
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 91
ISOPHORONE
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1995
This is a companion volume to Environmental Health Criteria
174: Isophorone
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the
United Nations Environment Programme, the International Labour
Organisation, and the World Health Organization)
This report contains the collective views of an international group
of experts and does not necessarily represent the decisions or the
stated policy of the United Nations Environment Programme, the
International Labour Organisation, or the World Health Organization
WHO Library Cataloguing in Publication Data
Isophorone: health and safety guide.
(Health and safety guide ; no. 91)
1.Solvents - standards 2.Solvents - toxicity
3.Hazardous substances - standards 4.Environmental exposure
I.Series
ISBN 92 4 151091 9 (NLM Classification: WA 240)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1995
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
INTRODUCTION
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1. Identity
1.2. Physical and chemical properties
1.3. Conversion factors
1.4. Analytical methods
1.5. Production and use
2. SUMMARY AND EVALUATION
2.1. Environmental transport, distribution, and transformation
2.2. Environmental levels and human exposure
2.3. Kinetics and metabolism in laboratory animals
and humans
2.4. Effects on laboratory mammals and in vitro
test systems
2.5. Effects on humans
2.6. Effects on other organisms in the laboratory and field
3. CONCLUSIONS AND RECOMMENDATIONS
3.1. Conclusions
3.1.1. General population exposure
3.1.2. Occupational exposure
3.1.3. The environment
3.2. Recommendations
3.2.1. General population and occupational exposure
3.2.2. Further studies
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1. Main human health hazards, prevention and protection,
first aid
4.1.1. First aid and medical treatment
4.1.1.1 Eye contact
4.1.1.2 Skin contact
4.1.1.3 Inhalation
4.1.1.4 Ingestion
4.1.2. Safe handling
4.1.2.1 Personal protection
4.2. Fire and explosion hazards
4.3. Storage
4.4. Transport
4.5. Spillage and disposal
4.5.1. Spillage
4.5.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
6.1. Previous evaluations by international bodies
6.2. Exposure limit values
6.3. Specific restrictions
6.4. Labelling, packaging, and transport
6.5. Waste disposal
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding
EHC. Section 4 includes advice on preventive and protective measures
and emergency action; health workers should be thoroughly familiar
with the medical information to ensure that they can act efficiently
in an emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those
in ministries, governmental agencies, industry, and trade unions who
are involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
1. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
1.1 Identity
Common name: Isophorone
Synonyms: 2-cyclohexen-1-one, 3,5,5,-trimethyl;
3,5,5-trimethyl-2-cyclohexene-1-one;
1,1,3-trimethyl-3-cyclohexene-5-one;
alpha-isophorone; isoacetophorone;
isoforone; izoforon;
1,5,5-trimethyl-3-oxo-cyclohexene
Empirical formula: C9H14O
Chemical structure:
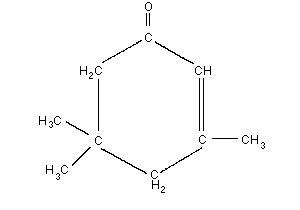 Relative molecular mass: 138.2
CAS registry number: 78-59-1
RTECs registry number: GW7700000
EEC number: 606-012-00-8
EINECS number: 1011260
1.2 Physical and Chemical Properties
Isophorone is a colourless liquid. Its odour has been described as
similar to those of peppermint and camphor. It is soluble in water
and is miscible in all proportions with aliphatic and aromatic
hydrocarbons, alcohols, ethers, esters, ketones, and chlorinated
hydrocarbons. Physical and chemical data are summarized in Table 1.
Table 1. Physical and chemical data of isophorone
Value
Specific gravity (20 °C/4 °C) 0.922
Boiling point at 1013 hPa 215 °C
Freezing point -8.1 °C
Refractive index 1.4775 n20D
Viscosity at 20 °C 2.6m Pas
Coefficient of cubic expansion 0.00085 °C-1
at 20 °C 0.00078 °C-1
Surface tension at 20 °C 30 mN/m
Vapour pressure 0.4 mbar (20 °C)
0.26 mmHg (25 °C)
Vapour density (air=1) 4.7
Concentration in saturated air approx. 1941 mg/m3
at 20 °C and 1013 hPa (340 ppm)
Solubility at 20 °C
Isophorone in water 12.0-17.5 g/litre
Water in isophorone 53 g/litre
Log Kow (20 °C) 1.67 (measured)
1.7 (estimated)
Solubility parameters (Hansen)
delta 19.2 (J/cm3)1/2
deltaD 16.6 (J/cm3)1/2
deltaP 8.2 (J/cm3)1/2
deltaH 7.4 (J/cm3)1/2
Hydrogen bonding parameter, gamma 14.9
Flashpoint, closed cup 85 °C
Explosion limits in air 0.8-3.8 vol-%
Ignition temperature 455-470 °C
Heat of evaporation at 215 °C 349.2 kJ/kg
Heat of combustion (p=const. at 20 °C) 38 100 kJ/kg
Relative permittivity at 20 °C 19.9
Specific resistivity 1 × 107 ohm × cm
A typical, commercial sample of isophorone may contain 1-3% of the
isomer beta-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) with
the sum of alpha- and beta-isomers exceeding 99%.
