
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES 40
Prepared by:
The forty-ninth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
SATURATED ALIPHATIC ACYCLIC BRANCHED-CHAIN PRIMARY ALCOHOLS,
ALDEHYDES, AND ACIDS
First draft prepared by
Dr G. Semino,
Institute of Pharmacological Sciences
University of Milan
Milan, Italy
1. Evaluation
1.1 Introduction
1.2 Estimated daily per capita intake
1.3 Absorption, metabolism and elimination
1.4 Application of the procedure for the safety evaluation of
flavouring agents
1.5 Consideration of combined intake
1.6 Conclusions
2. Relevant background information
2.1 Biological data
2.1.1 Absorption, distribution and excretion
2.1.1.1 Methyl-substituted aliphatic alcohols,
aldehydes and carboxylic acids
2.1.12 alpha-Ethyl-substituted aliphatic alcohols,
aldehydes and carboxylic acids
2.1.2 Biotransformation
2.1.2.1 Methyl-substituted aliphatic alcohols,
aldehydes and carboxylic acids
2.1.2.2 alpha-Ethyl-substituted aliphatic alcohols,
aldehydes and carboxylic acids
2.1.3 Toxicological studies
2.1.3.1 Acute toxicity
2.1.3.2 Short-term toxicity
2.1.3.3 Long-term toxicity/carcinogenicity
2.1.3.4 Genotoxicity
2.1.3.5 Reproductive toxicity
2.1.3.6 Developmental toxicity
2.1.3.7 Special studies on peroxisome proliferation
2.1.3.8 Special study on immunotoxicity
3. References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 25 flavouring agents that
includes selected saturated aliphatic acyclic branched-chain primary
alcohols, aldehydes and acids using the Procedure for the Safety
Evaluation of Flavouring Agents (the "Procedure") (see Figure 1 and
Table 1).
Twenty-two substances contain one or more methyl substituents and
the three remaining have ethyl substituents in the alpha position.
The Committee has evaluated two members of the group previously.
Isobutyl alcohol was evaluated at the twenty-third meeting, when an
ADI was not allocated because of a lack of information (Annex 1,
reference 50). An ADI of 0-0.5 mg/kg bw was allocated to
2-ethyl-1-hexanol at the forty-first meeting (Annex 1, reference 107).
1.2 Estimated daily per capita intake
The total annual production volume of the 22 methyl-substituted
saturated aliphatic branched-chain primary alcohols, aldehydes and
acids from their use as flavouring substances in the USA is
approximately 9.8 and 29 tonnes in Europe. In the USA seven substances
(isobutyl alcohol, isobutyraldehyde, isobutyric acid,
2-methylbutyraldehyde, 2-methylbutyric acid, 3-methylbutyraldehyde and
isovaleric acid) constitute greater than 80% of the total volume of
production. In Europe, greater than 85% of the total annual volume is
accounted for by five substances, four of which are the same as in the
USA (isobutyl alcohol, isobutyric acid, 2-methylbutyric acid,
isovaleric acid and 2-methylvaleric acid). In the unlikely event that
all 22 methyl-substituted alcohols, aldehydes and acids would be
consumed simultaneously on a daily basis, the estimated total daily
per capita intake of the 22 methyl-substituted substances from their
use as flavouring agents would be 1900 µg/person per day in the USA
and 410 µg/person per day in Europe.
The total reported annual production volume of use of the three 2-
ethyl substituted substances is 370 kg in the USA (NAS, 1987) and 1000
kg in Europe (IOFI, 1995). In the unlikely event that all three ethyl
substituted alcohols, aldehydes and acids would be consumed
simultaneously on a daily basis, the estimated total daily per
capita intake of the three ethyl-substituted substances would be
< 71 µg/person in the USA or < 145 µg/person in Europe.
Saturated aliphatic acyclic branched-chain primary alcohols, aldehydes
and acids have been detected in a wide variety of foods such as
cheese, fruits, vinegar and alcoholic beverages (CIVO-TNO, 1994).
Quantitative data on the natural occurrence of these flavouring agents
has been reported for 11 of the 25 substances in the group and
corresponds to a total of 1500 tonnes/year consumed in food (Stofberg
& Grundschober, 1987).
Table 1. Summary of results of the safety evaluations on saturated aliphatic acyclic branched-chain primary alcohols, aldehydes, and
acids
Step 1: All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person per day
Step 2: All of the substances in this group are metabolized to innocuous products
No. Substance Step A3 Comments Conclusion based on
(CAS No.) Does intake exceed the current levels of
human intake threshold?1 intake
Intake Estimates
(µg/person per day)
Structural class I: methyl-substituted saturated aliphatic branched-chain primary alcohols, aldehydes, and acids
0251 Isobutyl alcohol No
USA: 290 Europe: 530 No safety concern
0252 Isobutyraldehyde No
USA: 100 Europe: 130 No safety concern
0253 Isobutyric acid No
USA: 140 Europe: 820 No safety concern
0254 2-Methylbutyraldehyde No
USA: 370 Europe: 4.9 No safety concern
0255 2-Methylbutyric acid No
USA: 480 Europe: 1200 No safety concern
0258 3-Methylbutyraldehyde No
USA: 140 Europe: 110 No safety concern
0259 Isovaleric acid No
USA: 96 Europe: 480 No safety concern
0260 2-Methylpentanal No
USA: 8.5 Europe: 12 No safety concern
0261 2-Methylvaleric acid No
USA: 2.3 Europe: 680 No safety concern
0262 3-Methylpentanoic acid No
USA: 8.8 Europe: 2.9 No safety concern
0263 3-Methyl-1-pentanol No
USA: 4.2 Europe: 5.9 No safety concern
0264 4-Methylpentanoic acid No
USA: 55 Europe: 1.6 No safety concern
Table 1. Continued...
No. Substance Step A3 Comments Conclusion based on
(CAS No.) Does intake exceed the current levels of
human intake threshold?1 intake
Intake Estimates
(µg/person per day)
0265 2-Methylhexanoic acid No
USA: 2.3 Europe: 15 No safety concern
0266 5-Methylhexanoic acid No
USA: 8.6 Europe: 0.0 No safety concern
0268 3,5,5-Trimethyl-1-hexanol No
USA: 0.76 Europe: 13 No safety concern
0269 3,5,5-Trimethylhexanal No
USA: 150 Europe: 0.29 No safety concern
0270 2-Methyloctanal No
USA: 0.95 Europe: 0.14 No safety concern
0271 4-Methyloctanoic acid No
USA: 0.10 Europe: 11 No safety concern
0272 3,7-Dimethyl-1-octanol No
USA: 2.9 Europe: 94 No safety concern
0273 2,6-Dimethyloctanal No
USA: 6.7 Europe: 0.01 No safety concern
0274 4-Methylnonanoic acid No
USA: 1.5 Europe: 1.00 No safety concern
0275 2-Methylundecanal No
USA: 0.10 Europe: 0.61 No safety concern
Structural class II: ethyl-substituted saturated aliphatic acyclic branched-chain primary alcohols, aldehydes, and acids
0256 2-Ethylbutyraldehyde No 2-ethyl substituent inhibits the No safety concern
USA: 0.17 Europe: 0.57 œ-oxidation of aliphatic alcohols,
aldehydes and carboxylic acids. These
compounds undergo omega and omega-1
oxidation to yield polar metabolite
primarily excreted in the urine.
0257 2-Ethylbutyric acid No
USA: 31 Europe: 60 No safety concern
Table 1. Continued...
No. Substance Step A3 Comments Conclusion based on
(CAS No.) Does intake exceed the current levels of
human intake threshold?1 intake
Intake Estimates
(µg/person per day)
0267 2-Ethyl-1-hexanol No
USA: 40 Europe: 86 No safety concern
1 The human intake threshold for class I is 1800 µg per day; 540 µg per day for class II; and 90 µg per day for class III.
1.3 Absorption, metabolism and elimination
The metabolism of methyl and ethyl substituted saturated aliphatic
acyclic branched-chain alcohols, aldehydes and carboxylic acids is
discussed in the introduction to this chapter on flavouring agents.
1.4 Application of the procedure for the safety
evaluation of flavouring agents
Step 1. The 22 methyl-substituted saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes and acids were classified
in structural class I (Cramer et al., 1978). The three
ethyl-substituted saturated aliphatic acyclic branched-chain primary
alcohols, aldehydes and acids (2-ethylbutyraldehyde, 2-ethylbutyric
acid and 2-ethyl-1-hexanol) contain sterically hindered functional
groups and therefore were classified in structural class II.
Step 2. At current levels of intake from use as flavouring agents
(see Table 1) the 22 methyl-substituted alcohols, aldehydes and
carboxylic acids and the three 2-ethyl-substituted alcohols, aldehydes
and carboxylic acids would not be expected to saturate the metabolic
pathways and all the compounds were predicted to be metabolized to
innocuous products.
Step A3. All 22 class I substances in this group have estimated
USA and European daily per capita intake levels less than the human
intake threshold for this class (i.e. 1800 µg/person per day).
Therefore, the 22 methyl-substituted saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes and acids evaluated do not
pose a safety concern when used at current levels of estimated intake
as flavouring agents.
The three ethyl-substituted substances in class II
(2-ethylbutyraldehyde, 2-ethylbutyric acid and 2-ethyl-1-hexanol) have
estimated intake levels (0.6, 58 and 86 µg/person per day,
respectively) in the USA and Europe that are below the human intake
threshold for class II (i.e. 540 µg/person per day). Therefore, the
three ethyl-substituted saturated aliphatic acyclic branched-chain
primary alcohols, aldehydes and acids evaluated do not pose a safety
concern when used at current levels of estimated intake as flavouring
agents.
1.5 Consideration of combined intake
The stepwise evaluations of the 25 saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes and carboxylic acids used
as flavouring agents are summarized in Table 1.
In the unlikely event that all 25 saturated aliphatic acyclic
branched chain alcohols, aldehydes and carboxylic acids would be
consumed simultaneously on a daily basis, the Committee judged that
combined intake is of no safety concern, since all the substances in
this group are expected to be efficiently metabolized and the combined
intake level is not expected to saturate pathways.
1.6 Conclusions
The Committee concluded that the use of these substances as
flavouring agents would not present a safety concern at current levels
of intake.
No toxicity data were required for the application of the
Procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
Procedure. In cases where ADIs were previously established, these
ADIs were maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Biological data
2.1.1 Absorption, distribution and excretion
2.1.1.1 Methyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids (22)
In general, branched-chain aliphatic acyclic alcohols, aldehydes
and acids are rapidly absorbed from the gastrointestinal tract
(Gaillard & Derache, 1965; Dawson et al., 1964).
a) Branched-chain alcohols
In a study designed to measure the absorption, distribution and
excretion of alcohols and ketones, a mixture of isomers of amyl
alcohol, including the structurally related branched-chain
3-methylbutyl alcohol (isoamyl alcohol) and 2-methylbutyl alcohol, was
administered to rats by intraperitoneal (i.p.) injection. Complete
absorption of the primary alcohols from the peritoneum occurred within
1 hour, and disappearance from the blood occurred within 3´ to 9
hours. 3-Methylbutyraldehyde was detected as an intermediate in the
blood (Haggard et al., 1945).
b) Branched-chain carboxylic acids
1-14C-Isobutyric acid was administered by gavage to male Charles
River CD rats at doses of 4, 40 and 400 mg/kg bw and to female rats at
a single dose of 400 mg/kg bw. Rapid elimination in the breath as
expired 14CO2 was observed at all dose levels. Less than 5% of the
radioactive dose was detected in the urine and faeces. Similar
patterns of excretion were reported in all animals (DiVincenzo &
Hamilton, 1979). 1-14C-Isovaleric acid fed to rats was absorbed and
utilized in the formation of acetyl groups, fatty acids and
cholesterol (Zabin & Bloch, 1951).
2.1.1.2 alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids (3)
2-Ethyl[1-14C]hexanol orally administered to male rats was
readily absorbed. Within 28 hours, elimination of the radioactivity
occurred in the urine (80-82%), faeces (8-9%) and expired CO2 (6-7%)
(Albro, 1975).
2.1.2 Biotransformation
2.1.2.1 Methyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids (22)
Following absorption, primary alcohols are successively oxidized
to their corresponding aldehyde and carboxylic acid (Bosron &
Ting-Kai, 1980; Levi & Hodgson, 1989). The resulting
methyl-substituted carboxylic acids may undergo ß-oxidation
predominantly in the longer branched chain to yield ß-hydroxyacids
which may be further oxidized (ß-oxidation) and cleaved to yield
short-chain acids that are completely metabolized via the fatty acid
pathway or tricarboxylic acid cycles (Voet & Voet, 1990).
The position of the methyl substituent plays a role in metabolism.
Acids with a methyl substituent located at an even-numbered carbon
(e.g., 2-methyl-pentanoic acid or 4-methyldecanoic acid) are
extensively metabolized to CO2 via
ß-oxidative cleavage in the fatty acid pathway. If the methyl group is
located at the 3-position, ß-oxidation is inhibited and
omega-oxidation predominates, primarily leading to polar, acidic
metabolites capable of being further oxidized or conjugated and
excreted in the urine (Williams, 1959). As chain length and
lipophilicity increase, omega-oxidation competes with ß-oxidative
cleavage. Methyl substituted acids (e.g., 3-methylnonanoic,
2-methyldodecanoic, 4-methyldo-decanoic acids) are, to some extent,
omega-oxidized in animals to form diacids, which can be detected in
the urine (Williams, 1959).
a) Branched-chain alcohols and aldehydes
In human isoenzyme mixtures, oxidation of primary aliphatic
alcohols to aldehydes is catalysed by NAD+-dependent alcohol
dehydrogenase (ADH) (Pietruszko et al., 1973), and oxidation of
aldehydes to carboxylic acids is catalysed by NAD+-dependent
aldehyde dehydrogenase (ALD) (Weiner, 1980; Blair & Bodley, 1969).
Isobutyl alcohol and isobutyraldehyde have been reported to be
excellent substrates for ADH (Hedlund & Kiessling, 1969; Saito, 1975)
and ALD, respectively (Prunonosa et al., 1991). The rate of
oxidation by ADH is greater for isobutyl alcohol than for ethanol
(Lester & Benson, 1970), while the rate of oxidation by ALD is
approximately the same for isobutyraldehyde and acetaldehyde
(Walkenstein & Weinhouse, 1953). ALD-catalysed oxidation of low
molecular weight aldehydes such as isobutyraldehyde requires
glutathione (Eckfeldt & Yonetani, 1982), which implies that the
substrate for oxidation may be the thiohemiacetal formed by rapid
in vivo conjugation of aldehydes with glutathione (Brabec, 1993).
Isobutyraldehyde was oxidized by rat liver mitochondria in
vitro and was found to compete with acetaldehyde for ALD (Hedlund &
Kiessling, 1969). The metabolism of 3-methylbutyraldehyde and related
aldehydes was studied in male and female rat liver homogenate and in
rat liver in situ. 3-Methylbutyraldehyde was shown to be oxidized to
3-methylbutyric acid in an ADH-catalysed reaction at one half the rate
of acetaldehyde (Hedlund & Kiessling, 1969). 3-Methylbutyraldehyde was
reported to be oxidized to the corresponding acid in the mitochondria
of rat liver and kidney (Walkenstein, 1953). 3-Methylbutyraldehyde was
detected in the plasma of approximately 38 human subjects and was
shown to be a metabolite of ingested protein (Goldberg et al.,
1979).
b) Branched-chain carboxylic acids
The resulting methyl-substituted carboxylic acids are substrates
for ß-oxidation and cleavage in the amino acid and fatty acid pathways
(Voet & Voet, 1990). Isobutyric acid, isovaleric acid and
2-methylbutyric acid occur endogenously as intermediates in the human
metabolism of the amino acids valine (Kinnory et al., 1955), leucine
(Henning & Hird, 1970) and isoleucine (Voet & Voet, 1990),
respectively. For instance, in the leucine pathway isovaleryl CoA
undergoes successive dehydrogenation and carboxylation to yield
ß-methylglutaconyl CoA. ß-Methylglutaconyl CoA is hydrated to yield
ß-hydroxy ß-methylglutaryl CoA, which is finally cleaved to
acetoacetate and acetyl CoA (Voet & Voet, 1990).
The metabolism of isobutyric acid has been established in rodents.
Rats fed isobutyric acid excrete elevated levels of 2-methylmalonic
acid, which is an intermediate in the conversion of propionyl CoA to
succinyl CoA (Butenandt & Thomas, 1958). ß-Oxidation of 2-
methylbutyric acid has been observed in guinea-pigs in vivo
(Stokke et al., 1969). Isovaleric acid was identified as a urinary
metabolite in rabbits following oral administration of isobutyl
alcohol (Saito, 1975).
2-Methylvaleric acid is an alpha-substituted acid and, therefore,
undergoes ß-oxidation in the longer branched-chain followed by
cleavage to yield two propionyl CoA fragments. In rabbits,
2-methylvaleric acid is converted to propionyl CoA which is completely
metabolized (Deuel, 1957). 4-Methylpentanoic acid was identified in
the faeces of pigs fed a normal diet (Yasuhara et al., 1982).
5-Methylhexanoic acid was shown to conjugate with glucuronic acid
in phenobarbital-induced rat liver microsomes (Hamdoune et al.,
1995). Rabbits have been shown to excrete methyl-substituted
long-chain acids such as 2-methylnonanoic acid in the urine unchanged
(Deuel, 1957).
In conclusion, the metabolism of branched-chain alcohols,
aldehydes and carboxylic acids containing one or more methyl
substituents is determined primarily by the position of the methyl
group on the branched-chain. Alcohols are successively oxidized to the
corresponding aldehydes and carboxylic acids. The branched-chain acids
are metabolized via ß-oxidation in the longer branched-chain followed
by cleavage to yield linear acid fragments, which are completely
metabolized in the fatty acid pathway or the tricarboxylic acid cycle.
At high-dose levels, longer branched-chain acids may undergo
omega-oxidation to yield diacids, which may undergo further oxidation
and cleavage.
2.1.2.2 alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids (3)
The presence of an ethyl or propyl substituent at the position,
such as in 2-ethyl-1-hexanol, inhibits ß-oxidation (Deuel, 1957).
Detoxication pathways of omega- and omega-1 oxidation compete with
ß-oxidation of these sterically-hindered substances. In the principal
detoxication pathway, the parent alcohol or corresponding carboxylic
acid undergoes a combination of reactions including omega- or omega-1
oxidation and functional group oxidation leading to polar, acidic
metabolites capable of being excreted in the urine (Williams, 1959;
Deisinger et al., 1994). When the principal pathway is saturated,
the corresponding carboxylic acid conjugates with glucuronic acid and
is excreted primarily in the urine (Williams, 1959; Albro, 1975;
Deisinger et al., 1994).
The metabolism of 2-ethyl-1-hexanol has been studied in rats and
rabbits and it was shown that both species follow similar pathways.
Rats and mice were administered 0, 140, 350, 700, 1050 or 1750 mg/kg
bw/day 2-ethyl-1-hexanol by gavage for 14 days. As measured by the
rate of palmitoyl CoA oxidation, 2-ethyl-1-hexanol administration
resulted in a linear dose-related induction of peroxisomal ß-oxidation
(Keith et al., 1992).
The major urinary metabolites of 2-ethyl-1-hexanol in rats
include: the glucuronic acid conjugate of 2-ethylhexanoic acid; omega-
and omega-1 oxidation metabolites, 2-ethyl-1,6-dihexanoic acid,
5-hydroxy-2-ethylhexanoic acid and its corresponding delta-lactone;
and 6-hydroxy-2-ethylhexanoic acid. Minute quantities of the
omega-desaturation metabolite, 2-ethyl-5-hexenoic acid and metabolites
formed from ß-oxidation and oxidative decarboxylation, 2- and
4-heptanone, were also detected (Albro, 1975; Deisinger et al.,
1994).
2-Ethyl[1-14C]hexanol was administered orally to male rats. Using
acid extraction of the urine, the major urinary metabolite was
determined to be 2-ethylhexanoic acid, which most likely was excreted
as the glucuronic acid conjugate. According to the author,
2-ethylhexanoic acid can undergo partial ß-oxidation and
decarboxylation to produce CO2, and may also undergo omega- or
omega-1-oxidation. 2-Ethyl-5-hydroxyhexanoic acid,
2-ethyl-5-ketohexanoic acid, 2-ethyl-1,6-hexanedioic acid and 2- and
4-heptanone were also identified as urinary metabolites of
2-ethylhexanol. A small amount (3%) of the parent alcohol was excreted
unchanged in the urine (Albro, 1975).
Female Fischer rats were given doses of 50 or 500 mg/kg bw of
[14C]-2-ethyl-1-hexanol. At single or repeated low dose levels (i.e.
50 mg/kg bw) the omega-oxidation pathway predominated as observed in
the excretion of the principal urinary metabolites, 2-ethyl-1,6-acid
and its precursors. At the high-dose level of 2-ethyl-1-hexanol, the
principal metabolite observed was the glucuronide of the corresponding
acid, 2-ethylhexanoic acid (see Figure 1). The author indicated that
the data suggest the occurrence of metabolic saturation of the
omega-oxidation pathway at the high-dose level (Deisinger et al.,
1994).
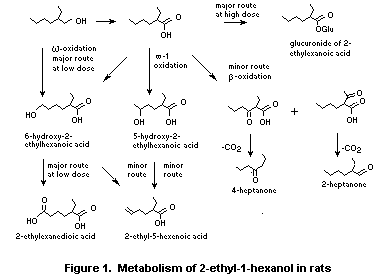 When rats and rabbits were administered 2-ethylhexanol-C14 or
2-ethylhexanol-C14 sulfate either orally or by intraperitoneal (i.p.)
injection, the principal metabolites included unchanged alcohol and
the sulfate ester, as well as hydroxylated 2-ethylhexanol and the
glucuronide conjugate of 2-ethylhexanoic acid (Knaak et al., 1966).
2-Ethylbutyric acid was administered orally and by subcutaneous
injection at a dose of approximately 1 gram to rabbits and 100 mg (as
the sodium salt) to rats. The acid was primarily excreted unchanged in
the urine as the glucuronic acid conjugate (Dziewiatowski et al.,
1949). In dogs, 2-ethylbutyric acid undergoes ß-oxidation and
decarboxylation to yield 2-pentanone (Deuel, 1957).
In conclusion, branched-chain alcohols, aldehydes and carboxylic
acids with bulky (ethyl, propyl, etc.) alpha-alkyl substituents are
metabolized by omega- and omega-1-oxidation to yield polar metabolites
capable of excretion in the urine. At high-dose levels these oxidation
pathways may be saturated, in which case, the corresponding acid may
undergo conjugation with glucuronic acid.
2.1.3 Toxicological studies
2.1.3.1 Acute toxicity
Acute toxicity studies have been reported for 20 of the 25
saturated aliphatic branched-chain primary alcohols, aldehydes and
acids in this group and are summarized in Table 2. The acute oral
toxicity of the group is demonstrated by oral LD50 values of >2000
mg/kg bw for the alcohols; >3200 mg/kg bw for the aldehydes; and
>1000 mg/kg bw for the carboxylic acids, except for isobutyric and
2-methylbutyric acid, which are 280 mg/kg bw, and 3-methylpentanoic
acid which is >700 mg/kg bw. The rat oral LD50 values for the three
alpha-ethyl-substituted substances 2-ethylbutyraldehdye,
2-ethylbutyric acid and 2-ethyl-1-hexanol are 3980 mg/kg bw, 2200
mg/kg bw and 2050-7100 mg/kg bw, respectively.
2.1.3.2 Short-term toxicity
The results of short-term toxicity studies with a
methyl-substituted alcohol (isobutyl alcohol), a structurally related
alcohol (isoamyl alcohol), and two methyl-substituted carboxylic acids
(isovaleric acid and 2-methylhexanoic acid) are summarized in Table 3
and described below. Short-term oral studies for 2-ethylbutyric acid
and 2-ethyl-1-hexanol are summarized in the section on
alpha-ethyl-substituted substances.
Table 2. Acute toxicity studies for saturated aliphatic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Isobutyl alcohol rat M/F oral 2640-3100 Smyth et al., 1954; WHO, 1987
M/F gavage 2650-3100 Purchase, 1969
mouse oral 3500 WHO, 1987
Isobutyraldehyde rat M/F oral 3730 Smyth et al., 1954
Isobutyric acid rat NR oral 280 Lewis, 1989
2-Methylbutyraldehyde rat NR oral 8570 Opdyke & Leitizia, 1982
2-Methylbutyric acid rat M/F oral 280-2200 Moreno et al., 1982; Lewis, 1989;
mouse M/F oral 1238 Schafer & Bowles, 1985
2-Ethylbutyraldehyde rat M/F oral 3980 Smyth et al., 1951
2-Ethylbutyric acid rat M/F oral 2200 Smyth et al., 1954
Isoamyl alcohol2 rat M/F oral 5700 Smyth et al., 1954
gavage 1300-4000 Purchase, 1969
3-Methylbutyraldehyde rat M/F oral 7166 Moreno, 1988
Isovaleric acid rat NR oral 2000; >3200 Fassett, 1963; NIOSH, 1976
2-Methylvaleric acid rat M/F oral 1600-3200 Smyth et al., 1954; ACGIH, 1989
3-Methylpentanoic acid rat NR oral >700 Vollmuth et al., 1989
3-Methyl-1-pentanol rat M/F oral >2000 Engler & Bahler, 1982
2-Ethyl-1-hexanol rat M/F oral 2050-7100 Hodge, 1943; Shaffer et al., 1945;
Smyth et al., 1969; Scala & Burtis, 1973;
Schmidt et al., 1973; Albro, 1975; Dave & Lidman, 1978
Table 2. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3,5,5-Trimethyl-1-hexanol rat M/F oral 2300 Moreno, 1977
3,5,5-Trimethylhexanal rat M/F oral 3240 Moreno, 1975; Opdyke & Leitizia, 1982
2-Methyloctanal rat M/F oral >5000 Moreno, 1977
3,7-Dimethyl-1-octanol rat M/F oral 5000 Shelanski & Moldovan, 1973; Moreno, 1977
4-Methylnonanoic acid rat NR oral 3700 IFREB, 1975
2-Methylundecanal rat M/F oral >5000 Owen & Meyer, 1971
4-Methylpentanoic acid rat NR oral 2050->3200 Smyth et al., 1954; Fassett, 1963; NIOSH, 1976;
Union Carbide, 1991;
mouse NR oral 5000 NIOSH, 1991
1 M = male; F = female; NR = Not reported.
2 Structurally related substance.
Table 3. Short-term and long-term toxicity studies for saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids
Substance Species (Sex) Route Duration NOEL Reference
(mg/kg bw
per day)
Short-term studies
Alcohols
Isobutyl alcohol rat (M/F) oral 90 days 1450 BASF, 1992
rat oral 4 months >0.126 nmol/g Hillbom et al., 1974
bw/day3 Johannsen &
rat (M/F) oral 53-56 weeks 2003 Purchase, 1969
Isoamyl alcohol2 rat (M/F) gavage 17 weeks 10003 Carpanini et al., 1973
rat (M/F) oral 56 weeks 20003 Johannsen & Purchase, 1969
2-Ethyl-1-hexanol mouse gavage 11 days 100 Astill et al., 1996a
mouse gavage 90 days 125 Astill et al., 1996a
mouse food 11 days 1150-4450 Astill et al., 1993
rat gavage 14 days 1303 Rhodes et al., 1984
NR Lake et al., 1975
rat oral 7 days 100 Astill et al., 1996a
rat gavage 11 days 125 Astill et al., 1996a
rat gavage 90 days <500-540 Astill et al., 1993
rat oral 11 days
Carboxylic Acid
2-Ethylbutyric acid rat oral 90 days 3003 Amoore et al., 1978
Isovaleric acid rat oral 90 days 25003 Amoore et al., 1978
2-Methylhexanoic acid rat (M/F) oral 90 days 33 Posternak et al., 1969
Long-term/ Carcinogenicity studies
2-Ethyl-1-hexanol mouse (M/F) gavage 540 days 200 Astill et al., 1996b
rat (M/F) gavage 730 days 50 Astill et al., 1996b
Table 3 (continued)
1 M = Male; F = Female; NR = not reported.
2 A structurally related branched-chain alcohol.
3 The study was performed at a single dose level or multiple dose levels that produced no adverse effects and, therefore, a NOEL was not
determined. The NOEL is probably higher than the reported dose level that produced no adverse effects.
a) Methyl substituted aliphatic alcohols, aldehydes and carboxylic
acids
i) 2-Methyl-1-propanol (isobutyl alcohol)
Groups of eleven 4-month-old male Wistar rats were given 1 M
solutions of isobutyl alcohol as their only drinking fluid for 4
months. Control animals received tap water. An ordinary diet was
provided for all animals. The level of isobutyl alcohol consumption
was reported to be 0.126 nmole/g bw/day at 120 days. At necropsy,
there was no evidence of hepatic steatosis, fibrosis or inflammation
(Hillbom et al., 1974).
Groups of 20 male and 20 female Wistar albino rats were given a
mixture of fusel oils including 0.2% isobutyl alcohol, which was
calculated (FDA, 1993) to provide an approximate daily intake of 200
mg/kg bw/day, as their sole drinking source for 53-56 weeks. Control
animals were given tap water. There were no statistically significant
differences in body or liver weights of treated animals as compared to
controls. Liver enzymes were determined at 2- to 4-week intervals and
revealed no significant differences in activity and protein content of
the liver between test and control animals. Histological examination
was performed on specimens of liver, kidney, heart, spleen and lung
and revealed no significant abnormalities (Johannsen & Purchase,
1969).
Groups of 10 male and 10 female Wistar rats were given 2-methyl-1-
propanol (i.e. isobutyl alcohol) in their drinking water for 3 months.
The test substance was administered in concentrations of 0, 1000, 4000
or 16 000 mg/litre, which was reported to correspond to approximate
dose levels of 0, 60, 340 or 1450 mg/kg bw per day. Food and drinking-
water consumption and body weight gain were not affected by the test
substance. All animals tested were free from adverse clinical effects.
Haematology and clinical chemistry parameters were measured and
revealed no treatment-related adverse effects. Gross pathology and
histopathological examinations were normal for all animals except for
testicular atrophy, which was observed in two males in the high-dose
group. Diffuse tubular generation and hyperplasia of the Leydig cells
were reported in the two animals. The authors concluded that the
absence of similar effects in other male rats indicated that the
effect was most likely unrelated to the test material. The authors
reported that the results of this study demonstrate a lack of toxicity
associated with administration of 2-methyl-1-propanol in the drinking-
water of rats, and that the NOEL is >1450 mg/kg bw per day (BASF,
1992). The NOEL is >100 000 times the estimated daily per capita
intake1 ("eaters only") of 4.85 g/kg bw of isobutyl alcohol from
its use as a flavouring substance in the USA and 8.79 g/kg bw of
isobutyl alcohol in Europe.
ii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage providing daily dose levels
of 0, 150, 500 or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight statistically significant reduction in body-weight
gain which was associated with reduced food intake. Over the entire
period of the study, however, there was no statistically significant
reduction in mean food intake. Examination of haematology, serum
analyses, urinalysis, renal concentration tests and organ weights
revealed no treatment-related effects. The animals were examined for
macroscopic abnormalities, and the major organs were weighed.
Microscopic examination was performed on several tissues of the
control and high-dose animals. No treatment-related abnormalities were
observed (Carpanini et al., 1973).
Groups of 20 male and 20 female Wistar albino rats were given a 2%
solution of isoamyl alcohol, which was calculated (FDA, 1993) to
provide an approximate daily intake of 2000 mg/kg bw per day, as their
sole drinking source for 53-56 weeks. Control animals were given tap
water. There were no statistically significant differences in body or
liver weights of treated animals as compared to controls. Liver
enzymes were determined at 2- to 4-week intervals and revealed no
significant differences in ADH, GOT or GPT activity and protein
content of the liver. Histological examination was performed on
specimens of liver, kidney, heart, spleen and lung and revealed no
significant abnormalities (Johannsen & Purchase, 1969).
iii) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0 or 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to provide a daily intake of 0 or 2500 mg/kg
bw. The rats were monitored for survival rates, body weight changes,
food intake, blood and urine analysis, organ weights and histology. At
2500 mg/kg bw per day, there were no observed changes in the rats
(Amoore et al., 1978).
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1995). Intake (mg/kg bw/day)
calculated as follows: [(mg/day)/body weight], where body weight = 60
kg. Slight variations may occur from rounding off.
iv) 2-Methylhexanoic acid
In a limited 90-day feeding study, 2-methylhexanoic acid was fed
to male and female Charles River CD rats at a dose of approximately 3
mg/kg bw/day. Observations included mortality rates, food intake, body
weight, haematology, blood urea, organ weights and histopathology. The
NOEL was 3 mg/kg bw dose level (Posternak et al., 1969) which is
>10 000 times the estimated daily per capita intake ("eaters only")
of 0.04 g/kg bw 2-methylhexanoic acid from use as a flavouring
substance in the USA and 0.24 g/kg bw from its use in Europe.
b) alpha-Ethyl substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
Eleven (11-) day and 90-day studies were conducted in B6C3F1 mice
as part of the dose selection process for an 18-month study (see
section 2.1.3.3). In the 11-day gavage study, groups of ten B6C3F1
mice of each sex were administrated nine applications of 0, 100, 330,
1000 or 1500 mg 2-ethylhexanol/kg bw per day. Mice in the high-dose
group exhibited effects such as ataxia, lethargy, loss of
consciousness, death in four females, increased absolute and relative
lever and stomach weights, macroscopic foci in the forestomach, and
microscopic lesions in the forestomach, liver, testes and kidneys.
These lesions consisted of hyperkeratosis and focal or multifocal
acanthosis and inflammatory oedema in the submucosa in most mice,
focal or multifocal ulceration of the mucous membrane in a few mice,
hypertrophy of hepatocytes in all mice, focal necrosis of liver cells
in one male and one female mouse, centrilobular fatty infiltration in
female mice that died intercurrently, tubular giant cells in
testicular tubules of two mice, and renal cortex tubular dilation and
nephrosis in mice that died intercurrently. Mice receiving the dose of
1000 mg/kg bw per day exhibited similar symptoms to those observed at
the highest dose (1500 mg/kg bw per day), but to a lesser degree,
while four mice (two male, two female) receiving 330 mg 2-ethyl
hexanol/kg bw per day had acanthosis in the mucous membrane of the
forestomach. No treatment-related adverse effects were observed in the
mice administered 100 mg 2-ethylhexanol/kg bw per day (Astill
et al., 1996a).
In the 90-day study, mice (number per group not reported) received
doses of 0, 25, 125, 250 or 500 mg 2-ethylhexanol/kg bw per day. At
the highest dose level, the treatment-related effects observed
included increased relative stomach weights in male mice and focal or
multifocal acanthosis of the forestomach mucosa in two male and one
female mouse. Increased relative stomach weights were observed in male
mice receiving 250 mg/kg bw per day. No treatment-related effects were
reported in mice of the 125 or 25 mg/kg bw per day dose level. Based
on the results of the 11- and 90-day studies, dose levels of 50, 200
or 750 mg 2-ethylhexanol/kg bw per day were chosen for the 18-month
mouse study (Astill et al., 1996a).
An 11-day mouse study was conducted using administration of
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of 10 male and 10 female B6C3F1 mice per group were used with
concentrations of microencapsulated material in the diet of 0.48,
0.96, 1.44 or 2.88%. The microcapsules contained 45.8% 2-ethylhexanol;
therefore, 2-ethylhexanol concentrations in the diet were 0.22, 0.44,
0.66 or 1.32%, respectively. These concentrations were reported to be
equal to doses of 550, 1150, 1800 or 4450 mg/kg bw per day for males
and 750, 1750, 2650 or 5750 mg/kg bw per day for females. The authors
reported that diet spillage did occur, but this was not quantified in
the treated groups of mice. As a result, the mg/kg bw per day dosage
calculations would not account for spillage and may represent
exaggerated 2-ethylhexanol intakes (Astill et al., 1993).
Significantly decreased body weight gains were observed at the
high-dose level for females throughout the test period and for males
at day 10 of the test period and at the 0.66% level in males at day 10
of the test period. No other treatment-related findings were reported.
