
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
SAFETY EVALUATION OF CERTAIN
FOOD ADDITIVES
WHO FOOD ADDITIVES SERIES: 42
Prepared by the Fifty-first meeting of the Joint FAO/WHO
Expert Committee on Food Additives (JECFA)
World Health Organization, Geneva, 1999
IPCS - International Programme on Chemical Safety
ALIPHATIC ACYCLIC AND ALICYCLIC TERPENOID TERTIARY ALCOHOLS AND
STRUCTURALLY RELATED SUBSTANCES
First draft prepared by
Dr Antonia Mattia
Division of Product Policy, Office of Premarket Approval (HFS-206)
Center for Food Safety and Applied Nutrition
US Food and Drug Administration
Washington DC, United States
Evaluation
Introduction
Estimated daily per capita intake
Absorption, metabolism, and elimination
Application of the Procedure for the Safety
Evaluation of Flavouring Substances
Consideration of combined intakes from use as
flavouring agents
Conclusions
Relevant background information
Explanation
Intake
Biological data
Absorption and metabolism
Toxicological studies
Acute toxicity
Short-term and long-term studies of toxicity
and carcinogenicity
Genotoxicity
Other relevant studies
References
1. EVALUATION
1.1 Introduction
The Committee evaluated 23 flavouring agents that include
selected tertiary alcohols and related esters (Table 1) using the
Procedure for the Safety Evaluation of Flavouring Agents (Figure 1, p.
222, and Annex 1, reference 131).
The Committee had evaluated two members of the group previously.
Linalool and linalyl acetate were evaluated with citral, citronellol,
and geranyl acetate at the eleventh meeting (Annex 1, reference 14).
The Committee recommended that at least one member of the group be
studied for effects after long-term exposure. Linalool and linalyl
acetate were re-evaluated at the twenty-third meeting (Annex 1,
reference 50). A group ADI of 0-0.5 mg/kg bw was established on the
basis of the clearly defined metabolism of these substances, their
rapid excretion, and their low toxicity in short-term studies.
At the forty-ninth meeting, the Committee evaluated a group of 26
geranyl, neryl, citronellyl, and rhodinyl esters formed from
branched-chain terpenoid primary alcohols and aliphatic acyclic linear
and branched-chain carboxylic acids using the procedure for the safety
evaluation of flavouring agents. The Committee concluded that these
substances pose no safety concerns on the basis of knowledge of their
metabolism and low levels of intake.
1.2 Estimated daily per capita intake
The total annual production volume of the 23 tertiary alcohols
and related esters is approximately 58 000 kg in Europe (International
Organization of the Flavor Industry, 1995) and 15 000 kg in the United
States (US National Academy of Sciences, 1987). Four substances in the
group, linalool (No. 356), linalyl acetate (No. 359), alpha-terpineol
(No. 366), and terpinyl acetate (No. 368) account for approximately
96% of the total annual volume in Europe and the United States. On the
basis of the reported annual volumes, the total estimated daily
per capita intake of linalool from its use and that of eight of its
esters as flavouring agents is about 4300 µg/person per day (72 µg/kg
bw per day) in Europe and 1300 µg/person per day (21 µg/kg bw per day)
in the United States. Similarly, the total estimated daily per
capita intake of alpha-terpineol from its use of and that of six of
its esters as flavouring agents is about 3200 µg/person per day (53
µg/kg bw per day) in Europe and 1400 µg/person per day (23 µg/kg bw
per day) in the United States.
Tertiary alcohols and related esters occur naturally in a wide
variety of foods, including fruits, spices, and tea. Thirteen of the
substances in this group have been reported to occur naturally in
foods (Maarse et al., 1994).
1.3 Absorption, metabolism, and elimination
The esters in this group would be readily hydrolysed to their
component alcohols and carboxylic acids. The hydrolysis products would
be detoxified primarily by conjugation with glucuronic acid and
excreted in the urine. Alternatively, alcohols with unsaturation may
be 4-oxidized at the allylic position to yield polar metabolites,
which may be conjugated and excreted. Metabolites of acyclic alcohols
may be further oxidized to eventually yield carbon dioxide. Ester
hydrolysis and metabolism of the hydrolysis products are further
discussed in the section 'General aspects of metabolism' in "Safety
Evaluations of Groups of Related Substances by the Procedure for the
Safety Evaluation of Flavouring Agents".
1.4 Application of the Procedure for the Safety Evaluation of
Flavouring Agents
Step 1A. All but one of the 23 terpenoid tertiary alcohols and
related substances is classified in structural class I
(Cramer et al., 1978). 2-Ethyl-1,3,3-trimethyl-2-norbornol
(No. 440) is a non-terpene bicyclic tertiary alcohol and is
therefore in structural class II.
Step 2A. Adequate data were available on the metabolism of both
linalool and alpha-terpineol to allow prediction of the
likely pathways of metabolism of all related compounds in
the same group. Although the position of oxidative
metabolism would differ between compounds, the structures of
linalool and alpha-terpineol would cover all of the
functional groups present in the other members of this group
of flavouring agents. On the basis of the toxicity of
linalool and alpha-terpineol, it was concluded that (with
one exception) the metabolism of compounds in this group
could be predicted to give rise to innocuous products.
Methyl 1-acetoxycyclohexylketone (No. 442) contains a
sterically hindered ketone group and cannot be considered
a priori to be similar metabolically or toxicologically to
other members of the group.
At current levels of intake, 22 of the substances in
this group (21 in class I and one in class II) would not be
expected to saturate the metabolic pathways, and these
substances are predicted to be metabolized to innocuous
products. The evaluation of these 22 substances thus
proceeds via the left side ('A') of the evaluation scheme
(steps A3-A5). Methyl 1-acetoxycyclohexyl-ketone (No. 442;
class I) would not be predicted to be metabolized to
innocuous products, so its evaluation proceeds via the right
side ('B') of the scheme (steps B3-B5).
Step A3. Twenty-two substances were evaluated at this step. As the
daily per capita intakes in Europe and the United States
of 18 of the 21 substances in class I are below the human
intake threshold for class I (1800 µg/person per day), these
substances pose no safety concern when used as flavouring
agents at their current levels of estimated intake.
In Europe, the intakes of three substances, linalool
(2600 µg/person per day), linalyl acetate (2100 µg/person
per day), and alpha-terpineol (3000 µg/person per day), are
greater than 1800 µg/person per day, and the total intakes
of linalool (No. 356) and alpha-terpineol (No. 366) from
their use and use of their esters (Nos 358-365 and 367-372,
respectively) are 4300 µg/person per day and 3200 µg/person
per day. Total intake in the United States of linalool and
its esters is 1300 µg/person per day, and that of
alpha-terpineol and its esters is 1400 µg/person per day.
Intake of the class II substance
2-ethyl-1,3,3-trimethyl-2-norbornol is 1 µg/person per day
in Europe and 0.02 µg/person per day in the United States --
below the human intake threshold for class II (540 µg/person
per day), indicating that this substance does not pose a
safety concern when used at current levels of estimated
intake as a flavouring agent.
Step A4. Linalool, linalyl acetate, and alpha-terpineol are not
endogenous in humans.
Step A5. NOEL values of > 50 mg/kg bw per day for linalool and
> 24 mg/kg bw per day for linalyl acetate have been found
in 90-day studies in rats. These NOELs provide safety
margins of > 1000, > 500, and > 500 for intake of
linalool (19 or 44 µg/kg bw per day), linalyl acetate (3 or
35 µg/kg bw per day), and total linalool (21 or 72 µg/kg bw
per day), respectively, in both Europe and the United
States. A NOEL of > 500 mg/kg bw per day terpinyl acetate
was found in a 20-week study in rats. This NOEL provides
safety margins of > 10 000 and > 500 for intake of
alpha-terpineol (18 or 50 µg/kg bw per day) and total
alpha-terpineol (23 or 53 µg/kg bw per day), respectively,
in both Europe and the United States. A bioassay of
carcinogenicity was conducted in rats given a mixture of the
terpenoid esters geranyl acetate and citronellyl acetate,
which together with linalool and linalyl acetate belong to
the group of terpenoid substances previously reviewed by the
Committee (Annex 1, reference 50). The NOEL of 1000 mg/kg bw
per day (710 mg/kg bw per day geranyl acetate and 290 mg/kg
bw per day citronellyl acetate) for the mixture provides a
safety margin of > 10 000 for total intake of linalool and
of alpha-terpineol.
Step B3. The daily per capita intake of the class I substance
methyl 1-acetoxy-cyclohexylketone (No. 442) in the United
States is 4 µg/person per day; no intake data were reported
for Europe. This intake is less than the human intake
threshold for class I (1800 µg/person per day).
Step B4. Adequate data were not available to determine a NOEL for
methyl 1-acetoxycyclohexylketone or structurally related
substances.
Step B5. The conditions of use of methyl 1-acetoxycyclohexylketone
result in an intake greater than 1.5 µg/day; therefore,
according to the procedure, additional information on this
substance is required for its evaluation.
The stepwise evaluations of the 23 aliphatic acyclic and
alicyclic terpenoid tertiary alcohols and structurally related
flavouring agents in this group are summarized in Table 1.
1.5 Consideration of combined intakes from use as flavouring agents
The data were adequate to complete an evaluation of 22 tertiary
alcohols and related esters in this group. In the unlikely event that
all 22 of these substances were consumed simultaneously on a daily
basis, the estimated combined intake would exceed the human intake
threshold for class I. These 22 substances are, however, expected to
be efficiently metabolized and would not saturate the metabolic
pathways. The consideration of combined intakes excludes intake of
methyl 1-acetoxycyclohexylketone because additional data on its
toxicity are required in order for its safety to be evaluated.
Consumption of four additional esters of linalool (linalyl
anthranilate, linalyl benzoate, linalyl cinnamate, and linalyl
phenylacetate) that were not evaluated as part of this group would
contribute an additional 10 µg/person per day (Europe) or 0.2
µg/person per day (United States) to the total intake of linalool.
Similarly, exposure to an additional ester of alpha-terpineol,
terpinyl cinnamate, would contribute an additional 0.008 µg/person per
day (Europe) or 0.5 µg/person per day (United States) to the total
intake of alpha-terpineol. The potential consumption of linalool and
alpha-terpineol from these additional esters is minor and would not
lead to saturation of metabolic pathways or alter the safety
evaluation.
1.6 Conclusions
The Committee concluded that 22 of the 23 terpenoid tertiary
alcohols and related substances in this group would not present safety
concerns when used at current levels of intake as flavouring agents.
Knowledge of the metabolism and toxicity of these flavouring agents
was required for application of the procedure. The Committee noted
that these and other available data are consistent with the results of
the safety evaluation by the procedure. For one substance, methyl
1-acetoxycyclohexylketone, the available metabolic data were
inadequate to predict that it would be metabolized to innocuous
products, a relevant NOEL was lacking, and intake exceeds 1.5 µg per
day. According to the procedure, the Committee concluded that
additional data are required for an evaluation of methyl
1-acetoxycyclohexylketone. All of the previously established ADIs were
maintained at the present meeting.
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes the key data relevant to the safety
evaluation of 23 tertiary alcohols and structurally related substances
(see Table 1). The group comprises the aliphatic unsaturated tertiary
alcohol linalool (No. 356) and eight esters of linalool (Nos 358-365);
the saturated homologue of linalool, tetrahydrolinalool (No. 357); the
alicyclic unsaturated tertiary alcohol alpha-terpineol (No. 366) and
six esters of alpha-terpineol (Nos 367-372); three unsaturated
alicyclic tertiary alcohols (Nos 373, 374, and 439); two saturated
bicyclic tertiary alcohols (Nos 440 and 441); and a saturated tertiary
ketoester (No. 442). The chemical structures of these substances are
given in Table 1.
Table 1. Results of safety evaluations of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally related substances
Step 1: All of the substances in the group are in structural class I, except for substance 440, which is in class II.
The human intake threshold is 1800 µg/day for class I and 540 µg/day for class II substances.
Step 2: All of the substances in this group are predicted to be metabolized to innocuous products, except methyl 1-acetoxycyclohexylketone.
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linaloola 356 78-70-6 2600/1100 Yes No Yes. The dose of Metabolized primarily No safety
50 mg/kg bw per by conjugation with concern
day that had no glucuronic acid and
adverse effects in excreted in urine.
a safety evaluation Oxidation of the allylic
(Oser, 1967) is methyl group may occur
> 1000 times the after repeated
intake of linalool. exposure.
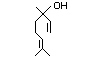 Tetrahydrolinalool 357 78-69-3 55/0.1 No N/R N/R See linalool. No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Tetrahydrolinalool 357 78-69-3 55/0.1 No N/R N/R See linalool. No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
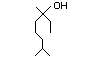 Linalyl formate 358 115-99-1 8/13 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl formate 358 115-99-1 8/13 No N/R N/R See linalyl acetate and No safety
linalool. concern
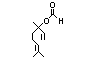 Linalyl acetatea 359 115-95-7 2100/180 Yes No Yes. The dose of Hydrolysed to linalool No safety
24 mg/kg bw per and acetic acid. See concern
day that had no also linalool.
adverse effects in
a safety evaluation
(Oser, 1967) is
> 500 times the
intake of linalyl
acetate.
Linalyl acetatea 359 115-95-7 2100/180 Yes No Yes. The dose of Hydrolysed to linalool No safety
24 mg/kg bw per and acetic acid. See concern
day that had no also linalool.
adverse effects in
a safety evaluation
(Oser, 1967) is
> 500 times the
intake of linalyl
acetate.
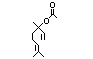 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl propionate 360 144-39-8 16/2 No N/R N/R See linalyl acetate and No safety
linalool. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl propionate 360 144-39-8 16/2 No N/R N/R See linalyl acetate and No safety
linalool. concern
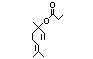 Linalyl butyrate 361 78-36-4 10/4 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl butyrate 361 78-36-4 10/4 No N/R N/R See linalyl acetate and No safety
linalool. concern
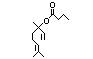 Linalyl isobutyrate 362 78-35-3 36/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl isobutyrate 362 78-35-3 36/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
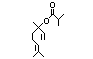 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl isovalerate 363 1118-27-0 5/6 No N/R N/R See linalyl acetate and No safety
linalool. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl isovalerate 363 1118-27-0 5/6 No N/R N/R See linalyl acetate and No safety
linalool. concern
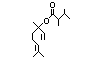 Linalyl hexanoate 364 7779-23-9 1/0.4 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl hexanoate 364 7779-23-9 1/0.4 No N/R N/R See linalyl acetate and No safety
linalool. concern
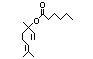 Linalyl octanoate 365 10024-64-3 0.1/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl octanoate 365 10024-64-3 0.1/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
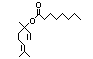 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
alpha-Terpineol 366 98-55-5 3000/1100 Yes No Yes. The dose of Metabolized primarily No safety
500 mg/kg bw per by conjugation with concern
day that had no glucuronic acid and
adverse effects excreted in urine.
(Hagan et al., Oxidation of the allylic
1967) is > 5000 methyl group followed by
times the intake hydrogenation to yield the
of the component corresponding saturated
alpha-terpineol. acid may occur
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
alpha-Terpineol 366 98-55-5 3000/1100 Yes No Yes. The dose of Metabolized primarily No safety
500 mg/kg bw per by conjugation with concern
day that had no glucuronic acid and
adverse effects excreted in urine.
(Hagan et al., Oxidation of the allylic
1967) is > 5000 methyl group followed by
times the intake hydrogenation to yield the
of the component corresponding saturated
alpha-terpineol. acid may occur
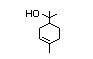 Terpinyl formate 367 2153-26-6 0.1/2 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl formate 367 2153-26-6 0.1/2 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
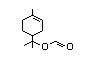 Terpinyl acetate 368 8007-35-0 260/390 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl acetate 368 8007-35-0 260/390 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
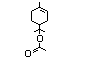 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl propionate 369 80-27-3 0.03/1 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl propionate 369 80-27-3 0.03/1 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
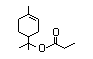 Terpinyl butyrate 370 80-26-6 6/6 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl butyrate 370 80-26-6 6/6 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
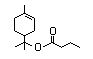 Terpinyl 371 7774-65-4 0.7/0.02 No N/R N/R See linalyl acetate and No safety
isobutyrate alpha-terpineol. concern
Terpinyl 371 7774-65-4 0.7/0.02 No N/R N/R See linalyl acetate and No safety
isobutyrate alpha-terpineol. concern
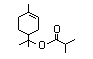 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl 372 1142-85-4 0.1/1 No N/R N/R See linalyl acetate and No safety
isovalerate alpha-terpineol. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl 372 1142-85-4 0.1/1 No N/R N/R See linalyl acetate and No safety
isovalerate alpha-terpineol. concern
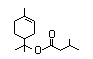 para-Menth-3-en-1-ol 373 586-82-3 41/0.4 No N/R N/R See alpha-terpineol. No safety
concern
para-Menth-3-en-1-ol 373 586-82-3 41/0.4 No N/R N/R See alpha-terpineol. No safety
concern
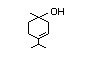 4-Carvomenthenol 439 562-74-3 170/51 No N/R N/R See alpha-terpineol. No safety
concern
4-Carvomenthenol 439 562-74-3 170/51 No N/R N/R See alpha-terpineol. No safety
concern
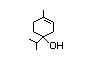 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
para-Menth-8-en-1-ol 374 138-87-4 2/21 No N/R N/R See alpha-terpineol. No safety
(ß-Terpineol) concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
para-Menth-8-en-1-ol 374 138-87-4 2/21 No N/R N/R See alpha-terpineol. No safety
(ß-Terpineol) concern
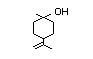 2-Ethyl-1,3,3- 440 18368-91-7 1/0.02 No N/R N/R The structurally related No safety
trimethyl-2- bicyclic tertiary concern
norbornanol alcohols thujyl alcohol
and ß-sante-nol
are conjugated with
glucuronic acid.
