
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
TOXICOLOGICAL EVALUATION OF CERTAIN
VETERINARY DRUG RESIDUES IN FOOD
WHO FOOD ADDITIVES SERIES 41
Prepared by:
The 50th meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1998
AZAPERONE (addendum)
First draft prepared by
G. Kirby, L. Ritter
Canadian Network of Toxicology Centres
Department of Environmental Biology
University of Guelph, Guelph, Ontario, Canada
and
Dr L.-E. Appelgren
Department of Pharmacology and Toxicology
Faculty of Veterinary Medicine
The Swedish University of Agricultural Science, Biomedical Centre,
Uppsala, Sweden
1. Explanation
2. Biological studies
2.1 Biotransformation
2.2 Structural similarity to known carcinogens
2.3 Reproductive toxicity
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Azaperone, a butyrophenone neuroleptic tranquillizer, was
reviewed at the thirty-eighth and forty-third meetings of the
Committee (Annex 1, references 97 and 113). A temporary ADI of 0-3
µg/kg bw was established at the forty-third meeting, which requested
the following information before subsequent review:
1. Results of studies to determine the genotoxic potential of the
metabolites of azaperone, which have been reported to be
mutagenic in Salmonella spp.
2. Evidence to support the claim that azaperone or its degradation
products are not structurally similar to known carcinogens
3. Results of a study to assess the effects of azaperone on
reproduction and fertility in male laboratory animals.
This monograph addendum summarizes the data that have become available
since the previous evaluation.
2. BIOLOGICAL STUDIES
2.1 Biotransformation
No further studies of mutagenicity were carried out, as the
results of tests in S. typhimurim with metabolic activation provided
by rat liver microsomes (Ames' test) were considered to reflect the
mutagenic potential of azaperone and its metabolites. To support this
statement, the biotransformation of azaperone was studied in vitro
with microsomal fractions from the livers of Arochlor 1254-treated
(500 mg/kg bw) male Wistar rats in order to identify the metabolites
formed under the conditions of Ames' test. Biotransformation was
assessed by incubating 14C-azaperone (0.01, 0.1, 0.5, or 2.0
mmol/plate) with rat liver fractions in buffer containing histidine,
biotin, nutrient broth and a NADPH generating system at 37°C for 30
and 120 min. Samples were analysed by radio-high-performance liquid
chromatography, and the major metabolites were identified by
comparison of the chromatograms with those for unlabelled reference
compounds and by liquid scintillation counter and mass spectrometry
of representative samples. Five metabolites were characterized, which,
with unmetabolized azaperone, corresponded to 91œ100% of the total
radiolabel applied. The main metabolite was an oxidized pyridinyl
derivative. The other metabolites, which occurred in small quantities,
were identified as the nor-piperazine derivative, the N-oxide, the
alcohol metabolite (azaperol), and a second oxidized pyridinyl
derivative (Vermeir, 1997). The Committee noted that almost all of the
primary metabolites previously found in vivo in rats and pigs were
produced under the conditions of Ames' test and concluded that the
mutagenic potential of azaperone and its metabolites in bacteria had
been adequately evaluated in the previously conducted tests and
particularly in the second, more throrough study described in the
previous evaluation, in which the presence of metabolites was
questioned. In view of this new information, the weight of evidence is
that azaperone is unlikely to be genotoxic.
2.2 Structural similarity to known carcinogens
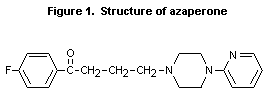
A brief discussion of the lack of similarity of azaperone and its
metabolites with known carcinogens was included in the previous
evaluation. It was noted that the only reactive subgroup on azaperone,
a neuroleptic of the butyro-phenone class, and its metabolites is
pyridine, a heterocyclic moiety (Sanderson & Earnshaw, 1991; see
Figure 1). Moreover, the report noted that human neuroleptics of the
same class had no structural similarity to known carcinogens. The
argument was reinforced by the lack of genotoxicity for azaperone and
other butyrophenones, including haloperidol, pimozide, bromperidol,
and bromperidol decanoate. Finally, apart from unspecified
prolactin-mediated effects on the pituitary and mammary gland, no
evidence of carcinogenicity had been found for the neuroleptics
haloperidol, pimozide, and bromperidol (references were not included).
