
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES AND CONTAMINANTS
WHO FOOD ADDITIVES SERIES 40
Prepared by:
The forty-ninth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
ESTERS DERIVED FROM BRANCHED-CHAIN TERPENOID ALCOHOLS AND ALIPHATIC
ACYCLIC LINEAR AND BRANCHED-CHAIN CARBOXYLIC ACIDS
First draft prepared by
Dr G.J.A. Speijers and Ms M.F.A. Wouters
National Institute of Public Health and
Environment Protection (RIVM)
Bilthoven, The Netherlands
1. Evaluation
1.1 Introduction
1.2 Estimated daily per capita intake
1.3 Absorption, metabolism and elimination
1.3.1 Terpenoid alcohols
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
1.5 Consideration of combined intake
1.6 Conclusions
2. Relevant background information
2.1 Explanation
2.2 Intake data
2.3 Biological data
2.3.1 Absorption, metabolism and elimination
2.3.1.1 Terpenoid alcohols
2.3.1.2 Aliphatic carboxylic acids
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
2.3.2.2 Short-term toxicity
2.3.2.3 Long-term toxicity/carcinogenicity studies
2.3.2.4 Genotoxicity
2.3.2.5 Reproductive toxicity
2.3.2.6 Other relevant studies
2.4 Observations in humans
2.4.1 Geranyl acetate
2.4.2 Neryl acetate
2.4.3 Citronellyl acetate
2.4.4 Rhodinyl acetate
2.4.5 Neryl isobutyrate
3. References
1. EVALUATION
1.1 Introduction
A safety evaluation of a group of 26 terpenoid esters was
conducted using the Procedure for Safety Evaluation of Flavouring
Agents (the "Procedure") (see Figure 1 and Table 1).
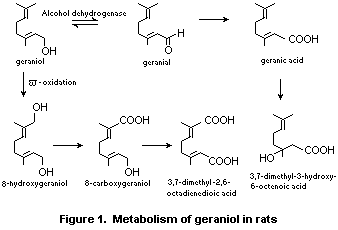 Table 1. Summary of results of the safety evaluations of esters of terpenoid alcohols
and aliphatic carboxylic acids
Step 1: All of the substances in the group are in structural class I, the human intake
threshold of which is 1800 µg per day.
Step 2: All of the substances in the group are metabolized to innocuous products.
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0053 Citronellyl formate No No safety concern
USA: 2.5 Europe: 103
0054 Geranyl formate No No safety concern
USA: 48 Europe: 330
0055 Neryl formate No No safety concern
USA: 0.04 Europe: 0.01
0056 Rhodinyl formate No No safety concern
USA: 0.10 Europe: unknown
0057 Citronellyl acetate No No safety concern
USA: 36 Europe: 217
0058 Geranyl acetate No No safety concern
USA: 205 Europe: 580
0059 Neryl acetate No No safety concern
USA: 63 Europe: 180
0060 Rhodinyl acetate No No safety concern
USA: 0.8 Europe: 1.1
0061 Citronellyl propionate No No safety concern
USA: 1.5 Europe: 41
0062 Geranyl propionate No No safety concern
USA: 11 Europe: 81
0063 Neryl propionate No No safety concern
USA: 1.1 Europe: 4.3
0064 Rhodinyl propionate No No safety concern
USA: 0.02 Europe: unknown
0065 Citronellyl butyrate No No safety concern
USA: 5 Europe: 32
Table 1. Continued...
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0066 Geranyl butyrate No No safety concern
USA: 25 Europe: 60
0067 Neryl butyrate No No safety concern
USA: 0.02 Europe: 0.4
0068 Rhodinyl butyrate No No safety concern
USA: 1.0 Europe: unknown
0069 Citronellyl valerate No No safety concern
USA: 4.0 Europe: 0.7
0070 Geranyl hexanoate No No safety concern
USA: 0.5 Europe: 0.07
0071 Citronellyl isobutyrate No No safety concern
USA: 1.3 Europe: 13
0072 Geranyl isobutyrate No No safety concern
USA: 3.0 Europe: 124
0073 Neryl isobutyrate No No safety concern
USA: 0.4 Europe: 2.0
0074 Rhodinyl isobutyrate No No safety concern
USA: 0.02 Europe: 0.03
0075 Geranyl isoverate No No safety concern
USA: 1.7 Europe: 43
0076 Neryl isovalerate No No safety concern
USA: 0.04 Europe: 0.03
0077 Rhodinyl isovalerate No No safety concern
USA: 0.02 Europe: 0.01
0078 3,7-Dimethyl-2,6-
octadien-1-yl 2-
ethylbutanoate No No safety concern
USA: 0 Europe: 0.6
1 The human intake threshold is 1800 µg per day for Class 1, 540 µg/day for Class 2 and
90 µg/day for Class 3
One member of the group, geranyl acetate, was previously evaluated
at the twenty-third meeting of the Committee. It was evaluated as part
of a group of other terpenoid flavouring substances, citral,
citronellol, and linalool, which have close chemical, biochemical and
toxicological relationships. The Committee allocated a group ADI of 0-
0.05 mg/kg bw based on the clearly defined metabolism of these
substances and their low toxicity in short-term toxicity studies
(Annex 1, reference 50).
Data relevant to the Procedure for the Safety Evaluation of 26
esters formed from the esterification of the terpenoid alcohols, (for
geraniol, nerol, (±)-citronellol and rhodinol) and saturated aliphatic
carboxylic acids (linear saturated aliphatic acids (C1-C6),
isobutyric acid isovaleric acid and 2-ethylbutyric acid) are
summarized.
1.2 Estimated daily per capita intake
The total annual production volume of the 26 terpenoid esters used
as flavouring agents is approximately 2100 kg in the USA and 12 600 kg
in Europe. In the USA and in Europe, approximately 60% of the total
annual volume is accounted for by the acetate and butyrate esters of
citronellol, geraniol and nerol. Based on the annual volume reported
in the USA and Europe, the total estimated daily per
capita intake of the 26 terpenoid esters used as flavouring
substances is 410 µg in the USA and 1800 µg in Europe. The total
estimated daily per capita intake of terpenoid alcohols (i.e.,
citronellol, geraniol, nerol and rhodinol) formed via hydrolysis of
these esters is 320 µg in the USA and 1400 µg in Europe.
Terpenoid esters are principal flavour components of citrus and
citrus peel oils, and have also been detected in wide variety of other
fruits, spices and vegetables. The terpenoid esters are usually found
at concentration of < 1 mg/kg in citrus fruit juices, < 20 000 mg/kg
in citrus peel oils, and < 50 000 mg/kg in spices (CIVO-TNO, 1994).
In the USA terpenoid esters are consumed predominantly as components
of traditional foods (Stofberg & Kirschman, 1985). In the USA, the
total annual consumption of seven of these terpenoid esters as natural
components of food is estimated to be approximately 300 tonnes
(Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism and elimination
The terpenoid esters are hydrolysed to the corresponding terpenoid
alcohols (geraniol, citronellol, nerol, and rhodinol) and aliphatic
carboxylic acids (formic, acetic, propionic, butyric, valeric,
hexanoic, isobutyric and isovaleric acids). Both the hydrolysis data
and the metabolism of aliphatic carboxylic acids are discussed in the
introduction to this chapter on flavouring agents.
1.3.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites, which are excreted primarily in the urine. Geraniol,
related terpenoid alcohols (citronellol and nerol), and the aldehydes
(geranial and neral) exhibit similar pathways of metabolic
detoxication in animals (Figure 1).
1.4 Application of the Procedure for Safety Evaluation of Flavouring
Agents
Step 1. All of the 26 terpenoid esters are in class I.
Step 2. It is expected that the esters in this group will be
readily hydrolysed to the component alcohols and carboxylic acids,
which are considered to be innocuous. The terpenoid alcohols are
expected to undergo omega-oxidation and functional group oxidation to
yield polar metabolites which are excreted as the glucuronic acid
conjugate in the urine. Eight of the 9 component carboxylic acids are
endogenous in humans, participating in the fatty acid ß-oxidation
pathway, amino acid pathways, the citric acid cycle, or the
C1 tetrahydrofolate pathway and eventually yielding CO2 and H2O.
The remaining carboxylic acid 2-ethylbutyric acid undergoes oxidation
to polar metabolites that are conjugated with glucuronic acid and
excreted. At current levels of intake these esters and their component
terpenoid alcohols and aliphatic carboxylic acids would not be
expected to saturate these metabolic pathways.
Step A3. None of the 26 terpenoid esters has a USA or European
daily per capita intake that exceeds 1800 µg/person per day.
Therefore, results of the Procedure indicate that none of the 26
terpenoid esters evaluated poses a safety concern when used at current
levels of intake as flavouring substances.
The stepwise evaluations of the 26 terpenoid esters used as
flavouring substances are summarized in Table 1.
1.5 Consideration of combined intake
In the unlikely event that these 26 terpenoid esters were to be
consumed simultaneously on a daily basis, the total daily intake would
still be within the human intake threshold of Class I (1800 µg/person
per day). The Committee noted that the terpene alcohols, geranoil,
citronellol and linalool are used as flavouring agents and that the
combined intakes of these alcohols and esters would be less than the
group ADI.
1.6 Conclusions
Applying the Procedure, the Committee concluded that for the
esters derived from branched-chain terpenoid alcohols and aliphatic
acyclic linear and branched-chain carboxylic acids there was no safety
concern at current intake levels.
No toxicity data were required for the application of the
procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
procedure.
The Committee noted that some of the esters are metabolized to
alpha,ß-unsaturated carbonyl compounds, but concluded that these had
been adequately evaluated previously (Annex 1, reference 50). The
Committee maintained the group ADI of 0-0.05 mg/kg bw for geranyl
acetate, citral, citronellol and linalool.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes all the available data relevant to the
safety evaluation of 26 esters formed from the esterification of the
terpenoid alcohols: geraniol, nerol, (±)-citronellol, and rhodinol and
saturated aliphatic carboxylic acids (linear saturated aliphatic acids
(C1-C6), isobutyric acid, isovaleric acid and 2-ethylbutyric acid).
The four terpenoid alcohols from which the 26 esters are formed are
close structural relatives. Geraniol and nerol are cis-trans (E-Z)
isomers of 3,7-dimethyl-2,6-octadien-1-ol; (±)-citronellol is
2,3-dihydrogeraniol (3,7-dimethyl-6-octen-1-ol); and rhodinol is a
mixture containing mainly l-citronellol and a small amount of the
isomer 3,7-dimethyl-7-octen-1-ol. As a commercial product, geraniol or
nerol may contain significant amounts of its (Z)- or (E)-isomer,
respectively, the monounsaturated alcohol (citronellol) and saturated
alcohol (3,7-dimethyl-1-octanol).
The basic structures of esters derived from branched-chain
terpenoid alcohols and saturated aliphatic acyclic carboxylic acids
are as follows:
Table 1. Summary of results of the safety evaluations of esters of terpenoid alcohols
and aliphatic carboxylic acids
Step 1: All of the substances in the group are in structural class I, the human intake
threshold of which is 1800 µg per day.
Step 2: All of the substances in the group are metabolized to innocuous products.
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0053 Citronellyl formate No No safety concern
USA: 2.5 Europe: 103
0054 Geranyl formate No No safety concern
USA: 48 Europe: 330
0055 Neryl formate No No safety concern
USA: 0.04 Europe: 0.01
0056 Rhodinyl formate No No safety concern
USA: 0.10 Europe: unknown
0057 Citronellyl acetate No No safety concern
USA: 36 Europe: 217
0058 Geranyl acetate No No safety concern
USA: 205 Europe: 580
0059 Neryl acetate No No safety concern
USA: 63 Europe: 180
0060 Rhodinyl acetate No No safety concern
USA: 0.8 Europe: 1.1
0061 Citronellyl propionate No No safety concern
USA: 1.5 Europe: 41
0062 Geranyl propionate No No safety concern
USA: 11 Europe: 81
0063 Neryl propionate No No safety concern
USA: 1.1 Europe: 4.3
0064 Rhodinyl propionate No No safety concern
USA: 0.02 Europe: unknown
0065 Citronellyl butyrate No No safety concern
USA: 5 Europe: 32
Table 1. Continued...
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0066 Geranyl butyrate No No safety concern
USA: 25 Europe: 60
0067 Neryl butyrate No No safety concern
USA: 0.02 Europe: 0.4
0068 Rhodinyl butyrate No No safety concern
USA: 1.0 Europe: unknown
0069 Citronellyl valerate No No safety concern
USA: 4.0 Europe: 0.7
0070 Geranyl hexanoate No No safety concern
USA: 0.5 Europe: 0.07
0071 Citronellyl isobutyrate No No safety concern
USA: 1.3 Europe: 13
0072 Geranyl isobutyrate No No safety concern
USA: 3.0 Europe: 124
0073 Neryl isobutyrate No No safety concern
USA: 0.4 Europe: 2.0
0074 Rhodinyl isobutyrate No No safety concern
USA: 0.02 Europe: 0.03
0075 Geranyl isoverate No No safety concern
USA: 1.7 Europe: 43
0076 Neryl isovalerate No No safety concern
USA: 0.04 Europe: 0.03
0077 Rhodinyl isovalerate No No safety concern
USA: 0.02 Europe: 0.01
0078 3,7-Dimethyl-2,6-
octadien-1-yl 2-
ethylbutanoate No No safety concern
USA: 0 Europe: 0.6
1 The human intake threshold is 1800 µg per day for Class 1, 540 µg/day for Class 2 and
90 µg/day for Class 3
One member of the group, geranyl acetate, was previously evaluated
at the twenty-third meeting of the Committee. It was evaluated as part
of a group of other terpenoid flavouring substances, citral,
citronellol, and linalool, which have close chemical, biochemical and
toxicological relationships. The Committee allocated a group ADI of 0-
0.05 mg/kg bw based on the clearly defined metabolism of these
substances and their low toxicity in short-term toxicity studies
(Annex 1, reference 50).
Data relevant to the Procedure for the Safety Evaluation of 26
esters formed from the esterification of the terpenoid alcohols, (for
geraniol, nerol, (±)-citronellol and rhodinol) and saturated aliphatic
carboxylic acids (linear saturated aliphatic acids (C1-C6),
isobutyric acid isovaleric acid and 2-ethylbutyric acid) are
summarized.
1.2 Estimated daily per capita intake
The total annual production volume of the 26 terpenoid esters used
as flavouring agents is approximately 2100 kg in the USA and 12 600 kg
in Europe. In the USA and in Europe, approximately 60% of the total
annual volume is accounted for by the acetate and butyrate esters of
citronellol, geraniol and nerol. Based on the annual volume reported
in the USA and Europe, the total estimated daily per
capita intake of the 26 terpenoid esters used as flavouring
substances is 410 µg in the USA and 1800 µg in Europe. The total
estimated daily per capita intake of terpenoid alcohols (i.e.,
citronellol, geraniol, nerol and rhodinol) formed via hydrolysis of
these esters is 320 µg in the USA and 1400 µg in Europe.
Terpenoid esters are principal flavour components of citrus and
citrus peel oils, and have also been detected in wide variety of other
fruits, spices and vegetables. The terpenoid esters are usually found
at concentration of < 1 mg/kg in citrus fruit juices, < 20 000 mg/kg
in citrus peel oils, and < 50 000 mg/kg in spices (CIVO-TNO, 1994).
In the USA terpenoid esters are consumed predominantly as components
of traditional foods (Stofberg & Kirschman, 1985). In the USA, the
total annual consumption of seven of these terpenoid esters as natural
components of food is estimated to be approximately 300 tonnes
(Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism and elimination
The terpenoid esters are hydrolysed to the corresponding terpenoid
alcohols (geraniol, citronellol, nerol, and rhodinol) and aliphatic
carboxylic acids (formic, acetic, propionic, butyric, valeric,
hexanoic, isobutyric and isovaleric acids). Both the hydrolysis data
and the metabolism of aliphatic carboxylic acids are discussed in the
introduction to this chapter on flavouring agents.
1.3.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites, which are excreted primarily in the urine. Geraniol,
related terpenoid alcohols (citronellol and nerol), and the aldehydes
(geranial and neral) exhibit similar pathways of metabolic
detoxication in animals (Figure 1).
1.4 Application of the Procedure for Safety Evaluation of Flavouring
Agents
Step 1. All of the 26 terpenoid esters are in class I.
Step 2. It is expected that the esters in this group will be
readily hydrolysed to the component alcohols and carboxylic acids,
which are considered to be innocuous. The terpenoid alcohols are
expected to undergo omega-oxidation and functional group oxidation to
yield polar metabolites which are excreted as the glucuronic acid
conjugate in the urine. Eight of the 9 component carboxylic acids are
endogenous in humans, participating in the fatty acid ß-oxidation
pathway, amino acid pathways, the citric acid cycle, or the
C1 tetrahydrofolate pathway and eventually yielding CO2 and H2O.
The remaining carboxylic acid 2-ethylbutyric acid undergoes oxidation
to polar metabolites that are conjugated with glucuronic acid and
excreted. At current levels of intake these esters and their component
terpenoid alcohols and aliphatic carboxylic acids would not be
expected to saturate these metabolic pathways.
Step A3. None of the 26 terpenoid esters has a USA or European
daily per capita intake that exceeds 1800 µg/person per day.
Therefore, results of the Procedure indicate that none of the 26
terpenoid esters evaluated poses a safety concern when used at current
levels of intake as flavouring substances.
The stepwise evaluations of the 26 terpenoid esters used as
flavouring substances are summarized in Table 1.
1.5 Consideration of combined intake
In the unlikely event that these 26 terpenoid esters were to be
consumed simultaneously on a daily basis, the total daily intake would
still be within the human intake threshold of Class I (1800 µg/person
per day). The Committee noted that the terpene alcohols, geranoil,
citronellol and linalool are used as flavouring agents and that the
combined intakes of these alcohols and esters would be less than the
group ADI.
1.6 Conclusions
Applying the Procedure, the Committee concluded that for the
esters derived from branched-chain terpenoid alcohols and aliphatic
acyclic linear and branched-chain carboxylic acids there was no safety
concern at current intake levels.
No toxicity data were required for the application of the
procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
procedure.
The Committee noted that some of the esters are metabolized to
alpha,ß-unsaturated carbonyl compounds, but concluded that these had
been adequately evaluated previously (Annex 1, reference 50). The
Committee maintained the group ADI of 0-0.05 mg/kg bw for geranyl
acetate, citral, citronellol and linalool.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes all the available data relevant to the
safety evaluation of 26 esters formed from the esterification of the
terpenoid alcohols: geraniol, nerol, (±)-citronellol, and rhodinol and
saturated aliphatic carboxylic acids (linear saturated aliphatic acids
(C1-C6), isobutyric acid, isovaleric acid and 2-ethylbutyric acid).
The four terpenoid alcohols from which the 26 esters are formed are
close structural relatives. Geraniol and nerol are cis-trans (E-Z)
isomers of 3,7-dimethyl-2,6-octadien-1-ol; (±)-citronellol is
2,3-dihydrogeraniol (3,7-dimethyl-6-octen-1-ol); and rhodinol is a
mixture containing mainly l-citronellol and a small amount of the
isomer 3,7-dimethyl-7-octen-1-ol. As a commercial product, geraniol or
nerol may contain significant amounts of its (Z)- or (E)-isomer,
respectively, the monounsaturated alcohol (citronellol) and saturated
alcohol (3,7-dimethyl-1-octanol).
The basic structures of esters derived from branched-chain
terpenoid alcohols and saturated aliphatic acyclic carboxylic acids
are as follows:
 2.2 Intake data
The 26 esters formed from terpenoid primary alcohols (4) and
aliphatic saturated carboxylic acids (9) are used as flavor
ingredients in the USA at average maximum use levels less than 100
mg/kg, except for geranyl isovalerate which is used up to 155 mg/kg.
The total annual volume of the 26 terpenoid esters used as flavouring
substances is appoximately 2.1 tonnes in the USA (NAS, 1987) and 12.6
tonnes in Europe (IOFI, 1996) (Table 2). In the USA and in Europe,
approximately 60% of the total annual volume (NAS, 1987; IOFI, 1996)
is accounted for by the acetate and butyrate esters of citronellol,
geraniol and nerol. Based on the annual volume reported in the USA
(NAS, 1987) and Europe (IOFI, 1996), the total estimated daily
per capita intake ('eaters only') of the 26 terpenoid esters used as
flavouring substances is 6.8 µg/kg bw in the USA and 29.9 µg/kg bw in
Europe. The total daily per capita intake ('eaters only') of
terpenoid alcohols (i.e., citronellol, geraniol, nerol and rhodinol)
formed via hydrolysis of these esters is 5.3 µg/kg bw in the USA and
13.3 µg/kg bw in Europe.
2.3 Biological data
2.3.1 Absorption, metabolism and elimination
In general, aliphatic esters are rapidly hydrolysed to their
component alcohols and carboxylic acids. For example, geranyl esters
are hydrolysed to geraniol and aliphatic carboxylic acids (see Figure
2). Hydrolysis is catalysed by classes of enzymes recognized as
carboxylesterases or esterases (Heymann, 1980), the most important of
which are the ß-esterases. In mammals, these enzymes occur in most
tissues throughout the body (Heymann, 1980; Anders, 1989) but
predominate in the hepatocytes (Heymann, 1980).
2.2 Intake data
The 26 esters formed from terpenoid primary alcohols (4) and
aliphatic saturated carboxylic acids (9) are used as flavor
ingredients in the USA at average maximum use levels less than 100
mg/kg, except for geranyl isovalerate which is used up to 155 mg/kg.
The total annual volume of the 26 terpenoid esters used as flavouring
substances is appoximately 2.1 tonnes in the USA (NAS, 1987) and 12.6
tonnes in Europe (IOFI, 1996) (Table 2). In the USA and in Europe,
approximately 60% of the total annual volume (NAS, 1987; IOFI, 1996)
is accounted for by the acetate and butyrate esters of citronellol,
geraniol and nerol. Based on the annual volume reported in the USA
(NAS, 1987) and Europe (IOFI, 1996), the total estimated daily
per capita intake ('eaters only') of the 26 terpenoid esters used as
flavouring substances is 6.8 µg/kg bw in the USA and 29.9 µg/kg bw in
Europe. The total daily per capita intake ('eaters only') of
terpenoid alcohols (i.e., citronellol, geraniol, nerol and rhodinol)
formed via hydrolysis of these esters is 5.3 µg/kg bw in the USA and
13.3 µg/kg bw in Europe.
2.3 Biological data
2.3.1 Absorption, metabolism and elimination
In general, aliphatic esters are rapidly hydrolysed to their
component alcohols and carboxylic acids. For example, geranyl esters
are hydrolysed to geraniol and aliphatic carboxylic acids (see Figure
2). Hydrolysis is catalysed by classes of enzymes recognized as
carboxylesterases or esterases (Heymann, 1980), the most important of
which are the ß-esterases. In mammals, these enzymes occur in most
tissues throughout the body (Heymann, 1980; Anders, 1989) but
predominate in the hepatocytes (Heymann, 1980).
