
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
STEVIOSIDE
First draft prepared by
Dr Josef Schlatter
Swiss Federal Office of Public Health, Switzerland
Explanation
Biological data
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Effects on enzymes and other biochemical parameters
Toxicological studies
Acute toxicity
Short-term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Developmental toxicity
Studies on metabolites: Steviol
Absorption, distribution, and excretion
Effects on enzymes and other biochemical
parameters
Acute toxicity
Genotoxicity
Developmental toxicity
Special studies
Cariogenicity
Renal function and vasodilatation
Observations in humans
Comments
Evaluation
References
1. EXPLANATION
Stevioside is a glycoside of the diterpene derivative steviol
(ent-13-hydroxykaur-16-en-19-oic acid). Steviol glycosides are natural
constituents of the plant Stevia rebaudiana Bertoni, belonging to
the Compositae family. The leaves of S. rebaudiana Bertoni contain
eight different steviol glycosides, the major constituent being
stevioside (triglucosylated steviol), constituting about 5-10% in dry
leaves. Other main constituents are rebaudioside A (tetraglucosylated
steviol), rebaudioside C, and dulcoside A. S. rebaudiana is native
to South America and has been used to sweeten beverages and food for
several centuries. The plant has also been distributed to Southeast
Asia. Stevioside has a sweetening potency 250-300 times that of
sucrose and is stable to heat. In a 62-year-old sample from a
herbarium, the intense sweetness of S. rebaudiana was conserved,
indicating the stability of stevioside to drying, preservation, and
storage (Soejarto et al., 1982; Hanson & De Oliveira, 1993).
Stevioside and its aglycone steviol may act in plants as a
feeding deterrent, e.g. against the aphid Schizaphis graminum. The
EC50 of stevioside was 650 mg/kg; steviol was more active, with an
EC50 of 150 mg/kg. Steviol lost its deterrent activity after
acetylation or glycosylation of the C-13 tertiary hydroxy group or
methylation of the C-19 carboxylic acid substituent, but the activity
of steviol was not greatly affected by modification of either the C-16
exomethylene group or its stereochemistry (Nanayakkara et al., 1987).
The biochemical pathway for the formation of steviol in
S. rebaudiana is partly known (Kim et al., 1996), and a simple,
efficient method for the extraction of steviol glycosides has been
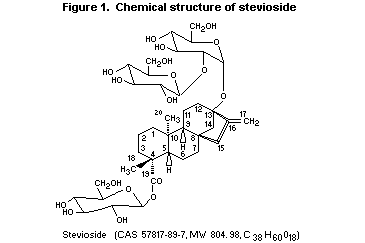
described (Liu et al., 1997). The chemical structure of stevioside
(Nanayakkara et al., 1987; Suttajit et al., 1993) is shown in Figure
1.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
3H-Stevioside (specific activity, 13 or 46 µCi/mg), administered
by gavage to groups of three to seven Wistar rats at a dose of 125
mg/kg bw (10-120 µCi/kg bw), was absorbed slowly, a maximal blood
concentration of 4.8 µg/ml being reached by 8 h. At 4 h, the highest
concentration was found in the caecum (280 µg/g stevioside
equivalent). At 24 h, the concentrations of radiolabel were low in
most organs, including blood, corresponding to about 2 µg/g or ml
stevioside equivalent, except in liver (5.7 µg/g), adrenal gland
(12 µg/g), small intestine (8.8 µg/g), caecum (40 µg/g), large
intestine (12 µg/g), and fat (12 µg/g). The elimination half-life was
24 h. At 48 h, 31% of the radiolabel remained in the body. By five
days, 68% had been excreted into the faeces, 24% in expired air, and
2.3% in the urine. In bile-duct cannulated rats, biliary excretion was
low up to 24 h, increasing rapidly thereafter to reach 41% of the dose
after three days. The authors concluded that stevioside is absorbed
from the gut very slowly, that enterohepatic circulation occurs in
rats, and that faecal excretion is the major route of elimination
(Nakayama et al., 1986).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The radiolabel in plasma decreased
rapidly, showing rapid distribution in the body. The highest
concentrations of radiolabel 10 and 120 min after injection were
observed in the liver (45 and 5% of the injected dose, respectively)
and the small intestine (18 and 66%). At 120 min after injection, the
radiolabel eliminated in the bile represented 52% of the original
dose; that excreted in the faeces and urine 24 h after injection
represented 35 and 35%, respectively, of the original dose (Cardoso et
al., 1996). The Committee considered that this study was of limited
value since introduction of a large 131I atom into stevioside might
significantly affect its absorption, distribution, metabolism, and
excretion in bile or urine.
The renal excretion of stevioside and its effect on the renal
excretion of several other substances was studied in groups of 10 male
Wistar rats, which received intravenous infusions of stevioside at
doses of 4, 8, 12, or 16 mg/kg bw per hour for 30 min, after a control
period of 30 min. No significant change in inulin clearance was
observed, but there was a significant increase in para-aminohippuric
acid clearance, fractional sodium excretion (FeNa+), urinary flow as
percent of glomerular filtration rate, and glucose clearance when
compared with controls at doses greater than 4 mg/kg bw per hour.
Stevioside clearance was greater than inulin clearance and smaller
than para-amino-hippuric acid clearance at all doses tested. The
authors concluded that stevioside is secreted by the renal tubular
epithelium and induces diuresis and natriuresis and a fall in renal
tubular reabsorption of glucose (Melis, 1992a).
2.1.2 Biotransformation
Thin-layer chromatography of the intestinal contents, faeces, and
bile of groups of three to seven Wistar rats given 3H-stevioside
(specific activity, 13 or 46 µCi/mg) by gavage at a dose of 125 mg/kg
bw (10-120 µCi/kg bw) revealed that stevioside is decomposed by rat
caecal flora to steviol and sugars. Stevioside was detected as the
major component in the stomach 1 h after administration. After 4 h in
the small intestine, stevioside, steviolbioside (produced by cleavage
of the glucose moiety at the C-19 position), and steviol accounted for
7.6, 8, and 7.5% of the radiolabel; in the caecum, these compounds
accounted for 39, 17, and 5.1% of the radiolabel, respectively. At 24
h, stevioside was not detectable in the caecum, but steviol and an
unidentified metabolite accounted for 16 and 68% of the radiolabel,
respectively. Steviol was found to be the major metabolite in faeces,
whereas stevioside and the unidentified metabolite were not
quantifiable. In bile, most of the radiolabel found up to 24 h was on
the unidentified metabolite, which was probably a steviol conjugate.
The authors concluded that orally administered stevioside is not
readily absorbed from the upper part of the small intestine, but
metabolites, formed primarily by the bacterial flora in the caecum,
are absorbed from the lower part of the intestine; they also concluded
that most of the stevioside is excreted as steviol in the faeces
(Nakayama et al., 1986). The Committee concluded further that the
faecal steviol could also have arisen from deconjugation of biliary
conjugates by the gut flora.
When 2.5 mg/ml stevioside were incubated under anaerobic
conditions with whole-cell suspensions of bacteria from rat caecum,
stevioside was completely degraded to steviol within two days. The
authors concluded that similar degradation of stevioside occurs in
humans (Wingard et al., 1980).
Mass spectral analysis of steviol and some analogues revealed
characteristic patterns reflecting differential stereochemistry and
variations in the nature of the substituents present. This information
was used to identify several metabolites of steviol (by gas
chromatography-mass spectrometry) which are known to produce a
mutagenic response in Salmonella typhimurium strain TM677 with
metabolic activation. After incubation with a 9000 × g fraction
derived from the livers of Aroclor 1254-pretreated rats, unchanged
steviol was the predominant compound, and nine metabolites were found.
The major pathways of mammalian metabolism of steviol proved to be
allylic oxidation and epoxidation. 15alpha-Hydroxysteviol represented
67% of the metabolites of steviol in vitro (Compadre et al., 1988).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The results of reverse-phase
high-performance liquid chromato-graphy (RP-HPLC) of the bile showed
that stevioside was degraded in vivo and that steviol was the major
metabolite (47% of radiolabel); 37% of the radiolabel was on
stevioside, and the remaining 15% was on an unidentified metabolite.
RP-HPLC analysis of urine 90 min after injection showed the presence
of stevioside and the same unidentified metabolite found in bile, but
no steviol. The authors concluded that stevioside is metabolized in
rat liver to steviol, which is excreted through the bile, and that
similar degradation occurs in humans (Cardoso et al., 1996). The
Committee concluded that there is an alternative explanation, namely
that stevioside is secreted into the bile, is degraded to steviol by
the gut flora, and is resorbed in the lower gut. The Committee
considered that this study was of limited value since introduction of
a large 131I atom into stevioside might significantly affect its
absorption, distribution, metabolism, and excretion in bile or urine.
Stevioside was perfused at a concentration of 0.2 or 0.5 mmol/L
(equivalent to 0.16 and 0.4 mg/ml) into rat livers and was
recirculated for 2 h. The concentration of stevioside remained
constant throughout the perfusion. The formation of hydrolysis
products, especially steviol, was investigated chromatographically,
with negative results. The authors concluded that the reported
metabolic transformation of intravenously injected 131I-stevioside is
either a specific characteristic of this derivative or depends on
factors that are absent in the isolated perfused rat liver
(Ishii-Iwamoto & Bracht, 1995). The Committee concluded that the most
likely explanation for the apparent discrepancy is the fact that
introduction of the large 131I atom into stevioside altered its
pharmacokinetic behaviour and that stevioside is secreted into the
bile in vivo and is degraded to steviol by the gut flora.
2.1.3 Effects on enzymes and other biochemical parameters
Stevioside given to female RCR/Ha mice did not induce glutathione
S-transferase activity in liver or intestinal mucosa (Pezzuto et
al., 1986).
Stevioside (1 mmol/L, equivalent to 0.8 mg/ml) inhibited
oxidative phosphorylation and the activity of ATPase (50% inhibition),
succinate oxidase (8% inhibition), and succinate dehydrogenase (10%
inhibition). No inhibition of NADH-oxidase or L-glutamate
dehydrogenase activity was seen. The ADP:O ratio was slightly
decreased. Substrate respiration was increased at low concentrations
(up to 0.5 mmol/L, equivalent to 0.4 mg/ml) and inhibited at higher
concentrations (1 mmol/L, equivalent to > 0.8 mg/ml). The authors
concluded that stevioside acts as a weak uncoupler of oxidative
phosphorylation (Kelmer-Bracht et al., 1985).
Stevioside inhibited oxidative phosphorylation in isolated rat
liver mitochondria. The concentration required for 50% inhibition of
ATP synthesis was 1.2 mmol/L, equivalent to 0.97 mg/ml (Vignais et
al., 1966).
The effect of stevioside, an inhibitor of long-chain fatty acid
transport, on ketogenesis and on 14C-carbon dioxide production from
[1-14C]-palmitate (100-300 µmol/L) was investigated in isolated and
haemoglobin-free perfused rat liver. Stevioside (2.5 mmol/L,
equivalent to 2 mg/ml) inhibited both parameters but had a smaller
effect on 14C-carbon dioxide production. At 300 µmol/L palmitate and
150 µmol/L albumin, ketogenesis was inhibited by 66%, whereas no
significant inhibition of 14C-carbon dioxide was seen. The authors
concluded that these results reflect different degrees of saturation
of the citric acid cycle and the ketogenic pathway and that changes in
the redox state of the mitochondrial NAD(+)-NADH complex occur after
infusion of stevioside (Constantin et al., 1991).
The Committee noted that the concentrations used in the studies
conducted in vitro were very high relative to those achieved in
blood after oral administration, when the major intestinal metabolite
that enters the circulation is steviol. These studies may therefore be
of limited significance.
When single doses of 200 µmol/L stevioside, equivalent to
650 mg/kg bw, were given orally to 24-h-fasted male Wistar rats,
either alone or simultaneously with fructose, stevioside increased the
initial glycogen deposition in the liver. When it was given to the
rats in the drinking-water at 1 or 2 mmol/L, equivalent to 81 and 160
mg/kg bw, at the beginning of a fasting period of 24 or 48 h,
increased hepatic glycogen concentrations were found at 48 h
(1 mmol/L) and at 24 h (2 mmol/L). The authors concluded that
stevioside stimulates hepatic glycogen synthesis under gluconeogenic
conditions (Hübler et al., 1994).
The effect of stevioside on the transport and metabolism of
D-glucose and D-fructose was investigated in isolated perfused rat
liver. The maximal exchange rate of D-glucose was 700 µmol/L per
min/ml, and the Km was 38 mmol/L. Stevioside inhibited D-glucose and
D-fructose transport across the cell membrane. The half-maximal effect
at 1 mmol/L D-glucose occurred at 0.8 mmol/L stevioside, equivalent to
0.65 mg/ml. Stevioside had no effect on D-glucose metabolism, except
to cause transient changes in D-glucose release, which reflected
changes in the intracellular concentration. D-Fructose consumption,
however, was specifically affected (half-maximal effect at 2.8 mmol/L,
equivalent to 2.3 mg/ml), as were all parameters that depend on
D-fructose transformation: D-glucose production, L-lactate and
pyruvate production, and extra oxygen uptake. In livers that released
D-glucose from endogenous glycogen, strong inhibition of transport
increased the intracellular:extracellular ratio of D-glucose
concentration. The control values for this ratio, representing an
average over the total intracellular water space, were all below unity
(Ishii et al., 1987).
Stevioside had no effect on gluconeogenesis or oxygen uptake in
isolated Wistar rat renal cortical tubules at concentrations up to
3 mmol/L, equivalent to 2.4 mg/ml. The authors concluded that the lack
of activity was due to the inability of stevioside to penetrate the
cell membrane (Yamamoto et al., 1985).
Intravenous infusion of stevioside at 150 mg/ml to male Wistar
rats at a dose of 100, 150, or 200 mg/kg bw per hour raised the plasma
glucose concentrations to 110, 140, and 130% of the control value
during and after infusion. The glucose turnover rate was not altered,
but glucose clearance was reduced by infusion of 200 mg/kg bw per hour
stevioside, from 6.5 to 5 ml/min per kg bw. The plasma insulin
concentration was unchanged. Pretreatment with angiotensin II and
arginine vasopressin had no effect, while prazosin, an
alpha-adrenergic blocker, attenuated the hyperglycaemic effect of
stevioside and infused insulin inhibited it. Oral administration of
stevioside at 2000 mg/kg bw had no effect on the plasma glucose
concentration. The authors concluded that the hyperglycaemic effect of
stevioside was due to an effect on glucose transport across the cell
(Suanarunsawat & Chaiyabutr, 1997).
The effects of stevioside at 1 and 5 mmol/L, equivalent to 0.8
and 4 mg/ml, on intestinal glucose absorption were examined in hamster
jejunum by the everted sac technique. Glucose absorption was not
inhibited (Toskulkao et al., 1995a,b).
Infusion of stevioside at 15 µmol/L, equivalent to 12 µg/ml, for
20 min did not significantly alter the arginine-induced secretion of
insulin or glucagon in the pancreas of male Wistar rats (Usami et al.,
1980).
Stevioside inhibited the action of atractyloside, a known
inhibitor of the adenine nucleotide carrier of mitochondria and in
consequence an inhibitor of energy metabolism, in isolated perfused
rat liver. It decreased the effects of atractyloside on glycolysis,
glycogenolysis, gluconeogenesis, and oxygen uptake. The concentration
for half-maximal action of stevioside was 0.5 mmol/L, equivalent to
0.4 mg/ml. The authors concluded that it acts on the outside of the
cell, as labelled stevioside did not penetrate the cell membranes
(Ishii & Bracht, 1986).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and stevioside did not
inhibit the induction of Epstein-Barr virus early antigen by TPA at
the highest concentration tested: 50 µg/ml (18% inhibition) (Okamoto
et al., 1983a).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the the toxicity of stevioside given as single doses
to rodents are summarized in Table 1. No lethality was seen within 14
days after administration, and no clinical signs of toxicity or
morphological or histopathological changes were found.
After intravenous administration of stevioside to
pentobarbital-anaesthetized dogs at a dose of 32.5 µmol/L per kg bw
(equivalent to 26 mg/kg bw), no significant changes were seen in any
parameters of whole blood, plasma, or renal function, and there was no
significant alteration of the renal ultrastructure. The authors
concluded that stevioside is totally devoid of acute extrarenal
effects (such as hypoxaemia, which could contribute to nephrotoxicity)
and direct renal effects during the 6-h period following intravenous
administration (Krejci & Koechel, 1992).
Table 1. Acute toxicity of stevioside (purity, 96%) given orally to
rodents
Species Sex LD50 (g/kg bw) Reference
Mouse Male and female > 15 Toskulkao et al. (1997)
Mouse Male > 2 Medon et al. (1982)
Rat Male and female > 15 Toskulkao et al. (1997)
Hamster Male and female > 15 Toskulkao et al. (1997)
2.2.2 Short-term studies of toxicity
Rats
A 13-week study of toxicity was carried out in Fischer 344 rats
given doses of 0, 0.31, 0.62, 1.25, 2.5, or 5% in the diet (equivalent
to 160, 310, 630, 1300, and 2500 mg/kg bw per day) to determine the
appropriate doses for a two-year study of carcinogenicity. The rats
were randomly allocated to six groups, each consisting of 10 males and
10 females. None of the animals died during the administration period,
and there was no difference in body-weight gain between the control
and treated groups during administration or in food consumption in the
later part of the study. The activity of lactic dehydrogenase and the
incidence of single-cell necrosis in the liver were increased in all
groups of treated males. The authors considered these effects to be
nonspecific because of the lack of a clear dose-response relationship,
the relatively low severity, and their limitation to males. Other
statistically significant differences in haematological and
biochemical parameters were also considered to be of minor
toxicological significance. The authors concluded that a concentration
of 5% in diet was a suitable maximum tolerable dose of stevioside for
a two-year study in rats (Aze et al., 1991).
2.2.3 Long-term studies of toxicity and carcinogenicity
Rats
Groups of 45 male and 45 female inbred Wistar rats were given
diets containing stevioside (purity, 85%) at 0, 0.2, 0.6, or 1.2%
(equivalent to 100, 300, and 600 mg/kg bw per day) for two years.
After 6, 12, and 24 months, blood was obtained from the tail vein of
five male and five female rats in each dose group for haematological
and clinical biochemical tests. One week later, these rats were housed
in metabolism cages for urine collection and were then killed for
further biochemical, pathological, and histopathological examination.
All surviving animals were killed at two years. Growth, food use and
consumption, general appearance, and mortality were similar in treated
and control groups. The mean life span of rats given stevioside was
not significantly different from that of the controls. No
treatment-related changes were observed in haematological, urinary, or
clinical biochemical values at any stage of the study. The incidence
and severity of non-neoplastic and neoplastic changes were unrelated
to the concentration of stevioside in the diet. The NOEL was 1.2%,
equivalent to 600 mg/kg bw per day. The authors suggested that the
acceptable daily intake of stevioside for humans was 7.9 mg/kg bw per
day, on the basis of the stevioside consumption of the rats during the
first three months (the average for males and females being 790 mg/kg
bw per day) and a safety factor of 100 (Xili et al., 1992).
Stevioside (purity, 95.6%) was added to powdered diet at
concentrations of 0, 2.5, or 5% (equal to 0, 970, and 2000 mg/kg bw
per day for males and 0, 1100, and 2400 for females) and pelleted
every three months. The doses were selected on the basis of the
results of the 13-week study and administered to groups of 50 male and
50 female Fischer 344/DuCrj rats ad libitum for 104 weeks.
Thereafter, all of the groups were maintained on basal diet for four
weeks. All surviving rats were killed at week 108. The body-weight
gain of treated animals was slightly depressed, and a relationship was
seen with the dose of stevioside: 2.3 and 4.4% in males at the low and
high dose and 2.4 and 9.2% in females at the low and high dose. Food
consumption did not differ between the groups. The final survival rate
of males at 5% was significantly decreased, with a rate of 60% versus
78% in controls. The absolute kidney weights were decreased in male
and female animals at the high dose; however, there was no significant
histopathological evidence of neoplastic or non-neoplastic lesions
attributable to treatment in any organ or tissue, except for a
decreased incidence of mammary adenomas in females and a reduced
severity of chronic nephropathy in males. The authors concluded that
stevioside is not carcinogenic in Fischer 344 rats under these
experimental conditions (Toyoda et al., 1995, 1997). The Committee
noted that the report of Toyoda et al. (1995) gives data only for
individual animals, with no summary tables or figures.
The effects of stevioside on urinary bladder carcinogenesis
initiated by N-nitrosobutyl- N-(4-hydroxybutyl)amine was evaluated
in male Fischer 344 rats given 0.01% of the nitrosamine in their
drinking-water for four weeks and then 5% stevioside in their diet,
equivalent to 5000 mg/kg bw per day, for 32 weeks. All surviving rats
were sacrificed after 36 weeks and examined histologically. Treatment
with 5% stevioside did not affect the incidence or extent of papillary
or nodular hyperplasia in nitrosamine-treated rats. No preneoplastic
or neoplastic lesions of the urinary bladder were observed in rats
treated with stevioside only. The authors concluded that stevioside
does not promote bladder carcinogenesis (Hagiwara et al., 1984; Ito et
al., 1984).
2.2.4 Genotoxicity
Studies of the genotoxicity of stevioside are summarized in
Table 2.
Table 2. Results of assays for the genotoxicity of stevioside
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98, TA100 50 mg/platea Negativeb Suttajit et al. (1993)
(purity, 99%)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platec Negative Matsui et al. (1996a)d
TA102, TA104, TA1535, TA1537 1 mg/platee Negative
(purity, 83%)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Matsui et al. (1996a)
Forward mutation S. typhimurium TM677 Not specifieda Negative Medon et al. (1982)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Pezzuto et al. (1985a)
umu Gene mutation S. typhimurium TA1535/pSK1002 5 mg/platea Negative Matsui et al. (1996a)
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 8 mg/mlc Negative Matsui et al. (1996a)
12 mg/mle
Chromosomal aberration Human lymphocytes 10 mg/ml Negative Suttajit et al. (1993)
Chromosomal aberration Chinese hamster lung fibroblasts 12 mg/mlc Negative Ishidate et al. (1984)
(purity, 85%)
a With and without metabolic activation
b A positive response towards TA98 was seen without metabolic activation at 50 mg/ml but not at lower concentrations
up to 20 mg/ml
c Without metabolic activation
d The same results were cited in an earlier abstract (Matsui et al., 1987).
e With metabolic activation
2.2.5 Reproductive toxicity
Hamsters
Groups of 10 male and 10 female one-month-old golden hamsters
(Mesocricetus auratus,) were force-fed with stevioside (purity, 90%)
at 0, 500, 1000, or 2500 mg/kg bw per day daily. Each female was mated
and allowed to bear three litters during the experiment. Females in
late gestation and while lactating (one month) received stevioside in
the drinking-water. Two weeks after the offspring had been weaned, the
females were mated again. No abnormalities were found in the growth or
fertility of animals of either sex. All of the males mated females
efficiently and successfully; the females showed normal four-day
oestrus cycles and became pregnant after mating. The duration of
gestation, number of fetuses, and number of offspring were not
significantly different from those of controls. Forty hamsters of each
sex from the first and second litters were divided into four groups
after weaning and force-fed stevioside at the same doses as their
parents. These animals also showed normal growth and fertility.
Histological examination of reproductive tissues from animals of all
three generations revealed no abnormality that could be linked to
treatment. The authors concluded that stevioside at doses up to 2500
mg/kg bw per day affected neither growth nor reproduction in hamsters
(Yodyingyuad & Bunyawong, 1991).
Rats
Groups of 11 male Wistar rats were given stevioside (purity, 96%)
in the diet at 0, 0.15, 0.75, or 3%, equivalent to 0, 150, 750, and
3000 mg/kg bw per day, for 60 days before and during mating, and
groups of 11 female Wistar rats received the same diet for 14 days
before mating and for seven days during gestation. Rats of each sex at
the highest dose had slightly decreased body-weight gain. There was no
treatment-related effect on mating performance or fertility, and no
malformations were seen in the fetuses. The authors concluded that
stevioside had no adverse effect on fertility or on the development of
fetuses (Mori et al., 1981). The Committee noted a slight but not
statistically significant increase in the number of dead or resorbed
fetuses at the highest dose.
A decoction of 5 g dry S. rebaudiana in 100 ml water was given
orally to inbred, adult female rats for 18 days, resulting in an
intake of approximately 40 ml/kg bw. They were mated with untreated
rats during the last six days. Fertility was reduced to 21% of that of
control rats and remained reduced (47%) after a 50-60-day recovery
period (Mazzei-Planas & Kuc, 1968).
The effects of aqueous S. rebaudiana extracts corresponding to
0.67 g dried leaves per ml, given at a dose of 2 ml/rat twice a day
for 60 days, were studied in prepubertal (25-30 days old) rats. The
end-points were glycaemia; serum concentrations of thyroxine and
tri-iodothyronine; available binding sites in thyroid hormone-binding
proteins; binding of 3H-methyltrienolone (a specific ligand of
androgen receptors) to prostate cytosol; zinc content of the prostate,
testis, submandibular salivary gland, and pancreas; water content of
testis and prostate; body-weight gain; and the final weights of the
testis, prostate, seminal vesicle, submandibular salivary gland, and
adrenal. None of these parameters was significantly different from
those in the control group, with the exception of the seminal vesicle
weight, which fell by about 60%. The authors concluded that if the
Stevia extract can decrease fertility in rats, the effect is almost
certainly not exerted on males (Oliveira-Filho et al., 1989).
2.2.6 Developmental toxicity
Rats
Stevioside (purity, 95.6%) dissolved in distilled water was given
to four groups of 25 or 26 pregnant Wistar rats by gavage once a day
on days 6-15 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw.
The rats were sacrificed on day 20 of gestation, and their fetuses
were examined for malformations. The end-points examined were maternal
and fetal body weight, number of live fetuses, sex distribution,
number of resorptions or dead fetuses, and incidence of malformations.
No treatment-related effect on general or reproductive toxicity was
observed up to the highest dose. The authors concluded that orally
administered stevioside is not teratogenic in rats (Takanaka et al.,
1991; Usami et al., 1995).
2.2.7 Studies on metabolites: Steviol
2.2.7.1 Absorption, distribution, and excretion: Steviol
Intact or bile-duct ligated rats were given [17-14C]-steviol
(specific activity, 2.9 µCi/mg, 1.7 µCi/rat, corresponding to
approximately 3 mg/kg bw) either orally or by intracaecal injection.
After oral administration, 1.5% of the radiolabel was excreted in the
urine of intact rats and 96% in that of bile-duct-ligated animals; the
corresponding amounts in faeces were 96 and 3.3%. After intracaecal
administration of 14C-steviol to bile-duct ligated rats, 94 and 6% of
the radiolabel was excreted in urine and faeces, respectively. When
bile was collected over 72 h, all of the intracaecally injected
radiolabel was recovered. Very little (0.02% of dose) was exhaled as
14C-carbon dioxide. The authors concluded that steviol is completely
absorbed from the rat lower bowel (Wingard et al., 1980).
2.2.7.2 Effects on enzymes and other biochemical parameters: Steviol
Steviol administered to female RCR/Ha mice did not induce
glutathione S-transferase activity in liver or intestinal mucosa
(Pezzuto et al., 1986).
Steviol at 0.5 mmol/L, equivalent to 0.16 mg/ml, inhibited
oxidative phosphorylation and the activity of ATPase (92%), NADH
oxidase (45%), succinate oxidase (42%), succinate dehydrogenase (46%),
and L-glutamate dehydrogenase (46%). The ADP:O ratio was decreased.
Substrate respiration was increased at concentrations up to
0.5 mmol/L, equivalent to 0.16 mg/ml, and inhibited at > 1 mmol/L,
equivalent to 0.32 mg/ml. Inhibition of substrate respiration was the
only effect observed in uncoupled mitochondria. Net proton ejection
induced by succinate and swelling induced by several substrates were
inhibited. The authors concluded that steviol acts as a uncoupler of
oxidative phosphorylation (Kelmer-Bracht et al., 1985).
Steviol decreased glucose production and inhibited oxygen uptake
in isolated Wistar rat renal cortical tubules (IC50, 0.3 mmol/L,
equivalent to 96 µg/ml). The authors concluded that this effect is
consistent with an inhibitory action on oxidative phosphorylation and
electron transport in mitochondria (Yamamoto et al., 1985).
Steviol inhibited oxidative phosphorylation in isolated rat liver
mitochondria. The concentration required for 50% inhibition of ATP
synthesis was 40 µmol/L, equivalent to 13 µg/ml. Steviol also
inhibited the 2,4-dinitro-phenol-stimulated ATPase, phosphorylation of
exogenous ADP, and exchange between exogenous 14C-ADP and endogenous
adenine nucleotides. The authors concluded that steviol does not act
at the level of the coupling mechanism but at the level of
mitochondrial translocation of adenine nucleotides (Vignais et al.,
1966).
The effects of steviol (purity, 90%) on intestinal glucose
absorption were examined in hamster jejunum by the everted sac
technique. Thus, 1 mmol/L steviol (equivalent to 318.5 µg/ml)
inhibited glucose absorption by 29-43%, and the inhibition was related
to the steviol concentration and incubation time. Reductions in the
intestinal mucosal ATP content and absorptive surface area were
responsible for the inhibition of glucose absorption. The decrease in
intestinal mucosal ATP content was accompanied by a decrease in the
activities of mitochondrial NADH cytochrome c reductase and cytochrome
oxidase. Steviol did not inhibit the activity of intestinal
Na+/K+-ATPase or glucose uptake in the intestinal brush-border
membrane vesicles. Steviol altered the morphology of the intestinal
absorptive cells. The authors concluded that inhibition of intestinal
glucose absorption by steviol in hamsters is due to a reduction in
mucosal ATP content and alteration of the morphology of the intestinal
absorptive cells (Toskulkao et al., 1995a,b).
Single doses of 200 µmol/L steviol, equivalent to 255 mg/kg bw,
were given orally to 24-h-fasted male Wistar rats, either alone or
simultaneously with fructose. Under these conditions, steviol
increased the initial glycogen deposition in the liver. When steviol
was given to the rats in drinking-water at 1 or 2 mmol/L, equivalent
to 32 and 64 mg/kg bw, at the beginning of a fasting period of 24 or
48 h, it had no effect on hepatic glycogen concentrations (Hübler et
al., 1994).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and steviol strongly
inhibited the induction of Epstein-Barr virus early antigen by TPA,
with 50% inhibition at 25 µg/ml (Okamoto et al., 1983a).
In a study of the effects of steviol at 0.2 µmol/L (equivalent to
64 ng/ml) on the induction of ornithine decarboxylase in mouse skin by
TPA, the activity in the epidermis had increased by about 300-fold
4-5 h after application of 17 nmol/L TPA. TPA-induced ornithine
decarboxylase activity was strongly decreased (63%) when steviol was
applied to mouse skin 1 h before TPA treatment, concurrently with TPA
(75%), or 1 h after TPA (71%). Steviol alone did not induce epidermal
ornithine decarboxylase activity. The authors concluded that steviol
interferes with the process of induction of this enzyme by TPA in
mouse skin (Okamoto et al., 1983b).
2.2.7.3 Acute toxicity: Steviol
In male and female mice and rats given steviol (purity, 90%)
orally, the LD50 was > 15 g/kg bw, and 1/15 animals died within 14
days of administration. The LD50 values in hamsters given steviol
orally were 5.2 g/kg bw in males and 6.1 g/kg bw in females.
Histopathological examination of the kidneys revealed severe
degeneration of the proximal tubular cells, and these structural
alterations were correlated with increased serum blood urea nitrogen
and creatinine. The authors concluded that the cause of death was
acute renal failure (Toskulkao et al., 1997).
2.2.7.4 Genotoxicity: Steviol
Studies of the genotoxicity of steviol are summarized in Table 3.
The major metabolite of steviol in vitro, 15alpha-hydroxysteviol,
was inactive at doses up to 7.5 mg/ml in the forward mutation assay in
S. typhimurium strain TM677 with metabolic activation.
15-Oxosteviol, a product of the oxidation of 15alpha-hydroxysteviol,
was a directly acting mutagen at 25-200 µg/ml and was highly toxic to
bacteria. Moreover, the expression of mutagenicity required the
presence of the 13-hydroxy group and the C-16 exomethylene group
(Compadre et al., 1988).
