
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
LINEAR AND BRANCHED-CHAIN ALIPHATIC, UNSATURATED, UNCONJUGATED ALCOHOLS,
ALDEHYDES, ACIDS, AND RELATED ESTERS
First draft prepared by
Dr Antonia Mattia
Division of Product Policy, Office of Premarket Approval (HFS-206)
Center for Food Safety and Applied Nutrition
US Food and Drug Administration
Washington DC, United States
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety
Evaluation of Flavouring Substances
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Explanation
Intake
Biological data
Absorption and metabolism
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
and carcinogenicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated 42 flavouring substances that include
linear and branched-chain aliphatic unsaturated and unconjugated
alcohols, aldehydes, acids, and related esters (Table 1) using the
Procedure for the Safety Evaluation of Flavouring Agents (Figure 1, p.
222, and Annex 1, reference 131). The Committee had evaluated one
member of the group, oleic acid, previously at its thirty-third
meeting (Annex 1, reference 83), when ADIs 'not specified' were
allocated to the calcium, potassium, and sodium salts of oleic acid.
At that meeting, the Committee noted that the safety of fatty acids,
including oleic acid, is based on knowledge about their metabolism and
excretion and on their occurrence in edible fats and oils that have a
long history of use as foods or food components.
1.2 Estimated daily per capita intake
The total annual volume of the 42 linear and branched-chain
aliphatic unsaturated primary alcohols and unconjugated aldehydes,
carboxylic acids, and related esters is approximately 39 000 kg in
Europe (International Organization of the Flavor Industry, 1995) and
13 000 kg in the United States (National Academy of Sciences, 1987).
Three substances in the group, cis-3-hexen-1-ol (No. 315), linoleic
acid (No. 332), and oleic acid (No. 330), account for about 96% of the
total annual volume in Europe. Four substances in the group,
cis-3-hexen-1-ol, oleic acid, 2,6-dimethyl-5-heptenal (No. 349), and
hexyl 2-methyl-3&4-pentenoate (No. 352) account for about 91% of the
total annual volume in the United States. On the basis of the reported
annual volume in Europe, the total estimated per capita intake is
4300 µg/person of cis-3-hexen-1-ol (71 µg/kg bw per day), 130
µg/person of linoleic acid (2 µg/kg bw per day), and 970 µg/person of
oleic acid (16 µg/kg bw per day). On the basis of the reported annual
volume in the United States, the total estimated per capita intake
is 440 µg/person of oleic acid (7 µg/kg bw per day), 250 µg/person of
2,6-dimethyl-5-heptenal (4 µg/kg bw per day), and 520 µg per person of
hexyl-2-methyl-3&4-pentenoate (9 µg/kg bw per day).
Twenty-seven of the 42 unsaturated aliphatic primary alcohols and
unconjugated aldehydes, acids, and related esters in this group are
reported to occur in a wide variety of foods, including fruits and
vegetables, dairy products, fish, and alcoholic beverages (Maarse et
al., 1994). Oleic and linoleic acids are some of the commonest
unsaturated fatty acids found in vegetable oils and animal fats, often
constituting more than 50% of the total fatty acid concentration.
1.3 Absorption, metabolism, and elimination
The metabolism of flavouring agents in this group involves common
pathways of intermediary metabolism, which are discussed in the
section 'General aspects of metabolism' in "Safety Evaluations of
Groups of Related Substances by the Procedure for the Safety
Evaluation of Flavouring Agents".
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step A1. All of the linear and branched-chain aliphatic unsaturated
primary alcohols and unconjugated aldehydes, acids, and
related esters are classified in structural class I (Cramer
et al., 1978).
Step A2. The metabolic fates of the linear and branched-chain
unsaturated primary alcohols and unconjugated aldehydes,
carboxylic acids, and related esters in this group of
flavouring agents can (with one exception) be predicted
readily because of their close structural relationship to
endogenous substrates and the broad substrate specificities
of the relevant enzymes. The prediction that the metabolites
would be innocuous was supported by available data on the
toxicity of members of this group. The Committee considered
that inadequate data were available to predict the
metabolism of ethyl 2-methyl-3,4-pentadienoate (No. 353),
because of its terminal diene.
At current levels of intake, the remaining 41
substances in this group would not be expected to saturate
the metabolic pathways, and all of them are predicted to be
metabolized to innocuous products (see section 4.2). The
evaluation of these 41 substances therefore proceeds via the
left side ('A') of the evaluation scheme (see Steps A3-A5).
The evaluation of ethyl 2-methyl-3,4-pentadienoate (No.
353) proceeds via the right side ('B') of the evaluation
scheme (see Steps B3 and B4).
Step A3. At current levels of intake, all 41 substances were
predicted to be metabolized to innocuous products and,
except for cis-3-hexen-1-ol, the intake is below the human
intake threshold for class I substances (1800 µg/person per
day). The 40 substances were considered to be of no safety
concern at this step on the basis of their structural class
and low levels of estimated intake. As the daily per
capita intakes of cis-3-hexen-1-ol in Europe and the
United States are 4300 µg/person per day (71 µg/kg bw per
day) and 1100 µg/person per day (18 µg/kg bw per day),
respectively, intake in Europe exceeds the human intake
threshold for class I substances.
Step A4. cis-3-Hexen-1-ol is not endogenous in humans.
Step A5. A NOEL of 120-150 mg/kg bw per day was reported for
cis-3-hexen-1-ol in a 98-day study in rats (Gaunt et al.,
1969). A safety margin of > 1000 exists between this NOEL
and the daily per capita intakes of 71 and 18 µg/kg bw per
day from use of cis-3-hexen-1-ol as a flavouring agent in
Europe and the United States, respectively.
Step B3. The daily per capita intakes of ethyl
2-methyl-3,4-pentadienoate in Europe and the United States
are 0.01 µg/person per day (< 0.001 µg/kg bw per day) and
10 µg/person per day (0.2 µg/kg bw per day), respectively;
therefore, intake does not exceed the human intake threshold
for class I substances.
Step B4. Adequate data to determine a NOEL for ethyl
2-methyl-3,4-pentadienoate were not available. The Committee
was aware of a study in rats given ethyl
2-methyl-3,4-pentadienoate in the diet at a concentration
equivalent to a daily intake of 1 mg/kg bw for 92 days (Cox
et al., 1978); however, a full report of the study was not
available.
The stepwise evaluations of the selected linear and
branched-chain aliphatic unsaturated and unconjugated alcohols,
aldehydes, acids, and related esters in this group of flavouring
agents, are summarized in Table 1.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all 41 of the linear and
branched-chain aliphatic unsaturated and unconjugated aldehydes,
acids, and related esters that were evaluated were consumed
concomitantly on a daily basis, the estimated combined intake would
exceed the human threshold for class I. All 41 substances are expected
to be efficiently metabolized, however, and would not saturate
metabolic pathways. On the basis of the evaluation of the collective
data, the combined intake was judged by the Committee not to raise
safety concerns. The consideration of combined intakes did not include
intake of ethyl 2-methyl-3,4-pentadienoate because the evaluation of
this substance was deferred pending review of the 90-day toxicity
study, which was not available to the Committee.
1.6 Conclusions
The Committee concluded that 41 of the linear and branched-chain
aliphatic, unsaturated, unconjugated alcohols, aldehydes, acids, and
related esters in this group would not present safety concerns when
used at current levels of intake as flavouring agents. Data on the
toxicity of cis-3-hexen-1-ol and on the metabolism of these
flavouring agents were required for application of the procedure. The
Committee noted that these and other available data on toxicity are
consistent with the results of the safety evaluation with the
procedure. The evaluation of one substance in the group, ethyl
2-methyl-3,4-pentadienoate, was deferred pending evaluation of a
90-day study in rats that was not available to the Committee. The ADI
'not specified' for the calcium, potassium, and sodium salts of oleic
acid established at the thirty-third meeting was maintained.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to the safety
evaluation of 42 linear and branched-chain aliphatic, unsaturated,
unconjugated alcohols, aldehydes, acids, and related esters (see
Table 1). This group does not include any alpha,ß-unsaturated (i.e.
conjugated) aldehydes, acids, or related esters. The group of
flavouring substances includes the following: five linear aliphatic
unsaturated primary alcohols (Nos 315, 318, 321, 322, and 324); a
branched-chain aliphatic unsaturated primary alcohol (No. 348); eight
linear aliphatic unsaturated unconjugated aldehydes (Nos 316, 319,
320, 323, 325, 326, 329, and 330); a branched-chain aliphatic
unsaturated unconjugated aldehyde (No. 349); seven linear aliphatic
unsaturated unconjugated carboxylic acids (Nos 314, 317, 327, 328, and
331-333); 13 esters of linear aliphatic unsaturated unconjugated
carboxylic acids (Nos 334-349); two branched-chain aliphatic
unsaturated unconjugated carboxylic acids (Nos 347 and 355); and five
esters of branched-chain aliphatic unsaturated unconjugated carboxylic
acids (Nos 350-354). The chemical structures of the substances in this
group are given in Table 1.
The substances in this group are structurally related because
they are primary aliphatic alcohols, aldehydes, carboxylic acids, and
related esters containing unsaturation that is not conjugated with the
functional group. Therefore, these unsaturated unconjugated substances
are predicted to have similar metabolic and toxicological profiles,
with one exception, as described below.
2.2 Intake
The most recent figures for the annual production volume in
Europe and the United States are given in Table 2 for the 42
substances in this group. Intake was estimated on the assumption that
the production was underestimated and consumption was by 10% of the
population, as indicated in the footnote to Table 2.
Quantitative data on natural occurrence have been reported for 11
of the 42 substances in the group, indicating that they are generally
consumed predominantly from traditional foods (Maarse et al., 1994).
Four of the 11 substa-nces (Nos 315, 317, 320, and 346) are consumed
in greater quantities when used as flavouring substances (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, short-chain (< C-8) linear and branched-chain
aliphatic alcohols, aldehydes, acids, and related esters are rapidly
absorbed from the gastrointestinal tract (Dawson et al., 1964;
Gaillard & Derache, 1965). Long-chain carboxylic acids such as
linoleic and oleic acid are readily absorbed as micelle aggregates,
esterified with glycerol in chylomicrons and very low density
lipoproteins, and transported via the lymphatic system (Borgstrom,
1974). Radiolabelled linoleic and oleic acids have been administered
to a variety of amphibians, mammals, and humans by various routes.
Fatty acid uptake occurred in all tissues, including the brain, by
passive and/or facilitated diffusion (Dhopeshwarkar & Mead, 1973;
Harris et al., 1980; Abumrad et al., 1984).
Hydrolysis of linear and branched-chain esters
The esters are hydrolysed in digestive fluids and in vivo to
yield the corresponding alcohols and unsaturated carboxylic acids, as
discussed in the section, 'General aspects of metabolism'. The results
of studies of hydrolysis in vitro indicate that the rate of
hydrolysis of straight-chain esters is approximately 100 times greater
than that of branched-chain esters (Drake et al., 1975).
Table 1. Summary of results of safety evaluations of linear and branched-chain aliphatic, unsaturated, unconjugated alcohols, aldehydes,
acids, and related esters
Step 1: All of the substances in this table are in structural class I.
The human intake threshold is 1800 µg/day for class I.
Step 2: All of the substances in this group are predicted to be metabolized to innocuous products, except ethyl 2-methyl-3,4-pentadienoate.
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
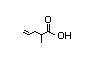
4-Pentenoic acid 314 5/3 No N/R N/R Converted to coenzyme No safety concern
A thioester which
undergoes dehydrogenation and
selective hydrogenation
and then participates in
the fatty acid and
tricarboxylic acid pathways
CAS No. 591-80-0
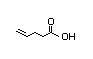 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexen-1-ol 315 4300/1100 Yes No Yes. The NOAEL Expected to be oxidized No safety concern
of 120-150 mg/kg to the corresponding
bw per day [Gaunt aldehyde and carboxylic
et al., 1969] is acid which is completely
>1000 times the metabolized in the
daily per capita fatty acid and tricarboxylic
intake. acid pathways
CAS No. 928-96-1
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexen-1-ol 315 4300/1100 Yes No Yes. The NOAEL Expected to be oxidized No safety concern
of 120-150 mg/kg to the corresponding
bw per day [Gaunt aldehyde and carboxylic
et al., 1969] is acid which is completely
>1000 times the metabolized in the
daily per capita fatty acid and tricarboxylic
intake. acid pathways
CAS No. 928-96-1
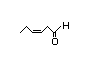
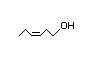 cis-3-Hexenal 316 5/3 No N/R N/R See cis-3-hexenol. No safety concern
CAS No. 6789-80-6
cis-3-Hexenal 316 5/3 No N/R N/R See cis-3-hexenol. No safety concern
CAS No. 6789-80-6
 3-Hexenoic acid 317 11/1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4219-24-3
3-Hexenoic acid 317 11/1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4219-24-3
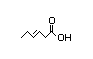 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Hexen-1-ol 318 3/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6126-50-7
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Hexen-1-ol 318 3/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6126-50-7
 cis-4-Hexenal 319 0.03/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4634-89-3
cis-4-Hexenal 319 0.03/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4634-89-3
 4-Heptenal 320 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6728-31-0
4-Heptenal 320 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6728-31-0
 cis-3-Octen-1-ol 321 6/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 20125-84-2
cis-3-Octen-1-ol 321 6/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 20125-84-2
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-5-Octen-1-ol 322 0.5/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 64275-73-6
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-5-Octen-1-ol 322 0.5/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 64275-73-6
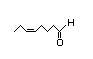
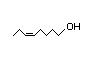 cis-5-Octenal 323 0.001/29 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 41547-22-2
cis-5-Octenal 323 0.001/29 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 41547-22-2
 cis-6-Nonen-1-ol 324 3/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 35854-86-5
cis-6-Nonen-1-ol 324 3/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 35854-86-5
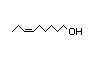 cis-6-Nonenal 325 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 2277-19-2
cis-6-Nonenal 325 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 2277-19-2
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Decenal 326 1/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 30390-50-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Decenal 326 1/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 30390-50-2
 5&6-Decenoic acid 327 4/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 72881-27-7
5&6-Decenoic acid 327 4/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 72881-27-7
 9-Decenoic acid 328 0.1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 14436-32-9
9-Decenoic acid 328 0.1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 14436-32-9
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
9-Undecenal 329 1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 143-14-6
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
9-Undecenal 329 1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 143-14-6
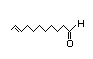 10-Undecenal 330 0.4/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-45-8
10-Undecenal 330 0.4/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-45-8
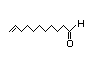 10-Undecenoic acid 331 30/0.8 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-38-9
10-Undecenoic acid 331 30/0.8 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-38-9
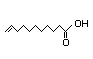 Linoleic acid 332 133/6 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 60-33-3
Linoleic acid 332 133/6 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 60-33-3
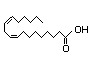 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Oleic acida 333 970/440 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 112-80-1
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Oleic acida 333 970/440 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 112-80-1
 Methyl 3-hexenoate 334 0.7/0.01 No N/R N/R Expected to be hydrolysed No safety
to its component concern
alcohol and acid
See also cis-3-hexenol.
CAS No. 2396-78-3
Methyl 3-hexenoate 334 0.7/0.01 No N/R N/R Expected to be hydrolysed No safety
to its component concern
alcohol and acid
See also cis-3-hexenol.
