
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
RIBOFLAVIN DERIVED BY FERMENTATION WITH GENETICALLY MODIFIED BACILLUS
SUBTILIS
First draft prepared by
M.E.V. Apeldoorn, H.V. Steeg, and G.J.A. Speijers
National Institute of Public Health and the Environment, Center of
Substances and Risk Assessment, Bilthoven, The Netherlands
Explanation
Biological data
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Genotoxicity
Methods of genetic modification
Molecular genetic methods used to clone and
express the riboflavin (rib) operon from
Bacillus subtilis
Genetic stability of the production strain
Foreign DNA present in the end-product
Comments
Evaluation
References
1. EXPLANATION
Riboflavin derived by a fermentation process with genetically
modified Bacillus subtilis has not been previously evaluated by the
Committee. Synthetic riboflavin was evaluated by the Committee at its
thirteenth meeting (Annex 1, reference 19), when an ADI of 0-0.5 mg/kg
bw was allocated on the basis of limited data. At the twenty-fifth
meeting (Annex 1, reference 56), a group ADI of 0-0.5 mg/kg bw was
allocated to riboflavin and riboflavin-5'-phosphate, expressed as
riboflavin, on the basis of a more extensive database including
information on reproductive toxicity.
Riboflavin (vitamin B2) is a water-soluble vitamin. It is
synthesized by all plants and many microorganisms, but it is not
produced by higher animals. Because it is a precursor of coenzymes
that are required for the enzymatic oxidation of carbohydrates,
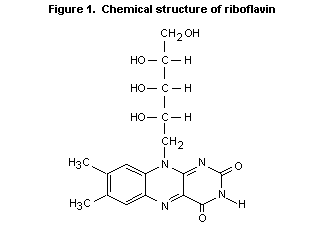
riboflavin is essential to basic metabolism. Its chemical structure is
given in Figure 1.
In higher animals, insufficient riboflavin can cause loss of
hair, inflammation of the skin, vision deterioration, and growth
failure. Riboflavin is therefore widely used as an additive to
foodstuffs and feedstuffs. It is used in foods predominantly for
fortification; however, quantities accounting for approximately 2% of
the total sales are used for colouring, e.g. for sweets. The foods to
which it is added for fortification or restoration are breakfast
cereals, soft drinks, slimming diet products, and baby food products.
These account, for example, for 82% of the riboflavin sold in the
United Kingdom for food use; a further 16% is used in ice cream, meat
and fish, sauces and soups, and food supplements.
 Although riboflavin can be produced by both synthetic and
fermentation processes, the latter was not found to be competitive for
production of very pure material. Recent developments in biotechnology
have resulted in microbiological processes that compete successfully
with the synthetic chemical process. Fermentation-produced riboflavin
is prepared by the controlled, submerged growth of a selected strain
of B. subtilis that has been genetically modified to produce
riboflavin. Manufacture involves fermentation resulting in a broth
containg riboflavin, recovery of riboflavin from the broth, and
further purification to a final product with a purity of 96 or 98%,
which is used in animal feed and human food, respectively (Pfister et
al., 1995).
* Fermentation is performed in vessels by aseptic, large-scale
microbiological techniques under controlled conditions of
temperature, aeration, agitation, and pH. The cultural purity of
the fermentation is routinely tested by checking growth behaviour
and colony identity. When the fermentation has proceeded to the
desired point, the broth is delivered for recovery.
* Riboflavin is recovered from the broth by centrifugation after
inactivation of the microorganisms by heat. Pasteurization of the
broth ensures that no viable cells of the production organism are
present in the final product. Differential centrifugation leads
to separation of cells and riboflavin crystals because of
differences in size and sedimentation behaviour.
* Mineral acid treatment (about 2% hydrochloric or sulfuric acid)
yields 96% riboflavin, which, after drying and packaging, is
either directly sold for application in feed or used for further
processing into a formulation. The acid treatment also ensures
destruction (via depurination) of DNA from the processing
organism. Recrystallization of the 96% pure material from
concentrated hydrochloric acid (> 25%) results in a food-quality
material containing 98% riboflavin. This last purification step
is identical to that currently used for purification of
riboflavin derived from chemical synthesis.
Three lots of 98% pure riboflavin derived by fermentation, four
lots of 96% pure material derived by fermentation, and one lot of 98%
pure material derived by synthesis were analysed by two separate
high-performance liquid chromatography (HPLC) methods in order to
identify and quantify any impurities. The riboflavin content of the
98% pure products derived by fermentation and synthesis ranged from 99
to 101%, and that of the 96% pure products derived by fermentation
from 97 to 99%. All of the peaks detected in riboflavin from
fermentation could be identified and appeared also to be present in
synthesized riboflavin, indicating that the impurities probably occur
during the purification steps (acidic treatment and crystallization)
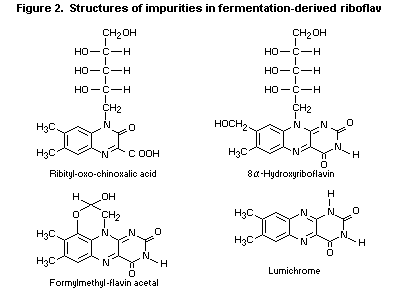
(Simon & Vogt, 1989; Ludwig et al., 1994). Four impurities were
identified in the fermentation-derived material (for chemical
structures, see Figure 2). Lumiflavin, a known degradation product of
riboflavin after basic treatment (Meinrad & Gerber, 1991), was present
in the synthesized product but not in the fermentation-derived
material.
Although riboflavin can be produced by both synthetic and
fermentation processes, the latter was not found to be competitive for
production of very pure material. Recent developments in biotechnology
have resulted in microbiological processes that compete successfully
with the synthetic chemical process. Fermentation-produced riboflavin
is prepared by the controlled, submerged growth of a selected strain
of B. subtilis that has been genetically modified to produce
riboflavin. Manufacture involves fermentation resulting in a broth
containg riboflavin, recovery of riboflavin from the broth, and
further purification to a final product with a purity of 96 or 98%,
which is used in animal feed and human food, respectively (Pfister et
al., 1995).
* Fermentation is performed in vessels by aseptic, large-scale
microbiological techniques under controlled conditions of
temperature, aeration, agitation, and pH. The cultural purity of
the fermentation is routinely tested by checking growth behaviour
and colony identity. When the fermentation has proceeded to the
desired point, the broth is delivered for recovery.
* Riboflavin is recovered from the broth by centrifugation after
inactivation of the microorganisms by heat. Pasteurization of the
broth ensures that no viable cells of the production organism are
present in the final product. Differential centrifugation leads
to separation of cells and riboflavin crystals because of
differences in size and sedimentation behaviour.
* Mineral acid treatment (about 2% hydrochloric or sulfuric acid)
yields 96% riboflavin, which, after drying and packaging, is
either directly sold for application in feed or used for further
processing into a formulation. The acid treatment also ensures
destruction (via depurination) of DNA from the processing
organism. Recrystallization of the 96% pure material from
concentrated hydrochloric acid (> 25%) results in a food-quality
material containing 98% riboflavin. This last purification step
is identical to that currently used for purification of
riboflavin derived from chemical synthesis.
Three lots of 98% pure riboflavin derived by fermentation, four
lots of 96% pure material derived by fermentation, and one lot of 98%
pure material derived by synthesis were analysed by two separate
high-performance liquid chromatography (HPLC) methods in order to
identify and quantify any impurities. The riboflavin content of the
98% pure products derived by fermentation and synthesis ranged from 99
to 101%, and that of the 96% pure products derived by fermentation
from 97 to 99%. All of the peaks detected in riboflavin from
fermentation could be identified and appeared also to be present in
synthesized riboflavin, indicating that the impurities probably occur
during the purification steps (acidic treatment and crystallization)
(Simon & Vogt, 1989; Ludwig et al., 1994). Four impurities were
identified in the fermentation-derived material (for chemical
structures, see Figure 2). Lumiflavin, a known degradation product of
riboflavin after basic treatment (Meinrad & Gerber, 1991), was present
in the synthesized product but not in the fermentation-derived
material.
 The presence of amino acids originating from fermentation was
analysed by the method of Spackman et al. (1958), and DNA was analysed
by polymerase chain reaction (PCR) techniques (Schurter & Hermann,
1995). The amounts of impurities in the different products are given
in Table 1. The concentration of 8alpha-hydroxymethylriboflavin, which
is a known degradation product after acid treatment of riboflavin, was
slightly increased in the fermentation-derived product, but only in
that of 98% purity. Since this product is derived from the 96% pure
material by crystallization from acidic media, this is to be expected;
however lumiflavin, which is a known degradation product after basic
treatment, was present in the synthesized riboflavin but not in the
fermentation-derived material.
Table 1. Composition of riboflavin products derived by fermentation and by synthesis
(average % weighed samples)
Component 96% ex 98% ex 96% ex synthesis
fermentation fermentation
HPLC method HPLC method HPLC method
I II I II I II
Riboflavin 98.4 98.3 100.1 99.7 98.8 98.6
Ribityl-oxo-chinoxalic acid Trace ND ND ND Trace Trace
8alpha-Hydroxy riboflavin Trace Trace 0.45 0.2 Trace Trace
Formylmethyl-flavin acetal Trace ND 0.11 Trace 0.52 ND
Lumichrome 0.14 0.11 ND Trace 0.18 Trace
Lumiflavin ND ND ND ND 0.56 0.5
Amino acids 0.9 0.9 0.06 0.06 - -
DNAa ND ND ND ND - -
Total 99.44 99.31 100.72 99.96 100.1 99.3
Trace, below limit of detection; ND, below limit for quantitative determination, 0.1%
a See also section 3.3, 'Foreign DNA present in the end product'
2. BIOLOGICAL DATA
2.1 Toxicological studies
2.1.1 Acute toxicity
No studies of the acute toxicity of riboflavin derived by
fermentation with genetically modified B. subtilis are available.
Table 2 gives the LD50 values for riboflavin (not derived from
fermentation), riboflavin phosphate, and some of the impurities
present in riboflavin. Except for the study with
8alpha-hydroxy-riboflavin, no full reports were available. The data in
Table 2 give an indication of the acute toxicity of synthetic
riboflavin (phosphate) and some of its impurities but are not relevant
for evaluating riboflavin derived by fermentation.
2.1.2 Short-term studies of toxicity
Rats
Groups of 10 male and 10 female six-week-old Wistar rats (the
males weighing 160-170 g and the females, 130-140 g) received diets
providing 20, 50, or 200 mg/kg bw of riboflavin -- either 99.4% pure
derived by fermentation from 96% pure riboflavin, 100.2% pure derived
by fermentation from 98% pure riboflavin, or 99.8% pure derived by
sunthesis from 98% pure riboflavin -- daily, on seven days per week
for 13 weeks. The study was performed according to good laboratory
practice (GLP) and OECD Guideline 408; a quality assurance (QA)
statement was included. A control group of 30 male and 30 female rats
was used. A satellite group of six male and six female animals was
attached to each group for determination of riboflavin concentrations
in blood and urine and for investigation of the reversibility of any
toxicological parameter. Feed and water were provided ad libitum.
The different assays were performed at one-week intervals. Samples of
the diet were taken at the start of treatment and at weeks 6 and 13 to
determine the content, homogeneity, and stability of the test compound
in the diet.
All animals were checked twice daily (once daily at weekends) for
behaviour and general condition. Ophthalmoscopy was performed on all
animals in the main control group and those at the three highest doses
before the start and at the end of the study. Body weight and food
intake were determined weekly. Water consumption was determined over
five consecutive days in weeks 1, 5, and 12. Haematological parameters
(haemoglobin concentration; haematocrit; erythrocyte, leukocyte, and
differential cell counts; mean corpuscular volume, haemoglobin, and
haemoglobin concentration; reticulocyte and thrombocyte counts; and
prothrombin time) were measured at week 6 and at autopsy in 10 rats of
each sex in the main control and treated groups. Haemoglobin
concentration; haematocrit; erythrocyte and leukocyte counts; mean
corpuscular volume, haemoglobin, and haemoglobin concentration; and
thrombocyte count were measured in all rats in the satellite groups at
the end of the recovery period. Clinical chemical parameters (alanine
and aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl
transferase, and lactic dehydrogenase activities; bilirubin, total
protein, albumin, albumin:globulin ratio, urea, creatinine, Na, K, Ca,
Cl, inorganic phosphate, cholesterol, triglycerides, and
phospholipids) were determined in all rats in the main treated groups
and in 15 rats of each sex in the main control group in weeks 7 and 13
after one night of fasting; the same parameters were measured in all
rats in the satellite groups at the end of the recovery period. In
weeks 7 and 13, all rats in the main treated groups and 15 rats of
each sex in the main control group underwent a renal concentration
test and urinalysis (appearance, pH, glucose, occult blood, ketones,
proteins, bilirubin, urobilinogen, sediment), except that sediment was
measured only in rats at the three highest doses. The sediment of
urine from all rats in the satellite groups was examined
microscopically for the presence of sperm at the end of the recovery
period. The weights (absolute and relative to body weight) of the
adrenals, kidneys, liver, spleen, brain, thymus, heart, and testes of
all rats were determined at the end of the treatment period in all of
the main groups and at the end of the recovery period in all satellite
groups. All rats in the main groups were examined macroscopically at
the end of treatment, and about 30 tissues from all animals in the
main control group and in the main three highest-dose groups were
examined microscopically The testes, epididymides, kidneys, liver,
lungs, and all gross lesions from all rats in the main groups were
examined microscopically. The riboflavin concentration in blood was
determined in all rats in the satellite groups before the start of the
study, at weeks 2, 6, and 13 of treatment, and at the end of the
recovery period. The riboflavin content of the urine was determined in
all rats in the satellite control group and in all rats in the
satellite groups treated with 96 and 98% pure riboflavin derived by
fermentation, before the study and at week 2 and 6. At the end of the
treatment period and at the end of the recovery period, the riboflavin
content of the urine was determined in all rats in all satellite
groups.