Isophorone is stable and may be stored in steel or aluminium
containers. Prolonged periods of storage may lead to slight
yellowing.
1.3 Conversion Factors
The following conversion factors have been calculated for 22 °C and
1013 hPa:
1 ppm = 5.71 mg/m3
1 mg/m3 = 0.175 ppm.
1.4 Analytical Methods
The purity of technical isophorone may be determined by capillary
gas chromatography (GC) with a flame ionization detector (FID).
Recommended conditions are shown in Table 2.
Earlier methods for the determination of isophorone in air were
based on adsorption on charcoal; however, it has been found that
isophorone adsorbed on charcoal decomposes during storage. More
recent methods involve adsorption on polymers, such as XAD resins or
Tenax-GC, followed by desorption and analysis by capillary GC with
FID.
The determination of isophorone in wastewater samples and fish
tissues may be achieved using solvent extraction, clean-up with gel
permeation chromatography, and analysis by GC/MS, in both the
electron impact and chemical ionization modes.
Table 2. Gas chromatographic conditions for the analysis of
technical isophorone
Column Fused silica capillary Macrobore
Coating OV - 1701 CP Wax 52CB
Dimensions 60 m/0.25 mm 25 m/0.53 mm
Injector temperature 240 °C 250 °C
Temperature-Programme 6 min 70°-220° 10 min 105°-120°
@ 4°/min @ 6°/min
2 min 120°-150°
@ 10°/min
1.5 Production and Use
Isophorone is produced commercially by the catalytic condensation of
acetone at elevated temperature and pressure and is purified by
distillation. Worldwide annual production capacity was estimated to
be 92 000 tonnes in 1988.
Isophorone is used as a chemical intermediate for the synthesis of a
variety of organic chemicals.
Isophorone is a solvent for a number of natural and synthetic resins
and polymers, such as polyvinyl chlorides and acetates, cellulose
derivatives, epoxy and alkyd resins, and polyacrylates. It is
therefore used as a high boiling solvent in industrial air drying
and stoving paints, nitro emulsion leather finishes, and the
manufacture of vinyl resin-based printing inks for plastic surfaces.
Isophorone is also used as a solvent for some pesticide
formulations, especially for emulsifiable concentrates of anilides
and carbamates.
2. SUMMARY AND EVALUATION
2.1 Environmental Transport, Distribution, and Transformation
Isophorone may enter the environment from numerous industries, waste
and waste-water disposal, and its use as a solvent and a pesticide
carrier. Following release into water or soil, environmental
concentrations decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded by approximately 70% in 14 days and 95% after 28 days.
The results of biodegradation studies are variable and limited.
Water solubility, soil adsorption coefficients, and polarity
indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and the
physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
2.2 Environmental Levels and Human Exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg (490 ppb) has been
reported. Isophorone has been identified in surface waters
(0.6-3 µg/litre), groundwater (10 µg/litre), urban run-off
(10 µg/litre), and landfill leachate (29 µg/litre).
Isophorone has been identified in industrial wastewater at
100 µg/litre. After classical secondary treatment, the concentration
of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6-12 µg/kg dry
weight).
It has also been identified in tissues from several species of fish
at concentrations of up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice, or sugar beet, following application as a pesticide carrier.
2.3 Kinetics and Metabolism in Laboratory Animals and Humans
Distribution studies on rats using 14C-isophorone showed that 93%
of orally administered radioactivity appeared mainly in the urine
and expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney, and
preputial glands.
The metabolites identified in the urine of rabbits after oral
administration of isophorone resulted from the oxidation of the
3-methyl group, reduction of the keto group, and hydrogenation of
the double link of the cyclohexene ring, and were eliminated as
such, or, in the case of the alcohols, as the glucuronide
derivatives.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
2.4 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, oral LD50 values being
>1500 mg/kg in the rat, >2200 mg/kg in the mouse, and >2000 mg/kg
in the rabbit. Dermal LD50 values were 1700 mg/kg in the rat and
>1200 mg/kg in the rabbit. Acute effects from dermal exposure in
rats and rabbits ranged from mild erythema to scabs. Conjunctivitis
and corneal damage have been reported on direct application to the
eye or exposure to high concentrations of isophorone. No skin
sensitization was reported in guinea-pigs using the
Magnusson-Kligman test.
In acute and short-term oral studies on rodents given high doses
(>1000 mg/kg), degenerative effects in the liver as well as CNS
depression were seen and there were some deaths. In 90-day studies,
the no-observed-effect levels (NOELs) in rats and mice were
evaluated to be 500 mg/kg body weight per day. In a 90-day oral
study on Beagle dogs (with limited numbers), no effects were seen at
doses of up to 150 mg/kg body weight per day.
In acute and short-term inhalation studies reviewed, irritation,
haematological effects, and decreased body weights were noted. Since
the study designs were inadequate, no NOEL could be determined and
no inference for human health could be made.