The authors concluded the NOEL for male mice was in the range of 0.96
to 1.44% microencapsulated material (0.44 to 0.66% 2-ethylhexanol or
reportedly 1150 to 1800 mg/kg bw per day) and the NOEL for female mice
was in the range of 1.44 to 2.88% microencapsulated material (0.66 to
1.32% 2-ethylhexanol or reportedly 1800 to 4450 mg/kg bw per day)
(Astill et al., 1993). The NOELs reported in this study are higher
than the NOEL of 100 to 330 mg 2-ethylhexanol/kg bw per day reported
in the 11-day gavage study in B6C3F1 mice (Astill et al., 1996a),
most likely as a result of the different modes of administration. The
lack of correction for diet spillage in the microencapsulation study
may also have been a factor.
To study the effect of 2-ethylhexanol on rat liver and testes,
Rhodes et al. (1984) administered 1 mmol 2-ethylhexanol/kg bw per
day (approximately 130 mg/kg bw per day) to five Wistar rats by gavage
for 14 days. 2-Ethylhexanol administration did not produce testicular
atrophy, hepatomegaly, peroxisome proliferation or hypolipidaemia in
the rats. Lake et al. (1992) orally administered 1335 mg
2-ethylhexanol/kg bw per day to six male Wistar rats for seven days.
The authors reported significantly increased relative liver weights,
increased microsomal biphenyl 4-hydroxylase activity, increased
alcohol dehydrogenase activity in the centrilobular area of the liver
lobule, increased number of microbodies, and dilatation of the smooth
endoplasmic reticulum in the livers. No effect on the activities of
aniline 4-hydroxylase or mitochondrial succinate dehydrogenase was
observed.
In an 11-day gavage study, groups of ten male and ten female
Fischer F344 rats were administered doses of 0, 100, 330, 1000 or 1500
mg 2-ethylhexanol/kg bw per day. Adverse effects, including reduction
in food consumption and body weight gain, ataxia, lethargy, changes in
blood chemistry, effects on absolute (increased liver and stomach,
decreased spleen, brain, and adrenal) and relative (increased stomach,
liver, kidney, brain, adrenal, and lung, decreased spleen) organ
weights, gross lesions in the forestomach and microscopic findings in
the forestomach, liver (high-dose only), spleen and thymus, were
observed in the rats at the highest two dose levels. At the 330 mg/kg
bw per day dose, female rats exhibited increased relative kidney
weights, as well as microscopic lesions in the forestomach of one
female rat and in the thymus of both sexes (it was not stated whether
or not these lesions were treatment-related). No treatment-related
adverse effects were observed at 100 mg/kg bw per day (Astill et
al., 1996a).
In the 90-day study, groups of ten male and ten female Fischer
F344 rats were administered doses of 0, 25, 125, 250 or 500 mg
2-ethylhexanol/kg bw per day by gavage. Reduced body weight and body
weight gain, changes in blood chemistry (consisting of reduced
cholesterol, glucose, alanine aminotransferase and alkaline
phosphatase, levels and increased reticulocytes, total protein and
albumin levels), increased absolute and relative liver and stomach
weights and gross lesions in the forestomach were observed in the rats
receiving 500 mg 2-ethylhexanol/kg bw per day. Also observed in the
high-dose rats were microscopic changes consisting of focal or
multifocal acanthosis in the mucosa of six rats (one male, five
female) and whole mucose acanthosis and submucosa degeneration and
oedema in one male rat. In addition, a number of high-dose rats with
fatty infiltration in the liver, as well as a lower grade of fat
deposition in the liver, were observed. At 250 mg/kg bw per day,
changes in the blood chemistry (consisting of reduced glucose,
alkaline phosphatase and alanine aminotransferase levels), increased
relative liver and stomach weights, and a lower grade of fat
deposition in the liver cells of the male rats were observed. No
treatment-related adverse effects were observed at the 25 or 125 mg/kg
bw per day dose levels. Based on the results of the 11- and 90-day
studies, dose levels of 50, 150 or 500 mg 2-ethylhexanol/kg bw per day
were chosen for a two-year study (Astill et al., 1996a).
An 11-day rat study was also conducted by administering
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of ten male and ten female Fischer rats per group were fed
concentrations of microencapsulated material in the diet of 1, 2, 3 or
6%. The microcapsules contained 45.8% 2-ethylhexanol; therefore,
2-ethylhexanol concentrations in the diet were 0.46, 0.92, 1.38 or
2.75%, respectively. These concentrations were reported to be equal to
doses of 500, 980, 1430 or 2590 mg/kg bw per day for males and 540,
1060, 1580 or 2820 mg/kg bw per day for females. A vehicle control
group receiving placebo microcapsules was employed. Decreased food and
water (top three doses only) consumption was observed at all dose
levels at some point in the study period with decreased body weights
reported for both sexes at the top dose and in females at day four in
the second highest dose. Changes in blood chemistry, consisting of
decreased cholesterol, triglycerides and alanine aminotransferase
levels (all doses), decreased glucose, reticulocyte and mean
corpuscular volume levels (high-dose only), decreased platelet level
(top two doses only), increased total protein level (top three doses),
increased erythrocyte level (high-dose only) and increased absolute
and relative stomach and/or liver weights were observed to some extent
in all dose groups. Varying degrees of hypertrophy of the hepatocytes
were reported in the top three dose groups. The authors reported the
NOEL to be less than 0.46% in the diet (500 or 540 mg/kg bw per day
for male and female rats, respectively) (Astill et al., 1993).
ii) 2-Ethylbutyric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0, 0.62, 1.25 or 10% 2-ethylbutyric acid in the
diet, equivalent to 0, 300, 625 or 5000 mg/kg bw per day,
respectively. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analysis, organ weights and
histology. At the 300 mg/kg bw dose level, there were no observed
changes in the rats. At both the 625 and 5000 mg/kg bw dose levels,
slight body weight changes were observed within the first 14 days of
the study (Amoore et al., 1978).
2.1.3.3 Long-term toxicity/carcinogenicity
The results of long-term toxicity/carcinogenicity studies on
2-ethyl-1-hexanol are summarized in Table 3 and described below.
a) Mice
Groups of 50 male and 50 female B6C3F1 mice were administered
2-ethylhexanol by gavage at doses of 0, 50, 200 or 750 mg/kg bw per
day, five days per week for 18 months. The vehicle consisted of doubly
distilled water containing approximately 5 mg Polyoxyl 35 Castor Oil
(Cremophor(R) EL) per 100 ml. Two control groups of 50 mice of each
sex were employed, one receiving doubly distilled water containing
approximately 5 mg Cremophor(R) EL/100 ml. The use of Cremophor(R)
EL was reported to have no effect on the outcome of the study. Food
consumption, body weights and haematological parameters were examined
at specific intervals during the study. At the end of the study, gross
and histopathological examinations were conducted.
No treatment-related adverse effects were observed in the mice
receiving 50 or 200 mg 2-ethylhexanol/kg bw per day. At the 750 mg/kg
bw per day dose level, body weight gain reductions of approximately 26
and 24% in males and females, respectively, were reported. These
weight gain reductions were associated with a significant reduction in
food consumption. Both male and female mice of the high-dose group
showed increased mortality compared to the vehicle control mice (i.e.
30% in the treated mice compared to 4 and 8% in the vehicle control
group male and female mice, respectively). With respect to
haematological parameters, both a slight increase in polymorphonuclear
neutrophils and a slight decrease in lymphocytes were observed in the
mice of the high-dose group. A slight, statistically insignificant,
increase in focal hyperplasia of the forestomach epithelium was also
observed in the high-dose group mice. A slight increase in
hepatocellular carcinomas in the female mice of the high-dose group
was determined to have no biological relevance. 2-Ethylhexanol showed
no evidence of carcinogenicity in this study (Astill et al., 1996b).
b) Rats
Groups of 50 male and 50 female Fischer F344 rats were
administered 2-ethylhexanol by gavage at doses of 0, 50, 150 or 500
mg/kg bw per day, 5 days per week for 2 years. The vehicle consisted
of doubly distilled water containing approximately 5 mg Polyoxyl 35
Castor Oil (Cremophor(R) EL) (as an emulsifier) per 100 ml. Two
control groups of 50 mice of each sex were used, one receiving doubly
distilled water containing 5 mg Cremophor(R) EL/100 ml. The use of
Cremophor(R) EL was reported to have no effect on the outcome of the
study. Food consumption, body weights, and haematological parameters
were examined at specific intervals during the study. At the end of
the study, gross and histopatho-logical examinations were conducted.
No treatment-related adverse effects were observed at the 50 mg/kg
bw per day dose level. At the 150 mg/kg bw per day dose level, rats
exhibited a body weight gain reduction of approximately 16% in males
and 12% in females. In addition, the rats also displayed a slightly
increased incidence of clinical signs, such as poor general condition,
laboured breathing, piloerection, and/or smearing of the genital
region with urine. A definite increase in the number of rats and/or a
definite increase in the incidence of clinical signs (same signs as
mentioned for the mid-dose group) was observed in the high-dose group
rats. Mortality was increased in the female rats of the high-dose
group (52% compared to 28% in the vehicle control) and both male and
female rats exhibited a reduction in the body weight gain (33% in
males and 31% in females). With respect to haematological parameters,
a slight increase in anisocytosis, predominantly microcytosis in
males, was observed after 12 months, but not after 18 or 24 months.
The incidence of bronchopneumonia was significantly increased in both
sexes at the high-dose level. This effect was found to be due to the
aspiration of the stomach contents, most likely resulting from the
poor general condition of the animals. 2-Ethylhexanol showed no
evidence of carcinogenicity in this study (Astill et al., 1996b).
2.1.3.4 Genotoxicity
The results of genotoxicity studies on saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes, and carboxylic acids are
summarized in Table 4.
Table 4. Genotoxicity studies of saturated aliphatic acyclic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Test system Test object Dose level Results Reference
Alcohols
Isobutyl alcohol Modified Ames test S. typhimurium TA98, TA100, 100 to 10 000 g/plate1,2 Negative Zeiger et
TA1535 TA97 and TA1537 al., 1988
Modified Ames test S. typhimurium TA100, TA98, 5 to 5000 mg/plate1 Negative Shimizu et
TA1535, TA1537 and TA1538; al., 1985
E. coli WP2 uvrA
2-Ethyl-1-hexanol 8-azaguanine resistance S. typhimurium TA100 0.5 to 1.5 mM Positive3 Seed, 1982
assay
Ames test4 S. typhimurium TA98, TA100, up to 2 ml in urine/plate Negative Divincenzo
TA1535, TA1537, TA1538 et al., 1985
Ames test S. typhimurium TA98, TA100, 0.01 to 1.0 µl/plate1 Negative Kirby et
TA1535, TA1537, TA1538 al., 1983
Ames test S. typhimurium TA98, TA100, 33 to 220 g/plate1,2 Negative Zeiger et
TA1535, TA1537 al., 1985
Ames test S. typhimurium TA98, TA100, 100 to 2000 g/plate1 Negative Agarwal et
TA1535, TA1537, TA1538, al., 1985
TA2637
L5178Y/TK+/- mouse L5178Y/TK+/- mouse 0.01-0.3 µl/ml1 Negative Kirby et
lymphoma assay lymphoma cells al., 1983
Rec-assay Bacillus subtilis 500 g/disk Negative Tomita et al.,
1982
CHO mutation assay Chinese hamster ovary cells 1.5-2.8 mM Negative Phillips et al.,
1982
Unscheduled DNA synthesis Primary rat hepatocytes not specified Negative Hodgson et
assay al., 1982
Aldehydes
Isobutyraldehyde Gradient plate technique S. typhimurium G46, TA1535, 0.1 to 1000 g/ml Positive5/ McMahon et
TA100, C3076, TA1537, D3052, Negative6 al., 1979
TA1538, and TA98; E. coli
WP2 and WP2 uvrA-
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Isobutyraldehyde Ames test S. typhimurium 3 µmol/plate1 Negative Florin et
(cont'd) al., 1980
Spot test TA98, TA100, TA1535, TA1537
Sister chromatid exchange adult human lymphocytes 0.002% Negative Obe & Beek,
1979
Paper-disk method E. coli Sd-4-73 0.01- 0.025 ml/disk Negative Szybalski,
1958
2-Methylbutyraldehyde Ames test S. typhimurium TA98, TA100, 0.03 to 30 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
Ames test S. typhimurium TA98, TA100 9 nmol to 0.9 mmol/plate1 Negative Aeschbacher
and TA102 et al., 1989
3-Methylbutyraldehyde Sister chromatid exchange Adult human lymphocytes 0.002 to 0.003% Negative Obe & Beek,
1979
Ames test S. typhimurium TA98, TA100 0.01 nmol to 1 mol/plate1 Negative Aeschbacher
and TA102 et al., 1989
Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
2-Methylpentanal Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
Spot test TA1535 and TA1537 al., 1980
2,6-Dimethyloctanal Ames test S. typhimurium TA98, TA100, up to 3.6 mg/plate Negative Wild et al.,
TA1535, TA1537, TA1538 1983
Carboxylic acids
Isobutyric acid Paper-disk method E. coli Sd-4-73 0.01 to 0.025 ml/disk Negative Szybalski,
1958
Cell mutagenesis assay mouse lymphoma 500 g/ml7,8 Positive Heck et al,
L5178Y TK +/- (weak) 1989
600 g/ml9,10 Negative
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Unscheduled DNA synthesis rat hepatocytes 1000 g/ml9,10 Negative Heck et al.,
assay 1989
Plate incorporation assay S. typhimurium TA98, 100, 75 000 g/plate9,10 Negative Heck et al.,
1535, 1537, 1538 1989
Rec-assay B. subtilis H17 rec+ and 19 g/disk Negative Oda et al.,
M45 rec- 1978
2-Ethyl-1-hexanol In vivo dominant lethal ICR/SIM mice 250, 500, 1000 mg/kg Negative Rushbrook et
assay bw/day for 5 days al., 1982
In vivo chromosomal Fischer 344 rat bone marrow 16.7, 58.4 or 167 mg/kg Negative Putman et
aberration assay cells bw/day for 5 days al., 1983
Isobutyraldehyde Sex linked recessive Drosophila melanogaster 80 000 ppm (feeding) Negative Woodruff et
lethal mutation test 50 000 ppm (injection) al., 1985
1 Both with and without metabolic activation
2 Metabolic activation in the form of S9 derived from rat and hamster
3 It is assumed that no S9 was used
4 Test performed on the metabolites of 2-ethyl-1-hexanol
5 Positive in G46, TA100, E. coli WP2, WP2 uvrA-
6 Negative in TA1535, TA1537, TA1538, TA98, C3076 and D3052
7 Without metabolic activation
8 Lowest active dose tested
9 With metabolic activation
10 Highest inactive dose tested
a) Methyl substituted aliphatic alcohols, aldehydes, and carboxylic
acids
i) Isobutyl alcohol
Isobutyl alcohol produced negative results in Ames tests with
Salmonella typhimurium strains TA97, TA98, TA100, TA1535, TA1537 at
concentrations of 100 to 10 000 g/plate (Zeiger et al., 1988).
Negative mutagenicity results were also obtained by Shimizu et al.
(1985) at dose levels of 5 to 5000 g/plate in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538, and
E. coli strain WP2 uvrA-.
ii) Branched-chain aldehydes
In Salmonella typhimurium, isobutyraldehyde (in strains TA98,
TA100, TA1535, TA1537), 2-methylbutyraldehyde (TA98, TA100, TA1535,
TA1537), 3-methylbutyraldehyde (TA98, TA100, TA1535, TA1537) and
2-methylpentanal (TA100, TA1535, TA1537) were negative in spot tests
at a concentration of 3 mol/plate with and without metabolic
activation (Florin et al., 1980). Similar results were reported for
2-methylbutyraldehyde and 3-methylbutyraldehyde in S. typhimurium
strains TA98, TA100 and TA102 at concentrations of 9.0 nmol/plate to
0.9 µmol/plate and 0.01 nmol/plate to 1 µmol/plate, respectively, with
and without metabolic activation (Aeschbacher, 1989). Florin et al.
(1980) subsequently subjected 2-methylbutyraldehyde to a quantitative
Ames test assessment at concentrations of 0.03 to 30 µmol/plate, and
found no evidence of mutagenicity in S. typhimurium strains TA98,
TA100 and TA1535, with and without metabolic activation.
Isobutyraldehyde gave negative results for frame-shift mutations in
S. typhimurium C3076, TA1537, D3052, TA1538 and TA98, and base
substitution mutations in S. typhimurium TA1535, and gave positive
results for base substitution mutations in S. typhimurium G46, TA100
and E. coli WP2 and WP2 uvrA-(McMahon et al., 1979). It was
non-mutagenic in E. coli Sd-4-73 (Szybalski, 1958). In an in vivo
study, isobutyraldehyde was negative for sex-linked recessive lethal
mutation in germ cells of Drosophila melanogaster by adult feeding
at 80 000 ppm and adult injection at 50 000 ppm (Woodruff et al.,
1985).
iii) Branched-chain carboxylic acids
Isobutyric acid was non-mutagenic in the Rec-assay with E.
coli Sd-4-73 at dose levels of 0.01 to 0.025 ml/disk (Szybalski,
1958) and in B. subtilis H17 and M45 at a dose of 19 g/ml (Oda,
1978). In a mouse lymphoma L5178YTK +/- assay, isobutyric acid had
negative results at a dose level of 600 g/ml with metabolic activation
and had weakly positive results at a dose of 500 g/ml without
metabolic activation (Heck et al., 1989). Isobutyric acid had
negative results in the Ames test plate incorporation assay with
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and
TA1538 at doses ranging up to 75 000 g/ml (Heck et al., 1989).
Isobutyric acid did not induce unscheduled DNA synthesis in rat
hepatocytes at doses of up to 1000 g/ml (Heck et al., 1989).
Based on a weight of evidence analysis, aliphatic acyclic
branched-chain primary alcohols, aldehydes and carboxylic acids are
not mutagenic or clastogenic in bacterial, in vitro or in vivo
systems. Sporadic positives were observed in some strains in three of
the approximately 20 bacterial assays conducted on members of this
group. Isobutyric acid was found in a single study to be mutagenic
in vitro in the presence of metabolic activation. However,
isobutyric acid was found to be negative in the rat UDS assay. The
mutagenic activity of isobutyric acid is not, therefore, likely to be
related to a mechanism involving direct binding to DNA. Consistent
with a lack of DNA damage, both isobutyraldehyde and
3-methylbutyraldehyde were negative in the sister chromatid exchange
assay. In addition, 2-ethyl-1-hexanol was found to be negative in two
assays for in vitro mutagenic activity (see 2-ethyl-1-hexanol
below). There is no evidence of mutagenic or clastogenic activity
in vivo in any of the compounds tested.
b) alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
There are several reports in the literature concerning the in
vitro testing of 2-ethylhexanol for mutagenicity in bacteria.
2-Ethylhexanol has shown no evidence of mutagenicity in the Ames test
with Salmonella typhimurium strains TA98, TA100, TA1535, TA 1537,
TA1538 or TA2637 with or without metabolic activation (Kirby et
al., 1983; Zeiger et al., 1985; Agarwal et al., 1985). Agarwal
et al. (1985) reported that 2-ethylhexanol was moderately cytotoxic
in most of the cultures tested, thereby having the potential to mask a
mutagenic effect. Weak dose-related mutagenic activity (maximum
increase in mutant frequency was only 3.5 times background) was
reported for 2-ethylhexanol in the Salmonella 8-azaguanine
resistance assay without activation at concentrations up to 1.5 mM.
2-Ethylhexanol was highly cytotoxic at concentrations that were
mutagenic (Seed, 1982).
Further Ames test results on the urinary metabolites of 2-ethyl-1-
hexanol, using S. typhimurium strains TA98, TA100, TA1537, TA1538
and TA1535, indicated no mutagenicity (Divincenzo et al.,
1985). The measurements were made with six male Sprague-Dawley rats
that were administered 1000 mg 2-ethyl-1-hexanol/kg bw/day by gavage
for 15 days. Urine samples were collected for use in the Ames assay at
doses of up to 2 ml per plate.
2-Ethylhexanol was not mutagenic in the L5178Y TK +/- mouse
lymphoma cell mutagenicity assay, either with or without metabolic
activation (Kirby et al., 1983), and produced negative results in
the rec-assay in Bacillus subtilis at a concentration of 500 g/disk
(Tomita et al., 1982). Negative results have also been reported for
2-ethylhexanol in a clastogenicity test on cultured Chinese hamster
ovary cells (Phillips et al., 1982) and in an unscheduled DNA
synthesis assay in primary rat hepatocytes (Hodgson et al., 1982).
In in vivo assays, negative results were reported in a dominant
lethal assay using oral doses of 250, 500 or 1000 mg 2-ethylhexanol/kg
bw administered for five consecutive days to ICR/SIM mice (Rushbrook
et al., 1982). 2-Ethylhexanol did not induce chromosomal aberrations
in bone marrow cells after oral administration of 0.02, 0.07 or 0.2
ml/kg bw per day (approximately 16.7, 58.4, 167 mg/kg bw per day
assuming a density of 0.8344) to groups of five Fischer 344 male rats
for five days (Putman et al., 1983).
The weight of evidence indicates that 2-ethylhexanol is not
mutagenic or genotoxic. Additionally, the urinary metabolites of
2-ethyl-1-hexanol were not mutagenic in the Ames tests. Negative
in vivo results were reported in a dominant lethal assay and in a
chromosomal aberration assay.
2.1.3.5 Reproductive toxicity
Gray & Beamand (1984) and Sjoberg et al. (1986) examined the
effect of 2-ethylhexanol on in vitro primary cultures of rat
testicular cells. No effect on the rate of germ-cell detachment from
Sertoli cells was observed at a 2-ethylhexanol concentration of
2x10-4 M.
Sjoberg et al. (1986) also examined the effect of 2-ethylhexanol
on rat testes in vivo. No testicular damage was observed in six
young male Sprague-Dawley rats orally administered 2.7 mmol 2-
ethylhexanol/kg bw per day (approximately 352 mg/kg bw per day) for 5
days.
2.1.3.6 Developmental toxicity
a) Mice
An oral developmental toxicity study was conducted using CD-1 mice
(NTP, 1991). Groups of 28 plug-positive mice were exposed to
microencapsulated 2-ethylhexanol at concentrations of 0, 0.009, 0.03
or 0.09% in the diet throughout gestation (days 0 to 17). These
concentrations were reported to be equal to 0, 17, 59 or 191 mg
2-ethylhexanol/kg bw per day, respectively, based on food consumption.
No evidence of maternal or developmental toxicity was observed in the
study. There were no treatment-related effects with respect to the
number of corpora lutea, uterine implantation sites, pre- and
post-implantation sites, pre- and post-implantation loss, sex ratio or
live fetal body weight, or to the incidence of individual, external,
visceral, skeletal or total malformations or variations.
Hardin et al. (1987) examined the developmental toxicity of
orally administered 2-ethylhexanol in pregnant mice. Fifty female CD-1
mice were administered by gavage a dose of 1525 mg 2-ethylhexanol/kg
bw per day on gestation days 6 to 13. A concurrent control group was
employed. Maternal mortality in the study was 35%, with significant
reductions in maternal weight gain and in the number of viable litters
being observed. Significant reductions in the treated group were also
reported for the following parameters: number of liveborn per litter,
percentage of survival of the pups, and the birth weight and weight
gain of the pups. The results of this study are of questionable
significance due to the presence of maternal toxicity and are
difficult to interpret due to the fact that only one dose level was
employed.
b) Rats
Groups of ten pregnant Wistar rats were administered by gavage
doses of 130, 650 or 1300 mg 2-ethylhexanol/kg bw per day on gestation
days 6 to 15. Two control groups were included, one that received
doubly distilled water and a second that received doubly distilled
water containing the emulsifier (0.005% Cremophor(R) EL) used as the
vehicle in the treated groups. On day 20 of gestation, the rats were
killed.
Significant maternal toxicity was observed in the high-dose rats.
These rats exhibited significant reductions in food consumption, body
weights and body weight gain. On days 6 to 10, a body weight loss was
reported. Six of the rats in the high-dose group were found dead on
days 9, 10 or 13. Other adverse effects reported in the high-dose rats
included severe clinical symptoms (e.g., unsteady gait, apathy), light
brown-gray discoloration of the liver in the rats that died during the
study, lung oedema and emphysema, and markedly decreased mean gravid
uterus weights. In the 650 mg/kg bw per day dose group, two dams were
reported to exhibit piloerection. Significant embryotoxic and
fetotoxic effects, such as an increase in the number of resorptions
resulting in increased post-implantation loss, decreased mean fetal
body weight, increased incidence of fetuses with dilated renal pelvis
and/or hydroureter, and a higher number of fetuses with skeletal
malformations, also occurred in the high-dose group. Variations and
retardation were also reported in the high-dose group. A slight
decrease in mean fetal body weight (statistically significant for all
viable fetuses combined and male fetuses separately, but
non-significant for female fetuses separately) and a statistically
significant increase in the frequency of fetuses with skeletal
variations and retardation were reported for the mid-dose group. The
authors reported that the only indications of significant adverse
effects on reproduction occurred in the high-dose group, which were
associated with maternal toxicity (Anonymous, 1991).
In an oral study, Ritter et al. (1987) exposed groups of
pregnant Wistar rats to doses of 6.25 or 12.5 mmoles 2-ethylhexanol/kg
bw (approximately 814 or 1628 mg/kg bw) on day 12 of gestation, the
rats being killed on day 20 of gestation. Several litters were
examined from each of the two treatment groups. For the low- and
high-dose groups of 2-ethylhexanol, the percentage of surviving pups
with malformation was 2 and 22.2, respectively. The malformations
observed in the high-dose group included tail and limb defects and
hydronephrosis. As indicated from the results of tests following oral
exposure (Anonymous, 1991) and dermal exposure (Tyl et al., 1992) in
rats, it would be expected that maternal toxicity could have occurred
at both dose levels employed in the study of Ritter et al. (1987),
even though the effect of treatment on the mother rats was not
discussed by the authors.
Tyl et al. (1992) conducted a range-finding study and a main
study examining the developmental toxicity of dermally applied
2-ethylhexanol in Fischer 344 rats. In the range-finding study, doses
of 0, 0.5, 1.0, 2.0 or 3.0 ml 2-ethylhexanol/kg bw per day (stated by
the authors to be equivalent to 0, 420, 840, 1680 or 2520 mg/kg bw per
day, respectively) were applied by occluded dermal application for 6
hours per day on gestation days 6 to 15 to groups of eight rats.
Naïve, sham (treated with 3.0 ml distilled water/kg bw per day) and
positive (treated with 420 or 1260 mg methoxyethanol/kg bw per day)
control groups were employed in the range-finding study. Valproic acid
was administered orally to a group of eight rats at a dose of 400
mg/kg bw per day as an oral reference compound.
In the main study, doses of 0, 0.3, 1.0 or 3.0 ml
2-ethylhexanol/kg bw per day (stated by authors to be equivalent to 0,
252, 840 or 2520 mg/kg bw per day, respectively) were applied to
groups of 25 rats by occluded dermal application for 6 hours per day
on gestation days 6 to 15. A sham control group receiving 3.0 ml/kg bw
per day distilled water and a positive control group receiving 840 mg
2-methoxyethanol/kg bw per day were included (Tyl et al., 1992).
Both positive control groups (valproic acid and methoxyethanol)
exhibited developmental toxicity and teratogenic effects. With respect
to 2-ethylhexanol, maternal weight gain was significantly reduced
during gestation days 6 to 15 at dose levels of 1680 and 2520 mg/kg bw
per day in the range-finding study. At the highest dose level in the
main study, maternal weight gain was significantly reduced during
gestation days 6 to 9, but was not significantly different from sham
controls for any other period examined (i.e. gestation days 0 to 6, 6
to 15, 15 to 21, or 0 to 21). In both studies, exfoliation and
encrustation at the site of application occurred at all dose levels.
However, this effect was reported to be typical of the effect of
alcohols on the skin. Erythema scores were mild to moderate at 840 and
2520 mg 2-ethylhexanol/kg bw per day. Oedema was not observed at any
treatment level. No treatment-related developmental or teratogenic
effects were observed in the fetuses of any of the dose levels in
either study. The authors reported no-observed-effect levels of 252 mg
2-ethylhexanol/kg bw per day based in skin irritation and 840 mg/kg
weight per day based on systemic toxicity (reduced maternal body
weight gain) (Tyl et al., 1992).
In a study designed to evaluate the association between the
pharma-cokinetics and teratogenicity of organic acids, pregnant
Sprague-Dawley rats were administered 14.1 mmoles/kg bw methylhexanoic
acid, or 12.5 or 15.625 mmoles/kg bw 2-ethylhexanoic acid undiluted by
oral gavage on day 12 of gestation. At various post-treatment
intervals (0.25-24 hours), embryos were dissected and exocoelomic
fluid and yolk sacs were analysed for the presence of the test
material. Samples of maternal blood and skeletal muscle were analysed
to determine the concentration of the test substance. The mothers were
killed on day 20 and fetuses were examined. Methylhexanoic acid
exhibited no signs of embryotoxicity except for a slight reduction in
fetal weight which was attributed to maternal toxicity. Ethylhexanoic
acid was toxic to the embryos removed on day 12, causing increased
death and malformation and a reduction of fetal weight. Both
methylhexanoic acid and ethylhexanoic acid reached a high
concentration in maternal plasma and embryo, but methylhexanoic acid
was eliminated more rapidly (Scott et al., 1994).
An in vivo developmental toxicity screen was conducted on 15
aliphatic acids including three substances in this group. Groups of 9
to 18 timed-pregnant Sprague-Dawley rats were treated by gavage once
daily on gestation days 6 to 15 with 2-ethylbutyric acid (0, 150 or
200 mg/kg bw per day), 2-methylvaleric acid (0, 187.5 or 250 mg/kg bw
per day) and 5-methylhexanoic acid (0, 300 or 400 mg/kg bw per day).
2-Ethylhexanoic acid, a metabolite of 2-ethylhexanol, was also tested
at dose levels of 0, 900 or 1200 mg/kg bw per day.
Maternal body weights were determined on gestational days 6, 8,
10, 13, 16 and 20. Clinical observations were made on all animals
throughout the study. Pups in each litter were examined and counted.
Skeletal examination was performed on two postnatal day 6 survivors
from each group. After postnatal day 6, the dams were killed and the
number of uterine implantation sites was recorded. 2-Methylvaleric
acid, 2-ethylbutyric acid and 5-methylhexanoic acid induced motor
depression in some mothers, which was associated with severe
respiratory effects (i.e. rales or dyspnoea). Perinatal mortality was
noted for 2-ethylbutyric acid but only in litters of dams that
exhibited severe respiratory effects. There was no delayed
parturition, decreased progeny viability, lumbar ribs, or any other
signs of developmental toxicity in pups of mothers treated with
2-ethylbutyric acid, 2-methylvaleric acid, or 5-methylhexanoic acid.
The highest dose levels of 200 mg/kg bw per day for 2-ethylbutyric
acid, 250 mg/kg bw/day for 2-methylvaleric acid, and 400 mg/kg bw/day
for 5-methylhexanoic that did not produce any signs of developmental
toxicity are >10 000 times their respective daily per capita
intakes ("eaters only") from use as flavouring substances in the USA
and Europe (see Table 1).
All dams treated with 2-ethylhexanoic acid exhibited motor
depression. Developmental effects were observed in pups of mothers
treated with 2-ethylhexanoic acid at both dose levels, including
reduced progeny viability, full-litter resorptions, apparent
stillbirths, postnatal deaths, reduced pup weights, external
malformations, skeletal malformations, and lumbar ribs (Narotsky et
al., 1994). 2-Ethylhexanoic acid produced developmental toxicity only
in the presence of maternal toxicity.
2.1.3.7 Special studies on peroxisome proliferation
Concentrations of 0.1 or 0.5 mM 2-ethylhexanol did not induce
palmitoyl CoA oxidase activity (a marker enzyme for peroxisome
proliferation) in rat hepatocytes in vitro (Rhodes et al., 1984).
A concentration of 1 mM 2-ethylhexanol was reported to induce
significantly (approximately 9 times the control level) the activity
of carnitine acetyltransferase (a peroxisomal enzyme) in cultured rat
hepatocytes in vitro, while a concentration of 0.2 mM did not induce
this enzyme (Gray et al., 1982).
In an in vivo study, Rhodes et al. (1984) reported that the
administration of 1 mmol 2-ethylhexanol/kg bw per day (approximately
130 mg/kg bw per day) to five male Wistar rats by gavage for 14 days
did not result in hepatic peroxisome proliferation in the rats.
Moody & Reddy (1978), however, reported that in five male F344
rats fed 2% 2-ethylhexanol in the diet (approximately 1000 mg/kg bw
per day) for 3 weeks, induction of hepatic peroxisome proliferation
occurred. Significant increases (using a Student's t test) in
activities of the enzymes hepatic catalase and carnitine
acetyltransferase also were reported in the rats treated with
2-ethylhexanol.
Hodgson (1987) conducted a peroxisome induction study in groups of
F344 male and female (five/sex/group) by administering doses of 100,
320 or 950 mg 2-ethylhexanol/kg bw per day by gavage for 21 days. A
significant reduction in body weight gain was observed in the
high-dose group rats with no effect on food consumption. Both sexes of
rats exhibited significant hepatomegaly at a dose of 950 mg
2-ethylhexanol/kg bw per day. The activity of cyanide-insensitive
palmitoyl CoA oxidation was increased in a dose-related manner in
males, with a 1.6-fold increase above controls at 320 mg/kg bw per day
and a 5.4-fold increase at 950 mg/kg bw per day. In females, a
3.1-fold increase in palmitoyl CoA activity was reported at the
high-dose level. Lauric acid hydroxylase activity of peroxisomes was
observed (using electron microscopy) in the hepatocytes of the rats of
the high-dose group.
Keith et al. (1992) examined the dose-response relationship for
2-ethylhexanol with respect to peroxisome proliferation in rats and
mice. Groups of five male and five female Alderley Park rats and mice
were administered doses of 0, 140, 350, 700, 1050 or 1750 mg
2-ethylhexanol/kg bw per day for 14 days by gavage. Rats in the
high-dose group exhibited toxic effects, leading to death of the rats.
A dose-related increase in the relative liver weight of both rats and
mice was observed, with the increase being significant in rats at the
700 and the 1050 mg/kg bw per day dose levels (rats in the high-dose
group died or were killed during the study), in male mice at the three
highest dose levels (700, 1050 and 1750 mg/kg bw per day), and in
female mice at the highest dose level (1750 mg/kg bw per day).
2-Ethylhexanol administration resulted in a virtually linear
dose-related induction of peroxisomal ß-oxidation (measured as
palmitoyl CoA oxidation activities) in both rats and mice. The level
of induction was greatest in the male mice. The dose at which this
effect became statistically significant was not stated. The increase
in rats and female mice was approximately 4-fold at the highest dose,
while for male mice, the increase was approximately 12-fold at the
highest dose.
2.1.3.8 Special study on immunotoxicity
3-Methylpentanoic acid was administered to 6- to 8-week-old female
CD-1 or B6C3F1 mice in corn oil by gavage for 5 days at dose levels
of 0, 175, 350 or 700 mg/kg bw per day. Cell-mediated immunity was
assessed by conducting a host resistance assay (Listeria
monocytogenes bacterial challenge). Humoral immunity was measured by
the antibody plaque-forming cell (PFC) response to sheep erythrocytes.
Body weights, lymphoid organ weights and spleen cellularity were also
measured and were normal as compared to controls. 3-Methylpentanoic
acid did not modulate the cell-mediated or humoral immune response
(Gaworski et al., 1994).
3. REFERENCES
Aeschbacher, H., Wolleb, U., Loliger, J., Spadone, J., & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food. Chem. Toxicicol., 27: 227-232.
Agarwal, D.K., Lawrence, W.H., Nunez, L.J., & Autian, J. (1985)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. Environ. Health, 16:
61-69.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5: 625-636.
American Conference of Governmental Industrial Hygienists, Inc.
(ACGIH) (1989) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, OH. USA.
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anonymous (1991) Study of the prenatal toxicity of isodecanol,
2-ethylhexanol, and 711 alcohols (T.C.) in rats after oral
administration (gavage) (Laboratory and authors associated with the
study were stated to be confidential) (Microfiche OTS0524015-2).
Astill, B.D., Gingell, R., Guest, D., Hodgson, J.R., Murphy, S.R., &
Tyler, T.R. (1993) Subacute and subchronic oral toxicity of
ethylhexanol to fischer 344 rats and B6C3F1 mice. Toxicologist, 13:
70.
Astill, B.D., Deckardt, K., Gembardt, Chr., Gingell, R., Guest, D.,
Hodgson, J.R., Mellert, W., Murphy, S.R., & Tyler, T.R. (1996a)
Prechronic toxicity studies on 2-ethylhexanol in F334 rats and B6C3F1
mice. Fundam. Appl. Toxicol., 29: 31-39.