The test substances were homogeneously distributed in the diets
and showed storage stability under simulated experimental conditions.
The actual measured concentrations in the diet were within the
tolerable range of ± 10% of the nominal concentration. Alopecic areas
were seen frequently in various groups, but these findings were
transient and were not related to dose. Ophthalmoscopy showed no
abnormalities. Excrement collected from rats at the highest doses of
the three test substances was yellowish throughout the study. No
dose-related difference in food and water intake was seen.
During treatment, females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation showed a slightly but usually statistically
significant decrease in growth of about 6%. Males and females at
50 mg/kg bw per day 98% pure riboflavin ex synthesis also had a slight
but statistically significant decrease in growth. These effects were
not considered to be toxicologically relevant because they were small
(< 10%) and food conversion was not affected. During the recovery
period, body weights were similar in all groups. No changes in
haematological parameters were seen after six weeks of treatment, but
at the end of treatment (week 13) females at 200 mg/kg bw per day 98%
pure riboflavin ex fermentation had a slightly but statistically
significantly lower mean haemoglobin value and erythrocyte count,
whereas the mean reticulocyte count was significantly increased. The
individual reticulocyte counts in these animals were markedly
increased in two of 10 females and moderately increased in another
two. Slight but statistically significant increases in mean
thrombocyte counts in females at the highest dose of 98% pure
riboflavin ex fermentation and in males at the highest dose of
riboflavin 98% ex synthesis were within the range of normal variation
and therefore considered not to be toxicologically relevant. At six
and 13 weeks, the total leukocyte counts were statistically
significantly decreased in several treated groups but with no
dose-response relationship and inconsistently within groups; these
changes were also not considered to be related to treatment.
Clinical chemical and urinary analyses showed no
treatment-related changes. At the end of treatment, a slight but
statistically significant increase in relative liver weight was seen
in females at 200 mg/kg bw per day 98% pure riboflavin ex
fermentation, and a slight but statistically significant increase in
relative spleen weight in males at 50 and 200 mg/kg bw per day 96%
pure riboflavin (ex fermentation), but with no dose-response
relationship. Macroscopic and microscopic examination did not reveal
any treatment-related abnormality. The changes in organ weights were
considered to be of questionable biological relevance because there
was no dose-response relationship and they were not accompanied by
histopathological changes or changes in clinical chemistry. At the end
of the recovery period, all of the haematological parameters were
normal, except for a statistically significant decrease in mean
thrombocyte counts in females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation, and there were no notable changes in organ
weights.
The riboflavin concentrations in blood and urine were not
reported.
The authors concluded that the NOEL was 200 mg/kg bw per day for
all three test substances. The decreases in mean haemoglobin
concentration and erythrocyte count in females at 200 mg/kg bw per day
98% pure riboflavin ex fermentation were considered to be fortuitous
findings because, except in two females, there was no overt
association between the low erythrocyte counts and the high
reticulocyte counts. Moreover, according to the authors, the measured
values did not exceed the expected range, and there was also no clear
dose-response relationship in any group. In addition, there were no
related changes (in e.g. haemolysis or weight and histopathology of
haematopoietic organs) that could be associated with variations in the
red blood cell profile (Buser et al., 1995).
The Committee concluded that the NOEL for 98% pure riboflavin ex
fermentation was 50 mg/kg bw per day, on the basis of the significant
decreases in mean haemoglobin concentration and erythrocyte count and
the significant increase in mean reticulocyte count in females at
200 mg/kg bw. For 96% pure riboflavin ex fermentation and 98% pure
riboflavin ex synthesis, the NOEL was 200 mg/kg bw per day, the
highest dose tested.
Table 2. Acute toxicity of synthetic riboflavin phosphate and some of its impurities
Compound Species Sex Route LD50 Remarks Reference
(mg/kg bw)
Riboflavin Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin Rat NR Intravenous 560 Bächtold (1980)
Riboflavin Rat NR Subcutaneous 5 000 Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intraperitoneal 890 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intravenous 780 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Oral > 20 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 1 030 Observation period, 24 h Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 560 Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intravenous 710 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Subcutaneous 790 Bächtold (1980)
8alpha-Hydroxyriboflavin Rat M&F Oral > 2 000 Limit test, by gavagea Schöni (1989)
(purity > 95%)
Lumichrome Mouse NR Oral > 9 000 Observation period, 10 days Bächtold (1980)
Lumichrome Mouse NR Intraperitoneal 11 000 Observation period, 10 days Bächtold (1980)
Lumiflavin Mouse NR Oral > 6 000 Observation period, 10 days Bächtold (1980)
NR, not reported; M&F, male and female
a Study performed according to OECD Guideline 401; no GLP statement or QA declaration was included.
Table 3. Results of assays for the genotoxicity of riboflavin derived by fermentation from B. subtilis and of 8alpha-hydroxyriboflavin
End-point Test object Concentration Results Reference
Riboflavin 96% Reverse mutationa S. typhimurium TA97, 50-5000 µg/plate Negativeb Albertini (1995a)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 99.4%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
Riboflavin 98% Reverse mutationa S. typhimurium TA 97, 50-5000 µg/plate Negativeb Albertini (1995b)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 100.2%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
8alpha-Hydroxy- Reverse mutationc S. typhimurium TA97, 100-5000 µg/plate Negativeb Albertini (1989)
riboflavin (purity TA98, TA100, TA102, in DMSO No toxicity; yellow-to-orange
not given) TA1535, TA1537, TA1538 precipitate at 2500 and 5000
µg/plate
DMSO, dimethyl sulfoxide
a Ames test; standard plate incorporation, preincubation modification assay. Study performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat experiment; a QA statement was included.
b With and without exogenous metabolic activation
c Ames test; standard plate incorporation, preincubation modification assay. Study not performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat assay; no QA statement was included.
2.1.3 Genotoxicity
Studies on the genotoxic potential of riboflavin derived by
fermentation from B. subtilis and of 8alpha-hydroxyriboflavin, an
impurity of the fermentation-derived material, are summarized in Table
3.
3. METHODS OF GENETIC MODIFICATION
3.1 Molecular genetic methods used to clone and express the riboflavin
(rib) operon from Bacillus subtilis
The riboflavin operon of B. subtilis containing the genes
ribT, ribH, ribA, and ribG was cloned into pUC19 as a 6.4-kb
NcoI/XbaI DNA fragment. In order to enhance expression of the rib
genes, a strong constitutive B. subtilis bacteriophage SPO1 promoter
was included. Two versions were made: (i) the SPO1-15 promoter was
inserted in front of the endogenous ribP2 promoter in a construct
later designated as pRF93 and (ii) the endogenous ribP1 promoter was
replaced by SPO1-15 in a construct later designated as pRF69. In order
to make the constructs selectable after integration into the B.
subtilis genome (the ß-lactamase gene of pUC19 is not expressed in
B. subtilis), the two constructs were provided with different
selectable genes, i.e. the tetracycline-resistant (tet) gene derived
from B. cereus was inserted into pRF93, and the chloramphenicol-
resistant (cat) gene derived from pUC194, origin S. aureus/
B. subtilis, into pRF69. Both constructs were integrated into the
genome of recipient B. subtilis strain RB50. The integration sites
were mapped for pRF69 at 209° and for pRF93 at 139° in the B.
subtilis genome.
RB50, a strain of B. subtilis that overproduces riboflavin, was
obtained by classical mutagenesis and selection procedures. With
various unknown mutations, RB50 contains a mutation that maps at the
ribC locus, which is present at 147° of the B. subtilis genome.
In order to further increase riboflavin production, the
RB50::[pRF69]: :[pRF93] was subjected to increasing concentrations of
the antibiotics tetracycline and chloramphenicol, resulting in
amplification of pRF69 and pRF93. In general, pRF69 was multiplied
20-30 times whereas pRF93 was multiplied 8-10 times, resulting in the
production strain RB50::[pRF69]n: :[pRF93]m. Although neither
antibiotic was present during the production stage, there was no
detectable loss of either pRF (Maruo & Yoshikawa, 1989; Kreneva &
Perumov, 1990; Mironov et al., 1994; Schurter, 1994). All of the
procedures were extensively and well described.
3.2 Genetic stability of the production strain
The production strain obtained after amplification was
RB50::[pRF69]n: :[pRF93]m. Samples were taken from the fermentor
during the process, and the numbers of copies of pRF69 and pRF93 were
determined by Southern blotting. There was no apparent loss of the
foreign DNA present in RB50: :[pRF69]n::[pRF93]m during the
process, which took 48 h, with samples taken at 6-h intervals
(Hümbelin & Hermann, 1995). As all of the experiments were well
described and appeared to be sound and convincing, it can be concluded
that the production strain is genetically stable during the
fermentation process.
3.3 Foreign DNA present in the end-product
One of the purification steps involved treatment of the product
with diluted hydrochloric acid for 60 min at 90°C. Under these
conditions, DNA is heavily depurinated and degraded (Hermann &
Schurter, 1994). A sensitive PCR method was used to detect any
residual transforming DNA (monitored as a 557-bp pRF69 PCR cat
fragment), with a detection limit of 0.5 ppb DNA per mg riboflavin. No
foreign DNA was detected. Furthermore, no transforming activity was
detected after only 5 min of hot acid treatment of crude riboflavin
(Schurter & Hermann, 1995). As all of the experiments were described
in detail, and the results are convincing, it can be concluded that
the riboflavin samples were free of any detectable transforming DNA.
4. COMMENTS
The strain used in riboflavin production has been genetically
modified to overproduce riboflavin by amplification of the chromosomal
region of the riboflavin operon containing suitable promoters and
flanked by pUC19 and antibiotic-resistance marker genes. The lack of
pathogenicity and toxicity of the strain of microorganism
(B. subtilis) from which the genetic information encoding riboflavin
was cloned is well documented, and the methods for genetic
modification have been well described. The production strain of
B. subtilis was shown to be genetically stable during fermentation.
It was convincingly established that the final product is riboflavin
of a purity comparable to, or greater than, that produced by
conventional methods and is free of DNA from the production organism.
Thus, antibiotic resistance marker genes are not present.
In a 90-day study of toxicity, rats were fed diets containing
food-grade riboflavin (98% pure) produced by fermentation from
genetically modified B. subtilis or riboflavin derived by chemical
synthesis, also of 98% purity. The doses tested were 0, 20, 50, or 200
mg/kg bw per day. Some animals at the high dose had reduced weight
gain, but generally by less than 10%, and food conversion efficiency
was not affected. In the group fed the high dose of
fermentation-derived riboflavin of 98% purity, some minor changes in
red blood cell parameters were observed, but they were not considered
relevant. The NOEL for 98% pure riboflavin produced by fermentation
was 200 mg/kg bw per day, which was the same as that for chemically
synthesized riboflavin.
Fermentation-derived riboflavin and the degradation product
8alpha-hydroxyriboflavin did not induce gene mutations in bacteria
in vitro in either the absence or the presence of metabolic
activation.
5. EVALUATION
The Committee concluded that the recombinant DNA techniques used
to derive the production strain of B. subtilis were well
characterized, providing assurance that no DNA is present in the
end-product. On the basis of molecular biological data and chemical
analytical research, it can be concluded that fermentation-derived
riboflavin from genetically modified B. subtilis is substantially
equivalent to synthetic riboflavin.
For 98% pure fermentation-derived riboflavin for use in food, the
NOEL in the 90-day study of toxicity in rats was 200 mg/kg bw per day,
the highest dose tested. Fermentation-derived riboflavin was evaluated
on the basis of its substantial equivalence to synthetic riboflavin.
Therefore, the Committee included riboflavin derived from a production
strain of genetically modified B. subtilis in the previously
established group ADI of 0-0.5 mg/kg bw for synthetic riboflavin and
riboflavin-5'-phosphate.