Isophorone does not induce gene mutations in bacteria, chromosomal
aberrations in vitro, DNA repair in primary rat hepatocytes, or
bone-marrow micronuclei in mice. Positive effects were observed only
in the absence of an exogenous metabolic system in L5178Y TK +/-
mouse lymphoma mutagenesis assays as well as in a sister chromatid
exchange assay. Isophorone induced morphological transformation in
vitro in the absence of an exogenous metabolism system. Isophorone
did not induce sex-linked recessive lethal mutations in Drosophila.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound. In
an in vivo assay, no DNA binding was observed in the liver and
kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies on mice and rats, male rats
showed several proliferative lesions of the kidney. The role of
alpha2u-globulin accumulation in the etiology of these lesions has
been recognized. Since significant amounts of alpha2u-globulin have
not been detected in humans, this mechanism of carcinogenesis
appears not to be relevant in humans. Preputial gland carcinomas
were observed in five high-dose male rats and two clitoral gland
adenomas were seen in low-dose female rats with exposure to
isophorone. These may also be related to alpha2u-globulin
accumulation. Isophorone exposure was associated with some
neoplastic lesions of the liver, and the integumentary and the
lymphoreticular systems of male mice, as well as nonneoplastic liver
and adrenal cortex lesions, but this was not observed in treated
female mice.
In the only available long-term inhalation study on rats and
rabbits, irritation of the eye and nasal mucosa as well as lung and
liver changes were observed at approx. 1347 mg/m3 (250 ppm).
However, this may have been because of limitations in the study.
Very limited studies on rats and mice indicate that isophorone does
not affect the fertility or cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural
despair swimming test.
2.5 Effects on Humans
The odour of isophorone can be detected at a concentration as low as
1.14 mg/m3 (0.2 ppm). Eye, nose, and throat irritation have been
reported at concentrations below 28.5 mg/m3 (5 ppm); above 1142
mg/m3 (200 ppm) nausea, headache, dizziness, faintness, and
inebriation have been reported.
2.6 Effects on Other Organisms in the Laboratory and Field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and marine
species. EC50s (96-h) (based on cell count and chlorophyll) ranged
from 105 to 126 mg/litre; 48-h LC50s for Daphnia magna ranged
from 117 to 120 mg/litre, and 96-h LC50s for freshwater fish
ranged from 145 to 255 mg/litre.
LC50s (96-h) for marine invertebrates ranged from 12.9 to 430
mg/litre. The 96-h LC50 for a single marine fish species was
between 170 and 300 mg/litre. Data from studies with measured
exposure concentrations did not differ from those of studies with
nominal concentrations. NOELs for Pimephales promelas, tested in
different laboratories, ranged from 14 to 45.4 mg/litre.
The available data suggest that the toxicity of isophorone for
aquatic organisms is low.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
3.1.1 General population exposure
Isophorone is used as a solvent for resins, polymers, and pesticide
formulations. Dermal and inhalation exposure may occur, but will
most likely be minimal. Data are available that show that isophorone
can occur in µg/litre concentrations in drinking-water and fish. In
view of the low toxicity of isophorone in experimental studies and
the low levels of exposure from environmental sources, the risks for
the general population appear to be minimal.
3.1.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin, and respiratory irritation;
other health effects may occur at higher concentrations. No studies
on long-term health effects in workers were available for review by
the Task Group.
3.1.3 The environment
Isophorone may be released into the environment following its use as
a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, though it has a low environmental persistence due to
biodegradation, volatilization, and photochemical oxidation
processes. The available data suggest that the toxicity of
isophorone for aquatic organisms is low.
3.2 Recommendations
3.2.1 General population and occupational exposure
Care should be taken to prevent contamination of groundwater and
air.
Workers involved in the manufacture or use of isophorone should be
protected from exposure by means of adequate engineering controls
and appropriate industrial hygiene measures. Levels of occupational
exposure should be kept within acceptable limits and should be
monitored regularly.
3.2.2 Further studies
Health surveillance of exposed workers should be conducted and
reported on.
Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
Adequate short-term/long-term inhalation studies on experimental
animals should be conducted, in order to determine safe levels of
occupational exposure.
Information is required on the anaerobic biodegradation of
isophorone, especially as it has been identified in landfill
leachate.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Isophorone vapours are irritating to the eyes and the respiratory
tract. Prolonged exposure may cause fatigue and malaise. Prolonged
contact with liquid isophorone may irritate the skin.
4.1.1 First aid and medical treatment
4.1.1.1 Eye contact
Immediately flush eyes with plenty of water. Ensure adequate
flushing of the eyes by separating the eyelids with fingers. Medical
attention should be obtained.
4.1.1.2 Skin contact
Contaminated clothing should be removed and the affected area of the
skin thoroughly flushed with water. If skin irritation occurs,
medical attention should be obtained.
4.1.1.3 Inhalation
Provide fresh air. Monitor breathing, and give oxygen if breathing
is difficult.
4.1.1.4 Ingestion
Seek medical advice immediately. Wash out mouth with water. Emesis
may be indicated soon after ingestion, unless the patient is
unconscious. Let the victim ingest charcoal (30-100 g in adults) as
a slurry, to limit absorption of isophorone from the intestine.