CAS No. 2396-78-3
 Ethyl 3-hexenoate 335 4/1 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 2396-83-0
Ethyl 3-hexenoate 335 4/1 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 2396-83-0
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexenyl 336 4/0.8 No N/R N/R See methyl 3-hexenoate. No safety
cis-3-hexenoate concern
CAS No. 61444-38-0
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexenyl 336 4/0.8 No N/R N/R See methyl 3-hexenoate. No safety
cis-3-hexenoate concern
CAS No. 61444-38-0
 Methyl 337 0.4/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 21063-71-8
Methyl 337 0.4/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 21063-71-8
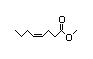 Ethyl 338 1/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 34495-71-1
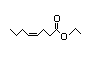
Ethyl 338 1/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 34495-71-1
 Ethyl 339 2/0.01 No N/R N/R See methyl 3-hexenoate. No safety
cis-4,7-octadienoate concern
CAS No. 69925-33-3
Ethyl 339 2/0.01 No N/R N/R See methyl 3-hexenoate. No safety
cis-4,7-octadienoate concern
CAS No. 69925-33-3
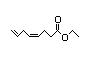 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3-nonenoate 340 2/76 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 13481-87-3
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3-nonenoate 340 2/76 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 13481-87-3
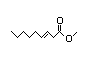 Ethyl 341 2/0.04 No N/R N/R See methyl 3-hexenoate. No safety
trans-4-decenoate concern
CAS No. 76649-16-6
Ethyl 341 2/0.04 No N/R N/R See methyl 3-hexenoate. No safety
trans-4-decenoate concern
CAS No. 76649-16-6
 Methyl 9-un-decenoate 342 39/0.4 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 5760-50-9
Methyl 9-un-decenoate 342 39/0.4 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 5760-50-9
 Ethyl 10-undecenoate 343 2/44 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 692-86-4
Ethyl 10-undecenoate 343 2/44 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 692-86-4
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Butyl 10-undecenoate 344 0.04/5 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 109-42-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Butyl 10-undecenoate 344 0.04/5 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 109-42-2
 Ethyl oleate 345 69/3 No N/R N/R See methyl 3-hexenoate No safety
and oleic acid. concern
CAS No. 111-62-6
Ethyl oleate 345 69/3 No N/R N/R See methyl 3-hexenoate No safety
and oleic acid. concern
CAS No. 111-62-6
 Methyl linoleate 346 N/D/23 No N/R N/R See methyl 3-hexenoate No safety
& Methyl linolenate and linoleic acid. concern
(mixture)
CAS No. 301-00-8
Methyl linoleate 346 N/D/23 No N/R N/R See methyl 3-hexenoate No safety
& Methyl linolenate and linoleic acid. concern
(mixture)
CAS No. 301-00-8
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
2-Methyl-3-pentenoic 347 1/2 No N/R N/R Undergoes ß oxidation to No safety
acid yield products that are concern
completely metabolized in
the tricarboxylic acid cycle
CAS No. 37674-63-8
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
2-Methyl-3-pentenoic 347 1/2 No N/R N/R Undergoes ß oxidation to No safety
acid yield products that are concern
completely metabolized in
the tricarboxylic acid cycle
CAS No. 37674-63-8
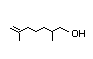
 2,6-Dimethyl- 348 N/D/0.1 No N/R N/R See 2-methyl-3-pentenoic No safety
6-hepten-1-ol acid. concern
CAS No. 36806-46-9
2,6-Dimethyl- 348 N/D/0.1 No N/R N/R See 2-methyl-3-pentenoic No safety
6-hepten-1-ol acid. concern
CAS No. 36806-46-9
 2,6-Dimethyl- 349 31/251 No N/R N/R See 2-methyl-3-pentenoic No safety
5-heptenal acid. concern
CAS No. 106-72-9
2,6-Dimethyl- 349 31/251 No N/R N/R See 2-methyl-3-pentenoic No safety
5-heptenal acid. concern
CAS No. 106-72-9
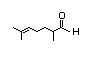 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
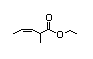
Ethyl 2-methyl- 350 6/6 No N/R N/R Expected to be hydrolysed No safety
3-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 1617-23-8
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Ethyl 2-methyl- 350 6/6 No N/R N/R Expected to be hydrolysed No safety
3-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 1617-23-8
 Ethyl 2-methyl- 351 0.3/0.02 No N/R N/R Expected to be hydrolysed No safety
4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 53399-81-8
Ethyl 2-methyl- 351 0.3/0.02 No N/R N/R Expected to be hydrolysed No safety
4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 53399-81-8
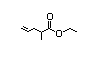 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Hexyl 2-methyl- 352 0.03/525 No N/R N/R Expected to be hydrolysed No safety
3&4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 58625-95-9
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Hexyl 2-methyl- 352 0.03/525 No N/R N/R Expected to be hydrolysed No safety
3&4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 58625-95-9
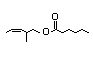
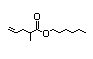 Ethyl 2-methyl- 353 0.01/10 - - - Cannot be predicted to Evaluation of this
3,4-pentadienoate be metabolized to innocuous substance was
products; therefore, , steps deferred pending
A3-5 are not applicable review of a 90-day
to this substance, which study not available
must be evaluated on at this meeting.
the right side ('B') of
the evaluation scheme.
CAS No. 60523-21-9
Ethyl 2-methyl- 353 0.01/10 - - - Cannot be predicted to Evaluation of this
3,4-pentadienoate be metabolized to innocuous substance was
products; therefore, , steps deferred pending
A3-5 are not applicable review of a 90-day
to this substance, which study not available
must be evaluated on at this meeting.
the right side ('B') of
the evaluation scheme.
CAS No. 60523-21-9
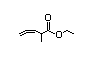 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3,7-dimethyl- 354 0.2/0.1 No N/R N/R Expected to be hydrolysed No safety concern
6-octenoate to its component
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 2270-60-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3,7-dimethyl- 354 0.2/0.1 No N/R N/R Expected to be hydrolysed No safety concern
6-octenoate to its component
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 2270-60-2
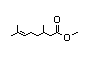 2-Methyl-4-pentenoic 355 N/D/0.04 No N/R N/R See 2-methyl-3-pentenoic No safety concern
acid acid.
CAS No. 1575-74-2
2-Methyl-4-pentenoic 355 N/D/0.04 No N/R N/R See 2-methyl-3-pentenoic No safety concern
acid acid.
CAS No. 1575-74-2
 N/R, Not required for evaluation because consumption was determined to be of no safety concern at Step 3A of the procedure
N/D, No intake data reported
a The ADI 'not specified' for the calcium, potassium, and sodium salts established at the thrity-third meeting of the Committee (Annex 1,
reference 83) was maintained.
Table 2. Most recent annual usage of linear and branched-chain aliphatic unsaturated
unconjugated alcohols, aldehydes, acids, and related esters as flavouring substances in Europe
and the United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Pentenoic acid (314)
Europe 32 5 0.08 NA
United States 15 3 0.05
cis-3-Hexen-1-ol (315)
Europe 30 000 4 300 71 NA
United States 5 600 1 100 18
cis-3-Hexenal (316)
Europe 34 5 0.08 NA
United States 18 3 0.06
3-Hexenoic acid (317)
Europe 77 11 0.2 NA
United States 5 1 0.02
4-Hexen-1-ol (318)
Europe 20 3 0.05 NA
United States 0.2 0.04 0.001
cis-4-Hexenal (319)
Europe 0.2 0.03 0.0005 NA
United States 0.5 0.1 0.002
4-Heptenal (320)
Europe 13 2 0.03 NA
United States 0.5 0.1 0.002
cis-3-Octen-1-ol (321)
Europe 39 6 0.1 NA
United States 0.05 0.01 0.0002
cis-5-Octen-1-ol (322)
Europe 3.3 0.5 0.01 NA
United States 0.2 0.04 0.001
cis-5-Octenal (323)
Europe 0.01 0.001 0.00002 NA
United States 150 29 0.5
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
cis-6-Nonen-1-ol (324 )
Europe 18 3 0.04 NA
United States 0.5 0.1 0.002
cis-6-Nonenal (325)
Europe 14 2 0.03 NA
United States 0.5 0.1 0.002
4-Decenal (326)
Europe 8 1 0.02 NA
United States 0.05 0.01 0.0002
5&6-Decenoic acid (327)
Europe 28 4 0.07 NA
United States 0.9 0.2 0.003
9-Decenoic acid (328)
Europe 0.8 0.1 0.002 NA
United States 0.9 0.2 0.003
9-Undecenal (329)
Europe 8 1 0.02 NA
United States 0.9 0.2 0.003
10-Undecenal (330)
Europe 2.6 0.4 0.01 NA
United States 0.2 0.04 0.001
10-Undecenoic acid (331)
Europe 210 30 0.5 NA
United States 4 0.8 0.01
Linoleic acid (332)
Europe 940 130 2 NA
United States 31 6 0.1
Oleic acid (333)
Europe 6 800 970 16 NA
United States 2 300 440 7
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3-hexenoate (334)
Europe 4.6 0.7 0.01 0.003
United States 0.05 0.01 0.0002
Ethyl 3-hexenoate (335)
Europe 26 4 0.1 0.03
United States 6 1 0.02
cis-3-Hexenyl cis-3-hexenoate (336)
Europe 26 4 0.1 0.05
United States 4 0.8 0.01
Methyl cis-4-octenoate (337)
Europe 3 0.4 0.01 0.002
United States 0.2 0.04 0.001
Ethyl cis-4-octenoate (338)
Europe 10 1 0.02 0.005
United States 0.2 0.04 0.001
Ethyl cis-4,7-octadienoate (339)
Europe 15 2 0.04 0.01
United States 0.04 0.01 0.0001
Methyl 3-nonenoate (340)
Europe 13 2 0.03 0.006
United States 400 76 1
Ethyl trans-4-octenoate (341)
Europe 15 2 0.04 0.009
United States 0.2 0.04 0.001
Methyl 9-undecenoate (342)
Europe 280 39 1 0.20
United States 2 0.4 0.01
Ethyl 10-undecenoate (343)
Europe 12 2 0.03 0.006
United States 230 44 1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Butyl 10-undecenoate (344)
Europe 0.3 0.04 0.0007 0.0002
United States 25 5 0.08
Ethyl oleate (345)
Europe 490 69 1 0.10
United States 17 3 0.1
Methyl linoleate & methyl
linolenate (mixture) (346)
Europe NR N/D
United States 120 23 0.4 0.046
2-Methyl-3-pentenoic acid (347)
Europe 10 1 0.02 NA
United States 9 2 0.03
2,6-Dimethyl-6-hepten-1-ol (348)
Europe NR N/D NA
United States 0.5 0.1 0.002
2,6-Dimethyl-5-heptenal (349)
Europe 220 31 1 NA
United States 1 300 250 4
Ethyl 2-methyl-3-pentenoate (350)
Europe 40 6 0.1 0.03
United States 31 6 0.1
Ethyl 2-methyl-4-pentenoate (351)
Europe 0.2 0.03 0.0005 0.0002
United States 0.9 0.2 0.003
Hexyl 2-methyl-3&4-pentenoate (352)
Europe 0.2 0.03 0.0005 0.0003
United States 2 760 520 9
Ethyl 2-methyl-3,4-pentadienoate (353)
Europe 0.1 0.01 0.0002 0.00007
United States 50 10 0.2
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3,7-dimethyl-6-octenoate (354)
Europe 1.1 0.2 0.003 0.0005
United States 0.3 0.1 0.001
2-Methyl-4-pentenoic acid (355)
Europe NR N/D NA
United States 0.2 0.04 0.001
Total
Europe 39 168 NA NA NA
United States 13 130 NA NA NA
NR, not reported; NA, not applicable
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor Industry,
1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
Linear unsaturated primary alcohols, aldehydes, and acids
The alcohols (including those formed from ester hydrolysis) and
aldehydes in this group are efficiently oxidized to the corresponding
unsaturated carboxylic acids by high-capacity enzyme pathways, as
discussed in the section 'General aspects of metabolism'. The
resulting linear unsaturated carboxylic acids participate in normal
fatty acid metabolism. Short-chain acids containing terminal
unsaturation, such as 4-pentenoic acid (No. 314), may be metabolized
by desaturation to yield a substrate that may participate in the fatty
acid pathway.
Alternative minor metabolic pathways have been characterized for
linear long-chain fatty acids (linoleic acid and oleic acid) and
short-chain acids containing unsaturation (i.e. 4-pentenoic acid).
While linoleic and oleic acids participate in ß-oxidation and normal
fatty acid metabolism in most tissues (Masoro, 1977), they may undergo
omega-oxidation in the liver and alpha-oxidation in the brain (Wakil &
Barnes, 1971; Gibson et al., 1982). In another minor pathway for the
oxidative metabolism of 4-pentenoic acid, trans-2,4-pentadienoyl
coenzyme A is produced, which is further metabolized via ß-oxidation
to yield 3-keto-4-pentenoyl coenzyme A (Schulz, 1983).
Branched-chain unsaturated primary alcohols, aldehydes, and acids
Generally, branched-chain aliphatic alcohols are oxidized to the
corresponding aldehydes, which, in turn, are oxidized to the
corresponding carboxylic acids (Bosron & Li, 1980; Levi & Hodgson,
1989). The aldehyde may also be reduced to the corresponding alcohol,
which is probably a short-lived intermediate in vivo. Like their
saturated analogues, unsaturated branched-chain aliphatic alcohols and
aldehydes are converted by the pathways cited above to the
corresponding acids. The acids may undergo ß-oxidative cleavage and
complete metabolism to carbon dioxide (Williams, 1959; Voet & Voet,
1990) in amino acid pathways, the fatty acid pathway, and the
tricarboxylic acid cycle. Alternatively, they may undergo a
combination of omega-, (omega-1)-, and ß-oxidation and selective
dehydrogenation and hydration to yield polar metabolites which are
excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Diliberto et al., 1990). The
principal metabolic pathways for detoxification of these
branched-chain substances is determined primarily by four structural
characteristics: carbon chain length and the position, number, and
size of the alkyl substituents.
These multiple pathways are illustrated by 2-ethylbutyric acid
and 2-ethyl-1-butanol. An oral dose of 2-ethyl-1-butanol was excreted
unchanged in the urine, principally as the glucuronic acid conjugate
(Kamil et al., 1953). 2-Ethylbutyric acid is metabolized by dogs to
2-pentanone, which is presumably derived from ß-oxidation and
decarboxylation (Deuel, 1957). Urinary metabolites of 2-ethylbutyric
acid reported in diabetic patients include the glucuronide conjugates
of 3-keto-2-ethylbutyric acid and 3,3'-diketo-2-ethylbutyric acid
formed by ß-oxidation of one or both ethyl chains (Granger et
al.,1954).
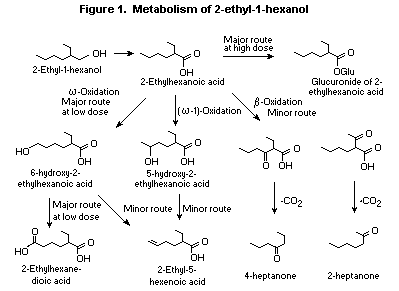
Higher homologues of 2-ethyl-1-butanol have a more complex
pattern of metabolism in which omega-oxidation competes with excretion
of the glucuronide conjugate of the corresponding acid. Female Fischer
344 rats were given a single oral dose of 50 or 500 mg/kg bw
[1-14C-ethyl]-2-ethylhexanol by gavage or repeated daily oral doses
of 50 mg/kg bw 2-ethyl-hexanol for 14 days. The major urinary
metabolites of the structurally related saturated branched-chain
alcohol 2-ethyl-1-hexanol collected over 0-24 h included the
glucuronic acid conjugate of 2-ethyl-hexanoic acid, omega- and
(omega-1)-oxidation metabolites, 2-ethyl-1,6-dihexanoic acid,
5-hydroxy-2-ethylhexanoic acid and its corresponding -lactone, and
6-hydroxy-2-ethylhexanoic acid. Minute quantities of the
omega-desaturation metabolite, 2-ethyl-5-hexenoic acid, and
metabolites formed from ß oxidation and oxidative decarboxylation, 2-
and 4-heptanone, were also detected (Albro, 1975; Deisinger et al.,
1994). After single or repeated doses of 50 mg/kg bw, the
omega-oxidation pathway predominated, as the principal urinary
metabolites excreted were 2-ethyl-1,6-hexane-dioic acid (30%) and its
precursors (8.5%). Only 6.8% of the dose was excreted in the urine as
the glucuronic acid conjugate of 2-ethylhexanoic acid. With 500 mg/kg
bw, 2-ethylhexanoic acid (25%) was the principal urinary metabolite,
indicating that saturation of the omega-desaturation pathway occurs at
this dose (Deisinger et al., 1994; Figure 1).
N/R, Not required for evaluation because consumption was determined to be of no safety concern at Step 3A of the procedure
N/D, No intake data reported
a The ADI 'not specified' for the calcium, potassium, and sodium salts established at the thrity-third meeting of the Committee (Annex 1,
reference 83) was maintained.