6. REFERENCES
Albertini, S. (1989) Mutagenicity evaluation of Ro 42-7491/000
(8alpha-hydroxy-riboflavin) in the Ames test (preincubation version).
Unpublished report from F. Hoffmann La Roche Ltd.dated 20 February
1989. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Albertini, S. (1995a) Mutagenicity evaluation of Ro 01-3131/055
(riboflavin 96% ex fermentation) in the Ames test (Study No. 139M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 September
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Albertini, S. (1995b) Mutagenicity evaluation of Ro 01-3131/054
(riboflavin 98% ex fermentation) in the Ames test (Study No. 138M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 Sepember
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Bächtold, H. (1980) Acute toxicity of riboflavine, some intermediates
and by-products of the synthesis, degradation products and
metabolites. Unpublished report No. 9024 from F. Hoffmann La Roche
dated 16 June 1980. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Buser, S., Hofmann, P., Lina, B., Forster, S. & Zabka, S. (1995)
Subchronic oral toxicity study with three different qualities of
riboflavin (Ro 01-3131/055 96% ex fermentation, Ro 01-3131/054 98% ex
fermentation and Ro 01-3131/000 98% ex synthesis) in rats (Project No.
920V94), parts I to III. Unpublished study from TNO Nutrition and Food
Institute, Zeist, Netherlands, dated 14 November 1995. Submitted to
WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Hermann, D. & Schurter, W. (1994) Effect of the riboflavin
purification process on DNA: Hot hydrochloric acid rapidly destroys
the biological activity of DNA. Unpublished report No. B-164'814 dated
29 December 1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Hümbelin, M. & Hermann, D. (1995) Genetic stability of the riboflavin
production strain RB50::[pRF69]n::[pRF93]m. Unpublished report No.
B-165 193 from Hoffmann La Roche Ltd. dated 30 October 1995. Submitted
to WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Kreneva, R.A. & Perumov, D.A. (1990) Genetic mapping of regulatory
mutations of Bacillus subtilis riboflavin operon. Mol. Gen.
Genet., 222, 467-469.
Ludwig, B., Bretzel, W., Manneberg, M., Bebniarz, J.C., Halter, V. &
Schöni, R. (1994) Analytical profile of riboflavin produced by
fermentation. Unpublished report No. B-163'358 from F. Hoffmann La
Roche Ltd dated 18 November 1994. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Maruo, B. & Yoshikawa, H., eds (1989) Topics in Secondary
Metabolism 1. Bacillus subtilis: Molecular Biology and Industrial
Application, Amsterdam, Elsevier, pp. 191-211.
Meinrad, J.L. & Gerber, C. (1991) Analytical profile of vitamin B2.
Part I. Unpublished report No. B-119'081 from F. Hoffmann La Roche Ltd
dated 14 August 1991. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Mironov, V.N., Kraev, A.S., Chikindas, M.L., Chernov, B.K., Stepanov,
A.I. & Skryabin, C.G. (1994) Functional organization of the riboflavin
biosynthesis operon from Bacillus subtilis SHgw. Mol. Gen. Genet.,
242, 201-208.
Pfister, M., Weber, W. & Hiraga, K. (1995) Riboflavin derived from
fermentation. Unpublished report No. B-332 023 from F. Hoffmann La
Roche Ltd dated 8 November 1995. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Schöni, R. (1989) Acute oral toxicity of Ro 42-7491/000 in male and
female rats after single administration. Unpublished report from F.
Hoffmann La Roche Ltd dated 22 March 1989. Submitted to WHO by F.
Hoffmann La Roche Ltd, Basel, Switzerland.
Schurter, W. (1994) Description of the riboflavin overproducer
B. subtilis strain RB50::[pRF69]n::[pRF93]m Ade+. Unpublished
report No. B-163'498 from F. Hoffmann La Roche Ltd dated 22 September
1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Schurter, W. & Hermann, D. (1995) PCR-analysis of riboflavin samples
from fermentation: Absence of production strain specific DNA.
Unpublished report No. B-165'177 from F. Hoffmann La Roche Ltd dated 9
May 1995. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Simon, W. & Vogt, R. (1989) [Riboflavin, Steps 6 and 7: Riboflavin
crude and pure. Isolation, identification, and synthesis of side
product.] Unpublished report No. BS/GCR 62'589 from F. Hoffmann La
Roche Ltd dated 5 September 1989. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland (in German).
Spackman, D.H., Stein, W.H. & Moore, S. (1958) Automatic recording
apparatus for use in the chromatography of amino acids. Anal. Chem.,
30, 1190-1206.
The presence of amino acids originating from fermentation was
analysed by the method of Spackman et al. (1958), and DNA was analysed
by polymerase chain reaction (PCR) techniques (Schurter & Hermann,
1995). The amounts of impurities in the different products are given
in Table 1. The concentration of 8alpha-hydroxymethylriboflavin, which
is a known degradation product after acid treatment of riboflavin, was
slightly increased in the fermentation-derived product, but only in
that of 98% purity. Since this product is derived from the 96% pure
material by crystallization from acidic media, this is to be expected;
however lumiflavin, which is a known degradation product after basic
treatment, was present in the synthesized riboflavin but not in the
fermentation-derived material.
Table 1. Composition of riboflavin products derived by fermentation and by synthesis
(average % weighed samples)
Component 96% ex 98% ex 96% ex synthesis
fermentation fermentation
HPLC method HPLC method HPLC method
I II I II I II
Riboflavin 98.4 98.3 100.1 99.7 98.8 98.6
Ribityl-oxo-chinoxalic acid Trace ND ND ND Trace Trace
8alpha-Hydroxy riboflavin Trace Trace 0.45 0.2 Trace Trace
Formylmethyl-flavin acetal Trace ND 0.11 Trace 0.52 ND
Lumichrome 0.14 0.11 ND Trace 0.18 Trace
Lumiflavin ND ND ND ND 0.56 0.5
Amino acids 0.9 0.9 0.06 0.06 - -
DNAa ND ND ND ND - -
Total 99.44 99.31 100.72 99.96 100.1 99.3
Trace, below limit of detection; ND, below limit for quantitative determination, 0.1%
a See also section 3.3, 'Foreign DNA present in the end product'
2. BIOLOGICAL DATA
2.1 Toxicological studies
2.1.1 Acute toxicity
No studies of the acute toxicity of riboflavin derived by
fermentation with genetically modified B. subtilis are available.
Table 2 gives the LD50 values for riboflavin (not derived from
fermentation), riboflavin phosphate, and some of the impurities
present in riboflavin. Except for the study with
8alpha-hydroxy-riboflavin, no full reports were available. The data in
Table 2 give an indication of the acute toxicity of synthetic
riboflavin (phosphate) and some of its impurities but are not relevant
for evaluating riboflavin derived by fermentation.
2.1.2 Short-term studies of toxicity
Rats
Groups of 10 male and 10 female six-week-old Wistar rats (the
males weighing 160-170 g and the females, 130-140 g) received diets
providing 20, 50, or 200 mg/kg bw of riboflavin -- either 99.4% pure
derived by fermentation from 96% pure riboflavin, 100.2% pure derived
by fermentation from 98% pure riboflavin, or 99.8% pure derived by
sunthesis from 98% pure riboflavin -- daily, on seven days per week
for 13 weeks. The study was performed according to good laboratory
practice (GLP) and OECD Guideline 408; a quality assurance (QA)
statement was included. A control group of 30 male and 30 female rats
was used. A satellite group of six male and six female animals was
attached to each group for determination of riboflavin concentrations
in blood and urine and for investigation of the reversibility of any
toxicological parameter. Feed and water were provided ad libitum.
The different assays were performed at one-week intervals. Samples of
the diet were taken at the start of treatment and at weeks 6 and 13 to
determine the content, homogeneity, and stability of the test compound
in the diet.
All animals were checked twice daily (once daily at weekends) for
behaviour and general condition. Ophthalmoscopy was performed on all
animals in the main control group and those at the three highest doses
before the start and at the end of the study. Body weight and food
intake were determined weekly. Water consumption was determined over
five consecutive days in weeks 1, 5, and 12. Haematological parameters
(haemoglobin concentration; haematocrit; erythrocyte, leukocyte, and
differential cell counts; mean corpuscular volume, haemoglobin, and
haemoglobin concentration; reticulocyte and thrombocyte counts; and
prothrombin time) were measured at week 6 and at autopsy in 10 rats of
each sex in the main control and treated groups. Haemoglobin
concentration; haematocrit; erythrocyte and leukocyte counts; mean
corpuscular volume, haemoglobin, and haemoglobin concentration; and
thrombocyte count were measured in all rats in the satellite groups at
the end of the recovery period. Clinical chemical parameters (alanine
and aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl
transferase, and lactic dehydrogenase activities; bilirubin, total
protein, albumin, albumin:globulin ratio, urea, creatinine, Na, K, Ca,
Cl, inorganic phosphate, cholesterol, triglycerides, and
phospholipids) were determined in all rats in the main treated groups
and in 15 rats of each sex in the main control group in weeks 7 and 13
after one night of fasting; the same parameters were measured in all
rats in the satellite groups at the end of the recovery period. In
weeks 7 and 13, all rats in the main treated groups and 15 rats of
each sex in the main control group underwent a renal concentration
test and urinalysis (appearance, pH, glucose, occult blood, ketones,
proteins, bilirubin, urobilinogen, sediment), except that sediment was
measured only in rats at the three highest doses. The sediment of
urine from all rats in the satellite groups was examined
microscopically for the presence of sperm at the end of the recovery
period. The weights (absolute and relative to body weight) of the
adrenals, kidneys, liver, spleen, brain, thymus, heart, and testes of
all rats were determined at the end of the treatment period in all of
the main groups and at the end of the recovery period in all satellite
groups. All rats in the main groups were examined macroscopically at
the end of treatment, and about 30 tissues from all animals in the
main control group and in the main three highest-dose groups were
examined microscopically The testes, epididymides, kidneys, liver,
lungs, and all gross lesions from all rats in the main groups were
examined microscopically. The riboflavin concentration in blood was
determined in all rats in the satellite groups before the start of the
study, at weeks 2, 6, and 13 of treatment, and at the end of the
recovery period. The riboflavin content of the urine was determined in
all rats in the satellite control group and in all rats in the
satellite groups treated with 96 and 98% pure riboflavin derived by
fermentation, before the study and at week 2 and 6. At the end of the
treatment period and at the end of the recovery period, the riboflavin
content of the urine was determined in all rats in all satellite
groups.
The test substances were homogeneously distributed in the diets
and showed storage stability under simulated experimental conditions.
The actual measured concentrations in the diet were within the
tolerable range of ± 10% of the nominal concentration. Alopecic areas
were seen frequently in various groups, but these findings were
transient and were not related to dose. Ophthalmoscopy showed no
abnormalities. Excrement collected from rats at the highest doses of
the three test substances was yellowish throughout the study. No
dose-related difference in food and water intake was seen.
During treatment, females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation showed a slightly but usually statistically
significant decrease in growth of about 6%. Males and females at
50 mg/kg bw per day 98% pure riboflavin ex synthesis also had a slight
but statistically significant decrease in growth. These effects were
not considered to be toxicologically relevant because they were small
(< 10%) and food conversion was not affected. During the recovery
period, body weights were similar in all groups. No changes in
haematological parameters were seen after six weeks of treatment, but
at the end of treatment (week 13) females at 200 mg/kg bw per day 98%
pure riboflavin ex fermentation had a slightly but statistically
significantly lower mean haemoglobin value and erythrocyte count,
whereas the mean reticulocyte count was significantly increased. The
individual reticulocyte counts in these animals were markedly
increased in two of 10 females and moderately increased in another
two. Slight but statistically significant increases in mean
thrombocyte counts in females at the highest dose of 98% pure
riboflavin ex fermentation and in males at the highest dose of
riboflavin 98% ex synthesis were within the range of normal variation
and therefore considered not to be toxicologically relevant. At six
and 13 weeks, the total leukocyte counts were statistically
significantly decreased in several treated groups but with no
dose-response relationship and inconsistently within groups; these
changes were also not considered to be related to treatment.
Clinical chemical and urinary analyses showed no
treatment-related changes. At the end of treatment, a slight but
statistically significant increase in relative liver weight was seen
in females at 200 mg/kg bw per day 98% pure riboflavin ex
fermentation, and a slight but statistically significant increase in
relative spleen weight in males at 50 and 200 mg/kg bw per day 96%
pure riboflavin (ex fermentation), but with no dose-response
relationship. Macroscopic and microscopic examination did not reveal
any treatment-related abnormality. The changes in organ weights were
considered to be of questionable biological relevance because there
was no dose-response relationship and they were not accompanied by
histopathological changes or changes in clinical chemistry. At the end
of the recovery period, all of the haematological parameters were
normal, except for a statistically significant decrease in mean
thrombocyte counts in females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation, and there were no notable changes in organ
weights.
The riboflavin concentrations in blood and urine were not
reported.
