on the invertebrate fauna of winter
wheat, Sittingbourne, Shell Research (SBGR.82.304).
SHIRES, S.W. (1983a) The use of small enclosures to assess
the toxic effects of cypermethrin in fish under field
conditions. Pestic. Sci., 14: 475-480.
SHIRES, S.W. (1983b) Pesticides and honey bees. Case studies
with RIPCORD and FASTAC. Span, 26(3): 118-120.
SHIRES, S.W. & BENNETT, D. (1982) Spray drift from aerial
application of RIPCORD to winter wheat in Kent, UK: fate and
effects in adjacent drainage ditches, Sittingbourne, Shell
Research (SBGR.82.274).
SHIRES, S.W. & BENNETT, D. (1985) Contamination and effects
in freshwater ditches, resulting from an aerial application of
cypermethrin. Ecotoxicol. environ. Saf., 9: 145-158.
SHIRES, S.W. & DEBRAY, P. (1982) Pyrethroids and the bee
problem. Shell Agric., May: 1-3.
SHIRES, S.W. & TIPTON, J.D. (1982) A study of the effects of
BIRLANE and RIPCORD on the hymenopterous parasites of white
flies on cotton in the Sudan, Sittingbourne, Shell Research
(SBGR.82.342).
SHIRES, S.W., BENNETT, D., & KANE, D.F. (1979) The effects
of WL 43467 on soil surface fauna, earthworms, and litter
composition, Sittingbourne, Shell Research (TLGR.79.074).
SHIRES, S.W., BENNETT, D., & CROSSLAND, N.O. (1980) Spray
drift from RIPCORD applications to arable crops in Suffolk,
UK: fate and effects in adjacent farm ponds, Sittingbourne,
Shell Research (TLGR.80.150).
SHONO, T. & CASIDA, J.E. (1978) Species-specificity in
enzymatic oxidation of pyrethroid insecticides:
3-phenoxybenzyl and alpha-cyano-3-phenoxybenzyl 3-(2,2-dihalo-
vinyl)-2,2-dimethylcyclopropanecarboxylates. J. Pestic. Sci.,
3: 165-168.
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of
trans- and cis-permethrin and trans- and cis-cypermethrin, and
decamethrin by microsomal enzymes. J. agric. food Chem.,
27(2): 316-325.
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation
of toxicity of pyrethroid insecticides to honey bees:
relevance to hazard in the field. Bee World, 63(4): 150-152.
SMIES, M., EVERS, R.H.J., PEIJNENBURG, F.H.M., & KOEMAN, J.H.
(1980) Environmental aspects of field trials with pyrethroids
to eradicate tsetse fly in Nigeria. Ecotoxicol. environ. Saf.,
4: 114-128.
SMITH, T.M. & STRATTON, G.W. (1986) Effects of synthetic
pyrethroid insecticides on non-target organisms. Residue Rev.,
97: 93-120.
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid
structure on rates of hydrolysis and oxidation by mouse liver
microsomal enzymes. Pestic. Biochem. Physiol., 7: 391-401.
SPIELBERGER, U., NA'ISA, B.K., KOCH, K., MANNO, A., SKIDMORE,
P.R., & COUHS, H.H. (1979) Field trials with the synthetic
pyrethroids permethrin, cypermethrin, and decamethrin against
Glossina (Diptera: gloninidae) in Nigeria. Bull. entomol.
Res., 69: 667-689.
STANDEN, M.E. (1977) The leaching and degradation of the
insecticide WL43467 when applied in sheep-dip solution to
soil, Sittingbourne, Shell Research (BLGR.0141.77)
(Unpublished report).
STELZER, K.J. & GORDON, M.A. (1984) Effects of pyrethroids
on lymphocyte mitogenic responsiveness. Res. Commun. chem.
Pathol. Pharmacol., 46(1): 137-150.
STEPHENSON, R.R. (1980a) The acute toxicity of WL 43467 to
some freshwater invertebrates in static water tests,
Sittingbourne, Shell Research (TLGR.80.040).
STEPHENSON, R.R. (1980b) The acute toxicity of cypermethrin
(WL 43467) to the freshwater shrimp (Gammarus pulex) and
larvae of the mayfly (Cloeon dipterum) in continuous-flow
tests, Sittingbourne, Shell Research (TLGR.80.079).
STEPHENSON, R.R. (1981a) RIPCORD: the acute toxicity of an
EC formulation to Tilapia nilotica in the laboratory,
Sittingbourne, Shell Research (SBGR.81.028).
STEPHENSON, R.R. (1981b) Cypermethrin: acute toxicity to
Tilapia nilotica in a continuous-flow test, Sittingbourne,
Shell Research (SBGR.81.080).
STEPHENSON, R.R. (1982a) RIPCORD: a laboratory study of the
acute toxicity of an EC formulation to Tilapia nilotica in the
presence of suspended solids, Sittingbourne, Shell Research
(SBGR.81.235).
STEPHENSON, R.R. (1982b) WL 85871 and cypermethrin: a
comparison of their acute toxicity to Salmo gairdneri, Daphnia
magna, and Selenastrum capricornutum, Sittingbourne, Shell
Research (SBGR.81.277).
STEPHENSON, R.R. (1982c) RIPCORD, BIRLANE, and FURADAN:
acute toxicity to common carp (Cyprinus carpio L.) in the
laboratory and in rice paddies, Sittingbourne, Shell Research
(SBGR.82.030).
STEPHENSON, R.R. (1982d) WL 85871 and cypermethrin: a
comparative study of their toxicity to the Fathead minnow
Pimephales promelas (Rafinesque), Sittingbourne, Shell
Research (SBGR.82.298).
STEPHENSON, R.R. (1982e) Aquatic toxicology of cypermethrin.
I. Acute toxicity to some freshwater fish and invertebrates in
laboratory tests. Aquat. Toxicol., 2: 175-185.
STEPHENSON, R.R. (1983) Pesticides and freshwater animals. A
case study with RIPCORD. Span, 26(3): 121-122.
STEPHENSON, R.R., CHOI, S.Y., & OLMOS-JEREZ, A. (1984)
Determining the toxicity and hazard to fish of a rice
insecticide. Crop Prot., 3(2): 151-165.
STEVENS, J.E.B. & HILL, I.R. (1980) Mobility of cypermethrin
and its degradation products in soil columns, Fernhurst,
Imperial Chemical Industries (Report No. RJ/0166-B)
(Unpublished ICI data).
SUHAS, Y. & DEVAIAH, M.C. (1985) Studies on the effect of
insecticides sprayed mulberry leaves to silkworm, Bombyx mori
L. Pesticides, 19(10): 53-54, 57.
SUNDARARAJAN, R. & CHAWLA, R.P. (1983) Simple, sensitive
technique for detection and separation of halogenated
synthetic pyrethroids by thin layer chromatography. J. Assoc.
Off. Anal. Chem., 66(4): 1009-1012.
SURULIVELU, T. & MENON, M.V. (1982) Contact toxicity of
synthetic pyrethroids, organophosphorus and carbamate
insecticides to adults of the parasite Chelonus blackburni
Cameron. J. Agric. Sci. Camb., 98: 331-334.
SUTHERS, J.R. & MARLOW, R.G. (1981) A study of the exposure
and health of Indian workers spraying RIPCORD on cotton over
five consecutive days using mistblower and knapsack
applicators, The Hague, Shell Internationale Research Mij (TOX
81-003).
SWAINE, H. & SAPIETS, A. (1980a) Residue transfer study with
dairy cows fed on a diet containing the insecticide.
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0186-B).
SWAINE, H. & SAPIETS, A. (1980b) Residue levels of the major
metabolites of the insecticide in the milk and tissues of
dairy cows fed on a diet containing cypermethrin at 50 mg/kg,
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0198-B).
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., & ASKAR,
A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46(1): 387-391.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985a) Hydrolysis of the pyrethroid insecticide cypermethrin
in aqueous media. J. Pestic. Sci., 10: 643-648.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985b) Photodegradation of the pyrethroid insecticide
cypermethrin in water and on soil surface. J. Pestic. Sci.,
10: 629-642.
TAYLOR, S.M., ELLIOTT, C.T., & BLANCHFLOWER, J. (1985)
Cypermethrin concentrations in hair of cattle after
application of impregnated ear tags. Vet. Rec., 116(23): 620.
TESH, J.M., TESH, S.A., & DAVIES, W. (1978) WL 43467:
effects upon the progress and outcome of pregnancy in rat,
Stock, Life Science Research (LSR Report No. 78/SHL2/364).
TEWARI, G.C. & KRISHNAMOORTHY, P.N. (1985) Selective
toxicity of some synthetic pyrethroids and conventional
insecticides to aphid predator, Menochilus sexmaculatus
Fabricius. Indian J. agric. Sci., 55(1): 40-43.
TRIGG, C.E., BUTTERWORTH, S., & HUNT, P.F. (1977)
Neurotoxicity of pyrethroids: a study of teased nerves from
rats fed WL 43467 for 12 months, Sittingbourne, Shell Research
(TLGR.0137.77).
TU, C.M. (1980) Influence of five pyrethroids insecticides
on microbial populations and activities in soil. Microbiol.
Ecol., 5: 321-327.
TU, C.M. (1982) Effects of some pesticides on Rhizobium
japonicum and on the seed germination and pathogens of
soybean. Chemosphere, 11(10): 1027-1033.
US EPA (1984) Cypermethrin: tolerances for residues in or on
raw agricultural commodities: final rule. Part III. Fed. Reg.,
49(117): 24865-24872.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the
peripheral nervous system of the frog. Pestic. Sci., 8:
692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Effects of
insecticides on the sensory system of Xenopus. Insect neurobiology
and pesticide action, London, Society of Chemical
Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M.
(1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effects of insecticides on the sensory nervous system.
In: Narashashi, T., ed. Neurotoxicology of insecticides and
pheromones, New York, London, Plenum Publishing Corporation,
pp. 183-210.
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985a)
Human oral dose-excretion study with RIPCORD, The Hague, Shell
Internationale Petroleum Maatschappy (HSE.85.008).
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985b)
Human dermal dose-excretion study with RIPCORD, The Hague,
Shell Internationale Petroleum Maatschappy (HSE.85.009).
VEKARIA, M.V. & VYAS, H.N. (1985) Studies on ovicidal
toxicity of certain insecticides against the eggs of Heliothis
armigera Hubner. Pesticides, 19(10): 43-44.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationships of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index. JMPR 1961-84, Geneva, World Health
Organization.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in
frog myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system.
Neuropathol. appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides
on the lateral-line sense organ and on peripheral nerves of
the clawed frog, Xenopus laevis. Pestic. Biochem. Physiol.,
18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on
sodium channel gating in myelinated nerves. Nature (Lond.),
295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature- and
structure-dependent interaction of pyrethroids with the sodium
channels in frog node of Ranvier. Biochim. biophys. Acta, 728:
73-82.
WADDILL, V.H. (1978) Contact toxicity of four synthetic
pyrethroids and methomyl to some adult insect parasites.
Florida Entomol., 61(1): 27-30.
WALLACE, B.G., ROBERTS, T.R., & MCKERRELL, E.H. (1982)
Cypermethrin. A residue transfer study with laying hens,
Sittingbourne, Shell Research (SBTR.82.059).
WATTERS, F.L., WHITE, N.D.G., & COTE, D. (1983) Effect of
temperature on toxicity and persistence of three pyrethroid
insecticides applied to fir plywood for the control of the red
flour beetle (Coleoptera: Tenebrionidae). J. econ. Entomol.,
76: 11-16.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23 .
WHO (1985) WHO Technical Report Series, No. 720 (Safe use of
pesticides. Ninth Report of the WHO Expert Committee on Vector
Biology and Control), pp. 14-19 .
WHO/FAO (1984) Cypermethrin, Geneva, World Health
Organization (Data Sheets on Pesticides, No. 84.58).
WILDE, G., KADOUM, A., & MIZE, T. (1984) Absence of
synergism with insecticides combinations used on chinch bugs
(Heteroptera: Lygaeridae). J. econ. Entomol., 77: 1297-1298.
WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic
pyrethoid insecticides to predaceous phytoseiid. Aust. J.
agric. Res., 30: 497-501.
WOOD, MACKENZIE, & CO. (1980) Pyrethroids, Agrochem. Monit.,
9: 3-14.
WOOD, MACKENZIE, & CO. (1981) Pyrethroids. Agrochem. Monit.,
15: 3-27.
WOOD, MACKENZIE, & CO. (1982) Pyrethroids. Agrochem. Monit.,
21: 3-17.
WOOD, MACKENZIE, & CO. (1983) Pyrethroids. Agrochem. Monit.,
27: 3-12.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual,
7th ed., Croydon, British Crop Protection Council, pp. 150-151.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of
pyrethroids. Gen. Pharmacol., 9: 387-398.
WRIGHT, A.N., ROBERTS, T.R., DUTTON, A.J., & DOIG, M.V.
(1980) The metabolism of cypermethrin in plants: the
conjugation of the cyclopropyl moiety. Pestic. Biochem.
Physiol., 13: 71-80.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G.
(1979) Toxicity of permethrin, decamethrin, and related
pyrethroids to salmon and lobster. Bull. environ. Contam.
Toxicol., 21: 338-343.
ZOHDY, G.I., OSMAN, A.A., & MOMEN, F.M. (1984) Toxicity of
some pyrethroid compounds to the predatory mite, Amblyseius
gossipi El-Badry. In: Griffiths, D.A. & Bowman, C.E., ed.
Acarology VI, Chichester, Ellis Horwood Ltd., Vol. 2, pp.
659-662.
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana esculenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3 - 10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittantly, cause large
depolarizing afterpotentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids
(allethrin, cismethrin, bioresmethrin, and 1R, cis-phenothrin)
caused moderate presynaptic repetitive activity, resulting in the
occurrence of multiple end-plate potentials (Ruigt & Van den
Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses.
The duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependent depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butyl-bicyclophosphoro-thionate [35S]-TBPS. Studies with 37
pyrethroids revealed an absolute correlation, without any false
positive or negative, between mouse intracerebral toxicity and in
vitro inhibition: all toxic cyano compounds including
deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin, and
[2S, alphaS]-fenvalerate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin
and [2S,alpha S]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA
receptorionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive muscle
action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids,
is not necessarily due to an exclusive central site of action. It
may be related to the long-lasting repetitive activity in sense
organs and possibly in other parts of the nervous system, which, in
a more advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established
that sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
 IUPAC chemical name (RS)-alpha-cyano-3-phenoxybenzyl(1RS)-
cis-, trans-3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylate
CAS chemical name (RS)-cyano(3-phenoxyphenyl)methyl(1RS)-
cis- trans-3-(2,2-dichloroethenyl)-2.2-
dimethylcyclopropane carboxylate
CAS registry number
IUPAC chemical name (RS)-alpha-cyano-3-phenoxybenzyl(1RS)-
cis-, trans-3-(2,2-dichlorovinyl)-2,2-
dimethylcyclopropane carboxylate
CAS chemical name (RS)-cyano(3-phenoxyphenyl)methyl(1RS)-
cis- trans-3-(2,2-dichloroethenyl)-2.2-
dimethylcyclopropane carboxylate
CAS registry number  Alternative procedures, based on high-performance liquid
chromatography with UV detection (HPLC/UV) and TLC with a
colorimetric end point, have been described, but have not been
widely adopted, because of the simplicity and sensitivity of the
GC/ECD methods. This is also true for more elaborate procedures
based on hydrolysis and derivatization.
Procedures have also been developed for the determination of
the more important cypermethrin metabolites, 3-phenoxybenzoic acid
(PBA), the cyclopropane carboxylic acid (CPA), and the amide.
Following extraction and clean-up, these materials are determined
by HPLC/UV or by GC procedures, after derivatization in the case of
the two acids.
The Codex Committee on Pesticide Residues lists recommended
methods for the determination of cypermethrin residues (FAO/WHO
1986).
The methods for residue, environmental, and product analysis
for cypermethrin are summarized in Table 2.
Table 2. Published analytical methods for cypermethrin
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Residue
analysis
Apple n-hexane:acetone extraction silica gel/ electron capture 0.01 Baker &
Pear (1:1) solvent:H2O CH2Cl2 detection-gas 0.01 Bottomley
Cabbage chromatography 0.01 (1982)
Potato 0.01
Apple n-hexane:acetone extraction silica gel/ high-performance liquid 0.2 Baker &
Pear (1:1) solvent:H2O CH2Cl2 chromatography 0.2 Bottomley
Cabbage 0.2 (1982)
Potato 0.2
Onion CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
Carrot (2:1) ether: n- detection-gas al. (1982)
hexane chromatography
Celery CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Wheat n-hexane:acetone 2% NaCl:extrac- Florisil/ electron capture 0.02 Joia et
grain (1:1) tion solvent benzene detection-gas al. (1981,
flour chromatography 1985a)
bran
middling
Beef CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
muscle (85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Egg yolk CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
(85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Milk CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Cotton n-hexane Florisil/ n- electron capture Estesen et
foliage hexane:EtOAc detection-gas al. (1982)
(dislodgable chromatography
residue)
Environmental
analysis
Fish n-hexane:acetone alumina/ n- electron capture McLeese et
Shrimp (1:1) hexane:benzene detection-gas al. (1980)
chromatography
Water XAD-2 resin: extraction sol- electron capture McLeese et
Seawater acetone vent: n-hexane detection-gas al. (1980)
chromatography
Soil acetone sat. Na2SO4: electron capture Harris et
n-hexane detection-gas al. (1981)
chromatography
Soil CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
(2:1) ether: n-hexane detection-gas al. (1982)
chromatography
Product
analysis
Technical n-hexane flame ionization Chapman &
grade detection-gas Simmons
chromatography (1977)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Technical methylene flame ionization Bland
and chloride (con- detection-capillary gas (1985)
formulated taining as chromatography
material internal stan-
dard dicyclo-
hexylphthalate)
---------------------------------------------------------------------------------------------------------
a GLC = gas-liquid chromatography.
HPLC = high-performance liquid chromatography.
b LD = limit of determination. (The lower practical limit of determination for most of the analytical
methods based on GLC is usually 0.01 mg/kg. The actual level achievable, however, depends to some
extent on the substrate and to a great extent on the intensity of the clean-up steps in the
procedure).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial Production
Cypermethrin was synthesized by Elliott et al. in 1974. It was
prepared by the esterification of a chloro analogue of chrysanthemic
acid (1R, 1S or 1RS, 3R, 3S, or cis-, trans-)-2,2-dimethyl-3-(2,2-
dichlorovinyl)cyclopropanecarboxylic acid with (alphaR, alphaS, or
alphaRS)-alpha-cyano-3-phenoxybenzyl alcohol. Today there are many
other methods of preparation.
Cypermethrin has been marketed since 1977. Recent global
production figures are given in Table 3.
Table 3. Global production of cypermethrin
-------------------------------------------------
Year Production Reference
(tonnes)
-------------------------------------------------
1979 200 Wood, Mackenzie, & Co. (1980)
1980 380 Wood, Mackenzie, & Co. (1981)
1981 375 Wood, Mackenzie, & Co. (1982)
1982 340 Wood, Mackenzie, & Co. (1983)
-------------------------------------------------
3.2. Use Patterns
Cypermethrin is a highly active synthetic pyrethroid
insecticide, effective against a wide range of pests in many crops.
According to Battelle (1982), global consumption of cypermethrin
amounted to 159 tonnes in 1980. Fifty-eight tonnes were consumed
in Africa and 9 tonnes in western Europe. Global production in
1982 was 340 tonnes. Cypermethrin was mainly (92.5%) used on
cotton, the major consumer areas being Turkey (47 tonnes), Central
America (44 tonnes), and Egypt (25 tonnes) (Battelle, 1982). Other
agricultural uses included the treatment of hop, vegetables, and
maize. Cypermethrin is also used for the control of veterinary and
public health insects, such as flies, lice, and mites and, in the
United Kingdom, it is used as a wood preservative.
Cypermethrin is formulated as emulsifiable concentrates (100
and 250 g/litre), ultra-low-volume concentrate (10 - 50 g/litre),
wettable powder (125 g/kg), and animal dip concentrate (5 - 15%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSPORTATION
4.1. Transport and Distribution between Media
Because of its physical and chemical characteristics,
cypermethrin is comparatively immobile in the outdoor environment
and transport between media is restricted. It has a very low
vapour pressure and water solubility and is strongly adsorbed from
aqueous solutions by solid surfaces. This drastically restricts
its movement in air and water, and particularly in soils.
4.1.1. Transport from soil to water
Kaufman et al. (1981), working in the laboratory with radio-
labelled cypermethrin in soil columns, down which a volume of water
equivalent to their moisture equivalent was allowed to percolate,
reported virtually no movement of radioactivity below the top
2.5 cm. Using the procedure introduced by Helling & Turner (1968),
where the mobility was studied using thin layer chromatographic
(TLC) plates, very little movement of cypermethrin occurred in
soils. However, radio-labelled PBA leached down the soil columns
to a level of about 8 cm and CPA reached a maximum concentration at
this level. On the basis of the Helling nomenclature for soil
TLC, CPA and PBA were of "intermediate mobility" to "mobile".
While the mobilities of CPA and PBA were relatively little affected
by the organic matter content of the soil, pH appeared to be a most
important factor, mobility being greatest in soils of highest pH,
presumably because of increased dissociation.
Stevens & Hill (1980) studied the leaching of cypermethrin in
the laboratory in 4 different soil types, a clay loam, a loamy
sand, a coarse sand, and a fen peat. The compound was incubated
for three weeks with each soil under aerobic conditions. The soils
were then packed into glass columns and leached with 67.5 cm water
over a 10-week period. At the end of the period of incubation, a
substantial proportion of the cypermethrin had been lost as 14CO2
(up to a third in one case), and only minor amounts of degradation
products had been formed. It was found that, after the leaching
period, more than 99% of the 14C residue remained within the top
5 cm in all the soils. Radioactivity in the leachate was below the
limit of determination in all cases.
In laboratory studies using labelled cypermethrin and soil
columns, Jackson (1977) found little penetration of cypermethrin
below the top 2-cm layer, even after the percolation of 1.35 metres
of water.
In a further study on the percolation of distilled water
through sandy loam soils containing 14C-benzyl cypermethrin from
spent sheep-dip baths, Standen (1977) reported that up to 0.3% of
the applied radioactivity was leached out. However, most of the
radioactivity was associated with fine soil particles in the
leachate and could not be extracted with organic solvents. The
water contained small amounts of unchanged cypermethrin and PBA.
Most (89%) of the radioactivity was contained in the top 14 cm of
the columns, mainly as cypermethrin itself.
Sakata et al. (1986) studied the leaching with distilled water
of radio-labelled cypermethrin through columns of 4 different types
of soil in the laboratory. The water flow was at a rate of 3 ml/h
and was continued for 3 weeks at 25 °C, so that the total flow
through the column was equivalent to about 3 metres. Cypermethrin
was relatively resistent to leaching but radioactivity was found in
the leachate, especially in one sandy soil where, after 30 days of
incubation, about 30% of the cyclopropyl label first added was
collected in the leachate. The major products associated with
radioactivity in the leachates were either CPA or PBA, depending on
the position of the label. Unchanged cypermethrin was present only
in trace amounts in sand containing less than 0.1% organic matter.
4.1.2. Transport within water bodies
Cypermethrin moves slowly in water bodies. In the experimental
overspraying of ponds carried out by Crossland (1982) and described
in detail in section 4.4.2, it was calculated that 48 h after
treatment with 100 g cypermethrin/ha, only about 8 - 16% of the
amount applied could be found underneath the surface film of 0.05
mm depth. In all of Crossland's studies, the levels of
cypermethrin residues in the sediment at the bottom of the ponds
were below 7 µg/kg.
With spray levels applied according to normal agricultural
practice, Crossland et al. (1982) found that water bodies adjacent
to sprayed arable fields in the United Kingdom received only four-
five orders of magnitude less cypermethrin per m2 than the land
itself and that the initial concentration in the surface film of
water was between 6 and 20 µg/litre. Residues in the water below
the surface film did not reach more than 0.1 µg/litre and within
24 h the levels had nearly all fallen to below the limit of
determination of 0.01 µg/litre. A similar study in French
vineyards showed comparable results, though the initial
concentrations reached in the surface films were higher, probably
because conditions in the area were more favourable for spray drift
than those in the British study.
Shires & Bennett (1985) reported similar results concerning
water in drainage ditches adjacent to cereal fields in the United
Kingdom treated with an aerial spray application of 25 g
cypermethrin/ha.
From the available studies, it can be concluded that
contamination of water bodies by overspray is likely to be very
superficial and of comparatively short duration.
4.2. Abiotic Degradation
4.2.1. Photodegradation
4.2.1.1. Basic studies
According to Ruzo et al. (1977), cypermethrin is one of the
more light-stable pyrethroids. Thus, when exposed in the solid
phase to sunlight for 30 h, no loss of cypermethrin was detected.
When exposed in methanol solution to light of wavelength > 290 nm
for about 2 days, 55% of cypermethrin was recovered, but no data on
the photodecomposition products formed were reported. According to
Ruzo & Casida (1980), the reaction quantum yield at 300 nm in
methanol was low, at 0.022. Ruzo (1983) further demonstrated the
comparative resistance of cypermethrin to irradiation in his
studies on the involvement of oxygen in the photodegradation of
pyrethroids.
Cypermethrin is more susceptible to radiation of lower
wavelengths; Lauren & Henzel (1977) reported that under ultra-
violet radiation, 90% of cypermethrin on a glass petri dish was
decomposed after 3 days, but only 45% was decomposed after 3 days
when the cypermethrin was deposited on grass and placed under an
UV-lamp.
4.2.1.2. Photodegradation
(a) Water
Day & Leahey (1980) studied the effects of sunlight on dilute
aqueous solutions of cypermethrin. In their studies, 14C-labelled
cis- or trans-isomers were used with the label in either the
cyclopropyl or the benzyl ring. They were dissolved in sterile
aqueous acetonitrile at a concentration of 1 mg/litre, irradiated
in sunlight for 32 days and the irradiated solutions compared with
controls stored for the same length of time in the dark. The
degree of photodegradation was very limited. At the end of the
study, 89.4% of the cypermethrin remained in the case of the
irradiated benzyl label, compared with 97.4% in the dark control.
Corresponding figures for the cyclopropyl label were 92.3 and
96.8%. Six of the 8 photodegradation products separated by
chromatography were positively identified; cis- and trans-CPA,
phenoxybenzyl alcohol, aldehyde and acid, and alpha-cyano-3-
phenoxybenzyl alcohol.
The effects of natural sunlight on aqueous solutions of the
(1R, cis-, alpha RS) and (1R, trans-, alpha RS) isomers were
studied by Takahashi et al. (1985a,b). The products were labelled
with 14C in either the cyclopropyl ring, the benzyl ring, or the
cyano carbon. The aqueous solutions were made from distilled
water, 2% acetone, aqueous humic acid, sea water, or natural river
water (both of which had been filtered). The isomers were added to
the water in the form of a stabilized suspension using Tween 20 to
give 50 µg/litre test suspension. The rates of degradation of the
isomers were very rapid compared with those reported by other
authors, however, a large part of the changes involved
transformation to other isomers. The degradation was more rapid in
river or sea water (half-life of cis-isomer, 0.6 - 0.7 days) than
in distilled water or humic acid (half-life of cis-isomers, 2.3 -
2.6 days), but the most rapid change of all occurred in the
presence of acetone. Presumably the differences were due to the
well known effect of photosensitizaton by the acetone or organic
constituents of the natural waters. The main degradation products,
in addition to the different isomers, were CPA, PBA together with
smaller amounts of the corresponding aldehyde, and carbon dioxide,
especially in the case of the cyano label. There was evidence of
further degradation of CPA and PBA.
(b) Soil
Hall et al. (1981) studied the photodegradation of cypermethrin
on the soil surface. Labelled cypermethrin, as used by Day &
Leahey, was applied to very thin soil plates (0.5 mm) at a rate
equivalent to about 200 g/ha. The plates were exposed to sunlight
in the open air protected against rain by polythene sheeting, when
necessary; the sheeting was transparent to the UV component of
sunlight. The plates were extracted after an exposure period of 32
days and the extracts chromatographed. In the case of the
cyclopropyl label, 63% of the radioactivity initially applied to
the irradiated plate was recovered, compared with 103.5% from the
plate that had been kept in the dark. The half-life of the
cyclopropyl-labelled cypermethrin was reduced from > 32 days to 8
- 16 days by irradiation with natural light. The main degradation
products appear to have been the amide, together with cis- and
trans-CPA, and some unidentified (partly volatile) products. In
the case of the benzyl label, the degradation products identified
were mainly the amide analogue of cypermethrin and various
phenoxybenzyl derivatives, such as the alcohol, aldehyde, and acid.
In these studies, the amide was the most prominent, even in the
unirradiated sample, and in this respect, the results differ
somewhat from those obtained in other soil incubation studies,
where the main metabolite from benzyl-labelled cypermethrin was
PBA, with the amide occurring only as a very minor product.
Takahashi et al. (1985a,b), working with the same products as
they used for their study on water, applied the labelled products
at 1.1 µg/cm2 to half-millimetre layers of 3 different soils and
found very rapid degradation in the irradiated soils compared with
those kept in the dark. The half-lives ranged from between 0.6 and
1.9 days with sunlight and > 7 days in the dark. With regard to
degradation products, the results were rather similar to those
reported by Hall et al. (1981) in that the main degradation product
was the amide of the otherwise intact isomers. In addition, they
found smaller amounts of PBA, virtually no CPA, but occasionally
small amounts of alpha carbamoyl- and alpha carboxyphenoxybenzyl
alcohol. In one of the soils in which degradation was the
highest, nearly half of the radio-labelled carbon was unextractable
at the end of the exposure period. In contrast with the water
study, there was very little evidence of isomerization of the
parent isomers.
There is no obvious explanation for the different rates of
degradation under the influence of irradiation and it is difficult
to extrapolate the results of these studies to the practical
situation. It appears likely that photochemical reactions will
hasten the degradation of deposits of cypermethrin on exposed
surfaces and possible residues in water, but there is little
indication that they greatly change the degradation pathways.
4.3. Biological Degradation in Soil
Cypermethrin degrades relatively quickly in soils, primarily by
biological processes involving cleavage of the ester linkages, to
give the two main degradation products, CPA and PBA. These
products are themselves subsequently mineralized. There is also
evidence for the formation, as an intermediate, of the amide of the
intact molecule and occasionally the 4-hydroxy phenoxy analogue.
Neither of the latter products appears to persist in the soil
(Leahey 1979; Sakata et al., 1986).
4.3.1. Mechanism
Chapman et al. (1981), who studied the effects of sterilization
of soils on the rate of cypermethrin degradation in the laboratory,
demonstrated that degradation in the soil was essentially a
biological process. Cypermethrin was added at 1 mg/kg to the soils
(either untreated or sterilized) and the soils incubated for 16
weeks, by which time the sterilized soils were considered to have
become contaminated. The experiment was conducted under strictly
aerobic conditions. It was found that 84% of the added material
had degraded in natural organic soil compared with only 8% in the
sterilized organic soil. The corresponding values for the mineral
soil were 96% and 7%. The small amounts of degradation in the
sterilized soils presumably resulted from residual microbial
activity, especially in the later stages of the study.
4.3.2. Degradation pathways (separate isomers)
In order to study degradation pathways, Roberts & Standen
(1977, 1981) carried out a series of soil studies in the laboratory
using either the racemic cis- or trans-isomers of cypermethrin or
mixtures of the two. The compounds were 14C-radio-labelled in
either the benzyl or the cyclopropyl ring and were added to the
soil at a rate of 2.5 mg/kg moist soil. Incubation with 3
different soils was carried out under either aerobic or anaerobic
conditions, initially for 16 weeks and subsequently for a total of
52 weeks. Experiments were also conducted using biometer flasks,
in order to measure the output of radio-labelled carbon dioxide.
In the case of the cis-isomer, the main degradation products
extracted from the soils were PBA, cis-CPA with small amounts of
trans-CPA, and limited amounts of the 4-hydroxy derivative of
cypermethrin. Between 25% and 30% of the added radioactivity could
not be extracted with acetonitrile/water. A similar spectrum of
degradation products was extracted in the trans-isomer experiments,
except that the cis-isomer was absent. Some of the remaining
radioactivity was identified in a further degradation product of
CPA, the dicarboxylic acid.
A further study was undertaken by Roberts & Standen (1981) in
which ring-labelled cis- and trans-isomers of CPA were added to a
sandy loam soil at 2.5 - 13.5 mg/kg. In most cases, the soils were
contained in loosely stoppered vessels but, in one experiment, a
biometer flask fitted with a caustic potash trap was used, in order
to measure carbon dioxide (CO2) production. In spite of the
production of labelled CO2 in the initial study with cyclopropyl-
labelled cypermethrin, very little was produced in the latter study
and most of the radioactivity was shown to be still present in the
soil. At the end of the 8-week exposure period, it was found that
the greater part of the radioactivity in the soil was still
associated with unchanged CPA, 33 - 65% in the case of the trans-
acid and 78% in the case of the cis-acid. There was also evidence
that some of the trans-CPA was transformed to the cis-isomer, but
not vice-versa. This finding was analogous to that with the parent
compound where a certain amount of cis-CPA was produced from trans-
cypermethrin.
A similar series of studies was carried out by Sakata et al.
(1986) who incubated 2 Japanese soils with the 1R cis-RS alpha and
1R trans-RS alpha isomers of cypermethrin for up to 168 days at
25 °C. While ester cleavage was the principal pathway of
degradation, limited production of the amides of the intact esters
and production of the 4-hydroxy derivatives (on the phenoxy group)
were also reported. The latter were often present in greater
amounts than the PBA or CPA fragments. The authors also reported
the presence of small amounts of the desphenoxy derivative derived
from ether cleavage, not previously reported for cypermethrin.
However, the level of 14C associated with extractable breakdown
products was low (1 - 17% of the amount initially added, at 56
days) compared with that of bound radio carbon (14 - 58% at 56
days), the actual levels being very dependent on the type of soil
under study. Since the trans-isomers degraded more readily than
the cis-isomers and since the level of free degradation products
was considerably lower for the trans- than the cis-isomers, it is
possible that a substantial proportion of the bound radio carbon
had reverted to the general carbon pool of the soil organic matter.
A major proportion of the added label was recovered as carbon
dioxide (16 - 48% at 56 days) and the amount was highest when the
label was on the benzyl carbon, indicating, as Roberts & Standen
had found, that the PBA was mineralized more readily than the CPA.
Sakata et al. also found that, under comparable circumstances, the
cis-isomers produced carbon dioxide more slowly than the trans-
isomers.
The principal degradation products in soils, prior to breakage
of the benzyl and cyclopropane rings, are shown in Fig. 2.
4.3.3. Rates of degradation
4.3.3.1. Laboratory studies
(a) Separate isomers
In the laboratory studies carried out by Roberts & Standen
(1977, 1981), the half-lives of the cis-isomers were around 4
weeks, except in the inactive Los Palacios soils, where the figure
was nearer to 10 - 12 weeks. The trans-isomer generally exhibited
a much shorter half-life of less than 2 weeks and less than 4 weeks
on the less active soil. After a year, the amounts of unchanged
material left in the soils were very low and nearly always below
10% of the amount applied. But, even at the low levels remaining
after such a long interval, residues of the trans- were still
substantially less than those of the cis-product.
Alternative procedures, based on high-performance liquid
chromatography with UV detection (HPLC/UV) and TLC with a
colorimetric end point, have been described, but have not been
widely adopted, because of the simplicity and sensitivity of the
GC/ECD methods. This is also true for more elaborate procedures
based on hydrolysis and derivatization.
Procedures have also been developed for the determination of
the more important cypermethrin metabolites, 3-phenoxybenzoic acid
(PBA), the cyclopropane carboxylic acid (CPA), and the amide.
Following extraction and clean-up, these materials are determined
by HPLC/UV or by GC procedures, after derivatization in the case of
the two acids.
The Codex Committee on Pesticide Residues lists recommended
methods for the determination of cypermethrin residues (FAO/WHO
1986).
The methods for residue, environmental, and product analysis
for cypermethrin are summarized in Table 2.
Table 2. Published analytical methods for cypermethrin
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Residue
analysis
Apple n-hexane:acetone extraction silica gel/ electron capture 0.01 Baker &
Pear (1:1) solvent:H2O CH2Cl2 detection-gas 0.01 Bottomley
Cabbage chromatography 0.01 (1982)
Potato 0.01
Apple n-hexane:acetone extraction silica gel/ high-performance liquid 0.2 Baker &
Pear (1:1) solvent:H2O CH2Cl2 chromatography 0.2 Bottomley
Cabbage 0.2 (1982)
Potato 0.2
Onion CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
Carrot (2:1) ether: n- detection-gas al. (1982)
hexane chromatography
Celery CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Wheat n-hexane:acetone 2% NaCl:extrac- Florisil/ electron capture 0.02 Joia et
grain (1:1) tion solvent benzene detection-gas al. (1981,
flour chromatography 1985a)
bran
middling
Beef CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
muscle (85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Egg yolk CH3CN:H2O n-hexane:2% Florisil/ electron capture 0.005 Braun &
(85:15) NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Milk CH3CN n-hexane:2% Florisil/ electron capture 0.005 Braun &
NaCl solution CH3CN/CH2Cl2: detection-gas Stanek
n-hexane chromatography (1982)
Cotton n-hexane Florisil/ n- electron capture Estesen et
foliage hexane:EtOAc detection-gas al. (1982)
(dislodgable chromatography
residue)
Environmental
analysis
Fish n-hexane:acetone alumina/ n- electron capture McLeese et
Shrimp (1:1) hexane:benzene detection-gas al. (1980)
chromatography
Water XAD-2 resin: extraction sol- electron capture McLeese et
Seawater acetone vent: n-hexane detection-gas al. (1980)
chromatography
Soil acetone sat. Na2SO4: electron capture Harris et
n-hexane detection-gas al. (1981)
chromatography
Soil CH3CN:H2O CH2Cl2 Florisil/ electron capture Frank et
(2:1) ether: n-hexane detection-gas al. (1982)
chromatography
Product
analysis
Technical n-hexane flame ionization Chapman &
grade detection-gas Simmons
chromatography (1977)
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Sample Sample preparation Method of determination LDb Reference
Extraction Partition Clean-up GLC or HPLC conditiona (mg/kg)
solvent Column/elution
---------------------------------------------------------------------------------------------------------
Technical methylene flame ionization Bland
and chloride (con- detection-capillary gas (1985)
formulated taining as chromatography
material internal stan-
dard dicyclo-
hexylphthalate)
---------------------------------------------------------------------------------------------------------
a GLC = gas-liquid chromatography.
HPLC = high-performance liquid chromatography.
b LD = limit of determination. (The lower practical limit of determination for most of the analytical
methods based on GLC is usually 0.01 mg/kg. The actual level achievable, however, depends to some
extent on the substrate and to a great extent on the intensity of the clean-up steps in the
procedure).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Industrial Production
Cypermethrin was synthesized by Elliott et al. in 1974. It was
prepared by the esterification of a chloro analogue of chrysanthemic
acid (1R, 1S or 1RS, 3R, 3S, or cis-, trans-)-2,2-dimethyl-3-(2,2-
dichlorovinyl)cyclopropanecarboxylic acid with (alphaR, alphaS, or
alphaRS)-alpha-cyano-3-phenoxybenzyl alcohol. Today there are many
other methods of preparation.
Cypermethrin has been marketed since 1977. Recent global
production figures are given in Table 3.
Table 3. Global production of cypermethrin
-------------------------------------------------
Year Production Reference
(tonnes)
-------------------------------------------------
1979 200 Wood, Mackenzie, & Co. (1980)
1980 380 Wood, Mackenzie, & Co. (1981)
1981 375 Wood, Mackenzie, & Co. (1982)
1982 340 Wood, Mackenzie, & Co. (1983)
-------------------------------------------------
3.2. Use Patterns
Cypermethrin is a highly active synthetic pyrethroid
insecticide, effective against a wide range of pests in many crops.
According to Battelle (1982), global consumption of cypermethrin
amounted to 159 tonnes in 1980. Fifty-eight tonnes were consumed
in Africa and 9 tonnes in western Europe. Global production in
1982 was 340 tonnes. Cypermethrin was mainly (92.5%) used on
cotton, the major consumer areas being Turkey (47 tonnes), Central
America (44 tonnes), and Egypt (25 tonnes) (Battelle, 1982). Other
agricultural uses included the treatment of hop, vegetables, and
maize. Cypermethrin is also used for the control of veterinary and
public health insects, such as flies, lice, and mites and, in the
United Kingdom, it is used as a wood preservative.
Cypermethrin is formulated as emulsifiable concentrates (100
and 250 g/litre), ultra-low-volume concentrate (10 - 50 g/litre),
wettable powder (125 g/kg), and animal dip concentrate (5 - 15%).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSPORTATION
4.1. Transport and Distribution between Media
Because of its physical and chemical characteristics,
cypermethrin is comparatively immobile in the outdoor environment
and transport between media is restricted. It has a very low
vapour pressure and water solubility and is strongly adsorbed from
aqueous solutions by solid surfaces. This drastically restricts
its movement in air and water, and particularly in soils.
4.1.1. Transport from soil to water
Kaufman et al. (1981), working in the laboratory with radio-
labelled cypermethrin in soil columns, down which a volume of water
equivalent to their moisture equivalent was allowed to percolate,
reported virtually no movement of radioactivity below the top
2.5 cm. Using the procedure introduced by Helling & Turner (1968),
where the mobility was studied using thin layer chromatographic
(TLC) plates, very little movement of cypermethrin occurred in
soils. However, radio-labelled PBA leached down the soil columns
to a level of about 8 cm and CPA reached a maximum concentration at
this level. On the basis of the Helling nomenclature for soil
TLC, CPA and PBA were of "intermediate mobility" to "mobile".
While the mobilities of CPA and PBA were relatively little affected
by the organic matter content of the soil, pH appeared to be a most
important factor, mobility being greatest in soils of highest pH,
presumably because of increased dissociation.
Stevens & Hill (1980) studied the leaching of cypermethrin in
the laboratory in 4 different soil types, a clay loam, a loamy
sand, a coarse sand, and a fen peat. The compound was incubated
for three weeks with each soil under aerobic conditions. The soils
were then packed into glass columns and leached with 67.5 cm water
over a 10-week period. At the end of the period of incubation, a
substantial proportion of the cypermethrin had been lost as 14CO2
(up to a third in one case), and only minor amounts of degradation
products had been formed. It was found that, after the leaching
period, more than 99% of the 14C residue remained within the top
5 cm in all the soils. Radioactivity in the leachate was below the
limit of determination in all cases.
In laboratory studies using labelled cypermethrin and soil
columns, Jackson (1977) found little penetration of cypermethrin
below the top 2-cm layer, even after the percolation of 1.35 metres
of water.
In a further study on the percolation of distilled water
through sandy loam soils containing 14C-benzyl cypermethrin from
spent sheep-dip baths, Standen (1977) reported that up to 0.3% of
the applied radioactivity was leached out. However, most of the
radioactivity was associated with fine soil particles in the
leachate and could not be extracted with organic solvents. The
water contained small amounts of unchanged cypermethrin and PBA.
Most (89%) of the radioactivity was contained in the top 14 cm of
the columns, mainly as cypermethrin itself.
Sakata et al. (1986) studied the leaching with distilled water
of radio-labelled cypermethrin through columns of 4 different types
of soil in the laboratory. The water flow was at a rate of 3 ml/h
and was continued for 3 weeks at 25 °C, so that the total flow
through the column was equivalent to about 3 metres. Cypermethrin
was relatively resistent to leaching but radioactivity was found in
the leachate, especially in one sandy soil where, after 30 days of
incubation, about 30% of the cyclopropyl label first added was
collected in the leachate. The major products associated with
radioactivity in the leachates were either CPA or PBA, depending on
the position of the label. Unchanged cypermethrin was present only
in trace amounts in sand containing less than 0.1% organic matter.
4.1.2. Transport within water bodies
Cypermethrin moves slowly in water bodies. In the experimental
overspraying of ponds carried out by Crossland (1982) and described
in detail in section 4.4.2, it was calculated that 48 h after
treatment with 100 g cypermethrin/ha, only about 8 - 16% of the
amount applied could be found underneath the surface film of 0.05
mm depth. In all of Crossland's studies, the levels of
cypermethrin residues in the sediment at the bottom of the ponds
were below 7 µg/kg.
With spray levels applied according to normal agricultural
practice, Crossland et al. (1982) found that water bodies adjacent
to sprayed arable fields in the United Kingdom received only four-
five orders of magnitude less cypermethrin per m2 than the land
itself and that the initial concentration in the surface film of
water was between 6 and 20 µg/litre. Residues in the water below
the surface film did not reach more than 0.1 µg/litre and within
24 h the levels had nearly all fallen to below the limit of
determination of 0.01 µg/litre. A similar study in French
vineyards showed comparable results, though the initial
concentrations reached in the surface films were higher, probably
because conditions in the area were more favourable for spray drift
than those in the British study.
Shires & Bennett (1985) reported similar results concerning
water in drainage ditches adjacent to cereal fields in the United
Kingdom treated with an aerial spray application of 25 g
cypermethrin/ha.
From the available studies, it can be concluded that
contamination of water bodies by overspray is likely to be very
superficial and of comparatively short duration.
4.2. Abiotic Degradation
4.2.1. Photodegradation
4.2.1.1. Basic studies
According to Ruzo et al. (1977), cypermethrin is one of the
more light-stable pyrethroids. Thus, when exposed in the solid
phase to sunlight for 30 h, no loss of cypermethrin was detected.
When exposed in methanol solution to light of wavelength > 290 nm
for about 2 days, 55% of cypermethrin was recovered, but no data on
the photodecomposition products formed were reported. According to
Ruzo & Casida (1980), the reaction quantum yield at 300 nm in
methanol was low, at 0.022. Ruzo (1983) further demonstrated the
comparative resistance of cypermethrin to irradiation in his
studies on the involvement of oxygen in the photodegradation of
pyrethroids.
Cypermethrin is more susceptible to radiation of lower
wavelengths; Lauren & Henzel (1977) reported that under ultra-
violet radiation, 90% of cypermethrin on a glass petri dish was
decomposed after 3 days, but only 45% was decomposed after 3 days
when the cypermethrin was deposited on grass and placed under an
UV-lamp.
4.2.1.2. Photodegradation
(a) Water
Day & Leahey (1980) studied the effects of sunlight on dilute
aqueous solutions of cypermethrin. In their studies, 14C-labelled
cis- or trans-isomers were used with the label in either the
cyclopropyl or the benzyl ring. They were dissolved in sterile
aqueous acetonitrile at a concentration of 1 mg/litre, irradiated
in sunlight for 32 days and the irradiated solutions compared with
controls stored for the same length of time in the dark. The
degree of photodegradation was very limited. At the end of the
study, 89.4% of the cypermethrin remained in the case of the
irradiated benzyl label, compared with 97.4% in the dark control.
Corresponding figures for the cyclopropyl label were 92.3 and
96.8%. Six of the 8 photodegradation products separated by
chromatography were positively identified; cis- and trans-CPA,
phenoxybenzyl alcohol, aldehyde and acid, and alpha-cyano-3-
phenoxybenzyl alcohol.
The effects of natural sunlight on aqueous solutions of the
(1R, cis-, alpha RS) and (1R, trans-, alpha RS) isomers were
studied by Takahashi et al. (1985a,b). The products were labelled
with 14C in either the cyclopropyl ring, the benzyl ring, or the
cyano carbon. The aqueous solutions were made from distilled
water, 2% acetone, aqueous humic acid, sea water, or natural river
water (both of which had been filtered). The isomers were added to
the water in the form of a stabilized suspension using Tween 20 to
give 50 µg/litre test suspension. The rates of degradation of the
isomers were very rapid compared with those reported by other
authors, however, a large part of the changes involved
transformation to other isomers. The degradation was more rapid in
river or sea water (half-life of cis-isomer, 0.6 - 0.7 days) than
in distilled water or humic acid (half-life of cis-isomers, 2.3 -
2.6 days), but the most rapid change of all occurred in the
presence of acetone. Presumably the differences were due to the
well known effect of photosensitizaton by the acetone or organic
constituents of the natural waters. The main degradation products,
in addition to the different isomers, were CPA, PBA together with
smaller amounts of the corresponding aldehyde, and carbon dioxide,
especially in the case of the cyano label. There was evidence of
further degradation of CPA and PBA.
(b) Soil
Hall et al. (1981) studied the photodegradation of cypermethrin
on the soil surface. Labelled cypermethrin, as used by Day &
Leahey, was applied to very thin soil plates (0.5 mm) at a rate
equivalent to about 200 g/ha. The plates were exposed to sunlight
in the open air protected against rain by polythene sheeting, when
necessary; the sheeting was transparent to the UV component of
sunlight. The plates were extracted after an exposure period of 32
days and the extracts chromatographed. In the case of the
cyclopropyl label, 63% of the radioactivity initially applied to
the irradiated plate was recovered, compared with 103.5% from the
plate that had been kept in the dark. The half-life of the
cyclopropyl-labelled cypermethrin was reduced from > 32 days to 8
- 16 days by irradiation with natural light. The main degradation
products appear to have been the amide, together with cis- and
trans-CPA, and some unidentified (partly volatile) products. In
the case of the benzyl label, the degradation products identified
were mainly the amide analogue of cypermethrin and various
phenoxybenzyl derivatives, such as the alcohol, aldehyde, and acid.
In these studies, the amide was the most prominent, even in the
unirradiated sample, and in this respect, the results differ
somewhat from those obtained in other soil incubation studies,
where the main metabolite from benzyl-labelled cypermethrin was
PBA, with the amide occurring only as a very minor product.
Takahashi et al. (1985a,b), working with the same products as
they used for their study on water, applied the labelled products
at 1.1 µg/cm2 to half-millimetre layers of 3 different soils and
found very rapid degradation in the irradiated soils compared with
those kept in the dark. The half-lives ranged from between 0.6 and
1.9 days with sunlight and > 7 days in the dark. With regard to
degradation products, the results were rather similar to those
reported by Hall et al. (1981) in that the main degradation product
was the amide of the otherwise intact isomers. In addition, they
found smaller amounts of PBA, virtually no CPA, but occasionally
small amounts of alpha carbamoyl- and alpha carboxyphenoxybenzyl
alcohol. In one of the soils in which degradation was the
highest, nearly half of the radio-labelled carbon was unextractable
at the end of the exposure period. In contrast with the water
study, there was very little evidence of isomerization of the
parent isomers.
There is no obvious explanation for the different rates of
degradation under the influence of irradiation and it is difficult
to extrapolate the results of these studies to the practical
situation. It appears likely that photochemical reactions will
hasten the degradation of deposits of cypermethrin on exposed
surfaces and possible residues in water, but there is little
indication that they greatly change the degradation pathways.
4.3. Biological Degradation in Soil
Cypermethrin degrades relatively quickly in soils, primarily by
biological processes involving cleavage of the ester linkages, to
give the two main degradation products, CPA and PBA. These
products are themselves subsequently mineralized. There is also
evidence for the formation, as an intermediate, of the amide of the
intact molecule and occasionally the 4-hydroxy phenoxy analogue.
Neither of the latter products appears to persist in the soil
(Leahey 1979; Sakata et al., 1986).
4.3.1. Mechanism
Chapman et al. (1981), who studied the effects of sterilization
of soils on the rate of cypermethrin degradation in the laboratory,
demonstrated that degradation in the soil was essentially a
biological process. Cypermethrin was added at 1 mg/kg to the soils
(either untreated or sterilized) and the soils incubated for 16
weeks, by which time the sterilized soils were considered to have
become contaminated. The experiment was conducted under strictly
aerobic conditions. It was found that 84% of the added material
had degraded in natural organic soil compared with only 8% in the
sterilized organic soil. The corresponding values for the mineral
soil were 96% and 7%. The small amounts of degradation in the
sterilized soils presumably resulted from residual microbial
activity, especially in the later stages of the study.
4.3.2. Degradation pathways (separate isomers)
In order to study degradation pathways, Roberts & Standen
(1977, 1981) carried out a series of soil studies in the laboratory
using either the racemic cis- or trans-isomers of cypermethrin or
mixtures of the two. The compounds were 14C-radio-labelled in
either the benzyl or the cyclopropyl ring and were added to the
soil at a rate of 2.5 mg/kg moist soil. Incubation with 3
different soils was carried out under either aerobic or anaerobic
conditions, initially for 16 weeks and subsequently for a total of
52 weeks. Experiments were also conducted using biometer flasks,
in order to measure the output of radio-labelled carbon dioxide.
In the case of the cis-isomer, the main degradation products
extracted from the soils were PBA, cis-CPA with small amounts of
trans-CPA, and limited amounts of the 4-hydroxy derivative of
cypermethrin. Between 25% and 30% of the added radioactivity could
not be extracted with acetonitrile/water. A similar spectrum of
degradation products was extracted in the trans-isomer experiments,
except that the cis-isomer was absent. Some of the remaining
radioactivity was identified in a further degradation product of
CPA, the dicarboxylic acid.
A further study was undertaken by Roberts & Standen (1981) in
which ring-labelled cis- and trans-isomers of CPA were added to a
sandy loam soil at 2.5 - 13.5 mg/kg. In most cases, the soils were
contained in loosely stoppered vessels but, in one experiment, a
biometer flask fitted with a caustic potash trap was used, in order
to measure carbon dioxide (CO2) production. In spite of the
production of labelled CO2 in the initial study with cyclopropyl-
labelled cypermethrin, very little was produced in the latter study
and most of the radioactivity was shown to be still present in the
soil. At the end of the 8-week exposure period, it was found that
the greater part of the radioactivity in the soil was still
associated with unchanged CPA, 33 - 65% in the case of the trans-
acid and 78% in the case of the cis-acid. There was also evidence
that some of the trans-CPA was transformed to the cis-isomer, but
not vice-versa. This finding was analogous to that with the parent
compound where a certain amount of cis-CPA was produced from trans-
cypermethrin.
A similar series of studies was carried out by Sakata et al.
(1986) who incubated 2 Japanese soils with the 1R cis-RS alpha and
1R trans-RS alpha isomers of cypermethrin for up to 168 days at
25 °C. While ester cleavage was the principal pathway of
degradation, limited production of the amides of the intact esters
and production of the 4-hydroxy derivatives (on the phenoxy group)
were also reported. The latter were often present in greater
amounts than the PBA or CPA fragments. The authors also reported
the presence of small amounts of the desphenoxy derivative derived
from ether cleavage, not previously reported for cypermethrin.
However, the level of 14C associated with extractable breakdown
products was low (1 - 17% of the amount initially added, at 56
days) compared with that of bound radio carbon (14 - 58% at 56
days), the actual levels being very dependent on the type of soil
under study. Since the trans-isomers degraded more readily than
the cis-isomers and since the level of free degradation products
was considerably lower for the trans- than the cis-isomers, it is
possible that a substantial proportion of the bound radio carbon
had reverted to the general carbon pool of the soil organic matter.
A major proportion of the added label was recovered as carbon
dioxide (16 - 48% at 56 days) and the amount was highest when the
label was on the benzyl carbon, indicating, as Roberts & Standen
had found, that the PBA was mineralized more readily than the CPA.
Sakata et al. also found that, under comparable circumstances, the
cis-isomers produced carbon dioxide more slowly than the trans-
isomers.
The principal degradation products in soils, prior to breakage
of the benzyl and cyclopropane rings, are shown in Fig. 2.
4.3.3. Rates of degradation
4.3.3.1. Laboratory studies
(a) Separate isomers
In the laboratory studies carried out by Roberts & Standen
(1977, 1981), the half-lives of the cis-isomers were around 4
weeks, except in the inactive Los Palacios soils, where the figure
was nearer to 10 - 12 weeks. The trans-isomer generally exhibited
a much shorter half-life of less than 2 weeks and less than 4 weeks
on the less active soil. After a year, the amounts of unchanged
material left in the soils were very low and nearly always below
10% of the amount applied. But, even at the low levels remaining
after such a long interval, residues of the trans- were still
substantially less than those of the cis-product.
 Sakata et al. (1986) in their incubation studies reported half-
lives of between 4.1 and 17.6 days for trans-cypermethrin and 12.5
and 56.4 days for the cis-isomer, under aerobic upland conditions.
Degradation was much slower in one of the soils than in the other,
as was also shown by Roberts & Standen (1977, 1981). Miyamoto &
Mikami (1983) reported data on the half-lives in soil incubation
tests for all 4 of the 1R isomers of cypermethrin. The alpha S
isomers of both cis- and trans-isomers degraded much more rapidly
than the alpha R isomers, sometimes nearly twice as fast. Again,
the cis-isomers were slower to degrade than the trans-isomers.
The greater readiness of the trans-isomers to degrade has been
observed extensively by other workers, i.e., Kaufman et al. (1978),
Chapman et al. (1981), Chapman & Harris, (1981), Harris et al.
(1981). The Japanese studies did not produce data for the 1S
isomers, but Chapman & Harris did not detect appreciable
differences between the rates of degradation of the 1R and 1S
isomers, either trans or cis. On the other hand, Harris et al.
(1981) reported a substantial decrease in the 1S/1R ratio for trans-
cypermethrin, as degradation in the soil proceeded suggesting
that, in these studies, the 1S trans-isomers degraded more quickly
than the 1R trans-isomers.
4.3.3.2. Field studies
(a) Cypermethrin, and separate isomers
Roberts & Standen (1981) showed that the rates of degradation
of cypermethrin observed in the laboratory and in the field did not
differ greatly. On the basis of their data, 2 - 4 weeks in the
growing season would appear to be a typical half-life for the
parent racemic cypermethrin, bearing in mind that the half-lives of
the cis-isomers were often approximately twice those of the trans-
isomers.
Shorter half-lives of less than 2 weeks on a mineral soil and
about 3 weeks on a peat soil were reported by Chapman & Harris
(1981). Harris et al. (1981) reported a half-life for cypermethrin
in Plainfield sand of about 2.5 weeks. The persistence of the
insecticidal activity of surface applications of cypermethrin, as
measured by toxicity for cutworms was studied by Cheng (1984).
Although these data cannot be expressed in terms of the half-life
of cypermethrin, it is interesting to note that initial
applications, giving 100% mortality, were only producing about 50%
mortality after 12 days.
However, Chapman & Harris (1981) warned that a simple half-life
expression was not necessarily a valid way of defining the rates of
degradation of cypermethrin, because these tend to decrease with
time. A possible explanation for this effect is that there is a
gradual increase in the proportion of cis-isomers in the residues.
Since these degrade more slowly, overall degradation rates are
bound to decrease with time. But the results of Harris et al.
(1981) cast doubt on whether this change in isomer ratio provides
the sole explanation. These authors reported that, in their
studies, the ratio of cis- to trans-isomers increased during the
early part of their studies, but decreased substantially
afterwards.
Chapman & Harris (1981) also reported that the degradation was
slowed down by high soil contents of organic matter or clay (c.f.,
the slow rates of degradation reported by Roberts & Standen (1977,
1981) on the very high clay soil, Los Palacios) and by anaerobic
conditions. Contrary to what might be expected in light of the
behaviour of other pesticides, they reported that cypermethrin
degraded more quickly on dry than on wet soils. They also
identified the level of cypermethrin in the soil as a very
important factor. Thus, degradation, expressed on a proportionate
basis, was 2 - 3 times slower with an initial concentration in the
soil of 10 mg/kg, than that with an initial concentration of
0.5 mg/kg. Kaufman et al. (1978) also reported faster degradation
with lower rates of application.
(b) Metabolites
In studies on the 2 metabolites (PBA and CPA), Roberts &
Standen (1977, 1981) reported that PBA was quicker to degrade than
CPA.
In the Leiston soil, only about 2% of applied radioactivity
was recovered as PBA after 16 weeks, though in the soil from Los
Palacios, the figure was just under 30% for the soil treated with
cis-cypermethrin and some 50% for the soil treated with trans-
cypermethrin. The higher figure for PBA derived from trans-
cypermethrin was, presumably, due to the more rapid rate of
degradation of this parent isomer.
The degradation of PBA is an oxidative process and, under
anaerobic conditions, its degradation was greatly retarded (Roberts
& Standen, 1977).
The data of Roberts & Standen (1977) on CPA showed that, in
Brenes soil treated with the parent cypermethrin cis-isomers,
radioactivity recovered as CPA reached a maximum (about 17% of the
total radioactivity initially added) at the 8th week. The maximum
level of CPA from the trans-cypermethrin was reached at about the
same time, but constituted nearly 50% of the radioactivity
originally applied. Moreover, by the 52nd week, whilst CPA from
the cis-product had practically disappeared, there was still a
residue of CPA from the trans-isomers, equivalent to some 10% of
the radioactivity originally applied.
The rate of decay of the unextractable radioactivity in soils
previously treated by Roberts & Standen (1977) with labelled
cypermethrin, as described above, was studied by incubating some of
the soils (Brenes & Leiston soils) for a further 26 weeks in
admixture with fresh soil. Substantial additional losses of radio
carbon were observed. At the end of this time, 25 - 45% of the
"bound" radioactivity initially present was lost. Perhaps
unexpectedly, the losses from cypermethrin labelled in the
cyclopropyl ring was almost double that from product labelled in
the benzyl ring. It is clear from these studies that the binding
of residues of breakdown products did not prevent their continued
degradation. Although some of the evidence of Roberts & Standen
relating to the rate of degradation of CPA itself appears to be
anomalous, it can be inferred that cypermethrin degrades rapidly in
the soil and that the subsequent degradation products are
mineralized, as shown by the liberation of labelled carbon dioxide
from cypermethrin labelled in either the cyclopropyl or benzyl
rings. As Miyamoto (1981) concluded, there appears to be little
likelihood of cypermethrin or its metabolites persisting for
lengthy periods in soils.
4.4. Degradation in Water and Sediments
4.4.1. Laboratory studies
(a) Cypermethrin and separate isomers
Camilleri (1984), using 10-5mol/litre solutions of the cis-2
isomer pair of enantiomers in dioxan-water, showed that, at
alkaline pH values, cypermethrin is readily degraded by ester
cleavage to give CPA and PBA. The alternative route of
degradation, hydrolysis of the cyano group to amide, required a
much higher energy of activation and could not be detected.