2-Ethyl-1,3,3- 440 18368-91-7 1/0.02 No N/R N/R The structurally related No safety
trimethyl-2- bicyclic tertiary concern
norbornanol alcohols thujyl alcohol
and ß-sante-nol
are conjugated with
glucuronic acid.
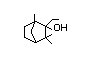 4-Thujanol 441 546-79-2 1/0.04 No N/R N/R The structurally related No safety
bicyclic tertiary concern
alcohols thujyl alcohol
and ß-sante-nol are
conjugated with
glucuronic acid.
4-Thujanol 441 546-79-2 1/0.04 No N/R N/R The structurally related No safety
bicyclic tertiary concern
alcohols thujyl alcohol
and ß-sante-nol are
conjugated with
glucuronic acid.
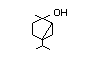 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
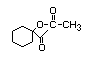
Methyl 1-acetoxy- 442 52789-73-8 N/D/4 - - - Cannot be predicted Additional
cyclohexyl to be metabolized data on
ketone to innocuous toxicity
products. Therefore, are required
Steps A3-A5 are not because a
applicable to this NOEL is not
substance, which must available
be evaluated on the for this or
right side ('B') of the a related
evaluation scheme. substance
(Step B4)
and intake
exceeds 1.5
µg/person
per day
(Step B5).
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Methyl 1-acetoxy- 442 52789-73-8 N/D/4 - - - Cannot be predicted Additional
cyclohexyl to be metabolized data on
ketone to innocuous toxicity
products. Therefore, are required
Steps A3-A5 are not because a
applicable to this NOEL is not
substance, which must available
be evaluated on the for this or
right side ('B') of the a related
evaluation scheme. substance
(Step B4)
and intake
exceeds 1.5
µg/person
per day
(Step B5).
 N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a The group ADI of 0-0.05 mg/kg bw established at the twenty-third meeting for citral, citronellol, linalool, and linalyl acetate as citral
was maintained.
The substances in this group are structurally related because
they each contain a tertiary alcohol function (without aryl
substitution); they therefore have similar metabolic and toxicological
profiles. Four esters of the tertiary alcohol linalool (linalyl
anthranilate, linalyl benzoate, linalyl cinnamate, and linalyl
phenylacetate) and one ester of alpha-terpineol (terpinyl cinnamate)
used as flavouring substances in Europe and the United States are not
included in this group because they may have different toxicological
profiles, owing to their carboxylic acid components. It would be
appropriate to evaluate these esters in subsequent groups, with
anthranilic acid, benzoic acid, cinnamic acid, phenylacetic acid, and
related substances used as flavouring substances. Any additional
intake of linalool and alpha-terpineol from these four esters is
minimal and would not have a significant impact on the total combined
intake of linalool, alpha-terpineol, and related tertiary alcohols and
esters (see section 1.5).
2.2 Intake
The total annual volume of the 23 tertiary alcohols and related
esters is approximately 58 000 kg in Europe (International
Organization of the Flavor Industry, 1995) and 15 000 kg in the United
States (US National Academy of Sciences, 1987). Production volumes and
intake levels of each flavouring substance are reported in Table 2.
Thirteen tertiary alcohols and related esters have been reported
to occur naturally in foods (Maarse et al., 1994); quantitative data
reported for eight of these substances (see Table 2) indicate that
they are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, esters are hydrolysed to their corresponding alcohol
and carboxylic acid. Hydrolysis is catalysed by classes of enzymes
recognized as carboxylesterases or esterases (Heymann, 1980), the most
important of which are the B-esterases. In mammals, these enzymes
occur in most tissues throughout the body (Anders, 1989; Heymann,
1980) but predominate in hepatocytes (Heymann, 1980).
Esters of linalool (Nos 358-365) and alpha-terpineol (Nos
367-372) are expected to be hydrolysed in humans to yield linalool and
alpha-terpineol, respectively, and the corresponding saturated
aliphatic carboxylic acid. Methyl 1-acetoxycyclohexyl ketone (No. 442)
is expected to be hydrolysed to acetic acid and methyl
1-hydroxycyclohexyl ketone.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic terpenoid tertiary
alcohols and structurally related substances as flavouring substances in Europe and the
United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Linalool (356)
Europe 18 000 2600 44 NA
United States 5 800 1100 19 NA
Tetrahydrolinalool (357)
Europe 390 55 0 NA
United States 0.5 0.1 0.002 NA
Linalyl formate (358)
Europe 57 8 0.14 0.1
United States 70 13 0.22 0.2
Linalyl acetate (359)
Europe 14 000 2100 35 27
United States 970 180 3 2
Linalyl propionate (360)
Europe 110 16 0.3 0.2
United States 8 2 0.03 0.02
Linalyl butyrate (361)
Europe 69 10 0.2 0.1
United States 22 4 0.07 0.05
Linalyl isobutyrate (362)
Europe 250 36 0.6 0.4
United States 3 0 0.00 0.00
Linalyl isovalerate (363)
Europe 38 5 0.0 0.06
United States 34 6 0.11 0.07
Linalyl hexanoate (364)
Europe 7 0 0.02 0.01
United States 2 0.4 0.00 0.004
Linalyl octanoate (365)
Europe 1 0.1 0.002 0.001
United States 5 0 0.02 0.00
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
alpha-Terpineol (366)
Europe 21 000 3000 50 NA
United States 5 500 1100 18 NA
Terpinyl formate (367)
Europe 1 0.1 0.002 0.002
United States 10 2 0.03 0.03
Terpinyl acetate (368)
Europe 1 800 260 4 3
United States 2 000 390 6 5
Terpinyl propionate (369)
Europe 0.2 0.03 0.0005 0.0004
United States 5 0 0.02 0.01
Terpinyl butyrate (370)
Europe 42 6 0.10 0.07
United States 33 6 0.10 0.07
Terpinyl isobutyrate (371)
Europe 5 0.7 0.01 0.00
United States 0.1 0.02 0.0003 0.0002
Terpinyl isovalerate (372)
Europe 1 0.1 0.002 0.002
United States 5 0 0.02 0.01
para-Menth-3-en-1-ol (373)
Europe 290 41 0 NA
United States 2 0.4 0.00 NA
4-Carvomenthenol (439)
Europe 1200 170 3 NA
United States 270 51 0 NA
para-Menth-8-en-1-ol (374)
Europe 11 2 0.03 NA
United States 110 21 0.3 NA
2-Ethyl-1,3,3-trimethyl-2-norbornol (440)
Europe 6.1 1 0.02 NA
United States 0.1 0.02 0.0003 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Thujanol (441)
Europe 7.5 1 0.02 NA
United States 0.2 0.04 0.0006 NA
Methyl 1-acetoxycyclohexyl-ketone (442)
Europe NR
United States 21 4 0.07 NA
Total
Europe 58 000 NA NA NA
United States 15 000 NA NA NA
Total linalool
Europe NA 4300 72 28
United States NA 1300 21 3
Total alpha-terpineol
Europe NA 3200 53 4
United States NA 1400 23 5
NR, not reported; NA, not applicable; +, reported to occur naturally in foods (Maase et al.,
1994), but no quantitative data available; -, not reported to occur naturally in foods
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor
Industry, 1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the
survey. Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where
body weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
In a study of hydrolysis in vitro, linalyl acetate was easily
hydrolysed in water and simulated gastric and pancreatic fluids, with
mean half-lives of 5.5 min in gastric fluid and 53 min in pancreatic
fluid (Hall, 1979). Terpenoid alcohols formed in the gastrointestinal
tract are rapidly absorbed (Phillips et al., 1976; Diliberto et al.,
1988).
In humans and animals, terpenoid tertiary alcohols are conjugated
primarily with glucuronic acid and are excreted in the urine and
faeces (Williams, 1959; Parke et al., 1974a,b; Horning et al., 1976;
Ventura et al., 1985). Unsaturated terpenoid alcohols may undergo
allylic oxidation to form polar diol metabolites, which may be
excreted either free or conjugated. If the diol contains a primary
alcohol function, it may under-go further oxidation to the
corresponding carboxylic acid (Madyastha & Srivatsan, 1988; Horning et
al., 1976; Ventura et al., 1985).
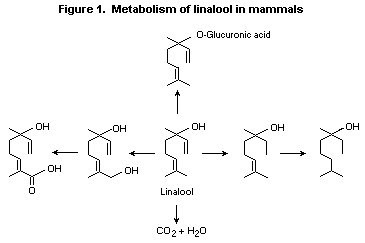
The metabolic fate of the aliphatic tertiary alcohol linalool
(No. 356) has been studied in mammals (Figure 1). Linalool labelled
with 14C in positions 1 and 2 was administered orally to rats at a
single dose of 500 mg/kg bw. The majority (55%) of the radioactivity
was excreted in the urine as the glucuronic acid conjugate, while 23%
was excreted as carbon dioxide in expired air, and 15% was excreted in
the faeces within 72 h of administration; only 3% of the radiolabel
was detected in tissues after 72 h, with 0.5% in the liver, 0.6% in
the gut, 0.8% in the skin, and 1.2% in skeletal muscle (Parke et al.,
1974a). Reduction metabolites such as dihydro- and tetrahydrolinalool
were identified in the urine after administration of a single dose of
linalool to rats (Rahman, 1974).
N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a The group ADI of 0-0.05 mg/kg bw established at the twenty-third meeting for citral, citronellol, linalool, and linalyl acetate as citral
was maintained.
The substances in this group are structurally related because
they each contain a tertiary alcohol function (without aryl
substitution); they therefore have similar metabolic and toxicological
profiles. Four esters of the tertiary alcohol linalool (linalyl
anthranilate, linalyl benzoate, linalyl cinnamate, and linalyl
phenylacetate) and one ester of alpha-terpineol (terpinyl cinnamate)
used as flavouring substances in Europe and the United States are not
included in this group because they may have different toxicological
profiles, owing to their carboxylic acid components. It would be
appropriate to evaluate these esters in subsequent groups, with
anthranilic acid, benzoic acid, cinnamic acid, phenylacetic acid, and
related substances used as flavouring substances. Any additional
intake of linalool and alpha-terpineol from these four esters is
minimal and would not have a significant impact on the total combined
intake of linalool, alpha-terpineol, and related tertiary alcohols and
esters (see section 1.5).
2.2 Intake
The total annual volume of the 23 tertiary alcohols and related
esters is approximately 58 000 kg in Europe (International
Organization of the Flavor Industry, 1995) and 15 000 kg in the United
States (US National Academy of Sciences, 1987). Production volumes and
intake levels of each flavouring substance are reported in Table 2.
Thirteen tertiary alcohols and related esters have been reported
to occur naturally in foods (Maarse et al., 1994); quantitative data
reported for eight of these substances (see Table 2) indicate that
they are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, esters are hydrolysed to their corresponding alcohol
and carboxylic acid. Hydrolysis is catalysed by classes of enzymes
recognized as carboxylesterases or esterases (Heymann, 1980), the most
important of which are the B-esterases. In mammals, these enzymes
occur in most tissues throughout the body (Anders, 1989; Heymann,
1980) but predominate in hepatocytes (Heymann, 1980).
Esters of linalool (Nos 358-365) and alpha-terpineol (Nos
367-372) are expected to be hydrolysed in humans to yield linalool and
alpha-terpineol, respectively, and the corresponding saturated
aliphatic carboxylic acid. Methyl 1-acetoxycyclohexyl ketone (No. 442)
is expected to be hydrolysed to acetic acid and methyl
1-hydroxycyclohexyl ketone.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic terpenoid tertiary
alcohols and structurally related substances as flavouring substances in Europe and the
United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Linalool (356)
Europe 18 000 2600 44 NA
United States 5 800 1100 19 NA
Tetrahydrolinalool (357)
Europe 390 55 0 NA
United States 0.5 0.1 0.002 NA
Linalyl formate (358)
Europe 57 8 0.14 0.1
United States 70 13 0.22 0.2
Linalyl acetate (359)
Europe 14 000 2100 35 27
United States 970 180 3 2
Linalyl propionate (360)
Europe 110 16 0.3 0.2
United States 8 2 0.03 0.02
Linalyl butyrate (361)
Europe 69 10 0.2 0.1
United States 22 4 0.07 0.05
Linalyl isobutyrate (362)
Europe 250 36 0.6 0.4
United States 3 0 0.00 0.00
Linalyl isovalerate (363)
Europe 38 5 0.0 0.06
United States 34 6 0.11 0.07
Linalyl hexanoate (364)
Europe 7 0 0.02 0.01
United States 2 0.4 0.00 0.004
Linalyl octanoate (365)
Europe 1 0.1 0.002 0.001
United States 5 0 0.02 0.00
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
alpha-Terpineol (366)
Europe 21 000 3000 50 NA
United States 5 500 1100 18 NA
Terpinyl formate (367)
Europe 1 0.1 0.002 0.002
United States 10 2 0.03 0.03
Terpinyl acetate (368)
Europe 1 800 260 4 3
United States 2 000 390 6 5
Terpinyl propionate (369)
Europe 0.2 0.03 0.0005 0.0004
United States 5 0 0.02 0.01
Terpinyl butyrate (370)
Europe 42 6 0.10 0.07
United States 33 6 0.10 0.07
Terpinyl isobutyrate (371)
Europe 5 0.7 0.01 0.00
United States 0.1 0.02 0.0003 0.0002
Terpinyl isovalerate (372)
Europe 1 0.1 0.002 0.002
United States 5 0 0.02 0.01
para-Menth-3-en-1-ol (373)
Europe 290 41 0 NA
United States 2 0.4 0.00 NA
4-Carvomenthenol (439)
Europe 1200 170 3 NA
United States 270 51 0 NA
para-Menth-8-en-1-ol (374)
Europe 11 2 0.03 NA
United States 110 21 0.3 NA
2-Ethyl-1,3,3-trimethyl-2-norbornol (440)
Europe 6.1 1 0.02 NA
United States 0.1 0.02 0.0003 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Thujanol (441)
Europe 7.5 1 0.02 NA
United States 0.2 0.04 0.0006 NA
Methyl 1-acetoxycyclohexyl-ketone (442)
Europe NR
United States 21 4 0.07 NA
Total
Europe 58 000 NA NA NA
United States 15 000 NA NA NA
Total linalool
Europe NA 4300 72 28
United States NA 1300 21 3
Total alpha-terpineol
Europe NA 3200 53 4
United States NA 1400 23 5
NR, not reported; NA, not applicable; +, reported to occur naturally in foods (Maase et al.,
1994), but no quantitative data available; -, not reported to occur naturally in foods
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor
Industry, 1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the
survey. Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where
body weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
In a study of hydrolysis in vitro, linalyl acetate was easily
hydrolysed in water and simulated gastric and pancreatic fluids, with
mean half-lives of 5.5 min in gastric fluid and 53 min in pancreatic
fluid (Hall, 1979). Terpenoid alcohols formed in the gastrointestinal
tract are rapidly absorbed (Phillips et al., 1976; Diliberto et al.,
1988).
In humans and animals, terpenoid tertiary alcohols are conjugated
primarily with glucuronic acid and are excreted in the urine and
faeces (Williams, 1959; Parke et al., 1974a,b; Horning et al., 1976;
Ventura et al., 1985). Unsaturated terpenoid alcohols may undergo
allylic oxidation to form polar diol metabolites, which may be
excreted either free or conjugated. If the diol contains a primary
alcohol function, it may under-go further oxidation to the
corresponding carboxylic acid (Madyastha & Srivatsan, 1988; Horning et
al., 1976; Ventura et al., 1985).
The metabolic fate of the aliphatic tertiary alcohol linalool
(No. 356) has been studied in mammals (Figure 1). Linalool labelled
with 14C in positions 1 and 2 was administered orally to rats at a
single dose of 500 mg/kg bw. The majority (55%) of the radioactivity
was excreted in the urine as the glucuronic acid conjugate, while 23%
was excreted as carbon dioxide in expired air, and 15% was excreted in
the faeces within 72 h of administration; only 3% of the radiolabel
was detected in tissues after 72 h, with 0.5% in the liver, 0.6% in
the gut, 0.8% in the skin, and 1.2% in skeletal muscle (Parke et al.,
1974a). Reduction metabolites such as dihydro- and tetrahydrolinalool
were identified in the urine after administration of a single dose of
linalool to rats (Rahman, 1974).
 In a separate study, male IISc strain rats were given daily oral
doses of 800 mg/kg bw linalool for 20 days. Urinary metabolites formed
by cytochrome P450 (CYP450)-induced allylic oxidation of linalool
included 8-hydroxylinalool and 8-carboxylinalool. CYP450 activity in
liver microsomes was increased by about 50% after three days, but the
activity had decreased to control values after six days (Chadha &
Madyastha, 1984). Linalool administered to four-week-old male Wistar
rats by gavage at a dose of 500 mg/kg bw per day for 64 days did not
induce CYP450 until the 30th day of treatment (Parke et al., 1974b).
These results suggest that glucuronic acid conjugation and
excretion are the primary route of metabolism of linalool and allylic
oxidation becomes an important pathway only after repeated dosing. It
has been suggested that biotransformation of the diol metabolite of
linalool to the corresponding aldehyde by the action of the
NAD+-dependent enzyme alcohol dehydrogenase is inhibited because of
the bulky nature of the neighbouring alkyl substituents and the
substrate specificity of the enzyme (Eder et al., 1982).