Table 2. Most recent annual usage of linear and branched-chain aliphatic unsaturated
unconjugated alcohols, aldehydes, acids, and related esters as flavouring substances in Europe
and the United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Pentenoic acid (314)
Europe 32 5 0.08 NA
United States 15 3 0.05
cis-3-Hexen-1-ol (315)
Europe 30 000 4 300 71 NA
United States 5 600 1 100 18
cis-3-Hexenal (316)
Europe 34 5 0.08 NA
United States 18 3 0.06
3-Hexenoic acid (317)
Europe 77 11 0.2 NA
United States 5 1 0.02
4-Hexen-1-ol (318)
Europe 20 3 0.05 NA
United States 0.2 0.04 0.001
cis-4-Hexenal (319)
Europe 0.2 0.03 0.0005 NA
United States 0.5 0.1 0.002
4-Heptenal (320)
Europe 13 2 0.03 NA
United States 0.5 0.1 0.002
cis-3-Octen-1-ol (321)
Europe 39 6 0.1 NA
United States 0.05 0.01 0.0002
cis-5-Octen-1-ol (322)
Europe 3.3 0.5 0.01 NA
United States 0.2 0.04 0.001
cis-5-Octenal (323)
Europe 0.01 0.001 0.00002 NA
United States 150 29 0.5
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
cis-6-Nonen-1-ol (324 )
Europe 18 3 0.04 NA
United States 0.5 0.1 0.002
cis-6-Nonenal (325)
Europe 14 2 0.03 NA
United States 0.5 0.1 0.002
4-Decenal (326)
Europe 8 1 0.02 NA
United States 0.05 0.01 0.0002
5&6-Decenoic acid (327)
Europe 28 4 0.07 NA
United States 0.9 0.2 0.003
9-Decenoic acid (328)
Europe 0.8 0.1 0.002 NA
United States 0.9 0.2 0.003
9-Undecenal (329)
Europe 8 1 0.02 NA
United States 0.9 0.2 0.003
10-Undecenal (330)
Europe 2.6 0.4 0.01 NA
United States 0.2 0.04 0.001
10-Undecenoic acid (331)
Europe 210 30 0.5 NA
United States 4 0.8 0.01
Linoleic acid (332)
Europe 940 130 2 NA
United States 31 6 0.1
Oleic acid (333)
Europe 6 800 970 16 NA
United States 2 300 440 7
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3-hexenoate (334)
Europe 4.6 0.7 0.01 0.003
United States 0.05 0.01 0.0002
Ethyl 3-hexenoate (335)
Europe 26 4 0.1 0.03
United States 6 1 0.02
cis-3-Hexenyl cis-3-hexenoate (336)
Europe 26 4 0.1 0.05
United States 4 0.8 0.01
Methyl cis-4-octenoate (337)
Europe 3 0.4 0.01 0.002
United States 0.2 0.04 0.001
Ethyl cis-4-octenoate (338)
Europe 10 1 0.02 0.005
United States 0.2 0.04 0.001
Ethyl cis-4,7-octadienoate (339)
Europe 15 2 0.04 0.01
United States 0.04 0.01 0.0001
Methyl 3-nonenoate (340)
Europe 13 2 0.03 0.006
United States 400 76 1
Ethyl trans-4-octenoate (341)
Europe 15 2 0.04 0.009
United States 0.2 0.04 0.001
Methyl 9-undecenoate (342)
Europe 280 39 1 0.20
United States 2 0.4 0.01
Ethyl 10-undecenoate (343)
Europe 12 2 0.03 0.006
United States 230 44 1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Butyl 10-undecenoate (344)
Europe 0.3 0.04 0.0007 0.0002
United States 25 5 0.08
Ethyl oleate (345)
Europe 490 69 1 0.10
United States 17 3 0.1
Methyl linoleate & methyl
linolenate (mixture) (346)
Europe NR N/D
United States 120 23 0.4 0.046
2-Methyl-3-pentenoic acid (347)
Europe 10 1 0.02 NA
United States 9 2 0.03
2,6-Dimethyl-6-hepten-1-ol (348)
Europe NR N/D NA
United States 0.5 0.1 0.002
2,6-Dimethyl-5-heptenal (349)
Europe 220 31 1 NA
United States 1 300 250 4
Ethyl 2-methyl-3-pentenoate (350)
Europe 40 6 0.1 0.03
United States 31 6 0.1
Ethyl 2-methyl-4-pentenoate (351)
Europe 0.2 0.03 0.0005 0.0002
United States 0.9 0.2 0.003
Hexyl 2-methyl-3&4-pentenoate (352)
Europe 0.2 0.03 0.0005 0.0003
United States 2 760 520 9
Ethyl 2-methyl-3,4-pentadienoate (353)
Europe 0.1 0.01 0.0002 0.00007
United States 50 10 0.2
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3,7-dimethyl-6-octenoate (354)
Europe 1.1 0.2 0.003 0.0005
United States 0.3 0.1 0.001
2-Methyl-4-pentenoic acid (355)
Europe NR N/D NA
United States 0.2 0.04 0.001
Total
Europe 39 168 NA NA NA
United States 13 130 NA NA NA
NR, not reported; NA, not applicable
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor Industry,
1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
Linear unsaturated primary alcohols, aldehydes, and acids
The alcohols (including those formed from ester hydrolysis) and
aldehydes in this group are efficiently oxidized to the corresponding
unsaturated carboxylic acids by high-capacity enzyme pathways, as
discussed in the section 'General aspects of metabolism'. The
resulting linear unsaturated carboxylic acids participate in normal
fatty acid metabolism. Short-chain acids containing terminal
unsaturation, such as 4-pentenoic acid (No. 314), may be metabolized
by desaturation to yield a substrate that may participate in the fatty
acid pathway.
Alternative minor metabolic pathways have been characterized for
linear long-chain fatty acids (linoleic acid and oleic acid) and
short-chain acids containing unsaturation (i.e. 4-pentenoic acid).
While linoleic and oleic acids participate in ß-oxidation and normal
fatty acid metabolism in most tissues (Masoro, 1977), they may undergo
omega-oxidation in the liver and alpha-oxidation in the brain (Wakil &
Barnes, 1971; Gibson et al., 1982). In another minor pathway for the
oxidative metabolism of 4-pentenoic acid, trans-2,4-pentadienoyl
coenzyme A is produced, which is further metabolized via ß-oxidation
to yield 3-keto-4-pentenoyl coenzyme A (Schulz, 1983).
Branched-chain unsaturated primary alcohols, aldehydes, and acids
Generally, branched-chain aliphatic alcohols are oxidized to the
corresponding aldehydes, which, in turn, are oxidized to the
corresponding carboxylic acids (Bosron & Li, 1980; Levi & Hodgson,
1989). The aldehyde may also be reduced to the corresponding alcohol,
which is probably a short-lived intermediate in vivo. Like their
saturated analogues, unsaturated branched-chain aliphatic alcohols and
aldehydes are converted by the pathways cited above to the
corresponding acids. The acids may undergo ß-oxidative cleavage and
complete metabolism to carbon dioxide (Williams, 1959; Voet & Voet,
1990) in amino acid pathways, the fatty acid pathway, and the
tricarboxylic acid cycle. Alternatively, they may undergo a
combination of omega-, (omega-1)-, and ß-oxidation and selective
dehydrogenation and hydration to yield polar metabolites which are
excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Diliberto et al., 1990). The
principal metabolic pathways for detoxification of these
branched-chain substances is determined primarily by four structural
characteristics: carbon chain length and the position, number, and
size of the alkyl substituents.
These multiple pathways are illustrated by 2-ethylbutyric acid
and 2-ethyl-1-butanol. An oral dose of 2-ethyl-1-butanol was excreted
unchanged in the urine, principally as the glucuronic acid conjugate
(Kamil et al., 1953). 2-Ethylbutyric acid is metabolized by dogs to
2-pentanone, which is presumably derived from ß-oxidation and
decarboxylation (Deuel, 1957). Urinary metabolites of 2-ethylbutyric
acid reported in diabetic patients include the glucuronide conjugates
of 3-keto-2-ethylbutyric acid and 3,3'-diketo-2-ethylbutyric acid
formed by ß-oxidation of one or both ethyl chains (Granger et
al.,1954).
Higher homologues of 2-ethyl-1-butanol have a more complex
pattern of metabolism in which omega-oxidation competes with excretion
of the glucuronide conjugate of the corresponding acid. Female Fischer
344 rats were given a single oral dose of 50 or 500 mg/kg bw
[1-14C-ethyl]-2-ethylhexanol by gavage or repeated daily oral doses
of 50 mg/kg bw 2-ethyl-hexanol for 14 days. The major urinary
metabolites of the structurally related saturated branched-chain
alcohol 2-ethyl-1-hexanol collected over 0-24 h included the
glucuronic acid conjugate of 2-ethyl-hexanoic acid, omega- and
(omega-1)-oxidation metabolites, 2-ethyl-1,6-dihexanoic acid,
5-hydroxy-2-ethylhexanoic acid and its corresponding -lactone, and
6-hydroxy-2-ethylhexanoic acid. Minute quantities of the
omega-desaturation metabolite, 2-ethyl-5-hexenoic acid, and
metabolites formed from ß oxidation and oxidative decarboxylation, 2-
and 4-heptanone, were also detected (Albro, 1975; Deisinger et al.,
1994). After single or repeated doses of 50 mg/kg bw, the
omega-oxidation pathway predominated, as the principal urinary
metabolites excreted were 2-ethyl-1,6-hexane-dioic acid (30%) and its
precursors (8.5%). Only 6.8% of the dose was excreted in the urine as
the glucuronic acid conjugate of 2-ethylhexanoic acid. With 500 mg/kg
bw, 2-ethylhexanoic acid (25%) was the principal urinary metabolite,
indicating that saturation of the omega-desaturation pathway occurs at
this dose (Deisinger et al., 1994; Figure 1).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for a number of the 42
substances in this group and are in the range 470-19 000 mg/kg bw,
demonstrating that the acute toxicity of linear and branched-chain
aliphatic unsaturated and unconjugated alcohols, aldehydes, acids, and
related esters is low when they are given orally (Jenner et al., 1964;
Briggs et al., 1976).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
cis-3-Hexen-1-ol (No. 315)
Groups of 15 male and 15 female weanling rats were given
drinking-water containing 0, 310, 1250, or 5000 ppm cis-3 hexen-1-ol
for 98 days. Weekly measurements of body weight, food intake, and
water consumption revealed no differences in body-weight gain or food
intake between treated and control animals, but a significant
(p < 0.01) increase in water intake was recorded for males at the
high dose. A significant (p < 0.001), transitory anaemia was seen
at six weeks in females given 5000 ppm; however, this effect was not
observed at 14 weeks or in the males and therefore was considered not
to be related to treatment. Kidney and liver function in animals at
the low and intermediate doses was comparable to that of controls.
After 98 days, males at the high dose had a statistically significant
increase in relative kidney (p < 0.05) and adrenal weights
(p < 0.01) and a slight increase in urine specific gravity during
the first 2 h after a standard water load; however, there were no
histological signs of renal damage and no indications of abnormal
kidney function associated with treatment. Histopathological
examination revealed lymphocyte infiltration in the lung, suggesting a
mild pulmonary infection, and foci of tubular degeneration and
dilatation in the kidney. The incidence of these findings was similar
in test and control animals and could not be attributed to treatment.
The NOEL of 1250 ppm, reported by the authors to be equivalent to an
intake of 120-150 mg/kg bw per day (Gaunt et al., 1969), is > 1000
times the current daily per capita intake of 71 and 18 µg/kg bw per
day from use of cis-3-hexen-1-ol as a flavouring substance in Europe
and the United States, respectively.
10-Undecenoic acid (No. 331)
In an abstract describing the results of a study in which 100,
200, or 400 mg/kg bw per day of 10-undecenoic acid were administered
to Sprague-Dawley rats by gavage for six months, no effects were
reported (Tislow et al., 1950). Because a full report of this study
was not available, it was considered inadequate for deriving a NOEL.
In another study of 10-undecenoic acid, Sprague-Dawley rats were
maintained on diets containing 0.5, 1, or 2.5% (approximately 500,
1000, and 2500 mg/kg bw per day (US Food & Drug Administration, 1993))
for eight weeks. An inhibitory effect on growth, possibly related to
decreased food consumption, was reported during the first five weeks
(Newell et al., 1949). These data, described by the authors as
preliminary, were not used to derive a NOEL.
Oleic acid (No. 333)
The Committee previously allocated an ADI 'not specified' to the
aluminum, ammonium, calcium, magnesium, potassium, and sodium salts of
oleic acid (Annex 1, reference 70). Recently, the results of a 13-week
and a two-year study of oleic acid diethanolamine condensate
administered dermally to Fischer 344/N rats and B6C3F1 mice were
reported (US National Toxicology Program, 1997). These studies were
not considered relevant to the safety evaluation of oleic acid used as
a flavouring substance.
Numerous other studies are available of the role of high
concentrations of oleic acid and other fatty acids in the diet. As the
effects observed are probably the result of the extremely high intake
of total dietary fat and fatty acids, these studies were not
considered relevant to the safety evaluation of oleic acid at its
current level of intake as a flavouring substance.
2,6-Dimethyl-5-heptenal (No. 349)
Groups of 15 male and 15 female rats were fed diets providing
2,6-dimethyl-5-heptenal at average intakes of 0, 9, 37, or 150 mg/kg
bw per day for at least 90 days. Statistically significant
(p < 0.05) increases in haemoglobin concentrations were found in
males at the high dose at weeks 6 and 14 and in males at the
intermediate dose at week 14; however, these effects were not
biologically significant and were not considered by the authors to be
adverse. Most of the absolute and relative weights of organs were
similar in treated and control animals, but statistically significant
(p < 0.05) increases in the relative and absolute spleen weights
were observed in males at the intermediate dose. A statistically
significant increase in serum glucose concentration was observed in
male and female rats at the high dose, which the authors noted was of
unknown origin. Also at the high dose, the renal concentrating ability
was reduced in males at week 6 and in females at week 14, resulting in
proteinuria. Slight, statistically significant increases (p < 0.05
by Student's t test) in relative kidney and liver weights were also
observed in females at this dose. Histological examination of rats at
the high dose showed no statistically significant difference between
the treated group and controls, other than alveolar thickening in
males (p < 0.05). Since the histopathological appearance was normal
in animals of each sex and the kidney weights were normal in males,
the proteinuria could not be associated with treatment. Additionally,
no structural kidney damage was seen by light microscopy. A functional
effect on the kidney might have been caused by treatment at the
highest level, however, because males and females at the high dose
failed to concentrate urine after a period of dehydration during weeks
6 and 13, respectively, and the kidney weights of females were
marginally increased. The NOEL for 2,6-dimethyl-5-heptenal was 37
mg/kg bw per day (Gaunt et al., 1983) which is > 1000 times the daily
per capita intakes of 1 and 4 g/kg bw per day from use of
2,6-dimethyl-5-heptenal as a flavouring substance in Europe and the
United States, respectively.
Ethyl 2-methyl-3,4-pentadienoate (No. 353)
The Committee was aware of a study on the toxicity of
ethyl-2-methyl-3,4-pentadienoate (Cox et al., 1978); however, a full
report of the study was not available.
2.3.2.3 Genotoxicity
Three representative linear and branched-chain aliphatic
unsaturated primary alcohols and unconjugated aldehydes, carboxylic
acids, and related esters in this group have been tested for
genotoxicity. The results of these tests are summarized in Table 3 and
described below.
No evidence of mutagenicity was reported for oleic acid, methyl
linoleate, methyl linolenate, or 2,6-dimethyl-5-heptenal in standard
and preincubation assays in Salmonella typhimurium strain TA98,
TA100, TA1535, TA1537, or TA1538 with or without the addition of
metabolic activation (Wild et al., 1983; Shimizu et al., 1985;
Mortelmans et al., 1986; Heck et al., 1989). The maximum
concentrations used in these studies ranged from 333 to 50 000
µg/plate. In further bacterial assays, such as the rec assay in
Bacillus subtilis incubated with oleic acid (Osawa & Namiki, 1982),
the his+ reversion assay in S. typhimurium incubated with methyl
linoleate or methyl linolenate (MacGregor et al., 1985), and a
modified Ames test in Escherichia coli WP2 uvrA incubated with
oleic acid (Shimizu et al., 1985), aliphatic unsaturated unconjugated
acids and esters were also shown to be non-mutagenic.
No unscheduled DNA synthesis was seen after exposure of rat
hepatocytes to 2,6-dimethyl-5-heptenal at concentrations up to 1 mg/ml
(Heck et al., 1989).
In S. typhimurium strain TA98, a concentration of 0.1 mmol/L
oleic acid was reported to inhibit (by approximately 50%) the
mutagenicity of known mutagens, including
3-amino-1,4-dimethyl-5 H-pyrido[4,3- b]indole,
2-amino-9 H-pyridol[2,3- b]indole,
2-amino-3-methylimidazo[4,5- d]quinoline, benzo[ a]-pyrene, and
aflatoxin B1, when tested in the presence or absence of metabolic
activation (Hayatsu et al., 1981).
Table 3. Results of assays for the genotoxicity studies for linear and branched-chain aliphatic unsaturated unconjugated alcohols, aldehydes,
acids and related esters
Substance No. End-point Test object Maximum Result Reference
concentration
Oleic acid 333 Reverse mutationa S. typhimurium TA1535, 5000 mg/plate Negativeb Shimizu et al. (1985)
TA1537, TA98, TA100, TA1538
Reverse mutationa S. typhimurium TA1535, 333 mg/plate Negativeb Mortelmans et al. (1986)
TA98, TA100, TA1537
Reverse mutationa E. coli 5000 mg/plate Negativeb Shimizu et al. (1985)
Rec assay B. subtilis 1 mg/plate Negativeb Osawa & Namiki (1982)
Methyl linoleate Part of His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
346 TA98, TA102, TA97, TA1537
His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
TA98, TA102, TA97, TA1537
2,6-Dimethyl-5-heptenal 349 Reverse mutation S. typhimurium TA1535, 3.6 mg/plate Negativeb Wild et al. (1983)
TA100, TA1537, TA1538, TA98
Reverse mutation S. typhimurium TA1535, 50 mg/plate Negativeb Heck et al. (1989)
TA100, TA1537, TA1538, TA98
Unscheduled DNA Rat hepatocytes 1 mg/ml Negativeb Heck et al. (1989)
synthesis
Micronucleus Mouse 1540 mg/kg bw Negative Wild et al. (1983)
formation
Basc test Drosophila melanogaster 25 mmol/L Negative Wild et al. (1983
a Modified Ames' assay, with preincubation
b With and without metabolic activation
2,6-Dimethyl-5-heptenal at a concentration of 25 mmol/L did not
cause Basc reversion in Drosophila melanogaster (Wild et al.,
1983).
In mice given a maximum single dose of 1540 mg/kg bw
2,6-dimethyl-5-heptenal, all of which survived treatment, the
incidence of polychromatic erythrocytes was not statistically
significantly increased (Wild et al., 1983).
Oleic acid did not induce oxidative damage in isolated DNA (de
Kok et al., 1994). In a three-week study in volunteers, ingestion of
oleic acid did not alter the frequency of micronucleated lymphocytes
in peripheral blood (Record et al., 1992).
These negative results indicate that the substances in this group
of linear and branched-chain aliphatic unsaturated and unconjugated
alcohols, aldehydes, acids, and related esters that are used as
flavouring substances are neither mutagenic nor genotoxic.
2.3.2.4 Other relevant studies
In a study of teratogenicity, two groups of 15 female NMRI mice
were mated with males for 2 h, and 4-pentenoic acid (No. 314) was
administered as the sodium salt in water by subcutaneous injection to
pregnant mice on day 8 of gestation as a single 600 mg/kg bw dose. A
group of control mice was included. Implantation sites were counted,
and each live fetus was weighed and inspected for neural tube defects.
Effects on the visceral or skeletal system were not examined.
4-Pentenoic acid had no effect on fetal weight, the number of live
fetuses, embryolethality, or exencephaly in comparison with control
animals (Nau & Loscher, 1986). These results could not be used to
derive a NOEL for oral intake of 4-pentenoic acid.
4. REFERENCES
Abumrad, N.A., Park, J.H. & Park, C.R. (1984) Permeation of long-chain
fatty acid into adipocytes. J. Biol. Chem., 259, 8945-8953.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Borgstrom, B. (1974) Fat digestion and absorption. In: Smyth, D.H.,
ed., Biomembranes--Intestinal Absorption, London, Plenum Press, Vol.