Takahashi et al. (1985a) demonstrated the effects of pH on the
hydrolysis of 1R cis- or 1R trans-cypermethrin in abiotic
buffered aqueous solutions. At acidic pH values, the half-life of
the isomers was one or more years, but it was appreciably shorter
at pH 7 and had fallen to a matter of minutes at pH 11 (all at
25 °C). In natural waters, sterilized by filtration and having a
pH of about 8, the half-life was about 3 weeks at 25 °C. The trans-
isomers were hydrolysed more readily than the cis-isomers.
The fate of cypermethrin under biotic conditions, simulating
those in rivers and ponds, was studied by Rapley et al. (1981)
using a radio-labelled product, with the label in either the
cyclopropyl or benzyl ring. Samples of water and sediments from 3
rivers and a pond were used in a laboratory experiment in which
mixtures of water and sediment were placed in pairs of glass
cylinders. The insecticide was added at a rate equivalent to 140
g/ha and the vessels incubated at 16 °C for up to 60 weeks,
periodic determinations being made of the level of cypermethrin
remaining and the amount of labelled CO2 evolved. One series of
vessels was aerated and the other left undisturbed. Degradation
was rapid in all cases, even in the non-aerated series. Some 50%
of cypermethrin was lost in less than 2 weeks and 90% within 2 - 9
weeks. After approximately one year, 40-70% of the 14C label from
the benzyl-labelled material was lost as 14CO2, but only 4% from
the cyclopropyl-labelled material, though this proportion rose to
10% after 63 weeks. In the case of the cyclopropyl-labelled
material, the main degradation product detected was CPA with a
small amount of dicarboxylic acid. Subsequent degradation of the
CPA was slow. When the label was in the benzyl ring, the main
product was PBA though, in the sediment, precursors (aldehyde and
to some extent the alcohol) were the most prominent, possibly
because aeration was defective.
Muir et al. (1985) studied the behaviour of cis- and trans-
cypermethrin isomers, labelled with 14C in the cyclopropyl ring, in
3 bottom sediments (sand, a river silty clay, and a pond bottom
clay). In each case, 0.064 or 0.64 mg of the trans-isomers/kg or
0.012, 0.017, or 0.17 mg of the cis-isomers/kg was added to the
sediment. Each sediment was covered with dechlorinated tap water
and allowed to equilibrate for 24 h. The system was sampled at 6
and 24 h and the level of radioactivity determined in the sediment,
pore water from the sediment (in a separate study), and in the
supernatant water. The radioactivity was much less strongly
absorbed on sediment treated with the trans-isomer than on that
treated with cis-isomer, indicating that a substantial proportion
of the radioactivity was associated with degradation products
rather than with the parent compound, because it is unlikely that
major differences in adsorption between the cis- and trans-isomers
of the parent molecule would have been noticed.
4.4.2. Field Studies
(a) Cypermethrin
Crossland (1982) studied the effects of deliberately
overspraying experimental ponds with cypermethrin at the rate of
100 g/ha. Water was sampled either from the surface (2.5 - 10 cm)
or from a depth of 50 cm. Approximately 4 h after treatment, the
concentration of cypermethrin in the surface was 0.1 mg/litre, but
it fell to about a tenth of this value in 24 h. By 13 days, the
surface concentration had fallen to 0.0007 mg/litre. Concentrations
at a depth of 50 cm rose to a plateau of 0.0023 - 0.0026 mg/litre,
4 h after treatment, and then started to fall. By 13 days after
treatment, the concentration had decreased to 0.0009 mg/litre.
Residues were also found in the sediment at the bottom of the pond;
these reached a concentration of 0.006 mg/kg by the thirteenth day.
In a second study with similar treatment, a procedure for
surface sampling was introduced that enabled water films of only
0.05 mm to be sampled. In this extremely thin surface film, the
initial concentration reached 24 mg/litre. There was a very rapid
fall to around 50 µg/litre after the first week, and by the third
week, none could be detected (the limit of determination was 1 - 2
µg/litre). In the subsurface water, where the limit of
determination was only 0.1 µg/litre, concentrations reached 1
µg/litre shortly after treatment but fell rapidly to about a fifth
of this value by the end of the first week. By the end of the
fourth week, the concentration was below the limit of
determination. Sporadic amounts were found in the sediments, but
most had disappeared by the end of the study (16 weeks).
The effects of overspraying ponds or streams adjacent to arable
fields in the United Kingdom and of treating vineyards in France
with cypermethrin were studied by Crossland et al. (1982). The
fields in the United Kingdom were treated with a tractor-drawn
sprayer at the rate of 70 g/ha and the French vineyards with
mistblowers at the rate of 30 - 45 g/ha. One objective of this
work was to determine the possible occurrence of the insecticide in
the water as a result of spray drift from the treated areas. In
the United Kingdom study, deposits on the soil where the spray had
been applied were in the range of 4 - 7 mg/m2, but those on the
surface of the water of the adjacent pond were 4 - 5 orders of
magnitude less. The concentration of cypermethrin in the surface
layer of water (0.06 mm) was between 6 and 20 µg/litre but, after
24 h, only one of the 14 surface samples showed any cypermethrin,
the concentration in this sample being 6 µg/litre. Residues in
the subsurface layers reached between 0.01 and 0.07 µg/litre after
5 h but then declined; after 24 h, levels in most samples were
below the limit of determination (0.01 µg/litre) with only the
occasional sample reaching 0.03 µg/litre.
In the French vineyards, deposits on the surface of the water
were considerably higher (0.04 - 0.5 mg/m2). Concentrations in the
surface water were initially between 0.14 and 1 mg/litre falling to
0.02 mg/litre within 3 h. Even in the subsurface samples,
concentrations of up to 2 µg/litre were occasionally reached, but
they fell rapidly and had generally decreased to 0.1 µg/litre or
less within a few hours.
Further experiments along similar lines were carried out by
Shires & Bennett (1985) who used a fixed wing aircraft to apply
cypermethrin at 25 g/ha to a large field of winter wheat that was
bordered on 3 sides by drainage ditches.
The deposit on the land was about 60% of the nominal rate of
application, while on the water it was only about a tenth of this
value (equivalent to 1.5 g/ha). Analysis of subsurface water
showed that any spray drift reaching the ditches resulted only in
very low levels ranging from below the level of determination of
0.01 µg/litre to a maximum of 0.03 µg/litre. By the fourth day,
none could be detected.
It appears unlikely that spray drift during properly conducted
spray operations will give rise to high concentrations of
cypermethrin in adjacent surface waters. It is also evident that
if cypermethrin residues do occur in natural waters, they are
relatively short lived.
4.5. Bioaccumulation and Biomagnification
4.5.1. n-Octanol/water partition coefficient
In common with those of other synthetic pyrethroids, the
n-octanol/water partition coefficient of cypermethrin is high; a
value of 2 x 106 (log Pow = 6.3) was obtained by extrapolation from
chromatographic data (Gray & Grayson, 1980). McLeese et al. (1980)
reported a calculated log n-octanol/water partition coefficient of
2.44.
4.5.2. Bioaccumulation in fish
The accumulation by fish of cypermethrin from water and its
subsequent elimination have been studied. In a preliminary study,
rainbow trout were exposed to 14C-benzyl-labelled cypermethrin in
water at 14 °C for a period of 22 days. The initial concentration
each day of 0.165 µg/litre decreased over the 24-h period to 0.064
µg/litre. Radioactivity in whole fish rose to a plateau equivalent
to 0.083 mg cypermethrin/kg wet weight after approximately 11 days.
During the steady state, at least 67% of the radioactivity was
unchanged cypermethrin, but unidentified materials were also
present. When the fish were transferred to clean water after 22
days, the concentration of radioactivity decreased to half the
plateau level in about 11 days. According to this study, allowing
for the cyclical nature of the exposure concentration, the best
estimate of the accumulation factor is approximately 1000 (Baldwin
& Lad, 1978b).
In a follow-up study, 2 groups of rainbow trout of different
sizes were exposed to steady, low concentrations of unlabelled
cypermethrin in a continuous-flow system. When exposed to a mean
concentration of 0.19 µg cypermethrin/litre, residues in small
trout (2-13 g) increased rapidly to approximately 0.15 mg/kg wet
weight over 10 days and 0.23 mg/kg wet weight in 10 - 18 days.
After 18 days, the fish were placed in clean water and depuration
followed. Using a one-compartment mathematical model, it was
calculated that the bioaccumulation factor at equilibrium was 1200.
The calculated depuration half-life was approximately 8 days. In
the larger trout (130 - 160 g), exposed to a mean concentration of
0.18 µg cypermethrin/litre in a continuous-flow system, the uptake
was slower, and residues in whole fish reached 0.12 mg/kg wet
weight after 24 days. Cypermethrin residues in fish were fairly
uniformly distributed (mean values 1 - 2 mg/kg tissue), when
expressed on a lipid-weight rather than a wet-weight basis, except
that the brain contained lower residues than the other tissues
(Bennett, 1981a).
Rainbow trout and common carp were exposed to cypermethrin
concentrations of 0.4 - 1.9 µg/litre in a continuous-flow study for
up to 21 days. It was found that residues in both species were
very similar on a wet- and on a lipid-weight basis, but that there
was only a small difference in residue burden between the fish that
died and those that survived. The mean residue concentrations
(mg/kg tissue) for trout and carp were respectively: 0.91 (died)
and 0.67 (survived), 0.68 (died) and 0.72 (survived), on a wet-
weight basis and 44 (died) and 29 (survived), and 43 (died) and 25
(survived) on a lipid-weight basis, (Bennett, 1981b).
McLeese et al. (1980) studied the concentration factors for
cypermethrin in salmon from various toxicity tests. The results
are given in Table 4.
Table 4. Concentration factors for
cypermethrin in salmona
-------------------------------------------
Concentration Exposure Concentration CFb
in water time fish
(µg/litre) (h) (mg/kg)
-------------------------------------------
12 12 0.04 3.5
7.8 21 0.02 3.6
3.0 62 0.02 6.7
1.4 96 0.01 7.1
-------------------------------------------
a From: McLeese et al. (1980).
b CF = Concentration in fish/concentration
in water.
The low CFs for cypermethrin may indicate rapid metabolism and
elimination of the compound by salmon.
Accumulation of cypermethrin by fish exposed under field
conditions was studied in rudd taken at various time intervals from
a pond treated with 100 g cypermethrin a.i./ha (Table 5).
These results show a rapid uptake of cypermethrin in the fish
followed by elimination from the fish as the compound is lost from
the water in the pond system. In such a dynamic situation, it is
not possible to give a definite accumulation factor (Crossland et
al., 1978).
Table 5. Residues of cypermethrin in rudd and water from a
pond treated at 100 g active ingredient/ha
---------------------------------------------------------------
Time after treatment Concentration of cypermethrin in:
subsurface water rudd (µg/kg wet weight)
(µg/litre) (individual values)
---------------------------------------------------------------
1 day 1.0 50 and 41
1 week 0.21 45 and 49
2 weeks 0.06 42 and 65
4 weeks 0.01 26 and 30
8 weeks 0.01 19 and 5
16 weeks - 5a
---------------------------------------------------------------
a Average of 8 fish.
In view of the very low concentrations of cypermethrin that are
likely to arise in water from normal agricultural use and the
rapidity with which concentrations decline, fish in the wild will
not contain measurable residues of cypermethrin, in spite of the
concentration factors reported.
4.5.3. Bioaccumulation in aquatic invertebrates
Muir et al. (1985) studied the accumulation of cypermethrin in
sediment-dwelling larvae of the midge Chironomus tentans. These
were allowed to establish themselves in the sediments or were kept
suspended in the water under the conditions of the study described
in section 4.4.1. Bioaccumulation factors were calculated for both
the water and sediment larvae; these varied from 43 to 245 for the
trans-compound and from 34 to 385 for the cis-, expressed as the
ratios of total radioactivity per gram of larvae to that per ml of
water.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air
No data are available.
5.1.2. Water
No data are available.
5.1.3. Soil
See section 4.3.
5.1.4. Food
Cypermethrin is used to control insect pests on a very wide
range of crops, and residues of the parent compound can sometimes
be found in agricultural commodities from treated crops. Foods of
animal origin can also contain limited residues arising either from
the use of the product for the control of ectoparasites or from the
occurrence of residues in the animal feed.
5.1.4.1. Residues in food commodities from treated crops
A large body of information on the levels of residues arising
in crop commodities where cypermethrin has been used according to
Good Agricultural Practice (GAP), was available to the Task Group.
The data had already been comprehensively reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues and summarized in their
published Monographs of the meetings in 1979, 1981, 1982, and 1984.
(FAO/WHO 1980b, 1982b, 1983b, and 1985c). As a consequence of
their reviews, the JMPR were able to propose a series of Maximum
Residue Limits (MRLs) for cypermethrin in a wide range of food
commodities (treated according to GAP) below which the actual
residue levels would be expected to fall. They range from 0.05 to
2 mg/kg. These MRLs are now at various steps in the Codex
procedure and many have already been fully adopted by the Codex
Alimentarious Commission, shown as step "CLX" in Table 6 (Codex
Alimentarius Commission, 1986).
Dried tea is an exception to this range of levels in food
commodities in that the level proposed is 20 mg/kg, but it was
shown that only 0.1% of the residues in dried tea enter the
infusion so that the brew, as drunk, will only contain negligible
amounts (FAO/WHO 1985b). Cereal straws also fall outside this
range in that the MRL is 5 mg/kg, but these are not foodstuffs.
Table 6. Codex limits for cypermethrin
residues in treated crops
--------------------------------------------
Crop MRL (mg/kg) Step
--------------------------------------------
Brassica leafy vegetables 1 CLX
Citrus 2 CLX
Lettuce 2 8
Oil seeds except peanuts 0.2 8
Peas 0.05 CLX
Root and tuber vegetables 0.05 CLX
Tomatoes 0.5 CLX
Wheat grain 0.2 8
--------------------------------------------
In addition to these data, limited information on residues have
been published by Lauren & Henzel (1977), Braun et al. (1982),
Frank et al. (1982), and Awasthi & Anand (1983).
Research has also been carried out on the fate of residues in
stored grain treated experimentally (Joia et al., 1985b; Noble &
Hamilton, 1985). Residues proved to be relatively persistent and a
knowledge of storage times and conditions would be required to
estimate the levels that would occur in the grain trade, should
this use of cypermethrin become accepted practice.
5.1.4.2. Residues in food of animal origin
Residues of cypermethrin can arise in foods of animal origin
(milk or milk products, eggs, meat or meat products), either from
topical application to livestock for the control of ecotoparasites
or from residues in livestock rations. In the USA, the actual
residues in meat and milk are expected to be less than the
tolerances of 0.05 mg/kg per litre product (US EPA, 1984). By
referring to available residue data, the JMPR was able to propose
MRLs for carcass meat and meat products, eggs, and milk.
Subsequently, the following Codex Limits (CLXs) were established
(Codex Alimentarius Commission-1986) (Table 7).
Table 7. Codex limits for cypermethrin residues
in foodstuffs
-------------------------------------------------
Commodity Maximum Residue Limit
mg/kg
-------------------------------------------------
Carcass meat (carcass fat) 0.2
Meat products 0.2
Eggs 0.05
Milk (whole milk) 0.01
-------------------------------------------------
5.2. General Population Exposure
Taking into consideration: (a) the levels of cypermethrin
residues that may occur in food commodities from crops or in foods
of animal origin, where cypermethrin has been used according to
GAP; (b) the contribution of the relevant commodities to the diet;
and (c) the losses that occur during the processing of these
commodities, it can confidently be inferred that the daily intake
of cypermethrin in the human diet will be well below the officially
adopted Acceptable Daily Intake. However, no total diet or market
basket studies are available.
5.3. Occupational Exposure
See section 9.2.2.
6. KINETICS AND METABOLISM
6.1. Absorption, Excretion, and Distribution
6.1.1. Oral
6.1.1.1. Rat
(a) Cypermethrin mixture
Three rats of each sex were given a single oral dose of 0.5 mg
(approximately 1.2 mg/kg body weight for males and 2.1 mg/kg body
weight for females) of a cis/trans mixture of 14C-cyclopropyl-
labelled cypermethrin. Three days after dosing, low concentrations
of radioactivity were found for both sexes in the kidneys, muscle,
brain, and blood. The level in the liver of male rats was 3 times
higher than that in the liver of female rats (0.37 and 0.12 mg/kg
tissue, respectively). The residues in the fat of the female rats
were 2 - 3 times higher than those in the male rats (0.72 and 0.31
mg/kg tissue respectively). Concentrations in muscle, brain, and
blood were < 0.05 mg/kg. The mean percentage recovery of the
administered dose was more than 100% (Crawford, 1977; Crawford et
al., 1981a).
Urinary excretion of the compound was rapid in both sexes;
approximately 50 - 65% of the dose being excreted in 48 h.
Elimination via the faeces was slower, the mean rate being
approximately 30% of the dose in 3 days. The amount of
radioactivity excreted via expired CO2, measured in a separate
study using one rat of each sex, was up to 0.1% of the dose in 15
days.
Studies with 14C-cyclopropyl-labelled cypermethrin indicated
that biliary excretion of the cyclopropyl moiety is a minor route
of elimination (up to 2% in 4 h) (Crawford et al., 1981a).
The metabolism of cypermethrin in maize oil was studied in male
and female Wistar rats following a single toxic oral dose of 200
mg/kg body weight of 2 radio-labelled forms (14C-benzyl and 14C-
cyclopropyl) of the insecticide. Minimal amounts of 14CO2 were
expired from both types of labelled cypermethrin: viz < 0.005 -
0.06% of dose. The elimination of radioactivity within 7 days was
29 - 33% (14C-benzyl label) and 41-56% (14C-cyclopropyl label) in
the urine and 55 - 59% and 34 - 46%, respectively, in the faeces.
The differences between the sexes were small (Rhodes et al., 1984).
The distribution and tissue retention of cypermethrin was
studied in 5 male and 5 female Wistar rats receiving daily oral
doses of 2 mg (14C-benzyl)-labelled cypermethrin/kg body weight for
28 days. Consistent with the lipophilic nature of cypermethrin,
the highest mean tissue concentration was found in the fat (4.1
mg/kg in males and 5.1 mg/kg in females). Concentrations in the
liver, kidneys, adrenals, gut, ovaries, and skin were of the order
of 0.4 - 0.9 mg/kg tissue. Small amounts of radioactivity (0.04 -
0.07 mg/kg) were detected in the muscle, spleen, and bone.
Negligible concentrations (< 0.01 mg/kg) were detected in the
brain (Rhodes et al., 1984). In a further study, the tissues
identified as containing the highest concentrations of 14C-benzyl-
labelled cypermethrin (fat, liver, kidneys, skin, and ovaries) as
well as whole blood and plasma were used to study the extent of
accumulation and rate of elimination of cypermethrin. A total of
60 female rats were dosed orally with 14C-benzyl-labelled
cypermethrin at 2 mg/kg body weight per day, for up to 70
consecutive days. Levels in all tissues reached a plateau after 56
days of dosing. The extent of accumulation, expressed as mg
equivalents of cypermethrin per kg tissue, was: fat, 3.91; liver,
0.97; kidneys, 0.69; ovaries, 0.03; skin, 1.89; whole blood, 0.35;
and plasma 0.64. Analysis of fat samples, 24 h after the final
dose, revealed that higher levels of the cis-isomer of cypermethrin
had been retained than of the trans-isomer. The rate of
elimination of radioactivity from fat was biphasic in nature, with
rapid elimination of trans-cypermethrin (half-life = 3.4 days) and
slower elimination of the less-readily hydrolysed cis-cypermethrin
(half-life = 18.9 days). Levels of 14C residues in the liver,
kidneys, and blood reached control background levels within 29, 8,
and 15 days, respectively, of the final dose. Apart from fat, the
only other tissue that contained radioactivity was the skin; the
rate of elimination of radioactivity from the skin was similar to
that for fat. Accumulation in the sciatic nerve was also studied
in rats dosed for 26 days. No appreciable bioaccumulation was
found to occur (Jones, 1981; Rhodes et al., 1984).
Three Wistar rats of each sex, given a single oral dose of
(14C-cyano)-cypermethrin (4.3 mg/kg body weight), eliminated 30 -
66% of the dose in the faeces over 3 days. Urinary excretion of
14CN-label was slow, accounting for 6 - 12% of the dose and
elimination of expired 14CO2 accounted for only 1.2 - 1.5% of the
dose. Tissue retention in major organs apart from fat, was higher
than that in similar studies involving 14C-benzyl or 14C-
cyclopropyl labelling, thus reflecting metabolism typical of the
14C-labelled cyanide moiety (Crawford et al., 1981a).
(b) Separate isomers
The fates of both cis- and trans-isomers have been studied
separately. Groups of 3 - 6 Wistar rats of each sex were given
single oral doses (approximately 2.5 mg/kg body weight) of either
the cis-isomer or the trans-isomer, both 14C-labelled in the
benzyl ring. Both isomers were rapidly eliminated. The greater
part of the administered dose was excreted in the urine; 40% and
60% for males and females, respectively, of the cis-isomer and 70%
and 80% of the trans-isomer within 48 h. Elimination of the cis-
isomer in the faeces amounted to 26% and 48% for male and females,
respectively; elimination of the trans-isomer was 24%. The
results for the cis-isomer show a clear sex difference in the route
of elimination. After 72 h, less than 5% of the administered dose
of either isomer remained in the animal tissues with the exception
of the intestines and skin. Fat and skin contained the highest
concentrations (Crawford, 1976a,b; Crawford et al., 1981a). It has
been demonstrated (Crawford & Hutson, 1977a, Crawford et al.,
1981a) that the residue derived from cis-cypermethrin is
eliminated more slowly from fat than from other tissues. In one
study, 8 female rats were given (14C-benzyl)- cis-cypermethrin at
2.5 mg/kg body weight orally, and elimination of radioactivity was
measured in fat samples from 8 up to 42 days after dosing. The
radioactivity was calculated to have a half-life of 11.7 (3.4 -
16.7) days. Ninety to 100% of the radioactivity still remaining in
the fat at 25 days was present as unchanged cypermethrin. The
residues in the liver and kidneys were much lower than those in the
fat but were eliminated at a similar rate (Crawford et al., 1981a).
6.1.1.2. Mouse
(a) Separate isomers
Elimination of radioactivity was measured in male Swiss-Webster
mice, dosed once orally with cis- or trans-cypermethrin, 14C-
labelled in either the benzyl (8 mg/kg body weight) or cyclopropyl
(7 mg/kg body weight) moiety. The 14C-benzyl-dosed mice eliminated
22% and 34% of the administered dose of cis-isomer in the urine
and faeces, respectively, in one day; values for the trans-isomer
were 41% and 16%, respectively. The 14C-cyclopropyl-dosed mice
eliminated 20% of the administered dose of cis-isomer in the urine
and 50% in the faeces in one day; the values for the trans-isomer
were 55% and 16%, respectively. Thus, radioactivity from the
trans-isomer was mainly eliminated in the urine and that from the
cis-isomer in the faeces. The 14C-benzyl-treated mice were killed
1, 3, or 8 days after dosing; the 14C-cyclopropyl-treated mice, 3
days after dosing. Residues of radioactivity from both labels, 3
days after dosing, were low in all tissues except for the fat. The
sequence of the residues in different organs was fat > liver ~
kidneys > blood ~ muscle > brain. Residues fell rapidly during
the 14C-benzyl study, with the exception of the residues derived
from the cis-isomer in fat, which did not decrease during the
study period (Hutson, 1978a; Hutson et al., 1981). However, in a
further study, radioactivity was measured in fat samples from 10
male mice taken up to 42 days after a single oral dose of
approximately 8.8 mg/kg body weight (14C-benzyl)- cis-cypermethrin.
The residue was eliminated exponentially with a half-life of 13.1
(3.6 - 18.4) days. At 8 and 22 days after dosing, approximately
90% of the radioactivity present in two pooled fat samples was
attributable to unchanged cis-cypermethrin (Crawford & Hutson,
1978; Crayford et al., 1980; Hutson et al., 1981).
6.1.1.3. Dog
(a) Cypermethrin mixture
Two male beagle dogs were given single oral doses of (14C-
cyclopropyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979a).
Elimination of labelled material was rapid in both dogs, though a
variable distribution between urine and faeces was observed between
the 2 dogs, i.e., 21 and 57% in urine and 78 and 48%, respectively,
in faeces. In a further study, one dog was dosed orally with
(14C-benzyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979b).
Over 4 days, 80% of the radioactivity was recovered in the faeces
and 11% in the urine. Analysis of tissues, 4 days after dosing,
revealed that the gall bladder (1.5 mg/kg tissue) and renal fat
(0.3 mg/kg tissue) contained the highest levels of radioactivity
expressed as cypermethrin. Negligible amounts were detected in the
brain (0.006 mg/kg tissue) and sciatic nerve (0.09 mg/kg tissue).
In the liver, adrenals, bone marrow, pituitary gland, and
mesenteric fat, levels of cypermethrin of 0.1 - 0.2 mg/kg tissue
were found.
(b) Separate isomers
Administration of (14C-benzyl)- cis-cypermethrin or
(14C-benzyl)- trans-cypermethrin separately to groups of 2 male
dogs as a single (2 mg/kg body weight) oral dose resulted in
83.4% of cis-isomer and 88% of trans-isomer being recovered in
the urine plus faeces over 6 - 7 days (Crawford, 1979b).
Quantitative differences existed between the amounts eliminated
via the 2 routes. As already mentioned, a variable distribution
was found. These data are consistent with the results of the study
involving 14C-cyclopropyl-labelled cypermethrin (Crawford, 1979a),
and the variation in amounts according to the route of elimination
probably reflects the inter-group differences in rates of
absorption of labelled material.
6.1.1.4. Cow
Three studies were carried out on lactating cows fed diets
containing 0.2, 5, or 10 mg 14C-benzyl and/or 14C-cyclo-propyl-
cypermethrin/kg feed, respectively, twice daily, for 7 or 21 days.
The estimated daily intake was 2, 50, or 100 mg cypermethrin/cow.
The radioactivity was rapidly eliminated following ingestion.
Equilibrium between ingestion and elimination was reached after
about 4 days. The amounts eliminated via the major routes were
similar for both labels, i.e., approximately 50% in the urine, and
approximately 40% in the faeces (mainly unchanged cypermethrin).
Polar and acidic components were found in the urine. Up to 0.2% of
the administered radioactivity was found in the milk, mainly in the
cream phase (about 88%). Feeding 0.2, 5, or 10 mg/kg feed, the
residues in the milk were 0.0006, 0.012, or 0.03 mg cypermethrin
/litre, respectively. Radioactivity (expressed as mg cypermethrin
/kg tissue) in the carcasses of the animals of the 3 groups at
slaughter was not detectable in muscle and brain (< 0.001- < 0.04
mg/kg). Levels in other tissues were: blood < 0.04 - 0.07 mg/kg,
liver 0.004 - 0.21 mg/kg, kidneys 0.003 - 0.11 mg/kg, and
subcutaneous and renal fat 0.01 - 0.1 mg/kg (Croucher et al.,
1985).
Swaine & Sapiets cf. FAO/WHO (1982b) dosed cows daily with 0.2,
5, or 50 mg cypermethrin (43% cis-isomers, 35% trans-isomers) per
kg feed for up to 29 days. Residues in milk and tissues were
comparable to those reported by Croucher et al. (1985).
6.1.1.5. Sheep
The elimination pattern in a single sheep, given one oral
dose of a mixture consisting of unlabelled cypermethrin with 14C-
benzyl- and 14C-cyclopropyl-labelled material (3.9 mg/kg body
weight) in a gelatin capsule, showed that 41% of the administered
dose was excreted in the urine and 20% was eliminated in faeces,
within 48 h. Tissue residues, 2 days after treatment, were muscle,
0.04 mg/kg; and liver, kidneys, and renal fat approximately 0.4
mg/kg tissue (Crawford & Hutson, 1977b).
6.1.1.6. Chicken
14C-phenoxy-labelled cypermethrin ( cis:trans, 55:45) was
administered orally to laying hens, daily for 14 days, at a rate
equivalent to 10 mg/kg diet (about 0.7 mg/kg body weight).
Radioactivity in the eggs reached a plateau, equivalent to about
0.05 mg cypermethrin/kg, after 8 days. Most of the radioactivity
was found in the yolk (up to 0.19 mg/kg) and about half of it was
identified as cypermethrin. The rest was closely associated with
neutral lipids and phosphatidyl cholines. Residues in the
carcasses, at slaughter, were low; values were between 0.01 and
0.02 mg/kg in muscle tissue, about 0.08 mg/kg in the subcutaneous
and peritoneal fat, and 0.37 mg/kg in the liver. The composition
of residues in the liver was not conclusively established. Apart
from small amounts of unchanged cypermethrin, the radioactivity was
also associated with highly polar material. However, it is evident
that the hen has a very effective mechanism for the metabolism of
cypermethrin (Hutson & Stoydin, 1987).
Comparable results were obtained from non-labelled studies with
laying hens in which dietary levels of up to 40 mg cypermethrin/kg
diet were fed for 28 days (Wallace et al., 1982).
6.1.1.7. Man
Male volunteers were each given a single oral dose of 0.25,
0.5, 1, or 1.5 mg cypermethrin in corn oil in a capsule. Urinary
excretion of cypermethrin metabolites was rapid. The subjects
excreted an average 78% of the dose of trans-isomer and 49% of the
cis-isomer within 24 h. These values did not differ from the
results in rats. The ester cleavage was a major route of
metabolism of cypermethrin in man. As reported in other animal
species, the trans-isomer was metabolized more readily than the
cis-isomer. Concentrations of both isomers excreted in the urine
between 2 and 5 days after dosing 0.5 or 1 mg cypermethrin were
below the limit of detection of 0.01 mg/litre (Eadsforth & Baldwin,
1983).
Groups of 2 male subjects were given cypermethrin in daily oral
doses of 0.25, 0.75, or 1.5 mg/man, by capsule, for 5 consecutive
days. During the dosing period and the following 5 days, 24-h
urine samples were collected daily and analysed for the
concentration of the cyclopropane carboxylic acid metabolite. The
results showed that the respective percent-ages of the cis- and
trans-isomers of cypermethrin, excreted in the 24-h period
following each of the oral doses, were similar to the percentage
excretion of these isomers measured in the single oral dose study.
Therefore, no accumulation in the body occurred (van Sittert et
al., 1985a).
6.1.2. Dermal
6.1.2.1. Cow
Two lactating cows were sprayed 3 times with 1.1 g
cypermethrin/animal, with 2-week intervals between treatments.
Milk samples were analysed during this period. Tissue samples
were analysed approximately three weeks after the final spraying.
The residues were: in whole milk, < 0.01 mg/litre; muscle, liver,
and kidneys, < 0.01 mg/kg tissue and in fat samples, 0.02 mg/kg
tissue or less (Baldwin et al., 1977).
Comparable results were obtained when 2 barns were sprayed with
either 0.05% or 0.1% of cypermethrin prepared from a 10% a.i.
formulation. Cows were present during spraying. Milk was
collected up to 4 weeks after spraying (0.05% application) or 4
days after spraying (0.1% application). Only the samples collected
4 days after the 0.05% treatment and 2 days after the 0.1%
treatment contained detectable residues (0.005 mg/kg milk). No
residues were found (< 0.002 mg/kg milk) in any of the other
samples (Baldwin & Lad, 1978a).
Cows were dipped twice in approximately 170 mg cypermethrin
/litre with a 10-week interval between treatments. The animals
were sacrificed 4 or 14 days after the second dipping. Residues in
muscle and liver did not exceed 0.01 mg/kg tissue. Fat samples
contained detectable residues. The highest was 0.13 mg/kg in renal
fat. The fat residue did not decline between 4 and 14 days after
treatment (Baldwin, 1977a).
Cattle sprayed once with 0.1 and 0.2% a.i. showed the same
level of residues (< 0.005 mg/kg tissue) in muscle, liver, and
kidneys, and a level of < 0.01 mg/kg in fat samples, 1, 3, 8, and
15 days after treatment. In cattle treated twice, fat samples
contained residues ranging from 0.01 to 0.05 mg/kg tissue (Bosio,
1979).
Many trials in which cows were sprayed with, or dipped in,
cypermethrin solutions were carried out in Australia. The milk
from cows sprayed with 0.1% cypermethrin did not contain any
detectable residues. The highest residue (0.03 mg/kg) in butterfat
was found one day after spraying. When the cows were dipped in a
dipwash containing 75 mg cypermethrin/litre, residues in the milk
determined 1, 3, and 7 days after dipping ranged from 0.01 to <
0.002 mg/litre. Omental fat contained the highest residue level
(0.02 mg/kg) 3 and 4 days after dipping. Liver, kidneys, and
muscle did not contain any detectable residues. A second dipping,
7 days after the first, did not cause any build-up of cypermethrin
in the tissues of the cattle (FAO/WHO, 1982b).
Detectable residues of cypermethrin of up to 0.01 mg/kg
butterfat were found in milk samples taken over 21 days from 5 of
10 cows wearing cypermethrin-integrated ear tags (Braun et al.,
1985).
Taylor et al. (1985) found cypermethrin in the hair of cattle,
in concentrations of up to 2.8 mg/kg, after application of
impregnated ear tags.
6.1.2.2. Sheep
Two sheep were each treated dermally with a mixture consisting
of unlabelled cypermethrin mixed with 14C-benzyl-and 14C-
cyclopropyl-labelled material at 22 mg/kg body weight. The
cypermethrin was slowly absorbed. Less than 0.5% of the dose was
excreted in the urine within 24 h and only 2% over a 6-day period.
Faecal elimination was also slow, 0.5% of the dose being eliminated
in 6 days. Approximately 30% of the dose was recovered from the
application area. Tissue residues, 6 days after treatment, were:
muscle, 0.04; renal fat, 0.3; and liver and kidneys 0.12 mg/kg
tissue (Crawford & Hutson, 1977b).
6.1.2.3. Man
A male subject was given a single dermal application of a ULV
formulation of cypermethrin (50 mg cypermethrin in hexylene
glycol/Shellsol AB) on the underside of the forearm. The majority
of this application (35 mg) was removed from the skin after 4 h.
Urine was monitored for residues of the acid metabolite [3-(2,2-
dichlorovinyl)-2,2-dimethylcyclo-propane-carboxylic acid] and its
glucuronide, for a 96-h period after dosing. The metabolites were
not detected over this period (Coveney & Eadsforth, 1982).
In a study by van Sittert et al. (1985b), 2 male volunteers
were given a single dermal application of a ULV formulation, 25 mg
cypermethrin in hexylene glycol/Shellsol A, on the underside of the
forearm. An average of 53% of the original amount of cypermethrin
applied was removed from the skin, 4 h after application.
Approximately 0.1% was excreted as the urinary metabolite,
cyclopropane carboxylic acid, during a 72-h period. Measurements
were made using gas liquid chromatography - mass spectrometry, a
method with a higher sensitivity and selectivity than gas liquid
chromatography - electron capture detection, which was used in the
previous study.
6.2. Metabolic Transformation
6.2.1. In vitro studies
In vitro studies on mouse liver homogenates have shown that
ester cleavage is more extensive for the trans-isomer than for the
cis-isomer. One mg of each of (1RS,trans)- and (1RS, cis)-
cypermethrin was incubated with 2.2 ml of approximately 10% mouse
microsome substrate at 37 °C for 30 min, under the following
conditions: (a) tetraethyl pyrophosphate (TEPP)-treated microsomes
(neither esterase nor oxidase activity); (b) normal microsomes
(esterase activity); (c) TEPP-treated microsomes plus NADPH
(oxidase activity); and (d) normal microsomes plus NADPH (esterase
plus oxidase activity). Each esterase preparation hydrolysed about
twice as much trans-cypermethrin as cis-cypermethrin. In contrast,
cis-cypermethrin was metabolized more rapidly in an oxidation
system than trans-cypermethrin. The major site of ring
hydroxylation was the 4' position and the secondary site was the 5
position. The trans-methyl group was an important site of
hydroxylation in the ester metabolites and cis-methyl oxidation was
predominant in the ester-cleaved acid metabolites. The
hydroxymethyl derivatives were further oxidized to the
corresponding aldehydes and carboxylic acids.
3-Phenoxybenzaldehyde-cyanohydrin was detected as a minor
metabolite. The preferred sites of hydroxylation were: with
trans-cypermethrin, cis-methyl > 4' position > trans-methyl > 5
position; with cis-cypermethrin, trans > cis > 4' position > 5
position (Shone & Casida, 1978; Shone et al., 1979). With cis-
cypermethrin, at least, cleavage of cypermethrin to cyanohydrin may
result from both hydrolytic and oxidative mechanisms, since large
amounts of the cleavage products were also evident in the oxidase
system, which lacks esterase activity (Shono & Casida, 1978; Shono
et al., 1979). However, at approximately 35-times higher substrate
levels, the hydrolysis rate of cypermethrin isomers was depressed
(Söderlund & Casida, 1977).
In studies on the metabolism of 14C-cypermethrin by rat liver
microsomes, the overall rates of metabolism of cis- and trans-
cypermethrin were similar, though their metabolic routes differed.
The cis-isomer was metabolized almost exclusively by an NADPH-
dependent oxidative pathway to 4'hydroxy- cis-cypermethrin with
subsequent oxidative ester cleavage. The predominant route for the
metabolism of the trans-isomer was hydrolysis to the trans-acid by
microsomal carboxylesterase (Crawford, 1979c). The in vitro
esteratic capacity was determined in rat, rabbit, and human liver
microsomes using p-nitrophenyl acetate and cypermethrin as
substrate. The relative ability to hydrolyse cypermethrin was
rabbit > man > rat. Rabbit and rat microsomes metabolized the
trans-isomer 6 times faster than the cis-isomer. Human
microsomes showed a similar capacity for metabolizing both cis-
and trans-isomers (Croucher et al., 1982a,b).
6.2.2. In vivo studies
The identification of the metabolites of cypermethrin has been
studied in mice (Hutson, 1978b, Casida et al., 1979; Hutson et al.,
1981), rats (Crawford & Hutson, 1977a; Casida et al., 1979; Hutson,
1979a,b; Crawford et al., 1981b; Rhodes et al., 1984), dogs
(Crawford, 1979d,e), and cows (Swaine & Sapiets, 1980a,b; Croucher
et al., 1985).
Overall, metabolism in these species is similar. Differences
that occur are related to the rate of metabolite formation rather
than to the nature of the metabolites formed. The only major
differences between species relate to conjugation reactions.
Cypermethrin (both isomers) is metabolized via cleavage of the
ester bond. The cyclopropane carboxylic acid moiety is mainly
excreted as the glucuronide conjugate; hydroxylation of the methyl
group occurs only to a limited extent (Crawford, 1979e; Rhodes et
al., 1984). The 3-phenoxy-benzyl product of the ester hydrolysis
is converted to PBA. The cyanide moiety is metabolized to
thiocyanate (Hutson, 1979b). The PBA moiety is mainly excreted as
a glutamic acid conjugate in the cow (Croucher et al., 1985), as a
taurine conjugate ( N-(3-phenoxy-benzoyl)taurine) in 2 strains of
mouse (Hutson & Casida, 1978; Hutson, 1978b, 1979a; Hutson et al.,
1981), and as a glycine conjugate in the rat and dog (Crawford &
Hutson, 1977a; Crawford, 1979d) and in the sheep, cat, and gerbil
(Huckle et al., 1981a). PBA is further metabolized (rat >
mouse > dog) via the 4'-hydroxylation to 3-(4'-hydroxyphenoxy)
benzoic acid and its sulfate conjugate (Crawford & Hutson, 1977a;
Hutson, 1978b; Crawford, 1979d). Glucuronic acid conjugates of PBA
and its 4'hydroxy derivative are the major urinary metabolites in
the marmoset, rabbit, guinea-pig, and hamster. The rat was unique
among the animal species tested in utilizing sulfuric acid for the
conjugation of the 4'-hydroxy derivative (Huckle et al., 1981a).
The major route of excretion for cypermethrin metabolites is via
the urine; unchanged cypermethrin accounted for the majority of
radioactivity found in faeces in radiolabel studies. The amount of
cyclopropyl-radioactivity eliminated in the bile (1%) suggests that
the biliary-intestinal-faecal route is of minor importance for this
moiety (Crawford & Hutson, 1977a; Crawford, 1979e; Rhodes et al.,
1984). Biliary excretion of PBA occurred as glucuronide and that
of 4'-OH-PBA as ether and ester glucuronic acid conjugates. Very
little was eliminated in the faeces, indicating that the biliary
glucuronides decompose and/or are enzymatically cleaved in the
gastro-intestinal tract to the respective benzoic acids. The
latter are subsequently reabsorbed and undergo further metabolism,
principally to the sulfate ester, which is excreted in the urine
(Huckle et al., 1981b). As already noted, the major urinary
metabolite of cypermethrin in cows is N-(3-phenoxy-benzoyl)glutamic
acid. This metabolite is also found in the organs and tissues with
only a small quantity of unchanged cypermethrin. The residues in
body fat consist mainly of cypermethrin. An unidentified polar
metabolite, present in the liver and kidneys, is suspected of being
a conjugate of 3-(4'-hydroxyphenoxy)benzoic acid. The small
portion of radioactivity appearing in milk was associated with
lipid components and consisted mainly of unchanged cypermethrin
(Croucher et al., 1985). The metabolic pathway of cypermethrin is
shown in Fig. 3.
As in other mammals, ester cleavage and elimination of the
cyclopropyl acid moieties in the free and glucuronidated form is a
major route of metabolism of cypermethrin in man (Eadsforth &
Baldwin, 1983).
6.2.3. Metabolism of the glucoside conjugate of 3-phenoxy-benzoic
acid
Studies have been carried out on rats on the metabolism of the
glucoside conjugate of 3-phenoxybenzoic acid, which occurs
occasionally as a metabolite in plants (Crayford, 1978). The
results indicated that the rat hydrolyses the glucoside and then
metabolizes the 3-phenoxybenzoic acid in virtually the same way as
it would metabolize PBA liberated during the metabolism of
cypermethrin.
Sakata et al. (1986) in their incubation studies reported half-
lives of between 4.1 and 17.6 days for trans-cypermethrin and 12.5
and 56.4 days for the cis-isomer, under aerobic upland conditions.
Degradation was much slower in one of the soils than in the other,
as was also shown by Roberts & Standen (1977, 1981). Miyamoto &
Mikami (1983) reported data on the half-lives in soil incubation
tests for all 4 of the 1R isomers of cypermethrin. The alpha S
isomers of both cis- and trans-isomers degraded much more rapidly
than the alpha R isomers, sometimes nearly twice as fast. Again,
the cis-isomers were slower to degrade than the trans-isomers.
The greater readiness of the trans-isomers to degrade has been
observed extensively by other workers, i.e., Kaufman et al. (1978),
Chapman et al. (1981), Chapman & Harris, (1981), Harris et al.
(1981). The Japanese studies did not produce data for the 1S
isomers, but Chapman & Harris did not detect appreciable
differences between the rates of degradation of the 1R and 1S
isomers, either trans or cis. On the other hand, Harris et al.
(1981) reported a substantial decrease in the 1S/1R ratio for trans-
cypermethrin, as degradation in the soil proceeded suggesting
that, in these studies, the 1S trans-isomers degraded more quickly
than the 1R trans-isomers.
4.3.3.2. Field studies
(a) Cypermethrin, and separate isomers
Roberts & Standen (1981) showed that the rates of degradation
of cypermethrin observed in the laboratory and in the field did not
differ greatly. On the basis of their data, 2 - 4 weeks in the
growing season would appear to be a typical half-life for the
parent racemic cypermethrin, bearing in mind that the half-lives of
the cis-isomers were often approximately twice those of the trans-
isomers.
Shorter half-lives of less than 2 weeks on a mineral soil and
about 3 weeks on a peat soil were reported by Chapman & Harris
(1981). Harris et al. (1981) reported a half-life for cypermethrin
in Plainfield sand of about 2.5 weeks. The persistence of the
insecticidal activity of surface applications of cypermethrin, as
measured by toxicity for cutworms was studied by Cheng (1984).
Although these data cannot be expressed in terms of the half-life
of cypermethrin, it is interesting to note that initial
applications, giving 100% mortality, were only producing about 50%
mortality after 12 days.
However, Chapman & Harris (1981) warned that a simple half-life
expression was not necessarily a valid way of defining the rates of
degradation of cypermethrin, because these tend to decrease with
time. A possible explanation for this effect is that there is a
gradual increase in the proportion of cis-isomers in the residues.
Since these degrade more slowly, overall degradation rates are
bound to decrease with time. But the results of Harris et al.
(1981) cast doubt on whether this change in isomer ratio provides
the sole explanation. These authors reported that, in their
studies, the ratio of cis- to trans-isomers increased during the
early part of their studies, but decreased substantially
afterwards.
Chapman & Harris (1981) also reported that the degradation was
slowed down by high soil contents of organic matter or clay (c.f.,
the slow rates of degradation reported by Roberts & Standen (1977,
1981) on the very high clay soil, Los Palacios) and by anaerobic
conditions. Contrary to what might be expected in light of the
behaviour of other pesticides, they reported that cypermethrin
degraded more quickly on dry than on wet soils. They also
identified the level of cypermethrin in the soil as a very
important factor. Thus, degradation, expressed on a proportionate
basis, was 2 - 3 times slower with an initial concentration in the
soil of 10 mg/kg, than that with an initial concentration of
0.5 mg/kg. Kaufman et al. (1978) also reported faster degradation
with lower rates of application.
(b) Metabolites
In studies on the 2 metabolites (PBA and CPA), Roberts &
Standen (1977, 1981) reported that PBA was quicker to degrade than
CPA.
In the Leiston soil, only about 2% of applied radioactivity
was recovered as PBA after 16 weeks, though in the soil from Los
Palacios, the figure was just under 30% for the soil treated with
cis-cypermethrin and some 50% for the soil treated with trans-
cypermethrin. The higher figure for PBA derived from trans-
cypermethrin was, presumably, due to the more rapid rate of
degradation of this parent isomer.
The degradation of PBA is an oxidative process and, under
anaerobic conditions, its degradation was greatly retarded (Roberts
& Standen, 1977).
The data of Roberts & Standen (1977) on CPA showed that, in
Brenes soil treated with the parent cypermethrin cis-isomers,
radioactivity recovered as CPA reached a maximum (about 17% of the
total radioactivity initially added) at the 8th week. The maximum
level of CPA from the trans-cypermethrin was reached at about the
same time, but constituted nearly 50% of the radioactivity
originally applied. Moreover, by the 52nd week, whilst CPA from
the cis-product had practically disappeared, there was still a
residue of CPA from the trans-isomers, equivalent to some 10% of
the radioactivity originally applied.
The rate of decay of the unextractable radioactivity in soils
previously treated by Roberts & Standen (1977) with labelled
cypermethrin, as described above, was studied by incubating some of
the soils (Brenes & Leiston soils) for a further 26 weeks in
admixture with fresh soil. Substantial additional losses of radio
carbon were observed. At the end of this time, 25 - 45% of the
"bound" radioactivity initially present was lost. Perhaps
unexpectedly, the losses from cypermethrin labelled in the
cyclopropyl ring was almost double that from product labelled in
the benzyl ring. It is clear from these studies that the binding
of residues of breakdown products did not prevent their continued
degradation. Although some of the evidence of Roberts & Standen
relating to the rate of degradation of CPA itself appears to be
anomalous, it can be inferred that cypermethrin degrades rapidly in
the soil and that the subsequent degradation products are
mineralized, as shown by the liberation of labelled carbon dioxide
from cypermethrin labelled in either the cyclopropyl or benzyl
rings. As Miyamoto (1981) concluded, there appears to be little
likelihood of cypermethrin or its metabolites persisting for
lengthy periods in soils.
4.4. Degradation in Water and Sediments
4.4.1. Laboratory studies
(a) Cypermethrin and separate isomers
Camilleri (1984), using 10-5mol/litre solutions of the cis-2
isomer pair of enantiomers in dioxan-water, showed that, at
alkaline pH values, cypermethrin is readily degraded by ester
cleavage to give CPA and PBA. The alternative route of
degradation, hydrolysis of the cyano group to amide, required a
much higher energy of activation and could not be detected.
Takahashi et al. (1985a) demonstrated the effects of pH on the
hydrolysis of 1R cis- or 1R trans-cypermethrin in abiotic
buffered aqueous solutions. At acidic pH values, the half-life of
the isomers was one or more years, but it was appreciably shorter
at pH 7 and had fallen to a matter of minutes at pH 11 (all at
25 °C). In natural waters, sterilized by filtration and having a
pH of about 8, the half-life was about 3 weeks at 25 °C. The trans-
isomers were hydrolysed more readily than the cis-isomers.
The fate of cypermethrin under biotic conditions, simulating
those in rivers and ponds, was studied by Rapley et al. (1981)
using a radio-labelled product, with the label in either the
cyclopropyl or benzyl ring. Samples of water and sediments from 3
rivers and a pond were used in a laboratory experiment in which
mixtures of water and sediment were placed in pairs of glass
cylinders. The insecticide was added at a rate equivalent to 140
g/ha and the vessels incubated at 16 °C for up to 60 weeks,
periodic determinations being made of the level of cypermethrin
remaining and the amount of labelled CO2 evolved. One series of
vessels was aerated and the other left undisturbed. Degradation
was rapid in all cases, even in the non-aerated series. Some 50%
of cypermethrin was lost in less than 2 weeks and 90% within 2 - 9
weeks. After approximately one year, 40-70% of the 14C label from
the benzyl-labelled material was lost as 14CO2, but only 4% from
the cyclopropyl-labelled material, though this proportion rose to
10% after 63 weeks. In the case of the cyclopropyl-labelled
material, the main degradation product detected was CPA with a
small amount of dicarboxylic acid. Subsequent degradation of the
CPA was slow. When the label was in the benzyl ring, the main
product was PBA though, in the sediment, precursors (aldehyde and
to some extent the alcohol) were the most prominent, possibly
because aeration was defective.
Muir et al. (1985) studied the behaviour of cis- and trans-
cypermethrin isomers, labelled with 14C in the cyclopropyl ring, in
3 bottom sediments (sand, a river silty clay, and a pond bottom
clay). In each case, 0.064 or 0.64 mg of the trans-isomers/kg or
0.012, 0.017, or 0.17 mg of the cis-isomers/kg was added to the
sediment. Each sediment was covered with dechlorinated tap water
and allowed to equilibrate for 24 h. The system was sampled at 6
and 24 h and the level of radioactivity determined in the sediment,
pore water from the sediment (in a separate study), and in the
supernatant water. The radioactivity was much less strongly
absorbed on sediment treated with the trans-isomer than on that
treated with cis-isomer, indicating that a substantial proportion
of the radioactivity was associated with degradation products
rather than with the parent compound, because it is unlikely that
major differences in adsorption between the cis- and trans-isomers
of the parent molecule would have been noticed.
4.4.2. Field Studies
(a) Cypermethrin
Crossland (1982) studied the effects of deliberately
overspraying experimental ponds with cypermethrin at the rate of
100 g/ha. Water was sampled either from the surface (2.5 - 10 cm)
or from a depth of 50 cm. Approximately 4 h after treatment, the
concentration of cypermethrin in the surface was 0.1 mg/litre, but
it fell to about a tenth of this value in 24 h. By 13 days, the
surface concentration had fallen to 0.0007 mg/litre. Concentrations
at a depth of 50 cm rose to a plateau of 0.0023 - 0.0026 mg/litre,
4 h after treatment, and then started to fall. By 13 days after
treatment, the concentration had decreased to 0.0009 mg/litre.
Residues were also found in the sediment at the bottom of the pond;
these reached a concentration of 0.006 mg/kg by the thirteenth day.
In a second study with similar treatment, a procedure for
surface sampling was introduced that enabled water films of only
0.05 mm to be sampled. In this extremely thin surface film, the
initial concentration reached 24 mg/litre. There was a very rapid
fall to around 50 µg/litre after the first week, and by the third
week, none could be detected (the limit of determination was 1 - 2
µg/litre). In the subsurface water, where the limit of
determination was only 0.1 µg/litre, concentrations reached 1
µg/litre shortly after treatment but fell rapidly to about a fifth
of this value by the end of the first week. By the end of the
fourth week, the concentration was below the limit of
determination. Sporadic amounts were found in the sediments, but
most had disappeared by the end of the study (16 weeks).
The effects of overspraying ponds or streams adjacent to arable
fields in the United Kingdom and of treating vineyards in France
with cypermethrin were studied by Crossland et al. (1982). The
fields in the United Kingdom were treated with a tractor-drawn
sprayer at the rate of 70 g/ha and the French vineyards with
mistblowers at the rate of 30 - 45 g/ha. One objective of this
work was to determine the possible occurrence of the insecticide in
the water as a result of spray drift from the treated areas. In
the United Kingdom study, deposits on the soil where the spray had
been applied were in the range of 4 - 7 mg/m2, but those on the
surface of the water of the adjacent pond were 4 - 5 orders of
magnitude less. The concentration of cypermethrin in the surface
layer of water (0.06 mm) was between 6 and 20 µg/litre but, after
24 h, only one of the 14 surface samples showed any cypermethrin,
the concentration in this sample being 6 µg/litre. Residues in
the subsurface layers reached between 0.01 and 0.07 µg/litre after
5 h but then declined; after 24 h, levels in most samples were
below the limit of determination (0.01 µg/litre) with only the
occasional sample reaching 0.03 µg/litre.
In the French vineyards, deposits on the surface of the water
were considerably higher (0.04 - 0.5 mg/m2). Concentrations in the
surface water were initially between 0.14 and 1 mg/litre falling to
0.02 mg/litre within 3 h. Even in the subsurface samples,
concentrations of up to 2 µg/litre were occasionally reached, but
they fell rapidly and had generally decreased to 0.1 µg/litre or
less within a few hours.
Further experiments along similar lines were carried out by
Shires & Bennett (1985) who used a fixed wing aircraft to apply
cypermethrin at 25 g/ha to a large field of winter wheat that was
bordered on 3 sides by drainage ditches.
The deposit on the land was about 60% of the nominal rate of
application, while on the water it was only about a tenth of this
value (equivalent to 1.5 g/ha). Analysis of subsurface water
showed that any spray drift reaching the ditches resulted only in
very low levels ranging from below the level of determination of
0.01 µg/litre to a maximum of 0.03 µg/litre. By the fourth day,
none could be detected.
It appears unlikely that spray drift during properly conducted
spray operations will give rise to high concentrations of
cypermethrin in adjacent surface waters. It is also evident that
if cypermethrin residues do occur in natural waters, they are
relatively short lived.
4.5. Bioaccumulation and Biomagnification
4.5.1. n-Octanol/water partition coefficient
In common with those of other synthetic pyrethroids, the
n-octanol/water partition coefficient of cypermethrin is high; a
value of 2 x 106 (log Pow = 6.3) was obtained by extrapolation from
chromatographic data (Gray & Grayson, 1980). McLeese et al. (1980)
reported a calculated log n-octanol/water partition coefficient of
2.44.
4.5.2. Bioaccumulation in fish
The accumulation by fish of cypermethrin from water and its
subsequent elimination have been studied. In a preliminary study,
rainbow trout were exposed to 14C-benzyl-labelled cypermethrin in
water at 14 °C for a period of 22 days. The initial concentration
each day of 0.165 µg/litre decreased over the 24-h period to 0.064
µg/litre. Radioactivity in whole fish rose to a plateau equivalent
to 0.083 mg cypermethrin/kg wet weight after approximately 11 days.
During the steady state, at least 67% of the radioactivity was
unchanged cypermethrin, but unidentified materials were also
present. When the fish were transferred to clean water after 22
days, the concentration of radioactivity decreased to half the
plateau level in about 11 days. According to this study, allowing
for the cyclical nature of the exposure concentration, the best
estimate of the accumulation factor is approximately 1000 (Baldwin
& Lad, 1978b).
In a follow-up study, 2 groups of rainbow trout of different
sizes were exposed to steady, low concentrations of unlabelled
cypermethrin in a continuous-flow system. When exposed to a mean
concentration of 0.19 µg cypermethrin/litre, residues in small
trout (2-13 g) increased rapidly to approximately 0.15 mg/kg wet
weight over 10 days and 0.23 mg/kg wet weight in 10 - 18 days.
After 18 days, the fish were placed in clean water and depuration
followed. Using a one-compartment mathematical model, it was
calculated that the bioaccumulation factor at equilibrium was 1200.
The calculated depuration half-life was approximately 8 days. In
the larger trout (130 - 160 g), exposed to a mean concentration of
0.18 µg cypermethrin/litre in a continuous-flow system, the uptake
was slower, and residues in whole fish reached 0.12 mg/kg wet
weight after 24 days. Cypermethrin residues in fish were fairly
uniformly distributed (mean values 1 - 2 mg/kg tissue), when
expressed on a lipid-weight rather than a wet-weight basis, except
that the brain contained lower residues than the other tissues
(Bennett, 1981a).
Rainbow trout and common carp were exposed to cypermethrin
concentrations of 0.4 - 1.9 µg/litre in a continuous-flow study for
up to 21 days. It was found that residues in both species were
very similar on a wet- and on a lipid-weight basis, but that there
was only a small difference in residue burden between the fish that
died and those that survived. The mean residue concentrations
(mg/kg tissue) for trout and carp were respectively: 0.91 (died)
and 0.67 (survived), 0.68 (died) and 0.72 (survived), on a wet-
weight basis and 44 (died) and 29 (survived), and 43 (died) and 25
(survived) on a lipid-weight basis, (Bennett, 1981b).
McLeese et al. (1980) studied the concentration factors for
cypermethrin in salmon from various toxicity tests. The results
are given in Table 4.
Table 4. Concentration factors for
cypermethrin in salmona
-------------------------------------------
Concentration Exposure Concentration CFb
in water time fish
(µg/litre) (h) (mg/kg)
-------------------------------------------
12 12 0.04 3.5
7.8 21 0.02 3.6
3.0 62 0.02 6.7
1.4 96 0.01 7.1
-------------------------------------------
a From: McLeese et al. (1980).
b CF = Concentration in fish/concentration
in water.
The low CFs for cypermethrin may indicate rapid metabolism and
elimination of the compound by salmon.
Accumulation of cypermethrin by fish exposed under field
conditions was studied in rudd taken at various time intervals from
a pond treated with 100 g cypermethrin a.i./ha (Table 5).
These results show a rapid uptake of cypermethrin in the fish
followed by elimination from the fish as the compound is lost from
the water in the pond system. In such a dynamic situation, it is
not possible to give a definite accumulation factor (Crossland et
al., 1978).
Table 5. Residues of cypermethrin in rudd and water from a
pond treated at 100 g active ingredient/ha
---------------------------------------------------------------
Time after treatment Concentration of cypermethrin in:
subsurface water rudd (µg/kg wet weight)
(µg/litre) (individual values)
---------------------------------------------------------------
1 day 1.0 50 and 41
1 week 0.21 45 and 49
2 weeks 0.06 42 and 65
4 weeks 0.01 26 and 30
8 weeks 0.01 19 and 5
16 weeks - 5a
---------------------------------------------------------------
a Average of 8 fish.
In view of the very low concentrations of cypermethrin that are
likely to arise in water from normal agricultural use and the
rapidity with which concentrations decline, fish in the wild will
not contain measurable residues of cypermethrin, in spite of the
concentration factors reported.
4.5.3. Bioaccumulation in aquatic invertebrates
Muir et al. (1985) studied the accumulation of cypermethrin in
sediment-dwelling larvae of the midge Chironomus tentans. These
were allowed to establish themselves in the sediments or were kept
suspended in the water under the conditions of the study described
in section 4.4.1. Bioaccumulation factors were calculated for both
the water and sediment larvae; these varied from 43 to 245 for the
trans-compound and from 34 to 385 for the cis-, expressed as the
ratios of total radioactivity per gram of larvae to that per ml of
water.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1. Air
No data are available.
5.1.2. Water
No data are available.
5.1.3. Soil
See section 4.3.
5.1.4. Food
Cypermethrin is used to control insect pests on a very wide
range of crops, and residues of the parent compound can sometimes
be found in agricultural commodities from treated crops. Foods of
animal origin can also contain limited residues arising either from
the use of the product for the control of ectoparasites or from the
occurrence of residues in the animal feed.
5.1.4.1. Residues in food commodities from treated crops
A large body of information on the levels of residues arising
in crop commodities where cypermethrin has been used according to
Good Agricultural Practice (GAP), was available to the Task Group.
The data had already been comprehensively reviewed by the FAO/WHO
Joint Meeting on Pesticide Residues and summarized in their
published Monographs of the meetings in 1979, 1981, 1982, and 1984.
(FAO/WHO 1980b, 1982b, 1983b, and 1985c). As a consequence of
their reviews, the JMPR were able to propose a series of Maximum
Residue Limits (MRLs) for cypermethrin in a wide range of food
commodities (treated according to GAP) below which the actual
residue levels would be expected to fall. They range from 0.05 to
2 mg/kg. These MRLs are now at various steps in the Codex
procedure and many have already been fully adopted by the Codex
Alimentarious Commission, shown as step "CLX" in Table 6 (Codex
Alimentarius Commission, 1986).
Dried tea is an exception to this range of levels in food
commodities in that the level proposed is 20 mg/kg, but it was
shown that only 0.1% of the residues in dried tea enter the
infusion so that the brew, as drunk, will only contain negligible
amounts (FAO/WHO 1985b). Cereal straws also fall outside this
range in that the MRL is 5 mg/kg, but these are not foodstuffs.
Table 6. Codex limits for cypermethrin
residues in treated crops
--------------------------------------------
Crop MRL (mg/kg) Step
--------------------------------------------
Brassica leafy vegetables 1 CLX
Citrus 2 CLX
Lettuce 2 8
Oil seeds except peanuts 0.2 8
Peas 0.05 CLX
Root and tuber vegetables 0.05 CLX
Tomatoes 0.5 CLX
Wheat grain 0.2 8
--------------------------------------------
In addition to these data, limited information on residues have
been published by Lauren & Henzel (1977), Braun et al. (1982),
Frank et al. (1982), and Awasthi & Anand (1983).
Research has also been carried out on the fate of residues in
stored grain treated experimentally (Joia et al., 1985b; Noble &
Hamilton, 1985). Residues proved to be relatively persistent and a
knowledge of storage times and conditions would be required to
estimate the levels that would occur in the grain trade, should
this use of cypermethrin become accepted practice.