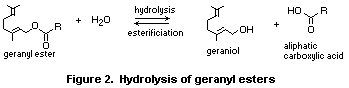 Each terpenoid ester in this group is hydrolysed to its
corresponding terpenoid alcohol (geraniol, citronellol, nerol and
rhodinol) and aliphatic carboxylic acid (formic, acetic, propionic,
butyric, valeric, hexanoic, isobutyric and isovaleric acids). A
concentration of 15 µl citronellyl acetate/litre was reported to be
completely hydrolysed within 2 hours by simulated intestinal fluid
containing pancreatin. A concentration <18 µl citronellyl
phenylacetate/litre was reported to be 60% hydrolysed within 2 hours
(Grundschober, 1977). Terpenoid alcohols formed in the
gastrointestinal tract are rapidly absorbed (Phillips et al., 1976;
Diliberto et al., 1988).
Table 2. Most recent annual usage of terpenoid alcohol esters in the USA and Europe
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl formate
USA 13 2.47 0.04 0.03
Europe 718 102.46 1.71 1.45
Geranyl formate
USA 250 47.56 0.79 0.67
Europe 2329 332.35 5.54 4.69
Neryl formate
USA 0.2 0.04 0.0006 0.003
Europe 0.054 0.01 0.0001 0.00004
Rhodinyl formate
USA 0.5 0.10 0.002 0.001
Citronellyl acetate
USA 190 36.15 0.60 0.47
Europe 1522 217.19 3.62 2.85
Geranyl acetate
USA 1070 203.58 3.39 2.66
Europe 3899 556.39 9.27 7.28
Neryl acetate
USA 333 63.36 1.06 0.83
Europe 1264 180.37 3.01 2.36
Rhodinyl acetate
USA 4 0.76 0.013 0.007
Europe 8 1.14 0.019 0.01
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl acetate
USA 8 1.52 0.03 0.02
Europe 286 40.81 0.68 0.49
Geranyl propionate
USA 60 11.42 0.19 0.14
Europe 565 80.63 1.34 0.98
Neryl propionate
USA 0.5 0.10 0.002 0.001
Europe 30 4.28 0.07 0.05
Rhodinyl propionate
USA 0.1 0.02 0.0003 0.0002
Citronellyl butyrate
USA 25 4.76 0.08 0.06
Europe 224 31.96 0.53 0.37
Geranyl butyrate
USA 130 24.73 0.41 0.28
Europe 423 60.36 1.01 0.69
Neryl butyrate
USA 0.1 0.02 0.0003 0.0002
Europe 2.9 0.41 0.007 0.004
Rhodinyl butyrate
USA 5 0.95 0.02 0.001
Europe 0
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl valerate
USA 20 3.81 0.06 0.04
Europe 5 0.71 0.01 0.01
Geranyl hexanoate
USA 3 0.57 0.01 0.01
Europe 0.5 0.07 0.001 0.0005
Citronellyl isobutyrate
USA 7 1.33 0.02 0.01
Europe 90 12.84 0.21 0.14
Geranyl isobutyrate
USA 16 3.04 0.05 0.03
Europe 866 123.58 2.06 1.41
Neryl isobutyrate
USA 2 0.38 0.01 0.01
Europe 14 2.00 0.03 0.02
Rhodinyl isobutyrate
USA 0.1 0.02 0.0003 0.002
Europe 0.2 0.03 0.0005 0.003
Geranyl isoverate
USA 9 1.71 0.03 0.02
Europe 339 48.38 0.81 0.52
Neryl isovalerate
USA 0.2 0.04 0.001 0.0005
Europe 0.2 0.03 0.0005 0.0003
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Rhodinyl isovalerate
USA 0.1 0.02 0.0003 0.0002
Europe 0.1 0.01 0.0002 0.0001
3,7-dimethylocta-2,6-
dienyl 2-ethylbutyrate
USA 0 0.00 0.0 0.0
Europe 4 0.57 0.010 0.005
Total USA 2146.8 408.45 6.82 5.31
Total Europe 12589.96 1798.59 29.94 13.33
1 USA: National Academy of Science (NAS, 1987) Evaluating the safety of food chemicals.
Washington DC. Europe: International Organization of the Flavour Industry (IOFI, 1996)
European Inquiry on volume of use. Private communication to FEMA
2 Intake calculated as follows: [[(anual volume, kg) × (1 x 109 mg/kg)]/[population × 0.6
× 365 days]], where population (10%, "eaters only") = 24 × 105 for the USA and 32 × 105 for
Europe; 0.6 represents the assumption that only 60% of the flavour volume was reported in
the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw/day) calculated as follows: [(mg/day)/body
weight], where body weight = 60 kg. Slight variation may occur from rounding off.
3 (Relative molecular mass of the component alcohol/relative molecular mass of the ester) x
(daily per capita intake "eaters only" of the ester)
2.3.1.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites which are excreted primarily in the urine. Alternately,
the corresponding carboxylic acids formed by oxidation of the alcohol
function may enter the ß-oxidation pathway and yield shorter chain
carboxylic acids which are completely metabolized to carbon dioxide
(Williams, 1959). Geraniol, related terpenoid alcohols (citronellol
and nerol), and the aldehydes (geranial and neral) exhibit similar
pathways of metabolic detoxication in animals (Figure 1).
Male rats were given single oral doses of 800 mg
[1-3H]-geraniol/kg bw by gavage daily for 20 days. Five urinary
metabolites were identified via two primary pathways. In one pathway,
the alcohol is oxidized to yield geranic acid
(3,7-dimethyl-2,6-octadienedioic acid), which is subsequently hydrated
to yield 3,7-dimethyl-3-hydroxy-6-octenoic acid. In a second pathway,
the alcohol undergoes omega-oxidation mediated by liver cytochrome
P-450 to yield 8-hydroxygeraniol. Selective oxidation at C-8 yields
8-carboxygeraniol, which undergoes further oxidation to the principal
urinary metabolite 3,7-dimethyl-2,6-octadienedioic acid
("Hildebrandt's acid") (Chadha & Madyastha, 1984) (see Figure 1). In
rat microsomes, the C-8 methyl group of geraniol or nerol utilizes
NADP+ and O2 and undergoes stereoselective omega-hydroxylation to
yield the (E)-isomer of the corresponding diol (Licht & Corsia, 1978).
In rats, the corresponding aldehyde, geranial and its (Z)-isomer,
neral, are metabolized via similar alcohol and omega-oxidation
pathways (Diliberto et al., 1990).
Geraniol, citronellol and rhodinol, the latter of which contains
mainly (-)-citronellol, exhibit a similar metabolic fate in rabbits.
Geraniol orally administered to rabbits by gavage is metabolized to
3,7-dimethyl-2,6-octadienedioic acid ("Hildebrandt's acid") and
3,7-dimethyl-2-octendioic acid ("reduced Hildebrandt's acid) which are
excreted in the urine (Fischer & Bielig, 1940). In rabbits,
(+)-citronellol is also metabolized to 3,7-dimethyl-2-octendioic acid
("reduced Hildebrandt's acid) (Asano & Yamakawa, 1950). An alcohol
precursor to "reduced Hildebrandt's acid
(8-hydroxy-3,7-dimethyl-6-octenoic acid) has been reported as a
urinary metabolite in rabbits given citronellol by gavage (Fischer &
Bielig, 1940). The corresponding aldehyde citronellal undergoes
omega-oxidation mediated by liver cytochrome P-450 (Chadha &
Madyastha, 1982) to yield "reduced Hildebrandt's acid" (Ishida
et al., 1989). Geraniol, as the pyrophosphate ester, is endogenous
in humans as an intermediate in the synthesis of cholesterol (Voet &
Voet, 1990).
2.3.1.2 Aliphatic carboxylic acids
With the exception of 2-ethylbutyric acid (see below), the
carboxylic acids formed via hydrolysis of the 26 terpenoid esters in
this group are endogenous in humans as intermediates either in the
fatty acid or amino acid pathways (Voet & Voet, 1990). In general, the
component linear saturated carboxylic acids (C1 - C6) participate in
fatty acid ß-oxidation and the citric acid cycle, or the C1
tetrahydrofolate pathway to eventually yield CO2 and H2O. The
component branched chain carboxylic acids (isobutyric acid and
isovaleric acid) formed during the oxidative deamination of the amino
acids valine and leucine, respectively, undergo oxidation,
preferentially in the longer branched chain, to yield linear
carboxylic acid fragments, which participate in the fatty acid pathway
and tricarboxylic acid cycle (Voet & Voet, 1990).
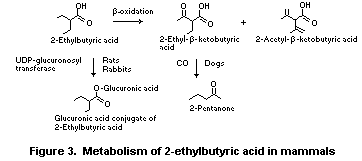
The non-endogenous substance 2-ethylbutyric acid contains an ethyl
substituent in the alpha position, which inhibits ß-oxidation and
complete metabolism to CO2 (Williams, 1959). Pathways of metabolic
detoxication include direct conjugation of the acid with glucuronic
acid, or ß-oxidation followed by conjugation. In rats and rabbits, 2-
ethylbutyric acid (Dziewiatowski et al., 1949) and 2-ethyl-1-butanol
(Kamil et al., 1953) are excreted unchanged in the urine principally
as the glucuronic acid conjugates. 2-Ethylbutyric acid is, in part,
metabolized by dogs to 2-pentanone, which is presumably derived from
ß-oxidation and subsequent decarboxylation of 2-ethyl-ß-ketobutyric
acid (Williams, 1959) (Figure 3).
Each terpenoid ester in this group is hydrolysed to its
corresponding terpenoid alcohol (geraniol, citronellol, nerol and
rhodinol) and aliphatic carboxylic acid (formic, acetic, propionic,
butyric, valeric, hexanoic, isobutyric and isovaleric acids). A
concentration of 15 µl citronellyl acetate/litre was reported to be
completely hydrolysed within 2 hours by simulated intestinal fluid
containing pancreatin. A concentration <18 µl citronellyl
phenylacetate/litre was reported to be 60% hydrolysed within 2 hours
(Grundschober, 1977). Terpenoid alcohols formed in the
gastrointestinal tract are rapidly absorbed (Phillips et al., 1976;
Diliberto et al., 1988).
Table 2. Most recent annual usage of terpenoid alcohol esters in the USA and Europe
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl formate
USA 13 2.47 0.04 0.03
Europe 718 102.46 1.71 1.45
Geranyl formate
USA 250 47.56 0.79 0.67
Europe 2329 332.35 5.54 4.69
Neryl formate
USA 0.2 0.04 0.0006 0.003
Europe 0.054 0.01 0.0001 0.00004
Rhodinyl formate
USA 0.5 0.10 0.002 0.001
Citronellyl acetate
USA 190 36.15 0.60 0.47
Europe 1522 217.19 3.62 2.85
Geranyl acetate
USA 1070 203.58 3.39 2.66
Europe 3899 556.39 9.27 7.28
Neryl acetate
USA 333 63.36 1.06 0.83
Europe 1264 180.37 3.01 2.36
Rhodinyl acetate
USA 4 0.76 0.013 0.007
Europe 8 1.14 0.019 0.01
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl acetate
USA 8 1.52 0.03 0.02
Europe 286 40.81 0.68 0.49
Geranyl propionate
USA 60 11.42 0.19 0.14
Europe 565 80.63 1.34 0.98
Neryl propionate
USA 0.5 0.10 0.002 0.001
Europe 30 4.28 0.07 0.05
Rhodinyl propionate
USA 0.1 0.02 0.0003 0.0002
Citronellyl butyrate
USA 25 4.76 0.08 0.06
Europe 224 31.96 0.53 0.37
Geranyl butyrate
USA 130 24.73 0.41 0.28
Europe 423 60.36 1.01 0.69
Neryl butyrate
USA 0.1 0.02 0.0003 0.0002
Europe 2.9 0.41 0.007 0.004
Rhodinyl butyrate
USA 5 0.95 0.02 0.001
Europe 0
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl valerate
USA 20 3.81 0.06 0.04
Europe 5 0.71 0.01 0.01
Geranyl hexanoate
USA 3 0.57 0.01 0.01
Europe 0.5 0.07 0.001 0.0005
Citronellyl isobutyrate
USA 7 1.33 0.02 0.01
Europe 90 12.84 0.21 0.14
Geranyl isobutyrate
USA 16 3.04 0.05 0.03
Europe 866 123.58 2.06 1.41
Neryl isobutyrate
USA 2 0.38 0.01 0.01
Europe 14 2.00 0.03 0.02
Rhodinyl isobutyrate
USA 0.1 0.02 0.0003 0.002
Europe 0.2 0.03 0.0005 0.003
Geranyl isoverate
USA 9 1.71 0.03 0.02
Europe 339 48.38 0.81 0.52
Neryl isovalerate
USA 0.2 0.04 0.001 0.0005
Europe 0.2 0.03 0.0005 0.0003
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Rhodinyl isovalerate
USA 0.1 0.02 0.0003 0.0002
Europe 0.1 0.01 0.0002 0.0001
3,7-dimethylocta-2,6-
dienyl 2-ethylbutyrate
USA 0 0.00 0.0 0.0
Europe 4 0.57 0.010 0.005
Total USA 2146.8 408.45 6.82 5.31
Total Europe 12589.96 1798.59 29.94 13.33
1 USA: National Academy of Science (NAS, 1987) Evaluating the safety of food chemicals.
Washington DC. Europe: International Organization of the Flavour Industry (IOFI, 1996)
European Inquiry on volume of use. Private communication to FEMA
2 Intake calculated as follows: [[(anual volume, kg) × (1 x 109 mg/kg)]/[population × 0.6
× 365 days]], where population (10%, "eaters only") = 24 × 105 for the USA and 32 × 105 for
Europe; 0.6 represents the assumption that only 60% of the flavour volume was reported in
the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw/day) calculated as follows: [(mg/day)/body
weight], where body weight = 60 kg. Slight variation may occur from rounding off.
3 (Relative molecular mass of the component alcohol/relative molecular mass of the ester) x
(daily per capita intake "eaters only" of the ester)
2.3.1.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites which are excreted primarily in the urine. Alternately,
the corresponding carboxylic acids formed by oxidation of the alcohol
function may enter the ß-oxidation pathway and yield shorter chain
carboxylic acids which are completely metabolized to carbon dioxide
(Williams, 1959). Geraniol, related terpenoid alcohols (citronellol
and nerol), and the aldehydes (geranial and neral) exhibit similar
pathways of metabolic detoxication in animals (Figure 1).
Male rats were given single oral doses of 800 mg
[1-3H]-geraniol/kg bw by gavage daily for 20 days. Five urinary
metabolites were identified via two primary pathways. In one pathway,
the alcohol is oxidized to yield geranic acid
(3,7-dimethyl-2,6-octadienedioic acid), which is subsequently hydrated
to yield 3,7-dimethyl-3-hydroxy-6-octenoic acid. In a second pathway,
the alcohol undergoes omega-oxidation mediated by liver cytochrome
P-450 to yield 8-hydroxygeraniol. Selective oxidation at C-8 yields
8-carboxygeraniol, which undergoes further oxidation to the principal
urinary metabolite 3,7-dimethyl-2,6-octadienedioic acid
("Hildebrandt's acid") (Chadha & Madyastha, 1984) (see Figure 1). In
rat microsomes, the C-8 methyl group of geraniol or nerol utilizes
NADP+ and O2 and undergoes stereoselective omega-hydroxylation to
yield the (E)-isomer of the corresponding diol (Licht & Corsia, 1978).
In rats, the corresponding aldehyde, geranial and its (Z)-isomer,
neral, are metabolized via similar alcohol and omega-oxidation
pathways (Diliberto et al., 1990).
Geraniol, citronellol and rhodinol, the latter of which contains
mainly (-)-citronellol, exhibit a similar metabolic fate in rabbits.
Geraniol orally administered to rabbits by gavage is metabolized to
3,7-dimethyl-2,6-octadienedioic acid ("Hildebrandt's acid") and
3,7-dimethyl-2-octendioic acid ("reduced Hildebrandt's acid) which are
excreted in the urine (Fischer & Bielig, 1940). In rabbits,
(+)-citronellol is also metabolized to 3,7-dimethyl-2-octendioic acid
("reduced Hildebrandt's acid) (Asano & Yamakawa, 1950). An alcohol
precursor to "reduced Hildebrandt's acid
(8-hydroxy-3,7-dimethyl-6-octenoic acid) has been reported as a
urinary metabolite in rabbits given citronellol by gavage (Fischer &
Bielig, 1940). The corresponding aldehyde citronellal undergoes
omega-oxidation mediated by liver cytochrome P-450 (Chadha &
Madyastha, 1982) to yield "reduced Hildebrandt's acid" (Ishida
et al., 1989). Geraniol, as the pyrophosphate ester, is endogenous
in humans as an intermediate in the synthesis of cholesterol (Voet &
Voet, 1990).
2.3.1.2 Aliphatic carboxylic acids
With the exception of 2-ethylbutyric acid (see below), the
carboxylic acids formed via hydrolysis of the 26 terpenoid esters in
this group are endogenous in humans as intermediates either in the
fatty acid or amino acid pathways (Voet & Voet, 1990). In general, the
component linear saturated carboxylic acids (C1 - C6) participate in
fatty acid ß-oxidation and the citric acid cycle, or the C1
tetrahydrofolate pathway to eventually yield CO2 and H2O. The
component branched chain carboxylic acids (isobutyric acid and
isovaleric acid) formed during the oxidative deamination of the amino
acids valine and leucine, respectively, undergo oxidation,
preferentially in the longer branched chain, to yield linear
carboxylic acid fragments, which participate in the fatty acid pathway
and tricarboxylic acid cycle (Voet & Voet, 1990).
The non-endogenous substance 2-ethylbutyric acid contains an ethyl
substituent in the alpha position, which inhibits ß-oxidation and
complete metabolism to CO2 (Williams, 1959). Pathways of metabolic
detoxication include direct conjugation of the acid with glucuronic
acid, or ß-oxidation followed by conjugation. In rats and rabbits, 2-
ethylbutyric acid (Dziewiatowski et al., 1949) and 2-ethyl-1-butanol
(Kamil et al., 1953) are excreted unchanged in the urine principally
as the glucuronic acid conjugates. 2-Ethylbutyric acid is, in part,
metabolized by dogs to 2-pentanone, which is presumably derived from
ß-oxidation and subsequent decarboxylation of 2-ethyl-ß-ketobutyric
acid (Williams, 1959) (Figure 3).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Acute toxicity studies have been conducted for 23 of the 26
terpenoid esters and are summarized in Table 3.
2.3.2.2 Short-term toxicity
The results of short-term toxicity studies with three terpenoid
esters (geranyl acetate, citronellyl acetate and citronellyl
isobutyrate) and two component alcohols (geraniol and citronellol) are
described below and are summarized in Table 4.
a) Mixture of geranyl acetate and citronellyl acetate
Groups of five male and five female B6C3F1 mice were administered
0, 125, 250, 500, 1000 or 2000 mg/kg bw per day of a mixture of
geranyl acetate (71% ) and citronellyl acetate (29% ) in corn oil by
gavage daily for 14 consecutive days. The animals were observed twice
daily and weighed on day 1, after one week, and at the end of the
study. All animals were necropsied at the end of the study. Three
female mice that received 2000 mg/kg bw per day died during the study.
All the other animals survived to the end of the study. Three of five
females receiving 2000 mg/kg bw per day exhibited a thickening of the
cardiac stomach and one of five males exhibited thickening of the
duodenal wall (NTP, 1987).
Table 3. Acute toxicity studies of terpenoid esters
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Citronellyl formate rat M & F gavage 8400 Calandra, 1973
Geranyl formate rat NR gavage 5460 Weir & Wong, 1971
Neryl formate rat NR oral 5000 Moreno, 1975
Rhodinyl formate rat NR oral 5000 Moreno, 1974
Citronellyl acetate rat M & F gavage 6800 Calandra, 1973
Geranyl acetate rat M & F gavage 6330 Jenner, 1964
Neryl acetate rat M & F gavage 4550 Opdyke, 1974
Rhodinyl acetate rat M & F gavage 5000 Opdyke, 1974
Citronellyl propionate rat NR oral 5000 Moreno, 1973
Geranyl propionate rat NR oral 5000 Russell, 1973
Neryl propionate rat NR oral 5000 Moreno, 1975
Rhodinyl propionate rat NR oral 5000 Moreno, 1976
Citronellyl butyrate rat M oral 5000 Moreno, 1972
Geranyl butyrate rat M & F gavage 10660 Jenner, 1964
Rhodinyl butyrate rat NR oral 5000 Moreno, 1975
Geranyl hexanoate rat NR oral 5000 Moreno, 1975
Citronellyl isobutyrate rat NR oral 5000 Denine & Palanker,
1973
Geranyl isobutyrate rat NR oral 5000 Shelanski & Moldovan,
1973
Neryl isobutyrate rat NR oral 5000 Moreno, 1980
Rhodinyl isobutyrate rat NR oral 5000 Moreno, 1975
Geranyl isovalerate rat NR oral 5000 Levenstein, 1975
Neryl isovalerate rat NR oral 5000 Moreno, 1976
Geranyl 2-ethylbutanoate mouse NR oral >8000 Givaudan Roure, 1971
1 M = males; F = females; NR = Not reported
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of B6C3F1 mice (10/sex/group) at dose levels of 0, 125,
250, 500, 1000 or 2000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and on animals dying during the test. Seven of 10 males and
9/10 females receiving 2000 mg/kg bw per day died during the study.
Gavage errors resulted in the death of three females at lower dose
levels. Mean body weights were comparable for dosed and control
animals. Male and female mice in the 2000 mg/kg bw per day dose groups
exhibited cytoplasmic vacuolization of the liver, kidney and
myocardium. Vacuolization was the result of lipid droplets that were
present throughout the liver lobule, particularly in the periportal
region. No treatment-related histopathological lesions or other
effects were observed in the 1000 mg/kg bw per day group (NTP, 1987).
Groups of five male and five female F344/N rats were administered
0, 62, 125, 250, 500 or 1000 mg/kg bw of a mixture of geranyl acetate
(71%) and citronellyl acetate (29%) in corn oil by gavage daily for 14
consecutive days. The animals were observed twice daily and weighed on
day 1, after one week, and at the end of the study. All animals were
necropsied at the end of the study. All animals survived to the end of
the dosing period and no compound-related effects were observed at
necropsy (NTP, 1987).
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of F344/N rats (10/sex/group) at dose levels of 0, 250,
500, 1000, 2000 or 4000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and in animals dying during the test. Two out of ten males
and one out of ten females receiving 4000 mg/kg bw per day died. Mean
body weights were comparable for dosed and control animals, except for
a decrease in mean body weight gain in males and females (19 % and 8%
relative to controls, respectively) at the 4000 mg/kg bw per day dose
level. No treatment-related histopathological effects were observed at
necropsy (NTP, 1987).