4.1.2 Safe handling
4.1.2.1 Personal protection
Atmospheric levels should be kept below the recommended occupational
exposure limits by local exhaust ventilation. Respirators should be
worn in areas where these limits are likely to be exceeded. Skin and
eye protection should be worn where exposure is likely to occur.
Nitrile rubber gloves are recommended.
4.2 Fire and Explosion Hazards
Isophorone is flammable. Suitable extinguishing media are water
spray, carbon dioxide, foam, or dry powder.
The explosion limits in air are 0.8-3.8 vol.%.
4.3 Storage
Containers should be kept tightly closed and stored in a
well-ventilated area.
4.4 Transport
Local requirements regarding the movement of hazardous goods or
wastes must be complied with. Containers should be checked to ensure
that they are sound and labels undamaged before despatch. For
regulations see section 6.4.
4.5 Spillage and Disposal
4.5.1 Spillage
The area should be evacuated and sources of ignition removed.
Self-contained breathing apparatus, gloves, goggles, face shield,
and boots should be worn. Liquid should be prevented from entering
sewers, basements, and workpits.
Small-scale spillages should be absorbed on paper towels and the
paper burnt, away from other combustible material.
For medium-scale spillages, the liquid should be absorbed with sand
or earth and all material should be removed to a safe place for
subsequent incineration. The contaminated area should be washed out
with plenty of water.
For large-scale spillages, the spilt liquid should be prevented from
spreading by the use of sand or earth. The liquid should be
transferred to a salvage tank if possible; otherwise it should be
treated as for medium-scale spillages. The local authorities should
be informed at once, if the spilt liquid enters the surface-water
drains.
4.5.2 Disposal
This combustible material may be burned in a chemical incinerator.
The product should not be buried or dumped in a landfill.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
The toxicity of isophorone for aquatic and terrestrial species is
low.
Industrial discharges and any spillage or unused product should be
prevented from polluting the environment and spreading to vegetation
or waterways and should be treated and disposed of properly (section
4.5.2).
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. The intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can only be fully understood
in the framework of the legislation of that country. Furthermore,
the regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate regulatory
authorities before application.
6.1 Previous Evaluations by International Bodies
None available.
6.2 Exposure Limit Values
Some exposure limit values are given in the table on the next page.
6.3 Specific Restrictions
In Germany, isophorone may not be handled by adolescents or
pregnant, or nursing, women.
6.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as an irritant
substance using the symbol:
The label must read:
Irritating to eyes, respiratory system and skin; in case of
contact with eyes, rinse immediately with plenty of water and
seek medical advice.
Regulations on the Transport of Dangerous Goods:
RID/ADR: Class 3, No. 32C
ADNR: Class 3, No. 4, category K3
GGVSee/IMDG-Code: Not classified
IATA-RAR: Article No. 1939
6.5 Waste Disposal
In the USA, isophorone is rated as a persistent compound, and any
non-domestic waste must be treated as a toxic pollutant. Specific
instructions are given for notification and incineration. Permits
are required for its discharge and discharge limits have been set.
Exposure limit values in some countries
Medium Specification Country/ Exposure Limit Description Value Effective
organization date
AIR Finland Threshold limit value (TLV) 28 mg/m3 (5 ppm) 1987
Germany Maximum worksite concentration (MAK)
- time-weighted average (TWA) 28.0 mg/m3 1980
Russian Federation Ceiling value 1.0 mg/m3 1978
Sweden Hygienic limit value 30.0 mg/m3 1985
United Kingdom Recommended limit (RECL)
- time-weighted average (TWA) 25 mg/m3 1985
- short-term exposure level (STEL) 25 mg/m3
(10-min-TWA)
USA Permissible Exposure Limit (OSHA)
- DOL/OSHA 8-h time-weighted average (TWA) 23.0 mg/m3 (4 ppm) 1989
- NIOSH Recommended Exposure Limit (REL)
up to a 10-h TWA 23 mg/m3 (4 ppm) 1988
- ACGIH Threshold limit value (TLV)
Ceiling value (15-min) 28 mg/m3 (5 ppm) 1992
BIBLIOGRAPHY
CEC (1987) Legislation in dangerous substances - Classification and
labelling in the European Communities - Vol. 1 & 2, Commission of
the European Communities, London, Graham & Trotman, Ltd.
CEC/IPCS (1991) International chemical safety card (ICSC) No. 0169
on isophorone, Luxembourg, Grand Duchy of Luxembourg, Commission
of the European Communities (CEC); Geneva, Switzerland,
International Programme on Chemical Safety (IPCS), World Health
Organization, 2 pp.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IPCS (in preparation) Environmental Health Criteria 174:
Isophorone, Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide
for safe warehousing of hazardous materials, Paris, United Nations
Environment Programme - Industry and Environment Office, 80 pp.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
Relative molecular mass: 138.2
CAS registry number: 78-59-1
RTECs registry number: GW7700000
EEC number: 606-012-00-8
EINECS number: 1011260
1.2 Physical and Chemical Properties
Isophorone is a colourless liquid. Its odour has been described as
similar to those of peppermint and camphor. It is soluble in water
and is miscible in all proportions with aliphatic and aromatic
hydrocarbons, alcohols, ethers, esters, ketones, and chlorinated
hydrocarbons. Physical and chemical data are summarized in Table 1.