5.1.4.2. Residues in food of animal origin
Residues of cypermethrin can arise in foods of animal origin
(milk or milk products, eggs, meat or meat products), either from
topical application to livestock for the control of ecotoparasites
or from residues in livestock rations. In the USA, the actual
residues in meat and milk are expected to be less than the
tolerances of 0.05 mg/kg per litre product (US EPA, 1984). By
referring to available residue data, the JMPR was able to propose
MRLs for carcass meat and meat products, eggs, and milk.
Subsequently, the following Codex Limits (CLXs) were established
(Codex Alimentarius Commission-1986) (Table 7).
Table 7. Codex limits for cypermethrin residues
in foodstuffs
-------------------------------------------------
Commodity Maximum Residue Limit
mg/kg
-------------------------------------------------
Carcass meat (carcass fat) 0.2
Meat products 0.2
Eggs 0.05
Milk (whole milk) 0.01
-------------------------------------------------
5.2. General Population Exposure
Taking into consideration: (a) the levels of cypermethrin
residues that may occur in food commodities from crops or in foods
of animal origin, where cypermethrin has been used according to
GAP; (b) the contribution of the relevant commodities to the diet;
and (c) the losses that occur during the processing of these
commodities, it can confidently be inferred that the daily intake
of cypermethrin in the human diet will be well below the officially
adopted Acceptable Daily Intake. However, no total diet or market
basket studies are available.
5.3. Occupational Exposure
See section 9.2.2.
6. KINETICS AND METABOLISM
6.1. Absorption, Excretion, and Distribution
6.1.1. Oral
6.1.1.1. Rat
(a) Cypermethrin mixture
Three rats of each sex were given a single oral dose of 0.5 mg
(approximately 1.2 mg/kg body weight for males and 2.1 mg/kg body
weight for females) of a cis/trans mixture of 14C-cyclopropyl-
labelled cypermethrin. Three days after dosing, low concentrations
of radioactivity were found for both sexes in the kidneys, muscle,
brain, and blood. The level in the liver of male rats was 3 times
higher than that in the liver of female rats (0.37 and 0.12 mg/kg
tissue, respectively). The residues in the fat of the female rats
were 2 - 3 times higher than those in the male rats (0.72 and 0.31
mg/kg tissue respectively). Concentrations in muscle, brain, and
blood were < 0.05 mg/kg. The mean percentage recovery of the
administered dose was more than 100% (Crawford, 1977; Crawford et
al., 1981a).
Urinary excretion of the compound was rapid in both sexes;
approximately 50 - 65% of the dose being excreted in 48 h.
Elimination via the faeces was slower, the mean rate being
approximately 30% of the dose in 3 days. The amount of
radioactivity excreted via expired CO2, measured in a separate
study using one rat of each sex, was up to 0.1% of the dose in 15
days.
Studies with 14C-cyclopropyl-labelled cypermethrin indicated
that biliary excretion of the cyclopropyl moiety is a minor route
of elimination (up to 2% in 4 h) (Crawford et al., 1981a).
The metabolism of cypermethrin in maize oil was studied in male
and female Wistar rats following a single toxic oral dose of 200
mg/kg body weight of 2 radio-labelled forms (14C-benzyl and 14C-
cyclopropyl) of the insecticide. Minimal amounts of 14CO2 were
expired from both types of labelled cypermethrin: viz < 0.005 -
0.06% of dose. The elimination of radioactivity within 7 days was
29 - 33% (14C-benzyl label) and 41-56% (14C-cyclopropyl label) in
the urine and 55 - 59% and 34 - 46%, respectively, in the faeces.
The differences between the sexes were small (Rhodes et al., 1984).
The distribution and tissue retention of cypermethrin was
studied in 5 male and 5 female Wistar rats receiving daily oral
doses of 2 mg (14C-benzyl)-labelled cypermethrin/kg body weight for
28 days. Consistent with the lipophilic nature of cypermethrin,
the highest mean tissue concentration was found in the fat (4.1
mg/kg in males and 5.1 mg/kg in females). Concentrations in the
liver, kidneys, adrenals, gut, ovaries, and skin were of the order
of 0.4 - 0.9 mg/kg tissue. Small amounts of radioactivity (0.04 -
0.07 mg/kg) were detected in the muscle, spleen, and bone.
Negligible concentrations (< 0.01 mg/kg) were detected in the
brain (Rhodes et al., 1984). In a further study, the tissues
identified as containing the highest concentrations of 14C-benzyl-
labelled cypermethrin (fat, liver, kidneys, skin, and ovaries) as
well as whole blood and plasma were used to study the extent of
accumulation and rate of elimination of cypermethrin. A total of
60 female rats were dosed orally with 14C-benzyl-labelled
cypermethrin at 2 mg/kg body weight per day, for up to 70
consecutive days. Levels in all tissues reached a plateau after 56
days of dosing. The extent of accumulation, expressed as mg
equivalents of cypermethrin per kg tissue, was: fat, 3.91; liver,
0.97; kidneys, 0.69; ovaries, 0.03; skin, 1.89; whole blood, 0.35;
and plasma 0.64. Analysis of fat samples, 24 h after the final
dose, revealed that higher levels of the cis-isomer of cypermethrin
had been retained than of the trans-isomer. The rate of
elimination of radioactivity from fat was biphasic in nature, with
rapid elimination of trans-cypermethrin (half-life = 3.4 days) and
slower elimination of the less-readily hydrolysed cis-cypermethrin
(half-life = 18.9 days). Levels of 14C residues in the liver,
kidneys, and blood reached control background levels within 29, 8,
and 15 days, respectively, of the final dose. Apart from fat, the
only other tissue that contained radioactivity was the skin; the
rate of elimination of radioactivity from the skin was similar to
that for fat. Accumulation in the sciatic nerve was also studied
in rats dosed for 26 days. No appreciable bioaccumulation was
found to occur (Jones, 1981; Rhodes et al., 1984).
Three Wistar rats of each sex, given a single oral dose of
(14C-cyano)-cypermethrin (4.3 mg/kg body weight), eliminated 30 -
66% of the dose in the faeces over 3 days. Urinary excretion of
14CN-label was slow, accounting for 6 - 12% of the dose and
elimination of expired 14CO2 accounted for only 1.2 - 1.5% of the
dose. Tissue retention in major organs apart from fat, was higher
than that in similar studies involving 14C-benzyl or 14C-
cyclopropyl labelling, thus reflecting metabolism typical of the
14C-labelled cyanide moiety (Crawford et al., 1981a).
(b) Separate isomers
The fates of both cis- and trans-isomers have been studied
separately. Groups of 3 - 6 Wistar rats of each sex were given
single oral doses (approximately 2.5 mg/kg body weight) of either
the cis-isomer or the trans-isomer, both 14C-labelled in the
benzyl ring. Both isomers were rapidly eliminated. The greater
part of the administered dose was excreted in the urine; 40% and
60% for males and females, respectively, of the cis-isomer and 70%
and 80% of the trans-isomer within 48 h. Elimination of the cis-
isomer in the faeces amounted to 26% and 48% for male and females,
respectively; elimination of the trans-isomer was 24%. The
results for the cis-isomer show a clear sex difference in the route
of elimination. After 72 h, less than 5% of the administered dose
of either isomer remained in the animal tissues with the exception
of the intestines and skin. Fat and skin contained the highest
concentrations (Crawford, 1976a,b; Crawford et al., 1981a). It has
been demonstrated (Crawford & Hutson, 1977a, Crawford et al.,
1981a) that the residue derived from cis-cypermethrin is
eliminated more slowly from fat than from other tissues. In one
study, 8 female rats were given (14C-benzyl)- cis-cypermethrin at
2.5 mg/kg body weight orally, and elimination of radioactivity was
measured in fat samples from 8 up to 42 days after dosing. The
radioactivity was calculated to have a half-life of 11.7 (3.4 -
16.7) days. Ninety to 100% of the radioactivity still remaining in
the fat at 25 days was present as unchanged cypermethrin. The
residues in the liver and kidneys were much lower than those in the
fat but were eliminated at a similar rate (Crawford et al., 1981a).
6.1.1.2. Mouse
(a) Separate isomers
Elimination of radioactivity was measured in male Swiss-Webster
mice, dosed once orally with cis- or trans-cypermethrin, 14C-
labelled in either the benzyl (8 mg/kg body weight) or cyclopropyl
(7 mg/kg body weight) moiety. The 14C-benzyl-dosed mice eliminated
22% and 34% of the administered dose of cis-isomer in the urine
and faeces, respectively, in one day; values for the trans-isomer
were 41% and 16%, respectively. The 14C-cyclopropyl-dosed mice
eliminated 20% of the administered dose of cis-isomer in the urine
and 50% in the faeces in one day; the values for the trans-isomer
were 55% and 16%, respectively. Thus, radioactivity from the
trans-isomer was mainly eliminated in the urine and that from the
cis-isomer in the faeces. The 14C-benzyl-treated mice were killed
1, 3, or 8 days after dosing; the 14C-cyclopropyl-treated mice, 3
days after dosing. Residues of radioactivity from both labels, 3
days after dosing, were low in all tissues except for the fat. The
sequence of the residues in different organs was fat > liver ~
kidneys > blood ~ muscle > brain. Residues fell rapidly during
the 14C-benzyl study, with the exception of the residues derived
from the cis-isomer in fat, which did not decrease during the
study period (Hutson, 1978a; Hutson et al., 1981). However, in a
further study, radioactivity was measured in fat samples from 10
male mice taken up to 42 days after a single oral dose of
approximately 8.8 mg/kg body weight (14C-benzyl)- cis-cypermethrin.
The residue was eliminated exponentially with a half-life of 13.1
(3.6 - 18.4) days. At 8 and 22 days after dosing, approximately
90% of the radioactivity present in two pooled fat samples was
attributable to unchanged cis-cypermethrin (Crawford & Hutson,
1978; Crayford et al., 1980; Hutson et al., 1981).
6.1.1.3. Dog
(a) Cypermethrin mixture
Two male beagle dogs were given single oral doses of (14C-
cyclopropyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979a).
Elimination of labelled material was rapid in both dogs, though a
variable distribution between urine and faeces was observed between
the 2 dogs, i.e., 21 and 57% in urine and 78 and 48%, respectively,
in faeces. In a further study, one dog was dosed orally with
(14C-benzyl)-cypermethrin at 2 mg/kg body weight (Crawford, 1979b).
Over 4 days, 80% of the radioactivity was recovered in the faeces
and 11% in the urine. Analysis of tissues, 4 days after dosing,
revealed that the gall bladder (1.5 mg/kg tissue) and renal fat
(0.3 mg/kg tissue) contained the highest levels of radioactivity
expressed as cypermethrin. Negligible amounts were detected in the
brain (0.006 mg/kg tissue) and sciatic nerve (0.09 mg/kg tissue).
In the liver, adrenals, bone marrow, pituitary gland, and
mesenteric fat, levels of cypermethrin of 0.1 - 0.2 mg/kg tissue
were found.
(b) Separate isomers
Administration of (14C-benzyl)- cis-cypermethrin or
(14C-benzyl)- trans-cypermethrin separately to groups of 2 male
dogs as a single (2 mg/kg body weight) oral dose resulted in
83.4% of cis-isomer and 88% of trans-isomer being recovered in
the urine plus faeces over 6 - 7 days (Crawford, 1979b).
Quantitative differences existed between the amounts eliminated
via the 2 routes. As already mentioned, a variable distribution
was found. These data are consistent with the results of the study
involving 14C-cyclopropyl-labelled cypermethrin (Crawford, 1979a),
and the variation in amounts according to the route of elimination
probably reflects the inter-group differences in rates of
absorption of labelled material.
6.1.1.4. Cow
Three studies were carried out on lactating cows fed diets
containing 0.2, 5, or 10 mg 14C-benzyl and/or 14C-cyclo-propyl-
cypermethrin/kg feed, respectively, twice daily, for 7 or 21 days.
The estimated daily intake was 2, 50, or 100 mg cypermethrin/cow.
The radioactivity was rapidly eliminated following ingestion.
Equilibrium between ingestion and elimination was reached after
about 4 days. The amounts eliminated via the major routes were
similar for both labels, i.e., approximately 50% in the urine, and
approximately 40% in the faeces (mainly unchanged cypermethrin).
Polar and acidic components were found in the urine. Up to 0.2% of
the administered radioactivity was found in the milk, mainly in the
cream phase (about 88%). Feeding 0.2, 5, or 10 mg/kg feed, the
residues in the milk were 0.0006, 0.012, or 0.03 mg cypermethrin
/litre, respectively. Radioactivity (expressed as mg cypermethrin
/kg tissue) in the carcasses of the animals of the 3 groups at
slaughter was not detectable in muscle and brain (< 0.001- < 0.04
mg/kg). Levels in other tissues were: blood < 0.04 - 0.07 mg/kg,
liver 0.004 - 0.21 mg/kg, kidneys 0.003 - 0.11 mg/kg, and
subcutaneous and renal fat 0.01 - 0.1 mg/kg (Croucher et al.,
1985).
Swaine & Sapiets cf. FAO/WHO (1982b) dosed cows daily with 0.2,
5, or 50 mg cypermethrin (43% cis-isomers, 35% trans-isomers) per
kg feed for up to 29 days. Residues in milk and tissues were
comparable to those reported by Croucher et al. (1985).
6.1.1.5. Sheep
The elimination pattern in a single sheep, given one oral
dose of a mixture consisting of unlabelled cypermethrin with 14C-
benzyl- and 14C-cyclopropyl-labelled material (3.9 mg/kg body
weight) in a gelatin capsule, showed that 41% of the administered
dose was excreted in the urine and 20% was eliminated in faeces,
within 48 h. Tissue residues, 2 days after treatment, were muscle,
0.04 mg/kg; and liver, kidneys, and renal fat approximately 0.4
mg/kg tissue (Crawford & Hutson, 1977b).
6.1.1.6. Chicken
14C-phenoxy-labelled cypermethrin ( cis:trans, 55:45) was
administered orally to laying hens, daily for 14 days, at a rate
equivalent to 10 mg/kg diet (about 0.7 mg/kg body weight).
Radioactivity in the eggs reached a plateau, equivalent to about
0.05 mg cypermethrin/kg, after 8 days. Most of the radioactivity
was found in the yolk (up to 0.19 mg/kg) and about half of it was
identified as cypermethrin. The rest was closely associated with
neutral lipids and phosphatidyl cholines. Residues in the
carcasses, at slaughter, were low; values were between 0.01 and
0.02 mg/kg in muscle tissue, about 0.08 mg/kg in the subcutaneous
and peritoneal fat, and 0.37 mg/kg in the liver. The composition
of residues in the liver was not conclusively established. Apart
from small amounts of unchanged cypermethrin, the radioactivity was
also associated with highly polar material. However, it is evident
that the hen has a very effective mechanism for the metabolism of
cypermethrin (Hutson & Stoydin, 1987).
Comparable results were obtained from non-labelled studies with
laying hens in which dietary levels of up to 40 mg cypermethrin/kg
diet were fed for 28 days (Wallace et al., 1982).
6.1.1.7. Man
Male volunteers were each given a single oral dose of 0.25,
0.5, 1, or 1.5 mg cypermethrin in corn oil in a capsule. Urinary
excretion of cypermethrin metabolites was rapid. The subjects
excreted an average 78% of the dose of trans-isomer and 49% of the
cis-isomer within 24 h. These values did not differ from the
results in rats. The ester cleavage was a major route of
metabolism of cypermethrin in man. As reported in other animal
species, the trans-isomer was metabolized more readily than the
cis-isomer. Concentrations of both isomers excreted in the urine
between 2 and 5 days after dosing 0.5 or 1 mg cypermethrin were
below the limit of detection of 0.01 mg/litre (Eadsforth & Baldwin,
1983).
Groups of 2 male subjects were given cypermethrin in daily oral
doses of 0.25, 0.75, or 1.5 mg/man, by capsule, for 5 consecutive
days. During the dosing period and the following 5 days, 24-h
urine samples were collected daily and analysed for the
concentration of the cyclopropane carboxylic acid metabolite. The
results showed that the respective percent-ages of the cis- and
trans-isomers of cypermethrin, excreted in the 24-h period
following each of the oral doses, were similar to the percentage
excretion of these isomers measured in the single oral dose study.
Therefore, no accumulation in the body occurred (van Sittert et
al., 1985a).
6.1.2. Dermal
6.1.2.1. Cow
Two lactating cows were sprayed 3 times with 1.1 g
cypermethrin/animal, with 2-week intervals between treatments.
Milk samples were analysed during this period. Tissue samples
were analysed approximately three weeks after the final spraying.
The residues were: in whole milk, < 0.01 mg/litre; muscle, liver,
and kidneys, < 0.01 mg/kg tissue and in fat samples, 0.02 mg/kg
tissue or less (Baldwin et al., 1977).
Comparable results were obtained when 2 barns were sprayed with
either 0.05% or 0.1% of cypermethrin prepared from a 10% a.i.
formulation. Cows were present during spraying. Milk was
collected up to 4 weeks after spraying (0.05% application) or 4
days after spraying (0.1% application). Only the samples collected
4 days after the 0.05% treatment and 2 days after the 0.1%
treatment contained detectable residues (0.005 mg/kg milk). No
residues were found (< 0.002 mg/kg milk) in any of the other
samples (Baldwin & Lad, 1978a).
Cows were dipped twice in approximately 170 mg cypermethrin
/litre with a 10-week interval between treatments. The animals
were sacrificed 4 or 14 days after the second dipping. Residues in
muscle and liver did not exceed 0.01 mg/kg tissue. Fat samples
contained detectable residues. The highest was 0.13 mg/kg in renal
fat. The fat residue did not decline between 4 and 14 days after
treatment (Baldwin, 1977a).
Cattle sprayed once with 0.1 and 0.2% a.i. showed the same
level of residues (< 0.005 mg/kg tissue) in muscle, liver, and
kidneys, and a level of < 0.01 mg/kg in fat samples, 1, 3, 8, and
15 days after treatment. In cattle treated twice, fat samples
contained residues ranging from 0.01 to 0.05 mg/kg tissue (Bosio,
1979).
Many trials in which cows were sprayed with, or dipped in,
cypermethrin solutions were carried out in Australia. The milk
from cows sprayed with 0.1% cypermethrin did not contain any
detectable residues. The highest residue (0.03 mg/kg) in butterfat
was found one day after spraying. When the cows were dipped in a
dipwash containing 75 mg cypermethrin/litre, residues in the milk
determined 1, 3, and 7 days after dipping ranged from 0.01 to <
0.002 mg/litre. Omental fat contained the highest residue level
(0.02 mg/kg) 3 and 4 days after dipping. Liver, kidneys, and
muscle did not contain any detectable residues. A second dipping,
7 days after the first, did not cause any build-up of cypermethrin
in the tissues of the cattle (FAO/WHO, 1982b).
Detectable residues of cypermethrin of up to 0.01 mg/kg
butterfat were found in milk samples taken over 21 days from 5 of
10 cows wearing cypermethrin-integrated ear tags (Braun et al.,
1985).
Taylor et al. (1985) found cypermethrin in the hair of cattle,
in concentrations of up to 2.8 mg/kg, after application of
impregnated ear tags.
6.1.2.2. Sheep
Two sheep were each treated dermally with a mixture consisting
of unlabelled cypermethrin mixed with 14C-benzyl-and 14C-
cyclopropyl-labelled material at 22 mg/kg body weight. The
cypermethrin was slowly absorbed. Less than 0.5% of the dose was
excreted in the urine within 24 h and only 2% over a 6-day period.
Faecal elimination was also slow, 0.5% of the dose being eliminated
in 6 days. Approximately 30% of the dose was recovered from the
application area. Tissue residues, 6 days after treatment, were:
muscle, 0.04; renal fat, 0.3; and liver and kidneys 0.12 mg/kg
tissue (Crawford & Hutson, 1977b).
6.1.2.3. Man
A male subject was given a single dermal application of a ULV
formulation of cypermethrin (50 mg cypermethrin in hexylene
glycol/Shellsol AB) on the underside of the forearm. The majority
of this application (35 mg) was removed from the skin after 4 h.
Urine was monitored for residues of the acid metabolite [3-(2,2-
dichlorovinyl)-2,2-dimethylcyclo-propane-carboxylic acid] and its
glucuronide, for a 96-h period after dosing. The metabolites were
not detected over this period (Coveney & Eadsforth, 1982).
In a study by van Sittert et al. (1985b), 2 male volunteers
were given a single dermal application of a ULV formulation, 25 mg
cypermethrin in hexylene glycol/Shellsol A, on the underside of the
forearm. An average of 53% of the original amount of cypermethrin
applied was removed from the skin, 4 h after application.
Approximately 0.1% was excreted as the urinary metabolite,
cyclopropane carboxylic acid, during a 72-h period. Measurements
were made using gas liquid chromatography - mass spectrometry, a
method with a higher sensitivity and selectivity than gas liquid
chromatography - electron capture detection, which was used in the
previous study.
6.2. Metabolic Transformation
6.2.1. In vitro studies
In vitro studies on mouse liver homogenates have shown that
ester cleavage is more extensive for the trans-isomer than for the
cis-isomer. One mg of each of (1RS,trans)- and (1RS, cis)-
cypermethrin was incubated with 2.2 ml of approximately 10% mouse
microsome substrate at 37 °C for 30 min, under the following
conditions: (a) tetraethyl pyrophosphate (TEPP)-treated microsomes
(neither esterase nor oxidase activity); (b) normal microsomes
(esterase activity); (c) TEPP-treated microsomes plus NADPH
(oxidase activity); and (d) normal microsomes plus NADPH (esterase
plus oxidase activity). Each esterase preparation hydrolysed about
twice as much trans-cypermethrin as cis-cypermethrin. In contrast,
cis-cypermethrin was metabolized more rapidly in an oxidation
system than trans-cypermethrin. The major site of ring
hydroxylation was the 4' position and the secondary site was the 5
position. The trans-methyl group was an important site of
hydroxylation in the ester metabolites and cis-methyl oxidation was
predominant in the ester-cleaved acid metabolites. The
hydroxymethyl derivatives were further oxidized to the
corresponding aldehydes and carboxylic acids.
3-Phenoxybenzaldehyde-cyanohydrin was detected as a minor
metabolite. The preferred sites of hydroxylation were: with
trans-cypermethrin, cis-methyl > 4' position > trans-methyl > 5
position; with cis-cypermethrin, trans > cis > 4' position > 5
position (Shone & Casida, 1978; Shone et al., 1979). With cis-
cypermethrin, at least, cleavage of cypermethrin to cyanohydrin may
result from both hydrolytic and oxidative mechanisms, since large
amounts of the cleavage products were also evident in the oxidase
system, which lacks esterase activity (Shono & Casida, 1978; Shono
et al., 1979). However, at approximately 35-times higher substrate
levels, the hydrolysis rate of cypermethrin isomers was depressed
(Söderlund & Casida, 1977).
In studies on the metabolism of 14C-cypermethrin by rat liver
microsomes, the overall rates of metabolism of cis- and trans-
cypermethrin were similar, though their metabolic routes differed.
The cis-isomer was metabolized almost exclusively by an NADPH-
dependent oxidative pathway to 4'hydroxy- cis-cypermethrin with
subsequent oxidative ester cleavage. The predominant route for the
metabolism of the trans-isomer was hydrolysis to the trans-acid by
microsomal carboxylesterase (Crawford, 1979c). The in vitro
esteratic capacity was determined in rat, rabbit, and human liver
microsomes using p-nitrophenyl acetate and cypermethrin as
substrate. The relative ability to hydrolyse cypermethrin was
rabbit > man > rat. Rabbit and rat microsomes metabolized the
trans-isomer 6 times faster than the cis-isomer. Human
microsomes showed a similar capacity for metabolizing both cis-
and trans-isomers (Croucher et al., 1982a,b).
6.2.2. In vivo studies
The identification of the metabolites of cypermethrin has been
studied in mice (Hutson, 1978b, Casida et al., 1979; Hutson et al.,
1981), rats (Crawford & Hutson, 1977a; Casida et al., 1979; Hutson,
1979a,b; Crawford et al., 1981b; Rhodes et al., 1984), dogs
(Crawford, 1979d,e), and cows (Swaine & Sapiets, 1980a,b; Croucher
et al., 1985).
Overall, metabolism in these species is similar. Differences
that occur are related to the rate of metabolite formation rather
than to the nature of the metabolites formed. The only major
differences between species relate to conjugation reactions.
Cypermethrin (both isomers) is metabolized via cleavage of the
ester bond. The cyclopropane carboxylic acid moiety is mainly
excreted as the glucuronide conjugate; hydroxylation of the methyl
group occurs only to a limited extent (Crawford, 1979e; Rhodes et
al., 1984). The 3-phenoxy-benzyl product of the ester hydrolysis
is converted to PBA. The cyanide moiety is metabolized to
thiocyanate (Hutson, 1979b). The PBA moiety is mainly excreted as
a glutamic acid conjugate in the cow (Croucher et al., 1985), as a
taurine conjugate ( N-(3-phenoxy-benzoyl)taurine) in 2 strains of
mouse (Hutson & Casida, 1978; Hutson, 1978b, 1979a; Hutson et al.,
1981), and as a glycine conjugate in the rat and dog (Crawford &
Hutson, 1977a; Crawford, 1979d) and in the sheep, cat, and gerbil
(Huckle et al., 1981a). PBA is further metabolized (rat >
mouse > dog) via the 4'-hydroxylation to 3-(4'-hydroxyphenoxy)
benzoic acid and its sulfate conjugate (Crawford & Hutson, 1977a;
Hutson, 1978b; Crawford, 1979d). Glucuronic acid conjugates of PBA
and its 4'hydroxy derivative are the major urinary metabolites in
the marmoset, rabbit, guinea-pig, and hamster. The rat was unique
among the animal species tested in utilizing sulfuric acid for the
conjugation of the 4'-hydroxy derivative (Huckle et al., 1981a).
The major route of excretion for cypermethrin metabolites is via
the urine; unchanged cypermethrin accounted for the majority of
radioactivity found in faeces in radiolabel studies. The amount of
cyclopropyl-radioactivity eliminated in the bile (1%) suggests that
the biliary-intestinal-faecal route is of minor importance for this
moiety (Crawford & Hutson, 1977a; Crawford, 1979e; Rhodes et al.,
1984). Biliary excretion of PBA occurred as glucuronide and that
of 4'-OH-PBA as ether and ester glucuronic acid conjugates. Very
little was eliminated in the faeces, indicating that the biliary
glucuronides decompose and/or are enzymatically cleaved in the
gastro-intestinal tract to the respective benzoic acids. The
latter are subsequently reabsorbed and undergo further metabolism,
principally to the sulfate ester, which is excreted in the urine
(Huckle et al., 1981b). As already noted, the major urinary
metabolite of cypermethrin in cows is N-(3-phenoxy-benzoyl)glutamic
acid. This metabolite is also found in the organs and tissues with
only a small quantity of unchanged cypermethrin. The residues in
body fat consist mainly of cypermethrin. An unidentified polar
metabolite, present in the liver and kidneys, is suspected of being
a conjugate of 3-(4'-hydroxyphenoxy)benzoic acid. The small
portion of radioactivity appearing in milk was associated with
lipid components and consisted mainly of unchanged cypermethrin
(Croucher et al., 1985). The metabolic pathway of cypermethrin is
shown in Fig. 3.
As in other mammals, ester cleavage and elimination of the
cyclopropyl acid moieties in the free and glucuronidated form is a
major route of metabolism of cypermethrin in man (Eadsforth &
Baldwin, 1983).
6.2.3. Metabolism of the glucoside conjugate of 3-phenoxy-benzoic
acid
Studies have been carried out on rats on the metabolism of the
glucoside conjugate of 3-phenoxybenzoic acid, which occurs
occasionally as a metabolite in plants (Crayford, 1978). The
results indicated that the rat hydrolyses the glucoside and then
metabolizes the 3-phenoxybenzoic acid in virtually the same way as
it would metabolize PBA liberated during the metabolism of
cypermethrin.
 The same conclusion was also reached by Mikami et al. (1985).
During this study involving the metabolism of the glucoside
conjugate of PBA, it was noticed that the skin and carcasses
contained high residues (4 - 7% of the administered dose) of
radioactivity. To characterize the metabolites of PBA in the skin
and carcasses, rats were given (14C-benzyl) 3-PBA in a single oral
dose (0.8 mg/kg body weight) or a higher dose (totalling
approximately 750 mg/kg body weight) for 7 consecutive days. Two
components were identified in the skin: unchanged PBA and a
mixture of 3-phenoxybenzoyl-dipalmitins. The components were
present in the skin of the high-dose animals in the approximate
ratio of 3:7 and in the carcasses at 9:1 (Crayford & Hutson, 1979,
1980).
6.3. Metabolism in Plants
Lettuce plants were sprayed outdoors with 14C cypermethrin
labelled in the cyclopropyl ring (Wright et al., 1980; Roberts,
1981). The plants were sprayed twice, at a rate equivalent to 0.3
kg/ha, and harvested for analysis 21 days after the last treatment.
Most of the residue was in the form of unchanged cypermethrin (33%
of the total label present) and polar materials (54%), which were
shown to be mainly conjugates of trans-CPA. One of these
conjugates was identified as the beta-D-glucopyranose ester.
Evidence for this was obtained from studies on abscised cotton
leaves. The acid, trans-CPA was shown to be readily converted
into a mixture of the beta-D-glucopyranose ester, an acidic
derivative of this, and disaccharide derivatives, including the
glucosyl arabinose ester and the glucosylxylose ester.
Small apple trees were grown in cages outdoors (Roberts, 1981)
and the cis- or trans-isomers, labelled in either the cyclopropyl
or the benzyl rings, were applied to either the leaves or fruit.
In the leaves, it was shown that the main component of the residue
was cypermethrin itself with smaller amounts of sugar conjugates
that gave rise on hydrolysis to the 3-phenoxybenzyl alcohol,
3-phenoxybenzyl aldehyde, or PBA. A small amount of 4-hydroxy
cypermethrin was also detected. There was also evidence that some
30% of the cis-cypermethrin was converted to the trans-isomer,
though the conversion of trans- to cis- was not observed. Less
extensive metabolism occurred in the apple fruit. More than 98% of
the total label recovered was associated with the peel. Of this,
up to 77% was cypermethrin. Small amounts of the other free
compounds, together with polar compounds, were detected (Roberts,
1981).
Furuzawa et al. (1986) treated young cabbage plants in the
greenhouse with known amounts of either cis- or trans-
cypermethrin labelled in either the cyclopropyl or benzyl rings.
After 42 days, 4 - 6% of the applied dose was found on the surface
of the leaves, 57 - 63% in the acetone extract of the whole leaves,
and 13 - 26% was present as a bound residue. The observed half-
lives were 4 - 5 days for the trans- and 7 - 8 days for the cis-
cypermethrin. Isomerization was carefully followed in these
studies. First, there was practically no change in the ratio of
alpha-R to alpha-S isomers. On the other hand, both of the 1R
isomers ( cis and trans) were converted, in part, to the
corresponding 1S isomers, though the transformation appeared
greater where cis- had been the starting material, compared with
trans-cypermethrin. However, the position was complicated by the
simultaneous conversion of trans to cis-isomers. Apparently, these
changes were much less in the case of the extracts from the whole
leaf and the authors considered that this isomerization, as well as
the 1R-1S epimerization, were probably photochemical. These
findings on the conversion of cis to trans are consistent with the
formation of trans-cypermethrin from cis- reported by Cole et al.
(1982) in bean and cotton leaves in the greenhouse. These authors
also considered this to be a photochemical reaction. Roberts
(1981) reported the conversion of cis- to trans-cypermethrin on
apple leaves, but not of trans to cis.
The results of studies by Furuzawa et al. (1986) showed that,
in the intact ester, there was some hydration of the cyano group to
the amide, and subsequently to the corresponding acid, as well as
hydroxylation at the 4-benzyl or one of the gem methyl groups in
the cyclopropyl moiety. However, by far the most important
metabolites were glycoside conjugates of 4'-OH-PBA and CPA (with a
limited degree of isomerization from cis to trans and vice versa).
Hydroxylated CPA, either free or conjugated, was a relatively minor
metabolite.
More et al. (1978) studied the uptake of 14C radio-labelled PBA
in the abscised leaves of a range of different plants, which were
dipped in aqueous solutions. The PBA was labelled in the benzyl
ring. Mainly cotton plants and vines were studied, but broad
beans, tomatoes, lettuce, peas, and soybeans were also included.
In the case of cotton, some of the leaves were exposed to 13C-
labelled CO2 and allowed to take up the labelled PBA after a 2-h
period of photosynthesis; the 13C label was used to assist
identification by mass spectroscopy. A large part of the PBA in
the cotton leaves was conjugated within a few hours; it was shown
that most of the conjugated material was the glucose ester. It was
also shown, in the 13CO2 study, that at least a part of the glucose
moiety had been derived from recent photosynthesis. On more
prolonged exposure, other conjugates were formed. In the case of
vine leaves, these proved to be a series of disaccharides that
contained pentose sugars as well as glucose. The evidence
suggested that these had been formed by the addition of the pentose
sugar to the glucose conjugate rather than by the PBA becoming
conjugated with the preformed disaccharide. The conjugates in the
other plant species were not always positively identified, but the
authors commented that the interspecies differences were likely to
be mainly of degree rather than of any fundamental differences in
process.
Mikami et al. (1984) studied the metabolism of PBA in abscised
leaves of cabbage, cotton, cucumber, kidney bean, and tomato
plants. PBA was rapidly converted into more polar products by
esterification with glucose, malonylglucose, gentiobiose,
cellobiose, glycosylxylose, and tri-glucose. The main metabolites
were malonyl glucoside in cabbage, kidney bean, and cucumber, and
the glucosylxylose ester in cotton. The gentiobiose and tri-glucose
esters were predominant in tomato. The metabolism of cypermethrin
in plants is summarized in Fig. 4.
6.4. Metabolism in Fish
The metabolism of cis-cypermethrin (14C-labelled in either the
acid or the alcohol moiety) in trout was studied by Edwards &
Millburn (1985a). In contrast with that in other animals (frogs,
mice, and quail), ester cleavage of cypermethrin was very slow,
and the main metabolic pathway was hydroxylation to give the 4-
hydroxyphenoxy derivative, which was excreted in the bile as the
glucuronide. Edwards & Millburn (1985b) identified the glucuronide
of CPA and the ether glucuronide and sulfate of 4-hydroxy PBA, but
only as minor metabolites.
The same conclusion was also reached by Mikami et al. (1985).
During this study involving the metabolism of the glucoside
conjugate of PBA, it was noticed that the skin and carcasses
contained high residues (4 - 7% of the administered dose) of
radioactivity. To characterize the metabolites of PBA in the skin
and carcasses, rats were given (14C-benzyl) 3-PBA in a single oral
dose (0.8 mg/kg body weight) or a higher dose (totalling
approximately 750 mg/kg body weight) for 7 consecutive days. Two
components were identified in the skin: unchanged PBA and a
mixture of 3-phenoxybenzoyl-dipalmitins. The components were
present in the skin of the high-dose animals in the approximate
ratio of 3:7 and in the carcasses at 9:1 (Crayford & Hutson, 1979,
1980).
6.3. Metabolism in Plants
Lettuce plants were sprayed outdoors with 14C cypermethrin
labelled in the cyclopropyl ring (Wright et al., 1980; Roberts,
1981). The plants were sprayed twice, at a rate equivalent to 0.3
kg/ha, and harvested for analysis 21 days after the last treatment.
Most of the residue was in the form of unchanged cypermethrin (33%
of the total label present) and polar materials (54%), which were
shown to be mainly conjugates of trans-CPA. One of these
conjugates was identified as the beta-D-glucopyranose ester.
Evidence for this was obtained from studies on abscised cotton
leaves. The acid, trans-CPA was shown to be readily converted
into a mixture of the beta-D-glucopyranose ester, an acidic
derivative of this, and disaccharide derivatives, including the
glucosyl arabinose ester and the glucosylxylose ester.
Small apple trees were grown in cages outdoors (Roberts, 1981)
and the cis- or trans-isomers, labelled in either the cyclopropyl
or the benzyl rings, were applied to either the leaves or fruit.
In the leaves, it was shown that the main component of the residue
was cypermethrin itself with smaller amounts of sugar conjugates
that gave rise on hydrolysis to the 3-phenoxybenzyl alcohol,
3-phenoxybenzyl aldehyde, or PBA. A small amount of 4-hydroxy
cypermethrin was also detected. There was also evidence that some
30% of the cis-cypermethrin was converted to the trans-isomer,
though the conversion of trans- to cis- was not observed. Less
extensive metabolism occurred in the apple fruit. More than 98% of
the total label recovered was associated with the peel. Of this,
up to 77% was cypermethrin. Small amounts of the other free
compounds, together with polar compounds, were detected (Roberts,
1981).
Furuzawa et al. (1986) treated young cabbage plants in the
greenhouse with known amounts of either cis- or trans-
cypermethrin labelled in either the cyclopropyl or benzyl rings.
After 42 days, 4 - 6% of the applied dose was found on the surface
of the leaves, 57 - 63% in the acetone extract of the whole leaves,
and 13 - 26% was present as a bound residue. The observed half-
lives were 4 - 5 days for the trans- and 7 - 8 days for the cis-
cypermethrin. Isomerization was carefully followed in these
studies. First, there was practically no change in the ratio of
alpha-R to alpha-S isomers. On the other hand, both of the 1R
isomers ( cis and trans) were converted, in part, to the
corresponding 1S isomers, though the transformation appeared
greater where cis- had been the starting material, compared with
trans-cypermethrin. However, the position was complicated by the
simultaneous conversion of trans to cis-isomers. Apparently, these
changes were much less in the case of the extracts from the whole
leaf and the authors considered that this isomerization, as well as
the 1R-1S epimerization, were probably photochemical. These
findings on the conversion of cis to trans are consistent with the
formation of trans-cypermethrin from cis- reported by Cole et al.
(1982) in bean and cotton leaves in the greenhouse. These authors
also considered this to be a photochemical reaction. Roberts
(1981) reported the conversion of cis- to trans-cypermethrin on
apple leaves, but not of trans to cis.
The results of studies by Furuzawa et al. (1986) showed that,
in the intact ester, there was some hydration of the cyano group to
the amide, and subsequently to the corresponding acid, as well as
hydroxylation at the 4-benzyl or one of the gem methyl groups in
the cyclopropyl moiety. However, by far the most important
metabolites were glycoside conjugates of 4'-OH-PBA and CPA (with a
limited degree of isomerization from cis to trans and vice versa).
Hydroxylated CPA, either free or conjugated, was a relatively minor
metabolite.
More et al. (1978) studied the uptake of 14C radio-labelled PBA
in the abscised leaves of a range of different plants, which were
dipped in aqueous solutions. The PBA was labelled in the benzyl
ring. Mainly cotton plants and vines were studied, but broad
beans, tomatoes, lettuce, peas, and soybeans were also included.
In the case of cotton, some of the leaves were exposed to 13C-
labelled CO2 and allowed to take up the labelled PBA after a 2-h
period of photosynthesis; the 13C label was used to assist
identification by mass spectroscopy. A large part of the PBA in
the cotton leaves was conjugated within a few hours; it was shown
that most of the conjugated material was the glucose ester. It was
also shown, in the 13CO2 study, that at least a part of the glucose
moiety had been derived from recent photosynthesis. On more
prolonged exposure, other conjugates were formed. In the case of
vine leaves, these proved to be a series of disaccharides that
contained pentose sugars as well as glucose. The evidence
suggested that these had been formed by the addition of the pentose
sugar to the glucose conjugate rather than by the PBA becoming
conjugated with the preformed disaccharide. The conjugates in the
other plant species were not always positively identified, but the
authors commented that the interspecies differences were likely to
be mainly of degree rather than of any fundamental differences in
process.
Mikami et al. (1984) studied the metabolism of PBA in abscised
leaves of cabbage, cotton, cucumber, kidney bean, and tomato
plants. PBA was rapidly converted into more polar products by
esterification with glucose, malonylglucose, gentiobiose,
cellobiose, glycosylxylose, and tri-glucose. The main metabolites
were malonyl glucoside in cabbage, kidney bean, and cucumber, and
the glucosylxylose ester in cotton. The gentiobiose and tri-glucose
esters were predominant in tomato. The metabolism of cypermethrin
in plants is summarized in Fig. 4.
6.4. Metabolism in Fish
The metabolism of cis-cypermethrin (14C-labelled in either the
acid or the alcohol moiety) in trout was studied by Edwards &
Millburn (1985a). In contrast with that in other animals (frogs,
mice, and quail), ester cleavage of cypermethrin was very slow,
and the main metabolic pathway was hydroxylation to give the 4-
hydroxyphenoxy derivative, which was excreted in the bile as the
glucuronide. Edwards & Millburn (1985b) identified the glucuronide
of CPA and the ether glucuronide and sulfate of 4-hydroxy PBA, but
only as minor metabolites.
 Trout liver microsomes metabolize both cis- and trans-isomers
of cypermethrin to the 4'-hydroxy derivatives of the intact esters
and their corresponding ether glucuronic acid conjugates at
comparable rates. There are indications, derived from studies with
other pyrethroids, that the liver microsomes of the carp are more
able to metabolize pyrethroids than those of the salmonids (Edwards
& Millburn, 1985b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Leahey (1985) and Smith & Stratton (1986) give extensive
reviews on this matter.
7.1. Microorganisms
Effects of cypermethrin on microbial activity in the soil have
been investigated under laboratory conditions. Cypermethrin was
applied to a sandy loam soil at a rate of 2.5 or 250 mg/kg soil.
No effect was found on the rate of carbon dioxide evolution (Cook,
1978a) or oxygen uptake (Loveridge & Cook, 1978a) at the lower
rate. With 250 mg/kg, slight, but significant, inhibition of the
CO2 evolution and a decrease in oxygen uptake were noted after long
incubation periods. Nitrogen fixation was assessed by the
acetylene reduction method (Loveridge & Cook, 1978b). No
significant effects were found. Ammonification and nitrification
were studied in soils, either alone, or amended with urea or
ammonium chloride. Again, no significant effects were found, even
at the high rate of application (Cook, 1978b). Finally, glucose
utilization was studied in the short term (2 days) and after pre-
incubation with cypermethrin for up to 38 weeks. No significant
effects were noted, even at the high rate (Bromley & Cook, 1979).
A second series of experiments, in which cypermethrin was
applied to a sandy loam at 0.5 or 5 mg/kg soil has been reported.
Antimicrobial activity was observed in the early stages of
incubation with fungal population numbers returning to normal
within 2 - 4 weeks, and with a subsequent stimulation of microbial
growth. There was no inhibition of acetylene reduction activity at
either dosage. Nitrification and microbial respiration were
increased at both dosages, suggesting microbial degradation of
cypermethrin. Dehydrogenase activity was not affected, while
urease activity was stimulated. This study indicates that
cypermethrin may exert transient effects on the populations and
activities of microflora, but they are short-lived and minor in
nature (Tu, 1980).
The toxicity of cypermethrin for 4 pathogens of soybean and for
the nodulation organism Rhizobium has been investigated. In a
paper disc inhibition test, cypermethrin proved non-toxic for
Rhizobium, the EC50 exceeding 10 000 mg/litre. The toxicity for
the pathogens was higher, EC50 values ranging from 390 to 1700
mg/litre (Tu, 1982).
7.2. Aquatic Organisms
7.2.1. Fish
7.2.1.1. Acute toxicity
In common with other pyrethroids, cypermethrin is very toxic
for fish in clean water under laboratory conditions. The available
data are summarized in Table 8. The data demonstrate a similar
high acute toxicity for both cold- and warm-water species of fish.
Certain of these data have been reviewed by Stephenson (1982e).
There is no evidence of a significant effect of temperature on
toxicity, though a negative temperature coefficient has been
claimed for certain pyrethroids (Kumaraguru & Beamish, 1981).
Furthermore, the toxicity of pyrethroids for fish was not
influenced by the hardness or pH of the water (Mauck et al., 1976).
To determine the acute toxicity of cypermethrin for Tilapia
nilotica, an EC formulation of cypermethrin (25 g/litre) was
sprayed on the surface of static water held in stainless steel
tanks in a glasshouse. The concentration of cypermethrin in the
water was determined over a 96-h period. Depending on the
application rate, the concentration of cypermethrin in the water
rose rapidly during the first 24 h after application and decreased
slowly over the following 3 days. The tanks each contained 5 fish
and water to a depth of 30 cm. Water temperatures were 23 - 26 °C.
No fish deaths occurred when the peak concentration of cypermethrin
was less than 1.5 µg/litre, while all fish died when the
concentration exceeded approximately 5 µg/litre (Stephenson,
1981a).
To study the influence of suspended solids on water
concentrations and the toxicity for fish of cypermethrin, Rainbow
trout were exposed to nominal concentrations of 2 or 5 µg
cypermethrin/litre, which was added to microfiltered mains water or
to pond water containing 14.5 mg suspended solids/litre. The
actual concentrations were measured for water samples taken 30 min
after initial mixing. Two samples of pond water were taken. One
was analysed as such while the second was centrifuged (60 min at
3000 rev/min) to precipitate the suspended solids; the clear
supernatant liquid was then analysed. The results are summarized
in Table 9.
The data from this study show that suspended solids absorb 40%
of the cypermethrin initially present. Fish exposed to an actual
concentration of 4.9 µg cypermethrin/litre in mains water died
within 24 h, those in pond water containing an actual concentration
of 2.5 - 4 µg/litre survived (Reiff, 1978a).
The high degree of adsorption of cypermethrin on soils was
demonstrated by Riley & Hill (1983) in their studies on the
toxicity of cypermethrin for Daphnia and other aquatic organisms.
The addition of soil to the system reduced the toxicity of the
aqueous solution by 200 - 350 times.
The stainless steel tank study with Tilapia nilotica was
repeated with the water initially containing 100 mg suspended
solids/litre. In this study too, the presence of suspended solids
reduced the toxic effects of cypermethrin (about 2-fold)
(Stephenson, 1982a).
Table 8. Acute toxicity of cypermethrin for fish
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Brown trout 5 - 8 technical, dispersed via 15 2 - 2.8 Reiff (1976)
(Salmo trutta) DMSO
5 - 8 technical, absorbed on 15 1.2a,b Reiff (1976)
pumice
Rainbow trout 1 - 11 technical, absorbed on 10 - 15 0.5a,b,c Reiff (1978b)
(Salmo gairdneri) pumice
1 - 2 technical, via acetone 10 0.5 Stephenson
(1982b)
3.3 technical, via acetone 15 2.8 Stephenson
(1982b)
3 emulsifiable concentrate 10 11d,g Coats &
(40% ai) O'Donnell-Jeffery
(1979)
3 technical, dispersed via 10 55d Coats &
acetone O'Donnell-Jeffery
(1979)
Common carp 4 - 10 technical, absorbed on 10 0.9a,b,c Reiff (1978b)
(Cyprinus carpio) pumice
4 - 10 technical, absorbed on 20 - 25 1.1a,b,c Reiff (1978b)
pumice
8 - 10 technical, via acetone 10 0.9 Stephenson
(1982c)
2.1 emulsifiable concentrate 22 - 26 3.4f Stephenson
(5% a.i.) (1982c)
Stephenson et
al. (1984)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Rudd (Scardinius 9 - 10 technical, absorbed on 15 0.4a,b,c Reiff (1978b)
erythrophthalmus) pumice
Atlantic salmon 5.3 technical, dispersed via 10 2 - 2.4b McLeese et al.
( Salmo salar) ethanol (1980)
9.4 1-R- cis- 10 0.74 Zitko et al.
(1979)
Tilapia nilotica 1 - 3 technical, absorbed on 25 2.0a,b,c Stephenson
pumice (1981b)
Stephenson et
al. (1984)
Fathead minnow 0.74 technical, absorbed on 23 - 25 1.2a,b Stephenson
(Pimephales (juvenile) pumice (1982d)
promelas)
Mugil cephalus 0.1 30% EC ? 24g Tag El-Din et al.
30 days (1981)
Gambusia affinis 4 weeks 30% EC 25 6.6g El-Sebae et al.
(1983)
---------------------------------------------------------------------------------------------------------
a Flow-through system. Otherwise, static test.
b Measured concentration. Otherwise, nominal.
c The data quoted have been recalculated from the primary data given in the original reports following
current statistical methods.
d 24-h LC50.
e Lethal threshold value (geometric mean of lowest concentration with and highest concentration without
mortality).
f 48-h LC50.
g Formulation.
Remark: In most tests, the pH of the water was 7.5 - 8.5; the hardness was 260 mg/litre as CaCO3 (except
for a few cases).
Table 9. Influence of suspended solids on the toxicity of cypermethrin
for Rainbow trout
-------------------------------------------------------------------------
Source Suspended Initial cypermethrin Response of Rainbow
solids concentration (µg/litre) trout
(mg/litre) actual
nominal total in water
-------------------------------------------------------------------------
Microfiltered 0 2 1.7 1.7 1/6 fish dead at 48 h
mains water 5 4.9 4.9 6/6 fish dead at 24 h
Pond water 14.5 2 1.2 0.65 no symptoms or deaths
5 4.0 2.5 at 7 days
-------------------------------------------------------------------------
7.2.1.2. Long-term toxicity
The effects of cypermethrin on the most sensitive stage in the
life cycle of the Fathead minnow (Pimephales promelas) were
investigated using a flow-through system. Total hardness, pH,
concentration of dissolved oxygen, and temperature were controlled.
Within 24 h of fertilization, eggs were exposed to nominal
concentrations of 0, 0.03, 0.1, 0.3, or 1.0 µg/litre (mean exposure
concentration 0, 0.03, 0.12, 0.17, and 0.79 µg cypermethrin/litre),
for a total of 34 days. Hatching occurred between the 3rd and 6th
day, while egg hatch was not affected at the highest concentration.
No fry survived day 34. Survival was reduced at concentrations of
0.3 and 0.1 µg/litre but not at 0.03 µg/litre. On the basis of the
most sensitive parameter, i.e., survival of young fry, the no-
observed-adverse-effect level for cypermethrin lay between 0.03 and
0.12 µg/litre (Stephenson, 1982d).
7.2.2. Invertebrates
7.2.2.1. Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cypermethrin. The data in Table 10 are from static, clean water
test systems and some of these data have been reviewed by
Stephenson (1982e). It can be seen that snails do not show any
effects at 5 µg/litre (close to the water solubility of
cypermethrin), while some insects show behavioural changes, but no
mortality at this level. Crustacea, particularly marine decapod
Crustacea, are highly susceptible to cypermethrin, mortality
occurring at levels below 0.05 µg/litre. Water mites are also very
susceptible (Zitko et al., 1979).
In addition to these static water tests, continuous-flow tests
in clean water have been undertaken with 2 sensitive species
(Stephenson, 1980b) (Table 11).
It can be concluded that effects can be expected when
concentrations of cypermethrin of the order of 0.01 µg/litre are
maintained in the water phase for more than 96 h.
It has been shown that, at 100 µg/litre, cypermethrin does not
affect the growth of the single-celled green alga Selenastrum
capricornutum, over a period of 2 - 4 days (Stephenson, 1982b).
7.2.2.2. Long-term toxicity
The effects of cypermethrin on the survival, growth, and
reproduction of Daphnia magna were investigated, over 21 days, in
a static water test with daily renewal of test solutions. The
nominal concentrations tested were 0, 0.003, 0.01, 0.03, 0.1, and
0.3 µg/litre. Cypermethrin affected all 3 parameters at a nominal
concentration of 0.3 µg/litre, but no effects were noted at 0.1
µg/litre. Chemical analysis suggested that the Daphnia were
exposed to about 50% of the nominal concentration, two-thirds in
solution, the remainder adsorbed on suspended solids. These
results show that the no-observed-adverse-effect level of
cypermethrin throughout the life cycle for Daphnia is of the order
of 0.05 µg/litre (Garforth, 1982).
7.2.3. Field studies
7.2.3.1. Deliberate overspraying
This subject has received wide attention, and various aspects
have been reviewed in a number of publications (Crossland &
Stephenson, 1979; Crossland, 1982; Crossland et al., 1982;
Crossland & Elgar, 1983; Shires, 1983a; Stephenson, 1983).
The effects of field applications of cypermethrin on fish were
studied in a preliminary study by Crossland & Bennett (1976). A
small pond was deliberately oversprayed with 100 g cypermethrin/ha
as an EC formulation. The peak concentration of cypermethrin in
the water was 2.6 µg/litre. Wild populations of fish and amphibia
were not affected by this treatment. Large numbers of young fish
seen during the 2 weeks following treatment did not appear to be
affected.
In a second study at the same rate of application, the peak
concentration of cypermethrin was 1.4 µg/litre. There was no
observed mortality among 12 small rudd that had been placed in the
pond 12 days before treatment, and no effects of treatment were
noted in wild populations of rudd or newts (Crossland et al.,
1978).
Table 10. Acute toxicity of cypermethrin for aquatic invertebrates in static tests
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Fresh-water
Crustacea
Water flea up to 24 h 22 acetone 4.2 Reiff (1977)
(Daphnia magna) old 18 water 2 2 Stephenson (1980a)b
20 acetone 1.2 - Stephenson (1982b)
20 acetone 0.3c - Stephenson (1982b)
Water hog louse 3 - 8 mm 15 water 0.02 0.2 Stephenson (1980a)
(Asellus spp.)
Freshwater shrimp 3 - 8 mm 15 id 0.04 0.1 Stephenson (1980a)
(Gammarus pulex)
Insecta
Mayfly larvae 15 id 0.07 0.6 Stephenson (1980a)
(Cloeon dipterum)
Whirligig beetle adult 15 id 0.07 > 5 Stephenson (1980a)
(Gyrinus natator)
Bloodworm larvae 15 id 0.2 > 5 Stephenson (1980a)
(Chironomus thummi)
Mosquito larvae 18 id 0.03 1 Stephenson (1980a)
(Aedes aegypti)
Midge (Chaoborus larvae 15 id 0.03 0.2 Stephenson (1980a)
crystallinus)
Water boatman adult 15 water 0.7 > 5 Stephenson (1980a)
(Corixa punctata)
Water boatman adult 15 id 0.3 > 5 Stephenson (1980a)
(Notonecta spp.)
---------------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Arachnida
Water mite adult 15 0.02 0.05 Stephenson (1980a)
(Piona carnea)
Mollusca
Snail (Lymnaea < 8 mm 15 id > 5 > 5 Stephenson (1980a)
peregra)
Marine
Crustacea
Lobster (Homarus 450 g 10 ethanol - 0.04g McLeese et al.
americanus) (1980)
450 g 10 id - 0.003d,f,g Zitko et al. (1979)
Shrimp (Crangon 1.3 g 10 id - 0.01d,e McLeese et al.
septemspinosa) (1980)
---------------------------------------------------------------------------------------------------------
a EC50 values are based on effects, usually immobilization, other than death.
b Stephenson (1980a) used cypermethrin (85% a.i.) dissolved in acetone and absorbed on pumice.
c 48-h EC50.
d 96-h LC50.
e Dispersed via ethanol.
f 1-R- cis-.
g Salinity, 30%.
Remark: In most tests, the pH of the water was 7.5 - 8.5; and the hardness, 260 mg/litre as CaCO3.
Table 11. Acute toxicity of cypermethrin for aquatic invertebrates in
continuous-flow tests
------------------------------------------------------------------------
Species Stage Temperature EC50 LC50
size (°C) (µg/litre) (µg/litre)
24-h 96-h 24-h 96-h
------------------------------------------------------------------------
Freshwater shrimp 3 - 8 mm 15 0.005 0.004 0.030 0.009
(Gammarus pulex)
Mayfly larvae 15 0.008 0.004 0.070a 0.020
(Cloeon dipterum) 0.5 - 1 cm
------------------------------------------------------------------------
a 48-h LC50.
Observations on invertebrates were included in these two pond
studies. In the first study, which lasted 2 weeks, populations of
Crustacea, mites, and insects were severely reduced. Surface
breathing insects were affected most rapidly, within hours of
treatment. Free-swimming dipterous larvae were not noticeably
affected for 24 h, while zooplankton were killed between 1 and 2
days after treatment. Bottom dwelling invertebrates, including
chironomid larvae, snails, leaches, and flatworms, did not appear
to be affected, though the numbers in the last 2 groups were low in
pre-treatment samples.
The second study was continued for 15 weeks after treatment.
Initial results were similar to those reported above. Macro-
invertebrates were markedly reduced in numbers, 2 weeks after
treatment. However, both numbers and diversity returned to normal
levels after 15 weeks. Snails and flat-worms (again numbers low in
this group in pretreatment counts) were unaffected, but no
arthropods were present in the samples taken at 2 weeks.
Recolonization by flying insects (beetles and chironomids)
commenced 4 weeks after treatment. The Crustacean Asellus had not
reappeared by the end of the study.
No daphnids or copepods were found in the zooplankton samples,
1 week after treatment, and they only reappeared in the 8-week
post-treatment sample. Populations returned to normal levels in
10 - 12 weeks. Some 2 weeks after treatment, an increase in
filamentous algae was noted, and this persisted until the end of
the study. It was inferred that this was a secondary effect
following from the elimination of known feeders on algae, for
instance, the mayfly, Cloeon dipterum, and the daphnid,
Simocephalus sp. (Crossland & Bennett, 1976; Crossland et al.,
1978).
7.2.3.2. Monitoring of drift from ground and aerial applications
Two field studies were undertaken to assess the fate and
effects in adjacent waters of spray drift from ground-based
agricultural applications of cypermethrin.
(a) Mistblower applications on vines (France)
Three sites were chosen, each with vines planted up to the
banks of adjacent streams. One application of cypermethrin was
monitored at each site and a second application at one of the
sites. A diluted EC formulation of cypermethrin was applied at
30 g a.i./ha using a backpack, swinging-head mistblower, and at
45 g a.i./ha using a tractor-mounted, fixed-head mistblower.
The deposition on the ground and an adjacent stream of 0.37 -
4.5 g/ha led to peak concentrations in the subsurface water ranging
from 0.17 to 1.7 µg/litre during the first hour after spraying; the
concentrations decreased to less than 0.1 µg/litre within a few
hours. No effects on fish, tadpoles, or frogs were observed. Some
aquatic invertebrates in adjacent streams were seen to be
hyperactive or immobilized during the first 2 h after application.
This was most obvious with mayfly larvae, water boatmen, water
beetles, pond skaters, and syrphid larvae. During the first 3 h
following application, there was an increase in the number of
invertebrates caught in drift nets. However, there were no
significant population effects in zooplankton or macro-
invertebrates (Bennett et al., 1980).
(b) Boom-and-nozzle applications to row crops (United Kingdom)
Two sites were chosen each with row crops (potatoes or
sugarbeet or both) planted up to within 1 - 3 m of the edge of
ponds. Two applications of cypermethrin were monitored at each of
3 ponds. In each case, a diluted EC formulation of cypermethrin
was applied at 70 g a.i./ha using tractor-mounted, boom-and-nozzle
equipment. Deposition on the ground and over the pond was
estimated. The deposition led to peak concentrations in subsurface
water in the range of 0.01 - 0.05 µg/litre. In one case, the
concentration in subsurface waters after 24 h was 0.02 - 0.03
µg/litre. In all other cases, this value was at, or below, 0.01
µg/litre, the limit of detection. No effects on fish were
observed. No residues of cypermethrin were found in fish (limit of
detection, 5 µg/kg wet weight). The only effects on invertebrates
in adjacent waters were noted in the corner of 1 pond. Some pond
skaters, one water-boatman, and 2 syrphid larvae were found
immobilized. They had recovered by the next day. There was no
evidence from zooplankton or sweep-net samples of any effects on
invertebrate populations (Shires et al., 1980).
(c) Aerial application to winter wheat (United Kingdom)
A large field of winter wheat was chosen, which was surrounded
on 3 sides by drainage ditches. An EC formulation of cypermethrin
was applied at 25 g a.i./ha by fixed-wing aircraft. Up to 6% of
the nominal dose was deposited on the water surfaces, resulting in
maximum levels in subsurface waters of 0.03 µg/litre, which
declined rapidly after spraying. No effects were observed on caged
or wild fish, and no significant residues of cypermethrin were
found in the fish. A few air-breathing water boatmen and water
mites, which are very susceptible, showed minor short-term
reductions in abundance (Shires & Bennett, 1982, 1985).
(d) Application for rice insect control (Korea, Spain)
An EC formulation of cypermethrin was applied to rice paddies
in Korea, in which carp had been caged, by hand-spraying at 15 or
40 g a.i./ha. Mortality at the higher rate was significantly
higher than that in the control, i.e., 15 and 7%, respectively
(Stephenson, 1982c).
Another field study was carried out in Spain. Cypermethrin was
applied by air to paddy rice and also to caged fish (Cyprinus
carpio) within the area. The application rate was 15 - 40 g
a.i./ha. The limited toxic effects of cypermethrin on fish under
field conditions were confirmed in this study. Aerial overspraying
of the rice paddies at 25 g a.i./ha did not produce any mortality
(Stephenson et al., 1984).
(e) Applications for tsetse fly control (Nigeria)
Field studies have been reported on the assessment of the
environmental impact of the application of cypermethrin for tsetse
fly control in Nigeria. Searches for affected fish were made in
areas that had been selectively sprayed from the ground with an EC
formulation of cypermethrin at 2 - 3 g a.i./litre, or sprayed from
the air with an oil-based solution of cypermethrin at 100 g a.i./ha
in 1977 and at 60 and 150 g a.i./ha in 1978. The ground spray
application was successful in controlling the tsetse fly, but only
the highest rate applied from the air approached a satisfactory
level of tsetse control. None of these searches revealed any dead
fish in the rivers within the areas sprayed with cypermethrin.
Pre- and post-treatment net samples of aquatic invertebrates were
taken in an area sprayed at 100/150 g a.i./ha. Acute mortality
occurred in terrestrial and aquatic arthropods, such as the water
beetle and Crustaceans. Shrimps and mayfly larvae disappeared from
river benthos after spraying, but reappeared one year later (Smies
et al., 1980).
7.3. Terrestrial Organisms
7.3.1. Laboratory studies
7.3.1.1. Acute toxicity
(a) Birds
The acute oral toxicity of cypermethrin for birds is summarized
in Table 12.
In the first study (Coombs et al., 1976), 4 birds of each
species were observed for a 3-week period after dosing; no deaths
or toxic signs were noted at the highest dosages applied. In the
second study (Rose, 1981), clinical signs of cypermethrin
intoxication, including ataxia and lethargy, were noted at dosages
of 3000 mg/kg body weight and above. One male duckling, out of 28
of each sex, died at the top dosage of 10 000 mg/kg body weight.