Table 4. Short- and long-term toxicity studies for terpenoid esters, component terpenoid alcohols and
component aliphatic saturated carboxylic acids
Substance Species, sex1 Route Duration Repeated dose Reference
study, NOEL2
(mg/kg bw
per day)
Terpenoid esters
Geranyl acetate3 mouse, M & F oral 13 weeks 1000 NTP, 1987
rat, M & F oral 13 weeks 2000 NTP, 1987
rat, M & F oral 17 weeks 5005 Hagan et al., 1967
Citronellyl isobutyrate rat, M & F oral 90 days 14.75 Damske, 1980
Component terpenoid
alcohols
Geraniol rat, M & F oral 16 weeks 5005 Hagan et al., 1967
rat, M & F oral 27-28 weeks 505 Hagan et al., 1967
Citronellol4 rat, M & F oral 12 weeks 505 Oser, 1967
1 M = male; F = female.
2 NOEL = No-observed-effect level.
3 The test material was composed of 79% geranyl acetate and 29% citronellyl acetate.
4 The test material was an equal mixture of citronellol and geraniol.
5 The study was performed at a single dose level or multiple dose levels, none of which produced
adverse effects. The reported dose level is, therefore, not an actual NOEL, but the highest level
that produced no adverse effects.
b) Geranyl acetate
Groups of ten male and ten female Osborne-Mendel rats were
provided geranyl acetate in the diet at concentrations of 0, 1000,
2500 or 10 000 mg/kg (equivalent to an average daily intake of 0, 50,
250 or 500 mg/kg bw) for 17 weeks. The diet was prepared weekly.
Determination of the dietary concentration of geranyl acetate revealed
a weekly loss of 4%. Measurement of body weight and food intake
recorded weekly showed no significant difference between test and
control animals at any intake level. At termination, haematological
examinations revealed no difference from controls. At necropsy, no
differences were reported between major organ weights of test and
control animals. Gross examination of tissue of all animals was
unremarkable and histopathological examination of 6-8 animals, equally
represented by gender, for the high-dose group and the control group
revealed no treatment-related lesions. The NOEL was 500 mg/kg bw per
day (Hagan et al., 1967).
c) Citronellyl isobutyrate
Citronellyl isobutyrate, prepared as a spray-dried material on gum
arabic, was provided in the diet to a group of 24 male and female
Charles River rats for 90 days at a level calculated to result in the
average daily intake of 14.7 mg/kg bw. Measurements of body weight and
food intake performed on a weekly basis showed no significant
differences between test and control animals. Blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed
normal values. At week 12, a significant decrease was recorded for
haemoglobin and haematocrit values in males. Organ weights at necropsy
revealed an increased relative heart weight in males, but there was no
significant difference in absolute heart weight between test and
control males. Gross examination and histopathology revealed no
treatment-related effects (Damske et al., 1980).
d) Component alcohols
i) Geraniol
The component alcohol geraniol, formed by hydrolysis of the
corresponding geranyl esters, was provided in the diet to groups of
five male and five female Osborne-Mendel rats for 16 weeks as a
mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol
(no further details) at a dietary concentration of 10 000 mg/kg
(equivalent to an average daily intake of 500 mg/kg bw). A second
study was conducted for 27-28 weeks using a dietary concentration of
1000 mg/kg (equivalent to an average daily intake of 50 mg/kg bw).
Measurement of body weight and food intake recorded weekly showed no
significant difference between test and control animals in either
study. At termination, haematological examinations revealed normal
values. At necropsy, no differences were reported between major organ
weights of test and control animals. Gross examination of the tissue
of all animals was unremarkable. Histopathological examination of 6-8
animals, equally represented by gender, revealed no treatment-related
lesions (Hagan et al., 1967).
ii) Citronellol
The parent alcohol citronellol, as a mixture with an equal weight
of linalool, was provided in the diet to groups of 10 male and 10
female weanling rats (strain not specified) at a level calculated to
result in the average daily intake of 50 mg/kg bw for a period of 12
weeks. A significant depression in body weight and food intake in male
rats was attributed to the impalatability of the test materials at the
level administered. The efficiency of food utilization showed no
significant difference between test and control animals. There were no
significant changes in appearance, urinalysis parameters, blood
haemoglobin, liver and kidney weights, or gross pathology (Oser,
1967).
2.3.2.3 Long-term toxicity/carcinogenicity studies
a) Mice
A carcinogenicity study was conducted in which groups of 50
B6C3F1 mice of each sex were administered 0, 500 or 1000 mg/kg bw of
a mixture of geranyl acetate (71%) and citronellyl acetate (29%) in
corn oil by gavage daily, 5 days/week for 103 weeks. Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and those
found dead during the study.
Mean body weights of high-dose male and female mice were lower
than those of control groups after week 18 of the study. Survival of
male mice in the high-dose group was significantly reduced (controls,
31/50; low dose 32/50; high dose, 0/50). Survival of the high- and
low-dose groups of female mice was significantly less (p<0.001; low
dose, 0.020) than that of the control group (controls, 28/50; low
dose, 15/50; high dose, 0/50). The mortality in females appeared
already from week 15 onwards. The probable cause of death of many
females was a genital tract infection. Inflammation of the vagina,
uterus, ovaries, or multiple organs occurred in 18 control, 14
low-dose, and 2 high-dose female mice. Although the etiological agent
was not isolated, Klebsiella pneumoniae was isolated from similarly
affected mice at this laboratory in subsequent chronic studies.
Surviving male (36) and female (11) mice in the high-dose groups were
killed in a moribund condition at week 91 after an inadvertent
overdose of the test substance. Eleven other animals (3 control males,
3 low-dose males, 3 low-dose females and 2 high-dose females) were
killed by gavage accidents during the course of the study.
There was no increase in the incidence of neoplastic lesions
associated with administration of the test substance. The incidence of
non-neoplastic lesions was significantly increased in high-dose male
and female mice only, and there was an increased incidence of
cytoplasmic vacuolization of the liver in male (control, 1/50; low
dose, 7/50; high dose, 47/50) and female mice (control, 1/50; low
dose, 27/50; high dose, 46/50) and the kidney or kidney tubule in male
(control, 0/50; low dose, 0/50; high dose, 41/50) and female mice
(control, 0/50; low dose, 24/49; high dose, 37/50). Under conditions
of this study, the mixture of geranyl acetate and citronellyl acetate
was not carcinogenic for either sex of B6C3F1 mice (NTP, 1987).
Owing to the low survival rate in both sexes and the early
mortality predominantly in female mice, this study should be
considered as having limited value for a safety evaluation.
b) Rats
A carcinogenicity study was conducted in which groups of 50 F344/N
rats of each sex were administered 0, 1000 or 2000 mg/kg bw of a
mixture of geranyl acetate (71%) and citronellyl acetate (29%) in corn
oil by gavage daily, 5 days/week for 103 weeks (2000 mg/kg bw
corresponds to estimated daily dose levels of 1420 mg geranyl
acetate/kg bw and 580 mg citronellyl acetate/kg bw). Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and on those
found dead during the study.
A statistically significant decrease in mean body weight was
reported for high-dose male rats throughout the study and low- and
high-dose female rats after week 40. Reduced mean body weight gains
were dose-related. Survival of the high-dose group (18/50) of male
rats was significantly less than those of the controls (34/50;
p=0.001) and the low-dose group (29/50; p=0.003). There was no other
significant difference in survival between any group of either sex.
A positive trend (controls, 6/50; low dose, 8/50; high dose, 9/50) in
the incidence of adrenal pheochromocytomas in male rats was not
statistically significant. There was no significant increase in the
incidence of any neoplasms in high-dose male or female rats compared
to the control groups. The overall incidence of these commonly
observed adrenal pheochromocytomas in paired control groups of male
rats has been reported to be 25.1% (Haseman et al., 1986).
Under conditions of this study, geranyl acetate was not carcinogenic
for either sex of F344/N rats. The NOEL based on body weight decreases
was 1000 mg/kg bw per day (NTP, 1987).
The toxicity of the component linear and branched-chain carboxylic
acid is discussed in the monographs on saturated
aliphatic acyclic linear primary alcohols aldehydes and acids and
on saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids.
2.3.2.4 Genotoxicity
Results of in vitro and in vivo genotoxicity studies carried
out on terpenoid esters, mainly geranyl acetate, are given in Table 5.
Table 5. Genotoxicity studies for terpenoid esters
Test system Substance name Test object Test concentration1 Results Reference
In vitro
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 2000 nl/plate2 negative3 Heck et al., 1989
TA1538
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 3333 µg/plate negative3 Mortelmans et al., 1986
TA1538
Rec assay Geranyl acetate B. subtilis 17 µg/disk4 negative Oda et al., 1978
Rec assay Geranyl acetate B. subtilis 20 µg/disk negative Yoo, 1986
Rec assay Geranyl formate B. subtilis 18 µg/disk4 negative Oda et al., 1978
HGPRT gene
mutation assay Geranyl acetate CHO cells no details negative3 Flowers & Li, 1988
Gene mutation assay Geranyl acetate Human lymphoblast TK6 320 µg/ml negative5 Caspary et al., 1988
500 µg/ml negative6
Chromosomal
aberrations Geranyl acetate Chinese hamster
ovary cells 100 µg/ml negative5 Galloway et al., 1987;
150 µg/ml negative6 Tennant et al., 1987
Sister chromatid Geranyl acetate Chinese hamster
exchange ovary cells 70 µg/ml positive5,7 Galloway et al., 1987;
299 µg/ml positive6,7 Tennant et al., 1987
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes no details negative Mirsalis et al., 1983
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes 100 nl/ml negative Heck et al., 1989
Alkaline elution Geranyl acetate Rat primary hepatocytes 0.30 mM negative Storer et al., 1996
PACE/PFGE8 Geranyl acetate Rat primary hepatocytes 0.25 mM positive9 Storer et al., 1996
Table 5. Continued...
Test system Substance name Test object Test concentration1 Results Reference
In vivo
Ses linked
recessive lethal
assay Geranyl acetate Drosophila melanogaster feeding 250 ppm; negative Foureman et al., 1994
injection 50 000 ppm negative
Micronucleus assay Geranyl acetate Marrow B6C3F1 mouse 1800 mg/kg bw/ day negative Shelby et al., 1993;
bone cells for 3 days, ip Shelby & Witt, 1995
Chromosomal Geranyl acetate Marrow B6C3F1 mouse no details, ip negative Shelby & Witt, 1995
aberrations bone cells
Unscheduled DNA
synthesis Geranyl acetate Fisher F344 male rats no details negative Mirsalis et al., 1983
1 Highest ineffective dose (negative) or lowest affective dose (positive).
2 Units based on information in Table 3.
3 Both with and without metabolic activation.
4 Single dose.
5 Without metabolic activation.
6 With metabolic activation.
7 Positive results were obtained at concentrations that were cytotoxic.
8 Programmed, autonomously-controlled electrode/pulsed-field gel electrophoresis.
9 DNA fragmentation greater than that seen for 40 Gy of gamma radiation.
2.3.2.5 Reproductive toxicity
No studies on reproduction and teratogenicity have been reported.
2.3.2.6 Other relevant studies
a) Citronellyl acetate
Citronellyl acetate applied full strength to intact and abraded
rabbit skin was irritating (Calandra, 1973).
b) Neryl acetate
Neryl acetate applied full strength to intact and abraded rabbit
skin for 24 hours under occlusion was not irritating (Opdyke, 1974).
c) Rhodinyl acetate
Rhodinyl acetate applied full strength to intact and abraded skin
for 24 h under occlusion was mildly irritating (Opdyke, 1974).
d) Neryl isobutyrate
As part of an acute dermal LD50 study, the undiluted material
produced slight irritant effects in rabbit patches tested for 24 h
under occlusion at a dose of 5 g/kg (Moreno, 1980).
2.4 Observations in humans
2.4.1 Geranyl acetate
A maximization test was carried out on 25 volunteers. Geranyl
acetate was tested at a concentration of 4% in petrolatum and produced
no sensitization reactions. Hypersensitivity occurred in some
individuals (Opdyke, 1974).
2.4.2 Neryl acetate
A maximization test was carried out on 25 volunteers. Neryl
acetate was tested at a concentration of 10% in petrolatum and
produced no senstitization reactions (Kligman, 1972).
When tested at 10% in petrolatum neryl acetate produced no
irritation in a 48-h closed patch test on human subjects (Opdyke,
1974).
2.4.3 Citronellyl acetate
When tested at a concentration of 4% in petrolatum, citronellyl
acetate produced a mild irritation in a 48-h closed patch test in 25
human subjects (Opdyke, 1974).
2.4.4 Rhodinyl acetate
When tested at 12% in petrolatum, rhodinyl acetate produced no
irritation in a 48-h closed patch test on human subjects (Kligman,
1974).
A maximization test was carried out on 25 volunteers. The material
was tested at 12% concentration in petrolatum and produced no
sensitization reactions (Opdyke, 1974).
2.4.5 Neryl isobutyrate
A 48-h closed patch test at a concentration of 5% in petrolatum on
the backs of 35 volunteers produced no irritation (Epstein, 1980).
A maximization test was carried out on 35 volunteers. The material
was tested at a concentration of 5% in petrolatum and produced no
sensitization reactions (Epstein, 1980).
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: intermediary xenobiotic metabolism in animals.
Taylor and Francis, New York, pp. 81-97.
Asano, M. & Yamakawa, T. (1950) The fate of branched chain fatty acids
in the body: I. A contribution to the problem of "Hildebrandt Acid".
J. Biochem. (Tokyo), 37: 321-327.
Calandra, J.C. (1973) In: Fragrance raw materials monographs. Food
Cosmet. Toxicol., 11: 1069.
Caspary, W.J., Langerbach, R., Penman B.W., Crespi, C., Myhr, B.C., &
A.D.Mitchell(1988) The mutagenic activity of selected compounds at the
TK locus: Rodent vs. human cells. Mutat. Res., 196(1): 61-81.
Chadha, A. & Madyastha, K.M. (1982) Omega-hydroxylation of acyclic
monoterpene alcohols by rat lung microsomes. Biochem. Biophys. Res.
Commun., 108: 1271-1277.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14: 365-374.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food. Cosmet.. Toxicol., 16:
255-276.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III, 7th
ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Damske, D.R., Mecler, F.J., & Craig D.K. (1980) 90-Day toxicity study
on citronellyl isobutyrate in rats. Unpublished report to FEMA.
Denine, E.P. & Palanker, A. (1973) Unpublished report to RIVM.
Diliberto, J.J., Usha, G., & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Dispos., 16: 721-727.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T.,
& Birnbaum, L.S. (1990) Metabolism of citral, an alpha,ß-unsaturated
aldehyde, in male F344 rats. Drug Metab. Dispos., 18: 866-875.
Dziewiatowski, D.D., Venkataraman, A., & Lewis, H.B. (1949) The
metabolism of some branched-chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Epstein, W.L. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol., 26(4): 273-415.
Fischer, F.G. & Bielig, H.J. (1940) On the hydrogenation of
unsaturated substances in the animal body. Physiol. Chem., 266(1/3):
73-98.
Flowers, L.J. & Li, A.P. (1988) Evaluation of 8 non-carcinogens in the
CHO-HGPRT gene mutation system. Environ. Mol. Mutagen, 11: 34.
Foureman, P., Mason, J.M., Valencia, R., & Zimmering, S. (1994)
Chemical mutagenesis testing in Drosophila melanogaster: IX. Results
of 50 coded compounds tested for the National Toxicology Program.
Environ. Mol. Mutagen., 23: 51-63.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., & Rimpo, J.
(1987) Chromosome aberration and sister chromatid exchanges in Chinese
hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10): 1-175.
Givaudan Roure Corporation (1971) Private communication to FEMA.
Greif, N. (1974) Cutaneous safety. In: Fragrance raw materials
monographs. Food Cosmet. Toxicol., 12: 885-887.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.
Taylor, J.M., Long, E.L., Nelson, A.A., & Brouwer, J.B. (1967) Food
flavorings and compounds of related structure. Subacute and chronic
toxicity. Food Chem. Toxicol., 5: 141-157.
Haseman, J.K., Winbush, J.S., & O'Donell, M.W. Jr. (1986) Use of dual
control groups to estimate false positive rates in laboratory animals
carcinogenicity studies. Fundam. Appl. Toxicol., 7: 573- 584.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9(1): 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
IOFI (1996) Private Communication to FEMA. 7/96.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and carvone in rabbits. Xenobiotica, 19:
843-855.
Jenner, P.M. (1964) Food flavorings and compounds of related
structure: Acute oral toxicity. Food Chem. Toxicol., 2: 327-343.
Kamil, I.A., Smith, J.N., & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem.J., 53:
129-136.
Keil, H. (1974) Contact dermatitis due to oil of citronella. In:
Fragrance raw materials monographs. Food Cosmet. Toxicol., 12:
885-887.
Kligman, A.M. (1974) In : Fragrance raw materials monographs. Food
Cosmet. Toxicol., 12: 885-887.
Levenstein, I. (1975) Private communication to FEMA.
Licht, H. & Corsia, C. J. (1978) Cytochrome P-450LM2 mediated
hydroxylation of monoterpene alcohols Biochemistry, 17: 5638-5646.
Meigs, T.E., Sherwood, S.W., & Simoni, R.D. (1995) Farnesyl acetate, a
derivative of an isoprenoid of the mevalonate pathway, inhibits DNA
replication in hamster and human cells. Exp. Cell Res., 219(2):
461-470.
Mirsalis, J., Tyson, K., Beck, J., Loh, E., Steinmetz, K., Contreras,
C., Austere, L., Martin, S., & Spalding, J. (1983) Induction of
unscheduled DNA synthesis (UDS) in hepatocytes following in vitro
and in vivo treatment. Environ. Mutagen., 5(3): 482.
Moreno, O.M. (1972) Private communication to FEMA.
Moreno, O.M. (1973) Private communication to FEMA.
Moreno, O.M. (1974) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M. (1976) Private communication to FEMA.
Moreno, O.M. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol. , 26(4): 273-415.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B., &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8(7): 1-119.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: a
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Myhr, B.C. & Caspary, W.J. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutagen., 18: 51-83.
National Academy of Sciences (1987) Evaluating the safety of food
chemicals. Washington, DC.
National Toxicology Program (NTP) (1987) Carcinogenesis studies of
food grade geranyl acetate (71%) and citronellyl acetate (29%).
Research Triangle Park, NC, USA (NTP-TR-252; PB-88-2508).
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Opdyke, D.L.J. (1974) Priate communication to FEMA.
Oser, B.L. (1967) Private communication to FEMA.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D., & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14: 537-540.
Russell, T. (1973) Unpublished report to FEMA.
Shelby, M.D., Erexson, G.L., Hook, G.L., & Tice, R.R. (1993)
Evaluation of a three-exposure mouse bone marrow micronucleus
protocol: Results with 49 chemicals. Environ. Mol. Mutagen., 21(2):
160-179.
Shelanski, M.V. & Moldovan, M. (1973) Unpublished report to RIVM.
Shelby, M.D. & Witt, K.L. (1995) Comparison of results from mouse bone
marrow chromosome aberration and micronucleus tests. Environ. Mol.
Mutagen., 25: 302-313.
Stanford, L.F. Jr., Aaron, C.S., Tuman, W.G., Godeh, E.G., Matthews,
R.J., & Naismith, R.W. (1988a) Detection of mammalian cell mutagenesis
in AS52 cells. EMS Abstr., 101: 246.
Stanford, L.F. Jnr., Godeh, E.G., Tuman, W.G., Biesczad, M.J., Miller,
A, Stec, E.E., Matthews, R.J., & Naismith, R.W. (1988b) Evaluation of
eight coded noncarcinogens in the CHO/WPRT and AS52/XPRT assays.
EMS Abstr., 101: 247.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Storer, R.D., McKelvey, T.M., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W., & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution/rat hepatocyte assay for DNA damage:
improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368: 59-101.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnick, M., & Stasiewicz, S. (1987)
Prediction of chemical carinogenicity in rodents from in vitro
genetic toxicity assays. Science, 236: 933-941.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T., &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA sythesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens screening of 22 known positives and 25
noncarcinogens. Mutat. Res., 320: 189-205.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Weir, R.J. & Wong, L.C.K. (1971) Private communication to FEMA.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed.Chapman and Hall
Ltd, London, pp. 88-113.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs . J. Osaka City Med. Center, 34(3-4):
267-288.
Yoshikawa, K. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspect., 104: 1, 40-46.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Acute toxicity studies have been conducted for 23 of the 26
terpenoid esters and are summarized in Table 3.
2.3.2.2 Short-term toxicity
The results of short-term toxicity studies with three terpenoid
esters (geranyl acetate, citronellyl acetate and citronellyl
isobutyrate) and two component alcohols (geraniol and citronellol) are
described below and are summarized in Table 4.
a) Mixture of geranyl acetate and citronellyl acetate
Groups of five male and five female B6C3F1 mice were administered
0, 125, 250, 500, 1000 or 2000 mg/kg bw per day of a mixture of
geranyl acetate (71% ) and citronellyl acetate (29% ) in corn oil by
gavage daily for 14 consecutive days. The animals were observed twice
daily and weighed on day 1, after one week, and at the end of the
study. All animals were necropsied at the end of the study. Three
female mice that received 2000 mg/kg bw per day died during the study.
All the other animals survived to the end of the study. Three of five
females receiving 2000 mg/kg bw per day exhibited a thickening of the
cardiac stomach and one of five males exhibited thickening of the
duodenal wall (NTP, 1987).
Table 3. Acute toxicity studies of terpenoid esters
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Citronellyl formate rat M & F gavage 8400 Calandra, 1973
Geranyl formate rat NR gavage 5460 Weir & Wong, 1971
Neryl formate rat NR oral 5000 Moreno, 1975
Rhodinyl formate rat NR oral 5000 Moreno, 1974
Citronellyl acetate rat M & F gavage 6800 Calandra, 1973
Geranyl acetate rat M & F gavage 6330 Jenner, 1964
Neryl acetate rat M & F gavage 4550 Opdyke, 1974
Rhodinyl acetate rat M & F gavage 5000 Opdyke, 1974
Citronellyl propionate rat NR oral 5000 Moreno, 1973
Geranyl propionate rat NR oral 5000 Russell, 1973
Neryl propionate rat NR oral 5000 Moreno, 1975
Rhodinyl propionate rat NR oral 5000 Moreno, 1976
Citronellyl butyrate rat M oral 5000 Moreno, 1972
Geranyl butyrate rat M & F gavage 10660 Jenner, 1964
Rhodinyl butyrate rat NR oral 5000 Moreno, 1975
Geranyl hexanoate rat NR oral 5000 Moreno, 1975
Citronellyl isobutyrate rat NR oral 5000 Denine & Palanker,
1973
Geranyl isobutyrate rat NR oral 5000 Shelanski & Moldovan,
1973
Neryl isobutyrate rat NR oral 5000 Moreno, 1980
Rhodinyl isobutyrate rat NR oral 5000 Moreno, 1975
Geranyl isovalerate rat NR oral 5000 Levenstein, 1975
Neryl isovalerate rat NR oral 5000 Moreno, 1976
Geranyl 2-ethylbutanoate mouse NR oral >8000 Givaudan Roure, 1971
1 M = males; F = females; NR = Not reported
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of B6C3F1 mice (10/sex/group) at dose levels of 0, 125,
250, 500, 1000 or 2000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and on animals dying during the test. Seven of 10 males and
9/10 females receiving 2000 mg/kg bw per day died during the study.
