
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES 40
Prepared by:
The forty-ninth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
ESTERS OF ALIPHATIC ACYCLIC PRIMARY ALCOHOLS WITH BRANCHED-CHAIN
ALIPHATIC ACYCLIC ACIDS
First draft prepared by
Dr G. Semino,
Institute of Pharmacological Sciences
University of Milan
Milan, Italy
1. Evaluation
1.1 Introduction
1.2 Estimated daily per capita intake
1.3 Absorption, metabolism and elimination
1.4 Application of the procedure for the safety evaluation of
flavouring agents
1.5 Consideration of combined intakes
1.6 Conclusions
2. Relevant background information
2.1 Explanation
2.2 Biological data
2.2.1 Absorption, distribution and excretion
2.2.2 Biotransformations
2.2.2.1 Branched-chain aliphatic acids
2.2.2.2 Aliphatic linear alcohols
2.2.3 Toxicological studies
2.2.3.1 Acute toxicity
2.2.3.2 Short-term toxicity studies
2.2.3.3 Long-term toxicity/carcinogenicity studies
2.2.3.4 Genotoxicity studies
2.2.3.5 Reproductive toxicity studies
3. References
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 32 flavouring agents that
includes selected esters of aliphatic acyclic primary alcohols with
branched-chain aliphatic acyclic acids using the Procedure for the
Safety Evaluation of Flavouring Agents (the "Procedure") (see Figure 1
and Table 1).
Table 1. Summary of results of the safety evaluations of esters of aliphatic acyclic primary alcohols and branched-chain aliphatic
acyclic acids
Step 1: All of the substances in the group are in structural class I, the human intake threshold of which is 1800 µg per person per day
Step 2: All substances in this group are metabolized to innocuous products
No. Substance Step A3 Step A4 Comments Conclusion based on
Does intake exceed Endogenous or current levels of
intake threshold? metabolized to intake
(µg/person per day) endogenous substances?1
0185 Methyl isobutyrate No N/R No safety concern
USA: 270 Europe: 23
0186 Ethyl isobutyrate No N/R No safety concern
USA: 470 Europe: 750
0187 Propyl isobutyrate No N/R No safety concern
USA: .08 Europe: 15
0188 Butyl isobutyrate No N/R No safety concern
USA: 1.9 Europe: 2.7
0189 Hexyl isobutyrate No N/R No safety concern
USA: 57 Europe: 3.00
0190 Heptyl isobutyrate No N/R No safety concern
USA: 3.0 Europe: 0.00
0191 trans-3-Heptenyl 2-
methylpropanoate No N/R No safety concern
USA: 2.3 Europe: 0.01
0192 Octyl isobutyrate No N/R No safety concern
USA: 5.0 Europe: 11
0193 Dodecyl isobutyrate No N/R No safety concern
USA: 0.76 Europe: 50
0194 Isobutyl isobutyrate No N/R No safety concern
USA: 2.3 Europe: 65
0195 Methyl isovalerate No N/R No safety concern
USA: 110 Europe: 7.8
0196 Ethyl isovalerate No N/R No safety concern
USA: 540 Europe: 760
Table 1. Continued...
No. Substance Step A3 Step A4 Comments Conclusion based on
Does intake exceed Endogenous or current levels of
intake threshold? metabolized to intake
(µg/person per day) endogenous substances?1
0197 Propyl isovalerate No N/R No safety concern
USA: 0.10 Europe: 2.00
0198 Butyl isovaletate No N/R No safety concern
USA: 500 Europe: 94
0199 Hexyl 3-methylbutanoate No N/R No safety concern
USA: 3.1 Europe: 2.3
0200 Octyl isovalerate No N/R No safety concern
USA: 0.57 Europe: 7.3
0201 Nonyl isovalerate No N/R No safety concern
USA: 0.08 Europe: 0.01
0202 3-Hexenyl 3-
methylbutanoate No N/R No safety concern
USA: 30 Europe: 9.4
0203 2-Methylpropyl 3-
methylbutyrate No N/R No safety concern
USA: 130 Europe: 78
0204 2-Methylbutyl 3-
methylbutanoate No N/R No safety concern
USA: 0.95 Europe: 0.86
0205 Methyl 2-methylbutyrate No N/R No safety concern
USA: 69 Europe: 390
0206 Ethyl 2-methylbutyrate Yes Yes The components ethyl alcohol No safety concern
USA: 560 Europe: 2200 and 2-methylbutyric acid are
endogenous. The acid is as
an intermediate in the
metabolism of the amino acid
isoleucine (Voet & Voet, 1990)
0207 n-Butyl 2-methylbutyrate No N/R No safety concern
USA: 0.02 Europe: 26
0208 Hexyl 2-methylbutanoate No N/R No safety concern
USA: 8.6 Europe: 4.9
Table 1. Continued...
No. Substance Step A3 Step A4 Comments Conclusion based on
Does intake exceed Endogenous or current levels of
intake threshold? metabolized to intake
(µg/person per day) endogenous substances?1
0209 Octyl 2-methylbutyrate No N/R No safety concern
USA: 0.10 Europe: 0.01
0210 Isopropyl 2-methylbutyrate No N/R No safety concern
USA: 0.10 Europe: 4.9
0211 3-Hexenyl 2-
methylbutanoate No N/R No safety concern
USA: 8.8 Europe: 5
0212 2-Methylbutyl 2-
methylbutyrate No N/R No safety concern
USA: 0.04 Europe: 3.6
0213 Methyl 2-methylpentanoate No N/R No safety concern
USA: 0.02 Europe: 0.17
0214 Ethyl 2-methylpentaoate No N/R No safety concern
USA: 320 Europe: 7.6
0215 Ethyl 3-methylpentanoate No N/R No safety concern
USA: 5.90 Europe: 0.31
0216 Methyl 4-methylvalerate No N/R No safety concern
USA: 0.10 Europe: 0.03
1 N/R: Not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3
of the Procedure
The Committee had previously evaluated one member of the group,
ethyl isovalerate, at its eleventh meeting (Annex 1, reference 14).
Because of a lack of data, the Committee was unable to allocate an ADI
to ethyl isovalerate.
1.2 Estimated daily per capita intake
The total annual production volume of the 32 esters of aliphatic
acyclic primary alcohols with branched-chain aliphatic acyclic acids
from their use as flavouring substances is approximately 16 tonnes in
the USA (NAS, 1987) and 32 tonnes in Europe (IOFI, 1995). In the USA,
approximately 67% of the total annual volume (NAS, 1987) is accounted
for by four esters: ethyl isobutyrate, ethyl isovalerate, butyl
isovalerate and ethyl 2-methylbutyrate. In Europe, more than 90% of
the total annual volume (IOFI, 1995) is accounted for by four esters:
ethyl isobutyrate, ethyl isovalerate, ethyl 2-methylbutyrate and
methyl 2-methylbutyrate. In the unlikely event that all of the
substances in this group were simultaneously consumed on a daily
basis, the estimated total daily per capita intake is 3 mg per
person per day in the USA and 4.6 mg/person per day in Europe. The
daily per capita intake of the branched-chain acids (i.e. isobutyric
acid, isovaleric acid, and 2-methylbutyric acid) formed via hydrolysis
of these esters is 2 mg/person per day in the USA and 3.1 mg/person
per day in Europe.
Esters of aliphatic acyclic primary alcohols and branched-chain
aliphatic acyclic acids have been detected in a wide variety of foods.
Quantitative data on the natural occurrence of these esters have been
reported for seven substances in the group and corresponds to a total
of 14 tonnes per year (CIVO-TNO, 1994). This estimate is approximately
equal to the estimated intake from their use as flavouring substances.
In the USA, the consumption of isobutyrate esters from natural
occurrence in food is equivalent to their use as flavouring substances
(Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987). The
consumption of isovalerate esters and 2-methylbutyrate esters from
natural food sources is several orders of magnitude higher than from
their use as flavouring agents.
1.3 Absorption, metabolism and elimination
It is expected that the esters in this group will be readily
hydrolysed to their component alcohols and carboxylic acids in the
intestinal tract, blood and liver. The metabolism of the hydrolysis
products is discussed in the introduction to this chapter on
flavouring agents.
1.4 Application of the Procedure for the Safety
Evaluation of Flavouring Agents
Step 1. All of the 32 esters of aliphatic acyclic primary alcohols
and branched-chain aliphatic acyclic acids were classified in
structural class I.
Step 2. At current levels of intake (see Table 1) these esters
would not be expected to saturate the metabolic pathways, and all the
compounds were predicted to be metabolized to innocuous products.
Step A3. All but one of the 32 esters of aliphatic acyclic primary
alcohols with branched-chain aliphatic acyclic acids have USA and
European estimated daily per capita intakes (see Table 1) that fall
below the human intake threshold for class I (1800 µg/person per day),
indicating that they pose no safety concern when used at current
levels of estimated intake as flavouring agents. Only ethyl
2-methylbutyrate has an estimated intake greater than 1800 µg/person
per day, which is 2200 µg/person per day in Europe.
Step A4. Ethyl 2-methylbutyrate is expected to be hydrolysed to
ethyl alcohol and 2-methylbutyric acid, which is endogenous.
Therefore, this substance was determined to be of no safety concern
based on its structural class and known metabolism.
1.5 Consideration of combined intakes
The stepwise evaluations of the 32 esters of aliphatic acyclic
primary alcohols with branched-chain aliphatic acyclic acids used as
flavouring substances are summarized in Table 1.
In the unlikely event that all 32 esters of aliphatic acyclic
primary alcohols with branched-chain aliphatic acyclic acids would be
consumed simultaneously on a daily basis, the estimated combined
intake will exceed the human intake threshold for class I. Since all
the 32 substances in this group are expected to be efficiently
metabolized, the combined intake level is not expected to saturate
metabolic pathways. On this basis of the evaluation of the collective
data, the Committee concluded that there were no safety concerns from
combined intake.
1.6 Conclusions
The Committee concluded that the substances in this group would
not present safety concerns at currently estimated levels of intake.
No toxicity data were required for the application of the
Procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
Procedure.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
The purpose of this monograph is to provide a safety evaluation of
a group of aliphatic acyclic esters formed from simple saturated
aliphatic branched-chain acids and linear and branched-chain aliphatic
alcohols using the safety evaluation procedure for flavouring
substances considered at the forty-fourth meeting and modified at the
forty-fifth meeting of JECFA. JECFA has previously evaluated one
member of the group, ethyl iso-valerate, at the JECFA meeting in
1967. Because of lack of data, the committee was unable to assign an
ADI for ethyl iso-valerate (Annex 1, reference 14).
This monograph summarizes the key data relevant to the safety
evaluation of 32 esters formed from the esterification of the
saturated aliphatic acyclic branched-chain acids, isobutyric acid,
isovaleric acid, 2-methylbutyric acid, and 2-, 3-, and
4-methylpentanoic acids with 9 aliphatic acyclic linear primary
alcohols (i.e., methanol, ethanol, propanol, butanol, hexanol,
heptanol, octanol, nonanol, and dodecyl alcohol), 2 unsaturated linear
primary alcohols (i.e., 3-hexenol and 3-heptenol), and 3 simple
aliphatic branched-chain alcohols (i.e., isopropyl alcohol, isobutyl
alcohol and 2-methylbutyl alcohol).
2.2 Biological data
2.2.1 Absorption, distribution and excretion
In general, aliphatic esters are rapidly hydrolysed to their
component alcohols and carboxylic acids (see Fig. 1). Hydrolysis is
catalysed by classes of enzymes recognized as carboxylesterases or
esterases. In mammals, these enzymes occur in most tissues throughout
the body (Heymann, 1980; Anders, 1989) but predominate in the
hepatocytes (Heymann, 1980). Select isoenzymes exhibit an increase in
enzyme binding (lower Km) and maximum velocity (Vmax) as the carbon
chain length of either the alcohol or carboxylic acid component of the
substrate increases (Heymann, 1980).
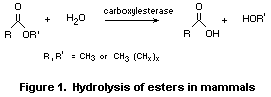 Branched-chain esters in this group are hydrolysed in vitro to
their corresponding branched-chain acids (isobutyric acid, isovaleric
acid or 2-methylbutyric acid) and corresponding aliphatic acyclic
alcohols. Table 2 summarizes the hydrolysis data for the five esters
containing a branched-chain alcohol and/or branched-chain acid (i.e.,
ethyl iso-valerate, iso-amyl caproate, benzyl iso-butyrate,
iso-amyl butyrate and iso-amyl iso-valerate). Following
hydrolysis, short-chain (<C6) branched-chain acids and alcohols
are rapidly absorbed from the gastrointestinal tract (Dawson et
al., 1964; Gaillard & Derache, 1965).
Table 2. In vitro ester hydrolysis data
Ester Artificial Artificial Rat liver Rat small % hydrolysis
gastric juice1 pancreatic juice1 preparation1 intestinal after 2 hours
t0.52 (min) t0.5 (min) t0.5 (sec) mucosa1
t0.5 (sec)
Ethyl iso-valerate 1390 198 2.35 133 63; 344
Isoamyl caproate7 146 37.8 NR NR NR
Benzyl isobutyrate7 577 17.8 0.0422 0.0707 NR
Isoamyl butyrate7 660 11.3 0.492 0.0713 123; 1004
Isoamyl isovalerate7 295 10.2 NR NR NR
Benzyl 2-methylbutanoate7 NR NR NR NR 1005
Isoamyl acetate7 NR NR NR NR 205; 1006
1 Longland et al., 1977
2 t1/2 = Half-life
3 In artificial gastric juice. Gangolli & Shilling, 1968
4 In artificial pancreatic juice. Gangolli & Shilling, 1968
5 By pancreatin. Leegwater & van Straten, 1974.
6 In whole homogenate of pig jejunum. Grundschober, 1977.
7 Structurally related ester
2.2.2 Biotransformations
2.2.2.1 Branched-chain aliphatic acids
The saturated branched-chain aliphatic acids isobutyric acid,
isovaleric acid and 2-methylbutyric acid formed via ester hydrolysis
are endogenous in humans as intermediary products in the metabolism of
the amino acids valine (Kinnory et al., 1955), leucine (Henning &
Hird, 1970), and isoleucine (Voet & Voet, 1990), respectively. These
acids participate in the fatty acid pathway and the tricarboxylic acid
cycle and are completely metabolized to CO2.
Short (<C6) branched-chain acids undergo ß-oxidation,
preferably in the longer chain. ß-Cleavage of the resulting acid
yields linear acid fragments which are sources of carbon in the fatty
acid pathway or tricarboxylic acid cycle (Voet & Voet, 1990). For
example, isobutyric acid (DiVincenzo & Hamilton, 1979) and methacrylic
acid (2-methylpropenoic acid) (Bratt & Hathway, 1977) given to rats by
gavage are rapidly eliminated almost exclusively as CO2. The CO2 is
presumed to arise from ß-oxidation and decarboxylation of isobutyric
acid or methacrylic acid to yield propionyl CoA, which, after
conversion to succinyl CoA, can participate in the tricarboxylic acid
cycle (Saito, 1975). Studies of the metabolism of the amino acid
leucine have shown that isovaleric acid is converted to acetyl
coenzyme A and acetoacetate (Voet & Voet, 1990).
The principal metabolic pathways utilized by the remaining 3
branched-chain acids in this group (i.e., 2-, 3-, and
4-methylpentanoic acid) are determined primarily by the position of
the methyl substituent. Acids with a methyl substituent located at an
even-numbered carbon (e.g., 2-methylpentanoic acid and
4-methylpentanoic acid) are extensively metabolized to CO2 via
ß-oxidation and cleavage in the fatty acid pathway. If the methyl
group is located at the ß-position (e.g., 3-methylpentanoic acid),
ß-oxidation is inhibited and alpha-oxidation predominates, primarily
leading to short-chain acid fragments capable of being completely
metabolized (Williams, 1959).
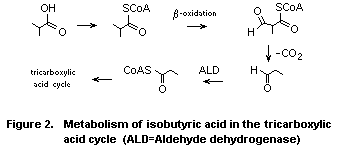
a) Isobutyric acid
Rats administered (1-14C)-isobutyric acid by gavage rapidly
eliminate 14CO2 (DiVincenzo & Hamilton, 1979). The CO2 is presumed
to arise from ß-oxidation and decarboxylation of isobutyric acid to
yield propionyl CoA which, after conversion to succinyl CoA, then
participates in the tricarboxylic acid cycle (Voet & Voet, 1990). The
formation of propionyl CoA is shown by the observation that rats fed
isobutyric acid excrete elevated levels of 2-methylmalonic acid, an
intermediate in the conversion of propionyl CoA to succinyl CoA (Voet
& Voet, 1990). It is anticipated that humans would metabolize
isobutyric acid by complete oxidation to CO2 via propionyl CoA and
succinyl CoA, components of the tricarboxylic acid cycle (Fig. 2).
Branched-chain esters in this group are hydrolysed in vitro to
their corresponding branched-chain acids (isobutyric acid, isovaleric
acid or 2-methylbutyric acid) and corresponding aliphatic acyclic
alcohols. Table 2 summarizes the hydrolysis data for the five esters
containing a branched-chain alcohol and/or branched-chain acid (i.e.,
ethyl iso-valerate, iso-amyl caproate, benzyl iso-butyrate,
iso-amyl butyrate and iso-amyl iso-valerate). Following
hydrolysis, short-chain (<C6) branched-chain acids and alcohols
are rapidly absorbed from the gastrointestinal tract (Dawson et
al., 1964; Gaillard & Derache, 1965).
Table 2. In vitro ester hydrolysis data
Ester Artificial Artificial Rat liver Rat small % hydrolysis
gastric juice1 pancreatic juice1 preparation1 intestinal after 2 hours
t0.52 (min) t0.5 (min) t0.5 (sec) mucosa1
t0.5 (sec)
Ethyl iso-valerate 1390 198 2.35 133 63; 344
Isoamyl caproate7 146 37.8 NR NR NR
Benzyl isobutyrate7 577 17.8 0.0422 0.0707 NR
Isoamyl butyrate7 660 11.3 0.492 0.0713 123; 1004
Isoamyl isovalerate7 295 10.2 NR NR NR
Benzyl 2-methylbutanoate7 NR NR NR NR 1005
Isoamyl acetate7 NR NR NR NR 205; 1006
1 Longland et al., 1977
2 t1/2 = Half-life
3 In artificial gastric juice. Gangolli & Shilling, 1968
4 In artificial pancreatic juice. Gangolli & Shilling, 1968
5 By pancreatin. Leegwater & van Straten, 1974.
6 In whole homogenate of pig jejunum. Grundschober, 1977.
7 Structurally related ester
2.2.2 Biotransformations
2.2.2.1 Branched-chain aliphatic acids
The saturated branched-chain aliphatic acids isobutyric acid,
isovaleric acid and 2-methylbutyric acid formed via ester hydrolysis
are endogenous in humans as intermediary products in the metabolism of
the amino acids valine (Kinnory et al., 1955), leucine (Henning &
Hird, 1970), and isoleucine (Voet & Voet, 1990), respectively. These
acids participate in the fatty acid pathway and the tricarboxylic acid
cycle and are completely metabolized to CO2.
Short (<C6) branched-chain acids undergo ß-oxidation,
preferably in the longer chain. ß-Cleavage of the resulting acid
yields linear acid fragments which are sources of carbon in the fatty
acid pathway or tricarboxylic acid cycle (Voet & Voet, 1990). For
example, isobutyric acid (DiVincenzo & Hamilton, 1979) and methacrylic
acid (2-methylpropenoic acid) (Bratt & Hathway, 1977) given to rats by
gavage are rapidly eliminated almost exclusively as CO2. The CO2 is
presumed to arise from ß-oxidation and decarboxylation of isobutyric
acid or methacrylic acid to yield propionyl CoA, which, after
conversion to succinyl CoA, can participate in the tricarboxylic acid
cycle (Saito, 1975). Studies of the metabolism of the amino acid
leucine have shown that isovaleric acid is converted to acetyl
coenzyme A and acetoacetate (Voet & Voet, 1990).