4B, pp. 555-620.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, New York, Academic Press,
Vol I, p. 231.
Briggs, G.B., Doyle, R.L. & Young, J.A. (1976) Safety studies on a
series of fatty acids. Am. Ind. Hyg. Ass. J., 37, 251-253.
Cox, G.E., Rucci, G. & Babish, J.G. (1978) 90-Day subacute dietary
toxicity study of ethyl-2-methyl-3,4-pentadienoate in Sprague-Dawley
rats. Unpublished report. Submitted to the Flavor and Extract
Manufactuers' Association of the United States, Washington DC, United
States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, A.M., Holdsworth, C.D. & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117, 97-100.
Deisinger, P.J., Boatman, R.J. & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24, 429-440.
Deuel, H.J., ed. (1957) The Lipids, Their Chemistry and
Biochemistry, New York, Wiley Interscience, Vol III.
Dhopeshwarkar, G.A. & Mead, J.F. (1973) Uptake and transport of fatty
acids into the brain and the role of the blood-brain barrier system.
In: Paoletti, R. & Kritchevsky, D., eds, Advances in Lipid
Research, New York, Academic Press, Vol. 11, pp. 109-142.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, B.L.T. &
Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated
aldehyde, in male F344 rats. Drug Metab. Disposition, 18, 868-875.
Drake, J.J.-P., Gaunt, I.F., Butterworth, K.R., Hooson, J., Hardy, J.
& Gangolli, S.D. (1975) Short-term toxicity of isoamyl salicylat in
rats. Food Cosmet. Toxicol., 13, 185-193.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montpellier, 25, 51-62.
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G. & Gangolli, S.D.
(1969) Acute (rat and mouse) and short-term (rat) toxicity studies on
cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7, 451-459.
Gaunt, I.F., Wright, M.G., Cottrell, R. & Gangolli, S.D. (1983)
Short-term toxicity of 2,6-dimethylhept-5-en-1-al in rats. Food
Chem. Toxicol., 21, 543-549.
Gibson, G.G., Orton, T.C. & Tamburini, P.P. (1982) Cytochrome P-450
induction by clofibrate. Purification and properties of a hepatic
cytochrome P-450 relatively specific for the 12- and 11-hydroxylation
of dodecanoic acid (lauric acid). Biochem. J., 203, 161-168.
Granger, R., Giroux, J. & Monnier, P. (1954) [Research on the
mechanism of action of calcium ethyl-2-butanoate.] Trav. Soc.
Pharm. Montpeller, 14, 335-341 (in French).
Harris, P., Gloster, J.A. & Ward, B.J. (1980) Transport of fatty acids
in the heart. Arch. Mal. Coeur, 73, 593-598.
Hayatsu, H., Inoue, K., Ohta, H., Namba, T., Togawa, K., Hayatsu, T.,
Makita, M. & Wataya, Y. (1981) Inhibition of the mutagenicity of
cooked-beef basic fraction by its acidic fraction. Mutat. Res., 91,
437-442.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Flavor
Manufacturers' Association of the United States, Washington DC, United
States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; the glucuronic acid conjugation of acyclic
aliphatic alcohols. J. Biochem., 53, 129-136.
de Kok, T.M., ten Vaarwerk, F., Zwingman, I., van Maanen, J.M. &
Kleinjans, J.C. (1994) Peroxidation of linoleic, arachidonic and oleic
acid in relation to the induction of oxidative DNA damage and
cytogenetic effects. Carcinogenesis, 15, 1399-1404.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London, Taylor &
Francis, pp. 119-138.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
MacGregor, J.T., Wilson, R.E., Neff, W.E. & Frankel, E.N. (1985)
Mutagenicity tests of lipid oxidation products in Salmonella
typhimurium: Monohydroperoxides and secondary oxidation products of
methyl linoleate and methyl linolenate. Food Chem. Toxicol., 23,
1041-1047.
Masoro, E.J. (1977) Lipids and lipid metabolism. Am. Rev. Physiol.,
39, 301-321.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nau, H. & Loscher, W. (1986) Pharmacologic evaluation of metabolites
and analogs of valproic acid: Teratogenic potencies in mice.
Fundam. Appli Toxicol., 6, 669-676.
Newell, G.W., Petretti, A.K. & Reiner, L. (1949) Studies of the acute
and chronic toxicity of undecylenic acid. J. Invest. Dermatol., 13,
145-149.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Arch.
Biochem., 46, 2299-2304.
Record, I.R., Konstantinopoulos, M. & Nestel, P.J. (1992) A
comparative study of micronucleus frequency in peripheral blood
lymphocytes of human subjects give dietary cis, trans and saturated
fat. Food Chem. Toxicol., 30, 585-588.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. J. Biochem., 22, 1827.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tislow, R., Margolin, S., Foley, E.J. & Lee, S.W. (1950) Toxicity of
undercylenic acid. J. Pharmacol. Exp. Ther., 98, 31-32.
US Food and Drug Administration (1993) Priority-based Assessment
of Food Additives (PAFA) Database, Center for Food Safety and
Applied Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1997) Toxicology and Carcinogenesis
Studies of Oleic Acid Diethanolamine Condensate (CAS No. 93-83-4)
in F344/N Rats and B6C3F1 Mice (Dermal Studies) (Technical Report
Series No. 481, NIH Publication No. 97-3971), Research Triangle Park,
North Carolina, US Department of Health and Human Services, Public
Health Service, National Institutes of Health.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Wakil, S.J. & Barnes, E.M. (1971) In: Florkin, M. & Stotz, E., eds,
Comprehensive Biochemistry, New York, Elsevier Publishing Co., Vol.
18S.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T. (1959) Detoxication Mechanisms, 2nd Ed., London,
Chapman & Hall, pp. 88-113.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for a number of the 42
substances in this group and are in the range 470-19 000 mg/kg bw,
demonstrating that the acute toxicity of linear and branched-chain
aliphatic unsaturated and unconjugated alcohols, aldehydes, acids, and
related esters is low when they are given orally (Jenner et al., 1964;
Briggs et al., 1976).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
cis-3-Hexen-1-ol (No. 315)
Groups of 15 male and 15 female weanling rats were given
drinking-water containing 0, 310, 1250, or 5000 ppm cis-3 hexen-1-ol
for 98 days. Weekly measurements of body weight, food intake, and
water consumption revealed no differences in body-weight gain or food
intake between treated and control animals, but a significant
(p < 0.01) increase in water intake was recorded for males at the
high dose. A significant (p < 0.001), transitory anaemia was seen
at six weeks in females given 5000 ppm; however, this effect was not
observed at 14 weeks or in the males and therefore was considered not
to be related to treatment. Kidney and liver function in animals at
the low and intermediate doses was comparable to that of controls.
After 98 days, males at the high dose had a statistically significant
increase in relative kidney (p < 0.05) and adrenal weights
(p < 0.01) and a slight increase in urine specific gravity during
the first 2 h after a standard water load; however, there were no
histological signs of renal damage and no indications of abnormal
kidney function associated with treatment. Histopathological
examination revealed lymphocyte infiltration in the lung, suggesting a
mild pulmonary infection, and foci of tubular degeneration and
dilatation in the kidney. The incidence of these findings was similar
in test and control animals and could not be attributed to treatment.
The NOEL of 1250 ppm, reported by the authors to be equivalent to an
intake of 120-150 mg/kg bw per day (Gaunt et al., 1969), is > 1000
times the current daily per capita intake of 71 and 18 µg/kg bw per
day from use of cis-3-hexen-1-ol as a flavouring substance in Europe
and the United States, respectively.
10-Undecenoic acid (No. 331)
In an abstract describing the results of a study in which 100,
200, or 400 mg/kg bw per day of 10-undecenoic acid were administered
to Sprague-Dawley rats by gavage for six months, no effects were
reported (Tislow et al., 1950). Because a full report of this study
was not available, it was considered inadequate for deriving a NOEL.
In another study of 10-undecenoic acid, Sprague-Dawley rats were
maintained on diets containing 0.5, 1, or 2.5% (approximately 500,
1000, and 2500 mg/kg bw per day (US Food & Drug Administration, 1993))
for eight weeks. An inhibitory effect on growth, possibly related to
decreased food consumption, was reported during the first five weeks
(Newell et al., 1949). These data, described by the authors as
preliminary, were not used to derive a NOEL.
Oleic acid (No. 333)
The Committee previously allocated an ADI 'not specified' to the
aluminum, ammonium, calcium, magnesium, potassium, and sodium salts of
oleic acid (Annex 1, reference 70). Recently, the results of a 13-week
and a two-year study of oleic acid diethanolamine condensate
administered dermally to Fischer 344/N rats and B6C3F1 mice were
reported (US National Toxicology Program, 1997). These studies were
not considered relevant to the safety evaluation of oleic acid used as
a flavouring substance.
Numerous other studies are available of the role of high
concentrations of oleic acid and other fatty acids in the diet. As the
effects observed are probably the result of the extremely high intake
of total dietary fat and fatty acids, these studies were not
considered relevant to the safety evaluation of oleic acid at its
current level of intake as a flavouring substance.
2,6-Dimethyl-5-heptenal (No. 349)
Groups of 15 male and 15 female rats were fed diets providing
2,6-dimethyl-5-heptenal at average intakes of 0, 9, 37, or 150 mg/kg
bw per day for at least 90 days. Statistically significant
(p < 0.05) increases in haemoglobin concentrations were found in
males at the high dose at weeks 6 and 14 and in males at the
intermediate dose at week 14; however, these effects were not
biologically significant and were not considered by the authors to be
adverse. Most of the absolute and relative weights of organs were
similar in treated and control animals, but statistically significant
(p < 0.05) increases in the relative and absolute spleen weights
were observed in males at the intermediate dose. A statistically
significant increase in serum glucose concentration was observed in
male and female rats at the high dose, which the authors noted was of
unknown origin. Also at the high dose, the renal concentrating ability
was reduced in males at week 6 and in females at week 14, resulting in
proteinuria. Slight, statistically significant increases (p < 0.05
by Student's t test) in relative kidney and liver weights were also
observed in females at this dose. Histological examination of rats at
the high dose showed no statistically significant difference between
the treated group and controls, other than alveolar thickening in
males (p < 0.05). Since the histopathological appearance was normal
in animals of each sex and the kidney weights were normal in males,
the proteinuria could not be associated with treatment. Additionally,
no structural kidney damage was seen by light microscopy. A functional
effect on the kidney might have been caused by treatment at the
highest level, however, because males and females at the high dose
failed to concentrate urine after a period of dehydration during weeks
6 and 13, respectively, and the kidney weights of females were
marginally increased. The NOEL for 2,6-dimethyl-5-heptenal was 37
mg/kg bw per day (Gaunt et al., 1983) which is > 1000 times the daily
per capita intakes of 1 and 4 g/kg bw per day from use of
2,6-dimethyl-5-heptenal as a flavouring substance in Europe and the
United States, respectively.
Ethyl 2-methyl-3,4-pentadienoate (No. 353)
The Committee was aware of a study on the toxicity of
ethyl-2-methyl-3,4-pentadienoate (Cox et al., 1978); however, a full
report of the study was not available.
2.3.2.3 Genotoxicity
Three representative linear and branched-chain aliphatic
unsaturated primary alcohols and unconjugated aldehydes, carboxylic
acids, and related esters in this group have been tested for
genotoxicity. The results of these tests are summarized in Table 3 and
described below.
No evidence of mutagenicity was reported for oleic acid, methyl
linoleate, methyl linolenate, or 2,6-dimethyl-5-heptenal in standard
and preincubation assays in Salmonella typhimurium strain TA98,
TA100, TA1535, TA1537, or TA1538 with or without the addition of
metabolic activation (Wild et al., 1983; Shimizu et al., 1985;
Mortelmans et al., 1986; Heck et al., 1989). The maximum
concentrations used in these studies ranged from 333 to 50 000
µg/plate. In further bacterial assays, such as the rec assay in
Bacillus subtilis incubated with oleic acid (Osawa & Namiki, 1982),
the his+ reversion assay in S. typhimurium incubated with methyl
linoleate or methyl linolenate (MacGregor et al., 1985), and a
modified Ames test in Escherichia coli WP2 uvrA incubated with
oleic acid (Shimizu et al., 1985), aliphatic unsaturated unconjugated
acids and esters were also shown to be non-mutagenic.
No unscheduled DNA synthesis was seen after exposure of rat
hepatocytes to 2,6-dimethyl-5-heptenal at concentrations up to 1 mg/ml
(Heck et al., 1989).
In S. typhimurium strain TA98, a concentration of 0.1 mmol/L
oleic acid was reported to inhibit (by approximately 50%) the
mutagenicity of known mutagens, including
3-amino-1,4-dimethyl-5 H-pyrido[4,3- b]indole,
2-amino-9 H-pyridol[2,3- b]indole,
2-amino-3-methylimidazo[4,5- d]quinoline, benzo[ a]-pyrene, and
aflatoxin B1, when tested in the presence or absence of metabolic
activation (Hayatsu et al., 1981).
Table 3. Results of assays for the genotoxicity studies for linear and branched-chain aliphatic unsaturated unconjugated alcohols, aldehydes,
acids and related esters
Substance No. End-point Test object Maximum Result Reference
concentration
Oleic acid 333 Reverse mutationa S. typhimurium TA1535, 5000 mg/plate Negativeb Shimizu et al. (1985)
TA1537, TA98, TA100, TA1538
Reverse mutationa S. typhimurium TA1535, 333 mg/plate Negativeb Mortelmans et al. (1986)
TA98, TA100, TA1537
Reverse mutationa E. coli 5000 mg/plate Negativeb Shimizu et al. (1985)
Rec assay B. subtilis 1 mg/plate Negativeb Osawa & Namiki (1982)
Methyl linoleate Part of His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
346 TA98, TA102, TA97, TA1537
His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
TA98, TA102, TA97, TA1537
2,6-Dimethyl-5-heptenal 349 Reverse mutation S. typhimurium TA1535, 3.6 mg/plate Negativeb Wild et al. (1983)
TA100, TA1537, TA1538, TA98
Reverse mutation S. typhimurium TA1535, 50 mg/plate Negativeb Heck et al. (1989)
TA100, TA1537, TA1538, TA98
Unscheduled DNA Rat hepatocytes 1 mg/ml Negativeb Heck et al. (1989)
synthesis
Micronucleus Mouse 1540 mg/kg bw Negative Wild et al. (1983)
formation
Basc test Drosophila melanogaster 25 mmol/L Negative Wild et al. (1983
a Modified Ames' assay, with preincubation
b With and without metabolic activation
2,6-Dimethyl-5-heptenal at a concentration of 25 mmol/L did not
cause Basc reversion in Drosophila melanogaster (Wild et al.,
1983).
In mice given a maximum single dose of 1540 mg/kg bw
2,6-dimethyl-5-heptenal, all of which survived treatment, the
incidence of polychromatic erythrocytes was not statistically
significantly increased (Wild et al., 1983).
Oleic acid did not induce oxidative damage in isolated DNA (de
Kok et al., 1994). In a three-week study in volunteers, ingestion of
oleic acid did not alter the frequency of micronucleated lymphocytes
in peripheral blood (Record et al., 1992).
These negative results indicate that the substances in this group
of linear and branched-chain aliphatic unsaturated and unconjugated
alcohols, aldehydes, acids, and related esters that are used as
flavouring substances are neither mutagenic nor genotoxic.
2.3.2.4 Other relevant studies
In a study of teratogenicity, two groups of 15 female NMRI mice
were mated with males for 2 h, and 4-pentenoic acid (No. 314) was
administered as the sodium salt in water by subcutaneous injection to
pregnant mice on day 8 of gestation as a single 600 mg/kg bw dose. A
group of control mice was included. Implantation sites were counted,
and each live fetus was weighed and inspected for neural tube defects.
Effects on the visceral or skeletal system were not examined.
4-Pentenoic acid had no effect on fetal weight, the number of live
fetuses, embryolethality, or exencephaly in comparison with control
animals (Nau & Loscher, 1986). These results could not be used to
derive a NOEL for oral intake of 4-pentenoic acid.
4. REFERENCES
Abumrad, N.A., Park, J.H. & Park, C.R. (1984) Permeation of long-chain
fatty acid into adipocytes. J. Biol. Chem., 259, 8945-8953.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Borgstrom, B. (1974) Fat digestion and absorption. In: Smyth, D.H.,
ed., Biomembranes--Intestinal Absorption, London, Plenum Press, Vol.
4B, pp. 555-620.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, New York, Academic Press,
Vol I, p. 231.
Briggs, G.B., Doyle, R.L. & Young, J.A. (1976) Safety studies on a
series of fatty acids. Am. Ind. Hyg. Ass. J., 37, 251-253.
Cox, G.E., Rucci, G. & Babish, J.G. (1978) 90-Day subacute dietary
toxicity study of ethyl-2-methyl-3,4-pentadienoate in Sprague-Dawley
rats. Unpublished report. Submitted to the Flavor and Extract
Manufactuers' Association of the United States, Washington DC, United
States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, A.M., Holdsworth, C.D. & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117, 97-100.
Deisinger, P.J., Boatman, R.J. & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24, 429-440.
Deuel, H.J., ed. (1957) The Lipids, Their Chemistry and
Biochemistry, New York, Wiley Interscience, Vol III.
Dhopeshwarkar, G.A. & Mead, J.F. (1973) Uptake and transport of fatty
acids into the brain and the role of the blood-brain barrier system.