Mammary gland stimulation is an epigenetic change induced by dopamine
antagonists at doses lower than those required for promotion in
prolactin-releasing tumours. Azaperone, however, is a relatively weak
prolactin-releasing agent in rats, stimulating the mammary gland at
doses > 40 mg/kg bw. There is therefore no evidence that azaperone
and its degradation products are similar to known carcinogens.
 2.3 Reproductive toxicity
Studies were conducted to evaluate the effect of azaperone on
reproduction and fertility in male rats. Groups of 24 male Wistar rats
were given doses of 5, 20, or 80 mg/kg bw daily by gavage for 74 days.
Signs of toxicity (severe sedation and ptosis, decreased body weight
and feed consumption) were observed at the highest dose throughout
treatment, and slight to moderate sedation and ptosis were also seen
at the intermediate dose. Mild, possibly drug-related haematological
changes (decreased thrombocytes) were seen, with alterations in serum
biochemistry (increased chloride and decreased calcium, total protein,
albumin, cholesterol, triglycerides, and phopholipids). The weight of
the thymus was slightly increased, and that of the adrenal glands was
decreased. Copulation and fertility rates and pre-coital intervals
were comparable in groups at different doses, and maternal and litter
parameters were not affected. No adverse effects on body weight, food
consumption, or number of corpora lutea were observed in untreated
females bred to treated males. Furthermore, embryofetal development
(i.e. numbers of live and dead fetuses, mean litter size, number of
implantations, number of resorptions, and pre- and post-implantation
loss) was not adversely affected. There were no dose-related
teratogenic effects in the offspring of untreated females mated with
male rats at the highest dose. It was concluded that azaperone had no
adverse effects on fertility in males or on embryofetal development in
the offspring of untreated females bred to treated males. The NOAEL
was 5 mg/kg bw per day on the basis of paternal toxicity (Dom, 1997).
3. COMMENTS
The Committee considered new studies to address the issues that
had been raised at the forty-third meeting. The studies were carried
out according to appropriate standards for study conduct and
protocol. A study was performed to address the issue of the genotoxic
potential of azaperone metabolites. Various concentrations of
radiolabelled azaperone were incubated with 9000 × g fractions
prepared from the livers of Arochlor-treated rats to determine the
metabolites formed under the conditions used in Ames' test. The
metabolites were isolated and found to be similar to those produced in
the target species. The Committee concluded that the results of the
Ames' tests with azaperone that were reviewed at the thirty-eighth and
forty-third meetings were adequate to assess the genotoxic potential
of azaperone and its metabolites in bacteria. Taking into account the
results of all of the genotoxicity studies performed, the Committee
concluded that azaperone is unlikely to be genotoxic. The Committee
reviewed a brief report on the lack of structural similarity of
azaperone and its metabolites with known carcinogens. It was noted
that azaperone, a neuroleptic of the butyrophenone class, and its
metabolites contain no reactive subgroups. The argument was reinforced
by the lack of evidence for genotoxicity in a battery of studies. The
Committee concluded that azaperone is unlikely to be carcinogenic.
Studies were conducted to evaluate the effects of azaperone on
reproduction and fertility in male rats. Azaperone was administered by
gavage daily to male rats for 74 days at doses of 5, 20, or 80 mg/kg
bw per day. Signs of toxicity, including severe sedation and ptosis,
decreased body weight and feed consumption, were observed in animals
at the highest dose throughout treatment. Slight to moderate sedation
and ptosis were seen at the intermediate dose. Copulation and
fertility rates and pre-coital intervals were comparable in all
groups. There were no adverse effects on male fertility or on
embryofetal development in the offspring of untreated females mated to
treated males. The NOEL was 5 mg/kg bw per day on the basis of
paternal toxicity.
4. EVALUATION
The Committee concluded that the pharmacological effects of
azaperone are the most relevant for determining an ADI. Therefore, an
ADI of 0œ6 µg/kg bw was established on the basis of the NOAEL of 630
µg/kg bw for neurobehavioural effects in dogs after oral
administration (as recommended by the forty-third meeting of the
Committee) and a safety factor of 100.
5. REFERENCES
Dom, P. (1987) Study on male fertility and on effects on embryo-foetal
development in SPF Wistar rats with R001929 by oral gavage (Report No.
R001929). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
Sanderson, D.M. & Earnshaw, C.G. (1991) Computer prediction of
possible toxic action from chemical structure; the DEREK system.
Hum. Exp. Toxicol., 10, 261-273.
Vermeir, M. (1997) A study on the metabolism of azaperone in liver
9000g supernatant fractions of Arochlor pretreated rats (Report No.