15-Oxosteviol was not mutagenic in various test systems.
Repetition of the experiment with S. typhimurium TM677 failed to
show significant induction of 8-azaguanine-resistant mutants, even
when the number of bacteria tested was greatly increased. The authors
concluded that the earlier positive result reported was due to a
common mishandling of data obtained in the TM677 system and that
15-oxosteviol is unlikely to be the active metabolite responsible for
the mutagenicity of steviol (Procinska et al., 1991).
Table 3. Results of assays for the genotoxicity of steviol
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98 and TA100 20 mg/platea Negative Suttajit et al. (1993)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platea Negative Matsui et al. (1996a)b
TA102, TA104, TA1535, and TA1537 (purity, 99%)
Forward mutation S. typhimurium TM677 10 mg/platec Negative Matsui et al. (1996a)
0.5-10 mg/plated Positive
Forward mutation S. typhimurium TM677 10 mg/platec Negative Pezzuto et al. (1985a)
10 mg/platee Positive
umu Gene mutation S. typhimurium TA1535/pSK1002 625-1250 µg/platec Positive Matsui et al. (1996a)
1259-2500 µg/plated Positive
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Gene mutation Chinese hamster lung fibroblasts 400 µg/mld Positivef Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 0.5 g/mlc Negative Matsui et al. (1996a)
1-1.5 mg/mld Positive
Chromosomal aberration Human lymphocytes 0.2 mg/ml Negative Suttajit et al. (1993)
Micronucleus formation MS/Ae mice 1000 mg/kg bwg Negative Matsui et al. (1996a)
a With and without metabolic activation
b The same results are cited in an earlier abstract (Matsui et al., 1987).
c Without metabolic activation
d With metabolic activation
e With metabolic activation derived from phenobarbital- or Aroclor 1254-pretreated rats; fractions from control or
3-methylcholanthrene-pretreated rats were ineffective.
f Diphtheria toxin-resistant colonies
g Toxic: 4/6 mice at highest dose given intraperitoneally died
The expression of the mutagenic activity of steviol in
S. typhimurium TM677 was dependent on both metabolic activation
(9000 × g fraction derived from phenobarbital- or Aroclor
1254-pretreated rats) and addition of NADPH. The authors concluded
that a cytochrome P450 mediates the metabolic activation of steviol to
a mutagenic species. As partially purified rat liver epoxide hydrolase
did not inhibit steviol-induced mutagenicity, the authors concluded
that the active metabolite is not an epoxide.
A species structurally related to steviol, isosteviol, was not
active in S. typhimurium TM677, regardless of whether metabolic
activation was provided. Similarly, chemical reduction of the
unsaturated bond linking the carbon-16 and -17 positions of steviol
resulted in the generation of two isomeric products, dihydrosteviol A
and B, which were not mutagenic. Ent-kaurenoic acid was also inactive.
A potential metabolite of steviol, steviol-16alpha,17-epoxide, was
synthesized chemically and found to be ineffective as a directly
acting mutagen. The authors concluded that it is a metabolite of an
integral component of stevioside that is mutagenic. The structural
features necessary for the expression of mutagenic activity include a
hydroxy group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 (Pezzuto et al., 1985a, 1986).
Steviol was mutagenic after metabolic activation in the forward
mutation assay with S. typhimurium TM677. The authors confirmed
first that the 8-aza-guanine resistance of the TM677 mutants resides
in the chromosomal guanine phosphoribosyltransferase (gpt) gene,
since it could be complemented by the gpt gene of Escherichia
coli. The chromosomal DNA of TM677 and TM677 mutants was digested by
several restriction enzymes (BamHI, Sau3AI, AluI, TaqI, HaeIII, HpaII,
and RsaI) and analysed by Southern blot hybridization with a probe for
the gpt gene in DNA of E. coli. No significant difference in DNA
fragment length was found between the wild type and spontaneous or
steviol-induced mutants (Matsui et al., 1988, 1989a).
pSV2-gpt plasmids were treated with metabolically activated
steviol (concentration not given), and the DNA was subsequently
analysed by polyacrylamide gel electrophoresis after digestion with
restriction endonucleases (Sau3AI, HhaI, HpaII). Steviol induced a
fivefold increase in mutation frequency, and seven mutants were
obtained, all showing deletions ranging from 20 bp to 2 kb (Matsui et
al., 1989b).
Steviol strongly induced mutations at the gpt gene of S.
typhimurium TM677 when a metabolic activation system was present,
but it had no activity in reverse mutation assays with E. coli
WP2uvrA/pKM101 or S. typhimurium TA strains. In order to
characterize the mutations induced by metabolically activated steviol,
the chromosomal gpt alleles of 24 induced (ST clones) and 16
spontaneous mutants (SP clones) of S. typhimurium TM677 were
sequenced, and the mutation spectra were compared. Nine out of 24 of
the mutations of ST clones were localized in the region between
nucleotides 280 and 330 from the starting codon ATG, whereas no
mutations of SP clones were found in that region. The mutations
identified included transitions (three clones), transversions (four
clones), a duplication, and a deletion. There were no other marked
differences between the ST and SP clones: base-change mutations
predominated over frameshifts and deletions (ST clones, 20 versus
three; SP clones, 16 versus two), and base-change mutations occurred
more frequently at G:C pairs than at A:T pairs (ST clones, 15 versus
five; SP clones, 12 versus four). The authors suggested that
metabolically activated steviol interrupts DNA synthesis around
nucleotide 280, thereby stimulating duplication, deletion, and
untargeted mutagenesis in the defined region of the gpt gene
downstream from the site of interruption (Matsui et al., 1996b).
19- O-ß-D-Glucopyranosyl steviol, a potential hydrolysis product
of stevioside, was mutagenic to S. typhimurium TM677 and
bactericidal in the presence of a metabolic activating system (Pezzuto
et al., 1986).
Microsomes derived from human liver mediated a mutagenic response
of steviol (Pezzuto et al., 1985b).
2.2.7.5 Developmental toxicity: Steviol
Groups of 20 pregnant golden hamsters were given steviol (purity,
90%) at doses of 0, 250, 500, 750, or 1000 mg/kg bw per day (only 12
animals at the highest dose) by gavage in corn oil on days 6-10 of
gestation. A significant decrease in body-weight gain and increased
mortality (1/20, 7/20, and 5/12) were observed at the three highest
doses, and the number of live fetuses per litter and mean fetal weight
decreased in parallel. Histopathological examination of the maternal
kidneys showed a dose-dependent increase in the severity of effects on
the convoluted tubules (dilatation, hyaline droplets). No
dose-dependent teratogenic effects were seen. The NOEL was 250 mg/kg
bw per day for both maternal and developmental toxicity (Wasuntarawat
et al., 1998).
2.2.8 Special studies
2.2.8.1 Cariogenicity
Groups of 15 albino Sprague-Dawley rat pups colonized with
Streptococcus sobrinus received 0.5% stevioside or 30% sucrose in
the basal diet or basal diet alone and were sacrificed after five
weeks, when S. sobrinus was counted and caries were evaluated. There
was no difference in food or water intake or in weight gain among the
groups, but significant differences in sulcal caries scores and
S. sobrinus counts were found between the group receiving sucrose
and the other groups. There was no significant difference between the
group receiving stevioside and the controls. The authors concluded
that stevioside was not cariogenic under the conditions of this study
(Das et al., 1992).
2.2.8.2 Renal function and vasodilatation
The effect of stevioside on renal function was evaluated by
clearance techniques in groups of seven pentobarbital-anesthetized
male Wistar rats simultaneously with the effect of indomethacin on the
renal action of stevioside, given at a priming dose of 4, 8, 12, or
16 mg/kg bw followed by an infusion rate of 4, 8, 12, or 16 mg/kg bw
per hour. Mean arterial pressure and renal function were measured.
Administration of stevioside resulted in a statistically significant,
dose-related decrease in mean arterial pressure (120 ± 2.3 with 4
mg/kg bw to 72 ± 4.8 mm Hg with 16 mg/kg bw) and an increase in renal
plasma flow (10 ± 1.2 with 4 mg/kg bw to 26 ± 2.9 ml/min per kg bw
with 16 mg/kg bw), with no change in glomerular filtration rate.
Stevioside also increased fractional sodium (FeNa+) and potassium
(FeK+) excretion and urine flow (volume/glomerular filtration rate).
The decrease in mean arterial pressure (control, 120 ± 0.93;
stevioside, 91 ± 2.5 mm Hg) and increase in renal plasma flow
(control, 14 ± 1.4; stevioside, 33 ± 2.8 ml/min per kg bw) induced by
stevioside at 16 mg/kg bw were inhibited by simultaneous
administration of indomethacin at 2 mg/kg bw, but the glomerular
filtration rate was not affected. The diuretic, natriuretic, and
kaliuretic effects of stevioside were also abolished by indomethacin.
The authors concluded that stevioside behaves like a typical
vasodilator, causing changes in mean arterial pressure, diuresis,
natriuresis, and kaliuresis per millilitre of glomerular filtration,
and that these effects probably depend on prostaglandins (Melis &
Sainati, 1991a).
The effects of intravenous administration of verapamil (0.015
mg/min) and calcium chloride (800 mEq/L, 0.025 ml/kg bw per min) on
renal function and mean arterial pressure were evaluated in groups of
10 pentobarbital-anaesthetized male Wistar rats weighing 280-320 g
during intravenous treatment with stevioside (16 mg/kg bw per min).
Verapamil significantly increased the hypotensive effect of stevioside
on mean arterial pressure (control, 120 ± 0.77; stevioside, 96 ± 1.5;
stevioside plus verapamil, 67 ± 0.70 mm Hg) and on fractional sodium
excretion (control, 0.76 ± 0.05; stevioside, 1.6 ± 0.10; stevioside
plus verapamil, 2.7 ± 0.25%). Urinary flow, reported as percent
glomerular filtration rate, and renal plasma flow were increased
slightly but not significantly during administration of stevioside
plus verapamil. In contrast, infusion of calcium chloride into rats
pretreated with stevioside resulted in a marked attenuation of mean
arterial pressure (control, 120 ± 1.8; stevioside, 70 ± 1.1;
stevioside plus calcium chloride, 110 ± 1.6 mm Hg) and renal plasma
flow (control, 17 ± 3.8; stevioside, 34 ± 2.6; stevioside plus calcium
chloride, 17 ± 2.9 ml/min per kg bw). The diuresis and natriuresis
induced by stevioside were also inhibited by simultaneous
administration of calcium chloride. The authors concluded that
stevioside acts on arterial pressure and renal function as a calcium
antagonist, as does verapamil (Melis, 1992b).
Classical clearance techniques and arterial pressure measurements
in pentobarbital-anaesthetized male Wistar rats showed that stevioside
at a priming dose of 8 or 16 mg/kg bw followed by an infusion rate of
8 or 16 mg/kg bw per h caused a fall in systemic blood pressure and in
diuresis and natriuresis per millilitre of glomerular filtration rate.
Verapamil tended to increase the renal and systemic effects of
stevioside. In contrast, an infusion of calcium chloride into rats
pretreated with stevioside induced marked attenuation of the
vasodilatatory responses to stevioside. The authors concluded that
stevioside, like verapamil, acts as a calcium antagonist (Melis &
Sainati, 1991b).
The effect of stevioside (purity, > 90%) at a priming dose of
16 mg/kg bw followed by an infusion rate of 16 mg/kg bw per h on renal
function in normal Wistar rats and rats with experimental renal
hypertension was evaluated by clearance techniques. Stevioside
provoked hypotension, diuresis, and natriuresis in both groups of
rats. The normal rats had increased renal plasma flow and a constant
glomerular filtration rate after stevioside administration, whereas
the hypertensive rats had increased renal plasma flow and glomerular
filtration rate. The authors concluded that stevioside impairs a renal
autoregulation mechanism in this model (Melis, 1992c).
The effects of administration of aqueous S. rebaudiana extracts
corresponding to 0.67 g/ml dried leaves given at 2 ml/rat twice a day
for 20, 40, or 60 days on renal function and mean arterial pressure
were studied in normal Wistar rats weighing 80-100 g. Rats treated for
20 days showed no significant difference from the controls, but
administration of the crude extract for 40 or 60 days induced
hypotension, diuresis, and natriuresis, with a constant glomerular
filtration rate. Increased renal plasma flow was seen only in the
group treated for 60 days. The authors concluded that oral
administration of an aqueous extract of dried leaves of Stevia to
rats induces systemic and renal vasodilation, causing hypotension,
diuresis, and natriuresis (Melis, 1995).
Normotensive and experimentally hypertensive male Wistar rats
(Goldblatt GII experimental hypertension induced by clipping the left
renal artery, leaving the contralateral kidney intact) received an
S. rebaudiana extract corresponding to 0.67 g/ml dried leaves given
at 2 ml/rat by gavage twice a day (2.7 g dry leaves per day) for 30
days. Administration of Stevia 10-12 weeks after clipping resulted in
a significant decrease in mean arterial pressure in both the
normotensive and hypertensive rats: normotensive, 110 ± 3.0 mm Hg in
controls versus 70 ± 4.0 mm Hg in those given Stevia; hypertensive,
160 ± 3.0 mm Hg in controls versus 110 ± 4.0 mm Hg with Stevia. The
glomerular filtration rate was constant in the normotensive rats but
increased significantly in the hypertensive rats after Stevia
treatment (16 ± 1.3 versus 14 ± 1.3 ml/min per kg bw in the controls
and Stevia groups, respectively). Both normotensive and hypertensive
rats had increased renal plasma flow after administration of Stevia:
normotensive, 16 ± 3.1 ml/min per kg bw in controls versus 33 ± 3.2
ml/min per kg bw in the Stevia group; hypertensive, 19 ± 2.5 ml/min
per kg bw in controls versus 37 ± 3.9 ml/min per kg bw in the
Stevia group. Stevia increased urinary flow in both normotensive
(1.4 ± 0.08% versus 2.3 ± 0.11%) and hypertensive animals (1.5 ± 0.07%
versus 3.0 ± 0.13%) and also increased sodium excretion
i(normotensive, 0.61 ± 0.07% in controls versus 1.6 ± 0.2% in the
Stevia group; hypertensive, 0.70 ± 0.1% in controls versus 2.2 ±
0.45% in the Stevia group). The authors concluded that Stevia
impairs renal autoregulation in this model (Melis, 1996).
2.3 Observations in humans
S. rebaudiana has been used by Indians in Paraguay as an oral
contraceptive (Mazzei-Planas & Kuc, 1968; Schvartzman et al., 1977).
Aqueous extracts of 5 g of S. rebaudiana leaves were
administered to 16 volunteers at 6-h intervals for three days, and
glucose tolerance tests were performed before and after
administration. Another six volunteers were given an aqueous solution
of arabinose in order to eliminate possible effects of stress. The
extract increased glucose tolerance and significantly decreased plasma
glucose concentrations during the test and after overnight fasting in
all volunteers (Curi et al., 1986).
3. COMMENTS
After oral administration to rats, stevioside is not readily
absorbed from the upper small intestine but is hydrolysed to the
aglycone, steviol, before absorption from the gut. Steviol per se is
completely absorbed and is excreted in the bile as conjugates; only a
very small fraction is detectable in urine. After biliary excretion,
the conjugates are hydrolysed, and steviol undergoes enterohepatic
circulation; its elimination half-life is 24 h. Steviol is the only
faecal metabolite of stevioside that has been identified, and
excretion in the faeces is the major route. After intravenous
administration, stevioside is rapidly distributed throughout the body,
partially secreted by the renal tubular epithelium, and excreted in
urine.
At high concentrations, stevioside affected a variety of
biochemical parameters in rat tissues in vitro. It weakly inhibited
oxidative phosphorylation, and steviol was about 30 times more potent
in this respect. The most likely mechanism is inhibition of the
mitochondrial translocation of adenine nucleotides. Steviol also
inhibited glucose absorption from rat gut by reducing the mucosal ATP
content. Stevioside may also act as a calcium antagonist, showing a
hypotensive effect and inducing diuresis, natriuresis, and a fall in
renal tubular reabsorption of glucose. Stevioside may not, however, be
able to penetrate cell membranes. Although most of these studies were
performed after intravenous injection of stevioside, orally
administered extracts of S. rebaudiana to rats had similar effects
(hypotension and diuresis).
Stevioside has very low acute oral toxicity. Oral administration
of stevioside at a dietary concentration of 2.5% to rats for two
years, equal to 970 and 1100 mg/kg bw per day in males and females,
respectively, had no significant effect. Reduced body-weight gain and
survival rate were observed at a dietary concentration of 5%
stevioside. There was no indication of carcinogenic potential in a
long-term study and no evidence of urinary bladder tumour promoting
potential in a separate bioassay.
In studies of reproductive toxicity, administration of stevioside
at doses up to 2500 mg/kg bw per day to hamsters and 3000 mg/kg bw per
day to rats had no effect. Although an aqueous infusion of
S. rebaudiana administered orally to female rats was reported to
cause a severe, long-lasting reduction in fertility, the contraceptive
effect of Stevia is probably not due to stevioside. Stevioside had
neither teratogenic nor embryotoxic effects in rats given up to 1000
mg/kg bw per day by gavage.
The results of tests for genotoxicity with stevioside in various
systems were uniformly negative.
The aglycone, steviol, was more acutely toxic than stevioside to
hamsters but not to rats. Steviol was clearly genotoxic after
metabolic activation, inducing forward mutations in bacteria and gene
mutations and chromosomal aberrations in Chinese hamster lung
fibroblasts. Several mechanistic studies indicated that the structural
features necessary for the expression of mutagenic activity include a
hydroxyl group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 of steviol. The fact that
stevioside is glycosylated at position 13 could explain the absence of
mutagenicity. The active metabolite of steviol responsible for its
mutagenic activity is not known. While some data suggest that
epoxidation may be involved in the metabolic activation of steviol,
other data indicate that the active metabolite is not an epoxide.
Preliminary data indicate that human liver microsomes may activate
steviol to a mutagenic metabolite.
4. EVALUATION
The Committee noted several shortcomings in the information
available on stevioside. In some studies, the material tested
(stevioside or steviol) was poorly specified or of variable quality,
and no information was available on other constituents or
contaminants. Furthermore, no studies of human metabolism of
stevioside and steviol were available. In addition, data on long-term
toxicity and carcinogenicity were available for stevioside in only one
species. The mutagenic potential of steviol has been tested
sufficiently only in vitro.
In view of the fact that no information was provided for
elaboration of specifications for stevioside and that the evaluation
of the available toxicological data revealed several limitations, the
Committee was unable to relate the results of the toxicological
investigations to the article of commerce and could not allocate an
ADI to stevioside.
Before the substance is reviewed again, specifications must be
developed to ensure that the material tested is representative of the
material of commerce, and further information should be made available
on the nature of the substance that was tested, on the human
metabolism of stevioside, and on the activity of steviol in suitable
studies of genotoxicity in vivo .
5. REFERENCES
Aze, Y., Toyoda, K., Imaida, K., Hayashi, S., Imazawa, T., Hayashi, Y.
& Takahashi, M. (1991) Subchronic oral toxicity study of stevioside in
F344 rats. Bull. Natl Inst. Hyg. Sci., 48-54 (in Japanese).
Cardoso, V.N., Barbosa, M.F., Muramoto, E., Mesquita, C.H. & Almeida,
M.A. (1996) Pharmacokinetic studies of 131I-stevioside and its
metabolites. Nucl. Med. Biol., 23, 97-100.
Compadre, C.M., Hussain, R.A., Nanayakkara, N.P., Pezzuto, J.M. &
Kinghorn, A.D. (1988) Mass spectral analysis of some derivatives and
in vitro metabolites of steviol, the aglycone of the natural
sweeteners, stevioside, rebaudioside A, and rubusoside. Biomed.
Environ. Mass Spectrom., 15, 211-222.
Constantin, J., Ishii-Iwamoto, E.L., Ferraresi-Filho, O.,
Kelmer-Bracht, A.M. & Bracht, A. (1991) Sensitivity of ketogenesis and
citric acid cycle to stevioside inhibition of palmitate transport
across the cell membrane. Braz. J. Med. Biol. Res., 24, 767-771.
Curi, R., Alvarez, M., Bazotte, R.B., Botion, L.M., Godoy, J.L. &
Bracht, A. (1986) Effect of Stevia rebaudiana on glucose tolerance
in normal adult humans. Braz.J. Med. Biol. Res., 19, 771-774
(English abstract only).
Das, S., Das, A.K., Murphy, R.A., Punwani, I.C., Nasution, M.P. &
Kinghorn, A.D. (1992) Evaluation of the cariogenic potential of the
intense natural sweeteners stevioside and rebaudioside A. Caries
Res., 26, 363-366.
Hagiwara, A., Fukushima, S., Kitaori, M., Shibata, M. & Ito, N. (1984)
Effects of three sweeteners on rat urinary bladder carcinogenesis
initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann, 75,
763-768 (English abstract only).
Hanson, J.R. & De Oliveira, B.H. (1993) Stevioside and related sweet
diterpenoid glycosides. Nat. Prod. Rep., 10, 301-309.
Hübler, M.O., Bracht, A. & Kelmer-Bracht, A.M. (1994) Influence of
stevioside on hepatic glycogen levels in fasted rats. Res. Commun.
Chem. Pathol. Pharmacol., 84, 111-118.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ishii, E.L. & Bracht, A. (1986) Stevioside, the sweet glycoside of
Stevia rebaudiana, inhibits the action of atractyloside in the
isolated perfused rat liver. Res. Commun. Chem. Pathol. Pharmacol.,
53, 79-91.
Ishii, E.L., Schwab, A.J. & Bracht, A. (1987) Inhibition of
monosaccharide transport in the intact rat liver by stevioside.
Biochem. Pharmacol., 36, 1417-1433.
Ishii-Iwamoto, E.L. & Bracht, A. (1995) Stevioside is not metabolized
in the isolated perfused rat liver. Res. Commun. Mol.
Pathol.Pharmacol., 87, 167-175.
Ito, N., Fukushima, S., Shirai, T., Hagiwara, A. & Imaida, K. (1984)
Drugs, food additives and natural products as promoters in rat urinary
bladder carcinogenesis. In: Börzsönyi, M., Day, N.E., Lapis, K. &
Yamasaki, H., eds, Models, Mechanisms and Etiology of Tumour
Promotion (IARC Scientific Publications No. 56), Lyon, International
Agency for Research on Cancer, pp. 399-407.
Kelmer-Bracht, A.M., Alvarez, M. & Bracht, A. (1985) Effects of
Stevia rebaudiana natural products on rat liver mitochondria.
Biochem. Pharmacol., 34, 873-882.
Kim, K.K., Sawa, Y. & Shibata, H. (1996) Hydroxylation of
ent-kaurenoic acid to steviol in Stevia rebaudiana Bertoni --
Purification and partial characterization of the enzyme. Arch.
Biochem. Biophys., 332, 223-230.
Krejci, M.E. & Koechel, D.A. (1992) Acute effects of
carboxyatractyloside and stevioside, inhibitors of mitochondrial
ADP/ATP translocation, on renal function and ultrastructure in
pentobarbital-anesthetized dogs. Toxicology, 72, 299-313.
Liu, J., Ong, C.P. & Li, S.F.Y. (1997) Subcritical fluid extraction of
Stevia sweeteners from Stevia rebaudiana. J. Chromatogr.Sci., 35,
446-450.
Matsui, M., Matsui, K., Kawasaki, Y., Sawada, M., Nohmi, T., Sofuni,
T., Yoshihira, K., Ishidate, M., Oda, Y., Noguchi, T., Komatsubara, S.
& Kitagawa, Y. (1987) Mutagenicity test on stevioside and its
aglycone, steviol. Mutat. Res., 182, 366.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1988)
Mutagenicity of steviol: An analytical approach using a Southern
blotting system. Mutat. Res., 203, 377.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989a)
Mutagenicity of steviol: An analytical approach using the Southern
blotting system. Bull. Natl Inst. Hyg.Sci., 83-87 (in Japanese).
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989b)
Detection of deletion mutations in pSV2-gpt plasmids induced by
metabolically activated steviol. Mutat. Res., 216, 367-368.
Matsui, M., Matsui, K., Kawasaki, Y., Oda, Y., Noguchi, T., Kitagawa,
Y., Sawada, M., Hayashi, M., Nohmi, T., Yoshihira, K., Ishidate, M. &
Sofuni, T. (1996a) Evaluation of the genotoxicity of stevioside and
steviol using six in vitro and one in vivo mutagenicity assays.
Mutagenesis, 11, 573-579.
Matsui, M., Sofuni, T. & Nohmi, T. (1996b) Regionally-targeted
mutagenesis by metabolically-activated steviol: DNA sequence analysis
of steviol-induced mutants of guanine phosphoribosyltransferase (gpt)
gene of Salmonella typhimurium TM677. Mutagenesis, 11, 565-572.
Mazzei-Planas, G. & Kuc, J. (1968) Contraceptive properties of
Stevia rebaudiana. Science, 162, 1007.
Medon, P.J., Pezzuto, J.M., Hovanec-Brown, J.M., Nanayakkara, N.P.,
Soejarto, D.D., Kamath, S.K. & Kinghorn, A.D. (1982) Safety assessment
of some Stevia rebaudiana sweet principles. Fed. Proc., 41, 1568.
Melis, M.S. (1992a) Renal excretion of stevioside in rats. J. Nat.
Prod., 55, 688-690.
Melis, M.S. (1992b) Influence of calcium on the blood pressure and
renal effects of stevioside. Braz. J. Med.Biol. Res., 25, 943-949.
Melis, M.S. (1992c) Stevioside effect on renal function of normal and
hypertensive rats. J. Ethnopharmacol., 36, 213-217.
Melis, M.S. (1995) Chronic administration of aqueous extract of
Stevia rebaudiana in rats: Renal effects. J. Ethnopharmacol., 47,
129-134.
Melis, M.S. (1996) A crude extract of Stevia rebaudiana increases
the renal plasma flow of normal and hypertensive rats. Braz. J.
Med.Biol. Res., 29, 669-675.
Melis, M.S. & Sainati, A.R. (1991a) Participation of prostaglandins in
the effect of stevioside on rat renal function and arterial pressure.
Braz. J. Med. Biol. Res., 24, 1269-1276.
Melis, M.S. & Sainati, A.R. (1991b) Effect of calcium and verapamil on
renal function of rats during treatment with stevioside. J.
Ethnopharmacol., 33, 257-262.
Mori, N., Sakanoue, M., Takeuchi, M., Shimpo, K. & Tanabe, T. (1981)
Effect of stevioside on fertility in rats. J. Food Hyg. Soc. Jpn.,
22, 409-414 (in Japanese).
Nakayama, K., Kasahara, D. & Yamamoto, F. (1986) Absorption,
distribution, metabolism and excretion of stevioside in rats.
J. Food Hyg. Soc. Jpn., 27, 1-8.
Nanayakkara, N.P., Klocke, J.A., Compadre, C.M., Hussain, R.A.,
Pezzuto, J.M. & Kinghorn, A.D. (1987) Characterization and feeding
deterrent effects on the aphid, Schizaphis graminum, of some
derivatives of the sweet compounds, stevioside and rebaudioside A.
J. Nat. Prod., 50, 434-441.
Okamoto, H., Yoshida, D. & Mizusaki, S. (1983a) Inhibition of
12-O-tetradecanoylphorbol-13-acetate-induced induction in Epstein-Barr
virus early antigen in Raji cells. Cancer Lett., 19, 47-53.
Okamoto, H., Yoshida, D., Saito, Y. & Mizusaki, S. (1983b) Inhibition
of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine
decarboxylase activity in mouse epidermis by sweetening agents and
related compounds. Cancer Lett., 21, 29-35.
Oliveira-Filho, R.M., Uehara, O.A., Minetti, C.A. & Valle, L.B. (1989)
Chronic administration of aqueous extract of Stevia rebaudiana
(Bert.) Bertoni in rats: Endocrine effects. Gen. Pharmacol., 20,
187-191.
Pezzuto, J.M., Compadre, C.M., Swanson, S.M., Nanayakkara, D. &
Kinghorn, A.D. (1985a) Metabolically activated steviol, the aglycone
of stevioside, is mutagenic. Proc. Natl Acad Sci. USA, 82,
2478-2482.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Lam, L.K., Sparnins, V.L. &
Wattenberg, L.W. (1985b) Evaluation of the potential of steviol
(13-hydroxy-ent-kaurenoic acid) and related diterpenes to mediate
bacterial mutagenesis and induce glutathione-S-transferase in mice.
Proc. Am. Assoc. Cancer Res., 26, 94.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Sparnins, V.L. & Lam, L.K. (1986)
Characterization of bacterial mutagenicity mediated by
13-hydroxy-ent-kaurenoic acid (steviol) and several
structurally-related derivatives and evaluation of potential to induce
glutathione S-transferase in mice. Mutat. Res., 169, 93-103.
Procinska, E., Bridges, B.A. & Hanson, J.R. (1991) Interpretation of
results with the 8-azaguanine resistance system in Salmonella
typhimurium: No evidence for direct acting mutagenesis by
15-oxosteviol, a possible metabolite of steviol. Mutagenesis, 6,
165-167.
Schvartzman, J.B., Krimer, D.B. & Moreno-Azorero, R. (1977)
Cytological effects of some medicinal plants used in the control of
fertility. Experientia, 33, 663-665.
Soejarto, D.D., Kinghorn, A.D. & Farnsworth, N.R. (1982) Potential
sweetening agents of plant origin. III. Organoleptic evaluation of
Stevia leaf herbarium samples for sweetness. J. Nat. Prod., 45,
590-599.
Suanarunsawat, T. & Chaiyabutr, N. (1997) The effect of stevioside on
glucose metabolism in the rat. Can. J. Physiol. Pharmacol., 75,
976-982.
Suttajit, M., Vinitketkaumnuen, U., Meevatee U. & Buddhasukh, D.
(1993) Mutagenicity and human chromosomal effect of stevioside, a
sweetener from Stevia rebaudiana Bertoni. Environ. Health
Perspectives, 101, 53-56.
Takanaka, T., Kawashima, K., Usami, M. & Sakami, K. (1991) A
teratological study of stevioside administered orally to rats.
Unpublished report from Department of Pharmacology, Biological Safety
Research Center, National Institute of Hygienic Sciences, Japan.
Submitted to WHO by Ministry of Health and Welfare, Food Chemistry
Division, Japan.
Toskulkao, C., Sutheerawatananon, M., Wanichanon, C., Saitongdee, P. &
Suttajit, M. (1995a) Effects of stevioside and steviol on intestinal
glucose absorption in hamsters. J. Nutr. Sci. Vitaminol., 41,
105-113.
Toskulkao, C., Sutheerawattananon, M. & Piyachaturawat, P. (1995b)
Inhibitory effect of steviol, a metabolite of stevioside, on glucose
absorption in everted hamster intestine in vitro. Toxicol. Lett.,
80, 153-159.
Toskulkao, C., Chaturat, L., Temcharoen, P. & Glinsukon, T. (1997)
Acute toxicity of stevioside, a natural sweetener, and its metabolite,
steviol, in several animal species. Drug Chem.Toxicol., 20, 31-44.
Toyoda, K., Kawanishi, T., Uneyama, C. & Takahashi, M. (1995)
Re-evaluation of the safety of a food additive (reported in fiscal
1994). A chronic toxicity/carcinogenicity study of stevioside (a
substance extracted from Stevia): Final report. Unpublished report
from Division of Pathology, Biological Safety Research Center,
National Institute of Health Sciences, Japan. Submitted to WHO by
Ministry of Health and Welfare, Food Chemistry Division, Japan.
Toyoda, K., Matsui, H., Shoda,T., Uneyama, C., Takada, K. & Takahashi,
M. (1997) Assessment of the carcinogenicity of stevioside in F344
rats. Food Chem. Toxicol., 35, 597-603.
Usami, M., Seino, Y., Takai, J., Nakahara, H., Seino, S., Ikeda, M. &
Imura, H. (1980) Effect of cyclamate sodium, saccharin sodium and
stevioside on arginine-induced insulin and glucagon secretion in the
isolated perfused rat pancreas. Horm. Metab. Res., 12, 705-706.
Usami, M., Sakemi, K., Kawashima, K., Tsuda, M. & Ohno, Y. (1995)
Teratogenicity study of stevioside in rats. Bull. Natl Inst. Hyg.