Table 1. Physical and chemical data of isophorone
Value
Specific gravity (20 °C/4 °C) 0.922
Boiling point at 1013 hPa 215 °C
Freezing point -8.1 °C
Refractive index 1.4775 n20D
Viscosity at 20 °C 2.6m Pas
Coefficient of cubic expansion 0.00085 °C-1
at 20 °C 0.00078 °C-1
Surface tension at 20 °C 30 mN/m
Vapour pressure 0.4 mbar (20 °C)
0.26 mmHg (25 °C)
Vapour density (air=1) 4.7
Concentration in saturated air approx. 1941 mg/m3
at 20 °C and 1013 hPa (340 ppm)
Solubility at 20 °C
Isophorone in water 12.0-17.5 g/litre
Water in isophorone 53 g/litre
Log Kow (20 °C) 1.67 (measured)
1.7 (estimated)
Solubility parameters (Hansen)
delta 19.2 (J/cm3)1/2
deltaD 16.6 (J/cm3)1/2
deltaP 8.2 (J/cm3)1/2
deltaH 7.4 (J/cm3)1/2
Hydrogen bonding parameter, gamma 14.9
Flashpoint, closed cup 85 °C
Explosion limits in air 0.8-3.8 vol-%
Ignition temperature 455-470 °C
Heat of evaporation at 215 °C 349.2 kJ/kg
Heat of combustion (p=const. at 20 °C) 38 100 kJ/kg
Relative permittivity at 20 °C 19.9
Specific resistivity 1 × 107 ohm × cm
A typical, commercial sample of isophorone may contain 1-3% of the
isomer beta-isophorone (3,5,5-trimethyl-3-cyclohexene-1-one) with
the sum of alpha- and beta-isomers exceeding 99%.
Isophorone is stable and may be stored in steel or aluminium
containers. Prolonged periods of storage may lead to slight
yellowing.
1.3 Conversion Factors
The following conversion factors have been calculated for 22 °C and
1013 hPa:
1 ppm = 5.71 mg/m3
1 mg/m3 = 0.175 ppm.
1.4 Analytical Methods
The purity of technical isophorone may be determined by capillary
gas chromatography (GC) with a flame ionization detector (FID).
Recommended conditions are shown in Table 2.
Earlier methods for the determination of isophorone in air were
based on adsorption on charcoal; however, it has been found that
isophorone adsorbed on charcoal decomposes during storage. More
recent methods involve adsorption on polymers, such as XAD resins or
Tenax-GC, followed by desorption and analysis by capillary GC with
FID.
The determination of isophorone in wastewater samples and fish
tissues may be achieved using solvent extraction, clean-up with gel
permeation chromatography, and analysis by GC/MS, in both the
electron impact and chemical ionization modes.
Table 2. Gas chromatographic conditions for the analysis of
technical isophorone
Column Fused silica capillary Macrobore
Coating OV - 1701 CP Wax 52CB
Dimensions 60 m/0.25 mm 25 m/0.53 mm
Injector temperature 240 °C 250 °C
Temperature-Programme 6 min 70°-220° 10 min 105°-120°
@ 4°/min @ 6°/min
2 min 120°-150°
@ 10°/min
1.5 Production and Use
Isophorone is produced commercially by the catalytic condensation of
acetone at elevated temperature and pressure and is purified by
distillation. Worldwide annual production capacity was estimated to
be 92 000 tonnes in 1988.
Isophorone is used as a chemical intermediate for the synthesis of a
variety of organic chemicals.
Isophorone is a solvent for a number of natural and synthetic resins
and polymers, such as polyvinyl chlorides and acetates, cellulose
derivatives, epoxy and alkyd resins, and polyacrylates. It is
therefore used as a high boiling solvent in industrial air drying
and stoving paints, nitro emulsion leather finishes, and the
manufacture of vinyl resin-based printing inks for plastic surfaces.
Isophorone is also used as a solvent for some pesticide
formulations, especially for emulsifiable concentrates of anilides
and carbamates.
2. SUMMARY AND EVALUATION
2.1 Environmental Transport, Distribution, and Transformation
Isophorone may enter the environment from numerous industries, waste
and waste-water disposal, and its use as a solvent and a pesticide
carrier. Following release into water or soil, environmental
concentrations decrease as a result of volatilization and
biodegradation. Isophorone in the atmosphere is removed by
photochemical processes with an estimated half-life of about 30 min
(based on a mathematical model). In a Die-away test, isophorone was
biodegraded by approximately 70% in 14 days and 95% after 28 days.
The results of biodegradation studies are variable and limited.
Water solubility, soil adsorption coefficients, and polarity
indicate that significant adsorption by suspended solids and
sediments is unlikely to occur.
Although isophorone has been found in fish tissues, the data and the
physical and chemical properties suggest that significant
bioconcentration is unlikely. A half-life of one day has been
measured in a single fish species.