When male albino IISc strain rats were given the alicyclic
tertiary alcohol alpha-terpineol (No. 366) orally at a daily dose of
600 mg/kg bw for 20 days, oxidation of the allylic methyl group was
observed to yield the corresponding carboxylic acid which was
hydrogenated to a small extent to yield the corresponding saturated
carboxylic acid (Madyastha & Srivatsan, 1988; Figure 2). When
alpha-terpineol was administered orally to rats, it increased the
liver microsomal P450 content and the activity of NADPH-cytochrome c
reductase (Madyastha & Srivatsan, 1988), suggesting that the oxidation
is mediated by CYP450.
In a separate study, male IISc strain rats were given daily oral
doses of 800 mg/kg bw linalool for 20 days. Urinary metabolites formed
by cytochrome P450 (CYP450)-induced allylic oxidation of linalool
included 8-hydroxylinalool and 8-carboxylinalool. CYP450 activity in
liver microsomes was increased by about 50% after three days, but the
activity had decreased to control values after six days (Chadha &
Madyastha, 1984). Linalool administered to four-week-old male Wistar
rats by gavage at a dose of 500 mg/kg bw per day for 64 days did not
induce CYP450 until the 30th day of treatment (Parke et al., 1974b).
These results suggest that glucuronic acid conjugation and
excretion are the primary route of metabolism of linalool and allylic
oxidation becomes an important pathway only after repeated dosing. It
has been suggested that biotransformation of the diol metabolite of
linalool to the corresponding aldehyde by the action of the
NAD+-dependent enzyme alcohol dehydrogenase is inhibited because of
the bulky nature of the neighbouring alkyl substituents and the
substrate specificity of the enzyme (Eder et al., 1982).
When male albino IISc strain rats were given the alicyclic
tertiary alcohol alpha-terpineol (No. 366) orally at a daily dose of
600 mg/kg bw for 20 days, oxidation of the allylic methyl group was
observed to yield the corresponding carboxylic acid which was
hydrogenated to a small extent to yield the corresponding saturated
carboxylic acid (Madyastha & Srivatsan, 1988; Figure 2). When
alpha-terpineol was administered orally to rats, it increased the
liver microsomal P450 content and the activity of NADPH-cytochrome c
reductase (Madyastha & Srivatsan, 1988), suggesting that the oxidation
is mediated by CYP450.
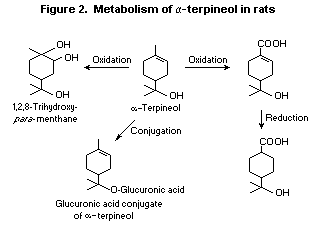 In a minor pathway, the endocyclic alkene of alpha-terpineol is
epoxidized and then hydrolysed to yield a triol metabolite
1,2,8-trihydroxy- para-menthane, which was also reported in humans
after inadvertent oral ingestion of a pine-oil disinfectant containing
alpha-terpineol (Horning et al., 1976). It is expected that after
single doses, alpha-terpineol would be metabolized like linalool
(Chadha & Madyastha, 1984), primarily by glucuronic acid conjugation
and excretion in the urine.
Bicyclic tertiary alcohols (Nos 440 and 441) are relatively
stable in vivo, but are eventually conjugated with glucuronic acid
and excreted. In rabbits, the structurally related bicyclic tertiary
alcohols thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo(2.2.1)heptan-2-ol) are conjugated with
glucuronic acid (Williams, 1959).
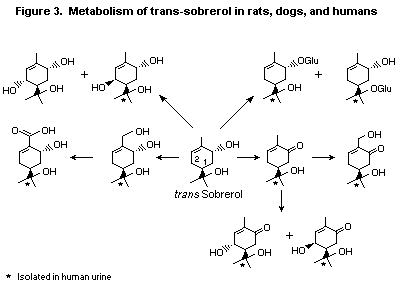
In a study of the metabolism of the structurally related
terpenoid tertiary alcohol trans-sobrerol in humans, dogs, and rats,
10 metabolites were isolated in urine, eight of which were found in
humans. Two principal modes of metabolism were observed: allylic
oxidation of the ring positions and alkyl substituents and conjugation
of the tertiary alcohol functions with glucuronic acid. These
metabolic patterns are common modes of converting tertiary (Ventura et
al., 1985) and secondary (Yamaguchi et al., 1994) terpenoid alcohols
to polar metabolites, which are easily excreted in the urine and
faeces (see Figure 3). Menthol forms similar oxidation and conjugation
products in rats (Yamaguchi et al., 1994).
In a minor pathway, the endocyclic alkene of alpha-terpineol is
epoxidized and then hydrolysed to yield a triol metabolite
1,2,8-trihydroxy- para-menthane, which was also reported in humans
after inadvertent oral ingestion of a pine-oil disinfectant containing
alpha-terpineol (Horning et al., 1976). It is expected that after
single doses, alpha-terpineol would be metabolized like linalool
(Chadha & Madyastha, 1984), primarily by glucuronic acid conjugation
and excretion in the urine.
Bicyclic tertiary alcohols (Nos 440 and 441) are relatively
stable in vivo, but are eventually conjugated with glucuronic acid
and excreted. In rabbits, the structurally related bicyclic tertiary
alcohols thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo(2.2.1)heptan-2-ol) are conjugated with
glucuronic acid (Williams, 1959).
In a study of the metabolism of the structurally related
terpenoid tertiary alcohol trans-sobrerol in humans, dogs, and rats,
10 metabolites were isolated in urine, eight of which were found in
humans. Two principal modes of metabolism were observed: allylic
oxidation of the ring positions and alkyl substituents and conjugation
of the tertiary alcohol functions with glucuronic acid. These
metabolic patterns are common modes of converting tertiary (Ventura et
al., 1985) and secondary (Yamaguchi et al., 1994) terpenoid alcohols
to polar metabolites, which are easily excreted in the urine and
faeces (see Figure 3). Menthol forms similar oxidation and conjugation
products in rats (Yamaguchi et al., 1994).
 After hydrolysis of linalyl and terpinyl esters, the component
aliphatic carboxylic acids undergo ß-oxidation and cleavage to
eventually yield carbon dioxide and water. Successive two-carbon units
are removed from the carbonyl end, which cleaves acids with even
numbers of carbons to acetyl coenzyme A and those with odd numbers of
carbons to acetyl and propionyl coenzyme A. Acetyl coenzyme A enters
the citric acid cycle directly or reacts with propionyl coenzyme A to
form succinyl coenzyme A, which also enters the citric acid cycle
(Voet & Voet, 1990).
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 16 of the 23 substances
in this group. They range from 1300 to > 36 000 mg/kg bw, indicating
that the acute toxicity of terpenoid tertiary alcohols and related
esters after oral exposure is low (Jenner et al., 1964; Moreno, 1977).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of studies with representative terpenoid tertiary
alcohols, esters, and related substances are summarized in Table 3 and
described below.
Linalool
In a safety evaluation, a 50:50 mixture of linalool and
citronellol was fed to male and female rats (numbers and strain
unspecified) in the diet. The daily intake was calculated to be 50
mg/kg bw of each substance. Haematological, clinical chemical, and
urinary analyses at weeks 6 and 12 showed no statistically significant
difference between treated and control groups. Histopathology revealed
no dose-related lesions. A slight retardation of growth was observed
in males, but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalool that had no adverse
effect (50 mg/kg bw) is > 1000 times the daily per capita intake of
44 or 19 µg/kg bw from use of linalool as a flavouring substance in
Europe and the United States, respectively, and > 500 times the total
daily per capita intake of 72 or 21 µg/kg bw from use of linalool
and its esters (i.e. alcohol equivalents) as flavouring substances in
Europe and the United States, respectively.
Linalyl esters
A mixture of linalyl acetate, linalyl isobutyrate, and geranyl
acetate was added to the diet of male and female rats (strain not
specified) at concentrations calculated to result in average daily
intakes of 24, 27, or 48 mg/kg bw, respectively, for 12 weeks.
Haematological, clinical chemical, and urinary analyses at weeks 6 and
12 showed normal values. Histopathological examination revealed no
dose-related lesions. Slight retardation of growth was observed in
females but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalyl acetate that had no
adverse effect (24 mg/kg bw per day) is > 500 times the daily
per capita intake of 34 µg/kg bw from use of linalyl acetate as a
flavouring substance in Europe and > 1000 times the daily per
capita intake of 3 µg/kg bw in the United States. Similarly, the
dose of linalyl isobutyrate that had no adverse effect (27 mg/kg bw
per day) is > 10 000 times the daily per capita intake of 1 µg/kg
bw from use of linalyl isobutyrate as a flavouring substance in Europe
and > 1 000 000 times the daily per capita intake of 0.01 µg/kg bw
in the United States.
Four groups of 10 male and 10 female Osborne-Mendel rats were fed
the structurally related ester of linalool, linalyl cinnamate, at
dietary concentrations of 0, 1000, 2500, or 10 000 mg/kg for 17 weeks.
These concentrations were calculated (US Food and Drug Administration,
1993) to provide intakes of 0, 50, 125, and 500 mg/kg bw per day,
respectively. Body weights were measured and haematological and gross
examinations were performed on all treated animals; only those at the
high dose were examined microscopically. No differences were found
between treated and control animals (Hagan et al., 1967).
Terpinyl acetate
Groups of 10 male and 10 female weanling Osborne-Mendel rats were
fed terpinyl acetate in the diet for 20 weeks at concentrations of 0,
1000, 2500, or 10 000 mg/kg (Hagan et al., 1967). These dietary levels
were calculated (US Food and Drug Administration, 1993) to result in
intakes of 0, 50, 125, and 500 mg/kg bw per day, respectively. All
animals were examined for changes in growth, haematological
parameters, and gross histological appearance. Microscopic examination
was performed on six to eight male and female animals at the high dose
and in the control groups. No statistically significant adverse
effects were reported (Hagan et al., 1967). The dose of terpinyl
acetate that had no adverse effects (500 mg/kg bw per day) is
> 50 000 times the daily per capita intake of 6 µg/kg bw from its
use as a flavouring substance in Europe and the United States. This
dose is > 5000 times the total daily per capita intakes of 53 or 23
µg/kg bw from use of alpha-terpineol and its esters (i.e. alcohol
equivalents) as flavouring substances in Europe and the United States.
Geranyl acetate and citronellyl acetate
The Committee previously evaluated linalool and linalyl acetate,
at the twenty-third meeting in 1979 (Annex 1, reference 50) as part of
a group of terpenoid flavouring substances including citral,
citronellol, and geranyl acetate. Although the Committee established a
group ADI of 0-0.5 mg/kg bw, they maintained that a long-term study
was required for at least one member of the group. Since that time,
long-term studies have been performed in rats and mice of a mixture of
the structurally related terpenoid ester geranyl acetate (71%) and
citronellyl acetate (29%) (US National Toxicology Program, 1987).
These studies were considered by the Committee at its forty-ninth
meeting (Annex 1, reference 131) in a safety evaluation of the group
of flavouring substances that includes 26 geranyl, neryl, citronellyl,
and rhodinyl esters formed from branched-chain terpenoid primary
alcohols and branched-chain carboxylic acids. The Committee determined
that the study of carcinogenicity in mice was inadequate owing to
dosing errors and the low survival associated with infections. The
NOEL of the mixture in rats was 1000 mg/kg bw per day, which
corresponds to estimated doses of 710 mg/kg bw per day geranyl acetate
and 290 mg/kg bw per day citronellyl acetate. This NOEL is > 10 000
times the estimated daily per capita intakes of 44 and 19 µg/kg bw
per day from use of the structurally related substance linalool as a
flavouring substance in Europe and the United States, respectively.
2.3.2.3 Genotoxicity
Five representative alcohols and esters in this group have been
tested for genotoxicity, including a complete battery of tests with
linalool in vitro. The results of these tests are summarized in
Table 4 and described below.
Linalool (No. 356) was inactive in Salmonella typhimurium
strains TA92, TA94, TA98, TA100, TA1535, TA1537, and TA1538 with and
without metabolic activation (Rockwell & Raw, 1979; Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
Linalool did not induce chromosomal aberrations when incubated with
Chinese hamster fibroblasts at a maximum concentration of 0.25 mg/ml
(Ishidate et al., 1984), nor did it induce unscheduled DNA synthesis
in rat hepatocytes at concentrations up to 50 µg/ml (Heck et al.,
1989).
Linalool did not induce mutation in E. coli WP2 uvrA at
concentrations of 0.125-1 mg/plate (Yoo, 1986). When incubated with
Bacillus subtilis H17 (rec+) and M45 (rec-), linalool was
not mutagenic at 17 µg/plate (Oda et al., 1978) but was mutagenic at
10 µl/disc (Yoo, 1986). Linalool was inactive in mouse lymphoma L5178Y
tk+/- cells with metabolic activation at 200 µg/ml but gave a
weakly positive response without activation at 150 µg/ml (Heck et al.,
1989).
The 24-h urine of Sprague-Dawley rats given 0.5 ml of linalool or
ß-terpineol (No. 374) was incubated with S. typhimurium strains TA98
and TA100 to observe mutagenicity. The following assays were
conducted: linalool or ß-terpineol (with metabolic activation),
aliquot of 24-h urine (with and without metabolic activation), ether
extract of 24-h urine (with and without metabolic activation), and
aqueous phase of 24-h urine ether extract (with and without metabolic
activation). The urine samples were diluted to 60 ml and incubated in
the presence of chloroform and ß-glu-curonidase before ether
extraction. The only positive response was found with the ether
extract of ß-terpineol.
Table 3. Short-term and long-term studies of toxicity and carcinogenicity and special studies of aliphatic acyclic and alicyclic
terpenoid tertiary alcohols and structurally related substances
Substance No. Species Sex No. groups/ Route Time NOEL Reference
no. per group (mg/kg bw
per day)
Linalool 356 Rat M/F 1/-- Dieta 84 days 50b Oser (1967)
Linalyl acetate 357 Rat M/F 1/NR Dietc 84 days 24b Oser (1967)
Linalyl isobutyrate 362 Rat M/F 1/NR Dietd 84 days 27b Oser (1967)
Terpinyl acetate 368 Rat M/F 3/20 Diete 140 days 500b Hagan et al. (1967)
Linalyl cinnamatee None Rat M/F 4/20 Dietf 17 weeks 500b Hagan et al. (1967)
Geranyl acetate/ None Mouse M/F 2/50 Gavage 103 weeks -g US National Toxicology
Citronellyl acetate Program(1987)
Geranyl acetate/ None Rat M/F 2/5 Gavage 103 weeks 1000 US National Toxicology
Citronellyl acetate (710 geranyl Program (1987)
acetate;
290 citronellyl
acetate)
Special study of immunotoxicity
Linalool 356 Mouse F 3/30 Gavage 5 375b Gaworski et al. (1994)
M, male; F, female; NR, not reported
a Administered with citronellol as part of a 50/50 mixture
b The study was performed at a single dose or multiple doses that had no adverse effects; therefore, no NOEL was determined. The NOEL
is probaby higher than the dose reported here, which is the highest dose that had no adverse effect.
c Administered with linalyl isobutyrate and geranyl acetate as part of a mixture
d Administered with linalyl acetate and geranyl acetate as part of a mixture
e Structurally related ester of linalool, not evaluated as part of this group
f Structurally related terpenoid esters administered as a mixture: geranyl acetate, 71%; citronellyl acetate, 29%
g There was no NOEL owing to a high incidence of gavage errors and low survival associated with pneumonia.
Table 4. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally
related substances
Substance No. End-point Test object Concentration Result Reference
Linalool 356 Reverse mutation S. typhimurium TA100 0.01-3 µl/2-ml Negativea Eder et al. (1980)
(modified test) incubation volume
Linalool 356 Reverse mutation S. typhimurium TA92, TA100, 1 mg/plateb Negativea Ishidate et al. (1984)
TA1535, TA1537, TA94, TA98
Linalool 356 Reverse mutation S. typhimurium TA98, TA100 0.05-100 µl/plate Negativea Rockwell & Raw (1979)
Linalool 356 Reverse mutation S. typhimurium TA100, TA1535, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1537, TA1538, TA98
Linalool 356 Gene mutation Mouse lymphoma L5178Y tk+/- 200 µg/ml Negativec Heck et al. (1989)
150 µg/ml Weakly
positived
Linalool 356 Unscheduled DNA Rat hepatocytes synthesis 50 µg/mlb Negativee Heck et al. (1989)
Linalool 356 Chromosomal Chinese hamster fibroblasts 0.25 mg/mlb Negatived Ishidate et al. (1984)
aberration
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 17 µg Negative Oda et al. (1978)
& M45 (rec-)
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 10 µl/discb Positive Yoo (1986)
& M45 (rec-)
Linalool 356 Gene mutation E. coli WP2 uvrA 0.125-1 mg/plate Negative Yoo (1986)
Linalyl acetate 359 Reverse mutation S. typhimurium TA1535, TA1537, 25 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
Linalyl acetate 359 Unscheduled DNA Fischer or Sprague-Dawley rat 300 µg/mlb Negativee Heck et al. (1989)
synthesis hepatocytes
Table 4. (continued)
Substance No. End-point Test object Concentration Result Reference
Linalyl acetate 359 Gene mutation Bacillus subtilis H17 (rec+) 18 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
alpha-Terpineol 366 Reverse mutation S. typhimurium TA1535, TA1537, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
alpha-Terpineol 366 Spot test S. typhimurium TA1535, TA1537, 3 µmol/plate Negativea Florin et al. (1980)
TA98, TA100
alpha-Terpineol 366 Gene mutation Mouse lymphoma L5178Y tk+/- 250 µg/mlb Negativec Heck et al. (1989)
300 µg/mlb Negatived
Terpinyl acetate 368 Gene mutation Bacillus subtilis H17 (rec+) 19 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
ß-Terpineol 374 Reverse mutation S. typhimurium TA100, TA98 0.05-100 µl/plate Negativec Rockwell & Raw (1979)
Terpineolf 366 Gene mutation S. cerevisiae Not reported Negativec Oda et al. (1978)
and/or
374
a With and without metabolic activation
b The highest inactive or lowest active concentration; range not specified
c With metabolic activation
d Without metabolic activation
e 75 or 150 cells analysed per dose
f Not specified whether alpha- or ß-terpineol
Linalyl acetate (No. 359) and alpha-terpineol (No. 366) were
inactive in S. typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538 with and without metabolic activation (Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
ß-Terpineol was inactive in S. typhimurium strains TA98 and TA100
with metabolic activation (Rockwell & Raw, 1979). When incubated with
B. subtilis H17 (rec+) and M45 (rec-), linalyl acetate and
terpinyl acetate were not mutagenic at 18 or 19 µg, respectively (Oda
et al., 1978).