Sci., 113, 31-35 (in Japanese).
Vignais, P.V., Duee, E.D., Vignais, P.M. & Huet, J. (1966) Effects of
atractyligenin and its structural analogues on oxidative
phosphorylation and on the translocation of adenine nucleotides in
mitochondria. Biochim. Biophys. Acta, 118, 465-483.
Wasuntarawat, C., Temcharoen, P., Toskulkao, C., Mungkornkarn, P.,
Suttajit, M. & Glinsukon, T. (1998) Developmental toxicity of steviol,
a metabilite of stevioside, in the hamster. Drug Chem.Toxicol., 21,
207-222.
Wingard, R.E., Brown, J.P., Enderlin, F.E., Dale, J.A., Hale, R.L. &
Seitz, C.T. (1980) Intestinal degradation and absorption of the
glycosidic sweeteners stevioside and rebaudioside A. Experientia,
36, 519-520.
Xili, L., Chengjiany, B., Eryi, X., Reiming, S., Yuengming, W.,
Haodong, S. & Zhiyian, H. (1992) Chronic oral toxicity and
carcinogenicity study of stevioside in rats. Food Chem. Toxicol.,
30, 957-965.
Yamamoto, N.S., Kelmer-Bracht, A.M., Ishii, E.L., Kemmelmeier, F.S.,
Alvarez, M. & Bracht, A. (1985) Effect of steviol and its structural
analogues on glucose production and oxygen uptake in rat renal
tubules. Experientia, 41, 55-57.
Yodyingyuad, V. & Bunyawong, S. (1991) Effect of stevioside on growth
and reproduction. Hum. Reprod., 6, 158-165.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
3H-Stevioside (specific activity, 13 or 46 µCi/mg), administered
by gavage to groups of three to seven Wistar rats at a dose of 125
mg/kg bw (10-120 µCi/kg bw), was absorbed slowly, a maximal blood
concentration of 4.8 µg/ml being reached by 8 h. At 4 h, the highest
concentration was found in the caecum (280 µg/g stevioside
equivalent). At 24 h, the concentrations of radiolabel were low in
most organs, including blood, corresponding to about 2 µg/g or ml
stevioside equivalent, except in liver (5.7 µg/g), adrenal gland
(12 µg/g), small intestine (8.8 µg/g), caecum (40 µg/g), large
intestine (12 µg/g), and fat (12 µg/g). The elimination half-life was
24 h. At 48 h, 31% of the radiolabel remained in the body. By five
days, 68% had been excreted into the faeces, 24% in expired air, and
2.3% in the urine. In bile-duct cannulated rats, biliary excretion was
low up to 24 h, increasing rapidly thereafter to reach 41% of the dose
after three days. The authors concluded that stevioside is absorbed
from the gut very slowly, that enterohepatic circulation occurs in
rats, and that faecal excretion is the major route of elimination
(Nakayama et al., 1986).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The radiolabel in plasma decreased
rapidly, showing rapid distribution in the body. The highest
concentrations of radiolabel 10 and 120 min after injection were
observed in the liver (45 and 5% of the injected dose, respectively)
and the small intestine (18 and 66%). At 120 min after injection, the
radiolabel eliminated in the bile represented 52% of the original
dose; that excreted in the faeces and urine 24 h after injection
represented 35 and 35%, respectively, of the original dose (Cardoso et
al., 1996). The Committee considered that this study was of limited
value since introduction of a large 131I atom into stevioside might
significantly affect its absorption, distribution, metabolism, and
excretion in bile or urine.
The renal excretion of stevioside and its effect on the renal
excretion of several other substances was studied in groups of 10 male
Wistar rats, which received intravenous infusions of stevioside at
doses of 4, 8, 12, or 16 mg/kg bw per hour for 30 min, after a control
period of 30 min. No significant change in inulin clearance was
observed, but there was a significant increase in para-aminohippuric
acid clearance, fractional sodium excretion (FeNa+), urinary flow as
percent of glomerular filtration rate, and glucose clearance when
compared with controls at doses greater than 4 mg/kg bw per hour.
Stevioside clearance was greater than inulin clearance and smaller
than para-amino-hippuric acid clearance at all doses tested. The
authors concluded that stevioside is secreted by the renal tubular
epithelium and induces diuresis and natriuresis and a fall in renal
tubular reabsorption of glucose (Melis, 1992a).
2.1.2 Biotransformation
Thin-layer chromatography of the intestinal contents, faeces, and
bile of groups of three to seven Wistar rats given 3H-stevioside
(specific activity, 13 or 46 µCi/mg) by gavage at a dose of 125 mg/kg
bw (10-120 µCi/kg bw) revealed that stevioside is decomposed by rat
caecal flora to steviol and sugars. Stevioside was detected as the
major component in the stomach 1 h after administration. After 4 h in
the small intestine, stevioside, steviolbioside (produced by cleavage
of the glucose moiety at the C-19 position), and steviol accounted for
7.6, 8, and 7.5% of the radiolabel; in the caecum, these compounds
accounted for 39, 17, and 5.1% of the radiolabel, respectively. At 24
h, stevioside was not detectable in the caecum, but steviol and an
unidentified metabolite accounted for 16 and 68% of the radiolabel,
respectively. Steviol was found to be the major metabolite in faeces,
whereas stevioside and the unidentified metabolite were not
quantifiable. In bile, most of the radiolabel found up to 24 h was on
the unidentified metabolite, which was probably a steviol conjugate.
The authors concluded that orally administered stevioside is not
readily absorbed from the upper part of the small intestine, but
metabolites, formed primarily by the bacterial flora in the caecum,
are absorbed from the lower part of the intestine; they also concluded
that most of the stevioside is excreted as steviol in the faeces
(Nakayama et al., 1986). The Committee concluded further that the
faecal steviol could also have arisen from deconjugation of biliary
conjugates by the gut flora.
When 2.5 mg/ml stevioside were incubated under anaerobic
conditions with whole-cell suspensions of bacteria from rat caecum,
stevioside was completely degraded to steviol within two days. The
authors concluded that similar degradation of stevioside occurs in
humans (Wingard et al., 1980).
Mass spectral analysis of steviol and some analogues revealed
characteristic patterns reflecting differential stereochemistry and
variations in the nature of the substituents present. This information
was used to identify several metabolites of steviol (by gas
chromatography-mass spectrometry) which are known to produce a
mutagenic response in Salmonella typhimurium strain TM677 with
metabolic activation. After incubation with a 9000 × g fraction
derived from the livers of Aroclor 1254-pretreated rats, unchanged
steviol was the predominant compound, and nine metabolites were found.
The major pathways of mammalian metabolism of steviol proved to be
allylic oxidation and epoxidation. 15alpha-Hydroxysteviol represented
67% of the metabolites of steviol in vitro (Compadre et al., 1988).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The results of reverse-phase
high-performance liquid chromato-graphy (RP-HPLC) of the bile showed
that stevioside was degraded in vivo and that steviol was the major
metabolite (47% of radiolabel); 37% of the radiolabel was on
stevioside, and the remaining 15% was on an unidentified metabolite.
RP-HPLC analysis of urine 90 min after injection showed the presence
of stevioside and the same unidentified metabolite found in bile, but
no steviol. The authors concluded that stevioside is metabolized in
rat liver to steviol, which is excreted through the bile, and that
similar degradation occurs in humans (Cardoso et al., 1996). The
Committee concluded that there is an alternative explanation, namely
that stevioside is secreted into the bile, is degraded to steviol by
the gut flora, and is resorbed in the lower gut. The Committee
considered that this study was of limited value since introduction of
a large 131I atom into stevioside might significantly affect its
absorption, distribution, metabolism, and excretion in bile or urine.
Stevioside was perfused at a concentration of 0.2 or 0.5 mmol/L
(equivalent to 0.16 and 0.4 mg/ml) into rat livers and was
recirculated for 2 h. The concentration of stevioside remained
constant throughout the perfusion. The formation of hydrolysis
products, especially steviol, was investigated chromatographically,
with negative results. The authors concluded that the reported
metabolic transformation of intravenously injected 131I-stevioside is
either a specific characteristic of this derivative or depends on
factors that are absent in the isolated perfused rat liver
(Ishii-Iwamoto & Bracht, 1995). The Committee concluded that the most
likely explanation for the apparent discrepancy is the fact that
introduction of the large 131I atom into stevioside altered its
pharmacokinetic behaviour and that stevioside is secreted into the
bile in vivo and is degraded to steviol by the gut flora.
2.1.3 Effects on enzymes and other biochemical parameters
Stevioside given to female RCR/Ha mice did not induce glutathione
S-transferase activity in liver or intestinal mucosa (Pezzuto et
al., 1986).
Stevioside (1 mmol/L, equivalent to 0.8 mg/ml) inhibited
oxidative phosphorylation and the activity of ATPase (50% inhibition),
succinate oxidase (8% inhibition), and succinate dehydrogenase (10%
inhibition). No inhibition of NADH-oxidase or L-glutamate
dehydrogenase activity was seen. The ADP:O ratio was slightly
decreased. Substrate respiration was increased at low concentrations
(up to 0.5 mmol/L, equivalent to 0.4 mg/ml) and inhibited at higher
concentrations (1 mmol/L, equivalent to > 0.8 mg/ml). The authors
concluded that stevioside acts as a weak uncoupler of oxidative
phosphorylation (Kelmer-Bracht et al., 1985).
Stevioside inhibited oxidative phosphorylation in isolated rat
liver mitochondria. The concentration required for 50% inhibition of
ATP synthesis was 1.2 mmol/L, equivalent to 0.97 mg/ml (Vignais et
al., 1966).
The effect of stevioside, an inhibitor of long-chain fatty acid
transport, on ketogenesis and on 14C-carbon dioxide production from
[1-14C]-palmitate (100-300 µmol/L) was investigated in isolated and
haemoglobin-free perfused rat liver. Stevioside (2.5 mmol/L,
equivalent to 2 mg/ml) inhibited both parameters but had a smaller
effect on 14C-carbon dioxide production. At 300 µmol/L palmitate and
150 µmol/L albumin, ketogenesis was inhibited by 66%, whereas no
significant inhibition of 14C-carbon dioxide was seen. The authors
concluded that these results reflect different degrees of saturation
of the citric acid cycle and the ketogenic pathway and that changes in
the redox state of the mitochondrial NAD(+)-NADH complex occur after
infusion of stevioside (Constantin et al., 1991).
The Committee noted that the concentrations used in the studies
conducted in vitro were very high relative to those achieved in
blood after oral administration, when the major intestinal metabolite
that enters the circulation is steviol. These studies may therefore be
of limited significance.
When single doses of 200 µmol/L stevioside, equivalent to
650 mg/kg bw, were given orally to 24-h-fasted male Wistar rats,
either alone or simultaneously with fructose, stevioside increased the
initial glycogen deposition in the liver. When it was given to the
rats in the drinking-water at 1 or 2 mmol/L, equivalent to 81 and 160
mg/kg bw, at the beginning of a fasting period of 24 or 48 h,
increased hepatic glycogen concentrations were found at 48 h
(1 mmol/L) and at 24 h (2 mmol/L). The authors concluded that
stevioside stimulates hepatic glycogen synthesis under gluconeogenic
conditions (Hübler et al., 1994).
The effect of stevioside on the transport and metabolism of
D-glucose and D-fructose was investigated in isolated perfused rat
liver. The maximal exchange rate of D-glucose was 700 µmol/L per
min/ml, and the Km was 38 mmol/L. Stevioside inhibited D-glucose and
D-fructose transport across the cell membrane. The half-maximal effect
at 1 mmol/L D-glucose occurred at 0.8 mmol/L stevioside, equivalent to
0.65 mg/ml. Stevioside had no effect on D-glucose metabolism, except
to cause transient changes in D-glucose release, which reflected
changes in the intracellular concentration. D-Fructose consumption,
however, was specifically affected (half-maximal effect at 2.8 mmol/L,
equivalent to 2.3 mg/ml), as were all parameters that depend on
D-fructose transformation: D-glucose production, L-lactate and
pyruvate production, and extra oxygen uptake. In livers that released
D-glucose from endogenous glycogen, strong inhibition of transport
increased the intracellular:extracellular ratio of D-glucose
concentration. The control values for this ratio, representing an
average over the total intracellular water space, were all below unity
(Ishii et al., 1987).
Stevioside had no effect on gluconeogenesis or oxygen uptake in
isolated Wistar rat renal cortical tubules at concentrations up to
3 mmol/L, equivalent to 2.4 mg/ml. The authors concluded that the lack
of activity was due to the inability of stevioside to penetrate the
cell membrane (Yamamoto et al., 1985).
Intravenous infusion of stevioside at 150 mg/ml to male Wistar
rats at a dose of 100, 150, or 200 mg/kg bw per hour raised the plasma
glucose concentrations to 110, 140, and 130% of the control value
during and after infusion. The glucose turnover rate was not altered,
but glucose clearance was reduced by infusion of 200 mg/kg bw per hour
stevioside, from 6.5 to 5 ml/min per kg bw. The plasma insulin
concentration was unchanged. Pretreatment with angiotensin II and
arginine vasopressin had no effect, while prazosin, an
alpha-adrenergic blocker, attenuated the hyperglycaemic effect of
stevioside and infused insulin inhibited it. Oral administration of
stevioside at 2000 mg/kg bw had no effect on the plasma glucose
concentration. The authors concluded that the hyperglycaemic effect of
stevioside was due to an effect on glucose transport across the cell
(Suanarunsawat & Chaiyabutr, 1997).
The effects of stevioside at 1 and 5 mmol/L, equivalent to 0.8
and 4 mg/ml, on intestinal glucose absorption were examined in hamster
jejunum by the everted sac technique. Glucose absorption was not
inhibited (Toskulkao et al., 1995a,b).
Infusion of stevioside at 15 µmol/L, equivalent to 12 µg/ml, for
20 min did not significantly alter the arginine-induced secretion of
insulin or glucagon in the pancreas of male Wistar rats (Usami et al.,
1980).
Stevioside inhibited the action of atractyloside, a known
inhibitor of the adenine nucleotide carrier of mitochondria and in
consequence an inhibitor of energy metabolism, in isolated perfused
rat liver. It decreased the effects of atractyloside on glycolysis,
glycogenolysis, gluconeogenesis, and oxygen uptake. The concentration
for half-maximal action of stevioside was 0.5 mmol/L, equivalent to
0.4 mg/ml. The authors concluded that it acts on the outside of the
cell, as labelled stevioside did not penetrate the cell membranes
(Ishii & Bracht, 1986).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and stevioside did not
inhibit the induction of Epstein-Barr virus early antigen by TPA at
the highest concentration tested: 50 µg/ml (18% inhibition) (Okamoto
et al., 1983a).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the the toxicity of stevioside given as single doses
to rodents are summarized in Table 1. No lethality was seen within 14
days after administration, and no clinical signs of toxicity or
morphological or histopathological changes were found.
After intravenous administration of stevioside to
pentobarbital-anaesthetized dogs at a dose of 32.5 µmol/L per kg bw
(equivalent to 26 mg/kg bw), no significant changes were seen in any
parameters of whole blood, plasma, or renal function, and there was no
significant alteration of the renal ultrastructure. The authors
concluded that stevioside is totally devoid of acute extrarenal
effects (such as hypoxaemia, which could contribute to nephrotoxicity)
and direct renal effects during the 6-h period following intravenous
administration (Krejci & Koechel, 1992).
Table 1. Acute toxicity of stevioside (purity, 96%) given orally to
rodents
Species Sex LD50 (g/kg bw) Reference
Mouse Male and female > 15 Toskulkao et al. (1997)
Mouse Male > 2 Medon et al. (1982)
Rat Male and female > 15 Toskulkao et al. (1997)
Hamster Male and female > 15 Toskulkao et al. (1997)
2.2.2 Short-term studies of toxicity
Rats
A 13-week study of toxicity was carried out in Fischer 344 rats
given doses of 0, 0.31, 0.62, 1.25, 2.5, or 5% in the diet (equivalent
to 160, 310, 630, 1300, and 2500 mg/kg bw per day) to determine the
appropriate doses for a two-year study of carcinogenicity. The rats
were randomly allocated to six groups, each consisting of 10 males and
10 females. None of the animals died during the administration period,
and there was no difference in body-weight gain between the control
and treated groups during administration or in food consumption in the
later part of the study. The activity of lactic dehydrogenase and the
incidence of single-cell necrosis in the liver were increased in all
groups of treated males. The authors considered these effects to be
nonspecific because of the lack of a clear dose-response relationship,
the relatively low severity, and their limitation to males. Other
statistically significant differences in haematological and
biochemical parameters were also considered to be of minor
toxicological significance. The authors concluded that a concentration
of 5% in diet was a suitable maximum tolerable dose of stevioside for
a two-year study in rats (Aze et al., 1991).
2.2.3 Long-term studies of toxicity and carcinogenicity
Rats
Groups of 45 male and 45 female inbred Wistar rats were given
diets containing stevioside (purity, 85%) at 0, 0.2, 0.6, or 1.2%
(equivalent to 100, 300, and 600 mg/kg bw per day) for two years.
After 6, 12, and 24 months, blood was obtained from the tail vein of
five male and five female rats in each dose group for haematological
and clinical biochemical tests. One week later, these rats were housed
in metabolism cages for urine collection and were then killed for
further biochemical, pathological, and histopathological examination.
All surviving animals were killed at two years. Growth, food use and
consumption, general appearance, and mortality were similar in treated
and control groups. The mean life span of rats given stevioside was
not significantly different from that of the controls. No
treatment-related changes were observed in haematological, urinary, or
clinical biochemical values at any stage of the study. The incidence
and severity of non-neoplastic and neoplastic changes were unrelated
to the concentration of stevioside in the diet. The NOEL was 1.2%,
equivalent to 600 mg/kg bw per day. The authors suggested that the
acceptable daily intake of stevioside for humans was 7.9 mg/kg bw per
day, on the basis of the stevioside consumption of the rats during the
first three months (the average for males and females being 790 mg/kg
bw per day) and a safety factor of 100 (Xili et al., 1992).
Stevioside (purity, 95.6%) was added to powdered diet at
concentrations of 0, 2.5, or 5% (equal to 0, 970, and 2000 mg/kg bw
per day for males and 0, 1100, and 2400 for females) and pelleted
every three months. The doses were selected on the basis of the
results of the 13-week study and administered to groups of 50 male and
50 female Fischer 344/DuCrj rats ad libitum for 104 weeks.
Thereafter, all of the groups were maintained on basal diet for four
weeks. All surviving rats were killed at week 108. The body-weight
gain of treated animals was slightly depressed, and a relationship was
seen with the dose of stevioside: 2.3 and 4.4% in males at the low and
high dose and 2.4 and 9.2% in females at the low and high dose. Food
consumption did not differ between the groups. The final survival rate
of males at 5% was significantly decreased, with a rate of 60% versus
78% in controls. The absolute kidney weights were decreased in male
and female animals at the high dose; however, there was no significant
histopathological evidence of neoplastic or non-neoplastic lesions
attributable to treatment in any organ or tissue, except for a
decreased incidence of mammary adenomas in females and a reduced
severity of chronic nephropathy in males. The authors concluded that
stevioside is not carcinogenic in Fischer 344 rats under these
experimental conditions (Toyoda et al., 1995, 1997). The Committee
noted that the report of Toyoda et al. (1995) gives data only for
individual animals, with no summary tables or figures.
The effects of stevioside on urinary bladder carcinogenesis
initiated by N-nitrosobutyl- N-(4-hydroxybutyl)amine was evaluated
in male Fischer 344 rats given 0.01% of the nitrosamine in their
drinking-water for four weeks and then 5% stevioside in their diet,
equivalent to 5000 mg/kg bw per day, for 32 weeks. All surviving rats
were sacrificed after 36 weeks and examined histologically. Treatment
with 5% stevioside did not affect the incidence or extent of papillary
or nodular hyperplasia in nitrosamine-treated rats. No preneoplastic
or neoplastic lesions of the urinary bladder were observed in rats
treated with stevioside only. The authors concluded that stevioside
does not promote bladder carcinogenesis (Hagiwara et al., 1984; Ito et
al., 1984).
2.2.4 Genotoxicity
Studies of the genotoxicity of stevioside are summarized in
Table 2.
Table 2. Results of assays for the genotoxicity of stevioside
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98, TA100 50 mg/platea Negativeb Suttajit et al. (1993)
(purity, 99%)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platec Negative Matsui et al. (1996a)d
TA102, TA104, TA1535, TA1537 1 mg/platee Negative
(purity, 83%)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Matsui et al. (1996a)
Forward mutation S. typhimurium TM677 Not specifieda Negative Medon et al. (1982)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Pezzuto et al. (1985a)
umu Gene mutation S. typhimurium TA1535/pSK1002 5 mg/platea Negative Matsui et al. (1996a)
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 8 mg/mlc Negative Matsui et al. (1996a)
12 mg/mle
Chromosomal aberration Human lymphocytes 10 mg/ml Negative Suttajit et al. (1993)
Chromosomal aberration Chinese hamster lung fibroblasts 12 mg/mlc Negative Ishidate et al. (1984)
(purity, 85%)
a With and without metabolic activation
b A positive response towards TA98 was seen without metabolic activation at 50 mg/ml but not at lower concentrations
up to 20 mg/ml
c Without metabolic activation
d The same results were cited in an earlier abstract (Matsui et al., 1987).
e With metabolic activation
2.2.5 Reproductive toxicity
Hamsters
Groups of 10 male and 10 female one-month-old golden hamsters
(Mesocricetus auratus,) were force-fed with stevioside (purity, 90%)
at 0, 500, 1000, or 2500 mg/kg bw per day daily. Each female was mated
and allowed to bear three litters during the experiment. Females in
late gestation and while lactating (one month) received stevioside in
the drinking-water. Two weeks after the offspring had been weaned, the
females were mated again. No abnormalities were found in the growth or
fertility of animals of either sex. All of the males mated females
efficiently and successfully; the females showed normal four-day
oestrus cycles and became pregnant after mating. The duration of
gestation, number of fetuses, and number of offspring were not
significantly different from those of controls. Forty hamsters of each
sex from the first and second litters were divided into four groups
after weaning and force-fed stevioside at the same doses as their
parents. These animals also showed normal growth and fertility.
Histological examination of reproductive tissues from animals of all
three generations revealed no abnormality that could be linked to
treatment. The authors concluded that stevioside at doses up to 2500
mg/kg bw per day affected neither growth nor reproduction in hamsters
(Yodyingyuad & Bunyawong, 1991).
Rats
Groups of 11 male Wistar rats were given stevioside (purity, 96%)
in the diet at 0, 0.15, 0.75, or 3%, equivalent to 0, 150, 750, and
3000 mg/kg bw per day, for 60 days before and during mating, and
groups of 11 female Wistar rats received the same diet for 14 days
before mating and for seven days during gestation. Rats of each sex at
the highest dose had slightly decreased body-weight gain. There was no
treatment-related effect on mating performance or fertility, and no
malformations were seen in the fetuses. The authors concluded that
stevioside had no adverse effect on fertility or on the development of
fetuses (Mori et al., 1981). The Committee noted a slight but not
statistically significant increase in the number of dead or resorbed
fetuses at the highest dose.
A decoction of 5 g dry S. rebaudiana in 100 ml water was given
orally to inbred, adult female rats for 18 days, resulting in an
intake of approximately 40 ml/kg bw. They were mated with untreated
rats during the last six days. Fertility was reduced to 21% of that of
control rats and remained reduced (47%) after a 50-60-day recovery
period (Mazzei-Planas & Kuc, 1968).
The effects of aqueous S. rebaudiana extracts corresponding to
0.67 g dried leaves per ml, given at a dose of 2 ml/rat twice a day
for 60 days, were studied in prepubertal (25-30 days old) rats. The
end-points were glycaemia; serum concentrations of thyroxine and
tri-iodothyronine; available binding sites in thyroid hormone-binding
proteins; binding of 3H-methyltrienolone (a specific ligand of
androgen receptors) to prostate cytosol; zinc content of the prostate,
testis, submandibular salivary gland, and pancreas; water content of
testis and prostate; body-weight gain; and the final weights of the
testis, prostate, seminal vesicle, submandibular salivary gland, and
adrenal. None of these parameters was significantly different from
those in the control group, with the exception of the seminal vesicle
weight, which fell by about 60%. The authors concluded that if the
Stevia extract can decrease fertility in rats, the effect is almost
certainly not exerted on males (Oliveira-Filho et al., 1989).
2.2.6 Developmental toxicity
Rats
Stevioside (purity, 95.6%) dissolved in distilled water was given
to four groups of 25 or 26 pregnant Wistar rats by gavage once a day
on days 6-15 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw.
The rats were sacrificed on day 20 of gestation, and their fetuses
were examined for malformations. The end-points examined were maternal
and fetal body weight, number of live fetuses, sex distribution,
number of resorptions or dead fetuses, and incidence of malformations.
No treatment-related effect on general or reproductive toxicity was
observed up to the highest dose. The authors concluded that orally
administered stevioside is not teratogenic in rats (Takanaka et al.,
1991; Usami et al., 1995).
2.2.7 Studies on metabolites: Steviol
2.2.7.1 Absorption, distribution, and excretion: Steviol
Intact or bile-duct ligated rats were given [17-14C]-steviol
(specific activity, 2.9 µCi/mg, 1.7 µCi/rat, corresponding to
approximately 3 mg/kg bw) either orally or by intracaecal injection.
After oral administration, 1.5% of the radiolabel was excreted in the
urine of intact rats and 96% in that of bile-duct-ligated animals; the
corresponding amounts in faeces were 96 and 3.3%. After intracaecal
administration of 14C-steviol to bile-duct ligated rats, 94 and 6% of
the radiolabel was excreted in urine and faeces, respectively. When
bile was collected over 72 h, all of the intracaecally injected
radiolabel was recovered. Very little (0.02% of dose) was exhaled as
14C-carbon dioxide. The authors concluded that steviol is completely
absorbed from the rat lower bowel (Wingard et al., 1980).
2.2.7.2 Effects on enzymes and other biochemical parameters: Steviol
Steviol administered to female RCR/Ha mice did not induce
glutathione S-transferase activity in liver or intestinal mucosa
(Pezzuto et al., 1986).
Steviol at 0.5 mmol/L, equivalent to 0.16 mg/ml, inhibited
oxidative phosphorylation and the activity of ATPase (92%), NADH
oxidase (45%), succinate oxidase (42%), succinate dehydrogenase (46%),
and L-glutamate dehydrogenase (46%). The ADP:O ratio was decreased.
Substrate respiration was increased at concentrations up to
0.5 mmol/L, equivalent to 0.16 mg/ml, and inhibited at > 1 mmol/L,
equivalent to 0.32 mg/ml. Inhibition of substrate respiration was the
only effect observed in uncoupled mitochondria. Net proton ejection
induced by succinate and swelling induced by several substrates were
inhibited. The authors concluded that steviol acts as a uncoupler of
oxidative phosphorylation (Kelmer-Bracht et al., 1985).
Steviol decreased glucose production and inhibited oxygen uptake
in isolated Wistar rat renal cortical tubules (IC50, 0.3 mmol/L,
equivalent to 96 µg/ml). The authors concluded that this effect is
consistent with an inhibitory action on oxidative phosphorylation and
electron transport in mitochondria (Yamamoto et al., 1985).
Steviol inhibited oxidative phosphorylation in isolated rat liver
mitochondria. The concentration required for 50% inhibition of ATP
synthesis was 40 µmol/L, equivalent to 13 µg/ml. Steviol also
inhibited the 2,4-dinitro-phenol-stimulated ATPase, phosphorylation of
exogenous ADP, and exchange between exogenous 14C-ADP and endogenous
adenine nucleotides. The authors concluded that steviol does not act
at the level of the coupling mechanism but at the level of
mitochondrial translocation of adenine nucleotides (Vignais et al.,
1966).
The effects of steviol (purity, 90%) on intestinal glucose
absorption were examined in hamster jejunum by the everted sac
technique. Thus, 1 mmol/L steviol (equivalent to 318.5 µg/ml)
inhibited glucose absorption by 29-43%, and the inhibition was related
to the steviol concentration and incubation time. Reductions in the
intestinal mucosal ATP content and absorptive surface area were
responsible for the inhibition of glucose absorption. The decrease in
intestinal mucosal ATP content was accompanied by a decrease in the
activities of mitochondrial NADH cytochrome c reductase and cytochrome
oxidase. Steviol did not inhibit the activity of intestinal
Na+/K+-ATPase or glucose uptake in the intestinal brush-border
membrane vesicles. Steviol altered the morphology of the intestinal
absorptive cells. The authors concluded that inhibition of intestinal
glucose absorption by steviol in hamsters is due to a reduction in
mucosal ATP content and alteration of the morphology of the intestinal
absorptive cells (Toskulkao et al., 1995a,b).
Single doses of 200 µmol/L steviol, equivalent to 255 mg/kg bw,
were given orally to 24-h-fasted male Wistar rats, either alone or
simultaneously with fructose. Under these conditions, steviol
increased the initial glycogen deposition in the liver. When steviol
was given to the rats in drinking-water at 1 or 2 mmol/L, equivalent
to 32 and 64 mg/kg bw, at the beginning of a fasting period of 24 or
48 h, it had no effect on hepatic glycogen concentrations (Hübler et
al., 1994).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and steviol strongly
inhibited the induction of Epstein-Barr virus early antigen by TPA,
with 50% inhibition at 25 µg/ml (Okamoto et al., 1983a).
In a study of the effects of steviol at 0.2 µmol/L (equivalent to
64 ng/ml) on the induction of ornithine decarboxylase in mouse skin by
TPA, the activity in the epidermis had increased by about 300-fold
4-5 h after application of 17 nmol/L TPA. TPA-induced ornithine
decarboxylase activity was strongly decreased (63%) when steviol was
applied to mouse skin 1 h before TPA treatment, concurrently with TPA
(75%), or 1 h after TPA (71%). Steviol alone did not induce epidermal
ornithine decarboxylase activity. The authors concluded that steviol
interferes with the process of induction of this enzyme by TPA in
mouse skin (Okamoto et al., 1983b).
2.2.7.3 Acute toxicity: Steviol
In male and female mice and rats given steviol (purity, 90%)
orally, the LD50 was > 15 g/kg bw, and 1/15 animals died within 14
days of administration. The LD50 values in hamsters given steviol
orally were 5.2 g/kg bw in males and 6.1 g/kg bw in females.
Histopathological examination of the kidneys revealed severe
degeneration of the proximal tubular cells, and these structural
alterations were correlated with increased serum blood urea nitrogen
and creatinine. The authors concluded that the cause of death was
acute renal failure (Toskulkao et al., 1997).
2.2.7.4 Genotoxicity: Steviol
Studies of the genotoxicity of steviol are summarized in Table 3.
The major metabolite of steviol in vitro, 15alpha-hydroxysteviol,
was inactive at doses up to 7.5 mg/ml in the forward mutation assay in
S. typhimurium strain TM677 with metabolic activation.
15-Oxosteviol, a product of the oxidation of 15alpha-hydroxysteviol,
was a directly acting mutagen at 25-200 µg/ml and was highly toxic to
bacteria. Moreover, the expression of mutagenicity required the
presence of the 13-hydroxy group and the C-16 exomethylene group
(Compadre et al., 1988).
15-Oxosteviol was not mutagenic in various test systems.
Repetition of the experiment with S. typhimurium TM677 failed to
show significant induction of 8-azaguanine-resistant mutants, even
when the number of bacteria tested was greatly increased. The authors
concluded that the earlier positive result reported was due to a
common mishandling of data obtained in the TM677 system and that
15-oxosteviol is unlikely to be the active metabolite responsible for
the mutagenicity of steviol (Procinska et al., 1991).