The authors concluded that the NOEL was 200 mg/kg bw per day for
all three test substances. The decreases in mean haemoglobin
concentration and erythrocyte count in females at 200 mg/kg bw per day
98% pure riboflavin ex fermentation were considered to be fortuitous
findings because, except in two females, there was no overt
association between the low erythrocyte counts and the high
reticulocyte counts. Moreover, according to the authors, the measured
values did not exceed the expected range, and there was also no clear
dose-response relationship in any group. In addition, there were no
related changes (in e.g. haemolysis or weight and histopathology of
haematopoietic organs) that could be associated with variations in the
red blood cell profile (Buser et al., 1995).
The Committee concluded that the NOEL for 98% pure riboflavin ex
fermentation was 50 mg/kg bw per day, on the basis of the significant
decreases in mean haemoglobin concentration and erythrocyte count and
the significant increase in mean reticulocyte count in females at
200 mg/kg bw. For 96% pure riboflavin ex fermentation and 98% pure
riboflavin ex synthesis, the NOEL was 200 mg/kg bw per day, the
highest dose tested.
Table 2. Acute toxicity of synthetic riboflavin phosphate and some of its impurities
Compound Species Sex Route LD50 Remarks Reference
(mg/kg bw)
Riboflavin Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin Rat NR Intravenous 560 Bächtold (1980)
Riboflavin Rat NR Subcutaneous 5 000 Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intraperitoneal 890 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intravenous 780 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Oral > 20 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 1 030 Observation period, 24 h Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 560 Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intravenous 710 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Subcutaneous 790 Bächtold (1980)
8alpha-Hydroxyriboflavin Rat M&F Oral > 2 000 Limit test, by gavagea Schöni (1989)
(purity > 95%)
Lumichrome Mouse NR Oral > 9 000 Observation period, 10 days Bächtold (1980)
Lumichrome Mouse NR Intraperitoneal 11 000 Observation period, 10 days Bächtold (1980)
Lumiflavin Mouse NR Oral > 6 000 Observation period, 10 days Bächtold (1980)
NR, not reported; M&F, male and female
a Study performed according to OECD Guideline 401; no GLP statement or QA declaration was included.
Table 3. Results of assays for the genotoxicity of riboflavin derived by fermentation from B. subtilis and of 8alpha-hydroxyriboflavin
End-point Test object Concentration Results Reference
Riboflavin 96% Reverse mutationa S. typhimurium TA97, 50-5000 µg/plate Negativeb Albertini (1995a)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 99.4%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
Riboflavin 98% Reverse mutationa S. typhimurium TA 97, 50-5000 µg/plate Negativeb Albertini (1995b)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 100.2%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
8alpha-Hydroxy- Reverse mutationc S. typhimurium TA97, 100-5000 µg/plate Negativeb Albertini (1989)
riboflavin (purity TA98, TA100, TA102, in DMSO No toxicity; yellow-to-orange
not given) TA1535, TA1537, TA1538 precipitate at 2500 and 5000
µg/plate
DMSO, dimethyl sulfoxide
a Ames test; standard plate incorporation, preincubation modification assay. Study performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat experiment; a QA statement was included.
b With and without exogenous metabolic activation
c Ames test; standard plate incorporation, preincubation modification assay. Study not performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat assay; no QA statement was included.
2.1.3 Genotoxicity
Studies on the genotoxic potential of riboflavin derived by
fermentation from B. subtilis and of 8alpha-hydroxyriboflavin, an
impurity of the fermentation-derived material, are summarized in Table
3.
3. METHODS OF GENETIC MODIFICATION
3.1 Molecular genetic methods used to clone and express the riboflavin
(rib) operon from Bacillus subtilis
The riboflavin operon of B. subtilis containing the genes
ribT, ribH, ribA, and ribG was cloned into pUC19 as a 6.4-kb
NcoI/XbaI DNA fragment. In order to enhance expression of the rib
genes, a strong constitutive B. subtilis bacteriophage SPO1 promoter
was included. Two versions were made: (i) the SPO1-15 promoter was
inserted in front of the endogenous ribP2 promoter in a construct
later designated as pRF93 and (ii) the endogenous ribP1 promoter was
replaced by SPO1-15 in a construct later designated as pRF69. In order
to make the constructs selectable after integration into the B.
subtilis genome (the ß-lactamase gene of pUC19 is not expressed in
B. subtilis), the two constructs were provided with different
selectable genes, i.e. the tetracycline-resistant (tet) gene derived
from B. cereus was inserted into pRF93, and the chloramphenicol-
resistant (cat) gene derived from pUC194, origin S. aureus/
B. subtilis, into pRF69. Both constructs were integrated into the
genome of recipient B. subtilis strain RB50. The integration sites
were mapped for pRF69 at 209° and for pRF93 at 139° in the B.
subtilis genome.
RB50, a strain of B. subtilis that overproduces riboflavin, was
obtained by classical mutagenesis and selection procedures. With
various unknown mutations, RB50 contains a mutation that maps at the
ribC locus, which is present at 147° of the B. subtilis genome.
In order to further increase riboflavin production, the
RB50::[pRF69]: :[pRF93] was subjected to increasing concentrations of
the antibiotics tetracycline and chloramphenicol, resulting in
amplification of pRF69 and pRF93. In general, pRF69 was multiplied
20-30 times whereas pRF93 was multiplied 8-10 times, resulting in the
production strain RB50::[pRF69]n: :[pRF93]m. Although neither
antibiotic was present during the production stage, there was no
detectable loss of either pRF (Maruo & Yoshikawa, 1989; Kreneva &
Perumov, 1990; Mironov et al., 1994; Schurter, 1994). All of the
procedures were extensively and well described.
3.2 Genetic stability of the production strain
The production strain obtained after amplification was
RB50::[pRF69]n: :[pRF93]m. Samples were taken from the fermentor
during the process, and the numbers of copies of pRF69 and pRF93 were
determined by Southern blotting. There was no apparent loss of the
foreign DNA present in RB50: :[pRF69]n::[pRF93]m during the
process, which took 48 h, with samples taken at 6-h intervals
(Hümbelin & Hermann, 1995). As all of the experiments were well
described and appeared to be sound and convincing, it can be concluded
that the production strain is genetically stable during the
fermentation process.
3.3 Foreign DNA present in the end-product
One of the purification steps involved treatment of the product
with diluted hydrochloric acid for 60 min at 90°C. Under these
conditions, DNA is heavily depurinated and degraded (Hermann &
Schurter, 1994). A sensitive PCR method was used to detect any
residual transforming DNA (monitored as a 557-bp pRF69 PCR cat
fragment), with a detection limit of 0.5 ppb DNA per mg riboflavin. No
foreign DNA was detected. Furthermore, no transforming activity was
detected after only 5 min of hot acid treatment of crude riboflavin
(Schurter & Hermann, 1995). As all of the experiments were described
in detail, and the results are convincing, it can be concluded that
the riboflavin samples were free of any detectable transforming DNA.
4. COMMENTS
The strain used in riboflavin production has been genetically
modified to overproduce riboflavin by amplification of the chromosomal
region of the riboflavin operon containing suitable promoters and
flanked by pUC19 and antibiotic-resistance marker genes. The lack of
pathogenicity and toxicity of the strain of microorganism
(B. subtilis) from which the genetic information encoding riboflavin
was cloned is well documented, and the methods for genetic
modification have been well described. The production strain of
B. subtilis was shown to be genetically stable during fermentation.
It was convincingly established that the final product is riboflavin
of a purity comparable to, or greater than, that produced by
conventional methods and is free of DNA from the production organism.
Thus, antibiotic resistance marker genes are not present.
In a 90-day study of toxicity, rats were fed diets containing
food-grade riboflavin (98% pure) produced by fermentation from
genetically modified B. subtilis or riboflavin derived by chemical
synthesis, also of 98% purity. The doses tested were 0, 20, 50, or 200
mg/kg bw per day. Some animals at the high dose had reduced weight
gain, but generally by less than 10%, and food conversion efficiency
was not affected. In the group fed the high dose of
fermentation-derived riboflavin of 98% purity, some minor changes in
red blood cell parameters were observed, but they were not considered
relevant. The NOEL for 98% pure riboflavin produced by fermentation
was 200 mg/kg bw per day, which was the same as that for chemically
synthesized riboflavin.
Fermentation-derived riboflavin and the degradation product
8alpha-hydroxyriboflavin did not induce gene mutations in bacteria
in vitro in either the absence or the presence of metabolic
activation.
5. EVALUATION
The Committee concluded that the recombinant DNA techniques used
to derive the production strain of B. subtilis were well
characterized, providing assurance that no DNA is present in the
end-product. On the basis of molecular biological data and chemical
analytical research, it can be concluded that fermentation-derived
riboflavin from genetically modified B. subtilis is substantially
equivalent to synthetic riboflavin.
For 98% pure fermentation-derived riboflavin for use in food, the
NOEL in the 90-day study of toxicity in rats was 200 mg/kg bw per day,
the highest dose tested. Fermentation-derived riboflavin was evaluated
on the basis of its substantial equivalence to synthetic riboflavin.
Therefore, the Committee included riboflavin derived from a production
strain of genetically modified B. subtilis in the previously
established group ADI of 0-0.5 mg/kg bw for synthetic riboflavin and
riboflavin-5'-phosphate.
6. REFERENCES
Albertini, S. (1989) Mutagenicity evaluation of Ro 42-7491/000
(8alpha-hydroxy-riboflavin) in the Ames test (preincubation version).
Unpublished report from F. Hoffmann La Roche Ltd.dated 20 February
1989. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Albertini, S. (1995a) Mutagenicity evaluation of Ro 01-3131/055
(riboflavin 96% ex fermentation) in the Ames test (Study No. 139M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 September
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Albertini, S. (1995b) Mutagenicity evaluation of Ro 01-3131/054
(riboflavin 98% ex fermentation) in the Ames test (Study No. 138M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 Sepember
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Bächtold, H. (1980) Acute toxicity of riboflavine, some intermediates
and by-products of the synthesis, degradation products and
metabolites. Unpublished report No. 9024 from F. Hoffmann La Roche
dated 16 June 1980. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Buser, S., Hofmann, P., Lina, B., Forster, S. & Zabka, S. (1995)
Subchronic oral toxicity study with three different qualities of
riboflavin (Ro 01-3131/055 96% ex fermentation, Ro 01-3131/054 98% ex
fermentation and Ro 01-3131/000 98% ex synthesis) in rats (Project No.
920V94), parts I to III. Unpublished study from TNO Nutrition and Food
Institute, Zeist, Netherlands, dated 14 November 1995. Submitted to
WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Hermann, D. & Schurter, W. (1994) Effect of the riboflavin
purification process on DNA: Hot hydrochloric acid rapidly destroys
the biological activity of DNA. Unpublished report No. B-164'814 dated
29 December 1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Hümbelin, M. & Hermann, D. (1995) Genetic stability of the riboflavin
production strain RB50::[pRF69]n::[pRF93]m. Unpublished report No.
B-165 193 from Hoffmann La Roche Ltd. dated 30 October 1995. Submitted
to WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Kreneva, R.A. & Perumov, D.A. (1990) Genetic mapping of regulatory
mutations of Bacillus subtilis riboflavin operon. Mol. Gen.
Genet., 222, 467-469.
Ludwig, B., Bretzel, W., Manneberg, M., Bebniarz, J.C., Halter, V. &
Schöni, R. (1994) Analytical profile of riboflavin produced by
fermentation. Unpublished report No. B-163'358 from F. Hoffmann La
Roche Ltd dated 18 November 1994. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Maruo, B. & Yoshikawa, H., eds (1989) Topics in Secondary
Metabolism 1. Bacillus subtilis: Molecular Biology and Industrial
Application, Amsterdam, Elsevier, pp. 191-211.
Meinrad, J.L. & Gerber, C. (1991) Analytical profile of vitamin B2.
Part I. Unpublished report No. B-119'081 from F. Hoffmann La Roche Ltd
dated 14 August 1991. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Mironov, V.N., Kraev, A.S., Chikindas, M.L., Chernov, B.K., Stepanov,
A.I. & Skryabin, C.G. (1994) Functional organization of the riboflavin
biosynthesis operon from Bacillus subtilis SHgw. Mol. Gen. Genet.,
242, 201-208.
Pfister, M., Weber, W. & Hiraga, K. (1995) Riboflavin derived from
fermentation. Unpublished report No. B-332 023 from F. Hoffmann La
Roche Ltd dated 8 November 1995. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Schöni, R. (1989) Acute oral toxicity of Ro 42-7491/000 in male and
female rats after single administration. Unpublished report from F.
Hoffmann La Roche Ltd dated 22 March 1989. Submitted to WHO by F.
Hoffmann La Roche Ltd, Basel, Switzerland.
