
IPCS INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Health and Safety Guide No. 104
ACRYLIC ACID
HEALTH AND SAFETY GUIDE
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
WORLD HEALTH ORGANIZATION, GENEVA 1997
This is a companion volume to
Environmental Health Criteria 191: Acrylic acid
Published by the World Health Organization for the International
Programme on Chemical Safety (a collaborative programme of the United
Nations Environment Programme, the International Labour Organisation,
and the World Health Organization) and produced within the framework
of the Inter-Organization Programme for the Sound Management of
Chemicals
WORLD HEALTH ORGANIZATION, GENEVA 1997
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
WHO Library Cataloguing in Publication Data
Acrylic acid: health and safety guide.
(Health and safety guide ; no. 104)
1.Acrylates - adverse effects - toxicity
2.Environmental exposure 3.Occupational exposure I.Series
ISBN 92 4 151104 4 (NLM Classification: QV 50)
ISSN 0259-7268
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1997
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in this
publication do not imply the expression of any opinion whatsoever on
the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
INTRODUCTION
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.1.1. Primary constituent
1.2. Physical and chemical properties
1.3. Analytical methods
1.4. Production and uses
2. SUMMARY AND EVALUATION
2.1. Human exposure
2.2. Kinetics and metabolism
2.3. Effects on animals
2.4. Effects on humans
2.5. Effects on the environment
3. CONCLUSIONS AND RECOMMENDATIONS
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first
aid
4.1.1. Advice to physicians
4.1.1.1 Symptoms of poisoning
4.1.1.2 Medical advice
4.1.2. Health surveillance advice
4.2. Safety in use
4.3. Explosion and fire hazards
4.4. Storage
4.5. Transport
4.6. Spillage and disposal
4.6.1. Spillage
4.6.2. Disposal
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
5.2. Prevention
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
7.1. Previous evaluations by international bodies
7.2. Exposure limit values
7.3. Specific restrictions
7.4. Labelling, packaging and transport
BIBLIOGRAPHY
INTRODUCTION
The Environmental Health Criteria (EHC) monographs produced by the
International Programme on Chemical Safety include an assessment of
the effects on the environment and on human health of exposure to a
chemical or combination of chemicals, or physical or biological
agents. They also provide guidelines for setting exposure limits.
The purpose of a Health and Safety Guide is to facilitate the
application of these guidelines in national chemical safety
programmes. The first three sections of a Health and Safety Guide
highlight the relevant technical information in the corresponding EHC.
Section 4 includes advice on preventive and protective measures and
emergency action; health workers should be thoroughly familiar with
the medical information to ensure that they can act efficiently in an
emergency. Within the Guide is a Summary of Chemical Safety
Information which should be readily available, and should be clearly
explained, to all who could come into contact with the chemical. The
section on regulatory information has been extracted from the legal
file of the International Register of Potentially Toxic Chemicals
(IRPTC) and from other United Nations sources.
The target readership includes occupational health services, those in
ministries, governmental agencies, industry, and trade unions who
are involved in the safe use of chemicals and the avoidance of
environmental health hazards, and those wanting more information on
this topic. An attempt has been made to use only terms that will be
familiar to the intended user. However, sections 1 and 2 inevitably
contain some technical terms. A bibliography has been included for
readers who require further background information.
Revision of the information in this Guide will take place in due
course, and the eventual aim is to use standardized terminology.
Comments on any difficulties encountered in using the Guide would be
very helpful and should be addressed to:
The Director
International Programme on Chemical Safety
World Health Organization
1211 Geneva 27
Switzerland
THE INFORMATION IN THIS GUIDE
SHOULD BE CONSIDERED AS A
STARTING POINT TO A COMPREHENSIVE
HEALTH AND SAFETY PROGRAMME
1. PRODUCT IDENTITY AND USES
1.1. Identity
1.1.1 Primary constituent
Common name: Acrylic acid
CAS name: 2-Propenoic acid
CAS registry number: 79-10-7
EEC No: 607-061-00-8
DOT UN: 22-18-29
RTECS Number: AS 4375000
Synonyms: acroleic acid
2-propenoic acid
vinylformic acid
propene acid
ethylenecarboxylic acid
UN 2218
propenoic acid
ethene carboxylic acid
Chemical formula: C3H4O2
Chemical structure:
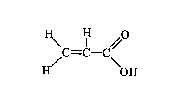 Relative molecular mass: 72.06
Conversion factors: 1 ppm = 3.0 mg/m3; 1 mg/m3 = 0.33 ppm
1.2 Physical and Chemical Properties
Acrylic acid is a colourless liquid with an irritating acrid
odour at room temperature and pressure. Its odour threshold is low
(0.20-3.14 mg/m3). It is miscible in water and most organic solvents.
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification, and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins. Acrylic acid polymerizes easily when exposed to heat, light or
metals, and so a polymerization inhibitor is added to commercial
acrylic acid to prevent the strong exothermic polymerization. The
inhibitors that are usually used in acrylic acid preparations are the
monomethyl ether of hydroquinone (methoxyphenol) at 200 ± 20 ppm,
phenothiazine at 0.1% and hydroquinone at 0.1%. Methylene blue at 0.5
to 1.0% and N,N'-diphenyl- p-phenylenediamine at 0.05% can also be
used.
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according
to the supplier's recommendations. Heat can cause vigorous
polymerization in some circumstances. Acrylic acid reacts readily with
free radicals and electrophilic or nucleophilic agents. It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C.
The presence of oxygen is required for the stabilizer to function
effectively. A head space containing sufficient air should always be
maintained above the monomer to ensure inhibitor effectiveness.
Dissolved oxygen takes part in the inhibition reaction and therefore
is gradually consumed. The level of dissolved oxygen should
periodically be replenished. This can be accomplished by thoroughly
aerating the liquid phase, i.e. recirculation of the inventory in
tanks or agitating drums (rotating).
Acrylic acid must never be handled under an inert atmosphere.
Freezing of acrylic acid occurs at 13°C. Rethawing under
inappropriate temperature conditions is another frequent reason for
acrylic acid polymerization. During the crystallization process the
inhibitor and oxygen concentrate in the mother liquor. Therefore no
mother liquor should be withdrawn from a partially frozen container.
This may result in a severe deficiency of the inhibitor system in the
crystalline matrix. If direct heat is applied, polymerization will
start immediately, often with great violence. Under no circumstances
must steam be used to thaw frozen acrylic acid, nor must thawing be
carried out at temperatures above 35°C.
Acrylic acid is a strong corrosive agent to many metals, such as
unalloyed steel, copper and brass. Frequently the hydrolysis of such
metalic materials generates a deep discoloration in acrylic acid.
Polyvalent metal salts formed during hydrolytic reactions could also
induce polymerization. Therefore, under no circumstances should
acrylic acid be stored or transported with equipment which contains
the above-mentioned metals. Acrylic acid does not affect stainless
steel.
1.3 Analytical Methods
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
was found to be 14 mg/m3 (14 ppm) in air and down to 1 mg/kg or
1 mg/litre (1 ppm) in other media.
1.4 Production and Uses
The worldwide production of acrylic acid in 1994 was estimated
to be approximately 2 million tonnes. Acrylic acid is used primarily
as a starting material in the production of acrylic esters; as a
monomer for polyacrylic acid and salts, as a co-monomer with
acrylamide for polymers used as flocculants, with ethylene for ion
exchange resin polymers, with methyl ester for polymers, and with
itatonic acid for other copolymers.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as polishes, paints and coatings, adhesives, rug backing,
plastics, textiles and paper finishes. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters. Acrylic acid also occurs in wastewater effluent
from its production. It is estimated that thousands of workers could
be exposed to acrylic acid, but exact figures are not available.
2.2 Kinetics and Metabolism
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxypropionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
2.3 Effects on Animals
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, and of moderate acute toxicity by the inhalation
and dermal routes.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg bw per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). For inhalation studies a lowest-observed-
adverse-effect level (LOAEL) of 15 mg/m3 (5 ppm) was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in in
vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay was negative, and no firm conclusions can be drawn
from an in vivo DNA binding study or from a dominant lethal assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
2.4 Effects on Humans
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
2.5 Effects of the Environment
No quantitative data on environmental levels of acrylic acid in
ambient air, drinking-water or soil have been reported.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Under soil
conditions, chemicals with low Henry's Law constants are essentially
non-volatile. However, the vapour pressure of acrylic acid would
suggest that it may volatilize from surfaces and dry soil. Acrylic
acid may be formed by hydrolysis of acrylamide monomer from industrial
waste in soil, especially under aerobic conditions.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. When added to water acrylic acid is rapidly oxidized, and
so it can potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
On the basis of the low octanol-water partition coefficient of
acrylic acid, bioconcentration in aquatic organisms is unlikely. There
have been no reports of biomagnification of acrylic acid in food
chains.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre,
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization)
for Daphnia magna were 54 mg/litre (24 h) and 95 mg/litre (48 h).
Acute toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre
(72-h LC50) for the golden orfe. The 96-h NOEC for acrylic acid
toxicity to rainbow trout was found to be 6.3 mg/litre based on a lack
of sublethal/behavioural responses.
No data on the effects of acrylic acid on terrestrial organisms
have been reported.
3. CONCLUSIONS AND RECOMMENDATIONS
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure to acrylic acid.
However, no obvious adverse effects in the general population have
been identified.
Acrylic acid poses minimal risk for the general environment,
except in the case of uncontrolled discharge.
It is recommended that exposure of the general public to acrylic
acid in the ambient air and drinking-water does not exceed the
guidance values given in the Environmental Health Criteria No. 19
Acrylic Acid (IPCS, 1997). These are as follows:
* Inhalation exposure: 54 µg/m3
* Oral exposure via drinking-water: 9.9 mg/litre.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first aid.
Additional details are given in the Summary on Chemical Safety
Information (section 6)
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The principal hazard of acrylic acid is its corrosive effect on
tissues. Both vapour and liquid can be irritating or corrosive to the
mucous membranes, skin and eyes. The severity of these effects is
dependent on the duration of contact, which, if prolonged, may result
in blisters and burns. Blister formation can appear as late as 24 h
after exposure. Severe corneal burns could occur to the eyes.
Permanent tissue damage may result if prompt and appropriate emergency
response is not provided. First aid recommendations are given in
section 6.
Inhalation of concentrated vapours and mist could produce
moderate to severe irritation of the respiratory tract. High
concentrations could result in pulmonary oedema while lower
concentrations could produce nasal and throat irritation. Lacrymation
may also result from inhalation exposure.
Although ingestion is not an expected route of human exposure,
swallowing of acrylic acid may cause severe irritation or burning of
the mouth, throat, oesophagus or stomach.
No serious health effects have been reported to result from
single exposure or repeated exposure at low concentrations of acrylic
acid.
4.1.1.2 Medical advice
In order to minimize possible consequences of accidents, all
personnel assigned to handle acrylic acid must be aware that prompt
and appropriate response (see following sections) is essential. First
aid must be rendered immediately. The installation of a sufficient
number of emergency showers and eye washes is a prerequisite for the
proper management of incidents.