Astill, B.D., Gingell, R., Guest, D., Hellwig, J., Hodgson, J.R.,
Kuettler, K., Mellert, W., Murphy, S.R., Sielken, R.L., & Tyler, T.R.
(1996b) Oncogenicity testing of 2-ethylhexanol in Fischer 344 rats and
B6C3F1 mice. Fundam. Appl. Toxicol., 31: 29-41.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months (Project No. 3350057).
Unpublished report.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
partial purification and properties. Can. J. Biochem., 47: 265-272.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B. ed. Enzymatic basis of detoxification. Academic Press, New York,
Vol. I, pp. 231-248.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E. ed Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, Vol. IIA, pp. 283-327.
Butenandt, A. & Thomas, K. (1958) Hoppe-Seyler's zeitschrift fur
physiologische chemie - Band 313. Walter Gruyter and Co., pp. 22-29.
Carpanini, F.M.B., Gaunt, I.F., Kiss, I.S., Grasso, P., & Gangolli,
S.D. (1973) Short-term toxicity of isoamyl alcohol in rats. Food
Cosmet. Toxicol., 11: 713-724.
Chen, T.H., Kavanagh, T.J., Chang, C.C., & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol.,
1: 155-171.
CIVO-TNO (1994) In: Maarse, H & Visscher, C.A. ed. Volatile components
in food: Qualitative and quantitative data - Volume III, 7th ed.
Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard-adecision tree approach. Food Cosmet. Toxicol., 16: 255-276.
Dave, G. & Lidman, U. (1978) Biological and toxicological effects of
solvent extraction chemicals: Range finding acute toxicity in the
rainbow trout. J. Hydrometall., 3: 210-216.
Dawson, A.M., Holdsworth, C.D., & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 17: 97-100.
Deisinger, P.J., Boatman, R J., & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
Deuel, H.J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley Interscience, New York.
DiVincenzo, G.D.& Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid the in the rat. Toxicol. Appl. Pharmacol., 47:
609-612.
DiVincenzo, G.D., Hamilton, M.L., Meuller, K.R., Donish, W.H., &
Barber, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
Dziewiatowski, D.D., Ventakaraman, A., & Lewis, H.B. (1949) The
metabolism of some branched chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Eckfeldt, J.H.& Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Wood, A. ed. Methods in
enzymology. Academic Press, New York, pp. 474-479.
Engler, H.W. & Bahler, B. (1982) Private communication to FEMA.
Fassett, D.W. (1963) Organic acids, anhydrides, lactones, acid
halides and amides, thioacids. In: Industrial hygene and toxicology,
2nd ed. Wiley-Interscience, New York, Vol. II, p. 1783.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
J. Toxicol., 15: 219-232.
Food and Drug Administration (FDA) (1993) Priority-based assessment of
food additives (PAFA) database. Center for Food Safety and Applied
Nutrition, p. 58.
Gaillard, D. & Derache, R. (1965) Metabolisation de differents
alcools, present dans les buissons alcooliques, chez le rat. Trav.
Soc. Pharm. Montp., 25: 51-62.
Gaworski, C., Vollmuth, T., Dozier, M., Heck, J., Dunn, L., Ratajczak,
H., & Thomas, P. (1994) An immunotoxicity assessment of food
flavouring ingredients. Food. Chem. Toxicol., 32: 409-415.
Goldberg, E.M., Sandler, S., & Blendis, L.M. (1979) A statistical
study of profiles of volatile metabolites in hepatic encephalopathy.
Modeling and Simulation - Volume 10, Part 1: Biomedical. In:
Proceedings of the Tenth Annual Conference, Pittsburgh, 25-27 April.
Instrumental Society of America, University of Pittsburgh.
Gray, T.J.B. & Beamand, J.A. (1984) Effect of some phthalate estsers
and other testicular toxins on primary cultures of testicular cells.
Food Chem. Toxicol., 22: 123-131.
Gray, T.J.B., Beamand, J.A., Lake, B.G., Foster, J.R., & Gangolli,
S.D. (1982) Peroxisome proliferation in cultured rat hepatocytes
produced by clofibrate and phthalate ester metabolites. Toxicol.
Lett., 10: 273-279.
Haggard, H.W., Miller, D.P., & Greenberg, L.A. (1945) The amyl
alcohols and their ketones: Their metabolic fates and comparative
toxicities. J. Ind. Hyg. Toxicol., 27: 1-14.
Hamdoune, M., Duclos, S., Mounie, J., Santona, L., Lhuguento, J.C.,
Magdalou, J., & Gouda, H. (1995) In vitro glucuronidation of
peroxisomal proliferators: 2-ethylhexanoic acid enantiomers and their
structural analogs. Toxicol. Appl. Pharmacol., 131: 235-243.
Hardin, B.D., Schuler, R.L., Burg, J.R., Booth, G.M., Hazelden, K.P.,
MacKenzie, K.M., Piccirillo, V.J., & Smith K.N. (1987) Evaluation of
60 chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen., 7: 29-48.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9: 257.
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. Acta Pharmacol. Toxico., 27: 381-396.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fermentative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Hillbom, M.E., Franssila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Jpn.
J. Stud. Alcohol, 9: 101-106.
Hodge, H.C. (1943) Acute toxicity for rats and mice of 2-ethyl hexanol
and 2-ethyl hexyl phthalate. J. Proc. Soc. Exp. Biol. Med., 53:
20-22.
Hodgson, J. R. (1987) Results of peroxisome induction studies on
tri(2-ethylhexyl)trimellitate and 2-ethylhexanol. Toxicol. Ind.
Health, 3: 49-60.
Hodgson, J.R., Myhr, B.C., McKeon, M., & Brusick, D.J. (1982)
Evaluation of di-(2-ethylhexyl)phthalate and its major metabolites in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 4: 388.
Institut Français de Recherches et Essais Biologiques (IFREB) (1975)
Acute toxicity of 4 methlynonanoic acid in rats. Private
communication to FEMA.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Johannsen, E. & Purchase, I. F. (1969) Kafficorn malting and brewing
studies: XXI. The effect of fusel oil of Bantu beer on rat liver
S. Afr. Med. J., 43: 326-328.
Keith, Y., Cornu, M.C., Canning, P.M., Foster, J., Lhuguennot, J.C., &
Elcombe, R.C. (1992) Peroxisome proliferation due to di(2-ethylhexyl)
adipate, 2-ethylhexanol and 2-ethylhexanoic acid. Arch. Toxicol.,
66: 321-326.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Kirby, P.E., Pizzarello, R.F., Lawlor, T.E., Haworth, S.R., & Hodgson,
J.R. (1983) Evaluation of di-(2ethylhexyl)phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
Knaak, J.B., Kozbelt, S.J., & Sullivan, L.J. (1966) Metabolism of 2-
ethylhexyl sulfate by the rat and rabbit. Toxicol. Appl.
Pharmacol., 8: 369-379.
Lake, B.G., Gangolli, S.D., Grasso, P., & Lloyd, A.G. (1992) Studies
on the hepatic effects of orally administered
di-(2-ethylhexyl)phthalate in the rat. Toxicol. Appl. Pharmacol.,
32: 355-367.
Lester, D. & Benson, G.D. (1970) Alcohol oxidation in rats inhibited
by pyrazole, oximes, and amides. Science, 169: 282-284.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J., & Paulson, G.D.
ed. Intermediary xenobiotic metabolism in animals. Taylor and
Francis, London, pp. 119-138..
Lewis, R.J. (1989) Food additives handbook. Van Nostrand Reinhold, New
York, p. 259.
McMahon, R.E., Cline, J.C., & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39: 682-693.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45: 497-504.
Moreno, O.M. (1988) Private communication to FEMA.
Moreno, O.M. (1977) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Unpublished
report to RIFM.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of sctrucural class with
no-observed-effect-levels: a proposal for establishing a threshold of
concern. In Press.
Narotsky, M.G., Francis, E.Z., & Kavlock, R.T. (1994) Developmental
toxicity and structure-activity relationships of aliphatic acids,
including dose-response assessment of valproic acid in mice and rats.
Fundam. Appl. Toxicol., 22: 251-265.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
National Institute for Occupational Safety and Health (NIOSH) (1976)
In: Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects
of chemical substances. NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Institute for Occupational Safety and Health (NIOSH) (1991)
Toxic Param. Ind. Tox. Chem. Under single exposure (1982). Cited in:
Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects of
chemical substances (1976). NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Toxicology Program (NTP) (1991) Final report on the
developmental toxicity of 2 ethylhexanol in CD-1-Swiss mice. National
Toxicology Program, Research Triangle Park, NC, USA (PB91-185900).
Obe, G. & Beek, B. (1979) Mutagenic activity of aldehydes. Drug
Alcohol Depend., 4: 91-94.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181
Opdyke, D.L.J. & Leitizia, C. (1982) Frangrance raw materials
monographs. Food Cosmet. Toxicol., 20: 739-740.
Owen, G. & Meyer, F.J. (1971) Private communication to FEMA.
Phillips, B.J., James, T.E.B., & Gangolli, S.D. (1982) Genotixicity
studies of di(2ethylhexyl)phalate and its metabolites in CHO cells.
Mutat. Res., 102: 297-304.
Pietruszko, R., Crawford, K., & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenase from human liver, horse
liver, and yeast towards saturated and 2-enoic alcohols and aldehydes.
Arch. Biochem. Biophys,. 159: 50-60.
Posternak, J.M. Linder, A., & Vodoz, C.A. (1969) Subchronic feeding
study of 2-methylhexanoic acid in rats. Food Chem. Toxicol., 7:
405-407.
Prunonosa, J., Sagrista, M.L., & Bozal, J. (1991) Inactivation
mechanism of low-Km rat liver mitochondrial aldehyde dehydrogenase
by cyanamide in vitro. Drug Metab. Dispos., 19: 787-792.
Purchase, I.F.H. (1969) Studies in kafficorn malting and brewing:
XXII. The acute toxicity of some fusel oils found in bantu beer.
S. Afr. Med. J., June 21(Suppl.).
Putman, D.L., Moore, W.A., Schechtman, L.M., & Hodgson, J.R. (1983)
Cytogenic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 5: 227-231.
Rhodes, C., Soames, T., Stonard, M.D., Simpson, M.G., Vernall, A.J., &
Elcombe, C.R. (1984) The absence of testicular atrophy and in vivo and
in vitro effects on hepatocyte morphology and peroxisomal enzyme
activ. in male rats following the administration of several alkanols.
Toxicol. Lett., 21: 103-109.
Ritter, E.J., Scott, W.J., Randall, J.L., & Ritter, J.M. (1987)
Teratogenicity of di(2-ethylhexyl) phthalate, 2-ethylhexanol,
2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine.
Teratology, 35: 41-46.
Rushbrook, C.J., Jorgenson, T.A., & Hodgson, J.R. (1982) Dominant
lethal study of di-(2-ehtylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
Saito, M. (1975) Studies on the metabolism of lower alcohols.
Nichidai Igaku Zasshi, 34: 569-585.
Scala, R.A. & Burtis, E.G. (1973) Acute toxicity of a homologous
series of branched-chain primary alcohols. Am. Ind. Hyg. Assoc. J.,
34: 493-499.
Schafer, E.W. Jr. & Bowles, W.A. Jr. (1985) The acute toxicity and
repellence of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14: 111-129.
Schmidt, P., Gohlke, R., & Rothe, R. (1973) Zur toxizitat einiger
C8-aldehyde und alkohole. Z. Gesamte Hyg. Grenzgeb., 19: 485-490.
Scott, W.J. Jr., Collinfs, M.D., & Heinz, N. (1994) Pharmacokinetic
determinants of embryotoxicity in rats associated with organic acids.
Environ. Health Perspect., 102: 97-101.
Seed, J.L. (1982) Mutagenic activity of phthalate esters in bacterial
liquid suspension assays. Environ. Health Perspect., 45: 111-114.
Shaffer, C.B., Carpenter, C.P., & Smyth, H.F. (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. Ind. Hyg. Toxicol., 27: 130-1135.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report to RIFM.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S., & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27: 400-419.
Sjöberg, P., Bondesson, U., Gray, T.J.B., & Ploen, L. (1986) Effects
of di-(2-ethylhexyl)phthalate and five of its metabolites on rat
testis in vivo and in vitro. Acta Pharmacol. Toxicol., 58:
225-233.
Smyth, H.F. Jr., Carpenter, C.P., & Weil, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4: 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S., & Possani U.C. (1954)
Range finding toxicity data: List V. Arch. Ind. Hyg., 10: 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, U.C., Striegel,
J.A., & Nycum, J.S. (1969) Range finding toxicity data: List VII.
Am. Ind. Hyg. Assoc. J., 30: 470-476.
Stofberg, J. & Gruncschober, F. (1987) The consumption ratio and Food
Predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Szybalski, W. (1958) Special microbial systems: II. Obeservations on
chemical mutagenesis in microorganisms. Ann. NY Acad. Sci., 76:
475-489.
Tomita, I., Nakamura, Y., Aoki, N., & Inui, N. (1982)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
Tyl, R.W., Fisher, L.C., Kubena, M.F., Vrbanic, M.A., Gingell, R.,
Guest, D. Hodgson, J.R., Murphy, S.R., Tyler, T.R. and Astill, B.D.
(1992) The developmental toxicity of 2-ethyl-1-hexanol applied
dermally to pregnant Fischer 344 rats. Fundam. Appl. Toxicol., 19:
176-185.
Union Carbide Data Sheet, 2/4/59. Cited in: NIOSH ed. Registry of
toxic effects of chemical substances (1991).
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V., & Thomas, P.T. (1989)
Immunotoxicity assessment of flavoring ingredients using a rapid and
economical screen. Toxicologist, 9: 206.
Walkenstein, S.S. & Weinhouse, S. (1953) Oxidation of aldehydes by
mitochondria of rat tissues. J. Biol. Chem., 200: 515-523.
Weiner, H. (1980) Aldehyde oxidizing enzymes. In: Jakoby, W.B. ed
Enzymatic basis of detoxication. Academic Press, New York, Vol. I,
pp.261-275.
Wild, D., King, M.T., Gocke, E., & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, basc and micronucleus tests. Food chem.
Toxicol., 21(6): 707-719.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall, Ltd, London, UK, pp. 4-5.
Woodruff, R.C., Mason, J.M., Valencia, R., & Zimmering, S. (1985)
Mutagenesis testing in Drosophilia: V. Results of 53 coded compounds
tested for the National Toxicology Program. J. Environ. Mutagen., 7:
677-702.
World Health Organization (1987) Environmental Health Criteria 65:
Butanols - Four isomers: 1-butanol, 2-butanol, tert-butanol, and
isobutanol. World Health Organization, International Programme on
Chemical Safety, Geneva
Yasuhara, A., Fuwa, K., & Jimbu, M. (1982) Variation in concentration
of odorous components of pig feces with growth. Agric. Biol. Chem.,
46: 1381-1383.
Zabin, I. & Bloch, K. (1951) Studies on the utilization of isovaleric
acid in cholesterol synthesis. J. Biol. Chem., 192: 267-273.
Zeiger, E., Haworth, S., Mortelmans, K., & Speck, W. (1985)
Mutagenicity testing of di(2 ethylhexyl)phthalate and related
chemicals in salmonella. Environ. Mutagen., 7: 213-232.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 2: 1-158.
When rats and rabbits were administered 2-ethylhexanol-C14 or
2-ethylhexanol-C14 sulfate either orally or by intraperitoneal (i.p.)
injection, the principal metabolites included unchanged alcohol and
the sulfate ester, as well as hydroxylated 2-ethylhexanol and the
glucuronide conjugate of 2-ethylhexanoic acid (Knaak et al., 1966).
2-Ethylbutyric acid was administered orally and by subcutaneous
injection at a dose of approximately 1 gram to rabbits and 100 mg (as
the sodium salt) to rats. The acid was primarily excreted unchanged in
the urine as the glucuronic acid conjugate (Dziewiatowski et al.,
1949). In dogs, 2-ethylbutyric acid undergoes ß-oxidation and
decarboxylation to yield 2-pentanone (Deuel, 1957).
In conclusion, branched-chain alcohols, aldehydes and carboxylic
acids with bulky (ethyl, propyl, etc.) alpha-alkyl substituents are
metabolized by omega- and omega-1-oxidation to yield polar metabolites
capable of excretion in the urine. At high-dose levels these oxidation
pathways may be saturated, in which case, the corresponding acid may
undergo conjugation with glucuronic acid.
2.1.3 Toxicological studies
2.1.3.1 Acute toxicity
Acute toxicity studies have been reported for 20 of the 25
saturated aliphatic branched-chain primary alcohols, aldehydes and
acids in this group and are summarized in Table 2. The acute oral
toxicity of the group is demonstrated by oral LD50 values of >2000
mg/kg bw for the alcohols; >3200 mg/kg bw for the aldehydes; and
>1000 mg/kg bw for the carboxylic acids, except for isobutyric and
2-methylbutyric acid, which are 280 mg/kg bw, and 3-methylpentanoic
acid which is >700 mg/kg bw. The rat oral LD50 values for the three
alpha-ethyl-substituted substances 2-ethylbutyraldehdye,
2-ethylbutyric acid and 2-ethyl-1-hexanol are 3980 mg/kg bw, 2200
mg/kg bw and 2050-7100 mg/kg bw, respectively.
2.1.3.2 Short-term toxicity
The results of short-term toxicity studies with a
methyl-substituted alcohol (isobutyl alcohol), a structurally related
alcohol (isoamyl alcohol), and two methyl-substituted carboxylic acids
(isovaleric acid and 2-methylhexanoic acid) are summarized in Table 3
and described below. Short-term oral studies for 2-ethylbutyric acid
and 2-ethyl-1-hexanol are summarized in the section on
alpha-ethyl-substituted substances.
Table 2. Acute toxicity studies for saturated aliphatic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Isobutyl alcohol rat M/F oral 2640-3100 Smyth et al., 1954; WHO, 1987
M/F gavage 2650-3100 Purchase, 1969
mouse oral 3500 WHO, 1987
Isobutyraldehyde rat M/F oral 3730 Smyth et al., 1954
Isobutyric acid rat NR oral 280 Lewis, 1989
2-Methylbutyraldehyde rat NR oral 8570 Opdyke & Leitizia, 1982
2-Methylbutyric acid rat M/F oral 280-2200 Moreno et al., 1982; Lewis, 1989;
mouse M/F oral 1238 Schafer & Bowles, 1985
2-Ethylbutyraldehyde rat M/F oral 3980 Smyth et al., 1951
2-Ethylbutyric acid rat M/F oral 2200 Smyth et al., 1954
Isoamyl alcohol2 rat M/F oral 5700 Smyth et al., 1954
gavage 1300-4000 Purchase, 1969
3-Methylbutyraldehyde rat M/F oral 7166 Moreno, 1988
Isovaleric acid rat NR oral 2000; >3200 Fassett, 1963; NIOSH, 1976
2-Methylvaleric acid rat M/F oral 1600-3200 Smyth et al., 1954; ACGIH, 1989
3-Methylpentanoic acid rat NR oral >700 Vollmuth et al., 1989
3-Methyl-1-pentanol rat M/F oral >2000 Engler & Bahler, 1982
2-Ethyl-1-hexanol rat M/F oral 2050-7100 Hodge, 1943; Shaffer et al., 1945;
Smyth et al., 1969; Scala & Burtis, 1973;
Schmidt et al., 1973; Albro, 1975; Dave & Lidman, 1978
Table 2. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3,5,5-Trimethyl-1-hexanol rat M/F oral 2300 Moreno, 1977
3,5,5-Trimethylhexanal rat M/F oral 3240 Moreno, 1975; Opdyke & Leitizia, 1982
2-Methyloctanal rat M/F oral >5000 Moreno, 1977
3,7-Dimethyl-1-octanol rat M/F oral 5000 Shelanski & Moldovan, 1973; Moreno, 1977
4-Methylnonanoic acid rat NR oral 3700 IFREB, 1975
2-Methylundecanal rat M/F oral >5000 Owen & Meyer, 1971
4-Methylpentanoic acid rat NR oral 2050->3200 Smyth et al., 1954; Fassett, 1963; NIOSH, 1976;
Union Carbide, 1991;
mouse NR oral 5000 NIOSH, 1991
1 M = male; F = female; NR = Not reported.
2 Structurally related substance.
Table 3. Short-term and long-term toxicity studies for saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids
Substance Species (Sex) Route Duration NOEL Reference
(mg/kg bw
per day)
Short-term studies
Alcohols
Isobutyl alcohol rat (M/F) oral 90 days 1450 BASF, 1992
rat oral 4 months >0.126 nmol/g Hillbom et al., 1974
bw/day3 Johannsen &
rat (M/F) oral 53-56 weeks 2003 Purchase, 1969
Isoamyl alcohol2 rat (M/F) gavage 17 weeks 10003 Carpanini et al., 1973
rat (M/F) oral 56 weeks 20003 Johannsen & Purchase, 1969
2-Ethyl-1-hexanol mouse gavage 11 days 100 Astill et al., 1996a
mouse gavage 90 days 125 Astill et al., 1996a
mouse food 11 days 1150-4450 Astill et al., 1993
rat gavage 14 days 1303 Rhodes et al., 1984
NR Lake et al., 1975
rat oral 7 days 100 Astill et al., 1996a
rat gavage 11 days 125 Astill et al., 1996a
rat gavage 90 days <500-540 Astill et al., 1993
rat oral 11 days
Carboxylic Acid
2-Ethylbutyric acid rat oral 90 days 3003 Amoore et al., 1978
Isovaleric acid rat oral 90 days 25003 Amoore et al., 1978
2-Methylhexanoic acid rat (M/F) oral 90 days 33 Posternak et al., 1969
Long-term/ Carcinogenicity studies
2-Ethyl-1-hexanol mouse (M/F) gavage 540 days 200 Astill et al., 1996b
rat (M/F) gavage 730 days 50 Astill et al., 1996b
Table 3 (continued)
1 M = Male; F = Female; NR = not reported.
2 A structurally related branched-chain alcohol.
3 The study was performed at a single dose level or multiple dose levels that produced no adverse effects and, therefore, a NOEL was not
determined. The NOEL is probably higher than the reported dose level that produced no adverse effects.
a) Methyl substituted aliphatic alcohols, aldehydes and carboxylic
acids
i) 2-Methyl-1-propanol (isobutyl alcohol)
Groups of eleven 4-month-old male Wistar rats were given 1 M
solutions of isobutyl alcohol as their only drinking fluid for 4
months. Control animals received tap water. An ordinary diet was
provided for all animals. The level of isobutyl alcohol consumption
was reported to be 0.126 nmole/g bw/day at 120 days. At necropsy,
there was no evidence of hepatic steatosis, fibrosis or inflammation
(Hillbom et al., 1974).
Groups of 20 male and 20 female Wistar albino rats were given a
mixture of fusel oils including 0.2% isobutyl alcohol, which was
calculated (FDA, 1993) to provide an approximate daily intake of 200
mg/kg bw/day, as their sole drinking source for 53-56 weeks. Control
animals were given tap water. There were no statistically significant
differences in body or liver weights of treated animals as compared to
controls. Liver enzymes were determined at 2- to 4-week intervals and
revealed no significant differences in activity and protein content of
the liver between test and control animals. Histological examination
was performed on specimens of liver, kidney, heart, spleen and lung
and revealed no significant abnormalities (Johannsen & Purchase,
1969).
Groups of 10 male and 10 female Wistar rats were given 2-methyl-1-
propanol (i.e. isobutyl alcohol) in their drinking water for 3 months.
The test substance was administered in concentrations of 0, 1000, 4000
or 16 000 mg/litre, which was reported to correspond to approximate
dose levels of 0, 60, 340 or 1450 mg/kg bw per day. Food and drinking-
water consumption and body weight gain were not affected by the test
substance. All animals tested were free from adverse clinical effects.
Haematology and clinical chemistry parameters were measured and
revealed no treatment-related adverse effects. Gross pathology and
histopathological examinations were normal for all animals except for
testicular atrophy, which was observed in two males in the high-dose
group. Diffuse tubular generation and hyperplasia of the Leydig cells
were reported in the two animals. The authors concluded that the
absence of similar effects in other male rats indicated that the
effect was most likely unrelated to the test material. The authors
reported that the results of this study demonstrate a lack of toxicity
associated with administration of 2-methyl-1-propanol in the drinking-
water of rats, and that the NOEL is >1450 mg/kg bw per day (BASF,
1992). The NOEL is >100 000 times the estimated daily per capita
intake1 ("eaters only") of 4.85 g/kg bw of isobutyl alcohol from
its use as a flavouring substance in the USA and 8.79 g/kg bw of
isobutyl alcohol in Europe.
ii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage providing daily dose levels
of 0, 150, 500 or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight statistically significant reduction in body-weight
gain which was associated with reduced food intake. Over the entire
period of the study, however, there was no statistically significant
reduction in mean food intake. Examination of haematology, serum
analyses, urinalysis, renal concentration tests and organ weights
revealed no treatment-related effects. The animals were examined for
macroscopic abnormalities, and the major organs were weighed.
Microscopic examination was performed on several tissues of the
control and high-dose animals. No treatment-related abnormalities were
observed (Carpanini et al., 1973).
Groups of 20 male and 20 female Wistar albino rats were given a 2%
solution of isoamyl alcohol, which was calculated (FDA, 1993) to
provide an approximate daily intake of 2000 mg/kg bw per day, as their
sole drinking source for 53-56 weeks. Control animals were given tap
water. There were no statistically significant differences in body or
liver weights of treated animals as compared to controls. Liver
enzymes were determined at 2- to 4-week intervals and revealed no
significant differences in ADH, GOT or GPT activity and protein
content of the liver. Histological examination was performed on
specimens of liver, kidney, heart, spleen and lung and revealed no
significant abnormalities (Johannsen & Purchase, 1969).
iii) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0 or 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to provide a daily intake of 0 or 2500 mg/kg
bw. The rats were monitored for survival rates, body weight changes,
food intake, blood and urine analysis, organ weights and histology. At
2500 mg/kg bw per day, there were no observed changes in the rats
(Amoore et al., 1978).
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1995). Intake (mg/kg bw/day)
calculated as follows: [(mg/day)/body weight], where body weight = 60
kg. Slight variations may occur from rounding off.
iv) 2-Methylhexanoic acid
In a limited 90-day feeding study, 2-methylhexanoic acid was fed
to male and female Charles River CD rats at a dose of approximately 3
mg/kg bw/day. Observations included mortality rates, food intake, body
weight, haematology, blood urea, organ weights and histopathology. The
NOEL was 3 mg/kg bw dose level (Posternak et al., 1969) which is
>10 000 times the estimated daily per capita intake ("eaters only")
of 0.04 g/kg bw 2-methylhexanoic acid from use as a flavouring
substance in the USA and 0.24 g/kg bw from its use in Europe.
b) alpha-Ethyl substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
Eleven (11-) day and 90-day studies were conducted in B6C3F1 mice
as part of the dose selection process for an 18-month study (see
section 2.1.3.3). In the 11-day gavage study, groups of ten B6C3F1
mice of each sex were administrated nine applications of 0, 100, 330,
1000 or 1500 mg 2-ethylhexanol/kg bw per day. Mice in the high-dose
group exhibited effects such as ataxia, lethargy, loss of
consciousness, death in four females, increased absolute and relative
lever and stomach weights, macroscopic foci in the forestomach, and
microscopic lesions in the forestomach, liver, testes and kidneys.
These lesions consisted of hyperkeratosis and focal or multifocal
acanthosis and inflammatory oedema in the submucosa in most mice,
focal or multifocal ulceration of the mucous membrane in a few mice,
hypertrophy of hepatocytes in all mice, focal necrosis of liver cells
in one male and one female mouse, centrilobular fatty infiltration in
female mice that died intercurrently, tubular giant cells in
testicular tubules of two mice, and renal cortex tubular dilation and
nephrosis in mice that died intercurrently. Mice receiving the dose of
1000 mg/kg bw per day exhibited similar symptoms to those observed at
the highest dose (1500 mg/kg bw per day), but to a lesser degree,
while four mice (two male, two female) receiving 330 mg 2-ethyl
hexanol/kg bw per day had acanthosis in the mucous membrane of the
forestomach. No treatment-related adverse effects were observed in the
mice administered 100 mg 2-ethylhexanol/kg bw per day (Astill
et al., 1996a).
In the 90-day study, mice (number per group not reported) received
doses of 0, 25, 125, 250 or 500 mg 2-ethylhexanol/kg bw per day. At
the highest dose level, the treatment-related effects observed
included increased relative stomach weights in male mice and focal or
multifocal acanthosis of the forestomach mucosa in two male and one
female mouse. Increased relative stomach weights were observed in male
mice receiving 250 mg/kg bw per day. No treatment-related effects were
reported in mice of the 125 or 25 mg/kg bw per day dose level. Based
on the results of the 11- and 90-day studies, dose levels of 50, 200
or 750 mg 2-ethylhexanol/kg bw per day were chosen for the 18-month
mouse study (Astill et al., 1996a).
An 11-day mouse study was conducted using administration of
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of 10 male and 10 female B6C3F1 mice per group were used with
concentrations of microencapsulated material in the diet of 0.48,
0.96, 1.44 or 2.88%. The microcapsules contained 45.8% 2-ethylhexanol;
therefore, 2-ethylhexanol concentrations in the diet were 0.22, 0.44,
0.66 or 1.32%, respectively. These concentrations were reported to be
equal to doses of 550, 1150, 1800 or 4450 mg/kg bw per day for males
and 750, 1750, 2650 or 5750 mg/kg bw per day for females. The authors
reported that diet spillage did occur, but this was not quantified in
the treated groups of mice. As a result, the mg/kg bw per day dosage
calculations would not account for spillage and may represent
exaggerated 2-ethylhexanol intakes (Astill et al., 1993).
Significantly decreased body weight gains were observed at the
high-dose level for females throughout the test period and for males
at day 10 of the test period and at the 0.66% level in males at day 10
of the test period. No other treatment-related findings were reported.
The authors concluded the NOEL for male mice was in the range of 0.96
to 1.44% microencapsulated material (0.44 to 0.66% 2-ethylhexanol or
reportedly 1150 to 1800 mg/kg bw per day) and the NOEL for female mice
was in the range of 1.44 to 2.88% microencapsulated material (0.66 to
1.32% 2-ethylhexanol or reportedly 1800 to 4450 mg/kg bw per day)
(Astill et al., 1993). The NOELs reported in this study are higher
than the NOEL of 100 to 330 mg 2-ethylhexanol/kg bw per day reported
in the 11-day gavage study in B6C3F1 mice (Astill et al., 1996a),
most likely as a result of the different modes of administration. The
lack of correction for diet spillage in the microencapsulation study
may also have been a factor.
To study the effect of 2-ethylhexanol on rat liver and testes,
Rhodes et al. (1984) administered 1 mmol 2-ethylhexanol/kg bw per
day (approximately 130 mg/kg bw per day) to five Wistar rats by gavage
for 14 days. 2-Ethylhexanol administration did not produce testicular
atrophy, hepatomegaly, peroxisome proliferation or hypolipidaemia in
the rats. Lake et al. (1992) orally administered 1335 mg
2-ethylhexanol/kg bw per day to six male Wistar rats for seven days.
The authors reported significantly increased relative liver weights,
increased microsomal biphenyl 4-hydroxylase activity, increased
alcohol dehydrogenase activity in the centrilobular area of the liver
lobule, increased number of microbodies, and dilatation of the smooth
endoplasmic reticulum in the livers. No effect on the activities of
aniline 4-hydroxylase or mitochondrial succinate dehydrogenase was
observed.
In an 11-day gavage study, groups of ten male and ten female
Fischer F344 rats were administered doses of 0, 100, 330, 1000 or 1500
mg 2-ethylhexanol/kg bw per day. Adverse effects, including reduction
in food consumption and body weight gain, ataxia, lethargy, changes in
blood chemistry, effects on absolute (increased liver and stomach,
decreased spleen, brain, and adrenal) and relative (increased stomach,
liver, kidney, brain, adrenal, and lung, decreased spleen) organ
weights, gross lesions in the forestomach and microscopic findings in
the forestomach, liver (high-dose only), spleen and thymus, were
observed in the rats at the highest two dose levels. At the 330 mg/kg
bw per day dose, female rats exhibited increased relative kidney
weights, as well as microscopic lesions in the forestomach of one
female rat and in the thymus of both sexes (it was not stated whether
or not these lesions were treatment-related). No treatment-related
adverse effects were observed at 100 mg/kg bw per day (Astill et
al., 1996a).
In the 90-day study, groups of ten male and ten female Fischer
F344 rats were administered doses of 0, 25, 125, 250 or 500 mg
2-ethylhexanol/kg bw per day by gavage. Reduced body weight and body
weight gain, changes in blood chemistry (consisting of reduced
cholesterol, glucose, alanine aminotransferase and alkaline
phosphatase, levels and increased reticulocytes, total protein and
albumin levels), increased absolute and relative liver and stomach
weights and gross lesions in the forestomach were observed in the rats
receiving 500 mg 2-ethylhexanol/kg bw per day. Also observed in the
high-dose rats were microscopic changes consisting of focal or
multifocal acanthosis in the mucosa of six rats (one male, five
female) and whole mucose acanthosis and submucosa degeneration and
oedema in one male rat. In addition, a number of high-dose rats with
fatty infiltration in the liver, as well as a lower grade of fat
deposition in the liver, were observed. At 250 mg/kg bw per day,
changes in the blood chemistry (consisting of reduced glucose,
alkaline phosphatase and alanine aminotransferase levels), increased
relative liver and stomach weights, and a lower grade of fat
deposition in the liver cells of the male rats were observed. No
treatment-related adverse effects were observed at the 25 or 125 mg/kg
bw per day dose levels. Based on the results of the 11- and 90-day
studies, dose levels of 50, 150 or 500 mg 2-ethylhexanol/kg bw per day
were chosen for a two-year study (Astill et al., 1996a).
An 11-day rat study was also conducted by administering
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of ten male and ten female Fischer rats per group were fed
concentrations of microencapsulated material in the diet of 1, 2, 3 or
6%. The microcapsules contained 45.8% 2-ethylhexanol; therefore,
2-ethylhexanol concentrations in the diet were 0.46, 0.92, 1.38 or
2.75%, respectively. These concentrations were reported to be equal to
doses of 500, 980, 1430 or 2590 mg/kg bw per day for males and 540,
1060, 1580 or 2820 mg/kg bw per day for females. A vehicle control
group receiving placebo microcapsules was employed. Decreased food and
water (top three doses only) consumption was observed at all dose
levels at some point in the study period with decreased body weights
reported for both sexes at the top dose and in females at day four in
the second highest dose. Changes in blood chemistry, consisting of
decreased cholesterol, triglycerides and alanine aminotransferase
levels (all doses), decreased glucose, reticulocyte and mean
corpuscular volume levels (high-dose only), decreased platelet level
(top two doses only), increased total protein level (top three doses),
increased erythrocyte level (high-dose only) and increased absolute
and relative stomach and/or liver weights were observed to some extent
in all dose groups. Varying degrees of hypertrophy of the hepatocytes
were reported in the top three dose groups. The authors reported the
NOEL to be less than 0.46% in the diet (500 or 540 mg/kg bw per day
for male and female rats, respectively) (Astill et al., 1993).
ii) 2-Ethylbutyric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0, 0.62, 1.25 or 10% 2-ethylbutyric acid in the
diet, equivalent to 0, 300, 625 or 5000 mg/kg bw per day,
respectively. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analysis, organ weights and
histology. At the 300 mg/kg bw dose level, there were no observed
changes in the rats. At both the 625 and 5000 mg/kg bw dose levels,
slight body weight changes were observed within the first 14 days of
the study (Amoore et al., 1978).
2.1.3.3 Long-term toxicity/carcinogenicity
The results of long-term toxicity/carcinogenicity studies on
2-ethyl-1-hexanol are summarized in Table 3 and described below.
a) Mice
Groups of 50 male and 50 female B6C3F1 mice were administered
2-ethylhexanol by gavage at doses of 0, 50, 200 or 750 mg/kg bw per
day, five days per week for 18 months. The vehicle consisted of doubly
distilled water containing approximately 5 mg Polyoxyl 35 Castor Oil
(Cremophor(R) EL) per 100 ml. Two control groups of 50 mice of each
sex were employed, one receiving doubly distilled water containing
approximately 5 mg Cremophor(R) EL/100 ml. The use of Cremophor(R)
EL was reported to have no effect on the outcome of the study. Food
consumption, body weights and haematological parameters were examined
at specific intervals during the study. At the end of the study, gross
and histopathological examinations were conducted.