Thus, all but two of 19 tests of the genotoxicity of terpenoid
tertiary alcohols and related esters in vitro gave negative results.
Linalool was active in a rec assay, in which differences in the
growth rates of two strains of B.subtilis are used as a measure of
DNA damage (Yoo, 1986). In contrast, no evidence of DNA damage was
observed in an assay for unscheduled DNA synthesis in rat hepatocytes
(Heck et al., 1989). The authors of a study in which linalool gave a
weak positive result in the mouse lymphoma assay (Heck et al., 1989)
emphasized that positive results are commonly observed for polar
substances in this assay in the presence of metabolic activation and
may be associated with changes in physiological culture conditions (pH
and osmolality). The weight of the evidence for terpenoid tertiary
alcohols and related esters indicates that this group of flavouring
substances would not be expected to have genotoxic potential
in vivo.
2.3.2.4 Other relevant studies
No adverse effects were reported at doses of linalool up to
375 mg/kg bw in a screening test in mice for humoral and cell-mediated
immune responses (see Table 4). Linalool in 1% methylcellulose was
administered intragastrically to 10-20 six- to eight-week-old female
B6C3F1 mice for five days at a dose of 94, 188, or 375 mg/kg bw per
day. Cell-mediated immunity was assessed in a host resistance assay
with a Listeria monocytogenes bacterial challenge. Humoral immunity
was measured by the antibody plaque-forming cell response to sheep
erythrocytes. Body weights, lymphoid organ weights, and spleen
cellularity were also measured. Cyclophosphamide was used as a
positive control. Linalool did not adversely modulate the immune
response in either assay (Gaworski et al., 1994).
4. REFERENCES
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard--A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro
mutagenic properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha,ß-unsaturated compounds: Considerations of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Gaworski, C.K., Vollmuth, T.A., Dozier, M.M., Heck, J.D., Dunn, L.T.,
Ratajczak, H.V. & Thomas, P.T. (1994) An immunotoxicity assessment of
food flavouring ingredients. Food Chem. Toxicol., 32, 409-415.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. J. Food Cosmet.
Toxicol., 5,141-147.
Hall, R.L. (1979) Unpublished report from McCormick & Co. Inc. to the
Flavor and Extracts Manufacturers' Association of the United States,
Washington DC, United States.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist., 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.J. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxide-diol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectroscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report from McCormick & Co. Inc.
to the Flavor and Extracts Manufacturers' Association of the United
States, Washington DC, United States.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., et al. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Srivatsan, V. (1988) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Moreno, O.M. (1977) Unpublished report to the Research Institute of
Fragrance Materials.Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen., 9, 177-181.
Oser, B.L. (1967) Unpublished report cited in FAO Nutrition Meeting
Report Series No. 44A, 1968 (Annex 1, reference 15).
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974a) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974b) Effect of linalool
on hepatic drug-metabolizing enzymes in the rat. Biochem. Soc.
Trans., 2 615-618.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D. & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14, 537-540.
Rahman, K.A.Q.M.Q. (1974) PhD thesis, University of Surrey. [Cited in
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.]
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs,
spices, and food additives. Nutr. Cancer, 1, 10-15.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC.
US National Toxicology Program (1987) Carcinogenesis Studies of
Food Grade Geranyl Acetate (71%) and Citronellyl Acetate (29%)
(NTP-TR-252; PB-88-2508), National Technical Information Services,
Research Triangle Park, North Carolina, United States.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Williams, R.T. (1959) Detoxication Mechanisms. The Metabolism and
Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
After hydrolysis of linalyl and terpinyl esters, the component
aliphatic carboxylic acids undergo ß-oxidation and cleavage to
eventually yield carbon dioxide and water. Successive two-carbon units
are removed from the carbonyl end, which cleaves acids with even
numbers of carbons to acetyl coenzyme A and those with odd numbers of
carbons to acetyl and propionyl coenzyme A. Acetyl coenzyme A enters
the citric acid cycle directly or reacts with propionyl coenzyme A to
form succinyl coenzyme A, which also enters the citric acid cycle
(Voet & Voet, 1990).
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 16 of the 23 substances
in this group. They range from 1300 to > 36 000 mg/kg bw, indicating
that the acute toxicity of terpenoid tertiary alcohols and related
esters after oral exposure is low (Jenner et al., 1964; Moreno, 1977).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of studies with representative terpenoid tertiary
alcohols, esters, and related substances are summarized in Table 3 and
described below.
Linalool
In a safety evaluation, a 50:50 mixture of linalool and
citronellol was fed to male and female rats (numbers and strain
unspecified) in the diet. The daily intake was calculated to be 50
mg/kg bw of each substance. Haematological, clinical chemical, and
urinary analyses at weeks 6 and 12 showed no statistically significant
difference between treated and control groups. Histopathology revealed
no dose-related lesions. A slight retardation of growth was observed
in males, but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalool that had no adverse
effect (50 mg/kg bw) is > 1000 times the daily per capita intake of
44 or 19 µg/kg bw from use of linalool as a flavouring substance in
Europe and the United States, respectively, and > 500 times the total
daily per capita intake of 72 or 21 µg/kg bw from use of linalool
and its esters (i.e. alcohol equivalents) as flavouring substances in
Europe and the United States, respectively.
Linalyl esters
A mixture of linalyl acetate, linalyl isobutyrate, and geranyl
acetate was added to the diet of male and female rats (strain not
specified) at concentrations calculated to result in average daily
intakes of 24, 27, or 48 mg/kg bw, respectively, for 12 weeks.
Haematological, clinical chemical, and urinary analyses at weeks 6 and
12 showed normal values. Histopathological examination revealed no
dose-related lesions. Slight retardation of growth was observed in
females but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalyl acetate that had no
adverse effect (24 mg/kg bw per day) is > 500 times the daily
per capita intake of 34 µg/kg bw from use of linalyl acetate as a
flavouring substance in Europe and > 1000 times the daily per
capita intake of 3 µg/kg bw in the United States. Similarly, the
dose of linalyl isobutyrate that had no adverse effect (27 mg/kg bw
per day) is > 10 000 times the daily per capita intake of 1 µg/kg
bw from use of linalyl isobutyrate as a flavouring substance in Europe
and > 1 000 000 times the daily per capita intake of 0.01 µg/kg bw
in the United States.
Four groups of 10 male and 10 female Osborne-Mendel rats were fed
the structurally related ester of linalool, linalyl cinnamate, at
dietary concentrations of 0, 1000, 2500, or 10 000 mg/kg for 17 weeks.
These concentrations were calculated (US Food and Drug Administration,
1993) to provide intakes of 0, 50, 125, and 500 mg/kg bw per day,
respectively. Body weights were measured and haematological and gross
examinations were performed on all treated animals; only those at the
high dose were examined microscopically. No differences were found
between treated and control animals (Hagan et al., 1967).
Terpinyl acetate
Groups of 10 male and 10 female weanling Osborne-Mendel rats were
fed terpinyl acetate in the diet for 20 weeks at concentrations of 0,
1000, 2500, or 10 000 mg/kg (Hagan et al., 1967). These dietary levels
were calculated (US Food and Drug Administration, 1993) to result in
intakes of 0, 50, 125, and 500 mg/kg bw per day, respectively. All
animals were examined for changes in growth, haematological
parameters, and gross histological appearance. Microscopic examination
was performed on six to eight male and female animals at the high dose
and in the control groups. No statistically significant adverse
effects were reported (Hagan et al., 1967). The dose of terpinyl
acetate that had no adverse effects (500 mg/kg bw per day) is
> 50 000 times the daily per capita intake of 6 µg/kg bw from its
use as a flavouring substance in Europe and the United States. This
dose is > 5000 times the total daily per capita intakes of 53 or 23
µg/kg bw from use of alpha-terpineol and its esters (i.e. alcohol
equivalents) as flavouring substances in Europe and the United States.
Geranyl acetate and citronellyl acetate
The Committee previously evaluated linalool and linalyl acetate,
at the twenty-third meeting in 1979 (Annex 1, reference 50) as part of
a group of terpenoid flavouring substances including citral,
citronellol, and geranyl acetate. Although the Committee established a
group ADI of 0-0.5 mg/kg bw, they maintained that a long-term study
was required for at least one member of the group. Since that time,
long-term studies have been performed in rats and mice of a mixture of
the structurally related terpenoid ester geranyl acetate (71%) and
citronellyl acetate (29%) (US National Toxicology Program, 1987).
These studies were considered by the Committee at its forty-ninth
meeting (Annex 1, reference 131) in a safety evaluation of the group
of flavouring substances that includes 26 geranyl, neryl, citronellyl,
and rhodinyl esters formed from branched-chain terpenoid primary
alcohols and branched-chain carboxylic acids. The Committee determined
that the study of carcinogenicity in mice was inadequate owing to
dosing errors and the low survival associated with infections. The
NOEL of the mixture in rats was 1000 mg/kg bw per day, which
corresponds to estimated doses of 710 mg/kg bw per day geranyl acetate
and 290 mg/kg bw per day citronellyl acetate. This NOEL is > 10 000
times the estimated daily per capita intakes of 44 and 19 µg/kg bw
per day from use of the structurally related substance linalool as a
flavouring substance in Europe and the United States, respectively.
2.3.2.3 Genotoxicity
Five representative alcohols and esters in this group have been
tested for genotoxicity, including a complete battery of tests with
linalool in vitro. The results of these tests are summarized in
Table 4 and described below.
Linalool (No. 356) was inactive in Salmonella typhimurium
strains TA92, TA94, TA98, TA100, TA1535, TA1537, and TA1538 with and
without metabolic activation (Rockwell & Raw, 1979; Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
Linalool did not induce chromosomal aberrations when incubated with
Chinese hamster fibroblasts at a maximum concentration of 0.25 mg/ml
(Ishidate et al., 1984), nor did it induce unscheduled DNA synthesis
in rat hepatocytes at concentrations up to 50 µg/ml (Heck et al.,
1989).
Linalool did not induce mutation in E. coli WP2 uvrA at
concentrations of 0.125-1 mg/plate (Yoo, 1986). When incubated with
Bacillus subtilis H17 (rec+) and M45 (rec-), linalool was
not mutagenic at 17 µg/plate (Oda et al., 1978) but was mutagenic at
10 µl/disc (Yoo, 1986). Linalool was inactive in mouse lymphoma L5178Y
tk+/- cells with metabolic activation at 200 µg/ml but gave a
weakly positive response without activation at 150 µg/ml (Heck et al.,
1989).
The 24-h urine of Sprague-Dawley rats given 0.5 ml of linalool or
ß-terpineol (No. 374) was incubated with S. typhimurium strains TA98
and TA100 to observe mutagenicity. The following assays were
conducted: linalool or ß-terpineol (with metabolic activation),
aliquot of 24-h urine (with and without metabolic activation), ether
extract of 24-h urine (with and without metabolic activation), and
aqueous phase of 24-h urine ether extract (with and without metabolic
activation). The urine samples were diluted to 60 ml and incubated in
the presence of chloroform and ß-glu-curonidase before ether
extraction. The only positive response was found with the ether
extract of ß-terpineol.
Table 3. Short-term and long-term studies of toxicity and carcinogenicity and special studies of aliphatic acyclic and alicyclic
terpenoid tertiary alcohols and structurally related substances
Substance No. Species Sex No. groups/ Route Time NOEL Reference
no. per group (mg/kg bw
per day)
Linalool 356 Rat M/F 1/-- Dieta 84 days 50b Oser (1967)
Linalyl acetate 357 Rat M/F 1/NR Dietc 84 days 24b Oser (1967)
Linalyl isobutyrate 362 Rat M/F 1/NR Dietd 84 days 27b Oser (1967)
Terpinyl acetate 368 Rat M/F 3/20 Diete 140 days 500b Hagan et al. (1967)
Linalyl cinnamatee None Rat M/F 4/20 Dietf 17 weeks 500b Hagan et al. (1967)
Geranyl acetate/ None Mouse M/F 2/50 Gavage 103 weeks -g US National Toxicology
Citronellyl acetate Program(1987)
Geranyl acetate/ None Rat M/F 2/5 Gavage 103 weeks 1000 US National Toxicology
Citronellyl acetate (710 geranyl Program (1987)
acetate;
290 citronellyl
acetate)
Special study of immunotoxicity
Linalool 356 Mouse F 3/30 Gavage 5 375b Gaworski et al. (1994)
M, male; F, female; NR, not reported
a Administered with citronellol as part of a 50/50 mixture
b The study was performed at a single dose or multiple doses that had no adverse effects; therefore, no NOEL was determined. The NOEL
is probaby higher than the dose reported here, which is the highest dose that had no adverse effect.
c Administered with linalyl isobutyrate and geranyl acetate as part of a mixture
d Administered with linalyl acetate and geranyl acetate as part of a mixture
e Structurally related ester of linalool, not evaluated as part of this group
f Structurally related terpenoid esters administered as a mixture: geranyl acetate, 71%; citronellyl acetate, 29%
g There was no NOEL owing to a high incidence of gavage errors and low survival associated with pneumonia.
Table 4. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally
related substances
Substance No. End-point Test object Concentration Result Reference
Linalool 356 Reverse mutation S. typhimurium TA100 0.01-3 µl/2-ml Negativea Eder et al. (1980)
(modified test) incubation volume
Linalool 356 Reverse mutation S. typhimurium TA92, TA100, 1 mg/plateb Negativea Ishidate et al. (1984)
TA1535, TA1537, TA94, TA98
Linalool 356 Reverse mutation S. typhimurium TA98, TA100 0.05-100 µl/plate Negativea Rockwell & Raw (1979)
Linalool 356 Reverse mutation S. typhimurium TA100, TA1535, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1537, TA1538, TA98
Linalool 356 Gene mutation Mouse lymphoma L5178Y tk+/- 200 µg/ml Negativec Heck et al. (1989)
150 µg/ml Weakly
positived
Linalool 356 Unscheduled DNA Rat hepatocytes synthesis 50 µg/mlb Negativee Heck et al. (1989)
Linalool 356 Chromosomal Chinese hamster fibroblasts 0.25 mg/mlb Negatived Ishidate et al. (1984)
aberration
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 17 µg Negative Oda et al. (1978)
& M45 (rec-)
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 10 µl/discb Positive Yoo (1986)
& M45 (rec-)
Linalool 356 Gene mutation E. coli WP2 uvrA 0.125-1 mg/plate Negative Yoo (1986)
Linalyl acetate 359 Reverse mutation S. typhimurium TA1535, TA1537, 25 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
Linalyl acetate 359 Unscheduled DNA Fischer or Sprague-Dawley rat 300 µg/mlb Negativee Heck et al. (1989)
synthesis hepatocytes
Table 4. (continued)
Substance No. End-point Test object Concentration Result Reference
Linalyl acetate 359 Gene mutation Bacillus subtilis H17 (rec+) 18 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
alpha-Terpineol 366 Reverse mutation S. typhimurium TA1535, TA1537, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
alpha-Terpineol 366 Spot test S. typhimurium TA1535, TA1537, 3 µmol/plate Negativea Florin et al. (1980)
TA98, TA100
alpha-Terpineol 366 Gene mutation Mouse lymphoma L5178Y tk+/- 250 µg/mlb Negativec Heck et al. (1989)
300 µg/mlb Negatived
Terpinyl acetate 368 Gene mutation Bacillus subtilis H17 (rec+) 19 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
ß-Terpineol 374 Reverse mutation S. typhimurium TA100, TA98 0.05-100 µl/plate Negativec Rockwell & Raw (1979)
Terpineolf 366 Gene mutation S. cerevisiae Not reported Negativec Oda et al. (1978)
and/or
374
a With and without metabolic activation
b The highest inactive or lowest active concentration; range not specified
c With metabolic activation
d Without metabolic activation
e 75 or 150 cells analysed per dose
f Not specified whether alpha- or ß-terpineol
Linalyl acetate (No. 359) and alpha-terpineol (No. 366) were
inactive in S. typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538 with and without metabolic activation (Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
ß-Terpineol was inactive in S. typhimurium strains TA98 and TA100
with metabolic activation (Rockwell & Raw, 1979). When incubated with
B. subtilis H17 (rec+) and M45 (rec-), linalyl acetate and
terpinyl acetate were not mutagenic at 18 or 19 µg, respectively (Oda
et al., 1978).
Thus, all but two of 19 tests of the genotoxicity of terpenoid
tertiary alcohols and related esters in vitro gave negative results.