If even minute quantities of acrylic acid enter the eyes, they
must be irrigated by means of an eye wash with copious amounts of
lukewarm water for at least 15 min. In order to secure the complete
removal of the acid, the assistance of a helper is strongly advised.
The eyelids should be held wide open and away from the eye balls.
Immediate help of an eye specialist should be sought. Oil or oily
ointments should not be applied unless recommended by a physician.
In the event that skin contact with acrylic acid has occurred,
all clothing should be completely removed under an emergency shower.
Washing with water should be continued until all odour has
disappeared, in any case not less than 15 min. The advice of a
physican should be sought. Contaminated clothing must never be reused
unless properly laundered. Leather wear must be disposed off.
A person who has suffered from inhalation of acid fumes should be
removed at once from the contaminated area and made to lie down in
fresh air without moving. If the patient is unconscious, he should be
placed on his side in a stable position. A physician and ambulance
should be summoned immediately. If available, pure oxygen should be
administered by means of a respirator, but only by a person who is
authorised for such duty by a physican.
If acrylic acid is ingested, the patient should be made to drink
large amounts of water. Vomiting should not be induced. A physican and
ambulance should be summoned immediately.
4.1.2 Health surveillance advice
Pre-employment and annual general medical examinations are
advised for regularly exposed workers.
4.2 Safety in Use
Acrylic acid should only be handled in well-aerated and well-
ventilated places. If exposure to concentrated vapour can not be
excluded (as in the case of an accident), self-contained breathing
apparatus or air supply masks must be worn. Care must be taken when
using filter-type masks to ensure that the filter capacity is not
exceeded for the intended time of use and expected concentration.
In areas where a release of acrylic acid is possible, eye
protection devices, face shields, neoprene gloves and rubber boots
should be worn. A chemical suit with a self-contained breathing
apparatus is strongly recommended if larger spills or emissions have
to be cleared. Appropriate protective clothing should be worn for work
involving breaking or entering into a closed acrylic acid system.
Owing to its vapour pressure, the concentration of acrylic acid in
closed rooms can reach high values.
If clothing or shoes have accidentally been contaminated with
acrylic acid, they must be removed immediately. Contaminated leather
shoes or other leather goods must be discarded.
For timely and appropriate emergency response, it is advisable to
provide complete sets of safety protection equipment near places where
accidents with acrylic acid are possible.
4.3 Explosion and Fire Hazards
Acrylic acid has a flash point of 54-68°C and does not form
explosible vapour mixtures at ordinary ambient temperatures. However,
ignition may occur if excessive amounts of mist or aerosols have
formed in air. Ignition sources can include spark discharges from
static electricity, and this can occur when acrylic acid is flowing
through or being discharged from a line. During transfer from one
container into another, the containers should be electrically
interconnected and properly grounded. Splashing into a tank should be
avoided by using a dip tube.
Since acrylic acid and water are miscible in any proportion,
water can be used to extingish fires. Small fires can be fought with
carbon dioxide or dry chemical extinguishers, whereas for larger fires
foam (alcohol or universal type) can be used.
If a fire occurs in or close to a tank farm containing acrylic
acid, tanks and pipes should be cooled by spraying with water in order
to prevent the acid from polymerizing.
4.4. Storage
Acrylic acid should be stored in a detached, cool, well-
ventilated, non-combustible place and its containers should be
protected against physical damage. Acrylic acid can be stored only in
vessels lined with glass, stainless steel, aluminum or polyethylene.
In order to inhibit polymerization during transport and storage,
200 ppm MeHQ (the monomethyl ether of hydroquinone) is commonly added
to acrylic acid by the manufacturer. The presence of oxygen is
required for the inhibitor to be effective. A major concern during the
storage of acrylic acid is the avoidance of elevated temperatures as
well as freezing, since both can lead to a failure of the inhibitor
system. Ideally acrylic acid should be stored within a temperature
range of 15 to 25°C.
Acrylic acid and its solutions should be kept out of reach of
children and unauthorized persons as well as away from food, drink and
animal feed. If any container in the store is leaking, appropriate
precautions should be taken (see section 6) and personal protective
equipment used.
4.5. Transport
Acrylic acid is shipped in containers in compliance with
regulations according to ADR/RID/GGVS/GGVE, Class 8 Packing Group B
specifications. Acrylic acid is commonly shipped in steel drums with
polyethylene inserts or in self-supporting high-density polyethylene
drums impermeable to ultraviolet light. White polyethylene container
are translucent to ultraviolet light and therefore may promote
polymerization. Stainless steel ISO containers are recommended for the
transport of quantities of acrylic acid up to 1 tonne.
4.6. Spillage and disposal
4.6.1. Spillage
Before dealing with any spillage, appropriate personal protective
equipment should be used (see section 6).
Small spills of up to 5 litres can be absorbed in commercially
available clean-up kits (using sand or clay). If a wastewater sewer is
close by, the spill can also be washed down with water provided that
it is not a storm-sewer or ditch that is routed to surface water.
Large spills should be contained, if possible, within a diked
area. A temporary dike can be arranged by stacking sand bags or
similar devices. Avoid run-off into storm sewers routed to public
surface water. If possible, the material should be recovered in
appropriate containers for reuse or disposal. If a wastewater sewer is
available, the acid or remainders can also be sparingly washed down
after dilution and neutralization prior to being discharged to a
water-treatment plant. During all handling operations of large spills
a chemical suit with a self-contained or air-supplied breathing device
must be worn.
In the event of accidental spillage of acrylic acid to surface
water or to a municipal sewer system, the pollution control agencies
must be notified promptly.
Spills of the monomer may be diluted and washed into a biological
treatment plant after notification of the person in charge. The
biodegradability of the material in diluted form is good (> 70% Zahn-
Wellens static test OECD 302 B). However, acrylic acid may be toxic to
the system if the bacteria have not been conditioned properly to this
material. Accordingly, the initial feed rate should be low with a
stepwise increase if a significant amount is to be fed into the
biological treatment plant. The maximum concentration should not
exceed 1000 mg per litre. It should be kept in mind, however, that
large quantities may affect the optimal acidity of the milieu and may
therefore need to be neutralized by the simultaneous addition of
sodium hydroxide.
4.6.2. Disposal
State laws and local regulations governing waste disposal make it
essential for producers, suppliers, hauliers and users of acrylic acid
to be fully aware of viable options for the ultimate disposal of
materials containing acrylic acid. Materials to be disposed of may be
residues from production or cleaning operations as well as waste
material from spills.
Acrylic acid is a highly corrosive material. Accordingly it
should always be handled with appropriate safety equipment.
Solid materials containing acrylic acid, such as absorbents or
polymeric material, can be disposed of by incineration. Disposal in
landfills must be thoroughly checked with the authorities and should
be practiced only as a last resort.
For the disposal of waste materials originating from laboratory
samples, great care must be taken to keep the monomer separated from
incompatible material, such as peroxides, which may initiate
polymerization.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
Most of the available data indicate that acrylic acid has low
toxicity for mammals and aquatic organisms. Algae are the most
sensitive group of aquatic organisms. Acrylic acid is miscible with
water and therefore would not be expected to adsorb significantly to
soil or sediment. If released on land or into water, acrylic acid
should readily biodegrade although no rate data are available. Acrylic
acid is unlikely to pose a problem in the general environment.
5.2. Prevention
Because of its action as a strong irritant to mucous membranes
and explosive properties of its mixtures with air, it is essential
that concentrations of acrylic acid in the ambient air be kept as low
as possible. Care should be taken during any manipulations with any
acrylic acid containers. Any effluent containing acrylic acid should
be properly treated, and any acrylic acid spillage should be protected
from all possible ignition sources.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, acrylic acid. It should be displayed
at, or near, entrances to areas where there is potential exposure
to acrylic acid, and on processing equipment and containers. The
summary should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Centre, and for local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
ACRYLIC ACID
CAS no. 79-10-7;
Chemical formula: C3H4O2
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 72.06 Watery, colourless liquid with an irritating acrid
Melting point (°C) 12.3-14 odour; polymerizes on heating or in the presence
Boiling point (°C) 141.3-141.6 of oxygen, acids, alkalis, amines, iron salts,
Flash point, open cup (°C) 54.4-68.3 light or other compounds forming peroxides or free
Autoignition temperature (°C) 390-446 radicals; during polymerization a large amount of
Flammable limits heat may be released; owing to photochemical
lower 2.0% attack, decomposes in the atmosphere; when heated,
upper 8.0% toxic gases are generated, which can form an
Specific gravity (20°C) 1.0497-1.0511 g/ml explosive mixture; miscible with water and soluble
Relative vapour density in many organic solvents; corrosive to many metals
(air = 1 at 20°C ) 2.5
Vapour pressure (39°C) 10 mm Hg
Octanol/water partition coefficient
(log Kow at 20-25°C) 0.161-0.46
Henry's law constant 3.2×10-7 atm × m3/mol
Solubility in water miscible
Solubility in organic solvents
alcohol miscible
chloroform miscible
benzene miscible
acetone soluble (> 10 %)
SUMMARY OF CHEMICAL SAFETY INFORMATION (Con't)
HAZARD/EFFECTS PREVENTION AND PROTECTION FIRST AID
GENERAL: Strong irritative liquid Prevent any spill; avoid inhalation of vapour;
and vapours if you feel unwell seek medical advice; keep
out of reach of children.
SKIN: liquid is a primary irritant, Avoid contact: wear neoprene gloves, acid Remove contaminated clothes and wash exposed
may cause burns by splash contact goggles or face shield; safety footwear of area thoroughly with plenty of water and soap;
chemical resistant material should be worn a physician should be consulted if irritation
over leather safety shoes; safety shower may or pain persists
be required
EYES: splashes may cause burns, Avoid contamination with liquid or exposure to If even minute quantities of acrylic acid enter
corneal damage and blindness; vapour; use acid goggles or face shield; safety the eyes, rinse them with copious amounts of
vapour may cause lacrimation and fountain may be required lukewarm water for at least 15 min; immediate
strong irritancy help of an eye specialist should be sought
INHALATION: mild inhalation Avoid exposure to vapour; wear chemical Move victim to fresh air, provide all emergency
effects may occur from acute respirator at ambient temperature to avoid medical care; if the victim is not breathing, give
exposure inhalation of noxious fumes; suitable artificial respiration; if breathing is difficult,
protective clothing and self-contained give humidified oxygen; call a physician and
respiratory protective apparatus should be ambulance immediately
available for those who may have to rescue
people overcome by fumes
INGESTION: strong irritant to Do not eat, drink or smoke during work If victim is conscious, give large amounts of
mucous membranes of alimentary water to drink; do not induce emesis as acrylic
tract acid is a corrosive material; obtain medical
advice immediately
ENVIRONMENT: High concentrations Avoid spillage into the environment
in surface waters may be toxic to
aquatic organisms; algae are the most
sensitive group of aquatic organisms
SUMMARY OF CHEMICAL SAFETY INFORMATION (Con't)
SPILLAGE STORAGE FIRE AND EXPLOSION
Absorb spilled liquid with sand, clay Store in detached, well-ventilated cool and Acrylic acid is flammable and may be ignited
or other non-combustible material; dry place; substance should be stored only in by heat, sparks or flames; when heated, toxic
alternatively dilute the spillage glass, stainless steel, aluminum or vapours are generated; use water spray,
with water and neutralize with sodium polyethylene-lined equipment; care should be alcohol foam, dry chemicals or carbon dioxide
hydrogen carbonate, crushed taken that uninhibited acrylic acid vapour to extinguish fires
limestone or lime; use appropriate does not polymerize in the vents of flame
personal protective equipment arresters of storage tanks, resulting in
(goggles, gloves, boots etc.) stoppage of vents
WASTE DISPOSAL NATIONAL INFORMATION
Burn at high temperature (up to National occupational exposure limit:
1600°C) in liquid injection National Poisons Control Centre:
incinerator, rotary kiln incinerator Local trade names
or fluidized bed incinerator
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines and standards. The reader should be aware that regulatory
decisions about chemicals taken in a certain country can only be fully
understood in the framework of the legislation of that country.