Table 3. Results of assays for the genotoxicity of steviol
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98 and TA100 20 mg/platea Negative Suttajit et al. (1993)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platea Negative Matsui et al. (1996a)b
TA102, TA104, TA1535, and TA1537 (purity, 99%)
Forward mutation S. typhimurium TM677 10 mg/platec Negative Matsui et al. (1996a)
0.5-10 mg/plated Positive
Forward mutation S. typhimurium TM677 10 mg/platec Negative Pezzuto et al. (1985a)
10 mg/platee Positive
umu Gene mutation S. typhimurium TA1535/pSK1002 625-1250 µg/platec Positive Matsui et al. (1996a)
1259-2500 µg/plated Positive
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Gene mutation Chinese hamster lung fibroblasts 400 µg/mld Positivef Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 0.5 g/mlc Negative Matsui et al. (1996a)
1-1.5 mg/mld Positive
Chromosomal aberration Human lymphocytes 0.2 mg/ml Negative Suttajit et al. (1993)
Micronucleus formation MS/Ae mice 1000 mg/kg bwg Negative Matsui et al. (1996a)
a With and without metabolic activation
b The same results are cited in an earlier abstract (Matsui et al., 1987).
c Without metabolic activation
d With metabolic activation
e With metabolic activation derived from phenobarbital- or Aroclor 1254-pretreated rats; fractions from control or
3-methylcholanthrene-pretreated rats were ineffective.
f Diphtheria toxin-resistant colonies
g Toxic: 4/6 mice at highest dose given intraperitoneally died
The expression of the mutagenic activity of steviol in
S. typhimurium TM677 was dependent on both metabolic activation
(9000 × g fraction derived from phenobarbital- or Aroclor
1254-pretreated rats) and addition of NADPH. The authors concluded
that a cytochrome P450 mediates the metabolic activation of steviol to
a mutagenic species. As partially purified rat liver epoxide hydrolase
did not inhibit steviol-induced mutagenicity, the authors concluded
that the active metabolite is not an epoxide.
A species structurally related to steviol, isosteviol, was not
active in S. typhimurium TM677, regardless of whether metabolic
activation was provided. Similarly, chemical reduction of the
unsaturated bond linking the carbon-16 and -17 positions of steviol
resulted in the generation of two isomeric products, dihydrosteviol A
and B, which were not mutagenic. Ent-kaurenoic acid was also inactive.
A potential metabolite of steviol, steviol-16alpha,17-epoxide, was
synthesized chemically and found to be ineffective as a directly
acting mutagen. The authors concluded that it is a metabolite of an
integral component of stevioside that is mutagenic. The structural
features necessary for the expression of mutagenic activity include a
hydroxy group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 (Pezzuto et al., 1985a, 1986).
Steviol was mutagenic after metabolic activation in the forward
mutation assay with S. typhimurium TM677. The authors confirmed
first that the 8-aza-guanine resistance of the TM677 mutants resides
in the chromosomal guanine phosphoribosyltransferase (gpt) gene,
since it could be complemented by the gpt gene of Escherichia
coli. The chromosomal DNA of TM677 and TM677 mutants was digested by
several restriction enzymes (BamHI, Sau3AI, AluI, TaqI, HaeIII, HpaII,
and RsaI) and analysed by Southern blot hybridization with a probe for
the gpt gene in DNA of E. coli. No significant difference in DNA
fragment length was found between the wild type and spontaneous or
steviol-induced mutants (Matsui et al., 1988, 1989a).
pSV2-gpt plasmids were treated with metabolically activated
steviol (concentration not given), and the DNA was subsequently
analysed by polyacrylamide gel electrophoresis after digestion with
restriction endonucleases (Sau3AI, HhaI, HpaII). Steviol induced a
fivefold increase in mutation frequency, and seven mutants were
obtained, all showing deletions ranging from 20 bp to 2 kb (Matsui et
al., 1989b).
Steviol strongly induced mutations at the gpt gene of S.
typhimurium TM677 when a metabolic activation system was present,
but it had no activity in reverse mutation assays with E. coli
WP2uvrA/pKM101 or S. typhimurium TA strains. In order to
characterize the mutations induced by metabolically activated steviol,
the chromosomal gpt alleles of 24 induced (ST clones) and 16
spontaneous mutants (SP clones) of S. typhimurium TM677 were
sequenced, and the mutation spectra were compared. Nine out of 24 of
the mutations of ST clones were localized in the region between
nucleotides 280 and 330 from the starting codon ATG, whereas no
mutations of SP clones were found in that region. The mutations
identified included transitions (three clones), transversions (four
clones), a duplication, and a deletion. There were no other marked
differences between the ST and SP clones: base-change mutations
predominated over frameshifts and deletions (ST clones, 20 versus
three; SP clones, 16 versus two), and base-change mutations occurred
more frequently at G:C pairs than at A:T pairs (ST clones, 15 versus
five; SP clones, 12 versus four). The authors suggested that
metabolically activated steviol interrupts DNA synthesis around
nucleotide 280, thereby stimulating duplication, deletion, and
untargeted mutagenesis in the defined region of the gpt gene
downstream from the site of interruption (Matsui et al., 1996b).
19- O-ß-D-Glucopyranosyl steviol, a potential hydrolysis product
of stevioside, was mutagenic to S. typhimurium TM677 and
bactericidal in the presence of a metabolic activating system (Pezzuto
et al., 1986).
Microsomes derived from human liver mediated a mutagenic response
of steviol (Pezzuto et al., 1985b).
2.2.7.5 Developmental toxicity: Steviol
Groups of 20 pregnant golden hamsters were given steviol (purity,
90%) at doses of 0, 250, 500, 750, or 1000 mg/kg bw per day (only 12
animals at the highest dose) by gavage in corn oil on days 6-10 of
gestation. A significant decrease in body-weight gain and increased
mortality (1/20, 7/20, and 5/12) were observed at the three highest
doses, and the number of live fetuses per litter and mean fetal weight
decreased in parallel. Histopathological examination of the maternal
kidneys showed a dose-dependent increase in the severity of effects on
the convoluted tubules (dilatation, hyaline droplets). No
dose-dependent teratogenic effects were seen. The NOEL was 250 mg/kg
bw per day for both maternal and developmental toxicity (Wasuntarawat
et al., 1998).
2.2.8 Special studies
2.2.8.1 Cariogenicity
Groups of 15 albino Sprague-Dawley rat pups colonized with
Streptococcus sobrinus received 0.5% stevioside or 30% sucrose in
the basal diet or basal diet alone and were sacrificed after five
weeks, when S. sobrinus was counted and caries were evaluated. There
was no difference in food or water intake or in weight gain among the
groups, but significant differences in sulcal caries scores and
S. sobrinus counts were found between the group receiving sucrose
and the other groups. There was no significant difference between the
group receiving stevioside and the controls. The authors concluded
that stevioside was not cariogenic under the conditions of this study
(Das et al., 1992).
2.2.8.2 Renal function and vasodilatation
The effect of stevioside on renal function was evaluated by
clearance techniques in groups of seven pentobarbital-anesthetized
male Wistar rats simultaneously with the effect of indomethacin on the
renal action of stevioside, given at a priming dose of 4, 8, 12, or
16 mg/kg bw followed by an infusion rate of 4, 8, 12, or 16 mg/kg bw
per hour. Mean arterial pressure and renal function were measured.
Administration of stevioside resulted in a statistically significant,
dose-related decrease in mean arterial pressure (120 ± 2.3 with 4
mg/kg bw to 72 ± 4.8 mm Hg with 16 mg/kg bw) and an increase in renal
plasma flow (10 ± 1.2 with 4 mg/kg bw to 26 ± 2.9 ml/min per kg bw
with 16 mg/kg bw), with no change in glomerular filtration rate.
Stevioside also increased fractional sodium (FeNa+) and potassium
(FeK+) excretion and urine flow (volume/glomerular filtration rate).
The decrease in mean arterial pressure (control, 120 ± 0.93;
stevioside, 91 ± 2.5 mm Hg) and increase in renal plasma flow
(control, 14 ± 1.4; stevioside, 33 ± 2.8 ml/min per kg bw) induced by
stevioside at 16 mg/kg bw were inhibited by simultaneous
administration of indomethacin at 2 mg/kg bw, but the glomerular
filtration rate was not affected. The diuretic, natriuretic, and
kaliuretic effects of stevioside were also abolished by indomethacin.
The authors concluded that stevioside behaves like a typical
vasodilator, causing changes in mean arterial pressure, diuresis,
natriuresis, and kaliuresis per millilitre of glomerular filtration,
and that these effects probably depend on prostaglandins (Melis &
Sainati, 1991a).
The effects of intravenous administration of verapamil (0.015
mg/min) and calcium chloride (800 mEq/L, 0.025 ml/kg bw per min) on
renal function and mean arterial pressure were evaluated in groups of
10 pentobarbital-anaesthetized male Wistar rats weighing 280-320 g
during intravenous treatment with stevioside (16 mg/kg bw per min).
Verapamil significantly increased the hypotensive effect of stevioside
on mean arterial pressure (control, 120 ± 0.77; stevioside, 96 ± 1.5;
stevioside plus verapamil, 67 ± 0.70 mm Hg) and on fractional sodium
excretion (control, 0.76 ± 0.05; stevioside, 1.6 ± 0.10; stevioside
plus verapamil, 2.7 ± 0.25%). Urinary flow, reported as percent
glomerular filtration rate, and renal plasma flow were increased
slightly but not significantly during administration of stevioside
plus verapamil. In contrast, infusion of calcium chloride into rats
pretreated with stevioside resulted in a marked attenuation of mean
arterial pressure (control, 120 ± 1.8; stevioside, 70 ± 1.1;
stevioside plus calcium chloride, 110 ± 1.6 mm Hg) and renal plasma
flow (control, 17 ± 3.8; stevioside, 34 ± 2.6; stevioside plus calcium
chloride, 17 ± 2.9 ml/min per kg bw). The diuresis and natriuresis
induced by stevioside were also inhibited by simultaneous
administration of calcium chloride. The authors concluded that
stevioside acts on arterial pressure and renal function as a calcium
antagonist, as does verapamil (Melis, 1992b).
Classical clearance techniques and arterial pressure measurements
in pentobarbital-anaesthetized male Wistar rats showed that stevioside
at a priming dose of 8 or 16 mg/kg bw followed by an infusion rate of
8 or 16 mg/kg bw per h caused a fall in systemic blood pressure and in
diuresis and natriuresis per millilitre of glomerular filtration rate.
Verapamil tended to increase the renal and systemic effects of
stevioside. In contrast, an infusion of calcium chloride into rats
pretreated with stevioside induced marked attenuation of the
vasodilatatory responses to stevioside. The authors concluded that
stevioside, like verapamil, acts as a calcium antagonist (Melis &
Sainati, 1991b).
The effect of stevioside (purity, > 90%) at a priming dose of
16 mg/kg bw followed by an infusion rate of 16 mg/kg bw per h on renal
function in normal Wistar rats and rats with experimental renal
hypertension was evaluated by clearance techniques. Stevioside
provoked hypotension, diuresis, and natriuresis in both groups of
rats. The normal rats had increased renal plasma flow and a constant
glomerular filtration rate after stevioside administration, whereas
the hypertensive rats had increased renal plasma flow and glomerular
filtration rate. The authors concluded that stevioside impairs a renal
autoregulation mechanism in this model (Melis, 1992c).
The effects of administration of aqueous S. rebaudiana extracts
corresponding to 0.67 g/ml dried leaves given at 2 ml/rat twice a day
for 20, 40, or 60 days on renal function and mean arterial pressure
were studied in normal Wistar rats weighing 80-100 g. Rats treated for
20 days showed no significant difference from the controls, but
administration of the crude extract for 40 or 60 days induced
hypotension, diuresis, and natriuresis, with a constant glomerular
filtration rate. Increased renal plasma flow was seen only in the
group treated for 60 days. The authors concluded that oral
administration of an aqueous extract of dried leaves of Stevia to
rats induces systemic and renal vasodilation, causing hypotension,
diuresis, and natriuresis (Melis, 1995).
Normotensive and experimentally hypertensive male Wistar rats
(Goldblatt GII experimental hypertension induced by clipping the left
renal artery, leaving the contralateral kidney intact) received an
S. rebaudiana extract corresponding to 0.67 g/ml dried leaves given
at 2 ml/rat by gavage twice a day (2.7 g dry leaves per day) for 30
days. Administration of Stevia 10-12 weeks after clipping resulted in
a significant decrease in mean arterial pressure in both the
normotensive and hypertensive rats: normotensive, 110 ± 3.0 mm Hg in
controls versus 70 ± 4.0 mm Hg in those given Stevia; hypertensive,
160 ± 3.0 mm Hg in controls versus 110 ± 4.0 mm Hg with Stevia. The
glomerular filtration rate was constant in the normotensive rats but
increased significantly in the hypertensive rats after Stevia
treatment (16 ± 1.3 versus 14 ± 1.3 ml/min per kg bw in the controls
and Stevia groups, respectively). Both normotensive and hypertensive
rats had increased renal plasma flow after administration of Stevia:
normotensive, 16 ± 3.1 ml/min per kg bw in controls versus 33 ± 3.2
ml/min per kg bw in the Stevia group; hypertensive, 19 ± 2.5 ml/min
per kg bw in controls versus 37 ± 3.9 ml/min per kg bw in the
Stevia group. Stevia increased urinary flow in both normotensive
(1.4 ± 0.08% versus 2.3 ± 0.11%) and hypertensive animals (1.5 ± 0.07%
versus 3.0 ± 0.13%) and also increased sodium excretion
i(normotensive, 0.61 ± 0.07% in controls versus 1.6 ± 0.2% in the
Stevia group; hypertensive, 0.70 ± 0.1% in controls versus 2.2 ±
0.45% in the Stevia group). The authors concluded that Stevia
impairs renal autoregulation in this model (Melis, 1996).
2.3 Observations in humans
S. rebaudiana has been used by Indians in Paraguay as an oral
contraceptive (Mazzei-Planas & Kuc, 1968; Schvartzman et al., 1977).
Aqueous extracts of 5 g of S. rebaudiana leaves were
administered to 16 volunteers at 6-h intervals for three days, and
glucose tolerance tests were performed before and after
administration. Another six volunteers were given an aqueous solution
of arabinose in order to eliminate possible effects of stress. The
extract increased glucose tolerance and significantly decreased plasma
glucose concentrations during the test and after overnight fasting in
all volunteers (Curi et al., 1986).
3. COMMENTS
After oral administration to rats, stevioside is not readily
absorbed from the upper small intestine but is hydrolysed to the
aglycone, steviol, before absorption from the gut. Steviol per se is
completely absorbed and is excreted in the bile as conjugates; only a
very small fraction is detectable in urine. After biliary excretion,
the conjugates are hydrolysed, and steviol undergoes enterohepatic
circulation; its elimination half-life is 24 h. Steviol is the only
faecal metabolite of stevioside that has been identified, and
excretion in the faeces is the major route. After intravenous
administration, stevioside is rapidly distributed throughout the body,
partially secreted by the renal tubular epithelium, and excreted in
urine.
At high concentrations, stevioside affected a variety of
biochemical parameters in rat tissues in vitro. It weakly inhibited
oxidative phosphorylation, and steviol was about 30 times more potent
in this respect. The most likely mechanism is inhibition of the
mitochondrial translocation of adenine nucleotides. Steviol also
inhibited glucose absorption from rat gut by reducing the mucosal ATP
content. Stevioside may also act as a calcium antagonist, showing a
hypotensive effect and inducing diuresis, natriuresis, and a fall in
renal tubular reabsorption of glucose. Stevioside may not, however, be
able to penetrate cell membranes. Although most of these studies were
performed after intravenous injection of stevioside, orally
administered extracts of S. rebaudiana to rats had similar effects
(hypotension and diuresis).
Stevioside has very low acute oral toxicity. Oral administration
of stevioside at a dietary concentration of 2.5% to rats for two
years, equal to 970 and 1100 mg/kg bw per day in males and females,
respectively, had no significant effect. Reduced body-weight gain and
survival rate were observed at a dietary concentration of 5%
stevioside. There was no indication of carcinogenic potential in a
long-term study and no evidence of urinary bladder tumour promoting
potential in a separate bioassay.
In studies of reproductive toxicity, administration of stevioside
at doses up to 2500 mg/kg bw per day to hamsters and 3000 mg/kg bw per
day to rats had no effect. Although an aqueous infusion of
S. rebaudiana administered orally to female rats was reported to
cause a severe, long-lasting reduction in fertility, the contraceptive
effect of Stevia is probably not due to stevioside. Stevioside had
neither teratogenic nor embryotoxic effects in rats given up to 1000
mg/kg bw per day by gavage.
The results of tests for genotoxicity with stevioside in various
systems were uniformly negative.
The aglycone, steviol, was more acutely toxic than stevioside to
hamsters but not to rats. Steviol was clearly genotoxic after
metabolic activation, inducing forward mutations in bacteria and gene
mutations and chromosomal aberrations in Chinese hamster lung
fibroblasts. Several mechanistic studies indicated that the structural
features necessary for the expression of mutagenic activity include a
hydroxyl group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 of steviol. The fact that
stevioside is glycosylated at position 13 could explain the absence of
mutagenicity. The active metabolite of steviol responsible for its
mutagenic activity is not known. While some data suggest that
epoxidation may be involved in the metabolic activation of steviol,
other data indicate that the active metabolite is not an epoxide.
Preliminary data indicate that human liver microsomes may activate
steviol to a mutagenic metabolite.
4. EVALUATION
The Committee noted several shortcomings in the information
available on stevioside. In some studies, the material tested
(stevioside or steviol) was poorly specified or of variable quality,
and no information was available on other constituents or
contaminants. Furthermore, no studies of human metabolism of
stevioside and steviol were available. In addition, data on long-term
toxicity and carcinogenicity were available for stevioside in only one
species. The mutagenic potential of steviol has been tested
sufficiently only in vitro.
In view of the fact that no information was provided for
elaboration of specifications for stevioside and that the evaluation
of the available toxicological data revealed several limitations, the
Committee was unable to relate the results of the toxicological
investigations to the article of commerce and could not allocate an
ADI to stevioside.
Before the substance is reviewed again, specifications must be
developed to ensure that the material tested is representative of the
material of commerce, and further information should be made available
on the nature of the substance that was tested, on the human
metabolism of stevioside, and on the activity of steviol in suitable
studies of genotoxicity in vivo .
5. REFERENCES
Aze, Y., Toyoda, K., Imaida, K., Hayashi, S., Imazawa, T., Hayashi, Y.
& Takahashi, M. (1991) Subchronic oral toxicity study of stevioside in
F344 rats. Bull. Natl Inst. Hyg. Sci., 48-54 (in Japanese).
Cardoso, V.N., Barbosa, M.F., Muramoto, E., Mesquita, C.H. & Almeida,
M.A. (1996) Pharmacokinetic studies of 131I-stevioside and its
metabolites. Nucl. Med. Biol., 23, 97-100.
Compadre, C.M., Hussain, R.A., Nanayakkara, N.P., Pezzuto, J.M. &
Kinghorn, A.D. (1988) Mass spectral analysis of some derivatives and
in vitro metabolites of steviol, the aglycone of the natural
sweeteners, stevioside, rebaudioside A, and rubusoside. Biomed.
Environ. Mass Spectrom., 15, 211-222.
Constantin, J., Ishii-Iwamoto, E.L., Ferraresi-Filho, O.,
Kelmer-Bracht, A.M. & Bracht, A. (1991) Sensitivity of ketogenesis and
citric acid cycle to stevioside inhibition of palmitate transport
across the cell membrane. Braz. J. Med. Biol. Res., 24, 767-771.
Curi, R., Alvarez, M., Bazotte, R.B., Botion, L.M., Godoy, J.L. &
Bracht, A. (1986) Effect of Stevia rebaudiana on glucose tolerance
in normal adult humans. Braz.J. Med. Biol. Res., 19, 771-774
(English abstract only).
Das, S., Das, A.K., Murphy, R.A., Punwani, I.C., Nasution, M.P. &
Kinghorn, A.D. (1992) Evaluation of the cariogenic potential of the
intense natural sweeteners stevioside and rebaudioside A. Caries
Res., 26, 363-366.
Hagiwara, A., Fukushima, S., Kitaori, M., Shibata, M. & Ito, N. (1984)
Effects of three sweeteners on rat urinary bladder carcinogenesis
initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann, 75,
763-768 (English abstract only).
Hanson, J.R. & De Oliveira, B.H. (1993) Stevioside and related sweet
diterpenoid glycosides. Nat. Prod. Rep., 10, 301-309.
Hübler, M.O., Bracht, A. & Kelmer-Bracht, A.M. (1994) Influence of
stevioside on hepatic glycogen levels in fasted rats. Res. Commun.
Chem. Pathol. Pharmacol., 84, 111-118.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ishii, E.L. & Bracht, A. (1986) Stevioside, the sweet glycoside of
Stevia rebaudiana, inhibits the action of atractyloside in the
isolated perfused rat liver. Res. Commun. Chem. Pathol. Pharmacol.,
53, 79-91.
Ishii, E.L., Schwab, A.J. & Bracht, A. (1987) Inhibition of
monosaccharide transport in the intact rat liver by stevioside.
Biochem. Pharmacol., 36, 1417-1433.
Ishii-Iwamoto, E.L. & Bracht, A. (1995) Stevioside is not metabolized
in the isolated perfused rat liver. Res. Commun. Mol.
Pathol.Pharmacol., 87, 167-175.
Ito, N., Fukushima, S., Shirai, T., Hagiwara, A. & Imaida, K. (1984)
Drugs, food additives and natural products as promoters in rat urinary
bladder carcinogenesis. In: Börzsönyi, M., Day, N.E., Lapis, K. &
Yamasaki, H., eds, Models, Mechanisms and Etiology of Tumour
Promotion (IARC Scientific Publications No. 56), Lyon, International
Agency for Research on Cancer, pp. 399-407.
Kelmer-Bracht, A.M., Alvarez, M. & Bracht, A. (1985) Effects of
Stevia rebaudiana natural products on rat liver mitochondria.
Biochem. Pharmacol., 34, 873-882.
Kim, K.K., Sawa, Y. & Shibata, H. (1996) Hydroxylation of
ent-kaurenoic acid to steviol in Stevia rebaudiana Bertoni --
Purification and partial characterization of the enzyme. Arch.
Biochem. Biophys., 332, 223-230.
Krejci, M.E. & Koechel, D.A. (1992) Acute effects of
carboxyatractyloside and stevioside, inhibitors of mitochondrial
ADP/ATP translocation, on renal function and ultrastructure in
pentobarbital-anesthetized dogs. Toxicology, 72, 299-313.
Liu, J., Ong, C.P. & Li, S.F.Y. (1997) Subcritical fluid extraction of
Stevia sweeteners from Stevia rebaudiana. J. Chromatogr.Sci., 35,
446-450.
Matsui, M., Matsui, K., Kawasaki, Y., Sawada, M., Nohmi, T., Sofuni,
T., Yoshihira, K., Ishidate, M., Oda, Y., Noguchi, T., Komatsubara, S.
& Kitagawa, Y. (1987) Mutagenicity test on stevioside and its
aglycone, steviol. Mutat. Res., 182, 366.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1988)
Mutagenicity of steviol: An analytical approach using a Southern
blotting system. Mutat. Res., 203, 377.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989a)
Mutagenicity of steviol: An analytical approach using the Southern
blotting system. Bull. Natl Inst. Hyg.Sci., 83-87 (in Japanese).
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989b)
Detection of deletion mutations in pSV2-gpt plasmids induced by
metabolically activated steviol. Mutat. Res., 216, 367-368.
Matsui, M., Matsui, K., Kawasaki, Y., Oda, Y., Noguchi, T., Kitagawa,
Y., Sawada, M., Hayashi, M., Nohmi, T., Yoshihira, K., Ishidate, M. &
Sofuni, T. (1996a) Evaluation of the genotoxicity of stevioside and
steviol using six in vitro and one in vivo mutagenicity assays.
Mutagenesis, 11, 573-579.
Matsui, M., Sofuni, T. & Nohmi, T. (1996b) Regionally-targeted
mutagenesis by metabolically-activated steviol: DNA sequence analysis
of steviol-induced mutants of guanine phosphoribosyltransferase (gpt)
gene of Salmonella typhimurium TM677. Mutagenesis, 11, 565-572.
Mazzei-Planas, G. & Kuc, J. (1968) Contraceptive properties of
Stevia rebaudiana. Science, 162, 1007.
Medon, P.J., Pezzuto, J.M., Hovanec-Brown, J.M., Nanayakkara, N.P.,
Soejarto, D.D., Kamath, S.K. & Kinghorn, A.D. (1982) Safety assessment
of some Stevia rebaudiana sweet principles. Fed. Proc., 41, 1568.
Melis, M.S. (1992a) Renal excretion of stevioside in rats. J. Nat.
Prod., 55, 688-690.
Melis, M.S. (1992b) Influence of calcium on the blood pressure and
renal effects of stevioside. Braz. J. Med.Biol. Res., 25, 943-949.
Melis, M.S. (1992c) Stevioside effect on renal function of normal and
hypertensive rats. J. Ethnopharmacol., 36, 213-217.
Melis, M.S. (1995) Chronic administration of aqueous extract of
Stevia rebaudiana in rats: Renal effects. J. Ethnopharmacol., 47,
129-134.
Melis, M.S. (1996) A crude extract of Stevia rebaudiana increases
the renal plasma flow of normal and hypertensive rats. Braz. J.
Med.Biol. Res., 29, 669-675.
Melis, M.S. & Sainati, A.R. (1991a) Participation of prostaglandins in
the effect of stevioside on rat renal function and arterial pressure.
Braz. J. Med. Biol. Res., 24, 1269-1276.
Melis, M.S. & Sainati, A.R. (1991b) Effect of calcium and verapamil on
renal function of rats during treatment with stevioside. J.
Ethnopharmacol., 33, 257-262.
Mori, N., Sakanoue, M., Takeuchi, M., Shimpo, K. & Tanabe, T. (1981)
Effect of stevioside on fertility in rats. J. Food Hyg. Soc. Jpn.,
22, 409-414 (in Japanese).
Nakayama, K., Kasahara, D. & Yamamoto, F. (1986) Absorption,
distribution, metabolism and excretion of stevioside in rats.
J. Food Hyg. Soc. Jpn., 27, 1-8.
Nanayakkara, N.P., Klocke, J.A., Compadre, C.M., Hussain, R.A.,
Pezzuto, J.M. & Kinghorn, A.D. (1987) Characterization and feeding
deterrent effects on the aphid, Schizaphis graminum, of some
derivatives of the sweet compounds, stevioside and rebaudioside A.
J. Nat. Prod., 50, 434-441.
Okamoto, H., Yoshida, D. & Mizusaki, S. (1983a) Inhibition of
12-O-tetradecanoylphorbol-13-acetate-induced induction in Epstein-Barr
virus early antigen in Raji cells. Cancer Lett., 19, 47-53.
Okamoto, H., Yoshida, D., Saito, Y. & Mizusaki, S. (1983b) Inhibition
of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine
decarboxylase activity in mouse epidermis by sweetening agents and
related compounds. Cancer Lett., 21, 29-35.
Oliveira-Filho, R.M., Uehara, O.A., Minetti, C.A. & Valle, L.B. (1989)
Chronic administration of aqueous extract of Stevia rebaudiana
(Bert.) Bertoni in rats: Endocrine effects. Gen. Pharmacol., 20,
187-191.
Pezzuto, J.M., Compadre, C.M., Swanson, S.M., Nanayakkara, D. &
Kinghorn, A.D. (1985a) Metabolically activated steviol, the aglycone
of stevioside, is mutagenic. Proc. Natl Acad Sci. USA, 82,
2478-2482.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Lam, L.K., Sparnins, V.L. &
Wattenberg, L.W. (1985b) Evaluation of the potential of steviol
(13-hydroxy-ent-kaurenoic acid) and related diterpenes to mediate
bacterial mutagenesis and induce glutathione-S-transferase in mice.
Proc. Am. Assoc. Cancer Res., 26, 94.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Sparnins, V.L. & Lam, L.K. (1986)
Characterization of bacterial mutagenicity mediated by
13-hydroxy-ent-kaurenoic acid (steviol) and several
structurally-related derivatives and evaluation of potential to induce
glutathione S-transferase in mice. Mutat. Res., 169, 93-103.
Procinska, E., Bridges, B.A. & Hanson, J.R. (1991) Interpretation of
results with the 8-azaguanine resistance system in Salmonella
typhimurium: No evidence for direct acting mutagenesis by
15-oxosteviol, a possible metabolite of steviol. Mutagenesis, 6,
165-167.
Schvartzman, J.B., Krimer, D.B. & Moreno-Azorero, R. (1977)
Cytological effects of some medicinal plants used in the control of
fertility. Experientia, 33, 663-665.
Soejarto, D.D., Kinghorn, A.D. & Farnsworth, N.R. (1982) Potential
sweetening agents of plant origin. III. Organoleptic evaluation of
Stevia leaf herbarium samples for sweetness. J. Nat. Prod., 45,
590-599.
Suanarunsawat, T. & Chaiyabutr, N. (1997) The effect of stevioside on
glucose metabolism in the rat. Can. J. Physiol. Pharmacol., 75,
976-982.
Suttajit, M., Vinitketkaumnuen, U., Meevatee U. & Buddhasukh, D.
(1993) Mutagenicity and human chromosomal effect of stevioside, a
sweetener from Stevia rebaudiana Bertoni. Environ. Health
Perspectives, 101, 53-56.
Takanaka, T., Kawashima, K., Usami, M. & Sakami, K. (1991) A
teratological study of stevioside administered orally to rats.
Unpublished report from Department of Pharmacology, Biological Safety
Research Center, National Institute of Hygienic Sciences, Japan.
Submitted to WHO by Ministry of Health and Welfare, Food Chemistry
Division, Japan.
Toskulkao, C., Sutheerawatananon, M., Wanichanon, C., Saitongdee, P. &
Suttajit, M. (1995a) Effects of stevioside and steviol on intestinal
glucose absorption in hamsters. J. Nutr. Sci. Vitaminol., 41,
105-113.
Toskulkao, C., Sutheerawattananon, M. & Piyachaturawat, P. (1995b)
Inhibitory effect of steviol, a metabolite of stevioside, on glucose
absorption in everted hamster intestine in vitro. Toxicol. Lett.,
80, 153-159.
Toskulkao, C., Chaturat, L., Temcharoen, P. & Glinsukon, T. (1997)
Acute toxicity of stevioside, a natural sweetener, and its metabolite,
steviol, in several animal species. Drug Chem.Toxicol., 20, 31-44.
Toyoda, K., Kawanishi, T., Uneyama, C. & Takahashi, M. (1995)
Re-evaluation of the safety of a food additive (reported in fiscal
1994). A chronic toxicity/carcinogenicity study of stevioside (a
substance extracted from Stevia): Final report. Unpublished report
from Division of Pathology, Biological Safety Research Center,
National Institute of Health Sciences, Japan. Submitted to WHO by
Ministry of Health and Welfare, Food Chemistry Division, Japan.
Toyoda, K., Matsui, H., Shoda,T., Uneyama, C., Takada, K. & Takahashi,
M. (1997) Assessment of the carcinogenicity of stevioside in F344
rats. Food Chem. Toxicol., 35, 597-603.
Usami, M., Seino, Y., Takai, J., Nakahara, H., Seino, S., Ikeda, M. &
Imura, H. (1980) Effect of cyclamate sodium, saccharin sodium and
stevioside on arginine-induced insulin and glucagon secretion in the
isolated perfused rat pancreas. Horm. Metab. Res., 12, 705-706.
Usami, M., Sakemi, K., Kawashima, K., Tsuda, M. & Ohno, Y. (1995)
Teratogenicity study of stevioside in rats. Bull. Natl Inst. Hyg.
Sci., 113, 31-35 (in Japanese).
Vignais, P.V., Duee, E.D., Vignais, P.M. & Huet, J. (1966) Effects of
atractyligenin and its structural analogues on oxidative
phosphorylation and on the translocation of adenine nucleotides in
mitochondria. Biochim. Biophys. Acta, 118, 465-483.
Wasuntarawat, C., Temcharoen, P., Toskulkao, C., Mungkornkarn, P.,
Suttajit, M. & Glinsukon, T. (1998) Developmental toxicity of steviol,
a metabilite of stevioside, in the hamster. Drug Chem.Toxicol., 21,
207-222.
Wingard, R.E., Brown, J.P., Enderlin, F.E., Dale, J.A., Hale, R.L. &
Seitz, C.T. (1980) Intestinal degradation and absorption of the
glycosidic sweeteners stevioside and rebaudioside A. Experientia,
36, 519-520.