2.2 Environmental Levels and Human Exposure
Isophorone has not been measured in ambient air. An isophorone
concentration in coal fly ash of 490 µg/kg (490 ppb) has been
reported. Isophorone has been identified in surface waters
(0.6-3 µg/litre), groundwater (10 µg/litre), urban run-off
(10 µg/litre), and landfill leachate (29 µg/litre).
Isophorone has been identified in industrial wastewater at
100 µg/litre. After classical secondary treatment, the concentration
of isophorone in the effluent was 10 µg/litre.
Isophorone has been identified in lake sediments (0.6-12 µg/kg dry
weight).
It has also been identified in tissues from several species of fish
at concentrations of up to 3.61 mg/kg wet weight.
Isophorone was not detected in the edible parts of bean plants,
rice, or sugar beet, following application as a pesticide carrier.
2.3 Kinetics and Metabolism in Laboratory Animals and Humans
Distribution studies on rats using 14C-isophorone showed that 93%
of orally administered radioactivity appeared mainly in the urine
and expired air within 24 h. The tissues retaining the highest
concentration after this period were the liver, kidney, and
preputial glands.
The metabolites identified in the urine of rabbits after oral
administration of isophorone resulted from the oxidation of the
3-methyl group, reduction of the keto group, and hydrogenation of
the double link of the cyclohexene ring, and were eliminated as
such, or, in the case of the alcohols, as the glucuronide
derivatives.
Percutaneous LD50 values indicate that isophorone is rapidly
absorbed through the skin.
2.4 Effects on laboratory mammals and in vitro test systems
The acute toxicity of isophorone is low, oral LD50 values being
>1500 mg/kg in the rat, >2200 mg/kg in the mouse, and >2000 mg/kg
in the rabbit. Dermal LD50 values were 1700 mg/kg in the rat and
>1200 mg/kg in the rabbit. Acute effects from dermal exposure in
rats and rabbits ranged from mild erythema to scabs. Conjunctivitis
and corneal damage have been reported on direct application to the
eye or exposure to high concentrations of isophorone. No skin
sensitization was reported in guinea-pigs using the
Magnusson-Kligman test.
In acute and short-term oral studies on rodents given high doses
(>1000 mg/kg), degenerative effects in the liver as well as CNS
depression were seen and there were some deaths. In 90-day studies,
the no-observed-effect levels (NOELs) in rats and mice were
evaluated to be 500 mg/kg body weight per day. In a 90-day oral
study on Beagle dogs (with limited numbers), no effects were seen at
doses of up to 150 mg/kg body weight per day.
In acute and short-term inhalation studies reviewed, irritation,
haematological effects, and decreased body weights were noted. Since
the study designs were inadequate, no NOEL could be determined and
no inference for human health could be made.
Isophorone does not induce gene mutations in bacteria, chromosomal
aberrations in vitro, DNA repair in primary rat hepatocytes, or
bone-marrow micronuclei in mice. Positive effects were observed only
in the absence of an exogenous metabolic system in L5178Y TK +/-
mouse lymphoma mutagenesis assays as well as in a sister chromatid
exchange assay. Isophorone induced morphological transformation in
vitro in the absence of an exogenous metabolism system. Isophorone
did not induce sex-linked recessive lethal mutations in Drosophila.
The weight of the evidence of all mutagenicity data supports the
contention that isophorone is not a potent DNA reactive compound. In
an in vivo assay, no DNA binding was observed in the liver and
kidneys (organs affected in the carcinogenicity bioassays).
In long-term oral toxicity studies on mice and rats, male rats
showed several proliferative lesions of the kidney. The role of
alpha2u-globulin accumulation in the etiology of these lesions has
been recognized. Since significant amounts of alpha2u-globulin have
not been detected in humans, this mechanism of carcinogenesis
appears not to be relevant in humans. Preputial gland carcinomas
were observed in five high-dose male rats and two clitoral gland
adenomas were seen in low-dose female rats with exposure to
isophorone. These may also be related to alpha2u-globulin
accumulation. Isophorone exposure was associated with some
neoplastic lesions of the liver, and the integumentary and the
lymphoreticular systems of male mice, as well as nonneoplastic liver
and adrenal cortex lesions, but this was not observed in treated
female mice.
In the only available long-term inhalation study on rats and
rabbits, irritation of the eye and nasal mucosa as well as lung and
liver changes were observed at approx. 1347 mg/m3 (250 ppm).
However, this may have been because of limitations in the study.
Very limited studies on rats and mice indicate that isophorone does
not affect the fertility or cause developmental toxicity in
experimental animals.
The fact that central nervous system depression occurs in
experimental animals could indicate a possible neurotoxic effect.
Isophorone also elicited a positive effect in the behavioural
despair swimming test.
2.5 Effects on Humans
The odour of isophorone can be detected at a concentration as low as
1.14 mg/m3 (0.2 ppm). Eye, nose, and throat irritation have been
reported at concentrations below 28.5 mg/m3 (5 ppm); above 1142
mg/m3 (200 ppm) nausea, headache, dizziness, faintness, and
inebriation have been reported.