7.3.1.2. Short-term toxicity
(a) Birds
Six laying hens were given 5 successive daily oral doses of
1000 mg cypermethrin/kg body weight in DMSO followed after 3 weeks
by a second 5-day dosing regime and were observed for a further 3
weeks. Control hens did not receive any treatment. No signs of
intoxication or histological changes in nervous tissue were noted
at any time (Owen & Butterworth, 1977).
Table 12. Acute oral toxicity of cypermethrin for birds
--------------------------------------------------------------------------
Species Age Vehicle Observation LD50 Reference
period (mg/kg
body
weight)
--------------------------------------------------------------------------
Domestic fowl adult 40% DMSO 21 days > 2000 Coombs et al.
(Gallus (1976)
domesticus)
French partridge adult 40% DMSO 21 days > 3000 Coombs et al.
(Allectoris (1976)
rufa)
Mallard duck 2 - 6 40% emul- 14 days > 10 000 Rose (1981)
(Anas months sifiable
platyrhynchos) concentrate
--------------------------------------------------------------------------
Cypermethrin was administered for 5 consecutive days to Mallard
ducks at dosages of 5000 or 10 000 mg/kg diet. No deaths or
clinical signs of intoxication were noted during the feeding period
or over a subsequent 40-day holding period. Food intake and body
weights were slightly depressed during the feeding period, though
most ducks had regained or exceeded their initial body weights by
the end of the holding period (Rose, 1981).
Riviere et al. (1983) studied the influence of cypermethrin on
the microsomal drug metabolizing enzymes in Japanese quail
(Coturnix coturnix). Cypermethrin had no or only a very weak
inducing effect.
(b) Honey bees
Cypermethrin was highly toxic for worker honey bees (Apis
mellifera) in laboratory tests (24-h oral LD50 0.035 µg a.i. per
bee) (Table 13). Cypermethrin applied on the dorsal side of the
thorax was more toxic for honey-bees at lower breeding
temperatures, i.e., at 12 - 20 °C, the toxic level was 0.01 - 0.02
µg/bee and at 32 °C, 0.02 - 0.03 µg/bee. In addition, the
sensitivity of the bees to cypermethrin increased with age (Delabie
et al., 1985).
An aqueous dispersion of cypermethrin was sprayed directly on
worker honey bees. Only 5% of the bees sprayed with a 0.01
g/litre solution were killed. All bees sprayed with 0.1 and 1
g/litre died (Harris & Turnbull, 1978).
First indications that cypermethrin might not be hazardous for
bees under field conditions were obtained in cage tests. Worker
honey bees were exposed for 2 h to residues produced by treating
flowering Solidago (Golden rod) to run-off with a spray containing
0.5 g cypermethrin/litre and aging the residue for 1 h. Some
knock-down of the bees (up to 9%) was noted during the first 8 h
following initial exposure. However, most bees recovered, and
mortality after 1 day and 10 days did not differ from that in
untreated control bees. Higher mortality had been noted in an
earlier study using the same technique but with a higher dosage
rate of 1.5 g cypermethrin/litre (Gerig, 1979, 1981).
Table 13. Toxicity of cypermethrin for worker honey bees
-------------------------------------------------------------------------
Formulation 24-h LD50 (mg/bee) Reference
Topical application Oral administration
-------------------------------------------------------------------------
Technical 0.02 0.035 Badmin & Twydell
(1976)
Technical 0.053 0.12 Knight (1982)
Technical 0.056 - Smart & Stevenson
(1982), Westlake
et al. (1985)
Emulsifiable - 0.031 Badmin & Twydell
concentrate (1976)
(40%)
-------------------------------------------------------------------------
(c) Predatory and parasitic species
Cypermethrin has also been evaluated against a number of
predatory or parasitic insects and mites. Laboratory data are
available for a number of predators (Coleoptera) and parasites
(Hymenoptera) of insect pests.
The available data indicate that cypermethrin can show useful
selectivity between insect predators and parasites and the relevant
pest species (Mulla et al., 1978; du Toit, 1978; Waddill, 1978;
Abdel-Aal et al., 1979; Coats et al., 1979; Holden, 1979; Leake et
al., 1979; McDonald, 1979; European Patent Office, 1980; Harris &
Turnbull, 1980; Hagley et al., 1981; Jordan & Chang, 1981; Saad et
al., 1981; Ascher et al., 1982; Baicu, 1982; Bayoumi, 1982; Chang &
Jordan, 1982; Osman et al., 1982; Rajakulendran & Plapp, 1982;
Surulivelu & Menon, 1982; Brempong-Yeboah et al., 1983; Chang &
Jordan, 1983; El-Guindy et al., 1983; El-Minshawy et al., 1983;
El-Sebae et al., 1983; Ho et al., 1983; Ishaaya et al., 1983;
Watters et al., 1983; Abbassy et al., 1984; Bariola & Lingren,
1984; Brempong-Yeboah et al., 1984a,b,c; Cheng & Hanlon, 1984; Dai
& Sun, 1984; El-Sayed & Knowles, 1984; Ewen et al., 1984; Fabellar
& Heinrichs, 1984; Hopkins et al., 1984; Liu et al., 1984; Mani &
Krishnamoorthy, 1984; Meisner et al., 1984; Le Patourel & Singh,
1984; Riskallah, 1984; Scott & Georghiou, 1984; Wilde et al., 1984;
Zohdy et al., 1984; Ahmed et al., 1985; Abou-Awad & El-Banhawy,
1985; Bostanian & Belanger, 1985; Bostanian et al., 1985; Corbitt
et al., 1985; Edwards et al., 1985; El-Sebae et al., 1985; Harris
et al., 1985; Knapp et al., 1985; Knowles & El-Sayed, 1985; McKee &
Knowles, 1985; Pree & Hagley, 1985; Suhas & Devaiah, 1985; Tewari &
Krishnamoorthy, 1985; Vekaria & Vyas, 1985; Barlow et al., 1986).
(d) Earthworms
The toxicity of cypermethrin for the earthworm (Eisenia
foetida) has been assessed in an artificial soil test system. Worms
were exposed to dosages of 0, 0.1, 1.0, 10, or 100 mg/kg soil for
14 days. No mortality was found (Inglesfield & Sherwood, 1983;
Inglesfield, 1984).
The LC50 for cypermethrin in Eisenia foetida was found to be
26.1 µg/cm2 (16.3 - 44.4 µg/cm2) (Roberts & Dorough, 1984).
(e) Higher plants
In studies on effects of cypermethrin on seed germination in
the soybean, seedling emergence and survival were not reduced by
levels of up to 5000 mg/litre (Tu, 1982). Cypermethrin has been
demonstrated not to be phytotoxic for crops when applied at
recommended dosages (Hargreaves & Cooper, 1979).
7.3.2. Field Studies
7.3.2.1. Applications for tsetse fly control in Nigeria
Field trials in Nigeria of cypermethrin against Tsetse flies
( Glossina palpalis (RD) and Glossina tachnoides Westw.) were
carried out, over a 2-year period by Spielberger et al. (1979).
Ground applications of cypermethrin (0.3%) were made on fly-resting
sites in vegetation using pressurized knapsack sprayers.
Populations of both species were eradicated after a single
application. Levels of over 150 g/ha were needed for complete
eradication by residual spraying from a helicopter.
The effects on the general insect population of aerial
applications of cypermethrin for tsetse fly control were studied by
Smies et al. (1980). Within a few hours of application, dead and
dying insects were found, exemplifying the rapid knockdown effect
of cypermethrin. Insect fallout, as assessed by funnel traps, was
markedly increased for 1 day by 100 g cypermethrin a.i./ha, and for
3 days by 150 g a.i./ha. General insect activity as assessed by
Malaise trap catches was not affected by 150 g cypermethrin a.i./ha
(Smies et al., 1980).
7.3.2.2. Honey bees
This subject has been reviewed by Shires & Debray (1982) and
Shires (1983b).
The hazard of practical field applications of cypermethrin for
worker honey bees was assessed in 2 field trials. In both trials,
cypermethrin was applied to flowering oilseed rape, by helicopter,
at a time when the crops were being actively worked by bees from
nearby colonies, thus representing a worst case exposure of the
bees. The rate of application in both trials was 25 g a.i./ha. Of
the 8 hives, placed adjacent to the treated crops, 6 had been
fitted with dead bee traps and 2 with pollen traps. Observations
were made on bee mortality, pollen collection, foraging activity,
hive populations, and brood areas. In addition, in the second
trial, cypermethrin residues were determined in the bees, pollen,
wax, honey, and in the leaves and flowers of the treated crop
(Pearson & Shires, 1981; Shires, 1982b).
In the first trial, only a small increase in bee mortality was
noted, following the cypermethrin treatment (Table 14).
Cypermethrin appeared to be repellent to honey bees foraging the
crop, but the duration of this effect could not be established
because of poor weather during the first few days following
treatment. Cypermethrin did not have any effects on hive
populations of adult bees or brood areas.
In the second trial, a slight increase in bee mortality was
found at the time of treatment (Table 14). Cypermethrin had a
repellent effect on honey bees for up to 24 h after application.
Following this period, foraging activity and pollen collection
returned to normal. Cypermethrin residues in dead bees, collected
on the day of spraying and on the following morning, were higher
than would be expected to be lethal. However, residue levels in
dead bees collected after this period were considerably lower than
those required to produce toxic effects, confirming that they
represented the natural mortality level. Very low levels of
cypermethrin were found in live bees and levels in honey and wax
were close to the limit of detection. Concentrations of
cypermethrin in flowers and pollen declined rapidly after
treatment. Again, cypermethrin did not have any effects on hive
populations of adult bees or of brood areas (Shires, 1982b).
Table 14. Mean number of dead bees per hive during large-scale
field trials with cypermethrina
-----------------------------------------------------------------
Treatment Time from treatment
compound -2 to -1 -1 to 0 0 to +4 +1 to +2 +3 to +4
(dosage) days days h days days
-----------------------------------------------------------------
Winter-sown rape; 65 6 260 40 2
cypermethrin
(25 g a.i./ha)
water control 75 17 0 26 15
Spring-sown rape 9 36 310 2 13
cypermethrin
(25 g a.i./ha)
-----------------------------------------------------------------
a From: Shires (1983b).
In glasshouse studies, in which insecticide treatment was
carried out during foraging by spraying the rape with 0.12 ml of
Cymbush in 100 ml water (equivalent to 50 g a.i./ha), high
mortality was observed 2 days after treatment, but no residual
mortality appeared during the following 2-month period. In this
study, oilseed rape flowers were not attractive to bees for at
least 2 days following treatment.
A field test was carried out on a 38-ha field of oilseed rape.
The insecticide was sprayed on a central area of 13 ha during the
morning, at a rate of 50 g a.i./ha. The weather was sunny and the
temperature about 18 °C. A high level of mortality occurred only
on the 3 days following treatment. The results also showed that the
bees avoided visiting the flowers as soon as the treatment was
made, especially during the first 2 days. From the third day after
the treatment, the repellent effect decreased, and the visits to
the rape flowers increased reaching normal on the fifth day. It
was suggested by the authors that the repellent effect appeared to
be due to the formulation ingredients.
No residues of cypermetherin were found in hive products
(pollen, wax, or honey) (Delabie et al., 1985).
In a study by Gerig (1981), cypermethrin was repellent to
worker honey bees for a short period after spraying. Flowering
Phaselia plants, within a flight tent, were treated with a spray
containing 0.5 g cypermethrin/litre. Honey bee visits to the
treated flowers were very few over the first 0.5 h following
treatment and remained at a reduced level during the remaining
5.5 h of observation on the day of treatment. Flower visits
returned to normal levels on the following day (Gerig, 1981).
7.3.2.3. Soil fauna
This topic has been reviewed by Shires (1980).
In an initial study on the effects of cypermethrin on the soil
surface fauna and earthworms, cypermethrin was applied at 100 g
a.i./ha to small plots of spring wheat. This treatment did not
have any effect on earthworm populations or on leaf litter
breakdown, which is an indirect indicator of earthworm activity.
The general population of soil surface predators, mainly beetles
and spiders, was reduced to about 50% of the control value soon
after treatment, but increased to a higher level than that in the
controls after 5 weeks. A subsequent secondary decrease in
predator populations was probably related to the efficiency of
control of cereal aphids by cypermethrin (Shires et al., 1979). In
a second study, cypermethrin was applied at 25 g a.i./ha to newly-
emerged winter barley to control the aphid vectors of barley yellow
dwarf virus. Populations of non-target arthropods were assessed
using pitfall and water traps. Populations of soil Collembola
were only slightly affected by cypermethrin. Populations of
predators (beetles, spiders, and centipedes) were already declining
because of the season, but there were significantly fewer predators
in the cypermethrin plots compared with the controls at the end of
the trial. Cypermethrin treatment did not affect the numbers of
invertebrates collected from the water traps (Sherwood & Shires,
1981).
Two studies have been reported on the summer application of
cypermethrin for aphid control in winter wheat. In France, ground
applications of cypermethrin at 50 and 75 g a.i./ha were made in
June and effects on the fauna in the air, on the crop, and on the
soil surface were studied. Cypermethrin proved effective against
the phytophagous (pest) species. Effects on non-target organisms
were only minor, with the exception of spiders. In the second
study in the United Kingdom, cypermethrin was applied in June using
a fixed-wing aircraft at 25 g a.i./ha. Again, cypermethrin proved
effective against the pest species, while, generally, effects on
non-target organisms were minor and short-lived (Shires, 1982a,c).
Similar results have been obtained in studies on the aerial
application of cypermethrin at 25 g a.i./ha on oilseed rape and at
50 and 75 g a.i./ha on maize, in France (Inglesfield, 1982;
Garforth, 1983).
7.3.2.4. Foliar predators and parasites
The relative toxicity of cypermethrin for pests and their
parasites and predators is such that the balance between host/prey
and parasites/predators may not be adversely affected in the field.
However, care should be taken where predatory mites are important
in pest management. The high toxicity of cypermethrin for
predatory mites has been confirmed under field conditions (Wong &
Chapman, 1979; Aliniazee & Cranham, 1980; Shires & Tipton, 1982).
The effects of cypermethrin treatments, included in a spray
programme for cotton insect control, on white fly parasitism have
been studied in the Sudan. The treatment regime that gave the
consistently highest level of control of parasitism included
cypermethrin at 40 g a.i./ha in 4 early season sprays (Shires &
Tipton, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Leahey (1985) and Smith & Stratton (1986) have reviewed the
available literature on this subject.
8.1. Single Exposures
8.1.1. Oral
The acute oral toxicity of cypermethrin (mixture of cis-/ trans-
isomers) in experimental animals is of a moderate order (Tables 15,
16). Signs of intoxication, indicative of an action on the central
nervous system consist of sedation, ataxia, splayed gait, tip-toe
walk, with occasional tremors and convulsions. These signs of
toxicity appear within a few hours following dosing, and survivors
show clinical recovery within 3 days (Coombs et al., 1976).
Table 16 shows the effects of the ratio of cis-: trans-isomers
on the acute oral toxicity (FAO/WHO, 1980b).
Factors known to influence the oral LD50 value of cypermethrin
include concentration, vehicle, temperature, age of the animals,
and the animal strain used (Coombs et al., 1976; Dewar & Owen,
1979). The acute toxicity of cypermethrin was approximately 3
times greater in 3-week-old, than in 12-week-old rats (Rose &
Dewar, 1978). The cis-: trans-ratio plays a role in determining
acute toxicity, the cis-isomers being more toxic than the trans-
isomers. The oral LD50 of the cis-isomers in rats (Carworth Farm
E strain) was 229 mg/kg body weight while, with the trans-isomers,
no deaths were found at 2000 mg/kg body weight (Brown, 1979a,b).
8.1.2. Dermal
The acute dermal toxicity of technical cypermethrin is of a low
order. There were no deaths in CFE rats at any dose level tested
up to 1600 mg/kg body weight using 20% w/v xylene as the vehicle
(Coombs et al., 1976). The dermal LD50 for the cis-isomer of
cypermethrin, applied to the skin of rats as a 10% solution in
DMSO, was 219 mg/kg body weight (Brown, 1979a).
The acute dermal toxicity of cypermethrin for the rabbit
is > 2460 mg/kg body weight (US EPA 1984).
8.1.3. Intraperitoneal
The intraperitoneal (ip) LD50 (20% w/v solution in corn oil)
for technical cypermethrin in CFI mice is 485 mg/kg body weight
(Coombs et al., 1976). In rats, the LD50 is between 198 and 315
mg/kg body weight (administered as 5% solution in DMSO) (Price,
1981a).
Table 15. Oral LD50 values for technical cypermethrin
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
-----------------------------------------------------------------------
rat 5% in corn oil 251 (203 - 295) Coombs et al.
(1976)
rat 5% in dimethylsulf- 303 (277 - 329) Coombs et al.
oxide (1976)
rat 5% in dimethylsulf- 570 (485 - 823) Price (1981a)
oxide
rat 40% in dimethylsulf- approximately 4000 Rose (1982)
oxide
rat 5% in glycerol 200 - 400 (male); Coombs et al.
formal approximately 200 (1976)
(female)
rat 10% in aqueous 400 - 800 (male); Coombs et al.
suspension approximately 400 (1976)
(female)
rat 50% in aqueous 3423 (2815 - 4328)a Rose (1982)
suspension
mouse 5% in corn oil 82 (68 - 116) Rose (1982)
mouse 5% in dimethylsulf- 138 (105 - 199) Coombs et al.
oxide (1976)
mouse 50% in aqueous 657 (439 - 1003) Rose (1982)
suspension
Syrian 10% in corn oil approximately 400 Coombs et al.
hamster (male and female) (1976)
Chinese 5% in corn oil 203 (144 - 255) Coombs et al.
hamster (1976)
guinea- 20% in corn oil approximately 500 Coombs et al.
pig (male); > 1000 (1976)
(female)
bovine 15% formulation in 142 - 284 (male) Cassidy (1979)
calves Shellsol A
piglets 15% formulation in > 600 (male) Cassidy (1979)
Shellsol A
-----------------------------------------------------------------------
Table 15. (contd.)
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
----------------------------------------------------------------------
lamb 15% formulation in 283 - 567 (male) Cassidy (1979)
Shellsol A
domestic 40% in dimethylsulf- > 2000 Coombs et al.
fowl oxide (1976)
partridge 40% in dimethylsulf- > 3000 Coombs et al.
oxide (1976)
-----------------------------------------------------------------------
a 40:60 cis/trans.
Table 16. Effects of cis-: trans-ratio on the acute toxicity of
cypermethrina
------------------------------------------------------------------
Species Sex Vehicle cis-: trans-ratio LD50 (mg/kg)
------------------------------------------------------------------
rat male, dimethylsulfoxide cis-only 160 - 300
female
rat male, dimethylsulfoxide trans-only > 2000
female
rat female corn oil 90:10 367
rat female corn oil 40:60 891
------------------------------------------------------------------
a From: FAO/WHO (1980b).
8.1.4. Inhalation
Groups of CD rats (4 of each sex) were exposed for 4 h to an
aqueous spray of cypermethrin as a 400 g/litre emulsifiable
concentrate. The liquid phase contained 3 g cypermethrin/litre and
the atmospheric concentration of droplets in the vicinity of the
rats was calculated to be 0.7 mg/litre. The median droplet size
was 130 µm with a geometric standard deviation of 1.6 µm,
comparable with the droplet spectrum of a field-spraying device.
(It should be noted that this droplet size is nearly not
inhalable). The rats became thoroughly soaked during the exposure,
thereby indicating a significant dermal exposure with probable oral
exposure during post-exposure grooming. Immediately after the
exposure, the female rats showed the abnormal gait and urinary
incontinence typical of pyrethroid intoxication. Recovery took
place within 3 days. No deaths or other adverse signs occurred
during the exposure period or the subsequent 14-day observation
period (Blair & Roderick, 1976).
In another study on possible effects on the peripheral nerves,
rats (8 of each sex) were exposed for 4 h to an aqueous spray of
cypermethrin as a 400 g/litre emulsifiable concentrate under
conditions similar to those in the previous study. Half of the
rats were necropsied immediately after exposure and the others
after 66 h. Examination of the sciatic nerves from all of the rats
did not reveal any abnormalities, even though signs of intoxication
had been observed (Blair et al., 1976).
8.1.5. Skin and eye irritation
Technical cypermethrin was moderately irritant to occluded
rabbit skin (both intact and abraded) and to the eyes of New
Zealand white rabbits. In both cases, recovery was complete in a
few days. The severity of the irritation depended on the
formulation and the solvent used (Hine & Zuidema, 1970; Coombs et
al., 1976).
The skin on the backs of guinea-pigs was treated with
cypermethrin. The animals responded by licking, rubbing,
scratching, or biting the sides tested. This reaction may be
associated with skin sensory effects characterized by transient
itching and tingling sensations (Cagen et al., 1984).
8.1.6. Sensitization
A skin sensitization test on guinea-pigs (maximization
procedure of Magnusson & Kligman) indicated that technical
cypermethrin may have a mild skin sensitizing potential (Coombs et
al., 1976; US EPA, 1984).
8.2. Short-Term Exposures
8.2.1. Oral
8.2.1.1. Rat
Groups of 6 male and 6 female Charles River rats were fed diets
containing 0, 25, 100, 250, 750, or 1500 mg cypermethrin/kg feed
for 5 weeks. In the 1500 mg/kg group, reduced body weight gain and
food intake, piloerection, nervousness, incoordinated movement,
increased liver weight, and increases in blood urea and haemoglobin
concentrations were observed, but there were no pathological
changes. No changes were detected in the groups receiving 750
mg/kg or less (Coombs et al., 1976).
Groups of Charles River rats, 12 of each sex (24 of each sex as
controls), were fed dietary concentrations of 0, 25, 100, 400, or
1600 mg cypermethrin/kg feed for 3 months. Signs of intoxication,
such as hypersensitivity and abnormal gait, were observed in the
1600 mg/kg group, during the first 5 weeks, and one animal died.
The survivors showed clinical recovery in the second half of the
study. However, body weight gain was reduced, liver and kidney
weights increased, and there were increases in plasma-urea
concentrations and plasma-alkaline phosphatase activity, and
decreases in haemoglobin concentrations and the red blood cell
count in females, and in the kaolin cephalin clothing time in males
and packed cell volume were observed. Two out of 4 male rats,
killed prematurely, showed axonal breaks and vacuolization of
myelin in the sciatic nerve. None of the survivors showed nerve
lesions, and no other pathological effects were found. In the
400 mg/kg group, males showed increased kidney weights but no
histopathological changes. No effects were found in the 100 mg/kg
group (Hend & Butterworth, 1976).
Male and female rats (20 in each group) were fed cypermethrin
( cis-: trans- = 44:56) with a purity of 92% in the diet at levels
of 0, 75, 150, or 1500 mg/kg diet, for 90 days. Haematology and
the results of urinalysis were normal. Both males and females
receiving 1500 mg/kg diet showed reduced body weight and reduced
food consumption during the first month of the study. Increased
liver microsomal oxidase activity was noted in both males and
females in the 1500 mg/kg group and in the males in the 150 mg/kg
group. These changes were substantially reversed within the 4-week
recovery period. Gross and microscopic (including electron
microscopic) examination of the tissues and organs did not reveal
any signficant differences between the treated groups and the
controls. Examination of the sciatic nerve of animals in the
control and the 1500 mg/kg groups did not reveal any changes that
could be directly attributed to cypermethrin (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats per group and 14 of
each sex as controls) were fed trans-isomer at concentrations of
0, 30, 100, 1000, or 3000 mg/kg diet, for 5 weeks. No gross or
microscopic findings were observed and mortality did not occur.
Changes in several haematological parameters, alkaline phosphatase
activity, and liver, spleen, and kidney weight were observed at the
2 highest dose levels. Special histopathological studies of the
sciatic nerve did not show any damage (Hend & Butterworth, 1977a).
Groups of rats (6 male and 6 female rats per group and 10 of
each sex used as controls) were fed concentrations of the cis-
isomer at 0, 30, 100, 300, 750, or 1500 mg/kg diet for 5 weeks
(protocol similar to that described above). Mortality was observed
at the 1500 mg/kg level as well as neurotoxic signs of poisoning.
Growth was reduced at the 750 mg/kg level. Significant reductions
in food intake were also noted at doses of 300 mg/kg or more,
during the initial phase of the study. Relative kidney weights
showed a statistically-significant increase at doses of 300 mg/kg
or more and an increase in liver weight was observed at 750 mg/kg.
Histopathological examinations revealed substantial degeneration
in both the liver and sciatic nerve at 1500 mg/kg. No lesions were
observed in the brain or spinal cord (Hend & Butterworth, 1977b).
8.2.1.2. Dog
Beagle hounds (4 of each sex per dose level) were fed diets
containing 0, 5, 50, 500, or 1500 mg cypermethrin/kg diet for 13
weeks. At 1500 mg/kg, severe signs of intoxication consisting of
diminished food intake, weight loss, diarrhoea, anorexia, licking
and chewing of the paws, whole body tremors, a stiff exaggerated
gait, ataxia, incoordination, and hyperaesthesia were observed.
Half of the dogs in this group were necropsied because of severe
clinical signs of intoxication. However, no changes in
haematology, organ weight, or histopathology were observed and
despite the severe signs of intoxication, no lesions of the sciatic
nerves were observed in the dogs. No effects were seen at 500
mg/kg feed (Buckwell & Butterworth, 1977).
8.2.2. Dermal
8.2.2.1. Rabbit
Groups of 10 male and 10 female New Zealand white rabbits
received occluded dermal applications (abraded and non-abraded
skin) of 2, 20, or 200 mg cypermethrin/kg body weight in
polyethylene glycol (PEG 300) for 6 h per day, 5 days per week,
for 3 weeks. A control group was similarly treated with the
vehicle only (PEG 300). Slight-to-moderate skin irritation was
observed in rabbits receiving 2 and 20 mg/kg body weight; those
receiving 200 mg/kg body weight showed slight-to-severe irritation.
In the rabbits treated at 200 mg/kg, reductions were observed in
food intake, body weight gain, and weight of gonads. No other
changes were noticed. No such effects were found at 20 mg/kg body
weight (Henderson & Parkinson, 1981).
8.2.3. Intraveneous
8.2.3.1. Rat
Lock & Berry (1981) found biochemical changes in the rat
cerebellum after a single iv administration of 25 mg
cypermethrin/kg body weight. Signs of toxicity included:
salivation, clonic convulsions, and sinuous writhing movements.
The concentrations of cerebellar amino acids and cyclic nucleotides
were determined at these stages of toxicity. Treatment with
cypermethrin resulted in an increase in cerebellar cyclic GMP at
the earliest stage, without changing cyclic AMP. Levels of blood-
and cerebellar-glucose, lactate, and ammonia were also increased in
most of the stages of toxicity. No changes were seen in
concentrations of glutamate, glutamine, gamma-aminobutyrate,
creatine, phosphate, or ATP in the cerebellum. The increase in
cerebellar cyclic GMP did not appear to be related to the
convulsive state of the animal or to the muscarinic cholinergic
stimulation.
8.3. Long-Term Exposures
8.3.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) were fed dietary concentrations of 0, 1, 10, 100, or
1000 mg cypermethrin/kg diet for 2 years. Additional groups (6 or
12 rats of each sex per dose level) were fed the diets for only 6,
12, or 18 months.
Throughout the 2-year study, the control and cypermethrin-
treated animals were similar in terms of behaviour; no compound-
related clinical signs were observed, except for a significantly-
reduced growth rate in both males and females fed 1000 mg/kg diet.
Survival was not affected by cypermethrin. No clinical-chemical,
haematological, or histopathological changes were observed. The
sciatic nerves from a number of animals of all groups, sacrificed
after 6, 12, 18, or 24 months, showed a small number of nerve
fibres exhibiting Wallerian degeneration. The incidence increased
with age, but there was no difference in severity between the
control and treated groups. It is concluded that doses of 100 mg
cypermethrin/kg diet or less did not produce significant
toxicological effects in rats over a 2-year period (McAusland et
al., 1978).
Assays of liver microsomal enzyme activity in 6 rats of each
sex fed 0 or 1000 mg cypermethrin/kg feed for 2 years showed
cypermethrin to be a weak inducer of the microsomal enzyme hepatic
p-nitroanisole- O-dimethylase (PNOD), used as an index of
monooxygenase activity (Potter & McAusland, 1980).
Five groups of Wistar rats, each composed of 52 males and 52
females, were given diets containing 0 (2 groups), 20, 150, or 1500
mg/kg diet (equivalent to 0, 1, 7.5, and 75 mg/kg body weight) for
2 years. Satellite groups of 12 male and 12 female rats were
sacrificed at 52 weeks. For the first 6 weeks of the study, the
rats in the 1500 mg/kg group received 1000 mg/kg. The purity of
the cypermethrin was between 88% and 93% ( cis-: trans-ratio;
55:45). After 104 weeks at the highest dose level, body weight
loss, increased liver weight, increased smooth endoplasmatic
reticulum in hepatocytes, and some haematological and other slight
clinical changes were observed. No other changes or increase in
the incidence of tumours were found. The dose of 150 mg/kg diet
was considered to be the no-observed-adverse-effect level in this
study (only a summary is given) (US-EPA, 1984).
8.3.2. Mouse
The effects of cypermethrin were investigated in groups of 70
male and 70 female Swiss mice fed diets containing 0 (2 groups),
100, 400, or 1600 mg/kg feed (equivalent to 0, 15, 60, or 240 mg/kg
body weight) for up to 101 weeks. The purity of the cypermethrin
was between 91.5 and 94.2%; cis-: trans-ratio; 53:47 or 54:46.
Ten males and 10 females per group were killed at 52 weeks for
interim haematological evaluation. Appearance, behaviour, and
survival were similar in all groups. Body weight gain was reduced
in mice fed 1600 mg cypermethrin/kg diet compared with the combined
control groups. Food intake of both males and females was not
significantly changed. Several haematological changes, consistent
with a mild anaemia, were found in the 1600 mg/kg group of animals
at the interim kill (decreased haemoglobin, haematocrit, and red
blood cell counts in males; decreased mean cell volume and mean
cell haemoglobin concentration in females) but were not found at
termination. Thrombocytosis and increased liver weight were also
observed in this group, at both the interim and terminal kills.
There were no accompanying histopathological changes (for
carcinogenic potential, see section 8.7). No effects were observed
at dose levels of 400 mg/kg diet or less (Lindsay et al., 1982).
8.3.3. Dog
Cypermethrin (dissolved in corn oil) was administered to 4
groups of 8 beagle dogs of each sex at dose levels of 0, 1, 5, or
15 mg/kg body weight per day, by capsule, for a period of 52 weeks.
The purity of the cypermethrin was 90.6% ( cis-: trans-ratio;
54:46). The dogs in the highest dose group exhibited loss of
appetite, tremors, gait changes, incoordination, disorientation,
and hypersensitivity. No other abnormalities were found in the
composition of the blood or urine or in organ weights. Dogs
receiving 5 mg/kg or more showed liquid stools by this mode of
administration. However, this effect was not found when
cypermethrin was fed in the diet for 2 years. No effects were seen
at 1 mg/kg (but only a summary is given) (US-EPA, 1984).
Groups of 4 male and 4 female Beagle hounds were fed diets
containing 0, 3, 30, or 300 mg cypermethrin/kg feed for 2 years.
An additional group received 1000 mg/kg feed but, because of severe
intoxication signs, the dose was decreased to 750 mg/kg. The signs
of intoxication consisted of licking and chewing of the paws, a
stiff high-stepping gait, whole body tremors, head shaking,
incoordination, ataxia, and convulsions. After 3 weeks of feeding
at 750 mg/kg, the animals received the control diet, until signs of
intoxication could no longer be seen. The dogs were then fed a
diet containing 600 mg cypermethrin/kg from week 8 until
termination at 2 years. No signs of intoxication were observed in
dogs fed diets containing 0, 3, 30, or 300 mg/kg during the course
of the study. There was reduced body weight gain in male dogs in
the 600 mg/kg group. Clinical chemistry and haematological
investigations, performed at 6-week intervals for 2 years, did not
reveal any consistent differences between treated groups and
controls. The minor changes in absolute organ weight observed in
the brain and thyroid in the 300 mg/kg group were not present when
the differences were corrected for terminal body weight.
Furthermore, a dose relationship was not apparent, and there were
no accompanying histopathological changes. Opthalmological
observations performed during the course of the study did not
reveal any ocular differences between treated groups and control.
No abnormalities were found in the sciatic nerves, brain, or spinal
cord in any of the treated groups. The feeding of diets containing
up to 600 mg cypermethrin/kg diet to dogs for 2 years did not
reveal any treatment-related gross or histopathological effects,
although the dogs receiving diets containing 1000 mg/kg decreased
to 600 mg cypermethrin/kg showed reduced body weight gain.
Cypermethrin did not produce any toxicological effects in dogs fed
dietary concentrations of 300 mg/kg feed or less, over 2 years
(Buckwell, 1981).
8.4. Special Studies
8.4.1. Synergism/potentiation studies
8.4.1.1. Organophosphate mixture
In a study on rats, designed to determine whether an
organophosphate insecticide, monocrotophos, potentiated the
neurotoxic effect of cypermethrin, 5 groups of 7 or 8 male and 7 or
8 female rats were given 7 consecutive oral doses of monocrotophos
(100 g/litre), cypermethrin (25g/litre), or a mixture of
monocrotophos and cypermethrin (100 g/litre and 25 g/litre,
respectively). Signs of intoxication and neurotoxic effects
(biochemical changes consistent with very sparse axonal
degeneration in sciatic/tibial nerves) were found to be wholly
attributable to the monocrotophos component of the mixture, and
there was no evidence of potentiation of neurotoxic effects (Rose &
Dewar, 1979a).
8.4.1.2. Organochlorine mixture
The oral and dermal toxicities of cypermethrin and endosulfan
for rats were determined both individually and combined. No
evidence for enhancement of toxicity was found for either
cypermethrin or endosulfan when administered as a 1:1 mixture in
corn oil (Price, 1981b).
8.4.2. Neurotoxicity
8.4.2.1. Characterization of the neurotoxic effects
During preliminary short-term studies on cypermethrin, 2
observations were made:
(a) high doses of cypermethrin given orally to rats
caused an unusual gait in intoxicated animals; and
(b) at lethal or near-lethal dermal or oral doses,
histopathological changes (swelling and/or
disintegration of axons of the sciatic nerve of
rats) were observed; such observations have been
reported to occur with other synthetic pyrethroids
(Okuno et al., 1976a) and natural pyrethrins (Okuno
et al., 1976b).
As a consequence of these findings, an extensive programme of
work has been undertaken to characterize the neurotoxic effects of
cypermethrin.
8.4.2.2. Neuropathological studies
(a) Rat
Single oral doses of cypermethrin in corn oil were given to
groups of 6 - 12 rats of each sex at 100, 200, or 400 mg/kg body
weight. All rats showed signs of intoxication, which varied in
severity according to the dose level. At 400 mg/kg, severe
clinical signs were seen within 2 days of dosing. Most of the
animals showed swelling of the myelin sheaths and breaks of some of
the axons of the sciatic nerves. At 200 mg/kg, 8 rats of each sex
died or were killed within 48 h of dosing, and the remaining 4 of
each sex survived the 9 days of the study. Nearly half of the
animals showed lesions of the sciatic nerve. At 100 mg/kg, all
rats survived 9 days of the trial. Only one female out of 12
showed minimal lesions of the sciatic nerve. Neuropathological
changes were found in a number of animals dosed with 200 mg
cypermethrin/kg body weight or more. Dose-related increases in
signs of intoxication and neuropathy were observed; however,
neuropathy was not detected in all the animals showing signs of
intoxication (Carter & Butterworth, 1976).
Groups of rats (10 males per group) were fed dietary levels of
cypermethrin ( cis-: trans- = 45:55) at 0, 1250, 2500, or 5000
mg/kg diet for 14 days. Mortality was observed at the 2 higher
doses and growth inhibition was seen in all treated groups.
Clinical signs of neurotoxicity were characterized by an impaired
ability to walk, splayed hind limbs, ataxia, and paralysis. Other
clinical signs were hypersensitivity, gross disorientation, and
convulsions. The neurotoxic signs of poisoning observed at 1250
mg/kg diet were reversible. Remission of ataxia at 2500 mg/kg diet
was also noted. Ultrastructural changes in the sciatic nerve were
observed in a small number of animals at the 2 highest dose levels.
Some evidence of axonal damage in the myelinated nerves was
observed, mainly in these groups (Glaister et al., 1977a).
In another study, rats were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg diet for 2 years (section 8.3).
At the 12-month interim kill, part of the sciatic nerve was removed
from 6 males and 6 females in both the control and 1000 mg/kg
group. The dissected nerves were divided and teased into a fan
shape, allowing each fibre to be examined. No significant
difference was found in the incidence of abnormal nerve fibres in
the control and the 1000 mg/kg group (Trigg et al., 1977; McAusland
et al., 1978).
(b) Hamster
Groups of 6 male and 6 female Syrian hamsters were given single
oral doses of cypermethrin in corn oil at 0, 794, 1000, and 1260
mg/kg body weight. Signs of intoxication (generally tiptoe
walking, tremors, irregular movements) occurred in all groups
receiving cypermethrin. Animals died or were killed at times
varying from a few hours to 9 days after dosing. Of the 31 animals
of the different test groups that were examined, 3 had nerve
lesions, axonal swelling, and axonal breaks in the sciatic and
posterior tibial nerves (many animals died within a few hours)
(Butterworth & Clark, 1977).
(c) Chicken
In a delayed neurotoxicity study on 6 adult domestic hens,
cypermethrin in DMSO did not cause histological lesions in the
nervous system (brain, spinal cord, and sciatic nerves) or signs of
intoxication at an oral dose of 1000 mg/kg body weight per day for
5 days, compared with a positive control group. No delayed
neurotoxicity was observed (Owen & Butterworth, 1977).
8.4.2.3. Biochemical and electrophysiological studies
Biochemical and electrophysiological studies have been carried
out because the sparse axonal degeneration described above is
difficult to demonstrate by conventional histopathological
techniques. Furthermore, evidence was required to quantify the
neurological dysfunction attributable to cypermethrin. The
rationale for measuring the activities of beta-glucuronidase and
beta-galactosidase in conjunction with an assessment of "mean slip
angle" and "mean landing foot spread" as markers for axonal
degeneration has been described by Dewar (1977a,b).
(a) Rat
Using biochemical markers, it has been shown that, at high oral
doses of cypermethrin, sparse axonal degeneration occurs in the
sciatic/posterior tibial nerves (Dewar, 1977b; Rose & Dewar, 1983),
and the trigeminal nerve and trigeminal ganglion of Wistar rats
(Dewar & Moffett, 1978a).
Three studies involving repeated oral administration of
cypermethrin to rats at 150 mg/kg body weight for 5 or 7 days and
0, 25, 50, 100, or 200 mg/kg body weight for 5 or 7 days have been
carried out. Mortality occurred from 100 mg/kg body weight
onwards. A dose-related transient functional impairment, assessed
by means of the inclined plane test, was found in the first week.
Significant increases in beta-glucuronidase or beta-galactosidase
activity of the sciatic, tibial, or trigeminal nerves only occurred
with 5 or 7 doses of 150 or 200 mg/kg body weight. The sensitivity
of these nerves is comparable. Complete functional recovery
occurred within 26 days. Increased activity of the enzymes in the
distal portion of nerves was found but, even in the most severely
intoxicated animals, the magnitude of the increases was less than
that induced by the known neurotoxic agent methylmercury chloride
(Dewar, 1977b; Dewar & Moffett, 1978a; Rose & Dewar, 1983).
Biochemical changes, consistent with primary axonal
degeneration, were detected in rats aged 3, 6, or 12 weeks.
Cypermethrin was more acutely toxic for 3-week-old rats than for
12-week-old rats and the dose required to cause a sparse axonal
degeneration, as demonstrated by biochemical changes, was
proportionally smaller for the younger rats (Rose & Dewar, 1978).
The time-course for development and recovery from axonopathy
was investigated in groups of rats (5 of each sex) at periods of
2 - 12 weeks after the start of dosing. The daily doses were 150
mg/kg body weight for the first 11 doses and 100 mg/kg body weight
for the subsequent 9 doses, over a period of 4 weeks. More than
half of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication, characterized by abnormal
gait, ataxia, salivation, lethargy, chromodacryorrhoea,
piloerection, and hypersensitivity to external stimuli. Maximum
enzyme activities in the sciatic posterior tibial nerves were found
after 5 weeks and had returned to control values by 12 weeks. In a
second phase of the study, 10 male and 10 female rats were given
20 oral doses of cypermethrin at 37.5, 75, or 150 mg/kg body weight
per day, (concurrent control group dosed with DMSO), over a 4-week
period. The animals given 75 and 150 mg/kg showed clinical signs
of intoxication. Mortality also occurred at the highest dose
level. The behaviour and appearance of animals in the 37.5 mg/kg
body weight per day group were similar to those of the controls.
Five weeks after the initial dose, biochemical changes, which were
indicative of a mild axonal degeneration, were observed in animals
in the highest dose group. No consistent or biologically
significant neurobiochemical changes were found in the 37.5 or 75
mg/kg body weight groups. The results of this study demonstrated
that 20 oral doses of cypermethrin at 75 mg/kg body weight per day
administered over a 4-week period, produced mild clinical signs of
intoxication; however, no biochemical changes which were indicative
of peripheral neuropathy were seen (Rose, 1983).
Further evidence to support the minor nature of the nerve
lesions has been afforded by electrophysiological studies on rats.
In these studies, measurements of the maximal motor conduction
velocities and conduction velocity of slower fibres in the sciatic
and tail nerves of Wistar rats were made before, and at intervals
of up to 5 weeks after, exposure to single (200 mg/kg body weight)
or repeated doses (7 doses of 150 mg/kg body weight) of
cypermethrin. Even at near-lethal doses, cypermethrin did not
cause any effects on these parameters (Dewar & Deacon, 1977).
The ability of the major metabolite of cypermethrin, PBA, to
produce axonal changes has been investigated. Four groups of 8
male and 8 female rats given 7 consecutive daily oral doses of 0,
25, 77, or 375 mg/kg body weight did not show any biochemical
changes indicative of peripheral nerve damage. The sparse axonal
degeneration observed with high doses of cypermethrin is,
therefore, not caused by PBA (Rose & Dewar, 1979b).
(b) Hamster
In 3 studies, Chinese hamsters were given oral doses of
5 - 30 mg cypermethrin/kg body weight per day, for 5 consecutive
days. Clinical signs of intoxication were observed and tests
made to assess whether the compound produced degenerative changes
in peripheral nerves and sensory ganglia. Beta-glucuronidase,
and beta-galactosidase activities were measured in the
sciatic/posterior tibial nerves and trigeminal ganglion. A
positive control group received 7.5 mg methylmercury chloride/kg
body weight per day, for 5 consecutive days.
The most striking feature in the hamsters treated with doses
exceeding 5 times 20 mg/kg body weight was a marked loss of fur in
the facial area, neck, and back, due to repeated scratching. The
results of the enzyme determinations suggest that the Chinese
hamster like the rat developed sparse axonal degeneration following
oral doses in excess of 5 times 10 mg/kg body weight (Dewar &
Moffet, 1978b)
(c) Rabbit
Rabbits were administered capsules with 0 (corn oil), 75,
150, or 300 mg cypermethrin (93.5%)/kg body weight in corn oil,
5 times a week, for 6 weeks. At the end of the 6th week,
electroencephalographic records were taken. The waves of the
complex EEG activity as well as the performance of the 6
constituting bands of different frequency were computer analysed.
No significant alterations were found (Desi et al., 1986a).
8.4.2.4. Appraisal
Repeated oral administration of cypermethrin to rats, at doses
sufficiently high to produce significant mortality in a group of
animals, produced biochemical changes in peripheral nerves that
were consistent with sparse axonal degeneration. The magnitude of
the biochemical changes was similar to that of changes produced
with either a single (Dewar, 1977b) or repeated doses of shorter
duration (Dewar & Moffet, 1978a), thus demonstrating a lack of
cumulative effect. The magnitude of change was substantially less
than that encountered with established neurotoxic agents and the
site of maximal enzyme change (distal portion of the nerve)
suggests that the degeneration is more likely to be of a Wallerian
type rather than segmental demyelination. The short time required
to produce ataxia and/or abnormal gait rules out a causal
relationship between ataxia and axonopathy. The clinical signs are
consistent with a pharmacologically-mediated effect whereas the
axonopathy is a minor reversible lesion that occurs several days
after exposure and has also been detected in animals that do not
show clinical signs.
Histopathological lesions of some fibres of the sciatic nerve
have only been demonstrated in rats or hamsters receiving extremely
high, lethal or near lethal, oral doses of cypermethrin (Carter &
Butterworth, 1976; Butterworth & Clark, 1977). Compound related
histopathological changes have not been observed in rats exposed to
cypermethrin through inhalation (Blair et al., 1976) or in rats fed
diets containing 1000 mg cypermethrin/kg over a 1 - 2 year period
(Trigg et al., 1977; McAusland et al., 1978). Furthermore, dogs
fed 1500 or 600 mg cypermethrin/kg diet for 3 or 24 months,
respectively, did not have any compound-related lesions of the
sciatic nerve (Buckwell & Butterworth, 1977; Buckwell, 1981).
The results of biochemical studies, used as sensitive
indicators of change, confirmed that minor changes that occur after
massive exposure to cypermethrin are reversible and are not
necessarily associated with clinical signs (Dewar, 1977b; Dewar &
Moffet, 1978a; Rose, 1983). There was no direct correlation
between the time course of the neuromuscular dysfunction and the
neurobiochemical changes (Rose & Dewar, 1983). The metabolite of
cypermethrin, PBA, did not produce any biochemical changes in
peripheral nerves (Rose & Dewar, 1979b).
8.4.3. Immunosuppressive action
Stelzer & Gordon (1984) studied the in vitro effects of
pyrethroids on the mitogenic responsiveness of murine splenic
lymphocytes to concornavalin A and lipopolysaccharide. The results
of these studies showed that at concentrations of the order of 1 -
10 µmol/litre, inhibition of murine splenic lymphocytes to both B
and T cell mitogens was found. These results support the
possibility of immune suppression by pyrethroid (cypermethrin)
exposure.
Desi et al., (1985, 1986a) studied the short-term effects of
6.25, 12.5 or 25 mg cypermethrin (93.5%)/kg body weight in rats, on
the immune system by measuring the autologous rosette formation of
T-lymphocytes and determining the ovalbumin titre. These studies
were performed over a period of 6 or 12 weeks.
The humoral immune response after vaccination with Salmonella
typhimurium and the cell-mediated immune response were determined
in rabbits. The antibody titre was measured by tube-agglutination
and complement-binding tests and the tuberculin skin test was used
for the immune response test. The dose levels in this study were
75, 150, or 300 mg/kg body weight, for 7 weeks. A significant
dose-dependent decrease was observed in both the anti-ovalbumin
titre of the blood-serum and in the autologous rosette formation of
the T-lymphocytes. In rabbits, the 2 highest dose levels induced a
significant dose-dependent decrease in serum antibody titres and
the tuberculin skin test showed the same dose-dependent tendency.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
Cypermethrin was fed to Wistar rats (30 male and 30 female per
group) at dietary concentrations of 0, 10, 100, or 500 mg/kg for 5
weeks, after which the males and females (10 weeks of age) from
each treatment group were mated. Two successive litters were
produced from each pair. The first of these litters was discarded,
and randomly-selected male and female pups of the second litters
were mated to produce the next generation. The study was continued
until 2 litters from each of 3 successive generations had been
bred. The parent animals in the 500 mg/kg group consumed less food
than the controls and this was accompanied by a reduction in body
weight. Otherwise, the parent animals of control and treatment
groups behaved similarly. Cypermethrin did not cause any adverse
effects on the reproductive performance of the rats or on the
survival of the offspring. No consistent changes were observed in
mean litter weight between birth and weaning in any treatment
group, with the exception of a reduction in total litter weights in
the 500 mg/kg F1a litters on days 4, 14, and 21. There was also a
statistically-significant decrease compared with controls in total
litter weights and size in the F1b litters at 500 mg
cypermethrin/kg diet. In the 500 mg/kg Fo group, one animal showed
a squamous cell carcinoma of the skin. However, this was
considered not to be related to the compound, because no increase
in tumour incidence was found in the long-term studies on rats and
mice. No changes were observed in rats administered 100 mg/kg diet
(Hend et al., 1978).
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1. Rat
Groups of pregnant female Sprague Dawley CD rats (25 animals
per group) were administered cypermethrin orally as a 1% solution
in corn oil at doses of 0, 17.5, 35, or 70 mg/kg body weight per
day, from days 6 to 15 (inclusive) of gestation. Cypermethrin at
17.5 mg/kg body weight per day did not affect maternal performance
or fetal survival and development. At the higher doses of 35 and
70 mg/kg body weight, respectively, slight and significant
retardation of maternal body weight gain was recorded. In
addition, at 70 mg/kg per day, slight to severe neurological
disturbances were observed in nearly half of the females including:
slight splaying of the hind legs while walking ranging to severe
splaying of all limbs, involuntary movements of the jaws,
convulsive spasms, and hypersensitivity to noise. Despite this
maternal toxicity, there were no indications of any embryotoxic or
teratogenic effects of cypermethrin (Tesh et al., 1978).
8.5.2.2. Rabbit
Groups of pregnant female Banded Dutch rabbits (30 controls and
20 for each dose group) were dosed orally with 0, 3, 10, or 30 mg
cypermethrin/kg body weight per day in corn oil (by gelatin
capsule) during days 6 - 18 (inclusive) of gestation. No influence
was found on growth, pre-implantation losses, resorptions, fetal
deaths, or numbers and sizes of fetuses. The incidence of fetal
visceral and/or skeletal abnormalities was comparable to that in
the vehicle control group, except for a slight increase in the mean
percentages of fetuses showing visceral and/or skeletal
abnormalities in the group receiving 30 mg/kg body weight per day.
No teratogenic effects were found in this study (Dix, 1978).
8.6. Mutagenicity and Related End-Points
8.6.1. In vitro studies
8.6.1.1. Microorganisms
Using bacterial assays, no increases in the reversion rates of
Escherichia coli WP2, E. coli WP2 uvrA, Salmonella typhimurium
TA 1535, TA 1537, TA 1538, TA 98, and TA 100 were detected with
cypermethrin (concentrations of up to 2 mg per plate) in the
presence or absence of a rat liver microsomal activation system.
Exposing Saccharomyces cerevisiae JD1 in liquid culture to
cypermethrin at concentrations of up to 5 mg/ml, both in the
presence and absence of a rat liver microsomal activation system,
did not result in any increase in the rate of mitotic gene
conversion (Brooks, 1980).
In a study in which cypermethrin was tested for mutagenicity in
Salmonella typhimurium TA 100 or TA 98, in the presence or absence
of a rat liver activation system, using the plate incorporation
assay, concentrations of up to 1 mg/plate gave negative results.
The same was true with the fluctuation test, with concentrations of
up to 10 µg/ml (Pluymen et al., 1984).
8.6.1.2. Mammalian cells
Cypermethrin was tested for mutagenicity in V79 Chinese hamster
cells. No cytotoxicity was observed when the assay was carried out
in the presence of rat hepatocytes. Cypermethrin was found not to
be mutagenic for either genetic locus (OUA' and TG') in V79 cells,
when tested in concentrations up to 20 µg/ml, in the presence or
absence of rat hepatocytes (Pluymen et al., 1984).
8.6.2. In vivo studies
8.6.2.1. Host-mediated assay
There was no increase in the rate of mitotic gene
conversion in Saccharomyces cerevisiae JD1 harvested from male
mice following a single oral dose of cypermethrin at 25 or 50 mg/kg
body weight for 2 days (Brooks, 1980).
8.6.2.2. Dominant lethal assay
No evidence of dominant lethality was found when male CD1 mice
(3 groups of 12 animals and a control group with 36 animals) were
given single oral doses of 6.25, 12.5, or 25 mg cypermethrin/kg
body weight. Two other groups were given 5 consecutive daily oral
doses of 2.5 or 5 mg/kg body weight (controls received the vehicle
DMSO). A significant reduction in fetal implants during the second
week of mating was noted and a marginal increase in early fetal
deaths was observed at 5 mg/kg per day. This effect was not
confirmed in a second study using oral doses of 2.5, 5, 7.5, or
10 mg/kg body weight for 5 consecutive days, when no effects were
found on reproductive capability or the histopathology of the
testes and epididymis. The marginal differences found in the
5 mg/kg per day group in the second study were considered not to
be related to the compound.
In summary, cypermethrin did not show detectable dominant
lethality after administration of doses of up to 10 mg/kg body
weight per day, for 5 days, or as a single oral dose of 25 mg/kg
body weight (Dean et al., 1977).
8.6.2.3. Bone marrow chromosome study
Chinese hamsters (12 of each sex per group) were orally dosed
with 20 or 40 mg cypermethrin/kg body weight, in DMSO, for 2
successive days. The incidence of chromosome abnormalities in
bone marrow cells, 8 and 24 h after dosing, did not differ from
that in the DMSO control animals. The positive control group
(100 mg/kg cyclophosphamide) showed many chromosomal aberrations
(Dean, 1977).
The effects of cypermethrin on sister chromatid exchange were
studied in the bone marrow cells of 3-month-old mice. Cypermethrin
was injected subcutaneously at 0.75, 1.5, or 3 mg/kg body weight
or 2.5, 5.0, or 10 mg Ripcord/kg body weight. Both the technical
and the formulated products showed a dose-related increase in
sister chromatid exchanges in the dividing cells at all dose
levels. The highest doses of both cypermethrin and Ripcord
completely inhibited mitotic division (Seehy et al., 1983).
8.6.2.4. Micronucleus test
The induction of micronuclei was studied in mouse bone marrow.
Cypermethrin was administered by 3 routes: intraperitoneal, oral,
and dermal. Cypermethrin showed mutagenic potential after oral
administration of 900 mg/kg diet for 7 and 14 consecutive days. No
effects were seen after ip administration of a single injection of
60 or 180 mg/kg body weight or double and triple injections of 60
mg/kg body weight.
Up to 4 dermal treatments with 360 mg cypermethrin/kg body
weight resulted in a significant increase in the frequency of
polychromatic erythrocytes with micronuclei (Amer & Aboul-Ela,
1985).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) (section 8.3) were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg for up to 2 years in a combined
long-term/carcinogenicity study. No evidence for carcinogenicity
was found in this study (McAusland et al., 1978) (section 8.3.1).
No increase in tumour incidence was seen when Wistar rats were
given diets containing 0, 20, 150, or 1500 mg cypermethrin/kg diet
(equivalent to 0, 1, 7.5, or 75 mg/kg body weight) for 2 years.
The purity of the cypermethrin ( cis-: trans- ratio; 55:45) was
between 88% and 93% (section 8.3.1) (US EPA, 1984).
8.7.1.2. Mouse
Swiss mice (70 male and 70 female) were fed diets containing 0
(2 groups), 100, 400, or 1600 mg cypermethrin/kg feed for up to 101
weeks. Effects consisted of increased liver weights at 400 and
1600 mg/kg diet and decreased body weight, thrombocytosis, and mild
anaemia at 1600 mg/kg diet (section 8.3.2).
There were no compound-related changes in non-neoplastic
histopathology or increases in tumours of types that are not
commonly associated with the mouse strain used. The incidence of
tumours was similar in all groups with the exception of a slightly
increased incidence of benign alveolar lung tumours in the females
in the 1600 mg/kg group. However, the magnitude of this increased
incidence was insufficient when compared with concurrent and
historical control incidence, to warrant concern. There was no
evidence for a decreased latency of benign alveolar lung tumours in
the female mice receiving the highest dose, and this tumour type
was not accompanied by any increase in malignancy. There was also
no evidence of a carcinogenic response in the male mice in this
study. Furthermore, benign alveolar lung tumours are know to occur
in both sexes at a high and variable incidence in this strain of
mouse. Therefore, it is considered that the occurrence of benign
alveolar lung tumours in the female mice receiving the highest dose
was not related to treatment with cypermethrin. Feeding
cypermethrin at levels of up to 1600 mg/kg diet to mice for a life-
time did not produce any evidence of carcinogenicity (Lindsay et
al., 1982).
8.8. Mechanisms of Toxicity - Mode of Action
In the classification in the Appendix, cypermethrin is
classified among the Type II compounds, which cause toxic signs of
choreoathetosis with salivation (CS-syndrome) in the rat
(Verschoyle & Aldridge, 1980) and bursts of spikes in the cercal
motor nerve of the cockroach (Gammon et al., 1981). Pyrethroids
having alpha-cyano-3-phenoxy-benzyl alcohol, such as cypermethrin,
do not induce repetitive firing in the cercal sensory nerves of the
cockroach in vivo or in vitro, but cause different signs including
a pronounced convulsive phase (Gammon et al., 1981).
The intravenous toxicity of 1RS- cis-, and 1RS- trans-
cypermethrin for the rat has been examined. Both compounds cause
the CS-syndrome, which is characterized by initial pawing and
burrowing behaviour, followed within 2 - 5 min by profuse
salivation, coarse whole-body tremor, increased startle response
and abnormal locomotion involving the hind limbs. The coarse
tremor progresses into sinuous writhing (choreoathetosis) of the
whole body, which gradually becomes more violent and is enhanced by
sensory stimuli. Clonic seizures are occasionally observed as a
terminal event. No increase in core temperature occurs and, in
fact, it may fall. In the electroencephalogram (EEG) during the
CS-syndrome, poisoned rats showed generalized spike and wave
discharges, prior to choreoathetosis (Verschoyle & Aldridge, 1980).
The primary site of action resulting in the CS-syndrome has not yet
been decided.
It has been suggested (Van den Bercken, personal communication,
1981) that the facial skin sensations experienced by people who
handle cypermethrin or other pyrethroids (section 9.2.2) are
brought about by repetitive firing of sensory nerve terminals in
the skin. In the opinion of the author, this is a strictly local
effect that may occur as soon as the pyrethroid concentration on or
in the skin reaches a certain level and is not a sign of general
intoxication. It is considered by the author that the occurrence
of these skin sensations are a warning signal indicating that
exposure has occurred. No undue hazard is likely, provided the
pyrethroid does not reach the blood in any significant
concentration. In this connection, it was stressed that, although
cypermethrin is among the least toxic insecticides when
administered orally, it may become highly toxic, whenever it
reaches the nervous system in sufficient concentrations.
The question remains whether there is a causal relation between
the intense repetitive activity induced by alpha-cyano pyrethroids
(of which cypermethrin is an example) and the nerve damage that
occurs in experimental animals after prolonged exposure to nearly
lethal concentrations of these compounds. Because of the large
number of different chemicals, most of which do not cause
repetitive activity in the nervous system but are known to cause
serious nerve damage, such a correlation is hardly expected.
Furthermore, the extensive literature on DDT (which causes the same
type of repetitive activity in sense organs and in sensory nerve
terminals as the non-cyano pyrethroids) does not contain any
indication that this insecticide may cause axonal degeneration.
However, in theory, there is a possibility that repeated occurrence
of intense repetitive firing will eventually lead to dysfunction of
sensory nerve terminals and sense organs, and, finally, to
degeneration of sensory nerve fibres (Van den Bercken, personal
communication, 1981; Van den Bercken & Vijverberg, 1980a).
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity: poisoning incidents
One report of accidental intoxication has been described. A
family who ate food cooked in 10% cypermethrin developed nausea,
prolonged vomiting with colicky pain, tenesmus, and diarrhoea
within minutes of ingestion. One male adult had convulsions,
passed into coma, and died due to respiratory paralysis. The
symptoms were less severe in other members of the family (Poulos et
al., 1982). There is some doubt whether this was a cypermethrin
intoxication (Suthers, personal communication).
9.1.2. Controlled human studies
Flannigan & Tucker (1985) studied the differences in the extent
of paraesthesia induced by a number of pyrethroids. Field strength
formulated cypermethrin (0.13 mg/cm2) was applied (0.05 ml) to a
4 cm2 area of the earlobe of volunteers on 5 occasions. Distilled
water was applied to the opposite earlobe. Participant evaluation
after each application continued for 48 h and involved description
of the cutaneous sensations. Each participant was treated after
each application with one of the other pyrethroids. Cypermethrin
(as the other pyrethroids) induced sensation. The paraesthesia
developed with a latency period of approximately 30 min, peaked by
8 h and deteriorated as early as 24 h. Dl-alpha tocopheryl acetate
markedly inhibited the occurrence of the paraesthesia.
9.1.3. Epidemiological studies
No information is available.
9.2. Occupational Exposure
9.2.1. Acute toxicity: poisoning incidents
No information is available.
9.2.2. Effects of short- and long-term exposure
Laboratory workers and field operators handling natural and
synthetic pyrethroids, including cypermethrin, have noticed a
transient sensation (described as "tingling" or "burning"
sensations) of the skin in the periorbital area of the face and of
other sites after direct skin exposure. The skin sensations have
been interpreted (section 8.8) as being caused by spontaneous
repetitive firing of local sensory nerve fibres or nerve endings,
with thresholds that have been transiently lowered by the compound
(Wouters & Van den Bercken, 1978). There is a delay of about
30 min before onset of these symptoms following pyrethroid
exposure; the sensation generally lasts only a few hours and does
not persist for more than one day after exposure (Van den Bercken &
Vijverberg, 1980a, Le Quesne et al., 1980).
In a clinical and electrophysiological assessment of these skin
sensations (paraesthesia), 23 laboratory workers, who had been
exposed to synthetic pyrethroids during the preceding months, were
examined. Nineteen of the 23 workers had experienced at least one
or more episodes of facial sensations. Neurological signs were not
observed and electrophysiological studies of selected motor and
sensory nerves in the legs and arms of the 23 subjects showed no
abnormalities (Le Quesne et al., 1980).
Field studies have been performed in the Côte d'Ivoire in which
cypermethrin was sprayed on cotton using a hand-held, ultra-low-
volume (ULV) commercial spraying machine. This method of
application represented a situation of high potential exposure.
The first study, in which 17 workers were involved, concerned a
single exposure to cypermethrin (Prinsen & Van Sittert, 1978),
while a second study was conducted in which 7 workers took part in
7 consecutive spray sessions (Prinsen & Van Sittert, 1979). Skin
exposure to cypermethrin was assessed by quantitative measurement
of the compound deposited on aluminium foil pads on the body of the
sprayer during the spraying operation. The rate of dermal exposure
of the operators during spraying ranged from 1.5 to 46.1 mg/h.