Xili, L., Chengjiany, B., Eryi, X., Reiming, S., Yuengming, W.,
Haodong, S. & Zhiyian, H. (1992) Chronic oral toxicity and
carcinogenicity study of stevioside in rats. Food Chem. Toxicol.,
30, 957-965.
Yamamoto, N.S., Kelmer-Bracht, A.M., Ishii, E.L., Kemmelmeier, F.S.,
Alvarez, M. & Bracht, A. (1985) Effect of steviol and its structural
analogues on glucose production and oxygen uptake in rat renal
tubules. Experientia, 41, 55-57.
Yodyingyuad, V. & Bunyawong, S. (1991) Effect of stevioside on growth
and reproduction. Hum. Reprod., 6, 158-165.
See Also:
Toxicological Abbreviations
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
3H-Stevioside (specific activity, 13 or 46 µCi/mg), administered
by gavage to groups of three to seven Wistar rats at a dose of 125
mg/kg bw (10-120 µCi/kg bw), was absorbed slowly, a maximal blood
concentration of 4.8 µg/ml being reached by 8 h. At 4 h, the highest
concentration was found in the caecum (280 µg/g stevioside
equivalent). At 24 h, the concentrations of radiolabel were low in
most organs, including blood, corresponding to about 2 µg/g or ml
stevioside equivalent, except in liver (5.7 µg/g), adrenal gland
(12 µg/g), small intestine (8.8 µg/g), caecum (40 µg/g), large
intestine (12 µg/g), and fat (12 µg/g). The elimination half-life was
24 h. At 48 h, 31% of the radiolabel remained in the body. By five
days, 68% had been excreted into the faeces, 24% in expired air, and
2.3% in the urine. In bile-duct cannulated rats, biliary excretion was
low up to 24 h, increasing rapidly thereafter to reach 41% of the dose
after three days. The authors concluded that stevioside is absorbed
from the gut very slowly, that enterohepatic circulation occurs in
rats, and that faecal excretion is the major route of elimination
(Nakayama et al., 1986).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The radiolabel in plasma decreased
rapidly, showing rapid distribution in the body. The highest
concentrations of radiolabel 10 and 120 min after injection were
observed in the liver (45 and 5% of the injected dose, respectively)
and the small intestine (18 and 66%). At 120 min after injection, the
radiolabel eliminated in the bile represented 52% of the original
dose; that excreted in the faeces and urine 24 h after injection
represented 35 and 35%, respectively, of the original dose (Cardoso et
al., 1996). The Committee considered that this study was of limited
value since introduction of a large 131I atom into stevioside might
significantly affect its absorption, distribution, metabolism, and
excretion in bile or urine.
The renal excretion of stevioside and its effect on the renal
excretion of several other substances was studied in groups of 10 male
Wistar rats, which received intravenous infusions of stevioside at
doses of 4, 8, 12, or 16 mg/kg bw per hour for 30 min, after a control
period of 30 min. No significant change in inulin clearance was
observed, but there was a significant increase in para-aminohippuric
acid clearance, fractional sodium excretion (FeNa+), urinary flow as
percent of glomerular filtration rate, and glucose clearance when
compared with controls at doses greater than 4 mg/kg bw per hour.
Stevioside clearance was greater than inulin clearance and smaller
than para-amino-hippuric acid clearance at all doses tested. The
authors concluded that stevioside is secreted by the renal tubular
epithelium and induces diuresis and natriuresis and a fall in renal
tubular reabsorption of glucose (Melis, 1992a).
2.1.2 Biotransformation
Thin-layer chromatography of the intestinal contents, faeces, and
bile of groups of three to seven Wistar rats given 3H-stevioside
(specific activity, 13 or 46 µCi/mg) by gavage at a dose of 125 mg/kg
bw (10-120 µCi/kg bw) revealed that stevioside is decomposed by rat
caecal flora to steviol and sugars. Stevioside was detected as the
major component in the stomach 1 h after administration. After 4 h in
the small intestine, stevioside, steviolbioside (produced by cleavage
of the glucose moiety at the C-19 position), and steviol accounted for
7.6, 8, and 7.5% of the radiolabel; in the caecum, these compounds
accounted for 39, 17, and 5.1% of the radiolabel, respectively. At 24
h, stevioside was not detectable in the caecum, but steviol and an
unidentified metabolite accounted for 16 and 68% of the radiolabel,
respectively. Steviol was found to be the major metabolite in faeces,
whereas stevioside and the unidentified metabolite were not
quantifiable. In bile, most of the radiolabel found up to 24 h was on
the unidentified metabolite, which was probably a steviol conjugate.
The authors concluded that orally administered stevioside is not
readily absorbed from the upper part of the small intestine, but
metabolites, formed primarily by the bacterial flora in the caecum,
are absorbed from the lower part of the intestine; they also concluded
that most of the stevioside is excreted as steviol in the faeces
(Nakayama et al., 1986). The Committee concluded further that the
faecal steviol could also have arisen from deconjugation of biliary
conjugates by the gut flora.
When 2.5 mg/ml stevioside were incubated under anaerobic
conditions with whole-cell suspensions of bacteria from rat caecum,
stevioside was completely degraded to steviol within two days. The
authors concluded that similar degradation of stevioside occurs in
humans (Wingard et al., 1980).
Mass spectral analysis of steviol and some analogues revealed
characteristic patterns reflecting differential stereochemistry and
variations in the nature of the substituents present. This information
was used to identify several metabolites of steviol (by gas
chromatography-mass spectrometry) which are known to produce a
mutagenic response in Salmonella typhimurium strain TM677 with
metabolic activation. After incubation with a 9000 × g fraction
derived from the livers of Aroclor 1254-pretreated rats, unchanged
steviol was the predominant compound, and nine metabolites were found.
The major pathways of mammalian metabolism of steviol proved to be
allylic oxidation and epoxidation. 15alpha-Hydroxysteviol represented
67% of the metabolites of steviol in vitro (Compadre et al., 1988).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The results of reverse-phase
high-performance liquid chromato-graphy (RP-HPLC) of the bile showed
that stevioside was degraded in vivo and that steviol was the major
metabolite (47% of radiolabel); 37% of the radiolabel was on
stevioside, and the remaining 15% was on an unidentified metabolite.
RP-HPLC analysis of urine 90 min after injection showed the presence
of stevioside and the same unidentified metabolite found in bile, but
no steviol. The authors concluded that stevioside is metabolized in
rat liver to steviol, which is excreted through the bile, and that
similar degradation occurs in humans (Cardoso et al., 1996). The
Committee concluded that there is an alternative explanation, namely
that stevioside is secreted into the bile, is degraded to steviol by
the gut flora, and is resorbed in the lower gut. The Committee
considered that this study was of limited value since introduction of
a large 131I atom into stevioside might significantly affect its
absorption, distribution, metabolism, and excretion in bile or urine.
Stevioside was perfused at a concentration of 0.2 or 0.5 mmol/L
(equivalent to 0.16 and 0.4 mg/ml) into rat livers and was
recirculated for 2 h. The concentration of stevioside remained
constant throughout the perfusion. The formation of hydrolysis
products, especially steviol, was investigated chromatographically,
with negative results. The authors concluded that the reported
metabolic transformation of intravenously injected 131I-stevioside is
either a specific characteristic of this derivative or depends on
factors that are absent in the isolated perfused rat liver
(Ishii-Iwamoto & Bracht, 1995). The Committee concluded that the most
likely explanation for the apparent discrepancy is the fact that
introduction of the large 131I atom into stevioside altered its
pharmacokinetic behaviour and that stevioside is secreted into the
bile in vivo and is degraded to steviol by the gut flora.
2.1.3 Effects on enzymes and other biochemical parameters
Stevioside given to female RCR/Ha mice did not induce glutathione
S-transferase activity in liver or intestinal mucosa (Pezzuto et
al., 1986).
Stevioside (1 mmol/L, equivalent to 0.8 mg/ml) inhibited
oxidative phosphorylation and the activity of ATPase (50% inhibition),
succinate oxidase (8% inhibition), and succinate dehydrogenase (10%
inhibition). No inhibition of NADH-oxidase or L-glutamate
dehydrogenase activity was seen. The ADP:O ratio was slightly
decreased. Substrate respiration was increased at low concentrations
(up to 0.5 mmol/L, equivalent to 0.4 mg/ml) and inhibited at higher
concentrations (1 mmol/L, equivalent to > 0.8 mg/ml). The authors
concluded that stevioside acts as a weak uncoupler of oxidative
phosphorylation (Kelmer-Bracht et al., 1985).
Stevioside inhibited oxidative phosphorylation in isolated rat
liver mitochondria. The concentration required for 50% inhibition of
ATP synthesis was 1.2 mmol/L, equivalent to 0.97 mg/ml (Vignais et
al., 1966).
The effect of stevioside, an inhibitor of long-chain fatty acid
transport, on ketogenesis and on 14C-carbon dioxide production from
[1-14C]-palmitate (100-300 µmol/L) was investigated in isolated and
haemoglobin-free perfused rat liver. Stevioside (2.5 mmol/L,
equivalent to 2 mg/ml) inhibited both parameters but had a smaller
effect on 14C-carbon dioxide production. At 300 µmol/L palmitate and
150 µmol/L albumin, ketogenesis was inhibited by 66%, whereas no
significant inhibition of 14C-carbon dioxide was seen. The authors
concluded that these results reflect different degrees of saturation
of the citric acid cycle and the ketogenic pathway and that changes in
the redox state of the mitochondrial NAD(+)-NADH complex occur after
infusion of stevioside (Constantin et al., 1991).
The Committee noted that the concentrations used in the studies
conducted in vitro were very high relative to those achieved in
blood after oral administration, when the major intestinal metabolite
that enters the circulation is steviol. These studies may therefore be
of limited significance.
When single doses of 200 µmol/L stevioside, equivalent to
650 mg/kg bw, were given orally to 24-h-fasted male Wistar rats,
either alone or simultaneously with fructose, stevioside increased the
initial glycogen deposition in the liver. When it was given to the
rats in the drinking-water at 1 or 2 mmol/L, equivalent to 81 and 160
mg/kg bw, at the beginning of a fasting period of 24 or 48 h,
increased hepatic glycogen concentrations were found at 48 h
(1 mmol/L) and at 24 h (2 mmol/L). The authors concluded that
stevioside stimulates hepatic glycogen synthesis under gluconeogenic
conditions (Hübler et al., 1994).
The effect of stevioside on the transport and metabolism of
D-glucose and D-fructose was investigated in isolated perfused rat
liver. The maximal exchange rate of D-glucose was 700 µmol/L per
min/ml, and the Km was 38 mmol/L. Stevioside inhibited D-glucose and
D-fructose transport across the cell membrane. The half-maximal effect
at 1 mmol/L D-glucose occurred at 0.8 mmol/L stevioside, equivalent to
0.65 mg/ml. Stevioside had no effect on D-glucose metabolism, except
to cause transient changes in D-glucose release, which reflected
changes in the intracellular concentration. D-Fructose consumption,
however, was specifically affected (half-maximal effect at 2.8 mmol/L,
equivalent to 2.3 mg/ml), as were all parameters that depend on
D-fructose transformation: D-glucose production, L-lactate and
pyruvate production, and extra oxygen uptake. In livers that released
D-glucose from endogenous glycogen, strong inhibition of transport
increased the intracellular:extracellular ratio of D-glucose
concentration. The control values for this ratio, representing an
average over the total intracellular water space, were all below unity
(Ishii et al., 1987).
Stevioside had no effect on gluconeogenesis or oxygen uptake in
isolated Wistar rat renal cortical tubules at concentrations up to
3 mmol/L, equivalent to 2.4 mg/ml. The authors concluded that the lack
of activity was due to the inability of stevioside to penetrate the
cell membrane (Yamamoto et al., 1985).
Intravenous infusion of stevioside at 150 mg/ml to male Wistar
rats at a dose of 100, 150, or 200 mg/kg bw per hour raised the plasma
glucose concentrations to 110, 140, and 130% of the control value
during and after infusion. The glucose turnover rate was not altered,
but glucose clearance was reduced by infusion of 200 mg/kg bw per hour
stevioside, from 6.5 to 5 ml/min per kg bw. The plasma insulin
concentration was unchanged. Pretreatment with angiotensin II and
arginine vasopressin had no effect, while prazosin, an
alpha-adrenergic blocker, attenuated the hyperglycaemic effect of
stevioside and infused insulin inhibited it. Oral administration of
stevioside at 2000 mg/kg bw had no effect on the plasma glucose
concentration. The authors concluded that the hyperglycaemic effect of
stevioside was due to an effect on glucose transport across the cell
(Suanarunsawat & Chaiyabutr, 1997).
The effects of stevioside at 1 and 5 mmol/L, equivalent to 0.8
and 4 mg/ml, on intestinal glucose absorption were examined in hamster
jejunum by the everted sac technique. Glucose absorption was not
inhibited (Toskulkao et al., 1995a,b).
Infusion of stevioside at 15 µmol/L, equivalent to 12 µg/ml, for
20 min did not significantly alter the arginine-induced secretion of
insulin or glucagon in the pancreas of male Wistar rats (Usami et al.,
1980).
Stevioside inhibited the action of atractyloside, a known
inhibitor of the adenine nucleotide carrier of mitochondria and in
consequence an inhibitor of energy metabolism, in isolated perfused
rat liver. It decreased the effects of atractyloside on glycolysis,
glycogenolysis, gluconeogenesis, and oxygen uptake. The concentration
for half-maximal action of stevioside was 0.5 mmol/L, equivalent to
0.4 mg/ml. The authors concluded that it acts on the outside of the
cell, as labelled stevioside did not penetrate the cell membranes
(Ishii & Bracht, 1986).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and stevioside did not
inhibit the induction of Epstein-Barr virus early antigen by TPA at
the highest concentration tested: 50 µg/ml (18% inhibition) (Okamoto
et al., 1983a).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the the toxicity of stevioside given as single doses
to rodents are summarized in Table 1. No lethality was seen within 14
days after administration, and no clinical signs of toxicity or
morphological or histopathological changes were found.
After intravenous administration of stevioside to
pentobarbital-anaesthetized dogs at a dose of 32.5 µmol/L per kg bw
(equivalent to 26 mg/kg bw), no significant changes were seen in any
parameters of whole blood, plasma, or renal function, and there was no
significant alteration of the renal ultrastructure. The authors
concluded that stevioside is totally devoid of acute extrarenal
effects (such as hypoxaemia, which could contribute to nephrotoxicity)
and direct renal effects during the 6-h period following intravenous
administration (Krejci & Koechel, 1992).
Table 1. Acute toxicity of stevioside (purity, 96%) given orally to
rodents
Species Sex LD50 (g/kg bw) Reference
Mouse Male and female > 15 Toskulkao et al. (1997)
Mouse Male > 2 Medon et al. (1982)
Rat Male and female > 15 Toskulkao et al. (1997)
Hamster Male and female > 15 Toskulkao et al. (1997)
2.2.2 Short-term studies of toxicity
Rats
A 13-week study of toxicity was carried out in Fischer 344 rats
given doses of 0, 0.31, 0.62, 1.25, 2.5, or 5% in the diet (equivalent
to 160, 310, 630, 1300, and 2500 mg/kg bw per day) to determine the
appropriate doses for a two-year study of carcinogenicity. The rats
were randomly allocated to six groups, each consisting of 10 males and
10 females. None of the animals died during the administration period,
and there was no difference in body-weight gain between the control
and treated groups during administration or in food consumption in the
later part of the study. The activity of lactic dehydrogenase and the
incidence of single-cell necrosis in the liver were increased in all
groups of treated males. The authors considered these effects to be
nonspecific because of the lack of a clear dose-response relationship,
the relatively low severity, and their limitation to males. Other
statistically significant differences in haematological and
biochemical parameters were also considered to be of minor
toxicological significance. The authors concluded that a concentration
of 5% in diet was a suitable maximum tolerable dose of stevioside for
a two-year study in rats (Aze et al., 1991).
2.2.3 Long-term studies of toxicity and carcinogenicity
Rats
Groups of 45 male and 45 female inbred Wistar rats were given
diets containing stevioside (purity, 85%) at 0, 0.2, 0.6, or 1.2%
(equivalent to 100, 300, and 600 mg/kg bw per day) for two years.
After 6, 12, and 24 months, blood was obtained from the tail vein of
five male and five female rats in each dose group for haematological
and clinical biochemical tests. One week later, these rats were housed
in metabolism cages for urine collection and were then killed for
further biochemical, pathological, and histopathological examination.
All surviving animals were killed at two years. Growth, food use and
consumption, general appearance, and mortality were similar in treated
and control groups. The mean life span of rats given stevioside was
not significantly different from that of the controls. No
treatment-related changes were observed in haematological, urinary, or
clinical biochemical values at any stage of the study. The incidence
and severity of non-neoplastic and neoplastic changes were unrelated
to the concentration of stevioside in the diet. The NOEL was 1.2%,
equivalent to 600 mg/kg bw per day. The authors suggested that the
acceptable daily intake of stevioside for humans was 7.9 mg/kg bw per
day, on the basis of the stevioside consumption of the rats during the
first three months (the average for males and females being 790 mg/kg
bw per day) and a safety factor of 100 (Xili et al., 1992).
Stevioside (purity, 95.6%) was added to powdered diet at
concentrations of 0, 2.5, or 5% (equal to 0, 970, and 2000 mg/kg bw
per day for males and 0, 1100, and 2400 for females) and pelleted
every three months. The doses were selected on the basis of the
results of the 13-week study and administered to groups of 50 male and
50 female Fischer 344/DuCrj rats ad libitum for 104 weeks.
Thereafter, all of the groups were maintained on basal diet for four
weeks. All surviving rats were killed at week 108. The body-weight
gain of treated animals was slightly depressed, and a relationship was
seen with the dose of stevioside: 2.3 and 4.4% in males at the low and
high dose and 2.4 and 9.2% in females at the low and high dose. Food
consumption did not differ between the groups. The final survival rate
of males at 5% was significantly decreased, with a rate of 60% versus
78% in controls. The absolute kidney weights were decreased in male
and female animals at the high dose; however, there was no significant
histopathological evidence of neoplastic or non-neoplastic lesions
attributable to treatment in any organ or tissue, except for a
decreased incidence of mammary adenomas in females and a reduced
severity of chronic nephropathy in males. The authors concluded that
stevioside is not carcinogenic in Fischer 344 rats under these
experimental conditions (Toyoda et al., 1995, 1997). The Committee
noted that the report of Toyoda et al. (1995) gives data only for
individual animals, with no summary tables or figures.
The effects of stevioside on urinary bladder carcinogenesis
initiated by N-nitrosobutyl- N-(4-hydroxybutyl)amine was evaluated
in male Fischer 344 rats given 0.01% of the nitrosamine in their
drinking-water for four weeks and then 5% stevioside in their diet,
equivalent to 5000 mg/kg bw per day, for 32 weeks. All surviving rats
were sacrificed after 36 weeks and examined histologically. Treatment
with 5% stevioside did not affect the incidence or extent of papillary
or nodular hyperplasia in nitrosamine-treated rats. No preneoplastic
or neoplastic lesions of the urinary bladder were observed in rats
treated with stevioside only. The authors concluded that stevioside
does not promote bladder carcinogenesis (Hagiwara et al., 1984; Ito et
al., 1984).
2.2.4 Genotoxicity
Studies of the genotoxicity of stevioside are summarized in
Table 2.
Table 2. Results of assays for the genotoxicity of stevioside
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98, TA100 50 mg/platea Negativeb Suttajit et al. (1993)
(purity, 99%)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platec Negative Matsui et al. (1996a)d
TA102, TA104, TA1535, TA1537 1 mg/platee Negative
(purity, 83%)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Matsui et al. (1996a)
Forward mutation S. typhimurium TM677 Not specifieda Negative Medon et al. (1982)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Pezzuto et al. (1985a)
umu Gene mutation S. typhimurium TA1535/pSK1002 5 mg/platea Negative Matsui et al. (1996a)
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 8 mg/mlc Negative Matsui et al. (1996a)
12 mg/mle
Chromosomal aberration Human lymphocytes 10 mg/ml Negative Suttajit et al. (1993)
Chromosomal aberration Chinese hamster lung fibroblasts 12 mg/mlc Negative Ishidate et al. (1984)
(purity, 85%)
a With and without metabolic activation
b A positive response towards TA98 was seen without metabolic activation at 50 mg/ml but not at lower concentrations
up to 20 mg/ml
c Without metabolic activation
d The same results were cited in an earlier abstract (Matsui et al., 1987).
e With metabolic activation
2.2.5 Reproductive toxicity
Hamsters
Groups of 10 male and 10 female one-month-old golden hamsters
(Mesocricetus auratus,) were force-fed with stevioside (purity, 90%)
at 0, 500, 1000, or 2500 mg/kg bw per day daily. Each female was mated
and allowed to bear three litters during the experiment. Females in
late gestation and while lactating (one month) received stevioside in
the drinking-water. Two weeks after the offspring had been weaned, the
females were mated again. No abnormalities were found in the growth or
fertility of animals of either sex. All of the males mated females
efficiently and successfully; the females showed normal four-day
oestrus cycles and became pregnant after mating. The duration of
gestation, number of fetuses, and number of offspring were not
significantly different from those of controls. Forty hamsters of each
sex from the first and second litters were divided into four groups
after weaning and force-fed stevioside at the same doses as their
parents. These animals also showed normal growth and fertility.
Histological examination of reproductive tissues from animals of all
three generations revealed no abnormality that could be linked to
treatment. The authors concluded that stevioside at doses up to 2500
mg/kg bw per day affected neither growth nor reproduction in hamsters
(Yodyingyuad & Bunyawong, 1991).
Rats
Groups of 11 male Wistar rats were given stevioside (purity, 96%)
in the diet at 0, 0.15, 0.75, or 3%, equivalent to 0, 150, 750, and
3000 mg/kg bw per day, for 60 days before and during mating, and
groups of 11 female Wistar rats received the same diet for 14 days
before mating and for seven days during gestation. Rats of each sex at
the highest dose had slightly decreased body-weight gain. There was no
treatment-related effect on mating performance or fertility, and no
malformations were seen in the fetuses. The authors concluded that
stevioside had no adverse effect on fertility or on the development of
fetuses (Mori et al., 1981). The Committee noted a slight but not
statistically significant increase in the number of dead or resorbed
fetuses at the highest dose.
A decoction of 5 g dry S. rebaudiana in 100 ml water was given
orally to inbred, adult female rats for 18 days, resulting in an
intake of approximately 40 ml/kg bw. They were mated with untreated
rats during the last six days. Fertility was reduced to 21% of that of
control rats and remained reduced (47%) after a 50-60-day recovery
period (Mazzei-Planas & Kuc, 1968).
The effects of aqueous S. rebaudiana extracts corresponding to
0.67 g dried leaves per ml, given at a dose of 2 ml/rat twice a day
for 60 days, were studied in prepubertal (25-30 days old) rats. The
end-points were glycaemia; serum concentrations of thyroxine and
tri-iodothyronine; available binding sites in thyroid hormone-binding
proteins; binding of 3H-methyltrienolone (a specific ligand of
androgen receptors) to prostate cytosol; zinc content of the prostate,
testis, submandibular salivary gland, and pancreas; water content of
testis and prostate; body-weight gain; and the final weights of the
testis, prostate, seminal vesicle, submandibular salivary gland, and
adrenal. None of these parameters was significantly different from
those in the control group, with the exception of the seminal vesicle
weight, which fell by about 60%. The authors concluded that if the
Stevia extract can decrease fertility in rats, the effect is almost
certainly not exerted on males (Oliveira-Filho et al., 1989).
2.2.6 Developmental toxicity
Rats
Stevioside (purity, 95.6%) dissolved in distilled water was given
to four groups of 25 or 26 pregnant Wistar rats by gavage once a day
on days 6-15 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw.
The rats were sacrificed on day 20 of gestation, and their fetuses
were examined for malformations. The end-points examined were maternal
and fetal body weight, number of live fetuses, sex distribution,
number of resorptions or dead fetuses, and incidence of malformations.
No treatment-related effect on general or reproductive toxicity was
observed up to the highest dose. The authors concluded that orally
administered stevioside is not teratogenic in rats (Takanaka et al.,
1991; Usami et al., 1995).
2.2.7 Studies on metabolites: Steviol
2.2.7.1 Absorption, distribution, and excretion: Steviol
Intact or bile-duct ligated rats were given [17-14C]-steviol
(specific activity, 2.9 µCi/mg, 1.7 µCi/rat, corresponding to
approximately 3 mg/kg bw) either orally or by intracaecal injection.
After oral administration, 1.5% of the radiolabel was excreted in the
urine of intact rats and 96% in that of bile-duct-ligated animals; the
corresponding amounts in faeces were 96 and 3.3%. After intracaecal
administration of 14C-steviol to bile-duct ligated rats, 94 and 6% of
the radiolabel was excreted in urine and faeces, respectively. When
bile was collected over 72 h, all of the intracaecally injected
radiolabel was recovered. Very little (0.02% of dose) was exhaled as
14C-carbon dioxide. The authors concluded that steviol is completely
absorbed from the rat lower bowel (Wingard et al., 1980).
2.2.7.2 Effects on enzymes and other biochemical parameters: Steviol
Steviol administered to female RCR/Ha mice did not induce
glutathione S-transferase activity in liver or intestinal mucosa
(Pezzuto et al., 1986).
Steviol at 0.5 mmol/L, equivalent to 0.16 mg/ml, inhibited
oxidative phosphorylation and the activity of ATPase (92%), NADH
oxidase (45%), succinate oxidase (42%), succinate dehydrogenase (46%),
and L-glutamate dehydrogenase (46%). The ADP:O ratio was decreased.
Substrate respiration was increased at concentrations up to
0.5 mmol/L, equivalent to 0.16 mg/ml, and inhibited at > 1 mmol/L,
equivalent to 0.32 mg/ml. Inhibition of substrate respiration was the
only effect observed in uncoupled mitochondria. Net proton ejection
induced by succinate and swelling induced by several substrates were
inhibited. The authors concluded that steviol acts as a uncoupler of
oxidative phosphorylation (Kelmer-Bracht et al., 1985).
Steviol decreased glucose production and inhibited oxygen uptake
in isolated Wistar rat renal cortical tubules (IC50, 0.3 mmol/L,
equivalent to 96 µg/ml). The authors concluded that this effect is
consistent with an inhibitory action on oxidative phosphorylation and
electron transport in mitochondria (Yamamoto et al., 1985).
Steviol inhibited oxidative phosphorylation in isolated rat liver
mitochondria. The concentration required for 50% inhibition of ATP
synthesis was 40 µmol/L, equivalent to 13 µg/ml. Steviol also
inhibited the 2,4-dinitro-phenol-stimulated ATPase, phosphorylation of
exogenous ADP, and exchange between exogenous 14C-ADP and endogenous
adenine nucleotides. The authors concluded that steviol does not act
at the level of the coupling mechanism but at the level of
mitochondrial translocation of adenine nucleotides (Vignais et al.,
1966).
The effects of steviol (purity, 90%) on intestinal glucose
absorption were examined in hamster jejunum by the everted sac
technique. Thus, 1 mmol/L steviol (equivalent to 318.5 µg/ml)
inhibited glucose absorption by 29-43%, and the inhibition was related
to the steviol concentration and incubation time. Reductions in the
intestinal mucosal ATP content and absorptive surface area were
responsible for the inhibition of glucose absorption. The decrease in
intestinal mucosal ATP content was accompanied by a decrease in the
activities of mitochondrial NADH cytochrome c reductase and cytochrome
oxidase. Steviol did not inhibit the activity of intestinal
Na+/K+-ATPase or glucose uptake in the intestinal brush-border
membrane vesicles. Steviol altered the morphology of the intestinal
absorptive cells. The authors concluded that inhibition of intestinal
glucose absorption by steviol in hamsters is due to a reduction in
mucosal ATP content and alteration of the morphology of the intestinal
absorptive cells (Toskulkao et al., 1995a,b).
Single doses of 200 µmol/L steviol, equivalent to 255 mg/kg bw,
were given orally to 24-h-fasted male Wistar rats, either alone or
simultaneously with fructose. Under these conditions, steviol
increased the initial glycogen deposition in the liver. When steviol
was given to the rats in drinking-water at 1 or 2 mmol/L, equivalent
to 32 and 64 mg/kg bw, at the beginning of a fasting period of 24 or
48 h, it had no effect on hepatic glycogen concentrations (Hübler et
al., 1994).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and steviol strongly
inhibited the induction of Epstein-Barr virus early antigen by TPA,
with 50% inhibition at 25 µg/ml (Okamoto et al., 1983a).
In a study of the effects of steviol at 0.2 µmol/L (equivalent to
64 ng/ml) on the induction of ornithine decarboxylase in mouse skin by
TPA, the activity in the epidermis had increased by about 300-fold
4-5 h after application of 17 nmol/L TPA. TPA-induced ornithine
decarboxylase activity was strongly decreased (63%) when steviol was
applied to mouse skin 1 h before TPA treatment, concurrently with TPA
(75%), or 1 h after TPA (71%). Steviol alone did not induce epidermal
ornithine decarboxylase activity. The authors concluded that steviol
interferes with the process of induction of this enzyme by TPA in
mouse skin (Okamoto et al., 1983b).
2.2.7.3 Acute toxicity: Steviol
In male and female mice and rats given steviol (purity, 90%)
orally, the LD50 was > 15 g/kg bw, and 1/15 animals died within 14
days of administration. The LD50 values in hamsters given steviol
orally were 5.2 g/kg bw in males and 6.1 g/kg bw in females.
Histopathological examination of the kidneys revealed severe
degeneration of the proximal tubular cells, and these structural
alterations were correlated with increased serum blood urea nitrogen
and creatinine. The authors concluded that the cause of death was
acute renal failure (Toskulkao et al., 1997).
2.2.7.4 Genotoxicity: Steviol
Studies of the genotoxicity of steviol are summarized in Table 3.
The major metabolite of steviol in vitro, 15alpha-hydroxysteviol,
was inactive at doses up to 7.5 mg/ml in the forward mutation assay in
S. typhimurium strain TM677 with metabolic activation.
15-Oxosteviol, a product of the oxidation of 15alpha-hydroxysteviol,
was a directly acting mutagen at 25-200 µg/ml and was highly toxic to
bacteria. Moreover, the expression of mutagenicity required the
presence of the 13-hydroxy group and the C-16 exomethylene group
(Compadre et al., 1988).
15-Oxosteviol was not mutagenic in various test systems.
Repetition of the experiment with S. typhimurium TM677 failed to
show significant induction of 8-azaguanine-resistant mutants, even
when the number of bacteria tested was greatly increased. The authors
concluded that the earlier positive result reported was due to a
common mishandling of data obtained in the TM677 system and that
15-oxosteviol is unlikely to be the active metabolite responsible for
the mutagenicity of steviol (Procinska et al., 1991).
Table 3. Results of assays for the genotoxicity of steviol
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98 and TA100 20 mg/platea Negative Suttajit et al. (1993)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platea Negative Matsui et al. (1996a)b
TA102, TA104, TA1535, and TA1537 (purity, 99%)
Forward mutation S. typhimurium TM677 10 mg/platec Negative Matsui et al. (1996a)
0.5-10 mg/plated Positive
Forward mutation S. typhimurium TM677 10 mg/platec Negative Pezzuto et al. (1985a)
10 mg/platee Positive
umu Gene mutation S. typhimurium TA1535/pSK1002 625-1250 µg/platec Positive Matsui et al. (1996a)
1259-2500 µg/plated Positive
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Gene mutation Chinese hamster lung fibroblasts 400 µg/mld Positivef Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 0.5 g/mlc Negative Matsui et al. (1996a)
1-1.5 mg/mld Positive
Chromosomal aberration Human lymphocytes 0.2 mg/ml Negative Suttajit et al. (1993)
Micronucleus formation MS/Ae mice 1000 mg/kg bwg Negative Matsui et al. (1996a)
a With and without metabolic activation
b The same results are cited in an earlier abstract (Matsui et al., 1987).
c Without metabolic activation
d With metabolic activation
e With metabolic activation derived from phenobarbital- or Aroclor 1254-pretreated rats; fractions from control or
3-methylcholanthrene-pretreated rats were ineffective.
f Diphtheria toxin-resistant colonies
g Toxic: 4/6 mice at highest dose given intraperitoneally died
The expression of the mutagenic activity of steviol in
S. typhimurium TM677 was dependent on both metabolic activation
(9000 × g fraction derived from phenobarbital- or Aroclor
1254-pretreated rats) and addition of NADPH. The authors concluded
that a cytochrome P450 mediates the metabolic activation of steviol to
a mutagenic species. As partially purified rat liver epoxide hydrolase
did not inhibit steviol-induced mutagenicity, the authors concluded
that the active metabolite is not an epoxide.