In: Paoletti, R. & Kritchevsky, D., eds, Advances in Lipid
Research, New York, Academic Press, Vol. 11, pp. 109-142.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, B.L.T. &
Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated
aldehyde, in male F344 rats. Drug Metab. Disposition, 18, 868-875.
Drake, J.J.-P., Gaunt, I.F., Butterworth, K.R., Hooson, J., Hardy, J.
& Gangolli, S.D. (1975) Short-term toxicity of isoamyl salicylat in
rats. Food Cosmet. Toxicol., 13, 185-193.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montpellier, 25, 51-62.
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G. & Gangolli, S.D.
(1969) Acute (rat and mouse) and short-term (rat) toxicity studies on
cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7, 451-459.
Gaunt, I.F., Wright, M.G., Cottrell, R. & Gangolli, S.D. (1983)
Short-term toxicity of 2,6-dimethylhept-5-en-1-al in rats. Food
Chem. Toxicol., 21, 543-549.
Gibson, G.G., Orton, T.C. & Tamburini, P.P. (1982) Cytochrome P-450
induction by clofibrate. Purification and properties of a hepatic
cytochrome P-450 relatively specific for the 12- and 11-hydroxylation
of dodecanoic acid (lauric acid). Biochem. J., 203, 161-168.
Granger, R., Giroux, J. & Monnier, P. (1954) [Research on the
mechanism of action of calcium ethyl-2-butanoate.] Trav. Soc.
Pharm. Montpeller, 14, 335-341 (in French).
Harris, P., Gloster, J.A. & Ward, B.J. (1980) Transport of fatty acids
in the heart. Arch. Mal. Coeur, 73, 593-598.
Hayatsu, H., Inoue, K., Ohta, H., Namba, T., Togawa, K., Hayatsu, T.,
Makita, M. & Wataya, Y. (1981) Inhibition of the mutagenicity of
cooked-beef basic fraction by its acidic fraction. Mutat. Res., 91,
437-442.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Flavor
Manufacturers' Association of the United States, Washington DC, United
States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; the glucuronic acid conjugation of acyclic
aliphatic alcohols. J. Biochem., 53, 129-136.
de Kok, T.M., ten Vaarwerk, F., Zwingman, I., van Maanen, J.M. &
Kleinjans, J.C. (1994) Peroxidation of linoleic, arachidonic and oleic
acid in relation to the induction of oxidative DNA damage and
cytogenetic effects. Carcinogenesis, 15, 1399-1404.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London, Taylor &
Francis, pp. 119-138.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
MacGregor, J.T., Wilson, R.E., Neff, W.E. & Frankel, E.N. (1985)
Mutagenicity tests of lipid oxidation products in Salmonella
typhimurium: Monohydroperoxides and secondary oxidation products of
methyl linoleate and methyl linolenate. Food Chem. Toxicol., 23,
1041-1047.
Masoro, E.J. (1977) Lipids and lipid metabolism. Am. Rev. Physiol.,
39, 301-321.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nau, H. & Loscher, W. (1986) Pharmacologic evaluation of metabolites
and analogs of valproic acid: Teratogenic potencies in mice.
Fundam. Appli Toxicol., 6, 669-676.
Newell, G.W., Petretti, A.K. & Reiner, L. (1949) Studies of the acute
and chronic toxicity of undecylenic acid. J. Invest. Dermatol., 13,
145-149.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Arch.
Biochem., 46, 2299-2304.
Record, I.R., Konstantinopoulos, M. & Nestel, P.J. (1992) A
comparative study of micronucleus frequency in peripheral blood
lymphocytes of human subjects give dietary cis, trans and saturated
fat. Food Chem. Toxicol., 30, 585-588.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. J. Biochem., 22, 1827.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tislow, R., Margolin, S., Foley, E.J. & Lee, S.W. (1950) Toxicity of
undercylenic acid. J. Pharmacol. Exp. Ther., 98, 31-32.
US Food and Drug Administration (1993) Priority-based Assessment
of Food Additives (PAFA) Database, Center for Food Safety and
Applied Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1997) Toxicology and Carcinogenesis
Studies of Oleic Acid Diethanolamine Condensate (CAS No. 93-83-4)
in F344/N Rats and B6C3F1 Mice (Dermal Studies) (Technical Report
Series No. 481, NIH Publication No. 97-3971), Research Triangle Park,
North Carolina, US Department of Health and Human Services, Public
Health Service, National Institutes of Health.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Wakil, S.J. & Barnes, E.M. (1971) In: Florkin, M. & Stotz, E., eds,
Comprehensive Biochemistry, New York, Elsevier Publishing Co., Vol.
18S.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T. (1959) Detoxication Mechanisms, 2nd Ed., London,
Chapman & Hall, pp. 88-113.
See Also:
Toxicological Abbreviations
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexen-1-ol 315 4300/1100 Yes No Yes. The NOAEL Expected to be oxidized No safety concern
of 120-150 mg/kg to the corresponding
bw per day [Gaunt aldehyde and carboxylic
et al., 1969] is acid which is completely
>1000 times the metabolized in the
daily per capita fatty acid and tricarboxylic
intake. acid pathways
CAS No. 928-96-1
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexen-1-ol 315 4300/1100 Yes No Yes. The NOAEL Expected to be oxidized No safety concern
of 120-150 mg/kg to the corresponding
bw per day [Gaunt aldehyde and carboxylic
et al., 1969] is acid which is completely
>1000 times the metabolized in the
daily per capita fatty acid and tricarboxylic
intake. acid pathways
CAS No. 928-96-1
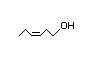 cis-3-Hexenal 316 5/3 No N/R N/R See cis-3-hexenol. No safety concern
CAS No. 6789-80-6
cis-3-Hexenal 316 5/3 No N/R N/R See cis-3-hexenol. No safety concern
CAS No. 6789-80-6
 3-Hexenoic acid 317 11/1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4219-24-3
3-Hexenoic acid 317 11/1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4219-24-3
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Hexen-1-ol 318 3/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6126-50-7
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Hexen-1-ol 318 3/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6126-50-7
 cis-4-Hexenal 319 0.03/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4634-89-3
cis-4-Hexenal 319 0.03/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 4634-89-3
 4-Heptenal 320 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6728-31-0
4-Heptenal 320 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 6728-31-0
 cis-3-Octen-1-ol 321 6/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 20125-84-2
cis-3-Octen-1-ol 321 6/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 20125-84-2
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-5-Octen-1-ol 322 0.5/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 64275-73-6
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-5-Octen-1-ol 322 0.5/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 64275-73-6
 cis-5-Octenal 323 0.001/29 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 41547-22-2
cis-5-Octenal 323 0.001/29 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 41547-22-2
 cis-6-Nonen-1-ol 324 3/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 35854-86-5
cis-6-Nonen-1-ol 324 3/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 35854-86-5
 cis-6-Nonenal 325 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 2277-19-2
cis-6-Nonenal 325 2/0.1 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 2277-19-2
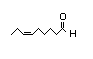 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Decenal 326 1/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 30390-50-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
4-Decenal 326 1/0.01 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 30390-50-2
 5&6-Decenoic acid 327 4/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 72881-27-7
5&6-Decenoic acid 327 4/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 72881-27-7

 9-Decenoic acid 328 0.1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 14436-32-9
9-Decenoic acid 328 0.1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 14436-32-9
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
9-Undecenal 329 1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 143-14-6
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
9-Undecenal 329 1/0.2 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 143-14-6
 10-Undecenal 330 0.4/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-45-8
10-Undecenal 330 0.4/0.04 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-45-8
 10-Undecenoic acid 331 30/0.8 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-38-9
10-Undecenoic acid 331 30/0.8 No N/R N/R See cis-3-hexenol. No safety
concern
CAS No. 112-38-9
 Linoleic acid 332 133/6 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 60-33-3
Linoleic acid 332 133/6 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 60-33-3
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Oleic acida 333 970/440 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 112-80-1
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Oleic acida 333 970/440 No N/R N/R Readily metabolized in No safety
the fatty acid oxidation concern
pathway
CAS No. 112-80-1
 Methyl 3-hexenoate 334 0.7/0.01 No N/R N/R Expected to be hydrolysed No safety
to its component concern
alcohol and acid
See also cis-3-hexenol.
CAS No. 2396-78-3
Methyl 3-hexenoate 334 0.7/0.01 No N/R N/R Expected to be hydrolysed No safety
to its component concern
alcohol and acid
See also cis-3-hexenol.
CAS No. 2396-78-3
 Ethyl 3-hexenoate 335 4/1 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 2396-83-0
Ethyl 3-hexenoate 335 4/1 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 2396-83-0
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexenyl 336 4/0.8 No N/R N/R See methyl 3-hexenoate. No safety
cis-3-hexenoate concern
CAS No. 61444-38-0
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
cis-3-Hexenyl 336 4/0.8 No N/R N/R See methyl 3-hexenoate. No safety
cis-3-hexenoate concern
CAS No. 61444-38-0
 Methyl 337 0.4/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 21063-71-8
Methyl 337 0.4/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 21063-71-8
 Ethyl 338 1/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 34495-71-1
Ethyl 338 1/0.04 No N/R N/R See methyl 3-hexenoate. No safety
cis-4-octenoate concern
CAS No. 34495-71-1
 Ethyl 339 2/0.01 No N/R N/R See methyl 3-hexenoate. No safety
cis-4,7-octadienoate concern
CAS No. 69925-33-3
Ethyl 339 2/0.01 No N/R N/R See methyl 3-hexenoate. No safety
cis-4,7-octadienoate concern
CAS No. 69925-33-3
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3-nonenoate 340 2/76 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 13481-87-3
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3-nonenoate 340 2/76 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 13481-87-3
 Ethyl 341 2/0.04 No N/R N/R See methyl 3-hexenoate. No safety
trans-4-decenoate concern
CAS No. 76649-16-6
Ethyl 341 2/0.04 No N/R N/R See methyl 3-hexenoate. No safety
trans-4-decenoate concern
CAS No. 76649-16-6
 Methyl 9-un-decenoate 342 39/0.4 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 5760-50-9
Methyl 9-un-decenoate 342 39/0.4 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 5760-50-9
 Ethyl 10-undecenoate 343 2/44 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 692-86-4
Ethyl 10-undecenoate 343 2/44 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 692-86-4
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Butyl 10-undecenoate 344 0.04/5 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 109-42-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Butyl 10-undecenoate 344 0.04/5 No N/R N/R See methyl 3-hexenoate. No safety
concern
CAS No. 109-42-2
 Ethyl oleate 345 69/3 No N/R N/R See methyl 3-hexenoate No safety
and oleic acid. concern
CAS No. 111-62-6
Ethyl oleate 345 69/3 No N/R N/R See methyl 3-hexenoate No safety
and oleic acid. concern
CAS No. 111-62-6
 Methyl linoleate 346 N/D/23 No N/R N/R See methyl 3-hexenoate No safety
& Methyl linolenate and linoleic acid. concern
(mixture)
CAS No. 301-00-8
Methyl linoleate 346 N/D/23 No N/R N/R See methyl 3-hexenoate No safety
& Methyl linolenate and linoleic acid. concern
(mixture)
CAS No. 301-00-8

 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
2-Methyl-3-pentenoic 347 1/2 No N/R N/R Undergoes ß oxidation to No safety
acid yield products that are concern
completely metabolized in
the tricarboxylic acid cycle
CAS No. 37674-63-8
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
2-Methyl-3-pentenoic 347 1/2 No N/R N/R Undergoes ß oxidation to No safety
acid yield products that are concern
completely metabolized in
the tricarboxylic acid cycle
CAS No. 37674-63-8
 2,6-Dimethyl- 348 N/D/0.1 No N/R N/R See 2-methyl-3-pentenoic No safety
6-hepten-1-ol acid. concern
CAS No. 36806-46-9
2,6-Dimethyl- 348 N/D/0.1 No N/R N/R See 2-methyl-3-pentenoic No safety
6-hepten-1-ol acid. concern
CAS No. 36806-46-9
 2,6-Dimethyl- 349 31/251 No N/R N/R See 2-methyl-3-pentenoic No safety
5-heptenal acid. concern
CAS No. 106-72-9
2,6-Dimethyl- 349 31/251 No N/R N/R See 2-methyl-3-pentenoic No safety
5-heptenal acid. concern
CAS No. 106-72-9
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Ethyl 2-methyl- 350 6/6 No N/R N/R Expected to be hydrolysed No safety
3-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 1617-23-8
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Ethyl 2-methyl- 350 6/6 No N/R N/R Expected to be hydrolysed No safety
3-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 1617-23-8
 Ethyl 2-methyl- 351 0.3/0.02 No N/R N/R Expected to be hydrolysed No safety
4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 53399-81-8
Ethyl 2-methyl- 351 0.3/0.02 No N/R N/R Expected to be hydrolysed No safety
4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 53399-81-8
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Hexyl 2-methyl- 352 0.03/525 No N/R N/R Expected to be hydrolysed No safety
3&4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 58625-95-9
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Hexyl 2-methyl- 352 0.03/525 No N/R N/R Expected to be hydrolysed No safety
3&4-pentenoate to its component concern
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 58625-95-9

 Ethyl 2-methyl- 353 0.01/10 - - - Cannot be predicted to Evaluation of this
3,4-pentadienoate be metabolized to innocuous substance was
products; therefore, , steps deferred pending
A3-5 are not applicable review of a 90-day
to this substance, which study not available
must be evaluated on at this meeting.
the right side ('B') of
the evaluation scheme.
CAS No. 60523-21-9
Ethyl 2-methyl- 353 0.01/10 - - - Cannot be predicted to Evaluation of this
3,4-pentadienoate be metabolized to innocuous substance was
products; therefore, , steps deferred pending
A3-5 are not applicable review of a 90-day
to this substance, which study not available
must be evaluated on at this meeting.
the right side ('B') of
the evaluation scheme.
CAS No. 60523-21-9
 Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3,7-dimethyl- 354 0.2/0.1 No N/R N/R Expected to be hydrolysed No safety concern
6-octenoate to its component
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 2270-60-2
Table 1. (continued)
Substance No. Estimated Step A3 Step A4 Step A5 Comments Conclusion
per capita Does Is the Adequate based on current
intake ('eaters intake substance NOEL for intake
only'), exceed or its substance
Europe/USA intake metabolites or related
(µg/day) threshold? endogenous? substance?
Methyl 3,7-dimethyl- 354 0.2/0.1 No N/R N/R Expected to be hydrolysed No safety concern
6-octenoate to its component
alcohol and carboxylic
acid. See also
2-methyl-3-pentenoic acid.
CAS No. 2270-60-2
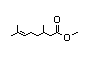 2-Methyl-4-pentenoic 355 N/D/0.04 No N/R N/R See 2-methyl-3-pentenoic No safety concern
acid acid.
CAS No. 1575-74-2
2-Methyl-4-pentenoic 355 N/D/0.04 No N/R N/R See 2-methyl-3-pentenoic No safety concern
acid acid.
CAS No. 1575-74-2
 N/R, Not required for evaluation because consumption was determined to be of no safety concern at Step 3A of the procedure
N/D, No intake data reported
a The ADI 'not specified' for the calcium, potassium, and sodium salts established at the thrity-third meeting of the Committee (Annex 1,
reference 83) was maintained.