No treatment-related adverse effects were observed in the mice
receiving 50 or 200 mg 2-ethylhexanol/kg bw per day. At the 750 mg/kg
bw per day dose level, body weight gain reductions of approximately 26
and 24% in males and females, respectively, were reported. These
weight gain reductions were associated with a significant reduction in
food consumption. Both male and female mice of the high-dose group
showed increased mortality compared to the vehicle control mice (i.e.
30% in the treated mice compared to 4 and 8% in the vehicle control
group male and female mice, respectively). With respect to
haematological parameters, both a slight increase in polymorphonuclear
neutrophils and a slight decrease in lymphocytes were observed in the
mice of the high-dose group. A slight, statistically insignificant,
increase in focal hyperplasia of the forestomach epithelium was also
observed in the high-dose group mice. A slight increase in
hepatocellular carcinomas in the female mice of the high-dose group
was determined to have no biological relevance. 2-Ethylhexanol showed
no evidence of carcinogenicity in this study (Astill et al., 1996b).
b) Rats
Groups of 50 male and 50 female Fischer F344 rats were
administered 2-ethylhexanol by gavage at doses of 0, 50, 150 or 500
mg/kg bw per day, 5 days per week for 2 years. The vehicle consisted
of doubly distilled water containing approximately 5 mg Polyoxyl 35
Castor Oil (Cremophor(R) EL) (as an emulsifier) per 100 ml. Two
control groups of 50 mice of each sex were used, one receiving doubly
distilled water containing 5 mg Cremophor(R) EL/100 ml. The use of
Cremophor(R) EL was reported to have no effect on the outcome of the
study. Food consumption, body weights, and haematological parameters
were examined at specific intervals during the study. At the end of
the study, gross and histopatho-logical examinations were conducted.
No treatment-related adverse effects were observed at the 50 mg/kg
bw per day dose level. At the 150 mg/kg bw per day dose level, rats
exhibited a body weight gain reduction of approximately 16% in males
and 12% in females. In addition, the rats also displayed a slightly
increased incidence of clinical signs, such as poor general condition,
laboured breathing, piloerection, and/or smearing of the genital
region with urine. A definite increase in the number of rats and/or a
definite increase in the incidence of clinical signs (same signs as
mentioned for the mid-dose group) was observed in the high-dose group
rats. Mortality was increased in the female rats of the high-dose
group (52% compared to 28% in the vehicle control) and both male and
female rats exhibited a reduction in the body weight gain (33% in
males and 31% in females). With respect to haematological parameters,
a slight increase in anisocytosis, predominantly microcytosis in
males, was observed after 12 months, but not after 18 or 24 months.
The incidence of bronchopneumonia was significantly increased in both
sexes at the high-dose level. This effect was found to be due to the
aspiration of the stomach contents, most likely resulting from the
poor general condition of the animals. 2-Ethylhexanol showed no
evidence of carcinogenicity in this study (Astill et al., 1996b).
2.1.3.4 Genotoxicity
The results of genotoxicity studies on saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes, and carboxylic acids are
summarized in Table 4.
Table 4. Genotoxicity studies of saturated aliphatic acyclic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Test system Test object Dose level Results Reference
Alcohols
Isobutyl alcohol Modified Ames test S. typhimurium TA98, TA100, 100 to 10 000 g/plate1,2 Negative Zeiger et
TA1535 TA97 and TA1537 al., 1988
Modified Ames test S. typhimurium TA100, TA98, 5 to 5000 mg/plate1 Negative Shimizu et
TA1535, TA1537 and TA1538; al., 1985
E. coli WP2 uvrA
2-Ethyl-1-hexanol 8-azaguanine resistance S. typhimurium TA100 0.5 to 1.5 mM Positive3 Seed, 1982
assay
Ames test4 S. typhimurium TA98, TA100, up to 2 ml in urine/plate Negative Divincenzo
TA1535, TA1537, TA1538 et al., 1985
Ames test S. typhimurium TA98, TA100, 0.01 to 1.0 µl/plate1 Negative Kirby et
TA1535, TA1537, TA1538 al., 1983
Ames test S. typhimurium TA98, TA100, 33 to 220 g/plate1,2 Negative Zeiger et
TA1535, TA1537 al., 1985
Ames test S. typhimurium TA98, TA100, 100 to 2000 g/plate1 Negative Agarwal et
TA1535, TA1537, TA1538, al., 1985
TA2637
L5178Y/TK+/- mouse L5178Y/TK+/- mouse 0.01-0.3 µl/ml1 Negative Kirby et
lymphoma assay lymphoma cells al., 1983
Rec-assay Bacillus subtilis 500 g/disk Negative Tomita et al.,
1982
CHO mutation assay Chinese hamster ovary cells 1.5-2.8 mM Negative Phillips et al.,
1982
Unscheduled DNA synthesis Primary rat hepatocytes not specified Negative Hodgson et
assay al., 1982
Aldehydes
Isobutyraldehyde Gradient plate technique S. typhimurium G46, TA1535, 0.1 to 1000 g/ml Positive5/ McMahon et
TA100, C3076, TA1537, D3052, Negative6 al., 1979
TA1538, and TA98; E. coli
WP2 and WP2 uvrA-
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Isobutyraldehyde Ames test S. typhimurium 3 µmol/plate1 Negative Florin et
(cont'd) al., 1980
Spot test TA98, TA100, TA1535, TA1537
Sister chromatid exchange adult human lymphocytes 0.002% Negative Obe & Beek,
1979
Paper-disk method E. coli Sd-4-73 0.01- 0.025 ml/disk Negative Szybalski,
1958
2-Methylbutyraldehyde Ames test S. typhimurium TA98, TA100, 0.03 to 30 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
Ames test S. typhimurium TA98, TA100 9 nmol to 0.9 mmol/plate1 Negative Aeschbacher
and TA102 et al., 1989
3-Methylbutyraldehyde Sister chromatid exchange Adult human lymphocytes 0.002 to 0.003% Negative Obe & Beek,
1979
Ames test S. typhimurium TA98, TA100 0.01 nmol to 1 mol/plate1 Negative Aeschbacher
and TA102 et al., 1989
Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
2-Methylpentanal Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
Spot test TA1535 and TA1537 al., 1980
2,6-Dimethyloctanal Ames test S. typhimurium TA98, TA100, up to 3.6 mg/plate Negative Wild et al.,
TA1535, TA1537, TA1538 1983
Carboxylic acids
Isobutyric acid Paper-disk method E. coli Sd-4-73 0.01 to 0.025 ml/disk Negative Szybalski,
1958
Cell mutagenesis assay mouse lymphoma 500 g/ml7,8 Positive Heck et al,
L5178Y TK +/- (weak) 1989
600 g/ml9,10 Negative
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Unscheduled DNA synthesis rat hepatocytes 1000 g/ml9,10 Negative Heck et al.,
assay 1989
Plate incorporation assay S. typhimurium TA98, 100, 75 000 g/plate9,10 Negative Heck et al.,
1535, 1537, 1538 1989
Rec-assay B. subtilis H17 rec+ and 19 g/disk Negative Oda et al.,
M45 rec- 1978
2-Ethyl-1-hexanol In vivo dominant lethal ICR/SIM mice 250, 500, 1000 mg/kg Negative Rushbrook et
assay bw/day for 5 days al., 1982
In vivo chromosomal Fischer 344 rat bone marrow 16.7, 58.4 or 167 mg/kg Negative Putman et
aberration assay cells bw/day for 5 days al., 1983
Isobutyraldehyde Sex linked recessive Drosophila melanogaster 80 000 ppm (feeding) Negative Woodruff et
lethal mutation test 50 000 ppm (injection) al., 1985
1 Both with and without metabolic activation
2 Metabolic activation in the form of S9 derived from rat and hamster
3 It is assumed that no S9 was used
4 Test performed on the metabolites of 2-ethyl-1-hexanol
5 Positive in G46, TA100, E. coli WP2, WP2 uvrA-
6 Negative in TA1535, TA1537, TA1538, TA98, C3076 and D3052
7 Without metabolic activation
8 Lowest active dose tested
9 With metabolic activation
10 Highest inactive dose tested
a) Methyl substituted aliphatic alcohols, aldehydes, and carboxylic
acids
i) Isobutyl alcohol
Isobutyl alcohol produced negative results in Ames tests with
Salmonella typhimurium strains TA97, TA98, TA100, TA1535, TA1537 at
concentrations of 100 to 10 000 g/plate (Zeiger et al., 1988).
Negative mutagenicity results were also obtained by Shimizu et al.
(1985) at dose levels of 5 to 5000 g/plate in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538, and
E. coli strain WP2 uvrA-.
ii) Branched-chain aldehydes
In Salmonella typhimurium, isobutyraldehyde (in strains TA98,
TA100, TA1535, TA1537), 2-methylbutyraldehyde (TA98, TA100, TA1535,
TA1537), 3-methylbutyraldehyde (TA98, TA100, TA1535, TA1537) and
2-methylpentanal (TA100, TA1535, TA1537) were negative in spot tests
at a concentration of 3 mol/plate with and without metabolic
activation (Florin et al., 1980). Similar results were reported for
2-methylbutyraldehyde and 3-methylbutyraldehyde in S. typhimurium
strains TA98, TA100 and TA102 at concentrations of 9.0 nmol/plate to
0.9 µmol/plate and 0.01 nmol/plate to 1 µmol/plate, respectively, with
and without metabolic activation (Aeschbacher, 1989). Florin et al.
(1980) subsequently subjected 2-methylbutyraldehyde to a quantitative
Ames test assessment at concentrations of 0.03 to 30 µmol/plate, and
found no evidence of mutagenicity in S. typhimurium strains TA98,
TA100 and TA1535, with and without metabolic activation.
Isobutyraldehyde gave negative results for frame-shift mutations in
S. typhimurium C3076, TA1537, D3052, TA1538 and TA98, and base
substitution mutations in S. typhimurium TA1535, and gave positive
results for base substitution mutations in S. typhimurium G46, TA100
and E. coli WP2 and WP2 uvrA-(McMahon et al., 1979). It was
non-mutagenic in E. coli Sd-4-73 (Szybalski, 1958). In an in vivo
study, isobutyraldehyde was negative for sex-linked recessive lethal
mutation in germ cells of Drosophila melanogaster by adult feeding
at 80 000 ppm and adult injection at 50 000 ppm (Woodruff et al.,
1985).
iii) Branched-chain carboxylic acids
Isobutyric acid was non-mutagenic in the Rec-assay with E.
coli Sd-4-73 at dose levels of 0.01 to 0.025 ml/disk (Szybalski,
1958) and in B. subtilis H17 and M45 at a dose of 19 g/ml (Oda,
1978). In a mouse lymphoma L5178YTK +/- assay, isobutyric acid had
negative results at a dose level of 600 g/ml with metabolic activation
and had weakly positive results at a dose of 500 g/ml without
metabolic activation (Heck et al., 1989). Isobutyric acid had
negative results in the Ames test plate incorporation assay with
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and
TA1538 at doses ranging up to 75 000 g/ml (Heck et al., 1989).
Isobutyric acid did not induce unscheduled DNA synthesis in rat
hepatocytes at doses of up to 1000 g/ml (Heck et al., 1989).
Based on a weight of evidence analysis, aliphatic acyclic
branched-chain primary alcohols, aldehydes and carboxylic acids are
not mutagenic or clastogenic in bacterial, in vitro or in vivo
systems. Sporadic positives were observed in some strains in three of
the approximately 20 bacterial assays conducted on members of this
group. Isobutyric acid was found in a single study to be mutagenic
in vitro in the presence of metabolic activation. However,
isobutyric acid was found to be negative in the rat UDS assay. The
mutagenic activity of isobutyric acid is not, therefore, likely to be
related to a mechanism involving direct binding to DNA. Consistent
with a lack of DNA damage, both isobutyraldehyde and
3-methylbutyraldehyde were negative in the sister chromatid exchange
assay. In addition, 2-ethyl-1-hexanol was found to be negative in two
assays for in vitro mutagenic activity (see 2-ethyl-1-hexanol
below). There is no evidence of mutagenic or clastogenic activity
in vivo in any of the compounds tested.
b) alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
There are several reports in the literature concerning the in
vitro testing of 2-ethylhexanol for mutagenicity in bacteria.
2-Ethylhexanol has shown no evidence of mutagenicity in the Ames test
with Salmonella typhimurium strains TA98, TA100, TA1535, TA 1537,
TA1538 or TA2637 with or without metabolic activation (Kirby et
al., 1983; Zeiger et al., 1985; Agarwal et al., 1985). Agarwal
et al. (1985) reported that 2-ethylhexanol was moderately cytotoxic
in most of the cultures tested, thereby having the potential to mask a
mutagenic effect. Weak dose-related mutagenic activity (maximum
increase in mutant frequency was only 3.5 times background) was
reported for 2-ethylhexanol in the Salmonella 8-azaguanine
resistance assay without activation at concentrations up to 1.5 mM.
2-Ethylhexanol was highly cytotoxic at concentrations that were
mutagenic (Seed, 1982).
Further Ames test results on the urinary metabolites of 2-ethyl-1-
hexanol, using S. typhimurium strains TA98, TA100, TA1537, TA1538
and TA1535, indicated no mutagenicity (Divincenzo et al.,
1985). The measurements were made with six male Sprague-Dawley rats
that were administered 1000 mg 2-ethyl-1-hexanol/kg bw/day by gavage
for 15 days. Urine samples were collected for use in the Ames assay at
doses of up to 2 ml per plate.
2-Ethylhexanol was not mutagenic in the L5178Y TK +/- mouse
lymphoma cell mutagenicity assay, either with or without metabolic
activation (Kirby et al., 1983), and produced negative results in
the rec-assay in Bacillus subtilis at a concentration of 500 g/disk
(Tomita et al., 1982). Negative results have also been reported for
2-ethylhexanol in a clastogenicity test on cultured Chinese hamster
ovary cells (Phillips et al., 1982) and in an unscheduled DNA
synthesis assay in primary rat hepatocytes (Hodgson et al., 1982).
In in vivo assays, negative results were reported in a dominant
lethal assay using oral doses of 250, 500 or 1000 mg 2-ethylhexanol/kg
bw administered for five consecutive days to ICR/SIM mice (Rushbrook
et al., 1982). 2-Ethylhexanol did not induce chromosomal aberrations
in bone marrow cells after oral administration of 0.02, 0.07 or 0.2
ml/kg bw per day (approximately 16.7, 58.4, 167 mg/kg bw per day
assuming a density of 0.8344) to groups of five Fischer 344 male rats
for five days (Putman et al., 1983).
The weight of evidence indicates that 2-ethylhexanol is not
mutagenic or genotoxic. Additionally, the urinary metabolites of
2-ethyl-1-hexanol were not mutagenic in the Ames tests. Negative
in vivo results were reported in a dominant lethal assay and in a
chromosomal aberration assay.
2.1.3.5 Reproductive toxicity
Gray & Beamand (1984) and Sjoberg et al. (1986) examined the
effect of 2-ethylhexanol on in vitro primary cultures of rat
testicular cells. No effect on the rate of germ-cell detachment from
Sertoli cells was observed at a 2-ethylhexanol concentration of
2x10-4 M.
Sjoberg et al. (1986) also examined the effect of 2-ethylhexanol
on rat testes in vivo. No testicular damage was observed in six
young male Sprague-Dawley rats orally administered 2.7 mmol 2-
ethylhexanol/kg bw per day (approximately 352 mg/kg bw per day) for 5
days.
2.1.3.6 Developmental toxicity
a) Mice
An oral developmental toxicity study was conducted using CD-1 mice
(NTP, 1991). Groups of 28 plug-positive mice were exposed to
microencapsulated 2-ethylhexanol at concentrations of 0, 0.009, 0.03
or 0.09% in the diet throughout gestation (days 0 to 17). These
concentrations were reported to be equal to 0, 17, 59 or 191 mg
2-ethylhexanol/kg bw per day, respectively, based on food consumption.
No evidence of maternal or developmental toxicity was observed in the
study. There were no treatment-related effects with respect to the
number of corpora lutea, uterine implantation sites, pre- and
post-implantation sites, pre- and post-implantation loss, sex ratio or
live fetal body weight, or to the incidence of individual, external,
visceral, skeletal or total malformations or variations.
Hardin et al. (1987) examined the developmental toxicity of
orally administered 2-ethylhexanol in pregnant mice. Fifty female CD-1
mice were administered by gavage a dose of 1525 mg 2-ethylhexanol/kg
bw per day on gestation days 6 to 13. A concurrent control group was
employed. Maternal mortality in the study was 35%, with significant
reductions in maternal weight gain and in the number of viable litters
being observed. Significant reductions in the treated group were also
reported for the following parameters: number of liveborn per litter,
percentage of survival of the pups, and the birth weight and weight
gain of the pups. The results of this study are of questionable
significance due to the presence of maternal toxicity and are
difficult to interpret due to the fact that only one dose level was
employed.
b) Rats
Groups of ten pregnant Wistar rats were administered by gavage
doses of 130, 650 or 1300 mg 2-ethylhexanol/kg bw per day on gestation
days 6 to 15. Two control groups were included, one that received
doubly distilled water and a second that received doubly distilled
water containing the emulsifier (0.005% Cremophor(R) EL) used as the
vehicle in the treated groups. On day 20 of gestation, the rats were
killed.
Significant maternal toxicity was observed in the high-dose rats.
These rats exhibited significant reductions in food consumption, body
weights and body weight gain. On days 6 to 10, a body weight loss was
reported. Six of the rats in the high-dose group were found dead on
days 9, 10 or 13. Other adverse effects reported in the high-dose rats
included severe clinical symptoms (e.g., unsteady gait, apathy), light
brown-gray discoloration of the liver in the rats that died during the
study, lung oedema and emphysema, and markedly decreased mean gravid
uterus weights. In the 650 mg/kg bw per day dose group, two dams were
reported to exhibit piloerection. Significant embryotoxic and
fetotoxic effects, such as an increase in the number of resorptions
resulting in increased post-implantation loss, decreased mean fetal
body weight, increased incidence of fetuses with dilated renal pelvis
and/or hydroureter, and a higher number of fetuses with skeletal
malformations, also occurred in the high-dose group. Variations and
retardation were also reported in the high-dose group. A slight
decrease in mean fetal body weight (statistically significant for all
viable fetuses combined and male fetuses separately, but
non-significant for female fetuses separately) and a statistically
significant increase in the frequency of fetuses with skeletal
variations and retardation were reported for the mid-dose group. The
authors reported that the only indications of significant adverse
effects on reproduction occurred in the high-dose group, which were
associated with maternal toxicity (Anonymous, 1991).
In an oral study, Ritter et al. (1987) exposed groups of
pregnant Wistar rats to doses of 6.25 or 12.5 mmoles 2-ethylhexanol/kg
bw (approximately 814 or 1628 mg/kg bw) on day 12 of gestation, the
rats being killed on day 20 of gestation. Several litters were
examined from each of the two treatment groups. For the low- and
high-dose groups of 2-ethylhexanol, the percentage of surviving pups
with malformation was 2 and 22.2, respectively. The malformations
observed in the high-dose group included tail and limb defects and
hydronephrosis. As indicated from the results of tests following oral
exposure (Anonymous, 1991) and dermal exposure (Tyl et al., 1992) in
rats, it would be expected that maternal toxicity could have occurred
at both dose levels employed in the study of Ritter et al. (1987),
even though the effect of treatment on the mother rats was not
discussed by the authors.
Tyl et al. (1992) conducted a range-finding study and a main
study examining the developmental toxicity of dermally applied
2-ethylhexanol in Fischer 344 rats. In the range-finding study, doses
of 0, 0.5, 1.0, 2.0 or 3.0 ml 2-ethylhexanol/kg bw per day (stated by
the authors to be equivalent to 0, 420, 840, 1680 or 2520 mg/kg bw per
day, respectively) were applied by occluded dermal application for 6
hours per day on gestation days 6 to 15 to groups of eight rats.
Naïve, sham (treated with 3.0 ml distilled water/kg bw per day) and
positive (treated with 420 or 1260 mg methoxyethanol/kg bw per day)
control groups were employed in the range-finding study. Valproic acid
was administered orally to a group of eight rats at a dose of 400
mg/kg bw per day as an oral reference compound.
In the main study, doses of 0, 0.3, 1.0 or 3.0 ml
2-ethylhexanol/kg bw per day (stated by authors to be equivalent to 0,
252, 840 or 2520 mg/kg bw per day, respectively) were applied to
groups of 25 rats by occluded dermal application for 6 hours per day
on gestation days 6 to 15. A sham control group receiving 3.0 ml/kg bw
per day distilled water and a positive control group receiving 840 mg
2-methoxyethanol/kg bw per day were included (Tyl et al., 1992).
Both positive control groups (valproic acid and methoxyethanol)
exhibited developmental toxicity and teratogenic effects. With respect
to 2-ethylhexanol, maternal weight gain was significantly reduced
during gestation days 6 to 15 at dose levels of 1680 and 2520 mg/kg bw
per day in the range-finding study. At the highest dose level in the
main study, maternal weight gain was significantly reduced during
gestation days 6 to 9, but was not significantly different from sham
controls for any other period examined (i.e. gestation days 0 to 6, 6
to 15, 15 to 21, or 0 to 21). In both studies, exfoliation and
encrustation at the site of application occurred at all dose levels.
However, this effect was reported to be typical of the effect of
alcohols on the skin. Erythema scores were mild to moderate at 840 and
2520 mg 2-ethylhexanol/kg bw per day. Oedema was not observed at any
treatment level. No treatment-related developmental or teratogenic
effects were observed in the fetuses of any of the dose levels in
either study. The authors reported no-observed-effect levels of 252 mg
2-ethylhexanol/kg bw per day based in skin irritation and 840 mg/kg
weight per day based on systemic toxicity (reduced maternal body
weight gain) (Tyl et al., 1992).
In a study designed to evaluate the association between the
pharma-cokinetics and teratogenicity of organic acids, pregnant
Sprague-Dawley rats were administered 14.1 mmoles/kg bw methylhexanoic
acid, or 12.5 or 15.625 mmoles/kg bw 2-ethylhexanoic acid undiluted by
oral gavage on day 12 of gestation. At various post-treatment
intervals (0.25-24 hours), embryos were dissected and exocoelomic
fluid and yolk sacs were analysed for the presence of the test
material. Samples of maternal blood and skeletal muscle were analysed
to determine the concentration of the test substance. The mothers were
killed on day 20 and fetuses were examined. Methylhexanoic acid
exhibited no signs of embryotoxicity except for a slight reduction in
fetal weight which was attributed to maternal toxicity. Ethylhexanoic
acid was toxic to the embryos removed on day 12, causing increased
death and malformation and a reduction of fetal weight. Both
methylhexanoic acid and ethylhexanoic acid reached a high
concentration in maternal plasma and embryo, but methylhexanoic acid
was eliminated more rapidly (Scott et al., 1994).
An in vivo developmental toxicity screen was conducted on 15
aliphatic acids including three substances in this group. Groups of 9
to 18 timed-pregnant Sprague-Dawley rats were treated by gavage once
daily on gestation days 6 to 15 with 2-ethylbutyric acid (0, 150 or
200 mg/kg bw per day), 2-methylvaleric acid (0, 187.5 or 250 mg/kg bw
per day) and 5-methylhexanoic acid (0, 300 or 400 mg/kg bw per day).
2-Ethylhexanoic acid, a metabolite of 2-ethylhexanol, was also tested
at dose levels of 0, 900 or 1200 mg/kg bw per day.
Maternal body weights were determined on gestational days 6, 8,
10, 13, 16 and 20. Clinical observations were made on all animals
throughout the study. Pups in each litter were examined and counted.
Skeletal examination was performed on two postnatal day 6 survivors
from each group. After postnatal day 6, the dams were killed and the
number of uterine implantation sites was recorded. 2-Methylvaleric
acid, 2-ethylbutyric acid and 5-methylhexanoic acid induced motor
depression in some mothers, which was associated with severe
respiratory effects (i.e. rales or dyspnoea). Perinatal mortality was
noted for 2-ethylbutyric acid but only in litters of dams that
exhibited severe respiratory effects. There was no delayed
parturition, decreased progeny viability, lumbar ribs, or any other
signs of developmental toxicity in pups of mothers treated with
2-ethylbutyric acid, 2-methylvaleric acid, or 5-methylhexanoic acid.
The highest dose levels of 200 mg/kg bw per day for 2-ethylbutyric
acid, 250 mg/kg bw/day for 2-methylvaleric acid, and 400 mg/kg bw/day
for 5-methylhexanoic that did not produce any signs of developmental
toxicity are >10 000 times their respective daily per capita
intakes ("eaters only") from use as flavouring substances in the USA
and Europe (see Table 1).
All dams treated with 2-ethylhexanoic acid exhibited motor
depression. Developmental effects were observed in pups of mothers
treated with 2-ethylhexanoic acid at both dose levels, including
reduced progeny viability, full-litter resorptions, apparent
stillbirths, postnatal deaths, reduced pup weights, external
malformations, skeletal malformations, and lumbar ribs (Narotsky et
al., 1994). 2-Ethylhexanoic acid produced developmental toxicity only
in the presence of maternal toxicity.
2.1.3.7 Special studies on peroxisome proliferation
Concentrations of 0.1 or 0.5 mM 2-ethylhexanol did not induce
palmitoyl CoA oxidase activity (a marker enzyme for peroxisome
proliferation) in rat hepatocytes in vitro (Rhodes et al., 1984).
A concentration of 1 mM 2-ethylhexanol was reported to induce
significantly (approximately 9 times the control level) the activity
of carnitine acetyltransferase (a peroxisomal enzyme) in cultured rat
hepatocytes in vitro, while a concentration of 0.2 mM did not induce
this enzyme (Gray et al., 1982).
In an in vivo study, Rhodes et al. (1984) reported that the
administration of 1 mmol 2-ethylhexanol/kg bw per day (approximately
130 mg/kg bw per day) to five male Wistar rats by gavage for 14 days
did not result in hepatic peroxisome proliferation in the rats.
Moody & Reddy (1978), however, reported that in five male F344
rats fed 2% 2-ethylhexanol in the diet (approximately 1000 mg/kg bw
per day) for 3 weeks, induction of hepatic peroxisome proliferation
occurred. Significant increases (using a Student's t test) in
activities of the enzymes hepatic catalase and carnitine
acetyltransferase also were reported in the rats treated with
2-ethylhexanol.
Hodgson (1987) conducted a peroxisome induction study in groups of
F344 male and female (five/sex/group) by administering doses of 100,
320 or 950 mg 2-ethylhexanol/kg bw per day by gavage for 21 days. A
significant reduction in body weight gain was observed in the
high-dose group rats with no effect on food consumption. Both sexes of
rats exhibited significant hepatomegaly at a dose of 950 mg
2-ethylhexanol/kg bw per day. The activity of cyanide-insensitive
palmitoyl CoA oxidation was increased in a dose-related manner in
males, with a 1.6-fold increase above controls at 320 mg/kg bw per day
and a 5.4-fold increase at 950 mg/kg bw per day. In females, a
3.1-fold increase in palmitoyl CoA activity was reported at the
high-dose level. Lauric acid hydroxylase activity of peroxisomes was
observed (using electron microscopy) in the hepatocytes of the rats of
the high-dose group.
Keith et al. (1992) examined the dose-response relationship for
2-ethylhexanol with respect to peroxisome proliferation in rats and
mice. Groups of five male and five female Alderley Park rats and mice
were administered doses of 0, 140, 350, 700, 1050 or 1750 mg
2-ethylhexanol/kg bw per day for 14 days by gavage. Rats in the
high-dose group exhibited toxic effects, leading to death of the rats.
A dose-related increase in the relative liver weight of both rats and
mice was observed, with the increase being significant in rats at the
700 and the 1050 mg/kg bw per day dose levels (rats in the high-dose
group died or were killed during the study), in male mice at the three
highest dose levels (700, 1050 and 1750 mg/kg bw per day), and in
female mice at the highest dose level (1750 mg/kg bw per day).
2-Ethylhexanol administration resulted in a virtually linear
dose-related induction of peroxisomal ß-oxidation (measured as
palmitoyl CoA oxidation activities) in both rats and mice. The level
of induction was greatest in the male mice. The dose at which this
effect became statistically significant was not stated. The increase
in rats and female mice was approximately 4-fold at the highest dose,
while for male mice, the increase was approximately 12-fold at the
highest dose.
2.1.3.8 Special study on immunotoxicity
3-Methylpentanoic acid was administered to 6- to 8-week-old female
CD-1 or B6C3F1 mice in corn oil by gavage for 5 days at dose levels
of 0, 175, 350 or 700 mg/kg bw per day. Cell-mediated immunity was
assessed by conducting a host resistance assay (Listeria
monocytogenes bacterial challenge). Humoral immunity was measured by
the antibody plaque-forming cell (PFC) response to sheep erythrocytes.
Body weights, lymphoid organ weights and spleen cellularity were also
measured and were normal as compared to controls. 3-Methylpentanoic
acid did not modulate the cell-mediated or humoral immune response
(Gaworski et al., 1994).
3. REFERENCES
Aeschbacher, H., Wolleb, U., Loliger, J., Spadone, J., & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food. Chem. Toxicicol., 27: 227-232.
Agarwal, D.K., Lawrence, W.H., Nunez, L.J., & Autian, J. (1985)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. Environ. Health, 16:
61-69.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5: 625-636.
American Conference of Governmental Industrial Hygienists, Inc.
(ACGIH) (1989) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, OH. USA.
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anonymous (1991) Study of the prenatal toxicity of isodecanol,
2-ethylhexanol, and 711 alcohols (T.C.) in rats after oral
administration (gavage) (Laboratory and authors associated with the
study were stated to be confidential) (Microfiche OTS0524015-2).
Astill, B.D., Gingell, R., Guest, D., Hodgson, J.R., Murphy, S.R., &
Tyler, T.R. (1993) Subacute and subchronic oral toxicity of
ethylhexanol to fischer 344 rats and B6C3F1 mice. Toxicologist, 13:
70.
Astill, B.D., Deckardt, K., Gembardt, Chr., Gingell, R., Guest, D.,
Hodgson, J.R., Mellert, W., Murphy, S.R., & Tyler, T.R. (1996a)
Prechronic toxicity studies on 2-ethylhexanol in F334 rats and B6C3F1
mice. Fundam. Appl. Toxicol., 29: 31-39.
Astill, B.D., Gingell, R., Guest, D., Hellwig, J., Hodgson, J.R.,
Kuettler, K., Mellert, W., Murphy, S.R., Sielken, R.L., & Tyler, T.R.
(1996b) Oncogenicity testing of 2-ethylhexanol in Fischer 344 rats and
B6C3F1 mice. Fundam. Appl. Toxicol., 31: 29-41.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months (Project No. 3350057).
Unpublished report.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
partial purification and properties. Can. J. Biochem., 47: 265-272.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B. ed. Enzymatic basis of detoxification. Academic Press, New York,
Vol. I, pp. 231-248.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E. ed Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, Vol. IIA, pp. 283-327.
Butenandt, A. & Thomas, K. (1958) Hoppe-Seyler's zeitschrift fur
physiologische chemie - Band 313. Walter Gruyter and Co., pp. 22-29.
Carpanini, F.M.B., Gaunt, I.F., Kiss, I.S., Grasso, P., & Gangolli,
S.D. (1973) Short-term toxicity of isoamyl alcohol in rats. Food
Cosmet. Toxicol., 11: 713-724.
Chen, T.H., Kavanagh, T.J., Chang, C.C., & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol.,
1: 155-171.
CIVO-TNO (1994) In: Maarse, H & Visscher, C.A. ed. Volatile components
in food: Qualitative and quantitative data - Volume III, 7th ed.
Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard-adecision tree approach. Food Cosmet. Toxicol., 16: 255-276.
Dave, G. & Lidman, U. (1978) Biological and toxicological effects of
solvent extraction chemicals: Range finding acute toxicity in the
rainbow trout. J. Hydrometall., 3: 210-216.
Dawson, A.M., Holdsworth, C.D., & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 17: 97-100.
Deisinger, P.J., Boatman, R J., & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
Deuel, H.J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley Interscience, New York.
DiVincenzo, G.D.& Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid the in the rat. Toxicol. Appl. Pharmacol., 47:
609-612.
DiVincenzo, G.D., Hamilton, M.L., Meuller, K.R., Donish, W.H., &
Barber, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
Dziewiatowski, D.D., Ventakaraman, A., & Lewis, H.B. (1949) The
metabolism of some branched chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Eckfeldt, J.H.& Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Wood, A. ed. Methods in
enzymology. Academic Press, New York, pp. 474-479.
Engler, H.W. & Bahler, B. (1982) Private communication to FEMA.
Fassett, D.W. (1963) Organic acids, anhydrides, lactones, acid
halides and amides, thioacids. In: Industrial hygene and toxicology,
2nd ed. Wiley-Interscience, New York, Vol. II, p. 1783.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
J. Toxicol., 15: 219-232.
Food and Drug Administration (FDA) (1993) Priority-based assessment of
food additives (PAFA) database. Center for Food Safety and Applied
Nutrition, p. 58.
Gaillard, D. & Derache, R. (1965) Metabolisation de differents
alcools, present dans les buissons alcooliques, chez le rat. Trav.
Soc. Pharm. Montp., 25: 51-62.
Gaworski, C., Vollmuth, T., Dozier, M., Heck, J., Dunn, L., Ratajczak,
H., & Thomas, P. (1994) An immunotoxicity assessment of food
flavouring ingredients. Food. Chem. Toxicol., 32: 409-415.
Goldberg, E.M., Sandler, S., & Blendis, L.M. (1979) A statistical
study of profiles of volatile metabolites in hepatic encephalopathy.
Modeling and Simulation - Volume 10, Part 1: Biomedical. In:
Proceedings of the Tenth Annual Conference, Pittsburgh, 25-27 April.
Instrumental Society of America, University of Pittsburgh.
Gray, T.J.B. & Beamand, J.A. (1984) Effect of some phthalate estsers
and other testicular toxins on primary cultures of testicular cells.
Food Chem. Toxicol., 22: 123-131.
Gray, T.J.B., Beamand, J.A., Lake, B.G., Foster, J.R., & Gangolli,
S.D. (1982) Peroxisome proliferation in cultured rat hepatocytes
produced by clofibrate and phthalate ester metabolites. Toxicol.
Lett., 10: 273-279.
Haggard, H.W., Miller, D.P., & Greenberg, L.A. (1945) The amyl
alcohols and their ketones: Their metabolic fates and comparative
toxicities. J. Ind. Hyg. Toxicol., 27: 1-14.
Hamdoune, M., Duclos, S., Mounie, J., Santona, L., Lhuguento, J.C.,
Magdalou, J., & Gouda, H. (1995) In vitro glucuronidation of
peroxisomal proliferators: 2-ethylhexanoic acid enantiomers and their
structural analogs. Toxicol. Appl. Pharmacol., 131: 235-243.
Hardin, B.D., Schuler, R.L., Burg, J.R., Booth, G.M., Hazelden, K.P.,
MacKenzie, K.M., Piccirillo, V.J., & Smith K.N. (1987) Evaluation of
60 chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen., 7: 29-48.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9: 257.
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. Acta Pharmacol. Toxico., 27: 381-396.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fermentative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Hillbom, M.E., Franssila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Jpn.
J. Stud. Alcohol, 9: 101-106.
Hodge, H.C. (1943) Acute toxicity for rats and mice of 2-ethyl hexanol
and 2-ethyl hexyl phthalate. J. Proc. Soc. Exp. Biol. Med., 53:
20-22.
Hodgson, J. R. (1987) Results of peroxisome induction studies on
tri(2-ethylhexyl)trimellitate and 2-ethylhexanol. Toxicol. Ind.
Health, 3: 49-60.
Hodgson, J.R., Myhr, B.C., McKeon, M., & Brusick, D.J. (1982)
Evaluation of di-(2-ethylhexyl)phthalate and its major metabolites in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 4: 388.
Institut Français de Recherches et Essais Biologiques (IFREB) (1975)
Acute toxicity of 4 methlynonanoic acid in rats. Private
communication to FEMA.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Johannsen, E. & Purchase, I. F. (1969) Kafficorn malting and brewing
studies: XXI. The effect of fusel oil of Bantu beer on rat liver
S. Afr. Med. J., 43: 326-328.
Keith, Y., Cornu, M.C., Canning, P.M., Foster, J., Lhuguennot, J.C., &
Elcombe, R.C. (1992) Peroxisome proliferation due to di(2-ethylhexyl)
adipate, 2-ethylhexanol and 2-ethylhexanoic acid. Arch. Toxicol.,
66: 321-326.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Kirby, P.E., Pizzarello, R.F., Lawlor, T.E., Haworth, S.R., & Hodgson,
J.R. (1983) Evaluation of di-(2ethylhexyl)phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
Knaak, J.B., Kozbelt, S.J., & Sullivan, L.J. (1966) Metabolism of 2-
ethylhexyl sulfate by the rat and rabbit. Toxicol. Appl.