Gavage errors resulted in the death of three females at lower dose
levels. Mean body weights were comparable for dosed and control
animals. Male and female mice in the 2000 mg/kg bw per day dose groups
exhibited cytoplasmic vacuolization of the liver, kidney and
myocardium. Vacuolization was the result of lipid droplets that were
present throughout the liver lobule, particularly in the periportal
region. No treatment-related histopathological lesions or other
effects were observed in the 1000 mg/kg bw per day group (NTP, 1987).
Groups of five male and five female F344/N rats were administered
0, 62, 125, 250, 500 or 1000 mg/kg bw of a mixture of geranyl acetate
(71%) and citronellyl acetate (29%) in corn oil by gavage daily for 14
consecutive days. The animals were observed twice daily and weighed on
day 1, after one week, and at the end of the study. All animals were
necropsied at the end of the study. All animals survived to the end of
the dosing period and no compound-related effects were observed at
necropsy (NTP, 1987).
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of F344/N rats (10/sex/group) at dose levels of 0, 250,
500, 1000, 2000 or 4000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and in animals dying during the test. Two out of ten males
and one out of ten females receiving 4000 mg/kg bw per day died. Mean
body weights were comparable for dosed and control animals, except for
a decrease in mean body weight gain in males and females (19 % and 8%
relative to controls, respectively) at the 4000 mg/kg bw per day dose
level. No treatment-related histopathological effects were observed at
necropsy (NTP, 1987).
Table 4. Short- and long-term toxicity studies for terpenoid esters, component terpenoid alcohols and
component aliphatic saturated carboxylic acids
Substance Species, sex1 Route Duration Repeated dose Reference
study, NOEL2
(mg/kg bw
per day)
Terpenoid esters
Geranyl acetate3 mouse, M & F oral 13 weeks 1000 NTP, 1987
rat, M & F oral 13 weeks 2000 NTP, 1987
rat, M & F oral 17 weeks 5005 Hagan et al., 1967
Citronellyl isobutyrate rat, M & F oral 90 days 14.75 Damske, 1980
Component terpenoid
alcohols
Geraniol rat, M & F oral 16 weeks 5005 Hagan et al., 1967
rat, M & F oral 27-28 weeks 505 Hagan et al., 1967
Citronellol4 rat, M & F oral 12 weeks 505 Oser, 1967
1 M = male; F = female.
2 NOEL = No-observed-effect level.
3 The test material was composed of 79% geranyl acetate and 29% citronellyl acetate.
4 The test material was an equal mixture of citronellol and geraniol.
5 The study was performed at a single dose level or multiple dose levels, none of which produced
adverse effects. The reported dose level is, therefore, not an actual NOEL, but the highest level
that produced no adverse effects.
b) Geranyl acetate
Groups of ten male and ten female Osborne-Mendel rats were
provided geranyl acetate in the diet at concentrations of 0, 1000,
2500 or 10 000 mg/kg (equivalent to an average daily intake of 0, 50,
250 or 500 mg/kg bw) for 17 weeks. The diet was prepared weekly.
Determination of the dietary concentration of geranyl acetate revealed
a weekly loss of 4%. Measurement of body weight and food intake
recorded weekly showed no significant difference between test and
control animals at any intake level. At termination, haematological
examinations revealed no difference from controls. At necropsy, no
differences were reported between major organ weights of test and
control animals. Gross examination of tissue of all animals was
unremarkable and histopathological examination of 6-8 animals, equally
represented by gender, for the high-dose group and the control group
revealed no treatment-related lesions. The NOEL was 500 mg/kg bw per
day (Hagan et al., 1967).
c) Citronellyl isobutyrate
Citronellyl isobutyrate, prepared as a spray-dried material on gum
arabic, was provided in the diet to a group of 24 male and female
Charles River rats for 90 days at a level calculated to result in the
average daily intake of 14.7 mg/kg bw. Measurements of body weight and
food intake performed on a weekly basis showed no significant
differences between test and control animals. Blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed
normal values. At week 12, a significant decrease was recorded for
haemoglobin and haematocrit values in males. Organ weights at necropsy
revealed an increased relative heart weight in males, but there was no
significant difference in absolute heart weight between test and
control males. Gross examination and histopathology revealed no
treatment-related effects (Damske et al., 1980).
d) Component alcohols
i) Geraniol
The component alcohol geraniol, formed by hydrolysis of the
corresponding geranyl esters, was provided in the diet to groups of
five male and five female Osborne-Mendel rats for 16 weeks as a
mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol
(no further details) at a dietary concentration of 10 000 mg/kg
(equivalent to an average daily intake of 500 mg/kg bw). A second
study was conducted for 27-28 weeks using a dietary concentration of
1000 mg/kg (equivalent to an average daily intake of 50 mg/kg bw).
Measurement of body weight and food intake recorded weekly showed no
significant difference between test and control animals in either
study. At termination, haematological examinations revealed normal
values. At necropsy, no differences were reported between major organ
weights of test and control animals. Gross examination of the tissue
of all animals was unremarkable. Histopathological examination of 6-8
animals, equally represented by gender, revealed no treatment-related
lesions (Hagan et al., 1967).
ii) Citronellol
The parent alcohol citronellol, as a mixture with an equal weight
of linalool, was provided in the diet to groups of 10 male and 10
female weanling rats (strain not specified) at a level calculated to
result in the average daily intake of 50 mg/kg bw for a period of 12
weeks. A significant depression in body weight and food intake in male
rats was attributed to the impalatability of the test materials at the
level administered. The efficiency of food utilization showed no
significant difference between test and control animals. There were no
significant changes in appearance, urinalysis parameters, blood
haemoglobin, liver and kidney weights, or gross pathology (Oser,
1967).
2.3.2.3 Long-term toxicity/carcinogenicity studies
a) Mice
A carcinogenicity study was conducted in which groups of 50
B6C3F1 mice of each sex were administered 0, 500 or 1000 mg/kg bw of
a mixture of geranyl acetate (71%) and citronellyl acetate (29%) in
corn oil by gavage daily, 5 days/week for 103 weeks. Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and those
found dead during the study.
Mean body weights of high-dose male and female mice were lower
than those of control groups after week 18 of the study. Survival of
male mice in the high-dose group was significantly reduced (controls,
31/50; low dose 32/50; high dose, 0/50). Survival of the high- and
low-dose groups of female mice was significantly less (p<0.001; low
dose, 0.020) than that of the control group (controls, 28/50; low
dose, 15/50; high dose, 0/50). The mortality in females appeared
already from week 15 onwards. The probable cause of death of many
females was a genital tract infection. Inflammation of the vagina,
uterus, ovaries, or multiple organs occurred in 18 control, 14
low-dose, and 2 high-dose female mice. Although the etiological agent
was not isolated, Klebsiella pneumoniae was isolated from similarly
affected mice at this laboratory in subsequent chronic studies.
Surviving male (36) and female (11) mice in the high-dose groups were
killed in a moribund condition at week 91 after an inadvertent
overdose of the test substance. Eleven other animals (3 control males,
3 low-dose males, 3 low-dose females and 2 high-dose females) were
killed by gavage accidents during the course of the study.
There was no increase in the incidence of neoplastic lesions
associated with administration of the test substance. The incidence of
non-neoplastic lesions was significantly increased in high-dose male
and female mice only, and there was an increased incidence of
cytoplasmic vacuolization of the liver in male (control, 1/50; low
dose, 7/50; high dose, 47/50) and female mice (control, 1/50; low
dose, 27/50; high dose, 46/50) and the kidney or kidney tubule in male
(control, 0/50; low dose, 0/50; high dose, 41/50) and female mice
(control, 0/50; low dose, 24/49; high dose, 37/50). Under conditions
of this study, the mixture of geranyl acetate and citronellyl acetate
was not carcinogenic for either sex of B6C3F1 mice (NTP, 1987).
Owing to the low survival rate in both sexes and the early
mortality predominantly in female mice, this study should be
considered as having limited value for a safety evaluation.
b) Rats
A carcinogenicity study was conducted in which groups of 50 F344/N
rats of each sex were administered 0, 1000 or 2000 mg/kg bw of a
mixture of geranyl acetate (71%) and citronellyl acetate (29%) in corn
oil by gavage daily, 5 days/week for 103 weeks (2000 mg/kg bw
corresponds to estimated daily dose levels of 1420 mg geranyl
acetate/kg bw and 580 mg citronellyl acetate/kg bw). Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and on those
found dead during the study.
A statistically significant decrease in mean body weight was
reported for high-dose male rats throughout the study and low- and
high-dose female rats after week 40. Reduced mean body weight gains
were dose-related. Survival of the high-dose group (18/50) of male
rats was significantly less than those of the controls (34/50;
p=0.001) and the low-dose group (29/50; p=0.003). There was no other
significant difference in survival between any group of either sex.
A positive trend (controls, 6/50; low dose, 8/50; high dose, 9/50) in
the incidence of adrenal pheochromocytomas in male rats was not
statistically significant. There was no significant increase in the
incidence of any neoplasms in high-dose male or female rats compared
to the control groups. The overall incidence of these commonly
observed adrenal pheochromocytomas in paired control groups of male
rats has been reported to be 25.1% (Haseman et al., 1986).
Under conditions of this study, geranyl acetate was not carcinogenic
for either sex of F344/N rats. The NOEL based on body weight decreases
was 1000 mg/kg bw per day (NTP, 1987).
The toxicity of the component linear and branched-chain carboxylic
acid is discussed in the monographs on saturated
aliphatic acyclic linear primary alcohols aldehydes and acids and
on saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids.
2.3.2.4 Genotoxicity
Results of in vitro and in vivo genotoxicity studies carried
out on terpenoid esters, mainly geranyl acetate, are given in Table 5.
Table 5. Genotoxicity studies for terpenoid esters
Test system Substance name Test object Test concentration1 Results Reference
In vitro
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 2000 nl/plate2 negative3 Heck et al., 1989
TA1538
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 3333 µg/plate negative3 Mortelmans et al., 1986
TA1538
Rec assay Geranyl acetate B. subtilis 17 µg/disk4 negative Oda et al., 1978
Rec assay Geranyl acetate B. subtilis 20 µg/disk negative Yoo, 1986
Rec assay Geranyl formate B. subtilis 18 µg/disk4 negative Oda et al., 1978
HGPRT gene
mutation assay Geranyl acetate CHO cells no details negative3 Flowers & Li, 1988
Gene mutation assay Geranyl acetate Human lymphoblast TK6 320 µg/ml negative5 Caspary et al., 1988
500 µg/ml negative6
Chromosomal
aberrations Geranyl acetate Chinese hamster
ovary cells 100 µg/ml negative5 Galloway et al., 1987;
150 µg/ml negative6 Tennant et al., 1987
Sister chromatid Geranyl acetate Chinese hamster
exchange ovary cells 70 µg/ml positive5,7 Galloway et al., 1987;
299 µg/ml positive6,7 Tennant et al., 1987
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes no details negative Mirsalis et al., 1983
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes 100 nl/ml negative Heck et al., 1989
Alkaline elution Geranyl acetate Rat primary hepatocytes 0.30 mM negative Storer et al., 1996
PACE/PFGE8 Geranyl acetate Rat primary hepatocytes 0.25 mM positive9 Storer et al., 1996
Table 5. Continued...
Test system Substance name Test object Test concentration1 Results Reference
In vivo
Ses linked
recessive lethal
assay Geranyl acetate Drosophila melanogaster feeding 250 ppm; negative Foureman et al., 1994
injection 50 000 ppm negative
Micronucleus assay Geranyl acetate Marrow B6C3F1 mouse 1800 mg/kg bw/ day negative Shelby et al., 1993;
bone cells for 3 days, ip Shelby & Witt, 1995
Chromosomal Geranyl acetate Marrow B6C3F1 mouse no details, ip negative Shelby & Witt, 1995
aberrations bone cells
Unscheduled DNA
synthesis Geranyl acetate Fisher F344 male rats no details negative Mirsalis et al., 1983
1 Highest ineffective dose (negative) or lowest affective dose (positive).
2 Units based on information in Table 3.
3 Both with and without metabolic activation.
4 Single dose.
5 Without metabolic activation.
6 With metabolic activation.
7 Positive results were obtained at concentrations that were cytotoxic.
8 Programmed, autonomously-controlled electrode/pulsed-field gel electrophoresis.
9 DNA fragmentation greater than that seen for 40 Gy of gamma radiation.
2.3.2.5 Reproductive toxicity
No studies on reproduction and teratogenicity have been reported.
2.3.2.6 Other relevant studies
a) Citronellyl acetate
Citronellyl acetate applied full strength to intact and abraded
rabbit skin was irritating (Calandra, 1973).
b) Neryl acetate
Neryl acetate applied full strength to intact and abraded rabbit
skin for 24 hours under occlusion was not irritating (Opdyke, 1974).
c) Rhodinyl acetate
Rhodinyl acetate applied full strength to intact and abraded skin
for 24 h under occlusion was mildly irritating (Opdyke, 1974).
d) Neryl isobutyrate
As part of an acute dermal LD50 study, the undiluted material
produced slight irritant effects in rabbit patches tested for 24 h
under occlusion at a dose of 5 g/kg (Moreno, 1980).
2.4 Observations in humans
2.4.1 Geranyl acetate
A maximization test was carried out on 25 volunteers. Geranyl
acetate was tested at a concentration of 4% in petrolatum and produced
no sensitization reactions. Hypersensitivity occurred in some
individuals (Opdyke, 1974).
2.4.2 Neryl acetate
A maximization test was carried out on 25 volunteers. Neryl
acetate was tested at a concentration of 10% in petrolatum and
produced no senstitization reactions (Kligman, 1972).
When tested at 10% in petrolatum neryl acetate produced no
irritation in a 48-h closed patch test on human subjects (Opdyke,
1974).
2.4.3 Citronellyl acetate
When tested at a concentration of 4% in petrolatum, citronellyl
acetate produced a mild irritation in a 48-h closed patch test in 25
human subjects (Opdyke, 1974).
2.4.4 Rhodinyl acetate
When tested at 12% in petrolatum, rhodinyl acetate produced no
irritation in a 48-h closed patch test on human subjects (Kligman,
1974).
A maximization test was carried out on 25 volunteers. The material
was tested at 12% concentration in petrolatum and produced no
sensitization reactions (Opdyke, 1974).
2.4.5 Neryl isobutyrate
A 48-h closed patch test at a concentration of 5% in petrolatum on
the backs of 35 volunteers produced no irritation (Epstein, 1980).
A maximization test was carried out on 35 volunteers. The material
was tested at a concentration of 5% in petrolatum and produced no
sensitization reactions (Epstein, 1980).
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: intermediary xenobiotic metabolism in animals.
Taylor and Francis, New York, pp. 81-97.
Asano, M. & Yamakawa, T. (1950) The fate of branched chain fatty acids
in the body: I. A contribution to the problem of "Hildebrandt Acid".
J. Biochem. (Tokyo), 37: 321-327.
Calandra, J.C. (1973) In: Fragrance raw materials monographs. Food
Cosmet. Toxicol., 11: 1069.
Caspary, W.J., Langerbach, R., Penman B.W., Crespi, C., Myhr, B.C., &
A.D.Mitchell(1988) The mutagenic activity of selected compounds at the
TK locus: Rodent vs. human cells. Mutat. Res., 196(1): 61-81.
Chadha, A. & Madyastha, K.M. (1982) Omega-hydroxylation of acyclic
monoterpene alcohols by rat lung microsomes. Biochem. Biophys. Res.
Commun., 108: 1271-1277.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14: 365-374.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food. Cosmet.. Toxicol., 16:
255-276.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III, 7th
ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Damske, D.R., Mecler, F.J., & Craig D.K. (1980) 90-Day toxicity study
on citronellyl isobutyrate in rats. Unpublished report to FEMA.
Denine, E.P. & Palanker, A. (1973) Unpublished report to RIVM.
Diliberto, J.J., Usha, G., & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Dispos., 16: 721-727.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T.,
& Birnbaum, L.S. (1990) Metabolism of citral, an alpha,ß-unsaturated
aldehyde, in male F344 rats. Drug Metab. Dispos., 18: 866-875.
Dziewiatowski, D.D., Venkataraman, A., & Lewis, H.B. (1949) The
metabolism of some branched-chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Epstein, W.L. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol., 26(4): 273-415.
Fischer, F.G. & Bielig, H.J. (1940) On the hydrogenation of
unsaturated substances in the animal body. Physiol. Chem., 266(1/3):
73-98.
Flowers, L.J. & Li, A.P. (1988) Evaluation of 8 non-carcinogens in the
CHO-HGPRT gene mutation system. Environ. Mol. Mutagen, 11: 34.
Foureman, P., Mason, J.M., Valencia, R., & Zimmering, S. (1994)
Chemical mutagenesis testing in Drosophila melanogaster: IX. Results
of 50 coded compounds tested for the National Toxicology Program.
Environ. Mol. Mutagen., 23: 51-63.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., & Rimpo, J.
(1987) Chromosome aberration and sister chromatid exchanges in Chinese
hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10): 1-175.
Givaudan Roure Corporation (1971) Private communication to FEMA.
Greif, N. (1974) Cutaneous safety. In: Fragrance raw materials
monographs. Food Cosmet. Toxicol., 12: 885-887.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.
Taylor, J.M., Long, E.L., Nelson, A.A., & Brouwer, J.B. (1967) Food
flavorings and compounds of related structure. Subacute and chronic
toxicity. Food Chem. Toxicol., 5: 141-157.
Haseman, J.K., Winbush, J.S., & O'Donell, M.W. Jr. (1986) Use of dual
control groups to estimate false positive rates in laboratory animals
carcinogenicity studies. Fundam. Appl. Toxicol., 7: 573- 584.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9(1): 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
IOFI (1996) Private Communication to FEMA. 7/96.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and carvone in rabbits. Xenobiotica, 19:
843-855.
Jenner, P.M. (1964) Food flavorings and compounds of related
structure: Acute oral toxicity. Food Chem. Toxicol., 2: 327-343.
Kamil, I.A., Smith, J.N., & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem.J., 53:
129-136.
Keil, H. (1974) Contact dermatitis due to oil of citronella. In:
Fragrance raw materials monographs. Food Cosmet. Toxicol., 12:
885-887.
Kligman, A.M. (1974) In : Fragrance raw materials monographs. Food
Cosmet. Toxicol., 12: 885-887.
Levenstein, I. (1975) Private communication to FEMA.
Licht, H. & Corsia, C. J. (1978) Cytochrome P-450LM2 mediated
hydroxylation of monoterpene alcohols Biochemistry, 17: 5638-5646.
Meigs, T.E., Sherwood, S.W., & Simoni, R.D. (1995) Farnesyl acetate, a
derivative of an isoprenoid of the mevalonate pathway, inhibits DNA
replication in hamster and human cells. Exp. Cell Res., 219(2):
461-470.
Mirsalis, J., Tyson, K., Beck, J., Loh, E., Steinmetz, K., Contreras,
C., Austere, L., Martin, S., & Spalding, J. (1983) Induction of
unscheduled DNA synthesis (UDS) in hepatocytes following in vitro
and in vivo treatment. Environ. Mutagen., 5(3): 482.
Moreno, O.M. (1972) Private communication to FEMA.
Moreno, O.M. (1973) Private communication to FEMA.
Moreno, O.M. (1974) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M. (1976) Private communication to FEMA.
Moreno, O.M. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol. , 26(4): 273-415.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B., &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8(7): 1-119.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: a
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Myhr, B.C. & Caspary, W.J. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutagen., 18: 51-83.
National Academy of Sciences (1987) Evaluating the safety of food
chemicals. Washington, DC.
National Toxicology Program (NTP) (1987) Carcinogenesis studies of
food grade geranyl acetate (71%) and citronellyl acetate (29%).
Research Triangle Park, NC, USA (NTP-TR-252; PB-88-2508).
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Opdyke, D.L.J. (1974) Priate communication to FEMA.
Oser, B.L. (1967) Private communication to FEMA.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D., & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14: 537-540.
Russell, T. (1973) Unpublished report to FEMA.
Shelby, M.D., Erexson, G.L., Hook, G.L., & Tice, R.R. (1993)
Evaluation of a three-exposure mouse bone marrow micronucleus
protocol: Results with 49 chemicals. Environ. Mol. Mutagen., 21(2):
160-179.
Shelanski, M.V. & Moldovan, M. (1973) Unpublished report to RIVM.
Shelby, M.D. & Witt, K.L. (1995) Comparison of results from mouse bone
marrow chromosome aberration and micronucleus tests. Environ. Mol.
Mutagen., 25: 302-313.
Stanford, L.F. Jr., Aaron, C.S., Tuman, W.G., Godeh, E.G., Matthews,
R.J., & Naismith, R.W. (1988a) Detection of mammalian cell mutagenesis
in AS52 cells. EMS Abstr., 101: 246.
Stanford, L.F. Jnr., Godeh, E.G., Tuman, W.G., Biesczad, M.J., Miller,
A, Stec, E.E., Matthews, R.J., & Naismith, R.W. (1988b) Evaluation of
eight coded noncarcinogens in the CHO/WPRT and AS52/XPRT assays.
EMS Abstr., 101: 247.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Storer, R.D., McKelvey, T.M., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W., & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution/rat hepatocyte assay for DNA damage:
improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368: 59-101.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnick, M., & Stasiewicz, S. (1987)
Prediction of chemical carinogenicity in rodents from in vitro
genetic toxicity assays. Science, 236: 933-941.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T., &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA sythesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens screening of 22 known positives and 25
noncarcinogens. Mutat. Res., 320: 189-205.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Weir, R.J. & Wong, L.C.K. (1971) Private communication to FEMA.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed.Chapman and Hall
Ltd, London, pp. 88-113.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs . J. Osaka City Med. Center, 34(3-4):
267-288.
Yoshikawa, K. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspect., 104: 1, 40-46.
See Also:
Toxicological Abbreviations
 Table 1. Summary of results of the safety evaluations of esters of terpenoid alcohols
and aliphatic carboxylic acids
Step 1: All of the substances in the group are in structural class I, the human intake
threshold of which is 1800 µg per day.
Step 2: All of the substances in the group are metabolized to innocuous products.
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0053 Citronellyl formate No No safety concern
USA: 2.5 Europe: 103
0054 Geranyl formate No No safety concern
USA: 48 Europe: 330
0055 Neryl formate No No safety concern
USA: 0.04 Europe: 0.01
0056 Rhodinyl formate No No safety concern
USA: 0.10 Europe: unknown
0057 Citronellyl acetate No No safety concern
USA: 36 Europe: 217
0058 Geranyl acetate No No safety concern
USA: 205 Europe: 580
0059 Neryl acetate No No safety concern
USA: 63 Europe: 180
0060 Rhodinyl acetate No No safety concern
USA: 0.8 Europe: 1.1
0061 Citronellyl propionate No No safety concern
USA: 1.5 Europe: 41
0062 Geranyl propionate No No safety concern
USA: 11 Europe: 81
0063 Neryl propionate No No safety concern
USA: 1.1 Europe: 4.3
0064 Rhodinyl propionate No No safety concern
USA: 0.02 Europe: unknown
0065 Citronellyl butyrate No No safety concern
USA: 5 Europe: 32
Table 1. Continued...