Furthermore, the regulations and guidelines of all countries are
subject to change and should always be verified with the appropriate
regulatory authorities before application.
7.1 Previous Evaluations by International Bodies
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer. Data on the
carcinogenicity of the compound for humans were considered inadequate.
There was inadequate evidence for carcinogenicity in animals.
Therefore, acrylic acid is not classifiable as to its carcinogenicity
to humans.
7.2 Exposure Limit Values
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the legal file of
the International Register of Potentially Toxic Chemicals. Exposure
limit values in some countries are shown in the table.
7.3 Specific Restrictions
In the USA acrylic acid as a commercial chemical product is
classified as a toxic waste subject to regulation and notification
requirements.
In the European Economic Community preparations that contain
acrylic acid at concentrations greater than 25% should be considered
as corrosive and at concentrations of 2-25% as irritant. Member States
should ensure that dangerous preparations containing acrylic acid are
not placed on the market unless their packages, fastenings and labels
comply with requirements laid down (section 7.4).
In Canada, the maximum amount of acrylic acid that may be
transported on a passenger aircraft, train or road vehicle is one
litre. The maximum amount that may be transported on a cargo aircraft
is 30 litres.
7.4 Labelling, Packaging and Transport
Acrylic acid has been classified in the European Community under
Council Directive 67/548/EEC (as amended for the seventh time in
Council Directive 92/32/EEC) and appears in Annex 1 under EEC No
607-061-00-8. Acrylic acid is classified as both flammable with risk
phrase RIC (flammable) and corrosive with symbol C and risk phrase R34
(causes burns). It is also labelled with the following safety phrases:
S26 (in case of contact with eyes, rinse immediately with plenty of
water and seek medical advice), S36 (wear suitable protective
clothing) and S45 (in case of accident or if you feel unwell seek
medical advice immediately, show the label where possible). The
following phrases also apply if the substance is sold to the general
public or is likely to be used in places where the public has access:
S1 (keep locked up) and S2 (keep out of reach of children).
CURRENT REGULATIONS, GUIDELINES AND STANDARDS
EXPOSURE LIMIT VALUES
Medium Specification Country/organization Exposure limit description Value
(mg/m3)
AIR Occupational Australia Threshold limit value (TLV)
Time-weighted average (8-h TWA) 30
Belgium Time-weighted average (TWA) 5.9
Denmark Time-weighted average (TWA) 30
France Threshold limit value (TLV) 5.9
Italy Time-weighted average (TWA) 5.9
Netherlands Time-weighted average (TWA) 5.9
Poland Maximum allowable concentration
- TWA 20
- Ceiling value 50
Sweden Hygienic limit value (HLV)
- Time-weighted average (8-h TWA) 30
- Short-term exposure limit (STEL) 45
Switzerland TWA 30
United Kingdom Time-weighted average (8-h TWA) 60
Short-term exposure limit (STEL)
(10-min TWA)
CURRENT REGULATIONS, GUIDELINES AND STANDARDS (Con't)
EXPOSURE LIMIT VALUES
Medium Specification Country/organization Exposure limit description Value
(mg/m3)
USA (OSHA) Permissible exposure limit (PEL) 30
- Time-weighted average (TWA) (skin)
USA (ACGIH) Threshold limit value (TLV) 59
- Time-weighted average (TWA) (skin)
Former USSR Maximum allowable concentration (MAC)
- Ceiling value (short-term) 5
BIBLIOGRAPHY
ACGIH (1990) 1990-1991 Threshold limit values for chemical substances
and physical agents and biological exposure indices. Cincinnati,
American Conference of Government Industrial Hygienists.
CEC/IPCS (1993) International Chemical Safety Card 688 Acrylic acid.
Luxembourg, Commission of the European Communities.
CHRIS (1989) CHRIS Hazardous Chemical Data, US Department of
Transportation, US Coast Guard, Washington, DC (CD-ROM Version).
Denver, Colorado, Micromedex, Inc.
Clayton G.D. and Clayton F.E. (Eds) (1982) Patty's industrial hygiene
and toxicology, Vol 2 Toxicology, 3rd ed, New York, John Wiley and
Sons.
Finkel A.J. (1983) Hamilton and Hardy's Industrial Toxicology, 4th
ed., Boston, John Wright, PSG Inc.
Fire Protection Guide on Hazardous Materials, 7th ed. (1978) Boston,
National Fire Protection Association.
Hamilton A. & Hardy H.L. (1974) Industrial Toxicology, 3rd ed.
Massachusetts, Publishing Sciences Group, Inc, Action.
HSDB (1989) Hazardous substances data bank. National Library of
Medicine, Bethesda, Maryland (CD-ROM Version). Denver, Colorado,
Micromedex, Inc.
IPCS (1997) Environmental Health Criteria 191: Acrylic acid. Geneva,
World Health Organization.
ITI (1985) Toxic and Hazardous Industrial Chemicals Safety Manual.
Tokyo, Japan, International Technical Information Institute.
Kirk-Othmer Encyclopedia of Chemical Technology (1978-1984) 3rd ed.
V.1-26, New York, J. Wiley and Sons.
NIOSH (1985) Pocket Guide to Chemical Hazards. Cincinnati, Ohio,
National Institute for Occupational Safety and Health.
OHM/TADS (1989) Oil and Hazardous Materials/Technical Assistance Data
System. US Environmental Protection Agency, Washington, DC (CD-ROM
Version). Denver, Colorado, Micromedex, Inc.
OSHA (1989) Department of Labour, Occupational Safety and Health
Administration: 29 CFR Part 1910; Air Contaminants; Final Rule.
Federal Register; 54(12): 2332-2983.
RTECS (1988) Registry of toxic effects of chemical substances.
National Institute for Occupational Safety and Health, Cincinnati,
Ohio (CD-ROM Version). Hamilton, Ontario, Canadian Centre for
Occupational Safety and Health.
Sax N.I. and Lewis R.J. (1987) Hawley's Condensed Chemical Dictionary,
11th ed. New York, Van Nostrand Reinhold Co, pp 18-19.
Sax N.I. and Lewis R.J. (1989) Dangerous properties of industrial
chemicals, 7th ed. New York, Van Nostrand Reinhold, pp 71-72.
Relative molecular mass: 72.06
Conversion factors: 1 ppm = 3.0 mg/m3; 1 mg/m3 = 0.33 ppm
1.2 Physical and Chemical Properties
Acrylic acid is a colourless liquid with an irritating acrid
odour at room temperature and pressure. Its odour threshold is low
(0.20-3.14 mg/m3). It is miscible in water and most organic solvents.
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification, and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins. Acrylic acid polymerizes easily when exposed to heat, light or
metals, and so a polymerization inhibitor is added to commercial
acrylic acid to prevent the strong exothermic polymerization. The
inhibitors that are usually used in acrylic acid preparations are the
monomethyl ether of hydroquinone (methoxyphenol) at 200 ± 20 ppm,
phenothiazine at 0.1% and hydroquinone at 0.1%. Methylene blue at 0.5
to 1.0% and N,N'-diphenyl- p-phenylenediamine at 0.05% can also be
used.
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according
to the supplier's recommendations. Heat can cause vigorous
polymerization in some circumstances. Acrylic acid reacts readily with
free radicals and electrophilic or nucleophilic agents. It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C.
The presence of oxygen is required for the stabilizer to function
effectively. A head space containing sufficient air should always be
maintained above the monomer to ensure inhibitor effectiveness.
Dissolved oxygen takes part in the inhibition reaction and therefore
is gradually consumed. The level of dissolved oxygen should
periodically be replenished. This can be accomplished by thoroughly
aerating the liquid phase, i.e. recirculation of the inventory in
tanks or agitating drums (rotating).
Acrylic acid must never be handled under an inert atmosphere.
Freezing of acrylic acid occurs at 13°C. Rethawing under
inappropriate temperature conditions is another frequent reason for
acrylic acid polymerization. During the crystallization process the
inhibitor and oxygen concentrate in the mother liquor. Therefore no
mother liquor should be withdrawn from a partially frozen container.
This may result in a severe deficiency of the inhibitor system in the
crystalline matrix. If direct heat is applied, polymerization will
start immediately, often with great violence. Under no circumstances
must steam be used to thaw frozen acrylic acid, nor must thawing be
carried out at temperatures above 35°C.
Acrylic acid is a strong corrosive agent to many metals, such as
unalloyed steel, copper and brass. Frequently the hydrolysis of such
metalic materials generates a deep discoloration in acrylic acid.
Polyvalent metal salts formed during hydrolytic reactions could also
induce polymerization. Therefore, under no circumstances should
acrylic acid be stored or transported with equipment which contains
the above-mentioned metals. Acrylic acid does not affect stainless
steel.
1.3 Analytical Methods
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
was found to be 14 mg/m3 (14 ppm) in air and down to 1 mg/kg or
1 mg/litre (1 ppm) in other media.
1.4 Production and Uses
The worldwide production of acrylic acid in 1994 was estimated
to be approximately 2 million tonnes. Acrylic acid is used primarily
as a starting material in the production of acrylic esters; as a
monomer for polyacrylic acid and salts, as a co-monomer with
acrylamide for polymers used as flocculants, with ethylene for ion
exchange resin polymers, with methyl ester for polymers, and with
itatonic acid for other copolymers.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as polishes, paints and coatings, adhesives, rug backing,
plastics, textiles and paper finishes. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters. Acrylic acid also occurs in wastewater effluent
from its production. It is estimated that thousands of workers could
be exposed to acrylic acid, but exact figures are not available.
2.2 Kinetics and Metabolism
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxypropionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
2.3 Effects on Animals
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, and of moderate acute toxicity by the inhalation
and dermal routes.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg bw per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). For inhalation studies a lowest-observed-
adverse-effect level (LOAEL) of 15 mg/m3 (5 ppm) was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in in
vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay was negative, and no firm conclusions can be drawn
from an in vivo DNA binding study or from a dominant lethal assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
2.4 Effects on Humans
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
2.5 Effects of the Environment
No quantitative data on environmental levels of acrylic acid in
ambient air, drinking-water or soil have been reported.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Under soil
conditions, chemicals with low Henry's Law constants are essentially
non-volatile. However, the vapour pressure of acrylic acid would
suggest that it may volatilize from surfaces and dry soil. Acrylic
acid may be formed by hydrolysis of acrylamide monomer from industrial
waste in soil, especially under aerobic conditions.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. When added to water acrylic acid is rapidly oxidized, and
so it can potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
On the basis of the low octanol-water partition coefficient of
acrylic acid, bioconcentration in aquatic organisms is unlikely. There
have been no reports of biomagnification of acrylic acid in food
chains.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre,
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization)
for Daphnia magna were 54 mg/litre (24 h) and 95 mg/litre (48 h).