A species structurally related to steviol, isosteviol, was not
active in S. typhimurium TM677, regardless of whether metabolic
activation was provided. Similarly, chemical reduction of the
unsaturated bond linking the carbon-16 and -17 positions of steviol
resulted in the generation of two isomeric products, dihydrosteviol A
and B, which were not mutagenic. Ent-kaurenoic acid was also inactive.
A potential metabolite of steviol, steviol-16alpha,17-epoxide, was
synthesized chemically and found to be ineffective as a directly
acting mutagen. The authors concluded that it is a metabolite of an
integral component of stevioside that is mutagenic. The structural
features necessary for the expression of mutagenic activity include a
hydroxy group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 (Pezzuto et al., 1985a, 1986).
Steviol was mutagenic after metabolic activation in the forward
mutation assay with S. typhimurium TM677. The authors confirmed
first that the 8-aza-guanine resistance of the TM677 mutants resides
in the chromosomal guanine phosphoribosyltransferase (gpt) gene,
since it could be complemented by the gpt gene of Escherichia
coli. The chromosomal DNA of TM677 and TM677 mutants was digested by
several restriction enzymes (BamHI, Sau3AI, AluI, TaqI, HaeIII, HpaII,
and RsaI) and analysed by Southern blot hybridization with a probe for
the gpt gene in DNA of E. coli. No significant difference in DNA
fragment length was found between the wild type and spontaneous or
steviol-induced mutants (Matsui et al., 1988, 1989a).
pSV2-gpt plasmids were treated with metabolically activated
steviol (concentration not given), and the DNA was subsequently
analysed by polyacrylamide gel electrophoresis after digestion with
restriction endonucleases (Sau3AI, HhaI, HpaII). Steviol induced a
fivefold increase in mutation frequency, and seven mutants were
obtained, all showing deletions ranging from 20 bp to 2 kb (Matsui et
al., 1989b).
Steviol strongly induced mutations at the gpt gene of S.
typhimurium TM677 when a metabolic activation system was present,
but it had no activity in reverse mutation assays with E. coli
WP2uvrA/pKM101 or S. typhimurium TA strains. In order to
characterize the mutations induced by metabolically activated steviol,
the chromosomal gpt alleles of 24 induced (ST clones) and 16
spontaneous mutants (SP clones) of S. typhimurium TM677 were
sequenced, and the mutation spectra were compared. Nine out of 24 of
the mutations of ST clones were localized in the region between
nucleotides 280 and 330 from the starting codon ATG, whereas no
mutations of SP clones were found in that region. The mutations
identified included transitions (three clones), transversions (four
clones), a duplication, and a deletion. There were no other marked
differences between the ST and SP clones: base-change mutations
predominated over frameshifts and deletions (ST clones, 20 versus
three; SP clones, 16 versus two), and base-change mutations occurred
more frequently at G:C pairs than at A:T pairs (ST clones, 15 versus
five; SP clones, 12 versus four). The authors suggested that
metabolically activated steviol interrupts DNA synthesis around
nucleotide 280, thereby stimulating duplication, deletion, and
untargeted mutagenesis in the defined region of the gpt gene
downstream from the site of interruption (Matsui et al., 1996b).
19- O-ß-D-Glucopyranosyl steviol, a potential hydrolysis product
of stevioside, was mutagenic to S. typhimurium TM677 and
bactericidal in the presence of a metabolic activating system (Pezzuto
et al., 1986).
Microsomes derived from human liver mediated a mutagenic response
of steviol (Pezzuto et al., 1985b).
2.2.7.5 Developmental toxicity: Steviol
Groups of 20 pregnant golden hamsters were given steviol (purity,
90%) at doses of 0, 250, 500, 750, or 1000 mg/kg bw per day (only 12
animals at the highest dose) by gavage in corn oil on days 6-10 of
gestation. A significant decrease in body-weight gain and increased
mortality (1/20, 7/20, and 5/12) were observed at the three highest
doses, and the number of live fetuses per litter and mean fetal weight
decreased in parallel. Histopathological examination of the maternal
kidneys showed a dose-dependent increase in the severity of effects on
the convoluted tubules (dilatation, hyaline droplets). No
dose-dependent teratogenic effects were seen. The NOEL was 250 mg/kg
bw per day for both maternal and developmental toxicity (Wasuntarawat
et al., 1998).
2.2.8 Special studies
2.2.8.1 Cariogenicity
Groups of 15 albino Sprague-Dawley rat pups colonized with
Streptococcus sobrinus received 0.5% stevioside or 30% sucrose in
the basal diet or basal diet alone and were sacrificed after five
weeks, when S. sobrinus was counted and caries were evaluated. There
was no difference in food or water intake or in weight gain among the
groups, but significant differences in sulcal caries scores and
S. sobrinus counts were found between the group receiving sucrose
and the other groups. There was no significant difference between the
group receiving stevioside and the controls. The authors concluded
that stevioside was not cariogenic under the conditions of this study
(Das et al., 1992).
2.2.8.2 Renal function and vasodilatation
The effect of stevioside on renal function was evaluated by
clearance techniques in groups of seven pentobarbital-anesthetized
male Wistar rats simultaneously with the effect of indomethacin on the
renal action of stevioside, given at a priming dose of 4, 8, 12, or
16 mg/kg bw followed by an infusion rate of 4, 8, 12, or 16 mg/kg bw
per hour. Mean arterial pressure and renal function were measured.
Administration of stevioside resulted in a statistically significant,
dose-related decrease in mean arterial pressure (120 ± 2.3 with 4
mg/kg bw to 72 ± 4.8 mm Hg with 16 mg/kg bw) and an increase in renal
plasma flow (10 ± 1.2 with 4 mg/kg bw to 26 ± 2.9 ml/min per kg bw
with 16 mg/kg bw), with no change in glomerular filtration rate.
Stevioside also increased fractional sodium (FeNa+) and potassium
(FeK+) excretion and urine flow (volume/glomerular filtration rate).
The decrease in mean arterial pressure (control, 120 ± 0.93;
stevioside, 91 ± 2.5 mm Hg) and increase in renal plasma flow
(control, 14 ± 1.4; stevioside, 33 ± 2.8 ml/min per kg bw) induced by
stevioside at 16 mg/kg bw were inhibited by simultaneous
administration of indomethacin at 2 mg/kg bw, but the glomerular
filtration rate was not affected. The diuretic, natriuretic, and
kaliuretic effects of stevioside were also abolished by indomethacin.
The authors concluded that stevioside behaves like a typical
vasodilator, causing changes in mean arterial pressure, diuresis,
natriuresis, and kaliuresis per millilitre of glomerular filtration,
and that these effects probably depend on prostaglandins (Melis &
Sainati, 1991a).
The effects of intravenous administration of verapamil (0.015
mg/min) and calcium chloride (800 mEq/L, 0.025 ml/kg bw per min) on
renal function and mean arterial pressure were evaluated in groups of
10 pentobarbital-anaesthetized male Wistar rats weighing 280-320 g
during intravenous treatment with stevioside (16 mg/kg bw per min).
Verapamil significantly increased the hypotensive effect of stevioside
on mean arterial pressure (control, 120 ± 0.77; stevioside, 96 ± 1.5;
stevioside plus verapamil, 67 ± 0.70 mm Hg) and on fractional sodium
excretion (control, 0.76 ± 0.05; stevioside, 1.6 ± 0.10; stevioside
plus verapamil, 2.7 ± 0.25%). Urinary flow, reported as percent
glomerular filtration rate, and renal plasma flow were increased
slightly but not significantly during administration of stevioside
plus verapamil. In contrast, infusion of calcium chloride into rats
pretreated with stevioside resulted in a marked attenuation of mean
arterial pressure (control, 120 ± 1.8; stevioside, 70 ± 1.1;
stevioside plus calcium chloride, 110 ± 1.6 mm Hg) and renal plasma
flow (control, 17 ± 3.8; stevioside, 34 ± 2.6; stevioside plus calcium
chloride, 17 ± 2.9 ml/min per kg bw). The diuresis and natriuresis
induced by stevioside were also inhibited by simultaneous
administration of calcium chloride. The authors concluded that
stevioside acts on arterial pressure and renal function as a calcium
antagonist, as does verapamil (Melis, 1992b).
Classical clearance techniques and arterial pressure measurements
in pentobarbital-anaesthetized male Wistar rats showed that stevioside
at a priming dose of 8 or 16 mg/kg bw followed by an infusion rate of
8 or 16 mg/kg bw per h caused a fall in systemic blood pressure and in
diuresis and natriuresis per millilitre of glomerular filtration rate.
Verapamil tended to increase the renal and systemic effects of
stevioside. In contrast, an infusion of calcium chloride into rats
pretreated with stevioside induced marked attenuation of the
vasodilatatory responses to stevioside. The authors concluded that
stevioside, like verapamil, acts as a calcium antagonist (Melis &
Sainati, 1991b).
The effect of stevioside (purity, > 90%) at a priming dose of
16 mg/kg bw followed by an infusion rate of 16 mg/kg bw per h on renal
function in normal Wistar rats and rats with experimental renal
hypertension was evaluated by clearance techniques. Stevioside
provoked hypotension, diuresis, and natriuresis in both groups of
rats. The normal rats had increased renal plasma flow and a constant
glomerular filtration rate after stevioside administration, whereas
the hypertensive rats had increased renal plasma flow and glomerular
filtration rate. The authors concluded that stevioside impairs a renal
autoregulation mechanism in this model (Melis, 1992c).
The effects of administration of aqueous S. rebaudiana extracts
corresponding to 0.67 g/ml dried leaves given at 2 ml/rat twice a day
for 20, 40, or 60 days on renal function and mean arterial pressure
were studied in normal Wistar rats weighing 80-100 g. Rats treated for
20 days showed no significant difference from the controls, but
administration of the crude extract for 40 or 60 days induced
hypotension, diuresis, and natriuresis, with a constant glomerular
filtration rate. Increased renal plasma flow was seen only in the
group treated for 60 days. The authors concluded that oral
administration of an aqueous extract of dried leaves of Stevia to
rats induces systemic and renal vasodilation, causing hypotension,
diuresis, and natriuresis (Melis, 1995).
Normotensive and experimentally hypertensive male Wistar rats
(Goldblatt GII experimental hypertension induced by clipping the left
renal artery, leaving the contralateral kidney intact) received an
S. rebaudiana extract corresponding to 0.67 g/ml dried leaves given
at 2 ml/rat by gavage twice a day (2.7 g dry leaves per day) for 30
days. Administration of Stevia 10-12 weeks after clipping resulted in
a significant decrease in mean arterial pressure in both the
normotensive and hypertensive rats: normotensive, 110 ± 3.0 mm Hg in
controls versus 70 ± 4.0 mm Hg in those given Stevia; hypertensive,
160 ± 3.0 mm Hg in controls versus 110 ± 4.0 mm Hg with Stevia. The
glomerular filtration rate was constant in the normotensive rats but
increased significantly in the hypertensive rats after Stevia
treatment (16 ± 1.3 versus 14 ± 1.3 ml/min per kg bw in the controls
and Stevia groups, respectively). Both normotensive and hypertensive
rats had increased renal plasma flow after administration of Stevia:
normotensive, 16 ± 3.1 ml/min per kg bw in controls versus 33 ± 3.2
ml/min per kg bw in the Stevia group; hypertensive, 19 ± 2.5 ml/min
per kg bw in controls versus 37 ± 3.9 ml/min per kg bw in the
Stevia group. Stevia increased urinary flow in both normotensive
(1.4 ± 0.08% versus 2.3 ± 0.11%) and hypertensive animals (1.5 ± 0.07%
versus 3.0 ± 0.13%) and also increased sodium excretion
i(normotensive, 0.61 ± 0.07% in controls versus 1.6 ± 0.2% in the
Stevia group; hypertensive, 0.70 ± 0.1% in controls versus 2.2 ±
0.45% in the Stevia group). The authors concluded that Stevia
impairs renal autoregulation in this model (Melis, 1996).
2.3 Observations in humans
S. rebaudiana has been used by Indians in Paraguay as an oral
contraceptive (Mazzei-Planas & Kuc, 1968; Schvartzman et al., 1977).
Aqueous extracts of 5 g of S. rebaudiana leaves were
administered to 16 volunteers at 6-h intervals for three days, and
glucose tolerance tests were performed before and after
administration. Another six volunteers were given an aqueous solution
of arabinose in order to eliminate possible effects of stress. The
extract increased glucose tolerance and significantly decreased plasma
glucose concentrations during the test and after overnight fasting in
all volunteers (Curi et al., 1986).
3. COMMENTS
After oral administration to rats, stevioside is not readily
absorbed from the upper small intestine but is hydrolysed to the
aglycone, steviol, before absorption from the gut. Steviol per se is
completely absorbed and is excreted in the bile as conjugates; only a
very small fraction is detectable in urine. After biliary excretion,
the conjugates are hydrolysed, and steviol undergoes enterohepatic
circulation; its elimination half-life is 24 h. Steviol is the only
faecal metabolite of stevioside that has been identified, and
excretion in the faeces is the major route. After intravenous
administration, stevioside is rapidly distributed throughout the body,
partially secreted by the renal tubular epithelium, and excreted in
urine.
At high concentrations, stevioside affected a variety of
biochemical parameters in rat tissues in vitro. It weakly inhibited
oxidative phosphorylation, and steviol was about 30 times more potent
in this respect. The most likely mechanism is inhibition of the
mitochondrial translocation of adenine nucleotides. Steviol also
inhibited glucose absorption from rat gut by reducing the mucosal ATP
content. Stevioside may also act as a calcium antagonist, showing a
hypotensive effect and inducing diuresis, natriuresis, and a fall in
renal tubular reabsorption of glucose. Stevioside may not, however, be
able to penetrate cell membranes. Although most of these studies were
performed after intravenous injection of stevioside, orally
administered extracts of S. rebaudiana to rats had similar effects
(hypotension and diuresis).
Stevioside has very low acute oral toxicity. Oral administration
of stevioside at a dietary concentration of 2.5% to rats for two
years, equal to 970 and 1100 mg/kg bw per day in males and females,
respectively, had no significant effect. Reduced body-weight gain and
survival rate were observed at a dietary concentration of 5%
stevioside. There was no indication of carcinogenic potential in a
long-term study and no evidence of urinary bladder tumour promoting
potential in a separate bioassay.
In studies of reproductive toxicity, administration of stevioside
at doses up to 2500 mg/kg bw per day to hamsters and 3000 mg/kg bw per
day to rats had no effect. Although an aqueous infusion of
S. rebaudiana administered orally to female rats was reported to
cause a severe, long-lasting reduction in fertility, the contraceptive
effect of Stevia is probably not due to stevioside. Stevioside had
neither teratogenic nor embryotoxic effects in rats given up to 1000
mg/kg bw per day by gavage.
The results of tests for genotoxicity with stevioside in various
systems were uniformly negative.
The aglycone, steviol, was more acutely toxic than stevioside to
hamsters but not to rats. Steviol was clearly genotoxic after
metabolic activation, inducing forward mutations in bacteria and gene
mutations and chromosomal aberrations in Chinese hamster lung
fibroblasts. Several mechanistic studies indicated that the structural
features necessary for the expression of mutagenic activity include a
hydroxyl group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 of steviol. The fact that
stevioside is glycosylated at position 13 could explain the absence of
mutagenicity. The active metabolite of steviol responsible for its
mutagenic activity is not known. While some data suggest that
epoxidation may be involved in the metabolic activation of steviol,
other data indicate that the active metabolite is not an epoxide.
Preliminary data indicate that human liver microsomes may activate
steviol to a mutagenic metabolite.
4. EVALUATION
The Committee noted several shortcomings in the information
available on stevioside. In some studies, the material tested
(stevioside or steviol) was poorly specified or of variable quality,
and no information was available on other constituents or
contaminants. Furthermore, no studies of human metabolism of
stevioside and steviol were available. In addition, data on long-term
toxicity and carcinogenicity were available for stevioside in only one
species. The mutagenic potential of steviol has been tested
sufficiently only in vitro.
In view of the fact that no information was provided for
elaboration of specifications for stevioside and that the evaluation
of the available toxicological data revealed several limitations, the
Committee was unable to relate the results of the toxicological
investigations to the article of commerce and could not allocate an
ADI to stevioside.
Before the substance is reviewed again, specifications must be
developed to ensure that the material tested is representative of the
material of commerce, and further information should be made available
on the nature of the substance that was tested, on the human
metabolism of stevioside, and on the activity of steviol in suitable
studies of genotoxicity in vivo .
5. REFERENCES
Aze, Y., Toyoda, K., Imaida, K., Hayashi, S., Imazawa, T., Hayashi, Y.
& Takahashi, M. (1991) Subchronic oral toxicity study of stevioside in
F344 rats. Bull. Natl Inst. Hyg. Sci., 48-54 (in Japanese).
Cardoso, V.N., Barbosa, M.F., Muramoto, E., Mesquita, C.H. & Almeida,
M.A. (1996) Pharmacokinetic studies of 131I-stevioside and its
metabolites. Nucl. Med. Biol., 23, 97-100.
Compadre, C.M., Hussain, R.A., Nanayakkara, N.P., Pezzuto, J.M. &
Kinghorn, A.D. (1988) Mass spectral analysis of some derivatives and
in vitro metabolites of steviol, the aglycone of the natural
sweeteners, stevioside, rebaudioside A, and rubusoside. Biomed.
Environ. Mass Spectrom., 15, 211-222.
Constantin, J., Ishii-Iwamoto, E.L., Ferraresi-Filho, O.,
Kelmer-Bracht, A.M. & Bracht, A. (1991) Sensitivity of ketogenesis and
citric acid cycle to stevioside inhibition of palmitate transport
across the cell membrane. Braz. J. Med. Biol. Res., 24, 767-771.
Curi, R., Alvarez, M., Bazotte, R.B., Botion, L.M., Godoy, J.L. &
Bracht, A. (1986) Effect of Stevia rebaudiana on glucose tolerance
in normal adult humans. Braz.J. Med. Biol. Res., 19, 771-774
(English abstract only).
Das, S., Das, A.K., Murphy, R.A., Punwani, I.C., Nasution, M.P. &
Kinghorn, A.D. (1992) Evaluation of the cariogenic potential of the
intense natural sweeteners stevioside and rebaudioside A. Caries
Res., 26, 363-366.
Hagiwara, A., Fukushima, S., Kitaori, M., Shibata, M. & Ito, N. (1984)
Effects of three sweeteners on rat urinary bladder carcinogenesis
initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann, 75,
763-768 (English abstract only).
Hanson, J.R. & De Oliveira, B.H. (1993) Stevioside and related sweet
diterpenoid glycosides. Nat. Prod. Rep., 10, 301-309.
Hübler, M.O., Bracht, A. & Kelmer-Bracht, A.M. (1994) Influence of
stevioside on hepatic glycogen levels in fasted rats. Res. Commun.
Chem. Pathol. Pharmacol., 84, 111-118.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ishii, E.L. & Bracht, A. (1986) Stevioside, the sweet glycoside of
Stevia rebaudiana, inhibits the action of atractyloside in the
isolated perfused rat liver. Res. Commun. Chem. Pathol. Pharmacol.,
53, 79-91.
Ishii, E.L., Schwab, A.J. & Bracht, A. (1987) Inhibition of
monosaccharide transport in the intact rat liver by stevioside.
Biochem. Pharmacol., 36, 1417-1433.
Ishii-Iwamoto, E.L. & Bracht, A. (1995) Stevioside is not metabolized
in the isolated perfused rat liver. Res. Commun. Mol.
Pathol.Pharmacol., 87, 167-175.
Ito, N., Fukushima, S., Shirai, T., Hagiwara, A. & Imaida, K. (1984)
Drugs, food additives and natural products as promoters in rat urinary
bladder carcinogenesis. In: Börzsönyi, M., Day, N.E., Lapis, K. &
Yamasaki, H., eds, Models, Mechanisms and Etiology of Tumour
Promotion (IARC Scientific Publications No. 56), Lyon, International
Agency for Research on Cancer, pp. 399-407.
Kelmer-Bracht, A.M., Alvarez, M. & Bracht, A. (1985) Effects of
Stevia rebaudiana natural products on rat liver mitochondria.
Biochem. Pharmacol., 34, 873-882.
Kim, K.K., Sawa, Y. & Shibata, H. (1996) Hydroxylation of
ent-kaurenoic acid to steviol in Stevia rebaudiana Bertoni --
Purification and partial characterization of the enzyme. Arch.
Biochem. Biophys., 332, 223-230.
Krejci, M.E. & Koechel, D.A. (1992) Acute effects of
carboxyatractyloside and stevioside, inhibitors of mitochondrial
ADP/ATP translocation, on renal function and ultrastructure in
pentobarbital-anesthetized dogs. Toxicology, 72, 299-313.
Liu, J., Ong, C.P. & Li, S.F.Y. (1997) Subcritical fluid extraction of
Stevia sweeteners from Stevia rebaudiana. J. Chromatogr.Sci., 35,
446-450.
Matsui, M., Matsui, K., Kawasaki, Y., Sawada, M., Nohmi, T., Sofuni,
T., Yoshihira, K., Ishidate, M., Oda, Y., Noguchi, T., Komatsubara, S.
& Kitagawa, Y. (1987) Mutagenicity test on stevioside and its
aglycone, steviol. Mutat. Res., 182, 366.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1988)
Mutagenicity of steviol: An analytical approach using a Southern
blotting system. Mutat. Res., 203, 377.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989a)
Mutagenicity of steviol: An analytical approach using the Southern
blotting system. Bull. Natl Inst. Hyg.Sci., 83-87 (in Japanese).
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989b)
Detection of deletion mutations in pSV2-gpt plasmids induced by
metabolically activated steviol. Mutat. Res., 216, 367-368.
Matsui, M., Matsui, K., Kawasaki, Y., Oda, Y., Noguchi, T., Kitagawa,
Y., Sawada, M., Hayashi, M., Nohmi, T., Yoshihira, K., Ishidate, M. &
Sofuni, T. (1996a) Evaluation of the genotoxicity of stevioside and
steviol using six in vitro and one in vivo mutagenicity assays.
Mutagenesis, 11, 573-579.
Matsui, M., Sofuni, T. & Nohmi, T. (1996b) Regionally-targeted
mutagenesis by metabolically-activated steviol: DNA sequence analysis
of steviol-induced mutants of guanine phosphoribosyltransferase (gpt)
gene of Salmonella typhimurium TM677. Mutagenesis, 11, 565-572.
Mazzei-Planas, G. & Kuc, J. (1968) Contraceptive properties of
Stevia rebaudiana. Science, 162, 1007.
Medon, P.J., Pezzuto, J.M., Hovanec-Brown, J.M., Nanayakkara, N.P.,
Soejarto, D.D., Kamath, S.K. & Kinghorn, A.D. (1982) Safety assessment
of some Stevia rebaudiana sweet principles. Fed. Proc., 41, 1568.
Melis, M.S. (1992a) Renal excretion of stevioside in rats. J. Nat.
Prod., 55, 688-690.
Melis, M.S. (1992b) Influence of calcium on the blood pressure and
renal effects of stevioside. Braz. J. Med.Biol. Res., 25, 943-949.
Melis, M.S. (1992c) Stevioside effect on renal function of normal and
hypertensive rats. J. Ethnopharmacol., 36, 213-217.
Melis, M.S. (1995) Chronic administration of aqueous extract of
Stevia rebaudiana in rats: Renal effects. J. Ethnopharmacol., 47,
129-134.
Melis, M.S. (1996) A crude extract of Stevia rebaudiana increases
the renal plasma flow of normal and hypertensive rats. Braz. J.
Med.Biol. Res., 29, 669-675.
Melis, M.S. & Sainati, A.R. (1991a) Participation of prostaglandins in
the effect of stevioside on rat renal function and arterial pressure.
Braz. J. Med. Biol. Res., 24, 1269-1276.
Melis, M.S. & Sainati, A.R. (1991b) Effect of calcium and verapamil on
renal function of rats during treatment with stevioside. J.
Ethnopharmacol., 33, 257-262.
Mori, N., Sakanoue, M., Takeuchi, M., Shimpo, K. & Tanabe, T. (1981)
Effect of stevioside on fertility in rats. J. Food Hyg. Soc. Jpn.,
22, 409-414 (in Japanese).
Nakayama, K., Kasahara, D. & Yamamoto, F. (1986) Absorption,
distribution, metabolism and excretion of stevioside in rats.
J. Food Hyg. Soc. Jpn., 27, 1-8.
Nanayakkara, N.P., Klocke, J.A., Compadre, C.M., Hussain, R.A.,
Pezzuto, J.M. & Kinghorn, A.D. (1987) Characterization and feeding
deterrent effects on the aphid, Schizaphis graminum, of some
derivatives of the sweet compounds, stevioside and rebaudioside A.
J. Nat. Prod., 50, 434-441.
Okamoto, H., Yoshida, D. & Mizusaki, S. (1983a) Inhibition of
12-O-tetradecanoylphorbol-13-acetate-induced induction in Epstein-Barr
virus early antigen in Raji cells. Cancer Lett., 19, 47-53.
Okamoto, H., Yoshida, D., Saito, Y. & Mizusaki, S. (1983b) Inhibition
of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine
decarboxylase activity in mouse epidermis by sweetening agents and
related compounds. Cancer Lett., 21, 29-35.
Oliveira-Filho, R.M., Uehara, O.A., Minetti, C.A. & Valle, L.B. (1989)
Chronic administration of aqueous extract of Stevia rebaudiana
(Bert.) Bertoni in rats: Endocrine effects. Gen. Pharmacol., 20,
187-191.
Pezzuto, J.M., Compadre, C.M., Swanson, S.M., Nanayakkara, D. &
Kinghorn, A.D. (1985a) Metabolically activated steviol, the aglycone
of stevioside, is mutagenic. Proc. Natl Acad Sci. USA, 82,
2478-2482.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Lam, L.K., Sparnins, V.L. &
Wattenberg, L.W. (1985b) Evaluation of the potential of steviol
(13-hydroxy-ent-kaurenoic acid) and related diterpenes to mediate
bacterial mutagenesis and induce glutathione-S-transferase in mice.
Proc. Am. Assoc. Cancer Res., 26, 94.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Sparnins, V.L. & Lam, L.K. (1986)
Characterization of bacterial mutagenicity mediated by
13-hydroxy-ent-kaurenoic acid (steviol) and several
structurally-related derivatives and evaluation of potential to induce
glutathione S-transferase in mice. Mutat. Res., 169, 93-103.
Procinska, E., Bridges, B.A. & Hanson, J.R. (1991) Interpretation of
results with the 8-azaguanine resistance system in Salmonella
typhimurium: No evidence for direct acting mutagenesis by
15-oxosteviol, a possible metabolite of steviol. Mutagenesis, 6,
165-167.
Schvartzman, J.B., Krimer, D.B. & Moreno-Azorero, R. (1977)
Cytological effects of some medicinal plants used in the control of
fertility. Experientia, 33, 663-665.
Soejarto, D.D., Kinghorn, A.D. & Farnsworth, N.R. (1982) Potential
sweetening agents of plant origin. III. Organoleptic evaluation of
Stevia leaf herbarium samples for sweetness. J. Nat. Prod., 45,
590-599.
Suanarunsawat, T. & Chaiyabutr, N. (1997) The effect of stevioside on
glucose metabolism in the rat. Can. J. Physiol. Pharmacol., 75,
976-982.
Suttajit, M., Vinitketkaumnuen, U., Meevatee U. & Buddhasukh, D.
(1993) Mutagenicity and human chromosomal effect of stevioside, a
sweetener from Stevia rebaudiana Bertoni. Environ. Health
Perspectives, 101, 53-56.
Takanaka, T., Kawashima, K., Usami, M. & Sakami, K. (1991) A
teratological study of stevioside administered orally to rats.
Unpublished report from Department of Pharmacology, Biological Safety
Research Center, National Institute of Hygienic Sciences, Japan.
Submitted to WHO by Ministry of Health and Welfare, Food Chemistry
Division, Japan.
Toskulkao, C., Sutheerawatananon, M., Wanichanon, C., Saitongdee, P. &
Suttajit, M. (1995a) Effects of stevioside and steviol on intestinal
glucose absorption in hamsters. J. Nutr. Sci. Vitaminol., 41,
105-113.
Toskulkao, C., Sutheerawattananon, M. & Piyachaturawat, P. (1995b)
Inhibitory effect of steviol, a metabolite of stevioside, on glucose
absorption in everted hamster intestine in vitro. Toxicol. Lett.,
80, 153-159.
Toskulkao, C., Chaturat, L., Temcharoen, P. & Glinsukon, T. (1997)
Acute toxicity of stevioside, a natural sweetener, and its metabolite,
steviol, in several animal species. Drug Chem.Toxicol., 20, 31-44.
Toyoda, K., Kawanishi, T., Uneyama, C. & Takahashi, M. (1995)
Re-evaluation of the safety of a food additive (reported in fiscal
1994). A chronic toxicity/carcinogenicity study of stevioside (a
substance extracted from Stevia): Final report. Unpublished report
from Division of Pathology, Biological Safety Research Center,
National Institute of Health Sciences, Japan. Submitted to WHO by
Ministry of Health and Welfare, Food Chemistry Division, Japan.
Toyoda, K., Matsui, H., Shoda,T., Uneyama, C., Takada, K. & Takahashi,
M. (1997) Assessment of the carcinogenicity of stevioside in F344
rats. Food Chem. Toxicol., 35, 597-603.
Usami, M., Seino, Y., Takai, J., Nakahara, H., Seino, S., Ikeda, M. &
Imura, H. (1980) Effect of cyclamate sodium, saccharin sodium and
stevioside on arginine-induced insulin and glucagon secretion in the
isolated perfused rat pancreas. Horm. Metab. Res., 12, 705-706.
Usami, M., Sakemi, K., Kawashima, K., Tsuda, M. & Ohno, Y. (1995)
Teratogenicity study of stevioside in rats. Bull. Natl Inst. Hyg.
Sci., 113, 31-35 (in Japanese).
Vignais, P.V., Duee, E.D., Vignais, P.M. & Huet, J. (1966) Effects of
atractyligenin and its structural analogues on oxidative
phosphorylation and on the translocation of adenine nucleotides in
mitochondria. Biochim. Biophys. Acta, 118, 465-483.
Wasuntarawat, C., Temcharoen, P., Toskulkao, C., Mungkornkarn, P.,
Suttajit, M. & Glinsukon, T. (1998) Developmental toxicity of steviol,
a metabilite of stevioside, in the hamster. Drug Chem.Toxicol., 21,
207-222.
Wingard, R.E., Brown, J.P., Enderlin, F.E., Dale, J.A., Hale, R.L. &
Seitz, C.T. (1980) Intestinal degradation and absorption of the
glycosidic sweeteners stevioside and rebaudioside A. Experientia,
36, 519-520.
Xili, L., Chengjiany, B., Eryi, X., Reiming, S., Yuengming, W.,
Haodong, S. & Zhiyian, H. (1992) Chronic oral toxicity and
carcinogenicity study of stevioside in rats. Food Chem. Toxicol.,
30, 957-965.
Yamamoto, N.S., Kelmer-Bracht, A.M., Ishii, E.L., Kemmelmeier, F.S.,
Alvarez, M. & Bracht, A. (1985) Effect of steviol and its structural
analogues on glucose production and oxygen uptake in rat renal
tubules. Experientia, 41, 55-57.