Total absorption of cypermethrin in the body was monitored by
urinary analysis for 2,2-dichlorovinyl-3,3-dimethyl-cyclopropane-
1-carboxylic acid, a metabolite of cypermethrin. In most of the
samples, the excretion was below the limit of detection
(0.05 mg/litre), indicating a low level of absorption. It was
estimated that approximately 3% of the total dermal dose was
absorbed. General medical, extensive clinical blood chemistry, and
electrophysiological examinations were carried out of selected
motor and sensory peripheral nerves in the legs and arms, of the
trigeminal nerve, and of the facial nerve. No effects were found.
In another field study in India, 18 workers, including spraymen
(spraying an emulsifiable concentrate formulation by mist-blower or
knap-sack), mixers, and loaders, handled cypermethrin daily for 5
consecutive days. Medical examination, with special attention to
the sensory function of the peripheral nervous system, was carried
out before, during, and after spraying (Suthers & Marlow, 1981).
No compound-related adverse clinical effects of peripheral
neuropathy were noticed in any of the workers. The urinary
excretion of the cypermethrin metabolite (methyl ester of the
cyclopropane carboxylic acid moiety) increased from day 1 to 5 of
the study, but decreased 24 h after spraying had ceased. On the
fifth day, concentrations of up to 0.18 mg (average 0.1 mg) were
found in the 24-h urine of workers using the mist-blower.
Concentrations were lower in workers using the knap-sack sprayer.
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
A field study was carried out on Indian workers in the Satara
district in India prior to, during, and after spraying cotton with
the synthetic pyrethroid formulations, permethrin (Ambush) and
cypermethrin (Cymbush). The formulations were applied using a
mist-blower or knap-sack spray. Exposure of 7 spraymen and 2
loaders/mixers was monitored by measuring the 24-h urinary
excretion of 3-(4'-hydroxyphenoxy)-benzoic acid, and medical
assessments were carried out by 4 medical doctors. The sensory
function of the peripheral nervous system was also assessed. The
formulations were applied at the recommended rates; Ambush, 150 g
a.i./ha and Cymbush, 70 g a.i./ha. Average exposure was
approximately 8 h per day for 5 days. No compound-related adverse
clinical effects were noticed. The average urinary excretion of
3-(4'-hydroxy-phenoxy)-benzoic acid increased from day 1 to day 5,
but then decreased 24 h after spraying had ceased (Hart et al.,
1982).
In another study, two agricultural pilots and five
mixer/loaders were monitored for dermal exposure during aerial
"ultra-low-volume" (ULV) applications of cypermethrin in vegetable
oil to cotton. This study was conducted in Greenwood, Mississippi.
The mixer/loaders wore protective equipment. The actual dermal
exposure for pilots was 0.67 mg/8 h and for mixer/loaders 2.43
mg/8h. The exposure of pilots was predominantly of the hands,
whereas that of mixer/loaders was more uniform (hands were
protected). The urinary excretion of cypermethrin metabolites was
very low; between 4 and 22 µg cypermethrin equivalents/day. This
study demonstrates that exposure of pilots and mixer/loaders during
the aerial ULV applications of cypermethrin is minimal and that
skin absorption is very slight (Chester et al., 1986).
Desi et al. (1986b) carried out a biological monitoring and
health surveillance study on 11 workers spraying organophosphate
carbamate and pyrethroid pesticides in greenhouses during the whole
year in comparison with 10 control persons. During the work,
protective clothing and masks were worn before and after a regular
spraying period with pyrethroids (including cypermethrin).
Extensive medical examinations, such as urinalysis, haematology,
immunoglobulin levels, whole blood cholinesterase activity, serum-
gamma-glutamyltransferase activity, chromosome analysis and
electro-cardiography were performed over a period of 3 months. The
amount of cypermethrin in the blood was just at the limit of
detection. No health injuries or other significant changes in the
parameters studied were found.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereo-isomers, is a highly active insecticide effective
against a wide range of pests in many food and non-food
commodities.
It is stable to light and heat, it has a low vapour pressure
and is more stable in acidic than in alkaline media. Sensitive
analytical methods for the determination of residues in food and
the environment are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly
rapid and residues do not accumulate in the environment.
Photodegradation is unlikely to play an important role. The main
route of degradation is cleavage of the ester linkage to give 2
main degradation products containing the cyclopropane, and the
phenoxybenzyl moiety. The half-lives in the soil are determined by
many factors, but are in the range of 2 - 4 weeks. Cypermethrin is
strongly adsorbed by soil and downward leaching is negligible.
Because of its rather fast breakdown forming less toxic products,
and the low dose rates used in good agricultural practice, it is
unlikely that cypermethrin will attain significant levels in the
environment.
Bioaccummulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the
levels of cypermethrin residues in food commodities are generally
low. Total diet studies are not available, but from the available
residue information, it can be inferred that the oral intake by man
is well below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are
generally rather resistant to cypermethrin, while fish and other
aquatic invertebrates are very sensitive. Because of its strong
adsorption on soil, only low levels of cypermethrin may leak into
surface water. These may have transient effects, mainly on surface
breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to
be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect for bees.
Absorption and elimination of cypermethrin has been rapid in
the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation
and conjugation of the cyclopropane- and phenoxybenzyl moiety. The
highest levels are found in body fat, which is consistent with the
lipophilic nature of cypermethrin. The half-life in the fat of the
rat is about 12 - 19 days for the cis-isomer and 3 - 4 days for the
trans-isomer. Breakdown products in plants are bound as
glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg
body weight. Short-term and long-term toxicity studies on rats,
mice, and dogs have shown effects on growth, on the liver and
kidneys, and the nervous system, and on haematology. A no-
observed-adverse-effect level of 7.5 mg/kg body weight has been
adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at
70 mg/kg body weight or rabbits at 30 mg/kg body weight. It was
also shown not to have any effects on reproductive performance
during a 3-generation reproduction study in rats administered
100 mg/kg diet. In a variety of mutagenicity studies, cypermethrin
was shown to be mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational
studies available, it seems that the skin sensation seen in workers
handling cypermethrin, generally lasts only a few hours and does
not persist for more than one day after exposure. Other
neurological signs were not observed. These skin sensations can be
considered to be an early warning that exposure has occurred and
that work practice should be reviewed. Cypermethrin may cause eye
irritation and may be a sensitizer for certain persons.
10.2. Conclusions
It can be concluded that:
General population: When applied according to good
agricultural practice, exposure of the general population to
cypermethrin is negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices,
hygiene measures, and safety precautions, the use of cypermethrin
is unlikely to present a hazard to those occupationally exposed to
it. The occurrence of "facial sensations" is an indication of
exposure. Under these circumstances work practice should be
reviewed.
Environment: With recommended application rates it is
unlikely that cypermethrin or its degradation products will attain
levels of environmental significance. Notwithstanding its high
toxicity for fish and honey bees, this is only likely to cause a
problem in the case of spillage and overspraying.
11. RECOMMENDATIONS
- Cypermethrin should be included among the residues looked for
in surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human
beings should be conducted, in order to better understand this
phenomenon.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cypermethrin was discussed at meetings of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) during the period 1979-84
(FAO/WHO, 1980a,b; 1982a,b; 1983a,b; 1984; 1985a,b,c; Vettorazzi &
Van den Hurk, 1984). In 1981, the JMPR established an Acceptable
Daily Intake (ADI) for cypermethrin of 0 - 0.05 mg/kg body weight.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, classified cypermethrin as "irritant to
eyes and sensitizer of skin" in the list of "technical products
unlikely to present an acute hazard in normal use" (Plestina, 1984;
WHO, 1979; WHO, 1985). The same division published a data sheet on
cypermethrin, No. 84.58 (WHO/FAO, 1975-85).
REFERENCES
ABBASSY, M.A., ASHRY, M., ADAM, F., KHALIL, F., & ABOU-SHLOU,
M.A. (1984) Toxicological and histopathological studies on
the cotton bollworm. ( Pectinophera gossypiella Saund). Meded.
Fac. Landbouwwet. Rijksuniv. Gent, 49(3a): 691-698.
ABDEL-AAL, Y.A.I., EL-SAYED, A.M.K., NEGM, A.A., HUSSEIN,
M.H., & EL-SEBAE, A.H. (1979) The relative toxicity of
certain insecticides to Spodoptera littoralis (Boisd) and
Coccinella undecimpunctata L. Int. Pest Control, 21(4): 79-80,
82.
ABOU-AWAD, B.A. & EL-BANHAWY, E.M. (1985) Comparison between
the toxicity of synthetic pyrethroids and other compounds to
the predacious mite Amblyseius gossipi (Mesostigmata:
Phytoseiidae). Exp. appl. Acarol., 1: 185-191.
AHMED, Y.M., MOSTAFA, A.M.A., & ELEWA, M.A. (1985) Toxicity
of certain dyes as insecticides and their joint action with
some pyrethroids. J. environ. Sci. Health, B20(6): 689-699.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four
synthetic pyrethroids on a predatory mite, Typhlodromus pyri,
and its prey, Panonychus ulmi, on apples in Southeast England.
Environ. Entomol., 9: 436-439.
AMER, S.M. & ABOUL-ELA, E.I. (1985) Cytogenetic effects of
pesticides. III. Induction of micronuclei in mouse bone marrow
by the insecticides cypermethrin and rotenone. Mutat. Res.,
155(3): 135-142.
ASCHER, K.R.S., ELIYAHU, M., NEMNY, N.E., & ISHAAYA, I.
(1982) The toxicity of some novel pesticides - synthetic
pyrethroids and benzoylphenylurea chitin synthesis inhibitors
- for eggs of Spodoptera littoralis (Boisd). Z. angew.
Entomol., 94: 504-509.
AWASTHI, M.D. & ANAND, L. (1983) Dissipation and persistence
of synthetic pyrethroids on fruits of okra. J. entomol. Res.,
7(1): 55-59.
BADMIN, J.S. & TWYDELL, R.S. (1976) Evaluation of the
insecticide WL 43467 against the honey bee Apis mellifera,
Sittingbourne, Shell Research (WKSR.0021.76).
BAICU, T. (1982) Toxicity of some pesticides to Trichoderma
viride pers., Crop Prot., 1(3): 349-358.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues
of synthetic pyrethroids in fruit and vegetables by gas-liquid
and high-performance liquid chromatography. Analyst, 107:
206-212.
BALDWIN, M.K. (1977a) Residues of the pyrethroid insecticide
WL43467 in tissues of cattle following a dip-bath application,
Sittingbourne, Shell Research (TLGR.0.115.77).
BALDWIN, M.K. & LAD, D.D. (1978a) Residue data for milk
following application of WL 43467 in a barn, Sittingbourne,
Shell Research (TLGR.0042.78).
BALDWIN, M.K. & LAD, D.D. (1978b) The accumulation and
elimination of WL 43467 by the Rainbow trout (Salmo
gairdneri), Sittingbourne, Shell Research (TLGR.0041.78).
BALDWIN, M.K., BUCKWELL, A.C., & LAD, D.D. (1977) Residue
data following the application of WL 43467 for nuisance fly
control on cattle, Sittingbourne, Shell Research (TGLR.0.112.
77).
BARIOLA, L.A. & LINGREN, P.D. (1984) Comparative toxicities
of selected insecticides against pink bollworm (Lepidoptera:
Gelechiidae) moths. J. econ. Entomol., 77: 207-210.
BARLOW, F., HADAWAY, A.B., FLOWER, L.S., & TURNER, C.R.
(1986) Residual contact toxicity of some insecticides to tse
tse flies in laboratory tests, London, Centre for Overseas
Pest Research, pp. 12.
BATTELLE (1982) Pesticide programme of research and market
planning. I. Insecticides, Geneva, Institute Battelle (October
1982).
BAYOUMI, O.C. (1982) The susceptibility of Myzus persicae
Sulz. to some insecticides in artificial diets. Parasitica,
38(4): 193-199.
BENNETT, D. (1981a) The accumulation, distribution, and
elimination of RIPCORD by Rainbow trout using a continuous-
flow procedure, Sittingbourne, Shell Research (SBGR.81.026 and
Addendum).
BENNETT, D. (1981b) Residues of RIPCORD in Rainbow trout
(Salmo gairdneri) and common carp (Cyprinus carpio) exposed to
lethal concentrations in water, Sittingbourne, Shell Research
(SBGR.81.075).
BENNETT, D., CROSSLAND, N.O., & SHIRES, S.W. (1980) Spray
drift from RIPCORD applications to vineyards in France: fate
and effects in adjacent streams, Sittingbourne, Shell Research
(TLGR.80.095).
BLAIR, D. & RODERICK, H.R. (1976) Toxicity studies on the
pyrethroid insecticide WL 43467, emulsifiable concentrate FX
3315. Acute inhalation exposure of rats to an aqueous spray,
Sittingbourne, Shell Research (TLGR.0001.76).
BLAIR, D., BUTTERWORTH, S.T.G., & RODERICK, H.R. (1976)
Toxicity studies on the pyrethroid insecticide WL 43467,
emulsifiable concentrate FX 3315. Histopathology of rats
exposed to an aqueous spray, Sittingbourne, Shell Research
(TLGR.0102.76).
BLAND, P.D. (1985) Capillary gas chromatographic
determination of cypermethrin in formulations: collaborative
study. J. Assoc. Off. Anal. Chem., 68(3): 592-595.
BOSIO, P.G. (1979) Residues of Barricade (cypermethrin) in
cattle from Australia, Berre, France, Shell Chimie (BEGR.79.
117) (Unpublished Shell data).
BOSTANIAN, N.J. & BELANGER, A. (1985) The toxicity of three
pyrethroids to Amblyseius fallacis (Garman) Acari.
Phytoseiidae and their residues on apple foliage. Agric.
Ecosyst. Environ., 14(3/4): 243-250.
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC
multi-residue method to determination of synthetic pyrethroid
residues in celery and animal products. J. Assoc. Off. Anal.
Chem., 65(3): 685-689.
BRAUN, H.E., FRANK, R., & MILLER, L.A. (1985) Residues of
cypermethrin in milk from cows wearing impregnated ear tags.
Bull. environ. Contam. Toxicol., 35: 61-64.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1983)
Injection toxicity of some pyrethroids in the armyworm.
J. Pestic. Sci., 8: 95-98.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984a)
Topical and injection toxicities of some pyrethroids in the
tobacco cutworm, Spodoptera litteralis Fabricius. J. Pestic.
Sci., 9: 481-487.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984b)
Topical and injection toxicities of some pyrethroids to the
german cockroach, Blattella germanica (Dictyoptera:
Blattellidae). Appl. Entomol. Zool., 19(3): 348-355.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984c) The
selective toxicity of some synthetic pyrethroids in the
armyworm. Pseudaletia separata (Walker). III Cuticle
permeabilities of some pyrethroids. Appl. Entomol. Zool.,
19(1): 87-94.
BROMLEY, S. & COOK, K.A. (1979) Determination of the effects
of WL 43467 on microbial activity in soil. V. Effects on
glucose utilization, Sittingbourne, Shell Research
(BLGR.79.099).
BROOKS, T.M. (1980) Toxicity studies with agricultural
chemicals: mutagenicity studies with RIPCORD in microorganisms
in vitro and in the host-mediated assay, Sittingbourne, Shell
Research (TLGR.80.059).
BROWN, V.K. (1979a) Toxicology of WL 43467 isomers: acute
toxicity of WL 43481 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.117).
BROWN, V.K. (1979b) Toxicology of WL 43467 isomers: acute
toxicity of WL 42641 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.116).
BUCKWELL, A.C. (1981) A 2-year feeding study in dogs on WL
43467, Sittingbourne, Shell Research (SBGR.81.126).
BUCKWELL, A.C. & BUTTERWORTH, S.T.G. (1977) Toxicity studies
on the pyrethroid insecticide WL 43467: a 13-week feeding
study in dogs, Sittingbourne, Shell Research (TLGR.0127.77).
BUTTERWORTH, S.T.G. & CLARK, D.G. (1977) Toxicity studies on
the insecticide WL 43467: acute oral toxicity and neuro-
pathological effects in Syrian hamsters, Sittingbourne, Shell
Research (TLGR.0094.76).
CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN
GELDER, G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin
sensory stimulation characterized by a new behavioral
paradigm. Toxicol. appl. Pharmacol., 76: 270-279.
CAMILLERI, P. (1984) Alkaline hydrolysis of some pyrethroid
insecticides. J. agric. food Chem., 32: 1122-1124.
CARTER, B.I. & BUTTERWORTH, S.T.G. (1976) Toxicity of
insecticides: the acute oral toxicity and neuropathological
effects of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0055.76).
CASIDA, J.E., GAUGHAN, L.C., & RUZO, L.O. (1979) Comparative
metabolism of pyrethroids derived from 3-phenoxybenzyl and
alpha-cyano-3-phenoxybenzyl alcohols. Adv. Pestic. Sci., 2:
182-189.
CASSIDY, S.L. (1979) Acute oral toxicity of the spray
formulation EF 5288 to calves, piglets, and lambs,
Sittingbourne, Shell Research (TLGR.0001.79).
CHANG, C.K. & JORDAN, T.W. (1982) Penetration and metabolism
of topically applied permethrin and cypermethrin in
pyrethroid-tolerant Wiscana cervinata larvae. Pestic. Biochem.
Physiol., 17: 196-204.
CHANG, C.K. & JORDAN, T.W. (1983) Insecticide handling
mechanisms in some New Zealand pasture pests. N.Z. J. Sci.,
26: 509-516.
CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four
pyrethroid insecticides in a mineral and an organic soil.
J. environ. Sci. Health, B16(5): 605-615.
CHAPMAN, R.A. & SIMMONS, H.S. (1977) Gas-liquid chromato-
graphy of picogram quantities of pyrethroid insecticides.
J. Assoc. Off. Anal. Chem., 60: 977-978.
CHAPMAN, R.A., TU, C.M., HARRIS, C.R., & COLE, C. (1981)
Persistence of five pyrethroid insecticides in sterile and
natural, mineral and organic soil. Bull. environ. Contam.
Toxicol., 26: 513-519.
CHENG, H.H. (1984) Residual toxicity of six pyrethroid and
two organophosphorus insecticides on the soil surface against
dark-sided cutworm (Lepidoptera: Noctuidae) on tobacco in
Ontario. Can. Entomol., 116: 11-17.
CHENG, H.H. & HANLON, J.J. (1984) Residual toxicity of six
insecticides and a herbicide applied sequentially or in tank
mix combinations on tobacco seedlings against dark-sided
cutworm (Lepidoptera: Noctuidae). Tob. Int., 186(22): 39-42.
CHESTER, G., HATFIELD, L.D., HART, T.B., LEPPERT, B.C.,
SWAINE, H., & TUMMON, O.J. (1986) Worker exposure to, and
absorption of cypermethrin during aerial application of an
"ultra low volume" formulation to cotton, Fernhurst, Imperial
Chemical Industries, Plant Protection Division (Unpublished
report).
COATS, S.A., COATS, J.R., & ELLIS, C.R. (1979) Selective
toxicity of three synthetic pyrethroids to eight coccinellids,
a eulophid parasitoid and two pest chrysomelids. Environ.
Entomol., 8(4): 720-722.
CODEX ALIMENTARIUS COMMISSION (1986) Guide to Codex
recommendations concerning pesticide residues. Part 2: Maximum
limits for pesticide residues, third preliminary issue, Rome,
Food and Agriculture Organization of the United Nations
(CAC/PR 2-1986).
COLE, L.M., CASIDA, J.E., & RUZO, L.O. (1982) Comparative
degradation of the pyrethroids tralomethrin, tralocythrin,
deltamethrin and cypermethrin on cotton and bean foliage.
J. agric. food Chem., 30: 916-920.
COOK, K.A. (1978a) Determination of the effects of WL 43467
on microbial activity in soil. I. Effects on carbon dioxide
evolution, Sittingbourne, Shell Research (BLGR.0003.78).
COOK, K.A. (1978b) Determination of the effects of WL 43467
on microbial activity in soil. IV. Effects on nitrification,
Sittingbourne, Shell Research (BLGR.0015.78).
COOMBS, A.D., CARTER, B.I., HEND, R.W., BUTTERWORTH, S.G., &
BUCKWELL, A.C. (1976) Toxicity studies on the insecticide WL
43467: Summary of results of preliminary experiments,
Sittingbourne, Shell Research (TLGR.0104.76).
CORBITT, T.S., WRIGHT, D.J., & GREEN, A.St.J. (1985) The
toxicity of abamectin (MK. 936) on cabbage to first and third
larval instars of Spodoptera littoralis (Boisd.). Meded. Fac.
Landbourwwet. Rijksuniv. Gent, 50(2b): 639-642.
COVENEY, P.C. & EADSFORTH, C.V. (1982) The metabolism of
cypermethrin in man (3). Urinary excretion following a single
dermal dose of cypermethrin, Sittingbourne, Shell Research
(SBGR.82.290).
CRAWFORD, M.J. (1976a) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of (14 C-benzyl)WL
43481 (cis-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0046.76).
CRAWFORD, M.J. (1976b) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of 14 C-WL 42641
(trans-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0077.76).
CRAWFORD, M.J. (1977) The metabolism of WL 43467 in mammals.
The fate of a single oral dose of (14 C-cyclopropyl)WL 43467 in
the rat, Sittingbourne, Shell Research (TLGR.0004.77).
CRAWFORD, M.J. (1979a) The metabolism of cypermethrin (WL
43467) in mammals. The fate of a single oral dose of
(14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne, Shell
Research (TLGR.79.029).
CRAWFORD, M.J. (1979b) The metabolism of cypermethrin (WL
43467) in mammals. The fate of single oral doses of cis- and
trans-(14 C-benzyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.0011.79).
CRAWFORD, M.J. (1979c) The metabolism of 14 C-cypermethrin by
rat liver microsomes, Sittingbourne, Shell Research
(TLGR.79.057).
CRAWFORD, M.J. (1979d) The metabolic fate of the cis- and
trans-isomers of WL 43467 (cypermethrin) and of 3-phenoxy-
benzoic acid in the dog, Sittingbourne, Shell Research
(TLGR.79.012).
CRAWFORD, M.J. (1979e) The metabolism of cypermethrin (WL
43467) in mammals. Metabolites derived from a single oral dose
of (14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.79.096).
CRAWFORD, M.J. & HUTSON, D.H. (1977a) The metabolic fate of
the cis- and trans-isomers of WL 43467 (cypermethrin).
Metabolism and elimination of 14 C-aryl-labelled cis- and
trans-isomers in rats, Sittingbourne, Shell Research
(TLGR.0131.77).
CRAWFORD, M.J. & HUTSON, D.H. (1977b) The elimination and
retention of WL 43467 when administered dermally or orally to
sheep, Sittingbourne, Shell Research (TLGR.0098.77).
CRAWFORD, M.J. & HUTSON, D.H. (1978) The elimination of
residues from the fat of mice following the oral
administration of (14 C-benzyl)WL 43481 (cis-WL 43467),
Sittingbourne, Shell Research (TLGR.0080.78).
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981a)
Metabolism of cis- and trans-cypermethrin in rats. Balance and
tissue retention study. J. agric. food Chem., 29: 130-135.
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981b) The
metabolism of the pyrethroid insecticide cypermethrin in rats:
excreted metabolites. Pestic. Sci., 12: 399-411.
CRAYFORD, J.V. (1978) A study of the metabolism of
3-phenoxybenzoic acid and its glucoside conjugate in rats,
Sittingbourne, Shell Research (TLGR.0186.78).
CRAYFORD, J.V. & HUTSON, D.H. (1979) The identification of
metabolites in the tissues of rats treated orally with
3- phenoxybenzoic acid, Sittingbourne, Shell Research (TLGR.0043.
79).
CRAYFORD, J.V. & HUTSON, D.H. (1980) Xenobiotic triglyceride
formation. Xenobiotica, 10(5): 349-354.
CRAYFORD, J.V., HUTSON, D.H., & THORPE, E. (1980) The
elimination of residues from the fat of mice following the
oral administration of 14 C-benzyl WL 43481 (cis-WL 43467),
Tunstall, Shell Toxicology Laboratory (TLGR.0080.78,
additional report).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin.
II. Fate and biological effects in pond experiments. Aquat.
Toxicol., 2: 205-222.
CROSSLAND, N.O. & BENNETT, D. (1976) A field trial to assess
the dispersion and toxicity of an EC formulation of the
insecticide WL 43467 in a pond system, Sittingbourne, Shell
Research (TLGR.0101.76).
CROSSLAND, N.O. & ELGAR, K.E. (1983) Fate and biological
effects of insecticides in ponds. In: Miyamoto, J. et al., ed.
IUPAC. Pesticide chemistry: human welfare and the environment,
Oxford, Pergamon Press, Vol. 3, pp. 551-556.
CROSSLAND, N.O. & STEPHENSON, R.R. (1979) The role of pond
studies in assessing the hazard of toxic chemicals to
freshwater ecosystems. In: Proceedings of the British Crop
Protection Conference, 1979: Pests and Diseases, Croydon,
British Crop Protection Council, Vol. 2, pp. 453-459.
CROSSLAND, N.O., BENNETT, D., KANE, D.F., & STEPHENSON, R.R.
(1978) The dispersion and toxic effects of the insecticide WL
43467 in a pond, Sittingbourne, Shell Research (TLGR.0076.78).
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic
toxicology of cypermethrin. III. Fate and biological effects
of spray drift deposits in freshwater adjacent to agricultural
land. Aquat. Toxicol., 2: 253-270.
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982a) Hepatic
esterases: characterization and quantitation in vitro of rat,
rabbit, and human liver esterases. Part I, Sittingbourne,
Shell Research (SBGR.82.204).
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982b) In vitro
metabolism of the pyrethroid insecticide cypermethrin by liver
esterases. In: Proceedings of the 5th International Congress
on Pesticide Chemicals, Vol. 4.
CROUCHER, A., HUTSON, D.H., & STOYDIN, G. (1985) Excretion
and residues of the pyrethroid insecticide cypermethrin in
lactating cows. Pestic. Sci., 16: 287-301.
DAI, S.M. & SUN, C.N. (1984) Pyrethroid resistance and
synergism in Nilaparvata lugens Stal (Homoptera: Delphacidae)
in Taiwan. J. econ. Entomol., 77: 891-897.
DAY, S.R. & LEAHEY, J.P. (1980) 14 C-cypermethrin: aqueous
photodegradation in sunlight, Fernhurst, Imperial Chemical
Industries (Unpublished ICI Report No. RJ0154B).
DEAN, B.J. (1977) Toxicity studies with SL 43467: chromosome
studies on bone marrow cells of Chinese hamsters after two
daily oral doses of WL 43467, Sittingbourne, Shell Research
(TLGR.0136.77).
DEAN, B.J., PAUW, C.L, VAN DER, & BUTTERWORTH, S.T.B. (1977)
Toxicity studies with WL 43467: dominant lethal assay in male
mice after single oral doses of WL 43467, Sittingbourne, Shell
Research (TLGR.0042.77).
DELABIE, J., BOS, C., FONTA, C., & MASSON, C. (1985) Toxic
and repellent effects of cypermethrin on the honey bee:
laboratory, glasshouse and field experiments. Pestic. Sci.,
16: 409-415.
DESI, I., VARGA, L., DOBRONYI, I., & SZKLENARIK, G. (1985)
Immunotoxicological investigation of the effects of a
pesticide: cypermethrin. Arch. Toxicol., Suppl. 8: 305-309.
DESI, I., DOBRONYI, I., & VARGA, L. (1986a) Immuno-, neuro-
and general toxicologic animal studies on a synthetic
pyrethroid: cypermethrin. Ecotoxicol. environ. Saf., 12:
220-232.
DESI, I., PALOTAS, M., VETRO, G., CSOLLE, I., NEHEZ, M.,
ZIMANYI, M., FERKE, A., HUSZTA, E., & NAGYMAJTENYI, L.
(1986b) Biological monitoring and health surveillance of a
group of greenhouse pesticide sprayers. Toxicol. Lett., 33:
91-105.
DEWAR, A.J. (1977a) The use of lysosomal enzyme measurements
as an indicator of chemically-induced peripheral neuropathy,
Sittingbourne, Shell Research (TLGR.0074.77).
DEWAR, A.J. (1977b) Toxicity studies on the insecticide WL
43467: biochemical and functional studies on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research (TLGR.0082.
77).
DEWAR, A.J. & DEACON, P.A. (1977) Toxicity studies on the
insecticide WL 43467: electrophysiological studies on the
neurotoxicity of WL 43467 to rats. I. The effect on motor
conduction velocity in the sciatic and tail nerves,
Sittingbourne, Shell Research (TLGR.0133.77).
DEWAR, A.J. & MOFFETT, B.J. (1978a) Toxicity studies on the
insecticide WL 43467: biochemical studies on the effect of WL
43467 on the rat trigeminal nerve and ganglion, Sittingbourne,
Shell Research (TLGR.0162.77).
DEWAR, A.J. & MOFFETT, B.J. (1978b) Toxicity studies on the
insecticide WL 43467: biochemical and functional studies on
the neurotoxicity of WL 43467 to Chinese hamsters,
Sittingbourne, Shell Research (TLGR.0038.78).
DEWAR, A.J. & OWEN, D.E. (1979) Toxicology of pyrethroids:
the acute oral toxicity to rats of a sample of ICI
cypermethrin, Sittingbourne, Shell Research (TLGR.79.019).
DIX, K.M. (1978) Toxicity of WL 43467: teratological studies
in rabbits given WL 43467 orally, Sittingbourne, Shell
Research (TLGR.0010.78).
DU TOIT, G.D.G. (1978) Evaluation by topical application of
selected insecticides against adult grass grub. In: Proceeding
of the 31st New Zealand Weed and Pest Control Conference, pp.
160-163.
EADSFORTH, C.V. & BALDWIN, M.K. (1983) Human dose-excretion
studies with the pyrethroid insecticide cypermethrin.
Xenobiotica, 13: 67-72.
EDWARDS, P.J., WILKINSON, W., & COULSON, M. (1985)
Laboratory toxicity test for carabid beetles. Proceedings of
the British Crop Protection Conference, 1984: Pests and
Diseases, Croydon, British Crop Protection Council, Vol. 1,
pp. 359-362.
EDWARDS, R. & MILLBURN, P. (1985a) Toxicity and metabolism
of cypermethrin in fish compared with other vertebrates.
Pestic. Sci., 16: 201-202.
EDWARDS, R. & MILLBURN, P. (1985b) The metabolism and
toxicity of insecticides in fish. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry and toxicology,
New York, John Wiley and Sons, Vol. 5, pp. 249-274.
ELLIOTT, M., FARNHAN, A.W., JONES, N.F., NEEDHAM, P.H., &
PULMAN, D.A. (1974) Synthetic insecticide with a new order
of activity. Nature (Lond.), 248: 710-711.
EL-MINSHAWY, A., MACKLAD, F., RAGAB, F., & DONIA, A. (1983)
Selective toxicity of certain pyrethroids to the cotton
leafworm Spodoptera littoralis (Boisd.) and to one of its
major parasites Microplitis rufiventris (Kok). Proceedings of
the International Conference on Environmental Hazards of
Agrochemicals, Alexandria, Egypt, 8-12 November, 1983, London,
Paris, New York, Harwood Academic Publishers, Vol. 1, pp. 551-
561.
EL-SAYED, G.N. & KNOWLES, C.O. (1984) Formamidine synergism
of pyrethroid toxicity to two-spotted spider mites (Acari,
Tetranychidae). J. econ. Entomol., 77: 23-30.
EL-SEBAE, A.H., EL-BAKARY, A.S., LE PATOUREL, J., KADOUS, E.,
& MACKLAD, M.F. (1983) Effect of photoperiodism on fish
susceptibility to insecticides. In: Zewail, A.H., ed.
Proceedings of the International Conference on Photochemistry
and Photobiology, Alexandria, Egypt, 8-12 November, 1983,
London, Paris, New York, Harwood Academic Publishers, pp. 960-
966.
EL-SEBAE, A.H., ENAN, E.E., DAOUD, A.S., & ZEID, M.I. (1985)
Selective toxicity of synthetic pyrethroids and some
synergists to mice and cotton leafworm in relation to some
biochemical enzyme activities. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 50(3a): 939-950.
ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1982) Dislodgable
insecticide residues on cotton foliage: carbaryl, cyper-
methrin, and methamidophos. Bull. environ. Contam. Toxicol.,
28: 490-493.
EUROPEAN PATENT OFFICE (1980) European patent application
(Publication No. 0015598(A2)).
EVANS, M.H. (1976) End-plate potentials in frog muscle
exposed to a synthetic pyrethroid. Pestic. Biochem. Physiol.,
6: 547-550.
EWEN, A.B., MUKERJI, M.K., & HINKS, C.F. (1984) Effect of
temperature on the toxicity of cypermethrin to nymphs of the
migratory grasshopper. Melanoplus sanguinipes (Orthoptera:
Acridoidea). Can. Entomol., 116(9): 1153-1156.
FABELLAR, L.T. & HEINRICHS, E.A. (1984) Toxicity of
insecticides to predators of rice brown planthoppers,
Nilaparvata lugens (Stal) (Homoptera: Delphacidae) Environ.
Entomol., 13: 832-837.
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20;
Suppl).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 56).
FAO/WHO (1985a) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985c) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Protection and Protection Paper No. 67).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
FRANK, R., BRAUN, H.E., RITCEY, G., MCEWEN, F.L., & SIRONS,
G.J. (1982) Pesticide residues in onions and carrots grown
on organic soils, Ontario, 1975-80. J. econ. Entomol., 75:
560-565.
FURUZAWA, K., MIKAMI, N., YAMADA, H., & MIYAMOTO, J. (1986)
Metabolism of the pyrethroid insecticide cypermethrin in
cabbages. J. Pestic. Sci., 11: 253-260.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes
of pyrethroid action in the cockroach. Pestic. Biochem.
Physiol., 15: 181-191.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most
potent class antagonize GABA action at the crayfish
neuromuscular junction. Neurosci. Letters, 40: 163-168.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982)
Pyrethroid toxicology: Protective effects of Diazepam and
phenobarbital in the mouse and the cockroach. Toxicol. applied
Pharmacol., 66:290-296.
GARFORTH, B.M. (1982) WL 85871 and cypermethrin: chronic
toxicity to Daphnia magna, Sittingbourne, Shell Research
(SBGR.82.119).
GARFORTH, B.M. (1983) The effect of RIPCORD on the non-
target fauna of maize, Sittingbourne, Shell Research (SBGR.83.
045).
GERIG, L. (1979) [The toxicity of synthetic pyrethrines to
foraging bees.] Schwei z. Bienen-Ztg, 101: 228-236 (in German).
GERIG, L. (1981) [The toxicity of synthetic pyrethrines to
foraging bees. Part 2.] Schweiz. Bienen-Ztg, 104: 155-174 (in
German).
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977a) Effects of
high dietary levels on clinical behaviour and structure of
sciatic nerves in the rat, Fernhurst, Imperial Chemical
Industries, Central Toxicology Laboratory, pp. 383
(Unpublished ICI report).
GLAISTER, J.R., GARE, C.W., MARSAT, G.J., PHILLIPS, C., &
PRATT, I. (1977b) 90-day feeding study in rats, Fernhurst,
Imperial Chemical Industries, Central Toxicology Laboratory,
pp. 383 (Unpublished ICI report).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4(6): 793-799.
GRAY, K. & GRAYSON, B.T. (1980) Determination of the
n-octanol/water partition coefficients of BIRLANE AND GARDONA
and a literature search for the values of other group
compounds, Sittingbourne, Shell Research (BLGR.80.103).
GRAYSON, B.T., LANGNER, E., & WELLS, D. (1982) Comparison of
two gas saturation methods for the determination of the vapour
pressure of cypermethrin. Pestic. Sci., 13: 552-556.
HAGLEY, E.A.C., PREE, D.J., & SIMPSON, C.M. (1981) Toxicity
of insecticides to parasites of the spotted tentiform
leafminer (Lepidoptera: Gracillariidae). Can. Entomol., 113:
899-906.
HALL, J.S., LEAHEY, J.P., & CURL, E.A. (1981) Cypermethrin:
photodegradation on a soil surface, Fernhurst, Imperial
Chemical Industries (Unpublished ICI Report No. RJ0192B).
HARGREAVES, J.R. & COOPER, L.P. (1979) Phytotoxicity tests
with pyrethroid insecticides on glasshouse grown tomato
seedlings. Queensland J. Agric. anim. Sci., 36(2): 151-154.
HARRIS, C.R. & TURNBULL, S.A. (1978) Laboratory studies on
the contact toxicity and activity in soil of four pyrethroid
insecticides. Can. Entomol., 110: 285-288.
HARRIS, C.R. & TURNBULL, S.A. (1980) Toxicity of some
insecticides to insecticide-susceptible strains of the onion,
cabbage and seedcorn maggots (Diptera: Anthomyiidae) and the
dark-sided cutworm (Lepidoptera: Nocturidae). Can. Entomol.,
112(10): 1029-1032.
HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory
studies on the persistence and behaviour in soil of 4
pyrethroid insecticides. Can. Entomol., 113: 685-694.
HARRIS, C.R., TURNBULL, S.A., & MCLEOD, D.G.R. (1985)
Contact toxicity of twenty-one insecticides to adults of the
carrot rust fly (Diptera: Psilidae). Can. Entomol., 117:
1025-1027.
HART, T.B., SPINKS, C.A., MARLOW, R.G., & SUTHERS, J.R.
(1982) A study of the exposure and health of Indian workers
spraying, Ambush and Cymbush on cotton using high volume
hand-held spray applicators, Fernhurst, Imperial Chemical
Industries, Plant Protection Division (Report TMF.1841-B).
HELLING, C.S. & TURNER, B.C. (1968) Pesticide mobility:
determination in soil by thin-layer chromatography. Science,
162: 562-563.
HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies on
the insecticide WL 43467: a three-month feeding study in rats,
Sittingbourne, Shell Research (TLGR.0027.76) (Unpublished
report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977a) Toxicity studies on
the insecticide WL 42641: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977b) Toxicity studies on
the insecticide WL 43481: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W., HENDY, R., & FLEMING, D.J. (1978) Toxicity
studies on the insecticide WL 43467: a three-generation
reproduction study in rats, Sittingbourne, Shell Research
(TLGR.0188.78).
HENDERSON, C. & PARKINSON, G.R. (1981) Cypermethrin
technical: subacute dermal toxicity study in rabbits, Alderley
Park, Imperial Chemical Industries (Report No. CTL/P/588).
HINE, C.H. & ZUIDEMA, H.H. (1970) The toxicological
properties of hydrocarbon solvents. Ind. Med., 39(5): 39-44.
HO, S.H., LEE, B.H., & SEE, D. (1983) Toxicity of
deltamethrin and cypermethrin to the larvae of the diamond-
back moth, Plutella xylostella L. Toxicol. Lett., 19: 127-131.
HOLDEN, J.S. (1979) Absorption and metabolism of permethrin
and cypermethrin in the cockroach and the cotton leaf-worm
larvae. Pestic. Sci., 10: 295-307.
HOPKINS, A.R., MOORE, R.F., & JAMES, W. (1984) Contact and
residual toxicities of pyrethroids and organophosphorus
compounds to the Boll weevil (Coleoptera: Curculionidae) J.
Georgia Entomol. Soc., 19(1): 27-34.
HUCKLE, K.R., HUTSON, D.H., & MILLBURN, P. (1981a) Species
differences in the metabolism of 3-phenoxybenzoic acid. Drug
Metab. Disp., 9: 352-359.
HUCKLE, K.R., CHIPMAN, J.K., HUTSON, D.H., & MILLBURN, P.
(1981b) Metabolism of 3-phenoxybenzoic acid and the
enterohepatorenal disposition of its metabolites in the rat.
Drug Metab. Disp., 9(4): 360-368.
HUTSON, D.H. (1978a) The elimination of radioactivity by
mice following oral dosing with 14 C-cis- and 14 C-trans-WL
43467 (cypermethrin), Sittingbourne, Shell Research
(TLGR.0079.78).
HUTSON, D.H. (1978b) The metabolites of cis- and trans-
cypermethrin (WL 43467) in mice, Sittingbourne, Shell Research
(TLGR.102.78).
HUTSON, D.H. (1979a) The metabolic fate of synthetic
pyrethroid insecticide in mammals. Prog. Drug Metab., 3:
215-252.
HUTSON, D.H. (1979b) The metabolism of WL 43467 in mammals.
Metabolites derived from (14 C-cyano)cypermethrin (WL 43467) in
rats, Sittingbourne, Shell Research (TLGR.79.183).
HUTSON, D.H. & CASIDA, J.E. (1978) Taurine conjugation in
metabolism of 3-phenoxybenzoic acid and the pyrethroid
insecticide cypermethrin in mouse. Xenobiotica, 8(9): 565-571.
HUTSON, D.H. & STOYDIN, G. (1987) Excretion and residues of
the pyrethroid insecticide cypermethrin in laying hens.
Pestic. Sci., 18: 157-168.
HUTSON, D.H., GAUGHAN, L.C., & CASIDA, J.E. (1981)
Metabolism of the cis- and trans-isomers of cypermethrin in
mice. Pestic. Sci., 12: 385-398.
INGLESFIELD, C. (1982) The effects of an aerial application
of RIPCORD on the non-target fauna of oil seed rape in France,
Sittingbourne, Shell Research (SBGR.82.364).
INGLESFIELD, C. (1984) Toxicity of the pyrethroid
insecticides cypermethrin and WL 85871 to the earthworm
Eisenia foetida Savigny. Bull. environ. Contam. Toxicol.,
33(5): 568-570.
INGLESFIELD, C. & SHERWOOD, C.M. (1983) Toxicity of
cypermethrin to the earthworm, Eisenia foetida L (Oligochaeta:
Lumbriculidae) in laboratory tests, Sittingbourne, Shell
Research (SBGR.83.070).
ISHAAYA, I., ASCHER, K.R.S., & CASIDA, J.E. (1983)
Pyrethroid synergism by esterase inhibition in Spodoptera
littoralis (Boisd.) larvae, Crop Prot., 2(3): 335-343.
JACKSON, C. (1977) The leaching of WL 43467 through
laboratory soil columns, Sittingbourne, Shell Research
(Unpublished Report BLGR.0150.77).
JOIA, B.S., LOSCHIAVO, S.R., & WEBSTER, G.R.B. (1981) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat. In: Proceedings of the 16th Annual Workshop
on Pesticide Residue Analysis, Western Canada, pp. 14-16.
JOIA, B.S., SARMA, L.P., & WEBSTER, G.R.B. (1985a) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat and milled fractions. J. environ. anal.
Chem., 21: 179-184.
JOIA, B.S., WEBSTER, G.R.B., & LOSCHIAVO, S.R. (1985b)
Cypermethrin and fenvalerate residues in stored wheat and
milled fractions. J. agric. food Chem., 33: 618-622.
JONES, B.J. (1981) Cypermethrin: bioaccumulation study in
the rat, Alderley Park, Imperial Chemical Industries (Report
No. CTL/P/599).
JORDAN, T.W. & CHANG, C.K. (1981) Pyrethroid resistance
mechanisms in Porina caterpillars. In: Proceedings of the 34th
New Zealand Weed and Pest Control Conference, pp. 167-169.
KAUFMAN, D.D., JORDAN, E.G., & KAYSER, A.J. (1978)
Degradation of cypermethrin and permethrin in soil. In:
Proceedings of the 175th Meeting on Pesticides of the American
Chemical Society, Washington, DC, American Chemical Society
(Abstract Paper No. 47).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J.
(1981) Movement of cypermethrin, decamethrin, permethrin, and
their degradation products in soil. J. agric. food Chem., 29:
239-245.
KNAPP, F.W., HERALD, F., & SCHWINGHAMMER, K.A. (1985)
Comparative toxicity of selected insecticides to laboratory-
reared and field-collected face flies (Diptera: Muscidae) J.
econ. Entomol., 78: 860-862.
KNIGHT, R.J. (1982) The toxicity of the pyrethroid WL 85871
against honey bee Apis mellifera, Sittingbourne, Shell
Research (SBGR.82.023).
KNOWLES, C.O. & EL-SAYED, G.N. (1985) Formanilide
enhancement of acaricide toxicity to Tetranychus urticae Koch
(Acari: Tetranychidae), J. econ. Entomol., 78: 308-310.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in
relation to body weight and temperature. Water Res., 15:
503-505.
LAUREN, D.R. & HENZEL, R.F. (1977) Residual lives of two
synthetic prethroid insecticides applied to pasture. In:
Proceedings of the 30th New Zealand Weed and Pest Control
Conference, pp. 207-210.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology:
mouse intracerebral structure-toxicity relationship. Pestic.
Biochem. Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action
of pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Inter-
actions of pyrethroid insecticides with chloride ionophore-
associated binding sites. Neurotoxicol., 6: 87-98.
LEAHEY, J.P. (1979) The metabolism and environmental
degradation of the pyrethroid insecticides. Outlook Agric.,
10(3): 135-142.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London,
Philadelphia, Taylor and Francis.
LEAKE, L.D., LAUCKNER, S.M., & FORD, M.G. (1979)
Relationship between neurophysiological effects of selected
pyrethroids and toxicity to the leech Haemopsis sanguisuga and
the locust Schistocerca gregaria. Neurobiol. Pest. Action.,
London, pp. 423-430.
LE PATOUREL, G.N.J. & SINGH, J. (1984) Toxicity of amorphous
silicas and silica-pyrethroid mixtures to Tribolium castaneum
(Herbst) (Coleoptera: Tenebrionidae). J. stored Prod. Res.,
20(4): 183-190.
LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to
synthetic pyrethroids: a clinical and electrophysiological
assessment. Neurotoxicology, 2: 1-11.
LINDSAY, S., BANHAM, P.B., CHART, I.S., CHALMERS, D.T.,
GODLEY, M.J., & TAYLOR, K. (1982) Cypermethrin: life-time
feeding study in mice, Alderley Park, Imperial Chemical
Industries (Report No. CTL/P/687).
LIU, M.Y., CHEN, J.S., & SUN, C.N. (1984) Synergism of
pyrethroids by several compounds in larvae of the Diamondback
moth (Lepidoptera: Plutellidae) J. econ. Entomol., 77: 851-856.
LOCK, E.A. & BERRY, P.N. (1981) Biochemical changes in the
rat cerebellum following cypermethrin administration. Toxicol.
appl. Pharmacol., 59(3): 508-514.
LOVERIDGE, D. & COOK, K.A. (1978a) Determination of the
effects of WL 43467 on microbial activity in soil. II. Effects
on oxygen uptake, Sittingbourne, Shell Research (BLGR.0004.78).
LOVERIDGE, D. & COOK, K.A. (1978b) Determination of the
effects of WL 43467 on microbial activity in soil. III.
Effects on nitrogen fixation, Sittingbourne, Shell Research
(BLGR.0007.78).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve
membrane effects of pyrethroids and DDT analogs. Pestic.
Biochem. Physiol., 20: 203-216.
MCAUSLAND, H.E., BUTTERWORTH, S.T.G., & HUNT, P.F. (1978)
Toxicity studies on the insecticide WL 43467: a two-year
feeding study in rats, Sittingbourne, Shell Research
(TLGR.0189.78).
MCDONALD, S. (1979) Evaluation of insecticides for control
of the army cutworm. J. econ. Entomol., 72(2): 277-280.
MCKEE, M.J. & KNOWLES, C.O. (1985) Pharmacokinetics of
pyrethroids in two-spotted spider mites. Pestic. Biochem.
Physiol., 24: 326-335.
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality
of permethrin, cypermethrin, and fenvalerate to salmon,
lobster, and shrimp. Bull. environ. Contam. Toxicol., 25:
950-955.
MANI, M. & KRISHNAMOORTHY, A. (1984) Toxicity of some
insecticides to Apanteles plutellae, a parasite of the
diamondback moth. Trop. Pestic. Manage., 30(2): 130-132.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th
ed., Croydon, British Crop Protection Council.
MAUCK, W.L., OLSEN, L.E., & MARKING, L.L. (1976) Toxicity of
natural pyrethrins and five pyrethroids to fish. Arch.
environ. Contam. Toxicol., 4: 18-29.
MEISNER, J., ASCHER, K.R.S., & EIZICK, C. (1984) Effect of
the commercial phagostimulants coax and gustol on the toxicity
of cypermethrin and deltamethrin against Spodoptera littoralis
(Lepidoptera: Noctuidae). J. econ. Entomol., 77: 1123-1126.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK,
J. (1983) Farm chemical handbook. C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, pp. C67.
MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J.
(1984) New conjugated metabolites of 3-phenoxybenzoic acid in
plants. Pestic. Sci., 15: 531-542.
MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO,
J. (1985) Metabolism in rats of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid glucoside conjugates formed in plants.
Pestic. Sci., 16: 33-45.
MIYAMOTO, J. (1981) The chemistry, metabolism, and residue
analysis of synthetic pyrethroids. Pure appl. Chem., 53:
1967-2022.
MIYAMOTO, J. & MIKAMI, N. (1983) Degradation of pyrethroid
insecticides in the field. In: IUPAC Pesticide chemistry,
human welfare and the environment, Oxford, Pergamon Press, pp.
193-200.
MORE, J.E., ROBERTS, T.R., & WRIGHT, A.N. (1978) Studies of
the metabolism of 3-phenoxybenzoic acid in plants. Pestic.
Biochem. Physiol., 9: 268-280.
MUIR, D.C.G., RAWN, G.P., TOWNSEND, B.E., LOCKHART, W.L., &
GREENHALGH, R. (1985) Bioconcentration of cypermethrin,
deltamethrin, fenvalerate, and permethrin by Chironomus
tentans larvae in sediment and water. Environ. Toxicol. Chem.,
4(1): 51-61.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some non-target insects. Mosq. News.,
38(1): 90-96.
NOBLE, R.M. & HAMILTON, D.J. (1985) Stability of
cypermethrin and cyfluthrin on wheat in storage. Pestic. Sci.,
16: 179-185.
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976a) Neurotoxic
effects of S 5602 and NRDC 149 by dermal application in rats,
Osaka, Sumitomo Chemical Company (AT-60-0047).
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976b) Neurotoxic
effects of natural pyrethrins and resmethrin by oral application
in rats, Osaka, Sumitomo Chemical Company (AT-60-0052).
OSMAN, A.A., ZOHDY, G.I., & MOMEN, F.M. (1982) Effect of
some pesticides on the food requirements of the predatory
mite, Amblyseius gossipiu-El-Badry. In: Griffiths, D.A. &
Bowman, C.E., ed. Acarology VI, Chichester, Ellis Horwood Ltd,
Vol. 2, pp. 669-672.
OWEN, D.E. & BUTTERWORTH, S.T.G. (1977) Toxicity of
pyrethroid insecticides: investigation of the neurotoxic
potential of WL 43467 to adult domestic hens, Sittingbourne,
Shell Research (TLGR.0134.77).
PEARSON, N. & SHIRES, S.W. (1981) A field study in France of
the effects on honey bees of an aerial application of RIPCORD
in winter-sown oil seed rape, Sittingbourne, Shell Research
(SBGR.81.304).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PLUYMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity
of synthetic pyrethroids in Salmonella typhimurium strains and
in V79 Chinese hamster cells. Mutat. Res., 137: 7-15.
POTTER, D. & MCAUSLAND, H.E. (1980) Toxicity studies on the
insecticide WL 43467: a study of liver microsomal enzyme
activity in rats fed WL 43467 for 2 years, Sittingbourne,
Shell Research (TLGR.80.057).
POULOS, L., ATHANASELIS, S., & COUTSELINIS, A. (1982) Acute
intoxication with cypermethrin (NRDC 149). J. Toxicol. clin.
Toxicol., 19(5): 519-520.
PREE, D.J. & HAGLEY, E.A.C. (1985) Toxicity of pesticides to
Chrysopa oculata Say (Neuroptera : Chrysopidae). J. econ.
Entomol., 78(1): 129-132.
PRICE, J.B. (1981a) Toxicology of pyrethroids: the acute
oral and intraperitoneal toxicity of cypermethrin,
Sittingbourne, Shell Research (SBGR.81.069).
PRICE, J.B. (1981b) Toxicology of pyrethroids: the acute
oral and percutaneous toxicities of RIPCORD and endosulfan,
individually or combined in a 1:1 mixture, Sittingbourne,
Shell Research (SBGR.81.121).
PRINSEN, G.H. & SITTERT, N.J., VAN (1978) Exposure and
medical monitoring study of the pyrethroid WL 43467 after
single application on cotton in Ivory Coast, The Hague, Shell
Internationale Research Mij (TOX 78-004).
PRINSEN, G.H. & SITTERT, N.J., VAN (1979) Exposure and
medical monitoring study of the pyrethroid RIPCORD (WL 43467)
after one season of spraying on cotton in Ivory Coast, The
Hague, Shell Internationale Research Mij (TOX 79-001).
RAJAKULENDRAN, S.V. & PLAPP, F.W., Jr (1982) Comparative
toxicities of five synthetic pyrethroids to the tobacco
budworm (Lepidoptera: Noctuidae), an Ichneumonid parasite,
Campoletis sonorensis and a predator, Chrysopa carnea. J.
econ. Entomol., 75: 769-772.
RAPLEY, J.H., ARNOLD, D.J., & VINCENT, J. (1981)
Cypermethrin: degradation in river and pond waters and
sediments, Fernhurst, Imperial Chemical Industries, Plant
Protection Division (Report No. RJ0175B) (Unpublished ICI
data).
REIFF, B. (1976) The acute toxicity of the pyrethroid
insecticide WL43467 to Brown trout (S. trutta), Sittingbourne,
Shell Research (TLBR.0096.76).
REIFF, B. (1977) The acute toxicity of the pyrethroid WL
43467 to Daphnia magna, Sittingbourne, Shell Research (TLGR.
0155.77).
REIFF, B. (1978a) The effect of suspended solids on the
toxicity of WL 43467 to Rainbow trout (Salmo gairdneri),
Sittingbourne, Shell Research (TLGR.0007.78).
REIFF, B. (1978b) The acute toxicity of the pyrethroid
insecticide WL 43467 to Rainbow trout (Salmo gairdneri),
Common carp (Cyprinus carpio), and Rudd (Scardinius
erythrophthalmus), Sittingbourne, Shell Research (TLGR.0067.
78).
RHODES, C., JONES, B.K., CROUCHER, A., HUTSON, D.H., LOGAN,
C.J., HOPKINS, R., HALL, B.E., & VICKERS, J.A. (1984) The
bioaccumulation and biotransformation of cis, trans-
cypermethrin in the rat. Pestic. Sci., 25: 471-480.
RILEY, D. & HILL, I.R. (1983) Adsorption reduces activity of
pesticides in soil and water. In: Proceedings of the 10th
International Congress on Plant Protection, Brighton, 20-25
November, 1983, Vol. 2, pp. 728 (4B-R18).
RISKALLAH, M.R. (1984) Influence of posttreatment
temperature on the toxicity of pyrethroid insecticides to
susceptible and resistant larvae of the Egyptian cotton
leafworm, Spodoptera littoralis (Boisd.) Experientia (Basel),
40(2): 188-190.
RIVIERE, J.L., BACH, J., & GROLLEAU, G. (1983) Effect of
pyrethroid insecticides and N-(3,5-dichlorophenyl)
dicarboximide fungicides on microsomal drug metabolizing
enzymes in the Japanese quail (Coturnix coturnix). Bull.
environ. Contam. Toxicol., 31: 479-485.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3(1): 67-68.
ROBERTS, T.R. (1981) The metabolism of the synthetic
pyrethroids in plants and soils. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry, New York, John
Wiley and Sons, Vol. 1, pp. 115-146.
ROBERTS, T.R. & STANDEN, M.E. (1977) Degradation of the
pyrethroid cypermethrin NRDC 149(±)-alpha-cyano-3-phenoxy-
benzyl(±)- cis,trans-3-(2,2-dichlorovinyl-2,2-dimethylcyclo-
propanecarboxylate and the respective cis-(NRDC 160) and the
trans-(NRDC 159) isomers in soils. Pestic. Sci., 8: 305-319.
ROBERTS, T.R. & STANDEN, M.E. (1981) Further studies of the
degradation of the pyrethroid insecticide cypermethrin in
soils. Pestic. Sci., 12: 285-296.
ROSE, B. (1981) A dietary toxicity study of WL 43467 in
Mallard ducks, Sittingbourne, Shell Research (TLGR.80.033).
ROSE, G.P. (1982) Toxicology of pyrethroids: the acute oral
and percutaneous toxicity of WL 85871 (cis-2-RIPCORD)
comparison with RIPCORD, Sittingbourne, Shell Research
(SBGR.82.130).
ROSE, G.P. (1983) Neurotoxicity of WL 85871 comparison with
WL 43467: the effect of twenty oral doses of WL 85871 or WL
43467 over a period of 4 weeks on the rat sciatic/posterior
tibial nerve, trigeminal nerve, and trigeminal ganglion,
Sittingbourne, Shell Research (SBGR.83.185).
ROSE, G.P. & DEWAR, A.J. (1978) Toxicity studies on the
insecticide WL 43467: the effect of age on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0039.78).
ROSE, G.P. & DEWAR, A.J. (1979a) Toxicity studies on the
RIPCORD/AZODRIN formulation EF 5254: biochemical and
functional studies on the neurotoxicity of the formulation EF
5254 in the rat, Sittingbourne, Shell Research (TLGR.79.027).
ROSE, G.P. & DEWAR, A.J. (1979b) A neurotoxicity study on
the pyrethroid metabolite 3-phenoxybenzoic acid (3-PBA),
Sittingbourne, Shell Research (TLGR.79.076).
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four
synthetic pyrethroids fails to show any correlation between
neuromuscular dysfunction and neurobiochemical abnormalities
in rats. Arch. Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of
pyrethroids on a nerve - muscle preparation of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 25: 176-187.
RUZO, L.O. (1983) Involvement of oxygen in the
photoreactions of cypermethrin and other halogenated
pyrethroids. J. agric. food Chem., 31: 1113-1115.
RUZO, L.O. & CASIDA, J.E. (1980) Pyrethroid photochemistry:
mechanistic aspects in reactions of the (dihalogenovinyl)-
cyclopropanecarboxylate substituent. J.C.S. Perkin Trans. I,
728-732.
RUZO, L.O., HOLMSTEAD, R.L., & CASIDA, J.E. (1977)
Pyrethroid photochemistry: decamethrin. J. agric. food Chem.,
25(6): 1385-1389.
SAAD, A.S.A., ELEWA, M.A., ALY, N.M., AUDA, M., & EL-SEBAE,
A.H. (1981) Toxicological studies on the Egyptian cotton
leafworm Spodoptera littoralis. I. Potentiation and antagonism
of synthetic pyrethroid, organophosphorus and urea derivative
insecticides. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(2):
559-571.
SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1986)
Degradation and leaching behaviour of the pyrethroid
insecticide cypermethrin in soils. J. Pestic. Sci., 11: 71-79.
SCOTT, J.G. & GEORGHIOU, G.P. (1984) Influence of
temperature on knockdown, toxicity and resistance to
pyrethroids in the housefly, Musca domestica. Pestic. Biochem.
Physiol., 21: 53-62.
SEEHY, M.A., SHALABI, H.G., SHAKER, N., & BADR, E. (1983) In
vivo induction of sister chromatid exchanges in mice by
cypermethrin. Proceedings of the International Conference on
Environmental Hazards of Agrochemicals, 1982, Vol. 1, 659-673.
SHERWOOD, C.M. & SHIRES, S.W. (1981) The effect of RIPCORD
on the invertebrate fauna of winter barley in France,
Sittingbourne, Shell Research (SBGR.81.070).
SHIRES, S.W. (1980) Soil surface predators in arable land:
the effects of farming practices. Span, 23(2): 62-64.
SHIRES, S.W. (1982a) The effect of RIPCORD on the
invertebrate fauna of winter wheat in France, Sittingbourne,
Shell Research (SBGR.81.303).
SHIRES, S.W. (1982b) A field study in France of the effects
on honey bees of an aerial application of RIPCORD in spring-
grown oil seed rape, Sittingbourne, Shell Research
(SBGR.82.066).
SHIRES, S.W. (1982c) A study of the effects of an aerial
application of RIPCORD on the invertebrate fauna of winter
wheat, Sittingbourne, Shell Research (SBGR.82.304).
SHIRES, S.W. (1983a) The use of small enclosures to assess
the toxic effects of cypermethrin in fish under field
conditions. Pestic. Sci., 14: 475-480.
SHIRES, S.W. (1983b) Pesticides and honey bees. Case studies
with RIPCORD and FASTAC. Span, 26(3): 118-120.
SHIRES, S.W. & BENNETT, D. (1982) Spray drift from aerial
application of RIPCORD to winter wheat in Kent, UK: fate and
effects in adjacent drainage ditches, Sittingbourne, Shell
Research (SBGR.82.274).
SHIRES, S.W. & BENNETT, D. (1985) Contamination and effects
in freshwater ditches, resulting from an aerial application of
cypermethrin. Ecotoxicol. environ. Saf., 9: 145-158.
SHIRES, S.W. & DEBRAY, P. (1982) Pyrethroids and the bee
problem. Shell Agric., May: 1-3.
SHIRES, S.W. & TIPTON, J.D. (1982) A study of the effects of
BIRLANE and RIPCORD on the hymenopterous parasites of white
flies on cotton in the Sudan, Sittingbourne, Shell Research
(SBGR.82.342).
SHIRES, S.W., BENNETT, D., & KANE, D.F. (1979) The effects
of WL 43467 on soil surface fauna, earthworms, and litter
composition, Sittingbourne, Shell Research (TLGR.79.074).
SHIRES, S.W., BENNETT, D., & CROSSLAND, N.O. (1980) Spray
drift from RIPCORD applications to arable crops in Suffolk,
UK: fate and effects in adjacent farm ponds, Sittingbourne,
Shell Research (TLGR.80.150).
SHONO, T. & CASIDA, J.E. (1978) Species-specificity in
enzymatic oxidation of pyrethroid insecticides:
3-phenoxybenzyl and alpha-cyano-3-phenoxybenzyl 3-(2,2-dihalo-
vinyl)-2,2-dimethylcyclopropanecarboxylates. J. Pestic. Sci.,
3: 165-168.
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of
trans- and cis-permethrin and trans- and cis-cypermethrin, and
decamethrin by microsomal enzymes. J. agric. food Chem.,
27(2): 316-325.