Acute toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre
(72-h LC50) for the golden orfe. The 96-h NOEC for acrylic acid
toxicity to rainbow trout was found to be 6.3 mg/litre based on a lack
of sublethal/behavioural responses.
No data on the effects of acrylic acid on terrestrial organisms
have been reported.
3. CONCLUSIONS AND RECOMMENDATIONS
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure to acrylic acid.
However, no obvious adverse effects in the general population have
been identified.
Acrylic acid poses minimal risk for the general environment,
except in the case of uncontrolled discharge.
It is recommended that exposure of the general public to acrylic
acid in the ambient air and drinking-water does not exceed the
guidance values given in the Environmental Health Criteria No. 19
Acrylic Acid (IPCS, 1997). These are as follows:
* Inhalation exposure: 54 µg/m3
* Oral exposure via drinking-water: 9.9 mg/litre.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first aid.
Additional details are given in the Summary on Chemical Safety
Information (section 6)
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The principal hazard of acrylic acid is its corrosive effect on
tissues. Both vapour and liquid can be irritating or corrosive to the
mucous membranes, skin and eyes. The severity of these effects is
dependent on the duration of contact, which, if prolonged, may result
in blisters and burns. Blister formation can appear as late as 24 h
after exposure. Severe corneal burns could occur to the eyes.
Permanent tissue damage may result if prompt and appropriate emergency
response is not provided. First aid recommendations are given in
section 6.
Inhalation of concentrated vapours and mist could produce
moderate to severe irritation of the respiratory tract. High
concentrations could result in pulmonary oedema while lower
concentrations could produce nasal and throat irritation. Lacrymation
may also result from inhalation exposure.
Although ingestion is not an expected route of human exposure,
swallowing of acrylic acid may cause severe irritation or burning of
the mouth, throat, oesophagus or stomach.
No serious health effects have been reported to result from
single exposure or repeated exposure at low concentrations of acrylic
acid.
4.1.1.2 Medical advice
In order to minimize possible consequences of accidents, all
personnel assigned to handle acrylic acid must be aware that prompt
and appropriate response (see following sections) is essential. First
aid must be rendered immediately. The installation of a sufficient
number of emergency showers and eye washes is a prerequisite for the
proper management of incidents.
If even minute quantities of acrylic acid enter the eyes, they
must be irrigated by means of an eye wash with copious amounts of
lukewarm water for at least 15 min. In order to secure the complete
removal of the acid, the assistance of a helper is strongly advised.
The eyelids should be held wide open and away from the eye balls.
Immediate help of an eye specialist should be sought. Oil or oily
ointments should not be applied unless recommended by a physician.
In the event that skin contact with acrylic acid has occurred,
all clothing should be completely removed under an emergency shower.
Washing with water should be continued until all odour has
disappeared, in any case not less than 15 min. The advice of a
physican should be sought. Contaminated clothing must never be reused
unless properly laundered. Leather wear must be disposed off.
A person who has suffered from inhalation of acid fumes should be
removed at once from the contaminated area and made to lie down in
fresh air without moving. If the patient is unconscious, he should be
placed on his side in a stable position. A physician and ambulance
should be summoned immediately. If available, pure oxygen should be
administered by means of a respirator, but only by a person who is
authorised for such duty by a physican.
If acrylic acid is ingested, the patient should be made to drink
large amounts of water. Vomiting should not be induced. A physican and
ambulance should be summoned immediately.
4.1.2 Health surveillance advice
Pre-employment and annual general medical examinations are
advised for regularly exposed workers.
4.2 Safety in Use
Acrylic acid should only be handled in well-aerated and well-
ventilated places. If exposure to concentrated vapour can not be
excluded (as in the case of an accident), self-contained breathing
apparatus or air supply masks must be worn. Care must be taken when
using filter-type masks to ensure that the filter capacity is not
exceeded for the intended time of use and expected concentration.
In areas where a release of acrylic acid is possible, eye
protection devices, face shields, neoprene gloves and rubber boots
should be worn. A chemical suit with a self-contained breathing
apparatus is strongly recommended if larger spills or emissions have
to be cleared. Appropriate protective clothing should be worn for work
involving breaking or entering into a closed acrylic acid system.
Owing to its vapour pressure, the concentration of acrylic acid in
closed rooms can reach high values.
If clothing or shoes have accidentally been contaminated with
acrylic acid, they must be removed immediately. Contaminated leather
shoes or other leather goods must be discarded.
For timely and appropriate emergency response, it is advisable to
provide complete sets of safety protection equipment near places where
accidents with acrylic acid are possible.
4.3 Explosion and Fire Hazards
Acrylic acid has a flash point of 54-68°C and does not form
explosible vapour mixtures at ordinary ambient temperatures. However,
ignition may occur if excessive amounts of mist or aerosols have
formed in air. Ignition sources can include spark discharges from
static electricity, and this can occur when acrylic acid is flowing
through or being discharged from a line. During transfer from one
container into another, the containers should be electrically
interconnected and properly grounded. Splashing into a tank should be
avoided by using a dip tube.
Since acrylic acid and water are miscible in any proportion,
water can be used to extingish fires. Small fires can be fought with
carbon dioxide or dry chemical extinguishers, whereas for larger fires
foam (alcohol or universal type) can be used.
If a fire occurs in or close to a tank farm containing acrylic
acid, tanks and pipes should be cooled by spraying with water in order
to prevent the acid from polymerizing.
4.4. Storage
Acrylic acid should be stored in a detached, cool, well-
ventilated, non-combustible place and its containers should be
protected against physical damage. Acrylic acid can be stored only in
vessels lined with glass, stainless steel, aluminum or polyethylene.
In order to inhibit polymerization during transport and storage,
200 ppm MeHQ (the monomethyl ether of hydroquinone) is commonly added
to acrylic acid by the manufacturer. The presence of oxygen is
required for the inhibitor to be effective. A major concern during the
storage of acrylic acid is the avoidance of elevated temperatures as
well as freezing, since both can lead to a failure of the inhibitor
system. Ideally acrylic acid should be stored within a temperature
range of 15 to 25°C.
Acrylic acid and its solutions should be kept out of reach of
children and unauthorized persons as well as away from food, drink and
animal feed. If any container in the store is leaking, appropriate
precautions should be taken (see section 6) and personal protective
equipment used.
4.5. Transport
Acrylic acid is shipped in containers in compliance with
regulations according to ADR/RID/GGVS/GGVE, Class 8 Packing Group B
specifications. Acrylic acid is commonly shipped in steel drums with
polyethylene inserts or in self-supporting high-density polyethylene
drums impermeable to ultraviolet light. White polyethylene container
are translucent to ultraviolet light and therefore may promote
polymerization. Stainless steel ISO containers are recommended for the
transport of quantities of acrylic acid up to 1 tonne.
4.6. Spillage and disposal
4.6.1. Spillage
Before dealing with any spillage, appropriate personal protective
equipment should be used (see section 6).
Small spills of up to 5 litres can be absorbed in commercially
available clean-up kits (using sand or clay). If a wastewater sewer is
close by, the spill can also be washed down with water provided that
it is not a storm-sewer or ditch that is routed to surface water.
Large spills should be contained, if possible, within a diked
area. A temporary dike can be arranged by stacking sand bags or
similar devices. Avoid run-off into storm sewers routed to public
surface water. If possible, the material should be recovered in
appropriate containers for reuse or disposal. If a wastewater sewer is
available, the acid or remainders can also be sparingly washed down
after dilution and neutralization prior to being discharged to a
water-treatment plant. During all handling operations of large spills
a chemical suit with a self-contained or air-supplied breathing device
must be worn.
In the event of accidental spillage of acrylic acid to surface
water or to a municipal sewer system, the pollution control agencies
must be notified promptly.
Spills of the monomer may be diluted and washed into a biological
treatment plant after notification of the person in charge. The
biodegradability of the material in diluted form is good (> 70% Zahn-
Wellens static test OECD 302 B). However, acrylic acid may be toxic to
the system if the bacteria have not been conditioned properly to this
material. Accordingly, the initial feed rate should be low with a
stepwise increase if a significant amount is to be fed into the
biological treatment plant. The maximum concentration should not
exceed 1000 mg per litre. It should be kept in mind, however, that
large quantities may affect the optimal acidity of the milieu and may
therefore need to be neutralized by the simultaneous addition of
sodium hydroxide.
4.6.2. Disposal
State laws and local regulations governing waste disposal make it
essential for producers, suppliers, hauliers and users of acrylic acid
to be fully aware of viable options for the ultimate disposal of
materials containing acrylic acid. Materials to be disposed of may be
residues from production or cleaning operations as well as waste
material from spills.
Acrylic acid is a highly corrosive material. Accordingly it
should always be handled with appropriate safety equipment.
Solid materials containing acrylic acid, such as absorbents or
polymeric material, can be disposed of by incineration. Disposal in
landfills must be thoroughly checked with the authorities and should
be practiced only as a last resort.
For the disposal of waste materials originating from laboratory
samples, great care must be taken to keep the monomer separated from
incompatible material, such as peroxides, which may initiate
polymerization.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
Most of the available data indicate that acrylic acid has low
toxicity for mammals and aquatic organisms. Algae are the most
sensitive group of aquatic organisms. Acrylic acid is miscible with
water and therefore would not be expected to adsorb significantly to
soil or sediment. If released on land or into water, acrylic acid
should readily biodegrade although no rate data are available. Acrylic
acid is unlikely to pose a problem in the general environment.
5.2. Prevention
Because of its action as a strong irritant to mucous membranes
and explosive properties of its mixtures with air, it is essential
that concentrations of acrylic acid in the ambient air be kept as low
as possible. Care should be taken during any manipulations with any
acrylic acid containers. Any effluent containing acrylic acid should
be properly treated, and any acrylic acid spillage should be protected
from all possible ignition sources.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, acrylic acid. It should be displayed
at, or near, entrances to areas where there is potential exposure
to acrylic acid, and on processing equipment and containers. The
summary should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Centre, and for local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
ACRYLIC ACID
CAS no. 79-10-7;
Chemical formula: C3H4O2
PHYSICAL PROPERTIES OTHER CHARACTERISTICS
Relative molecular mass 72.06 Watery, colourless liquid with an irritating acrid
Melting point (°C) 12.3-14 odour; polymerizes on heating or in the presence
Boiling point (°C) 141.3-141.6 of oxygen, acids, alkalis, amines, iron salts,
Flash point, open cup (°C) 54.4-68.3 light or other compounds forming peroxides or free
Autoignition temperature (°C) 390-446 radicals; during polymerization a large amount of
Flammable limits heat may be released; owing to photochemical
lower 2.0% attack, decomposes in the atmosphere; when heated,
upper 8.0% toxic gases are generated, which can form an
Specific gravity (20°C) 1.0497-1.0511 g/ml explosive mixture; miscible with water and soluble
Relative vapour density in many organic solvents; corrosive to many metals
(air = 1 at 20°C ) 2.5
Vapour pressure (39°C) 10 mm Hg
Octanol/water partition coefficient
(log Kow at 20-25°C) 0.161-0.46
Henry's law constant 3.2×10-7 atm × m3/mol
Solubility in water miscible
Solubility in organic solvents
alcohol miscible
chloroform miscible
benzene miscible
acetone soluble (> 10 %)
SUMMARY OF CHEMICAL SAFETY INFORMATION (Con't)
HAZARD/EFFECTS PREVENTION AND PROTECTION FIRST AID
GENERAL: Strong irritative liquid Prevent any spill; avoid inhalation of vapour;
and vapours if you feel unwell seek medical advice; keep
out of reach of children.