Yodyingyuad, V. & Bunyawong, S. (1991) Effect of stevioside on growth
and reproduction. Hum. Reprod., 6, 158-165.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Rats
3H-Stevioside (specific activity, 13 or 46 µCi/mg), administered
by gavage to groups of three to seven Wistar rats at a dose of 125
mg/kg bw (10-120 µCi/kg bw), was absorbed slowly, a maximal blood
concentration of 4.8 µg/ml being reached by 8 h. At 4 h, the highest
concentration was found in the caecum (280 µg/g stevioside
equivalent). At 24 h, the concentrations of radiolabel were low in
most organs, including blood, corresponding to about 2 µg/g or ml
stevioside equivalent, except in liver (5.7 µg/g), adrenal gland
(12 µg/g), small intestine (8.8 µg/g), caecum (40 µg/g), large
intestine (12 µg/g), and fat (12 µg/g). The elimination half-life was
24 h. At 48 h, 31% of the radiolabel remained in the body. By five
days, 68% had been excreted into the faeces, 24% in expired air, and
2.3% in the urine. In bile-duct cannulated rats, biliary excretion was
low up to 24 h, increasing rapidly thereafter to reach 41% of the dose
after three days. The authors concluded that stevioside is absorbed
from the gut very slowly, that enterohepatic circulation occurs in
rats, and that faecal excretion is the major route of elimination
(Nakayama et al., 1986).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The radiolabel in plasma decreased
rapidly, showing rapid distribution in the body. The highest
concentrations of radiolabel 10 and 120 min after injection were
observed in the liver (45 and 5% of the injected dose, respectively)
and the small intestine (18 and 66%). At 120 min after injection, the
radiolabel eliminated in the bile represented 52% of the original
dose; that excreted in the faeces and urine 24 h after injection
represented 35 and 35%, respectively, of the original dose (Cardoso et
al., 1996). The Committee considered that this study was of limited
value since introduction of a large 131I atom into stevioside might
significantly affect its absorption, distribution, metabolism, and
excretion in bile or urine.
The renal excretion of stevioside and its effect on the renal
excretion of several other substances was studied in groups of 10 male
Wistar rats, which received intravenous infusions of stevioside at
doses of 4, 8, 12, or 16 mg/kg bw per hour for 30 min, after a control
period of 30 min. No significant change in inulin clearance was
observed, but there was a significant increase in para-aminohippuric
acid clearance, fractional sodium excretion (FeNa+), urinary flow as
percent of glomerular filtration rate, and glucose clearance when
compared with controls at doses greater than 4 mg/kg bw per hour.
Stevioside clearance was greater than inulin clearance and smaller
than para-amino-hippuric acid clearance at all doses tested. The
authors concluded that stevioside is secreted by the renal tubular
epithelium and induces diuresis and natriuresis and a fall in renal
tubular reabsorption of glucose (Melis, 1992a).
2.1.2 Biotransformation
Thin-layer chromatography of the intestinal contents, faeces, and
bile of groups of three to seven Wistar rats given 3H-stevioside
(specific activity, 13 or 46 µCi/mg) by gavage at a dose of 125 mg/kg
bw (10-120 µCi/kg bw) revealed that stevioside is decomposed by rat
caecal flora to steviol and sugars. Stevioside was detected as the
major component in the stomach 1 h after administration. After 4 h in
the small intestine, stevioside, steviolbioside (produced by cleavage
of the glucose moiety at the C-19 position), and steviol accounted for
7.6, 8, and 7.5% of the radiolabel; in the caecum, these compounds
accounted for 39, 17, and 5.1% of the radiolabel, respectively. At 24
h, stevioside was not detectable in the caecum, but steviol and an
unidentified metabolite accounted for 16 and 68% of the radiolabel,
respectively. Steviol was found to be the major metabolite in faeces,
whereas stevioside and the unidentified metabolite were not
quantifiable. In bile, most of the radiolabel found up to 24 h was on
the unidentified metabolite, which was probably a steviol conjugate.
The authors concluded that orally administered stevioside is not
readily absorbed from the upper part of the small intestine, but
metabolites, formed primarily by the bacterial flora in the caecum,
are absorbed from the lower part of the intestine; they also concluded
that most of the stevioside is excreted as steviol in the faeces
(Nakayama et al., 1986). The Committee concluded further that the
faecal steviol could also have arisen from deconjugation of biliary
conjugates by the gut flora.
When 2.5 mg/ml stevioside were incubated under anaerobic
conditions with whole-cell suspensions of bacteria from rat caecum,
stevioside was completely degraded to steviol within two days. The
authors concluded that similar degradation of stevioside occurs in
humans (Wingard et al., 1980).
Mass spectral analysis of steviol and some analogues revealed
characteristic patterns reflecting differential stereochemistry and
variations in the nature of the substituents present. This information
was used to identify several metabolites of steviol (by gas
chromatography-mass spectrometry) which are known to produce a
mutagenic response in Salmonella typhimurium strain TM677 with
metabolic activation. After incubation with a 9000 × g fraction
derived from the livers of Aroclor 1254-pretreated rats, unchanged
steviol was the predominant compound, and nine metabolites were found.
The major pathways of mammalian metabolism of steviol proved to be
allylic oxidation and epoxidation. 15alpha-Hydroxysteviol represented
67% of the metabolites of steviol in vitro (Compadre et al., 1988).
131I-Stevioside (specific activity, 3.7 MBq/mg, equivalent to
100 µCi/mg; 1.1 MBq, 30 µCi, equivalent to 1 mg/kg bw) was injected
intravenously to male Wistar rats. The results of reverse-phase
high-performance liquid chromato-graphy (RP-HPLC) of the bile showed
that stevioside was degraded in vivo and that steviol was the major
metabolite (47% of radiolabel); 37% of the radiolabel was on
stevioside, and the remaining 15% was on an unidentified metabolite.
RP-HPLC analysis of urine 90 min after injection showed the presence
of stevioside and the same unidentified metabolite found in bile, but
no steviol. The authors concluded that stevioside is metabolized in
rat liver to steviol, which is excreted through the bile, and that
similar degradation occurs in humans (Cardoso et al., 1996). The
Committee concluded that there is an alternative explanation, namely
that stevioside is secreted into the bile, is degraded to steviol by
the gut flora, and is resorbed in the lower gut. The Committee
considered that this study was of limited value since introduction of
a large 131I atom into stevioside might significantly affect its
absorption, distribution, metabolism, and excretion in bile or urine.
Stevioside was perfused at a concentration of 0.2 or 0.5 mmol/L
(equivalent to 0.16 and 0.4 mg/ml) into rat livers and was
recirculated for 2 h. The concentration of stevioside remained
constant throughout the perfusion. The formation of hydrolysis
products, especially steviol, was investigated chromatographically,
with negative results. The authors concluded that the reported
metabolic transformation of intravenously injected 131I-stevioside is
either a specific characteristic of this derivative or depends on
factors that are absent in the isolated perfused rat liver
(Ishii-Iwamoto & Bracht, 1995). The Committee concluded that the most
likely explanation for the apparent discrepancy is the fact that
introduction of the large 131I atom into stevioside altered its
pharmacokinetic behaviour and that stevioside is secreted into the
bile in vivo and is degraded to steviol by the gut flora.
2.1.3 Effects on enzymes and other biochemical parameters
Stevioside given to female RCR/Ha mice did not induce glutathione
S-transferase activity in liver or intestinal mucosa (Pezzuto et
al., 1986).
Stevioside (1 mmol/L, equivalent to 0.8 mg/ml) inhibited
oxidative phosphorylation and the activity of ATPase (50% inhibition),
succinate oxidase (8% inhibition), and succinate dehydrogenase (10%
inhibition). No inhibition of NADH-oxidase or L-glutamate
dehydrogenase activity was seen. The ADP:O ratio was slightly
decreased. Substrate respiration was increased at low concentrations
(up to 0.5 mmol/L, equivalent to 0.4 mg/ml) and inhibited at higher
concentrations (1 mmol/L, equivalent to > 0.8 mg/ml). The authors
concluded that stevioside acts as a weak uncoupler of oxidative
phosphorylation (Kelmer-Bracht et al., 1985).
Stevioside inhibited oxidative phosphorylation in isolated rat
liver mitochondria. The concentration required for 50% inhibition of
ATP synthesis was 1.2 mmol/L, equivalent to 0.97 mg/ml (Vignais et
al., 1966).
The effect of stevioside, an inhibitor of long-chain fatty acid
transport, on ketogenesis and on 14C-carbon dioxide production from
[1-14C]-palmitate (100-300 µmol/L) was investigated in isolated and
haemoglobin-free perfused rat liver. Stevioside (2.5 mmol/L,
equivalent to 2 mg/ml) inhibited both parameters but had a smaller
effect on 14C-carbon dioxide production. At 300 µmol/L palmitate and
150 µmol/L albumin, ketogenesis was inhibited by 66%, whereas no
significant inhibition of 14C-carbon dioxide was seen. The authors
concluded that these results reflect different degrees of saturation
of the citric acid cycle and the ketogenic pathway and that changes in
the redox state of the mitochondrial NAD(+)-NADH complex occur after
infusion of stevioside (Constantin et al., 1991).
The Committee noted that the concentrations used in the studies
conducted in vitro were very high relative to those achieved in
blood after oral administration, when the major intestinal metabolite
that enters the circulation is steviol. These studies may therefore be
of limited significance.
When single doses of 200 µmol/L stevioside, equivalent to
650 mg/kg bw, were given orally to 24-h-fasted male Wistar rats,
either alone or simultaneously with fructose, stevioside increased the
initial glycogen deposition in the liver. When it was given to the
rats in the drinking-water at 1 or 2 mmol/L, equivalent to 81 and 160
mg/kg bw, at the beginning of a fasting period of 24 or 48 h,
increased hepatic glycogen concentrations were found at 48 h
(1 mmol/L) and at 24 h (2 mmol/L). The authors concluded that
stevioside stimulates hepatic glycogen synthesis under gluconeogenic
conditions (Hübler et al., 1994).
The effect of stevioside on the transport and metabolism of
D-glucose and D-fructose was investigated in isolated perfused rat
liver. The maximal exchange rate of D-glucose was 700 µmol/L per
min/ml, and the Km was 38 mmol/L. Stevioside inhibited D-glucose and
D-fructose transport across the cell membrane. The half-maximal effect
at 1 mmol/L D-glucose occurred at 0.8 mmol/L stevioside, equivalent to
0.65 mg/ml. Stevioside had no effect on D-glucose metabolism, except
to cause transient changes in D-glucose release, which reflected
changes in the intracellular concentration. D-Fructose consumption,
however, was specifically affected (half-maximal effect at 2.8 mmol/L,
equivalent to 2.3 mg/ml), as were all parameters that depend on
D-fructose transformation: D-glucose production, L-lactate and
pyruvate production, and extra oxygen uptake. In livers that released
D-glucose from endogenous glycogen, strong inhibition of transport
increased the intracellular:extracellular ratio of D-glucose
concentration. The control values for this ratio, representing an
average over the total intracellular water space, were all below unity
(Ishii et al., 1987).
Stevioside had no effect on gluconeogenesis or oxygen uptake in
isolated Wistar rat renal cortical tubules at concentrations up to
3 mmol/L, equivalent to 2.4 mg/ml. The authors concluded that the lack
of activity was due to the inability of stevioside to penetrate the
cell membrane (Yamamoto et al., 1985).
Intravenous infusion of stevioside at 150 mg/ml to male Wistar
rats at a dose of 100, 150, or 200 mg/kg bw per hour raised the plasma
glucose concentrations to 110, 140, and 130% of the control value
during and after infusion. The glucose turnover rate was not altered,
but glucose clearance was reduced by infusion of 200 mg/kg bw per hour
stevioside, from 6.5 to 5 ml/min per kg bw. The plasma insulin
concentration was unchanged. Pretreatment with angiotensin II and
arginine vasopressin had no effect, while prazosin, an
alpha-adrenergic blocker, attenuated the hyperglycaemic effect of
stevioside and infused insulin inhibited it. Oral administration of
stevioside at 2000 mg/kg bw had no effect on the plasma glucose
concentration. The authors concluded that the hyperglycaemic effect of
stevioside was due to an effect on glucose transport across the cell
(Suanarunsawat & Chaiyabutr, 1997).
The effects of stevioside at 1 and 5 mmol/L, equivalent to 0.8
and 4 mg/ml, on intestinal glucose absorption were examined in hamster
jejunum by the everted sac technique. Glucose absorption was not
inhibited (Toskulkao et al., 1995a,b).
Infusion of stevioside at 15 µmol/L, equivalent to 12 µg/ml, for
20 min did not significantly alter the arginine-induced secretion of
insulin or glucagon in the pancreas of male Wistar rats (Usami et al.,
1980).
Stevioside inhibited the action of atractyloside, a known
inhibitor of the adenine nucleotide carrier of mitochondria and in
consequence an inhibitor of energy metabolism, in isolated perfused
rat liver. It decreased the effects of atractyloside on glycolysis,
glycogenolysis, gluconeogenesis, and oxygen uptake. The concentration
for half-maximal action of stevioside was 0.5 mmol/L, equivalent to
0.4 mg/ml. The authors concluded that it acts on the outside of the
cell, as labelled stevioside did not penetrate the cell membranes
(Ishii & Bracht, 1986).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and stevioside did not
inhibit the induction of Epstein-Barr virus early antigen by TPA at
the highest concentration tested: 50 µg/ml (18% inhibition) (Okamoto
et al., 1983a).
2.2 Toxicological studies
2.2.1 Acute toxicity
Studies of the the toxicity of stevioside given as single doses
to rodents are summarized in Table 1. No lethality was seen within 14
days after administration, and no clinical signs of toxicity or
morphological or histopathological changes were found.
After intravenous administration of stevioside to
pentobarbital-anaesthetized dogs at a dose of 32.5 µmol/L per kg bw
(equivalent to 26 mg/kg bw), no significant changes were seen in any
parameters of whole blood, plasma, or renal function, and there was no
significant alteration of the renal ultrastructure. The authors
concluded that stevioside is totally devoid of acute extrarenal
effects (such as hypoxaemia, which could contribute to nephrotoxicity)
and direct renal effects during the 6-h period following intravenous
administration (Krejci & Koechel, 1992).
Table 1. Acute toxicity of stevioside (purity, 96%) given orally to
rodents
Species Sex LD50 (g/kg bw) Reference
Mouse Male and female > 15 Toskulkao et al. (1997)
Mouse Male > 2 Medon et al. (1982)
Rat Male and female > 15 Toskulkao et al. (1997)
Hamster Male and female > 15 Toskulkao et al. (1997)
2.2.2 Short-term studies of toxicity
Rats
A 13-week study of toxicity was carried out in Fischer 344 rats
given doses of 0, 0.31, 0.62, 1.25, 2.5, or 5% in the diet (equivalent
to 160, 310, 630, 1300, and 2500 mg/kg bw per day) to determine the
appropriate doses for a two-year study of carcinogenicity. The rats
were randomly allocated to six groups, each consisting of 10 males and
10 females. None of the animals died during the administration period,
and there was no difference in body-weight gain between the control
and treated groups during administration or in food consumption in the
later part of the study. The activity of lactic dehydrogenase and the
incidence of single-cell necrosis in the liver were increased in all
groups of treated males. The authors considered these effects to be
nonspecific because of the lack of a clear dose-response relationship,
the relatively low severity, and their limitation to males. Other
statistically significant differences in haematological and
biochemical parameters were also considered to be of minor
toxicological significance. The authors concluded that a concentration
of 5% in diet was a suitable maximum tolerable dose of stevioside for
a two-year study in rats (Aze et al., 1991).
2.2.3 Long-term studies of toxicity and carcinogenicity
Rats
Groups of 45 male and 45 female inbred Wistar rats were given
diets containing stevioside (purity, 85%) at 0, 0.2, 0.6, or 1.2%
(equivalent to 100, 300, and 600 mg/kg bw per day) for two years.
After 6, 12, and 24 months, blood was obtained from the tail vein of
five male and five female rats in each dose group for haematological
and clinical biochemical tests. One week later, these rats were housed
in metabolism cages for urine collection and were then killed for
further biochemical, pathological, and histopathological examination.
All surviving animals were killed at two years. Growth, food use and
consumption, general appearance, and mortality were similar in treated
and control groups. The mean life span of rats given stevioside was
not significantly different from that of the controls. No
treatment-related changes were observed in haematological, urinary, or
clinical biochemical values at any stage of the study. The incidence
and severity of non-neoplastic and neoplastic changes were unrelated
to the concentration of stevioside in the diet. The NOEL was 1.2%,
equivalent to 600 mg/kg bw per day. The authors suggested that the
acceptable daily intake of stevioside for humans was 7.9 mg/kg bw per
day, on the basis of the stevioside consumption of the rats during the
first three months (the average for males and females being 790 mg/kg
bw per day) and a safety factor of 100 (Xili et al., 1992).
Stevioside (purity, 95.6%) was added to powdered diet at
concentrations of 0, 2.5, or 5% (equal to 0, 970, and 2000 mg/kg bw
per day for males and 0, 1100, and 2400 for females) and pelleted
every three months. The doses were selected on the basis of the
results of the 13-week study and administered to groups of 50 male and
50 female Fischer 344/DuCrj rats ad libitum for 104 weeks.
Thereafter, all of the groups were maintained on basal diet for four
weeks. All surviving rats were killed at week 108. The body-weight
gain of treated animals was slightly depressed, and a relationship was
seen with the dose of stevioside: 2.3 and 4.4% in males at the low and
high dose and 2.4 and 9.2% in females at the low and high dose. Food
consumption did not differ between the groups. The final survival rate
of males at 5% was significantly decreased, with a rate of 60% versus
78% in controls. The absolute kidney weights were decreased in male
and female animals at the high dose; however, there was no significant
histopathological evidence of neoplastic or non-neoplastic lesions
attributable to treatment in any organ or tissue, except for a
decreased incidence of mammary adenomas in females and a reduced
severity of chronic nephropathy in males. The authors concluded that
stevioside is not carcinogenic in Fischer 344 rats under these
experimental conditions (Toyoda et al., 1995, 1997). The Committee
noted that the report of Toyoda et al. (1995) gives data only for
individual animals, with no summary tables or figures.
The effects of stevioside on urinary bladder carcinogenesis
initiated by N-nitrosobutyl- N-(4-hydroxybutyl)amine was evaluated
in male Fischer 344 rats given 0.01% of the nitrosamine in their
drinking-water for four weeks and then 5% stevioside in their diet,
equivalent to 5000 mg/kg bw per day, for 32 weeks. All surviving rats
were sacrificed after 36 weeks and examined histologically. Treatment
with 5% stevioside did not affect the incidence or extent of papillary
or nodular hyperplasia in nitrosamine-treated rats. No preneoplastic
or neoplastic lesions of the urinary bladder were observed in rats
treated with stevioside only. The authors concluded that stevioside
does not promote bladder carcinogenesis (Hagiwara et al., 1984; Ito et
al., 1984).
2.2.4 Genotoxicity
Studies of the genotoxicity of stevioside are summarized in
Table 2.
Table 2. Results of assays for the genotoxicity of stevioside
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98, TA100 50 mg/platea Negativeb Suttajit et al. (1993)
(purity, 99%)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platec Negative Matsui et al. (1996a)d
TA102, TA104, TA1535, TA1537 1 mg/platee Negative
(purity, 83%)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Matsui et al. (1996a)
Forward mutation S. typhimurium TM677 Not specifieda Negative Medon et al. (1982)
Forward mutation S. typhimurium TM677 10 mg/platea Negative Pezzuto et al. (1985a)
umu Gene mutation S. typhimurium TA1535/pSK1002 5 mg/platea Negative Matsui et al. (1996a)
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 8 mg/mlc Negative Matsui et al. (1996a)
12 mg/mle
Chromosomal aberration Human lymphocytes 10 mg/ml Negative Suttajit et al. (1993)
Chromosomal aberration Chinese hamster lung fibroblasts 12 mg/mlc Negative Ishidate et al. (1984)
(purity, 85%)
a With and without metabolic activation
b A positive response towards TA98 was seen without metabolic activation at 50 mg/ml but not at lower concentrations
up to 20 mg/ml
c Without metabolic activation
d The same results were cited in an earlier abstract (Matsui et al., 1987).
e With metabolic activation
2.2.5 Reproductive toxicity
Hamsters
Groups of 10 male and 10 female one-month-old golden hamsters
(Mesocricetus auratus,) were force-fed with stevioside (purity, 90%)
at 0, 500, 1000, or 2500 mg/kg bw per day daily. Each female was mated
and allowed to bear three litters during the experiment. Females in
late gestation and while lactating (one month) received stevioside in
the drinking-water. Two weeks after the offspring had been weaned, the
females were mated again. No abnormalities were found in the growth or
fertility of animals of either sex. All of the males mated females
efficiently and successfully; the females showed normal four-day
oestrus cycles and became pregnant after mating. The duration of
gestation, number of fetuses, and number of offspring were not
significantly different from those of controls. Forty hamsters of each
sex from the first and second litters were divided into four groups
after weaning and force-fed stevioside at the same doses as their
parents. These animals also showed normal growth and fertility.
Histological examination of reproductive tissues from animals of all
three generations revealed no abnormality that could be linked to
treatment. The authors concluded that stevioside at doses up to 2500
mg/kg bw per day affected neither growth nor reproduction in hamsters
(Yodyingyuad & Bunyawong, 1991).
Rats
Groups of 11 male Wistar rats were given stevioside (purity, 96%)
in the diet at 0, 0.15, 0.75, or 3%, equivalent to 0, 150, 750, and
3000 mg/kg bw per day, for 60 days before and during mating, and
groups of 11 female Wistar rats received the same diet for 14 days
before mating and for seven days during gestation. Rats of each sex at
the highest dose had slightly decreased body-weight gain. There was no
treatment-related effect on mating performance or fertility, and no
malformations were seen in the fetuses. The authors concluded that
stevioside had no adverse effect on fertility or on the development of
fetuses (Mori et al., 1981). The Committee noted a slight but not
statistically significant increase in the number of dead or resorbed
fetuses at the highest dose.
A decoction of 5 g dry S. rebaudiana in 100 ml water was given
orally to inbred, adult female rats for 18 days, resulting in an
intake of approximately 40 ml/kg bw. They were mated with untreated
rats during the last six days. Fertility was reduced to 21% of that of
control rats and remained reduced (47%) after a 50-60-day recovery
period (Mazzei-Planas & Kuc, 1968).
The effects of aqueous S. rebaudiana extracts corresponding to
0.67 g dried leaves per ml, given at a dose of 2 ml/rat twice a day
for 60 days, were studied in prepubertal (25-30 days old) rats. The
end-points were glycaemia; serum concentrations of thyroxine and
tri-iodothyronine; available binding sites in thyroid hormone-binding
proteins; binding of 3H-methyltrienolone (a specific ligand of
androgen receptors) to prostate cytosol; zinc content of the prostate,
testis, submandibular salivary gland, and pancreas; water content of
testis and prostate; body-weight gain; and the final weights of the
testis, prostate, seminal vesicle, submandibular salivary gland, and
adrenal. None of these parameters was significantly different from
those in the control group, with the exception of the seminal vesicle
weight, which fell by about 60%. The authors concluded that if the
Stevia extract can decrease fertility in rats, the effect is almost
certainly not exerted on males (Oliveira-Filho et al., 1989).
2.2.6 Developmental toxicity
Rats
Stevioside (purity, 95.6%) dissolved in distilled water was given
to four groups of 25 or 26 pregnant Wistar rats by gavage once a day
on days 6-15 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw.
The rats were sacrificed on day 20 of gestation, and their fetuses
were examined for malformations. The end-points examined were maternal
and fetal body weight, number of live fetuses, sex distribution,
number of resorptions or dead fetuses, and incidence of malformations.
No treatment-related effect on general or reproductive toxicity was
observed up to the highest dose. The authors concluded that orally
administered stevioside is not teratogenic in rats (Takanaka et al.,
1991; Usami et al., 1995).
2.2.7 Studies on metabolites: Steviol
2.2.7.1 Absorption, distribution, and excretion: Steviol
Intact or bile-duct ligated rats were given [17-14C]-steviol
(specific activity, 2.9 µCi/mg, 1.7 µCi/rat, corresponding to
approximately 3 mg/kg bw) either orally or by intracaecal injection.
After oral administration, 1.5% of the radiolabel was excreted in the
urine of intact rats and 96% in that of bile-duct-ligated animals; the
corresponding amounts in faeces were 96 and 3.3%. After intracaecal
administration of 14C-steviol to bile-duct ligated rats, 94 and 6% of
the radiolabel was excreted in urine and faeces, respectively. When
bile was collected over 72 h, all of the intracaecally injected
radiolabel was recovered. Very little (0.02% of dose) was exhaled as
14C-carbon dioxide. The authors concluded that steviol is completely
absorbed from the rat lower bowel (Wingard et al., 1980).
2.2.7.2 Effects on enzymes and other biochemical parameters: Steviol
Steviol administered to female RCR/Ha mice did not induce
glutathione S-transferase activity in liver or intestinal mucosa
(Pezzuto et al., 1986).
Steviol at 0.5 mmol/L, equivalent to 0.16 mg/ml, inhibited
oxidative phosphorylation and the activity of ATPase (92%), NADH
oxidase (45%), succinate oxidase (42%), succinate dehydrogenase (46%),
and L-glutamate dehydrogenase (46%). The ADP:O ratio was decreased.
Substrate respiration was increased at concentrations up to
0.5 mmol/L, equivalent to 0.16 mg/ml, and inhibited at > 1 mmol/L,
equivalent to 0.32 mg/ml. Inhibition of substrate respiration was the
only effect observed in uncoupled mitochondria. Net proton ejection
induced by succinate and swelling induced by several substrates were
inhibited. The authors concluded that steviol acts as a uncoupler of
oxidative phosphorylation (Kelmer-Bracht et al., 1985).
Steviol decreased glucose production and inhibited oxygen uptake
in isolated Wistar rat renal cortical tubules (IC50, 0.3 mmol/L,
equivalent to 96 µg/ml). The authors concluded that this effect is
consistent with an inhibitory action on oxidative phosphorylation and
electron transport in mitochondria (Yamamoto et al., 1985).
Steviol inhibited oxidative phosphorylation in isolated rat liver
mitochondria. The concentration required for 50% inhibition of ATP
synthesis was 40 µmol/L, equivalent to 13 µg/ml. Steviol also
inhibited the 2,4-dinitro-phenol-stimulated ATPase, phosphorylation of
exogenous ADP, and exchange between exogenous 14C-ADP and endogenous
adenine nucleotides. The authors concluded that steviol does not act
at the level of the coupling mechanism but at the level of
mitochondrial translocation of adenine nucleotides (Vignais et al.,
1966).
The effects of steviol (purity, 90%) on intestinal glucose
absorption were examined in hamster jejunum by the everted sac
technique. Thus, 1 mmol/L steviol (equivalent to 318.5 µg/ml)
inhibited glucose absorption by 29-43%, and the inhibition was related
to the steviol concentration and incubation time. Reductions in the
intestinal mucosal ATP content and absorptive surface area were
responsible for the inhibition of glucose absorption. The decrease in
intestinal mucosal ATP content was accompanied by a decrease in the
activities of mitochondrial NADH cytochrome c reductase and cytochrome
oxidase. Steviol did not inhibit the activity of intestinal
Na+/K+-ATPase or glucose uptake in the intestinal brush-border
membrane vesicles. Steviol altered the morphology of the intestinal
absorptive cells. The authors concluded that inhibition of intestinal
glucose absorption by steviol in hamsters is due to a reduction in
mucosal ATP content and alteration of the morphology of the intestinal
absorptive cells (Toskulkao et al., 1995a,b).
Single doses of 200 µmol/L steviol, equivalent to 255 mg/kg bw,
were given orally to 24-h-fasted male Wistar rats, either alone or
simultaneously with fructose. Under these conditions, steviol
increased the initial glycogen deposition in the liver. When steviol
was given to the rats in drinking-water at 1 or 2 mmol/L, equivalent
to 32 and 64 mg/kg bw, at the beginning of a fasting period of 24 or
48 h, it had no effect on hepatic glycogen concentrations (Hübler et
al., 1994).
Concomitant treatment of Raji cells (human lymphoblastoid cells
carrying the Epstein-Barr viral genome) with
12- O-tetradecanoylphorbol 13-acetate (TPA) and steviol strongly
inhibited the induction of Epstein-Barr virus early antigen by TPA,
with 50% inhibition at 25 µg/ml (Okamoto et al., 1983a).
In a study of the effects of steviol at 0.2 µmol/L (equivalent to
64 ng/ml) on the induction of ornithine decarboxylase in mouse skin by
TPA, the activity in the epidermis had increased by about 300-fold
4-5 h after application of 17 nmol/L TPA. TPA-induced ornithine
decarboxylase activity was strongly decreased (63%) when steviol was
applied to mouse skin 1 h before TPA treatment, concurrently with TPA
(75%), or 1 h after TPA (71%). Steviol alone did not induce epidermal
ornithine decarboxylase activity. The authors concluded that steviol
interferes with the process of induction of this enzyme by TPA in
mouse skin (Okamoto et al., 1983b).
2.2.7.3 Acute toxicity: Steviol
In male and female mice and rats given steviol (purity, 90%)
orally, the LD50 was > 15 g/kg bw, and 1/15 animals died within 14
days of administration. The LD50 values in hamsters given steviol
orally were 5.2 g/kg bw in males and 6.1 g/kg bw in females.
Histopathological examination of the kidneys revealed severe
degeneration of the proximal tubular cells, and these structural
alterations were correlated with increased serum blood urea nitrogen
and creatinine. The authors concluded that the cause of death was
acute renal failure (Toskulkao et al., 1997).
2.2.7.4 Genotoxicity: Steviol
Studies of the genotoxicity of steviol are summarized in Table 3.
The major metabolite of steviol in vitro, 15alpha-hydroxysteviol,
was inactive at doses up to 7.5 mg/ml in the forward mutation assay in
S. typhimurium strain TM677 with metabolic activation.
15-Oxosteviol, a product of the oxidation of 15alpha-hydroxysteviol,
was a directly acting mutagen at 25-200 µg/ml and was highly toxic to
bacteria. Moreover, the expression of mutagenicity required the
presence of the 13-hydroxy group and the C-16 exomethylene group
(Compadre et al., 1988).
15-Oxosteviol was not mutagenic in various test systems.
Repetition of the experiment with S. typhimurium TM677 failed to
show significant induction of 8-azaguanine-resistant mutants, even
when the number of bacteria tested was greatly increased. The authors
concluded that the earlier positive result reported was due to a
common mishandling of data obtained in the TM677 system and that
15-oxosteviol is unlikely to be the active metabolite responsible for
the mutagenicity of steviol (Procinska et al., 1991).
Table 3. Results of assays for the genotoxicity of steviol
End-point Test object Concentration Results Reference
Reverse mutation S. typhimurium TA98 and TA100 20 mg/platea Negative Suttajit et al. (1993)
Reverse mutation S. typhimurium TA97, TA98, TA100, 5 mg/platea Negative Matsui et al. (1996a)b
TA102, TA104, TA1535, and TA1537 (purity, 99%)
Forward mutation S. typhimurium TM677 10 mg/platec Negative Matsui et al. (1996a)
0.5-10 mg/plated Positive
Forward mutation S. typhimurium TM677 10 mg/platec Negative Pezzuto et al. (1985a)
10 mg/platee Positive
umu Gene mutation S. typhimurium TA1535/pSK1002 625-1250 µg/platec Positive Matsui et al. (1996a)
1259-2500 µg/plated Positive
Gene mutation B. subtilis H17 rec+, M45 rec- 10 mg/disca Negative Matsui et al. (1996a)
Gene mutation Chinese hamster lung fibroblasts 400 µg/mld Positivef Matsui et al. (1996a)
Chromosomal aberration Chinese hamster lung fibroblasts 0.5 g/mlc Negative Matsui et al. (1996a)
1-1.5 mg/mld Positive
Chromosomal aberration Human lymphocytes 0.2 mg/ml Negative Suttajit et al. (1993)
Micronucleus formation MS/Ae mice 1000 mg/kg bwg Negative Matsui et al. (1996a)
a With and without metabolic activation
b The same results are cited in an earlier abstract (Matsui et al., 1987).
c Without metabolic activation
d With metabolic activation
e With metabolic activation derived from phenobarbital- or Aroclor 1254-pretreated rats; fractions from control or
3-methylcholanthrene-pretreated rats were ineffective.
f Diphtheria toxin-resistant colonies
g Toxic: 4/6 mice at highest dose given intraperitoneally died
The expression of the mutagenic activity of steviol in
S. typhimurium TM677 was dependent on both metabolic activation
(9000 × g fraction derived from phenobarbital- or Aroclor
1254-pretreated rats) and addition of NADPH. The authors concluded
that a cytochrome P450 mediates the metabolic activation of steviol to
a mutagenic species. As partially purified rat liver epoxide hydrolase
did not inhibit steviol-induced mutagenicity, the authors concluded
that the active metabolite is not an epoxide.