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation
of toxicity of pyrethroid insecticides to honey bees:
relevance to hazard in the field. Bee World, 63(4): 150-152.
SMIES, M., EVERS, R.H.J., PEIJNENBURG, F.H.M., & KOEMAN, J.H.
(1980) Environmental aspects of field trials with pyrethroids
to eradicate tsetse fly in Nigeria. Ecotoxicol. environ. Saf.,
4: 114-128.
SMITH, T.M. & STRATTON, G.W. (1986) Effects of synthetic
pyrethroid insecticides on non-target organisms. Residue Rev.,
97: 93-120.
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid
structure on rates of hydrolysis and oxidation by mouse liver
microsomal enzymes. Pestic. Biochem. Physiol., 7: 391-401.
SPIELBERGER, U., NA'ISA, B.K., KOCH, K., MANNO, A., SKIDMORE,
P.R., & COUHS, H.H. (1979) Field trials with the synthetic
pyrethroids permethrin, cypermethrin, and decamethrin against
Glossina (Diptera: gloninidae) in Nigeria. Bull. entomol.
Res., 69: 667-689.
STANDEN, M.E. (1977) The leaching and degradation of the
insecticide WL43467 when applied in sheep-dip solution to
soil, Sittingbourne, Shell Research (BLGR.0141.77)
(Unpublished report).
STELZER, K.J. & GORDON, M.A. (1984) Effects of pyrethroids
on lymphocyte mitogenic responsiveness. Res. Commun. chem.
Pathol. Pharmacol., 46(1): 137-150.
STEPHENSON, R.R. (1980a) The acute toxicity of WL 43467 to
some freshwater invertebrates in static water tests,
Sittingbourne, Shell Research (TLGR.80.040).
STEPHENSON, R.R. (1980b) The acute toxicity of cypermethrin
(WL 43467) to the freshwater shrimp (Gammarus pulex) and
larvae of the mayfly (Cloeon dipterum) in continuous-flow
tests, Sittingbourne, Shell Research (TLGR.80.079).
STEPHENSON, R.R. (1981a) RIPCORD: the acute toxicity of an
EC formulation to Tilapia nilotica in the laboratory,
Sittingbourne, Shell Research (SBGR.81.028).
STEPHENSON, R.R. (1981b) Cypermethrin: acute toxicity to
Tilapia nilotica in a continuous-flow test, Sittingbourne,
Shell Research (SBGR.81.080).
STEPHENSON, R.R. (1982a) RIPCORD: a laboratory study of the
acute toxicity of an EC formulation to Tilapia nilotica in the
presence of suspended solids, Sittingbourne, Shell Research
(SBGR.81.235).
STEPHENSON, R.R. (1982b) WL 85871 and cypermethrin: a
comparison of their acute toxicity to Salmo gairdneri, Daphnia
magna, and Selenastrum capricornutum, Sittingbourne, Shell
Research (SBGR.81.277).
STEPHENSON, R.R. (1982c) RIPCORD, BIRLANE, and FURADAN:
acute toxicity to common carp (Cyprinus carpio L.) in the
laboratory and in rice paddies, Sittingbourne, Shell Research
(SBGR.82.030).
STEPHENSON, R.R. (1982d) WL 85871 and cypermethrin: a
comparative study of their toxicity to the Fathead minnow
Pimephales promelas (Rafinesque), Sittingbourne, Shell
Research (SBGR.82.298).
STEPHENSON, R.R. (1982e) Aquatic toxicology of cypermethrin.
I. Acute toxicity to some freshwater fish and invertebrates in
laboratory tests. Aquat. Toxicol., 2: 175-185.
STEPHENSON, R.R. (1983) Pesticides and freshwater animals. A
case study with RIPCORD. Span, 26(3): 121-122.
STEPHENSON, R.R., CHOI, S.Y., & OLMOS-JEREZ, A. (1984)
Determining the toxicity and hazard to fish of a rice
insecticide. Crop Prot., 3(2): 151-165.
STEVENS, J.E.B. & HILL, I.R. (1980) Mobility of cypermethrin
and its degradation products in soil columns, Fernhurst,
Imperial Chemical Industries (Report No. RJ/0166-B)
(Unpublished ICI data).
SUHAS, Y. & DEVAIAH, M.C. (1985) Studies on the effect of
insecticides sprayed mulberry leaves to silkworm, Bombyx mori
L. Pesticides, 19(10): 53-54, 57.
SUNDARARAJAN, R. & CHAWLA, R.P. (1983) Simple, sensitive
technique for detection and separation of halogenated
synthetic pyrethroids by thin layer chromatography. J. Assoc.
Off. Anal. Chem., 66(4): 1009-1012.
SURULIVELU, T. & MENON, M.V. (1982) Contact toxicity of
synthetic pyrethroids, organophosphorus and carbamate
insecticides to adults of the parasite Chelonus blackburni
Cameron. J. Agric. Sci. Camb., 98: 331-334.
SUTHERS, J.R. & MARLOW, R.G. (1981) A study of the exposure
and health of Indian workers spraying RIPCORD on cotton over
five consecutive days using mistblower and knapsack
applicators, The Hague, Shell Internationale Research Mij (TOX
81-003).
SWAINE, H. & SAPIETS, A. (1980a) Residue transfer study with
dairy cows fed on a diet containing the insecticide.
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0186-B).
SWAINE, H. & SAPIETS, A. (1980b) Residue levels of the major
metabolites of the insecticide in the milk and tissues of
dairy cows fed on a diet containing cypermethrin at 50 mg/kg,
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0198-B).
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., & ASKAR,
A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46(1): 387-391.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985a) Hydrolysis of the pyrethroid insecticide cypermethrin
in aqueous media. J. Pestic. Sci., 10: 643-648.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985b) Photodegradation of the pyrethroid insecticide
cypermethrin in water and on soil surface. J. Pestic. Sci.,
10: 629-642.
TAYLOR, S.M., ELLIOTT, C.T., & BLANCHFLOWER, J. (1985)
Cypermethrin concentrations in hair of cattle after
application of impregnated ear tags. Vet. Rec., 116(23): 620.
TESH, J.M., TESH, S.A., & DAVIES, W. (1978) WL 43467:
effects upon the progress and outcome of pregnancy in rat,
Stock, Life Science Research (LSR Report No. 78/SHL2/364).
TEWARI, G.C. & KRISHNAMOORTHY, P.N. (1985) Selective
toxicity of some synthetic pyrethroids and conventional
insecticides to aphid predator, Menochilus sexmaculatus
Fabricius. Indian J. agric. Sci., 55(1): 40-43.
TRIGG, C.E., BUTTERWORTH, S., & HUNT, P.F. (1977)
Neurotoxicity of pyrethroids: a study of teased nerves from
rats fed WL 43467 for 12 months, Sittingbourne, Shell Research
(TLGR.0137.77).
TU, C.M. (1980) Influence of five pyrethroids insecticides
on microbial populations and activities in soil. Microbiol.
Ecol., 5: 321-327.
TU, C.M. (1982) Effects of some pesticides on Rhizobium
japonicum and on the seed germination and pathogens of
soybean. Chemosphere, 11(10): 1027-1033.
US EPA (1984) Cypermethrin: tolerances for residues in or on
raw agricultural commodities: final rule. Part III. Fed. Reg.,
49(117): 24865-24872.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the
peripheral nervous system of the frog. Pestic. Sci., 8:
692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Effects of
insecticides on the sensory system of Xenopus. Insect neurobiology
and pesticide action, London, Society of Chemical
Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M.
(1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effects of insecticides on the sensory nervous system.
In: Narashashi, T., ed. Neurotoxicology of insecticides and
pheromones, New York, London, Plenum Publishing Corporation,
pp. 183-210.
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985a)
Human oral dose-excretion study with RIPCORD, The Hague, Shell
Internationale Petroleum Maatschappy (HSE.85.008).
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985b)
Human dermal dose-excretion study with RIPCORD, The Hague,
Shell Internationale Petroleum Maatschappy (HSE.85.009).
VEKARIA, M.V. & VYAS, H.N. (1985) Studies on ovicidal
toxicity of certain insecticides against the eggs of Heliothis
armigera Hubner. Pesticides, 19(10): 43-44.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationships of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index. JMPR 1961-84, Geneva, World Health
Organization.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in
frog myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system.
Neuropathol. appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides
on the lateral-line sense organ and on peripheral nerves of
the clawed frog, Xenopus laevis. Pestic. Biochem. Physiol.,
18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on
sodium channel gating in myelinated nerves. Nature (Lond.),
295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature- and
structure-dependent interaction of pyrethroids with the sodium
channels in frog node of Ranvier. Biochim. biophys. Acta, 728:
73-82.
WADDILL, V.H. (1978) Contact toxicity of four synthetic
pyrethroids and methomyl to some adult insect parasites.
Florida Entomol., 61(1): 27-30.
WALLACE, B.G., ROBERTS, T.R., & MCKERRELL, E.H. (1982)
Cypermethrin. A residue transfer study with laying hens,
Sittingbourne, Shell Research (SBTR.82.059).
WATTERS, F.L., WHITE, N.D.G., & COTE, D. (1983) Effect of
temperature on toxicity and persistence of three pyrethroid
insecticides applied to fir plywood for the control of the red
flour beetle (Coleoptera: Tenebrionidae). J. econ. Entomol.,
76: 11-16.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23 .
WHO (1985) WHO Technical Report Series, No. 720 (Safe use of
pesticides. Ninth Report of the WHO Expert Committee on Vector
Biology and Control), pp. 14-19 .
WHO/FAO (1984) Cypermethrin, Geneva, World Health
Organization (Data Sheets on Pesticides, No. 84.58).
WILDE, G., KADOUM, A., & MIZE, T. (1984) Absence of
synergism with insecticides combinations used on chinch bugs
(Heteroptera: Lygaeridae). J. econ. Entomol., 77: 1297-1298.
WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic
pyrethoid insecticides to predaceous phytoseiid. Aust. J.
agric. Res., 30: 497-501.
WOOD, MACKENZIE, & CO. (1980) Pyrethroids, Agrochem. Monit.,
9: 3-14.
WOOD, MACKENZIE, & CO. (1981) Pyrethroids. Agrochem. Monit.,
15: 3-27.
WOOD, MACKENZIE, & CO. (1982) Pyrethroids. Agrochem. Monit.,
21: 3-17.
WOOD, MACKENZIE, & CO. (1983) Pyrethroids. Agrochem. Monit.,
27: 3-12.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual,
7th ed., Croydon, British Crop Protection Council, pp. 150-151.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of
pyrethroids. Gen. Pharmacol., 9: 387-398.
WRIGHT, A.N., ROBERTS, T.R., DUTTON, A.J., & DOIG, M.V.
(1980) The metabolism of cypermethrin in plants: the
conjugation of the cyclopropyl moiety. Pestic. Biochem.
Physiol., 13: 71-80.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G.
(1979) Toxicity of permethrin, decamethrin, and related
pyrethroids to salmon and lobster. Bull. environ. Contam.
Toxicol., 21: 338-343.
ZOHDY, G.I., OSMAN, A.A., & MOMEN, F.M. (1984) Toxicity of
some pyrethroid compounds to the predatory mite, Amblyseius
gossipi El-Badry. In: Griffiths, D.A. & Bowman, C.E., ed.
Acarology VI, Chichester, Ellis Horwood Ltd., Vol. 2, pp.
659-662.
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana esculenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3 - 10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittantly, cause large
depolarizing afterpotentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids
(allethrin, cismethrin, bioresmethrin, and 1R, cis-phenothrin)
caused moderate presynaptic repetitive activity, resulting in the
occurrence of multiple end-plate potentials (Ruigt & Van den
Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses.
The duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependent depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butyl-bicyclophosphoro-thionate [35S]-TBPS. Studies with 37
pyrethroids revealed an absolute correlation, without any false
positive or negative, between mouse intracerebral toxicity and in
vitro inhibition: all toxic cyano compounds including
deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin, and
[2S, alphaS]-fenvalerate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin
and [2S,alpha S]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA
receptorionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive muscle
action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids,
is not necessarily due to an exclusive central site of action. It
may be related to the long-lasting repetitive activity in sense
organs and possibly in other parts of the nervous system, which, in
a more advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established
that sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
Trout liver microsomes metabolize both cis- and trans-isomers
of cypermethrin to the 4'-hydroxy derivatives of the intact esters
and their corresponding ether glucuronic acid conjugates at
comparable rates. There are indications, derived from studies with
other pyrethroids, that the liver microsomes of the carp are more
able to metabolize pyrethroids than those of the salmonids (Edwards
& Millburn, 1985b).
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
Leahey (1985) and Smith & Stratton (1986) give extensive
reviews on this matter.
7.1. Microorganisms
Effects of cypermethrin on microbial activity in the soil have
been investigated under laboratory conditions. Cypermethrin was
applied to a sandy loam soil at a rate of 2.5 or 250 mg/kg soil.
No effect was found on the rate of carbon dioxide evolution (Cook,
1978a) or oxygen uptake (Loveridge & Cook, 1978a) at the lower
rate. With 250 mg/kg, slight, but significant, inhibition of the
CO2 evolution and a decrease in oxygen uptake were noted after long
incubation periods. Nitrogen fixation was assessed by the
acetylene reduction method (Loveridge & Cook, 1978b). No
significant effects were found. Ammonification and nitrification
were studied in soils, either alone, or amended with urea or
ammonium chloride. Again, no significant effects were found, even
at the high rate of application (Cook, 1978b). Finally, glucose
utilization was studied in the short term (2 days) and after pre-
incubation with cypermethrin for up to 38 weeks. No significant
effects were noted, even at the high rate (Bromley & Cook, 1979).
A second series of experiments, in which cypermethrin was
applied to a sandy loam at 0.5 or 5 mg/kg soil has been reported.
Antimicrobial activity was observed in the early stages of
incubation with fungal population numbers returning to normal
within 2 - 4 weeks, and with a subsequent stimulation of microbial
growth. There was no inhibition of acetylene reduction activity at
either dosage. Nitrification and microbial respiration were
increased at both dosages, suggesting microbial degradation of
cypermethrin. Dehydrogenase activity was not affected, while
urease activity was stimulated. This study indicates that
cypermethrin may exert transient effects on the populations and
activities of microflora, but they are short-lived and minor in
nature (Tu, 1980).
The toxicity of cypermethrin for 4 pathogens of soybean and for
the nodulation organism Rhizobium has been investigated. In a
paper disc inhibition test, cypermethrin proved non-toxic for
Rhizobium, the EC50 exceeding 10 000 mg/litre. The toxicity for
the pathogens was higher, EC50 values ranging from 390 to 1700
mg/litre (Tu, 1982).
7.2. Aquatic Organisms
7.2.1. Fish
7.2.1.1. Acute toxicity
In common with other pyrethroids, cypermethrin is very toxic
for fish in clean water under laboratory conditions. The available
data are summarized in Table 8. The data demonstrate a similar
high acute toxicity for both cold- and warm-water species of fish.
Certain of these data have been reviewed by Stephenson (1982e).
There is no evidence of a significant effect of temperature on
toxicity, though a negative temperature coefficient has been
claimed for certain pyrethroids (Kumaraguru & Beamish, 1981).
Furthermore, the toxicity of pyrethroids for fish was not
influenced by the hardness or pH of the water (Mauck et al., 1976).
To determine the acute toxicity of cypermethrin for Tilapia
nilotica, an EC formulation of cypermethrin (25 g/litre) was
sprayed on the surface of static water held in stainless steel
tanks in a glasshouse. The concentration of cypermethrin in the
water was determined over a 96-h period. Depending on the
application rate, the concentration of cypermethrin in the water
rose rapidly during the first 24 h after application and decreased
slowly over the following 3 days. The tanks each contained 5 fish
and water to a depth of 30 cm. Water temperatures were 23 - 26 °C.
No fish deaths occurred when the peak concentration of cypermethrin
was less than 1.5 µg/litre, while all fish died when the
concentration exceeded approximately 5 µg/litre (Stephenson,
1981a).
To study the influence of suspended solids on water
concentrations and the toxicity for fish of cypermethrin, Rainbow
trout were exposed to nominal concentrations of 2 or 5 µg
cypermethrin/litre, which was added to microfiltered mains water or
to pond water containing 14.5 mg suspended solids/litre. The
actual concentrations were measured for water samples taken 30 min
after initial mixing. Two samples of pond water were taken. One
was analysed as such while the second was centrifuged (60 min at
3000 rev/min) to precipitate the suspended solids; the clear
supernatant liquid was then analysed. The results are summarized
in Table 9.
The data from this study show that suspended solids absorb 40%
of the cypermethrin initially present. Fish exposed to an actual
concentration of 4.9 µg cypermethrin/litre in mains water died
within 24 h, those in pond water containing an actual concentration
of 2.5 - 4 µg/litre survived (Reiff, 1978a).
The high degree of adsorption of cypermethrin on soils was
demonstrated by Riley & Hill (1983) in their studies on the
toxicity of cypermethrin for Daphnia and other aquatic organisms.
The addition of soil to the system reduced the toxicity of the
aqueous solution by 200 - 350 times.
The stainless steel tank study with Tilapia nilotica was
repeated with the water initially containing 100 mg suspended
solids/litre. In this study too, the presence of suspended solids
reduced the toxic effects of cypermethrin (about 2-fold)
(Stephenson, 1982a).
Table 8. Acute toxicity of cypermethrin for fish
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Brown trout 5 - 8 technical, dispersed via 15 2 - 2.8 Reiff (1976)
(Salmo trutta) DMSO
5 - 8 technical, absorbed on 15 1.2a,b Reiff (1976)
pumice
Rainbow trout 1 - 11 technical, absorbed on 10 - 15 0.5a,b,c Reiff (1978b)
(Salmo gairdneri) pumice
1 - 2 technical, via acetone 10 0.5 Stephenson
(1982b)
3.3 technical, via acetone 15 2.8 Stephenson
(1982b)
3 emulsifiable concentrate 10 11d,g Coats &
(40% ai) O'Donnell-Jeffery
(1979)
3 technical, dispersed via 10 55d Coats &
acetone O'Donnell-Jeffery
(1979)
Common carp 4 - 10 technical, absorbed on 10 0.9a,b,c Reiff (1978b)
(Cyprinus carpio) pumice
4 - 10 technical, absorbed on 20 - 25 1.1a,b,c Reiff (1978b)
pumice
8 - 10 technical, via acetone 10 0.9 Stephenson
(1982c)
2.1 emulsifiable concentrate 22 - 26 3.4f Stephenson
(5% a.i.) (1982c)
Stephenson et
al. (1984)
---------------------------------------------------------------------------------------------------------
Table 8. (contd.)
---------------------------------------------------------------------------------------------------------
Species Weight (g) Vehicle Temperature 96-h LC50 Reference
(or age) (°C) (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Rudd (Scardinius 9 - 10 technical, absorbed on 15 0.4a,b,c Reiff (1978b)
erythrophthalmus) pumice
Atlantic salmon 5.3 technical, dispersed via 10 2 - 2.4b McLeese et al.
( Salmo salar) ethanol (1980)
9.4 1-R- cis- 10 0.74 Zitko et al.
(1979)
Tilapia nilotica 1 - 3 technical, absorbed on 25 2.0a,b,c Stephenson
pumice (1981b)
Stephenson et
al. (1984)
Fathead minnow 0.74 technical, absorbed on 23 - 25 1.2a,b Stephenson
(Pimephales (juvenile) pumice (1982d)
promelas)
Mugil cephalus 0.1 30% EC ? 24g Tag El-Din et al.
30 days (1981)
Gambusia affinis 4 weeks 30% EC 25 6.6g El-Sebae et al.
(1983)
---------------------------------------------------------------------------------------------------------
a Flow-through system. Otherwise, static test.
b Measured concentration. Otherwise, nominal.
c The data quoted have been recalculated from the primary data given in the original reports following
current statistical methods.
d 24-h LC50.
e Lethal threshold value (geometric mean of lowest concentration with and highest concentration without
mortality).
f 48-h LC50.
g Formulation.
Remark: In most tests, the pH of the water was 7.5 - 8.5; the hardness was 260 mg/litre as CaCO3 (except
for a few cases).
Table 9. Influence of suspended solids on the toxicity of cypermethrin
for Rainbow trout
-------------------------------------------------------------------------
Source Suspended Initial cypermethrin Response of Rainbow
solids concentration (µg/litre) trout
(mg/litre) actual
nominal total in water
-------------------------------------------------------------------------
Microfiltered 0 2 1.7 1.7 1/6 fish dead at 48 h
mains water 5 4.9 4.9 6/6 fish dead at 24 h
Pond water 14.5 2 1.2 0.65 no symptoms or deaths
5 4.0 2.5 at 7 days
-------------------------------------------------------------------------
7.2.1.2. Long-term toxicity
The effects of cypermethrin on the most sensitive stage in the
life cycle of the Fathead minnow (Pimephales promelas) were
investigated using a flow-through system. Total hardness, pH,
concentration of dissolved oxygen, and temperature were controlled.
Within 24 h of fertilization, eggs were exposed to nominal
concentrations of 0, 0.03, 0.1, 0.3, or 1.0 µg/litre (mean exposure
concentration 0, 0.03, 0.12, 0.17, and 0.79 µg cypermethrin/litre),
for a total of 34 days. Hatching occurred between the 3rd and 6th
day, while egg hatch was not affected at the highest concentration.
No fry survived day 34. Survival was reduced at concentrations of
0.3 and 0.1 µg/litre but not at 0.03 µg/litre. On the basis of the
most sensitive parameter, i.e., survival of young fry, the no-
observed-adverse-effect level for cypermethrin lay between 0.03 and
0.12 µg/litre (Stephenson, 1982d).
7.2.2. Invertebrates
7.2.2.1. Acute toxicity
Aquatic invertebrates show a wide range of susceptibility to
cypermethrin. The data in Table 10 are from static, clean water
test systems and some of these data have been reviewed by
Stephenson (1982e). It can be seen that snails do not show any
effects at 5 µg/litre (close to the water solubility of
cypermethrin), while some insects show behavioural changes, but no
mortality at this level. Crustacea, particularly marine decapod
Crustacea, are highly susceptible to cypermethrin, mortality
occurring at levels below 0.05 µg/litre. Water mites are also very
susceptible (Zitko et al., 1979).
In addition to these static water tests, continuous-flow tests
in clean water have been undertaken with 2 sensitive species
(Stephenson, 1980b) (Table 11).
It can be concluded that effects can be expected when
concentrations of cypermethrin of the order of 0.01 µg/litre are
maintained in the water phase for more than 96 h.
It has been shown that, at 100 µg/litre, cypermethrin does not
affect the growth of the single-celled green alga Selenastrum
capricornutum, over a period of 2 - 4 days (Stephenson, 1982b).
7.2.2.2. Long-term toxicity
The effects of cypermethrin on the survival, growth, and
reproduction of Daphnia magna were investigated, over 21 days, in
a static water test with daily renewal of test solutions. The
nominal concentrations tested were 0, 0.003, 0.01, 0.03, 0.1, and
0.3 µg/litre. Cypermethrin affected all 3 parameters at a nominal
concentration of 0.3 µg/litre, but no effects were noted at 0.1
µg/litre. Chemical analysis suggested that the Daphnia were
exposed to about 50% of the nominal concentration, two-thirds in
solution, the remainder adsorbed on suspended solids. These
results show that the no-observed-adverse-effect level of
cypermethrin throughout the life cycle for Daphnia is of the order
of 0.05 µg/litre (Garforth, 1982).
7.2.3. Field studies
7.2.3.1. Deliberate overspraying
This subject has received wide attention, and various aspects
have been reviewed in a number of publications (Crossland &
Stephenson, 1979; Crossland, 1982; Crossland et al., 1982;
Crossland & Elgar, 1983; Shires, 1983a; Stephenson, 1983).
The effects of field applications of cypermethrin on fish were
studied in a preliminary study by Crossland & Bennett (1976). A
small pond was deliberately oversprayed with 100 g cypermethrin/ha
as an EC formulation. The peak concentration of cypermethrin in
the water was 2.6 µg/litre. Wild populations of fish and amphibia
were not affected by this treatment. Large numbers of young fish
seen during the 2 weeks following treatment did not appear to be
affected.
In a second study at the same rate of application, the peak
concentration of cypermethrin was 1.4 µg/litre. There was no
observed mortality among 12 small rudd that had been placed in the
pond 12 days before treatment, and no effects of treatment were
noted in wild populations of rudd or newts (Crossland et al.,
1978).
Table 10. Acute toxicity of cypermethrin for aquatic invertebrates in static tests
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Fresh-water
Crustacea
Water flea up to 24 h 22 acetone 4.2 Reiff (1977)
(Daphnia magna) old 18 water 2 2 Stephenson (1980a)b
20 acetone 1.2 - Stephenson (1982b)
20 acetone 0.3c - Stephenson (1982b)
Water hog louse 3 - 8 mm 15 water 0.02 0.2 Stephenson (1980a)
(Asellus spp.)
Freshwater shrimp 3 - 8 mm 15 id 0.04 0.1 Stephenson (1980a)
(Gammarus pulex)
Insecta
Mayfly larvae 15 id 0.07 0.6 Stephenson (1980a)
(Cloeon dipterum)
Whirligig beetle adult 15 id 0.07 > 5 Stephenson (1980a)
(Gyrinus natator)
Bloodworm larvae 15 id 0.2 > 5 Stephenson (1980a)
(Chironomus thummi)
Mosquito larvae 18 id 0.03 1 Stephenson (1980a)
(Aedes aegypti)
Midge (Chaoborus larvae 15 id 0.03 0.2 Stephenson (1980a)
crystallinus)
Water boatman adult 15 water 0.7 > 5 Stephenson (1980a)
(Corixa punctata)
Water boatman adult 15 id 0.3 > 5 Stephenson (1980a)
(Notonecta spp.)
---------------------------------------------------------------------------------------------------------
Table 10. (contd.)
---------------------------------------------------------------------------------------------------------
Species Stage Temperature Solvent 24-h EC50 24-h LC50 Reference
(°C) (µg/litre)a (µg a.i./litre)
---------------------------------------------------------------------------------------------------------
Arachnida
Water mite adult 15 0.02 0.05 Stephenson (1980a)
(Piona carnea)
Mollusca
Snail (Lymnaea < 8 mm 15 id > 5 > 5 Stephenson (1980a)
peregra)
Marine
Crustacea
Lobster (Homarus 450 g 10 ethanol - 0.04g McLeese et al.
americanus) (1980)
450 g 10 id - 0.003d,f,g Zitko et al. (1979)
Shrimp (Crangon 1.3 g 10 id - 0.01d,e McLeese et al.
septemspinosa) (1980)
---------------------------------------------------------------------------------------------------------
a EC50 values are based on effects, usually immobilization, other than death.
b Stephenson (1980a) used cypermethrin (85% a.i.) dissolved in acetone and absorbed on pumice.
c 48-h EC50.
d 96-h LC50.
e Dispersed via ethanol.
f 1-R- cis-.
g Salinity, 30%.
Remark: In most tests, the pH of the water was 7.5 - 8.5; and the hardness, 260 mg/litre as CaCO3.
Table 11. Acute toxicity of cypermethrin for aquatic invertebrates in
continuous-flow tests
------------------------------------------------------------------------
Species Stage Temperature EC50 LC50
size (°C) (µg/litre) (µg/litre)
24-h 96-h 24-h 96-h
------------------------------------------------------------------------
Freshwater shrimp 3 - 8 mm 15 0.005 0.004 0.030 0.009
(Gammarus pulex)
Mayfly larvae 15 0.008 0.004 0.070a 0.020
(Cloeon dipterum) 0.5 - 1 cm
------------------------------------------------------------------------
a 48-h LC50.
Observations on invertebrates were included in these two pond
studies. In the first study, which lasted 2 weeks, populations of
Crustacea, mites, and insects were severely reduced. Surface
breathing insects were affected most rapidly, within hours of
treatment. Free-swimming dipterous larvae were not noticeably
affected for 24 h, while zooplankton were killed between 1 and 2
days after treatment. Bottom dwelling invertebrates, including
chironomid larvae, snails, leaches, and flatworms, did not appear
to be affected, though the numbers in the last 2 groups were low in
pre-treatment samples.
The second study was continued for 15 weeks after treatment.
Initial results were similar to those reported above. Macro-
invertebrates were markedly reduced in numbers, 2 weeks after
treatment. However, both numbers and diversity returned to normal
levels after 15 weeks. Snails and flat-worms (again numbers low in
this group in pretreatment counts) were unaffected, but no
arthropods were present in the samples taken at 2 weeks.
Recolonization by flying insects (beetles and chironomids)
commenced 4 weeks after treatment. The Crustacean Asellus had not
reappeared by the end of the study.
No daphnids or copepods were found in the zooplankton samples,
1 week after treatment, and they only reappeared in the 8-week
post-treatment sample. Populations returned to normal levels in
10 - 12 weeks. Some 2 weeks after treatment, an increase in
filamentous algae was noted, and this persisted until the end of
the study. It was inferred that this was a secondary effect
following from the elimination of known feeders on algae, for
instance, the mayfly, Cloeon dipterum, and the daphnid,
Simocephalus sp. (Crossland & Bennett, 1976; Crossland et al.,
1978).
7.2.3.2. Monitoring of drift from ground and aerial applications
Two field studies were undertaken to assess the fate and
effects in adjacent waters of spray drift from ground-based
agricultural applications of cypermethrin.
(a) Mistblower applications on vines (France)
Three sites were chosen, each with vines planted up to the
banks of adjacent streams. One application of cypermethrin was
monitored at each site and a second application at one of the
sites. A diluted EC formulation of cypermethrin was applied at
30 g a.i./ha using a backpack, swinging-head mistblower, and at
45 g a.i./ha using a tractor-mounted, fixed-head mistblower.
The deposition on the ground and an adjacent stream of 0.37 -
4.5 g/ha led to peak concentrations in the subsurface water ranging
from 0.17 to 1.7 µg/litre during the first hour after spraying; the
concentrations decreased to less than 0.1 µg/litre within a few
hours. No effects on fish, tadpoles, or frogs were observed. Some
aquatic invertebrates in adjacent streams were seen to be
hyperactive or immobilized during the first 2 h after application.
This was most obvious with mayfly larvae, water boatmen, water
beetles, pond skaters, and syrphid larvae. During the first 3 h
following application, there was an increase in the number of
invertebrates caught in drift nets. However, there were no
significant population effects in zooplankton or macro-
invertebrates (Bennett et al., 1980).
(b) Boom-and-nozzle applications to row crops (United Kingdom)
Two sites were chosen each with row crops (potatoes or
sugarbeet or both) planted up to within 1 - 3 m of the edge of
ponds. Two applications of cypermethrin were monitored at each of
3 ponds. In each case, a diluted EC formulation of cypermethrin
was applied at 70 g a.i./ha using tractor-mounted, boom-and-nozzle
equipment. Deposition on the ground and over the pond was
estimated. The deposition led to peak concentrations in subsurface
water in the range of 0.01 - 0.05 µg/litre. In one case, the
concentration in subsurface waters after 24 h was 0.02 - 0.03
µg/litre. In all other cases, this value was at, or below, 0.01
µg/litre, the limit of detection. No effects on fish were
observed. No residues of cypermethrin were found in fish (limit of
detection, 5 µg/kg wet weight). The only effects on invertebrates
in adjacent waters were noted in the corner of 1 pond. Some pond
skaters, one water-boatman, and 2 syrphid larvae were found
immobilized. They had recovered by the next day. There was no
evidence from zooplankton or sweep-net samples of any effects on
invertebrate populations (Shires et al., 1980).
(c) Aerial application to winter wheat (United Kingdom)
A large field of winter wheat was chosen, which was surrounded
on 3 sides by drainage ditches. An EC formulation of cypermethrin
was applied at 25 g a.i./ha by fixed-wing aircraft. Up to 6% of
the nominal dose was deposited on the water surfaces, resulting in
maximum levels in subsurface waters of 0.03 µg/litre, which
declined rapidly after spraying. No effects were observed on caged
or wild fish, and no significant residues of cypermethrin were
found in the fish. A few air-breathing water boatmen and water
mites, which are very susceptible, showed minor short-term
reductions in abundance (Shires & Bennett, 1982, 1985).
(d) Application for rice insect control (Korea, Spain)
An EC formulation of cypermethrin was applied to rice paddies
in Korea, in which carp had been caged, by hand-spraying at 15 or
40 g a.i./ha. Mortality at the higher rate was significantly
higher than that in the control, i.e., 15 and 7%, respectively
(Stephenson, 1982c).
Another field study was carried out in Spain. Cypermethrin was
applied by air to paddy rice and also to caged fish (Cyprinus
carpio) within the area. The application rate was 15 - 40 g
a.i./ha. The limited toxic effects of cypermethrin on fish under
field conditions were confirmed in this study. Aerial overspraying
of the rice paddies at 25 g a.i./ha did not produce any mortality
(Stephenson et al., 1984).
(e) Applications for tsetse fly control (Nigeria)
Field studies have been reported on the assessment of the
environmental impact of the application of cypermethrin for tsetse
fly control in Nigeria. Searches for affected fish were made in
areas that had been selectively sprayed from the ground with an EC
formulation of cypermethrin at 2 - 3 g a.i./litre, or sprayed from
the air with an oil-based solution of cypermethrin at 100 g a.i./ha
in 1977 and at 60 and 150 g a.i./ha in 1978. The ground spray
application was successful in controlling the tsetse fly, but only
the highest rate applied from the air approached a satisfactory
level of tsetse control. None of these searches revealed any dead
fish in the rivers within the areas sprayed with cypermethrin.
Pre- and post-treatment net samples of aquatic invertebrates were
taken in an area sprayed at 100/150 g a.i./ha. Acute mortality
occurred in terrestrial and aquatic arthropods, such as the water
beetle and Crustaceans. Shrimps and mayfly larvae disappeared from
river benthos after spraying, but reappeared one year later (Smies
et al., 1980).
7.3. Terrestrial Organisms
7.3.1. Laboratory studies
7.3.1.1. Acute toxicity
(a) Birds
The acute oral toxicity of cypermethrin for birds is summarized
in Table 12.
In the first study (Coombs et al., 1976), 4 birds of each
species were observed for a 3-week period after dosing; no deaths
or toxic signs were noted at the highest dosages applied. In the
second study (Rose, 1981), clinical signs of cypermethrin
intoxication, including ataxia and lethargy, were noted at dosages
of 3000 mg/kg body weight and above. One male duckling, out of 28
of each sex, died at the top dosage of 10 000 mg/kg body weight.
7.3.1.2. Short-term toxicity
(a) Birds
Six laying hens were given 5 successive daily oral doses of
1000 mg cypermethrin/kg body weight in DMSO followed after 3 weeks
by a second 5-day dosing regime and were observed for a further 3
weeks. Control hens did not receive any treatment. No signs of
intoxication or histological changes in nervous tissue were noted
at any time (Owen & Butterworth, 1977).
Table 12. Acute oral toxicity of cypermethrin for birds
--------------------------------------------------------------------------
Species Age Vehicle Observation LD50 Reference
period (mg/kg
body
weight)
--------------------------------------------------------------------------
Domestic fowl adult 40% DMSO 21 days > 2000 Coombs et al.
(Gallus (1976)
domesticus)
French partridge adult 40% DMSO 21 days > 3000 Coombs et al.
(Allectoris (1976)
rufa)
Mallard duck 2 - 6 40% emul- 14 days > 10 000 Rose (1981)
(Anas months sifiable
platyrhynchos) concentrate
--------------------------------------------------------------------------
Cypermethrin was administered for 5 consecutive days to Mallard
ducks at dosages of 5000 or 10 000 mg/kg diet. No deaths or
clinical signs of intoxication were noted during the feeding period
or over a subsequent 40-day holding period. Food intake and body
weights were slightly depressed during the feeding period, though
most ducks had regained or exceeded their initial body weights by
the end of the holding period (Rose, 1981).
Riviere et al. (1983) studied the influence of cypermethrin on
the microsomal drug metabolizing enzymes in Japanese quail
(Coturnix coturnix). Cypermethrin had no or only a very weak
inducing effect.
(b) Honey bees
Cypermethrin was highly toxic for worker honey bees (Apis
mellifera) in laboratory tests (24-h oral LD50 0.035 µg a.i. per
bee) (Table 13). Cypermethrin applied on the dorsal side of the
thorax was more toxic for honey-bees at lower breeding
temperatures, i.e., at 12 - 20 °C, the toxic level was 0.01 - 0.02
µg/bee and at 32 °C, 0.02 - 0.03 µg/bee. In addition, the
sensitivity of the bees to cypermethrin increased with age (Delabie
et al., 1985).
An aqueous dispersion of cypermethrin was sprayed directly on
worker honey bees. Only 5% of the bees sprayed with a 0.01
g/litre solution were killed. All bees sprayed with 0.1 and 1
g/litre died (Harris & Turnbull, 1978).
First indications that cypermethrin might not be hazardous for
bees under field conditions were obtained in cage tests. Worker
honey bees were exposed for 2 h to residues produced by treating
flowering Solidago (Golden rod) to run-off with a spray containing
0.5 g cypermethrin/litre and aging the residue for 1 h. Some
knock-down of the bees (up to 9%) was noted during the first 8 h
following initial exposure. However, most bees recovered, and
mortality after 1 day and 10 days did not differ from that in
untreated control bees. Higher mortality had been noted in an
earlier study using the same technique but with a higher dosage
rate of 1.5 g cypermethrin/litre (Gerig, 1979, 1981).
Table 13. Toxicity of cypermethrin for worker honey bees
-------------------------------------------------------------------------
Formulation 24-h LD50 (mg/bee) Reference
Topical application Oral administration
-------------------------------------------------------------------------
Technical 0.02 0.035 Badmin & Twydell
(1976)
Technical 0.053 0.12 Knight (1982)
Technical 0.056 - Smart & Stevenson
(1982), Westlake
et al. (1985)
Emulsifiable - 0.031 Badmin & Twydell
concentrate (1976)
(40%)
-------------------------------------------------------------------------
(c) Predatory and parasitic species
Cypermethrin has also been evaluated against a number of
predatory or parasitic insects and mites. Laboratory data are
available for a number of predators (Coleoptera) and parasites
(Hymenoptera) of insect pests.
The available data indicate that cypermethrin can show useful
selectivity between insect predators and parasites and the relevant
pest species (Mulla et al., 1978; du Toit, 1978; Waddill, 1978;
Abdel-Aal et al., 1979; Coats et al., 1979; Holden, 1979; Leake et
al., 1979; McDonald, 1979; European Patent Office, 1980; Harris &
Turnbull, 1980; Hagley et al., 1981; Jordan & Chang, 1981; Saad et
al., 1981; Ascher et al., 1982; Baicu, 1982; Bayoumi, 1982; Chang &
Jordan, 1982; Osman et al., 1982; Rajakulendran & Plapp, 1982;
Surulivelu & Menon, 1982; Brempong-Yeboah et al., 1983; Chang &
Jordan, 1983; El-Guindy et al., 1983; El-Minshawy et al., 1983;
El-Sebae et al., 1983; Ho et al., 1983; Ishaaya et al., 1983;
Watters et al., 1983; Abbassy et al., 1984; Bariola & Lingren,
1984; Brempong-Yeboah et al., 1984a,b,c; Cheng & Hanlon, 1984; Dai
& Sun, 1984; El-Sayed & Knowles, 1984; Ewen et al., 1984; Fabellar
& Heinrichs, 1984; Hopkins et al., 1984; Liu et al., 1984; Mani &
Krishnamoorthy, 1984; Meisner et al., 1984; Le Patourel & Singh,
1984; Riskallah, 1984; Scott & Georghiou, 1984; Wilde et al., 1984;
Zohdy et al., 1984; Ahmed et al., 1985; Abou-Awad & El-Banhawy,
1985; Bostanian & Belanger, 1985; Bostanian et al., 1985; Corbitt
et al., 1985; Edwards et al., 1985; El-Sebae et al., 1985; Harris
et al., 1985; Knapp et al., 1985; Knowles & El-Sayed, 1985; McKee &
Knowles, 1985; Pree & Hagley, 1985; Suhas & Devaiah, 1985; Tewari &
Krishnamoorthy, 1985; Vekaria & Vyas, 1985; Barlow et al., 1986).
(d) Earthworms
The toxicity of cypermethrin for the earthworm (Eisenia
foetida) has been assessed in an artificial soil test system. Worms
were exposed to dosages of 0, 0.1, 1.0, 10, or 100 mg/kg soil for
14 days. No mortality was found (Inglesfield & Sherwood, 1983;
Inglesfield, 1984).
The LC50 for cypermethrin in Eisenia foetida was found to be
26.1 µg/cm2 (16.3 - 44.4 µg/cm2) (Roberts & Dorough, 1984).
(e) Higher plants
In studies on effects of cypermethrin on seed germination in
the soybean, seedling emergence and survival were not reduced by
levels of up to 5000 mg/litre (Tu, 1982). Cypermethrin has been
demonstrated not to be phytotoxic for crops when applied at
recommended dosages (Hargreaves & Cooper, 1979).
7.3.2. Field Studies
7.3.2.1. Applications for tsetse fly control in Nigeria
Field trials in Nigeria of cypermethrin against Tsetse flies
( Glossina palpalis (RD) and Glossina tachnoides Westw.) were
carried out, over a 2-year period by Spielberger et al. (1979).
Ground applications of cypermethrin (0.3%) were made on fly-resting
sites in vegetation using pressurized knapsack sprayers.
Populations of both species were eradicated after a single
application. Levels of over 150 g/ha were needed for complete
eradication by residual spraying from a helicopter.
The effects on the general insect population of aerial
applications of cypermethrin for tsetse fly control were studied by
Smies et al. (1980). Within a few hours of application, dead and
dying insects were found, exemplifying the rapid knockdown effect
of cypermethrin. Insect fallout, as assessed by funnel traps, was
markedly increased for 1 day by 100 g cypermethrin a.i./ha, and for
3 days by 150 g a.i./ha. General insect activity as assessed by
Malaise trap catches was not affected by 150 g cypermethrin a.i./ha
(Smies et al., 1980).
7.3.2.2. Honey bees
This subject has been reviewed by Shires & Debray (1982) and
Shires (1983b).
The hazard of practical field applications of cypermethrin for
worker honey bees was assessed in 2 field trials. In both trials,
cypermethrin was applied to flowering oilseed rape, by helicopter,
at a time when the crops were being actively worked by bees from
nearby colonies, thus representing a worst case exposure of the
bees. The rate of application in both trials was 25 g a.i./ha. Of
the 8 hives, placed adjacent to the treated crops, 6 had been
fitted with dead bee traps and 2 with pollen traps. Observations
were made on bee mortality, pollen collection, foraging activity,
hive populations, and brood areas. In addition, in the second
trial, cypermethrin residues were determined in the bees, pollen,
wax, honey, and in the leaves and flowers of the treated crop
(Pearson & Shires, 1981; Shires, 1982b).
In the first trial, only a small increase in bee mortality was
noted, following the cypermethrin treatment (Table 14).
Cypermethrin appeared to be repellent to honey bees foraging the
crop, but the duration of this effect could not be established
because of poor weather during the first few days following
treatment. Cypermethrin did not have any effects on hive
populations of adult bees or brood areas.
In the second trial, a slight increase in bee mortality was
found at the time of treatment (Table 14). Cypermethrin had a
repellent effect on honey bees for up to 24 h after application.
Following this period, foraging activity and pollen collection
returned to normal. Cypermethrin residues in dead bees, collected
on the day of spraying and on the following morning, were higher
than would be expected to be lethal. However, residue levels in
dead bees collected after this period were considerably lower than
those required to produce toxic effects, confirming that they
represented the natural mortality level. Very low levels of
cypermethrin were found in live bees and levels in honey and wax
were close to the limit of detection. Concentrations of
cypermethrin in flowers and pollen declined rapidly after
treatment. Again, cypermethrin did not have any effects on hive
populations of adult bees or of brood areas (Shires, 1982b).
Table 14. Mean number of dead bees per hive during large-scale
field trials with cypermethrina
-----------------------------------------------------------------
Treatment Time from treatment
compound -2 to -1 -1 to 0 0 to +4 +1 to +2 +3 to +4
(dosage) days days h days days
-----------------------------------------------------------------
Winter-sown rape; 65 6 260 40 2
cypermethrin
(25 g a.i./ha)
water control 75 17 0 26 15
Spring-sown rape 9 36 310 2 13
cypermethrin
(25 g a.i./ha)
-----------------------------------------------------------------
a From: Shires (1983b).
In glasshouse studies, in which insecticide treatment was
carried out during foraging by spraying the rape with 0.12 ml of
Cymbush in 100 ml water (equivalent to 50 g a.i./ha), high
mortality was observed 2 days after treatment, but no residual
mortality appeared during the following 2-month period. In this
study, oilseed rape flowers were not attractive to bees for at
least 2 days following treatment.
A field test was carried out on a 38-ha field of oilseed rape.
The insecticide was sprayed on a central area of 13 ha during the
morning, at a rate of 50 g a.i./ha. The weather was sunny and the
temperature about 18 °C. A high level of mortality occurred only
on the 3 days following treatment. The results also showed that the
bees avoided visiting the flowers as soon as the treatment was
made, especially during the first 2 days. From the third day after
the treatment, the repellent effect decreased, and the visits to
the rape flowers increased reaching normal on the fifth day. It
was suggested by the authors that the repellent effect appeared to
be due to the formulation ingredients.
No residues of cypermetherin were found in hive products
(pollen, wax, or honey) (Delabie et al., 1985).
In a study by Gerig (1981), cypermethrin was repellent to
worker honey bees for a short period after spraying. Flowering
Phaselia plants, within a flight tent, were treated with a spray
containing 0.5 g cypermethrin/litre. Honey bee visits to the
treated flowers were very few over the first 0.5 h following
treatment and remained at a reduced level during the remaining
5.5 h of observation on the day of treatment. Flower visits
returned to normal levels on the following day (Gerig, 1981).
7.3.2.3. Soil fauna
This topic has been reviewed by Shires (1980).
In an initial study on the effects of cypermethrin on the soil
surface fauna and earthworms, cypermethrin was applied at 100 g
a.i./ha to small plots of spring wheat. This treatment did not
have any effect on earthworm populations or on leaf litter
breakdown, which is an indirect indicator of earthworm activity.
The general population of soil surface predators, mainly beetles
and spiders, was reduced to about 50% of the control value soon
after treatment, but increased to a higher level than that in the
controls after 5 weeks. A subsequent secondary decrease in
predator populations was probably related to the efficiency of
control of cereal aphids by cypermethrin (Shires et al., 1979). In
a second study, cypermethrin was applied at 25 g a.i./ha to newly-
emerged winter barley to control the aphid vectors of barley yellow
dwarf virus. Populations of non-target arthropods were assessed
using pitfall and water traps. Populations of soil Collembola
were only slightly affected by cypermethrin. Populations of
predators (beetles, spiders, and centipedes) were already declining
because of the season, but there were significantly fewer predators
in the cypermethrin plots compared with the controls at the end of
the trial. Cypermethrin treatment did not affect the numbers of
invertebrates collected from the water traps (Sherwood & Shires,
1981).
Two studies have been reported on the summer application of
cypermethrin for aphid control in winter wheat. In France, ground
applications of cypermethrin at 50 and 75 g a.i./ha were made in
June and effects on the fauna in the air, on the crop, and on the
soil surface were studied. Cypermethrin proved effective against
the phytophagous (pest) species. Effects on non-target organisms
were only minor, with the exception of spiders. In the second
study in the United Kingdom, cypermethrin was applied in June using
a fixed-wing aircraft at 25 g a.i./ha. Again, cypermethrin proved
effective against the pest species, while, generally, effects on
non-target organisms were minor and short-lived (Shires, 1982a,c).
Similar results have been obtained in studies on the aerial
application of cypermethrin at 25 g a.i./ha on oilseed rape and at
50 and 75 g a.i./ha on maize, in France (Inglesfield, 1982;
Garforth, 1983).
7.3.2.4. Foliar predators and parasites
The relative toxicity of cypermethrin for pests and their
parasites and predators is such that the balance between host/prey
and parasites/predators may not be adversely affected in the field.
However, care should be taken where predatory mites are important
in pest management. The high toxicity of cypermethrin for
predatory mites has been confirmed under field conditions (Wong &
Chapman, 1979; Aliniazee & Cranham, 1980; Shires & Tipton, 1982).
The effects of cypermethrin treatments, included in a spray
programme for cotton insect control, on white fly parasitism have
been studied in the Sudan. The treatment regime that gave the
consistently highest level of control of parasitism included
cypermethrin at 40 g a.i./ha in 4 early season sprays (Shires &
Tipton, 1982).
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Leahey (1985) and Smith & Stratton (1986) have reviewed the
available literature on this subject.
8.1. Single Exposures
8.1.1. Oral
The acute oral toxicity of cypermethrin (mixture of cis-/ trans-
isomers) in experimental animals is of a moderate order (Tables 15,
16). Signs of intoxication, indicative of an action on the central
nervous system consist of sedation, ataxia, splayed gait, tip-toe
walk, with occasional tremors and convulsions. These signs of
toxicity appear within a few hours following dosing, and survivors
show clinical recovery within 3 days (Coombs et al., 1976).
Table 16 shows the effects of the ratio of cis-: trans-isomers
on the acute oral toxicity (FAO/WHO, 1980b).
Factors known to influence the oral LD50 value of cypermethrin
include concentration, vehicle, temperature, age of the animals,
and the animal strain used (Coombs et al., 1976; Dewar & Owen,
1979). The acute toxicity of cypermethrin was approximately 3
times greater in 3-week-old, than in 12-week-old rats (Rose &
Dewar, 1978). The cis-: trans-ratio plays a role in determining
acute toxicity, the cis-isomers being more toxic than the trans-
isomers. The oral LD50 of the cis-isomers in rats (Carworth Farm
E strain) was 229 mg/kg body weight while, with the trans-isomers,
no deaths were found at 2000 mg/kg body weight (Brown, 1979a,b).
8.1.2. Dermal
The acute dermal toxicity of technical cypermethrin is of a low
order. There were no deaths in CFE rats at any dose level tested
up to 1600 mg/kg body weight using 20% w/v xylene as the vehicle
(Coombs et al., 1976). The dermal LD50 for the cis-isomer of
cypermethrin, applied to the skin of rats as a 10% solution in
DMSO, was 219 mg/kg body weight (Brown, 1979a).
The acute dermal toxicity of cypermethrin for the rabbit
is > 2460 mg/kg body weight (US EPA 1984).
8.1.3. Intraperitoneal
The intraperitoneal (ip) LD50 (20% w/v solution in corn oil)
for technical cypermethrin in CFI mice is 485 mg/kg body weight
(Coombs et al., 1976). In rats, the LD50 is between 198 and 315
mg/kg body weight (administered as 5% solution in DMSO) (Price,
1981a).
Table 15. Oral LD50 values for technical cypermethrin
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
-----------------------------------------------------------------------
rat 5% in corn oil 251 (203 - 295) Coombs et al.
(1976)
rat 5% in dimethylsulf- 303 (277 - 329) Coombs et al.
oxide (1976)
rat 5% in dimethylsulf- 570 (485 - 823) Price (1981a)
oxide
rat 40% in dimethylsulf- approximately 4000 Rose (1982)
oxide
rat 5% in glycerol 200 - 400 (male); Coombs et al.
formal approximately 200 (1976)
(female)
rat 10% in aqueous 400 - 800 (male); Coombs et al.
suspension approximately 400 (1976)
(female)
rat 50% in aqueous 3423 (2815 - 4328)a Rose (1982)
suspension
mouse 5% in corn oil 82 (68 - 116) Rose (1982)
mouse 5% in dimethylsulf- 138 (105 - 199) Coombs et al.
oxide (1976)
mouse 50% in aqueous 657 (439 - 1003) Rose (1982)
suspension
Syrian 10% in corn oil approximately 400 Coombs et al.
hamster (male and female) (1976)
Chinese 5% in corn oil 203 (144 - 255) Coombs et al.
hamster (1976)
guinea- 20% in corn oil approximately 500 Coombs et al.
pig (male); > 1000 (1976)
(female)
bovine 15% formulation in 142 - 284 (male) Cassidy (1979)
calves Shellsol A
piglets 15% formulation in > 600 (male) Cassidy (1979)
Shellsol A
-----------------------------------------------------------------------
Table 15. (contd.)
-----------------------------------------------------------------------
Species Concentration and LD50 value (mg a.i./ Reference
vehicle kg body weight) (with
95% confidence limits)
----------------------------------------------------------------------
lamb 15% formulation in 283 - 567 (male) Cassidy (1979)
Shellsol A
domestic 40% in dimethylsulf- > 2000 Coombs et al.
fowl oxide (1976)
partridge 40% in dimethylsulf- > 3000 Coombs et al.
oxide (1976)
-----------------------------------------------------------------------
a 40:60 cis/trans.
Table 16. Effects of cis-: trans-ratio on the acute toxicity of
cypermethrina
------------------------------------------------------------------
Species Sex Vehicle cis-: trans-ratio LD50 (mg/kg)
------------------------------------------------------------------
rat male, dimethylsulfoxide cis-only 160 - 300
female
rat male, dimethylsulfoxide trans-only > 2000
female
rat female corn oil 90:10 367
rat female corn oil 40:60 891
------------------------------------------------------------------
a From: FAO/WHO (1980b).
8.1.4. Inhalation
Groups of CD rats (4 of each sex) were exposed for 4 h to an
aqueous spray of cypermethrin as a 400 g/litre emulsifiable
concentrate. The liquid phase contained 3 g cypermethrin/litre and
the atmospheric concentration of droplets in the vicinity of the
rats was calculated to be 0.7 mg/litre. The median droplet size
was 130 µm with a geometric standard deviation of 1.6 µm,
comparable with the droplet spectrum of a field-spraying device.
(It should be noted that this droplet size is nearly not
inhalable). The rats became thoroughly soaked during the exposure,
thereby indicating a significant dermal exposure with probable oral
exposure during post-exposure grooming. Immediately after the
exposure, the female rats showed the abnormal gait and urinary
incontinence typical of pyrethroid intoxication. Recovery took
place within 3 days. No deaths or other adverse signs occurred
during the exposure period or the subsequent 14-day observation
period (Blair & Roderick, 1976).
In another study on possible effects on the peripheral nerves,
rats (8 of each sex) were exposed for 4 h to an aqueous spray of
cypermethrin as a 400 g/litre emulsifiable concentrate under
conditions similar to those in the previous study. Half of the
rats were necropsied immediately after exposure and the others
after 66 h. Examination of the sciatic nerves from all of the rats
did not reveal any abnormalities, even though signs of intoxication
had been observed (Blair et al., 1976).
8.1.5. Skin and eye irritation
Technical cypermethrin was moderately irritant to occluded
rabbit skin (both intact and abraded) and to the eyes of New
Zealand white rabbits. In both cases, recovery was complete in a
few days. The severity of the irritation depended on the
formulation and the solvent used (Hine & Zuidema, 1970; Coombs et
al., 1976).
The skin on the backs of guinea-pigs was treated with
cypermethrin. The animals responded by licking, rubbing,
scratching, or biting the sides tested. This reaction may be
associated with skin sensory effects characterized by transient
itching and tingling sensations (Cagen et al., 1984).
8.1.6. Sensitization
A skin sensitization test on guinea-pigs (maximization
procedure of Magnusson & Kligman) indicated that technical
cypermethrin may have a mild skin sensitizing potential (Coombs et
al., 1976; US EPA, 1984).
8.2. Short-Term Exposures
8.2.1. Oral
8.2.1.1. Rat
Groups of 6 male and 6 female Charles River rats were fed diets
containing 0, 25, 100, 250, 750, or 1500 mg cypermethrin/kg feed
for 5 weeks. In the 1500 mg/kg group, reduced body weight gain and
food intake, piloerection, nervousness, incoordinated movement,
increased liver weight, and increases in blood urea and haemoglobin
concentrations were observed, but there were no pathological
changes. No changes were detected in the groups receiving 750
mg/kg or less (Coombs et al., 1976).
Groups of Charles River rats, 12 of each sex (24 of each sex as
controls), were fed dietary concentrations of 0, 25, 100, 400, or
1600 mg cypermethrin/kg feed for 3 months. Signs of intoxication,
such as hypersensitivity and abnormal gait, were observed in the
1600 mg/kg group, during the first 5 weeks, and one animal died.
The survivors showed clinical recovery in the second half of the
study. However, body weight gain was reduced, liver and kidney
weights increased, and there were increases in plasma-urea
concentrations and plasma-alkaline phosphatase activity, and
decreases in haemoglobin concentrations and the red blood cell
count in females, and in the kaolin cephalin clothing time in males
and packed cell volume were observed. Two out of 4 male rats,
killed prematurely, showed axonal breaks and vacuolization of
myelin in the sciatic nerve. None of the survivors showed nerve
lesions, and no other pathological effects were found. In the
400 mg/kg group, males showed increased kidney weights but no
histopathological changes. No effects were found in the 100 mg/kg
group (Hend & Butterworth, 1976).
Male and female rats (20 in each group) were fed cypermethrin
( cis-: trans- = 44:56) with a purity of 92% in the diet at levels
of 0, 75, 150, or 1500 mg/kg diet, for 90 days. Haematology and
the results of urinalysis were normal. Both males and females
receiving 1500 mg/kg diet showed reduced body weight and reduced
food consumption during the first month of the study. Increased
liver microsomal oxidase activity was noted in both males and
females in the 1500 mg/kg group and in the males in the 150 mg/kg
group. These changes were substantially reversed within the 4-week
recovery period. Gross and microscopic (including electron
microscopic) examination of the tissues and organs did not reveal
any signficant differences between the treated groups and the
controls. Examination of the sciatic nerve of animals in the
control and the 1500 mg/kg groups did not reveal any changes that
could be directly attributed to cypermethrin (Glaister et al.,
1977b).
Groups of rats (6 male and 6 female rats per group and 14 of
each sex as controls) were fed trans-isomer at concentrations of
0, 30, 100, 1000, or 3000 mg/kg diet, for 5 weeks. No gross or
microscopic findings were observed and mortality did not occur.
Changes in several haematological parameters, alkaline phosphatase
activity, and liver, spleen, and kidney weight were observed at the
2 highest dose levels. Special histopathological studies of the
sciatic nerve did not show any damage (Hend & Butterworth, 1977a).
Groups of rats (6 male and 6 female rats per group and 10 of
each sex used as controls) were fed concentrations of the cis-
isomer at 0, 30, 100, 300, 750, or 1500 mg/kg diet for 5 weeks
(protocol similar to that described above). Mortality was observed
at the 1500 mg/kg level as well as neurotoxic signs of poisoning.
Growth was reduced at the 750 mg/kg level. Significant reductions
in food intake were also noted at doses of 300 mg/kg or more,
during the initial phase of the study. Relative kidney weights
showed a statistically-significant increase at doses of 300 mg/kg
or more and an increase in liver weight was observed at 750 mg/kg.
Histopathological examinations revealed substantial degeneration
in both the liver and sciatic nerve at 1500 mg/kg. No lesions were
observed in the brain or spinal cord (Hend & Butterworth, 1977b).
8.2.1.2. Dog
Beagle hounds (4 of each sex per dose level) were fed diets
containing 0, 5, 50, 500, or 1500 mg cypermethrin/kg diet for 13
weeks. At 1500 mg/kg, severe signs of intoxication consisting of
diminished food intake, weight loss, diarrhoea, anorexia, licking
and chewing of the paws, whole body tremors, a stiff exaggerated
gait, ataxia, incoordination, and hyperaesthesia were observed.
Half of the dogs in this group were necropsied because of severe
clinical signs of intoxication. However, no changes in
haematology, organ weight, or histopathology were observed and
despite the severe signs of intoxication, no lesions of the sciatic
nerves were observed in the dogs. No effects were seen at 500
mg/kg feed (Buckwell & Butterworth, 1977).
8.2.2. Dermal
8.2.2.1. Rabbit
Groups of 10 male and 10 female New Zealand white rabbits
received occluded dermal applications (abraded and non-abraded
skin) of 2, 20, or 200 mg cypermethrin/kg body weight in
polyethylene glycol (PEG 300) for 6 h per day, 5 days per week,
for 3 weeks. A control group was similarly treated with the
vehicle only (PEG 300). Slight-to-moderate skin irritation was
observed in rabbits receiving 2 and 20 mg/kg body weight; those
receiving 200 mg/kg body weight showed slight-to-severe irritation.
In the rabbits treated at 200 mg/kg, reductions were observed in
food intake, body weight gain, and weight of gonads. No other
changes were noticed. No such effects were found at 20 mg/kg body
weight (Henderson & Parkinson, 1981).
8.2.3. Intraveneous
8.2.3.1. Rat
Lock & Berry (1981) found biochemical changes in the rat
cerebellum after a single iv administration of 25 mg
cypermethrin/kg body weight. Signs of toxicity included:
salivation, clonic convulsions, and sinuous writhing movements.
The concentrations of cerebellar amino acids and cyclic nucleotides
were determined at these stages of toxicity. Treatment with
cypermethrin resulted in an increase in cerebellar cyclic GMP at
the earliest stage, without changing cyclic AMP. Levels of blood-
and cerebellar-glucose, lactate, and ammonia were also increased in
most of the stages of toxicity. No changes were seen in
concentrations of glutamate, glutamine, gamma-aminobutyrate,
creatine, phosphate, or ATP in the cerebellum. The increase in
cerebellar cyclic GMP did not appear to be related to the
convulsive state of the animal or to the muscarinic cholinergic
stimulation.
8.3. Long-Term Exposures
8.3.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) were fed dietary concentrations of 0, 1, 10, 100, or
1000 mg cypermethrin/kg diet for 2 years. Additional groups (6 or
12 rats of each sex per dose level) were fed the diets for only 6,
12, or 18 months.
Throughout the 2-year study, the control and cypermethrin-
treated animals were similar in terms of behaviour; no compound-
related clinical signs were observed, except for a significantly-
reduced growth rate in both males and females fed 1000 mg/kg diet.
Survival was not affected by cypermethrin. No clinical-chemical,
haematological, or histopathological changes were observed. The
sciatic nerves from a number of animals of all groups, sacrificed
after 6, 12, 18, or 24 months, showed a small number of nerve
fibres exhibiting Wallerian degeneration. The incidence increased
with age, but there was no difference in severity between the
control and treated groups. It is concluded that doses of 100 mg
cypermethrin/kg diet or less did not produce significant
toxicological effects in rats over a 2-year period (McAusland et
al., 1978).