Schurter, W. (1994) Description of the riboflavin overproducer
B. subtilis strain RB50::[pRF69]n::[pRF93]m Ade+. Unpublished
report No. B-163'498 from F. Hoffmann La Roche Ltd dated 22 September
1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Schurter, W. & Hermann, D. (1995) PCR-analysis of riboflavin samples
from fermentation: Absence of production strain specific DNA.
Unpublished report No. B-165'177 from F. Hoffmann La Roche Ltd dated 9
May 1995. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Simon, W. & Vogt, R. (1989) [Riboflavin, Steps 6 and 7: Riboflavin
crude and pure. Isolation, identification, and synthesis of side
product.] Unpublished report No. BS/GCR 62'589 from F. Hoffmann La
Roche Ltd dated 5 September 1989. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland (in German).
Spackman, D.H., Stein, W.H. & Moore, S. (1958) Automatic recording
apparatus for use in the chromatography of amino acids. Anal. Chem.,
30, 1190-1206.
See Also:
Toxicological Abbreviations
 Although riboflavin can be produced by both synthetic and
fermentation processes, the latter was not found to be competitive for
production of very pure material. Recent developments in biotechnology
have resulted in microbiological processes that compete successfully
with the synthetic chemical process. Fermentation-produced riboflavin
is prepared by the controlled, submerged growth of a selected strain
of B. subtilis that has been genetically modified to produce
riboflavin. Manufacture involves fermentation resulting in a broth
containg riboflavin, recovery of riboflavin from the broth, and
further purification to a final product with a purity of 96 or 98%,
which is used in animal feed and human food, respectively (Pfister et
al., 1995).
* Fermentation is performed in vessels by aseptic, large-scale
microbiological techniques under controlled conditions of
temperature, aeration, agitation, and pH. The cultural purity of
the fermentation is routinely tested by checking growth behaviour
and colony identity. When the fermentation has proceeded to the
desired point, the broth is delivered for recovery.
* Riboflavin is recovered from the broth by centrifugation after
inactivation of the microorganisms by heat. Pasteurization of the
broth ensures that no viable cells of the production organism are
present in the final product. Differential centrifugation leads
to separation of cells and riboflavin crystals because of
differences in size and sedimentation behaviour.
* Mineral acid treatment (about 2% hydrochloric or sulfuric acid)
yields 96% riboflavin, which, after drying and packaging, is
either directly sold for application in feed or used for further
processing into a formulation. The acid treatment also ensures
destruction (via depurination) of DNA from the processing
organism. Recrystallization of the 96% pure material from
concentrated hydrochloric acid (> 25%) results in a food-quality
material containing 98% riboflavin. This last purification step
is identical to that currently used for purification of
riboflavin derived from chemical synthesis.
Three lots of 98% pure riboflavin derived by fermentation, four
lots of 96% pure material derived by fermentation, and one lot of 98%
pure material derived by synthesis were analysed by two separate
high-performance liquid chromatography (HPLC) methods in order to
identify and quantify any impurities. The riboflavin content of the
98% pure products derived by fermentation and synthesis ranged from 99
to 101%, and that of the 96% pure products derived by fermentation
from 97 to 99%. All of the peaks detected in riboflavin from
fermentation could be identified and appeared also to be present in
synthesized riboflavin, indicating that the impurities probably occur
during the purification steps (acidic treatment and crystallization)
(Simon & Vogt, 1989; Ludwig et al., 1994). Four impurities were
identified in the fermentation-derived material (for chemical
structures, see Figure 2). Lumiflavin, a known degradation product of
riboflavin after basic treatment (Meinrad & Gerber, 1991), was present
in the synthesized product but not in the fermentation-derived
material.
Although riboflavin can be produced by both synthetic and
fermentation processes, the latter was not found to be competitive for
production of very pure material. Recent developments in biotechnology
have resulted in microbiological processes that compete successfully
with the synthetic chemical process. Fermentation-produced riboflavin
is prepared by the controlled, submerged growth of a selected strain
of B. subtilis that has been genetically modified to produce
riboflavin. Manufacture involves fermentation resulting in a broth
containg riboflavin, recovery of riboflavin from the broth, and
further purification to a final product with a purity of 96 or 98%,
which is used in animal feed and human food, respectively (Pfister et
al., 1995).
* Fermentation is performed in vessels by aseptic, large-scale
microbiological techniques under controlled conditions of
temperature, aeration, agitation, and pH. The cultural purity of
the fermentation is routinely tested by checking growth behaviour
and colony identity. When the fermentation has proceeded to the
desired point, the broth is delivered for recovery.
* Riboflavin is recovered from the broth by centrifugation after
inactivation of the microorganisms by heat. Pasteurization of the
broth ensures that no viable cells of the production organism are
present in the final product. Differential centrifugation leads
to separation of cells and riboflavin crystals because of
differences in size and sedimentation behaviour.
* Mineral acid treatment (about 2% hydrochloric or sulfuric acid)
yields 96% riboflavin, which, after drying and packaging, is
either directly sold for application in feed or used for further
processing into a formulation. The acid treatment also ensures
destruction (via depurination) of DNA from the processing
organism. Recrystallization of the 96% pure material from
concentrated hydrochloric acid (> 25%) results in a food-quality
material containing 98% riboflavin. This last purification step
is identical to that currently used for purification of
riboflavin derived from chemical synthesis.
Three lots of 98% pure riboflavin derived by fermentation, four
lots of 96% pure material derived by fermentation, and one lot of 98%
pure material derived by synthesis were analysed by two separate
high-performance liquid chromatography (HPLC) methods in order to
identify and quantify any impurities. The riboflavin content of the
98% pure products derived by fermentation and synthesis ranged from 99
to 101%, and that of the 96% pure products derived by fermentation
from 97 to 99%. All of the peaks detected in riboflavin from
fermentation could be identified and appeared also to be present in
synthesized riboflavin, indicating that the impurities probably occur
during the purification steps (acidic treatment and crystallization)
(Simon & Vogt, 1989; Ludwig et al., 1994). Four impurities were
identified in the fermentation-derived material (for chemical
structures, see Figure 2). Lumiflavin, a known degradation product of
riboflavin after basic treatment (Meinrad & Gerber, 1991), was present
in the synthesized product but not in the fermentation-derived
material.
 The presence of amino acids originating from fermentation was
analysed by the method of Spackman et al. (1958), and DNA was analysed
by polymerase chain reaction (PCR) techniques (Schurter & Hermann,
1995). The amounts of impurities in the different products are given
in Table 1. The concentration of 8alpha-hydroxymethylriboflavin, which
is a known degradation product after acid treatment of riboflavin, was
slightly increased in the fermentation-derived product, but only in
that of 98% purity. Since this product is derived from the 96% pure
material by crystallization from acidic media, this is to be expected;
however lumiflavin, which is a known degradation product after basic
treatment, was present in the synthesized riboflavin but not in the
fermentation-derived material.
Table 1. Composition of riboflavin products derived by fermentation and by synthesis
(average % weighed samples)
Component 96% ex 98% ex 96% ex synthesis
fermentation fermentation
HPLC method HPLC method HPLC method
I II I II I II
Riboflavin 98.4 98.3 100.1 99.7 98.8 98.6
Ribityl-oxo-chinoxalic acid Trace ND ND ND Trace Trace
8alpha-Hydroxy riboflavin Trace Trace 0.45 0.2 Trace Trace
Formylmethyl-flavin acetal Trace ND 0.11 Trace 0.52 ND
Lumichrome 0.14 0.11 ND Trace 0.18 Trace
Lumiflavin ND ND ND ND 0.56 0.5
Amino acids 0.9 0.9 0.06 0.06 - -
DNAa ND ND ND ND - -
Total 99.44 99.31 100.72 99.96 100.1 99.3
Trace, below limit of detection; ND, below limit for quantitative determination, 0.1%
a See also section 3.3, 'Foreign DNA present in the end product'
2. BIOLOGICAL DATA
2.1 Toxicological studies
2.1.1 Acute toxicity
No studies of the acute toxicity of riboflavin derived by
fermentation with genetically modified B. subtilis are available.
Table 2 gives the LD50 values for riboflavin (not derived from
fermentation), riboflavin phosphate, and some of the impurities
present in riboflavin. Except for the study with
8alpha-hydroxy-riboflavin, no full reports were available. The data in
Table 2 give an indication of the acute toxicity of synthetic
riboflavin (phosphate) and some of its impurities but are not relevant
for evaluating riboflavin derived by fermentation.
2.1.2 Short-term studies of toxicity
Rats
Groups of 10 male and 10 female six-week-old Wistar rats (the
males weighing 160-170 g and the females, 130-140 g) received diets
providing 20, 50, or 200 mg/kg bw of riboflavin -- either 99.4% pure
derived by fermentation from 96% pure riboflavin, 100.2% pure derived
by fermentation from 98% pure riboflavin, or 99.8% pure derived by
sunthesis from 98% pure riboflavin -- daily, on seven days per week
for 13 weeks. The study was performed according to good laboratory
practice (GLP) and OECD Guideline 408; a quality assurance (QA)
statement was included. A control group of 30 male and 30 female rats
was used. A satellite group of six male and six female animals was
attached to each group for determination of riboflavin concentrations
in blood and urine and for investigation of the reversibility of any
toxicological parameter. Feed and water were provided ad libitum.
The different assays were performed at one-week intervals. Samples of
the diet were taken at the start of treatment and at weeks 6 and 13 to
determine the content, homogeneity, and stability of the test compound
in the diet.
All animals were checked twice daily (once daily at weekends) for
behaviour and general condition. Ophthalmoscopy was performed on all
animals in the main control group and those at the three highest doses
before the start and at the end of the study. Body weight and food
intake were determined weekly. Water consumption was determined over
five consecutive days in weeks 1, 5, and 12. Haematological parameters
(haemoglobin concentration; haematocrit; erythrocyte, leukocyte, and
differential cell counts; mean corpuscular volume, haemoglobin, and
haemoglobin concentration; reticulocyte and thrombocyte counts; and
prothrombin time) were measured at week 6 and at autopsy in 10 rats of
each sex in the main control and treated groups. Haemoglobin
concentration; haematocrit; erythrocyte and leukocyte counts; mean
corpuscular volume, haemoglobin, and haemoglobin concentration; and
thrombocyte count were measured in all rats in the satellite groups at
the end of the recovery period. Clinical chemical parameters (alanine
and aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl
transferase, and lactic dehydrogenase activities; bilirubin, total
protein, albumin, albumin:globulin ratio, urea, creatinine, Na, K, Ca,
Cl, inorganic phosphate, cholesterol, triglycerides, and
phospholipids) were determined in all rats in the main treated groups
and in 15 rats of each sex in the main control group in weeks 7 and 13
after one night of fasting; the same parameters were measured in all
rats in the satellite groups at the end of the recovery period. In
weeks 7 and 13, all rats in the main treated groups and 15 rats of
each sex in the main control group underwent a renal concentration
test and urinalysis (appearance, pH, glucose, occult blood, ketones,
proteins, bilirubin, urobilinogen, sediment), except that sediment was
measured only in rats at the three highest doses. The sediment of
urine from all rats in the satellite groups was examined
microscopically for the presence of sperm at the end of the recovery
period. The weights (absolute and relative to body weight) of the
adrenals, kidneys, liver, spleen, brain, thymus, heart, and testes of
all rats were determined at the end of the treatment period in all of
the main groups and at the end of the recovery period in all satellite
groups. All rats in the main groups were examined macroscopically at
the end of treatment, and about 30 tissues from all animals in the
main control group and in the main three highest-dose groups were
examined microscopically The testes, epididymides, kidneys, liver,
lungs, and all gross lesions from all rats in the main groups were
examined microscopically. The riboflavin concentration in blood was
determined in all rats in the satellite groups before the start of the
study, at weeks 2, 6, and 13 of treatment, and at the end of the
recovery period. The riboflavin content of the urine was determined in
all rats in the satellite control group and in all rats in the
satellite groups treated with 96 and 98% pure riboflavin derived by
fermentation, before the study and at week 2 and 6. At the end of the
treatment period and at the end of the recovery period, the riboflavin
content of the urine was determined in all rats in all satellite
groups.
The test substances were homogeneously distributed in the diets
and showed storage stability under simulated experimental conditions.
The actual measured concentrations in the diet were within the
tolerable range of ± 10% of the nominal concentration. Alopecic areas
were seen frequently in various groups, but these findings were
transient and were not related to dose. Ophthalmoscopy showed no
abnormalities. Excrement collected from rats at the highest doses of
the three test substances was yellowish throughout the study. No
dose-related difference in food and water intake was seen.