R001929/FK2533). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
2.3 Reproductive toxicity
Studies were conducted to evaluate the effect of azaperone on
reproduction and fertility in male rats. Groups of 24 male Wistar rats
were given doses of 5, 20, or 80 mg/kg bw daily by gavage for 74 days.
Signs of toxicity (severe sedation and ptosis, decreased body weight
and feed consumption) were observed at the highest dose throughout
treatment, and slight to moderate sedation and ptosis were also seen
at the intermediate dose. Mild, possibly drug-related haematological
changes (decreased thrombocytes) were seen, with alterations in serum
biochemistry (increased chloride and decreased calcium, total protein,
albumin, cholesterol, triglycerides, and phopholipids). The weight of
the thymus was slightly increased, and that of the adrenal glands was
decreased. Copulation and fertility rates and pre-coital intervals
were comparable in groups at different doses, and maternal and litter
parameters were not affected. No adverse effects on body weight, food
consumption, or number of corpora lutea were observed in untreated
females bred to treated males. Furthermore, embryofetal development
(i.e. numbers of live and dead fetuses, mean litter size, number of
implantations, number of resorptions, and pre- and post-implantation
loss) was not adversely affected. There were no dose-related
teratogenic effects in the offspring of untreated females mated with
male rats at the highest dose. It was concluded that azaperone had no
adverse effects on fertility in males or on embryofetal development in
the offspring of untreated females bred to treated males. The NOAEL
was 5 mg/kg bw per day on the basis of paternal toxicity (Dom, 1997).
3. COMMENTS
The Committee considered new studies to address the issues that
had been raised at the forty-third meeting. The studies were carried
out according to appropriate standards for study conduct and
protocol. A study was performed to address the issue of the genotoxic
potential of azaperone metabolites. Various concentrations of
radiolabelled azaperone were incubated with 9000 × g fractions
prepared from the livers of Arochlor-treated rats to determine the
metabolites formed under the conditions used in Ames' test. The
metabolites were isolated and found to be similar to those produced in
the target species. The Committee concluded that the results of the
Ames' tests with azaperone that were reviewed at the thirty-eighth and
forty-third meetings were adequate to assess the genotoxic potential
of azaperone and its metabolites in bacteria. Taking into account the
results of all of the genotoxicity studies performed, the Committee
concluded that azaperone is unlikely to be genotoxic. The Committee
reviewed a brief report on the lack of structural similarity of
azaperone and its metabolites with known carcinogens. It was noted
that azaperone, a neuroleptic of the butyrophenone class, and its
metabolites contain no reactive subgroups. The argument was reinforced
by the lack of evidence for genotoxicity in a battery of studies. The
Committee concluded that azaperone is unlikely to be carcinogenic.
Studies were conducted to evaluate the effects of azaperone on
reproduction and fertility in male rats. Azaperone was administered by
gavage daily to male rats for 74 days at doses of 5, 20, or 80 mg/kg
bw per day. Signs of toxicity, including severe sedation and ptosis,
decreased body weight and feed consumption, were observed in animals
at the highest dose throughout treatment. Slight to moderate sedation
and ptosis were seen at the intermediate dose. Copulation and
fertility rates and pre-coital intervals were comparable in all
groups. There were no adverse effects on male fertility or on
embryofetal development in the offspring of untreated females mated to
treated males. The NOEL was 5 mg/kg bw per day on the basis of
paternal toxicity.
4. EVALUATION
The Committee concluded that the pharmacological effects of
azaperone are the most relevant for determining an ADI. Therefore, an
ADI of 0œ6 µg/kg bw was established on the basis of the NOAEL of 630
µg/kg bw for neurobehavioural effects in dogs after oral
administration (as recommended by the forty-third meeting of the
Committee) and a safety factor of 100.
5. REFERENCES
Dom, P. (1987) Study on male fertility and on effects on embryo-foetal
development in SPF Wistar rats with R001929 by oral gavage (Report No.
R001929). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
Sanderson, D.M. & Earnshaw, C.G. (1991) Computer prediction of
possible toxic action from chemical structure; the DEREK system.
Hum. Exp. Toxicol., 10, 261-273.
Vermeir, M. (1997) A study on the metabolism of azaperone in liver
9000g supernatant fractions of Arochlor pretreated rats (Report No.