Pharmacol., 8: 369-379.
Lake, B.G., Gangolli, S.D., Grasso, P., & Lloyd, A.G. (1992) Studies
on the hepatic effects of orally administered
di-(2-ethylhexyl)phthalate in the rat. Toxicol. Appl. Pharmacol.,
32: 355-367.
Lester, D. & Benson, G.D. (1970) Alcohol oxidation in rats inhibited
by pyrazole, oximes, and amides. Science, 169: 282-284.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J., & Paulson, G.D.
ed. Intermediary xenobiotic metabolism in animals. Taylor and
Francis, London, pp. 119-138..
Lewis, R.J. (1989) Food additives handbook. Van Nostrand Reinhold, New
York, p. 259.
McMahon, R.E., Cline, J.C., & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39: 682-693.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45: 497-504.
Moreno, O.M. (1988) Private communication to FEMA.
Moreno, O.M. (1977) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Unpublished
report to RIFM.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of sctrucural class with
no-observed-effect-levels: a proposal for establishing a threshold of
concern. In Press.
Narotsky, M.G., Francis, E.Z., & Kavlock, R.T. (1994) Developmental
toxicity and structure-activity relationships of aliphatic acids,
including dose-response assessment of valproic acid in mice and rats.
Fundam. Appl. Toxicol., 22: 251-265.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
National Institute for Occupational Safety and Health (NIOSH) (1976)
In: Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects
of chemical substances. NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Institute for Occupational Safety and Health (NIOSH) (1991)
Toxic Param. Ind. Tox. Chem. Under single exposure (1982). Cited in:
Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects of
chemical substances (1976). NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Toxicology Program (NTP) (1991) Final report on the
developmental toxicity of 2 ethylhexanol in CD-1-Swiss mice. National
Toxicology Program, Research Triangle Park, NC, USA (PB91-185900).
Obe, G. & Beek, B. (1979) Mutagenic activity of aldehydes. Drug
Alcohol Depend., 4: 91-94.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181
Opdyke, D.L.J. & Leitizia, C. (1982) Frangrance raw materials
monographs. Food Cosmet. Toxicol., 20: 739-740.
Owen, G. & Meyer, F.J. (1971) Private communication to FEMA.
Phillips, B.J., James, T.E.B., & Gangolli, S.D. (1982) Genotixicity
studies of di(2ethylhexyl)phalate and its metabolites in CHO cells.
Mutat. Res., 102: 297-304.
Pietruszko, R., Crawford, K., & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenase from human liver, horse
liver, and yeast towards saturated and 2-enoic alcohols and aldehydes.
Arch. Biochem. Biophys,. 159: 50-60.
Posternak, J.M. Linder, A., & Vodoz, C.A. (1969) Subchronic feeding
study of 2-methylhexanoic acid in rats. Food Chem. Toxicol., 7:
405-407.
Prunonosa, J., Sagrista, M.L., & Bozal, J. (1991) Inactivation
mechanism of low-Km rat liver mitochondrial aldehyde dehydrogenase
by cyanamide in vitro. Drug Metab. Dispos., 19: 787-792.
Purchase, I.F.H. (1969) Studies in kafficorn malting and brewing:
XXII. The acute toxicity of some fusel oils found in bantu beer.
S. Afr. Med. J., June 21(Suppl.).
Putman, D.L., Moore, W.A., Schechtman, L.M., & Hodgson, J.R. (1983)
Cytogenic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 5: 227-231.
Rhodes, C., Soames, T., Stonard, M.D., Simpson, M.G., Vernall, A.J., &
Elcombe, C.R. (1984) The absence of testicular atrophy and in vivo and
in vitro effects on hepatocyte morphology and peroxisomal enzyme
activ. in male rats following the administration of several alkanols.
Toxicol. Lett., 21: 103-109.
Ritter, E.J., Scott, W.J., Randall, J.L., & Ritter, J.M. (1987)
Teratogenicity of di(2-ethylhexyl) phthalate, 2-ethylhexanol,
2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine.
Teratology, 35: 41-46.
Rushbrook, C.J., Jorgenson, T.A., & Hodgson, J.R. (1982) Dominant
lethal study of di-(2-ehtylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
Saito, M. (1975) Studies on the metabolism of lower alcohols.
Nichidai Igaku Zasshi, 34: 569-585.
Scala, R.A. & Burtis, E.G. (1973) Acute toxicity of a homologous
series of branched-chain primary alcohols. Am. Ind. Hyg. Assoc. J.,
34: 493-499.
Schafer, E.W. Jr. & Bowles, W.A. Jr. (1985) The acute toxicity and
repellence of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14: 111-129.
Schmidt, P., Gohlke, R., & Rothe, R. (1973) Zur toxizitat einiger
C8-aldehyde und alkohole. Z. Gesamte Hyg. Grenzgeb., 19: 485-490.
Scott, W.J. Jr., Collinfs, M.D., & Heinz, N. (1994) Pharmacokinetic
determinants of embryotoxicity in rats associated with organic acids.
Environ. Health Perspect., 102: 97-101.
Seed, J.L. (1982) Mutagenic activity of phthalate esters in bacterial
liquid suspension assays. Environ. Health Perspect., 45: 111-114.
Shaffer, C.B., Carpenter, C.P., & Smyth, H.F. (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. Ind. Hyg. Toxicol., 27: 130-1135.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report to RIFM.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S., & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27: 400-419.
Sjöberg, P., Bondesson, U., Gray, T.J.B., & Ploen, L. (1986) Effects
of di-(2-ethylhexyl)phthalate and five of its metabolites on rat
testis in vivo and in vitro. Acta Pharmacol. Toxicol., 58:
225-233.
Smyth, H.F. Jr., Carpenter, C.P., & Weil, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4: 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S., & Possani U.C. (1954)
Range finding toxicity data: List V. Arch. Ind. Hyg., 10: 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, U.C., Striegel,
J.A., & Nycum, J.S. (1969) Range finding toxicity data: List VII.
Am. Ind. Hyg. Assoc. J., 30: 470-476.
Stofberg, J. & Gruncschober, F. (1987) The consumption ratio and Food
Predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Szybalski, W. (1958) Special microbial systems: II. Obeservations on
chemical mutagenesis in microorganisms. Ann. NY Acad. Sci., 76:
475-489.
Tomita, I., Nakamura, Y., Aoki, N., & Inui, N. (1982)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
Tyl, R.W., Fisher, L.C., Kubena, M.F., Vrbanic, M.A., Gingell, R.,
Guest, D. Hodgson, J.R., Murphy, S.R., Tyler, T.R. and Astill, B.D.
(1992) The developmental toxicity of 2-ethyl-1-hexanol applied
dermally to pregnant Fischer 344 rats. Fundam. Appl. Toxicol., 19:
176-185.
Union Carbide Data Sheet, 2/4/59. Cited in: NIOSH ed. Registry of
toxic effects of chemical substances (1991).
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V., & Thomas, P.T. (1989)
Immunotoxicity assessment of flavoring ingredients using a rapid and
economical screen. Toxicologist, 9: 206.
Walkenstein, S.S. & Weinhouse, S. (1953) Oxidation of aldehydes by
mitochondria of rat tissues. J. Biol. Chem., 200: 515-523.
Weiner, H. (1980) Aldehyde oxidizing enzymes. In: Jakoby, W.B. ed
Enzymatic basis of detoxication. Academic Press, New York, Vol. I,
pp.261-275.
Wild, D., King, M.T., Gocke, E., & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, basc and micronucleus tests. Food chem.
Toxicol., 21(6): 707-719.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall, Ltd, London, UK, pp. 4-5.
Woodruff, R.C., Mason, J.M., Valencia, R., & Zimmering, S. (1985)
Mutagenesis testing in Drosophilia: V. Results of 53 coded compounds
tested for the National Toxicology Program. J. Environ. Mutagen., 7:
677-702.
World Health Organization (1987) Environmental Health Criteria 65:
Butanols - Four isomers: 1-butanol, 2-butanol, tert-butanol, and
isobutanol. World Health Organization, International Programme on
Chemical Safety, Geneva
Yasuhara, A., Fuwa, K., & Jimbu, M. (1982) Variation in concentration
of odorous components of pig feces with growth. Agric. Biol. Chem.,
46: 1381-1383.
Zabin, I. & Bloch, K. (1951) Studies on the utilization of isovaleric
acid in cholesterol synthesis. J. Biol. Chem., 192: 267-273.
Zeiger, E., Haworth, S., Mortelmans, K., & Speck, W. (1985)
Mutagenicity testing of di(2 ethylhexyl)phthalate and related
chemicals in salmonella. Environ. Mutagen., 7: 213-232.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 2: 1-158.
See Also:
Toxicological Abbreviations
 When rats and rabbits were administered 2-ethylhexanol-C14 or
2-ethylhexanol-C14 sulfate either orally or by intraperitoneal (i.p.)
injection, the principal metabolites included unchanged alcohol and
the sulfate ester, as well as hydroxylated 2-ethylhexanol and the
glucuronide conjugate of 2-ethylhexanoic acid (Knaak et al., 1966).
2-Ethylbutyric acid was administered orally and by subcutaneous
injection at a dose of approximately 1 gram to rabbits and 100 mg (as
the sodium salt) to rats. The acid was primarily excreted unchanged in
the urine as the glucuronic acid conjugate (Dziewiatowski et al.,
1949). In dogs, 2-ethylbutyric acid undergoes ß-oxidation and
decarboxylation to yield 2-pentanone (Deuel, 1957).
In conclusion, branched-chain alcohols, aldehydes and carboxylic
acids with bulky (ethyl, propyl, etc.) alpha-alkyl substituents are
metabolized by omega- and omega-1-oxidation to yield polar metabolites
capable of excretion in the urine. At high-dose levels these oxidation
pathways may be saturated, in which case, the corresponding acid may
undergo conjugation with glucuronic acid.
2.1.3 Toxicological studies
2.1.3.1 Acute toxicity
Acute toxicity studies have been reported for 20 of the 25
saturated aliphatic branched-chain primary alcohols, aldehydes and
acids in this group and are summarized in Table 2. The acute oral
toxicity of the group is demonstrated by oral LD50 values of >2000
mg/kg bw for the alcohols; >3200 mg/kg bw for the aldehydes; and
>1000 mg/kg bw for the carboxylic acids, except for isobutyric and
2-methylbutyric acid, which are 280 mg/kg bw, and 3-methylpentanoic
acid which is >700 mg/kg bw. The rat oral LD50 values for the three
alpha-ethyl-substituted substances 2-ethylbutyraldehdye,
2-ethylbutyric acid and 2-ethyl-1-hexanol are 3980 mg/kg bw, 2200
mg/kg bw and 2050-7100 mg/kg bw, respectively.
2.1.3.2 Short-term toxicity
The results of short-term toxicity studies with a
methyl-substituted alcohol (isobutyl alcohol), a structurally related
alcohol (isoamyl alcohol), and two methyl-substituted carboxylic acids
(isovaleric acid and 2-methylhexanoic acid) are summarized in Table 3
and described below. Short-term oral studies for 2-ethylbutyric acid
and 2-ethyl-1-hexanol are summarized in the section on
alpha-ethyl-substituted substances.
Table 2. Acute toxicity studies for saturated aliphatic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Isobutyl alcohol rat M/F oral 2640-3100 Smyth et al., 1954; WHO, 1987
M/F gavage 2650-3100 Purchase, 1969
mouse oral 3500 WHO, 1987
Isobutyraldehyde rat M/F oral 3730 Smyth et al., 1954
Isobutyric acid rat NR oral 280 Lewis, 1989
2-Methylbutyraldehyde rat NR oral 8570 Opdyke & Leitizia, 1982
2-Methylbutyric acid rat M/F oral 280-2200 Moreno et al., 1982; Lewis, 1989;
mouse M/F oral 1238 Schafer & Bowles, 1985
2-Ethylbutyraldehyde rat M/F oral 3980 Smyth et al., 1951
2-Ethylbutyric acid rat M/F oral 2200 Smyth et al., 1954
Isoamyl alcohol2 rat M/F oral 5700 Smyth et al., 1954
gavage 1300-4000 Purchase, 1969
3-Methylbutyraldehyde rat M/F oral 7166 Moreno, 1988
Isovaleric acid rat NR oral 2000; >3200 Fassett, 1963; NIOSH, 1976
2-Methylvaleric acid rat M/F oral 1600-3200 Smyth et al., 1954; ACGIH, 1989
3-Methylpentanoic acid rat NR oral >700 Vollmuth et al., 1989
3-Methyl-1-pentanol rat M/F oral >2000 Engler & Bahler, 1982
2-Ethyl-1-hexanol rat M/F oral 2050-7100 Hodge, 1943; Shaffer et al., 1945;
Smyth et al., 1969; Scala & Burtis, 1973;
Schmidt et al., 1973; Albro, 1975; Dave & Lidman, 1978
Table 2. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3,5,5-Trimethyl-1-hexanol rat M/F oral 2300 Moreno, 1977
3,5,5-Trimethylhexanal rat M/F oral 3240 Moreno, 1975; Opdyke & Leitizia, 1982
2-Methyloctanal rat M/F oral >5000 Moreno, 1977
3,7-Dimethyl-1-octanol rat M/F oral 5000 Shelanski & Moldovan, 1973; Moreno, 1977
4-Methylnonanoic acid rat NR oral 3700 IFREB, 1975
2-Methylundecanal rat M/F oral >5000 Owen & Meyer, 1971
4-Methylpentanoic acid rat NR oral 2050->3200 Smyth et al., 1954; Fassett, 1963; NIOSH, 1976;
Union Carbide, 1991;
mouse NR oral 5000 NIOSH, 1991
1 M = male; F = female; NR = Not reported.
2 Structurally related substance.
Table 3. Short-term and long-term toxicity studies for saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids
Substance Species (Sex) Route Duration NOEL Reference
(mg/kg bw
per day)
Short-term studies
Alcohols
Isobutyl alcohol rat (M/F) oral 90 days 1450 BASF, 1992
rat oral 4 months >0.126 nmol/g Hillbom et al., 1974
bw/day3 Johannsen &
rat (M/F) oral 53-56 weeks 2003 Purchase, 1969
Isoamyl alcohol2 rat (M/F) gavage 17 weeks 10003 Carpanini et al., 1973
rat (M/F) oral 56 weeks 20003 Johannsen & Purchase, 1969
2-Ethyl-1-hexanol mouse gavage 11 days 100 Astill et al., 1996a
mouse gavage 90 days 125 Astill et al., 1996a
mouse food 11 days 1150-4450 Astill et al., 1993
rat gavage 14 days 1303 Rhodes et al., 1984
NR Lake et al., 1975
rat oral 7 days 100 Astill et al., 1996a
rat gavage 11 days 125 Astill et al., 1996a
rat gavage 90 days <500-540 Astill et al., 1993
rat oral 11 days
Carboxylic Acid
2-Ethylbutyric acid rat oral 90 days 3003 Amoore et al., 1978
Isovaleric acid rat oral 90 days 25003 Amoore et al., 1978
2-Methylhexanoic acid rat (M/F) oral 90 days 33 Posternak et al., 1969
Long-term/ Carcinogenicity studies
2-Ethyl-1-hexanol mouse (M/F) gavage 540 days 200 Astill et al., 1996b
rat (M/F) gavage 730 days 50 Astill et al., 1996b
Table 3 (continued)
1 M = Male; F = Female; NR = not reported.
2 A structurally related branched-chain alcohol.
3 The study was performed at a single dose level or multiple dose levels that produced no adverse effects and, therefore, a NOEL was not
determined. The NOEL is probably higher than the reported dose level that produced no adverse effects.
a) Methyl substituted aliphatic alcohols, aldehydes and carboxylic
acids
i) 2-Methyl-1-propanol (isobutyl alcohol)
Groups of eleven 4-month-old male Wistar rats were given 1 M
solutions of isobutyl alcohol as their only drinking fluid for 4
months. Control animals received tap water. An ordinary diet was
provided for all animals. The level of isobutyl alcohol consumption
was reported to be 0.126 nmole/g bw/day at 120 days. At necropsy,
there was no evidence of hepatic steatosis, fibrosis or inflammation
(Hillbom et al., 1974).
Groups of 20 male and 20 female Wistar albino rats were given a
mixture of fusel oils including 0.2% isobutyl alcohol, which was
calculated (FDA, 1993) to provide an approximate daily intake of 200
mg/kg bw/day, as their sole drinking source for 53-56 weeks. Control
animals were given tap water. There were no statistically significant
differences in body or liver weights of treated animals as compared to
controls. Liver enzymes were determined at 2- to 4-week intervals and
revealed no significant differences in activity and protein content of
the liver between test and control animals. Histological examination
was performed on specimens of liver, kidney, heart, spleen and lung
and revealed no significant abnormalities (Johannsen & Purchase,
1969).
Groups of 10 male and 10 female Wistar rats were given 2-methyl-1-
propanol (i.e. isobutyl alcohol) in their drinking water for 3 months.
The test substance was administered in concentrations of 0, 1000, 4000
or 16 000 mg/litre, which was reported to correspond to approximate
dose levels of 0, 60, 340 or 1450 mg/kg bw per day. Food and drinking-
water consumption and body weight gain were not affected by the test
substance. All animals tested were free from adverse clinical effects.
Haematology and clinical chemistry parameters were measured and
revealed no treatment-related adverse effects. Gross pathology and
histopathological examinations were normal for all animals except for
testicular atrophy, which was observed in two males in the high-dose
group. Diffuse tubular generation and hyperplasia of the Leydig cells
were reported in the two animals. The authors concluded that the
absence of similar effects in other male rats indicated that the
effect was most likely unrelated to the test material. The authors
reported that the results of this study demonstrate a lack of toxicity
associated with administration of 2-methyl-1-propanol in the drinking-
water of rats, and that the NOEL is >1450 mg/kg bw per day (BASF,
1992). The NOEL is >100 000 times the estimated daily per capita
intake1 ("eaters only") of 4.85 g/kg bw of isobutyl alcohol from
its use as a flavouring substance in the USA and 8.79 g/kg bw of
isobutyl alcohol in Europe.
ii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage providing daily dose levels
of 0, 150, 500 or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight statistically significant reduction in body-weight
gain which was associated with reduced food intake. Over the entire
period of the study, however, there was no statistically significant
reduction in mean food intake. Examination of haematology, serum
analyses, urinalysis, renal concentration tests and organ weights
revealed no treatment-related effects. The animals were examined for
macroscopic abnormalities, and the major organs were weighed.
Microscopic examination was performed on several tissues of the
control and high-dose animals. No treatment-related abnormalities were
observed (Carpanini et al., 1973).
Groups of 20 male and 20 female Wistar albino rats were given a 2%
solution of isoamyl alcohol, which was calculated (FDA, 1993) to
provide an approximate daily intake of 2000 mg/kg bw per day, as their
sole drinking source for 53-56 weeks. Control animals were given tap
water. There were no statistically significant differences in body or
liver weights of treated animals as compared to controls. Liver
enzymes were determined at 2- to 4-week intervals and revealed no
significant differences in ADH, GOT or GPT activity and protein
content of the liver. Histological examination was performed on
specimens of liver, kidney, heart, spleen and lung and revealed no
significant abnormalities (Johannsen & Purchase, 1969).
iii) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0 or 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to provide a daily intake of 0 or 2500 mg/kg
bw. The rats were monitored for survival rates, body weight changes,
food intake, blood and urine analysis, organ weights and histology. At
2500 mg/kg bw per day, there were no observed changes in the rats
(Amoore et al., 1978).
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1995). Intake (mg/kg bw/day)
calculated as follows: [(mg/day)/body weight], where body weight = 60
kg. Slight variations may occur from rounding off.
iv) 2-Methylhexanoic acid
In a limited 90-day feeding study, 2-methylhexanoic acid was fed
to male and female Charles River CD rats at a dose of approximately 3
mg/kg bw/day. Observations included mortality rates, food intake, body
weight, haematology, blood urea, organ weights and histopathology. The
NOEL was 3 mg/kg bw dose level (Posternak et al., 1969) which is
>10 000 times the estimated daily per capita intake ("eaters only")
of 0.04 g/kg bw 2-methylhexanoic acid from use as a flavouring
substance in the USA and 0.24 g/kg bw from its use in Europe.
b) alpha-Ethyl substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
Eleven (11-) day and 90-day studies were conducted in B6C3F1 mice
as part of the dose selection process for an 18-month study (see
section 2.1.3.3). In the 11-day gavage study, groups of ten B6C3F1
mice of each sex were administrated nine applications of 0, 100, 330,
1000 or 1500 mg 2-ethylhexanol/kg bw per day. Mice in the high-dose
group exhibited effects such as ataxia, lethargy, loss of
consciousness, death in four females, increased absolute and relative
lever and stomach weights, macroscopic foci in the forestomach, and
microscopic lesions in the forestomach, liver, testes and kidneys.
These lesions consisted of hyperkeratosis and focal or multifocal
acanthosis and inflammatory oedema in the submucosa in most mice,
focal or multifocal ulceration of the mucous membrane in a few mice,
hypertrophy of hepatocytes in all mice, focal necrosis of liver cells
in one male and one female mouse, centrilobular fatty infiltration in
female mice that died intercurrently, tubular giant cells in
testicular tubules of two mice, and renal cortex tubular dilation and
nephrosis in mice that died intercurrently. Mice receiving the dose of
1000 mg/kg bw per day exhibited similar symptoms to those observed at
the highest dose (1500 mg/kg bw per day), but to a lesser degree,
while four mice (two male, two female) receiving 330 mg 2-ethyl
hexanol/kg bw per day had acanthosis in the mucous membrane of the
forestomach. No treatment-related adverse effects were observed in the
mice administered 100 mg 2-ethylhexanol/kg bw per day (Astill
et al., 1996a).
In the 90-day study, mice (number per group not reported) received
doses of 0, 25, 125, 250 or 500 mg 2-ethylhexanol/kg bw per day. At
the highest dose level, the treatment-related effects observed
included increased relative stomach weights in male mice and focal or
multifocal acanthosis of the forestomach mucosa in two male and one
female mouse. Increased relative stomach weights were observed in male
mice receiving 250 mg/kg bw per day. No treatment-related effects were
reported in mice of the 125 or 25 mg/kg bw per day dose level. Based
on the results of the 11- and 90-day studies, dose levels of 50, 200
or 750 mg 2-ethylhexanol/kg bw per day were chosen for the 18-month
mouse study (Astill et al., 1996a).
An 11-day mouse study was conducted using administration of
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of 10 male and 10 female B6C3F1 mice per group were used with
concentrations of microencapsulated material in the diet of 0.48,
0.96, 1.44 or 2.88%. The microcapsules contained 45.8% 2-ethylhexanol;
therefore, 2-ethylhexanol concentrations in the diet were 0.22, 0.44,
0.66 or 1.32%, respectively. These concentrations were reported to be
equal to doses of 550, 1150, 1800 or 4450 mg/kg bw per day for males
and 750, 1750, 2650 or 5750 mg/kg bw per day for females. The authors
reported that diet spillage did occur, but this was not quantified in
the treated groups of mice. As a result, the mg/kg bw per day dosage
calculations would not account for spillage and may represent
exaggerated 2-ethylhexanol intakes (Astill et al., 1993).
Significantly decreased body weight gains were observed at the
high-dose level for females throughout the test period and for males
at day 10 of the test period and at the 0.66% level in males at day 10
of the test period. No other treatment-related findings were reported.
The authors concluded the NOEL for male mice was in the range of 0.96
to 1.44% microencapsulated material (0.44 to 0.66% 2-ethylhexanol or
reportedly 1150 to 1800 mg/kg bw per day) and the NOEL for female mice
was in the range of 1.44 to 2.88% microencapsulated material (0.66 to
1.32% 2-ethylhexanol or reportedly 1800 to 4450 mg/kg bw per day)
(Astill et al., 1993). The NOELs reported in this study are higher
than the NOEL of 100 to 330 mg 2-ethylhexanol/kg bw per day reported
in the 11-day gavage study in B6C3F1 mice (Astill et al., 1996a),
most likely as a result of the different modes of administration. The
lack of correction for diet spillage in the microencapsulation study
may also have been a factor.
To study the effect of 2-ethylhexanol on rat liver and testes,
Rhodes et al. (1984) administered 1 mmol 2-ethylhexanol/kg bw per
day (approximately 130 mg/kg bw per day) to five Wistar rats by gavage
for 14 days. 2-Ethylhexanol administration did not produce testicular
atrophy, hepatomegaly, peroxisome proliferation or hypolipidaemia in
the rats. Lake et al. (1992) orally administered 1335 mg
2-ethylhexanol/kg bw per day to six male Wistar rats for seven days.
The authors reported significantly increased relative liver weights,
increased microsomal biphenyl 4-hydroxylase activity, increased
alcohol dehydrogenase activity in the centrilobular area of the liver
lobule, increased number of microbodies, and dilatation of the smooth
endoplasmic reticulum in the livers. No effect on the activities of
aniline 4-hydroxylase or mitochondrial succinate dehydrogenase was
observed.
In an 11-day gavage study, groups of ten male and ten female
Fischer F344 rats were administered doses of 0, 100, 330, 1000 or 1500
mg 2-ethylhexanol/kg bw per day. Adverse effects, including reduction
in food consumption and body weight gain, ataxia, lethargy, changes in
blood chemistry, effects on absolute (increased liver and stomach,
decreased spleen, brain, and adrenal) and relative (increased stomach,
liver, kidney, brain, adrenal, and lung, decreased spleen) organ
weights, gross lesions in the forestomach and microscopic findings in
the forestomach, liver (high-dose only), spleen and thymus, were
observed in the rats at the highest two dose levels. At the 330 mg/kg
bw per day dose, female rats exhibited increased relative kidney
weights, as well as microscopic lesions in the forestomach of one
female rat and in the thymus of both sexes (it was not stated whether
or not these lesions were treatment-related). No treatment-related
adverse effects were observed at 100 mg/kg bw per day (Astill et
al., 1996a).
In the 90-day study, groups of ten male and ten female Fischer
F344 rats were administered doses of 0, 25, 125, 250 or 500 mg
2-ethylhexanol/kg bw per day by gavage. Reduced body weight and body
weight gain, changes in blood chemistry (consisting of reduced
cholesterol, glucose, alanine aminotransferase and alkaline
phosphatase, levels and increased reticulocytes, total protein and
albumin levels), increased absolute and relative liver and stomach
weights and gross lesions in the forestomach were observed in the rats
receiving 500 mg 2-ethylhexanol/kg bw per day. Also observed in the
high-dose rats were microscopic changes consisting of focal or
multifocal acanthosis in the mucosa of six rats (one male, five
female) and whole mucose acanthosis and submucosa degeneration and
oedema in one male rat. In addition, a number of high-dose rats with
fatty infiltration in the liver, as well as a lower grade of fat
deposition in the liver, were observed. At 250 mg/kg bw per day,
changes in the blood chemistry (consisting of reduced glucose,
alkaline phosphatase and alanine aminotransferase levels), increased
relative liver and stomach weights, and a lower grade of fat
deposition in the liver cells of the male rats were observed. No
treatment-related adverse effects were observed at the 25 or 125 mg/kg
bw per day dose levels. Based on the results of the 11- and 90-day
studies, dose levels of 50, 150 or 500 mg 2-ethylhexanol/kg bw per day
were chosen for a two-year study (Astill et al., 1996a).
An 11-day rat study was also conducted by administering
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of ten male and ten female Fischer rats per group were fed
concentrations of microencapsulated material in the diet of 1, 2, 3 or
6%. The microcapsules contained 45.8% 2-ethylhexanol; therefore,
2-ethylhexanol concentrations in the diet were 0.46, 0.92, 1.38 or
2.75%, respectively. These concentrations were reported to be equal to
doses of 500, 980, 1430 or 2590 mg/kg bw per day for males and 540,
1060, 1580 or 2820 mg/kg bw per day for females. A vehicle control
group receiving placebo microcapsules was employed. Decreased food and
water (top three doses only) consumption was observed at all dose
levels at some point in the study period with decreased body weights
reported for both sexes at the top dose and in females at day four in
the second highest dose. Changes in blood chemistry, consisting of
decreased cholesterol, triglycerides and alanine aminotransferase
levels (all doses), decreased glucose, reticulocyte and mean
corpuscular volume levels (high-dose only), decreased platelet level
(top two doses only), increased total protein level (top three doses),
increased erythrocyte level (high-dose only) and increased absolute
and relative stomach and/or liver weights were observed to some extent
in all dose groups. Varying degrees of hypertrophy of the hepatocytes
were reported in the top three dose groups. The authors reported the
NOEL to be less than 0.46% in the diet (500 or 540 mg/kg bw per day
for male and female rats, respectively) (Astill et al., 1993).
ii) 2-Ethylbutyric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0, 0.62, 1.25 or 10% 2-ethylbutyric acid in the
diet, equivalent to 0, 300, 625 or 5000 mg/kg bw per day,
respectively. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analysis, organ weights and
histology. At the 300 mg/kg bw dose level, there were no observed
changes in the rats. At both the 625 and 5000 mg/kg bw dose levels,
slight body weight changes were observed within the first 14 days of
the study (Amoore et al., 1978).
2.1.3.3 Long-term toxicity/carcinogenicity
The results of long-term toxicity/carcinogenicity studies on
2-ethyl-1-hexanol are summarized in Table 3 and described below.
a) Mice
Groups of 50 male and 50 female B6C3F1 mice were administered
2-ethylhexanol by gavage at doses of 0, 50, 200 or 750 mg/kg bw per
day, five days per week for 18 months. The vehicle consisted of doubly
distilled water containing approximately 5 mg Polyoxyl 35 Castor Oil
(Cremophor(R) EL) per 100 ml. Two control groups of 50 mice of each
sex were employed, one receiving doubly distilled water containing
approximately 5 mg Cremophor(R) EL/100 ml. The use of Cremophor(R)
EL was reported to have no effect on the outcome of the study. Food
consumption, body weights and haematological parameters were examined
at specific intervals during the study. At the end of the study, gross
and histopathological examinations were conducted.
No treatment-related adverse effects were observed in the mice
receiving 50 or 200 mg 2-ethylhexanol/kg bw per day. At the 750 mg/kg
bw per day dose level, body weight gain reductions of approximately 26
and 24% in males and females, respectively, were reported. These
weight gain reductions were associated with a significant reduction in
food consumption. Both male and female mice of the high-dose group
showed increased mortality compared to the vehicle control mice (i.e.
30% in the treated mice compared to 4 and 8% in the vehicle control
group male and female mice, respectively). With respect to
haematological parameters, both a slight increase in polymorphonuclear
neutrophils and a slight decrease in lymphocytes were observed in the
mice of the high-dose group. A slight, statistically insignificant,
increase in focal hyperplasia of the forestomach epithelium was also
observed in the high-dose group mice. A slight increase in
hepatocellular carcinomas in the female mice of the high-dose group
was determined to have no biological relevance. 2-Ethylhexanol showed
no evidence of carcinogenicity in this study (Astill et al., 1996b).
b) Rats
Groups of 50 male and 50 female Fischer F344 rats were
administered 2-ethylhexanol by gavage at doses of 0, 50, 150 or 500
mg/kg bw per day, 5 days per week for 2 years. The vehicle consisted
of doubly distilled water containing approximately 5 mg Polyoxyl 35
Castor Oil (Cremophor(R) EL) (as an emulsifier) per 100 ml. Two
control groups of 50 mice of each sex were used, one receiving doubly
distilled water containing 5 mg Cremophor(R) EL/100 ml. The use of
Cremophor(R) EL was reported to have no effect on the outcome of the
study. Food consumption, body weights, and haematological parameters
were examined at specific intervals during the study. At the end of
the study, gross and histopatho-logical examinations were conducted.
No treatment-related adverse effects were observed at the 50 mg/kg
bw per day dose level. At the 150 mg/kg bw per day dose level, rats
exhibited a body weight gain reduction of approximately 16% in males
and 12% in females. In addition, the rats also displayed a slightly
increased incidence of clinical signs, such as poor general condition,
laboured breathing, piloerection, and/or smearing of the genital
region with urine. A definite increase in the number of rats and/or a
definite increase in the incidence of clinical signs (same signs as
mentioned for the mid-dose group) was observed in the high-dose group
rats. Mortality was increased in the female rats of the high-dose
group (52% compared to 28% in the vehicle control) and both male and
female rats exhibited a reduction in the body weight gain (33% in
males and 31% in females). With respect to haematological parameters,
a slight increase in anisocytosis, predominantly microcytosis in
males, was observed after 12 months, but not after 18 or 24 months.
The incidence of bronchopneumonia was significantly increased in both
sexes at the high-dose level. This effect was found to be due to the
aspiration of the stomach contents, most likely resulting from the
poor general condition of the animals. 2-Ethylhexanol showed no
evidence of carcinogenicity in this study (Astill et al., 1996b).
2.1.3.4 Genotoxicity
The results of genotoxicity studies on saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes, and carboxylic acids are
summarized in Table 4.
Table 4. Genotoxicity studies of saturated aliphatic acyclic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Test system Test object Dose level Results Reference
Alcohols
Isobutyl alcohol Modified Ames test S. typhimurium TA98, TA100, 100 to 10 000 g/plate1,2 Negative Zeiger et
TA1535 TA97 and TA1537 al., 1988
Modified Ames test S. typhimurium TA100, TA98, 5 to 5000 mg/plate1 Negative Shimizu et
TA1535, TA1537 and TA1538; al., 1985
E. coli WP2 uvrA
2-Ethyl-1-hexanol 8-azaguanine resistance S. typhimurium TA100 0.5 to 1.5 mM Positive3 Seed, 1982
assay
Ames test4 S. typhimurium TA98, TA100, up to 2 ml in urine/plate Negative Divincenzo
TA1535, TA1537, TA1538 et al., 1985
Ames test S. typhimurium TA98, TA100, 0.01 to 1.0 µl/plate1 Negative Kirby et
TA1535, TA1537, TA1538 al., 1983
Ames test S. typhimurium TA98, TA100, 33 to 220 g/plate1,2 Negative Zeiger et
TA1535, TA1537 al., 1985
Ames test S. typhimurium TA98, TA100, 100 to 2000 g/plate1 Negative Agarwal et
TA1535, TA1537, TA1538, al., 1985
TA2637
L5178Y/TK+/- mouse L5178Y/TK+/- mouse 0.01-0.3 µl/ml1 Negative Kirby et
lymphoma assay lymphoma cells al., 1983
Rec-assay Bacillus subtilis 500 g/disk Negative Tomita et al.,
1982
CHO mutation assay Chinese hamster ovary cells 1.5-2.8 mM Negative Phillips et al.,
1982
Unscheduled DNA synthesis Primary rat hepatocytes not specified Negative Hodgson et
assay al., 1982
Aldehydes
Isobutyraldehyde Gradient plate technique S. typhimurium G46, TA1535, 0.1 to 1000 g/ml Positive5/ McMahon et
TA100, C3076, TA1537, D3052, Negative6 al., 1979
TA1538, and TA98; E. coli
WP2 and WP2 uvrA-
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Isobutyraldehyde Ames test S. typhimurium 3 µmol/plate1 Negative Florin et
(cont'd) al., 1980
Spot test TA98, TA100, TA1535, TA1537
Sister chromatid exchange adult human lymphocytes 0.002% Negative Obe & Beek,
1979
Paper-disk method E. coli Sd-4-73 0.01- 0.025 ml/disk Negative Szybalski,
1958
2-Methylbutyraldehyde Ames test S. typhimurium TA98, TA100, 0.03 to 30 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
Ames test S. typhimurium TA98, TA100 9 nmol to 0.9 mmol/plate1 Negative Aeschbacher
and TA102 et al., 1989
3-Methylbutyraldehyde Sister chromatid exchange Adult human lymphocytes 0.002 to 0.003% Negative Obe & Beek,
1979
Ames test S. typhimurium TA98, TA100 0.01 nmol to 1 mol/plate1 Negative Aeschbacher
and TA102 et al., 1989
Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
2-Methylpentanal Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
Spot test TA1535 and TA1537 al., 1980
2,6-Dimethyloctanal Ames test S. typhimurium TA98, TA100, up to 3.6 mg/plate Negative Wild et al.,
TA1535, TA1537, TA1538 1983
Carboxylic acids
Isobutyric acid Paper-disk method E. coli Sd-4-73 0.01 to 0.025 ml/disk Negative Szybalski,
1958
Cell mutagenesis assay mouse lymphoma 500 g/ml7,8 Positive Heck et al,
L5178Y TK +/- (weak) 1989
600 g/ml9,10 Negative
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Unscheduled DNA synthesis rat hepatocytes 1000 g/ml9,10 Negative Heck et al.,
assay 1989
Plate incorporation assay S. typhimurium TA98, 100, 75 000 g/plate9,10 Negative Heck et al.,
1535, 1537, 1538 1989
Rec-assay B. subtilis H17 rec+ and 19 g/disk Negative Oda et al.,
M45 rec- 1978
2-Ethyl-1-hexanol In vivo dominant lethal ICR/SIM mice 250, 500, 1000 mg/kg Negative Rushbrook et
assay bw/day for 5 days al., 1982
In vivo chromosomal Fischer 344 rat bone marrow 16.7, 58.4 or 167 mg/kg Negative Putman et
aberration assay cells bw/day for 5 days al., 1983
Isobutyraldehyde Sex linked recessive Drosophila melanogaster 80 000 ppm (feeding) Negative Woodruff et
lethal mutation test 50 000 ppm (injection) al., 1985
1 Both with and without metabolic activation
2 Metabolic activation in the form of S9 derived from rat and hamster
3 It is assumed that no S9 was used
4 Test performed on the metabolites of 2-ethyl-1-hexanol
5 Positive in G46, TA100, E. coli WP2, WP2 uvrA-
6 Negative in TA1535, TA1537, TA1538, TA98, C3076 and D3052
7 Without metabolic activation
8 Lowest active dose tested
9 With metabolic activation
10 Highest inactive dose tested
a) Methyl substituted aliphatic alcohols, aldehydes, and carboxylic
acids
i) Isobutyl alcohol
Isobutyl alcohol produced negative results in Ames tests with
Salmonella typhimurium strains TA97, TA98, TA100, TA1535, TA1537 at
concentrations of 100 to 10 000 g/plate (Zeiger et al., 1988).