Linalool was active in a rec assay, in which differences in the
growth rates of two strains of B.subtilis are used as a measure of
DNA damage (Yoo, 1986). In contrast, no evidence of DNA damage was
observed in an assay for unscheduled DNA synthesis in rat hepatocytes
(Heck et al., 1989). The authors of a study in which linalool gave a
weak positive result in the mouse lymphoma assay (Heck et al., 1989)
emphasized that positive results are commonly observed for polar
substances in this assay in the presence of metabolic activation and
may be associated with changes in physiological culture conditions (pH
and osmolality). The weight of the evidence for terpenoid tertiary
alcohols and related esters indicates that this group of flavouring
substances would not be expected to have genotoxic potential
in vivo.
2.3.2.4 Other relevant studies
No adverse effects were reported at doses of linalool up to
375 mg/kg bw in a screening test in mice for humoral and cell-mediated
immune responses (see Table 4). Linalool in 1% methylcellulose was
administered intragastrically to 10-20 six- to eight-week-old female
B6C3F1 mice for five days at a dose of 94, 188, or 375 mg/kg bw per
day. Cell-mediated immunity was assessed in a host resistance assay
with a Listeria monocytogenes bacterial challenge. Humoral immunity
was measured by the antibody plaque-forming cell response to sheep
erythrocytes. Body weights, lymphoid organ weights, and spleen
cellularity were also measured. Cyclophosphamide was used as a
positive control. Linalool did not adversely modulate the immune
response in either assay (Gaworski et al., 1994).
4. REFERENCES
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard--A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro
mutagenic properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha,ß-unsaturated compounds: Considerations of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Gaworski, C.K., Vollmuth, T.A., Dozier, M.M., Heck, J.D., Dunn, L.T.,
Ratajczak, H.V. & Thomas, P.T. (1994) An immunotoxicity assessment of
food flavouring ingredients. Food Chem. Toxicol., 32, 409-415.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. J. Food Cosmet.
Toxicol., 5,141-147.
Hall, R.L. (1979) Unpublished report from McCormick & Co. Inc. to the
Flavor and Extracts Manufacturers' Association of the United States,
Washington DC, United States.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist., 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.J. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxide-diol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectroscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report from McCormick & Co. Inc.
to the Flavor and Extracts Manufacturers' Association of the United
States, Washington DC, United States.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., et al. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Srivatsan, V. (1988) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Moreno, O.M. (1977) Unpublished report to the Research Institute of
Fragrance Materials.Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen., 9, 177-181.
Oser, B.L. (1967) Unpublished report cited in FAO Nutrition Meeting
Report Series No. 44A, 1968 (Annex 1, reference 15).
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974a) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974b) Effect of linalool
on hepatic drug-metabolizing enzymes in the rat. Biochem. Soc.
Trans., 2 615-618.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D. & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14, 537-540.
Rahman, K.A.Q.M.Q. (1974) PhD thesis, University of Surrey. [Cited in
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.]
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs,
spices, and food additives. Nutr. Cancer, 1, 10-15.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC.
US National Toxicology Program (1987) Carcinogenesis Studies of
Food Grade Geranyl Acetate (71%) and Citronellyl Acetate (29%)
(NTP-TR-252; PB-88-2508), National Technical Information Services,
Research Triangle Park, North Carolina, United States.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Williams, R.T. (1959) Detoxication Mechanisms. The Metabolism and
Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
See Also:
Toxicological Abbreviations
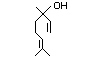 Tetrahydrolinalool 357 78-69-3 55/0.1 No N/R N/R See linalool. No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Tetrahydrolinalool 357 78-69-3 55/0.1 No N/R N/R See linalool. No safety
concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
 Linalyl formate 358 115-99-1 8/13 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl formate 358 115-99-1 8/13 No N/R N/R See linalyl acetate and No safety
linalool. concern
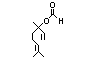 Linalyl acetatea 359 115-95-7 2100/180 Yes No Yes. The dose of Hydrolysed to linalool No safety
24 mg/kg bw per and acetic acid. See concern
day that had no also linalool.
adverse effects in
a safety evaluation
(Oser, 1967) is
> 500 times the
intake of linalyl
acetate.
Linalyl acetatea 359 115-95-7 2100/180 Yes No Yes. The dose of Hydrolysed to linalool No safety
24 mg/kg bw per and acetic acid. See concern
day that had no also linalool.
adverse effects in
a safety evaluation
(Oser, 1967) is
> 500 times the
intake of linalyl
acetate.
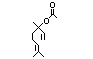 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl propionate 360 144-39-8 16/2 No N/R N/R See linalyl acetate and No safety
linalool. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl propionate 360 144-39-8 16/2 No N/R N/R See linalyl acetate and No safety
linalool. concern
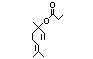 Linalyl butyrate 361 78-36-4 10/4 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl butyrate 361 78-36-4 10/4 No N/R N/R See linalyl acetate and No safety
linalool. concern
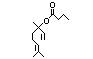 Linalyl isobutyrate 362 78-35-3 36/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl isobutyrate 362 78-35-3 36/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
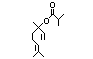 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl isovalerate 363 1118-27-0 5/6 No N/R N/R See linalyl acetate and No safety
linalool. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Linalyl isovalerate 363 1118-27-0 5/6 No N/R N/R See linalyl acetate and No safety
linalool. concern
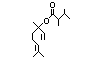 Linalyl hexanoate 364 7779-23-9 1/0.4 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl hexanoate 364 7779-23-9 1/0.4 No N/R N/R See linalyl acetate and No safety
linalool. concern
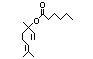 Linalyl octanoate 365 10024-64-3 0.1/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
Linalyl octanoate 365 10024-64-3 0.1/1 No N/R N/R See linalyl acetate and No safety
linalool. concern
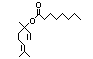 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
alpha-Terpineol 366 98-55-5 3000/1100 Yes No Yes. The dose of Metabolized primarily No safety
500 mg/kg bw per by conjugation with concern
day that had no glucuronic acid and
adverse effects excreted in urine.
(Hagan et al., Oxidation of the allylic
1967) is > 5000 methyl group followed by
times the intake hydrogenation to yield the
of the component corresponding saturated
alpha-terpineol. acid may occur
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
alpha-Terpineol 366 98-55-5 3000/1100 Yes No Yes. The dose of Metabolized primarily No safety
500 mg/kg bw per by conjugation with concern
day that had no glucuronic acid and
adverse effects excreted in urine.
(Hagan et al., Oxidation of the allylic
1967) is > 5000 methyl group followed by
times the intake hydrogenation to yield the
of the component corresponding saturated
alpha-terpineol. acid may occur
 Terpinyl formate 367 2153-26-6 0.1/2 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl formate 367 2153-26-6 0.1/2 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
 Terpinyl acetate 368 8007-35-0 260/390 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl acetate 368 8007-35-0 260/390 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl propionate 369 80-27-3 0.03/1 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl propionate 369 80-27-3 0.03/1 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
 Terpinyl butyrate 370 80-26-6 6/6 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
Terpinyl butyrate 370 80-26-6 6/6 No N/R N/R See linalyl acetate and No safety
alpha-terpineol. concern
 Terpinyl 371 7774-65-4 0.7/0.02 No N/R N/R See linalyl acetate and No safety
isobutyrate alpha-terpineol. concern
Terpinyl 371 7774-65-4 0.7/0.02 No N/R N/R See linalyl acetate and No safety
isobutyrate alpha-terpineol. concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl 372 1142-85-4 0.1/1 No N/R N/R See linalyl acetate and No safety
isovalerate alpha-terpineol. concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Terpinyl 372 1142-85-4 0.1/1 No N/R N/R See linalyl acetate and No safety
isovalerate alpha-terpineol. concern
 para-Menth-3-en-1-ol 373 586-82-3 41/0.4 No N/R N/R See alpha-terpineol. No safety
concern
para-Menth-3-en-1-ol 373 586-82-3 41/0.4 No N/R N/R See alpha-terpineol. No safety
concern
 4-Carvomenthenol 439 562-74-3 170/51 No N/R N/R See alpha-terpineol. No safety
concern
4-Carvomenthenol 439 562-74-3 170/51 No N/R N/R See alpha-terpineol. No safety
concern
 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
para-Menth-8-en-1-ol 374 138-87-4 2/21 No N/R N/R See alpha-terpineol. No safety
(ß-Terpineol) concern
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
para-Menth-8-en-1-ol 374 138-87-4 2/21 No N/R N/R See alpha-terpineol. No safety
(ß-Terpineol) concern
 2-Ethyl-1,3,3- 440 18368-91-7 1/0.02 No N/R N/R The structurally related No safety
trimethyl-2- bicyclic tertiary concern
norbornanol alcohols thujyl alcohol
and ß-sante-nol
are conjugated with
glucuronic acid.
2-Ethyl-1,3,3- 440 18368-91-7 1/0.02 No N/R N/R The structurally related No safety
trimethyl-2- bicyclic tertiary concern
norbornanol alcohols thujyl alcohol
and ß-sante-nol
are conjugated with
glucuronic acid.
 4-Thujanol 441 546-79-2 1/0.04 No N/R N/R The structurally related No safety
bicyclic tertiary concern
alcohols thujyl alcohol
and ß-sante-nol are
conjugated with
glucuronic acid.
4-Thujanol 441 546-79-2 1/0.04 No N/R N/R The structurally related No safety
bicyclic tertiary concern
alcohols thujyl alcohol
and ß-sante-nol are
conjugated with
glucuronic acid.
 Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Methyl 1-acetoxy- 442 52789-73-8 N/D/4 - - - Cannot be predicted Additional
cyclohexyl to be metabolized data on
ketone to innocuous toxicity
products. Therefore, are required
Steps A3-A5 are not because a
applicable to this NOEL is not
substance, which must available
be evaluated on the for this or
right side ('B') of the a related
evaluation scheme. substance
(Step B4)
and intake
exceeds 1.5
µg/person
per day
(Step B5).
Table 1. (continued)
Substance No. CAS No. Estimated per Step A3 Step A4 Step A5 Comments Conclusion
capita intake, Does Is the Adequate NOEL based on
Europe/USA intake substance for substance current
(µg/day) exceed or its or related intake
threshold? human metabolites
intake substance?
endogenous?
Methyl 1-acetoxy- 442 52789-73-8 N/D/4 - - - Cannot be predicted Additional
cyclohexyl to be metabolized data on
ketone to innocuous toxicity
products. Therefore, are required
Steps A3-A5 are not because a
applicable to this NOEL is not
substance, which must available
be evaluated on the for this or
right side ('B') of the a related
evaluation scheme. substance
(Step B4)
and intake
exceeds 1.5
µg/person
per day
(Step B5).
 N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a The group ADI of 0-0.05 mg/kg bw established at the twenty-third meeting for citral, citronellol, linalool, and linalyl acetate as citral
was maintained.
The substances in this group are structurally related because
they each contain a tertiary alcohol function (without aryl
substitution); they therefore have similar metabolic and toxicological
profiles. Four esters of the tertiary alcohol linalool (linalyl
anthranilate, linalyl benzoate, linalyl cinnamate, and linalyl
phenylacetate) and one ester of alpha-terpineol (terpinyl cinnamate)
used as flavouring substances in Europe and the United States are not
included in this group because they may have different toxicological
profiles, owing to their carboxylic acid components. It would be
appropriate to evaluate these esters in subsequent groups, with
anthranilic acid, benzoic acid, cinnamic acid, phenylacetic acid, and
related substances used as flavouring substances. Any additional
intake of linalool and alpha-terpineol from these four esters is
minimal and would not have a significant impact on the total combined
intake of linalool, alpha-terpineol, and related tertiary alcohols and
esters (see section 1.5).
2.2 Intake
The total annual volume of the 23 tertiary alcohols and related
esters is approximately 58 000 kg in Europe (International
Organization of the Flavor Industry, 1995) and 15 000 kg in the United
States (US National Academy of Sciences, 1987). Production volumes and
intake levels of each flavouring substance are reported in Table 2.
Thirteen tertiary alcohols and related esters have been reported
to occur naturally in foods (Maarse et al., 1994); quantitative data
reported for eight of these substances (see Table 2) indicate that
they are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, esters are hydrolysed to their corresponding alcohol
and carboxylic acid. Hydrolysis is catalysed by classes of enzymes
recognized as carboxylesterases or esterases (Heymann, 1980), the most
important of which are the B-esterases. In mammals, these enzymes
occur in most tissues throughout the body (Anders, 1989; Heymann,
1980) but predominate in hepatocytes (Heymann, 1980).
Esters of linalool (Nos 358-365) and alpha-terpineol (Nos
367-372) are expected to be hydrolysed in humans to yield linalool and
alpha-terpineol, respectively, and the corresponding saturated
aliphatic carboxylic acid. Methyl 1-acetoxycyclohexyl ketone (No. 442)
is expected to be hydrolysed to acetic acid and methyl
1-hydroxycyclohexyl ketone.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic terpenoid tertiary
alcohols and structurally related substances as flavouring substances in Europe and the
United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Linalool (356)
Europe 18 000 2600 44 NA
United States 5 800 1100 19 NA
Tetrahydrolinalool (357)
Europe 390 55 0 NA
United States 0.5 0.1 0.002 NA
Linalyl formate (358)
Europe 57 8 0.14 0.1
United States 70 13 0.22 0.2
Linalyl acetate (359)
Europe 14 000 2100 35 27
United States 970 180 3 2
Linalyl propionate (360)
Europe 110 16 0.3 0.2
United States 8 2 0.03 0.02
Linalyl butyrate (361)
Europe 69 10 0.2 0.1
United States 22 4 0.07 0.05
Linalyl isobutyrate (362)
Europe 250 36 0.6 0.4
United States 3 0 0.00 0.00
Linalyl isovalerate (363)
Europe 38 5 0.0 0.06
United States 34 6 0.11 0.07
Linalyl hexanoate (364)
Europe 7 0 0.02 0.01
United States 2 0.4 0.00 0.004
Linalyl octanoate (365)
Europe 1 0.1 0.002 0.001
United States 5 0 0.02 0.00
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
alpha-Terpineol (366)
Europe 21 000 3000 50 NA
United States 5 500 1100 18 NA
Terpinyl formate (367)
Europe 1 0.1 0.002 0.002
United States 10 2 0.03 0.03
Terpinyl acetate (368)
Europe 1 800 260 4 3
United States 2 000 390 6 5
Terpinyl propionate (369)
Europe 0.2 0.03 0.0005 0.0004
United States 5 0 0.02 0.01
Terpinyl butyrate (370)
Europe 42 6 0.10 0.07
United States 33 6 0.10 0.07
Terpinyl isobutyrate (371)
Europe 5 0.7 0.01 0.00
United States 0.1 0.02 0.0003 0.0002
Terpinyl isovalerate (372)
Europe 1 0.1 0.002 0.002
United States 5 0 0.02 0.01
para-Menth-3-en-1-ol (373)
Europe 290 41 0 NA
United States 2 0.4 0.00 NA
4-Carvomenthenol (439)
Europe 1200 170 3 NA
United States 270 51 0 NA
para-Menth-8-en-1-ol (374)
Europe 11 2 0.03 NA
United States 110 21 0.3 NA
2-Ethyl-1,3,3-trimethyl-2-norbornol (440)
Europe 6.1 1 0.02 NA
United States 0.1 0.02 0.0003 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Thujanol (441)
Europe 7.5 1 0.02 NA
United States 0.2 0.04 0.0006 NA
Methyl 1-acetoxycyclohexyl-ketone (442)
Europe NR
United States 21 4 0.07 NA
Total
Europe 58 000 NA NA NA
United States 15 000 NA NA NA
Total linalool
Europe NA 4300 72 28
United States NA 1300 21 3
Total alpha-terpineol
Europe NA 3200 53 4
United States NA 1400 23 5
NR, not reported; NA, not applicable; +, reported to occur naturally in foods (Maase et al.,
1994), but no quantitative data available; -, not reported to occur naturally in foods
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor
Industry, 1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the
survey. Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where
body weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
In a study of hydrolysis in vitro, linalyl acetate was easily
hydrolysed in water and simulated gastric and pancreatic fluids, with
mean half-lives of 5.5 min in gastric fluid and 53 min in pancreatic
fluid (Hall, 1979). Terpenoid alcohols formed in the gastrointestinal
tract are rapidly absorbed (Phillips et al., 1976; Diliberto et al.,
1988).
In humans and animals, terpenoid tertiary alcohols are conjugated
primarily with glucuronic acid and are excreted in the urine and
faeces (Williams, 1959; Parke et al., 1974a,b; Horning et al., 1976;
Ventura et al., 1985). Unsaturated terpenoid alcohols may undergo
allylic oxidation to form polar diol metabolites, which may be
excreted either free or conjugated. If the diol contains a primary
alcohol function, it may under-go further oxidation to the
corresponding carboxylic acid (Madyastha & Srivatsan, 1988; Horning et
al., 1976; Ventura et al., 1985).
The metabolic fate of the aliphatic tertiary alcohol linalool
(No. 356) has been studied in mammals (Figure 1). Linalool labelled
with 14C in positions 1 and 2 was administered orally to rats at a
single dose of 500 mg/kg bw. The majority (55%) of the radioactivity
was excreted in the urine as the glucuronic acid conjugate, while 23%
was excreted as carbon dioxide in expired air, and 15% was excreted in
the faeces within 72 h of administration; only 3% of the radiolabel
was detected in tissues after 72 h, with 0.5% in the liver, 0.6% in
the gut, 0.8% in the skin, and 1.2% in skeletal muscle (Parke et al.,
1974a). Reduction metabolites such as dihydro- and tetrahydrolinalool
were identified in the urine after administration of a single dose of
linalool to rats (Rahman, 1974).