The principal metabolic pathways utilized by the remaining 3
branched-chain acids in this group (i.e., 2-, 3-, and
4-methylpentanoic acid) are determined primarily by the position of
the methyl substituent. Acids with a methyl substituent located at an
even-numbered carbon (e.g., 2-methylpentanoic acid and
4-methylpentanoic acid) are extensively metabolized to CO2 via
ß-oxidation and cleavage in the fatty acid pathway. If the methyl
group is located at the ß-position (e.g., 3-methylpentanoic acid),
ß-oxidation is inhibited and alpha-oxidation predominates, primarily
leading to short-chain acid fragments capable of being completely
metabolized (Williams, 1959).
a) Isobutyric acid
Rats administered (1-14C)-isobutyric acid by gavage rapidly
eliminate 14CO2 (DiVincenzo & Hamilton, 1979). The CO2 is presumed
to arise from ß-oxidation and decarboxylation of isobutyric acid to
yield propionyl CoA which, after conversion to succinyl CoA, then
participates in the tricarboxylic acid cycle (Voet & Voet, 1990). The
formation of propionyl CoA is shown by the observation that rats fed
isobutyric acid excrete elevated levels of 2-methylmalonic acid, an
intermediate in the conversion of propionyl CoA to succinyl CoA (Voet
& Voet, 1990). It is anticipated that humans would metabolize
isobutyric acid by complete oxidation to CO2 via propionyl CoA and
succinyl CoA, components of the tricarboxylic acid cycle (Fig. 2).
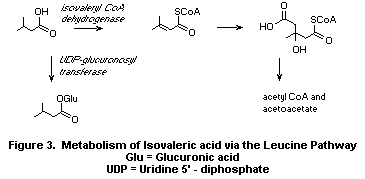
 b) Isovaleric acid
Isovaleric acid as the CoA thioester undergoes successive
dehydrogenation and carboxylation in the leucine pathway to yield
ß-methylglutaconyl CoA. ß-Methylglutaconyl CoA is hydrated to yield
ß-hydroxy ß-methylglutaryl CoA which is finally cleaved to
acetoacetate and acetyl CoA (Voet & Voet, 1990) (Fig. 3).
b) Isovaleric acid
Isovaleric acid as the CoA thioester undergoes successive
dehydrogenation and carboxylation in the leucine pathway to yield
ß-methylglutaconyl CoA. ß-Methylglutaconyl CoA is hydrated to yield
ß-hydroxy ß-methylglutaryl CoA which is finally cleaved to
acetoacetate and acetyl CoA (Voet & Voet, 1990) (Fig. 3).
 c) 2-Methylbutyric acid, 2-methylpentanoic acid, and 4-methylpentanoic
acid
In general, branched-chain acids with an alpha-methyl or
4-methyl substituent are metabolized via oxidative cleavage to yield
linear acid fragments (Deuel, 1957), which are completely oxidized to
CO2 in the fatty acid pathway and tricarboxylic acid cycle. 2-
Methylbutyric acid undergoes ß-oxidation and cleavage to yield acetyl
CoA and propionyl CoA. Propionyl CoA is converted to succinyl CoA,
which, together with acetyl CoA, can be completely metabolized to CO2
in the tricarboxylic acid cycle (Deuel, 1957). ß-Oxidation of
2-methylbutyric acid has been observed in guinea-pigs in vivo
(Stokke et al., 1969) (Fig. 4).
c) 2-Methylbutyric acid, 2-methylpentanoic acid, and 4-methylpentanoic
acid
In general, branched-chain acids with an alpha-methyl or
4-methyl substituent are metabolized via oxidative cleavage to yield
linear acid fragments (Deuel, 1957), which are completely oxidized to
CO2 in the fatty acid pathway and tricarboxylic acid cycle. 2-
Methylbutyric acid undergoes ß-oxidation and cleavage to yield acetyl
CoA and propionyl CoA. Propionyl CoA is converted to succinyl CoA,
which, together with acetyl CoA, can be completely metabolized to CO2
in the tricarboxylic acid cycle (Deuel, 1957). ß-Oxidation of
2-methylbutyric acid has been observed in guinea-pigs in vivo
(Stokke et al., 1969) (Fig. 4).
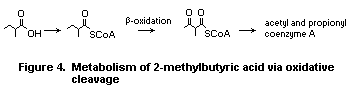 The metabolism of 2-methylpentanoic acid has been studied in
mammals. 2-Methypentanoic acid undergoes ß-oxidation in the longer
branched-chain followed by cleavage to yield two propionyl CoA
fragments. In rabbits, 2-methylpentanoic acid is converted to
propionyl CoA, which is completely metabolized (Deuel, 1957).
4-Methylpentanoic acid, a gamma-methyl substituted acid, is expected
to undergo ß-oxidative cleavage to acetyl CoA and isobutyl CoA which
would be completely metabolized (Voet & Voet, 1990).
d) 3-Methylpentanoic acid
In general, ß-oxidation of branched-chain acids with a ß-methyl is
inhibited. However, ß-substituted acids may undergo alpha-oxidative
cleavage to yield acid fragments (Deuel, 1957; Williams, 1959) which
are completely metabolized in the fatty acid and tricarboxylic acid
cycles. In addition, they may undergo partial ß-oxidation to yield
ß-hydroxy acids which are further oxidized (omega-oxidation) and
fragmented to yield short-chain acids.
The metabolism of 3-methylpentanoic acid has been studied in
mammals. alpha-Oxidation and decarboxylation of 3-methylpentanoic acid
would yield 2-methylbutyric acid followed by ß-oxidative cleavage to
yield acetyl CoA and acetone. In guinea-pig liver homogenate,
3-methylpentanoic acid is converted to 2-methylbutyric acid which is
further metabolized via ß-oxidative cleavage (Stokke, 1969). In
rabbits, 3-methylpentanoic acid is converted to ß-hydroxybutyric acid
(Williams, 1959).
2.2.2.2 Aliphatic linear alcohols
Linear saturated and unsaturated alcohols are oxidized
successively to their corresponding aldehydes and carboxylic acids,
which enter the fatty acid ß-oxidation pathway. Branched-chain
aliphatic alcohols are converted by similar oxidation reactions to
their corresponding carboxylic acid, which undergoes metabolism via
ß-oxidation and cleavage to yield CO2 in amino acid pathways, the
fatty acid pathway, and the tricarboxylic acid cycle.
Based on the above information, it is anticipated that the 32
esters of aliphatic acyclic primary alcohols and branched-chain
aliphatic acyclic acids will be hydrolysed in the intestinal tract,
blood and liver to yield the corresponding branched-chain acids and
aliphatic alcohols. The resulting branched-chain acids and aliphatic
alcohols would be rapidly absorbed from the gastrointestinal tract and
completely metabolized to CO2 in the tricarboxylic acid cycle and
fatty acid pathway. The resulting linear alcohols would be
successively oxidized to their corresponding aldehyde and acid. The
resulting branched-chain aliphatic alcohols are converted by similar
oxidation reactions to their corresponding carboxylic acid, which
undergoes complete metabolism to CO2.
2.2.3 Toxicological studies
2.2.3.1 Acute toxicity
The results of acute toxicity studies for 24 of the 32 esters of
aliphatic acyclic primary alcohols and branched-chain aliphatic
acyclic acids are summarized in Table 3. The low acute oral toxicity
of the group is demonstrated by oral LD50 values >2300 mg/kg bw,
with the majority being >5000 mg/kg bw.
2.2.3.2 Short-term toxicity studies
The results of short-term toxicity studies with 4 branched-chain
esters (ethyl isobutyrate, isobutyl isobutyrate, ethyl isovalerate and
isoamyl isovalerate), one component acid (isovaleric acid) and 7
component alcohols (propyl alcohol, butyl alcohol, hexanol, octanol,
3-hexen-1-ol, isobutanol and isoamyl alcohol) are summarized in Table
4.
a) Branched-chain Esters
i) Ethyl isobutyrate
Ethyl isobutyrate was added to the diet of 12 male rats and 12
female rats at a level calculated to provide an average daily intake
of 29.2 mg/kg bw for 90 days. Weekly measurement of body weights and
food intake revealed no significant difference between test and
control animals. Haematological examination, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Determination of mean organ weights
revealed a slight increase in both absolute and relative adrenal gland
weights in females compared to controls, but these values were not
statistically significant. Gross and histopathological examination
failed to reveal any dose-related effects on the adrenal gland or any
other organs (Mecler & Craig, 1980). The NOEL of 29.2 mg ethyl
isobutyrate/kg bw per day in rats is >1000 times the estimated daily
per capita intake1 ("eaters only") of 7.74 µg/kg bw from use of
ethyl isobutyrate as a flavouring substance in the USA and 12.44 µg/kg
bw from its use as a flavouring substance in Europe.
ii) Ethyl isovalerate
For 90 days, ethyl isovalerate on gum arabic (10%) was added to the
diet of 12 male rats and 12 female rats at a level calculated to
provide an average daily intake of 12.1 mg/kg bw for males and 13.6
mg/kg bw for females. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed.
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw per
day) calculated as follows: [(mg per day)/body weight], where body
weight = 60 kg. Slight variations may occur from rounding off.
Table 3. Acute toxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
iso-Butyrates
Methyl iso-butyrate Rats NR Oral 16 000 Sandmeyer & Kirwin, 1981
Ethyl iso-butyrate Rats NR Oral >5000 Moreno, 1975
Propyl iso-butyrate Rats NR Oral 15 000 Jenner et al., 1964
Butyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
Hexyl iso-butyrate Rats NR Oral >5000 Moreno, 1977
Trans-3-heptenyl 2-
methylpropanoate Rats NR Oral >25 000 Food and Drug Research Labs, 1975
Octyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
iso-Butyl iso-butyrate Rats
and mice NR Oral 12 800 Sandmeyer & Kirwin, 1981
iso-Valerates
Methyl iso-valerate Rabbits NR Oral 5690 Munch, 1972
Ethyl iso-valerate Rats NR Oral >5000 Levenstein, 1976; BASF, 1980
Rabbits NR Oral 7030 Munch, 1972
Propyl iso-valerate Rabbits NR Oral 8220 Munch, 1972
Butyl iso-valerate Rats NR Oral >5000 Moreno, 1978
Rabbits NR Oral 8230 Munch, 1972
Hexyl 3-methylbutanoate Rats NR Oral >5000 Moreno et al., 1981
2-Methylbutyrates
2-Methylpropyl Rabbits NR Oral >5000 Munch, 1972
3-methylbutyrate Rats NR Oral 6970 Moreno, 1978
2-Methylbutyl
3-methylbutanoate Rats NR Oral >5000 Moreno, 1978
Methyl 2-methylbutyrate Rats NR Oral >5000 Moreno et al., 1982
Hexyl 2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
iso-Propyl
2-methylbutyrate Rats NR Oral >20 000 Griffiths & Giessinger, 1979
Table 3. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3-Hexenyl
2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
Ethyl 3-methylpentanoate Rats NR Oral >4000 Biosphere Research Center, Inc., 1981
Methyl 2-methylpentanoate Rats NR Oral >5000 Pharmakon Inc.,1980
1 The study was performed at a single dose level or multiple dose levels that produced no effects and,
therefore, a NOEL was not determined.
The NOEL is probably much higher than the reported dose level that produced no adverse effects
Table 4. Short-term studies for branched-chain esters and their component alcohols and acids
Substance Species, sex Route Duration NOEL Reference
(mg/kg bw per day)
Branched esters
Isobutyl isobutyrate Rats, M & F Oral and gavage 18 weeks 1000 Drake et al., 1978
Ethyl isobutyrate Rats, M & F Oral 90 days 29.21 Mecler & Craig, 1980
Ethyl isovalerate Rats, M & F Oral 13 weeks 12.11 Mecler & Craig, 1980
Isoamyl isovalerate2 Rats, M & F Oral 90 days 2201 Damske et al., 1980
Component acids
Isovaleric acid Rats Oral 90 days 25001 Amoore et al., 1978
Component alcohols
Propyl alcohol Rat, male Oral 4 months 601 Hillbom et al., 1974
Butyl alcohol Rats, male Oral 13 weeks 5.61 Wakabayashi et al., 1984
Rats, M & F Oral 14 days 1380 PPG, 1991
Rats, NR Oral 28 days 9401 Bio-Fax, 1969
Hexyl alcohol Beagles, M & F Oral 13 weeks 230 Eibert, 1992
Rat, M & F Oral 13 weeks 577 Eibert, 1992
Octanol Mice Oral 1 month 1791 Voskovofnikova, 1966
3-Hexen-1-ol Rats, M & F Oral 90 days 150 Gaunt et al., 1969
Isopropanol Adult Human Oral 6 weeks 6.4 Wills, 1969
Isobutanol Rats, M & F Oral 90 days 1450 BASF, 1992
Isoamyl alcohol2 Rats M & F Gavage 17 weeks 1000 Carpanini et al., 1973
1 The study was performed at a single dose level or multiple dose levels that produced no effects and, therefore, a NOEL was not
determined. The NOEL is probably much higher than the reported dose level that produced no adverse effects.
2 Structurally related substance
Haematological examination, blood chemical determinations and
urinalysis conducted at weeks 6 and 12 revealed no difference from
controls. At necropsy increases in absolute and relative mean adrenal
gland and thyroid gland weights were observed, but the results were
not statistically significant. Gross and histopathological examination
did not support relative weight changes, and revealed no dose-related
lesions (Mecler & Craig, 1980). The NOEL of 12.1 mg ethyl
isovalerate/kg bw per day in rats is >1000 times the estimated daily
per capita intake ("eaters only") of 9.01µg/kg bw from use of ethyl
isovalerate as a flavouring substance in the USA, and 12.75 µg/kg bw
from its use in Europe.
iii) Isobutyl isobutyrate
Groups of 15 male and 15 female rats were given daily doses of 0,
10, 100 or 1000 mg isobutyl isobutyrate/kg bw by gavage for 18 weeks.
Body weights, food consumption and water intake were measured and were
not different from controls. Haematology, urine analyses and serum
chemistry parameters were evaluated and did not reveal any
treatment-related effects. At necropsy gross examinations were
performed on all animals and organ weights were obtained. No
significant effects were reported. Histological examination was
conducted on all tissues from rats in the high-dose group and 50% of
the control animals. Histopathological examination of the major organs
was also performed on rats given 10 and 100 mg/kg bw per day. No
evidence of histopathological effects was reported (Drake et al.,
1978). The highest level of 1000 mg isobutyl isobutyrate/kg bw per day
which produced no adverse effects in rats is >100 000 times the
estimated daily per capita intake1 ("eaters only") of 0.04 µg/kg bw
per day from use of isobutyl isobutyrate as a flavouring substance in
the USA and 1.09 µg/kg bw per day from its use in Europe.
iv) Isoamyl isovalerate
Isoamyl isovalerate was added to the diet of male and female rats
at levels calculated to provide average daily intakes of 22, 69 or 220
mg/kg bw for 90 days. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed. Haematological examinations, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Organ weights at necropsy were normal
compared to controls. Gross and histopathological examination revealed
no dose-related lesions (Damske et al., 1980). The intake level of
220 mg isoamyl isovalerate/kg bw per day that produced no effects in
rats is approximately 10 000 times the estimated daily per capita
intake ("eaters only") of 13 µg/kg bw from use of isoamyl isovalerate
as a flavouring substance in the USA and 23.91 µg/kg bw from its use
as a flavouring substance in Europe (see footnote in section
2.2.3.2a i).
b) Component alcohols and acids
Ester hydrolysis yields the component branched-chain acid and
linear or branched-chain alcohol. The results of subchronic studies
for the component acids and alcohols of esters of aliphatic acyclic
primary alcohols and branched-chain aliphatic acyclic acids has
revealed no evidence of toxicity at levels up to 2500 mg/kg bw per
day. These short-term studies are described below and summarized in
Table 4.
i) 1-Propanol
Four-month-old male Wistar rats were given a 1M solution of
1-propanol, corresponding to 60 mg/kg bw per day, as the sole drinking
fluid for 4 months. Control animals received tap water. Consumption of
food and alcohol solution and the weight gain of each animal were
recorded. A lower ratio of weight gain to caloric intake was observed
in the rats given the 1-propanol, indicating that this group utilized
food less efficiently than those untreated. However, this action is
not indicative of toxicity. Histological examination of the livers of
the experimental animals revealed no difference from controls. No
hepatic steatosis was observed. Neither inflammation nor cirrhosis was
seen in any of the livers (Hillbom et al., 1974).
ii) Butanol
No adverse effects were reported when 6.9% butanol with 25%
sucrose was added to the drinking-water of male rats for 13 weeks
(Wakabayashi et al., 1984) at an estimated daily intake level of 5.6
mg butanol/kg bw.
A 14-day study was conducted using rats to evaluate the effects of
butanol on gross and microscopic pathology, body weight, clinical
signs and clinical chemistry. Levels of 1.38%, 2.75% or 5.5% butanol
were administered orally to rats, which corresponds to daily intake
levels of 1380-5500 mg butanol/kg bw. A statistically significant
increase in the liver-to-body weight ratio resulted in males at all
levels and females at the 5.5% level (PPG, 1991).
No deaths or unusual symptoms were observed when rats (sex was not
reported) were fed butyl alcohol in a 2% corn oil solution blended
with the diet at concentrations of 0, 1000, 3500 or 10 000 mg/kg
(equal to 0, 90, 300 or 940 mg/kg bw per day, respectively) for 28
days. At necropsy, no significant gross lesions were observed. Liver
and kidney weights showed no significant differences from those of the
controls (Bio-Fax, 1969).
iii) Hexyl alcohol
Two groups of male and female rats were fed hexyl alcohol for 13
weeks at dietary levels of 0.25 or 0.50%; a third group was fed 1% for
weeks 1-10 and 2%, 4% and 6% for weeks 11, 12 and 13, respectively.
Decreased food consumption was observed in females at the high-dose
level, but body weights for all animals were normal. No significant
haematological changes or differences in urine analyses were observed
for the test and control groups. Gross pathology and microscopic
evaluations were performed and revealed no treatment-related effects.
The 1% level was reported to be equivalent to an intake of 577 mg/kg
bw per day (Eibert, 1992).
Three groups of 2 male and 2 female purebred beagle dogs were fed
hexyl alcohol for 13 weeks at levels of 0.50%, 1.0% or 1000 mg/kg bw
per day via gelatin capsules. A fourth group of 4 males and 4 females
served as controls. Body weight, organ weight, and food consumption
for the treated animals did not differ from controls. All animals in
the high-dose group displayed gross signs of toxicity intermittently
after treatment, including salivation, excitation, ataxia, tremors and
anaesthesia. The animals generally returned to normal within 4 hours
of treatment. One female dog died on the first day of treatment and
was replaced by another female. Three of the four remaining animals
died on either the 23rd or 38th day of the study. No signs of toxicity
were observed in the other test animals. Haematology, serum chemistry
and urinalysis was performed and showed no significant difference as
compared to controls. All animals were necropsied at the end of the
study.
Animals in the high-dose group exhibited gastrointestinal
inflammation and congestion of other visceral organs. Some gastric
irritation was observed in the mid-dose group. Both males treated at
the 1000 mg/kg bw per day level exhibited significant testicular
atrophy (Eibert, 1992). Effects on the testes and other reproductive
tissue have been observed with other aliphatic alcohols at high dose
levels (Lington & Bevan, 1994). A finding of nodules on the lung
surface of some animals was reported to be non-treatment-related. The
1% NOEL corresponds to a daily intake level ranging from 230 to 695
mg/kg bw (Eibert, 1992).
iv) Octanol
Seventeen groups of 10 mice each received oral doses of 1-octanol
at a level of 179 mg/kg bw per day for one month. 1-Octanol was
administered intragastrically in the form of a solution or suspension.
No cumulative effects were observed (Voskovofnikova, 1966).
v) Cis-3-hexen-1-ol
Short-term toxicity studies have been reported for
cis-3-hexen-1-ol, which is the alcohol component for esters of
unsaturated alcohols in this group. Groups of 15 male and 15 female
weanling rats were given drinking-water containing 0, 310, 1250 or
5000 mg/litre cis-3-hexen-1-ol for 98 days. Body weight, food intake
and water consumption were recorded weekly. A reduction in water
intake was reported in male rats in the high-dose group only.