Negative mutagenicity results were also obtained by Shimizu et al.
(1985) at dose levels of 5 to 5000 g/plate in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538, and
E. coli strain WP2 uvrA-.
ii) Branched-chain aldehydes
In Salmonella typhimurium, isobutyraldehyde (in strains TA98,
TA100, TA1535, TA1537), 2-methylbutyraldehyde (TA98, TA100, TA1535,
TA1537), 3-methylbutyraldehyde (TA98, TA100, TA1535, TA1537) and
2-methylpentanal (TA100, TA1535, TA1537) were negative in spot tests
at a concentration of 3 mol/plate with and without metabolic
activation (Florin et al., 1980). Similar results were reported for
2-methylbutyraldehyde and 3-methylbutyraldehyde in S. typhimurium
strains TA98, TA100 and TA102 at concentrations of 9.0 nmol/plate to
0.9 µmol/plate and 0.01 nmol/plate to 1 µmol/plate, respectively, with
and without metabolic activation (Aeschbacher, 1989). Florin et al.
(1980) subsequently subjected 2-methylbutyraldehyde to a quantitative
Ames test assessment at concentrations of 0.03 to 30 µmol/plate, and
found no evidence of mutagenicity in S. typhimurium strains TA98,
TA100 and TA1535, with and without metabolic activation.
Isobutyraldehyde gave negative results for frame-shift mutations in
S. typhimurium C3076, TA1537, D3052, TA1538 and TA98, and base
substitution mutations in S. typhimurium TA1535, and gave positive
results for base substitution mutations in S. typhimurium G46, TA100
and E. coli WP2 and WP2 uvrA-(McMahon et al., 1979). It was
non-mutagenic in E. coli Sd-4-73 (Szybalski, 1958). In an in vivo
study, isobutyraldehyde was negative for sex-linked recessive lethal
mutation in germ cells of Drosophila melanogaster by adult feeding
at 80 000 ppm and adult injection at 50 000 ppm (Woodruff et al.,
1985).
iii) Branched-chain carboxylic acids
Isobutyric acid was non-mutagenic in the Rec-assay with E.
coli Sd-4-73 at dose levels of 0.01 to 0.025 ml/disk (Szybalski,
1958) and in B. subtilis H17 and M45 at a dose of 19 g/ml (Oda,
1978). In a mouse lymphoma L5178YTK +/- assay, isobutyric acid had
negative results at a dose level of 600 g/ml with metabolic activation
and had weakly positive results at a dose of 500 g/ml without
metabolic activation (Heck et al., 1989). Isobutyric acid had
negative results in the Ames test plate incorporation assay with
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and
TA1538 at doses ranging up to 75 000 g/ml (Heck et al., 1989).
Isobutyric acid did not induce unscheduled DNA synthesis in rat
hepatocytes at doses of up to 1000 g/ml (Heck et al., 1989).
Based on a weight of evidence analysis, aliphatic acyclic
branched-chain primary alcohols, aldehydes and carboxylic acids are
not mutagenic or clastogenic in bacterial, in vitro or in vivo
systems. Sporadic positives were observed in some strains in three of
the approximately 20 bacterial assays conducted on members of this
group. Isobutyric acid was found in a single study to be mutagenic
in vitro in the presence of metabolic activation. However,
isobutyric acid was found to be negative in the rat UDS assay. The
mutagenic activity of isobutyric acid is not, therefore, likely to be
related to a mechanism involving direct binding to DNA. Consistent
with a lack of DNA damage, both isobutyraldehyde and
3-methylbutyraldehyde were negative in the sister chromatid exchange
assay. In addition, 2-ethyl-1-hexanol was found to be negative in two
assays for in vitro mutagenic activity (see 2-ethyl-1-hexanol
below). There is no evidence of mutagenic or clastogenic activity
in vivo in any of the compounds tested.
b) alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
There are several reports in the literature concerning the in
vitro testing of 2-ethylhexanol for mutagenicity in bacteria.
2-Ethylhexanol has shown no evidence of mutagenicity in the Ames test
with Salmonella typhimurium strains TA98, TA100, TA1535, TA 1537,
TA1538 or TA2637 with or without metabolic activation (Kirby et
al., 1983; Zeiger et al., 1985; Agarwal et al., 1985). Agarwal
et al. (1985) reported that 2-ethylhexanol was moderately cytotoxic
in most of the cultures tested, thereby having the potential to mask a
mutagenic effect. Weak dose-related mutagenic activity (maximum
increase in mutant frequency was only 3.5 times background) was
reported for 2-ethylhexanol in the Salmonella 8-azaguanine
resistance assay without activation at concentrations up to 1.5 mM.
2-Ethylhexanol was highly cytotoxic at concentrations that were
mutagenic (Seed, 1982).
Further Ames test results on the urinary metabolites of 2-ethyl-1-
hexanol, using S. typhimurium strains TA98, TA100, TA1537, TA1538
and TA1535, indicated no mutagenicity (Divincenzo et al.,
1985). The measurements were made with six male Sprague-Dawley rats
that were administered 1000 mg 2-ethyl-1-hexanol/kg bw/day by gavage
for 15 days. Urine samples were collected for use in the Ames assay at
doses of up to 2 ml per plate.
2-Ethylhexanol was not mutagenic in the L5178Y TK +/- mouse
lymphoma cell mutagenicity assay, either with or without metabolic
activation (Kirby et al., 1983), and produced negative results in
the rec-assay in Bacillus subtilis at a concentration of 500 g/disk
(Tomita et al., 1982). Negative results have also been reported for
2-ethylhexanol in a clastogenicity test on cultured Chinese hamster
ovary cells (Phillips et al., 1982) and in an unscheduled DNA
synthesis assay in primary rat hepatocytes (Hodgson et al., 1982).
In in vivo assays, negative results were reported in a dominant
lethal assay using oral doses of 250, 500 or 1000 mg 2-ethylhexanol/kg
bw administered for five consecutive days to ICR/SIM mice (Rushbrook
et al., 1982). 2-Ethylhexanol did not induce chromosomal aberrations
in bone marrow cells after oral administration of 0.02, 0.07 or 0.2
ml/kg bw per day (approximately 16.7, 58.4, 167 mg/kg bw per day
assuming a density of 0.8344) to groups of five Fischer 344 male rats
for five days (Putman et al., 1983).
The weight of evidence indicates that 2-ethylhexanol is not
mutagenic or genotoxic. Additionally, the urinary metabolites of
2-ethyl-1-hexanol were not mutagenic in the Ames tests. Negative
in vivo results were reported in a dominant lethal assay and in a
chromosomal aberration assay.
2.1.3.5 Reproductive toxicity
Gray & Beamand (1984) and Sjoberg et al. (1986) examined the
effect of 2-ethylhexanol on in vitro primary cultures of rat
testicular cells. No effect on the rate of germ-cell detachment from
Sertoli cells was observed at a 2-ethylhexanol concentration of
2x10-4 M.
Sjoberg et al. (1986) also examined the effect of 2-ethylhexanol
on rat testes in vivo. No testicular damage was observed in six
young male Sprague-Dawley rats orally administered 2.7 mmol 2-
ethylhexanol/kg bw per day (approximately 352 mg/kg bw per day) for 5
days.
2.1.3.6 Developmental toxicity
a) Mice
An oral developmental toxicity study was conducted using CD-1 mice
(NTP, 1991). Groups of 28 plug-positive mice were exposed to
microencapsulated 2-ethylhexanol at concentrations of 0, 0.009, 0.03
or 0.09% in the diet throughout gestation (days 0 to 17). These
concentrations were reported to be equal to 0, 17, 59 or 191 mg
2-ethylhexanol/kg bw per day, respectively, based on food consumption.
No evidence of maternal or developmental toxicity was observed in the
study. There were no treatment-related effects with respect to the
number of corpora lutea, uterine implantation sites, pre- and
post-implantation sites, pre- and post-implantation loss, sex ratio or
live fetal body weight, or to the incidence of individual, external,
visceral, skeletal or total malformations or variations.
Hardin et al. (1987) examined the developmental toxicity of
orally administered 2-ethylhexanol in pregnant mice. Fifty female CD-1
mice were administered by gavage a dose of 1525 mg 2-ethylhexanol/kg
bw per day on gestation days 6 to 13. A concurrent control group was
employed. Maternal mortality in the study was 35%, with significant
reductions in maternal weight gain and in the number of viable litters
being observed. Significant reductions in the treated group were also
reported for the following parameters: number of liveborn per litter,
percentage of survival of the pups, and the birth weight and weight
gain of the pups. The results of this study are of questionable
significance due to the presence of maternal toxicity and are
difficult to interpret due to the fact that only one dose level was
employed.
b) Rats
Groups of ten pregnant Wistar rats were administered by gavage
doses of 130, 650 or 1300 mg 2-ethylhexanol/kg bw per day on gestation
days 6 to 15. Two control groups were included, one that received
doubly distilled water and a second that received doubly distilled
water containing the emulsifier (0.005% Cremophor(R) EL) used as the
vehicle in the treated groups. On day 20 of gestation, the rats were
killed.
Significant maternal toxicity was observed in the high-dose rats.
These rats exhibited significant reductions in food consumption, body
weights and body weight gain. On days 6 to 10, a body weight loss was
reported. Six of the rats in the high-dose group were found dead on
days 9, 10 or 13. Other adverse effects reported in the high-dose rats
included severe clinical symptoms (e.g., unsteady gait, apathy), light
brown-gray discoloration of the liver in the rats that died during the
study, lung oedema and emphysema, and markedly decreased mean gravid
uterus weights. In the 650 mg/kg bw per day dose group, two dams were
reported to exhibit piloerection. Significant embryotoxic and
fetotoxic effects, such as an increase in the number of resorptions
resulting in increased post-implantation loss, decreased mean fetal
body weight, increased incidence of fetuses with dilated renal pelvis
and/or hydroureter, and a higher number of fetuses with skeletal
malformations, also occurred in the high-dose group. Variations and
retardation were also reported in the high-dose group. A slight
decrease in mean fetal body weight (statistically significant for all
viable fetuses combined and male fetuses separately, but
non-significant for female fetuses separately) and a statistically
significant increase in the frequency of fetuses with skeletal
variations and retardation were reported for the mid-dose group. The
authors reported that the only indications of significant adverse
effects on reproduction occurred in the high-dose group, which were
associated with maternal toxicity (Anonymous, 1991).
In an oral study, Ritter et al. (1987) exposed groups of
pregnant Wistar rats to doses of 6.25 or 12.5 mmoles 2-ethylhexanol/kg
bw (approximately 814 or 1628 mg/kg bw) on day 12 of gestation, the
rats being killed on day 20 of gestation. Several litters were
examined from each of the two treatment groups. For the low- and
high-dose groups of 2-ethylhexanol, the percentage of surviving pups
with malformation was 2 and 22.2, respectively. The malformations
observed in the high-dose group included tail and limb defects and
hydronephrosis. As indicated from the results of tests following oral
exposure (Anonymous, 1991) and dermal exposure (Tyl et al., 1992) in
rats, it would be expected that maternal toxicity could have occurred
at both dose levels employed in the study of Ritter et al. (1987),
even though the effect of treatment on the mother rats was not
discussed by the authors.
Tyl et al. (1992) conducted a range-finding study and a main
study examining the developmental toxicity of dermally applied
2-ethylhexanol in Fischer 344 rats. In the range-finding study, doses
of 0, 0.5, 1.0, 2.0 or 3.0 ml 2-ethylhexanol/kg bw per day (stated by
the authors to be equivalent to 0, 420, 840, 1680 or 2520 mg/kg bw per
day, respectively) were applied by occluded dermal application for 6
hours per day on gestation days 6 to 15 to groups of eight rats.
Naïve, sham (treated with 3.0 ml distilled water/kg bw per day) and
positive (treated with 420 or 1260 mg methoxyethanol/kg bw per day)
control groups were employed in the range-finding study. Valproic acid
was administered orally to a group of eight rats at a dose of 400
mg/kg bw per day as an oral reference compound.
In the main study, doses of 0, 0.3, 1.0 or 3.0 ml
2-ethylhexanol/kg bw per day (stated by authors to be equivalent to 0,
252, 840 or 2520 mg/kg bw per day, respectively) were applied to
groups of 25 rats by occluded dermal application for 6 hours per day
on gestation days 6 to 15. A sham control group receiving 3.0 ml/kg bw
per day distilled water and a positive control group receiving 840 mg
2-methoxyethanol/kg bw per day were included (Tyl et al., 1992).
Both positive control groups (valproic acid and methoxyethanol)
exhibited developmental toxicity and teratogenic effects. With respect
to 2-ethylhexanol, maternal weight gain was significantly reduced
during gestation days 6 to 15 at dose levels of 1680 and 2520 mg/kg bw
per day in the range-finding study. At the highest dose level in the
main study, maternal weight gain was significantly reduced during
gestation days 6 to 9, but was not significantly different from sham
controls for any other period examined (i.e. gestation days 0 to 6, 6
to 15, 15 to 21, or 0 to 21). In both studies, exfoliation and
encrustation at the site of application occurred at all dose levels.
However, this effect was reported to be typical of the effect of
alcohols on the skin. Erythema scores were mild to moderate at 840 and
2520 mg 2-ethylhexanol/kg bw per day. Oedema was not observed at any
treatment level. No treatment-related developmental or teratogenic
effects were observed in the fetuses of any of the dose levels in
either study. The authors reported no-observed-effect levels of 252 mg
2-ethylhexanol/kg bw per day based in skin irritation and 840 mg/kg
weight per day based on systemic toxicity (reduced maternal body
weight gain) (Tyl et al., 1992).
In a study designed to evaluate the association between the
pharma-cokinetics and teratogenicity of organic acids, pregnant
Sprague-Dawley rats were administered 14.1 mmoles/kg bw methylhexanoic
acid, or 12.5 or 15.625 mmoles/kg bw 2-ethylhexanoic acid undiluted by
oral gavage on day 12 of gestation. At various post-treatment
intervals (0.25-24 hours), embryos were dissected and exocoelomic
fluid and yolk sacs were analysed for the presence of the test
material. Samples of maternal blood and skeletal muscle were analysed
to determine the concentration of the test substance. The mothers were
killed on day 20 and fetuses were examined. Methylhexanoic acid
exhibited no signs of embryotoxicity except for a slight reduction in
fetal weight which was attributed to maternal toxicity. Ethylhexanoic
acid was toxic to the embryos removed on day 12, causing increased
death and malformation and a reduction of fetal weight. Both
methylhexanoic acid and ethylhexanoic acid reached a high
concentration in maternal plasma and embryo, but methylhexanoic acid
was eliminated more rapidly (Scott et al., 1994).
An in vivo developmental toxicity screen was conducted on 15
aliphatic acids including three substances in this group. Groups of 9
to 18 timed-pregnant Sprague-Dawley rats were treated by gavage once
daily on gestation days 6 to 15 with 2-ethylbutyric acid (0, 150 or
200 mg/kg bw per day), 2-methylvaleric acid (0, 187.5 or 250 mg/kg bw
per day) and 5-methylhexanoic acid (0, 300 or 400 mg/kg bw per day).
2-Ethylhexanoic acid, a metabolite of 2-ethylhexanol, was also tested
at dose levels of 0, 900 or 1200 mg/kg bw per day.
Maternal body weights were determined on gestational days 6, 8,
10, 13, 16 and 20. Clinical observations were made on all animals
throughout the study. Pups in each litter were examined and counted.
Skeletal examination was performed on two postnatal day 6 survivors
from each group. After postnatal day 6, the dams were killed and the
number of uterine implantation sites was recorded. 2-Methylvaleric
acid, 2-ethylbutyric acid and 5-methylhexanoic acid induced motor
depression in some mothers, which was associated with severe
respiratory effects (i.e. rales or dyspnoea). Perinatal mortality was
noted for 2-ethylbutyric acid but only in litters of dams that
exhibited severe respiratory effects. There was no delayed
parturition, decreased progeny viability, lumbar ribs, or any other
signs of developmental toxicity in pups of mothers treated with
2-ethylbutyric acid, 2-methylvaleric acid, or 5-methylhexanoic acid.
The highest dose levels of 200 mg/kg bw per day for 2-ethylbutyric
acid, 250 mg/kg bw/day for 2-methylvaleric acid, and 400 mg/kg bw/day
for 5-methylhexanoic that did not produce any signs of developmental
toxicity are >10 000 times their respective daily per capita
intakes ("eaters only") from use as flavouring substances in the USA
and Europe (see Table 1).
All dams treated with 2-ethylhexanoic acid exhibited motor
depression. Developmental effects were observed in pups of mothers
treated with 2-ethylhexanoic acid at both dose levels, including
reduced progeny viability, full-litter resorptions, apparent
stillbirths, postnatal deaths, reduced pup weights, external
malformations, skeletal malformations, and lumbar ribs (Narotsky et
al., 1994). 2-Ethylhexanoic acid produced developmental toxicity only
in the presence of maternal toxicity.
2.1.3.7 Special studies on peroxisome proliferation
Concentrations of 0.1 or 0.5 mM 2-ethylhexanol did not induce
palmitoyl CoA oxidase activity (a marker enzyme for peroxisome
proliferation) in rat hepatocytes in vitro (Rhodes et al., 1984).
A concentration of 1 mM 2-ethylhexanol was reported to induce
significantly (approximately 9 times the control level) the activity
of carnitine acetyltransferase (a peroxisomal enzyme) in cultured rat
hepatocytes in vitro, while a concentration of 0.2 mM did not induce
this enzyme (Gray et al., 1982).
In an in vivo study, Rhodes et al. (1984) reported that the
administration of 1 mmol 2-ethylhexanol/kg bw per day (approximately
130 mg/kg bw per day) to five male Wistar rats by gavage for 14 days
did not result in hepatic peroxisome proliferation in the rats.
Moody & Reddy (1978), however, reported that in five male F344
rats fed 2% 2-ethylhexanol in the diet (approximately 1000 mg/kg bw
per day) for 3 weeks, induction of hepatic peroxisome proliferation
occurred. Significant increases (using a Student's t test) in
activities of the enzymes hepatic catalase and carnitine
acetyltransferase also were reported in the rats treated with
2-ethylhexanol.
Hodgson (1987) conducted a peroxisome induction study in groups of
F344 male and female (five/sex/group) by administering doses of 100,
320 or 950 mg 2-ethylhexanol/kg bw per day by gavage for 21 days. A
significant reduction in body weight gain was observed in the
high-dose group rats with no effect on food consumption. Both sexes of
rats exhibited significant hepatomegaly at a dose of 950 mg
2-ethylhexanol/kg bw per day. The activity of cyanide-insensitive
palmitoyl CoA oxidation was increased in a dose-related manner in
males, with a 1.6-fold increase above controls at 320 mg/kg bw per day
and a 5.4-fold increase at 950 mg/kg bw per day. In females, a
3.1-fold increase in palmitoyl CoA activity was reported at the
high-dose level. Lauric acid hydroxylase activity of peroxisomes was
observed (using electron microscopy) in the hepatocytes of the rats of
the high-dose group.
Keith et al. (1992) examined the dose-response relationship for
2-ethylhexanol with respect to peroxisome proliferation in rats and
mice. Groups of five male and five female Alderley Park rats and mice
were administered doses of 0, 140, 350, 700, 1050 or 1750 mg
2-ethylhexanol/kg bw per day for 14 days by gavage. Rats in the
high-dose group exhibited toxic effects, leading to death of the rats.
A dose-related increase in the relative liver weight of both rats and
mice was observed, with the increase being significant in rats at the
700 and the 1050 mg/kg bw per day dose levels (rats in the high-dose
group died or were killed during the study), in male mice at the three
highest dose levels (700, 1050 and 1750 mg/kg bw per day), and in
female mice at the highest dose level (1750 mg/kg bw per day).
2-Ethylhexanol administration resulted in a virtually linear
dose-related induction of peroxisomal ß-oxidation (measured as
palmitoyl CoA oxidation activities) in both rats and mice. The level
of induction was greatest in the male mice. The dose at which this
effect became statistically significant was not stated. The increase
in rats and female mice was approximately 4-fold at the highest dose,
while for male mice, the increase was approximately 12-fold at the
highest dose.
2.1.3.8 Special study on immunotoxicity
3-Methylpentanoic acid was administered to 6- to 8-week-old female
CD-1 or B6C3F1 mice in corn oil by gavage for 5 days at dose levels
of 0, 175, 350 or 700 mg/kg bw per day. Cell-mediated immunity was
assessed by conducting a host resistance assay (Listeria
monocytogenes bacterial challenge). Humoral immunity was measured by
the antibody plaque-forming cell (PFC) response to sheep erythrocytes.
Body weights, lymphoid organ weights and spleen cellularity were also
measured and were normal as compared to controls. 3-Methylpentanoic
acid did not modulate the cell-mediated or humoral immune response
(Gaworski et al., 1994).
3. REFERENCES
Aeschbacher, H., Wolleb, U., Loliger, J., Spadone, J., & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food. Chem. Toxicicol., 27: 227-232.
Agarwal, D.K., Lawrence, W.H., Nunez, L.J., & Autian, J. (1985)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. Environ. Health, 16:
61-69.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5: 625-636.
American Conference of Governmental Industrial Hygienists, Inc.
(ACGIH) (1989) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, OH. USA.
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anonymous (1991) Study of the prenatal toxicity of isodecanol,
2-ethylhexanol, and 711 alcohols (T.C.) in rats after oral
administration (gavage) (Laboratory and authors associated with the
study were stated to be confidential) (Microfiche OTS0524015-2).
Astill, B.D., Gingell, R., Guest, D., Hodgson, J.R., Murphy, S.R., &
Tyler, T.R. (1993) Subacute and subchronic oral toxicity of
ethylhexanol to fischer 344 rats and B6C3F1 mice. Toxicologist, 13:
70.
Astill, B.D., Deckardt, K., Gembardt, Chr., Gingell, R., Guest, D.,
Hodgson, J.R., Mellert, W., Murphy, S.R., & Tyler, T.R. (1996a)
Prechronic toxicity studies on 2-ethylhexanol in F334 rats and B6C3F1
mice. Fundam. Appl. Toxicol., 29: 31-39.
Astill, B.D., Gingell, R., Guest, D., Hellwig, J., Hodgson, J.R.,
Kuettler, K., Mellert, W., Murphy, S.R., Sielken, R.L., & Tyler, T.R.
(1996b) Oncogenicity testing of 2-ethylhexanol in Fischer 344 rats and
B6C3F1 mice. Fundam. Appl. Toxicol., 31: 29-41.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months (Project No. 3350057).
Unpublished report.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
partial purification and properties. Can. J. Biochem., 47: 265-272.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B. ed. Enzymatic basis of detoxification. Academic Press, New York,
Vol. I, pp. 231-248.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E. ed Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, Vol. IIA, pp. 283-327.
Butenandt, A. & Thomas, K. (1958) Hoppe-Seyler's zeitschrift fur
physiologische chemie - Band 313. Walter Gruyter and Co., pp. 22-29.
Carpanini, F.M.B., Gaunt, I.F., Kiss, I.S., Grasso, P., & Gangolli,
S.D. (1973) Short-term toxicity of isoamyl alcohol in rats. Food
Cosmet. Toxicol., 11: 713-724.
Chen, T.H., Kavanagh, T.J., Chang, C.C., & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol.,
1: 155-171.
CIVO-TNO (1994) In: Maarse, H & Visscher, C.A. ed. Volatile components
in food: Qualitative and quantitative data - Volume III, 7th ed.
Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard-adecision tree approach. Food Cosmet. Toxicol., 16: 255-276.
Dave, G. & Lidman, U. (1978) Biological and toxicological effects of
solvent extraction chemicals: Range finding acute toxicity in the
rainbow trout. J. Hydrometall., 3: 210-216.
Dawson, A.M., Holdsworth, C.D., & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 17: 97-100.
Deisinger, P.J., Boatman, R J., & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
Deuel, H.J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley Interscience, New York.
DiVincenzo, G.D.& Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid the in the rat. Toxicol. Appl. Pharmacol., 47:
609-612.
DiVincenzo, G.D., Hamilton, M.L., Meuller, K.R., Donish, W.H., &
Barber, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
Dziewiatowski, D.D., Ventakaraman, A., & Lewis, H.B. (1949) The
metabolism of some branched chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Eckfeldt, J.H.& Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Wood, A. ed. Methods in
enzymology. Academic Press, New York, pp. 474-479.
Engler, H.W. & Bahler, B. (1982) Private communication to FEMA.
Fassett, D.W. (1963) Organic acids, anhydrides, lactones, acid
halides and amides, thioacids. In: Industrial hygene and toxicology,
2nd ed. Wiley-Interscience, New York, Vol. II, p. 1783.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
J. Toxicol., 15: 219-232.
Food and Drug Administration (FDA) (1993) Priority-based assessment of
food additives (PAFA) database. Center for Food Safety and Applied
Nutrition, p. 58.
Gaillard, D. & Derache, R. (1965) Metabolisation de differents
alcools, present dans les buissons alcooliques, chez le rat. Trav.
Soc. Pharm. Montp., 25: 51-62.
Gaworski, C., Vollmuth, T., Dozier, M., Heck, J., Dunn, L., Ratajczak,
H., & Thomas, P. (1994) An immunotoxicity assessment of food
flavouring ingredients. Food. Chem. Toxicol., 32: 409-415.
Goldberg, E.M., Sandler, S., & Blendis, L.M. (1979) A statistical
study of profiles of volatile metabolites in hepatic encephalopathy.
Modeling and Simulation - Volume 10, Part 1: Biomedical. In:
Proceedings of the Tenth Annual Conference, Pittsburgh, 25-27 April.
Instrumental Society of America, University of Pittsburgh.
Gray, T.J.B. & Beamand, J.A. (1984) Effect of some phthalate estsers
and other testicular toxins on primary cultures of testicular cells.
Food Chem. Toxicol., 22: 123-131.
Gray, T.J.B., Beamand, J.A., Lake, B.G., Foster, J.R., & Gangolli,
S.D. (1982) Peroxisome proliferation in cultured rat hepatocytes
produced by clofibrate and phthalate ester metabolites. Toxicol.
Lett., 10: 273-279.
Haggard, H.W., Miller, D.P., & Greenberg, L.A. (1945) The amyl
alcohols and their ketones: Their metabolic fates and comparative
toxicities. J. Ind. Hyg. Toxicol., 27: 1-14.
Hamdoune, M., Duclos, S., Mounie, J., Santona, L., Lhuguento, J.C.,
Magdalou, J., & Gouda, H. (1995) In vitro glucuronidation of
peroxisomal proliferators: 2-ethylhexanoic acid enantiomers and their
structural analogs. Toxicol. Appl. Pharmacol., 131: 235-243.
Hardin, B.D., Schuler, R.L., Burg, J.R., Booth, G.M., Hazelden, K.P.,
MacKenzie, K.M., Piccirillo, V.J., & Smith K.N. (1987) Evaluation of
60 chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen., 7: 29-48.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9: 257.
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. Acta Pharmacol. Toxico., 27: 381-396.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fermentative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Hillbom, M.E., Franssila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Jpn.
J. Stud. Alcohol, 9: 101-106.
Hodge, H.C. (1943) Acute toxicity for rats and mice of 2-ethyl hexanol
and 2-ethyl hexyl phthalate. J. Proc. Soc. Exp. Biol. Med., 53:
20-22.
Hodgson, J. R. (1987) Results of peroxisome induction studies on
tri(2-ethylhexyl)trimellitate and 2-ethylhexanol. Toxicol. Ind.
Health, 3: 49-60.
Hodgson, J.R., Myhr, B.C., McKeon, M., & Brusick, D.J. (1982)
Evaluation of di-(2-ethylhexyl)phthalate and its major metabolites in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 4: 388.
Institut Français de Recherches et Essais Biologiques (IFREB) (1975)
Acute toxicity of 4 methlynonanoic acid in rats. Private
communication to FEMA.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Johannsen, E. & Purchase, I. F. (1969) Kafficorn malting and brewing
studies: XXI. The effect of fusel oil of Bantu beer on rat liver
S. Afr. Med. J., 43: 326-328.
Keith, Y., Cornu, M.C., Canning, P.M., Foster, J., Lhuguennot, J.C., &
Elcombe, R.C. (1992) Peroxisome proliferation due to di(2-ethylhexyl)
adipate, 2-ethylhexanol and 2-ethylhexanoic acid. Arch. Toxicol.,
66: 321-326.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Kirby, P.E., Pizzarello, R.F., Lawlor, T.E., Haworth, S.R., & Hodgson,
J.R. (1983) Evaluation of di-(2ethylhexyl)phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
Knaak, J.B., Kozbelt, S.J., & Sullivan, L.J. (1966) Metabolism of 2-
ethylhexyl sulfate by the rat and rabbit. Toxicol. Appl.
Pharmacol., 8: 369-379.
Lake, B.G., Gangolli, S.D., Grasso, P., & Lloyd, A.G. (1992) Studies
on the hepatic effects of orally administered
di-(2-ethylhexyl)phthalate in the rat. Toxicol. Appl. Pharmacol.,
32: 355-367.
Lester, D. & Benson, G.D. (1970) Alcohol oxidation in rats inhibited
by pyrazole, oximes, and amides. Science, 169: 282-284.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J., & Paulson, G.D.
ed. Intermediary xenobiotic metabolism in animals. Taylor and
Francis, London, pp. 119-138..
Lewis, R.J. (1989) Food additives handbook. Van Nostrand Reinhold, New
York, p. 259.
McMahon, R.E., Cline, J.C., & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39: 682-693.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45: 497-504.
Moreno, O.M. (1988) Private communication to FEMA.
Moreno, O.M. (1977) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Unpublished
report to RIFM.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of sctrucural class with
no-observed-effect-levels: a proposal for establishing a threshold of
concern. In Press.
Narotsky, M.G., Francis, E.Z., & Kavlock, R.T. (1994) Developmental
toxicity and structure-activity relationships of aliphatic acids,
including dose-response assessment of valproic acid in mice and rats.
Fundam. Appl. Toxicol., 22: 251-265.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
National Institute for Occupational Safety and Health (NIOSH) (1976)
In: Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects
of chemical substances. NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Institute for Occupational Safety and Health (NIOSH) (1991)
Toxic Param. Ind. Tox. Chem. Under single exposure (1982). Cited in:
Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects of
chemical substances (1976). NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Toxicology Program (NTP) (1991) Final report on the
developmental toxicity of 2 ethylhexanol in CD-1-Swiss mice. National
Toxicology Program, Research Triangle Park, NC, USA (PB91-185900).
Obe, G. & Beek, B. (1979) Mutagenic activity of aldehydes. Drug
Alcohol Depend., 4: 91-94.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181
Opdyke, D.L.J. & Leitizia, C. (1982) Frangrance raw materials
monographs. Food Cosmet. Toxicol., 20: 739-740.
Owen, G. & Meyer, F.J. (1971) Private communication to FEMA.
Phillips, B.J., James, T.E.B., & Gangolli, S.D. (1982) Genotixicity
studies of di(2ethylhexyl)phalate and its metabolites in CHO cells.
Mutat. Res., 102: 297-304.
Pietruszko, R., Crawford, K., & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenase from human liver, horse
liver, and yeast towards saturated and 2-enoic alcohols and aldehydes.
Arch. Biochem. Biophys,. 159: 50-60.
Posternak, J.M. Linder, A., & Vodoz, C.A. (1969) Subchronic feeding
study of 2-methylhexanoic acid in rats. Food Chem. Toxicol., 7:
405-407.
Prunonosa, J., Sagrista, M.L., & Bozal, J. (1991) Inactivation
mechanism of low-Km rat liver mitochondrial aldehyde dehydrogenase
by cyanamide in vitro. Drug Metab. Dispos., 19: 787-792.
Purchase, I.F.H. (1969) Studies in kafficorn malting and brewing:
XXII. The acute toxicity of some fusel oils found in bantu beer.
S. Afr. Med. J., June 21(Suppl.).
Putman, D.L., Moore, W.A., Schechtman, L.M., & Hodgson, J.R. (1983)
Cytogenic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 5: 227-231.
Rhodes, C., Soames, T., Stonard, M.D., Simpson, M.G., Vernall, A.J., &
Elcombe, C.R. (1984) The absence of testicular atrophy and in vivo and
in vitro effects on hepatocyte morphology and peroxisomal enzyme
activ. in male rats following the administration of several alkanols.
Toxicol. Lett., 21: 103-109.
Ritter, E.J., Scott, W.J., Randall, J.L., & Ritter, J.M. (1987)
Teratogenicity of di(2-ethylhexyl) phthalate, 2-ethylhexanol,
2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine.
Teratology, 35: 41-46.
Rushbrook, C.J., Jorgenson, T.A., & Hodgson, J.R. (1982) Dominant
lethal study of di-(2-ehtylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
Saito, M. (1975) Studies on the metabolism of lower alcohols.
Nichidai Igaku Zasshi, 34: 569-585.
Scala, R.A. & Burtis, E.G. (1973) Acute toxicity of a homologous
series of branched-chain primary alcohols. Am. Ind. Hyg. Assoc. J.,
34: 493-499.
Schafer, E.W. Jr. & Bowles, W.A. Jr. (1985) The acute toxicity and
repellence of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14: 111-129.
Schmidt, P., Gohlke, R., & Rothe, R. (1973) Zur toxizitat einiger
C8-aldehyde und alkohole. Z. Gesamte Hyg. Grenzgeb., 19: 485-490.
Scott, W.J. Jr., Collinfs, M.D., & Heinz, N. (1994) Pharmacokinetic
determinants of embryotoxicity in rats associated with organic acids.
Environ. Health Perspect., 102: 97-101.
Seed, J.L. (1982) Mutagenic activity of phthalate esters in bacterial
liquid suspension assays. Environ. Health Perspect., 45: 111-114.
Shaffer, C.B., Carpenter, C.P., & Smyth, H.F. (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. Ind. Hyg. Toxicol., 27: 130-1135.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report to RIFM.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S., & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27: 400-419.
Sjöberg, P., Bondesson, U., Gray, T.J.B., & Ploen, L. (1986) Effects
of di-(2-ethylhexyl)phthalate and five of its metabolites on rat
testis in vivo and in vitro. Acta Pharmacol. Toxicol., 58:
225-233.
Smyth, H.F. Jr., Carpenter, C.P., & Weil, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4: 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S., & Possani U.C. (1954)
Range finding toxicity data: List V. Arch. Ind. Hyg., 10: 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, U.C., Striegel,
J.A., & Nycum, J.S. (1969) Range finding toxicity data: List VII.
Am. Ind. Hyg. Assoc. J., 30: 470-476.