N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step A3 of the procedure;
N/D, no intake data reported
a The group ADI of 0-0.05 mg/kg bw established at the twenty-third meeting for citral, citronellol, linalool, and linalyl acetate as citral
was maintained.
The substances in this group are structurally related because
they each contain a tertiary alcohol function (without aryl
substitution); they therefore have similar metabolic and toxicological
profiles. Four esters of the tertiary alcohol linalool (linalyl
anthranilate, linalyl benzoate, linalyl cinnamate, and linalyl
phenylacetate) and one ester of alpha-terpineol (terpinyl cinnamate)
used as flavouring substances in Europe and the United States are not
included in this group because they may have different toxicological
profiles, owing to their carboxylic acid components. It would be
appropriate to evaluate these esters in subsequent groups, with
anthranilic acid, benzoic acid, cinnamic acid, phenylacetic acid, and
related substances used as flavouring substances. Any additional
intake of linalool and alpha-terpineol from these four esters is
minimal and would not have a significant impact on the total combined
intake of linalool, alpha-terpineol, and related tertiary alcohols and
esters (see section 1.5).
2.2 Intake
The total annual volume of the 23 tertiary alcohols and related
esters is approximately 58 000 kg in Europe (International
Organization of the Flavor Industry, 1995) and 15 000 kg in the United
States (US National Academy of Sciences, 1987). Production volumes and
intake levels of each flavouring substance are reported in Table 2.
Thirteen tertiary alcohols and related esters have been reported
to occur naturally in foods (Maarse et al., 1994); quantitative data
reported for eight of these substances (see Table 2) indicate that
they are consumed predominantly from traditional foods (Stofberg &
Kirschman, 1985; Stofberg & Grundschober, 1987).
2.3 Biological data
2.3.1 Absorption and metabolism
In general, esters are hydrolysed to their corresponding alcohol
and carboxylic acid. Hydrolysis is catalysed by classes of enzymes
recognized as carboxylesterases or esterases (Heymann, 1980), the most
important of which are the B-esterases. In mammals, these enzymes
occur in most tissues throughout the body (Anders, 1989; Heymann,
1980) but predominate in hepatocytes (Heymann, 1980).
Esters of linalool (Nos 358-365) and alpha-terpineol (Nos
367-372) are expected to be hydrolysed in humans to yield linalool and
alpha-terpineol, respectively, and the corresponding saturated
aliphatic carboxylic acid. Methyl 1-acetoxycyclohexyl ketone (No. 442)
is expected to be hydrolysed to acetic acid and methyl
1-hydroxycyclohexyl ketone.
Table 2. Most recent annual usage of aliphatic acyclic and alicyclic terpenoid tertiary
alcohols and structurally related substances as flavouring substances in Europe and the
United States
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
Linalool (356)
Europe 18 000 2600 44 NA
United States 5 800 1100 19 NA
Tetrahydrolinalool (357)
Europe 390 55 0 NA
United States 0.5 0.1 0.002 NA
Linalyl formate (358)
Europe 57 8 0.14 0.1
United States 70 13 0.22 0.2
Linalyl acetate (359)
Europe 14 000 2100 35 27
United States 970 180 3 2
Linalyl propionate (360)
Europe 110 16 0.3 0.2
United States 8 2 0.03 0.02
Linalyl butyrate (361)
Europe 69 10 0.2 0.1
United States 22 4 0.07 0.05
Linalyl isobutyrate (362)
Europe 250 36 0.6 0.4
United States 3 0 0.00 0.00
Linalyl isovalerate (363)
Europe 38 5 0.0 0.06
United States 34 6 0.11 0.07
Linalyl hexanoate (364)
Europe 7 0 0.02 0.01
United States 2 0.4 0.00 0.004
Linalyl octanoate (365)
Europe 1 0.1 0.002 0.001
United States 5 0 0.02 0.00
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
alpha-Terpineol (366)
Europe 21 000 3000 50 NA
United States 5 500 1100 18 NA
Terpinyl formate (367)
Europe 1 0.1 0.002 0.002
United States 10 2 0.03 0.03
Terpinyl acetate (368)
Europe 1 800 260 4 3
United States 2 000 390 6 5
Terpinyl propionate (369)
Europe 0.2 0.03 0.0005 0.0004
United States 5 0 0.02 0.01
Terpinyl butyrate (370)
Europe 42 6 0.10 0.07
United States 33 6 0.10 0.07
Terpinyl isobutyrate (371)
Europe 5 0.7 0.01 0.00
United States 0.1 0.02 0.0003 0.0002
Terpinyl isovalerate (372)
Europe 1 0.1 0.002 0.002
United States 5 0 0.02 0.01
para-Menth-3-en-1-ol (373)
Europe 290 41 0 NA
United States 2 0.4 0.00 NA
4-Carvomenthenol (439)
Europe 1200 170 3 NA
United States 270 51 0 NA
para-Menth-8-en-1-ol (374)
Europe 11 2 0.03 NA
United States 110 21 0.3 NA
2-Ethyl-1,3,3-trimethyl-2-norbornol (440)
Europe 6.1 1 0.02 NA
United States 0.1 0.02 0.0003 NA
Table 2. (continued)
Substance (No.) Most recent Per capita intakea Per capita intakeb,
annual volume alcohol equivalents
(kg) µg/day µg/kg bw (µg/kg bw per day)
per day
4-Thujanol (441)
Europe 7.5 1 0.02 NA
United States 0.2 0.04 0.0006 NA
Methyl 1-acetoxycyclohexyl-ketone (442)
Europe NR
United States 21 4 0.07 NA
Total
Europe 58 000 NA NA NA
United States 15 000 NA NA NA
Total linalool
Europe NA 4300 72 28
United States NA 1300 21 3
Total alpha-terpineol
Europe NA 3200 53 4
United States NA 1400 23 5
NR, not reported; NA, not applicable; +, reported to occur naturally in foods (Maase et al.,
1994), but no quantitative data available; -, not reported to occur naturally in foods
a Intake (µg/day) calculated as follows:
[(annual volume, kg) × (1 × 109 µg/kg)]/[population × 0.6 × 365 days], where population
(10% 'eaters only') = 32 × 106 for Europe (International Organization of the Flavor
Industry, 1995) and 24 × 106 for the United States (US National Academy of Sciences, 1989);
0.6 represents the assumption that only 60% of the flavour volume was reported in the
survey. Intake (µg/kg bw per day) calculated as follows: [(µg/day)/body weight], where
body weight = 60 kg. Slight variations may occur due to rounding off.
b Calculated as follows: (molecular mass alcohol/molecular mass ester) × daily per capita
intake ester
In a study of hydrolysis in vitro, linalyl acetate was easily
hydrolysed in water and simulated gastric and pancreatic fluids, with
mean half-lives of 5.5 min in gastric fluid and 53 min in pancreatic
fluid (Hall, 1979). Terpenoid alcohols formed in the gastrointestinal
tract are rapidly absorbed (Phillips et al., 1976; Diliberto et al.,
1988).
In humans and animals, terpenoid tertiary alcohols are conjugated
primarily with glucuronic acid and are excreted in the urine and
faeces (Williams, 1959; Parke et al., 1974a,b; Horning et al., 1976;
Ventura et al., 1985). Unsaturated terpenoid alcohols may undergo
allylic oxidation to form polar diol metabolites, which may be
excreted either free or conjugated. If the diol contains a primary
alcohol function, it may under-go further oxidation to the
corresponding carboxylic acid (Madyastha & Srivatsan, 1988; Horning et
al., 1976; Ventura et al., 1985).
The metabolic fate of the aliphatic tertiary alcohol linalool
(No. 356) has been studied in mammals (Figure 1). Linalool labelled
with 14C in positions 1 and 2 was administered orally to rats at a
single dose of 500 mg/kg bw. The majority (55%) of the radioactivity
was excreted in the urine as the glucuronic acid conjugate, while 23%
was excreted as carbon dioxide in expired air, and 15% was excreted in
the faeces within 72 h of administration; only 3% of the radiolabel
was detected in tissues after 72 h, with 0.5% in the liver, 0.6% in
the gut, 0.8% in the skin, and 1.2% in skeletal muscle (Parke et al.,
1974a). Reduction metabolites such as dihydro- and tetrahydrolinalool
were identified in the urine after administration of a single dose of
linalool to rats (Rahman, 1974).
 In a separate study, male IISc strain rats were given daily oral
doses of 800 mg/kg bw linalool for 20 days. Urinary metabolites formed
by cytochrome P450 (CYP450)-induced allylic oxidation of linalool
included 8-hydroxylinalool and 8-carboxylinalool. CYP450 activity in
liver microsomes was increased by about 50% after three days, but the
activity had decreased to control values after six days (Chadha &
Madyastha, 1984). Linalool administered to four-week-old male Wistar
rats by gavage at a dose of 500 mg/kg bw per day for 64 days did not
induce CYP450 until the 30th day of treatment (Parke et al., 1974b).
These results suggest that glucuronic acid conjugation and
excretion are the primary route of metabolism of linalool and allylic
oxidation becomes an important pathway only after repeated dosing. It
has been suggested that biotransformation of the diol metabolite of
linalool to the corresponding aldehyde by the action of the
NAD+-dependent enzyme alcohol dehydrogenase is inhibited because of
the bulky nature of the neighbouring alkyl substituents and the
substrate specificity of the enzyme (Eder et al., 1982).
When male albino IISc strain rats were given the alicyclic
tertiary alcohol alpha-terpineol (No. 366) orally at a daily dose of
600 mg/kg bw for 20 days, oxidation of the allylic methyl group was
observed to yield the corresponding carboxylic acid which was
hydrogenated to a small extent to yield the corresponding saturated
carboxylic acid (Madyastha & Srivatsan, 1988; Figure 2). When
alpha-terpineol was administered orally to rats, it increased the
liver microsomal P450 content and the activity of NADPH-cytochrome c
reductase (Madyastha & Srivatsan, 1988), suggesting that the oxidation
is mediated by CYP450.
In a separate study, male IISc strain rats were given daily oral
doses of 800 mg/kg bw linalool for 20 days. Urinary metabolites formed
by cytochrome P450 (CYP450)-induced allylic oxidation of linalool
included 8-hydroxylinalool and 8-carboxylinalool. CYP450 activity in
liver microsomes was increased by about 50% after three days, but the
activity had decreased to control values after six days (Chadha &
Madyastha, 1984). Linalool administered to four-week-old male Wistar
rats by gavage at a dose of 500 mg/kg bw per day for 64 days did not
induce CYP450 until the 30th day of treatment (Parke et al., 1974b).
These results suggest that glucuronic acid conjugation and
excretion are the primary route of metabolism of linalool and allylic
oxidation becomes an important pathway only after repeated dosing. It
has been suggested that biotransformation of the diol metabolite of
linalool to the corresponding aldehyde by the action of the
NAD+-dependent enzyme alcohol dehydrogenase is inhibited because of
the bulky nature of the neighbouring alkyl substituents and the
substrate specificity of the enzyme (Eder et al., 1982).
When male albino IISc strain rats were given the alicyclic
tertiary alcohol alpha-terpineol (No. 366) orally at a daily dose of
600 mg/kg bw for 20 days, oxidation of the allylic methyl group was
observed to yield the corresponding carboxylic acid which was
hydrogenated to a small extent to yield the corresponding saturated
carboxylic acid (Madyastha & Srivatsan, 1988; Figure 2). When
alpha-terpineol was administered orally to rats, it increased the
liver microsomal P450 content and the activity of NADPH-cytochrome c
reductase (Madyastha & Srivatsan, 1988), suggesting that the oxidation
is mediated by CYP450.
 In a minor pathway, the endocyclic alkene of alpha-terpineol is
epoxidized and then hydrolysed to yield a triol metabolite
1,2,8-trihydroxy- para-menthane, which was also reported in humans
after inadvertent oral ingestion of a pine-oil disinfectant containing
alpha-terpineol (Horning et al., 1976). It is expected that after
single doses, alpha-terpineol would be metabolized like linalool
(Chadha & Madyastha, 1984), primarily by glucuronic acid conjugation
and excretion in the urine.
Bicyclic tertiary alcohols (Nos 440 and 441) are relatively
stable in vivo, but are eventually conjugated with glucuronic acid
and excreted. In rabbits, the structurally related bicyclic tertiary
alcohols thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo(2.2.1)heptan-2-ol) are conjugated with
glucuronic acid (Williams, 1959).
In a study of the metabolism of the structurally related
terpenoid tertiary alcohol trans-sobrerol in humans, dogs, and rats,
10 metabolites were isolated in urine, eight of which were found in
humans. Two principal modes of metabolism were observed: allylic
oxidation of the ring positions and alkyl substituents and conjugation
of the tertiary alcohol functions with glucuronic acid. These
metabolic patterns are common modes of converting tertiary (Ventura et
al., 1985) and secondary (Yamaguchi et al., 1994) terpenoid alcohols
to polar metabolites, which are easily excreted in the urine and
faeces (see Figure 3). Menthol forms similar oxidation and conjugation
products in rats (Yamaguchi et al., 1994).
In a minor pathway, the endocyclic alkene of alpha-terpineol is
epoxidized and then hydrolysed to yield a triol metabolite
1,2,8-trihydroxy- para-menthane, which was also reported in humans
after inadvertent oral ingestion of a pine-oil disinfectant containing
alpha-terpineol (Horning et al., 1976). It is expected that after
single doses, alpha-terpineol would be metabolized like linalool
(Chadha & Madyastha, 1984), primarily by glucuronic acid conjugation
and excretion in the urine.
Bicyclic tertiary alcohols (Nos 440 and 441) are relatively
stable in vivo, but are eventually conjugated with glucuronic acid
and excreted. In rabbits, the structurally related bicyclic tertiary
alcohols thujyl alcohol and ß-santenol
(2,3,7-trimethylbicyclo(2.2.1)heptan-2-ol) are conjugated with
glucuronic acid (Williams, 1959).
In a study of the metabolism of the structurally related
terpenoid tertiary alcohol trans-sobrerol in humans, dogs, and rats,
10 metabolites were isolated in urine, eight of which were found in
humans. Two principal modes of metabolism were observed: allylic
oxidation of the ring positions and alkyl substituents and conjugation
of the tertiary alcohol functions with glucuronic acid. These
metabolic patterns are common modes of converting tertiary (Ventura et
al., 1985) and secondary (Yamaguchi et al., 1994) terpenoid alcohols
to polar metabolites, which are easily excreted in the urine and
faeces (see Figure 3). Menthol forms similar oxidation and conjugation
products in rats (Yamaguchi et al., 1994).
 After hydrolysis of linalyl and terpinyl esters, the component
aliphatic carboxylic acids undergo ß-oxidation and cleavage to
eventually yield carbon dioxide and water. Successive two-carbon units
are removed from the carbonyl end, which cleaves acids with even
numbers of carbons to acetyl coenzyme A and those with odd numbers of
carbons to acetyl and propionyl coenzyme A. Acetyl coenzyme A enters
the citric acid cycle directly or reacts with propionyl coenzyme A to
form succinyl coenzyme A, which also enters the citric acid cycle
(Voet & Voet, 1990).
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 16 of the 23 substances
in this group. They range from 1300 to > 36 000 mg/kg bw, indicating
that the acute toxicity of terpenoid tertiary alcohols and related
esters after oral exposure is low (Jenner et al., 1964; Moreno, 1977).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of studies with representative terpenoid tertiary
alcohols, esters, and related substances are summarized in Table 3 and
described below.
Linalool
In a safety evaluation, a 50:50 mixture of linalool and
citronellol was fed to male and female rats (numbers and strain
unspecified) in the diet. The daily intake was calculated to be 50
mg/kg bw of each substance. Haematological, clinical chemical, and
urinary analyses at weeks 6 and 12 showed no statistically significant
difference between treated and control groups. Histopathology revealed
no dose-related lesions. A slight retardation of growth was observed
in males, but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalool that had no adverse
effect (50 mg/kg bw) is > 1000 times the daily per capita intake of
44 or 19 µg/kg bw from use of linalool as a flavouring substance in
Europe and the United States, respectively, and > 500 times the total
daily per capita intake of 72 or 21 µg/kg bw from use of linalool
and its esters (i.e. alcohol equivalents) as flavouring substances in
Europe and the United States, respectively.
Linalyl esters
A mixture of linalyl acetate, linalyl isobutyrate, and geranyl
acetate was added to the diet of male and female rats (strain not
specified) at concentrations calculated to result in average daily
intakes of 24, 27, or 48 mg/kg bw, respectively, for 12 weeks.
Haematological, clinical chemical, and urinary analyses at weeks 6 and
12 showed normal values. Histopathological examination revealed no
dose-related lesions. Slight retardation of growth was observed in
females but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalyl acetate that had no
adverse effect (24 mg/kg bw per day) is > 500 times the daily
per capita intake of 34 µg/kg bw from use of linalyl acetate as a
flavouring substance in Europe and > 1000 times the daily per
capita intake of 3 µg/kg bw in the United States. Similarly, the
dose of linalyl isobutyrate that had no adverse effect (27 mg/kg bw
per day) is > 10 000 times the daily per capita intake of 1 µg/kg
bw from use of linalyl isobutyrate as a flavouring substance in Europe
and > 1 000 000 times the daily per capita intake of 0.01 µg/kg bw
in the United States.
Four groups of 10 male and 10 female Osborne-Mendel rats were fed
the structurally related ester of linalool, linalyl cinnamate, at
dietary concentrations of 0, 1000, 2500, or 10 000 mg/kg for 17 weeks.
These concentrations were calculated (US Food and Drug Administration,
1993) to provide intakes of 0, 50, 125, and 500 mg/kg bw per day,
respectively. Body weights were measured and haematological and gross
examinations were performed on all treated animals; only those at the
high dose were examined microscopically. No differences were found
between treated and control animals (Hagan et al., 1967).