Table 2. Most recent annual usage of linear and branched-chain aliphatic unsaturated
unconjugated alcohols, aldehydes, acids, and related esters as flavouring substances in Europe
and the United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Pentenoic acid (314)
Europe 32 5 0.08 NA
United States 15 3 0.05
cis-3-Hexen-1-ol (315)
Europe 30 000 4 300 71 NA
United States 5 600 1 100 18
cis-3-Hexenal (316)
Europe 34 5 0.08 NA
United States 18 3 0.06
3-Hexenoic acid (317)
Europe 77 11 0.2 NA
United States 5 1 0.02
4-Hexen-1-ol (318)
Europe 20 3 0.05 NA
United States 0.2 0.04 0.001
cis-4-Hexenal (319)
Europe 0.2 0.03 0.0005 NA
United States 0.5 0.1 0.002
4-Heptenal (320)
Europe 13 2 0.03 NA
United States 0.5 0.1 0.002
cis-3-Octen-1-ol (321)
Europe 39 6 0.1 NA
United States 0.05 0.01 0.0002
cis-5-Octen-1-ol (322)
Europe 3.3 0.5 0.01 NA
United States 0.2 0.04 0.001
cis-5-Octenal (323)
Europe 0.01 0.001 0.00002 NA
United States 150 29 0.5
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
cis-6-Nonen-1-ol (324 )
Europe 18 3 0.04 NA
United States 0.5 0.1 0.002
cis-6-Nonenal (325)
Europe 14 2 0.03 NA
United States 0.5 0.1 0.002
4-Decenal (326)
Europe 8 1 0.02 NA
United States 0.05 0.01 0.0002
5&6-Decenoic acid (327)
Europe 28 4 0.07 NA
United States 0.9 0.2 0.003
9-Decenoic acid (328)
Europe 0.8 0.1 0.002 NA
United States 0.9 0.2 0.003
9-Undecenal (329)
Europe 8 1 0.02 NA
United States 0.9 0.2 0.003
10-Undecenal (330)
Europe 2.6 0.4 0.01 NA
United States 0.2 0.04 0.001
10-Undecenoic acid (331)
Europe 210 30 0.5 NA
United States 4 0.8 0.01
Linoleic acid (332)
Europe 940 130 2 NA
United States 31 6 0.1
Oleic acid (333)
Europe 6 800 970 16 NA
United States 2 300 440 7
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3-hexenoate (334)
Europe 4.6 0.7 0.01 0.003
United States 0.05 0.01 0.0002
Ethyl 3-hexenoate (335)
Europe 26 4 0.1 0.03
United States 6 1 0.02
cis-3-Hexenyl cis-3-hexenoate (336)
Europe 26 4 0.1 0.05
United States 4 0.8 0.01
Methyl cis-4-octenoate (337)
Europe 3 0.4 0.01 0.002
United States 0.2 0.04 0.001
Ethyl cis-4-octenoate (338)
Europe 10 1 0.02 0.005
United States 0.2 0.04 0.001
Ethyl cis-4,7-octadienoate (339)
Europe 15 2 0.04 0.01
United States 0.04 0.01 0.0001
Methyl 3-nonenoate (340)
Europe 13 2 0.03 0.006
United States 400 76 1
Ethyl trans-4-octenoate (341)
Europe 15 2 0.04 0.009
United States 0.2 0.04 0.001
Methyl 9-undecenoate (342)
Europe 280 39 1 0.20
United States 2 0.4 0.01
Ethyl 10-undecenoate (343)
Europe 12 2 0.03 0.006
United States 230 44 1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Butyl 10-undecenoate (344)
Europe 0.3 0.04 0.0007 0.0002
United States 25 5 0.08
Ethyl oleate (345)
Europe 490 69 1 0.10
United States 17 3 0.1
Methyl linoleate & methyl
linolenate (mixture) (346)
Europe NR N/D
United States 120 23 0.4 0.046
2-Methyl-3-pentenoic acid (347)
Europe 10 1 0.02 NA
United States 9 2 0.03
2,6-Dimethyl-6-hepten-1-ol (348)
Europe NR N/D NA
United States 0.5 0.1 0.002
2,6-Dimethyl-5-heptenal (349)
Europe 220 31 1 NA
United States 1 300 250 4
Ethyl 2-methyl-3-pentenoate (350)
Europe 40 6 0.1 0.03
United States 31 6 0.1
Ethyl 2-methyl-4-pentenoate (351)
Europe 0.2 0.03 0.0005 0.0002
United States 0.9 0.2 0.003
Hexyl 2-methyl-3&4-pentenoate (352)
Europe 0.2 0.03 0.0005 0.0003
United States 2 760 520 9
Ethyl 2-methyl-3,4-pentadienoate (353)
Europe 0.1 0.01 0.0002 0.00007
United States 50 10 0.2
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3,7-dimethyl-6-octenoate (354)
Europe 1.1 0.2 0.003 0.0005
United States 0.3 0.1 0.001
2-Methyl-4-pentenoic acid (355)
Europe NR N/D NA
United States 0.2 0.04 0.001
Total
Europe 39 168 NA NA NA
United States 13 130 NA NA NA
NR, not reported; NA, not applicable
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor Industry,
1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
Linear unsaturated primary alcohols, aldehydes, and acids
The alcohols (including those formed from ester hydrolysis) and
aldehydes in this group are efficiently oxidized to the corresponding
unsaturated carboxylic acids by high-capacity enzyme pathways, as
discussed in the section 'General aspects of metabolism'. The
resulting linear unsaturated carboxylic acids participate in normal
fatty acid metabolism. Short-chain acids containing terminal
unsaturation, such as 4-pentenoic acid (No. 314), may be metabolized
by desaturation to yield a substrate that may participate in the fatty
acid pathway.
Alternative minor metabolic pathways have been characterized for
linear long-chain fatty acids (linoleic acid and oleic acid) and
short-chain acids containing unsaturation (i.e. 4-pentenoic acid).
While linoleic and oleic acids participate in ß-oxidation and normal
fatty acid metabolism in most tissues (Masoro, 1977), they may undergo
omega-oxidation in the liver and alpha-oxidation in the brain (Wakil &
Barnes, 1971; Gibson et al., 1982). In another minor pathway for the
oxidative metabolism of 4-pentenoic acid, trans-2,4-pentadienoyl
coenzyme A is produced, which is further metabolized via ß-oxidation
to yield 3-keto-4-pentenoyl coenzyme A (Schulz, 1983).
Branched-chain unsaturated primary alcohols, aldehydes, and acids
Generally, branched-chain aliphatic alcohols are oxidized to the
corresponding aldehydes, which, in turn, are oxidized to the
corresponding carboxylic acids (Bosron & Li, 1980; Levi & Hodgson,
1989). The aldehyde may also be reduced to the corresponding alcohol,
which is probably a short-lived intermediate in vivo. Like their
saturated analogues, unsaturated branched-chain aliphatic alcohols and
aldehydes are converted by the pathways cited above to the
corresponding acids. The acids may undergo ß-oxidative cleavage and
complete metabolism to carbon dioxide (Williams, 1959; Voet & Voet,
1990) in amino acid pathways, the fatty acid pathway, and the
tricarboxylic acid cycle. Alternatively, they may undergo a
combination of omega-, (omega-1)-, and ß-oxidation and selective
dehydrogenation and hydration to yield polar metabolites which are
excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Diliberto et al., 1990). The
principal metabolic pathways for detoxification of these
branched-chain substances is determined primarily by four structural
characteristics: carbon chain length and the position, number, and
size of the alkyl substituents.
These multiple pathways are illustrated by 2-ethylbutyric acid
and 2-ethyl-1-butanol. An oral dose of 2-ethyl-1-butanol was excreted
unchanged in the urine, principally as the glucuronic acid conjugate
(Kamil et al., 1953). 2-Ethylbutyric acid is metabolized by dogs to
2-pentanone, which is presumably derived from ß-oxidation and
decarboxylation (Deuel, 1957). Urinary metabolites of 2-ethylbutyric
acid reported in diabetic patients include the glucuronide conjugates
of 3-keto-2-ethylbutyric acid and 3,3'-diketo-2-ethylbutyric acid
formed by ß-oxidation of one or both ethyl chains (Granger et
al.,1954).
Higher homologues of 2-ethyl-1-butanol have a more complex
pattern of metabolism in which omega-oxidation competes with excretion
of the glucuronide conjugate of the corresponding acid. Female Fischer
344 rats were given a single oral dose of 50 or 500 mg/kg bw
[1-14C-ethyl]-2-ethylhexanol by gavage or repeated daily oral doses
of 50 mg/kg bw 2-ethyl-hexanol for 14 days. The major urinary
metabolites of the structurally related saturated branched-chain
alcohol 2-ethyl-1-hexanol collected over 0-24 h included the
glucuronic acid conjugate of 2-ethyl-hexanoic acid, omega- and
(omega-1)-oxidation metabolites, 2-ethyl-1,6-dihexanoic acid,
5-hydroxy-2-ethylhexanoic acid and its corresponding -lactone, and
6-hydroxy-2-ethylhexanoic acid. Minute quantities of the
omega-desaturation metabolite, 2-ethyl-5-hexenoic acid, and
metabolites formed from ß oxidation and oxidative decarboxylation, 2-
and 4-heptanone, were also detected (Albro, 1975; Deisinger et al.,
1994). After single or repeated doses of 50 mg/kg bw, the
omega-oxidation pathway predominated, as the principal urinary
metabolites excreted were 2-ethyl-1,6-hexane-dioic acid (30%) and its
precursors (8.5%). Only 6.8% of the dose was excreted in the urine as
the glucuronic acid conjugate of 2-ethylhexanoic acid. With 500 mg/kg
bw, 2-ethylhexanoic acid (25%) was the principal urinary metabolite,
indicating that saturation of the omega-desaturation pathway occurs at
this dose (Deisinger et al., 1994; Figure 1).
N/R, Not required for evaluation because consumption was determined to be of no safety concern at Step 3A of the procedure
N/D, No intake data reported
a The ADI 'not specified' for the calcium, potassium, and sodium salts established at the thrity-third meeting of the Committee (Annex 1,
reference 83) was maintained.
Table 2. Most recent annual usage of linear and branched-chain aliphatic unsaturated
unconjugated alcohols, aldehydes, acids, and related esters as flavouring substances in Europe
and the United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Pentenoic acid (314)
Europe 32 5 0.08 NA
United States 15 3 0.05
cis-3-Hexen-1-ol (315)
Europe 30 000 4 300 71 NA
United States 5 600 1 100 18
cis-3-Hexenal (316)
Europe 34 5 0.08 NA
United States 18 3 0.06
3-Hexenoic acid (317)
Europe 77 11 0.2 NA
United States 5 1 0.02
4-Hexen-1-ol (318)
Europe 20 3 0.05 NA
United States 0.2 0.04 0.001
cis-4-Hexenal (319)
Europe 0.2 0.03 0.0005 NA
United States 0.5 0.1 0.002
4-Heptenal (320)
Europe 13 2 0.03 NA
United States 0.5 0.1 0.002
cis-3-Octen-1-ol (321)
Europe 39 6 0.1 NA
United States 0.05 0.01 0.0002
cis-5-Octen-1-ol (322)
Europe 3.3 0.5 0.01 NA
United States 0.2 0.04 0.001
cis-5-Octenal (323)
Europe 0.01 0.001 0.00002 NA
United States 150 29 0.5
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
cis-6-Nonen-1-ol (324 )
Europe 18 3 0.04 NA
United States 0.5 0.1 0.002
cis-6-Nonenal (325)
Europe 14 2 0.03 NA
United States 0.5 0.1 0.002
4-Decenal (326)
Europe 8 1 0.02 NA
United States 0.05 0.01 0.0002
5&6-Decenoic acid (327)
Europe 28 4 0.07 NA
United States 0.9 0.2 0.003
9-Decenoic acid (328)
Europe 0.8 0.1 0.002 NA
United States 0.9 0.2 0.003
9-Undecenal (329)
Europe 8 1 0.02 NA
United States 0.9 0.2 0.003
10-Undecenal (330)
Europe 2.6 0.4 0.01 NA
United States 0.2 0.04 0.001
10-Undecenoic acid (331)
Europe 210 30 0.5 NA
United States 4 0.8 0.01
Linoleic acid (332)
Europe 940 130 2 NA
United States 31 6 0.1
Oleic acid (333)
Europe 6 800 970 16 NA
United States 2 300 440 7
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3-hexenoate (334)
Europe 4.6 0.7 0.01 0.003
United States 0.05 0.01 0.0002
Ethyl 3-hexenoate (335)
Europe 26 4 0.1 0.03
United States 6 1 0.02
cis-3-Hexenyl cis-3-hexenoate (336)
Europe 26 4 0.1 0.05
United States 4 0.8 0.01
Methyl cis-4-octenoate (337)
Europe 3 0.4 0.01 0.002
United States 0.2 0.04 0.001
Ethyl cis-4-octenoate (338)
Europe 10 1 0.02 0.005
United States 0.2 0.04 0.001
Ethyl cis-4,7-octadienoate (339)
Europe 15 2 0.04 0.01
United States 0.04 0.01 0.0001
Methyl 3-nonenoate (340)
Europe 13 2 0.03 0.006
United States 400 76 1
Ethyl trans-4-octenoate (341)
Europe 15 2 0.04 0.009
United States 0.2 0.04 0.001
Methyl 9-undecenoate (342)
Europe 280 39 1 0.20
United States 2 0.4 0.01
Ethyl 10-undecenoate (343)
Europe 12 2 0.03 0.006
United States 230 44 1
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Butyl 10-undecenoate (344)
Europe 0.3 0.04 0.0007 0.0002
United States 25 5 0.08
Ethyl oleate (345)
Europe 490 69 1 0.10
United States 17 3 0.1
Methyl linoleate & methyl
linolenate (mixture) (346)
Europe NR N/D
United States 120 23 0.4 0.046
2-Methyl-3-pentenoic acid (347)
Europe 10 1 0.02 NA
United States 9 2 0.03
2,6-Dimethyl-6-hepten-1-ol (348)
Europe NR N/D NA
United States 0.5 0.1 0.002
2,6-Dimethyl-5-heptenal (349)
Europe 220 31 1 NA
United States 1 300 250 4
Ethyl 2-methyl-3-pentenoate (350)
Europe 40 6 0.1 0.03
United States 31 6 0.1
Ethyl 2-methyl-4-pentenoate (351)
Europe 0.2 0.03 0.0005 0.0002
United States 0.9 0.2 0.003
Hexyl 2-methyl-3&4-pentenoate (352)
Europe 0.2 0.03 0.0005 0.0003
United States 2 760 520 9
Ethyl 2-methyl-3,4-pentadienoate (353)
Europe 0.1 0.01 0.0002 0.00007
United States 50 10 0.2
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Methyl 3,7-dimethyl-6-octenoate (354)
Europe 1.1 0.2 0.003 0.0005
United States 0.3 0.1 0.001
2-Methyl-4-pentenoic acid (355)
Europe NR N/D NA
United States 0.2 0.04 0.001
Total
Europe 39 168 NA NA NA
United States 13 130 NA NA NA
NR, not reported; NA, not applicable
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor Industry,
1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the survey.
Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where body
weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
Linear unsaturated primary alcohols, aldehydes, and acids
The alcohols (including those formed from ester hydrolysis) and
aldehydes in this group are efficiently oxidized to the corresponding
unsaturated carboxylic acids by high-capacity enzyme pathways, as
discussed in the section 'General aspects of metabolism'. The
resulting linear unsaturated carboxylic acids participate in normal
fatty acid metabolism. Short-chain acids containing terminal
unsaturation, such as 4-pentenoic acid (No. 314), may be metabolized
by desaturation to yield a substrate that may participate in the fatty
acid pathway.
Alternative minor metabolic pathways have been characterized for
linear long-chain fatty acids (linoleic acid and oleic acid) and
short-chain acids containing unsaturation (i.e. 4-pentenoic acid).
While linoleic and oleic acids participate in ß-oxidation and normal
fatty acid metabolism in most tissues (Masoro, 1977), they may undergo
omega-oxidation in the liver and alpha-oxidation in the brain (Wakil &
Barnes, 1971; Gibson et al., 1982). In another minor pathway for the
oxidative metabolism of 4-pentenoic acid, trans-2,4-pentadienoyl
coenzyme A is produced, which is further metabolized via ß-oxidation
to yield 3-keto-4-pentenoyl coenzyme A (Schulz, 1983).
Branched-chain unsaturated primary alcohols, aldehydes, and acids
Generally, branched-chain aliphatic alcohols are oxidized to the
corresponding aldehydes, which, in turn, are oxidized to the
corresponding carboxylic acids (Bosron & Li, 1980; Levi & Hodgson,
1989). The aldehyde may also be reduced to the corresponding alcohol,
which is probably a short-lived intermediate in vivo. Like their
saturated analogues, unsaturated branched-chain aliphatic alcohols and
aldehydes are converted by the pathways cited above to the
corresponding acids. The acids may undergo ß-oxidative cleavage and
complete metabolism to carbon dioxide (Williams, 1959; Voet & Voet,
1990) in amino acid pathways, the fatty acid pathway, and the
tricarboxylic acid cycle. Alternatively, they may undergo a
combination of omega-, (omega-1)-, and ß-oxidation and selective
dehydrogenation and hydration to yield polar metabolites which are
excreted as the glucuronic acid or sulfate conjugates in the urine
and, to a lesser extent, in the faeces (Diliberto et al., 1990). The
principal metabolic pathways for detoxification of these
branched-chain substances is determined primarily by four structural
characteristics: carbon chain length and the position, number, and
size of the alkyl substituents.
These multiple pathways are illustrated by 2-ethylbutyric acid
and 2-ethyl-1-butanol. An oral dose of 2-ethyl-1-butanol was excreted
unchanged in the urine, principally as the glucuronic acid conjugate
(Kamil et al., 1953). 2-Ethylbutyric acid is metabolized by dogs to
2-pentanone, which is presumably derived from ß-oxidation and
decarboxylation (Deuel, 1957). Urinary metabolites of 2-ethylbutyric
acid reported in diabetic patients include the glucuronide conjugates
of 3-keto-2-ethylbutyric acid and 3,3'-diketo-2-ethylbutyric acid
formed by ß-oxidation of one or both ethyl chains (Granger et
al.,1954).
Higher homologues of 2-ethyl-1-butanol have a more complex
pattern of metabolism in which omega-oxidation competes with excretion
of the glucuronide conjugate of the corresponding acid. Female Fischer
344 rats were given a single oral dose of 50 or 500 mg/kg bw
[1-14C-ethyl]-2-ethylhexanol by gavage or repeated daily oral doses
of 50 mg/kg bw 2-ethyl-hexanol for 14 days. The major urinary
metabolites of the structurally related saturated branched-chain
alcohol 2-ethyl-1-hexanol collected over 0-24 h included the
glucuronic acid conjugate of 2-ethyl-hexanoic acid, omega- and
(omega-1)-oxidation metabolites, 2-ethyl-1,6-dihexanoic acid,
5-hydroxy-2-ethylhexanoic acid and its corresponding -lactone, and
6-hydroxy-2-ethylhexanoic acid. Minute quantities of the
omega-desaturation metabolite, 2-ethyl-5-hexenoic acid, and
metabolites formed from ß oxidation and oxidative decarboxylation, 2-
and 4-heptanone, were also detected (Albro, 1975; Deisinger et al.,
1994). After single or repeated doses of 50 mg/kg bw, the
omega-oxidation pathway predominated, as the principal urinary
metabolites excreted were 2-ethyl-1,6-hexane-dioic acid (30%) and its
precursors (8.5%). Only 6.8% of the dose was excreted in the urine as
the glucuronic acid conjugate of 2-ethylhexanoic acid. With 500 mg/kg
bw, 2-ethylhexanoic acid (25%) was the principal urinary metabolite,
indicating that saturation of the omega-desaturation pathway occurs at
this dose (Deisinger et al., 1994; Figure 1).