A species structurally related to steviol, isosteviol, was not
active in S. typhimurium TM677, regardless of whether metabolic
activation was provided. Similarly, chemical reduction of the
unsaturated bond linking the carbon-16 and -17 positions of steviol
resulted in the generation of two isomeric products, dihydrosteviol A
and B, which were not mutagenic. Ent-kaurenoic acid was also inactive.
A potential metabolite of steviol, steviol-16alpha,17-epoxide, was
synthesized chemically and found to be ineffective as a directly
acting mutagen. The authors concluded that it is a metabolite of an
integral component of stevioside that is mutagenic. The structural
features necessary for the expression of mutagenic activity include a
hydroxy group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 (Pezzuto et al., 1985a, 1986).
Steviol was mutagenic after metabolic activation in the forward
mutation assay with S. typhimurium TM677. The authors confirmed
first that the 8-aza-guanine resistance of the TM677 mutants resides
in the chromosomal guanine phosphoribosyltransferase (gpt) gene,
since it could be complemented by the gpt gene of Escherichia
coli. The chromosomal DNA of TM677 and TM677 mutants was digested by
several restriction enzymes (BamHI, Sau3AI, AluI, TaqI, HaeIII, HpaII,
and RsaI) and analysed by Southern blot hybridization with a probe for
the gpt gene in DNA of E. coli. No significant difference in DNA
fragment length was found between the wild type and spontaneous or
steviol-induced mutants (Matsui et al., 1988, 1989a).
pSV2-gpt plasmids were treated with metabolically activated
steviol (concentration not given), and the DNA was subsequently
analysed by polyacrylamide gel electrophoresis after digestion with
restriction endonucleases (Sau3AI, HhaI, HpaII). Steviol induced a
fivefold increase in mutation frequency, and seven mutants were
obtained, all showing deletions ranging from 20 bp to 2 kb (Matsui et
al., 1989b).
Steviol strongly induced mutations at the gpt gene of S.
typhimurium TM677 when a metabolic activation system was present,
but it had no activity in reverse mutation assays with E. coli
WP2uvrA/pKM101 or S. typhimurium TA strains. In order to
characterize the mutations induced by metabolically activated steviol,
the chromosomal gpt alleles of 24 induced (ST clones) and 16
spontaneous mutants (SP clones) of S. typhimurium TM677 were
sequenced, and the mutation spectra were compared. Nine out of 24 of
the mutations of ST clones were localized in the region between
nucleotides 280 and 330 from the starting codon ATG, whereas no
mutations of SP clones were found in that region. The mutations
identified included transitions (three clones), transversions (four
clones), a duplication, and a deletion. There were no other marked
differences between the ST and SP clones: base-change mutations
predominated over frameshifts and deletions (ST clones, 20 versus
three; SP clones, 16 versus two), and base-change mutations occurred
more frequently at G:C pairs than at A:T pairs (ST clones, 15 versus
five; SP clones, 12 versus four). The authors suggested that
metabolically activated steviol interrupts DNA synthesis around
nucleotide 280, thereby stimulating duplication, deletion, and
untargeted mutagenesis in the defined region of the gpt gene
downstream from the site of interruption (Matsui et al., 1996b).
19- O-ß-D-Glucopyranosyl steviol, a potential hydrolysis product
of stevioside, was mutagenic to S. typhimurium TM677 and
bactericidal in the presence of a metabolic activating system (Pezzuto
et al., 1986).
Microsomes derived from human liver mediated a mutagenic response
of steviol (Pezzuto et al., 1985b).
2.2.7.5 Developmental toxicity: Steviol
Groups of 20 pregnant golden hamsters were given steviol (purity,
90%) at doses of 0, 250, 500, 750, or 1000 mg/kg bw per day (only 12
animals at the highest dose) by gavage in corn oil on days 6-10 of
gestation. A significant decrease in body-weight gain and increased
mortality (1/20, 7/20, and 5/12) were observed at the three highest
doses, and the number of live fetuses per litter and mean fetal weight
decreased in parallel. Histopathological examination of the maternal
kidneys showed a dose-dependent increase in the severity of effects on
the convoluted tubules (dilatation, hyaline droplets). No
dose-dependent teratogenic effects were seen. The NOEL was 250 mg/kg
bw per day for both maternal and developmental toxicity (Wasuntarawat
et al., 1998).
2.2.8 Special studies
2.2.8.1 Cariogenicity
Groups of 15 albino Sprague-Dawley rat pups colonized with
Streptococcus sobrinus received 0.5% stevioside or 30% sucrose in
the basal diet or basal diet alone and were sacrificed after five
weeks, when S. sobrinus was counted and caries were evaluated. There
was no difference in food or water intake or in weight gain among the
groups, but significant differences in sulcal caries scores and
S. sobrinus counts were found between the group receiving sucrose
and the other groups. There was no significant difference between the
group receiving stevioside and the controls. The authors concluded
that stevioside was not cariogenic under the conditions of this study
(Das et al., 1992).
2.2.8.2 Renal function and vasodilatation
The effect of stevioside on renal function was evaluated by
clearance techniques in groups of seven pentobarbital-anesthetized
male Wistar rats simultaneously with the effect of indomethacin on the
renal action of stevioside, given at a priming dose of 4, 8, 12, or
16 mg/kg bw followed by an infusion rate of 4, 8, 12, or 16 mg/kg bw
per hour. Mean arterial pressure and renal function were measured.
Administration of stevioside resulted in a statistically significant,
dose-related decrease in mean arterial pressure (120 ± 2.3 with 4
mg/kg bw to 72 ± 4.8 mm Hg with 16 mg/kg bw) and an increase in renal
plasma flow (10 ± 1.2 with 4 mg/kg bw to 26 ± 2.9 ml/min per kg bw
with 16 mg/kg bw), with no change in glomerular filtration rate.
Stevioside also increased fractional sodium (FeNa+) and potassium
(FeK+) excretion and urine flow (volume/glomerular filtration rate).
The decrease in mean arterial pressure (control, 120 ± 0.93;
stevioside, 91 ± 2.5 mm Hg) and increase in renal plasma flow
(control, 14 ± 1.4; stevioside, 33 ± 2.8 ml/min per kg bw) induced by
stevioside at 16 mg/kg bw were inhibited by simultaneous
administration of indomethacin at 2 mg/kg bw, but the glomerular
filtration rate was not affected. The diuretic, natriuretic, and
kaliuretic effects of stevioside were also abolished by indomethacin.
The authors concluded that stevioside behaves like a typical
vasodilator, causing changes in mean arterial pressure, diuresis,
natriuresis, and kaliuresis per millilitre of glomerular filtration,
and that these effects probably depend on prostaglandins (Melis &
Sainati, 1991a).
The effects of intravenous administration of verapamil (0.015
mg/min) and calcium chloride (800 mEq/L, 0.025 ml/kg bw per min) on
renal function and mean arterial pressure were evaluated in groups of
10 pentobarbital-anaesthetized male Wistar rats weighing 280-320 g
during intravenous treatment with stevioside (16 mg/kg bw per min).
Verapamil significantly increased the hypotensive effect of stevioside
on mean arterial pressure (control, 120 ± 0.77; stevioside, 96 ± 1.5;
stevioside plus verapamil, 67 ± 0.70 mm Hg) and on fractional sodium
excretion (control, 0.76 ± 0.05; stevioside, 1.6 ± 0.10; stevioside
plus verapamil, 2.7 ± 0.25%). Urinary flow, reported as percent
glomerular filtration rate, and renal plasma flow were increased
slightly but not significantly during administration of stevioside
plus verapamil. In contrast, infusion of calcium chloride into rats
pretreated with stevioside resulted in a marked attenuation of mean
arterial pressure (control, 120 ± 1.8; stevioside, 70 ± 1.1;
stevioside plus calcium chloride, 110 ± 1.6 mm Hg) and renal plasma
flow (control, 17 ± 3.8; stevioside, 34 ± 2.6; stevioside plus calcium
chloride, 17 ± 2.9 ml/min per kg bw). The diuresis and natriuresis
induced by stevioside were also inhibited by simultaneous
administration of calcium chloride. The authors concluded that
stevioside acts on arterial pressure and renal function as a calcium
antagonist, as does verapamil (Melis, 1992b).
Classical clearance techniques and arterial pressure measurements
in pentobarbital-anaesthetized male Wistar rats showed that stevioside
at a priming dose of 8 or 16 mg/kg bw followed by an infusion rate of
8 or 16 mg/kg bw per h caused a fall in systemic blood pressure and in
diuresis and natriuresis per millilitre of glomerular filtration rate.
Verapamil tended to increase the renal and systemic effects of
stevioside. In contrast, an infusion of calcium chloride into rats
pretreated with stevioside induced marked attenuation of the
vasodilatatory responses to stevioside. The authors concluded that
stevioside, like verapamil, acts as a calcium antagonist (Melis &
Sainati, 1991b).
The effect of stevioside (purity, > 90%) at a priming dose of
16 mg/kg bw followed by an infusion rate of 16 mg/kg bw per h on renal
function in normal Wistar rats and rats with experimental renal
hypertension was evaluated by clearance techniques. Stevioside
provoked hypotension, diuresis, and natriuresis in both groups of
rats. The normal rats had increased renal plasma flow and a constant
glomerular filtration rate after stevioside administration, whereas
the hypertensive rats had increased renal plasma flow and glomerular
filtration rate. The authors concluded that stevioside impairs a renal
autoregulation mechanism in this model (Melis, 1992c).
The effects of administration of aqueous S. rebaudiana extracts
corresponding to 0.67 g/ml dried leaves given at 2 ml/rat twice a day
for 20, 40, or 60 days on renal function and mean arterial pressure
were studied in normal Wistar rats weighing 80-100 g. Rats treated for
20 days showed no significant difference from the controls, but
administration of the crude extract for 40 or 60 days induced
hypotension, diuresis, and natriuresis, with a constant glomerular
filtration rate. Increased renal plasma flow was seen only in the
group treated for 60 days. The authors concluded that oral
administration of an aqueous extract of dried leaves of Stevia to
rats induces systemic and renal vasodilation, causing hypotension,
diuresis, and natriuresis (Melis, 1995).
Normotensive and experimentally hypertensive male Wistar rats
(Goldblatt GII experimental hypertension induced by clipping the left
renal artery, leaving the contralateral kidney intact) received an
S. rebaudiana extract corresponding to 0.67 g/ml dried leaves given
at 2 ml/rat by gavage twice a day (2.7 g dry leaves per day) for 30
days. Administration of Stevia 10-12 weeks after clipping resulted in
a significant decrease in mean arterial pressure in both the
normotensive and hypertensive rats: normotensive, 110 ± 3.0 mm Hg in
controls versus 70 ± 4.0 mm Hg in those given Stevia; hypertensive,
160 ± 3.0 mm Hg in controls versus 110 ± 4.0 mm Hg with Stevia. The
glomerular filtration rate was constant in the normotensive rats but
increased significantly in the hypertensive rats after Stevia
treatment (16 ± 1.3 versus 14 ± 1.3 ml/min per kg bw in the controls
and Stevia groups, respectively). Both normotensive and hypertensive
rats had increased renal plasma flow after administration of Stevia:
normotensive, 16 ± 3.1 ml/min per kg bw in controls versus 33 ± 3.2
ml/min per kg bw in the Stevia group; hypertensive, 19 ± 2.5 ml/min
per kg bw in controls versus 37 ± 3.9 ml/min per kg bw in the
Stevia group. Stevia increased urinary flow in both normotensive
(1.4 ± 0.08% versus 2.3 ± 0.11%) and hypertensive animals (1.5 ± 0.07%
versus 3.0 ± 0.13%) and also increased sodium excretion
i(normotensive, 0.61 ± 0.07% in controls versus 1.6 ± 0.2% in the
Stevia group; hypertensive, 0.70 ± 0.1% in controls versus 2.2 ±
0.45% in the Stevia group). The authors concluded that Stevia
impairs renal autoregulation in this model (Melis, 1996).
2.3 Observations in humans
S. rebaudiana has been used by Indians in Paraguay as an oral
contraceptive (Mazzei-Planas & Kuc, 1968; Schvartzman et al., 1977).
Aqueous extracts of 5 g of S. rebaudiana leaves were
administered to 16 volunteers at 6-h intervals for three days, and
glucose tolerance tests were performed before and after
administration. Another six volunteers were given an aqueous solution
of arabinose in order to eliminate possible effects of stress. The
extract increased glucose tolerance and significantly decreased plasma
glucose concentrations during the test and after overnight fasting in
all volunteers (Curi et al., 1986).
3. COMMENTS
After oral administration to rats, stevioside is not readily
absorbed from the upper small intestine but is hydrolysed to the
aglycone, steviol, before absorption from the gut. Steviol per se is
completely absorbed and is excreted in the bile as conjugates; only a
very small fraction is detectable in urine. After biliary excretion,
the conjugates are hydrolysed, and steviol undergoes enterohepatic
circulation; its elimination half-life is 24 h. Steviol is the only
faecal metabolite of stevioside that has been identified, and
excretion in the faeces is the major route. After intravenous
administration, stevioside is rapidly distributed throughout the body,
partially secreted by the renal tubular epithelium, and excreted in
urine.
At high concentrations, stevioside affected a variety of
biochemical parameters in rat tissues in vitro. It weakly inhibited
oxidative phosphorylation, and steviol was about 30 times more potent
in this respect. The most likely mechanism is inhibition of the
mitochondrial translocation of adenine nucleotides. Steviol also
inhibited glucose absorption from rat gut by reducing the mucosal ATP
content. Stevioside may also act as a calcium antagonist, showing a
hypotensive effect and inducing diuresis, natriuresis, and a fall in
renal tubular reabsorption of glucose. Stevioside may not, however, be
able to penetrate cell membranes. Although most of these studies were
performed after intravenous injection of stevioside, orally
administered extracts of S. rebaudiana to rats had similar effects
(hypotension and diuresis).
Stevioside has very low acute oral toxicity. Oral administration
of stevioside at a dietary concentration of 2.5% to rats for two
years, equal to 970 and 1100 mg/kg bw per day in males and females,
respectively, had no significant effect. Reduced body-weight gain and
survival rate were observed at a dietary concentration of 5%
stevioside. There was no indication of carcinogenic potential in a
long-term study and no evidence of urinary bladder tumour promoting
potential in a separate bioassay.
In studies of reproductive toxicity, administration of stevioside
at doses up to 2500 mg/kg bw per day to hamsters and 3000 mg/kg bw per
day to rats had no effect. Although an aqueous infusion of
S. rebaudiana administered orally to female rats was reported to
cause a severe, long-lasting reduction in fertility, the contraceptive
effect of Stevia is probably not due to stevioside. Stevioside had
neither teratogenic nor embryotoxic effects in rats given up to 1000
mg/kg bw per day by gavage.
The results of tests for genotoxicity with stevioside in various
systems were uniformly negative.
The aglycone, steviol, was more acutely toxic than stevioside to
hamsters but not to rats. Steviol was clearly genotoxic after
metabolic activation, inducing forward mutations in bacteria and gene
mutations and chromosomal aberrations in Chinese hamster lung
fibroblasts. Several mechanistic studies indicated that the structural
features necessary for the expression of mutagenic activity include a
hydroxyl group at position 13 and an unsaturated bond joining the
carbon atoms at positions 16 and 17 of steviol. The fact that
stevioside is glycosylated at position 13 could explain the absence of
mutagenicity. The active metabolite of steviol responsible for its
mutagenic activity is not known. While some data suggest that
epoxidation may be involved in the metabolic activation of steviol,
other data indicate that the active metabolite is not an epoxide.
Preliminary data indicate that human liver microsomes may activate
steviol to a mutagenic metabolite.
4. EVALUATION
The Committee noted several shortcomings in the information
available on stevioside. In some studies, the material tested
(stevioside or steviol) was poorly specified or of variable quality,
and no information was available on other constituents or
contaminants. Furthermore, no studies of human metabolism of
stevioside and steviol were available. In addition, data on long-term
toxicity and carcinogenicity were available for stevioside in only one
species. The mutagenic potential of steviol has been tested
sufficiently only in vitro.
In view of the fact that no information was provided for
elaboration of specifications for stevioside and that the evaluation
of the available toxicological data revealed several limitations, the
Committee was unable to relate the results of the toxicological
investigations to the article of commerce and could not allocate an
ADI to stevioside.
Before the substance is reviewed again, specifications must be
developed to ensure that the material tested is representative of the
material of commerce, and further information should be made available
on the nature of the substance that was tested, on the human
metabolism of stevioside, and on the activity of steviol in suitable
studies of genotoxicity in vivo .
5. REFERENCES
Aze, Y., Toyoda, K., Imaida, K., Hayashi, S., Imazawa, T., Hayashi, Y.
& Takahashi, M. (1991) Subchronic oral toxicity study of stevioside in
F344 rats. Bull. Natl Inst. Hyg. Sci., 48-54 (in Japanese).
Cardoso, V.N., Barbosa, M.F., Muramoto, E., Mesquita, C.H. & Almeida,
M.A. (1996) Pharmacokinetic studies of 131I-stevioside and its
metabolites. Nucl. Med. Biol., 23, 97-100.
Compadre, C.M., Hussain, R.A., Nanayakkara, N.P., Pezzuto, J.M. &
Kinghorn, A.D. (1988) Mass spectral analysis of some derivatives and
in vitro metabolites of steviol, the aglycone of the natural
sweeteners, stevioside, rebaudioside A, and rubusoside. Biomed.
Environ. Mass Spectrom., 15, 211-222.
Constantin, J., Ishii-Iwamoto, E.L., Ferraresi-Filho, O.,
Kelmer-Bracht, A.M. & Bracht, A. (1991) Sensitivity of ketogenesis and
citric acid cycle to stevioside inhibition of palmitate transport
across the cell membrane. Braz. J. Med. Biol. Res., 24, 767-771.
Curi, R., Alvarez, M., Bazotte, R.B., Botion, L.M., Godoy, J.L. &
Bracht, A. (1986) Effect of Stevia rebaudiana on glucose tolerance
in normal adult humans. Braz.J. Med. Biol. Res., 19, 771-774
(English abstract only).
Das, S., Das, A.K., Murphy, R.A., Punwani, I.C., Nasution, M.P. &
Kinghorn, A.D. (1992) Evaluation of the cariogenic potential of the
intense natural sweeteners stevioside and rebaudioside A. Caries
Res., 26, 363-366.
Hagiwara, A., Fukushima, S., Kitaori, M., Shibata, M. & Ito, N. (1984)
Effects of three sweeteners on rat urinary bladder carcinogenesis
initiated by N-butyl-N-(4-hydroxybutyl)nitrosamine. Gann, 75,
763-768 (English abstract only).
Hanson, J.R. & De Oliveira, B.H. (1993) Stevioside and related sweet
diterpenoid glycosides. Nat. Prod. Rep., 10, 301-309.
Hübler, M.O., Bracht, A. & Kelmer-Bracht, A.M. (1994) Influence of
stevioside on hepatic glycogen levels in fasted rats. Res. Commun.
Chem. Pathol. Pharmacol., 84, 111-118.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M. & Matsuoka, A. (1984) Primary mutagenicity screening of
food additives currently used in Japan. Food Chem. Toxicol., 22,
623-636.
Ishii, E.L. & Bracht, A. (1986) Stevioside, the sweet glycoside of
Stevia rebaudiana, inhibits the action of atractyloside in the
isolated perfused rat liver. Res. Commun. Chem. Pathol. Pharmacol.,
53, 79-91.
Ishii, E.L., Schwab, A.J. & Bracht, A. (1987) Inhibition of
monosaccharide transport in the intact rat liver by stevioside.
Biochem. Pharmacol., 36, 1417-1433.
Ishii-Iwamoto, E.L. & Bracht, A. (1995) Stevioside is not metabolized
in the isolated perfused rat liver. Res. Commun. Mol.
Pathol.Pharmacol., 87, 167-175.
Ito, N., Fukushima, S., Shirai, T., Hagiwara, A. & Imaida, K. (1984)
Drugs, food additives and natural products as promoters in rat urinary
bladder carcinogenesis. In: Börzsönyi, M., Day, N.E., Lapis, K. &
Yamasaki, H., eds, Models, Mechanisms and Etiology of Tumour
Promotion (IARC Scientific Publications No. 56), Lyon, International
Agency for Research on Cancer, pp. 399-407.
Kelmer-Bracht, A.M., Alvarez, M. & Bracht, A. (1985) Effects of
Stevia rebaudiana natural products on rat liver mitochondria.
Biochem. Pharmacol., 34, 873-882.
Kim, K.K., Sawa, Y. & Shibata, H. (1996) Hydroxylation of
ent-kaurenoic acid to steviol in Stevia rebaudiana Bertoni --
Purification and partial characterization of the enzyme. Arch.
Biochem. Biophys., 332, 223-230.
Krejci, M.E. & Koechel, D.A. (1992) Acute effects of
carboxyatractyloside and stevioside, inhibitors of mitochondrial
ADP/ATP translocation, on renal function and ultrastructure in
pentobarbital-anesthetized dogs. Toxicology, 72, 299-313.
Liu, J., Ong, C.P. & Li, S.F.Y. (1997) Subcritical fluid extraction of
Stevia sweeteners from Stevia rebaudiana. J. Chromatogr.Sci., 35,
446-450.
Matsui, M., Matsui, K., Kawasaki, Y., Sawada, M., Nohmi, T., Sofuni,
T., Yoshihira, K., Ishidate, M., Oda, Y., Noguchi, T., Komatsubara, S.
& Kitagawa, Y. (1987) Mutagenicity test on stevioside and its
aglycone, steviol. Mutat. Res., 182, 366.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1988)
Mutagenicity of steviol: An analytical approach using a Southern
blotting system. Mutat. Res., 203, 377.
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989a)
Mutagenicity of steviol: An analytical approach using the Southern
blotting system. Bull. Natl Inst. Hyg.Sci., 83-87 (in Japanese).
Matsui, M., Matsui, K., Nohmi, T., Mizusawa, H. & Ishidate, M. (1989b)
Detection of deletion mutations in pSV2-gpt plasmids induced by
metabolically activated steviol. Mutat. Res., 216, 367-368.
Matsui, M., Matsui, K., Kawasaki, Y., Oda, Y., Noguchi, T., Kitagawa,
Y., Sawada, M., Hayashi, M., Nohmi, T., Yoshihira, K., Ishidate, M. &
Sofuni, T. (1996a) Evaluation of the genotoxicity of stevioside and
steviol using six in vitro and one in vivo mutagenicity assays.
Mutagenesis, 11, 573-579.
Matsui, M., Sofuni, T. & Nohmi, T. (1996b) Regionally-targeted
mutagenesis by metabolically-activated steviol: DNA sequence analysis
of steviol-induced mutants of guanine phosphoribosyltransferase (gpt)
gene of Salmonella typhimurium TM677. Mutagenesis, 11, 565-572.
Mazzei-Planas, G. & Kuc, J. (1968) Contraceptive properties of
Stevia rebaudiana. Science, 162, 1007.
Medon, P.J., Pezzuto, J.M., Hovanec-Brown, J.M., Nanayakkara, N.P.,
Soejarto, D.D., Kamath, S.K. & Kinghorn, A.D. (1982) Safety assessment
of some Stevia rebaudiana sweet principles. Fed. Proc., 41, 1568.
Melis, M.S. (1992a) Renal excretion of stevioside in rats. J. Nat.
Prod., 55, 688-690.
Melis, M.S. (1992b) Influence of calcium on the blood pressure and
renal effects of stevioside. Braz. J. Med.Biol. Res., 25, 943-949.
Melis, M.S. (1992c) Stevioside effect on renal function of normal and
hypertensive rats. J. Ethnopharmacol., 36, 213-217.
Melis, M.S. (1995) Chronic administration of aqueous extract of
Stevia rebaudiana in rats: Renal effects. J. Ethnopharmacol., 47,
129-134.
Melis, M.S. (1996) A crude extract of Stevia rebaudiana increases
the renal plasma flow of normal and hypertensive rats. Braz. J.
Med.Biol. Res., 29, 669-675.
Melis, M.S. & Sainati, A.R. (1991a) Participation of prostaglandins in
the effect of stevioside on rat renal function and arterial pressure.
Braz. J. Med. Biol. Res., 24, 1269-1276.
Melis, M.S. & Sainati, A.R. (1991b) Effect of calcium and verapamil on
renal function of rats during treatment with stevioside. J.
Ethnopharmacol., 33, 257-262.
Mori, N., Sakanoue, M., Takeuchi, M., Shimpo, K. & Tanabe, T. (1981)
Effect of stevioside on fertility in rats. J. Food Hyg. Soc. Jpn.,
22, 409-414 (in Japanese).
Nakayama, K., Kasahara, D. & Yamamoto, F. (1986) Absorption,
distribution, metabolism and excretion of stevioside in rats.
J. Food Hyg. Soc. Jpn., 27, 1-8.
Nanayakkara, N.P., Klocke, J.A., Compadre, C.M., Hussain, R.A.,
Pezzuto, J.M. & Kinghorn, A.D. (1987) Characterization and feeding
deterrent effects on the aphid, Schizaphis graminum, of some
derivatives of the sweet compounds, stevioside and rebaudioside A.
J. Nat. Prod., 50, 434-441.
Okamoto, H., Yoshida, D. & Mizusaki, S. (1983a) Inhibition of
12-O-tetradecanoylphorbol-13-acetate-induced induction in Epstein-Barr
virus early antigen in Raji cells. Cancer Lett., 19, 47-53.
Okamoto, H., Yoshida, D., Saito, Y. & Mizusaki, S. (1983b) Inhibition
of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine
decarboxylase activity in mouse epidermis by sweetening agents and
related compounds. Cancer Lett., 21, 29-35.
Oliveira-Filho, R.M., Uehara, O.A., Minetti, C.A. & Valle, L.B. (1989)
Chronic administration of aqueous extract of Stevia rebaudiana
(Bert.) Bertoni in rats: Endocrine effects. Gen. Pharmacol., 20,
187-191.
Pezzuto, J.M., Compadre, C.M., Swanson, S.M., Nanayakkara, D. &
Kinghorn, A.D. (1985a) Metabolically activated steviol, the aglycone
of stevioside, is mutagenic. Proc. Natl Acad Sci. USA, 82,
2478-2482.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Lam, L.K., Sparnins, V.L. &
Wattenberg, L.W. (1985b) Evaluation of the potential of steviol
(13-hydroxy-ent-kaurenoic acid) and related diterpenes to mediate
bacterial mutagenesis and induce glutathione-S-transferase in mice.
Proc. Am. Assoc. Cancer Res., 26, 94.
Pezzuto, J.M., Nanayakkara, N.P., Compadre, C.M., Swanson, S.M.,
Kinghorn, A.D., Guenthner, T.M., Sparnins, V.L. & Lam, L.K. (1986)
Characterization of bacterial mutagenicity mediated by
13-hydroxy-ent-kaurenoic acid (steviol) and several
structurally-related derivatives and evaluation of potential to induce
glutathione S-transferase in mice. Mutat. Res., 169, 93-103.
Procinska, E., Bridges, B.A. & Hanson, J.R. (1991) Interpretation of
results with the 8-azaguanine resistance system in Salmonella
typhimurium: No evidence for direct acting mutagenesis by
15-oxosteviol, a possible metabolite of steviol. Mutagenesis, 6,
165-167.
Schvartzman, J.B., Krimer, D.B. & Moreno-Azorero, R. (1977)
Cytological effects of some medicinal plants used in the control of
fertility. Experientia, 33, 663-665.
Soejarto, D.D., Kinghorn, A.D. & Farnsworth, N.R. (1982) Potential
sweetening agents of plant origin. III. Organoleptic evaluation of
Stevia leaf herbarium samples for sweetness. J. Nat. Prod., 45,
590-599.
Suanarunsawat, T. & Chaiyabutr, N. (1997) The effect of stevioside on
glucose metabolism in the rat. Can. J. Physiol. Pharmacol., 75,
976-982.
Suttajit, M., Vinitketkaumnuen, U., Meevatee U. & Buddhasukh, D.
(1993) Mutagenicity and human chromosomal effect of stevioside, a
sweetener from Stevia rebaudiana Bertoni. Environ. Health
Perspectives, 101, 53-56.
Takanaka, T., Kawashima, K., Usami, M. & Sakami, K. (1991) A
teratological study of stevioside administered orally to rats.
Unpublished report from Department of Pharmacology, Biological Safety
Research Center, National Institute of Hygienic Sciences, Japan.
Submitted to WHO by Ministry of Health and Welfare, Food Chemistry
Division, Japan.
Toskulkao, C., Sutheerawatananon, M., Wanichanon, C., Saitongdee, P. &
Suttajit, M. (1995a) Effects of stevioside and steviol on intestinal
glucose absorption in hamsters. J. Nutr. Sci. Vitaminol., 41,
105-113.
Toskulkao, C., Sutheerawattananon, M. & Piyachaturawat, P. (1995b)
Inhibitory effect of steviol, a metabolite of stevioside, on glucose
absorption in everted hamster intestine in vitro. Toxicol. Lett.,
80, 153-159.
Toskulkao, C., Chaturat, L., Temcharoen, P. & Glinsukon, T. (1997)
Acute toxicity of stevioside, a natural sweetener, and its metabolite,
steviol, in several animal species. Drug Chem.Toxicol., 20, 31-44.
Toyoda, K., Kawanishi, T., Uneyama, C. & Takahashi, M. (1995)
Re-evaluation of the safety of a food additive (reported in fiscal
1994). A chronic toxicity/carcinogenicity study of stevioside (a
substance extracted from Stevia): Final report. Unpublished report
from Division of Pathology, Biological Safety Research Center,
National Institute of Health Sciences, Japan. Submitted to WHO by
Ministry of Health and Welfare, Food Chemistry Division, Japan.
Toyoda, K., Matsui, H., Shoda,T., Uneyama, C., Takada, K. & Takahashi,
M. (1997) Assessment of the carcinogenicity of stevioside in F344
rats. Food Chem. Toxicol., 35, 597-603.
Usami, M., Seino, Y., Takai, J., Nakahara, H., Seino, S., Ikeda, M. &
Imura, H. (1980) Effect of cyclamate sodium, saccharin sodium and
stevioside on arginine-induced insulin and glucagon secretion in the
isolated perfused rat pancreas. Horm. Metab. Res., 12, 705-706.
Usami, M., Sakemi, K., Kawashima, K., Tsuda, M. & Ohno, Y. (1995)
Teratogenicity study of stevioside in rats. Bull. Natl Inst. Hyg.
Sci., 113, 31-35 (in Japanese).
Vignais, P.V., Duee, E.D., Vignais, P.M. & Huet, J. (1966) Effects of
atractyligenin and its structural analogues on oxidative
phosphorylation and on the translocation of adenine nucleotides in
mitochondria. Biochim. Biophys. Acta, 118, 465-483.
Wasuntarawat, C., Temcharoen, P., Toskulkao, C., Mungkornkarn, P.,
Suttajit, M. & Glinsukon, T. (1998) Developmental toxicity of steviol,
a metabilite of stevioside, in the hamster. Drug Chem.Toxicol., 21,
207-222.
Wingard, R.E., Brown, J.P., Enderlin, F.E., Dale, J.A., Hale, R.L. &
Seitz, C.T. (1980) Intestinal degradation and absorption of the
glycosidic sweeteners stevioside and rebaudioside A. Experientia,
36, 519-520.
Xili, L., Chengjiany, B., Eryi, X., Reiming, S., Yuengming, W.,
Haodong, S. & Zhiyian, H. (1992) Chronic oral toxicity and
carcinogenicity study of stevioside in rats. Food Chem. Toxicol.,
30, 957-965.
Yamamoto, N.S., Kelmer-Bracht, A.M., Ishii, E.L., Kemmelmeier, F.S.,
Alvarez, M. & Bracht, A. (1985) Effect of steviol and its structural
analogues on glucose production and oxygen uptake in rat renal
tubules. Experientia, 41, 55-57.
Yodyingyuad, V. & Bunyawong, S. (1991) Effect of stevioside on growth
and reproduction. Hum. Reprod., 6, 158-165.