Assays of liver microsomal enzyme activity in 6 rats of each
sex fed 0 or 1000 mg cypermethrin/kg feed for 2 years showed
cypermethrin to be a weak inducer of the microsomal enzyme hepatic
p-nitroanisole- O-dimethylase (PNOD), used as an index of
monooxygenase activity (Potter & McAusland, 1980).
Five groups of Wistar rats, each composed of 52 males and 52
females, were given diets containing 0 (2 groups), 20, 150, or 1500
mg/kg diet (equivalent to 0, 1, 7.5, and 75 mg/kg body weight) for
2 years. Satellite groups of 12 male and 12 female rats were
sacrificed at 52 weeks. For the first 6 weeks of the study, the
rats in the 1500 mg/kg group received 1000 mg/kg. The purity of
the cypermethrin was between 88% and 93% ( cis-: trans-ratio;
55:45). After 104 weeks at the highest dose level, body weight
loss, increased liver weight, increased smooth endoplasmatic
reticulum in hepatocytes, and some haematological and other slight
clinical changes were observed. No other changes or increase in
the incidence of tumours were found. The dose of 150 mg/kg diet
was considered to be the no-observed-adverse-effect level in this
study (only a summary is given) (US-EPA, 1984).
8.3.2. Mouse
The effects of cypermethrin were investigated in groups of 70
male and 70 female Swiss mice fed diets containing 0 (2 groups),
100, 400, or 1600 mg/kg feed (equivalent to 0, 15, 60, or 240 mg/kg
body weight) for up to 101 weeks. The purity of the cypermethrin
was between 91.5 and 94.2%; cis-: trans-ratio; 53:47 or 54:46.
Ten males and 10 females per group were killed at 52 weeks for
interim haematological evaluation. Appearance, behaviour, and
survival were similar in all groups. Body weight gain was reduced
in mice fed 1600 mg cypermethrin/kg diet compared with the combined
control groups. Food intake of both males and females was not
significantly changed. Several haematological changes, consistent
with a mild anaemia, were found in the 1600 mg/kg group of animals
at the interim kill (decreased haemoglobin, haematocrit, and red
blood cell counts in males; decreased mean cell volume and mean
cell haemoglobin concentration in females) but were not found at
termination. Thrombocytosis and increased liver weight were also
observed in this group, at both the interim and terminal kills.
There were no accompanying histopathological changes (for
carcinogenic potential, see section 8.7). No effects were observed
at dose levels of 400 mg/kg diet or less (Lindsay et al., 1982).
8.3.3. Dog
Cypermethrin (dissolved in corn oil) was administered to 4
groups of 8 beagle dogs of each sex at dose levels of 0, 1, 5, or
15 mg/kg body weight per day, by capsule, for a period of 52 weeks.
The purity of the cypermethrin was 90.6% ( cis-: trans-ratio;
54:46). The dogs in the highest dose group exhibited loss of
appetite, tremors, gait changes, incoordination, disorientation,
and hypersensitivity. No other abnormalities were found in the
composition of the blood or urine or in organ weights. Dogs
receiving 5 mg/kg or more showed liquid stools by this mode of
administration. However, this effect was not found when
cypermethrin was fed in the diet for 2 years. No effects were seen
at 1 mg/kg (but only a summary is given) (US-EPA, 1984).
Groups of 4 male and 4 female Beagle hounds were fed diets
containing 0, 3, 30, or 300 mg cypermethrin/kg feed for 2 years.
An additional group received 1000 mg/kg feed but, because of severe
intoxication signs, the dose was decreased to 750 mg/kg. The signs
of intoxication consisted of licking and chewing of the paws, a
stiff high-stepping gait, whole body tremors, head shaking,
incoordination, ataxia, and convulsions. After 3 weeks of feeding
at 750 mg/kg, the animals received the control diet, until signs of
intoxication could no longer be seen. The dogs were then fed a
diet containing 600 mg cypermethrin/kg from week 8 until
termination at 2 years. No signs of intoxication were observed in
dogs fed diets containing 0, 3, 30, or 300 mg/kg during the course
of the study. There was reduced body weight gain in male dogs in
the 600 mg/kg group. Clinical chemistry and haematological
investigations, performed at 6-week intervals for 2 years, did not
reveal any consistent differences between treated groups and
controls. The minor changes in absolute organ weight observed in
the brain and thyroid in the 300 mg/kg group were not present when
the differences were corrected for terminal body weight.
Furthermore, a dose relationship was not apparent, and there were
no accompanying histopathological changes. Opthalmological
observations performed during the course of the study did not
reveal any ocular differences between treated groups and control.
No abnormalities were found in the sciatic nerves, brain, or spinal
cord in any of the treated groups. The feeding of diets containing
up to 600 mg cypermethrin/kg diet to dogs for 2 years did not
reveal any treatment-related gross or histopathological effects,
although the dogs receiving diets containing 1000 mg/kg decreased
to 600 mg cypermethrin/kg showed reduced body weight gain.
Cypermethrin did not produce any toxicological effects in dogs fed
dietary concentrations of 300 mg/kg feed or less, over 2 years
(Buckwell, 1981).
8.4. Special Studies
8.4.1. Synergism/potentiation studies
8.4.1.1. Organophosphate mixture
In a study on rats, designed to determine whether an
organophosphate insecticide, monocrotophos, potentiated the
neurotoxic effect of cypermethrin, 5 groups of 7 or 8 male and 7 or
8 female rats were given 7 consecutive oral doses of monocrotophos
(100 g/litre), cypermethrin (25g/litre), or a mixture of
monocrotophos and cypermethrin (100 g/litre and 25 g/litre,
respectively). Signs of intoxication and neurotoxic effects
(biochemical changes consistent with very sparse axonal
degeneration in sciatic/tibial nerves) were found to be wholly
attributable to the monocrotophos component of the mixture, and
there was no evidence of potentiation of neurotoxic effects (Rose &
Dewar, 1979a).
8.4.1.2. Organochlorine mixture
The oral and dermal toxicities of cypermethrin and endosulfan
for rats were determined both individually and combined. No
evidence for enhancement of toxicity was found for either
cypermethrin or endosulfan when administered as a 1:1 mixture in
corn oil (Price, 1981b).
8.4.2. Neurotoxicity
8.4.2.1. Characterization of the neurotoxic effects
During preliminary short-term studies on cypermethrin, 2
observations were made:
(a) high doses of cypermethrin given orally to rats
caused an unusual gait in intoxicated animals; and
(b) at lethal or near-lethal dermal or oral doses,
histopathological changes (swelling and/or
disintegration of axons of the sciatic nerve of
rats) were observed; such observations have been
reported to occur with other synthetic pyrethroids
(Okuno et al., 1976a) and natural pyrethrins (Okuno
et al., 1976b).
As a consequence of these findings, an extensive programme of
work has been undertaken to characterize the neurotoxic effects of
cypermethrin.
8.4.2.2. Neuropathological studies
(a) Rat
Single oral doses of cypermethrin in corn oil were given to
groups of 6 - 12 rats of each sex at 100, 200, or 400 mg/kg body
weight. All rats showed signs of intoxication, which varied in
severity according to the dose level. At 400 mg/kg, severe
clinical signs were seen within 2 days of dosing. Most of the
animals showed swelling of the myelin sheaths and breaks of some of
the axons of the sciatic nerves. At 200 mg/kg, 8 rats of each sex
died or were killed within 48 h of dosing, and the remaining 4 of
each sex survived the 9 days of the study. Nearly half of the
animals showed lesions of the sciatic nerve. At 100 mg/kg, all
rats survived 9 days of the trial. Only one female out of 12
showed minimal lesions of the sciatic nerve. Neuropathological
changes were found in a number of animals dosed with 200 mg
cypermethrin/kg body weight or more. Dose-related increases in
signs of intoxication and neuropathy were observed; however,
neuropathy was not detected in all the animals showing signs of
intoxication (Carter & Butterworth, 1976).
Groups of rats (10 males per group) were fed dietary levels of
cypermethrin ( cis-: trans- = 45:55) at 0, 1250, 2500, or 5000
mg/kg diet for 14 days. Mortality was observed at the 2 higher
doses and growth inhibition was seen in all treated groups.
Clinical signs of neurotoxicity were characterized by an impaired
ability to walk, splayed hind limbs, ataxia, and paralysis. Other
clinical signs were hypersensitivity, gross disorientation, and
convulsions. The neurotoxic signs of poisoning observed at 1250
mg/kg diet were reversible. Remission of ataxia at 2500 mg/kg diet
was also noted. Ultrastructural changes in the sciatic nerve were
observed in a small number of animals at the 2 highest dose levels.
Some evidence of axonal damage in the myelinated nerves was
observed, mainly in these groups (Glaister et al., 1977a).
In another study, rats were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg diet for 2 years (section 8.3).
At the 12-month interim kill, part of the sciatic nerve was removed
from 6 males and 6 females in both the control and 1000 mg/kg
group. The dissected nerves were divided and teased into a fan
shape, allowing each fibre to be examined. No significant
difference was found in the incidence of abnormal nerve fibres in
the control and the 1000 mg/kg group (Trigg et al., 1977; McAusland
et al., 1978).
(b) Hamster
Groups of 6 male and 6 female Syrian hamsters were given single
oral doses of cypermethrin in corn oil at 0, 794, 1000, and 1260
mg/kg body weight. Signs of intoxication (generally tiptoe
walking, tremors, irregular movements) occurred in all groups
receiving cypermethrin. Animals died or were killed at times
varying from a few hours to 9 days after dosing. Of the 31 animals
of the different test groups that were examined, 3 had nerve
lesions, axonal swelling, and axonal breaks in the sciatic and
posterior tibial nerves (many animals died within a few hours)
(Butterworth & Clark, 1977).
(c) Chicken
In a delayed neurotoxicity study on 6 adult domestic hens,
cypermethrin in DMSO did not cause histological lesions in the
nervous system (brain, spinal cord, and sciatic nerves) or signs of
intoxication at an oral dose of 1000 mg/kg body weight per day for
5 days, compared with a positive control group. No delayed
neurotoxicity was observed (Owen & Butterworth, 1977).
8.4.2.3. Biochemical and electrophysiological studies
Biochemical and electrophysiological studies have been carried
out because the sparse axonal degeneration described above is
difficult to demonstrate by conventional histopathological
techniques. Furthermore, evidence was required to quantify the
neurological dysfunction attributable to cypermethrin. The
rationale for measuring the activities of beta-glucuronidase and
beta-galactosidase in conjunction with an assessment of "mean slip
angle" and "mean landing foot spread" as markers for axonal
degeneration has been described by Dewar (1977a,b).
(a) Rat
Using biochemical markers, it has been shown that, at high oral
doses of cypermethrin, sparse axonal degeneration occurs in the
sciatic/posterior tibial nerves (Dewar, 1977b; Rose & Dewar, 1983),
and the trigeminal nerve and trigeminal ganglion of Wistar rats
(Dewar & Moffett, 1978a).
Three studies involving repeated oral administration of
cypermethrin to rats at 150 mg/kg body weight for 5 or 7 days and
0, 25, 50, 100, or 200 mg/kg body weight for 5 or 7 days have been
carried out. Mortality occurred from 100 mg/kg body weight
onwards. A dose-related transient functional impairment, assessed
by means of the inclined plane test, was found in the first week.
Significant increases in beta-glucuronidase or beta-galactosidase
activity of the sciatic, tibial, or trigeminal nerves only occurred
with 5 or 7 doses of 150 or 200 mg/kg body weight. The sensitivity
of these nerves is comparable. Complete functional recovery
occurred within 26 days. Increased activity of the enzymes in the
distal portion of nerves was found but, even in the most severely
intoxicated animals, the magnitude of the increases was less than
that induced by the known neurotoxic agent methylmercury chloride
(Dewar, 1977b; Dewar & Moffett, 1978a; Rose & Dewar, 1983).
Biochemical changes, consistent with primary axonal
degeneration, were detected in rats aged 3, 6, or 12 weeks.
Cypermethrin was more acutely toxic for 3-week-old rats than for
12-week-old rats and the dose required to cause a sparse axonal
degeneration, as demonstrated by biochemical changes, was
proportionally smaller for the younger rats (Rose & Dewar, 1978).
The time-course for development and recovery from axonopathy
was investigated in groups of rats (5 of each sex) at periods of
2 - 12 weeks after the start of dosing. The daily doses were 150
mg/kg body weight for the first 11 doses and 100 mg/kg body weight
for the subsequent 9 doses, over a period of 4 weeks. More than
half of the animals died and more than 80% of the treated animals
showed clinical signs of intoxication, characterized by abnormal
gait, ataxia, salivation, lethargy, chromodacryorrhoea,
piloerection, and hypersensitivity to external stimuli. Maximum
enzyme activities in the sciatic posterior tibial nerves were found
after 5 weeks and had returned to control values by 12 weeks. In a
second phase of the study, 10 male and 10 female rats were given
20 oral doses of cypermethrin at 37.5, 75, or 150 mg/kg body weight
per day, (concurrent control group dosed with DMSO), over a 4-week
period. The animals given 75 and 150 mg/kg showed clinical signs
of intoxication. Mortality also occurred at the highest dose
level. The behaviour and appearance of animals in the 37.5 mg/kg
body weight per day group were similar to those of the controls.
Five weeks after the initial dose, biochemical changes, which were
indicative of a mild axonal degeneration, were observed in animals
in the highest dose group. No consistent or biologically
significant neurobiochemical changes were found in the 37.5 or 75
mg/kg body weight groups. The results of this study demonstrated
that 20 oral doses of cypermethrin at 75 mg/kg body weight per day
administered over a 4-week period, produced mild clinical signs of
intoxication; however, no biochemical changes which were indicative
of peripheral neuropathy were seen (Rose, 1983).
Further evidence to support the minor nature of the nerve
lesions has been afforded by electrophysiological studies on rats.
In these studies, measurements of the maximal motor conduction
velocities and conduction velocity of slower fibres in the sciatic
and tail nerves of Wistar rats were made before, and at intervals
of up to 5 weeks after, exposure to single (200 mg/kg body weight)
or repeated doses (7 doses of 150 mg/kg body weight) of
cypermethrin. Even at near-lethal doses, cypermethrin did not
cause any effects on these parameters (Dewar & Deacon, 1977).
The ability of the major metabolite of cypermethrin, PBA, to
produce axonal changes has been investigated. Four groups of 8
male and 8 female rats given 7 consecutive daily oral doses of 0,
25, 77, or 375 mg/kg body weight did not show any biochemical
changes indicative of peripheral nerve damage. The sparse axonal
degeneration observed with high doses of cypermethrin is,
therefore, not caused by PBA (Rose & Dewar, 1979b).
(b) Hamster
In 3 studies, Chinese hamsters were given oral doses of
5 - 30 mg cypermethrin/kg body weight per day, for 5 consecutive
days. Clinical signs of intoxication were observed and tests
made to assess whether the compound produced degenerative changes
in peripheral nerves and sensory ganglia. Beta-glucuronidase,
and beta-galactosidase activities were measured in the
sciatic/posterior tibial nerves and trigeminal ganglion. A
positive control group received 7.5 mg methylmercury chloride/kg
body weight per day, for 5 consecutive days.
The most striking feature in the hamsters treated with doses
exceeding 5 times 20 mg/kg body weight was a marked loss of fur in
the facial area, neck, and back, due to repeated scratching. The
results of the enzyme determinations suggest that the Chinese
hamster like the rat developed sparse axonal degeneration following
oral doses in excess of 5 times 10 mg/kg body weight (Dewar &
Moffet, 1978b)
(c) Rabbit
Rabbits were administered capsules with 0 (corn oil), 75,
150, or 300 mg cypermethrin (93.5%)/kg body weight in corn oil,
5 times a week, for 6 weeks. At the end of the 6th week,
electroencephalographic records were taken. The waves of the
complex EEG activity as well as the performance of the 6
constituting bands of different frequency were computer analysed.
No significant alterations were found (Desi et al., 1986a).
8.4.2.4. Appraisal
Repeated oral administration of cypermethrin to rats, at doses
sufficiently high to produce significant mortality in a group of
animals, produced biochemical changes in peripheral nerves that
were consistent with sparse axonal degeneration. The magnitude of
the biochemical changes was similar to that of changes produced
with either a single (Dewar, 1977b) or repeated doses of shorter
duration (Dewar & Moffet, 1978a), thus demonstrating a lack of
cumulative effect. The magnitude of change was substantially less
than that encountered with established neurotoxic agents and the
site of maximal enzyme change (distal portion of the nerve)
suggests that the degeneration is more likely to be of a Wallerian
type rather than segmental demyelination. The short time required
to produce ataxia and/or abnormal gait rules out a causal
relationship between ataxia and axonopathy. The clinical signs are
consistent with a pharmacologically-mediated effect whereas the
axonopathy is a minor reversible lesion that occurs several days
after exposure and has also been detected in animals that do not
show clinical signs.
Histopathological lesions of some fibres of the sciatic nerve
have only been demonstrated in rats or hamsters receiving extremely
high, lethal or near lethal, oral doses of cypermethrin (Carter &
Butterworth, 1976; Butterworth & Clark, 1977). Compound related
histopathological changes have not been observed in rats exposed to
cypermethrin through inhalation (Blair et al., 1976) or in rats fed
diets containing 1000 mg cypermethrin/kg over a 1 - 2 year period
(Trigg et al., 1977; McAusland et al., 1978). Furthermore, dogs
fed 1500 or 600 mg cypermethrin/kg diet for 3 or 24 months,
respectively, did not have any compound-related lesions of the
sciatic nerve (Buckwell & Butterworth, 1977; Buckwell, 1981).
The results of biochemical studies, used as sensitive
indicators of change, confirmed that minor changes that occur after
massive exposure to cypermethrin are reversible and are not
necessarily associated with clinical signs (Dewar, 1977b; Dewar &
Moffet, 1978a; Rose, 1983). There was no direct correlation
between the time course of the neuromuscular dysfunction and the
neurobiochemical changes (Rose & Dewar, 1983). The metabolite of
cypermethrin, PBA, did not produce any biochemical changes in
peripheral nerves (Rose & Dewar, 1979b).
8.4.3. Immunosuppressive action
Stelzer & Gordon (1984) studied the in vitro effects of
pyrethroids on the mitogenic responsiveness of murine splenic
lymphocytes to concornavalin A and lipopolysaccharide. The results
of these studies showed that at concentrations of the order of 1 -
10 µmol/litre, inhibition of murine splenic lymphocytes to both B
and T cell mitogens was found. These results support the
possibility of immune suppression by pyrethroid (cypermethrin)
exposure.
Desi et al., (1985, 1986a) studied the short-term effects of
6.25, 12.5 or 25 mg cypermethrin (93.5%)/kg body weight in rats, on
the immune system by measuring the autologous rosette formation of
T-lymphocytes and determining the ovalbumin titre. These studies
were performed over a period of 6 or 12 weeks.
The humoral immune response after vaccination with Salmonella
typhimurium and the cell-mediated immune response were determined
in rabbits. The antibody titre was measured by tube-agglutination
and complement-binding tests and the tuberculin skin test was used
for the immune response test. The dose levels in this study were
75, 150, or 300 mg/kg body weight, for 7 weeks. A significant
dose-dependent decrease was observed in both the anti-ovalbumin
titre of the blood-serum and in the autologous rosette formation of
the T-lymphocytes. In rabbits, the 2 highest dose levels induced a
significant dose-dependent decrease in serum antibody titres and
the tuberculin skin test showed the same dose-dependent tendency.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
Cypermethrin was fed to Wistar rats (30 male and 30 female per
group) at dietary concentrations of 0, 10, 100, or 500 mg/kg for 5
weeks, after which the males and females (10 weeks of age) from
each treatment group were mated. Two successive litters were
produced from each pair. The first of these litters was discarded,
and randomly-selected male and female pups of the second litters
were mated to produce the next generation. The study was continued
until 2 litters from each of 3 successive generations had been
bred. The parent animals in the 500 mg/kg group consumed less food
than the controls and this was accompanied by a reduction in body
weight. Otherwise, the parent animals of control and treatment
groups behaved similarly. Cypermethrin did not cause any adverse
effects on the reproductive performance of the rats or on the
survival of the offspring. No consistent changes were observed in
mean litter weight between birth and weaning in any treatment
group, with the exception of a reduction in total litter weights in
the 500 mg/kg F1a litters on days 4, 14, and 21. There was also a
statistically-significant decrease compared with controls in total
litter weights and size in the F1b litters at 500 mg
cypermethrin/kg diet. In the 500 mg/kg Fo group, one animal showed
a squamous cell carcinoma of the skin. However, this was
considered not to be related to the compound, because no increase
in tumour incidence was found in the long-term studies on rats and
mice. No changes were observed in rats administered 100 mg/kg diet
(Hend et al., 1978).
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1. Rat
Groups of pregnant female Sprague Dawley CD rats (25 animals
per group) were administered cypermethrin orally as a 1% solution
in corn oil at doses of 0, 17.5, 35, or 70 mg/kg body weight per
day, from days 6 to 15 (inclusive) of gestation. Cypermethrin at
17.5 mg/kg body weight per day did not affect maternal performance
or fetal survival and development. At the higher doses of 35 and
70 mg/kg body weight, respectively, slight and significant
retardation of maternal body weight gain was recorded. In
addition, at 70 mg/kg per day, slight to severe neurological
disturbances were observed in nearly half of the females including:
slight splaying of the hind legs while walking ranging to severe
splaying of all limbs, involuntary movements of the jaws,
convulsive spasms, and hypersensitivity to noise. Despite this
maternal toxicity, there were no indications of any embryotoxic or
teratogenic effects of cypermethrin (Tesh et al., 1978).
8.5.2.2. Rabbit
Groups of pregnant female Banded Dutch rabbits (30 controls and
20 for each dose group) were dosed orally with 0, 3, 10, or 30 mg
cypermethrin/kg body weight per day in corn oil (by gelatin
capsule) during days 6 - 18 (inclusive) of gestation. No influence
was found on growth, pre-implantation losses, resorptions, fetal
deaths, or numbers and sizes of fetuses. The incidence of fetal
visceral and/or skeletal abnormalities was comparable to that in
the vehicle control group, except for a slight increase in the mean
percentages of fetuses showing visceral and/or skeletal
abnormalities in the group receiving 30 mg/kg body weight per day.
No teratogenic effects were found in this study (Dix, 1978).
8.6. Mutagenicity and Related End-Points
8.6.1. In vitro studies
8.6.1.1. Microorganisms
Using bacterial assays, no increases in the reversion rates of
Escherichia coli WP2, E. coli WP2 uvrA, Salmonella typhimurium
TA 1535, TA 1537, TA 1538, TA 98, and TA 100 were detected with
cypermethrin (concentrations of up to 2 mg per plate) in the
presence or absence of a rat liver microsomal activation system.
Exposing Saccharomyces cerevisiae JD1 in liquid culture to
cypermethrin at concentrations of up to 5 mg/ml, both in the
presence and absence of a rat liver microsomal activation system,
did not result in any increase in the rate of mitotic gene
conversion (Brooks, 1980).
In a study in which cypermethrin was tested for mutagenicity in
Salmonella typhimurium TA 100 or TA 98, in the presence or absence
of a rat liver activation system, using the plate incorporation
assay, concentrations of up to 1 mg/plate gave negative results.
The same was true with the fluctuation test, with concentrations of
up to 10 µg/ml (Pluymen et al., 1984).
8.6.1.2. Mammalian cells
Cypermethrin was tested for mutagenicity in V79 Chinese hamster
cells. No cytotoxicity was observed when the assay was carried out
in the presence of rat hepatocytes. Cypermethrin was found not to
be mutagenic for either genetic locus (OUA' and TG') in V79 cells,
when tested in concentrations up to 20 µg/ml, in the presence or
absence of rat hepatocytes (Pluymen et al., 1984).
8.6.2. In vivo studies
8.6.2.1. Host-mediated assay
There was no increase in the rate of mitotic gene
conversion in Saccharomyces cerevisiae JD1 harvested from male
mice following a single oral dose of cypermethrin at 25 or 50 mg/kg
body weight for 2 days (Brooks, 1980).
8.6.2.2. Dominant lethal assay
No evidence of dominant lethality was found when male CD1 mice
(3 groups of 12 animals and a control group with 36 animals) were
given single oral doses of 6.25, 12.5, or 25 mg cypermethrin/kg
body weight. Two other groups were given 5 consecutive daily oral
doses of 2.5 or 5 mg/kg body weight (controls received the vehicle
DMSO). A significant reduction in fetal implants during the second
week of mating was noted and a marginal increase in early fetal
deaths was observed at 5 mg/kg per day. This effect was not
confirmed in a second study using oral doses of 2.5, 5, 7.5, or
10 mg/kg body weight for 5 consecutive days, when no effects were
found on reproductive capability or the histopathology of the
testes and epididymis. The marginal differences found in the
5 mg/kg per day group in the second study were considered not to
be related to the compound.
In summary, cypermethrin did not show detectable dominant
lethality after administration of doses of up to 10 mg/kg body
weight per day, for 5 days, or as a single oral dose of 25 mg/kg
body weight (Dean et al., 1977).
8.6.2.3. Bone marrow chromosome study
Chinese hamsters (12 of each sex per group) were orally dosed
with 20 or 40 mg cypermethrin/kg body weight, in DMSO, for 2
successive days. The incidence of chromosome abnormalities in
bone marrow cells, 8 and 24 h after dosing, did not differ from
that in the DMSO control animals. The positive control group
(100 mg/kg cyclophosphamide) showed many chromosomal aberrations
(Dean, 1977).
The effects of cypermethrin on sister chromatid exchange were
studied in the bone marrow cells of 3-month-old mice. Cypermethrin
was injected subcutaneously at 0.75, 1.5, or 3 mg/kg body weight
or 2.5, 5.0, or 10 mg Ripcord/kg body weight. Both the technical
and the formulated products showed a dose-related increase in
sister chromatid exchanges in the dividing cells at all dose
levels. The highest doses of both cypermethrin and Ripcord
completely inhibited mitotic division (Seehy et al., 1983).
8.6.2.4. Micronucleus test
The induction of micronuclei was studied in mouse bone marrow.
Cypermethrin was administered by 3 routes: intraperitoneal, oral,
and dermal. Cypermethrin showed mutagenic potential after oral
administration of 900 mg/kg diet for 7 and 14 consecutive days. No
effects were seen after ip administration of a single injection of
60 or 180 mg/kg body weight or double and triple injections of 60
mg/kg body weight.
Up to 4 dermal treatments with 360 mg cypermethrin/kg body
weight resulted in a significant increase in the frequency of
polychromatic erythrocytes with micronuclei (Amer & Aboul-Ela,
1985).
8.7. Carcinogenicity
8.7.1. Oral
8.7.1.1. Rat
Wistar rats (24 of each sex per dose level and 48 of each sex
as controls) (section 8.3) were fed dietary concentrations of 0, 1,
10, 100, or 1000 mg cypermethrin/kg for up to 2 years in a combined
long-term/carcinogenicity study. No evidence for carcinogenicity
was found in this study (McAusland et al., 1978) (section 8.3.1).
No increase in tumour incidence was seen when Wistar rats were
given diets containing 0, 20, 150, or 1500 mg cypermethrin/kg diet
(equivalent to 0, 1, 7.5, or 75 mg/kg body weight) for 2 years.
The purity of the cypermethrin ( cis-: trans- ratio; 55:45) was
between 88% and 93% (section 8.3.1) (US EPA, 1984).
8.7.1.2. Mouse
Swiss mice (70 male and 70 female) were fed diets containing 0
(2 groups), 100, 400, or 1600 mg cypermethrin/kg feed for up to 101
weeks. Effects consisted of increased liver weights at 400 and
1600 mg/kg diet and decreased body weight, thrombocytosis, and mild
anaemia at 1600 mg/kg diet (section 8.3.2).
There were no compound-related changes in non-neoplastic
histopathology or increases in tumours of types that are not
commonly associated with the mouse strain used. The incidence of
tumours was similar in all groups with the exception of a slightly
increased incidence of benign alveolar lung tumours in the females
in the 1600 mg/kg group. However, the magnitude of this increased
incidence was insufficient when compared with concurrent and
historical control incidence, to warrant concern. There was no
evidence for a decreased latency of benign alveolar lung tumours in
the female mice receiving the highest dose, and this tumour type
was not accompanied by any increase in malignancy. There was also
no evidence of a carcinogenic response in the male mice in this
study. Furthermore, benign alveolar lung tumours are know to occur
in both sexes at a high and variable incidence in this strain of
mouse. Therefore, it is considered that the occurrence of benign
alveolar lung tumours in the female mice receiving the highest dose
was not related to treatment with cypermethrin. Feeding
cypermethrin at levels of up to 1600 mg/kg diet to mice for a life-
time did not produce any evidence of carcinogenicity (Lindsay et
al., 1982).
8.8. Mechanisms of Toxicity - Mode of Action
In the classification in the Appendix, cypermethrin is
classified among the Type II compounds, which cause toxic signs of
choreoathetosis with salivation (CS-syndrome) in the rat
(Verschoyle & Aldridge, 1980) and bursts of spikes in the cercal
motor nerve of the cockroach (Gammon et al., 1981). Pyrethroids
having alpha-cyano-3-phenoxy-benzyl alcohol, such as cypermethrin,
do not induce repetitive firing in the cercal sensory nerves of the
cockroach in vivo or in vitro, but cause different signs including
a pronounced convulsive phase (Gammon et al., 1981).
The intravenous toxicity of 1RS- cis-, and 1RS- trans-
cypermethrin for the rat has been examined. Both compounds cause
the CS-syndrome, which is characterized by initial pawing and
burrowing behaviour, followed within 2 - 5 min by profuse
salivation, coarse whole-body tremor, increased startle response
and abnormal locomotion involving the hind limbs. The coarse
tremor progresses into sinuous writhing (choreoathetosis) of the
whole body, which gradually becomes more violent and is enhanced by
sensory stimuli. Clonic seizures are occasionally observed as a
terminal event. No increase in core temperature occurs and, in
fact, it may fall. In the electroencephalogram (EEG) during the
CS-syndrome, poisoned rats showed generalized spike and wave
discharges, prior to choreoathetosis (Verschoyle & Aldridge, 1980).
The primary site of action resulting in the CS-syndrome has not yet
been decided.
It has been suggested (Van den Bercken, personal communication,
1981) that the facial skin sensations experienced by people who
handle cypermethrin or other pyrethroids (section 9.2.2) are
brought about by repetitive firing of sensory nerve terminals in
the skin. In the opinion of the author, this is a strictly local
effect that may occur as soon as the pyrethroid concentration on or
in the skin reaches a certain level and is not a sign of general
intoxication. It is considered by the author that the occurrence
of these skin sensations are a warning signal indicating that
exposure has occurred. No undue hazard is likely, provided the
pyrethroid does not reach the blood in any significant
concentration. In this connection, it was stressed that, although
cypermethrin is among the least toxic insecticides when
administered orally, it may become highly toxic, whenever it
reaches the nervous system in sufficient concentrations.
The question remains whether there is a causal relation between
the intense repetitive activity induced by alpha-cyano pyrethroids
(of which cypermethrin is an example) and the nerve damage that
occurs in experimental animals after prolonged exposure to nearly
lethal concentrations of these compounds. Because of the large
number of different chemicals, most of which do not cause
repetitive activity in the nervous system but are known to cause
serious nerve damage, such a correlation is hardly expected.
Furthermore, the extensive literature on DDT (which causes the same
type of repetitive activity in sense organs and in sensory nerve
terminals as the non-cyano pyrethroids) does not contain any
indication that this insecticide may cause axonal degeneration.
However, in theory, there is a possibility that repeated occurrence
of intense repetitive firing will eventually lead to dysfunction of
sensory nerve terminals and sense organs, and, finally, to
degeneration of sensory nerve fibres (Van den Bercken, personal
communication, 1981; Van den Bercken & Vijverberg, 1980a).
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
9. EFFECTS ON MAN
9.1. General Population Exposure
9.1.1. Acute toxicity: poisoning incidents
One report of accidental intoxication has been described. A
family who ate food cooked in 10% cypermethrin developed nausea,
prolonged vomiting with colicky pain, tenesmus, and diarrhoea
within minutes of ingestion. One male adult had convulsions,
passed into coma, and died due to respiratory paralysis. The
symptoms were less severe in other members of the family (Poulos et
al., 1982). There is some doubt whether this was a cypermethrin
intoxication (Suthers, personal communication).
9.1.2. Controlled human studies
Flannigan & Tucker (1985) studied the differences in the extent
of paraesthesia induced by a number of pyrethroids. Field strength
formulated cypermethrin (0.13 mg/cm2) was applied (0.05 ml) to a
4 cm2 area of the earlobe of volunteers on 5 occasions. Distilled
water was applied to the opposite earlobe. Participant evaluation
after each application continued for 48 h and involved description
of the cutaneous sensations. Each participant was treated after
each application with one of the other pyrethroids. Cypermethrin
(as the other pyrethroids) induced sensation. The paraesthesia
developed with a latency period of approximately 30 min, peaked by
8 h and deteriorated as early as 24 h. Dl-alpha tocopheryl acetate
markedly inhibited the occurrence of the paraesthesia.
9.1.3. Epidemiological studies
No information is available.
9.2. Occupational Exposure
9.2.1. Acute toxicity: poisoning incidents
No information is available.
9.2.2. Effects of short- and long-term exposure
Laboratory workers and field operators handling natural and
synthetic pyrethroids, including cypermethrin, have noticed a
transient sensation (described as "tingling" or "burning"
sensations) of the skin in the periorbital area of the face and of
other sites after direct skin exposure. The skin sensations have
been interpreted (section 8.8) as being caused by spontaneous
repetitive firing of local sensory nerve fibres or nerve endings,
with thresholds that have been transiently lowered by the compound
(Wouters & Van den Bercken, 1978). There is a delay of about
30 min before onset of these symptoms following pyrethroid
exposure; the sensation generally lasts only a few hours and does
not persist for more than one day after exposure (Van den Bercken &
Vijverberg, 1980a, Le Quesne et al., 1980).
In a clinical and electrophysiological assessment of these skin
sensations (paraesthesia), 23 laboratory workers, who had been
exposed to synthetic pyrethroids during the preceding months, were
examined. Nineteen of the 23 workers had experienced at least one
or more episodes of facial sensations. Neurological signs were not
observed and electrophysiological studies of selected motor and
sensory nerves in the legs and arms of the 23 subjects showed no
abnormalities (Le Quesne et al., 1980).
Field studies have been performed in the Côte d'Ivoire in which
cypermethrin was sprayed on cotton using a hand-held, ultra-low-
volume (ULV) commercial spraying machine. This method of
application represented a situation of high potential exposure.
The first study, in which 17 workers were involved, concerned a
single exposure to cypermethrin (Prinsen & Van Sittert, 1978),
while a second study was conducted in which 7 workers took part in
7 consecutive spray sessions (Prinsen & Van Sittert, 1979). Skin
exposure to cypermethrin was assessed by quantitative measurement
of the compound deposited on aluminium foil pads on the body of the
sprayer during the spraying operation. The rate of dermal exposure
of the operators during spraying ranged from 1.5 to 46.1 mg/h.
Total absorption of cypermethrin in the body was monitored by
urinary analysis for 2,2-dichlorovinyl-3,3-dimethyl-cyclopropane-
1-carboxylic acid, a metabolite of cypermethrin. In most of the
samples, the excretion was below the limit of detection
(0.05 mg/litre), indicating a low level of absorption. It was
estimated that approximately 3% of the total dermal dose was
absorbed. General medical, extensive clinical blood chemistry, and
electrophysiological examinations were carried out of selected
motor and sensory peripheral nerves in the legs and arms, of the
trigeminal nerve, and of the facial nerve. No effects were found.
In another field study in India, 18 workers, including spraymen
(spraying an emulsifiable concentrate formulation by mist-blower or
knap-sack), mixers, and loaders, handled cypermethrin daily for 5
consecutive days. Medical examination, with special attention to
the sensory function of the peripheral nervous system, was carried
out before, during, and after spraying (Suthers & Marlow, 1981).
No compound-related adverse clinical effects of peripheral
neuropathy were noticed in any of the workers. The urinary
excretion of the cypermethrin metabolite (methyl ester of the
cyclopropane carboxylic acid moiety) increased from day 1 to 5 of
the study, but decreased 24 h after spraying had ceased. On the
fifth day, concentrations of up to 0.18 mg (average 0.1 mg) were
found in the 24-h urine of workers using the mist-blower.
Concentrations were lower in workers using the knap-sack sprayer.
-------------------------------------------------------------------
a Van den Bercken, J. & Vijverberg, H.P.M. (1980) Letter
(personal communication) to WHO concerning "Mode of action of
pyrethroid insecticides in the vertebrate nervous system"
(dated April, 1980).
A field study was carried out on Indian workers in the Satara
district in India prior to, during, and after spraying cotton with
the synthetic pyrethroid formulations, permethrin (Ambush) and
cypermethrin (Cymbush). The formulations were applied using a
mist-blower or knap-sack spray. Exposure of 7 spraymen and 2
loaders/mixers was monitored by measuring the 24-h urinary
excretion of 3-(4'-hydroxyphenoxy)-benzoic acid, and medical
assessments were carried out by 4 medical doctors. The sensory
function of the peripheral nervous system was also assessed. The
formulations were applied at the recommended rates; Ambush, 150 g
a.i./ha and Cymbush, 70 g a.i./ha. Average exposure was
approximately 8 h per day for 5 days. No compound-related adverse
clinical effects were noticed. The average urinary excretion of
3-(4'-hydroxy-phenoxy)-benzoic acid increased from day 1 to day 5,
but then decreased 24 h after spraying had ceased (Hart et al.,
1982).
In another study, two agricultural pilots and five
mixer/loaders were monitored for dermal exposure during aerial
"ultra-low-volume" (ULV) applications of cypermethrin in vegetable
oil to cotton. This study was conducted in Greenwood, Mississippi.
The mixer/loaders wore protective equipment. The actual dermal
exposure for pilots was 0.67 mg/8 h and for mixer/loaders 2.43
mg/8h. The exposure of pilots was predominantly of the hands,
whereas that of mixer/loaders was more uniform (hands were
protected). The urinary excretion of cypermethrin metabolites was
very low; between 4 and 22 µg cypermethrin equivalents/day. This
study demonstrates that exposure of pilots and mixer/loaders during
the aerial ULV applications of cypermethrin is minimal and that
skin absorption is very slight (Chester et al., 1986).
Desi et al. (1986b) carried out a biological monitoring and
health surveillance study on 11 workers spraying organophosphate
carbamate and pyrethroid pesticides in greenhouses during the whole
year in comparison with 10 control persons. During the work,
protective clothing and masks were worn before and after a regular
spraying period with pyrethroids (including cypermethrin).
Extensive medical examinations, such as urinalysis, haematology,
immunoglobulin levels, whole blood cholinesterase activity, serum-
gamma-glutamyltransferase activity, chromosome analysis and
electro-cardiography were performed over a period of 3 months. The
amount of cypermethrin in the blood was just at the limit of
detection. No health injuries or other significant changes in the
parameters studied were found.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation
Cypermethrin, an alpha-cyano pyrethroid consisting of a mixture
of 8 stereo-isomers, is a highly active insecticide effective
against a wide range of pests in many food and non-food
commodities.
It is stable to light and heat, it has a low vapour pressure
and is more stable in acidic than in alkaline media. Sensitive
analytical methods for the determination of residues in food and
the environment are available.
When cypermethrin is applied to crops, residues may occur in
soils and surface waters, but biological degradation is fairly
rapid and residues do not accumulate in the environment.
Photodegradation is unlikely to play an important role. The main
route of degradation is cleavage of the ester linkage to give 2
main degradation products containing the cyclopropane, and the
phenoxybenzyl moiety. The half-lives in the soil are determined by
many factors, but are in the range of 2 - 4 weeks. Cypermethrin is
strongly adsorbed by soil and downward leaching is negligible.
Because of its rather fast breakdown forming less toxic products,
and the low dose rates used in good agricultural practice, it is
unlikely that cypermethrin will attain significant levels in the
environment.
Bioaccummulation in certain organisms, such as fish, took place
under laboratory conditions, but levels declined on cessation of
exposure and there are indications that, under natural conditions,
fish will not contain measurable residues.
When applied according to good agricultural practice, the
levels of cypermethrin residues in food commodities are generally
low. Total diet studies are not available, but from the available
residue information, it can be inferred that the oral intake by man
is well below the ADI.
High dose levels of cypermethrin may exert transient effects on
the soil microflora. Earthworms and other soil organisms are
generally rather resistant to cypermethrin, while fish and other
aquatic invertebrates are very sensitive. Because of its strong
adsorption on soil, only low levels of cypermethrin may leak into
surface water. These may have transient effects, mainly on surface
breathing insects.
The toxicity of cypermethrin for birds is low. Bees appear to
be very sensitive in laboratory tests. Under field conditions, the
effect on bees is minimal, because cypermethrin seems to have a
repellent effect for bees.
Absorption and elimination of cypermethrin has been rapid in
the different mammalian species tested. The major metabolic
reaction is cleavage of the ester bond followed by hydroxylation
and conjugation of the cyclopropane- and phenoxybenzyl moiety. The
highest levels are found in body fat, which is consistent with the
lipophilic nature of cypermethrin. The half-life in the fat of the
rat is about 12 - 19 days for the cis-isomer and 3 - 4 days for the
trans-isomer. Breakdown products in plants are bound as
glucosides.
The acute toxicity of cypermethrin for mammals is of a moderate
order. The oral LD50 for the rat ranges from 200 to 4000 mg/kg
body weight. Short-term and long-term toxicity studies on rats,
mice, and dogs have shown effects on growth, on the liver and
kidneys, and the nervous system, and on haematology. A no-
observed-adverse-effect level of 7.5 mg/kg body weight has been
adopted by the Task Group.
Cypermethrin was not carcinogenic for mice or rats fed diets
containing high levels of the material over a 2-year period.
Cypermethrin did not induce teratogenic effects in either rats at
70 mg/kg body weight or rabbits at 30 mg/kg body weight. It was
also shown not to have any effects on reproductive performance
during a 3-generation reproduction study in rats administered
100 mg/kg diet. In a variety of mutagenicity studies, cypermethrin
was shown to be mainly without mutagenic activity.
The mechanism of the action on the nervous system has been
extensively studied. From these studies and the occupational
studies available, it seems that the skin sensation seen in workers
handling cypermethrin, generally lasts only a few hours and does
not persist for more than one day after exposure. Other
neurological signs were not observed. These skin sensations can be
considered to be an early warning that exposure has occurred and
that work practice should be reviewed. Cypermethrin may cause eye
irritation and may be a sensitizer for certain persons.
10.2. Conclusions
It can be concluded that:
General population: When applied according to good
agricultural practice, exposure of the general population to
cypermethrin is negligible and is unlikely to present a hazard.
Occupational exposure: With reasonable work practices,
hygiene measures, and safety precautions, the use of cypermethrin
is unlikely to present a hazard to those occupationally exposed to
it. The occurrence of "facial sensations" is an indication of
exposure. Under these circumstances work practice should be
reviewed.
Environment: With recommended application rates it is
unlikely that cypermethrin or its degradation products will attain
levels of environmental significance. Notwithstanding its high
toxicity for fish and honey bees, this is only likely to cause a
problem in the case of spillage and overspraying.
11. RECOMMENDATIONS
- Cypermethrin should be included among the residues looked for
in surveillance, market-basket, or total diet studies.
- Attention should be paid to the implications for the welfare of
human beings of animal studies indicating immune suppression.
- Further follow-up studies into the facial effects in human
beings should be conducted, in order to better understand this
phenomenon.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Cypermethrin was discussed at meetings of the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) during the period 1979-84
(FAO/WHO, 1980a,b; 1982a,b; 1983a,b; 1984; 1985a,b,c; Vettorazzi &
Van den Hurk, 1984). In 1981, the JMPR established an Acceptable
Daily Intake (ADI) for cypermethrin of 0 - 0.05 mg/kg body weight.
The Pesticide Development and Safe Use Unit, Division of Vector
Biology and Control, WHO, classified cypermethrin as "irritant to
eyes and sensitizer of skin" in the list of "technical products
unlikely to present an acute hazard in normal use" (Plestina, 1984;
WHO, 1979; WHO, 1985). The same division published a data sheet on
cypermethrin, No. 84.58 (WHO/FAO, 1975-85).
REFERENCES
ABBASSY, M.A., ASHRY, M., ADAM, F., KHALIL, F., & ABOU-SHLOU,
M.A. (1984) Toxicological and histopathological studies on
the cotton bollworm. ( Pectinophera gossypiella Saund). Meded.
Fac. Landbouwwet. Rijksuniv. Gent, 49(3a): 691-698.
ABDEL-AAL, Y.A.I., EL-SAYED, A.M.K., NEGM, A.A., HUSSEIN,
M.H., & EL-SEBAE, A.H. (1979) The relative toxicity of
certain insecticides to Spodoptera littoralis (Boisd) and
Coccinella undecimpunctata L. Int. Pest Control, 21(4): 79-80,
82.
ABOU-AWAD, B.A. & EL-BANHAWY, E.M. (1985) Comparison between
the toxicity of synthetic pyrethroids and other compounds to
the predacious mite Amblyseius gossipi (Mesostigmata:
Phytoseiidae). Exp. appl. Acarol., 1: 185-191.
AHMED, Y.M., MOSTAFA, A.M.A., & ELEWA, M.A. (1985) Toxicity
of certain dyes as insecticides and their joint action with
some pyrethroids. J. environ. Sci. Health, B20(6): 689-699.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four
synthetic pyrethroids on a predatory mite, Typhlodromus pyri,
and its prey, Panonychus ulmi, on apples in Southeast England.
Environ. Entomol., 9: 436-439.
AMER, S.M. & ABOUL-ELA, E.I. (1985) Cytogenetic effects of
pesticides. III. Induction of micronuclei in mouse bone marrow
by the insecticides cypermethrin and rotenone. Mutat. Res.,
155(3): 135-142.
ASCHER, K.R.S., ELIYAHU, M., NEMNY, N.E., & ISHAAYA, I.
(1982) The toxicity of some novel pesticides - synthetic
pyrethroids and benzoylphenylurea chitin synthesis inhibitors
- for eggs of Spodoptera littoralis (Boisd). Z. angew.
Entomol., 94: 504-509.
AWASTHI, M.D. & ANAND, L. (1983) Dissipation and persistence
of synthetic pyrethroids on fruits of okra. J. entomol. Res.,
7(1): 55-59.
BADMIN, J.S. & TWYDELL, R.S. (1976) Evaluation of the
insecticide WL 43467 against the honey bee Apis mellifera,
Sittingbourne, Shell Research (WKSR.0021.76).
BAICU, T. (1982) Toxicity of some pesticides to Trichoderma
viride pers., Crop Prot., 1(3): 349-358.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues
of synthetic pyrethroids in fruit and vegetables by gas-liquid
and high-performance liquid chromatography. Analyst, 107:
206-212.
BALDWIN, M.K. (1977a) Residues of the pyrethroid insecticide
WL43467 in tissues of cattle following a dip-bath application,
Sittingbourne, Shell Research (TLGR.0.115.77).
BALDWIN, M.K. & LAD, D.D. (1978a) Residue data for milk
following application of WL 43467 in a barn, Sittingbourne,
Shell Research (TLGR.0042.78).
BALDWIN, M.K. & LAD, D.D. (1978b) The accumulation and
elimination of WL 43467 by the Rainbow trout (Salmo
gairdneri), Sittingbourne, Shell Research (TLGR.0041.78).
BALDWIN, M.K., BUCKWELL, A.C., & LAD, D.D. (1977) Residue
data following the application of WL 43467 for nuisance fly
control on cattle, Sittingbourne, Shell Research (TGLR.0.112.
77).
BARIOLA, L.A. & LINGREN, P.D. (1984) Comparative toxicities
of selected insecticides against pink bollworm (Lepidoptera:
Gelechiidae) moths. J. econ. Entomol., 77: 207-210.
BARLOW, F., HADAWAY, A.B., FLOWER, L.S., & TURNER, C.R.
(1986) Residual contact toxicity of some insecticides to tse
tse flies in laboratory tests, London, Centre for Overseas
Pest Research, pp. 12.
BATTELLE (1982) Pesticide programme of research and market
planning. I. Insecticides, Geneva, Institute Battelle (October
1982).
BAYOUMI, O.C. (1982) The susceptibility of Myzus persicae
Sulz. to some insecticides in artificial diets. Parasitica,
38(4): 193-199.
BENNETT, D. (1981a) The accumulation, distribution, and
elimination of RIPCORD by Rainbow trout using a continuous-
flow procedure, Sittingbourne, Shell Research (SBGR.81.026 and
Addendum).
BENNETT, D. (1981b) Residues of RIPCORD in Rainbow trout
(Salmo gairdneri) and common carp (Cyprinus carpio) exposed to
lethal concentrations in water, Sittingbourne, Shell Research
(SBGR.81.075).
BENNETT, D., CROSSLAND, N.O., & SHIRES, S.W. (1980) Spray
drift from RIPCORD applications to vineyards in France: fate
and effects in adjacent streams, Sittingbourne, Shell Research
(TLGR.80.095).
BLAIR, D. & RODERICK, H.R. (1976) Toxicity studies on the
pyrethroid insecticide WL 43467, emulsifiable concentrate FX
3315. Acute inhalation exposure of rats to an aqueous spray,
Sittingbourne, Shell Research (TLGR.0001.76).
BLAIR, D., BUTTERWORTH, S.T.G., & RODERICK, H.R. (1976)
Toxicity studies on the pyrethroid insecticide WL 43467,
emulsifiable concentrate FX 3315. Histopathology of rats
exposed to an aqueous spray, Sittingbourne, Shell Research
(TLGR.0102.76).
BLAND, P.D. (1985) Capillary gas chromatographic
determination of cypermethrin in formulations: collaborative
study. J. Assoc. Off. Anal. Chem., 68(3): 592-595.
BOSIO, P.G. (1979) Residues of Barricade (cypermethrin) in
cattle from Australia, Berre, France, Shell Chimie (BEGR.79.
117) (Unpublished Shell data).
BOSTANIAN, N.J. & BELANGER, A. (1985) The toxicity of three
pyrethroids to Amblyseius fallacis (Garman) Acari.
Phytoseiidae and their residues on apple foliage. Agric.
Ecosyst. Environ., 14(3/4): 243-250.
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC
multi-residue method to determination of synthetic pyrethroid
residues in celery and animal products. J. Assoc. Off. Anal.
Chem., 65(3): 685-689.
BRAUN, H.E., FRANK, R., & MILLER, L.A. (1985) Residues of
cypermethrin in milk from cows wearing impregnated ear tags.
Bull. environ. Contam. Toxicol., 35: 61-64.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1983)
Injection toxicity of some pyrethroids in the armyworm.
J. Pestic. Sci., 8: 95-98.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984a)
Topical and injection toxicities of some pyrethroids in the
tobacco cutworm, Spodoptera litteralis Fabricius. J. Pestic.
Sci., 9: 481-487.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984b)
Topical and injection toxicities of some pyrethroids to the
german cockroach, Blattella germanica (Dictyoptera:
Blattellidae). Appl. Entomol. Zool., 19(3): 348-355.
BREMPONG-YEBOAH, C.Y., SAITO, T., & MIYATA, T. (1984c) The
selective toxicity of some synthetic pyrethroids in the
armyworm. Pseudaletia separata (Walker). III Cuticle
permeabilities of some pyrethroids. Appl. Entomol. Zool.,
19(1): 87-94.
BROMLEY, S. & COOK, K.A. (1979) Determination of the effects
of WL 43467 on microbial activity in soil. V. Effects on
glucose utilization, Sittingbourne, Shell Research
(BLGR.79.099).
BROOKS, T.M. (1980) Toxicity studies with agricultural
chemicals: mutagenicity studies with RIPCORD in microorganisms
in vitro and in the host-mediated assay, Sittingbourne, Shell
Research (TLGR.80.059).
BROWN, V.K. (1979a) Toxicology of WL 43467 isomers: acute
toxicity of WL 43481 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.117).
BROWN, V.K. (1979b) Toxicology of WL 43467 isomers: acute
toxicity of WL 42641 in DMSO to rats, Sittingbourne, Shell
Research (TLGR.79.116).
BUCKWELL, A.C. (1981) A 2-year feeding study in dogs on WL
43467, Sittingbourne, Shell Research (SBGR.81.126).
BUCKWELL, A.C. & BUTTERWORTH, S.T.G. (1977) Toxicity studies
on the pyrethroid insecticide WL 43467: a 13-week feeding
study in dogs, Sittingbourne, Shell Research (TLGR.0127.77).
BUTTERWORTH, S.T.G. & CLARK, D.G. (1977) Toxicity studies on
the insecticide WL 43467: acute oral toxicity and neuro-
pathological effects in Syrian hamsters, Sittingbourne, Shell
Research (TLGR.0094.76).
CAGEN, S.Z., MALLEY, L.A., PARKER, C.M., GARDINER, T.H., VAN
GELDER, G.A., & JUD, V.A. (1984) Pyrethroid-mediated skin
sensory stimulation characterized by a new behavioral
paradigm. Toxicol. appl. Pharmacol., 76: 270-279.
CAMILLERI, P. (1984) Alkaline hydrolysis of some pyrethroid
insecticides. J. agric. food Chem., 32: 1122-1124.
CARTER, B.I. & BUTTERWORTH, S.T.G. (1976) Toxicity of
insecticides: the acute oral toxicity and neuropathological
effects of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0055.76).
CASIDA, J.E., GAUGHAN, L.C., & RUZO, L.O. (1979) Comparative
metabolism of pyrethroids derived from 3-phenoxybenzyl and
alpha-cyano-3-phenoxybenzyl alcohols. Adv. Pestic. Sci., 2:
182-189.
CASSIDY, S.L. (1979) Acute oral toxicity of the spray
formulation EF 5288 to calves, piglets, and lambs,
Sittingbourne, Shell Research (TLGR.0001.79).
CHANG, C.K. & JORDAN, T.W. (1982) Penetration and metabolism
of topically applied permethrin and cypermethrin in
pyrethroid-tolerant Wiscana cervinata larvae. Pestic. Biochem.
Physiol., 17: 196-204.
CHANG, C.K. & JORDAN, T.W. (1983) Insecticide handling
mechanisms in some New Zealand pasture pests. N.Z. J. Sci.,
26: 509-516.
CHAPMAN, R.A. & HARRIS, C.R. (1981) Persistence of four
pyrethroid insecticides in a mineral and an organic soil.
J. environ. Sci. Health, B16(5): 605-615.
CHAPMAN, R.A. & SIMMONS, H.S. (1977) Gas-liquid chromato-
graphy of picogram quantities of pyrethroid insecticides.
J. Assoc. Off. Anal. Chem., 60: 977-978.
CHAPMAN, R.A., TU, C.M., HARRIS, C.R., & COLE, C. (1981)
Persistence of five pyrethroid insecticides in sterile and
natural, mineral and organic soil. Bull. environ. Contam.
Toxicol., 26: 513-519.
CHENG, H.H. (1984) Residual toxicity of six pyrethroid and
two organophosphorus insecticides on the soil surface against
dark-sided cutworm (Lepidoptera: Noctuidae) on tobacco in
Ontario. Can. Entomol., 116: 11-17.
CHENG, H.H. & HANLON, J.J. (1984) Residual toxicity of six
insecticides and a herbicide applied sequentially or in tank
mix combinations on tobacco seedlings against dark-sided
cutworm (Lepidoptera: Noctuidae). Tob. Int., 186(22): 39-42.
CHESTER, G., HATFIELD, L.D., HART, T.B., LEPPERT, B.C.,
SWAINE, H., & TUMMON, O.J. (1986) Worker exposure to, and
absorption of cypermethrin during aerial application of an
"ultra low volume" formulation to cotton, Fernhurst, Imperial
Chemical Industries, Plant Protection Division (Unpublished
report).
COATS, S.A., COATS, J.R., & ELLIS, C.R. (1979) Selective
toxicity of three synthetic pyrethroids to eight coccinellids,
a eulophid parasitoid and two pest chrysomelids. Environ.
Entomol., 8(4): 720-722.
CODEX ALIMENTARIUS COMMISSION (1986) Guide to Codex
recommendations concerning pesticide residues. Part 2: Maximum
limits for pesticide residues, third preliminary issue, Rome,
Food and Agriculture Organization of the United Nations
(CAC/PR 2-1986).
COLE, L.M., CASIDA, J.E., & RUZO, L.O. (1982) Comparative
degradation of the pyrethroids tralomethrin, tralocythrin,
deltamethrin and cypermethrin on cotton and bean foliage.
J. agric. food Chem., 30: 916-920.
COOK, K.A. (1978a) Determination of the effects of WL 43467
on microbial activity in soil. I. Effects on carbon dioxide
evolution, Sittingbourne, Shell Research (BLGR.0003.78).
COOK, K.A. (1978b) Determination of the effects of WL 43467
on microbial activity in soil. IV. Effects on nitrification,
Sittingbourne, Shell Research (BLGR.0015.78).
COOMBS, A.D., CARTER, B.I., HEND, R.W., BUTTERWORTH, S.G., &
BUCKWELL, A.C. (1976) Toxicity studies on the insecticide WL
43467: Summary of results of preliminary experiments,
Sittingbourne, Shell Research (TLGR.0104.76).
CORBITT, T.S., WRIGHT, D.J., & GREEN, A.St.J. (1985) The
toxicity of abamectin (MK. 936) on cabbage to first and third
larval instars of Spodoptera littoralis (Boisd.). Meded. Fac.
Landbourwwet. Rijksuniv. Gent, 50(2b): 639-642.
COVENEY, P.C. & EADSFORTH, C.V. (1982) The metabolism of
cypermethrin in man (3). Urinary excretion following a single
dermal dose of cypermethrin, Sittingbourne, Shell Research
(SBGR.82.290).
CRAWFORD, M.J. (1976a) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of (14 C-benzyl)WL
43481 (cis-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0046.76).
CRAWFORD, M.J. (1976b) The metabolism of WL 43467 in
mammals. The fate of a single oral dose of 14 C-WL 42641
(trans-WL 43467) in the rat, Sittingbourne, Shell Research
(TLGR.0077.76).
CRAWFORD, M.J. (1977) The metabolism of WL 43467 in mammals.
The fate of a single oral dose of (14 C-cyclopropyl)WL 43467 in
the rat, Sittingbourne, Shell Research (TLGR.0004.77).
CRAWFORD, M.J. (1979a) The metabolism of cypermethrin (WL
43467) in mammals. The fate of a single oral dose of
(14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne, Shell
Research (TLGR.79.029).
CRAWFORD, M.J. (1979b) The metabolism of cypermethrin (WL
43467) in mammals. The fate of single oral doses of cis- and
trans-(14 C-benzyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.0011.79).
CRAWFORD, M.J. (1979c) The metabolism of 14 C-cypermethrin by
rat liver microsomes, Sittingbourne, Shell Research
(TLGR.79.057).
CRAWFORD, M.J. (1979d) The metabolic fate of the cis- and
trans-isomers of WL 43467 (cypermethrin) and of 3-phenoxy-
benzoic acid in the dog, Sittingbourne, Shell Research
(TLGR.79.012).
CRAWFORD, M.J. (1979e) The metabolism of cypermethrin (WL
43467) in mammals. Metabolites derived from a single oral dose
of (14 C-cyclopropyl)cypermethrin in the dog, Sittingbourne,
Shell Research (TLGR.79.096).
CRAWFORD, M.J. & HUTSON, D.H. (1977a) The metabolic fate of
the cis- and trans-isomers of WL 43467 (cypermethrin).
Metabolism and elimination of 14 C-aryl-labelled cis- and
trans-isomers in rats, Sittingbourne, Shell Research
(TLGR.0131.77).
CRAWFORD, M.J. & HUTSON, D.H. (1977b) The elimination and
retention of WL 43467 when administered dermally or orally to
sheep, Sittingbourne, Shell Research (TLGR.0098.77).
CRAWFORD, M.J. & HUTSON, D.H. (1978) The elimination of
residues from the fat of mice following the oral
administration of (14 C-benzyl)WL 43481 (cis-WL 43467),
Sittingbourne, Shell Research (TLGR.0080.78).
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981a)
Metabolism of cis- and trans-cypermethrin in rats. Balance and
tissue retention study. J. agric. food Chem., 29: 130-135.
CRAWFORD, M.J., CROUCHER, A., & HUTSON, D.H. (1981b) The
metabolism of the pyrethroid insecticide cypermethrin in rats:
excreted metabolites. Pestic. Sci., 12: 399-411.
CRAYFORD, J.V. (1978) A study of the metabolism of
3-phenoxybenzoic acid and its glucoside conjugate in rats,
Sittingbourne, Shell Research (TLGR.0186.78).
CRAYFORD, J.V. & HUTSON, D.H. (1979) The identification of
metabolites in the tissues of rats treated orally with
3- phenoxybenzoic acid, Sittingbourne, Shell Research (TLGR.0043.
79).
CRAYFORD, J.V. & HUTSON, D.H. (1980) Xenobiotic triglyceride
formation. Xenobiotica, 10(5): 349-354.
CRAYFORD, J.V., HUTSON, D.H., & THORPE, E. (1980) The
elimination of residues from the fat of mice following the
oral administration of 14 C-benzyl WL 43481 (cis-WL 43467),
Tunstall, Shell Toxicology Laboratory (TLGR.0080.78,
additional report).
CROSSLAND, N.O. (1982) Aquatic toxicology of cypermethrin.
II. Fate and biological effects in pond experiments. Aquat.
Toxicol., 2: 205-222.
CROSSLAND, N.O. & BENNETT, D. (1976) A field trial to assess
the dispersion and toxicity of an EC formulation of the
insecticide WL 43467 in a pond system, Sittingbourne, Shell
Research (TLGR.0101.76).
CROSSLAND, N.O. & ELGAR, K.E. (1983) Fate and biological
effects of insecticides in ponds. In: Miyamoto, J. et al., ed.
IUPAC. Pesticide chemistry: human welfare and the environment,
Oxford, Pergamon Press, Vol. 3, pp. 551-556.
CROSSLAND, N.O. & STEPHENSON, R.R. (1979) The role of pond
studies in assessing the hazard of toxic chemicals to
freshwater ecosystems. In: Proceedings of the British Crop
Protection Conference, 1979: Pests and Diseases, Croydon,
British Crop Protection Council, Vol. 2, pp. 453-459.