SKIN: liquid is a primary irritant, Avoid contact: wear neoprene gloves, acid Remove contaminated clothes and wash exposed
may cause burns by splash contact goggles or face shield; safety footwear of area thoroughly with plenty of water and soap;
chemical resistant material should be worn a physician should be consulted if irritation
over leather safety shoes; safety shower may or pain persists
be required
EYES: splashes may cause burns, Avoid contamination with liquid or exposure to If even minute quantities of acrylic acid enter
corneal damage and blindness; vapour; use acid goggles or face shield; safety the eyes, rinse them with copious amounts of
vapour may cause lacrimation and fountain may be required lukewarm water for at least 15 min; immediate
strong irritancy help of an eye specialist should be sought
INHALATION: mild inhalation Avoid exposure to vapour; wear chemical Move victim to fresh air, provide all emergency
effects may occur from acute respirator at ambient temperature to avoid medical care; if the victim is not breathing, give
exposure inhalation of noxious fumes; suitable artificial respiration; if breathing is difficult,
protective clothing and self-contained give humidified oxygen; call a physician and
respiratory protective apparatus should be ambulance immediately
available for those who may have to rescue
people overcome by fumes
INGESTION: strong irritant to Do not eat, drink or smoke during work If victim is conscious, give large amounts of
mucous membranes of alimentary water to drink; do not induce emesis as acrylic
tract acid is a corrosive material; obtain medical
advice immediately
ENVIRONMENT: High concentrations Avoid spillage into the environment
in surface waters may be toxic to
aquatic organisms; algae are the most
sensitive group of aquatic organisms
SUMMARY OF CHEMICAL SAFETY INFORMATION (Con't)
SPILLAGE STORAGE FIRE AND EXPLOSION
Absorb spilled liquid with sand, clay Store in detached, well-ventilated cool and Acrylic acid is flammable and may be ignited
or other non-combustible material; dry place; substance should be stored only in by heat, sparks or flames; when heated, toxic
alternatively dilute the spillage glass, stainless steel, aluminum or vapours are generated; use water spray,
with water and neutralize with sodium polyethylene-lined equipment; care should be alcohol foam, dry chemicals or carbon dioxide
hydrogen carbonate, crushed taken that uninhibited acrylic acid vapour to extinguish fires
limestone or lime; use appropriate does not polymerize in the vents of flame
personal protective equipment arresters of storage tanks, resulting in
(goggles, gloves, boots etc.) stoppage of vents
WASTE DISPOSAL NATIONAL INFORMATION
Burn at high temperature (up to National occupational exposure limit:
1600°C) in liquid injection National Poisons Control Centre:
incinerator, rotary kiln incinerator Local trade names
or fluidized bed incinerator
7. CURRENT REGULATIONS, GUIDELINES AND STANDARDS
The information given in this section has been extracted from the
International Register of Potentially Toxic Chemicals (IRPTC) legal
file and other UN sources. The intention is to give the reader a
representative but non-exhaustive overview of current regulations,
guidelines and standards. The reader should be aware that regulatory
decisions about chemicals taken in a certain country can only be fully
understood in the framework of the legislation of that country.
Furthermore, the regulations and guidelines of all countries are
subject to change and should always be verified with the appropriate
regulatory authorities before application.
7.1 Previous Evaluations by International Bodies
The carcinogenicity of acrylic acid has been evaluated by the
International Agency for Research on Cancer. Data on the
carcinogenicity of the compound for humans were considered inadequate.
There was inadequate evidence for carcinogenicity in animals.
Therefore, acrylic acid is not classifiable as to its carcinogenicity
to humans.
7.2 Exposure Limit Values
Regulatory standards established by national bodies in different
countries and the European Union are summarized in the legal file of
the International Register of Potentially Toxic Chemicals. Exposure
limit values in some countries are shown in the table.
7.3 Specific Restrictions
In the USA acrylic acid as a commercial chemical product is
classified as a toxic waste subject to regulation and notification
requirements.
In the European Economic Community preparations that contain
acrylic acid at concentrations greater than 25% should be considered
as corrosive and at concentrations of 2-25% as irritant. Member States
should ensure that dangerous preparations containing acrylic acid are
not placed on the market unless their packages, fastenings and labels
comply with requirements laid down (section 7.4).
In Canada, the maximum amount of acrylic acid that may be
transported on a passenger aircraft, train or road vehicle is one
litre. The maximum amount that may be transported on a cargo aircraft
is 30 litres.
7.4 Labelling, Packaging and Transport
Acrylic acid has been classified in the European Community under
Council Directive 67/548/EEC (as amended for the seventh time in
Council Directive 92/32/EEC) and appears in Annex 1 under EEC No
607-061-00-8. Acrylic acid is classified as both flammable with risk
phrase RIC (flammable) and corrosive with symbol C and risk phrase R34
(causes burns). It is also labelled with the following safety phrases:
S26 (in case of contact with eyes, rinse immediately with plenty of
water and seek medical advice), S36 (wear suitable protective
clothing) and S45 (in case of accident or if you feel unwell seek
medical advice immediately, show the label where possible). The
following phrases also apply if the substance is sold to the general
public or is likely to be used in places where the public has access:
S1 (keep locked up) and S2 (keep out of reach of children).
CURRENT REGULATIONS, GUIDELINES AND STANDARDS
EXPOSURE LIMIT VALUES
Medium Specification Country/organization Exposure limit description Value
(mg/m3)
AIR Occupational Australia Threshold limit value (TLV)
Time-weighted average (8-h TWA) 30
Belgium Time-weighted average (TWA) 5.9
Denmark Time-weighted average (TWA) 30
France Threshold limit value (TLV) 5.9
Italy Time-weighted average (TWA) 5.9
Netherlands Time-weighted average (TWA) 5.9
Poland Maximum allowable concentration
- TWA 20
- Ceiling value 50
Sweden Hygienic limit value (HLV)
- Time-weighted average (8-h TWA) 30
- Short-term exposure limit (STEL) 45
Switzerland TWA 30
United Kingdom Time-weighted average (8-h TWA) 60
Short-term exposure limit (STEL)
(10-min TWA)
CURRENT REGULATIONS, GUIDELINES AND STANDARDS (Con't)
EXPOSURE LIMIT VALUES
Medium Specification Country/organization Exposure limit description Value
(mg/m3)
USA (OSHA) Permissible exposure limit (PEL) 30
- Time-weighted average (TWA) (skin)
USA (ACGIH) Threshold limit value (TLV) 59
- Time-weighted average (TWA) (skin)
Former USSR Maximum allowable concentration (MAC)
- Ceiling value (short-term) 5
BIBLIOGRAPHY
ACGIH (1990) 1990-1991 Threshold limit values for chemical substances
and physical agents and biological exposure indices. Cincinnati,
American Conference of Government Industrial Hygienists.
CEC/IPCS (1993) International Chemical Safety Card 688 Acrylic acid.
Luxembourg, Commission of the European Communities.
CHRIS (1989) CHRIS Hazardous Chemical Data, US Department of
Transportation, US Coast Guard, Washington, DC (CD-ROM Version).
Denver, Colorado, Micromedex, Inc.
Clayton G.D. and Clayton F.E. (Eds) (1982) Patty's industrial hygiene
and toxicology, Vol 2 Toxicology, 3rd ed, New York, John Wiley and
Sons.
Finkel A.J. (1983) Hamilton and Hardy's Industrial Toxicology, 4th
ed., Boston, John Wright, PSG Inc.
Fire Protection Guide on Hazardous Materials, 7th ed. (1978) Boston,
National Fire Protection Association.
Hamilton A. & Hardy H.L. (1974) Industrial Toxicology, 3rd ed.
Massachusetts, Publishing Sciences Group, Inc, Action.
HSDB (1989) Hazardous substances data bank. National Library of
Medicine, Bethesda, Maryland (CD-ROM Version). Denver, Colorado,
Micromedex, Inc.
IPCS (1997) Environmental Health Criteria 191: Acrylic acid. Geneva,
World Health Organization.
ITI (1985) Toxic and Hazardous Industrial Chemicals Safety Manual.
Tokyo, Japan, International Technical Information Institute.
Kirk-Othmer Encyclopedia of Chemical Technology (1978-1984) 3rd ed.
V.1-26, New York, J. Wiley and Sons.
NIOSH (1985) Pocket Guide to Chemical Hazards. Cincinnati, Ohio,
National Institute for Occupational Safety and Health.
OHM/TADS (1989) Oil and Hazardous Materials/Technical Assistance Data
System. US Environmental Protection Agency, Washington, DC (CD-ROM
Version). Denver, Colorado, Micromedex, Inc.
OSHA (1989) Department of Labour, Occupational Safety and Health
Administration: 29 CFR Part 1910; Air Contaminants; Final Rule.
Federal Register; 54(12): 2332-2983.
RTECS (1988) Registry of toxic effects of chemical substances.
National Institute for Occupational Safety and Health, Cincinnati,
Ohio (CD-ROM Version). Hamilton, Ontario, Canadian Centre for
Occupational Safety and Health.
Sax N.I. and Lewis R.J. (1987) Hawley's Condensed Chemical Dictionary,
11th ed. New York, Van Nostrand Reinhold Co, pp 18-19.
Sax N.I. and Lewis R.J. (1989) Dangerous properties of industrial
chemicals, 7th ed. New York, Van Nostrand Reinhold, pp 71-72.
 Relative molecular mass: 72.06
Conversion factors: 1 ppm = 3.0 mg/m3; 1 mg/m3 = 0.33 ppm
1.2 Physical and Chemical Properties
Acrylic acid is a colourless liquid with an irritating acrid
odour at room temperature and pressure. Its odour threshold is low
(0.20-3.14 mg/m3). It is miscible in water and most organic solvents.
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification, and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins. Acrylic acid polymerizes easily when exposed to heat, light or
metals, and so a polymerization inhibitor is added to commercial
acrylic acid to prevent the strong exothermic polymerization. The
inhibitors that are usually used in acrylic acid preparations are the
monomethyl ether of hydroquinone (methoxyphenol) at 200 ± 20 ppm,
phenothiazine at 0.1% and hydroquinone at 0.1%. Methylene blue at 0.5
to 1.0% and N,N'-diphenyl- p-phenylenediamine at 0.05% can also be
used.