Stofberg, J. & Gruncschober, F. (1987) The consumption ratio and Food
Predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Szybalski, W. (1958) Special microbial systems: II. Obeservations on
chemical mutagenesis in microorganisms. Ann. NY Acad. Sci., 76:
475-489.
Tomita, I., Nakamura, Y., Aoki, N., & Inui, N. (1982)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
Tyl, R.W., Fisher, L.C., Kubena, M.F., Vrbanic, M.A., Gingell, R.,
Guest, D. Hodgson, J.R., Murphy, S.R., Tyler, T.R. and Astill, B.D.
(1992) The developmental toxicity of 2-ethyl-1-hexanol applied
dermally to pregnant Fischer 344 rats. Fundam. Appl. Toxicol., 19:
176-185.
Union Carbide Data Sheet, 2/4/59. Cited in: NIOSH ed. Registry of
toxic effects of chemical substances (1991).
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V., & Thomas, P.T. (1989)
Immunotoxicity assessment of flavoring ingredients using a rapid and
economical screen. Toxicologist, 9: 206.
Walkenstein, S.S. & Weinhouse, S. (1953) Oxidation of aldehydes by
mitochondria of rat tissues. J. Biol. Chem., 200: 515-523.
Weiner, H. (1980) Aldehyde oxidizing enzymes. In: Jakoby, W.B. ed
Enzymatic basis of detoxication. Academic Press, New York, Vol. I,
pp.261-275.
Wild, D., King, M.T., Gocke, E., & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, basc and micronucleus tests. Food chem.
Toxicol., 21(6): 707-719.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall, Ltd, London, UK, pp. 4-5.
Woodruff, R.C., Mason, J.M., Valencia, R., & Zimmering, S. (1985)
Mutagenesis testing in Drosophilia: V. Results of 53 coded compounds
tested for the National Toxicology Program. J. Environ. Mutagen., 7:
677-702.
World Health Organization (1987) Environmental Health Criteria 65:
Butanols - Four isomers: 1-butanol, 2-butanol, tert-butanol, and
isobutanol. World Health Organization, International Programme on
Chemical Safety, Geneva
Yasuhara, A., Fuwa, K., & Jimbu, M. (1982) Variation in concentration
of odorous components of pig feces with growth. Agric. Biol. Chem.,
46: 1381-1383.
Zabin, I. & Bloch, K. (1951) Studies on the utilization of isovaleric
acid in cholesterol synthesis. J. Biol. Chem., 192: 267-273.
Zeiger, E., Haworth, S., Mortelmans, K., & Speck, W. (1985)
Mutagenicity testing of di(2 ethylhexyl)phthalate and related
chemicals in salmonella. Environ. Mutagen., 7: 213-232.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 2: 1-158.
When rats and rabbits were administered 2-ethylhexanol-C14 or
2-ethylhexanol-C14 sulfate either orally or by intraperitoneal (i.p.)
injection, the principal metabolites included unchanged alcohol and
the sulfate ester, as well as hydroxylated 2-ethylhexanol and the
glucuronide conjugate of 2-ethylhexanoic acid (Knaak et al., 1966).
2-Ethylbutyric acid was administered orally and by subcutaneous
injection at a dose of approximately 1 gram to rabbits and 100 mg (as
the sodium salt) to rats. The acid was primarily excreted unchanged in
the urine as the glucuronic acid conjugate (Dziewiatowski et al.,
1949). In dogs, 2-ethylbutyric acid undergoes ß-oxidation and
decarboxylation to yield 2-pentanone (Deuel, 1957).
In conclusion, branched-chain alcohols, aldehydes and carboxylic
acids with bulky (ethyl, propyl, etc.) alpha-alkyl substituents are
metabolized by omega- and omega-1-oxidation to yield polar metabolites
capable of excretion in the urine. At high-dose levels these oxidation
pathways may be saturated, in which case, the corresponding acid may
undergo conjugation with glucuronic acid.
2.1.3 Toxicological studies
2.1.3.1 Acute toxicity
Acute toxicity studies have been reported for 20 of the 25
saturated aliphatic branched-chain primary alcohols, aldehydes and
acids in this group and are summarized in Table 2. The acute oral
toxicity of the group is demonstrated by oral LD50 values of >2000
mg/kg bw for the alcohols; >3200 mg/kg bw for the aldehydes; and
>1000 mg/kg bw for the carboxylic acids, except for isobutyric and
2-methylbutyric acid, which are 280 mg/kg bw, and 3-methylpentanoic
acid which is >700 mg/kg bw. The rat oral LD50 values for the three
alpha-ethyl-substituted substances 2-ethylbutyraldehdye,
2-ethylbutyric acid and 2-ethyl-1-hexanol are 3980 mg/kg bw, 2200
mg/kg bw and 2050-7100 mg/kg bw, respectively.
2.1.3.2 Short-term toxicity
The results of short-term toxicity studies with a
methyl-substituted alcohol (isobutyl alcohol), a structurally related
alcohol (isoamyl alcohol), and two methyl-substituted carboxylic acids
(isovaleric acid and 2-methylhexanoic acid) are summarized in Table 3
and described below. Short-term oral studies for 2-ethylbutyric acid
and 2-ethyl-1-hexanol are summarized in the section on
alpha-ethyl-substituted substances.
Table 2. Acute toxicity studies for saturated aliphatic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Isobutyl alcohol rat M/F oral 2640-3100 Smyth et al., 1954; WHO, 1987
M/F gavage 2650-3100 Purchase, 1969
mouse oral 3500 WHO, 1987
Isobutyraldehyde rat M/F oral 3730 Smyth et al., 1954
Isobutyric acid rat NR oral 280 Lewis, 1989
2-Methylbutyraldehyde rat NR oral 8570 Opdyke & Leitizia, 1982
2-Methylbutyric acid rat M/F oral 280-2200 Moreno et al., 1982; Lewis, 1989;
mouse M/F oral 1238 Schafer & Bowles, 1985
2-Ethylbutyraldehyde rat M/F oral 3980 Smyth et al., 1951
2-Ethylbutyric acid rat M/F oral 2200 Smyth et al., 1954
Isoamyl alcohol2 rat M/F oral 5700 Smyth et al., 1954
gavage 1300-4000 Purchase, 1969
3-Methylbutyraldehyde rat M/F oral 7166 Moreno, 1988
Isovaleric acid rat NR oral 2000; >3200 Fassett, 1963; NIOSH, 1976
2-Methylvaleric acid rat M/F oral 1600-3200 Smyth et al., 1954; ACGIH, 1989
3-Methylpentanoic acid rat NR oral >700 Vollmuth et al., 1989
3-Methyl-1-pentanol rat M/F oral >2000 Engler & Bahler, 1982
2-Ethyl-1-hexanol rat M/F oral 2050-7100 Hodge, 1943; Shaffer et al., 1945;
Smyth et al., 1969; Scala & Burtis, 1973;
Schmidt et al., 1973; Albro, 1975; Dave & Lidman, 1978
Table 2. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3,5,5-Trimethyl-1-hexanol rat M/F oral 2300 Moreno, 1977
3,5,5-Trimethylhexanal rat M/F oral 3240 Moreno, 1975; Opdyke & Leitizia, 1982
2-Methyloctanal rat M/F oral >5000 Moreno, 1977
3,7-Dimethyl-1-octanol rat M/F oral 5000 Shelanski & Moldovan, 1973; Moreno, 1977
4-Methylnonanoic acid rat NR oral 3700 IFREB, 1975
2-Methylundecanal rat M/F oral >5000 Owen & Meyer, 1971
4-Methylpentanoic acid rat NR oral 2050->3200 Smyth et al., 1954; Fassett, 1963; NIOSH, 1976;
Union Carbide, 1991;
mouse NR oral 5000 NIOSH, 1991
1 M = male; F = female; NR = Not reported.
2 Structurally related substance.
Table 3. Short-term and long-term toxicity studies for saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids
Substance Species (Sex) Route Duration NOEL Reference
(mg/kg bw
per day)
Short-term studies
Alcohols
Isobutyl alcohol rat (M/F) oral 90 days 1450 BASF, 1992
rat oral 4 months >0.126 nmol/g Hillbom et al., 1974
bw/day3 Johannsen &
rat (M/F) oral 53-56 weeks 2003 Purchase, 1969
Isoamyl alcohol2 rat (M/F) gavage 17 weeks 10003 Carpanini et al., 1973
rat (M/F) oral 56 weeks 20003 Johannsen & Purchase, 1969
2-Ethyl-1-hexanol mouse gavage 11 days 100 Astill et al., 1996a
mouse gavage 90 days 125 Astill et al., 1996a
mouse food 11 days 1150-4450 Astill et al., 1993
rat gavage 14 days 1303 Rhodes et al., 1984
NR Lake et al., 1975
rat oral 7 days 100 Astill et al., 1996a
rat gavage 11 days 125 Astill et al., 1996a
rat gavage 90 days <500-540 Astill et al., 1993
rat oral 11 days
Carboxylic Acid
2-Ethylbutyric acid rat oral 90 days 3003 Amoore et al., 1978
Isovaleric acid rat oral 90 days 25003 Amoore et al., 1978
2-Methylhexanoic acid rat (M/F) oral 90 days 33 Posternak et al., 1969
Long-term/ Carcinogenicity studies
2-Ethyl-1-hexanol mouse (M/F) gavage 540 days 200 Astill et al., 1996b
rat (M/F) gavage 730 days 50 Astill et al., 1996b
Table 3 (continued)
1 M = Male; F = Female; NR = not reported.
2 A structurally related branched-chain alcohol.
3 The study was performed at a single dose level or multiple dose levels that produced no adverse effects and, therefore, a NOEL was not
determined. The NOEL is probably higher than the reported dose level that produced no adverse effects.
a) Methyl substituted aliphatic alcohols, aldehydes and carboxylic
acids
i) 2-Methyl-1-propanol (isobutyl alcohol)
Groups of eleven 4-month-old male Wistar rats were given 1 M
solutions of isobutyl alcohol as their only drinking fluid for 4
months. Control animals received tap water. An ordinary diet was
provided for all animals. The level of isobutyl alcohol consumption
was reported to be 0.126 nmole/g bw/day at 120 days. At necropsy,
there was no evidence of hepatic steatosis, fibrosis or inflammation
(Hillbom et al., 1974).
Groups of 20 male and 20 female Wistar albino rats were given a
mixture of fusel oils including 0.2% isobutyl alcohol, which was
calculated (FDA, 1993) to provide an approximate daily intake of 200
mg/kg bw/day, as their sole drinking source for 53-56 weeks. Control
animals were given tap water. There were no statistically significant
differences in body or liver weights of treated animals as compared to
controls. Liver enzymes were determined at 2- to 4-week intervals and
revealed no significant differences in activity and protein content of
the liver between test and control animals. Histological examination
was performed on specimens of liver, kidney, heart, spleen and lung
and revealed no significant abnormalities (Johannsen & Purchase,
1969).
Groups of 10 male and 10 female Wistar rats were given 2-methyl-1-
propanol (i.e. isobutyl alcohol) in their drinking water for 3 months.
The test substance was administered in concentrations of 0, 1000, 4000
or 16 000 mg/litre, which was reported to correspond to approximate
dose levels of 0, 60, 340 or 1450 mg/kg bw per day. Food and drinking-
water consumption and body weight gain were not affected by the test
substance. All animals tested were free from adverse clinical effects.
Haematology and clinical chemistry parameters were measured and
revealed no treatment-related adverse effects. Gross pathology and
histopathological examinations were normal for all animals except for
testicular atrophy, which was observed in two males in the high-dose
group. Diffuse tubular generation and hyperplasia of the Leydig cells
were reported in the two animals. The authors concluded that the
absence of similar effects in other male rats indicated that the
effect was most likely unrelated to the test material. The authors
reported that the results of this study demonstrate a lack of toxicity
associated with administration of 2-methyl-1-propanol in the drinking-
water of rats, and that the NOEL is >1450 mg/kg bw per day (BASF,
1992). The NOEL is >100 000 times the estimated daily per capita
intake1 ("eaters only") of 4.85 g/kg bw of isobutyl alcohol from
its use as a flavouring substance in the USA and 8.79 g/kg bw of
isobutyl alcohol in Europe.
ii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage providing daily dose levels
of 0, 150, 500 or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight statistically significant reduction in body-weight
gain which was associated with reduced food intake. Over the entire
period of the study, however, there was no statistically significant
reduction in mean food intake. Examination of haematology, serum
analyses, urinalysis, renal concentration tests and organ weights
revealed no treatment-related effects. The animals were examined for
macroscopic abnormalities, and the major organs were weighed.
Microscopic examination was performed on several tissues of the
control and high-dose animals. No treatment-related abnormalities were
observed (Carpanini et al., 1973).
Groups of 20 male and 20 female Wistar albino rats were given a 2%
solution of isoamyl alcohol, which was calculated (FDA, 1993) to
provide an approximate daily intake of 2000 mg/kg bw per day, as their
sole drinking source for 53-56 weeks. Control animals were given tap
water. There were no statistically significant differences in body or
liver weights of treated animals as compared to controls. Liver
enzymes were determined at 2- to 4-week intervals and revealed no
significant differences in ADH, GOT or GPT activity and protein
content of the liver. Histological examination was performed on
specimens of liver, kidney, heart, spleen and lung and revealed no
significant abnormalities (Johannsen & Purchase, 1969).
iii) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0 or 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to provide a daily intake of 0 or 2500 mg/kg
bw. The rats were monitored for survival rates, body weight changes,
food intake, blood and urine analysis, organ weights and histology. At
2500 mg/kg bw per day, there were no observed changes in the rats
(Amoore et al., 1978).
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1995). Intake (mg/kg bw/day)
calculated as follows: [(mg/day)/body weight], where body weight = 60
kg. Slight variations may occur from rounding off.
iv) 2-Methylhexanoic acid
In a limited 90-day feeding study, 2-methylhexanoic acid was fed
to male and female Charles River CD rats at a dose of approximately 3
mg/kg bw/day. Observations included mortality rates, food intake, body
weight, haematology, blood urea, organ weights and histopathology. The
NOEL was 3 mg/kg bw dose level (Posternak et al., 1969) which is
>10 000 times the estimated daily per capita intake ("eaters only")
of 0.04 g/kg bw 2-methylhexanoic acid from use as a flavouring
substance in the USA and 0.24 g/kg bw from its use in Europe.
b) alpha-Ethyl substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
Eleven (11-) day and 90-day studies were conducted in B6C3F1 mice
as part of the dose selection process for an 18-month study (see
section 2.1.3.3). In the 11-day gavage study, groups of ten B6C3F1
mice of each sex were administrated nine applications of 0, 100, 330,
1000 or 1500 mg 2-ethylhexanol/kg bw per day. Mice in the high-dose
group exhibited effects such as ataxia, lethargy, loss of
consciousness, death in four females, increased absolute and relative
lever and stomach weights, macroscopic foci in the forestomach, and
microscopic lesions in the forestomach, liver, testes and kidneys.
These lesions consisted of hyperkeratosis and focal or multifocal
acanthosis and inflammatory oedema in the submucosa in most mice,
focal or multifocal ulceration of the mucous membrane in a few mice,
hypertrophy of hepatocytes in all mice, focal necrosis of liver cells
in one male and one female mouse, centrilobular fatty infiltration in
female mice that died intercurrently, tubular giant cells in
testicular tubules of two mice, and renal cortex tubular dilation and
nephrosis in mice that died intercurrently. Mice receiving the dose of
1000 mg/kg bw per day exhibited similar symptoms to those observed at
the highest dose (1500 mg/kg bw per day), but to a lesser degree,
while four mice (two male, two female) receiving 330 mg 2-ethyl
hexanol/kg bw per day had acanthosis in the mucous membrane of the
forestomach. No treatment-related adverse effects were observed in the
mice administered 100 mg 2-ethylhexanol/kg bw per day (Astill
et al., 1996a).
In the 90-day study, mice (number per group not reported) received
doses of 0, 25, 125, 250 or 500 mg 2-ethylhexanol/kg bw per day. At
the highest dose level, the treatment-related effects observed
included increased relative stomach weights in male mice and focal or
multifocal acanthosis of the forestomach mucosa in two male and one
female mouse. Increased relative stomach weights were observed in male
mice receiving 250 mg/kg bw per day. No treatment-related effects were
reported in mice of the 125 or 25 mg/kg bw per day dose level. Based
on the results of the 11- and 90-day studies, dose levels of 50, 200
or 750 mg 2-ethylhexanol/kg bw per day were chosen for the 18-month
mouse study (Astill et al., 1996a).
An 11-day mouse study was conducted using administration of
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of 10 male and 10 female B6C3F1 mice per group were used with
concentrations of microencapsulated material in the diet of 0.48,
0.96, 1.44 or 2.88%. The microcapsules contained 45.8% 2-ethylhexanol;
therefore, 2-ethylhexanol concentrations in the diet were 0.22, 0.44,
0.66 or 1.32%, respectively. These concentrations were reported to be
equal to doses of 550, 1150, 1800 or 4450 mg/kg bw per day for males
and 750, 1750, 2650 or 5750 mg/kg bw per day for females. The authors
reported that diet spillage did occur, but this was not quantified in
the treated groups of mice. As a result, the mg/kg bw per day dosage
calculations would not account for spillage and may represent
exaggerated 2-ethylhexanol intakes (Astill et al., 1993).
Significantly decreased body weight gains were observed at the
high-dose level for females throughout the test period and for males
at day 10 of the test period and at the 0.66% level in males at day 10
of the test period. No other treatment-related findings were reported.
The authors concluded the NOEL for male mice was in the range of 0.96
to 1.44% microencapsulated material (0.44 to 0.66% 2-ethylhexanol or
reportedly 1150 to 1800 mg/kg bw per day) and the NOEL for female mice
was in the range of 1.44 to 2.88% microencapsulated material (0.66 to
1.32% 2-ethylhexanol or reportedly 1800 to 4450 mg/kg bw per day)
(Astill et al., 1993). The NOELs reported in this study are higher
than the NOEL of 100 to 330 mg 2-ethylhexanol/kg bw per day reported
in the 11-day gavage study in B6C3F1 mice (Astill et al., 1996a),
most likely as a result of the different modes of administration. The
lack of correction for diet spillage in the microencapsulation study
may also have been a factor.
To study the effect of 2-ethylhexanol on rat liver and testes,
Rhodes et al. (1984) administered 1 mmol 2-ethylhexanol/kg bw per
day (approximately 130 mg/kg bw per day) to five Wistar rats by gavage
for 14 days. 2-Ethylhexanol administration did not produce testicular
atrophy, hepatomegaly, peroxisome proliferation or hypolipidaemia in
the rats. Lake et al. (1992) orally administered 1335 mg
2-ethylhexanol/kg bw per day to six male Wistar rats for seven days.
The authors reported significantly increased relative liver weights,
increased microsomal biphenyl 4-hydroxylase activity, increased
alcohol dehydrogenase activity in the centrilobular area of the liver
lobule, increased number of microbodies, and dilatation of the smooth
endoplasmic reticulum in the livers. No effect on the activities of
aniline 4-hydroxylase or mitochondrial succinate dehydrogenase was
observed.
In an 11-day gavage study, groups of ten male and ten female
Fischer F344 rats were administered doses of 0, 100, 330, 1000 or 1500
mg 2-ethylhexanol/kg bw per day. Adverse effects, including reduction
in food consumption and body weight gain, ataxia, lethargy, changes in
blood chemistry, effects on absolute (increased liver and stomach,
decreased spleen, brain, and adrenal) and relative (increased stomach,
liver, kidney, brain, adrenal, and lung, decreased spleen) organ
weights, gross lesions in the forestomach and microscopic findings in
the forestomach, liver (high-dose only), spleen and thymus, were
observed in the rats at the highest two dose levels. At the 330 mg/kg
bw per day dose, female rats exhibited increased relative kidney
weights, as well as microscopic lesions in the forestomach of one
female rat and in the thymus of both sexes (it was not stated whether
or not these lesions were treatment-related). No treatment-related
adverse effects were observed at 100 mg/kg bw per day (Astill et
al., 1996a).
In the 90-day study, groups of ten male and ten female Fischer
F344 rats were administered doses of 0, 25, 125, 250 or 500 mg
2-ethylhexanol/kg bw per day by gavage. Reduced body weight and body
weight gain, changes in blood chemistry (consisting of reduced
cholesterol, glucose, alanine aminotransferase and alkaline
phosphatase, levels and increased reticulocytes, total protein and
albumin levels), increased absolute and relative liver and stomach
weights and gross lesions in the forestomach were observed in the rats
receiving 500 mg 2-ethylhexanol/kg bw per day. Also observed in the
high-dose rats were microscopic changes consisting of focal or
multifocal acanthosis in the mucosa of six rats (one male, five
female) and whole mucose acanthosis and submucosa degeneration and
oedema in one male rat. In addition, a number of high-dose rats with
fatty infiltration in the liver, as well as a lower grade of fat
deposition in the liver, were observed. At 250 mg/kg bw per day,
changes in the blood chemistry (consisting of reduced glucose,
alkaline phosphatase and alanine aminotransferase levels), increased
relative liver and stomach weights, and a lower grade of fat
deposition in the liver cells of the male rats were observed. No
treatment-related adverse effects were observed at the 25 or 125 mg/kg
bw per day dose levels. Based on the results of the 11- and 90-day
studies, dose levels of 50, 150 or 500 mg 2-ethylhexanol/kg bw per day
were chosen for a two-year study (Astill et al., 1996a).
An 11-day rat study was also conducted by administering
2-ethylhexanol as microencapsulated material in the diet. Four dose
groups of ten male and ten female Fischer rats per group were fed
concentrations of microencapsulated material in the diet of 1, 2, 3 or
6%. The microcapsules contained 45.8% 2-ethylhexanol; therefore,
2-ethylhexanol concentrations in the diet were 0.46, 0.92, 1.38 or
2.75%, respectively. These concentrations were reported to be equal to
doses of 500, 980, 1430 or 2590 mg/kg bw per day for males and 540,
1060, 1580 or 2820 mg/kg bw per day for females. A vehicle control
group receiving placebo microcapsules was employed. Decreased food and
water (top three doses only) consumption was observed at all dose
levels at some point in the study period with decreased body weights
reported for both sexes at the top dose and in females at day four in
the second highest dose. Changes in blood chemistry, consisting of
decreased cholesterol, triglycerides and alanine aminotransferase
levels (all doses), decreased glucose, reticulocyte and mean
corpuscular volume levels (high-dose only), decreased platelet level
(top two doses only), increased total protein level (top three doses),
increased erythrocyte level (high-dose only) and increased absolute
and relative stomach and/or liver weights were observed to some extent
in all dose groups. Varying degrees of hypertrophy of the hepatocytes
were reported in the top three dose groups. The authors reported the
NOEL to be less than 0.46% in the diet (500 or 540 mg/kg bw per day
for male and female rats, respectively) (Astill et al., 1993).
ii) 2-Ethylbutyric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 0, 0.62, 1.25 or 10% 2-ethylbutyric acid in the
diet, equivalent to 0, 300, 625 or 5000 mg/kg bw per day,
respectively. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analysis, organ weights and
histology. At the 300 mg/kg bw dose level, there were no observed
changes in the rats. At both the 625 and 5000 mg/kg bw dose levels,
slight body weight changes were observed within the first 14 days of
the study (Amoore et al., 1978).
2.1.3.3 Long-term toxicity/carcinogenicity
The results of long-term toxicity/carcinogenicity studies on
2-ethyl-1-hexanol are summarized in Table 3 and described below.
a) Mice
Groups of 50 male and 50 female B6C3F1 mice were administered
2-ethylhexanol by gavage at doses of 0, 50, 200 or 750 mg/kg bw per
day, five days per week for 18 months. The vehicle consisted of doubly
distilled water containing approximately 5 mg Polyoxyl 35 Castor Oil
(Cremophor(R) EL) per 100 ml. Two control groups of 50 mice of each
sex were employed, one receiving doubly distilled water containing
approximately 5 mg Cremophor(R) EL/100 ml. The use of Cremophor(R)
EL was reported to have no effect on the outcome of the study. Food
consumption, body weights and haematological parameters were examined
at specific intervals during the study. At the end of the study, gross
and histopathological examinations were conducted.
No treatment-related adverse effects were observed in the mice
receiving 50 or 200 mg 2-ethylhexanol/kg bw per day. At the 750 mg/kg
bw per day dose level, body weight gain reductions of approximately 26
and 24% in males and females, respectively, were reported. These
weight gain reductions were associated with a significant reduction in
food consumption. Both male and female mice of the high-dose group
showed increased mortality compared to the vehicle control mice (i.e.
30% in the treated mice compared to 4 and 8% in the vehicle control
group male and female mice, respectively). With respect to
haematological parameters, both a slight increase in polymorphonuclear
neutrophils and a slight decrease in lymphocytes were observed in the
mice of the high-dose group. A slight, statistically insignificant,
increase in focal hyperplasia of the forestomach epithelium was also
observed in the high-dose group mice. A slight increase in
hepatocellular carcinomas in the female mice of the high-dose group
was determined to have no biological relevance. 2-Ethylhexanol showed
no evidence of carcinogenicity in this study (Astill et al., 1996b).
b) Rats
Groups of 50 male and 50 female Fischer F344 rats were
administered 2-ethylhexanol by gavage at doses of 0, 50, 150 or 500
mg/kg bw per day, 5 days per week for 2 years. The vehicle consisted
of doubly distilled water containing approximately 5 mg Polyoxyl 35
Castor Oil (Cremophor(R) EL) (as an emulsifier) per 100 ml. Two
control groups of 50 mice of each sex were used, one receiving doubly
distilled water containing 5 mg Cremophor(R) EL/100 ml. The use of
Cremophor(R) EL was reported to have no effect on the outcome of the
study. Food consumption, body weights, and haematological parameters
were examined at specific intervals during the study. At the end of
the study, gross and histopatho-logical examinations were conducted.
No treatment-related adverse effects were observed at the 50 mg/kg
bw per day dose level. At the 150 mg/kg bw per day dose level, rats
exhibited a body weight gain reduction of approximately 16% in males
and 12% in females. In addition, the rats also displayed a slightly
increased incidence of clinical signs, such as poor general condition,
laboured breathing, piloerection, and/or smearing of the genital
region with urine. A definite increase in the number of rats and/or a
definite increase in the incidence of clinical signs (same signs as
mentioned for the mid-dose group) was observed in the high-dose group
rats. Mortality was increased in the female rats of the high-dose
group (52% compared to 28% in the vehicle control) and both male and
female rats exhibited a reduction in the body weight gain (33% in
males and 31% in females). With respect to haematological parameters,
a slight increase in anisocytosis, predominantly microcytosis in
males, was observed after 12 months, but not after 18 or 24 months.
The incidence of bronchopneumonia was significantly increased in both
sexes at the high-dose level. This effect was found to be due to the
aspiration of the stomach contents, most likely resulting from the
poor general condition of the animals. 2-Ethylhexanol showed no
evidence of carcinogenicity in this study (Astill et al., 1996b).
2.1.3.4 Genotoxicity
The results of genotoxicity studies on saturated aliphatic acyclic
branched-chain primary alcohols, aldehydes, and carboxylic acids are
summarized in Table 4.
Table 4. Genotoxicity studies of saturated aliphatic acyclic branched-chain primary alcohols, aldehydes and carboxylic acids
Substance Test system Test object Dose level Results Reference
Alcohols
Isobutyl alcohol Modified Ames test S. typhimurium TA98, TA100, 100 to 10 000 g/plate1,2 Negative Zeiger et
TA1535 TA97 and TA1537 al., 1988
Modified Ames test S. typhimurium TA100, TA98, 5 to 5000 mg/plate1 Negative Shimizu et
TA1535, TA1537 and TA1538; al., 1985
E. coli WP2 uvrA
2-Ethyl-1-hexanol 8-azaguanine resistance S. typhimurium TA100 0.5 to 1.5 mM Positive3 Seed, 1982
assay
Ames test4 S. typhimurium TA98, TA100, up to 2 ml in urine/plate Negative Divincenzo
TA1535, TA1537, TA1538 et al., 1985
Ames test S. typhimurium TA98, TA100, 0.01 to 1.0 µl/plate1 Negative Kirby et
TA1535, TA1537, TA1538 al., 1983
Ames test S. typhimurium TA98, TA100, 33 to 220 g/plate1,2 Negative Zeiger et
TA1535, TA1537 al., 1985
Ames test S. typhimurium TA98, TA100, 100 to 2000 g/plate1 Negative Agarwal et
TA1535, TA1537, TA1538, al., 1985
TA2637
L5178Y/TK+/- mouse L5178Y/TK+/- mouse 0.01-0.3 µl/ml1 Negative Kirby et
lymphoma assay lymphoma cells al., 1983
Rec-assay Bacillus subtilis 500 g/disk Negative Tomita et al.,
1982
CHO mutation assay Chinese hamster ovary cells 1.5-2.8 mM Negative Phillips et al.,
1982
Unscheduled DNA synthesis Primary rat hepatocytes not specified Negative Hodgson et
assay al., 1982
Aldehydes
Isobutyraldehyde Gradient plate technique S. typhimurium G46, TA1535, 0.1 to 1000 g/ml Positive5/ McMahon et
TA100, C3076, TA1537, D3052, Negative6 al., 1979
TA1538, and TA98; E. coli
WP2 and WP2 uvrA-
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Isobutyraldehyde Ames test S. typhimurium 3 µmol/plate1 Negative Florin et
(cont'd) al., 1980
Spot test TA98, TA100, TA1535, TA1537
Sister chromatid exchange adult human lymphocytes 0.002% Negative Obe & Beek,
1979
Paper-disk method E. coli Sd-4-73 0.01- 0.025 ml/disk Negative Szybalski,
1958
2-Methylbutyraldehyde Ames test S. typhimurium TA98, TA100, 0.03 to 30 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
Ames test S. typhimurium TA98, TA100 9 nmol to 0.9 mmol/plate1 Negative Aeschbacher
and TA102 et al., 1989
3-Methylbutyraldehyde Sister chromatid exchange Adult human lymphocytes 0.002 to 0.003% Negative Obe & Beek,
1979
Ames test S. typhimurium TA98, TA100 0.01 nmol to 1 mol/plate1 Negative Aeschbacher
and TA102 et al., 1989
Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
TA1535 and TA1537 al., 1980
2-Methylpentanal Ames test S. typhimurium TA98, TA100, 3 mol/plate1 Negative Florin et
Spot test TA1535 and TA1537 al., 1980
2,6-Dimethyloctanal Ames test S. typhimurium TA98, TA100, up to 3.6 mg/plate Negative Wild et al.,
TA1535, TA1537, TA1538 1983
Carboxylic acids
Isobutyric acid Paper-disk method E. coli Sd-4-73 0.01 to 0.025 ml/disk Negative Szybalski,
1958
Cell mutagenesis assay mouse lymphoma 500 g/ml7,8 Positive Heck et al,
L5178Y TK +/- (weak) 1989
600 g/ml9,10 Negative
Table 4. Continued...
Substance Test system Test object Dose level Results Reference
Unscheduled DNA synthesis rat hepatocytes 1000 g/ml9,10 Negative Heck et al.,
assay 1989
Plate incorporation assay S. typhimurium TA98, 100, 75 000 g/plate9,10 Negative Heck et al.,
1535, 1537, 1538 1989
Rec-assay B. subtilis H17 rec+ and 19 g/disk Negative Oda et al.,
M45 rec- 1978
2-Ethyl-1-hexanol In vivo dominant lethal ICR/SIM mice 250, 500, 1000 mg/kg Negative Rushbrook et
assay bw/day for 5 days al., 1982
In vivo chromosomal Fischer 344 rat bone marrow 16.7, 58.4 or 167 mg/kg Negative Putman et
aberration assay cells bw/day for 5 days al., 1983
Isobutyraldehyde Sex linked recessive Drosophila melanogaster 80 000 ppm (feeding) Negative Woodruff et
lethal mutation test 50 000 ppm (injection) al., 1985
1 Both with and without metabolic activation
2 Metabolic activation in the form of S9 derived from rat and hamster
3 It is assumed that no S9 was used
4 Test performed on the metabolites of 2-ethyl-1-hexanol
5 Positive in G46, TA100, E. coli WP2, WP2 uvrA-
6 Negative in TA1535, TA1537, TA1538, TA98, C3076 and D3052
7 Without metabolic activation
8 Lowest active dose tested
9 With metabolic activation
10 Highest inactive dose tested
a) Methyl substituted aliphatic alcohols, aldehydes, and carboxylic
acids
i) Isobutyl alcohol
Isobutyl alcohol produced negative results in Ames tests with
Salmonella typhimurium strains TA97, TA98, TA100, TA1535, TA1537 at
concentrations of 100 to 10 000 g/plate (Zeiger et al., 1988).
Negative mutagenicity results were also obtained by Shimizu et al.
(1985) at dose levels of 5 to 5000 g/plate in Salmonella
typhimurium strains TA98, TA100, TA1535, TA1537, TA1538, and
E. coli strain WP2 uvrA-.
ii) Branched-chain aldehydes
In Salmonella typhimurium, isobutyraldehyde (in strains TA98,
TA100, TA1535, TA1537), 2-methylbutyraldehyde (TA98, TA100, TA1535,
TA1537), 3-methylbutyraldehyde (TA98, TA100, TA1535, TA1537) and
2-methylpentanal (TA100, TA1535, TA1537) were negative in spot tests
at a concentration of 3 mol/plate with and without metabolic
activation (Florin et al., 1980). Similar results were reported for
2-methylbutyraldehyde and 3-methylbutyraldehyde in S. typhimurium
strains TA98, TA100 and TA102 at concentrations of 9.0 nmol/plate to
0.9 µmol/plate and 0.01 nmol/plate to 1 µmol/plate, respectively, with
and without metabolic activation (Aeschbacher, 1989). Florin et al.
(1980) subsequently subjected 2-methylbutyraldehyde to a quantitative
Ames test assessment at concentrations of 0.03 to 30 µmol/plate, and
found no evidence of mutagenicity in S. typhimurium strains TA98,
TA100 and TA1535, with and without metabolic activation.
Isobutyraldehyde gave negative results for frame-shift mutations in
S. typhimurium C3076, TA1537, D3052, TA1538 and TA98, and base
substitution mutations in S. typhimurium TA1535, and gave positive
results for base substitution mutations in S. typhimurium G46, TA100
and E. coli WP2 and WP2 uvrA-(McMahon et al., 1979). It was
non-mutagenic in E. coli Sd-4-73 (Szybalski, 1958). In an in vivo
study, isobutyraldehyde was negative for sex-linked recessive lethal
mutation in germ cells of Drosophila melanogaster by adult feeding
at 80 000 ppm and adult injection at 50 000 ppm (Woodruff et al.,
1985).
iii) Branched-chain carboxylic acids
Isobutyric acid was non-mutagenic in the Rec-assay with E.
coli Sd-4-73 at dose levels of 0.01 to 0.025 ml/disk (Szybalski,
1958) and in B. subtilis H17 and M45 at a dose of 19 g/ml (Oda,
1978). In a mouse lymphoma L5178YTK +/- assay, isobutyric acid had
negative results at a dose level of 600 g/ml with metabolic activation
and had weakly positive results at a dose of 500 g/ml without
metabolic activation (Heck et al., 1989). Isobutyric acid had
negative results in the Ames test plate incorporation assay with
Salmonella typhimurium strains TA98, TA100, TA1535, TA1537 and
TA1538 at doses ranging up to 75 000 g/ml (Heck et al., 1989).
Isobutyric acid did not induce unscheduled DNA synthesis in rat
hepatocytes at doses of up to 1000 g/ml (Heck et al., 1989).
Based on a weight of evidence analysis, aliphatic acyclic
branched-chain primary alcohols, aldehydes and carboxylic acids are
not mutagenic or clastogenic in bacterial, in vitro or in vivo
systems. Sporadic positives were observed in some strains in three of
the approximately 20 bacterial assays conducted on members of this
group. Isobutyric acid was found in a single study to be mutagenic
in vitro in the presence of metabolic activation. However,
isobutyric acid was found to be negative in the rat UDS assay. The
mutagenic activity of isobutyric acid is not, therefore, likely to be
related to a mechanism involving direct binding to DNA. Consistent
with a lack of DNA damage, both isobutyraldehyde and
3-methylbutyraldehyde were negative in the sister chromatid exchange
assay. In addition, 2-ethyl-1-hexanol was found to be negative in two
assays for in vitro mutagenic activity (see 2-ethyl-1-hexanol
below). There is no evidence of mutagenic or clastogenic activity
in vivo in any of the compounds tested.
b) alpha-Ethyl-substituted aliphatic alcohols, aldehydes and
carboxylic acids
i) 2-Ethyl-1-hexanol
There are several reports in the literature concerning the in
vitro testing of 2-ethylhexanol for mutagenicity in bacteria.