Terpinyl acetate
Groups of 10 male and 10 female weanling Osborne-Mendel rats were
fed terpinyl acetate in the diet for 20 weeks at concentrations of 0,
1000, 2500, or 10 000 mg/kg (Hagan et al., 1967). These dietary levels
were calculated (US Food and Drug Administration, 1993) to result in
intakes of 0, 50, 125, and 500 mg/kg bw per day, respectively. All
animals were examined for changes in growth, haematological
parameters, and gross histological appearance. Microscopic examination
was performed on six to eight male and female animals at the high dose
and in the control groups. No statistically significant adverse
effects were reported (Hagan et al., 1967). The dose of terpinyl
acetate that had no adverse effects (500 mg/kg bw per day) is
> 50 000 times the daily per capita intake of 6 µg/kg bw from its
use as a flavouring substance in Europe and the United States. This
dose is > 5000 times the total daily per capita intakes of 53 or 23
µg/kg bw from use of alpha-terpineol and its esters (i.e. alcohol
equivalents) as flavouring substances in Europe and the United States.
Geranyl acetate and citronellyl acetate
The Committee previously evaluated linalool and linalyl acetate,
at the twenty-third meeting in 1979 (Annex 1, reference 50) as part of
a group of terpenoid flavouring substances including citral,
citronellol, and geranyl acetate. Although the Committee established a
group ADI of 0-0.5 mg/kg bw, they maintained that a long-term study
was required for at least one member of the group. Since that time,
long-term studies have been performed in rats and mice of a mixture of
the structurally related terpenoid ester geranyl acetate (71%) and
citronellyl acetate (29%) (US National Toxicology Program, 1987).
These studies were considered by the Committee at its forty-ninth
meeting (Annex 1, reference 131) in a safety evaluation of the group
of flavouring substances that includes 26 geranyl, neryl, citronellyl,
and rhodinyl esters formed from branched-chain terpenoid primary
alcohols and branched-chain carboxylic acids. The Committee determined
that the study of carcinogenicity in mice was inadequate owing to
dosing errors and the low survival associated with infections. The
NOEL of the mixture in rats was 1000 mg/kg bw per day, which
corresponds to estimated doses of 710 mg/kg bw per day geranyl acetate
and 290 mg/kg bw per day citronellyl acetate. This NOEL is > 10 000
times the estimated daily per capita intakes of 44 and 19 µg/kg bw
per day from use of the structurally related substance linalool as a
flavouring substance in Europe and the United States, respectively.
2.3.2.3 Genotoxicity
Five representative alcohols and esters in this group have been
tested for genotoxicity, including a complete battery of tests with
linalool in vitro. The results of these tests are summarized in
Table 4 and described below.
Linalool (No. 356) was inactive in Salmonella typhimurium
strains TA92, TA94, TA98, TA100, TA1535, TA1537, and TA1538 with and
without metabolic activation (Rockwell & Raw, 1979; Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
Linalool did not induce chromosomal aberrations when incubated with
Chinese hamster fibroblasts at a maximum concentration of 0.25 mg/ml
(Ishidate et al., 1984), nor did it induce unscheduled DNA synthesis
in rat hepatocytes at concentrations up to 50 µg/ml (Heck et al.,
1989).
Linalool did not induce mutation in E. coli WP2 uvrA at
concentrations of 0.125-1 mg/plate (Yoo, 1986). When incubated with
Bacillus subtilis H17 (rec+) and M45 (rec-), linalool was
not mutagenic at 17 µg/plate (Oda et al., 1978) but was mutagenic at
10 µl/disc (Yoo, 1986). Linalool was inactive in mouse lymphoma L5178Y
tk+/- cells with metabolic activation at 200 µg/ml but gave a
weakly positive response without activation at 150 µg/ml (Heck et al.,
1989).
The 24-h urine of Sprague-Dawley rats given 0.5 ml of linalool or
ß-terpineol (No. 374) was incubated with S. typhimurium strains TA98
and TA100 to observe mutagenicity. The following assays were
conducted: linalool or ß-terpineol (with metabolic activation),
aliquot of 24-h urine (with and without metabolic activation), ether
extract of 24-h urine (with and without metabolic activation), and
aqueous phase of 24-h urine ether extract (with and without metabolic
activation). The urine samples were diluted to 60 ml and incubated in
the presence of chloroform and ß-glu-curonidase before ether
extraction. The only positive response was found with the ether
extract of ß-terpineol.
Table 3. Short-term and long-term studies of toxicity and carcinogenicity and special studies of aliphatic acyclic and alicyclic
terpenoid tertiary alcohols and structurally related substances
Substance No. Species Sex No. groups/ Route Time NOEL Reference
no. per group (mg/kg bw
per day)
Linalool 356 Rat M/F 1/-- Dieta 84 days 50b Oser (1967)
Linalyl acetate 357 Rat M/F 1/NR Dietc 84 days 24b Oser (1967)
Linalyl isobutyrate 362 Rat M/F 1/NR Dietd 84 days 27b Oser (1967)
Terpinyl acetate 368 Rat M/F 3/20 Diete 140 days 500b Hagan et al. (1967)
Linalyl cinnamatee None Rat M/F 4/20 Dietf 17 weeks 500b Hagan et al. (1967)
Geranyl acetate/ None Mouse M/F 2/50 Gavage 103 weeks -g US National Toxicology
Citronellyl acetate Program(1987)
Geranyl acetate/ None Rat M/F 2/5 Gavage 103 weeks 1000 US National Toxicology
Citronellyl acetate (710 geranyl Program (1987)
acetate;
290 citronellyl
acetate)
Special study of immunotoxicity
Linalool 356 Mouse F 3/30 Gavage 5 375b Gaworski et al. (1994)
M, male; F, female; NR, not reported
a Administered with citronellol as part of a 50/50 mixture
b The study was performed at a single dose or multiple doses that had no adverse effects; therefore, no NOEL was determined. The NOEL
is probaby higher than the dose reported here, which is the highest dose that had no adverse effect.
c Administered with linalyl isobutyrate and geranyl acetate as part of a mixture
d Administered with linalyl acetate and geranyl acetate as part of a mixture
e Structurally related ester of linalool, not evaluated as part of this group
f Structurally related terpenoid esters administered as a mixture: geranyl acetate, 71%; citronellyl acetate, 29%
g There was no NOEL owing to a high incidence of gavage errors and low survival associated with pneumonia.
Table 4. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally
related substances
Substance No. End-point Test object Concentration Result Reference
Linalool 356 Reverse mutation S. typhimurium TA100 0.01-3 µl/2-ml Negativea Eder et al. (1980)
(modified test) incubation volume
Linalool 356 Reverse mutation S. typhimurium TA92, TA100, 1 mg/plateb Negativea Ishidate et al. (1984)
TA1535, TA1537, TA94, TA98
Linalool 356 Reverse mutation S. typhimurium TA98, TA100 0.05-100 µl/plate Negativea Rockwell & Raw (1979)
Linalool 356 Reverse mutation S. typhimurium TA100, TA1535, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1537, TA1538, TA98
Linalool 356 Gene mutation Mouse lymphoma L5178Y tk+/- 200 µg/ml Negativec Heck et al. (1989)
150 µg/ml Weakly
positived
Linalool 356 Unscheduled DNA Rat hepatocytes synthesis 50 µg/mlb Negativee Heck et al. (1989)
Linalool 356 Chromosomal Chinese hamster fibroblasts 0.25 mg/mlb Negatived Ishidate et al. (1984)
aberration
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 17 µg Negative Oda et al. (1978)
& M45 (rec-)
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 10 µl/discb Positive Yoo (1986)
& M45 (rec-)
Linalool 356 Gene mutation E. coli WP2 uvrA 0.125-1 mg/plate Negative Yoo (1986)
Linalyl acetate 359 Reverse mutation S. typhimurium TA1535, TA1537, 25 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
Linalyl acetate 359 Unscheduled DNA Fischer or Sprague-Dawley rat 300 µg/mlb Negativee Heck et al. (1989)
synthesis hepatocytes
Table 4. (continued)
Substance No. End-point Test object Concentration Result Reference
Linalyl acetate 359 Gene mutation Bacillus subtilis H17 (rec+) 18 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
alpha-Terpineol 366 Reverse mutation S. typhimurium TA1535, TA1537, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
alpha-Terpineol 366 Spot test S. typhimurium TA1535, TA1537, 3 µmol/plate Negativea Florin et al. (1980)
TA98, TA100
alpha-Terpineol 366 Gene mutation Mouse lymphoma L5178Y tk+/- 250 µg/mlb Negativec Heck et al. (1989)
300 µg/mlb Negatived
Terpinyl acetate 368 Gene mutation Bacillus subtilis H17 (rec+) 19 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
ß-Terpineol 374 Reverse mutation S. typhimurium TA100, TA98 0.05-100 µl/plate Negativec Rockwell & Raw (1979)
Terpineolf 366 Gene mutation S. cerevisiae Not reported Negativec Oda et al. (1978)
and/or
374
a With and without metabolic activation
b The highest inactive or lowest active concentration; range not specified
c With metabolic activation
d Without metabolic activation
e 75 or 150 cells analysed per dose
f Not specified whether alpha- or ß-terpineol
Linalyl acetate (No. 359) and alpha-terpineol (No. 366) were
inactive in S. typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538 with and without metabolic activation (Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
ß-Terpineol was inactive in S. typhimurium strains TA98 and TA100
with metabolic activation (Rockwell & Raw, 1979). When incubated with
B. subtilis H17 (rec+) and M45 (rec-), linalyl acetate and
terpinyl acetate were not mutagenic at 18 or 19 µg, respectively (Oda
et al., 1978).
Thus, all but two of 19 tests of the genotoxicity of terpenoid
tertiary alcohols and related esters in vitro gave negative results.
Linalool was active in a rec assay, in which differences in the
growth rates of two strains of B.subtilis are used as a measure of
DNA damage (Yoo, 1986). In contrast, no evidence of DNA damage was
observed in an assay for unscheduled DNA synthesis in rat hepatocytes
(Heck et al., 1989). The authors of a study in which linalool gave a
weak positive result in the mouse lymphoma assay (Heck et al., 1989)
emphasized that positive results are commonly observed for polar
substances in this assay in the presence of metabolic activation and
may be associated with changes in physiological culture conditions (pH
and osmolality). The weight of the evidence for terpenoid tertiary
alcohols and related esters indicates that this group of flavouring
substances would not be expected to have genotoxic potential
in vivo.
2.3.2.4 Other relevant studies
No adverse effects were reported at doses of linalool up to
375 mg/kg bw in a screening test in mice for humoral and cell-mediated
immune responses (see Table 4). Linalool in 1% methylcellulose was
administered intragastrically to 10-20 six- to eight-week-old female
B6C3F1 mice for five days at a dose of 94, 188, or 375 mg/kg bw per
day. Cell-mediated immunity was assessed in a host resistance assay
with a Listeria monocytogenes bacterial challenge. Humoral immunity
was measured by the antibody plaque-forming cell response to sheep
erythrocytes. Body weights, lymphoid organ weights, and spleen
cellularity were also measured. Cyclophosphamide was used as a
positive control. Linalool did not adversely modulate the immune
response in either assay (Gaworski et al., 1994).
4. REFERENCES
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard--A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro
mutagenic properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha,ß-unsaturated compounds: Considerations of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Gaworski, C.K., Vollmuth, T.A., Dozier, M.M., Heck, J.D., Dunn, L.T.,
Ratajczak, H.V. & Thomas, P.T. (1994) An immunotoxicity assessment of
food flavouring ingredients. Food Chem. Toxicol., 32, 409-415.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. J. Food Cosmet.
Toxicol., 5,141-147.
Hall, R.L. (1979) Unpublished report from McCormick & Co. Inc. to the
Flavor and Extracts Manufacturers' Association of the United States,
Washington DC, United States.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist., 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.J. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxide-diol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectroscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report from McCormick & Co. Inc.
to the Flavor and Extracts Manufacturers' Association of the United
States, Washington DC, United States.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., et al. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Srivatsan, V. (1988) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Moreno, O.M. (1977) Unpublished report to the Research Institute of
Fragrance Materials.Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen., 9, 177-181.
Oser, B.L. (1967) Unpublished report cited in FAO Nutrition Meeting
Report Series No. 44A, 1968 (Annex 1, reference 15).
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974a) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974b) Effect of linalool
on hepatic drug-metabolizing enzymes in the rat. Biochem. Soc.
Trans., 2 615-618.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D. & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14, 537-540.
Rahman, K.A.Q.M.Q. (1974) PhD thesis, University of Surrey. [Cited in
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.]
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs,
spices, and food additives. Nutr. Cancer, 1, 10-15.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC.
US National Toxicology Program (1987) Carcinogenesis Studies of
Food Grade Geranyl Acetate (71%) and Citronellyl Acetate (29%)
(NTP-TR-252; PB-88-2508), National Technical Information Services,
Research Triangle Park, North Carolina, United States.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Williams, R.T. (1959) Detoxication Mechanisms. The Metabolism and
Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
After hydrolysis of linalyl and terpinyl esters, the component
aliphatic carboxylic acids undergo ß-oxidation and cleavage to
eventually yield carbon dioxide and water. Successive two-carbon units
are removed from the carbonyl end, which cleaves acids with even
numbers of carbons to acetyl coenzyme A and those with odd numbers of
carbons to acetyl and propionyl coenzyme A. Acetyl coenzyme A enters
the citric acid cycle directly or reacts with propionyl coenzyme A to
form succinyl coenzyme A, which also enters the citric acid cycle
(Voet & Voet, 1990).
2.3.2 Toxicological studies
2.3.2.1 Acute toxicity
Oral LD50 values have been reported for 16 of the 23 substances
in this group. They range from 1300 to > 36 000 mg/kg bw, indicating
that the acute toxicity of terpenoid tertiary alcohols and related
esters after oral exposure is low (Jenner et al., 1964; Moreno, 1977).
2.3.2.2 Short-term and long-term studies of toxicity and
carcinogenicity
The results of studies with representative terpenoid tertiary
alcohols, esters, and related substances are summarized in Table 3 and
described below.
Linalool
In a safety evaluation, a 50:50 mixture of linalool and
citronellol was fed to male and female rats (numbers and strain
unspecified) in the diet. The daily intake was calculated to be 50
mg/kg bw of each substance. Haematological, clinical chemical, and
urinary analyses at weeks 6 and 12 showed no statistically significant
difference between treated and control groups. Histopathology revealed
no dose-related lesions. A slight retardation of growth was observed
in males, but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalool that had no adverse
effect (50 mg/kg bw) is > 1000 times the daily per capita intake of
44 or 19 µg/kg bw from use of linalool as a flavouring substance in
Europe and the United States, respectively, and > 500 times the total
daily per capita intake of 72 or 21 µg/kg bw from use of linalool
and its esters (i.e. alcohol equivalents) as flavouring substances in
Europe and the United States, respectively.
Linalyl esters
A mixture of linalyl acetate, linalyl isobutyrate, and geranyl
acetate was added to the diet of male and female rats (strain not
specified) at concentrations calculated to result in average daily
intakes of 24, 27, or 48 mg/kg bw, respectively, for 12 weeks.
Haematological, clinical chemical, and urinary analyses at weeks 6 and
12 showed normal values. Histopathological examination revealed no
dose-related lesions. Slight retardation of growth was observed in
females but was considered by the authors to be biologically
insignificant (Oser, 1967). The dose of linalyl acetate that had no
adverse effect (24 mg/kg bw per day) is > 500 times the daily
per capita intake of 34 µg/kg bw from use of linalyl acetate as a
flavouring substance in Europe and > 1000 times the daily per
capita intake of 3 µg/kg bw in the United States. Similarly, the
dose of linalyl isobutyrate that had no adverse effect (27 mg/kg bw
per day) is > 10 000 times the daily per capita intake of 1 µg/kg
bw from use of linalyl isobutyrate as a flavouring substance in Europe
and > 1 000 000 times the daily per capita intake of 0.01 µg/kg bw
in the United States.
Four groups of 10 male and 10 female Osborne-Mendel rats were fed
the structurally related ester of linalool, linalyl cinnamate, at
dietary concentrations of 0, 1000, 2500, or 10 000 mg/kg for 17 weeks.
These concentrations were calculated (US Food and Drug Administration,
1993) to provide intakes of 0, 50, 125, and 500 mg/kg bw per day,
respectively. Body weights were measured and haematological and gross
examinations were performed on all treated animals; only those at the
high dose were examined microscopically. No differences were found
between treated and control animals (Hagan et al., 1967).
Terpinyl acetate
Groups of 10 male and 10 female weanling Osborne-Mendel rats were
fed terpinyl acetate in the diet for 20 weeks at concentrations of 0,
1000, 2500, or 10 000 mg/kg (Hagan et al., 1967). These dietary levels
were calculated (US Food and Drug Administration, 1993) to result in
intakes of 0, 50, 125, and 500 mg/kg bw per day, respectively. All
animals were examined for changes in growth, haematological
parameters, and gross histological appearance. Microscopic examination
was performed on six to eight male and female animals at the high dose
and in the control groups. No statistically significant adverse
effects were reported (Hagan et al., 1967). The dose of terpinyl
acetate that had no adverse effects (500 mg/kg bw per day) is
> 50 000 times the daily per capita intake of 6 µg/kg bw from its
use as a flavouring substance in Europe and the United States. This
dose is > 5000 times the total daily per capita intakes of 53 or 23
µg/kg bw from use of alpha-terpineol and its esters (i.e. alcohol
equivalents) as flavouring substances in Europe and the United States.