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0066 Geranyl butyrate No No safety concern
USA: 25 Europe: 60
0067 Neryl butyrate No No safety concern
USA: 0.02 Europe: 0.4
0068 Rhodinyl butyrate No No safety concern
USA: 1.0 Europe: unknown
0069 Citronellyl valerate No No safety concern
USA: 4.0 Europe: 0.7
0070 Geranyl hexanoate No No safety concern
USA: 0.5 Europe: 0.07
0071 Citronellyl isobutyrate No No safety concern
USA: 1.3 Europe: 13
0072 Geranyl isobutyrate No No safety concern
USA: 3.0 Europe: 124
0073 Neryl isobutyrate No No safety concern
USA: 0.4 Europe: 2.0
0074 Rhodinyl isobutyrate No No safety concern
USA: 0.02 Europe: 0.03
0075 Geranyl isoverate No No safety concern
USA: 1.7 Europe: 43
0076 Neryl isovalerate No No safety concern
USA: 0.04 Europe: 0.03
0077 Rhodinyl isovalerate No No safety concern
USA: 0.02 Europe: 0.01
0078 3,7-Dimethyl-2,6-
octadien-1-yl 2-
ethylbutanoate No No safety concern
USA: 0 Europe: 0.6
1 The human intake threshold is 1800 µg per day for Class 1, 540 µg/day for Class 2 and
90 µg/day for Class 3
One member of the group, geranyl acetate, was previously evaluated
at the twenty-third meeting of the Committee. It was evaluated as part
of a group of other terpenoid flavouring substances, citral,
citronellol, and linalool, which have close chemical, biochemical and
toxicological relationships. The Committee allocated a group ADI of 0-
0.05 mg/kg bw based on the clearly defined metabolism of these
substances and their low toxicity in short-term toxicity studies
(Annex 1, reference 50).
Data relevant to the Procedure for the Safety Evaluation of 26
esters formed from the esterification of the terpenoid alcohols, (for
geraniol, nerol, (±)-citronellol and rhodinol) and saturated aliphatic
carboxylic acids (linear saturated aliphatic acids (C1-C6),
isobutyric acid isovaleric acid and 2-ethylbutyric acid) are
summarized.
1.2 Estimated daily per capita intake
The total annual production volume of the 26 terpenoid esters used
as flavouring agents is approximately 2100 kg in the USA and 12 600 kg
in Europe. In the USA and in Europe, approximately 60% of the total
annual volume is accounted for by the acetate and butyrate esters of
citronellol, geraniol and nerol. Based on the annual volume reported
in the USA and Europe, the total estimated daily per
capita intake of the 26 terpenoid esters used as flavouring
substances is 410 µg in the USA and 1800 µg in Europe. The total
estimated daily per capita intake of terpenoid alcohols (i.e.,
citronellol, geraniol, nerol and rhodinol) formed via hydrolysis of
these esters is 320 µg in the USA and 1400 µg in Europe.
Terpenoid esters are principal flavour components of citrus and
citrus peel oils, and have also been detected in wide variety of other
fruits, spices and vegetables. The terpenoid esters are usually found
at concentration of < 1 mg/kg in citrus fruit juices, < 20 000 mg/kg
in citrus peel oils, and < 50 000 mg/kg in spices (CIVO-TNO, 1994).
In the USA terpenoid esters are consumed predominantly as components
of traditional foods (Stofberg & Kirschman, 1985). In the USA, the
total annual consumption of seven of these terpenoid esters as natural
components of food is estimated to be approximately 300 tonnes
(Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism and elimination
The terpenoid esters are hydrolysed to the corresponding terpenoid
alcohols (geraniol, citronellol, nerol, and rhodinol) and aliphatic
carboxylic acids (formic, acetic, propionic, butyric, valeric,
hexanoic, isobutyric and isovaleric acids). Both the hydrolysis data
and the metabolism of aliphatic carboxylic acids are discussed in the
introduction to this chapter on flavouring agents.
1.3.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites, which are excreted primarily in the urine. Geraniol,
related terpenoid alcohols (citronellol and nerol), and the aldehydes
(geranial and neral) exhibit similar pathways of metabolic
detoxication in animals (Figure 1).
1.4 Application of the Procedure for Safety Evaluation of Flavouring
Agents
Step 1. All of the 26 terpenoid esters are in class I.
Step 2. It is expected that the esters in this group will be
readily hydrolysed to the component alcohols and carboxylic acids,
which are considered to be innocuous. The terpenoid alcohols are
expected to undergo omega-oxidation and functional group oxidation to
yield polar metabolites which are excreted as the glucuronic acid
conjugate in the urine. Eight of the 9 component carboxylic acids are
endogenous in humans, participating in the fatty acid ß-oxidation
pathway, amino acid pathways, the citric acid cycle, or the
C1 tetrahydrofolate pathway and eventually yielding CO2 and H2O.
The remaining carboxylic acid 2-ethylbutyric acid undergoes oxidation
to polar metabolites that are conjugated with glucuronic acid and
excreted. At current levels of intake these esters and their component
terpenoid alcohols and aliphatic carboxylic acids would not be
expected to saturate these metabolic pathways.
Step A3. None of the 26 terpenoid esters has a USA or European
daily per capita intake that exceeds 1800 µg/person per day.
Therefore, results of the Procedure indicate that none of the 26
terpenoid esters evaluated poses a safety concern when used at current
levels of intake as flavouring substances.
The stepwise evaluations of the 26 terpenoid esters used as
flavouring substances are summarized in Table 1.
1.5 Consideration of combined intake
In the unlikely event that these 26 terpenoid esters were to be
consumed simultaneously on a daily basis, the total daily intake would
still be within the human intake threshold of Class I (1800 µg/person
per day). The Committee noted that the terpene alcohols, geranoil,
citronellol and linalool are used as flavouring agents and that the
combined intakes of these alcohols and esters would be less than the
group ADI.
1.6 Conclusions
Applying the Procedure, the Committee concluded that for the
esters derived from branched-chain terpenoid alcohols and aliphatic
acyclic linear and branched-chain carboxylic acids there was no safety
concern at current intake levels.
No toxicity data were required for the application of the
procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
procedure.
The Committee noted that some of the esters are metabolized to
alpha,ß-unsaturated carbonyl compounds, but concluded that these had
been adequately evaluated previously (Annex 1, reference 50). The
Committee maintained the group ADI of 0-0.05 mg/kg bw for geranyl
acetate, citral, citronellol and linalool.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes all the available data relevant to the
safety evaluation of 26 esters formed from the esterification of the
terpenoid alcohols: geraniol, nerol, (±)-citronellol, and rhodinol and
saturated aliphatic carboxylic acids (linear saturated aliphatic acids
(C1-C6), isobutyric acid, isovaleric acid and 2-ethylbutyric acid).
The four terpenoid alcohols from which the 26 esters are formed are
close structural relatives. Geraniol and nerol are cis-trans (E-Z)
isomers of 3,7-dimethyl-2,6-octadien-1-ol; (±)-citronellol is
2,3-dihydrogeraniol (3,7-dimethyl-6-octen-1-ol); and rhodinol is a
mixture containing mainly l-citronellol and a small amount of the
isomer 3,7-dimethyl-7-octen-1-ol. As a commercial product, geraniol or
nerol may contain significant amounts of its (Z)- or (E)-isomer,
respectively, the monounsaturated alcohol (citronellol) and saturated
alcohol (3,7-dimethyl-1-octanol).
The basic structures of esters derived from branched-chain
terpenoid alcohols and saturated aliphatic acyclic carboxylic acids
are as follows:
Table 1. Summary of results of the safety evaluations of esters of terpenoid alcohols
and aliphatic carboxylic acids
Step 1: All of the substances in the group are in structural class I, the human intake
threshold of which is 1800 µg per day.
Step 2: All of the substances in the group are metabolized to innocuous products.
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0053 Citronellyl formate No No safety concern
USA: 2.5 Europe: 103
0054 Geranyl formate No No safety concern
USA: 48 Europe: 330
0055 Neryl formate No No safety concern
USA: 0.04 Europe: 0.01
0056 Rhodinyl formate No No safety concern
USA: 0.10 Europe: unknown
0057 Citronellyl acetate No No safety concern
USA: 36 Europe: 217
0058 Geranyl acetate No No safety concern
USA: 205 Europe: 580
0059 Neryl acetate No No safety concern
USA: 63 Europe: 180
0060 Rhodinyl acetate No No safety concern
USA: 0.8 Europe: 1.1
0061 Citronellyl propionate No No safety concern
USA: 1.5 Europe: 41
0062 Geranyl propionate No No safety concern
USA: 11 Europe: 81
0063 Neryl propionate No No safety concern
USA: 1.1 Europe: 4.3
0064 Rhodinyl propionate No No safety concern
USA: 0.02 Europe: unknown
0065 Citronellyl butyrate No No safety concern
USA: 5 Europe: 32
Table 1. Continued...
No. Substance Step A3 Conclusions based on
(CAS No.) Does intake exceed intake current intake
threshold? Intake estimates
(µg/person per day1)
0066 Geranyl butyrate No No safety concern
USA: 25 Europe: 60
0067 Neryl butyrate No No safety concern
USA: 0.02 Europe: 0.4
0068 Rhodinyl butyrate No No safety concern
USA: 1.0 Europe: unknown
0069 Citronellyl valerate No No safety concern
USA: 4.0 Europe: 0.7
0070 Geranyl hexanoate No No safety concern
USA: 0.5 Europe: 0.07
0071 Citronellyl isobutyrate No No safety concern
USA: 1.3 Europe: 13
0072 Geranyl isobutyrate No No safety concern
USA: 3.0 Europe: 124
0073 Neryl isobutyrate No No safety concern
USA: 0.4 Europe: 2.0
0074 Rhodinyl isobutyrate No No safety concern
USA: 0.02 Europe: 0.03
0075 Geranyl isoverate No No safety concern
USA: 1.7 Europe: 43
0076 Neryl isovalerate No No safety concern
USA: 0.04 Europe: 0.03
0077 Rhodinyl isovalerate No No safety concern
USA: 0.02 Europe: 0.01
0078 3,7-Dimethyl-2,6-
octadien-1-yl 2-
ethylbutanoate No No safety concern
USA: 0 Europe: 0.6
1 The human intake threshold is 1800 µg per day for Class 1, 540 µg/day for Class 2 and
90 µg/day for Class 3
One member of the group, geranyl acetate, was previously evaluated
at the twenty-third meeting of the Committee. It was evaluated as part
of a group of other terpenoid flavouring substances, citral,
citronellol, and linalool, which have close chemical, biochemical and
toxicological relationships. The Committee allocated a group ADI of 0-
0.05 mg/kg bw based on the clearly defined metabolism of these
substances and their low toxicity in short-term toxicity studies
(Annex 1, reference 50).
Data relevant to the Procedure for the Safety Evaluation of 26
esters formed from the esterification of the terpenoid alcohols, (for
geraniol, nerol, (±)-citronellol and rhodinol) and saturated aliphatic
carboxylic acids (linear saturated aliphatic acids (C1-C6),
isobutyric acid isovaleric acid and 2-ethylbutyric acid) are
summarized.
1.2 Estimated daily per capita intake
The total annual production volume of the 26 terpenoid esters used
as flavouring agents is approximately 2100 kg in the USA and 12 600 kg
in Europe. In the USA and in Europe, approximately 60% of the total
annual volume is accounted for by the acetate and butyrate esters of
citronellol, geraniol and nerol. Based on the annual volume reported
in the USA and Europe, the total estimated daily per
capita intake of the 26 terpenoid esters used as flavouring
substances is 410 µg in the USA and 1800 µg in Europe. The total
estimated daily per capita intake of terpenoid alcohols (i.e.,
citronellol, geraniol, nerol and rhodinol) formed via hydrolysis of
these esters is 320 µg in the USA and 1400 µg in Europe.
Terpenoid esters are principal flavour components of citrus and
citrus peel oils, and have also been detected in wide variety of other
fruits, spices and vegetables. The terpenoid esters are usually found
at concentration of < 1 mg/kg in citrus fruit juices, < 20 000 mg/kg
in citrus peel oils, and < 50 000 mg/kg in spices (CIVO-TNO, 1994).
In the USA terpenoid esters are consumed predominantly as components
of traditional foods (Stofberg & Kirschman, 1985). In the USA, the
total annual consumption of seven of these terpenoid esters as natural
components of food is estimated to be approximately 300 tonnes
(Stofberg & Grundschober, 1987).
1.3 Absorption, metabolism and elimination
The terpenoid esters are hydrolysed to the corresponding terpenoid
alcohols (geraniol, citronellol, nerol, and rhodinol) and aliphatic
carboxylic acids (formic, acetic, propionic, butyric, valeric,
hexanoic, isobutyric and isovaleric acids). Both the hydrolysis data
and the metabolism of aliphatic carboxylic acids are discussed in the
introduction to this chapter on flavouring agents.
1.3.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites, which are excreted primarily in the urine. Geraniol,
related terpenoid alcohols (citronellol and nerol), and the aldehydes
(geranial and neral) exhibit similar pathways of metabolic
detoxication in animals (Figure 1).
1.4 Application of the Procedure for Safety Evaluation of Flavouring
Agents
Step 1. All of the 26 terpenoid esters are in class I.
Step 2. It is expected that the esters in this group will be
readily hydrolysed to the component alcohols and carboxylic acids,
which are considered to be innocuous. The terpenoid alcohols are
expected to undergo omega-oxidation and functional group oxidation to
yield polar metabolites which are excreted as the glucuronic acid
conjugate in the urine. Eight of the 9 component carboxylic acids are
endogenous in humans, participating in the fatty acid ß-oxidation
pathway, amino acid pathways, the citric acid cycle, or the
C1 tetrahydrofolate pathway and eventually yielding CO2 and H2O.
The remaining carboxylic acid 2-ethylbutyric acid undergoes oxidation
to polar metabolites that are conjugated with glucuronic acid and
excreted. At current levels of intake these esters and their component
terpenoid alcohols and aliphatic carboxylic acids would not be
expected to saturate these metabolic pathways.
Step A3. None of the 26 terpenoid esters has a USA or European
daily per capita intake that exceeds 1800 µg/person per day.
Therefore, results of the Procedure indicate that none of the 26
terpenoid esters evaluated poses a safety concern when used at current
levels of intake as flavouring substances.
The stepwise evaluations of the 26 terpenoid esters used as
flavouring substances are summarized in Table 1.
1.5 Consideration of combined intake
In the unlikely event that these 26 terpenoid esters were to be
consumed simultaneously on a daily basis, the total daily intake would
still be within the human intake threshold of Class I (1800 µg/person
per day). The Committee noted that the terpene alcohols, geranoil,
citronellol and linalool are used as flavouring agents and that the
combined intakes of these alcohols and esters would be less than the
group ADI.
1.6 Conclusions
Applying the Procedure, the Committee concluded that for the
esters derived from branched-chain terpenoid alcohols and aliphatic
acyclic linear and branched-chain carboxylic acids there was no safety
concern at current intake levels.
No toxicity data were required for the application of the
procedure. The Committee noted that the available toxicity data were
consistent with the results of the safety evaluation using the
procedure.
The Committee noted that some of the esters are metabolized to
alpha,ß-unsaturated carbonyl compounds, but concluded that these had
been adequately evaluated previously (Annex 1, reference 50). The
Committee maintained the group ADI of 0-0.05 mg/kg bw for geranyl
acetate, citral, citronellol and linalool.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes all the available data relevant to the
safety evaluation of 26 esters formed from the esterification of the
terpenoid alcohols: geraniol, nerol, (±)-citronellol, and rhodinol and
saturated aliphatic carboxylic acids (linear saturated aliphatic acids
(C1-C6), isobutyric acid, isovaleric acid and 2-ethylbutyric acid).
The four terpenoid alcohols from which the 26 esters are formed are
close structural relatives. Geraniol and nerol are cis-trans (E-Z)
isomers of 3,7-dimethyl-2,6-octadien-1-ol; (±)-citronellol is
2,3-dihydrogeraniol (3,7-dimethyl-6-octen-1-ol); and rhodinol is a
mixture containing mainly l-citronellol and a small amount of the
isomer 3,7-dimethyl-7-octen-1-ol. As a commercial product, geraniol or
nerol may contain significant amounts of its (Z)- or (E)-isomer,
respectively, the monounsaturated alcohol (citronellol) and saturated
alcohol (3,7-dimethyl-1-octanol).
The basic structures of esters derived from branched-chain
terpenoid alcohols and saturated aliphatic acyclic carboxylic acids
are as follows:
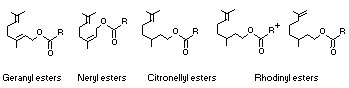 2.2 Intake data
The 26 esters formed from terpenoid primary alcohols (4) and
aliphatic saturated carboxylic acids (9) are used as flavor
ingredients in the USA at average maximum use levels less than 100
mg/kg, except for geranyl isovalerate which is used up to 155 mg/kg.
The total annual volume of the 26 terpenoid esters used as flavouring
substances is appoximately 2.1 tonnes in the USA (NAS, 1987) and 12.6
tonnes in Europe (IOFI, 1996) (Table 2). In the USA and in Europe,
approximately 60% of the total annual volume (NAS, 1987; IOFI, 1996)
is accounted for by the acetate and butyrate esters of citronellol,
geraniol and nerol. Based on the annual volume reported in the USA
(NAS, 1987) and Europe (IOFI, 1996), the total estimated daily
per capita intake ('eaters only') of the 26 terpenoid esters used as
flavouring substances is 6.8 µg/kg bw in the USA and 29.9 µg/kg bw in
Europe. The total daily per capita intake ('eaters only') of
terpenoid alcohols (i.e., citronellol, geraniol, nerol and rhodinol)
formed via hydrolysis of these esters is 5.3 µg/kg bw in the USA and
13.3 µg/kg bw in Europe.
2.3 Biological data
2.3.1 Absorption, metabolism and elimination
In general, aliphatic esters are rapidly hydrolysed to their
component alcohols and carboxylic acids. For example, geranyl esters
are hydrolysed to geraniol and aliphatic carboxylic acids (see Figure
2). Hydrolysis is catalysed by classes of enzymes recognized as
carboxylesterases or esterases (Heymann, 1980), the most important of
which are the ß-esterases. In mammals, these enzymes occur in most
tissues throughout the body (Heymann, 1980; Anders, 1989) but
predominate in the hepatocytes (Heymann, 1980).
2.2 Intake data
The 26 esters formed from terpenoid primary alcohols (4) and
aliphatic saturated carboxylic acids (9) are used as flavor
ingredients in the USA at average maximum use levels less than 100
mg/kg, except for geranyl isovalerate which is used up to 155 mg/kg.
The total annual volume of the 26 terpenoid esters used as flavouring
substances is appoximately 2.1 tonnes in the USA (NAS, 1987) and 12.6
tonnes in Europe (IOFI, 1996) (Table 2). In the USA and in Europe,
approximately 60% of the total annual volume (NAS, 1987; IOFI, 1996)
is accounted for by the acetate and butyrate esters of citronellol,
geraniol and nerol. Based on the annual volume reported in the USA
(NAS, 1987) and Europe (IOFI, 1996), the total estimated daily
per capita intake ('eaters only') of the 26 terpenoid esters used as
flavouring substances is 6.8 µg/kg bw in the USA and 29.9 µg/kg bw in
Europe. The total daily per capita intake ('eaters only') of
terpenoid alcohols (i.e., citronellol, geraniol, nerol and rhodinol)
formed via hydrolysis of these esters is 5.3 µg/kg bw in the USA and
13.3 µg/kg bw in Europe.
2.3 Biological data
2.3.1 Absorption, metabolism and elimination
In general, aliphatic esters are rapidly hydrolysed to their
component alcohols and carboxylic acids. For example, geranyl esters
are hydrolysed to geraniol and aliphatic carboxylic acids (see Figure
2). Hydrolysis is catalysed by classes of enzymes recognized as
carboxylesterases or esterases (Heymann, 1980), the most important of
which are the ß-esterases. In mammals, these enzymes occur in most
tissues throughout the body (Heymann, 1980; Anders, 1989) but
predominate in the hepatocytes (Heymann, 1980).
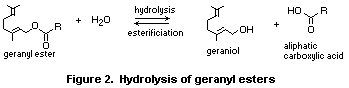 Each terpenoid ester in this group is hydrolysed to its
corresponding terpenoid alcohol (geraniol, citronellol, nerol and
rhodinol) and aliphatic carboxylic acid (formic, acetic, propionic,
butyric, valeric, hexanoic, isobutyric and isovaleric acids). A
concentration of 15 µl citronellyl acetate/litre was reported to be
completely hydrolysed within 2 hours by simulated intestinal fluid
containing pancreatin. A concentration <18 µl citronellyl
phenylacetate/litre was reported to be 60% hydrolysed within 2 hours
(Grundschober, 1977). Terpenoid alcohols formed in the
gastrointestinal tract are rapidly absorbed (Phillips et al., 1976;
Diliberto et al., 1988).