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according
to the supplier's recommendations. Heat can cause vigorous
polymerization in some circumstances. Acrylic acid reacts readily with
free radicals and electrophilic or nucleophilic agents. It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C.
The presence of oxygen is required for the stabilizer to function
effectively. A head space containing sufficient air should always be
maintained above the monomer to ensure inhibitor effectiveness.
Dissolved oxygen takes part in the inhibition reaction and therefore
is gradually consumed. The level of dissolved oxygen should
periodically be replenished. This can be accomplished by thoroughly
aerating the liquid phase, i.e. recirculation of the inventory in
tanks or agitating drums (rotating).
Acrylic acid must never be handled under an inert atmosphere.
Freezing of acrylic acid occurs at 13°C. Rethawing under
inappropriate temperature conditions is another frequent reason for
acrylic acid polymerization. During the crystallization process the
inhibitor and oxygen concentrate in the mother liquor. Therefore no
mother liquor should be withdrawn from a partially frozen container.
This may result in a severe deficiency of the inhibitor system in the
crystalline matrix. If direct heat is applied, polymerization will
start immediately, often with great violence. Under no circumstances
must steam be used to thaw frozen acrylic acid, nor must thawing be
carried out at temperatures above 35°C.
Acrylic acid is a strong corrosive agent to many metals, such as
unalloyed steel, copper and brass. Frequently the hydrolysis of such
metalic materials generates a deep discoloration in acrylic acid.
Polyvalent metal salts formed during hydrolytic reactions could also
induce polymerization. Therefore, under no circumstances should
acrylic acid be stored or transported with equipment which contains
the above-mentioned metals. Acrylic acid does not affect stainless
steel.
1.3 Analytical Methods
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
was found to be 14 mg/m3 (14 ppm) in air and down to 1 mg/kg or
1 mg/litre (1 ppm) in other media.
1.4 Production and Uses
The worldwide production of acrylic acid in 1994 was estimated
to be approximately 2 million tonnes. Acrylic acid is used primarily
as a starting material in the production of acrylic esters; as a
monomer for polyacrylic acid and salts, as a co-monomer with
acrylamide for polymers used as flocculants, with ethylene for ion
exchange resin polymers, with methyl ester for polymers, and with
itatonic acid for other copolymers.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as polishes, paints and coatings, adhesives, rug backing,
plastics, textiles and paper finishes. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters. Acrylic acid also occurs in wastewater effluent
from its production. It is estimated that thousands of workers could
be exposed to acrylic acid, but exact figures are not available.
2.2 Kinetics and Metabolism
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxypropionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
2.3 Effects on Animals
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, and of moderate acute toxicity by the inhalation
and dermal routes.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg bw per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). For inhalation studies a lowest-observed-
adverse-effect level (LOAEL) of 15 mg/m3 (5 ppm) was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in in
vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay was negative, and no firm conclusions can be drawn
from an in vivo DNA binding study or from a dominant lethal assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
2.4 Effects on Humans
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
2.5 Effects of the Environment
No quantitative data on environmental levels of acrylic acid in
ambient air, drinking-water or soil have been reported.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Under soil
conditions, chemicals with low Henry's Law constants are essentially
non-volatile. However, the vapour pressure of acrylic acid would
suggest that it may volatilize from surfaces and dry soil. Acrylic
acid may be formed by hydrolysis of acrylamide monomer from industrial
waste in soil, especially under aerobic conditions.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. When added to water acrylic acid is rapidly oxidized, and
so it can potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
On the basis of the low octanol-water partition coefficient of
acrylic acid, bioconcentration in aquatic organisms is unlikely. There
have been no reports of biomagnification of acrylic acid in food
chains.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre,
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization)
for Daphnia magna were 54 mg/litre (24 h) and 95 mg/litre (48 h).
Acute toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre
(72-h LC50) for the golden orfe. The 96-h NOEC for acrylic acid
toxicity to rainbow trout was found to be 6.3 mg/litre based on a lack
of sublethal/behavioural responses.
No data on the effects of acrylic acid on terrestrial organisms
have been reported.
3. CONCLUSIONS AND RECOMMENDATIONS
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure to acrylic acid.
However, no obvious adverse effects in the general population have
been identified.
Acrylic acid poses minimal risk for the general environment,
except in the case of uncontrolled discharge.
It is recommended that exposure of the general public to acrylic
acid in the ambient air and drinking-water does not exceed the
guidance values given in the Environmental Health Criteria No. 19
Acrylic Acid (IPCS, 1997). These are as follows:
* Inhalation exposure: 54 µg/m3
* Oral exposure via drinking-water: 9.9 mg/litre.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first aid.
Additional details are given in the Summary on Chemical Safety
Information (section 6)
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The principal hazard of acrylic acid is its corrosive effect on
tissues. Both vapour and liquid can be irritating or corrosive to the
mucous membranes, skin and eyes. The severity of these effects is
dependent on the duration of contact, which, if prolonged, may result
in blisters and burns. Blister formation can appear as late as 24 h
after exposure. Severe corneal burns could occur to the eyes.
Permanent tissue damage may result if prompt and appropriate emergency
response is not provided. First aid recommendations are given in
section 6.
Inhalation of concentrated vapours and mist could produce
moderate to severe irritation of the respiratory tract. High
concentrations could result in pulmonary oedema while lower
concentrations could produce nasal and throat irritation. Lacrymation
may also result from inhalation exposure.
Although ingestion is not an expected route of human exposure,
swallowing of acrylic acid may cause severe irritation or burning of
the mouth, throat, oesophagus or stomach.
No serious health effects have been reported to result from
single exposure or repeated exposure at low concentrations of acrylic
acid.
4.1.1.2 Medical advice
In order to minimize possible consequences of accidents, all
personnel assigned to handle acrylic acid must be aware that prompt
and appropriate response (see following sections) is essential. First
aid must be rendered immediately. The installation of a sufficient
number of emergency showers and eye washes is a prerequisite for the
proper management of incidents.
If even minute quantities of acrylic acid enter the eyes, they
must be irrigated by means of an eye wash with copious amounts of
lukewarm water for at least 15 min. In order to secure the complete
removal of the acid, the assistance of a helper is strongly advised.
The eyelids should be held wide open and away from the eye balls.
Immediate help of an eye specialist should be sought. Oil or oily
ointments should not be applied unless recommended by a physician.
In the event that skin contact with acrylic acid has occurred,
all clothing should be completely removed under an emergency shower.
Washing with water should be continued until all odour has
disappeared, in any case not less than 15 min. The advice of a
physican should be sought. Contaminated clothing must never be reused
unless properly laundered. Leather wear must be disposed off.
A person who has suffered from inhalation of acid fumes should be
removed at once from the contaminated area and made to lie down in
fresh air without moving. If the patient is unconscious, he should be
placed on his side in a stable position. A physician and ambulance
should be summoned immediately. If available, pure oxygen should be
administered by means of a respirator, but only by a person who is
authorised for such duty by a physican.
If acrylic acid is ingested, the patient should be made to drink
large amounts of water. Vomiting should not be induced. A physican and
ambulance should be summoned immediately.
4.1.2 Health surveillance advice
Pre-employment and annual general medical examinations are
advised for regularly exposed workers.
4.2 Safety in Use
Acrylic acid should only be handled in well-aerated and well-
ventilated places. If exposure to concentrated vapour can not be
excluded (as in the case of an accident), self-contained breathing
apparatus or air supply masks must be worn. Care must be taken when
using filter-type masks to ensure that the filter capacity is not
exceeded for the intended time of use and expected concentration.
In areas where a release of acrylic acid is possible, eye
protection devices, face shields, neoprene gloves and rubber boots
should be worn. A chemical suit with a self-contained breathing
apparatus is strongly recommended if larger spills or emissions have
to be cleared. Appropriate protective clothing should be worn for work
involving breaking or entering into a closed acrylic acid system.
Owing to its vapour pressure, the concentration of acrylic acid in
closed rooms can reach high values.
If clothing or shoes have accidentally been contaminated with
acrylic acid, they must be removed immediately. Contaminated leather
shoes or other leather goods must be discarded.
For timely and appropriate emergency response, it is advisable to
provide complete sets of safety protection equipment near places where
accidents with acrylic acid are possible.
4.3 Explosion and Fire Hazards
Acrylic acid has a flash point of 54-68°C and does not form
explosible vapour mixtures at ordinary ambient temperatures. However,
ignition may occur if excessive amounts of mist or aerosols have
formed in air. Ignition sources can include spark discharges from
static electricity, and this can occur when acrylic acid is flowing
through or being discharged from a line. During transfer from one
container into another, the containers should be electrically
interconnected and properly grounded. Splashing into a tank should be
avoided by using a dip tube.
Since acrylic acid and water are miscible in any proportion,
water can be used to extingish fires. Small fires can be fought with
carbon dioxide or dry chemical extinguishers, whereas for larger fires
foam (alcohol or universal type) can be used.
If a fire occurs in or close to a tank farm containing acrylic
acid, tanks and pipes should be cooled by spraying with water in order
to prevent the acid from polymerizing.
4.4. Storage
Acrylic acid should be stored in a detached, cool, well-
ventilated, non-combustible place and its containers should be
protected against physical damage. Acrylic acid can be stored only in
vessels lined with glass, stainless steel, aluminum or polyethylene.
In order to inhibit polymerization during transport and storage,
200 ppm MeHQ (the monomethyl ether of hydroquinone) is commonly added
to acrylic acid by the manufacturer. The presence of oxygen is
required for the inhibitor to be effective. A major concern during the
storage of acrylic acid is the avoidance of elevated temperatures as
well as freezing, since both can lead to a failure of the inhibitor
system. Ideally acrylic acid should be stored within a temperature
range of 15 to 25°C.
Acrylic acid and its solutions should be kept out of reach of
children and unauthorized persons as well as away from food, drink and
animal feed. If any container in the store is leaking, appropriate
precautions should be taken (see section 6) and personal protective
equipment used.
4.5. Transport
Acrylic acid is shipped in containers in compliance with
regulations according to ADR/RID/GGVS/GGVE, Class 8 Packing Group B
specifications. Acrylic acid is commonly shipped in steel drums with
polyethylene inserts or in self-supporting high-density polyethylene
drums impermeable to ultraviolet light. White polyethylene container
are translucent to ultraviolet light and therefore may promote
polymerization. Stainless steel ISO containers are recommended for the
transport of quantities of acrylic acid up to 1 tonne.
4.6. Spillage and disposal
4.6.1. Spillage
Before dealing with any spillage, appropriate personal protective
equipment should be used (see section 6).
Small spills of up to 5 litres can be absorbed in commercially
available clean-up kits (using sand or clay). If a wastewater sewer is
close by, the spill can also be washed down with water provided that
it is not a storm-sewer or ditch that is routed to surface water.
Large spills should be contained, if possible, within a diked
area. A temporary dike can be arranged by stacking sand bags or
similar devices. Avoid run-off into storm sewers routed to public
surface water. If possible, the material should be recovered in
appropriate containers for reuse or disposal. If a wastewater sewer is
available, the acid or remainders can also be sparingly washed down
after dilution and neutralization prior to being discharged to a
water-treatment plant. During all handling operations of large spills
a chemical suit with a self-contained or air-supplied breathing device
must be worn.