Haematology studies were conducted on eight rats from each sex in the
control, 1250 and 5000 mg/litre groups during the sixth week.
Urinalysis, including pH, microscopic constituents and content of
bile, blood and glucose, was performed for eight animals of each sex
from each group during week 6, and from week 12 until the end of the
study. Kidney function was assessed regularly. Necropsies were
performed on all animals at the end of the study. Serum chemistry
parameters were measured for all animals at necropsy. All tissues were
examined for macroscopic abnormalities, and organ weights were
obtained for the brain, pituitary, thyroid, heart, liver, spleen,
kidneys, adrenals and gonads. Tissues from all rats in the high-dose
group and from 50% of the controls were examined microscopically.
Slightly increased relative kidney and adrenal gland weights observed
in males only at the 5000 mg/litre level were not accompanied by any
evidence of histopathology. These increases were not considered to be
statistically significant. No treatment-related abnormalities were
reported (Gaunt et al., 1969).
vi) Isopropanol
Daily doses of 2.6 or 6.4 mg isopropanol/kg bw were given to adult
human males for a period of six weeks. Studies of the microscopic and
chemical characteristics of blood and urine were repeated on the
first, third and seventh days and at weekly intervals thereafter.
Removal of BSP from blood was estimated once before initiation of
ingestion of isopropyl alcohol and at the end of the experimental
period. Retention of BSP in serum at the end of the experiment was not
significantly different from that before the start in any of the men.
No significant, persistent changes that are clearly attributable to
the ingestion of isopropanol were evident (Wills, 1963).
vii) Isobutanol
Groups of 10 male and 10 female Wistar rats were given isobutyl
alcohol in their drinking-water for 3 months. The test substance was
administered in concentrations of 0, 1000, 4000 or 16 000 mg/litre,
which was reported to correspond to approximate dose levels of 0, 60
340 or 1450 mg/kg bw per day. Food and water consumption and body
weight gain were not affected by the test substance. All animals
tested were free from adverse clinical effects. Haematology and
clinical chemistry parameters were measured and revealed no
treatment-related adverse effects. Gross pathology and
histopathological examinations were no different from controls. The
authors concluded that results of this study demonstrate a lack of
toxicity associated with administration of isobutyl alcohol in the
drinking-water of rats, and that the NOEL is >1450 mg/kg bw per day
(BASF, 1992).
viii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage, providing daily dose levels
of 0, 150 500, or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight reduction in body-weight gain, which was associated
with reduced food intake. Over the entire period of the study,
however, there was no statistically significant reduction in mean food
intake. Examination of haematology, serum analyses, urinalysis, renal
concentration tests, and organ weights revealed no treatment-related
effects. At necropsy, the animals were examined for macroscopic
abnormalities, and the major organs were weighed. Microscopic
examination was performed on several tissues of the control and
high-dose animals. No treatment-related abnormalities were observed
(Carpanini et al., 1973).
ix) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to be equivalent to 2500 mg isovaleric acid/kg
bw per day. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analyses, organ weights and
histology. At the 2500 mg/kg bw dose level, there were no
treatment-related effects on the measured parameters (Amoore et
al., 1978).
2.2.3.3 Long-term toxicity/carcinogenicity studies
The results of long-term toxicity/carcinogenicity studies with two
of the component alcohols are described below and summarized in Table
5.
a) Isobutanol
Twenty males and 20 female Wistar rats were given 0.2% isobutanol
in drinking-water for 53 to 56 weeks. Clinical chemistry, including
alcohol dehydrogenase, glutamic oxaloacetate transaminase glutamic
pyruviuc transaminase, and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isobutanol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 200 mg/kg bw. This is >10 000 times the daily per capita
intake ("eaters only") of 4.85 µg/kg bw isobutanol from use as a
flavouring substance in the USA and of 8.79 µg/kg bw isobutanol from
its use in Europe (see footnote in section 2.2.3.2a i).
Table 5. Long-term toxicity studies for esters of aliphatic acyclic primary alcohols and
branched-chain aliphatic acyclic acids
Substance Species, Route Duration NOEL Reference
sex (weeks) (mg/kg bw per day)
Component Alcohols
Isobutyl alcohol Rats, M&F Drinking-water 53-56 2001 Johannsen & Purchase, 1969
Isoamyl alcohol2 Rats, M&F Drinking-water 53-56 20001 Johannsen & Purchase, 1969
1 The study was performed at a single dose level or multiple dose levels that produced no
effects and, therefore, a NOEL was not determined. The NOEL is probably much higher than
the reported dose level that produced no effects
2 A structually related branched-chain alcohol
b) Isoamyl alcohol
Twenty males and 20 female Wistar rats were given drinking-water
containing 2% isoamyl alcohol for 53 to 56 weeks. Clinical chemistry,
including alcohol dehydrogenase, glutamic oxaloacetate transaminase,
glutamic pyruviuc transaminase and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isoamyl alcohol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 2000 mg/kg bw. This is > 10 000 times the daily per
capita intake ("eaters only") of 26.32 µg isoamyl alcohol/kg bw from
use as a flavouring substance in the USA and of 30.47 µg isoamyl
alcohol/kg bw from its use as a flavouring substance in Europe (see
footnote in section 2.2.3.2a i).
2.2.3.4 Genotoxicity studies
The results of genotoxicity studies with the group of 32 branched-
chain esters are summarized in Table 6. Mutagenicity testing of ethyl
isovalerate in vitro by means of the Ames test, at concentrations up
to 10 mg/plate has shown no evidence of mutagenicity in Salmonella
typhimurium strains TA92, TA94, TA97, TA98, TA100, TA102, TA1535,
TA1537 or in TA2637 with or without metabolic activation (Ishidate et
al., 1984; Fujita & Sasaki, 1987). There was no evidence of
mutagenicity in Chinese hamster fibroblast cells when the chromosomal
aberration test was performed with ethyl isovalerate at concentrations
up to 2 mg/ml (Ishidate et al., 1984). When incubated with Bacillus
subtilis, at concentrations up to 20 µl/disk (Yoo, 1986), and at 17
µg/plate (Oda et al., 1978), ethyl isovalerate was reported to be
non-mutagenic. At high concentrations (100-200 µl/ml) of ethyl
isovalerate, Bacillus subtilis was reported to show signs positive
for mutagenicity in the rec assay (Koruda, 1984a). However, these
results are not supported by the results of other rec assays using
similar bacterial strains (Yoo, 1986; Oda et al., 1978). Considering
the results of the rec assay, the Ames test and the chromosomal aberration
test, it is concluded that the 32 branched-chain esters are not genotoxic.
2.2.3.5 Reproductive toxicity studies
No reproduction studies have been reported for esters of aliphatic
acyclic primary alcohols and branched-chain aliphatic acyclic acids.
Table 6. Genotoxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance name Test system Test object Concentration of Results Reference
in vitro substance
Ethyl isovalerate Ames test S. typhimurium TA92, 0.01-1 mg/plate Negative1 Fujita & Sasaki, 1987
TA1535
Ames test TA100, TA1537, TA94, 10 mg/plate Negative1 Ishidate et al., 1984
TA98 and TA2637
Rec assay B. subtilis 100-200 µl/ml Positive Kuroda et al., 1984
Rec assay B. subtilis M45(rec-) up to 20 µl/disk Negative Yoo, 1986
and H17(rec+)
S. typhimurium TA97
and TA102
Chromosomal
aberration test Chinese hamster up to 2 mg/ml Negative Ishidate et al., 1984
fibroblasts cells
Rec assay B. subtilis H17 17µg/plate Negative Oda et al., 1978
and M45
1 Both with and without S-9 activation
3. REFERENCES
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: Efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D. ed.
Intermediary xenobiotic metabolism in animals. Taylor and Francis, New
York, pp. 81-97.
BASF (1980) Private communication to RIFM. Unpublished document.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months. Private communication to
FEMA.
Bio-Fax Industrial Bio-test Lab. Inc. (1969) Data sheet No. 2-5/69:
n-Butyl alcohol. Northbrook, Illinois, USA.
Biophere Research Center, Inc. (1981) Private communication to FEMA.
Browning, E. (1965) Toxicity and metabolism of industrial solvents.
Elsevier Publishing Co., New York.
Caldwell, J. (1989) Endogenous ethanol. Report to FEMA.
Carpanini, I.F., Kiss I.S., Grasso P., & Gangolli, S.D. (1973)
Short-term toxicity of isoamyl alcohol in rats. Food Cosmet.
Toxicol., 11: 713-724.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III,
6th ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food Cosmet. Toxicol., 16:
255-276.
Damske, D.R., Mecler, F.J., Beliles, R.P., & Liverman, J. L. (1980)
iso-Amyl iso-valerate. Report to FEMA. Unpublished document (Submitted
to WHO by FEMA).
Dawson, A.M., Holdsworth, C.D., & Webb J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117: 97-100.
Deuel, H. J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley-Interscience, New York.
DiVincenzo, G.D. & Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid in the rat. Toxicol. Appl. Pharmacol., 47: 609-612.
Drake, J.J.P., Butterworth, K.R., Gaunt, I.F., & Grasso, P. (1978)
Short-term toxicity study of isobutyl isobutyrate in rats. Food
Cosmet. Toxicol., 16: 337-342.
Eibert, J Jr. (1992) Private communication to FEMA. Unpublished
report. Cited in: Food and Drug Administration (FDA) (1993)
Priority-based assessment of food additives (PAFA) database. Center
for Food Safety and Applied Nutrition, p. 58.
FDA (1993) Priority-based assessment of food additives (PAFA)
database. US Food and Drug Administration, Center for Food Safety and
Applied Nutrition, p. 58.
Food and Drug Research Laboratory (1975) Unpublished report to RIFM.
Fujita H. & Sasaki, M. (1987) Mutagenicity test of food additives with
Salmonella typhimurium TA97 and TA102. Annual Report of the Tokyo
Metropolitan Research Laboratory P.H., 3.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montp., 25(1): 51.
Gangolli, S.D. & Shilling, W.H. (1968) Hydrolysis of esters by
artificial gastric and pancreatic juices. Analytical Chemistry
Research Report No. 11/1968. Unpublished study (Submitted to WHO by
FEMA).
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G., & Gangolli,
S.D. (1969) Acute (rat and mouse) and short-term (rat) toxicity
studies on cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7: 451-459.
Gibel, W.K., Lohs, K., & Wildner, G.P. (1975) Carcinogenic activity of
propanol, 2-methyl-1-propanol, and 3-methyl-1-butanol. Arch.
Geschwulstforsch., 45: 19-24.
Givaudan-Roure Corporation (1971) Private communication to FEMA.
Griffiths, P.J. & Giessinger, M. (1979) Private communication to FEMA.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fementative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
Hillbom, M.E., Fransaila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Research
Laboratories of the State Alcohol Monopoly (Alko), Helsinki, Finland.
Ishidate, M. Jr., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22(8):
623-636.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Jenner, P.M.,Hagan, E.C., Taylor, J.M., Cook, E.L., & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure: I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
Johannsen, E. & Purchase, I.F.H. (1969) Kaffircorn malting and brewing
studies: XXI. The effect of fuel oils of bantu beer on rat liver.
S.Afr. Med. J., 43(12): 326-328.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Krebs, H.A. & Perkins, J.R. (1970) The physiological role of liver
alcohol dehydrogenase. Biochem. J., 118: 635-644.
Kuroda, K., Tanaka, S., Yu, Y.S., & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31(6): 277.
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the
hydrolysis of twenty-six organic esters by pancreatin. Centraal
Instituut Voor Voedingsonderzoek, Zeist, The Netherlands (TNO Report
No. R 4319).
Levenstein, I. (1974) Report to RIFM. Unpublished document.
Levenstein, I. (1976) Report to RIFM. Unpublished document.
Liebich, H.M., Buelow, H.J., & Kallmayer, R. (1982) Quantification of
endogenous aliphatic alcohols in serum and urine. J. Chromatogr.,
239: 343-349.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, p. 2613.
Longland, R.C., Shilling, W.H., & Gangolli, S.D. (1977) The hydrolysis
of flavouring esters by artificial gastrointestinal juices and rat
tissue preparations. Toxicology, 8: 197-204.
Mecler, F.J. & Craig, D.K. (1980) Three month toxicity study on ethyl
isobutyrate in rat. Report to FEMA. Unpublished document.
Moreno, O.M. (1977) Report to RIFM. Unpublished document.
Moreno, O.M. (1978) Report to RIFM. Unpublished document.
Moreno, O.M. (1975) Report to RIFM. Unpublished document.
Moreno, O M., Cerven D.R., & Altenbach, E.J. (1981) Report to RIFM.
Unpublished document.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Report to RIFM.
Unpublished document.
Munch, J. C. (1972) Aliphatic alcohols and alkyl esters: Narcotic and
lethal potencies to tadpoles and rabbits. Ind. Med. Surg., 41:
31-33.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of structural class with
no-observed-effect levels: a proposal for establishing a threshold of
concern. In Press.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Pharmakon Inc. (1980) Private communication to FEMA.
Plaut, G.W.E. & Lardy, H.A. (1951) Enzymatic incorporation of
C14-bicarbonate into acetoacetate in the presence of various
substrates. J. Biol. Chem., 192: 435-445.
PPG Industries Inc. (1991) Submission to EPA. Unpublished document.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34(8-9): 569-85.
Sandmeyer, E.E. & Kirwin, C.J. (1981) Esters. In. Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology - Volume
2A, 3rd ed. John Wiley and Sons, Inc., New York, pp. 2259-2412.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Voskovofnikova, V.B. (1966) Substantiation of the maximum permissible
concentration of the flotation reagent IM-68 and its component
alcohols (hexyl, heptyl and octyl alcohol) in bodies of water. Gig.
i Sanit., 31: 310-316.
Wakabayashi, T., Horiuchi, M., Sakaguchi, M., Onda, H., & Iijima, M.
(1984) Induction of megamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta Pathol. Jpn., 34: 471-480.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall Ltd, London, pp. 88-113.
Wills, J.H., Jameson, E.M., & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15: 560-565.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34(3-4):
267-288.
The metabolism of 2-methylpentanoic acid has been studied in
mammals. 2-Methypentanoic acid undergoes ß-oxidation in the longer
branched-chain followed by cleavage to yield two propionyl CoA
fragments. In rabbits, 2-methylpentanoic acid is converted to
propionyl CoA, which is completely metabolized (Deuel, 1957).
4-Methylpentanoic acid, a gamma-methyl substituted acid, is expected
to undergo ß-oxidative cleavage to acetyl CoA and isobutyl CoA which
would be completely metabolized (Voet & Voet, 1990).
d) 3-Methylpentanoic acid
In general, ß-oxidation of branched-chain acids with a ß-methyl is
inhibited. However, ß-substituted acids may undergo alpha-oxidative
cleavage to yield acid fragments (Deuel, 1957; Williams, 1959) which
are completely metabolized in the fatty acid and tricarboxylic acid
cycles. In addition, they may undergo partial ß-oxidation to yield
ß-hydroxy acids which are further oxidized (omega-oxidation) and
fragmented to yield short-chain acids.
The metabolism of 3-methylpentanoic acid has been studied in
mammals. alpha-Oxidation and decarboxylation of 3-methylpentanoic acid
would yield 2-methylbutyric acid followed by ß-oxidative cleavage to
yield acetyl CoA and acetone. In guinea-pig liver homogenate,
3-methylpentanoic acid is converted to 2-methylbutyric acid which is
further metabolized via ß-oxidative cleavage (Stokke, 1969). In
rabbits, 3-methylpentanoic acid is converted to ß-hydroxybutyric acid
(Williams, 1959).
2.2.2.2 Aliphatic linear alcohols
Linear saturated and unsaturated alcohols are oxidized
successively to their corresponding aldehydes and carboxylic acids,
which enter the fatty acid ß-oxidation pathway. Branched-chain
aliphatic alcohols are converted by similar oxidation reactions to
their corresponding carboxylic acid, which undergoes metabolism via
ß-oxidation and cleavage to yield CO2 in amino acid pathways, the
fatty acid pathway, and the tricarboxylic acid cycle.
Based on the above information, it is anticipated that the 32
esters of aliphatic acyclic primary alcohols and branched-chain
aliphatic acyclic acids will be hydrolysed in the intestinal tract,
blood and liver to yield the corresponding branched-chain acids and
aliphatic alcohols. The resulting branched-chain acids and aliphatic
alcohols would be rapidly absorbed from the gastrointestinal tract and
completely metabolized to CO2 in the tricarboxylic acid cycle and
fatty acid pathway. The resulting linear alcohols would be
successively oxidized to their corresponding aldehyde and acid. The
resulting branched-chain aliphatic alcohols are converted by similar
oxidation reactions to their corresponding carboxylic acid, which
undergoes complete metabolism to CO2.
2.2.3 Toxicological studies
2.2.3.1 Acute toxicity
The results of acute toxicity studies for 24 of the 32 esters of
aliphatic acyclic primary alcohols and branched-chain aliphatic
acyclic acids are summarized in Table 3. The low acute oral toxicity
of the group is demonstrated by oral LD50 values >2300 mg/kg bw,
with the majority being >5000 mg/kg bw.
2.2.3.2 Short-term toxicity studies
The results of short-term toxicity studies with 4 branched-chain
esters (ethyl isobutyrate, isobutyl isobutyrate, ethyl isovalerate and
isoamyl isovalerate), one component acid (isovaleric acid) and 7
component alcohols (propyl alcohol, butyl alcohol, hexanol, octanol,
3-hexen-1-ol, isobutanol and isoamyl alcohol) are summarized in Table
4.
a) Branched-chain Esters
i) Ethyl isobutyrate
Ethyl isobutyrate was added to the diet of 12 male rats and 12
female rats at a level calculated to provide an average daily intake
of 29.2 mg/kg bw for 90 days. Weekly measurement of body weights and
food intake revealed no significant difference between test and
control animals. Haematological examination, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Determination of mean organ weights
revealed a slight increase in both absolute and relative adrenal gland
weights in females compared to controls, but these values were not
statistically significant. Gross and histopathological examination
failed to reveal any dose-related effects on the adrenal gland or any
other organs (Mecler & Craig, 1980). The NOEL of 29.2 mg ethyl
isobutyrate/kg bw per day in rats is >1000 times the estimated daily
per capita intake1 ("eaters only") of 7.74 µg/kg bw from use of
ethyl isobutyrate as a flavouring substance in the USA and 12.44 µg/kg
bw from its use as a flavouring substance in Europe.
ii) Ethyl isovalerate
For 90 days, ethyl isovalerate on gum arabic (10%) was added to the
diet of 12 male rats and 12 female rats at a level calculated to
provide an average daily intake of 12.1 mg/kg bw for males and 13.6
mg/kg bw for females. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed.
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw per
day) calculated as follows: [(mg per day)/body weight], where body
weight = 60 kg. Slight variations may occur from rounding off.