 2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for a number of the 42
substances in this group and are in the range 470-19 000 mg/kg bw,
demonstrating that the acute toxicity of linear and branched-chain
aliphatic unsaturated and unconjugated alcohols, aldehydes, acids, and
related esters is low when they are given orally (Jenner et al., 1964;
Briggs et al., 1976).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
cis-3-Hexen-1-ol (No. 315)
Groups of 15 male and 15 female weanling rats were given
drinking-water containing 0, 310, 1250, or 5000 ppm cis-3 hexen-1-ol
for 98 days. Weekly measurements of body weight, food intake, and
water consumption revealed no differences in body-weight gain or food
intake between treated and control animals, but a significant
(p < 0.01) increase in water intake was recorded for males at the
high dose. A significant (p < 0.001), transitory anaemia was seen
at six weeks in females given 5000 ppm; however, this effect was not
observed at 14 weeks or in the males and therefore was considered not
to be related to treatment. Kidney and liver function in animals at
the low and intermediate doses was comparable to that of controls.
After 98 days, males at the high dose had a statistically significant
increase in relative kidney (p < 0.05) and adrenal weights
(p < 0.01) and a slight increase in urine specific gravity during
the first 2 h after a standard water load; however, there were no
histological signs of renal damage and no indications of abnormal
kidney function associated with treatment. Histopathological
examination revealed lymphocyte infiltration in the lung, suggesting a
mild pulmonary infection, and foci of tubular degeneration and
dilatation in the kidney. The incidence of these findings was similar
in test and control animals and could not be attributed to treatment.
The NOEL of 1250 ppm, reported by the authors to be equivalent to an
intake of 120-150 mg/kg bw per day (Gaunt et al., 1969), is > 1000
times the current daily per capita intake of 71 and 18 µg/kg bw per
day from use of cis-3-hexen-1-ol as a flavouring substance in Europe
and the United States, respectively.
10-Undecenoic acid (No. 331)
In an abstract describing the results of a study in which 100,
200, or 400 mg/kg bw per day of 10-undecenoic acid were administered
to Sprague-Dawley rats by gavage for six months, no effects were
reported (Tislow et al., 1950). Because a full report of this study
was not available, it was considered inadequate for deriving a NOEL.
In another study of 10-undecenoic acid, Sprague-Dawley rats were
maintained on diets containing 0.5, 1, or 2.5% (approximately 500,
1000, and 2500 mg/kg bw per day (US Food & Drug Administration, 1993))
for eight weeks. An inhibitory effect on growth, possibly related to
decreased food consumption, was reported during the first five weeks
(Newell et al., 1949). These data, described by the authors as
preliminary, were not used to derive a NOEL.
Oleic acid (No. 333)
The Committee previously allocated an ADI 'not specified' to the
aluminum, ammonium, calcium, magnesium, potassium, and sodium salts of
oleic acid (Annex 1, reference 70). Recently, the results of a 13-week
and a two-year study of oleic acid diethanolamine condensate
administered dermally to Fischer 344/N rats and B6C3F1 mice were
reported (US National Toxicology Program, 1997). These studies were
not considered relevant to the safety evaluation of oleic acid used as
a flavouring substance.
Numerous other studies are available of the role of high
concentrations of oleic acid and other fatty acids in the diet. As the
effects observed are probably the result of the extremely high intake
of total dietary fat and fatty acids, these studies were not
considered relevant to the safety evaluation of oleic acid at its
current level of intake as a flavouring substance.
2,6-Dimethyl-5-heptenal (No. 349)
Groups of 15 male and 15 female rats were fed diets providing
2,6-dimethyl-5-heptenal at average intakes of 0, 9, 37, or 150 mg/kg
bw per day for at least 90 days. Statistically significant
(p < 0.05) increases in haemoglobin concentrations were found in
males at the high dose at weeks 6 and 14 and in males at the
intermediate dose at week 14; however, these effects were not
biologically significant and were not considered by the authors to be
adverse. Most of the absolute and relative weights of organs were
similar in treated and control animals, but statistically significant
(p < 0.05) increases in the relative and absolute spleen weights
were observed in males at the intermediate dose. A statistically
significant increase in serum glucose concentration was observed in
male and female rats at the high dose, which the authors noted was of
unknown origin. Also at the high dose, the renal concentrating ability
was reduced in males at week 6 and in females at week 14, resulting in
proteinuria. Slight, statistically significant increases (p < 0.05
by Student's t test) in relative kidney and liver weights were also
observed in females at this dose. Histological examination of rats at
the high dose showed no statistically significant difference between
the treated group and controls, other than alveolar thickening in
males (p < 0.05). Since the histopathological appearance was normal
in animals of each sex and the kidney weights were normal in males,
the proteinuria could not be associated with treatment. Additionally,
no structural kidney damage was seen by light microscopy. A functional
effect on the kidney might have been caused by treatment at the
highest level, however, because males and females at the high dose
failed to concentrate urine after a period of dehydration during weeks
6 and 13, respectively, and the kidney weights of females were
marginally increased. The NOEL for 2,6-dimethyl-5-heptenal was 37
mg/kg bw per day (Gaunt et al., 1983) which is > 1000 times the daily
per capita intakes of 1 and 4 g/kg bw per day from use of
2,6-dimethyl-5-heptenal as a flavouring substance in Europe and the
United States, respectively.
Ethyl 2-methyl-3,4-pentadienoate (No. 353)
The Committee was aware of a study on the toxicity of
ethyl-2-methyl-3,4-pentadienoate (Cox et al., 1978); however, a full
report of the study was not available.
2.3.2.3 Genotoxicity
Three representative linear and branched-chain aliphatic
unsaturated primary alcohols and unconjugated aldehydes, carboxylic
acids, and related esters in this group have been tested for
genotoxicity. The results of these tests are summarized in Table 3 and
described below.
No evidence of mutagenicity was reported for oleic acid, methyl
linoleate, methyl linolenate, or 2,6-dimethyl-5-heptenal in standard
and preincubation assays in Salmonella typhimurium strain TA98,
TA100, TA1535, TA1537, or TA1538 with or without the addition of
metabolic activation (Wild et al., 1983; Shimizu et al., 1985;
Mortelmans et al., 1986; Heck et al., 1989). The maximum
concentrations used in these studies ranged from 333 to 50 000
µg/plate. In further bacterial assays, such as the rec assay in
Bacillus subtilis incubated with oleic acid (Osawa & Namiki, 1982),
the his+ reversion assay in S. typhimurium incubated with methyl
linoleate or methyl linolenate (MacGregor et al., 1985), and a
modified Ames test in Escherichia coli WP2 uvrA incubated with
oleic acid (Shimizu et al., 1985), aliphatic unsaturated unconjugated
acids and esters were also shown to be non-mutagenic.
No unscheduled DNA synthesis was seen after exposure of rat
hepatocytes to 2,6-dimethyl-5-heptenal at concentrations up to 1 mg/ml
(Heck et al., 1989).
In S. typhimurium strain TA98, a concentration of 0.1 mmol/L
oleic acid was reported to inhibit (by approximately 50%) the
mutagenicity of known mutagens, including
3-amino-1,4-dimethyl-5 H-pyrido[4,3- b]indole,
2-amino-9 H-pyridol[2,3- b]indole,
2-amino-3-methylimidazo[4,5- d]quinoline, benzo[ a]-pyrene, and
aflatoxin B1, when tested in the presence or absence of metabolic
activation (Hayatsu et al., 1981).
Table 3. Results of assays for the genotoxicity studies for linear and branched-chain aliphatic unsaturated unconjugated alcohols, aldehydes,
acids and related esters
Substance No. End-point Test object Maximum Result Reference
concentration
Oleic acid 333 Reverse mutationa S. typhimurium TA1535, 5000 mg/plate Negativeb Shimizu et al. (1985)
TA1537, TA98, TA100, TA1538
Reverse mutationa S. typhimurium TA1535, 333 mg/plate Negativeb Mortelmans et al. (1986)
TA98, TA100, TA1537
Reverse mutationa E. coli 5000 mg/plate Negativeb Shimizu et al. (1985)
Rec assay B. subtilis 1 mg/plate Negativeb Osawa & Namiki (1982)
Methyl linoleate Part of His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
346 TA98, TA102, TA97, TA1537
His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
TA98, TA102, TA97, TA1537
2,6-Dimethyl-5-heptenal 349 Reverse mutation S. typhimurium TA1535, 3.6 mg/plate Negativeb Wild et al. (1983)
TA100, TA1537, TA1538, TA98
Reverse mutation S. typhimurium TA1535, 50 mg/plate Negativeb Heck et al. (1989)
TA100, TA1537, TA1538, TA98
Unscheduled DNA Rat hepatocytes 1 mg/ml Negativeb Heck et al. (1989)
synthesis
Micronucleus Mouse 1540 mg/kg bw Negative Wild et al. (1983)
formation
Basc test Drosophila melanogaster 25 mmol/L Negative Wild et al. (1983
a Modified Ames' assay, with preincubation
b With and without metabolic activation
2,6-Dimethyl-5-heptenal at a concentration of 25 mmol/L did not
cause Basc reversion in Drosophila melanogaster (Wild et al.,
1983).
In mice given a maximum single dose of 1540 mg/kg bw
2,6-dimethyl-5-heptenal, all of which survived treatment, the
incidence of polychromatic erythrocytes was not statistically
significantly increased (Wild et al., 1983).
Oleic acid did not induce oxidative damage in isolated DNA (de
Kok et al., 1994). In a three-week study in volunteers, ingestion of
oleic acid did not alter the frequency of micronucleated lymphocytes
in peripheral blood (Record et al., 1992).
These negative results indicate that the substances in this group
of linear and branched-chain aliphatic unsaturated and unconjugated
alcohols, aldehydes, acids, and related esters that are used as
flavouring substances are neither mutagenic nor genotoxic.
2.3.2.4 Other relevant studies
In a study of teratogenicity, two groups of 15 female NMRI mice
were mated with males for 2 h, and 4-pentenoic acid (No. 314) was
administered as the sodium salt in water by subcutaneous injection to
pregnant mice on day 8 of gestation as a single 600 mg/kg bw dose. A
group of control mice was included. Implantation sites were counted,
and each live fetus was weighed and inspected for neural tube defects.
Effects on the visceral or skeletal system were not examined.
4-Pentenoic acid had no effect on fetal weight, the number of live
fetuses, embryolethality, or exencephaly in comparison with control
animals (Nau & Loscher, 1986). These results could not be used to
derive a NOEL for oral intake of 4-pentenoic acid.
4. REFERENCES
Abumrad, N.A., Park, J.H. & Park, C.R. (1984) Permeation of long-chain
fatty acid into adipocytes. J. Biol. Chem., 259, 8945-8953.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Borgstrom, B. (1974) Fat digestion and absorption. In: Smyth, D.H.,
ed., Biomembranes--Intestinal Absorption, London, Plenum Press, Vol.
4B, pp. 555-620.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, New York, Academic Press,
Vol I, p. 231.
Briggs, G.B., Doyle, R.L. & Young, J.A. (1976) Safety studies on a
series of fatty acids. Am. Ind. Hyg. Ass. J., 37, 251-253.
Cox, G.E., Rucci, G. & Babish, J.G. (1978) 90-Day subacute dietary
toxicity study of ethyl-2-methyl-3,4-pentadienoate in Sprague-Dawley
rats. Unpublished report. Submitted to the Flavor and Extract
Manufactuers' Association of the United States, Washington DC, United
States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, A.M., Holdsworth, C.D. & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117, 97-100.
Deisinger, P.J., Boatman, R.J. & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24, 429-440.
Deuel, H.J., ed. (1957) The Lipids, Their Chemistry and
Biochemistry, New York, Wiley Interscience, Vol III.
Dhopeshwarkar, G.A. & Mead, J.F. (1973) Uptake and transport of fatty
acids into the brain and the role of the blood-brain barrier system.
In: Paoletti, R. & Kritchevsky, D., eds, Advances in Lipid
Research, New York, Academic Press, Vol. 11, pp. 109-142.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, B.L.T. &
Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated
aldehyde, in male F344 rats. Drug Metab. Disposition, 18, 868-875.
Drake, J.J.-P., Gaunt, I.F., Butterworth, K.R., Hooson, J., Hardy, J.
& Gangolli, S.D. (1975) Short-term toxicity of isoamyl salicylat in
rats. Food Cosmet. Toxicol., 13, 185-193.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montpellier, 25, 51-62.
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G. & Gangolli, S.D.
(1969) Acute (rat and mouse) and short-term (rat) toxicity studies on
cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7, 451-459.
Gaunt, I.F., Wright, M.G., Cottrell, R. & Gangolli, S.D. (1983)
Short-term toxicity of 2,6-dimethylhept-5-en-1-al in rats. Food
Chem. Toxicol., 21, 543-549.
Gibson, G.G., Orton, T.C. & Tamburini, P.P. (1982) Cytochrome P-450
induction by clofibrate. Purification and properties of a hepatic
cytochrome P-450 relatively specific for the 12- and 11-hydroxylation
of dodecanoic acid (lauric acid). Biochem. J., 203, 161-168.
Granger, R., Giroux, J. & Monnier, P. (1954) [Research on the
mechanism of action of calcium ethyl-2-butanoate.] Trav. Soc.
Pharm. Montpeller, 14, 335-341 (in French).
Harris, P., Gloster, J.A. & Ward, B.J. (1980) Transport of fatty acids
in the heart. Arch. Mal. Coeur, 73, 593-598.
Hayatsu, H., Inoue, K., Ohta, H., Namba, T., Togawa, K., Hayatsu, T.,
Makita, M. & Wataya, Y. (1981) Inhibition of the mutagenicity of
cooked-beef basic fraction by its acidic fraction. Mutat. Res., 91,
437-442.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Flavor
Manufacturers' Association of the United States, Washington DC, United
States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; the glucuronic acid conjugation of acyclic
aliphatic alcohols. J. Biochem., 53, 129-136.
de Kok, T.M., ten Vaarwerk, F., Zwingman, I., van Maanen, J.M. &
Kleinjans, J.C. (1994) Peroxidation of linoleic, arachidonic and oleic
acid in relation to the induction of oxidative DNA damage and
cytogenetic effects. Carcinogenesis, 15, 1399-1404.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London, Taylor &
Francis, pp. 119-138.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
MacGregor, J.T., Wilson, R.E., Neff, W.E. & Frankel, E.N. (1985)
Mutagenicity tests of lipid oxidation products in Salmonella
typhimurium: Monohydroperoxides and secondary oxidation products of
methyl linoleate and methyl linolenate. Food Chem. Toxicol., 23,
1041-1047.
Masoro, E.J. (1977) Lipids and lipid metabolism. Am. Rev. Physiol.,
39, 301-321.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nau, H. & Loscher, W. (1986) Pharmacologic evaluation of metabolites
and analogs of valproic acid: Teratogenic potencies in mice.
Fundam. Appli Toxicol., 6, 669-676.
Newell, G.W., Petretti, A.K. & Reiner, L. (1949) Studies of the acute
and chronic toxicity of undecylenic acid. J. Invest. Dermatol., 13,
145-149.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Arch.
Biochem., 46, 2299-2304.
Record, I.R., Konstantinopoulos, M. & Nestel, P.J. (1992) A
comparative study of micronucleus frequency in peripheral blood
lymphocytes of human subjects give dietary cis, trans and saturated
fat. Food Chem. Toxicol., 30, 585-588.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. J. Biochem., 22, 1827.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tislow, R., Margolin, S., Foley, E.J. & Lee, S.W. (1950) Toxicity of
undercylenic acid. J. Pharmacol. Exp. Ther., 98, 31-32.
US Food and Drug Administration (1993) Priority-based Assessment
of Food Additives (PAFA) Database, Center for Food Safety and
Applied Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1997) Toxicology and Carcinogenesis
Studies of Oleic Acid Diethanolamine Condensate (CAS No. 93-83-4)
in F344/N Rats and B6C3F1 Mice (Dermal Studies) (Technical Report
Series No. 481, NIH Publication No. 97-3971), Research Triangle Park,
North Carolina, US Department of Health and Human Services, Public
Health Service, National Institutes of Health.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Wakil, S.J. & Barnes, E.M. (1971) In: Florkin, M. & Stotz, E., eds,
Comprehensive Biochemistry, New York, Elsevier Publishing Co., Vol.
18S.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T. (1959) Detoxication Mechanisms, 2nd Ed., London,
Chapman & Hall, pp. 88-113.
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for a number of the 42
substances in this group and are in the range 470-19 000 mg/kg bw,
demonstrating that the acute toxicity of linear and branched-chain
aliphatic unsaturated and unconjugated alcohols, aldehydes, acids, and
related esters is low when they are given orally (Jenner et al., 1964;
Briggs et al., 1976).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
cis-3-Hexen-1-ol (No. 315)
Groups of 15 male and 15 female weanling rats were given
drinking-water containing 0, 310, 1250, or 5000 ppm cis-3 hexen-1-ol
for 98 days. Weekly measurements of body weight, food intake, and
water consumption revealed no differences in body-weight gain or food
intake between treated and control animals, but a significant
(p < 0.01) increase in water intake was recorded for males at the
high dose. A significant (p < 0.001), transitory anaemia was seen
at six weeks in females given 5000 ppm; however, this effect was not
observed at 14 weeks or in the males and therefore was considered not
to be related to treatment. Kidney and liver function in animals at
the low and intermediate doses was comparable to that of controls.
After 98 days, males at the high dose had a statistically significant
increase in relative kidney (p < 0.05) and adrenal weights
(p < 0.01) and a slight increase in urine specific gravity during
the first 2 h after a standard water load; however, there were no
histological signs of renal damage and no indications of abnormal
kidney function associated with treatment. Histopathological
examination revealed lymphocyte infiltration in the lung, suggesting a
mild pulmonary infection, and foci of tubular degeneration and
dilatation in the kidney. The incidence of these findings was similar
in test and control animals and could not be attributed to treatment.