2.6 Effects on Other Organisms in the Laboratory and Field
No data on terrestrial animals were available.
Acute LC50 values are available for several freshwater and marine
species. EC50s (96-h) (based on cell count and chlorophyll) ranged
from 105 to 126 mg/litre; 48-h LC50s for Daphnia magna ranged
from 117 to 120 mg/litre, and 96-h LC50s for freshwater fish
ranged from 145 to 255 mg/litre.
LC50s (96-h) for marine invertebrates ranged from 12.9 to 430
mg/litre. The 96-h LC50 for a single marine fish species was
between 170 and 300 mg/litre. Data from studies with measured
exposure concentrations did not differ from those of studies with
nominal concentrations. NOELs for Pimephales promelas, tested in
different laboratories, ranged from 14 to 45.4 mg/litre.
The available data suggest that the toxicity of isophorone for
aquatic organisms is low.
3. CONCLUSIONS AND RECOMMENDATIONS
3.1 Conclusions
3.1.1 General population exposure
Isophorone is used as a solvent for resins, polymers, and pesticide
formulations. Dermal and inhalation exposure may occur, but will
most likely be minimal. Data are available that show that isophorone
can occur in µg/litre concentrations in drinking-water and fish. In
view of the low toxicity of isophorone in experimental studies and
the low levels of exposure from environmental sources, the risks for
the general population appear to be minimal.
3.1.2 Occupational exposure
In the absence of adequate engineering controls and industrial
hygiene measures, occupational exposure to isophorone may exceed
acceptable levels and cause eye, skin, and respiratory irritation;
other health effects may occur at higher concentrations. No studies
on long-term health effects in workers were available for review by
the Task Group.
3.1.3 The environment
Isophorone may be released into the environment following its use as
a pesticide carrier and its ubiquitous use as a solvent. Low
concentrations have been identified in several environmental
compartments, though it has a low environmental persistence due to
biodegradation, volatilization, and photochemical oxidation
processes. The available data suggest that the toxicity of
isophorone for aquatic organisms is low.
3.2 Recommendations
3.2.1 General population and occupational exposure
Care should be taken to prevent contamination of groundwater and
air.
Workers involved in the manufacture or use of isophorone should be
protected from exposure by means of adequate engineering controls
and appropriate industrial hygiene measures. Levels of occupational
exposure should be kept within acceptable limits and should be
monitored regularly.
3.2.2 Further studies
Health surveillance of exposed workers should be conducted and
reported on.
Actual levels of isophorone in the waters surrounding industrial
areas should be determined.
Adequate short-term/long-term inhalation studies on experimental
animals should be conducted, in order to determine safe levels of
occupational exposure.
Information is required on the anaerobic biodegradation of
isophorone, especially as it has been identified in landfill
leachate.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY
ACTION
4.1 Main Human Health Hazards, Prevention and Protection, First Aid
Isophorone vapours are irritating to the eyes and the respiratory
tract. Prolonged exposure may cause fatigue and malaise. Prolonged
contact with liquid isophorone may irritate the skin.
4.1.1 First aid and medical treatment
4.1.1.1 Eye contact
Immediately flush eyes with plenty of water. Ensure adequate
flushing of the eyes by separating the eyelids with fingers. Medical
attention should be obtained.
4.1.1.2 Skin contact
Contaminated clothing should be removed and the affected area of the
skin thoroughly flushed with water. If skin irritation occurs,
medical attention should be obtained.
4.1.1.3 Inhalation
Provide fresh air. Monitor breathing, and give oxygen if breathing
is difficult.
4.1.1.4 Ingestion
Seek medical advice immediately. Wash out mouth with water. Emesis
may be indicated soon after ingestion, unless the patient is
unconscious. Let the victim ingest charcoal (30-100 g in adults) as
a slurry, to limit absorption of isophorone from the intestine.
4.1.2 Safe handling
4.1.2.1 Personal protection
Atmospheric levels should be kept below the recommended occupational
exposure limits by local exhaust ventilation. Respirators should be
worn in areas where these limits are likely to be exceeded. Skin and
eye protection should be worn where exposure is likely to occur.
Nitrile rubber gloves are recommended.
4.2 Fire and Explosion Hazards
Isophorone is flammable. Suitable extinguishing media are water
spray, carbon dioxide, foam, or dry powder.
The explosion limits in air are 0.8-3.8 vol.%.
4.3 Storage
Containers should be kept tightly closed and stored in a
well-ventilated area.
4.4 Transport
Local requirements regarding the movement of hazardous goods or
wastes must be complied with. Containers should be checked to ensure
that they are sound and labels undamaged before despatch. For
regulations see section 6.4.
4.5 Spillage and Disposal
4.5.1 Spillage
The area should be evacuated and sources of ignition removed.
Self-contained breathing apparatus, gloves, goggles, face shield,
and boots should be worn. Liquid should be prevented from entering
sewers, basements, and workpits.
Small-scale spillages should be absorbed on paper towels and the
paper burnt, away from other combustible material.