Table 3. Acute toxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
iso-Butyrates
Methyl iso-butyrate Rats NR Oral 16 000 Sandmeyer & Kirwin, 1981
Ethyl iso-butyrate Rats NR Oral >5000 Moreno, 1975
Propyl iso-butyrate Rats NR Oral 15 000 Jenner et al., 1964
Butyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
Hexyl iso-butyrate Rats NR Oral >5000 Moreno, 1977
Trans-3-heptenyl 2-
methylpropanoate Rats NR Oral >25 000 Food and Drug Research Labs, 1975
Octyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
iso-Butyl iso-butyrate Rats
and mice NR Oral 12 800 Sandmeyer & Kirwin, 1981
iso-Valerates
Methyl iso-valerate Rabbits NR Oral 5690 Munch, 1972
Ethyl iso-valerate Rats NR Oral >5000 Levenstein, 1976; BASF, 1980
Rabbits NR Oral 7030 Munch, 1972
Propyl iso-valerate Rabbits NR Oral 8220 Munch, 1972
Butyl iso-valerate Rats NR Oral >5000 Moreno, 1978
Rabbits NR Oral 8230 Munch, 1972
Hexyl 3-methylbutanoate Rats NR Oral >5000 Moreno et al., 1981
2-Methylbutyrates
2-Methylpropyl Rabbits NR Oral >5000 Munch, 1972
3-methylbutyrate Rats NR Oral 6970 Moreno, 1978
2-Methylbutyl
3-methylbutanoate Rats NR Oral >5000 Moreno, 1978
Methyl 2-methylbutyrate Rats NR Oral >5000 Moreno et al., 1982
Hexyl 2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
iso-Propyl
2-methylbutyrate Rats NR Oral >20 000 Griffiths & Giessinger, 1979
Table 3. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3-Hexenyl
2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
Ethyl 3-methylpentanoate Rats NR Oral >4000 Biosphere Research Center, Inc., 1981
Methyl 2-methylpentanoate Rats NR Oral >5000 Pharmakon Inc.,1980
1 The study was performed at a single dose level or multiple dose levels that produced no effects and,
therefore, a NOEL was not determined.
The NOEL is probably much higher than the reported dose level that produced no adverse effects
Table 4. Short-term studies for branched-chain esters and their component alcohols and acids
Substance Species, sex Route Duration NOEL Reference
(mg/kg bw per day)
Branched esters
Isobutyl isobutyrate Rats, M & F Oral and gavage 18 weeks 1000 Drake et al., 1978
Ethyl isobutyrate Rats, M & F Oral 90 days 29.21 Mecler & Craig, 1980
Ethyl isovalerate Rats, M & F Oral 13 weeks 12.11 Mecler & Craig, 1980
Isoamyl isovalerate2 Rats, M & F Oral 90 days 2201 Damske et al., 1980
Component acids
Isovaleric acid Rats Oral 90 days 25001 Amoore et al., 1978
Component alcohols
Propyl alcohol Rat, male Oral 4 months 601 Hillbom et al., 1974
Butyl alcohol Rats, male Oral 13 weeks 5.61 Wakabayashi et al., 1984
Rats, M & F Oral 14 days 1380 PPG, 1991
Rats, NR Oral 28 days 9401 Bio-Fax, 1969
Hexyl alcohol Beagles, M & F Oral 13 weeks 230 Eibert, 1992
Rat, M & F Oral 13 weeks 577 Eibert, 1992
Octanol Mice Oral 1 month 1791 Voskovofnikova, 1966
3-Hexen-1-ol Rats, M & F Oral 90 days 150 Gaunt et al., 1969
Isopropanol Adult Human Oral 6 weeks 6.4 Wills, 1969
Isobutanol Rats, M & F Oral 90 days 1450 BASF, 1992
Isoamyl alcohol2 Rats M & F Gavage 17 weeks 1000 Carpanini et al., 1973
1 The study was performed at a single dose level or multiple dose levels that produced no effects and, therefore, a NOEL was not
determined. The NOEL is probably much higher than the reported dose level that produced no adverse effects.
2 Structurally related substance
Haematological examination, blood chemical determinations and
urinalysis conducted at weeks 6 and 12 revealed no difference from
controls. At necropsy increases in absolute and relative mean adrenal
gland and thyroid gland weights were observed, but the results were
not statistically significant. Gross and histopathological examination
did not support relative weight changes, and revealed no dose-related
lesions (Mecler & Craig, 1980). The NOEL of 12.1 mg ethyl
isovalerate/kg bw per day in rats is >1000 times the estimated daily
per capita intake ("eaters only") of 9.01µg/kg bw from use of ethyl
isovalerate as a flavouring substance in the USA, and 12.75 µg/kg bw
from its use in Europe.
iii) Isobutyl isobutyrate
Groups of 15 male and 15 female rats were given daily doses of 0,
10, 100 or 1000 mg isobutyl isobutyrate/kg bw by gavage for 18 weeks.
Body weights, food consumption and water intake were measured and were
not different from controls. Haematology, urine analyses and serum
chemistry parameters were evaluated and did not reveal any
treatment-related effects. At necropsy gross examinations were
performed on all animals and organ weights were obtained. No
significant effects were reported. Histological examination was
conducted on all tissues from rats in the high-dose group and 50% of
the control animals. Histopathological examination of the major organs
was also performed on rats given 10 and 100 mg/kg bw per day. No
evidence of histopathological effects was reported (Drake et al.,
1978). The highest level of 1000 mg isobutyl isobutyrate/kg bw per day
which produced no adverse effects in rats is >100 000 times the
estimated daily per capita intake1 ("eaters only") of 0.04 µg/kg bw
per day from use of isobutyl isobutyrate as a flavouring substance in
the USA and 1.09 µg/kg bw per day from its use in Europe.
iv) Isoamyl isovalerate
Isoamyl isovalerate was added to the diet of male and female rats
at levels calculated to provide average daily intakes of 22, 69 or 220
mg/kg bw for 90 days. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed. Haematological examinations, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Organ weights at necropsy were normal
compared to controls. Gross and histopathological examination revealed
no dose-related lesions (Damske et al., 1980). The intake level of
220 mg isoamyl isovalerate/kg bw per day that produced no effects in
rats is approximately 10 000 times the estimated daily per capita
intake ("eaters only") of 13 µg/kg bw from use of isoamyl isovalerate
as a flavouring substance in the USA and 23.91 µg/kg bw from its use
as a flavouring substance in Europe (see footnote in section
2.2.3.2a i).
b) Component alcohols and acids
Ester hydrolysis yields the component branched-chain acid and
linear or branched-chain alcohol. The results of subchronic studies
for the component acids and alcohols of esters of aliphatic acyclic
primary alcohols and branched-chain aliphatic acyclic acids has
revealed no evidence of toxicity at levels up to 2500 mg/kg bw per
day. These short-term studies are described below and summarized in
Table 4.
i) 1-Propanol
Four-month-old male Wistar rats were given a 1M solution of
1-propanol, corresponding to 60 mg/kg bw per day, as the sole drinking
fluid for 4 months. Control animals received tap water. Consumption of
food and alcohol solution and the weight gain of each animal were
recorded. A lower ratio of weight gain to caloric intake was observed
in the rats given the 1-propanol, indicating that this group utilized
food less efficiently than those untreated. However, this action is
not indicative of toxicity. Histological examination of the livers of
the experimental animals revealed no difference from controls. No
hepatic steatosis was observed. Neither inflammation nor cirrhosis was
seen in any of the livers (Hillbom et al., 1974).
ii) Butanol
No adverse effects were reported when 6.9% butanol with 25%
sucrose was added to the drinking-water of male rats for 13 weeks
(Wakabayashi et al., 1984) at an estimated daily intake level of 5.6
mg butanol/kg bw.
A 14-day study was conducted using rats to evaluate the effects of
butanol on gross and microscopic pathology, body weight, clinical
signs and clinical chemistry. Levels of 1.38%, 2.75% or 5.5% butanol
were administered orally to rats, which corresponds to daily intake
levels of 1380-5500 mg butanol/kg bw. A statistically significant
increase in the liver-to-body weight ratio resulted in males at all
levels and females at the 5.5% level (PPG, 1991).
No deaths or unusual symptoms were observed when rats (sex was not
reported) were fed butyl alcohol in a 2% corn oil solution blended
with the diet at concentrations of 0, 1000, 3500 or 10 000 mg/kg
(equal to 0, 90, 300 or 940 mg/kg bw per day, respectively) for 28
days. At necropsy, no significant gross lesions were observed. Liver
and kidney weights showed no significant differences from those of the
controls (Bio-Fax, 1969).
iii) Hexyl alcohol
Two groups of male and female rats were fed hexyl alcohol for 13
weeks at dietary levels of 0.25 or 0.50%; a third group was fed 1% for
weeks 1-10 and 2%, 4% and 6% for weeks 11, 12 and 13, respectively.
Decreased food consumption was observed in females at the high-dose
level, but body weights for all animals were normal. No significant
haematological changes or differences in urine analyses were observed
for the test and control groups. Gross pathology and microscopic
evaluations were performed and revealed no treatment-related effects.
The 1% level was reported to be equivalent to an intake of 577 mg/kg
bw per day (Eibert, 1992).
Three groups of 2 male and 2 female purebred beagle dogs were fed
hexyl alcohol for 13 weeks at levels of 0.50%, 1.0% or 1000 mg/kg bw
per day via gelatin capsules. A fourth group of 4 males and 4 females
served as controls. Body weight, organ weight, and food consumption
for the treated animals did not differ from controls. All animals in
the high-dose group displayed gross signs of toxicity intermittently
after treatment, including salivation, excitation, ataxia, tremors and
anaesthesia. The animals generally returned to normal within 4 hours
of treatment. One female dog died on the first day of treatment and
was replaced by another female. Three of the four remaining animals
died on either the 23rd or 38th day of the study. No signs of toxicity
were observed in the other test animals. Haematology, serum chemistry
and urinalysis was performed and showed no significant difference as
compared to controls. All animals were necropsied at the end of the
study.
Animals in the high-dose group exhibited gastrointestinal
inflammation and congestion of other visceral organs. Some gastric
irritation was observed in the mid-dose group. Both males treated at
the 1000 mg/kg bw per day level exhibited significant testicular
atrophy (Eibert, 1992). Effects on the testes and other reproductive
tissue have been observed with other aliphatic alcohols at high dose
levels (Lington & Bevan, 1994). A finding of nodules on the lung
surface of some animals was reported to be non-treatment-related. The
1% NOEL corresponds to a daily intake level ranging from 230 to 695
mg/kg bw (Eibert, 1992).
iv) Octanol
Seventeen groups of 10 mice each received oral doses of 1-octanol
at a level of 179 mg/kg bw per day for one month. 1-Octanol was
administered intragastrically in the form of a solution or suspension.
No cumulative effects were observed (Voskovofnikova, 1966).
v) Cis-3-hexen-1-ol
Short-term toxicity studies have been reported for
cis-3-hexen-1-ol, which is the alcohol component for esters of
unsaturated alcohols in this group. Groups of 15 male and 15 female
weanling rats were given drinking-water containing 0, 310, 1250 or
5000 mg/litre cis-3-hexen-1-ol for 98 days. Body weight, food intake
and water consumption were recorded weekly. A reduction in water
intake was reported in male rats in the high-dose group only.
Haematology studies were conducted on eight rats from each sex in the
control, 1250 and 5000 mg/litre groups during the sixth week.
Urinalysis, including pH, microscopic constituents and content of
bile, blood and glucose, was performed for eight animals of each sex
from each group during week 6, and from week 12 until the end of the
study. Kidney function was assessed regularly. Necropsies were
performed on all animals at the end of the study. Serum chemistry
parameters were measured for all animals at necropsy. All tissues were
examined for macroscopic abnormalities, and organ weights were
obtained for the brain, pituitary, thyroid, heart, liver, spleen,
kidneys, adrenals and gonads. Tissues from all rats in the high-dose
group and from 50% of the controls were examined microscopically.
Slightly increased relative kidney and adrenal gland weights observed
in males only at the 5000 mg/litre level were not accompanied by any
evidence of histopathology. These increases were not considered to be
statistically significant. No treatment-related abnormalities were
reported (Gaunt et al., 1969).
vi) Isopropanol
Daily doses of 2.6 or 6.4 mg isopropanol/kg bw were given to adult
human males for a period of six weeks. Studies of the microscopic and
chemical characteristics of blood and urine were repeated on the
first, third and seventh days and at weekly intervals thereafter.
Removal of BSP from blood was estimated once before initiation of
ingestion of isopropyl alcohol and at the end of the experimental
period. Retention of BSP in serum at the end of the experiment was not
significantly different from that before the start in any of the men.
No significant, persistent changes that are clearly attributable to
the ingestion of isopropanol were evident (Wills, 1963).
vii) Isobutanol
Groups of 10 male and 10 female Wistar rats were given isobutyl
alcohol in their drinking-water for 3 months. The test substance was
administered in concentrations of 0, 1000, 4000 or 16 000 mg/litre,
which was reported to correspond to approximate dose levels of 0, 60
340 or 1450 mg/kg bw per day. Food and water consumption and body
weight gain were not affected by the test substance. All animals
tested were free from adverse clinical effects. Haematology and
clinical chemistry parameters were measured and revealed no
treatment-related adverse effects. Gross pathology and
histopathological examinations were no different from controls. The
authors concluded that results of this study demonstrate a lack of
toxicity associated with administration of isobutyl alcohol in the
drinking-water of rats, and that the NOEL is >1450 mg/kg bw per day
(BASF, 1992).
viii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage, providing daily dose levels
of 0, 150 500, or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight reduction in body-weight gain, which was associated
with reduced food intake. Over the entire period of the study,
however, there was no statistically significant reduction in mean food
intake. Examination of haematology, serum analyses, urinalysis, renal
concentration tests, and organ weights revealed no treatment-related
effects. At necropsy, the animals were examined for macroscopic
abnormalities, and the major organs were weighed. Microscopic
examination was performed on several tissues of the control and
high-dose animals. No treatment-related abnormalities were observed
(Carpanini et al., 1973).
ix) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to be equivalent to 2500 mg isovaleric acid/kg
bw per day. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analyses, organ weights and
histology. At the 2500 mg/kg bw dose level, there were no
treatment-related effects on the measured parameters (Amoore et
al., 1978).
2.2.3.3 Long-term toxicity/carcinogenicity studies
The results of long-term toxicity/carcinogenicity studies with two
of the component alcohols are described below and summarized in Table
5.
a) Isobutanol
Twenty males and 20 female Wistar rats were given 0.2% isobutanol
in drinking-water for 53 to 56 weeks. Clinical chemistry, including
alcohol dehydrogenase, glutamic oxaloacetate transaminase glutamic
pyruviuc transaminase, and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isobutanol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 200 mg/kg bw. This is >10 000 times the daily per capita
intake ("eaters only") of 4.85 µg/kg bw isobutanol from use as a
flavouring substance in the USA and of 8.79 µg/kg bw isobutanol from
its use in Europe (see footnote in section 2.2.3.2a i).
Table 5. Long-term toxicity studies for esters of aliphatic acyclic primary alcohols and
branched-chain aliphatic acyclic acids
Substance Species, Route Duration NOEL Reference
sex (weeks) (mg/kg bw per day)
Component Alcohols
Isobutyl alcohol Rats, M&F Drinking-water 53-56 2001 Johannsen & Purchase, 1969
Isoamyl alcohol2 Rats, M&F Drinking-water 53-56 20001 Johannsen & Purchase, 1969
1 The study was performed at a single dose level or multiple dose levels that produced no
effects and, therefore, a NOEL was not determined. The NOEL is probably much higher than
the reported dose level that produced no effects
2 A structually related branched-chain alcohol
b) Isoamyl alcohol
Twenty males and 20 female Wistar rats were given drinking-water
containing 2% isoamyl alcohol for 53 to 56 weeks. Clinical chemistry,
including alcohol dehydrogenase, glutamic oxaloacetate transaminase,
glutamic pyruviuc transaminase and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isoamyl alcohol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 2000 mg/kg bw. This is > 10 000 times the daily per
capita intake ("eaters only") of 26.32 µg isoamyl alcohol/kg bw from
use as a flavouring substance in the USA and of 30.47 µg isoamyl
alcohol/kg bw from its use as a flavouring substance in Europe (see
footnote in section 2.2.3.2a i).
2.2.3.4 Genotoxicity studies
The results of genotoxicity studies with the group of 32 branched-
chain esters are summarized in Table 6. Mutagenicity testing of ethyl
isovalerate in vitro by means of the Ames test, at concentrations up
to 10 mg/plate has shown no evidence of mutagenicity in Salmonella
typhimurium strains TA92, TA94, TA97, TA98, TA100, TA102, TA1535,
TA1537 or in TA2637 with or without metabolic activation (Ishidate et
al., 1984; Fujita & Sasaki, 1987). There was no evidence of
mutagenicity in Chinese hamster fibroblast cells when the chromosomal
aberration test was performed with ethyl isovalerate at concentrations
up to 2 mg/ml (Ishidate et al., 1984). When incubated with Bacillus
subtilis, at concentrations up to 20 µl/disk (Yoo, 1986), and at 17
µg/plate (Oda et al., 1978), ethyl isovalerate was reported to be
non-mutagenic. At high concentrations (100-200 µl/ml) of ethyl
isovalerate, Bacillus subtilis was reported to show signs positive
for mutagenicity in the rec assay (Koruda, 1984a). However, these
results are not supported by the results of other rec assays using
similar bacterial strains (Yoo, 1986; Oda et al., 1978). Considering
the results of the rec assay, the Ames test and the chromosomal aberration
test, it is concluded that the 32 branched-chain esters are not genotoxic.
2.2.3.5 Reproductive toxicity studies
No reproduction studies have been reported for esters of aliphatic
acyclic primary alcohols and branched-chain aliphatic acyclic acids.
Table 6. Genotoxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance name Test system Test object Concentration of Results Reference
in vitro substance
Ethyl isovalerate Ames test S. typhimurium TA92, 0.01-1 mg/plate Negative1 Fujita & Sasaki, 1987
TA1535
Ames test TA100, TA1537, TA94, 10 mg/plate Negative1 Ishidate et al., 1984
TA98 and TA2637
Rec assay B. subtilis 100-200 µl/ml Positive Kuroda et al., 1984
Rec assay B. subtilis M45(rec-) up to 20 µl/disk Negative Yoo, 1986
and H17(rec+)
S. typhimurium TA97
and TA102
Chromosomal
aberration test Chinese hamster up to 2 mg/ml Negative Ishidate et al., 1984
fibroblasts cells
Rec assay B. subtilis H17 17µg/plate Negative Oda et al., 1978
and M45
1 Both with and without S-9 activation
3. REFERENCES
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: Efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D. ed.
Intermediary xenobiotic metabolism in animals. Taylor and Francis, New
York, pp. 81-97.
BASF (1980) Private communication to RIFM. Unpublished document.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months. Private communication to
FEMA.
Bio-Fax Industrial Bio-test Lab. Inc. (1969) Data sheet No. 2-5/69:
n-Butyl alcohol. Northbrook, Illinois, USA.
Biophere Research Center, Inc. (1981) Private communication to FEMA.
Browning, E. (1965) Toxicity and metabolism of industrial solvents.
Elsevier Publishing Co., New York.
Caldwell, J. (1989) Endogenous ethanol. Report to FEMA.
Carpanini, I.F., Kiss I.S., Grasso P., & Gangolli, S.D. (1973)
Short-term toxicity of isoamyl alcohol in rats. Food Cosmet.
Toxicol., 11: 713-724.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III,
6th ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food Cosmet. Toxicol., 16:
255-276.
Damske, D.R., Mecler, F.J., Beliles, R.P., & Liverman, J. L. (1980)
iso-Amyl iso-valerate. Report to FEMA. Unpublished document (Submitted
to WHO by FEMA).
Dawson, A.M., Holdsworth, C.D., & Webb J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117: 97-100.
Deuel, H. J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley-Interscience, New York.
DiVincenzo, G.D. & Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid in the rat. Toxicol. Appl. Pharmacol., 47: 609-612.
Drake, J.J.P., Butterworth, K.R., Gaunt, I.F., & Grasso, P. (1978)
Short-term toxicity study of isobutyl isobutyrate in rats. Food
Cosmet. Toxicol., 16: 337-342.
Eibert, J Jr. (1992) Private communication to FEMA. Unpublished
report. Cited in: Food and Drug Administration (FDA) (1993)
Priority-based assessment of food additives (PAFA) database. Center
for Food Safety and Applied Nutrition, p. 58.
FDA (1993) Priority-based assessment of food additives (PAFA)
database. US Food and Drug Administration, Center for Food Safety and
Applied Nutrition, p. 58.
Food and Drug Research Laboratory (1975) Unpublished report to RIFM.
Fujita H. & Sasaki, M. (1987) Mutagenicity test of food additives with
Salmonella typhimurium TA97 and TA102. Annual Report of the Tokyo
Metropolitan Research Laboratory P.H., 3.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montp., 25(1): 51.