R001929/FK2533). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
See Also:
Toxicological Abbreviations
Azaperone (WHO Food Additives Series 29)
Azaperone (WHO Food Additives Series 34)
AZAPERONE (JECFA Evaluation)
 2.3 Reproductive toxicity
Studies were conducted to evaluate the effect of azaperone on
reproduction and fertility in male rats. Groups of 24 male Wistar rats
were given doses of 5, 20, or 80 mg/kg bw daily by gavage for 74 days.
Signs of toxicity (severe sedation and ptosis, decreased body weight
and feed consumption) were observed at the highest dose throughout
treatment, and slight to moderate sedation and ptosis were also seen
at the intermediate dose. Mild, possibly drug-related haematological
changes (decreased thrombocytes) were seen, with alterations in serum
biochemistry (increased chloride and decreased calcium, total protein,
albumin, cholesterol, triglycerides, and phopholipids). The weight of
the thymus was slightly increased, and that of the adrenal glands was
decreased. Copulation and fertility rates and pre-coital intervals
were comparable in groups at different doses, and maternal and litter
parameters were not affected. No adverse effects on body weight, food
consumption, or number of corpora lutea were observed in untreated
females bred to treated males. Furthermore, embryofetal development
(i.e. numbers of live and dead fetuses, mean litter size, number of
implantations, number of resorptions, and pre- and post-implantation
loss) was not adversely affected. There were no dose-related
teratogenic effects in the offspring of untreated females mated with
male rats at the highest dose. It was concluded that azaperone had no
adverse effects on fertility in males or on embryofetal development in
the offspring of untreated females bred to treated males. The NOAEL
was 5 mg/kg bw per day on the basis of paternal toxicity (Dom, 1997).
3. COMMENTS
The Committee considered new studies to address the issues that
had been raised at the forty-third meeting. The studies were carried
out according to appropriate standards for study conduct and
protocol. A study was performed to address the issue of the genotoxic
potential of azaperone metabolites. Various concentrations of
radiolabelled azaperone were incubated with 9000 × g fractions
prepared from the livers of Arochlor-treated rats to determine the
metabolites formed under the conditions used in Ames' test. The
metabolites were isolated and found to be similar to those produced in
the target species. The Committee concluded that the results of the
Ames' tests with azaperone that were reviewed at the thirty-eighth and
forty-third meetings were adequate to assess the genotoxic potential
of azaperone and its metabolites in bacteria. Taking into account the
results of all of the genotoxicity studies performed, the Committee
concluded that azaperone is unlikely to be genotoxic. The Committee
reviewed a brief report on the lack of structural similarity of
azaperone and its metabolites with known carcinogens. It was noted
that azaperone, a neuroleptic of the butyrophenone class, and its
metabolites contain no reactive subgroups. The argument was reinforced
by the lack of evidence for genotoxicity in a battery of studies. The
Committee concluded that azaperone is unlikely to be carcinogenic.
Studies were conducted to evaluate the effects of azaperone on
reproduction and fertility in male rats. Azaperone was administered by
gavage daily to male rats for 74 days at doses of 5, 20, or 80 mg/kg
bw per day. Signs of toxicity, including severe sedation and ptosis,
decreased body weight and feed consumption, were observed in animals
at the highest dose throughout treatment. Slight to moderate sedation
and ptosis were seen at the intermediate dose. Copulation and
fertility rates and pre-coital intervals were comparable in all
groups. There were no adverse effects on male fertility or on
embryofetal development in the offspring of untreated females mated to
treated males. The NOEL was 5 mg/kg bw per day on the basis of
paternal toxicity.
4. EVALUATION
The Committee concluded that the pharmacological effects of
azaperone are the most relevant for determining an ADI. Therefore, an
ADI of 0œ6 µg/kg bw was established on the basis of the NOAEL of 630
µg/kg bw for neurobehavioural effects in dogs after oral
administration (as recommended by the forty-third meeting of the
Committee) and a safety factor of 100.
5. REFERENCES
Dom, P. (1987) Study on male fertility and on effects on embryo-foetal
development in SPF Wistar rats with R001929 by oral gavage (Report No.
R001929). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
Sanderson, D.M. & Earnshaw, C.G. (1991) Computer prediction of
possible toxic action from chemical structure; the DEREK system.
Hum. Exp. Toxicol., 10, 261-273.
Vermeir, M. (1997) A study on the metabolism of azaperone in liver
9000g supernatant fractions of Arochlor pretreated rats (Report No.