CROSSLAND, N.O., BENNETT, D., KANE, D.F., & STEPHENSON, R.R.
(1978) The dispersion and toxic effects of the insecticide WL
43467 in a pond, Sittingbourne, Shell Research (TLGR.0076.78).
CROSSLAND, N.O., SHIRES, S.W., & BENNETT, D. (1982) Aquatic
toxicology of cypermethrin. III. Fate and biological effects
of spray drift deposits in freshwater adjacent to agricultural
land. Aquat. Toxicol., 2: 253-270.
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982a) Hepatic
esterases: characterization and quantitation in vitro of rat,
rabbit, and human liver esterases. Part I, Sittingbourne,
Shell Research (SBGR.82.204).
CROUCHER, A., HUTSON, D.H., & LOGAN, C.J. (1982b) In vitro
metabolism of the pyrethroid insecticide cypermethrin by liver
esterases. In: Proceedings of the 5th International Congress
on Pesticide Chemicals, Vol. 4.
CROUCHER, A., HUTSON, D.H., & STOYDIN, G. (1985) Excretion
and residues of the pyrethroid insecticide cypermethrin in
lactating cows. Pestic. Sci., 16: 287-301.
DAI, S.M. & SUN, C.N. (1984) Pyrethroid resistance and
synergism in Nilaparvata lugens Stal (Homoptera: Delphacidae)
in Taiwan. J. econ. Entomol., 77: 891-897.
DAY, S.R. & LEAHEY, J.P. (1980) 14 C-cypermethrin: aqueous
photodegradation in sunlight, Fernhurst, Imperial Chemical
Industries (Unpublished ICI Report No. RJ0154B).
DEAN, B.J. (1977) Toxicity studies with SL 43467: chromosome
studies on bone marrow cells of Chinese hamsters after two
daily oral doses of WL 43467, Sittingbourne, Shell Research
(TLGR.0136.77).
DEAN, B.J., PAUW, C.L, VAN DER, & BUTTERWORTH, S.T.B. (1977)
Toxicity studies with WL 43467: dominant lethal assay in male
mice after single oral doses of WL 43467, Sittingbourne, Shell
Research (TLGR.0042.77).
DELABIE, J., BOS, C., FONTA, C., & MASSON, C. (1985) Toxic
and repellent effects of cypermethrin on the honey bee:
laboratory, glasshouse and field experiments. Pestic. Sci.,
16: 409-415.
DESI, I., VARGA, L., DOBRONYI, I., & SZKLENARIK, G. (1985)
Immunotoxicological investigation of the effects of a
pesticide: cypermethrin. Arch. Toxicol., Suppl. 8: 305-309.
DESI, I., DOBRONYI, I., & VARGA, L. (1986a) Immuno-, neuro-
and general toxicologic animal studies on a synthetic
pyrethroid: cypermethrin. Ecotoxicol. environ. Saf., 12:
220-232.
DESI, I., PALOTAS, M., VETRO, G., CSOLLE, I., NEHEZ, M.,
ZIMANYI, M., FERKE, A., HUSZTA, E., & NAGYMAJTENYI, L.
(1986b) Biological monitoring and health surveillance of a
group of greenhouse pesticide sprayers. Toxicol. Lett., 33:
91-105.
DEWAR, A.J. (1977a) The use of lysosomal enzyme measurements
as an indicator of chemically-induced peripheral neuropathy,
Sittingbourne, Shell Research (TLGR.0074.77).
DEWAR, A.J. (1977b) Toxicity studies on the insecticide WL
43467: biochemical and functional studies on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research (TLGR.0082.
77).
DEWAR, A.J. & DEACON, P.A. (1977) Toxicity studies on the
insecticide WL 43467: electrophysiological studies on the
neurotoxicity of WL 43467 to rats. I. The effect on motor
conduction velocity in the sciatic and tail nerves,
Sittingbourne, Shell Research (TLGR.0133.77).
DEWAR, A.J. & MOFFETT, B.J. (1978a) Toxicity studies on the
insecticide WL 43467: biochemical studies on the effect of WL
43467 on the rat trigeminal nerve and ganglion, Sittingbourne,
Shell Research (TLGR.0162.77).
DEWAR, A.J. & MOFFETT, B.J. (1978b) Toxicity studies on the
insecticide WL 43467: biochemical and functional studies on
the neurotoxicity of WL 43467 to Chinese hamsters,
Sittingbourne, Shell Research (TLGR.0038.78).
DEWAR, A.J. & OWEN, D.E. (1979) Toxicology of pyrethroids:
the acute oral toxicity to rats of a sample of ICI
cypermethrin, Sittingbourne, Shell Research (TLGR.79.019).
DIX, K.M. (1978) Toxicity of WL 43467: teratological studies
in rabbits given WL 43467 orally, Sittingbourne, Shell
Research (TLGR.0010.78).
DU TOIT, G.D.G. (1978) Evaluation by topical application of
selected insecticides against adult grass grub. In: Proceeding
of the 31st New Zealand Weed and Pest Control Conference, pp.
160-163.
EADSFORTH, C.V. & BALDWIN, M.K. (1983) Human dose-excretion
studies with the pyrethroid insecticide cypermethrin.
Xenobiotica, 13: 67-72.
EDWARDS, P.J., WILKINSON, W., & COULSON, M. (1985)
Laboratory toxicity test for carabid beetles. Proceedings of
the British Crop Protection Conference, 1984: Pests and
Diseases, Croydon, British Crop Protection Council, Vol. 1,
pp. 359-362.
EDWARDS, R. & MILLBURN, P. (1985a) Toxicity and metabolism
of cypermethrin in fish compared with other vertebrates.
Pestic. Sci., 16: 201-202.
EDWARDS, R. & MILLBURN, P. (1985b) The metabolism and
toxicity of insecticides in fish. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry and toxicology,
New York, John Wiley and Sons, Vol. 5, pp. 249-274.
ELLIOTT, M., FARNHAN, A.W., JONES, N.F., NEEDHAM, P.H., &
PULMAN, D.A. (1974) Synthetic insecticide with a new order
of activity. Nature (Lond.), 248: 710-711.
EL-MINSHAWY, A., MACKLAD, F., RAGAB, F., & DONIA, A. (1983)
Selective toxicity of certain pyrethroids to the cotton
leafworm Spodoptera littoralis (Boisd.) and to one of its
major parasites Microplitis rufiventris (Kok). Proceedings of
the International Conference on Environmental Hazards of
Agrochemicals, Alexandria, Egypt, 8-12 November, 1983, London,
Paris, New York, Harwood Academic Publishers, Vol. 1, pp. 551-
561.
EL-SAYED, G.N. & KNOWLES, C.O. (1984) Formamidine synergism
of pyrethroid toxicity to two-spotted spider mites (Acari,
Tetranychidae). J. econ. Entomol., 77: 23-30.
EL-SEBAE, A.H., EL-BAKARY, A.S., LE PATOUREL, J., KADOUS, E.,
& MACKLAD, M.F. (1983) Effect of photoperiodism on fish
susceptibility to insecticides. In: Zewail, A.H., ed.
Proceedings of the International Conference on Photochemistry
and Photobiology, Alexandria, Egypt, 8-12 November, 1983,
London, Paris, New York, Harwood Academic Publishers, pp. 960-
966.
EL-SEBAE, A.H., ENAN, E.E., DAOUD, A.S., & ZEID, M.I. (1985)
Selective toxicity of synthetic pyrethroids and some
synergists to mice and cotton leafworm in relation to some
biochemical enzyme activities. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 50(3a): 939-950.
ESTESEN, B.J., BUCK, N.A., & WARE, G.W. (1982) Dislodgable
insecticide residues on cotton foliage: carbaryl, cyper-
methrin, and methamidophos. Bull. environ. Contam. Toxicol.,
28: 490-493.
EUROPEAN PATENT OFFICE (1980) European patent application
(Publication No. 0015598(A2)).
EVANS, M.H. (1976) End-plate potentials in frog muscle
exposed to a synthetic pyrethroid. Pestic. Biochem. Physiol.,
6: 547-550.
EWEN, A.B., MUKERJI, M.K., & HINKS, C.F. (1984) Effect of
temperature on the toxicity of cypermethrin to nymphs of the
migratory grasshopper. Melanoplus sanguinipes (Orthoptera:
Acridoidea). Can. Entomol., 116(9): 1153-1156.
FABELLAR, L.T. & HEINRICHS, E.A. (1984) Toxicity of
insecticides to predators of rice brown planthoppers,
Nilaparvata lugens (Stal) (Homoptera: Delphacidae) Environ.
Entomol., 13: 832-837.
FAO/WHO (1980a) Pesticide residues in food. Report of the
1979 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 20;
Suppl).
FAO/WHO (1982a) Pesticide residues in food. Report of the
1981 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the
1982 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 49).
FAO/WHO (1984) Pesticide residues in food. Report of the
1983 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 56).
FAO/WHO (1985a) 1983 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper No. 61).
FAO/WHO (1985b) Pesticide residues in food. Report of the
1984 Joint Meeting of the FAO Panel of Experts on Pesticide
Residues in Food and the Environment and the WHO Expert Group
on Pesticide Residues, Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection
Paper No. 62).
FAO/WHO (1985c) 1984 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Protection and Protection Paper No. 67).
FAO/WHO (1986) Guide to Codex recommendations concerning
pesticide residues. Part 8. Recommendations for methods of
analysis of pesticide residues, 3rd ed., Rome, Codex Committee
on Pesticide Residues.
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact
Dermatitis, 13: 140-147.
FRANK, R., BRAUN, H.E., RITCEY, G., MCEWEN, F.L., & SIRONS,
G.J. (1982) Pesticide residues in onions and carrots grown
on organic soils, Ontario, 1975-80. J. econ. Entomol., 75:
560-565.
FURUZAWA, K., MIKAMI, N., YAMADA, H., & MIYAMOTO, J. (1986)
Metabolism of the pyrethroid insecticide cypermethrin in
cabbages. J. Pestic. Sci., 11: 253-260.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes
of pyrethroid action in the cockroach. Pestic. Biochem.
Physiol., 15: 181-191.
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most
potent class antagonize GABA action at the crayfish
neuromuscular junction. Neurosci. Letters, 40: 163-168.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982)
Pyrethroid toxicology: Protective effects of Diazepam and
phenobarbital in the mouse and the cockroach. Toxicol. applied
Pharmacol., 66:290-296.
GARFORTH, B.M. (1982) WL 85871 and cypermethrin: chronic
toxicity to Daphnia magna, Sittingbourne, Shell Research
(SBGR.82.119).
GARFORTH, B.M. (1983) The effect of RIPCORD on the non-
target fauna of maize, Sittingbourne, Shell Research (SBGR.83.
045).
GERIG, L. (1979) [The toxicity of synthetic pyrethrines to
foraging bees.] Schwei z. Bienen-Ztg, 101: 228-236 (in German).
GERIG, L. (1981) [The toxicity of synthetic pyrethrines to
foraging bees. Part 2.] Schweiz. Bienen-Ztg, 104: 155-174 (in
German).
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977a) Effects of
high dietary levels on clinical behaviour and structure of
sciatic nerves in the rat, Fernhurst, Imperial Chemical
Industries, Central Toxicology Laboratory, pp. 383
(Unpublished ICI report).
GLAISTER, J.R., GARE, C.W., MARSAT, G.J., PHILLIPS, C., &
PRATT, I. (1977b) 90-day feeding study in rats, Fernhurst,
Imperial Chemical Industries, Central Toxicology Laboratory,
pp. 383 (Unpublished ICI report).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural
variations affecting pyrethroid neurotoxicity. Neurobehav.
Toxicol. Teratol., 4(6): 793-799.
GRAY, K. & GRAYSON, B.T. (1980) Determination of the
n-octanol/water partition coefficients of BIRLANE AND GARDONA
and a literature search for the values of other group
compounds, Sittingbourne, Shell Research (BLGR.80.103).
GRAYSON, B.T., LANGNER, E., & WELLS, D. (1982) Comparison of
two gas saturation methods for the determination of the vapour
pressure of cypermethrin. Pestic. Sci., 13: 552-556.
HAGLEY, E.A.C., PREE, D.J., & SIMPSON, C.M. (1981) Toxicity
of insecticides to parasites of the spotted tentiform
leafminer (Lepidoptera: Gracillariidae). Can. Entomol., 113:
899-906.
HALL, J.S., LEAHEY, J.P., & CURL, E.A. (1981) Cypermethrin:
photodegradation on a soil surface, Fernhurst, Imperial
Chemical Industries (Unpublished ICI Report No. RJ0192B).
HARGREAVES, J.R. & COOPER, L.P. (1979) Phytotoxicity tests
with pyrethroid insecticides on glasshouse grown tomato
seedlings. Queensland J. Agric. anim. Sci., 36(2): 151-154.
HARRIS, C.R. & TURNBULL, S.A. (1978) Laboratory studies on
the contact toxicity and activity in soil of four pyrethroid
insecticides. Can. Entomol., 110: 285-288.
HARRIS, C.R. & TURNBULL, S.A. (1980) Toxicity of some
insecticides to insecticide-susceptible strains of the onion,
cabbage and seedcorn maggots (Diptera: Anthomyiidae) and the
dark-sided cutworm (Lepidoptera: Nocturidae). Can. Entomol.,
112(10): 1029-1032.
HARRIS, C.R., CHAPMAN, R.A., & HARRIS, C. (1981) Laboratory
studies on the persistence and behaviour in soil of 4
pyrethroid insecticides. Can. Entomol., 113: 685-694.
HARRIS, C.R., TURNBULL, S.A., & MCLEOD, D.G.R. (1985)
Contact toxicity of twenty-one insecticides to adults of the
carrot rust fly (Diptera: Psilidae). Can. Entomol., 117:
1025-1027.
HART, T.B., SPINKS, C.A., MARLOW, R.G., & SUTHERS, J.R.
(1982) A study of the exposure and health of Indian workers
spraying, Ambush and Cymbush on cotton using high volume
hand-held spray applicators, Fernhurst, Imperial Chemical
Industries, Plant Protection Division (Report TMF.1841-B).
HELLING, C.S. & TURNER, B.C. (1968) Pesticide mobility:
determination in soil by thin-layer chromatography. Science,
162: 562-563.
HEND, R.W. & BUTTERWORTH, S.T.G. (1976) Toxicity studies on
the insecticide WL 43467: a three-month feeding study in rats,
Sittingbourne, Shell Research (TLGR.0027.76) (Unpublished
report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977a) Toxicity studies on
the insecticide WL 42641: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W. & BUTTERWORTH, S.T.G. (1977b) Toxicity studies on
the insecticide WL 43481: a five-week feeding study in rats,
Sittingbourne, Shell Research (Unpublished report).
HEND, R.W., HENDY, R., & FLEMING, D.J. (1978) Toxicity
studies on the insecticide WL 43467: a three-generation
reproduction study in rats, Sittingbourne, Shell Research
(TLGR.0188.78).
HENDERSON, C. & PARKINSON, G.R. (1981) Cypermethrin
technical: subacute dermal toxicity study in rabbits, Alderley
Park, Imperial Chemical Industries (Report No. CTL/P/588).
HINE, C.H. & ZUIDEMA, H.H. (1970) The toxicological
properties of hydrocarbon solvents. Ind. Med., 39(5): 39-44.
HO, S.H., LEE, B.H., & SEE, D. (1983) Toxicity of
deltamethrin and cypermethrin to the larvae of the diamond-
back moth, Plutella xylostella L. Toxicol. Lett., 19: 127-131.
HOLDEN, J.S. (1979) Absorption and metabolism of permethrin
and cypermethrin in the cockroach and the cotton leaf-worm
larvae. Pestic. Sci., 10: 295-307.
HOPKINS, A.R., MOORE, R.F., & JAMES, W. (1984) Contact and
residual toxicities of pyrethroids and organophosphorus
compounds to the Boll weevil (Coleoptera: Curculionidae) J.
Georgia Entomol. Soc., 19(1): 27-34.
HUCKLE, K.R., HUTSON, D.H., & MILLBURN, P. (1981a) Species
differences in the metabolism of 3-phenoxybenzoic acid. Drug
Metab. Disp., 9: 352-359.
HUCKLE, K.R., CHIPMAN, J.K., HUTSON, D.H., & MILLBURN, P.
(1981b) Metabolism of 3-phenoxybenzoic acid and the
enterohepatorenal disposition of its metabolites in the rat.
Drug Metab. Disp., 9(4): 360-368.
HUTSON, D.H. (1978a) The elimination of radioactivity by
mice following oral dosing with 14 C-cis- and 14 C-trans-WL
43467 (cypermethrin), Sittingbourne, Shell Research
(TLGR.0079.78).
HUTSON, D.H. (1978b) The metabolites of cis- and trans-
cypermethrin (WL 43467) in mice, Sittingbourne, Shell Research
(TLGR.102.78).
HUTSON, D.H. (1979a) The metabolic fate of synthetic
pyrethroid insecticide in mammals. Prog. Drug Metab., 3:
215-252.
HUTSON, D.H. (1979b) The metabolism of WL 43467 in mammals.
Metabolites derived from (14 C-cyano)cypermethrin (WL 43467) in
rats, Sittingbourne, Shell Research (TLGR.79.183).
HUTSON, D.H. & CASIDA, J.E. (1978) Taurine conjugation in
metabolism of 3-phenoxybenzoic acid and the pyrethroid
insecticide cypermethrin in mouse. Xenobiotica, 8(9): 565-571.
HUTSON, D.H. & STOYDIN, G. (1987) Excretion and residues of
the pyrethroid insecticide cypermethrin in laying hens.
Pestic. Sci., 18: 157-168.
HUTSON, D.H., GAUGHAN, L.C., & CASIDA, J.E. (1981)
Metabolism of the cis- and trans-isomers of cypermethrin in
mice. Pestic. Sci., 12: 385-398.
INGLESFIELD, C. (1982) The effects of an aerial application
of RIPCORD on the non-target fauna of oil seed rape in France,
Sittingbourne, Shell Research (SBGR.82.364).
INGLESFIELD, C. (1984) Toxicity of the pyrethroid
insecticides cypermethrin and WL 85871 to the earthworm
Eisenia foetida Savigny. Bull. environ. Contam. Toxicol.,
33(5): 568-570.
INGLESFIELD, C. & SHERWOOD, C.M. (1983) Toxicity of
cypermethrin to the earthworm, Eisenia foetida L (Oligochaeta:
Lumbriculidae) in laboratory tests, Sittingbourne, Shell
Research (SBGR.83.070).
ISHAAYA, I., ASCHER, K.R.S., & CASIDA, J.E. (1983)
Pyrethroid synergism by esterase inhibition in Spodoptera
littoralis (Boisd.) larvae, Crop Prot., 2(3): 335-343.
JACKSON, C. (1977) The leaching of WL 43467 through
laboratory soil columns, Sittingbourne, Shell Research
(Unpublished Report BLGR.0150.77).
JOIA, B.S., LOSCHIAVO, S.R., & WEBSTER, G.R.B. (1981) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat. In: Proceedings of the 16th Annual Workshop
on Pesticide Residue Analysis, Western Canada, pp. 14-16.
JOIA, B.S., SARMA, L.P., & WEBSTER, G.R.B. (1985a) Gas
chromatographic determination of cypermethrin and fenvalerate
residues in wheat and milled fractions. J. environ. anal.
Chem., 21: 179-184.
JOIA, B.S., WEBSTER, G.R.B., & LOSCHIAVO, S.R. (1985b)
Cypermethrin and fenvalerate residues in stored wheat and
milled fractions. J. agric. food Chem., 33: 618-622.
JONES, B.J. (1981) Cypermethrin: bioaccumulation study in
the rat, Alderley Park, Imperial Chemical Industries (Report
No. CTL/P/599).
JORDAN, T.W. & CHANG, C.K. (1981) Pyrethroid resistance
mechanisms in Porina caterpillars. In: Proceedings of the 34th
New Zealand Weed and Pest Control Conference, pp. 167-169.
KAUFMAN, D.D., JORDAN, E.G., & KAYSER, A.J. (1978)
Degradation of cypermethrin and permethrin in soil. In:
Proceedings of the 175th Meeting on Pesticides of the American
Chemical Society, Washington, DC, American Chemical Society
(Abstract Paper No. 47).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J.
(1981) Movement of cypermethrin, decamethrin, permethrin, and
their degradation products in soil. J. agric. food Chem., 29:
239-245.
KNAPP, F.W., HERALD, F., & SCHWINGHAMMER, K.A. (1985)
Comparative toxicity of selected insecticides to laboratory-
reared and field-collected face flies (Diptera: Muscidae) J.
econ. Entomol., 78: 860-862.
KNIGHT, R.J. (1982) The toxicity of the pyrethroid WL 85871
against honey bee Apis mellifera, Sittingbourne, Shell
Research (SBGR.82.023).
KNOWLES, C.O. & EL-SAYED, G.N. (1985) Formanilide
enhancement of acaricide toxicity to Tetranychus urticae Koch
(Acari: Tetranychidae), J. econ. Entomol., 78: 308-310.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in
relation to body weight and temperature. Water Res., 15:
503-505.
LAUREN, D.R. & HENZEL, R.F. (1977) Residual lives of two
synthetic prethroid insecticides applied to pasture. In:
Proceedings of the 30th New Zealand Weed and Pest Control
Conference, pp. 207-210.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology:
mouse intracerebral structure-toxicity relationship. Pestic.
Biochem. Physiol., 18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action
of pyrethroid insecticides on the gamma-aminobutyric acid
receptor-ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Inter-
actions of pyrethroid insecticides with chloride ionophore-
associated binding sites. Neurotoxicol., 6: 87-98.
LEAHEY, J.P. (1979) The metabolism and environmental
degradation of the pyrethroid insecticides. Outlook Agric.,
10(3): 135-142.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London,
Philadelphia, Taylor and Francis.
LEAKE, L.D., LAUCKNER, S.M., & FORD, M.G. (1979)
Relationship between neurophysiological effects of selected
pyrethroids and toxicity to the leech Haemopsis sanguisuga and
the locust Schistocerca gregaria. Neurobiol. Pest. Action.,
London, pp. 423-430.
LE PATOUREL, G.N.J. & SINGH, J. (1984) Toxicity of amorphous
silicas and silica-pyrethroid mixtures to Tribolium castaneum
(Herbst) (Coleoptera: Tenebrionidae). J. stored Prod. Res.,
20(4): 183-190.
LE QUESNE, P.M., MAXWELL, I.C., & BUTTERWORTH, S.T.G. (1980)
Transient facial sensory symptoms following exposure to
synthetic pyrethroids: a clinical and electrophysiological
assessment. Neurotoxicology, 2: 1-11.
LINDSAY, S., BANHAM, P.B., CHART, I.S., CHALMERS, D.T.,
GODLEY, M.J., & TAYLOR, K. (1982) Cypermethrin: life-time
feeding study in mice, Alderley Park, Imperial Chemical
Industries (Report No. CTL/P/687).
LIU, M.Y., CHEN, J.S., & SUN, C.N. (1984) Synergism of
pyrethroids by several compounds in larvae of the Diamondback
moth (Lepidoptera: Plutellidae) J. econ. Entomol., 77: 851-856.
LOCK, E.A. & BERRY, P.N. (1981) Biochemical changes in the
rat cerebellum following cypermethrin administration. Toxicol.
appl. Pharmacol., 59(3): 508-514.
LOVERIDGE, D. & COOK, K.A. (1978a) Determination of the
effects of WL 43467 on microbial activity in soil. II. Effects
on oxygen uptake, Sittingbourne, Shell Research (BLGR.0004.78).
LOVERIDGE, D. & COOK, K.A. (1978b) Determination of the
effects of WL 43467 on microbial activity in soil. III.
Effects on nitrogen fixation, Sittingbourne, Shell Research
(BLGR.0007.78).
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve
membrane effects of pyrethroids and DDT analogs. Pestic.
Biochem. Physiol., 20: 203-216.
MCAUSLAND, H.E., BUTTERWORTH, S.T.G., & HUNT, P.F. (1978)
Toxicity studies on the insecticide WL 43467: a two-year
feeding study in rats, Sittingbourne, Shell Research
(TLGR.0189.78).
MCDONALD, S. (1979) Evaluation of insecticides for control
of the army cutworm. J. econ. Entomol., 72(2): 277-280.
MCKEE, M.J. & KNOWLES, C.O. (1985) Pharmacokinetics of
pyrethroids in two-spotted spider mites. Pestic. Biochem.
Physiol., 24: 326-335.
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality
of permethrin, cypermethrin, and fenvalerate to salmon,
lobster, and shrimp. Bull. environ. Contam. Toxicol., 25:
950-955.
MANI, M. & KRISHNAMOORTHY, A. (1984) Toxicity of some
insecticides to Apanteles plutellae, a parasite of the
diamondback moth. Trop. Pestic. Manage., 30(2): 130-132.
MARTIN, H. & WORTHING, C.R. (1977) The pesticide manual, 5th
ed., Croydon, British Crop Protection Council.
MAUCK, W.L., OLSEN, L.E., & MARKING, L.L. (1976) Toxicity of
natural pyrethrins and five pyrethroids to fish. Arch.
environ. Contam. Toxicol., 4: 18-29.
MEISNER, J., ASCHER, K.R.S., & EIZICK, C. (1984) Effect of
the commercial phagostimulants coax and gustol on the toxicity
of cypermethrin and deltamethrin against Spodoptera littoralis
(Lepidoptera: Noctuidae). J. econ. Entomol., 77: 1123-1126.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK,
J. (1983) Farm chemical handbook. C. Pesticide dictionary,
Willoughby, Ohio, Meister Publishing Company, pp. C67.
MIKAMI, N., WAKABAYASHI, N., YAMADA, H., & MIYAMOTO, J.
(1984) New conjugated metabolites of 3-phenoxybenzoic acid in
plants. Pestic. Sci., 15: 531-542.
MIKAMI, N., YOSHIMURA, J., KANEKO, H., YAMADA, H., & MIYAMOTO,
J. (1985) Metabolism in rats of 3-phenoxybenzyl alcohol and
3-phenoxybenzoic acid glucoside conjugates formed in plants.
Pestic. Sci., 16: 33-45.
MIYAMOTO, J. (1981) The chemistry, metabolism, and residue
analysis of synthetic pyrethroids. Pure appl. Chem., 53:
1967-2022.
MIYAMOTO, J. & MIKAMI, N. (1983) Degradation of pyrethroid
insecticides in the field. In: IUPAC Pesticide chemistry,
human welfare and the environment, Oxford, Pergamon Press, pp.
193-200.
MORE, J.E., ROBERTS, T.R., & WRIGHT, A.N. (1978) Studies of
the metabolism of 3-phenoxybenzoic acid in plants. Pestic.
Biochem. Physiol., 9: 268-280.
MUIR, D.C.G., RAWN, G.P., TOWNSEND, B.E., LOCKHART, W.L., &
GREENHALGH, R. (1985) Bioconcentration of cypermethrin,
deltamethrin, fenvalerate, and permethrin by Chironomus
tentans larvae in sediment and water. Environ. Toxicol. Chem.,
4(1): 51-61.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978)
Biological activity and longevity of new synthetic pyrethroids
against mosquitoes and some non-target insects. Mosq. News.,
38(1): 90-96.
NOBLE, R.M. & HAMILTON, D.J. (1985) Stability of
cypermethrin and cyfluthrin on wheat in storage. Pestic. Sci.,
16: 179-185.
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976a) Neurotoxic
effects of S 5602 and NRDC 149 by dermal application in rats,
Osaka, Sumitomo Chemical Company (AT-60-0047).
OKUNO, Y., KOHDA, H., & KADOTA, T. (1976b) Neurotoxic
effects of natural pyrethrins and resmethrin by oral application
in rats, Osaka, Sumitomo Chemical Company (AT-60-0052).
OSMAN, A.A., ZOHDY, G.I., & MOMEN, F.M. (1982) Effect of
some pesticides on the food requirements of the predatory
mite, Amblyseius gossipiu-El-Badry. In: Griffiths, D.A. &
Bowman, C.E., ed. Acarology VI, Chichester, Ellis Horwood Ltd,
Vol. 2, pp. 669-672.
OWEN, D.E. & BUTTERWORTH, S.T.G. (1977) Toxicity of
pyrethroid insecticides: investigation of the neurotoxic
potential of WL 43467 to adult domestic hens, Sittingbourne,
Shell Research (TLGR.0134.77).
PEARSON, N. & SHIRES, S.W. (1981) A field study in France of
the effects on honey bees of an aerial application of RIPCORD
in winter-sown oil seed rape, Sittingbourne, Shell Research
(SBGR.81.304).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning, Geneva, World Health Organization
(Report No. VBC/84.889).
PLUYMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity
of synthetic pyrethroids in Salmonella typhimurium strains and
in V79 Chinese hamster cells. Mutat. Res., 137: 7-15.
POTTER, D. & MCAUSLAND, H.E. (1980) Toxicity studies on the
insecticide WL 43467: a study of liver microsomal enzyme
activity in rats fed WL 43467 for 2 years, Sittingbourne,
Shell Research (TLGR.80.057).
POULOS, L., ATHANASELIS, S., & COUTSELINIS, A. (1982) Acute
intoxication with cypermethrin (NRDC 149). J. Toxicol. clin.
Toxicol., 19(5): 519-520.
PREE, D.J. & HAGLEY, E.A.C. (1985) Toxicity of pesticides to
Chrysopa oculata Say (Neuroptera : Chrysopidae). J. econ.
Entomol., 78(1): 129-132.
PRICE, J.B. (1981a) Toxicology of pyrethroids: the acute
oral and intraperitoneal toxicity of cypermethrin,
Sittingbourne, Shell Research (SBGR.81.069).
PRICE, J.B. (1981b) Toxicology of pyrethroids: the acute
oral and percutaneous toxicities of RIPCORD and endosulfan,
individually or combined in a 1:1 mixture, Sittingbourne,
Shell Research (SBGR.81.121).
PRINSEN, G.H. & SITTERT, N.J., VAN (1978) Exposure and
medical monitoring study of the pyrethroid WL 43467 after
single application on cotton in Ivory Coast, The Hague, Shell
Internationale Research Mij (TOX 78-004).
PRINSEN, G.H. & SITTERT, N.J., VAN (1979) Exposure and
medical monitoring study of the pyrethroid RIPCORD (WL 43467)
after one season of spraying on cotton in Ivory Coast, The
Hague, Shell Internationale Research Mij (TOX 79-001).
RAJAKULENDRAN, S.V. & PLAPP, F.W., Jr (1982) Comparative
toxicities of five synthetic pyrethroids to the tobacco
budworm (Lepidoptera: Noctuidae), an Ichneumonid parasite,
Campoletis sonorensis and a predator, Chrysopa carnea. J.
econ. Entomol., 75: 769-772.
RAPLEY, J.H., ARNOLD, D.J., & VINCENT, J. (1981)
Cypermethrin: degradation in river and pond waters and
sediments, Fernhurst, Imperial Chemical Industries, Plant
Protection Division (Report No. RJ0175B) (Unpublished ICI
data).
REIFF, B. (1976) The acute toxicity of the pyrethroid
insecticide WL43467 to Brown trout (S. trutta), Sittingbourne,
Shell Research (TLBR.0096.76).
REIFF, B. (1977) The acute toxicity of the pyrethroid WL
43467 to Daphnia magna, Sittingbourne, Shell Research (TLGR.
0155.77).
REIFF, B. (1978a) The effect of suspended solids on the
toxicity of WL 43467 to Rainbow trout (Salmo gairdneri),
Sittingbourne, Shell Research (TLGR.0007.78).
REIFF, B. (1978b) The acute toxicity of the pyrethroid
insecticide WL 43467 to Rainbow trout (Salmo gairdneri),
Common carp (Cyprinus carpio), and Rudd (Scardinius
erythrophthalmus), Sittingbourne, Shell Research (TLGR.0067.
78).
RHODES, C., JONES, B.K., CROUCHER, A., HUTSON, D.H., LOGAN,
C.J., HOPKINS, R., HALL, B.E., & VICKERS, J.A. (1984) The
bioaccumulation and biotransformation of cis, trans-
cypermethrin in the rat. Pestic. Sci., 25: 471-480.
RILEY, D. & HILL, I.R. (1983) Adsorption reduces activity of
pesticides in soil and water. In: Proceedings of the 10th
International Congress on Plant Protection, Brighton, 20-25
November, 1983, Vol. 2, pp. 728 (4B-R18).
RISKALLAH, M.R. (1984) Influence of posttreatment
temperature on the toxicity of pyrethroid insecticides to
susceptible and resistant larvae of the Egyptian cotton
leafworm, Spodoptera littoralis (Boisd.) Experientia (Basel),
40(2): 188-190.
RIVIERE, J.L., BACH, J., & GROLLEAU, G. (1983) Effect of
pyrethroid insecticides and N-(3,5-dichlorophenyl)
dicarboximide fungicides on microsomal drug metabolizing
enzymes in the Japanese quail (Coturnix coturnix). Bull.
environ. Contam. Toxicol., 31: 479-485.
ROBERTS, B.L. & DOROUGH, H.W. (1984) Relative toxicities of
chemicals to the earthworm Eisenia foetida. Environ. Toxicol.
Chem., 3(1): 67-68.
ROBERTS, T.R. (1981) The metabolism of the synthetic
pyrethroids in plants and soils. In: Hutson, D.H. & Roberts,
T.R., ed. Progress in pesticide biochemistry, New York, John
Wiley and Sons, Vol. 1, pp. 115-146.
ROBERTS, T.R. & STANDEN, M.E. (1977) Degradation of the
pyrethroid cypermethrin NRDC 149(±)-alpha-cyano-3-phenoxy-
benzyl(±)- cis,trans-3-(2,2-dichlorovinyl-2,2-dimethylcyclo-
propanecarboxylate and the respective cis-(NRDC 160) and the
trans-(NRDC 159) isomers in soils. Pestic. Sci., 8: 305-319.
ROBERTS, T.R. & STANDEN, M.E. (1981) Further studies of the
degradation of the pyrethroid insecticide cypermethrin in
soils. Pestic. Sci., 12: 285-296.
ROSE, B. (1981) A dietary toxicity study of WL 43467 in
Mallard ducks, Sittingbourne, Shell Research (TLGR.80.033).
ROSE, G.P. (1982) Toxicology of pyrethroids: the acute oral
and percutaneous toxicity of WL 85871 (cis-2-RIPCORD)
comparison with RIPCORD, Sittingbourne, Shell Research
(SBGR.82.130).
ROSE, G.P. (1983) Neurotoxicity of WL 85871 comparison with
WL 43467: the effect of twenty oral doses of WL 85871 or WL
43467 over a period of 4 weeks on the rat sciatic/posterior
tibial nerve, trigeminal nerve, and trigeminal ganglion,
Sittingbourne, Shell Research (SBGR.83.185).
ROSE, G.P. & DEWAR, A.J. (1978) Toxicity studies on the
insecticide WL 43467: the effect of age on the neurotoxicity
of WL 43467 to rats, Sittingbourne, Shell Research
(TLGR.0039.78).
ROSE, G.P. & DEWAR, A.J. (1979a) Toxicity studies on the
RIPCORD/AZODRIN formulation EF 5254: biochemical and
functional studies on the neurotoxicity of the formulation EF
5254 in the rat, Sittingbourne, Shell Research (TLGR.79.027).
ROSE, G.P. & DEWAR, A.J. (1979b) A neurotoxicity study on
the pyrethroid metabolite 3-phenoxybenzoic acid (3-PBA),
Sittingbourne, Shell Research (TLGR.79.076).
ROSE, G.P. & DEWAR, A.J. (1983) Intoxication with four
synthetic pyrethroids fails to show any correlation between
neuromuscular dysfunction and neurobiochemical abnormalities
in rats. Arch. Toxicol., 53: 297-316.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of
pyrethroids on a nerve - muscle preparation of the clawed
frog, Xenopus laevis. Pestic. Biochem. Physiol., 25: 176-187.
RUZO, L.O. (1983) Involvement of oxygen in the
photoreactions of cypermethrin and other halogenated
pyrethroids. J. agric. food Chem., 31: 1113-1115.
RUZO, L.O. & CASIDA, J.E. (1980) Pyrethroid photochemistry:
mechanistic aspects in reactions of the (dihalogenovinyl)-
cyclopropanecarboxylate substituent. J.C.S. Perkin Trans. I,
728-732.
RUZO, L.O., HOLMSTEAD, R.L., & CASIDA, J.E. (1977)
Pyrethroid photochemistry: decamethrin. J. agric. food Chem.,
25(6): 1385-1389.
SAAD, A.S.A., ELEWA, M.A., ALY, N.M., AUDA, M., & EL-SEBAE,
A.H. (1981) Toxicological studies on the Egyptian cotton
leafworm Spodoptera littoralis. I. Potentiation and antagonism
of synthetic pyrethroid, organophosphorus and urea derivative
insecticides. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 46(2):
559-571.
SAKATA, S., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J. (1986)
Degradation and leaching behaviour of the pyrethroid
insecticide cypermethrin in soils. J. Pestic. Sci., 11: 71-79.
SCOTT, J.G. & GEORGHIOU, G.P. (1984) Influence of
temperature on knockdown, toxicity and resistance to
pyrethroids in the housefly, Musca domestica. Pestic. Biochem.
Physiol., 21: 53-62.
SEEHY, M.A., SHALABI, H.G., SHAKER, N., & BADR, E. (1983) In
vivo induction of sister chromatid exchanges in mice by
cypermethrin. Proceedings of the International Conference on
Environmental Hazards of Agrochemicals, 1982, Vol. 1, 659-673.
SHERWOOD, C.M. & SHIRES, S.W. (1981) The effect of RIPCORD
on the invertebrate fauna of winter barley in France,
Sittingbourne, Shell Research (SBGR.81.070).
SHIRES, S.W. (1980) Soil surface predators in arable land:
the effects of farming practices. Span, 23(2): 62-64.
SHIRES, S.W. (1982a) The effect of RIPCORD on the
invertebrate fauna of winter wheat in France, Sittingbourne,
Shell Research (SBGR.81.303).
SHIRES, S.W. (1982b) A field study in France of the effects
on honey bees of an aerial application of RIPCORD in spring-
grown oil seed rape, Sittingbourne, Shell Research
(SBGR.82.066).
SHIRES, S.W. (1982c) A study of the effects of an aerial
application of RIPCORD on the invertebrate fauna of winter
wheat, Sittingbourne, Shell Research (SBGR.82.304).
SHIRES, S.W. (1983a) The use of small enclosures to assess
the toxic effects of cypermethrin in fish under field
conditions. Pestic. Sci., 14: 475-480.
SHIRES, S.W. (1983b) Pesticides and honey bees. Case studies
with RIPCORD and FASTAC. Span, 26(3): 118-120.
SHIRES, S.W. & BENNETT, D. (1982) Spray drift from aerial
application of RIPCORD to winter wheat in Kent, UK: fate and
effects in adjacent drainage ditches, Sittingbourne, Shell
Research (SBGR.82.274).
SHIRES, S.W. & BENNETT, D. (1985) Contamination and effects
in freshwater ditches, resulting from an aerial application of
cypermethrin. Ecotoxicol. environ. Saf., 9: 145-158.
SHIRES, S.W. & DEBRAY, P. (1982) Pyrethroids and the bee
problem. Shell Agric., May: 1-3.
SHIRES, S.W. & TIPTON, J.D. (1982) A study of the effects of
BIRLANE and RIPCORD on the hymenopterous parasites of white
flies on cotton in the Sudan, Sittingbourne, Shell Research
(SBGR.82.342).
SHIRES, S.W., BENNETT, D., & KANE, D.F. (1979) The effects
of WL 43467 on soil surface fauna, earthworms, and litter
composition, Sittingbourne, Shell Research (TLGR.79.074).
SHIRES, S.W., BENNETT, D., & CROSSLAND, N.O. (1980) Spray
drift from RIPCORD applications to arable crops in Suffolk,
UK: fate and effects in adjacent farm ponds, Sittingbourne,
Shell Research (TLGR.80.150).
SHONO, T. & CASIDA, J.E. (1978) Species-specificity in
enzymatic oxidation of pyrethroid insecticides:
3-phenoxybenzyl and alpha-cyano-3-phenoxybenzyl 3-(2,2-dihalo-
vinyl)-2,2-dimethylcyclopropanecarboxylates. J. Pestic. Sci.,
3: 165-168.
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of
trans- and cis-permethrin and trans- and cis-cypermethrin, and
decamethrin by microsomal enzymes. J. agric. food Chem.,
27(2): 316-325.
SMART, L.E. & STEVENSON, J.H. (1982) Laboratory estimation
of toxicity of pyrethroid insecticides to honey bees:
relevance to hazard in the field. Bee World, 63(4): 150-152.
SMIES, M., EVERS, R.H.J., PEIJNENBURG, F.H.M., & KOEMAN, J.H.
(1980) Environmental aspects of field trials with pyrethroids
to eradicate tsetse fly in Nigeria. Ecotoxicol. environ. Saf.,
4: 114-128.
SMITH, T.M. & STRATTON, G.W. (1986) Effects of synthetic
pyrethroid insecticides on non-target organisms. Residue Rev.,
97: 93-120.
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid
structure on rates of hydrolysis and oxidation by mouse liver
microsomal enzymes. Pestic. Biochem. Physiol., 7: 391-401.
SPIELBERGER, U., NA'ISA, B.K., KOCH, K., MANNO, A., SKIDMORE,
P.R., & COUHS, H.H. (1979) Field trials with the synthetic
pyrethroids permethrin, cypermethrin, and decamethrin against
Glossina (Diptera: gloninidae) in Nigeria. Bull. entomol.
Res., 69: 667-689.
STANDEN, M.E. (1977) The leaching and degradation of the
insecticide WL43467 when applied in sheep-dip solution to
soil, Sittingbourne, Shell Research (BLGR.0141.77)
(Unpublished report).
STELZER, K.J. & GORDON, M.A. (1984) Effects of pyrethroids
on lymphocyte mitogenic responsiveness. Res. Commun. chem.
Pathol. Pharmacol., 46(1): 137-150.
STEPHENSON, R.R. (1980a) The acute toxicity of WL 43467 to
some freshwater invertebrates in static water tests,
Sittingbourne, Shell Research (TLGR.80.040).
STEPHENSON, R.R. (1980b) The acute toxicity of cypermethrin
(WL 43467) to the freshwater shrimp (Gammarus pulex) and
larvae of the mayfly (Cloeon dipterum) in continuous-flow
tests, Sittingbourne, Shell Research (TLGR.80.079).
STEPHENSON, R.R. (1981a) RIPCORD: the acute toxicity of an
EC formulation to Tilapia nilotica in the laboratory,
Sittingbourne, Shell Research (SBGR.81.028).
STEPHENSON, R.R. (1981b) Cypermethrin: acute toxicity to
Tilapia nilotica in a continuous-flow test, Sittingbourne,
Shell Research (SBGR.81.080).
STEPHENSON, R.R. (1982a) RIPCORD: a laboratory study of the
acute toxicity of an EC formulation to Tilapia nilotica in the
presence of suspended solids, Sittingbourne, Shell Research
(SBGR.81.235).
STEPHENSON, R.R. (1982b) WL 85871 and cypermethrin: a
comparison of their acute toxicity to Salmo gairdneri, Daphnia
magna, and Selenastrum capricornutum, Sittingbourne, Shell
Research (SBGR.81.277).
STEPHENSON, R.R. (1982c) RIPCORD, BIRLANE, and FURADAN:
acute toxicity to common carp (Cyprinus carpio L.) in the
laboratory and in rice paddies, Sittingbourne, Shell Research
(SBGR.82.030).
STEPHENSON, R.R. (1982d) WL 85871 and cypermethrin: a
comparative study of their toxicity to the Fathead minnow
Pimephales promelas (Rafinesque), Sittingbourne, Shell
Research (SBGR.82.298).
STEPHENSON, R.R. (1982e) Aquatic toxicology of cypermethrin.
I. Acute toxicity to some freshwater fish and invertebrates in
laboratory tests. Aquat. Toxicol., 2: 175-185.
STEPHENSON, R.R. (1983) Pesticides and freshwater animals. A
case study with RIPCORD. Span, 26(3): 121-122.
STEPHENSON, R.R., CHOI, S.Y., & OLMOS-JEREZ, A. (1984)
Determining the toxicity and hazard to fish of a rice
insecticide. Crop Prot., 3(2): 151-165.
STEVENS, J.E.B. & HILL, I.R. (1980) Mobility of cypermethrin
and its degradation products in soil columns, Fernhurst,
Imperial Chemical Industries (Report No. RJ/0166-B)
(Unpublished ICI data).
SUHAS, Y. & DEVAIAH, M.C. (1985) Studies on the effect of
insecticides sprayed mulberry leaves to silkworm, Bombyx mori
L. Pesticides, 19(10): 53-54, 57.
SUNDARARAJAN, R. & CHAWLA, R.P. (1983) Simple, sensitive
technique for detection and separation of halogenated
synthetic pyrethroids by thin layer chromatography. J. Assoc.
Off. Anal. Chem., 66(4): 1009-1012.
SURULIVELU, T. & MENON, M.V. (1982) Contact toxicity of
synthetic pyrethroids, organophosphorus and carbamate
insecticides to adults of the parasite Chelonus blackburni
Cameron. J. Agric. Sci. Camb., 98: 331-334.
SUTHERS, J.R. & MARLOW, R.G. (1981) A study of the exposure
and health of Indian workers spraying RIPCORD on cotton over
five consecutive days using mistblower and knapsack
applicators, The Hague, Shell Internationale Research Mij (TOX
81-003).
SWAINE, H. & SAPIETS, A. (1980a) Residue transfer study with
dairy cows fed on a diet containing the insecticide.
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0186-B).
SWAINE, H. & SAPIETS, A. (1980b) Residue levels of the major
metabolites of the insecticide in the milk and tissues of
dairy cows fed on a diet containing cypermethrin at 50 mg/kg,
Fernhurst, Imperial Chemical Industries (Unpublished Report
No. RJ/0198-B).
TAG EL-DIN, A., ABBAS, M.M., ALY, H.A., TANTAWY, G., & ASKAR,
A. (1981) Acute toxicities to Mugil cephalus fry caused by
some herbicides and new pyrethroids. Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 46(1): 387-391.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985a) Hydrolysis of the pyrethroid insecticide cypermethrin
in aqueous media. J. Pestic. Sci., 10: 643-648.
TAKAHASHI, N., MIKAMI, N., MATSUDA, T., & MIYAMOTO, J.
(1985b) Photodegradation of the pyrethroid insecticide
cypermethrin in water and on soil surface. J. Pestic. Sci.,
10: 629-642.
TAYLOR, S.M., ELLIOTT, C.T., & BLANCHFLOWER, J. (1985)
Cypermethrin concentrations in hair of cattle after
application of impregnated ear tags. Vet. Rec., 116(23): 620.
TESH, J.M., TESH, S.A., & DAVIES, W. (1978) WL 43467:
effects upon the progress and outcome of pregnancy in rat,
Stock, Life Science Research (LSR Report No. 78/SHL2/364).
TEWARI, G.C. & KRISHNAMOORTHY, P.N. (1985) Selective
toxicity of some synthetic pyrethroids and conventional
insecticides to aphid predator, Menochilus sexmaculatus
Fabricius. Indian J. agric. Sci., 55(1): 40-43.
TRIGG, C.E., BUTTERWORTH, S., & HUNT, P.F. (1977)
Neurotoxicity of pyrethroids: a study of teased nerves from
rats fed WL 43467 for 12 months, Sittingbourne, Shell Research
(TLGR.0137.77).
TU, C.M. (1980) Influence of five pyrethroids insecticides
on microbial populations and activities in soil. Microbiol.
Ecol., 5: 321-327.
TU, C.M. (1982) Effects of some pesticides on Rhizobium
japonicum and on the seed germination and pathogens of
soybean. Chemosphere, 11(10): 1027-1033.
US EPA (1984) Cypermethrin: tolerances for residues in or on
raw agricultural commodities: final rule. Part III. Fed. Reg.,
49(117): 24865-24872.
VAN DEN BERCKEN, J. (1977) The action of allethrin on the
peripheral nervous system of the frog. Pestic. Sci., 8:
692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980) Effects of
insecticides on the sensory system of Xenopus. Insect neurobiology
and pesticide action, London, Society of Chemical
Industry, pp. 79-85.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM, J.M.
(1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effects of insecticides on the sensory nervous system.
In: Narashashi, T., ed. Neurotoxicology of insecticides and
pheromones, New York, London, Plenum Publishing Corporation,
pp. 183-210.
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985a)
Human oral dose-excretion study with RIPCORD, The Hague, Shell
Internationale Petroleum Maatschappy (HSE.85.008).
VAN SITTERT, N.J., EADSFORTH, C.V., & BRAGT, P.C. (1985b)
Human dermal dose-excretion study with RIPCORD, The Hague,
Shell Internationale Petroleum Maatschappy (HSE.85.009).
VEKARIA, M.V. & VYAS, H.N. (1985) Studies on ovicidal
toxicity of certain insecticides against the eggs of Heliothis
armigera Hubner. Pesticides, 19(10): 43-44.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationships of some pyrethroids in rats. Arch. Toxicol., 45:
325-329.
VETTORAZZI, G. & VAN DEN HURK, G.W. (1984) Pesticides
reference index. JMPR 1961-84, Geneva, World Health
Organization.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in
frog myelinated nerve fibres. Eur. J. Pharmacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system.
Neuropathol. appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J.
(1982a) Structure-related effects of pyrethroid insecticides
on the lateral-line sense organ and on peripheral nerves of
the clawed frog, Xenopus laevis. Pestic. Biochem. Physiol.,
18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on
sodium channel gating in myelinated nerves. Nature (Lond.),
295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature- and
structure-dependent interaction of pyrethroids with the sodium
channels in frog node of Ranvier. Biochim. biophys. Acta, 728:
73-82.
WADDILL, V.H. (1978) Contact toxicity of four synthetic
pyrethroids and methomyl to some adult insect parasites.
Florida Entomol., 61(1): 27-30.
WALLACE, B.G., ROBERTS, T.R., & MCKERRELL, E.H. (1982)
Cypermethrin. A residue transfer study with laying hens,
Sittingbourne, Shell Research (SBTR.82.059).
WATTERS, F.L., WHITE, N.D.G., & COTE, D. (1983) Effect of
temperature on toxicity and persistence of three pyrethroid
insecticides applied to fir plywood for the control of the red
flour beetle (Coleoptera: Tenebrionidae). J. econ. Entomol.,
76: 11-16.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector
Biology and Control), pp. 18-23 .
WHO (1985) WHO Technical Report Series, No. 720 (Safe use of
pesticides. Ninth Report of the WHO Expert Committee on Vector
Biology and Control), pp. 14-19 .
WHO/FAO (1984) Cypermethrin, Geneva, World Health
Organization (Data Sheets on Pesticides, No. 84.58).
WILDE, G., KADOUM, A., & MIZE, T. (1984) Absence of
synergism with insecticides combinations used on chinch bugs
(Heteroptera: Lygaeridae). J. econ. Entomol., 77: 1297-1298.
WONG, S.W. & CHAPMAN, R.B. (1979) Toxicity of synthetic
pyrethoid insecticides to predaceous phytoseiid. Aust. J.
agric. Res., 30: 497-501.
WOOD, MACKENZIE, & CO. (1980) Pyrethroids, Agrochem. Monit.,
9: 3-14.
WOOD, MACKENZIE, & CO. (1981) Pyrethroids. Agrochem. Monit.,
15: 3-27.
WOOD, MACKENZIE, & CO. (1982) Pyrethroids. Agrochem. Monit.,
21: 3-17.
WOOD, MACKENZIE, & CO. (1983) Pyrethroids. Agrochem. Monit.,
27: 3-12.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual,
7th ed., Croydon, British Crop Protection Council, pp. 150-151.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of
pyrethroids. Gen. Pharmacol., 9: 387-398.
WRIGHT, A.N., ROBERTS, T.R., DUTTON, A.J., & DOIG, M.V.
(1980) The metabolism of cypermethrin in plants: the
conjugation of the cyclopropyl moiety. Pestic. Biochem.
Physiol., 13: 71-80.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G.
(1979) Toxicity of permethrin, decamethrin, and related
pyrethroids to salmon and lobster. Bull. environ. Contam.
Toxicol., 21: 338-343.
ZOHDY, G.I., OSMAN, A.A., & MOMEN, F.M. (1984) Toxicity of
some pyrethroid compounds to the predatory mite, Amblyseius
gossipi El-Badry. In: Griffiths, D.A. & Bowman, C.E., ed.
Acarology VI, Chichester, Ellis Horwood Ltd., Vol. 2, pp.
659-662.
APPENDIX
On the basis of electrophysiological studies with peripheral
nerve preparations of frogs (Xenopus laevis; Rana temporaria, and
Rana esculenta), it is possible to distinguish between 2 classes
of pyrethroid insecticides: (Type I and Type II). A similar
distinction between these 2 classes of pyrethroids has been made on
the basis of the symptoms of toxicity in mammals and insects (Van
den Bercken et al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980;
Glickman & Casida, 1982; Lawrence & Casida, 1982). The same
distinction was found in studies on cockroaches (Gammon et al.,
1981).
Based on the binding assay on the gamma-aminobutyric acid
(GABA) receptor-ionophore complex, synthetic pyrethroids can also
be classified into two types: the alpha-cyano-3-phenoxy-benzyl
pyrethroids and the non-cyano pyrethroids (Gammon et al., 1982;
Gammon & Casida, 1983; Lawrence & Casida, 1983; Lawrence et al.,
1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and
bioresmethrin) (Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give
rise to pronounced repetitive activity in sense organs and in
sensory nerve fibres (Van den Bercken et al., 1973). At room
temperature, this repetitive activity usually consists of trains of
3 - 10 impulses and occasionally up to 25 impulses. Train duration
is between 10 and 5 milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van
den Bercken, 1977). There was no significant effect of the
insecticide on neurotransmitter release or on the sensitivity of
the subsynaptic membrane, nor on the muscle fibre membrane.
Presynaptic repetitive firing was also observed in the sympathetic
ganglion treated with these pyrethroids.
In the lateral-line sense organ and in the motor nerve
terminal, but not in the cutaneous touch receptor or in sensory
nerve fibres, the pyrethroid-induced repetitive activity increases
dramatically as the temperature is lowered, and a decrease of 5 °C
in temperature may cause a more than 3-fold increase in the number
of repetitive impulses per train. This effect is easily reversed
by raising the temperature. The origin of this "negative
temperature coefficient" is not clear (Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through
interference with the sodium channel gating mechanism that
underlies the generation and conduction of each nerve impulse. The
transitional state of the sodium channel is controlled by 2
separately acting gating mechanisms, referred to as the activation
gate and the inactivation gate. Since pyrethroids only appear to
affect the sodium current during depolarization, the rapid opening
of the activation gate and the slow closing of the inactivation
gate proceed normally. However, once the sodium channel is open,
the activation gate is restrained in the open position by the
pyrethroid molecule. While all pyrethroids have essentially the
same basic mechanism of action, however, the rate of relaxation
differs substantially for the various pyrethroids (Flannigan &
Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation
of the transient increase in sodium permeability of the nerve
membrane during excitation (Van den Bercken & Vijverberg, 1980).
Evidence so far available indicates that allethrin selectively
slows down the closing of the activation gate of a fraction of the
sodium channels that open during depolarization of the membrane.
The time constant of closing of the activation gate in the
allethrin-affected channels is about 100 milliseconds compared with
less than 100 microseconds in the normal sodium channel, i.e., it
is slowed down by a factor of more than 100. This results in a
marked prolongation of the sodium current across the nerve membrane
during excitation, and this prolonged sodium current is directly
responsible for the repetitive activity induced by allethrin
(Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive
firing, and no significant effects on transmitter release or on the
subsynaptic membrane.
Interestingly, the action of these pyrethroids closely
resembles that of the insecticide DDT in the peripheral nervous
system of the frog. DDT also causes pronounced repetitive activity
in sense organs, in sensory nerve fibres, and in motor nerve
terminals, due to a prolongation of the transient increase in
sodium permeability of the nerve membrane during excitation.
Recently, it was demonstrated that allethrin and DDT have
essentially the same effect on sodium channels in frog myelinated
nerve membrane. Both compounds slow down the rate of closing of a
fraction of the sodium channels that open on depolarization of the
membrane (Van den Bercken et al., 1973, 1979; Vijverberg et al.,
1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittantly, cause large
depolarizing afterpotentials, and evoke repetitive firing with
minimal effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin,
like allethrin, primarily affect the sodium channels in the nerve
membrane and cause a prolongation of the transient increase in
sodium permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type I pyrethroids
(allethrin, cismethrin, bioresmethrin, and 1R, cis-phenothrin)
caused moderate presynaptic repetitive activity, resulting in the
occurrence of multiple end-plate potentials (Ruigt & Van den
Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl
alcohol (deltamethrin, cypermethrin, fenvalerate, and fenpropanate)
(Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense
repetitive activity in the lateral line organ in the form of long-
lasting trains of impulses (Vijverberg et al., 1982a). Such a
train may last for up to 1 min and contains thousands of impulses.
The duration of the trains and the number of impulses per train
increase markedly on lowering the temperature. Cypermethrin does
not cause repetitive activity in myelinated nerve fibres. Instead,
this pyrethroid causes a frequency-dependent depression of the
nervous impulse, brought about by a progressive depolarization of
the nerve membrane as a result of the summation of depolarizing
after-potentials during train stimulation (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in
sodium permeability during excitation, presumably by slowing down
the closing of the activation gate of the sodium channel
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983). The
time constant of closing of the activation gate in the
cypermethrin-affected channels is prolonged to more than 100
milliseconds. Apparently, the amplitude of the prolonged sodium
current after cypermethrin is too small to induce repetitive
activity in nerve fibres, but is sufficient to cause the long-
lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily
affect the sodium channels in the nerve membrane and cause a long-
lasting prolongation of the transient increase in sodium
permeability of the membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a
modified continuous open state persistently, depolarize the
membrane, and block the action potential without causing repetitive
firing (Lund & Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of
the Type II pyrethroid syndrome include action at the GABA receptor
complex or a closely linked class of neuroreceptor (Gammon et al.,
1982).
The Type II syndrome of intracerebrally administered
pyrethroids closely approximates that of the convulsant picrotoxin
(PTX). Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin
to rat brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible
relation between the Type II pyrethroid action and the GABA
receptor complex. The stereospecific correlation between the
toxicity of Type II pyrethroids and their potency to inhibit the
[35S]-TBPS binding was established using a radioligand, [35S]- t-
butyl-bicyclophosphoro-thionate [35S]-TBPS. Studies with 37
pyrethroids revealed an absolute correlation, without any false
positive or negative, between mouse intracerebral toxicity and in
vitro inhibition: all toxic cyano compounds including
deltamethrin, 1R, cis-cypermethrin, 1R, trans-cypermethrin, and
[2S, alphaS]-fenvalerate were inhibitors, but their non-toxic
stereoisomers were not; non-cyano pyrethroids were much less potent
or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies
of pyrethroids were closely related to their mammalian toxicities.
The most toxic pyrethroids of Type II were the most potent
inhibitors of [3H]-Ro 5-4864 specific binding to rat brain
membranes. The [3H]-dihydropicrotoxin and [35S]-TBPS binding
studies with pyrethroids strongly indicated that Type II effects of
pyrethroids are mediated, at least in part, through an interaction
with a GABA-regulated chloride ionophore-associated binding site.
Moreover, studies with [3H]-Ro 5-4864 support this hypothesis and,
in addition, indicate that the pyrethroid-binding site may be very
closely related to the convulsant benzodiazepine site of action
(Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin
and [2S,alpha S]-fenvalerate) increased the input resistance of
crayfish claw opener muscle fibres bathed in GABA. In contrast,
two non-insecticidal stereoisomers and Type I pyrethroids
(permethrin, resmethrin, allethrin) were inactive. Therefore,
cyanophenoxybenzyl pyrethroids appear to act on the GABA
receptorionophore complex (Gammon & Casida, 1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation
of the clawed frog (Xenopus laevis). Type II pyrethroids
(cypermethrin and deltamethrin) induced trains of repetitive muscle
action potentials without presynaptic repetitive activity.
However, an intermediate group of pyrethroids (1R-permethrin,
cyphenothrin, and fenvalerate) caused both types of effect. Thus,
in muscle or nerve membrane the pyrethroid induced repetitive
activities due to a prolongation of the sodium current. But no
clear distinction was observed between non-cyano and alpha-cyano
pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary
target site of pyrethroid insecticides in the vertebrate nervous
system is the sodium channel in the nerve membrane. Pyrethroids
without an alpha-cyano group (allethrin, d-phenothrin, permethrin,
and cismethrin) cause a moderate prolongation of the transient
increase in sodium permeability of the nerve membrane during
excitation. This results in relatively short trains of repetitive
nerve impulses in sense organs, sensory (afferent) nerve fibres,
and, in effect, nerve terminals. On the other hand, the alpha-
cyano pyrethroids cause a long-lasting prolongation of the
transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of
repetitive impulses in sense organs and a frequency-dependent
depression of the nerve impulse in nerve fibres. The difference in
effects between permethrin and cypermethrin, which have identical
molecular structures except for the presence of an alpha-cyano
group on the phenoxybenzyl alcohol, indicates that it is this
alpha-cyano group that is responsible for the long-lasting
prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation
and conduction are basically the same throughout the entire nervous
system, pyrethroids may also induce repetitive activity in various
parts of the brain. The difference in symptoms of poisoning by
alpha-cyano pyrethroids, compared with the classical pyrethroids,
is not necessarily due to an exclusive central site of action. It
may be related to the long-lasting repetitive activity in sense
organs and possibly in other parts of the nervous system, which, in
a more advance state of poisoning, may be accompanied by a
frequency-dependent depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of
the nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane
is also the most important target site of pyrethroids in the
invertebrate nervous system (Wouters & Van den Bercken, 1978; WHO,
1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be
considered restricted to particular animal species, or to a certain
region of the nervous system. Although it has been established
that sense organs and nerve endings are the most vulnerable to the
action of pyrethroids, the ultimate lesion that causes death will
depend on the animal species, environmental conditions, and on the
chemical structure and physical characteristics of the pyrethroid
molecule (Vijverberg & Van den Bercken, 1982).