Gangolli, S.D. & Shilling, W.H. (1968) Hydrolysis of esters by
artificial gastric and pancreatic juices. Analytical Chemistry
Research Report No. 11/1968. Unpublished study (Submitted to WHO by
FEMA).
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G., & Gangolli,
S.D. (1969) Acute (rat and mouse) and short-term (rat) toxicity
studies on cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7: 451-459.
Gibel, W.K., Lohs, K., & Wildner, G.P. (1975) Carcinogenic activity of
propanol, 2-methyl-1-propanol, and 3-methyl-1-butanol. Arch.
Geschwulstforsch., 45: 19-24.
Givaudan-Roure Corporation (1971) Private communication to FEMA.
Griffiths, P.J. & Giessinger, M. (1979) Private communication to FEMA.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fementative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
Hillbom, M.E., Fransaila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Research
Laboratories of the State Alcohol Monopoly (Alko), Helsinki, Finland.
Ishidate, M. Jr., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22(8):
623-636.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Jenner, P.M.,Hagan, E.C., Taylor, J.M., Cook, E.L., & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure: I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
Johannsen, E. & Purchase, I.F.H. (1969) Kaffircorn malting and brewing
studies: XXI. The effect of fuel oils of bantu beer on rat liver.
S.Afr. Med. J., 43(12): 326-328.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Krebs, H.A. & Perkins, J.R. (1970) The physiological role of liver
alcohol dehydrogenase. Biochem. J., 118: 635-644.
Kuroda, K., Tanaka, S., Yu, Y.S., & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31(6): 277.
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the
hydrolysis of twenty-six organic esters by pancreatin. Centraal
Instituut Voor Voedingsonderzoek, Zeist, The Netherlands (TNO Report
No. R 4319).
Levenstein, I. (1974) Report to RIFM. Unpublished document.
Levenstein, I. (1976) Report to RIFM. Unpublished document.
Liebich, H.M., Buelow, H.J., & Kallmayer, R. (1982) Quantification of
endogenous aliphatic alcohols in serum and urine. J. Chromatogr.,
239: 343-349.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, p. 2613.
Longland, R.C., Shilling, W.H., & Gangolli, S.D. (1977) The hydrolysis
of flavouring esters by artificial gastrointestinal juices and rat
tissue preparations. Toxicology, 8: 197-204.
Mecler, F.J. & Craig, D.K. (1980) Three month toxicity study on ethyl
isobutyrate in rat. Report to FEMA. Unpublished document.
Moreno, O.M. (1977) Report to RIFM. Unpublished document.
Moreno, O.M. (1978) Report to RIFM. Unpublished document.
Moreno, O.M. (1975) Report to RIFM. Unpublished document.
Moreno, O M., Cerven D.R., & Altenbach, E.J. (1981) Report to RIFM.
Unpublished document.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Report to RIFM.
Unpublished document.
Munch, J. C. (1972) Aliphatic alcohols and alkyl esters: Narcotic and
lethal potencies to tadpoles and rabbits. Ind. Med. Surg., 41:
31-33.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of structural class with
no-observed-effect levels: a proposal for establishing a threshold of
concern. In Press.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Pharmakon Inc. (1980) Private communication to FEMA.
Plaut, G.W.E. & Lardy, H.A. (1951) Enzymatic incorporation of
C14-bicarbonate into acetoacetate in the presence of various
substrates. J. Biol. Chem., 192: 435-445.
PPG Industries Inc. (1991) Submission to EPA. Unpublished document.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34(8-9): 569-85.
Sandmeyer, E.E. & Kirwin, C.J. (1981) Esters. In. Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology - Volume
2A, 3rd ed. John Wiley and Sons, Inc., New York, pp. 2259-2412.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Voskovofnikova, V.B. (1966) Substantiation of the maximum permissible
concentration of the flotation reagent IM-68 and its component
alcohols (hexyl, heptyl and octyl alcohol) in bodies of water. Gig.
i Sanit., 31: 310-316.
Wakabayashi, T., Horiuchi, M., Sakaguchi, M., Onda, H., & Iijima, M.
(1984) Induction of megamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta Pathol. Jpn., 34: 471-480.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall Ltd, London, pp. 88-113.
Wills, J.H., Jameson, E.M., & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15: 560-565.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34(3-4):
267-288.
See Also:
Toxicological Abbreviations
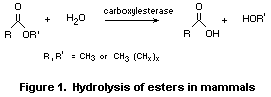 Branched-chain esters in this group are hydrolysed in vitro to
their corresponding branched-chain acids (isobutyric acid, isovaleric
acid or 2-methylbutyric acid) and corresponding aliphatic acyclic
alcohols. Table 2 summarizes the hydrolysis data for the five esters
containing a branched-chain alcohol and/or branched-chain acid (i.e.,
ethyl iso-valerate, iso-amyl caproate, benzyl iso-butyrate,
iso-amyl butyrate and iso-amyl iso-valerate). Following
hydrolysis, short-chain (<C6) branched-chain acids and alcohols
are rapidly absorbed from the gastrointestinal tract (Dawson et
al., 1964; Gaillard & Derache, 1965).
Table 2. In vitro ester hydrolysis data
Ester Artificial Artificial Rat liver Rat small % hydrolysis
gastric juice1 pancreatic juice1 preparation1 intestinal after 2 hours
t0.52 (min) t0.5 (min) t0.5 (sec) mucosa1
t0.5 (sec)
Ethyl iso-valerate 1390 198 2.35 133 63; 344
Isoamyl caproate7 146 37.8 NR NR NR
Benzyl isobutyrate7 577 17.8 0.0422 0.0707 NR
Isoamyl butyrate7 660 11.3 0.492 0.0713 123; 1004
Isoamyl isovalerate7 295 10.2 NR NR NR
Benzyl 2-methylbutanoate7 NR NR NR NR 1005
Isoamyl acetate7 NR NR NR NR 205; 1006
1 Longland et al., 1977
2 t1/2 = Half-life
3 In artificial gastric juice. Gangolli & Shilling, 1968
4 In artificial pancreatic juice. Gangolli & Shilling, 1968
5 By pancreatin. Leegwater & van Straten, 1974.
6 In whole homogenate of pig jejunum. Grundschober, 1977.
7 Structurally related ester
2.2.2 Biotransformations
2.2.2.1 Branched-chain aliphatic acids
The saturated branched-chain aliphatic acids isobutyric acid,
isovaleric acid and 2-methylbutyric acid formed via ester hydrolysis
are endogenous in humans as intermediary products in the metabolism of
the amino acids valine (Kinnory et al., 1955), leucine (Henning &
Hird, 1970), and isoleucine (Voet & Voet, 1990), respectively. These
acids participate in the fatty acid pathway and the tricarboxylic acid
cycle and are completely metabolized to CO2.
Short (<C6) branched-chain acids undergo ß-oxidation,
preferably in the longer chain. ß-Cleavage of the resulting acid
yields linear acid fragments which are sources of carbon in the fatty
acid pathway or tricarboxylic acid cycle (Voet & Voet, 1990). For
example, isobutyric acid (DiVincenzo & Hamilton, 1979) and methacrylic
acid (2-methylpropenoic acid) (Bratt & Hathway, 1977) given to rats by
gavage are rapidly eliminated almost exclusively as CO2. The CO2 is
presumed to arise from ß-oxidation and decarboxylation of isobutyric
acid or methacrylic acid to yield propionyl CoA, which, after
conversion to succinyl CoA, can participate in the tricarboxylic acid
cycle (Saito, 1975). Studies of the metabolism of the amino acid
leucine have shown that isovaleric acid is converted to acetyl
coenzyme A and acetoacetate (Voet & Voet, 1990).
The principal metabolic pathways utilized by the remaining 3
branched-chain acids in this group (i.e., 2-, 3-, and
4-methylpentanoic acid) are determined primarily by the position of
the methyl substituent. Acids with a methyl substituent located at an
even-numbered carbon (e.g., 2-methylpentanoic acid and
4-methylpentanoic acid) are extensively metabolized to CO2 via
ß-oxidation and cleavage in the fatty acid pathway. If the methyl
group is located at the ß-position (e.g., 3-methylpentanoic acid),
ß-oxidation is inhibited and alpha-oxidation predominates, primarily
leading to short-chain acid fragments capable of being completely
metabolized (Williams, 1959).
a) Isobutyric acid
Rats administered (1-14C)-isobutyric acid by gavage rapidly
eliminate 14CO2 (DiVincenzo & Hamilton, 1979). The CO2 is presumed
to arise from ß-oxidation and decarboxylation of isobutyric acid to
yield propionyl CoA which, after conversion to succinyl CoA, then
participates in the tricarboxylic acid cycle (Voet & Voet, 1990). The
formation of propionyl CoA is shown by the observation that rats fed
isobutyric acid excrete elevated levels of 2-methylmalonic acid, an
intermediate in the conversion of propionyl CoA to succinyl CoA (Voet
& Voet, 1990). It is anticipated that humans would metabolize
isobutyric acid by complete oxidation to CO2 via propionyl CoA and
succinyl CoA, components of the tricarboxylic acid cycle (Fig. 2).
Branched-chain esters in this group are hydrolysed in vitro to
their corresponding branched-chain acids (isobutyric acid, isovaleric
acid or 2-methylbutyric acid) and corresponding aliphatic acyclic
alcohols. Table 2 summarizes the hydrolysis data for the five esters
containing a branched-chain alcohol and/or branched-chain acid (i.e.,
ethyl iso-valerate, iso-amyl caproate, benzyl iso-butyrate,
iso-amyl butyrate and iso-amyl iso-valerate). Following
hydrolysis, short-chain (<C6) branched-chain acids and alcohols
are rapidly absorbed from the gastrointestinal tract (Dawson et
al., 1964; Gaillard & Derache, 1965).
Table 2. In vitro ester hydrolysis data
Ester Artificial Artificial Rat liver Rat small % hydrolysis
gastric juice1 pancreatic juice1 preparation1 intestinal after 2 hours
t0.52 (min) t0.5 (min) t0.5 (sec) mucosa1
t0.5 (sec)
Ethyl iso-valerate 1390 198 2.35 133 63; 344
Isoamyl caproate7 146 37.8 NR NR NR
Benzyl isobutyrate7 577 17.8 0.0422 0.0707 NR
Isoamyl butyrate7 660 11.3 0.492 0.0713 123; 1004
Isoamyl isovalerate7 295 10.2 NR NR NR
Benzyl 2-methylbutanoate7 NR NR NR NR 1005
Isoamyl acetate7 NR NR NR NR 205; 1006
1 Longland et al., 1977
2 t1/2 = Half-life
3 In artificial gastric juice. Gangolli & Shilling, 1968
4 In artificial pancreatic juice. Gangolli & Shilling, 1968
5 By pancreatin. Leegwater & van Straten, 1974.
6 In whole homogenate of pig jejunum. Grundschober, 1977.
7 Structurally related ester
2.2.2 Biotransformations
2.2.2.1 Branched-chain aliphatic acids
The saturated branched-chain aliphatic acids isobutyric acid,
isovaleric acid and 2-methylbutyric acid formed via ester hydrolysis
are endogenous in humans as intermediary products in the metabolism of
the amino acids valine (Kinnory et al., 1955), leucine (Henning &
Hird, 1970), and isoleucine (Voet & Voet, 1990), respectively. These
acids participate in the fatty acid pathway and the tricarboxylic acid
cycle and are completely metabolized to CO2.
Short (<C6) branched-chain acids undergo ß-oxidation,
preferably in the longer chain. ß-Cleavage of the resulting acid
yields linear acid fragments which are sources of carbon in the fatty
acid pathway or tricarboxylic acid cycle (Voet & Voet, 1990). For
example, isobutyric acid (DiVincenzo & Hamilton, 1979) and methacrylic
acid (2-methylpropenoic acid) (Bratt & Hathway, 1977) given to rats by
gavage are rapidly eliminated almost exclusively as CO2. The CO2 is
presumed to arise from ß-oxidation and decarboxylation of isobutyric
acid or methacrylic acid to yield propionyl CoA, which, after
conversion to succinyl CoA, can participate in the tricarboxylic acid
cycle (Saito, 1975). Studies of the metabolism of the amino acid
leucine have shown that isovaleric acid is converted to acetyl
coenzyme A and acetoacetate (Voet & Voet, 1990).
The principal metabolic pathways utilized by the remaining 3
branched-chain acids in this group (i.e., 2-, 3-, and
4-methylpentanoic acid) are determined primarily by the position of
the methyl substituent. Acids with a methyl substituent located at an
even-numbered carbon (e.g., 2-methylpentanoic acid and
4-methylpentanoic acid) are extensively metabolized to CO2 via
ß-oxidation and cleavage in the fatty acid pathway. If the methyl
group is located at the ß-position (e.g., 3-methylpentanoic acid),
ß-oxidation is inhibited and alpha-oxidation predominates, primarily
leading to short-chain acid fragments capable of being completely
metabolized (Williams, 1959).
a) Isobutyric acid
Rats administered (1-14C)-isobutyric acid by gavage rapidly
eliminate 14CO2 (DiVincenzo & Hamilton, 1979). The CO2 is presumed
to arise from ß-oxidation and decarboxylation of isobutyric acid to
yield propionyl CoA which, after conversion to succinyl CoA, then
participates in the tricarboxylic acid cycle (Voet & Voet, 1990). The
formation of propionyl CoA is shown by the observation that rats fed
isobutyric acid excrete elevated levels of 2-methylmalonic acid, an
intermediate in the conversion of propionyl CoA to succinyl CoA (Voet
& Voet, 1990). It is anticipated that humans would metabolize
isobutyric acid by complete oxidation to CO2 via propionyl CoA and
succinyl CoA, components of the tricarboxylic acid cycle (Fig. 2).
 b) Isovaleric acid
Isovaleric acid as the CoA thioester undergoes successive
dehydrogenation and carboxylation in the leucine pathway to yield
ß-methylglutaconyl CoA. ß-Methylglutaconyl CoA is hydrated to yield
ß-hydroxy ß-methylglutaryl CoA which is finally cleaved to
acetoacetate and acetyl CoA (Voet & Voet, 1990) (Fig. 3).
b) Isovaleric acid
Isovaleric acid as the CoA thioester undergoes successive
dehydrogenation and carboxylation in the leucine pathway to yield
ß-methylglutaconyl CoA. ß-Methylglutaconyl CoA is hydrated to yield
ß-hydroxy ß-methylglutaryl CoA which is finally cleaved to
acetoacetate and acetyl CoA (Voet & Voet, 1990) (Fig. 3).
 c) 2-Methylbutyric acid, 2-methylpentanoic acid, and 4-methylpentanoic
acid
In general, branched-chain acids with an alpha-methyl or
4-methyl substituent are metabolized via oxidative cleavage to yield
linear acid fragments (Deuel, 1957), which are completely oxidized to
CO2 in the fatty acid pathway and tricarboxylic acid cycle. 2-
Methylbutyric acid undergoes ß-oxidation and cleavage to yield acetyl
CoA and propionyl CoA. Propionyl CoA is converted to succinyl CoA,
which, together with acetyl CoA, can be completely metabolized to CO2
in the tricarboxylic acid cycle (Deuel, 1957). ß-Oxidation of
2-methylbutyric acid has been observed in guinea-pigs in vivo
(Stokke et al., 1969) (Fig. 4).
c) 2-Methylbutyric acid, 2-methylpentanoic acid, and 4-methylpentanoic
acid
In general, branched-chain acids with an alpha-methyl or
4-methyl substituent are metabolized via oxidative cleavage to yield
linear acid fragments (Deuel, 1957), which are completely oxidized to
CO2 in the fatty acid pathway and tricarboxylic acid cycle. 2-
Methylbutyric acid undergoes ß-oxidation and cleavage to yield acetyl
CoA and propionyl CoA. Propionyl CoA is converted to succinyl CoA,
which, together with acetyl CoA, can be completely metabolized to CO2
in the tricarboxylic acid cycle (Deuel, 1957). ß-Oxidation of
2-methylbutyric acid has been observed in guinea-pigs in vivo
(Stokke et al., 1969) (Fig. 4).
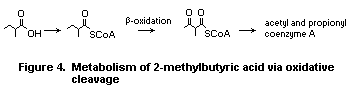 The metabolism of 2-methylpentanoic acid has been studied in
mammals. 2-Methypentanoic acid undergoes ß-oxidation in the longer
branched-chain followed by cleavage to yield two propionyl CoA
fragments. In rabbits, 2-methylpentanoic acid is converted to
propionyl CoA, which is completely metabolized (Deuel, 1957).
4-Methylpentanoic acid, a gamma-methyl substituted acid, is expected
to undergo ß-oxidative cleavage to acetyl CoA and isobutyl CoA which
would be completely metabolized (Voet & Voet, 1990).
d) 3-Methylpentanoic acid
In general, ß-oxidation of branched-chain acids with a ß-methyl is
inhibited. However, ß-substituted acids may undergo alpha-oxidative
cleavage to yield acid fragments (Deuel, 1957; Williams, 1959) which
are completely metabolized in the fatty acid and tricarboxylic acid
cycles. In addition, they may undergo partial ß-oxidation to yield
ß-hydroxy acids which are further oxidized (omega-oxidation) and
fragmented to yield short-chain acids.
The metabolism of 3-methylpentanoic acid has been studied in
mammals. alpha-Oxidation and decarboxylation of 3-methylpentanoic acid
would yield 2-methylbutyric acid followed by ß-oxidative cleavage to
yield acetyl CoA and acetone. In guinea-pig liver homogenate,
3-methylpentanoic acid is converted to 2-methylbutyric acid which is
further metabolized via ß-oxidative cleavage (Stokke, 1969). In
rabbits, 3-methylpentanoic acid is converted to ß-hydroxybutyric acid
(Williams, 1959).
2.2.2.2 Aliphatic linear alcohols
Linear saturated and unsaturated alcohols are oxidized
successively to their corresponding aldehydes and carboxylic acids,
which enter the fatty acid ß-oxidation pathway. Branched-chain
aliphatic alcohols are converted by similar oxidation reactions to
their corresponding carboxylic acid, which undergoes metabolism via
ß-oxidation and cleavage to yield CO2 in amino acid pathways, the
fatty acid pathway, and the tricarboxylic acid cycle.
Based on the above information, it is anticipated that the 32
esters of aliphatic acyclic primary alcohols and branched-chain
aliphatic acyclic acids will be hydrolysed in the intestinal tract,
blood and liver to yield the corresponding branched-chain acids and
aliphatic alcohols. The resulting branched-chain acids and aliphatic
alcohols would be rapidly absorbed from the gastrointestinal tract and
completely metabolized to CO2 in the tricarboxylic acid cycle and
fatty acid pathway. The resulting linear alcohols would be
successively oxidized to their corresponding aldehyde and acid. The
resulting branched-chain aliphatic alcohols are converted by similar
oxidation reactions to their corresponding carboxylic acid, which
undergoes complete metabolism to CO2.
2.2.3 Toxicological studies
2.2.3.1 Acute toxicity
The results of acute toxicity studies for 24 of the 32 esters of
aliphatic acyclic primary alcohols and branched-chain aliphatic
acyclic acids are summarized in Table 3. The low acute oral toxicity
of the group is demonstrated by oral LD50 values >2300 mg/kg bw,
with the majority being >5000 mg/kg bw.