During treatment, females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation showed a slightly but usually statistically
significant decrease in growth of about 6%. Males and females at
50 mg/kg bw per day 98% pure riboflavin ex synthesis also had a slight
but statistically significant decrease in growth. These effects were
not considered to be toxicologically relevant because they were small
(< 10%) and food conversion was not affected. During the recovery
period, body weights were similar in all groups. No changes in
haematological parameters were seen after six weeks of treatment, but
at the end of treatment (week 13) females at 200 mg/kg bw per day 98%
pure riboflavin ex fermentation had a slightly but statistically
significantly lower mean haemoglobin value and erythrocyte count,
whereas the mean reticulocyte count was significantly increased. The
individual reticulocyte counts in these animals were markedly
increased in two of 10 females and moderately increased in another
two. Slight but statistically significant increases in mean
thrombocyte counts in females at the highest dose of 98% pure
riboflavin ex fermentation and in males at the highest dose of
riboflavin 98% ex synthesis were within the range of normal variation
and therefore considered not to be toxicologically relevant. At six
and 13 weeks, the total leukocyte counts were statistically
significantly decreased in several treated groups but with no
dose-response relationship and inconsistently within groups; these
changes were also not considered to be related to treatment.
Clinical chemical and urinary analyses showed no
treatment-related changes. At the end of treatment, a slight but
statistically significant increase in relative liver weight was seen
in females at 200 mg/kg bw per day 98% pure riboflavin ex
fermentation, and a slight but statistically significant increase in
relative spleen weight in males at 50 and 200 mg/kg bw per day 96%
pure riboflavin (ex fermentation), but with no dose-response
relationship. Macroscopic and microscopic examination did not reveal
any treatment-related abnormality. The changes in organ weights were
considered to be of questionable biological relevance because there
was no dose-response relationship and they were not accompanied by
histopathological changes or changes in clinical chemistry. At the end
of the recovery period, all of the haematological parameters were
normal, except for a statistically significant decrease in mean
thrombocyte counts in females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation, and there were no notable changes in organ
weights.
The riboflavin concentrations in blood and urine were not
reported.
The authors concluded that the NOEL was 200 mg/kg bw per day for
all three test substances. The decreases in mean haemoglobin
concentration and erythrocyte count in females at 200 mg/kg bw per day
98% pure riboflavin ex fermentation were considered to be fortuitous
findings because, except in two females, there was no overt
association between the low erythrocyte counts and the high
reticulocyte counts. Moreover, according to the authors, the measured
values did not exceed the expected range, and there was also no clear
dose-response relationship in any group. In addition, there were no
related changes (in e.g. haemolysis or weight and histopathology of
haematopoietic organs) that could be associated with variations in the
red blood cell profile (Buser et al., 1995).
The Committee concluded that the NOEL for 98% pure riboflavin ex
fermentation was 50 mg/kg bw per day, on the basis of the significant
decreases in mean haemoglobin concentration and erythrocyte count and
the significant increase in mean reticulocyte count in females at
200 mg/kg bw. For 96% pure riboflavin ex fermentation and 98% pure
riboflavin ex synthesis, the NOEL was 200 mg/kg bw per day, the
highest dose tested.
Table 2. Acute toxicity of synthetic riboflavin phosphate and some of its impurities
Compound Species Sex Route LD50 Remarks Reference
(mg/kg bw)
Riboflavin Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin Rat NR Intravenous 560 Bächtold (1980)
Riboflavin Rat NR Subcutaneous 5 000 Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intraperitoneal 890 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intravenous 780 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Oral > 20 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 1 030 Observation period, 24 h Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 560 Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intravenous 710 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Subcutaneous 790 Bächtold (1980)
8alpha-Hydroxyriboflavin Rat M&F Oral > 2 000 Limit test, by gavagea Schöni (1989)
(purity > 95%)
Lumichrome Mouse NR Oral > 9 000 Observation period, 10 days Bächtold (1980)
Lumichrome Mouse NR Intraperitoneal 11 000 Observation period, 10 days Bächtold (1980)
Lumiflavin Mouse NR Oral > 6 000 Observation period, 10 days Bächtold (1980)
NR, not reported; M&F, male and female
a Study performed according to OECD Guideline 401; no GLP statement or QA declaration was included.
Table 3. Results of assays for the genotoxicity of riboflavin derived by fermentation from B. subtilis and of 8alpha-hydroxyriboflavin
End-point Test object Concentration Results Reference
Riboflavin 96% Reverse mutationa S. typhimurium TA97, 50-5000 µg/plate Negativeb Albertini (1995a)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 99.4%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
Riboflavin 98% Reverse mutationa S. typhimurium TA 97, 50-5000 µg/plate Negativeb Albertini (1995b)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 100.2%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
8alpha-Hydroxy- Reverse mutationc S. typhimurium TA97, 100-5000 µg/plate Negativeb Albertini (1989)
riboflavin (purity TA98, TA100, TA102, in DMSO No toxicity; yellow-to-orange
not given) TA1535, TA1537, TA1538 precipitate at 2500 and 5000
µg/plate
DMSO, dimethyl sulfoxide
a Ames test; standard plate incorporation, preincubation modification assay. Study performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat experiment; a QA statement was included.
b With and without exogenous metabolic activation
c Ames test; standard plate incorporation, preincubation modification assay. Study not performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat assay; no QA statement was included.
2.1.3 Genotoxicity
Studies on the genotoxic potential of riboflavin derived by
fermentation from B. subtilis and of 8alpha-hydroxyriboflavin, an
impurity of the fermentation-derived material, are summarized in Table
3.
3. METHODS OF GENETIC MODIFICATION
3.1 Molecular genetic methods used to clone and express the riboflavin
(rib) operon from Bacillus subtilis
The riboflavin operon of B. subtilis containing the genes
ribT, ribH, ribA, and ribG was cloned into pUC19 as a 6.4-kb
NcoI/XbaI DNA fragment. In order to enhance expression of the rib
genes, a strong constitutive B. subtilis bacteriophage SPO1 promoter
was included. Two versions were made: (i) the SPO1-15 promoter was
inserted in front of the endogenous ribP2 promoter in a construct
later designated as pRF93 and (ii) the endogenous ribP1 promoter was
replaced by SPO1-15 in a construct later designated as pRF69. In order
to make the constructs selectable after integration into the B.
subtilis genome (the ß-lactamase gene of pUC19 is not expressed in
B. subtilis), the two constructs were provided with different
selectable genes, i.e. the tetracycline-resistant (tet) gene derived
from B. cereus was inserted into pRF93, and the chloramphenicol-
resistant (cat) gene derived from pUC194, origin S. aureus/
B. subtilis, into pRF69. Both constructs were integrated into the
genome of recipient B. subtilis strain RB50. The integration sites
were mapped for pRF69 at 209° and for pRF93 at 139° in the B.
subtilis genome.
RB50, a strain of B. subtilis that overproduces riboflavin, was
obtained by classical mutagenesis and selection procedures. With
various unknown mutations, RB50 contains a mutation that maps at the
ribC locus, which is present at 147° of the B. subtilis genome.
In order to further increase riboflavin production, the
RB50::[pRF69]: :[pRF93] was subjected to increasing concentrations of
the antibiotics tetracycline and chloramphenicol, resulting in
amplification of pRF69 and pRF93. In general, pRF69 was multiplied
20-30 times whereas pRF93 was multiplied 8-10 times, resulting in the
production strain RB50::[pRF69]n: :[pRF93]m. Although neither
antibiotic was present during the production stage, there was no
detectable loss of either pRF (Maruo & Yoshikawa, 1989; Kreneva &
Perumov, 1990; Mironov et al., 1994; Schurter, 1994). All of the
procedures were extensively and well described.
3.2 Genetic stability of the production strain
The production strain obtained after amplification was
RB50::[pRF69]n: :[pRF93]m. Samples were taken from the fermentor
during the process, and the numbers of copies of pRF69 and pRF93 were
determined by Southern blotting. There was no apparent loss of the
foreign DNA present in RB50: :[pRF69]n::[pRF93]m during the
process, which took 48 h, with samples taken at 6-h intervals
(Hümbelin & Hermann, 1995). As all of the experiments were well
described and appeared to be sound and convincing, it can be concluded
that the production strain is genetically stable during the
fermentation process.
3.3 Foreign DNA present in the end-product
One of the purification steps involved treatment of the product
with diluted hydrochloric acid for 60 min at 90°C. Under these
conditions, DNA is heavily depurinated and degraded (Hermann &
Schurter, 1994). A sensitive PCR method was used to detect any
residual transforming DNA (monitored as a 557-bp pRF69 PCR cat
fragment), with a detection limit of 0.5 ppb DNA per mg riboflavin. No
foreign DNA was detected. Furthermore, no transforming activity was
detected after only 5 min of hot acid treatment of crude riboflavin
(Schurter & Hermann, 1995). As all of the experiments were described
in detail, and the results are convincing, it can be concluded that
the riboflavin samples were free of any detectable transforming DNA.
4. COMMENTS
The strain used in riboflavin production has been genetically
modified to overproduce riboflavin by amplification of the chromosomal
region of the riboflavin operon containing suitable promoters and
flanked by pUC19 and antibiotic-resistance marker genes. The lack of
pathogenicity and toxicity of the strain of microorganism
(B. subtilis) from which the genetic information encoding riboflavin
was cloned is well documented, and the methods for genetic
modification have been well described. The production strain of
B. subtilis was shown to be genetically stable during fermentation.
It was convincingly established that the final product is riboflavin
of a purity comparable to, or greater than, that produced by
conventional methods and is free of DNA from the production organism.
Thus, antibiotic resistance marker genes are not present.
In a 90-day study of toxicity, rats were fed diets containing
food-grade riboflavin (98% pure) produced by fermentation from
genetically modified B. subtilis or riboflavin derived by chemical
synthesis, also of 98% purity. The doses tested were 0, 20, 50, or 200
mg/kg bw per day. Some animals at the high dose had reduced weight
gain, but generally by less than 10%, and food conversion efficiency
was not affected. In the group fed the high dose of
fermentation-derived riboflavin of 98% purity, some minor changes in
red blood cell parameters were observed, but they were not considered
relevant. The NOEL for 98% pure riboflavin produced by fermentation
was 200 mg/kg bw per day, which was the same as that for chemically
synthesized riboflavin.
Fermentation-derived riboflavin and the degradation product
8alpha-hydroxyriboflavin did not induce gene mutations in bacteria
in vitro in either the absence or the presence of metabolic
activation.
5. EVALUATION
The Committee concluded that the recombinant DNA techniques used
to derive the production strain of B. subtilis were well
characterized, providing assurance that no DNA is present in the
end-product. On the basis of molecular biological data and chemical
analytical research, it can be concluded that fermentation-derived
riboflavin from genetically modified B. subtilis is substantially
equivalent to synthetic riboflavin.
For 98% pure fermentation-derived riboflavin for use in food, the
NOEL in the 90-day study of toxicity in rats was 200 mg/kg bw per day,
the highest dose tested. Fermentation-derived riboflavin was evaluated
on the basis of its substantial equivalence to synthetic riboflavin.
Therefore, the Committee included riboflavin derived from a production
strain of genetically modified B. subtilis in the previously
established group ADI of 0-0.5 mg/kg bw for synthetic riboflavin and
riboflavin-5'-phosphate.
6. REFERENCES
Albertini, S. (1989) Mutagenicity evaluation of Ro 42-7491/000
(8alpha-hydroxy-riboflavin) in the Ames test (preincubation version).
Unpublished report from F. Hoffmann La Roche Ltd.dated 20 February
1989. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Albertini, S. (1995a) Mutagenicity evaluation of Ro 01-3131/055
(riboflavin 96% ex fermentation) in the Ames test (Study No. 139M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 September
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Albertini, S. (1995b) Mutagenicity evaluation of Ro 01-3131/054
(riboflavin 98% ex fermentation) in the Ames test (Study No. 138M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 Sepember
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Bächtold, H. (1980) Acute toxicity of riboflavine, some intermediates
and by-products of the synthesis, degradation products and
metabolites. Unpublished report No. 9024 from F. Hoffmann La Roche
dated 16 June 1980. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Buser, S., Hofmann, P., Lina, B., Forster, S. & Zabka, S. (1995)
Subchronic oral toxicity study with three different qualities of
riboflavin (Ro 01-3131/055 96% ex fermentation, Ro 01-3131/054 98% ex
fermentation and Ro 01-3131/000 98% ex synthesis) in rats (Project No.
920V94), parts I to III. Unpublished study from TNO Nutrition and Food
Institute, Zeist, Netherlands, dated 14 November 1995. Submitted to
WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Hermann, D. & Schurter, W. (1994) Effect of the riboflavin
purification process on DNA: Hot hydrochloric acid rapidly destroys
the biological activity of DNA. Unpublished report No. B-164'814 dated
29 December 1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Hümbelin, M. & Hermann, D. (1995) Genetic stability of the riboflavin
production strain RB50::[pRF69]n::[pRF93]m. Unpublished report No.
B-165 193 from Hoffmann La Roche Ltd. dated 30 October 1995. Submitted
to WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Kreneva, R.A. & Perumov, D.A. (1990) Genetic mapping of regulatory
mutations of Bacillus subtilis riboflavin operon. Mol. Gen.
Genet., 222, 467-469.