In the event of accidental spillage of acrylic acid to surface
water or to a municipal sewer system, the pollution control agencies
must be notified promptly.
Spills of the monomer may be diluted and washed into a biological
treatment plant after notification of the person in charge. The
biodegradability of the material in diluted form is good (> 70% Zahn-
Wellens static test OECD 302 B). However, acrylic acid may be toxic to
the system if the bacteria have not been conditioned properly to this
material. Accordingly, the initial feed rate should be low with a
stepwise increase if a significant amount is to be fed into the
biological treatment plant. The maximum concentration should not
exceed 1000 mg per litre. It should be kept in mind, however, that
large quantities may affect the optimal acidity of the milieu and may
therefore need to be neutralized by the simultaneous addition of
sodium hydroxide.
4.6.2. Disposal
State laws and local regulations governing waste disposal make it
essential for producers, suppliers, hauliers and users of acrylic acid
to be fully aware of viable options for the ultimate disposal of
materials containing acrylic acid. Materials to be disposed of may be
residues from production or cleaning operations as well as waste
material from spills.
Acrylic acid is a highly corrosive material. Accordingly it
should always be handled with appropriate safety equipment.
Solid materials containing acrylic acid, such as absorbents or
polymeric material, can be disposed of by incineration. Disposal in
landfills must be thoroughly checked with the authorities and should
be practiced only as a last resort.
For the disposal of waste materials originating from laboratory
samples, great care must be taken to keep the monomer separated from
incompatible material, such as peroxides, which may initiate
polymerization.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
Most of the available data indicate that acrylic acid has low
toxicity for mammals and aquatic organisms. Algae are the most
sensitive group of aquatic organisms. Acrylic acid is miscible with
water and therefore would not be expected to adsorb significantly to
soil or sediment. If released on land or into water, acrylic acid
should readily biodegrade although no rate data are available. Acrylic
acid is unlikely to pose a problem in the general environment.
5.2. Prevention
Because of its action as a strong irritant to mucous membranes
and explosive properties of its mixtures with air, it is essential
that concentrations of acrylic acid in the ambient air be kept as low
as possible. Care should be taken during any manipulations with any
acrylic acid containers. Any effluent containing acrylic acid should
be properly treated, and any acrylic acid spillage should be protected
from all possible ignition sources.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, acrylic acid. It should be displayed
at, or near, entrances to areas where there is potential exposure
to acrylic acid, and on processing equipment and containers. The
summary should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Centre, and for local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
ACRYLIC ACID
CAS no.
Relative molecular mass: 72.06
Conversion factors: 1 ppm = 3.0 mg/m3; 1 mg/m3 = 0.33 ppm
1.2 Physical and Chemical Properties
Acrylic acid is a colourless liquid with an irritating acrid
odour at room temperature and pressure. Its odour threshold is low
(0.20-3.14 mg/m3). It is miscible in water and most organic solvents.
Acrylic acid is commercially available in two grades: technical
grade (94%) for esterification, and glacial grade (98-99.5% by weight
and a maximum of 0.3% water by weight) for production of water-soluble
resins. Acrylic acid polymerizes easily when exposed to heat, light or
metals, and so a polymerization inhibitor is added to commercial
acrylic acid to prevent the strong exothermic polymerization. The
inhibitors that are usually used in acrylic acid preparations are the
monomethyl ether of hydroquinone (methoxyphenol) at 200 ± 20 ppm,
phenothiazine at 0.1% and hydroquinone at 0.1%. Methylene blue at 0.5
to 1.0% and N,N'-diphenyl- p-phenylenediamine at 0.05% can also be
used.
Acrylic acid preparations containing polymerization inhibitors
are reasonably stable when stored at 15-25°C and handled according
to the supplier's recommendations. Heat can cause vigorous
polymerization in some circumstances. Acrylic acid reacts readily with
free radicals and electrophilic or nucleophilic agents. It may
polymerize in the presence of acids (sulfuric acid, chlorosulfonic
acid), alkalis (ammonium hydroxide), amines (ethylenediamine,
ethyleneimine, 2-aminoethanol), iron salts, elevated temperature,
light, peroxides, and other compounds that form peroxides or free
radicals. In the absence of inhibitor, peroxides are formed when
oxygen is sparged into acrylic acid. This mixture can undergo violent
polymerization if heated to 60°C.
The presence of oxygen is required for the stabilizer to function
effectively. A head space containing sufficient air should always be
maintained above the monomer to ensure inhibitor effectiveness.
Dissolved oxygen takes part in the inhibition reaction and therefore
is gradually consumed. The level of dissolved oxygen should
periodically be replenished. This can be accomplished by thoroughly
aerating the liquid phase, i.e. recirculation of the inventory in
tanks or agitating drums (rotating).
Acrylic acid must never be handled under an inert atmosphere.
Freezing of acrylic acid occurs at 13°C. Rethawing under
inappropriate temperature conditions is another frequent reason for
acrylic acid polymerization. During the crystallization process the
inhibitor and oxygen concentrate in the mother liquor. Therefore no
mother liquor should be withdrawn from a partially frozen container.
This may result in a severe deficiency of the inhibitor system in the
crystalline matrix. If direct heat is applied, polymerization will
start immediately, often with great violence. Under no circumstances
must steam be used to thaw frozen acrylic acid, nor must thawing be
carried out at temperatures above 35°C.
Acrylic acid is a strong corrosive agent to many metals, such as
unalloyed steel, copper and brass. Frequently the hydrolysis of such
metalic materials generates a deep discoloration in acrylic acid.
Polyvalent metal salts formed during hydrolytic reactions could also
induce polymerization. Therefore, under no circumstances should
acrylic acid be stored or transported with equipment which contains
the above-mentioned metals. Acrylic acid does not affect stainless
steel.
1.3 Analytical Methods
Acrylic acid residues in air and other media can be quantified by
means of gas chromatographic, high performance liquid chromatographic
and polarographic techniques. The detection limits of these methods
was found to be 14 mg/m3 (14 ppm) in air and down to 1 mg/kg or
1 mg/litre (1 ppm) in other media.
1.4 Production and Uses
The worldwide production of acrylic acid in 1994 was estimated
to be approximately 2 million tonnes. Acrylic acid is used primarily
as a starting material in the production of acrylic esters; as a
monomer for polyacrylic acid and salts, as a co-monomer with
acrylamide for polymers used as flocculants, with ethylene for ion
exchange resin polymers, with methyl ester for polymers, and with
itatonic acid for other copolymers.
2. SUMMARY AND EVALUATION
2.1 Human Exposure
No data on general population exposure are available. However,
consumers may be exposed to unreacted acrylic acid in household goods
such as polishes, paints and coatings, adhesives, rug backing,
plastics, textiles and paper finishes. A potential source of internal
exposure to acrylic acid may result from metabolism of absorbed
acrylic acid esters. Acrylic acid also occurs in wastewater effluent
from its production. It is estimated that thousands of workers could
be exposed to acrylic acid, but exact figures are not available.
2.2 Kinetics and Metabolism
Inhalation and contact with skin are important routes of
occupational exposure.
Regardless of the route of exposure, acrylic acid is rapidly
absorbed and metabolized. It is extensively metabolized, mainly to
3-hydroxypropionic acid, CO2 and mercapturic acid, which are
eliminated in the expired air and urine. Owing to its rapid metabolism
and elimination, the half-life of acrylic acid is short (minutes) and
therefore it has no potential for bioaccumulation.
2.3 Effects on Animals
Although a wide range of LD50 values has been reported, most
data indicate that acrylic acid is of low to moderate acute toxicity
by the oral route, and of moderate acute toxicity by the inhalation
and dermal routes.
Acrylic acid is corrosive or irritant to skin and eyes. It is
unclear what concentration is non-irritant. It is also a strong
irritant to the respiratory tract.
Positive and negative skin sensitization results have been
reported with acrylic acid, but it appears that the positive results
may be due to an impurity.
In drinking-water studies on rats the no-observed-adverse-effect
level (NOAEL) was 140 mg/kg body weight per day for decreased body
weight gain in a 12-month study and 240 mg/kg bw per day for
histopathological changes in the stomach. A chronic drinking-water
study on rats showed no effect at the highest dose tested (78 mg/kg
body weight per day). For inhalation studies a lowest-observed-
adverse-effect level (LOAEL) of 15 mg/m3 (5 ppm) was observed in mice
exposed to acrylic acid for 90 days, based on very mild nasal lesions
in females at this level. Nasal effects in rats were observed at
225 mg/m3 (75 ppm), but not at 15 or 75 mg/m3 (5 or 25 ppm).
Available reproduction studies indicate that acrylic acid is not
teratogenic and has no effect on reproduction.
Both positive and negative results have been obtained in in
vitro genotoxicity tests. An in vivo bone marrow chromosome
aberration assay was negative, and no firm conclusions can be drawn
from an in vivo DNA binding study or from a dominant lethal assay.
Available data do not provide evidence for an indication of
carcinogenicity of acrylic acid, but the data are inadequate to
conclude that no carcinogenic hazard exist.
2.4 Effects on Humans
There have been no reports of poisoning incidents in the general
population. No occupational epidemiological studies have been
reported.
Because acrylic acid toxicity occurs at the site of contact,
separate guidance values are recommended for oral and inhalation
exposure. Guidance values of 9.9 mg/litre for drinking-water and
54 µg/m3 for ambient air for the general population are proposed.
2.5 Effects of the Environment
No quantitative data on environmental levels of acrylic acid in
ambient air, drinking-water or soil have been reported.
Acrylic acid is miscible with water and, therefore, would not be
expected to adsorb significantly to soil or sediment. Under soil
conditions, chemicals with low Henry's Law constants are essentially
non-volatile. However, the vapour pressure of acrylic acid would
suggest that it may volatilize from surfaces and dry soil. Acrylic
acid may be formed by hydrolysis of acrylamide monomer from industrial
waste in soil, especially under aerobic conditions.
The toxicity of acrylic acid to bacteria and soil microorganisms
is low.
Acrylic acid emitted into the atmosphere will react with
photochemically produced hydroxyl radicals and ozone, resulting in
rapid degradation. There is no potential for long-range atmospheric
transport of acrylic acid because it has an atmospheric lifetime of
less than one month.
When released into water, acrylic acid readily biodegrades. The
fate of acrylic acid in water depends on chemical and microbial
degradation. When added to water acrylic acid is rapidly oxidized, and
so it can potentially deplete oxygen if discharged in large quantities
into a body of water. Acrylic acid has been shown to be degraded under
both aerobic and anaerobic conditions.
On the basis of the low octanol-water partition coefficient of
acrylic acid, bioconcentration in aquatic organisms is unlikely. There
have been no reports of biomagnification of acrylic acid in food
chains.
Algae are the most sensitive group of aquatic organisms studied,
with EC50 values, based on growth, ranging from 0.04 to 63 mg/litre,
and a no-observed-effect concentration (NOEC) for the most sensitive
species of 0.008 mg/litre. EC50 values (based on immobilization)
for Daphnia magna were 54 mg/litre (24 h) and 95 mg/litre (48 h).