2.2.3.2 Short-term toxicity studies
The results of short-term toxicity studies with 4 branched-chain
esters (ethyl isobutyrate, isobutyl isobutyrate, ethyl isovalerate and
isoamyl isovalerate), one component acid (isovaleric acid) and 7
component alcohols (propyl alcohol, butyl alcohol, hexanol, octanol,
3-hexen-1-ol, isobutanol and isoamyl alcohol) are summarized in Table
4.
a) Branched-chain Esters
i) Ethyl isobutyrate
Ethyl isobutyrate was added to the diet of 12 male rats and 12
female rats at a level calculated to provide an average daily intake
of 29.2 mg/kg bw for 90 days. Weekly measurement of body weights and
food intake revealed no significant difference between test and
control animals. Haematological examination, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Determination of mean organ weights
revealed a slight increase in both absolute and relative adrenal gland
weights in females compared to controls, but these values were not
statistically significant. Gross and histopathological examination
failed to reveal any dose-related effects on the adrenal gland or any
other organs (Mecler & Craig, 1980). The NOEL of 29.2 mg ethyl
isobutyrate/kg bw per day in rats is >1000 times the estimated daily
per capita intake1 ("eaters only") of 7.74 µg/kg bw from use of
ethyl isobutyrate as a flavouring substance in the USA and 12.44 µg/kg
bw from its use as a flavouring substance in Europe.
ii) Ethyl isovalerate
For 90 days, ethyl isovalerate on gum arabic (10%) was added to the
diet of 12 male rats and 12 female rats at a level calculated to
provide an average daily intake of 12.1 mg/kg bw for males and 13.6
mg/kg bw for females. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed.
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw per
day) calculated as follows: [(mg per day)/body weight], where body
weight = 60 kg. Slight variations may occur from rounding off.
Table 3. Acute toxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
iso-Butyrates
Methyl iso-butyrate Rats NR Oral 16 000 Sandmeyer & Kirwin, 1981
Ethyl iso-butyrate Rats NR Oral >5000 Moreno, 1975
Propyl iso-butyrate Rats NR Oral 15 000 Jenner et al., 1964
Butyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
Hexyl iso-butyrate Rats NR Oral >5000 Moreno, 1977
Trans-3-heptenyl 2-
methylpropanoate Rats NR Oral >25 000 Food and Drug Research Labs, 1975
Octyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
iso-Butyl iso-butyrate Rats
and mice NR Oral 12 800 Sandmeyer & Kirwin, 1981
iso-Valerates
Methyl iso-valerate Rabbits NR Oral 5690 Munch, 1972
Ethyl iso-valerate Rats NR Oral >5000 Levenstein, 1976; BASF, 1980
Rabbits NR Oral 7030 Munch, 1972
Propyl iso-valerate Rabbits NR Oral 8220 Munch, 1972
Butyl iso-valerate Rats NR Oral >5000 Moreno, 1978
Rabbits NR Oral 8230 Munch, 1972
Hexyl 3-methylbutanoate Rats NR Oral >5000 Moreno et al., 1981
2-Methylbutyrates
2-Methylpropyl Rabbits NR Oral >5000 Munch, 1972
3-methylbutyrate Rats NR Oral 6970 Moreno, 1978
2-Methylbutyl
3-methylbutanoate Rats NR Oral >5000 Moreno, 1978
Methyl 2-methylbutyrate Rats NR Oral >5000 Moreno et al., 1982
Hexyl 2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
iso-Propyl
2-methylbutyrate Rats NR Oral >20 000 Griffiths & Giessinger, 1979
Table 3. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3-Hexenyl
2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
Ethyl 3-methylpentanoate Rats NR Oral >4000 Biosphere Research Center, Inc., 1981
Methyl 2-methylpentanoate Rats NR Oral >5000 Pharmakon Inc.,1980
1 The study was performed at a single dose level or multiple dose levels that produced no effects and,
therefore, a NOEL was not determined.
The NOEL is probably much higher than the reported dose level that produced no adverse effects
Table 4. Short-term studies for branched-chain esters and their component alcohols and acids
Substance Species, sex Route Duration NOEL Reference
(mg/kg bw per day)
Branched esters
Isobutyl isobutyrate Rats, M & F Oral and gavage 18 weeks 1000 Drake et al., 1978
Ethyl isobutyrate Rats, M & F Oral 90 days 29.21 Mecler & Craig, 1980
Ethyl isovalerate Rats, M & F Oral 13 weeks 12.11 Mecler & Craig, 1980
Isoamyl isovalerate2 Rats, M & F Oral 90 days 2201 Damske et al., 1980
Component acids
Isovaleric acid Rats Oral 90 days 25001 Amoore et al., 1978
Component alcohols
Propyl alcohol Rat, male Oral 4 months 601 Hillbom et al., 1974
Butyl alcohol Rats, male Oral 13 weeks 5.61 Wakabayashi et al., 1984
Rats, M & F Oral 14 days 1380 PPG, 1991
Rats, NR Oral 28 days 9401 Bio-Fax, 1969
Hexyl alcohol Beagles, M & F Oral 13 weeks 230 Eibert, 1992
Rat, M & F Oral 13 weeks 577 Eibert, 1992
Octanol Mice Oral 1 month 1791 Voskovofnikova, 1966
3-Hexen-1-ol Rats, M & F Oral 90 days 150 Gaunt et al., 1969
Isopropanol Adult Human Oral 6 weeks 6.4 Wills, 1969
Isobutanol Rats, M & F Oral 90 days 1450 BASF, 1992
Isoamyl alcohol2 Rats M & F Gavage 17 weeks 1000 Carpanini et al., 1973
1 The study was performed at a single dose level or multiple dose levels that produced no effects and, therefore, a NOEL was not
determined. The NOEL is probably much higher than the reported dose level that produced no adverse effects.
2 Structurally related substance
Haematological examination, blood chemical determinations and
urinalysis conducted at weeks 6 and 12 revealed no difference from
controls. At necropsy increases in absolute and relative mean adrenal
gland and thyroid gland weights were observed, but the results were
not statistically significant. Gross and histopathological examination
did not support relative weight changes, and revealed no dose-related
lesions (Mecler & Craig, 1980). The NOEL of 12.1 mg ethyl
isovalerate/kg bw per day in rats is >1000 times the estimated daily
per capita intake ("eaters only") of 9.01µg/kg bw from use of ethyl
isovalerate as a flavouring substance in the USA, and 12.75 µg/kg bw
from its use in Europe.
iii) Isobutyl isobutyrate
Groups of 15 male and 15 female rats were given daily doses of 0,
10, 100 or 1000 mg isobutyl isobutyrate/kg bw by gavage for 18 weeks.
Body weights, food consumption and water intake were measured and were
not different from controls. Haematology, urine analyses and serum
chemistry parameters were evaluated and did not reveal any
treatment-related effects. At necropsy gross examinations were
performed on all animals and organ weights were obtained. No
significant effects were reported. Histological examination was
conducted on all tissues from rats in the high-dose group and 50% of
the control animals. Histopathological examination of the major organs
was also performed on rats given 10 and 100 mg/kg bw per day. No
evidence of histopathological effects was reported (Drake et al.,
1978). The highest level of 1000 mg isobutyl isobutyrate/kg bw per day
which produced no adverse effects in rats is >100 000 times the
estimated daily per capita intake1 ("eaters only") of 0.04 µg/kg bw
per day from use of isobutyl isobutyrate as a flavouring substance in
the USA and 1.09 µg/kg bw per day from its use in Europe.
iv) Isoamyl isovalerate
Isoamyl isovalerate was added to the diet of male and female rats
at levels calculated to provide average daily intakes of 22, 69 or 220
mg/kg bw for 90 days. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed. Haematological examinations, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Organ weights at necropsy were normal
compared to controls. Gross and histopathological examination revealed
no dose-related lesions (Damske et al., 1980). The intake level of
220 mg isoamyl isovalerate/kg bw per day that produced no effects in
rats is approximately 10 000 times the estimated daily per capita
intake ("eaters only") of 13 µg/kg bw from use of isoamyl isovalerate
as a flavouring substance in the USA and 23.91 µg/kg bw from its use
as a flavouring substance in Europe (see footnote in section
2.2.3.2a i).
b) Component alcohols and acids
Ester hydrolysis yields the component branched-chain acid and
linear or branched-chain alcohol. The results of subchronic studies
for the component acids and alcohols of esters of aliphatic acyclic
primary alcohols and branched-chain aliphatic acyclic acids has
revealed no evidence of toxicity at levels up to 2500 mg/kg bw per
day. These short-term studies are described below and summarized in
Table 4.
i) 1-Propanol
Four-month-old male Wistar rats were given a 1M solution of
1-propanol, corresponding to 60 mg/kg bw per day, as the sole drinking
fluid for 4 months. Control animals received tap water. Consumption of
food and alcohol solution and the weight gain of each animal were
recorded. A lower ratio of weight gain to caloric intake was observed
in the rats given the 1-propanol, indicating that this group utilized
food less efficiently than those untreated. However, this action is
not indicative of toxicity. Histological examination of the livers of
the experimental animals revealed no difference from controls. No
hepatic steatosis was observed. Neither inflammation nor cirrhosis was
seen in any of the livers (Hillbom et al., 1974).
ii) Butanol
No adverse effects were reported when 6.9% butanol with 25%
sucrose was added to the drinking-water of male rats for 13 weeks
(Wakabayashi et al., 1984) at an estimated daily intake level of 5.6
mg butanol/kg bw.
A 14-day study was conducted using rats to evaluate the effects of
butanol on gross and microscopic pathology, body weight, clinical
signs and clinical chemistry. Levels of 1.38%, 2.75% or 5.5% butanol
were administered orally to rats, which corresponds to daily intake
levels of 1380-5500 mg butanol/kg bw. A statistically significant
increase in the liver-to-body weight ratio resulted in males at all
levels and females at the 5.5% level (PPG, 1991).
No deaths or unusual symptoms were observed when rats (sex was not
reported) were fed butyl alcohol in a 2% corn oil solution blended
with the diet at concentrations of 0, 1000, 3500 or 10 000 mg/kg
(equal to 0, 90, 300 or 940 mg/kg bw per day, respectively) for 28
days. At necropsy, no significant gross lesions were observed. Liver
and kidney weights showed no significant differences from those of the
controls (Bio-Fax, 1969).
iii) Hexyl alcohol
Two groups of male and female rats were fed hexyl alcohol for 13
weeks at dietary levels of 0.25 or 0.50%; a third group was fed 1% for
weeks 1-10 and 2%, 4% and 6% for weeks 11, 12 and 13, respectively.
Decreased food consumption was observed in females at the high-dose
level, but body weights for all animals were normal. No significant
haematological changes or differences in urine analyses were observed
for the test and control groups. Gross pathology and microscopic
evaluations were performed and revealed no treatment-related effects.
The 1% level was reported to be equivalent to an intake of 577 mg/kg
bw per day (Eibert, 1992).
Three groups of 2 male and 2 female purebred beagle dogs were fed
hexyl alcohol for 13 weeks at levels of 0.50%, 1.0% or 1000 mg/kg bw
per day via gelatin capsules. A fourth group of 4 males and 4 females
served as controls. Body weight, organ weight, and food consumption
for the treated animals did not differ from controls. All animals in
the high-dose group displayed gross signs of toxicity intermittently
after treatment, including salivation, excitation, ataxia, tremors and
anaesthesia. The animals generally returned to normal within 4 hours
of treatment. One female dog died on the first day of treatment and
was replaced by another female. Three of the four remaining animals
died on either the 23rd or 38th day of the study. No signs of toxicity
were observed in the other test animals. Haematology, serum chemistry
and urinalysis was performed and showed no significant difference as
compared to controls. All animals were necropsied at the end of the
study.
Animals in the high-dose group exhibited gastrointestinal
inflammation and congestion of other visceral organs. Some gastric
irritation was observed in the mid-dose group. Both males treated at
the 1000 mg/kg bw per day level exhibited significant testicular
atrophy (Eibert, 1992). Effects on the testes and other reproductive
tissue have been observed with other aliphatic alcohols at high dose
levels (Lington & Bevan, 1994). A finding of nodules on the lung
surface of some animals was reported to be non-treatment-related. The
1% NOEL corresponds to a daily intake level ranging from 230 to 695
mg/kg bw (Eibert, 1992).
iv) Octanol
Seventeen groups of 10 mice each received oral doses of 1-octanol
at a level of 179 mg/kg bw per day for one month. 1-Octanol was
administered intragastrically in the form of a solution or suspension.
No cumulative effects were observed (Voskovofnikova, 1966).
v) Cis-3-hexen-1-ol
Short-term toxicity studies have been reported for
cis-3-hexen-1-ol, which is the alcohol component for esters of
unsaturated alcohols in this group. Groups of 15 male and 15 female
weanling rats were given drinking-water containing 0, 310, 1250 or
5000 mg/litre cis-3-hexen-1-ol for 98 days. Body weight, food intake
and water consumption were recorded weekly. A reduction in water
intake was reported in male rats in the high-dose group only.
Haematology studies were conducted on eight rats from each sex in the
control, 1250 and 5000 mg/litre groups during the sixth week.
Urinalysis, including pH, microscopic constituents and content of
bile, blood and glucose, was performed for eight animals of each sex
from each group during week 6, and from week 12 until the end of the
study. Kidney function was assessed regularly. Necropsies were
performed on all animals at the end of the study. Serum chemistry
parameters were measured for all animals at necropsy. All tissues were
examined for macroscopic abnormalities, and organ weights were
obtained for the brain, pituitary, thyroid, heart, liver, spleen,
kidneys, adrenals and gonads. Tissues from all rats in the high-dose
group and from 50% of the controls were examined microscopically.
Slightly increased relative kidney and adrenal gland weights observed
in males only at the 5000 mg/litre level were not accompanied by any
evidence of histopathology. These increases were not considered to be
statistically significant. No treatment-related abnormalities were
reported (Gaunt et al., 1969).
vi) Isopropanol
Daily doses of 2.6 or 6.4 mg isopropanol/kg bw were given to adult
human males for a period of six weeks. Studies of the microscopic and
chemical characteristics of blood and urine were repeated on the
first, third and seventh days and at weekly intervals thereafter.
Removal of BSP from blood was estimated once before initiation of
ingestion of isopropyl alcohol and at the end of the experimental
period. Retention of BSP in serum at the end of the experiment was not
significantly different from that before the start in any of the men.
No significant, persistent changes that are clearly attributable to
the ingestion of isopropanol were evident (Wills, 1963).
vii) Isobutanol
Groups of 10 male and 10 female Wistar rats were given isobutyl
alcohol in their drinking-water for 3 months. The test substance was
administered in concentrations of 0, 1000, 4000 or 16 000 mg/litre,
which was reported to correspond to approximate dose levels of 0, 60
340 or 1450 mg/kg bw per day. Food and water consumption and body
weight gain were not affected by the test substance. All animals
tested were free from adverse clinical effects. Haematology and
clinical chemistry parameters were measured and revealed no
treatment-related adverse effects. Gross pathology and
histopathological examinations were no different from controls. The
authors concluded that results of this study demonstrate a lack of
toxicity associated with administration of isobutyl alcohol in the
drinking-water of rats, and that the NOEL is >1450 mg/kg bw per day
(BASF, 1992).
viii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage, providing daily dose levels
of 0, 150 500, or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight reduction in body-weight gain, which was associated
with reduced food intake. Over the entire period of the study,
however, there was no statistically significant reduction in mean food
intake. Examination of haematology, serum analyses, urinalysis, renal
concentration tests, and organ weights revealed no treatment-related
effects. At necropsy, the animals were examined for macroscopic
abnormalities, and the major organs were weighed. Microscopic
examination was performed on several tissues of the control and
high-dose animals. No treatment-related abnormalities were observed
(Carpanini et al., 1973).
ix) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to be equivalent to 2500 mg isovaleric acid/kg
bw per day. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analyses, organ weights and
histology. At the 2500 mg/kg bw dose level, there were no
treatment-related effects on the measured parameters (Amoore et
al., 1978).
2.2.3.3 Long-term toxicity/carcinogenicity studies
The results of long-term toxicity/carcinogenicity studies with two
of the component alcohols are described below and summarized in Table
5.
a) Isobutanol
Twenty males and 20 female Wistar rats were given 0.2% isobutanol
in drinking-water for 53 to 56 weeks. Clinical chemistry, including
alcohol dehydrogenase, glutamic oxaloacetate transaminase glutamic
pyruviuc transaminase, and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isobutanol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 200 mg/kg bw. This is >10 000 times the daily per capita
intake ("eaters only") of 4.85 µg/kg bw isobutanol from use as a
flavouring substance in the USA and of 8.79 µg/kg bw isobutanol from
its use in Europe (see footnote in section 2.2.3.2a i).
Table 5. Long-term toxicity studies for esters of aliphatic acyclic primary alcohols and
branched-chain aliphatic acyclic acids
Substance Species, Route Duration NOEL Reference
sex (weeks) (mg/kg bw per day)
Component Alcohols
Isobutyl alcohol Rats, M&F Drinking-water 53-56 2001 Johannsen & Purchase, 1969
Isoamyl alcohol2 Rats, M&F Drinking-water 53-56 20001 Johannsen & Purchase, 1969
1 The study was performed at a single dose level or multiple dose levels that produced no
effects and, therefore, a NOEL was not determined. The NOEL is probably much higher than
the reported dose level that produced no effects
2 A structually related branched-chain alcohol
b) Isoamyl alcohol
Twenty males and 20 female Wistar rats were given drinking-water
containing 2% isoamyl alcohol for 53 to 56 weeks. Clinical chemistry,
including alcohol dehydrogenase, glutamic oxaloacetate transaminase,
glutamic pyruviuc transaminase and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isoamyl alcohol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 2000 mg/kg bw. This is > 10 000 times the daily per
capita intake ("eaters only") of 26.32 µg isoamyl alcohol/kg bw from
use as a flavouring substance in the USA and of 30.47 µg isoamyl
alcohol/kg bw from its use as a flavouring substance in Europe (see
footnote in section 2.2.3.2a i).
2.2.3.4 Genotoxicity studies
The results of genotoxicity studies with the group of 32 branched-
chain esters are summarized in Table 6. Mutagenicity testing of ethyl
isovalerate in vitro by means of the Ames test, at concentrations up
to 10 mg/plate has shown no evidence of mutagenicity in Salmonella
typhimurium strains TA92, TA94, TA97, TA98, TA100, TA102, TA1535,
TA1537 or in TA2637 with or without metabolic activation (Ishidate et
al., 1984; Fujita & Sasaki, 1987). There was no evidence of
mutagenicity in Chinese hamster fibroblast cells when the chromosomal
aberration test was performed with ethyl isovalerate at concentrations
up to 2 mg/ml (Ishidate et al., 1984). When incubated with Bacillus
subtilis, at concentrations up to 20 µl/disk (Yoo, 1986), and at 17
µg/plate (Oda et al., 1978), ethyl isovalerate was reported to be
non-mutagenic. At high concentrations (100-200 µl/ml) of ethyl
isovalerate, Bacillus subtilis was reported to show signs positive
for mutagenicity in the rec assay (Koruda, 1984a). However, these
results are not supported by the results of other rec assays using
similar bacterial strains (Yoo, 1986; Oda et al., 1978). Considering
the results of the rec assay, the Ames test and the chromosomal aberration
test, it is concluded that the 32 branched-chain esters are not genotoxic.
2.2.3.5 Reproductive toxicity studies
No reproduction studies have been reported for esters of aliphatic
acyclic primary alcohols and branched-chain aliphatic acyclic acids.
Table 6. Genotoxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance name Test system Test object Concentration of Results Reference
in vitro substance
Ethyl isovalerate Ames test S. typhimurium TA92, 0.01-1 mg/plate Negative1 Fujita & Sasaki, 1987
TA1535
Ames test TA100, TA1537, TA94, 10 mg/plate Negative1 Ishidate et al., 1984
TA98 and TA2637
Rec assay B. subtilis 100-200 µl/ml Positive Kuroda et al., 1984
Rec assay B. subtilis M45(rec-) up to 20 µl/disk Negative Yoo, 1986
and H17(rec+)
S. typhimurium TA97
and TA102
Chromosomal
aberration test Chinese hamster up to 2 mg/ml Negative Ishidate et al., 1984
fibroblasts cells
Rec assay B. subtilis H17 17µg/plate Negative Oda et al., 1978
and M45
1 Both with and without S-9 activation
3. REFERENCES
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: Efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D. ed.
Intermediary xenobiotic metabolism in animals. Taylor and Francis, New
York, pp. 81-97.
BASF (1980) Private communication to RIFM. Unpublished document.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months. Private communication to
FEMA.