Ludwig, B., Bretzel, W., Manneberg, M., Bebniarz, J.C., Halter, V. &
Schöni, R. (1994) Analytical profile of riboflavin produced by
fermentation. Unpublished report No. B-163'358 from F. Hoffmann La
Roche Ltd dated 18 November 1994. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Maruo, B. & Yoshikawa, H., eds (1989) Topics in Secondary
Metabolism 1. Bacillus subtilis: Molecular Biology and Industrial
Application, Amsterdam, Elsevier, pp. 191-211.
Meinrad, J.L. & Gerber, C. (1991) Analytical profile of vitamin B2.
Part I. Unpublished report No. B-119'081 from F. Hoffmann La Roche Ltd
dated 14 August 1991. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Mironov, V.N., Kraev, A.S., Chikindas, M.L., Chernov, B.K., Stepanov,
A.I. & Skryabin, C.G. (1994) Functional organization of the riboflavin
biosynthesis operon from Bacillus subtilis SHgw. Mol. Gen. Genet.,
242, 201-208.
Pfister, M., Weber, W. & Hiraga, K. (1995) Riboflavin derived from
fermentation. Unpublished report No. B-332 023 from F. Hoffmann La
Roche Ltd dated 8 November 1995. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Schöni, R. (1989) Acute oral toxicity of Ro 42-7491/000 in male and
female rats after single administration. Unpublished report from F.
Hoffmann La Roche Ltd dated 22 March 1989. Submitted to WHO by F.
Hoffmann La Roche Ltd, Basel, Switzerland.
Schurter, W. (1994) Description of the riboflavin overproducer
B. subtilis strain RB50::[pRF69]n::[pRF93]m Ade+. Unpublished
report No. B-163'498 from F. Hoffmann La Roche Ltd dated 22 September
1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Schurter, W. & Hermann, D. (1995) PCR-analysis of riboflavin samples
from fermentation: Absence of production strain specific DNA.
Unpublished report No. B-165'177 from F. Hoffmann La Roche Ltd dated 9
May 1995. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Simon, W. & Vogt, R. (1989) [Riboflavin, Steps 6 and 7: Riboflavin
crude and pure. Isolation, identification, and synthesis of side
product.] Unpublished report No. BS/GCR 62'589 from F. Hoffmann La
Roche Ltd dated 5 September 1989. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland (in German).
Spackman, D.H., Stein, W.H. & Moore, S. (1958) Automatic recording
apparatus for use in the chromatography of amino acids. Anal. Chem.,
30, 1190-1206.
The presence of amino acids originating from fermentation was
analysed by the method of Spackman et al. (1958), and DNA was analysed
by polymerase chain reaction (PCR) techniques (Schurter & Hermann,
1995). The amounts of impurities in the different products are given
in Table 1. The concentration of 8alpha-hydroxymethylriboflavin, which
is a known degradation product after acid treatment of riboflavin, was
slightly increased in the fermentation-derived product, but only in
that of 98% purity. Since this product is derived from the 96% pure
material by crystallization from acidic media, this is to be expected;
however lumiflavin, which is a known degradation product after basic
treatment, was present in the synthesized riboflavin but not in the
fermentation-derived material.
Table 1. Composition of riboflavin products derived by fermentation and by synthesis
(average % weighed samples)
Component 96% ex 98% ex 96% ex synthesis
fermentation fermentation
HPLC method HPLC method HPLC method
I II I II I II
Riboflavin 98.4 98.3 100.1 99.7 98.8 98.6
Ribityl-oxo-chinoxalic acid Trace ND ND ND Trace Trace
8alpha-Hydroxy riboflavin Trace Trace 0.45 0.2 Trace Trace
Formylmethyl-flavin acetal Trace ND 0.11 Trace 0.52 ND
Lumichrome 0.14 0.11 ND Trace 0.18 Trace
Lumiflavin ND ND ND ND 0.56 0.5
Amino acids 0.9 0.9 0.06 0.06 - -
DNAa ND ND ND ND - -
Total 99.44 99.31 100.72 99.96 100.1 99.3
Trace, below limit of detection; ND, below limit for quantitative determination, 0.1%
a See also section 3.3, 'Foreign DNA present in the end product'
2. BIOLOGICAL DATA
2.1 Toxicological studies
2.1.1 Acute toxicity
No studies of the acute toxicity of riboflavin derived by
fermentation with genetically modified B. subtilis are available.
Table 2 gives the LD50 values for riboflavin (not derived from
fermentation), riboflavin phosphate, and some of the impurities
present in riboflavin. Except for the study with
8alpha-hydroxy-riboflavin, no full reports were available. The data in
Table 2 give an indication of the acute toxicity of synthetic
riboflavin (phosphate) and some of its impurities but are not relevant
for evaluating riboflavin derived by fermentation.
2.1.2 Short-term studies of toxicity
Rats
Groups of 10 male and 10 female six-week-old Wistar rats (the
males weighing 160-170 g and the females, 130-140 g) received diets
providing 20, 50, or 200 mg/kg bw of riboflavin -- either 99.4% pure
derived by fermentation from 96% pure riboflavin, 100.2% pure derived
by fermentation from 98% pure riboflavin, or 99.8% pure derived by
sunthesis from 98% pure riboflavin -- daily, on seven days per week
for 13 weeks. The study was performed according to good laboratory
practice (GLP) and OECD Guideline 408; a quality assurance (QA)
statement was included. A control group of 30 male and 30 female rats
was used. A satellite group of six male and six female animals was
attached to each group for determination of riboflavin concentrations
in blood and urine and for investigation of the reversibility of any
toxicological parameter. Feed and water were provided ad libitum.
The different assays were performed at one-week intervals. Samples of
the diet were taken at the start of treatment and at weeks 6 and 13 to
determine the content, homogeneity, and stability of the test compound
in the diet.
All animals were checked twice daily (once daily at weekends) for
behaviour and general condition. Ophthalmoscopy was performed on all
animals in the main control group and those at the three highest doses
before the start and at the end of the study. Body weight and food
intake were determined weekly. Water consumption was determined over
five consecutive days in weeks 1, 5, and 12. Haematological parameters
(haemoglobin concentration; haematocrit; erythrocyte, leukocyte, and
differential cell counts; mean corpuscular volume, haemoglobin, and
haemoglobin concentration; reticulocyte and thrombocyte counts; and
prothrombin time) were measured at week 6 and at autopsy in 10 rats of
each sex in the main control and treated groups. Haemoglobin
concentration; haematocrit; erythrocyte and leukocyte counts; mean
corpuscular volume, haemoglobin, and haemoglobin concentration; and
thrombocyte count were measured in all rats in the satellite groups at
the end of the recovery period. Clinical chemical parameters (alanine
and aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl
transferase, and lactic dehydrogenase activities; bilirubin, total
protein, albumin, albumin:globulin ratio, urea, creatinine, Na, K, Ca,
Cl, inorganic phosphate, cholesterol, triglycerides, and
phospholipids) were determined in all rats in the main treated groups
and in 15 rats of each sex in the main control group in weeks 7 and 13
after one night of fasting; the same parameters were measured in all
rats in the satellite groups at the end of the recovery period. In
weeks 7 and 13, all rats in the main treated groups and 15 rats of
each sex in the main control group underwent a renal concentration
test and urinalysis (appearance, pH, glucose, occult blood, ketones,
proteins, bilirubin, urobilinogen, sediment), except that sediment was
measured only in rats at the three highest doses. The sediment of
urine from all rats in the satellite groups was examined
microscopically for the presence of sperm at the end of the recovery
period. The weights (absolute and relative to body weight) of the
adrenals, kidneys, liver, spleen, brain, thymus, heart, and testes of
all rats were determined at the end of the treatment period in all of
the main groups and at the end of the recovery period in all satellite
groups. All rats in the main groups were examined macroscopically at
the end of treatment, and about 30 tissues from all animals in the
main control group and in the main three highest-dose groups were
examined microscopically The testes, epididymides, kidneys, liver,
lungs, and all gross lesions from all rats in the main groups were
examined microscopically. The riboflavin concentration in blood was
determined in all rats in the satellite groups before the start of the
study, at weeks 2, 6, and 13 of treatment, and at the end of the
recovery period. The riboflavin content of the urine was determined in
all rats in the satellite control group and in all rats in the
satellite groups treated with 96 and 98% pure riboflavin derived by
fermentation, before the study and at week 2 and 6. At the end of the
treatment period and at the end of the recovery period, the riboflavin
content of the urine was determined in all rats in all satellite
groups.
The test substances were homogeneously distributed in the diets
and showed storage stability under simulated experimental conditions.
The actual measured concentrations in the diet were within the
tolerable range of ± 10% of the nominal concentration. Alopecic areas
were seen frequently in various groups, but these findings were
transient and were not related to dose. Ophthalmoscopy showed no
abnormalities. Excrement collected from rats at the highest doses of
the three test substances was yellowish throughout the study. No
dose-related difference in food and water intake was seen.
During treatment, females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation showed a slightly but usually statistically
significant decrease in growth of about 6%. Males and females at
50 mg/kg bw per day 98% pure riboflavin ex synthesis also had a slight
but statistically significant decrease in growth. These effects were
not considered to be toxicologically relevant because they were small
(< 10%) and food conversion was not affected. During the recovery
period, body weights were similar in all groups. No changes in
haematological parameters were seen after six weeks of treatment, but
at the end of treatment (week 13) females at 200 mg/kg bw per day 98%
pure riboflavin ex fermentation had a slightly but statistically
significantly lower mean haemoglobin value and erythrocyte count,
whereas the mean reticulocyte count was significantly increased. The
individual reticulocyte counts in these animals were markedly
increased in two of 10 females and moderately increased in another
two. Slight but statistically significant increases in mean
thrombocyte counts in females at the highest dose of 98% pure
riboflavin ex fermentation and in males at the highest dose of
riboflavin 98% ex synthesis were within the range of normal variation
and therefore considered not to be toxicologically relevant. At six
and 13 weeks, the total leukocyte counts were statistically
significantly decreased in several treated groups but with no
dose-response relationship and inconsistently within groups; these
changes were also not considered to be related to treatment.
Clinical chemical and urinary analyses showed no
treatment-related changes. At the end of treatment, a slight but
statistically significant increase in relative liver weight was seen
in females at 200 mg/kg bw per day 98% pure riboflavin ex
fermentation, and a slight but statistically significant increase in
relative spleen weight in males at 50 and 200 mg/kg bw per day 96%
pure riboflavin (ex fermentation), but with no dose-response
relationship. Macroscopic and microscopic examination did not reveal
any treatment-related abnormality. The changes in organ weights were
considered to be of questionable biological relevance because there
was no dose-response relationship and they were not accompanied by
histopathological changes or changes in clinical chemistry. At the end
of the recovery period, all of the haematological parameters were
normal, except for a statistically significant decrease in mean
thrombocyte counts in females at 200 mg/kg bw per day 98% pure
riboflavin ex fermentation, and there were no notable changes in organ
weights.
The riboflavin concentrations in blood and urine were not
reported.
The authors concluded that the NOEL was 200 mg/kg bw per day for
all three test substances. The decreases in mean haemoglobin
concentration and erythrocyte count in females at 200 mg/kg bw per day
98% pure riboflavin ex fermentation were considered to be fortuitous
findings because, except in two females, there was no overt
association between the low erythrocyte counts and the high
reticulocyte counts. Moreover, according to the authors, the measured
values did not exceed the expected range, and there was also no clear
dose-response relationship in any group. In addition, there were no
related changes (in e.g. haemolysis or weight and histopathology of
haematopoietic organs) that could be associated with variations in the
red blood cell profile (Buser et al., 1995).
The Committee concluded that the NOEL for 98% pure riboflavin ex
fermentation was 50 mg/kg bw per day, on the basis of the significant
decreases in mean haemoglobin concentration and erythrocyte count and
the significant increase in mean reticulocyte count in females at
200 mg/kg bw. For 96% pure riboflavin ex fermentation and 98% pure
riboflavin ex synthesis, the NOEL was 200 mg/kg bw per day, the
highest dose tested.
Table 2. Acute toxicity of synthetic riboflavin phosphate and some of its impurities
Compound Species Sex Route LD50 Remarks Reference
(mg/kg bw)
Riboflavin Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin Rat NR Intravenous 560 Bächtold (1980)
Riboflavin Rat NR Subcutaneous 5 000 Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Oral > 40 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intraperitoneal 890 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Mouse NR Intravenous 780 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Oral > 20 000 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 1 030 Observation period, 24 h Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intraperitoneal 560 Bächtold (1980)
Riboflavin phosphate sodium Rat NR Intravenous 710 Observation period, 10 days Bächtold (1980)
Riboflavin phosphate sodium Rat NR Subcutaneous 790 Bächtold (1980)
8alpha-Hydroxyriboflavin Rat M&F Oral > 2 000 Limit test, by gavagea Schöni (1989)
(purity > 95%)
Lumichrome Mouse NR Oral > 9 000 Observation period, 10 days Bächtold (1980)
Lumichrome Mouse NR Intraperitoneal 11 000 Observation period, 10 days Bächtold (1980)
Lumiflavin Mouse NR Oral > 6 000 Observation period, 10 days Bächtold (1980)
NR, not reported; M&F, male and female
a Study performed according to OECD Guideline 401; no GLP statement or QA declaration was included.