R001929/FK2533). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
2.3 Reproductive toxicity
Studies were conducted to evaluate the effect of azaperone on
reproduction and fertility in male rats. Groups of 24 male Wistar rats
were given doses of 5, 20, or 80 mg/kg bw daily by gavage for 74 days.
Signs of toxicity (severe sedation and ptosis, decreased body weight
and feed consumption) were observed at the highest dose throughout
treatment, and slight to moderate sedation and ptosis were also seen
at the intermediate dose. Mild, possibly drug-related haematological
changes (decreased thrombocytes) were seen, with alterations in serum
biochemistry (increased chloride and decreased calcium, total protein,
albumin, cholesterol, triglycerides, and phopholipids). The weight of
the thymus was slightly increased, and that of the adrenal glands was
decreased. Copulation and fertility rates and pre-coital intervals
were comparable in groups at different doses, and maternal and litter
parameters were not affected. No adverse effects on body weight, food
consumption, or number of corpora lutea were observed in untreated
females bred to treated males. Furthermore, embryofetal development
(i.e. numbers of live and dead fetuses, mean litter size, number of
implantations, number of resorptions, and pre- and post-implantation
loss) was not adversely affected. There were no dose-related
teratogenic effects in the offspring of untreated females mated with
male rats at the highest dose. It was concluded that azaperone had no
adverse effects on fertility in males or on embryofetal development in
the offspring of untreated females bred to treated males. The NOAEL
was 5 mg/kg bw per day on the basis of paternal toxicity (Dom, 1997).
3. COMMENTS
The Committee considered new studies to address the issues that
had been raised at the forty-third meeting. The studies were carried
out according to appropriate standards for study conduct and
protocol. A study was performed to address the issue of the genotoxic
potential of azaperone metabolites. Various concentrations of
radiolabelled azaperone were incubated with 9000 × g fractions
prepared from the livers of Arochlor-treated rats to determine the
metabolites formed under the conditions used in Ames' test. The
metabolites were isolated and found to be similar to those produced in
the target species. The Committee concluded that the results of the
Ames' tests with azaperone that were reviewed at the thirty-eighth and
forty-third meetings were adequate to assess the genotoxic potential
of azaperone and its metabolites in bacteria. Taking into account the
results of all of the genotoxicity studies performed, the Committee
concluded that azaperone is unlikely to be genotoxic. The Committee
reviewed a brief report on the lack of structural similarity of
azaperone and its metabolites with known carcinogens. It was noted
that azaperone, a neuroleptic of the butyrophenone class, and its
metabolites contain no reactive subgroups. The argument was reinforced
by the lack of evidence for genotoxicity in a battery of studies. The
Committee concluded that azaperone is unlikely to be carcinogenic.
Studies were conducted to evaluate the effects of azaperone on
reproduction and fertility in male rats. Azaperone was administered by
gavage daily to male rats for 74 days at doses of 5, 20, or 80 mg/kg
bw per day. Signs of toxicity, including severe sedation and ptosis,
decreased body weight and feed consumption, were observed in animals
at the highest dose throughout treatment. Slight to moderate sedation
and ptosis were seen at the intermediate dose. Copulation and
fertility rates and pre-coital intervals were comparable in all
groups. There were no adverse effects on male fertility or on
embryofetal development in the offspring of untreated females mated to
treated males. The NOEL was 5 mg/kg bw per day on the basis of
paternal toxicity.
4. EVALUATION
The Committee concluded that the pharmacological effects of
azaperone are the most relevant for determining an ADI. Therefore, an
ADI of 0œ6 µg/kg bw was established on the basis of the NOAEL of 630
µg/kg bw for neurobehavioural effects in dogs after oral
administration (as recommended by the forty-third meeting of the
Committee) and a safety factor of 100.
5. REFERENCES
Dom, P. (1987) Study on male fertility and on effects on embryo-foetal
development in SPF Wistar rats with R001929 by oral gavage (Report No.
R001929). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
Sanderson, D.M. & Earnshaw, C.G. (1991) Computer prediction of
possible toxic action from chemical structure; the DEREK system.
Hum. Exp. Toxicol., 10, 261-273.
Vermeir, M. (1997) A study on the metabolism of azaperone in liver
9000g supernatant fractions of Arochlor pretreated rats (Report No.
R001929/FK2533). Unpublished report from Janssen Research Foundation.
Submitted to WHO by Janssen Animal Health, Beerse, Belgium.