The NOEL of 1250 ppm, reported by the authors to be equivalent to an
intake of 120-150 mg/kg bw per day (Gaunt et al., 1969), is > 1000
times the current daily per capita intake of 71 and 18 µg/kg bw per
day from use of cis-3-hexen-1-ol as a flavouring substance in Europe
and the United States, respectively.
10-Undecenoic acid (No. 331)
In an abstract describing the results of a study in which 100,
200, or 400 mg/kg bw per day of 10-undecenoic acid were administered
to Sprague-Dawley rats by gavage for six months, no effects were
reported (Tislow et al., 1950). Because a full report of this study
was not available, it was considered inadequate for deriving a NOEL.
In another study of 10-undecenoic acid, Sprague-Dawley rats were
maintained on diets containing 0.5, 1, or 2.5% (approximately 500,
1000, and 2500 mg/kg bw per day (US Food & Drug Administration, 1993))
for eight weeks. An inhibitory effect on growth, possibly related to
decreased food consumption, was reported during the first five weeks
(Newell et al., 1949). These data, described by the authors as
preliminary, were not used to derive a NOEL.
Oleic acid (No. 333)
The Committee previously allocated an ADI 'not specified' to the
aluminum, ammonium, calcium, magnesium, potassium, and sodium salts of
oleic acid (Annex 1, reference 70). Recently, the results of a 13-week
and a two-year study of oleic acid diethanolamine condensate
administered dermally to Fischer 344/N rats and B6C3F1 mice were
reported (US National Toxicology Program, 1997). These studies were
not considered relevant to the safety evaluation of oleic acid used as
a flavouring substance.
Numerous other studies are available of the role of high
concentrations of oleic acid and other fatty acids in the diet. As the
effects observed are probably the result of the extremely high intake
of total dietary fat and fatty acids, these studies were not
considered relevant to the safety evaluation of oleic acid at its
current level of intake as a flavouring substance.
2,6-Dimethyl-5-heptenal (No. 349)
Groups of 15 male and 15 female rats were fed diets providing
2,6-dimethyl-5-heptenal at average intakes of 0, 9, 37, or 150 mg/kg
bw per day for at least 90 days. Statistically significant
(p < 0.05) increases in haemoglobin concentrations were found in
males at the high dose at weeks 6 and 14 and in males at the
intermediate dose at week 14; however, these effects were not
biologically significant and were not considered by the authors to be
adverse. Most of the absolute and relative weights of organs were
similar in treated and control animals, but statistically significant
(p < 0.05) increases in the relative and absolute spleen weights
were observed in males at the intermediate dose. A statistically
significant increase in serum glucose concentration was observed in
male and female rats at the high dose, which the authors noted was of
unknown origin. Also at the high dose, the renal concentrating ability
was reduced in males at week 6 and in females at week 14, resulting in
proteinuria. Slight, statistically significant increases (p < 0.05
by Student's t test) in relative kidney and liver weights were also
observed in females at this dose. Histological examination of rats at
the high dose showed no statistically significant difference between
the treated group and controls, other than alveolar thickening in
males (p < 0.05). Since the histopathological appearance was normal
in animals of each sex and the kidney weights were normal in males,
the proteinuria could not be associated with treatment. Additionally,
no structural kidney damage was seen by light microscopy. A functional
effect on the kidney might have been caused by treatment at the
highest level, however, because males and females at the high dose
failed to concentrate urine after a period of dehydration during weeks
6 and 13, respectively, and the kidney weights of females were
marginally increased. The NOEL for 2,6-dimethyl-5-heptenal was 37
mg/kg bw per day (Gaunt et al., 1983) which is > 1000 times the daily
per capita intakes of 1 and 4 g/kg bw per day from use of
2,6-dimethyl-5-heptenal as a flavouring substance in Europe and the
United States, respectively.
Ethyl 2-methyl-3,4-pentadienoate (No. 353)
The Committee was aware of a study on the toxicity of
ethyl-2-methyl-3,4-pentadienoate (Cox et al., 1978); however, a full
report of the study was not available.
2.3.2.3 Genotoxicity
Three representative linear and branched-chain aliphatic
unsaturated primary alcohols and unconjugated aldehydes, carboxylic
acids, and related esters in this group have been tested for
genotoxicity. The results of these tests are summarized in Table 3 and
described below.
No evidence of mutagenicity was reported for oleic acid, methyl
linoleate, methyl linolenate, or 2,6-dimethyl-5-heptenal in standard
and preincubation assays in Salmonella typhimurium strain TA98,
TA100, TA1535, TA1537, or TA1538 with or without the addition of
metabolic activation (Wild et al., 1983; Shimizu et al., 1985;
Mortelmans et al., 1986; Heck et al., 1989). The maximum
concentrations used in these studies ranged from 333 to 50 000
µg/plate. In further bacterial assays, such as the rec assay in
Bacillus subtilis incubated with oleic acid (Osawa & Namiki, 1982),
the his+ reversion assay in S. typhimurium incubated with methyl
linoleate or methyl linolenate (MacGregor et al., 1985), and a
modified Ames test in Escherichia coli WP2 uvrA incubated with
oleic acid (Shimizu et al., 1985), aliphatic unsaturated unconjugated
acids and esters were also shown to be non-mutagenic.
No unscheduled DNA synthesis was seen after exposure of rat
hepatocytes to 2,6-dimethyl-5-heptenal at concentrations up to 1 mg/ml
(Heck et al., 1989).
In S. typhimurium strain TA98, a concentration of 0.1 mmol/L
oleic acid was reported to inhibit (by approximately 50%) the
mutagenicity of known mutagens, including
3-amino-1,4-dimethyl-5 H-pyrido[4,3- b]indole,
2-amino-9 H-pyridol[2,3- b]indole,
2-amino-3-methylimidazo[4,5- d]quinoline, benzo[ a]-pyrene, and
aflatoxin B1, when tested in the presence or absence of metabolic
activation (Hayatsu et al., 1981).
Table 3. Results of assays for the genotoxicity studies for linear and branched-chain aliphatic unsaturated unconjugated alcohols, aldehydes,
acids and related esters
Substance No. End-point Test object Maximum Result Reference
concentration
Oleic acid 333 Reverse mutationa S. typhimurium TA1535, 5000 mg/plate Negativeb Shimizu et al. (1985)
TA1537, TA98, TA100, TA1538
Reverse mutationa S. typhimurium TA1535, 333 mg/plate Negativeb Mortelmans et al. (1986)
TA98, TA100, TA1537
Reverse mutationa E. coli 5000 mg/plate Negativeb Shimizu et al. (1985)
Rec assay B. subtilis 1 mg/plate Negativeb Osawa & Namiki (1982)
Methyl linoleate Part of His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
346 TA98, TA102, TA97, TA1537
His+ reversion S. typhimurium TA100, 1 mg/plate Negativeb MacGregor et al. (1985)
TA98, TA102, TA97, TA1537
2,6-Dimethyl-5-heptenal 349 Reverse mutation S. typhimurium TA1535, 3.6 mg/plate Negativeb Wild et al. (1983)
TA100, TA1537, TA1538, TA98
Reverse mutation S. typhimurium TA1535, 50 mg/plate Negativeb Heck et al. (1989)
TA100, TA1537, TA1538, TA98
Unscheduled DNA Rat hepatocytes 1 mg/ml Negativeb Heck et al. (1989)
synthesis
Micronucleus Mouse 1540 mg/kg bw Negative Wild et al. (1983)
formation
Basc test Drosophila melanogaster 25 mmol/L Negative Wild et al. (1983
a Modified Ames' assay, with preincubation
b With and without metabolic activation
2,6-Dimethyl-5-heptenal at a concentration of 25 mmol/L did not
cause Basc reversion in Drosophila melanogaster (Wild et al.,
1983).
In mice given a maximum single dose of 1540 mg/kg bw
2,6-dimethyl-5-heptenal, all of which survived treatment, the
incidence of polychromatic erythrocytes was not statistically
significantly increased (Wild et al., 1983).
Oleic acid did not induce oxidative damage in isolated DNA (de
Kok et al., 1994). In a three-week study in volunteers, ingestion of
oleic acid did not alter the frequency of micronucleated lymphocytes
in peripheral blood (Record et al., 1992).
These negative results indicate that the substances in this group
of linear and branched-chain aliphatic unsaturated and unconjugated
alcohols, aldehydes, acids, and related esters that are used as
flavouring substances are neither mutagenic nor genotoxic.
2.3.2.4 Other relevant studies
In a study of teratogenicity, two groups of 15 female NMRI mice
were mated with males for 2 h, and 4-pentenoic acid (No. 314) was
administered as the sodium salt in water by subcutaneous injection to
pregnant mice on day 8 of gestation as a single 600 mg/kg bw dose. A
group of control mice was included. Implantation sites were counted,
and each live fetus was weighed and inspected for neural tube defects.
Effects on the visceral or skeletal system were not examined.
4-Pentenoic acid had no effect on fetal weight, the number of live
fetuses, embryolethality, or exencephaly in comparison with control
animals (Nau & Loscher, 1986). These results could not be used to
derive a NOEL for oral intake of 4-pentenoic acid.
4. REFERENCES
Abumrad, N.A., Park, J.H. & Park, C.R. (1984) Permeation of long-chain
fatty acid into adipocytes. J. Biol. Chem., 259, 8945-8953.
Albro, P.W. (1975) The metabolism of 2-ethylhexanol in rats.
Xenobiotica, 5, 625-636.
Borgstrom, B. (1974) Fat digestion and absorption. In: Smyth, D.H.,
ed., Biomembranes--Intestinal Absorption, London, Plenum Press, Vol.
4B, pp. 555-620.
Bosron, W.F. & Li, T.-K. (1980) Alcohol dehydrogenase. In: Jacoby, W.,
ed., Enzymatic Basis of Detoxification, New York, Academic Press,
Vol I, p. 231.
Briggs, G.B., Doyle, R.L. & Young, J.A. (1976) Safety studies on a
series of fatty acids. Am. Ind. Hyg. Ass. J., 37, 251-253.
Cox, G.E., Rucci, G. & Babish, J.G. (1978) 90-Day subacute dietary
toxicity study of ethyl-2-methyl-3,4-pentadienoate in Sprague-Dawley
rats. Unpublished report. Submitted to the Flavor and Extract
Manufactuers' Association of the United States, Washington DC, United
States.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard: A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Dawson, A.M., Holdsworth, C.D. & Webb, J. (1964) Absorption of short
chain fatty acids in man. Proc. Soc. Exp. Biol. Med., 117, 97-100.
Deisinger, P.J., Boatman, R.J. & Guest, D. (1994) Metabolism of
2-ethylhexanol administered orally and dermally to the female Fischer
344 rat. Xenobiotica, 24, 429-440.
Deuel, H.J., ed. (1957) The Lipids, Their Chemistry and
Biochemistry, New York, Wiley Interscience, Vol III.
Dhopeshwarkar, G.A. & Mead, J.F. (1973) Uptake and transport of fatty
acids into the brain and the role of the blood-brain barrier system.
In: Paoletti, R. & Kritchevsky, D., eds, Advances in Lipid
Research, New York, Academic Press, Vol. 11, pp. 109-142.
Diliberto, J.J., Srinivas, P., Overstreet, D., Usha, B.L.T. &
Birnbaum, L.S. (1990) Metabolism of citral, an alpha,beta-unsaturated
aldehyde, in male F344 rats. Drug Metab. Disposition, 18, 868-875.
Drake, J.J.-P., Gaunt, I.F., Butterworth, K.R., Hooson, J., Hardy, J.
& Gangolli, S.D. (1975) Short-term toxicity of isoamyl salicylat in
rats. Food Cosmet. Toxicol., 13, 185-193.
Gaillard, D. & Derache, R. (1965) Metabolism of different alcohols,
present in alcoholic beverages, in the rat. Trav. Soc. Pharm.
Montpellier, 25, 51-62.
Gaunt, I.F., Colley, J., Grasso, P., Lansdown, A.B.G. & Gangolli, S.D.
(1969) Acute (rat and mouse) and short-term (rat) toxicity studies on
cis-3-hexen-1-ol. Food Cosmet. Toxicol., 7, 451-459.
Gaunt, I.F., Wright, M.G., Cottrell, R. & Gangolli, S.D. (1983)
Short-term toxicity of 2,6-dimethylhept-5-en-1-al in rats. Food
Chem. Toxicol., 21, 543-549.
Gibson, G.G., Orton, T.C. & Tamburini, P.P. (1982) Cytochrome P-450
induction by clofibrate. Purification and properties of a hepatic
cytochrome P-450 relatively specific for the 12- and 11-hydroxylation
of dodecanoic acid (lauric acid). Biochem. J., 203, 161-168.
Granger, R., Giroux, J. & Monnier, P. (1954) [Research on the
mechanism of action of calcium ethyl-2-butanoate.] Trav. Soc.
Pharm. Montpeller, 14, 335-341 (in French).
Harris, P., Gloster, J.A. & Ward, B.J. (1980) Transport of fatty acids
in the heart. Arch. Mal. Coeur, 73, 593-598.
Hayatsu, H., Inoue, K., Ohta, H., Namba, T., Togawa, K., Hayatsu, T.,
Makita, M. & Wataya, Y. (1981) Inhibition of the mutagenicity of
cooked-beef basic fraction by its acidic fraction. Mutat. Res., 91,
437-442.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist, 9, 257.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report to the Flavor
Manufacturers' Association of the United States, Washington DC, United
States.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavorings and compounds of related structure I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Kamil, A.I., Smith, J.N. & Williams, R.T. (1953) The metabolism of
aliphatic alcohols; the glucuronic acid conjugation of acyclic
aliphatic alcohols. J. Biochem., 53, 129-136.
de Kok, T.M., ten Vaarwerk, F., Zwingman, I., van Maanen, J.M. &
Kleinjans, J.C. (1994) Peroxidation of linoleic, arachidonic and oleic
acid in relation to the induction of oxidative DNA damage and
cytogenetic effects. Carcinogenesis, 15, 1399-1404.
Levi, E. & Hodgson, E. (1989) Metabolites resulting from oxidative and
reductive processes. In: Hutson, D.H., Caldwell, J. & Paulson, G.D.,
eds, Intermediary Xenobiotic Metabolism in Animals, London, Taylor &
Francis, pp. 119-138.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
MacGregor, J.T., Wilson, R.E., Neff, W.E. & Frankel, E.N. (1985)
Mutagenicity tests of lipid oxidation products in Salmonella
typhimurium: Monohydroperoxides and secondary oxidation products of
methyl linoleate and methyl linolenate. Food Chem. Toxicol., 23,
1041-1047.
Masoro, E.J. (1977) Lipids and lipid metabolism. Am. Rev. Physiol.,
39, 301-321.
Mortelmans, K., Haworth, S., Lawlor, T., Speck, W., Tainer, B. &
Zeiger, E. (1986) Salmonella mutagenicity tests: II. Results from the
testing of 270 chemicals. Environ. Mutag., 8, 1-119.
Nau, H. & Loscher, W. (1986) Pharmacologic evaluation of metabolites
and analogs of valproic acid: Teratogenic potencies in mice.
Fundam. Appli Toxicol., 6, 669-676.
Newell, G.W., Petretti, A.K. & Reiner, L. (1949) Studies of the acute
and chronic toxicity of undecylenic acid. J. Invest. Dermatol., 13,
145-149.
Osawa, T. & Namiki, M. (1982) Mutagen formation in the reaction of
nitrite with the food components analogous to sorbic acid. Arch.
Biochem., 46, 2299-2304.
Record, I.R., Konstantinopoulos, M. & Nestel, P.J. (1992) A
comparative study of micronucleus frequency in peripheral blood
lymphocytes of human subjects give dietary cis, trans and saturated
fat. Food Chem. Toxicol., 30, 585-588.
Schulz, H. (1983) Metabolism of 4-pentenoic acid and inhibition of
thiolase by metabolites of 4-pentenoic acid. J. Biochem., 22, 1827.
Shimizu, H., Suzuki, Y., Takemura, N., Goto, S. & Matshushita, H.
(1985) The results of microbial mutation test for forty-three
industrial chemicals. Jpn. J. Ind. Health, 27, 400-419.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist ,12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
Tislow, R., Margolin, S., Foley, E.J. & Lee, S.W. (1950) Toxicity of
undercylenic acid. J. Pharmacol. Exp. Ther., 98, 31-32.
US Food and Drug Administration (1993) Priority-based Assessment
of Food Additives (PAFA) Database, Center for Food Safety and
Applied Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC, United States.
US National Toxicology Program (1997) Toxicology and Carcinogenesis
Studies of Oleic Acid Diethanolamine Condensate (CAS No. 93-83-4)
in F344/N Rats and B6C3F1 Mice (Dermal Studies) (Technical Report
Series No. 481, NIH Publication No. 97-3971), Research Triangle Park,
North Carolina, US Department of Health and Human Services, Public
Health Service, National Institutes of Health.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Wakil, S.J. & Barnes, E.M. (1971) In: Florkin, M. & Stotz, E., eds,
Comprehensive Biochemistry, New York, Elsevier Publishing Co., Vol.
18S.
Wild, D., King, M.T., Gocke, E. & Eckhardt, K. (1983) Study of
artificial flavouring substances for mutagenicity in the
Salmonella/microsome, basc and micronucleus tests. Food Chem.
Toxicol., 21, 707-719.
Williams, R.T. (1959) Detoxication Mechanisms, 2nd Ed., London,
Chapman & Hall, pp. 88-113.