Acute toxicity studies with fish have yielded results ranging from
27 mg/litre (96-h LC50) for the rainbow trout to 315 mg/litre
(72-h LC50) for the golden orfe. The 96-h NOEC for acrylic acid
toxicity to rainbow trout was found to be 6.3 mg/litre based on a lack
of sublethal/behavioural responses.
No data on the effects of acrylic acid on terrestrial organisms
have been reported.
3. CONCLUSIONS AND RECOMMENDATIONS
The risks associated with occupational exposure to acrylic acid
are low, as long as good industrial practice is followed. There is a
lack of quantitative data on the levels of exposure to acrylic acid.
However, no obvious adverse effects in the general population have
been identified.
Acrylic acid poses minimal risk for the general environment,
except in the case of uncontrolled discharge.
It is recommended that exposure of the general public to acrylic
acid in the ambient air and drinking-water does not exceed the
guidance values given in the Environmental Health Criteria No. 19
Acrylic Acid (IPCS, 1997). These are as follows:
* Inhalation exposure: 54 µg/m3
* Oral exposure via drinking-water: 9.9 mg/litre.
4. HUMAN HEALTH HAZARDS, PREVENTION AND PROTECTION, EMERGENCY ACTION
4.1. Main human health hazards, prevention and protection, first aid.
Additional details are given in the Summary on Chemical Safety
Information (section 6)
4.1.1 Advice to physicians
4.1.1.1 Symptoms of poisoning
The principal hazard of acrylic acid is its corrosive effect on
tissues. Both vapour and liquid can be irritating or corrosive to the
mucous membranes, skin and eyes. The severity of these effects is
dependent on the duration of contact, which, if prolonged, may result
in blisters and burns. Blister formation can appear as late as 24 h
after exposure. Severe corneal burns could occur to the eyes.
Permanent tissue damage may result if prompt and appropriate emergency
response is not provided. First aid recommendations are given in
section 6.
Inhalation of concentrated vapours and mist could produce
moderate to severe irritation of the respiratory tract. High
concentrations could result in pulmonary oedema while lower
concentrations could produce nasal and throat irritation. Lacrymation
may also result from inhalation exposure.
Although ingestion is not an expected route of human exposure,
swallowing of acrylic acid may cause severe irritation or burning of
the mouth, throat, oesophagus or stomach.
No serious health effects have been reported to result from
single exposure or repeated exposure at low concentrations of acrylic
acid.
4.1.1.2 Medical advice
In order to minimize possible consequences of accidents, all
personnel assigned to handle acrylic acid must be aware that prompt
and appropriate response (see following sections) is essential. First
aid must be rendered immediately. The installation of a sufficient
number of emergency showers and eye washes is a prerequisite for the
proper management of incidents.
If even minute quantities of acrylic acid enter the eyes, they
must be irrigated by means of an eye wash with copious amounts of
lukewarm water for at least 15 min. In order to secure the complete
removal of the acid, the assistance of a helper is strongly advised.
The eyelids should be held wide open and away from the eye balls.
Immediate help of an eye specialist should be sought. Oil or oily
ointments should not be applied unless recommended by a physician.
In the event that skin contact with acrylic acid has occurred,
all clothing should be completely removed under an emergency shower.
Washing with water should be continued until all odour has
disappeared, in any case not less than 15 min. The advice of a
physican should be sought. Contaminated clothing must never be reused
unless properly laundered. Leather wear must be disposed off.
A person who has suffered from inhalation of acid fumes should be
removed at once from the contaminated area and made to lie down in
fresh air without moving. If the patient is unconscious, he should be
placed on his side in a stable position. A physician and ambulance
should be summoned immediately. If available, pure oxygen should be
administered by means of a respirator, but only by a person who is
authorised for such duty by a physican.
If acrylic acid is ingested, the patient should be made to drink
large amounts of water. Vomiting should not be induced. A physican and
ambulance should be summoned immediately.
4.1.2 Health surveillance advice
Pre-employment and annual general medical examinations are
advised for regularly exposed workers.
4.2 Safety in Use
Acrylic acid should only be handled in well-aerated and well-
ventilated places. If exposure to concentrated vapour can not be
excluded (as in the case of an accident), self-contained breathing
apparatus or air supply masks must be worn. Care must be taken when
using filter-type masks to ensure that the filter capacity is not
exceeded for the intended time of use and expected concentration.
In areas where a release of acrylic acid is possible, eye
protection devices, face shields, neoprene gloves and rubber boots
should be worn. A chemical suit with a self-contained breathing
apparatus is strongly recommended if larger spills or emissions have
to be cleared. Appropriate protective clothing should be worn for work
involving breaking or entering into a closed acrylic acid system.
Owing to its vapour pressure, the concentration of acrylic acid in
closed rooms can reach high values.
If clothing or shoes have accidentally been contaminated with
acrylic acid, they must be removed immediately. Contaminated leather
shoes or other leather goods must be discarded.
For timely and appropriate emergency response, it is advisable to
provide complete sets of safety protection equipment near places where
accidents with acrylic acid are possible.
4.3 Explosion and Fire Hazards
Acrylic acid has a flash point of 54-68°C and does not form
explosible vapour mixtures at ordinary ambient temperatures. However,
ignition may occur if excessive amounts of mist or aerosols have
formed in air. Ignition sources can include spark discharges from
static electricity, and this can occur when acrylic acid is flowing
through or being discharged from a line. During transfer from one
container into another, the containers should be electrically
interconnected and properly grounded. Splashing into a tank should be
avoided by using a dip tube.
Since acrylic acid and water are miscible in any proportion,
water can be used to extingish fires. Small fires can be fought with
carbon dioxide or dry chemical extinguishers, whereas for larger fires
foam (alcohol or universal type) can be used.
If a fire occurs in or close to a tank farm containing acrylic
acid, tanks and pipes should be cooled by spraying with water in order
to prevent the acid from polymerizing.
4.4. Storage
Acrylic acid should be stored in a detached, cool, well-
ventilated, non-combustible place and its containers should be
protected against physical damage. Acrylic acid can be stored only in
vessels lined with glass, stainless steel, aluminum or polyethylene.
In order to inhibit polymerization during transport and storage,
200 ppm MeHQ (the monomethyl ether of hydroquinone) is commonly added
to acrylic acid by the manufacturer. The presence of oxygen is
required for the inhibitor to be effective. A major concern during the
storage of acrylic acid is the avoidance of elevated temperatures as
well as freezing, since both can lead to a failure of the inhibitor
system. Ideally acrylic acid should be stored within a temperature
range of 15 to 25°C.
Acrylic acid and its solutions should be kept out of reach of
children and unauthorized persons as well as away from food, drink and
animal feed. If any container in the store is leaking, appropriate
precautions should be taken (see section 6) and personal protective
equipment used.
4.5. Transport
Acrylic acid is shipped in containers in compliance with
regulations according to ADR/RID/GGVS/GGVE, Class 8 Packing Group B
specifications. Acrylic acid is commonly shipped in steel drums with
polyethylene inserts or in self-supporting high-density polyethylene
drums impermeable to ultraviolet light. White polyethylene container
are translucent to ultraviolet light and therefore may promote
polymerization. Stainless steel ISO containers are recommended for the
transport of quantities of acrylic acid up to 1 tonne.
4.6. Spillage and disposal
4.6.1. Spillage
Before dealing with any spillage, appropriate personal protective
equipment should be used (see section 6).
Small spills of up to 5 litres can be absorbed in commercially
available clean-up kits (using sand or clay). If a wastewater sewer is
close by, the spill can also be washed down with water provided that
it is not a storm-sewer or ditch that is routed to surface water.
Large spills should be contained, if possible, within a diked
area. A temporary dike can be arranged by stacking sand bags or
similar devices. Avoid run-off into storm sewers routed to public
surface water. If possible, the material should be recovered in
appropriate containers for reuse or disposal. If a wastewater sewer is
available, the acid or remainders can also be sparingly washed down
after dilution and neutralization prior to being discharged to a
water-treatment plant. During all handling operations of large spills
a chemical suit with a self-contained or air-supplied breathing device
must be worn.
In the event of accidental spillage of acrylic acid to surface
water or to a municipal sewer system, the pollution control agencies
must be notified promptly.
Spills of the monomer may be diluted and washed into a biological
treatment plant after notification of the person in charge. The
biodegradability of the material in diluted form is good (> 70% Zahn-
Wellens static test OECD 302 B). However, acrylic acid may be toxic to
the system if the bacteria have not been conditioned properly to this
material. Accordingly, the initial feed rate should be low with a
stepwise increase if a significant amount is to be fed into the
biological treatment plant. The maximum concentration should not
exceed 1000 mg per litre. It should be kept in mind, however, that
large quantities may affect the optimal acidity of the milieu and may
therefore need to be neutralized by the simultaneous addition of
sodium hydroxide.
4.6.2. Disposal
State laws and local regulations governing waste disposal make it
essential for producers, suppliers, hauliers and users of acrylic acid
to be fully aware of viable options for the ultimate disposal of
materials containing acrylic acid. Materials to be disposed of may be
residues from production or cleaning operations as well as waste
material from spills.
Acrylic acid is a highly corrosive material. Accordingly it
should always be handled with appropriate safety equipment.
Solid materials containing acrylic acid, such as absorbents or
polymeric material, can be disposed of by incineration. Disposal in
landfills must be thoroughly checked with the authorities and should
be practiced only as a last resort.
For the disposal of waste materials originating from laboratory
samples, great care must be taken to keep the monomer separated from
incompatible material, such as peroxides, which may initiate
polymerization.
5. HAZARDS FOR THE ENVIRONMENT AND THEIR PREVENTION
5.1. Hazards
Most of the available data indicate that acrylic acid has low
toxicity for mammals and aquatic organisms. Algae are the most
sensitive group of aquatic organisms. Acrylic acid is miscible with
water and therefore would not be expected to adsorb significantly to
soil or sediment. If released on land or into water, acrylic acid
should readily biodegrade although no rate data are available. Acrylic
acid is unlikely to pose a problem in the general environment.
5.2. Prevention
Because of its action as a strong irritant to mucous membranes
and explosive properties of its mixtures with air, it is essential
that concentrations of acrylic acid in the ambient air be kept as low
as possible. Care should be taken during any manipulations with any
acrylic acid containers. Any effluent containing acrylic acid should
be properly treated, and any acrylic acid spillage should be protected
from all possible ignition sources.
6. SUMMARY OF CHEMICAL SAFETY INFORMATION
This summary should be easily available to all health workers
concerned with, and users of, acrylic acid. It should be displayed
at, or near, entrances to areas where there is potential exposure
to acrylic acid, and on processing equipment and containers. The
summary should be translated into the appropriate language(s). All
persons potentially exposed to the chemical should also have the
instructions in the summary clearly explained.
Space is available for insertion of the National Occupational
Exposure Limit, the address and telephone number of the National
Poison Centre, and for local trade names.
SUMMARY OF CHEMICAL SAFETY INFORMATION
ACRYLIC ACID
CAS no. 