Geranyl acetate and citronellyl acetate
The Committee previously evaluated linalool and linalyl acetate,
at the twenty-third meeting in 1979 (Annex 1, reference 50) as part of
a group of terpenoid flavouring substances including citral,
citronellol, and geranyl acetate. Although the Committee established a
group ADI of 0-0.5 mg/kg bw, they maintained that a long-term study
was required for at least one member of the group. Since that time,
long-term studies have been performed in rats and mice of a mixture of
the structurally related terpenoid ester geranyl acetate (71%) and
citronellyl acetate (29%) (US National Toxicology Program, 1987).
These studies were considered by the Committee at its forty-ninth
meeting (Annex 1, reference 131) in a safety evaluation of the group
of flavouring substances that includes 26 geranyl, neryl, citronellyl,
and rhodinyl esters formed from branched-chain terpenoid primary
alcohols and branched-chain carboxylic acids. The Committee determined
that the study of carcinogenicity in mice was inadequate owing to
dosing errors and the low survival associated with infections. The
NOEL of the mixture in rats was 1000 mg/kg bw per day, which
corresponds to estimated doses of 710 mg/kg bw per day geranyl acetate
and 290 mg/kg bw per day citronellyl acetate. This NOEL is > 10 000
times the estimated daily per capita intakes of 44 and 19 µg/kg bw
per day from use of the structurally related substance linalool as a
flavouring substance in Europe and the United States, respectively.
2.3.2.3 Genotoxicity
Five representative alcohols and esters in this group have been
tested for genotoxicity, including a complete battery of tests with
linalool in vitro. The results of these tests are summarized in
Table 4 and described below.
Linalool (No. 356) was inactive in Salmonella typhimurium
strains TA92, TA94, TA98, TA100, TA1535, TA1537, and TA1538 with and
without metabolic activation (Rockwell & Raw, 1979; Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
Linalool did not induce chromosomal aberrations when incubated with
Chinese hamster fibroblasts at a maximum concentration of 0.25 mg/ml
(Ishidate et al., 1984), nor did it induce unscheduled DNA synthesis
in rat hepatocytes at concentrations up to 50 µg/ml (Heck et al.,
1989).
Linalool did not induce mutation in E. coli WP2 uvrA at
concentrations of 0.125-1 mg/plate (Yoo, 1986). When incubated with
Bacillus subtilis H17 (rec+) and M45 (rec-), linalool was
not mutagenic at 17 µg/plate (Oda et al., 1978) but was mutagenic at
10 µl/disc (Yoo, 1986). Linalool was inactive in mouse lymphoma L5178Y
tk+/- cells with metabolic activation at 200 µg/ml but gave a
weakly positive response without activation at 150 µg/ml (Heck et al.,
1989).
The 24-h urine of Sprague-Dawley rats given 0.5 ml of linalool or
ß-terpineol (No. 374) was incubated with S. typhimurium strains TA98
and TA100 to observe mutagenicity. The following assays were
conducted: linalool or ß-terpineol (with metabolic activation),
aliquot of 24-h urine (with and without metabolic activation), ether
extract of 24-h urine (with and without metabolic activation), and
aqueous phase of 24-h urine ether extract (with and without metabolic
activation). The urine samples were diluted to 60 ml and incubated in
the presence of chloroform and ß-glu-curonidase before ether
extraction. The only positive response was found with the ether
extract of ß-terpineol.
Table 3. Short-term and long-term studies of toxicity and carcinogenicity and special studies of aliphatic acyclic and alicyclic
terpenoid tertiary alcohols and structurally related substances
Substance No. Species Sex No. groups/ Route Time NOEL Reference
no. per group (mg/kg bw
per day)
Linalool 356 Rat M/F 1/-- Dieta 84 days 50b Oser (1967)
Linalyl acetate 357 Rat M/F 1/NR Dietc 84 days 24b Oser (1967)
Linalyl isobutyrate 362 Rat M/F 1/NR Dietd 84 days 27b Oser (1967)
Terpinyl acetate 368 Rat M/F 3/20 Diete 140 days 500b Hagan et al. (1967)
Linalyl cinnamatee None Rat M/F 4/20 Dietf 17 weeks 500b Hagan et al. (1967)
Geranyl acetate/ None Mouse M/F 2/50 Gavage 103 weeks -g US National Toxicology
Citronellyl acetate Program(1987)
Geranyl acetate/ None Rat M/F 2/5 Gavage 103 weeks 1000 US National Toxicology
Citronellyl acetate (710 geranyl Program (1987)
acetate;
290 citronellyl
acetate)
Special study of immunotoxicity
Linalool 356 Mouse F 3/30 Gavage 5 375b Gaworski et al. (1994)
M, male; F, female; NR, not reported
a Administered with citronellol as part of a 50/50 mixture
b The study was performed at a single dose or multiple doses that had no adverse effects; therefore, no NOEL was determined. The NOEL
is probaby higher than the dose reported here, which is the highest dose that had no adverse effect.
c Administered with linalyl isobutyrate and geranyl acetate as part of a mixture
d Administered with linalyl acetate and geranyl acetate as part of a mixture
e Structurally related ester of linalool, not evaluated as part of this group
f Structurally related terpenoid esters administered as a mixture: geranyl acetate, 71%; citronellyl acetate, 29%
g There was no NOEL owing to a high incidence of gavage errors and low survival associated with pneumonia.
Table 4. Results of assays for the genotoxicity of aliphatic acyclic and alicyclic terpenoid tertiary alcohols and structurally
related substances
Substance No. End-point Test object Concentration Result Reference
Linalool 356 Reverse mutation S. typhimurium TA100 0.01-3 µl/2-ml Negativea Eder et al. (1980)
(modified test) incubation volume
Linalool 356 Reverse mutation S. typhimurium TA92, TA100, 1 mg/plateb Negativea Ishidate et al. (1984)
TA1535, TA1537, TA94, TA98
Linalool 356 Reverse mutation S. typhimurium TA98, TA100 0.05-100 µl/plate Negativea Rockwell & Raw (1979)
Linalool 356 Reverse mutation S. typhimurium TA100, TA1535, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1537, TA1538, TA98
Linalool 356 Gene mutation Mouse lymphoma L5178Y tk+/- 200 µg/ml Negativec Heck et al. (1989)
150 µg/ml Weakly
positived
Linalool 356 Unscheduled DNA Rat hepatocytes synthesis 50 µg/mlb Negativee Heck et al. (1989)
Linalool 356 Chromosomal Chinese hamster fibroblasts 0.25 mg/mlb Negatived Ishidate et al. (1984)
aberration
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 17 µg Negative Oda et al. (1978)
& M45 (rec-)
Linalool 356 Gene mutation Bacillus subtilis H17 (rec+) 10 µl/discb Positive Yoo (1986)
& M45 (rec-)
Linalool 356 Gene mutation E. coli WP2 uvrA 0.125-1 mg/plate Negative Yoo (1986)
Linalyl acetate 359 Reverse mutation S. typhimurium TA1535, TA1537, 25 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
Linalyl acetate 359 Unscheduled DNA Fischer or Sprague-Dawley rat 300 µg/mlb Negativee Heck et al. (1989)
synthesis hepatocytes
Table 4. (continued)
Substance No. End-point Test object Concentration Result Reference
Linalyl acetate 359 Gene mutation Bacillus subtilis H17 (rec+) 18 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
alpha-Terpineol 366 Reverse mutation S. typhimurium TA1535, TA1537, 10 000 µg/plateb Negativea Heck et al. (1989)
TA1538, TA98, TA100
alpha-Terpineol 366 Spot test S. typhimurium TA1535, TA1537, 3 µmol/plate Negativea Florin et al. (1980)
TA98, TA100
alpha-Terpineol 366 Gene mutation Mouse lymphoma L5178Y tk+/- 250 µg/mlb Negativec Heck et al. (1989)
300 µg/mlb Negatived
Terpinyl acetate 368 Gene mutation Bacillus subtilis H17 (rec+) 19 µg/plate Negative Oda et al. (1978)
& M45 (rec-)
ß-Terpineol 374 Reverse mutation S. typhimurium TA100, TA98 0.05-100 µl/plate Negativec Rockwell & Raw (1979)
Terpineolf 366 Gene mutation S. cerevisiae Not reported Negativec Oda et al. (1978)
and/or
374
a With and without metabolic activation
b The highest inactive or lowest active concentration; range not specified
c With metabolic activation
d Without metabolic activation
e 75 or 150 cells analysed per dose
f Not specified whether alpha- or ß-terpineol
Linalyl acetate (No. 359) and alpha-terpineol (No. 366) were
inactive in S. typhimurium strains TA98, TA100, TA1535, TA1537, and
TA1538 with and without metabolic activation (Eder et al., 1980;
Florin et al., 1980; Ishidate et al., 1984; Heck et al., 1989).
ß-Terpineol was inactive in S. typhimurium strains TA98 and TA100
with metabolic activation (Rockwell & Raw, 1979). When incubated with
B. subtilis H17 (rec+) and M45 (rec-), linalyl acetate and
terpinyl acetate were not mutagenic at 18 or 19 µg, respectively (Oda
et al., 1978).
Thus, all but two of 19 tests of the genotoxicity of terpenoid
tertiary alcohols and related esters in vitro gave negative results.
Linalool was active in a rec assay, in which differences in the
growth rates of two strains of B.subtilis are used as a measure of
DNA damage (Yoo, 1986). In contrast, no evidence of DNA damage was
observed in an assay for unscheduled DNA synthesis in rat hepatocytes
(Heck et al., 1989). The authors of a study in which linalool gave a
weak positive result in the mouse lymphoma assay (Heck et al., 1989)
emphasized that positive results are commonly observed for polar
substances in this assay in the presence of metabolic activation and
may be associated with changes in physiological culture conditions (pH
and osmolality). The weight of the evidence for terpenoid tertiary
alcohols and related esters indicates that this group of flavouring
substances would not be expected to have genotoxic potential
in vivo.
2.3.2.4 Other relevant studies
No adverse effects were reported at doses of linalool up to
375 mg/kg bw in a screening test in mice for humoral and cell-mediated
immune responses (see Table 4). Linalool in 1% methylcellulose was
administered intragastrically to 10-20 six- to eight-week-old female
B6C3F1 mice for five days at a dose of 94, 188, or 375 mg/kg bw per
day. Cell-mediated immunity was assessed in a host resistance assay
with a Listeria monocytogenes bacterial challenge. Humoral immunity
was measured by the antibody plaque-forming cell response to sheep
erythrocytes. Body weights, lymphoid organ weights, and spleen
cellularity were also measured. Cyclophosphamide was used as a
positive control. Linalool did not adversely modulate the immune
response in either assay (Gaworski et al., 1994).
4. REFERENCES
Anders, M. W. (1989) Biotransformation and bioactivation of
xenobiotics by the kidney. In: Hutson, D.H., Caldwell, J. & Paulson,
G.D., eds, Intermediary Xenobiotic Metabolism in Animals, New York,
Taylor & Francis, pp. 81-97.
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic
hazard--A decision tree approach. Food Cosmet. Toxicol., 16,
255-276.
Diliberto, J.J., Usha, G. & Birnbaum, L.S. (1988) Disposition of
citral in male Fischer rats. Drug Metab. Disposition, 16, 721-727.
Eder, E., Neudecker, T., Lutz, D. & Henschler, D. (1980) Mutagenic
potential of allyl and allylic compounds. Structure-activity
relationship as determined by alkylating and direct in vitro
mutagenic properties. Biochem. Pharmacol., 29, 993-998.
Eder, E., Henschler, D. & Neudecker, T. (1982) Mutagenic properties of
allylic and alpha,ß-unsaturated compounds: Considerations of
alkylating mechanisms. Xenobiotica, 12, 831-848.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Gaworski, C.K., Vollmuth, T.A., Dozier, M.M., Heck, J.D., Dunn, L.T.,
Ratajczak, H.V. & Thomas, P.T. (1994) An immunotoxicity assessment of
food flavouring ingredients. Food Chem. Toxicol., 32, 409-415.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I. &
Taylor, J.M. (1967) Food flavorings and compounds of related
structure. II. Subacute and chronic toxicity. J. Food Cosmet.
Toxicol., 5,141-147.
Hall, R.L. (1979) Unpublished report from McCormick & Co. Inc. to the
Flavor and Extracts Manufacturers' Association of the United States,
Washington DC, United States.
Heck, J.D., Vollmuth, T.A., Cifone, M.A., Jagannath, D.R., Myhr, B. &
Curren, R.D. (1989) An evaluation of food flavoring ingredients in a
genetic toxicity screening battery. Toxicologist., 9, 257.
Heymann, E. (1980) Carboxylesterases and amidases. In: Jacoby, W.B.,
ed., Enzymatic Basis of Detoxication, 2nd Ed., New York, Academic
Press, pp. 291-323.
Horning, M.G., Butler, C.M., Stafford, M., Stillwell, R.N., Hill,
R.M., Zion, T.E., Harvey, D.J. & Stillwell, W.G. (1976) Metabolism of
drugs by the epoxide-diol pathway. In: Frigerio, A. & Catagnoli, N.,
eds, Advances in Mass Spectroscopy in Biochemistry and Medicine, New
York, Spectrum Publications, Vol. I, pp. 91-108.
International Organization of the Flavor Industry (1995) European
inquiry on volume of use. Unpublished report from McCormick & Co. Inc.
to the Flavor and Extracts Manufacturers' Association of the United
States, Washington DC, United States.
Ishidate, M., Sofuni, T., Yoshikawa, K., Hayashi, M., Nohmi, T.,
Sawada, M., et al. (1984) Primary mutagenicity screening of food
additives currently used in Japan. Food Chem. Toxicol., 22, 623-636.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G.
(1964) Food flavourings and compounds of related structure. I. Acute
oral toxicity. Food Cosmet. Toxicol., 2, 327-343.
Maarse, H., Visscher, C.A., Willimsens, L.C., Nijssen, L.M. & Boelens,
M.H., eds (1994) Volatile Components in Food: Qualitative and
Quantitative Data, 7th Ed., Zeist, Centraal Instituut voor
Voedingsonderzoek TNO, Vol. III.
Madyastha, K.M. & Srivatsan, V. (1988) Biotransformations of
alpha-terpineol in the rat: Its effects on the liver microsomal
cytochrome P-450 system. Bull. Environ. Contam. Toxicol., 41, 17-25.
Moreno, O.M. (1977) Unpublished report to the Research Institute of
Fragrance Materials.Submitted to WHO by the Flavor and Extract
Manufacturers' Association of the United States, Washington DC, United
States
Oda, Y., Hamono, Y., Inoue, K., Yamamoto, H., Niihara, T. & Kunita, N.
(1978) Mutagenicity of food flavors in bacteria. Shokuhin Eisei
Hen., 9, 177-181.
Oser, B.L. (1967) Unpublished report cited in FAO Nutrition Meeting
Report Series No. 44A, 1968 (Annex 1, reference 15).
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974a) The absorption,
distribution and excretion of linalool in the rat. Biochem. Soc.
Trans., 2, 612-615.
Parke, D.V., Rahman, K.H.M.Q. & Walker, R. (1974b) Effect of linalool
on hepatic drug-metabolizing enzymes in the rat. Biochem. Soc.
Trans., 2 615-618.
Phillips, J.C. Kingsnorth, J., Gangolli, S.D. & Gaunt, I.F. (1976)
Studies on the absorption, distribution and excretion of citral in the
rat and the mouse. Food Chem. Toxicol., 14, 537-540.
Rahman, K.A.Q.M.Q. (1974) PhD thesis, University of Surrey. [Cited in
Chadha, A. & Madyastha, K.M. (1984) Metabolism of geraniol and
linalool in the rat and effects on liver and lung microsomal enzymes.
Xenobiotica, 14, 365-374.]
Rockwell, P. & Raw, I. (1979) A mutagenic screening of various herbs,
spices, and food additives. Nutr. Cancer, 1, 10-15.
Stofberg, J. & Grundschober, F. (1987) The consumption ratio and food
predominance of flavoring materials. Perfum. Flavorist, 12, 27-56.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of
flavoring materials: A mechanism for setting priorities for safety
evaluation. Food Chem. Toxicol., 23, 857-860.
US Food and Drug Administration (1993) Priority-based Assessment of
Food Additives (PAFA) Database, Center for Food Safety and Applied
Nutrition, Washington DC, United States, p. 58.
US National Academy of Sciences (1987) Evaluating the Safety of
Food Chemicals, Washington DC.
US National Toxicology Program (1987) Carcinogenesis Studies of
Food Grade Geranyl Acetate (71%) and Citronellyl Acetate (29%)
(NTP-TR-252; PB-88-2508), National Technical Information Services,
Research Triangle Park, North Carolina, United States.
Ventura, P., Schiavi, M., Serafini, S. & Selva, S. (1985) Further
studies of trans-sobrerol metabolism: Rat, dog, and human urine.
Xenobiotica, 15, 317-325.
Voet, D. & Voet, J.G. (1990) Biochemistry, New York, John Wiley &
Sons.
Williams, R.T. (1959) Detoxication Mechanisms. The Metabolism and
Detoxication of Drugs, Toxic Substances, and Other Organic
Compounds, 2nd Ed., London, Chapman & Hall, pp. 119-120.
Yamaguchi, T., Caldwell, J. & Farmer, P.B. (1994) Metabolic fate of
[3H]- l-menthol in the rat. Drug Metab. Disposition, 22, 616-624.
Yoo, Y.S. (1986) Mutagenic and antimutagenic activities of flavoring
agents used in foodstuffs. J. Osaka City Med. Center, 34, 267-288.