Table 2. Most recent annual usage of terpenoid alcohol esters in the USA and Europe
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl formate
USA 13 2.47 0.04 0.03
Europe 718 102.46 1.71 1.45
Geranyl formate
USA 250 47.56 0.79 0.67
Europe 2329 332.35 5.54 4.69
Neryl formate
USA 0.2 0.04 0.0006 0.003
Europe 0.054 0.01 0.0001 0.00004
Rhodinyl formate
USA 0.5 0.10 0.002 0.001
Citronellyl acetate
USA 190 36.15 0.60 0.47
Europe 1522 217.19 3.62 2.85
Geranyl acetate
USA 1070 203.58 3.39 2.66
Europe 3899 556.39 9.27 7.28
Neryl acetate
USA 333 63.36 1.06 0.83
Europe 1264 180.37 3.01 2.36
Rhodinyl acetate
USA 4 0.76 0.013 0.007
Europe 8 1.14 0.019 0.01
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl acetate
USA 8 1.52 0.03 0.02
Europe 286 40.81 0.68 0.49
Geranyl propionate
USA 60 11.42 0.19 0.14
Europe 565 80.63 1.34 0.98
Neryl propionate
USA 0.5 0.10 0.002 0.001
Europe 30 4.28 0.07 0.05
Rhodinyl propionate
USA 0.1 0.02 0.0003 0.0002
Citronellyl butyrate
USA 25 4.76 0.08 0.06
Europe 224 31.96 0.53 0.37
Geranyl butyrate
USA 130 24.73 0.41 0.28
Europe 423 60.36 1.01 0.69
Neryl butyrate
USA 0.1 0.02 0.0003 0.0002
Europe 2.9 0.41 0.007 0.004
Rhodinyl butyrate
USA 5 0.95 0.02 0.001
Europe 0
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl valerate
USA 20 3.81 0.06 0.04
Europe 5 0.71 0.01 0.01
Geranyl hexanoate
USA 3 0.57 0.01 0.01
Europe 0.5 0.07 0.001 0.0005
Citronellyl isobutyrate
USA 7 1.33 0.02 0.01
Europe 90 12.84 0.21 0.14
Geranyl isobutyrate
USA 16 3.04 0.05 0.03
Europe 866 123.58 2.06 1.41
Neryl isobutyrate
USA 2 0.38 0.01 0.01
Europe 14 2.00 0.03 0.02
Rhodinyl isobutyrate
USA 0.1 0.02 0.0003 0.002
Europe 0.2 0.03 0.0005 0.003
Geranyl isoverate
USA 9 1.71 0.03 0.02
Europe 339 48.38 0.81 0.52
Neryl isovalerate
USA 0.2 0.04 0.001 0.0005
Europe 0.2 0.03 0.0005 0.0003
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Rhodinyl isovalerate
USA 0.1 0.02 0.0003 0.0002
Europe 0.1 0.01 0.0002 0.0001
3,7-dimethylocta-2,6-
dienyl 2-ethylbutyrate
USA 0 0.00 0.0 0.0
Europe 4 0.57 0.010 0.005
Total USA 2146.8 408.45 6.82 5.31
Total Europe 12589.96 1798.59 29.94 13.33
1 USA: National Academy of Science (NAS, 1987) Evaluating the safety of food chemicals.
Washington DC. Europe: International Organization of the Flavour Industry (IOFI, 1996)
European Inquiry on volume of use. Private communication to FEMA
2 Intake calculated as follows: [[(anual volume, kg) × (1 x 109 mg/kg)]/[population × 0.6
× 365 days]], where population (10%, "eaters only") = 24 × 105 for the USA and 32 × 105 for
Europe; 0.6 represents the assumption that only 60% of the flavour volume was reported in
the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw/day) calculated as follows: [(mg/day)/body
weight], where body weight = 60 kg. Slight variation may occur from rounding off.
3 (Relative molecular mass of the component alcohol/relative molecular mass of the ester) x
(daily per capita intake "eaters only" of the ester)
2.3.1.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites which are excreted primarily in the urine. Alternately,
the corresponding carboxylic acids formed by oxidation of the alcohol
function may enter the ß-oxidation pathway and yield shorter chain
carboxylic acids which are completely metabolized to carbon dioxide
(Williams, 1959). Geraniol, related terpenoid alcohols (citronellol
and nerol), and the aldehydes (geranial and neral) exhibit similar
pathways of metabolic detoxication in animals (Figure 1).
Male rats were given single oral doses of 800 mg
[1-3H]-geraniol/kg bw by gavage daily for 20 days. Five urinary
metabolites were identified via two primary pathways. In one pathway,
the alcohol is oxidized to yield geranic acid
(3,7-dimethyl-2,6-octadienedioic acid), which is subsequently hydrated
to yield 3,7-dimethyl-3-hydroxy-6-octenoic acid. In a second pathway,
the alcohol undergoes omega-oxidation mediated by liver cytochrome
P-450 to yield 8-hydroxygeraniol. Selective oxidation at C-8 yields
8-carboxygeraniol, which undergoes further oxidation to the principal
urinary metabolite 3,7-dimethyl-2,6-octadienedioic acid
("Hildebrandt's acid") (Chadha & Madyastha, 1984) (see Figure 1). In
rat microsomes, the C-8 methyl group of geraniol or nerol utilizes
NADP+ and O2 and undergoes stereoselective omega-hydroxylation to
yield the (E)-isomer of the corresponding diol (Licht & Corsia, 1978).
In rats, the corresponding aldehyde, geranial and its (Z)-isomer,
neral, are metabolized via similar alcohol and omega-oxidation
pathways (Diliberto et al., 1990).
Geraniol, citronellol and rhodinol, the latter of which contains
mainly (-)-citronellol, exhibit a similar metabolic fate in rabbits.
Geraniol orally administered to rabbits by gavage is metabolized to
3,7-dimethyl-2,6-octadienedioic acid ("Hildebrandt's acid") and
3,7-dimethyl-2-octendioic acid ("reduced Hildebrandt's acid) which are
excreted in the urine (Fischer & Bielig, 1940). In rabbits,
(+)-citronellol is also metabolized to 3,7-dimethyl-2-octendioic acid
("reduced Hildebrandt's acid) (Asano & Yamakawa, 1950). An alcohol
precursor to "reduced Hildebrandt's acid
(8-hydroxy-3,7-dimethyl-6-octenoic acid) has been reported as a
urinary metabolite in rabbits given citronellol by gavage (Fischer &
Bielig, 1940). The corresponding aldehyde citronellal undergoes
omega-oxidation mediated by liver cytochrome P-450 (Chadha &
Madyastha, 1982) to yield "reduced Hildebrandt's acid" (Ishida
et al., 1989). Geraniol, as the pyrophosphate ester, is endogenous
in humans as an intermediate in the synthesis of cholesterol (Voet &
Voet, 1990).
2.3.1.2 Aliphatic carboxylic acids
With the exception of 2-ethylbutyric acid (see below), the
carboxylic acids formed via hydrolysis of the 26 terpenoid esters in
this group are endogenous in humans as intermediates either in the
fatty acid or amino acid pathways (Voet & Voet, 1990). In general, the
component linear saturated carboxylic acids (C1 - C6) participate in
fatty acid ß-oxidation and the citric acid cycle, or the C1
tetrahydrofolate pathway to eventually yield CO2 and H2O. The
component branched chain carboxylic acids (isobutyric acid and
isovaleric acid) formed during the oxidative deamination of the amino
acids valine and leucine, respectively, undergo oxidation,
preferentially in the longer branched chain, to yield linear
carboxylic acid fragments, which participate in the fatty acid pathway
and tricarboxylic acid cycle (Voet & Voet, 1990).
The non-endogenous substance 2-ethylbutyric acid contains an ethyl
substituent in the alpha position, which inhibits ß-oxidation and
complete metabolism to CO2 (Williams, 1959). Pathways of metabolic
detoxication include direct conjugation of the acid with glucuronic
acid, or ß-oxidation followed by conjugation. In rats and rabbits, 2-
ethylbutyric acid (Dziewiatowski et al., 1949) and 2-ethyl-1-butanol
(Kamil et al., 1953) are excreted unchanged in the urine principally
as the glucuronic acid conjugates. 2-Ethylbutyric acid is, in part,
metabolized by dogs to 2-pentanone, which is presumably derived from
ß-oxidation and subsequent decarboxylation of 2-ethyl-ß-ketobutyric
acid (Williams, 1959) (Figure 3).
Each terpenoid ester in this group is hydrolysed to its
corresponding terpenoid alcohol (geraniol, citronellol, nerol and
rhodinol) and aliphatic carboxylic acid (formic, acetic, propionic,
butyric, valeric, hexanoic, isobutyric and isovaleric acids). A
concentration of 15 µl citronellyl acetate/litre was reported to be
completely hydrolysed within 2 hours by simulated intestinal fluid
containing pancreatin. A concentration <18 µl citronellyl
phenylacetate/litre was reported to be 60% hydrolysed within 2 hours
(Grundschober, 1977). Terpenoid alcohols formed in the
gastrointestinal tract are rapidly absorbed (Phillips et al., 1976;
Diliberto et al., 1988).
Table 2. Most recent annual usage of terpenoid alcohol esters in the USA and Europe
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl formate
USA 13 2.47 0.04 0.03
Europe 718 102.46 1.71 1.45
Geranyl formate
USA 250 47.56 0.79 0.67
Europe 2329 332.35 5.54 4.69
Neryl formate
USA 0.2 0.04 0.0006 0.003
Europe 0.054 0.01 0.0001 0.00004
Rhodinyl formate
USA 0.5 0.10 0.002 0.001
Citronellyl acetate
USA 190 36.15 0.60 0.47
Europe 1522 217.19 3.62 2.85
Geranyl acetate
USA 1070 203.58 3.39 2.66
Europe 3899 556.39 9.27 7.28
Neryl acetate
USA 333 63.36 1.06 0.83
Europe 1264 180.37 3.01 2.36
Rhodinyl acetate
USA 4 0.76 0.013 0.007
Europe 8 1.14 0.019 0.01
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl acetate
USA 8 1.52 0.03 0.02
Europe 286 40.81 0.68 0.49
Geranyl propionate
USA 60 11.42 0.19 0.14
Europe 565 80.63 1.34 0.98
Neryl propionate
USA 0.5 0.10 0.002 0.001
Europe 30 4.28 0.07 0.05
Rhodinyl propionate
USA 0.1 0.02 0.0003 0.0002
Citronellyl butyrate
USA 25 4.76 0.08 0.06
Europe 224 31.96 0.53 0.37
Geranyl butyrate
USA 130 24.73 0.41 0.28
Europe 423 60.36 1.01 0.69
Neryl butyrate
USA 0.1 0.02 0.0003 0.0002
Europe 2.9 0.41 0.007 0.004
Rhodinyl butyrate
USA 5 0.95 0.02 0.001
Europe 0
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Citronellyl valerate
USA 20 3.81 0.06 0.04
Europe 5 0.71 0.01 0.01
Geranyl hexanoate
USA 3 0.57 0.01 0.01
Europe 0.5 0.07 0.001 0.0005
Citronellyl isobutyrate
USA 7 1.33 0.02 0.01
Europe 90 12.84 0.21 0.14
Geranyl isobutyrate
USA 16 3.04 0.05 0.03
Europe 866 123.58 2.06 1.41
Neryl isobutyrate
USA 2 0.38 0.01 0.01
Europe 14 2.00 0.03 0.02
Rhodinyl isobutyrate
USA 0.1 0.02 0.0003 0.002
Europe 0.2 0.03 0.0005 0.003
Geranyl isoverate
USA 9 1.71 0.03 0.02
Europe 339 48.38 0.81 0.52
Neryl isovalerate
USA 0.2 0.04 0.001 0.0005
Europe 0.2 0.03 0.0005 0.0003
Table 2. Continued...
Substance Most recent Daily per capita intake2 Daily per capita
annual volume, ("eaters only") intake3 ("eaters only"),
(kg1) alcohol equivalents,
µg/g µg/kg bw/day (µg/kg bw/day)
Rhodinyl isovalerate
USA 0.1 0.02 0.0003 0.0002
Europe 0.1 0.01 0.0002 0.0001
3,7-dimethylocta-2,6-
dienyl 2-ethylbutyrate
USA 0 0.00 0.0 0.0
Europe 4 0.57 0.010 0.005
Total USA 2146.8 408.45 6.82 5.31
Total Europe 12589.96 1798.59 29.94 13.33
1 USA: National Academy of Science (NAS, 1987) Evaluating the safety of food chemicals.
Washington DC. Europe: International Organization of the Flavour Industry (IOFI, 1996)
European Inquiry on volume of use. Private communication to FEMA
2 Intake calculated as follows: [[(anual volume, kg) × (1 x 109 mg/kg)]/[population × 0.6
× 365 days]], where population (10%, "eaters only") = 24 × 105 for the USA and 32 × 105 for
Europe; 0.6 represents the assumption that only 60% of the flavour volume was reported in
the survey (NAS, 1987; IOFI, 1996). Intake (mg/kg bw/day) calculated as follows: [(mg/day)/body
weight], where body weight = 60 kg. Slight variation may occur from rounding off.
3 (Relative molecular mass of the component alcohol/relative molecular mass of the ester) x
(daily per capita intake "eaters only" of the ester)
2.3.1.1 Terpenoid alcohols
Following hydrolysis, the terpenoid alcohols undergo a complex
pattern of alcohol oxidation, omega-oxidation, hydration, selective
hydrogenation and subsequent conjugation to form oxygenated polar
metabolites which are excreted primarily in the urine. Alternately,
the corresponding carboxylic acids formed by oxidation of the alcohol
function may enter the ß-oxidation pathway and yield shorter chain
carboxylic acids which are completely metabolized to carbon dioxide
(Williams, 1959). Geraniol, related terpenoid alcohols (citronellol
and nerol), and the aldehydes (geranial and neral) exhibit similar
pathways of metabolic detoxication in animals (Figure 1).
Male rats were given single oral doses of 800 mg
[1-3H]-geraniol/kg bw by gavage daily for 20 days. Five urinary
metabolites were identified via two primary pathways. In one pathway,
the alcohol is oxidized to yield geranic acid
(3,7-dimethyl-2,6-octadienedioic acid), which is subsequently hydrated
to yield 3,7-dimethyl-3-hydroxy-6-octenoic acid. In a second pathway,
the alcohol undergoes omega-oxidation mediated by liver cytochrome
P-450 to yield 8-hydroxygeraniol. Selective oxidation at C-8 yields
8-carboxygeraniol, which undergoes further oxidation to the principal
urinary metabolite 3,7-dimethyl-2,6-octadienedioic acid
("Hildebrandt's acid") (Chadha & Madyastha, 1984) (see Figure 1). In
rat microsomes, the C-8 methyl group of geraniol or nerol utilizes
NADP+ and O2 and undergoes stereoselective omega-hydroxylation to
yield the (E)-isomer of the corresponding diol (Licht & Corsia, 1978).
In rats, the corresponding aldehyde, geranial and its (Z)-isomer,
neral, are metabolized via similar alcohol and omega-oxidation
pathways (Diliberto et al., 1990).
Geraniol, citronellol and rhodinol, the latter of which contains
mainly (-)-citronellol, exhibit a similar metabolic fate in rabbits.
Geraniol orally administered to rabbits by gavage is metabolized to
3,7-dimethyl-2,6-octadienedioic acid ("Hildebrandt's acid") and
3,7-dimethyl-2-octendioic acid ("reduced Hildebrandt's acid) which are
excreted in the urine (Fischer & Bielig, 1940). In rabbits,
(+)-citronellol is also metabolized to 3,7-dimethyl-2-octendioic acid
("reduced Hildebrandt's acid) (Asano & Yamakawa, 1950). An alcohol
precursor to "reduced Hildebrandt's acid
(8-hydroxy-3,7-dimethyl-6-octenoic acid) has been reported as a
urinary metabolite in rabbits given citronellol by gavage (Fischer &
Bielig, 1940). The corresponding aldehyde citronellal undergoes
omega-oxidation mediated by liver cytochrome P-450 (Chadha &
Madyastha, 1982) to yield "reduced Hildebrandt's acid" (Ishida
et al., 1989). Geraniol, as the pyrophosphate ester, is endogenous
in humans as an intermediate in the synthesis of cholesterol (Voet &
Voet, 1990).
2.3.1.2 Aliphatic carboxylic acids
With the exception of 2-ethylbutyric acid (see below), the
carboxylic acids formed via hydrolysis of the 26 terpenoid esters in
this group are endogenous in humans as intermediates either in the
fatty acid or amino acid pathways (Voet & Voet, 1990). In general, the
component linear saturated carboxylic acids (C1 - C6) participate in
fatty acid ß-oxidation and the citric acid cycle, or the C1
tetrahydrofolate pathway to eventually yield CO2 and H2O. The
component branched chain carboxylic acids (isobutyric acid and
isovaleric acid) formed during the oxidative deamination of the amino
acids valine and leucine, respectively, undergo oxidation,
preferentially in the longer branched chain, to yield linear
carboxylic acid fragments, which participate in the fatty acid pathway
and tricarboxylic acid cycle (Voet & Voet, 1990).
The non-endogenous substance 2-ethylbutyric acid contains an ethyl
substituent in the alpha position, which inhibits ß-oxidation and
complete metabolism to CO2 (Williams, 1959). Pathways of metabolic
detoxication include direct conjugation of the acid with glucuronic
acid, or ß-oxidation followed by conjugation. In rats and rabbits, 2-
ethylbutyric acid (Dziewiatowski et al., 1949) and 2-ethyl-1-butanol
(Kamil et al., 1953) are excreted unchanged in the urine principally
as the glucuronic acid conjugates. 2-Ethylbutyric acid is, in part,
metabolized by dogs to 2-pentanone, which is presumably derived from
ß-oxidation and subsequent decarboxylation of 2-ethyl-ß-ketobutyric
acid (Williams, 1959) (Figure 3).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Acute toxicity studies have been conducted for 23 of the 26
terpenoid esters and are summarized in Table 3.
2.3.2.2 Short-term toxicity
The results of short-term toxicity studies with three terpenoid
esters (geranyl acetate, citronellyl acetate and citronellyl
isobutyrate) and two component alcohols (geraniol and citronellol) are
described below and are summarized in Table 4.
a) Mixture of geranyl acetate and citronellyl acetate
Groups of five male and five female B6C3F1 mice were administered
0, 125, 250, 500, 1000 or 2000 mg/kg bw per day of a mixture of
geranyl acetate (71% ) and citronellyl acetate (29% ) in corn oil by
gavage daily for 14 consecutive days. The animals were observed twice
daily and weighed on day 1, after one week, and at the end of the
study. All animals were necropsied at the end of the study. Three
female mice that received 2000 mg/kg bw per day died during the study.
All the other animals survived to the end of the study. Three of five
females receiving 2000 mg/kg bw per day exhibited a thickening of the
cardiac stomach and one of five males exhibited thickening of the
duodenal wall (NTP, 1987).
Table 3. Acute toxicity studies of terpenoid esters
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Citronellyl formate rat M & F gavage 8400 Calandra, 1973
Geranyl formate rat NR gavage 5460 Weir & Wong, 1971
Neryl formate rat NR oral 5000 Moreno, 1975
Rhodinyl formate rat NR oral 5000 Moreno, 1974
Citronellyl acetate rat M & F gavage 6800 Calandra, 1973
Geranyl acetate rat M & F gavage 6330 Jenner, 1964
Neryl acetate rat M & F gavage 4550 Opdyke, 1974
Rhodinyl acetate rat M & F gavage 5000 Opdyke, 1974
Citronellyl propionate rat NR oral 5000 Moreno, 1973
Geranyl propionate rat NR oral 5000 Russell, 1973
Neryl propionate rat NR oral 5000 Moreno, 1975
Rhodinyl propionate rat NR oral 5000 Moreno, 1976
Citronellyl butyrate rat M oral 5000 Moreno, 1972
Geranyl butyrate rat M & F gavage 10660 Jenner, 1964
Rhodinyl butyrate rat NR oral 5000 Moreno, 1975
Geranyl hexanoate rat NR oral 5000 Moreno, 1975
Citronellyl isobutyrate rat NR oral 5000 Denine & Palanker,
1973
Geranyl isobutyrate rat NR oral 5000 Shelanski & Moldovan,
1973
Neryl isobutyrate rat NR oral 5000 Moreno, 1980
Rhodinyl isobutyrate rat NR oral 5000 Moreno, 1975
Geranyl isovalerate rat NR oral 5000 Levenstein, 1975
Neryl isovalerate rat NR oral 5000 Moreno, 1976
Geranyl 2-ethylbutanoate mouse NR oral >8000 Givaudan Roure, 1971
1 M = males; F = females; NR = Not reported
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of B6C3F1 mice (10/sex/group) at dose levels of 0, 125,
250, 500, 1000 or 2000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and on animals dying during the test. Seven of 10 males and
9/10 females receiving 2000 mg/kg bw per day died during the study.
Gavage errors resulted in the death of three females at lower dose
levels. Mean body weights were comparable for dosed and control
animals. Male and female mice in the 2000 mg/kg bw per day dose groups
exhibited cytoplasmic vacuolization of the liver, kidney and
myocardium. Vacuolization was the result of lipid droplets that were
present throughout the liver lobule, particularly in the periportal
region. No treatment-related histopathological lesions or other
effects were observed in the 1000 mg/kg bw per day group (NTP, 1987).
Groups of five male and five female F344/N rats were administered
0, 62, 125, 250, 500 or 1000 mg/kg bw of a mixture of geranyl acetate
(71%) and citronellyl acetate (29%) in corn oil by gavage daily for 14
consecutive days. The animals were observed twice daily and weighed on
day 1, after one week, and at the end of the study. All animals were
necropsied at the end of the study. All animals survived to the end of
the dosing period and no compound-related effects were observed at
necropsy (NTP, 1987).
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of F344/N rats (10/sex/group) at dose levels of 0, 250,
500, 1000, 2000 or 4000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and in animals dying during the test. Two out of ten males
and one out of ten females receiving 4000 mg/kg bw per day died. Mean
body weights were comparable for dosed and control animals, except for
a decrease in mean body weight gain in males and females (19 % and 8%
relative to controls, respectively) at the 4000 mg/kg bw per day dose
level. No treatment-related histopathological effects were observed at
necropsy (NTP, 1987).
Table 4. Short- and long-term toxicity studies for terpenoid esters, component terpenoid alcohols and
component aliphatic saturated carboxylic acids
Substance Species, sex1 Route Duration Repeated dose Reference
study, NOEL2
(mg/kg bw
per day)
Terpenoid esters
Geranyl acetate3 mouse, M & F oral 13 weeks 1000 NTP, 1987
rat, M & F oral 13 weeks 2000 NTP, 1987
rat, M & F oral 17 weeks 5005 Hagan et al., 1967
Citronellyl isobutyrate rat, M & F oral 90 days 14.75 Damske, 1980
Component terpenoid
alcohols
Geraniol rat, M & F oral 16 weeks 5005 Hagan et al., 1967
rat, M & F oral 27-28 weeks 505 Hagan et al., 1967
Citronellol4 rat, M & F oral 12 weeks 505 Oser, 1967
1 M = male; F = female.
2 NOEL = No-observed-effect level.
3 The test material was composed of 79% geranyl acetate and 29% citronellyl acetate.
4 The test material was an equal mixture of citronellol and geraniol.
5 The study was performed at a single dose level or multiple dose levels, none of which produced
adverse effects. The reported dose level is, therefore, not an actual NOEL, but the highest level
that produced no adverse effects.
b) Geranyl acetate
Groups of ten male and ten female Osborne-Mendel rats were
provided geranyl acetate in the diet at concentrations of 0, 1000,
2500 or 10 000 mg/kg (equivalent to an average daily intake of 0, 50,
250 or 500 mg/kg bw) for 17 weeks. The diet was prepared weekly.