2-Ethylhexanol has shown no evidence of mutagenicity in the Ames test
with Salmonella typhimurium strains TA98, TA100, TA1535, TA 1537,
TA1538 or TA2637 with or without metabolic activation (Kirby et
al., 1983; Zeiger et al., 1985; Agarwal et al., 1985). Agarwal
et al. (1985) reported that 2-ethylhexanol was moderately cytotoxic
in most of the cultures tested, thereby having the potential to mask a
mutagenic effect. Weak dose-related mutagenic activity (maximum
increase in mutant frequency was only 3.5 times background) was
reported for 2-ethylhexanol in the Salmonella 8-azaguanine
resistance assay without activation at concentrations up to 1.5 mM.
2-Ethylhexanol was highly cytotoxic at concentrations that were
mutagenic (Seed, 1982).
Further Ames test results on the urinary metabolites of 2-ethyl-1-
hexanol, using S. typhimurium strains TA98, TA100, TA1537, TA1538
and TA1535, indicated no mutagenicity (Divincenzo et al.,
1985). The measurements were made with six male Sprague-Dawley rats
that were administered 1000 mg 2-ethyl-1-hexanol/kg bw/day by gavage
for 15 days. Urine samples were collected for use in the Ames assay at
doses of up to 2 ml per plate.
2-Ethylhexanol was not mutagenic in the L5178Y TK +/- mouse
lymphoma cell mutagenicity assay, either with or without metabolic
activation (Kirby et al., 1983), and produced negative results in
the rec-assay in Bacillus subtilis at a concentration of 500 g/disk
(Tomita et al., 1982). Negative results have also been reported for
2-ethylhexanol in a clastogenicity test on cultured Chinese hamster
ovary cells (Phillips et al., 1982) and in an unscheduled DNA
synthesis assay in primary rat hepatocytes (Hodgson et al., 1982).
In in vivo assays, negative results were reported in a dominant
lethal assay using oral doses of 250, 500 or 1000 mg 2-ethylhexanol/kg
bw administered for five consecutive days to ICR/SIM mice (Rushbrook
et al., 1982). 2-Ethylhexanol did not induce chromosomal aberrations
in bone marrow cells after oral administration of 0.02, 0.07 or 0.2
ml/kg bw per day (approximately 16.7, 58.4, 167 mg/kg bw per day
assuming a density of 0.8344) to groups of five Fischer 344 male rats
for five days (Putman et al., 1983).
The weight of evidence indicates that 2-ethylhexanol is not
mutagenic or genotoxic. Additionally, the urinary metabolites of
2-ethyl-1-hexanol were not mutagenic in the Ames tests. Negative
in vivo results were reported in a dominant lethal assay and in a
chromosomal aberration assay.
2.1.3.5 Reproductive toxicity
Gray & Beamand (1984) and Sjoberg et al. (1986) examined the
effect of 2-ethylhexanol on in vitro primary cultures of rat
testicular cells. No effect on the rate of germ-cell detachment from
Sertoli cells was observed at a 2-ethylhexanol concentration of
2x10-4 M.
Sjoberg et al. (1986) also examined the effect of 2-ethylhexanol
on rat testes in vivo. No testicular damage was observed in six
young male Sprague-Dawley rats orally administered 2.7 mmol 2-
ethylhexanol/kg bw per day (approximately 352 mg/kg bw per day) for 5
days.
2.1.3.6 Developmental toxicity
a) Mice
An oral developmental toxicity study was conducted using CD-1 mice
(NTP, 1991). Groups of 28 plug-positive mice were exposed to
microencapsulated 2-ethylhexanol at concentrations of 0, 0.009, 0.03
or 0.09% in the diet throughout gestation (days 0 to 17). These
concentrations were reported to be equal to 0, 17, 59 or 191 mg
2-ethylhexanol/kg bw per day, respectively, based on food consumption.
No evidence of maternal or developmental toxicity was observed in the
study. There were no treatment-related effects with respect to the
number of corpora lutea, uterine implantation sites, pre- and
post-implantation sites, pre- and post-implantation loss, sex ratio or
live fetal body weight, or to the incidence of individual, external,
visceral, skeletal or total malformations or variations.
Hardin et al. (1987) examined the developmental toxicity of
orally administered 2-ethylhexanol in pregnant mice. Fifty female CD-1
mice were administered by gavage a dose of 1525 mg 2-ethylhexanol/kg
bw per day on gestation days 6 to 13. A concurrent control group was
employed. Maternal mortality in the study was 35%, with significant
reductions in maternal weight gain and in the number of viable litters
being observed. Significant reductions in the treated group were also
reported for the following parameters: number of liveborn per litter,
percentage of survival of the pups, and the birth weight and weight
gain of the pups. The results of this study are of questionable
significance due to the presence of maternal toxicity and are
difficult to interpret due to the fact that only one dose level was
employed.
b) Rats
Groups of ten pregnant Wistar rats were administered by gavage
doses of 130, 650 or 1300 mg 2-ethylhexanol/kg bw per day on gestation
days 6 to 15. Two control groups were included, one that received
doubly distilled water and a second that received doubly distilled
water containing the emulsifier (0.005% Cremophor(R) EL) used as the
vehicle in the treated groups. On day 20 of gestation, the rats were
killed.
Significant maternal toxicity was observed in the high-dose rats.
These rats exhibited significant reductions in food consumption, body
weights and body weight gain. On days 6 to 10, a body weight loss was
reported. Six of the rats in the high-dose group were found dead on
days 9, 10 or 13. Other adverse effects reported in the high-dose rats
included severe clinical symptoms (e.g., unsteady gait, apathy), light
brown-gray discoloration of the liver in the rats that died during the
study, lung oedema and emphysema, and markedly decreased mean gravid
uterus weights. In the 650 mg/kg bw per day dose group, two dams were
reported to exhibit piloerection. Significant embryotoxic and
fetotoxic effects, such as an increase in the number of resorptions
resulting in increased post-implantation loss, decreased mean fetal
body weight, increased incidence of fetuses with dilated renal pelvis
and/or hydroureter, and a higher number of fetuses with skeletal
malformations, also occurred in the high-dose group. Variations and
retardation were also reported in the high-dose group. A slight
decrease in mean fetal body weight (statistically significant for all
viable fetuses combined and male fetuses separately, but
non-significant for female fetuses separately) and a statistically
significant increase in the frequency of fetuses with skeletal
variations and retardation were reported for the mid-dose group. The
authors reported that the only indications of significant adverse
effects on reproduction occurred in the high-dose group, which were
associated with maternal toxicity (Anonymous, 1991).
In an oral study, Ritter et al. (1987) exposed groups of
pregnant Wistar rats to doses of 6.25 or 12.5 mmoles 2-ethylhexanol/kg
bw (approximately 814 or 1628 mg/kg bw) on day 12 of gestation, the
rats being killed on day 20 of gestation. Several litters were
examined from each of the two treatment groups. For the low- and
high-dose groups of 2-ethylhexanol, the percentage of surviving pups
with malformation was 2 and 22.2, respectively. The malformations
observed in the high-dose group included tail and limb defects and
hydronephrosis. As indicated from the results of tests following oral
exposure (Anonymous, 1991) and dermal exposure (Tyl et al., 1992) in
rats, it would be expected that maternal toxicity could have occurred
at both dose levels employed in the study of Ritter et al. (1987),
even though the effect of treatment on the mother rats was not
discussed by the authors.
Tyl et al. (1992) conducted a range-finding study and a main
study examining the developmental toxicity of dermally applied
2-ethylhexanol in Fischer 344 rats. In the range-finding study, doses
of 0, 0.5, 1.0, 2.0 or 3.0 ml 2-ethylhexanol/kg bw per day (stated by
the authors to be equivalent to 0, 420, 840, 1680 or 2520 mg/kg bw per
day, respectively) were applied by occluded dermal application for 6
hours per day on gestation days 6 to 15 to groups of eight rats.
Naïve, sham (treated with 3.0 ml distilled water/kg bw per day) and
positive (treated with 420 or 1260 mg methoxyethanol/kg bw per day)
control groups were employed in the range-finding study. Valproic acid
was administered orally to a group of eight rats at a dose of 400
mg/kg bw per day as an oral reference compound.
In the main study, doses of 0, 0.3, 1.0 or 3.0 ml
2-ethylhexanol/kg bw per day (stated by authors to be equivalent to 0,
252, 840 or 2520 mg/kg bw per day, respectively) were applied to
groups of 25 rats by occluded dermal application for 6 hours per day
on gestation days 6 to 15. A sham control group receiving 3.0 ml/kg bw
per day distilled water and a positive control group receiving 840 mg
2-methoxyethanol/kg bw per day were included (Tyl et al., 1992).
Both positive control groups (valproic acid and methoxyethanol)
exhibited developmental toxicity and teratogenic effects. With respect
to 2-ethylhexanol, maternal weight gain was significantly reduced
during gestation days 6 to 15 at dose levels of 1680 and 2520 mg/kg bw
per day in the range-finding study. At the highest dose level in the
main study, maternal weight gain was significantly reduced during
gestation days 6 to 9, but was not significantly different from sham
controls for any other period examined (i.e. gestation days 0 to 6, 6
to 15, 15 to 21, or 0 to 21). In both studies, exfoliation and
encrustation at the site of application occurred at all dose levels.
However, this effect was reported to be typical of the effect of
alcohols on the skin. Erythema scores were mild to moderate at 840 and
2520 mg 2-ethylhexanol/kg bw per day. Oedema was not observed at any
treatment level. No treatment-related developmental or teratogenic
effects were observed in the fetuses of any of the dose levels in
either study. The authors reported no-observed-effect levels of 252 mg
2-ethylhexanol/kg bw per day based in skin irritation and 840 mg/kg
weight per day based on systemic toxicity (reduced maternal body
weight gain) (Tyl et al., 1992).
In a study designed to evaluate the association between the
pharma-cokinetics and teratogenicity of organic acids, pregnant
Sprague-Dawley rats were administered 14.1 mmoles/kg bw methylhexanoic
acid, or 12.5 or 15.625 mmoles/kg bw 2-ethylhexanoic acid undiluted by
oral gavage on day 12 of gestation. At various post-treatment
intervals (0.25-24 hours), embryos were dissected and exocoelomic
fluid and yolk sacs were analysed for the presence of the test
material. Samples of maternal blood and skeletal muscle were analysed
to determine the concentration of the test substance. The mothers were
killed on day 20 and fetuses were examined. Methylhexanoic acid
exhibited no signs of embryotoxicity except for a slight reduction in
fetal weight which was attributed to maternal toxicity. Ethylhexanoic
acid was toxic to the embryos removed on day 12, causing increased
death and malformation and a reduction of fetal weight. Both
methylhexanoic acid and ethylhexanoic acid reached a high
concentration in maternal plasma and embryo, but methylhexanoic acid
was eliminated more rapidly (Scott et al., 1994).
An in vivo developmental toxicity screen was conducted on 15
aliphatic acids including three substances in this group. Groups of 9
to 18 timed-pregnant Sprague-Dawley rats were treated by gavage once
daily on gestation days 6 to 15 with 2-ethylbutyric acid (0, 150 or
200 mg/kg bw per day), 2-methylvaleric acid (0, 187.5 or 250 mg/kg bw
per day) and 5-methylhexanoic acid (0, 300 or 400 mg/kg bw per day).
2-Ethylhexanoic acid, a metabolite of 2-ethylhexanol, was also tested
at dose levels of 0, 900 or 1200 mg/kg bw per day.
Maternal body weights were determined on gestational days 6, 8,
10, 13, 16 and 20. Clinical observations were made on all animals
throughout the study. Pups in each litter were examined and counted.
Skeletal examination was performed on two postnatal day 6 survivors
from each group. After postnatal day 6, the dams were killed and the
number of uterine implantation sites was recorded. 2-Methylvaleric
acid, 2-ethylbutyric acid and 5-methylhexanoic acid induced motor
depression in some mothers, which was associated with severe
respiratory effects (i.e. rales or dyspnoea). Perinatal mortality was
noted for 2-ethylbutyric acid but only in litters of dams that
exhibited severe respiratory effects. There was no delayed
parturition, decreased progeny viability, lumbar ribs, or any other
signs of developmental toxicity in pups of mothers treated with
2-ethylbutyric acid, 2-methylvaleric acid, or 5-methylhexanoic acid.
The highest dose levels of 200 mg/kg bw per day for 2-ethylbutyric
acid, 250 mg/kg bw/day for 2-methylvaleric acid, and 400 mg/kg bw/day
for 5-methylhexanoic that did not produce any signs of developmental
toxicity are >10 000 times their respective daily per capita
intakes ("eaters only") from use as flavouring substances in the USA
and Europe (see Table 1).
All dams treated with 2-ethylhexanoic acid exhibited motor
depression. Developmental effects were observed in pups of mothers
treated with 2-ethylhexanoic acid at both dose levels, including
reduced progeny viability, full-litter resorptions, apparent
stillbirths, postnatal deaths, reduced pup weights, external
malformations, skeletal malformations, and lumbar ribs (Narotsky et
al., 1994). 2-Ethylhexanoic acid produced developmental toxicity only
in the presence of maternal toxicity.
2.1.3.7 Special studies on peroxisome proliferation
Concentrations of 0.1 or 0.5 mM 2-ethylhexanol did not induce
palmitoyl CoA oxidase activity (a marker enzyme for peroxisome
proliferation) in rat hepatocytes in vitro (Rhodes et al., 1984).
A concentration of 1 mM 2-ethylhexanol was reported to induce
significantly (approximately 9 times the control level) the activity
of carnitine acetyltransferase (a peroxisomal enzyme) in cultured rat
hepatocytes in vitro, while a concentration of 0.2 mM did not induce
this enzyme (Gray et al., 1982).
In an in vivo study, Rhodes et al. (1984) reported that the
administration of 1 mmol 2-ethylhexanol/kg bw per day (approximately
130 mg/kg bw per day) to five male Wistar rats by gavage for 14 days
did not result in hepatic peroxisome proliferation in the rats.
Moody & Reddy (1978), however, reported that in five male F344
rats fed 2% 2-ethylhexanol in the diet (approximately 1000 mg/kg bw
per day) for 3 weeks, induction of hepatic peroxisome proliferation
occurred. Significant increases (using a Student's t test) in
activities of the enzymes hepatic catalase and carnitine
acetyltransferase also were reported in the rats treated with
2-ethylhexanol.
Hodgson (1987) conducted a peroxisome induction study in groups of
F344 male and female (five/sex/group) by administering doses of 100,
320 or 950 mg 2-ethylhexanol/kg bw per day by gavage for 21 days. A
significant reduction in body weight gain was observed in the
high-dose group rats with no effect on food consumption. Both sexes of
rats exhibited significant hepatomegaly at a dose of 950 mg
2-ethylhexanol/kg bw per day. The activity of cyanide-insensitive
palmitoyl CoA oxidation was increased in a dose-related manner in
males, with a 1.6-fold increase above controls at 320 mg/kg bw per day
and a 5.4-fold increase at 950 mg/kg bw per day. In females, a
3.1-fold increase in palmitoyl CoA activity was reported at the
high-dose level. Lauric acid hydroxylase activity of peroxisomes was
observed (using electron microscopy) in the hepatocytes of the rats of
the high-dose group.
Keith et al. (1992) examined the dose-response relationship for
2-ethylhexanol with respect to peroxisome proliferation in rats and
mice. Groups of five male and five female Alderley Park rats and mice
were administered doses of 0, 140, 350, 700, 1050 or 1750 mg
2-ethylhexanol/kg bw per day for 14 days by gavage. Rats in the
high-dose group exhibited toxic effects, leading to death of the rats.
A dose-related increase in the relative liver weight of both rats and
mice was observed, with the increase being significant in rats at the
700 and the 1050 mg/kg bw per day dose levels (rats in the high-dose
group died or were killed during the study), in male mice at the three
highest dose levels (700, 1050 and 1750 mg/kg bw per day), and in
female mice at the highest dose level (1750 mg/kg bw per day).
2-Ethylhexanol administration resulted in a virtually linear
dose-related induction of peroxisomal ß-oxidation (measured as
palmitoyl CoA oxidation activities) in both rats and mice. The level
of induction was greatest in the male mice. The dose at which this
effect became statistically significant was not stated. The increase
in rats and female mice was approximately 4-fold at the highest dose,
while for male mice, the increase was approximately 12-fold at the
highest dose.
2.1.3.8 Special study on immunotoxicity
3-Methylpentanoic acid was administered to 6- to 8-week-old female
CD-1 or B6C3F1 mice in corn oil by gavage for 5 days at dose levels
of 0, 175, 350 or 700 mg/kg bw per day. Cell-mediated immunity was
assessed by conducting a host resistance assay (Listeria
monocytogenes bacterial challenge). Humoral immunity was measured by
the antibody plaque-forming cell (PFC) response to sheep erythrocytes.
Body weights, lymphoid organ weights and spleen cellularity were also
measured and were normal as compared to controls. 3-Methylpentanoic
acid did not modulate the cell-mediated or humoral immune response
(Gaworski et al., 1994).
3. REFERENCES
Aeschbacher, H., Wolleb, U., Loliger, J., Spadone, J., & Liardon, R.
(1989) Contribution of coffee aroma constituents to the mutagenicity
of coffee. Food. Chem. Toxicicol., 27: 227-232.
Agarwal, D.K., Lawrence, W.H., Nunez, L.J., & Autian, J. (1985)
Mutagenicity evaluation of phthalic acid esters and metabolites in
Salmonella typhimurium cultures. J. Toxicol. Environ. Health, 16:
61-69.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5: 625-636.
American Conference of Governmental Industrial Hygienists, Inc.
(ACGIH) (1989) Documentation of the threshold limit values and
biological exposure indices. Cincinnati, OH. USA.
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anonymous (1991) Study of the prenatal toxicity of isodecanol,
2-ethylhexanol, and 711 alcohols (T.C.) in rats after oral
administration (gavage) (Laboratory and authors associated with the
study were stated to be confidential) (Microfiche OTS0524015-2).
Astill, B.D., Gingell, R., Guest, D., Hodgson, J.R., Murphy, S.R., &
Tyler, T.R. (1993) Subacute and subchronic oral toxicity of
ethylhexanol to fischer 344 rats and B6C3F1 mice. Toxicologist, 13:
70.
Astill, B.D., Deckardt, K., Gembardt, Chr., Gingell, R., Guest, D.,
Hodgson, J.R., Mellert, W., Murphy, S.R., & Tyler, T.R. (1996a)
Prechronic toxicity studies on 2-ethylhexanol in F334 rats and B6C3F1
mice. Fundam. Appl. Toxicol., 29: 31-39.
Astill, B.D., Gingell, R., Guest, D., Hellwig, J., Hodgson, J.R.,
Kuettler, K., Mellert, W., Murphy, S.R., Sielken, R.L., & Tyler, T.R.
(1996b) Oncogenicity testing of 2-ethylhexanol in Fischer 344 rats and
B6C3F1 mice. Fundam. Appl. Toxicol., 31: 29-41.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months (Project No. 3350057).
Unpublished report.
Blair, A.H. & Bodley, F.H. (1969) Human liver aldehyde dehydrogenase:
partial purification and properties. Can. J. Biochem., 47: 265-272.
Bosron, W.F. & Ting-Kai, L. (1980) Alcohol dehydrogenase. In: Jacoby,
W.B. ed. Enzymatic basis of detoxification. Academic Press, New York,
Vol. I, pp. 231-248.
Brabec, M.J. (1993) Aldehydes and acetals. In: Clayton, G.D. &
Clayton, F.E. ed Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, Vol. IIA, pp. 283-327.
Butenandt, A. & Thomas, K. (1958) Hoppe-Seyler's zeitschrift fur
physiologische chemie - Band 313. Walter Gruyter and Co., pp. 22-29.
Carpanini, F.M.B., Gaunt, I.F., Kiss, I.S., Grasso, P., & Gangolli,
S.D. (1973) Short-term toxicity of isoamyl alcohol in rats. Food
Cosmet. Toxicol., 11: 713-724.
Chen, T.H., Kavanagh, T.J., Chang, C.C., & Trosko, J.E. (1984)
Inhibition of metabolic cooperation in Chinese hamster V79 cells by
various organic solvents and simple compounds. Cell Biol. Toxicol.,
1: 155-171.
CIVO-TNO (1994) In: Maarse, H & Visscher, C.A. ed. Volatile components
in food: Qualitative and quantitative data - Volume III, 7th ed.
Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard-adecision tree approach. Food Cosmet. Toxicol., 16: 255-276.
Dave, G. & Lidman, U. (1978) Biological and toxicological effects of
solvent extraction chemicals: Range finding acute toxicity in the
rainbow trout. J. Hydrometall., 3: 210-216.
Dawson, A.M., Holdsworth, C.D., & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 17: 97-100.
Deisinger, P.J., Boatman, R J., & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24: 429-440.
Deuel, H.J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley Interscience, New York.
DiVincenzo, G.D.& Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid the in the rat. Toxicol. Appl. Pharmacol., 47:
609-612.
DiVincenzo, G.D., Hamilton, M.L., Meuller, K.R., Donish, W.H., &
Barber, E.D. (1985) Bacterial mutagenicity testing of urine from rats
dosed with 2-ethylhexanol derived plasticizers. Toxicology, 34:
247-259.
Dziewiatowski, D.D., Ventakaraman, A., & Lewis, H.B. (1949) The
metabolism of some branched chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Eckfeldt, J.H.& Yonetani, Y. (1982) Isoenzymes of aldehyde
dehydrogenase from horse liver. In: Wood, A. ed. Methods in
enzymology. Academic Press, New York, pp. 474-479.
Engler, H.W. & Bahler, B. (1982) Private communication to FEMA.
Fassett, D.W. (1963) Organic acids, anhydrides, lactones, acid
halides and amides, thioacids. In: Industrial hygene and toxicology,
2nd ed. Wiley-Interscience, New York, Vol. II, p. 1783.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames test.
J. Toxicol., 15: 219-232.
Food and Drug Administration (FDA) (1993) Priority-based assessment of
food additives (PAFA) database. Center for Food Safety and Applied
Nutrition, p. 58.
Gaillard, D. & Derache, R. (1965) Metabolisation de differents
alcools, present dans les buissons alcooliques, chez le rat. Trav.
Soc. Pharm. Montp., 25: 51-62.
Gaworski, C., Vollmuth, T., Dozier, M., Heck, J., Dunn, L., Ratajczak,
H., & Thomas, P. (1994) An immunotoxicity assessment of food
flavouring ingredients. Food. Chem. Toxicol., 32: 409-415.
Goldberg, E.M., Sandler, S., & Blendis, L.M. (1979) A statistical
study of profiles of volatile metabolites in hepatic encephalopathy.
Modeling and Simulation - Volume 10, Part 1: Biomedical. In:
Proceedings of the Tenth Annual Conference, Pittsburgh, 25-27 April.
Instrumental Society of America, University of Pittsburgh.
Gray, T.J.B. & Beamand, J.A. (1984) Effect of some phthalate estsers
and other testicular toxins on primary cultures of testicular cells.
Food Chem. Toxicol., 22: 123-131.
Gray, T.J.B., Beamand, J.A., Lake, B.G., Foster, J.R., & Gangolli,
S.D. (1982) Peroxisome proliferation in cultured rat hepatocytes
produced by clofibrate and phthalate ester metabolites. Toxicol.
Lett., 10: 273-279.
Haggard, H.W., Miller, D.P., & Greenberg, L.A. (1945) The amyl
alcohols and their ketones: Their metabolic fates and comparative
toxicities. J. Ind. Hyg. Toxicol., 27: 1-14.
Hamdoune, M., Duclos, S., Mounie, J., Santona, L., Lhuguento, J.C.,
Magdalou, J., & Gouda, H. (1995) In vitro glucuronidation of
peroxisomal proliferators: 2-ethylhexanoic acid enantiomers and their
structural analogs. Toxicol. Appl. Pharmacol., 131: 235-243.
Hardin, B.D., Schuler, R.L., Burg, J.R., Booth, G.M., Hazelden, K.P.,
MacKenzie, K.M., Piccirillo, V.J., & Smith K.N. (1987) Evaluation of
60 chemicals in a preliminary developmental toxicity test. Teratog.
Carcinog. Mutagen., 7: 29-48.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9: 257.
Hedlund, S.G. & Kiessling, K.H. (1969) The physiological mechanism
involved in hangover. Acta Pharmacol. Toxico., 27: 381-396.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fermentative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Hillbom, M.E., Franssila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Jpn.
J. Stud. Alcohol, 9: 101-106.
Hodge, H.C. (1943) Acute toxicity for rats and mice of 2-ethyl hexanol
and 2-ethyl hexyl phthalate. J. Proc. Soc. Exp. Biol. Med., 53:
20-22.
Hodgson, J. R. (1987) Results of peroxisome induction studies on
tri(2-ethylhexyl)trimellitate and 2-ethylhexanol. Toxicol. Ind.
Health, 3: 49-60.
Hodgson, J.R., Myhr, B.C., McKeon, M., & Brusick, D.J. (1982)
Evaluation of di-(2-ethylhexyl)phthalate and its major metabolites in
the primary rat hepatocyte unscheduled DNA synthesis assay.
Environ. Mutagen., 4: 388.
Institut Français de Recherches et Essais Biologiques (IFREB) (1975)
Acute toxicity of 4 methlynonanoic acid in rats. Private
communication to FEMA.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Johannsen, E. & Purchase, I. F. (1969) Kafficorn malting and brewing
studies: XXI. The effect of fusel oil of Bantu beer on rat liver
S. Afr. Med. J., 43: 326-328.
Keith, Y., Cornu, M.C., Canning, P.M., Foster, J., Lhuguennot, J.C., &
Elcombe, R.C. (1992) Peroxisome proliferation due to di(2-ethylhexyl)
adipate, 2-ethylhexanol and 2-ethylhexanoic acid. Arch. Toxicol.,
66: 321-326.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Kirby, P.E., Pizzarello, R.F., Lawlor, T.E., Haworth, S.R., & Hodgson,
J.R. (1983) Evaluation of di-(2ethylhexyl)phthalate and its major
metabolites in the Ames test and L5178Y mouse lymphoma mutagenicity
assay. Environ. Mutagen., 5: 657-663.
Knaak, J.B., Kozbelt, S.J., & Sullivan, L.J. (1966) Metabolism of 2-
ethylhexyl sulfate by the rat and rabbit. Toxicol. Appl.
Pharmacol., 8: 369-379.
Lake, B.G., Gangolli, S.D., Grasso, P., & Lloyd, A.G. (1992) Studies
on the hepatic effects of orally administered
di-(2-ethylhexyl)phthalate in the rat. Toxicol. Appl. Pharmacol.,
32: 355-367.
Lester, D. & Benson, G.D. (1970) Alcohol oxidation in rats inhibited
by pyrazole, oximes, and amides. Science, 169: 282-284.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J., & Paulson, G.D.
ed. Intermediary xenobiotic metabolism in animals. Taylor and
Francis, London, pp. 119-138..
Lewis, R.J. (1989) Food additives handbook. Van Nostrand Reinhold, New
York, p. 259.
McMahon, R.E., Cline, J.C., & Thompson, C.Z. (1979) Assay of 855 test
chemicals in ten tester strains using a new modification of the Ames
test for bacterial mutagens. Cancer Res., 39: 682-693.
Moody, D.E. & Reddy, J.K. (1978) Hepatic peroxisome (microbody)
proliferation in rats fed plasticizers and related compounds.
Toxicol. Appl. Pharmacol., 45: 497-504.
Moreno, O.M. (1988) Private communication to FEMA.
Moreno, O.M. (1977) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Unpublished
report to RIFM.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of sctrucural class with
no-observed-effect-levels: a proposal for establishing a threshold of
concern. In Press.
Narotsky, M.G., Francis, E.Z., & Kavlock, R.T. (1994) Developmental
toxicity and structure-activity relationships of aliphatic acids,
including dose-response assessment of valproic acid in mice and rats.
Fundam. Appl. Toxicol., 22: 251-265.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
National Institute for Occupational Safety and Health (NIOSH) (1976)
In: Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects
of chemical substances. NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Institute for Occupational Safety and Health (NIOSH) (1991)
Toxic Param. Ind. Tox. Chem. Under single exposure (1982). Cited in:
Christensen, H.E. & Fairchild, E.J. ed. Registry of toxic effects of
chemical substances (1976). NIOSH, Washington, DC, p. 649 (Entry NY
14000).
National Toxicology Program (NTP) (1991) Final report on the
developmental toxicity of 2 ethylhexanol in CD-1-Swiss mice. National
Toxicology Program, Research Triangle Park, NC, USA (PB91-185900).
Obe, G. & Beek, B. (1979) Mutagenic activity of aldehydes. Drug
Alcohol Depend., 4: 91-94.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181
Opdyke, D.L.J. & Leitizia, C. (1982) Frangrance raw materials
monographs. Food Cosmet. Toxicol., 20: 739-740.
Owen, G. & Meyer, F.J. (1971) Private communication to FEMA.
Phillips, B.J., James, T.E.B., & Gangolli, S.D. (1982) Genotixicity
studies of di(2ethylhexyl)phalate and its metabolites in CHO cells.
Mutat. Res., 102: 297-304.
Pietruszko, R., Crawford, K., & Lester, D. (1973) Comparison of
substrate specificity of alcohol dehydrogenase from human liver, horse
liver, and yeast towards saturated and 2-enoic alcohols and aldehydes.
Arch. Biochem. Biophys,. 159: 50-60.
Posternak, J.M. Linder, A., & Vodoz, C.A. (1969) Subchronic feeding
study of 2-methylhexanoic acid in rats. Food Chem. Toxicol., 7:
405-407.
Prunonosa, J., Sagrista, M.L., & Bozal, J. (1991) Inactivation
mechanism of low-Km rat liver mitochondrial aldehyde dehydrogenase
by cyanamide in vitro. Drug Metab. Dispos., 19: 787-792.
Purchase, I.F.H. (1969) Studies in kafficorn malting and brewing:
XXII. The acute toxicity of some fusel oils found in bantu beer.
S. Afr. Med. J., June 21(Suppl.).
Putman, D.L., Moore, W.A., Schechtman, L.M., & Hodgson, J.R. (1983)
Cytogenic evaluation of di-(2-ethylhexyl)phthalate and its major
metabolites in Fischer 344 rats. Environ. Mutagen., 5: 227-231.
Rhodes, C., Soames, T., Stonard, M.D., Simpson, M.G., Vernall, A.J., &
Elcombe, C.R. (1984) The absence of testicular atrophy and in vivo and
in vitro effects on hepatocyte morphology and peroxisomal enzyme
activ. in male rats following the administration of several alkanols.
Toxicol. Lett., 21: 103-109.
Ritter, E.J., Scott, W.J., Randall, J.L., & Ritter, J.M. (1987)
Teratogenicity of di(2-ethylhexyl) phthalate, 2-ethylhexanol,
2-ethylhexanoic acid, and valproic acid, and potentiation by caffeine.
Teratology, 35: 41-46.
Rushbrook, C.J., Jorgenson, T.A., & Hodgson, J.R. (1982) Dominant
lethal study of di-(2-ehtylhexyl)phthalate and its major metabolites
in ICR/SIM mice. Environ. Mutagen., 4: 387.
Saito, M. (1975) Studies on the metabolism of lower alcohols.
Nichidai Igaku Zasshi, 34: 569-585.
Scala, R.A. & Burtis, E.G. (1973) Acute toxicity of a homologous
series of branched-chain primary alcohols. Am. Ind. Hyg. Assoc. J.,
34: 493-499.
Schafer, E.W. Jr. & Bowles, W.A. Jr. (1985) The acute toxicity and
repellence of 933 chemicals to house and deer mice. Arch. Environ.
Contam. Toxicol., 14: 111-129.
Schmidt, P., Gohlke, R., & Rothe, R. (1973) Zur toxizitat einiger
C8-aldehyde und alkohole. Z. Gesamte Hyg. Grenzgeb., 19: 485-490.
Scott, W.J. Jr., Collinfs, M.D., & Heinz, N. (1994) Pharmacokinetic
determinants of embryotoxicity in rats associated with organic acids.
Environ. Health Perspect., 102: 97-101.
Seed, J.L. (1982) Mutagenic activity of phthalate esters in bacterial
liquid suspension assays. Environ. Health Perspect., 45: 111-114.
Shaffer, C.B., Carpenter, C.P., & Smyth, H.F. (1945) Acute and
subacute toxicity of di(2-ethylhexyl) phthalate with note upon its
metabolism. J. Ind. Hyg. Toxicol., 27: 130-1135.
Shelanski, M.V. & Moldovan, M. (1973) Acute oral and dermal toxicity
studies. Unpublished report to RIFM.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S., & Matsushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27: 400-419.
Sjöberg, P., Bondesson, U., Gray, T.J.B., & Ploen, L. (1986) Effects
of di-(2-ethylhexyl)phthalate and five of its metabolites on rat
testis in vivo and in vitro. Acta Pharmacol. Toxicol., 58:
225-233.
Smyth, H.F. Jr., Carpenter, C.P., & Weil, C.S. (1951) Range finding
toxicity data: List IV. Arch. Ind. Hyg. Occup. Med., 4: 119-122.
Smyth, H.F., Carpenter, C.P., Weil, C.S., & Possani U.C. (1954)
Range finding toxicity data: List V. Arch. Ind. Hyg., 10: 61-68.
Smyth, H.F., Carpenter, C.P., Weil, C.S., Pozzani, U.C., Striegel,
J.A., & Nycum, J.S. (1969) Range finding toxicity data: List VII.
Am. Ind. Hyg. Assoc. J., 30: 470-476.
Stofberg, J. & Gruncschober, F. (1987) The consumption ratio and Food
Predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Szybalski, W. (1958) Special microbial systems: II. Obeservations on
chemical mutagenesis in microorganisms. Ann. NY Acad. Sci., 76:
475-489.
Tomita, I., Nakamura, Y., Aoki, N., & Inui, N. (1982)
Mutagenic/carcinogenic potential of DEHP and MEHP. Environ. Health
Perspect., 45: 119-125.
Tyl, R.W., Fisher, L.C., Kubena, M.F., Vrbanic, M.A., Gingell, R.,
Guest, D. Hodgson, J.R., Murphy, S.R., Tyler, T.R. and Astill, B.D.
(1992) The developmental toxicity of 2-ethyl-1-hexanol applied
dermally to pregnant Fischer 344 rats. Fundam. Appl. Toxicol., 19:
176-185.
Union Carbide Data Sheet, 2/4/59. Cited in: NIOSH ed. Registry of
toxic effects of chemical substances (1991).
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Vollmuth, T.A., Heck, J.D., Ratajczak, H.V., & Thomas, P.T. (1989)
Immunotoxicity assessment of flavoring ingredients using a rapid and
economical screen. Toxicologist, 9: 206.
Walkenstein, S.S. & Weinhouse, S. (1953) Oxidation of aldehydes by
mitochondria of rat tissues. J. Biol. Chem., 200: 515-523.
Weiner, H. (1980) Aldehyde oxidizing enzymes. In: Jakoby, W.B. ed
Enzymatic basis of detoxication. Academic Press, New York, Vol. I,
pp.261-275.
Wild, D., King, M.T., Gocke, E., & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
salmonella/microsome, basc and micronucleus tests. Food chem.
Toxicol., 21(6): 707-719.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall, Ltd, London, UK, pp. 4-5.
Woodruff, R.C., Mason, J.M., Valencia, R., & Zimmering, S. (1985)
Mutagenesis testing in Drosophilia: V. Results of 53 coded compounds
tested for the National Toxicology Program. J. Environ. Mutagen., 7:
677-702.
World Health Organization (1987) Environmental Health Criteria 65:
Butanols - Four isomers: 1-butanol, 2-butanol, tert-butanol, and
isobutanol. World Health Organization, International Programme on
Chemical Safety, Geneva
Yasuhara, A., Fuwa, K., & Jimbu, M. (1982) Variation in concentration
of odorous components of pig feces with growth. Agric. Biol. Chem.,
46: 1381-1383.
Zabin, I. & Bloch, K. (1951) Studies on the utilization of isovaleric
acid in cholesterol synthesis. J. Biol. Chem., 192: 267-273.
Zeiger, E., Haworth, S., Mortelmans, K., & Speck, W. (1985)
Mutagenicity testing of di(2 ethylhexyl)phthalate and related
chemicals in salmonella. Environ. Mutagen., 7: 213-232.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988) Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 2: 1-158.