Bio-Fax Industrial Bio-test Lab. Inc. (1969) Data sheet No. 2-5/69:
n-Butyl alcohol. Northbrook, Illinois, USA.
Biophere Research Center, Inc. (1981) Private communication to FEMA.
Browning, E. (1965) Toxicity and metabolism of industrial solvents.
Elsevier Publishing Co., New York.
Caldwell, J. (1989) Endogenous ethanol. Report to FEMA.
Carpanini, I.F., Kiss I.S., Grasso P., & Gangolli, S.D. (1973)
Short-term toxicity of isoamyl alcohol in rats. Food Cosmet.
Toxicol., 11: 713-724.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III,
6th ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food Cosmet. Toxicol., 16:
255-276.
Damske, D.R., Mecler, F.J., Beliles, R.P., & Liverman, J. L. (1980)
iso-Amyl iso-valerate. Report to FEMA. Unpublished document (Submitted
to WHO by FEMA).
Dawson, A.M., Holdsworth, C.D., & Webb J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117: 97-100.
Deuel, H. J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley-Interscience, New York.
DiVincenzo, G.D. & Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid in the rat. Toxicol. Appl. Pharmacol., 47: 609-612.
Drake, J.J.P., Butterworth, K.R., Gaunt, I.F., & Grasso, P. (1978)
Short-term toxicity study of isobutyl isobutyrate in rats. Food
Cosmet. Toxicol., 16: 337-342.
Eibert, J Jr. (1992) Private communication to FEMA. Unpublished
report. Cited in: Food and Drug Administration (FDA) (1993)
Priority-based assessment of food additives (PAFA) database. Center
for Food Safety and Applied Nutrition, p. 58.
FDA (1993) Priority-based assessment of food additives (PAFA)
database. US Food and Drug Administration, Center for Food Safety and
Applied Nutrition, p. 58.
Food and Drug Research Laboratory (1975) Unpublished report to RIFM.
Fujita H. & Sasaki, M. (1987) Mutagenicity test of food additives with
Salmonella typhimurium TA97 and TA102. Annual Report of the Tokyo
Metropolitan Research Laboratory P.H., 3.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montp., 25(1): 51.
Gangolli, S.D. & Shilling, W.H. (1968) Hydrolysis of esters by
artificial gastric and pancreatic juices. Analytical Chemistry
Research Report No. 11/1968. Unpublished study (Submitted to WHO by
FEMA).
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G., & Gangolli,
S.D. (1969) Acute (rat and mouse) and short-term (rat) toxicity
studies on cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7: 451-459.
Gibel, W.K., Lohs, K., & Wildner, G.P. (1975) Carcinogenic activity of
propanol, 2-methyl-1-propanol, and 3-methyl-1-butanol. Arch.
Geschwulstforsch., 45: 19-24.
Givaudan-Roure Corporation (1971) Private communication to FEMA.
Griffiths, P.J. & Giessinger, M. (1979) Private communication to FEMA.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fementative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
Hillbom, M.E., Fransaila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Research
Laboratories of the State Alcohol Monopoly (Alko), Helsinki, Finland.
Ishidate, M. Jr., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22(8):
623-636.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Jenner, P.M.,Hagan, E.C., Taylor, J.M., Cook, E.L., & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure: I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
Johannsen, E. & Purchase, I.F.H. (1969) Kaffircorn malting and brewing
studies: XXI. The effect of fuel oils of bantu beer on rat liver.
S.Afr. Med. J., 43(12): 326-328.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Krebs, H.A. & Perkins, J.R. (1970) The physiological role of liver
alcohol dehydrogenase. Biochem. J., 118: 635-644.
Kuroda, K., Tanaka, S., Yu, Y.S., & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31(6): 277.
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the
hydrolysis of twenty-six organic esters by pancreatin. Centraal
Instituut Voor Voedingsonderzoek, Zeist, The Netherlands (TNO Report
No. R 4319).
Levenstein, I. (1974) Report to RIFM. Unpublished document.
Levenstein, I. (1976) Report to RIFM. Unpublished document.
Liebich, H.M., Buelow, H.J., & Kallmayer, R. (1982) Quantification of
endogenous aliphatic alcohols in serum and urine. J. Chromatogr.,
239: 343-349.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, p. 2613.
Longland, R.C., Shilling, W.H., & Gangolli, S.D. (1977) The hydrolysis
of flavouring esters by artificial gastrointestinal juices and rat
tissue preparations. Toxicology, 8: 197-204.
Mecler, F.J. & Craig, D.K. (1980) Three month toxicity study on ethyl
isobutyrate in rat. Report to FEMA. Unpublished document.
Moreno, O.M. (1977) Report to RIFM. Unpublished document.
Moreno, O.M. (1978) Report to RIFM. Unpublished document.
Moreno, O.M. (1975) Report to RIFM. Unpublished document.
Moreno, O M., Cerven D.R., & Altenbach, E.J. (1981) Report to RIFM.
Unpublished document.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Report to RIFM.
Unpublished document.
Munch, J. C. (1972) Aliphatic alcohols and alkyl esters: Narcotic and
lethal potencies to tadpoles and rabbits. Ind. Med. Surg., 41:
31-33.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of structural class with
no-observed-effect levels: a proposal for establishing a threshold of
concern. In Press.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Pharmakon Inc. (1980) Private communication to FEMA.
Plaut, G.W.E. & Lardy, H.A. (1951) Enzymatic incorporation of
C14-bicarbonate into acetoacetate in the presence of various
substrates. J. Biol. Chem., 192: 435-445.
PPG Industries Inc. (1991) Submission to EPA. Unpublished document.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34(8-9): 569-85.
Sandmeyer, E.E. & Kirwin, C.J. (1981) Esters. In. Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology - Volume
2A, 3rd ed. John Wiley and Sons, Inc., New York, pp. 2259-2412.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Voskovofnikova, V.B. (1966) Substantiation of the maximum permissible
concentration of the flotation reagent IM-68 and its component
alcohols (hexyl, heptyl and octyl alcohol) in bodies of water. Gig.
i Sanit., 31: 310-316.
Wakabayashi, T., Horiuchi, M., Sakaguchi, M., Onda, H., & Iijima, M.
(1984) Induction of megamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta Pathol. Jpn., 34: 471-480.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall Ltd, London, pp. 88-113.
Wills, J.H., Jameson, E.M., & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15: 560-565.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34(3-4):
267-288.
The metabolism of 2-methylpentanoic acid has been studied in
mammals. 2-Methypentanoic acid undergoes ß-oxidation in the longer
branched-chain followed by cleavage to yield two propionyl CoA
fragments. In rabbits, 2-methylpentanoic acid is converted to
propionyl CoA, which is completely metabolized (Deuel, 1957).
4-Methylpentanoic acid, a gamma-methyl substituted acid, is expected
to undergo ß-oxidative cleavage to acetyl CoA and isobutyl CoA which
would be completely metabolized (Voet & Voet, 1990).
d) 3-Methylpentanoic acid
In general, ß-oxidation of branched-chain acids with a ß-methyl is
inhibited. However, ß-substituted acids may undergo alpha-oxidative
cleavage to yield acid fragments (Deuel, 1957; Williams, 1959) which
are completely metabolized in the fatty acid and tricarboxylic acid
cycles. In addition, they may undergo partial ß-oxidation to yield
ß-hydroxy acids which are further oxidized (omega-oxidation) and
fragmented to yield short-chain acids.
The metabolism of 3-methylpentanoic acid has been studied in
mammals. alpha-Oxidation and decarboxylation of 3-methylpentanoic acid
would yield 2-methylbutyric acid followed by ß-oxidative cleavage to
yield acetyl CoA and acetone. In guinea-pig liver homogenate,
3-methylpentanoic acid is converted to 2-methylbutyric acid which is
further metabolized via ß-oxidative cleavage (Stokke, 1969). In
rabbits, 3-methylpentanoic acid is converted to ß-hydroxybutyric acid
(Williams, 1959).
2.2.2.2 Aliphatic linear alcohols
Linear saturated and unsaturated alcohols are oxidized
successively to their corresponding aldehydes and carboxylic acids,
which enter the fatty acid ß-oxidation pathway. Branched-chain
aliphatic alcohols are converted by similar oxidation reactions to
their corresponding carboxylic acid, which undergoes metabolism via
ß-oxidation and cleavage to yield CO2 in amino acid pathways, the
fatty acid pathway, and the tricarboxylic acid cycle.
Based on the above information, it is anticipated that the 32
esters of aliphatic acyclic primary alcohols and branched-chain
aliphatic acyclic acids will be hydrolysed in the intestinal tract,
blood and liver to yield the corresponding branched-chain acids and
aliphatic alcohols. The resulting branched-chain acids and aliphatic
alcohols would be rapidly absorbed from the gastrointestinal tract and
completely metabolized to CO2 in the tricarboxylic acid cycle and
fatty acid pathway. The resulting linear alcohols would be
successively oxidized to their corresponding aldehyde and acid. The
resulting branched-chain aliphatic alcohols are converted by similar
oxidation reactions to their corresponding carboxylic acid, which
undergoes complete metabolism to CO2.
2.2.3 Toxicological studies
2.2.3.1 Acute toxicity
The results of acute toxicity studies for 24 of the 32 esters of
aliphatic acyclic primary alcohols and branched-chain aliphatic
acyclic acids are summarized in Table 3. The low acute oral toxicity
of the group is demonstrated by oral LD50 values >2300 mg/kg bw,
with the majority being >5000 mg/kg bw.
2.2.3.2 Short-term toxicity studies
The results of short-term toxicity studies with 4 branched-chain
esters (ethyl isobutyrate, isobutyl isobutyrate, ethyl isovalerate and
isoamyl isovalerate), one component acid (isovaleric acid) and 7
component alcohols (propyl alcohol, butyl alcohol, hexanol, octanol,
3-hexen-1-ol, isobutanol and isoamyl alcohol) are summarized in Table
4.
a) Branched-chain Esters
i) Ethyl isobutyrate
Ethyl isobutyrate was added to the diet of 12 male rats and 12
female rats at a level calculated to provide an average daily intake
of 29.2 mg/kg bw for 90 days. Weekly measurement of body weights and
food intake revealed no significant difference between test and
control animals. Haematological examination, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Determination of mean organ weights
revealed a slight increase in both absolute and relative adrenal gland
weights in females compared to controls, but these values were not
statistically significant. Gross and histopathological examination
failed to reveal any dose-related effects on the adrenal gland or any
other organs (Mecler & Craig, 1980). The NOEL of 29.2 mg ethyl
isobutyrate/kg bw per day in rats is >1000 times the estimated daily
per capita intake1 ("eaters only") of 7.74 µg/kg bw from use of
ethyl isobutyrate as a flavouring substance in the USA and 12.44 µg/kg
bw from its use as a flavouring substance in Europe.
ii) Ethyl isovalerate
For 90 days, ethyl isovalerate on gum arabic (10%) was added to the
diet of 12 male rats and 12 female rats at a level calculated to
provide an average daily intake of 12.1 mg/kg bw for males and 13.6
mg/kg bw for females. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed.
1 Intake calculated as follows: [[(annual volume, kg) x (1 x 109
mg/kg)]/[population x 0.6 x 365 days]], where population (10%, "eaters
only") = 24 x 106 for the USA and 32 x 106 for Europe; 0.6
represents the assumption that only 60% of the flavour volume was
reported in the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw per
day) calculated as follows: [(mg per day)/body weight], where body
weight = 60 kg. Slight variations may occur from rounding off.
Table 3. Acute toxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
iso-Butyrates
Methyl iso-butyrate Rats NR Oral 16 000 Sandmeyer & Kirwin, 1981
Ethyl iso-butyrate Rats NR Oral >5000 Moreno, 1975
Propyl iso-butyrate Rats NR Oral 15 000 Jenner et al., 1964
Butyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
Hexyl iso-butyrate Rats NR Oral >5000 Moreno, 1977
Trans-3-heptenyl 2-
methylpropanoate Rats NR Oral >25 000 Food and Drug Research Labs, 1975
Octyl iso-butyrate Rats NR Oral >5000 Levenstein, 1974
iso-Butyl iso-butyrate Rats
and mice NR Oral 12 800 Sandmeyer & Kirwin, 1981
iso-Valerates
Methyl iso-valerate Rabbits NR Oral 5690 Munch, 1972
Ethyl iso-valerate Rats NR Oral >5000 Levenstein, 1976; BASF, 1980
Rabbits NR Oral 7030 Munch, 1972
Propyl iso-valerate Rabbits NR Oral 8220 Munch, 1972
Butyl iso-valerate Rats NR Oral >5000 Moreno, 1978
Rabbits NR Oral 8230 Munch, 1972
Hexyl 3-methylbutanoate Rats NR Oral >5000 Moreno et al., 1981
2-Methylbutyrates
2-Methylpropyl Rabbits NR Oral >5000 Munch, 1972
3-methylbutyrate Rats NR Oral 6970 Moreno, 1978
2-Methylbutyl
3-methylbutanoate Rats NR Oral >5000 Moreno, 1978
Methyl 2-methylbutyrate Rats NR Oral >5000 Moreno et al., 1982
Hexyl 2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
iso-Propyl
2-methylbutyrate Rats NR Oral >20 000 Griffiths & Giessinger, 1979
Table 3. Continued...
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
3-Hexenyl
2-methylbutanoate Rats NR Oral >5000 Moreno, 1977
Ethyl 3-methylpentanoate Rats NR Oral >4000 Biosphere Research Center, Inc., 1981
Methyl 2-methylpentanoate Rats NR Oral >5000 Pharmakon Inc.,1980
1 The study was performed at a single dose level or multiple dose levels that produced no effects and,
therefore, a NOEL was not determined.
The NOEL is probably much higher than the reported dose level that produced no adverse effects
Table 4. Short-term studies for branched-chain esters and their component alcohols and acids
Substance Species, sex Route Duration NOEL Reference
(mg/kg bw per day)
Branched esters
Isobutyl isobutyrate Rats, M & F Oral and gavage 18 weeks 1000 Drake et al., 1978
Ethyl isobutyrate Rats, M & F Oral 90 days 29.21 Mecler & Craig, 1980
Ethyl isovalerate Rats, M & F Oral 13 weeks 12.11 Mecler & Craig, 1980
Isoamyl isovalerate2 Rats, M & F Oral 90 days 2201 Damske et al., 1980
Component acids
Isovaleric acid Rats Oral 90 days 25001 Amoore et al., 1978
Component alcohols
Propyl alcohol Rat, male Oral 4 months 601 Hillbom et al., 1974
Butyl alcohol Rats, male Oral 13 weeks 5.61 Wakabayashi et al., 1984
Rats, M & F Oral 14 days 1380 PPG, 1991
Rats, NR Oral 28 days 9401 Bio-Fax, 1969
Hexyl alcohol Beagles, M & F Oral 13 weeks 230 Eibert, 1992
Rat, M & F Oral 13 weeks 577 Eibert, 1992
Octanol Mice Oral 1 month 1791 Voskovofnikova, 1966
3-Hexen-1-ol Rats, M & F Oral 90 days 150 Gaunt et al., 1969
Isopropanol Adult Human Oral 6 weeks 6.4 Wills, 1969
Isobutanol Rats, M & F Oral 90 days 1450 BASF, 1992
Isoamyl alcohol2 Rats M & F Gavage 17 weeks 1000 Carpanini et al., 1973
1 The study was performed at a single dose level or multiple dose levels that produced no effects and, therefore, a NOEL was not
determined. The NOEL is probably much higher than the reported dose level that produced no adverse effects.
2 Structurally related substance
Haematological examination, blood chemical determinations and
urinalysis conducted at weeks 6 and 12 revealed no difference from
controls. At necropsy increases in absolute and relative mean adrenal
gland and thyroid gland weights were observed, but the results were
not statistically significant. Gross and histopathological examination
did not support relative weight changes, and revealed no dose-related
lesions (Mecler & Craig, 1980). The NOEL of 12.1 mg ethyl
isovalerate/kg bw per day in rats is >1000 times the estimated daily
per capita intake ("eaters only") of 9.01µg/kg bw from use of ethyl
isovalerate as a flavouring substance in the USA, and 12.75 µg/kg bw
from its use in Europe.
iii) Isobutyl isobutyrate
Groups of 15 male and 15 female rats were given daily doses of 0,
10, 100 or 1000 mg isobutyl isobutyrate/kg bw by gavage for 18 weeks.
Body weights, food consumption and water intake were measured and were
not different from controls. Haematology, urine analyses and serum
chemistry parameters were evaluated and did not reveal any
treatment-related effects. At necropsy gross examinations were
performed on all animals and organ weights were obtained. No
significant effects were reported. Histological examination was
conducted on all tissues from rats in the high-dose group and 50% of
the control animals. Histopathological examination of the major organs
was also performed on rats given 10 and 100 mg/kg bw per day. No
evidence of histopathological effects was reported (Drake et al.,
1978). The highest level of 1000 mg isobutyl isobutyrate/kg bw per day
which produced no adverse effects in rats is >100 000 times the
estimated daily per capita intake1 ("eaters only") of 0.04 µg/kg bw
per day from use of isobutyl isobutyrate as a flavouring substance in
the USA and 1.09 µg/kg bw per day from its use in Europe.
iv) Isoamyl isovalerate
Isoamyl isovalerate was added to the diet of male and female rats
at levels calculated to provide average daily intakes of 22, 69 or 220
mg/kg bw for 90 days. Body weights and food intake were measured
weekly. No significant differences between test and control animals
were observed. Haematological examinations, blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed no
differences from controls. Organ weights at necropsy were normal
compared to controls. Gross and histopathological examination revealed
no dose-related lesions (Damske et al., 1980). The intake level of
220 mg isoamyl isovalerate/kg bw per day that produced no effects in
rats is approximately 10 000 times the estimated daily per capita
intake ("eaters only") of 13 µg/kg bw from use of isoamyl isovalerate
as a flavouring substance in the USA and 23.91 µg/kg bw from its use
as a flavouring substance in Europe (see footnote in section
2.2.3.2a i).
b) Component alcohols and acids
Ester hydrolysis yields the component branched-chain acid and
linear or branched-chain alcohol. The results of subchronic studies
for the component acids and alcohols of esters of aliphatic acyclic
primary alcohols and branched-chain aliphatic acyclic acids has
revealed no evidence of toxicity at levels up to 2500 mg/kg bw per
day. These short-term studies are described below and summarized in
Table 4.
i) 1-Propanol
Four-month-old male Wistar rats were given a 1M solution of
1-propanol, corresponding to 60 mg/kg bw per day, as the sole drinking
fluid for 4 months. Control animals received tap water. Consumption of
food and alcohol solution and the weight gain of each animal were
recorded. A lower ratio of weight gain to caloric intake was observed
in the rats given the 1-propanol, indicating that this group utilized
food less efficiently than those untreated. However, this action is
not indicative of toxicity. Histological examination of the livers of
the experimental animals revealed no difference from controls. No
hepatic steatosis was observed. Neither inflammation nor cirrhosis was
seen in any of the livers (Hillbom et al., 1974).
ii) Butanol
No adverse effects were reported when 6.9% butanol with 25%
sucrose was added to the drinking-water of male rats for 13 weeks
(Wakabayashi et al., 1984) at an estimated daily intake level of 5.6
mg butanol/kg bw.
A 14-day study was conducted using rats to evaluate the effects of
butanol on gross and microscopic pathology, body weight, clinical
signs and clinical chemistry. Levels of 1.38%, 2.75% or 5.5% butanol
were administered orally to rats, which corresponds to daily intake
levels of 1380-5500 mg butanol/kg bw. A statistically significant
increase in the liver-to-body weight ratio resulted in males at all
levels and females at the 5.5% level (PPG, 1991).