Determination of the dietary concentration of geranyl acetate revealed
a weekly loss of 4%. Measurement of body weight and food intake
recorded weekly showed no significant difference between test and
control animals at any intake level. At termination, haematological
examinations revealed no difference from controls. At necropsy, no
differences were reported between major organ weights of test and
control animals. Gross examination of tissue of all animals was
unremarkable and histopathological examination of 6-8 animals, equally
represented by gender, for the high-dose group and the control group
revealed no treatment-related lesions. The NOEL was 500 mg/kg bw per
day (Hagan et al., 1967).
c) Citronellyl isobutyrate
Citronellyl isobutyrate, prepared as a spray-dried material on gum
arabic, was provided in the diet to a group of 24 male and female
Charles River rats for 90 days at a level calculated to result in the
average daily intake of 14.7 mg/kg bw. Measurements of body weight and
food intake performed on a weekly basis showed no significant
differences between test and control animals. Blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed
normal values. At week 12, a significant decrease was recorded for
haemoglobin and haematocrit values in males. Organ weights at necropsy
revealed an increased relative heart weight in males, but there was no
significant difference in absolute heart weight between test and
control males. Gross examination and histopathology revealed no
treatment-related effects (Damske et al., 1980).
d) Component alcohols
i) Geraniol
The component alcohol geraniol, formed by hydrolysis of the
corresponding geranyl esters, was provided in the diet to groups of
five male and five female Osborne-Mendel rats for 16 weeks as a
mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol
(no further details) at a dietary concentration of 10 000 mg/kg
(equivalent to an average daily intake of 500 mg/kg bw). A second
study was conducted for 27-28 weeks using a dietary concentration of
1000 mg/kg (equivalent to an average daily intake of 50 mg/kg bw).
Measurement of body weight and food intake recorded weekly showed no
significant difference between test and control animals in either
study. At termination, haematological examinations revealed normal
values. At necropsy, no differences were reported between major organ
weights of test and control animals. Gross examination of the tissue
of all animals was unremarkable. Histopathological examination of 6-8
animals, equally represented by gender, revealed no treatment-related
lesions (Hagan et al., 1967).
ii) Citronellol
The parent alcohol citronellol, as a mixture with an equal weight
of linalool, was provided in the diet to groups of 10 male and 10
female weanling rats (strain not specified) at a level calculated to
result in the average daily intake of 50 mg/kg bw for a period of 12
weeks. A significant depression in body weight and food intake in male
rats was attributed to the impalatability of the test materials at the
level administered. The efficiency of food utilization showed no
significant difference between test and control animals. There were no
significant changes in appearance, urinalysis parameters, blood
haemoglobin, liver and kidney weights, or gross pathology (Oser,
1967).
2.3.2.3 Long-term toxicity/carcinogenicity studies
a) Mice
A carcinogenicity study was conducted in which groups of 50
B6C3F1 mice of each sex were administered 0, 500 or 1000 mg/kg bw of
a mixture of geranyl acetate (71%) and citronellyl acetate (29%) in
corn oil by gavage daily, 5 days/week for 103 weeks. Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and those
found dead during the study.
Mean body weights of high-dose male and female mice were lower
than those of control groups after week 18 of the study. Survival of
male mice in the high-dose group was significantly reduced (controls,
31/50; low dose 32/50; high dose, 0/50). Survival of the high- and
low-dose groups of female mice was significantly less (p<0.001; low
dose, 0.020) than that of the control group (controls, 28/50; low
dose, 15/50; high dose, 0/50). The mortality in females appeared
already from week 15 onwards. The probable cause of death of many
females was a genital tract infection. Inflammation of the vagina,
uterus, ovaries, or multiple organs occurred in 18 control, 14
low-dose, and 2 high-dose female mice. Although the etiological agent
was not isolated, Klebsiella pneumoniae was isolated from similarly
affected mice at this laboratory in subsequent chronic studies.
Surviving male (36) and female (11) mice in the high-dose groups were
killed in a moribund condition at week 91 after an inadvertent
overdose of the test substance. Eleven other animals (3 control males,
3 low-dose males, 3 low-dose females and 2 high-dose females) were
killed by gavage accidents during the course of the study.
There was no increase in the incidence of neoplastic lesions
associated with administration of the test substance. The incidence of
non-neoplastic lesions was significantly increased in high-dose male
and female mice only, and there was an increased incidence of
cytoplasmic vacuolization of the liver in male (control, 1/50; low
dose, 7/50; high dose, 47/50) and female mice (control, 1/50; low
dose, 27/50; high dose, 46/50) and the kidney or kidney tubule in male
(control, 0/50; low dose, 0/50; high dose, 41/50) and female mice
(control, 0/50; low dose, 24/49; high dose, 37/50). Under conditions
of this study, the mixture of geranyl acetate and citronellyl acetate
was not carcinogenic for either sex of B6C3F1 mice (NTP, 1987).
Owing to the low survival rate in both sexes and the early
mortality predominantly in female mice, this study should be
considered as having limited value for a safety evaluation.
b) Rats
A carcinogenicity study was conducted in which groups of 50 F344/N
rats of each sex were administered 0, 1000 or 2000 mg/kg bw of a
mixture of geranyl acetate (71%) and citronellyl acetate (29%) in corn
oil by gavage daily, 5 days/week for 103 weeks (2000 mg/kg bw
corresponds to estimated daily dose levels of 1420 mg geranyl
acetate/kg bw and 580 mg citronellyl acetate/kg bw). Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and on those
found dead during the study.
A statistically significant decrease in mean body weight was
reported for high-dose male rats throughout the study and low- and
high-dose female rats after week 40. Reduced mean body weight gains
were dose-related. Survival of the high-dose group (18/50) of male
rats was significantly less than those of the controls (34/50;
p=0.001) and the low-dose group (29/50; p=0.003). There was no other
significant difference in survival between any group of either sex.
A positive trend (controls, 6/50; low dose, 8/50; high dose, 9/50) in
the incidence of adrenal pheochromocytomas in male rats was not
statistically significant. There was no significant increase in the
incidence of any neoplasms in high-dose male or female rats compared
to the control groups. The overall incidence of these commonly
observed adrenal pheochromocytomas in paired control groups of male
rats has been reported to be 25.1% (Haseman et al., 1986).
Under conditions of this study, geranyl acetate was not carcinogenic
for either sex of F344/N rats. The NOEL based on body weight decreases
was 1000 mg/kg bw per day (NTP, 1987).
The toxicity of the component linear and branched-chain carboxylic
acid is discussed in the monographs on saturated
aliphatic acyclic linear primary alcohols aldehydes and acids and
on saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids.
2.3.2.4 Genotoxicity
Results of in vitro and in vivo genotoxicity studies carried
out on terpenoid esters, mainly geranyl acetate, are given in Table 5.
Table 5. Genotoxicity studies for terpenoid esters
Test system Substance name Test object Test concentration1 Results Reference
In vitro
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 2000 nl/plate2 negative3 Heck et al., 1989
TA1538
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 3333 µg/plate negative3 Mortelmans et al., 1986
TA1538
Rec assay Geranyl acetate B. subtilis 17 µg/disk4 negative Oda et al., 1978
Rec assay Geranyl acetate B. subtilis 20 µg/disk negative Yoo, 1986
Rec assay Geranyl formate B. subtilis 18 µg/disk4 negative Oda et al., 1978
HGPRT gene
mutation assay Geranyl acetate CHO cells no details negative3 Flowers & Li, 1988
Gene mutation assay Geranyl acetate Human lymphoblast TK6 320 µg/ml negative5 Caspary et al., 1988
500 µg/ml negative6
Chromosomal
aberrations Geranyl acetate Chinese hamster
ovary cells 100 µg/ml negative5 Galloway et al., 1987;
150 µg/ml negative6 Tennant et al., 1987
Sister chromatid Geranyl acetate Chinese hamster
exchange ovary cells 70 µg/ml positive5,7 Galloway et al., 1987;
299 µg/ml positive6,7 Tennant et al., 1987
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes no details negative Mirsalis et al., 1983
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes 100 nl/ml negative Heck et al., 1989
Alkaline elution Geranyl acetate Rat primary hepatocytes 0.30 mM negative Storer et al., 1996
PACE/PFGE8 Geranyl acetate Rat primary hepatocytes 0.25 mM positive9 Storer et al., 1996
Table 5. Continued...
Test system Substance name Test object Test concentration1 Results Reference
In vivo
Ses linked
recessive lethal
assay Geranyl acetate Drosophila melanogaster feeding 250 ppm; negative Foureman et al., 1994
injection 50 000 ppm negative
Micronucleus assay Geranyl acetate Marrow B6C3F1 mouse 1800 mg/kg bw/ day negative Shelby et al., 1993;
bone cells for 3 days, ip Shelby & Witt, 1995
Chromosomal Geranyl acetate Marrow B6C3F1 mouse no details, ip negative Shelby & Witt, 1995
aberrations bone cells
Unscheduled DNA
synthesis Geranyl acetate Fisher F344 male rats no details negative Mirsalis et al., 1983
1 Highest ineffective dose (negative) or lowest affective dose (positive).
2 Units based on information in Table 3.
3 Both with and without metabolic activation.
4 Single dose.
5 Without metabolic activation.
6 With metabolic activation.
7 Positive results were obtained at concentrations that were cytotoxic.
8 Programmed, autonomously-controlled electrode/pulsed-field gel electrophoresis.
9 DNA fragmentation greater than that seen for 40 Gy of gamma radiation.
2.3.2.5 Reproductive toxicity
No studies on reproduction and teratogenicity have been reported.
2.3.2.6 Other relevant studies
a) Citronellyl acetate
Citronellyl acetate applied full strength to intact and abraded
rabbit skin was irritating (Calandra, 1973).
b) Neryl acetate
Neryl acetate applied full strength to intact and abraded rabbit
skin for 24 hours under occlusion was not irritating (Opdyke, 1974).
c) Rhodinyl acetate
Rhodinyl acetate applied full strength to intact and abraded skin
for 24 h under occlusion was mildly irritating (Opdyke, 1974).
d) Neryl isobutyrate
As part of an acute dermal LD50 study, the undiluted material
produced slight irritant effects in rabbit patches tested for 24 h
under occlusion at a dose of 5 g/kg (Moreno, 1980).
2.4 Observations in humans
2.4.1 Geranyl acetate
A maximization test was carried out on 25 volunteers. Geranyl
acetate was tested at a concentration of 4% in petrolatum and produced
no sensitization reactions. Hypersensitivity occurred in some
individuals (Opdyke, 1974).
2.4.2 Neryl acetate
A maximization test was carried out on 25 volunteers. Neryl
acetate was tested at a concentration of 10% in petrolatum and
produced no senstitization reactions (Kligman, 1972).
When tested at 10% in petrolatum neryl acetate produced no
irritation in a 48-h closed patch test on human subjects (Opdyke,
1974).
2.4.3 Citronellyl acetate
When tested at a concentration of 4% in petrolatum, citronellyl
acetate produced a mild irritation in a 48-h closed patch test in 25
human subjects (Opdyke, 1974).
2.4.4 Rhodinyl acetate
When tested at 12% in petrolatum, rhodinyl acetate produced no
irritation in a 48-h closed patch test on human subjects (Kligman,
1974).
A maximization test was carried out on 25 volunteers. The material
was tested at 12% concentration in petrolatum and produced no
sensitization reactions (Opdyke, 1974).
2.4.5 Neryl isobutyrate
A 48-h closed patch test at a concentration of 5% in petrolatum on
the backs of 35 volunteers produced no irritation (Epstein, 1980).
A maximization test was carried out on 35 volunteers. The material
was tested at a concentration of 5% in petrolatum and produced no
sensitization reactions (Epstein, 1980).
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: intermediary xenobiotic metabolism in animals.
Taylor and Francis, New York, pp. 81-97.
Asano, M. & Yamakawa, T. (1950) The fate of branched chain fatty acids
in the body: I. A contribution to the problem of "Hildebrandt Acid".
J. Biochem. (Tokyo), 37: 321-327.
Calandra, J.C. (1973) In: Fragrance raw materials monographs. Food
Cosmet. Toxicol., 11: 1069.
Caspary, W.J., Langerbach, R., Penman B.W., Crespi, C., Myhr, B.C., &
A.D.Mitchell(1988) The mutagenic activity of selected compounds at the
TK locus: Rodent vs. human cells. Mutat. Res., 196(1): 61-81.
Chadha, A. & Madyastha, K.M. (1982) Omega-hydroxylation of acyclic
monoterpene alcohols by rat lung microsomes. Biochem. Biophys. Res.
Commun., 108: 1271-1277.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14: 365-374.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food. Cosmet.. Toxicol., 16:
255-276.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III, 7th
ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Damske, D.R., Mecler, F.J., & Craig D.K. (1980) 90-Day toxicity study
on citronellyl isobutyrate in rats. Unpublished report to FEMA.
Denine, E.P. & Palanker, A. (1973) Unpublished report to RIVM.
Diliberto, J.J., Usha, G., & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Dispos., 16: 721-727.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T.,
& Birnbaum, L.S. (1990) Metabolism of citral, an alpha,ß-unsaturated
aldehyde, in male F344 rats. Drug Metab. Dispos., 18: 866-875.
Dziewiatowski, D.D., Venkataraman, A., & Lewis, H.B. (1949) The
metabolism of some branched-chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Epstein, W.L. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol., 26(4): 273-415.
Fischer, F.G. & Bielig, H.J. (1940) On the hydrogenation of
unsaturated substances in the animal body. Physiol. Chem., 266(1/3):
73-98.
Flowers, L.J. & Li, A.P. (1988) Evaluation of 8 non-carcinogens in the
CHO-HGPRT gene mutation system. Environ. Mol. Mutagen, 11: 34.
Foureman, P., Mason, J.M., Valencia, R., & Zimmering, S. (1994)
Chemical mutagenesis testing in Drosophila melanogaster: IX. Results
of 50 coded compounds tested for the National Toxicology Program.
Environ. Mol. Mutagen., 23: 51-63.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., & Rimpo, J.
(1987) Chromosome aberration and sister chromatid exchanges in Chinese
hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10): 1-175.
Givaudan Roure Corporation (1971) Private communication to FEMA.
Greif, N. (1974) Cutaneous safety. In: Fragrance raw materials
monographs. Food Cosmet. Toxicol., 12: 885-887.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.
Taylor, J.M., Long, E.L., Nelson, A.A., & Brouwer, J.B. (1967) Food
flavorings and compounds of related structure. Subacute and chronic
toxicity. Food Chem. Toxicol., 5: 141-157.
Haseman, J.K., Winbush, J.S., & O'Donell, M.W. Jr. (1986) Use of dual
control groups to estimate false positive rates in laboratory animals
carcinogenicity studies. Fundam. Appl. Toxicol., 7: 573- 584.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9(1): 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
IOFI (1996) Private Communication to FEMA. 7/96.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and carvone in rabbits. Xenobiotica, 19:
843-855.
Jenner, P.M. (1964) Food flavorings and compounds of related
structure: Acute oral toxicity. Food Chem. Toxicol., 2: 327-343.
Kamil, I.A., Smith, J.N., & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem.J., 53:
129-136.
Keil, H. (1974) Contact dermatitis due to oil of citronella. In:
Fragrance raw materials monographs. Food Cosmet. Toxicol., 12:
885-887.
Kligman, A.M. (1974) In : Fragrance raw materials monographs. Food
Cosmet. Toxicol., 12: 885-887.
Levenstein, I. (1975) Private communication to FEMA.
Licht, H. & Corsia, C. J. (1978) Cytochrome P-450LM2 mediated
hydroxylation of monoterpene alcohols Biochemistry, 17: 5638-5646.
Meigs, T.E., Sherwood, S.W., & Simoni, R.D. (1995) Farnesyl acetate, a
derivative of an isoprenoid of the mevalonate pathway, inhibits DNA
replication in hamster and human cells. Exp. Cell Res., 219(2):
461-470.
Mirsalis, J., Tyson, K., Beck, J., Loh, E., Steinmetz, K., Contreras,
C., Austere, L., Martin, S., & Spalding, J. (1983) Induction of
unscheduled DNA synthesis (UDS) in hepatocytes following in vitro
and in vivo treatment. Environ. Mutagen., 5(3): 482.
Moreno, O.M. (1972) Private communication to FEMA.
Moreno, O.M. (1973) Private communication to FEMA.
Moreno, O.M. (1974) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M. (1976) Private communication to FEMA.
Moreno, O.M. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol. , 26(4): 273-415.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B., &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8(7): 1-119.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: a
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Myhr, B.C. & Caspary, W.J. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutagen., 18: 51-83.
National Academy of Sciences (1987) Evaluating the safety of food
chemicals. Washington, DC.
National Toxicology Program (NTP) (1987) Carcinogenesis studies of
food grade geranyl acetate (71%) and citronellyl acetate (29%).
Research Triangle Park, NC, USA (NTP-TR-252; PB-88-2508).
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Opdyke, D.L.J. (1974) Priate communication to FEMA.
Oser, B.L. (1967) Private communication to FEMA.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D., & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14: 537-540.
Russell, T. (1973) Unpublished report to FEMA.
Shelby, M.D., Erexson, G.L., Hook, G.L., & Tice, R.R. (1993)
Evaluation of a three-exposure mouse bone marrow micronucleus
protocol: Results with 49 chemicals. Environ. Mol. Mutagen., 21(2):
160-179.
Shelanski, M.V. & Moldovan, M. (1973) Unpublished report to RIVM.
Shelby, M.D. & Witt, K.L. (1995) Comparison of results from mouse bone
marrow chromosome aberration and micronucleus tests. Environ. Mol.
Mutagen., 25: 302-313.
Stanford, L.F. Jr., Aaron, C.S., Tuman, W.G., Godeh, E.G., Matthews,
R.J., & Naismith, R.W. (1988a) Detection of mammalian cell mutagenesis
in AS52 cells. EMS Abstr., 101: 246.
Stanford, L.F. Jnr., Godeh, E.G., Tuman, W.G., Biesczad, M.J., Miller,
A, Stec, E.E., Matthews, R.J., & Naismith, R.W. (1988b) Evaluation of
eight coded noncarcinogens in the CHO/WPRT and AS52/XPRT assays.
EMS Abstr., 101: 247.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Storer, R.D., McKelvey, T.M., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W., & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution/rat hepatocyte assay for DNA damage:
improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368: 59-101.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnick, M., & Stasiewicz, S. (1987)
Prediction of chemical carinogenicity in rodents from in vitro
genetic toxicity assays. Science, 236: 933-941.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T., &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA sythesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens screening of 22 known positives and 25
noncarcinogens. Mutat. Res., 320: 189-205.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Weir, R.J. & Wong, L.C.K. (1971) Private communication to FEMA.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed.Chapman and Hall
Ltd, London, pp. 88-113.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs . J. Osaka City Med. Center, 34(3-4):
267-288.
Yoshikawa, K. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspect., 104: 1, 40-46.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Acute toxicity studies have been conducted for 23 of the 26
terpenoid esters and are summarized in Table 3.
2.3.2.2 Short-term toxicity
The results of short-term toxicity studies with three terpenoid
esters (geranyl acetate, citronellyl acetate and citronellyl
isobutyrate) and two component alcohols (geraniol and citronellol) are
described below and are summarized in Table 4.
a) Mixture of geranyl acetate and citronellyl acetate
Groups of five male and five female B6C3F1 mice were administered
0, 125, 250, 500, 1000 or 2000 mg/kg bw per day of a mixture of
geranyl acetate (71% ) and citronellyl acetate (29% ) in corn oil by
gavage daily for 14 consecutive days. The animals were observed twice
daily and weighed on day 1, after one week, and at the end of the
study. All animals were necropsied at the end of the study. Three
female mice that received 2000 mg/kg bw per day died during the study.
All the other animals survived to the end of the study. Three of five
females receiving 2000 mg/kg bw per day exhibited a thickening of the
cardiac stomach and one of five males exhibited thickening of the
duodenal wall (NTP, 1987).
Table 3. Acute toxicity studies of terpenoid esters
Substance Species Sex1 Route LD50 Reference
(mg/kg bw)
Citronellyl formate rat M & F gavage 8400 Calandra, 1973
Geranyl formate rat NR gavage 5460 Weir & Wong, 1971
Neryl formate rat NR oral 5000 Moreno, 1975
Rhodinyl formate rat NR oral 5000 Moreno, 1974
Citronellyl acetate rat M & F gavage 6800 Calandra, 1973
Geranyl acetate rat M & F gavage 6330 Jenner, 1964
Neryl acetate rat M & F gavage 4550 Opdyke, 1974
Rhodinyl acetate rat M & F gavage 5000 Opdyke, 1974
Citronellyl propionate rat NR oral 5000 Moreno, 1973
Geranyl propionate rat NR oral 5000 Russell, 1973
Neryl propionate rat NR oral 5000 Moreno, 1975
Rhodinyl propionate rat NR oral 5000 Moreno, 1976
Citronellyl butyrate rat M oral 5000 Moreno, 1972
Geranyl butyrate rat M & F gavage 10660 Jenner, 1964
Rhodinyl butyrate rat NR oral 5000 Moreno, 1975
Geranyl hexanoate rat NR oral 5000 Moreno, 1975
Citronellyl isobutyrate rat NR oral 5000 Denine & Palanker,
1973
Geranyl isobutyrate rat NR oral 5000 Shelanski & Moldovan,
1973
Neryl isobutyrate rat NR oral 5000 Moreno, 1980
Rhodinyl isobutyrate rat NR oral 5000 Moreno, 1975
Geranyl isovalerate rat NR oral 5000 Levenstein, 1975
Neryl isovalerate rat NR oral 5000 Moreno, 1976
Geranyl 2-ethylbutanoate mouse NR oral >8000 Givaudan Roure, 1971
1 M = males; F = females; NR = Not reported
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of B6C3F1 mice (10/sex/group) at dose levels of 0, 125,
250, 500, 1000 or 2000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and on animals dying during the test. Seven of 10 males and
9/10 females receiving 2000 mg/kg bw per day died during the study.
Gavage errors resulted in the death of three females at lower dose
levels. Mean body weights were comparable for dosed and control
animals. Male and female mice in the 2000 mg/kg bw per day dose groups
exhibited cytoplasmic vacuolization of the liver, kidney and
myocardium. Vacuolization was the result of lipid droplets that were
present throughout the liver lobule, particularly in the periportal
region. No treatment-related histopathological lesions or other
effects were observed in the 1000 mg/kg bw per day group (NTP, 1987).
Groups of five male and five female F344/N rats were administered
0, 62, 125, 250, 500 or 1000 mg/kg bw of a mixture of geranyl acetate
(71%) and citronellyl acetate (29%) in corn oil by gavage daily for 14
consecutive days. The animals were observed twice daily and weighed on
day 1, after one week, and at the end of the study. All animals were
necropsied at the end of the study. All animals survived to the end of
the dosing period and no compound-related effects were observed at
necropsy (NTP, 1987).
In a 13-week study, a mixture of geranyl acetate (71%) and
citronellyl acetate (29%) was administered by gavage in corn oil to
six groups of F344/N rats (10/sex/group) at dose levels of 0, 250,
500, 1000, 2000 or 4000 mg/kg bw daily (5 days/week). Animals were
checked twice daily for signs of morbidity, and body weight data were
collected weekly. Histopathological examinations were performed on
animals in the vehicle control group, animals in the 2000 mg/kg bw per
day group, and in animals dying during the test. Two out of ten males
and one out of ten females receiving 4000 mg/kg bw per day died. Mean
body weights were comparable for dosed and control animals, except for
a decrease in mean body weight gain in males and females (19 % and 8%
relative to controls, respectively) at the 4000 mg/kg bw per day dose
level. No treatment-related histopathological effects were observed at
necropsy (NTP, 1987).