For medium-scale spillages, the liquid should be absorbed with sand
or earth and all material should be removed to a safe place for
subsequent incineration. The contaminated area should be washed out
with plenty of water.
For large-scale spillages, the spilt liquid should be prevented from
spreading by the use of sand or earth. The liquid should be
transferred to a salvage tank if possible; otherwise it should be
treated as for medium-scale spillages. The local authorities should
be informed at once, if the spilt liquid enters the surface-water
drains.
4.5.2 Disposal
This combustible material may be burned in a chemical incinerator.
The product should not be buried or dumped in a landfill.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
The toxicity of isophorone for aquatic and terrestrial species is
low.
Industrial discharges and any spillage or unused product should be
prevented from polluting the environment and spreading to vegetation
or waterways and should be treated and disposed of properly (section
4.5.2).
6. CURRENT REGULATIONS, GUIDELINES, AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other United Nations sources. The intention is to give the
reader a representative but non-exhaustive overview of current
regulations, guidelines, and standards.
The reader should be aware that regulatory decisions about
chemicals, taken in a certain country, can only be fully understood
in the framework of the legislation of that country. Furthermore,
the regulations and guidelines of all countries are subject to
change and should always be verified with the appropriate regulatory
authorities before application.
6.1 Previous Evaluations by International Bodies
None available.
6.2 Exposure Limit Values
Some exposure limit values are given in the table on the next page.
6.3 Specific Restrictions
In Germany, isophorone may not be handled by adolescents or
pregnant, or nursing, women.
6.4 Labelling, Packaging, and Transport
The European Community legislation requires labelling as an irritant
substance using the symbol:
The label must read:
Irritating to eyes, respiratory system and skin; in case of
contact with eyes, rinse immediately with plenty of water and
seek medical advice.
Regulations on the Transport of Dangerous Goods:
RID/ADR: Class 3, No. 32C
ADNR: Class 3, No. 4, category K3
GGVSee/IMDG-Code: Not classified
IATA-RAR: Article No. 1939
6.5 Waste Disposal
In the USA, isophorone is rated as a persistent compound, and any
non-domestic waste must be treated as a toxic pollutant. Specific
instructions are given for notification and incineration. Permits
are required for its discharge and discharge limits have been set.
Exposure limit values in some countries
Medium Specification Country/ Exposure Limit Description Value Effective
organization date
AIR Finland Threshold limit value (TLV) 28 mg/m3 (5 ppm) 1987
Germany Maximum worksite concentration (MAK)
- time-weighted average (TWA) 28.0 mg/m3 1980
Russian Federation Ceiling value 1.0 mg/m3 1978
Sweden Hygienic limit value 30.0 mg/m3 1985
United Kingdom Recommended limit (RECL)
- time-weighted average (TWA) 25 mg/m3 1985
- short-term exposure level (STEL) 25 mg/m3
(10-min-TWA)
USA Permissible Exposure Limit (OSHA)
- DOL/OSHA 8-h time-weighted average (TWA) 23.0 mg/m3 (4 ppm) 1989
- NIOSH Recommended Exposure Limit (REL)
up to a 10-h TWA 23 mg/m3 (4 ppm) 1988
- ACGIH Threshold limit value (TLV)
Ceiling value (15-min) 28 mg/m3 (5 ppm) 1992
BIBLIOGRAPHY
CEC (1987) Legislation in dangerous substances - Classification and
labelling in the European Communities - Vol. 1 & 2, Commission of
the European Communities, London, Graham & Trotman, Ltd.
CEC/IPCS (1991) International chemical safety card (ICSC) No. 0169
on isophorone, Luxembourg, Grand Duchy of Luxembourg, Commission
of the European Communities (CEC); Geneva, Switzerland,
International Programme on Chemical Safety (IPCS), World Health
Organization, 2 pp.
IARC (1972-present) IARC monographs on the evaluation of
carcinogenic risk of chemicals to man, Lyon, International Agency
for Research on Cancer.
IPCS (in preparation) Environmental Health Criteria 174:
Isophorone, Geneva, World Health Organization.
IRPTC (1985) IRPTC file on treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme.
IRPTC (1986) IRPTC legal file 1986, Geneva, International Register
of Potentially Toxic Chemicals, United Nations Environment
Programme.
SAX, N.I. (1984) Dangerous properties of industrial materials, New
York, Van Nostrand Reinhold Company, Inc.
UNEP/IEO (1990) Storage of hazardous materials: a technical guide
for safe warehousing of hazardous materials, Paris, United Nations
Environment Programme - Industry and Environment Office, 80 pp.
UNITED NATIONS (1986) Recommendations on the transport of dangerous
goods, 4th ed., New York, United Nations.
US NIOSH/OSHA (1981) Occupational health guidelines for chemical
hazards, 3 Vol., Washington DC, US Department of Health and Human
Services, US Department of Labor (Publication No. DHHS (NIOSH)
01-123).
 Relative molecular mass: 138.2
CAS registry number:
Relative molecular mass: 138.2
CAS registry number: 
 Relative molecular mass: 138.2
CAS registry number:
Relative molecular mass: 138.2
CAS registry number: