
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
MENTHOL
First draft prepared by Dr G.J.A. Speijers
National Institute of Public Health and the Environment,
Center of Substances and Risk Assessment, Bilthoven,
The Netherlands
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Feeding studies
Other studies
Genotoxicity
Developmental toxicity
Special studies
Cellular and biochemical effects
Immunotoxicity and sensitivity
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Menthol was first evaluated at the eleventh meeting of the
Committee (Annex 1, reference 14), when it was allocated an
unconditional ADI of 0-0.2 mg/kg bw and a conditional ADI of 0.2-2
mg/kg bw. At the eighteenth meeting, an ADI of 0-0.2 mg/kg bw was
established (Annex 1, reference 35). The Committee reevaluated menthol
at its twentieth meeting (Annex 1, reference 41), when the previous
ADI was maintained. The desired information identified (Annex 1,
reference 42) consisted of the results of long-term studies of
toxicity and carcinogenicity in rats; information on the average and
likely maximum intake levels of menthol; clinical observations of
subjects with higher than average intake of menthol; and studies on
metabolism. Since that time, new studies have become available,
principally, two-year studies of carcinogenicity in mice and rats.
Menthol exists in four geometrical forms with three asymmetric
carbon atoms. The principal division of menthol forms is into optical
isomers, (+)-menthol, (-)-menthol, and the mixture (±)-menthol. The
(-) and (±) forms are used for flavour applications.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Absorbed menthol is largely eliminated as glucuronides. Thus, 79%
of a 1-g (Quick, 1928) and 78% of a 10-20-mg (Atzl et al., 1972) oral
dose of menthol was eliminated as the glucuronic acid conjugate within
6 h after administration to volunteers. When 750 mg (-)-menthol were
given orally to three human volunteers, followed by oral or
intravenous administration of 200 mg [6-13C]-glucuronolactone or
[6-13C]-sodium glucuronate, menthyl glucuronide was excreted for two
days, in average daily yields ranging from approximately 27 to 84%
(Eisenberg et al., 1955).
Of a dose of 47 mg/kg bw [3-3H]-(-)-menthol, 82% was eliminated
in the urine 17 h after administration. Smaller amounts were
distributed in the faeces and ileum; only 1% of the activity remained
in the liver (Clegg et al., 1982).
The human capacity to eliminate menthol is indicated by research
on patients with chronic liver disease. Doses of 2 g menthol were
given to evaluate the glucuronization capacity of patients with
alcohol-induced cirrhosis or steatosis when compared with normal
subjects. While the mean excretion of menthol glucuronide was slightly
lower in patients with liver disease, they retained a significant
capacity to metabolize menthol (Horvath et al., 1984).
The yield of menthol glucuronidation products is sufficiently
high that menthol glucuronide output has been used as a marker in
pharmacokinetic studies on bioavailability from drug formulations. In
a pharmacokinetic study of treatment with peppermint oil in
enteric-coated capsules (containing 91-97 mg menthol) or soft gelatin
capsules as a carminative and antispasmodic, human urinary excretion
of menthol glucuronide represented 17% of the dose from two coated
capsules and 29% of the dose from the capsules 24 h after
administration (Somerville et al., 1984). In a comparison of two
delay-release peppermint oil preparations, 13 subjects ingested 0.6 ml
peppermint oil providing 110 and 117 mg menthol. Peak urinary
excretion occurred at 3 and < 9 h, the latter from a coated
capsule. The average total 24-h urinary excretion of menthol
glucuronide was 82% of the ingested dose of menthol from the coated
tablet and 119% of the ingested dose from the other preparation (White
et al., 1987). When an enteric-coated capsule containing 130 mg
peppermint oil was fed to four subjects, the average 14-h urinary
excretion of menthol glucuronide was 40% of the dose (range, 20-64%)
(Kaffenberger & Doyle, 1990).
2.1.2 Biotransformation
In rabbits, orally administered menthol is conjugated with
glucuronic acid and eliminated in the urine (Quick, 1924; Williams,
1938, 1939; Deichmann & Thomas, 1943). The maximum amount of menthol
glucuronide excreted by a 2-kg rabbit was about 3 g after 10 h of
feeding of 3.5 g menthol, resulting in a yield of 3/3.5 × 100% = 86%
elimination by glucuronidation, even when this maximum toxic dose was
fed (Quick, 1924). After single daily doses of 2 g menthol for
24 days, 90% was excreted as menthol glucuronide within 6 h. The
glucuronide is only a minor urinary excretion product in dogs,
suggesting that other metabolic routes, e.g. oxidation, are more
important in this species (Williams, 1938).
In rats, the vast majority of orally administered menthol is
elimiminated in the urine or faeces as the glucuronic acid conjugate
or various oxidation products (Madyastha & Srivatsan, 1988; Yamaguchi
et al., 1994).
Groups of five male intact and five male bile duct-cannulated
male Fischer 344 rats were given a single dose of 500 mg/kg bw
[3-3H]-(-)-menthol. Urine and faeces were collected over the next 24
and 48 h from the intact rats, and bile was collected from the
cannulated rats at 2-h intervals for the first 6 h and then at 24 h.
Urine was collected at 24 h. Total recovery of radiolabelled substance
in the urine and faeces of the intact rats was 72%, with 45% of the
dose recovered within the first 24 h; 38% of the radiolabel was
excreted in the urine, with equal amounts at the first and second
24 h, and 34% was excreted in the faeces, with 27% at the first 24 h.
In the bile duct-cannulated rats, total recovery of radiolabelled
menthol and metabolites in the urine and bile was 74%, the majority
(67%) being recovered in the bile. The major metabolite found in the
bile was menthol glucuronide; various oxidation products were found in
the urine (Yamaguchi et al., 1994).
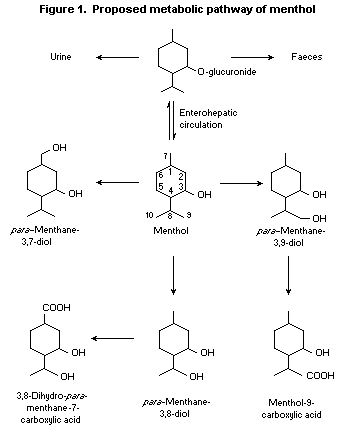
Menthol glucuronide formed in the liver passes into the bile,
with subsequent elimination or entry into the enterohepatic
circulation. It undergoes various oxidation reactions upon each
passage through the liver. The oxidation products of menthol include
para-menthane-3,8-diol, para-menthane-3,9-diol, and
3,8-dihydroxy- para-menthane-7-carboxylic acid (Madyastha &
Srivatsan, 1988; Yamaguchi et al., 1994; see Figure 1). Additional
oxidation metabolites that have been identified include a primary
alcohol, a triol, and hydroxy acids (Yamaguchi et al., 1994).
Humans metabolize menthol primarily by conjugation with
glucuronic acid and elimination in the urine. It is anticipated that
cytochrome P450-mediated oxidation (see section 2.1.3) occurs in
humans, yielding various alcohol and hydroxy acid derivatives, which
would also be eliminated in the urine unchanged or conjugated with
glucuronic acid. Menthol would not be expected to form menthofuran, a
reactive metabolite of the structurally related substance pulegone,
because it lacks the 2-isopropylidene side-chain required for
formation of the menthofuran ring. Additionally, there are no data
which suggest that menthol dehydrogenates in vivo to form an
isopropylidene substituent.
2.1.3 Effects on enzymes and other biochemical parameters
Gluronidase activity was measured in the organs of mice after
administration of menthol in 20-mg doses three times per day on days
1-4 and twice on the fifth day. Glucuronidase activity was increased
in liver, kidney, and spleen (Fishman, 1940).
The results of a study with rat liver microsomes in vitro
suggest that oxidation of (-)-menthol is mediated by cytochrome P450
(Madyastha & Srivatsan, 1988). Rats receiving repeated oral doses of
800 mg/kg bw (-)-menthol for one to seven days had increased
activities of hepatic microsomal cytochrome C and NADPH-cytochrome
P450 reductase. There was no effect on two other P450 enzyme
complexes, cytochrome b5 and NADH-cytochrome C reductase. Rat liver
microsomes readily convert (-)-menthol to para-menthane-3,8-diol in
the presence of NADPH and oxygen. This activity was significantly
greater in microsomes obtained from phenobarbital-induced microsomal
preparations than from controls, whereas 3-methylcholanthrene-induced
microsomes failed to convert (-)-menthol to 1-menthane-3,8-diol in the
presence of NADPH and oxygen.
Menthol administered intraperitoneally to rats at a dose of
40 mg/kg bw for three days had no effect on the total content of
cytochrome P450. Mild induction of a fraction of the P450 complex,
designated P450IIB, was observed. Menthol induced a twofold increase
over normal levels of P450IIB, in comparison with a 20-fold increase
with phenobarbitol. The P450IIB family comprises as little as 5% of
the total P450 material (Austin et al., 1988).
In earlier studies, menthol was used to examine the inducibility
of the glucuronide-adding enzyme, hepatic UDP-glucuronosyl transferase
(UDPGT), by 3-methylcholanthrane (group 1 substrates) and
phenobarbital (group 2 substrates). When liver microsomes from C57BI/6
mice induced by 3-methylcholanthrane or phenobarbital were treated
in vitro with menthol, only the phenobarbital-induced microsomal
fraction stimulated glucuronidation of menthol (Batt et al., 1981).
The activity of hepatic UDPGT in tissue isolated from castrated male
Large White pigs was tested after administration of 0.3 mmol/L
menthol. Menthol slightly increased the activity (110% of control
response) of the phenobarbital-induced UDPGT enzyme fraction. The
pattern of UDPGT inducibility in pig resembles that in isolated human
liver tissue (Boutin et al., 1981). The effect of menthol on the
inducibility of UDPGT was also examined in Wistar rats, Gunn rats, and
guinea-pigs. Menthol glucuronidation was associated with the
phenobarbital-inducible microsomal fraction in all three species,
although these species contain a UDPGT specific for monoterpenoid
alcohols, such as menthol (Boutin et al., 1985).
 Menthol has been used to detect and confirm the presence of a rat
liver DNA fraction coding for glucuronide transferase activity (Green
et al., 1995).
The efficient glucuronidation of menthol was used as a model in a
competitive reaction to evaluate oestradiol glucuronide binding sites
in rat liver plasma membranes. Menthol had a stronger affinity for
plasma binding sites than oestradiol glucuronide (Takacs & Vore,
1987).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the toxicity of single doses of menthol are summarized
in Table 1. It has also been reported that the LD50 values in rats
and mice are > 4000 mg/kg bw (Mengs & Stotzem, 1989).
Table 1. Studies of the acute toxicity of menthol
Species Route LD50 (mg/kg bw) Reference
(-)-Menthol
Mouse Oral 4 380 Litton Bionetics, Inc. (1975)
Rat Oral 940 Litton Bionetics, Inc. (1975)
3 300 JECFA (1976)
Cat Oral 800-1 000 JECFA (1976)
Mouse Subcutaneous 5 000-6 000 JECFA (1976)
Intraperitoneal 2 000 JECFA (1976)
Guinea-pig Intraperitoneal 4 000 JECFA (1976)
Rat Subcutaneous 1 000-2 500 JECFA (1976)
Intraperitoneal 710 JECFA (1976)
Cat Intraperitoneal 800-1 000 JECFA (1976)
Intravenous 34 JECFA (1976)
(±)-Menthol
Mouse Oral 3 100 Wokes (1932)
Rat Oral 2 900 JECFA (1976)
3 180 Jenner et al. (1964)
Cat Oral 1 500-1 600 JECFA (1976)
Mouse Subcutaneous 14 000-16 000 JECFA (1976)
Rat Intraperitoneal 750 JECFA (1976)
Cat Intraperitoneal 1 500-1 600 JECFA (1976)
Rabbit Intraperitoneal 2 000 JECFA (1976)
2.2.2 Short-term studies of toxicity
Mice
Groups of six male mice were given (-)-menthol at doses of 2000,
2500, 3200, 4000, or 5000 mg/kg bw by gavage for five days and
examined for 14 days. Gross necropsy of animals that died or were
killed at termination revealed no abnormal finding. The LD50 was
calculated to be 2600 mg/kg bw (Litton Bionetics, Inc., 1975).
Groups of 10 male and 10 female B6C3F1 mice were maintained on
diets containing (±)-menthol at concentrations of 0, 930, 1870, 3750,
7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 140, 280,
560, 1100, and 2300 mg/kg bw per day, respectively. Necropsies were
performed on all animals at the end of the study; histopathological
examination was performed on tissues from the control animals and
those at 2300 mg/kg bw per day and on selected tissues from animals at
1100 mg/kg bw per day. Six mice (sex not specified) died during the
study, but the deaths could not be attributed to treatment. The final
mean body weights of the treated mice were not statistically
significantly different from those of the controls, except for females
at the high dose which had statistically significant decreased body
weights. Slight increases in the incidences of perivascular lymphoid
hyperplasia and interstitial nephritis were reported for female mice
at the two highest doses. The NOEL was 560 mg/kg bw per day (US
National Cancer Institute, 1979).
Rats
No adverse effects on weight gain, excretion of glucuronides,
water, or electrolytes, or interference with central nervous system
reactions to stimulants were observed when groups of 40 rats of each
sex were fed (-)-or (±)-menthol in the diet for 5.5 weeks at doses of
0, 100, or 200 mg/kg bw per day (Herken, 1961).
In groups of three to four adult male Wistar rats given 1%
menthol in the diet for two weeks, increased serum cholesterol and
serum triglycerides were observed, but there was no effect on apo A-1
lipids, an indicator of high-density lipoprotein status. Body weight
and liver weight were unaffected (Imaizumi et al., 1985).
In a series of studies, groups of 10 rats of each sex were given
menthol in a soybean oil solution at 5 ml/kg bw at doses of 0, 200,
400, or 800 mg/kg bw per day by gavage for 28 days. Animals were
housed two to a cage. Body weight and consumption of food and water
were recorded weekly, and blood samples were collected for
determination of haemoglobin, packed cell volume, erythrocyte,
leukocyte, and reticulocyte counts, glucose, creatinine, and urea
concentrations, and the activity of aspartate aminotransferase. Urine
was examined for blood, ketones, glucose, and proteins. A conventional
selection of tissues was examined at termination.
Significant increases in the absolute and relative weights of the
liver were seen in males at all doses and in females at the
intermediate and high doses. Vacuolization of hepatocytes was seen in
0/20 controls, 4/20 rats at 200 mg/kg bw per day, 5/19 at 400 mg/kg bw
per day, and 4/17 at 800 mg/kg bw per day. No effects were seen on
other parameters measured. The vacuolization was not dose related and
may have reflected adaptation (Thorup et al.,1983a,b; Madsen et al.,
1986).
While the major constituent of peppermint oil is menthol, it also
contains other constituents. Although its effects cannot be assigned
entirely to menthol, dose-proportionate menthol-attributable effects
can be expected. The studies of peppermint oil administered by gavage
include a 28-day study in Wistar rats (Thorup et al., 1983b) at 0, 10,
40, or 100 mg/kg bw per day; a five-week study in Wistar rats and dogs
at 0, 25, or 125 mg/kg bw per day (Mengs & Stotzem, 1989); and a
90-day study in Wistar rats at 0, 10, 40, or 100 mg/kg bw per day
(Spindler & Madsen, 1992). Significant effects were seen on organ
weights and liver morphology, but no histopathological changes were
observed at these doses.
Feeding of terpenoid substances present in mint oils (see the
safety evaluation of substances structurally related to menthol, p.
381) affected the morphology of rat cerebellum. Similar effects were
not seen in the 28-day study of menthol, however, and a subsequent
review of the slides indicated that the observations were artefacts
(Adams et al., 1996), as supported by the results of a recent study
(Mœlck et al., 1998).
Groups of 10 female and 10 male Fischer 344 rats were maintained
on diets containing (±)-menthol at concentrations of 0, 930, 1900,
3800, 7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 93,
190, 380, 750, or 1500 mg/kg bw per day. Necropsies were performed on
all animals at the end of the study; histopathological examination was
performed on tissues from the control animals and those at 1500 mg/kg
bw per day and on selected tissues from animals at 750 mg/kg bw per
day. The final mean body weights of treated rats were similar to those
of the controls. A slight increase in the incidence of interstitial
nephritis was observed in male rats at the highest dose. The NOEL was
750 mg/kg bw per day (US National Cancer Institute, 1979).
Because of its pharmacological properties, menthol has also been
tested by intraperitoneal, intravenous, inhalation, and dermal
administration. As noted above, oral administration results in
substantial biotransformation, whereas these processes are largely
by-passed when it is given by the other routes, and the results of
these studies are not applicable to this safety evaluation.
Furthermore, some short-term studies result in biological end-points
such as antispasmodic activity, which are not of primary relevance to
the food uses of menthol. Studies relevant to intake of menthol from
food are summarized below.
The previous monograph summarized studies on the antispasmodic
activity of pharmaceutical preparations on intestinal tissue (Annex 1,
reference 42), although they contained peppermint oil rather than
menthol per se (Bowen & Cubbin, 1992). The doses of menthol
necessary to obtain this effect are significantly higher than the
amounts present in foods.
The pharmacological effect of menthol on the respiratory tract
has also been studied. In a 71-79-day study with young albino Sherman
rats, inhalation of (-)-menthol vapour at 0.087, 0.15, or 0.26 ppm
caused no gross changes. Histological examination showed effects on
lung tissue only at the highest dose, at which irritation also
occurred (Rakieten et al., 1954).
2.2.3 Long-term studies of toxicity and carcinogenicity
2.2.3.1 Feeding studies
Mice
Groups of 50 B6C3F1 mice of each sex were given (±)-menthol at
doses of 0, 2000, or 4000 mg/kg diet daily for 103 weeks, equivalent
to 300 or 600 mg/kg bw per day, respectively. Animals were housed five
per cage and were observed twice daily for signs of toxicity. Body
weights and food consumption was recorded every two weeks for the
first 12 weeks and once a month thereafter. Necropsies and
histological examinations were performed on all animals at termination
of the study and on those found dead during the study.
The mean body weights of the treated mice were slightly lower
than those of the controls. The survival of males was similar to that
of controls, but females at the high dose had statistically
significantly worse survival rates; subsequent evaluation (Haseman et
al., 1985) showed, however, that the survival of all female mice was
within the range for historical controls, and the survival rate of the
control group in this study was at the high end of the range. The
survival of animals at the high dose was in fact closer to the
historical average, and there was no evidence of toxicity in this
group. An increased incidence of hepatocellular carcinoma was observed
in males at the high dose, but the increase was not significantly
different from that of concurrent or historical control mice of that
age and strain (Haseman et al., 1986). A low incidence of alveolar or
bronchiolar adenomas of the lung was observed in treated females, but
the rate was not significantly different from that in historical
controls. It was concluded that (±)-menthol is not carcinogenic and
has no organ-specific toxicity in B6C3F1 mice of either sex at the
doses tested (US National Cancer Institute, 1979).
Rats
Groups of 50 Fischer 344 rats of each sex were given 0, 3750, or
7500 mg/kg diet (±)-menthol in their feed daily for 103 weeks,
equivalent to 190, and 380 mg/kg bw per day, respectively. Animals
were housed five per cage until week 48, when the male rats were
divided into groups of two to three per cage. The animals were
observed twice daily for signs of toxicity. Body weight and food
consumption were recorded every two weeks for the first 12 weeks and
once a month thereafter. Necropsies and histological examinations were
performed on all animals at the end of the study and on those found
dead during the study.
The mean body weights of treated rats were slightly lower than
those of the controls. Survival of treated rats was similar to that of
controls. Chronic inflammation of the kidney was observed in the older
treated males, but was not considered to be related to administration
of menthol since the effect is commonly observed in aged male Fischer
344 rats. The incidence of neoplasms was not increased in treated
females, and, in fact, fibroadenomas of the mammary glands occurred at
lower incidences in treated (10/49 at the low dose and 7/49 at the
high dose) than in control animals (20/50). Alveolar or bronchiolar
adenomas or carcinomas were reported only in female controls. It was
concluded that (±)-menthol is neither carcinogenic nor toxic for
Fischer 344 rats of either sex at the doses tested (US National Cancer
Institute, 1979).
2.2.3.2 Other studies
Groups of 30 female A/He strain mice received 2000 mg/kg bw
menthol dissolved in tricaprylin, the maximum dose tolerated after six
intraperitoneal injections over two weeks, and one-quarter this dose,
500 mg/kg bw, three times weekly for eight weeks. The animals were
observed for an additional 16 weeks. A group of 24 control female mice
received a similar number of injections of tricaprylin. All animals
that survived treatment were killed after 24 weeks and the numbers of
pulmonary adenomas counted. No increase in the incidence of
non-neoplastic or neoplastic lesions was reported in the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands of
treated animals. Approximately 30-45% of the menthol-treated animals
and 15% of the control animals died before the end of the study. The
authors reported that tricaprylin is an unsuitable vehicle for
bioassays, as it caused a 3- or 4-g weight loss in the control animals
during the first week of the study, a high mortality rate, and a
higher mean tumour rate. In the same test system, urethane (total
dose, 10 or 20 mg) and several alkylating agents induced marked
increases in the incidence of pulmonary adenomas, but other substances
shown to be carcinogenic in other test systems, e.g. safrole, caused
no increase (Stoner et al., 1973; Annex 1, reference 42). A
retrospective evaluation of the lung tumour response in strain A mice
is provided by Stoner (1991).
Menthol has been studied for its chemopreventive effect on
ras-mediated tumour growth in four types of rat liver cell
in vitro, with lovastatin as the positive control. Concentrations of
0.1-2.5 mmol/L of menthol inhibited tumour growth, but the authors
concluded that the mechanism of the chemopreventive effect of menthol
is different from that of lovastatin (Ruch & Sigler, 1994).
In a study of the preventive activity of menthol against
7,12-dimethylbenz [a]anthracene (DMBA)-induced mammary tumours in
rats, a diet containing 1% menthol was fed from two weeks before DMBA
treatment to up to 22 weeks after treatment. A reduction in mammary
tumour incidence was seen. A diet containing 0.5% menthol fed from two
weeks before DMBA treatment until one week after treatment also
resulted in a reduction in tumour incidence (Russin et al., 1989;
Gould et al., 1990).
In studies of mentholated cigarettes in animals and humans, the
presence of menthol in cigarettes did not enhance the incidence of
lung cancer over that due to smoking unmentholated cigarettes (Kabat &
Hebert, 1991; Gaworski et al., 1997).
2.2.4 Genotoxicity
The results of tests for genotoxicity with menthol are presented
in Table 2.
Menthol was administered at 725 mg/kg bw or the maximum tolerated
dose of 1450 mg/kg bw to male Fischer 344 rats and male B6C3F1 mice.
Hepatocytes were removed at 24, 39, and 49 h, and replicative DNA
synthesis was measured. Synthesis was increased in 6% of the rats and
1.7% of the mice. This assay indicates cell replication (i.e.
mitogenesis), however, and not genotoxicity (Uno et al., 1994;
Yoshikawa, 1996).
2.2.5 Developmental toxicity
Mice
(-)-Menthol was administered in corn oil by gavage to 22 or 23
pregnant CD-1 mice at 0, 1.9, 8.6, 40, or 190 mg/kg bw per day on days
6-15 of gestation; to 22 or 23 pregnant Wistar rats at 0, 2.2, 10, 47,
or 220 mg/kg bw per day on days 6-15 of gestation; to 21-23 golden
hamsters at 0, 4.1, 21, 98, or 400 mg/kg bw per day on days 6-10 of
gestation; and to 11 to 14 Dutch-belt rabbits at 0, 4.3, 20, 92, or
430 mg/kg bw per day on days 6-18 of gestation. Control groups for
each species were sham treated; positive control groups for each
species were given 150 or 250 mg/kg bw per day aspirin. Body weights
were recorded on three or four days during the gestation period. All
animals were observed daily for appearance, behaviour, and food
consumption. On the scheduled day, the fetuses were removed from all
dams and dams and fetuses examined. One-third of the fetuses from each
group underwent detailed visceral examination; the other two-thirds
were examined for skeletal defects. There were no effects on nidation,
maternal survival, fetal survival, or fetal abnormalities. The numbers
of abnormalities seen in soft or skeletal tissues of treated animals
did not differ from those occurring spontaneously in the sham-treated
controls (Food and Drug Research Labs, Inc., 1973).
2.2.6 Special studies
2.2.6.1 Cellular and biochemical effects
Menthol has been tested for potential bactericidal and fungicidal
effects on microorganisms that are foodborne or found in the oral
cavity. Menthol at concentrations of 0.1-5 mmol/L was cytotoxic and
affected tissue processes in trachea from chick embryos, ascites
sarcoma BP8 cells, isolated hamster brown adipocytes, and rat liver
mitochondria (Bernson & Pettersson, 1983). Menthol had no cytotoxic
effect on human HeLa cells in vitro at concentrations of 1, 10, and
100 µg/ml (Nachev et al., 1967).
Menthol was lethal to E. coli at a concentration of 0.5%
(Wokes, 1932) or 0.05% (Morris et al., 1979; Megalla et al., 1980; Jay
& Rivers, 1984); to Salmonella typhimurium at a concentration of
0.05% (Karapinar & Aktug, 1987); to Clostridium at 0.05% (Ueda et
al., 1982); to Staphylococcus at 0.05% (Wokes, 1932; Morris et al.,
1979; Karapinar & Aktug, 1987; Moleyar & Narasimham 1992) or 0.003%
(Jay & Rivers, 1984); to Vibrio spp. at 0.05% (Karapinar & Aktug;
1987); and to Bacillus spp. at 0.05% (Morris et al., 1979; Megalla
et al., 1980; Ueda et al., 1982; Moleyar & Narasimham, 1992). It was
cytotoxic to spoilage fungi at 0.08-0.3% (Yousef et al., 1978; Kurita
et al., 1981), 0.05% (Morris et al., 1979; Megalla et al., 1980;
Mahmoud, 1994; Muller-Reibau et al., 1995), or 0.025% (Jay & Rivers,
1984).
The results of these studies are difficult to extrapolate to the
situation in experimental animal in vivo, and cannot be used
directly in the safety assessment of menthol. Furthermore, the
concentration at which cytotoxicity was observed is considerably
higher than those present in the diets tested.
2.2.6.2 Immunotoxicity and sensitivity
The presence of menthol and menthol-containing flavour and
fragrance oils at high concentrations in consumer products such as
cigarettes, toothpaste, and topical medications has led to sensitivity
reactions in the oral and nasal cavity (e.g. Millard, 1973;
Dooms-Goossens et al., 1977; Lewis et al., 1995; Morton et al., 1995;
Shah et al., 1996).
The preferred method for testing sensitivity or food intolerance
is the double-blind placebo-controlled food challenge. In a study on
the prevalence of food allergy and food intolerance in 1483 Dutch
adults by this method, it was estimated that the prevalence of
sensiivity or intolerance was 0.8-2.4% of the adult population. Within
the sampled population, 73 subjects reported such effects, and 12 of
the responses were confirmed. Only one of these responders had a
reaction to menthol challenge; the subject reported 'aggravation of
aphthae' (whitish spots in the mouth that characterize apthous
stomatitis) 1 h after administration (Niestijl Janson et al., 1994).
2.3 Observations in humans
Menthol has been tested in humans mainly for its potential
pharmaceutical properties, such as enhancement of lung and airway
volume (e.g. Bowen & Cubbin, 1992). The usual human oral dose is
60-120 mg menthol per person. It can be estimated from unreferenced
citations in pharmaceutical texts, such as Gleason et al. (1969), that
the lethal human dose is 50-500 mg/kg bw. In section 2.1.1, the
maximum doses tested were 180 mg (Kaffenberger & Doyle, 1990) and 1 g
(Quick, 1928).
The airway hyperresponsiveness of 23 human subjects with chronic
mild asthma was tested by use of a nebulizer containing menthol twice
a day for four weeks. As measured by expiratory flow rates, vital
capacity, and forced expiratory volume, menthol improved airway
hyperresponsiveness at doses as low as 20 mg (Tamaoki et al., 1995).
The efficient hepatic glucuronidation of menthol has been
investigated as a possible basis for a test of liver function. In a
study of the output of menthol glucuronide by normal subjects and
subjects with alcohol-induced cirrhosis and steatosis of the liver, it
was concluded that the group differences were not sufficient to
justify use of menthol glucuronide output as a test for liver disease.
The results demonstrate that even a compromised liver has sufficient
capacity to handle menthol (Szabo & Ebrey, 1963; Horvath et al.,
1984).
A controlled study of the effects of a mentholated powder was
carried out on 60 consecutive glucose-6-phosphate
dehydrogenase-deficient babies. The umbilical cords of 30 babies were
treated daily with the powder, while the remainder served as controls.
Significantly more of the treated babies developed severe jaundice
than the controls. The inability of neonates to conjugate menthol was
probably responsible for the increased severity of the jaundice
developed by the deficient babies. It was concluded that the use of
menthol-containing products on neonates should be discontinued,
especially in communities where the incidence of glucose-6-phosphate
dehydrogenase deficiency is high (Olowe & Ransome-Kuti, 1980). As
neonates do not have an oral intake of menthol, the relevance of these
results is limited. No data were available on the possible effects of
menthol in older children or in adults with glucose-6-phosphate
dehydrogenase deficiency.
Table 2. Results of assays for the genotoxicity of menthol
End-point Test object Concentration Results Reference
In vitro
(±)-Menthol
Reverse mutation S. typhimurium TA92, TA1535, < 5 mg/plate Negativea Ishidate et al. (1984)
TA100, TA1537, TA94, TA98
Reverse mutation S. typhimurium 666 µg/plate Negativea Tennant et al. (1987)
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)
TA98
Antimutagenicity S. typhimurium TA98 < 200 µg/ml Negative Ohta et al. (1986)
Antimutagenicity Escherichia coli < 200 µg/ml Negative Ohta et al. (1986)
Chromosomal aberration Chinese hamster fibroblasts < 0.2 mg/ml Negative Ishidate et al. (1984)
Chromosomal aberration Chinese hamster lung fibroblasts < 0.3 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Chinese hamster ovary cells < 250 µg/ml Negative Tennant et al. (1987)
and sister chromatid
exchange
Chromosomal aberration Chinese hamster ovary cells < 167 µg/ml Negative Ivett et al. (1989)
and sister chromatid
exchange
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Tennant et al. (1987)
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Myhr & Caspary (1991)
(-)-Menthol
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)TA98
Reverse mutation S. typhimurium TA98, TA100, 6.4-800 µg/plate Negativea Andersen & Jennies (1984)
TA1535, TA1537
Reverse mutation E. coli WP2 uvrA 0.1-0.8 mg/plate Negative Yoo (1986)
Reverse mutation S. typhimurium TA1530, G46 0.25 ml/plate Negative Litton Bionetics, Inc. (1975)
Antimutagenicity E. coli WP2 uvrA 0.5-2.0 mg/ml Negative Yoo (1986)
DNA repair Bacillus subtilis < 10 mg/disk Negative Yoo (1986)
Gene mutation Bacillus subtilis < 20 mg/plate Negative Oda et al. (1978)
Gene mutation Saccharomyces cerevisiae D3 0.2 ml/plate Equivocal Litton Bionetics, Inc. (1975)
Table 2. (continued)
End-point Test object Concentration Results Reference
Chromosomal aberration Chinese hamster lung fibroblasts < 0.125 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Human WI-38 embryonic lung cells 10 mg/ml Negative Litton Bionetics, Inc. (1975)
Chromosomal aberration Human peripheral blood lymphocytes 0.1-10 mmol/L Negativea Murthy et al. (1991)
and sister chromatid
exchange
(+)-Menthol
DNA damage Rat hepatocytes 0.7-1.3 mmpl/L Positive Storer et al. (1996)
DNA damage Chinese hamster V79 cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
DNA damage Human blood cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
In vivo
(±)-Menthol
Micronucleus formation Male B6C3F1 mouse bone marrow < 1 g/kg bw Negative Shelby et al. (1993)
(-)-Menthol
Host-mediated S. typhimurium TA1530, G46/ < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity ICR mouse host
Host-mediated S. cerevisiae D3/ICR mouse host < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity
Chromosomal aberration Albino rat bone marrow < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
Dominant lethal mutation Rat < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
a With and without an exogenous metabolic activation system
3. COMMENTS
Menthol exists as two optical isomers, (+)-menthol and
(-)-menthol; the racemic mixture is (±)-menthol. The (-) and (±) forms
are used in flavour applications.
Menthol is readily absorbed. Up to 100% of an ingested dose
appeared to be absorbed, on the basis of the elimination of menthol
metabolites in faeces and urine. Absorbed menthol is known to be
largely eliminated as glucuronides; 70-80% is eliminated in urine and
faeces within 48 h. Metabolic studies indicate that oral doses of
menthol are metabolized mainly in the liver and excreted via the
kidneys and in the bile. Menthol is efficiently metabolized by normal
processes. The metabolites are simple glucuronic acid conjugates and
oxidation products. Mammals can efficiently handle menthol by
processes that do not create hazardous products.
The NOEL in 13-week studies of toxicity with (±)-menthol in the
diet was 560 mg/kg bw per day in mice and 750 mg/kg bw per day in rats
on the basis of slightly increased incidences of interstitial
nephritis at the next highest dose.
In a two-year study of toxicity and carcinogenicity, mice were
fed (±)-menthol in the diet at concentrations equivalent to 300 or 600
mg/kg bw per day. The incidences of hepatocellular tumours in males
and lung tumours in females at the highest dose were not significantly
different from those in concurrent or historical controls. The
survival rate was decreased in female mice but remained within the
range of that of historical controls. The NOEL was 600 mg/kg bw per
day. In a two-year study of toxicity and carcinogenicity in rats given
(±)-menthol in the diet at concentrations equivalent to 190 or
380 mg/kg bw per day, the NOEL was 380 mg/kg bw per day.
Neither menthol nor its metabolites were genotoxic in vitro or
in vivo.
While no studies of reproductive toxicity were available with
menthol, (-)-menthol was tested at maximum doses of 190-430 mg/kg bw
per day for teratogenicity in mice, rats, hamsters, and rabbits; no
teratogenic effects were observed.
4. EVALUATION
The limited data that allow comparisons of metabolism and
toxicity provide no indication of a difference in the toxicity of
(-)-menthol and (±)-menthol. Therefore, the Committee concluded that
an ADI could be established for the two optical isomers of menthol.
The Committee noted that the highest dose of (±)-menthol tested
in the long-term studies in mice and rats had no specific toxic
effect. As the survival of mice was reduced at the high dose of
600 mg/kg bw per day, the Committee allocated an ADI of 0-4 mg/kg bw
on the basis of the NOEL of 380 mg/kg bw per day in the long-term
study in rats, applying a safety factor of 100 and rounding to one
significant figure.
5. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Andersen, P.H. & Jennies, N.J. (1984) Mutagenic investigation of
peppermint oil in the Salmonella/mammalian-microsome test.
Mutat. Res., 138, 17-20.
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Austin, C.A., Shephard, E.A., Pike, S.F., Rabin, B.R. & Phillips, I.R.
(1988) The effect of terpenoid compounds on cytochrome P-450 levels in
rat liver. Biochem. Pharmacol., 37, 2223-2229.
Batt, A.M., Martin, N. & Siest, G. (1981) Induction of group-1 and
group-2 UDP-glucuronosyl transferase in microsomes from the livers of
C57 Bl/6 mice. Toxicol. Lett., 9, 355-360.
Bernson, V.S.M. & Pettersson, B. (1983) The toxicity of menthol in
short-term bioassays. Chem.-Biol. Interactions, 46, 233-246.
Boutin, J.A., Lepage, C., Batt, A.M. & Siest, G. (1981) The activity
of hepatic UDP-glucuronosyltransferase from control and induced pigs
toward 17 hydroxylated aglycones. IRCS Med. Sci., 9, 633-634.
Boutin, J.A., Thomassin, G., Siest, G. & Cartier, A. (1985)
Heterogeneity of hepatic microsomal UDP-glucuronosyltransferase
activities. Conjugations of phenolic and monoterpenoid aglycons in
control and induced rats and guinea pigs. Biochem. Pharmacol., 34,
2235-2249.
Bowen, I.H. & Cubbin, I.J. (1992) Mentha piperita and Mentha spicata.
In: Adverse Effects of Herbal Drugs, pp. 171-178.
Clegg, R.J., Middleton, B., Bell, G.D. & White, D.A. (1982) The
mechanism of cyclic monoterpene inhibition of hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase in vivo in the rat.
J. Biol. Chem., 257, 2294-2299.
Deichmann, W. & Thomas, G. (1943) Glucuronic acid in the urine as a
measure of the absorption of certain organic compounds. J. Ind.
Hyg. Toxicol., 25, 286-292.
Dooms-Goossens, A., Degreef, H., Holvoet, C. & Maertens, M. (1977)
Turpentine-induced hypersensitivity to peppermint oil. Contact
Derm., 3, 304-308.
Eisenberg, F., Field, J.B. & Stetten, D. (1955) Studies on glyceronide
conjugation in man. Arch. Biochem. Biophys., 59, 297-299.
Fishman, W.H. (1940) Studies on beta-glucuronidase. III. The increase
in beta-glucuronidase activity of mammalian tissues induced by feeding
glucuronidogenic substances. J. Biol. Chem., 136, 229-236.
Food & Drug Research Labs, Inc. (1973) Teratologic evaluation of FDA
71-57 (menthol natural Brazilian), June 1973. US National Technical
Information Service report No. PB-223815. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Gaworski, C.L., Dozier, M.M., Gerhart, J.M., Rajendrans, N.,
Brennecke, L.H., Aranyi, C. & Heck, J.D. (1997) 13-Week inhalation
toxicity study of menthol cigarette smoke. Food Chem. Toxicol., 35,
683-692.
Gleason, M.N., Gosselin,R.E., Hodge, H.C. & Smith, R.P. (1969)
Clinical Toxicology of Commercial Products, 3rd Ed., Philadelphia,
Williams & Wilkins Co.
Gould, W.D., Wacker, P. & Maltzman, T.H. (1990) Chemoprevention and
chemotherapy of mammary tumors by monoterpenoids. Prog. Clin. Biol.
Res., 347, 255-268.
Green, M.D., Clarke, D.J., Oturu, E.M., Styczynski, P.B., Jackson,
M.R., Burchell, B. & Tephly, T.R. (1995) Cloning and expression of a
rat liver phenobarbital-inducible UDP-glucuronosyl-transferase (2B12)
with specificity for monoterpenoid alcohols. Arch. Biochem.
Biophys., 322, 460-468.
Hartmann, A. & Speit, G. (1997) The contribution of cytotoxicity to
DNA-effects in the single cell gel test (Comet assay). Toxicol.
Lett., 90, 183-188.
Haseman, J.K., Huff, J.E., Rav, G.N., Arnold, J.E., Boorman, G.A. &
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57Bl/6N × C3H/HeN) F1
(B6C3F1) mice. J. Natl Cancer Inst., 75, 975-984.
Haseman, J.K., Tharrington, E.C., Huff, J.U.E. & McConnell, E.E.
(1986) Comparison of site-specific and overall tumor incidence
analysis for 81 recent National Toxicology Program carcinogenicity
studies. Reg. Toxicol. Pharmacol., 6, 155-170.
Herken, H. (1961) [Pharmacological expertise on tolerance to natural
and synthetic menthol.] Unpublished report from Pharmakologischen
Institut der Freien Universität, Berlin, Duhlen. Submitted to WHO by
Schering AG, Berlin, Germany (in German).
Horvath, T., Past, T., Tatui, Z., Par, A., Kadas, I., Tapsonyi, Z. &
Javor, J. (1984) Investigation of biotransformation capacity in
patients with chronic liver diseases by pharmacokinetic methods:
Experimental trials. Pol. J. Pharmacol. Pharm., 36, 361-371.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ivett, J.L., Brown, B.M., Rodgers, C., Anderson, B.E., Resnick, M.A. &
Zeiger, E. (1989) Chromosomal aberrations and sister chromatid
exchange tests in Chinese hamster ovary cells in vitro. IV. Results
with 15 chemicals. Environ. Mol. Mutag., 14, 165-187.
Jay, J.J. & Rivers, G.M. (1984) Antimicrobial activity of some food
flavoring compounds. J. Food Saf., 6, 129-139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kabat, G.C. & Hebert, J.R. (1991) Use of mentholated cigarettes and
lung cancer risk. Cancer Res., 51, 6510-6513.
Kaffenberger, R.M. & Doyle, M.J. (1990) Determination of menthol and
menthol glucuronide in human urine by gas chromatography using an
enzyme-sensitive internal standard and flame ionization detection.
J. Chromatogr., 527, 59-66.
Karapinar, M. & Aktug, S.E. (1987) Inhibition of food borne pathogens
by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol.,
4, 161-166.
Kurita, N., Miyaji, M., Kurane, R. & Takahara, Y. (1981) Antifungal
activity of components of essential oil. Agric. Biol. Chem., 45,
945-952.
Lewis, F.M., Shah., M. & Gawkrodger, D.J. (1995) Contact sensitivity
to food additives can cause oral and perioral symptoms. Contact
Derm., 33, 429-430.
Litton Bionetics, Inc. (1975) Mutagenic evaluation of compound FDA
71-57, menthol, 14 January 1975. US National Technical Information
Service report No. PB-245444. Submitted to WHO by International
Federation of Chewing Gum Associations, Keller and Hechman LLP,
Washington DC, United States
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Madyastha, K.M. & Srivatsan, V. (1988) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Mahmoud, A.L.E. (1994) Antifungal action and antiaflatoxigenic
properties of some essential oil constituents. Lett. Appl.
Microbiol., 19, 110-113
Megalla, S.E., El-Keltawi, N.E. & Ross, S.A. (1980) A study of
antimicrobial action of some essential oil constituents. Herba
Pol., 26, 181-186.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Millard, L.G. (1973) Contact sensitivity to toothpaste. Br. Med.
J., i, 676.
Mœlck, A.M., Poulsen, M., Lauridsen, S.T. & Olsen, P. (1998) Lack of
histological cerebellar changes in Wistar rats given pulegone for 28
days. Comparison of immersion and perfusion tissue fixation.
Toxicol. Lett., 95, 117-122.
Moleyar, V & Narasimham, P. (1992) Antibacterial activity of essential
oil components. Int. J. Food Microbiol., 16, 337-342.
Morris, J.A., Khettry, A. & Seitz, E.W. (1979) Antimicrobial activity
of aroma chemicals and essential oils. J. Am. Oil Chem. Soc., 56,
595-603.
Morton, C.A., Garioch, J., Todd, P., Lamey, P.J. & Forsyth, A. (1995)
Contact sensitivity to menthol and peppermint in patients with
intra-oral symptoms. Contact Derm., 32, 281-284.
Muller-Riebau, F., Berger, B. & Yegen, O. (1995) Chemical composition
and fungitoxic properties to phytopathogenic fungi of essential oils
of selected aromatic plants growing wild in Turkey. J. Agric. Food
Chem., 43, 2262-2266.
Murthy, P.B., Ahmed, M.M. & Regu, K. (1991) Lack of genotoxicity of
menthol in chromosome aberration and sister chromatid exchange assays
using human lymphocytes in vitro. Toxicol. In Vitro, 5, 337-340.
Myhr, B.C. & Caspary, W.C. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutag., 18, 51-83.
Nachev, C., Zolotovitch, G., Siljanovska, K. & Stojcev, S. (1967)
Cytotoxic effect of alcohols isolated from essential oils. C.R.
Acad. Bulg. Sci., 20, 1081-1084.
Niestijl Janson, J.J., Kardinaal, A.F.M., Huijbers, G.,
Vlieg-Boerstra, B.J., Martens, B.P.M. & Ockhuizen, T (1994) Prevalence
of food allergy and intolerance in the adult Dutch population.
J. Allergy Clin. Immunol., 93, 446-456.
Nohmi, T., Miyata, R., Yoshilawa, K. & Ishidate, M., Jr (1985)
Mutagenicity tests on organic chemical contaminants in city water and
related compounds. I. Bacterial mutagenicity tests. Eisei Shikenjo
Hokoku, 103, 60-64.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Nihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria (1st report).
Shokunin Eisei Hen, 9, 177-181.
Ohta, T., Watanabe, M., Watanabe, K., Shirasu, Y. & Kada, T. (1986)
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24, 51-54.
Olowe, S.A. & Ransome-Kuti, O. (1980) The risk of jaundice in
glucose-6-phospate dehydrogenase deficient babies to menthol.
Acta Paediatr. Scand., 69, 341-345.
Quick, A.J. (1924) The synthesis of menthol glycuronic acid in the
rabbit. J. Biol. Chem., 61, 679-683.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Rakieten, N., Rakieten, M.L. & Boykin, M. (1954) Effects of menthol
vapor on the intact animal with special reference to the upper
respiratory tract. J. Am. Pharm. Assoc. Sci. Ed., 43, 390-392.
Ruch, R.J. & Sigler, K. (1994) Growth inhibition of rat liver
epithelial tumor cells by monoterpenes does not involve rat plasma
membrane association. Carcinogenesis, 15, 787-789.
Russin, W.A., Hoesly, J.D., Elson, C.E., Tanner, M.A. & Gould, M.
(1989) Inhibition of rat mammary carcinogenesis by monoterpenoids.
Carcinogenesis, 10, 2161-2164.
Shah, M., Lewis, F.M. & Gawkrodger, D.J. (1996) Contact allergy in
patients with oral symptoms: A study of 47 patients. Am. J.
Contact Derm., 7, 146-151.
Shelby, M.D., Erexson, G., Hook, G.L. & Tice, R.R. (1993) Evaluation
of a three-exposure mouse bone marrow micronucleus protocol: Results
with 49 chemicals. Environ. Mol. Mutag., 21, 160-179.
Sofuni, T., Hayashi, M., Matsuoka, A., Sawada, M., Hatanaka, M. &
Ishidate, M. (1985) Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells. Eisei Shikenjo
Hokoku, 103, 64-75.
Somerville, K.W., Richmond, C.R. & Bell, G.D. (1984) Delayed release
peppermint oil capsules (Colpermin) for the spastic colon syndrome: A
pharmacokinetic study. Br. J. Clin. Pharmacol., 18, 638-640.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stoner, G.D. (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Exp. Lung Res., 17,
405-423.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Storer, R.D., McKelvey, T.W., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W. & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution rat hepatocyte assay for DNA damage:
Improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368, 59-101.
Szabo, L., & Ebrey, P. (1963) Studies on the inheritance of
Crigler-Najjar's syndrome by the menthol test. Acta Pediatr. Acad.
Sci. Hung., 4, 153-158.
Takacs, A.L. & Vore, M. (1987) Binding of 3H-estradiol-17B
(B-D-glucuronide), a cholestatic organic anion, to rat liver plasma
membranes. Evidence consonant with identification of organic anion
carriers. Mol. Pharmacol., 32, 511-518.
Tamaoki, J., Chiyotani, A., Sakai, A., Takemura, H. & Konno, K. (1995)
Effect of menthol vapour on airway hyper responsiveness in patients
with mild asthma. Respir. Med., 89, 503-504.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnik, M., Stasiewicz, S., Anderson,
B. & Minor, R. (1987) Prediction of chemical carcinogenicity in
rodents from in vitro genetic toxicity assays. Science, 236,
933-941.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Ueda, S., Yamashita, H. & Kuwabara, Y. (1982) Inhibition of
Clostridium botulinum and Bacillus sp. by spices and flavoring
compounds. Nippon Shokuhin Kogyo Gakkaishi, 29, 389-392.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T. &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA synthesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens, screening of 22 known positives and
25 noncarcinogens. Mutat. Res., 320, 189-205.
US National Cancer Institute (1979) Bioassay of DL-menthol for
possible carcinogenicity. Report NCI-CG-TR-98. US National Technical
Information Service report No. PB-288761, Bethesda, Maryland, United
States.
White, D.A., Thompson, S.A., Wilson, C.G. & Bell, G.D. (1987) A
pharmacokinetic comparison of 2 delayed-release peppermint oil
preparations, Colpermin and Mintec, for treatment of the irritable
bowel syndrome. Int. J. Pharmacol., 40, 151-155.
WHO (1987) Principles for the Safety Assessment of Food Additives
and Contaminants in Food (Environmental Health Criteria 70), Geneva
Williams, R.T. (1938) Studies in detoxication. II. (a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol and dl-isomenthol in the rabbit; (b)
d-isoMenthylglucuronide, a new conjugated glucuronic acid. Biochem.
J., 32, 1849-1855.
Williams, R.T. (1939) CLXXXVI. Studies in detoxication. III. The use
of the glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 32, 1519-1524.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Cent., 34, 267.
Yoshikawa, R.T. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: Nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspectives, 104, 40-46.
Yousef, R.T., Aggag, M.E. & Tawil, G.G. (1978) Evaluation of the
antifungal activity of some components of volatile oils against
dermatophytes. Mykosen, 21, 190-193.
Menthol has been used to detect and confirm the presence of a rat
liver DNA fraction coding for glucuronide transferase activity (Green
et al., 1995).
The efficient glucuronidation of menthol was used as a model in a
competitive reaction to evaluate oestradiol glucuronide binding sites
in rat liver plasma membranes. Menthol had a stronger affinity for
plasma binding sites than oestradiol glucuronide (Takacs & Vore,
1987).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the toxicity of single doses of menthol are summarized
in Table 1. It has also been reported that the LD50 values in rats
and mice are > 4000 mg/kg bw (Mengs & Stotzem, 1989).
Table 1. Studies of the acute toxicity of menthol
Species Route LD50 (mg/kg bw) Reference
(-)-Menthol
Mouse Oral 4 380 Litton Bionetics, Inc. (1975)
Rat Oral 940 Litton Bionetics, Inc. (1975)
3 300 JECFA (1976)
Cat Oral 800-1 000 JECFA (1976)
Mouse Subcutaneous 5 000-6 000 JECFA (1976)
Intraperitoneal 2 000 JECFA (1976)
Guinea-pig Intraperitoneal 4 000 JECFA (1976)
Rat Subcutaneous 1 000-2 500 JECFA (1976)
Intraperitoneal 710 JECFA (1976)
Cat Intraperitoneal 800-1 000 JECFA (1976)
Intravenous 34 JECFA (1976)
(±)-Menthol
Mouse Oral 3 100 Wokes (1932)
Rat Oral 2 900 JECFA (1976)
3 180 Jenner et al. (1964)
Cat Oral 1 500-1 600 JECFA (1976)
Mouse Subcutaneous 14 000-16 000 JECFA (1976)
Rat Intraperitoneal 750 JECFA (1976)
Cat Intraperitoneal 1 500-1 600 JECFA (1976)
Rabbit Intraperitoneal 2 000 JECFA (1976)
2.2.2 Short-term studies of toxicity
Mice
Groups of six male mice were given (-)-menthol at doses of 2000,
2500, 3200, 4000, or 5000 mg/kg bw by gavage for five days and
examined for 14 days. Gross necropsy of animals that died or were
killed at termination revealed no abnormal finding. The LD50 was
calculated to be 2600 mg/kg bw (Litton Bionetics, Inc., 1975).
Groups of 10 male and 10 female B6C3F1 mice were maintained on
diets containing (±)-menthol at concentrations of 0, 930, 1870, 3750,
7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 140, 280,
560, 1100, and 2300 mg/kg bw per day, respectively. Necropsies were
performed on all animals at the end of the study; histopathological
examination was performed on tissues from the control animals and
those at 2300 mg/kg bw per day and on selected tissues from animals at
1100 mg/kg bw per day. Six mice (sex not specified) died during the
study, but the deaths could not be attributed to treatment. The final
mean body weights of the treated mice were not statistically
significantly different from those of the controls, except for females
at the high dose which had statistically significant decreased body
weights. Slight increases in the incidences of perivascular lymphoid
hyperplasia and interstitial nephritis were reported for female mice
at the two highest doses. The NOEL was 560 mg/kg bw per day (US
National Cancer Institute, 1979).
Rats
No adverse effects on weight gain, excretion of glucuronides,
water, or electrolytes, or interference with central nervous system
reactions to stimulants were observed when groups of 40 rats of each
sex were fed (-)-or (±)-menthol in the diet for 5.5 weeks at doses of
0, 100, or 200 mg/kg bw per day (Herken, 1961).
In groups of three to four adult male Wistar rats given 1%
menthol in the diet for two weeks, increased serum cholesterol and
serum triglycerides were observed, but there was no effect on apo A-1
lipids, an indicator of high-density lipoprotein status. Body weight
and liver weight were unaffected (Imaizumi et al., 1985).
In a series of studies, groups of 10 rats of each sex were given
menthol in a soybean oil solution at 5 ml/kg bw at doses of 0, 200,
400, or 800 mg/kg bw per day by gavage for 28 days. Animals were
housed two to a cage. Body weight and consumption of food and water
were recorded weekly, and blood samples were collected for
determination of haemoglobin, packed cell volume, erythrocyte,
leukocyte, and reticulocyte counts, glucose, creatinine, and urea
concentrations, and the activity of aspartate aminotransferase. Urine
was examined for blood, ketones, glucose, and proteins. A conventional
selection of tissues was examined at termination.
Significant increases in the absolute and relative weights of the
liver were seen in males at all doses and in females at the
intermediate and high doses. Vacuolization of hepatocytes was seen in
0/20 controls, 4/20 rats at 200 mg/kg bw per day, 5/19 at 400 mg/kg bw
per day, and 4/17 at 800 mg/kg bw per day. No effects were seen on
other parameters measured. The vacuolization was not dose related and
may have reflected adaptation (Thorup et al.,1983a,b; Madsen et al.,
1986).
While the major constituent of peppermint oil is menthol, it also
contains other constituents. Although its effects cannot be assigned
entirely to menthol, dose-proportionate menthol-attributable effects
can be expected. The studies of peppermint oil administered by gavage
include a 28-day study in Wistar rats (Thorup et al., 1983b) at 0, 10,
40, or 100 mg/kg bw per day; a five-week study in Wistar rats and dogs
at 0, 25, or 125 mg/kg bw per day (Mengs & Stotzem, 1989); and a
90-day study in Wistar rats at 0, 10, 40, or 100 mg/kg bw per day
(Spindler & Madsen, 1992). Significant effects were seen on organ
weights and liver morphology, but no histopathological changes were
observed at these doses.
Feeding of terpenoid substances present in mint oils (see the
safety evaluation of substances structurally related to menthol, p.
381) affected the morphology of rat cerebellum. Similar effects were
not seen in the 28-day study of menthol, however, and a subsequent
review of the slides indicated that the observations were artefacts
(Adams et al., 1996), as supported by the results of a recent study
(Mœlck et al., 1998).
Groups of 10 female and 10 male Fischer 344 rats were maintained
on diets containing (±)-menthol at concentrations of 0, 930, 1900,
3800, 7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 93,
190, 380, 750, or 1500 mg/kg bw per day. Necropsies were performed on
all animals at the end of the study; histopathological examination was
performed on tissues from the control animals and those at 1500 mg/kg
bw per day and on selected tissues from animals at 750 mg/kg bw per
day. The final mean body weights of treated rats were similar to those
of the controls. A slight increase in the incidence of interstitial
nephritis was observed in male rats at the highest dose. The NOEL was
750 mg/kg bw per day (US National Cancer Institute, 1979).
Because of its pharmacological properties, menthol has also been
tested by intraperitoneal, intravenous, inhalation, and dermal
administration. As noted above, oral administration results in
substantial biotransformation, whereas these processes are largely
by-passed when it is given by the other routes, and the results of
these studies are not applicable to this safety evaluation.
Furthermore, some short-term studies result in biological end-points
such as antispasmodic activity, which are not of primary relevance to
the food uses of menthol. Studies relevant to intake of menthol from
food are summarized below.
The previous monograph summarized studies on the antispasmodic
activity of pharmaceutical preparations on intestinal tissue (Annex 1,
reference 42), although they contained peppermint oil rather than
menthol per se (Bowen & Cubbin, 1992). The doses of menthol
necessary to obtain this effect are significantly higher than the
amounts present in foods.
The pharmacological effect of menthol on the respiratory tract
has also been studied. In a 71-79-day study with young albino Sherman
rats, inhalation of (-)-menthol vapour at 0.087, 0.15, or 0.26 ppm
caused no gross changes. Histological examination showed effects on
lung tissue only at the highest dose, at which irritation also
occurred (Rakieten et al., 1954).
2.2.3 Long-term studies of toxicity and carcinogenicity
2.2.3.1 Feeding studies
Mice
Groups of 50 B6C3F1 mice of each sex were given (±)-menthol at
doses of 0, 2000, or 4000 mg/kg diet daily for 103 weeks, equivalent
to 300 or 600 mg/kg bw per day, respectively. Animals were housed five
per cage and were observed twice daily for signs of toxicity. Body
weights and food consumption was recorded every two weeks for the
first 12 weeks and once a month thereafter. Necropsies and
histological examinations were performed on all animals at termination
of the study and on those found dead during the study.
The mean body weights of the treated mice were slightly lower
than those of the controls. The survival of males was similar to that
of controls, but females at the high dose had statistically
significantly worse survival rates; subsequent evaluation (Haseman et
al., 1985) showed, however, that the survival of all female mice was
within the range for historical controls, and the survival rate of the
control group in this study was at the high end of the range. The
survival of animals at the high dose was in fact closer to the
historical average, and there was no evidence of toxicity in this
group. An increased incidence of hepatocellular carcinoma was observed
in males at the high dose, but the increase was not significantly
different from that of concurrent or historical control mice of that
age and strain (Haseman et al., 1986). A low incidence of alveolar or
bronchiolar adenomas of the lung was observed in treated females, but
the rate was not significantly different from that in historical
controls. It was concluded that (±)-menthol is not carcinogenic and
has no organ-specific toxicity in B6C3F1 mice of either sex at the
doses tested (US National Cancer Institute, 1979).
Rats
Groups of 50 Fischer 344 rats of each sex were given 0, 3750, or
7500 mg/kg diet (±)-menthol in their feed daily for 103 weeks,
equivalent to 190, and 380 mg/kg bw per day, respectively. Animals
were housed five per cage until week 48, when the male rats were
divided into groups of two to three per cage. The animals were
observed twice daily for signs of toxicity. Body weight and food
consumption were recorded every two weeks for the first 12 weeks and
once a month thereafter. Necropsies and histological examinations were
performed on all animals at the end of the study and on those found
dead during the study.
The mean body weights of treated rats were slightly lower than
those of the controls. Survival of treated rats was similar to that of
controls. Chronic inflammation of the kidney was observed in the older
treated males, but was not considered to be related to administration
of menthol since the effect is commonly observed in aged male Fischer
344 rats. The incidence of neoplasms was not increased in treated
females, and, in fact, fibroadenomas of the mammary glands occurred at
lower incidences in treated (10/49 at the low dose and 7/49 at the
high dose) than in control animals (20/50). Alveolar or bronchiolar
adenomas or carcinomas were reported only in female controls. It was
concluded that (±)-menthol is neither carcinogenic nor toxic for
Fischer 344 rats of either sex at the doses tested (US National Cancer
Institute, 1979).
2.2.3.2 Other studies
Groups of 30 female A/He strain mice received 2000 mg/kg bw
menthol dissolved in tricaprylin, the maximum dose tolerated after six
intraperitoneal injections over two weeks, and one-quarter this dose,
500 mg/kg bw, three times weekly for eight weeks. The animals were
observed for an additional 16 weeks. A group of 24 control female mice
received a similar number of injections of tricaprylin. All animals
that survived treatment were killed after 24 weeks and the numbers of
pulmonary adenomas counted. No increase in the incidence of
non-neoplastic or neoplastic lesions was reported in the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands of
treated animals. Approximately 30-45% of the menthol-treated animals
and 15% of the control animals died before the end of the study. The
authors reported that tricaprylin is an unsuitable vehicle for
bioassays, as it caused a 3- or 4-g weight loss in the control animals
during the first week of the study, a high mortality rate, and a
higher mean tumour rate. In the same test system, urethane (total
dose, 10 or 20 mg) and several alkylating agents induced marked
increases in the incidence of pulmonary adenomas, but other substances
shown to be carcinogenic in other test systems, e.g. safrole, caused
no increase (Stoner et al., 1973; Annex 1, reference 42). A
retrospective evaluation of the lung tumour response in strain A mice
is provided by Stoner (1991).
Menthol has been studied for its chemopreventive effect on
ras-mediated tumour growth in four types of rat liver cell
in vitro, with lovastatin as the positive control. Concentrations of
0.1-2.5 mmol/L of menthol inhibited tumour growth, but the authors
concluded that the mechanism of the chemopreventive effect of menthol
is different from that of lovastatin (Ruch & Sigler, 1994).
In a study of the preventive activity of menthol against
7,12-dimethylbenz [a]anthracene (DMBA)-induced mammary tumours in
rats, a diet containing 1% menthol was fed from two weeks before DMBA
treatment to up to 22 weeks after treatment. A reduction in mammary
tumour incidence was seen. A diet containing 0.5% menthol fed from two
weeks before DMBA treatment until one week after treatment also
resulted in a reduction in tumour incidence (Russin et al., 1989;
Gould et al., 1990).
In studies of mentholated cigarettes in animals and humans, the
presence of menthol in cigarettes did not enhance the incidence of
lung cancer over that due to smoking unmentholated cigarettes (Kabat &
Hebert, 1991; Gaworski et al., 1997).
2.2.4 Genotoxicity
The results of tests for genotoxicity with menthol are presented
in Table 2.
Menthol was administered at 725 mg/kg bw or the maximum tolerated
dose of 1450 mg/kg bw to male Fischer 344 rats and male B6C3F1 mice.
Hepatocytes were removed at 24, 39, and 49 h, and replicative DNA
synthesis was measured. Synthesis was increased in 6% of the rats and
1.7% of the mice. This assay indicates cell replication (i.e.
mitogenesis), however, and not genotoxicity (Uno et al., 1994;
Yoshikawa, 1996).
2.2.5 Developmental toxicity
Mice
(-)-Menthol was administered in corn oil by gavage to 22 or 23
pregnant CD-1 mice at 0, 1.9, 8.6, 40, or 190 mg/kg bw per day on days
6-15 of gestation; to 22 or 23 pregnant Wistar rats at 0, 2.2, 10, 47,
or 220 mg/kg bw per day on days 6-15 of gestation; to 21-23 golden
hamsters at 0, 4.1, 21, 98, or 400 mg/kg bw per day on days 6-10 of
gestation; and to 11 to 14 Dutch-belt rabbits at 0, 4.3, 20, 92, or
430 mg/kg bw per day on days 6-18 of gestation. Control groups for
each species were sham treated; positive control groups for each
species were given 150 or 250 mg/kg bw per day aspirin. Body weights
were recorded on three or four days during the gestation period. All
animals were observed daily for appearance, behaviour, and food
consumption. On the scheduled day, the fetuses were removed from all
dams and dams and fetuses examined. One-third of the fetuses from each
group underwent detailed visceral examination; the other two-thirds
were examined for skeletal defects. There were no effects on nidation,
maternal survival, fetal survival, or fetal abnormalities. The numbers
of abnormalities seen in soft or skeletal tissues of treated animals
did not differ from those occurring spontaneously in the sham-treated
controls (Food and Drug Research Labs, Inc., 1973).
2.2.6 Special studies
2.2.6.1 Cellular and biochemical effects
Menthol has been tested for potential bactericidal and fungicidal
effects on microorganisms that are foodborne or found in the oral
cavity. Menthol at concentrations of 0.1-5 mmol/L was cytotoxic and
affected tissue processes in trachea from chick embryos, ascites
sarcoma BP8 cells, isolated hamster brown adipocytes, and rat liver
mitochondria (Bernson & Pettersson, 1983). Menthol had no cytotoxic
effect on human HeLa cells in vitro at concentrations of 1, 10, and
100 µg/ml (Nachev et al., 1967).
Menthol was lethal to E. coli at a concentration of 0.5%
(Wokes, 1932) or 0.05% (Morris et al., 1979; Megalla et al., 1980; Jay
& Rivers, 1984); to Salmonella typhimurium at a concentration of
0.05% (Karapinar & Aktug, 1987); to Clostridium at 0.05% (Ueda et
al., 1982); to Staphylococcus at 0.05% (Wokes, 1932; Morris et al.,
1979; Karapinar & Aktug, 1987; Moleyar & Narasimham 1992) or 0.003%
(Jay & Rivers, 1984); to Vibrio spp. at 0.05% (Karapinar & Aktug;
1987); and to Bacillus spp. at 0.05% (Morris et al., 1979; Megalla
et al., 1980; Ueda et al., 1982; Moleyar & Narasimham, 1992). It was
cytotoxic to spoilage fungi at 0.08-0.3% (Yousef et al., 1978; Kurita
et al., 1981), 0.05% (Morris et al., 1979; Megalla et al., 1980;
Mahmoud, 1994; Muller-Reibau et al., 1995), or 0.025% (Jay & Rivers,
1984).
The results of these studies are difficult to extrapolate to the
situation in experimental animal in vivo, and cannot be used
directly in the safety assessment of menthol. Furthermore, the
concentration at which cytotoxicity was observed is considerably
higher than those present in the diets tested.
2.2.6.2 Immunotoxicity and sensitivity
The presence of menthol and menthol-containing flavour and
fragrance oils at high concentrations in consumer products such as
cigarettes, toothpaste, and topical medications has led to sensitivity
reactions in the oral and nasal cavity (e.g. Millard, 1973;
Dooms-Goossens et al., 1977; Lewis et al., 1995; Morton et al., 1995;
Shah et al., 1996).
The preferred method for testing sensitivity or food intolerance
is the double-blind placebo-controlled food challenge. In a study on
the prevalence of food allergy and food intolerance in 1483 Dutch
adults by this method, it was estimated that the prevalence of
sensiivity or intolerance was 0.8-2.4% of the adult population. Within
the sampled population, 73 subjects reported such effects, and 12 of
the responses were confirmed. Only one of these responders had a
reaction to menthol challenge; the subject reported 'aggravation of
aphthae' (whitish spots in the mouth that characterize apthous
stomatitis) 1 h after administration (Niestijl Janson et al., 1994).
2.3 Observations in humans
Menthol has been tested in humans mainly for its potential
pharmaceutical properties, such as enhancement of lung and airway
volume (e.g. Bowen & Cubbin, 1992). The usual human oral dose is
60-120 mg menthol per person. It can be estimated from unreferenced
citations in pharmaceutical texts, such as Gleason et al. (1969), that
the lethal human dose is 50-500 mg/kg bw. In section 2.1.1, the
maximum doses tested were 180 mg (Kaffenberger & Doyle, 1990) and 1 g
(Quick, 1928).
The airway hyperresponsiveness of 23 human subjects with chronic
mild asthma was tested by use of a nebulizer containing menthol twice
a day for four weeks. As measured by expiratory flow rates, vital
capacity, and forced expiratory volume, menthol improved airway
hyperresponsiveness at doses as low as 20 mg (Tamaoki et al., 1995).
The efficient hepatic glucuronidation of menthol has been
investigated as a possible basis for a test of liver function. In a
study of the output of menthol glucuronide by normal subjects and
subjects with alcohol-induced cirrhosis and steatosis of the liver, it
was concluded that the group differences were not sufficient to
justify use of menthol glucuronide output as a test for liver disease.
The results demonstrate that even a compromised liver has sufficient
capacity to handle menthol (Szabo & Ebrey, 1963; Horvath et al.,
1984).
A controlled study of the effects of a mentholated powder was
carried out on 60 consecutive glucose-6-phosphate
dehydrogenase-deficient babies. The umbilical cords of 30 babies were
treated daily with the powder, while the remainder served as controls.
Significantly more of the treated babies developed severe jaundice
than the controls. The inability of neonates to conjugate menthol was
probably responsible for the increased severity of the jaundice
developed by the deficient babies. It was concluded that the use of
menthol-containing products on neonates should be discontinued,
especially in communities where the incidence of glucose-6-phosphate
dehydrogenase deficiency is high (Olowe & Ransome-Kuti, 1980). As
neonates do not have an oral intake of menthol, the relevance of these
results is limited. No data were available on the possible effects of
menthol in older children or in adults with glucose-6-phosphate
dehydrogenase deficiency.
Table 2. Results of assays for the genotoxicity of menthol
End-point Test object Concentration Results Reference
In vitro
(±)-Menthol
Reverse mutation S. typhimurium TA92, TA1535, < 5 mg/plate Negativea Ishidate et al. (1984)
TA100, TA1537, TA94, TA98
Reverse mutation S. typhimurium 666 µg/plate Negativea Tennant et al. (1987)
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)
TA98
Antimutagenicity S. typhimurium TA98 < 200 µg/ml Negative Ohta et al. (1986)
Antimutagenicity Escherichia coli < 200 µg/ml Negative Ohta et al. (1986)
Chromosomal aberration Chinese hamster fibroblasts < 0.2 mg/ml Negative Ishidate et al. (1984)
Chromosomal aberration Chinese hamster lung fibroblasts < 0.3 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Chinese hamster ovary cells < 250 µg/ml Negative Tennant et al. (1987)
and sister chromatid
exchange
Chromosomal aberration Chinese hamster ovary cells < 167 µg/ml Negative Ivett et al. (1989)
and sister chromatid
exchange
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Tennant et al. (1987)
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Myhr & Caspary (1991)
(-)-Menthol
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)TA98
Reverse mutation S. typhimurium TA98, TA100, 6.4-800 µg/plate Negativea Andersen & Jennies (1984)
TA1535, TA1537
Reverse mutation E. coli WP2 uvrA 0.1-0.8 mg/plate Negative Yoo (1986)
Reverse mutation S. typhimurium TA1530, G46 0.25 ml/plate Negative Litton Bionetics, Inc. (1975)
Antimutagenicity E. coli WP2 uvrA 0.5-2.0 mg/ml Negative Yoo (1986)
DNA repair Bacillus subtilis < 10 mg/disk Negative Yoo (1986)
Gene mutation Bacillus subtilis < 20 mg/plate Negative Oda et al. (1978)
Gene mutation Saccharomyces cerevisiae D3 0.2 ml/plate Equivocal Litton Bionetics, Inc. (1975)
Table 2. (continued)
End-point Test object Concentration Results Reference
Chromosomal aberration Chinese hamster lung fibroblasts < 0.125 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Human WI-38 embryonic lung cells 10 mg/ml Negative Litton Bionetics, Inc. (1975)
Chromosomal aberration Human peripheral blood lymphocytes 0.1-10 mmol/L Negativea Murthy et al. (1991)
and sister chromatid
exchange
(+)-Menthol
DNA damage Rat hepatocytes 0.7-1.3 mmpl/L Positive Storer et al. (1996)
DNA damage Chinese hamster V79 cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
DNA damage Human blood cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
In vivo
(±)-Menthol
Micronucleus formation Male B6C3F1 mouse bone marrow < 1 g/kg bw Negative Shelby et al. (1993)
(-)-Menthol
Host-mediated S. typhimurium TA1530, G46/ < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity ICR mouse host
Host-mediated S. cerevisiae D3/ICR mouse host < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity
Chromosomal aberration Albino rat bone marrow < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
Dominant lethal mutation Rat < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
a With and without an exogenous metabolic activation system
3. COMMENTS
Menthol exists as two optical isomers, (+)-menthol and
(-)-menthol; the racemic mixture is (±)-menthol. The (-) and (±) forms
are used in flavour applications.
Menthol is readily absorbed. Up to 100% of an ingested dose
appeared to be absorbed, on the basis of the elimination of menthol
metabolites in faeces and urine. Absorbed menthol is known to be
largely eliminated as glucuronides; 70-80% is eliminated in urine and
faeces within 48 h. Metabolic studies indicate that oral doses of
menthol are metabolized mainly in the liver and excreted via the
kidneys and in the bile. Menthol is efficiently metabolized by normal
processes. The metabolites are simple glucuronic acid conjugates and
oxidation products. Mammals can efficiently handle menthol by
processes that do not create hazardous products.
The NOEL in 13-week studies of toxicity with (±)-menthol in the
diet was 560 mg/kg bw per day in mice and 750 mg/kg bw per day in rats
on the basis of slightly increased incidences of interstitial
nephritis at the next highest dose.
In a two-year study of toxicity and carcinogenicity, mice were
fed (±)-menthol in the diet at concentrations equivalent to 300 or 600
mg/kg bw per day. The incidences of hepatocellular tumours in males
and lung tumours in females at the highest dose were not significantly
different from those in concurrent or historical controls. The
survival rate was decreased in female mice but remained within the
range of that of historical controls. The NOEL was 600 mg/kg bw per
day. In a two-year study of toxicity and carcinogenicity in rats given
(±)-menthol in the diet at concentrations equivalent to 190 or
380 mg/kg bw per day, the NOEL was 380 mg/kg bw per day.
Neither menthol nor its metabolites were genotoxic in vitro or
in vivo.
While no studies of reproductive toxicity were available with
menthol, (-)-menthol was tested at maximum doses of 190-430 mg/kg bw
per day for teratogenicity in mice, rats, hamsters, and rabbits; no
teratogenic effects were observed.
4. EVALUATION
The limited data that allow comparisons of metabolism and
toxicity provide no indication of a difference in the toxicity of
(-)-menthol and (±)-menthol. Therefore, the Committee concluded that
an ADI could be established for the two optical isomers of menthol.
The Committee noted that the highest dose of (±)-menthol tested
in the long-term studies in mice and rats had no specific toxic
effect. As the survival of mice was reduced at the high dose of
600 mg/kg bw per day, the Committee allocated an ADI of 0-4 mg/kg bw
on the basis of the NOEL of 380 mg/kg bw per day in the long-term
study in rats, applying a safety factor of 100 and rounding to one
significant figure.
5. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Andersen, P.H. & Jennies, N.J. (1984) Mutagenic investigation of
peppermint oil in the Salmonella/mammalian-microsome test.
Mutat. Res., 138, 17-20.
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Austin, C.A., Shephard, E.A., Pike, S.F., Rabin, B.R. & Phillips, I.R.
(1988) The effect of terpenoid compounds on cytochrome P-450 levels in
rat liver. Biochem. Pharmacol., 37, 2223-2229.
Batt, A.M., Martin, N. & Siest, G. (1981) Induction of group-1 and
group-2 UDP-glucuronosyl transferase in microsomes from the livers of
C57 Bl/6 mice. Toxicol. Lett., 9, 355-360.
Bernson, V.S.M. & Pettersson, B. (1983) The toxicity of menthol in
short-term bioassays. Chem.-Biol. Interactions, 46, 233-246.
Boutin, J.A., Lepage, C., Batt, A.M. & Siest, G. (1981) The activity
of hepatic UDP-glucuronosyltransferase from control and induced pigs
toward 17 hydroxylated aglycones. IRCS Med. Sci., 9, 633-634.
Boutin, J.A., Thomassin, G., Siest, G. & Cartier, A. (1985)
Heterogeneity of hepatic microsomal UDP-glucuronosyltransferase
activities. Conjugations of phenolic and monoterpenoid aglycons in
control and induced rats and guinea pigs. Biochem. Pharmacol., 34,
2235-2249.
Bowen, I.H. & Cubbin, I.J. (1992) Mentha piperita and Mentha spicata.
In: Adverse Effects of Herbal Drugs, pp. 171-178.
Clegg, R.J., Middleton, B., Bell, G.D. & White, D.A. (1982) The
mechanism of cyclic monoterpene inhibition of hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase in vivo in the rat.
J. Biol. Chem., 257, 2294-2299.
Deichmann, W. & Thomas, G. (1943) Glucuronic acid in the urine as a
measure of the absorption of certain organic compounds. J. Ind.
Hyg. Toxicol., 25, 286-292.
Dooms-Goossens, A., Degreef, H., Holvoet, C. & Maertens, M. (1977)
Turpentine-induced hypersensitivity to peppermint oil. Contact
Derm., 3, 304-308.
Eisenberg, F., Field, J.B. & Stetten, D. (1955) Studies on glyceronide
conjugation in man. Arch. Biochem. Biophys., 59, 297-299.
Fishman, W.H. (1940) Studies on beta-glucuronidase. III. The increase
in beta-glucuronidase activity of mammalian tissues induced by feeding
glucuronidogenic substances. J. Biol. Chem., 136, 229-236.
Food & Drug Research Labs, Inc. (1973) Teratologic evaluation of FDA
71-57 (menthol natural Brazilian), June 1973. US National Technical
Information Service report No. PB-223815. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Gaworski, C.L., Dozier, M.M., Gerhart, J.M., Rajendrans, N.,
Brennecke, L.H., Aranyi, C. & Heck, J.D. (1997) 13-Week inhalation
toxicity study of menthol cigarette smoke. Food Chem. Toxicol., 35,
683-692.
Gleason, M.N., Gosselin,R.E., Hodge, H.C. & Smith, R.P. (1969)
Clinical Toxicology of Commercial Products, 3rd Ed., Philadelphia,
Williams & Wilkins Co.
Gould, W.D., Wacker, P. & Maltzman, T.H. (1990) Chemoprevention and
chemotherapy of mammary tumors by monoterpenoids. Prog. Clin. Biol.
Res., 347, 255-268.
Green, M.D., Clarke, D.J., Oturu, E.M., Styczynski, P.B., Jackson,
M.R., Burchell, B. & Tephly, T.R. (1995) Cloning and expression of a
rat liver phenobarbital-inducible UDP-glucuronosyl-transferase (2B12)
with specificity for monoterpenoid alcohols. Arch. Biochem.
Biophys., 322, 460-468.
Hartmann, A. & Speit, G. (1997) The contribution of cytotoxicity to
DNA-effects in the single cell gel test (Comet assay). Toxicol.
Lett., 90, 183-188.
Haseman, J.K., Huff, J.E., Rav, G.N., Arnold, J.E., Boorman, G.A. &
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57Bl/6N × C3H/HeN) F1
(B6C3F1) mice. J. Natl Cancer Inst., 75, 975-984.
Haseman, J.K., Tharrington, E.C., Huff, J.U.E. & McConnell, E.E.
(1986) Comparison of site-specific and overall tumor incidence
analysis for 81 recent National Toxicology Program carcinogenicity
studies. Reg. Toxicol. Pharmacol., 6, 155-170.
Herken, H. (1961) [Pharmacological expertise on tolerance to natural
and synthetic menthol.] Unpublished report from Pharmakologischen
Institut der Freien Universität, Berlin, Duhlen. Submitted to WHO by
Schering AG, Berlin, Germany (in German).
Horvath, T., Past, T., Tatui, Z., Par, A., Kadas, I., Tapsonyi, Z. &
Javor, J. (1984) Investigation of biotransformation capacity in
patients with chronic liver diseases by pharmacokinetic methods:
Experimental trials. Pol. J. Pharmacol. Pharm., 36, 361-371.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ivett, J.L., Brown, B.M., Rodgers, C., Anderson, B.E., Resnick, M.A. &
Zeiger, E. (1989) Chromosomal aberrations and sister chromatid
exchange tests in Chinese hamster ovary cells in vitro. IV. Results
with 15 chemicals. Environ. Mol. Mutag., 14, 165-187.
Jay, J.J. & Rivers, G.M. (1984) Antimicrobial activity of some food
flavoring compounds. J. Food Saf., 6, 129-139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kabat, G.C. & Hebert, J.R. (1991) Use of mentholated cigarettes and
lung cancer risk. Cancer Res., 51, 6510-6513.
Kaffenberger, R.M. & Doyle, M.J. (1990) Determination of menthol and
menthol glucuronide in human urine by gas chromatography using an
enzyme-sensitive internal standard and flame ionization detection.
J. Chromatogr., 527, 59-66.
Karapinar, M. & Aktug, S.E. (1987) Inhibition of food borne pathogens
by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol.,
4, 161-166.
Kurita, N., Miyaji, M., Kurane, R. & Takahara, Y. (1981) Antifungal
activity of components of essential oil. Agric. Biol. Chem., 45,
945-952.
Lewis, F.M., Shah., M. & Gawkrodger, D.J. (1995) Contact sensitivity
to food additives can cause oral and perioral symptoms. Contact
Derm., 33, 429-430.
Litton Bionetics, Inc. (1975) Mutagenic evaluation of compound FDA
71-57, menthol, 14 January 1975. US National Technical Information
Service report No. PB-245444. Submitted to WHO by International
Federation of Chewing Gum Associations, Keller and Hechman LLP,
Washington DC, United States
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Madyastha, K.M. & Srivatsan, V. (1988) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Mahmoud, A.L.E. (1994) Antifungal action and antiaflatoxigenic
properties of some essential oil constituents. Lett. Appl.
Microbiol., 19, 110-113
Megalla, S.E., El-Keltawi, N.E. & Ross, S.A. (1980) A study of
antimicrobial action of some essential oil constituents. Herba
Pol., 26, 181-186.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Millard, L.G. (1973) Contact sensitivity to toothpaste. Br. Med.
J., i, 676.
Mœlck, A.M., Poulsen, M., Lauridsen, S.T. & Olsen, P. (1998) Lack of
histological cerebellar changes in Wistar rats given pulegone for 28
days. Comparison of immersion and perfusion tissue fixation.
Toxicol. Lett., 95, 117-122.
Moleyar, V & Narasimham, P. (1992) Antibacterial activity of essential
oil components. Int. J. Food Microbiol., 16, 337-342.
Morris, J.A., Khettry, A. & Seitz, E.W. (1979) Antimicrobial activity
of aroma chemicals and essential oils. J. Am. Oil Chem. Soc., 56,
595-603.
Morton, C.A., Garioch, J., Todd, P., Lamey, P.J. & Forsyth, A. (1995)
Contact sensitivity to menthol and peppermint in patients with
intra-oral symptoms. Contact Derm., 32, 281-284.
Muller-Riebau, F., Berger, B. & Yegen, O. (1995) Chemical composition
and fungitoxic properties to phytopathogenic fungi of essential oils
of selected aromatic plants growing wild in Turkey. J. Agric. Food
Chem., 43, 2262-2266.
Murthy, P.B., Ahmed, M.M. & Regu, K. (1991) Lack of genotoxicity of
menthol in chromosome aberration and sister chromatid exchange assays
using human lymphocytes in vitro. Toxicol. In Vitro, 5, 337-340.
Myhr, B.C. & Caspary, W.C. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutag., 18, 51-83.
Nachev, C., Zolotovitch, G., Siljanovska, K. & Stojcev, S. (1967)
Cytotoxic effect of alcohols isolated from essential oils. C.R.
Acad. Bulg. Sci., 20, 1081-1084.
Niestijl Janson, J.J., Kardinaal, A.F.M., Huijbers, G.,
Vlieg-Boerstra, B.J., Martens, B.P.M. & Ockhuizen, T (1994) Prevalence
of food allergy and intolerance in the adult Dutch population.
J. Allergy Clin. Immunol., 93, 446-456.
Nohmi, T., Miyata, R., Yoshilawa, K. & Ishidate, M., Jr (1985)
Mutagenicity tests on organic chemical contaminants in city water and
related compounds. I. Bacterial mutagenicity tests. Eisei Shikenjo
Hokoku, 103, 60-64.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Nihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria (1st report).
Shokunin Eisei Hen, 9, 177-181.
Ohta, T., Watanabe, M., Watanabe, K., Shirasu, Y. & Kada, T. (1986)
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24, 51-54.
Olowe, S.A. & Ransome-Kuti, O. (1980) The risk of jaundice in
glucose-6-phospate dehydrogenase deficient babies to menthol.
Acta Paediatr. Scand., 69, 341-345.
Quick, A.J. (1924) The synthesis of menthol glycuronic acid in the
rabbit. J. Biol. Chem., 61, 679-683.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Rakieten, N., Rakieten, M.L. & Boykin, M. (1954) Effects of menthol
vapor on the intact animal with special reference to the upper
respiratory tract. J. Am. Pharm. Assoc. Sci. Ed., 43, 390-392.
Ruch, R.J. & Sigler, K. (1994) Growth inhibition of rat liver
epithelial tumor cells by monoterpenes does not involve rat plasma
membrane association. Carcinogenesis, 15, 787-789.
Russin, W.A., Hoesly, J.D., Elson, C.E., Tanner, M.A. & Gould, M.
(1989) Inhibition of rat mammary carcinogenesis by monoterpenoids.
Carcinogenesis, 10, 2161-2164.
Shah, M., Lewis, F.M. & Gawkrodger, D.J. (1996) Contact allergy in
patients with oral symptoms: A study of 47 patients. Am. J.
Contact Derm., 7, 146-151.
Shelby, M.D., Erexson, G., Hook, G.L. & Tice, R.R. (1993) Evaluation
of a three-exposure mouse bone marrow micronucleus protocol: Results
with 49 chemicals. Environ. Mol. Mutag., 21, 160-179.
Sofuni, T., Hayashi, M., Matsuoka, A., Sawada, M., Hatanaka, M. &
Ishidate, M. (1985) Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells. Eisei Shikenjo
Hokoku, 103, 64-75.
Somerville, K.W., Richmond, C.R. & Bell, G.D. (1984) Delayed release
peppermint oil capsules (Colpermin) for the spastic colon syndrome: A
pharmacokinetic study. Br. J. Clin. Pharmacol., 18, 638-640.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stoner, G.D. (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Exp. Lung Res., 17,
405-423.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Storer, R.D., McKelvey, T.W., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W. & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution rat hepatocyte assay for DNA damage:
Improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368, 59-101.
Szabo, L., & Ebrey, P. (1963) Studies on the inheritance of
Crigler-Najjar's syndrome by the menthol test. Acta Pediatr. Acad.
Sci. Hung., 4, 153-158.
Takacs, A.L. & Vore, M. (1987) Binding of 3H-estradiol-17B
(B-D-glucuronide), a cholestatic organic anion, to rat liver plasma
membranes. Evidence consonant with identification of organic anion
carriers. Mol. Pharmacol., 32, 511-518.
Tamaoki, J., Chiyotani, A., Sakai, A., Takemura, H. & Konno, K. (1995)
Effect of menthol vapour on airway hyper responsiveness in patients
with mild asthma. Respir. Med., 89, 503-504.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnik, M., Stasiewicz, S., Anderson,
B. & Minor, R. (1987) Prediction of chemical carcinogenicity in
rodents from in vitro genetic toxicity assays. Science, 236,
933-941.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Ueda, S., Yamashita, H. & Kuwabara, Y. (1982) Inhibition of
Clostridium botulinum and Bacillus sp. by spices and flavoring
compounds. Nippon Shokuhin Kogyo Gakkaishi, 29, 389-392.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T. &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA synthesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens, screening of 22 known positives and
25 noncarcinogens. Mutat. Res., 320, 189-205.
US National Cancer Institute (1979) Bioassay of DL-menthol for
possible carcinogenicity. Report NCI-CG-TR-98. US National Technical
Information Service report No. PB-288761, Bethesda, Maryland, United
States.
White, D.A., Thompson, S.A., Wilson, C.G. & Bell, G.D. (1987) A
pharmacokinetic comparison of 2 delayed-release peppermint oil
preparations, Colpermin and Mintec, for treatment of the irritable
bowel syndrome. Int. J. Pharmacol., 40, 151-155.
WHO (1987) Principles for the Safety Assessment of Food Additives
and Contaminants in Food (Environmental Health Criteria 70), Geneva
Williams, R.T. (1938) Studies in detoxication. II. (a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol and dl-isomenthol in the rabbit; (b)
d-isoMenthylglucuronide, a new conjugated glucuronic acid. Biochem.
J., 32, 1849-1855.
Williams, R.T. (1939) CLXXXVI. Studies in detoxication. III. The use
of the glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 32, 1519-1524.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Cent., 34, 267.
Yoshikawa, R.T. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: Nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspectives, 104, 40-46.
Yousef, R.T., Aggag, M.E. & Tawil, G.G. (1978) Evaluation of the
antifungal activity of some components of volatile oils against
dermatophytes. Mykosen, 21, 190-193.
See Also:
Toxicological Abbreviations
Menthol (WHO Food Additives Series 10)
MENTHOL (JECFA Evaluation)
 Menthol has been used to detect and confirm the presence of a rat
liver DNA fraction coding for glucuronide transferase activity (Green
et al., 1995).
The efficient glucuronidation of menthol was used as a model in a
competitive reaction to evaluate oestradiol glucuronide binding sites
in rat liver plasma membranes. Menthol had a stronger affinity for
plasma binding sites than oestradiol glucuronide (Takacs & Vore,
1987).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the toxicity of single doses of menthol are summarized
in Table 1. It has also been reported that the LD50 values in rats
and mice are > 4000 mg/kg bw (Mengs & Stotzem, 1989).
Table 1. Studies of the acute toxicity of menthol
Species Route LD50 (mg/kg bw) Reference
(-)-Menthol
Mouse Oral 4 380 Litton Bionetics, Inc. (1975)
Rat Oral 940 Litton Bionetics, Inc. (1975)
3 300 JECFA (1976)
Cat Oral 800-1 000 JECFA (1976)
Mouse Subcutaneous 5 000-6 000 JECFA (1976)
Intraperitoneal 2 000 JECFA (1976)
Guinea-pig Intraperitoneal 4 000 JECFA (1976)
Rat Subcutaneous 1 000-2 500 JECFA (1976)
Intraperitoneal 710 JECFA (1976)
Cat Intraperitoneal 800-1 000 JECFA (1976)
Intravenous 34 JECFA (1976)
(±)-Menthol
Mouse Oral 3 100 Wokes (1932)
Rat Oral 2 900 JECFA (1976)
3 180 Jenner et al. (1964)
Cat Oral 1 500-1 600 JECFA (1976)
Mouse Subcutaneous 14 000-16 000 JECFA (1976)
Rat Intraperitoneal 750 JECFA (1976)
Cat Intraperitoneal 1 500-1 600 JECFA (1976)
Rabbit Intraperitoneal 2 000 JECFA (1976)
2.2.2 Short-term studies of toxicity
Mice
Groups of six male mice were given (-)-menthol at doses of 2000,
2500, 3200, 4000, or 5000 mg/kg bw by gavage for five days and
examined for 14 days. Gross necropsy of animals that died or were
killed at termination revealed no abnormal finding. The LD50 was
calculated to be 2600 mg/kg bw (Litton Bionetics, Inc., 1975).
Groups of 10 male and 10 female B6C3F1 mice were maintained on
diets containing (±)-menthol at concentrations of 0, 930, 1870, 3750,
7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 140, 280,
560, 1100, and 2300 mg/kg bw per day, respectively. Necropsies were
performed on all animals at the end of the study; histopathological
examination was performed on tissues from the control animals and
those at 2300 mg/kg bw per day and on selected tissues from animals at
1100 mg/kg bw per day. Six mice (sex not specified) died during the
study, but the deaths could not be attributed to treatment. The final
mean body weights of the treated mice were not statistically
significantly different from those of the controls, except for females
at the high dose which had statistically significant decreased body
weights. Slight increases in the incidences of perivascular lymphoid
hyperplasia and interstitial nephritis were reported for female mice
at the two highest doses. The NOEL was 560 mg/kg bw per day (US
National Cancer Institute, 1979).
Rats
No adverse effects on weight gain, excretion of glucuronides,
water, or electrolytes, or interference with central nervous system
reactions to stimulants were observed when groups of 40 rats of each
sex were fed (-)-or (±)-menthol in the diet for 5.5 weeks at doses of
0, 100, or 200 mg/kg bw per day (Herken, 1961).
In groups of three to four adult male Wistar rats given 1%
menthol in the diet for two weeks, increased serum cholesterol and
serum triglycerides were observed, but there was no effect on apo A-1
lipids, an indicator of high-density lipoprotein status. Body weight
and liver weight were unaffected (Imaizumi et al., 1985).
In a series of studies, groups of 10 rats of each sex were given
menthol in a soybean oil solution at 5 ml/kg bw at doses of 0, 200,
400, or 800 mg/kg bw per day by gavage for 28 days. Animals were
housed two to a cage. Body weight and consumption of food and water
were recorded weekly, and blood samples were collected for
determination of haemoglobin, packed cell volume, erythrocyte,
leukocyte, and reticulocyte counts, glucose, creatinine, and urea
concentrations, and the activity of aspartate aminotransferase. Urine
was examined for blood, ketones, glucose, and proteins. A conventional
selection of tissues was examined at termination.
Significant increases in the absolute and relative weights of the
liver were seen in males at all doses and in females at the
intermediate and high doses. Vacuolization of hepatocytes was seen in
0/20 controls, 4/20 rats at 200 mg/kg bw per day, 5/19 at 400 mg/kg bw
per day, and 4/17 at 800 mg/kg bw per day. No effects were seen on
other parameters measured. The vacuolization was not dose related and
may have reflected adaptation (Thorup et al.,1983a,b; Madsen et al.,
1986).
While the major constituent of peppermint oil is menthol, it also
contains other constituents. Although its effects cannot be assigned
entirely to menthol, dose-proportionate menthol-attributable effects
can be expected. The studies of peppermint oil administered by gavage
include a 28-day study in Wistar rats (Thorup et al., 1983b) at 0, 10,
40, or 100 mg/kg bw per day; a five-week study in Wistar rats and dogs
at 0, 25, or 125 mg/kg bw per day (Mengs & Stotzem, 1989); and a
90-day study in Wistar rats at 0, 10, 40, or 100 mg/kg bw per day
(Spindler & Madsen, 1992). Significant effects were seen on organ
weights and liver morphology, but no histopathological changes were
observed at these doses.
Feeding of terpenoid substances present in mint oils (see the
safety evaluation of substances structurally related to menthol, p.
381) affected the morphology of rat cerebellum. Similar effects were
not seen in the 28-day study of menthol, however, and a subsequent
review of the slides indicated that the observations were artefacts
(Adams et al., 1996), as supported by the results of a recent study
(Mœlck et al., 1998).
Groups of 10 female and 10 male Fischer 344 rats were maintained
on diets containing (±)-menthol at concentrations of 0, 930, 1900,
3800, 7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 93,
190, 380, 750, or 1500 mg/kg bw per day. Necropsies were performed on
all animals at the end of the study; histopathological examination was
performed on tissues from the control animals and those at 1500 mg/kg
bw per day and on selected tissues from animals at 750 mg/kg bw per
day. The final mean body weights of treated rats were similar to those
of the controls. A slight increase in the incidence of interstitial
nephritis was observed in male rats at the highest dose. The NOEL was
750 mg/kg bw per day (US National Cancer Institute, 1979).
Because of its pharmacological properties, menthol has also been
tested by intraperitoneal, intravenous, inhalation, and dermal
administration. As noted above, oral administration results in
substantial biotransformation, whereas these processes are largely
by-passed when it is given by the other routes, and the results of
these studies are not applicable to this safety evaluation.
Furthermore, some short-term studies result in biological end-points
such as antispasmodic activity, which are not of primary relevance to
the food uses of menthol. Studies relevant to intake of menthol from
food are summarized below.
The previous monograph summarized studies on the antispasmodic
activity of pharmaceutical preparations on intestinal tissue (Annex 1,
reference 42), although they contained peppermint oil rather than
menthol per se (Bowen & Cubbin, 1992). The doses of menthol
necessary to obtain this effect are significantly higher than the
amounts present in foods.
The pharmacological effect of menthol on the respiratory tract
has also been studied. In a 71-79-day study with young albino Sherman
rats, inhalation of (-)-menthol vapour at 0.087, 0.15, or 0.26 ppm
caused no gross changes. Histological examination showed effects on
lung tissue only at the highest dose, at which irritation also
occurred (Rakieten et al., 1954).
2.2.3 Long-term studies of toxicity and carcinogenicity
2.2.3.1 Feeding studies
Mice
Groups of 50 B6C3F1 mice of each sex were given (±)-menthol at
doses of 0, 2000, or 4000 mg/kg diet daily for 103 weeks, equivalent
to 300 or 600 mg/kg bw per day, respectively. Animals were housed five
per cage and were observed twice daily for signs of toxicity. Body
weights and food consumption was recorded every two weeks for the
first 12 weeks and once a month thereafter. Necropsies and
histological examinations were performed on all animals at termination
of the study and on those found dead during the study.
The mean body weights of the treated mice were slightly lower
than those of the controls. The survival of males was similar to that
of controls, but females at the high dose had statistically
significantly worse survival rates; subsequent evaluation (Haseman et
al., 1985) showed, however, that the survival of all female mice was
within the range for historical controls, and the survival rate of the
control group in this study was at the high end of the range. The
survival of animals at the high dose was in fact closer to the
historical average, and there was no evidence of toxicity in this
group. An increased incidence of hepatocellular carcinoma was observed
in males at the high dose, but the increase was not significantly
different from that of concurrent or historical control mice of that
age and strain (Haseman et al., 1986). A low incidence of alveolar or
bronchiolar adenomas of the lung was observed in treated females, but
the rate was not significantly different from that in historical
controls. It was concluded that (±)-menthol is not carcinogenic and
has no organ-specific toxicity in B6C3F1 mice of either sex at the
doses tested (US National Cancer Institute, 1979).
Rats
Groups of 50 Fischer 344 rats of each sex were given 0, 3750, or
7500 mg/kg diet (±)-menthol in their feed daily for 103 weeks,
equivalent to 190, and 380 mg/kg bw per day, respectively. Animals
were housed five per cage until week 48, when the male rats were
divided into groups of two to three per cage. The animals were
observed twice daily for signs of toxicity. Body weight and food
consumption were recorded every two weeks for the first 12 weeks and
once a month thereafter. Necropsies and histological examinations were
performed on all animals at the end of the study and on those found
dead during the study.
The mean body weights of treated rats were slightly lower than
those of the controls. Survival of treated rats was similar to that of
controls. Chronic inflammation of the kidney was observed in the older
treated males, but was not considered to be related to administration
of menthol since the effect is commonly observed in aged male Fischer
344 rats. The incidence of neoplasms was not increased in treated
females, and, in fact, fibroadenomas of the mammary glands occurred at
lower incidences in treated (10/49 at the low dose and 7/49 at the
high dose) than in control animals (20/50). Alveolar or bronchiolar
adenomas or carcinomas were reported only in female controls. It was
concluded that (±)-menthol is neither carcinogenic nor toxic for
Fischer 344 rats of either sex at the doses tested (US National Cancer
Institute, 1979).
2.2.3.2 Other studies
Groups of 30 female A/He strain mice received 2000 mg/kg bw
menthol dissolved in tricaprylin, the maximum dose tolerated after six
intraperitoneal injections over two weeks, and one-quarter this dose,
500 mg/kg bw, three times weekly for eight weeks. The animals were
observed for an additional 16 weeks. A group of 24 control female mice
received a similar number of injections of tricaprylin. All animals
that survived treatment were killed after 24 weeks and the numbers of
pulmonary adenomas counted. No increase in the incidence of
non-neoplastic or neoplastic lesions was reported in the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands of
treated animals. Approximately 30-45% of the menthol-treated animals
and 15% of the control animals died before the end of the study. The
authors reported that tricaprylin is an unsuitable vehicle for
bioassays, as it caused a 3- or 4-g weight loss in the control animals
during the first week of the study, a high mortality rate, and a
higher mean tumour rate. In the same test system, urethane (total
dose, 10 or 20 mg) and several alkylating agents induced marked
increases in the incidence of pulmonary adenomas, but other substances
shown to be carcinogenic in other test systems, e.g. safrole, caused
no increase (Stoner et al., 1973; Annex 1, reference 42). A
retrospective evaluation of the lung tumour response in strain A mice
is provided by Stoner (1991).
Menthol has been studied for its chemopreventive effect on
ras-mediated tumour growth in four types of rat liver cell
in vitro, with lovastatin as the positive control. Concentrations of
0.1-2.5 mmol/L of menthol inhibited tumour growth, but the authors
concluded that the mechanism of the chemopreventive effect of menthol
is different from that of lovastatin (Ruch & Sigler, 1994).
In a study of the preventive activity of menthol against
7,12-dimethylbenz [a]anthracene (DMBA)-induced mammary tumours in
rats, a diet containing 1% menthol was fed from two weeks before DMBA
treatment to up to 22 weeks after treatment. A reduction in mammary
tumour incidence was seen. A diet containing 0.5% menthol fed from two
weeks before DMBA treatment until one week after treatment also
resulted in a reduction in tumour incidence (Russin et al., 1989;
Gould et al., 1990).
In studies of mentholated cigarettes in animals and humans, the
presence of menthol in cigarettes did not enhance the incidence of
lung cancer over that due to smoking unmentholated cigarettes (Kabat &
Hebert, 1991; Gaworski et al., 1997).
2.2.4 Genotoxicity
The results of tests for genotoxicity with menthol are presented
in Table 2.
Menthol was administered at 725 mg/kg bw or the maximum tolerated
dose of 1450 mg/kg bw to male Fischer 344 rats and male B6C3F1 mice.
Hepatocytes were removed at 24, 39, and 49 h, and replicative DNA
synthesis was measured. Synthesis was increased in 6% of the rats and
1.7% of the mice. This assay indicates cell replication (i.e.
mitogenesis), however, and not genotoxicity (Uno et al., 1994;
Yoshikawa, 1996).
2.2.5 Developmental toxicity
Mice
(-)-Menthol was administered in corn oil by gavage to 22 or 23
pregnant CD-1 mice at 0, 1.9, 8.6, 40, or 190 mg/kg bw per day on days
6-15 of gestation; to 22 or 23 pregnant Wistar rats at 0, 2.2, 10, 47,
or 220 mg/kg bw per day on days 6-15 of gestation; to 21-23 golden
hamsters at 0, 4.1, 21, 98, or 400 mg/kg bw per day on days 6-10 of
gestation; and to 11 to 14 Dutch-belt rabbits at 0, 4.3, 20, 92, or
430 mg/kg bw per day on days 6-18 of gestation. Control groups for
each species were sham treated; positive control groups for each
species were given 150 or 250 mg/kg bw per day aspirin. Body weights
were recorded on three or four days during the gestation period. All
animals were observed daily for appearance, behaviour, and food
consumption. On the scheduled day, the fetuses were removed from all
dams and dams and fetuses examined. One-third of the fetuses from each
group underwent detailed visceral examination; the other two-thirds
were examined for skeletal defects. There were no effects on nidation,
maternal survival, fetal survival, or fetal abnormalities. The numbers
of abnormalities seen in soft or skeletal tissues of treated animals
did not differ from those occurring spontaneously in the sham-treated
controls (Food and Drug Research Labs, Inc., 1973).
2.2.6 Special studies
2.2.6.1 Cellular and biochemical effects
Menthol has been tested for potential bactericidal and fungicidal
effects on microorganisms that are foodborne or found in the oral
cavity. Menthol at concentrations of 0.1-5 mmol/L was cytotoxic and
affected tissue processes in trachea from chick embryos, ascites
sarcoma BP8 cells, isolated hamster brown adipocytes, and rat liver
mitochondria (Bernson & Pettersson, 1983). Menthol had no cytotoxic
effect on human HeLa cells in vitro at concentrations of 1, 10, and
100 µg/ml (Nachev et al., 1967).
Menthol was lethal to E. coli at a concentration of 0.5%
(Wokes, 1932) or 0.05% (Morris et al., 1979; Megalla et al., 1980; Jay
& Rivers, 1984); to Salmonella typhimurium at a concentration of
0.05% (Karapinar & Aktug, 1987); to Clostridium at 0.05% (Ueda et
al., 1982); to Staphylococcus at 0.05% (Wokes, 1932; Morris et al.,
1979; Karapinar & Aktug, 1987; Moleyar & Narasimham 1992) or 0.003%
(Jay & Rivers, 1984); to Vibrio spp. at 0.05% (Karapinar & Aktug;
1987); and to Bacillus spp. at 0.05% (Morris et al., 1979; Megalla
et al., 1980; Ueda et al., 1982; Moleyar & Narasimham, 1992). It was
cytotoxic to spoilage fungi at 0.08-0.3% (Yousef et al., 1978; Kurita
et al., 1981), 0.05% (Morris et al., 1979; Megalla et al., 1980;
Mahmoud, 1994; Muller-Reibau et al., 1995), or 0.025% (Jay & Rivers,
1984).
The results of these studies are difficult to extrapolate to the
situation in experimental animal in vivo, and cannot be used
directly in the safety assessment of menthol. Furthermore, the
concentration at which cytotoxicity was observed is considerably
higher than those present in the diets tested.
2.2.6.2 Immunotoxicity and sensitivity
The presence of menthol and menthol-containing flavour and
fragrance oils at high concentrations in consumer products such as
cigarettes, toothpaste, and topical medications has led to sensitivity
reactions in the oral and nasal cavity (e.g. Millard, 1973;
Dooms-Goossens et al., 1977; Lewis et al., 1995; Morton et al., 1995;
Shah et al., 1996).
The preferred method for testing sensitivity or food intolerance
is the double-blind placebo-controlled food challenge. In a study on
the prevalence of food allergy and food intolerance in 1483 Dutch
adults by this method, it was estimated that the prevalence of
sensiivity or intolerance was 0.8-2.4% of the adult population. Within
the sampled population, 73 subjects reported such effects, and 12 of
the responses were confirmed. Only one of these responders had a
reaction to menthol challenge; the subject reported 'aggravation of
aphthae' (whitish spots in the mouth that characterize apthous
stomatitis) 1 h after administration (Niestijl Janson et al., 1994).
2.3 Observations in humans
Menthol has been tested in humans mainly for its potential
pharmaceutical properties, such as enhancement of lung and airway
volume (e.g. Bowen & Cubbin, 1992). The usual human oral dose is
60-120 mg menthol per person. It can be estimated from unreferenced
citations in pharmaceutical texts, such as Gleason et al. (1969), that
the lethal human dose is 50-500 mg/kg bw. In section 2.1.1, the
maximum doses tested were 180 mg (Kaffenberger & Doyle, 1990) and 1 g
(Quick, 1928).
The airway hyperresponsiveness of 23 human subjects with chronic
mild asthma was tested by use of a nebulizer containing menthol twice
a day for four weeks. As measured by expiratory flow rates, vital
capacity, and forced expiratory volume, menthol improved airway
hyperresponsiveness at doses as low as 20 mg (Tamaoki et al., 1995).
The efficient hepatic glucuronidation of menthol has been
investigated as a possible basis for a test of liver function. In a
study of the output of menthol glucuronide by normal subjects and
subjects with alcohol-induced cirrhosis and steatosis of the liver, it
was concluded that the group differences were not sufficient to
justify use of menthol glucuronide output as a test for liver disease.
The results demonstrate that even a compromised liver has sufficient
capacity to handle menthol (Szabo & Ebrey, 1963; Horvath et al.,
1984).
A controlled study of the effects of a mentholated powder was
carried out on 60 consecutive glucose-6-phosphate
dehydrogenase-deficient babies. The umbilical cords of 30 babies were
treated daily with the powder, while the remainder served as controls.
Significantly more of the treated babies developed severe jaundice
than the controls. The inability of neonates to conjugate menthol was
probably responsible for the increased severity of the jaundice
developed by the deficient babies. It was concluded that the use of
menthol-containing products on neonates should be discontinued,
especially in communities where the incidence of glucose-6-phosphate
dehydrogenase deficiency is high (Olowe & Ransome-Kuti, 1980). As
neonates do not have an oral intake of menthol, the relevance of these
results is limited. No data were available on the possible effects of
menthol in older children or in adults with glucose-6-phosphate
dehydrogenase deficiency.
Table 2. Results of assays for the genotoxicity of menthol
End-point Test object Concentration Results Reference
In vitro
(±)-Menthol
Reverse mutation S. typhimurium TA92, TA1535, < 5 mg/plate Negativea Ishidate et al. (1984)
TA100, TA1537, TA94, TA98
Reverse mutation S. typhimurium 666 µg/plate Negativea Tennant et al. (1987)
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)
TA98
Antimutagenicity S. typhimurium TA98 < 200 µg/ml Negative Ohta et al. (1986)
Antimutagenicity Escherichia coli < 200 µg/ml Negative Ohta et al. (1986)
Chromosomal aberration Chinese hamster fibroblasts < 0.2 mg/ml Negative Ishidate et al. (1984)
Chromosomal aberration Chinese hamster lung fibroblasts < 0.3 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Chinese hamster ovary cells < 250 µg/ml Negative Tennant et al. (1987)
and sister chromatid
exchange
Chromosomal aberration Chinese hamster ovary cells < 167 µg/ml Negative Ivett et al. (1989)
and sister chromatid
exchange
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Tennant et al. (1987)
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Myhr & Caspary (1991)
(-)-Menthol
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)TA98
Reverse mutation S. typhimurium TA98, TA100, 6.4-800 µg/plate Negativea Andersen & Jennies (1984)
TA1535, TA1537
Reverse mutation E. coli WP2 uvrA 0.1-0.8 mg/plate Negative Yoo (1986)
Reverse mutation S. typhimurium TA1530, G46 0.25 ml/plate Negative Litton Bionetics, Inc. (1975)
Antimutagenicity E. coli WP2 uvrA 0.5-2.0 mg/ml Negative Yoo (1986)
DNA repair Bacillus subtilis < 10 mg/disk Negative Yoo (1986)
Gene mutation Bacillus subtilis < 20 mg/plate Negative Oda et al. (1978)
Gene mutation Saccharomyces cerevisiae D3 0.2 ml/plate Equivocal Litton Bionetics, Inc. (1975)
Table 2. (continued)
End-point Test object Concentration Results Reference
Chromosomal aberration Chinese hamster lung fibroblasts < 0.125 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Human WI-38 embryonic lung cells 10 mg/ml Negative Litton Bionetics, Inc. (1975)
Chromosomal aberration Human peripheral blood lymphocytes 0.1-10 mmol/L Negativea Murthy et al. (1991)
and sister chromatid
exchange
(+)-Menthol
DNA damage Rat hepatocytes 0.7-1.3 mmpl/L Positive Storer et al. (1996)
DNA damage Chinese hamster V79 cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
DNA damage Human blood cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
In vivo
(±)-Menthol
Micronucleus formation Male B6C3F1 mouse bone marrow < 1 g/kg bw Negative Shelby et al. (1993)
(-)-Menthol
Host-mediated S. typhimurium TA1530, G46/ < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity ICR mouse host
Host-mediated S. cerevisiae D3/ICR mouse host < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity
Chromosomal aberration Albino rat bone marrow < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
Dominant lethal mutation Rat < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
a With and without an exogenous metabolic activation system
3. COMMENTS
Menthol exists as two optical isomers, (+)-menthol and
(-)-menthol; the racemic mixture is (±)-menthol. The (-) and (±) forms
are used in flavour applications.
Menthol is readily absorbed. Up to 100% of an ingested dose
appeared to be absorbed, on the basis of the elimination of menthol
metabolites in faeces and urine. Absorbed menthol is known to be
largely eliminated as glucuronides; 70-80% is eliminated in urine and
faeces within 48 h. Metabolic studies indicate that oral doses of
menthol are metabolized mainly in the liver and excreted via the
kidneys and in the bile. Menthol is efficiently metabolized by normal
processes. The metabolites are simple glucuronic acid conjugates and
oxidation products. Mammals can efficiently handle menthol by
processes that do not create hazardous products.
The NOEL in 13-week studies of toxicity with (±)-menthol in the
diet was 560 mg/kg bw per day in mice and 750 mg/kg bw per day in rats
on the basis of slightly increased incidences of interstitial
nephritis at the next highest dose.
In a two-year study of toxicity and carcinogenicity, mice were
fed (±)-menthol in the diet at concentrations equivalent to 300 or 600
mg/kg bw per day. The incidences of hepatocellular tumours in males
and lung tumours in females at the highest dose were not significantly
different from those in concurrent or historical controls. The
survival rate was decreased in female mice but remained within the
range of that of historical controls. The NOEL was 600 mg/kg bw per
day. In a two-year study of toxicity and carcinogenicity in rats given
(±)-menthol in the diet at concentrations equivalent to 190 or
380 mg/kg bw per day, the NOEL was 380 mg/kg bw per day.
Neither menthol nor its metabolites were genotoxic in vitro or
in vivo.
While no studies of reproductive toxicity were available with
menthol, (-)-menthol was tested at maximum doses of 190-430 mg/kg bw
per day for teratogenicity in mice, rats, hamsters, and rabbits; no
teratogenic effects were observed.
4. EVALUATION
The limited data that allow comparisons of metabolism and
toxicity provide no indication of a difference in the toxicity of
(-)-menthol and (±)-menthol. Therefore, the Committee concluded that
an ADI could be established for the two optical isomers of menthol.
The Committee noted that the highest dose of (±)-menthol tested
in the long-term studies in mice and rats had no specific toxic
effect. As the survival of mice was reduced at the high dose of
600 mg/kg bw per day, the Committee allocated an ADI of 0-4 mg/kg bw
on the basis of the NOEL of 380 mg/kg bw per day in the long-term
study in rats, applying a safety factor of 100 and rounding to one
significant figure.
5. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Andersen, P.H. & Jennies, N.J. (1984) Mutagenic investigation of
peppermint oil in the Salmonella/mammalian-microsome test.
Mutat. Res., 138, 17-20.
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Austin, C.A., Shephard, E.A., Pike, S.F., Rabin, B.R. & Phillips, I.R.
(1988) The effect of terpenoid compounds on cytochrome P-450 levels in
rat liver. Biochem. Pharmacol., 37, 2223-2229.
Batt, A.M., Martin, N. & Siest, G. (1981) Induction of group-1 and
group-2 UDP-glucuronosyl transferase in microsomes from the livers of
C57 Bl/6 mice. Toxicol. Lett., 9, 355-360.
Bernson, V.S.M. & Pettersson, B. (1983) The toxicity of menthol in
short-term bioassays. Chem.-Biol. Interactions, 46, 233-246.
Boutin, J.A., Lepage, C., Batt, A.M. & Siest, G. (1981) The activity
of hepatic UDP-glucuronosyltransferase from control and induced pigs
toward 17 hydroxylated aglycones. IRCS Med. Sci., 9, 633-634.
Boutin, J.A., Thomassin, G., Siest, G. & Cartier, A. (1985)
Heterogeneity of hepatic microsomal UDP-glucuronosyltransferase
activities. Conjugations of phenolic and monoterpenoid aglycons in
control and induced rats and guinea pigs. Biochem. Pharmacol., 34,
2235-2249.
Bowen, I.H. & Cubbin, I.J. (1992) Mentha piperita and Mentha spicata.
In: Adverse Effects of Herbal Drugs, pp. 171-178.
Clegg, R.J., Middleton, B., Bell, G.D. & White, D.A. (1982) The
mechanism of cyclic monoterpene inhibition of hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase in vivo in the rat.
J. Biol. Chem., 257, 2294-2299.
Deichmann, W. & Thomas, G. (1943) Glucuronic acid in the urine as a
measure of the absorption of certain organic compounds. J. Ind.
Hyg. Toxicol., 25, 286-292.
Dooms-Goossens, A., Degreef, H., Holvoet, C. & Maertens, M. (1977)
Turpentine-induced hypersensitivity to peppermint oil. Contact
Derm., 3, 304-308.
Eisenberg, F., Field, J.B. & Stetten, D. (1955) Studies on glyceronide
conjugation in man. Arch. Biochem. Biophys., 59, 297-299.
Fishman, W.H. (1940) Studies on beta-glucuronidase. III. The increase
in beta-glucuronidase activity of mammalian tissues induced by feeding
glucuronidogenic substances. J. Biol. Chem., 136, 229-236.
Food & Drug Research Labs, Inc. (1973) Teratologic evaluation of FDA
71-57 (menthol natural Brazilian), June 1973. US National Technical
Information Service report No. PB-223815. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Gaworski, C.L., Dozier, M.M., Gerhart, J.M., Rajendrans, N.,
Brennecke, L.H., Aranyi, C. & Heck, J.D. (1997) 13-Week inhalation
toxicity study of menthol cigarette smoke. Food Chem. Toxicol., 35,
683-692.
Gleason, M.N., Gosselin,R.E., Hodge, H.C. & Smith, R.P. (1969)
Clinical Toxicology of Commercial Products, 3rd Ed., Philadelphia,
Williams & Wilkins Co.
Gould, W.D., Wacker, P. & Maltzman, T.H. (1990) Chemoprevention and
chemotherapy of mammary tumors by monoterpenoids. Prog. Clin. Biol.
Res., 347, 255-268.
Green, M.D., Clarke, D.J., Oturu, E.M., Styczynski, P.B., Jackson,
M.R., Burchell, B. & Tephly, T.R. (1995) Cloning and expression of a
rat liver phenobarbital-inducible UDP-glucuronosyl-transferase (2B12)
with specificity for monoterpenoid alcohols. Arch. Biochem.
Biophys., 322, 460-468.
Hartmann, A. & Speit, G. (1997) The contribution of cytotoxicity to
DNA-effects in the single cell gel test (Comet assay). Toxicol.
Lett., 90, 183-188.
Haseman, J.K., Huff, J.E., Rav, G.N., Arnold, J.E., Boorman, G.A. &
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57Bl/6N × C3H/HeN) F1
(B6C3F1) mice. J. Natl Cancer Inst., 75, 975-984.
Haseman, J.K., Tharrington, E.C., Huff, J.U.E. & McConnell, E.E.
(1986) Comparison of site-specific and overall tumor incidence
analysis for 81 recent National Toxicology Program carcinogenicity
studies. Reg. Toxicol. Pharmacol., 6, 155-170.
Herken, H. (1961) [Pharmacological expertise on tolerance to natural
and synthetic menthol.] Unpublished report from Pharmakologischen
Institut der Freien Universität, Berlin, Duhlen. Submitted to WHO by
Schering AG, Berlin, Germany (in German).
Horvath, T., Past, T., Tatui, Z., Par, A., Kadas, I., Tapsonyi, Z. &
Javor, J. (1984) Investigation of biotransformation capacity in
patients with chronic liver diseases by pharmacokinetic methods:
Experimental trials. Pol. J. Pharmacol. Pharm., 36, 361-371.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ivett, J.L., Brown, B.M., Rodgers, C., Anderson, B.E., Resnick, M.A. &
Zeiger, E. (1989) Chromosomal aberrations and sister chromatid
exchange tests in Chinese hamster ovary cells in vitro. IV. Results
with 15 chemicals. Environ. Mol. Mutag., 14, 165-187.
Jay, J.J. & Rivers, G.M. (1984) Antimicrobial activity of some food
flavoring compounds. J. Food Saf., 6, 129-139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kabat, G.C. & Hebert, J.R. (1991) Use of mentholated cigarettes and
lung cancer risk. Cancer Res., 51, 6510-6513.
Kaffenberger, R.M. & Doyle, M.J. (1990) Determination of menthol and
menthol glucuronide in human urine by gas chromatography using an
enzyme-sensitive internal standard and flame ionization detection.
J. Chromatogr., 527, 59-66.
Karapinar, M. & Aktug, S.E. (1987) Inhibition of food borne pathogens
by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol.,
4, 161-166.
Kurita, N., Miyaji, M., Kurane, R. & Takahara, Y. (1981) Antifungal
activity of components of essential oil. Agric. Biol. Chem., 45,
945-952.
Lewis, F.M., Shah., M. & Gawkrodger, D.J. (1995) Contact sensitivity
to food additives can cause oral and perioral symptoms. Contact
Derm., 33, 429-430.
Litton Bionetics, Inc. (1975) Mutagenic evaluation of compound FDA
71-57, menthol, 14 January 1975. US National Technical Information
Service report No. PB-245444. Submitted to WHO by International
Federation of Chewing Gum Associations, Keller and Hechman LLP,
Washington DC, United States
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Madyastha, K.M. & Srivatsan, V. (1988) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Mahmoud, A.L.E. (1994) Antifungal action and antiaflatoxigenic
properties of some essential oil constituents. Lett. Appl.
Microbiol., 19, 110-113
Megalla, S.E., El-Keltawi, N.E. & Ross, S.A. (1980) A study of
antimicrobial action of some essential oil constituents. Herba
Pol., 26, 181-186.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Millard, L.G. (1973) Contact sensitivity to toothpaste. Br. Med.
J., i, 676.
Mœlck, A.M., Poulsen, M., Lauridsen, S.T. & Olsen, P. (1998) Lack of
histological cerebellar changes in Wistar rats given pulegone for 28
days. Comparison of immersion and perfusion tissue fixation.
Toxicol. Lett., 95, 117-122.
Moleyar, V & Narasimham, P. (1992) Antibacterial activity of essential
oil components. Int. J. Food Microbiol., 16, 337-342.
Morris, J.A., Khettry, A. & Seitz, E.W. (1979) Antimicrobial activity
of aroma chemicals and essential oils. J. Am. Oil Chem. Soc., 56,
595-603.
Morton, C.A., Garioch, J., Todd, P., Lamey, P.J. & Forsyth, A. (1995)
Contact sensitivity to menthol and peppermint in patients with
intra-oral symptoms. Contact Derm., 32, 281-284.
Muller-Riebau, F., Berger, B. & Yegen, O. (1995) Chemical composition
and fungitoxic properties to phytopathogenic fungi of essential oils
of selected aromatic plants growing wild in Turkey. J. Agric. Food
Chem., 43, 2262-2266.
Murthy, P.B., Ahmed, M.M. & Regu, K. (1991) Lack of genotoxicity of
menthol in chromosome aberration and sister chromatid exchange assays
using human lymphocytes in vitro. Toxicol. In Vitro, 5, 337-340.
Myhr, B.C. & Caspary, W.C. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutag., 18, 51-83.
Nachev, C., Zolotovitch, G., Siljanovska, K. & Stojcev, S. (1967)
Cytotoxic effect of alcohols isolated from essential oils. C.R.
Acad. Bulg. Sci., 20, 1081-1084.
Niestijl Janson, J.J., Kardinaal, A.F.M., Huijbers, G.,
Vlieg-Boerstra, B.J., Martens, B.P.M. & Ockhuizen, T (1994) Prevalence
of food allergy and intolerance in the adult Dutch population.
J. Allergy Clin. Immunol., 93, 446-456.
Nohmi, T., Miyata, R., Yoshilawa, K. & Ishidate, M., Jr (1985)
Mutagenicity tests on organic chemical contaminants in city water and
related compounds. I. Bacterial mutagenicity tests. Eisei Shikenjo
Hokoku, 103, 60-64.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Nihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria (1st report).
Shokunin Eisei Hen, 9, 177-181.
Ohta, T., Watanabe, M., Watanabe, K., Shirasu, Y. & Kada, T. (1986)
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24, 51-54.
Olowe, S.A. & Ransome-Kuti, O. (1980) The risk of jaundice in
glucose-6-phospate dehydrogenase deficient babies to menthol.
Acta Paediatr. Scand., 69, 341-345.
Quick, A.J. (1924) The synthesis of menthol glycuronic acid in the
rabbit. J. Biol. Chem., 61, 679-683.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Rakieten, N., Rakieten, M.L. & Boykin, M. (1954) Effects of menthol
vapor on the intact animal with special reference to the upper
respiratory tract. J. Am. Pharm. Assoc. Sci. Ed., 43, 390-392.
Ruch, R.J. & Sigler, K. (1994) Growth inhibition of rat liver
epithelial tumor cells by monoterpenes does not involve rat plasma
membrane association. Carcinogenesis, 15, 787-789.
Russin, W.A., Hoesly, J.D., Elson, C.E., Tanner, M.A. & Gould, M.
(1989) Inhibition of rat mammary carcinogenesis by monoterpenoids.
Carcinogenesis, 10, 2161-2164.
Shah, M., Lewis, F.M. & Gawkrodger, D.J. (1996) Contact allergy in
patients with oral symptoms: A study of 47 patients. Am. J.
Contact Derm., 7, 146-151.
Shelby, M.D., Erexson, G., Hook, G.L. & Tice, R.R. (1993) Evaluation
of a three-exposure mouse bone marrow micronucleus protocol: Results
with 49 chemicals. Environ. Mol. Mutag., 21, 160-179.
Sofuni, T., Hayashi, M., Matsuoka, A., Sawada, M., Hatanaka, M. &
Ishidate, M. (1985) Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells. Eisei Shikenjo
Hokoku, 103, 64-75.
Somerville, K.W., Richmond, C.R. & Bell, G.D. (1984) Delayed release
peppermint oil capsules (Colpermin) for the spastic colon syndrome: A
pharmacokinetic study. Br. J. Clin. Pharmacol., 18, 638-640.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stoner, G.D. (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Exp. Lung Res., 17,
405-423.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Storer, R.D., McKelvey, T.W., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W. & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution rat hepatocyte assay for DNA damage:
Improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368, 59-101.
Szabo, L., & Ebrey, P. (1963) Studies on the inheritance of
Crigler-Najjar's syndrome by the menthol test. Acta Pediatr. Acad.
Sci. Hung., 4, 153-158.
Takacs, A.L. & Vore, M. (1987) Binding of 3H-estradiol-17B
(B-D-glucuronide), a cholestatic organic anion, to rat liver plasma
membranes. Evidence consonant with identification of organic anion
carriers. Mol. Pharmacol., 32, 511-518.
Tamaoki, J., Chiyotani, A., Sakai, A., Takemura, H. & Konno, K. (1995)
Effect of menthol vapour on airway hyper responsiveness in patients
with mild asthma. Respir. Med., 89, 503-504.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnik, M., Stasiewicz, S., Anderson,
B. & Minor, R. (1987) Prediction of chemical carcinogenicity in
rodents from in vitro genetic toxicity assays. Science, 236,
933-941.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Ueda, S., Yamashita, H. & Kuwabara, Y. (1982) Inhibition of
Clostridium botulinum and Bacillus sp. by spices and flavoring
compounds. Nippon Shokuhin Kogyo Gakkaishi, 29, 389-392.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T. &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA synthesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens, screening of 22 known positives and
25 noncarcinogens. Mutat. Res., 320, 189-205.
US National Cancer Institute (1979) Bioassay of DL-menthol for
possible carcinogenicity. Report NCI-CG-TR-98. US National Technical
Information Service report No. PB-288761, Bethesda, Maryland, United
States.
White, D.A., Thompson, S.A., Wilson, C.G. & Bell, G.D. (1987) A
pharmacokinetic comparison of 2 delayed-release peppermint oil
preparations, Colpermin and Mintec, for treatment of the irritable
bowel syndrome. Int. J. Pharmacol., 40, 151-155.
WHO (1987) Principles for the Safety Assessment of Food Additives
and Contaminants in Food (Environmental Health Criteria 70), Geneva
Williams, R.T. (1938) Studies in detoxication. II. (a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol and dl-isomenthol in the rabbit; (b)
d-isoMenthylglucuronide, a new conjugated glucuronic acid. Biochem.
J., 32, 1849-1855.
Williams, R.T. (1939) CLXXXVI. Studies in detoxication. III. The use
of the glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 32, 1519-1524.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Cent., 34, 267.
Yoshikawa, R.T. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: Nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspectives, 104, 40-46.
Yousef, R.T., Aggag, M.E. & Tawil, G.G. (1978) Evaluation of the
antifungal activity of some components of volatile oils against
dermatophytes. Mykosen, 21, 190-193.
Menthol has been used to detect and confirm the presence of a rat
liver DNA fraction coding for glucuronide transferase activity (Green
et al., 1995).
The efficient glucuronidation of menthol was used as a model in a
competitive reaction to evaluate oestradiol glucuronide binding sites
in rat liver plasma membranes. Menthol had a stronger affinity for
plasma binding sites than oestradiol glucuronide (Takacs & Vore,
1987).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the toxicity of single doses of menthol are summarized
in Table 1. It has also been reported that the LD50 values in rats
and mice are > 4000 mg/kg bw (Mengs & Stotzem, 1989).
Table 1. Studies of the acute toxicity of menthol
Species Route LD50 (mg/kg bw) Reference
(-)-Menthol
Mouse Oral 4 380 Litton Bionetics, Inc. (1975)
Rat Oral 940 Litton Bionetics, Inc. (1975)
3 300 JECFA (1976)
Cat Oral 800-1 000 JECFA (1976)
Mouse Subcutaneous 5 000-6 000 JECFA (1976)
Intraperitoneal 2 000 JECFA (1976)
Guinea-pig Intraperitoneal 4 000 JECFA (1976)
Rat Subcutaneous 1 000-2 500 JECFA (1976)
Intraperitoneal 710 JECFA (1976)
Cat Intraperitoneal 800-1 000 JECFA (1976)
Intravenous 34 JECFA (1976)
(±)-Menthol
Mouse Oral 3 100 Wokes (1932)
Rat Oral 2 900 JECFA (1976)
3 180 Jenner et al. (1964)
Cat Oral 1 500-1 600 JECFA (1976)
Mouse Subcutaneous 14 000-16 000 JECFA (1976)
Rat Intraperitoneal 750 JECFA (1976)
Cat Intraperitoneal 1 500-1 600 JECFA (1976)
Rabbit Intraperitoneal 2 000 JECFA (1976)
2.2.2 Short-term studies of toxicity
Mice
Groups of six male mice were given (-)-menthol at doses of 2000,
2500, 3200, 4000, or 5000 mg/kg bw by gavage for five days and
examined for 14 days. Gross necropsy of animals that died or were
killed at termination revealed no abnormal finding. The LD50 was
calculated to be 2600 mg/kg bw (Litton Bionetics, Inc., 1975).
Groups of 10 male and 10 female B6C3F1 mice were maintained on
diets containing (±)-menthol at concentrations of 0, 930, 1870, 3750,
7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 140, 280,
560, 1100, and 2300 mg/kg bw per day, respectively. Necropsies were
performed on all animals at the end of the study; histopathological
examination was performed on tissues from the control animals and
those at 2300 mg/kg bw per day and on selected tissues from animals at
1100 mg/kg bw per day. Six mice (sex not specified) died during the
study, but the deaths could not be attributed to treatment. The final
mean body weights of the treated mice were not statistically
significantly different from those of the controls, except for females
at the high dose which had statistically significant decreased body
weights. Slight increases in the incidences of perivascular lymphoid
hyperplasia and interstitial nephritis were reported for female mice
at the two highest doses. The NOEL was 560 mg/kg bw per day (US
National Cancer Institute, 1979).
Rats
No adverse effects on weight gain, excretion of glucuronides,
water, or electrolytes, or interference with central nervous system
reactions to stimulants were observed when groups of 40 rats of each
sex were fed (-)-or (±)-menthol in the diet for 5.5 weeks at doses of
0, 100, or 200 mg/kg bw per day (Herken, 1961).
In groups of three to four adult male Wistar rats given 1%
menthol in the diet for two weeks, increased serum cholesterol and
serum triglycerides were observed, but there was no effect on apo A-1
lipids, an indicator of high-density lipoprotein status. Body weight
and liver weight were unaffected (Imaizumi et al., 1985).
In a series of studies, groups of 10 rats of each sex were given
menthol in a soybean oil solution at 5 ml/kg bw at doses of 0, 200,
400, or 800 mg/kg bw per day by gavage for 28 days. Animals were
housed two to a cage. Body weight and consumption of food and water
were recorded weekly, and blood samples were collected for
determination of haemoglobin, packed cell volume, erythrocyte,
leukocyte, and reticulocyte counts, glucose, creatinine, and urea
concentrations, and the activity of aspartate aminotransferase. Urine
was examined for blood, ketones, glucose, and proteins. A conventional
selection of tissues was examined at termination.
Significant increases in the absolute and relative weights of the
liver were seen in males at all doses and in females at the
intermediate and high doses. Vacuolization of hepatocytes was seen in
0/20 controls, 4/20 rats at 200 mg/kg bw per day, 5/19 at 400 mg/kg bw
per day, and 4/17 at 800 mg/kg bw per day. No effects were seen on
other parameters measured. The vacuolization was not dose related and
may have reflected adaptation (Thorup et al.,1983a,b; Madsen et al.,
1986).
While the major constituent of peppermint oil is menthol, it also
contains other constituents. Although its effects cannot be assigned
entirely to menthol, dose-proportionate menthol-attributable effects
can be expected. The studies of peppermint oil administered by gavage
include a 28-day study in Wistar rats (Thorup et al., 1983b) at 0, 10,
40, or 100 mg/kg bw per day; a five-week study in Wistar rats and dogs
at 0, 25, or 125 mg/kg bw per day (Mengs & Stotzem, 1989); and a
90-day study in Wistar rats at 0, 10, 40, or 100 mg/kg bw per day
(Spindler & Madsen, 1992). Significant effects were seen on organ
weights and liver morphology, but no histopathological changes were
observed at these doses.
Feeding of terpenoid substances present in mint oils (see the
safety evaluation of substances structurally related to menthol, p.
381) affected the morphology of rat cerebellum. Similar effects were
not seen in the 28-day study of menthol, however, and a subsequent
review of the slides indicated that the observations were artefacts
(Adams et al., 1996), as supported by the results of a recent study
(Mœlck et al., 1998).
Groups of 10 female and 10 male Fischer 344 rats were maintained
on diets containing (±)-menthol at concentrations of 0, 930, 1900,
3800, 7500, or 15 000 mg/kg diet for 13 weeks, equivalent to 0, 93,
190, 380, 750, or 1500 mg/kg bw per day. Necropsies were performed on
all animals at the end of the study; histopathological examination was
performed on tissues from the control animals and those at 1500 mg/kg
bw per day and on selected tissues from animals at 750 mg/kg bw per
day. The final mean body weights of treated rats were similar to those
of the controls. A slight increase in the incidence of interstitial
nephritis was observed in male rats at the highest dose. The NOEL was
750 mg/kg bw per day (US National Cancer Institute, 1979).
Because of its pharmacological properties, menthol has also been
tested by intraperitoneal, intravenous, inhalation, and dermal
administration. As noted above, oral administration results in
substantial biotransformation, whereas these processes are largely
by-passed when it is given by the other routes, and the results of
these studies are not applicable to this safety evaluation.
Furthermore, some short-term studies result in biological end-points
such as antispasmodic activity, which are not of primary relevance to
the food uses of menthol. Studies relevant to intake of menthol from
food are summarized below.
The previous monograph summarized studies on the antispasmodic
activity of pharmaceutical preparations on intestinal tissue (Annex 1,
reference 42), although they contained peppermint oil rather than
menthol per se (Bowen & Cubbin, 1992). The doses of menthol
necessary to obtain this effect are significantly higher than the
amounts present in foods.
The pharmacological effect of menthol on the respiratory tract
has also been studied. In a 71-79-day study with young albino Sherman
rats, inhalation of (-)-menthol vapour at 0.087, 0.15, or 0.26 ppm
caused no gross changes. Histological examination showed effects on
lung tissue only at the highest dose, at which irritation also
occurred (Rakieten et al., 1954).
2.2.3 Long-term studies of toxicity and carcinogenicity
2.2.3.1 Feeding studies
Mice
Groups of 50 B6C3F1 mice of each sex were given (±)-menthol at
doses of 0, 2000, or 4000 mg/kg diet daily for 103 weeks, equivalent
to 300 or 600 mg/kg bw per day, respectively. Animals were housed five
per cage and were observed twice daily for signs of toxicity. Body
weights and food consumption was recorded every two weeks for the
first 12 weeks and once a month thereafter. Necropsies and
histological examinations were performed on all animals at termination
of the study and on those found dead during the study.
The mean body weights of the treated mice were slightly lower
than those of the controls. The survival of males was similar to that
of controls, but females at the high dose had statistically
significantly worse survival rates; subsequent evaluation (Haseman et
al., 1985) showed, however, that the survival of all female mice was
within the range for historical controls, and the survival rate of the
control group in this study was at the high end of the range. The
survival of animals at the high dose was in fact closer to the
historical average, and there was no evidence of toxicity in this
group. An increased incidence of hepatocellular carcinoma was observed
in males at the high dose, but the increase was not significantly
different from that of concurrent or historical control mice of that
age and strain (Haseman et al., 1986). A low incidence of alveolar or
bronchiolar adenomas of the lung was observed in treated females, but
the rate was not significantly different from that in historical
controls. It was concluded that (±)-menthol is not carcinogenic and
has no organ-specific toxicity in B6C3F1 mice of either sex at the
doses tested (US National Cancer Institute, 1979).
Rats
Groups of 50 Fischer 344 rats of each sex were given 0, 3750, or
7500 mg/kg diet (±)-menthol in their feed daily for 103 weeks,
equivalent to 190, and 380 mg/kg bw per day, respectively. Animals
were housed five per cage until week 48, when the male rats were
divided into groups of two to three per cage. The animals were
observed twice daily for signs of toxicity. Body weight and food
consumption were recorded every two weeks for the first 12 weeks and
once a month thereafter. Necropsies and histological examinations were
performed on all animals at the end of the study and on those found
dead during the study.
The mean body weights of treated rats were slightly lower than
those of the controls. Survival of treated rats was similar to that of
controls. Chronic inflammation of the kidney was observed in the older
treated males, but was not considered to be related to administration
of menthol since the effect is commonly observed in aged male Fischer
344 rats. The incidence of neoplasms was not increased in treated
females, and, in fact, fibroadenomas of the mammary glands occurred at
lower incidences in treated (10/49 at the low dose and 7/49 at the
high dose) than in control animals (20/50). Alveolar or bronchiolar
adenomas or carcinomas were reported only in female controls. It was
concluded that (±)-menthol is neither carcinogenic nor toxic for
Fischer 344 rats of either sex at the doses tested (US National Cancer
Institute, 1979).
2.2.3.2 Other studies
Groups of 30 female A/He strain mice received 2000 mg/kg bw
menthol dissolved in tricaprylin, the maximum dose tolerated after six
intraperitoneal injections over two weeks, and one-quarter this dose,
500 mg/kg bw, three times weekly for eight weeks. The animals were
observed for an additional 16 weeks. A group of 24 control female mice
received a similar number of injections of tricaprylin. All animals
that survived treatment were killed after 24 weeks and the numbers of
pulmonary adenomas counted. No increase in the incidence of
non-neoplastic or neoplastic lesions was reported in the lung, liver,
kidney, spleen, thymus, intestine, or salivary or endocrine glands of
treated animals. Approximately 30-45% of the menthol-treated animals
and 15% of the control animals died before the end of the study. The
authors reported that tricaprylin is an unsuitable vehicle for
bioassays, as it caused a 3- or 4-g weight loss in the control animals
during the first week of the study, a high mortality rate, and a
higher mean tumour rate. In the same test system, urethane (total
dose, 10 or 20 mg) and several alkylating agents induced marked
increases in the incidence of pulmonary adenomas, but other substances
shown to be carcinogenic in other test systems, e.g. safrole, caused
no increase (Stoner et al., 1973; Annex 1, reference 42). A
retrospective evaluation of the lung tumour response in strain A mice
is provided by Stoner (1991).
Menthol has been studied for its chemopreventive effect on
ras-mediated tumour growth in four types of rat liver cell
in vitro, with lovastatin as the positive control. Concentrations of
0.1-2.5 mmol/L of menthol inhibited tumour growth, but the authors
concluded that the mechanism of the chemopreventive effect of menthol
is different from that of lovastatin (Ruch & Sigler, 1994).
In a study of the preventive activity of menthol against
7,12-dimethylbenz [a]anthracene (DMBA)-induced mammary tumours in
rats, a diet containing 1% menthol was fed from two weeks before DMBA
treatment to up to 22 weeks after treatment. A reduction in mammary
tumour incidence was seen. A diet containing 0.5% menthol fed from two
weeks before DMBA treatment until one week after treatment also
resulted in a reduction in tumour incidence (Russin et al., 1989;
Gould et al., 1990).
In studies of mentholated cigarettes in animals and humans, the
presence of menthol in cigarettes did not enhance the incidence of
lung cancer over that due to smoking unmentholated cigarettes (Kabat &
Hebert, 1991; Gaworski et al., 1997).
2.2.4 Genotoxicity
The results of tests for genotoxicity with menthol are presented
in Table 2.
Menthol was administered at 725 mg/kg bw or the maximum tolerated
dose of 1450 mg/kg bw to male Fischer 344 rats and male B6C3F1 mice.
Hepatocytes were removed at 24, 39, and 49 h, and replicative DNA
synthesis was measured. Synthesis was increased in 6% of the rats and
1.7% of the mice. This assay indicates cell replication (i.e.
mitogenesis), however, and not genotoxicity (Uno et al., 1994;
Yoshikawa, 1996).
2.2.5 Developmental toxicity
Mice
(-)-Menthol was administered in corn oil by gavage to 22 or 23
pregnant CD-1 mice at 0, 1.9, 8.6, 40, or 190 mg/kg bw per day on days
6-15 of gestation; to 22 or 23 pregnant Wistar rats at 0, 2.2, 10, 47,
or 220 mg/kg bw per day on days 6-15 of gestation; to 21-23 golden
hamsters at 0, 4.1, 21, 98, or 400 mg/kg bw per day on days 6-10 of
gestation; and to 11 to 14 Dutch-belt rabbits at 0, 4.3, 20, 92, or
430 mg/kg bw per day on days 6-18 of gestation. Control groups for
each species were sham treated; positive control groups for each
species were given 150 or 250 mg/kg bw per day aspirin. Body weights
were recorded on three or four days during the gestation period. All
animals were observed daily for appearance, behaviour, and food
consumption. On the scheduled day, the fetuses were removed from all
dams and dams and fetuses examined. One-third of the fetuses from each
group underwent detailed visceral examination; the other two-thirds
were examined for skeletal defects. There were no effects on nidation,
maternal survival, fetal survival, or fetal abnormalities. The numbers
of abnormalities seen in soft or skeletal tissues of treated animals
did not differ from those occurring spontaneously in the sham-treated
controls (Food and Drug Research Labs, Inc., 1973).
2.2.6 Special studies
2.2.6.1 Cellular and biochemical effects
Menthol has been tested for potential bactericidal and fungicidal
effects on microorganisms that are foodborne or found in the oral
cavity. Menthol at concentrations of 0.1-5 mmol/L was cytotoxic and
affected tissue processes in trachea from chick embryos, ascites
sarcoma BP8 cells, isolated hamster brown adipocytes, and rat liver
mitochondria (Bernson & Pettersson, 1983). Menthol had no cytotoxic
effect on human HeLa cells in vitro at concentrations of 1, 10, and
100 µg/ml (Nachev et al., 1967).
Menthol was lethal to E. coli at a concentration of 0.5%
(Wokes, 1932) or 0.05% (Morris et al., 1979; Megalla et al., 1980; Jay
& Rivers, 1984); to Salmonella typhimurium at a concentration of
0.05% (Karapinar & Aktug, 1987); to Clostridium at 0.05% (Ueda et
al., 1982); to Staphylococcus at 0.05% (Wokes, 1932; Morris et al.,
1979; Karapinar & Aktug, 1987; Moleyar & Narasimham 1992) or 0.003%
(Jay & Rivers, 1984); to Vibrio spp. at 0.05% (Karapinar & Aktug;
1987); and to Bacillus spp. at 0.05% (Morris et al., 1979; Megalla
et al., 1980; Ueda et al., 1982; Moleyar & Narasimham, 1992). It was
cytotoxic to spoilage fungi at 0.08-0.3% (Yousef et al., 1978; Kurita
et al., 1981), 0.05% (Morris et al., 1979; Megalla et al., 1980;
Mahmoud, 1994; Muller-Reibau et al., 1995), or 0.025% (Jay & Rivers,
1984).
The results of these studies are difficult to extrapolate to the
situation in experimental animal in vivo, and cannot be used
directly in the safety assessment of menthol. Furthermore, the
concentration at which cytotoxicity was observed is considerably
higher than those present in the diets tested.
2.2.6.2 Immunotoxicity and sensitivity
The presence of menthol and menthol-containing flavour and
fragrance oils at high concentrations in consumer products such as
cigarettes, toothpaste, and topical medications has led to sensitivity
reactions in the oral and nasal cavity (e.g. Millard, 1973;
Dooms-Goossens et al., 1977; Lewis et al., 1995; Morton et al., 1995;
Shah et al., 1996).
The preferred method for testing sensitivity or food intolerance
is the double-blind placebo-controlled food challenge. In a study on
the prevalence of food allergy and food intolerance in 1483 Dutch
adults by this method, it was estimated that the prevalence of
sensiivity or intolerance was 0.8-2.4% of the adult population. Within
the sampled population, 73 subjects reported such effects, and 12 of
the responses were confirmed. Only one of these responders had a
reaction to menthol challenge; the subject reported 'aggravation of
aphthae' (whitish spots in the mouth that characterize apthous
stomatitis) 1 h after administration (Niestijl Janson et al., 1994).
2.3 Observations in humans
Menthol has been tested in humans mainly for its potential
pharmaceutical properties, such as enhancement of lung and airway
volume (e.g. Bowen & Cubbin, 1992). The usual human oral dose is
60-120 mg menthol per person. It can be estimated from unreferenced
citations in pharmaceutical texts, such as Gleason et al. (1969), that
the lethal human dose is 50-500 mg/kg bw. In section 2.1.1, the
maximum doses tested were 180 mg (Kaffenberger & Doyle, 1990) and 1 g
(Quick, 1928).
The airway hyperresponsiveness of 23 human subjects with chronic
mild asthma was tested by use of a nebulizer containing menthol twice
a day for four weeks. As measured by expiratory flow rates, vital
capacity, and forced expiratory volume, menthol improved airway
hyperresponsiveness at doses as low as 20 mg (Tamaoki et al., 1995).
The efficient hepatic glucuronidation of menthol has been
investigated as a possible basis for a test of liver function. In a
study of the output of menthol glucuronide by normal subjects and
subjects with alcohol-induced cirrhosis and steatosis of the liver, it
was concluded that the group differences were not sufficient to
justify use of menthol glucuronide output as a test for liver disease.
The results demonstrate that even a compromised liver has sufficient
capacity to handle menthol (Szabo & Ebrey, 1963; Horvath et al.,
1984).
A controlled study of the effects of a mentholated powder was
carried out on 60 consecutive glucose-6-phosphate
dehydrogenase-deficient babies. The umbilical cords of 30 babies were
treated daily with the powder, while the remainder served as controls.
Significantly more of the treated babies developed severe jaundice
than the controls. The inability of neonates to conjugate menthol was
probably responsible for the increased severity of the jaundice
developed by the deficient babies. It was concluded that the use of
menthol-containing products on neonates should be discontinued,
especially in communities where the incidence of glucose-6-phosphate
dehydrogenase deficiency is high (Olowe & Ransome-Kuti, 1980). As
neonates do not have an oral intake of menthol, the relevance of these
results is limited. No data were available on the possible effects of
menthol in older children or in adults with glucose-6-phosphate
dehydrogenase deficiency.
Table 2. Results of assays for the genotoxicity of menthol
End-point Test object Concentration Results Reference
In vitro
(±)-Menthol
Reverse mutation S. typhimurium TA92, TA1535, < 5 mg/plate Negativea Ishidate et al. (1984)
TA100, TA1537, TA94, TA98
Reverse mutation S. typhimurium 666 µg/plate Negativea Tennant et al. (1987)
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)
TA98
Antimutagenicity S. typhimurium TA98 < 200 µg/ml Negative Ohta et al. (1986)
Antimutagenicity Escherichia coli < 200 µg/ml Negative Ohta et al. (1986)
Chromosomal aberration Chinese hamster fibroblasts < 0.2 mg/ml Negative Ishidate et al. (1984)
Chromosomal aberration Chinese hamster lung fibroblasts < 0.3 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Chinese hamster ovary cells < 250 µg/ml Negative Tennant et al. (1987)
and sister chromatid
exchange
Chromosomal aberration Chinese hamster ovary cells < 167 µg/ml Negative Ivett et al. (1989)
and sister chromatid
exchange
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Tennant et al. (1987)
Forward mutation L5178Y mouse lymphoma cells < 200 µg/ml Negative Myhr & Caspary (1991)
(-)-Menthol
Reverse mutation S. typhimurium TA100, TA2637, < 0.5 mg/plate Negativea Nohmi et al. (1985)TA98
Reverse mutation S. typhimurium TA98, TA100, 6.4-800 µg/plate Negativea Andersen & Jennies (1984)
TA1535, TA1537
Reverse mutation E. coli WP2 uvrA 0.1-0.8 mg/plate Negative Yoo (1986)
Reverse mutation S. typhimurium TA1530, G46 0.25 ml/plate Negative Litton Bionetics, Inc. (1975)
Antimutagenicity E. coli WP2 uvrA 0.5-2.0 mg/ml Negative Yoo (1986)
DNA repair Bacillus subtilis < 10 mg/disk Negative Yoo (1986)
Gene mutation Bacillus subtilis < 20 mg/plate Negative Oda et al. (1978)
Gene mutation Saccharomyces cerevisiae D3 0.2 ml/plate Equivocal Litton Bionetics, Inc. (1975)
Table 2. (continued)
End-point Test object Concentration Results Reference
Chromosomal aberration Chinese hamster lung fibroblasts < 0.125 mg/ml Negative Sofuni et al. (1985)
Chromosomal aberration Human WI-38 embryonic lung cells 10 mg/ml Negative Litton Bionetics, Inc. (1975)
Chromosomal aberration Human peripheral blood lymphocytes 0.1-10 mmol/L Negativea Murthy et al. (1991)
and sister chromatid
exchange
(+)-Menthol
DNA damage Rat hepatocytes 0.7-1.3 mmpl/L Positive Storer et al. (1996)
DNA damage Chinese hamster V79 cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
DNA damage Human blood cells 0.5-2 mmol/L Negative Hartmann & Speit (1997)
In vivo
(±)-Menthol
Micronucleus formation Male B6C3F1 mouse bone marrow < 1 g/kg bw Negative Shelby et al. (1993)
(-)-Menthol
Host-mediated S. typhimurium TA1530, G46/ < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity ICR mouse host
Host-mediated S. cerevisiae D3/ICR mouse host < 5000 mg/kg bw Negative Litton Bionetics, Inc. (1975)
mutagenicity
Chromosomal aberration Albino rat bone marrow < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
Dominant lethal mutation Rat < 3 g/kg bw Negative Litton Bionetics, Inc. (1975)
a With and without an exogenous metabolic activation system
3. COMMENTS
Menthol exists as two optical isomers, (+)-menthol and
(-)-menthol; the racemic mixture is (±)-menthol. The (-) and (±) forms
are used in flavour applications.
Menthol is readily absorbed. Up to 100% of an ingested dose
appeared to be absorbed, on the basis of the elimination of menthol
metabolites in faeces and urine. Absorbed menthol is known to be
largely eliminated as glucuronides; 70-80% is eliminated in urine and
faeces within 48 h. Metabolic studies indicate that oral doses of
menthol are metabolized mainly in the liver and excreted via the
kidneys and in the bile. Menthol is efficiently metabolized by normal
processes. The metabolites are simple glucuronic acid conjugates and
oxidation products. Mammals can efficiently handle menthol by
processes that do not create hazardous products.
The NOEL in 13-week studies of toxicity with (±)-menthol in the
diet was 560 mg/kg bw per day in mice and 750 mg/kg bw per day in rats
on the basis of slightly increased incidences of interstitial
nephritis at the next highest dose.
In a two-year study of toxicity and carcinogenicity, mice were
fed (±)-menthol in the diet at concentrations equivalent to 300 or 600
mg/kg bw per day. The incidences of hepatocellular tumours in males
and lung tumours in females at the highest dose were not significantly
different from those in concurrent or historical controls. The
survival rate was decreased in female mice but remained within the
range of that of historical controls. The NOEL was 600 mg/kg bw per
day. In a two-year study of toxicity and carcinogenicity in rats given
(±)-menthol in the diet at concentrations equivalent to 190 or
380 mg/kg bw per day, the NOEL was 380 mg/kg bw per day.
Neither menthol nor its metabolites were genotoxic in vitro or
in vivo.
While no studies of reproductive toxicity were available with
menthol, (-)-menthol was tested at maximum doses of 190-430 mg/kg bw
per day for teratogenicity in mice, rats, hamsters, and rabbits; no
teratogenic effects were observed.
4. EVALUATION
The limited data that allow comparisons of metabolism and
toxicity provide no indication of a difference in the toxicity of
(-)-menthol and (±)-menthol. Therefore, the Committee concluded that
an ADI could be established for the two optical isomers of menthol.
The Committee noted that the highest dose of (±)-menthol tested
in the long-term studies in mice and rats had no specific toxic
effect. As the survival of mice was reduced at the high dose of
600 mg/kg bw per day, the Committee allocated an ADI of 0-4 mg/kg bw
on the basis of the NOEL of 380 mg/kg bw per day in the long-term
study in rats, applying a safety factor of 100 and rounding to one
significant figure.
5. REFERENCES
Adams, T.B., Hallagan, J.B., Putman, J.M., Gierke, T.L., Doull, J.,
Munro, I.C., Newberne, P., Portoghese, P.S., Smith, R.L., Wagner,
B.M., Weil, C.S., Woods, L.A. & Ford, R.A. (1996) The FEMA GRAS
assessment of alicyclic substances used as flavour ingredients.
Food Chem. Toxicol., 34, 763-828.
Andersen, P.H. & Jennies, N.J. (1984) Mutagenic investigation of
peppermint oil in the Salmonella/mammalian-microsome test.
Mutat. Res., 138, 17-20.
Atzl, G., Bertl, M., Daxenbichler, G. & Gleispach, H. (1972)
Determination of etheral oils from the urine by gas-liquid
chromatography. Chromatographia, 5, 250-255.
Austin, C.A., Shephard, E.A., Pike, S.F., Rabin, B.R. & Phillips, I.R.
(1988) The effect of terpenoid compounds on cytochrome P-450 levels in
rat liver. Biochem. Pharmacol., 37, 2223-2229.
Batt, A.M., Martin, N. & Siest, G. (1981) Induction of group-1 and
group-2 UDP-glucuronosyl transferase in microsomes from the livers of
C57 Bl/6 mice. Toxicol. Lett., 9, 355-360.
Bernson, V.S.M. & Pettersson, B. (1983) The toxicity of menthol in
short-term bioassays. Chem.-Biol. Interactions, 46, 233-246.
Boutin, J.A., Lepage, C., Batt, A.M. & Siest, G. (1981) The activity
of hepatic UDP-glucuronosyltransferase from control and induced pigs
toward 17 hydroxylated aglycones. IRCS Med. Sci., 9, 633-634.
Boutin, J.A., Thomassin, G., Siest, G. & Cartier, A. (1985)
Heterogeneity of hepatic microsomal UDP-glucuronosyltransferase
activities. Conjugations of phenolic and monoterpenoid aglycons in
control and induced rats and guinea pigs. Biochem. Pharmacol., 34,
2235-2249.
Bowen, I.H. & Cubbin, I.J. (1992) Mentha piperita and Mentha spicata.
In: Adverse Effects of Herbal Drugs, pp. 171-178.
Clegg, R.J., Middleton, B., Bell, G.D. & White, D.A. (1982) The
mechanism of cyclic monoterpene inhibition of hepatic
3-hydroxy-3-methylglutaryl coenzyme A reductase in vivo in the rat.
J. Biol. Chem., 257, 2294-2299.
Deichmann, W. & Thomas, G. (1943) Glucuronic acid in the urine as a
measure of the absorption of certain organic compounds. J. Ind.
Hyg. Toxicol., 25, 286-292.
Dooms-Goossens, A., Degreef, H., Holvoet, C. & Maertens, M. (1977)
Turpentine-induced hypersensitivity to peppermint oil. Contact
Derm., 3, 304-308.
Eisenberg, F., Field, J.B. & Stetten, D. (1955) Studies on glyceronide
conjugation in man. Arch. Biochem. Biophys., 59, 297-299.
Fishman, W.H. (1940) Studies on beta-glucuronidase. III. The increase
in beta-glucuronidase activity of mammalian tissues induced by feeding
glucuronidogenic substances. J. Biol. Chem., 136, 229-236.
Food & Drug Research Labs, Inc. (1973) Teratologic evaluation of FDA
71-57 (menthol natural Brazilian), June 1973. US National Technical
Information Service report No. PB-223815. Submitted to WHO by the
Flavor and Extract Manufacturers' Association of the United States,
Washington DC, United States.
Gaworski, C.L., Dozier, M.M., Gerhart, J.M., Rajendrans, N.,
Brennecke, L.H., Aranyi, C. & Heck, J.D. (1997) 13-Week inhalation
toxicity study of menthol cigarette smoke. Food Chem. Toxicol., 35,
683-692.
Gleason, M.N., Gosselin,R.E., Hodge, H.C. & Smith, R.P. (1969)
Clinical Toxicology of Commercial Products, 3rd Ed., Philadelphia,
Williams & Wilkins Co.
Gould, W.D., Wacker, P. & Maltzman, T.H. (1990) Chemoprevention and
chemotherapy of mammary tumors by monoterpenoids. Prog. Clin. Biol.
Res., 347, 255-268.
Green, M.D., Clarke, D.J., Oturu, E.M., Styczynski, P.B., Jackson,
M.R., Burchell, B. & Tephly, T.R. (1995) Cloning and expression of a
rat liver phenobarbital-inducible UDP-glucuronosyl-transferase (2B12)
with specificity for monoterpenoid alcohols. Arch. Biochem.
Biophys., 322, 460-468.
Hartmann, A. & Speit, G. (1997) The contribution of cytotoxicity to
DNA-effects in the single cell gel test (Comet assay). Toxicol.
Lett., 90, 183-188.
Haseman, J.K., Huff, J.E., Rav, G.N., Arnold, J.E., Boorman, G.A. &
McConnell, E.E. (1985) Neoplasms observed in untreated and corn oil
gavage control groups of F344/N rats and (C57Bl/6N × C3H/HeN) F1
(B6C3F1) mice. J. Natl Cancer Inst., 75, 975-984.
Haseman, J.K., Tharrington, E.C., Huff, J.U.E. & McConnell, E.E.
(1986) Comparison of site-specific and overall tumor incidence
analysis for 81 recent National Toxicology Program carcinogenicity
studies. Reg. Toxicol. Pharmacol., 6, 155-170.
Herken, H. (1961) [Pharmacological expertise on tolerance to natural
and synthetic menthol.] Unpublished report from Pharmakologischen
Institut der Freien Universität, Berlin, Duhlen. Submitted to WHO by
Schering AG, Berlin, Germany (in German).
Horvath, T., Past, T., Tatui, Z., Par, A., Kadas, I., Tapsonyi, Z. &
Javor, J. (1984) Investigation of biotransformation capacity in
patients with chronic liver diseases by pharmacokinetic methods:
Experimental trials. Pol. J. Pharmacol. Pharm., 36, 361-371.
Imaizumi, K., Hanada, K., Mawatari, K. & Sugano, M. (1985) Effect of
essential oils on the concentration of serum lipids and
apolipoproteins in rats. Agric. Biol. Chem., 49, 2795-2796.
Ishidate, M., Jr, Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ivett, J.L., Brown, B.M., Rodgers, C., Anderson, B.E., Resnick, M.A. &
Zeiger, E. (1989) Chromosomal aberrations and sister chromatid
exchange tests in Chinese hamster ovary cells in vitro. IV. Results
with 15 chemicals. Environ. Mol. Mutag., 14, 165-187.
Jay, J.J. & Rivers, G.M. (1984) Antimicrobial activity of some food
flavoring compounds. J. Food Saf., 6, 129-139.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kabat, G.C. & Hebert, J.R. (1991) Use of mentholated cigarettes and
lung cancer risk. Cancer Res., 51, 6510-6513.
Kaffenberger, R.M. & Doyle, M.J. (1990) Determination of menthol and
menthol glucuronide in human urine by gas chromatography using an
enzyme-sensitive internal standard and flame ionization detection.
J. Chromatogr., 527, 59-66.
Karapinar, M. & Aktug, S.E. (1987) Inhibition of food borne pathogens
by thymol, eugenol, menthol and anethole. Int. J. Food Microbiol.,
4, 161-166.
Kurita, N., Miyaji, M., Kurane, R. & Takahara, Y. (1981) Antifungal
activity of components of essential oil. Agric. Biol. Chem., 45,
945-952.
Lewis, F.M., Shah., M. & Gawkrodger, D.J. (1995) Contact sensitivity
to food additives can cause oral and perioral symptoms. Contact
Derm., 33, 429-430.
Litton Bionetics, Inc. (1975) Mutagenic evaluation of compound FDA
71-57, menthol, 14 January 1975. US National Technical Information
Service report No. PB-245444. Submitted to WHO by International
Federation of Chewing Gum Associations, Keller and Hechman LLP,
Washington DC, United States
Madsen, C., Wurtzen, G. & Carstensen, J. (1986) Short-term toxicity
study in rats dosed with menthone. Toxicol. Lett., 32, 147-152.
Madyastha, K.M. & Srivatsan, V. (1988) Studies on the metabolism of
l-menthol in rats. Drug Metab. Disposition, 16, 765-772.
Mahmoud, A.L.E. (1994) Antifungal action and antiaflatoxigenic
properties of some essential oil constituents. Lett. Appl.
Microbiol., 19, 110-113
Megalla, S.E., El-Keltawi, N.E. & Ross, S.A. (1980) A study of
antimicrobial action of some essential oil constituents. Herba
Pol., 26, 181-186.
Mengs, U. & Stotzem, C.D. (1989) Toxicological evaluation of
peppermint oil in rodents and dogs. Med. Sci. Res., 17, 499-500.
Millard, L.G. (1973) Contact sensitivity to toothpaste. Br. Med.
J., i, 676.
Mœlck, A.M., Poulsen, M., Lauridsen, S.T. & Olsen, P. (1998) Lack of
histological cerebellar changes in Wistar rats given pulegone for 28
days. Comparison of immersion and perfusion tissue fixation.
Toxicol. Lett., 95, 117-122.
Moleyar, V & Narasimham, P. (1992) Antibacterial activity of essential
oil components. Int. J. Food Microbiol., 16, 337-342.
Morris, J.A., Khettry, A. & Seitz, E.W. (1979) Antimicrobial activity
of aroma chemicals and essential oils. J. Am. Oil Chem. Soc., 56,
595-603.
Morton, C.A., Garioch, J., Todd, P., Lamey, P.J. & Forsyth, A. (1995)
Contact sensitivity to menthol and peppermint in patients with
intra-oral symptoms. Contact Derm., 32, 281-284.
Muller-Riebau, F., Berger, B. & Yegen, O. (1995) Chemical composition
and fungitoxic properties to phytopathogenic fungi of essential oils
of selected aromatic plants growing wild in Turkey. J. Agric. Food
Chem., 43, 2262-2266.
Murthy, P.B., Ahmed, M.M. & Regu, K. (1991) Lack of genotoxicity of
menthol in chromosome aberration and sister chromatid exchange assays
using human lymphocytes in vitro. Toxicol. In Vitro, 5, 337-340.
Myhr, B.C. & Caspary, W.C. (1991) Chemical mutagenesis at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Results for 31
coded compounds in the National Toxicology Program. Environ. Mol.
Mutag., 18, 51-83.
Nachev, C., Zolotovitch, G., Siljanovska, K. & Stojcev, S. (1967)
Cytotoxic effect of alcohols isolated from essential oils. C.R.
Acad. Bulg. Sci., 20, 1081-1084.
Niestijl Janson, J.J., Kardinaal, A.F.M., Huijbers, G.,
Vlieg-Boerstra, B.J., Martens, B.P.M. & Ockhuizen, T (1994) Prevalence
of food allergy and intolerance in the adult Dutch population.
J. Allergy Clin. Immunol., 93, 446-456.
Nohmi, T., Miyata, R., Yoshilawa, K. & Ishidate, M., Jr (1985)
Mutagenicity tests on organic chemical contaminants in city water and
related compounds. I. Bacterial mutagenicity tests. Eisei Shikenjo
Hokoku, 103, 60-64.
Oda, Y., Hamano, Y., Inoue, K., Yamamoto, H., Nihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria (1st report).
Shokunin Eisei Hen, 9, 177-181.
Ohta, T., Watanabe, M., Watanabe, K., Shirasu, Y. & Kada, T. (1986)
Inhibitory effects of flavourings on mutagenesis induced by chemicals
in bacteria. Food Chem. Toxicol., 24, 51-54.
Olowe, S.A. & Ransome-Kuti, O. (1980) The risk of jaundice in
glucose-6-phospate dehydrogenase deficient babies to menthol.
Acta Paediatr. Scand., 69, 341-345.
Quick, A.J. (1924) The synthesis of menthol glycuronic acid in the
rabbit. J. Biol. Chem., 61, 679-683.
Quick, A.J. (1928) Quantitative studies of beta-oxidation. IV. The
metabolism of conjugated glycuronic acids. J. Biol. Chem., 80,
535-541.
Rakieten, N., Rakieten, M.L. & Boykin, M. (1954) Effects of menthol
vapor on the intact animal with special reference to the upper
respiratory tract. J. Am. Pharm. Assoc. Sci. Ed., 43, 390-392.
Ruch, R.J. & Sigler, K. (1994) Growth inhibition of rat liver
epithelial tumor cells by monoterpenes does not involve rat plasma
membrane association. Carcinogenesis, 15, 787-789.
Russin, W.A., Hoesly, J.D., Elson, C.E., Tanner, M.A. & Gould, M.
(1989) Inhibition of rat mammary carcinogenesis by monoterpenoids.
Carcinogenesis, 10, 2161-2164.
Shah, M., Lewis, F.M. & Gawkrodger, D.J. (1996) Contact allergy in
patients with oral symptoms: A study of 47 patients. Am. J.
Contact Derm., 7, 146-151.
Shelby, M.D., Erexson, G., Hook, G.L. & Tice, R.R. (1993) Evaluation
of a three-exposure mouse bone marrow micronucleus protocol: Results
with 49 chemicals. Environ. Mol. Mutag., 21, 160-179.
Sofuni, T., Hayashi, M., Matsuoka, A., Sawada, M., Hatanaka, M. &
Ishidate, M. (1985) Mutagenicity tests on organic chemical
contaminants in city water and related compounds. II. Chromosome
aberration tests in cultured mammalian cells. Eisei Shikenjo
Hokoku, 103, 64-75.
Somerville, K.W., Richmond, C.R. & Bell, G.D. (1984) Delayed release
peppermint oil capsules (Colpermin) for the spastic colon syndrome: A
pharmacokinetic study. Br. J. Clin. Pharmacol., 18, 638-640.
Spindler, P. & Madsen, C. (1992) Subchronic toxicity study of
peppermint oil in rats. Toxicol. Lett., 62, 215-220.
Stoner, G.D. (1991) Lung tumors in strain A mice as a bioassay for
carcinogenicity of environmental chemicals. Exp. Lung Res., 17,
405-423.
Stoner, G.D., Shimkin, M.B., Kniazeff, A., Weisburger, J.H.,
Weisburger, E.K. & Go, G.K. (1973) Test for carcinogenicity of food
additives and chemotherapeutic agents by the pulmonary tumor response
in strain A mice. Cancer Res., 33, 3069-3085.
Storer, R.D., McKelvey, T.W., Kraynak, A.R., Elia, M.C., Barnum, J.E.,
Harmon, L.S., Nichols, W.W. & DeLuca, J.G. (1996) Revalidation of the
in vitro alkaline elution rat hepatocyte assay for DNA damage:
Improved criteria for assessment of cytotoxicity and genotoxicity and
results for 81 compounds. Mutat. Res., 368, 59-101.
Szabo, L., & Ebrey, P. (1963) Studies on the inheritance of
Crigler-Najjar's syndrome by the menthol test. Acta Pediatr. Acad.
Sci. Hung., 4, 153-158.
Takacs, A.L. & Vore, M. (1987) Binding of 3H-estradiol-17B
(B-D-glucuronide), a cholestatic organic anion, to rat liver plasma
membranes. Evidence consonant with identification of organic anion
carriers. Mol. Pharmacol., 32, 511-518.
Tamaoki, J., Chiyotani, A., Sakai, A., Takemura, H. & Konno, K. (1995)
Effect of menthol vapour on airway hyper responsiveness in patients
with mild asthma. Respir. Med., 89, 503-504.
Tennant, R.W., Margolin, B.H., Shelby, M.D., Zeiger, E., Haseman,
J.K., Spalding, J., Caspary, W., Resnik, M., Stasiewicz, S., Anderson,
B. & Minor, R. (1987) Prediction of chemical carcinogenicity in
rodents from in vitro genetic toxicity assays. Science, 236,
933-941.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983a) Short term
toxicity study in rats dosed with pulegone and menthol. Toxicol.
Lett., 19, 207-210.
Thorup, I., Wurtzen, G., Carstensen, J. & Olsen, P. (1983b) Short term
toxicity study in rats dosed with peppermint oil. Toxicol. Lett.,
19, 211-215.
Ueda, S., Yamashita, H. & Kuwabara, Y. (1982) Inhibition of
Clostridium botulinum and Bacillus sp. by spices and flavoring
compounds. Nippon Shokuhin Kogyo Gakkaishi, 29, 389-392.
Uno, Y., Takasawa, H., Miyagawa, M., Inoue, Y., Murata, T. &
Yoshikawa, K. (1994) An in vivo-in vitro replicative DNA synthesis
(RDS) test using rat hepatocytes as an early prediction assay for
non-genotoxic hepatocarcinogens, screening of 22 known positives and
25 noncarcinogens. Mutat. Res., 320, 189-205.
US National Cancer Institute (1979) Bioassay of DL-menthol for
possible carcinogenicity. Report NCI-CG-TR-98. US National Technical
Information Service report No. PB-288761, Bethesda, Maryland, United
States.
White, D.A., Thompson, S.A., Wilson, C.G. & Bell, G.D. (1987) A
pharmacokinetic comparison of 2 delayed-release peppermint oil
preparations, Colpermin and Mintec, for treatment of the irritable
bowel syndrome. Int. J. Pharmacol., 40, 151-155.
WHO (1987) Principles for the Safety Assessment of Food Additives
and Contaminants in Food (Environmental Health Criteria 70), Geneva
Williams, R.T. (1938) Studies in detoxication. II. (a) The conjugation
of isomeric 3-menthanols with glucuronic acid and the asymmetric
conjugation of dl-menthol and dl-isomenthol in the rabbit; (b)
d-isoMenthylglucuronide, a new conjugated glucuronic acid. Biochem.
J., 32, 1849-1855.
Williams, R.T. (1939) CLXXXVI. Studies in detoxication. III. The use
of the glucuronic acid detoxication mechanism of the rabbit for the
resolution of dl-menthol. Biochem. J., 32, 1519-1524.
Wokes, F. (1932) The antiseptic value and toxicity of menthol isomers.
Q. J. Pharm. Pharmacol., 5, 233-244.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]-l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Cent., 34, 267.
Yoshikawa, R.T. (1996) Anomalous nonidentity between Salmonella
genotoxicants and rodent carcinogens: Nongenotoxic carcinogens and
genotoxic noncarcinogens. Environ. Health Perspectives, 104, 40-46.
Yousef, R.T., Aggag, M.E. & Tawil, G.G. (1978) Evaluation of the
antifungal activity of some components of volatile oils against
dermatophytes. Mykosen, 21, 190-193.