Table 3. Results of assays for the genotoxicity of riboflavin derived by fermentation from B. subtilis and of 8alpha-hydroxyriboflavin
End-point Test object Concentration Results Reference
Riboflavin 96% Reverse mutationa S. typhimurium TA97, 50-5000 µg/plate Negativeb Albertini (1995a)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 99.4%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
Riboflavin 98% Reverse mutationa S. typhimurium TA 97, 50-5000 µg/plate Negativeb Albertini (1995b)
ex fermentation TA98, TA100, TA102, in DMSO No toxicity; increasingly
(purity, 100.2%) TA1535 milky suspensions at
> 50 µg/plate. At 1666 and
5000 µg/plate, precipitation
prevented colony counting
in preincubation assay.
8alpha-Hydroxy- Reverse mutationc S. typhimurium TA97, 100-5000 µg/plate Negativeb Albertini (1989)
riboflavin (purity TA98, TA100, TA102, in DMSO No toxicity; yellow-to-orange
not given) TA1535, TA1537, TA1538 precipitate at 2500 and 5000
µg/plate
DMSO, dimethyl sulfoxide
a Ames test; standard plate incorporation, preincubation modification assay. Study performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat experiment; a QA statement was included.
b With and without exogenous metabolic activation
c Ames test; standard plate incorporation, preincubation modification assay. Study not performed according to GLP; protocol resembled OECD
Guideline 471 except for the performance of an independent repeat assay; no QA statement was included.
2.1.3 Genotoxicity
Studies on the genotoxic potential of riboflavin derived by
fermentation from B. subtilis and of 8alpha-hydroxyriboflavin, an
impurity of the fermentation-derived material, are summarized in Table
3.
3. METHODS OF GENETIC MODIFICATION
3.1 Molecular genetic methods used to clone and express the riboflavin
(rib) operon from Bacillus subtilis
The riboflavin operon of B. subtilis containing the genes
ribT, ribH, ribA, and ribG was cloned into pUC19 as a 6.4-kb
NcoI/XbaI DNA fragment. In order to enhance expression of the rib
genes, a strong constitutive B. subtilis bacteriophage SPO1 promoter
was included. Two versions were made: (i) the SPO1-15 promoter was
inserted in front of the endogenous ribP2 promoter in a construct
later designated as pRF93 and (ii) the endogenous ribP1 promoter was
replaced by SPO1-15 in a construct later designated as pRF69. In order
to make the constructs selectable after integration into the B.
subtilis genome (the ß-lactamase gene of pUC19 is not expressed in
B. subtilis), the two constructs were provided with different
selectable genes, i.e. the tetracycline-resistant (tet) gene derived
from B. cereus was inserted into pRF93, and the chloramphenicol-
resistant (cat) gene derived from pUC194, origin S. aureus/
B. subtilis, into pRF69. Both constructs were integrated into the
genome of recipient B. subtilis strain RB50. The integration sites
were mapped for pRF69 at 209° and for pRF93 at 139° in the B.
subtilis genome.
RB50, a strain of B. subtilis that overproduces riboflavin, was
obtained by classical mutagenesis and selection procedures. With
various unknown mutations, RB50 contains a mutation that maps at the
ribC locus, which is present at 147° of the B. subtilis genome.
In order to further increase riboflavin production, the
RB50::[pRF69]: :[pRF93] was subjected to increasing concentrations of
the antibiotics tetracycline and chloramphenicol, resulting in
amplification of pRF69 and pRF93. In general, pRF69 was multiplied
20-30 times whereas pRF93 was multiplied 8-10 times, resulting in the
production strain RB50::[pRF69]n: :[pRF93]m. Although neither
antibiotic was present during the production stage, there was no
detectable loss of either pRF (Maruo & Yoshikawa, 1989; Kreneva &
Perumov, 1990; Mironov et al., 1994; Schurter, 1994). All of the
procedures were extensively and well described.
3.2 Genetic stability of the production strain
The production strain obtained after amplification was
RB50::[pRF69]n: :[pRF93]m. Samples were taken from the fermentor
during the process, and the numbers of copies of pRF69 and pRF93 were
determined by Southern blotting. There was no apparent loss of the
foreign DNA present in RB50: :[pRF69]n::[pRF93]m during the
process, which took 48 h, with samples taken at 6-h intervals
(Hümbelin & Hermann, 1995). As all of the experiments were well
described and appeared to be sound and convincing, it can be concluded
that the production strain is genetically stable during the
fermentation process.
3.3 Foreign DNA present in the end-product
One of the purification steps involved treatment of the product
with diluted hydrochloric acid for 60 min at 90°C. Under these
conditions, DNA is heavily depurinated and degraded (Hermann &
Schurter, 1994). A sensitive PCR method was used to detect any
residual transforming DNA (monitored as a 557-bp pRF69 PCR cat
fragment), with a detection limit of 0.5 ppb DNA per mg riboflavin. No
foreign DNA was detected. Furthermore, no transforming activity was
detected after only 5 min of hot acid treatment of crude riboflavin
(Schurter & Hermann, 1995). As all of the experiments were described
in detail, and the results are convincing, it can be concluded that
the riboflavin samples were free of any detectable transforming DNA.
4. COMMENTS
The strain used in riboflavin production has been genetically
modified to overproduce riboflavin by amplification of the chromosomal
region of the riboflavin operon containing suitable promoters and
flanked by pUC19 and antibiotic-resistance marker genes. The lack of
pathogenicity and toxicity of the strain of microorganism
(B. subtilis) from which the genetic information encoding riboflavin
was cloned is well documented, and the methods for genetic
modification have been well described. The production strain of
B. subtilis was shown to be genetically stable during fermentation.
It was convincingly established that the final product is riboflavin
of a purity comparable to, or greater than, that produced by
conventional methods and is free of DNA from the production organism.
Thus, antibiotic resistance marker genes are not present.
In a 90-day study of toxicity, rats were fed diets containing
food-grade riboflavin (98% pure) produced by fermentation from
genetically modified B. subtilis or riboflavin derived by chemical
synthesis, also of 98% purity. The doses tested were 0, 20, 50, or 200
mg/kg bw per day. Some animals at the high dose had reduced weight
gain, but generally by less than 10%, and food conversion efficiency
was not affected. In the group fed the high dose of
fermentation-derived riboflavin of 98% purity, some minor changes in
red blood cell parameters were observed, but they were not considered
relevant. The NOEL for 98% pure riboflavin produced by fermentation
was 200 mg/kg bw per day, which was the same as that for chemically
synthesized riboflavin.
Fermentation-derived riboflavin and the degradation product
8alpha-hydroxyriboflavin did not induce gene mutations in bacteria
in vitro in either the absence or the presence of metabolic
activation.
5. EVALUATION
The Committee concluded that the recombinant DNA techniques used
to derive the production strain of B. subtilis were well
characterized, providing assurance that no DNA is present in the
end-product. On the basis of molecular biological data and chemical
analytical research, it can be concluded that fermentation-derived
riboflavin from genetically modified B. subtilis is substantially
equivalent to synthetic riboflavin.
For 98% pure fermentation-derived riboflavin for use in food, the
NOEL in the 90-day study of toxicity in rats was 200 mg/kg bw per day,
the highest dose tested. Fermentation-derived riboflavin was evaluated
on the basis of its substantial equivalence to synthetic riboflavin.
Therefore, the Committee included riboflavin derived from a production
strain of genetically modified B. subtilis in the previously
established group ADI of 0-0.5 mg/kg bw for synthetic riboflavin and
riboflavin-5'-phosphate.
6. REFERENCES
Albertini, S. (1989) Mutagenicity evaluation of Ro 42-7491/000
(8alpha-hydroxy-riboflavin) in the Ames test (preincubation version).
Unpublished report from F. Hoffmann La Roche Ltd.dated 20 February
1989. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Albertini, S. (1995a) Mutagenicity evaluation of Ro 01-3131/055
(riboflavin 96% ex fermentation) in the Ames test (Study No. 139M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 September
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Albertini, S. (1995b) Mutagenicity evaluation of Ro 01-3131/054
(riboflavin 98% ex fermentation) in the Ames test (Study No. 138M95).
Unpublished report from F. Hoffmann La Roche Ltd. dated 8 Sepember
1995. Submitted to WHO by F.Hoffmann La Roche Ltd, Basel, Switzerland.
Bächtold, H. (1980) Acute toxicity of riboflavine, some intermediates
and by-products of the synthesis, degradation products and
metabolites. Unpublished report No. 9024 from F. Hoffmann La Roche
dated 16 June 1980. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Buser, S., Hofmann, P., Lina, B., Forster, S. & Zabka, S. (1995)
Subchronic oral toxicity study with three different qualities of
riboflavin (Ro 01-3131/055 96% ex fermentation, Ro 01-3131/054 98% ex
fermentation and Ro 01-3131/000 98% ex synthesis) in rats (Project No.
920V94), parts I to III. Unpublished study from TNO Nutrition and Food
Institute, Zeist, Netherlands, dated 14 November 1995. Submitted to
WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Hermann, D. & Schurter, W. (1994) Effect of the riboflavin
purification process on DNA: Hot hydrochloric acid rapidly destroys
the biological activity of DNA. Unpublished report No. B-164'814 dated
29 December 1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Hümbelin, M. & Hermann, D. (1995) Genetic stability of the riboflavin
production strain RB50::[pRF69]n::[pRF93]m. Unpublished report No.
B-165 193 from Hoffmann La Roche Ltd. dated 30 October 1995. Submitted
to WHO by F. Hoffmann La Roche Ltd, Basel, Switzerland.
Kreneva, R.A. & Perumov, D.A. (1990) Genetic mapping of regulatory
mutations of Bacillus subtilis riboflavin operon. Mol. Gen.
Genet., 222, 467-469.
Ludwig, B., Bretzel, W., Manneberg, M., Bebniarz, J.C., Halter, V. &
Schöni, R. (1994) Analytical profile of riboflavin produced by
fermentation. Unpublished report No. B-163'358 from F. Hoffmann La
Roche Ltd dated 18 November 1994. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Maruo, B. & Yoshikawa, H., eds (1989) Topics in Secondary
Metabolism 1. Bacillus subtilis: Molecular Biology and Industrial
Application, Amsterdam, Elsevier, pp. 191-211.
Meinrad, J.L. & Gerber, C. (1991) Analytical profile of vitamin B2.
Part I. Unpublished report No. B-119'081 from F. Hoffmann La Roche Ltd
dated 14 August 1991. Submitted to WHO by F. Hoffmann La Roche Ltd,
Basel, Switzerland.
Mironov, V.N., Kraev, A.S., Chikindas, M.L., Chernov, B.K., Stepanov,
A.I. & Skryabin, C.G. (1994) Functional organization of the riboflavin
biosynthesis operon from Bacillus subtilis SHgw. Mol. Gen. Genet.,
242, 201-208.
Pfister, M., Weber, W. & Hiraga, K. (1995) Riboflavin derived from
fermentation. Unpublished report No. B-332 023 from F. Hoffmann La
Roche Ltd dated 8 November 1995. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland.
Schöni, R. (1989) Acute oral toxicity of Ro 42-7491/000 in male and
female rats after single administration. Unpublished report from F.
Hoffmann La Roche Ltd dated 22 March 1989. Submitted to WHO by F.
Hoffmann La Roche Ltd, Basel, Switzerland.
Schurter, W. (1994) Description of the riboflavin overproducer
B. subtilis strain RB50::[pRF69]n::[pRF93]m Ade+. Unpublished
report No. B-163'498 from F. Hoffmann La Roche Ltd dated 22 September
1994. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Schurter, W. & Hermann, D. (1995) PCR-analysis of riboflavin samples
from fermentation: Absence of production strain specific DNA.
Unpublished report No. B-165'177 from F. Hoffmann La Roche Ltd dated 9
May 1995. Submitted to WHO by F. Hoffmann La Roche Ltd, Basel,
Switzerland.
Simon, W. & Vogt, R. (1989) [Riboflavin, Steps 6 and 7: Riboflavin
crude and pure. Isolation, identification, and synthesis of side
product.] Unpublished report No. BS/GCR 62'589 from F. Hoffmann La
Roche Ltd dated 5 September 1989. Submitted to WHO by F. Hoffmann La
Roche Ltd, Basel, Switzerland (in German).
Spackman, D.H., Stein, W.H. & Moore, S. (1958) Automatic recording
apparatus for use in the chromatography of amino acids. Anal. Chem.,
30, 1190-1206.