Table 4. Short- and long-term toxicity studies for terpenoid esters, component terpenoid alcohols and
component aliphatic saturated carboxylic acids
Substance Species, sex1 Route Duration Repeated dose Reference
study, NOEL2
(mg/kg bw
per day)
Terpenoid esters
Geranyl acetate3 mouse, M & F oral 13 weeks 1000 NTP, 1987
rat, M & F oral 13 weeks 2000 NTP, 1987
rat, M & F oral 17 weeks 5005 Hagan et al., 1967
Citronellyl isobutyrate rat, M & F oral 90 days 14.75 Damske, 1980
Component terpenoid
alcohols
Geraniol rat, M & F oral 16 weeks 5005 Hagan et al., 1967
rat, M & F oral 27-28 weeks 505 Hagan et al., 1967
Citronellol4 rat, M & F oral 12 weeks 505 Oser, 1967
1 M = male; F = female.
2 NOEL = No-observed-effect level.
3 The test material was composed of 79% geranyl acetate and 29% citronellyl acetate.
4 The test material was an equal mixture of citronellol and geraniol.
5 The study was performed at a single dose level or multiple dose levels, none of which produced
adverse effects. The reported dose level is, therefore, not an actual NOEL, but the highest level
that produced no adverse effects.
b) Geranyl acetate
Groups of ten male and ten female Osborne-Mendel rats were
provided geranyl acetate in the diet at concentrations of 0, 1000,
2500 or 10 000 mg/kg (equivalent to an average daily intake of 0, 50,
250 or 500 mg/kg bw) for 17 weeks. The diet was prepared weekly.
Determination of the dietary concentration of geranyl acetate revealed
a weekly loss of 4%. Measurement of body weight and food intake
recorded weekly showed no significant difference between test and
control animals at any intake level. At termination, haematological
examinations revealed no difference from controls. At necropsy, no
differences were reported between major organ weights of test and
control animals. Gross examination of tissue of all animals was
unremarkable and histopathological examination of 6-8 animals, equally
represented by gender, for the high-dose group and the control group
revealed no treatment-related lesions. The NOEL was 500 mg/kg bw per
day (Hagan et al., 1967).
c) Citronellyl isobutyrate
Citronellyl isobutyrate, prepared as a spray-dried material on gum
arabic, was provided in the diet to a group of 24 male and female
Charles River rats for 90 days at a level calculated to result in the
average daily intake of 14.7 mg/kg bw. Measurements of body weight and
food intake performed on a weekly basis showed no significant
differences between test and control animals. Blood chemical
determinations and urinalysis conducted at weeks 6 and 12 revealed
normal values. At week 12, a significant decrease was recorded for
haemoglobin and haematocrit values in males. Organ weights at necropsy
revealed an increased relative heart weight in males, but there was no
significant difference in absolute heart weight between test and
control males. Gross examination and histopathology revealed no
treatment-related effects (Damske et al., 1980).
d) Component alcohols
i) Geraniol
The component alcohol geraniol, formed by hydrolysis of the
corresponding geranyl esters, was provided in the diet to groups of
five male and five female Osborne-Mendel rats for 16 weeks as a
mixture of 3,7-dimethyl-2,6-octadienol and 3,7-dimethyl-1,6-octadienol
(no further details) at a dietary concentration of 10 000 mg/kg
(equivalent to an average daily intake of 500 mg/kg bw). A second
study was conducted for 27-28 weeks using a dietary concentration of
1000 mg/kg (equivalent to an average daily intake of 50 mg/kg bw).
Measurement of body weight and food intake recorded weekly showed no
significant difference between test and control animals in either
study. At termination, haematological examinations revealed normal
values. At necropsy, no differences were reported between major organ
weights of test and control animals. Gross examination of the tissue
of all animals was unremarkable. Histopathological examination of 6-8
animals, equally represented by gender, revealed no treatment-related
lesions (Hagan et al., 1967).
ii) Citronellol
The parent alcohol citronellol, as a mixture with an equal weight
of linalool, was provided in the diet to groups of 10 male and 10
female weanling rats (strain not specified) at a level calculated to
result in the average daily intake of 50 mg/kg bw for a period of 12
weeks. A significant depression in body weight and food intake in male
rats was attributed to the impalatability of the test materials at the
level administered. The efficiency of food utilization showed no
significant difference between test and control animals. There were no
significant changes in appearance, urinalysis parameters, blood
haemoglobin, liver and kidney weights, or gross pathology (Oser,
1967).
2.3.2.3 Long-term toxicity/carcinogenicity studies
a) Mice
A carcinogenicity study was conducted in which groups of 50
B6C3F1 mice of each sex were administered 0, 500 or 1000 mg/kg bw of
a mixture of geranyl acetate (71%) and citronellyl acetate (29%) in
corn oil by gavage daily, 5 days/week for 103 weeks. Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and those
found dead during the study.
Mean body weights of high-dose male and female mice were lower
than those of control groups after week 18 of the study. Survival of
male mice in the high-dose group was significantly reduced (controls,
31/50; low dose 32/50; high dose, 0/50). Survival of the high- and
low-dose groups of female mice was significantly less (p<0.001; low
dose, 0.020) than that of the control group (controls, 28/50; low
dose, 15/50; high dose, 0/50). The mortality in females appeared
already from week 15 onwards. The probable cause of death of many
females was a genital tract infection. Inflammation of the vagina,
uterus, ovaries, or multiple organs occurred in 18 control, 14
low-dose, and 2 high-dose female mice. Although the etiological agent
was not isolated, Klebsiella pneumoniae was isolated from similarly
affected mice at this laboratory in subsequent chronic studies.
Surviving male (36) and female (11) mice in the high-dose groups were
killed in a moribund condition at week 91 after an inadvertent
overdose of the test substance. Eleven other animals (3 control males,
3 low-dose males, 3 low-dose females and 2 high-dose females) were
killed by gavage accidents during the course of the study.
There was no increase in the incidence of neoplastic lesions
associated with administration of the test substance. The incidence of
non-neoplastic lesions was significantly increased in high-dose male
and female mice only, and there was an increased incidence of
cytoplasmic vacuolization of the liver in male (control, 1/50; low
dose, 7/50; high dose, 47/50) and female mice (control, 1/50; low
dose, 27/50; high dose, 46/50) and the kidney or kidney tubule in male
(control, 0/50; low dose, 0/50; high dose, 41/50) and female mice
(control, 0/50; low dose, 24/49; high dose, 37/50). Under conditions
of this study, the mixture of geranyl acetate and citronellyl acetate
was not carcinogenic for either sex of B6C3F1 mice (NTP, 1987).
Owing to the low survival rate in both sexes and the early
mortality predominantly in female mice, this study should be
considered as having limited value for a safety evaluation.
b) Rats
A carcinogenicity study was conducted in which groups of 50 F344/N
rats of each sex were administered 0, 1000 or 2000 mg/kg bw of a
mixture of geranyl acetate (71%) and citronellyl acetate (29%) in corn
oil by gavage daily, 5 days/week for 103 weeks (2000 mg/kg bw
corresponds to estimated daily dose levels of 1420 mg geranyl
acetate/kg bw and 580 mg citronellyl acetate/kg bw). Body weights were
recorded weekly for the first 12 weeks and monthly thereafter.
Necropsies were performed on all animals at termination and on those
found dead during the study.
A statistically significant decrease in mean body weight was
reported for high-dose male rats throughout the study and low- and
high-dose female rats after week 40. Reduced mean body weight gains
were dose-related. Survival of the high-dose group (18/50) of male
rats was significantly less than those of the controls (34/50;
p=0.001) and the low-dose group (29/50; p=0.003). There was no other
significant difference in survival between any group of either sex.
A positive trend (controls, 6/50; low dose, 8/50; high dose, 9/50) in
the incidence of adrenal pheochromocytomas in male rats was not
statistically significant. There was no significant increase in the
incidence of any neoplasms in high-dose male or female rats compared
to the control groups. The overall incidence of these commonly
observed adrenal pheochromocytomas in paired control groups of male
rats has been reported to be 25.1% (Haseman et al., 1986).
Under conditions of this study, geranyl acetate was not carcinogenic
for either sex of F344/N rats. The NOEL based on body weight decreases
was 1000 mg/kg bw per day (NTP, 1987).
The toxicity of the component linear and branched-chain carboxylic
acid is discussed in the monographs on saturated
aliphatic acyclic linear primary alcohols aldehydes and acids and
on saturated aliphatic acyclic branched-chain primary alcohols,
aldehydes and acids.
2.3.2.4 Genotoxicity
Results of in vitro and in vivo genotoxicity studies carried
out on terpenoid esters, mainly geranyl acetate, are given in Table 5.
Table 5. Genotoxicity studies for terpenoid esters
Test system Substance name Test object Test concentration1 Results Reference
In vitro
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 2000 nl/plate2 negative3 Heck et al., 1989
TA1538
Ames test Geranyl acetate S. typhimurium TA98,
TA100, TA1535, TA1537, 3333 µg/plate negative3 Mortelmans et al., 1986
TA1538
Rec assay Geranyl acetate B. subtilis 17 µg/disk4 negative Oda et al., 1978
Rec assay Geranyl acetate B. subtilis 20 µg/disk negative Yoo, 1986
Rec assay Geranyl formate B. subtilis 18 µg/disk4 negative Oda et al., 1978
HGPRT gene
mutation assay Geranyl acetate CHO cells no details negative3 Flowers & Li, 1988
Gene mutation assay Geranyl acetate Human lymphoblast TK6 320 µg/ml negative5 Caspary et al., 1988
500 µg/ml negative6
Chromosomal
aberrations Geranyl acetate Chinese hamster
ovary cells 100 µg/ml negative5 Galloway et al., 1987;
150 µg/ml negative6 Tennant et al., 1987
Sister chromatid Geranyl acetate Chinese hamster
exchange ovary cells 70 µg/ml positive5,7 Galloway et al., 1987;
299 µg/ml positive6,7 Tennant et al., 1987
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes no details negative Mirsalis et al., 1983
Unscheduled DNA
synthesis Geranyl acetate Rat primary hepatocytes 100 nl/ml negative Heck et al., 1989
Alkaline elution Geranyl acetate Rat primary hepatocytes 0.30 mM negative Storer et al., 1996
PACE/PFGE8 Geranyl acetate Rat primary hepatocytes 0.25 mM positive9 Storer et al., 1996
Table 5. Continued...
Test system Substance name Test object Test concentration1 Results Reference
In vivo
Ses linked
recessive lethal
assay Geranyl acetate Drosophila melanogaster feeding 250 ppm; negative Foureman et al., 1994
injection 50 000 ppm negative
Micronucleus assay Geranyl acetate Marrow B6C3F1 mouse 1800 mg/kg bw/ day negative Shelby et al., 1993;
bone cells for 3 days, ip Shelby & Witt, 1995
Chromosomal Geranyl acetate Marrow B6C3F1 mouse no details, ip negative Shelby & Witt, 1995
aberrations bone cells
Unscheduled DNA
synthesis Geranyl acetate Fisher F344 male rats no details negative Mirsalis et al., 1983
1 Highest ineffective dose (negative) or lowest affective dose (positive).
2 Units based on information in Table 3.
3 Both with and without metabolic activation.
4 Single dose.
5 Without metabolic activation.
6 With metabolic activation.
7 Positive results were obtained at concentrations that were cytotoxic.
8 Programmed, autonomously-controlled electrode/pulsed-field gel electrophoresis.
9 DNA fragmentation greater than that seen for 40 Gy of gamma radiation.
2.3.2.5 Reproductive toxicity
No studies on reproduction and teratogenicity have been reported.
2.3.2.6 Other relevant studies
a) Citronellyl acetate
Citronellyl acetate applied full strength to intact and abraded
rabbit skin was irritating (Calandra, 1973).
b) Neryl acetate
Neryl acetate applied full strength to intact and abraded rabbit
skin for 24 hours under occlusion was not irritating (Opdyke, 1974).
c) Rhodinyl acetate
Rhodinyl acetate applied full strength to intact and abraded skin
for 24 h under occlusion was mildly irritating (Opdyke, 1974).
d) Neryl isobutyrate
As part of an acute dermal LD50 study, the undiluted material
produced slight irritant effects in rabbit patches tested for 24 h
under occlusion at a dose of 5 g/kg (Moreno, 1980).
2.4 Observations in humans
2.4.1 Geranyl acetate
A maximization test was carried out on 25 volunteers. Geranyl
acetate was tested at a concentration of 4% in petrolatum and produced
no sensitization reactions. Hypersensitivity occurred in some
individuals (Opdyke, 1974).
2.4.2 Neryl acetate
A maximization test was carried out on 25 volunteers. Neryl
acetate was tested at a concentration of 10% in petrolatum and
produced no senstitization reactions (Kligman, 1972).
When tested at 10% in petrolatum neryl acetate produced no
irritation in a 48-h closed patch test on human subjects (Opdyke,
1974).
2.4.3 Citronellyl acetate
When tested at a concentration of 4% in petrolatum, citronellyl
acetate produced a mild irritation in a 48-h closed patch test in 25
human subjects (Opdyke, 1974).
2.4.4 Rhodinyl acetate
When tested at 12% in petrolatum, rhodinyl acetate produced no
irritation in a 48-h closed patch test on human subjects (Kligman,
1974).
A maximization test was carried out on 25 volunteers. The material
was tested at 12% concentration in petrolatum and produced no
sensitization reactions (Opdyke, 1974).
2.4.5 Neryl isobutyrate
A 48-h closed patch test at a concentration of 5% in petrolatum on
the backs of 35 volunteers produced no irritation (Epstein, 1980).
A maximization test was carried out on 35 volunteers. The material
was tested at a concentration of 5% in petrolatum and produced no
sensitization reactions (Epstein, 1980).
3. REFERENCES
Anders, M.W. (1989) Biotransformation and bioactivation of xenobiotics
by the kidney. In: intermediary xenobiotic metabolism in animals.
Taylor and Francis, New York, pp. 81-97.
Asano, M. & Yamakawa, T. (1950) The fate of branched chain fatty acids
in the body: I. A contribution to the problem of "Hildebrandt Acid".
J. Biochem. (Tokyo), 37: 321-327.
Calandra, J.C. (1973) In: Fragrance raw materials monographs. Food
Cosmet. Toxicol., 11: 1069.
Caspary, W.J., Langerbach, R., Penman B.W., Crespi, C., Myhr, B.C., &
A.D.Mitchell(1988) The mutagenic activity of selected compounds at the
TK locus: Rodent vs. human cells. Mutat. Res., 196(1): 61-81.
Chadha, A. & Madyastha, K.M. (1982) Omega-hydroxylation of acyclic
monoterpene alcohols by rat lung microsomes. Biochem. Biophys. Res.
Commun., 108: 1271-1277.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14: 365-374.
Cramer, G.M., Ford, R.A., & Hall, R.L. (1978) Estimation of toxic
hazard - a decision tree approach. Food. Cosmet.. Toxicol., 16:
255-276.
CIVO-TNO (1994) In: Maarse, H. & Visscher, C.A. ed. Volatile
components in food: Qualitative and quantitative data, Volume III, 7th
ed. Centraal Instituut Voor Voedingsonderzoek TNO, Zeist, The
Netherlands.
Damske, D.R., Mecler, F.J., & Craig D.K. (1980) 90-Day toxicity study
on citronellyl isobutyrate in rats. Unpublished report to FEMA.
Denine, E.P. & Palanker, A. (1973) Unpublished report to RIVM.
Diliberto, J.J., Usha, G., & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Dispos., 16: 721-727.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, G., Burka, L.T.,
& Birnbaum, L.S. (1990) Metabolism of citral, an alpha,ß-unsaturated
aldehyde, in male F344 rats. Drug Metab. Dispos., 18: 866-875.
Dziewiatowski, D.D., Venkataraman, A., & Lewis, H.B. (1949) The
metabolism of some branched-chain aliphatic acids. J. Biol. Chem.,
178: 169-177.
Epstein, W.L. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol., 26(4): 273-415.
Fischer, F.G. & Bielig, H.J. (1940) On the hydrogenation of
unsaturated substances in the animal body. Physiol. Chem., 266(1/3):
73-98.
Flowers, L.J. & Li, A.P. (1988) Evaluation of 8 non-carcinogens in the
CHO-HGPRT gene mutation system. Environ. Mol. Mutagen, 11: 34.
Foureman, P., Mason, J.M., Valencia, R., & Zimmering, S. (1994)
Chemical mutagenesis testing in Drosophila melanogaster: IX. Results
of 50 coded compounds tested for the National Toxicology Program.
Environ. Mol. Mutagen., 23: 51-63.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon, C., Bloom, A.D., Nakamura, F., Ahmed, M., Duk, S., & Rimpo, J.
(1987) Chromosome aberration and sister chromatid exchanges in Chinese
hamster ovary cells: Evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10): 1-175.
Givaudan Roure Corporation (1971) Private communication to FEMA.
Greif, N. (1974) Cutaneous safety. In: Fragrance raw materials
monographs. Food Cosmet. Toxicol., 12: 885-887.
Grundschober, F. (1977) Toxicological assessment of flavoring esters.
Toxicology, 8: 387-390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I.
Taylor, J.M., Long, E.L., Nelson, A.A., & Brouwer, J.B. (1967) Food
flavorings and compounds of related structure. Subacute and chronic
toxicity. Food Chem. Toxicol., 5: 141-157.
Haseman, J.K., Winbush, J.S., & O'Donell, M.W. Jr. (1986) Use of dual
control groups to estimate false positive rates in laboratory animals
carcinogenicity studies. Fundam. Appl. Toxicol., 7: 573- 584.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr B., &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9(1): 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jakoby, W.B.
ed. Enzymatic basis of detoxication, 2nd ed. Academic Press, New York,
pp. 291-323.
IOFI (1996) Private Communication to FEMA. 7/96.
Ishida, T., Toyota, M., & Asakawa, Y. (1989) Terpenoid
biotransformation in mammals. V. Metabolism of (+)-citronellal,
7-hydroxycitronellal, citral, (-)-perillaldehyde, (-)-myrtenal,
cuminaldehyde, thujone, and carvone in rabbits. Xenobiotica, 19:
843-855.
Jenner, P.M. (1964) Food flavorings and compounds of related
structure: Acute oral toxicity. Food Chem. Toxicol., 2: 327-343.
Kamil, I.A., Smith, J.N., & Williams, R.T. (1953) Studies in
detoxication. 46. The metabolism of aliphatic alcohols. The glucuronic
acid conjugation of acyclic aliphatic alcohols. Biochem.J., 53:
129-136.
Keil, H. (1974) Contact dermatitis due to oil of citronella. In:
Fragrance raw materials monographs. Food Cosmet. Toxicol., 12:
885-887.
Kligman, A.M. (1974) In : Fragrance raw materials monographs. Food
Cosmet. Toxicol., 12: 885-887.
Levenstein, I. (1975) Private communication to FEMA.
Licht, H. & Corsia, C. J. (1978) Cytochrome P-450LM2 mediated
hydroxylation of monoterpene alcohols Biochemistry, 17: 5638-5646.
Meigs, T.E., Sherwood, S.W., & Simoni, R.D. (1995) Farnesyl acetate, a
derivative of an isoprenoid of the mevalonate pathway, inhibits DNA
replication in hamster and human cells. Exp. Cell Res., 219(2):
461-470.
Mirsalis, J., Tyson, K., Beck, J., Loh, E., Steinmetz, K., Contreras,
C., Austere, L., Martin, S., & Spalding, J. (1983) Induction of
unscheduled DNA synthesis (UDS) in hepatocytes following in vitro
and in vivo treatment. Environ. Mutagen., 5(3): 482.
Moreno, O.M. (1972) Private communication to FEMA.
Moreno, O.M. (1973) Private communication to FEMA.
Moreno, O.M. (1974) Private communication to FEMA.
Moreno, O.M. (1975) Private communication to FEMA.
Moreno, O.M. (1976) Private communication to FEMA.
Moreno, O.M. (1980) Neryl isobutyrate. Cited in Ford, R.A., Letizia,
C., & Api, A.M. (1988) Monographs on fragrance raw materials. Food
Chem. Toxicol. , 26(4): 273-415.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B., &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutagen., 8(7): 1-119.
Munro, I.C., Ford, R.A., Kennepohl, E., & Spenger, J.G. (1996)
Correlation of structural class with no-observed-effect levels: a
proposal for establishing a threshold of concern. Food Chem.
Toxicol., 34: 829-867.
Myhr, B.C. & Caspary, W.J. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutagen., 18: 51-83.
National Academy of Sciences (1987) Evaluating the safety of food
chemicals. Washington, DC.
National Toxicology Program (NTP) (1987) Carcinogenesis studies of
food grade geranyl acetate (71%) and citronellyl acetate (29%).
Research Triangle Park, NC, USA (NTP-TR-252; PB-88-2508).
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T., & Kunita,
N. (1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen, 9: 177-181.
Opdyke, D.L.J. (1974) Priate communication to FEMA.
Oser, B.L. (1967) Private communication to FEMA.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D., & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14: 537-540.
Russell, T. (1973) Unpublished report to FEMA.
Shelby, M.D., Erexson, G.L., Hook, G.L., & Tice, R.R. (1993)
Evaluation of a three-exposure mouse bone marrow micronucleus
protocol: Results with 49 chemicals. Environ. Mol. Mutagen., 21(2):
160-179.
Shelanski, M.V. & Moldovan, M. (1973) Unpublished report to RIVM.
Shelby, M.D. & Witt, K.L. (1995) Comparison of results from mouse bone
marrow chromosome aberration and micronucleus tests. Environ. Mol.
Mutagen., 25: 302-313.
Stanford, L.F. Jr., Aaron, C.S., Tuman, W.G., Godeh, E.G., Matthews,
R.J., & Naismith, R.W. (1988a) Detection of mammalian cell mutagenesis
in AS52 cells. EMS Abstr., 101: 246.
Stanford, L.F. Jnr., Godeh, E.G., Tuman, W.G., Biesczad, M.J., Miller,
A, Stec, E.E., Matthews, R.J., & Naismith, R.W. (1988b) Evaluation of
eight coded noncarcinogens in the CHO/WPRT and AS52/XPRT assays.
EMS Abstr., 101: 247.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food
predominance of flavoring materials. Perfum. Flavor., 12: 27.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23: 857-860.
Storer, R.D., McKelvey, T.M., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W., & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution/rat hepatocyte assay for DNA damage:
improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368: 59-101.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnick, M., & Stasiewicz, S. (1987)
Prediction of chemical carinogenicity in rodents from in vitro
genetic toxicity assays. Science, 236: 933-941.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T., &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA sythesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens screening of 22 known positives and 25
noncarcinogens. Mutat. Res., 320: 189-205.
Voet, D. & Voet, J.G. (1990) Biochemistry. John Wiley & Sons, New
York.
Weir, R.J. & Wong, L.C.K. (1971) Private communication to FEMA.
Williams, R.T. (1959) Detoxication mechanisms, 2nd ed.Chapman and Hall
Ltd, London, pp. 88-113.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs . J. Osaka City Med. Center, 34(3-4):
267-288.
Yoshikawa, K. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspect., 104: 1, 40-46.