No deaths or unusual symptoms were observed when rats (sex was not
reported) were fed butyl alcohol in a 2% corn oil solution blended
with the diet at concentrations of 0, 1000, 3500 or 10 000 mg/kg
(equal to 0, 90, 300 or 940 mg/kg bw per day, respectively) for 28
days. At necropsy, no significant gross lesions were observed. Liver
and kidney weights showed no significant differences from those of the
controls (Bio-Fax, 1969).
iii) Hexyl alcohol
Two groups of male and female rats were fed hexyl alcohol for 13
weeks at dietary levels of 0.25 or 0.50%; a third group was fed 1% for
weeks 1-10 and 2%, 4% and 6% for weeks 11, 12 and 13, respectively.
Decreased food consumption was observed in females at the high-dose
level, but body weights for all animals were normal. No significant
haematological changes or differences in urine analyses were observed
for the test and control groups. Gross pathology and microscopic
evaluations were performed and revealed no treatment-related effects.
The 1% level was reported to be equivalent to an intake of 577 mg/kg
bw per day (Eibert, 1992).
Three groups of 2 male and 2 female purebred beagle dogs were fed
hexyl alcohol for 13 weeks at levels of 0.50%, 1.0% or 1000 mg/kg bw
per day via gelatin capsules. A fourth group of 4 males and 4 females
served as controls. Body weight, organ weight, and food consumption
for the treated animals did not differ from controls. All animals in
the high-dose group displayed gross signs of toxicity intermittently
after treatment, including salivation, excitation, ataxia, tremors and
anaesthesia. The animals generally returned to normal within 4 hours
of treatment. One female dog died on the first day of treatment and
was replaced by another female. Three of the four remaining animals
died on either the 23rd or 38th day of the study. No signs of toxicity
were observed in the other test animals. Haematology, serum chemistry
and urinalysis was performed and showed no significant difference as
compared to controls. All animals were necropsied at the end of the
study.
Animals in the high-dose group exhibited gastrointestinal
inflammation and congestion of other visceral organs. Some gastric
irritation was observed in the mid-dose group. Both males treated at
the 1000 mg/kg bw per day level exhibited significant testicular
atrophy (Eibert, 1992). Effects on the testes and other reproductive
tissue have been observed with other aliphatic alcohols at high dose
levels (Lington & Bevan, 1994). A finding of nodules on the lung
surface of some animals was reported to be non-treatment-related. The
1% NOEL corresponds to a daily intake level ranging from 230 to 695
mg/kg bw (Eibert, 1992).
iv) Octanol
Seventeen groups of 10 mice each received oral doses of 1-octanol
at a level of 179 mg/kg bw per day for one month. 1-Octanol was
administered intragastrically in the form of a solution or suspension.
No cumulative effects were observed (Voskovofnikova, 1966).
v) Cis-3-hexen-1-ol
Short-term toxicity studies have been reported for
cis-3-hexen-1-ol, which is the alcohol component for esters of
unsaturated alcohols in this group. Groups of 15 male and 15 female
weanling rats were given drinking-water containing 0, 310, 1250 or
5000 mg/litre cis-3-hexen-1-ol for 98 days. Body weight, food intake
and water consumption were recorded weekly. A reduction in water
intake was reported in male rats in the high-dose group only.
Haematology studies were conducted on eight rats from each sex in the
control, 1250 and 5000 mg/litre groups during the sixth week.
Urinalysis, including pH, microscopic constituents and content of
bile, blood and glucose, was performed for eight animals of each sex
from each group during week 6, and from week 12 until the end of the
study. Kidney function was assessed regularly. Necropsies were
performed on all animals at the end of the study. Serum chemistry
parameters were measured for all animals at necropsy. All tissues were
examined for macroscopic abnormalities, and organ weights were
obtained for the brain, pituitary, thyroid, heart, liver, spleen,
kidneys, adrenals and gonads. Tissues from all rats in the high-dose
group and from 50% of the controls were examined microscopically.
Slightly increased relative kidney and adrenal gland weights observed
in males only at the 5000 mg/litre level were not accompanied by any
evidence of histopathology. These increases were not considered to be
statistically significant. No treatment-related abnormalities were
reported (Gaunt et al., 1969).
vi) Isopropanol
Daily doses of 2.6 or 6.4 mg isopropanol/kg bw were given to adult
human males for a period of six weeks. Studies of the microscopic and
chemical characteristics of blood and urine were repeated on the
first, third and seventh days and at weekly intervals thereafter.
Removal of BSP from blood was estimated once before initiation of
ingestion of isopropyl alcohol and at the end of the experimental
period. Retention of BSP in serum at the end of the experiment was not
significantly different from that before the start in any of the men.
No significant, persistent changes that are clearly attributable to
the ingestion of isopropanol were evident (Wills, 1963).
vii) Isobutanol
Groups of 10 male and 10 female Wistar rats were given isobutyl
alcohol in their drinking-water for 3 months. The test substance was
administered in concentrations of 0, 1000, 4000 or 16 000 mg/litre,
which was reported to correspond to approximate dose levels of 0, 60
340 or 1450 mg/kg bw per day. Food and water consumption and body
weight gain were not affected by the test substance. All animals
tested were free from adverse clinical effects. Haematology and
clinical chemistry parameters were measured and revealed no
treatment-related adverse effects. Gross pathology and
histopathological examinations were no different from controls. The
authors concluded that results of this study demonstrate a lack of
toxicity associated with administration of isobutyl alcohol in the
drinking-water of rats, and that the NOEL is >1450 mg/kg bw per day
(BASF, 1992).
viii) Isoamyl alcohol
Isoamyl alcohol was administered to groups of 15 male and 15
female Ash/CSE rats in corn oil by gavage, providing daily dose levels
of 0, 150 500, or 1000 mg/kg bw per day for 17 weeks. High-dose males
exhibited a slight reduction in body-weight gain, which was associated
with reduced food intake. Over the entire period of the study,
however, there was no statistically significant reduction in mean food
intake. Examination of haematology, serum analyses, urinalysis, renal
concentration tests, and organ weights revealed no treatment-related
effects. At necropsy, the animals were examined for macroscopic
abnormalities, and the major organs were weighed. Microscopic
examination was performed on several tissues of the control and
high-dose animals. No treatment-related abnormalities were observed
(Carpanini et al., 1973).
ix) Isovaleric acid
In a limited 90-day feeding study, Sprague-Dawley rats were
administered doses of 5% isovaleric acid in the diet, which was
calculated (FDA, 1993) to be equivalent to 2500 mg isovaleric acid/kg
bw per day. The rats were monitored for survival rates, body weight
changes, food intake, blood and urine analyses, organ weights and
histology. At the 2500 mg/kg bw dose level, there were no
treatment-related effects on the measured parameters (Amoore et
al., 1978).
2.2.3.3 Long-term toxicity/carcinogenicity studies
The results of long-term toxicity/carcinogenicity studies with two
of the component alcohols are described below and summarized in Table
5.
a) Isobutanol
Twenty males and 20 female Wistar rats were given 0.2% isobutanol
in drinking-water for 53 to 56 weeks. Clinical chemistry, including
alcohol dehydrogenase, glutamic oxaloacetate transaminase glutamic
pyruviuc transaminase, and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isobutanol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 200 mg/kg bw. This is >10 000 times the daily per capita
intake ("eaters only") of 4.85 µg/kg bw isobutanol from use as a
flavouring substance in the USA and of 8.79 µg/kg bw isobutanol from
its use in Europe (see footnote in section 2.2.3.2a i).
Table 5. Long-term toxicity studies for esters of aliphatic acyclic primary alcohols and
branched-chain aliphatic acyclic acids
Substance Species, Route Duration NOEL Reference
sex (weeks) (mg/kg bw per day)
Component Alcohols
Isobutyl alcohol Rats, M&F Drinking-water 53-56 2001 Johannsen & Purchase, 1969
Isoamyl alcohol2 Rats, M&F Drinking-water 53-56 20001 Johannsen & Purchase, 1969
1 The study was performed at a single dose level or multiple dose levels that produced no
effects and, therefore, a NOEL was not determined. The NOEL is probably much higher than
the reported dose level that produced no effects
2 A structually related branched-chain alcohol
b) Isoamyl alcohol
Twenty males and 20 female Wistar rats were given drinking-water
containing 2% isoamyl alcohol for 53 to 56 weeks. Clinical chemistry,
including alcohol dehydrogenase, glutamic oxaloacetate transaminase,
glutamic pyruviuc transaminase and protein content, were conducted and
revealed no difference from controls. At necropsy, no abnormalities
were observed during histological examination (Johannsen & Purchase,
1969). The concentration of isoamyl alcohol that produced no adverse
effects was calculated (FDA, 1993) to provide an approximate daily
intake of 2000 mg/kg bw. This is > 10 000 times the daily per
capita intake ("eaters only") of 26.32 µg isoamyl alcohol/kg bw from
use as a flavouring substance in the USA and of 30.47 µg isoamyl
alcohol/kg bw from its use as a flavouring substance in Europe (see
footnote in section 2.2.3.2a i).
2.2.3.4 Genotoxicity studies
The results of genotoxicity studies with the group of 32 branched-
chain esters are summarized in Table 6. Mutagenicity testing of ethyl
isovalerate in vitro by means of the Ames test, at concentrations up
to 10 mg/plate has shown no evidence of mutagenicity in Salmonella
typhimurium strains TA92, TA94, TA97, TA98, TA100, TA102, TA1535,
TA1537 or in TA2637 with or without metabolic activation (Ishidate et
al., 1984; Fujita & Sasaki, 1987). There was no evidence of
mutagenicity in Chinese hamster fibroblast cells when the chromosomal
aberration test was performed with ethyl isovalerate at concentrations
up to 2 mg/ml (Ishidate et al., 1984). When incubated with Bacillus
subtilis, at concentrations up to 20 µl/disk (Yoo, 1986), and at 17
µg/plate (Oda et al., 1978), ethyl isovalerate was reported to be
non-mutagenic. At high concentrations (100-200 µl/ml) of ethyl
isovalerate, Bacillus subtilis was reported to show signs positive
for mutagenicity in the rec assay (Koruda, 1984a). However, these
results are not supported by the results of other rec assays using
similar bacterial strains (Yoo, 1986; Oda et al., 1978). Considering
the results of the rec assay, the Ames test and the chromosomal aberration
test, it is concluded that the 32 branched-chain esters are not genotoxic.
2.2.3.5 Reproductive toxicity studies
No reproduction studies have been reported for esters of aliphatic
acyclic primary alcohols and branched-chain aliphatic acyclic acids.
Table 6. Genotoxicity studies for esters of aliphatic acyclic primary alcohols and branched-chain aliphatic acyclic acids
Substance name Test system Test object Concentration of Results Reference
in vitro substance
Ethyl isovalerate Ames test S. typhimurium TA92, 0.01-1 mg/plate Negative1 Fujita & Sasaki, 1987
TA1535
Ames test TA100, TA1537, TA94, 10 mg/plate Negative1 Ishidate et al., 1984
TA98 and TA2637
Rec assay B. subtilis 100-200 µl/ml Positive Kuroda et al., 1984
Rec assay B. subtilis M45(rec-) up to 20 µl/disk Negative Yoo, 1986
and H17(rec+)
S. typhimurium TA97
and TA102
Chromosomal
aberration test Chinese hamster up to 2 mg/ml Negative Ishidate et al., 1984
fibroblasts cells
Rec assay B. subtilis H17 17µg/plate Negative Oda et al., 1978
and M45
1 Both with and without S-9 activation
3. REFERENCES
Amoore, J.E., Gumbmann, M.R., Booth, A.N., & Gould, D.H. (1978)
Synthetic flavors: Efficiency and safety factors for sweaty and fishy
odorants. Chem. Senses Flavour, 3: 307-317.
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: Hutson, D.H., Caldwell, J., & Paulson, G.D. ed.
Intermediary xenobiotic metabolism in animals. Taylor and Francis, New
York, pp. 81-97.
BASF (1980) Private communication to RIFM. Unpublished document.
BASF (1992) Study on the oral toxicity of 2-methyl-1-propanol in rats
via the drinking water over three months. Private communication to
FEMA.
Bio-Fax Industrial Bio-test Lab. Inc. (1969) Data sheet No. 2-5/69:
n-Butyl alcohol. Northbrook, Illinois, USA.
Biophere Research Center, Inc. (1981) Private communication to FEMA.
Browning, E. (1965) Toxicity and metabolism of industrial solvents.
Elsevier Publishing Co., New York.
Caldwell, J. (1989) Endogenous ethanol. Report to FEMA.
Carpanini, I.F., Kiss I.S., Grasso P., & Gangolli, S.D. (1973)
Short-term toxicity of isoamyl alcohol in rats. Food Cosmet.
Toxicol., 11: 713-724.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III,
6th ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food Cosmet. Toxicol., 16:
255-276.
Damske, D.R., Mecler, F.J., Beliles, R.P., & Liverman, J. L. (1980)
iso-Amyl iso-valerate. Report to FEMA. Unpublished document (Submitted
to WHO by FEMA).
Dawson, A.M., Holdsworth, C.D., & Webb J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117: 97-100.
Deuel, H. J. ed. (1957) The lipids, their chemistry and biochemistry -
Volume III. Wiley-Interscience, New York.
DiVincenzo, G.D. & Hamilton, M.L. (1979) Metabolic fate of [1-14C]
isobutyric acid in the rat. Toxicol. Appl. Pharmacol., 47: 609-612.
Drake, J.J.P., Butterworth, K.R., Gaunt, I.F., & Grasso, P. (1978)
Short-term toxicity study of isobutyl isobutyrate in rats. Food
Cosmet. Toxicol., 16: 337-342.
Eibert, J Jr. (1992) Private communication to FEMA. Unpublished
report. Cited in: Food and Drug Administration (FDA) (1993)
Priority-based assessment of food additives (PAFA) database. Center
for Food Safety and Applied Nutrition, p. 58.
FDA (1993) Priority-based assessment of food additives (PAFA)
database. US Food and Drug Administration, Center for Food Safety and
Applied Nutrition, p. 58.
Food and Drug Research Laboratory (1975) Unpublished report to RIFM.
Fujita H. & Sasaki, M. (1987) Mutagenicity test of food additives with
Salmonella typhimurium TA97 and TA102. Annual Report of the Tokyo
Metropolitan Research Laboratory P.H., 3.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montp., 25(1): 51.
Gangolli, S.D. & Shilling, W.H. (1968) Hydrolysis of esters by
artificial gastric and pancreatic juices. Analytical Chemistry
Research Report No. 11/1968. Unpublished study (Submitted to WHO by
FEMA).
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G., & Gangolli,
S.D. (1969) Acute (rat and mouse) and short-term (rat) toxicity
studies on cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7: 451-459.
Gibel, W.K., Lohs, K., & Wildner, G.P. (1975) Carcinogenic activity of
propanol, 2-methyl-1-propanol, and 3-methyl-1-butanol. Arch.
Geschwulstforsch., 45: 19-24.
Givaudan-Roure Corporation (1971) Private communication to FEMA.
Griffiths, P.J. & Giessinger, M. (1979) Private communication to FEMA.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Henning, S.J. & Hird, F.J.R. (1970) The concentration and metabolism
of volatile fatty acids in the fementative organs of two species of
kangaroo and the guinea pig. Br. J. Nutr., 24: 145-155.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
Hillbom, M.E., Fransaila, K., & Forsander, O.A. (1974) Effects of
chronic ingestion of some lower aliphatic alcohols in rats. Research
Laboratories of the State Alcohol Monopoly (Alko), Helsinki, Finland.
Ishidate, M. Jr., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., & Matsu, A. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22(8):
623-636.
International Organization of the Flavor Industry (IOFI) (1995)
European inquiry on volume of use. Private communication to FEMA.
Jenner, P.M.,Hagan, E.C., Taylor, J.M., Cook, E.L., & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure: I. Acute
oral toxicity. Food Cosmet. Toxicol., 2: 327-343.
Johannsen, E. & Purchase, I.F.H. (1969) Kaffircorn malting and brewing
studies: XXI. The effect of fuel oils of bantu beer on rat liver.
S.Afr. Med. J., 43(12): 326-328.
Kinnory, D.S., Takeda, Y., & Greenberg, D.M. (1955) Isotope studies on
the metabolism of valine. J. Biol. Chem., 212: 385-396.
Krebs, H.A. & Perkins, J.R. (1970) The physiological role of liver
alcohol dehydrogenase. Biochem. J., 118: 635-644.
Kuroda, K., Tanaka, S., Yu, Y.S., & Ishibashi, T. (1984) Rec-assay of
food additives. Nippon Kosnu Eisei Zasshi, 31(6): 277.
Leegwater, D.C. & van Straten, S. (1974) In vitro study on the
hydrolysis of twenty-six organic esters by pancreatin. Centraal
Instituut Voor Voedingsonderzoek, Zeist, The Netherlands (TNO Report
No. R 4319).
Levenstein, I. (1974) Report to RIFM. Unpublished document.
Levenstein, I. (1976) Report to RIFM. Unpublished document.
Liebich, H.M., Buelow, H.J., & Kallmayer, R. (1982) Quantification of
endogenous aliphatic alcohols in serum and urine. J. Chromatogr.,
239: 343-349.
Lington, A.W. & Bevan, C. (1994) Alcohols. In: Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology, 4th ed.
John Wiley and Sons, Inc., New York, p. 2613.
Longland, R.C., Shilling, W.H., & Gangolli, S.D. (1977) The hydrolysis
of flavouring esters by artificial gastrointestinal juices and rat
tissue preparations. Toxicology, 8: 197-204.
Mecler, F.J. & Craig, D.K. (1980) Three month toxicity study on ethyl
isobutyrate in rat. Report to FEMA. Unpublished document.
Moreno, O.M. (1977) Report to RIFM. Unpublished document.
Moreno, O.M. (1978) Report to RIFM. Unpublished document.
Moreno, O.M. (1975) Report to RIFM. Unpublished document.
Moreno, O M., Cerven D.R., & Altenbach, E.J. (1981) Report to RIFM.
Unpublished document.
Moreno, O.M., Cerven, D.R., & Altenbach, E.J. (1982) Report to RIFM.
Unpublished document.
Munch, J. C. (1972) Aliphatic alcohols and alkyl esters: Narcotic and
lethal potencies to tadpoles and rabbits. Ind. Med. Surg., 41:
31-33.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Unpublished report to RIFM: Correlation of structural class with
no-observed-effect levels: a proposal for establishing a threshold of
concern. In Press.
National Academy of Sciences (NAS) (1987) Evaluating the safety of
food chemicals. Washington, DC.
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Pharmakon Inc. (1980) Private communication to FEMA.
Plaut, G.W.E. & Lardy, H.A. (1951) Enzymatic incorporation of
C14-bicarbonate into acetoacetate in the presence of various
substrates. J. Biol. Chem., 192: 435-445.
PPG Industries Inc. (1991) Submission to EPA. Unpublished document.
Saito, M. (1975) Metabolism of lower alcohols. Nichidai Igaku
Zasshi, 34(8-9): 569-85.
Sandmeyer, E.E. & Kirwin, C.J. (1981) Esters. In. Clayton, G.D. &
Clayton, F.E. ed. Patty's industrial hygiene and toxicology - Volume
2A, 3rd ed. John Wiley and Sons, Inc., New York, pp. 2259-2412.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Stokke, O. (1969) Degradation of a branched-chain fatty acid by
alternations between alpha- and beta-oxidations. Biochem. Biophys.
Acta, 176: 54-59.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Voskovofnikova, V.B. (1966) Substantiation of the maximum permissible
concentration of the flotation reagent IM-68 and its component
alcohols (hexyl, heptyl and octyl alcohol) in bodies of water. Gig.
i Sanit., 31: 310-316.
Wakabayashi, T., Horiuchi, M., Sakaguchi, M., Onda, H., & Iijima, M.
(1984) Induction of megamitochondria in the rat liver by n-propyl
alcohol and n-butyl alcohol. Acta Pathol. Jpn., 34: 471-480.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed. Chapman and
Hall Ltd, London, pp. 88-113.
Wills, J.H., Jameson, E.M., & Coulston, F. (1969) Effects on man of
daily ingestion of small doses of isopropyl alcohol. Toxicol. Appl.
Pharmacol., 15: 560-565.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34(3-4):
267-288.
